User login
Hip pain in active patients: What you may be missing
• Consider both musculoskeletal and nonmusculoskeletal causes in patients with vague complaints of hip and groin pain. B
• Use imaging studies to confirm a hip pain diagnosis. B
• Refer patients who fail to respond to nonsurgical treatment to a sports medicine specialist or an orthopedic surgeon. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hip pain is a common complaint, and commonly misunderstood. Although the pain can be associated with a broad spectrum of conditions, the presentation is often vague and nonspecific.
Thus, hip pain and injury are frequently attributed, often incorrectly, to a “hip pointer”—a contusion of soft tissues against the iliac crest. It’s not unusual for patients who receive this diagnosis to be treated conservatively for prolonged periods, leading some previously active individuals to abandon their favorite sport or self-impose limits on the activities they engage in.1
But it doesn’t have to be this way.
Minimally invasive hip arthroscopy and advances in imaging, instrumentation, and devices have made it easier to identify and address underlying pathology associated with hip pain, helping patients return to their previous level of activity more rapidly.2,3 And, while many conditions associated with hip pain can be treated conservatively, family physicians—whom patients often go to first—should not hesitate to provide a referral when more aggressive treatment or diagnostic confirmation is needed.
We created this guide with family physicians in mind. Our focus here is primarily on anterior hip pain—the most common presentation—in active, or athletic, patients.
When did the pain begin? Where does it hurt?
Before performing a physical examination, find out as much as possible about the onset of pain and when and under what circumstances it occurs. (A review of hip anatomy is provided here.) Did it begin suddenly, after an acute injury or a particular physical maneuver? Or is the pain insidious, as was the case with one of our patients?
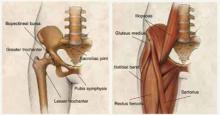
Osseous morphology of the hip includes the anterior superior iliac spine, the origin of the sartorius muscle and the ilioinguinal ligament. The anterior inferior iliac spine attaches to the rectus femoris, a major hip flexor and knee extender. The adductors of the hip originate in the anterior pelvic region.
The inguinal canal contains the ilioinguinal nerve, which is responsible for radiation of pain to the anterior hip. The hip joint itself is a spheroid comprising the femoral head and acetabulum, with most of the articular hip innervated by the femoral or obturator nerves.
Most intra-articular conditions radiate to the anterior groin or hip, although there are cases in which the pain is referred to either the lateral aspect of the hip or the buttocks. The iliopsoas muscle is the major hip flexor, and crosses under the ilioinguinal ligament to insert on the lesser tuberosity after crossing over the anterior capsule of the hip. A large bursa surrounds it, helping the tendon glide smoothly over the hip.
CASE Mack Q, a 27-year-old man with an 8-month history of right hip pain, sought care at our medical center for an achy pain in his right groin; he also described an occasional “clicking and popping sensation” in his groin but denied any trauma. The pain worsened with prolonged sitting and certain activities, such as squatting, twisting, and putting on shoes and socks. Our patient had stopped playing soccer because it hurt too much. He had tried physical therapy, oral anti-inflammatories, and a corticosteroid injection, with little relief.
Start with a gait assessment
The physical examination should begin with a gait assessment. Consider the patient’s ability to bear weight and his or her foot angle.
An individual with a stress fracture will have difficulty bearing weight on the affected side, resulting in a limp, or antalgic gait. A patient with femoral acetabular impingement (FAI) will often exhibit greater external rotation of the foot on the affected side compared with the other foot. And a patient with weakened abductor muscles, typically because of severe osteoarthritis, will exhibit the Trendelenburg sign—a pelvic tilt when the weight is shifted to the affected extremity.
Although most individuals with hip pain will not have an obvious gait abnormality, any patient who walks with a limp or needs crutches requires an immediate referral to an orthopedic surgeon.
Include these elements in the physical exam
Examine the hip with the patient sitting on the side of the exam table. Assess range of motion (ROM), comparing the range of flexion, extension, and internal/external rotation on the affected and unaffected sides. Include the following maneuvers:
Impingement testing. In patients with FAI and osteoarthritis, impingement testing—encompassing Flexion, ADDuction, and Internal Rotation (FADDIR)—will elicit pain. The maneuver can be tested starting at 45° of hip flexion, increasing to approximately 120°. Pain with <45° of hip flexion indicates that the impingement is severe.
Such testing can also reveal labral tears, which may be caused by FAI or other structural abnormalities. In a patient with anterior labral tears, FADDIR will produce groin pain; posterior labral tears will produce pain when the patient is sitting with legs hanging off the exam table and the contralateral leg is brought to the chest and the affected limb fully extended.
In patients with hip pain and bursitis, applying downward pressure will elicit a snapping sound as the iliopsoas snaps over the iliopectineal eminence or femoral head. Flexion, ABduction, and External Rotation (FABER) can also be used to diagnose iliopsoas tendonitis: The test is positive if it elicits pain in the affected extremity or in the sacroiliac joint on the opposite side.
Log roll. A painful response to this test, which involves internally and externally rotating the affected hip while it is relaxed and the knee fully extended, is an indication of synovitis of the hip caused by intra-articular pathology. To test hip stability, externally rotate the leg while it is extended. If the hip is stable, the leg will return to a neutral position; microinstability of the hip is likely if the leg remains in the rotated position.
Muscular strength testing. To assess for tendinopathy in the hip area, the patient should be in a seated position and contract the internal and external rotators and the adductor muscles while you apply resistance. To test abductor strength, have the patient assume a lateral position and hold and abduct the leg on the affected side while you apply resistance.
Hip flexion strength should be tested with the patient in both supine and seated positions. A patient with quadriceps tendonitis will have much greater pain with resisted hip flexion in the supine position vs the seated position; the opposite is true for a patient with iliopsoas tendonitis. (See “Did you know…? Hip pain facts and findings” on for additional diagnostic tips.)
- A patient with quadriceps tendonitis will have much greater pain with resisted hip flexion in a supine position vs a seated position. The opposite is true for a patient with iliopsoas tendonitis.
- Patients with femoral neck stress fractures typically present with activity-related anterior groin pain that is relieved by rest. Initially, they may be only mildly affected, but the condition worsens in those who continue to “work through the pain.”
- Plain radiography can confirm a diagnosis of osteonecrosis in patients with advanced disease, but magnetic resonance imaging is useful for evaluating earlier clinical presentations.
- Patients with labral tears often exhibit what has been called the “c-sign”—so named for the shape patients make with their hand as they grip their hip just above the greater trochanter to indicate where it hurts.
- Athletes who experience adductor strains often play sports in which kicking or frequent changes in direction are required, such as football, hockey, and soccer, and are generally able to tell you exactly what they were doing when the injury occurred.
- Unlike other hernias, a sports hernia (athletic pubalgia) does not involve a bulge of tissue protruding through one part of the body into another. Instead, it occurs when the oblique abdominal muscles strain or completely tear away from the pubis.
Perform a neurologic evaluation to rule out a back condition that might radiate pain into the anterior hip; ask the patient to do a sit-up while you apply resistance to test for abdominal wall pathology, as well.
Hip palpation. This aspect of the physical exam is important regardless of the cause of the pain but especially crucial for pediatric and adolescent patients, whose anterior hip pain may be related to apophyseal injury. Palpate the superior iliac spine (and over the inferior iliac spine in thin patients) to determine if the sartorius or rectus femoris has been injured. The area just lateral to the symphysis will be tender to palpation in patients with osteitis pubis.
Refer or treat? Here’s what to consider
While the history and physical should provide ample information for a differential diagnosis, imaging studies are generally required for confirmation. Clinical assessment— including physical exam, imaging, and intra-articular injection—of patients with hip pain is up to 98% accurate in identifying hip abnormalities, with arthroscopy as the gold standard.4
CASE On physical examination, Mr. Q had right hip extension to 0°, flexion to 110°, external rotation to 50°, and internal rotation to neutral; he also had positive impingement and subspine impingement tests, a painful arc of motion from 12 to 4 o’clock, tenderness over the hip adductor, and pain with resisted hip adduction. He did not walk with a limp.
Diagnostic studies included plain radiographs, which demonstrated that the joint space was well preserved. We identified subtle anatomical abnormalities on the femoral head-neck junction, known as a cam deformity. Magnetic resonance imaging (MRI) revealed an anterior superior labral tear with cartilage delamination.
Stress fractures affect runners, military recruits
In addition to long-distance runners who have recently increased the frequency, duration, or intensity of training,5,6 military recruits have a higher incidence of stress fractures due to the rapid onset of intensive training. Stress fractures can also occur in patients who do not have a history of intense activity but have metabolically weakened bone, in some cases as a result of an eating disorder.7
Patients with femoral neck stress fractures typically present with activity-related anterior groin pain that is relieved by rest; initially, they may be only mildly affected, but the condition worsens in those who continue to “work through the pain.” By the time such individuals seek treatment, they almost always have pain with weight bearing and an antalgic gait.
Symptoms consistent with a femoral neck stress fracture can be further evaluated with plain radiographs. However, x-rays are often negative for up to 4 weeks after the onset of pain.8 In cases in which radiographs are negative but the physical exam is suggestive of a stress fracture, MRI—which can detect an abnormality within a day or 2 of injury8,9—should be used to confirm the diagnosis (FIGURE 1).
FIGURE 1
MRI reveals a femoral neck stress fracture
Treatment. A complete femoral neck fracture portends impending displacement and requires emergent evaluation by an orthopedist, and superior neck changes, also known as tension-sided stress fractures, require urgent treatment with percutaneous screw fixation.9 However, compression-sided, or inferior, stress fractures can be treated with restricted weight bearing and activity modification. Gradual resumption of activity is allowed only after the patient has been asymptomatic for 6 weeks; recurrent pain indicates residual stress reaction, and signals that activities should be abated.
Osteonecrosis has many causes
Necrosis of the femoral head is a debilitating and progressive condition primarily affecting patients between the ages of 20 and 50 years.10 It has multiple (and diverse) causes, including trauma, steroids, alcohol, smoking, lupus, sickle cell anemia, and coagulopathies, as well as scuba diving. But about 20% of cases have no apparent cause.11,12
Patients with osteonecrosis of the hip typically present with groin pain, often described as a deep, intermittent ache that interferes with activities of daily living. Exam findings depend on the stage of presentation. Early on, pain will occur only with extreme ROM; in advanced cases, ROM is restricted and pain occurs even with limited motion.
Femoral head collapse due to loss of the structural integrity of the subchondral bone—which occurs in 80% of cases12—is thought to be caused by decreased blood flow. Plain radiography can confirm a diagnosis of osteonecrosis in patients with advanced disease, but MRI is useful for evaluating patients with earlier clinical presentations.
Treatment of osteonecrosis is dictated by the stage of the disease, but remains controversial because no intervention has been shown to prevent progression in all cases.12 All patients should be referred to a specialist. Those without collapse or cartilage damage can be treated surgically with core decompression, possibly with additional vascularized bone grafting,13,14 while those with more advanced disease typically require a total hip replacement at a relatively young age. Results for total hip replacement in patients with osteonecrosis are thought to be inferior to hip replacement in patients with osteoarthritis, although comparison is difficult because of the differences in age and activity levels in these 2 groups.15,16
Femoral acetabular impingement can occur on the cam or pincer side
FAI pathology can exist on either the femoral (cam) or acetabular (pincer) side,17 or both.18 In pure cam impingement, the anterior femoral neck loses its normal concave anatomy and develops a “bump,” which impinges on the anterosuperior labrum during hip flexion, causing labral tears and delamination of the adjacent cartilage.
Pure pincer impingement arises from a prominent acetabular rim, causing overcoverage of the femoral head. Acetabular labral tears result from the repetitive impaction with flexion and internal rotation.
Patients report an insidious onset of groin pain that is exacerbated by flexion-type sports, such as hockey, football, and golf,19 as well as activities of daily living. In patients with cartilage damage, even walking can be painful. Physical examination of patients with FAI reveals findings that are similar to those of patients with acetabular labral tears. Abnormally large cam lesions or acetabular overcoverage will result in restriction of hip ROM, especially internal rotation and flexion due to a mechanical block.
Radiographs (FIGURE 2) are essential to diagnose FAI and to distinguish this condition from an isolated labral tear.20 Cam impingement will be best demonstrated on a cross-table lateral radiograph, which shows an asphericity of the femoral head/neck junction anteriorly, while pincer impingement will show overcoverage of the femoral head on an AP radiograph. MRI or magnetic resonance arthrography (MRA) is frequently obtained to see whether any cartilage deterioration has occurred. Computed tomography, which can provide a 3-dimensional reproduction of the hip morphology, is often used for preoperative planning when surgical intervention is required.
FIGURE 2
Femoral acetabular impingement with a prominent pincer lesion
Treatment. Surgical intervention is often needed to correct or remove the abnormal anatomy, and both arthroscopic and open surgery are recommended.20 Both methods include osteoplasty at the femoral head/neck junction and/or the acetabular rim to allow the proximal femur to articulate with the acetabulum without injury to the labrum with flexion and internal rotation.21
Results of both open and arthroscopic osteoplasty of the femur and acetabulum are still preliminary, with only a few studies reporting mid-term results. Open surgery typically has longer recovery and rehabilitation, but advocates emphasize the improved ability to contour the femur or acetabulum. Both open and arthroscopic procedures have about an 8% to 13% rate of revision in short-term follow-up.17
Labral tears occur with trauma and certain sports
In addition to FAI, causes of labral tears include dysplasia, instability, trauma, and degeneration, as well as sports that require repetitive hip flexion and/or pivoting, such as hockey, soccer, and football.22,23
Patients with labral tears typically present with anterior hip pain radiating to the groin, worsening with twisting motions, running, walking, and sitting for prolonged periods. Clicking or catching may occur, as well. Patients may exhibit what one researcher called the “c-sign”—so named for the shape patients make with their hand as they grip their hip just above the greater trochanter to indicate where it hurts.4 The work-up for labral tears includes radiographs and, often, MRA, which is nearly 100% specific.24
Treatment. Conservative treatment, which may include activity modification or rest and ice, nonsteroidal anti-inflammatory drugs (NSAIDs), and physical therapy, is often effective for labral tears; when such measures fail, surgical intervention is indicated. A systematic review found a 67% satisfaction rate after 3.5 years in patients who had undergone labral debridement, and complete resolution of mechanical symptoms in nearly 50%.25 Another study showed similar results for hip arthroscopy, with symptom relief continuing for 4.8 years after surgery, on average, and 84% of patients able to return to their previous level of activity.26
The long-term results of labral debridement are unknown, however, and the possibility of an association between this procedure and the development of arthritis remains. Most specialists prefer anatomic repair to restore normal hip kinematics and, potentially, long-term hip function,27,28 but structural abnormalities must also be addressed to prevent failure of the repair or recurrent tears.
Iliopsoas tendonitis: You know the snap
Often referred to as internal snapping of the hip or internal coxa saltans, iliopsoas tendonitis/bursitis can be a recalcitrant cause of anterior hip pain. Snapping of the iliopsoas leading to bursitis or tendonitis can occur at the iliopectineal eminence, the femoral head, or the lesser trochanter.29 Runners and ballet dancers are often affected.30,31
Snapping in itself is not an indication of pathology, but chronicity of symptoms is. Patients with relatively acute symptoms typically have only bursitis, while a longer duration of symptoms leads to tendonitis or tendinopathy.32
Treatment. First-line therapy is nonoperative, and includes activity modification, rest, ice, NSAIDs, and physical therapy. Advise patients to refrain from activities causing pain, and to apply ice to the affected every 20 minutes (with a 20- to 30-minute off period) for one to 2 hours. Physical therapy focuses on stretching the iliopsoas and rectus femoris muscles and strengthening the hamstring muscles to relieve the stress on the anterior pelvis. If such treatment is unsuccessful, ultrasound can be used to guide a therapeutic injection of cortisone.33 If this fails to bring relief, fractional lengthening of the iliopsoas tendon can be performed to eliminate snapping and relieve pain.34
Muscular strains/avulsion fractures: Sports and age play a role
Although strains can affect any of the anterior muscles around the hip, in active individuals the adductors are most commonly affected. Skeletally immature patients are an exception: apophyseal fractures at the origin of the sartorius and rectus femoris muscles are more common than muscular strains in this patient population.
Athletes who experience adductor strains often play sports in which kicking or frequent changes in direction are required—eg, football, hockey, and soccer35—and generally are able to tell you exactly what they were doing when the injury occurred. Physical examination can reveal focal findings, with swelling and tenderness confined to the anteromedial aspect of the hip along the adductor muscle group. MRI can help differentiate the site of true pathology.36
Treatment of adductor strains is nonoperative, with rest, ice, and activity modification until the tendon heals. In the rare case in which complete tendon avulsion is found, surgical reattachment is needed.
Apophyseal fracture in skeletally immature patients typically occurs during participation in a sport that requires rapid acceleration and deceleration with the hip in an extended position. In such patients, stretching the affected muscle should reproduce the pain. Radiographs are diagnostic and will often show minimal displacement of the apophysis. Treatment is almost always nonoperative. Surgical intervention is rarely needed, and only indicated with displacement >2 cm.37
Athletic pubalgia: A challenging Dx
Also referred to as sports hernia, athletic pubalgia is an enigmatic cause of anterior hip pain in athletes. Diagnosis can be especially challenging, and patients may have lingering symptoms for years before the cause is discovered.38 A sports hernia, unlike other hernias, does not involve a bulge of tissue protruding through one body part into another. In contrast, a sports hernia occurs when the oblique abdominal muscles strain or completely tear away from the pubis. A recent systematic review found that the underlying etiology involves posterior inguinal wall weakening, which can be a result of poorly balanced hip adductor and abdominal muscle activation.39
Patients with sports hernia will often present with anterior hip and/or groin pain, especially with hip extension, twisting, and turning. In addition, patients can have pain in the lower abdomen and, in males, in the testicles. Physical examination will usually show pubic point tenderness, which is exacerbated by resisted hip adduction.40 MRI and ultrasound are extremely helpful in diagnosing and forming a treatment plan.39
The initial treatment of choice for sports hernias is nonoperative, and the first step is always activity modification or temporary avoidance of symptom-producing activities. Additional modalities include NSAIDs, ice, and physical therapy to strengthen the surrounding muscles. Surgical intervention, if needed, may be done laparoscopically or via an open approach with direct repair.40,41
Less common causes to consider
While the conditions detailed here account for most anterior hip etiologies, there are other less common causes to consider. One such cause is osteitis pubis, an umbrella term for conditions that affect the area surrounding the symphysis pubis. Patients with osteitis pubis present with pain over the anterior aspect of the pelvis that is worse with sit-ups, rising from a chair, or any activity where contraction of the rectus muscles occurs.29 Tenderness is found directly over and just lateral to the pubic symphysis. Radiographs are frequently negative, but occasionally chronic degenerative changes at the symphysis are present in addition to symphyseal narrowing. Additional imaging is often necessary for diagnosis.
Neuropathies. When history, physical examination, and imaging studies have ruled out other causes, neuropathies (ilioinguinal, genitofemoral, and obturator) should be considered, particularly in patients with vague, radiating anterior hip and/or groin pain.42 In pediatric patients, Legg-Calve-Perthes disease and slipped capital femoral epiphysis are possibilities, as well.
Getting patients back on track
Rehabilitation after hip injury resulting in anterior hip pain will be determined by the site, type, and mechanism of injury, as well as the severity. Restrictions in weight bearing and the use of an assistive device may be needed to prevent excessive stress on bone and supporting soft-tissue structures in the early stages of healing. Physical therapy, as needed, should initially focus on early controlled ROM of the hip joint to prevent both intra- and extra-articular adhesions and excessive scar tissue formation.2
For patients who undergo surgery, much of the focus will be on strengthening the supporting musculature—the hip abductor group, anterior and posterior thigh musculature, and core stabilizing muscles. Neuromuscular training may be needed to promote normal biomechanics and minimize compensatory movement patterns. For athletes, cardiovascular training and a return-to-play program should be implemented, as well.2,43,44
CASE Mr. Q was diagnosed with right hip pain due to a labral tear secondary to a cam femoral acetabular impingement. Given that he had failed nonoperative treatment and had long-standing pain, we recommended surgery for this patient. He underwent right hip arthroscopic labral repair, acetabular rim trimming, acetabular microfracture, femoral osteochondroplasty with capsular plication. At 12-month follow-up, he was doing well, with resolution of the presurgical pain and return to all athletic activities.
CORRESPONDENCE Rachel M. Frank, MD, Department of Orthopedic Surgery, Rush University Medical Center, 1611 West Harrison Street, Suite 300, Chicago, IL 60612; rmfrank3@gmail.com
1. Margo K, Drezner J, Motzkin D. Evaluation and management of hip pain: an algorithmic approach. J Fam Pract. 2003;52:607-617.
2. Leunig M, Beaule PE, Ganz R. The concept of femoroacetabular impingement: current status and future perspectives. Clin Orthop Relat Res. 2009;467:616-622.
3. Enseki KR, Martin RL, Draovitch P, et al. The hip joint: arthroscopic procedures and postoperative rehabilitation J Orthop Sports Phys Ther. 2006;36:516-525.
4. Byrd JW, Jones KS. Diagnostic accuracy of clinical assessment, magnetic resonance imaging, magnetic resonance arthrography, and intra-articular injection in hip arthroscopy patients. Am J Sports Med. 2004;32:1668-1674.
5. Fredericson M, Jennings F, Beaulieu C, et al. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17:309-325.
6. Matheson GO, Clement DB, McKenzie DC, et al. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15:46-58.
7. Stanitski CL, McMaster JH, Scranton PE. On the nature of stress fractures. Am J Sports Med. 1978;6:391-396.
8. Sofka CM. Imaging of stress fractures. Clin Sports Med. 2006;25:53-62, viii.
9. Shin AY, Gillingham BL. Fatigue fractures of the femoral neck in athletes. J Am Acad Orthop Surg. 1997;5:293-302.
10. Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459-474.
11. Lavernia CJ, Sierra RJ, Gomez-Marin O. Smoking and joint replacement: resource consumption and short-term outcome. Clin Orthop Relat Res. 1999;(367):172-180.
12. Lavernia CJ, Sierra RJ, Grieco FR. Osteonecrosis of the femoral head. J Am Acad Orthop Surg. 1999;7:250-261.
13. Smith SW, Fehring TK, Griffin WL, Beaver WB. Core decompression of the osteonecrotic femoral head. J Bone Joint Surg Am. 1995;77:674-680.
14. Fairbank AC, Bhatia D, Jinnah RH, et al. Long-term results of core decompression for ischaemic necrosis of the femoral head. J Bone Joint Surg Br. 1995;77:42-49.
15. Chandler HP, Reineck FT, Wixson RL, et al. Total hip replacement in patients younger than thirty years old. A five-year follow-up study. J Bone Joint Surg Am. 1981;63:1426-1434.
16. Wei SY, Klimkiewicz JJ, Lai M, et al. Revision total hip arthroplasty in patients with avascular necrosis. Orthopedics. 1999;22:747-757.
17. Bedi A, Chen N, Robertson W, et al. The management of labral tears and femoroacetabular impingement of the hip in the young, active patient. Arthroscopy. 2008;24:1135-1145.
18. Guanche CA, Bare AA. Arthroscopic treatment of femoroacetabular impingement. Arthroscopy. 2006;22:95-106.
19. Philippon M, Schenker M, Briggs K, et al. Femoroacetabular impingement in 45 professional athletes: Knee Surg Sports Traumatol Arthrosc. 2007;15:908-914.
20. Sierra RJ, Trousdale RT, Ganz R, et al. Hip disease in the young, active patient: evaluation and nonarthroplasty surgical options. J Am Acad Orthop Surg. 2008;16:689-703.
21. Byrd JW, Jones KS. Prospective analysis of hip arthroscopy with 10-year followup. Clin Orthop Relat Res. 2009;468:741-746.
22. Burnett RS, Della Rocca GJ, Prather H, et al. Clinical presentation of patients with tears of the acetabular labrum. J Bone Joint Surg Am. 2006;88:1448-1457.
23. Bare AA, Guanche CA. Hip impingement: the role of arthroscopy. Orthopedics. 2005;28:266-273.
24. Toomayan GA, Holman WR, Major NM, et al. Sensitivity of MR arthrography in the evaluation of acetabular labral tears. AJR Am J Roentgenol. 2006;186:449-453.
25. Robertson WJ, Kadrmas WR, Kelly BT. Arthroscopic management of labral tears in the hip: a systematic review of the literature. Clin Orthop Relat Res. 2007;455:88-92.
26. Kamath AF, Componovo R, Baldwin K, et al. Hip arthroscopy for labral tears: review of clinical outcomes with 4.8-year mean follow-up. Am J Sports Med. 2009;37:1721-1727.
27. Larson CM, Giveans MR. Arthroscopic debridement versus refixation of the acetabular labrum associated with femoroacetabular impingement. Arthroscopy. 2009;25:369-376.
28. Larson CM, Guanche CA, Kelly BT, et al. Advanced techniques in hip arthroscopy. Instr Course Lect. 2009;58:423-436.
29. Tibor LM, Sekiya JK. Differential diagnosis of pain around the hip joint. Arthroscopy. 2008;24:1407-1421.
30. Holmich P. Long-standing groin pain in sportspeople falls into three primary patterns, a “clinical entity” approach: a prospective study of 207 patients. Br J Sports Med. 2007;41:247-252.
31. Winston P, Awan R, Cassidy JD, et al. Clinical examination and ultrasound of self-reported snapping hip syndrome in elite ballet dancers. Am J Sports Med. 2007;35:118-126.
32. Blankenbaker DG, De Smet AA, Keene JS. Sonography of the iliopsoas tendon and injection of the iliopsoas bursa for diagnosis and management of the painful snapping hip. Skeletal Radiol. 2006;35:565-571.
33. Adler RS, Buly R, Ambrose R, et al. Diagnostic and therapeutic use of sonography-guided iliopsoas peritendinous injections. AJR Am J Roentgenol. 2005;185:940-943.
34. Anderson SA, Keene JS. Results of arthroscopic iliopsoas tendon release in competitive and recreational athletes. Am J Sports Med. 2008;36:2363-2371.
35. Maffey L, Emery C. What are the risk factors for groin strain injury in sport? Sports Med. 2007;37:881-894.
36. Verrall GM, Slavotinek JP, Fon GT, et al. Outcome of conservative management of athletic chronic groin injury diagnosed as pubic bone stress injury. Am J Sports Med. 2007;35:467-474.
37. Pointinger H, Munk P, Poeschl GP. Avulsion fracture of the anterior superior iliac spine following apophysitis. Br J Sports Med. 2003;37:361-362.
38. Unverzagt CA, Schuemann T, Mathisen J. Differential diagnosis of a sports hernia in a high-school athlete. J Orthop Sports Phys Ther. 2008;38:63-70.
39. Caudill P, Nyland J, Smith C, et al. Sports hernias: a systematic literature review. Br J Sports Med. 2008;42:954-964.
40. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55:393-396.
41. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29:521-533.
42. Petchprapa CN, Rosenberg ZS, Sconfienza LM, et al. MR imaging of entrapment neuropathies of the lower extremity. Part I. Radiographs. 2010;30:983-1000.
43. Voight M, Robinson K, Gill L, et al. Postoperative guidelines for hip arthroscopy in the active population. Sports Health. 2010;2:222-230.
44. Stalzer S, Wahoff M, Scanlan M. Rehabilitation following hip arthroscopy. Clin Sports Med. 2006;25:337-357.
• Consider both musculoskeletal and nonmusculoskeletal causes in patients with vague complaints of hip and groin pain. B
• Use imaging studies to confirm a hip pain diagnosis. B
• Refer patients who fail to respond to nonsurgical treatment to a sports medicine specialist or an orthopedic surgeon. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hip pain is a common complaint, and commonly misunderstood. Although the pain can be associated with a broad spectrum of conditions, the presentation is often vague and nonspecific.
Thus, hip pain and injury are frequently attributed, often incorrectly, to a “hip pointer”—a contusion of soft tissues against the iliac crest. It’s not unusual for patients who receive this diagnosis to be treated conservatively for prolonged periods, leading some previously active individuals to abandon their favorite sport or self-impose limits on the activities they engage in.1
But it doesn’t have to be this way.
Minimally invasive hip arthroscopy and advances in imaging, instrumentation, and devices have made it easier to identify and address underlying pathology associated with hip pain, helping patients return to their previous level of activity more rapidly.2,3 And, while many conditions associated with hip pain can be treated conservatively, family physicians—whom patients often go to first—should not hesitate to provide a referral when more aggressive treatment or diagnostic confirmation is needed.
We created this guide with family physicians in mind. Our focus here is primarily on anterior hip pain—the most common presentation—in active, or athletic, patients.
When did the pain begin? Where does it hurt?
Before performing a physical examination, find out as much as possible about the onset of pain and when and under what circumstances it occurs. (A review of hip anatomy is provided here.) Did it begin suddenly, after an acute injury or a particular physical maneuver? Or is the pain insidious, as was the case with one of our patients?
Osseous morphology of the hip includes the anterior superior iliac spine, the origin of the sartorius muscle and the ilioinguinal ligament. The anterior inferior iliac spine attaches to the rectus femoris, a major hip flexor and knee extender. The adductors of the hip originate in the anterior pelvic region.
The inguinal canal contains the ilioinguinal nerve, which is responsible for radiation of pain to the anterior hip. The hip joint itself is a spheroid comprising the femoral head and acetabulum, with most of the articular hip innervated by the femoral or obturator nerves.
Most intra-articular conditions radiate to the anterior groin or hip, although there are cases in which the pain is referred to either the lateral aspect of the hip or the buttocks. The iliopsoas muscle is the major hip flexor, and crosses under the ilioinguinal ligament to insert on the lesser tuberosity after crossing over the anterior capsule of the hip. A large bursa surrounds it, helping the tendon glide smoothly over the hip.
CASE Mack Q, a 27-year-old man with an 8-month history of right hip pain, sought care at our medical center for an achy pain in his right groin; he also described an occasional “clicking and popping sensation” in his groin but denied any trauma. The pain worsened with prolonged sitting and certain activities, such as squatting, twisting, and putting on shoes and socks. Our patient had stopped playing soccer because it hurt too much. He had tried physical therapy, oral anti-inflammatories, and a corticosteroid injection, with little relief.
Start with a gait assessment
The physical examination should begin with a gait assessment. Consider the patient’s ability to bear weight and his or her foot angle.
An individual with a stress fracture will have difficulty bearing weight on the affected side, resulting in a limp, or antalgic gait. A patient with femoral acetabular impingement (FAI) will often exhibit greater external rotation of the foot on the affected side compared with the other foot. And a patient with weakened abductor muscles, typically because of severe osteoarthritis, will exhibit the Trendelenburg sign—a pelvic tilt when the weight is shifted to the affected extremity.
Although most individuals with hip pain will not have an obvious gait abnormality, any patient who walks with a limp or needs crutches requires an immediate referral to an orthopedic surgeon.
Include these elements in the physical exam
Examine the hip with the patient sitting on the side of the exam table. Assess range of motion (ROM), comparing the range of flexion, extension, and internal/external rotation on the affected and unaffected sides. Include the following maneuvers:
Impingement testing. In patients with FAI and osteoarthritis, impingement testing—encompassing Flexion, ADDuction, and Internal Rotation (FADDIR)—will elicit pain. The maneuver can be tested starting at 45° of hip flexion, increasing to approximately 120°. Pain with <45° of hip flexion indicates that the impingement is severe.
Such testing can also reveal labral tears, which may be caused by FAI or other structural abnormalities. In a patient with anterior labral tears, FADDIR will produce groin pain; posterior labral tears will produce pain when the patient is sitting with legs hanging off the exam table and the contralateral leg is brought to the chest and the affected limb fully extended.
In patients with hip pain and bursitis, applying downward pressure will elicit a snapping sound as the iliopsoas snaps over the iliopectineal eminence or femoral head. Flexion, ABduction, and External Rotation (FABER) can also be used to diagnose iliopsoas tendonitis: The test is positive if it elicits pain in the affected extremity or in the sacroiliac joint on the opposite side.
Log roll. A painful response to this test, which involves internally and externally rotating the affected hip while it is relaxed and the knee fully extended, is an indication of synovitis of the hip caused by intra-articular pathology. To test hip stability, externally rotate the leg while it is extended. If the hip is stable, the leg will return to a neutral position; microinstability of the hip is likely if the leg remains in the rotated position.
Muscular strength testing. To assess for tendinopathy in the hip area, the patient should be in a seated position and contract the internal and external rotators and the adductor muscles while you apply resistance. To test abductor strength, have the patient assume a lateral position and hold and abduct the leg on the affected side while you apply resistance.
Hip flexion strength should be tested with the patient in both supine and seated positions. A patient with quadriceps tendonitis will have much greater pain with resisted hip flexion in the supine position vs the seated position; the opposite is true for a patient with iliopsoas tendonitis. (See “Did you know…? Hip pain facts and findings” on for additional diagnostic tips.)
- A patient with quadriceps tendonitis will have much greater pain with resisted hip flexion in a supine position vs a seated position. The opposite is true for a patient with iliopsoas tendonitis.
- Patients with femoral neck stress fractures typically present with activity-related anterior groin pain that is relieved by rest. Initially, they may be only mildly affected, but the condition worsens in those who continue to “work through the pain.”
- Plain radiography can confirm a diagnosis of osteonecrosis in patients with advanced disease, but magnetic resonance imaging is useful for evaluating earlier clinical presentations.
- Patients with labral tears often exhibit what has been called the “c-sign”—so named for the shape patients make with their hand as they grip their hip just above the greater trochanter to indicate where it hurts.
- Athletes who experience adductor strains often play sports in which kicking or frequent changes in direction are required, such as football, hockey, and soccer, and are generally able to tell you exactly what they were doing when the injury occurred.
- Unlike other hernias, a sports hernia (athletic pubalgia) does not involve a bulge of tissue protruding through one part of the body into another. Instead, it occurs when the oblique abdominal muscles strain or completely tear away from the pubis.
Perform a neurologic evaluation to rule out a back condition that might radiate pain into the anterior hip; ask the patient to do a sit-up while you apply resistance to test for abdominal wall pathology, as well.
Hip palpation. This aspect of the physical exam is important regardless of the cause of the pain but especially crucial for pediatric and adolescent patients, whose anterior hip pain may be related to apophyseal injury. Palpate the superior iliac spine (and over the inferior iliac spine in thin patients) to determine if the sartorius or rectus femoris has been injured. The area just lateral to the symphysis will be tender to palpation in patients with osteitis pubis.
Refer or treat? Here’s what to consider
While the history and physical should provide ample information for a differential diagnosis, imaging studies are generally required for confirmation. Clinical assessment— including physical exam, imaging, and intra-articular injection—of patients with hip pain is up to 98% accurate in identifying hip abnormalities, with arthroscopy as the gold standard.4
CASE On physical examination, Mr. Q had right hip extension to 0°, flexion to 110°, external rotation to 50°, and internal rotation to neutral; he also had positive impingement and subspine impingement tests, a painful arc of motion from 12 to 4 o’clock, tenderness over the hip adductor, and pain with resisted hip adduction. He did not walk with a limp.
Diagnostic studies included plain radiographs, which demonstrated that the joint space was well preserved. We identified subtle anatomical abnormalities on the femoral head-neck junction, known as a cam deformity. Magnetic resonance imaging (MRI) revealed an anterior superior labral tear with cartilage delamination.
Stress fractures affect runners, military recruits
In addition to long-distance runners who have recently increased the frequency, duration, or intensity of training,5,6 military recruits have a higher incidence of stress fractures due to the rapid onset of intensive training. Stress fractures can also occur in patients who do not have a history of intense activity but have metabolically weakened bone, in some cases as a result of an eating disorder.7
Patients with femoral neck stress fractures typically present with activity-related anterior groin pain that is relieved by rest; initially, they may be only mildly affected, but the condition worsens in those who continue to “work through the pain.” By the time such individuals seek treatment, they almost always have pain with weight bearing and an antalgic gait.
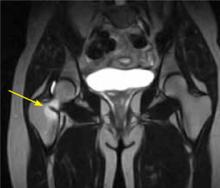
Symptoms consistent with a femoral neck stress fracture can be further evaluated with plain radiographs. However, x-rays are often negative for up to 4 weeks after the onset of pain.8 In cases in which radiographs are negative but the physical exam is suggestive of a stress fracture, MRI—which can detect an abnormality within a day or 2 of injury8,9—should be used to confirm the diagnosis (FIGURE 1).
FIGURE 1
MRI reveals a femoral neck stress fracture
Treatment. A complete femoral neck fracture portends impending displacement and requires emergent evaluation by an orthopedist, and superior neck changes, also known as tension-sided stress fractures, require urgent treatment with percutaneous screw fixation.9 However, compression-sided, or inferior, stress fractures can be treated with restricted weight bearing and activity modification. Gradual resumption of activity is allowed only after the patient has been asymptomatic for 6 weeks; recurrent pain indicates residual stress reaction, and signals that activities should be abated.
Osteonecrosis has many causes
Necrosis of the femoral head is a debilitating and progressive condition primarily affecting patients between the ages of 20 and 50 years.10 It has multiple (and diverse) causes, including trauma, steroids, alcohol, smoking, lupus, sickle cell anemia, and coagulopathies, as well as scuba diving. But about 20% of cases have no apparent cause.11,12
Patients with osteonecrosis of the hip typically present with groin pain, often described as a deep, intermittent ache that interferes with activities of daily living. Exam findings depend on the stage of presentation. Early on, pain will occur only with extreme ROM; in advanced cases, ROM is restricted and pain occurs even with limited motion.
Femoral head collapse due to loss of the structural integrity of the subchondral bone—which occurs in 80% of cases12—is thought to be caused by decreased blood flow. Plain radiography can confirm a diagnosis of osteonecrosis in patients with advanced disease, but MRI is useful for evaluating patients with earlier clinical presentations.
Treatment of osteonecrosis is dictated by the stage of the disease, but remains controversial because no intervention has been shown to prevent progression in all cases.12 All patients should be referred to a specialist. Those without collapse or cartilage damage can be treated surgically with core decompression, possibly with additional vascularized bone grafting,13,14 while those with more advanced disease typically require a total hip replacement at a relatively young age. Results for total hip replacement in patients with osteonecrosis are thought to be inferior to hip replacement in patients with osteoarthritis, although comparison is difficult because of the differences in age and activity levels in these 2 groups.15,16
Femoral acetabular impingement can occur on the cam or pincer side
FAI pathology can exist on either the femoral (cam) or acetabular (pincer) side,17 or both.18 In pure cam impingement, the anterior femoral neck loses its normal concave anatomy and develops a “bump,” which impinges on the anterosuperior labrum during hip flexion, causing labral tears and delamination of the adjacent cartilage.
Pure pincer impingement arises from a prominent acetabular rim, causing overcoverage of the femoral head. Acetabular labral tears result from the repetitive impaction with flexion and internal rotation.
Patients report an insidious onset of groin pain that is exacerbated by flexion-type sports, such as hockey, football, and golf,19 as well as activities of daily living. In patients with cartilage damage, even walking can be painful. Physical examination of patients with FAI reveals findings that are similar to those of patients with acetabular labral tears. Abnormally large cam lesions or acetabular overcoverage will result in restriction of hip ROM, especially internal rotation and flexion due to a mechanical block.
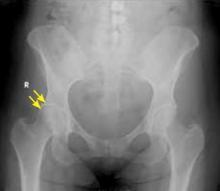
Radiographs (FIGURE 2) are essential to diagnose FAI and to distinguish this condition from an isolated labral tear.20 Cam impingement will be best demonstrated on a cross-table lateral radiograph, which shows an asphericity of the femoral head/neck junction anteriorly, while pincer impingement will show overcoverage of the femoral head on an AP radiograph. MRI or magnetic resonance arthrography (MRA) is frequently obtained to see whether any cartilage deterioration has occurred. Computed tomography, which can provide a 3-dimensional reproduction of the hip morphology, is often used for preoperative planning when surgical intervention is required.
FIGURE 2
Femoral acetabular impingement with a prominent pincer lesion
Treatment. Surgical intervention is often needed to correct or remove the abnormal anatomy, and both arthroscopic and open surgery are recommended.20 Both methods include osteoplasty at the femoral head/neck junction and/or the acetabular rim to allow the proximal femur to articulate with the acetabulum without injury to the labrum with flexion and internal rotation.21
Results of both open and arthroscopic osteoplasty of the femur and acetabulum are still preliminary, with only a few studies reporting mid-term results. Open surgery typically has longer recovery and rehabilitation, but advocates emphasize the improved ability to contour the femur or acetabulum. Both open and arthroscopic procedures have about an 8% to 13% rate of revision in short-term follow-up.17
Labral tears occur with trauma and certain sports
In addition to FAI, causes of labral tears include dysplasia, instability, trauma, and degeneration, as well as sports that require repetitive hip flexion and/or pivoting, such as hockey, soccer, and football.22,23
Patients with labral tears typically present with anterior hip pain radiating to the groin, worsening with twisting motions, running, walking, and sitting for prolonged periods. Clicking or catching may occur, as well. Patients may exhibit what one researcher called the “c-sign”—so named for the shape patients make with their hand as they grip their hip just above the greater trochanter to indicate where it hurts.4 The work-up for labral tears includes radiographs and, often, MRA, which is nearly 100% specific.24
Treatment. Conservative treatment, which may include activity modification or rest and ice, nonsteroidal anti-inflammatory drugs (NSAIDs), and physical therapy, is often effective for labral tears; when such measures fail, surgical intervention is indicated. A systematic review found a 67% satisfaction rate after 3.5 years in patients who had undergone labral debridement, and complete resolution of mechanical symptoms in nearly 50%.25 Another study showed similar results for hip arthroscopy, with symptom relief continuing for 4.8 years after surgery, on average, and 84% of patients able to return to their previous level of activity.26
The long-term results of labral debridement are unknown, however, and the possibility of an association between this procedure and the development of arthritis remains. Most specialists prefer anatomic repair to restore normal hip kinematics and, potentially, long-term hip function,27,28 but structural abnormalities must also be addressed to prevent failure of the repair or recurrent tears.
Iliopsoas tendonitis: You know the snap
Often referred to as internal snapping of the hip or internal coxa saltans, iliopsoas tendonitis/bursitis can be a recalcitrant cause of anterior hip pain. Snapping of the iliopsoas leading to bursitis or tendonitis can occur at the iliopectineal eminence, the femoral head, or the lesser trochanter.29 Runners and ballet dancers are often affected.30,31
Snapping in itself is not an indication of pathology, but chronicity of symptoms is. Patients with relatively acute symptoms typically have only bursitis, while a longer duration of symptoms leads to tendonitis or tendinopathy.32
Treatment. First-line therapy is nonoperative, and includes activity modification, rest, ice, NSAIDs, and physical therapy. Advise patients to refrain from activities causing pain, and to apply ice to the affected every 20 minutes (with a 20- to 30-minute off period) for one to 2 hours. Physical therapy focuses on stretching the iliopsoas and rectus femoris muscles and strengthening the hamstring muscles to relieve the stress on the anterior pelvis. If such treatment is unsuccessful, ultrasound can be used to guide a therapeutic injection of cortisone.33 If this fails to bring relief, fractional lengthening of the iliopsoas tendon can be performed to eliminate snapping and relieve pain.34
Muscular strains/avulsion fractures: Sports and age play a role
Although strains can affect any of the anterior muscles around the hip, in active individuals the adductors are most commonly affected. Skeletally immature patients are an exception: apophyseal fractures at the origin of the sartorius and rectus femoris muscles are more common than muscular strains in this patient population.
Athletes who experience adductor strains often play sports in which kicking or frequent changes in direction are required—eg, football, hockey, and soccer35—and generally are able to tell you exactly what they were doing when the injury occurred. Physical examination can reveal focal findings, with swelling and tenderness confined to the anteromedial aspect of the hip along the adductor muscle group. MRI can help differentiate the site of true pathology.36
Treatment of adductor strains is nonoperative, with rest, ice, and activity modification until the tendon heals. In the rare case in which complete tendon avulsion is found, surgical reattachment is needed.
Apophyseal fracture in skeletally immature patients typically occurs during participation in a sport that requires rapid acceleration and deceleration with the hip in an extended position. In such patients, stretching the affected muscle should reproduce the pain. Radiographs are diagnostic and will often show minimal displacement of the apophysis. Treatment is almost always nonoperative. Surgical intervention is rarely needed, and only indicated with displacement >2 cm.37
Athletic pubalgia: A challenging Dx
Also referred to as sports hernia, athletic pubalgia is an enigmatic cause of anterior hip pain in athletes. Diagnosis can be especially challenging, and patients may have lingering symptoms for years before the cause is discovered.38 A sports hernia, unlike other hernias, does not involve a bulge of tissue protruding through one body part into another. In contrast, a sports hernia occurs when the oblique abdominal muscles strain or completely tear away from the pubis. A recent systematic review found that the underlying etiology involves posterior inguinal wall weakening, which can be a result of poorly balanced hip adductor and abdominal muscle activation.39
Patients with sports hernia will often present with anterior hip and/or groin pain, especially with hip extension, twisting, and turning. In addition, patients can have pain in the lower abdomen and, in males, in the testicles. Physical examination will usually show pubic point tenderness, which is exacerbated by resisted hip adduction.40 MRI and ultrasound are extremely helpful in diagnosing and forming a treatment plan.39
The initial treatment of choice for sports hernias is nonoperative, and the first step is always activity modification or temporary avoidance of symptom-producing activities. Additional modalities include NSAIDs, ice, and physical therapy to strengthen the surrounding muscles. Surgical intervention, if needed, may be done laparoscopically or via an open approach with direct repair.40,41
Less common causes to consider
While the conditions detailed here account for most anterior hip etiologies, there are other less common causes to consider. One such cause is osteitis pubis, an umbrella term for conditions that affect the area surrounding the symphysis pubis. Patients with osteitis pubis present with pain over the anterior aspect of the pelvis that is worse with sit-ups, rising from a chair, or any activity where contraction of the rectus muscles occurs.29 Tenderness is found directly over and just lateral to the pubic symphysis. Radiographs are frequently negative, but occasionally chronic degenerative changes at the symphysis are present in addition to symphyseal narrowing. Additional imaging is often necessary for diagnosis.
Neuropathies. When history, physical examination, and imaging studies have ruled out other causes, neuropathies (ilioinguinal, genitofemoral, and obturator) should be considered, particularly in patients with vague, radiating anterior hip and/or groin pain.42 In pediatric patients, Legg-Calve-Perthes disease and slipped capital femoral epiphysis are possibilities, as well.
Getting patients back on track
Rehabilitation after hip injury resulting in anterior hip pain will be determined by the site, type, and mechanism of injury, as well as the severity. Restrictions in weight bearing and the use of an assistive device may be needed to prevent excessive stress on bone and supporting soft-tissue structures in the early stages of healing. Physical therapy, as needed, should initially focus on early controlled ROM of the hip joint to prevent both intra- and extra-articular adhesions and excessive scar tissue formation.2
For patients who undergo surgery, much of the focus will be on strengthening the supporting musculature—the hip abductor group, anterior and posterior thigh musculature, and core stabilizing muscles. Neuromuscular training may be needed to promote normal biomechanics and minimize compensatory movement patterns. For athletes, cardiovascular training and a return-to-play program should be implemented, as well.2,43,44
CASE Mr. Q was diagnosed with right hip pain due to a labral tear secondary to a cam femoral acetabular impingement. Given that he had failed nonoperative treatment and had long-standing pain, we recommended surgery for this patient. He underwent right hip arthroscopic labral repair, acetabular rim trimming, acetabular microfracture, femoral osteochondroplasty with capsular plication. At 12-month follow-up, he was doing well, with resolution of the presurgical pain and return to all athletic activities.
CORRESPONDENCE Rachel M. Frank, MD, Department of Orthopedic Surgery, Rush University Medical Center, 1611 West Harrison Street, Suite 300, Chicago, IL 60612; rmfrank3@gmail.com
• Consider both musculoskeletal and nonmusculoskeletal causes in patients with vague complaints of hip and groin pain. B
• Use imaging studies to confirm a hip pain diagnosis. B
• Refer patients who fail to respond to nonsurgical treatment to a sports medicine specialist or an orthopedic surgeon. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hip pain is a common complaint, and commonly misunderstood. Although the pain can be associated with a broad spectrum of conditions, the presentation is often vague and nonspecific.
Thus, hip pain and injury are frequently attributed, often incorrectly, to a “hip pointer”—a contusion of soft tissues against the iliac crest. It’s not unusual for patients who receive this diagnosis to be treated conservatively for prolonged periods, leading some previously active individuals to abandon their favorite sport or self-impose limits on the activities they engage in.1
But it doesn’t have to be this way.
Minimally invasive hip arthroscopy and advances in imaging, instrumentation, and devices have made it easier to identify and address underlying pathology associated with hip pain, helping patients return to their previous level of activity more rapidly.2,3 And, while many conditions associated with hip pain can be treated conservatively, family physicians—whom patients often go to first—should not hesitate to provide a referral when more aggressive treatment or diagnostic confirmation is needed.
We created this guide with family physicians in mind. Our focus here is primarily on anterior hip pain—the most common presentation—in active, or athletic, patients.
When did the pain begin? Where does it hurt?
Before performing a physical examination, find out as much as possible about the onset of pain and when and under what circumstances it occurs. (A review of hip anatomy is provided here.) Did it begin suddenly, after an acute injury or a particular physical maneuver? Or is the pain insidious, as was the case with one of our patients?
Osseous morphology of the hip includes the anterior superior iliac spine, the origin of the sartorius muscle and the ilioinguinal ligament. The anterior inferior iliac spine attaches to the rectus femoris, a major hip flexor and knee extender. The adductors of the hip originate in the anterior pelvic region.
The inguinal canal contains the ilioinguinal nerve, which is responsible for radiation of pain to the anterior hip. The hip joint itself is a spheroid comprising the femoral head and acetabulum, with most of the articular hip innervated by the femoral or obturator nerves.
Most intra-articular conditions radiate to the anterior groin or hip, although there are cases in which the pain is referred to either the lateral aspect of the hip or the buttocks. The iliopsoas muscle is the major hip flexor, and crosses under the ilioinguinal ligament to insert on the lesser tuberosity after crossing over the anterior capsule of the hip. A large bursa surrounds it, helping the tendon glide smoothly over the hip.
CASE Mack Q, a 27-year-old man with an 8-month history of right hip pain, sought care at our medical center for an achy pain in his right groin; he also described an occasional “clicking and popping sensation” in his groin but denied any trauma. The pain worsened with prolonged sitting and certain activities, such as squatting, twisting, and putting on shoes and socks. Our patient had stopped playing soccer because it hurt too much. He had tried physical therapy, oral anti-inflammatories, and a corticosteroid injection, with little relief.
Start with a gait assessment
The physical examination should begin with a gait assessment. Consider the patient’s ability to bear weight and his or her foot angle.
An individual with a stress fracture will have difficulty bearing weight on the affected side, resulting in a limp, or antalgic gait. A patient with femoral acetabular impingement (FAI) will often exhibit greater external rotation of the foot on the affected side compared with the other foot. And a patient with weakened abductor muscles, typically because of severe osteoarthritis, will exhibit the Trendelenburg sign—a pelvic tilt when the weight is shifted to the affected extremity.
Although most individuals with hip pain will not have an obvious gait abnormality, any patient who walks with a limp or needs crutches requires an immediate referral to an orthopedic surgeon.
Include these elements in the physical exam
Examine the hip with the patient sitting on the side of the exam table. Assess range of motion (ROM), comparing the range of flexion, extension, and internal/external rotation on the affected and unaffected sides. Include the following maneuvers:
Impingement testing. In patients with FAI and osteoarthritis, impingement testing—encompassing Flexion, ADDuction, and Internal Rotation (FADDIR)—will elicit pain. The maneuver can be tested starting at 45° of hip flexion, increasing to approximately 120°. Pain with <45° of hip flexion indicates that the impingement is severe.
Such testing can also reveal labral tears, which may be caused by FAI or other structural abnormalities. In a patient with anterior labral tears, FADDIR will produce groin pain; posterior labral tears will produce pain when the patient is sitting with legs hanging off the exam table and the contralateral leg is brought to the chest and the affected limb fully extended.
In patients with hip pain and bursitis, applying downward pressure will elicit a snapping sound as the iliopsoas snaps over the iliopectineal eminence or femoral head. Flexion, ABduction, and External Rotation (FABER) can also be used to diagnose iliopsoas tendonitis: The test is positive if it elicits pain in the affected extremity or in the sacroiliac joint on the opposite side.
Log roll. A painful response to this test, which involves internally and externally rotating the affected hip while it is relaxed and the knee fully extended, is an indication of synovitis of the hip caused by intra-articular pathology. To test hip stability, externally rotate the leg while it is extended. If the hip is stable, the leg will return to a neutral position; microinstability of the hip is likely if the leg remains in the rotated position.
Muscular strength testing. To assess for tendinopathy in the hip area, the patient should be in a seated position and contract the internal and external rotators and the adductor muscles while you apply resistance. To test abductor strength, have the patient assume a lateral position and hold and abduct the leg on the affected side while you apply resistance.
Hip flexion strength should be tested with the patient in both supine and seated positions. A patient with quadriceps tendonitis will have much greater pain with resisted hip flexion in the supine position vs the seated position; the opposite is true for a patient with iliopsoas tendonitis. (See “Did you know…? Hip pain facts and findings” on for additional diagnostic tips.)
- A patient with quadriceps tendonitis will have much greater pain with resisted hip flexion in a supine position vs a seated position. The opposite is true for a patient with iliopsoas tendonitis.
- Patients with femoral neck stress fractures typically present with activity-related anterior groin pain that is relieved by rest. Initially, they may be only mildly affected, but the condition worsens in those who continue to “work through the pain.”
- Plain radiography can confirm a diagnosis of osteonecrosis in patients with advanced disease, but magnetic resonance imaging is useful for evaluating earlier clinical presentations.
- Patients with labral tears often exhibit what has been called the “c-sign”—so named for the shape patients make with their hand as they grip their hip just above the greater trochanter to indicate where it hurts.
- Athletes who experience adductor strains often play sports in which kicking or frequent changes in direction are required, such as football, hockey, and soccer, and are generally able to tell you exactly what they were doing when the injury occurred.
- Unlike other hernias, a sports hernia (athletic pubalgia) does not involve a bulge of tissue protruding through one part of the body into another. Instead, it occurs when the oblique abdominal muscles strain or completely tear away from the pubis.
Perform a neurologic evaluation to rule out a back condition that might radiate pain into the anterior hip; ask the patient to do a sit-up while you apply resistance to test for abdominal wall pathology, as well.
Hip palpation. This aspect of the physical exam is important regardless of the cause of the pain but especially crucial for pediatric and adolescent patients, whose anterior hip pain may be related to apophyseal injury. Palpate the superior iliac spine (and over the inferior iliac spine in thin patients) to determine if the sartorius or rectus femoris has been injured. The area just lateral to the symphysis will be tender to palpation in patients with osteitis pubis.
Refer or treat? Here’s what to consider
While the history and physical should provide ample information for a differential diagnosis, imaging studies are generally required for confirmation. Clinical assessment— including physical exam, imaging, and intra-articular injection—of patients with hip pain is up to 98% accurate in identifying hip abnormalities, with arthroscopy as the gold standard.4
CASE On physical examination, Mr. Q had right hip extension to 0°, flexion to 110°, external rotation to 50°, and internal rotation to neutral; he also had positive impingement and subspine impingement tests, a painful arc of motion from 12 to 4 o’clock, tenderness over the hip adductor, and pain with resisted hip adduction. He did not walk with a limp.
Diagnostic studies included plain radiographs, which demonstrated that the joint space was well preserved. We identified subtle anatomical abnormalities on the femoral head-neck junction, known as a cam deformity. Magnetic resonance imaging (MRI) revealed an anterior superior labral tear with cartilage delamination.
Stress fractures affect runners, military recruits
In addition to long-distance runners who have recently increased the frequency, duration, or intensity of training,5,6 military recruits have a higher incidence of stress fractures due to the rapid onset of intensive training. Stress fractures can also occur in patients who do not have a history of intense activity but have metabolically weakened bone, in some cases as a result of an eating disorder.7
Patients with femoral neck stress fractures typically present with activity-related anterior groin pain that is relieved by rest; initially, they may be only mildly affected, but the condition worsens in those who continue to “work through the pain.” By the time such individuals seek treatment, they almost always have pain with weight bearing and an antalgic gait.
Symptoms consistent with a femoral neck stress fracture can be further evaluated with plain radiographs. However, x-rays are often negative for up to 4 weeks after the onset of pain.8 In cases in which radiographs are negative but the physical exam is suggestive of a stress fracture, MRI—which can detect an abnormality within a day or 2 of injury8,9—should be used to confirm the diagnosis (FIGURE 1).
FIGURE 1
MRI reveals a femoral neck stress fracture
Treatment. A complete femoral neck fracture portends impending displacement and requires emergent evaluation by an orthopedist, and superior neck changes, also known as tension-sided stress fractures, require urgent treatment with percutaneous screw fixation.9 However, compression-sided, or inferior, stress fractures can be treated with restricted weight bearing and activity modification. Gradual resumption of activity is allowed only after the patient has been asymptomatic for 6 weeks; recurrent pain indicates residual stress reaction, and signals that activities should be abated.
Osteonecrosis has many causes
Necrosis of the femoral head is a debilitating and progressive condition primarily affecting patients between the ages of 20 and 50 years.10 It has multiple (and diverse) causes, including trauma, steroids, alcohol, smoking, lupus, sickle cell anemia, and coagulopathies, as well as scuba diving. But about 20% of cases have no apparent cause.11,12
Patients with osteonecrosis of the hip typically present with groin pain, often described as a deep, intermittent ache that interferes with activities of daily living. Exam findings depend on the stage of presentation. Early on, pain will occur only with extreme ROM; in advanced cases, ROM is restricted and pain occurs even with limited motion.
Femoral head collapse due to loss of the structural integrity of the subchondral bone—which occurs in 80% of cases12—is thought to be caused by decreased blood flow. Plain radiography can confirm a diagnosis of osteonecrosis in patients with advanced disease, but MRI is useful for evaluating patients with earlier clinical presentations.
Treatment of osteonecrosis is dictated by the stage of the disease, but remains controversial because no intervention has been shown to prevent progression in all cases.12 All patients should be referred to a specialist. Those without collapse or cartilage damage can be treated surgically with core decompression, possibly with additional vascularized bone grafting,13,14 while those with more advanced disease typically require a total hip replacement at a relatively young age. Results for total hip replacement in patients with osteonecrosis are thought to be inferior to hip replacement in patients with osteoarthritis, although comparison is difficult because of the differences in age and activity levels in these 2 groups.15,16
Femoral acetabular impingement can occur on the cam or pincer side
FAI pathology can exist on either the femoral (cam) or acetabular (pincer) side,17 or both.18 In pure cam impingement, the anterior femoral neck loses its normal concave anatomy and develops a “bump,” which impinges on the anterosuperior labrum during hip flexion, causing labral tears and delamination of the adjacent cartilage.
Pure pincer impingement arises from a prominent acetabular rim, causing overcoverage of the femoral head. Acetabular labral tears result from the repetitive impaction with flexion and internal rotation.
Patients report an insidious onset of groin pain that is exacerbated by flexion-type sports, such as hockey, football, and golf,19 as well as activities of daily living. In patients with cartilage damage, even walking can be painful. Physical examination of patients with FAI reveals findings that are similar to those of patients with acetabular labral tears. Abnormally large cam lesions or acetabular overcoverage will result in restriction of hip ROM, especially internal rotation and flexion due to a mechanical block.
Radiographs (FIGURE 2) are essential to diagnose FAI and to distinguish this condition from an isolated labral tear.20 Cam impingement will be best demonstrated on a cross-table lateral radiograph, which shows an asphericity of the femoral head/neck junction anteriorly, while pincer impingement will show overcoverage of the femoral head on an AP radiograph. MRI or magnetic resonance arthrography (MRA) is frequently obtained to see whether any cartilage deterioration has occurred. Computed tomography, which can provide a 3-dimensional reproduction of the hip morphology, is often used for preoperative planning when surgical intervention is required.
FIGURE 2
Femoral acetabular impingement with a prominent pincer lesion
Treatment. Surgical intervention is often needed to correct or remove the abnormal anatomy, and both arthroscopic and open surgery are recommended.20 Both methods include osteoplasty at the femoral head/neck junction and/or the acetabular rim to allow the proximal femur to articulate with the acetabulum without injury to the labrum with flexion and internal rotation.21
Results of both open and arthroscopic osteoplasty of the femur and acetabulum are still preliminary, with only a few studies reporting mid-term results. Open surgery typically has longer recovery and rehabilitation, but advocates emphasize the improved ability to contour the femur or acetabulum. Both open and arthroscopic procedures have about an 8% to 13% rate of revision in short-term follow-up.17
Labral tears occur with trauma and certain sports
In addition to FAI, causes of labral tears include dysplasia, instability, trauma, and degeneration, as well as sports that require repetitive hip flexion and/or pivoting, such as hockey, soccer, and football.22,23
Patients with labral tears typically present with anterior hip pain radiating to the groin, worsening with twisting motions, running, walking, and sitting for prolonged periods. Clicking or catching may occur, as well. Patients may exhibit what one researcher called the “c-sign”—so named for the shape patients make with their hand as they grip their hip just above the greater trochanter to indicate where it hurts.4 The work-up for labral tears includes radiographs and, often, MRA, which is nearly 100% specific.24
Treatment. Conservative treatment, which may include activity modification or rest and ice, nonsteroidal anti-inflammatory drugs (NSAIDs), and physical therapy, is often effective for labral tears; when such measures fail, surgical intervention is indicated. A systematic review found a 67% satisfaction rate after 3.5 years in patients who had undergone labral debridement, and complete resolution of mechanical symptoms in nearly 50%.25 Another study showed similar results for hip arthroscopy, with symptom relief continuing for 4.8 years after surgery, on average, and 84% of patients able to return to their previous level of activity.26
The long-term results of labral debridement are unknown, however, and the possibility of an association between this procedure and the development of arthritis remains. Most specialists prefer anatomic repair to restore normal hip kinematics and, potentially, long-term hip function,27,28 but structural abnormalities must also be addressed to prevent failure of the repair or recurrent tears.
Iliopsoas tendonitis: You know the snap
Often referred to as internal snapping of the hip or internal coxa saltans, iliopsoas tendonitis/bursitis can be a recalcitrant cause of anterior hip pain. Snapping of the iliopsoas leading to bursitis or tendonitis can occur at the iliopectineal eminence, the femoral head, or the lesser trochanter.29 Runners and ballet dancers are often affected.30,31
Snapping in itself is not an indication of pathology, but chronicity of symptoms is. Patients with relatively acute symptoms typically have only bursitis, while a longer duration of symptoms leads to tendonitis or tendinopathy.32
Treatment. First-line therapy is nonoperative, and includes activity modification, rest, ice, NSAIDs, and physical therapy. Advise patients to refrain from activities causing pain, and to apply ice to the affected every 20 minutes (with a 20- to 30-minute off period) for one to 2 hours. Physical therapy focuses on stretching the iliopsoas and rectus femoris muscles and strengthening the hamstring muscles to relieve the stress on the anterior pelvis. If such treatment is unsuccessful, ultrasound can be used to guide a therapeutic injection of cortisone.33 If this fails to bring relief, fractional lengthening of the iliopsoas tendon can be performed to eliminate snapping and relieve pain.34
Muscular strains/avulsion fractures: Sports and age play a role
Although strains can affect any of the anterior muscles around the hip, in active individuals the adductors are most commonly affected. Skeletally immature patients are an exception: apophyseal fractures at the origin of the sartorius and rectus femoris muscles are more common than muscular strains in this patient population.
Athletes who experience adductor strains often play sports in which kicking or frequent changes in direction are required—eg, football, hockey, and soccer35—and generally are able to tell you exactly what they were doing when the injury occurred. Physical examination can reveal focal findings, with swelling and tenderness confined to the anteromedial aspect of the hip along the adductor muscle group. MRI can help differentiate the site of true pathology.36
Treatment of adductor strains is nonoperative, with rest, ice, and activity modification until the tendon heals. In the rare case in which complete tendon avulsion is found, surgical reattachment is needed.
Apophyseal fracture in skeletally immature patients typically occurs during participation in a sport that requires rapid acceleration and deceleration with the hip in an extended position. In such patients, stretching the affected muscle should reproduce the pain. Radiographs are diagnostic and will often show minimal displacement of the apophysis. Treatment is almost always nonoperative. Surgical intervention is rarely needed, and only indicated with displacement >2 cm.37
Athletic pubalgia: A challenging Dx
Also referred to as sports hernia, athletic pubalgia is an enigmatic cause of anterior hip pain in athletes. Diagnosis can be especially challenging, and patients may have lingering symptoms for years before the cause is discovered.38 A sports hernia, unlike other hernias, does not involve a bulge of tissue protruding through one body part into another. In contrast, a sports hernia occurs when the oblique abdominal muscles strain or completely tear away from the pubis. A recent systematic review found that the underlying etiology involves posterior inguinal wall weakening, which can be a result of poorly balanced hip adductor and abdominal muscle activation.39
Patients with sports hernia will often present with anterior hip and/or groin pain, especially with hip extension, twisting, and turning. In addition, patients can have pain in the lower abdomen and, in males, in the testicles. Physical examination will usually show pubic point tenderness, which is exacerbated by resisted hip adduction.40 MRI and ultrasound are extremely helpful in diagnosing and forming a treatment plan.39
The initial treatment of choice for sports hernias is nonoperative, and the first step is always activity modification or temporary avoidance of symptom-producing activities. Additional modalities include NSAIDs, ice, and physical therapy to strengthen the surrounding muscles. Surgical intervention, if needed, may be done laparoscopically or via an open approach with direct repair.40,41
Less common causes to consider
While the conditions detailed here account for most anterior hip etiologies, there are other less common causes to consider. One such cause is osteitis pubis, an umbrella term for conditions that affect the area surrounding the symphysis pubis. Patients with osteitis pubis present with pain over the anterior aspect of the pelvis that is worse with sit-ups, rising from a chair, or any activity where contraction of the rectus muscles occurs.29 Tenderness is found directly over and just lateral to the pubic symphysis. Radiographs are frequently negative, but occasionally chronic degenerative changes at the symphysis are present in addition to symphyseal narrowing. Additional imaging is often necessary for diagnosis.
Neuropathies. When history, physical examination, and imaging studies have ruled out other causes, neuropathies (ilioinguinal, genitofemoral, and obturator) should be considered, particularly in patients with vague, radiating anterior hip and/or groin pain.42 In pediatric patients, Legg-Calve-Perthes disease and slipped capital femoral epiphysis are possibilities, as well.
Getting patients back on track
Rehabilitation after hip injury resulting in anterior hip pain will be determined by the site, type, and mechanism of injury, as well as the severity. Restrictions in weight bearing and the use of an assistive device may be needed to prevent excessive stress on bone and supporting soft-tissue structures in the early stages of healing. Physical therapy, as needed, should initially focus on early controlled ROM of the hip joint to prevent both intra- and extra-articular adhesions and excessive scar tissue formation.2
For patients who undergo surgery, much of the focus will be on strengthening the supporting musculature—the hip abductor group, anterior and posterior thigh musculature, and core stabilizing muscles. Neuromuscular training may be needed to promote normal biomechanics and minimize compensatory movement patterns. For athletes, cardiovascular training and a return-to-play program should be implemented, as well.2,43,44
CASE Mr. Q was diagnosed with right hip pain due to a labral tear secondary to a cam femoral acetabular impingement. Given that he had failed nonoperative treatment and had long-standing pain, we recommended surgery for this patient. He underwent right hip arthroscopic labral repair, acetabular rim trimming, acetabular microfracture, femoral osteochondroplasty with capsular plication. At 12-month follow-up, he was doing well, with resolution of the presurgical pain and return to all athletic activities.
CORRESPONDENCE Rachel M. Frank, MD, Department of Orthopedic Surgery, Rush University Medical Center, 1611 West Harrison Street, Suite 300, Chicago, IL 60612; rmfrank3@gmail.com
1. Margo K, Drezner J, Motzkin D. Evaluation and management of hip pain: an algorithmic approach. J Fam Pract. 2003;52:607-617.
2. Leunig M, Beaule PE, Ganz R. The concept of femoroacetabular impingement: current status and future perspectives. Clin Orthop Relat Res. 2009;467:616-622.
3. Enseki KR, Martin RL, Draovitch P, et al. The hip joint: arthroscopic procedures and postoperative rehabilitation J Orthop Sports Phys Ther. 2006;36:516-525.
4. Byrd JW, Jones KS. Diagnostic accuracy of clinical assessment, magnetic resonance imaging, magnetic resonance arthrography, and intra-articular injection in hip arthroscopy patients. Am J Sports Med. 2004;32:1668-1674.
5. Fredericson M, Jennings F, Beaulieu C, et al. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17:309-325.
6. Matheson GO, Clement DB, McKenzie DC, et al. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15:46-58.
7. Stanitski CL, McMaster JH, Scranton PE. On the nature of stress fractures. Am J Sports Med. 1978;6:391-396.
8. Sofka CM. Imaging of stress fractures. Clin Sports Med. 2006;25:53-62, viii.
9. Shin AY, Gillingham BL. Fatigue fractures of the femoral neck in athletes. J Am Acad Orthop Surg. 1997;5:293-302.
10. Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459-474.
11. Lavernia CJ, Sierra RJ, Gomez-Marin O. Smoking and joint replacement: resource consumption and short-term outcome. Clin Orthop Relat Res. 1999;(367):172-180.
12. Lavernia CJ, Sierra RJ, Grieco FR. Osteonecrosis of the femoral head. J Am Acad Orthop Surg. 1999;7:250-261.
13. Smith SW, Fehring TK, Griffin WL, Beaver WB. Core decompression of the osteonecrotic femoral head. J Bone Joint Surg Am. 1995;77:674-680.
14. Fairbank AC, Bhatia D, Jinnah RH, et al. Long-term results of core decompression for ischaemic necrosis of the femoral head. J Bone Joint Surg Br. 1995;77:42-49.
15. Chandler HP, Reineck FT, Wixson RL, et al. Total hip replacement in patients younger than thirty years old. A five-year follow-up study. J Bone Joint Surg Am. 1981;63:1426-1434.
16. Wei SY, Klimkiewicz JJ, Lai M, et al. Revision total hip arthroplasty in patients with avascular necrosis. Orthopedics. 1999;22:747-757.
17. Bedi A, Chen N, Robertson W, et al. The management of labral tears and femoroacetabular impingement of the hip in the young, active patient. Arthroscopy. 2008;24:1135-1145.
18. Guanche CA, Bare AA. Arthroscopic treatment of femoroacetabular impingement. Arthroscopy. 2006;22:95-106.
19. Philippon M, Schenker M, Briggs K, et al. Femoroacetabular impingement in 45 professional athletes: Knee Surg Sports Traumatol Arthrosc. 2007;15:908-914.
20. Sierra RJ, Trousdale RT, Ganz R, et al. Hip disease in the young, active patient: evaluation and nonarthroplasty surgical options. J Am Acad Orthop Surg. 2008;16:689-703.
21. Byrd JW, Jones KS. Prospective analysis of hip arthroscopy with 10-year followup. Clin Orthop Relat Res. 2009;468:741-746.
22. Burnett RS, Della Rocca GJ, Prather H, et al. Clinical presentation of patients with tears of the acetabular labrum. J Bone Joint Surg Am. 2006;88:1448-1457.
23. Bare AA, Guanche CA. Hip impingement: the role of arthroscopy. Orthopedics. 2005;28:266-273.
24. Toomayan GA, Holman WR, Major NM, et al. Sensitivity of MR arthrography in the evaluation of acetabular labral tears. AJR Am J Roentgenol. 2006;186:449-453.
25. Robertson WJ, Kadrmas WR, Kelly BT. Arthroscopic management of labral tears in the hip: a systematic review of the literature. Clin Orthop Relat Res. 2007;455:88-92.
26. Kamath AF, Componovo R, Baldwin K, et al. Hip arthroscopy for labral tears: review of clinical outcomes with 4.8-year mean follow-up. Am J Sports Med. 2009;37:1721-1727.
27. Larson CM, Giveans MR. Arthroscopic debridement versus refixation of the acetabular labrum associated with femoroacetabular impingement. Arthroscopy. 2009;25:369-376.
28. Larson CM, Guanche CA, Kelly BT, et al. Advanced techniques in hip arthroscopy. Instr Course Lect. 2009;58:423-436.
29. Tibor LM, Sekiya JK. Differential diagnosis of pain around the hip joint. Arthroscopy. 2008;24:1407-1421.
30. Holmich P. Long-standing groin pain in sportspeople falls into three primary patterns, a “clinical entity” approach: a prospective study of 207 patients. Br J Sports Med. 2007;41:247-252.
31. Winston P, Awan R, Cassidy JD, et al. Clinical examination and ultrasound of self-reported snapping hip syndrome in elite ballet dancers. Am J Sports Med. 2007;35:118-126.
32. Blankenbaker DG, De Smet AA, Keene JS. Sonography of the iliopsoas tendon and injection of the iliopsoas bursa for diagnosis and management of the painful snapping hip. Skeletal Radiol. 2006;35:565-571.
33. Adler RS, Buly R, Ambrose R, et al. Diagnostic and therapeutic use of sonography-guided iliopsoas peritendinous injections. AJR Am J Roentgenol. 2005;185:940-943.
34. Anderson SA, Keene JS. Results of arthroscopic iliopsoas tendon release in competitive and recreational athletes. Am J Sports Med. 2008;36:2363-2371.
35. Maffey L, Emery C. What are the risk factors for groin strain injury in sport? Sports Med. 2007;37:881-894.
36. Verrall GM, Slavotinek JP, Fon GT, et al. Outcome of conservative management of athletic chronic groin injury diagnosed as pubic bone stress injury. Am J Sports Med. 2007;35:467-474.
37. Pointinger H, Munk P, Poeschl GP. Avulsion fracture of the anterior superior iliac spine following apophysitis. Br J Sports Med. 2003;37:361-362.
38. Unverzagt CA, Schuemann T, Mathisen J. Differential diagnosis of a sports hernia in a high-school athlete. J Orthop Sports Phys Ther. 2008;38:63-70.
39. Caudill P, Nyland J, Smith C, et al. Sports hernias: a systematic literature review. Br J Sports Med. 2008;42:954-964.
40. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55:393-396.
41. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29:521-533.
42. Petchprapa CN, Rosenberg ZS, Sconfienza LM, et al. MR imaging of entrapment neuropathies of the lower extremity. Part I. Radiographs. 2010;30:983-1000.
43. Voight M, Robinson K, Gill L, et al. Postoperative guidelines for hip arthroscopy in the active population. Sports Health. 2010;2:222-230.
44. Stalzer S, Wahoff M, Scanlan M. Rehabilitation following hip arthroscopy. Clin Sports Med. 2006;25:337-357.
1. Margo K, Drezner J, Motzkin D. Evaluation and management of hip pain: an algorithmic approach. J Fam Pract. 2003;52:607-617.
2. Leunig M, Beaule PE, Ganz R. The concept of femoroacetabular impingement: current status and future perspectives. Clin Orthop Relat Res. 2009;467:616-622.
3. Enseki KR, Martin RL, Draovitch P, et al. The hip joint: arthroscopic procedures and postoperative rehabilitation J Orthop Sports Phys Ther. 2006;36:516-525.
4. Byrd JW, Jones KS. Diagnostic accuracy of clinical assessment, magnetic resonance imaging, magnetic resonance arthrography, and intra-articular injection in hip arthroscopy patients. Am J Sports Med. 2004;32:1668-1674.
5. Fredericson M, Jennings F, Beaulieu C, et al. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17:309-325.
6. Matheson GO, Clement DB, McKenzie DC, et al. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15:46-58.
7. Stanitski CL, McMaster JH, Scranton PE. On the nature of stress fractures. Am J Sports Med. 1978;6:391-396.
8. Sofka CM. Imaging of stress fractures. Clin Sports Med. 2006;25:53-62, viii.
9. Shin AY, Gillingham BL. Fatigue fractures of the femoral neck in athletes. J Am Acad Orthop Surg. 1997;5:293-302.
10. Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459-474.
11. Lavernia CJ, Sierra RJ, Gomez-Marin O. Smoking and joint replacement: resource consumption and short-term outcome. Clin Orthop Relat Res. 1999;(367):172-180.
12. Lavernia CJ, Sierra RJ, Grieco FR. Osteonecrosis of the femoral head. J Am Acad Orthop Surg. 1999;7:250-261.
13. Smith SW, Fehring TK, Griffin WL, Beaver WB. Core decompression of the osteonecrotic femoral head. J Bone Joint Surg Am. 1995;77:674-680.
14. Fairbank AC, Bhatia D, Jinnah RH, et al. Long-term results of core decompression for ischaemic necrosis of the femoral head. J Bone Joint Surg Br. 1995;77:42-49.
15. Chandler HP, Reineck FT, Wixson RL, et al. Total hip replacement in patients younger than thirty years old. A five-year follow-up study. J Bone Joint Surg Am. 1981;63:1426-1434.
16. Wei SY, Klimkiewicz JJ, Lai M, et al. Revision total hip arthroplasty in patients with avascular necrosis. Orthopedics. 1999;22:747-757.
17. Bedi A, Chen N, Robertson W, et al. The management of labral tears and femoroacetabular impingement of the hip in the young, active patient. Arthroscopy. 2008;24:1135-1145.
18. Guanche CA, Bare AA. Arthroscopic treatment of femoroacetabular impingement. Arthroscopy. 2006;22:95-106.
19. Philippon M, Schenker M, Briggs K, et al. Femoroacetabular impingement in 45 professional athletes: Knee Surg Sports Traumatol Arthrosc. 2007;15:908-914.
20. Sierra RJ, Trousdale RT, Ganz R, et al. Hip disease in the young, active patient: evaluation and nonarthroplasty surgical options. J Am Acad Orthop Surg. 2008;16:689-703.
21. Byrd JW, Jones KS. Prospective analysis of hip arthroscopy with 10-year followup. Clin Orthop Relat Res. 2009;468:741-746.
22. Burnett RS, Della Rocca GJ, Prather H, et al. Clinical presentation of patients with tears of the acetabular labrum. J Bone Joint Surg Am. 2006;88:1448-1457.
23. Bare AA, Guanche CA. Hip impingement: the role of arthroscopy. Orthopedics. 2005;28:266-273.
24. Toomayan GA, Holman WR, Major NM, et al. Sensitivity of MR arthrography in the evaluation of acetabular labral tears. AJR Am J Roentgenol. 2006;186:449-453.
25. Robertson WJ, Kadrmas WR, Kelly BT. Arthroscopic management of labral tears in the hip: a systematic review of the literature. Clin Orthop Relat Res. 2007;455:88-92.
26. Kamath AF, Componovo R, Baldwin K, et al. Hip arthroscopy for labral tears: review of clinical outcomes with 4.8-year mean follow-up. Am J Sports Med. 2009;37:1721-1727.
27. Larson CM, Giveans MR. Arthroscopic debridement versus refixation of the acetabular labrum associated with femoroacetabular impingement. Arthroscopy. 2009;25:369-376.
28. Larson CM, Guanche CA, Kelly BT, et al. Advanced techniques in hip arthroscopy. Instr Course Lect. 2009;58:423-436.
29. Tibor LM, Sekiya JK. Differential diagnosis of pain around the hip joint. Arthroscopy. 2008;24:1407-1421.
30. Holmich P. Long-standing groin pain in sportspeople falls into three primary patterns, a “clinical entity” approach: a prospective study of 207 patients. Br J Sports Med. 2007;41:247-252.
31. Winston P, Awan R, Cassidy JD, et al. Clinical examination and ultrasound of self-reported snapping hip syndrome in elite ballet dancers. Am J Sports Med. 2007;35:118-126.
32. Blankenbaker DG, De Smet AA, Keene JS. Sonography of the iliopsoas tendon and injection of the iliopsoas bursa for diagnosis and management of the painful snapping hip. Skeletal Radiol. 2006;35:565-571.
33. Adler RS, Buly R, Ambrose R, et al. Diagnostic and therapeutic use of sonography-guided iliopsoas peritendinous injections. AJR Am J Roentgenol. 2005;185:940-943.
34. Anderson SA, Keene JS. Results of arthroscopic iliopsoas tendon release in competitive and recreational athletes. Am J Sports Med. 2008;36:2363-2371.
35. Maffey L, Emery C. What are the risk factors for groin strain injury in sport? Sports Med. 2007;37:881-894.
36. Verrall GM, Slavotinek JP, Fon GT, et al. Outcome of conservative management of athletic chronic groin injury diagnosed as pubic bone stress injury. Am J Sports Med. 2007;35:467-474.
37. Pointinger H, Munk P, Poeschl GP. Avulsion fracture of the anterior superior iliac spine following apophysitis. Br J Sports Med. 2003;37:361-362.
38. Unverzagt CA, Schuemann T, Mathisen J. Differential diagnosis of a sports hernia in a high-school athlete. J Orthop Sports Phys Ther. 2008;38:63-70.
39. Caudill P, Nyland J, Smith C, et al. Sports hernias: a systematic literature review. Br J Sports Med. 2008;42:954-964.
40. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55:393-396.
41. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29:521-533.
42. Petchprapa CN, Rosenberg ZS, Sconfienza LM, et al. MR imaging of entrapment neuropathies of the lower extremity. Part I. Radiographs. 2010;30:983-1000.
43. Voight M, Robinson K, Gill L, et al. Postoperative guidelines for hip arthroscopy in the active population. Sports Health. 2010;2:222-230.
44. Stalzer S, Wahoff M, Scanlan M. Rehabilitation following hip arthroscopy. Clin Sports Med. 2006;25:337-357.
Carpal tunnel syndrome—try these diagnostic maneuvers
• Before considering surgery, offer patients with mild-to-moderate carpal tunnel syndrome (CTS) a trial of conservative therapy such as splinting or corticosteroids. A
• Order electrodiagnostic studies (EDS) as needed, to rule out other conditions with a similar presentation, confirm an uncertain diagnosis, and gauge the severity of CTS C, or when surgery is being considered. B
• Recommend carpal tunnel release for patients who have severe CTS or have failed to respond to nonsurgical t0reatment. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane K, 52, comes to see you because of discomfort in her right wrist and tingling in her hand. The symptoms began 3 months ago, but have been getting progressively worse, and have started to interfere with her sleep. Ms. K often awakens with “pins and needles” in her hand, and says that she often has the urge to “shake it out.” Her sister has carpal tunnel syndrome (CTS), and Ms. K suspects that she does, too. On exam, you find that Ms. K has a positive Phalen’s and Durkan’s compression test, but normal Tinel’s test. She has normal strength and sensation in her hands. Her neck and upper extremity exam is otherwise unremarkable. You note that her hypothyroidism is well controlled, with a recent thyroid-stimulating hormone level of 1.2 mIU/L.
The patient has tried acetaminophen and ibuprofen, with little relief. She has researched CTS on the Internet and read about cold laser therapy, and wants to know whether you think it will work. What should you tell her?
Carpal tunnel syndrome is one of the most common disorders of the upper extremities and the most prevalent compression neuropathy.1 About 3% of US adults are affected, typically those between the ages of 40 and 60 years.2 Women are almost 3 times more likely than men to develop CTS.1
Other risk factors include diabetes, hypothyroidism, rheumatoid arthritis, pregnancy, obesity, family history, and trauma. A history of hand-related repetitive motions also increases the risk.3-5 Evidence does not support a definite link between keyboard or mouse use and CTS; however, occupations that require use of hand-operated vibratory tools or repeated and forceful movements of the hand/wrist (such as assembly work and food processing or packaging) are associated with CTS.6
The optimal diagnostic approach incorporates history and physical exam findings, including the results of a number of provocative maneuvers, as well as electrodiagnostic studies (EDS) in some cases.7 While surgery is the definitive treatment for CTS, numerous nonsurgical options exist, including splinting, corticosteroids, and a variety of alternative therapies, some of which (eg, chiropractic and cold laser therapy) have little evidence to support them.
Because family physicians are often the first to see patients with symptoms associated with CTS, you need to know what to look for, when to test, and whether to provide treatment or a referral. Here’s what to keep in mind.
Clinical presentation of CTS
Increased pressure in the carpal tunnel compresses the median nerve, leading to numbness, tingling, or pain in the palmar aspect of the first 3 fingers and the radial half of the fourth (FIGURE). Symptoms vary widely, with pain or numbness localized to the hand or wrist in some cases and pain radiating into the forearm or shoulder in others.
Figure
Compressed median nerve leads to numbness and tingling
Early in the course of CTS, symptoms are often most bothersome at night. In a scenario like that reported by Ms. K, patients are often awakened by numbness or tingling and the desire to shake out the affected hand—a phenomenon known as the flick sign.8 Pain and numbness may occur intermittently at first, especially with repetitive wrist motion. Activities such as driving or holding a telephone often aggravate symptoms.
As CTS progresses, the intensity and duration of symptoms increase. Patients may complain of weakness in the hand and report that they often drop things. Paradoxically, patients with more severe CTS sometimes have less pain, rather than more, because of increasing sensory loss.9
Late in the course of CTS, physical exam findings typically include decreased sensation in the fingers innervated by the median nerve, sparing the thenar eminence. (A loss of sensation in the thenar eminence suggests the presence of a lesion proximal to the carpal tunnel, rather than CTS itself.10) In advanced cases, weakness of thumb abduction and opposition may occur, as well as atrophy of the thenar eminence.11
Sudden onset of severe symptoms with minimal trauma to the wrist should raise suspicion of a hematoma in the carpal tunnel—a particular risk for patients who have a clotting disorder or are being treated with newer anticoagulants such as dabigatran. Prompt surgical decompression is required to prevent permanent median nerve damage in such cases.12
Include these maneuvers in the physical exam
A thorough evaluation of the neck, shoulder, elbow, and wrist is crucial for all patients with signs and symptoms associated with CTS. Provocative maneuvers (TABLE 1)7,13 are also important as an aid to diagnosis. The results of the following tests should be viewed with caution, however, as studies have found wide variations in their sensitivity and specificity:
TABLE 1
Diagnosing carpal tunnel syndrome, using physical maneuvers7,13
| Test | Technique | Positive test | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| Phalen’s | Patient holds wrist flexed 90° with elbow in full extension | Pain or paresthesia ≤60 sec | 68 | 73 |
| Tinel’s | Clinician repetitively taps wrist over transverse carpal ligament | Pain or paresthesia | 50 | 77 |
| Median nerve compression* (MNC) | Clinician applies direct pressure over the transverse carpal ligament | Pain or paresthesia ≤30 sec | 64 | 83 |
| MNC + Phalen’s | Same as above | Same as above | 80 | 92 |
| *also known as Durkan’s test. | ||||
Phalen’s maneuver. The patient flexes his or her wrist with the elbow in full extension to increase pressure on the median nerve, and holds the position for 60 seconds. The onset of pain or paresthesia is a positive test. A meta-analysis found the sensitivity and specificity of a positive Phalen’s sign to be 68% and 73%, respectively.7
Tinel’s test. Tap the volar surface of the patient’s wrist just proximal to, or on top of, the carpal tunnel. Pain or paresthesia in the fingers innervated by the median nerve as a result of the percussion constitutes a positive result. Tinel’s test is less sensitive than the Phalen’s maneuver, but has a similar specificity.13
The median nerve (Durkan’s) compression test. Apply pressure over the transverse carpal ligament; the test is positive if pain or paresthesia develops within 30 seconds.7
The hand elevation test. The patient raises both hands overhead for 60 seconds; here, too, pain or paresthesia is a positive result.14
Combining results of provocative maneuvers may increase sensitivity and specificity. Positive results in both the Phalen’s and median nerve compression tests, for example, have a collective sensitivity and specificity of 80% and 92%, respectively.13
When (or whether) to order electrodiagnostic studies
While some clinicians consider EDS to be the gold standard in CTS diagnosis,6 evidence is limited. One issue is the lack of universally accepted reference standards; another is that most studies have been affected by “spectrum bias.”15 What’s more, EDS—which include nerve conduction studies (NCS) and electromyography (EMG)—do not always correlate directly with symptoms, and 16% to 34% of mild cases can be missed.16
EDS are useful in many instances, however. EMG can rule out other causes of CTS symptoms (TABLE 2 details the differential diagnosis),7,11 while NCS can aid in diagnosing CTS, gauging its severity, and arriving at a prognosis. Specifically, NCS can detect delayed distal latencies and slowed conduction velocities that can occur when the myelin sheath is damaged by prolonged compression of the median nerve.17 With more severe compression, axonal damage occurs, as evidenced by reduced action potential amplitudes on NCS. Results of the nerve conduction tests are compared to age-dependent normal values and to results from other nerves on either the same or the contralateral hand. In a 2002 systemic review, the sensitivity of NCS for CTS was 56% to 85% and the specificity was 94% to 99%.18
TABLE 2
Differential diagnosis for CTS7,11
| Condition | Characteristics |
|---|---|
| Carpometacarpal arthritis of thumb | Thumb is painful when in motion; radiographic findings |
| Cervical radiculopathy | Neck pain, nerve root distribution (eg, C6), positive Spurling’s test |
| DeQuervain’s tenosynovitis | Painful resisted thumb dorsiflexion, tender at base of thumb |
| Hypothyroidism | Fatigue, cold intolerance, dry skin, hair loss, abnormal thyroid function tests |
| Peripheral neuropathy | History of DM, lower extremity involvement |
| Pronator syndrome (median nerve compression at the elbow) | Tenderness at proximal forearm |
| Ulnar compressive neuropathy | Compression and positive Tinel’s sign: ulnar nerve at elbow or wrist produces pain or paresthesias in 4th and 5th fingers |
| Vibration white finger | History of use of power drill or other hand-held vibratory tool; symptoms of Raynaud’s syndrome |
| Wrist arthritis | Painful wrist ROM, radiographic findings |
| CTS, carpal tunnel syndrome; DM, diabetes mellitus; ROM, range of motion. | |
Before and after surgery. The American Academy of Orthopedic Surgeons (AAOS) recommends EDS when CTS surgery is being considered. 7 EDS may also be used after surgery, to verify neurologic improvement.
Ultrasound. In patients with CTS, ultrasound reveals an increased cross-sectional area of the median nerve, a finding that has prompted studies of this modality as a diagnostic tool.19 Although evidence suggests that ultrasound’s sensitivity and specificity for CTS would be similar to that of EDS, the optimal cutoff for an abnormal test has not been defined,19 and ultrasound does not provide information on prognosis or alternate causes.
Thus, AAOS does not currently recommend ultrasound for CTS diagnosis.7 Magnetic resonance imaging is inappropriate for routine CTS diagnosis, as well.7
Treatment: Start conservatively
Multiple nonsurgical options are available, but the best evidence supports splinting, steroid injection, and oral steroids. Splinting or steroids alone may bring long-term relief for patients with mild to moderate cases;20 in fact, about a third of mild cases improve spontaneously.21
Conservative therapy can also provide relief for those who wish to avoid or delay surgery and for cases of transient CTS (pediatric patients, for example, or those whose condition is associated with pregnancy or hypothyroidism).18 A successful response to therapy can also help to confirm a CTS diagnosis.
Most conservative treatments begin providing relief within 2 to 6 weeks and reach the maximal benefit at 3 months.22 If there is no response after 6 weeks, it’s time to consider another approach.
In initiating splinting or corticosteroids, here’s a look at what to keep in mind:
Splinting. A splint can be used to maintain the wrist in a position with the least intracanal pressure, thereby limiting pressure on the median nerve. Splinting is equally effective whether used continually or only at night.23
Splinting can relieve symptoms and improve functional status within 2 weeks and the effects can last for 3 to 6 months, eliminating the need for surgery for some patients with mild CTS.19,20 Nerve gliding exercises, (see image at left), have been evaluated in combination with splinting. While evidence is limited, an at-home program involving these simple exercises may be a beneficial adjunctive treatment with minimal cost or harm.24,25
Local corticosteroid injection. A Cochrane meta-analysis found significant improvement in symptoms and function at one month among patients with CTS who were treated with steroid injection.26 In many cases, the effects last for many months.
A recent trial found that nearly half of patients with mild to moderate CTS who were treated with steroid injections had improved symptoms and EDS results at the 12-month follow-up.20 However, while patients with severe CTS experienced improvement at 4 weeks postinjection, most eventually required surgery.20
Evidence does not support one particular steroid dose or formulation over another, or one particular injection site.22 Injecting 4 cm proximal to the wrist flexion crease is as effective as a more distal injection.26,27
Caution is required, however, as risks associated with local injections include tendon rupture and median nerve injury. If a patient experiences intense pain or paresthesia in the median nerve distribution when the needle is inserted, redirect the needle away from the median nerve immediately. For patients who respond well to this treatment, one additional injection can be given after 6 months if symptoms recur.
Oral corticosteroids. Oral prednisone at a dose of 20 mg/d for 2 weeks improves symptoms and function in patients with CTS, but is less effective than steroid injections.28 Treatment for 2 weeks is as effective as treatment for 4 weeks; the effects tend to wane after 8 weeks in both cases.29 Nonsteroidal anti-inflammatory drugs, diuretics, and vitamin B6 have not been found to be effective.30
CASE Ms. K also asks about “those needle tests”—a reference to EDS—which her sister had to diagnose her CTS. You explain that these studies are not necessary at this time because her symptoms are mild and there is no need for other causes to be ruled out.
Instead, you offer her a neutral wrist splint for night-time use and recommend home-based nerve glide exercises. There is no evidence that cold laser therapy is effective, you explain to Ms. K, and it is expensive. She agrees to try the splint and the exercises, and you schedule a follow-up visit in 6 weeks.
A look at alternative therapies
There are many nontraditional treatments for CTS, with yoga, carpal bone mobilization, ergonomic keyboards, and ultrasound therapy among them. Some have limited evidence to suggest that they may have a therapeutic effect;30 others have little or no evidence to support them.
Yoga. Stretching and improved joint posture with specific yoga exercises may lead to decreased compression within the carpal tunnel and increased blood flow to the median nerve. One small study found that yoga was more effective than nocturnal wrist splinting for pain relief, and had similar improvement for nocturnal symptoms and grip strength.31
Carpal bone mobilization. One small study found this physical therapy technique, which involves movement of the bones in the wrist, to improve symptoms such as numbness and tingling after 3 weeks of therapy. Yet carpal bone mobilization did not relieve pain or help restore function.32
Ergonomic keyboard. Patients who use computers at work may find that an ergonomic keyboard helps to relieve pain associated with CTS, compared with a standard keyboard.33
Therapeutic ultrasound. A recent meta-analysis found that there is only poor-quality evidence for ultrasound as an effective treatment for CTS—a process in which a round-headed instrument applied to the skin delivers sound waves that are absorbed by underlying tissues in the carpal tunnel. And there is insufficient evidence for one type of ultrasound over another, or to suggest that ultrasound is more effective than other nonsurgical treatments.34 Notably, ultrasound takes several weeks to provide a therapeutic benefit.
What about acupuncture? A recent trial found that acupuncture was no more effective than sham acupuncture in relieving symptoms of CTS in patients wearing wrist splints.35 Magnet therapy, chiropractic, and cold laser therapy are not supported by evidence either.28
Is the patient a candidate for surgery?
Carpal tunnel release provides good long-term outcomes for 70% to 90% of patients and is a cost-effective treatment.36,37 Evidence supports a trial of conservative therapy, however, before considering surgery for patients with mild-to-moderate CTS.22 Future studies are needed to identify prognostic characteristics of patients most likely to respond to each type of intervention, and the optimal timing for surgical release.
Patients with severe CTS—with findings such as thenar atrophy, diminished hand function, and median nerve denervation—should be referred for surgery without delay. This recommendation is based on expert opinion, however, as most clinical trials comparing surgical vs nonsurgical treatment exclude those with severe CTS.38
3 surgical techniques, and a novel approach
Surgical techniques include open, endoscopic, and minimal incision carpal tunnel release, with benefits and drawbacks for each. Compared with open release, for example, patients who undergo endoscopic release have less postoperative pain at 12 weeks, quicker return to work, and fewer wound complications, but are more likely to require surgical revision. And minimal incision release is associated with improved symptoms and function compared with open release.38 However, there is no long-term evidence that any one of these 3 surgical approaches is more effective than another.39
Percutaneous carpal tunnel release is a novel approach that may be offered in outpatient settings, with local anesthesia and ultrasound guidance to avoid median nerve damage.40 Because studies of the safety and efficacy of percutaneous carpal tunnel release are limited, however, this approach is considered experimental.41 Percutaneous release is not a treatment recommended by the AAOS.38
What to tell patients about postop care
Regardless of the method used for carpal tunnel release, most complications are minor—eg, a painful or hypertrophic scar, stiffness, swelling, and pain or tenderness on either side of the incision—and resolve within a few months.42 Advise patients not to continue to wear a wrist splint after surgery; doing so can cause stiffness or adhesions and may compromise surgical outcomes.41 Postoperatively, patients should be instructed to do nerve gliding exercises and to massage their scars, both of which they can safely do at home.43
Patients can expect significant symptomatic improvement within 1 week of surgery, and most will be able to return to normal activities in 2 weeks.44 Those with severe CTS should be warned, however, that it could take up to a year to determine the extent of recovery.22 Evidence suggests that from 3% to 19% of patients may have persistent or recurrent symptoms even after carpal tunnel release, with up to 12% requiring surgical revision.45
CASE When Ms. K returns, she reports that while there has been some improvement, some activities—such as driving long distances and talking on the phone—still cause numbness and tingling. And, if she doesn’t wear the splint at night, she awakens with tingling in her hands. You discuss 2 options—continued conservative treatment with a local steroid injection, or EDS and surgical referral. The patient opts for the injection and continued use of the nocturnal wrist splint and exercises. When she returns in another 6 weeks, Ms. K reports significant improvement. You agree to stop the wrist splint and exercises and advise her to follow-up on an as-needed basis if the symptoms return.
CORRESPONDENCE Jennifer Wipperman, MD, MPH, Via Christi Family Medicine, 1121 S. Clifton, Wichita, KS 67218; jennifer.wipperman@viachristi.org
1. Atroshi I, Gummesson C, Johnsson, et al. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282:153-158.
2. Luckhaupt SE, Dahlhamer JM, Ward BW, et al. Prevalence and work-relatedness of carpal tunnel syndrome in the working population, United States, 2010 national health interview survey. Am J Ind Med. 2012 April 12. [Epub ahead of print.]
3. van Dijk MA, Reitsma JB, Fischer JC, et al. Indications for requesting laboratory tests for concurrent diseases in patients with carpal tunnel syndrome: a systematic review. Clin Chem. 2003;49:1437-1444.
4. Padua L, Di Pasquale A, Pazzaglia C, et al. Systematic review of pregnancy-related carpal tunnel syndrome. Muscle Nerve. 2010;42:697-702.
5. Bland JD. The relationship of obesity, age, and carpal tunnel syndrome: more complex than was thought? Muscle Nerve. 2005;32:527-532.
6. Prime MS, Palmer J, Khan WS, et al. Is there light at the end of the tunnel? Controversies in the diagnosis and management of carpal tunnel syndrome. Hand. 2010;5:354-360.
7. Keith MW, Masear V, Chung KC, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on diagnosis of carpal tunnel syndrome. J Bone Joint Surg Am. 2009;91:2478-2479.
8. Hansen PA, Micklesen P, Robinson LR. Clinical utility of the flick maneuver in diagnosing carpal tunnel syndrome. Am J Phys Med Rehabil. 2004;83:363-367.
9. Padua L, Padua R, Aprile I, et al. Carpal tunnel syndrome: relationship between clinical and patient-oriented assessment. Clin Orthop Relat Res. 2002;395:128-134.
10. Bland JD. Carpal tunnel syndrome. BMJ. 2007;335:343-346.
11. Wright PE. Carpal tunnel syndrome. In: Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, Pa: Mosby, Inc; 2008:4285–4291.
12. Sibley PA, Mandel RJ. Atraumatic acute carpal tunnel syndrome in a patient taking dabigatran. Orthopedics. 2012;35:e1286-e1289.
13. MacDermid JC, Wessel J. Clinical diagnosis of carpal tunnel syndrome: a systematic review. J Hand Ther. 2004;17:309-319.
14. Ahn DS. Hand elevation: a new test for carpal tunnel syndrome. Ann Plastic Surg. 2001;46:120-124.
15. Boyer K, Wies J, Turkelson CM. Effects of bias on the results of diagnostic studies of carpal tunnel syndrome. J Hand Surg. 2009;34:1006-1013.
16. Witt JC, Hentz JG, Stevens JC. Carpal tunnel syndrome with normal nerve conduction studies. Muscle Nerve. 2004;29:515-522.
17. Graham B. The value added by electrodiagnostic testing in the diagnosis of carpal tunnel syndrome. J Bone Joint Surg. 2008;90:2587-2593.
18. Jablecki CK, Andary MT, Floeter MK, et al. Practice parameter: electrodiagnostic studies in carpal tunnel syndrome. Report of the American Association of Electrodiagnostic Medicine, American Academy of Neurology, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2002;58:1589-1592.
19. Descatha A, Huard L, Aubert F, et al. Meta-analysis on the performance of sonography for the diagnosis of carpal tunnel syndrome. Semin Arthritis Rheum. 2012;41:914-922.
20. Visser LH, Ngo Q, Groeneweg SJ, et al. Long term effect of local corticosteroid injection for carpal tunnel syndrome: a relation with electrodiagnostic severity. Clin Neurophysiol. 2012;123:838-841.
21. Padua L, Padua R, Aprile I, et al. Multiperspective follow-up of untreated carpal tunnel syndrome: a multicenter study. Neurology. 2001;56:1459-1466.
22. Shi Q, MacDermid JC. Is surgical intervention more effective than non-surgical treatment for carpal tunnel syndrome? A systematic review. J Orthop Surg Res. 2011;6:17.-
23. Walker WC, Metzler M, Cifu DX, et al. Neutral wrist splinting in carpal tunnel syndrome: a comparison of night-only versus full-time wear instructions. Arch Phys Med Rehabil. 2000;81:424-429.
24. Brininger TL, Rogers JC, Holm MB, et al. Efficacy of a fabricated customized splint and tendon and nerve gliding exercises for the treatment of carpal tunnel syndrome: a randomized controlled trial. Arch Phys Med Rehabil. 2007;88:1429-1435.
25. Schmid AB, Elliott JM, Strudwick MW, et al. Effect of splinting and exercise on intraneural edema of the median nerve in carpal tunnel syndrome-an MRI study to reveal therapeutic mechanisms. J Orthop Res. 2012;30:1343-1350.
26. Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(2):CD001554.-
27. Kamanli A, Bezgincan M, Kaya A. Comparison of local steroid injection into carpal tunnel via proximal and distal approach in patients with carpal tunnel syndrome. Bratislavske Lek Listy. 2011;112:337-341.
28. Huisstede BM, Hoogvliet P, Randsdorp MS, et al. Carpal tunnel syndrome. Part I: effectiveness of nonsurgical treatments—a systematic review. Arch Phys Med Rehabil. 2010;91:981-1004.
29. Chang MH, Ger LP, Hsieh PF, et al. A randomised clinical trial of oral steroids in the treatment of carpal tunnel syndrome: a long term follow up. J Neurol Neurosurg Psychiatry. 2002;73:710-714.
30. O’Connor D, Marshall S, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. 2003;(1):CD003219.-
31. Garfinkel MS, Singhal A, Katz WA, et al. Yoga-based intervention for carpal tunnel syndrome: a randomized trial. JAMA. 1998;280:1601-1603.
32. Tal-Akabi A, Rushton A. An investigation to compare the effectiveness of carpal bone mobilisation and neurodynamic mobilisation as methods of treatment for carpal tunnel syndrome. Man Ther. 2000;5:214-222.
33. O’Connor D, Page MJ, Marshall SC, et al. Ergonomic positioning or equipment for treating carpal tunnel syndrome. Cochrane Database Syst Rev. 2012;(1):CD009600.-
34. Page MJ, O’Connor D, Pitt V, et al. Therapeutic ultrasound for carpal tunnel syndrome. Cochrane Database Syst Rev. 2012;(1):CD009601.-
35. Yao E, Gerritz PK, Henricson E, et al. Randomized controlled trial comparing acupuncture with placebo acupuncture for the treatment of carpal tunnel syndrome. PMR. 2012;4:367-373.
36. Pomerance J, Zurakowski D, Fine I. The cost-effectiveness of nonsurgical versus surgical treatment for carpal tunnel syndrome. J Hand Surg. 2009;34:1193-1200.
37. Turner A, Kimble F, Gulyas K, et al. Can the outcome of open carpal tunnel release be predicted? A review of the literature. ANZ J Surg. 2010;80:50-54.
38. Keith MW, Masear V, Chung KC, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of carpal tunnel syndrome. J Bone Joint Surg. 2010;92:218-219.
39. Scholten RJ, Mink van der Molen A, Uitdehaag BM, et al. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(4):CD003905.-
40. Nakamichi K, Tachibana S, Yamamoto S, et al. Percutaneous carpal tunnel release compared with mini-open release using ultrasonographic guidance for both techniques. J Hand Surg Am. 2010;35:437-445.
41. Huisstede BM, Randsdorp MS, Coert JH, et al. Carpal tunnel syndrome. Part II: effectiveness of surgical treatments—a systematic review. Arch Phys Med Rehabil. 2010;91:1005-1024.
42. Ludlow KS, Merla JL, Cox JA, et al. Pillar pain as a postoperative complication of carpal tunnel release: a review of the literature. J Hand Ther. 1997;10:277-282.
43. Pomerance J, Fine I. Outcomes of carpal tunnel surgery with and without supervised postoperative therapy. J Hand Surg. 2007;32:1159-1163.
44. Acharya AD, Auchincloss JM. Return to functional hand use and work following open carpal tunnel surgery. J Hand Surg Br. 2005;30:607-610.
45. Dahlin LB, Salo M, Thomsen N, et al. Carpal tunnel syndrome and treatment of recurrent symptoms. Scand J Plast Reconstr Surg Hand Surg. 2010;44:4-11.
• Before considering surgery, offer patients with mild-to-moderate carpal tunnel syndrome (CTS) a trial of conservative therapy such as splinting or corticosteroids. A
• Order electrodiagnostic studies (EDS) as needed, to rule out other conditions with a similar presentation, confirm an uncertain diagnosis, and gauge the severity of CTS C, or when surgery is being considered. B
• Recommend carpal tunnel release for patients who have severe CTS or have failed to respond to nonsurgical t0reatment. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane K, 52, comes to see you because of discomfort in her right wrist and tingling in her hand. The symptoms began 3 months ago, but have been getting progressively worse, and have started to interfere with her sleep. Ms. K often awakens with “pins and needles” in her hand, and says that she often has the urge to “shake it out.” Her sister has carpal tunnel syndrome (CTS), and Ms. K suspects that she does, too. On exam, you find that Ms. K has a positive Phalen’s and Durkan’s compression test, but normal Tinel’s test. She has normal strength and sensation in her hands. Her neck and upper extremity exam is otherwise unremarkable. You note that her hypothyroidism is well controlled, with a recent thyroid-stimulating hormone level of 1.2 mIU/L.
The patient has tried acetaminophen and ibuprofen, with little relief. She has researched CTS on the Internet and read about cold laser therapy, and wants to know whether you think it will work. What should you tell her?
Carpal tunnel syndrome is one of the most common disorders of the upper extremities and the most prevalent compression neuropathy.1 About 3% of US adults are affected, typically those between the ages of 40 and 60 years.2 Women are almost 3 times more likely than men to develop CTS.1
Other risk factors include diabetes, hypothyroidism, rheumatoid arthritis, pregnancy, obesity, family history, and trauma. A history of hand-related repetitive motions also increases the risk.3-5 Evidence does not support a definite link between keyboard or mouse use and CTS; however, occupations that require use of hand-operated vibratory tools or repeated and forceful movements of the hand/wrist (such as assembly work and food processing or packaging) are associated with CTS.6
The optimal diagnostic approach incorporates history and physical exam findings, including the results of a number of provocative maneuvers, as well as electrodiagnostic studies (EDS) in some cases.7 While surgery is the definitive treatment for CTS, numerous nonsurgical options exist, including splinting, corticosteroids, and a variety of alternative therapies, some of which (eg, chiropractic and cold laser therapy) have little evidence to support them.
Because family physicians are often the first to see patients with symptoms associated with CTS, you need to know what to look for, when to test, and whether to provide treatment or a referral. Here’s what to keep in mind.
Clinical presentation of CTS
Increased pressure in the carpal tunnel compresses the median nerve, leading to numbness, tingling, or pain in the palmar aspect of the first 3 fingers and the radial half of the fourth (FIGURE). Symptoms vary widely, with pain or numbness localized to the hand or wrist in some cases and pain radiating into the forearm or shoulder in others.
Figure
Compressed median nerve leads to numbness and tingling
Early in the course of CTS, symptoms are often most bothersome at night. In a scenario like that reported by Ms. K, patients are often awakened by numbness or tingling and the desire to shake out the affected hand—a phenomenon known as the flick sign.8 Pain and numbness may occur intermittently at first, especially with repetitive wrist motion. Activities such as driving or holding a telephone often aggravate symptoms.
As CTS progresses, the intensity and duration of symptoms increase. Patients may complain of weakness in the hand and report that they often drop things. Paradoxically, patients with more severe CTS sometimes have less pain, rather than more, because of increasing sensory loss.9
Late in the course of CTS, physical exam findings typically include decreased sensation in the fingers innervated by the median nerve, sparing the thenar eminence. (A loss of sensation in the thenar eminence suggests the presence of a lesion proximal to the carpal tunnel, rather than CTS itself.10) In advanced cases, weakness of thumb abduction and opposition may occur, as well as atrophy of the thenar eminence.11
Sudden onset of severe symptoms with minimal trauma to the wrist should raise suspicion of a hematoma in the carpal tunnel—a particular risk for patients who have a clotting disorder or are being treated with newer anticoagulants such as dabigatran. Prompt surgical decompression is required to prevent permanent median nerve damage in such cases.12
Include these maneuvers in the physical exam
A thorough evaluation of the neck, shoulder, elbow, and wrist is crucial for all patients with signs and symptoms associated with CTS. Provocative maneuvers (TABLE 1)7,13 are also important as an aid to diagnosis. The results of the following tests should be viewed with caution, however, as studies have found wide variations in their sensitivity and specificity:
TABLE 1
Diagnosing carpal tunnel syndrome, using physical maneuvers7,13
| Test | Technique | Positive test | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| Phalen’s | Patient holds wrist flexed 90° with elbow in full extension | Pain or paresthesia ≤60 sec | 68 | 73 |
| Tinel’s | Clinician repetitively taps wrist over transverse carpal ligament | Pain or paresthesia | 50 | 77 |
| Median nerve compression* (MNC) | Clinician applies direct pressure over the transverse carpal ligament | Pain or paresthesia ≤30 sec | 64 | 83 |
| MNC + Phalen’s | Same as above | Same as above | 80 | 92 |
| *also known as Durkan’s test. | ||||
Phalen’s maneuver. The patient flexes his or her wrist with the elbow in full extension to increase pressure on the median nerve, and holds the position for 60 seconds. The onset of pain or paresthesia is a positive test. A meta-analysis found the sensitivity and specificity of a positive Phalen’s sign to be 68% and 73%, respectively.7
Tinel’s test. Tap the volar surface of the patient’s wrist just proximal to, or on top of, the carpal tunnel. Pain or paresthesia in the fingers innervated by the median nerve as a result of the percussion constitutes a positive result. Tinel’s test is less sensitive than the Phalen’s maneuver, but has a similar specificity.13
The median nerve (Durkan’s) compression test. Apply pressure over the transverse carpal ligament; the test is positive if pain or paresthesia develops within 30 seconds.7
The hand elevation test. The patient raises both hands overhead for 60 seconds; here, too, pain or paresthesia is a positive result.14
Combining results of provocative maneuvers may increase sensitivity and specificity. Positive results in both the Phalen’s and median nerve compression tests, for example, have a collective sensitivity and specificity of 80% and 92%, respectively.13
When (or whether) to order electrodiagnostic studies
While some clinicians consider EDS to be the gold standard in CTS diagnosis,6 evidence is limited. One issue is the lack of universally accepted reference standards; another is that most studies have been affected by “spectrum bias.”15 What’s more, EDS—which include nerve conduction studies (NCS) and electromyography (EMG)—do not always correlate directly with symptoms, and 16% to 34% of mild cases can be missed.16
EDS are useful in many instances, however. EMG can rule out other causes of CTS symptoms (TABLE 2 details the differential diagnosis),7,11 while NCS can aid in diagnosing CTS, gauging its severity, and arriving at a prognosis. Specifically, NCS can detect delayed distal latencies and slowed conduction velocities that can occur when the myelin sheath is damaged by prolonged compression of the median nerve.17 With more severe compression, axonal damage occurs, as evidenced by reduced action potential amplitudes on NCS. Results of the nerve conduction tests are compared to age-dependent normal values and to results from other nerves on either the same or the contralateral hand. In a 2002 systemic review, the sensitivity of NCS for CTS was 56% to 85% and the specificity was 94% to 99%.18
TABLE 2
Differential diagnosis for CTS7,11
| Condition | Characteristics |
|---|---|
| Carpometacarpal arthritis of thumb | Thumb is painful when in motion; radiographic findings |
| Cervical radiculopathy | Neck pain, nerve root distribution (eg, C6), positive Spurling’s test |
| DeQuervain’s tenosynovitis | Painful resisted thumb dorsiflexion, tender at base of thumb |
| Hypothyroidism | Fatigue, cold intolerance, dry skin, hair loss, abnormal thyroid function tests |
| Peripheral neuropathy | History of DM, lower extremity involvement |
| Pronator syndrome (median nerve compression at the elbow) | Tenderness at proximal forearm |
| Ulnar compressive neuropathy | Compression and positive Tinel’s sign: ulnar nerve at elbow or wrist produces pain or paresthesias in 4th and 5th fingers |
| Vibration white finger | History of use of power drill or other hand-held vibratory tool; symptoms of Raynaud’s syndrome |
| Wrist arthritis | Painful wrist ROM, radiographic findings |
| CTS, carpal tunnel syndrome; DM, diabetes mellitus; ROM, range of motion. | |
Before and after surgery. The American Academy of Orthopedic Surgeons (AAOS) recommends EDS when CTS surgery is being considered. 7 EDS may also be used after surgery, to verify neurologic improvement.
Ultrasound. In patients with CTS, ultrasound reveals an increased cross-sectional area of the median nerve, a finding that has prompted studies of this modality as a diagnostic tool.19 Although evidence suggests that ultrasound’s sensitivity and specificity for CTS would be similar to that of EDS, the optimal cutoff for an abnormal test has not been defined,19 and ultrasound does not provide information on prognosis or alternate causes.
Thus, AAOS does not currently recommend ultrasound for CTS diagnosis.7 Magnetic resonance imaging is inappropriate for routine CTS diagnosis, as well.7
Treatment: Start conservatively
Multiple nonsurgical options are available, but the best evidence supports splinting, steroid injection, and oral steroids. Splinting or steroids alone may bring long-term relief for patients with mild to moderate cases;20 in fact, about a third of mild cases improve spontaneously.21
Conservative therapy can also provide relief for those who wish to avoid or delay surgery and for cases of transient CTS (pediatric patients, for example, or those whose condition is associated with pregnancy or hypothyroidism).18 A successful response to therapy can also help to confirm a CTS diagnosis.
Most conservative treatments begin providing relief within 2 to 6 weeks and reach the maximal benefit at 3 months.22 If there is no response after 6 weeks, it’s time to consider another approach.
In initiating splinting or corticosteroids, here’s a look at what to keep in mind:
Splinting. A splint can be used to maintain the wrist in a position with the least intracanal pressure, thereby limiting pressure on the median nerve. Splinting is equally effective whether used continually or only at night.23
Splinting can relieve symptoms and improve functional status within 2 weeks and the effects can last for 3 to 6 months, eliminating the need for surgery for some patients with mild CTS.19,20 Nerve gliding exercises, (see image at left), have been evaluated in combination with splinting. While evidence is limited, an at-home program involving these simple exercises may be a beneficial adjunctive treatment with minimal cost or harm.24,25
Local corticosteroid injection. A Cochrane meta-analysis found significant improvement in symptoms and function at one month among patients with CTS who were treated with steroid injection.26 In many cases, the effects last for many months.
A recent trial found that nearly half of patients with mild to moderate CTS who were treated with steroid injections had improved symptoms and EDS results at the 12-month follow-up.20 However, while patients with severe CTS experienced improvement at 4 weeks postinjection, most eventually required surgery.20
Evidence does not support one particular steroid dose or formulation over another, or one particular injection site.22 Injecting 4 cm proximal to the wrist flexion crease is as effective as a more distal injection.26,27
Caution is required, however, as risks associated with local injections include tendon rupture and median nerve injury. If a patient experiences intense pain or paresthesia in the median nerve distribution when the needle is inserted, redirect the needle away from the median nerve immediately. For patients who respond well to this treatment, one additional injection can be given after 6 months if symptoms recur.
Oral corticosteroids. Oral prednisone at a dose of 20 mg/d for 2 weeks improves symptoms and function in patients with CTS, but is less effective than steroid injections.28 Treatment for 2 weeks is as effective as treatment for 4 weeks; the effects tend to wane after 8 weeks in both cases.29 Nonsteroidal anti-inflammatory drugs, diuretics, and vitamin B6 have not been found to be effective.30
CASE Ms. K also asks about “those needle tests”—a reference to EDS—which her sister had to diagnose her CTS. You explain that these studies are not necessary at this time because her symptoms are mild and there is no need for other causes to be ruled out.
Instead, you offer her a neutral wrist splint for night-time use and recommend home-based nerve glide exercises. There is no evidence that cold laser therapy is effective, you explain to Ms. K, and it is expensive. She agrees to try the splint and the exercises, and you schedule a follow-up visit in 6 weeks.
A look at alternative therapies
There are many nontraditional treatments for CTS, with yoga, carpal bone mobilization, ergonomic keyboards, and ultrasound therapy among them. Some have limited evidence to suggest that they may have a therapeutic effect;30 others have little or no evidence to support them.
Yoga. Stretching and improved joint posture with specific yoga exercises may lead to decreased compression within the carpal tunnel and increased blood flow to the median nerve. One small study found that yoga was more effective than nocturnal wrist splinting for pain relief, and had similar improvement for nocturnal symptoms and grip strength.31
Carpal bone mobilization. One small study found this physical therapy technique, which involves movement of the bones in the wrist, to improve symptoms such as numbness and tingling after 3 weeks of therapy. Yet carpal bone mobilization did not relieve pain or help restore function.32
Ergonomic keyboard. Patients who use computers at work may find that an ergonomic keyboard helps to relieve pain associated with CTS, compared with a standard keyboard.33
Therapeutic ultrasound. A recent meta-analysis found that there is only poor-quality evidence for ultrasound as an effective treatment for CTS—a process in which a round-headed instrument applied to the skin delivers sound waves that are absorbed by underlying tissues in the carpal tunnel. And there is insufficient evidence for one type of ultrasound over another, or to suggest that ultrasound is more effective than other nonsurgical treatments.34 Notably, ultrasound takes several weeks to provide a therapeutic benefit.
What about acupuncture? A recent trial found that acupuncture was no more effective than sham acupuncture in relieving symptoms of CTS in patients wearing wrist splints.35 Magnet therapy, chiropractic, and cold laser therapy are not supported by evidence either.28
Is the patient a candidate for surgery?
Carpal tunnel release provides good long-term outcomes for 70% to 90% of patients and is a cost-effective treatment.36,37 Evidence supports a trial of conservative therapy, however, before considering surgery for patients with mild-to-moderate CTS.22 Future studies are needed to identify prognostic characteristics of patients most likely to respond to each type of intervention, and the optimal timing for surgical release.
Patients with severe CTS—with findings such as thenar atrophy, diminished hand function, and median nerve denervation—should be referred for surgery without delay. This recommendation is based on expert opinion, however, as most clinical trials comparing surgical vs nonsurgical treatment exclude those with severe CTS.38
3 surgical techniques, and a novel approach
Surgical techniques include open, endoscopic, and minimal incision carpal tunnel release, with benefits and drawbacks for each. Compared with open release, for example, patients who undergo endoscopic release have less postoperative pain at 12 weeks, quicker return to work, and fewer wound complications, but are more likely to require surgical revision. And minimal incision release is associated with improved symptoms and function compared with open release.38 However, there is no long-term evidence that any one of these 3 surgical approaches is more effective than another.39
Percutaneous carpal tunnel release is a novel approach that may be offered in outpatient settings, with local anesthesia and ultrasound guidance to avoid median nerve damage.40 Because studies of the safety and efficacy of percutaneous carpal tunnel release are limited, however, this approach is considered experimental.41 Percutaneous release is not a treatment recommended by the AAOS.38
What to tell patients about postop care
Regardless of the method used for carpal tunnel release, most complications are minor—eg, a painful or hypertrophic scar, stiffness, swelling, and pain or tenderness on either side of the incision—and resolve within a few months.42 Advise patients not to continue to wear a wrist splint after surgery; doing so can cause stiffness or adhesions and may compromise surgical outcomes.41 Postoperatively, patients should be instructed to do nerve gliding exercises and to massage their scars, both of which they can safely do at home.43
Patients can expect significant symptomatic improvement within 1 week of surgery, and most will be able to return to normal activities in 2 weeks.44 Those with severe CTS should be warned, however, that it could take up to a year to determine the extent of recovery.22 Evidence suggests that from 3% to 19% of patients may have persistent or recurrent symptoms even after carpal tunnel release, with up to 12% requiring surgical revision.45
CASE When Ms. K returns, she reports that while there has been some improvement, some activities—such as driving long distances and talking on the phone—still cause numbness and tingling. And, if she doesn’t wear the splint at night, she awakens with tingling in her hands. You discuss 2 options—continued conservative treatment with a local steroid injection, or EDS and surgical referral. The patient opts for the injection and continued use of the nocturnal wrist splint and exercises. When she returns in another 6 weeks, Ms. K reports significant improvement. You agree to stop the wrist splint and exercises and advise her to follow-up on an as-needed basis if the symptoms return.
CORRESPONDENCE Jennifer Wipperman, MD, MPH, Via Christi Family Medicine, 1121 S. Clifton, Wichita, KS 67218; jennifer.wipperman@viachristi.org
• Before considering surgery, offer patients with mild-to-moderate carpal tunnel syndrome (CTS) a trial of conservative therapy such as splinting or corticosteroids. A
• Order electrodiagnostic studies (EDS) as needed, to rule out other conditions with a similar presentation, confirm an uncertain diagnosis, and gauge the severity of CTS C, or when surgery is being considered. B
• Recommend carpal tunnel release for patients who have severe CTS or have failed to respond to nonsurgical t0reatment. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane K, 52, comes to see you because of discomfort in her right wrist and tingling in her hand. The symptoms began 3 months ago, but have been getting progressively worse, and have started to interfere with her sleep. Ms. K often awakens with “pins and needles” in her hand, and says that she often has the urge to “shake it out.” Her sister has carpal tunnel syndrome (CTS), and Ms. K suspects that she does, too. On exam, you find that Ms. K has a positive Phalen’s and Durkan’s compression test, but normal Tinel’s test. She has normal strength and sensation in her hands. Her neck and upper extremity exam is otherwise unremarkable. You note that her hypothyroidism is well controlled, with a recent thyroid-stimulating hormone level of 1.2 mIU/L.
The patient has tried acetaminophen and ibuprofen, with little relief. She has researched CTS on the Internet and read about cold laser therapy, and wants to know whether you think it will work. What should you tell her?
Carpal tunnel syndrome is one of the most common disorders of the upper extremities and the most prevalent compression neuropathy.1 About 3% of US adults are affected, typically those between the ages of 40 and 60 years.2 Women are almost 3 times more likely than men to develop CTS.1
Other risk factors include diabetes, hypothyroidism, rheumatoid arthritis, pregnancy, obesity, family history, and trauma. A history of hand-related repetitive motions also increases the risk.3-5 Evidence does not support a definite link between keyboard or mouse use and CTS; however, occupations that require use of hand-operated vibratory tools or repeated and forceful movements of the hand/wrist (such as assembly work and food processing or packaging) are associated with CTS.6
The optimal diagnostic approach incorporates history and physical exam findings, including the results of a number of provocative maneuvers, as well as electrodiagnostic studies (EDS) in some cases.7 While surgery is the definitive treatment for CTS, numerous nonsurgical options exist, including splinting, corticosteroids, and a variety of alternative therapies, some of which (eg, chiropractic and cold laser therapy) have little evidence to support them.
Because family physicians are often the first to see patients with symptoms associated with CTS, you need to know what to look for, when to test, and whether to provide treatment or a referral. Here’s what to keep in mind.
Clinical presentation of CTS
Increased pressure in the carpal tunnel compresses the median nerve, leading to numbness, tingling, or pain in the palmar aspect of the first 3 fingers and the radial half of the fourth (FIGURE). Symptoms vary widely, with pain or numbness localized to the hand or wrist in some cases and pain radiating into the forearm or shoulder in others.
Figure
Compressed median nerve leads to numbness and tingling
Early in the course of CTS, symptoms are often most bothersome at night. In a scenario like that reported by Ms. K, patients are often awakened by numbness or tingling and the desire to shake out the affected hand—a phenomenon known as the flick sign.8 Pain and numbness may occur intermittently at first, especially with repetitive wrist motion. Activities such as driving or holding a telephone often aggravate symptoms.
As CTS progresses, the intensity and duration of symptoms increase. Patients may complain of weakness in the hand and report that they often drop things. Paradoxically, patients with more severe CTS sometimes have less pain, rather than more, because of increasing sensory loss.9
Late in the course of CTS, physical exam findings typically include decreased sensation in the fingers innervated by the median nerve, sparing the thenar eminence. (A loss of sensation in the thenar eminence suggests the presence of a lesion proximal to the carpal tunnel, rather than CTS itself.10) In advanced cases, weakness of thumb abduction and opposition may occur, as well as atrophy of the thenar eminence.11
Sudden onset of severe symptoms with minimal trauma to the wrist should raise suspicion of a hematoma in the carpal tunnel—a particular risk for patients who have a clotting disorder or are being treated with newer anticoagulants such as dabigatran. Prompt surgical decompression is required to prevent permanent median nerve damage in such cases.12
Include these maneuvers in the physical exam
A thorough evaluation of the neck, shoulder, elbow, and wrist is crucial for all patients with signs and symptoms associated with CTS. Provocative maneuvers (TABLE 1)7,13 are also important as an aid to diagnosis. The results of the following tests should be viewed with caution, however, as studies have found wide variations in their sensitivity and specificity:
TABLE 1
Diagnosing carpal tunnel syndrome, using physical maneuvers7,13
| Test | Technique | Positive test | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| Phalen’s | Patient holds wrist flexed 90° with elbow in full extension | Pain or paresthesia ≤60 sec | 68 | 73 |
| Tinel’s | Clinician repetitively taps wrist over transverse carpal ligament | Pain or paresthesia | 50 | 77 |
| Median nerve compression* (MNC) | Clinician applies direct pressure over the transverse carpal ligament | Pain or paresthesia ≤30 sec | 64 | 83 |
| MNC + Phalen’s | Same as above | Same as above | 80 | 92 |
| *also known as Durkan’s test. | ||||
Phalen’s maneuver. The patient flexes his or her wrist with the elbow in full extension to increase pressure on the median nerve, and holds the position for 60 seconds. The onset of pain or paresthesia is a positive test. A meta-analysis found the sensitivity and specificity of a positive Phalen’s sign to be 68% and 73%, respectively.7
Tinel’s test. Tap the volar surface of the patient’s wrist just proximal to, or on top of, the carpal tunnel. Pain or paresthesia in the fingers innervated by the median nerve as a result of the percussion constitutes a positive result. Tinel’s test is less sensitive than the Phalen’s maneuver, but has a similar specificity.13
The median nerve (Durkan’s) compression test. Apply pressure over the transverse carpal ligament; the test is positive if pain or paresthesia develops within 30 seconds.7
The hand elevation test. The patient raises both hands overhead for 60 seconds; here, too, pain or paresthesia is a positive result.14
Combining results of provocative maneuvers may increase sensitivity and specificity. Positive results in both the Phalen’s and median nerve compression tests, for example, have a collective sensitivity and specificity of 80% and 92%, respectively.13
When (or whether) to order electrodiagnostic studies
While some clinicians consider EDS to be the gold standard in CTS diagnosis,6 evidence is limited. One issue is the lack of universally accepted reference standards; another is that most studies have been affected by “spectrum bias.”15 What’s more, EDS—which include nerve conduction studies (NCS) and electromyography (EMG)—do not always correlate directly with symptoms, and 16% to 34% of mild cases can be missed.16
EDS are useful in many instances, however. EMG can rule out other causes of CTS symptoms (TABLE 2 details the differential diagnosis),7,11 while NCS can aid in diagnosing CTS, gauging its severity, and arriving at a prognosis. Specifically, NCS can detect delayed distal latencies and slowed conduction velocities that can occur when the myelin sheath is damaged by prolonged compression of the median nerve.17 With more severe compression, axonal damage occurs, as evidenced by reduced action potential amplitudes on NCS. Results of the nerve conduction tests are compared to age-dependent normal values and to results from other nerves on either the same or the contralateral hand. In a 2002 systemic review, the sensitivity of NCS for CTS was 56% to 85% and the specificity was 94% to 99%.18
TABLE 2
Differential diagnosis for CTS7,11
| Condition | Characteristics |
|---|---|
| Carpometacarpal arthritis of thumb | Thumb is painful when in motion; radiographic findings |
| Cervical radiculopathy | Neck pain, nerve root distribution (eg, C6), positive Spurling’s test |
| DeQuervain’s tenosynovitis | Painful resisted thumb dorsiflexion, tender at base of thumb |
| Hypothyroidism | Fatigue, cold intolerance, dry skin, hair loss, abnormal thyroid function tests |
| Peripheral neuropathy | History of DM, lower extremity involvement |
| Pronator syndrome (median nerve compression at the elbow) | Tenderness at proximal forearm |
| Ulnar compressive neuropathy | Compression and positive Tinel’s sign: ulnar nerve at elbow or wrist produces pain or paresthesias in 4th and 5th fingers |
| Vibration white finger | History of use of power drill or other hand-held vibratory tool; symptoms of Raynaud’s syndrome |
| Wrist arthritis | Painful wrist ROM, radiographic findings |
| CTS, carpal tunnel syndrome; DM, diabetes mellitus; ROM, range of motion. | |
Before and after surgery. The American Academy of Orthopedic Surgeons (AAOS) recommends EDS when CTS surgery is being considered. 7 EDS may also be used after surgery, to verify neurologic improvement.
Ultrasound. In patients with CTS, ultrasound reveals an increased cross-sectional area of the median nerve, a finding that has prompted studies of this modality as a diagnostic tool.19 Although evidence suggests that ultrasound’s sensitivity and specificity for CTS would be similar to that of EDS, the optimal cutoff for an abnormal test has not been defined,19 and ultrasound does not provide information on prognosis or alternate causes.
Thus, AAOS does not currently recommend ultrasound for CTS diagnosis.7 Magnetic resonance imaging is inappropriate for routine CTS diagnosis, as well.7
Treatment: Start conservatively
Multiple nonsurgical options are available, but the best evidence supports splinting, steroid injection, and oral steroids. Splinting or steroids alone may bring long-term relief for patients with mild to moderate cases;20 in fact, about a third of mild cases improve spontaneously.21
Conservative therapy can also provide relief for those who wish to avoid or delay surgery and for cases of transient CTS (pediatric patients, for example, or those whose condition is associated with pregnancy or hypothyroidism).18 A successful response to therapy can also help to confirm a CTS diagnosis.
Most conservative treatments begin providing relief within 2 to 6 weeks and reach the maximal benefit at 3 months.22 If there is no response after 6 weeks, it’s time to consider another approach.
In initiating splinting or corticosteroids, here’s a look at what to keep in mind:
Splinting. A splint can be used to maintain the wrist in a position with the least intracanal pressure, thereby limiting pressure on the median nerve. Splinting is equally effective whether used continually or only at night.23
Splinting can relieve symptoms and improve functional status within 2 weeks and the effects can last for 3 to 6 months, eliminating the need for surgery for some patients with mild CTS.19,20 Nerve gliding exercises, (see image at left), have been evaluated in combination with splinting. While evidence is limited, an at-home program involving these simple exercises may be a beneficial adjunctive treatment with minimal cost or harm.24,25
Local corticosteroid injection. A Cochrane meta-analysis found significant improvement in symptoms and function at one month among patients with CTS who were treated with steroid injection.26 In many cases, the effects last for many months.
A recent trial found that nearly half of patients with mild to moderate CTS who were treated with steroid injections had improved symptoms and EDS results at the 12-month follow-up.20 However, while patients with severe CTS experienced improvement at 4 weeks postinjection, most eventually required surgery.20
Evidence does not support one particular steroid dose or formulation over another, or one particular injection site.22 Injecting 4 cm proximal to the wrist flexion crease is as effective as a more distal injection.26,27
Caution is required, however, as risks associated with local injections include tendon rupture and median nerve injury. If a patient experiences intense pain or paresthesia in the median nerve distribution when the needle is inserted, redirect the needle away from the median nerve immediately. For patients who respond well to this treatment, one additional injection can be given after 6 months if symptoms recur.
Oral corticosteroids. Oral prednisone at a dose of 20 mg/d for 2 weeks improves symptoms and function in patients with CTS, but is less effective than steroid injections.28 Treatment for 2 weeks is as effective as treatment for 4 weeks; the effects tend to wane after 8 weeks in both cases.29 Nonsteroidal anti-inflammatory drugs, diuretics, and vitamin B6 have not been found to be effective.30
CASE Ms. K also asks about “those needle tests”—a reference to EDS—which her sister had to diagnose her CTS. You explain that these studies are not necessary at this time because her symptoms are mild and there is no need for other causes to be ruled out.
Instead, you offer her a neutral wrist splint for night-time use and recommend home-based nerve glide exercises. There is no evidence that cold laser therapy is effective, you explain to Ms. K, and it is expensive. She agrees to try the splint and the exercises, and you schedule a follow-up visit in 6 weeks.
A look at alternative therapies
There are many nontraditional treatments for CTS, with yoga, carpal bone mobilization, ergonomic keyboards, and ultrasound therapy among them. Some have limited evidence to suggest that they may have a therapeutic effect;30 others have little or no evidence to support them.
Yoga. Stretching and improved joint posture with specific yoga exercises may lead to decreased compression within the carpal tunnel and increased blood flow to the median nerve. One small study found that yoga was more effective than nocturnal wrist splinting for pain relief, and had similar improvement for nocturnal symptoms and grip strength.31
Carpal bone mobilization. One small study found this physical therapy technique, which involves movement of the bones in the wrist, to improve symptoms such as numbness and tingling after 3 weeks of therapy. Yet carpal bone mobilization did not relieve pain or help restore function.32
Ergonomic keyboard. Patients who use computers at work may find that an ergonomic keyboard helps to relieve pain associated with CTS, compared with a standard keyboard.33
Therapeutic ultrasound. A recent meta-analysis found that there is only poor-quality evidence for ultrasound as an effective treatment for CTS—a process in which a round-headed instrument applied to the skin delivers sound waves that are absorbed by underlying tissues in the carpal tunnel. And there is insufficient evidence for one type of ultrasound over another, or to suggest that ultrasound is more effective than other nonsurgical treatments.34 Notably, ultrasound takes several weeks to provide a therapeutic benefit.
What about acupuncture? A recent trial found that acupuncture was no more effective than sham acupuncture in relieving symptoms of CTS in patients wearing wrist splints.35 Magnet therapy, chiropractic, and cold laser therapy are not supported by evidence either.28
Is the patient a candidate for surgery?
Carpal tunnel release provides good long-term outcomes for 70% to 90% of patients and is a cost-effective treatment.36,37 Evidence supports a trial of conservative therapy, however, before considering surgery for patients with mild-to-moderate CTS.22 Future studies are needed to identify prognostic characteristics of patients most likely to respond to each type of intervention, and the optimal timing for surgical release.
Patients with severe CTS—with findings such as thenar atrophy, diminished hand function, and median nerve denervation—should be referred for surgery without delay. This recommendation is based on expert opinion, however, as most clinical trials comparing surgical vs nonsurgical treatment exclude those with severe CTS.38
3 surgical techniques, and a novel approach
Surgical techniques include open, endoscopic, and minimal incision carpal tunnel release, with benefits and drawbacks for each. Compared with open release, for example, patients who undergo endoscopic release have less postoperative pain at 12 weeks, quicker return to work, and fewer wound complications, but are more likely to require surgical revision. And minimal incision release is associated with improved symptoms and function compared with open release.38 However, there is no long-term evidence that any one of these 3 surgical approaches is more effective than another.39
Percutaneous carpal tunnel release is a novel approach that may be offered in outpatient settings, with local anesthesia and ultrasound guidance to avoid median nerve damage.40 Because studies of the safety and efficacy of percutaneous carpal tunnel release are limited, however, this approach is considered experimental.41 Percutaneous release is not a treatment recommended by the AAOS.38
What to tell patients about postop care
Regardless of the method used for carpal tunnel release, most complications are minor—eg, a painful or hypertrophic scar, stiffness, swelling, and pain or tenderness on either side of the incision—and resolve within a few months.42 Advise patients not to continue to wear a wrist splint after surgery; doing so can cause stiffness or adhesions and may compromise surgical outcomes.41 Postoperatively, patients should be instructed to do nerve gliding exercises and to massage their scars, both of which they can safely do at home.43
Patients can expect significant symptomatic improvement within 1 week of surgery, and most will be able to return to normal activities in 2 weeks.44 Those with severe CTS should be warned, however, that it could take up to a year to determine the extent of recovery.22 Evidence suggests that from 3% to 19% of patients may have persistent or recurrent symptoms even after carpal tunnel release, with up to 12% requiring surgical revision.45
CASE When Ms. K returns, she reports that while there has been some improvement, some activities—such as driving long distances and talking on the phone—still cause numbness and tingling. And, if she doesn’t wear the splint at night, she awakens with tingling in her hands. You discuss 2 options—continued conservative treatment with a local steroid injection, or EDS and surgical referral. The patient opts for the injection and continued use of the nocturnal wrist splint and exercises. When she returns in another 6 weeks, Ms. K reports significant improvement. You agree to stop the wrist splint and exercises and advise her to follow-up on an as-needed basis if the symptoms return.
CORRESPONDENCE Jennifer Wipperman, MD, MPH, Via Christi Family Medicine, 1121 S. Clifton, Wichita, KS 67218; jennifer.wipperman@viachristi.org
1. Atroshi I, Gummesson C, Johnsson, et al. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282:153-158.
2. Luckhaupt SE, Dahlhamer JM, Ward BW, et al. Prevalence and work-relatedness of carpal tunnel syndrome in the working population, United States, 2010 national health interview survey. Am J Ind Med. 2012 April 12. [Epub ahead of print.]
3. van Dijk MA, Reitsma JB, Fischer JC, et al. Indications for requesting laboratory tests for concurrent diseases in patients with carpal tunnel syndrome: a systematic review. Clin Chem. 2003;49:1437-1444.
4. Padua L, Di Pasquale A, Pazzaglia C, et al. Systematic review of pregnancy-related carpal tunnel syndrome. Muscle Nerve. 2010;42:697-702.
5. Bland JD. The relationship of obesity, age, and carpal tunnel syndrome: more complex than was thought? Muscle Nerve. 2005;32:527-532.
6. Prime MS, Palmer J, Khan WS, et al. Is there light at the end of the tunnel? Controversies in the diagnosis and management of carpal tunnel syndrome. Hand. 2010;5:354-360.
7. Keith MW, Masear V, Chung KC, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on diagnosis of carpal tunnel syndrome. J Bone Joint Surg Am. 2009;91:2478-2479.
8. Hansen PA, Micklesen P, Robinson LR. Clinical utility of the flick maneuver in diagnosing carpal tunnel syndrome. Am J Phys Med Rehabil. 2004;83:363-367.
9. Padua L, Padua R, Aprile I, et al. Carpal tunnel syndrome: relationship between clinical and patient-oriented assessment. Clin Orthop Relat Res. 2002;395:128-134.
10. Bland JD. Carpal tunnel syndrome. BMJ. 2007;335:343-346.
11. Wright PE. Carpal tunnel syndrome. In: Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, Pa: Mosby, Inc; 2008:4285–4291.
12. Sibley PA, Mandel RJ. Atraumatic acute carpal tunnel syndrome in a patient taking dabigatran. Orthopedics. 2012;35:e1286-e1289.
13. MacDermid JC, Wessel J. Clinical diagnosis of carpal tunnel syndrome: a systematic review. J Hand Ther. 2004;17:309-319.
14. Ahn DS. Hand elevation: a new test for carpal tunnel syndrome. Ann Plastic Surg. 2001;46:120-124.
15. Boyer K, Wies J, Turkelson CM. Effects of bias on the results of diagnostic studies of carpal tunnel syndrome. J Hand Surg. 2009;34:1006-1013.
16. Witt JC, Hentz JG, Stevens JC. Carpal tunnel syndrome with normal nerve conduction studies. Muscle Nerve. 2004;29:515-522.
17. Graham B. The value added by electrodiagnostic testing in the diagnosis of carpal tunnel syndrome. J Bone Joint Surg. 2008;90:2587-2593.
18. Jablecki CK, Andary MT, Floeter MK, et al. Practice parameter: electrodiagnostic studies in carpal tunnel syndrome. Report of the American Association of Electrodiagnostic Medicine, American Academy of Neurology, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2002;58:1589-1592.
19. Descatha A, Huard L, Aubert F, et al. Meta-analysis on the performance of sonography for the diagnosis of carpal tunnel syndrome. Semin Arthritis Rheum. 2012;41:914-922.
20. Visser LH, Ngo Q, Groeneweg SJ, et al. Long term effect of local corticosteroid injection for carpal tunnel syndrome: a relation with electrodiagnostic severity. Clin Neurophysiol. 2012;123:838-841.
21. Padua L, Padua R, Aprile I, et al. Multiperspective follow-up of untreated carpal tunnel syndrome: a multicenter study. Neurology. 2001;56:1459-1466.
22. Shi Q, MacDermid JC. Is surgical intervention more effective than non-surgical treatment for carpal tunnel syndrome? A systematic review. J Orthop Surg Res. 2011;6:17.-
23. Walker WC, Metzler M, Cifu DX, et al. Neutral wrist splinting in carpal tunnel syndrome: a comparison of night-only versus full-time wear instructions. Arch Phys Med Rehabil. 2000;81:424-429.
24. Brininger TL, Rogers JC, Holm MB, et al. Efficacy of a fabricated customized splint and tendon and nerve gliding exercises for the treatment of carpal tunnel syndrome: a randomized controlled trial. Arch Phys Med Rehabil. 2007;88:1429-1435.
25. Schmid AB, Elliott JM, Strudwick MW, et al. Effect of splinting and exercise on intraneural edema of the median nerve in carpal tunnel syndrome-an MRI study to reveal therapeutic mechanisms. J Orthop Res. 2012;30:1343-1350.
26. Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(2):CD001554.-
27. Kamanli A, Bezgincan M, Kaya A. Comparison of local steroid injection into carpal tunnel via proximal and distal approach in patients with carpal tunnel syndrome. Bratislavske Lek Listy. 2011;112:337-341.
28. Huisstede BM, Hoogvliet P, Randsdorp MS, et al. Carpal tunnel syndrome. Part I: effectiveness of nonsurgical treatments—a systematic review. Arch Phys Med Rehabil. 2010;91:981-1004.
29. Chang MH, Ger LP, Hsieh PF, et al. A randomised clinical trial of oral steroids in the treatment of carpal tunnel syndrome: a long term follow up. J Neurol Neurosurg Psychiatry. 2002;73:710-714.
30. O’Connor D, Marshall S, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. 2003;(1):CD003219.-
31. Garfinkel MS, Singhal A, Katz WA, et al. Yoga-based intervention for carpal tunnel syndrome: a randomized trial. JAMA. 1998;280:1601-1603.
32. Tal-Akabi A, Rushton A. An investigation to compare the effectiveness of carpal bone mobilisation and neurodynamic mobilisation as methods of treatment for carpal tunnel syndrome. Man Ther. 2000;5:214-222.
33. O’Connor D, Page MJ, Marshall SC, et al. Ergonomic positioning or equipment for treating carpal tunnel syndrome. Cochrane Database Syst Rev. 2012;(1):CD009600.-
34. Page MJ, O’Connor D, Pitt V, et al. Therapeutic ultrasound for carpal tunnel syndrome. Cochrane Database Syst Rev. 2012;(1):CD009601.-
35. Yao E, Gerritz PK, Henricson E, et al. Randomized controlled trial comparing acupuncture with placebo acupuncture for the treatment of carpal tunnel syndrome. PMR. 2012;4:367-373.
36. Pomerance J, Zurakowski D, Fine I. The cost-effectiveness of nonsurgical versus surgical treatment for carpal tunnel syndrome. J Hand Surg. 2009;34:1193-1200.
37. Turner A, Kimble F, Gulyas K, et al. Can the outcome of open carpal tunnel release be predicted? A review of the literature. ANZ J Surg. 2010;80:50-54.
38. Keith MW, Masear V, Chung KC, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of carpal tunnel syndrome. J Bone Joint Surg. 2010;92:218-219.
39. Scholten RJ, Mink van der Molen A, Uitdehaag BM, et al. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(4):CD003905.-
40. Nakamichi K, Tachibana S, Yamamoto S, et al. Percutaneous carpal tunnel release compared with mini-open release using ultrasonographic guidance for both techniques. J Hand Surg Am. 2010;35:437-445.
41. Huisstede BM, Randsdorp MS, Coert JH, et al. Carpal tunnel syndrome. Part II: effectiveness of surgical treatments—a systematic review. Arch Phys Med Rehabil. 2010;91:1005-1024.
42. Ludlow KS, Merla JL, Cox JA, et al. Pillar pain as a postoperative complication of carpal tunnel release: a review of the literature. J Hand Ther. 1997;10:277-282.
43. Pomerance J, Fine I. Outcomes of carpal tunnel surgery with and without supervised postoperative therapy. J Hand Surg. 2007;32:1159-1163.
44. Acharya AD, Auchincloss JM. Return to functional hand use and work following open carpal tunnel surgery. J Hand Surg Br. 2005;30:607-610.
45. Dahlin LB, Salo M, Thomsen N, et al. Carpal tunnel syndrome and treatment of recurrent symptoms. Scand J Plast Reconstr Surg Hand Surg. 2010;44:4-11.
1. Atroshi I, Gummesson C, Johnsson, et al. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282:153-158.
2. Luckhaupt SE, Dahlhamer JM, Ward BW, et al. Prevalence and work-relatedness of carpal tunnel syndrome in the working population, United States, 2010 national health interview survey. Am J Ind Med. 2012 April 12. [Epub ahead of print.]
3. van Dijk MA, Reitsma JB, Fischer JC, et al. Indications for requesting laboratory tests for concurrent diseases in patients with carpal tunnel syndrome: a systematic review. Clin Chem. 2003;49:1437-1444.
4. Padua L, Di Pasquale A, Pazzaglia C, et al. Systematic review of pregnancy-related carpal tunnel syndrome. Muscle Nerve. 2010;42:697-702.
5. Bland JD. The relationship of obesity, age, and carpal tunnel syndrome: more complex than was thought? Muscle Nerve. 2005;32:527-532.
6. Prime MS, Palmer J, Khan WS, et al. Is there light at the end of the tunnel? Controversies in the diagnosis and management of carpal tunnel syndrome. Hand. 2010;5:354-360.
7. Keith MW, Masear V, Chung KC, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on diagnosis of carpal tunnel syndrome. J Bone Joint Surg Am. 2009;91:2478-2479.
8. Hansen PA, Micklesen P, Robinson LR. Clinical utility of the flick maneuver in diagnosing carpal tunnel syndrome. Am J Phys Med Rehabil. 2004;83:363-367.
9. Padua L, Padua R, Aprile I, et al. Carpal tunnel syndrome: relationship between clinical and patient-oriented assessment. Clin Orthop Relat Res. 2002;395:128-134.
10. Bland JD. Carpal tunnel syndrome. BMJ. 2007;335:343-346.
11. Wright PE. Carpal tunnel syndrome. In: Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, Pa: Mosby, Inc; 2008:4285–4291.
12. Sibley PA, Mandel RJ. Atraumatic acute carpal tunnel syndrome in a patient taking dabigatran. Orthopedics. 2012;35:e1286-e1289.
13. MacDermid JC, Wessel J. Clinical diagnosis of carpal tunnel syndrome: a systematic review. J Hand Ther. 2004;17:309-319.
14. Ahn DS. Hand elevation: a new test for carpal tunnel syndrome. Ann Plastic Surg. 2001;46:120-124.
15. Boyer K, Wies J, Turkelson CM. Effects of bias on the results of diagnostic studies of carpal tunnel syndrome. J Hand Surg. 2009;34:1006-1013.
16. Witt JC, Hentz JG, Stevens JC. Carpal tunnel syndrome with normal nerve conduction studies. Muscle Nerve. 2004;29:515-522.
17. Graham B. The value added by electrodiagnostic testing in the diagnosis of carpal tunnel syndrome. J Bone Joint Surg. 2008;90:2587-2593.
18. Jablecki CK, Andary MT, Floeter MK, et al. Practice parameter: electrodiagnostic studies in carpal tunnel syndrome. Report of the American Association of Electrodiagnostic Medicine, American Academy of Neurology, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2002;58:1589-1592.
19. Descatha A, Huard L, Aubert F, et al. Meta-analysis on the performance of sonography for the diagnosis of carpal tunnel syndrome. Semin Arthritis Rheum. 2012;41:914-922.
20. Visser LH, Ngo Q, Groeneweg SJ, et al. Long term effect of local corticosteroid injection for carpal tunnel syndrome: a relation with electrodiagnostic severity. Clin Neurophysiol. 2012;123:838-841.
21. Padua L, Padua R, Aprile I, et al. Multiperspective follow-up of untreated carpal tunnel syndrome: a multicenter study. Neurology. 2001;56:1459-1466.
22. Shi Q, MacDermid JC. Is surgical intervention more effective than non-surgical treatment for carpal tunnel syndrome? A systematic review. J Orthop Surg Res. 2011;6:17.-
23. Walker WC, Metzler M, Cifu DX, et al. Neutral wrist splinting in carpal tunnel syndrome: a comparison of night-only versus full-time wear instructions. Arch Phys Med Rehabil. 2000;81:424-429.
24. Brininger TL, Rogers JC, Holm MB, et al. Efficacy of a fabricated customized splint and tendon and nerve gliding exercises for the treatment of carpal tunnel syndrome: a randomized controlled trial. Arch Phys Med Rehabil. 2007;88:1429-1435.
25. Schmid AB, Elliott JM, Strudwick MW, et al. Effect of splinting and exercise on intraneural edema of the median nerve in carpal tunnel syndrome-an MRI study to reveal therapeutic mechanisms. J Orthop Res. 2012;30:1343-1350.
26. Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(2):CD001554.-
27. Kamanli A, Bezgincan M, Kaya A. Comparison of local steroid injection into carpal tunnel via proximal and distal approach in patients with carpal tunnel syndrome. Bratislavske Lek Listy. 2011;112:337-341.
28. Huisstede BM, Hoogvliet P, Randsdorp MS, et al. Carpal tunnel syndrome. Part I: effectiveness of nonsurgical treatments—a systematic review. Arch Phys Med Rehabil. 2010;91:981-1004.
29. Chang MH, Ger LP, Hsieh PF, et al. A randomised clinical trial of oral steroids in the treatment of carpal tunnel syndrome: a long term follow up. J Neurol Neurosurg Psychiatry. 2002;73:710-714.
30. O’Connor D, Marshall S, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. 2003;(1):CD003219.-
31. Garfinkel MS, Singhal A, Katz WA, et al. Yoga-based intervention for carpal tunnel syndrome: a randomized trial. JAMA. 1998;280:1601-1603.
32. Tal-Akabi A, Rushton A. An investigation to compare the effectiveness of carpal bone mobilisation and neurodynamic mobilisation as methods of treatment for carpal tunnel syndrome. Man Ther. 2000;5:214-222.
33. O’Connor D, Page MJ, Marshall SC, et al. Ergonomic positioning or equipment for treating carpal tunnel syndrome. Cochrane Database Syst Rev. 2012;(1):CD009600.-
34. Page MJ, O’Connor D, Pitt V, et al. Therapeutic ultrasound for carpal tunnel syndrome. Cochrane Database Syst Rev. 2012;(1):CD009601.-
35. Yao E, Gerritz PK, Henricson E, et al. Randomized controlled trial comparing acupuncture with placebo acupuncture for the treatment of carpal tunnel syndrome. PMR. 2012;4:367-373.
36. Pomerance J, Zurakowski D, Fine I. The cost-effectiveness of nonsurgical versus surgical treatment for carpal tunnel syndrome. J Hand Surg. 2009;34:1193-1200.
37. Turner A, Kimble F, Gulyas K, et al. Can the outcome of open carpal tunnel release be predicted? A review of the literature. ANZ J Surg. 2010;80:50-54.
38. Keith MW, Masear V, Chung KC, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of carpal tunnel syndrome. J Bone Joint Surg. 2010;92:218-219.
39. Scholten RJ, Mink van der Molen A, Uitdehaag BM, et al. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(4):CD003905.-
40. Nakamichi K, Tachibana S, Yamamoto S, et al. Percutaneous carpal tunnel release compared with mini-open release using ultrasonographic guidance for both techniques. J Hand Surg Am. 2010;35:437-445.
41. Huisstede BM, Randsdorp MS, Coert JH, et al. Carpal tunnel syndrome. Part II: effectiveness of surgical treatments—a systematic review. Arch Phys Med Rehabil. 2010;91:1005-1024.
42. Ludlow KS, Merla JL, Cox JA, et al. Pillar pain as a postoperative complication of carpal tunnel release: a review of the literature. J Hand Ther. 1997;10:277-282.
43. Pomerance J, Fine I. Outcomes of carpal tunnel surgery with and without supervised postoperative therapy. J Hand Surg. 2007;32:1159-1163.
44. Acharya AD, Auchincloss JM. Return to functional hand use and work following open carpal tunnel surgery. J Hand Surg Br. 2005;30:607-610.
45. Dahlin LB, Salo M, Thomsen N, et al. Carpal tunnel syndrome and treatment of recurrent symptoms. Scand J Plast Reconstr Surg Hand Surg. 2010;44:4-11.
Gynecomastia: When is treatment indicated?
• Examine enlarged male breasts to differentiate between true gynecomastia and pseudogynecomastia (seen with obesity) or a mass suggestive of tumor activity. C
• Ask patients about the use of medications associated with gynecomastia, such as some antihypertensives, antibiotics, psychotropic agents, or hormones. C
• Order renal function tests and measure levels of liver enzymes, testosterone, and other hormones when initial history and examination findings are insufficient for a diagnosis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Harry J is a 57-year-old man who came to us for evaluation and management of hypertension. He also complained of chronic headaches. Our initial examination revealed a body mass index (BMI) of 29 kg/m2 and blood pressure (BP) of 150/100 mm Hg. The hypertension responded well to a combination of valsartan and hydrochlorothiazide. A few months later, he developed left breast soreness, as well as decreased libido. Examination revealed a round movable subareolar nodule 2 cm in diameter, with no associated skin changes or lymphadenopathy. Laboratory results were: total testosterone, 106 ng/dL (normal, 241-827); free testosterone, 23 pg/mL (47-244); thyroid-stimulating hormone (TSH), 2.222 mIU/mL (0.350-5.500); and prolactin, 102.7 ng/mL (2.1-17.7). Magnetic resonance imaging (MRI) of the brain revealed a nodular density <10 mm in the pituitary gland with minimal displacement of the stalk, consistent with a microadenoma.
Enlargement of the male breasts—gynecomastia—is caused by a benign proliferation of the ductal epithelium, due to a relative increase in the ratio of free estrogen to androgen locally in the breast. Gynecomastia of recent onset is often associated with pain and tenderness, as was the case with our patient.
Often self-limiting, age-related influences. Gynecomastia is common in newborns, during adolescence, and in old age.1 In both male and female newborns, maternal and placental estrogens induce bilateral proliferation of breast tissue. This resolves within a few weeks after birth. During the early stages of male puberty, there is a relative increase in estrogens derived mostly from peripheral aromatization of testicular and adrenal androgens. If gynecomastia results, it usually regresses spontaneously as testicular testosterone production increases in late puberty.2 Gynecomastia is also common in elderly men due to a decrease in testosterone production and an increase in sex hormone binding globulin (SHBG) that lowers free testosterone levels.
Deleterious contributing factors. Several other potential causes of gynecomastia exist (TABLE 1),3,4 and these can usually be identified with a systematic approach using a careful history, physical examination, and selected laboratory studies. Many medications are associated with gynecomastia (TABLE 2),5 one of the most common being spironolactone due to its antiandrogenic activity at the receptor level.5 Some drugs, although associated with gynecomastia, cannot be linked to a direct cause-and-effect mechanism. These factors are compounded in elderly, obese men who take medications such as spironolactone, known to cause gynecomastia.
TABLE 1
Causes of gynecomastia3,4
| Physiologic |
| Neonatal Adolescent Aging-related |
| Drug induced |
| Antiandrogens Antibiotics Antihypertensive agents GI agents Hormones Illicit drugs Psychiatric drugs |
| Decreased androgen production |
| Primary (testicular) hypogonadism Secondary (central) hypogonadism |
| Decreased androgen effect or synthesis |
| Androgen insensitivity syndrome 5α-Reductase deficiency 17-β-Hydroxysteroid dehydrogenase deficiency |
| Increased estrogen production |
| Adrenal tumor Testicular tumor hCG-secreting tumor Familial aromatase excess syndrome |
| Other |
| Liver disease Thyrotoxicosis Obesity Renal disease Malnutrition |
| GI, gastrointestinal; hCG, human chorionic gonadotropin. |
TABLE 2
Drugs associated with gynecomastia5
| Antiandrogens | Bicalutamide, flutamide, finasteride, spironolactone |
| Antibiotics | Isoniazid, ketoconazole, metronidazole |
| Antihypertensive agents | Amlodipine, diltiazem, nifedipine, verapamil, captopril, enalapril |
| GI agents | Cimetidine, ranitidine, omeprazole |
| Hormones | Anabolic steroids, estrogens, hCG, growth hormone, GnRH agonists |
| Illicit drugs, alcohol | Marijuana, methadone |
| Psychiatric drugs | Psychotropic agents, tricyclic antidepressants |
| Other | Antiretroviral agents, digitalis, fibrates, methotrexate, statins |
| GI, gastrointestinal; GnRH, gonadotropin-releasing hormone; hCG, human chorionic gonadotropin. | |
A patient’s medical history may reveal chronic conditions associated with gynecomastia. Such disorders include cirrhosis, hyperthyroidism, malnutrition, and chronic kidney disease. Rarely, gynecomastia can be a manifestation of a testicular, adrenal, or other neoplasm.
Despite a thorough evaluation, no detectable abnormality is found initially in 25% of gynecomastia cases.6 Close observation and monitoring is necessary in such instances, to ensure the earliest possible identification of the underlying cause and initiation of appropriate medical or surgical therapy.
First steps in the clinical evaluation
In cases of male breast enlargement, first determine whether you are dealing with true gynecomastia or “pseudogynecomastia,” which involves increased fat deposits typically seen in obese individuals.3 In cases of pseudogynecomastia, the tissue is uniformly enlarged and soft, with the same consistency as adipose tissue.
In about half of the cases of gynecomastia, the condition is bilateral.3 It is characteristically a rubbery or firm mass concentric with the nipple-areolar complex.
Clues to look for in the history. When examination suggests true gynecomastia, conduct a focused history to determine if medications or other substances might be causing the problem. (See “A case where drug therapy was to blame”) Some plant-derived oils used as skin care products have also been associated with gynecomastia due to weak estrogenic or anti-androgenic activity.7
Jed G is a 61-year-old man who reported decreased libido and erectile dysfunction. Examination revealed normal male external genitalia and prostate. Gynecomastia was not present. Laboratory results were: total testosterone, 159 ng/dL (normal, 241-827); free testosterone, 40 pg/mL (47-244); follicle-stimulating hormone (FSH), 9.1 mIU/mL (1.4-18.1); luteinizing hormone (LH), 3.4 mIU/mL (1.5-9.3); prolactin, 2.8 ng/mL (2.1-17.7); and normal values for ferritin and iron. His prostate-specific antigen (PSA) level was 0.8 ng/mL (normal, 0.00-4.00 ng/mL).
Mr. G was started on testosterone 1% gel at 5 g/d. The repeat total testosterone measurement was 215 ng/dL, and free testosterone was 82 pg/mL. The patient discontinued the testosterone gel a few months later due to the medication’s high cost.
Several years later, his total testosterone level had fallen to 110 ng/dL, and he continued to complain of fatigue, decreased libido, and erectile dysfunction. We initiated testosterone enanthate 100 mg IM every 3 weeks, which increased his testosterone level to 285 mg/dL. However, hemoglobin increased to 18.3 g/dL, and he noted bilateral nipple tenderness since the start of the injections. Small bilateral gynecomastia about 1 cm in diameter was noted. Testosterone injections were discontinued due to the erythrocytosis. The breast tenderness and gynecomastia resolved 4 months later.
Mr. G had idiopathic hypogonadism. The breast tenderness and gynecomastia he developed were most likely a result of peripheral aromatization of testosterone. This is similar to gynecomastia commonly observed during early puberty and would likely have regressed with continued therapy. However, as noted above, the testosterone injections had to be stopped due to significant erythrocytosis.
The history may also uncover significant weight gain, because obesity is associated with increased aromatase activity resulting in a relative increase in estrogens systemically and locally in the breast. When obesity is the cause of gynecomastia, the breast examination reveals firm, rubbery tissue (unlike the findings in pseudogynecomastia, where there is a soft enlargement of the breast). Alternatively, a history of weight loss is important because it can lead to hypothalamic dysfunction and a decrease in gonadotropin (follicle-stimulating hormone [FSH], luteinizing hormone [LH]) secretion, resulting in decreased testosterone levels.8
Also inquire about prior diagnoses of liver cirrhosis or thyrotoxicosis or the presence of symptoms suggestive of these disorders, such as fatigue, jaundice, bloating, heat intolerance, or heart palpitations. These conditions can alter the metabolism of sex steroids and their binding proteins. A history of decreased libido and erectile dysfunction is suggestive of low testosterone levels, also known as hypogonadism. Headaches, visual disturbances, and behavioral abnormalities suggest a hypothalamic or pituitary disorder resulting in decreased FSH and LH levels and secondary hypogonadism. A family history of gynecomastia is elicited in half the patients with persistent pubertal gynecomastia.9
Physical examination. For all patients (except newborns), calculate the BMI and measure arm span and upper and lower body segments. A eunuchoid proportion—arm span 2 cm or greater than height—is associated with early-onset hypogonadism that precedes fusion of the epiphyses.3 Thus, you’ll need to consider congenital disorders of the testes, such as Klinefelter syndrome, as well as hypothalamic or pituitary disease, such as Kallmann syndrome, resulting in deficient FSH and LH production.
As noted earlier, you’ll need to examine the breasts to determine if true gynecomastia exists, as opposed to increased adipose tissue or the presence of a suspicious mass. A hard or irregular mass outside the areola, especially if associated with skin changes such as dimpling or retraction, should raise the possibility of breast carcinoma. Promptly arrange for diagnostic mammography and possible biopsy in this setting.
Carefully examine the secondary sexual characteristics, including body hair distribution and muscle mass. Inspect the external genitalia, penile development, and position of the urethral meatus. Note testicular size and consistency. Small, firm testes are suggestive of dysgenetic gonads found in patients with Klinefelter syndrome (47 XXY), whereas small, soft testes suggest secondary hypogonadism. A unilateral testicular mass raises suspicion of a neoplasm. Palpate the prostate in older men, especially if contemplating androgen therapy, which could exacerbate a preexisting focal prostate cancer.
Look for signs of hyperthyroidism, such as goiter, exophthalmos, tachycardia, and hyper-reflexia. Examine the abdomen for masses, hepato- or splenomegaly, and signs of cirrhosis, such as ascites and venous congestion. The examination should also include visual fields, cranial nerves, and fundoscopy for possible pituitary (or other) central nervous system lesions. Look for spider angiomas and palmar erythema (as occur in cirrhosis); warm, moist skin and myxedema (as in Graves’ disease); and mucocutaneous lentigines (as in Peutz-Jeghers syndrome).10
When laboratory and radiologic testing may help
Most adolescents with gynecomastia are best managed by reassurance and observation11 (ALGORITHM),3 and no laboratory or radiographic studies are recommended in most cases. Exceptions would be gynecomastia that develops before the onset of puberty; evidence of undervirilization on physical examination; a testicular mass; or persistence of gynecomastia beyond the usual observation period of 12 to 18 months.11
ALGORITHM
Evaluating gynecomastia3
CT, computed tomography; hCG, human chorionic gonadotropin; LH, luteinizing hormone; MRI, magnetic resonance imaging; TSH, thyroid-stimulating hormone.
↑ = elevated; ↓ = lowered; ↔ = normal.
If findings on physical examination are consistent with a breast neoplasm, arrange for mammography immediately. The sensitivity and specificity of mammography for benign and malignant conditions exceed 90%.12 A biopsy may be necessary if uncertainty remains after imaging.
No specific tests are necessary when gynecomastia is clearly associated with intake of a medication known to be associated with the condition, especially if the history and examination are otherwise negative. A prompt regression of gynecomastia after discontinuation of the offending drug will confirm the diagnosis.
If the condition persists in an adolescent or adult and the cause is still unclear, perform renal function tests and measure levels of liver enzymes, early-morning serum human chorionic gonadotropin (hCG), LH, total testosterone, estradiol, TSH, and prolactin.
What lab results may mean. If the total testosterone level is borderline or low-normal (200-350 ng/dL), repeat the test and measure the free testosterone level.
If an elevated hCG level is found, repeat the testicular examination carefully and order ultrasonography. In the absence of a testicular tumor, consider an MRI of the brain and computed tomography (CT) of the abdomen and chest to help identify an extragonadal hCG-secreting tumor.
An elevated LH level and low testosterone level are diagnostic of primary testicular hypogonadism. A karyotype may be necessary in some individuals to diagnose Klinefelter syndrome. Elevated LH and testosterone levels are seen in patients with androgen insensitivity syndromes. These conditions are caused by abnormalities in the androgen receptor with a wide range of possible phenotypes, including ambiguous genitalia.
A low testosterone level with a low or normal LH level indicates secondary hypogonadism of hypothalamic or pituitary origin. An elevated prolactin level in such cases (as was seen in Mr. J.’s case) is usually due to a prolactin secreting pituitary adenoma.
Hereditary hemochromatosis is an important and often overlooked cause of hypogonadism. Obtain iron studies and ferritin levels in this setting.13 Unrecognized hemochromatosis may result in fibrosis and multiple organ failure.
Patients with secondary hypogonadism are best managed by a referral to an endocrinologist, as the potential list of causes is extensive.4,14,15
A low TSH level is consistent with thyrotoxicosis, which may result in increased levels of SHBG and altered metabolism of estrogens and androgens.16 Thus, about 10% of men with thyrotoxicosis present with gynecomastia and erectile dysfunction.16 If the estradiol level is elevated, a testicular ultrasound as well as an adrenal CT scan will help identify a neoplasm.
In a significant number of patients, the diagnostic tests are normal, leading to a diagnosis of idiopathic gynecomastia. In these cases, the alteration in androgen and estrogen levels can be subtle and intermittent.17 Continue surveillance and periodically reevaluate the patient.
Management of gynecomastia
Gynecomastia often results from transient hormonal imbalance and regresses spontaneously. Therefore, no specific treatment is necessary for neonatal, pubertal, or drug-induced gynecomastia. In other situations, prompt diagnosis and treatment are important to maximize the likelihood of successful medical therapy. It has been shown that fibrosis develops 6 to 12 months after the onset of gynecomastia, making it unlikely that medical treatments beyond that stage will result in significant regression of the breast enlargement.18 In such long-standing cases, surgical intervention with subcutaneous mastectomy or liposuction can be considered for patients who have significant psychological problems or esthetic issues. Indications for surgery also include continued growth and tenderness of breast tissue or malignancy.
Available medications include those aimed at decreasing estrogen production or estrogen effect on target breast tissue. Aromatase inhibitors such as testolactone, anastrozole, and letrozole can decrease the synthesis of estrogen by inhibiting aromatization of androgens. Although theoretically promising, results of the few controlled trials with aromatase inhibitors have been generally disappointing.19
Selective estrogen receptor modulators that alter the effect of estrogen on breast tissue are tamoxifen and raloxifene. Tamoxifen is not yet approved for treatment of gynecomastia, but has proven effective in randomized trials.20 At a dose of 20 mg/d for 3 or more months, tamoxifen resulted in complete regression of gynecomastia in 60% of patients and partial regression in 20% of patients.20 Tamoxifen also prevents gynecomastia after medial prostatectomy and treatment with the antiandrogen, bicalutamide.
CASE Mr. J had a pituitary prolactin-secreting microadenoma causing secondary hypogonadism and gynecomastia. He was started on cabergoline (a dopamine agonist) 0.5 mg orally once a week. Four months later, his total testosterone level was 291 ng/dL, and prolactin was 9.3 ng/mL. His headaches and gynecomastia had significantly decreased. He continued to do well on the same regimen and, 6 years later, his prolactin level was 1.4 ng/mL, indicating that treatment had been effective.
CORRESPONDENCE
Roy N. Morcos, MD, Department of Family Medicine, St. Elizabeth Health Center, 1044 Belmont Avenue, Youngstown, OH 44501; roymorcos@gmail.com
1. Haynes B, Mookadem F. Male gynecomastia. Mayo Clin Proc. 2009;84:672.-
2. Nordt C, Divanta A. Gynecomastia in adolescents. Curr Opin Pediatr. 2008;20:375-382.
3. Braunstein G. Gynecomastia. N Engl J Med. 2007;357:1229-1237.
4. Bhasin SI. Testicular disorders. In: Kronenberg HM, Melmed S, Polonsky KS, et al, eds. Williams Textbook of Endocrinology. 11th ed. Philadelphia, Pa: Saunders-Elsevier; 2008:569–671.
5. Eckman A, Dobs A. Drug-induced gynecomastia. Expert Opin Drug Saf. 2008;7:691-702.
6. Derkacz M, Chmiel-Perzyriska I, Nowakowski A. Gynecomastia – a difficult diagnostic problem. Endokrynol Pol. 2011;62:190-202.
7. Henley D, Lipson N, Kovach K, et al. Pubertal gynecomastia linked to lavender and tea tree oils. N Engl J Med. 2007;356:479-485.
8. Ma N, Geffnes M. Gynecomastia in prepubertal and pubertal boys. Curr Opin Pediatr. 2008;20:465-470.
9. Eberle AJ, Sparrow JT, Keenan BS. Treatment of persistent pubertal gynecomastia with dihydrotestosterone heptanoate. J Pediatr. 1986;109:144-149.
10. Kapoor S. Cutaneous manifestations of systemic condition associated with gynecomastia. Skinmed. 2010;8:87-92.
11. Johnson RE, Murad MH. Gynecomastia: pathophysiology, evaluation, and management. Mayo Clin Proc. 2009;84:1010-1015.
12. Evans GF, Anthony T, Turnage RH, et al. The diagnostic accuracy of mammography in the evaluation of male breast disease. Am J Surg. 2001;181:96-102.
13. Allen KJ, Gurrin LC, Constantine CC, et al. Iron-overload related disease in HFE hereditary hemochromatosis. N Engl J Med. 2008;358:221-230.
14. Sedlmeyer IL, Palmert MR. Delayed puberty: analysis of a large case series from an academic center. J Clin Endocrinol Metab. 2002;87:1613-1620.
15. Bhasin SI, Jameson JL. Disorders of the testes and male reproductive system. In: Longo D, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012:3019–3020.
16. Meikle AW. The interrelationships between thyroid dysfunction and hypogonadism in men and boys. Thyroid. 2004;14(suppl 1):S17-S25.
17. Wu FCW, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123-135.
18. Di Lorenzo G, Autorino R, Perdona S, et al. Management of gynaecomastia in patients with prostate cancer: a systematic review. Lancet Oncol. 2005;6:972-979.
19. Mauras N, Bishop K, Merinbaum D, et al. Pharmacokinetics and pharmacodynamics of anastrozole in pubertal boys with recent onset gynecomastia. J Clin Endocrinol Metab. 2009;94:2975-2978.
20. Derman O, Kanbur N, Kilic I, et al. Long-term follow-up of tamoxifen treatment in adolescents with gynecomastia. J Pediatr Endocrinol Metab. 2008;21:449-453.
• Examine enlarged male breasts to differentiate between true gynecomastia and pseudogynecomastia (seen with obesity) or a mass suggestive of tumor activity. C
• Ask patients about the use of medications associated with gynecomastia, such as some antihypertensives, antibiotics, psychotropic agents, or hormones. C
• Order renal function tests and measure levels of liver enzymes, testosterone, and other hormones when initial history and examination findings are insufficient for a diagnosis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Harry J is a 57-year-old man who came to us for evaluation and management of hypertension. He also complained of chronic headaches. Our initial examination revealed a body mass index (BMI) of 29 kg/m2 and blood pressure (BP) of 150/100 mm Hg. The hypertension responded well to a combination of valsartan and hydrochlorothiazide. A few months later, he developed left breast soreness, as well as decreased libido. Examination revealed a round movable subareolar nodule 2 cm in diameter, with no associated skin changes or lymphadenopathy. Laboratory results were: total testosterone, 106 ng/dL (normal, 241-827); free testosterone, 23 pg/mL (47-244); thyroid-stimulating hormone (TSH), 2.222 mIU/mL (0.350-5.500); and prolactin, 102.7 ng/mL (2.1-17.7). Magnetic resonance imaging (MRI) of the brain revealed a nodular density <10 mm in the pituitary gland with minimal displacement of the stalk, consistent with a microadenoma.
Enlargement of the male breasts—gynecomastia—is caused by a benign proliferation of the ductal epithelium, due to a relative increase in the ratio of free estrogen to androgen locally in the breast. Gynecomastia of recent onset is often associated with pain and tenderness, as was the case with our patient.
Often self-limiting, age-related influences. Gynecomastia is common in newborns, during adolescence, and in old age.1 In both male and female newborns, maternal and placental estrogens induce bilateral proliferation of breast tissue. This resolves within a few weeks after birth. During the early stages of male puberty, there is a relative increase in estrogens derived mostly from peripheral aromatization of testicular and adrenal androgens. If gynecomastia results, it usually regresses spontaneously as testicular testosterone production increases in late puberty.2 Gynecomastia is also common in elderly men due to a decrease in testosterone production and an increase in sex hormone binding globulin (SHBG) that lowers free testosterone levels.
Deleterious contributing factors. Several other potential causes of gynecomastia exist (TABLE 1),3,4 and these can usually be identified with a systematic approach using a careful history, physical examination, and selected laboratory studies. Many medications are associated with gynecomastia (TABLE 2),5 one of the most common being spironolactone due to its antiandrogenic activity at the receptor level.5 Some drugs, although associated with gynecomastia, cannot be linked to a direct cause-and-effect mechanism. These factors are compounded in elderly, obese men who take medications such as spironolactone, known to cause gynecomastia.
TABLE 1
Causes of gynecomastia3,4
| Physiologic |
| Neonatal Adolescent Aging-related |
| Drug induced |
| Antiandrogens Antibiotics Antihypertensive agents GI agents Hormones Illicit drugs Psychiatric drugs |
| Decreased androgen production |
| Primary (testicular) hypogonadism Secondary (central) hypogonadism |
| Decreased androgen effect or synthesis |
| Androgen insensitivity syndrome 5α-Reductase deficiency 17-β-Hydroxysteroid dehydrogenase deficiency |
| Increased estrogen production |
| Adrenal tumor Testicular tumor hCG-secreting tumor Familial aromatase excess syndrome |
| Other |
| Liver disease Thyrotoxicosis Obesity Renal disease Malnutrition |
| GI, gastrointestinal; hCG, human chorionic gonadotropin. |
TABLE 2
Drugs associated with gynecomastia5
| Antiandrogens | Bicalutamide, flutamide, finasteride, spironolactone |
| Antibiotics | Isoniazid, ketoconazole, metronidazole |
| Antihypertensive agents | Amlodipine, diltiazem, nifedipine, verapamil, captopril, enalapril |
| GI agents | Cimetidine, ranitidine, omeprazole |
| Hormones | Anabolic steroids, estrogens, hCG, growth hormone, GnRH agonists |
| Illicit drugs, alcohol | Marijuana, methadone |
| Psychiatric drugs | Psychotropic agents, tricyclic antidepressants |
| Other | Antiretroviral agents, digitalis, fibrates, methotrexate, statins |
| GI, gastrointestinal; GnRH, gonadotropin-releasing hormone; hCG, human chorionic gonadotropin. | |
A patient’s medical history may reveal chronic conditions associated with gynecomastia. Such disorders include cirrhosis, hyperthyroidism, malnutrition, and chronic kidney disease. Rarely, gynecomastia can be a manifestation of a testicular, adrenal, or other neoplasm.
Despite a thorough evaluation, no detectable abnormality is found initially in 25% of gynecomastia cases.6 Close observation and monitoring is necessary in such instances, to ensure the earliest possible identification of the underlying cause and initiation of appropriate medical or surgical therapy.
First steps in the clinical evaluation
In cases of male breast enlargement, first determine whether you are dealing with true gynecomastia or “pseudogynecomastia,” which involves increased fat deposits typically seen in obese individuals.3 In cases of pseudogynecomastia, the tissue is uniformly enlarged and soft, with the same consistency as adipose tissue.
In about half of the cases of gynecomastia, the condition is bilateral.3 It is characteristically a rubbery or firm mass concentric with the nipple-areolar complex.
Clues to look for in the history. When examination suggests true gynecomastia, conduct a focused history to determine if medications or other substances might be causing the problem. (See “A case where drug therapy was to blame”) Some plant-derived oils used as skin care products have also been associated with gynecomastia due to weak estrogenic or anti-androgenic activity.7
Jed G is a 61-year-old man who reported decreased libido and erectile dysfunction. Examination revealed normal male external genitalia and prostate. Gynecomastia was not present. Laboratory results were: total testosterone, 159 ng/dL (normal, 241-827); free testosterone, 40 pg/mL (47-244); follicle-stimulating hormone (FSH), 9.1 mIU/mL (1.4-18.1); luteinizing hormone (LH), 3.4 mIU/mL (1.5-9.3); prolactin, 2.8 ng/mL (2.1-17.7); and normal values for ferritin and iron. His prostate-specific antigen (PSA) level was 0.8 ng/mL (normal, 0.00-4.00 ng/mL).
Mr. G was started on testosterone 1% gel at 5 g/d. The repeat total testosterone measurement was 215 ng/dL, and free testosterone was 82 pg/mL. The patient discontinued the testosterone gel a few months later due to the medication’s high cost.
Several years later, his total testosterone level had fallen to 110 ng/dL, and he continued to complain of fatigue, decreased libido, and erectile dysfunction. We initiated testosterone enanthate 100 mg IM every 3 weeks, which increased his testosterone level to 285 mg/dL. However, hemoglobin increased to 18.3 g/dL, and he noted bilateral nipple tenderness since the start of the injections. Small bilateral gynecomastia about 1 cm in diameter was noted. Testosterone injections were discontinued due to the erythrocytosis. The breast tenderness and gynecomastia resolved 4 months later.
Mr. G had idiopathic hypogonadism. The breast tenderness and gynecomastia he developed were most likely a result of peripheral aromatization of testosterone. This is similar to gynecomastia commonly observed during early puberty and would likely have regressed with continued therapy. However, as noted above, the testosterone injections had to be stopped due to significant erythrocytosis.
The history may also uncover significant weight gain, because obesity is associated with increased aromatase activity resulting in a relative increase in estrogens systemically and locally in the breast. When obesity is the cause of gynecomastia, the breast examination reveals firm, rubbery tissue (unlike the findings in pseudogynecomastia, where there is a soft enlargement of the breast). Alternatively, a history of weight loss is important because it can lead to hypothalamic dysfunction and a decrease in gonadotropin (follicle-stimulating hormone [FSH], luteinizing hormone [LH]) secretion, resulting in decreased testosterone levels.8
Also inquire about prior diagnoses of liver cirrhosis or thyrotoxicosis or the presence of symptoms suggestive of these disorders, such as fatigue, jaundice, bloating, heat intolerance, or heart palpitations. These conditions can alter the metabolism of sex steroids and their binding proteins. A history of decreased libido and erectile dysfunction is suggestive of low testosterone levels, also known as hypogonadism. Headaches, visual disturbances, and behavioral abnormalities suggest a hypothalamic or pituitary disorder resulting in decreased FSH and LH levels and secondary hypogonadism. A family history of gynecomastia is elicited in half the patients with persistent pubertal gynecomastia.9
Physical examination. For all patients (except newborns), calculate the BMI and measure arm span and upper and lower body segments. A eunuchoid proportion—arm span 2 cm or greater than height—is associated with early-onset hypogonadism that precedes fusion of the epiphyses.3 Thus, you’ll need to consider congenital disorders of the testes, such as Klinefelter syndrome, as well as hypothalamic or pituitary disease, such as Kallmann syndrome, resulting in deficient FSH and LH production.
As noted earlier, you’ll need to examine the breasts to determine if true gynecomastia exists, as opposed to increased adipose tissue or the presence of a suspicious mass. A hard or irregular mass outside the areola, especially if associated with skin changes such as dimpling or retraction, should raise the possibility of breast carcinoma. Promptly arrange for diagnostic mammography and possible biopsy in this setting.
Carefully examine the secondary sexual characteristics, including body hair distribution and muscle mass. Inspect the external genitalia, penile development, and position of the urethral meatus. Note testicular size and consistency. Small, firm testes are suggestive of dysgenetic gonads found in patients with Klinefelter syndrome (47 XXY), whereas small, soft testes suggest secondary hypogonadism. A unilateral testicular mass raises suspicion of a neoplasm. Palpate the prostate in older men, especially if contemplating androgen therapy, which could exacerbate a preexisting focal prostate cancer.
Look for signs of hyperthyroidism, such as goiter, exophthalmos, tachycardia, and hyper-reflexia. Examine the abdomen for masses, hepato- or splenomegaly, and signs of cirrhosis, such as ascites and venous congestion. The examination should also include visual fields, cranial nerves, and fundoscopy for possible pituitary (or other) central nervous system lesions. Look for spider angiomas and palmar erythema (as occur in cirrhosis); warm, moist skin and myxedema (as in Graves’ disease); and mucocutaneous lentigines (as in Peutz-Jeghers syndrome).10
When laboratory and radiologic testing may help
Most adolescents with gynecomastia are best managed by reassurance and observation11 (ALGORITHM),3 and no laboratory or radiographic studies are recommended in most cases. Exceptions would be gynecomastia that develops before the onset of puberty; evidence of undervirilization on physical examination; a testicular mass; or persistence of gynecomastia beyond the usual observation period of 12 to 18 months.11
ALGORITHM
Evaluating gynecomastia3
CT, computed tomography; hCG, human chorionic gonadotropin; LH, luteinizing hormone; MRI, magnetic resonance imaging; TSH, thyroid-stimulating hormone.
↑ = elevated; ↓ = lowered; ↔ = normal.
If findings on physical examination are consistent with a breast neoplasm, arrange for mammography immediately. The sensitivity and specificity of mammography for benign and malignant conditions exceed 90%.12 A biopsy may be necessary if uncertainty remains after imaging.
No specific tests are necessary when gynecomastia is clearly associated with intake of a medication known to be associated with the condition, especially if the history and examination are otherwise negative. A prompt regression of gynecomastia after discontinuation of the offending drug will confirm the diagnosis.
If the condition persists in an adolescent or adult and the cause is still unclear, perform renal function tests and measure levels of liver enzymes, early-morning serum human chorionic gonadotropin (hCG), LH, total testosterone, estradiol, TSH, and prolactin.
What lab results may mean. If the total testosterone level is borderline or low-normal (200-350 ng/dL), repeat the test and measure the free testosterone level.
If an elevated hCG level is found, repeat the testicular examination carefully and order ultrasonography. In the absence of a testicular tumor, consider an MRI of the brain and computed tomography (CT) of the abdomen and chest to help identify an extragonadal hCG-secreting tumor.
An elevated LH level and low testosterone level are diagnostic of primary testicular hypogonadism. A karyotype may be necessary in some individuals to diagnose Klinefelter syndrome. Elevated LH and testosterone levels are seen in patients with androgen insensitivity syndromes. These conditions are caused by abnormalities in the androgen receptor with a wide range of possible phenotypes, including ambiguous genitalia.
A low testosterone level with a low or normal LH level indicates secondary hypogonadism of hypothalamic or pituitary origin. An elevated prolactin level in such cases (as was seen in Mr. J.’s case) is usually due to a prolactin secreting pituitary adenoma.
Hereditary hemochromatosis is an important and often overlooked cause of hypogonadism. Obtain iron studies and ferritin levels in this setting.13 Unrecognized hemochromatosis may result in fibrosis and multiple organ failure.
Patients with secondary hypogonadism are best managed by a referral to an endocrinologist, as the potential list of causes is extensive.4,14,15
A low TSH level is consistent with thyrotoxicosis, which may result in increased levels of SHBG and altered metabolism of estrogens and androgens.16 Thus, about 10% of men with thyrotoxicosis present with gynecomastia and erectile dysfunction.16 If the estradiol level is elevated, a testicular ultrasound as well as an adrenal CT scan will help identify a neoplasm.
In a significant number of patients, the diagnostic tests are normal, leading to a diagnosis of idiopathic gynecomastia. In these cases, the alteration in androgen and estrogen levels can be subtle and intermittent.17 Continue surveillance and periodically reevaluate the patient.
Management of gynecomastia
Gynecomastia often results from transient hormonal imbalance and regresses spontaneously. Therefore, no specific treatment is necessary for neonatal, pubertal, or drug-induced gynecomastia. In other situations, prompt diagnosis and treatment are important to maximize the likelihood of successful medical therapy. It has been shown that fibrosis develops 6 to 12 months after the onset of gynecomastia, making it unlikely that medical treatments beyond that stage will result in significant regression of the breast enlargement.18 In such long-standing cases, surgical intervention with subcutaneous mastectomy or liposuction can be considered for patients who have significant psychological problems or esthetic issues. Indications for surgery also include continued growth and tenderness of breast tissue or malignancy.
Available medications include those aimed at decreasing estrogen production or estrogen effect on target breast tissue. Aromatase inhibitors such as testolactone, anastrozole, and letrozole can decrease the synthesis of estrogen by inhibiting aromatization of androgens. Although theoretically promising, results of the few controlled trials with aromatase inhibitors have been generally disappointing.19
Selective estrogen receptor modulators that alter the effect of estrogen on breast tissue are tamoxifen and raloxifene. Tamoxifen is not yet approved for treatment of gynecomastia, but has proven effective in randomized trials.20 At a dose of 20 mg/d for 3 or more months, tamoxifen resulted in complete regression of gynecomastia in 60% of patients and partial regression in 20% of patients.20 Tamoxifen also prevents gynecomastia after medial prostatectomy and treatment with the antiandrogen, bicalutamide.
CASE Mr. J had a pituitary prolactin-secreting microadenoma causing secondary hypogonadism and gynecomastia. He was started on cabergoline (a dopamine agonist) 0.5 mg orally once a week. Four months later, his total testosterone level was 291 ng/dL, and prolactin was 9.3 ng/mL. His headaches and gynecomastia had significantly decreased. He continued to do well on the same regimen and, 6 years later, his prolactin level was 1.4 ng/mL, indicating that treatment had been effective.
CORRESPONDENCE
Roy N. Morcos, MD, Department of Family Medicine, St. Elizabeth Health Center, 1044 Belmont Avenue, Youngstown, OH 44501; roymorcos@gmail.com
• Examine enlarged male breasts to differentiate between true gynecomastia and pseudogynecomastia (seen with obesity) or a mass suggestive of tumor activity. C
• Ask patients about the use of medications associated with gynecomastia, such as some antihypertensives, antibiotics, psychotropic agents, or hormones. C
• Order renal function tests and measure levels of liver enzymes, testosterone, and other hormones when initial history and examination findings are insufficient for a diagnosis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Harry J is a 57-year-old man who came to us for evaluation and management of hypertension. He also complained of chronic headaches. Our initial examination revealed a body mass index (BMI) of 29 kg/m2 and blood pressure (BP) of 150/100 mm Hg. The hypertension responded well to a combination of valsartan and hydrochlorothiazide. A few months later, he developed left breast soreness, as well as decreased libido. Examination revealed a round movable subareolar nodule 2 cm in diameter, with no associated skin changes or lymphadenopathy. Laboratory results were: total testosterone, 106 ng/dL (normal, 241-827); free testosterone, 23 pg/mL (47-244); thyroid-stimulating hormone (TSH), 2.222 mIU/mL (0.350-5.500); and prolactin, 102.7 ng/mL (2.1-17.7). Magnetic resonance imaging (MRI) of the brain revealed a nodular density <10 mm in the pituitary gland with minimal displacement of the stalk, consistent with a microadenoma.
Enlargement of the male breasts—gynecomastia—is caused by a benign proliferation of the ductal epithelium, due to a relative increase in the ratio of free estrogen to androgen locally in the breast. Gynecomastia of recent onset is often associated with pain and tenderness, as was the case with our patient.
Often self-limiting, age-related influences. Gynecomastia is common in newborns, during adolescence, and in old age.1 In both male and female newborns, maternal and placental estrogens induce bilateral proliferation of breast tissue. This resolves within a few weeks after birth. During the early stages of male puberty, there is a relative increase in estrogens derived mostly from peripheral aromatization of testicular and adrenal androgens. If gynecomastia results, it usually regresses spontaneously as testicular testosterone production increases in late puberty.2 Gynecomastia is also common in elderly men due to a decrease in testosterone production and an increase in sex hormone binding globulin (SHBG) that lowers free testosterone levels.
Deleterious contributing factors. Several other potential causes of gynecomastia exist (TABLE 1),3,4 and these can usually be identified with a systematic approach using a careful history, physical examination, and selected laboratory studies. Many medications are associated with gynecomastia (TABLE 2),5 one of the most common being spironolactone due to its antiandrogenic activity at the receptor level.5 Some drugs, although associated with gynecomastia, cannot be linked to a direct cause-and-effect mechanism. These factors are compounded in elderly, obese men who take medications such as spironolactone, known to cause gynecomastia.
TABLE 1
Causes of gynecomastia3,4
| Physiologic |
| Neonatal Adolescent Aging-related |
| Drug induced |
| Antiandrogens Antibiotics Antihypertensive agents GI agents Hormones Illicit drugs Psychiatric drugs |
| Decreased androgen production |
| Primary (testicular) hypogonadism Secondary (central) hypogonadism |
| Decreased androgen effect or synthesis |
| Androgen insensitivity syndrome 5α-Reductase deficiency 17-β-Hydroxysteroid dehydrogenase deficiency |
| Increased estrogen production |
| Adrenal tumor Testicular tumor hCG-secreting tumor Familial aromatase excess syndrome |
| Other |
| Liver disease Thyrotoxicosis Obesity Renal disease Malnutrition |
| GI, gastrointestinal; hCG, human chorionic gonadotropin. |
TABLE 2
Drugs associated with gynecomastia5
| Antiandrogens | Bicalutamide, flutamide, finasteride, spironolactone |
| Antibiotics | Isoniazid, ketoconazole, metronidazole |
| Antihypertensive agents | Amlodipine, diltiazem, nifedipine, verapamil, captopril, enalapril |
| GI agents | Cimetidine, ranitidine, omeprazole |
| Hormones | Anabolic steroids, estrogens, hCG, growth hormone, GnRH agonists |
| Illicit drugs, alcohol | Marijuana, methadone |
| Psychiatric drugs | Psychotropic agents, tricyclic antidepressants |
| Other | Antiretroviral agents, digitalis, fibrates, methotrexate, statins |
| GI, gastrointestinal; GnRH, gonadotropin-releasing hormone; hCG, human chorionic gonadotropin. | |
A patient’s medical history may reveal chronic conditions associated with gynecomastia. Such disorders include cirrhosis, hyperthyroidism, malnutrition, and chronic kidney disease. Rarely, gynecomastia can be a manifestation of a testicular, adrenal, or other neoplasm.
Despite a thorough evaluation, no detectable abnormality is found initially in 25% of gynecomastia cases.6 Close observation and monitoring is necessary in such instances, to ensure the earliest possible identification of the underlying cause and initiation of appropriate medical or surgical therapy.
First steps in the clinical evaluation
In cases of male breast enlargement, first determine whether you are dealing with true gynecomastia or “pseudogynecomastia,” which involves increased fat deposits typically seen in obese individuals.3 In cases of pseudogynecomastia, the tissue is uniformly enlarged and soft, with the same consistency as adipose tissue.
In about half of the cases of gynecomastia, the condition is bilateral.3 It is characteristically a rubbery or firm mass concentric with the nipple-areolar complex.
Clues to look for in the history. When examination suggests true gynecomastia, conduct a focused history to determine if medications or other substances might be causing the problem. (See “A case where drug therapy was to blame”) Some plant-derived oils used as skin care products have also been associated with gynecomastia due to weak estrogenic or anti-androgenic activity.7
Jed G is a 61-year-old man who reported decreased libido and erectile dysfunction. Examination revealed normal male external genitalia and prostate. Gynecomastia was not present. Laboratory results were: total testosterone, 159 ng/dL (normal, 241-827); free testosterone, 40 pg/mL (47-244); follicle-stimulating hormone (FSH), 9.1 mIU/mL (1.4-18.1); luteinizing hormone (LH), 3.4 mIU/mL (1.5-9.3); prolactin, 2.8 ng/mL (2.1-17.7); and normal values for ferritin and iron. His prostate-specific antigen (PSA) level was 0.8 ng/mL (normal, 0.00-4.00 ng/mL).
Mr. G was started on testosterone 1% gel at 5 g/d. The repeat total testosterone measurement was 215 ng/dL, and free testosterone was 82 pg/mL. The patient discontinued the testosterone gel a few months later due to the medication’s high cost.
Several years later, his total testosterone level had fallen to 110 ng/dL, and he continued to complain of fatigue, decreased libido, and erectile dysfunction. We initiated testosterone enanthate 100 mg IM every 3 weeks, which increased his testosterone level to 285 mg/dL. However, hemoglobin increased to 18.3 g/dL, and he noted bilateral nipple tenderness since the start of the injections. Small bilateral gynecomastia about 1 cm in diameter was noted. Testosterone injections were discontinued due to the erythrocytosis. The breast tenderness and gynecomastia resolved 4 months later.
Mr. G had idiopathic hypogonadism. The breast tenderness and gynecomastia he developed were most likely a result of peripheral aromatization of testosterone. This is similar to gynecomastia commonly observed during early puberty and would likely have regressed with continued therapy. However, as noted above, the testosterone injections had to be stopped due to significant erythrocytosis.
The history may also uncover significant weight gain, because obesity is associated with increased aromatase activity resulting in a relative increase in estrogens systemically and locally in the breast. When obesity is the cause of gynecomastia, the breast examination reveals firm, rubbery tissue (unlike the findings in pseudogynecomastia, where there is a soft enlargement of the breast). Alternatively, a history of weight loss is important because it can lead to hypothalamic dysfunction and a decrease in gonadotropin (follicle-stimulating hormone [FSH], luteinizing hormone [LH]) secretion, resulting in decreased testosterone levels.8
Also inquire about prior diagnoses of liver cirrhosis or thyrotoxicosis or the presence of symptoms suggestive of these disorders, such as fatigue, jaundice, bloating, heat intolerance, or heart palpitations. These conditions can alter the metabolism of sex steroids and their binding proteins. A history of decreased libido and erectile dysfunction is suggestive of low testosterone levels, also known as hypogonadism. Headaches, visual disturbances, and behavioral abnormalities suggest a hypothalamic or pituitary disorder resulting in decreased FSH and LH levels and secondary hypogonadism. A family history of gynecomastia is elicited in half the patients with persistent pubertal gynecomastia.9
Physical examination. For all patients (except newborns), calculate the BMI and measure arm span and upper and lower body segments. A eunuchoid proportion—arm span 2 cm or greater than height—is associated with early-onset hypogonadism that precedes fusion of the epiphyses.3 Thus, you’ll need to consider congenital disorders of the testes, such as Klinefelter syndrome, as well as hypothalamic or pituitary disease, such as Kallmann syndrome, resulting in deficient FSH and LH production.
As noted earlier, you’ll need to examine the breasts to determine if true gynecomastia exists, as opposed to increased adipose tissue or the presence of a suspicious mass. A hard or irregular mass outside the areola, especially if associated with skin changes such as dimpling or retraction, should raise the possibility of breast carcinoma. Promptly arrange for diagnostic mammography and possible biopsy in this setting.
Carefully examine the secondary sexual characteristics, including body hair distribution and muscle mass. Inspect the external genitalia, penile development, and position of the urethral meatus. Note testicular size and consistency. Small, firm testes are suggestive of dysgenetic gonads found in patients with Klinefelter syndrome (47 XXY), whereas small, soft testes suggest secondary hypogonadism. A unilateral testicular mass raises suspicion of a neoplasm. Palpate the prostate in older men, especially if contemplating androgen therapy, which could exacerbate a preexisting focal prostate cancer.
Look for signs of hyperthyroidism, such as goiter, exophthalmos, tachycardia, and hyper-reflexia. Examine the abdomen for masses, hepato- or splenomegaly, and signs of cirrhosis, such as ascites and venous congestion. The examination should also include visual fields, cranial nerves, and fundoscopy for possible pituitary (or other) central nervous system lesions. Look for spider angiomas and palmar erythema (as occur in cirrhosis); warm, moist skin and myxedema (as in Graves’ disease); and mucocutaneous lentigines (as in Peutz-Jeghers syndrome).10
When laboratory and radiologic testing may help
Most adolescents with gynecomastia are best managed by reassurance and observation11 (ALGORITHM),3 and no laboratory or radiographic studies are recommended in most cases. Exceptions would be gynecomastia that develops before the onset of puberty; evidence of undervirilization on physical examination; a testicular mass; or persistence of gynecomastia beyond the usual observation period of 12 to 18 months.11
ALGORITHM
Evaluating gynecomastia3
CT, computed tomography; hCG, human chorionic gonadotropin; LH, luteinizing hormone; MRI, magnetic resonance imaging; TSH, thyroid-stimulating hormone.
↑ = elevated; ↓ = lowered; ↔ = normal.
If findings on physical examination are consistent with a breast neoplasm, arrange for mammography immediately. The sensitivity and specificity of mammography for benign and malignant conditions exceed 90%.12 A biopsy may be necessary if uncertainty remains after imaging.
No specific tests are necessary when gynecomastia is clearly associated with intake of a medication known to be associated with the condition, especially if the history and examination are otherwise negative. A prompt regression of gynecomastia after discontinuation of the offending drug will confirm the diagnosis.
If the condition persists in an adolescent or adult and the cause is still unclear, perform renal function tests and measure levels of liver enzymes, early-morning serum human chorionic gonadotropin (hCG), LH, total testosterone, estradiol, TSH, and prolactin.
What lab results may mean. If the total testosterone level is borderline or low-normal (200-350 ng/dL), repeat the test and measure the free testosterone level.
If an elevated hCG level is found, repeat the testicular examination carefully and order ultrasonography. In the absence of a testicular tumor, consider an MRI of the brain and computed tomography (CT) of the abdomen and chest to help identify an extragonadal hCG-secreting tumor.
An elevated LH level and low testosterone level are diagnostic of primary testicular hypogonadism. A karyotype may be necessary in some individuals to diagnose Klinefelter syndrome. Elevated LH and testosterone levels are seen in patients with androgen insensitivity syndromes. These conditions are caused by abnormalities in the androgen receptor with a wide range of possible phenotypes, including ambiguous genitalia.
A low testosterone level with a low or normal LH level indicates secondary hypogonadism of hypothalamic or pituitary origin. An elevated prolactin level in such cases (as was seen in Mr. J.’s case) is usually due to a prolactin secreting pituitary adenoma.
Hereditary hemochromatosis is an important and often overlooked cause of hypogonadism. Obtain iron studies and ferritin levels in this setting.13 Unrecognized hemochromatosis may result in fibrosis and multiple organ failure.
Patients with secondary hypogonadism are best managed by a referral to an endocrinologist, as the potential list of causes is extensive.4,14,15
A low TSH level is consistent with thyrotoxicosis, which may result in increased levels of SHBG and altered metabolism of estrogens and androgens.16 Thus, about 10% of men with thyrotoxicosis present with gynecomastia and erectile dysfunction.16 If the estradiol level is elevated, a testicular ultrasound as well as an adrenal CT scan will help identify a neoplasm.
In a significant number of patients, the diagnostic tests are normal, leading to a diagnosis of idiopathic gynecomastia. In these cases, the alteration in androgen and estrogen levels can be subtle and intermittent.17 Continue surveillance and periodically reevaluate the patient.
Management of gynecomastia
Gynecomastia often results from transient hormonal imbalance and regresses spontaneously. Therefore, no specific treatment is necessary for neonatal, pubertal, or drug-induced gynecomastia. In other situations, prompt diagnosis and treatment are important to maximize the likelihood of successful medical therapy. It has been shown that fibrosis develops 6 to 12 months after the onset of gynecomastia, making it unlikely that medical treatments beyond that stage will result in significant regression of the breast enlargement.18 In such long-standing cases, surgical intervention with subcutaneous mastectomy or liposuction can be considered for patients who have significant psychological problems or esthetic issues. Indications for surgery also include continued growth and tenderness of breast tissue or malignancy.
Available medications include those aimed at decreasing estrogen production or estrogen effect on target breast tissue. Aromatase inhibitors such as testolactone, anastrozole, and letrozole can decrease the synthesis of estrogen by inhibiting aromatization of androgens. Although theoretically promising, results of the few controlled trials with aromatase inhibitors have been generally disappointing.19
Selective estrogen receptor modulators that alter the effect of estrogen on breast tissue are tamoxifen and raloxifene. Tamoxifen is not yet approved for treatment of gynecomastia, but has proven effective in randomized trials.20 At a dose of 20 mg/d for 3 or more months, tamoxifen resulted in complete regression of gynecomastia in 60% of patients and partial regression in 20% of patients.20 Tamoxifen also prevents gynecomastia after medial prostatectomy and treatment with the antiandrogen, bicalutamide.
CASE Mr. J had a pituitary prolactin-secreting microadenoma causing secondary hypogonadism and gynecomastia. He was started on cabergoline (a dopamine agonist) 0.5 mg orally once a week. Four months later, his total testosterone level was 291 ng/dL, and prolactin was 9.3 ng/mL. His headaches and gynecomastia had significantly decreased. He continued to do well on the same regimen and, 6 years later, his prolactin level was 1.4 ng/mL, indicating that treatment had been effective.
CORRESPONDENCE
Roy N. Morcos, MD, Department of Family Medicine, St. Elizabeth Health Center, 1044 Belmont Avenue, Youngstown, OH 44501; roymorcos@gmail.com
1. Haynes B, Mookadem F. Male gynecomastia. Mayo Clin Proc. 2009;84:672.-
2. Nordt C, Divanta A. Gynecomastia in adolescents. Curr Opin Pediatr. 2008;20:375-382.
3. Braunstein G. Gynecomastia. N Engl J Med. 2007;357:1229-1237.
4. Bhasin SI. Testicular disorders. In: Kronenberg HM, Melmed S, Polonsky KS, et al, eds. Williams Textbook of Endocrinology. 11th ed. Philadelphia, Pa: Saunders-Elsevier; 2008:569–671.
5. Eckman A, Dobs A. Drug-induced gynecomastia. Expert Opin Drug Saf. 2008;7:691-702.
6. Derkacz M, Chmiel-Perzyriska I, Nowakowski A. Gynecomastia – a difficult diagnostic problem. Endokrynol Pol. 2011;62:190-202.
7. Henley D, Lipson N, Kovach K, et al. Pubertal gynecomastia linked to lavender and tea tree oils. N Engl J Med. 2007;356:479-485.
8. Ma N, Geffnes M. Gynecomastia in prepubertal and pubertal boys. Curr Opin Pediatr. 2008;20:465-470.
9. Eberle AJ, Sparrow JT, Keenan BS. Treatment of persistent pubertal gynecomastia with dihydrotestosterone heptanoate. J Pediatr. 1986;109:144-149.
10. Kapoor S. Cutaneous manifestations of systemic condition associated with gynecomastia. Skinmed. 2010;8:87-92.
11. Johnson RE, Murad MH. Gynecomastia: pathophysiology, evaluation, and management. Mayo Clin Proc. 2009;84:1010-1015.
12. Evans GF, Anthony T, Turnage RH, et al. The diagnostic accuracy of mammography in the evaluation of male breast disease. Am J Surg. 2001;181:96-102.
13. Allen KJ, Gurrin LC, Constantine CC, et al. Iron-overload related disease in HFE hereditary hemochromatosis. N Engl J Med. 2008;358:221-230.
14. Sedlmeyer IL, Palmert MR. Delayed puberty: analysis of a large case series from an academic center. J Clin Endocrinol Metab. 2002;87:1613-1620.
15. Bhasin SI, Jameson JL. Disorders of the testes and male reproductive system. In: Longo D, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012:3019–3020.
16. Meikle AW. The interrelationships between thyroid dysfunction and hypogonadism in men and boys. Thyroid. 2004;14(suppl 1):S17-S25.
17. Wu FCW, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123-135.
18. Di Lorenzo G, Autorino R, Perdona S, et al. Management of gynaecomastia in patients with prostate cancer: a systematic review. Lancet Oncol. 2005;6:972-979.
19. Mauras N, Bishop K, Merinbaum D, et al. Pharmacokinetics and pharmacodynamics of anastrozole in pubertal boys with recent onset gynecomastia. J Clin Endocrinol Metab. 2009;94:2975-2978.
20. Derman O, Kanbur N, Kilic I, et al. Long-term follow-up of tamoxifen treatment in adolescents with gynecomastia. J Pediatr Endocrinol Metab. 2008;21:449-453.
1. Haynes B, Mookadem F. Male gynecomastia. Mayo Clin Proc. 2009;84:672.-
2. Nordt C, Divanta A. Gynecomastia in adolescents. Curr Opin Pediatr. 2008;20:375-382.
3. Braunstein G. Gynecomastia. N Engl J Med. 2007;357:1229-1237.
4. Bhasin SI. Testicular disorders. In: Kronenberg HM, Melmed S, Polonsky KS, et al, eds. Williams Textbook of Endocrinology. 11th ed. Philadelphia, Pa: Saunders-Elsevier; 2008:569–671.
5. Eckman A, Dobs A. Drug-induced gynecomastia. Expert Opin Drug Saf. 2008;7:691-702.
6. Derkacz M, Chmiel-Perzyriska I, Nowakowski A. Gynecomastia – a difficult diagnostic problem. Endokrynol Pol. 2011;62:190-202.
7. Henley D, Lipson N, Kovach K, et al. Pubertal gynecomastia linked to lavender and tea tree oils. N Engl J Med. 2007;356:479-485.
8. Ma N, Geffnes M. Gynecomastia in prepubertal and pubertal boys. Curr Opin Pediatr. 2008;20:465-470.
9. Eberle AJ, Sparrow JT, Keenan BS. Treatment of persistent pubertal gynecomastia with dihydrotestosterone heptanoate. J Pediatr. 1986;109:144-149.
10. Kapoor S. Cutaneous manifestations of systemic condition associated with gynecomastia. Skinmed. 2010;8:87-92.
11. Johnson RE, Murad MH. Gynecomastia: pathophysiology, evaluation, and management. Mayo Clin Proc. 2009;84:1010-1015.
12. Evans GF, Anthony T, Turnage RH, et al. The diagnostic accuracy of mammography in the evaluation of male breast disease. Am J Surg. 2001;181:96-102.
13. Allen KJ, Gurrin LC, Constantine CC, et al. Iron-overload related disease in HFE hereditary hemochromatosis. N Engl J Med. 2008;358:221-230.
14. Sedlmeyer IL, Palmert MR. Delayed puberty: analysis of a large case series from an academic center. J Clin Endocrinol Metab. 2002;87:1613-1620.
15. Bhasin SI, Jameson JL. Disorders of the testes and male reproductive system. In: Longo D, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012:3019–3020.
16. Meikle AW. The interrelationships between thyroid dysfunction and hypogonadism in men and boys. Thyroid. 2004;14(suppl 1):S17-S25.
17. Wu FCW, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123-135.
18. Di Lorenzo G, Autorino R, Perdona S, et al. Management of gynaecomastia in patients with prostate cancer: a systematic review. Lancet Oncol. 2005;6:972-979.
19. Mauras N, Bishop K, Merinbaum D, et al. Pharmacokinetics and pharmacodynamics of anastrozole in pubertal boys with recent onset gynecomastia. J Clin Endocrinol Metab. 2009;94:2975-2978.
20. Derman O, Kanbur N, Kilic I, et al. Long-term follow-up of tamoxifen treatment in adolescents with gynecomastia. J Pediatr Endocrinol Metab. 2008;21:449-453.
Ectopic pregnancy: Zero in on these lab and imaging clues
• Administer a urine pregnancy test for women of childbearing age who present with abdominal pain or vaginal bleeding. C
• Initiate quantitative beta-human chorionic gonadotropin testing and order transvaginal ultrasound for women with abdominal pain or vaginal bleeding and a positive urine pregnancy test, but no confirmation of intrauterine pregnancy by abdominal ultrasound. B
• Refer hemodynamically stable patients with ectopic pregnancy for laparoscopic salpingostomy. For selected patients, an alternative is medical treatment with methotrexate. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 Helen, who is 31 years old and G1P0, comes in to the office with a 10-day history of intermittent vaginal spotting without pelvic pain. A home pregnancy test 2 weeks earlier was positive, and this is a desired pregnancy. She has had no gynecologic disorders. It has been 6 weeks since her last menstrual period. Her vital signs are normal and her abdominal and pelvic exams are unremarkable. The cervical os is closed and there is a small amount of blood in the vaginal vault. Her family physician (FP) draws blood to measure the level of beta-human chorionic gonadotropin (β-hCG) and orders a transvaginal ultrasound (TVUS).
CASE 2 Mary is 28 years old and G2P1. She has experienced intermittent vaginal spotting and moderate pelvic discomfort for 3 days. She fears a return of pelvic inflammatory disease (PID). Her period is one week late and the office pregnancy test is positive. Her vital signs are normal. She has no cervical motion tenderness, but there is mild right adnexal tenderness to palpation. Her FP draws blood for a serum β-hCG level and orders a TVUS.
Assess physical and history findings for perspective
Abdominal or pelvic pain and vaginal bleeding in the first trimester are the most common presenting symptoms of ectopic pregnancies.1 Physical examination will often elicit lateral or bilateral abdominal or pelvic tenderness, peritoneal signs, and cervical motion tenderness. But such findings (or their absence) cannot confirm (or exclude) the diagnosis with a high level of reliability.2 A woman with a positive pregnancy test and pelvic pain or vaginal bleeding may instead have a normal pregnancy, spontaneous abortion (failing intrauterine pregnancy), or a disorder such as PID, acute appendicitis, tubo-ovarian abscess, or ovarian torsion.
In an early ectopic pregnancy, vital signs are usually normal. Even in cases of ruptured ectopic pregnancy, hypotension or tachycardia is present in <40% of cases.3
Factors conferring a relative risk ratio >2 for ectopic pregnancy are a previous ectopic pregnancy; documented tubal pathology or tubal instrumentation (eg, tubal sterilization or tubal corrective surgery); assisted reproductive technology such as in vitro fertilization; history of infertility; smoking; or a history of PID.4-11
Proceed with a laboratory and imaging strategy
When a woman who has tested positive for pregnancy presents with abdominal pain or vaginal bleeding and a normal intrauterine pregnancy (IUP) has not been confirmed by abdominal ultrasound, request a quantitative measurement of the β-hCG level and arrange for urgent TVUS.12,13 If pregnancy has been unsuspected in a patient with these symptoms, perform a urine test for pregnancy immediately and follow up with ultrasound.14
If TVUS reveals either IUP or ectopic pregnancy, management is relatively straightforward. However, an inconclusive TVUS result indicates a “pregnancy of unknown location” (PUL) and necessitates further testing and follow-up to achieve a final diagnosis.15
Monitor β-hCG levels
Valuable diagnostic measures include documenting the initial serum level of β-hCG, monitoring the subsequent rise-or-fall pattern in the level, and making use of the “discriminatory cutoff” value.
β-hCG, made by placental cells, can be detected in the mother’s blood approximately 11 days after conception, and in the urine 12 to 14 days after conception. The serum β-hCG level normally doubles every 48 to 72 hours until it reaches its peak in the first 8 to 11 weeks of pregnancy. The level then declines and plateaus.
”Discriminatory cutoff” is a widely accepted concept signifying the level of β-hCG at which a normal IUP can be visualized by ultrasonography with sensitivity approaching 100%.16 Generally an intrauterine sac can by visualized by abdominal ultrasound when the serum β-hCG level is >6500 mIU/mL.17 Visualization with TVUS (the preferred modality) has been demonstrated when the serum β-hCG level is as low as 1000 mIU/mL.17 However, the generally accepted cutoff range is 1500 to 2500 mIU/mL, based on several studies.13,18-20 The absence of an IUP in a pregnant woman with pain or bleeding and a β-hCG level above the cutoff implies an ectopic pregnancy18 or a failing IUP (spontaneous abortion).
Serial ß-hCG levels. When the β-hCG level is below the discriminatory cutoff, serial β-hCG measurements every 2 to 3 days are needed to assess viability of the pregnancy. A “normal rise” of β-hCG indicates early viable pregnancy and “normal fall” indicates spontaneous abortion. An analysis of 287 women with abdominal pain or vaginal bleeding who ultimately had normal uterine pregnancies found that the median slope for rise of β-hCG was 1.5 times (50% increase) in 1 day, and 2.24 times (124% rise) in 2 days.21 A rapid fall in β-hCG is consistent with a miscarriage that may resolve spontaneously. However, if the β-hCG level does not decline by 21% to 35% in 2 days, suspect ectopic pregnancy.21
Arrange for transvaginal ultrasound
TVUS is the imaging modality of choice for diagnosis of ectopic pregnancy, with a sensitivity of 87.0% to 99.0% and specificity of 94.0% to 99.9%.22 Arrange for TVUS when a women has abdominal pain or vaginal bleeding and a positive urine pregnancy test, even if the β-hCG level is lower than the discriminatory cutoff of 1500 to 2500 mIU/mL.13,18-20 Ordering TVUS and β-hCG level at the same time yields the best outcome for diagnosis,19 while varying the discriminatory zone alone has not improved diagnosis.18,23
Other novel markers
The use of serum progesterone and other novel markers such as inhibin A, activin A, creatinine kinase, vascular endothelial growth factor, and cancer antigen 125 in the diagnosis of ectopic pregnancy has been studied extensively. To date, no single marker has demonstrated high sensitivity and specificity in differentiating ectopic pregnancy.24 However, when the initial progesterone level is ≤10 nmol/L (equivalent to 31.4 ng/mL) in a woman with a PUL, the probability that she will require any intervention is reported to be low (4 cases out of 227 PUL cases).25 Multiplex tools to combine multiple biomarkers may become available in the future.
Evacuation of uterine contents
When the β-hCG level is above the discriminatory cutoff but no evidence of an extrauterine or intrauterine pregnancy can be found by TVUS, the patient likely has a failing IUP or impending abortion. Some experts suggest considering evaluation of the uterine contents by dilation and curettage (D&C) or manual vacuum extraction at this time, to differentiate an abnormal intrauterine gestation from an ectopic pregnancy. Barnhart found that more than one-third of such cases were due to a failed uterine pregnancy, not ectopic pregnancy.26
If, after a D&C or manual extraction, chorionic villi are not confirmed by pathologic examination of the uterine contents, then treat as an ectopic pregnancy. Some clinicians alternatively recommend checking the β-hCG level again in 12 to 24 hours, expecting ≥15% decline with a spontaneous abortion.27 Alternatively, some recommend using methotrexate (MTX) without D&C to avoid unnecessary medical and surgical treatment.26
CASE 1 Helen’s serum β-hCG level is 4500 mIU/mL, and the TVUS image the next day shows an echogenic mass next to the right ovary—highly suspicious for ectopic pregnancy.
CASE 2 Mary’s TVUS does not show any evidence of IUP or any abnormality in either adnexa. Her serum β-hCG level is 650 mIU/mL. She has a PUL. Her FP informs her that she may have an early normal pregnancy, a failed IUP, or an ectopic pregnancy. She agrees to have her serum β-hCG measured every 2 days. Her β-hCG level increases to 1100, 2000, and 3500 mIU/mL, in 2, 4, and 6 days, respectively. TVUS on the sixth day is still nondiagnostic.
Treatment of ectopic pregnancy: Surgical vs medical
For hemodynamically unstable patients, laparotomy is still the mainstay of therapy. However, with early diagnosis and a stable patient, options are minimally invasive surgical intervention via laparostomy or medical management with MTX in a single or multidose regimen. Surgical and medical treatments have comparable outcomes, as documented by a Cochrane review.28
The risk of recurrent ectopic pregnancy after MTX treatment and salpingostomy is similar—about 10%.29 Ipsilateral tubal patency as documented by hysterosalpingography after MTX treatment or salpingostomy was reported to be equal.28 Reproductive outcomes after either treatment were similar, as well.30
We recommend urgent referral for OB/GYN consultation if the diagnosis of ectopic pregnancy is made by TVUS, since the recommended treatment is laparoscopic salpingostomy. In the case of a PUL, we recommend referral to an OB/GYN when the serum β-hCG level is above the discriminatory cutoff of 1500 to 2500 mIU/mL without signs of IUP as seen by a gestational sac via TVUS. When an urgent referral is not possible, initiate medical treatment. Regardless of the treatment method, give anti-D immunoglobulin to any woman whose blood is Rh negative (no D-antigen) and who has not been sensitized to D-antigen.
Surgical management
Laparoscopic salpingostomy is the preferred surgical treatment for ectopic pregnancy. A Cochrane review meta-analysis of 35 randomized controlled trials (RCTs) on intervention of ectopic pregnancy concluded that, compared with laparotomy, laparoscopy results in shorter operative time, less blood loss, less analgesia, shorter hospital stays, and greater cost effectiveness.28 Another meta-analysis of 15 RCTs concluded that laparoscopic salpingostomy is the most cost-effective treatment for ectopic pregnancy. 31
Medical management with methotrexate
This folic acid antagonist is highly effective in treating ectopic pregnancy, and is usually given intramuscularly for this indication. Clinicians who use this chemotherapeutic agent must be familiar with its dosing regimen, contraindications, and possible adverse effects. Multidose MTX is more effective than surgery, but more expensive.32 Single-dose MTX has a higher failure rate than laparoscopic salpingostomy, especially in patients with higher β-hCG levels.32
The best candidate for medical therapy is the woman who is asymptomatic, motivated, and compliant. Absolute contraindications to single-dose MTX include the following:
- breastfeeding
- overt or lab evidence of immunodeficiency
- alcoholism, alcoholic liver disease, or other chronic liver disease
- preexisting blood dyscrasias, such as bone marrow hypoplasia, leucopenia, thrombocytopenia, or significant anemia
- known sensitivity for methotrexate
- acute pulmonary disease
- peptic ulcer disease
- hepatic, renal, or hematologic dysfunction, and several metabolic diseases.33
Dosing regimen. The 3 general dosing schemes of single dose, 2-dose, and multidose (up to 4 doses) are shown in the TABLE. These were recommended by the American College of Obstetrician and Gynecologists (ACOG).33
Single dose vs multidose. The single-dose treatment is easier to administer and monitor and is most cost effective, but it may have a higher failure rate than the multidose regimens.28 The best prognostic indicator of successful treatment with single-dose MTX is the initial β-hCG level. The lower the initial level, the higher the success rate. The reported failure rate is 1.5% if the initial β-hCG level is <1000 mIU/mL; 5.6% with 1000 to 2000 mIU/mL; 3.8% with 2000 to 5000 mIU/mL; and 14.3% with 5000 to 10,000 mIU/mL.34 ACOG has outlined relative contraindications to single-dose MTX: ectopic pregnancy larger than 3.5 cm and the presence of fetal cardiac activity. Both correlate with an increased failure rate. Patients with PUL and low β-hCG levels are good candidates for single-dose MTX treatment.
Monitoring efficacy of treatment
Serum β-hCG levels indicate response to medical and surgical therapy. After salpingostomy, the serum β-hCG level declines rapidly within the first 4 days, and then more gradually, with mean resolution occurring at about 20 days. In contrast, after single-dose MTX, the mean serum β-hCG level increases for the first 4 days and then gradually declines, with a mean resolution at 27 days.35 The guideline for surveillance is shown in the TABLE.
CASE 1 The FP counsels Helen on the risks and benefits of surgery and MTX treatment for her ectopic pregnancy, and she elects to have a laparoscopic salpingostomy. The FP refers Helen to an OB/GYN via the emergency department on the same day. Helen does well. After the surgery, her β-hCG is monitored every 2 days until it decreases to 1000 mIU/mL, then every week until it is negative.
CASE 2 The FP advises Mary that an OB/GYN would likely recommend a D&C for her PUL, as her β-hCG level is above the discriminatory cutoff and the TVUS does not show a viable IUP. After discussing MTX treatment and manual vacuum aspiration of the uterine contents, Mary elects to have the MTX treatment and receives the 2-dose protocol. Her β-hCG level is 4210 mIU/mL on Day 1—higher than her level prior to the methotrexate treatment, but expected. Levels drop to 3635, 3102, and 2214 mIU/mL on Days 4, 7, and 10, respectively. Mary receives weekly surveillance until her level decreases to 0, which it did in a month.
TABLE
Monitoring methotrexate therapy for ectopic pregnancy
| Regimen | Surveillance |
|---|---|
| Single dose* Methotrexate, 50 mg/m2 IM | Measure β-hCG level on Days 4 and 7: If difference ≥15%, repeat weekly until undetectable
|
| 2 dose Methotrexate, 50 mg/m2 IM, Days 0, 4 | Follow up as for single-dose regimen |
| Multidose (up to 4 doses) Methotrexate, 1 mg/kg IM, Days 1, 3, 5, 7 Leucovorin, 0.1 mg/kg IM, Days 2, 4, 6, 8 | Measure β-hCG level on Days 1, 3, 5, and 7
|
| β-hCG, beta-human chorionic gonadotropin; IM, intramuscularly. *Preferred treatment if low initial β-hCG level. Adapted from: Seeber BE, et al. Obstet Gynecol. 2006.27 | |
CORRESPONDENCE Sahoko H. Little, MD, Room 2300, Lobby H, 24 Frank Lloyd Wright Drive, Ann Arbor, MI 48105-9755; sahoko@med.umich.edu
1. Aboud E, Chaliha C. Nine year survey of 138 ectopic pregnancies. Arch Gynecol Obstet. 1998;261:83-87.
2. Dart GD, Kaplan B, Varaklis K. Predictive value of history and physical examination in patients with suspected ectopic pregnancy. Ann Emerg Med. 1999;33:283-290.
3. Birkhahn RH, Gaeta TJ, Van Deusen SK, et al. The ability of traditional vital signs and shock index to identify ruptured ectopic pregnancy. Am J Obstet Gynecol. 2003;189:1293-1296.
4. Cunningham FG, Leveno KJ, Bloom SL, et al. Ectopic pregnancy. In: Cunningham FG, Leveno KJ, Bloom SL, et al, eds. Williams Obstetrics. 23rd ed. New York, NY: McGraw-Hill; 2010:238–256.
5. Bakken IJ, Skjeldestad FE, Lydersen S, et al. Births and ectopic pregnancies in a large cohort of women tested for Chlamydia trachomatis. Sex Transm Dis. 2007;34:739-743.
6. Bakken IJ, Skjeldestad FE, Nordbo SA. Chlamydia trachomatis infections increase the risk for ectopic pregnancy: a population-based, nested case–control study. Sex Transm Dis. 2007;34:166-169.
7. Barnhart KT, Sammel MD, Gracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic first-trimester pregnancies. Fertil Steril. 2006;86:36-43.
8. Bouyer J, Coste J, Shojaei T, et al. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol. 2003;157:185-194.
9. Gala RB. Ectopic pregnancy. In: Schorge JO, Schaffer JI, Halvorson LM, et al, eds. Williams Gynecology. New York, NY: McGraw-Hill; 2008:160-175.
10. Karaer A, Avsar FA, Batioglu S. Risk factors for ectopic pregnancy: a case-control study. Aust N Z J Obstet Gynaecol. 2006;46:521-527.
11. Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357:648-653.
12. Dart RG, Kaplan B, Cox C. Transvaginal ultrasound in patients with low beta-human chorionic gonadotropin values: how often is the study diagnostic? Ann Emerg Med. 1997;30:135-140.
13. Kaplan BC, Dart RG, Moskos M, et al. Ectopic pregnancy: prospective study with improved diagnostic accuracy. Ann Emerg Med. 1996;28:10-17.
14. Clinical policy: critical issues for the initial evaluation and management of patients presenting with a chief complaint of nontraumatic acute abdominal pain Ann Emerg Med. 2000;36:406-415.
15. Barnhart K, van Mello NM, Bourne T, et al. Pregnancy of unknown location: a consensus statement of nomenclature, definitions, and outcome. Fertil Steril. 2011;95:857-866.
16. Barnhart K, Mennuti MT, Benjamin I, et al. Prompt diagnosis of ectopic pregnancy in an emergency department setting. Obstet Gynecol. 1994;84:1010-1015.
17. Aleem FA, DeFazio M, Gintautas J. Endovaginal sonography for the early diagnosis of intrauterine and ectopic pregnancies. Hum Reprod. 1990;5:755-758.
18. Coundous G, Kirk E, Lu C, et al. Diagnostic accuracy of varying discriminatory zones for the prediction of ectopic pregnancy in women with a pregnancy of unknown location. Ultrasound Obstet Gynecol. 2005;26:770-775.
19. Gracia CR, Kurt TB. Diagnosing ectopic pregnancy: decision analysis comparing six strategies. Obstet Gynecol. 2001;97:464-470.
20. Kohn MA, Kerr K, Malkevich D, et al. Beta-human chorionic gonadotropin levels and the likelihood of ectopic pregnancy in emergency department patients with abdominal pain or vaginal bleeding. Acad Emerg Med. 2003;10:119-126.
21. Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104:975-981.
22. Kirk E, Bourne T. Diagnosis of ectopic pregnancy with ultrasound. Best Pract Res Clin Obstet Gynaecol. 2009;23:501-508.
23. van Mello NM, Mol F, Opmeer BC, et al. Diagnostic value of serum hCG on the outcome of pregnancy of unknown location: a systematic review and meta-analysis. Hum Reprod Update. 2012;18:603-617.
24. Segal S, Mercado R, Rivnay B. Ectopic pregnancy early diagnosis markers. Minerva Ginecol. 2010;62:49-62.
25. Cordina M, Schramm-Gajraj K, Ross JA, et al. Introduction of a single visit protocol in the management of selected patients with pregnancy of unknown location: a prospective study. BJOG. 2011;118:693-697.
26. Barnhart KT, Katz I, Hummel A. Presumed diagnosis of ectopic pregnancy. Obstet Gynecol. 2002;100:505-510.
27. Seeber BE, Barnhart KT. Suspected ectopic pregnancy. Obstet Gynecol. 2006;107:399-413.
28. Hajenius PJ, Mol F, Mol BW, et al. Interventions for tubal ectopic pregnancy. Cochrane Database Syst Rev. 2007;(1):CD000324.-
29. Stovall TG. Medical management should be routinely used as primary therapy for ectopic pregnancy. Clin Obstet Gynecol. 1995;38:346-352.
30. Fernandez H, Yves Vincent SC, Pauthier S, et al. Randomized trial of conservative laparoscopic treatment and methotrexate administration in ectopic pregnancy and subsequent fertility. Hum Reprod. 1998;13:3239-3243.
31. Mol F, Strandell A, Jurkovic D, et al. The ESEP study: salpingostomy versus salpingectomy for tubal ectopic pregnancy; the impact on future fertility: a randomised controlled trial. BMC Womens Health. 2008;8:11.-
32. Mol F, Mol BW, Ankum WM, et al. Current evidence on surgery, systemic methotrexate and expectant management in the treatment of tubal ectopic pregnancy: a systematic review and meta-analysis. Hum Reprod Update. 2008;14:309-319.
33. American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 94. Medical management of ectopic pregnancy. Obstet Gynecol. 2008;111:1479-1485.
34. Menon S, Colins J, Barnhart KT. Establishing a human chorionic gonadotropin cutoff to guide methotrexate treatment of ectopic pregnancy: a systematic review. Fertil Steril. 2007;87:481-484.
35. Saraj AJ, Wilcox JG, Najmabadi S, et al. Resolution of hormonal markers of ectopic gestation: a randomized trial comparing single-dose intramuscular methotrexate with salpingostomy. Obstet Gynecol. 1998;92:989-994.
• Administer a urine pregnancy test for women of childbearing age who present with abdominal pain or vaginal bleeding. C
• Initiate quantitative beta-human chorionic gonadotropin testing and order transvaginal ultrasound for women with abdominal pain or vaginal bleeding and a positive urine pregnancy test, but no confirmation of intrauterine pregnancy by abdominal ultrasound. B
• Refer hemodynamically stable patients with ectopic pregnancy for laparoscopic salpingostomy. For selected patients, an alternative is medical treatment with methotrexate. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 Helen, who is 31 years old and G1P0, comes in to the office with a 10-day history of intermittent vaginal spotting without pelvic pain. A home pregnancy test 2 weeks earlier was positive, and this is a desired pregnancy. She has had no gynecologic disorders. It has been 6 weeks since her last menstrual period. Her vital signs are normal and her abdominal and pelvic exams are unremarkable. The cervical os is closed and there is a small amount of blood in the vaginal vault. Her family physician (FP) draws blood to measure the level of beta-human chorionic gonadotropin (β-hCG) and orders a transvaginal ultrasound (TVUS).
CASE 2 Mary is 28 years old and G2P1. She has experienced intermittent vaginal spotting and moderate pelvic discomfort for 3 days. She fears a return of pelvic inflammatory disease (PID). Her period is one week late and the office pregnancy test is positive. Her vital signs are normal. She has no cervical motion tenderness, but there is mild right adnexal tenderness to palpation. Her FP draws blood for a serum β-hCG level and orders a TVUS.
Assess physical and history findings for perspective
Abdominal or pelvic pain and vaginal bleeding in the first trimester are the most common presenting symptoms of ectopic pregnancies.1 Physical examination will often elicit lateral or bilateral abdominal or pelvic tenderness, peritoneal signs, and cervical motion tenderness. But such findings (or their absence) cannot confirm (or exclude) the diagnosis with a high level of reliability.2 A woman with a positive pregnancy test and pelvic pain or vaginal bleeding may instead have a normal pregnancy, spontaneous abortion (failing intrauterine pregnancy), or a disorder such as PID, acute appendicitis, tubo-ovarian abscess, or ovarian torsion.
In an early ectopic pregnancy, vital signs are usually normal. Even in cases of ruptured ectopic pregnancy, hypotension or tachycardia is present in <40% of cases.3
Factors conferring a relative risk ratio >2 for ectopic pregnancy are a previous ectopic pregnancy; documented tubal pathology or tubal instrumentation (eg, tubal sterilization or tubal corrective surgery); assisted reproductive technology such as in vitro fertilization; history of infertility; smoking; or a history of PID.4-11
Proceed with a laboratory and imaging strategy
When a woman who has tested positive for pregnancy presents with abdominal pain or vaginal bleeding and a normal intrauterine pregnancy (IUP) has not been confirmed by abdominal ultrasound, request a quantitative measurement of the β-hCG level and arrange for urgent TVUS.12,13 If pregnancy has been unsuspected in a patient with these symptoms, perform a urine test for pregnancy immediately and follow up with ultrasound.14
If TVUS reveals either IUP or ectopic pregnancy, management is relatively straightforward. However, an inconclusive TVUS result indicates a “pregnancy of unknown location” (PUL) and necessitates further testing and follow-up to achieve a final diagnosis.15
Monitor β-hCG levels
Valuable diagnostic measures include documenting the initial serum level of β-hCG, monitoring the subsequent rise-or-fall pattern in the level, and making use of the “discriminatory cutoff” value.
β-hCG, made by placental cells, can be detected in the mother’s blood approximately 11 days after conception, and in the urine 12 to 14 days after conception. The serum β-hCG level normally doubles every 48 to 72 hours until it reaches its peak in the first 8 to 11 weeks of pregnancy. The level then declines and plateaus.
”Discriminatory cutoff” is a widely accepted concept signifying the level of β-hCG at which a normal IUP can be visualized by ultrasonography with sensitivity approaching 100%.16 Generally an intrauterine sac can by visualized by abdominal ultrasound when the serum β-hCG level is >6500 mIU/mL.17 Visualization with TVUS (the preferred modality) has been demonstrated when the serum β-hCG level is as low as 1000 mIU/mL.17 However, the generally accepted cutoff range is 1500 to 2500 mIU/mL, based on several studies.13,18-20 The absence of an IUP in a pregnant woman with pain or bleeding and a β-hCG level above the cutoff implies an ectopic pregnancy18 or a failing IUP (spontaneous abortion).
Serial ß-hCG levels. When the β-hCG level is below the discriminatory cutoff, serial β-hCG measurements every 2 to 3 days are needed to assess viability of the pregnancy. A “normal rise” of β-hCG indicates early viable pregnancy and “normal fall” indicates spontaneous abortion. An analysis of 287 women with abdominal pain or vaginal bleeding who ultimately had normal uterine pregnancies found that the median slope for rise of β-hCG was 1.5 times (50% increase) in 1 day, and 2.24 times (124% rise) in 2 days.21 A rapid fall in β-hCG is consistent with a miscarriage that may resolve spontaneously. However, if the β-hCG level does not decline by 21% to 35% in 2 days, suspect ectopic pregnancy.21
Arrange for transvaginal ultrasound
TVUS is the imaging modality of choice for diagnosis of ectopic pregnancy, with a sensitivity of 87.0% to 99.0% and specificity of 94.0% to 99.9%.22 Arrange for TVUS when a women has abdominal pain or vaginal bleeding and a positive urine pregnancy test, even if the β-hCG level is lower than the discriminatory cutoff of 1500 to 2500 mIU/mL.13,18-20 Ordering TVUS and β-hCG level at the same time yields the best outcome for diagnosis,19 while varying the discriminatory zone alone has not improved diagnosis.18,23
Other novel markers
The use of serum progesterone and other novel markers such as inhibin A, activin A, creatinine kinase, vascular endothelial growth factor, and cancer antigen 125 in the diagnosis of ectopic pregnancy has been studied extensively. To date, no single marker has demonstrated high sensitivity and specificity in differentiating ectopic pregnancy.24 However, when the initial progesterone level is ≤10 nmol/L (equivalent to 31.4 ng/mL) in a woman with a PUL, the probability that she will require any intervention is reported to be low (4 cases out of 227 PUL cases).25 Multiplex tools to combine multiple biomarkers may become available in the future.
Evacuation of uterine contents
When the β-hCG level is above the discriminatory cutoff but no evidence of an extrauterine or intrauterine pregnancy can be found by TVUS, the patient likely has a failing IUP or impending abortion. Some experts suggest considering evaluation of the uterine contents by dilation and curettage (D&C) or manual vacuum extraction at this time, to differentiate an abnormal intrauterine gestation from an ectopic pregnancy. Barnhart found that more than one-third of such cases were due to a failed uterine pregnancy, not ectopic pregnancy.26
If, after a D&C or manual extraction, chorionic villi are not confirmed by pathologic examination of the uterine contents, then treat as an ectopic pregnancy. Some clinicians alternatively recommend checking the β-hCG level again in 12 to 24 hours, expecting ≥15% decline with a spontaneous abortion.27 Alternatively, some recommend using methotrexate (MTX) without D&C to avoid unnecessary medical and surgical treatment.26
CASE 1 Helen’s serum β-hCG level is 4500 mIU/mL, and the TVUS image the next day shows an echogenic mass next to the right ovary—highly suspicious for ectopic pregnancy.
CASE 2 Mary’s TVUS does not show any evidence of IUP or any abnormality in either adnexa. Her serum β-hCG level is 650 mIU/mL. She has a PUL. Her FP informs her that she may have an early normal pregnancy, a failed IUP, or an ectopic pregnancy. She agrees to have her serum β-hCG measured every 2 days. Her β-hCG level increases to 1100, 2000, and 3500 mIU/mL, in 2, 4, and 6 days, respectively. TVUS on the sixth day is still nondiagnostic.
Treatment of ectopic pregnancy: Surgical vs medical
For hemodynamically unstable patients, laparotomy is still the mainstay of therapy. However, with early diagnosis and a stable patient, options are minimally invasive surgical intervention via laparostomy or medical management with MTX in a single or multidose regimen. Surgical and medical treatments have comparable outcomes, as documented by a Cochrane review.28
The risk of recurrent ectopic pregnancy after MTX treatment and salpingostomy is similar—about 10%.29 Ipsilateral tubal patency as documented by hysterosalpingography after MTX treatment or salpingostomy was reported to be equal.28 Reproductive outcomes after either treatment were similar, as well.30
We recommend urgent referral for OB/GYN consultation if the diagnosis of ectopic pregnancy is made by TVUS, since the recommended treatment is laparoscopic salpingostomy. In the case of a PUL, we recommend referral to an OB/GYN when the serum β-hCG level is above the discriminatory cutoff of 1500 to 2500 mIU/mL without signs of IUP as seen by a gestational sac via TVUS. When an urgent referral is not possible, initiate medical treatment. Regardless of the treatment method, give anti-D immunoglobulin to any woman whose blood is Rh negative (no D-antigen) and who has not been sensitized to D-antigen.
Surgical management
Laparoscopic salpingostomy is the preferred surgical treatment for ectopic pregnancy. A Cochrane review meta-analysis of 35 randomized controlled trials (RCTs) on intervention of ectopic pregnancy concluded that, compared with laparotomy, laparoscopy results in shorter operative time, less blood loss, less analgesia, shorter hospital stays, and greater cost effectiveness.28 Another meta-analysis of 15 RCTs concluded that laparoscopic salpingostomy is the most cost-effective treatment for ectopic pregnancy. 31
Medical management with methotrexate
This folic acid antagonist is highly effective in treating ectopic pregnancy, and is usually given intramuscularly for this indication. Clinicians who use this chemotherapeutic agent must be familiar with its dosing regimen, contraindications, and possible adverse effects. Multidose MTX is more effective than surgery, but more expensive.32 Single-dose MTX has a higher failure rate than laparoscopic salpingostomy, especially in patients with higher β-hCG levels.32
The best candidate for medical therapy is the woman who is asymptomatic, motivated, and compliant. Absolute contraindications to single-dose MTX include the following:
- breastfeeding
- overt or lab evidence of immunodeficiency
- alcoholism, alcoholic liver disease, or other chronic liver disease
- preexisting blood dyscrasias, such as bone marrow hypoplasia, leucopenia, thrombocytopenia, or significant anemia
- known sensitivity for methotrexate
- acute pulmonary disease
- peptic ulcer disease
- hepatic, renal, or hematologic dysfunction, and several metabolic diseases.33
Dosing regimen. The 3 general dosing schemes of single dose, 2-dose, and multidose (up to 4 doses) are shown in the TABLE. These were recommended by the American College of Obstetrician and Gynecologists (ACOG).33
Single dose vs multidose. The single-dose treatment is easier to administer and monitor and is most cost effective, but it may have a higher failure rate than the multidose regimens.28 The best prognostic indicator of successful treatment with single-dose MTX is the initial β-hCG level. The lower the initial level, the higher the success rate. The reported failure rate is 1.5% if the initial β-hCG level is <1000 mIU/mL; 5.6% with 1000 to 2000 mIU/mL; 3.8% with 2000 to 5000 mIU/mL; and 14.3% with 5000 to 10,000 mIU/mL.34 ACOG has outlined relative contraindications to single-dose MTX: ectopic pregnancy larger than 3.5 cm and the presence of fetal cardiac activity. Both correlate with an increased failure rate. Patients with PUL and low β-hCG levels are good candidates for single-dose MTX treatment.
Monitoring efficacy of treatment
Serum β-hCG levels indicate response to medical and surgical therapy. After salpingostomy, the serum β-hCG level declines rapidly within the first 4 days, and then more gradually, with mean resolution occurring at about 20 days. In contrast, after single-dose MTX, the mean serum β-hCG level increases for the first 4 days and then gradually declines, with a mean resolution at 27 days.35 The guideline for surveillance is shown in the TABLE.
CASE 1 The FP counsels Helen on the risks and benefits of surgery and MTX treatment for her ectopic pregnancy, and she elects to have a laparoscopic salpingostomy. The FP refers Helen to an OB/GYN via the emergency department on the same day. Helen does well. After the surgery, her β-hCG is monitored every 2 days until it decreases to 1000 mIU/mL, then every week until it is negative.
CASE 2 The FP advises Mary that an OB/GYN would likely recommend a D&C for her PUL, as her β-hCG level is above the discriminatory cutoff and the TVUS does not show a viable IUP. After discussing MTX treatment and manual vacuum aspiration of the uterine contents, Mary elects to have the MTX treatment and receives the 2-dose protocol. Her β-hCG level is 4210 mIU/mL on Day 1—higher than her level prior to the methotrexate treatment, but expected. Levels drop to 3635, 3102, and 2214 mIU/mL on Days 4, 7, and 10, respectively. Mary receives weekly surveillance until her level decreases to 0, which it did in a month.
TABLE
Monitoring methotrexate therapy for ectopic pregnancy
| Regimen | Surveillance |
|---|---|
| Single dose* Methotrexate, 50 mg/m2 IM | Measure β-hCG level on Days 4 and 7: If difference ≥15%, repeat weekly until undetectable
|
| 2 dose Methotrexate, 50 mg/m2 IM, Days 0, 4 | Follow up as for single-dose regimen |
| Multidose (up to 4 doses) Methotrexate, 1 mg/kg IM, Days 1, 3, 5, 7 Leucovorin, 0.1 mg/kg IM, Days 2, 4, 6, 8 | Measure β-hCG level on Days 1, 3, 5, and 7
|
| β-hCG, beta-human chorionic gonadotropin; IM, intramuscularly. *Preferred treatment if low initial β-hCG level. Adapted from: Seeber BE, et al. Obstet Gynecol. 2006.27 | |
CORRESPONDENCE Sahoko H. Little, MD, Room 2300, Lobby H, 24 Frank Lloyd Wright Drive, Ann Arbor, MI 48105-9755; sahoko@med.umich.edu
• Administer a urine pregnancy test for women of childbearing age who present with abdominal pain or vaginal bleeding. C
• Initiate quantitative beta-human chorionic gonadotropin testing and order transvaginal ultrasound for women with abdominal pain or vaginal bleeding and a positive urine pregnancy test, but no confirmation of intrauterine pregnancy by abdominal ultrasound. B
• Refer hemodynamically stable patients with ectopic pregnancy for laparoscopic salpingostomy. For selected patients, an alternative is medical treatment with methotrexate. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 Helen, who is 31 years old and G1P0, comes in to the office with a 10-day history of intermittent vaginal spotting without pelvic pain. A home pregnancy test 2 weeks earlier was positive, and this is a desired pregnancy. She has had no gynecologic disorders. It has been 6 weeks since her last menstrual period. Her vital signs are normal and her abdominal and pelvic exams are unremarkable. The cervical os is closed and there is a small amount of blood in the vaginal vault. Her family physician (FP) draws blood to measure the level of beta-human chorionic gonadotropin (β-hCG) and orders a transvaginal ultrasound (TVUS).
CASE 2 Mary is 28 years old and G2P1. She has experienced intermittent vaginal spotting and moderate pelvic discomfort for 3 days. She fears a return of pelvic inflammatory disease (PID). Her period is one week late and the office pregnancy test is positive. Her vital signs are normal. She has no cervical motion tenderness, but there is mild right adnexal tenderness to palpation. Her FP draws blood for a serum β-hCG level and orders a TVUS.
Assess physical and history findings for perspective
Abdominal or pelvic pain and vaginal bleeding in the first trimester are the most common presenting symptoms of ectopic pregnancies.1 Physical examination will often elicit lateral or bilateral abdominal or pelvic tenderness, peritoneal signs, and cervical motion tenderness. But such findings (or their absence) cannot confirm (or exclude) the diagnosis with a high level of reliability.2 A woman with a positive pregnancy test and pelvic pain or vaginal bleeding may instead have a normal pregnancy, spontaneous abortion (failing intrauterine pregnancy), or a disorder such as PID, acute appendicitis, tubo-ovarian abscess, or ovarian torsion.
In an early ectopic pregnancy, vital signs are usually normal. Even in cases of ruptured ectopic pregnancy, hypotension or tachycardia is present in <40% of cases.3
Factors conferring a relative risk ratio >2 for ectopic pregnancy are a previous ectopic pregnancy; documented tubal pathology or tubal instrumentation (eg, tubal sterilization or tubal corrective surgery); assisted reproductive technology such as in vitro fertilization; history of infertility; smoking; or a history of PID.4-11
Proceed with a laboratory and imaging strategy
When a woman who has tested positive for pregnancy presents with abdominal pain or vaginal bleeding and a normal intrauterine pregnancy (IUP) has not been confirmed by abdominal ultrasound, request a quantitative measurement of the β-hCG level and arrange for urgent TVUS.12,13 If pregnancy has been unsuspected in a patient with these symptoms, perform a urine test for pregnancy immediately and follow up with ultrasound.14
If TVUS reveals either IUP or ectopic pregnancy, management is relatively straightforward. However, an inconclusive TVUS result indicates a “pregnancy of unknown location” (PUL) and necessitates further testing and follow-up to achieve a final diagnosis.15
Monitor β-hCG levels
Valuable diagnostic measures include documenting the initial serum level of β-hCG, monitoring the subsequent rise-or-fall pattern in the level, and making use of the “discriminatory cutoff” value.
β-hCG, made by placental cells, can be detected in the mother’s blood approximately 11 days after conception, and in the urine 12 to 14 days after conception. The serum β-hCG level normally doubles every 48 to 72 hours until it reaches its peak in the first 8 to 11 weeks of pregnancy. The level then declines and plateaus.
”Discriminatory cutoff” is a widely accepted concept signifying the level of β-hCG at which a normal IUP can be visualized by ultrasonography with sensitivity approaching 100%.16 Generally an intrauterine sac can by visualized by abdominal ultrasound when the serum β-hCG level is >6500 mIU/mL.17 Visualization with TVUS (the preferred modality) has been demonstrated when the serum β-hCG level is as low as 1000 mIU/mL.17 However, the generally accepted cutoff range is 1500 to 2500 mIU/mL, based on several studies.13,18-20 The absence of an IUP in a pregnant woman with pain or bleeding and a β-hCG level above the cutoff implies an ectopic pregnancy18 or a failing IUP (spontaneous abortion).
Serial ß-hCG levels. When the β-hCG level is below the discriminatory cutoff, serial β-hCG measurements every 2 to 3 days are needed to assess viability of the pregnancy. A “normal rise” of β-hCG indicates early viable pregnancy and “normal fall” indicates spontaneous abortion. An analysis of 287 women with abdominal pain or vaginal bleeding who ultimately had normal uterine pregnancies found that the median slope for rise of β-hCG was 1.5 times (50% increase) in 1 day, and 2.24 times (124% rise) in 2 days.21 A rapid fall in β-hCG is consistent with a miscarriage that may resolve spontaneously. However, if the β-hCG level does not decline by 21% to 35% in 2 days, suspect ectopic pregnancy.21
Arrange for transvaginal ultrasound
TVUS is the imaging modality of choice for diagnosis of ectopic pregnancy, with a sensitivity of 87.0% to 99.0% and specificity of 94.0% to 99.9%.22 Arrange for TVUS when a women has abdominal pain or vaginal bleeding and a positive urine pregnancy test, even if the β-hCG level is lower than the discriminatory cutoff of 1500 to 2500 mIU/mL.13,18-20 Ordering TVUS and β-hCG level at the same time yields the best outcome for diagnosis,19 while varying the discriminatory zone alone has not improved diagnosis.18,23
Other novel markers
The use of serum progesterone and other novel markers such as inhibin A, activin A, creatinine kinase, vascular endothelial growth factor, and cancer antigen 125 in the diagnosis of ectopic pregnancy has been studied extensively. To date, no single marker has demonstrated high sensitivity and specificity in differentiating ectopic pregnancy.24 However, when the initial progesterone level is ≤10 nmol/L (equivalent to 31.4 ng/mL) in a woman with a PUL, the probability that she will require any intervention is reported to be low (4 cases out of 227 PUL cases).25 Multiplex tools to combine multiple biomarkers may become available in the future.
Evacuation of uterine contents
When the β-hCG level is above the discriminatory cutoff but no evidence of an extrauterine or intrauterine pregnancy can be found by TVUS, the patient likely has a failing IUP or impending abortion. Some experts suggest considering evaluation of the uterine contents by dilation and curettage (D&C) or manual vacuum extraction at this time, to differentiate an abnormal intrauterine gestation from an ectopic pregnancy. Barnhart found that more than one-third of such cases were due to a failed uterine pregnancy, not ectopic pregnancy.26
If, after a D&C or manual extraction, chorionic villi are not confirmed by pathologic examination of the uterine contents, then treat as an ectopic pregnancy. Some clinicians alternatively recommend checking the β-hCG level again in 12 to 24 hours, expecting ≥15% decline with a spontaneous abortion.27 Alternatively, some recommend using methotrexate (MTX) without D&C to avoid unnecessary medical and surgical treatment.26
CASE 1 Helen’s serum β-hCG level is 4500 mIU/mL, and the TVUS image the next day shows an echogenic mass next to the right ovary—highly suspicious for ectopic pregnancy.
CASE 2 Mary’s TVUS does not show any evidence of IUP or any abnormality in either adnexa. Her serum β-hCG level is 650 mIU/mL. She has a PUL. Her FP informs her that she may have an early normal pregnancy, a failed IUP, or an ectopic pregnancy. She agrees to have her serum β-hCG measured every 2 days. Her β-hCG level increases to 1100, 2000, and 3500 mIU/mL, in 2, 4, and 6 days, respectively. TVUS on the sixth day is still nondiagnostic.
Treatment of ectopic pregnancy: Surgical vs medical
For hemodynamically unstable patients, laparotomy is still the mainstay of therapy. However, with early diagnosis and a stable patient, options are minimally invasive surgical intervention via laparostomy or medical management with MTX in a single or multidose regimen. Surgical and medical treatments have comparable outcomes, as documented by a Cochrane review.28
The risk of recurrent ectopic pregnancy after MTX treatment and salpingostomy is similar—about 10%.29 Ipsilateral tubal patency as documented by hysterosalpingography after MTX treatment or salpingostomy was reported to be equal.28 Reproductive outcomes after either treatment were similar, as well.30
We recommend urgent referral for OB/GYN consultation if the diagnosis of ectopic pregnancy is made by TVUS, since the recommended treatment is laparoscopic salpingostomy. In the case of a PUL, we recommend referral to an OB/GYN when the serum β-hCG level is above the discriminatory cutoff of 1500 to 2500 mIU/mL without signs of IUP as seen by a gestational sac via TVUS. When an urgent referral is not possible, initiate medical treatment. Regardless of the treatment method, give anti-D immunoglobulin to any woman whose blood is Rh negative (no D-antigen) and who has not been sensitized to D-antigen.
Surgical management
Laparoscopic salpingostomy is the preferred surgical treatment for ectopic pregnancy. A Cochrane review meta-analysis of 35 randomized controlled trials (RCTs) on intervention of ectopic pregnancy concluded that, compared with laparotomy, laparoscopy results in shorter operative time, less blood loss, less analgesia, shorter hospital stays, and greater cost effectiveness.28 Another meta-analysis of 15 RCTs concluded that laparoscopic salpingostomy is the most cost-effective treatment for ectopic pregnancy. 31
Medical management with methotrexate
This folic acid antagonist is highly effective in treating ectopic pregnancy, and is usually given intramuscularly for this indication. Clinicians who use this chemotherapeutic agent must be familiar with its dosing regimen, contraindications, and possible adverse effects. Multidose MTX is more effective than surgery, but more expensive.32 Single-dose MTX has a higher failure rate than laparoscopic salpingostomy, especially in patients with higher β-hCG levels.32
The best candidate for medical therapy is the woman who is asymptomatic, motivated, and compliant. Absolute contraindications to single-dose MTX include the following:
- breastfeeding
- overt or lab evidence of immunodeficiency
- alcoholism, alcoholic liver disease, or other chronic liver disease
- preexisting blood dyscrasias, such as bone marrow hypoplasia, leucopenia, thrombocytopenia, or significant anemia
- known sensitivity for methotrexate
- acute pulmonary disease
- peptic ulcer disease
- hepatic, renal, or hematologic dysfunction, and several metabolic diseases.33
Dosing regimen. The 3 general dosing schemes of single dose, 2-dose, and multidose (up to 4 doses) are shown in the TABLE. These were recommended by the American College of Obstetrician and Gynecologists (ACOG).33
Single dose vs multidose. The single-dose treatment is easier to administer and monitor and is most cost effective, but it may have a higher failure rate than the multidose regimens.28 The best prognostic indicator of successful treatment with single-dose MTX is the initial β-hCG level. The lower the initial level, the higher the success rate. The reported failure rate is 1.5% if the initial β-hCG level is <1000 mIU/mL; 5.6% with 1000 to 2000 mIU/mL; 3.8% with 2000 to 5000 mIU/mL; and 14.3% with 5000 to 10,000 mIU/mL.34 ACOG has outlined relative contraindications to single-dose MTX: ectopic pregnancy larger than 3.5 cm and the presence of fetal cardiac activity. Both correlate with an increased failure rate. Patients with PUL and low β-hCG levels are good candidates for single-dose MTX treatment.
Monitoring efficacy of treatment
Serum β-hCG levels indicate response to medical and surgical therapy. After salpingostomy, the serum β-hCG level declines rapidly within the first 4 days, and then more gradually, with mean resolution occurring at about 20 days. In contrast, after single-dose MTX, the mean serum β-hCG level increases for the first 4 days and then gradually declines, with a mean resolution at 27 days.35 The guideline for surveillance is shown in the TABLE.
CASE 1 The FP counsels Helen on the risks and benefits of surgery and MTX treatment for her ectopic pregnancy, and she elects to have a laparoscopic salpingostomy. The FP refers Helen to an OB/GYN via the emergency department on the same day. Helen does well. After the surgery, her β-hCG is monitored every 2 days until it decreases to 1000 mIU/mL, then every week until it is negative.
CASE 2 The FP advises Mary that an OB/GYN would likely recommend a D&C for her PUL, as her β-hCG level is above the discriminatory cutoff and the TVUS does not show a viable IUP. After discussing MTX treatment and manual vacuum aspiration of the uterine contents, Mary elects to have the MTX treatment and receives the 2-dose protocol. Her β-hCG level is 4210 mIU/mL on Day 1—higher than her level prior to the methotrexate treatment, but expected. Levels drop to 3635, 3102, and 2214 mIU/mL on Days 4, 7, and 10, respectively. Mary receives weekly surveillance until her level decreases to 0, which it did in a month.
TABLE
Monitoring methotrexate therapy for ectopic pregnancy
| Regimen | Surveillance |
|---|---|
| Single dose* Methotrexate, 50 mg/m2 IM | Measure β-hCG level on Days 4 and 7: If difference ≥15%, repeat weekly until undetectable
|
| 2 dose Methotrexate, 50 mg/m2 IM, Days 0, 4 | Follow up as for single-dose regimen |
| Multidose (up to 4 doses) Methotrexate, 1 mg/kg IM, Days 1, 3, 5, 7 Leucovorin, 0.1 mg/kg IM, Days 2, 4, 6, 8 | Measure β-hCG level on Days 1, 3, 5, and 7
|
| β-hCG, beta-human chorionic gonadotropin; IM, intramuscularly. *Preferred treatment if low initial β-hCG level. Adapted from: Seeber BE, et al. Obstet Gynecol. 2006.27 | |
CORRESPONDENCE Sahoko H. Little, MD, Room 2300, Lobby H, 24 Frank Lloyd Wright Drive, Ann Arbor, MI 48105-9755; sahoko@med.umich.edu
1. Aboud E, Chaliha C. Nine year survey of 138 ectopic pregnancies. Arch Gynecol Obstet. 1998;261:83-87.
2. Dart GD, Kaplan B, Varaklis K. Predictive value of history and physical examination in patients with suspected ectopic pregnancy. Ann Emerg Med. 1999;33:283-290.
3. Birkhahn RH, Gaeta TJ, Van Deusen SK, et al. The ability of traditional vital signs and shock index to identify ruptured ectopic pregnancy. Am J Obstet Gynecol. 2003;189:1293-1296.
4. Cunningham FG, Leveno KJ, Bloom SL, et al. Ectopic pregnancy. In: Cunningham FG, Leveno KJ, Bloom SL, et al, eds. Williams Obstetrics. 23rd ed. New York, NY: McGraw-Hill; 2010:238–256.
5. Bakken IJ, Skjeldestad FE, Lydersen S, et al. Births and ectopic pregnancies in a large cohort of women tested for Chlamydia trachomatis. Sex Transm Dis. 2007;34:739-743.
6. Bakken IJ, Skjeldestad FE, Nordbo SA. Chlamydia trachomatis infections increase the risk for ectopic pregnancy: a population-based, nested case–control study. Sex Transm Dis. 2007;34:166-169.
7. Barnhart KT, Sammel MD, Gracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic first-trimester pregnancies. Fertil Steril. 2006;86:36-43.
8. Bouyer J, Coste J, Shojaei T, et al. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol. 2003;157:185-194.
9. Gala RB. Ectopic pregnancy. In: Schorge JO, Schaffer JI, Halvorson LM, et al, eds. Williams Gynecology. New York, NY: McGraw-Hill; 2008:160-175.
10. Karaer A, Avsar FA, Batioglu S. Risk factors for ectopic pregnancy: a case-control study. Aust N Z J Obstet Gynaecol. 2006;46:521-527.
11. Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357:648-653.
12. Dart RG, Kaplan B, Cox C. Transvaginal ultrasound in patients with low beta-human chorionic gonadotropin values: how often is the study diagnostic? Ann Emerg Med. 1997;30:135-140.
13. Kaplan BC, Dart RG, Moskos M, et al. Ectopic pregnancy: prospective study with improved diagnostic accuracy. Ann Emerg Med. 1996;28:10-17.
14. Clinical policy: critical issues for the initial evaluation and management of patients presenting with a chief complaint of nontraumatic acute abdominal pain Ann Emerg Med. 2000;36:406-415.
15. Barnhart K, van Mello NM, Bourne T, et al. Pregnancy of unknown location: a consensus statement of nomenclature, definitions, and outcome. Fertil Steril. 2011;95:857-866.
16. Barnhart K, Mennuti MT, Benjamin I, et al. Prompt diagnosis of ectopic pregnancy in an emergency department setting. Obstet Gynecol. 1994;84:1010-1015.
17. Aleem FA, DeFazio M, Gintautas J. Endovaginal sonography for the early diagnosis of intrauterine and ectopic pregnancies. Hum Reprod. 1990;5:755-758.
18. Coundous G, Kirk E, Lu C, et al. Diagnostic accuracy of varying discriminatory zones for the prediction of ectopic pregnancy in women with a pregnancy of unknown location. Ultrasound Obstet Gynecol. 2005;26:770-775.
19. Gracia CR, Kurt TB. Diagnosing ectopic pregnancy: decision analysis comparing six strategies. Obstet Gynecol. 2001;97:464-470.
20. Kohn MA, Kerr K, Malkevich D, et al. Beta-human chorionic gonadotropin levels and the likelihood of ectopic pregnancy in emergency department patients with abdominal pain or vaginal bleeding. Acad Emerg Med. 2003;10:119-126.
21. Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104:975-981.
22. Kirk E, Bourne T. Diagnosis of ectopic pregnancy with ultrasound. Best Pract Res Clin Obstet Gynaecol. 2009;23:501-508.
23. van Mello NM, Mol F, Opmeer BC, et al. Diagnostic value of serum hCG on the outcome of pregnancy of unknown location: a systematic review and meta-analysis. Hum Reprod Update. 2012;18:603-617.
24. Segal S, Mercado R, Rivnay B. Ectopic pregnancy early diagnosis markers. Minerva Ginecol. 2010;62:49-62.
25. Cordina M, Schramm-Gajraj K, Ross JA, et al. Introduction of a single visit protocol in the management of selected patients with pregnancy of unknown location: a prospective study. BJOG. 2011;118:693-697.
26. Barnhart KT, Katz I, Hummel A. Presumed diagnosis of ectopic pregnancy. Obstet Gynecol. 2002;100:505-510.
27. Seeber BE, Barnhart KT. Suspected ectopic pregnancy. Obstet Gynecol. 2006;107:399-413.
28. Hajenius PJ, Mol F, Mol BW, et al. Interventions for tubal ectopic pregnancy. Cochrane Database Syst Rev. 2007;(1):CD000324.-
29. Stovall TG. Medical management should be routinely used as primary therapy for ectopic pregnancy. Clin Obstet Gynecol. 1995;38:346-352.
30. Fernandez H, Yves Vincent SC, Pauthier S, et al. Randomized trial of conservative laparoscopic treatment and methotrexate administration in ectopic pregnancy and subsequent fertility. Hum Reprod. 1998;13:3239-3243.
31. Mol F, Strandell A, Jurkovic D, et al. The ESEP study: salpingostomy versus salpingectomy for tubal ectopic pregnancy; the impact on future fertility: a randomised controlled trial. BMC Womens Health. 2008;8:11.-
32. Mol F, Mol BW, Ankum WM, et al. Current evidence on surgery, systemic methotrexate and expectant management in the treatment of tubal ectopic pregnancy: a systematic review and meta-analysis. Hum Reprod Update. 2008;14:309-319.
33. American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 94. Medical management of ectopic pregnancy. Obstet Gynecol. 2008;111:1479-1485.
34. Menon S, Colins J, Barnhart KT. Establishing a human chorionic gonadotropin cutoff to guide methotrexate treatment of ectopic pregnancy: a systematic review. Fertil Steril. 2007;87:481-484.
35. Saraj AJ, Wilcox JG, Najmabadi S, et al. Resolution of hormonal markers of ectopic gestation: a randomized trial comparing single-dose intramuscular methotrexate with salpingostomy. Obstet Gynecol. 1998;92:989-994.
1. Aboud E, Chaliha C. Nine year survey of 138 ectopic pregnancies. Arch Gynecol Obstet. 1998;261:83-87.
2. Dart GD, Kaplan B, Varaklis K. Predictive value of history and physical examination in patients with suspected ectopic pregnancy. Ann Emerg Med. 1999;33:283-290.
3. Birkhahn RH, Gaeta TJ, Van Deusen SK, et al. The ability of traditional vital signs and shock index to identify ruptured ectopic pregnancy. Am J Obstet Gynecol. 2003;189:1293-1296.
4. Cunningham FG, Leveno KJ, Bloom SL, et al. Ectopic pregnancy. In: Cunningham FG, Leveno KJ, Bloom SL, et al, eds. Williams Obstetrics. 23rd ed. New York, NY: McGraw-Hill; 2010:238–256.
5. Bakken IJ, Skjeldestad FE, Lydersen S, et al. Births and ectopic pregnancies in a large cohort of women tested for Chlamydia trachomatis. Sex Transm Dis. 2007;34:739-743.
6. Bakken IJ, Skjeldestad FE, Nordbo SA. Chlamydia trachomatis infections increase the risk for ectopic pregnancy: a population-based, nested case–control study. Sex Transm Dis. 2007;34:166-169.
7. Barnhart KT, Sammel MD, Gracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic first-trimester pregnancies. Fertil Steril. 2006;86:36-43.
8. Bouyer J, Coste J, Shojaei T, et al. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol. 2003;157:185-194.
9. Gala RB. Ectopic pregnancy. In: Schorge JO, Schaffer JI, Halvorson LM, et al, eds. Williams Gynecology. New York, NY: McGraw-Hill; 2008:160-175.
10. Karaer A, Avsar FA, Batioglu S. Risk factors for ectopic pregnancy: a case-control study. Aust N Z J Obstet Gynaecol. 2006;46:521-527.
11. Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357:648-653.
12. Dart RG, Kaplan B, Cox C. Transvaginal ultrasound in patients with low beta-human chorionic gonadotropin values: how often is the study diagnostic? Ann Emerg Med. 1997;30:135-140.
13. Kaplan BC, Dart RG, Moskos M, et al. Ectopic pregnancy: prospective study with improved diagnostic accuracy. Ann Emerg Med. 1996;28:10-17.
14. Clinical policy: critical issues for the initial evaluation and management of patients presenting with a chief complaint of nontraumatic acute abdominal pain Ann Emerg Med. 2000;36:406-415.
15. Barnhart K, van Mello NM, Bourne T, et al. Pregnancy of unknown location: a consensus statement of nomenclature, definitions, and outcome. Fertil Steril. 2011;95:857-866.
16. Barnhart K, Mennuti MT, Benjamin I, et al. Prompt diagnosis of ectopic pregnancy in an emergency department setting. Obstet Gynecol. 1994;84:1010-1015.
17. Aleem FA, DeFazio M, Gintautas J. Endovaginal sonography for the early diagnosis of intrauterine and ectopic pregnancies. Hum Reprod. 1990;5:755-758.
18. Coundous G, Kirk E, Lu C, et al. Diagnostic accuracy of varying discriminatory zones for the prediction of ectopic pregnancy in women with a pregnancy of unknown location. Ultrasound Obstet Gynecol. 2005;26:770-775.
19. Gracia CR, Kurt TB. Diagnosing ectopic pregnancy: decision analysis comparing six strategies. Obstet Gynecol. 2001;97:464-470.
20. Kohn MA, Kerr K, Malkevich D, et al. Beta-human chorionic gonadotropin levels and the likelihood of ectopic pregnancy in emergency department patients with abdominal pain or vaginal bleeding. Acad Emerg Med. 2003;10:119-126.
21. Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104:975-981.
22. Kirk E, Bourne T. Diagnosis of ectopic pregnancy with ultrasound. Best Pract Res Clin Obstet Gynaecol. 2009;23:501-508.
23. van Mello NM, Mol F, Opmeer BC, et al. Diagnostic value of serum hCG on the outcome of pregnancy of unknown location: a systematic review and meta-analysis. Hum Reprod Update. 2012;18:603-617.
24. Segal S, Mercado R, Rivnay B. Ectopic pregnancy early diagnosis markers. Minerva Ginecol. 2010;62:49-62.
25. Cordina M, Schramm-Gajraj K, Ross JA, et al. Introduction of a single visit protocol in the management of selected patients with pregnancy of unknown location: a prospective study. BJOG. 2011;118:693-697.
26. Barnhart KT, Katz I, Hummel A. Presumed diagnosis of ectopic pregnancy. Obstet Gynecol. 2002;100:505-510.
27. Seeber BE, Barnhart KT. Suspected ectopic pregnancy. Obstet Gynecol. 2006;107:399-413.
28. Hajenius PJ, Mol F, Mol BW, et al. Interventions for tubal ectopic pregnancy. Cochrane Database Syst Rev. 2007;(1):CD000324.-
29. Stovall TG. Medical management should be routinely used as primary therapy for ectopic pregnancy. Clin Obstet Gynecol. 1995;38:346-352.
30. Fernandez H, Yves Vincent SC, Pauthier S, et al. Randomized trial of conservative laparoscopic treatment and methotrexate administration in ectopic pregnancy and subsequent fertility. Hum Reprod. 1998;13:3239-3243.
31. Mol F, Strandell A, Jurkovic D, et al. The ESEP study: salpingostomy versus salpingectomy for tubal ectopic pregnancy; the impact on future fertility: a randomised controlled trial. BMC Womens Health. 2008;8:11.-
32. Mol F, Mol BW, Ankum WM, et al. Current evidence on surgery, systemic methotrexate and expectant management in the treatment of tubal ectopic pregnancy: a systematic review and meta-analysis. Hum Reprod Update. 2008;14:309-319.
33. American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 94. Medical management of ectopic pregnancy. Obstet Gynecol. 2008;111:1479-1485.
34. Menon S, Colins J, Barnhart KT. Establishing a human chorionic gonadotropin cutoff to guide methotrexate treatment of ectopic pregnancy: a systematic review. Fertil Steril. 2007;87:481-484.
35. Saraj AJ, Wilcox JG, Najmabadi S, et al. Resolution of hormonal markers of ectopic gestation: a randomized trial comparing single-dose intramuscular methotrexate with salpingostomy. Obstet Gynecol. 1998;92:989-994.
AGING: Are these 4 pain myths complicating care?
Beliefs about aging itself can also have dramatic consequences, both positive and negative. In one longitudinal study, those who had positive self-perceptions of aging when they were 50 had better health during 2 decades of follow-up and lived, on average, 7½ years longer than those who had negative self-perceptions at the age of 50.4
Although little research has focused specifically on pain-related stereotypes held by older adults, their importance has long been recognized.
Twenty years ago, a review found that the failure to incorporate older patients’ beliefs about pain could have a negative effect on pain management.5 And in 2011, an Institute of Medicine report found a critical need for public education to counter the myths, misunderstandings, stereotypes, and stigma that hinder pain management in patients across the lifespan.6
We set out to identify widely held stereotypes that older adults and physicians have about pain—and to report on primary studies that support or refute them. We focused on noncancer pain. In the pages that follow, we identify 4 key stereotypes that misrepresent the experience of older adults with regard to pain, and present evidence to debunk them.
Stereotype #1: Pain is a natural part of getting older
Chronic pain is often perceived as an age-related condition. In in-depth interviews, older adults with osteoarthritis reported pain as a normal, even essential, part of life. As one patient put it, “That’s how you know you’re alive … you ache.”7
Among primary care patients with osteoarthritis, those older than 70 years were more likely than younger patients to believe that people should expect to live with pain as they get older.8 And more than half of older adults who responded to a community-based survey considered arthritis to be a natural part of getting old.9
Physicians, too, often view pain as an inevitable part of the aging process, giving patients feedback such as “What do you expect? You’re just getting older.”10
Are they right?
Is pain inevitable? No
In fact, chronic pain is common in older adults, occurring in more than half of those assessed, according to some studies.11 In addition, some epidemiological studies have found an age-related increase in the prevalence of pain,12-14 with older age predicting a more likely onset of, and failure to recover from, persistent pain.15 But numerous studies have failed to find a direct relationship between pain and age.
A National Center for Health Statistics report found that 29% of adults between the ages of 45 and 64 years vs 21% of those 65 or older reported pain lasting >24 hours in the month before the survey.16 And a meta-analysis comparing age-related differences in pain perception found that the highest prevalence of chronic pain occurred at about age 65; a slight decline with advancing age followed, even beyond the age of 85.17
Chronic pain disorders are less frequent. In fact, many chronic pain disorders occur less frequently with advancing age. Population-based studies have found a lower prevalence of low back, neck, and face pain among older adults compared with their younger counterparts;16 evidence has also found lower rates of headache and abdominal pain.18 Other epidemiological studies suggest that the prevalence of musculoskeletal pain generally declines with advancing age,19 and a study of patients in their last 2 years of life found pain to be inversely correlated with age.20 These findings refute the stereotype that advancing age inexorably involves pain, and challenge the notion that pain in later life is normal and expected, and unworthy of treatment.
Stereotype #2: Pain worsens
over time
Some patients and physicians expect that as people age, their pain will increase in intensity. In one study of community-dwelling older adults, 87% of those surveyed rated the belief that more aches and pains are an accepted part of aging as definitely or somewhat true.21 Indeed, patients of all ages have expressed the belief that older age confers greater susceptibility to, and suffering from, painful conditions like arthritis.22 Many common causes of pain in older adults, especially osteoarthritis, are seen as resulting from degenerative changes, which worsen over time.23
Does pain intensify? Not necessarily
Some studies have linked older age to a worse prognosis for patients with musculoskeletal pain, but a greater number have found that aging has no effect on it.24
Pain does not always progress. In a large cohort of patients with peripheral joint osteoarthritis, radiographic joint space narrowing worsened over 3 years, but this did not correlate consistently with worsening pain.25 When the same cohort was assessed after 8 years, there was significant variability in pain, with no clear progression.26
In another study involving older patients with restrictive back pain, the pain was frequently short-lived and episodic and did not increase with age.27 And in a population sample in Norway, the mean number of pain sites decreased slightly over 14 years in those older than 60 years, while increasing in those aged 44 to 60.28 Another study of patients with knee osteoarthritis identified factors that were protective against a decline in pain-related function: These included good mental health, self-efficacy, social support, and greater activity—but not younger age.29 The enormous heterogeneity in both the experience and the course of pain suggests that age-related pain progression is neither universal nor expected—and contradicts a purely biological paradigm in which pain inevitably worsens over time.
Stereotype #3: Stoicism leads to pain tolerance
Some patients believe that the inability to deal with pain is a sign of being soft or weak, and that a “tough it out” approach makes pain easier to tolerate.7 In one survey, older adults were more likely than their younger counterparts to express such stoicism, frequently agreeing with statements like, “I maintain my pride and keep a stiff upper lip when in pain,” “I go on as if nothing had happened …,” and “Pain is something that should be ignored.” 30
Unfortunately, some physicians reinforce such attitudes, telling older patients, in effect, that they’d better “get used to it.”10 And family and friends may make it worse. Patients taking opioids reported that it wasn’t unusual for those close to them to view their use of these analgesics as a sign of weakness.31
Does stoicism help? Probably not
Older adults seem less likely than younger adults to label a sensation as painful, suggesting a more stoic approach in general.30 While some research has found that nociception—the perception of pain in response to painful stimuli—decreases with advancing age,32 other studies have found the opposite.33 And population-based studies focusing on the consequences of pain indicate that it continues to have powerful negative effects, especially depression and insomnia, in older patients.
The degree of pain experienced is more strongly associated with depression in older patients compared with younger adults,34 and greater pain reduces the likelihood that depression will improve with treatment.35 Pain also continues to interfere with sleep. In one national sample, 25% of those with arthritis said they suffered from insomnia, roughly twice the prevalence of insomnia found in those without arthritis.36 In another study, individuals with arthritis were 3 times more likely to have sleep problems compared with individuals without arthritis37—an association independent of age. Being stoic about pain, it appears, does not diminish its consequences over time or help patients better tolerate it.
Stereotype #4: Prescription analgesics are highly addictive
Patients often think that prescription analgesics, especially opioids, are highly addictive or harmful—and older adults may refuse to take them for fear of becoming addicted.7 The stereotype is often shared by family and friends, as well as clinicians.
In one study, one-third of physicians said they hesitated to prescribe opioid medications to older adults because of the risk of addiction (a concern that no clinician with training in geriatrics shared).38 What’s more, 16% of the physicians estimated that about one in 4 older patients receiving chronic opioid therapy becomes addicted. The actual risk is far lower. (More on that below.) News reports of an epidemic of prescription opioid addictions and fatalities,39 including the assertion that opioids are replacing heroin as the primary drug of choice on the street,40 may reinforce such stereotypes.
How great is the risk of addiction? For older adults, it’s very low
While rates of aberrant opioid use vary widely depending on the context, one consistent theme is that older age is associated with decreased risk.41 In one retrospective cohort study of older patients who had recently been started on an opioid medication for the treatment of chronic pain, only 3% showed evidence of behaviors associated with abuse or misuse.42
What’s more, long-term opioid use among older patients with painful conditions is relatively uncommon, and prescription patterns suggest that most older adults discontinue opioids after one or 2 prescriptions.42-44 Decades of research have found that, although opioid medications can cause physiological dependence, addiction is rare in patients treated with them.45,46 (To learn more, see “Diagnosing and treating opioid dependence,” J Fam Pract. 2012;61: 588-597.)
Debunking myths: Implications for practice
Our findings—that pain is not a natural part of aging and often improves or remains stable over time, stoicism does not lead to acclimation, and pain medications are not highly addictive in older adults—make it clear that the stereotypes we identified are misconceptions of pain in later life. Debunking these stereotypes has several implications for clinical practice. We recommend the following:
Identify and counter these stereotypes. Avoid reinforcing stereotypes; counter them by summarizing these evidence-based findings for older patients. We believe patients would be receptive.
In one study, more than 80% of patients with osteoarthritis said they wanted prognostic information about the course of the disease, but only about one-third had received it.47 Presenting the research findings would challenge patients’ stereotypes and help them reframe their expectations.
Elicit patients’ perspectives. Ask patients about age- and pain-related stereotypes and their expectations and perspectives of what constitutes successful treatment. Research shows that patients often wish to discuss lifestyle changes and nonmedical approaches to pain, for example, but that clinicians typically focus on medications instead.48
Emphasize the positive. Frame discussions of pain and aging in a positive light, offering encouragement rather than supporting stoicism or resignation. Attention to protective factors, including good mental health, self-efficacy, social support, and greater activity, may enable older patients to adapt better to any pain they experience.
CORRESPONDENCE
Stephen Thielke, MD, MSPH, MA, University of Washington, Psychiatry and Behavioral Sciences, Box 356560, Seattle, WA 98195; sthielke@u.washington.edu
1. Herr K. Pain in the older adult: an imperative across all health care settings. Pain Manag Nurs. 2010;11(2 suppl):S1-S10.
2. Pitkala KH, Strandberg TE, Tilvis RS. Management of nonmalignant pain in home-dwelling older people: a population-based survey. J Am Geriatr Soc. 2002;50:1861-1865.
3. Levy B. Stereotype embodiment: a psychosocial approach to aging. Curr Dir Psychol Sci. 2009;18:332-336.
4. Levy BR, Slade MD, Kasl SV. Longitudinal benefit of positive self-perceptions of aging on functional health. J Gerontol B Psychol Sci Soc Sci. 2002;57:409-417.
5. Hofland SL. Elder beliefs: blocks to pain management. J Gerontol Nurs. 1992;18:19-23.
6. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
7. Sale J, Gignac M, Hawker G. How “bad” does the pain have to be? A qualitative study examining adherence to pain medication in older adults with osteoarthritis. Arthritis Rheum. 2006;55:272-278.
8. Appelt CJ, Burant BC, Siminoff LA, et al. Health beliefs related to aging among older male patients with knee and/or hip osteoarthritis. J Gerontol A Biol Sci Med Sci. 2007;62:184-190.
9. Goodwin JS, Black SA, Satish S. Aging versus disease: the opinions of older black, Hispanic, and non-Hispanic white Americans about the causes and treatment of common medical conditions. J Am Geriatr Soc. 1999;47:973-979.
10. Gignac M, Davis A, Hawker G, et al. “What do you expect? You’re just getting older”: a comparison of perceived osteoarthritis-related and aging-related health experiences in middle- and older-age adults. Arthritis Rheum. 2006;55:905-912.
11. Helme RD, Gibson SJ. Pain in the elderly. In: Jensen TS, Turner JA, Weisenfeld-Hallin Z, eds. Progress in Pain Research and Management. Proceedings of the 8th World Congress on Pain. Vol 8. Seattle, Wash: IASP Press; 1997:919–944.
12. Badley EM, Tennant A. Changing profile of joint disorders with age: findings from a postal survey of the population of Calderdale, West Yorkshire, United Kingdom. Ann Rheumatic Dis. 1992;51:366-371.
13. Brattberg G, Parker MG, Thorslund M. A longitudinal study of pain: reported pain from middle age to old age. Clin J Pain. 1997;13:144-149.
14. Crook J, Rideout E, Browne G. The prevalence of pain complaints in a general population. Pain. 1984;18:299-314.
15. Gureje O, Simon GE, Von Korff M. A cross-national study of the course of persistent pain in primary care. Pain. 2001;92:195-200.
16. National Center for Health Statistics. Special feature: pain. In: Health, United States, 2006 with Chartbook on Trends in the Health of Americans. Hyattsville, Md: Centers for Disease Control and Prevention; 2006:68–87. Available at: http://www.cdc.gov/nchs/data/hus/hus06.pdf. Accessed October 16, 2012.
17. Gibson SJ, Helme RD. Age differences in pain perception and report: a review of physiological, psychological, laboratory and clinical studies. Pain Rev. 1995;2:111-137.
18. Gallagher RM, Verma S, Mossey J. Chronic pain. Sources of late-life pain and risk factors for disability. Geriatrics. 2000;55:40-44, 47.
19. Picavet HS, Schouten JS. Musculoskeletal pain in the Netherlands: prevalences, consequences and risk groups, the DMC(3)-study. Pain. 2003;102:167-178.
20. Smith AK, Cenzer IS, Knight SJ, et al. The epidemiology of pain during the last 2 years of life. Ann Intern Med. 2010;153:563-569.
21. Sarkisian CA, Hays RD, Mangione CM. Do older adults expect to age successfully? The association between expectations regarding aging and beliefs regarding healthcare seeking among older adults. J Am Geriatr Soc. 2002;50:1837-1843.
22. Keller ML, Leventhal H, Prohaska TR, et al. Beliefs about aging and illness in a community sample. Res Nurs Health. 1989;12:247-255.
23. Dougados M, Gueguen A, Nguyen M, et al. Longitudinal radiologic evaluation of osteoarthritis of the knee. J Rheumatology. 1992;19:378-384.
24. Mallen CD, Peat G, Thomas E, et al. Prognostic factors for musculoskeletal pain in primary care: a systematic review. Br J Gen Pract. 2007;57:655-661.
25. Dieppe PA, Cushnaghan J, Shepstone L. The Bristol ‘OA500’ study: progression of osteoarthritis (OA) over 3 years and the relationship between clinical and radiographic changes at the knee joint. Osteoarthritis Cartilage. 1997;5:87-97.
26. Dieppe P, Cushnaghan J, Tucker M, et al. The Bristol ‘OA500 study’: progression and impact of the disease after 8 years. Osteoarthritis Cartilage. 2000;8:63-68.
27. Makris UE, Fraenkel L, Han L, et al. Epidemiology of restricting back pain in community-living older persons. J Am Geriatr Soc. 2011;59:610-614.
28. Kamaleri Y, Natvig B, Ihlebaek CM, et al. Change in the number of musculoskeletal pain sites: a 14-year prospective study. Pain. 2009;141:25-30.
29. Sharma L, Cahue S, Song J, et al. Physical functioning over three years in knee osteoarthritis: role of psychosocial, local mechanical, and neuromuscular factors. Arthritis Rheum. 2003;48:3359-3370.
30. Yong HH, Gibson SJ, Horne DJ, et al. Development of a pin attitudes questionnaire to assess stoicism and cautiousness for possible age differences. J Gerontol B Psychol Sci Soc Sci. 2001;56:279-284.
31. Vallerand A, Nowak L. Chronic opioid therapy for nonmalignant pain: the patient’s perspective. Part II—barriers to chronic opioid therapy. Pain manag nurs. 2010;11:126-131.
32. Gibson SJ, Farrell M. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin J Pain. 2004;20:227-239.
33. Woodrow KM, Friedman GD, Siegelaub AB, et al. Pain tolerance: differences according to age, sex and race. Psychosom Med. 1972;34:548-556.
34. Turk DC, Okifuji A, Scharff L. Chronic pain and depression: role of perceived impact and perceived control in different age cohorts. Pain. 1995;61:93-101.
35. Thielke SM, Fan MY, Sullivan M, et al. Pain limits the effectiveness of collaborative care for depression. Am J Geriatr Psychiatry. 2007;15:699-707.
36. Power JD, Perruccio AV, Badley EM. Pain as a mediator of sleep problems in arthritis and other chronic conditions. Arthritis Rheum. 2005;53:911-919.
37. Louie GH, Tektonidou MG, Caban-Martizen AJ, et al. Sleep disturbances in adults with arthritis: prevalence, mediators, and subgroups at greatest risk. Arthritis Care Res. 2011;63:247-260.
38. Lin JJ, Alfandre D, Moore C. Physician attitudes toward opioid prescribing for patients with persistent noncancer pain. Clin J Pain. 2007;23:799-803.
39. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613-2620.
40. Fischer B, Gittins J, Kendall P, et al. Thinking the unthinkable: could the increasing misuse of prescription opioids among street drug users offer benefits for public health? Public Health. 2009;123:145-146.
41. Fleming MF, Davis J, Passik SD. Reported lifetime aberrant drug-taking behaviors are predictive of current substance use and mental health problems in primary care patients. Pain Med. 2008;9:1098-1106.
42. Reid MC, Henderson CR, Jr, Papaleontiou M, et al. Characteristics of older adults receiving opioids in primary care: treatment duration and outcomes. Pain Med. 2010;11:1063-1071.
43. Solomon DH, Rassen JA, Glynn RJ, et al. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med. 2010;170:1979-1986.
44. Thielke SM, Simoni-Wastila L, Edlund MJ, et al. Age and sex trends in long-term opioid use in two large American health systems between 2000 and 2005. Pain Med. 2010;11:248-256.
45. Soden K, Ali S, Alloway L, et al. How do nurses assess and manage breakthrough pain in specialist palliative care inpatient units? A multicentre study. Palliat Med. 2010;24:294-298.
46. Papaleontiou M, Henderson CR, Jr, Turner BJ, et al. Outcomes associated with opioid use in the treatment of chronic noncancer pain in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2010;58:1353-1369.
47. Mallen CD, Peat G. Discussing prognosis with older people with musculoskeletal pain: a cross-sectional study in general practice. BMC Fam Pract. 2009;10:50.-
48. Rosemann T, Wensing M, Joest K, et al. Problems and needs for improving primary care of osteoarthritis patients: the views of patients, general practitioners and practice nurses. BMC Musculoskelet Disord. 2006;7:48.
Beliefs about aging itself can also have dramatic consequences, both positive and negative. In one longitudinal study, those who had positive self-perceptions of aging when they were 50 had better health during 2 decades of follow-up and lived, on average, 7½ years longer than those who had negative self-perceptions at the age of 50.4
Although little research has focused specifically on pain-related stereotypes held by older adults, their importance has long been recognized.
Twenty years ago, a review found that the failure to incorporate older patients’ beliefs about pain could have a negative effect on pain management.5 And in 2011, an Institute of Medicine report found a critical need for public education to counter the myths, misunderstandings, stereotypes, and stigma that hinder pain management in patients across the lifespan.6
We set out to identify widely held stereotypes that older adults and physicians have about pain—and to report on primary studies that support or refute them. We focused on noncancer pain. In the pages that follow, we identify 4 key stereotypes that misrepresent the experience of older adults with regard to pain, and present evidence to debunk them.
Stereotype #1: Pain is a natural part of getting older
Chronic pain is often perceived as an age-related condition. In in-depth interviews, older adults with osteoarthritis reported pain as a normal, even essential, part of life. As one patient put it, “That’s how you know you’re alive … you ache.”7
Among primary care patients with osteoarthritis, those older than 70 years were more likely than younger patients to believe that people should expect to live with pain as they get older.8 And more than half of older adults who responded to a community-based survey considered arthritis to be a natural part of getting old.9
Physicians, too, often view pain as an inevitable part of the aging process, giving patients feedback such as “What do you expect? You’re just getting older.”10
Are they right?
Is pain inevitable? No
In fact, chronic pain is common in older adults, occurring in more than half of those assessed, according to some studies.11 In addition, some epidemiological studies have found an age-related increase in the prevalence of pain,12-14 with older age predicting a more likely onset of, and failure to recover from, persistent pain.15 But numerous studies have failed to find a direct relationship between pain and age.
A National Center for Health Statistics report found that 29% of adults between the ages of 45 and 64 years vs 21% of those 65 or older reported pain lasting >24 hours in the month before the survey.16 And a meta-analysis comparing age-related differences in pain perception found that the highest prevalence of chronic pain occurred at about age 65; a slight decline with advancing age followed, even beyond the age of 85.17
Chronic pain disorders are less frequent. In fact, many chronic pain disorders occur less frequently with advancing age. Population-based studies have found a lower prevalence of low back, neck, and face pain among older adults compared with their younger counterparts;16 evidence has also found lower rates of headache and abdominal pain.18 Other epidemiological studies suggest that the prevalence of musculoskeletal pain generally declines with advancing age,19 and a study of patients in their last 2 years of life found pain to be inversely correlated with age.20 These findings refute the stereotype that advancing age inexorably involves pain, and challenge the notion that pain in later life is normal and expected, and unworthy of treatment.
Stereotype #2: Pain worsens
over time
Some patients and physicians expect that as people age, their pain will increase in intensity. In one study of community-dwelling older adults, 87% of those surveyed rated the belief that more aches and pains are an accepted part of aging as definitely or somewhat true.21 Indeed, patients of all ages have expressed the belief that older age confers greater susceptibility to, and suffering from, painful conditions like arthritis.22 Many common causes of pain in older adults, especially osteoarthritis, are seen as resulting from degenerative changes, which worsen over time.23
Does pain intensify? Not necessarily
Some studies have linked older age to a worse prognosis for patients with musculoskeletal pain, but a greater number have found that aging has no effect on it.24
Pain does not always progress. In a large cohort of patients with peripheral joint osteoarthritis, radiographic joint space narrowing worsened over 3 years, but this did not correlate consistently with worsening pain.25 When the same cohort was assessed after 8 years, there was significant variability in pain, with no clear progression.26
In another study involving older patients with restrictive back pain, the pain was frequently short-lived and episodic and did not increase with age.27 And in a population sample in Norway, the mean number of pain sites decreased slightly over 14 years in those older than 60 years, while increasing in those aged 44 to 60.28 Another study of patients with knee osteoarthritis identified factors that were protective against a decline in pain-related function: These included good mental health, self-efficacy, social support, and greater activity—but not younger age.29 The enormous heterogeneity in both the experience and the course of pain suggests that age-related pain progression is neither universal nor expected—and contradicts a purely biological paradigm in which pain inevitably worsens over time.
Stereotype #3: Stoicism leads to pain tolerance
Some patients believe that the inability to deal with pain is a sign of being soft or weak, and that a “tough it out” approach makes pain easier to tolerate.7 In one survey, older adults were more likely than their younger counterparts to express such stoicism, frequently agreeing with statements like, “I maintain my pride and keep a stiff upper lip when in pain,” “I go on as if nothing had happened …,” and “Pain is something that should be ignored.” 30
Unfortunately, some physicians reinforce such attitudes, telling older patients, in effect, that they’d better “get used to it.”10 And family and friends may make it worse. Patients taking opioids reported that it wasn’t unusual for those close to them to view their use of these analgesics as a sign of weakness.31
Does stoicism help? Probably not
Older adults seem less likely than younger adults to label a sensation as painful, suggesting a more stoic approach in general.30 While some research has found that nociception—the perception of pain in response to painful stimuli—decreases with advancing age,32 other studies have found the opposite.33 And population-based studies focusing on the consequences of pain indicate that it continues to have powerful negative effects, especially depression and insomnia, in older patients.
The degree of pain experienced is more strongly associated with depression in older patients compared with younger adults,34 and greater pain reduces the likelihood that depression will improve with treatment.35 Pain also continues to interfere with sleep. In one national sample, 25% of those with arthritis said they suffered from insomnia, roughly twice the prevalence of insomnia found in those without arthritis.36 In another study, individuals with arthritis were 3 times more likely to have sleep problems compared with individuals without arthritis37—an association independent of age. Being stoic about pain, it appears, does not diminish its consequences over time or help patients better tolerate it.
Stereotype #4: Prescription analgesics are highly addictive
Patients often think that prescription analgesics, especially opioids, are highly addictive or harmful—and older adults may refuse to take them for fear of becoming addicted.7 The stereotype is often shared by family and friends, as well as clinicians.
In one study, one-third of physicians said they hesitated to prescribe opioid medications to older adults because of the risk of addiction (a concern that no clinician with training in geriatrics shared).38 What’s more, 16% of the physicians estimated that about one in 4 older patients receiving chronic opioid therapy becomes addicted. The actual risk is far lower. (More on that below.) News reports of an epidemic of prescription opioid addictions and fatalities,39 including the assertion that opioids are replacing heroin as the primary drug of choice on the street,40 may reinforce such stereotypes.
How great is the risk of addiction? For older adults, it’s very low
While rates of aberrant opioid use vary widely depending on the context, one consistent theme is that older age is associated with decreased risk.41 In one retrospective cohort study of older patients who had recently been started on an opioid medication for the treatment of chronic pain, only 3% showed evidence of behaviors associated with abuse or misuse.42
What’s more, long-term opioid use among older patients with painful conditions is relatively uncommon, and prescription patterns suggest that most older adults discontinue opioids after one or 2 prescriptions.42-44 Decades of research have found that, although opioid medications can cause physiological dependence, addiction is rare in patients treated with them.45,46 (To learn more, see “Diagnosing and treating opioid dependence,” J Fam Pract. 2012;61: 588-597.)
Debunking myths: Implications for practice
Our findings—that pain is not a natural part of aging and often improves or remains stable over time, stoicism does not lead to acclimation, and pain medications are not highly addictive in older adults—make it clear that the stereotypes we identified are misconceptions of pain in later life. Debunking these stereotypes has several implications for clinical practice. We recommend the following:
Identify and counter these stereotypes. Avoid reinforcing stereotypes; counter them by summarizing these evidence-based findings for older patients. We believe patients would be receptive.
In one study, more than 80% of patients with osteoarthritis said they wanted prognostic information about the course of the disease, but only about one-third had received it.47 Presenting the research findings would challenge patients’ stereotypes and help them reframe their expectations.
Elicit patients’ perspectives. Ask patients about age- and pain-related stereotypes and their expectations and perspectives of what constitutes successful treatment. Research shows that patients often wish to discuss lifestyle changes and nonmedical approaches to pain, for example, but that clinicians typically focus on medications instead.48
Emphasize the positive. Frame discussions of pain and aging in a positive light, offering encouragement rather than supporting stoicism or resignation. Attention to protective factors, including good mental health, self-efficacy, social support, and greater activity, may enable older patients to adapt better to any pain they experience.
CORRESPONDENCE
Stephen Thielke, MD, MSPH, MA, University of Washington, Psychiatry and Behavioral Sciences, Box 356560, Seattle, WA 98195; sthielke@u.washington.edu
Beliefs about aging itself can also have dramatic consequences, both positive and negative. In one longitudinal study, those who had positive self-perceptions of aging when they were 50 had better health during 2 decades of follow-up and lived, on average, 7½ years longer than those who had negative self-perceptions at the age of 50.4
Although little research has focused specifically on pain-related stereotypes held by older adults, their importance has long been recognized.
Twenty years ago, a review found that the failure to incorporate older patients’ beliefs about pain could have a negative effect on pain management.5 And in 2011, an Institute of Medicine report found a critical need for public education to counter the myths, misunderstandings, stereotypes, and stigma that hinder pain management in patients across the lifespan.6
We set out to identify widely held stereotypes that older adults and physicians have about pain—and to report on primary studies that support or refute them. We focused on noncancer pain. In the pages that follow, we identify 4 key stereotypes that misrepresent the experience of older adults with regard to pain, and present evidence to debunk them.
Stereotype #1: Pain is a natural part of getting older
Chronic pain is often perceived as an age-related condition. In in-depth interviews, older adults with osteoarthritis reported pain as a normal, even essential, part of life. As one patient put it, “That’s how you know you’re alive … you ache.”7
Among primary care patients with osteoarthritis, those older than 70 years were more likely than younger patients to believe that people should expect to live with pain as they get older.8 And more than half of older adults who responded to a community-based survey considered arthritis to be a natural part of getting old.9
Physicians, too, often view pain as an inevitable part of the aging process, giving patients feedback such as “What do you expect? You’re just getting older.”10
Are they right?
Is pain inevitable? No
In fact, chronic pain is common in older adults, occurring in more than half of those assessed, according to some studies.11 In addition, some epidemiological studies have found an age-related increase in the prevalence of pain,12-14 with older age predicting a more likely onset of, and failure to recover from, persistent pain.15 But numerous studies have failed to find a direct relationship between pain and age.
A National Center for Health Statistics report found that 29% of adults between the ages of 45 and 64 years vs 21% of those 65 or older reported pain lasting >24 hours in the month before the survey.16 And a meta-analysis comparing age-related differences in pain perception found that the highest prevalence of chronic pain occurred at about age 65; a slight decline with advancing age followed, even beyond the age of 85.17
Chronic pain disorders are less frequent. In fact, many chronic pain disorders occur less frequently with advancing age. Population-based studies have found a lower prevalence of low back, neck, and face pain among older adults compared with their younger counterparts;16 evidence has also found lower rates of headache and abdominal pain.18 Other epidemiological studies suggest that the prevalence of musculoskeletal pain generally declines with advancing age,19 and a study of patients in their last 2 years of life found pain to be inversely correlated with age.20 These findings refute the stereotype that advancing age inexorably involves pain, and challenge the notion that pain in later life is normal and expected, and unworthy of treatment.
Stereotype #2: Pain worsens
over time
Some patients and physicians expect that as people age, their pain will increase in intensity. In one study of community-dwelling older adults, 87% of those surveyed rated the belief that more aches and pains are an accepted part of aging as definitely or somewhat true.21 Indeed, patients of all ages have expressed the belief that older age confers greater susceptibility to, and suffering from, painful conditions like arthritis.22 Many common causes of pain in older adults, especially osteoarthritis, are seen as resulting from degenerative changes, which worsen over time.23
Does pain intensify? Not necessarily
Some studies have linked older age to a worse prognosis for patients with musculoskeletal pain, but a greater number have found that aging has no effect on it.24
Pain does not always progress. In a large cohort of patients with peripheral joint osteoarthritis, radiographic joint space narrowing worsened over 3 years, but this did not correlate consistently with worsening pain.25 When the same cohort was assessed after 8 years, there was significant variability in pain, with no clear progression.26
In another study involving older patients with restrictive back pain, the pain was frequently short-lived and episodic and did not increase with age.27 And in a population sample in Norway, the mean number of pain sites decreased slightly over 14 years in those older than 60 years, while increasing in those aged 44 to 60.28 Another study of patients with knee osteoarthritis identified factors that were protective against a decline in pain-related function: These included good mental health, self-efficacy, social support, and greater activity—but not younger age.29 The enormous heterogeneity in both the experience and the course of pain suggests that age-related pain progression is neither universal nor expected—and contradicts a purely biological paradigm in which pain inevitably worsens over time.
Stereotype #3: Stoicism leads to pain tolerance
Some patients believe that the inability to deal with pain is a sign of being soft or weak, and that a “tough it out” approach makes pain easier to tolerate.7 In one survey, older adults were more likely than their younger counterparts to express such stoicism, frequently agreeing with statements like, “I maintain my pride and keep a stiff upper lip when in pain,” “I go on as if nothing had happened …,” and “Pain is something that should be ignored.” 30
Unfortunately, some physicians reinforce such attitudes, telling older patients, in effect, that they’d better “get used to it.”10 And family and friends may make it worse. Patients taking opioids reported that it wasn’t unusual for those close to them to view their use of these analgesics as a sign of weakness.31
Does stoicism help? Probably not
Older adults seem less likely than younger adults to label a sensation as painful, suggesting a more stoic approach in general.30 While some research has found that nociception—the perception of pain in response to painful stimuli—decreases with advancing age,32 other studies have found the opposite.33 And population-based studies focusing on the consequences of pain indicate that it continues to have powerful negative effects, especially depression and insomnia, in older patients.
The degree of pain experienced is more strongly associated with depression in older patients compared with younger adults,34 and greater pain reduces the likelihood that depression will improve with treatment.35 Pain also continues to interfere with sleep. In one national sample, 25% of those with arthritis said they suffered from insomnia, roughly twice the prevalence of insomnia found in those without arthritis.36 In another study, individuals with arthritis were 3 times more likely to have sleep problems compared with individuals without arthritis37—an association independent of age. Being stoic about pain, it appears, does not diminish its consequences over time or help patients better tolerate it.
Stereotype #4: Prescription analgesics are highly addictive
Patients often think that prescription analgesics, especially opioids, are highly addictive or harmful—and older adults may refuse to take them for fear of becoming addicted.7 The stereotype is often shared by family and friends, as well as clinicians.
In one study, one-third of physicians said they hesitated to prescribe opioid medications to older adults because of the risk of addiction (a concern that no clinician with training in geriatrics shared).38 What’s more, 16% of the physicians estimated that about one in 4 older patients receiving chronic opioid therapy becomes addicted. The actual risk is far lower. (More on that below.) News reports of an epidemic of prescription opioid addictions and fatalities,39 including the assertion that opioids are replacing heroin as the primary drug of choice on the street,40 may reinforce such stereotypes.
How great is the risk of addiction? For older adults, it’s very low
While rates of aberrant opioid use vary widely depending on the context, one consistent theme is that older age is associated with decreased risk.41 In one retrospective cohort study of older patients who had recently been started on an opioid medication for the treatment of chronic pain, only 3% showed evidence of behaviors associated with abuse or misuse.42
What’s more, long-term opioid use among older patients with painful conditions is relatively uncommon, and prescription patterns suggest that most older adults discontinue opioids after one or 2 prescriptions.42-44 Decades of research have found that, although opioid medications can cause physiological dependence, addiction is rare in patients treated with them.45,46 (To learn more, see “Diagnosing and treating opioid dependence,” J Fam Pract. 2012;61: 588-597.)
Debunking myths: Implications for practice
Our findings—that pain is not a natural part of aging and often improves or remains stable over time, stoicism does not lead to acclimation, and pain medications are not highly addictive in older adults—make it clear that the stereotypes we identified are misconceptions of pain in later life. Debunking these stereotypes has several implications for clinical practice. We recommend the following:
Identify and counter these stereotypes. Avoid reinforcing stereotypes; counter them by summarizing these evidence-based findings for older patients. We believe patients would be receptive.
In one study, more than 80% of patients with osteoarthritis said they wanted prognostic information about the course of the disease, but only about one-third had received it.47 Presenting the research findings would challenge patients’ stereotypes and help them reframe their expectations.
Elicit patients’ perspectives. Ask patients about age- and pain-related stereotypes and their expectations and perspectives of what constitutes successful treatment. Research shows that patients often wish to discuss lifestyle changes and nonmedical approaches to pain, for example, but that clinicians typically focus on medications instead.48
Emphasize the positive. Frame discussions of pain and aging in a positive light, offering encouragement rather than supporting stoicism or resignation. Attention to protective factors, including good mental health, self-efficacy, social support, and greater activity, may enable older patients to adapt better to any pain they experience.
CORRESPONDENCE
Stephen Thielke, MD, MSPH, MA, University of Washington, Psychiatry and Behavioral Sciences, Box 356560, Seattle, WA 98195; sthielke@u.washington.edu
1. Herr K. Pain in the older adult: an imperative across all health care settings. Pain Manag Nurs. 2010;11(2 suppl):S1-S10.
2. Pitkala KH, Strandberg TE, Tilvis RS. Management of nonmalignant pain in home-dwelling older people: a population-based survey. J Am Geriatr Soc. 2002;50:1861-1865.
3. Levy B. Stereotype embodiment: a psychosocial approach to aging. Curr Dir Psychol Sci. 2009;18:332-336.
4. Levy BR, Slade MD, Kasl SV. Longitudinal benefit of positive self-perceptions of aging on functional health. J Gerontol B Psychol Sci Soc Sci. 2002;57:409-417.
5. Hofland SL. Elder beliefs: blocks to pain management. J Gerontol Nurs. 1992;18:19-23.
6. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
7. Sale J, Gignac M, Hawker G. How “bad” does the pain have to be? A qualitative study examining adherence to pain medication in older adults with osteoarthritis. Arthritis Rheum. 2006;55:272-278.
8. Appelt CJ, Burant BC, Siminoff LA, et al. Health beliefs related to aging among older male patients with knee and/or hip osteoarthritis. J Gerontol A Biol Sci Med Sci. 2007;62:184-190.
9. Goodwin JS, Black SA, Satish S. Aging versus disease: the opinions of older black, Hispanic, and non-Hispanic white Americans about the causes and treatment of common medical conditions. J Am Geriatr Soc. 1999;47:973-979.
10. Gignac M, Davis A, Hawker G, et al. “What do you expect? You’re just getting older”: a comparison of perceived osteoarthritis-related and aging-related health experiences in middle- and older-age adults. Arthritis Rheum. 2006;55:905-912.
11. Helme RD, Gibson SJ. Pain in the elderly. In: Jensen TS, Turner JA, Weisenfeld-Hallin Z, eds. Progress in Pain Research and Management. Proceedings of the 8th World Congress on Pain. Vol 8. Seattle, Wash: IASP Press; 1997:919–944.
12. Badley EM, Tennant A. Changing profile of joint disorders with age: findings from a postal survey of the population of Calderdale, West Yorkshire, United Kingdom. Ann Rheumatic Dis. 1992;51:366-371.
13. Brattberg G, Parker MG, Thorslund M. A longitudinal study of pain: reported pain from middle age to old age. Clin J Pain. 1997;13:144-149.
14. Crook J, Rideout E, Browne G. The prevalence of pain complaints in a general population. Pain. 1984;18:299-314.
15. Gureje O, Simon GE, Von Korff M. A cross-national study of the course of persistent pain in primary care. Pain. 2001;92:195-200.
16. National Center for Health Statistics. Special feature: pain. In: Health, United States, 2006 with Chartbook on Trends in the Health of Americans. Hyattsville, Md: Centers for Disease Control and Prevention; 2006:68–87. Available at: http://www.cdc.gov/nchs/data/hus/hus06.pdf. Accessed October 16, 2012.
17. Gibson SJ, Helme RD. Age differences in pain perception and report: a review of physiological, psychological, laboratory and clinical studies. Pain Rev. 1995;2:111-137.
18. Gallagher RM, Verma S, Mossey J. Chronic pain. Sources of late-life pain and risk factors for disability. Geriatrics. 2000;55:40-44, 47.
19. Picavet HS, Schouten JS. Musculoskeletal pain in the Netherlands: prevalences, consequences and risk groups, the DMC(3)-study. Pain. 2003;102:167-178.
20. Smith AK, Cenzer IS, Knight SJ, et al. The epidemiology of pain during the last 2 years of life. Ann Intern Med. 2010;153:563-569.
21. Sarkisian CA, Hays RD, Mangione CM. Do older adults expect to age successfully? The association between expectations regarding aging and beliefs regarding healthcare seeking among older adults. J Am Geriatr Soc. 2002;50:1837-1843.
22. Keller ML, Leventhal H, Prohaska TR, et al. Beliefs about aging and illness in a community sample. Res Nurs Health. 1989;12:247-255.
23. Dougados M, Gueguen A, Nguyen M, et al. Longitudinal radiologic evaluation of osteoarthritis of the knee. J Rheumatology. 1992;19:378-384.
24. Mallen CD, Peat G, Thomas E, et al. Prognostic factors for musculoskeletal pain in primary care: a systematic review. Br J Gen Pract. 2007;57:655-661.
25. Dieppe PA, Cushnaghan J, Shepstone L. The Bristol ‘OA500’ study: progression of osteoarthritis (OA) over 3 years and the relationship between clinical and radiographic changes at the knee joint. Osteoarthritis Cartilage. 1997;5:87-97.
26. Dieppe P, Cushnaghan J, Tucker M, et al. The Bristol ‘OA500 study’: progression and impact of the disease after 8 years. Osteoarthritis Cartilage. 2000;8:63-68.
27. Makris UE, Fraenkel L, Han L, et al. Epidemiology of restricting back pain in community-living older persons. J Am Geriatr Soc. 2011;59:610-614.
28. Kamaleri Y, Natvig B, Ihlebaek CM, et al. Change in the number of musculoskeletal pain sites: a 14-year prospective study. Pain. 2009;141:25-30.
29. Sharma L, Cahue S, Song J, et al. Physical functioning over three years in knee osteoarthritis: role of psychosocial, local mechanical, and neuromuscular factors. Arthritis Rheum. 2003;48:3359-3370.
30. Yong HH, Gibson SJ, Horne DJ, et al. Development of a pin attitudes questionnaire to assess stoicism and cautiousness for possible age differences. J Gerontol B Psychol Sci Soc Sci. 2001;56:279-284.
31. Vallerand A, Nowak L. Chronic opioid therapy for nonmalignant pain: the patient’s perspective. Part II—barriers to chronic opioid therapy. Pain manag nurs. 2010;11:126-131.
32. Gibson SJ, Farrell M. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin J Pain. 2004;20:227-239.
33. Woodrow KM, Friedman GD, Siegelaub AB, et al. Pain tolerance: differences according to age, sex and race. Psychosom Med. 1972;34:548-556.
34. Turk DC, Okifuji A, Scharff L. Chronic pain and depression: role of perceived impact and perceived control in different age cohorts. Pain. 1995;61:93-101.
35. Thielke SM, Fan MY, Sullivan M, et al. Pain limits the effectiveness of collaborative care for depression. Am J Geriatr Psychiatry. 2007;15:699-707.
36. Power JD, Perruccio AV, Badley EM. Pain as a mediator of sleep problems in arthritis and other chronic conditions. Arthritis Rheum. 2005;53:911-919.
37. Louie GH, Tektonidou MG, Caban-Martizen AJ, et al. Sleep disturbances in adults with arthritis: prevalence, mediators, and subgroups at greatest risk. Arthritis Care Res. 2011;63:247-260.
38. Lin JJ, Alfandre D, Moore C. Physician attitudes toward opioid prescribing for patients with persistent noncancer pain. Clin J Pain. 2007;23:799-803.
39. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613-2620.
40. Fischer B, Gittins J, Kendall P, et al. Thinking the unthinkable: could the increasing misuse of prescription opioids among street drug users offer benefits for public health? Public Health. 2009;123:145-146.
41. Fleming MF, Davis J, Passik SD. Reported lifetime aberrant drug-taking behaviors are predictive of current substance use and mental health problems in primary care patients. Pain Med. 2008;9:1098-1106.
42. Reid MC, Henderson CR, Jr, Papaleontiou M, et al. Characteristics of older adults receiving opioids in primary care: treatment duration and outcomes. Pain Med. 2010;11:1063-1071.
43. Solomon DH, Rassen JA, Glynn RJ, et al. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med. 2010;170:1979-1986.
44. Thielke SM, Simoni-Wastila L, Edlund MJ, et al. Age and sex trends in long-term opioid use in two large American health systems between 2000 and 2005. Pain Med. 2010;11:248-256.
45. Soden K, Ali S, Alloway L, et al. How do nurses assess and manage breakthrough pain in specialist palliative care inpatient units? A multicentre study. Palliat Med. 2010;24:294-298.
46. Papaleontiou M, Henderson CR, Jr, Turner BJ, et al. Outcomes associated with opioid use in the treatment of chronic noncancer pain in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2010;58:1353-1369.
47. Mallen CD, Peat G. Discussing prognosis with older people with musculoskeletal pain: a cross-sectional study in general practice. BMC Fam Pract. 2009;10:50.-
48. Rosemann T, Wensing M, Joest K, et al. Problems and needs for improving primary care of osteoarthritis patients: the views of patients, general practitioners and practice nurses. BMC Musculoskelet Disord. 2006;7:48.
1. Herr K. Pain in the older adult: an imperative across all health care settings. Pain Manag Nurs. 2010;11(2 suppl):S1-S10.
2. Pitkala KH, Strandberg TE, Tilvis RS. Management of nonmalignant pain in home-dwelling older people: a population-based survey. J Am Geriatr Soc. 2002;50:1861-1865.
3. Levy B. Stereotype embodiment: a psychosocial approach to aging. Curr Dir Psychol Sci. 2009;18:332-336.
4. Levy BR, Slade MD, Kasl SV. Longitudinal benefit of positive self-perceptions of aging on functional health. J Gerontol B Psychol Sci Soc Sci. 2002;57:409-417.
5. Hofland SL. Elder beliefs: blocks to pain management. J Gerontol Nurs. 1992;18:19-23.
6. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
7. Sale J, Gignac M, Hawker G. How “bad” does the pain have to be? A qualitative study examining adherence to pain medication in older adults with osteoarthritis. Arthritis Rheum. 2006;55:272-278.
8. Appelt CJ, Burant BC, Siminoff LA, et al. Health beliefs related to aging among older male patients with knee and/or hip osteoarthritis. J Gerontol A Biol Sci Med Sci. 2007;62:184-190.
9. Goodwin JS, Black SA, Satish S. Aging versus disease: the opinions of older black, Hispanic, and non-Hispanic white Americans about the causes and treatment of common medical conditions. J Am Geriatr Soc. 1999;47:973-979.
10. Gignac M, Davis A, Hawker G, et al. “What do you expect? You’re just getting older”: a comparison of perceived osteoarthritis-related and aging-related health experiences in middle- and older-age adults. Arthritis Rheum. 2006;55:905-912.
11. Helme RD, Gibson SJ. Pain in the elderly. In: Jensen TS, Turner JA, Weisenfeld-Hallin Z, eds. Progress in Pain Research and Management. Proceedings of the 8th World Congress on Pain. Vol 8. Seattle, Wash: IASP Press; 1997:919–944.
12. Badley EM, Tennant A. Changing profile of joint disorders with age: findings from a postal survey of the population of Calderdale, West Yorkshire, United Kingdom. Ann Rheumatic Dis. 1992;51:366-371.
13. Brattberg G, Parker MG, Thorslund M. A longitudinal study of pain: reported pain from middle age to old age. Clin J Pain. 1997;13:144-149.
14. Crook J, Rideout E, Browne G. The prevalence of pain complaints in a general population. Pain. 1984;18:299-314.
15. Gureje O, Simon GE, Von Korff M. A cross-national study of the course of persistent pain in primary care. Pain. 2001;92:195-200.
16. National Center for Health Statistics. Special feature: pain. In: Health, United States, 2006 with Chartbook on Trends in the Health of Americans. Hyattsville, Md: Centers for Disease Control and Prevention; 2006:68–87. Available at: http://www.cdc.gov/nchs/data/hus/hus06.pdf. Accessed October 16, 2012.
17. Gibson SJ, Helme RD. Age differences in pain perception and report: a review of physiological, psychological, laboratory and clinical studies. Pain Rev. 1995;2:111-137.
18. Gallagher RM, Verma S, Mossey J. Chronic pain. Sources of late-life pain and risk factors for disability. Geriatrics. 2000;55:40-44, 47.
19. Picavet HS, Schouten JS. Musculoskeletal pain in the Netherlands: prevalences, consequences and risk groups, the DMC(3)-study. Pain. 2003;102:167-178.
20. Smith AK, Cenzer IS, Knight SJ, et al. The epidemiology of pain during the last 2 years of life. Ann Intern Med. 2010;153:563-569.
21. Sarkisian CA, Hays RD, Mangione CM. Do older adults expect to age successfully? The association between expectations regarding aging and beliefs regarding healthcare seeking among older adults. J Am Geriatr Soc. 2002;50:1837-1843.
22. Keller ML, Leventhal H, Prohaska TR, et al. Beliefs about aging and illness in a community sample. Res Nurs Health. 1989;12:247-255.
23. Dougados M, Gueguen A, Nguyen M, et al. Longitudinal radiologic evaluation of osteoarthritis of the knee. J Rheumatology. 1992;19:378-384.
24. Mallen CD, Peat G, Thomas E, et al. Prognostic factors for musculoskeletal pain in primary care: a systematic review. Br J Gen Pract. 2007;57:655-661.
25. Dieppe PA, Cushnaghan J, Shepstone L. The Bristol ‘OA500’ study: progression of osteoarthritis (OA) over 3 years and the relationship between clinical and radiographic changes at the knee joint. Osteoarthritis Cartilage. 1997;5:87-97.
26. Dieppe P, Cushnaghan J, Tucker M, et al. The Bristol ‘OA500 study’: progression and impact of the disease after 8 years. Osteoarthritis Cartilage. 2000;8:63-68.
27. Makris UE, Fraenkel L, Han L, et al. Epidemiology of restricting back pain in community-living older persons. J Am Geriatr Soc. 2011;59:610-614.
28. Kamaleri Y, Natvig B, Ihlebaek CM, et al. Change in the number of musculoskeletal pain sites: a 14-year prospective study. Pain. 2009;141:25-30.
29. Sharma L, Cahue S, Song J, et al. Physical functioning over three years in knee osteoarthritis: role of psychosocial, local mechanical, and neuromuscular factors. Arthritis Rheum. 2003;48:3359-3370.
30. Yong HH, Gibson SJ, Horne DJ, et al. Development of a pin attitudes questionnaire to assess stoicism and cautiousness for possible age differences. J Gerontol B Psychol Sci Soc Sci. 2001;56:279-284.
31. Vallerand A, Nowak L. Chronic opioid therapy for nonmalignant pain: the patient’s perspective. Part II—barriers to chronic opioid therapy. Pain manag nurs. 2010;11:126-131.
32. Gibson SJ, Farrell M. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin J Pain. 2004;20:227-239.
33. Woodrow KM, Friedman GD, Siegelaub AB, et al. Pain tolerance: differences according to age, sex and race. Psychosom Med. 1972;34:548-556.
34. Turk DC, Okifuji A, Scharff L. Chronic pain and depression: role of perceived impact and perceived control in different age cohorts. Pain. 1995;61:93-101.
35. Thielke SM, Fan MY, Sullivan M, et al. Pain limits the effectiveness of collaborative care for depression. Am J Geriatr Psychiatry. 2007;15:699-707.
36. Power JD, Perruccio AV, Badley EM. Pain as a mediator of sleep problems in arthritis and other chronic conditions. Arthritis Rheum. 2005;53:911-919.
37. Louie GH, Tektonidou MG, Caban-Martizen AJ, et al. Sleep disturbances in adults with arthritis: prevalence, mediators, and subgroups at greatest risk. Arthritis Care Res. 2011;63:247-260.
38. Lin JJ, Alfandre D, Moore C. Physician attitudes toward opioid prescribing for patients with persistent noncancer pain. Clin J Pain. 2007;23:799-803.
39. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613-2620.
40. Fischer B, Gittins J, Kendall P, et al. Thinking the unthinkable: could the increasing misuse of prescription opioids among street drug users offer benefits for public health? Public Health. 2009;123:145-146.
41. Fleming MF, Davis J, Passik SD. Reported lifetime aberrant drug-taking behaviors are predictive of current substance use and mental health problems in primary care patients. Pain Med. 2008;9:1098-1106.
42. Reid MC, Henderson CR, Jr, Papaleontiou M, et al. Characteristics of older adults receiving opioids in primary care: treatment duration and outcomes. Pain Med. 2010;11:1063-1071.
43. Solomon DH, Rassen JA, Glynn RJ, et al. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med. 2010;170:1979-1986.
44. Thielke SM, Simoni-Wastila L, Edlund MJ, et al. Age and sex trends in long-term opioid use in two large American health systems between 2000 and 2005. Pain Med. 2010;11:248-256.
45. Soden K, Ali S, Alloway L, et al. How do nurses assess and manage breakthrough pain in specialist palliative care inpatient units? A multicentre study. Palliat Med. 2010;24:294-298.
46. Papaleontiou M, Henderson CR, Jr, Turner BJ, et al. Outcomes associated with opioid use in the treatment of chronic noncancer pain in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2010;58:1353-1369.
47. Mallen CD, Peat G. Discussing prognosis with older people with musculoskeletal pain: a cross-sectional study in general practice. BMC Fam Pract. 2009;10:50.-
48. Rosemann T, Wensing M, Joest K, et al. Problems and needs for improving primary care of osteoarthritis patients: the views of patients, general practitioners and practice nurses. BMC Musculoskelet Disord. 2006;7:48.
AGING: Is your patient taking too many pills?
• Consider the possibility that an adverse drug effect—rather than a new condition—is at play when a patient taking multiple medications develops a new symptom. C
• Use an online interaction checker, which can be accessed via a smart phone or tablet, to check for potential drug-drug interactions in patients on multiple medications. C
• Cross-check patients’ medications with a list of their medical problems, with the goal of discontinuing any drug that duplicates the action of another or is age-inappropriate, ineffective, or not indicated for the condition for which it was prescribed. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Older adults are taking more medications than ever before. Nearly 9 out of 10 US residents who are 60 years of age or older take at least one prescription drug, more than a third take 5 to 9 medications, and 12% take 10 or more.1
The increase is largely driven by newer medications to effectively treat a variety of medical conditions, and by practice guidelines that often recommend multidrug regimens.2
As a result, the term “polypharmacy,” which once referred to a specific number of medications, is now used more broadly to mean “a large number” of drugs.
From a safety standpoint, the number of medications a patient takes matters. The risk of adverse drug effects and dangerous drug-drug interactions increases significantly when an individual takes ≥5 medications.3
More than 4.5 million adverse drug effects occur each year in the United States, and nearly three quarters of them are initially evaluated in outpatient settings.4 Research suggests that about 80% of the time, these adverse effects are not recognized as such by the patient’s physician. So instead of discontinuing the offending medication, physicians treat the drug-related symptoms by adding yet another medication—a phenomenon known as “the prescribing cascade.”5
This review can help you safeguard older patients taking multiple medications by recognizing and responding to drug-related problems, identifying drugs that can be safely eliminated (or, in some cases, drugs that should be added), and checking regularly to ensure that the medication regimen is appropriate and up to date.
CASE Mrs. R, a 79-year-old woman who recently moved to town, is brought to your office by her daughter and son-in-law. The patient has a hard time reporting her medical history, but her daughter tells you her mother has chronic obstructive pulmonary disease (COPD), heart failure, type 2 diabetes, and mild urinary incontinence, and was recently diagnosed with early dementia.
Mrs. R’s daughter has brought in a bagful of medications, but she’s not sure which ones her mother takes regularly. The medications are an albuterol inhaler, alprazolam, digoxin, diphenhydramine, donepezil, furosemide, glargine insulin, guaifenesin, levothyroxine, metformin, extended-release metoprolol, naproxen, omeprazole, simvastatin, tolterodine, and zolpidem—a total of 16 different drugs.
If Mrs. R were your patient, how would you manage her multidrug regimen?
Start with a medication review
The first step in evaluating a patient’s medication regimen is to find out whether the drugs in the patient’s possession and/or in the medical record are the ones he or she is actually taking. Ask older patients who haven’t brought in their medications, or the caregiver of a confused patient, to bring them to the next visit.
The next step: Determine whether the medication regimen is right for the patient.
Polypharmacy may be indicated
Despite the risks associated with polypharmacy, do not assume that it is inappropriate. For some conditions, multiple medications are routinely recommended. Patients with heart failure, for example, have been shown to have better outcomes when they take 3 to 5 medications, including beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, and diuretics.2
Some treatment guidelines also call for multiple medications. Achieving the more stringent blood pressure goals recommended in the Seventh Report of the Joint National Committee on Prevention, for instance, often requires 2 or more antihypertensive agents.6 In many cases, however, patients end up taking more drugs than necessary.
Is the patient taking the right drugs?
Medication reconciliation (determining whether the treatment regimen is appropriate for the patient’s diagnoses) is the way to find out.
The most widely recommended approach to medication reconciliation is to create a table and do a systematic review.7 List all the patient’s medical conditions in the first column and all current medications in the second column. Use the third column to note whether each medication is one the patient should be on, based not only on his or her medical conditions and other drugs being taken but also on current renal and hepatic function and body size, and contraindications.
A medication may be inappropriate if it duplicates, cancels out the action of, or otherwise interacts with another drug the patient is taking; is contraindicated in older patients; or is ineffective for the condition for which it was prescribed. In one key study of nearly 200 patients 65 years and older who took 5 or more medications, more than half had been prescribed at least one drug that was ineffective for the patient’s condition or that duplicated the action of another medication.8
In addition to finding drugs that the patient should not be taking, medication reconciliation may also reveal that the patient is not receiving optimal therapy and that one or more drugs should be added to his or her treatment regimen.
Check meds after transitions. A move from home to hospital, from emergency department to home, or any other transition relating to patient care should prompt a medication reconciliation. Medications are often added or inadvertently discontinued at such times,9,10 and instructions relating to medication are often misunderstood.11 In one study of 384 frail elderly patients being discharged from a hospital, for example, 44% were found to have been given at least one unnecessary prescription—most commonly for a medication that was neither indicated nor effective for any of the patient’s medical problems.12 It was also common for patients to be given drugs that duplicated the action of others they were already taking.
Even in the absence of such transitions, medication reconciliation should occur at regular intervals. Many physicians do a medication reconciliation at every visit to ensure that the medical record is accurate and the patient’s medication regimen is optimal.
Managing polypharmacy: These resources can help
Numerous tools are available to help you evaluate and monitor patients’ medication regimens, including some that were developed specifically for older patients.
START (Screening Tool to Alert doctors to Right Treatment) identifies drugs and drug classes that are underused with older patients.13 START criteria (TABLE 1)13-17 focus on medications that should be used yet are often omitted in older patients who have the appropriate indications.
TABLE 1
START criteria: Drug therapy that should be given to older patients13-17
Cardiovascular
|
Endocrine
|
Gastrointestinal
|
Musculoskeletal
|
Nervous system
|
Respiratory
|
| ACE, angiotensin-converting enzyme; BP, blood pressure; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; FEV1, forced expiratory volume in 1 second; GI, gastrointestinal; MI, myocardial infarction; PPI, proton-pump inhibitor; START, Screening Tool to alert doctors to right Treatment. |
In using START or any other drug-related tool, it is important to keep in mind that therapy should be individualized. Not all the medications in the START criteria are appropriate for every patient, and a medication that is indicated for a given medical condition may or may not provide real benefit for a particular patient. That would depend on the individual’s overall health and life expectancy, the goals of treatment, and how long it would take for the patient to realize any benefit from the drug in question.18 A vigorous 79-year-old might benefit from statin therapy for prevention of cardiovascular events, for instance, while a patient like Mrs. R, who is also 79 but has dementia and multiple other medical problems, would be unlikely to live long enough to realize such a benefit.
”Age” assessment tool. One criterion in deciding whether medication(s) are appropriate for an older patient is his or her “physiologic age”—calculated on the basis of the individual’s chronological age and self-reported health status (TABLE 2).19
TABLE 2
Calculating your patient’s “real” age19
| Actual age (y) | Physiologic age (y) | |||||||
|---|---|---|---|---|---|---|---|---|
| Self-reported health | ||||||||
| Excellent | Good | Fair | Poor | |||||
| Male | Female | Male | Female | Male | Female | Male | Female | |
| 65 | 58 | 60 | 64 | 64 | 68 | 66 | 73 | 72 |
| 70 | 62 | 65 | 69 | 69 | 73 | 71 | 78 | 77 |
| 75 | 67 | 70 | 74 | 74 | 78 | 76 | 83 | 82 |
| 80 | 72 | 75 | 79 | 79 | 83 | 81 | 85+ | 85+ |
Flagging drugs that may be inappropriate
Several tools have been developed to aid clinicians in identifying medications that are potentially inappropriate for older adults, although here, too, decisions about their use must be individualized. Two of the most widely used tools are the Beers criteria and STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions).
Beers criteria were developed by Mark Beers et al in 199120 and have been updated at regular intervals, most recently by the American Geriatrics Society in 2012.21 The drugs and drug classes included in the Beers criteria should not be prescribed for older patients in most cases, either because the risk of using them outweighs the benefit or because safer alternatives are available. Key components are listed in TABLE 3.21
TABLE 3
Beers criteria:* Drug classes that may be inappropriate for older adults21
| Drug class | Concern |
|---|---|
| Alpha-blockers with peripheral activity | Orthostatic hypotension |
| Anticholinergics | Cognitive impairment, urinary retention |
| Antipsychotics | Increased death rate when used for behavior control in patients with dementia |
| NSAIDs | Renal dysfunction, GI bleeding, fluid retention, exacerbation of heart failure |
| Sedative hypnotics | Cognitive impairment, delirium |
| Tricyclic antidepressants | Cognitive impairment, delirium, urinary retention |
| GI, gastrointestinal; NSAIDs, nonsteroidal anti-inflammatory drugs. *The full Beers criteria contains 53 drugs and drug classes that are generally inappropriate for older adults. The full list is available from the American Geriatrics Society at: www.americangeriatrics.org/files/documents/beers/2012BeersCriteria_JAGS.pdf. | |
One limitation of the Beers criteria has been its all-or-nothing approach, with many of the medications on the list deemed inappropriate for all older adults regardless of their circumstances. The 2012 update does a better job of individualizing recommendations: Medications are now categorized as those that should be avoided in older patients regardless of their diseases or conditions, those that should be avoided only in patients with certain diseases or conditions, and those that may be used for this patient population but require caution.21
STOPP is similar to the Beers criteria, but uses a different approach: Most medications on this list are considered in the context of specific medical problems.22 While the Beers criteria classify digoxin >0.125 mg/d as generally inappropriate for older adults, for example, STOPP criteria state that long-term dosing at that level is inappropriate only for those with impaired renal function.22 A list of medications identified by STOPP as contributing to hospitalization due to adverse drug effects is available at http://ageing.oxfordjournals.org/content/37/6/673.
Both tools address this drug category. Cumulative anticholinergic burden is a concept applied to the use of anticholinergic medications, which are included in both the Beers and STOPP criteria. Although isolated short-term exposure to a drug with anticholinergic properties may be tolerated by a healthy and cognitively intact older patient, repetitive exposure to such drugs, even if separated in time, has negative effects. One study evaluated more than 500 community-dwelling older adults and found that the more exposure an individual had to anticholinergic medications over the course of a year, the greater the impairment in short-term memory and activities of daily living.23 Another study, this one involving more than 13,000 community-dwelling and institutionalized patients, showed that the longer an older patient takes an anticholinergic medication, the more likely there is to be a measurable decline in performance on the Mini-Mental State Examination.24
Programs that flag potential interactions
Drug-drug interactions are a key concern of polypharmacy, and electronic medical records and prescribing systems that flag potential drug-drug interactions when a new medication is ordered are designed to help physicians avoid them. Unfortunately, clinicians only react to 3% to 9% of such notifications, overriding them because computerized systems often fail to distinguish between important and unimportant interactions.25-27 Thus, clinicians often must decide whether to react to or override warnings, an often difficult decision with patient safety and medicolegal implications. The best advice we can offer is to carefully evaluate drug interaction warnings using common sense, and seek consultation with a clinical pharmacist when uncertainty exists. This approach should prevent prescribing medications that have potentially harmful interactions with drugs the patient is already taking.
For physicians who do not have access to an electronic prescribing system that provides such notification, several online resources are available, some by subscription (eg, Lexicomp, www.lexi.com; Micromedex, www.micromedex.com/index.html; and Pepid, www.pepid.com) and others with free access (eg, AARP, healthtools.aarp.org/drug-interactions; Drugs.com (www.drugs.com/drug_interactions.php; and HealthLine, www.healthline.com/druginteractions).
CASE After doing a medication reconciliation for Mrs. R, you find that she is taking tolterodine, an anticholinergic medication for urge urinary incontinence, and donepezil, a procholinergic medication for dementia. This type of drug-drug interaction, in which the action of one drug effectively cancels out the effect of another, should not be ignored.
Overall, you identify 8 of her medications that could be discontinued: The list includes guaifenesin (a nonessential medication of questionable efficacy); naproxen (inappropriate per Beers criteria; inappropriate in patients with heart failure, according to STOPP); alprazolam, zolpidem, and diphenhydramine (duplicate medications that are all on the Beers criteria as inappropriate for chronic use and ill-advised in patients with cognitive impairment); and omeprazole and levothyroxine (for which nothing in the patient’s history suggests a need), as well as tolterodine. Depending on dose, digoxin is yet another candidate for discontinuation.
Discontinuing medications: Proceed carefully
Physicians are often reluctant to discontinue chronic medications in older patients—even in those with advanced disease who are not likely to benefit from treatment. Focus groups have identified a number of reasons for their hesitation, including:
- the assumption that patients have no problem taking large numbers of drugs
- the fear that patients may misinterpret a plan to discontinue medications as evidence that the physician is giving up on them
- the belief that physicians must comply with practice guidelines that recommend multiple drug treatments
- concern that proposing discontinuation of medications often leads to a discussion of life expectancy and end-of-life care.28
Physicians may also fear that discontinuation of certain drugs will increase the risk of adverse outcomes. More than 30 studies have evaluated discontinuation of chronic medications in older adults, however, and found that drugs as diverse as antihypertensives, antipsychotics, benzodiazepines, and selective serotonin reuptake inhibitors (SSRIs) can often be discontinued without adverse outcomes. In many cases, improvement in patient function results.29 Medications that present the most difficulty are those that patients often become physically or psychologically dependent on, such as benzodiazepines, guaifenesin, proton-pump inhibitors, nonsteroidal anti-inflammatory drugs, and SSRIs. Some (eg, benzodiazepines, SSRIs) require a gradual reduction; for others, no taper is required
(TABLE 4).30-37
TABLE 4
Recommendations for discontinuing hard-to-stop drugs
| Medication or drug class | Discontinuation regimen | Comments |
|---|---|---|
| Benzodiazepines30 | Taper dose by 25% q 2 wk | No withdrawal symptoms reported with this taper regimen. Subtle cognitive improvement noted over a period of months |
| Guaifenesin31 | Can be discontinued without tapering if not combined with opioids or other medications. Elimination half-life is approximately 1 hour | Guaifenesin is often marketed as a combination product with opioids; such combination products require tapering |
| PPIs32-34 | Decrease dose by 50% q 2 wk; supplement with H2 blocker if needed, but tapering of H2 blocker may be required | Abrupt discontinuation after long-term use causes rebound gastric acid hypersecretion and lowers rate of success. Higher success rates with taper regimen and in patients who do not have documented GERD |
| NSAIDs35 | No taper required | Short-term use (<3 mo) acceptable for patients with no contraindications |
| SSRIs36,37 | Gradual reduction in dose over 6-8 wk | Highest rate of success in patients without a clear diagnosis of depression |
| GERD, gastroesophageal reflux disease; NSAIDs, nonsteroidal anti-inflammatory drugs; PPIs, proton-pump inhibitors; SSRIs, selective serotonin reuptake inhibitors. | ||
CASE You trim down Mrs. R’s regimen by discontinuing each of the 8 drugs, one at a time, and carefully monitor the patient during the withdrawal period. Because she had been taking alprazolam daily, the dose is tapered slowly to avoid withdrawal. Omeprazole also requires a gradual taper to avoid rebound hyperacidity.3
After confirming that Mrs. R has heart failure and COPD, you identify 2 medications that should be added to her drug regimen—an ACE inhibitor for heart failure and an inhaled anticholinergic for COPD.
Going from 16 medications to 10 saves money, decreases the likelihood of adverse events and drug-drug interactions, and helps with adherence. Mrs. R’s new drug regimen is expected to lead to improvements in memory and overall quality of life, as well.
CORRESPONDENCE
Barry D. Weiss, MD, Department of Family and Community Medicine, University of Arizona College of Medicine, Tucson, AZ 85724; bdweiss@email.arizona.edu
1. Gu Q, Dillon CF, Burt V. Prescription drug use continued to increase: US prescription drug data for 2007-2008. CDC/NCHS Data Brief. 2010;42:1-2.
2. Jessup K, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;119:1977-2016.
3. Johnell K, Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30:911-918.
4. Sarkar U, Lopez A, Maselli JH, et al. Adverse drug events in US adult ambulatory medical care. Health Services Res. 2011;46:1517-1533.
5. Rollason V, Vogt N. Reduction of polypharmacy in the elderly. A systematic review of the role of the pharmacist. Drugs Aging. 2003;20:817-832.
6. National Heart, Lung, and Blood Institute. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Available at: www.nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf. Accessed October 11, 2012.
7. Steinman MA, Hanlon JT. Managing medications in clinically complex elders. JAMA. 2010;304:1592-1601.
8. Steinman MA, Landefeld CS, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54:1516-23.
9. Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuations of medications for chronic disease. JAMA. 2011;306:840-847.
10. Moore C, Wisnivesky J, Williams S, et al. Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med. 2003;18:646-651.
11. Ziaeian B, Arauho KL, Van Ness PH, et al. Medication reconciliation accuracy and patient understanding of intended medication changes on hospital discharge. J Gen Intern Med. 2012 July 12. ePub ahead of print.
12. Hajjar ER, Hanlon JT, Sloane RJ, et al. Unnecessary drug use in frail older people at hospital discharge. J Am Geriatr Soc. 2005;53:1518-1523.
13. O’Mahony D, Gallagher P, Ryan C, et al. STOPP & START criteria: a new approach to detecting potentially inappropriate prescribing in old age. Eur Geriatr Med. 2010;1:45-51.
14. Denneboom W, Dautzenberg KGH, Grol R, et al. Analysis of polypharmacy in older patients in primary acre using a multidisciplinary expert panel. Br J Gen Pract. 2006;56:504-510.
15. Ko DT, Mamdani M, Alter DA. Lipid-lowering therapy with statins in high-risk elderly patients. JAMA. 2004;291:1864-70.
16. Wright RM, Sloane R, Pieper CF, et al. Underuse of indicated medications among physically frail older US veterans at the time of hospital discharge: results of a cross-sectional analysis of data from the Geriatric Evaluation and Management Drug Study. Am J Geriatr Pharmacother. 2009;7:271-280.
17. Garwood CL. Use of anticoagulation in elderly patients with atrial fibrillation who are risk for falls. Ann Pharmacother. 2008;42:523-532.
18. Holmes HM, Hayley DC, Alexander GC, et al. Reconsidering medication appropriateness for patients late in life. Arch Intern Med. 2006;166:605-609.
19. Simplified Methods for Estimating Life Expectancy. Available at: http://painconsortium.nih.gov/symptomresearch/chapter_14/Part_3/sec4/chspt3s4pg1.htm. Accessed October 9, 2012.
20. Beers MH, Ouslander JG, Rollingher I, et al. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med. 1991;151:1825-1832.
21. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society Update Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616-631.
22. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers’ criteria. Age Aging. 2008;37:673-379.
23. Han L, Agostini JV, Allore HG. Cumulative anticholinergic exposure is associated with poor memory and executive function in older men. J Am Geriatr Soc. 2008;56:2203-2210.
24. Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59:1477-1483.
25. Knight A, Falade O, Maygers J, et al. Factors associated with medication warning acceptance [abstract]. J Hosp Med. 2012;7(suppl 2):515.-
26. Isaac T, Weissman JS, Davis RB, et al. Overrides of medication alerts in ambulatory care. Arch Intern Med. 2009;169:305-311.
27. Van Der Sijs H, Aarts J, Vulto A, et al. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;12:138-147.
28. Schuling J, Gebben H, Veehof LJG, et al. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Family Practice. 2012;13:56. http://www.biomedcentral.com/1471-2296/13/56.
29. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older. A systematic review. Drugs Aging. 2008;25:1021-1031.
30. Curran HV, Collins R, Fletcher S, et al. Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med. 2003;33:1223-1237.
31. Krinsky DL, Berardi RR, Ferreris SP, et al. Handbook of Nonprescription Drugs: An Interactive Approach to Self-Care. Washington, DC: American Pharmacists Association; 2012:209.
32. Bjornsson E, Abrahamsson H, Simren M, et al. Discontinuation of proton pump inhibitors in patients on long-term therapy: a double-blind, placebo-controlled trial. Aliment Pharmacol Ther. 2006;24:945-954.
33. Inadomi JM, Jamai R, Murata GH, et al. Step-down management of gastroesophageal reflux disease. Gastroenterology. 2001;131:1095-1100.
34. Hester SA. Proton pump inhibitors and rebound acid hypersecretion. Pharm Lett. 2009;25:250920.-
35. Taylor R, Jr, Lemtouni S, Weiss K, et al. Pain management in the elderly: an FDA safe use initiative expert panel’s view on preventable harm associated with NSAID therapy. Curr Gerontol Geriatr Res. 2012;196159.-
36. Ulfvarson J, Adami J, Wredling R, et al. Controlled withdrawal of selective serotonin reuptake inhibitor drugs in elderly patients in nursing homes with no indication of depression. Eur J Clin Pharmacol. 2003;59:735-740.
37. Lindstrom K, Ekedahl A, Carlsten A, et al. Can selective serotonin inhibitor drugs in elderly patients in nursing homes be reduced? Scand J Prim Health Care. 2007;25:3-8.
• Consider the possibility that an adverse drug effect—rather than a new condition—is at play when a patient taking multiple medications develops a new symptom. C
• Use an online interaction checker, which can be accessed via a smart phone or tablet, to check for potential drug-drug interactions in patients on multiple medications. C
• Cross-check patients’ medications with a list of their medical problems, with the goal of discontinuing any drug that duplicates the action of another or is age-inappropriate, ineffective, or not indicated for the condition for which it was prescribed. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Older adults are taking more medications than ever before. Nearly 9 out of 10 US residents who are 60 years of age or older take at least one prescription drug, more than a third take 5 to 9 medications, and 12% take 10 or more.1
The increase is largely driven by newer medications to effectively treat a variety of medical conditions, and by practice guidelines that often recommend multidrug regimens.2
As a result, the term “polypharmacy,” which once referred to a specific number of medications, is now used more broadly to mean “a large number” of drugs.
From a safety standpoint, the number of medications a patient takes matters. The risk of adverse drug effects and dangerous drug-drug interactions increases significantly when an individual takes ≥5 medications.3
More than 4.5 million adverse drug effects occur each year in the United States, and nearly three quarters of them are initially evaluated in outpatient settings.4 Research suggests that about 80% of the time, these adverse effects are not recognized as such by the patient’s physician. So instead of discontinuing the offending medication, physicians treat the drug-related symptoms by adding yet another medication—a phenomenon known as “the prescribing cascade.”5
This review can help you safeguard older patients taking multiple medications by recognizing and responding to drug-related problems, identifying drugs that can be safely eliminated (or, in some cases, drugs that should be added), and checking regularly to ensure that the medication regimen is appropriate and up to date.
CASE Mrs. R, a 79-year-old woman who recently moved to town, is brought to your office by her daughter and son-in-law. The patient has a hard time reporting her medical history, but her daughter tells you her mother has chronic obstructive pulmonary disease (COPD), heart failure, type 2 diabetes, and mild urinary incontinence, and was recently diagnosed with early dementia.
Mrs. R’s daughter has brought in a bagful of medications, but she’s not sure which ones her mother takes regularly. The medications are an albuterol inhaler, alprazolam, digoxin, diphenhydramine, donepezil, furosemide, glargine insulin, guaifenesin, levothyroxine, metformin, extended-release metoprolol, naproxen, omeprazole, simvastatin, tolterodine, and zolpidem—a total of 16 different drugs.
If Mrs. R were your patient, how would you manage her multidrug regimen?
Start with a medication review
The first step in evaluating a patient’s medication regimen is to find out whether the drugs in the patient’s possession and/or in the medical record are the ones he or she is actually taking. Ask older patients who haven’t brought in their medications, or the caregiver of a confused patient, to bring them to the next visit.
The next step: Determine whether the medication regimen is right for the patient.
Polypharmacy may be indicated
Despite the risks associated with polypharmacy, do not assume that it is inappropriate. For some conditions, multiple medications are routinely recommended. Patients with heart failure, for example, have been shown to have better outcomes when they take 3 to 5 medications, including beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, and diuretics.2
Some treatment guidelines also call for multiple medications. Achieving the more stringent blood pressure goals recommended in the Seventh Report of the Joint National Committee on Prevention, for instance, often requires 2 or more antihypertensive agents.6 In many cases, however, patients end up taking more drugs than necessary.
Is the patient taking the right drugs?
Medication reconciliation (determining whether the treatment regimen is appropriate for the patient’s diagnoses) is the way to find out.
The most widely recommended approach to medication reconciliation is to create a table and do a systematic review.7 List all the patient’s medical conditions in the first column and all current medications in the second column. Use the third column to note whether each medication is one the patient should be on, based not only on his or her medical conditions and other drugs being taken but also on current renal and hepatic function and body size, and contraindications.
A medication may be inappropriate if it duplicates, cancels out the action of, or otherwise interacts with another drug the patient is taking; is contraindicated in older patients; or is ineffective for the condition for which it was prescribed. In one key study of nearly 200 patients 65 years and older who took 5 or more medications, more than half had been prescribed at least one drug that was ineffective for the patient’s condition or that duplicated the action of another medication.8
In addition to finding drugs that the patient should not be taking, medication reconciliation may also reveal that the patient is not receiving optimal therapy and that one or more drugs should be added to his or her treatment regimen.
Check meds after transitions. A move from home to hospital, from emergency department to home, or any other transition relating to patient care should prompt a medication reconciliation. Medications are often added or inadvertently discontinued at such times,9,10 and instructions relating to medication are often misunderstood.11 In one study of 384 frail elderly patients being discharged from a hospital, for example, 44% were found to have been given at least one unnecessary prescription—most commonly for a medication that was neither indicated nor effective for any of the patient’s medical problems.12 It was also common for patients to be given drugs that duplicated the action of others they were already taking.
Even in the absence of such transitions, medication reconciliation should occur at regular intervals. Many physicians do a medication reconciliation at every visit to ensure that the medical record is accurate and the patient’s medication regimen is optimal.
Managing polypharmacy: These resources can help
Numerous tools are available to help you evaluate and monitor patients’ medication regimens, including some that were developed specifically for older patients.
START (Screening Tool to Alert doctors to Right Treatment) identifies drugs and drug classes that are underused with older patients.13 START criteria (TABLE 1)13-17 focus on medications that should be used yet are often omitted in older patients who have the appropriate indications.
TABLE 1
START criteria: Drug therapy that should be given to older patients13-17
Cardiovascular
|
Endocrine
|
Gastrointestinal
|
Musculoskeletal
|
Nervous system
|
Respiratory
|
| ACE, angiotensin-converting enzyme; BP, blood pressure; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; FEV1, forced expiratory volume in 1 second; GI, gastrointestinal; MI, myocardial infarction; PPI, proton-pump inhibitor; START, Screening Tool to alert doctors to right Treatment. |
In using START or any other drug-related tool, it is important to keep in mind that therapy should be individualized. Not all the medications in the START criteria are appropriate for every patient, and a medication that is indicated for a given medical condition may or may not provide real benefit for a particular patient. That would depend on the individual’s overall health and life expectancy, the goals of treatment, and how long it would take for the patient to realize any benefit from the drug in question.18 A vigorous 79-year-old might benefit from statin therapy for prevention of cardiovascular events, for instance, while a patient like Mrs. R, who is also 79 but has dementia and multiple other medical problems, would be unlikely to live long enough to realize such a benefit.
”Age” assessment tool. One criterion in deciding whether medication(s) are appropriate for an older patient is his or her “physiologic age”—calculated on the basis of the individual’s chronological age and self-reported health status (TABLE 2).19
TABLE 2
Calculating your patient’s “real” age19
| Actual age (y) | Physiologic age (y) | |||||||
|---|---|---|---|---|---|---|---|---|
| Self-reported health | ||||||||
| Excellent | Good | Fair | Poor | |||||
| Male | Female | Male | Female | Male | Female | Male | Female | |
| 65 | 58 | 60 | 64 | 64 | 68 | 66 | 73 | 72 |
| 70 | 62 | 65 | 69 | 69 | 73 | 71 | 78 | 77 |
| 75 | 67 | 70 | 74 | 74 | 78 | 76 | 83 | 82 |
| 80 | 72 | 75 | 79 | 79 | 83 | 81 | 85+ | 85+ |
Flagging drugs that may be inappropriate
Several tools have been developed to aid clinicians in identifying medications that are potentially inappropriate for older adults, although here, too, decisions about their use must be individualized. Two of the most widely used tools are the Beers criteria and STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions).
Beers criteria were developed by Mark Beers et al in 199120 and have been updated at regular intervals, most recently by the American Geriatrics Society in 2012.21 The drugs and drug classes included in the Beers criteria should not be prescribed for older patients in most cases, either because the risk of using them outweighs the benefit or because safer alternatives are available. Key components are listed in TABLE 3.21
TABLE 3
Beers criteria:* Drug classes that may be inappropriate for older adults21
| Drug class | Concern |
|---|---|
| Alpha-blockers with peripheral activity | Orthostatic hypotension |
| Anticholinergics | Cognitive impairment, urinary retention |
| Antipsychotics | Increased death rate when used for behavior control in patients with dementia |
| NSAIDs | Renal dysfunction, GI bleeding, fluid retention, exacerbation of heart failure |
| Sedative hypnotics | Cognitive impairment, delirium |
| Tricyclic antidepressants | Cognitive impairment, delirium, urinary retention |
| GI, gastrointestinal; NSAIDs, nonsteroidal anti-inflammatory drugs. *The full Beers criteria contains 53 drugs and drug classes that are generally inappropriate for older adults. The full list is available from the American Geriatrics Society at: www.americangeriatrics.org/files/documents/beers/2012BeersCriteria_JAGS.pdf. | |
One limitation of the Beers criteria has been its all-or-nothing approach, with many of the medications on the list deemed inappropriate for all older adults regardless of their circumstances. The 2012 update does a better job of individualizing recommendations: Medications are now categorized as those that should be avoided in older patients regardless of their diseases or conditions, those that should be avoided only in patients with certain diseases or conditions, and those that may be used for this patient population but require caution.21
STOPP is similar to the Beers criteria, but uses a different approach: Most medications on this list are considered in the context of specific medical problems.22 While the Beers criteria classify digoxin >0.125 mg/d as generally inappropriate for older adults, for example, STOPP criteria state that long-term dosing at that level is inappropriate only for those with impaired renal function.22 A list of medications identified by STOPP as contributing to hospitalization due to adverse drug effects is available at http://ageing.oxfordjournals.org/content/37/6/673.
Both tools address this drug category. Cumulative anticholinergic burden is a concept applied to the use of anticholinergic medications, which are included in both the Beers and STOPP criteria. Although isolated short-term exposure to a drug with anticholinergic properties may be tolerated by a healthy and cognitively intact older patient, repetitive exposure to such drugs, even if separated in time, has negative effects. One study evaluated more than 500 community-dwelling older adults and found that the more exposure an individual had to anticholinergic medications over the course of a year, the greater the impairment in short-term memory and activities of daily living.23 Another study, this one involving more than 13,000 community-dwelling and institutionalized patients, showed that the longer an older patient takes an anticholinergic medication, the more likely there is to be a measurable decline in performance on the Mini-Mental State Examination.24
Programs that flag potential interactions
Drug-drug interactions are a key concern of polypharmacy, and electronic medical records and prescribing systems that flag potential drug-drug interactions when a new medication is ordered are designed to help physicians avoid them. Unfortunately, clinicians only react to 3% to 9% of such notifications, overriding them because computerized systems often fail to distinguish between important and unimportant interactions.25-27 Thus, clinicians often must decide whether to react to or override warnings, an often difficult decision with patient safety and medicolegal implications. The best advice we can offer is to carefully evaluate drug interaction warnings using common sense, and seek consultation with a clinical pharmacist when uncertainty exists. This approach should prevent prescribing medications that have potentially harmful interactions with drugs the patient is already taking.
For physicians who do not have access to an electronic prescribing system that provides such notification, several online resources are available, some by subscription (eg, Lexicomp, www.lexi.com; Micromedex, www.micromedex.com/index.html; and Pepid, www.pepid.com) and others with free access (eg, AARP, healthtools.aarp.org/drug-interactions; Drugs.com (www.drugs.com/drug_interactions.php; and HealthLine, www.healthline.com/druginteractions).
CASE After doing a medication reconciliation for Mrs. R, you find that she is taking tolterodine, an anticholinergic medication for urge urinary incontinence, and donepezil, a procholinergic medication for dementia. This type of drug-drug interaction, in which the action of one drug effectively cancels out the effect of another, should not be ignored.
Overall, you identify 8 of her medications that could be discontinued: The list includes guaifenesin (a nonessential medication of questionable efficacy); naproxen (inappropriate per Beers criteria; inappropriate in patients with heart failure, according to STOPP); alprazolam, zolpidem, and diphenhydramine (duplicate medications that are all on the Beers criteria as inappropriate for chronic use and ill-advised in patients with cognitive impairment); and omeprazole and levothyroxine (for which nothing in the patient’s history suggests a need), as well as tolterodine. Depending on dose, digoxin is yet another candidate for discontinuation.
Discontinuing medications: Proceed carefully
Physicians are often reluctant to discontinue chronic medications in older patients—even in those with advanced disease who are not likely to benefit from treatment. Focus groups have identified a number of reasons for their hesitation, including:
- the assumption that patients have no problem taking large numbers of drugs
- the fear that patients may misinterpret a plan to discontinue medications as evidence that the physician is giving up on them
- the belief that physicians must comply with practice guidelines that recommend multiple drug treatments
- concern that proposing discontinuation of medications often leads to a discussion of life expectancy and end-of-life care.28
Physicians may also fear that discontinuation of certain drugs will increase the risk of adverse outcomes. More than 30 studies have evaluated discontinuation of chronic medications in older adults, however, and found that drugs as diverse as antihypertensives, antipsychotics, benzodiazepines, and selective serotonin reuptake inhibitors (SSRIs) can often be discontinued without adverse outcomes. In many cases, improvement in patient function results.29 Medications that present the most difficulty are those that patients often become physically or psychologically dependent on, such as benzodiazepines, guaifenesin, proton-pump inhibitors, nonsteroidal anti-inflammatory drugs, and SSRIs. Some (eg, benzodiazepines, SSRIs) require a gradual reduction; for others, no taper is required
(TABLE 4).30-37
TABLE 4
Recommendations for discontinuing hard-to-stop drugs
| Medication or drug class | Discontinuation regimen | Comments |
|---|---|---|
| Benzodiazepines30 | Taper dose by 25% q 2 wk | No withdrawal symptoms reported with this taper regimen. Subtle cognitive improvement noted over a period of months |
| Guaifenesin31 | Can be discontinued without tapering if not combined with opioids or other medications. Elimination half-life is approximately 1 hour | Guaifenesin is often marketed as a combination product with opioids; such combination products require tapering |
| PPIs32-34 | Decrease dose by 50% q 2 wk; supplement with H2 blocker if needed, but tapering of H2 blocker may be required | Abrupt discontinuation after long-term use causes rebound gastric acid hypersecretion and lowers rate of success. Higher success rates with taper regimen and in patients who do not have documented GERD |
| NSAIDs35 | No taper required | Short-term use (<3 mo) acceptable for patients with no contraindications |
| SSRIs36,37 | Gradual reduction in dose over 6-8 wk | Highest rate of success in patients without a clear diagnosis of depression |
| GERD, gastroesophageal reflux disease; NSAIDs, nonsteroidal anti-inflammatory drugs; PPIs, proton-pump inhibitors; SSRIs, selective serotonin reuptake inhibitors. | ||
CASE You trim down Mrs. R’s regimen by discontinuing each of the 8 drugs, one at a time, and carefully monitor the patient during the withdrawal period. Because she had been taking alprazolam daily, the dose is tapered slowly to avoid withdrawal. Omeprazole also requires a gradual taper to avoid rebound hyperacidity.3
After confirming that Mrs. R has heart failure and COPD, you identify 2 medications that should be added to her drug regimen—an ACE inhibitor for heart failure and an inhaled anticholinergic for COPD.
Going from 16 medications to 10 saves money, decreases the likelihood of adverse events and drug-drug interactions, and helps with adherence. Mrs. R’s new drug regimen is expected to lead to improvements in memory and overall quality of life, as well.
CORRESPONDENCE
Barry D. Weiss, MD, Department of Family and Community Medicine, University of Arizona College of Medicine, Tucson, AZ 85724; bdweiss@email.arizona.edu
• Consider the possibility that an adverse drug effect—rather than a new condition—is at play when a patient taking multiple medications develops a new symptom. C
• Use an online interaction checker, which can be accessed via a smart phone or tablet, to check for potential drug-drug interactions in patients on multiple medications. C
• Cross-check patients’ medications with a list of their medical problems, with the goal of discontinuing any drug that duplicates the action of another or is age-inappropriate, ineffective, or not indicated for the condition for which it was prescribed. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Older adults are taking more medications than ever before. Nearly 9 out of 10 US residents who are 60 years of age or older take at least one prescription drug, more than a third take 5 to 9 medications, and 12% take 10 or more.1
The increase is largely driven by newer medications to effectively treat a variety of medical conditions, and by practice guidelines that often recommend multidrug regimens.2
As a result, the term “polypharmacy,” which once referred to a specific number of medications, is now used more broadly to mean “a large number” of drugs.
From a safety standpoint, the number of medications a patient takes matters. The risk of adverse drug effects and dangerous drug-drug interactions increases significantly when an individual takes ≥5 medications.3
More than 4.5 million adverse drug effects occur each year in the United States, and nearly three quarters of them are initially evaluated in outpatient settings.4 Research suggests that about 80% of the time, these adverse effects are not recognized as such by the patient’s physician. So instead of discontinuing the offending medication, physicians treat the drug-related symptoms by adding yet another medication—a phenomenon known as “the prescribing cascade.”5
This review can help you safeguard older patients taking multiple medications by recognizing and responding to drug-related problems, identifying drugs that can be safely eliminated (or, in some cases, drugs that should be added), and checking regularly to ensure that the medication regimen is appropriate and up to date.
CASE Mrs. R, a 79-year-old woman who recently moved to town, is brought to your office by her daughter and son-in-law. The patient has a hard time reporting her medical history, but her daughter tells you her mother has chronic obstructive pulmonary disease (COPD), heart failure, type 2 diabetes, and mild urinary incontinence, and was recently diagnosed with early dementia.
Mrs. R’s daughter has brought in a bagful of medications, but she’s not sure which ones her mother takes regularly. The medications are an albuterol inhaler, alprazolam, digoxin, diphenhydramine, donepezil, furosemide, glargine insulin, guaifenesin, levothyroxine, metformin, extended-release metoprolol, naproxen, omeprazole, simvastatin, tolterodine, and zolpidem—a total of 16 different drugs.
If Mrs. R were your patient, how would you manage her multidrug regimen?
Start with a medication review
The first step in evaluating a patient’s medication regimen is to find out whether the drugs in the patient’s possession and/or in the medical record are the ones he or she is actually taking. Ask older patients who haven’t brought in their medications, or the caregiver of a confused patient, to bring them to the next visit.
The next step: Determine whether the medication regimen is right for the patient.
Polypharmacy may be indicated
Despite the risks associated with polypharmacy, do not assume that it is inappropriate. For some conditions, multiple medications are routinely recommended. Patients with heart failure, for example, have been shown to have better outcomes when they take 3 to 5 medications, including beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, and diuretics.2
Some treatment guidelines also call for multiple medications. Achieving the more stringent blood pressure goals recommended in the Seventh Report of the Joint National Committee on Prevention, for instance, often requires 2 or more antihypertensive agents.6 In many cases, however, patients end up taking more drugs than necessary.
Is the patient taking the right drugs?
Medication reconciliation (determining whether the treatment regimen is appropriate for the patient’s diagnoses) is the way to find out.
The most widely recommended approach to medication reconciliation is to create a table and do a systematic review.7 List all the patient’s medical conditions in the first column and all current medications in the second column. Use the third column to note whether each medication is one the patient should be on, based not only on his or her medical conditions and other drugs being taken but also on current renal and hepatic function and body size, and contraindications.
A medication may be inappropriate if it duplicates, cancels out the action of, or otherwise interacts with another drug the patient is taking; is contraindicated in older patients; or is ineffective for the condition for which it was prescribed. In one key study of nearly 200 patients 65 years and older who took 5 or more medications, more than half had been prescribed at least one drug that was ineffective for the patient’s condition or that duplicated the action of another medication.8
In addition to finding drugs that the patient should not be taking, medication reconciliation may also reveal that the patient is not receiving optimal therapy and that one or more drugs should be added to his or her treatment regimen.
Check meds after transitions. A move from home to hospital, from emergency department to home, or any other transition relating to patient care should prompt a medication reconciliation. Medications are often added or inadvertently discontinued at such times,9,10 and instructions relating to medication are often misunderstood.11 In one study of 384 frail elderly patients being discharged from a hospital, for example, 44% were found to have been given at least one unnecessary prescription—most commonly for a medication that was neither indicated nor effective for any of the patient’s medical problems.12 It was also common for patients to be given drugs that duplicated the action of others they were already taking.
Even in the absence of such transitions, medication reconciliation should occur at regular intervals. Many physicians do a medication reconciliation at every visit to ensure that the medical record is accurate and the patient’s medication regimen is optimal.
Managing polypharmacy: These resources can help
Numerous tools are available to help you evaluate and monitor patients’ medication regimens, including some that were developed specifically for older patients.
START (Screening Tool to Alert doctors to Right Treatment) identifies drugs and drug classes that are underused with older patients.13 START criteria (TABLE 1)13-17 focus on medications that should be used yet are often omitted in older patients who have the appropriate indications.
TABLE 1
START criteria: Drug therapy that should be given to older patients13-17
Cardiovascular
|
Endocrine
|
Gastrointestinal
|
Musculoskeletal
|
Nervous system
|
Respiratory
|
| ACE, angiotensin-converting enzyme; BP, blood pressure; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; FEV1, forced expiratory volume in 1 second; GI, gastrointestinal; MI, myocardial infarction; PPI, proton-pump inhibitor; START, Screening Tool to alert doctors to right Treatment. |
In using START or any other drug-related tool, it is important to keep in mind that therapy should be individualized. Not all the medications in the START criteria are appropriate for every patient, and a medication that is indicated for a given medical condition may or may not provide real benefit for a particular patient. That would depend on the individual’s overall health and life expectancy, the goals of treatment, and how long it would take for the patient to realize any benefit from the drug in question.18 A vigorous 79-year-old might benefit from statin therapy for prevention of cardiovascular events, for instance, while a patient like Mrs. R, who is also 79 but has dementia and multiple other medical problems, would be unlikely to live long enough to realize such a benefit.
”Age” assessment tool. One criterion in deciding whether medication(s) are appropriate for an older patient is his or her “physiologic age”—calculated on the basis of the individual’s chronological age and self-reported health status (TABLE 2).19
TABLE 2
Calculating your patient’s “real” age19
| Actual age (y) | Physiologic age (y) | |||||||
|---|---|---|---|---|---|---|---|---|
| Self-reported health | ||||||||
| Excellent | Good | Fair | Poor | |||||
| Male | Female | Male | Female | Male | Female | Male | Female | |
| 65 | 58 | 60 | 64 | 64 | 68 | 66 | 73 | 72 |
| 70 | 62 | 65 | 69 | 69 | 73 | 71 | 78 | 77 |
| 75 | 67 | 70 | 74 | 74 | 78 | 76 | 83 | 82 |
| 80 | 72 | 75 | 79 | 79 | 83 | 81 | 85+ | 85+ |
Flagging drugs that may be inappropriate
Several tools have been developed to aid clinicians in identifying medications that are potentially inappropriate for older adults, although here, too, decisions about their use must be individualized. Two of the most widely used tools are the Beers criteria and STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions).
Beers criteria were developed by Mark Beers et al in 199120 and have been updated at regular intervals, most recently by the American Geriatrics Society in 2012.21 The drugs and drug classes included in the Beers criteria should not be prescribed for older patients in most cases, either because the risk of using them outweighs the benefit or because safer alternatives are available. Key components are listed in TABLE 3.21
TABLE 3
Beers criteria:* Drug classes that may be inappropriate for older adults21
| Drug class | Concern |
|---|---|
| Alpha-blockers with peripheral activity | Orthostatic hypotension |
| Anticholinergics | Cognitive impairment, urinary retention |
| Antipsychotics | Increased death rate when used for behavior control in patients with dementia |
| NSAIDs | Renal dysfunction, GI bleeding, fluid retention, exacerbation of heart failure |
| Sedative hypnotics | Cognitive impairment, delirium |
| Tricyclic antidepressants | Cognitive impairment, delirium, urinary retention |
| GI, gastrointestinal; NSAIDs, nonsteroidal anti-inflammatory drugs. *The full Beers criteria contains 53 drugs and drug classes that are generally inappropriate for older adults. The full list is available from the American Geriatrics Society at: www.americangeriatrics.org/files/documents/beers/2012BeersCriteria_JAGS.pdf. | |
One limitation of the Beers criteria has been its all-or-nothing approach, with many of the medications on the list deemed inappropriate for all older adults regardless of their circumstances. The 2012 update does a better job of individualizing recommendations: Medications are now categorized as those that should be avoided in older patients regardless of their diseases or conditions, those that should be avoided only in patients with certain diseases or conditions, and those that may be used for this patient population but require caution.21
STOPP is similar to the Beers criteria, but uses a different approach: Most medications on this list are considered in the context of specific medical problems.22 While the Beers criteria classify digoxin >0.125 mg/d as generally inappropriate for older adults, for example, STOPP criteria state that long-term dosing at that level is inappropriate only for those with impaired renal function.22 A list of medications identified by STOPP as contributing to hospitalization due to adverse drug effects is available at http://ageing.oxfordjournals.org/content/37/6/673.
Both tools address this drug category. Cumulative anticholinergic burden is a concept applied to the use of anticholinergic medications, which are included in both the Beers and STOPP criteria. Although isolated short-term exposure to a drug with anticholinergic properties may be tolerated by a healthy and cognitively intact older patient, repetitive exposure to such drugs, even if separated in time, has negative effects. One study evaluated more than 500 community-dwelling older adults and found that the more exposure an individual had to anticholinergic medications over the course of a year, the greater the impairment in short-term memory and activities of daily living.23 Another study, this one involving more than 13,000 community-dwelling and institutionalized patients, showed that the longer an older patient takes an anticholinergic medication, the more likely there is to be a measurable decline in performance on the Mini-Mental State Examination.24
Programs that flag potential interactions
Drug-drug interactions are a key concern of polypharmacy, and electronic medical records and prescribing systems that flag potential drug-drug interactions when a new medication is ordered are designed to help physicians avoid them. Unfortunately, clinicians only react to 3% to 9% of such notifications, overriding them because computerized systems often fail to distinguish between important and unimportant interactions.25-27 Thus, clinicians often must decide whether to react to or override warnings, an often difficult decision with patient safety and medicolegal implications. The best advice we can offer is to carefully evaluate drug interaction warnings using common sense, and seek consultation with a clinical pharmacist when uncertainty exists. This approach should prevent prescribing medications that have potentially harmful interactions with drugs the patient is already taking.
For physicians who do not have access to an electronic prescribing system that provides such notification, several online resources are available, some by subscription (eg, Lexicomp, www.lexi.com; Micromedex, www.micromedex.com/index.html; and Pepid, www.pepid.com) and others with free access (eg, AARP, healthtools.aarp.org/drug-interactions; Drugs.com (www.drugs.com/drug_interactions.php; and HealthLine, www.healthline.com/druginteractions).
CASE After doing a medication reconciliation for Mrs. R, you find that she is taking tolterodine, an anticholinergic medication for urge urinary incontinence, and donepezil, a procholinergic medication for dementia. This type of drug-drug interaction, in which the action of one drug effectively cancels out the effect of another, should not be ignored.
Overall, you identify 8 of her medications that could be discontinued: The list includes guaifenesin (a nonessential medication of questionable efficacy); naproxen (inappropriate per Beers criteria; inappropriate in patients with heart failure, according to STOPP); alprazolam, zolpidem, and diphenhydramine (duplicate medications that are all on the Beers criteria as inappropriate for chronic use and ill-advised in patients with cognitive impairment); and omeprazole and levothyroxine (for which nothing in the patient’s history suggests a need), as well as tolterodine. Depending on dose, digoxin is yet another candidate for discontinuation.
Discontinuing medications: Proceed carefully
Physicians are often reluctant to discontinue chronic medications in older patients—even in those with advanced disease who are not likely to benefit from treatment. Focus groups have identified a number of reasons for their hesitation, including:
- the assumption that patients have no problem taking large numbers of drugs
- the fear that patients may misinterpret a plan to discontinue medications as evidence that the physician is giving up on them
- the belief that physicians must comply with practice guidelines that recommend multiple drug treatments
- concern that proposing discontinuation of medications often leads to a discussion of life expectancy and end-of-life care.28
Physicians may also fear that discontinuation of certain drugs will increase the risk of adverse outcomes. More than 30 studies have evaluated discontinuation of chronic medications in older adults, however, and found that drugs as diverse as antihypertensives, antipsychotics, benzodiazepines, and selective serotonin reuptake inhibitors (SSRIs) can often be discontinued without adverse outcomes. In many cases, improvement in patient function results.29 Medications that present the most difficulty are those that patients often become physically or psychologically dependent on, such as benzodiazepines, guaifenesin, proton-pump inhibitors, nonsteroidal anti-inflammatory drugs, and SSRIs. Some (eg, benzodiazepines, SSRIs) require a gradual reduction; for others, no taper is required
(TABLE 4).30-37
TABLE 4
Recommendations for discontinuing hard-to-stop drugs
| Medication or drug class | Discontinuation regimen | Comments |
|---|---|---|
| Benzodiazepines30 | Taper dose by 25% q 2 wk | No withdrawal symptoms reported with this taper regimen. Subtle cognitive improvement noted over a period of months |
| Guaifenesin31 | Can be discontinued without tapering if not combined with opioids or other medications. Elimination half-life is approximately 1 hour | Guaifenesin is often marketed as a combination product with opioids; such combination products require tapering |
| PPIs32-34 | Decrease dose by 50% q 2 wk; supplement with H2 blocker if needed, but tapering of H2 blocker may be required | Abrupt discontinuation after long-term use causes rebound gastric acid hypersecretion and lowers rate of success. Higher success rates with taper regimen and in patients who do not have documented GERD |
| NSAIDs35 | No taper required | Short-term use (<3 mo) acceptable for patients with no contraindications |
| SSRIs36,37 | Gradual reduction in dose over 6-8 wk | Highest rate of success in patients without a clear diagnosis of depression |
| GERD, gastroesophageal reflux disease; NSAIDs, nonsteroidal anti-inflammatory drugs; PPIs, proton-pump inhibitors; SSRIs, selective serotonin reuptake inhibitors. | ||
CASE You trim down Mrs. R’s regimen by discontinuing each of the 8 drugs, one at a time, and carefully monitor the patient during the withdrawal period. Because she had been taking alprazolam daily, the dose is tapered slowly to avoid withdrawal. Omeprazole also requires a gradual taper to avoid rebound hyperacidity.3
After confirming that Mrs. R has heart failure and COPD, you identify 2 medications that should be added to her drug regimen—an ACE inhibitor for heart failure and an inhaled anticholinergic for COPD.
Going from 16 medications to 10 saves money, decreases the likelihood of adverse events and drug-drug interactions, and helps with adherence. Mrs. R’s new drug regimen is expected to lead to improvements in memory and overall quality of life, as well.
CORRESPONDENCE
Barry D. Weiss, MD, Department of Family and Community Medicine, University of Arizona College of Medicine, Tucson, AZ 85724; bdweiss@email.arizona.edu
1. Gu Q, Dillon CF, Burt V. Prescription drug use continued to increase: US prescription drug data for 2007-2008. CDC/NCHS Data Brief. 2010;42:1-2.
2. Jessup K, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;119:1977-2016.
3. Johnell K, Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30:911-918.
4. Sarkar U, Lopez A, Maselli JH, et al. Adverse drug events in US adult ambulatory medical care. Health Services Res. 2011;46:1517-1533.
5. Rollason V, Vogt N. Reduction of polypharmacy in the elderly. A systematic review of the role of the pharmacist. Drugs Aging. 2003;20:817-832.
6. National Heart, Lung, and Blood Institute. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Available at: www.nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf. Accessed October 11, 2012.
7. Steinman MA, Hanlon JT. Managing medications in clinically complex elders. JAMA. 2010;304:1592-1601.
8. Steinman MA, Landefeld CS, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54:1516-23.
9. Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuations of medications for chronic disease. JAMA. 2011;306:840-847.
10. Moore C, Wisnivesky J, Williams S, et al. Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med. 2003;18:646-651.
11. Ziaeian B, Arauho KL, Van Ness PH, et al. Medication reconciliation accuracy and patient understanding of intended medication changes on hospital discharge. J Gen Intern Med. 2012 July 12. ePub ahead of print.
12. Hajjar ER, Hanlon JT, Sloane RJ, et al. Unnecessary drug use in frail older people at hospital discharge. J Am Geriatr Soc. 2005;53:1518-1523.
13. O’Mahony D, Gallagher P, Ryan C, et al. STOPP & START criteria: a new approach to detecting potentially inappropriate prescribing in old age. Eur Geriatr Med. 2010;1:45-51.
14. Denneboom W, Dautzenberg KGH, Grol R, et al. Analysis of polypharmacy in older patients in primary acre using a multidisciplinary expert panel. Br J Gen Pract. 2006;56:504-510.
15. Ko DT, Mamdani M, Alter DA. Lipid-lowering therapy with statins in high-risk elderly patients. JAMA. 2004;291:1864-70.
16. Wright RM, Sloane R, Pieper CF, et al. Underuse of indicated medications among physically frail older US veterans at the time of hospital discharge: results of a cross-sectional analysis of data from the Geriatric Evaluation and Management Drug Study. Am J Geriatr Pharmacother. 2009;7:271-280.
17. Garwood CL. Use of anticoagulation in elderly patients with atrial fibrillation who are risk for falls. Ann Pharmacother. 2008;42:523-532.
18. Holmes HM, Hayley DC, Alexander GC, et al. Reconsidering medication appropriateness for patients late in life. Arch Intern Med. 2006;166:605-609.
19. Simplified Methods for Estimating Life Expectancy. Available at: http://painconsortium.nih.gov/symptomresearch/chapter_14/Part_3/sec4/chspt3s4pg1.htm. Accessed October 9, 2012.
20. Beers MH, Ouslander JG, Rollingher I, et al. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med. 1991;151:1825-1832.
21. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society Update Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616-631.
22. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers’ criteria. Age Aging. 2008;37:673-379.
23. Han L, Agostini JV, Allore HG. Cumulative anticholinergic exposure is associated with poor memory and executive function in older men. J Am Geriatr Soc. 2008;56:2203-2210.
24. Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59:1477-1483.
25. Knight A, Falade O, Maygers J, et al. Factors associated with medication warning acceptance [abstract]. J Hosp Med. 2012;7(suppl 2):515.-
26. Isaac T, Weissman JS, Davis RB, et al. Overrides of medication alerts in ambulatory care. Arch Intern Med. 2009;169:305-311.
27. Van Der Sijs H, Aarts J, Vulto A, et al. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;12:138-147.
28. Schuling J, Gebben H, Veehof LJG, et al. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Family Practice. 2012;13:56. http://www.biomedcentral.com/1471-2296/13/56.
29. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older. A systematic review. Drugs Aging. 2008;25:1021-1031.
30. Curran HV, Collins R, Fletcher S, et al. Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med. 2003;33:1223-1237.
31. Krinsky DL, Berardi RR, Ferreris SP, et al. Handbook of Nonprescription Drugs: An Interactive Approach to Self-Care. Washington, DC: American Pharmacists Association; 2012:209.
32. Bjornsson E, Abrahamsson H, Simren M, et al. Discontinuation of proton pump inhibitors in patients on long-term therapy: a double-blind, placebo-controlled trial. Aliment Pharmacol Ther. 2006;24:945-954.
33. Inadomi JM, Jamai R, Murata GH, et al. Step-down management of gastroesophageal reflux disease. Gastroenterology. 2001;131:1095-1100.
34. Hester SA. Proton pump inhibitors and rebound acid hypersecretion. Pharm Lett. 2009;25:250920.-
35. Taylor R, Jr, Lemtouni S, Weiss K, et al. Pain management in the elderly: an FDA safe use initiative expert panel’s view on preventable harm associated with NSAID therapy. Curr Gerontol Geriatr Res. 2012;196159.-
36. Ulfvarson J, Adami J, Wredling R, et al. Controlled withdrawal of selective serotonin reuptake inhibitor drugs in elderly patients in nursing homes with no indication of depression. Eur J Clin Pharmacol. 2003;59:735-740.
37. Lindstrom K, Ekedahl A, Carlsten A, et al. Can selective serotonin inhibitor drugs in elderly patients in nursing homes be reduced? Scand J Prim Health Care. 2007;25:3-8.
1. Gu Q, Dillon CF, Burt V. Prescription drug use continued to increase: US prescription drug data for 2007-2008. CDC/NCHS Data Brief. 2010;42:1-2.
2. Jessup K, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;119:1977-2016.
3. Johnell K, Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30:911-918.
4. Sarkar U, Lopez A, Maselli JH, et al. Adverse drug events in US adult ambulatory medical care. Health Services Res. 2011;46:1517-1533.
5. Rollason V, Vogt N. Reduction of polypharmacy in the elderly. A systematic review of the role of the pharmacist. Drugs Aging. 2003;20:817-832.
6. National Heart, Lung, and Blood Institute. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Available at: www.nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf. Accessed October 11, 2012.
7. Steinman MA, Hanlon JT. Managing medications in clinically complex elders. JAMA. 2010;304:1592-1601.
8. Steinman MA, Landefeld CS, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54:1516-23.
9. Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuations of medications for chronic disease. JAMA. 2011;306:840-847.
10. Moore C, Wisnivesky J, Williams S, et al. Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med. 2003;18:646-651.
11. Ziaeian B, Arauho KL, Van Ness PH, et al. Medication reconciliation accuracy and patient understanding of intended medication changes on hospital discharge. J Gen Intern Med. 2012 July 12. ePub ahead of print.
12. Hajjar ER, Hanlon JT, Sloane RJ, et al. Unnecessary drug use in frail older people at hospital discharge. J Am Geriatr Soc. 2005;53:1518-1523.
13. O’Mahony D, Gallagher P, Ryan C, et al. STOPP & START criteria: a new approach to detecting potentially inappropriate prescribing in old age. Eur Geriatr Med. 2010;1:45-51.
14. Denneboom W, Dautzenberg KGH, Grol R, et al. Analysis of polypharmacy in older patients in primary acre using a multidisciplinary expert panel. Br J Gen Pract. 2006;56:504-510.
15. Ko DT, Mamdani M, Alter DA. Lipid-lowering therapy with statins in high-risk elderly patients. JAMA. 2004;291:1864-70.
16. Wright RM, Sloane R, Pieper CF, et al. Underuse of indicated medications among physically frail older US veterans at the time of hospital discharge: results of a cross-sectional analysis of data from the Geriatric Evaluation and Management Drug Study. Am J Geriatr Pharmacother. 2009;7:271-280.
17. Garwood CL. Use of anticoagulation in elderly patients with atrial fibrillation who are risk for falls. Ann Pharmacother. 2008;42:523-532.
18. Holmes HM, Hayley DC, Alexander GC, et al. Reconsidering medication appropriateness for patients late in life. Arch Intern Med. 2006;166:605-609.
19. Simplified Methods for Estimating Life Expectancy. Available at: http://painconsortium.nih.gov/symptomresearch/chapter_14/Part_3/sec4/chspt3s4pg1.htm. Accessed October 9, 2012.
20. Beers MH, Ouslander JG, Rollingher I, et al. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med. 1991;151:1825-1832.
21. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society Update Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616-631.
22. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers’ criteria. Age Aging. 2008;37:673-379.
23. Han L, Agostini JV, Allore HG. Cumulative anticholinergic exposure is associated with poor memory and executive function in older men. J Am Geriatr Soc. 2008;56:2203-2210.
24. Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59:1477-1483.
25. Knight A, Falade O, Maygers J, et al. Factors associated with medication warning acceptance [abstract]. J Hosp Med. 2012;7(suppl 2):515.-
26. Isaac T, Weissman JS, Davis RB, et al. Overrides of medication alerts in ambulatory care. Arch Intern Med. 2009;169:305-311.
27. Van Der Sijs H, Aarts J, Vulto A, et al. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;12:138-147.
28. Schuling J, Gebben H, Veehof LJG, et al. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Family Practice. 2012;13:56. http://www.biomedcentral.com/1471-2296/13/56.
29. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older. A systematic review. Drugs Aging. 2008;25:1021-1031.
30. Curran HV, Collins R, Fletcher S, et al. Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med. 2003;33:1223-1237.
31. Krinsky DL, Berardi RR, Ferreris SP, et al. Handbook of Nonprescription Drugs: An Interactive Approach to Self-Care. Washington, DC: American Pharmacists Association; 2012:209.
32. Bjornsson E, Abrahamsson H, Simren M, et al. Discontinuation of proton pump inhibitors in patients on long-term therapy: a double-blind, placebo-controlled trial. Aliment Pharmacol Ther. 2006;24:945-954.
33. Inadomi JM, Jamai R, Murata GH, et al. Step-down management of gastroesophageal reflux disease. Gastroenterology. 2001;131:1095-1100.
34. Hester SA. Proton pump inhibitors and rebound acid hypersecretion. Pharm Lett. 2009;25:250920.-
35. Taylor R, Jr, Lemtouni S, Weiss K, et al. Pain management in the elderly: an FDA safe use initiative expert panel’s view on preventable harm associated with NSAID therapy. Curr Gerontol Geriatr Res. 2012;196159.-
36. Ulfvarson J, Adami J, Wredling R, et al. Controlled withdrawal of selective serotonin reuptake inhibitor drugs in elderly patients in nursing homes with no indication of depression. Eur J Clin Pharmacol. 2003;59:735-740.
37. Lindstrom K, Ekedahl A, Carlsten A, et al. Can selective serotonin inhibitor drugs in elderly patients in nursing homes be reduced? Scand J Prim Health Care. 2007;25:3-8.
What’s new in type 2 diabetes?
In April 2012, the American Diabetes Association (ADA) updated its guidelines for evaluating and treating type 2 diabetes mellitus (T2DM). In particular, the ADA acknowledges the value of an individualized, patient-centered approach that is less formulaic than its earlier guidelines. In this article, we highlight these and other recently published developments in the context of a case study. To help ensure follow-through on these newest recommendations, we also frame our review with the mnemonic, “ABCD IS diabetes.”
CASE JR is a 57-year-old man being seen for a regular follow-up appointment. His medical history includes T2DM, hypertension, and obesity. He is taking metformin 1000 mg twice daily, lisinopril 40 mg each morning, and amlodipine 10 mg each morning. He is current on his influenza and pneumococcal vaccinations. He does not smoke cigarettes. His physical exam and lab results reveal the following:
- blood pressure (BP), 132/70 mm Hg
- body mass index (BMI), 33 kg/m2
- glycosylated hemoglobin (A1C), 7.6%
- lipid profile: Total cholesterol, 185 mg/dL; high-density lipoprotein (HDL), 40 mg/dL; triglycerides (TG), 145 mg/dL; low-density lipoprotein (LDL), 90 mg/dL
Applying the “ABCD IS diabetes” mnemonic leads us through the following assessments.
Antiplatelets
In the past, guidelines have recommended that most patients with diabetes be placed on aspirin therapy. However, 2 trials published in 2008 failed to demonstrate significant reduction in cardiovascular disease (CVD) end points with aspirin use, raising questions about its effectiveness for primary CVD prevention in patients with diabetes.1,2 In 2010, the ADA, American Heart Association, and American College of Cardiology Foundation modified their recommendations for primary prevention,3 which remain unchanged in the 2012 ADA guidelines.4
Antiplatelet agents continue to play a role in primary prevention of CVD for patients with T2DM, but only after appropriate risk stratification.4 Consider low-dose aspirin therapy (75-162 mg/d) for patients with diabetes who have a 10-year Framingham risk >10%.4 (To calculate a patient’s 10-year risk, go to http://hp2010.nhlbihin.net/atpiii/calculator.asp.)
Many patients with T2DM seen in the primary care setting will reach this risk level and qualify for aspirin—in particular, men older than 50 years and women older than 60 with a family history of CVD, hypertension, smoking, dyslipidemia, or albuminuria.4 Aspirin therapy is not recommended for primary prevention in adults with diabetes at low risk for CVD (10-year Framingham risk <5%)—eg, men <50 and women <60 years without additional CVD risk factors.4 For patients with a 10-year Framingham risk between 5% and 10%, a decision to treat rests with the physician.
CASE Should JR be started on aspirin therapy for primary prevention of CVD? Initiating low-dose aspirin is recommended, assuming no contraindications, because his 10-year Framingham risk assessment is 11%.
A (antiplatelets): Consider low-dose aspirin therapy (75-162 mg/d) for diabetes patients with a 10-year Framingham risk >10%.
B (blood pressure): Individualize a patient’s goal for systolic blood pressure, aiming higher or lower than the customary systolic target of <130 mm Hg, as appropriate.
C (cholesterol): Recommend lifestyle changes and prescribe a statin, as needed, to achieve LDL goals in T2DM patients.
D (drug management): Use a patient-centered approach to achieve an individualized A1C goal. Metformin is the initial medication of choice. Select additional drug classes to balance adverse effects, cost, and effectiveness.
I (immunizations): Ensure that each T2DM patient receives influenza and pneumococcal vaccines, and the hepatitis B vaccine if <60 years.
S (surveillance): Confirm at each visit that annual surveillance testing for nephropathy, retinopathy, and peripheral neuropathy has been completed.
Blood pressure
The benefits of lowering BP in diabetes to <140 mm Hg systolic and <80 mm Hg diastolic have been established in randomized control trials.5-8 However, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial demonstrated that, in patients with T2DM, intensive BP lowering to <120 mm Hg systolic yielded no significant differences in fatal and nonfatal cardiovascular events compared with BP maintained between 130 and 140 mm Hg.9 Moreover, aggressive BP lowering may be associated with serious adverse events.10 The 2012 ADA guidelines state that a systolic BP goal of <130 mm Hg is appropriate for most patients; however, higher or lower BP targets may be individualized.4
Recommendations for adding a second antihypertensive agent and timing medication administration. For T2DM patients with hypertension, the 2012 guidelines recommend that you treat initially with either an angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB), if tolerated.4 When adding a second agent, the Avoiding Cardiovascular Events through COMbination therapy in Patients LIving with Systolic Hypertension (ACCOMPLISH) trial demonstrated reduced morbidity and mortality in patients receiving benazepril and amlodipine compared with those receiving benazepril and hydrochlorothiazide.11 As a result, amlodipine has joined diuretics as a preferred second oral antihypertensive agent after an ACEI or ARB.
If a patient is taking multiple BP medications, one or more should be taken at bedtime.4 Administering an antihypertensive at night results in better ambulatory BP control and reduces cardiovascular mortality.12
CASE JR is on maximal doses of 2 antihypertensive agents, and his BP is 132/70 mm Hg. His physician must individualize care and decide if adding a third agent is worth the risk of another medication when clear benefit has not been demonstrated. It is reasonable to continue his current regimen with the exception of changing his lisinopril dose to the evening and reassessing his BP control at his next visit.
Cholesterol
Controlling LDL remains the top priority of T2DM lipid management. In addition to lifestyle changes, statins are the primary means of achieving LDL goals. All patients with overt CVD should receive a statin.4 Also prescribe a statin for patients with diabetes who do not have CVD but who are older than 40 and have one or more cardiac risk factors, regardless of their baseline LDL cholesterol.4 The recommended LDL goal in T2DM patients continues to be <100 mg/dL. However, <70 mg/dL is a reasonable goal for those with known CVD.13
Using additional lipid-lowering agents besides a statin may improve cholesterol numbers, but not CVD outcomes. In the ACCORD study, adding fenofibrate to simvastatin did not decrease fatal cardiovascular events or nonfatal myocardial infarction and stroke compared with simvastatin given alone.14 The AIM-High study showed no difference in cardiovascular outcomes and a possible increase in ischemic stroke with combination niacin and statin compared with statin therapy alone.15 For now, lifestyle changes and statins remain the ideal modalities to achieve LDL goals.
CASE Should a statin be initiated for our patient? Since JR is over 40 without known CVD and has a cardiac risk factor of hypertension, he should be started on statin therapy regardless of his baseline LDL (90 mg/dL), which is already at goal (<100 mg/dL).
Drug management
Let the glycemic goal for each patient guide your medication management. The 2012 ADA recommendation for most adults is an A1C of <7%.4 More strict control (A1C <6.5%) may be appropriate for certain individuals with a long life expectancy, short duration of diabetes, and no significant micro- or macrovascular disease.4 Less strict control (A1C <8%) may be appropriate for individuals with significant comorbidities, shorter life expectancy, severe hypoglycemia, or long-standing T2DM that’s been difficult to control despite multiple medications, including insulin.4
Individualize treatment. In April 2012, the ADA released a position statement encouraging a patient-centered approach to managing hyperglycemia in T2DM.16 This statement contains a new treatment algorithm (available at: http://care.diabetesjournals.org/content/early/2012/04/17/dc12-0413.full.pdf+html) that is less prescriptive than the previous 2009 algorithm and balances provider judgment, patient preference, and susceptibility to adverse effects in order to attain an individualized A1C target.16 Although a comprehensive review of T2DM pharmacotherapy is beyond the scope of this article, we will discuss the importance of metformin, familiarize prescribers with incretin-based therapy, and highlight recent safety concerns regarding thiazolidinediones (TZDs).
Metformin is first line. The 2012 ADA guidelines recommend prescribing metformin at the time of diagnosis of T2DM, in addition to advising lifestyle changes.4,16 The American College of Physicians (ACP) also recommends metformin as the first agent in diabetes management, citing the benefits of weight loss, improved lipid profiles, and decreased cardiovascular mortality.17 Adding a second medication to metformin at the time of diagnosis may be considered if the initial A1C value is >9%.16 Because robust comparative trials are lacking, the selection of additional medications beyond metformin depends on a patient-centered approach, with consideration of efficacy, adverse effect profile, and cost.16 The TABLE provides a succinct review of the key properties of diabetic medications that clinicians may discuss with their patients. All of the listed agents are valid second-line treatments, and you should select one based on the individual’s needs.
Incretin-based therapy. Among newer antihyperglycemic agents, incretins have drawn much attention and thus warrant special focus. The emphasis on these agents should not be interpreted as an implied endorsement for their second-line use. There are 2 main classes: dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 (GLP-1) agonists. Both act on the gut peptide GLP-1 to enhance glucose-stimulated insulin secretion and glucagon suppression.16
DPP-4 inhibitors promote the effects of endogenous GLP-1 by inhibiting its breakdown by the enzyme DPP-4. By increasing GLP-1, these agents achieve mild glucose lowering while remaining weight neutral.16 DPP-4 inhibitors can be combined with metformin and other oral agents and are not associated with hypoglycemia.16
Injectable GLP-1 receptor agonists provide supraphysiologic levels of GLP-1, resulting in increased insulin secretion, reduced glucagon secretion, delayed gastric emptying, increased satiety, and weight loss.16 Research has shown that exenatide can decrease mean weight by 7 kg over 2.4 years.18,19 Exenatide is dosed subcutaneously twice daily, while liraglutide is administered once daily. Once-weekly exenatide was approved by the US Food and Drug Administration (FDA) in February 2012. A recent study showed once-weekly exenatide lowered A1C levels, reduced weight, and caused fewer episodes of hypoglycemia compared with adding insulin glargine to the regimen when diabetes was uncontrolled on metformin (with or without a sulfonylurea).20 Patients may experience nausea, vomiting, and diarrhea at the onset of use of GLP-1 agonists.21 Slow titration and forewarning the patient of these adverse effects will help with compliance.
In October 2011, the FDA approved the use of exenatide with basal insulin. For patients already taking basal insulin with or without metformin or pioglitazone, adding exenatide resulted in improved A1C values and weight loss over a 30-week period.22 Reducing the dose of basal insulin at the initiation of exenatide helps decrease the incidence of hypoglycemia when considering this combination.22 Basal insulin lowers fasting glucose levels, while exenatide reduces postprandial glucose.
Although gaining in popularity, incretin therapy is being monitored for long-term safety. Cases of pancreatitis have been reported in both classes of medicines.4 Liraglutide has been associated with medullary thyroid cancer (MTC) in rodents.23,24 The FDA has recommended against using liraglutide and extended-release exenatide in patients with a personal or family history of MTC.16 Although the long-term safety of GLP-1 agonists and DPP-4 inhibitors is unknown, their novel mechanisms of action can prove useful for the right patient.
Concerns over TZDs. In addition to the FDA recommendation to avoid TZDs in patients with symptomatic heart failure, 2 studies have recently found that pioglitazone may be associated with an increased risk of bladder cancer.25,26 The FDA recommends avoiding use of pioglitazone in patients with active bladder cancer, and that it should be used with caution in patients with a history of cured bladder cancer. The European Medicines Agency also recommends against pioglitazone use in patients with uninvestigated macroscopic hematuria.27 The potential association between pioglitazone and bladder cancer requires further study. At this point, TZDs remain a valid second- or third-line treatment option in patients only after they are made aware of the potential risks and benefits.
CASE JR’s A1C of 7.6% is above his individualized goal of 7%. He feels he has maximized his efforts in the realm of lifestyle changes and is interested in another medication. Using the recommended patient-centered approach, we discuss with him the risks and benefits of each medication in the TABLE and we select the medication best suited to him based on adverse-effect profile.
TABLE
Matching diabetic medication attributes to patient needs
| Class | Medications | Actions | Benefits | Possible adverse effects and disadvantages | A1C-lowering (%) | Cost* |
|---|---|---|---|---|---|---|
| Biguanides | Metformin | ↓ Hepatic glucose production | Weight neutral or loss No hypoglycemia ↓ CV mortality | GI side effects Lactic acidosis Impaired B12 absorption Use caution or avoid in renal dysfunction | 1-2 | $ |
| Sulfonylureas | Gliclazide Glimepiride Glipizide Glyburide | ↑ Insulin secretion | Fast-onset glucose lowering | Hypoglycemia Lack of durable glycemic control Weight gain | 1-2 | $ |
| Meglitinides | Repaglinide Nateglinide | ↑ Insulin secretion | Improve meal-related insulin release and postprandial glucose | Hypoglycemia Weight gain | 0.1-2.1 | $$-$$$ |
| Thiazolidinediones | Pioglitazone | ↑ Insulin sensitivity | No hypoglycemia ↑ HDL ↓ Triglycerides | Bladder cancer concerns Edema Fracture risk Heart failure Weight gain | 0.5-1.4 | $$$ |
| GLP-1 receptor agonists | Exenatide Liraglutide | ↑ Insulin secretion ↓ Glucagon secretion Delayed gastric emptying Early satiety | Possible beta-cell preservation Weight loss | GI (nausea, vomiting, diarrhea) Injectable Medullary thyroid tumors in rodents Pancreatitis | 0.5-1.5 | $$$ |
| DPP-4 inhibitors | Linagliptin Saxagliptin Sitagliptin Vildagliptin | ↓ Glucagon secretion ↑ Insulin secretion | No hypoglycemia Weight neutral | Angioedema Pancreatitis | 0.5-0.8 | $$$ |
| Alpha-glucosidase inhibitors | Acarbose Miglitol | Delays carbohydrate absorption | Nonsystemic medication Reduces postprandial glucose | Frequent dosing GI side effects (abdominal cramping, flatulence) | 0.5-0.8 | $$ |
| Insulin | Aspart Detemir Glargine Lispro NPH Regular | Replaces endogenous insulin | Mimics physiology Rapidly effective | Hypoglycemia Weight gain | 1.5-3.5 | $-$$$ |
| CV, cardiovascular; DPP, dipeptidyl peptidase; GI, gastrointestinal; GLP, glucagon-like peptide, HDL, high-density lipoprotein. *Monthly cost of an average daily maintenance dose of available products: $, <$50; $$, $50.01-$100; $$$, >$100. Source: www.drugstore.com; accessed October 10, 2012. Adapted from: Reid TS. Options for intensifying diabetes treatment. J Fam Pract. 2011;9(suppl 1):S7-S10; American Diabetes Association Position Statement. Standards of Medical Care in Type 2 Diabetes-2012. Diabetes Care. 2012;35(suppl 1):S11-S63. | ||||||
Immunizations
An often overlooked but important part of the diabetes visit is reviewing the patient’s immunization history. Unless there are contraindications, all individuals with diabetes should receive the pneumococcal and annual influenza vaccines.4 In addition, the Advisory Committee on Immunization Practices now recommends hepatitis B virus (HBV) vaccine for unvaccinated adults with diabetes from ages 19 to 59.28 Unvaccinated adults with diabetes over age 60 should be vaccinated at the discretion of the provider after risk assessment.28 Patients may be at risk of contracting HBV in long-term care facilities where assisted blood sugar monitoring commonly occurs.28 Studies have shown that patients with diabetes may progress to chronic hepatitis B infection more often than patients without diabetes, and are at higher risk for nonalcoholic liver disease and hepatocellular carcinoma.29
CASE JR’s history shows that he is current on his influenza and pneumococcal vaccines. However, he doesn’t recall whether he’s been vaccinated against HBV. Serum testing reveals no previous immunization, and recommending HBV vaccine is appropriate.
Surveillance
The 2012 ADA recommendations do not include any new surveillance practices for microvascular disease. Providers should continue to offer the following screening to T2DM patients annually: urine albumin excretion testing and serum creatinine to assess for nephropathy, a comprehensive dilated eye exam to assess for retinopathy, and a foot exam to assess for distal symmetric polyneuropathy.4
CASE Each of these tests were performed (or ordered) for JR. We’ll see him again in 2 to 3 months for diabetes follow-up.
Acknowledgement
The authors thank Pamela Williams, MD, and Brent Smith, MD, for their guidance in the preparation of this article.
CORRESPONDENCE Jason C. McCarthy, MD, 101 Bodin Circle, Travis Air Force Base, CA 94535; Jason.McCarthy.6@us.af.mil
1. Ogawa H, Nakayama M, Morimoto T, et al. Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300:2134-2141.
2. Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840.-
3. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395-1402.
4. American Diabetes Association. Standards of medical care in diabetes–2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
5. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
6. Turner R, Holman R, Stratton I, et al. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703-713.
7. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755-1762.
8. Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412-419.
9. Cushman WC, Evans GW, Byington RP, et al. ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
10. Bangalore S, Kumar S, Lobach I, et al. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and Bayesian random-effects meta-analyses of randomized trials. Circulation. 2011;123:2799-2810.
11. Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417-2428.
12. Hermida R, Ayala D, Mojon A, et al. Influence of time of day of blood-pressure lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2001;34:1270-1276.
13. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227-239.
14. Cushman W, Evans G, Byington R, et al. The ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
15. Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
16. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379.
17. Qaseem A, Humphrey L, Sweet D, et al. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2012;156:218-231.
18. Varanasi A, Chaudhuri A, Dhindsa S, et al. Durability of effects of exenatide treatment on glycemic control, body weight, systolic blood pressure, C-reactive protein, and triglyceride concentrations. Endocr Pract. 2011;17:192-200.
19. Vilsboll T, Christensen M, Junker A, et al. Effects of glucagon-like peptide-1 receptor agonist on weight loss: systemic review and meta-analyses of randomized controlled trials. BMJ. 2012;344:d7771.-
20. Diamant M, Van Gaal L, Stranks S, et al. Safety and efficacy of once-weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes over 84 weeks. Diabetes Care. 2012;35:683-689.
21. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39-47.
22. Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized controlled trial. Ann Intern Med. 2011;154:103-112.
23. Drucker DJ, Sherman SI, Bergenstal RM, et al. The safety of incretin-based therapies—review of the scientific evidence. J Clin Endocrinol Metab. 2011;96:2027-2031.
24. Elashoff M, Matveyenko A, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141:150-156.
25. Piccinni C, Motola D, Marchesini G, et al. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care. 2011;34:1369-1371.
26. Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922.
27. European Medicines Agency. European Medicines Agency recommends new contra-indications and warnings for pioglitazone to reduce small increased risk of bladder cancer, updated July 7, 2011. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2011/07. Accessed March 22, 2012.
28. Sawyer M, Hoerger T. Use of hepatitis B vaccination for adults with diabetes mellitus: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2011;60:1709-1711.
29. El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-468.
In April 2012, the American Diabetes Association (ADA) updated its guidelines for evaluating and treating type 2 diabetes mellitus (T2DM). In particular, the ADA acknowledges the value of an individualized, patient-centered approach that is less formulaic than its earlier guidelines. In this article, we highlight these and other recently published developments in the context of a case study. To help ensure follow-through on these newest recommendations, we also frame our review with the mnemonic, “ABCD IS diabetes.”
CASE JR is a 57-year-old man being seen for a regular follow-up appointment. His medical history includes T2DM, hypertension, and obesity. He is taking metformin 1000 mg twice daily, lisinopril 40 mg each morning, and amlodipine 10 mg each morning. He is current on his influenza and pneumococcal vaccinations. He does not smoke cigarettes. His physical exam and lab results reveal the following:
- blood pressure (BP), 132/70 mm Hg
- body mass index (BMI), 33 kg/m2
- glycosylated hemoglobin (A1C), 7.6%
- lipid profile: Total cholesterol, 185 mg/dL; high-density lipoprotein (HDL), 40 mg/dL; triglycerides (TG), 145 mg/dL; low-density lipoprotein (LDL), 90 mg/dL
Applying the “ABCD IS diabetes” mnemonic leads us through the following assessments.
Antiplatelets
In the past, guidelines have recommended that most patients with diabetes be placed on aspirin therapy. However, 2 trials published in 2008 failed to demonstrate significant reduction in cardiovascular disease (CVD) end points with aspirin use, raising questions about its effectiveness for primary CVD prevention in patients with diabetes.1,2 In 2010, the ADA, American Heart Association, and American College of Cardiology Foundation modified their recommendations for primary prevention,3 which remain unchanged in the 2012 ADA guidelines.4
Antiplatelet agents continue to play a role in primary prevention of CVD for patients with T2DM, but only after appropriate risk stratification.4 Consider low-dose aspirin therapy (75-162 mg/d) for patients with diabetes who have a 10-year Framingham risk >10%.4 (To calculate a patient’s 10-year risk, go to http://hp2010.nhlbihin.net/atpiii/calculator.asp.)
Many patients with T2DM seen in the primary care setting will reach this risk level and qualify for aspirin—in particular, men older than 50 years and women older than 60 with a family history of CVD, hypertension, smoking, dyslipidemia, or albuminuria.4 Aspirin therapy is not recommended for primary prevention in adults with diabetes at low risk for CVD (10-year Framingham risk <5%)—eg, men <50 and women <60 years without additional CVD risk factors.4 For patients with a 10-year Framingham risk between 5% and 10%, a decision to treat rests with the physician.
CASE Should JR be started on aspirin therapy for primary prevention of CVD? Initiating low-dose aspirin is recommended, assuming no contraindications, because his 10-year Framingham risk assessment is 11%.
A (antiplatelets): Consider low-dose aspirin therapy (75-162 mg/d) for diabetes patients with a 10-year Framingham risk >10%.
B (blood pressure): Individualize a patient’s goal for systolic blood pressure, aiming higher or lower than the customary systolic target of <130 mm Hg, as appropriate.
C (cholesterol): Recommend lifestyle changes and prescribe a statin, as needed, to achieve LDL goals in T2DM patients.
D (drug management): Use a patient-centered approach to achieve an individualized A1C goal. Metformin is the initial medication of choice. Select additional drug classes to balance adverse effects, cost, and effectiveness.
I (immunizations): Ensure that each T2DM patient receives influenza and pneumococcal vaccines, and the hepatitis B vaccine if <60 years.
S (surveillance): Confirm at each visit that annual surveillance testing for nephropathy, retinopathy, and peripheral neuropathy has been completed.
Blood pressure
The benefits of lowering BP in diabetes to <140 mm Hg systolic and <80 mm Hg diastolic have been established in randomized control trials.5-8 However, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial demonstrated that, in patients with T2DM, intensive BP lowering to <120 mm Hg systolic yielded no significant differences in fatal and nonfatal cardiovascular events compared with BP maintained between 130 and 140 mm Hg.9 Moreover, aggressive BP lowering may be associated with serious adverse events.10 The 2012 ADA guidelines state that a systolic BP goal of <130 mm Hg is appropriate for most patients; however, higher or lower BP targets may be individualized.4
Recommendations for adding a second antihypertensive agent and timing medication administration. For T2DM patients with hypertension, the 2012 guidelines recommend that you treat initially with either an angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB), if tolerated.4 When adding a second agent, the Avoiding Cardiovascular Events through COMbination therapy in Patients LIving with Systolic Hypertension (ACCOMPLISH) trial demonstrated reduced morbidity and mortality in patients receiving benazepril and amlodipine compared with those receiving benazepril and hydrochlorothiazide.11 As a result, amlodipine has joined diuretics as a preferred second oral antihypertensive agent after an ACEI or ARB.
If a patient is taking multiple BP medications, one or more should be taken at bedtime.4 Administering an antihypertensive at night results in better ambulatory BP control and reduces cardiovascular mortality.12
CASE JR is on maximal doses of 2 antihypertensive agents, and his BP is 132/70 mm Hg. His physician must individualize care and decide if adding a third agent is worth the risk of another medication when clear benefit has not been demonstrated. It is reasonable to continue his current regimen with the exception of changing his lisinopril dose to the evening and reassessing his BP control at his next visit.
Cholesterol
Controlling LDL remains the top priority of T2DM lipid management. In addition to lifestyle changes, statins are the primary means of achieving LDL goals. All patients with overt CVD should receive a statin.4 Also prescribe a statin for patients with diabetes who do not have CVD but who are older than 40 and have one or more cardiac risk factors, regardless of their baseline LDL cholesterol.4 The recommended LDL goal in T2DM patients continues to be <100 mg/dL. However, <70 mg/dL is a reasonable goal for those with known CVD.13
Using additional lipid-lowering agents besides a statin may improve cholesterol numbers, but not CVD outcomes. In the ACCORD study, adding fenofibrate to simvastatin did not decrease fatal cardiovascular events or nonfatal myocardial infarction and stroke compared with simvastatin given alone.14 The AIM-High study showed no difference in cardiovascular outcomes and a possible increase in ischemic stroke with combination niacin and statin compared with statin therapy alone.15 For now, lifestyle changes and statins remain the ideal modalities to achieve LDL goals.
CASE Should a statin be initiated for our patient? Since JR is over 40 without known CVD and has a cardiac risk factor of hypertension, he should be started on statin therapy regardless of his baseline LDL (90 mg/dL), which is already at goal (<100 mg/dL).
Drug management
Let the glycemic goal for each patient guide your medication management. The 2012 ADA recommendation for most adults is an A1C of <7%.4 More strict control (A1C <6.5%) may be appropriate for certain individuals with a long life expectancy, short duration of diabetes, and no significant micro- or macrovascular disease.4 Less strict control (A1C <8%) may be appropriate for individuals with significant comorbidities, shorter life expectancy, severe hypoglycemia, or long-standing T2DM that’s been difficult to control despite multiple medications, including insulin.4
Individualize treatment. In April 2012, the ADA released a position statement encouraging a patient-centered approach to managing hyperglycemia in T2DM.16 This statement contains a new treatment algorithm (available at: http://care.diabetesjournals.org/content/early/2012/04/17/dc12-0413.full.pdf+html) that is less prescriptive than the previous 2009 algorithm and balances provider judgment, patient preference, and susceptibility to adverse effects in order to attain an individualized A1C target.16 Although a comprehensive review of T2DM pharmacotherapy is beyond the scope of this article, we will discuss the importance of metformin, familiarize prescribers with incretin-based therapy, and highlight recent safety concerns regarding thiazolidinediones (TZDs).
Metformin is first line. The 2012 ADA guidelines recommend prescribing metformin at the time of diagnosis of T2DM, in addition to advising lifestyle changes.4,16 The American College of Physicians (ACP) also recommends metformin as the first agent in diabetes management, citing the benefits of weight loss, improved lipid profiles, and decreased cardiovascular mortality.17 Adding a second medication to metformin at the time of diagnosis may be considered if the initial A1C value is >9%.16 Because robust comparative trials are lacking, the selection of additional medications beyond metformin depends on a patient-centered approach, with consideration of efficacy, adverse effect profile, and cost.16 The TABLE provides a succinct review of the key properties of diabetic medications that clinicians may discuss with their patients. All of the listed agents are valid second-line treatments, and you should select one based on the individual’s needs.
Incretin-based therapy. Among newer antihyperglycemic agents, incretins have drawn much attention and thus warrant special focus. The emphasis on these agents should not be interpreted as an implied endorsement for their second-line use. There are 2 main classes: dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 (GLP-1) agonists. Both act on the gut peptide GLP-1 to enhance glucose-stimulated insulin secretion and glucagon suppression.16
DPP-4 inhibitors promote the effects of endogenous GLP-1 by inhibiting its breakdown by the enzyme DPP-4. By increasing GLP-1, these agents achieve mild glucose lowering while remaining weight neutral.16 DPP-4 inhibitors can be combined with metformin and other oral agents and are not associated with hypoglycemia.16
Injectable GLP-1 receptor agonists provide supraphysiologic levels of GLP-1, resulting in increased insulin secretion, reduced glucagon secretion, delayed gastric emptying, increased satiety, and weight loss.16 Research has shown that exenatide can decrease mean weight by 7 kg over 2.4 years.18,19 Exenatide is dosed subcutaneously twice daily, while liraglutide is administered once daily. Once-weekly exenatide was approved by the US Food and Drug Administration (FDA) in February 2012. A recent study showed once-weekly exenatide lowered A1C levels, reduced weight, and caused fewer episodes of hypoglycemia compared with adding insulin glargine to the regimen when diabetes was uncontrolled on metformin (with or without a sulfonylurea).20 Patients may experience nausea, vomiting, and diarrhea at the onset of use of GLP-1 agonists.21 Slow titration and forewarning the patient of these adverse effects will help with compliance.
In October 2011, the FDA approved the use of exenatide with basal insulin. For patients already taking basal insulin with or without metformin or pioglitazone, adding exenatide resulted in improved A1C values and weight loss over a 30-week period.22 Reducing the dose of basal insulin at the initiation of exenatide helps decrease the incidence of hypoglycemia when considering this combination.22 Basal insulin lowers fasting glucose levels, while exenatide reduces postprandial glucose.
Although gaining in popularity, incretin therapy is being monitored for long-term safety. Cases of pancreatitis have been reported in both classes of medicines.4 Liraglutide has been associated with medullary thyroid cancer (MTC) in rodents.23,24 The FDA has recommended against using liraglutide and extended-release exenatide in patients with a personal or family history of MTC.16 Although the long-term safety of GLP-1 agonists and DPP-4 inhibitors is unknown, their novel mechanisms of action can prove useful for the right patient.
Concerns over TZDs. In addition to the FDA recommendation to avoid TZDs in patients with symptomatic heart failure, 2 studies have recently found that pioglitazone may be associated with an increased risk of bladder cancer.25,26 The FDA recommends avoiding use of pioglitazone in patients with active bladder cancer, and that it should be used with caution in patients with a history of cured bladder cancer. The European Medicines Agency also recommends against pioglitazone use in patients with uninvestigated macroscopic hematuria.27 The potential association between pioglitazone and bladder cancer requires further study. At this point, TZDs remain a valid second- or third-line treatment option in patients only after they are made aware of the potential risks and benefits.
CASE JR’s A1C of 7.6% is above his individualized goal of 7%. He feels he has maximized his efforts in the realm of lifestyle changes and is interested in another medication. Using the recommended patient-centered approach, we discuss with him the risks and benefits of each medication in the TABLE and we select the medication best suited to him based on adverse-effect profile.
TABLE
Matching diabetic medication attributes to patient needs
| Class | Medications | Actions | Benefits | Possible adverse effects and disadvantages | A1C-lowering (%) | Cost* |
|---|---|---|---|---|---|---|
| Biguanides | Metformin | ↓ Hepatic glucose production | Weight neutral or loss No hypoglycemia ↓ CV mortality | GI side effects Lactic acidosis Impaired B12 absorption Use caution or avoid in renal dysfunction | 1-2 | $ |
| Sulfonylureas | Gliclazide Glimepiride Glipizide Glyburide | ↑ Insulin secretion | Fast-onset glucose lowering | Hypoglycemia Lack of durable glycemic control Weight gain | 1-2 | $ |
| Meglitinides | Repaglinide Nateglinide | ↑ Insulin secretion | Improve meal-related insulin release and postprandial glucose | Hypoglycemia Weight gain | 0.1-2.1 | $$-$$$ |
| Thiazolidinediones | Pioglitazone | ↑ Insulin sensitivity | No hypoglycemia ↑ HDL ↓ Triglycerides | Bladder cancer concerns Edema Fracture risk Heart failure Weight gain | 0.5-1.4 | $$$ |
| GLP-1 receptor agonists | Exenatide Liraglutide | ↑ Insulin secretion ↓ Glucagon secretion Delayed gastric emptying Early satiety | Possible beta-cell preservation Weight loss | GI (nausea, vomiting, diarrhea) Injectable Medullary thyroid tumors in rodents Pancreatitis | 0.5-1.5 | $$$ |
| DPP-4 inhibitors | Linagliptin Saxagliptin Sitagliptin Vildagliptin | ↓ Glucagon secretion ↑ Insulin secretion | No hypoglycemia Weight neutral | Angioedema Pancreatitis | 0.5-0.8 | $$$ |
| Alpha-glucosidase inhibitors | Acarbose Miglitol | Delays carbohydrate absorption | Nonsystemic medication Reduces postprandial glucose | Frequent dosing GI side effects (abdominal cramping, flatulence) | 0.5-0.8 | $$ |
| Insulin | Aspart Detemir Glargine Lispro NPH Regular | Replaces endogenous insulin | Mimics physiology Rapidly effective | Hypoglycemia Weight gain | 1.5-3.5 | $-$$$ |
| CV, cardiovascular; DPP, dipeptidyl peptidase; GI, gastrointestinal; GLP, glucagon-like peptide, HDL, high-density lipoprotein. *Monthly cost of an average daily maintenance dose of available products: $, <$50; $$, $50.01-$100; $$$, >$100. Source: www.drugstore.com; accessed October 10, 2012. Adapted from: Reid TS. Options for intensifying diabetes treatment. J Fam Pract. 2011;9(suppl 1):S7-S10; American Diabetes Association Position Statement. Standards of Medical Care in Type 2 Diabetes-2012. Diabetes Care. 2012;35(suppl 1):S11-S63. | ||||||
Immunizations
An often overlooked but important part of the diabetes visit is reviewing the patient’s immunization history. Unless there are contraindications, all individuals with diabetes should receive the pneumococcal and annual influenza vaccines.4 In addition, the Advisory Committee on Immunization Practices now recommends hepatitis B virus (HBV) vaccine for unvaccinated adults with diabetes from ages 19 to 59.28 Unvaccinated adults with diabetes over age 60 should be vaccinated at the discretion of the provider after risk assessment.28 Patients may be at risk of contracting HBV in long-term care facilities where assisted blood sugar monitoring commonly occurs.28 Studies have shown that patients with diabetes may progress to chronic hepatitis B infection more often than patients without diabetes, and are at higher risk for nonalcoholic liver disease and hepatocellular carcinoma.29
CASE JR’s history shows that he is current on his influenza and pneumococcal vaccines. However, he doesn’t recall whether he’s been vaccinated against HBV. Serum testing reveals no previous immunization, and recommending HBV vaccine is appropriate.
Surveillance
The 2012 ADA recommendations do not include any new surveillance practices for microvascular disease. Providers should continue to offer the following screening to T2DM patients annually: urine albumin excretion testing and serum creatinine to assess for nephropathy, a comprehensive dilated eye exam to assess for retinopathy, and a foot exam to assess for distal symmetric polyneuropathy.4
CASE Each of these tests were performed (or ordered) for JR. We’ll see him again in 2 to 3 months for diabetes follow-up.
Acknowledgement
The authors thank Pamela Williams, MD, and Brent Smith, MD, for their guidance in the preparation of this article.
CORRESPONDENCE Jason C. McCarthy, MD, 101 Bodin Circle, Travis Air Force Base, CA 94535; Jason.McCarthy.6@us.af.mil
In April 2012, the American Diabetes Association (ADA) updated its guidelines for evaluating and treating type 2 diabetes mellitus (T2DM). In particular, the ADA acknowledges the value of an individualized, patient-centered approach that is less formulaic than its earlier guidelines. In this article, we highlight these and other recently published developments in the context of a case study. To help ensure follow-through on these newest recommendations, we also frame our review with the mnemonic, “ABCD IS diabetes.”
CASE JR is a 57-year-old man being seen for a regular follow-up appointment. His medical history includes T2DM, hypertension, and obesity. He is taking metformin 1000 mg twice daily, lisinopril 40 mg each morning, and amlodipine 10 mg each morning. He is current on his influenza and pneumococcal vaccinations. He does not smoke cigarettes. His physical exam and lab results reveal the following:
- blood pressure (BP), 132/70 mm Hg
- body mass index (BMI), 33 kg/m2
- glycosylated hemoglobin (A1C), 7.6%
- lipid profile: Total cholesterol, 185 mg/dL; high-density lipoprotein (HDL), 40 mg/dL; triglycerides (TG), 145 mg/dL; low-density lipoprotein (LDL), 90 mg/dL
Applying the “ABCD IS diabetes” mnemonic leads us through the following assessments.
Antiplatelets
In the past, guidelines have recommended that most patients with diabetes be placed on aspirin therapy. However, 2 trials published in 2008 failed to demonstrate significant reduction in cardiovascular disease (CVD) end points with aspirin use, raising questions about its effectiveness for primary CVD prevention in patients with diabetes.1,2 In 2010, the ADA, American Heart Association, and American College of Cardiology Foundation modified their recommendations for primary prevention,3 which remain unchanged in the 2012 ADA guidelines.4
Antiplatelet agents continue to play a role in primary prevention of CVD for patients with T2DM, but only after appropriate risk stratification.4 Consider low-dose aspirin therapy (75-162 mg/d) for patients with diabetes who have a 10-year Framingham risk >10%.4 (To calculate a patient’s 10-year risk, go to http://hp2010.nhlbihin.net/atpiii/calculator.asp.)
Many patients with T2DM seen in the primary care setting will reach this risk level and qualify for aspirin—in particular, men older than 50 years and women older than 60 with a family history of CVD, hypertension, smoking, dyslipidemia, or albuminuria.4 Aspirin therapy is not recommended for primary prevention in adults with diabetes at low risk for CVD (10-year Framingham risk <5%)—eg, men <50 and women <60 years without additional CVD risk factors.4 For patients with a 10-year Framingham risk between 5% and 10%, a decision to treat rests with the physician.
CASE Should JR be started on aspirin therapy for primary prevention of CVD? Initiating low-dose aspirin is recommended, assuming no contraindications, because his 10-year Framingham risk assessment is 11%.
A (antiplatelets): Consider low-dose aspirin therapy (75-162 mg/d) for diabetes patients with a 10-year Framingham risk >10%.
B (blood pressure): Individualize a patient’s goal for systolic blood pressure, aiming higher or lower than the customary systolic target of <130 mm Hg, as appropriate.
C (cholesterol): Recommend lifestyle changes and prescribe a statin, as needed, to achieve LDL goals in T2DM patients.
D (drug management): Use a patient-centered approach to achieve an individualized A1C goal. Metformin is the initial medication of choice. Select additional drug classes to balance adverse effects, cost, and effectiveness.
I (immunizations): Ensure that each T2DM patient receives influenza and pneumococcal vaccines, and the hepatitis B vaccine if <60 years.
S (surveillance): Confirm at each visit that annual surveillance testing for nephropathy, retinopathy, and peripheral neuropathy has been completed.
Blood pressure
The benefits of lowering BP in diabetes to <140 mm Hg systolic and <80 mm Hg diastolic have been established in randomized control trials.5-8 However, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial demonstrated that, in patients with T2DM, intensive BP lowering to <120 mm Hg systolic yielded no significant differences in fatal and nonfatal cardiovascular events compared with BP maintained between 130 and 140 mm Hg.9 Moreover, aggressive BP lowering may be associated with serious adverse events.10 The 2012 ADA guidelines state that a systolic BP goal of <130 mm Hg is appropriate for most patients; however, higher or lower BP targets may be individualized.4
Recommendations for adding a second antihypertensive agent and timing medication administration. For T2DM patients with hypertension, the 2012 guidelines recommend that you treat initially with either an angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB), if tolerated.4 When adding a second agent, the Avoiding Cardiovascular Events through COMbination therapy in Patients LIving with Systolic Hypertension (ACCOMPLISH) trial demonstrated reduced morbidity and mortality in patients receiving benazepril and amlodipine compared with those receiving benazepril and hydrochlorothiazide.11 As a result, amlodipine has joined diuretics as a preferred second oral antihypertensive agent after an ACEI or ARB.
If a patient is taking multiple BP medications, one or more should be taken at bedtime.4 Administering an antihypertensive at night results in better ambulatory BP control and reduces cardiovascular mortality.12
CASE JR is on maximal doses of 2 antihypertensive agents, and his BP is 132/70 mm Hg. His physician must individualize care and decide if adding a third agent is worth the risk of another medication when clear benefit has not been demonstrated. It is reasonable to continue his current regimen with the exception of changing his lisinopril dose to the evening and reassessing his BP control at his next visit.
Cholesterol
Controlling LDL remains the top priority of T2DM lipid management. In addition to lifestyle changes, statins are the primary means of achieving LDL goals. All patients with overt CVD should receive a statin.4 Also prescribe a statin for patients with diabetes who do not have CVD but who are older than 40 and have one or more cardiac risk factors, regardless of their baseline LDL cholesterol.4 The recommended LDL goal in T2DM patients continues to be <100 mg/dL. However, <70 mg/dL is a reasonable goal for those with known CVD.13
Using additional lipid-lowering agents besides a statin may improve cholesterol numbers, but not CVD outcomes. In the ACCORD study, adding fenofibrate to simvastatin did not decrease fatal cardiovascular events or nonfatal myocardial infarction and stroke compared with simvastatin given alone.14 The AIM-High study showed no difference in cardiovascular outcomes and a possible increase in ischemic stroke with combination niacin and statin compared with statin therapy alone.15 For now, lifestyle changes and statins remain the ideal modalities to achieve LDL goals.
CASE Should a statin be initiated for our patient? Since JR is over 40 without known CVD and has a cardiac risk factor of hypertension, he should be started on statin therapy regardless of his baseline LDL (90 mg/dL), which is already at goal (<100 mg/dL).
Drug management
Let the glycemic goal for each patient guide your medication management. The 2012 ADA recommendation for most adults is an A1C of <7%.4 More strict control (A1C <6.5%) may be appropriate for certain individuals with a long life expectancy, short duration of diabetes, and no significant micro- or macrovascular disease.4 Less strict control (A1C <8%) may be appropriate for individuals with significant comorbidities, shorter life expectancy, severe hypoglycemia, or long-standing T2DM that’s been difficult to control despite multiple medications, including insulin.4
Individualize treatment. In April 2012, the ADA released a position statement encouraging a patient-centered approach to managing hyperglycemia in T2DM.16 This statement contains a new treatment algorithm (available at: http://care.diabetesjournals.org/content/early/2012/04/17/dc12-0413.full.pdf+html) that is less prescriptive than the previous 2009 algorithm and balances provider judgment, patient preference, and susceptibility to adverse effects in order to attain an individualized A1C target.16 Although a comprehensive review of T2DM pharmacotherapy is beyond the scope of this article, we will discuss the importance of metformin, familiarize prescribers with incretin-based therapy, and highlight recent safety concerns regarding thiazolidinediones (TZDs).
Metformin is first line. The 2012 ADA guidelines recommend prescribing metformin at the time of diagnosis of T2DM, in addition to advising lifestyle changes.4,16 The American College of Physicians (ACP) also recommends metformin as the first agent in diabetes management, citing the benefits of weight loss, improved lipid profiles, and decreased cardiovascular mortality.17 Adding a second medication to metformin at the time of diagnosis may be considered if the initial A1C value is >9%.16 Because robust comparative trials are lacking, the selection of additional medications beyond metformin depends on a patient-centered approach, with consideration of efficacy, adverse effect profile, and cost.16 The TABLE provides a succinct review of the key properties of diabetic medications that clinicians may discuss with their patients. All of the listed agents are valid second-line treatments, and you should select one based on the individual’s needs.
Incretin-based therapy. Among newer antihyperglycemic agents, incretins have drawn much attention and thus warrant special focus. The emphasis on these agents should not be interpreted as an implied endorsement for their second-line use. There are 2 main classes: dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 (GLP-1) agonists. Both act on the gut peptide GLP-1 to enhance glucose-stimulated insulin secretion and glucagon suppression.16
DPP-4 inhibitors promote the effects of endogenous GLP-1 by inhibiting its breakdown by the enzyme DPP-4. By increasing GLP-1, these agents achieve mild glucose lowering while remaining weight neutral.16 DPP-4 inhibitors can be combined with metformin and other oral agents and are not associated with hypoglycemia.16
Injectable GLP-1 receptor agonists provide supraphysiologic levels of GLP-1, resulting in increased insulin secretion, reduced glucagon secretion, delayed gastric emptying, increased satiety, and weight loss.16 Research has shown that exenatide can decrease mean weight by 7 kg over 2.4 years.18,19 Exenatide is dosed subcutaneously twice daily, while liraglutide is administered once daily. Once-weekly exenatide was approved by the US Food and Drug Administration (FDA) in February 2012. A recent study showed once-weekly exenatide lowered A1C levels, reduced weight, and caused fewer episodes of hypoglycemia compared with adding insulin glargine to the regimen when diabetes was uncontrolled on metformin (with or without a sulfonylurea).20 Patients may experience nausea, vomiting, and diarrhea at the onset of use of GLP-1 agonists.21 Slow titration and forewarning the patient of these adverse effects will help with compliance.
In October 2011, the FDA approved the use of exenatide with basal insulin. For patients already taking basal insulin with or without metformin or pioglitazone, adding exenatide resulted in improved A1C values and weight loss over a 30-week period.22 Reducing the dose of basal insulin at the initiation of exenatide helps decrease the incidence of hypoglycemia when considering this combination.22 Basal insulin lowers fasting glucose levels, while exenatide reduces postprandial glucose.
Although gaining in popularity, incretin therapy is being monitored for long-term safety. Cases of pancreatitis have been reported in both classes of medicines.4 Liraglutide has been associated with medullary thyroid cancer (MTC) in rodents.23,24 The FDA has recommended against using liraglutide and extended-release exenatide in patients with a personal or family history of MTC.16 Although the long-term safety of GLP-1 agonists and DPP-4 inhibitors is unknown, their novel mechanisms of action can prove useful for the right patient.
Concerns over TZDs. In addition to the FDA recommendation to avoid TZDs in patients with symptomatic heart failure, 2 studies have recently found that pioglitazone may be associated with an increased risk of bladder cancer.25,26 The FDA recommends avoiding use of pioglitazone in patients with active bladder cancer, and that it should be used with caution in patients with a history of cured bladder cancer. The European Medicines Agency also recommends against pioglitazone use in patients with uninvestigated macroscopic hematuria.27 The potential association between pioglitazone and bladder cancer requires further study. At this point, TZDs remain a valid second- or third-line treatment option in patients only after they are made aware of the potential risks and benefits.
CASE JR’s A1C of 7.6% is above his individualized goal of 7%. He feels he has maximized his efforts in the realm of lifestyle changes and is interested in another medication. Using the recommended patient-centered approach, we discuss with him the risks and benefits of each medication in the TABLE and we select the medication best suited to him based on adverse-effect profile.
TABLE
Matching diabetic medication attributes to patient needs
| Class | Medications | Actions | Benefits | Possible adverse effects and disadvantages | A1C-lowering (%) | Cost* |
|---|---|---|---|---|---|---|
| Biguanides | Metformin | ↓ Hepatic glucose production | Weight neutral or loss No hypoglycemia ↓ CV mortality | GI side effects Lactic acidosis Impaired B12 absorption Use caution or avoid in renal dysfunction | 1-2 | $ |
| Sulfonylureas | Gliclazide Glimepiride Glipizide Glyburide | ↑ Insulin secretion | Fast-onset glucose lowering | Hypoglycemia Lack of durable glycemic control Weight gain | 1-2 | $ |
| Meglitinides | Repaglinide Nateglinide | ↑ Insulin secretion | Improve meal-related insulin release and postprandial glucose | Hypoglycemia Weight gain | 0.1-2.1 | $$-$$$ |
| Thiazolidinediones | Pioglitazone | ↑ Insulin sensitivity | No hypoglycemia ↑ HDL ↓ Triglycerides | Bladder cancer concerns Edema Fracture risk Heart failure Weight gain | 0.5-1.4 | $$$ |
| GLP-1 receptor agonists | Exenatide Liraglutide | ↑ Insulin secretion ↓ Glucagon secretion Delayed gastric emptying Early satiety | Possible beta-cell preservation Weight loss | GI (nausea, vomiting, diarrhea) Injectable Medullary thyroid tumors in rodents Pancreatitis | 0.5-1.5 | $$$ |
| DPP-4 inhibitors | Linagliptin Saxagliptin Sitagliptin Vildagliptin | ↓ Glucagon secretion ↑ Insulin secretion | No hypoglycemia Weight neutral | Angioedema Pancreatitis | 0.5-0.8 | $$$ |
| Alpha-glucosidase inhibitors | Acarbose Miglitol | Delays carbohydrate absorption | Nonsystemic medication Reduces postprandial glucose | Frequent dosing GI side effects (abdominal cramping, flatulence) | 0.5-0.8 | $$ |
| Insulin | Aspart Detemir Glargine Lispro NPH Regular | Replaces endogenous insulin | Mimics physiology Rapidly effective | Hypoglycemia Weight gain | 1.5-3.5 | $-$$$ |
| CV, cardiovascular; DPP, dipeptidyl peptidase; GI, gastrointestinal; GLP, glucagon-like peptide, HDL, high-density lipoprotein. *Monthly cost of an average daily maintenance dose of available products: $, <$50; $$, $50.01-$100; $$$, >$100. Source: www.drugstore.com; accessed October 10, 2012. Adapted from: Reid TS. Options for intensifying diabetes treatment. J Fam Pract. 2011;9(suppl 1):S7-S10; American Diabetes Association Position Statement. Standards of Medical Care in Type 2 Diabetes-2012. Diabetes Care. 2012;35(suppl 1):S11-S63. | ||||||
Immunizations
An often overlooked but important part of the diabetes visit is reviewing the patient’s immunization history. Unless there are contraindications, all individuals with diabetes should receive the pneumococcal and annual influenza vaccines.4 In addition, the Advisory Committee on Immunization Practices now recommends hepatitis B virus (HBV) vaccine for unvaccinated adults with diabetes from ages 19 to 59.28 Unvaccinated adults with diabetes over age 60 should be vaccinated at the discretion of the provider after risk assessment.28 Patients may be at risk of contracting HBV in long-term care facilities where assisted blood sugar monitoring commonly occurs.28 Studies have shown that patients with diabetes may progress to chronic hepatitis B infection more often than patients without diabetes, and are at higher risk for nonalcoholic liver disease and hepatocellular carcinoma.29
CASE JR’s history shows that he is current on his influenza and pneumococcal vaccines. However, he doesn’t recall whether he’s been vaccinated against HBV. Serum testing reveals no previous immunization, and recommending HBV vaccine is appropriate.
Surveillance
The 2012 ADA recommendations do not include any new surveillance practices for microvascular disease. Providers should continue to offer the following screening to T2DM patients annually: urine albumin excretion testing and serum creatinine to assess for nephropathy, a comprehensive dilated eye exam to assess for retinopathy, and a foot exam to assess for distal symmetric polyneuropathy.4
CASE Each of these tests were performed (or ordered) for JR. We’ll see him again in 2 to 3 months for diabetes follow-up.
Acknowledgement
The authors thank Pamela Williams, MD, and Brent Smith, MD, for their guidance in the preparation of this article.
CORRESPONDENCE Jason C. McCarthy, MD, 101 Bodin Circle, Travis Air Force Base, CA 94535; Jason.McCarthy.6@us.af.mil
1. Ogawa H, Nakayama M, Morimoto T, et al. Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300:2134-2141.
2. Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840.-
3. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395-1402.
4. American Diabetes Association. Standards of medical care in diabetes–2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
5. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
6. Turner R, Holman R, Stratton I, et al. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703-713.
7. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755-1762.
8. Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412-419.
9. Cushman WC, Evans GW, Byington RP, et al. ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
10. Bangalore S, Kumar S, Lobach I, et al. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and Bayesian random-effects meta-analyses of randomized trials. Circulation. 2011;123:2799-2810.
11. Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417-2428.
12. Hermida R, Ayala D, Mojon A, et al. Influence of time of day of blood-pressure lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2001;34:1270-1276.
13. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227-239.
14. Cushman W, Evans G, Byington R, et al. The ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
15. Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
16. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379.
17. Qaseem A, Humphrey L, Sweet D, et al. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2012;156:218-231.
18. Varanasi A, Chaudhuri A, Dhindsa S, et al. Durability of effects of exenatide treatment on glycemic control, body weight, systolic blood pressure, C-reactive protein, and triglyceride concentrations. Endocr Pract. 2011;17:192-200.
19. Vilsboll T, Christensen M, Junker A, et al. Effects of glucagon-like peptide-1 receptor agonist on weight loss: systemic review and meta-analyses of randomized controlled trials. BMJ. 2012;344:d7771.-
20. Diamant M, Van Gaal L, Stranks S, et al. Safety and efficacy of once-weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes over 84 weeks. Diabetes Care. 2012;35:683-689.
21. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39-47.
22. Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized controlled trial. Ann Intern Med. 2011;154:103-112.
23. Drucker DJ, Sherman SI, Bergenstal RM, et al. The safety of incretin-based therapies—review of the scientific evidence. J Clin Endocrinol Metab. 2011;96:2027-2031.
24. Elashoff M, Matveyenko A, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141:150-156.
25. Piccinni C, Motola D, Marchesini G, et al. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care. 2011;34:1369-1371.
26. Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922.
27. European Medicines Agency. European Medicines Agency recommends new contra-indications and warnings for pioglitazone to reduce small increased risk of bladder cancer, updated July 7, 2011. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2011/07. Accessed March 22, 2012.
28. Sawyer M, Hoerger T. Use of hepatitis B vaccination for adults with diabetes mellitus: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2011;60:1709-1711.
29. El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-468.
1. Ogawa H, Nakayama M, Morimoto T, et al. Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300:2134-2141.
2. Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840.-
3. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395-1402.
4. American Diabetes Association. Standards of medical care in diabetes–2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
5. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
6. Turner R, Holman R, Stratton I, et al. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703-713.
7. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755-1762.
8. Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412-419.
9. Cushman WC, Evans GW, Byington RP, et al. ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
10. Bangalore S, Kumar S, Lobach I, et al. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and Bayesian random-effects meta-analyses of randomized trials. Circulation. 2011;123:2799-2810.
11. Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417-2428.
12. Hermida R, Ayala D, Mojon A, et al. Influence of time of day of blood-pressure lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2001;34:1270-1276.
13. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227-239.
14. Cushman W, Evans G, Byington R, et al. The ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
15. Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
16. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379.
17. Qaseem A, Humphrey L, Sweet D, et al. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2012;156:218-231.
18. Varanasi A, Chaudhuri A, Dhindsa S, et al. Durability of effects of exenatide treatment on glycemic control, body weight, systolic blood pressure, C-reactive protein, and triglyceride concentrations. Endocr Pract. 2011;17:192-200.
19. Vilsboll T, Christensen M, Junker A, et al. Effects of glucagon-like peptide-1 receptor agonist on weight loss: systemic review and meta-analyses of randomized controlled trials. BMJ. 2012;344:d7771.-
20. Diamant M, Van Gaal L, Stranks S, et al. Safety and efficacy of once-weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes over 84 weeks. Diabetes Care. 2012;35:683-689.
21. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39-47.
22. Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized controlled trial. Ann Intern Med. 2011;154:103-112.
23. Drucker DJ, Sherman SI, Bergenstal RM, et al. The safety of incretin-based therapies—review of the scientific evidence. J Clin Endocrinol Metab. 2011;96:2027-2031.
24. Elashoff M, Matveyenko A, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141:150-156.
25. Piccinni C, Motola D, Marchesini G, et al. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care. 2011;34:1369-1371.
26. Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922.
27. European Medicines Agency. European Medicines Agency recommends new contra-indications and warnings for pioglitazone to reduce small increased risk of bladder cancer, updated July 7, 2011. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2011/07. Accessed March 22, 2012.
28. Sawyer M, Hoerger T. Use of hepatitis B vaccination for adults with diabetes mellitus: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2011;60:1709-1711.
29. El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-468.
Postmenopausal bleeding: First steps in the workup
• Screen all women with postmenopausal vaginal bleeding (PMB) for endometrial cancer. A
• Use transvaginal ultrasound for the initial study for patients at low risk for endometrial cancer, and endometrial biopsy for those at higher risk. B
• Use saline infusion sonography as a second step in the evaluation of PMB if the diagnosis remains unclear after a biopsy or the bleeding persists despite a normal initial workup. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Irene R, age 55, comes to see you because of vaginal bleeding, which started 7 days ago. The patient reports that she stopped menstruating about 4 years ago and is not on hormone replacement therapy or taking any medication. Irene, who is married and in a monogamous relationship with her husband of 20 years, denies any vaginal irritation, discharge, or dyspareunia. Her uterus is intact and she had a Pap smear about a year ago.
What will you include in a workup to determine the cause of her bleeding?
Endometrial cancer is the most common malignancy of the female reproductive organs, with more than 43,000 new cases detected in the United States in 2010 alone.1 More than half of all cases of endometrial cancer are diagnosed in women between the ages of 50 and 69 years.1,2
Vaginal bleeding, which more than 90% of women with endometrial cancer experience,3 is often the first sign of malignancy. Thus, all women who present with postmenopausal bleeding (PMB)—defined as any vaginal bleeding occurring ≥1 year after cessation of menses or any unscheduled bleeding in women on hormone replacement therapy (HRT)—require further evaluation.
Prognosis for endometrial cancer depends on the extent of the disease at the time of diagnosis. Most cases are diagnosed in the early stages and have a 5-year survival rate greater than 96%.1 Surgery alone can be curative if the malignancy is contained within the uterus.1,2
What are the essential elements of a workup for a woman with PMB? Which lab tests should be ordered and which procedures performed? You’ll find the answers in the at-a-glance ALGORITHM4-6 we created, and in the additional information provided in this evidence-based review.
ALGORITHM
Postmenopausal bleeding: An evidence-based workup4-6
CBC, complete blood count; EMB, endometrial biopsy; ET, endometrial thickness; H&P, history and physical; Pap, Papanicolaou smear; SIS, saline infusion sonography; STD, sexually transmitted disease; TVUS, transvaginal ultrasonography.
*Laboratory tests are generally not helpful in evaluating postmenopausal bleeding, but a complete blood count is warranted if bleeding is prolonged or heavy and a test for sexually transmitted diseases may be appropriate based on patient history or physical exam.
Endometrial cancer is the key concern
While endometrial cancer is the most serious cause of PMB, it is not the most common. Atrophic endometrium is the culprit 60% to 80% of the time, while endometrial cancer accounts for up to 10% of cases. Endometrial polyps or hyperplasia, HRT, and cervical cancer are among the conditions included in the differential diagnosis (TABLE).7
A workup for PMB starts with a thorough medical history and a physical examination, including a Pap smear to screen for cervical cancer. Results from the Pap smear may suggest other pathology, such as benign endometrial cells, atypical endometrial cells, or atypical glandular cells.
Is lab work necessary? Laboratory tests are generally not helpful in evaluating PMB itself. A complete blood count is warranted if the bleeding is prolonged or heavy, however, and testing for sexually transmitted diseases may be appropriate, based on the patient’s history and/or physical exam.5,8
TABLE
Postmenopausal bleeding: The differential diagnosis7
| Cause | Incidence (%) |
|---|---|
| Atrophic endometrium | 60-80 |
| HRT | 15-25 |
| Endometrial cancer | 7-10 |
| Endometrial hyperplasia | 5-10 |
| Polyp(s) (endometrial or cervical) | 2-12 |
| Miscellaneous (uterine leiomyomas, cervicitis, atrophic vaginitis, tamoxifen therapy, trauma, anticoagulation) | <10 |
| HRT, hormone replacement therapy. | |
Endometrial biopsy or transvaginal ultrasound: Which test is better?
For many years, dilatation and curettage (D&C) of the endometrium was standard practice in the evaluation of patients with PMB. The Society of Radiologists in Ultrasound (SRU) and the American College of Obstetricians and Gynecologists (ACOG) now advise starting with either endometrial biopsy (EMB) or transvaginal ultrasound (TVUS).4,6 Both procedures are more advantageous than D&C for evaluating PMB because they can be done in an outpatient setting, are less expensive, provide faster results, and correlate with surgical findings more than 95% of the time.9
Although numerous studies have attempted to define the roles of EMB with Pipelle and TVUS, the literature is unclear as to which initial test is preferable.
Endometrial biopsy. Many physicians prefer to start with EMB, because it provides tissue samples for a histological diagnosis, is easily performed, and causes minimal cramping. The test does have limitations, such as difficulty in obtaining adequate tissue samples.
In a large cohort study (n=1535), EMB failed to provide an adequate tissue sample as much as 16% of the time.10 EMB’s sensitivity in detecting endometrial hyperplasia and cancer was 84%, the researchers reported, with a specificity of 99%; both the positive and negative predictive value were 94%. In a smaller study in which 97 women underwent TVUS and EMB was attempted, researchers reported that while no cases of endometrial cancer were missed when EMB sampling was successful, there was only a 27% probability of obtaining an adequate endometrial sample in women with an endometrial thickness (ET) <5 mm.11 EMB does a poorer job of detecting focal pathologies—with the potential to miss up to 18% of focal lesions, such as endometrial polyps, according to another study.12
Transvaginal ultrasound. TVUS is a safe, noninvasive, and cost-effective way to evaluate the endometrium, both to visualize focal lesions and assess ET. The technique, as defined by the SRU, involves scanning the uterus in a sagittal view and measuring the double-layer ET in the anteroposterior dimension from one basalis layer to the other.4
Reports of the sensitivity of TVUS in detecting endometrial cancer vary, depending on what cut point is used to rule it out. The SRU recommends an ET cutoff of ≤5 mm;4 ACOG recommends ≤4 mm.6 The consensus statements of both groups are based on a meta-analysis of 35 prospective studies that included data from nearly 6000 women with PMB. The sensitivity of TVUS in detecting endometrial cancer was 96%, whether the ≤4 or ≤5 mm cutoff was used, but specificity differed (53% for ≤4 mm vs 61% for ≤5 mm).13
In numerous studies with cut points of ≤4 or ≤5 mm, TVUS had a negative predictive value >99%.14-18 Because of this, ACOG states in an opinion issued in 2009 and reaffirmed in 2011, that TVUS is a “reasonable first approach.” The opinion further notes that in patients with an ET ≤4 mm, endometrial sampling is not required.6 In a meta-analysis of 3813 women, 3096 of whom were postmenopausal, researchers came to a different conclusion. An ET measurement on TVUS does not reduce the need for invasive diagnostic testing, the authors reported, because 4% of endometrial cancers would be missed even when a low threshold was used for reporting suspicious results.19
Type 2 endometrial cancer may be missed
A thin or indistinct endometrial lining on TVUS does not reliably exclude type 2 endometrial lesions20—which are not related to estrogen exposure or endometrial hyperplasia and typically present later in life, are diagnosed at a more advanced stage, and occur less frequently than type 1 endometrial cancer. A retrospective review of 52 patients with type 2 endometrial cancer found that 17% had an ET <4 mm, and another 17% had an indistinct endometrium.20
Factor risk level into decision-making
Researchers who conducted a decision analysis found that EMB is a more cost-effective initial diagnostic test for populations with a prevalence of endometrial cancer ≥15%.21 Overall, the prevalence among postmenopausal women in the United States is roughly 0.7%,22 but it is considerably higher among women with polycystic ovarian syndrome, obesity, diabetes, early menarche, late menopause, nulliparity, a history of tamoxifen use, or hereditary nonpolyposis colorectal cancer.5 For women with PMB and any of these risk factors, physicians should consider EMB as the first diagnostic test.
Because EMB is a substandard test for diagnosing benign endometrial abnormalities, such as polyps and submucosa leiomyomas, TVUS may be a better starting point for women at lower risk for endometrial cancer. In any case, an ET >4 mm requires further investigation using EMB, saline infusion sonography (SIS), or hysteroscopy with biopsy.9 Patients with uterine pathology noted with TVUS should be referred for treatment.
Next step? Consider saline infusion sonography
SIS is a procedure in which sterile saline is infused into the endometrial cavity, then TVUS is performed. The saline solution distends the uterus, promoting visualization and thus providing more detail than a conventional ultrasound.
Because SIS is expensive and uncomfortable, it is used mainly as a second step in the evaluation of PMB. It is useful when:
- a diagnosis remains unclear after biopsy
- TVUS finds evidence of a focal lesion
- bleeding persists despite a normal initial workup
- the patient has a relative contraindication for hysteroscopy with D&C.23,24
SIS is contraindicated in cases in which cancer cells were detected with either EMB or TVUS, as the procedure has been associated with a small but real risk of malignant cell dissemination.25
CASE Irene’s history did not reveal any significant risk factors for endometrial cancer. Physical exam revealed a cervical polyp. We obtained a Pap smear, which was normal, and removed the polyp, which was benign. Irene also underwent TVUS because of her low risk status. The test revealed an endometrial stripe of <4 mm and an endometrial polyp, prompting referral to a specialist.
The patient underwent a hysteroscopy and D&C. Her endometrial polyp was benign and the endometrial scrapings revealed atrophic squamous mucosa. Irene has had no further bleeding and is doing well at this time.
CORRESPONDENCE Danette B. Null, MD, 2411 Fox Hollow, Lake Charles, LA 70605; dnull@lcmh.com
1. American Cancer Society. Cancer facts and figures 2012. Atlanta: American Cancer Society; 2012.
2. Blair AR, Casas CM. Gynecologic cancers. Prim Care Clin Office Pract. 2009;36:115-130.
3. Doubilet PM. Society of Radiologists in Ultrasound consensus conference statement on postmenopausal bleeding. J Ultrasound Med. 2001;20:1037-1042.
4. Goldstein RB, Bree RL, Benson CB, et al. Evaluation of the woman with postmenopausal bleeding: Society of Radiologists in Ultrasound-Sponsored Consensus Conference statement. J Ultrasound Med. 2001;20:1025.-
5. Buchanan EM, Weinstein LC, Hillson C. Endometrial cancer. Am Fam Physician. 2009;80:1075-1080.
6. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 440: the role of transvaginal ultrasonography in the evaluation of postmenopausal bleeding. Obstet Gynecol. 2009;114:409-411.Available at: http://journals.lww.com/greenjournal/Citation/2009/08000/ACOG_Committee_Opinion_No__440__The_Role_of.33.aspx. Accessed September 19, 2012.
7. Hsu C, Chen C, Wang K. Assessment of postmenopausal bleeding. Int J Gerontol. 2008;2:55-59.
8. Mounsey AL. Postmenopausal bleeding evaluation and management. Clinics Fam Pract. 2002;4:173-192.
9. O’Connell L, Fries M, Zeringue E, et al. Triage of abnormal postmenopausal bleeding: a comparison of endometrial biopsy and transvaginal sonohysterography versus factional curettage with hysteroscopy. Am J Obstet Gynecol. 1998;178:956-961
10. Machado F, Moreno J, Carazo M, et al. Accuracy of endometrial biopsy with the Cornier Pipelle for diagnosis of endometrial cancer and a typical hyperplasia. Eur J Gynaecol Oncol. 2003;23:279-281.
11. Elsandabesee D, Greenwood P. The performance of Pipelle endometrial sampling in a dedicated postmenopausal bleeding clinic. J Obstet Gynaecol. 2005;25:32-34.
12. Goldstein SR, Zeltser I, Horan CK, et al. Ultrasonography-based triage for perimenopausal patients with abnormal uterine bleeding. Am J Obstet Gynecol. 1997;177:102-108.
13. Smith-Bindman R, Kerlikowske K, Feldstein VA, et al. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA. 1998;280:1510-1517.
14. Karlsson B, Granberg S, Wikland M, et al. Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding—a Nordic multicenter study. Am J Obstet Gynecol. 1995;172:1488-1494.
15. Ferrazi E, Torri V, Trio D, et al. Sonographic endometrial thickness: a useful test to predict atrophy in patients with postmenopausal bleeding. An Italian multicenter study. Ultrasound Obstet Gynecol. 1996;7:315-321.
16. Gull B, Carlsson K, Karlsson B, et al. Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding; is it always necessary to perform an endometrial biopsy? Am J Obstet Gynecol. 2000;182:509-515.
17. Epstein E, Valentin L. Rebleeding and endometrial growth in women with postmenopausal bleeding and endometrial thickness <5 mm managed by dilatation and curettage or ultrasound follow-up: a randomized controlled study. Ultrasound Obstet Gynecol. 2001;18:499-504.
18. Gull B, Karlsson B, Milsom I, et al. Can ultrasound replace dilatation and curettage? A longitudinal evaluation of postmenopausal bleeding and transvaginal sonographic measurement of the endometrium as predictors of endometrial cancer. Am J Obstet Gynecol. 2003;18:401-408.
19. Tabor A, Watt HC, Wald NJ. Endometrial thickness as a test for endometrial cancer in women with postmenopausal vaginal bleeding. Obstet Gynecol. 2002;99:663-670.
20. Wang J, Wieslander C, Hansen G, et al. Thin endometrial echo complex on ultrasound does not reliably exclude type 2 endometrial cancers. Gynecol Oncol. 2006;101:120-125.
21. Dijkhuizen FP, Mol BW, Brolmann HA, et al. Cost-effectiveness of the use of transvaginal sonography in the evaluation of postmenopausal bleeding. Maturitas. 2003;45:275-282.
22. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2008. Bethesda, Md: National Cancer Institute. Available at: http://seer.cancer.gov/csr/1975_2008, based on November 2010 SEER data submission, posted to SEER web site, 2011.
23. Kamel HS, Darwish AM, Mohamed SA. Comparison of transvaginal ultrasonography and vaginal sonohysterography in the detection of endometrial polyps. Acta Obstet Gynecol Scand. 2000;79:60-64.
24. Krampl E, Bourne T, Hurlen-Solbakken H, et al. Transvaginal ultrasonography sonohysterography and operative hysteroscopy for the evaluation of abnormal uterine bleeding. Acta Obstet Gynecol Scand. 2001;80:616-622.
25. Alcazar JL, Errasti T, Zornoza A. Saline infusion sonohysterography in endometrial cancer: assessment of malignant cells dissemination risk. Acta Obstet Gynecol Scand. 2000;79:321-322.
• Screen all women with postmenopausal vaginal bleeding (PMB) for endometrial cancer. A
• Use transvaginal ultrasound for the initial study for patients at low risk for endometrial cancer, and endometrial biopsy for those at higher risk. B
• Use saline infusion sonography as a second step in the evaluation of PMB if the diagnosis remains unclear after a biopsy or the bleeding persists despite a normal initial workup. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Irene R, age 55, comes to see you because of vaginal bleeding, which started 7 days ago. The patient reports that she stopped menstruating about 4 years ago and is not on hormone replacement therapy or taking any medication. Irene, who is married and in a monogamous relationship with her husband of 20 years, denies any vaginal irritation, discharge, or dyspareunia. Her uterus is intact and she had a Pap smear about a year ago.
What will you include in a workup to determine the cause of her bleeding?
Endometrial cancer is the most common malignancy of the female reproductive organs, with more than 43,000 new cases detected in the United States in 2010 alone.1 More than half of all cases of endometrial cancer are diagnosed in women between the ages of 50 and 69 years.1,2
Vaginal bleeding, which more than 90% of women with endometrial cancer experience,3 is often the first sign of malignancy. Thus, all women who present with postmenopausal bleeding (PMB)—defined as any vaginal bleeding occurring ≥1 year after cessation of menses or any unscheduled bleeding in women on hormone replacement therapy (HRT)—require further evaluation.
Prognosis for endometrial cancer depends on the extent of the disease at the time of diagnosis. Most cases are diagnosed in the early stages and have a 5-year survival rate greater than 96%.1 Surgery alone can be curative if the malignancy is contained within the uterus.1,2
What are the essential elements of a workup for a woman with PMB? Which lab tests should be ordered and which procedures performed? You’ll find the answers in the at-a-glance ALGORITHM4-6 we created, and in the additional information provided in this evidence-based review.
ALGORITHM
Postmenopausal bleeding: An evidence-based workup4-6
CBC, complete blood count; EMB, endometrial biopsy; ET, endometrial thickness; H&P, history and physical; Pap, Papanicolaou smear; SIS, saline infusion sonography; STD, sexually transmitted disease; TVUS, transvaginal ultrasonography.
*Laboratory tests are generally not helpful in evaluating postmenopausal bleeding, but a complete blood count is warranted if bleeding is prolonged or heavy and a test for sexually transmitted diseases may be appropriate based on patient history or physical exam.
Endometrial cancer is the key concern
While endometrial cancer is the most serious cause of PMB, it is not the most common. Atrophic endometrium is the culprit 60% to 80% of the time, while endometrial cancer accounts for up to 10% of cases. Endometrial polyps or hyperplasia, HRT, and cervical cancer are among the conditions included in the differential diagnosis (TABLE).7
A workup for PMB starts with a thorough medical history and a physical examination, including a Pap smear to screen for cervical cancer. Results from the Pap smear may suggest other pathology, such as benign endometrial cells, atypical endometrial cells, or atypical glandular cells.
Is lab work necessary? Laboratory tests are generally not helpful in evaluating PMB itself. A complete blood count is warranted if the bleeding is prolonged or heavy, however, and testing for sexually transmitted diseases may be appropriate, based on the patient’s history and/or physical exam.5,8
TABLE
Postmenopausal bleeding: The differential diagnosis7
| Cause | Incidence (%) |
|---|---|
| Atrophic endometrium | 60-80 |
| HRT | 15-25 |
| Endometrial cancer | 7-10 |
| Endometrial hyperplasia | 5-10 |
| Polyp(s) (endometrial or cervical) | 2-12 |
| Miscellaneous (uterine leiomyomas, cervicitis, atrophic vaginitis, tamoxifen therapy, trauma, anticoagulation) | <10 |
| HRT, hormone replacement therapy. | |
Endometrial biopsy or transvaginal ultrasound: Which test is better?
For many years, dilatation and curettage (D&C) of the endometrium was standard practice in the evaluation of patients with PMB. The Society of Radiologists in Ultrasound (SRU) and the American College of Obstetricians and Gynecologists (ACOG) now advise starting with either endometrial biopsy (EMB) or transvaginal ultrasound (TVUS).4,6 Both procedures are more advantageous than D&C for evaluating PMB because they can be done in an outpatient setting, are less expensive, provide faster results, and correlate with surgical findings more than 95% of the time.9
Although numerous studies have attempted to define the roles of EMB with Pipelle and TVUS, the literature is unclear as to which initial test is preferable.
Endometrial biopsy. Many physicians prefer to start with EMB, because it provides tissue samples for a histological diagnosis, is easily performed, and causes minimal cramping. The test does have limitations, such as difficulty in obtaining adequate tissue samples.
In a large cohort study (n=1535), EMB failed to provide an adequate tissue sample as much as 16% of the time.10 EMB’s sensitivity in detecting endometrial hyperplasia and cancer was 84%, the researchers reported, with a specificity of 99%; both the positive and negative predictive value were 94%. In a smaller study in which 97 women underwent TVUS and EMB was attempted, researchers reported that while no cases of endometrial cancer were missed when EMB sampling was successful, there was only a 27% probability of obtaining an adequate endometrial sample in women with an endometrial thickness (ET) <5 mm.11 EMB does a poorer job of detecting focal pathologies—with the potential to miss up to 18% of focal lesions, such as endometrial polyps, according to another study.12
Transvaginal ultrasound. TVUS is a safe, noninvasive, and cost-effective way to evaluate the endometrium, both to visualize focal lesions and assess ET. The technique, as defined by the SRU, involves scanning the uterus in a sagittal view and measuring the double-layer ET in the anteroposterior dimension from one basalis layer to the other.4
Reports of the sensitivity of TVUS in detecting endometrial cancer vary, depending on what cut point is used to rule it out. The SRU recommends an ET cutoff of ≤5 mm;4 ACOG recommends ≤4 mm.6 The consensus statements of both groups are based on a meta-analysis of 35 prospective studies that included data from nearly 6000 women with PMB. The sensitivity of TVUS in detecting endometrial cancer was 96%, whether the ≤4 or ≤5 mm cutoff was used, but specificity differed (53% for ≤4 mm vs 61% for ≤5 mm).13
In numerous studies with cut points of ≤4 or ≤5 mm, TVUS had a negative predictive value >99%.14-18 Because of this, ACOG states in an opinion issued in 2009 and reaffirmed in 2011, that TVUS is a “reasonable first approach.” The opinion further notes that in patients with an ET ≤4 mm, endometrial sampling is not required.6 In a meta-analysis of 3813 women, 3096 of whom were postmenopausal, researchers came to a different conclusion. An ET measurement on TVUS does not reduce the need for invasive diagnostic testing, the authors reported, because 4% of endometrial cancers would be missed even when a low threshold was used for reporting suspicious results.19
Type 2 endometrial cancer may be missed
A thin or indistinct endometrial lining on TVUS does not reliably exclude type 2 endometrial lesions20—which are not related to estrogen exposure or endometrial hyperplasia and typically present later in life, are diagnosed at a more advanced stage, and occur less frequently than type 1 endometrial cancer. A retrospective review of 52 patients with type 2 endometrial cancer found that 17% had an ET <4 mm, and another 17% had an indistinct endometrium.20
Factor risk level into decision-making
Researchers who conducted a decision analysis found that EMB is a more cost-effective initial diagnostic test for populations with a prevalence of endometrial cancer ≥15%.21 Overall, the prevalence among postmenopausal women in the United States is roughly 0.7%,22 but it is considerably higher among women with polycystic ovarian syndrome, obesity, diabetes, early menarche, late menopause, nulliparity, a history of tamoxifen use, or hereditary nonpolyposis colorectal cancer.5 For women with PMB and any of these risk factors, physicians should consider EMB as the first diagnostic test.
Because EMB is a substandard test for diagnosing benign endometrial abnormalities, such as polyps and submucosa leiomyomas, TVUS may be a better starting point for women at lower risk for endometrial cancer. In any case, an ET >4 mm requires further investigation using EMB, saline infusion sonography (SIS), or hysteroscopy with biopsy.9 Patients with uterine pathology noted with TVUS should be referred for treatment.
Next step? Consider saline infusion sonography
SIS is a procedure in which sterile saline is infused into the endometrial cavity, then TVUS is performed. The saline solution distends the uterus, promoting visualization and thus providing more detail than a conventional ultrasound.
Because SIS is expensive and uncomfortable, it is used mainly as a second step in the evaluation of PMB. It is useful when:
- a diagnosis remains unclear after biopsy
- TVUS finds evidence of a focal lesion
- bleeding persists despite a normal initial workup
- the patient has a relative contraindication for hysteroscopy with D&C.23,24
SIS is contraindicated in cases in which cancer cells were detected with either EMB or TVUS, as the procedure has been associated with a small but real risk of malignant cell dissemination.25
CASE Irene’s history did not reveal any significant risk factors for endometrial cancer. Physical exam revealed a cervical polyp. We obtained a Pap smear, which was normal, and removed the polyp, which was benign. Irene also underwent TVUS because of her low risk status. The test revealed an endometrial stripe of <4 mm and an endometrial polyp, prompting referral to a specialist.
The patient underwent a hysteroscopy and D&C. Her endometrial polyp was benign and the endometrial scrapings revealed atrophic squamous mucosa. Irene has had no further bleeding and is doing well at this time.
CORRESPONDENCE Danette B. Null, MD, 2411 Fox Hollow, Lake Charles, LA 70605; dnull@lcmh.com
• Screen all women with postmenopausal vaginal bleeding (PMB) for endometrial cancer. A
• Use transvaginal ultrasound for the initial study for patients at low risk for endometrial cancer, and endometrial biopsy for those at higher risk. B
• Use saline infusion sonography as a second step in the evaluation of PMB if the diagnosis remains unclear after a biopsy or the bleeding persists despite a normal initial workup. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Irene R, age 55, comes to see you because of vaginal bleeding, which started 7 days ago. The patient reports that she stopped menstruating about 4 years ago and is not on hormone replacement therapy or taking any medication. Irene, who is married and in a monogamous relationship with her husband of 20 years, denies any vaginal irritation, discharge, or dyspareunia. Her uterus is intact and she had a Pap smear about a year ago.
What will you include in a workup to determine the cause of her bleeding?
Endometrial cancer is the most common malignancy of the female reproductive organs, with more than 43,000 new cases detected in the United States in 2010 alone.1 More than half of all cases of endometrial cancer are diagnosed in women between the ages of 50 and 69 years.1,2
Vaginal bleeding, which more than 90% of women with endometrial cancer experience,3 is often the first sign of malignancy. Thus, all women who present with postmenopausal bleeding (PMB)—defined as any vaginal bleeding occurring ≥1 year after cessation of menses or any unscheduled bleeding in women on hormone replacement therapy (HRT)—require further evaluation.
Prognosis for endometrial cancer depends on the extent of the disease at the time of diagnosis. Most cases are diagnosed in the early stages and have a 5-year survival rate greater than 96%.1 Surgery alone can be curative if the malignancy is contained within the uterus.1,2
What are the essential elements of a workup for a woman with PMB? Which lab tests should be ordered and which procedures performed? You’ll find the answers in the at-a-glance ALGORITHM4-6 we created, and in the additional information provided in this evidence-based review.
ALGORITHM
Postmenopausal bleeding: An evidence-based workup4-6
CBC, complete blood count; EMB, endometrial biopsy; ET, endometrial thickness; H&P, history and physical; Pap, Papanicolaou smear; SIS, saline infusion sonography; STD, sexually transmitted disease; TVUS, transvaginal ultrasonography.
*Laboratory tests are generally not helpful in evaluating postmenopausal bleeding, but a complete blood count is warranted if bleeding is prolonged or heavy and a test for sexually transmitted diseases may be appropriate based on patient history or physical exam.
Endometrial cancer is the key concern
While endometrial cancer is the most serious cause of PMB, it is not the most common. Atrophic endometrium is the culprit 60% to 80% of the time, while endometrial cancer accounts for up to 10% of cases. Endometrial polyps or hyperplasia, HRT, and cervical cancer are among the conditions included in the differential diagnosis (TABLE).7
A workup for PMB starts with a thorough medical history and a physical examination, including a Pap smear to screen for cervical cancer. Results from the Pap smear may suggest other pathology, such as benign endometrial cells, atypical endometrial cells, or atypical glandular cells.
Is lab work necessary? Laboratory tests are generally not helpful in evaluating PMB itself. A complete blood count is warranted if the bleeding is prolonged or heavy, however, and testing for sexually transmitted diseases may be appropriate, based on the patient’s history and/or physical exam.5,8
TABLE
Postmenopausal bleeding: The differential diagnosis7
| Cause | Incidence (%) |
|---|---|
| Atrophic endometrium | 60-80 |
| HRT | 15-25 |
| Endometrial cancer | 7-10 |
| Endometrial hyperplasia | 5-10 |
| Polyp(s) (endometrial or cervical) | 2-12 |
| Miscellaneous (uterine leiomyomas, cervicitis, atrophic vaginitis, tamoxifen therapy, trauma, anticoagulation) | <10 |
| HRT, hormone replacement therapy. | |
Endometrial biopsy or transvaginal ultrasound: Which test is better?
For many years, dilatation and curettage (D&C) of the endometrium was standard practice in the evaluation of patients with PMB. The Society of Radiologists in Ultrasound (SRU) and the American College of Obstetricians and Gynecologists (ACOG) now advise starting with either endometrial biopsy (EMB) or transvaginal ultrasound (TVUS).4,6 Both procedures are more advantageous than D&C for evaluating PMB because they can be done in an outpatient setting, are less expensive, provide faster results, and correlate with surgical findings more than 95% of the time.9
Although numerous studies have attempted to define the roles of EMB with Pipelle and TVUS, the literature is unclear as to which initial test is preferable.
Endometrial biopsy. Many physicians prefer to start with EMB, because it provides tissue samples for a histological diagnosis, is easily performed, and causes minimal cramping. The test does have limitations, such as difficulty in obtaining adequate tissue samples.
In a large cohort study (n=1535), EMB failed to provide an adequate tissue sample as much as 16% of the time.10 EMB’s sensitivity in detecting endometrial hyperplasia and cancer was 84%, the researchers reported, with a specificity of 99%; both the positive and negative predictive value were 94%. In a smaller study in which 97 women underwent TVUS and EMB was attempted, researchers reported that while no cases of endometrial cancer were missed when EMB sampling was successful, there was only a 27% probability of obtaining an adequate endometrial sample in women with an endometrial thickness (ET) <5 mm.11 EMB does a poorer job of detecting focal pathologies—with the potential to miss up to 18% of focal lesions, such as endometrial polyps, according to another study.12
Transvaginal ultrasound. TVUS is a safe, noninvasive, and cost-effective way to evaluate the endometrium, both to visualize focal lesions and assess ET. The technique, as defined by the SRU, involves scanning the uterus in a sagittal view and measuring the double-layer ET in the anteroposterior dimension from one basalis layer to the other.4
Reports of the sensitivity of TVUS in detecting endometrial cancer vary, depending on what cut point is used to rule it out. The SRU recommends an ET cutoff of ≤5 mm;4 ACOG recommends ≤4 mm.6 The consensus statements of both groups are based on a meta-analysis of 35 prospective studies that included data from nearly 6000 women with PMB. The sensitivity of TVUS in detecting endometrial cancer was 96%, whether the ≤4 or ≤5 mm cutoff was used, but specificity differed (53% for ≤4 mm vs 61% for ≤5 mm).13
In numerous studies with cut points of ≤4 or ≤5 mm, TVUS had a negative predictive value >99%.14-18 Because of this, ACOG states in an opinion issued in 2009 and reaffirmed in 2011, that TVUS is a “reasonable first approach.” The opinion further notes that in patients with an ET ≤4 mm, endometrial sampling is not required.6 In a meta-analysis of 3813 women, 3096 of whom were postmenopausal, researchers came to a different conclusion. An ET measurement on TVUS does not reduce the need for invasive diagnostic testing, the authors reported, because 4% of endometrial cancers would be missed even when a low threshold was used for reporting suspicious results.19
Type 2 endometrial cancer may be missed
A thin or indistinct endometrial lining on TVUS does not reliably exclude type 2 endometrial lesions20—which are not related to estrogen exposure or endometrial hyperplasia and typically present later in life, are diagnosed at a more advanced stage, and occur less frequently than type 1 endometrial cancer. A retrospective review of 52 patients with type 2 endometrial cancer found that 17% had an ET <4 mm, and another 17% had an indistinct endometrium.20
Factor risk level into decision-making
Researchers who conducted a decision analysis found that EMB is a more cost-effective initial diagnostic test for populations with a prevalence of endometrial cancer ≥15%.21 Overall, the prevalence among postmenopausal women in the United States is roughly 0.7%,22 but it is considerably higher among women with polycystic ovarian syndrome, obesity, diabetes, early menarche, late menopause, nulliparity, a history of tamoxifen use, or hereditary nonpolyposis colorectal cancer.5 For women with PMB and any of these risk factors, physicians should consider EMB as the first diagnostic test.
Because EMB is a substandard test for diagnosing benign endometrial abnormalities, such as polyps and submucosa leiomyomas, TVUS may be a better starting point for women at lower risk for endometrial cancer. In any case, an ET >4 mm requires further investigation using EMB, saline infusion sonography (SIS), or hysteroscopy with biopsy.9 Patients with uterine pathology noted with TVUS should be referred for treatment.
Next step? Consider saline infusion sonography
SIS is a procedure in which sterile saline is infused into the endometrial cavity, then TVUS is performed. The saline solution distends the uterus, promoting visualization and thus providing more detail than a conventional ultrasound.
Because SIS is expensive and uncomfortable, it is used mainly as a second step in the evaluation of PMB. It is useful when:
- a diagnosis remains unclear after biopsy
- TVUS finds evidence of a focal lesion
- bleeding persists despite a normal initial workup
- the patient has a relative contraindication for hysteroscopy with D&C.23,24
SIS is contraindicated in cases in which cancer cells were detected with either EMB or TVUS, as the procedure has been associated with a small but real risk of malignant cell dissemination.25
CASE Irene’s history did not reveal any significant risk factors for endometrial cancer. Physical exam revealed a cervical polyp. We obtained a Pap smear, which was normal, and removed the polyp, which was benign. Irene also underwent TVUS because of her low risk status. The test revealed an endometrial stripe of <4 mm and an endometrial polyp, prompting referral to a specialist.
The patient underwent a hysteroscopy and D&C. Her endometrial polyp was benign and the endometrial scrapings revealed atrophic squamous mucosa. Irene has had no further bleeding and is doing well at this time.
CORRESPONDENCE Danette B. Null, MD, 2411 Fox Hollow, Lake Charles, LA 70605; dnull@lcmh.com
1. American Cancer Society. Cancer facts and figures 2012. Atlanta: American Cancer Society; 2012.
2. Blair AR, Casas CM. Gynecologic cancers. Prim Care Clin Office Pract. 2009;36:115-130.
3. Doubilet PM. Society of Radiologists in Ultrasound consensus conference statement on postmenopausal bleeding. J Ultrasound Med. 2001;20:1037-1042.
4. Goldstein RB, Bree RL, Benson CB, et al. Evaluation of the woman with postmenopausal bleeding: Society of Radiologists in Ultrasound-Sponsored Consensus Conference statement. J Ultrasound Med. 2001;20:1025.-
5. Buchanan EM, Weinstein LC, Hillson C. Endometrial cancer. Am Fam Physician. 2009;80:1075-1080.
6. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 440: the role of transvaginal ultrasonography in the evaluation of postmenopausal bleeding. Obstet Gynecol. 2009;114:409-411.Available at: http://journals.lww.com/greenjournal/Citation/2009/08000/ACOG_Committee_Opinion_No__440__The_Role_of.33.aspx. Accessed September 19, 2012.
7. Hsu C, Chen C, Wang K. Assessment of postmenopausal bleeding. Int J Gerontol. 2008;2:55-59.
8. Mounsey AL. Postmenopausal bleeding evaluation and management. Clinics Fam Pract. 2002;4:173-192.
9. O’Connell L, Fries M, Zeringue E, et al. Triage of abnormal postmenopausal bleeding: a comparison of endometrial biopsy and transvaginal sonohysterography versus factional curettage with hysteroscopy. Am J Obstet Gynecol. 1998;178:956-961
10. Machado F, Moreno J, Carazo M, et al. Accuracy of endometrial biopsy with the Cornier Pipelle for diagnosis of endometrial cancer and a typical hyperplasia. Eur J Gynaecol Oncol. 2003;23:279-281.
11. Elsandabesee D, Greenwood P. The performance of Pipelle endometrial sampling in a dedicated postmenopausal bleeding clinic. J Obstet Gynaecol. 2005;25:32-34.
12. Goldstein SR, Zeltser I, Horan CK, et al. Ultrasonography-based triage for perimenopausal patients with abnormal uterine bleeding. Am J Obstet Gynecol. 1997;177:102-108.
13. Smith-Bindman R, Kerlikowske K, Feldstein VA, et al. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA. 1998;280:1510-1517.
14. Karlsson B, Granberg S, Wikland M, et al. Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding—a Nordic multicenter study. Am J Obstet Gynecol. 1995;172:1488-1494.
15. Ferrazi E, Torri V, Trio D, et al. Sonographic endometrial thickness: a useful test to predict atrophy in patients with postmenopausal bleeding. An Italian multicenter study. Ultrasound Obstet Gynecol. 1996;7:315-321.
16. Gull B, Carlsson K, Karlsson B, et al. Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding; is it always necessary to perform an endometrial biopsy? Am J Obstet Gynecol. 2000;182:509-515.
17. Epstein E, Valentin L. Rebleeding and endometrial growth in women with postmenopausal bleeding and endometrial thickness <5 mm managed by dilatation and curettage or ultrasound follow-up: a randomized controlled study. Ultrasound Obstet Gynecol. 2001;18:499-504.
18. Gull B, Karlsson B, Milsom I, et al. Can ultrasound replace dilatation and curettage? A longitudinal evaluation of postmenopausal bleeding and transvaginal sonographic measurement of the endometrium as predictors of endometrial cancer. Am J Obstet Gynecol. 2003;18:401-408.
19. Tabor A, Watt HC, Wald NJ. Endometrial thickness as a test for endometrial cancer in women with postmenopausal vaginal bleeding. Obstet Gynecol. 2002;99:663-670.
20. Wang J, Wieslander C, Hansen G, et al. Thin endometrial echo complex on ultrasound does not reliably exclude type 2 endometrial cancers. Gynecol Oncol. 2006;101:120-125.
21. Dijkhuizen FP, Mol BW, Brolmann HA, et al. Cost-effectiveness of the use of transvaginal sonography in the evaluation of postmenopausal bleeding. Maturitas. 2003;45:275-282.
22. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2008. Bethesda, Md: National Cancer Institute. Available at: http://seer.cancer.gov/csr/1975_2008, based on November 2010 SEER data submission, posted to SEER web site, 2011.
23. Kamel HS, Darwish AM, Mohamed SA. Comparison of transvaginal ultrasonography and vaginal sonohysterography in the detection of endometrial polyps. Acta Obstet Gynecol Scand. 2000;79:60-64.
24. Krampl E, Bourne T, Hurlen-Solbakken H, et al. Transvaginal ultrasonography sonohysterography and operative hysteroscopy for the evaluation of abnormal uterine bleeding. Acta Obstet Gynecol Scand. 2001;80:616-622.
25. Alcazar JL, Errasti T, Zornoza A. Saline infusion sonohysterography in endometrial cancer: assessment of malignant cells dissemination risk. Acta Obstet Gynecol Scand. 2000;79:321-322.
1. American Cancer Society. Cancer facts and figures 2012. Atlanta: American Cancer Society; 2012.
2. Blair AR, Casas CM. Gynecologic cancers. Prim Care Clin Office Pract. 2009;36:115-130.
3. Doubilet PM. Society of Radiologists in Ultrasound consensus conference statement on postmenopausal bleeding. J Ultrasound Med. 2001;20:1037-1042.
4. Goldstein RB, Bree RL, Benson CB, et al. Evaluation of the woman with postmenopausal bleeding: Society of Radiologists in Ultrasound-Sponsored Consensus Conference statement. J Ultrasound Med. 2001;20:1025.-
5. Buchanan EM, Weinstein LC, Hillson C. Endometrial cancer. Am Fam Physician. 2009;80:1075-1080.
6. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 440: the role of transvaginal ultrasonography in the evaluation of postmenopausal bleeding. Obstet Gynecol. 2009;114:409-411.Available at: http://journals.lww.com/greenjournal/Citation/2009/08000/ACOG_Committee_Opinion_No__440__The_Role_of.33.aspx. Accessed September 19, 2012.
7. Hsu C, Chen C, Wang K. Assessment of postmenopausal bleeding. Int J Gerontol. 2008;2:55-59.
8. Mounsey AL. Postmenopausal bleeding evaluation and management. Clinics Fam Pract. 2002;4:173-192.
9. O’Connell L, Fries M, Zeringue E, et al. Triage of abnormal postmenopausal bleeding: a comparison of endometrial biopsy and transvaginal sonohysterography versus factional curettage with hysteroscopy. Am J Obstet Gynecol. 1998;178:956-961
10. Machado F, Moreno J, Carazo M, et al. Accuracy of endometrial biopsy with the Cornier Pipelle for diagnosis of endometrial cancer and a typical hyperplasia. Eur J Gynaecol Oncol. 2003;23:279-281.
11. Elsandabesee D, Greenwood P. The performance of Pipelle endometrial sampling in a dedicated postmenopausal bleeding clinic. J Obstet Gynaecol. 2005;25:32-34.
12. Goldstein SR, Zeltser I, Horan CK, et al. Ultrasonography-based triage for perimenopausal patients with abnormal uterine bleeding. Am J Obstet Gynecol. 1997;177:102-108.
13. Smith-Bindman R, Kerlikowske K, Feldstein VA, et al. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA. 1998;280:1510-1517.
14. Karlsson B, Granberg S, Wikland M, et al. Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding—a Nordic multicenter study. Am J Obstet Gynecol. 1995;172:1488-1494.
15. Ferrazi E, Torri V, Trio D, et al. Sonographic endometrial thickness: a useful test to predict atrophy in patients with postmenopausal bleeding. An Italian multicenter study. Ultrasound Obstet Gynecol. 1996;7:315-321.
16. Gull B, Carlsson K, Karlsson B, et al. Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding; is it always necessary to perform an endometrial biopsy? Am J Obstet Gynecol. 2000;182:509-515.
17. Epstein E, Valentin L. Rebleeding and endometrial growth in women with postmenopausal bleeding and endometrial thickness <5 mm managed by dilatation and curettage or ultrasound follow-up: a randomized controlled study. Ultrasound Obstet Gynecol. 2001;18:499-504.
18. Gull B, Karlsson B, Milsom I, et al. Can ultrasound replace dilatation and curettage? A longitudinal evaluation of postmenopausal bleeding and transvaginal sonographic measurement of the endometrium as predictors of endometrial cancer. Am J Obstet Gynecol. 2003;18:401-408.
19. Tabor A, Watt HC, Wald NJ. Endometrial thickness as a test for endometrial cancer in women with postmenopausal vaginal bleeding. Obstet Gynecol. 2002;99:663-670.
20. Wang J, Wieslander C, Hansen G, et al. Thin endometrial echo complex on ultrasound does not reliably exclude type 2 endometrial cancers. Gynecol Oncol. 2006;101:120-125.
21. Dijkhuizen FP, Mol BW, Brolmann HA, et al. Cost-effectiveness of the use of transvaginal sonography in the evaluation of postmenopausal bleeding. Maturitas. 2003;45:275-282.
22. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2008. Bethesda, Md: National Cancer Institute. Available at: http://seer.cancer.gov/csr/1975_2008, based on November 2010 SEER data submission, posted to SEER web site, 2011.
23. Kamel HS, Darwish AM, Mohamed SA. Comparison of transvaginal ultrasonography and vaginal sonohysterography in the detection of endometrial polyps. Acta Obstet Gynecol Scand. 2000;79:60-64.
24. Krampl E, Bourne T, Hurlen-Solbakken H, et al. Transvaginal ultrasonography sonohysterography and operative hysteroscopy for the evaluation of abnormal uterine bleeding. Acta Obstet Gynecol Scand. 2001;80:616-622.
25. Alcazar JL, Errasti T, Zornoza A. Saline infusion sonohysterography in endometrial cancer: assessment of malignant cells dissemination risk. Acta Obstet Gynecol Scand. 2000;79:321-322.
Diagnosing and treating opioid dependence
• Ask all patients about the inappropriate use of substances, including prescription opioids. A
• Recommend pharmacotherapy for patients entering treatment for opioid dependence. A
• Warn patients who are opioid dependent about the risk of accidental fatal overdose, particularly with relapse. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Sam M, age 48, is in your office for the first time in more than 2 years. He has gained a considerable amount of weight and appears a bit sluggish, and you wonder whether he’s depressed. While taking a history, Sam reminds you that he was laid off 16 months ago and had been caring for his wife, who sustained a debilitating back injury. When you saw her recently, she told you she’s back to work and pain-free. So you’re taken aback when Sam asks you to refill his wife’s oxycodone prescription for lingering pain that often keeps her up at night.
If Sam were your patient, would you suspect opioid dependence?
Dependence on opioid analgesics and the adverse consequences associated with it have steadily increased during the past decade. Consider the following:
- Between 2004 and 2008, the number of emergency department visits related to nonmedical prescription opioid use more than doubled, rising by 111%.1
- The increasing prevalence of opioid abuse has led to a recent spike in unintentional deaths,2 with the number of lives lost to opioid analgesic overdose now exceeding that of heroin or cocaine.3
- More than 75% of opioids used for nonmedical purposes were prescribed for someone else.4
The course of opioid use is highly variable. Some people start with a legitimate medical prescription for an opioid analgesic, then continue taking it after the pain subsides. Others experiment briefly with nonmedical prescription opioids or use them intermittently without adverse effect. Some progress from prescription opioids to heroin, despite its dangers.5 Still others have a catastrophic outcome, such as an overdose or severe accident, the first time they use opioids.6 Rapid progression from misuse of opioids to dependence is most likely in vulnerable populations, such as those with concurrent mental illness, other substance use disorders, or increased sensitivity to pain.7
Understanding the terms. Before we continue, a word about terminology is in order. “Misuse” generally refers to the use of a medication in a manner (ie, purpose, dose, or frequency) other than its intended use, while “drug addiction” is the repeated use of a drug despite resulting harm. Here we will use “opioid dependence” to mean a pattern of increasing use characterized by significant impairment and distress and an inability to stop, and “opioid withdrawal” to reflect a constellation of symptoms, such as insomnia, nausea, diarrhea, and muscle aches, that can follow physiological dependence (though not necessarily opioid dependence). Our definitions of these terms are consistent with those of the American Psychiatric Association (APA).8 Worth noting, however, is the fact that as the APA prepares for the publication of the 5th edition of its Diagnostic and Statistical Manual of Mental Disorders, its Substance Disorder Work Group has proposed replacing the term “opioid dependence” with “opioid use disorder” to reduce the confusion associated with these definitions.9
Assessing illicit opioid use: Start with a targeted question
Most patients who are opioid dependent do not seek treatment for it,10 and are typically free of medical sequelae associated with drug addiction when they see family practitioners. The absence of self-reporting and obvious physical signs and symptoms, coupled with the increase in illicit use of prescription opioids, underscores the need for family physicians to identify patients who are abusing opioids and ensure that they get the help they need.
Screening tools. There are a number of screening tools you can use for this purpose—eg, CAGE-Adapted to Include Drugs (CAGE-AID) and Drug Abuse Screening Test (DAST)11,12—but they have not been found to be significantly better than a careful substance abuse history.13
Straightforward questions. You can start by asking, “Do you take any medications for pain?” If the answer is Yes, get the name of the drug and inquire about the frequency of use and the route, the amount typically taken, and the duration of the current use pattern. Ask specifically about opioids when taking a substance abuse history. After a question about alcohol use, you can say, “Do you use any other drugs in a serious way? Marijuana? Opioids like Percocet, Vicodin, or Oxycontin?” Although it can be very difficult to detect opioid dependence if the patient is not forthcoming, other likely indicators of drug-seeking behavior should trigger additional questions. (See “Opioid dependence: Red flags to keep in mind”.14-16)
“Brief” protocols. Recent studies of Screening, Brief Intervention, and Referral to Treatment (SBIRT) programs have found that the simple, time-limited interventions they offer (visit http://www.samhsa.gov/prevention/sbirt/SBIRTwhitepaper.pdf to learn more) lead to a reduction in self-reported illicit opioid use.17,18 Family physicians can readily incorporate SBIRT protocols into routine practice, as an evidence-based and often reimbursable approach to substance abuse.17
Suspect opioid dependence in a patient who:
- describes pain resulting from back or orthopedic injuries without corresponding documentation or imaging
- requests a specific opioid for pain management
- shows little interest in a physical exam, diagnostic testing, or nonpharmacological remedies
- talks about changes in work or relationship status
- ceases to participate in activities or hobbies that previously occupied a considerable amount of his or her time. This may signal social isolation or indicate that the patient is spending a great deal of time in pursuit of opioids.
Additional steps before initiating treatment
After screening and diagnostic evaluation provide evidence that a patient is opioid dependent, you can take several steps to guide him or her to the appropriate treatment.
A thorough biopsychosocial assessment covering co-occurring psychiatric illnesses, pain, psychosocial stressors contributing to opioid use, and infectious disease screening is required to gain a clear picture of the patient’s situation. In every case, acute emergencies such as suicidal ideation require immediate intervention, which may involve hospitalization.19
Assess the patient’s desire for help. After the initial assessment, it is often helpful to categorize the patient’s “stage of change” (precontemplation, contemplation, preparation, action, or maintenance),20 and to tailor your next step accordingly. A patient who denies that opioid use is a problem or is clearly ambivalent about seeking treatment may require a conversation that uses principles of motivational interviewing—a collaborative approach that aims to evoke and strengthen personal motivation for change.21 Consider a question that encourages him or her to express reasons for change, such as: “How would you like your current situation to be different?” As almost everyone abusing opioids has thoughts about stopping, such a question may help the patient focus on specific changes.
CASE When you question Sam about his interest in oxycodone, he breaks down. He’s been unable to find work or to lose the excess weight he gained during the many months he cared for his wife. He tells you that soon after his wife stopped taking the pain pills, he started taking them. At first, he took one occasionally. Then he started taking the opioids every day, and finally, whenever he awakened at night. Now, Sam says, he has no more pills, and he’s nauseous, depressed, and unable to sleep—and looking to you for help.
Sam fits the criteria for opioid withdrawal as a result of physiological dependence; further questioning reveals that he also suffers from opioid dependence, and that he is receptive to treatment.
Recommending treatment and following up
Several options are available for patients who, like Sam, have signs and symptoms of opioid withdrawal as a result of physiological dependence. You can provide a referral to a physician specializing in addiction, recommend detoxification and/or treatment in an inpatient facility, or initiate pharmacological treatment and provide a referral to a behavioral therapist. Whatever the initial approach, most patients will ultimately be treated as outpatients, with a combination of pharmacotherapy and behavioral therapy—often, with monitoring and oversight by a primary care physician. Which approach to pursue should be guided by evidence-based recommendations (TABLE)17,22-27 and jointly decided by physician and patient.
TABLE
Treating opioid dependence: Key clinical recommendations
| Recommendation | Evidence (SOR) | Comments |
|---|---|---|
| Screen all patients for substance use, including opioids. Brief interventions and referral to treatment when appropriate may reduce opioid use17,22 | Consistent findings from RCTs; evidence-based guideline (A) | SBIRT reduces self-reported opioid use; efforts to replicate such reports with objective evidence (eg, toxicology screens) are underway |
| Recommend maintenance medication (ie, buprenorphine, naltrexone, methadone) for all patients entering treatment for opioid dependence with physiological dependence; methadone is the safest for pregnant women23-25 | Consistent findings from RCTs; evidence-based guideline (A) | Methadone is the gold standard for pregnant women; further studies are needed to determine the safety of in utero exposure to buprenorphine and naltrexone |
| Keep patients on maintenance medication for ≥3 months; higher relapse rates are noted when medication is discontinued in <3 months23,24 | Consistent findings from RCTs (A) | Relapse rates are higher when maintenance medication is discontinued in <3 months |
| Caution patients with opioid dependence of the risk for accidental overdose and death with relapse and take action—eg, offering naloxone rescue kits to patients and families, as appropriate26 | Consistent findings from RCTs and prospective cohort studies; evidence-based guideline (A) | |
| Take steps to prevent diversion and accidental ingestion of agonist therapies, using tools such as frequent toxicology screens, random pill counts, and designated pharmacies, and monitoring adherence to psychosocial treatment26,27 | Practice guideline (consensus) (C) | |
| RCTs, randomized clinical trials; SBIRT, Screening, Brief Intervention, and Referral to Treatment; SOR, strength of recommendation. | ||
Medication plays a key role in recovery
Recommend medication-assisted treatment, either with an agonist (buprenorphine or methadone) or an antagonist (naltrexone), for every patient with physiological opioid dependence. The goals of pharmacotherapy are to prevent or reduce withdrawal symptoms and craving, avoid relapse, and restore to a normal state any physiological functions (eg, sleep, bowel movements) that have been disrupted by opioid use.28 When continued for ≥3 months, medication has been shown to improve outcomes.23,24,29 In one recent study, 49% of opioid-dependent participants who were still taking buprenorphine-naloxone at 12 weeks had successful outcomes (minimal or no opioid use), vs 7% of those undergoing a brief buprenorphine-naloxone taper.24
There are risks associated with medication-assisted therapy, however. The ones of greatest concern are a potential increase in drug-drug interactions, the risk of diversion (a concern with both buprenorphine and methadone), and the potential for accidental overdose.2,30
Buprenorphine, a partial mu-opioid receptor agonist, is a Schedule III controlled substance and can be dispensed by a pharmacy, making inpatient opioid detoxification unnecessary for many opioid-dependent patients. Physicians who wish to prescribe buprenorphine for the treatment of opioid dependence must complete an 8-hour course, offered by the American Medical Association and the APA, among other medical groups, and obtain a Drug Enforcement Administration code (“X”) license. 31
Buprenorphine has a high affinity for, and a slow dissociation from, mu-opioid receptors, resulting in the displacement of other opioids from the mu receptor and less severe withdrawal.32 As a partial agonist, buprenorphine attenuates opioid withdrawal symptoms with a ceiling, or near maximal, effect at 16 mg, thereby lowering the risk for overdose.33 A sublingual formulation that combines buprenorphine with naloxone, an opioid antagonist that exerts its full effect when injected but is minimally absorbed sublingually, reduces the potential for abuse of buprenorphine without interfering with its effectiveness.34
Compared with methadone, buprenorphine is less likely to interact with antiretroviral medications or to cause QTc prolongation, erectile dysfunction, or cognitive or psychomotor impairment.31,35-37 Limitations include the ceiling effect, which can be a problem for cases in which more agonist is needed; cost (approximately $12/d), and the lack of approval by the US Food and Drug Administration (FDA) for use during pregnancy.
Buprenorphine maintenance involves 3 phases: induction, stabilization, and maintenance.38 Induction takes place in a clinician’s office at the time the patient experiences opioid withdrawal symptoms, typically 6 to 48 hours after taking the last opioid. Extended treatment improves clinical outcomes,23,24 and longer-term maintenance (of indefinite duration) is frequently required.
Naltrexone is a mu-receptor antagonist, and therefore does not cause physical dependence or have agonist effects such as euphoria and sedation. As a result, it has no diversion value and may appeal to those who view opioid-agonist pharmacotherapy as simply trading one drug for another.39 Naltrexone is not a controlled substance and is not subject to the regulatory requirements that buprenorphine and methadone face.
Although agonists can be started in the first day or 2 after a patient decides to stop using opioids, patients must be opioid-free for ≥7 days before starting naltrexone. That’s because its antagonist properties will precipitate withdrawal if another opioid is present on the opioid receptors. During the 7-day “washout” period, you can treat opioid withdrawal symptoms with medications such as clonidine and dicyclomine, but such symptoms make patients especially vulnerable to relapse while waiting to start naltrexone.
Oral naltrexone’s effectiveness as a treatment for opioid dependence has been limited by poor adherence. But a long-acting intramuscular form of the drug, approved by the FDA in 2010 and requiring once-a-month injection, mitigates this concern.40,41
Methadone is a full mu-opioid agonist, administered daily at specialized clinics, as a maintenance therapy for opioid dependence. Although office-based physicians can prescribe methadone for pain, the drug can only be used for opioid dependence under the auspices of state- and federally regulated opioid treatment programs (http://findtreatment.samhsa.gov/TreatmentLocator/faces/quickSearch.jspx; a mobile phone application is also available at http://www.samhsa.gov/mobile/treatmentlocator.aspx).
Methadone, a Schedule III controlled substance with a half-life averaging 24 to 36 hours, requires daily dosing.42 Its slow metabolism and long half-life increase the risk for overdose.
Methadone is best for patients who are highly dependent on opioids and likely to benefit from a structured treatment environment with daily supervision (although patients who are doing well may earn take-home privileges so they don’t have to come to the clinic every day).43 New patients should receive an initial dose of 30 mg or less, and a maximum first-day dose of 40 mg.44
Methadone remains the standard of care for pregnant women being treated for opioid dependence, while studies of the effects of buprenorphine and naltrexone on a developing fetus continue. Although methadone’s efficacy, particularly in lower doses, is similar to that of buprenorphine,45 its adverse effect profile is worse. Adverse effects include drug-drug interactions, the potential for respiratory depression (especially when combined with alcohol or sedatives), QTc prolongation (which requires monitoring by electrocardiogram), sedation, and weight gain, and should be considered before selecting methadone as a maintenance pharmacotherapy.30,37,46 And, because relapse rates within 12 months of tapering off methadone have been reported to exceed 80%,47 both the clinician and the patient need to consider the likelihood of long-term, even lifelong, maintenance before initiating treatment.
Behavioral interventions are a vital part of the picture
Studies evaluating the extent to which various types and amounts of counseling improve outcomes compared with pharmacotherapy alone have had conflicting results.24,48 Nonetheless, most clinicians consider counseling to be a critical component of treatment for opioid dependence and recommend, at a minimum, either individual or group counseling (various modalities have been shown to be effective) and regular attendance at a self-help group like Narcotics Anonymous. Contingency management, a type of therapy that uses prizes as incentives for desired behaviors; and family therapy, individual counseling, and community-based programs have all been found to improve outcomes.6,49
CASE You refer Sam to an addiction psychiatrist, who stabilizes him on 16 mg buprenorphine/naloxone daily as part of an outpatient treatment program. Sam is enrolled in a weekly buprenorphine stabilization group, where he gives a urine sample each week. He also begins seeing a social worker weekly for counseling and attends Narcotics Anonymous meetings 2 to 3 times a week. At a follow-up appointment with you 6 months later, he reports that he has been abstinent from oxycodone for 6 months, his sleep is improved, and he feels better about his chances of finding another job.
Your role in safeguarding the patient
With the rising prevalence of opioid overdose, patient education aimed at crisis prevention is crucial, as well. Warn patients of the risk of accidental overdose, often associated with relapse, stressing the importance of continuing treatment and taking their maintenance medication exactly as prescribed.
There are other steps you can take to safeguard patients—eg, providing naloxone rescue kits to patients and their families when appropriate. You can also institute diversion and overdose prevention measures for patients taking buprenorphine or methadone—providing a lock box for take-home medication, implementing treatment contracts, and using a designated pharmacy to dispense buprenorphine, for example.26,27,50
Regular monitoring, urine drug screens (see TABLE W1), and random pill counts, in which patients are typically given 24 hours to bring in their prescribed medication so it can be counted, can also help keep patients on track. Treatment for concurrent psychiatric disorders—depression, anxiety, and personality disorders are common among patients with opioid dependence—is likely to improve the outcome of treatment, as well.
TABLE W1
Pharmacokinetics of common opioids: Time detectable in urine*
| Drug (half-life) | Time detectable in urine | Comment |
|---|---|---|
| Codeine (2.5-3 h) | 48 h | Pharmacogenetic-dependent effects may affect detection |
| Fentanyl Transdermal (17 h) Submucosal (7 h) | Not usually detected in urine (lack of metabolites) | Excretion of transdermal fentanyl can last days |
| Hydromorphone IR (2.3 h) ER (18.6 h) | 2-4 d | Significant interpatient variability |
| Methadone (8-59 h) | 3 d | |
| Morphine (1.5-2 h) | 48-72 h | 90% eliminated within 24 h |
| Oxycodone IR (3.2 h) ER (4.5 h) | Often not detected in urine | High-fat meals may increase serum concentrations of ER formulation |
| Propoxyphene Parent drug (6-12 h) Metabolite (30-36 h) | 6-48 h | |
| ER, extended release; IR, immediate release. *Previously appeared in: McBane S, Weige N. Is it time to drug test your chronic pain patient? J Fam Pract. 2010;59:628-633. Sources: Clinical Pharmacology [online]. Tampa, FL: Gold Standard Inc; 2010. Available at: http://cp.gsm.com. Accessed March 5, 2010; Drug Facts and Comparisons [online]. 2010. Available at: http://www.factsandcomparisons.com/. Accessed March 5, 2010. | ||
CORRESPONDENCE Kevin P. Hill, MD, MHS, McLean Hospital, 115 Mill Street, Belmont, MA 02478; khill@mclean.harvard.edu
1. Centers for Disease Control and Prevention (CDC). Emergency department visits involving nonmedical use of selected prescription drugs - United States, 2004-2008. MMWR Morb Mortal Wkly Rep. 2010;59:705-709.
2. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315-1321.
3. Warner M, Chen LH, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999-2006. NCHS Data Brief. 2009;(22):1-8.4.
4. Substance Abuse and Mental Health Services Administration. Results From the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. Rockville, Md: SAMHSA, Office of Applied Studies; 2010. NSDUH Series H-38A, HHS publication SMA 10-4856. Available at: http://www.samhsa.gov/data/NSDUH/2k9NSDUH/2k9Results.htm. Accessed August 22, 2012.
5. Hser YI, Huang D, Brecht ML, et al. Contrasting trajectories of heroin, cocaine, and methamphetamine use. J Addict Dis. 2008;27:13-21.
6. Veilleux JC, Colvin PJ, Anderson J, et al. A review of opioid dependence treatment: pharmacological and psychosocial interventions to treat opioid addiction. Clin Psychol Rev. 2011;30:155-166.
7. George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2011;35:232-247.
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text rev (DSM-IV-TR). Arlington, Va: American Psychiatric Association; 2000.
9. American Psychiatric Association. R 19 opioid use disorder. http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=460. Updated April 30, 2012. Accessed June 20, 2012.
10. Substance Abuse and Mental Health Services Administration. Results From the 2008 National Survey on Drug Use and Health: National Findings. Rockville, Md: SAMHSA, Office of Applied Studies; 2009. NSDUH Series H-36, HHS publication SMA 09-4434. Available at: http://www.samhsa.gov/data/nsduh/2k8nsduh/2k8Results.htm. Accessed August 22, 2012.
11. Brown RL, Rounds LA. Conjoint screening questionnaires for alcohol and other drug abuse: criterion validity in a primary care practice. Wis Med J. 1995;94:135-140.
12. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7:363-371.
13. US Preventive Services Task Force. Screening for Illicit Drug Use: U.S. Preventive Services Task Force Recommendation Statement. January 2008. Available at: http://www.uspreventiveservicestaskforce.org/uspstf08/druguse/drugrs.htm. Accessed May 7, 2012.
14. Gourlay D, Caplan Y, Heit H. Urine Drug Testing in Clinical Practice: Dispelling the Myths and Designing Strategies. San Francisco, Calif: California Academy of Family Physicians; 2006.
15. Jackman R, Purvis J, Mallett B. Chronic nonmalignant pain in primary care. Am Fam Physician. 2008;78:1155-1162.
16. McBane S, Weigle N. Is it time to drug test your chronic pain patient? J Fam Pract. 2010;59:628-633.
17. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
18. The InSight Project Research Group. SBIRT outcomes in Houston: final report on InSight, a hospital district-based program for patients at risk for alcohol or drug use problems. Alcohol Clin Exp Res. 2009;33:1374-1381.
19. Borges G, Walters EE, Kessler RC. Associations of substance use, abuse, and dependence with subsequent suicidal behavior. Am J Epidemiol. 2000;151:781-789.
20. Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51:390-395.
21. Smedslund G, Berg RC, Hammerstrom KT, et al. Motivational interviewing for substance abuse. Cochrane Database Syst Rev. 2011;(5):CD008063.-
22. Gryczynski J, Mitchell SG, Peterson TR, et al. The relationship between services delivered and substance use outcomes in New Mexico’s Screening, Brief Intervention, Referral and Treatment (SBIRT) Initiative. Drug Alcohol Depend. 2011;118:152-157.
23. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300:2003-2011.
24. Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68:1238-1246.
25. Johansson BA, Berglund M, Lindgren A. Efficacy of maintenance treatment with naltrexone for opioid dependence: a meta-analytical review. Addiction. 2006;101:491-503.
26. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613-2620.
27. Zacny J, Bigelow G, Compton P, et al. College on Problems of Drug Dependence taskforce on prescription opioid non-medical use and abuse: position statement. Drug Alcohol Depend. 2003;69:215-232.
28. Kreek MJ. Rationale for maintenance pharmacotherapy of opiate dependence. Res Publ Assoc Res Nerv Ment Dis. 1992;70:205-230.
29. Mattick RP, Breen C, Kimber J, et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;(3):CD002209.-
30. McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010;19:4-16.
31. Office of National Drug Control Policy Reauthorization Act of 2006 (ONDCPRA), HR 6344, 109th Cong, 2nd Sess (2006).
32. Lewis JW, Walter D. Buprenorphine—background to its development as a treatment for opiate dependence. In: Blaine JD, ed. Buprenorphine: An Alternative Treatment for Opioid Dependence. Rockville, Md: National Institute on Drug Abuse; 1992:5-11. NIDA Research Monograph, No. 121. Available at: http://archives.drugabuse.gov/pdf/monographs/121.pdf. Accessed August 22, 2012.
33. Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569-580.
34. Alho H, Sinclair D, Vuori E, et al. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88:75-78.
35. Hallinan R, Byrne A, Agho K, et al. Erectile dysfunction in men receiving methadone and buprenorphine maintenance treatment. J Sex Med. 2008;5:684-692.
36. Rapeli P, Fabritius C, Alho H, et al. Methadone vs. buprenorphine/naloxone during early opioid substitution treatment: a naturalistic comparison of cognitive performance relative to healthy controls. BMC Clin Pharmacol. 2007;7:5.-
37. Wedam EF, Bigelow GE, Johnson RE, et al. QT-interval effects of methadone, levomethadyl, and buprenorphine in a randomized trial. Arch Intern Med. 2007;167:2469-2475.
38. Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Rockville, Md: Substance Abuse and Mental Health Services Administration; 2004. Treatment Improvement Protocol (TIP) Series 40. DHHS publication SMA 04-3939.
39. Kleber HD. Methadone maintenance 4 decades later: thousands of lives saved but still controversial. JAMA. 2008;300:2303-2305.
40. Hulse GK, Morris N, Arnold-Reed D, et al. Improving clinical outcomes in treating heroin dependence: randomized, controlled trial of oral or implant naltrexone. Arch Gen Psychiatry. 2009;66:1108-1115.
41. US Food and Drug Administration. FDA approves injectable drug to treat opioid-dependent patients. October 12, 2010. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2010/ucm229109.htm. Accessed September 11, 2012.
42. Inturrisi CE, Verebely K. The levels of methadone in the plasma in methadone maintenance. Clin Pharmacol Ther. 1972;13 (5 pt 1):633-637.
43. Stitzer M, Bigelow G, Lawrence C, et al. Medication take-home as a reinforcer in a methadone maintenance program. Addict Behav. 1977;2:9-14.
44. Code of Federal Regulations. Title 42.8.12. Federal Opioid Treatment Standards. October 2010.
45. Johnson RE, Chutuape MA, Strain EC, et al. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343:1290-1297.
46. Krantz MJ, Martin J, Stimmel B, et al. QTc interval screening in methadone treatment. Ann Intern Med. 2009;150:387-395.
47. Ball JC, Lange WR, Myers CP, et al. Reducing the risk of AIDS through methadone maintenance treatment. J Health Soc Behav. 1988;29:214-226.
48. Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355:365-374.
49. Defulio A, Everly JJ, Leoutsakos JM, et al. Employment-based reinforcement of adherence to an FDA approved extended release formulation of naltrexone in opioid-dependent adults: a randomized controlled trial. Drug Alcohol Depend. 2012;120:48-54.
50. Savage SR. Management of opioid medications in patients with chronic pain and risk of substance misuse. Curr Psychiatry Rep. 2009;11:377-384.
• Ask all patients about the inappropriate use of substances, including prescription opioids. A
• Recommend pharmacotherapy for patients entering treatment for opioid dependence. A
• Warn patients who are opioid dependent about the risk of accidental fatal overdose, particularly with relapse. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Sam M, age 48, is in your office for the first time in more than 2 years. He has gained a considerable amount of weight and appears a bit sluggish, and you wonder whether he’s depressed. While taking a history, Sam reminds you that he was laid off 16 months ago and had been caring for his wife, who sustained a debilitating back injury. When you saw her recently, she told you she’s back to work and pain-free. So you’re taken aback when Sam asks you to refill his wife’s oxycodone prescription for lingering pain that often keeps her up at night.
If Sam were your patient, would you suspect opioid dependence?
Dependence on opioid analgesics and the adverse consequences associated with it have steadily increased during the past decade. Consider the following:
- Between 2004 and 2008, the number of emergency department visits related to nonmedical prescription opioid use more than doubled, rising by 111%.1
- The increasing prevalence of opioid abuse has led to a recent spike in unintentional deaths,2 with the number of lives lost to opioid analgesic overdose now exceeding that of heroin or cocaine.3
- More than 75% of opioids used for nonmedical purposes were prescribed for someone else.4
The course of opioid use is highly variable. Some people start with a legitimate medical prescription for an opioid analgesic, then continue taking it after the pain subsides. Others experiment briefly with nonmedical prescription opioids or use them intermittently without adverse effect. Some progress from prescription opioids to heroin, despite its dangers.5 Still others have a catastrophic outcome, such as an overdose or severe accident, the first time they use opioids.6 Rapid progression from misuse of opioids to dependence is most likely in vulnerable populations, such as those with concurrent mental illness, other substance use disorders, or increased sensitivity to pain.7
Understanding the terms. Before we continue, a word about terminology is in order. “Misuse” generally refers to the use of a medication in a manner (ie, purpose, dose, or frequency) other than its intended use, while “drug addiction” is the repeated use of a drug despite resulting harm. Here we will use “opioid dependence” to mean a pattern of increasing use characterized by significant impairment and distress and an inability to stop, and “opioid withdrawal” to reflect a constellation of symptoms, such as insomnia, nausea, diarrhea, and muscle aches, that can follow physiological dependence (though not necessarily opioid dependence). Our definitions of these terms are consistent with those of the American Psychiatric Association (APA).8 Worth noting, however, is the fact that as the APA prepares for the publication of the 5th edition of its Diagnostic and Statistical Manual of Mental Disorders, its Substance Disorder Work Group has proposed replacing the term “opioid dependence” with “opioid use disorder” to reduce the confusion associated with these definitions.9
Assessing illicit opioid use: Start with a targeted question
Most patients who are opioid dependent do not seek treatment for it,10 and are typically free of medical sequelae associated with drug addiction when they see family practitioners. The absence of self-reporting and obvious physical signs and symptoms, coupled with the increase in illicit use of prescription opioids, underscores the need for family physicians to identify patients who are abusing opioids and ensure that they get the help they need.
Screening tools. There are a number of screening tools you can use for this purpose—eg, CAGE-Adapted to Include Drugs (CAGE-AID) and Drug Abuse Screening Test (DAST)11,12—but they have not been found to be significantly better than a careful substance abuse history.13
Straightforward questions. You can start by asking, “Do you take any medications for pain?” If the answer is Yes, get the name of the drug and inquire about the frequency of use and the route, the amount typically taken, and the duration of the current use pattern. Ask specifically about opioids when taking a substance abuse history. After a question about alcohol use, you can say, “Do you use any other drugs in a serious way? Marijuana? Opioids like Percocet, Vicodin, or Oxycontin?” Although it can be very difficult to detect opioid dependence if the patient is not forthcoming, other likely indicators of drug-seeking behavior should trigger additional questions. (See “Opioid dependence: Red flags to keep in mind”.14-16)
“Brief” protocols. Recent studies of Screening, Brief Intervention, and Referral to Treatment (SBIRT) programs have found that the simple, time-limited interventions they offer (visit http://www.samhsa.gov/prevention/sbirt/SBIRTwhitepaper.pdf to learn more) lead to a reduction in self-reported illicit opioid use.17,18 Family physicians can readily incorporate SBIRT protocols into routine practice, as an evidence-based and often reimbursable approach to substance abuse.17
Suspect opioid dependence in a patient who:
- describes pain resulting from back or orthopedic injuries without corresponding documentation or imaging
- requests a specific opioid for pain management
- shows little interest in a physical exam, diagnostic testing, or nonpharmacological remedies
- talks about changes in work or relationship status
- ceases to participate in activities or hobbies that previously occupied a considerable amount of his or her time. This may signal social isolation or indicate that the patient is spending a great deal of time in pursuit of opioids.
Additional steps before initiating treatment
After screening and diagnostic evaluation provide evidence that a patient is opioid dependent, you can take several steps to guide him or her to the appropriate treatment.
A thorough biopsychosocial assessment covering co-occurring psychiatric illnesses, pain, psychosocial stressors contributing to opioid use, and infectious disease screening is required to gain a clear picture of the patient’s situation. In every case, acute emergencies such as suicidal ideation require immediate intervention, which may involve hospitalization.19
Assess the patient’s desire for help. After the initial assessment, it is often helpful to categorize the patient’s “stage of change” (precontemplation, contemplation, preparation, action, or maintenance),20 and to tailor your next step accordingly. A patient who denies that opioid use is a problem or is clearly ambivalent about seeking treatment may require a conversation that uses principles of motivational interviewing—a collaborative approach that aims to evoke and strengthen personal motivation for change.21 Consider a question that encourages him or her to express reasons for change, such as: “How would you like your current situation to be different?” As almost everyone abusing opioids has thoughts about stopping, such a question may help the patient focus on specific changes.
CASE When you question Sam about his interest in oxycodone, he breaks down. He’s been unable to find work or to lose the excess weight he gained during the many months he cared for his wife. He tells you that soon after his wife stopped taking the pain pills, he started taking them. At first, he took one occasionally. Then he started taking the opioids every day, and finally, whenever he awakened at night. Now, Sam says, he has no more pills, and he’s nauseous, depressed, and unable to sleep—and looking to you for help.
Sam fits the criteria for opioid withdrawal as a result of physiological dependence; further questioning reveals that he also suffers from opioid dependence, and that he is receptive to treatment.
Recommending treatment and following up
Several options are available for patients who, like Sam, have signs and symptoms of opioid withdrawal as a result of physiological dependence. You can provide a referral to a physician specializing in addiction, recommend detoxification and/or treatment in an inpatient facility, or initiate pharmacological treatment and provide a referral to a behavioral therapist. Whatever the initial approach, most patients will ultimately be treated as outpatients, with a combination of pharmacotherapy and behavioral therapy—often, with monitoring and oversight by a primary care physician. Which approach to pursue should be guided by evidence-based recommendations (TABLE)17,22-27 and jointly decided by physician and patient.
TABLE
Treating opioid dependence: Key clinical recommendations
| Recommendation | Evidence (SOR) | Comments |
|---|---|---|
| Screen all patients for substance use, including opioids. Brief interventions and referral to treatment when appropriate may reduce opioid use17,22 | Consistent findings from RCTs; evidence-based guideline (A) | SBIRT reduces self-reported opioid use; efforts to replicate such reports with objective evidence (eg, toxicology screens) are underway |
| Recommend maintenance medication (ie, buprenorphine, naltrexone, methadone) for all patients entering treatment for opioid dependence with physiological dependence; methadone is the safest for pregnant women23-25 | Consistent findings from RCTs; evidence-based guideline (A) | Methadone is the gold standard for pregnant women; further studies are needed to determine the safety of in utero exposure to buprenorphine and naltrexone |
| Keep patients on maintenance medication for ≥3 months; higher relapse rates are noted when medication is discontinued in <3 months23,24 | Consistent findings from RCTs (A) | Relapse rates are higher when maintenance medication is discontinued in <3 months |
| Caution patients with opioid dependence of the risk for accidental overdose and death with relapse and take action—eg, offering naloxone rescue kits to patients and families, as appropriate26 | Consistent findings from RCTs and prospective cohort studies; evidence-based guideline (A) | |
| Take steps to prevent diversion and accidental ingestion of agonist therapies, using tools such as frequent toxicology screens, random pill counts, and designated pharmacies, and monitoring adherence to psychosocial treatment26,27 | Practice guideline (consensus) (C) | |
| RCTs, randomized clinical trials; SBIRT, Screening, Brief Intervention, and Referral to Treatment; SOR, strength of recommendation. | ||
Medication plays a key role in recovery
Recommend medication-assisted treatment, either with an agonist (buprenorphine or methadone) or an antagonist (naltrexone), for every patient with physiological opioid dependence. The goals of pharmacotherapy are to prevent or reduce withdrawal symptoms and craving, avoid relapse, and restore to a normal state any physiological functions (eg, sleep, bowel movements) that have been disrupted by opioid use.28 When continued for ≥3 months, medication has been shown to improve outcomes.23,24,29 In one recent study, 49% of opioid-dependent participants who were still taking buprenorphine-naloxone at 12 weeks had successful outcomes (minimal or no opioid use), vs 7% of those undergoing a brief buprenorphine-naloxone taper.24
There are risks associated with medication-assisted therapy, however. The ones of greatest concern are a potential increase in drug-drug interactions, the risk of diversion (a concern with both buprenorphine and methadone), and the potential for accidental overdose.2,30
Buprenorphine, a partial mu-opioid receptor agonist, is a Schedule III controlled substance and can be dispensed by a pharmacy, making inpatient opioid detoxification unnecessary for many opioid-dependent patients. Physicians who wish to prescribe buprenorphine for the treatment of opioid dependence must complete an 8-hour course, offered by the American Medical Association and the APA, among other medical groups, and obtain a Drug Enforcement Administration code (“X”) license. 31
Buprenorphine has a high affinity for, and a slow dissociation from, mu-opioid receptors, resulting in the displacement of other opioids from the mu receptor and less severe withdrawal.32 As a partial agonist, buprenorphine attenuates opioid withdrawal symptoms with a ceiling, or near maximal, effect at 16 mg, thereby lowering the risk for overdose.33 A sublingual formulation that combines buprenorphine with naloxone, an opioid antagonist that exerts its full effect when injected but is minimally absorbed sublingually, reduces the potential for abuse of buprenorphine without interfering with its effectiveness.34
Compared with methadone, buprenorphine is less likely to interact with antiretroviral medications or to cause QTc prolongation, erectile dysfunction, or cognitive or psychomotor impairment.31,35-37 Limitations include the ceiling effect, which can be a problem for cases in which more agonist is needed; cost (approximately $12/d), and the lack of approval by the US Food and Drug Administration (FDA) for use during pregnancy.
Buprenorphine maintenance involves 3 phases: induction, stabilization, and maintenance.38 Induction takes place in a clinician’s office at the time the patient experiences opioid withdrawal symptoms, typically 6 to 48 hours after taking the last opioid. Extended treatment improves clinical outcomes,23,24 and longer-term maintenance (of indefinite duration) is frequently required.
Naltrexone is a mu-receptor antagonist, and therefore does not cause physical dependence or have agonist effects such as euphoria and sedation. As a result, it has no diversion value and may appeal to those who view opioid-agonist pharmacotherapy as simply trading one drug for another.39 Naltrexone is not a controlled substance and is not subject to the regulatory requirements that buprenorphine and methadone face.
Although agonists can be started in the first day or 2 after a patient decides to stop using opioids, patients must be opioid-free for ≥7 days before starting naltrexone. That’s because its antagonist properties will precipitate withdrawal if another opioid is present on the opioid receptors. During the 7-day “washout” period, you can treat opioid withdrawal symptoms with medications such as clonidine and dicyclomine, but such symptoms make patients especially vulnerable to relapse while waiting to start naltrexone.
Oral naltrexone’s effectiveness as a treatment for opioid dependence has been limited by poor adherence. But a long-acting intramuscular form of the drug, approved by the FDA in 2010 and requiring once-a-month injection, mitigates this concern.40,41
Methadone is a full mu-opioid agonist, administered daily at specialized clinics, as a maintenance therapy for opioid dependence. Although office-based physicians can prescribe methadone for pain, the drug can only be used for opioid dependence under the auspices of state- and federally regulated opioid treatment programs (http://findtreatment.samhsa.gov/TreatmentLocator/faces/quickSearch.jspx; a mobile phone application is also available at http://www.samhsa.gov/mobile/treatmentlocator.aspx).
Methadone, a Schedule III controlled substance with a half-life averaging 24 to 36 hours, requires daily dosing.42 Its slow metabolism and long half-life increase the risk for overdose.
Methadone is best for patients who are highly dependent on opioids and likely to benefit from a structured treatment environment with daily supervision (although patients who are doing well may earn take-home privileges so they don’t have to come to the clinic every day).43 New patients should receive an initial dose of 30 mg or less, and a maximum first-day dose of 40 mg.44
Methadone remains the standard of care for pregnant women being treated for opioid dependence, while studies of the effects of buprenorphine and naltrexone on a developing fetus continue. Although methadone’s efficacy, particularly in lower doses, is similar to that of buprenorphine,45 its adverse effect profile is worse. Adverse effects include drug-drug interactions, the potential for respiratory depression (especially when combined with alcohol or sedatives), QTc prolongation (which requires monitoring by electrocardiogram), sedation, and weight gain, and should be considered before selecting methadone as a maintenance pharmacotherapy.30,37,46 And, because relapse rates within 12 months of tapering off methadone have been reported to exceed 80%,47 both the clinician and the patient need to consider the likelihood of long-term, even lifelong, maintenance before initiating treatment.
Behavioral interventions are a vital part of the picture
Studies evaluating the extent to which various types and amounts of counseling improve outcomes compared with pharmacotherapy alone have had conflicting results.24,48 Nonetheless, most clinicians consider counseling to be a critical component of treatment for opioid dependence and recommend, at a minimum, either individual or group counseling (various modalities have been shown to be effective) and regular attendance at a self-help group like Narcotics Anonymous. Contingency management, a type of therapy that uses prizes as incentives for desired behaviors; and family therapy, individual counseling, and community-based programs have all been found to improve outcomes.6,49
CASE You refer Sam to an addiction psychiatrist, who stabilizes him on 16 mg buprenorphine/naloxone daily as part of an outpatient treatment program. Sam is enrolled in a weekly buprenorphine stabilization group, where he gives a urine sample each week. He also begins seeing a social worker weekly for counseling and attends Narcotics Anonymous meetings 2 to 3 times a week. At a follow-up appointment with you 6 months later, he reports that he has been abstinent from oxycodone for 6 months, his sleep is improved, and he feels better about his chances of finding another job.
Your role in safeguarding the patient
With the rising prevalence of opioid overdose, patient education aimed at crisis prevention is crucial, as well. Warn patients of the risk of accidental overdose, often associated with relapse, stressing the importance of continuing treatment and taking their maintenance medication exactly as prescribed.
There are other steps you can take to safeguard patients—eg, providing naloxone rescue kits to patients and their families when appropriate. You can also institute diversion and overdose prevention measures for patients taking buprenorphine or methadone—providing a lock box for take-home medication, implementing treatment contracts, and using a designated pharmacy to dispense buprenorphine, for example.26,27,50
Regular monitoring, urine drug screens (see TABLE W1), and random pill counts, in which patients are typically given 24 hours to bring in their prescribed medication so it can be counted, can also help keep patients on track. Treatment for concurrent psychiatric disorders—depression, anxiety, and personality disorders are common among patients with opioid dependence—is likely to improve the outcome of treatment, as well.
TABLE W1
Pharmacokinetics of common opioids: Time detectable in urine*
| Drug (half-life) | Time detectable in urine | Comment |
|---|---|---|
| Codeine (2.5-3 h) | 48 h | Pharmacogenetic-dependent effects may affect detection |
| Fentanyl Transdermal (17 h) Submucosal (7 h) | Not usually detected in urine (lack of metabolites) | Excretion of transdermal fentanyl can last days |
| Hydromorphone IR (2.3 h) ER (18.6 h) | 2-4 d | Significant interpatient variability |
| Methadone (8-59 h) | 3 d | |
| Morphine (1.5-2 h) | 48-72 h | 90% eliminated within 24 h |
| Oxycodone IR (3.2 h) ER (4.5 h) | Often not detected in urine | High-fat meals may increase serum concentrations of ER formulation |
| Propoxyphene Parent drug (6-12 h) Metabolite (30-36 h) | 6-48 h | |
| ER, extended release; IR, immediate release. *Previously appeared in: McBane S, Weige N. Is it time to drug test your chronic pain patient? J Fam Pract. 2010;59:628-633. Sources: Clinical Pharmacology [online]. Tampa, FL: Gold Standard Inc; 2010. Available at: http://cp.gsm.com. Accessed March 5, 2010; Drug Facts and Comparisons [online]. 2010. Available at: http://www.factsandcomparisons.com/. Accessed March 5, 2010. | ||
CORRESPONDENCE Kevin P. Hill, MD, MHS, McLean Hospital, 115 Mill Street, Belmont, MA 02478; khill@mclean.harvard.edu
• Ask all patients about the inappropriate use of substances, including prescription opioids. A
• Recommend pharmacotherapy for patients entering treatment for opioid dependence. A
• Warn patients who are opioid dependent about the risk of accidental fatal overdose, particularly with relapse. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Sam M, age 48, is in your office for the first time in more than 2 years. He has gained a considerable amount of weight and appears a bit sluggish, and you wonder whether he’s depressed. While taking a history, Sam reminds you that he was laid off 16 months ago and had been caring for his wife, who sustained a debilitating back injury. When you saw her recently, she told you she’s back to work and pain-free. So you’re taken aback when Sam asks you to refill his wife’s oxycodone prescription for lingering pain that often keeps her up at night.
If Sam were your patient, would you suspect opioid dependence?
Dependence on opioid analgesics and the adverse consequences associated with it have steadily increased during the past decade. Consider the following:
- Between 2004 and 2008, the number of emergency department visits related to nonmedical prescription opioid use more than doubled, rising by 111%.1
- The increasing prevalence of opioid abuse has led to a recent spike in unintentional deaths,2 with the number of lives lost to opioid analgesic overdose now exceeding that of heroin or cocaine.3
- More than 75% of opioids used for nonmedical purposes were prescribed for someone else.4
The course of opioid use is highly variable. Some people start with a legitimate medical prescription for an opioid analgesic, then continue taking it after the pain subsides. Others experiment briefly with nonmedical prescription opioids or use them intermittently without adverse effect. Some progress from prescription opioids to heroin, despite its dangers.5 Still others have a catastrophic outcome, such as an overdose or severe accident, the first time they use opioids.6 Rapid progression from misuse of opioids to dependence is most likely in vulnerable populations, such as those with concurrent mental illness, other substance use disorders, or increased sensitivity to pain.7
Understanding the terms. Before we continue, a word about terminology is in order. “Misuse” generally refers to the use of a medication in a manner (ie, purpose, dose, or frequency) other than its intended use, while “drug addiction” is the repeated use of a drug despite resulting harm. Here we will use “opioid dependence” to mean a pattern of increasing use characterized by significant impairment and distress and an inability to stop, and “opioid withdrawal” to reflect a constellation of symptoms, such as insomnia, nausea, diarrhea, and muscle aches, that can follow physiological dependence (though not necessarily opioid dependence). Our definitions of these terms are consistent with those of the American Psychiatric Association (APA).8 Worth noting, however, is the fact that as the APA prepares for the publication of the 5th edition of its Diagnostic and Statistical Manual of Mental Disorders, its Substance Disorder Work Group has proposed replacing the term “opioid dependence” with “opioid use disorder” to reduce the confusion associated with these definitions.9
Assessing illicit opioid use: Start with a targeted question
Most patients who are opioid dependent do not seek treatment for it,10 and are typically free of medical sequelae associated with drug addiction when they see family practitioners. The absence of self-reporting and obvious physical signs and symptoms, coupled with the increase in illicit use of prescription opioids, underscores the need for family physicians to identify patients who are abusing opioids and ensure that they get the help they need.
Screening tools. There are a number of screening tools you can use for this purpose—eg, CAGE-Adapted to Include Drugs (CAGE-AID) and Drug Abuse Screening Test (DAST)11,12—but they have not been found to be significantly better than a careful substance abuse history.13
Straightforward questions. You can start by asking, “Do you take any medications for pain?” If the answer is Yes, get the name of the drug and inquire about the frequency of use and the route, the amount typically taken, and the duration of the current use pattern. Ask specifically about opioids when taking a substance abuse history. After a question about alcohol use, you can say, “Do you use any other drugs in a serious way? Marijuana? Opioids like Percocet, Vicodin, or Oxycontin?” Although it can be very difficult to detect opioid dependence if the patient is not forthcoming, other likely indicators of drug-seeking behavior should trigger additional questions. (See “Opioid dependence: Red flags to keep in mind”.14-16)
“Brief” protocols. Recent studies of Screening, Brief Intervention, and Referral to Treatment (SBIRT) programs have found that the simple, time-limited interventions they offer (visit http://www.samhsa.gov/prevention/sbirt/SBIRTwhitepaper.pdf to learn more) lead to a reduction in self-reported illicit opioid use.17,18 Family physicians can readily incorporate SBIRT protocols into routine practice, as an evidence-based and often reimbursable approach to substance abuse.17
Suspect opioid dependence in a patient who:
- describes pain resulting from back or orthopedic injuries without corresponding documentation or imaging
- requests a specific opioid for pain management
- shows little interest in a physical exam, diagnostic testing, or nonpharmacological remedies
- talks about changes in work or relationship status
- ceases to participate in activities or hobbies that previously occupied a considerable amount of his or her time. This may signal social isolation or indicate that the patient is spending a great deal of time in pursuit of opioids.
Additional steps before initiating treatment
After screening and diagnostic evaluation provide evidence that a patient is opioid dependent, you can take several steps to guide him or her to the appropriate treatment.
A thorough biopsychosocial assessment covering co-occurring psychiatric illnesses, pain, psychosocial stressors contributing to opioid use, and infectious disease screening is required to gain a clear picture of the patient’s situation. In every case, acute emergencies such as suicidal ideation require immediate intervention, which may involve hospitalization.19
Assess the patient’s desire for help. After the initial assessment, it is often helpful to categorize the patient’s “stage of change” (precontemplation, contemplation, preparation, action, or maintenance),20 and to tailor your next step accordingly. A patient who denies that opioid use is a problem or is clearly ambivalent about seeking treatment may require a conversation that uses principles of motivational interviewing—a collaborative approach that aims to evoke and strengthen personal motivation for change.21 Consider a question that encourages him or her to express reasons for change, such as: “How would you like your current situation to be different?” As almost everyone abusing opioids has thoughts about stopping, such a question may help the patient focus on specific changes.
CASE When you question Sam about his interest in oxycodone, he breaks down. He’s been unable to find work or to lose the excess weight he gained during the many months he cared for his wife. He tells you that soon after his wife stopped taking the pain pills, he started taking them. At first, he took one occasionally. Then he started taking the opioids every day, and finally, whenever he awakened at night. Now, Sam says, he has no more pills, and he’s nauseous, depressed, and unable to sleep—and looking to you for help.
Sam fits the criteria for opioid withdrawal as a result of physiological dependence; further questioning reveals that he also suffers from opioid dependence, and that he is receptive to treatment.
Recommending treatment and following up
Several options are available for patients who, like Sam, have signs and symptoms of opioid withdrawal as a result of physiological dependence. You can provide a referral to a physician specializing in addiction, recommend detoxification and/or treatment in an inpatient facility, or initiate pharmacological treatment and provide a referral to a behavioral therapist. Whatever the initial approach, most patients will ultimately be treated as outpatients, with a combination of pharmacotherapy and behavioral therapy—often, with monitoring and oversight by a primary care physician. Which approach to pursue should be guided by evidence-based recommendations (TABLE)17,22-27 and jointly decided by physician and patient.
TABLE
Treating opioid dependence: Key clinical recommendations
| Recommendation | Evidence (SOR) | Comments |
|---|---|---|
| Screen all patients for substance use, including opioids. Brief interventions and referral to treatment when appropriate may reduce opioid use17,22 | Consistent findings from RCTs; evidence-based guideline (A) | SBIRT reduces self-reported opioid use; efforts to replicate such reports with objective evidence (eg, toxicology screens) are underway |
| Recommend maintenance medication (ie, buprenorphine, naltrexone, methadone) for all patients entering treatment for opioid dependence with physiological dependence; methadone is the safest for pregnant women23-25 | Consistent findings from RCTs; evidence-based guideline (A) | Methadone is the gold standard for pregnant women; further studies are needed to determine the safety of in utero exposure to buprenorphine and naltrexone |
| Keep patients on maintenance medication for ≥3 months; higher relapse rates are noted when medication is discontinued in <3 months23,24 | Consistent findings from RCTs (A) | Relapse rates are higher when maintenance medication is discontinued in <3 months |
| Caution patients with opioid dependence of the risk for accidental overdose and death with relapse and take action—eg, offering naloxone rescue kits to patients and families, as appropriate26 | Consistent findings from RCTs and prospective cohort studies; evidence-based guideline (A) | |
| Take steps to prevent diversion and accidental ingestion of agonist therapies, using tools such as frequent toxicology screens, random pill counts, and designated pharmacies, and monitoring adherence to psychosocial treatment26,27 | Practice guideline (consensus) (C) | |
| RCTs, randomized clinical trials; SBIRT, Screening, Brief Intervention, and Referral to Treatment; SOR, strength of recommendation. | ||
Medication plays a key role in recovery
Recommend medication-assisted treatment, either with an agonist (buprenorphine or methadone) or an antagonist (naltrexone), for every patient with physiological opioid dependence. The goals of pharmacotherapy are to prevent or reduce withdrawal symptoms and craving, avoid relapse, and restore to a normal state any physiological functions (eg, sleep, bowel movements) that have been disrupted by opioid use.28 When continued for ≥3 months, medication has been shown to improve outcomes.23,24,29 In one recent study, 49% of opioid-dependent participants who were still taking buprenorphine-naloxone at 12 weeks had successful outcomes (minimal or no opioid use), vs 7% of those undergoing a brief buprenorphine-naloxone taper.24
There are risks associated with medication-assisted therapy, however. The ones of greatest concern are a potential increase in drug-drug interactions, the risk of diversion (a concern with both buprenorphine and methadone), and the potential for accidental overdose.2,30
Buprenorphine, a partial mu-opioid receptor agonist, is a Schedule III controlled substance and can be dispensed by a pharmacy, making inpatient opioid detoxification unnecessary for many opioid-dependent patients. Physicians who wish to prescribe buprenorphine for the treatment of opioid dependence must complete an 8-hour course, offered by the American Medical Association and the APA, among other medical groups, and obtain a Drug Enforcement Administration code (“X”) license. 31
Buprenorphine has a high affinity for, and a slow dissociation from, mu-opioid receptors, resulting in the displacement of other opioids from the mu receptor and less severe withdrawal.32 As a partial agonist, buprenorphine attenuates opioid withdrawal symptoms with a ceiling, or near maximal, effect at 16 mg, thereby lowering the risk for overdose.33 A sublingual formulation that combines buprenorphine with naloxone, an opioid antagonist that exerts its full effect when injected but is minimally absorbed sublingually, reduces the potential for abuse of buprenorphine without interfering with its effectiveness.34
Compared with methadone, buprenorphine is less likely to interact with antiretroviral medications or to cause QTc prolongation, erectile dysfunction, or cognitive or psychomotor impairment.31,35-37 Limitations include the ceiling effect, which can be a problem for cases in which more agonist is needed; cost (approximately $12/d), and the lack of approval by the US Food and Drug Administration (FDA) for use during pregnancy.
Buprenorphine maintenance involves 3 phases: induction, stabilization, and maintenance.38 Induction takes place in a clinician’s office at the time the patient experiences opioid withdrawal symptoms, typically 6 to 48 hours after taking the last opioid. Extended treatment improves clinical outcomes,23,24 and longer-term maintenance (of indefinite duration) is frequently required.
Naltrexone is a mu-receptor antagonist, and therefore does not cause physical dependence or have agonist effects such as euphoria and sedation. As a result, it has no diversion value and may appeal to those who view opioid-agonist pharmacotherapy as simply trading one drug for another.39 Naltrexone is not a controlled substance and is not subject to the regulatory requirements that buprenorphine and methadone face.
Although agonists can be started in the first day or 2 after a patient decides to stop using opioids, patients must be opioid-free for ≥7 days before starting naltrexone. That’s because its antagonist properties will precipitate withdrawal if another opioid is present on the opioid receptors. During the 7-day “washout” period, you can treat opioid withdrawal symptoms with medications such as clonidine and dicyclomine, but such symptoms make patients especially vulnerable to relapse while waiting to start naltrexone.
Oral naltrexone’s effectiveness as a treatment for opioid dependence has been limited by poor adherence. But a long-acting intramuscular form of the drug, approved by the FDA in 2010 and requiring once-a-month injection, mitigates this concern.40,41
Methadone is a full mu-opioid agonist, administered daily at specialized clinics, as a maintenance therapy for opioid dependence. Although office-based physicians can prescribe methadone for pain, the drug can only be used for opioid dependence under the auspices of state- and federally regulated opioid treatment programs (http://findtreatment.samhsa.gov/TreatmentLocator/faces/quickSearch.jspx; a mobile phone application is also available at http://www.samhsa.gov/mobile/treatmentlocator.aspx).
Methadone, a Schedule III controlled substance with a half-life averaging 24 to 36 hours, requires daily dosing.42 Its slow metabolism and long half-life increase the risk for overdose.
Methadone is best for patients who are highly dependent on opioids and likely to benefit from a structured treatment environment with daily supervision (although patients who are doing well may earn take-home privileges so they don’t have to come to the clinic every day).43 New patients should receive an initial dose of 30 mg or less, and a maximum first-day dose of 40 mg.44
Methadone remains the standard of care for pregnant women being treated for opioid dependence, while studies of the effects of buprenorphine and naltrexone on a developing fetus continue. Although methadone’s efficacy, particularly in lower doses, is similar to that of buprenorphine,45 its adverse effect profile is worse. Adverse effects include drug-drug interactions, the potential for respiratory depression (especially when combined with alcohol or sedatives), QTc prolongation (which requires monitoring by electrocardiogram), sedation, and weight gain, and should be considered before selecting methadone as a maintenance pharmacotherapy.30,37,46 And, because relapse rates within 12 months of tapering off methadone have been reported to exceed 80%,47 both the clinician and the patient need to consider the likelihood of long-term, even lifelong, maintenance before initiating treatment.
Behavioral interventions are a vital part of the picture
Studies evaluating the extent to which various types and amounts of counseling improve outcomes compared with pharmacotherapy alone have had conflicting results.24,48 Nonetheless, most clinicians consider counseling to be a critical component of treatment for opioid dependence and recommend, at a minimum, either individual or group counseling (various modalities have been shown to be effective) and regular attendance at a self-help group like Narcotics Anonymous. Contingency management, a type of therapy that uses prizes as incentives for desired behaviors; and family therapy, individual counseling, and community-based programs have all been found to improve outcomes.6,49
CASE You refer Sam to an addiction psychiatrist, who stabilizes him on 16 mg buprenorphine/naloxone daily as part of an outpatient treatment program. Sam is enrolled in a weekly buprenorphine stabilization group, where he gives a urine sample each week. He also begins seeing a social worker weekly for counseling and attends Narcotics Anonymous meetings 2 to 3 times a week. At a follow-up appointment with you 6 months later, he reports that he has been abstinent from oxycodone for 6 months, his sleep is improved, and he feels better about his chances of finding another job.
Your role in safeguarding the patient
With the rising prevalence of opioid overdose, patient education aimed at crisis prevention is crucial, as well. Warn patients of the risk of accidental overdose, often associated with relapse, stressing the importance of continuing treatment and taking their maintenance medication exactly as prescribed.
There are other steps you can take to safeguard patients—eg, providing naloxone rescue kits to patients and their families when appropriate. You can also institute diversion and overdose prevention measures for patients taking buprenorphine or methadone—providing a lock box for take-home medication, implementing treatment contracts, and using a designated pharmacy to dispense buprenorphine, for example.26,27,50
Regular monitoring, urine drug screens (see TABLE W1), and random pill counts, in which patients are typically given 24 hours to bring in their prescribed medication so it can be counted, can also help keep patients on track. Treatment for concurrent psychiatric disorders—depression, anxiety, and personality disorders are common among patients with opioid dependence—is likely to improve the outcome of treatment, as well.
TABLE W1
Pharmacokinetics of common opioids: Time detectable in urine*
| Drug (half-life) | Time detectable in urine | Comment |
|---|---|---|
| Codeine (2.5-3 h) | 48 h | Pharmacogenetic-dependent effects may affect detection |
| Fentanyl Transdermal (17 h) Submucosal (7 h) | Not usually detected in urine (lack of metabolites) | Excretion of transdermal fentanyl can last days |
| Hydromorphone IR (2.3 h) ER (18.6 h) | 2-4 d | Significant interpatient variability |
| Methadone (8-59 h) | 3 d | |
| Morphine (1.5-2 h) | 48-72 h | 90% eliminated within 24 h |
| Oxycodone IR (3.2 h) ER (4.5 h) | Often not detected in urine | High-fat meals may increase serum concentrations of ER formulation |
| Propoxyphene Parent drug (6-12 h) Metabolite (30-36 h) | 6-48 h | |
| ER, extended release; IR, immediate release. *Previously appeared in: McBane S, Weige N. Is it time to drug test your chronic pain patient? J Fam Pract. 2010;59:628-633. Sources: Clinical Pharmacology [online]. Tampa, FL: Gold Standard Inc; 2010. Available at: http://cp.gsm.com. Accessed March 5, 2010; Drug Facts and Comparisons [online]. 2010. Available at: http://www.factsandcomparisons.com/. Accessed March 5, 2010. | ||
CORRESPONDENCE Kevin P. Hill, MD, MHS, McLean Hospital, 115 Mill Street, Belmont, MA 02478; khill@mclean.harvard.edu
1. Centers for Disease Control and Prevention (CDC). Emergency department visits involving nonmedical use of selected prescription drugs - United States, 2004-2008. MMWR Morb Mortal Wkly Rep. 2010;59:705-709.
2. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315-1321.
3. Warner M, Chen LH, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999-2006. NCHS Data Brief. 2009;(22):1-8.4.
4. Substance Abuse and Mental Health Services Administration. Results From the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. Rockville, Md: SAMHSA, Office of Applied Studies; 2010. NSDUH Series H-38A, HHS publication SMA 10-4856. Available at: http://www.samhsa.gov/data/NSDUH/2k9NSDUH/2k9Results.htm. Accessed August 22, 2012.
5. Hser YI, Huang D, Brecht ML, et al. Contrasting trajectories of heroin, cocaine, and methamphetamine use. J Addict Dis. 2008;27:13-21.
6. Veilleux JC, Colvin PJ, Anderson J, et al. A review of opioid dependence treatment: pharmacological and psychosocial interventions to treat opioid addiction. Clin Psychol Rev. 2011;30:155-166.
7. George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2011;35:232-247.
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text rev (DSM-IV-TR). Arlington, Va: American Psychiatric Association; 2000.
9. American Psychiatric Association. R 19 opioid use disorder. http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=460. Updated April 30, 2012. Accessed June 20, 2012.
10. Substance Abuse and Mental Health Services Administration. Results From the 2008 National Survey on Drug Use and Health: National Findings. Rockville, Md: SAMHSA, Office of Applied Studies; 2009. NSDUH Series H-36, HHS publication SMA 09-4434. Available at: http://www.samhsa.gov/data/nsduh/2k8nsduh/2k8Results.htm. Accessed August 22, 2012.
11. Brown RL, Rounds LA. Conjoint screening questionnaires for alcohol and other drug abuse: criterion validity in a primary care practice. Wis Med J. 1995;94:135-140.
12. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7:363-371.
13. US Preventive Services Task Force. Screening for Illicit Drug Use: U.S. Preventive Services Task Force Recommendation Statement. January 2008. Available at: http://www.uspreventiveservicestaskforce.org/uspstf08/druguse/drugrs.htm. Accessed May 7, 2012.
14. Gourlay D, Caplan Y, Heit H. Urine Drug Testing in Clinical Practice: Dispelling the Myths and Designing Strategies. San Francisco, Calif: California Academy of Family Physicians; 2006.
15. Jackman R, Purvis J, Mallett B. Chronic nonmalignant pain in primary care. Am Fam Physician. 2008;78:1155-1162.
16. McBane S, Weigle N. Is it time to drug test your chronic pain patient? J Fam Pract. 2010;59:628-633.
17. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
18. The InSight Project Research Group. SBIRT outcomes in Houston: final report on InSight, a hospital district-based program for patients at risk for alcohol or drug use problems. Alcohol Clin Exp Res. 2009;33:1374-1381.
19. Borges G, Walters EE, Kessler RC. Associations of substance use, abuse, and dependence with subsequent suicidal behavior. Am J Epidemiol. 2000;151:781-789.
20. Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51:390-395.
21. Smedslund G, Berg RC, Hammerstrom KT, et al. Motivational interviewing for substance abuse. Cochrane Database Syst Rev. 2011;(5):CD008063.-
22. Gryczynski J, Mitchell SG, Peterson TR, et al. The relationship between services delivered and substance use outcomes in New Mexico’s Screening, Brief Intervention, Referral and Treatment (SBIRT) Initiative. Drug Alcohol Depend. 2011;118:152-157.
23. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300:2003-2011.
24. Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68:1238-1246.
25. Johansson BA, Berglund M, Lindgren A. Efficacy of maintenance treatment with naltrexone for opioid dependence: a meta-analytical review. Addiction. 2006;101:491-503.
26. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613-2620.
27. Zacny J, Bigelow G, Compton P, et al. College on Problems of Drug Dependence taskforce on prescription opioid non-medical use and abuse: position statement. Drug Alcohol Depend. 2003;69:215-232.
28. Kreek MJ. Rationale for maintenance pharmacotherapy of opiate dependence. Res Publ Assoc Res Nerv Ment Dis. 1992;70:205-230.
29. Mattick RP, Breen C, Kimber J, et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;(3):CD002209.-
30. McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010;19:4-16.
31. Office of National Drug Control Policy Reauthorization Act of 2006 (ONDCPRA), HR 6344, 109th Cong, 2nd Sess (2006).
32. Lewis JW, Walter D. Buprenorphine—background to its development as a treatment for opiate dependence. In: Blaine JD, ed. Buprenorphine: An Alternative Treatment for Opioid Dependence. Rockville, Md: National Institute on Drug Abuse; 1992:5-11. NIDA Research Monograph, No. 121. Available at: http://archives.drugabuse.gov/pdf/monographs/121.pdf. Accessed August 22, 2012.
33. Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569-580.
34. Alho H, Sinclair D, Vuori E, et al. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88:75-78.
35. Hallinan R, Byrne A, Agho K, et al. Erectile dysfunction in men receiving methadone and buprenorphine maintenance treatment. J Sex Med. 2008;5:684-692.
36. Rapeli P, Fabritius C, Alho H, et al. Methadone vs. buprenorphine/naloxone during early opioid substitution treatment: a naturalistic comparison of cognitive performance relative to healthy controls. BMC Clin Pharmacol. 2007;7:5.-
37. Wedam EF, Bigelow GE, Johnson RE, et al. QT-interval effects of methadone, levomethadyl, and buprenorphine in a randomized trial. Arch Intern Med. 2007;167:2469-2475.
38. Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Rockville, Md: Substance Abuse and Mental Health Services Administration; 2004. Treatment Improvement Protocol (TIP) Series 40. DHHS publication SMA 04-3939.
39. Kleber HD. Methadone maintenance 4 decades later: thousands of lives saved but still controversial. JAMA. 2008;300:2303-2305.
40. Hulse GK, Morris N, Arnold-Reed D, et al. Improving clinical outcomes in treating heroin dependence: randomized, controlled trial of oral or implant naltrexone. Arch Gen Psychiatry. 2009;66:1108-1115.
41. US Food and Drug Administration. FDA approves injectable drug to treat opioid-dependent patients. October 12, 2010. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2010/ucm229109.htm. Accessed September 11, 2012.
42. Inturrisi CE, Verebely K. The levels of methadone in the plasma in methadone maintenance. Clin Pharmacol Ther. 1972;13 (5 pt 1):633-637.
43. Stitzer M, Bigelow G, Lawrence C, et al. Medication take-home as a reinforcer in a methadone maintenance program. Addict Behav. 1977;2:9-14.
44. Code of Federal Regulations. Title 42.8.12. Federal Opioid Treatment Standards. October 2010.
45. Johnson RE, Chutuape MA, Strain EC, et al. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343:1290-1297.
46. Krantz MJ, Martin J, Stimmel B, et al. QTc interval screening in methadone treatment. Ann Intern Med. 2009;150:387-395.
47. Ball JC, Lange WR, Myers CP, et al. Reducing the risk of AIDS through methadone maintenance treatment. J Health Soc Behav. 1988;29:214-226.
48. Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355:365-374.
49. Defulio A, Everly JJ, Leoutsakos JM, et al. Employment-based reinforcement of adherence to an FDA approved extended release formulation of naltrexone in opioid-dependent adults: a randomized controlled trial. Drug Alcohol Depend. 2012;120:48-54.
50. Savage SR. Management of opioid medications in patients with chronic pain and risk of substance misuse. Curr Psychiatry Rep. 2009;11:377-384.
1. Centers for Disease Control and Prevention (CDC). Emergency department visits involving nonmedical use of selected prescription drugs - United States, 2004-2008. MMWR Morb Mortal Wkly Rep. 2010;59:705-709.
2. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315-1321.
3. Warner M, Chen LH, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999-2006. NCHS Data Brief. 2009;(22):1-8.4.
4. Substance Abuse and Mental Health Services Administration. Results From the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. Rockville, Md: SAMHSA, Office of Applied Studies; 2010. NSDUH Series H-38A, HHS publication SMA 10-4856. Available at: http://www.samhsa.gov/data/NSDUH/2k9NSDUH/2k9Results.htm. Accessed August 22, 2012.
5. Hser YI, Huang D, Brecht ML, et al. Contrasting trajectories of heroin, cocaine, and methamphetamine use. J Addict Dis. 2008;27:13-21.
6. Veilleux JC, Colvin PJ, Anderson J, et al. A review of opioid dependence treatment: pharmacological and psychosocial interventions to treat opioid addiction. Clin Psychol Rev. 2011;30:155-166.
7. George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2011;35:232-247.
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text rev (DSM-IV-TR). Arlington, Va: American Psychiatric Association; 2000.
9. American Psychiatric Association. R 19 opioid use disorder. http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=460. Updated April 30, 2012. Accessed June 20, 2012.
10. Substance Abuse and Mental Health Services Administration. Results From the 2008 National Survey on Drug Use and Health: National Findings. Rockville, Md: SAMHSA, Office of Applied Studies; 2009. NSDUH Series H-36, HHS publication SMA 09-4434. Available at: http://www.samhsa.gov/data/nsduh/2k8nsduh/2k8Results.htm. Accessed August 22, 2012.
11. Brown RL, Rounds LA. Conjoint screening questionnaires for alcohol and other drug abuse: criterion validity in a primary care practice. Wis Med J. 1995;94:135-140.
12. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7:363-371.
13. US Preventive Services Task Force. Screening for Illicit Drug Use: U.S. Preventive Services Task Force Recommendation Statement. January 2008. Available at: http://www.uspreventiveservicestaskforce.org/uspstf08/druguse/drugrs.htm. Accessed May 7, 2012.
14. Gourlay D, Caplan Y, Heit H. Urine Drug Testing in Clinical Practice: Dispelling the Myths and Designing Strategies. San Francisco, Calif: California Academy of Family Physicians; 2006.
15. Jackman R, Purvis J, Mallett B. Chronic nonmalignant pain in primary care. Am Fam Physician. 2008;78:1155-1162.
16. McBane S, Weigle N. Is it time to drug test your chronic pain patient? J Fam Pract. 2010;59:628-633.
17. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
18. The InSight Project Research Group. SBIRT outcomes in Houston: final report on InSight, a hospital district-based program for patients at risk for alcohol or drug use problems. Alcohol Clin Exp Res. 2009;33:1374-1381.
19. Borges G, Walters EE, Kessler RC. Associations of substance use, abuse, and dependence with subsequent suicidal behavior. Am J Epidemiol. 2000;151:781-789.
20. Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51:390-395.
21. Smedslund G, Berg RC, Hammerstrom KT, et al. Motivational interviewing for substance abuse. Cochrane Database Syst Rev. 2011;(5):CD008063.-
22. Gryczynski J, Mitchell SG, Peterson TR, et al. The relationship between services delivered and substance use outcomes in New Mexico’s Screening, Brief Intervention, Referral and Treatment (SBIRT) Initiative. Drug Alcohol Depend. 2011;118:152-157.
23. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300:2003-2011.
24. Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68:1238-1246.
25. Johansson BA, Berglund M, Lindgren A. Efficacy of maintenance treatment with naltrexone for opioid dependence: a meta-analytical review. Addiction. 2006;101:491-503.
26. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613-2620.
27. Zacny J, Bigelow G, Compton P, et al. College on Problems of Drug Dependence taskforce on prescription opioid non-medical use and abuse: position statement. Drug Alcohol Depend. 2003;69:215-232.
28. Kreek MJ. Rationale for maintenance pharmacotherapy of opiate dependence. Res Publ Assoc Res Nerv Ment Dis. 1992;70:205-230.
29. Mattick RP, Breen C, Kimber J, et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;(3):CD002209.-
30. McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010;19:4-16.
31. Office of National Drug Control Policy Reauthorization Act of 2006 (ONDCPRA), HR 6344, 109th Cong, 2nd Sess (2006).
32. Lewis JW, Walter D. Buprenorphine—background to its development as a treatment for opiate dependence. In: Blaine JD, ed. Buprenorphine: An Alternative Treatment for Opioid Dependence. Rockville, Md: National Institute on Drug Abuse; 1992:5-11. NIDA Research Monograph, No. 121. Available at: http://archives.drugabuse.gov/pdf/monographs/121.pdf. Accessed August 22, 2012.
33. Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569-580.
34. Alho H, Sinclair D, Vuori E, et al. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88:75-78.
35. Hallinan R, Byrne A, Agho K, et al. Erectile dysfunction in men receiving methadone and buprenorphine maintenance treatment. J Sex Med. 2008;5:684-692.
36. Rapeli P, Fabritius C, Alho H, et al. Methadone vs. buprenorphine/naloxone during early opioid substitution treatment: a naturalistic comparison of cognitive performance relative to healthy controls. BMC Clin Pharmacol. 2007;7:5.-
37. Wedam EF, Bigelow GE, Johnson RE, et al. QT-interval effects of methadone, levomethadyl, and buprenorphine in a randomized trial. Arch Intern Med. 2007;167:2469-2475.
38. Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Rockville, Md: Substance Abuse and Mental Health Services Administration; 2004. Treatment Improvement Protocol (TIP) Series 40. DHHS publication SMA 04-3939.
39. Kleber HD. Methadone maintenance 4 decades later: thousands of lives saved but still controversial. JAMA. 2008;300:2303-2305.
40. Hulse GK, Morris N, Arnold-Reed D, et al. Improving clinical outcomes in treating heroin dependence: randomized, controlled trial of oral or implant naltrexone. Arch Gen Psychiatry. 2009;66:1108-1115.
41. US Food and Drug Administration. FDA approves injectable drug to treat opioid-dependent patients. October 12, 2010. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2010/ucm229109.htm. Accessed September 11, 2012.
42. Inturrisi CE, Verebely K. The levels of methadone in the plasma in methadone maintenance. Clin Pharmacol Ther. 1972;13 (5 pt 1):633-637.
43. Stitzer M, Bigelow G, Lawrence C, et al. Medication take-home as a reinforcer in a methadone maintenance program. Addict Behav. 1977;2:9-14.
44. Code of Federal Regulations. Title 42.8.12. Federal Opioid Treatment Standards. October 2010.
45. Johnson RE, Chutuape MA, Strain EC, et al. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343:1290-1297.
46. Krantz MJ, Martin J, Stimmel B, et al. QTc interval screening in methadone treatment. Ann Intern Med. 2009;150:387-395.
47. Ball JC, Lange WR, Myers CP, et al. Reducing the risk of AIDS through methadone maintenance treatment. J Health Soc Behav. 1988;29:214-226.
48. Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355:365-374.
49. Defulio A, Everly JJ, Leoutsakos JM, et al. Employment-based reinforcement of adherence to an FDA approved extended release formulation of naltrexone in opioid-dependent adults: a randomized controlled trial. Drug Alcohol Depend. 2012;120:48-54.
50. Savage SR. Management of opioid medications in patients with chronic pain and risk of substance misuse. Curr Psychiatry Rep. 2009;11:377-384.
Rhabdomyolysis after spin class?
Primary care physicians frequently encourage patients to lead a more active, healthy lifestyle. The rise in popularity of endurance events, yoga, and organized gym-based fitness classes has, no doubt, improved the health of those who participate. But what happens when an individual moves too quickly from a sedentary existence to a more physically active one?
In this article we describe 2 clinical cases of rhabdomyolysis that occurred after healthy individuals participated for the first time in a class involving high-intensity stationary cycling, known as “spinning.” This exercise activity originated in California around 1989 when a competitive cyclist introduced variable resistance and speed training to stationary cycle workouts.1 Over the last 10 years, spinning has gained a worldwide following as a means of building cardiovascular endurance while achieving a significant calorie burn.
CASE 1: Lack of conditioning, improper hydration spell trouble
A previously healthy 38-year-old white man presented with left lower extremity pain and dark urine. Three days earlier, he had participated in a spin class for the first time. Despite a sedentary lifestyle, he had no difficulty completing the session and felt fine during the class. He did feel mildly fatigued afterward. The next day, he played 18 holes of golf in hot, humid weather. He admitted to poor fluid intake, stating he “drank a few beers” during the round. The next day, he began noticing discomfort and swelling in his left knee, which progressed to his anterior thigh. That evening, he became concerned because of a dark red tint to his urine. He was not taking any medications.
The physical exam was unremarkable except for a moderately swollen, tender knee with reduced range of motion. An x-ray of the knee showed a moderate suprapatellar effusion, but no fracture or dislocation. Urinalysis was remarkable for blood and myoglobin. The CPK value was 149,985 U/L (normal, 24-170 U/L), AST was 2234 U/L (normal, 9-25 U/L), ALT was 570 U/L (normal, 7-30 U/L), and BMI was 26.6 kg/m2. Renal function was normal, as evidenced by a BUN of 17 mg/dL and a creatinine level of 1.0 mg/dL. He was afebrile and his WBC count was 9.6 x 103/mm3.
We hospitalized the patient with a diagnosis of rhabdomyolysis and started him on aggressive intravenous (IV) hydration. The patient’s CPK and transaminase levels started trending down the next day, urine output (UOP) remained at goal, and renal function remained stable. Pain and swelling diminished over the next 3 days. He was discharged home on Day 4. At discharge, his CPK level was 26,180 U/L, BUN 10 mg/dL, and creatinine 0.8 mg/dL. At 1 month follow-up, his CPK was within normal limits.
CASE 2: Even those who exercise regularly can overdo it
A previously healthy 26-year-old white woman sought care at our clinic complaining of bilateral leg pain and dark urine. Despite being overweight, she regularly engaged in moderate exercise, and 2 days prior had participated in her first spin class. She felt some discomfort 30 minutes into the class, and the next day noted discomfort in her anterior thighs, which progressively worsened. Two days after the workout, her pain was worse and her urine became reddish-brown. She was not taking any medications.
The physical exam was unremarkable except for antalgic gait and tenderness of the anterior thighs, which were also moderately firm and warm to the touch. Urinalysis showed a large blood concentration and was positive for myoglobin. ALT was 366 U/L, AST was 1383 U/L, CPK was 86,592 U/L, and BMI was 33.36 kg/m2. A BUN level of 11 mg/dL and creatinine level of 0.8 mg/dL suggested normal renal function. Her WBC count was 12.2 x 103/mm3.
We hospitalized the patient for a presumptive diagnosis of rhabdomyolysis, and initiated aggressive IV hydration to achieve a UOP of at least 200 mL/h. CPK levels and renal and liver function were closely monitored. On hospital Day 2, the patient’s thighs were tender and tight, so we consulted orthopedics about possible compartment syndrome. The consultant believed that intervention was unwarranted.
By Day 3, the swelling and pain began to resolve. UOP remained at target, and CPK and transaminase levels continued to trend down. Renal function remained stable. The patient was discharged home on Day 4 with a CPK of 11,388 U/L, BUN of 8 mg/dL, and creatinine of 0.7 mg/dL. At her 2-week follow-up, CPK was down to 772 U/L, and transaminases were within normal limits.
Discussion
Rhabdomyolysis occurs as a result of damage to the striated muscle cell membranes. Such injury releases into the systemic circulation calcium, potassium, phosphate, urate myoglobin, CPK, aldolase, lactate dehydrogenase, AST, and ALT. In the presence of excess calcium, further muscle fiber necrosis occurs and can lead to acute renal failure.2,3 Serum haptoglobin binding capacity becomes overly saturated. This results in free myoglobin, causing renal tubular obstruction. Myoglobin then dissociates into ferrihemate and globulin. Ferrihemate further exacerbates failure of the renal tubular transport system, eventually resulting in cell death and renal failure.2
Military trainees and casual athletes comprise many of the cases of exercise- induced rhabdomyolysis.4-6 People who exercise regularly are less likely to develop the condition than their more sedentary counterparts. As with our cases, a sudden increase in the intensity and duration of vigorous exercise, without proper training, may increase the likelihood of rhabdomyolysis.6
Other potential underlying causes. In addition to exercise and dehydration as depicted in our cases, rhabdomyolysis can result from burns, shock, acidosis, infections, crush trauma, immobility, malignancy, medications, toxins, abuse of drugs, or pre-existing illness such as sickle cell trait or other metabolic conditions.7,8
Clinical presentation varies. Regardless of the cause, patients typically present with muscle pain, weakness and cramping, and discolored urine.4,8 However, many patients will have dark urine associated with other symptoms, such as general malaise, visceral pain, swelling, muscle stiffness and tightness, fever, tachycardia, nausea, and vomiting.2,3 A careful history may help elucidate the cause.
Laboratory clues. Diagnostic guidelines commonly specify a serum CPK level 5 times the upper limit of normal as an indication of rhabdomyolysis, specifically in the exertional variety.9 Typically the level of this is around 1000 U/L.3 However, there is no agreement on what CPK level is diagnostic of rhabdomyolysis. Suggestions range from 1000 to 20,000 U/L.3,8 A CPK level in excess of 5000 U/L increases the risk for acute renal failure and renal cell death.3,10 In athletes, an elevated CPK after working out is not uncommon and may be much higher than in other individuals.6,8 Endurance exercises such as marathon running or cycling have been noted to elevate CPK for up to 2 hours postexercise.8
Myoglobin becomes detectable in urine when it exceeds 1.5 mg/dL.10 Urine becomes tea-colored or reddish-brown when myoglobin is >100 mg/dL.10
Complications from rhabdomyolysis include compartment syndrome, hyperkalemia, disseminated intravascular coagulation, coagulopathies, and acute renal failure.
Treatment for rhabdomyolysis consists of aggressive IV hydration with normal saline (with variable rate) or crystalloids to maintain a UOP of 200 to 300 mL/h.2,3,11 Avoid fluid overload in the elderly and those with renal or cardiac disease.2 As CPK and myoglobin continue to trend down, it’s important to adjust IV fluids and electrolyte replacement.2,11 Using bicarbonate to alkalinize the urine is controversial, with no studies showing any benefit.3,10 In severe situations, consider a nephrology consult for hemodialysis to bring down CPK, which may be secondary to renal failure and hyperkalemia.2,10 However, renal failure is less likely to occur in physically active, healthy athletes.
Advice after recovery. After an episode of acute rhabdomyolysis, conditioned athletes can return to physical training with resolution of their symptoms or a CPK level from 1000 to 5000 U/L, usually within a week.6 A more judicious approach may be needed for less fit individuals. Regardless of their fitness level, advise patients to avoid diuretics and alcohol before exercise, remain hydrated during and after exercise, and avoid overheating to decrease the likelihood of developing rhabdomyolysis.4 However, in patients with sickle cell trait, exertional sickling can occur with intensity of exercise without overheating.7
In the case of our male patient, poor physical conditioning and intensive, prolonged exercise followed by poor hydration and the diuretic effect of alcohol created the perfect storm for the development of rhabdomyolysis. On the other hand, our female patient routinely exercised, but still pushed herself beyond her limit and went too far too fast. Although BMI may play a role in the development of rhabdomyolysis, it does not appear to be as significant a factor as hydration status and overall physical conditioning.
Our patients’ prompt attention to the need for medical help and the recognition of the problem by their clinicians contributed to good outcomes in both cases.
CORRESPONDENCE Jacqueline DuBose, MD, Department of Family Medicine, Georgia Health Sciences University, 1120 15th Street, Augusta, GA 30912; jdubose@georgiahealth.edu
1. Metzker G. The man who put a new spin on stationary bikes. Los Angeles Times. April 17, 2000. Available at: http://articles.latimes.com/2000/apr/17/health/he-20459. Accessed February 7, 2012.
2. Sauret JM, Marinides G, Wang GK. Rhabdomyolysis. Am Fam Physician. 2002;65:907-912.
3. Cervellin G, Comelli I, Lippi G. Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med. 2010;48:749-756.
4. Sayers SP, Clarkson PM. Excercise-induced rhabdomyolysis. Curr Sports Med Rep. 2002;1:59-60.
5. Alpers JP, Jones LK. Natural history of exertional rhabdomyolysis: a population-based analysis. Muscle Nerve. 2010;42:487-491.
6. Eichner ER. Exertional rhabdomyolysis. Curr Sports Med Rep. 2008;7:3-4.
7. Eichner ER. Pearls and pitfalls: exertional sickling. Curr Sports Med Rep. 2010;9:3-4.
8. Clarkson PM, Eichner ER. Exertional rhabdomyolysis: does elevated blood creatine kinase foretell renal failure? Curr Sports Med Rep. 2006;5:57-60.
9. Capaccchione JF, Muldoon SM. The relationship between exertional heat illness, exertional rhabdomyolysis, and malignant hyperthermia. Anesth Analg. 2009;109:1065-1069.
10. Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9:158-169.
11. Young IM, Thomson K. Spinning-induced rhabdomyolysis: a case report. Eur J Emerg Med. 2004;11:358-359.
Primary care physicians frequently encourage patients to lead a more active, healthy lifestyle. The rise in popularity of endurance events, yoga, and organized gym-based fitness classes has, no doubt, improved the health of those who participate. But what happens when an individual moves too quickly from a sedentary existence to a more physically active one?
In this article we describe 2 clinical cases of rhabdomyolysis that occurred after healthy individuals participated for the first time in a class involving high-intensity stationary cycling, known as “spinning.” This exercise activity originated in California around 1989 when a competitive cyclist introduced variable resistance and speed training to stationary cycle workouts.1 Over the last 10 years, spinning has gained a worldwide following as a means of building cardiovascular endurance while achieving a significant calorie burn.
CASE 1: Lack of conditioning, improper hydration spell trouble
A previously healthy 38-year-old white man presented with left lower extremity pain and dark urine. Three days earlier, he had participated in a spin class for the first time. Despite a sedentary lifestyle, he had no difficulty completing the session and felt fine during the class. He did feel mildly fatigued afterward. The next day, he played 18 holes of golf in hot, humid weather. He admitted to poor fluid intake, stating he “drank a few beers” during the round. The next day, he began noticing discomfort and swelling in his left knee, which progressed to his anterior thigh. That evening, he became concerned because of a dark red tint to his urine. He was not taking any medications.
The physical exam was unremarkable except for a moderately swollen, tender knee with reduced range of motion. An x-ray of the knee showed a moderate suprapatellar effusion, but no fracture or dislocation. Urinalysis was remarkable for blood and myoglobin. The CPK value was 149,985 U/L (normal, 24-170 U/L), AST was 2234 U/L (normal, 9-25 U/L), ALT was 570 U/L (normal, 7-30 U/L), and BMI was 26.6 kg/m2. Renal function was normal, as evidenced by a BUN of 17 mg/dL and a creatinine level of 1.0 mg/dL. He was afebrile and his WBC count was 9.6 x 103/mm3.
We hospitalized the patient with a diagnosis of rhabdomyolysis and started him on aggressive intravenous (IV) hydration. The patient’s CPK and transaminase levels started trending down the next day, urine output (UOP) remained at goal, and renal function remained stable. Pain and swelling diminished over the next 3 days. He was discharged home on Day 4. At discharge, his CPK level was 26,180 U/L, BUN 10 mg/dL, and creatinine 0.8 mg/dL. At 1 month follow-up, his CPK was within normal limits.
CASE 2: Even those who exercise regularly can overdo it
A previously healthy 26-year-old white woman sought care at our clinic complaining of bilateral leg pain and dark urine. Despite being overweight, she regularly engaged in moderate exercise, and 2 days prior had participated in her first spin class. She felt some discomfort 30 minutes into the class, and the next day noted discomfort in her anterior thighs, which progressively worsened. Two days after the workout, her pain was worse and her urine became reddish-brown. She was not taking any medications.
The physical exam was unremarkable except for antalgic gait and tenderness of the anterior thighs, which were also moderately firm and warm to the touch. Urinalysis showed a large blood concentration and was positive for myoglobin. ALT was 366 U/L, AST was 1383 U/L, CPK was 86,592 U/L, and BMI was 33.36 kg/m2. A BUN level of 11 mg/dL and creatinine level of 0.8 mg/dL suggested normal renal function. Her WBC count was 12.2 x 103/mm3.
We hospitalized the patient for a presumptive diagnosis of rhabdomyolysis, and initiated aggressive IV hydration to achieve a UOP of at least 200 mL/h. CPK levels and renal and liver function were closely monitored. On hospital Day 2, the patient’s thighs were tender and tight, so we consulted orthopedics about possible compartment syndrome. The consultant believed that intervention was unwarranted.
By Day 3, the swelling and pain began to resolve. UOP remained at target, and CPK and transaminase levels continued to trend down. Renal function remained stable. The patient was discharged home on Day 4 with a CPK of 11,388 U/L, BUN of 8 mg/dL, and creatinine of 0.7 mg/dL. At her 2-week follow-up, CPK was down to 772 U/L, and transaminases were within normal limits.
Discussion
Rhabdomyolysis occurs as a result of damage to the striated muscle cell membranes. Such injury releases into the systemic circulation calcium, potassium, phosphate, urate myoglobin, CPK, aldolase, lactate dehydrogenase, AST, and ALT. In the presence of excess calcium, further muscle fiber necrosis occurs and can lead to acute renal failure.2,3 Serum haptoglobin binding capacity becomes overly saturated. This results in free myoglobin, causing renal tubular obstruction. Myoglobin then dissociates into ferrihemate and globulin. Ferrihemate further exacerbates failure of the renal tubular transport system, eventually resulting in cell death and renal failure.2
Military trainees and casual athletes comprise many of the cases of exercise- induced rhabdomyolysis.4-6 People who exercise regularly are less likely to develop the condition than their more sedentary counterparts. As with our cases, a sudden increase in the intensity and duration of vigorous exercise, without proper training, may increase the likelihood of rhabdomyolysis.6
Other potential underlying causes. In addition to exercise and dehydration as depicted in our cases, rhabdomyolysis can result from burns, shock, acidosis, infections, crush trauma, immobility, malignancy, medications, toxins, abuse of drugs, or pre-existing illness such as sickle cell trait or other metabolic conditions.7,8
Clinical presentation varies. Regardless of the cause, patients typically present with muscle pain, weakness and cramping, and discolored urine.4,8 However, many patients will have dark urine associated with other symptoms, such as general malaise, visceral pain, swelling, muscle stiffness and tightness, fever, tachycardia, nausea, and vomiting.2,3 A careful history may help elucidate the cause.
Laboratory clues. Diagnostic guidelines commonly specify a serum CPK level 5 times the upper limit of normal as an indication of rhabdomyolysis, specifically in the exertional variety.9 Typically the level of this is around 1000 U/L.3 However, there is no agreement on what CPK level is diagnostic of rhabdomyolysis. Suggestions range from 1000 to 20,000 U/L.3,8 A CPK level in excess of 5000 U/L increases the risk for acute renal failure and renal cell death.3,10 In athletes, an elevated CPK after working out is not uncommon and may be much higher than in other individuals.6,8 Endurance exercises such as marathon running or cycling have been noted to elevate CPK for up to 2 hours postexercise.8
Myoglobin becomes detectable in urine when it exceeds 1.5 mg/dL.10 Urine becomes tea-colored or reddish-brown when myoglobin is >100 mg/dL.10
Complications from rhabdomyolysis include compartment syndrome, hyperkalemia, disseminated intravascular coagulation, coagulopathies, and acute renal failure.
Treatment for rhabdomyolysis consists of aggressive IV hydration with normal saline (with variable rate) or crystalloids to maintain a UOP of 200 to 300 mL/h.2,3,11 Avoid fluid overload in the elderly and those with renal or cardiac disease.2 As CPK and myoglobin continue to trend down, it’s important to adjust IV fluids and electrolyte replacement.2,11 Using bicarbonate to alkalinize the urine is controversial, with no studies showing any benefit.3,10 In severe situations, consider a nephrology consult for hemodialysis to bring down CPK, which may be secondary to renal failure and hyperkalemia.2,10 However, renal failure is less likely to occur in physically active, healthy athletes.
Advice after recovery. After an episode of acute rhabdomyolysis, conditioned athletes can return to physical training with resolution of their symptoms or a CPK level from 1000 to 5000 U/L, usually within a week.6 A more judicious approach may be needed for less fit individuals. Regardless of their fitness level, advise patients to avoid diuretics and alcohol before exercise, remain hydrated during and after exercise, and avoid overheating to decrease the likelihood of developing rhabdomyolysis.4 However, in patients with sickle cell trait, exertional sickling can occur with intensity of exercise without overheating.7
In the case of our male patient, poor physical conditioning and intensive, prolonged exercise followed by poor hydration and the diuretic effect of alcohol created the perfect storm for the development of rhabdomyolysis. On the other hand, our female patient routinely exercised, but still pushed herself beyond her limit and went too far too fast. Although BMI may play a role in the development of rhabdomyolysis, it does not appear to be as significant a factor as hydration status and overall physical conditioning.
Our patients’ prompt attention to the need for medical help and the recognition of the problem by their clinicians contributed to good outcomes in both cases.
CORRESPONDENCE Jacqueline DuBose, MD, Department of Family Medicine, Georgia Health Sciences University, 1120 15th Street, Augusta, GA 30912; jdubose@georgiahealth.edu
Primary care physicians frequently encourage patients to lead a more active, healthy lifestyle. The rise in popularity of endurance events, yoga, and organized gym-based fitness classes has, no doubt, improved the health of those who participate. But what happens when an individual moves too quickly from a sedentary existence to a more physically active one?
In this article we describe 2 clinical cases of rhabdomyolysis that occurred after healthy individuals participated for the first time in a class involving high-intensity stationary cycling, known as “spinning.” This exercise activity originated in California around 1989 when a competitive cyclist introduced variable resistance and speed training to stationary cycle workouts.1 Over the last 10 years, spinning has gained a worldwide following as a means of building cardiovascular endurance while achieving a significant calorie burn.
CASE 1: Lack of conditioning, improper hydration spell trouble
A previously healthy 38-year-old white man presented with left lower extremity pain and dark urine. Three days earlier, he had participated in a spin class for the first time. Despite a sedentary lifestyle, he had no difficulty completing the session and felt fine during the class. He did feel mildly fatigued afterward. The next day, he played 18 holes of golf in hot, humid weather. He admitted to poor fluid intake, stating he “drank a few beers” during the round. The next day, he began noticing discomfort and swelling in his left knee, which progressed to his anterior thigh. That evening, he became concerned because of a dark red tint to his urine. He was not taking any medications.
The physical exam was unremarkable except for a moderately swollen, tender knee with reduced range of motion. An x-ray of the knee showed a moderate suprapatellar effusion, but no fracture or dislocation. Urinalysis was remarkable for blood and myoglobin. The CPK value was 149,985 U/L (normal, 24-170 U/L), AST was 2234 U/L (normal, 9-25 U/L), ALT was 570 U/L (normal, 7-30 U/L), and BMI was 26.6 kg/m2. Renal function was normal, as evidenced by a BUN of 17 mg/dL and a creatinine level of 1.0 mg/dL. He was afebrile and his WBC count was 9.6 x 103/mm3.
We hospitalized the patient with a diagnosis of rhabdomyolysis and started him on aggressive intravenous (IV) hydration. The patient’s CPK and transaminase levels started trending down the next day, urine output (UOP) remained at goal, and renal function remained stable. Pain and swelling diminished over the next 3 days. He was discharged home on Day 4. At discharge, his CPK level was 26,180 U/L, BUN 10 mg/dL, and creatinine 0.8 mg/dL. At 1 month follow-up, his CPK was within normal limits.
CASE 2: Even those who exercise regularly can overdo it
A previously healthy 26-year-old white woman sought care at our clinic complaining of bilateral leg pain and dark urine. Despite being overweight, she regularly engaged in moderate exercise, and 2 days prior had participated in her first spin class. She felt some discomfort 30 minutes into the class, and the next day noted discomfort in her anterior thighs, which progressively worsened. Two days after the workout, her pain was worse and her urine became reddish-brown. She was not taking any medications.
The physical exam was unremarkable except for antalgic gait and tenderness of the anterior thighs, which were also moderately firm and warm to the touch. Urinalysis showed a large blood concentration and was positive for myoglobin. ALT was 366 U/L, AST was 1383 U/L, CPK was 86,592 U/L, and BMI was 33.36 kg/m2. A BUN level of 11 mg/dL and creatinine level of 0.8 mg/dL suggested normal renal function. Her WBC count was 12.2 x 103/mm3.
We hospitalized the patient for a presumptive diagnosis of rhabdomyolysis, and initiated aggressive IV hydration to achieve a UOP of at least 200 mL/h. CPK levels and renal and liver function were closely monitored. On hospital Day 2, the patient’s thighs were tender and tight, so we consulted orthopedics about possible compartment syndrome. The consultant believed that intervention was unwarranted.
By Day 3, the swelling and pain began to resolve. UOP remained at target, and CPK and transaminase levels continued to trend down. Renal function remained stable. The patient was discharged home on Day 4 with a CPK of 11,388 U/L, BUN of 8 mg/dL, and creatinine of 0.7 mg/dL. At her 2-week follow-up, CPK was down to 772 U/L, and transaminases were within normal limits.
Discussion
Rhabdomyolysis occurs as a result of damage to the striated muscle cell membranes. Such injury releases into the systemic circulation calcium, potassium, phosphate, urate myoglobin, CPK, aldolase, lactate dehydrogenase, AST, and ALT. In the presence of excess calcium, further muscle fiber necrosis occurs and can lead to acute renal failure.2,3 Serum haptoglobin binding capacity becomes overly saturated. This results in free myoglobin, causing renal tubular obstruction. Myoglobin then dissociates into ferrihemate and globulin. Ferrihemate further exacerbates failure of the renal tubular transport system, eventually resulting in cell death and renal failure.2
Military trainees and casual athletes comprise many of the cases of exercise- induced rhabdomyolysis.4-6 People who exercise regularly are less likely to develop the condition than their more sedentary counterparts. As with our cases, a sudden increase in the intensity and duration of vigorous exercise, without proper training, may increase the likelihood of rhabdomyolysis.6
Other potential underlying causes. In addition to exercise and dehydration as depicted in our cases, rhabdomyolysis can result from burns, shock, acidosis, infections, crush trauma, immobility, malignancy, medications, toxins, abuse of drugs, or pre-existing illness such as sickle cell trait or other metabolic conditions.7,8
Clinical presentation varies. Regardless of the cause, patients typically present with muscle pain, weakness and cramping, and discolored urine.4,8 However, many patients will have dark urine associated with other symptoms, such as general malaise, visceral pain, swelling, muscle stiffness and tightness, fever, tachycardia, nausea, and vomiting.2,3 A careful history may help elucidate the cause.
Laboratory clues. Diagnostic guidelines commonly specify a serum CPK level 5 times the upper limit of normal as an indication of rhabdomyolysis, specifically in the exertional variety.9 Typically the level of this is around 1000 U/L.3 However, there is no agreement on what CPK level is diagnostic of rhabdomyolysis. Suggestions range from 1000 to 20,000 U/L.3,8 A CPK level in excess of 5000 U/L increases the risk for acute renal failure and renal cell death.3,10 In athletes, an elevated CPK after working out is not uncommon and may be much higher than in other individuals.6,8 Endurance exercises such as marathon running or cycling have been noted to elevate CPK for up to 2 hours postexercise.8
Myoglobin becomes detectable in urine when it exceeds 1.5 mg/dL.10 Urine becomes tea-colored or reddish-brown when myoglobin is >100 mg/dL.10
Complications from rhabdomyolysis include compartment syndrome, hyperkalemia, disseminated intravascular coagulation, coagulopathies, and acute renal failure.
Treatment for rhabdomyolysis consists of aggressive IV hydration with normal saline (with variable rate) or crystalloids to maintain a UOP of 200 to 300 mL/h.2,3,11 Avoid fluid overload in the elderly and those with renal or cardiac disease.2 As CPK and myoglobin continue to trend down, it’s important to adjust IV fluids and electrolyte replacement.2,11 Using bicarbonate to alkalinize the urine is controversial, with no studies showing any benefit.3,10 In severe situations, consider a nephrology consult for hemodialysis to bring down CPK, which may be secondary to renal failure and hyperkalemia.2,10 However, renal failure is less likely to occur in physically active, healthy athletes.
Advice after recovery. After an episode of acute rhabdomyolysis, conditioned athletes can return to physical training with resolution of their symptoms or a CPK level from 1000 to 5000 U/L, usually within a week.6 A more judicious approach may be needed for less fit individuals. Regardless of their fitness level, advise patients to avoid diuretics and alcohol before exercise, remain hydrated during and after exercise, and avoid overheating to decrease the likelihood of developing rhabdomyolysis.4 However, in patients with sickle cell trait, exertional sickling can occur with intensity of exercise without overheating.7
In the case of our male patient, poor physical conditioning and intensive, prolonged exercise followed by poor hydration and the diuretic effect of alcohol created the perfect storm for the development of rhabdomyolysis. On the other hand, our female patient routinely exercised, but still pushed herself beyond her limit and went too far too fast. Although BMI may play a role in the development of rhabdomyolysis, it does not appear to be as significant a factor as hydration status and overall physical conditioning.
Our patients’ prompt attention to the need for medical help and the recognition of the problem by their clinicians contributed to good outcomes in both cases.
CORRESPONDENCE Jacqueline DuBose, MD, Department of Family Medicine, Georgia Health Sciences University, 1120 15th Street, Augusta, GA 30912; jdubose@georgiahealth.edu
1. Metzker G. The man who put a new spin on stationary bikes. Los Angeles Times. April 17, 2000. Available at: http://articles.latimes.com/2000/apr/17/health/he-20459. Accessed February 7, 2012.
2. Sauret JM, Marinides G, Wang GK. Rhabdomyolysis. Am Fam Physician. 2002;65:907-912.
3. Cervellin G, Comelli I, Lippi G. Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med. 2010;48:749-756.
4. Sayers SP, Clarkson PM. Excercise-induced rhabdomyolysis. Curr Sports Med Rep. 2002;1:59-60.
5. Alpers JP, Jones LK. Natural history of exertional rhabdomyolysis: a population-based analysis. Muscle Nerve. 2010;42:487-491.
6. Eichner ER. Exertional rhabdomyolysis. Curr Sports Med Rep. 2008;7:3-4.
7. Eichner ER. Pearls and pitfalls: exertional sickling. Curr Sports Med Rep. 2010;9:3-4.
8. Clarkson PM, Eichner ER. Exertional rhabdomyolysis: does elevated blood creatine kinase foretell renal failure? Curr Sports Med Rep. 2006;5:57-60.
9. Capaccchione JF, Muldoon SM. The relationship between exertional heat illness, exertional rhabdomyolysis, and malignant hyperthermia. Anesth Analg. 2009;109:1065-1069.
10. Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9:158-169.
11. Young IM, Thomson K. Spinning-induced rhabdomyolysis: a case report. Eur J Emerg Med. 2004;11:358-359.
1. Metzker G. The man who put a new spin on stationary bikes. Los Angeles Times. April 17, 2000. Available at: http://articles.latimes.com/2000/apr/17/health/he-20459. Accessed February 7, 2012.
2. Sauret JM, Marinides G, Wang GK. Rhabdomyolysis. Am Fam Physician. 2002;65:907-912.
3. Cervellin G, Comelli I, Lippi G. Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med. 2010;48:749-756.
4. Sayers SP, Clarkson PM. Excercise-induced rhabdomyolysis. Curr Sports Med Rep. 2002;1:59-60.
5. Alpers JP, Jones LK. Natural history of exertional rhabdomyolysis: a population-based analysis. Muscle Nerve. 2010;42:487-491.
6. Eichner ER. Exertional rhabdomyolysis. Curr Sports Med Rep. 2008;7:3-4.
7. Eichner ER. Pearls and pitfalls: exertional sickling. Curr Sports Med Rep. 2010;9:3-4.
8. Clarkson PM, Eichner ER. Exertional rhabdomyolysis: does elevated blood creatine kinase foretell renal failure? Curr Sports Med Rep. 2006;5:57-60.
9. Capaccchione JF, Muldoon SM. The relationship between exertional heat illness, exertional rhabdomyolysis, and malignant hyperthermia. Anesth Analg. 2009;109:1065-1069.
10. Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9:158-169.
11. Young IM, Thomson K. Spinning-induced rhabdomyolysis: a case report. Eur J Emerg Med. 2004;11:358-359.