User login
Acoustic neuroma: What the evidence says about evaluation and treatment
• The gold standard for diagnosis of acoustic neuroma is contrast magnetic resonance imaging. A
• Consider watchful waiting for tumors <1 cm in the elderly, medically infirm, or patients with serviceable hearing who opt for a more conservative approach. C
• The best treatment option for a tumor >1 cm and <3 cm varies. Base your decision on tumor size and the patient’s age, comorbidities, and preference. B
• Recommend microsurgery for tumors >3 cm. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
When a patient presents with unilateral hearing loss—especially with a report of gradual onset— that is accompanied by tinnitus, consider an acoustic neuroma (AN). Also known as vestibular schwannoma, ANs represent just 9% of all intracranial tumors. But its relatively slow growth rate can insidiously lead to impaired quality of life and even complete hearing loss.1 Vestibular symptoms, such as vertigo, may be present. And in some instances, tinnitus may be absent. Even without hearing loss or other symptoms, ANs may be detected incidentally on magnetic resonance imaging (MRI) performed for other reasons. In this article, I describe the diagnostic work-up for AN and 3 options for treatment.
How acoustic neuroma arises
ANs evolve from the abnormal growth and proliferation of Schwann cells, or neurolemmocytes, at their junction with glial cells surrounding the vestibular nerve. ANs represent 80% of all cerebellopontine angle tumors.1 The incidence in the general population is about 1 in 100,000 people per year, with equal distribution between men and women.1 Among adults with sensorineural hearing loss, about 1% have ANs.2 Tumor growth within the internal auditory canal and resultant compression of cranial nerves VII and VIII causes associated symptoms. Unabated growth can lead to prolapse into the cerebellopontine angle with compression of the brainstem and cerebellum.1
There are 2 major types of AN. The sporadic type, which will be the focus of this discussion, occurs in 95% of all cases, is unilateral, and usually affects individuals 40 to 60 years old. AN associated with neurofibromatosis type 2 is typically bilateral, autosomal dominant, and usually affects teens and young adults.1
The only known possible risk factor for the development of AN is the use of cell phones, but data are conflicting. Although 2 meta-analyses did find a correlation between AN and ipsilateral cell phone use >10 years, these studies were limited by possible recall bias and misclassification.3,4
How acoustic neuroma can present
Slowly progressive, high-frequency, sensori-neural hearing loss is typical for AN. In most cases, hearing loss is unilateral. Nerve compression and stretching from the growing neuroma cause hearing loss of gradual onset.1 However, up to 25% of patients may present with sudden onset hearing loss due to total occlusion of the internal auditory canal and the artery supplying the cochlea.1 Tinnitus is reported in up to 70% of patients with AN.1 The mechanism of injury is the same as for hearing loss.
A feeling of imbalance or unsteadiness occurs in more than 50% of patients with AN.1 One research group found that vertigo was the more common symptom in patients with smaller tumors, whereas patients with larger tumors complained more of dysequilibrium.1
Other physical findings can include trigeminal nerve dysfunction (found in 50% of patients with AN, but patients rarely note the absent corneal reflex), facial nerve motor dysfunction (2%), increased intracranial pressure (rare in tumors <3 cm), and brainstem and cerebellar symptoms (rare).1
Asymptomatic AN. Small tumors may be detected by MRI incidentally before the onset of hearing loss. Such a finding accounts for about 12% of all patients diagnosed with AN.5 A 2005 study by Lin et al found the prevalence of incidental AN to be about 2 per 10,000 people.6 This is higher than the 1 in 100,000 suggested by epidemiologic studies,6 but Lin’s study was performed at a large tertiary center.
Diagnostic evaluation
Contrast MRI is the gold standard for diagnosing AN.2 One study did find that a heavily T2-weighted noncontrast scan in the hands of an experienced radiologist reduced the procedural cost and was as effective as contrast MRI in evaluating the VII and VIII cranial nerves within the cerebellopontine angle and internal auditory canal.7
Although not as sensitive for small tumors, the auditory brainstem response can be used in certain circumstances. During the initial evaluation, other diagnoses to consider are facial neuroma and jugular foramen tumors.1
Three options for treatment
The goal of treatment is to slow or eliminate both tumor growth and deterioration in hearing and neurological function. Lin’s study found that 43% of patients with incidental AN had abnormal audiometry findings.6 A hearing evaluation may therefore be helpful in guiding patients through a meaningful discussion about prognosis, further testing and consultations, and the 3 therapeutic options—watchful waiting, microsurgery, and stereotactic radiosurgery.8
Watchful waiting has been recommended for the elderly and for infirm patients with tumors <1 cm who would be poor candidates for surgery or radiation. In up to 57% of AN cases, no further tumor growth occurs after diagnosis; in about 8% of these cases, tumor regression is noted.9 A little more than half of patients will experience further hearing loss.9
Unfortunately, there are no prediction rules for determining who is most at risk for tumor growth and hearing loss. A recent study found that conservative management was a cost-effective approach for tumors <1.5 cm in any age group,10 provided there was no increase in complications from continued tumor growth. This, then, is a third group of patients for whom watchful waiting might be an option.10 Follow-up MRI can be used as a surveillance tool.8,9,11
Microsurgery. This option is the oldest and best-studied treatment for AN. Micro-surgery appears to provide the best tumor control, although morbidity and mortality remain risks. A systematic review by Yamakami in 2003 showed that microsurgery completely removed 96% of ANs, with tumor recurrence, mortality, and major disability rates of 1.8%, 0.63%, and 2.9%, respectively.12 More recent reviews have shown mortality rates of approximately 0.1%.13 Surgery usually involves removal of cranial nerve VIII, with the risk of damage to cranial nerve VII. Nerve-sparing procedures are available. Cerebral spinal fluid leaks and meningitis are occasional adverse events. The experience of the surgical team can affect outcome, including complications and cost.14
Stereotactic radiosurgery. Through the use of sophisticated imaging devices and 3-dimensional treatment-planning computers, stereotactic irradiation allows much more specific targeting of the AN, with significantly less radiation delivered to surrounding healthy tissues.15 Dynamic beam shaping and intensity modulation provide flexibility and enable delivery of much higher radiation doses to the tumor, resulting in greater control rates and decreased complications. There are 3 delivery technologies: Gamma Knife, proton beam, and specially modified linear accelerators.
Whereas older studies did not provide sufficient evidence to support the use of low-dose over high-dose radiation for long-term control of ANs,16 a more recent systematic analysis by Yang et al seems to indicate that patients treated with the lower dose (12.5 Gy) did equally as well with better preservation of hearing.17
Applying the evidence in practice
A large randomized controlled trial comparing these treatment options has yet to be done and, indeed, would be difficult to conduct due to the small number of AN cases, varying surgical expertise among centers, the different treatment goals inherent in the 3 therapies, and the risk involved in each. Evidence to date generally indicates that observation is appropriate for small intracanalicular tumors (<1 cm) in the elderly, medically infirm, or asymptomatic patients who understand and opt for this management approach. For tumors ≥3 cm, evidence supports microsurgery as optimal management.13,18 Tumors falling between these extremes pose the real challenge.
Over the last 10 years, numerous studies have demonstrated good tumor control with either microsurgery or radiosurgery, but with varying degrees of hearing preservation and permanent nerve injury to the facial and trigeminal nerve. There is also a concern for malignant transformation of AN after radiosurgery, with 8 cases reported in the literature.8,13,19
Three evidence-based studies in the last 6 years have compared the 2 interventions. In 2002, Nikolopoulos et al reviewed 111 studies and concluded there was insufficient evidence to support one approach over the other.18 Pollock conducted a prospective cohort study in 2008 that showed superior outcomes in facial movement, hearing preservation, and Health Status Questionnaire subscales for patients undergoing stereotactic radiosurgery.13 This study was limited to nonfractionated radiosurgery, and follow-up varied from 12 to 62 months.
In 2009, a Norwegian prospective study of 91 patients reported better facial nerve and hearing outcomes from radiosurgery for medium and small tumors. This study was well performed, but it looked at only a small, non-randomized population.20 The same author in 2005 had found that, from the patient perspective, cranial nerve function and overall outcomes were better in the radiosurgery group.21
Stereotactic radiosurgery does confer lower risks for acute treatment complications than microsurgery, and therefore can be advantageous for patients who are older, infirm, require anticoagulant therapy, are otherwise poor candidates for surgery or anesthesia, or have serviceable hearing and opt for a more conservative approach. Other advantages of stereotactic radiosurgery over microsurgery are its lower cost and its preferred use in patients with permanent hearing loss in the unaffected ear. These advantages of stereotactic radiosurgery may, however, be offset in the long term by cranial neuropathy and eventual hearing loss, which can be comparable to the experiences of patients after microsurgery.8
Based on these limited studies, patient-oriented outcomes can be comparable between stereotactic radiosurgery and micro-surgery in medium-sized tumors (1-2.9 cm), depending on the patient’s clinical presentation. Hearing and preservation of nerve VII are more likely with radiosurgery than with microsurgery. But several real and potentially large confounding factors limit this interpretation. How does one define tumor control in light of AN’s inherently slow growth? What about reports of less than optimal microsurgery outcomes if previous radiosurgery has failed? And, although it is small, a definite risk of malignant transformation exists after irradiation.8,13
For patients to make good decisions, family physicians need to be aware of these issues when discussing treatment options. To aid in patient education and decision making, there is a helpful algorithm from the International RadioSurgery Association in Stereotactic radiosurgery for patients with vestibular schwannomas (available online at http://www.irsa.org/ANGuideline.pdf).15
Follow-up
Follow-up depends on the treatment modality, but usually relies on MRI to document tumor behavior. MRI studies are typically performed at predetermined intervals such as 6 months and 1, 2, and 4 years. For patients with preserved serviceable hearing, audiograms are recommended at intervals coinciding with clinical and neuroimaging re-evaluations. Tumors proven to be stable over 4 to 5 years can subsequently be reassessed at 2- to 4-year intervals.15
Enabling recovery. One of the most important educational opportunities involves early vestibular rehabilitation to facilitate recovery of postural control after treatment. Research has shown benefit from customized vestibular rehabilitation in addition to instructions that stress the need to engage in some type of activity, such as walking or other modes of exercise.22
CORRESPONDENCE
Robert McDonald, MD, Spartanburg Family Medicine Residency Program, 853 N. Church Street, Suite 510, Spartanburg, SC 29303; rmcdonald@srhs.com
1. Ho SY, Kveton JF. Acoustic neuroma assessment and management. Otolaryngol Clin North Am. 2002;35:393-404.
2. Zealley IA, Cooper RC, Clifford KM, et al. MRI screening for acoustic neuroma: a comparison of fast spin echo and contrast enhanced imaging in 1233 patients. Br J Radiol. 2000;73:242-247.
3. Hardell L, Carlberg M, Söderqvist F, et al. Meta-analysis of long-term mobile phone use and the association with brain tumors. Int J Oncol. 2008;32:1097-1103.
4. Khurana VG, Teo C, Kundi M, et al. Cell phones and brain tumors: a review including the long-term epidemiologic data. Surg Neurol. 2009;72:205-214.
5. DynaMed [database online]. Acoustic neuroma. Updated August 18, 2009. Available at http://www.ebscohost.com/dynamed. Accessed August 24, 2009.
6. Lin D, Hegarty JL, Fischbein NJ, et al. The prevalence of “incidental” acoustic neuroma. Arch Otolaryngol Head Neck Surg. 2005;131:241-244.
7. Fortnum H, O’Neill C, Taylor R, et al. The role of magnetic resonance imaging in the identification of suspected acoustic neuroma: a systematic review of clinical and cost effectiveness and natural history. Health Technol Assess. 2009;13:iii-iv,ix-xi,1-154.
8. Diaz R, Brodie H. Gamma knife and other stereotactic radiotherapies for acoustic neuroma. April 10, 2009. Available at http://emedicine.medscape.com/article/857604-overview. Accessed August 24, 2009.
9. Smouha EE, Yoo M, Mohr K, et al. Conservative management of acoustic neuroma: a meta-analysis and proposed treatment algorithm. Laryngoscope. 2005;115:450-454.
10. Verma S, Anthony R, Tsai V, et al. Evaluation of cost effectiveness and active management strategies for acoustic neuroma. Clin Otolaryngol. 2009;34:438-446.
11. Yoshimoto Y. Systematic review of the natural history of vestibular schwannoma. J Neurosurg. 2005;103:59-63.
12. Yamakami I, Uchino Y, Kobayashi E, et al. Conservative management, gamma-knife radiosurgery, and microsurgery for acoustic neuromas: a systematic review of outcome and risk of three therapeutic options. Neurol Res. 2003;25:682-690.
13. Pollock BE. Vestibular schwannoma management: an evidence-based comparison of stereotactic radiosurgery and microsurgical resection. Prog Neurol Surg. 2008;21:222-227.
14. Slattery WH, Schwartz MS, Fisher LM, et al. Acoustic neuroma surgical cost and outcome by hospital volume in California. Otolaryngol Head Neck Surg. 2004;130:726-735.
15. International RadioSurgery Association (IRSA). Stereotactic radiosurgery for patients with vestibular schwannomas. Harrisburg, PA: International RadioSurgery Association; 2006. Radio-surgery practice guideline report, no. 4-06. Available at: http://www.irsa.org/AN%20Guideline.pdf. Accessed August 24, 2009.
16. Weil RS, Cohen JM, Portarena I, et al. Optimal dose of stereotactic radiosurgery for acoustic neuromas: a systematic review. Br J Neurosurg. 2006;20:195-202.
17. Yang I, Aranda D, Han SJ, et al. Hearing preservation after radio-surgery for vestibular schwannoma: a systematic review. J Clin Neurosci. 2009;16:742-747.
18. Nikolopoulos TP, O’Donoghue GM. Acoustic neuroma management: an evidence-based medicine approach. Otol Neurotol. 2002;23:534-541.
19. Shin M, Ueki K, Kurita H, et al. Malignant transformation of a vestibular schwannoma after gamma knife surgery. Lancet. 2002;360:309-310.
20. Myrseth E, Moller P, Pedersen PH, et al. Vestibular schwannoma: surgery or gamma knife radiosurgery? A prospective, nonrandomized study. Neurosurgery. 2009;64:654-661.
21. Myrseth E, Moller P, Pedersen PH, et al. Vestibular schwannomas: clinical results and quality of life after microsurgery or gamma knife radiosurgery. Neurosurgery. 2005;56:927-935.
22. Vereeck L, Wuyts FL, Truijen S, et al. The effect of early customized vestibular rehabilitation on balance after acoustic neuroma resection. Clin Rehabil. 2008;22:698-713.
• The gold standard for diagnosis of acoustic neuroma is contrast magnetic resonance imaging. A
• Consider watchful waiting for tumors <1 cm in the elderly, medically infirm, or patients with serviceable hearing who opt for a more conservative approach. C
• The best treatment option for a tumor >1 cm and <3 cm varies. Base your decision on tumor size and the patient’s age, comorbidities, and preference. B
• Recommend microsurgery for tumors >3 cm. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
When a patient presents with unilateral hearing loss—especially with a report of gradual onset— that is accompanied by tinnitus, consider an acoustic neuroma (AN). Also known as vestibular schwannoma, ANs represent just 9% of all intracranial tumors. But its relatively slow growth rate can insidiously lead to impaired quality of life and even complete hearing loss.1 Vestibular symptoms, such as vertigo, may be present. And in some instances, tinnitus may be absent. Even without hearing loss or other symptoms, ANs may be detected incidentally on magnetic resonance imaging (MRI) performed for other reasons. In this article, I describe the diagnostic work-up for AN and 3 options for treatment.
How acoustic neuroma arises
ANs evolve from the abnormal growth and proliferation of Schwann cells, or neurolemmocytes, at their junction with glial cells surrounding the vestibular nerve. ANs represent 80% of all cerebellopontine angle tumors.1 The incidence in the general population is about 1 in 100,000 people per year, with equal distribution between men and women.1 Among adults with sensorineural hearing loss, about 1% have ANs.2 Tumor growth within the internal auditory canal and resultant compression of cranial nerves VII and VIII causes associated symptoms. Unabated growth can lead to prolapse into the cerebellopontine angle with compression of the brainstem and cerebellum.1
There are 2 major types of AN. The sporadic type, which will be the focus of this discussion, occurs in 95% of all cases, is unilateral, and usually affects individuals 40 to 60 years old. AN associated with neurofibromatosis type 2 is typically bilateral, autosomal dominant, and usually affects teens and young adults.1
The only known possible risk factor for the development of AN is the use of cell phones, but data are conflicting. Although 2 meta-analyses did find a correlation between AN and ipsilateral cell phone use >10 years, these studies were limited by possible recall bias and misclassification.3,4
How acoustic neuroma can present
Slowly progressive, high-frequency, sensori-neural hearing loss is typical for AN. In most cases, hearing loss is unilateral. Nerve compression and stretching from the growing neuroma cause hearing loss of gradual onset.1 However, up to 25% of patients may present with sudden onset hearing loss due to total occlusion of the internal auditory canal and the artery supplying the cochlea.1 Tinnitus is reported in up to 70% of patients with AN.1 The mechanism of injury is the same as for hearing loss.
A feeling of imbalance or unsteadiness occurs in more than 50% of patients with AN.1 One research group found that vertigo was the more common symptom in patients with smaller tumors, whereas patients with larger tumors complained more of dysequilibrium.1
Other physical findings can include trigeminal nerve dysfunction (found in 50% of patients with AN, but patients rarely note the absent corneal reflex), facial nerve motor dysfunction (2%), increased intracranial pressure (rare in tumors <3 cm), and brainstem and cerebellar symptoms (rare).1
Asymptomatic AN. Small tumors may be detected by MRI incidentally before the onset of hearing loss. Such a finding accounts for about 12% of all patients diagnosed with AN.5 A 2005 study by Lin et al found the prevalence of incidental AN to be about 2 per 10,000 people.6 This is higher than the 1 in 100,000 suggested by epidemiologic studies,6 but Lin’s study was performed at a large tertiary center.
Diagnostic evaluation
Contrast MRI is the gold standard for diagnosing AN.2 One study did find that a heavily T2-weighted noncontrast scan in the hands of an experienced radiologist reduced the procedural cost and was as effective as contrast MRI in evaluating the VII and VIII cranial nerves within the cerebellopontine angle and internal auditory canal.7
Although not as sensitive for small tumors, the auditory brainstem response can be used in certain circumstances. During the initial evaluation, other diagnoses to consider are facial neuroma and jugular foramen tumors.1
Three options for treatment
The goal of treatment is to slow or eliminate both tumor growth and deterioration in hearing and neurological function. Lin’s study found that 43% of patients with incidental AN had abnormal audiometry findings.6 A hearing evaluation may therefore be helpful in guiding patients through a meaningful discussion about prognosis, further testing and consultations, and the 3 therapeutic options—watchful waiting, microsurgery, and stereotactic radiosurgery.8
Watchful waiting has been recommended for the elderly and for infirm patients with tumors <1 cm who would be poor candidates for surgery or radiation. In up to 57% of AN cases, no further tumor growth occurs after diagnosis; in about 8% of these cases, tumor regression is noted.9 A little more than half of patients will experience further hearing loss.9
Unfortunately, there are no prediction rules for determining who is most at risk for tumor growth and hearing loss. A recent study found that conservative management was a cost-effective approach for tumors <1.5 cm in any age group,10 provided there was no increase in complications from continued tumor growth. This, then, is a third group of patients for whom watchful waiting might be an option.10 Follow-up MRI can be used as a surveillance tool.8,9,11
Microsurgery. This option is the oldest and best-studied treatment for AN. Micro-surgery appears to provide the best tumor control, although morbidity and mortality remain risks. A systematic review by Yamakami in 2003 showed that microsurgery completely removed 96% of ANs, with tumor recurrence, mortality, and major disability rates of 1.8%, 0.63%, and 2.9%, respectively.12 More recent reviews have shown mortality rates of approximately 0.1%.13 Surgery usually involves removal of cranial nerve VIII, with the risk of damage to cranial nerve VII. Nerve-sparing procedures are available. Cerebral spinal fluid leaks and meningitis are occasional adverse events. The experience of the surgical team can affect outcome, including complications and cost.14
Stereotactic radiosurgery. Through the use of sophisticated imaging devices and 3-dimensional treatment-planning computers, stereotactic irradiation allows much more specific targeting of the AN, with significantly less radiation delivered to surrounding healthy tissues.15 Dynamic beam shaping and intensity modulation provide flexibility and enable delivery of much higher radiation doses to the tumor, resulting in greater control rates and decreased complications. There are 3 delivery technologies: Gamma Knife, proton beam, and specially modified linear accelerators.
Whereas older studies did not provide sufficient evidence to support the use of low-dose over high-dose radiation for long-term control of ANs,16 a more recent systematic analysis by Yang et al seems to indicate that patients treated with the lower dose (12.5 Gy) did equally as well with better preservation of hearing.17
Applying the evidence in practice
A large randomized controlled trial comparing these treatment options has yet to be done and, indeed, would be difficult to conduct due to the small number of AN cases, varying surgical expertise among centers, the different treatment goals inherent in the 3 therapies, and the risk involved in each. Evidence to date generally indicates that observation is appropriate for small intracanalicular tumors (<1 cm) in the elderly, medically infirm, or asymptomatic patients who understand and opt for this management approach. For tumors ≥3 cm, evidence supports microsurgery as optimal management.13,18 Tumors falling between these extremes pose the real challenge.
Over the last 10 years, numerous studies have demonstrated good tumor control with either microsurgery or radiosurgery, but with varying degrees of hearing preservation and permanent nerve injury to the facial and trigeminal nerve. There is also a concern for malignant transformation of AN after radiosurgery, with 8 cases reported in the literature.8,13,19
Three evidence-based studies in the last 6 years have compared the 2 interventions. In 2002, Nikolopoulos et al reviewed 111 studies and concluded there was insufficient evidence to support one approach over the other.18 Pollock conducted a prospective cohort study in 2008 that showed superior outcomes in facial movement, hearing preservation, and Health Status Questionnaire subscales for patients undergoing stereotactic radiosurgery.13 This study was limited to nonfractionated radiosurgery, and follow-up varied from 12 to 62 months.
In 2009, a Norwegian prospective study of 91 patients reported better facial nerve and hearing outcomes from radiosurgery for medium and small tumors. This study was well performed, but it looked at only a small, non-randomized population.20 The same author in 2005 had found that, from the patient perspective, cranial nerve function and overall outcomes were better in the radiosurgery group.21
Stereotactic radiosurgery does confer lower risks for acute treatment complications than microsurgery, and therefore can be advantageous for patients who are older, infirm, require anticoagulant therapy, are otherwise poor candidates for surgery or anesthesia, or have serviceable hearing and opt for a more conservative approach. Other advantages of stereotactic radiosurgery over microsurgery are its lower cost and its preferred use in patients with permanent hearing loss in the unaffected ear. These advantages of stereotactic radiosurgery may, however, be offset in the long term by cranial neuropathy and eventual hearing loss, which can be comparable to the experiences of patients after microsurgery.8
Based on these limited studies, patient-oriented outcomes can be comparable between stereotactic radiosurgery and micro-surgery in medium-sized tumors (1-2.9 cm), depending on the patient’s clinical presentation. Hearing and preservation of nerve VII are more likely with radiosurgery than with microsurgery. But several real and potentially large confounding factors limit this interpretation. How does one define tumor control in light of AN’s inherently slow growth? What about reports of less than optimal microsurgery outcomes if previous radiosurgery has failed? And, although it is small, a definite risk of malignant transformation exists after irradiation.8,13
For patients to make good decisions, family physicians need to be aware of these issues when discussing treatment options. To aid in patient education and decision making, there is a helpful algorithm from the International RadioSurgery Association in Stereotactic radiosurgery for patients with vestibular schwannomas (available online at http://www.irsa.org/ANGuideline.pdf).15
Follow-up
Follow-up depends on the treatment modality, but usually relies on MRI to document tumor behavior. MRI studies are typically performed at predetermined intervals such as 6 months and 1, 2, and 4 years. For patients with preserved serviceable hearing, audiograms are recommended at intervals coinciding with clinical and neuroimaging re-evaluations. Tumors proven to be stable over 4 to 5 years can subsequently be reassessed at 2- to 4-year intervals.15
Enabling recovery. One of the most important educational opportunities involves early vestibular rehabilitation to facilitate recovery of postural control after treatment. Research has shown benefit from customized vestibular rehabilitation in addition to instructions that stress the need to engage in some type of activity, such as walking or other modes of exercise.22
CORRESPONDENCE
Robert McDonald, MD, Spartanburg Family Medicine Residency Program, 853 N. Church Street, Suite 510, Spartanburg, SC 29303; rmcdonald@srhs.com
• The gold standard for diagnosis of acoustic neuroma is contrast magnetic resonance imaging. A
• Consider watchful waiting for tumors <1 cm in the elderly, medically infirm, or patients with serviceable hearing who opt for a more conservative approach. C
• The best treatment option for a tumor >1 cm and <3 cm varies. Base your decision on tumor size and the patient’s age, comorbidities, and preference. B
• Recommend microsurgery for tumors >3 cm. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
When a patient presents with unilateral hearing loss—especially with a report of gradual onset— that is accompanied by tinnitus, consider an acoustic neuroma (AN). Also known as vestibular schwannoma, ANs represent just 9% of all intracranial tumors. But its relatively slow growth rate can insidiously lead to impaired quality of life and even complete hearing loss.1 Vestibular symptoms, such as vertigo, may be present. And in some instances, tinnitus may be absent. Even without hearing loss or other symptoms, ANs may be detected incidentally on magnetic resonance imaging (MRI) performed for other reasons. In this article, I describe the diagnostic work-up for AN and 3 options for treatment.
How acoustic neuroma arises
ANs evolve from the abnormal growth and proliferation of Schwann cells, or neurolemmocytes, at their junction with glial cells surrounding the vestibular nerve. ANs represent 80% of all cerebellopontine angle tumors.1 The incidence in the general population is about 1 in 100,000 people per year, with equal distribution between men and women.1 Among adults with sensorineural hearing loss, about 1% have ANs.2 Tumor growth within the internal auditory canal and resultant compression of cranial nerves VII and VIII causes associated symptoms. Unabated growth can lead to prolapse into the cerebellopontine angle with compression of the brainstem and cerebellum.1
There are 2 major types of AN. The sporadic type, which will be the focus of this discussion, occurs in 95% of all cases, is unilateral, and usually affects individuals 40 to 60 years old. AN associated with neurofibromatosis type 2 is typically bilateral, autosomal dominant, and usually affects teens and young adults.1
The only known possible risk factor for the development of AN is the use of cell phones, but data are conflicting. Although 2 meta-analyses did find a correlation between AN and ipsilateral cell phone use >10 years, these studies were limited by possible recall bias and misclassification.3,4
How acoustic neuroma can present
Slowly progressive, high-frequency, sensori-neural hearing loss is typical for AN. In most cases, hearing loss is unilateral. Nerve compression and stretching from the growing neuroma cause hearing loss of gradual onset.1 However, up to 25% of patients may present with sudden onset hearing loss due to total occlusion of the internal auditory canal and the artery supplying the cochlea.1 Tinnitus is reported in up to 70% of patients with AN.1 The mechanism of injury is the same as for hearing loss.
A feeling of imbalance or unsteadiness occurs in more than 50% of patients with AN.1 One research group found that vertigo was the more common symptom in patients with smaller tumors, whereas patients with larger tumors complained more of dysequilibrium.1
Other physical findings can include trigeminal nerve dysfunction (found in 50% of patients with AN, but patients rarely note the absent corneal reflex), facial nerve motor dysfunction (2%), increased intracranial pressure (rare in tumors <3 cm), and brainstem and cerebellar symptoms (rare).1
Asymptomatic AN. Small tumors may be detected by MRI incidentally before the onset of hearing loss. Such a finding accounts for about 12% of all patients diagnosed with AN.5 A 2005 study by Lin et al found the prevalence of incidental AN to be about 2 per 10,000 people.6 This is higher than the 1 in 100,000 suggested by epidemiologic studies,6 but Lin’s study was performed at a large tertiary center.
Diagnostic evaluation
Contrast MRI is the gold standard for diagnosing AN.2 One study did find that a heavily T2-weighted noncontrast scan in the hands of an experienced radiologist reduced the procedural cost and was as effective as contrast MRI in evaluating the VII and VIII cranial nerves within the cerebellopontine angle and internal auditory canal.7
Although not as sensitive for small tumors, the auditory brainstem response can be used in certain circumstances. During the initial evaluation, other diagnoses to consider are facial neuroma and jugular foramen tumors.1
Three options for treatment
The goal of treatment is to slow or eliminate both tumor growth and deterioration in hearing and neurological function. Lin’s study found that 43% of patients with incidental AN had abnormal audiometry findings.6 A hearing evaluation may therefore be helpful in guiding patients through a meaningful discussion about prognosis, further testing and consultations, and the 3 therapeutic options—watchful waiting, microsurgery, and stereotactic radiosurgery.8
Watchful waiting has been recommended for the elderly and for infirm patients with tumors <1 cm who would be poor candidates for surgery or radiation. In up to 57% of AN cases, no further tumor growth occurs after diagnosis; in about 8% of these cases, tumor regression is noted.9 A little more than half of patients will experience further hearing loss.9
Unfortunately, there are no prediction rules for determining who is most at risk for tumor growth and hearing loss. A recent study found that conservative management was a cost-effective approach for tumors <1.5 cm in any age group,10 provided there was no increase in complications from continued tumor growth. This, then, is a third group of patients for whom watchful waiting might be an option.10 Follow-up MRI can be used as a surveillance tool.8,9,11
Microsurgery. This option is the oldest and best-studied treatment for AN. Micro-surgery appears to provide the best tumor control, although morbidity and mortality remain risks. A systematic review by Yamakami in 2003 showed that microsurgery completely removed 96% of ANs, with tumor recurrence, mortality, and major disability rates of 1.8%, 0.63%, and 2.9%, respectively.12 More recent reviews have shown mortality rates of approximately 0.1%.13 Surgery usually involves removal of cranial nerve VIII, with the risk of damage to cranial nerve VII. Nerve-sparing procedures are available. Cerebral spinal fluid leaks and meningitis are occasional adverse events. The experience of the surgical team can affect outcome, including complications and cost.14
Stereotactic radiosurgery. Through the use of sophisticated imaging devices and 3-dimensional treatment-planning computers, stereotactic irradiation allows much more specific targeting of the AN, with significantly less radiation delivered to surrounding healthy tissues.15 Dynamic beam shaping and intensity modulation provide flexibility and enable delivery of much higher radiation doses to the tumor, resulting in greater control rates and decreased complications. There are 3 delivery technologies: Gamma Knife, proton beam, and specially modified linear accelerators.
Whereas older studies did not provide sufficient evidence to support the use of low-dose over high-dose radiation for long-term control of ANs,16 a more recent systematic analysis by Yang et al seems to indicate that patients treated with the lower dose (12.5 Gy) did equally as well with better preservation of hearing.17
Applying the evidence in practice
A large randomized controlled trial comparing these treatment options has yet to be done and, indeed, would be difficult to conduct due to the small number of AN cases, varying surgical expertise among centers, the different treatment goals inherent in the 3 therapies, and the risk involved in each. Evidence to date generally indicates that observation is appropriate for small intracanalicular tumors (<1 cm) in the elderly, medically infirm, or asymptomatic patients who understand and opt for this management approach. For tumors ≥3 cm, evidence supports microsurgery as optimal management.13,18 Tumors falling between these extremes pose the real challenge.
Over the last 10 years, numerous studies have demonstrated good tumor control with either microsurgery or radiosurgery, but with varying degrees of hearing preservation and permanent nerve injury to the facial and trigeminal nerve. There is also a concern for malignant transformation of AN after radiosurgery, with 8 cases reported in the literature.8,13,19
Three evidence-based studies in the last 6 years have compared the 2 interventions. In 2002, Nikolopoulos et al reviewed 111 studies and concluded there was insufficient evidence to support one approach over the other.18 Pollock conducted a prospective cohort study in 2008 that showed superior outcomes in facial movement, hearing preservation, and Health Status Questionnaire subscales for patients undergoing stereotactic radiosurgery.13 This study was limited to nonfractionated radiosurgery, and follow-up varied from 12 to 62 months.
In 2009, a Norwegian prospective study of 91 patients reported better facial nerve and hearing outcomes from radiosurgery for medium and small tumors. This study was well performed, but it looked at only a small, non-randomized population.20 The same author in 2005 had found that, from the patient perspective, cranial nerve function and overall outcomes were better in the radiosurgery group.21
Stereotactic radiosurgery does confer lower risks for acute treatment complications than microsurgery, and therefore can be advantageous for patients who are older, infirm, require anticoagulant therapy, are otherwise poor candidates for surgery or anesthesia, or have serviceable hearing and opt for a more conservative approach. Other advantages of stereotactic radiosurgery over microsurgery are its lower cost and its preferred use in patients with permanent hearing loss in the unaffected ear. These advantages of stereotactic radiosurgery may, however, be offset in the long term by cranial neuropathy and eventual hearing loss, which can be comparable to the experiences of patients after microsurgery.8
Based on these limited studies, patient-oriented outcomes can be comparable between stereotactic radiosurgery and micro-surgery in medium-sized tumors (1-2.9 cm), depending on the patient’s clinical presentation. Hearing and preservation of nerve VII are more likely with radiosurgery than with microsurgery. But several real and potentially large confounding factors limit this interpretation. How does one define tumor control in light of AN’s inherently slow growth? What about reports of less than optimal microsurgery outcomes if previous radiosurgery has failed? And, although it is small, a definite risk of malignant transformation exists after irradiation.8,13
For patients to make good decisions, family physicians need to be aware of these issues when discussing treatment options. To aid in patient education and decision making, there is a helpful algorithm from the International RadioSurgery Association in Stereotactic radiosurgery for patients with vestibular schwannomas (available online at http://www.irsa.org/ANGuideline.pdf).15
Follow-up
Follow-up depends on the treatment modality, but usually relies on MRI to document tumor behavior. MRI studies are typically performed at predetermined intervals such as 6 months and 1, 2, and 4 years. For patients with preserved serviceable hearing, audiograms are recommended at intervals coinciding with clinical and neuroimaging re-evaluations. Tumors proven to be stable over 4 to 5 years can subsequently be reassessed at 2- to 4-year intervals.15
Enabling recovery. One of the most important educational opportunities involves early vestibular rehabilitation to facilitate recovery of postural control after treatment. Research has shown benefit from customized vestibular rehabilitation in addition to instructions that stress the need to engage in some type of activity, such as walking or other modes of exercise.22
CORRESPONDENCE
Robert McDonald, MD, Spartanburg Family Medicine Residency Program, 853 N. Church Street, Suite 510, Spartanburg, SC 29303; rmcdonald@srhs.com
1. Ho SY, Kveton JF. Acoustic neuroma assessment and management. Otolaryngol Clin North Am. 2002;35:393-404.
2. Zealley IA, Cooper RC, Clifford KM, et al. MRI screening for acoustic neuroma: a comparison of fast spin echo and contrast enhanced imaging in 1233 patients. Br J Radiol. 2000;73:242-247.
3. Hardell L, Carlberg M, Söderqvist F, et al. Meta-analysis of long-term mobile phone use and the association with brain tumors. Int J Oncol. 2008;32:1097-1103.
4. Khurana VG, Teo C, Kundi M, et al. Cell phones and brain tumors: a review including the long-term epidemiologic data. Surg Neurol. 2009;72:205-214.
5. DynaMed [database online]. Acoustic neuroma. Updated August 18, 2009. Available at http://www.ebscohost.com/dynamed. Accessed August 24, 2009.
6. Lin D, Hegarty JL, Fischbein NJ, et al. The prevalence of “incidental” acoustic neuroma. Arch Otolaryngol Head Neck Surg. 2005;131:241-244.
7. Fortnum H, O’Neill C, Taylor R, et al. The role of magnetic resonance imaging in the identification of suspected acoustic neuroma: a systematic review of clinical and cost effectiveness and natural history. Health Technol Assess. 2009;13:iii-iv,ix-xi,1-154.
8. Diaz R, Brodie H. Gamma knife and other stereotactic radiotherapies for acoustic neuroma. April 10, 2009. Available at http://emedicine.medscape.com/article/857604-overview. Accessed August 24, 2009.
9. Smouha EE, Yoo M, Mohr K, et al. Conservative management of acoustic neuroma: a meta-analysis and proposed treatment algorithm. Laryngoscope. 2005;115:450-454.
10. Verma S, Anthony R, Tsai V, et al. Evaluation of cost effectiveness and active management strategies for acoustic neuroma. Clin Otolaryngol. 2009;34:438-446.
11. Yoshimoto Y. Systematic review of the natural history of vestibular schwannoma. J Neurosurg. 2005;103:59-63.
12. Yamakami I, Uchino Y, Kobayashi E, et al. Conservative management, gamma-knife radiosurgery, and microsurgery for acoustic neuromas: a systematic review of outcome and risk of three therapeutic options. Neurol Res. 2003;25:682-690.
13. Pollock BE. Vestibular schwannoma management: an evidence-based comparison of stereotactic radiosurgery and microsurgical resection. Prog Neurol Surg. 2008;21:222-227.
14. Slattery WH, Schwartz MS, Fisher LM, et al. Acoustic neuroma surgical cost and outcome by hospital volume in California. Otolaryngol Head Neck Surg. 2004;130:726-735.
15. International RadioSurgery Association (IRSA). Stereotactic radiosurgery for patients with vestibular schwannomas. Harrisburg, PA: International RadioSurgery Association; 2006. Radio-surgery practice guideline report, no. 4-06. Available at: http://www.irsa.org/AN%20Guideline.pdf. Accessed August 24, 2009.
16. Weil RS, Cohen JM, Portarena I, et al. Optimal dose of stereotactic radiosurgery for acoustic neuromas: a systematic review. Br J Neurosurg. 2006;20:195-202.
17. Yang I, Aranda D, Han SJ, et al. Hearing preservation after radio-surgery for vestibular schwannoma: a systematic review. J Clin Neurosci. 2009;16:742-747.
18. Nikolopoulos TP, O’Donoghue GM. Acoustic neuroma management: an evidence-based medicine approach. Otol Neurotol. 2002;23:534-541.
19. Shin M, Ueki K, Kurita H, et al. Malignant transformation of a vestibular schwannoma after gamma knife surgery. Lancet. 2002;360:309-310.
20. Myrseth E, Moller P, Pedersen PH, et al. Vestibular schwannoma: surgery or gamma knife radiosurgery? A prospective, nonrandomized study. Neurosurgery. 2009;64:654-661.
21. Myrseth E, Moller P, Pedersen PH, et al. Vestibular schwannomas: clinical results and quality of life after microsurgery or gamma knife radiosurgery. Neurosurgery. 2005;56:927-935.
22. Vereeck L, Wuyts FL, Truijen S, et al. The effect of early customized vestibular rehabilitation on balance after acoustic neuroma resection. Clin Rehabil. 2008;22:698-713.
1. Ho SY, Kveton JF. Acoustic neuroma assessment and management. Otolaryngol Clin North Am. 2002;35:393-404.
2. Zealley IA, Cooper RC, Clifford KM, et al. MRI screening for acoustic neuroma: a comparison of fast spin echo and contrast enhanced imaging in 1233 patients. Br J Radiol. 2000;73:242-247.
3. Hardell L, Carlberg M, Söderqvist F, et al. Meta-analysis of long-term mobile phone use and the association with brain tumors. Int J Oncol. 2008;32:1097-1103.
4. Khurana VG, Teo C, Kundi M, et al. Cell phones and brain tumors: a review including the long-term epidemiologic data. Surg Neurol. 2009;72:205-214.
5. DynaMed [database online]. Acoustic neuroma. Updated August 18, 2009. Available at http://www.ebscohost.com/dynamed. Accessed August 24, 2009.
6. Lin D, Hegarty JL, Fischbein NJ, et al. The prevalence of “incidental” acoustic neuroma. Arch Otolaryngol Head Neck Surg. 2005;131:241-244.
7. Fortnum H, O’Neill C, Taylor R, et al. The role of magnetic resonance imaging in the identification of suspected acoustic neuroma: a systematic review of clinical and cost effectiveness and natural history. Health Technol Assess. 2009;13:iii-iv,ix-xi,1-154.
8. Diaz R, Brodie H. Gamma knife and other stereotactic radiotherapies for acoustic neuroma. April 10, 2009. Available at http://emedicine.medscape.com/article/857604-overview. Accessed August 24, 2009.
9. Smouha EE, Yoo M, Mohr K, et al. Conservative management of acoustic neuroma: a meta-analysis and proposed treatment algorithm. Laryngoscope. 2005;115:450-454.
10. Verma S, Anthony R, Tsai V, et al. Evaluation of cost effectiveness and active management strategies for acoustic neuroma. Clin Otolaryngol. 2009;34:438-446.
11. Yoshimoto Y. Systematic review of the natural history of vestibular schwannoma. J Neurosurg. 2005;103:59-63.
12. Yamakami I, Uchino Y, Kobayashi E, et al. Conservative management, gamma-knife radiosurgery, and microsurgery for acoustic neuromas: a systematic review of outcome and risk of three therapeutic options. Neurol Res. 2003;25:682-690.
13. Pollock BE. Vestibular schwannoma management: an evidence-based comparison of stereotactic radiosurgery and microsurgical resection. Prog Neurol Surg. 2008;21:222-227.
14. Slattery WH, Schwartz MS, Fisher LM, et al. Acoustic neuroma surgical cost and outcome by hospital volume in California. Otolaryngol Head Neck Surg. 2004;130:726-735.
15. International RadioSurgery Association (IRSA). Stereotactic radiosurgery for patients with vestibular schwannomas. Harrisburg, PA: International RadioSurgery Association; 2006. Radio-surgery practice guideline report, no. 4-06. Available at: http://www.irsa.org/AN%20Guideline.pdf. Accessed August 24, 2009.
16. Weil RS, Cohen JM, Portarena I, et al. Optimal dose of stereotactic radiosurgery for acoustic neuromas: a systematic review. Br J Neurosurg. 2006;20:195-202.
17. Yang I, Aranda D, Han SJ, et al. Hearing preservation after radio-surgery for vestibular schwannoma: a systematic review. J Clin Neurosci. 2009;16:742-747.
18. Nikolopoulos TP, O’Donoghue GM. Acoustic neuroma management: an evidence-based medicine approach. Otol Neurotol. 2002;23:534-541.
19. Shin M, Ueki K, Kurita H, et al. Malignant transformation of a vestibular schwannoma after gamma knife surgery. Lancet. 2002;360:309-310.
20. Myrseth E, Moller P, Pedersen PH, et al. Vestibular schwannoma: surgery or gamma knife radiosurgery? A prospective, nonrandomized study. Neurosurgery. 2009;64:654-661.
21. Myrseth E, Moller P, Pedersen PH, et al. Vestibular schwannomas: clinical results and quality of life after microsurgery or gamma knife radiosurgery. Neurosurgery. 2005;56:927-935.
22. Vereeck L, Wuyts FL, Truijen S, et al. The effect of early customized vestibular rehabilitation on balance after acoustic neuroma resection. Clin Rehabil. 2008;22:698-713.
When to suspect interstitial cystitis
• Suspect interstitial cystitis (IC) in a patient who has had suprapubic pain, pressure, or discomfort and frequency of urination for >3 months in the absence of a urinary tract infection or other pelvic condition with similar symptoms. A
• Mild symptoms of IC can be largely contained with dietary changes, off-label oral agents such as amitriptyline or hydroxyzine, and muscle relaxants to reduce pelvic floor muscle spasm. B
• Use pentosan polysulfate with caution; although the drug is approved for the treatment of IC, recent studies indicate it has little benefit. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jan D, a 27-year-old woman, comes in requesting treatment for pelvic pain and urinary frequency, symptoms she’s had for about 6 months. She describes a feeling of pressure over the suprapubic area that’s relieved by voiding, sensitivity over the vulvar area, and both daytime frequency and nocturia. The patient has a history of allergies and chronic fatigue syndrome (CFS) of 2 years’ duration. When you inquire about her prior medical history, Jan reports that she had frequent urinary tract infections (UTIs) during adolescence.
You order a urinalysis and culture, both of which are negative. If Jan were your patient, what would your next step be?
Interstitial cystitis (IC) is a painful bladder disorder that predominantly affects young and middle-aged women, with an average age of onset of 40 years.1,2 But men can also develop IC, as can women of any age.2 Estimates of prevalence among US women range from less than 1% to more than 6%.2,3 In recent years, however, the number of cases reported has multiplied, the combined result of greater awareness of IC and population surveys based on symptoms rather than on established criteria alone.4
Because the disorder is recognized as a major source of chronic pelvic pain and disability, the term interstitial cystitis/bladder pain syndrome (IC/BPS) is now used by the American Urological Association and many experts to describe it.1,5
Early diagnosis and management of IC/BPS are keys to substantial symptom reduction and improved quality of life. Yet it is often under- or misdiagnosed, both because of the many comorbidities found in patients with the disorder and because its symptoms overlap with those of other common conditions.5
Family physicians are often the first practitioners whom patients with IC/BPS turn to for help. Yet a recent survey of physician practices found significant knowledge gaps with regard to IC/BPS among primary care physicians.6 This evidence-based review is designed to raise awareness of this chronic condition and better prepare you to diagnose and treat it.
IC/BPS: An overview
IC/BPS is characterized by at least 3 to 6 months of pain, pressure, or discomfort over the suprapubic area or the bladder, accompanied by frequency of urination during the day and night in a patient who does not have a UTI.1 There is no known etiology or cure. While evidence suggests that about 90% of those affected are female, some urologists consider chronic bacterial prostatitis to be the male equivalent of IC/BPS, and therefore maintain that the proportion of men with IC/BPS may be considerably higher. 2,3
Chronic pain—the most common symptom—is regional and diffuse over the lower pelvic area, and can be severe. In one study of more than 600 patients with IC/BPS, the most common locations of the pain were the lower abdomen, cited by 80% of those surveyed; the urethral area, cited by 74%; and the low back, by 65%.7 (Dyspareunia is also common, and contributes to the poor quality of life associated with this condition.8)
For about 40% of female patients, the pain and urinary frequency are highest perimenstrually.8 Physical or psychological stress, spicy foods, and smoking can exacerbate symptoms.9,10
Genetics may play a role. Some evidence suggests a genetic predisposition to IC/BPS. In one study, 5 of 8 monozygotic twins of patients with the condition (but 0 of 8 dizygotic twins) were found to have either probable or confirmed IC/BPS. In addition, IC/BPS was 17 times more common in first-degree relatives of patients with the disorder than in the general population.11
There is no established pathogenesis. No infectious organism (bacterial, fungal, or viral) has been identified as a cause for IC/BPS.12 However, an increased number of activated bladder mast cells has been documented in patients with IC/BPS—a possible reason for the pain and some of the histology associated with the condition.9 Inflammation is present to variable degrees and not in all patients.
Some studies suggest that bladder glycosaminoglycans—which form a coating on the luminal surface of the bladder that creates an impermeable, protective barrier—may be compromised in patients with IC/BPS,13 which makes it possible for noxious molecules in the urine to activate sensory nerve endings and lead to chronic pelvic pain.
Overlapping symptoms, comorbidities are common
Symptoms associated with IC/BPS overlap with those of a number of other conditions, including UTIs, sensory urgency, recurrent cystitis, and overactive bladder (OAB), as well as chronic nonbacterial prostatitis in men.4 Comorbidities further complicate the picture.14,15
IC/BPS patients often have a history of allergies,14,15 although they may have negative results on radioallergosorbent (RAST) or skin prick tests, and a number of other comorbidities (TABLE 1). Studies have shown a high correlation between IC/BPS and chronic fatigue syndrome, irritable bowel syndrome, vulvodynia, fibromyalgia, endometriosis, and panic disorder.16-20
TABLE 1
Interstitial cystitis/bladder pain syndrome: Common comorbidities14-20
| Condition | Frequency of comorbidity |
|---|---|
| Allergies | 40%-60% |
| Chronic fatigue syndrome | 35% |
| Endometriosis | 50% |
| Fibromyalgia | 35% |
| Irritable bowel syndrome | 35% |
| Vulvodynia | 20% |
Rule out UTIs and overactive bladder
IC/BPS is largely a diagnosis of exclusion: When a patient presents with suprapubic pain, pressure, or discomfort related to bladder filling and increased urinary frequency lasting for several months, other related conditions—most notably, UTI and OAB—must be ruled out. Often, this can be done with urinalysis and culture, a complete medical history, and symptom assessment. But when doubt remains, a trial of antibiotics (for a UTI) or an anticholinergic agent (for OAB) may be appropriate.
A targeted history and symptom assessment
A history of allergic, gastrointestinal, gynecologic, and/or musculoskeletal disease is often significant.4 In addition, bladder problems in childhood and adolescence are notable, as they are far more common in women with IC/BPS than in the general population.21,22
Identify voiding problems. Question the patient not only about how often she voids, but also about the extent to which the frequency is affecting her life. The severity of the persistent need to void is more significant for an IC/BPS diagnosis than the sudden urge to void for fear of leakage, which is typical of OAB.23
Ask about abuse. Evidence suggests that 50% of women with IC/BPS have been abused, half of them sexually,24 so it is important to include questions about past and present physical, emotional, and sexual abuse in the medical history. Physical or sexual trauma in childhood appears to increase an individual’s lifetime risk for chronic pain syndromes.25
Use these tools to gauge symptoms and severity. Two tools that can aid in diagnosing—or ruling out—IC/BPS are the O’Leary-Sant Symptom and Problem Index, and the Pelvic Pain and Urgency/Frequency (PUF) questionnaire. Both are available at http://www.ichelp.org/Page.aspx?pid=444.
The O’Leary-Sant Index is a measure of urinary and pain symptoms, and of how problematic the symptoms are for the patient.26 The PUF questionnaire also incorporates an assessment of sexual function and the impact of the pain and urinary symptoms,27 but it has not been validated.
The medical work-up
Perform a full gynecologic evaluation of female patients and a rectal examination of men. Include the following laboratory tests in your evaluation: complete blood count (CBC) with differential, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), total immunoglobulin E, and liver and thyroid function; test leuteinizing hormone and follicle-stimulating hormone levels for women, as well. Also include urine culture and sensitivity tests in the work-up.
Referral to a urologist is indicated if microscopic hematuria and pyuria are present or the patient’s symptoms are severe. The urologist may conduct a number of tests, for further evaluation or to confirm an IC/BPS diagnosis. These include:
Digital and manometric pelvic floor muscle examination. Manometry is performed using a vaginal or rectal pressure-sensitive probe that measures the strength of contraction and the ability to relax the pelvic floor muscles. In one study, 87% of women with IC/BPS were found to have levator muscle pain described as “consistent with pelvic floor dysfunction.”28
Kaufman Q-tip touch sensitivity test. This involves touching all 4 quadrants of the vulvar and vestibular Skene’s gland ostia to evaluate for vestibulodynia, using a visual analog scale to document the level of pain and sensitivity the patient is experiencing.
Potassium sensitivity test. The physician instills a high concentration of potassium chloride into the bladder to evaluate how much pain it elicits.27 (Although this test is frequently included in the evaluation of patients suspected of having IC/BPS, its use is controversial because it is unnecessarily painful, while its sensitivity and specificity are low.1)
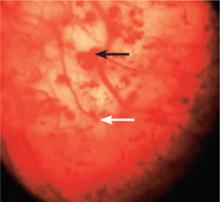
Cystoscopy after hydrodistension, preferably with isotonic saline or isotonic glycine to avoid hypotonic damage to the urothelium, is performed under anesthesia (FIGURE 1). This is the most common procedure performed on patients with IC/BPS29 because it allows visualization of the urothelial glomerulations, or petechiae, and submucosal hemorrhages, found in most patients with this condition. The test would also reveal the presence of the mucosal lesions (Hunner’s ulcers) found in those with “classic” IC/BPS, which represents 5% to 15% of all cases.4
FIGURE 1
IC/BPS: A cystoscopic image
Visualization of the bladder during cystoscopy after hydrodistension reveals submucosal hemorrhages (black arrow) and glomerulations (white arrow), found in most patients with IC/BPS.
Glomerulations can occur in other bladder conditions, however, and may even be found in normal bladders.30 Thus, glomerulations are not diagnostic of IC/BPS in and of themselves, but this finding is often used to confirm the diagnosis.
CASE After Jan’s initial urinalysis, culture, and sensitivity tests, you follow up with a number of other laboratory tests, including CBC with differential, ESR, CRP, and hormonal and immune indexes. All are within the normal range. You also perform a gynecologic examination and use the O’Leary-Sant Symptom and Problem Index to diagnose IC/BPS, and refer the patient to a urologist for further evaluation. The urologist performs cystoscopy with bladder hydrodistension, which reveals multiple submucosal hemorrhages and glomerulations.
Treatment of IC/BPS is multimodal
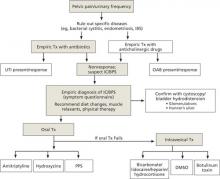
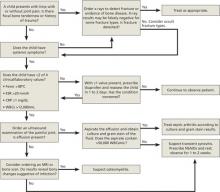
In January 2011, the American Urological Association (AUA) approved diagnostic and treatment recommendations for IC/BPS (available at http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=ic-bps). The AUA recommends starting with the most conservative treatments, such as stress management, patient education, and self-care. Interventions may include dietary modification (eliminating bladder irritants such as caffeine and alcohol), self-guided imagery, meditation, yoga, deep breathing, self-hypnosis, and manual physical therapy to the pelvic floor myofascial trigger points, with oral or intravesical medications and other procedures added, as needed (FIGURE 2). Pain management, the AUA notes, should be considered throughout the course of therapy.31
FIGURE 2
Treatment algorithm for interstitial cystitis/bladder pain syndrome (IC/BPS)
DMSO, dimethylsulfoxide; IBS, irritable bowel syndrome; PPS, pentosan polysulfate; UTI, urinary tract infection.
Sources: American Urological Association31; International Incontinence Society (http://www.icsoffice.org/Documents/Documents.aspx?DocumentID=494).
The other professional society with treatment recommendations for IC/BPS is the International Continence Society (http://www.icsoffice.org/Documents/Documents.aspx?DocumentID=494). Because there is no known cure for IC/BPS, the Society focuses on alleviating symptoms and improving the patient’s quality of life. Treatment is highly individual, the Society states, and may consist of diet modification, oral drugs, bladder instillations or injections, and neuromodulation or surgical interventions, as a last resort. Multiple approaches may be used, often together. 29
Both mild discomfort/pain and urinary frequency in newly diagnosed patients may be treated with a number of oral drugs used off label (TABLE 2), as well as with muscle relaxants, such as diazepam. Pentosan polysulfate—the only oral drug with US Food and Drug Administration approval for treatment of IC/BPS—was initially shown to be “modestly beneficial.”32 However, in 2 recent randomized studies, including one multicenter trial funded by the National Institute of Diabetes and Digestive and Kidney Diseases, it was found to be little (or no) better than placebo.33,34 Thus, we recommend that the drug be tried for no more than 4 to 6 months. If there is no benefit or adverse effects such as GI problems or hair loss develop, the drug should be discontinued.
TABLE 2
Frequently used medications for mild to moderate symptoms
| Agent | Usual dose |
|---|---|
| Amitriptyline | 50-75 mg at bedtime* |
| Diazepam | 2-5 mg up to 4 times per day prn |
| Hydroxyzine | 50-75 mg at bedtime* |
| Pentosan polysulfate | 100 mg tid |
| Pain control | |
| Doxepin cream† | Apply 2-3 times per day |
| Gabapentin | 200 mg tid (starting dose) |
| Tramadol | 100 mg bid |
| *Titrated up over 3-4 weeks. †For vulvodynia. | |
The tricyclic antidepressant amitriptyline is often used to relieve symptoms—both urinary frequency and pain/discomfort—that are mild to moderate. In one small clinical trial, amitriptyline (self-titrated up to 100 mg/d for 4 months) produced a 64% response rate. But nearly a third of the patients in the intervention group (and many more on placebo) dropped out due to nonresponse.35 A recent multicenter, randomized, placebo-controlled trial of amitriptyline showed that only patients who took >50 mg daily had a significantly higher response rate (P=.01) compared with the placebo group.36
Amitriptyline may also be combined with hydroxyzine, especially in patients with allergies, but the combination could result in considerable sedation. The antidepressant doxepin, which is both a histamine-1 (H1) and histamine-2 (H2) receptor antagonist (RA), is an alternative that has been shown to reduce chronic neuropathic pain.37 A doxepin cream may also be used locally for vulvodynia.
Although no comparative studies have been conducted, nontricyclic antidepressants do not appear to have the same benefit for IC symptoms. In an open-label study of 48 women with IC/BPS, the antidepressant duloxetine (titrated to 40 mg bid for 5 weeks) showed no significant improvement of symptoms.38
Hydroxyzine is an H1 RA with mild anxiolytic and antiallergy properties. In an open-label study of IC/BPS patients with allergies (n=37), it was found to reduce symptoms by 55%.39
Is there a role for dietary supplements? In developing its new recommendations for IC/BPS, the AUA did not review studies of dietary supplements.31 The Interstitial Cystitis Association (ICA), however, includes information on its Web site about a number of dietary supplements that may be helpful in controlling symptoms (http://www.ichelp.org/Page.aspx?pid=635).
One such product contains aloe vera, which the ICA describes as having anti-inflammatory actions that have been found to reduce IC/BPS symptoms.40 Another is a dietary supplement that the author (TCT) developed, which contains quercetin, a flavonoid with anti-inflammatory properties, as well as chondroitin and hyaluronate—components of the glycosaminoglycan protective layer in the bladder that may be damaged in patients with ICS. In an open label study of 127 patients with refractory symptoms of IC/BPS, this supplement produced a 51% response rate (P<.0001).41
Pain management is particularly challenging
Intense chronic pain is the most difficult aspect of IC/BPS to treat. Tramadol, an opioid with weaker addiction potential and fewer adverse effects than morphine, is often helpful. Gabapentin, an antiseizure drug, and pregabalin, a similar drug recently approved for fibromyalgia, may also be useful. A fentanyl patch, as well as belladonna and opium suppositories, may be used under the care of a pain management specialist.
If these pain regimens fail, urologists often try intravesical approaches, such as bladder hydrodistension under anesthesia, which has been found to provide short-term (up to 5.3 months) symptom relief in 30% to 50% of patients.4,42,43 Intravesical treatments, in which medication is directly instilled into the bladder, are frequently used, especially in patients with severe symptoms.44
Intravesical dimethylsulfoxide (DMSO) may be given once a week for 6 weeks, but instillation often hurts and DMSO causes the patients to smell of garlic, which severely limits compliance. In one randomized double-blind study involving 11 patients with classic IC/BPS (ie, with Hunner’s ulcers) and 10 IC/BPS patients without Hunner’s ulcers, DMSO reduced urinary frequency and pain only in those with classic cases.45
Intravesical hyaluronate sodium, given in weekly instillations for 4 weeks, resulted in some pain reduction in 2 open-label studies,4 but in a subsequent randomized, double-blind, placebo-controlled, multicenter study, instilling 10 times the amount of hyaluronate failed to show any benefit and was terminated by the sponsor (Seikagaku Corp., written correspondence, March 2004). In one small study, intravesical hyaluronate and chondroitin, given weekly for 20 weeks and then monthly for 3 months, led to significant improvement in frequency, urgency, and pain.46
In a multicenter trial, intravesical instillation of lidocaine, together with sodium bicarbonate, led to 30% improvement, compared with the controls.47 (Such intravesical “cocktails” are often supplemented with other agents, such as heparin or hydrocortisone.)
Other options for refractory pain include intravesical laser ablation, fulguration of bladder lesions, intravesical injections of botulinum toxin, and neuromodulation of the sacral or pudendal nerve via an implanted impulse generator.
CASE After the urologist confirmed Jan’s diagnosis, she returned to your office for treatment. Jan was started on dietary modification, hydroxyzine (50 mg at bedtime), and physical therapy. She had a 50% reduction in symptoms after 3 months of therapy.
CORRESPONDENCE
Theoharis C. Theoharides, MD, PhD, FAAAAI, Tufts University School of Medicine, 136 Harrison Avenue, Boston, MA 02111; theoharis.theoharides@tufts.edu
1. Hanno P, Lin A, Nordling J, et al. Bladder Pain Syndrome Committee of the International Consultation on Incontinence. Neurourol Urodyn. 2010;29:191-198.
2. Association of Reproductive Health Professionals. Screening, tratment, and management of IC/PBS. May 2008. Available at: http://www.arhp.org/Publications-and-Resources/Clinical-Proceedings/Screening-Treatment-and-Management-of-ICPBS/Definition. Accessed May 9, 2011.
3. Interstitial Cystitis Association. 4 to 12 million may have IC. http://www.ichelp.org/Page.aspx?pid=917. Posted January 12, 2010. Accessed May 9, 2011.
4. Hanno PM. Painful bladder syndrome. In: Wein AJ, Kavossi LR, Novick AC, et al, eds. Campbell’s Urology. 9th ed. Philadelphia, Pa: Elsevier; 2007:330-370.
5. American Urological Association. First-ever clinical guidance on interstitial cystitis/bladder pain syndrome released [press release]. March 1, 2011. Available at: http://www.auanet.org/content/press/press_releases/article.cfm?articleNo=224. Accessed May 9, 2011.
6. Clemens JQ, Calhoun EA, Litwin MS, et al. A survey of primary care physician practices in the diagnosis and management of women with interstitial cystitis/painful bladder syndrome. Urology. 2010;76:323-328.
7. FitzGerald MP, Brensinger C, Brubaker L, et al. What is the pain of interstitial cystitis like? Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:69-72.
8. Powell-Boone T, Ness TJ, Cannon R, et al. Menstrual cycle affects bladder pain sensation in subjects with interstitial cystitis. J Urol. 2005;174:1832-1836.
9. Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1-12.
10. Shorter B, Lesser M, Moldwin RM, et al. Effect of comestibles on symptoms of interstitial cystitis. J Urol. 2007;178:145-152.
11. Warren JW, Jackson TL, Langenberg P, et al. Prevalence of interstitial cystitis in first-degree relatives of patients with interstitial cystitis. Urology. 2004;63:17-21.
12. Al-Hadithi HN, Williams H, Hart CA, et al. Absence of bacterial and viral DNA in bladder biopsies from patients with interstitial cystitis/chronic pelvic pain syndrome. J Urol. 2005;174:151-154.
13. Parsons CL, Lilly JD, Stein P. Epithelial dysfunction in nonbacterial cystitis (interstitial cystitis). J Urol. 1991;145:732-735.
14. Erickson DR, Morgan KC, Ordille S, et al. Nonbladder related symptoms in patients with interstitial cystitis. J Urol. 2001;166:557-562.
15. Theoharides TC, Whitmore K, Stanford E, et al. Interstitial cystitis: bladder pain and beyond. Expert Opin Pharmacother. 2008;9:2979-2994.
16. Nickel JC, Tripp DA, Pontari M, et al. Interstitial cystitis/painful bladder syndrome and associated medical conditions with an emphasis on irritable bowel syndrome, fibromyalgia and chronic fatigue syndrome. J Urol. 2010;184:1358-1363.
17. Novi JM, Jeronis S, Srinivas S, et al. Risk of irritable bowel syndrome and depression in women with interstitial cystitis: a case-control study. J Urol. 2005;174:937-940.
18. Weissman MM, Gross R, Fyer A, et al. Interstitial cystitis and panic disorder: a potential genetic syndrome. Arch Gen Psychiatr. 2004;61:273-279.
19. Wu EQ, Birnbaum H, Mareva M, et al. Interstitial cystitis: cost, treatment and comorbidities in an employed population. Pharmacoeconomics. 2006;24:55-65.
20. Stanford EJ, Koziol J, Feng A. The prevalence of interstitial cystitis, endometriosis, adhesions, and vulvar pain in women with chronic pelvic pain. J Minim Invasive Gynecol. 2005;12:43-49.
21. Peters KM, Killinger KA, Ibrahim IA. Childhood symptoms and events in women with interstitial cystitis/painful bladder syndrome. Urology. 2009;73:258-262.
22. Rackow BW, Novi JM, Arya LA, Pfeifer SM. Interstitial cystitis is an etiology of chronic pelvic pain in young women. J Pediatr Adolesc Gynecol. 2009;22:181-185.
23. Diggs C, Meyer WA, Langenberg P, et al. Assessing urgency in interstitial cystitis/painful bladder syndrome. Urology. 2007;69:210-214.
24. Peters KM, Kalinowski SE, Carrico DJ, et al. Fact or fiction—is abuse prevalent in patients with interstitial cystitis? Results from a community survey and clinic population. J Urol. 2007;178(3 Pt 1):891-895.
25. Mayson BE, Teichman JM. The relationship between sexual abuse and interstitial cystitis/painful bladder syndrome. Curr Urol Rep. 2009;10:441-447.
26. O’Leary MP, Sant GR, Fowler FJ, Jr, et al. The interstitial cystitis symptom index and problem index. Urology. 1997;49(suppl 5A):S58-S63.
27. Parsons CL, Dell J, Stanford EJ, et al. Increased prevalence of interstitial cystitis: previously unrecognized urologic and gynecologic cases identified using a new symptom questionnaire and intravesical potassium sensitivity. Urology. 2002;60:573-578.
28. Peters KM, Carrico DJ, Kalinowski SE, et al. Prevalence of pelvic floor dysfunction in patients with interstitial cystitis. Urology. 2007;70:16-18.
29. Nordling J, Anjum FH, Bade JJ, et al. Primary evaluation of patients suspected of having interstitial cystitis (IC). Eur Urol. 2004;45:662-669.
30. Waxman JA, Sulak PJ, Kuehl TJ. Cystoscopic findings consistent with interstitial cystitis in normal women undergoing tubal ligation. J Urol. 1998;160:1663-1667.
31. American Urological Association. Guideline on the diagnosis and treatment of interstitial cystitis/bladder pain syndrome (2011). Available at: http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=ic.bps. Accessed May 10, 2011.
32. Dimitrakov J, Kroenke K, Steers WD, et al. Pharmacologic management of painful bladder syndrome/interstitial cystitis: a systematic review. Arch Intern Med. 2007;167:1922-1929.
33. Sant GR, Propert KJ, Hanno PM, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol. 2003;170:810-815.
34. Buffington CA. Re: cyclosporine A and pentosan polysulfate sodium for the treatment of interstitial cystitis: a randomized comparative study. J Urol. 2006;176:838.-
35. van Ophoven A, Hertle L. Long-term results of amitriptyline treatment for interstitial cystitis. J Urol. 2005;174:1837-1840.
36. Foster HE, Jr, Hanno PM, Nickel JC, et al. Effect of amitriptyline on symptoms in treatment naive patients with interstitial cystitis/painful bladder syndrome. J Urol. 2010;183:1853-1858.
37. Hameroff SR, Weiss JL, Lerman JC, et al. Doxepin’s effects on chronic pain and depression: a controlled study. J Clin Psychiatry. 1984;45(3 Pt 2):PMID 6321454.-
38. van Ophoven A, Hertle L. The dual serotonin and noradrenaline reuptake inhibitor duloxetine for the treatment of interstitial cystitis: results of an observational study. J Urol. 2007;177:552-555.
39. Theoharides TC, Sant GR. Hydroxyzine therapy for interstitial cystitis. Urology. 1997;49(suppl):S108-S110.
40. Interstitial Cystitis Association. IC supplements. Revised April 11, 2011. Available at: http://www.ichelp.org/Page.aspx?pid=635. Accessed May 9, 2011.
41. Theoharides TC, Kempuraj D, Vakali S, et al. Treatment of refractory interstitial cystitis/painful bladder syndrome with CystoProtek—an oral multi-agent natural supplement. Can J Urol. 2008;15:4410-4414.
42. Phatak S, Foster HE, Jr. The management of interstitial cystitis: an update. Nat Clin Pract Urol. 2006;3:45-53.
43. Erickson DR, Kunselman AR, Bentley CM, et al. Changes in urine markers and symptoms after bladder distension for interstitial cystitis. J Urol. 2007;117:556-560.
44. Dawson TE, Jamison J. Intravesical treatments for painful bladder syndrome/interstitial cystitis. Cochrane Database Syst Rev. 2007;(4):CD006113.-
45. Peeker R, Haghsheno MA, Holmang S, et al. Intravesical bacillus Calmette-Guerin and dimethyl sulfoxide for treatment of classic and nonulcer interstitial cystitis:a prospective, randomized double-blind study. J Urol. 2000;164:1912-1916.
46. Cervigni M, Natale F, Nasta L, et al. A combined intravesical therapy with hyaluronic acid and chondroitin for refractory painful bladder syndrome/interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:943-947.
47. Nickel JC, Moldwin R, Lee S, et al. Intravesical alkalinized lidocaine (PSD597) offers sustained relief from symptoms of interstitial cystitis and painful bladder syndrome. BJU Int. 2009;103:910-918.
• Suspect interstitial cystitis (IC) in a patient who has had suprapubic pain, pressure, or discomfort and frequency of urination for >3 months in the absence of a urinary tract infection or other pelvic condition with similar symptoms. A
• Mild symptoms of IC can be largely contained with dietary changes, off-label oral agents such as amitriptyline or hydroxyzine, and muscle relaxants to reduce pelvic floor muscle spasm. B
• Use pentosan polysulfate with caution; although the drug is approved for the treatment of IC, recent studies indicate it has little benefit. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jan D, a 27-year-old woman, comes in requesting treatment for pelvic pain and urinary frequency, symptoms she’s had for about 6 months. She describes a feeling of pressure over the suprapubic area that’s relieved by voiding, sensitivity over the vulvar area, and both daytime frequency and nocturia. The patient has a history of allergies and chronic fatigue syndrome (CFS) of 2 years’ duration. When you inquire about her prior medical history, Jan reports that she had frequent urinary tract infections (UTIs) during adolescence.
You order a urinalysis and culture, both of which are negative. If Jan were your patient, what would your next step be?
Interstitial cystitis (IC) is a painful bladder disorder that predominantly affects young and middle-aged women, with an average age of onset of 40 years.1,2 But men can also develop IC, as can women of any age.2 Estimates of prevalence among US women range from less than 1% to more than 6%.2,3 In recent years, however, the number of cases reported has multiplied, the combined result of greater awareness of IC and population surveys based on symptoms rather than on established criteria alone.4
Because the disorder is recognized as a major source of chronic pelvic pain and disability, the term interstitial cystitis/bladder pain syndrome (IC/BPS) is now used by the American Urological Association and many experts to describe it.1,5
Early diagnosis and management of IC/BPS are keys to substantial symptom reduction and improved quality of life. Yet it is often under- or misdiagnosed, both because of the many comorbidities found in patients with the disorder and because its symptoms overlap with those of other common conditions.5
Family physicians are often the first practitioners whom patients with IC/BPS turn to for help. Yet a recent survey of physician practices found significant knowledge gaps with regard to IC/BPS among primary care physicians.6 This evidence-based review is designed to raise awareness of this chronic condition and better prepare you to diagnose and treat it.
IC/BPS: An overview
IC/BPS is characterized by at least 3 to 6 months of pain, pressure, or discomfort over the suprapubic area or the bladder, accompanied by frequency of urination during the day and night in a patient who does not have a UTI.1 There is no known etiology or cure. While evidence suggests that about 90% of those affected are female, some urologists consider chronic bacterial prostatitis to be the male equivalent of IC/BPS, and therefore maintain that the proportion of men with IC/BPS may be considerably higher. 2,3
Chronic pain—the most common symptom—is regional and diffuse over the lower pelvic area, and can be severe. In one study of more than 600 patients with IC/BPS, the most common locations of the pain were the lower abdomen, cited by 80% of those surveyed; the urethral area, cited by 74%; and the low back, by 65%.7 (Dyspareunia is also common, and contributes to the poor quality of life associated with this condition.8)
For about 40% of female patients, the pain and urinary frequency are highest perimenstrually.8 Physical or psychological stress, spicy foods, and smoking can exacerbate symptoms.9,10
Genetics may play a role. Some evidence suggests a genetic predisposition to IC/BPS. In one study, 5 of 8 monozygotic twins of patients with the condition (but 0 of 8 dizygotic twins) were found to have either probable or confirmed IC/BPS. In addition, IC/BPS was 17 times more common in first-degree relatives of patients with the disorder than in the general population.11
There is no established pathogenesis. No infectious organism (bacterial, fungal, or viral) has been identified as a cause for IC/BPS.12 However, an increased number of activated bladder mast cells has been documented in patients with IC/BPS—a possible reason for the pain and some of the histology associated with the condition.9 Inflammation is present to variable degrees and not in all patients.
Some studies suggest that bladder glycosaminoglycans—which form a coating on the luminal surface of the bladder that creates an impermeable, protective barrier—may be compromised in patients with IC/BPS,13 which makes it possible for noxious molecules in the urine to activate sensory nerve endings and lead to chronic pelvic pain.
Overlapping symptoms, comorbidities are common
Symptoms associated with IC/BPS overlap with those of a number of other conditions, including UTIs, sensory urgency, recurrent cystitis, and overactive bladder (OAB), as well as chronic nonbacterial prostatitis in men.4 Comorbidities further complicate the picture.14,15
IC/BPS patients often have a history of allergies,14,15 although they may have negative results on radioallergosorbent (RAST) or skin prick tests, and a number of other comorbidities (TABLE 1). Studies have shown a high correlation between IC/BPS and chronic fatigue syndrome, irritable bowel syndrome, vulvodynia, fibromyalgia, endometriosis, and panic disorder.16-20
TABLE 1
Interstitial cystitis/bladder pain syndrome: Common comorbidities14-20
| Condition | Frequency of comorbidity |
|---|---|
| Allergies | 40%-60% |
| Chronic fatigue syndrome | 35% |
| Endometriosis | 50% |
| Fibromyalgia | 35% |
| Irritable bowel syndrome | 35% |
| Vulvodynia | 20% |
Rule out UTIs and overactive bladder
IC/BPS is largely a diagnosis of exclusion: When a patient presents with suprapubic pain, pressure, or discomfort related to bladder filling and increased urinary frequency lasting for several months, other related conditions—most notably, UTI and OAB—must be ruled out. Often, this can be done with urinalysis and culture, a complete medical history, and symptom assessment. But when doubt remains, a trial of antibiotics (for a UTI) or an anticholinergic agent (for OAB) may be appropriate.
A targeted history and symptom assessment
A history of allergic, gastrointestinal, gynecologic, and/or musculoskeletal disease is often significant.4 In addition, bladder problems in childhood and adolescence are notable, as they are far more common in women with IC/BPS than in the general population.21,22
Identify voiding problems. Question the patient not only about how often she voids, but also about the extent to which the frequency is affecting her life. The severity of the persistent need to void is more significant for an IC/BPS diagnosis than the sudden urge to void for fear of leakage, which is typical of OAB.23
Ask about abuse. Evidence suggests that 50% of women with IC/BPS have been abused, half of them sexually,24 so it is important to include questions about past and present physical, emotional, and sexual abuse in the medical history. Physical or sexual trauma in childhood appears to increase an individual’s lifetime risk for chronic pain syndromes.25
Use these tools to gauge symptoms and severity. Two tools that can aid in diagnosing—or ruling out—IC/BPS are the O’Leary-Sant Symptom and Problem Index, and the Pelvic Pain and Urgency/Frequency (PUF) questionnaire. Both are available at http://www.ichelp.org/Page.aspx?pid=444.
The O’Leary-Sant Index is a measure of urinary and pain symptoms, and of how problematic the symptoms are for the patient.26 The PUF questionnaire also incorporates an assessment of sexual function and the impact of the pain and urinary symptoms,27 but it has not been validated.
The medical work-up
Perform a full gynecologic evaluation of female patients and a rectal examination of men. Include the following laboratory tests in your evaluation: complete blood count (CBC) with differential, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), total immunoglobulin E, and liver and thyroid function; test leuteinizing hormone and follicle-stimulating hormone levels for women, as well. Also include urine culture and sensitivity tests in the work-up.
Referral to a urologist is indicated if microscopic hematuria and pyuria are present or the patient’s symptoms are severe. The urologist may conduct a number of tests, for further evaluation or to confirm an IC/BPS diagnosis. These include:
Digital and manometric pelvic floor muscle examination. Manometry is performed using a vaginal or rectal pressure-sensitive probe that measures the strength of contraction and the ability to relax the pelvic floor muscles. In one study, 87% of women with IC/BPS were found to have levator muscle pain described as “consistent with pelvic floor dysfunction.”28
Kaufman Q-tip touch sensitivity test. This involves touching all 4 quadrants of the vulvar and vestibular Skene’s gland ostia to evaluate for vestibulodynia, using a visual analog scale to document the level of pain and sensitivity the patient is experiencing.
Potassium sensitivity test. The physician instills a high concentration of potassium chloride into the bladder to evaluate how much pain it elicits.27 (Although this test is frequently included in the evaluation of patients suspected of having IC/BPS, its use is controversial because it is unnecessarily painful, while its sensitivity and specificity are low.1)
Cystoscopy after hydrodistension, preferably with isotonic saline or isotonic glycine to avoid hypotonic damage to the urothelium, is performed under anesthesia (FIGURE 1). This is the most common procedure performed on patients with IC/BPS29 because it allows visualization of the urothelial glomerulations, or petechiae, and submucosal hemorrhages, found in most patients with this condition. The test would also reveal the presence of the mucosal lesions (Hunner’s ulcers) found in those with “classic” IC/BPS, which represents 5% to 15% of all cases.4
FIGURE 1
IC/BPS: A cystoscopic image
Visualization of the bladder during cystoscopy after hydrodistension reveals submucosal hemorrhages (black arrow) and glomerulations (white arrow), found in most patients with IC/BPS.
Glomerulations can occur in other bladder conditions, however, and may even be found in normal bladders.30 Thus, glomerulations are not diagnostic of IC/BPS in and of themselves, but this finding is often used to confirm the diagnosis.
CASE After Jan’s initial urinalysis, culture, and sensitivity tests, you follow up with a number of other laboratory tests, including CBC with differential, ESR, CRP, and hormonal and immune indexes. All are within the normal range. You also perform a gynecologic examination and use the O’Leary-Sant Symptom and Problem Index to diagnose IC/BPS, and refer the patient to a urologist for further evaluation. The urologist performs cystoscopy with bladder hydrodistension, which reveals multiple submucosal hemorrhages and glomerulations.
Treatment of IC/BPS is multimodal
In January 2011, the American Urological Association (AUA) approved diagnostic and treatment recommendations for IC/BPS (available at http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=ic-bps). The AUA recommends starting with the most conservative treatments, such as stress management, patient education, and self-care. Interventions may include dietary modification (eliminating bladder irritants such as caffeine and alcohol), self-guided imagery, meditation, yoga, deep breathing, self-hypnosis, and manual physical therapy to the pelvic floor myofascial trigger points, with oral or intravesical medications and other procedures added, as needed (FIGURE 2). Pain management, the AUA notes, should be considered throughout the course of therapy.31
FIGURE 2
Treatment algorithm for interstitial cystitis/bladder pain syndrome (IC/BPS)
DMSO, dimethylsulfoxide; IBS, irritable bowel syndrome; PPS, pentosan polysulfate; UTI, urinary tract infection.
Sources: American Urological Association31; International Incontinence Society (http://www.icsoffice.org/Documents/Documents.aspx?DocumentID=494).
The other professional society with treatment recommendations for IC/BPS is the International Continence Society (http://www.icsoffice.org/Documents/Documents.aspx?DocumentID=494). Because there is no known cure for IC/BPS, the Society focuses on alleviating symptoms and improving the patient’s quality of life. Treatment is highly individual, the Society states, and may consist of diet modification, oral drugs, bladder instillations or injections, and neuromodulation or surgical interventions, as a last resort. Multiple approaches may be used, often together. 29
Both mild discomfort/pain and urinary frequency in newly diagnosed patients may be treated with a number of oral drugs used off label (TABLE 2), as well as with muscle relaxants, such as diazepam. Pentosan polysulfate—the only oral drug with US Food and Drug Administration approval for treatment of IC/BPS—was initially shown to be “modestly beneficial.”32 However, in 2 recent randomized studies, including one multicenter trial funded by the National Institute of Diabetes and Digestive and Kidney Diseases, it was found to be little (or no) better than placebo.33,34 Thus, we recommend that the drug be tried for no more than 4 to 6 months. If there is no benefit or adverse effects such as GI problems or hair loss develop, the drug should be discontinued.
TABLE 2
Frequently used medications for mild to moderate symptoms
| Agent | Usual dose |
|---|---|
| Amitriptyline | 50-75 mg at bedtime* |
| Diazepam | 2-5 mg up to 4 times per day prn |
| Hydroxyzine | 50-75 mg at bedtime* |
| Pentosan polysulfate | 100 mg tid |
| Pain control | |
| Doxepin cream† | Apply 2-3 times per day |
| Gabapentin | 200 mg tid (starting dose) |
| Tramadol | 100 mg bid |
| *Titrated up over 3-4 weeks. †For vulvodynia. | |
The tricyclic antidepressant amitriptyline is often used to relieve symptoms—both urinary frequency and pain/discomfort—that are mild to moderate. In one small clinical trial, amitriptyline (self-titrated up to 100 mg/d for 4 months) produced a 64% response rate. But nearly a third of the patients in the intervention group (and many more on placebo) dropped out due to nonresponse.35 A recent multicenter, randomized, placebo-controlled trial of amitriptyline showed that only patients who took >50 mg daily had a significantly higher response rate (P=.01) compared with the placebo group.36
Amitriptyline may also be combined with hydroxyzine, especially in patients with allergies, but the combination could result in considerable sedation. The antidepressant doxepin, which is both a histamine-1 (H1) and histamine-2 (H2) receptor antagonist (RA), is an alternative that has been shown to reduce chronic neuropathic pain.37 A doxepin cream may also be used locally for vulvodynia.
Although no comparative studies have been conducted, nontricyclic antidepressants do not appear to have the same benefit for IC symptoms. In an open-label study of 48 women with IC/BPS, the antidepressant duloxetine (titrated to 40 mg bid for 5 weeks) showed no significant improvement of symptoms.38
Hydroxyzine is an H1 RA with mild anxiolytic and antiallergy properties. In an open-label study of IC/BPS patients with allergies (n=37), it was found to reduce symptoms by 55%.39
Is there a role for dietary supplements? In developing its new recommendations for IC/BPS, the AUA did not review studies of dietary supplements.31 The Interstitial Cystitis Association (ICA), however, includes information on its Web site about a number of dietary supplements that may be helpful in controlling symptoms (http://www.ichelp.org/Page.aspx?pid=635).
One such product contains aloe vera, which the ICA describes as having anti-inflammatory actions that have been found to reduce IC/BPS symptoms.40 Another is a dietary supplement that the author (TCT) developed, which contains quercetin, a flavonoid with anti-inflammatory properties, as well as chondroitin and hyaluronate—components of the glycosaminoglycan protective layer in the bladder that may be damaged in patients with ICS. In an open label study of 127 patients with refractory symptoms of IC/BPS, this supplement produced a 51% response rate (P<.0001).41
Pain management is particularly challenging
Intense chronic pain is the most difficult aspect of IC/BPS to treat. Tramadol, an opioid with weaker addiction potential and fewer adverse effects than morphine, is often helpful. Gabapentin, an antiseizure drug, and pregabalin, a similar drug recently approved for fibromyalgia, may also be useful. A fentanyl patch, as well as belladonna and opium suppositories, may be used under the care of a pain management specialist.
If these pain regimens fail, urologists often try intravesical approaches, such as bladder hydrodistension under anesthesia, which has been found to provide short-term (up to 5.3 months) symptom relief in 30% to 50% of patients.4,42,43 Intravesical treatments, in which medication is directly instilled into the bladder, are frequently used, especially in patients with severe symptoms.44
Intravesical dimethylsulfoxide (DMSO) may be given once a week for 6 weeks, but instillation often hurts and DMSO causes the patients to smell of garlic, which severely limits compliance. In one randomized double-blind study involving 11 patients with classic IC/BPS (ie, with Hunner’s ulcers) and 10 IC/BPS patients without Hunner’s ulcers, DMSO reduced urinary frequency and pain only in those with classic cases.45
Intravesical hyaluronate sodium, given in weekly instillations for 4 weeks, resulted in some pain reduction in 2 open-label studies,4 but in a subsequent randomized, double-blind, placebo-controlled, multicenter study, instilling 10 times the amount of hyaluronate failed to show any benefit and was terminated by the sponsor (Seikagaku Corp., written correspondence, March 2004). In one small study, intravesical hyaluronate and chondroitin, given weekly for 20 weeks and then monthly for 3 months, led to significant improvement in frequency, urgency, and pain.46
In a multicenter trial, intravesical instillation of lidocaine, together with sodium bicarbonate, led to 30% improvement, compared with the controls.47 (Such intravesical “cocktails” are often supplemented with other agents, such as heparin or hydrocortisone.)
Other options for refractory pain include intravesical laser ablation, fulguration of bladder lesions, intravesical injections of botulinum toxin, and neuromodulation of the sacral or pudendal nerve via an implanted impulse generator.
CASE After the urologist confirmed Jan’s diagnosis, she returned to your office for treatment. Jan was started on dietary modification, hydroxyzine (50 mg at bedtime), and physical therapy. She had a 50% reduction in symptoms after 3 months of therapy.
CORRESPONDENCE
Theoharis C. Theoharides, MD, PhD, FAAAAI, Tufts University School of Medicine, 136 Harrison Avenue, Boston, MA 02111; theoharis.theoharides@tufts.edu
• Suspect interstitial cystitis (IC) in a patient who has had suprapubic pain, pressure, or discomfort and frequency of urination for >3 months in the absence of a urinary tract infection or other pelvic condition with similar symptoms. A
• Mild symptoms of IC can be largely contained with dietary changes, off-label oral agents such as amitriptyline or hydroxyzine, and muscle relaxants to reduce pelvic floor muscle spasm. B
• Use pentosan polysulfate with caution; although the drug is approved for the treatment of IC, recent studies indicate it has little benefit. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jan D, a 27-year-old woman, comes in requesting treatment for pelvic pain and urinary frequency, symptoms she’s had for about 6 months. She describes a feeling of pressure over the suprapubic area that’s relieved by voiding, sensitivity over the vulvar area, and both daytime frequency and nocturia. The patient has a history of allergies and chronic fatigue syndrome (CFS) of 2 years’ duration. When you inquire about her prior medical history, Jan reports that she had frequent urinary tract infections (UTIs) during adolescence.
You order a urinalysis and culture, both of which are negative. If Jan were your patient, what would your next step be?
Interstitial cystitis (IC) is a painful bladder disorder that predominantly affects young and middle-aged women, with an average age of onset of 40 years.1,2 But men can also develop IC, as can women of any age.2 Estimates of prevalence among US women range from less than 1% to more than 6%.2,3 In recent years, however, the number of cases reported has multiplied, the combined result of greater awareness of IC and population surveys based on symptoms rather than on established criteria alone.4
Because the disorder is recognized as a major source of chronic pelvic pain and disability, the term interstitial cystitis/bladder pain syndrome (IC/BPS) is now used by the American Urological Association and many experts to describe it.1,5
Early diagnosis and management of IC/BPS are keys to substantial symptom reduction and improved quality of life. Yet it is often under- or misdiagnosed, both because of the many comorbidities found in patients with the disorder and because its symptoms overlap with those of other common conditions.5
Family physicians are often the first practitioners whom patients with IC/BPS turn to for help. Yet a recent survey of physician practices found significant knowledge gaps with regard to IC/BPS among primary care physicians.6 This evidence-based review is designed to raise awareness of this chronic condition and better prepare you to diagnose and treat it.
IC/BPS: An overview
IC/BPS is characterized by at least 3 to 6 months of pain, pressure, or discomfort over the suprapubic area or the bladder, accompanied by frequency of urination during the day and night in a patient who does not have a UTI.1 There is no known etiology or cure. While evidence suggests that about 90% of those affected are female, some urologists consider chronic bacterial prostatitis to be the male equivalent of IC/BPS, and therefore maintain that the proportion of men with IC/BPS may be considerably higher. 2,3
Chronic pain—the most common symptom—is regional and diffuse over the lower pelvic area, and can be severe. In one study of more than 600 patients with IC/BPS, the most common locations of the pain were the lower abdomen, cited by 80% of those surveyed; the urethral area, cited by 74%; and the low back, by 65%.7 (Dyspareunia is also common, and contributes to the poor quality of life associated with this condition.8)
For about 40% of female patients, the pain and urinary frequency are highest perimenstrually.8 Physical or psychological stress, spicy foods, and smoking can exacerbate symptoms.9,10
Genetics may play a role. Some evidence suggests a genetic predisposition to IC/BPS. In one study, 5 of 8 monozygotic twins of patients with the condition (but 0 of 8 dizygotic twins) were found to have either probable or confirmed IC/BPS. In addition, IC/BPS was 17 times more common in first-degree relatives of patients with the disorder than in the general population.11
There is no established pathogenesis. No infectious organism (bacterial, fungal, or viral) has been identified as a cause for IC/BPS.12 However, an increased number of activated bladder mast cells has been documented in patients with IC/BPS—a possible reason for the pain and some of the histology associated with the condition.9 Inflammation is present to variable degrees and not in all patients.
Some studies suggest that bladder glycosaminoglycans—which form a coating on the luminal surface of the bladder that creates an impermeable, protective barrier—may be compromised in patients with IC/BPS,13 which makes it possible for noxious molecules in the urine to activate sensory nerve endings and lead to chronic pelvic pain.
Overlapping symptoms, comorbidities are common
Symptoms associated with IC/BPS overlap with those of a number of other conditions, including UTIs, sensory urgency, recurrent cystitis, and overactive bladder (OAB), as well as chronic nonbacterial prostatitis in men.4 Comorbidities further complicate the picture.14,15
IC/BPS patients often have a history of allergies,14,15 although they may have negative results on radioallergosorbent (RAST) or skin prick tests, and a number of other comorbidities (TABLE 1). Studies have shown a high correlation between IC/BPS and chronic fatigue syndrome, irritable bowel syndrome, vulvodynia, fibromyalgia, endometriosis, and panic disorder.16-20
TABLE 1
Interstitial cystitis/bladder pain syndrome: Common comorbidities14-20
| Condition | Frequency of comorbidity |
|---|---|
| Allergies | 40%-60% |
| Chronic fatigue syndrome | 35% |
| Endometriosis | 50% |
| Fibromyalgia | 35% |
| Irritable bowel syndrome | 35% |
| Vulvodynia | 20% |
Rule out UTIs and overactive bladder
IC/BPS is largely a diagnosis of exclusion: When a patient presents with suprapubic pain, pressure, or discomfort related to bladder filling and increased urinary frequency lasting for several months, other related conditions—most notably, UTI and OAB—must be ruled out. Often, this can be done with urinalysis and culture, a complete medical history, and symptom assessment. But when doubt remains, a trial of antibiotics (for a UTI) or an anticholinergic agent (for OAB) may be appropriate.
A targeted history and symptom assessment
A history of allergic, gastrointestinal, gynecologic, and/or musculoskeletal disease is often significant.4 In addition, bladder problems in childhood and adolescence are notable, as they are far more common in women with IC/BPS than in the general population.21,22
Identify voiding problems. Question the patient not only about how often she voids, but also about the extent to which the frequency is affecting her life. The severity of the persistent need to void is more significant for an IC/BPS diagnosis than the sudden urge to void for fear of leakage, which is typical of OAB.23
Ask about abuse. Evidence suggests that 50% of women with IC/BPS have been abused, half of them sexually,24 so it is important to include questions about past and present physical, emotional, and sexual abuse in the medical history. Physical or sexual trauma in childhood appears to increase an individual’s lifetime risk for chronic pain syndromes.25
Use these tools to gauge symptoms and severity. Two tools that can aid in diagnosing—or ruling out—IC/BPS are the O’Leary-Sant Symptom and Problem Index, and the Pelvic Pain and Urgency/Frequency (PUF) questionnaire. Both are available at http://www.ichelp.org/Page.aspx?pid=444.
The O’Leary-Sant Index is a measure of urinary and pain symptoms, and of how problematic the symptoms are for the patient.26 The PUF questionnaire also incorporates an assessment of sexual function and the impact of the pain and urinary symptoms,27 but it has not been validated.
The medical work-up
Perform a full gynecologic evaluation of female patients and a rectal examination of men. Include the following laboratory tests in your evaluation: complete blood count (CBC) with differential, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), total immunoglobulin E, and liver and thyroid function; test leuteinizing hormone and follicle-stimulating hormone levels for women, as well. Also include urine culture and sensitivity tests in the work-up.
Referral to a urologist is indicated if microscopic hematuria and pyuria are present or the patient’s symptoms are severe. The urologist may conduct a number of tests, for further evaluation or to confirm an IC/BPS diagnosis. These include:
Digital and manometric pelvic floor muscle examination. Manometry is performed using a vaginal or rectal pressure-sensitive probe that measures the strength of contraction and the ability to relax the pelvic floor muscles. In one study, 87% of women with IC/BPS were found to have levator muscle pain described as “consistent with pelvic floor dysfunction.”28
Kaufman Q-tip touch sensitivity test. This involves touching all 4 quadrants of the vulvar and vestibular Skene’s gland ostia to evaluate for vestibulodynia, using a visual analog scale to document the level of pain and sensitivity the patient is experiencing.
Potassium sensitivity test. The physician instills a high concentration of potassium chloride into the bladder to evaluate how much pain it elicits.27 (Although this test is frequently included in the evaluation of patients suspected of having IC/BPS, its use is controversial because it is unnecessarily painful, while its sensitivity and specificity are low.1)
Cystoscopy after hydrodistension, preferably with isotonic saline or isotonic glycine to avoid hypotonic damage to the urothelium, is performed under anesthesia (FIGURE 1). This is the most common procedure performed on patients with IC/BPS29 because it allows visualization of the urothelial glomerulations, or petechiae, and submucosal hemorrhages, found in most patients with this condition. The test would also reveal the presence of the mucosal lesions (Hunner’s ulcers) found in those with “classic” IC/BPS, which represents 5% to 15% of all cases.4
FIGURE 1
IC/BPS: A cystoscopic image
Visualization of the bladder during cystoscopy after hydrodistension reveals submucosal hemorrhages (black arrow) and glomerulations (white arrow), found in most patients with IC/BPS.
Glomerulations can occur in other bladder conditions, however, and may even be found in normal bladders.30 Thus, glomerulations are not diagnostic of IC/BPS in and of themselves, but this finding is often used to confirm the diagnosis.
CASE After Jan’s initial urinalysis, culture, and sensitivity tests, you follow up with a number of other laboratory tests, including CBC with differential, ESR, CRP, and hormonal and immune indexes. All are within the normal range. You also perform a gynecologic examination and use the O’Leary-Sant Symptom and Problem Index to diagnose IC/BPS, and refer the patient to a urologist for further evaluation. The urologist performs cystoscopy with bladder hydrodistension, which reveals multiple submucosal hemorrhages and glomerulations.
Treatment of IC/BPS is multimodal
In January 2011, the American Urological Association (AUA) approved diagnostic and treatment recommendations for IC/BPS (available at http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=ic-bps). The AUA recommends starting with the most conservative treatments, such as stress management, patient education, and self-care. Interventions may include dietary modification (eliminating bladder irritants such as caffeine and alcohol), self-guided imagery, meditation, yoga, deep breathing, self-hypnosis, and manual physical therapy to the pelvic floor myofascial trigger points, with oral or intravesical medications and other procedures added, as needed (FIGURE 2). Pain management, the AUA notes, should be considered throughout the course of therapy.31
FIGURE 2
Treatment algorithm for interstitial cystitis/bladder pain syndrome (IC/BPS)
DMSO, dimethylsulfoxide; IBS, irritable bowel syndrome; PPS, pentosan polysulfate; UTI, urinary tract infection.
Sources: American Urological Association31; International Incontinence Society (http://www.icsoffice.org/Documents/Documents.aspx?DocumentID=494).
The other professional society with treatment recommendations for IC/BPS is the International Continence Society (http://www.icsoffice.org/Documents/Documents.aspx?DocumentID=494). Because there is no known cure for IC/BPS, the Society focuses on alleviating symptoms and improving the patient’s quality of life. Treatment is highly individual, the Society states, and may consist of diet modification, oral drugs, bladder instillations or injections, and neuromodulation or surgical interventions, as a last resort. Multiple approaches may be used, often together. 29
Both mild discomfort/pain and urinary frequency in newly diagnosed patients may be treated with a number of oral drugs used off label (TABLE 2), as well as with muscle relaxants, such as diazepam. Pentosan polysulfate—the only oral drug with US Food and Drug Administration approval for treatment of IC/BPS—was initially shown to be “modestly beneficial.”32 However, in 2 recent randomized studies, including one multicenter trial funded by the National Institute of Diabetes and Digestive and Kidney Diseases, it was found to be little (or no) better than placebo.33,34 Thus, we recommend that the drug be tried for no more than 4 to 6 months. If there is no benefit or adverse effects such as GI problems or hair loss develop, the drug should be discontinued.
TABLE 2
Frequently used medications for mild to moderate symptoms
| Agent | Usual dose |
|---|---|
| Amitriptyline | 50-75 mg at bedtime* |
| Diazepam | 2-5 mg up to 4 times per day prn |
| Hydroxyzine | 50-75 mg at bedtime* |
| Pentosan polysulfate | 100 mg tid |
| Pain control | |
| Doxepin cream† | Apply 2-3 times per day |
| Gabapentin | 200 mg tid (starting dose) |
| Tramadol | 100 mg bid |
| *Titrated up over 3-4 weeks. †For vulvodynia. | |
The tricyclic antidepressant amitriptyline is often used to relieve symptoms—both urinary frequency and pain/discomfort—that are mild to moderate. In one small clinical trial, amitriptyline (self-titrated up to 100 mg/d for 4 months) produced a 64% response rate. But nearly a third of the patients in the intervention group (and many more on placebo) dropped out due to nonresponse.35 A recent multicenter, randomized, placebo-controlled trial of amitriptyline showed that only patients who took >50 mg daily had a significantly higher response rate (P=.01) compared with the placebo group.36
Amitriptyline may also be combined with hydroxyzine, especially in patients with allergies, but the combination could result in considerable sedation. The antidepressant doxepin, which is both a histamine-1 (H1) and histamine-2 (H2) receptor antagonist (RA), is an alternative that has been shown to reduce chronic neuropathic pain.37 A doxepin cream may also be used locally for vulvodynia.
Although no comparative studies have been conducted, nontricyclic antidepressants do not appear to have the same benefit for IC symptoms. In an open-label study of 48 women with IC/BPS, the antidepressant duloxetine (titrated to 40 mg bid for 5 weeks) showed no significant improvement of symptoms.38
Hydroxyzine is an H1 RA with mild anxiolytic and antiallergy properties. In an open-label study of IC/BPS patients with allergies (n=37), it was found to reduce symptoms by 55%.39
Is there a role for dietary supplements? In developing its new recommendations for IC/BPS, the AUA did not review studies of dietary supplements.31 The Interstitial Cystitis Association (ICA), however, includes information on its Web site about a number of dietary supplements that may be helpful in controlling symptoms (http://www.ichelp.org/Page.aspx?pid=635).
One such product contains aloe vera, which the ICA describes as having anti-inflammatory actions that have been found to reduce IC/BPS symptoms.40 Another is a dietary supplement that the author (TCT) developed, which contains quercetin, a flavonoid with anti-inflammatory properties, as well as chondroitin and hyaluronate—components of the glycosaminoglycan protective layer in the bladder that may be damaged in patients with ICS. In an open label study of 127 patients with refractory symptoms of IC/BPS, this supplement produced a 51% response rate (P<.0001).41
Pain management is particularly challenging
Intense chronic pain is the most difficult aspect of IC/BPS to treat. Tramadol, an opioid with weaker addiction potential and fewer adverse effects than morphine, is often helpful. Gabapentin, an antiseizure drug, and pregabalin, a similar drug recently approved for fibromyalgia, may also be useful. A fentanyl patch, as well as belladonna and opium suppositories, may be used under the care of a pain management specialist.
If these pain regimens fail, urologists often try intravesical approaches, such as bladder hydrodistension under anesthesia, which has been found to provide short-term (up to 5.3 months) symptom relief in 30% to 50% of patients.4,42,43 Intravesical treatments, in which medication is directly instilled into the bladder, are frequently used, especially in patients with severe symptoms.44
Intravesical dimethylsulfoxide (DMSO) may be given once a week for 6 weeks, but instillation often hurts and DMSO causes the patients to smell of garlic, which severely limits compliance. In one randomized double-blind study involving 11 patients with classic IC/BPS (ie, with Hunner’s ulcers) and 10 IC/BPS patients without Hunner’s ulcers, DMSO reduced urinary frequency and pain only in those with classic cases.45
Intravesical hyaluronate sodium, given in weekly instillations for 4 weeks, resulted in some pain reduction in 2 open-label studies,4 but in a subsequent randomized, double-blind, placebo-controlled, multicenter study, instilling 10 times the amount of hyaluronate failed to show any benefit and was terminated by the sponsor (Seikagaku Corp., written correspondence, March 2004). In one small study, intravesical hyaluronate and chondroitin, given weekly for 20 weeks and then monthly for 3 months, led to significant improvement in frequency, urgency, and pain.46
In a multicenter trial, intravesical instillation of lidocaine, together with sodium bicarbonate, led to 30% improvement, compared with the controls.47 (Such intravesical “cocktails” are often supplemented with other agents, such as heparin or hydrocortisone.)
Other options for refractory pain include intravesical laser ablation, fulguration of bladder lesions, intravesical injections of botulinum toxin, and neuromodulation of the sacral or pudendal nerve via an implanted impulse generator.
CASE After the urologist confirmed Jan’s diagnosis, she returned to your office for treatment. Jan was started on dietary modification, hydroxyzine (50 mg at bedtime), and physical therapy. She had a 50% reduction in symptoms after 3 months of therapy.
CORRESPONDENCE
Theoharis C. Theoharides, MD, PhD, FAAAAI, Tufts University School of Medicine, 136 Harrison Avenue, Boston, MA 02111; theoharis.theoharides@tufts.edu
1. Hanno P, Lin A, Nordling J, et al. Bladder Pain Syndrome Committee of the International Consultation on Incontinence. Neurourol Urodyn. 2010;29:191-198.
2. Association of Reproductive Health Professionals. Screening, tratment, and management of IC/PBS. May 2008. Available at: http://www.arhp.org/Publications-and-Resources/Clinical-Proceedings/Screening-Treatment-and-Management-of-ICPBS/Definition. Accessed May 9, 2011.
3. Interstitial Cystitis Association. 4 to 12 million may have IC. http://www.ichelp.org/Page.aspx?pid=917. Posted January 12, 2010. Accessed May 9, 2011.
4. Hanno PM. Painful bladder syndrome. In: Wein AJ, Kavossi LR, Novick AC, et al, eds. Campbell’s Urology. 9th ed. Philadelphia, Pa: Elsevier; 2007:330-370.
5. American Urological Association. First-ever clinical guidance on interstitial cystitis/bladder pain syndrome released [press release]. March 1, 2011. Available at: http://www.auanet.org/content/press/press_releases/article.cfm?articleNo=224. Accessed May 9, 2011.
6. Clemens JQ, Calhoun EA, Litwin MS, et al. A survey of primary care physician practices in the diagnosis and management of women with interstitial cystitis/painful bladder syndrome. Urology. 2010;76:323-328.
7. FitzGerald MP, Brensinger C, Brubaker L, et al. What is the pain of interstitial cystitis like? Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:69-72.
8. Powell-Boone T, Ness TJ, Cannon R, et al. Menstrual cycle affects bladder pain sensation in subjects with interstitial cystitis. J Urol. 2005;174:1832-1836.
9. Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1-12.
10. Shorter B, Lesser M, Moldwin RM, et al. Effect of comestibles on symptoms of interstitial cystitis. J Urol. 2007;178:145-152.
11. Warren JW, Jackson TL, Langenberg P, et al. Prevalence of interstitial cystitis in first-degree relatives of patients with interstitial cystitis. Urology. 2004;63:17-21.
12. Al-Hadithi HN, Williams H, Hart CA, et al. Absence of bacterial and viral DNA in bladder biopsies from patients with interstitial cystitis/chronic pelvic pain syndrome. J Urol. 2005;174:151-154.
13. Parsons CL, Lilly JD, Stein P. Epithelial dysfunction in nonbacterial cystitis (interstitial cystitis). J Urol. 1991;145:732-735.
14. Erickson DR, Morgan KC, Ordille S, et al. Nonbladder related symptoms in patients with interstitial cystitis. J Urol. 2001;166:557-562.
15. Theoharides TC, Whitmore K, Stanford E, et al. Interstitial cystitis: bladder pain and beyond. Expert Opin Pharmacother. 2008;9:2979-2994.
16. Nickel JC, Tripp DA, Pontari M, et al. Interstitial cystitis/painful bladder syndrome and associated medical conditions with an emphasis on irritable bowel syndrome, fibromyalgia and chronic fatigue syndrome. J Urol. 2010;184:1358-1363.
17. Novi JM, Jeronis S, Srinivas S, et al. Risk of irritable bowel syndrome and depression in women with interstitial cystitis: a case-control study. J Urol. 2005;174:937-940.
18. Weissman MM, Gross R, Fyer A, et al. Interstitial cystitis and panic disorder: a potential genetic syndrome. Arch Gen Psychiatr. 2004;61:273-279.
19. Wu EQ, Birnbaum H, Mareva M, et al. Interstitial cystitis: cost, treatment and comorbidities in an employed population. Pharmacoeconomics. 2006;24:55-65.
20. Stanford EJ, Koziol J, Feng A. The prevalence of interstitial cystitis, endometriosis, adhesions, and vulvar pain in women with chronic pelvic pain. J Minim Invasive Gynecol. 2005;12:43-49.
21. Peters KM, Killinger KA, Ibrahim IA. Childhood symptoms and events in women with interstitial cystitis/painful bladder syndrome. Urology. 2009;73:258-262.
22. Rackow BW, Novi JM, Arya LA, Pfeifer SM. Interstitial cystitis is an etiology of chronic pelvic pain in young women. J Pediatr Adolesc Gynecol. 2009;22:181-185.
23. Diggs C, Meyer WA, Langenberg P, et al. Assessing urgency in interstitial cystitis/painful bladder syndrome. Urology. 2007;69:210-214.
24. Peters KM, Kalinowski SE, Carrico DJ, et al. Fact or fiction—is abuse prevalent in patients with interstitial cystitis? Results from a community survey and clinic population. J Urol. 2007;178(3 Pt 1):891-895.
25. Mayson BE, Teichman JM. The relationship between sexual abuse and interstitial cystitis/painful bladder syndrome. Curr Urol Rep. 2009;10:441-447.
26. O’Leary MP, Sant GR, Fowler FJ, Jr, et al. The interstitial cystitis symptom index and problem index. Urology. 1997;49(suppl 5A):S58-S63.
27. Parsons CL, Dell J, Stanford EJ, et al. Increased prevalence of interstitial cystitis: previously unrecognized urologic and gynecologic cases identified using a new symptom questionnaire and intravesical potassium sensitivity. Urology. 2002;60:573-578.
28. Peters KM, Carrico DJ, Kalinowski SE, et al. Prevalence of pelvic floor dysfunction in patients with interstitial cystitis. Urology. 2007;70:16-18.
29. Nordling J, Anjum FH, Bade JJ, et al. Primary evaluation of patients suspected of having interstitial cystitis (IC). Eur Urol. 2004;45:662-669.
30. Waxman JA, Sulak PJ, Kuehl TJ. Cystoscopic findings consistent with interstitial cystitis in normal women undergoing tubal ligation. J Urol. 1998;160:1663-1667.
31. American Urological Association. Guideline on the diagnosis and treatment of interstitial cystitis/bladder pain syndrome (2011). Available at: http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=ic.bps. Accessed May 10, 2011.
32. Dimitrakov J, Kroenke K, Steers WD, et al. Pharmacologic management of painful bladder syndrome/interstitial cystitis: a systematic review. Arch Intern Med. 2007;167:1922-1929.
33. Sant GR, Propert KJ, Hanno PM, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol. 2003;170:810-815.
34. Buffington CA. Re: cyclosporine A and pentosan polysulfate sodium for the treatment of interstitial cystitis: a randomized comparative study. J Urol. 2006;176:838.-
35. van Ophoven A, Hertle L. Long-term results of amitriptyline treatment for interstitial cystitis. J Urol. 2005;174:1837-1840.
36. Foster HE, Jr, Hanno PM, Nickel JC, et al. Effect of amitriptyline on symptoms in treatment naive patients with interstitial cystitis/painful bladder syndrome. J Urol. 2010;183:1853-1858.
37. Hameroff SR, Weiss JL, Lerman JC, et al. Doxepin’s effects on chronic pain and depression: a controlled study. J Clin Psychiatry. 1984;45(3 Pt 2):PMID 6321454.-
38. van Ophoven A, Hertle L. The dual serotonin and noradrenaline reuptake inhibitor duloxetine for the treatment of interstitial cystitis: results of an observational study. J Urol. 2007;177:552-555.
39. Theoharides TC, Sant GR. Hydroxyzine therapy for interstitial cystitis. Urology. 1997;49(suppl):S108-S110.
40. Interstitial Cystitis Association. IC supplements. Revised April 11, 2011. Available at: http://www.ichelp.org/Page.aspx?pid=635. Accessed May 9, 2011.
41. Theoharides TC, Kempuraj D, Vakali S, et al. Treatment of refractory interstitial cystitis/painful bladder syndrome with CystoProtek—an oral multi-agent natural supplement. Can J Urol. 2008;15:4410-4414.
42. Phatak S, Foster HE, Jr. The management of interstitial cystitis: an update. Nat Clin Pract Urol. 2006;3:45-53.
43. Erickson DR, Kunselman AR, Bentley CM, et al. Changes in urine markers and symptoms after bladder distension for interstitial cystitis. J Urol. 2007;117:556-560.
44. Dawson TE, Jamison J. Intravesical treatments for painful bladder syndrome/interstitial cystitis. Cochrane Database Syst Rev. 2007;(4):CD006113.-
45. Peeker R, Haghsheno MA, Holmang S, et al. Intravesical bacillus Calmette-Guerin and dimethyl sulfoxide for treatment of classic and nonulcer interstitial cystitis:a prospective, randomized double-blind study. J Urol. 2000;164:1912-1916.
46. Cervigni M, Natale F, Nasta L, et al. A combined intravesical therapy with hyaluronic acid and chondroitin for refractory painful bladder syndrome/interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:943-947.
47. Nickel JC, Moldwin R, Lee S, et al. Intravesical alkalinized lidocaine (PSD597) offers sustained relief from symptoms of interstitial cystitis and painful bladder syndrome. BJU Int. 2009;103:910-918.
1. Hanno P, Lin A, Nordling J, et al. Bladder Pain Syndrome Committee of the International Consultation on Incontinence. Neurourol Urodyn. 2010;29:191-198.
2. Association of Reproductive Health Professionals. Screening, tratment, and management of IC/PBS. May 2008. Available at: http://www.arhp.org/Publications-and-Resources/Clinical-Proceedings/Screening-Treatment-and-Management-of-ICPBS/Definition. Accessed May 9, 2011.
3. Interstitial Cystitis Association. 4 to 12 million may have IC. http://www.ichelp.org/Page.aspx?pid=917. Posted January 12, 2010. Accessed May 9, 2011.
4. Hanno PM. Painful bladder syndrome. In: Wein AJ, Kavossi LR, Novick AC, et al, eds. Campbell’s Urology. 9th ed. Philadelphia, Pa: Elsevier; 2007:330-370.
5. American Urological Association. First-ever clinical guidance on interstitial cystitis/bladder pain syndrome released [press release]. March 1, 2011. Available at: http://www.auanet.org/content/press/press_releases/article.cfm?articleNo=224. Accessed May 9, 2011.
6. Clemens JQ, Calhoun EA, Litwin MS, et al. A survey of primary care physician practices in the diagnosis and management of women with interstitial cystitis/painful bladder syndrome. Urology. 2010;76:323-328.
7. FitzGerald MP, Brensinger C, Brubaker L, et al. What is the pain of interstitial cystitis like? Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:69-72.
8. Powell-Boone T, Ness TJ, Cannon R, et al. Menstrual cycle affects bladder pain sensation in subjects with interstitial cystitis. J Urol. 2005;174:1832-1836.
9. Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1-12.
10. Shorter B, Lesser M, Moldwin RM, et al. Effect of comestibles on symptoms of interstitial cystitis. J Urol. 2007;178:145-152.
11. Warren JW, Jackson TL, Langenberg P, et al. Prevalence of interstitial cystitis in first-degree relatives of patients with interstitial cystitis. Urology. 2004;63:17-21.
12. Al-Hadithi HN, Williams H, Hart CA, et al. Absence of bacterial and viral DNA in bladder biopsies from patients with interstitial cystitis/chronic pelvic pain syndrome. J Urol. 2005;174:151-154.
13. Parsons CL, Lilly JD, Stein P. Epithelial dysfunction in nonbacterial cystitis (interstitial cystitis). J Urol. 1991;145:732-735.
14. Erickson DR, Morgan KC, Ordille S, et al. Nonbladder related symptoms in patients with interstitial cystitis. J Urol. 2001;166:557-562.
15. Theoharides TC, Whitmore K, Stanford E, et al. Interstitial cystitis: bladder pain and beyond. Expert Opin Pharmacother. 2008;9:2979-2994.
16. Nickel JC, Tripp DA, Pontari M, et al. Interstitial cystitis/painful bladder syndrome and associated medical conditions with an emphasis on irritable bowel syndrome, fibromyalgia and chronic fatigue syndrome. J Urol. 2010;184:1358-1363.
17. Novi JM, Jeronis S, Srinivas S, et al. Risk of irritable bowel syndrome and depression in women with interstitial cystitis: a case-control study. J Urol. 2005;174:937-940.
18. Weissman MM, Gross R, Fyer A, et al. Interstitial cystitis and panic disorder: a potential genetic syndrome. Arch Gen Psychiatr. 2004;61:273-279.
19. Wu EQ, Birnbaum H, Mareva M, et al. Interstitial cystitis: cost, treatment and comorbidities in an employed population. Pharmacoeconomics. 2006;24:55-65.
20. Stanford EJ, Koziol J, Feng A. The prevalence of interstitial cystitis, endometriosis, adhesions, and vulvar pain in women with chronic pelvic pain. J Minim Invasive Gynecol. 2005;12:43-49.
21. Peters KM, Killinger KA, Ibrahim IA. Childhood symptoms and events in women with interstitial cystitis/painful bladder syndrome. Urology. 2009;73:258-262.
22. Rackow BW, Novi JM, Arya LA, Pfeifer SM. Interstitial cystitis is an etiology of chronic pelvic pain in young women. J Pediatr Adolesc Gynecol. 2009;22:181-185.
23. Diggs C, Meyer WA, Langenberg P, et al. Assessing urgency in interstitial cystitis/painful bladder syndrome. Urology. 2007;69:210-214.
24. Peters KM, Kalinowski SE, Carrico DJ, et al. Fact or fiction—is abuse prevalent in patients with interstitial cystitis? Results from a community survey and clinic population. J Urol. 2007;178(3 Pt 1):891-895.
25. Mayson BE, Teichman JM. The relationship between sexual abuse and interstitial cystitis/painful bladder syndrome. Curr Urol Rep. 2009;10:441-447.
26. O’Leary MP, Sant GR, Fowler FJ, Jr, et al. The interstitial cystitis symptom index and problem index. Urology. 1997;49(suppl 5A):S58-S63.
27. Parsons CL, Dell J, Stanford EJ, et al. Increased prevalence of interstitial cystitis: previously unrecognized urologic and gynecologic cases identified using a new symptom questionnaire and intravesical potassium sensitivity. Urology. 2002;60:573-578.
28. Peters KM, Carrico DJ, Kalinowski SE, et al. Prevalence of pelvic floor dysfunction in patients with interstitial cystitis. Urology. 2007;70:16-18.
29. Nordling J, Anjum FH, Bade JJ, et al. Primary evaluation of patients suspected of having interstitial cystitis (IC). Eur Urol. 2004;45:662-669.
30. Waxman JA, Sulak PJ, Kuehl TJ. Cystoscopic findings consistent with interstitial cystitis in normal women undergoing tubal ligation. J Urol. 1998;160:1663-1667.
31. American Urological Association. Guideline on the diagnosis and treatment of interstitial cystitis/bladder pain syndrome (2011). Available at: http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=ic.bps. Accessed May 10, 2011.
32. Dimitrakov J, Kroenke K, Steers WD, et al. Pharmacologic management of painful bladder syndrome/interstitial cystitis: a systematic review. Arch Intern Med. 2007;167:1922-1929.
33. Sant GR, Propert KJ, Hanno PM, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol. 2003;170:810-815.
34. Buffington CA. Re: cyclosporine A and pentosan polysulfate sodium for the treatment of interstitial cystitis: a randomized comparative study. J Urol. 2006;176:838.-
35. van Ophoven A, Hertle L. Long-term results of amitriptyline treatment for interstitial cystitis. J Urol. 2005;174:1837-1840.
36. Foster HE, Jr, Hanno PM, Nickel JC, et al. Effect of amitriptyline on symptoms in treatment naive patients with interstitial cystitis/painful bladder syndrome. J Urol. 2010;183:1853-1858.
37. Hameroff SR, Weiss JL, Lerman JC, et al. Doxepin’s effects on chronic pain and depression: a controlled study. J Clin Psychiatry. 1984;45(3 Pt 2):PMID 6321454.-
38. van Ophoven A, Hertle L. The dual serotonin and noradrenaline reuptake inhibitor duloxetine for the treatment of interstitial cystitis: results of an observational study. J Urol. 2007;177:552-555.
39. Theoharides TC, Sant GR. Hydroxyzine therapy for interstitial cystitis. Urology. 1997;49(suppl):S108-S110.
40. Interstitial Cystitis Association. IC supplements. Revised April 11, 2011. Available at: http://www.ichelp.org/Page.aspx?pid=635. Accessed May 9, 2011.
41. Theoharides TC, Kempuraj D, Vakali S, et al. Treatment of refractory interstitial cystitis/painful bladder syndrome with CystoProtek—an oral multi-agent natural supplement. Can J Urol. 2008;15:4410-4414.
42. Phatak S, Foster HE, Jr. The management of interstitial cystitis: an update. Nat Clin Pract Urol. 2006;3:45-53.
43. Erickson DR, Kunselman AR, Bentley CM, et al. Changes in urine markers and symptoms after bladder distension for interstitial cystitis. J Urol. 2007;117:556-560.
44. Dawson TE, Jamison J. Intravesical treatments for painful bladder syndrome/interstitial cystitis. Cochrane Database Syst Rev. 2007;(4):CD006113.-
45. Peeker R, Haghsheno MA, Holmang S, et al. Intravesical bacillus Calmette-Guerin and dimethyl sulfoxide for treatment of classic and nonulcer interstitial cystitis:a prospective, randomized double-blind study. J Urol. 2000;164:1912-1916.
46. Cervigni M, Natale F, Nasta L, et al. A combined intravesical therapy with hyaluronic acid and chondroitin for refractory painful bladder syndrome/interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:943-947.
47. Nickel JC, Moldwin R, Lee S, et al. Intravesical alkalinized lidocaine (PSD597) offers sustained relief from symptoms of interstitial cystitis and painful bladder syndrome. BJU Int. 2009;103:910-918.
Strategies for managing hot flashes
• Use hormone therapy (HT) at the lowest effective dose and for the shortest duration possible (preferably ≤5 years) in women for whom the potential benefits outweigh the potential risks. A
• Counsel patients that the effectiveness of phytoestrogens (soy), exercise routines, yoga, acupuncture, vitamin E, evening primrose oil, and other herbal preparations has not been established. B
• When HT is refused or contraindicated by a patient’s risk profile, consider antidepressants (selective norepinephrine reuptake inhibitors and selective serotonin reuptake inhibitors), gabapentin, or clonidine. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hot flashes are the most prevalent and most bothersome symptoms of the menopausal transition and the leading cause for seeking medical attention during that period of a woman’s life.1 They may last for a few seconds or for several minutes and may occur as frequently as every hour to several times per week. On average, women experience hot flashes for a period of 6 months to 2 years, but the symptoms may last up to 10 years or more.2,3
Hot flashes have been reported by up to 70% of women undergoing natural menopause, and by almost all women undergoing surgical menopause.4 For many women, these symptoms are mild and can be managed with reassurance and counseling. For others, the symptoms are severe, overwhelming, last for many years, and impair the quality of life.
According to a community-based survey of 16,000 women, hot flashes occur most often in late perimenopause and among those with a body mass index ≥27. Hot flashes are also more common among African Americans and women who are less physically active and have a lower income.5
Since the publication of the Women’s Health Initiative study in 2002 raised concerns about the long-term safety of hormone therapy (HT), nonhormonal remedies have emerged as potential alternative treatments.6,7 A wealth of evidence has accumulated on the efficacy and safety of these, and various other approaches to the management of hot flashes. This review will summarize that evidence to help you provide optimal care and assist patients in making informed choices about their treatment.
Hormone replacement therapy
HT, given as estrogen alone in women without a uterus or estrogen plus progestin in women with a uterus, is the most studied and most effective therapy for vasomotor symptoms attributable to menopause. Data from one Cochrane review showed a significant reduction in the frequency of weekly hot flashes for oral estrogen compared with placebo, with a weighted mean difference (WMD) of -17.92 (95% confidence interval [CI], -22.86 to -12.99).8 This was equivalent to a 75% reduction in frequency (95% CI, 64.3-82.3) for HT relative to placebo. Results were similar for both opposed and unopposed estrogen regimens.8
Transdermal vs oral therapy. Another review compared oral estradiol, transdermal estradiol, and placebo in terms of reduction of hot flash frequency or severity, or both.9 The review revealed a pooled WMD in hot flashes of -16.8 per week (95% CI, -23.4 to -10.2) for oral estradiol and -22.4 per week (95% CI, -35.9 to -10.4) for transdermal estradiol. Results were similar for opposed and unopposed estrogen regimens.9
Transdermal delivery of estrogen as patches, gels, and sprays delivers unmetabolized estradiol directly to the blood stream, so that lower doses can achieve similar efficacy to doses administered orally.10 Thus, the transdermal route would be in keeping with current guidelines to prescribe the lowest effective dose that relieves symptoms. Emerging research should provide more insight regarding safety and the potential for fewer health risks with transdermal HT compared with oral therapy.
Best way to discontinue? When HT is discontinued, hot flashes may return—sometimes immediately, sometimes after a few months. No evidence-based guidelines exist on the best way to discontinue HT with the least recurrence and severity of hot flashes. No optimal tapering regimen (either by dose or number of days per week that HT is taken) has yet been described in any studies, nor have any randomized controlled trials (RCTs)revealed a significant difference between tapered or abrupt discontinuation.11,12
The breast cancer connection. The relationship between HT and breast cancer has generated considerable controversy. In the Women’s Health Initiative (WHI) trial, which included participants on estrogen-only and estrogen plus progesterone regimens, an overall increased risk (hazard ratio [HR]=1.26; 95% CI, 1.00-1.59) was reported. The increased risk fell short of statistical significance, and varied with the duration of exposure.6
In subsequent studies, the magnitude of the associated risk was substantially greater for the estrogen-progestogen preparation, and also higher for longer-term exposure.13 Additionally, a meta-analysis of 8 studies of the risk of breast cancer with combination HT resulted in an odds ratio (OR) of 1.39 (95% CI, 1.12-1.72). Estimates were higher for more than 5 years of use (OR=1.63; 95% CI, 1.22-2.18) when compared with estimates for less than 5 years of use (OR=1.35; CI, 1.16-1.57).14 Recent studies have reported a decline in the incidence of breast cancer in the United States, which has been attributed to a parallel reduction in HT use.15
In contrast to the findings for women taking combination HT, the estrogen-only arm of the WHI study showed a decrease in the overall risk of breast cancer.16 A new analysis of data on participants in the estrogen-only arm of the study shows that, 10 years after the intervention ended, a decreased risk of breast cancer persists. In addition, the increased risk of stroke and deep vein thrombosis found in the original study had dissipated, and the decreased risk of hip fracture was not maintained.
Age matters. Health outcomes in this new analysis were more favorable for younger women for coronary heart disease, heart attack, colorectal cancer, total mortality, and a global index of chronic diseases.17 Pooled results of systematic reviews and meta-analyses on HT risks are available in TABLE 1.14,16,18-21
Progestins
Progesterone in the form of injections (Depo-Provera 150 mg, for example) or oral medroxyprogesterone acetate 20 mg daily has shown a significant reduction in hot flashes compared with placebo.22 However, associated side effects (withdrawal bleeding and weight gain) and concerns about breast cancer often limit the use of this medication.23 Because of the paucity of evidence, transdermal progesterone creams should not be recommended.24
Tibolone
Tibolone is a synthetic steroid that is structurally related to 19-nortestosterone derivatives. It has weak estrogenic, progestogenic, and androgenic properties and has shown significant reduction in hot flashes and night sweating compared with placebo.25 Tibolone has also been shown to enhance mood and sexual function.26 However, evidence of its safety on outcomes such as breast cancer and cardiovascular events is obscure and has shown conflicting results, especially for breast cancer.25,26
A recent double-blinded trial on women with breast cancer found that although tibolone significantly improved vasomotor symptoms, it was associated with an increased risk of breast cancer recurrence (HR=1.40; 95% CI, 1.14-1.70]; P=.001).27 Tibolone is not approved for sale in the United States.
Nonhormonal options
Lifestyle modifications
According to recent reviews, exercise is less effective than HT in relieving hot flashes, but does seems to have beneficial effects on mood, sleep, and overall quality of life.28,29 Other lifestyle modifications such as smoking cessation and weight loss may also be of use.5
Antidepressants
Data from one meta-analysis showed that selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors (SNRIs) were significantly more effective than placebo in reducing daily frequency of hot flashes (WMD=-1.13; 95% CI, -1.70 to -0.57).30 Efficacy varied for individual drugs.
Venlafaxine, an SNRI, has been shown to be more effective than placebo in managing hot flashes.31 The effect on hot flashes was noticed after 4 weeks of treatment with the 75- and 150-mg doses, but the higher dosage was associated with more adverse effects such as dry mouth, sleeplessness, and decreased appetite.32 Even so, 93% of the participants in the venlafaxine group chose to continue treatment, because the reduction in hot flashes had significantly improved their daily lives.31 Desvenlafaxine, a metabolite of venlafaxine, has been shown to reduce the number of hot flashes by almost 65% from baseline at Weeks 4 and 12, with dosages of 100 and 150 mg/d.33
The SSRIs fluoxetine, citalopram, and paroxetine in 10-, 20-, and 30-mg doses and paroxetine CR (12.5 and 25 mg) have been studied in many RCTs. All have demonstrated a significant decrease in hot flashes compared with placebo with various dosages used. SSRIs reduce hot flashes by as much as 50% to 60%, compared with 80% for estrogen.34 The duration of treatment ranged from 4 weeks to 6 months.
Gabapentin
Gabapentin is approved by the FDA for the treatment of partial seizures and postherpetic neuralgia.35 A meta-analysis of multiple studies published in 2006 showed that gabapentin reduced the mean number of daily hot flashes by 2.05 (95% CI, -2.80 to -1.30).30 Two recent reviews evaluated the efficacy and safety of gabapentin in the treatment of hot flashes in menopausal women and reported that gabapentin in daily doses ranging from 900 to 2400 mg and titration periods lasting 3 to12 days was well tolerated and effective.35,36
A meta-analysis published in 2009 that included 4 RCTs reported significant heterogeneity from one study to another, but comparisons of gabapentin and placebo showed reductions of 20% to 30% in the frequency and severity of hot flashes with gabapentin.36 The most commonly reported adverse effects included somnolence, dizziness, ataxia, fatigue, nystagmus, and peripheral edema.
Clonidine
Clonidine has been studied in oral and transdermal forms for the treatment of hot flashes in menopausal women, especially in women with breast cancer.37-39 Data from one meta-analysis revealed significant reductions in daily hot flashes in the clonidine group compared with placebo at 4 weeks (mean difference [MD]=-0.95; 95% CI, -1.44 to -0.47) and at 8 weeks (MD=-1.63; 95% CI, -2.76 to -0.50).30 Adverse effects included dry mouth, drowsiness, and dizziness. The transdermal route may avoid some of these side effects.40
Alternative remedies
Phytoestrogen and isoflavones. Phytoestrogens are sterol molecules produced by plants. They are similar in structure to human estrogens and have been shown to have estrogen-like activity.40 They are available as dietary soy, soy extract, and red clover extracts. Isoflavones are a type of phytoestrogen.
Comparing trials of effects of soy or isoflavone is difficult, as various formulations and amounts of these products have been used. Combining the data from these trials yielded nonsignificant results for Promensil, a red clover extract (WMD=-0.6; 95% CI, -1.8 to 0.6), and inconsistent results (sometimes favoring the intervention, other times the placebo) for soy food and soy extracts.41-43
There is no evidence of estrogenic stimulation of the endometrium with phytoestrogens used for up to 2 years.41 Nevertheless, in the absence of evidence on the safety of long-term use, women with a personal or strong family history of hormone-dependent cancers (breast, uterine, or ovarian) or thromboembolic events should be cautious about using soy-based therapies.42 Long-term safety of these products has to be established before any evidence-based recommendations can be made.
Black cohosh. Actaea racemosa (formerly Cimicifuga racemosa) is the most studied and perhaps the most widely used herbal remedy for hot flashes. It is commonly known as black cohosh and has been used traditionally by Native Americans for the treatment of various medical conditions, including amenorrhea and menopause.44,45
Remifemin is an available standardized extract. Evidence for effectiveness is limited and contradictory. Data from a recent meta-analysis showed that although there was significant heterogeneity between included trials, preparations containing black cohosh improved vasomotor symptoms overall by 26% (95% CI, 11%-40%).45
A recent well-conducted RCT concluded that neither black cohosh nor red clover significantly reduced the frequency of symptoms compared with placebo.46 The same study found that both botanicals were safe as administered for a 12-month period. Some case reports have identified serious adverse events including acute hepatocellular damage, which warrants further investigation, although no causal relationship has been established.47
Evidence for safety and efficacy of antidepressants, gabapentin, clonidine, isoflavones, black cohosh, yoga, acupuncture, and herbal remedies is summarized in TABLE 2.30,36,41,47-55
For more on the treatment of hot flashes, see “Clinical approach to managing hot flashes”.40
Start with a detailed history: Ask about the nature of your patient’s symptoms, her past gynecologic and medical history, and her family history. Take a baseline blood pressure, measure body mass index (BMI), and order a lipid profile. Exclude other possible causes of hot flashes: hyperthyroidism, panic disorder, diabetes, and medications such as antiestrogens or selective estrogen receptor modulators.
Assess the severity of hot flashes and explain their typical clinical course. Discuss lifestyle modifications that may help: losing weight, quitting smoking, wearing lighter clothing, and cutting down on caffeine intake, alcohol, and spicy foods. Tell your patient that hormone therapy (HT) has been shown to be the most effective treatment for women without contraindications. These include current, past, or suspected breast cancer, other estrogen-sensitive malignant conditions, undiagnosed genital bleeding, untreated endometrial hyperplasia, venous thromboembolism, angina, myocardial infarction, uncontrolled hypertension, liver disease, porphyria cutanea tarda (absolute contraindication), or hypersensitivity to the active substances of HT.40
If contraindications can be ruled out, find out whether she is receptive to HT or would prefer alternatives. If she is interested in HT, discuss the risks and benefits involved and the different dosages and routes of administration that are available. If she prefers to explore nonhormonal remedies, discuss the various options and present the evidence for their safety and efficacy. Tell her that the safety of some herbal remedies that contain estrogenic compounds has not been established.
What the future holds
The selective estrogen receptor agonist MF-101, which can induce tissue-specific estrogen-like effects, has been shown to be effective in reducing menopausal hot flashes compared with placebo in a phase II trial.56 A larger phase III trial is in progress.
Lower and ultra-lower doses of systemic estrogen are now available and approved by the FDA, and were found effective in relieving vasomotor symptoms.57-59 The drawback of these preparations is that they may take longer than standard-dose estrogen to achieve maximum relief of symptoms (8-12 weeks vs 4 weeks, respectively). The lower doses have been associated with fewer adverse effects (such as vaginal bleeding and breast tenderness) compared with the standard doses.58,59 Their long-term effects on the cardiovascular system, bone, and breast are still being tested and need to be established.
Newer selective estrogen receptor modulators, especially in combination with estrogen, are another approach to menopausal symptoms currently in testing.60
CORRESPONDENCE
Ghufran A. Jassim, MD, ABMS, MSc, Department of Family and Community Medicine, Royal College of Surgeons in Ireland-Medical University of Bahrain, PO Box 15503, Adliya, Bahrain; gjassim@rcsi-mub.com
1. Williams RE, Kalilani L, DiBenedetti DB. Healthcare seeking and treatment for menopausal symptoms in the US. Maturitas. 2007;58:348-358.
2. North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of the North American Menopause Society. Menopause. 2010;17:242-256.
3. National Institutes of Health. NIH state-of-the-science conference statement: management of menopause-related symptoms. Ann Intern Med. 2005;143(12 pt 2):1003-1013.
4. Berecki-Gisolf J, Begum N, Dobson AJ. Symptoms reported by women in midlife: menopausal transition or aging? Menopause. 2009;16:1021-1029.
5. Gold EB, Sternfeld B, Kelsey JL. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol. 2000;152:463-473.
6. Rossouw JE, Anderson GL, Prentice RL, et al. Writing group for the Women’s Health Initiative investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA. 2002;288:321-333.
7. Lukes A. Evolving issues in the clinical and managed care settings on the management of menopause following the Women’s Health Initiative. J Manag Care Pharm. 2008;14(3 suppl):7-13.
8. MacLennan AH, Broadbent JL, Lester W, et al. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database Syst Rev. 2004;(4):CD002978.-
9. Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610-1620.
10. Carroll N. A review of transdermal nonpatch estrogen therapy for the management of menopausal symptoms. J Womens Health (Larchmt). 2010;19:47-55.
11. Suffoletto JA, Hess R. Tapering vs cold turkey: symptoms versus successful discontinuation of menopausal hormone therapy. Menopause. 2009;16:436-437.
12. Lindh-Astrand L, Bixo M, Hirschberg AL, et al. A randomized controlled study of taper-down or abrupt discontinuation of hormone therapy in women treated for vasomotor symptoms. Menopause. 2010;17:72-29.
13. Banks E, Canfell K, Reeves G. HRT and breast cancer. Womens Health. 2008;4:427-431.
14. Shah NR, Borenstein J, Dubois RW. Postmenopausal hormone therapy and breast cancer. Menopause. 2005;12:668-678.
15. Krieger N, Chen JT, Waterman PD. Decline in US breast cancer rates after the Women’s Health Initiative. Am J Public Health. 2010;100 (suppl 1):S132-S139.
16. Nelson HD, Humphrey LL, Nygren P, et al. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288:872-881.
17. LaCroix AZ, Chiebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy. JAMA. 2011;305:1305-1314.
18. Canonico M, Plu-Bureau G, Lowe GD, et al. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women. BMJ. 2008;336:1227-1231.
19. Gabriel SR, Carmona L, Roque M, et al. Hormone replacement therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2005;(2):CD002229.-
20. Sare GM, Gray LJ, Bath PM. Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta-analysis. Eur Heart J. 2008;29:2031-2041.
21. Bath PM, Gray LJ. Association between hormone replacement therapy and subsequent stroke. BMJ. 2005;330:342.-
22. Boothby LA, Doering PL, Kipersztok S. Bioidentical hormone therapy: a review. Menopause. 2004;11:356-367.
23. Pasqualini JR. Progestins and breast cancer. Gynecol Endocrinol. 2007;23(suppl 1):32-41.
24. Wren BG. Transdermal progesterone creams for postmenopausal women. Med J Aust. 2005;182:237-225.
25. Kenemans P, Speroff L. Tibolone: clinical recommendations and practical guidelines: a report of the International Tibolone Consensus Group. Maturitas. 2005;51:21-28.
26. Wang PH, Cheng MH, Chao HT, et al. Effects of tibolone on the breast of postmenopausal women. Taiwan J Obstet Gynecol. 2007;46:121-126.
27. Kenemans P, Bundred NJ, Foidart JM, et al. Safety and efficacy of tibolone in breast-cancer patients with vasomotor symptoms. Lancet Oncol. 2009;10:135-146.
28. Daley A, Stokes-Lampard HJ, Macarthur C. Exercise to reduce vasomotor and other menopausal symptoms: a review. Maturitas. 2009;63:176-180.
29. Daley A, MacArthur C, Mutrie N, et al. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2007;(4):CD006108.-
30. Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes. JAMA. 2006;295:2057-2072.
31. Evans ML, Pritts E, Vittinghof E, et al. Management of postmenopausal hot flushes with venlafaxine hydrochloride. Obstet Gynecol. 2005;105:161-166.
32. Barton D, La Vasseur B, Loprinzi C, et al. Venlafaxine for the control of hot flashes. Oncol Nurs Forum. 2002;29:33-40.
33. Lilue M, Palacios S. Non-hormonal treatment for vasomotor symptoms during menopause: role of desvenlafaxine. Ginecol Obstet Mex. 2009;77:475-481.
34. Albertazzi P. Non-estrogenic approaches for the treatment of climacteric symptoms. Climacteric. 2007;10(suppl 2):115-120.
35. Brown JN, Wright BR. Use of gabapentin in patients experiencing hot flashes. Pharmacotherapy. 2009;29:74-81.
36. Toulis KA, Tzellas T, Kouvelas D, et al. Gabapentin for the treatment of hot flashes in women with natural or tamoxifen-induced menopause: a systematic review and meta-analysis. Clin Ther. 2009;31:221-235.
37. Pandya KJ, Raubertas AF, Flynn PJ, et al. Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes. Ann Intern Med. 2000;132:788-793.
38. Goldberg RM, Loprinzi CL, O’Fallon JR. Transdermal clonidine for ameliorating tamoxifen-induced hot flashes. J Clin Oncol. 1994;12:155-158.
39. Buijs C, Mom CH, Willemse PH, et al. Venlafaxine versus clonidine for the treatment of hot flashes in breast cancer patients. Breast Cancer Res Treat. 2009;115:573-580.
40. Cobin RH, Futterweit W, Ginzburg SB, et al. AACE Menopause Guidelines Revision Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of menopause. Endocr Pract. 2006;12:315-337.
41. Lethaby AE, Brown J, Marjoribanks J. Phytoestrogens for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2007;(4):CD001395.-
42. Tempfer CB, Bentz EH, Leodolter S, et al. Phytoestrogens in clinical practice. Fertil Steril. 2007;87:1243-1249.
43. Krebs EE, Ensrud KE, MacDonald R, et al. Phytoestrogens for treatment of menopausal symptoms: a systematic review. Obstet Gynecol. 2004;104:824-836.
44. McKenna DJ, Jones K, Humphrey S, et al. Black cohosh. Altern Ther Health Med. 2001;7:93-100.
45. Shams T, Setia MS, Hemmings R, et al. Efficacy of black cohosh-containing preparations on menopausal symptoms: a meta-analysis. Altern Ther Health Med. 2010;16:36-44.
46. Geller SE, Shulman LP, van Breeman RB, et al. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms. Menopause. 2009;16:1156-1166.
47. Borrelli F, Ernst E. Black cohosh (Cimicifuga racemosa): a systematic review of adverse events. Am J Obstet Gynecol. 2008;199:455-466.
48. Nelson HD. Menopause. Lancet. 2008;371:760-770.
49. Borrelli F, Ernst E. Black cohosh (Cimicifuga racemosa) for menopausal symptoms. Pharmacol Res. 2008;58:8-14.
50. Huntley A, Ernst E. A systematic review of the safety of black cohosh. Menopause. 2003;10:58-64.
51. Kanadys WM, Leszczynska-Gorselak B, Oleszczuk J. Efficacy and safety of black cohosh in the treatment of vasomotor symptoms. Ginekol Pol. 2008;79:287-296.
52. Huntley AL, Ernst E. A systematic review of herbal medicinal products for the treatment of menopausal symptoms. Menopause. 2003;10:465-476.
53. Lee MS, Shin BC, Ernst E. Acupuncture for treating menopausal hot flushes: a systematic review. Climacteric. 2009;12:16-25.
54. Lee MS, Kim JI, Ha JY, et al. Yoga for menopausal symptoms: a systematic review. Menopause. 2009;16:602-608.
55. Cho SH, Whang WW. Acupuncture for vasomotor menopausal symptoms. Menopause. 2009;16:1065-1073.
56. Stovall DW, Pinkerton JV. MF-101, an estrogen receptor beta agonist for the treatment of vasomotor symptoms in peri- and postmenopausal women. Curr Opin Investig Drugs. 2009;10:365-371.
57. Peeyananjarassri K, Baber R. Effects of low-dose hormone therapy on menopausal symptoms, bone mineral density, endometrium, and the cardiovascular system. Climacteric. 2004;8:13-23.
58. Ettinger B. Vasomotor symptom relief versus unwanted effects: role of estrogen dosage. Am J Med. 2005;118(suppl 12B):74-78.
59. Ettinger B. Rationale for use of lower estrogen doses for postmenopausal hormone therapy. Maturitas. 2007;57:81-84.
60. Jain N, Xu J, Kanojia RM, et al. Identification and structure-activity relationships of chromene-derived selective estrogen receptor modulators for treatment of postmenopausal symptoms. J Med Chem. 2009;52:7544-7569.
• Use hormone therapy (HT) at the lowest effective dose and for the shortest duration possible (preferably ≤5 years) in women for whom the potential benefits outweigh the potential risks. A
• Counsel patients that the effectiveness of phytoestrogens (soy), exercise routines, yoga, acupuncture, vitamin E, evening primrose oil, and other herbal preparations has not been established. B
• When HT is refused or contraindicated by a patient’s risk profile, consider antidepressants (selective norepinephrine reuptake inhibitors and selective serotonin reuptake inhibitors), gabapentin, or clonidine. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hot flashes are the most prevalent and most bothersome symptoms of the menopausal transition and the leading cause for seeking medical attention during that period of a woman’s life.1 They may last for a few seconds or for several minutes and may occur as frequently as every hour to several times per week. On average, women experience hot flashes for a period of 6 months to 2 years, but the symptoms may last up to 10 years or more.2,3
Hot flashes have been reported by up to 70% of women undergoing natural menopause, and by almost all women undergoing surgical menopause.4 For many women, these symptoms are mild and can be managed with reassurance and counseling. For others, the symptoms are severe, overwhelming, last for many years, and impair the quality of life.
According to a community-based survey of 16,000 women, hot flashes occur most often in late perimenopause and among those with a body mass index ≥27. Hot flashes are also more common among African Americans and women who are less physically active and have a lower income.5
Since the publication of the Women’s Health Initiative study in 2002 raised concerns about the long-term safety of hormone therapy (HT), nonhormonal remedies have emerged as potential alternative treatments.6,7 A wealth of evidence has accumulated on the efficacy and safety of these, and various other approaches to the management of hot flashes. This review will summarize that evidence to help you provide optimal care and assist patients in making informed choices about their treatment.
Hormone replacement therapy
HT, given as estrogen alone in women without a uterus or estrogen plus progestin in women with a uterus, is the most studied and most effective therapy for vasomotor symptoms attributable to menopause. Data from one Cochrane review showed a significant reduction in the frequency of weekly hot flashes for oral estrogen compared with placebo, with a weighted mean difference (WMD) of -17.92 (95% confidence interval [CI], -22.86 to -12.99).8 This was equivalent to a 75% reduction in frequency (95% CI, 64.3-82.3) for HT relative to placebo. Results were similar for both opposed and unopposed estrogen regimens.8
Transdermal vs oral therapy. Another review compared oral estradiol, transdermal estradiol, and placebo in terms of reduction of hot flash frequency or severity, or both.9 The review revealed a pooled WMD in hot flashes of -16.8 per week (95% CI, -23.4 to -10.2) for oral estradiol and -22.4 per week (95% CI, -35.9 to -10.4) for transdermal estradiol. Results were similar for opposed and unopposed estrogen regimens.9
Transdermal delivery of estrogen as patches, gels, and sprays delivers unmetabolized estradiol directly to the blood stream, so that lower doses can achieve similar efficacy to doses administered orally.10 Thus, the transdermal route would be in keeping with current guidelines to prescribe the lowest effective dose that relieves symptoms. Emerging research should provide more insight regarding safety and the potential for fewer health risks with transdermal HT compared with oral therapy.
Best way to discontinue? When HT is discontinued, hot flashes may return—sometimes immediately, sometimes after a few months. No evidence-based guidelines exist on the best way to discontinue HT with the least recurrence and severity of hot flashes. No optimal tapering regimen (either by dose or number of days per week that HT is taken) has yet been described in any studies, nor have any randomized controlled trials (RCTs)revealed a significant difference between tapered or abrupt discontinuation.11,12
The breast cancer connection. The relationship between HT and breast cancer has generated considerable controversy. In the Women’s Health Initiative (WHI) trial, which included participants on estrogen-only and estrogen plus progesterone regimens, an overall increased risk (hazard ratio [HR]=1.26; 95% CI, 1.00-1.59) was reported. The increased risk fell short of statistical significance, and varied with the duration of exposure.6
In subsequent studies, the magnitude of the associated risk was substantially greater for the estrogen-progestogen preparation, and also higher for longer-term exposure.13 Additionally, a meta-analysis of 8 studies of the risk of breast cancer with combination HT resulted in an odds ratio (OR) of 1.39 (95% CI, 1.12-1.72). Estimates were higher for more than 5 years of use (OR=1.63; 95% CI, 1.22-2.18) when compared with estimates for less than 5 years of use (OR=1.35; CI, 1.16-1.57).14 Recent studies have reported a decline in the incidence of breast cancer in the United States, which has been attributed to a parallel reduction in HT use.15
In contrast to the findings for women taking combination HT, the estrogen-only arm of the WHI study showed a decrease in the overall risk of breast cancer.16 A new analysis of data on participants in the estrogen-only arm of the study shows that, 10 years after the intervention ended, a decreased risk of breast cancer persists. In addition, the increased risk of stroke and deep vein thrombosis found in the original study had dissipated, and the decreased risk of hip fracture was not maintained.
Age matters. Health outcomes in this new analysis were more favorable for younger women for coronary heart disease, heart attack, colorectal cancer, total mortality, and a global index of chronic diseases.17 Pooled results of systematic reviews and meta-analyses on HT risks are available in TABLE 1.14,16,18-21
Progestins
Progesterone in the form of injections (Depo-Provera 150 mg, for example) or oral medroxyprogesterone acetate 20 mg daily has shown a significant reduction in hot flashes compared with placebo.22 However, associated side effects (withdrawal bleeding and weight gain) and concerns about breast cancer often limit the use of this medication.23 Because of the paucity of evidence, transdermal progesterone creams should not be recommended.24
Tibolone
Tibolone is a synthetic steroid that is structurally related to 19-nortestosterone derivatives. It has weak estrogenic, progestogenic, and androgenic properties and has shown significant reduction in hot flashes and night sweating compared with placebo.25 Tibolone has also been shown to enhance mood and sexual function.26 However, evidence of its safety on outcomes such as breast cancer and cardiovascular events is obscure and has shown conflicting results, especially for breast cancer.25,26
A recent double-blinded trial on women with breast cancer found that although tibolone significantly improved vasomotor symptoms, it was associated with an increased risk of breast cancer recurrence (HR=1.40; 95% CI, 1.14-1.70]; P=.001).27 Tibolone is not approved for sale in the United States.
Nonhormonal options
Lifestyle modifications
According to recent reviews, exercise is less effective than HT in relieving hot flashes, but does seems to have beneficial effects on mood, sleep, and overall quality of life.28,29 Other lifestyle modifications such as smoking cessation and weight loss may also be of use.5
Antidepressants
Data from one meta-analysis showed that selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors (SNRIs) were significantly more effective than placebo in reducing daily frequency of hot flashes (WMD=-1.13; 95% CI, -1.70 to -0.57).30 Efficacy varied for individual drugs.
Venlafaxine, an SNRI, has been shown to be more effective than placebo in managing hot flashes.31 The effect on hot flashes was noticed after 4 weeks of treatment with the 75- and 150-mg doses, but the higher dosage was associated with more adverse effects such as dry mouth, sleeplessness, and decreased appetite.32 Even so, 93% of the participants in the venlafaxine group chose to continue treatment, because the reduction in hot flashes had significantly improved their daily lives.31 Desvenlafaxine, a metabolite of venlafaxine, has been shown to reduce the number of hot flashes by almost 65% from baseline at Weeks 4 and 12, with dosages of 100 and 150 mg/d.33
The SSRIs fluoxetine, citalopram, and paroxetine in 10-, 20-, and 30-mg doses and paroxetine CR (12.5 and 25 mg) have been studied in many RCTs. All have demonstrated a significant decrease in hot flashes compared with placebo with various dosages used. SSRIs reduce hot flashes by as much as 50% to 60%, compared with 80% for estrogen.34 The duration of treatment ranged from 4 weeks to 6 months.
Gabapentin
Gabapentin is approved by the FDA for the treatment of partial seizures and postherpetic neuralgia.35 A meta-analysis of multiple studies published in 2006 showed that gabapentin reduced the mean number of daily hot flashes by 2.05 (95% CI, -2.80 to -1.30).30 Two recent reviews evaluated the efficacy and safety of gabapentin in the treatment of hot flashes in menopausal women and reported that gabapentin in daily doses ranging from 900 to 2400 mg and titration periods lasting 3 to12 days was well tolerated and effective.35,36
A meta-analysis published in 2009 that included 4 RCTs reported significant heterogeneity from one study to another, but comparisons of gabapentin and placebo showed reductions of 20% to 30% in the frequency and severity of hot flashes with gabapentin.36 The most commonly reported adverse effects included somnolence, dizziness, ataxia, fatigue, nystagmus, and peripheral edema.
Clonidine
Clonidine has been studied in oral and transdermal forms for the treatment of hot flashes in menopausal women, especially in women with breast cancer.37-39 Data from one meta-analysis revealed significant reductions in daily hot flashes in the clonidine group compared with placebo at 4 weeks (mean difference [MD]=-0.95; 95% CI, -1.44 to -0.47) and at 8 weeks (MD=-1.63; 95% CI, -2.76 to -0.50).30 Adverse effects included dry mouth, drowsiness, and dizziness. The transdermal route may avoid some of these side effects.40
Alternative remedies
Phytoestrogen and isoflavones. Phytoestrogens are sterol molecules produced by plants. They are similar in structure to human estrogens and have been shown to have estrogen-like activity.40 They are available as dietary soy, soy extract, and red clover extracts. Isoflavones are a type of phytoestrogen.
Comparing trials of effects of soy or isoflavone is difficult, as various formulations and amounts of these products have been used. Combining the data from these trials yielded nonsignificant results for Promensil, a red clover extract (WMD=-0.6; 95% CI, -1.8 to 0.6), and inconsistent results (sometimes favoring the intervention, other times the placebo) for soy food and soy extracts.41-43
There is no evidence of estrogenic stimulation of the endometrium with phytoestrogens used for up to 2 years.41 Nevertheless, in the absence of evidence on the safety of long-term use, women with a personal or strong family history of hormone-dependent cancers (breast, uterine, or ovarian) or thromboembolic events should be cautious about using soy-based therapies.42 Long-term safety of these products has to be established before any evidence-based recommendations can be made.
Black cohosh. Actaea racemosa (formerly Cimicifuga racemosa) is the most studied and perhaps the most widely used herbal remedy for hot flashes. It is commonly known as black cohosh and has been used traditionally by Native Americans for the treatment of various medical conditions, including amenorrhea and menopause.44,45
Remifemin is an available standardized extract. Evidence for effectiveness is limited and contradictory. Data from a recent meta-analysis showed that although there was significant heterogeneity between included trials, preparations containing black cohosh improved vasomotor symptoms overall by 26% (95% CI, 11%-40%).45
A recent well-conducted RCT concluded that neither black cohosh nor red clover significantly reduced the frequency of symptoms compared with placebo.46 The same study found that both botanicals were safe as administered for a 12-month period. Some case reports have identified serious adverse events including acute hepatocellular damage, which warrants further investigation, although no causal relationship has been established.47
Evidence for safety and efficacy of antidepressants, gabapentin, clonidine, isoflavones, black cohosh, yoga, acupuncture, and herbal remedies is summarized in TABLE 2.30,36,41,47-55
For more on the treatment of hot flashes, see “Clinical approach to managing hot flashes”.40
Start with a detailed history: Ask about the nature of your patient’s symptoms, her past gynecologic and medical history, and her family history. Take a baseline blood pressure, measure body mass index (BMI), and order a lipid profile. Exclude other possible causes of hot flashes: hyperthyroidism, panic disorder, diabetes, and medications such as antiestrogens or selective estrogen receptor modulators.
Assess the severity of hot flashes and explain their typical clinical course. Discuss lifestyle modifications that may help: losing weight, quitting smoking, wearing lighter clothing, and cutting down on caffeine intake, alcohol, and spicy foods. Tell your patient that hormone therapy (HT) has been shown to be the most effective treatment for women without contraindications. These include current, past, or suspected breast cancer, other estrogen-sensitive malignant conditions, undiagnosed genital bleeding, untreated endometrial hyperplasia, venous thromboembolism, angina, myocardial infarction, uncontrolled hypertension, liver disease, porphyria cutanea tarda (absolute contraindication), or hypersensitivity to the active substances of HT.40
If contraindications can be ruled out, find out whether she is receptive to HT or would prefer alternatives. If she is interested in HT, discuss the risks and benefits involved and the different dosages and routes of administration that are available. If she prefers to explore nonhormonal remedies, discuss the various options and present the evidence for their safety and efficacy. Tell her that the safety of some herbal remedies that contain estrogenic compounds has not been established.
What the future holds
The selective estrogen receptor agonist MF-101, which can induce tissue-specific estrogen-like effects, has been shown to be effective in reducing menopausal hot flashes compared with placebo in a phase II trial.56 A larger phase III trial is in progress.
Lower and ultra-lower doses of systemic estrogen are now available and approved by the FDA, and were found effective in relieving vasomotor symptoms.57-59 The drawback of these preparations is that they may take longer than standard-dose estrogen to achieve maximum relief of symptoms (8-12 weeks vs 4 weeks, respectively). The lower doses have been associated with fewer adverse effects (such as vaginal bleeding and breast tenderness) compared with the standard doses.58,59 Their long-term effects on the cardiovascular system, bone, and breast are still being tested and need to be established.
Newer selective estrogen receptor modulators, especially in combination with estrogen, are another approach to menopausal symptoms currently in testing.60
CORRESPONDENCE
Ghufran A. Jassim, MD, ABMS, MSc, Department of Family and Community Medicine, Royal College of Surgeons in Ireland-Medical University of Bahrain, PO Box 15503, Adliya, Bahrain; gjassim@rcsi-mub.com
• Use hormone therapy (HT) at the lowest effective dose and for the shortest duration possible (preferably ≤5 years) in women for whom the potential benefits outweigh the potential risks. A
• Counsel patients that the effectiveness of phytoestrogens (soy), exercise routines, yoga, acupuncture, vitamin E, evening primrose oil, and other herbal preparations has not been established. B
• When HT is refused or contraindicated by a patient’s risk profile, consider antidepressants (selective norepinephrine reuptake inhibitors and selective serotonin reuptake inhibitors), gabapentin, or clonidine. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hot flashes are the most prevalent and most bothersome symptoms of the menopausal transition and the leading cause for seeking medical attention during that period of a woman’s life.1 They may last for a few seconds or for several minutes and may occur as frequently as every hour to several times per week. On average, women experience hot flashes for a period of 6 months to 2 years, but the symptoms may last up to 10 years or more.2,3
Hot flashes have been reported by up to 70% of women undergoing natural menopause, and by almost all women undergoing surgical menopause.4 For many women, these symptoms are mild and can be managed with reassurance and counseling. For others, the symptoms are severe, overwhelming, last for many years, and impair the quality of life.
According to a community-based survey of 16,000 women, hot flashes occur most often in late perimenopause and among those with a body mass index ≥27. Hot flashes are also more common among African Americans and women who are less physically active and have a lower income.5
Since the publication of the Women’s Health Initiative study in 2002 raised concerns about the long-term safety of hormone therapy (HT), nonhormonal remedies have emerged as potential alternative treatments.6,7 A wealth of evidence has accumulated on the efficacy and safety of these, and various other approaches to the management of hot flashes. This review will summarize that evidence to help you provide optimal care and assist patients in making informed choices about their treatment.
Hormone replacement therapy
HT, given as estrogen alone in women without a uterus or estrogen plus progestin in women with a uterus, is the most studied and most effective therapy for vasomotor symptoms attributable to menopause. Data from one Cochrane review showed a significant reduction in the frequency of weekly hot flashes for oral estrogen compared with placebo, with a weighted mean difference (WMD) of -17.92 (95% confidence interval [CI], -22.86 to -12.99).8 This was equivalent to a 75% reduction in frequency (95% CI, 64.3-82.3) for HT relative to placebo. Results were similar for both opposed and unopposed estrogen regimens.8
Transdermal vs oral therapy. Another review compared oral estradiol, transdermal estradiol, and placebo in terms of reduction of hot flash frequency or severity, or both.9 The review revealed a pooled WMD in hot flashes of -16.8 per week (95% CI, -23.4 to -10.2) for oral estradiol and -22.4 per week (95% CI, -35.9 to -10.4) for transdermal estradiol. Results were similar for opposed and unopposed estrogen regimens.9
Transdermal delivery of estrogen as patches, gels, and sprays delivers unmetabolized estradiol directly to the blood stream, so that lower doses can achieve similar efficacy to doses administered orally.10 Thus, the transdermal route would be in keeping with current guidelines to prescribe the lowest effective dose that relieves symptoms. Emerging research should provide more insight regarding safety and the potential for fewer health risks with transdermal HT compared with oral therapy.
Best way to discontinue? When HT is discontinued, hot flashes may return—sometimes immediately, sometimes after a few months. No evidence-based guidelines exist on the best way to discontinue HT with the least recurrence and severity of hot flashes. No optimal tapering regimen (either by dose or number of days per week that HT is taken) has yet been described in any studies, nor have any randomized controlled trials (RCTs)revealed a significant difference between tapered or abrupt discontinuation.11,12
The breast cancer connection. The relationship between HT and breast cancer has generated considerable controversy. In the Women’s Health Initiative (WHI) trial, which included participants on estrogen-only and estrogen plus progesterone regimens, an overall increased risk (hazard ratio [HR]=1.26; 95% CI, 1.00-1.59) was reported. The increased risk fell short of statistical significance, and varied with the duration of exposure.6
In subsequent studies, the magnitude of the associated risk was substantially greater for the estrogen-progestogen preparation, and also higher for longer-term exposure.13 Additionally, a meta-analysis of 8 studies of the risk of breast cancer with combination HT resulted in an odds ratio (OR) of 1.39 (95% CI, 1.12-1.72). Estimates were higher for more than 5 years of use (OR=1.63; 95% CI, 1.22-2.18) when compared with estimates for less than 5 years of use (OR=1.35; CI, 1.16-1.57).14 Recent studies have reported a decline in the incidence of breast cancer in the United States, which has been attributed to a parallel reduction in HT use.15
In contrast to the findings for women taking combination HT, the estrogen-only arm of the WHI study showed a decrease in the overall risk of breast cancer.16 A new analysis of data on participants in the estrogen-only arm of the study shows that, 10 years after the intervention ended, a decreased risk of breast cancer persists. In addition, the increased risk of stroke and deep vein thrombosis found in the original study had dissipated, and the decreased risk of hip fracture was not maintained.
Age matters. Health outcomes in this new analysis were more favorable for younger women for coronary heart disease, heart attack, colorectal cancer, total mortality, and a global index of chronic diseases.17 Pooled results of systematic reviews and meta-analyses on HT risks are available in TABLE 1.14,16,18-21
Progestins
Progesterone in the form of injections (Depo-Provera 150 mg, for example) or oral medroxyprogesterone acetate 20 mg daily has shown a significant reduction in hot flashes compared with placebo.22 However, associated side effects (withdrawal bleeding and weight gain) and concerns about breast cancer often limit the use of this medication.23 Because of the paucity of evidence, transdermal progesterone creams should not be recommended.24
Tibolone
Tibolone is a synthetic steroid that is structurally related to 19-nortestosterone derivatives. It has weak estrogenic, progestogenic, and androgenic properties and has shown significant reduction in hot flashes and night sweating compared with placebo.25 Tibolone has also been shown to enhance mood and sexual function.26 However, evidence of its safety on outcomes such as breast cancer and cardiovascular events is obscure and has shown conflicting results, especially for breast cancer.25,26
A recent double-blinded trial on women with breast cancer found that although tibolone significantly improved vasomotor symptoms, it was associated with an increased risk of breast cancer recurrence (HR=1.40; 95% CI, 1.14-1.70]; P=.001).27 Tibolone is not approved for sale in the United States.
Nonhormonal options
Lifestyle modifications
According to recent reviews, exercise is less effective than HT in relieving hot flashes, but does seems to have beneficial effects on mood, sleep, and overall quality of life.28,29 Other lifestyle modifications such as smoking cessation and weight loss may also be of use.5
Antidepressants
Data from one meta-analysis showed that selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors (SNRIs) were significantly more effective than placebo in reducing daily frequency of hot flashes (WMD=-1.13; 95% CI, -1.70 to -0.57).30 Efficacy varied for individual drugs.
Venlafaxine, an SNRI, has been shown to be more effective than placebo in managing hot flashes.31 The effect on hot flashes was noticed after 4 weeks of treatment with the 75- and 150-mg doses, but the higher dosage was associated with more adverse effects such as dry mouth, sleeplessness, and decreased appetite.32 Even so, 93% of the participants in the venlafaxine group chose to continue treatment, because the reduction in hot flashes had significantly improved their daily lives.31 Desvenlafaxine, a metabolite of venlafaxine, has been shown to reduce the number of hot flashes by almost 65% from baseline at Weeks 4 and 12, with dosages of 100 and 150 mg/d.33
The SSRIs fluoxetine, citalopram, and paroxetine in 10-, 20-, and 30-mg doses and paroxetine CR (12.5 and 25 mg) have been studied in many RCTs. All have demonstrated a significant decrease in hot flashes compared with placebo with various dosages used. SSRIs reduce hot flashes by as much as 50% to 60%, compared with 80% for estrogen.34 The duration of treatment ranged from 4 weeks to 6 months.
Gabapentin
Gabapentin is approved by the FDA for the treatment of partial seizures and postherpetic neuralgia.35 A meta-analysis of multiple studies published in 2006 showed that gabapentin reduced the mean number of daily hot flashes by 2.05 (95% CI, -2.80 to -1.30).30 Two recent reviews evaluated the efficacy and safety of gabapentin in the treatment of hot flashes in menopausal women and reported that gabapentin in daily doses ranging from 900 to 2400 mg and titration periods lasting 3 to12 days was well tolerated and effective.35,36
A meta-analysis published in 2009 that included 4 RCTs reported significant heterogeneity from one study to another, but comparisons of gabapentin and placebo showed reductions of 20% to 30% in the frequency and severity of hot flashes with gabapentin.36 The most commonly reported adverse effects included somnolence, dizziness, ataxia, fatigue, nystagmus, and peripheral edema.
Clonidine
Clonidine has been studied in oral and transdermal forms for the treatment of hot flashes in menopausal women, especially in women with breast cancer.37-39 Data from one meta-analysis revealed significant reductions in daily hot flashes in the clonidine group compared with placebo at 4 weeks (mean difference [MD]=-0.95; 95% CI, -1.44 to -0.47) and at 8 weeks (MD=-1.63; 95% CI, -2.76 to -0.50).30 Adverse effects included dry mouth, drowsiness, and dizziness. The transdermal route may avoid some of these side effects.40
Alternative remedies
Phytoestrogen and isoflavones. Phytoestrogens are sterol molecules produced by plants. They are similar in structure to human estrogens and have been shown to have estrogen-like activity.40 They are available as dietary soy, soy extract, and red clover extracts. Isoflavones are a type of phytoestrogen.
Comparing trials of effects of soy or isoflavone is difficult, as various formulations and amounts of these products have been used. Combining the data from these trials yielded nonsignificant results for Promensil, a red clover extract (WMD=-0.6; 95% CI, -1.8 to 0.6), and inconsistent results (sometimes favoring the intervention, other times the placebo) for soy food and soy extracts.41-43
There is no evidence of estrogenic stimulation of the endometrium with phytoestrogens used for up to 2 years.41 Nevertheless, in the absence of evidence on the safety of long-term use, women with a personal or strong family history of hormone-dependent cancers (breast, uterine, or ovarian) or thromboembolic events should be cautious about using soy-based therapies.42 Long-term safety of these products has to be established before any evidence-based recommendations can be made.
Black cohosh. Actaea racemosa (formerly Cimicifuga racemosa) is the most studied and perhaps the most widely used herbal remedy for hot flashes. It is commonly known as black cohosh and has been used traditionally by Native Americans for the treatment of various medical conditions, including amenorrhea and menopause.44,45
Remifemin is an available standardized extract. Evidence for effectiveness is limited and contradictory. Data from a recent meta-analysis showed that although there was significant heterogeneity between included trials, preparations containing black cohosh improved vasomotor symptoms overall by 26% (95% CI, 11%-40%).45
A recent well-conducted RCT concluded that neither black cohosh nor red clover significantly reduced the frequency of symptoms compared with placebo.46 The same study found that both botanicals were safe as administered for a 12-month period. Some case reports have identified serious adverse events including acute hepatocellular damage, which warrants further investigation, although no causal relationship has been established.47
Evidence for safety and efficacy of antidepressants, gabapentin, clonidine, isoflavones, black cohosh, yoga, acupuncture, and herbal remedies is summarized in TABLE 2.30,36,41,47-55
For more on the treatment of hot flashes, see “Clinical approach to managing hot flashes”.40
Start with a detailed history: Ask about the nature of your patient’s symptoms, her past gynecologic and medical history, and her family history. Take a baseline blood pressure, measure body mass index (BMI), and order a lipid profile. Exclude other possible causes of hot flashes: hyperthyroidism, panic disorder, diabetes, and medications such as antiestrogens or selective estrogen receptor modulators.
Assess the severity of hot flashes and explain their typical clinical course. Discuss lifestyle modifications that may help: losing weight, quitting smoking, wearing lighter clothing, and cutting down on caffeine intake, alcohol, and spicy foods. Tell your patient that hormone therapy (HT) has been shown to be the most effective treatment for women without contraindications. These include current, past, or suspected breast cancer, other estrogen-sensitive malignant conditions, undiagnosed genital bleeding, untreated endometrial hyperplasia, venous thromboembolism, angina, myocardial infarction, uncontrolled hypertension, liver disease, porphyria cutanea tarda (absolute contraindication), or hypersensitivity to the active substances of HT.40
If contraindications can be ruled out, find out whether she is receptive to HT or would prefer alternatives. If she is interested in HT, discuss the risks and benefits involved and the different dosages and routes of administration that are available. If she prefers to explore nonhormonal remedies, discuss the various options and present the evidence for their safety and efficacy. Tell her that the safety of some herbal remedies that contain estrogenic compounds has not been established.
What the future holds
The selective estrogen receptor agonist MF-101, which can induce tissue-specific estrogen-like effects, has been shown to be effective in reducing menopausal hot flashes compared with placebo in a phase II trial.56 A larger phase III trial is in progress.
Lower and ultra-lower doses of systemic estrogen are now available and approved by the FDA, and were found effective in relieving vasomotor symptoms.57-59 The drawback of these preparations is that they may take longer than standard-dose estrogen to achieve maximum relief of symptoms (8-12 weeks vs 4 weeks, respectively). The lower doses have been associated with fewer adverse effects (such as vaginal bleeding and breast tenderness) compared with the standard doses.58,59 Their long-term effects on the cardiovascular system, bone, and breast are still being tested and need to be established.
Newer selective estrogen receptor modulators, especially in combination with estrogen, are another approach to menopausal symptoms currently in testing.60
CORRESPONDENCE
Ghufran A. Jassim, MD, ABMS, MSc, Department of Family and Community Medicine, Royal College of Surgeons in Ireland-Medical University of Bahrain, PO Box 15503, Adliya, Bahrain; gjassim@rcsi-mub.com
1. Williams RE, Kalilani L, DiBenedetti DB. Healthcare seeking and treatment for menopausal symptoms in the US. Maturitas. 2007;58:348-358.
2. North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of the North American Menopause Society. Menopause. 2010;17:242-256.
3. National Institutes of Health. NIH state-of-the-science conference statement: management of menopause-related symptoms. Ann Intern Med. 2005;143(12 pt 2):1003-1013.
4. Berecki-Gisolf J, Begum N, Dobson AJ. Symptoms reported by women in midlife: menopausal transition or aging? Menopause. 2009;16:1021-1029.
5. Gold EB, Sternfeld B, Kelsey JL. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol. 2000;152:463-473.
6. Rossouw JE, Anderson GL, Prentice RL, et al. Writing group for the Women’s Health Initiative investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA. 2002;288:321-333.
7. Lukes A. Evolving issues in the clinical and managed care settings on the management of menopause following the Women’s Health Initiative. J Manag Care Pharm. 2008;14(3 suppl):7-13.
8. MacLennan AH, Broadbent JL, Lester W, et al. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database Syst Rev. 2004;(4):CD002978.-
9. Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610-1620.
10. Carroll N. A review of transdermal nonpatch estrogen therapy for the management of menopausal symptoms. J Womens Health (Larchmt). 2010;19:47-55.
11. Suffoletto JA, Hess R. Tapering vs cold turkey: symptoms versus successful discontinuation of menopausal hormone therapy. Menopause. 2009;16:436-437.
12. Lindh-Astrand L, Bixo M, Hirschberg AL, et al. A randomized controlled study of taper-down or abrupt discontinuation of hormone therapy in women treated for vasomotor symptoms. Menopause. 2010;17:72-29.
13. Banks E, Canfell K, Reeves G. HRT and breast cancer. Womens Health. 2008;4:427-431.
14. Shah NR, Borenstein J, Dubois RW. Postmenopausal hormone therapy and breast cancer. Menopause. 2005;12:668-678.
15. Krieger N, Chen JT, Waterman PD. Decline in US breast cancer rates after the Women’s Health Initiative. Am J Public Health. 2010;100 (suppl 1):S132-S139.
16. Nelson HD, Humphrey LL, Nygren P, et al. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288:872-881.
17. LaCroix AZ, Chiebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy. JAMA. 2011;305:1305-1314.
18. Canonico M, Plu-Bureau G, Lowe GD, et al. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women. BMJ. 2008;336:1227-1231.
19. Gabriel SR, Carmona L, Roque M, et al. Hormone replacement therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2005;(2):CD002229.-
20. Sare GM, Gray LJ, Bath PM. Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta-analysis. Eur Heart J. 2008;29:2031-2041.
21. Bath PM, Gray LJ. Association between hormone replacement therapy and subsequent stroke. BMJ. 2005;330:342.-
22. Boothby LA, Doering PL, Kipersztok S. Bioidentical hormone therapy: a review. Menopause. 2004;11:356-367.
23. Pasqualini JR. Progestins and breast cancer. Gynecol Endocrinol. 2007;23(suppl 1):32-41.
24. Wren BG. Transdermal progesterone creams for postmenopausal women. Med J Aust. 2005;182:237-225.
25. Kenemans P, Speroff L. Tibolone: clinical recommendations and practical guidelines: a report of the International Tibolone Consensus Group. Maturitas. 2005;51:21-28.
26. Wang PH, Cheng MH, Chao HT, et al. Effects of tibolone on the breast of postmenopausal women. Taiwan J Obstet Gynecol. 2007;46:121-126.
27. Kenemans P, Bundred NJ, Foidart JM, et al. Safety and efficacy of tibolone in breast-cancer patients with vasomotor symptoms. Lancet Oncol. 2009;10:135-146.
28. Daley A, Stokes-Lampard HJ, Macarthur C. Exercise to reduce vasomotor and other menopausal symptoms: a review. Maturitas. 2009;63:176-180.
29. Daley A, MacArthur C, Mutrie N, et al. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2007;(4):CD006108.-
30. Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes. JAMA. 2006;295:2057-2072.
31. Evans ML, Pritts E, Vittinghof E, et al. Management of postmenopausal hot flushes with venlafaxine hydrochloride. Obstet Gynecol. 2005;105:161-166.
32. Barton D, La Vasseur B, Loprinzi C, et al. Venlafaxine for the control of hot flashes. Oncol Nurs Forum. 2002;29:33-40.
33. Lilue M, Palacios S. Non-hormonal treatment for vasomotor symptoms during menopause: role of desvenlafaxine. Ginecol Obstet Mex. 2009;77:475-481.
34. Albertazzi P. Non-estrogenic approaches for the treatment of climacteric symptoms. Climacteric. 2007;10(suppl 2):115-120.
35. Brown JN, Wright BR. Use of gabapentin in patients experiencing hot flashes. Pharmacotherapy. 2009;29:74-81.
36. Toulis KA, Tzellas T, Kouvelas D, et al. Gabapentin for the treatment of hot flashes in women with natural or tamoxifen-induced menopause: a systematic review and meta-analysis. Clin Ther. 2009;31:221-235.
37. Pandya KJ, Raubertas AF, Flynn PJ, et al. Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes. Ann Intern Med. 2000;132:788-793.
38. Goldberg RM, Loprinzi CL, O’Fallon JR. Transdermal clonidine for ameliorating tamoxifen-induced hot flashes. J Clin Oncol. 1994;12:155-158.
39. Buijs C, Mom CH, Willemse PH, et al. Venlafaxine versus clonidine for the treatment of hot flashes in breast cancer patients. Breast Cancer Res Treat. 2009;115:573-580.
40. Cobin RH, Futterweit W, Ginzburg SB, et al. AACE Menopause Guidelines Revision Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of menopause. Endocr Pract. 2006;12:315-337.
41. Lethaby AE, Brown J, Marjoribanks J. Phytoestrogens for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2007;(4):CD001395.-
42. Tempfer CB, Bentz EH, Leodolter S, et al. Phytoestrogens in clinical practice. Fertil Steril. 2007;87:1243-1249.
43. Krebs EE, Ensrud KE, MacDonald R, et al. Phytoestrogens for treatment of menopausal symptoms: a systematic review. Obstet Gynecol. 2004;104:824-836.
44. McKenna DJ, Jones K, Humphrey S, et al. Black cohosh. Altern Ther Health Med. 2001;7:93-100.
45. Shams T, Setia MS, Hemmings R, et al. Efficacy of black cohosh-containing preparations on menopausal symptoms: a meta-analysis. Altern Ther Health Med. 2010;16:36-44.
46. Geller SE, Shulman LP, van Breeman RB, et al. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms. Menopause. 2009;16:1156-1166.
47. Borrelli F, Ernst E. Black cohosh (Cimicifuga racemosa): a systematic review of adverse events. Am J Obstet Gynecol. 2008;199:455-466.
48. Nelson HD. Menopause. Lancet. 2008;371:760-770.
49. Borrelli F, Ernst E. Black cohosh (Cimicifuga racemosa) for menopausal symptoms. Pharmacol Res. 2008;58:8-14.
50. Huntley A, Ernst E. A systematic review of the safety of black cohosh. Menopause. 2003;10:58-64.
51. Kanadys WM, Leszczynska-Gorselak B, Oleszczuk J. Efficacy and safety of black cohosh in the treatment of vasomotor symptoms. Ginekol Pol. 2008;79:287-296.
52. Huntley AL, Ernst E. A systematic review of herbal medicinal products for the treatment of menopausal symptoms. Menopause. 2003;10:465-476.
53. Lee MS, Shin BC, Ernst E. Acupuncture for treating menopausal hot flushes: a systematic review. Climacteric. 2009;12:16-25.
54. Lee MS, Kim JI, Ha JY, et al. Yoga for menopausal symptoms: a systematic review. Menopause. 2009;16:602-608.
55. Cho SH, Whang WW. Acupuncture for vasomotor menopausal symptoms. Menopause. 2009;16:1065-1073.
56. Stovall DW, Pinkerton JV. MF-101, an estrogen receptor beta agonist for the treatment of vasomotor symptoms in peri- and postmenopausal women. Curr Opin Investig Drugs. 2009;10:365-371.
57. Peeyananjarassri K, Baber R. Effects of low-dose hormone therapy on menopausal symptoms, bone mineral density, endometrium, and the cardiovascular system. Climacteric. 2004;8:13-23.
58. Ettinger B. Vasomotor symptom relief versus unwanted effects: role of estrogen dosage. Am J Med. 2005;118(suppl 12B):74-78.
59. Ettinger B. Rationale for use of lower estrogen doses for postmenopausal hormone therapy. Maturitas. 2007;57:81-84.
60. Jain N, Xu J, Kanojia RM, et al. Identification and structure-activity relationships of chromene-derived selective estrogen receptor modulators for treatment of postmenopausal symptoms. J Med Chem. 2009;52:7544-7569.
1. Williams RE, Kalilani L, DiBenedetti DB. Healthcare seeking and treatment for menopausal symptoms in the US. Maturitas. 2007;58:348-358.
2. North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of the North American Menopause Society. Menopause. 2010;17:242-256.
3. National Institutes of Health. NIH state-of-the-science conference statement: management of menopause-related symptoms. Ann Intern Med. 2005;143(12 pt 2):1003-1013.
4. Berecki-Gisolf J, Begum N, Dobson AJ. Symptoms reported by women in midlife: menopausal transition or aging? Menopause. 2009;16:1021-1029.
5. Gold EB, Sternfeld B, Kelsey JL. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol. 2000;152:463-473.
6. Rossouw JE, Anderson GL, Prentice RL, et al. Writing group for the Women’s Health Initiative investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA. 2002;288:321-333.
7. Lukes A. Evolving issues in the clinical and managed care settings on the management of menopause following the Women’s Health Initiative. J Manag Care Pharm. 2008;14(3 suppl):7-13.
8. MacLennan AH, Broadbent JL, Lester W, et al. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database Syst Rev. 2004;(4):CD002978.-
9. Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610-1620.
10. Carroll N. A review of transdermal nonpatch estrogen therapy for the management of menopausal symptoms. J Womens Health (Larchmt). 2010;19:47-55.
11. Suffoletto JA, Hess R. Tapering vs cold turkey: symptoms versus successful discontinuation of menopausal hormone therapy. Menopause. 2009;16:436-437.
12. Lindh-Astrand L, Bixo M, Hirschberg AL, et al. A randomized controlled study of taper-down or abrupt discontinuation of hormone therapy in women treated for vasomotor symptoms. Menopause. 2010;17:72-29.
13. Banks E, Canfell K, Reeves G. HRT and breast cancer. Womens Health. 2008;4:427-431.
14. Shah NR, Borenstein J, Dubois RW. Postmenopausal hormone therapy and breast cancer. Menopause. 2005;12:668-678.
15. Krieger N, Chen JT, Waterman PD. Decline in US breast cancer rates after the Women’s Health Initiative. Am J Public Health. 2010;100 (suppl 1):S132-S139.
16. Nelson HD, Humphrey LL, Nygren P, et al. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288:872-881.
17. LaCroix AZ, Chiebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy. JAMA. 2011;305:1305-1314.
18. Canonico M, Plu-Bureau G, Lowe GD, et al. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women. BMJ. 2008;336:1227-1231.
19. Gabriel SR, Carmona L, Roque M, et al. Hormone replacement therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2005;(2):CD002229.-
20. Sare GM, Gray LJ, Bath PM. Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta-analysis. Eur Heart J. 2008;29:2031-2041.
21. Bath PM, Gray LJ. Association between hormone replacement therapy and subsequent stroke. BMJ. 2005;330:342.-
22. Boothby LA, Doering PL, Kipersztok S. Bioidentical hormone therapy: a review. Menopause. 2004;11:356-367.
23. Pasqualini JR. Progestins and breast cancer. Gynecol Endocrinol. 2007;23(suppl 1):32-41.
24. Wren BG. Transdermal progesterone creams for postmenopausal women. Med J Aust. 2005;182:237-225.
25. Kenemans P, Speroff L. Tibolone: clinical recommendations and practical guidelines: a report of the International Tibolone Consensus Group. Maturitas. 2005;51:21-28.
26. Wang PH, Cheng MH, Chao HT, et al. Effects of tibolone on the breast of postmenopausal women. Taiwan J Obstet Gynecol. 2007;46:121-126.
27. Kenemans P, Bundred NJ, Foidart JM, et al. Safety and efficacy of tibolone in breast-cancer patients with vasomotor symptoms. Lancet Oncol. 2009;10:135-146.
28. Daley A, Stokes-Lampard HJ, Macarthur C. Exercise to reduce vasomotor and other menopausal symptoms: a review. Maturitas. 2009;63:176-180.
29. Daley A, MacArthur C, Mutrie N, et al. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2007;(4):CD006108.-
30. Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes. JAMA. 2006;295:2057-2072.
31. Evans ML, Pritts E, Vittinghof E, et al. Management of postmenopausal hot flushes with venlafaxine hydrochloride. Obstet Gynecol. 2005;105:161-166.
32. Barton D, La Vasseur B, Loprinzi C, et al. Venlafaxine for the control of hot flashes. Oncol Nurs Forum. 2002;29:33-40.
33. Lilue M, Palacios S. Non-hormonal treatment for vasomotor symptoms during menopause: role of desvenlafaxine. Ginecol Obstet Mex. 2009;77:475-481.
34. Albertazzi P. Non-estrogenic approaches for the treatment of climacteric symptoms. Climacteric. 2007;10(suppl 2):115-120.
35. Brown JN, Wright BR. Use of gabapentin in patients experiencing hot flashes. Pharmacotherapy. 2009;29:74-81.
36. Toulis KA, Tzellas T, Kouvelas D, et al. Gabapentin for the treatment of hot flashes in women with natural or tamoxifen-induced menopause: a systematic review and meta-analysis. Clin Ther. 2009;31:221-235.
37. Pandya KJ, Raubertas AF, Flynn PJ, et al. Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes. Ann Intern Med. 2000;132:788-793.
38. Goldberg RM, Loprinzi CL, O’Fallon JR. Transdermal clonidine for ameliorating tamoxifen-induced hot flashes. J Clin Oncol. 1994;12:155-158.
39. Buijs C, Mom CH, Willemse PH, et al. Venlafaxine versus clonidine for the treatment of hot flashes in breast cancer patients. Breast Cancer Res Treat. 2009;115:573-580.
40. Cobin RH, Futterweit W, Ginzburg SB, et al. AACE Menopause Guidelines Revision Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of menopause. Endocr Pract. 2006;12:315-337.
41. Lethaby AE, Brown J, Marjoribanks J. Phytoestrogens for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2007;(4):CD001395.-
42. Tempfer CB, Bentz EH, Leodolter S, et al. Phytoestrogens in clinical practice. Fertil Steril. 2007;87:1243-1249.
43. Krebs EE, Ensrud KE, MacDonald R, et al. Phytoestrogens for treatment of menopausal symptoms: a systematic review. Obstet Gynecol. 2004;104:824-836.
44. McKenna DJ, Jones K, Humphrey S, et al. Black cohosh. Altern Ther Health Med. 2001;7:93-100.
45. Shams T, Setia MS, Hemmings R, et al. Efficacy of black cohosh-containing preparations on menopausal symptoms: a meta-analysis. Altern Ther Health Med. 2010;16:36-44.
46. Geller SE, Shulman LP, van Breeman RB, et al. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms. Menopause. 2009;16:1156-1166.
47. Borrelli F, Ernst E. Black cohosh (Cimicifuga racemosa): a systematic review of adverse events. Am J Obstet Gynecol. 2008;199:455-466.
48. Nelson HD. Menopause. Lancet. 2008;371:760-770.
49. Borrelli F, Ernst E. Black cohosh (Cimicifuga racemosa) for menopausal symptoms. Pharmacol Res. 2008;58:8-14.
50. Huntley A, Ernst E. A systematic review of the safety of black cohosh. Menopause. 2003;10:58-64.
51. Kanadys WM, Leszczynska-Gorselak B, Oleszczuk J. Efficacy and safety of black cohosh in the treatment of vasomotor symptoms. Ginekol Pol. 2008;79:287-296.
52. Huntley AL, Ernst E. A systematic review of herbal medicinal products for the treatment of menopausal symptoms. Menopause. 2003;10:465-476.
53. Lee MS, Shin BC, Ernst E. Acupuncture for treating menopausal hot flushes: a systematic review. Climacteric. 2009;12:16-25.
54. Lee MS, Kim JI, Ha JY, et al. Yoga for menopausal symptoms: a systematic review. Menopause. 2009;16:602-608.
55. Cho SH, Whang WW. Acupuncture for vasomotor menopausal symptoms. Menopause. 2009;16:1065-1073.
56. Stovall DW, Pinkerton JV. MF-101, an estrogen receptor beta agonist for the treatment of vasomotor symptoms in peri- and postmenopausal women. Curr Opin Investig Drugs. 2009;10:365-371.
57. Peeyananjarassri K, Baber R. Effects of low-dose hormone therapy on menopausal symptoms, bone mineral density, endometrium, and the cardiovascular system. Climacteric. 2004;8:13-23.
58. Ettinger B. Vasomotor symptom relief versus unwanted effects: role of estrogen dosage. Am J Med. 2005;118(suppl 12B):74-78.
59. Ettinger B. Rationale for use of lower estrogen doses for postmenopausal hormone therapy. Maturitas. 2007;57:81-84.
60. Jain N, Xu J, Kanojia RM, et al. Identification and structure-activity relationships of chromene-derived selective estrogen receptor modulators for treatment of postmenopausal symptoms. J Med Chem. 2009;52:7544-7569.
“Doctor, my thumb hurts”
• Do not treat de Quervain’s tenosynovitis with a corticosteroid injection plus a nonsteroidal anti-inflammatory drug; the combination is no more effective than the injection alone. B
• Resection arthroplasty of the carpometacarpal (CMC) joint is the gold standard for surgical treatment of thumb CMC osteoarthritis, but should be offered only if conservative measures fail. C
• Percutaneous release of trigger thumb combined with a corticosteroid injection provides greater symptom relief than the injection alone. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Among the many possible causes of nontraumatic thumb pain are 3 conditions that primary care physicians are likely to encounter again and again: de Quervain’s tenosynovitis (dQT), first carpometacarpal osteoarthritis (CMC OA), and trigger thumb (TT). Common as they are, however, there are no consensus guidelines for the treatment of these conditions.
With that in mind, we did a literature search for studies of treatments for common causes of nontraumatic thumb pain. After reviewing the findings, we developed this evidence-based summary—and the “bottom line” treatment guide—as an aid to clinical decision making.
de Quervain’s tenosynovitis: An overuse injury
dQT is characterized by a gradual onset of pain in the first dorsal compartment of the wrist. The pain is reproduced on physical exam with clenched fist ulnar deviation of the wrist (Finkelstein test) (FIGURE). The suspected cause is overuse, leading to thickening of the tendons of the first dorsal compartment and subsequent resisted gliding of the tendons in their fibro-osseous canal.1
FIGURE
Finkelstein test for de Quervain’s tenosynovitis
With elbows flexed to 90°, the forearms parallel to each other and the floor, and the thumb clenched gently inside a fist (A), the patient drops the hand down (adduction) at the wrist (B). Pain over the first dorsal compartment is considered a positive test.
NSAIDs and injection: No better than injection alone
Conservative treatment of dQT consists of topical or oral nonsteroidal anti-inflammatory drugs (NSAIDs), splinting, and corticosteroid injection.1 We identified 2 studies using such conservative modalities. The first was a randomized double-blind, placebo-controlled trial, which found that oral NSAIDs combined with corticosteroid injection provided no statistically significant benefit compared with corticosteroid injection alone (P=.69).2 The second study was a pooled qualitative analysis and showed that 83% (n=495) of patients were asymptomatic after corticosteroid injection alone.3 Treatment failure in the remaining 17% of patients was attributed to poor technique and anatomic variation within the first dorsal compartment.
Another arm of the study compared the combination of corticosteroid injection and splinting with splinting alone, which yielded 61% and 14% success rates, respectively. Some patients were treated with NSAIDs and rest alone, but this intervention had a 0% success rate.3
Surgery has a high “cure rate”
Symptoms of dQT of >9 months’ duration may not respond as well to conservative therapy.4 In such cases—and for patients for whom conservative measures bring only short-term relief—a surgical referral may be the best approach.
Surgery for dQT, a relatively simple procedure in which the sheaths surrounding the inflamed tendons at the base of the thumb are released to relieve the pain and swelling, has uniformly positive results. The “cure rate”—resolution of symptoms without complications—is reported to be >90%.1 One researcher found a positive correlation between a longer duration (>9 months) of preoperative symptoms and increased postoperative satisfaction (P<.4). 4
First carpometacarpal OA: Pain, deformity, functional impairment
In a study of patients with joint-specific arthritis of the hand, the prevalence of first CMC OA was reported at 21%.5 Symptoms include pain and deformity that may result in significant functional impairment of the thumb. Physical findings may include pain with palpation and swelling and warmth over the dorsal aspect of the CMC joint. The “grind test”—axial compression with internal and external rotation of the CMC joint—should reproduce the pain and may demonstrate crepitus.6 As with osteoarthritis in general, CMC OA radiographic findings do not directly correlate with the physical exam.
Splinting and physical therapy bring considerable relief
Conservative treatment options for CMC OA include NSAIDs, physical therapy, splinting, and corticosteroid injection. American College of Rheumatology guidelines support NSAIDs or acetaminophen as a first-line treatment for osteoarthritis pain of the knees and hips, but no guidelines specifically address CMC OA.7 Nor have there been any studies focused on NSAID therapy for CMC OA.
One retrospective study (n=130) evaluated splinting the thumb in abduction, and found that it reduced symptoms of CMC OA by an average of 54% to 61% at 6-month follow-up.8 The researchers studied the results of splinting in patients with stage 1 or 2 (mild to moderate) CMC OA vs those with stage 3 or 4 (moderate to severe) CMC OA, and found no significant difference in levels of improvement. In another study of patients with first CMC OA who were treated with splinting and physical therapy for 7 months, 70% of those who underwent treatment declined subsequent surgery, suggesting symptom improvement.9
Corticosteroid injections alone for CMC OA have had mixed results. One study compared corticosteroid injection with saline injection (n=40) and reported no difference at 24 weeks’ posttreatment.10 Another found short-term improvement from a corticosteroid injection (n=25), as measured on a visual analog scale at 1 month (P<.001), but no significant improvement in symptoms after 3 months.11
Consider surgery if conservative measures fail
As with most cases of osteoarthritis, surgery for CMC OA should be considered only after failure of conservative treatment. Surgical treatment options should be individualized, depending on the extent of disease.
Resection arthroplasty of the CMC joint is the gold standard for surgical treatment of thumb CMC OA.6 In one small study (n=24), researchers found that 90% of patients were satisfied with the outcome after 15 years.12 There are numerous surgical alternatives, however, and research addressing resurfacing, synthetic implants, and spacer materials is ongoing.6
Trigger thumb: Swelling, pain, limited motion
TT, also known as stenosing tenosynovitis, is characterized by swelling, limitation of thumb range of motion, and a “catching” sensation when the thumb is flexed. Pain is usually referred to the first dorsal compartment of the hand. The primary pathology is thickening of the A1 pulley, with resultant entrapment of the flexor tendon, thus forming a triggering mechanism.13
Early treatment leads to better response
Conservative treatment options for TT include splinting and corticosteroid injection; NSAIDs alone have not been found to provide any benefit.14 One study found that corticosteroid injection followed by splinting in 10° to 15° flexion for 3 to 12 weeks relieved symptoms for 66% of those with any trigger digit—but only 50% of patients with TT reported an improvement in symptoms.15
Overall, patients with TT symptoms for <4 months have been found to respond significantly better to any treatment (P=.01).16 This finding may be related to repeat injury to the tendon sheath, which leads to chronic inflammation and permanent sheath hypertrophy and scarring,16 and highlights the importance of early diagnosis and treatment.
Limited research has been done on the effect of corticosteroid injection alone on TT. Maneerit et al performed a prospective study (n=115) comparing steroid injection alone with percutaneous release combined with corticosteroid injection, and found that the injection alone was successful in improving symptoms in 47% of patients.17 (The combination of percutaneous release and steroid injection, discussed below, had a much higher success rate.)
A retrospective study of treatment for trigger digits demonstrated significant improvement with corticosteroid injection in patients who did not have diabetes; 52% had full resolution and 47% had improvement in symptoms (P=.04).18 In contrast, corticosteroid injection led to symptom resolution for only 32% of patients with diabetes.
Surgery for TT: Percutaneous or open release
Surgical treatment options for TT include percutaneous or open release. Complications of surgical intervention for trigger digits include infection, digital nerve injury, scarring, tenderness, and joint contractures. Nimigan et al reported a 99% improvement in symptoms and return to activity with open surgical release for patients with TT (n=72).18
In the study by Maneerit et al cited earlier, percutaneous release combined with corticosteroid injection had a success rate (indicated by decreased pain and triggering) of 91%, vs a 47% response rate for the group who received corticosteroid injection alone (P=.001).17 In another study, 25 patients with TT that had failed to respond to conservative treatment underwent percutaneous release. The result: An 84% success rate, as shown by a decrease in reported pain on a visual analog scale (P<.001), with no digital nerve damage reported.13
Digital nerve damage is more of a concern with percutaneous release than with open release, because of the proximity of the digital nerves to the A1 pulley.13 Success rates for percutaneous release vary from 38% to 100%, with improvement shown after appropriate physician training.13
de Quervain’s tenosynovitis. Initially, corticosteroid injection has been found to be the most appropriate first-line treatment for dQT;1,4,5 the addition of an oral nonsteroidal anti-inflammatory drug (NSAID) does not result in any additional benefit.5 What’s more, oral NSAIDs and thumb splinting are not effective.3 Overall, surgical repair has demonstrated the greatest success, but it is invasive and costly.1,4
First carpometacarpal osteoarthritis. There are few valid clinical trials for CMC OA. The available evidence, however, suggests starting with NSAIDs and progressing to splinting and physical therapy, as needed. Corticosteroid injections provide no long-term pain relief.10,11 As with osteoarthritis in general, surgery for CMC OA is usually reserved for patients who fail to respond to conservative treatments.
Trigger thumb. There are various methods and levels of success for trigger digit treatment, but few studies specifically examining treatment of TT. The evidence suggests starting with conservative treatment—corticosteroid injection and splinting—in patients who are opposed to surgery.15 Both open and percutaneous surgical release of TT have high success rates, however, and can be offered at any time.13
Acknowledgement
The authors thank Joshua Hodge, MD, for his constructive critique of this article.
CORRESPONDENCE
Christopher W. Bunt, MD, Major, USAF, MC, FAAFP, 2501 Capehart Road, Offutt AFB, NE 68113; Christopher.Bunt@offutt.af.mil
1. Ilyas AM, Ast M, Schaffer AA, Thoder J. De Quervain tenosynovitis of the wrist. J Am Acad Orthop Surg. 2007;15:757-764.
2. Jirarattanaphochai K, Saengnipanthkul S, Vipulakorn K, et al. Treatment of de Quervain disease with triamcinolone injection with or without nimesulide. A randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am. 2004;86A:2700-2706.
3. Richie A, Briner W. Corticosteroid injection for treatment of de Quervain’s tenosynovitis: a pooled quantitative literature evaluation. J Am Board Fam Pract. 2003;16:102-106.
4. Ta KT, Eidelman D, Thomson JG. Patient satisfaction and outcomes of surgery for De Quervain’s tenosynovitis. J Hand Surg Am. 1999;24:1071-1077.
5. Wilder FV, Barrett JP, Farina EJ. Joint-specific prevalence of osteoarthritis of the hand. Osteoarthritis Cartilage. 2006;14:953-957.
6. Van Heest AE, Kallemeier P. Thumb carpal metacarpal arthritis. J Am Acad Orthop Surg. 2008;16:140-151.
7. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee. Arthritis Rheum. 2000;43:1905-1915.
8. Swigart CR, Eaton RG, Glickel SZ, et al. Splinting in the treatment of arthritis of the first carpometacarpal joint. J Hand Surg Am. 1999;24:86-91.
9. Berggren M, Joost-Davidsson A, Lindstrand J, et al. Reduction in the need for operation after conservative treatment of osteoarthritis of the first carpometacarpal joint: a seven year prospective study. Scand J Plast Reconstr Surg Hand Surg. 2001;35:415-417.
10. Meenagh GK, Patton J, Kynes C, et al. A randomized controlled trial of intra-articular corticosteroid injection of the carpometacarpal joint of the thumb in osteoarthritis. Ann Rheum Dis. 2004;63:1260-1263.
11. Joshi R. Intraarticular corticosteroid injection for first carpometacarpal osteoarthritis. J Rheumatol. 2005;32:1305-1306.
12. Freedman DM, Clickel SZ, Eaton RG. Long-term follow-up of volar ligament reconstruction of the thumb. J Hand Surg Am. 2000;25A:297-304.
13. Cebesoy O, Kose KC, Baltaci ET, et al. Percutaneous release of the trigger thumb: is it safe, cheap and effective? Int Orthop. 2007;31:345-349.
14. Akhtar S, Bradley MJ, Quinton DN, et al. Management and referral for trigger finger/thumb. BMJ. 2005;331:30-33.
15. Patel MR, Bassini L. Trigger fingers and thumb: when to splint, inject, or operate. J Hand Surg Am. 1992;17:110>-113.
16. Rhoades CE, Gelberman RH, Manjarris JF. Stenosing tenosynovitis of the fingers and thumb. Results of a prospective trial of steroid injection and splinting. Clin Orthop Relat Res. 1984;190:236-238.
17. Maneerit J, Sriworakun C, Budhraja N, et al. Trigger thumb: results of a prospective randomized study of percutaneous release with steroid injection versus steroid injection alone. J Hand Surg Br. 2003;28:586-589.
18. Nimigan AS, Ross DC, Gan BS. Steroid injections in the management of trigger fingers. Am J Phys Med Rehabil. 2006;85:36-43.
• Do not treat de Quervain’s tenosynovitis with a corticosteroid injection plus a nonsteroidal anti-inflammatory drug; the combination is no more effective than the injection alone. B
• Resection arthroplasty of the carpometacarpal (CMC) joint is the gold standard for surgical treatment of thumb CMC osteoarthritis, but should be offered only if conservative measures fail. C
• Percutaneous release of trigger thumb combined with a corticosteroid injection provides greater symptom relief than the injection alone. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Among the many possible causes of nontraumatic thumb pain are 3 conditions that primary care physicians are likely to encounter again and again: de Quervain’s tenosynovitis (dQT), first carpometacarpal osteoarthritis (CMC OA), and trigger thumb (TT). Common as they are, however, there are no consensus guidelines for the treatment of these conditions.
With that in mind, we did a literature search for studies of treatments for common causes of nontraumatic thumb pain. After reviewing the findings, we developed this evidence-based summary—and the “bottom line” treatment guide—as an aid to clinical decision making.
de Quervain’s tenosynovitis: An overuse injury
dQT is characterized by a gradual onset of pain in the first dorsal compartment of the wrist. The pain is reproduced on physical exam with clenched fist ulnar deviation of the wrist (Finkelstein test) (FIGURE). The suspected cause is overuse, leading to thickening of the tendons of the first dorsal compartment and subsequent resisted gliding of the tendons in their fibro-osseous canal.1
FIGURE
Finkelstein test for de Quervain’s tenosynovitis
With elbows flexed to 90°, the forearms parallel to each other and the floor, and the thumb clenched gently inside a fist (A), the patient drops the hand down (adduction) at the wrist (B). Pain over the first dorsal compartment is considered a positive test.
NSAIDs and injection: No better than injection alone
Conservative treatment of dQT consists of topical or oral nonsteroidal anti-inflammatory drugs (NSAIDs), splinting, and corticosteroid injection.1 We identified 2 studies using such conservative modalities. The first was a randomized double-blind, placebo-controlled trial, which found that oral NSAIDs combined with corticosteroid injection provided no statistically significant benefit compared with corticosteroid injection alone (P=.69).2 The second study was a pooled qualitative analysis and showed that 83% (n=495) of patients were asymptomatic after corticosteroid injection alone.3 Treatment failure in the remaining 17% of patients was attributed to poor technique and anatomic variation within the first dorsal compartment.
Another arm of the study compared the combination of corticosteroid injection and splinting with splinting alone, which yielded 61% and 14% success rates, respectively. Some patients were treated with NSAIDs and rest alone, but this intervention had a 0% success rate.3
Surgery has a high “cure rate”
Symptoms of dQT of >9 months’ duration may not respond as well to conservative therapy.4 In such cases—and for patients for whom conservative measures bring only short-term relief—a surgical referral may be the best approach.
Surgery for dQT, a relatively simple procedure in which the sheaths surrounding the inflamed tendons at the base of the thumb are released to relieve the pain and swelling, has uniformly positive results. The “cure rate”—resolution of symptoms without complications—is reported to be >90%.1 One researcher found a positive correlation between a longer duration (>9 months) of preoperative symptoms and increased postoperative satisfaction (P<.4). 4
First carpometacarpal OA: Pain, deformity, functional impairment
In a study of patients with joint-specific arthritis of the hand, the prevalence of first CMC OA was reported at 21%.5 Symptoms include pain and deformity that may result in significant functional impairment of the thumb. Physical findings may include pain with palpation and swelling and warmth over the dorsal aspect of the CMC joint. The “grind test”—axial compression with internal and external rotation of the CMC joint—should reproduce the pain and may demonstrate crepitus.6 As with osteoarthritis in general, CMC OA radiographic findings do not directly correlate with the physical exam.
Splinting and physical therapy bring considerable relief
Conservative treatment options for CMC OA include NSAIDs, physical therapy, splinting, and corticosteroid injection. American College of Rheumatology guidelines support NSAIDs or acetaminophen as a first-line treatment for osteoarthritis pain of the knees and hips, but no guidelines specifically address CMC OA.7 Nor have there been any studies focused on NSAID therapy for CMC OA.
One retrospective study (n=130) evaluated splinting the thumb in abduction, and found that it reduced symptoms of CMC OA by an average of 54% to 61% at 6-month follow-up.8 The researchers studied the results of splinting in patients with stage 1 or 2 (mild to moderate) CMC OA vs those with stage 3 or 4 (moderate to severe) CMC OA, and found no significant difference in levels of improvement. In another study of patients with first CMC OA who were treated with splinting and physical therapy for 7 months, 70% of those who underwent treatment declined subsequent surgery, suggesting symptom improvement.9
Corticosteroid injections alone for CMC OA have had mixed results. One study compared corticosteroid injection with saline injection (n=40) and reported no difference at 24 weeks’ posttreatment.10 Another found short-term improvement from a corticosteroid injection (n=25), as measured on a visual analog scale at 1 month (P<.001), but no significant improvement in symptoms after 3 months.11
Consider surgery if conservative measures fail
As with most cases of osteoarthritis, surgery for CMC OA should be considered only after failure of conservative treatment. Surgical treatment options should be individualized, depending on the extent of disease.
Resection arthroplasty of the CMC joint is the gold standard for surgical treatment of thumb CMC OA.6 In one small study (n=24), researchers found that 90% of patients were satisfied with the outcome after 15 years.12 There are numerous surgical alternatives, however, and research addressing resurfacing, synthetic implants, and spacer materials is ongoing.6
Trigger thumb: Swelling, pain, limited motion
TT, also known as stenosing tenosynovitis, is characterized by swelling, limitation of thumb range of motion, and a “catching” sensation when the thumb is flexed. Pain is usually referred to the first dorsal compartment of the hand. The primary pathology is thickening of the A1 pulley, with resultant entrapment of the flexor tendon, thus forming a triggering mechanism.13
Early treatment leads to better response
Conservative treatment options for TT include splinting and corticosteroid injection; NSAIDs alone have not been found to provide any benefit.14 One study found that corticosteroid injection followed by splinting in 10° to 15° flexion for 3 to 12 weeks relieved symptoms for 66% of those with any trigger digit—but only 50% of patients with TT reported an improvement in symptoms.15
Overall, patients with TT symptoms for <4 months have been found to respond significantly better to any treatment (P=.01).16 This finding may be related to repeat injury to the tendon sheath, which leads to chronic inflammation and permanent sheath hypertrophy and scarring,16 and highlights the importance of early diagnosis and treatment.
Limited research has been done on the effect of corticosteroid injection alone on TT. Maneerit et al performed a prospective study (n=115) comparing steroid injection alone with percutaneous release combined with corticosteroid injection, and found that the injection alone was successful in improving symptoms in 47% of patients.17 (The combination of percutaneous release and steroid injection, discussed below, had a much higher success rate.)
A retrospective study of treatment for trigger digits demonstrated significant improvement with corticosteroid injection in patients who did not have diabetes; 52% had full resolution and 47% had improvement in symptoms (P=.04).18 In contrast, corticosteroid injection led to symptom resolution for only 32% of patients with diabetes.
Surgery for TT: Percutaneous or open release
Surgical treatment options for TT include percutaneous or open release. Complications of surgical intervention for trigger digits include infection, digital nerve injury, scarring, tenderness, and joint contractures. Nimigan et al reported a 99% improvement in symptoms and return to activity with open surgical release for patients with TT (n=72).18
In the study by Maneerit et al cited earlier, percutaneous release combined with corticosteroid injection had a success rate (indicated by decreased pain and triggering) of 91%, vs a 47% response rate for the group who received corticosteroid injection alone (P=.001).17 In another study, 25 patients with TT that had failed to respond to conservative treatment underwent percutaneous release. The result: An 84% success rate, as shown by a decrease in reported pain on a visual analog scale (P<.001), with no digital nerve damage reported.13
Digital nerve damage is more of a concern with percutaneous release than with open release, because of the proximity of the digital nerves to the A1 pulley.13 Success rates for percutaneous release vary from 38% to 100%, with improvement shown after appropriate physician training.13
de Quervain’s tenosynovitis. Initially, corticosteroid injection has been found to be the most appropriate first-line treatment for dQT;1,4,5 the addition of an oral nonsteroidal anti-inflammatory drug (NSAID) does not result in any additional benefit.5 What’s more, oral NSAIDs and thumb splinting are not effective.3 Overall, surgical repair has demonstrated the greatest success, but it is invasive and costly.1,4
First carpometacarpal osteoarthritis. There are few valid clinical trials for CMC OA. The available evidence, however, suggests starting with NSAIDs and progressing to splinting and physical therapy, as needed. Corticosteroid injections provide no long-term pain relief.10,11 As with osteoarthritis in general, surgery for CMC OA is usually reserved for patients who fail to respond to conservative treatments.
Trigger thumb. There are various methods and levels of success for trigger digit treatment, but few studies specifically examining treatment of TT. The evidence suggests starting with conservative treatment—corticosteroid injection and splinting—in patients who are opposed to surgery.15 Both open and percutaneous surgical release of TT have high success rates, however, and can be offered at any time.13
Acknowledgement
The authors thank Joshua Hodge, MD, for his constructive critique of this article.
CORRESPONDENCE
Christopher W. Bunt, MD, Major, USAF, MC, FAAFP, 2501 Capehart Road, Offutt AFB, NE 68113; Christopher.Bunt@offutt.af.mil
• Do not treat de Quervain’s tenosynovitis with a corticosteroid injection plus a nonsteroidal anti-inflammatory drug; the combination is no more effective than the injection alone. B
• Resection arthroplasty of the carpometacarpal (CMC) joint is the gold standard for surgical treatment of thumb CMC osteoarthritis, but should be offered only if conservative measures fail. C
• Percutaneous release of trigger thumb combined with a corticosteroid injection provides greater symptom relief than the injection alone. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Among the many possible causes of nontraumatic thumb pain are 3 conditions that primary care physicians are likely to encounter again and again: de Quervain’s tenosynovitis (dQT), first carpometacarpal osteoarthritis (CMC OA), and trigger thumb (TT). Common as they are, however, there are no consensus guidelines for the treatment of these conditions.
With that in mind, we did a literature search for studies of treatments for common causes of nontraumatic thumb pain. After reviewing the findings, we developed this evidence-based summary—and the “bottom line” treatment guide—as an aid to clinical decision making.
de Quervain’s tenosynovitis: An overuse injury
dQT is characterized by a gradual onset of pain in the first dorsal compartment of the wrist. The pain is reproduced on physical exam with clenched fist ulnar deviation of the wrist (Finkelstein test) (FIGURE). The suspected cause is overuse, leading to thickening of the tendons of the first dorsal compartment and subsequent resisted gliding of the tendons in their fibro-osseous canal.1
FIGURE
Finkelstein test for de Quervain’s tenosynovitis
With elbows flexed to 90°, the forearms parallel to each other and the floor, and the thumb clenched gently inside a fist (A), the patient drops the hand down (adduction) at the wrist (B). Pain over the first dorsal compartment is considered a positive test.
NSAIDs and injection: No better than injection alone
Conservative treatment of dQT consists of topical or oral nonsteroidal anti-inflammatory drugs (NSAIDs), splinting, and corticosteroid injection.1 We identified 2 studies using such conservative modalities. The first was a randomized double-blind, placebo-controlled trial, which found that oral NSAIDs combined with corticosteroid injection provided no statistically significant benefit compared with corticosteroid injection alone (P=.69).2 The second study was a pooled qualitative analysis and showed that 83% (n=495) of patients were asymptomatic after corticosteroid injection alone.3 Treatment failure in the remaining 17% of patients was attributed to poor technique and anatomic variation within the first dorsal compartment.
Another arm of the study compared the combination of corticosteroid injection and splinting with splinting alone, which yielded 61% and 14% success rates, respectively. Some patients were treated with NSAIDs and rest alone, but this intervention had a 0% success rate.3
Surgery has a high “cure rate”
Symptoms of dQT of >9 months’ duration may not respond as well to conservative therapy.4 In such cases—and for patients for whom conservative measures bring only short-term relief—a surgical referral may be the best approach.
Surgery for dQT, a relatively simple procedure in which the sheaths surrounding the inflamed tendons at the base of the thumb are released to relieve the pain and swelling, has uniformly positive results. The “cure rate”—resolution of symptoms without complications—is reported to be >90%.1 One researcher found a positive correlation between a longer duration (>9 months) of preoperative symptoms and increased postoperative satisfaction (P<.4). 4
First carpometacarpal OA: Pain, deformity, functional impairment
In a study of patients with joint-specific arthritis of the hand, the prevalence of first CMC OA was reported at 21%.5 Symptoms include pain and deformity that may result in significant functional impairment of the thumb. Physical findings may include pain with palpation and swelling and warmth over the dorsal aspect of the CMC joint. The “grind test”—axial compression with internal and external rotation of the CMC joint—should reproduce the pain and may demonstrate crepitus.6 As with osteoarthritis in general, CMC OA radiographic findings do not directly correlate with the physical exam.
Splinting and physical therapy bring considerable relief
Conservative treatment options for CMC OA include NSAIDs, physical therapy, splinting, and corticosteroid injection. American College of Rheumatology guidelines support NSAIDs or acetaminophen as a first-line treatment for osteoarthritis pain of the knees and hips, but no guidelines specifically address CMC OA.7 Nor have there been any studies focused on NSAID therapy for CMC OA.
One retrospective study (n=130) evaluated splinting the thumb in abduction, and found that it reduced symptoms of CMC OA by an average of 54% to 61% at 6-month follow-up.8 The researchers studied the results of splinting in patients with stage 1 or 2 (mild to moderate) CMC OA vs those with stage 3 or 4 (moderate to severe) CMC OA, and found no significant difference in levels of improvement. In another study of patients with first CMC OA who were treated with splinting and physical therapy for 7 months, 70% of those who underwent treatment declined subsequent surgery, suggesting symptom improvement.9
Corticosteroid injections alone for CMC OA have had mixed results. One study compared corticosteroid injection with saline injection (n=40) and reported no difference at 24 weeks’ posttreatment.10 Another found short-term improvement from a corticosteroid injection (n=25), as measured on a visual analog scale at 1 month (P<.001), but no significant improvement in symptoms after 3 months.11
Consider surgery if conservative measures fail
As with most cases of osteoarthritis, surgery for CMC OA should be considered only after failure of conservative treatment. Surgical treatment options should be individualized, depending on the extent of disease.
Resection arthroplasty of the CMC joint is the gold standard for surgical treatment of thumb CMC OA.6 In one small study (n=24), researchers found that 90% of patients were satisfied with the outcome after 15 years.12 There are numerous surgical alternatives, however, and research addressing resurfacing, synthetic implants, and spacer materials is ongoing.6
Trigger thumb: Swelling, pain, limited motion
TT, also known as stenosing tenosynovitis, is characterized by swelling, limitation of thumb range of motion, and a “catching” sensation when the thumb is flexed. Pain is usually referred to the first dorsal compartment of the hand. The primary pathology is thickening of the A1 pulley, with resultant entrapment of the flexor tendon, thus forming a triggering mechanism.13
Early treatment leads to better response
Conservative treatment options for TT include splinting and corticosteroid injection; NSAIDs alone have not been found to provide any benefit.14 One study found that corticosteroid injection followed by splinting in 10° to 15° flexion for 3 to 12 weeks relieved symptoms for 66% of those with any trigger digit—but only 50% of patients with TT reported an improvement in symptoms.15
Overall, patients with TT symptoms for <4 months have been found to respond significantly better to any treatment (P=.01).16 This finding may be related to repeat injury to the tendon sheath, which leads to chronic inflammation and permanent sheath hypertrophy and scarring,16 and highlights the importance of early diagnosis and treatment.
Limited research has been done on the effect of corticosteroid injection alone on TT. Maneerit et al performed a prospective study (n=115) comparing steroid injection alone with percutaneous release combined with corticosteroid injection, and found that the injection alone was successful in improving symptoms in 47% of patients.17 (The combination of percutaneous release and steroid injection, discussed below, had a much higher success rate.)
A retrospective study of treatment for trigger digits demonstrated significant improvement with corticosteroid injection in patients who did not have diabetes; 52% had full resolution and 47% had improvement in symptoms (P=.04).18 In contrast, corticosteroid injection led to symptom resolution for only 32% of patients with diabetes.
Surgery for TT: Percutaneous or open release
Surgical treatment options for TT include percutaneous or open release. Complications of surgical intervention for trigger digits include infection, digital nerve injury, scarring, tenderness, and joint contractures. Nimigan et al reported a 99% improvement in symptoms and return to activity with open surgical release for patients with TT (n=72).18
In the study by Maneerit et al cited earlier, percutaneous release combined with corticosteroid injection had a success rate (indicated by decreased pain and triggering) of 91%, vs a 47% response rate for the group who received corticosteroid injection alone (P=.001).17 In another study, 25 patients with TT that had failed to respond to conservative treatment underwent percutaneous release. The result: An 84% success rate, as shown by a decrease in reported pain on a visual analog scale (P<.001), with no digital nerve damage reported.13
Digital nerve damage is more of a concern with percutaneous release than with open release, because of the proximity of the digital nerves to the A1 pulley.13 Success rates for percutaneous release vary from 38% to 100%, with improvement shown after appropriate physician training.13
de Quervain’s tenosynovitis. Initially, corticosteroid injection has been found to be the most appropriate first-line treatment for dQT;1,4,5 the addition of an oral nonsteroidal anti-inflammatory drug (NSAID) does not result in any additional benefit.5 What’s more, oral NSAIDs and thumb splinting are not effective.3 Overall, surgical repair has demonstrated the greatest success, but it is invasive and costly.1,4
First carpometacarpal osteoarthritis. There are few valid clinical trials for CMC OA. The available evidence, however, suggests starting with NSAIDs and progressing to splinting and physical therapy, as needed. Corticosteroid injections provide no long-term pain relief.10,11 As with osteoarthritis in general, surgery for CMC OA is usually reserved for patients who fail to respond to conservative treatments.
Trigger thumb. There are various methods and levels of success for trigger digit treatment, but few studies specifically examining treatment of TT. The evidence suggests starting with conservative treatment—corticosteroid injection and splinting—in patients who are opposed to surgery.15 Both open and percutaneous surgical release of TT have high success rates, however, and can be offered at any time.13
Acknowledgement
The authors thank Joshua Hodge, MD, for his constructive critique of this article.
CORRESPONDENCE
Christopher W. Bunt, MD, Major, USAF, MC, FAAFP, 2501 Capehart Road, Offutt AFB, NE 68113; Christopher.Bunt@offutt.af.mil
1. Ilyas AM, Ast M, Schaffer AA, Thoder J. De Quervain tenosynovitis of the wrist. J Am Acad Orthop Surg. 2007;15:757-764.
2. Jirarattanaphochai K, Saengnipanthkul S, Vipulakorn K, et al. Treatment of de Quervain disease with triamcinolone injection with or without nimesulide. A randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am. 2004;86A:2700-2706.
3. Richie A, Briner W. Corticosteroid injection for treatment of de Quervain’s tenosynovitis: a pooled quantitative literature evaluation. J Am Board Fam Pract. 2003;16:102-106.
4. Ta KT, Eidelman D, Thomson JG. Patient satisfaction and outcomes of surgery for De Quervain’s tenosynovitis. J Hand Surg Am. 1999;24:1071-1077.
5. Wilder FV, Barrett JP, Farina EJ. Joint-specific prevalence of osteoarthritis of the hand. Osteoarthritis Cartilage. 2006;14:953-957.
6. Van Heest AE, Kallemeier P. Thumb carpal metacarpal arthritis. J Am Acad Orthop Surg. 2008;16:140-151.
7. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee. Arthritis Rheum. 2000;43:1905-1915.
8. Swigart CR, Eaton RG, Glickel SZ, et al. Splinting in the treatment of arthritis of the first carpometacarpal joint. J Hand Surg Am. 1999;24:86-91.
9. Berggren M, Joost-Davidsson A, Lindstrand J, et al. Reduction in the need for operation after conservative treatment of osteoarthritis of the first carpometacarpal joint: a seven year prospective study. Scand J Plast Reconstr Surg Hand Surg. 2001;35:415-417.
10. Meenagh GK, Patton J, Kynes C, et al. A randomized controlled trial of intra-articular corticosteroid injection of the carpometacarpal joint of the thumb in osteoarthritis. Ann Rheum Dis. 2004;63:1260-1263.
11. Joshi R. Intraarticular corticosteroid injection for first carpometacarpal osteoarthritis. J Rheumatol. 2005;32:1305-1306.
12. Freedman DM, Clickel SZ, Eaton RG. Long-term follow-up of volar ligament reconstruction of the thumb. J Hand Surg Am. 2000;25A:297-304.
13. Cebesoy O, Kose KC, Baltaci ET, et al. Percutaneous release of the trigger thumb: is it safe, cheap and effective? Int Orthop. 2007;31:345-349.
14. Akhtar S, Bradley MJ, Quinton DN, et al. Management and referral for trigger finger/thumb. BMJ. 2005;331:30-33.
15. Patel MR, Bassini L. Trigger fingers and thumb: when to splint, inject, or operate. J Hand Surg Am. 1992;17:110>-113.
16. Rhoades CE, Gelberman RH, Manjarris JF. Stenosing tenosynovitis of the fingers and thumb. Results of a prospective trial of steroid injection and splinting. Clin Orthop Relat Res. 1984;190:236-238.
17. Maneerit J, Sriworakun C, Budhraja N, et al. Trigger thumb: results of a prospective randomized study of percutaneous release with steroid injection versus steroid injection alone. J Hand Surg Br. 2003;28:586-589.
18. Nimigan AS, Ross DC, Gan BS. Steroid injections in the management of trigger fingers. Am J Phys Med Rehabil. 2006;85:36-43.
1. Ilyas AM, Ast M, Schaffer AA, Thoder J. De Quervain tenosynovitis of the wrist. J Am Acad Orthop Surg. 2007;15:757-764.
2. Jirarattanaphochai K, Saengnipanthkul S, Vipulakorn K, et al. Treatment of de Quervain disease with triamcinolone injection with or without nimesulide. A randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am. 2004;86A:2700-2706.
3. Richie A, Briner W. Corticosteroid injection for treatment of de Quervain’s tenosynovitis: a pooled quantitative literature evaluation. J Am Board Fam Pract. 2003;16:102-106.
4. Ta KT, Eidelman D, Thomson JG. Patient satisfaction and outcomes of surgery for De Quervain’s tenosynovitis. J Hand Surg Am. 1999;24:1071-1077.
5. Wilder FV, Barrett JP, Farina EJ. Joint-specific prevalence of osteoarthritis of the hand. Osteoarthritis Cartilage. 2006;14:953-957.
6. Van Heest AE, Kallemeier P. Thumb carpal metacarpal arthritis. J Am Acad Orthop Surg. 2008;16:140-151.
7. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee. Arthritis Rheum. 2000;43:1905-1915.
8. Swigart CR, Eaton RG, Glickel SZ, et al. Splinting in the treatment of arthritis of the first carpometacarpal joint. J Hand Surg Am. 1999;24:86-91.
9. Berggren M, Joost-Davidsson A, Lindstrand J, et al. Reduction in the need for operation after conservative treatment of osteoarthritis of the first carpometacarpal joint: a seven year prospective study. Scand J Plast Reconstr Surg Hand Surg. 2001;35:415-417.
10. Meenagh GK, Patton J, Kynes C, et al. A randomized controlled trial of intra-articular corticosteroid injection of the carpometacarpal joint of the thumb in osteoarthritis. Ann Rheum Dis. 2004;63:1260-1263.
11. Joshi R. Intraarticular corticosteroid injection for first carpometacarpal osteoarthritis. J Rheumatol. 2005;32:1305-1306.
12. Freedman DM, Clickel SZ, Eaton RG. Long-term follow-up of volar ligament reconstruction of the thumb. J Hand Surg Am. 2000;25A:297-304.
13. Cebesoy O, Kose KC, Baltaci ET, et al. Percutaneous release of the trigger thumb: is it safe, cheap and effective? Int Orthop. 2007;31:345-349.
14. Akhtar S, Bradley MJ, Quinton DN, et al. Management and referral for trigger finger/thumb. BMJ. 2005;331:30-33.
15. Patel MR, Bassini L. Trigger fingers and thumb: when to splint, inject, or operate. J Hand Surg Am. 1992;17:110>-113.
16. Rhoades CE, Gelberman RH, Manjarris JF. Stenosing tenosynovitis of the fingers and thumb. Results of a prospective trial of steroid injection and splinting. Clin Orthop Relat Res. 1984;190:236-238.
17. Maneerit J, Sriworakun C, Budhraja N, et al. Trigger thumb: results of a prospective randomized study of percutaneous release with steroid injection versus steroid injection alone. J Hand Surg Br. 2003;28:586-589.
18. Nimigan AS, Ross DC, Gan BS. Steroid injections in the management of trigger fingers. Am J Phys Med Rehabil. 2006;85:36-43.
Rotavirus infection: Optimal treatment and prevention
• Patients with rotavirus infection require oral, enteral, or intravenous fluids to treat dehydration. A
• Give the first dose of rotavirus (RV) vaccine between the ages of 6 weeks and 14 weeks 6 days; give subsequent doses at 4- to 10-week intervals, completing by 8 months. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Rotavirus is the most common cause of severe gastroenteritis in infants and children younger than 5 years of age, and it accounts for approximately 5% of childhood deaths worldwide.1 In the United States, rotavirus causes numerous cases of dehydrating diarrhea and vomiting, and is responsible for direct and indirect healthcare costs of approximately $1 billion per year. Infection during childhood is almost universal.2
Improved personal hygiene and community sanitation have steadily reduced the prevalence of bacterial and parasitic disease. But these measures have had little effect on the spread of rotavirus and its potential complications of severe dehydration, hospitalization, and even death.1 Importantly, we now have the means to vaccinate against rotavirus infection and dramatically reduce the incidence of disease. In this article, I describe the available vaccines and the vaccination recommendations endorsed by the Advisory Committee on Immunization Practices (ACIP), the American Academy of Pediatrics (AAP), and the American Academy of Family Physicians (AAFP). I also review supportive treatment for rotavirus infection, which entails both do’s and don’ts.
Who is at risk of rotavirus disease?
For most term neonates, rotavirus disease is mild, perhaps because of partial protection from maternal antibodies.3 However, premature infants lacking full maternal antibody protection often suffer from more serious gastroenteritis. The most severe infections usually strike children between the ages of 4 months, when maternally derived antibody protection wanes, and 23 months, when dehydration risk lessens.4-6
The virus spreads from person to person via the fecal–oral route.6,7 Thirty percent to 50% of family members of an infected child may also become infected, but disease in older children and adults is usually subclinical or mild.6 Outbreaks of rotavirus are common in childcare centers and in children’s hospitals.7,8
How the disease presents
Rotavirus gastroenteritis peaks during the winter. With mild cases, a watery diarrhea will last a few days. In severe cases, onset is usually abrupt with fever, abdominal pain, and vomiting, which can precede diarrhea. A third of patients have a temperature higher than 102°F (38.9°C).6 There is a risk of dehydration, shock, and even, occasionally, infant death.9
Typically, the incubation period is 1 to 4 days, and the infection lasts 3 to 7 days. However, damage to the brush border of the intestinal villi can produce persistent disaccharide malabsorption, resulting in prolonged diarrhea even after resolution of infection.10,11 Stools generally do not contain blood or leukocytes. Ultrasound examination during rotavirus infections has shown thickening of the distal ileum and lymphadenopathy, which may predispose to intussusception.12 Other problems possibly linked to wild-type rotavirus infection are Kawasaki disease and sudden infant death syndrome. Recurrent rotavirus infection with one of the many different serotypes is common during childhood.
More than 25 different assays can detect rotavirus in stool, but the most reliable method is direct electron microscopy. A suitable clinically available alternative is enzyme immunoassay testing of stool samples. In mild cases, testing to detect rotavirus is not necessary. But for bloody, severe, or persistent diarrhea, stool testing for rotavirus and other entities is warranted.
Supportive treatment: Do’s and don’ts
No specific antiviral treatment is available for rotavirus infection. That said, the do’s and don’ts that follow will help guide your care.
DO administer oral, enteral, or intravenous (IV) fluids to prevent or correct dehydration. Oral rehydration therapy is the standard treatment for dehydration in anyone with acute gastroenteritis, including that caused by rotavirus. The recommended World Health Organization (WHO) oral rehydration solution contains sodium, chloride, and electrolytes (TABLE 1).13 Rice-based oral rehydration solution is an easily metabolized carbohydrate formulation that helps repair damaged tissues and enhances electrolyte absorption.9 WHO has endorsed guidelines that base fluid replacement on the patient’s age and weight, and that recommend oral zinc intake (10 mg/d for 10-14 days up to age 6 months; 20 mg/d for 10-14 days for older children) for all episodes of diarrhea (http://hetv.org/pdf/diarrhoea-guidelines.pdf). Oral glucose electrolyte solutions containing less sodium and chloride are also effective treatments.
DO recommend frequent small doses of oral rehydration solution, even if the patient is vomiting.14 Rehydration volumes are suggested in TABLE 1. Alternatively, give 10 to 20 mL/kg for each diarrheal episode, and 2 mL/kg for each bout of emesis. Feeding frequent small volumes (30 mL every 5-10 minutes) reduces the risk of emesis.
Although oral rehydration solutions are contraindicated for infants and young children with depressed consciousness, vomiting is not a contraindication to oral intake. About half of the oral intake stays in the stomach, even after vomiting. A single dose of ondansetron may safely reduce vomiting.15
Patients with mainly diarrhea can take fluids or feed at will. With children who refuse to drink, oral rehydration solutions can be administered via nasogastric tube at approximately 5 mL/min to limit vomiting and maintain hydration.14 In dehydrated infants and toddlers with collapsed veins, nasogastric intubation has been shown to be less traumatic than repetitive attempts at placing IV catheters.
DO encourage nursing mothers to con tinue breastfeeding during rehydration treatments. If a mother is bottle feeding, keep this in mind: Rotavirus can cause temporary lactase deficiency for some non-breastfeeding infants; lactose-free formulas may help.
DON’T assume that parents know how to provide proper supplementation. Tell them to avoid fluids containing mostly sugar that lack significant electrolyte supplementation (eg, cola) unless no other fluid alternative is available. Advise caregivers to avoid juices and other liquids high in complex or simple sugars because the osmotic load may worsen diarrhea.14
DON’T give antidiarrheal agents for acute treatment in infants and young children. Such treatment has resulted in death.14
TABLE 1
Prevent or correct dehydration using the WHO-recommended oral rehydration salts solution13
| With this formulation… | …rehydrate per these specifications… | …at this rate | ||
|---|---|---|---|---|
| Component (mmol/L) | Age | Weight (kg) | mL solution/4 h | |
| Sodium (75); chloride (65); glucose (75); potassium (20); citrate (10) | ≤4 mo 4-12 mo 12 mo-2 y 2-5 y | <6 6 to <10 10 to <12 12 to 19 | 200-400 400-700 700-900 900-1400 | |
| WHO, World Health Organization. | ||||
KEEP IN MIND
Hospitalization may be needed to replace fluids via IV or interosseous supplementation. For the severely dehydrated child, 20 mL/kg isotonic fluid can be administered as a rapid bolus.14 It may be necessary to repeat a rapid fluid infusion of 10 to 20 mL/kg every 20 to 30 minutes. For less severely ill infants who require IV rehydration, standard references such as the Harriet Lane Handbook16 provide excellent guidance.
Probiotics may help. Consider probiotics with Lactobacillus or Bifida bacterium to reduce the severity of diarrhea in infants and children who are mildly to moderately ill.17,18 Their usefulness in the severely ill patient has not been demonstrated.
Available vaccines and clinical recommendations
In February 2006, the US Food and Drug Administration (FDA) licensed a 3-dose, oral pentavalent rotavirus vaccine (RV5, RotaTeq) for use among infants. The vaccine contains live reassortant rotaviruses19—4 human rotavirus G outer-surface proteins and 1 human P attachment protein reassorted into a bovine rotavirus not infectious to humans.
In February 2008, the FDA approved a 2-dose, oral monovalent rotavirus vaccine (RV1, Rotarix), an attenuated live human rotavirus containing 1 G protein and 1 P protein. Both vaccines have proven to be clinically effective in rotavirus prevention trials, but effectiveness may depend on which rotavirus serotypes circulate each season.
ACIP, AAP, and AAFP recommend that all infants be routinely vaccinated with either RV5 or RV1.6,20–22 Vaccination should be complete by the time infants reach the age of 8 months (TABLE 2). Guidelines for vaccination emphasize the following points:
Timing. According to the ACIP, the first dose of either vaccine must be administered between the ages of 6 weeks and 14 weeks 6 days (the RV5 manufacturer [Merck] states a maximum age of 12 weeks). Give subsequent doses at 4- to 10-week intervals, as long as all doses are administered by 8 months of age. The RV1 manufacturer (GlaxoSmithKline) suggests completing the second (final) dose of its vaccine by age 24 weeks.
If an infant 15 weeks of age or older accidentally receives a first dose of RV vaccine, the series should be continued, as long as the last dose can be given by 8 months of age. Either vaccine can be administered concurrently with all other vaccines.
Contraindications. The only absolute contraindications to RV5 administration are a demonstrated hypersensitivity to any component of the vaccine and severe combined immunodeficiency disease (SCID). Contraindications to RV1 vaccine are vaccine component hypersensitivity, SCID, latex-induced allergy (anaphylaxis), and uncorrected malformation of the gastrointestinal (GI) tract that might predispose to intussusception.
Precautions. Precautions for vaccines include other forms of primary or secondary immunocompromised or immunodeficiency states, including cancer and acute or chronic GI disorders such as ongoing gastroenteritis or intussusception. Infants with transient mild illness with or without low-grade fever and infants who are breastfeeding can receive either vaccine. RV5 is shed in 9% of recipients and RV1 in 26% of recipients after Dose 1, but transmission of vaccine virus is not known to occur. Likewise, reversion of vaccine virus to more virulent pathogens is not known to occur. A household member with an immuno-compromised condition does not preclude giving either RV vaccine to an infant. The risk of transmitting vaccine virus is much smaller than the risk of acquiring infectious wild-type rotavirus.
Regurgitation of a vaccine dose is uncommon. When it does occur, the RV5 vaccine should not be repeated; some of the vaccine dose is retained and the safety of the additional vaccine from a second dose is unknown. Readministration of a dose of RV1 is not recommended, although not contraindicated.
TABLE 2
Recommended rotavirus live virus vaccine dosing6
| Patient age (mo) | RV5 (RotaTeq) | RV1 (Rotarix) |
|---|---|---|
| 2 | 2 mL | 1 mL |
| 4 | 2 mL | 1 mL* |
| 6 | 2 mlL* | — |
| *The final dose of either vaccine must be given by no later than 8 months of age. | ||
Vaccine efficacy
The safety and efficacy of live rotavirus vaccines were demonstrated in large studies that enrolled 71,725 children in RV5 vaccine trials23 and 24,163 children in RV1 vaccine trials.21 The pivotal RV5 study included a nested substudy to evaluate efficacy against any G1–G4 rotavirus gastroenteritis.
RV5 (RotaTeq) vaccine. In double-blind, placebo-controlled clinical trials, for the first rotavirus season, live RV5 vaccine effectively prevented severe rotavirus infection in 98% of cases, and reduced hospitalization by 95%, emergency department visits by 94%, physician office visits by 86%, and all rotavirus cases by 74% for infants who received all 3 doses of vaccine according to protocol.23 Hospitalization for any-cause gastroenteritis was reduced by 63%. Second-season data showed persistence of antibody protection. All 3 doses of vaccine are required for maximum protection.23
Both preterm and term infants received their first dose of vaccine between 6 and 12 weeks of life. For preterm infants who are experiencing medical difficulties, the first dose of vaccine may be delayed until the patient is stable, if it can be given before 15 weeks of age.
RV1 (Rotarix) vaccine. In double-blind, placebo-controlled clinical trials, for the first rotavirus season, live RV1 vaccine was 85% (Latin America) to 96% (Europe) effective in preventing severe rotavirus infection. It reduced hospitalization due to rotavirus by 85% (Latin America) to 100% (Europe), and all rotavirus cases by 87% (Europe) for infants who received both doses of the vaccine according to protocol. For the second season, the vaccine reduced severe rotavirus disease by 70% to 96%, and any rotavirus disease by 73% to 89%, showing persistence of antibody protection.6
Adverse events
With both vaccines, common side effects include irritability, flatulence, fever, vomiting, diarrhea, cough, runny nose, and loss of appetite. The RV5 vaccine has been shown not to increase the risk of intussusception compared with placebo.24,25 The RV1 vaccine should not be used in children with an uncorrected bowel malformation, due to unproven increased risk of intussusception. Risk of death from complications after administration of either vaccine did not differ from that among children receiving placebo.
Postmarketing surveillance of vaccination outcomes
Even though rotavirus vaccine coverage with RV5 RotaTeq in the 2007-2008 and 2008-2009 seasons was far less than that with other childhood immunizations, the number of rotavirus infections dropped by >60% in both 2008 and 2009.26 The number of stool tests for rotavirus and the percentage of positive results also dropped dramatically.
Additionally, the rotavirus peak incidence was delayed 2 to 4 months until April 2008 and March 2009.26 Incidence was reduced in all age groups, suggesting the possibility of herd immunity despite a low vaccine coverage rate (estimates are 57% for ≥1 dose) that would not be expected to provide herd immunity.27 Hospitalizations in the United States for rotavirus gastroenteritis dropped by as much as 85%,28 markedly reducing costs for gastroenteritis.
In a 2010 report from an emergency department in Houston, a complete RV5 vaccine series conferred 82% protection against acute gastroenteritis, 96% against severe rotavirus disease requiring IV rehydration, and 100% against hospitalization.29 For more on the vaccine, see the report by Yen et al.30
Reports of the effectiveness of Rotarix in postmarketing surveillance are limited, but the vaccine does seem to provide broad coverage.31
As of April 11, 2011, RV5 costs $59.76/$69.59 per dose in the public/private sectors, respectively (3-dose series: $179.28/$208.77); RV1 costs $89.25/$102.50 per dose (2-dose series: $178.50/$205). routine vaccination costs about $138 per case averted and $3024 per serious case averted. neither vaccine contains thimerosal. Both vaccines are available in 10-dose packs.
Source: Centers for Disease Control and Prevention. CDC vaccine price list. Prices last reviewed/updated: April 8, 2011. Available at: http://www.cdc.gov/vaccines/programs/vfc/cdc-vac-price-list.htm. Accessed April 11, 2011.
Incorporating rotavirus vaccine into a family medicine practice
Given inadequately reimbursed costs including the cost of stocking RV vaccine (see “Costs of RV vaccines” above), family physicians who treat relatively few infants must determine whether offering RV vaccine fits within their practices.
For family physicians who do treat infants, offering RV vaccination makes sense. These oral vaccines are highly effective, safe, and easy to administer, and will prevent a great deal of worry and calls regarding infants who have a fever or diarrhea or are vomiting. Due to the costs of stocking all vaccines, private practitioners are wise to purchase vaccine loss insurance. Many insurance agencies provide a rider on office insurance policies to cover vaccine supplies.
CORRESPONDENCE
Donald B. Middleton, MD, UPMC St. Margaret, 815 Freeport Road, Pittsburgh, PA 15215; middletondb@upmc.edu
1. Parashar UD, Gibson CJ, Bresee JS, et al. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304-306.
2. Malek MA, Curns AT, Holman RC, et al. Diarrhea- and rotavirus-associated hospitalizations among children less than 5 years of age: United States, 1997 and 2000. Pediatrics. 2006;117:1887-1892.
3. Xu J, Dennehy P, Keyserling H, et al. Serum antibody responses in children with rotavirus diarrhea can serve as proxy for protection. Clin Diagn Lab Immunol. 2005;12:273-279.
4. World Health Organization. Rotavirus vaccines. Wkly Epidemiol Rec. 2007;82:285-295.
5. Ward RL, Bernstein DI, Staat MA. Rotaviruses. In: Feigin RD, Cherry JD, eds. Textbook of Pediatric Infectious Disease. Vol 2, 6th ed. New York, NY: Saunders; 2009:2245-2270.
6. Centers for Disease Control and Prevention. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2009;58(RR-2):1-25.
7. Butz AM, Fosarelli P, Dick J, et al. Prevalence of rotavirus on high-risk fomites in day-care facilities. Pediatrics. 1993;92:202-205.
8. Fischer TK, Bresee JS, Glass RI. Rotavirus vaccines and the prevention of hospital-acquired diarrhea in children. Vaccine. 2004;22(suppl):S49-S54.
9. Kapikian AZ, Hoshino Y, Chanock RM. Rotaviruses. In: Knipe DM, Howley PM, Griffin DE, et al, eds. Fields Virology. 4th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2001: 1787-1825.
10. Lorrot M, Vasseur M. How do the rotavirus NSP4 and bacterial enterotoxins lead differently to diarrhea? Virol J. 2007;4:31.-
11. Ramig RF. Pathogenesis of intestinal and systemic rotavirus infection. J Virol. 2004;78:10213-10220.
12. Robinson CG, Hernanz-Schulman M, Zhu Y, et al. Evaluation of anatomic changes in young children with natural rotavirus infection: is intussusception biologically plausible? J Infect Dis. 2004;189:1382-1387.
13. World Health Organization. Oral Rehydration Salts: Production of the New ORS. Geneva, Switzerland: WHO Document Production Services; 2006. Available at: http://whqlibdoc.who.int/hq/2006/WHO_FCH_CAH_06.1.pdf. Accessed April 11, 2011.
14. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52(RR-16):1-16.
15. DeCamp LR, Byerley JS, Doshi N, et al. Use of antiemetic agents in acute gastroenteritis: a systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2008;162:858-865.
16. The Johns Hopkins Hospital, Custer JW, Rau RE. Harriet Lane Handbook: A Manual for Pediatric House Officers. 18th ed. St. Louis, Mo: Mosby/Elsevier; 2008.
17. Canani RB, Cirillo P, Terrin G, et al. Probiotics for treatment of acute diarrhoea in children: randomised clinical trial of five different preparations. BMJ. 2007;335:340.-
18. Van Niel CW, Feudtner C, Garrison MM, et al. Lactobacillus therapy for acute infectious diarrhea in children: a meta- analysis. Pediatrics. 2002;109:678-684.
19. Heaton PM, Goveia MG, Miller JM, et al. Development of a pentavalent rotavirus vaccine against prevalent serotypes of rotavirus gastroenteritis. J Infect Dis. 2005;192(suppl 1):S17-S21.
20. Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0 through 18 years — United States, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(5):1-4.
21. American Academy of Pediatrics (AAP) Committee on Infectious Diseases. Prevention of rotavirus disease: updated guidelines for use of rotavirus vaccine. Pediatrics. 2009;123:1412-1420.
22. Temte JL. Practice guidelines. ACIP releases 2009 child and adolescent immunization schedules. Am Fam Physician. 2009;79:56.-Available at: http://www.aafp.org/afp/2009/0101/p56.html. Accessed January 4, 2010.
23. Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23-33.
24. Centers for Disease Control and Prevention. Intussusception among recipients of rotavirus vaccine: United States, 1998– 1999. MMWR Morb Mortal Wkly Rep. 1999;48:577-581.
25. Centers for Disease Control and Prevention. Postmarketing monitoring of intussusception after RotaTeq vaccine: United States. February 1, 2006–February 15, 2007. MMWR Morb Mortal Wkly Rep. 2007;56:218-222.
26. Centers for Disease Control and Prevention. Reduction in rotavirus after vaccine introduction — United States, 2000-2009. MMWR Morb Mortal Wkly Rep. 2009;58:1146-1149.
27. Clark HF, Lawley D, Mallette LA, et al. Decline in cases of rotavirus gastroenteritis presenting to The Children’s Hospital of Philadelphia after introduction of a pentavalent rotavirus vaccine. Clin Vaccine Immunol. 2009;16:382-386.
28. Chang HG, Smith P, Tserenpuntsag B, et al. Reduction in New York hospitalizations for diarrhea and rotavirus. Presented at: 43rd National Immunization Conference; March 30-April 2, 2009; Dallas, Tex. Abstract 41. Available at: http://cdc.confex.com/cdc/nic2009/webprogram/Paper18073.html. Accessed April 15, 2011.
29. Boom JA, Tate JE, Sahni LC, et al. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics. 2010;125:e199-e207.
30. Yen C, Tate JE, Wenk JD, et al. Diarrhea-associated hospitalizations among US children over 2 rotavirus seasons after vaccine introduction. Pediatrics. 2011;127:e9-e15.
31. Correia JB, Patel MM, Nakagomi O, et al. Effectiveness of monovalent rotavirus vaccine (Rotarix) against severe diarrhea caused by serotypically unrelated G2P[4] strains in Brazil. J Infect Dis. 2010;201:363-369.
• Patients with rotavirus infection require oral, enteral, or intravenous fluids to treat dehydration. A
• Give the first dose of rotavirus (RV) vaccine between the ages of 6 weeks and 14 weeks 6 days; give subsequent doses at 4- to 10-week intervals, completing by 8 months. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Rotavirus is the most common cause of severe gastroenteritis in infants and children younger than 5 years of age, and it accounts for approximately 5% of childhood deaths worldwide.1 In the United States, rotavirus causes numerous cases of dehydrating diarrhea and vomiting, and is responsible for direct and indirect healthcare costs of approximately $1 billion per year. Infection during childhood is almost universal.2
Improved personal hygiene and community sanitation have steadily reduced the prevalence of bacterial and parasitic disease. But these measures have had little effect on the spread of rotavirus and its potential complications of severe dehydration, hospitalization, and even death.1 Importantly, we now have the means to vaccinate against rotavirus infection and dramatically reduce the incidence of disease. In this article, I describe the available vaccines and the vaccination recommendations endorsed by the Advisory Committee on Immunization Practices (ACIP), the American Academy of Pediatrics (AAP), and the American Academy of Family Physicians (AAFP). I also review supportive treatment for rotavirus infection, which entails both do’s and don’ts.
Who is at risk of rotavirus disease?
For most term neonates, rotavirus disease is mild, perhaps because of partial protection from maternal antibodies.3 However, premature infants lacking full maternal antibody protection often suffer from more serious gastroenteritis. The most severe infections usually strike children between the ages of 4 months, when maternally derived antibody protection wanes, and 23 months, when dehydration risk lessens.4-6
The virus spreads from person to person via the fecal–oral route.6,7 Thirty percent to 50% of family members of an infected child may also become infected, but disease in older children and adults is usually subclinical or mild.6 Outbreaks of rotavirus are common in childcare centers and in children’s hospitals.7,8
How the disease presents
Rotavirus gastroenteritis peaks during the winter. With mild cases, a watery diarrhea will last a few days. In severe cases, onset is usually abrupt with fever, abdominal pain, and vomiting, which can precede diarrhea. A third of patients have a temperature higher than 102°F (38.9°C).6 There is a risk of dehydration, shock, and even, occasionally, infant death.9
Typically, the incubation period is 1 to 4 days, and the infection lasts 3 to 7 days. However, damage to the brush border of the intestinal villi can produce persistent disaccharide malabsorption, resulting in prolonged diarrhea even after resolution of infection.10,11 Stools generally do not contain blood or leukocytes. Ultrasound examination during rotavirus infections has shown thickening of the distal ileum and lymphadenopathy, which may predispose to intussusception.12 Other problems possibly linked to wild-type rotavirus infection are Kawasaki disease and sudden infant death syndrome. Recurrent rotavirus infection with one of the many different serotypes is common during childhood.
More than 25 different assays can detect rotavirus in stool, but the most reliable method is direct electron microscopy. A suitable clinically available alternative is enzyme immunoassay testing of stool samples. In mild cases, testing to detect rotavirus is not necessary. But for bloody, severe, or persistent diarrhea, stool testing for rotavirus and other entities is warranted.
Supportive treatment: Do’s and don’ts
No specific antiviral treatment is available for rotavirus infection. That said, the do’s and don’ts that follow will help guide your care.
DO administer oral, enteral, or intravenous (IV) fluids to prevent or correct dehydration. Oral rehydration therapy is the standard treatment for dehydration in anyone with acute gastroenteritis, including that caused by rotavirus. The recommended World Health Organization (WHO) oral rehydration solution contains sodium, chloride, and electrolytes (TABLE 1).13 Rice-based oral rehydration solution is an easily metabolized carbohydrate formulation that helps repair damaged tissues and enhances electrolyte absorption.9 WHO has endorsed guidelines that base fluid replacement on the patient’s age and weight, and that recommend oral zinc intake (10 mg/d for 10-14 days up to age 6 months; 20 mg/d for 10-14 days for older children) for all episodes of diarrhea (http://hetv.org/pdf/diarrhoea-guidelines.pdf). Oral glucose electrolyte solutions containing less sodium and chloride are also effective treatments.
DO recommend frequent small doses of oral rehydration solution, even if the patient is vomiting.14 Rehydration volumes are suggested in TABLE 1. Alternatively, give 10 to 20 mL/kg for each diarrheal episode, and 2 mL/kg for each bout of emesis. Feeding frequent small volumes (30 mL every 5-10 minutes) reduces the risk of emesis.
Although oral rehydration solutions are contraindicated for infants and young children with depressed consciousness, vomiting is not a contraindication to oral intake. About half of the oral intake stays in the stomach, even after vomiting. A single dose of ondansetron may safely reduce vomiting.15
Patients with mainly diarrhea can take fluids or feed at will. With children who refuse to drink, oral rehydration solutions can be administered via nasogastric tube at approximately 5 mL/min to limit vomiting and maintain hydration.14 In dehydrated infants and toddlers with collapsed veins, nasogastric intubation has been shown to be less traumatic than repetitive attempts at placing IV catheters.
DO encourage nursing mothers to con tinue breastfeeding during rehydration treatments. If a mother is bottle feeding, keep this in mind: Rotavirus can cause temporary lactase deficiency for some non-breastfeeding infants; lactose-free formulas may help.
DON’T assume that parents know how to provide proper supplementation. Tell them to avoid fluids containing mostly sugar that lack significant electrolyte supplementation (eg, cola) unless no other fluid alternative is available. Advise caregivers to avoid juices and other liquids high in complex or simple sugars because the osmotic load may worsen diarrhea.14
DON’T give antidiarrheal agents for acute treatment in infants and young children. Such treatment has resulted in death.14
TABLE 1
Prevent or correct dehydration using the WHO-recommended oral rehydration salts solution13
| With this formulation… | …rehydrate per these specifications… | …at this rate | ||
|---|---|---|---|---|
| Component (mmol/L) | Age | Weight (kg) | mL solution/4 h | |
| Sodium (75); chloride (65); glucose (75); potassium (20); citrate (10) | ≤4 mo 4-12 mo 12 mo-2 y 2-5 y | <6 6 to <10 10 to <12 12 to 19 | 200-400 400-700 700-900 900-1400 | |
| WHO, World Health Organization. | ||||
KEEP IN MIND
Hospitalization may be needed to replace fluids via IV or interosseous supplementation. For the severely dehydrated child, 20 mL/kg isotonic fluid can be administered as a rapid bolus.14 It may be necessary to repeat a rapid fluid infusion of 10 to 20 mL/kg every 20 to 30 minutes. For less severely ill infants who require IV rehydration, standard references such as the Harriet Lane Handbook16 provide excellent guidance.
Probiotics may help. Consider probiotics with Lactobacillus or Bifida bacterium to reduce the severity of diarrhea in infants and children who are mildly to moderately ill.17,18 Their usefulness in the severely ill patient has not been demonstrated.
Available vaccines and clinical recommendations
In February 2006, the US Food and Drug Administration (FDA) licensed a 3-dose, oral pentavalent rotavirus vaccine (RV5, RotaTeq) for use among infants. The vaccine contains live reassortant rotaviruses19—4 human rotavirus G outer-surface proteins and 1 human P attachment protein reassorted into a bovine rotavirus not infectious to humans.
In February 2008, the FDA approved a 2-dose, oral monovalent rotavirus vaccine (RV1, Rotarix), an attenuated live human rotavirus containing 1 G protein and 1 P protein. Both vaccines have proven to be clinically effective in rotavirus prevention trials, but effectiveness may depend on which rotavirus serotypes circulate each season.
ACIP, AAP, and AAFP recommend that all infants be routinely vaccinated with either RV5 or RV1.6,20–22 Vaccination should be complete by the time infants reach the age of 8 months (TABLE 2). Guidelines for vaccination emphasize the following points:
Timing. According to the ACIP, the first dose of either vaccine must be administered between the ages of 6 weeks and 14 weeks 6 days (the RV5 manufacturer [Merck] states a maximum age of 12 weeks). Give subsequent doses at 4- to 10-week intervals, as long as all doses are administered by 8 months of age. The RV1 manufacturer (GlaxoSmithKline) suggests completing the second (final) dose of its vaccine by age 24 weeks.
If an infant 15 weeks of age or older accidentally receives a first dose of RV vaccine, the series should be continued, as long as the last dose can be given by 8 months of age. Either vaccine can be administered concurrently with all other vaccines.
Contraindications. The only absolute contraindications to RV5 administration are a demonstrated hypersensitivity to any component of the vaccine and severe combined immunodeficiency disease (SCID). Contraindications to RV1 vaccine are vaccine component hypersensitivity, SCID, latex-induced allergy (anaphylaxis), and uncorrected malformation of the gastrointestinal (GI) tract that might predispose to intussusception.
Precautions. Precautions for vaccines include other forms of primary or secondary immunocompromised or immunodeficiency states, including cancer and acute or chronic GI disorders such as ongoing gastroenteritis or intussusception. Infants with transient mild illness with or without low-grade fever and infants who are breastfeeding can receive either vaccine. RV5 is shed in 9% of recipients and RV1 in 26% of recipients after Dose 1, but transmission of vaccine virus is not known to occur. Likewise, reversion of vaccine virus to more virulent pathogens is not known to occur. A household member with an immuno-compromised condition does not preclude giving either RV vaccine to an infant. The risk of transmitting vaccine virus is much smaller than the risk of acquiring infectious wild-type rotavirus.
Regurgitation of a vaccine dose is uncommon. When it does occur, the RV5 vaccine should not be repeated; some of the vaccine dose is retained and the safety of the additional vaccine from a second dose is unknown. Readministration of a dose of RV1 is not recommended, although not contraindicated.
TABLE 2
Recommended rotavirus live virus vaccine dosing6
| Patient age (mo) | RV5 (RotaTeq) | RV1 (Rotarix) |
|---|---|---|
| 2 | 2 mL | 1 mL |
| 4 | 2 mL | 1 mL* |
| 6 | 2 mlL* | — |
| *The final dose of either vaccine must be given by no later than 8 months of age. | ||
Vaccine efficacy
The safety and efficacy of live rotavirus vaccines were demonstrated in large studies that enrolled 71,725 children in RV5 vaccine trials23 and 24,163 children in RV1 vaccine trials.21 The pivotal RV5 study included a nested substudy to evaluate efficacy against any G1–G4 rotavirus gastroenteritis.
RV5 (RotaTeq) vaccine. In double-blind, placebo-controlled clinical trials, for the first rotavirus season, live RV5 vaccine effectively prevented severe rotavirus infection in 98% of cases, and reduced hospitalization by 95%, emergency department visits by 94%, physician office visits by 86%, and all rotavirus cases by 74% for infants who received all 3 doses of vaccine according to protocol.23 Hospitalization for any-cause gastroenteritis was reduced by 63%. Second-season data showed persistence of antibody protection. All 3 doses of vaccine are required for maximum protection.23
Both preterm and term infants received their first dose of vaccine between 6 and 12 weeks of life. For preterm infants who are experiencing medical difficulties, the first dose of vaccine may be delayed until the patient is stable, if it can be given before 15 weeks of age.
RV1 (Rotarix) vaccine. In double-blind, placebo-controlled clinical trials, for the first rotavirus season, live RV1 vaccine was 85% (Latin America) to 96% (Europe) effective in preventing severe rotavirus infection. It reduced hospitalization due to rotavirus by 85% (Latin America) to 100% (Europe), and all rotavirus cases by 87% (Europe) for infants who received both doses of the vaccine according to protocol. For the second season, the vaccine reduced severe rotavirus disease by 70% to 96%, and any rotavirus disease by 73% to 89%, showing persistence of antibody protection.6
Adverse events
With both vaccines, common side effects include irritability, flatulence, fever, vomiting, diarrhea, cough, runny nose, and loss of appetite. The RV5 vaccine has been shown not to increase the risk of intussusception compared with placebo.24,25 The RV1 vaccine should not be used in children with an uncorrected bowel malformation, due to unproven increased risk of intussusception. Risk of death from complications after administration of either vaccine did not differ from that among children receiving placebo.
Postmarketing surveillance of vaccination outcomes
Even though rotavirus vaccine coverage with RV5 RotaTeq in the 2007-2008 and 2008-2009 seasons was far less than that with other childhood immunizations, the number of rotavirus infections dropped by >60% in both 2008 and 2009.26 The number of stool tests for rotavirus and the percentage of positive results also dropped dramatically.
Additionally, the rotavirus peak incidence was delayed 2 to 4 months until April 2008 and March 2009.26 Incidence was reduced in all age groups, suggesting the possibility of herd immunity despite a low vaccine coverage rate (estimates are 57% for ≥1 dose) that would not be expected to provide herd immunity.27 Hospitalizations in the United States for rotavirus gastroenteritis dropped by as much as 85%,28 markedly reducing costs for gastroenteritis.
In a 2010 report from an emergency department in Houston, a complete RV5 vaccine series conferred 82% protection against acute gastroenteritis, 96% against severe rotavirus disease requiring IV rehydration, and 100% against hospitalization.29 For more on the vaccine, see the report by Yen et al.30
Reports of the effectiveness of Rotarix in postmarketing surveillance are limited, but the vaccine does seem to provide broad coverage.31
As of April 11, 2011, RV5 costs $59.76/$69.59 per dose in the public/private sectors, respectively (3-dose series: $179.28/$208.77); RV1 costs $89.25/$102.50 per dose (2-dose series: $178.50/$205). routine vaccination costs about $138 per case averted and $3024 per serious case averted. neither vaccine contains thimerosal. Both vaccines are available in 10-dose packs.
Source: Centers for Disease Control and Prevention. CDC vaccine price list. Prices last reviewed/updated: April 8, 2011. Available at: http://www.cdc.gov/vaccines/programs/vfc/cdc-vac-price-list.htm. Accessed April 11, 2011.
Incorporating rotavirus vaccine into a family medicine practice
Given inadequately reimbursed costs including the cost of stocking RV vaccine (see “Costs of RV vaccines” above), family physicians who treat relatively few infants must determine whether offering RV vaccine fits within their practices.
For family physicians who do treat infants, offering RV vaccination makes sense. These oral vaccines are highly effective, safe, and easy to administer, and will prevent a great deal of worry and calls regarding infants who have a fever or diarrhea or are vomiting. Due to the costs of stocking all vaccines, private practitioners are wise to purchase vaccine loss insurance. Many insurance agencies provide a rider on office insurance policies to cover vaccine supplies.
CORRESPONDENCE
Donald B. Middleton, MD, UPMC St. Margaret, 815 Freeport Road, Pittsburgh, PA 15215; middletondb@upmc.edu
• Patients with rotavirus infection require oral, enteral, or intravenous fluids to treat dehydration. A
• Give the first dose of rotavirus (RV) vaccine between the ages of 6 weeks and 14 weeks 6 days; give subsequent doses at 4- to 10-week intervals, completing by 8 months. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Rotavirus is the most common cause of severe gastroenteritis in infants and children younger than 5 years of age, and it accounts for approximately 5% of childhood deaths worldwide.1 In the United States, rotavirus causes numerous cases of dehydrating diarrhea and vomiting, and is responsible for direct and indirect healthcare costs of approximately $1 billion per year. Infection during childhood is almost universal.2
Improved personal hygiene and community sanitation have steadily reduced the prevalence of bacterial and parasitic disease. But these measures have had little effect on the spread of rotavirus and its potential complications of severe dehydration, hospitalization, and even death.1 Importantly, we now have the means to vaccinate against rotavirus infection and dramatically reduce the incidence of disease. In this article, I describe the available vaccines and the vaccination recommendations endorsed by the Advisory Committee on Immunization Practices (ACIP), the American Academy of Pediatrics (AAP), and the American Academy of Family Physicians (AAFP). I also review supportive treatment for rotavirus infection, which entails both do’s and don’ts.
Who is at risk of rotavirus disease?
For most term neonates, rotavirus disease is mild, perhaps because of partial protection from maternal antibodies.3 However, premature infants lacking full maternal antibody protection often suffer from more serious gastroenteritis. The most severe infections usually strike children between the ages of 4 months, when maternally derived antibody protection wanes, and 23 months, when dehydration risk lessens.4-6
The virus spreads from person to person via the fecal–oral route.6,7 Thirty percent to 50% of family members of an infected child may also become infected, but disease in older children and adults is usually subclinical or mild.6 Outbreaks of rotavirus are common in childcare centers and in children’s hospitals.7,8
How the disease presents
Rotavirus gastroenteritis peaks during the winter. With mild cases, a watery diarrhea will last a few days. In severe cases, onset is usually abrupt with fever, abdominal pain, and vomiting, which can precede diarrhea. A third of patients have a temperature higher than 102°F (38.9°C).6 There is a risk of dehydration, shock, and even, occasionally, infant death.9
Typically, the incubation period is 1 to 4 days, and the infection lasts 3 to 7 days. However, damage to the brush border of the intestinal villi can produce persistent disaccharide malabsorption, resulting in prolonged diarrhea even after resolution of infection.10,11 Stools generally do not contain blood or leukocytes. Ultrasound examination during rotavirus infections has shown thickening of the distal ileum and lymphadenopathy, which may predispose to intussusception.12 Other problems possibly linked to wild-type rotavirus infection are Kawasaki disease and sudden infant death syndrome. Recurrent rotavirus infection with one of the many different serotypes is common during childhood.
More than 25 different assays can detect rotavirus in stool, but the most reliable method is direct electron microscopy. A suitable clinically available alternative is enzyme immunoassay testing of stool samples. In mild cases, testing to detect rotavirus is not necessary. But for bloody, severe, or persistent diarrhea, stool testing for rotavirus and other entities is warranted.
Supportive treatment: Do’s and don’ts
No specific antiviral treatment is available for rotavirus infection. That said, the do’s and don’ts that follow will help guide your care.
DO administer oral, enteral, or intravenous (IV) fluids to prevent or correct dehydration. Oral rehydration therapy is the standard treatment for dehydration in anyone with acute gastroenteritis, including that caused by rotavirus. The recommended World Health Organization (WHO) oral rehydration solution contains sodium, chloride, and electrolytes (TABLE 1).13 Rice-based oral rehydration solution is an easily metabolized carbohydrate formulation that helps repair damaged tissues and enhances electrolyte absorption.9 WHO has endorsed guidelines that base fluid replacement on the patient’s age and weight, and that recommend oral zinc intake (10 mg/d for 10-14 days up to age 6 months; 20 mg/d for 10-14 days for older children) for all episodes of diarrhea (http://hetv.org/pdf/diarrhoea-guidelines.pdf). Oral glucose electrolyte solutions containing less sodium and chloride are also effective treatments.
DO recommend frequent small doses of oral rehydration solution, even if the patient is vomiting.14 Rehydration volumes are suggested in TABLE 1. Alternatively, give 10 to 20 mL/kg for each diarrheal episode, and 2 mL/kg for each bout of emesis. Feeding frequent small volumes (30 mL every 5-10 minutes) reduces the risk of emesis.
Although oral rehydration solutions are contraindicated for infants and young children with depressed consciousness, vomiting is not a contraindication to oral intake. About half of the oral intake stays in the stomach, even after vomiting. A single dose of ondansetron may safely reduce vomiting.15
Patients with mainly diarrhea can take fluids or feed at will. With children who refuse to drink, oral rehydration solutions can be administered via nasogastric tube at approximately 5 mL/min to limit vomiting and maintain hydration.14 In dehydrated infants and toddlers with collapsed veins, nasogastric intubation has been shown to be less traumatic than repetitive attempts at placing IV catheters.
DO encourage nursing mothers to con tinue breastfeeding during rehydration treatments. If a mother is bottle feeding, keep this in mind: Rotavirus can cause temporary lactase deficiency for some non-breastfeeding infants; lactose-free formulas may help.
DON’T assume that parents know how to provide proper supplementation. Tell them to avoid fluids containing mostly sugar that lack significant electrolyte supplementation (eg, cola) unless no other fluid alternative is available. Advise caregivers to avoid juices and other liquids high in complex or simple sugars because the osmotic load may worsen diarrhea.14
DON’T give antidiarrheal agents for acute treatment in infants and young children. Such treatment has resulted in death.14
TABLE 1
Prevent or correct dehydration using the WHO-recommended oral rehydration salts solution13
| With this formulation… | …rehydrate per these specifications… | …at this rate | ||
|---|---|---|---|---|
| Component (mmol/L) | Age | Weight (kg) | mL solution/4 h | |
| Sodium (75); chloride (65); glucose (75); potassium (20); citrate (10) | ≤4 mo 4-12 mo 12 mo-2 y 2-5 y | <6 6 to <10 10 to <12 12 to 19 | 200-400 400-700 700-900 900-1400 | |
| WHO, World Health Organization. | ||||
KEEP IN MIND
Hospitalization may be needed to replace fluids via IV or interosseous supplementation. For the severely dehydrated child, 20 mL/kg isotonic fluid can be administered as a rapid bolus.14 It may be necessary to repeat a rapid fluid infusion of 10 to 20 mL/kg every 20 to 30 minutes. For less severely ill infants who require IV rehydration, standard references such as the Harriet Lane Handbook16 provide excellent guidance.
Probiotics may help. Consider probiotics with Lactobacillus or Bifida bacterium to reduce the severity of diarrhea in infants and children who are mildly to moderately ill.17,18 Their usefulness in the severely ill patient has not been demonstrated.
Available vaccines and clinical recommendations
In February 2006, the US Food and Drug Administration (FDA) licensed a 3-dose, oral pentavalent rotavirus vaccine (RV5, RotaTeq) for use among infants. The vaccine contains live reassortant rotaviruses19—4 human rotavirus G outer-surface proteins and 1 human P attachment protein reassorted into a bovine rotavirus not infectious to humans.
In February 2008, the FDA approved a 2-dose, oral monovalent rotavirus vaccine (RV1, Rotarix), an attenuated live human rotavirus containing 1 G protein and 1 P protein. Both vaccines have proven to be clinically effective in rotavirus prevention trials, but effectiveness may depend on which rotavirus serotypes circulate each season.
ACIP, AAP, and AAFP recommend that all infants be routinely vaccinated with either RV5 or RV1.6,20–22 Vaccination should be complete by the time infants reach the age of 8 months (TABLE 2). Guidelines for vaccination emphasize the following points:
Timing. According to the ACIP, the first dose of either vaccine must be administered between the ages of 6 weeks and 14 weeks 6 days (the RV5 manufacturer [Merck] states a maximum age of 12 weeks). Give subsequent doses at 4- to 10-week intervals, as long as all doses are administered by 8 months of age. The RV1 manufacturer (GlaxoSmithKline) suggests completing the second (final) dose of its vaccine by age 24 weeks.
If an infant 15 weeks of age or older accidentally receives a first dose of RV vaccine, the series should be continued, as long as the last dose can be given by 8 months of age. Either vaccine can be administered concurrently with all other vaccines.
Contraindications. The only absolute contraindications to RV5 administration are a demonstrated hypersensitivity to any component of the vaccine and severe combined immunodeficiency disease (SCID). Contraindications to RV1 vaccine are vaccine component hypersensitivity, SCID, latex-induced allergy (anaphylaxis), and uncorrected malformation of the gastrointestinal (GI) tract that might predispose to intussusception.
Precautions. Precautions for vaccines include other forms of primary or secondary immunocompromised or immunodeficiency states, including cancer and acute or chronic GI disorders such as ongoing gastroenteritis or intussusception. Infants with transient mild illness with or without low-grade fever and infants who are breastfeeding can receive either vaccine. RV5 is shed in 9% of recipients and RV1 in 26% of recipients after Dose 1, but transmission of vaccine virus is not known to occur. Likewise, reversion of vaccine virus to more virulent pathogens is not known to occur. A household member with an immuno-compromised condition does not preclude giving either RV vaccine to an infant. The risk of transmitting vaccine virus is much smaller than the risk of acquiring infectious wild-type rotavirus.
Regurgitation of a vaccine dose is uncommon. When it does occur, the RV5 vaccine should not be repeated; some of the vaccine dose is retained and the safety of the additional vaccine from a second dose is unknown. Readministration of a dose of RV1 is not recommended, although not contraindicated.
TABLE 2
Recommended rotavirus live virus vaccine dosing6
| Patient age (mo) | RV5 (RotaTeq) | RV1 (Rotarix) |
|---|---|---|
| 2 | 2 mL | 1 mL |
| 4 | 2 mL | 1 mL* |
| 6 | 2 mlL* | — |
| *The final dose of either vaccine must be given by no later than 8 months of age. | ||
Vaccine efficacy
The safety and efficacy of live rotavirus vaccines were demonstrated in large studies that enrolled 71,725 children in RV5 vaccine trials23 and 24,163 children in RV1 vaccine trials.21 The pivotal RV5 study included a nested substudy to evaluate efficacy against any G1–G4 rotavirus gastroenteritis.
RV5 (RotaTeq) vaccine. In double-blind, placebo-controlled clinical trials, for the first rotavirus season, live RV5 vaccine effectively prevented severe rotavirus infection in 98% of cases, and reduced hospitalization by 95%, emergency department visits by 94%, physician office visits by 86%, and all rotavirus cases by 74% for infants who received all 3 doses of vaccine according to protocol.23 Hospitalization for any-cause gastroenteritis was reduced by 63%. Second-season data showed persistence of antibody protection. All 3 doses of vaccine are required for maximum protection.23
Both preterm and term infants received their first dose of vaccine between 6 and 12 weeks of life. For preterm infants who are experiencing medical difficulties, the first dose of vaccine may be delayed until the patient is stable, if it can be given before 15 weeks of age.
RV1 (Rotarix) vaccine. In double-blind, placebo-controlled clinical trials, for the first rotavirus season, live RV1 vaccine was 85% (Latin America) to 96% (Europe) effective in preventing severe rotavirus infection. It reduced hospitalization due to rotavirus by 85% (Latin America) to 100% (Europe), and all rotavirus cases by 87% (Europe) for infants who received both doses of the vaccine according to protocol. For the second season, the vaccine reduced severe rotavirus disease by 70% to 96%, and any rotavirus disease by 73% to 89%, showing persistence of antibody protection.6
Adverse events
With both vaccines, common side effects include irritability, flatulence, fever, vomiting, diarrhea, cough, runny nose, and loss of appetite. The RV5 vaccine has been shown not to increase the risk of intussusception compared with placebo.24,25 The RV1 vaccine should not be used in children with an uncorrected bowel malformation, due to unproven increased risk of intussusception. Risk of death from complications after administration of either vaccine did not differ from that among children receiving placebo.
Postmarketing surveillance of vaccination outcomes
Even though rotavirus vaccine coverage with RV5 RotaTeq in the 2007-2008 and 2008-2009 seasons was far less than that with other childhood immunizations, the number of rotavirus infections dropped by >60% in both 2008 and 2009.26 The number of stool tests for rotavirus and the percentage of positive results also dropped dramatically.
Additionally, the rotavirus peak incidence was delayed 2 to 4 months until April 2008 and March 2009.26 Incidence was reduced in all age groups, suggesting the possibility of herd immunity despite a low vaccine coverage rate (estimates are 57% for ≥1 dose) that would not be expected to provide herd immunity.27 Hospitalizations in the United States for rotavirus gastroenteritis dropped by as much as 85%,28 markedly reducing costs for gastroenteritis.
In a 2010 report from an emergency department in Houston, a complete RV5 vaccine series conferred 82% protection against acute gastroenteritis, 96% against severe rotavirus disease requiring IV rehydration, and 100% against hospitalization.29 For more on the vaccine, see the report by Yen et al.30
Reports of the effectiveness of Rotarix in postmarketing surveillance are limited, but the vaccine does seem to provide broad coverage.31
As of April 11, 2011, RV5 costs $59.76/$69.59 per dose in the public/private sectors, respectively (3-dose series: $179.28/$208.77); RV1 costs $89.25/$102.50 per dose (2-dose series: $178.50/$205). routine vaccination costs about $138 per case averted and $3024 per serious case averted. neither vaccine contains thimerosal. Both vaccines are available in 10-dose packs.
Source: Centers for Disease Control and Prevention. CDC vaccine price list. Prices last reviewed/updated: April 8, 2011. Available at: http://www.cdc.gov/vaccines/programs/vfc/cdc-vac-price-list.htm. Accessed April 11, 2011.
Incorporating rotavirus vaccine into a family medicine practice
Given inadequately reimbursed costs including the cost of stocking RV vaccine (see “Costs of RV vaccines” above), family physicians who treat relatively few infants must determine whether offering RV vaccine fits within their practices.
For family physicians who do treat infants, offering RV vaccination makes sense. These oral vaccines are highly effective, safe, and easy to administer, and will prevent a great deal of worry and calls regarding infants who have a fever or diarrhea or are vomiting. Due to the costs of stocking all vaccines, private practitioners are wise to purchase vaccine loss insurance. Many insurance agencies provide a rider on office insurance policies to cover vaccine supplies.
CORRESPONDENCE
Donald B. Middleton, MD, UPMC St. Margaret, 815 Freeport Road, Pittsburgh, PA 15215; middletondb@upmc.edu
1. Parashar UD, Gibson CJ, Bresee JS, et al. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304-306.
2. Malek MA, Curns AT, Holman RC, et al. Diarrhea- and rotavirus-associated hospitalizations among children less than 5 years of age: United States, 1997 and 2000. Pediatrics. 2006;117:1887-1892.
3. Xu J, Dennehy P, Keyserling H, et al. Serum antibody responses in children with rotavirus diarrhea can serve as proxy for protection. Clin Diagn Lab Immunol. 2005;12:273-279.
4. World Health Organization. Rotavirus vaccines. Wkly Epidemiol Rec. 2007;82:285-295.
5. Ward RL, Bernstein DI, Staat MA. Rotaviruses. In: Feigin RD, Cherry JD, eds. Textbook of Pediatric Infectious Disease. Vol 2, 6th ed. New York, NY: Saunders; 2009:2245-2270.
6. Centers for Disease Control and Prevention. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2009;58(RR-2):1-25.
7. Butz AM, Fosarelli P, Dick J, et al. Prevalence of rotavirus on high-risk fomites in day-care facilities. Pediatrics. 1993;92:202-205.
8. Fischer TK, Bresee JS, Glass RI. Rotavirus vaccines and the prevention of hospital-acquired diarrhea in children. Vaccine. 2004;22(suppl):S49-S54.
9. Kapikian AZ, Hoshino Y, Chanock RM. Rotaviruses. In: Knipe DM, Howley PM, Griffin DE, et al, eds. Fields Virology. 4th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2001: 1787-1825.
10. Lorrot M, Vasseur M. How do the rotavirus NSP4 and bacterial enterotoxins lead differently to diarrhea? Virol J. 2007;4:31.-
11. Ramig RF. Pathogenesis of intestinal and systemic rotavirus infection. J Virol. 2004;78:10213-10220.
12. Robinson CG, Hernanz-Schulman M, Zhu Y, et al. Evaluation of anatomic changes in young children with natural rotavirus infection: is intussusception biologically plausible? J Infect Dis. 2004;189:1382-1387.
13. World Health Organization. Oral Rehydration Salts: Production of the New ORS. Geneva, Switzerland: WHO Document Production Services; 2006. Available at: http://whqlibdoc.who.int/hq/2006/WHO_FCH_CAH_06.1.pdf. Accessed April 11, 2011.
14. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52(RR-16):1-16.
15. DeCamp LR, Byerley JS, Doshi N, et al. Use of antiemetic agents in acute gastroenteritis: a systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2008;162:858-865.
16. The Johns Hopkins Hospital, Custer JW, Rau RE. Harriet Lane Handbook: A Manual for Pediatric House Officers. 18th ed. St. Louis, Mo: Mosby/Elsevier; 2008.
17. Canani RB, Cirillo P, Terrin G, et al. Probiotics for treatment of acute diarrhoea in children: randomised clinical trial of five different preparations. BMJ. 2007;335:340.-
18. Van Niel CW, Feudtner C, Garrison MM, et al. Lactobacillus therapy for acute infectious diarrhea in children: a meta- analysis. Pediatrics. 2002;109:678-684.
19. Heaton PM, Goveia MG, Miller JM, et al. Development of a pentavalent rotavirus vaccine against prevalent serotypes of rotavirus gastroenteritis. J Infect Dis. 2005;192(suppl 1):S17-S21.
20. Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0 through 18 years — United States, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(5):1-4.
21. American Academy of Pediatrics (AAP) Committee on Infectious Diseases. Prevention of rotavirus disease: updated guidelines for use of rotavirus vaccine. Pediatrics. 2009;123:1412-1420.
22. Temte JL. Practice guidelines. ACIP releases 2009 child and adolescent immunization schedules. Am Fam Physician. 2009;79:56.-Available at: http://www.aafp.org/afp/2009/0101/p56.html. Accessed January 4, 2010.
23. Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23-33.
24. Centers for Disease Control and Prevention. Intussusception among recipients of rotavirus vaccine: United States, 1998– 1999. MMWR Morb Mortal Wkly Rep. 1999;48:577-581.
25. Centers for Disease Control and Prevention. Postmarketing monitoring of intussusception after RotaTeq vaccine: United States. February 1, 2006–February 15, 2007. MMWR Morb Mortal Wkly Rep. 2007;56:218-222.
26. Centers for Disease Control and Prevention. Reduction in rotavirus after vaccine introduction — United States, 2000-2009. MMWR Morb Mortal Wkly Rep. 2009;58:1146-1149.
27. Clark HF, Lawley D, Mallette LA, et al. Decline in cases of rotavirus gastroenteritis presenting to The Children’s Hospital of Philadelphia after introduction of a pentavalent rotavirus vaccine. Clin Vaccine Immunol. 2009;16:382-386.
28. Chang HG, Smith P, Tserenpuntsag B, et al. Reduction in New York hospitalizations for diarrhea and rotavirus. Presented at: 43rd National Immunization Conference; March 30-April 2, 2009; Dallas, Tex. Abstract 41. Available at: http://cdc.confex.com/cdc/nic2009/webprogram/Paper18073.html. Accessed April 15, 2011.
29. Boom JA, Tate JE, Sahni LC, et al. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics. 2010;125:e199-e207.
30. Yen C, Tate JE, Wenk JD, et al. Diarrhea-associated hospitalizations among US children over 2 rotavirus seasons after vaccine introduction. Pediatrics. 2011;127:e9-e15.
31. Correia JB, Patel MM, Nakagomi O, et al. Effectiveness of monovalent rotavirus vaccine (Rotarix) against severe diarrhea caused by serotypically unrelated G2P[4] strains in Brazil. J Infect Dis. 2010;201:363-369.
1. Parashar UD, Gibson CJ, Bresee JS, et al. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304-306.
2. Malek MA, Curns AT, Holman RC, et al. Diarrhea- and rotavirus-associated hospitalizations among children less than 5 years of age: United States, 1997 and 2000. Pediatrics. 2006;117:1887-1892.
3. Xu J, Dennehy P, Keyserling H, et al. Serum antibody responses in children with rotavirus diarrhea can serve as proxy for protection. Clin Diagn Lab Immunol. 2005;12:273-279.
4. World Health Organization. Rotavirus vaccines. Wkly Epidemiol Rec. 2007;82:285-295.
5. Ward RL, Bernstein DI, Staat MA. Rotaviruses. In: Feigin RD, Cherry JD, eds. Textbook of Pediatric Infectious Disease. Vol 2, 6th ed. New York, NY: Saunders; 2009:2245-2270.
6. Centers for Disease Control and Prevention. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2009;58(RR-2):1-25.
7. Butz AM, Fosarelli P, Dick J, et al. Prevalence of rotavirus on high-risk fomites in day-care facilities. Pediatrics. 1993;92:202-205.
8. Fischer TK, Bresee JS, Glass RI. Rotavirus vaccines and the prevention of hospital-acquired diarrhea in children. Vaccine. 2004;22(suppl):S49-S54.
9. Kapikian AZ, Hoshino Y, Chanock RM. Rotaviruses. In: Knipe DM, Howley PM, Griffin DE, et al, eds. Fields Virology. 4th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2001: 1787-1825.
10. Lorrot M, Vasseur M. How do the rotavirus NSP4 and bacterial enterotoxins lead differently to diarrhea? Virol J. 2007;4:31.-
11. Ramig RF. Pathogenesis of intestinal and systemic rotavirus infection. J Virol. 2004;78:10213-10220.
12. Robinson CG, Hernanz-Schulman M, Zhu Y, et al. Evaluation of anatomic changes in young children with natural rotavirus infection: is intussusception biologically plausible? J Infect Dis. 2004;189:1382-1387.
13. World Health Organization. Oral Rehydration Salts: Production of the New ORS. Geneva, Switzerland: WHO Document Production Services; 2006. Available at: http://whqlibdoc.who.int/hq/2006/WHO_FCH_CAH_06.1.pdf. Accessed April 11, 2011.
14. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52(RR-16):1-16.
15. DeCamp LR, Byerley JS, Doshi N, et al. Use of antiemetic agents in acute gastroenteritis: a systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2008;162:858-865.
16. The Johns Hopkins Hospital, Custer JW, Rau RE. Harriet Lane Handbook: A Manual for Pediatric House Officers. 18th ed. St. Louis, Mo: Mosby/Elsevier; 2008.
17. Canani RB, Cirillo P, Terrin G, et al. Probiotics for treatment of acute diarrhoea in children: randomised clinical trial of five different preparations. BMJ. 2007;335:340.-
18. Van Niel CW, Feudtner C, Garrison MM, et al. Lactobacillus therapy for acute infectious diarrhea in children: a meta- analysis. Pediatrics. 2002;109:678-684.
19. Heaton PM, Goveia MG, Miller JM, et al. Development of a pentavalent rotavirus vaccine against prevalent serotypes of rotavirus gastroenteritis. J Infect Dis. 2005;192(suppl 1):S17-S21.
20. Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0 through 18 years — United States, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(5):1-4.
21. American Academy of Pediatrics (AAP) Committee on Infectious Diseases. Prevention of rotavirus disease: updated guidelines for use of rotavirus vaccine. Pediatrics. 2009;123:1412-1420.
22. Temte JL. Practice guidelines. ACIP releases 2009 child and adolescent immunization schedules. Am Fam Physician. 2009;79:56.-Available at: http://www.aafp.org/afp/2009/0101/p56.html. Accessed January 4, 2010.
23. Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23-33.
24. Centers for Disease Control and Prevention. Intussusception among recipients of rotavirus vaccine: United States, 1998– 1999. MMWR Morb Mortal Wkly Rep. 1999;48:577-581.
25. Centers for Disease Control and Prevention. Postmarketing monitoring of intussusception after RotaTeq vaccine: United States. February 1, 2006–February 15, 2007. MMWR Morb Mortal Wkly Rep. 2007;56:218-222.
26. Centers for Disease Control and Prevention. Reduction in rotavirus after vaccine introduction — United States, 2000-2009. MMWR Morb Mortal Wkly Rep. 2009;58:1146-1149.
27. Clark HF, Lawley D, Mallette LA, et al. Decline in cases of rotavirus gastroenteritis presenting to The Children’s Hospital of Philadelphia after introduction of a pentavalent rotavirus vaccine. Clin Vaccine Immunol. 2009;16:382-386.
28. Chang HG, Smith P, Tserenpuntsag B, et al. Reduction in New York hospitalizations for diarrhea and rotavirus. Presented at: 43rd National Immunization Conference; March 30-April 2, 2009; Dallas, Tex. Abstract 41. Available at: http://cdc.confex.com/cdc/nic2009/webprogram/Paper18073.html. Accessed April 15, 2011.
29. Boom JA, Tate JE, Sahni LC, et al. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics. 2010;125:e199-e207.
30. Yen C, Tate JE, Wenk JD, et al. Diarrhea-associated hospitalizations among US children over 2 rotavirus seasons after vaccine introduction. Pediatrics. 2011;127:e9-e15.
31. Correia JB, Patel MM, Nakagomi O, et al. Effectiveness of monovalent rotavirus vaccine (Rotarix) against severe diarrhea caused by serotypically unrelated G2P[4] strains in Brazil. J Infect Dis. 2010;201:363-369.
It's time to abandon the sliding scale
� To prevent wide fluctuations in glucose levels, combine basal insulin coverage with a bolus of insulin given with each meal. A
� Use 2-hour postprandial blood glucose levels to adjust doses of rapid-acting insulin. A
� Use a basal/bolus regimen for hospitalized patients with insulin-dependent diabetes, adjusted for weight, age, IV glucose amount, meal size, and prehospital insulin regimen. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Among the many insulin management systems that have been developed, none has been as widely used as the sliding scale. Despite its acceptance by physicians and patients throughout the world, however, there is little evidence of the sliding scale�s efficacy.1-11
A number of studies have focused on potential problems associated with the sliding scale, primarily related to the �roller coaster� blood glucose levels that often result. The authors of a recent literature review concluded that fluctuating glucose levels are more harmful physiologically than levels that are continuously elevated, even when the elevation is mild.12
In this review, we take a closer look at the failings of the sliding scale system and the advantages of adopting an insulin regimen that more closely resembles a natural physiologic state. To enable you to provide optimal support for patients with insulin-dependent diabetes, we highlight 5 principles of insulin management.
Premeal glucose levels do not reflect insulin need
In a sliding scale system, insulin administration is based primarily�and in some cases, entirely�on a single point in time. Blood glucose levels are tested before meals and at bedtime or every 6 hours, and the amount of insulin administered at that time is based on the test result.
But a premeal blood glucose level is not an accurate predictor of the insulin needed at that time; it is simply a reflection of the insulin previously administered. Insulin given in response to the current blood glucose, then, may compound a prior dosing error, leading to serious drops or spikes in blood sugar. These wide fluctuations present challenges in both inpatient and outpatient settings, although each uses a different sliding scale system.
In the hospital, basal insulin is often withheld
In an inpatient setting, an IV insulin protocol is typically used, and patients may be placed on a sliding scale regimen even if their prehospital HbA1c was in a satisfactory range. The sliding scale is usually dependent on blood glucose levels obtained by bedside monitoring,13 tested every 6 hours. Hospitalized patients often receive no basal insulin, and, because rapid-acting insulin lasts only 3 to 4 hours, the results of their glucose tests are based on the intake of short-acting (regular) insulin alone. Short-acting insulin lasts about 6 to 8 hours, depending on dosage.
A sliding scale regimen generally has a cutoff point, below which no insulin is given. But skipping a dose because a patient�s glucose levels dip below the cut point can leave him or her without insulin for many hours, resulting in a spike in blood sugar.
Standing orders: One size does not �fit� all. Failure to individualize the insulin protocol is another problem with the use of sliding scales in an inpatient setting.7 Typically the sliding scale protocol is preprinted on standing orders, which are the same for all patients who require insulin and are under the care of a particular physician. Rarely are the orders adjusted for factors such as age, weight, IV glucose intake, time of day, size of the upcoming meal (or absence of food, at midnight), or type of diabetes. Outcomes studies of a sliding scale protocol in a hospital setting found that its use did not consistently achieve the desired results.7
Outpatient sliding scale fails to normalize blood glucose levels
Unlike the sliding scale regimen commonly used in hospitals, outpatient protocols often incorporate basal insulin. But that fact alone is not enough to eliminate the peaks and dips in blood glucose levels associated with this approach.
The dose of basal insulin�a long-acting or twice-a-day intermediate-acting insulin�is sometimes given on a sliding scale. More commonly, the sliding scale is used to determine the dose of rapid- or short-acting insulin administered prior to meals, based on the preprandial blood glucose reading and/or the expected caloric or carbohydrate intake. The wide fluctuations and excessive spikes in blood glucose levels associated with sliding scale management may cause reactive oxidative stress�a trigger for vascular damage, especially in patients with type 2 diabetes.14,15
CASE Louis C, an 8-year-old boy diagnosed with type 1 diabetes at age 4, was under the care of a pediatric endocrinologist, who used a �pattern� insulin management system to achieve blood glucose control. Louis was maintained on a 4-dose-per-day schedule: Rapid-acting insulin was administered before each meal (with the dosage adjusted based on the pattern of postprandial glucose values over the last 2 or 3 days and by his expected caloric intake and activity level) and a basal insulin given once a day. His HbA1c was 7.2%. He rarely experienced even mild hypoglycemia.
When his family relocated, Louis was taken to another specialist�a pediatric endocrinologist at a medical school affiliated with a children�s hospital. This physician thought that pattern management was out of date, and insisted that sliding scale was the only acceptable approach.
Thus, Louis was put on a regimen of intermediate-acting insulin before breakfast and at bedtime and rapid-acting insulin, given on a sliding scale, at mealtimes. The rapid-acting insulin was withheld if his blood glucose level was ?100 mg/dL; one unit was administered if his level was 101-150 mg/dL, 2 units for a reading of 151-200 mg/dL, and so on. Within 3 months, Louis� HbA1c had risen to 9.8%, and he developed significant hypoglycemia, especially in the afternoon and during the night. Louis� mother reported that he was tired all the time and wondered if it was because his blood glucose levels were �on a roller coaster.�
Adjust�don�t skip�insulin doses
The best medical care aims to reproduce the physiologic state to the extent possible. For patients with insulin-dependent diabetes, that means receiving 24-hour basal insulin coverage, as well as a bolus of insulin with each meal, to mimic normal insulin secretion.16,17
Pharmacodynamically, short-acting insulin lasts 6 to 8 hours. It should be given every 6 hours, in 4 equal doses, without ever skipping a dose. Rapid-acting insulin, too, should never be skipped. Because food or caloric intake requires insulin at the time of ingestion to facilitate glucose transport across the cell membrane, rapid-acting insulin should be administered before each meal (in 3 daily doses if the patient is also taking a long-acting or intermediate-acting insulin, or 6 times a day if used without basal insulin).
Rapid-acting insulin is essentially out of the system by the time of the next preprandial blood glucose test. Therefore, premeal levels mainly measure the action of the basal (long- or intermediate-acting) insulin. The package inserts for rapid-acting insulin state that a 2-hour postprandial blood glucose level should be used to adjust the next dose of rapid-acting insulin.18,19 (See �5 principles of insulin management�)
These principles can be used in the hospital for professional education and in the outpatient setting for patient education:
- Advise health care professionals (and patients) not to skip insulin doses. To avoid high blood glucose levels caused by low or missed doses, stress the importance of administering short-acting insulin every 6 hours, in 4 equal doses, or rapid-acting insulin before each meal with a long-acting basal insulin.
- Teach providers and patients that on an outpatient basis, routine daily regimens should reflect the pattern of postprandial blood glucose levels over the previous 2 or 3 days.
- Explain that rapid-acting insulin doses should be based primarily on the amount to be eaten, rather than on premeal glucose levels (although abnormally elevated or depressed levels may require a correction).
- Set parameters for glucose levels and instruct patients to call (or to administer a correction dose) if the value falls above or below a predetermined range.
- Consider providing insulin-dependent patients (or their parents, school nurse, or hospital staff) with an algorithm that uses a basal insulin dose and premeal rapid-acting insulin doses, adjusted for caloric or carbohydrate intake. Examples of insulin algorithms, which can help keep problems and telephone calls to a minimum, are available for both type 1 and type 2 diabetes from the Texas Diabetes Council at http://www.tdctoolkit.org/algorithms_and_guidelines.asp.
A closer look at pattern management
Measuring�and recording�both fasting and 1- or 2-hour postprandial blood glucose levels over a 2- to 3-day period is the first step in pattern management. The patient�s insulin intake is determined by the pattern of these values, with adjustments made for anticipated need. Here�s how it works in an outpatient setting:
- In the morning, the patient receives short- or rapid-acting insulin; the number of units is determined by his or her previous after-breakfast blood glucose levels, with adjustments depending on the caloric intake expected at breakfast and any deviation in the patient�s normal activity level to follow.
- At noon, the short- or rapid-acting insulin is adjusted for the size of the lunch and the patient�s recorded blood glucose levels 1 to 2 hours after lunch.
- At suppertime, the amount of insulin depends on the meal and the patient�s previous after-supper blood glucose levels.
- At bedtime, intermediate- or long-acting insulin is administered in an amount adjusted according to the patient�s fasting and/or premeal blood glucose levels. If the values are elevated because of extra eating or decreased activity, a correction dose (See �Calculating a correction dose�) may be needed to restore his or her blood sugar to within an acceptable range.20
If a patient�s blood glucose level rises above a predetermined value, he or she may need an insulin bolus to bring it down. Calculating the supplemental dose is a 2-step process: First, an insulin sensitivity factor (ISF) is calculated; then, the desired blood glucose level is subtracted from the actual blood glucose reading and divided by the ISF.
Step 1: Divide 1700 by the total daily dose (eg, 50 units)
![]()
Step 2: Subtract the desired blood glucose level (110 mg/dL) from the actual blood glucose reading (eg, 240 mg/dL) and divide by the ISF
Commentary: For an adult of average size,* an additional 3.8 (or 4) units of rapid-acting insulin should bring the patient�s blood glucose level down to 110 mg/dL. If the elevated blood glucose level occurs at this same time on subsequent days, it is an indication that the dose at the previous meal should be increased.
*An insulin-sensitive child might require less insulin, and an overweight adult might require more. When in doubt, give half of the calculated correction dose and repeat in an hour, if needed.
Other factors that may influence blood glucose levels include the injection site (insulin is absorbed faster when administered in the abdomen vs the leg); the type, or combination, of insulin used, ie, long-acting basal insulin vs intermediate-acting vs (or combined with) short- or rapid-acting insulin; and lifestyle (sedentary, active, very active, or suddenly becoming very active), which also affects the absorption of insulin.21
CASE After 5 months, Louis and his family returned to their former home. But by then, Louis had an HbA1c of 10.2%. On the advice of the pediatric endocrinologist who had treated him initially, Louis was put back on a pattern management system, using both rapid- and long-acting insulin. Three months later, his HbA1c was down to 7.6%�close to its previous level�and he no longer had problems with hypoglycemia.
CORRESPONDENCE
Diana W. Guthrie, PhD, Mid-America Diabetes Associates, 200 South Hillside, Wichita, KS 672111; dguthrie@kumc.edu
1. Clement S, Braithwaite SS, Magee MF, et al. for the American Diabetes Association Diabetes in Hospitals Writing Committee. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;21:553-591.
2. Jolobe O. The use of the sliding scale also needs to be reviewed [letter]. QJM. 2008;101:593-594.
3. Lorber DL. Sliding scale insulin [letter]. Diabetes Care. 2001;24:2011-2012.
4. MacMillan DR. Insulin adjustment by the sliding scale method�a straw man who won�t stay down? J Ky Med Assoc. 1991;89:211-212.
5. MacMillan DR. The fallacy of insulin adjustment by the sliding scale. J Ky Med Assoc. 1970;68:577-579.
6. Robbins L. Let�s get the sliding scale out of medicine. Med Rec Ann. 1963;56:201.-
7. Umpierrez GE, Palacio A, Smiley D. Sliding scale insulin use: myth or insanity? Am J Med. 2007;120:563-567.
8. Umpierrez GE. Basal versus sliding-scale regular insulin in hospitalized patients with hyperglycemia during enteral nutrition therapy [editorial]. Diabetes Care. 2009;32:751-753.
9. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30:2181-2186.
10. Katz CM. How efficient is sliding-scale insulin therapy? Problems with a �cookbook� approach in hospitalized patients. Postgrad Med. 1991;89:46-48,51-54, 57.
11. Hirsch IB. Sliding scale insulin�time to stop sliding [commentary]. JAMA. 2009;301:213-214.
12. Nalysnyk L, Hernandez-Medina M, Krishnarajah G. Glycaemic variability and complications in patients with diabetes mellitus: evidence from a systematic review of the literature. Diabetes Obes Metab. 2010;12:288-298.
13. Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157:545-552.
14. Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Rad Biol Med. 2011;50:567-575.
15. Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681-1687.
16. Bergenstal RM, Kendall DM, Franz MJ, et al. Management of type 2 diabetes: a systematic approach to meeting the standards of care. In: DeGroot LJ, Jamison JL, eds. Endocrinology. 4th ed. Philadelphia, Pa: WB Saunders Co; 2001:281.
17. Leahy JL. Intensive insulin therapy in type 1 diabetes mellitus. In: Leahy JL, Cefalu WT, eds. Insulin Therapy. New York: Marcel Dekker, Inc; 2002:87-112.
18. Humalog [package insert]. Indianapolis, Ind: Eli Lilly and Company; 2009.
19. Apidra [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2009.
20. Guthrie DW, Guthrie RA. Management of Diabetes. New York: Springer; 2009:83-195.
21. Albisser AM, Sperlich M. Adjusting insulins. Diabetes Educ. 1992;18:211-219.
� To prevent wide fluctuations in glucose levels, combine basal insulin coverage with a bolus of insulin given with each meal. A
� Use 2-hour postprandial blood glucose levels to adjust doses of rapid-acting insulin. A
� Use a basal/bolus regimen for hospitalized patients with insulin-dependent diabetes, adjusted for weight, age, IV glucose amount, meal size, and prehospital insulin regimen. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Among the many insulin management systems that have been developed, none has been as widely used as the sliding scale. Despite its acceptance by physicians and patients throughout the world, however, there is little evidence of the sliding scale�s efficacy.1-11
A number of studies have focused on potential problems associated with the sliding scale, primarily related to the �roller coaster� blood glucose levels that often result. The authors of a recent literature review concluded that fluctuating glucose levels are more harmful physiologically than levels that are continuously elevated, even when the elevation is mild.12
In this review, we take a closer look at the failings of the sliding scale system and the advantages of adopting an insulin regimen that more closely resembles a natural physiologic state. To enable you to provide optimal support for patients with insulin-dependent diabetes, we highlight 5 principles of insulin management.
Premeal glucose levels do not reflect insulin need
In a sliding scale system, insulin administration is based primarily�and in some cases, entirely�on a single point in time. Blood glucose levels are tested before meals and at bedtime or every 6 hours, and the amount of insulin administered at that time is based on the test result.
But a premeal blood glucose level is not an accurate predictor of the insulin needed at that time; it is simply a reflection of the insulin previously administered. Insulin given in response to the current blood glucose, then, may compound a prior dosing error, leading to serious drops or spikes in blood sugar. These wide fluctuations present challenges in both inpatient and outpatient settings, although each uses a different sliding scale system.
In the hospital, basal insulin is often withheld
In an inpatient setting, an IV insulin protocol is typically used, and patients may be placed on a sliding scale regimen even if their prehospital HbA1c was in a satisfactory range. The sliding scale is usually dependent on blood glucose levels obtained by bedside monitoring,13 tested every 6 hours. Hospitalized patients often receive no basal insulin, and, because rapid-acting insulin lasts only 3 to 4 hours, the results of their glucose tests are based on the intake of short-acting (regular) insulin alone. Short-acting insulin lasts about 6 to 8 hours, depending on dosage.
A sliding scale regimen generally has a cutoff point, below which no insulin is given. But skipping a dose because a patient�s glucose levels dip below the cut point can leave him or her without insulin for many hours, resulting in a spike in blood sugar.
Standing orders: One size does not �fit� all. Failure to individualize the insulin protocol is another problem with the use of sliding scales in an inpatient setting.7 Typically the sliding scale protocol is preprinted on standing orders, which are the same for all patients who require insulin and are under the care of a particular physician. Rarely are the orders adjusted for factors such as age, weight, IV glucose intake, time of day, size of the upcoming meal (or absence of food, at midnight), or type of diabetes. Outcomes studies of a sliding scale protocol in a hospital setting found that its use did not consistently achieve the desired results.7
Outpatient sliding scale fails to normalize blood glucose levels
Unlike the sliding scale regimen commonly used in hospitals, outpatient protocols often incorporate basal insulin. But that fact alone is not enough to eliminate the peaks and dips in blood glucose levels associated with this approach.
The dose of basal insulin�a long-acting or twice-a-day intermediate-acting insulin�is sometimes given on a sliding scale. More commonly, the sliding scale is used to determine the dose of rapid- or short-acting insulin administered prior to meals, based on the preprandial blood glucose reading and/or the expected caloric or carbohydrate intake. The wide fluctuations and excessive spikes in blood glucose levels associated with sliding scale management may cause reactive oxidative stress�a trigger for vascular damage, especially in patients with type 2 diabetes.14,15
CASE Louis C, an 8-year-old boy diagnosed with type 1 diabetes at age 4, was under the care of a pediatric endocrinologist, who used a �pattern� insulin management system to achieve blood glucose control. Louis was maintained on a 4-dose-per-day schedule: Rapid-acting insulin was administered before each meal (with the dosage adjusted based on the pattern of postprandial glucose values over the last 2 or 3 days and by his expected caloric intake and activity level) and a basal insulin given once a day. His HbA1c was 7.2%. He rarely experienced even mild hypoglycemia.
When his family relocated, Louis was taken to another specialist�a pediatric endocrinologist at a medical school affiliated with a children�s hospital. This physician thought that pattern management was out of date, and insisted that sliding scale was the only acceptable approach.
Thus, Louis was put on a regimen of intermediate-acting insulin before breakfast and at bedtime and rapid-acting insulin, given on a sliding scale, at mealtimes. The rapid-acting insulin was withheld if his blood glucose level was ?100 mg/dL; one unit was administered if his level was 101-150 mg/dL, 2 units for a reading of 151-200 mg/dL, and so on. Within 3 months, Louis� HbA1c had risen to 9.8%, and he developed significant hypoglycemia, especially in the afternoon and during the night. Louis� mother reported that he was tired all the time and wondered if it was because his blood glucose levels were �on a roller coaster.�
Adjust�don�t skip�insulin doses
The best medical care aims to reproduce the physiologic state to the extent possible. For patients with insulin-dependent diabetes, that means receiving 24-hour basal insulin coverage, as well as a bolus of insulin with each meal, to mimic normal insulin secretion.16,17
Pharmacodynamically, short-acting insulin lasts 6 to 8 hours. It should be given every 6 hours, in 4 equal doses, without ever skipping a dose. Rapid-acting insulin, too, should never be skipped. Because food or caloric intake requires insulin at the time of ingestion to facilitate glucose transport across the cell membrane, rapid-acting insulin should be administered before each meal (in 3 daily doses if the patient is also taking a long-acting or intermediate-acting insulin, or 6 times a day if used without basal insulin).
Rapid-acting insulin is essentially out of the system by the time of the next preprandial blood glucose test. Therefore, premeal levels mainly measure the action of the basal (long- or intermediate-acting) insulin. The package inserts for rapid-acting insulin state that a 2-hour postprandial blood glucose level should be used to adjust the next dose of rapid-acting insulin.18,19 (See �5 principles of insulin management�)
These principles can be used in the hospital for professional education and in the outpatient setting for patient education:
- Advise health care professionals (and patients) not to skip insulin doses. To avoid high blood glucose levels caused by low or missed doses, stress the importance of administering short-acting insulin every 6 hours, in 4 equal doses, or rapid-acting insulin before each meal with a long-acting basal insulin.
- Teach providers and patients that on an outpatient basis, routine daily regimens should reflect the pattern of postprandial blood glucose levels over the previous 2 or 3 days.
- Explain that rapid-acting insulin doses should be based primarily on the amount to be eaten, rather than on premeal glucose levels (although abnormally elevated or depressed levels may require a correction).
- Set parameters for glucose levels and instruct patients to call (or to administer a correction dose) if the value falls above or below a predetermined range.
- Consider providing insulin-dependent patients (or their parents, school nurse, or hospital staff) with an algorithm that uses a basal insulin dose and premeal rapid-acting insulin doses, adjusted for caloric or carbohydrate intake. Examples of insulin algorithms, which can help keep problems and telephone calls to a minimum, are available for both type 1 and type 2 diabetes from the Texas Diabetes Council at http://www.tdctoolkit.org/algorithms_and_guidelines.asp.
A closer look at pattern management
Measuring�and recording�both fasting and 1- or 2-hour postprandial blood glucose levels over a 2- to 3-day period is the first step in pattern management. The patient�s insulin intake is determined by the pattern of these values, with adjustments made for anticipated need. Here�s how it works in an outpatient setting:
- In the morning, the patient receives short- or rapid-acting insulin; the number of units is determined by his or her previous after-breakfast blood glucose levels, with adjustments depending on the caloric intake expected at breakfast and any deviation in the patient�s normal activity level to follow.
- At noon, the short- or rapid-acting insulin is adjusted for the size of the lunch and the patient�s recorded blood glucose levels 1 to 2 hours after lunch.
- At suppertime, the amount of insulin depends on the meal and the patient�s previous after-supper blood glucose levels.
- At bedtime, intermediate- or long-acting insulin is administered in an amount adjusted according to the patient�s fasting and/or premeal blood glucose levels. If the values are elevated because of extra eating or decreased activity, a correction dose (See �Calculating a correction dose�) may be needed to restore his or her blood sugar to within an acceptable range.20
If a patient�s blood glucose level rises above a predetermined value, he or she may need an insulin bolus to bring it down. Calculating the supplemental dose is a 2-step process: First, an insulin sensitivity factor (ISF) is calculated; then, the desired blood glucose level is subtracted from the actual blood glucose reading and divided by the ISF.
Step 1: Divide 1700 by the total daily dose (eg, 50 units)
![]()
Step 2: Subtract the desired blood glucose level (110 mg/dL) from the actual blood glucose reading (eg, 240 mg/dL) and divide by the ISF
Commentary: For an adult of average size,* an additional 3.8 (or 4) units of rapid-acting insulin should bring the patient�s blood glucose level down to 110 mg/dL. If the elevated blood glucose level occurs at this same time on subsequent days, it is an indication that the dose at the previous meal should be increased.
*An insulin-sensitive child might require less insulin, and an overweight adult might require more. When in doubt, give half of the calculated correction dose and repeat in an hour, if needed.
Other factors that may influence blood glucose levels include the injection site (insulin is absorbed faster when administered in the abdomen vs the leg); the type, or combination, of insulin used, ie, long-acting basal insulin vs intermediate-acting vs (or combined with) short- or rapid-acting insulin; and lifestyle (sedentary, active, very active, or suddenly becoming very active), which also affects the absorption of insulin.21
CASE After 5 months, Louis and his family returned to their former home. But by then, Louis had an HbA1c of 10.2%. On the advice of the pediatric endocrinologist who had treated him initially, Louis was put back on a pattern management system, using both rapid- and long-acting insulin. Three months later, his HbA1c was down to 7.6%�close to its previous level�and he no longer had problems with hypoglycemia.
CORRESPONDENCE
Diana W. Guthrie, PhD, Mid-America Diabetes Associates, 200 South Hillside, Wichita, KS 672111; dguthrie@kumc.edu
� To prevent wide fluctuations in glucose levels, combine basal insulin coverage with a bolus of insulin given with each meal. A
� Use 2-hour postprandial blood glucose levels to adjust doses of rapid-acting insulin. A
� Use a basal/bolus regimen for hospitalized patients with insulin-dependent diabetes, adjusted for weight, age, IV glucose amount, meal size, and prehospital insulin regimen. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Among the many insulin management systems that have been developed, none has been as widely used as the sliding scale. Despite its acceptance by physicians and patients throughout the world, however, there is little evidence of the sliding scale�s efficacy.1-11
A number of studies have focused on potential problems associated with the sliding scale, primarily related to the �roller coaster� blood glucose levels that often result. The authors of a recent literature review concluded that fluctuating glucose levels are more harmful physiologically than levels that are continuously elevated, even when the elevation is mild.12
In this review, we take a closer look at the failings of the sliding scale system and the advantages of adopting an insulin regimen that more closely resembles a natural physiologic state. To enable you to provide optimal support for patients with insulin-dependent diabetes, we highlight 5 principles of insulin management.
Premeal glucose levels do not reflect insulin need
In a sliding scale system, insulin administration is based primarily�and in some cases, entirely�on a single point in time. Blood glucose levels are tested before meals and at bedtime or every 6 hours, and the amount of insulin administered at that time is based on the test result.
But a premeal blood glucose level is not an accurate predictor of the insulin needed at that time; it is simply a reflection of the insulin previously administered. Insulin given in response to the current blood glucose, then, may compound a prior dosing error, leading to serious drops or spikes in blood sugar. These wide fluctuations present challenges in both inpatient and outpatient settings, although each uses a different sliding scale system.
In the hospital, basal insulin is often withheld
In an inpatient setting, an IV insulin protocol is typically used, and patients may be placed on a sliding scale regimen even if their prehospital HbA1c was in a satisfactory range. The sliding scale is usually dependent on blood glucose levels obtained by bedside monitoring,13 tested every 6 hours. Hospitalized patients often receive no basal insulin, and, because rapid-acting insulin lasts only 3 to 4 hours, the results of their glucose tests are based on the intake of short-acting (regular) insulin alone. Short-acting insulin lasts about 6 to 8 hours, depending on dosage.
A sliding scale regimen generally has a cutoff point, below which no insulin is given. But skipping a dose because a patient�s glucose levels dip below the cut point can leave him or her without insulin for many hours, resulting in a spike in blood sugar.
Standing orders: One size does not �fit� all. Failure to individualize the insulin protocol is another problem with the use of sliding scales in an inpatient setting.7 Typically the sliding scale protocol is preprinted on standing orders, which are the same for all patients who require insulin and are under the care of a particular physician. Rarely are the orders adjusted for factors such as age, weight, IV glucose intake, time of day, size of the upcoming meal (or absence of food, at midnight), or type of diabetes. Outcomes studies of a sliding scale protocol in a hospital setting found that its use did not consistently achieve the desired results.7
Outpatient sliding scale fails to normalize blood glucose levels
Unlike the sliding scale regimen commonly used in hospitals, outpatient protocols often incorporate basal insulin. But that fact alone is not enough to eliminate the peaks and dips in blood glucose levels associated with this approach.
The dose of basal insulin�a long-acting or twice-a-day intermediate-acting insulin�is sometimes given on a sliding scale. More commonly, the sliding scale is used to determine the dose of rapid- or short-acting insulin administered prior to meals, based on the preprandial blood glucose reading and/or the expected caloric or carbohydrate intake. The wide fluctuations and excessive spikes in blood glucose levels associated with sliding scale management may cause reactive oxidative stress�a trigger for vascular damage, especially in patients with type 2 diabetes.14,15
CASE Louis C, an 8-year-old boy diagnosed with type 1 diabetes at age 4, was under the care of a pediatric endocrinologist, who used a �pattern� insulin management system to achieve blood glucose control. Louis was maintained on a 4-dose-per-day schedule: Rapid-acting insulin was administered before each meal (with the dosage adjusted based on the pattern of postprandial glucose values over the last 2 or 3 days and by his expected caloric intake and activity level) and a basal insulin given once a day. His HbA1c was 7.2%. He rarely experienced even mild hypoglycemia.
When his family relocated, Louis was taken to another specialist�a pediatric endocrinologist at a medical school affiliated with a children�s hospital. This physician thought that pattern management was out of date, and insisted that sliding scale was the only acceptable approach.
Thus, Louis was put on a regimen of intermediate-acting insulin before breakfast and at bedtime and rapid-acting insulin, given on a sliding scale, at mealtimes. The rapid-acting insulin was withheld if his blood glucose level was ?100 mg/dL; one unit was administered if his level was 101-150 mg/dL, 2 units for a reading of 151-200 mg/dL, and so on. Within 3 months, Louis� HbA1c had risen to 9.8%, and he developed significant hypoglycemia, especially in the afternoon and during the night. Louis� mother reported that he was tired all the time and wondered if it was because his blood glucose levels were �on a roller coaster.�
Adjust�don�t skip�insulin doses
The best medical care aims to reproduce the physiologic state to the extent possible. For patients with insulin-dependent diabetes, that means receiving 24-hour basal insulin coverage, as well as a bolus of insulin with each meal, to mimic normal insulin secretion.16,17
Pharmacodynamically, short-acting insulin lasts 6 to 8 hours. It should be given every 6 hours, in 4 equal doses, without ever skipping a dose. Rapid-acting insulin, too, should never be skipped. Because food or caloric intake requires insulin at the time of ingestion to facilitate glucose transport across the cell membrane, rapid-acting insulin should be administered before each meal (in 3 daily doses if the patient is also taking a long-acting or intermediate-acting insulin, or 6 times a day if used without basal insulin).
Rapid-acting insulin is essentially out of the system by the time of the next preprandial blood glucose test. Therefore, premeal levels mainly measure the action of the basal (long- or intermediate-acting) insulin. The package inserts for rapid-acting insulin state that a 2-hour postprandial blood glucose level should be used to adjust the next dose of rapid-acting insulin.18,19 (See �5 principles of insulin management�)
These principles can be used in the hospital for professional education and in the outpatient setting for patient education:
- Advise health care professionals (and patients) not to skip insulin doses. To avoid high blood glucose levels caused by low or missed doses, stress the importance of administering short-acting insulin every 6 hours, in 4 equal doses, or rapid-acting insulin before each meal with a long-acting basal insulin.
- Teach providers and patients that on an outpatient basis, routine daily regimens should reflect the pattern of postprandial blood glucose levels over the previous 2 or 3 days.
- Explain that rapid-acting insulin doses should be based primarily on the amount to be eaten, rather than on premeal glucose levels (although abnormally elevated or depressed levels may require a correction).
- Set parameters for glucose levels and instruct patients to call (or to administer a correction dose) if the value falls above or below a predetermined range.
- Consider providing insulin-dependent patients (or their parents, school nurse, or hospital staff) with an algorithm that uses a basal insulin dose and premeal rapid-acting insulin doses, adjusted for caloric or carbohydrate intake. Examples of insulin algorithms, which can help keep problems and telephone calls to a minimum, are available for both type 1 and type 2 diabetes from the Texas Diabetes Council at http://www.tdctoolkit.org/algorithms_and_guidelines.asp.
A closer look at pattern management
Measuring�and recording�both fasting and 1- or 2-hour postprandial blood glucose levels over a 2- to 3-day period is the first step in pattern management. The patient�s insulin intake is determined by the pattern of these values, with adjustments made for anticipated need. Here�s how it works in an outpatient setting:
- In the morning, the patient receives short- or rapid-acting insulin; the number of units is determined by his or her previous after-breakfast blood glucose levels, with adjustments depending on the caloric intake expected at breakfast and any deviation in the patient�s normal activity level to follow.
- At noon, the short- or rapid-acting insulin is adjusted for the size of the lunch and the patient�s recorded blood glucose levels 1 to 2 hours after lunch.
- At suppertime, the amount of insulin depends on the meal and the patient�s previous after-supper blood glucose levels.
- At bedtime, intermediate- or long-acting insulin is administered in an amount adjusted according to the patient�s fasting and/or premeal blood glucose levels. If the values are elevated because of extra eating or decreased activity, a correction dose (See �Calculating a correction dose�) may be needed to restore his or her blood sugar to within an acceptable range.20
If a patient�s blood glucose level rises above a predetermined value, he or she may need an insulin bolus to bring it down. Calculating the supplemental dose is a 2-step process: First, an insulin sensitivity factor (ISF) is calculated; then, the desired blood glucose level is subtracted from the actual blood glucose reading and divided by the ISF.
Step 1: Divide 1700 by the total daily dose (eg, 50 units)
![]()
Step 2: Subtract the desired blood glucose level (110 mg/dL) from the actual blood glucose reading (eg, 240 mg/dL) and divide by the ISF
Commentary: For an adult of average size,* an additional 3.8 (or 4) units of rapid-acting insulin should bring the patient�s blood glucose level down to 110 mg/dL. If the elevated blood glucose level occurs at this same time on subsequent days, it is an indication that the dose at the previous meal should be increased.
*An insulin-sensitive child might require less insulin, and an overweight adult might require more. When in doubt, give half of the calculated correction dose and repeat in an hour, if needed.
Other factors that may influence blood glucose levels include the injection site (insulin is absorbed faster when administered in the abdomen vs the leg); the type, or combination, of insulin used, ie, long-acting basal insulin vs intermediate-acting vs (or combined with) short- or rapid-acting insulin; and lifestyle (sedentary, active, very active, or suddenly becoming very active), which also affects the absorption of insulin.21
CASE After 5 months, Louis and his family returned to their former home. But by then, Louis had an HbA1c of 10.2%. On the advice of the pediatric endocrinologist who had treated him initially, Louis was put back on a pattern management system, using both rapid- and long-acting insulin. Three months later, his HbA1c was down to 7.6%�close to its previous level�and he no longer had problems with hypoglycemia.
CORRESPONDENCE
Diana W. Guthrie, PhD, Mid-America Diabetes Associates, 200 South Hillside, Wichita, KS 672111; dguthrie@kumc.edu
1. Clement S, Braithwaite SS, Magee MF, et al. for the American Diabetes Association Diabetes in Hospitals Writing Committee. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;21:553-591.
2. Jolobe O. The use of the sliding scale also needs to be reviewed [letter]. QJM. 2008;101:593-594.
3. Lorber DL. Sliding scale insulin [letter]. Diabetes Care. 2001;24:2011-2012.
4. MacMillan DR. Insulin adjustment by the sliding scale method�a straw man who won�t stay down? J Ky Med Assoc. 1991;89:211-212.
5. MacMillan DR. The fallacy of insulin adjustment by the sliding scale. J Ky Med Assoc. 1970;68:577-579.
6. Robbins L. Let�s get the sliding scale out of medicine. Med Rec Ann. 1963;56:201.-
7. Umpierrez GE, Palacio A, Smiley D. Sliding scale insulin use: myth or insanity? Am J Med. 2007;120:563-567.
8. Umpierrez GE. Basal versus sliding-scale regular insulin in hospitalized patients with hyperglycemia during enteral nutrition therapy [editorial]. Diabetes Care. 2009;32:751-753.
9. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30:2181-2186.
10. Katz CM. How efficient is sliding-scale insulin therapy? Problems with a �cookbook� approach in hospitalized patients. Postgrad Med. 1991;89:46-48,51-54, 57.
11. Hirsch IB. Sliding scale insulin�time to stop sliding [commentary]. JAMA. 2009;301:213-214.
12. Nalysnyk L, Hernandez-Medina M, Krishnarajah G. Glycaemic variability and complications in patients with diabetes mellitus: evidence from a systematic review of the literature. Diabetes Obes Metab. 2010;12:288-298.
13. Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157:545-552.
14. Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Rad Biol Med. 2011;50:567-575.
15. Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681-1687.
16. Bergenstal RM, Kendall DM, Franz MJ, et al. Management of type 2 diabetes: a systematic approach to meeting the standards of care. In: DeGroot LJ, Jamison JL, eds. Endocrinology. 4th ed. Philadelphia, Pa: WB Saunders Co; 2001:281.
17. Leahy JL. Intensive insulin therapy in type 1 diabetes mellitus. In: Leahy JL, Cefalu WT, eds. Insulin Therapy. New York: Marcel Dekker, Inc; 2002:87-112.
18. Humalog [package insert]. Indianapolis, Ind: Eli Lilly and Company; 2009.
19. Apidra [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2009.
20. Guthrie DW, Guthrie RA. Management of Diabetes. New York: Springer; 2009:83-195.
21. Albisser AM, Sperlich M. Adjusting insulins. Diabetes Educ. 1992;18:211-219.
1. Clement S, Braithwaite SS, Magee MF, et al. for the American Diabetes Association Diabetes in Hospitals Writing Committee. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;21:553-591.
2. Jolobe O. The use of the sliding scale also needs to be reviewed [letter]. QJM. 2008;101:593-594.
3. Lorber DL. Sliding scale insulin [letter]. Diabetes Care. 2001;24:2011-2012.
4. MacMillan DR. Insulin adjustment by the sliding scale method�a straw man who won�t stay down? J Ky Med Assoc. 1991;89:211-212.
5. MacMillan DR. The fallacy of insulin adjustment by the sliding scale. J Ky Med Assoc. 1970;68:577-579.
6. Robbins L. Let�s get the sliding scale out of medicine. Med Rec Ann. 1963;56:201.-
7. Umpierrez GE, Palacio A, Smiley D. Sliding scale insulin use: myth or insanity? Am J Med. 2007;120:563-567.
8. Umpierrez GE. Basal versus sliding-scale regular insulin in hospitalized patients with hyperglycemia during enteral nutrition therapy [editorial]. Diabetes Care. 2009;32:751-753.
9. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30:2181-2186.
10. Katz CM. How efficient is sliding-scale insulin therapy? Problems with a �cookbook� approach in hospitalized patients. Postgrad Med. 1991;89:46-48,51-54, 57.
11. Hirsch IB. Sliding scale insulin�time to stop sliding [commentary]. JAMA. 2009;301:213-214.
12. Nalysnyk L, Hernandez-Medina M, Krishnarajah G. Glycaemic variability and complications in patients with diabetes mellitus: evidence from a systematic review of the literature. Diabetes Obes Metab. 2010;12:288-298.
13. Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157:545-552.
14. Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Rad Biol Med. 2011;50:567-575.
15. Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681-1687.
16. Bergenstal RM, Kendall DM, Franz MJ, et al. Management of type 2 diabetes: a systematic approach to meeting the standards of care. In: DeGroot LJ, Jamison JL, eds. Endocrinology. 4th ed. Philadelphia, Pa: WB Saunders Co; 2001:281.
17. Leahy JL. Intensive insulin therapy in type 1 diabetes mellitus. In: Leahy JL, Cefalu WT, eds. Insulin Therapy. New York: Marcel Dekker, Inc; 2002:87-112.
18. Humalog [package insert]. Indianapolis, Ind: Eli Lilly and Company; 2009.
19. Apidra [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2009.
20. Guthrie DW, Guthrie RA. Management of Diabetes. New York: Springer; 2009:83-195.
21. Albisser AM, Sperlich M. Adjusting insulins. Diabetes Educ. 1992;18:211-219.
PPI therapy: When to worry about fracture risk
• For most patients with chronic heartburn and regurgitation, step-down therapy to the lowest effective dose of proton pump inhibitors (PPIs) or treatment with a histamine-2 receptor antagonist (H2RA) is a reasonable, cost-effective approach. A
• Advise elderly patients who require long-term, high-dose PPI therapy to increase their dietary and/or supplemental calcium intake. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 Damian F,* a 39-year-old construction worker who takes omeprazole for chronic gastroesophageal reflux disease (GERD), comes in to request a refill. He’s had several accidents in recent years—he fell off a ladder on one occasion, and went down a flight of stairs on another—but none that resulted in significant trauma. Damian admits that he could better control his GERD symptoms by avoiding spicy and fatty foods, limiting alcohol consumption, and quitting smoking, but takes omeprazole nearly every day instead.
CASE 2 Estella G,* a 71-year-old retiree, has been on continuous proton pump inhibitor (PPI) therapy for chronic GERD and erosive esophagitis for nearly 20 years. The patient is a frail woman (body mass index=19.8 kg/m2) and a former smoker (1½ packs a day), both of which increase her risk of osteoporosis. But she has never had a dual energy x-ray absorptiometry (DEXA) scan.
*These cases are based on real patients in my practice, but their names and details have been changed to protect their identity.
Proton pump inhibitors (PPIs) are one of the most commonly used prescription drug categories in the United States,1 but they have been associated with an increase in fracture risk. A US Food and Drug Administration (FDA) safety update issued in March 2011 noted that there is little problem with the lower doses and shorter duration for which over-the-counter PPIs are intended, but patients who take higher-dose prescription PPIs or take prescription PPIs for more than a year may be at greater risk.2
If Damian and Estella were your patients, would you continue to prescribe PPI therapy or offer them alternatives? How should you treat other patients with chronic upper gastrointestinal (GI) distress? The evidence review that follows can help you answer those questions.
How high is the risk? Evidence is mixed (or lacking)
Several retrospective studies have demonstrated a modest increased risk for hip, spine, and wrist fractures in men and women taking PPIs, with the highest risk in patients who have taken higher than standard doses for >4 years.3-6 Concomitant risk factors (alcohol abuse, cigarette smoking, diabetes, and neurologic or renal disease) may increase fracture risk.6 But other retrospective studies, as well as prospective studies, have found no significant increase in fracture risk in patients taking PPIs,7-9 even after 5 years of therapy.7 However, some studies that failed to find an increased risk of osteoporosis with PPI use had a small number of subjects,8,9 resulting in a wide range in confidence intervals.
These findings, based on 6 retrospective case-control, cohort, and cross-sectional studies and 2 prospective cohort studies, are summarized in TABLE 1. No prospective randomized, blinded, controlled trials have examined the potential increased fracture risk associated with PPI use.
Do PPIs interfere with calcium metabolism?
Here, too, the findings are mixed. PPIs are known to inhibit the production and secretion of intragastric hydrochloric acid, which mediates small intestinal absorption of calcium,10 but evidence is conflicting about the role of intragastric hydrochloric acid in calcium absorption. Osteoclasts also have proton pumps, and some researchers have suggested that PPIs have the potential to limit the activity of these proton pumps, leading to reduced bone resorption.11
To date, the only studies that have examined the impact of PPIs on intestinal calcium absorption were limited by the health status of the participants—all either had renal failure and were on hemodialysis or had hypo- or achlorhydria, chronic conditions known to adversely affect calcium metabolism.12 Long-term randomized, double-blinded, placebo-controlled trials are needed to determine whether PPIs adversely affect intestinal calcium absorption and result in bone resorption abnormalities and increased fracture risk.
A closer look at the data
The varying responses associated with PPI dose and duration and the possibility that acid inhibition may decrease calcium absorption support a causal association between PPI use and fracture risk. But the low magnitude of the proposed association (most odds ratios <2) and the lack of data assessing potentially confounding factors limit evidence of causality.3,5,6,9 One key limitation of the earlier studies is that they were not designed to define the specific mechanism underlying the association between PPI therapy and fracture risk.
Older studies suggest a causal relationship
Two case-control studies3,4 found a causal association between PPI use and fracture risk, but one of them failed to identify either a dose-response or a duration-response effect.4 And neither study was designed to define underlying mechanisms to explain the potential association between fracture risk and PPI therapy.
A retrospective matched cohort study5 found an increase in the overall risk of fracture among patients with ≥7 years of PPI therapy and an in-creased risk of hip fracture with ≥5 years of therapy, but short-term risk of fracture was not found to be significant. The results of this study suggest that the risk of osteoporotic fracture increases with duration of exposure to PPI therapy, but not in a dose-dependent fashion.
Newer data are less worrisome
The results of a retrospective cross-sectional trial, published last year, are more reassuring. The researchers determined via univariate analysis that PPI use was associated with a lower risk of osteoporosis, both at the lumbar spine (for all levels of PPI use) and the hip (in patients who had taken more than 1500 standard PPI doses over the previous 5 years).7
This finding—that increasing intensity (both longer duration and higher dosage) of PPI exposure is not associated with an increased risk of osteoporosis—contrasts with results of the authors’ earlier study.5 This may be because they monitored annualized changes in BMD and were able to detect significant changes in other medications participants were taking that might affect bone loss or gain. That allowed them to validate their findings regarding a lack of true association between bone loss and PPI use, the authors reported.
A matched, nested case-control trial8 determined that the use of PPIs does not increase the risk of hip fracture in patients without associated major risk factors (ie, alcohol dependence, underlying neurologic disease, accidental falls, and senility). The researchers suggested that the difference between their findings and those of an earlier nested case-control study3 could mean that the increased risk of hip fracture found in the older study occurred only among PPI users with definable risk factors for hip fracture.
Recent results from the Women’s Health Initiative (WHI) suggest that in postmenopausal women, PPI use is not associated with hip fractures. The WHI did, however, find a modest association between PPI use and clinical spine, forearm, or wrist fracture, as well as total fractures.13 Compared with previous trials, this large cohort study had a large number of fracture events and assessed confounding factors that had not been addressed, including calcium intake. It also was the first trial to assess associations between BMD and fracture risk relative to PPI dosing. Although no specific conclusion was reported, the researchers did not find evidence of dose dependence.
A reasonable approach to PPI use
A consensus statement from the FDA2 and the authors of 2 meta-analyses14,15 recommend that PPIs be used only for appropriate indications—GERD, peptic ulcer disease, dyspepsia, and treatment of Helicobacter pylori—and not in higher doses or for longer periods than are necessary to achieve the desired results.
Whenever possible, implement step-down therapy to the lowest effective dose or prescribe an H2RA rather than a PPI. Both are cost-effective ways to treat most patients with upper GI symptoms.2 It is important, too, to advise elderly patients who require long-term, high-dose PPI therapy to increase their dietary and/or supplemental calcium intake, to recommend DEXA scans for individuals at risk for osteoporosis, and to counsel patients who suffer from GI distress to avoid foods that are known to exacerbate symptoms (TABLE 2).16
TABLE 2
GERD and diet: Foods that worsen symptoms16
| Alcohol |
| Caffeine-containing beverages |
| Citrus fruits |
| Chocolate |
| Fried and fatty foods |
| Garlic and onions |
| Mint flavorings |
| Spicy foods |
| Tomato-based foods (eg, chili, pizza, spaghetti sauce, salsa) |
CASE 1 Damian
You talk to Damian about the association between prolonged PPI therapy and fracture risk and stress the need for dietary changes and lifestyle modifications, particularly smoking cessation. On a return visit several months later, he reports that he has stopped smoking and cut way back on alcohol consumption, and eats fast food less frequently. As a result, he no longer requires chronic use of PPI therapy, and now takes omeprazole only when he has symptoms of GERD—usually, after indulging in fried or fatty foods.
CASE 2 Estella
Estella has severe GERD and erosive esophagitis and will probably need lifelong PPI therapy to adequately control her symptoms. After a detailed discussion of potential risks vs benefits of PPIs, she agrees to a DEXA scan to evaluate for osteoporosis. Her test results show osteopenia in the lumbar spine and femoral neck, but no evidence of osteoporosis. You advise her to increase her consumption of calcium and to undergo DEXA scanning in another 2 years.
CORRESPONDENCE
Joel J. Heidelbaugh, MD, FAAFP, FACG, Ypsilanti Health Center, 200 Arnet Suite 200, Ypsilanti, MI 48198; jheidel@med.umich.edu
1. Bartholow M. Top 200 prescription drugs of 2009. May 11, 2010. Pharmacy Times. Available at: http://www.pharmacytimes. http://www.pharmacytimes.com/publications/issue/2010/May2010/RxFocusTopDrugs-0510. Accessed April 8, 2011.
2. US Food and Drug Administration. FDA Drug Safety Communication: Possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. May 25, 2010; March 23, 2011 update. Available at: http://www.fda.gov/Drugs/DrugSafety/postmarketdrugsafetyInformationforpatientsandproviders/ucm213206.htm#SafetyAnnouncement. Accessed March 24, 2011.
3. Yang YX, Lewis JD, Epstein S, et al. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947-2953.
4. Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine h(2) receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int. 2006;79:76-83.
5. Targownik LE, Lix LM, Metge CJ. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ. 2008;179:319-326.
6. Corley DA, Kubo A, Zhao W, et al. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology. 2010;139:93-101.
7. Targownik LE, Lix LM, Leung S, et al. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology. 2010;138:896-904.
8. Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy. 2008;28:951-959.
9. Yu EW, Blackwell T, Ensrud KE, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int. 2008;83:251-259.
10. Bo-Linn GW, Davis GR, Buddrus DJ, et al. An evaluation of the importance of gastric acid secretion in the absorption of dietary calcium. J Clin Invest. 1984;73:640-647.
11. Farina C, Gagliardi S. Selective inhibition of osteoclast vacuolar H+-ATPase. Curr Pharm Des. 2002;8:2033-2048.
12. Insogna KL. The effect of proton pump-inhibiting drugs on mineral metabolism. Am J Gastroenterol. 2009;104(suppl 2):S2-S4.
13. Gray SL, LaCroix AZ, Larson L, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women. Arch Intern Med. 2010;170:765-771.
14. Laine L. Proton pump inhibitors and bone fractures? Am J Gastroenterol. 2009;104(suppl 2):S21-S26.
15. Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors: a review of cost-effectiveness and risk. Am J Gastroenterol.;2009;104(suppl 2):S27-S32.
16. National Digestive Diseases Information Clearinghouse. Heartburn, gastroesophageal reflux (GER), and gastroesophageal reflux disease (GERD). Available at: http://digestive.niddk.nih.gov/ddiseases/pubs/gerd. Accessed April 18, 2011.
• For most patients with chronic heartburn and regurgitation, step-down therapy to the lowest effective dose of proton pump inhibitors (PPIs) or treatment with a histamine-2 receptor antagonist (H2RA) is a reasonable, cost-effective approach. A
• Advise elderly patients who require long-term, high-dose PPI therapy to increase their dietary and/or supplemental calcium intake. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 Damian F,* a 39-year-old construction worker who takes omeprazole for chronic gastroesophageal reflux disease (GERD), comes in to request a refill. He’s had several accidents in recent years—he fell off a ladder on one occasion, and went down a flight of stairs on another—but none that resulted in significant trauma. Damian admits that he could better control his GERD symptoms by avoiding spicy and fatty foods, limiting alcohol consumption, and quitting smoking, but takes omeprazole nearly every day instead.
CASE 2 Estella G,* a 71-year-old retiree, has been on continuous proton pump inhibitor (PPI) therapy for chronic GERD and erosive esophagitis for nearly 20 years. The patient is a frail woman (body mass index=19.8 kg/m2) and a former smoker (1½ packs a day), both of which increase her risk of osteoporosis. But she has never had a dual energy x-ray absorptiometry (DEXA) scan.
*These cases are based on real patients in my practice, but their names and details have been changed to protect their identity.
Proton pump inhibitors (PPIs) are one of the most commonly used prescription drug categories in the United States,1 but they have been associated with an increase in fracture risk. A US Food and Drug Administration (FDA) safety update issued in March 2011 noted that there is little problem with the lower doses and shorter duration for which over-the-counter PPIs are intended, but patients who take higher-dose prescription PPIs or take prescription PPIs for more than a year may be at greater risk.2
If Damian and Estella were your patients, would you continue to prescribe PPI therapy or offer them alternatives? How should you treat other patients with chronic upper gastrointestinal (GI) distress? The evidence review that follows can help you answer those questions.
How high is the risk? Evidence is mixed (or lacking)
Several retrospective studies have demonstrated a modest increased risk for hip, spine, and wrist fractures in men and women taking PPIs, with the highest risk in patients who have taken higher than standard doses for >4 years.3-6 Concomitant risk factors (alcohol abuse, cigarette smoking, diabetes, and neurologic or renal disease) may increase fracture risk.6 But other retrospective studies, as well as prospective studies, have found no significant increase in fracture risk in patients taking PPIs,7-9 even after 5 years of therapy.7 However, some studies that failed to find an increased risk of osteoporosis with PPI use had a small number of subjects,8,9 resulting in a wide range in confidence intervals.
These findings, based on 6 retrospective case-control, cohort, and cross-sectional studies and 2 prospective cohort studies, are summarized in TABLE 1. No prospective randomized, blinded, controlled trials have examined the potential increased fracture risk associated with PPI use.
Do PPIs interfere with calcium metabolism?
Here, too, the findings are mixed. PPIs are known to inhibit the production and secretion of intragastric hydrochloric acid, which mediates small intestinal absorption of calcium,10 but evidence is conflicting about the role of intragastric hydrochloric acid in calcium absorption. Osteoclasts also have proton pumps, and some researchers have suggested that PPIs have the potential to limit the activity of these proton pumps, leading to reduced bone resorption.11
To date, the only studies that have examined the impact of PPIs on intestinal calcium absorption were limited by the health status of the participants—all either had renal failure and were on hemodialysis or had hypo- or achlorhydria, chronic conditions known to adversely affect calcium metabolism.12 Long-term randomized, double-blinded, placebo-controlled trials are needed to determine whether PPIs adversely affect intestinal calcium absorption and result in bone resorption abnormalities and increased fracture risk.
A closer look at the data
The varying responses associated with PPI dose and duration and the possibility that acid inhibition may decrease calcium absorption support a causal association between PPI use and fracture risk. But the low magnitude of the proposed association (most odds ratios <2) and the lack of data assessing potentially confounding factors limit evidence of causality.3,5,6,9 One key limitation of the earlier studies is that they were not designed to define the specific mechanism underlying the association between PPI therapy and fracture risk.
Older studies suggest a causal relationship
Two case-control studies3,4 found a causal association between PPI use and fracture risk, but one of them failed to identify either a dose-response or a duration-response effect.4 And neither study was designed to define underlying mechanisms to explain the potential association between fracture risk and PPI therapy.
A retrospective matched cohort study5 found an increase in the overall risk of fracture among patients with ≥7 years of PPI therapy and an in-creased risk of hip fracture with ≥5 years of therapy, but short-term risk of fracture was not found to be significant. The results of this study suggest that the risk of osteoporotic fracture increases with duration of exposure to PPI therapy, but not in a dose-dependent fashion.
Newer data are less worrisome
The results of a retrospective cross-sectional trial, published last year, are more reassuring. The researchers determined via univariate analysis that PPI use was associated with a lower risk of osteoporosis, both at the lumbar spine (for all levels of PPI use) and the hip (in patients who had taken more than 1500 standard PPI doses over the previous 5 years).7
This finding—that increasing intensity (both longer duration and higher dosage) of PPI exposure is not associated with an increased risk of osteoporosis—contrasts with results of the authors’ earlier study.5 This may be because they monitored annualized changes in BMD and were able to detect significant changes in other medications participants were taking that might affect bone loss or gain. That allowed them to validate their findings regarding a lack of true association between bone loss and PPI use, the authors reported.
A matched, nested case-control trial8 determined that the use of PPIs does not increase the risk of hip fracture in patients without associated major risk factors (ie, alcohol dependence, underlying neurologic disease, accidental falls, and senility). The researchers suggested that the difference between their findings and those of an earlier nested case-control study3 could mean that the increased risk of hip fracture found in the older study occurred only among PPI users with definable risk factors for hip fracture.
Recent results from the Women’s Health Initiative (WHI) suggest that in postmenopausal women, PPI use is not associated with hip fractures. The WHI did, however, find a modest association between PPI use and clinical spine, forearm, or wrist fracture, as well as total fractures.13 Compared with previous trials, this large cohort study had a large number of fracture events and assessed confounding factors that had not been addressed, including calcium intake. It also was the first trial to assess associations between BMD and fracture risk relative to PPI dosing. Although no specific conclusion was reported, the researchers did not find evidence of dose dependence.
A reasonable approach to PPI use
A consensus statement from the FDA2 and the authors of 2 meta-analyses14,15 recommend that PPIs be used only for appropriate indications—GERD, peptic ulcer disease, dyspepsia, and treatment of Helicobacter pylori—and not in higher doses or for longer periods than are necessary to achieve the desired results.
Whenever possible, implement step-down therapy to the lowest effective dose or prescribe an H2RA rather than a PPI. Both are cost-effective ways to treat most patients with upper GI symptoms.2 It is important, too, to advise elderly patients who require long-term, high-dose PPI therapy to increase their dietary and/or supplemental calcium intake, to recommend DEXA scans for individuals at risk for osteoporosis, and to counsel patients who suffer from GI distress to avoid foods that are known to exacerbate symptoms (TABLE 2).16
TABLE 2
GERD and diet: Foods that worsen symptoms16
| Alcohol |
| Caffeine-containing beverages |
| Citrus fruits |
| Chocolate |
| Fried and fatty foods |
| Garlic and onions |
| Mint flavorings |
| Spicy foods |
| Tomato-based foods (eg, chili, pizza, spaghetti sauce, salsa) |
CASE 1 Damian
You talk to Damian about the association between prolonged PPI therapy and fracture risk and stress the need for dietary changes and lifestyle modifications, particularly smoking cessation. On a return visit several months later, he reports that he has stopped smoking and cut way back on alcohol consumption, and eats fast food less frequently. As a result, he no longer requires chronic use of PPI therapy, and now takes omeprazole only when he has symptoms of GERD—usually, after indulging in fried or fatty foods.
CASE 2 Estella
Estella has severe GERD and erosive esophagitis and will probably need lifelong PPI therapy to adequately control her symptoms. After a detailed discussion of potential risks vs benefits of PPIs, she agrees to a DEXA scan to evaluate for osteoporosis. Her test results show osteopenia in the lumbar spine and femoral neck, but no evidence of osteoporosis. You advise her to increase her consumption of calcium and to undergo DEXA scanning in another 2 years.
CORRESPONDENCE
Joel J. Heidelbaugh, MD, FAAFP, FACG, Ypsilanti Health Center, 200 Arnet Suite 200, Ypsilanti, MI 48198; jheidel@med.umich.edu
• For most patients with chronic heartburn and regurgitation, step-down therapy to the lowest effective dose of proton pump inhibitors (PPIs) or treatment with a histamine-2 receptor antagonist (H2RA) is a reasonable, cost-effective approach. A
• Advise elderly patients who require long-term, high-dose PPI therapy to increase their dietary and/or supplemental calcium intake. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 Damian F,* a 39-year-old construction worker who takes omeprazole for chronic gastroesophageal reflux disease (GERD), comes in to request a refill. He’s had several accidents in recent years—he fell off a ladder on one occasion, and went down a flight of stairs on another—but none that resulted in significant trauma. Damian admits that he could better control his GERD symptoms by avoiding spicy and fatty foods, limiting alcohol consumption, and quitting smoking, but takes omeprazole nearly every day instead.
CASE 2 Estella G,* a 71-year-old retiree, has been on continuous proton pump inhibitor (PPI) therapy for chronic GERD and erosive esophagitis for nearly 20 years. The patient is a frail woman (body mass index=19.8 kg/m2) and a former smoker (1½ packs a day), both of which increase her risk of osteoporosis. But she has never had a dual energy x-ray absorptiometry (DEXA) scan.
*These cases are based on real patients in my practice, but their names and details have been changed to protect their identity.
Proton pump inhibitors (PPIs) are one of the most commonly used prescription drug categories in the United States,1 but they have been associated with an increase in fracture risk. A US Food and Drug Administration (FDA) safety update issued in March 2011 noted that there is little problem with the lower doses and shorter duration for which over-the-counter PPIs are intended, but patients who take higher-dose prescription PPIs or take prescription PPIs for more than a year may be at greater risk.2
If Damian and Estella were your patients, would you continue to prescribe PPI therapy or offer them alternatives? How should you treat other patients with chronic upper gastrointestinal (GI) distress? The evidence review that follows can help you answer those questions.
How high is the risk? Evidence is mixed (or lacking)
Several retrospective studies have demonstrated a modest increased risk for hip, spine, and wrist fractures in men and women taking PPIs, with the highest risk in patients who have taken higher than standard doses for >4 years.3-6 Concomitant risk factors (alcohol abuse, cigarette smoking, diabetes, and neurologic or renal disease) may increase fracture risk.6 But other retrospective studies, as well as prospective studies, have found no significant increase in fracture risk in patients taking PPIs,7-9 even after 5 years of therapy.7 However, some studies that failed to find an increased risk of osteoporosis with PPI use had a small number of subjects,8,9 resulting in a wide range in confidence intervals.
These findings, based on 6 retrospective case-control, cohort, and cross-sectional studies and 2 prospective cohort studies, are summarized in TABLE 1. No prospective randomized, blinded, controlled trials have examined the potential increased fracture risk associated with PPI use.
Do PPIs interfere with calcium metabolism?
Here, too, the findings are mixed. PPIs are known to inhibit the production and secretion of intragastric hydrochloric acid, which mediates small intestinal absorption of calcium,10 but evidence is conflicting about the role of intragastric hydrochloric acid in calcium absorption. Osteoclasts also have proton pumps, and some researchers have suggested that PPIs have the potential to limit the activity of these proton pumps, leading to reduced bone resorption.11
To date, the only studies that have examined the impact of PPIs on intestinal calcium absorption were limited by the health status of the participants—all either had renal failure and were on hemodialysis or had hypo- or achlorhydria, chronic conditions known to adversely affect calcium metabolism.12 Long-term randomized, double-blinded, placebo-controlled trials are needed to determine whether PPIs adversely affect intestinal calcium absorption and result in bone resorption abnormalities and increased fracture risk.
A closer look at the data
The varying responses associated with PPI dose and duration and the possibility that acid inhibition may decrease calcium absorption support a causal association between PPI use and fracture risk. But the low magnitude of the proposed association (most odds ratios <2) and the lack of data assessing potentially confounding factors limit evidence of causality.3,5,6,9 One key limitation of the earlier studies is that they were not designed to define the specific mechanism underlying the association between PPI therapy and fracture risk.
Older studies suggest a causal relationship
Two case-control studies3,4 found a causal association between PPI use and fracture risk, but one of them failed to identify either a dose-response or a duration-response effect.4 And neither study was designed to define underlying mechanisms to explain the potential association between fracture risk and PPI therapy.
A retrospective matched cohort study5 found an increase in the overall risk of fracture among patients with ≥7 years of PPI therapy and an in-creased risk of hip fracture with ≥5 years of therapy, but short-term risk of fracture was not found to be significant. The results of this study suggest that the risk of osteoporotic fracture increases with duration of exposure to PPI therapy, but not in a dose-dependent fashion.
Newer data are less worrisome
The results of a retrospective cross-sectional trial, published last year, are more reassuring. The researchers determined via univariate analysis that PPI use was associated with a lower risk of osteoporosis, both at the lumbar spine (for all levels of PPI use) and the hip (in patients who had taken more than 1500 standard PPI doses over the previous 5 years).7
This finding—that increasing intensity (both longer duration and higher dosage) of PPI exposure is not associated with an increased risk of osteoporosis—contrasts with results of the authors’ earlier study.5 This may be because they monitored annualized changes in BMD and were able to detect significant changes in other medications participants were taking that might affect bone loss or gain. That allowed them to validate their findings regarding a lack of true association between bone loss and PPI use, the authors reported.
A matched, nested case-control trial8 determined that the use of PPIs does not increase the risk of hip fracture in patients without associated major risk factors (ie, alcohol dependence, underlying neurologic disease, accidental falls, and senility). The researchers suggested that the difference between their findings and those of an earlier nested case-control study3 could mean that the increased risk of hip fracture found in the older study occurred only among PPI users with definable risk factors for hip fracture.
Recent results from the Women’s Health Initiative (WHI) suggest that in postmenopausal women, PPI use is not associated with hip fractures. The WHI did, however, find a modest association between PPI use and clinical spine, forearm, or wrist fracture, as well as total fractures.13 Compared with previous trials, this large cohort study had a large number of fracture events and assessed confounding factors that had not been addressed, including calcium intake. It also was the first trial to assess associations between BMD and fracture risk relative to PPI dosing. Although no specific conclusion was reported, the researchers did not find evidence of dose dependence.
A reasonable approach to PPI use
A consensus statement from the FDA2 and the authors of 2 meta-analyses14,15 recommend that PPIs be used only for appropriate indications—GERD, peptic ulcer disease, dyspepsia, and treatment of Helicobacter pylori—and not in higher doses or for longer periods than are necessary to achieve the desired results.
Whenever possible, implement step-down therapy to the lowest effective dose or prescribe an H2RA rather than a PPI. Both are cost-effective ways to treat most patients with upper GI symptoms.2 It is important, too, to advise elderly patients who require long-term, high-dose PPI therapy to increase their dietary and/or supplemental calcium intake, to recommend DEXA scans for individuals at risk for osteoporosis, and to counsel patients who suffer from GI distress to avoid foods that are known to exacerbate symptoms (TABLE 2).16
TABLE 2
GERD and diet: Foods that worsen symptoms16
| Alcohol |
| Caffeine-containing beverages |
| Citrus fruits |
| Chocolate |
| Fried and fatty foods |
| Garlic and onions |
| Mint flavorings |
| Spicy foods |
| Tomato-based foods (eg, chili, pizza, spaghetti sauce, salsa) |
CASE 1 Damian
You talk to Damian about the association between prolonged PPI therapy and fracture risk and stress the need for dietary changes and lifestyle modifications, particularly smoking cessation. On a return visit several months later, he reports that he has stopped smoking and cut way back on alcohol consumption, and eats fast food less frequently. As a result, he no longer requires chronic use of PPI therapy, and now takes omeprazole only when he has symptoms of GERD—usually, after indulging in fried or fatty foods.
CASE 2 Estella
Estella has severe GERD and erosive esophagitis and will probably need lifelong PPI therapy to adequately control her symptoms. After a detailed discussion of potential risks vs benefits of PPIs, she agrees to a DEXA scan to evaluate for osteoporosis. Her test results show osteopenia in the lumbar spine and femoral neck, but no evidence of osteoporosis. You advise her to increase her consumption of calcium and to undergo DEXA scanning in another 2 years.
CORRESPONDENCE
Joel J. Heidelbaugh, MD, FAAFP, FACG, Ypsilanti Health Center, 200 Arnet Suite 200, Ypsilanti, MI 48198; jheidel@med.umich.edu
1. Bartholow M. Top 200 prescription drugs of 2009. May 11, 2010. Pharmacy Times. Available at: http://www.pharmacytimes. http://www.pharmacytimes.com/publications/issue/2010/May2010/RxFocusTopDrugs-0510. Accessed April 8, 2011.
2. US Food and Drug Administration. FDA Drug Safety Communication: Possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. May 25, 2010; March 23, 2011 update. Available at: http://www.fda.gov/Drugs/DrugSafety/postmarketdrugsafetyInformationforpatientsandproviders/ucm213206.htm#SafetyAnnouncement. Accessed March 24, 2011.
3. Yang YX, Lewis JD, Epstein S, et al. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947-2953.
4. Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine h(2) receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int. 2006;79:76-83.
5. Targownik LE, Lix LM, Metge CJ. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ. 2008;179:319-326.
6. Corley DA, Kubo A, Zhao W, et al. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology. 2010;139:93-101.
7. Targownik LE, Lix LM, Leung S, et al. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology. 2010;138:896-904.
8. Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy. 2008;28:951-959.
9. Yu EW, Blackwell T, Ensrud KE, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int. 2008;83:251-259.
10. Bo-Linn GW, Davis GR, Buddrus DJ, et al. An evaluation of the importance of gastric acid secretion in the absorption of dietary calcium. J Clin Invest. 1984;73:640-647.
11. Farina C, Gagliardi S. Selective inhibition of osteoclast vacuolar H+-ATPase. Curr Pharm Des. 2002;8:2033-2048.
12. Insogna KL. The effect of proton pump-inhibiting drugs on mineral metabolism. Am J Gastroenterol. 2009;104(suppl 2):S2-S4.
13. Gray SL, LaCroix AZ, Larson L, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women. Arch Intern Med. 2010;170:765-771.
14. Laine L. Proton pump inhibitors and bone fractures? Am J Gastroenterol. 2009;104(suppl 2):S21-S26.
15. Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors: a review of cost-effectiveness and risk. Am J Gastroenterol.;2009;104(suppl 2):S27-S32.
16. National Digestive Diseases Information Clearinghouse. Heartburn, gastroesophageal reflux (GER), and gastroesophageal reflux disease (GERD). Available at: http://digestive.niddk.nih.gov/ddiseases/pubs/gerd. Accessed April 18, 2011.
1. Bartholow M. Top 200 prescription drugs of 2009. May 11, 2010. Pharmacy Times. Available at: http://www.pharmacytimes. http://www.pharmacytimes.com/publications/issue/2010/May2010/RxFocusTopDrugs-0510. Accessed April 8, 2011.
2. US Food and Drug Administration. FDA Drug Safety Communication: Possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. May 25, 2010; March 23, 2011 update. Available at: http://www.fda.gov/Drugs/DrugSafety/postmarketdrugsafetyInformationforpatientsandproviders/ucm213206.htm#SafetyAnnouncement. Accessed March 24, 2011.
3. Yang YX, Lewis JD, Epstein S, et al. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947-2953.
4. Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine h(2) receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int. 2006;79:76-83.
5. Targownik LE, Lix LM, Metge CJ. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ. 2008;179:319-326.
6. Corley DA, Kubo A, Zhao W, et al. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology. 2010;139:93-101.
7. Targownik LE, Lix LM, Leung S, et al. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology. 2010;138:896-904.
8. Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy. 2008;28:951-959.
9. Yu EW, Blackwell T, Ensrud KE, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int. 2008;83:251-259.
10. Bo-Linn GW, Davis GR, Buddrus DJ, et al. An evaluation of the importance of gastric acid secretion in the absorption of dietary calcium. J Clin Invest. 1984;73:640-647.
11. Farina C, Gagliardi S. Selective inhibition of osteoclast vacuolar H+-ATPase. Curr Pharm Des. 2002;8:2033-2048.
12. Insogna KL. The effect of proton pump-inhibiting drugs on mineral metabolism. Am J Gastroenterol. 2009;104(suppl 2):S2-S4.
13. Gray SL, LaCroix AZ, Larson L, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women. Arch Intern Med. 2010;170:765-771.
14. Laine L. Proton pump inhibitors and bone fractures? Am J Gastroenterol. 2009;104(suppl 2):S21-S26.
15. Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors: a review of cost-effectiveness and risk. Am J Gastroenterol.;2009;104(suppl 2):S27-S32.
16. National Digestive Diseases Information Clearinghouse. Heartburn, gastroesophageal reflux (GER), and gastroesophageal reflux disease (GERD). Available at: http://digestive.niddk.nih.gov/ddiseases/pubs/gerd. Accessed April 18, 2011.
Managing ADHD in children: Are you doing enough?
• Side effects of psychostimulants can often be managed with monitoring, dose adjustment, a switch to another drug, or adjunctive therapy. A
• Weigh and measure a child being treated for ADHD twice a year; aberrant growth may indicate a need for a change in medication regimen. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Untreated attention deficit hyperactivity disorder (ADHD) can have serious academic, social, and psychological consequences, both for young patients and their parents. Diagnosis is based on criteria detailed in the Diagnostic and Statistical Manual of Mental Health Disorders, Fourth Edition Text Revision (DSM-IV-TR), with observations of the child’s behavior obtained from more than one setting.
Physicians should also consider the possibility of coexisting conditions, which could complicate diagnosis and subsequent attempts to treat the signs and symptoms of ADHD. Treatment is multifaceted, and will vary depending on severity, comorbidities, and the degree of compliance with nonpharmacologic modalities.
A comprehensive approach is called for
Managing pediatric ADHD in a primary care setting requires a comprehensive, goal-oriented treatment plan. The primary goal, as noted in the American Academy of Child and Adolescent Psychiatry (AACAP)’s ADHD guideline,1 is to maximize the child’s functioning, both in terms of an improvement in relationships and academic performance and a reduction of disruptive behavior. Parents and children should be integrated into community supports and school resources, the guideline recommends1 (strength of recommendation [SOR]: A).
Additional recommendations focus on patient (and parental) education, and on medication, monitoring, and follow-up (SOR: A). Physicians should:
Educate parents and patients about common ADHD symptoms and treatment strategies.
Initiate pharmacotherapy. Select an agent that is approved by the US Food and Drug Administration (FDA) for ADHD. These include the psychostimulants dextroamphetamine, D- and DL-methylphenidate, and mixed salts amphetamine; and atomoxetine, a noradrenergic reuptake inhibitor. (Central nervous system stimulants should be avoided in children with cardiac abnormalities, who are at increased risk of experiencing sympathomimetic effects.)
Familiarize themselves with medication side effects. Decreased appetite, insomnia, headache, abdominal pain, and irritable mood are the most common side effects of psychostimulants. Common side effects of atomoxetine include somnolence, anorexia, nausea, skin rash, and a mild increase in blood pressure or heart rate. Notably, there is a small risk of suicide associated with atomoxetine.
Monitor patients for the emergence and severity of side effects. Many of the side effects of stimulants are transient and can be managed through monitoring, as long as it does not compromise the patient’s health or interfere with daily living. Side effects can also be managed with dose adjustment, change of drug treatment, or adjunctive therapy.
Measure height and weight of the patient twice yearly. If a child’s height or weight crosses 2 percentiles on his or her growth curve, it may be an indication of aberrant growth—and a drug holiday or switching to a different medication should be considered.
Evaluate treatment success several times a year. The review should include behavior, academic progress, emergence of comorbid disorders, and the need for behavioral therapy and continuing pharmacotherapy. A lack of response to one psychostimulant is not predictive of the patient’s response to another, the AACAP emphasizes, and it is important to keep trying to find another medication until treatment goals are reached.1
If none of the FDA-approved ADHD medications has the desired results, the AACAP recommends (SOR: B):
- a referral to a cognitive behavioral therapist or child psychologist
- a trial with a medication that is not FDA-approved for ADHD, such as bupropion, a tricyclic antidepressant, or an alpha-agonist
- a reevaluation of the ADHD diagnosis, adherence to the treatment plan, and the presence of comorbid conditions.1
AAP stresses hands-on behavioral intervention
The American Academy of Pediatrics (AAP) also has a clinical practice guideline for the treatment of ADHD, issued in 2001.2 Its recommendations are similar to those of the AACAP. But AAP puts additional emphasis on parental training in behavioral therapy and classroom behavioral interventions, and considers both to be more effective than cognitive behavioral therapy (CBT).2
Virtual reality: A viable option?
Although conventional treatment of childhood ADHD has had considerable clinical success, other forms of treatment may be needed in some cases—if a child’s parents reject psychopharmacologic treatment, for example, or medication trials and traditional behavioral therapies, such as CBT, fail to bring the desired results.
Virtual reality (VR), a computer-generated 3-dimensional interactive system, is an emerging clinical tool. VR programs such as The Virtual Classroom3,4—in which a child is “immersed” in a simulated classroom setting—have shown promise for ADHD assessment and treatment.
Perhaps the biggest benefit of VR as an ADHD intervention is the opportunity for a clinician to place a patient in a virtual classroom, with tasks that require the child’s attention as well as distractors, such as conversation, ambient noise, and moving objects. Another advantage is the ability to integrate traditional assessment tools (Continuous Performance Tasks, for example) and treatment modalities, such as CBT.5 This can be accomplished through a graphic display of a child’s performance during a VR session, which the therapist can use as part of the therapeutic process.3 And VR has no side effects.
Several facilities are either using or experimenting with VR for ADHD. More information is available from the Virtual Reality Medical Center at http://www.vrphobia.com/adhd.htm.
CORRESPONDENCE
Keith B. Holten, MD, Berger Health System, 600 North Pickaway Street, Circleville, OH 43113; keith.holten@bergerhealth.com
1. Pliszka S. AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894-921.
2. American Academy of Pediatrics. Subcommittee on Attention-Deficit/Hyperactivity Disorder. Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108:1033-1044.
3. Rizzo AA, Buckwalter JG, Humphrey L, et al. The virtual classroom: a virtual environment for the assessment and rehabilitation of attention deficits. CyberPsych Behav. 2000;3:483-499.
4. Rizzo AA, Klimchuk D, Mitura R, et al. A virtual reality scenario for all seasons: the virtual classroom. CNS Spectr. 2006;11:35-44.
5. Pollak Y, Weiss PL, Rizzo AA, et al. The utility of a continuous performance test embedded in virtual reality in measuring ADHD-related deficits. J Dev Behav Pediatr. 2009;30:2-6.
• Side effects of psychostimulants can often be managed with monitoring, dose adjustment, a switch to another drug, or adjunctive therapy. A
• Weigh and measure a child being treated for ADHD twice a year; aberrant growth may indicate a need for a change in medication regimen. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Untreated attention deficit hyperactivity disorder (ADHD) can have serious academic, social, and psychological consequences, both for young patients and their parents. Diagnosis is based on criteria detailed in the Diagnostic and Statistical Manual of Mental Health Disorders, Fourth Edition Text Revision (DSM-IV-TR), with observations of the child’s behavior obtained from more than one setting.
Physicians should also consider the possibility of coexisting conditions, which could complicate diagnosis and subsequent attempts to treat the signs and symptoms of ADHD. Treatment is multifaceted, and will vary depending on severity, comorbidities, and the degree of compliance with nonpharmacologic modalities.
A comprehensive approach is called for
Managing pediatric ADHD in a primary care setting requires a comprehensive, goal-oriented treatment plan. The primary goal, as noted in the American Academy of Child and Adolescent Psychiatry (AACAP)’s ADHD guideline,1 is to maximize the child’s functioning, both in terms of an improvement in relationships and academic performance and a reduction of disruptive behavior. Parents and children should be integrated into community supports and school resources, the guideline recommends1 (strength of recommendation [SOR]: A).
Additional recommendations focus on patient (and parental) education, and on medication, monitoring, and follow-up (SOR: A). Physicians should:
Educate parents and patients about common ADHD symptoms and treatment strategies.
Initiate pharmacotherapy. Select an agent that is approved by the US Food and Drug Administration (FDA) for ADHD. These include the psychostimulants dextroamphetamine, D- and DL-methylphenidate, and mixed salts amphetamine; and atomoxetine, a noradrenergic reuptake inhibitor. (Central nervous system stimulants should be avoided in children with cardiac abnormalities, who are at increased risk of experiencing sympathomimetic effects.)
Familiarize themselves with medication side effects. Decreased appetite, insomnia, headache, abdominal pain, and irritable mood are the most common side effects of psychostimulants. Common side effects of atomoxetine include somnolence, anorexia, nausea, skin rash, and a mild increase in blood pressure or heart rate. Notably, there is a small risk of suicide associated with atomoxetine.
Monitor patients for the emergence and severity of side effects. Many of the side effects of stimulants are transient and can be managed through monitoring, as long as it does not compromise the patient’s health or interfere with daily living. Side effects can also be managed with dose adjustment, change of drug treatment, or adjunctive therapy.
Measure height and weight of the patient twice yearly. If a child’s height or weight crosses 2 percentiles on his or her growth curve, it may be an indication of aberrant growth—and a drug holiday or switching to a different medication should be considered.
Evaluate treatment success several times a year. The review should include behavior, academic progress, emergence of comorbid disorders, and the need for behavioral therapy and continuing pharmacotherapy. A lack of response to one psychostimulant is not predictive of the patient’s response to another, the AACAP emphasizes, and it is important to keep trying to find another medication until treatment goals are reached.1
If none of the FDA-approved ADHD medications has the desired results, the AACAP recommends (SOR: B):
- a referral to a cognitive behavioral therapist or child psychologist
- a trial with a medication that is not FDA-approved for ADHD, such as bupropion, a tricyclic antidepressant, or an alpha-agonist
- a reevaluation of the ADHD diagnosis, adherence to the treatment plan, and the presence of comorbid conditions.1
AAP stresses hands-on behavioral intervention
The American Academy of Pediatrics (AAP) also has a clinical practice guideline for the treatment of ADHD, issued in 2001.2 Its recommendations are similar to those of the AACAP. But AAP puts additional emphasis on parental training in behavioral therapy and classroom behavioral interventions, and considers both to be more effective than cognitive behavioral therapy (CBT).2
Virtual reality: A viable option?
Although conventional treatment of childhood ADHD has had considerable clinical success, other forms of treatment may be needed in some cases—if a child’s parents reject psychopharmacologic treatment, for example, or medication trials and traditional behavioral therapies, such as CBT, fail to bring the desired results.
Virtual reality (VR), a computer-generated 3-dimensional interactive system, is an emerging clinical tool. VR programs such as The Virtual Classroom3,4—in which a child is “immersed” in a simulated classroom setting—have shown promise for ADHD assessment and treatment.
Perhaps the biggest benefit of VR as an ADHD intervention is the opportunity for a clinician to place a patient in a virtual classroom, with tasks that require the child’s attention as well as distractors, such as conversation, ambient noise, and moving objects. Another advantage is the ability to integrate traditional assessment tools (Continuous Performance Tasks, for example) and treatment modalities, such as CBT.5 This can be accomplished through a graphic display of a child’s performance during a VR session, which the therapist can use as part of the therapeutic process.3 And VR has no side effects.
Several facilities are either using or experimenting with VR for ADHD. More information is available from the Virtual Reality Medical Center at http://www.vrphobia.com/adhd.htm.
CORRESPONDENCE
Keith B. Holten, MD, Berger Health System, 600 North Pickaway Street, Circleville, OH 43113; keith.holten@bergerhealth.com
• Side effects of psychostimulants can often be managed with monitoring, dose adjustment, a switch to another drug, or adjunctive therapy. A
• Weigh and measure a child being treated for ADHD twice a year; aberrant growth may indicate a need for a change in medication regimen. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Untreated attention deficit hyperactivity disorder (ADHD) can have serious academic, social, and psychological consequences, both for young patients and their parents. Diagnosis is based on criteria detailed in the Diagnostic and Statistical Manual of Mental Health Disorders, Fourth Edition Text Revision (DSM-IV-TR), with observations of the child’s behavior obtained from more than one setting.
Physicians should also consider the possibility of coexisting conditions, which could complicate diagnosis and subsequent attempts to treat the signs and symptoms of ADHD. Treatment is multifaceted, and will vary depending on severity, comorbidities, and the degree of compliance with nonpharmacologic modalities.
A comprehensive approach is called for
Managing pediatric ADHD in a primary care setting requires a comprehensive, goal-oriented treatment plan. The primary goal, as noted in the American Academy of Child and Adolescent Psychiatry (AACAP)’s ADHD guideline,1 is to maximize the child’s functioning, both in terms of an improvement in relationships and academic performance and a reduction of disruptive behavior. Parents and children should be integrated into community supports and school resources, the guideline recommends1 (strength of recommendation [SOR]: A).
Additional recommendations focus on patient (and parental) education, and on medication, monitoring, and follow-up (SOR: A). Physicians should:
Educate parents and patients about common ADHD symptoms and treatment strategies.
Initiate pharmacotherapy. Select an agent that is approved by the US Food and Drug Administration (FDA) for ADHD. These include the psychostimulants dextroamphetamine, D- and DL-methylphenidate, and mixed salts amphetamine; and atomoxetine, a noradrenergic reuptake inhibitor. (Central nervous system stimulants should be avoided in children with cardiac abnormalities, who are at increased risk of experiencing sympathomimetic effects.)
Familiarize themselves with medication side effects. Decreased appetite, insomnia, headache, abdominal pain, and irritable mood are the most common side effects of psychostimulants. Common side effects of atomoxetine include somnolence, anorexia, nausea, skin rash, and a mild increase in blood pressure or heart rate. Notably, there is a small risk of suicide associated with atomoxetine.
Monitor patients for the emergence and severity of side effects. Many of the side effects of stimulants are transient and can be managed through monitoring, as long as it does not compromise the patient’s health or interfere with daily living. Side effects can also be managed with dose adjustment, change of drug treatment, or adjunctive therapy.
Measure height and weight of the patient twice yearly. If a child’s height or weight crosses 2 percentiles on his or her growth curve, it may be an indication of aberrant growth—and a drug holiday or switching to a different medication should be considered.
Evaluate treatment success several times a year. The review should include behavior, academic progress, emergence of comorbid disorders, and the need for behavioral therapy and continuing pharmacotherapy. A lack of response to one psychostimulant is not predictive of the patient’s response to another, the AACAP emphasizes, and it is important to keep trying to find another medication until treatment goals are reached.1
If none of the FDA-approved ADHD medications has the desired results, the AACAP recommends (SOR: B):
- a referral to a cognitive behavioral therapist or child psychologist
- a trial with a medication that is not FDA-approved for ADHD, such as bupropion, a tricyclic antidepressant, or an alpha-agonist
- a reevaluation of the ADHD diagnosis, adherence to the treatment plan, and the presence of comorbid conditions.1
AAP stresses hands-on behavioral intervention
The American Academy of Pediatrics (AAP) also has a clinical practice guideline for the treatment of ADHD, issued in 2001.2 Its recommendations are similar to those of the AACAP. But AAP puts additional emphasis on parental training in behavioral therapy and classroom behavioral interventions, and considers both to be more effective than cognitive behavioral therapy (CBT).2
Virtual reality: A viable option?
Although conventional treatment of childhood ADHD has had considerable clinical success, other forms of treatment may be needed in some cases—if a child’s parents reject psychopharmacologic treatment, for example, or medication trials and traditional behavioral therapies, such as CBT, fail to bring the desired results.
Virtual reality (VR), a computer-generated 3-dimensional interactive system, is an emerging clinical tool. VR programs such as The Virtual Classroom3,4—in which a child is “immersed” in a simulated classroom setting—have shown promise for ADHD assessment and treatment.
Perhaps the biggest benefit of VR as an ADHD intervention is the opportunity for a clinician to place a patient in a virtual classroom, with tasks that require the child’s attention as well as distractors, such as conversation, ambient noise, and moving objects. Another advantage is the ability to integrate traditional assessment tools (Continuous Performance Tasks, for example) and treatment modalities, such as CBT.5 This can be accomplished through a graphic display of a child’s performance during a VR session, which the therapist can use as part of the therapeutic process.3 And VR has no side effects.
Several facilities are either using or experimenting with VR for ADHD. More information is available from the Virtual Reality Medical Center at http://www.vrphobia.com/adhd.htm.
CORRESPONDENCE
Keith B. Holten, MD, Berger Health System, 600 North Pickaway Street, Circleville, OH 43113; keith.holten@bergerhealth.com
1. Pliszka S. AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894-921.
2. American Academy of Pediatrics. Subcommittee on Attention-Deficit/Hyperactivity Disorder. Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108:1033-1044.
3. Rizzo AA, Buckwalter JG, Humphrey L, et al. The virtual classroom: a virtual environment for the assessment and rehabilitation of attention deficits. CyberPsych Behav. 2000;3:483-499.
4. Rizzo AA, Klimchuk D, Mitura R, et al. A virtual reality scenario for all seasons: the virtual classroom. CNS Spectr. 2006;11:35-44.
5. Pollak Y, Weiss PL, Rizzo AA, et al. The utility of a continuous performance test embedded in virtual reality in measuring ADHD-related deficits. J Dev Behav Pediatr. 2009;30:2-6.
1. Pliszka S. AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894-921.
2. American Academy of Pediatrics. Subcommittee on Attention-Deficit/Hyperactivity Disorder. Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108:1033-1044.
3. Rizzo AA, Buckwalter JG, Humphrey L, et al. The virtual classroom: a virtual environment for the assessment and rehabilitation of attention deficits. CyberPsych Behav. 2000;3:483-499.
4. Rizzo AA, Klimchuk D, Mitura R, et al. A virtual reality scenario for all seasons: the virtual classroom. CNS Spectr. 2006;11:35-44.
5. Pollak Y, Weiss PL, Rizzo AA, et al. The utility of a continuous performance test embedded in virtual reality in measuring ADHD-related deficits. J Dev Behav Pediatr. 2009;30:2-6.
Opioids for osteoarthritis? Weighing benefits and risks: A Cochrane Musculoskeletal Group review
Osteoarthritis (OA) affects nearly 27 million Americans, or about 12% of US adults.1 As the average age of the population increases, the prevalence and burden of this debilitating disorder continue to rise.2
The American College of Rheumatology (ACR)’s guidelines for the medical management of OA of the hip and knee, last updated in 2000,3 focus on controlling pain and improving function and health-related quality of life while minimizing the toxic effects of therapy. The guidelines recommend tramadol—an atypical opioid with 2 distinct mechanisms of action4—for moderate-to-severe pain in OA patients who either have contraindications to COX-2 inhibitors and non steroidal anti-inflammatory drugs (NSAIDs) or have failed to respond to previous oral therapy. Patients with severe pain who don’t respond to or are unable to tolerate tramadol may be candidates for more traditional opioid therapy, the guidelines indicate.3
In recent years, however, the use (and abuse) of opioids has skyrocketed. Between 1997 and 2007, US per capita retail purchases of hydrocodone and oxycodone increased 4-fold and 9-fold, respectively.5 In a similar time frame (1996-2006), the number of deaths from opioid overdose more than tripled, going from 4000 to 13,800 annually.6 Not surprisingly, the use of narcotics for noncancer pain remains controversial.7,8 But inadequately treated pain continues to be a serious public health problem, as well.9
This is the third in a series of articles based on the findings of the Cochrane Musculoskeletal Group (CMSG). One of the largest groups in the Cochrane Collaboration, the CMSG synthesizes the results of clinical trials to determine whether interventions for the prevention, treatment, and rehabilitation of musculoskeletal disorders are safe and effective. In this installment, the reviewers use detailed analysis, as well as a case study, to bring their findings to the attention of family physicians in a practical, clinically relevant context.
In 2006 and 2009, respectively, the Cochrane Collaboration published systematic reviews of tramadol (for OA in any joint)10 and other oral and transdermal opioids (for OA of the hip or knee).11 The reviewers’ findings, presented here along with data from more recent trials, can help ensure that you prescribe opioids for patients with OA only when their use is clinically appropriate and evidence-based. We’ve also included a case study (see page 211), so you can assess your knowledge and clinical skills.
CASE Carol J, an active 72-year-old, was diagnosed with OA in her right hip 5 years ago. Now she reports that the pain is getting progressively worse, making it harder and harder to turn over in bed at night or get in and out of the car. The pain is particularly bad at night, Carol says, and she’s had interrupted sleep for months. The patient has taken acetaminophen for the pain since her OA diagnosis, but now finds the analgesic is ineffective, even at the maximum dose of 4 g per day.
Carol has hypertension, which was difficult to manage until she began taking a combination ACE inhibitor/diuretic. She also has moderate renal impairment and mild chronic obstructive pulmonary disease, which limits her exercise tolerance. Nonetheless, she continues to smoke. The patient lives with and cares for her husband, who has Alzheimer’s disease, and worries about her ability to continue to care for him.
What are her treatment options?
Full-dose acetaminophen is no longer helping Carol, and NSAIDs are contraindicated because she takes an ACE inhibitor/diuretic and has moderate renal impairment. Increasing exercise will be a challenge. You strongly encourage her to stop smoking, emphasizing that this is particularly important to reduce the risk involved with any future joint replacement surgery.
Oral dosing options for the patient include:
- prescribing tramadol, starting with a low-dose immediate-release formulation taken one hour before bedtime (The controlled-release formulation is not advisable, given her age and renal function.) or
- adding a traditional opioid, eg, codeine 30 to 60 mg every 6 hours as needed, to her regular acetaminophen regimen.
Codeine and hydrocodone are available in combination preparations with acetaminophen, which may be convenient for some patients. However, hydrocodone was not one of the opioids tested in the trials included in the Cochrane reviews, and evidence of its use in OA is lacking.
Intra-articular corticosteroid injection, performed under imaging guidance, is another option for Carol. You explain that although there have been no studies of intra-articular corticosteroid injections for OA of the hip, these are used occasionally and may provide short-term symptom relief.7
You emphasize that surgery is likely to give her the best long-term outcome. In view of the patient’s circumstances and the need to care for her husband, however, you prescribe tramadol 50 mg at night. (Because of Carol’s age, renal impairment, and the possible adverse effects, it’s wise to start with a low dose and titrate upwards.) You warn her of the risks associated with opioids and advise her to alert your office staff if she experiences any adverse effects.
Before the patient leaves, you arrange an orthopedic consult and schedule a return visit for the following week. At your urging, she agrees to look into respite options for her husband.
Tramadol produces modest results—or none at all
The tramadol review10 included 11 randomized controlled trials (RCTs) with a total of 1019 participants who took tramadol or tramadol/acetaminophen (paracetamol) and 920 controls. In 6 of the 11 studies, the controls received placebo; the remaining 5 trials used “active controls,” with the control group for each RCT receiving a different analgesic. (To learn more about the methodology, see “How the reviews were conducted”.)
Placebo-controlled trials. Compared with patients on placebo, those receiving tramadol had an average absolute reduction in pain of 8.5 mm on a 0-100 mm visual analog scale (VAS) (95% confidence interval [CI], -12.05 to -4.9). That small benefit, however, did not reach the level defined as the minimal perceptible clinical improvement—a reduction of 9.7 mm on Western Ontario and McMaster Universities (WOMAC)’s OA pain subscale.12
Active-controlled trials. In the 5 RCTs comparing tramadol with another active agent, tramadol proved to be no better than the control drug. In fact, in a study of tramadol vs acetaminophen, 500 mg acetaminophen 3 times a day provided more pain relief than 50 mg tramadol 3 times a day.13 Although this was a small (N=20), short-term (7-day) study, this finding is notable because participants took less than the usual acetaminophen dose of 1 g up to 4 times a day.
Nor was tramadol superior to the agents it was compared with in the 4 other active-controlled trials—dihydrocodeine,14 dextropropoxyphene,15 pentazocine,16 and diclofenac17—in reducing pain intensity. It is important to keep in mind, however, that in each of these studies, both the quantity and quality of the evidence was limited. (Two studies did not use numerical scales,14,16 for example; all had methodological issues; and none lasted longer than 28 days.)
The Cochrane Musculoskeletal Group conducted a review of tramadol and a review of other oral opioids and transdermal fentanyl for the treatment of osteoarthritis (OA). Both reviews featured pain, function, and safety as primary outcomes. The tramadol review included randomized controlled trials (RCTs) for OA in any joint, while the oral and transdermal opioid review included randomized and quasi-randomized trials of treatment for OA of the hip or knee. Other parameters follow:
The tramadol review included 11 RCTs, with a total of 1019 participants receiving either tramadol alone or tramadol/acetaminophen (paracetamol) and 920 controls. In 6 of the 11 studies, the controls received placebo; the remaining 5 studies featured “active control.” That is, the control groups received acetaminophen 500 mg 3 times daily, diclofenac (25-50 mg up to 3 times daily on demand), dihydrocodeine 60 mg twice daily, dextropropoxyphene 100 mg 3 times daily, or pentazocine 50 mg 4 times per day. Because each of these agents was used in only one trial, the reviewers could not reach definitive conclusions about tramadol’s performance relative to other medications. The average number of participants in the tramadol and control groups was 91 and 80, respectively. The average length of follow-up was 35 days.
The 11 RCTs included in this review used a variety of pain scales to assess the results of tramadol, active control medications, and placebo. For comparative purposes, the reviewers pooled the results from studies that used numerical scales (0 to 100 and 0 to 10) to assess pain intensity. As a reference, we have used 9.7 and 9.3, respectively, determined by other researchers to be the minimal perceptible clinical improvements on the Western Ontario and McMaster Universities (WOMAC) pain and physical function 0-100 mm visual analog scales.12
The review of oral and transdermal opioids included 10 studies, with a total of 1541 patients receiving opioids and 727 receiving placebo.17 There were 3 trials of codeine (in 2 of the 3, a simple analgesic [acetaminophen 3000 mg/d or ibuprofen 1200 mg/d] was co-administered to both the treatment and control groups); other opioids included in the trials were oxycodone (4 trials), oxymorphone (2 trials), morphine (1 trial), and transdermal fentanyl (1 trial).
A modest boost in well-being
The reviewers measured function in 2 ways, focusing on both global improvement and improvement in physical function.
Global assessment. For the global assessment, the reviewers defined a treatment response as achieving at least a moderate improvement. By that standard, tramadol may improve overall well-being more than placebo. In the placebo-controlled trials, the number needed to treat (NNT) to elicit one treatment response was 6.
Three of the trials with active controls included global/functional assessments, and the results—bearing in mind the reduced quality and quantity of the evidence—were mixed. In a comparison of tramadol with dextropropoxyphene, tramadol increased the likelihood of moderate improvement by 38% (relative risk, 1.38 (95% CI, 1.15-1.67).10 In a trial of tramadol vs pentazocine, tramadol was more effective in reducing the duration of morning stiffness (by about 10 minutes), but not its severity. Tramadol was comparable with pentazocine in the 7 other measures of OA and function.16 In the tramadol-diclofenac study, both drugs were equally effective.17
Physical function. Four of the 6 placebo-controlled tramadol studies included in the Cochrane review used the WOMAC Index score, which included the physical function subscale. The tramadol group had a larger reduction in the score than the placebo group, by 0.34 mm (95% CI, -0.49 to -0.19). While this was equivalent to an 8.5% relative reduction in mean baseline score, it is still small compared with the minimal perceptible clinical improvement level of 9.3 mm on a 0-100 scale needed for the WOMAC physical function subscale. A similar improvement was reported for those taking tramadol compared with diclofenac—the only one of the active-controlled studies to report on physical function.17
Other opioids relieve pain, improve function—but how much?
The review of oral and transdermal opioids for OA11 encompassed 10 trials, with a total of 1541 patients receiving opioids and 727 on placebo. The opioids used in the trials were codeine, oxycodone, oxymorphone, morphine, and transdermal fentanyl. (For more details, see “How the reviews were conducted”.)
Pain. The trials included in the review used a variety of scales to measure pain, so the reviewers gauged results by the proportion of patients responding to treatment. Response was defined as a 50% improvement in pain score.
In the overall analysis, 35% of patients taking opioids responded to treatment, vs 31% of those on placebo—or 4 more patients in 100. That represents an NNT of 25. (A subgroup analysis did not demonstrate any significant differences in effect size among the opioids tested. In addition, the effect size was similar regardless of the potency of the opioid or the administration route.)
Function. Seven of the 10 trials (1794 participants, including both the treatment groups and controls) used validated function scores to measure physical function after 4 weeks of treatment. Here, too, the reviewers defined a treatment response as a 50% improvement in score.
Their finding? Opioids had a greater effect on function compared with placebo, equaling 0.7 on a WOMAC disability scale of 1 to 10. This means that about 3 more patients in 100 responded to treatment with opioids vs placebo—an NNT of 30.
But what about safety?
Opioids, including tramadol, are associated with adverse events (AEs), which may be minor or major. To determine when, or whether, the benefits outweigh the risks for treating patients with OA, both reviews reported on AEs and the number of participants who stopped taking the drug because of AEs.
AEs limit tramadol’s usefulness
While tramadol was more effective than placebo at reducing pain intensity, relieving symptoms, and improving function, the benefits were small—with an overall NNT of 6 (TABLE 1). This is similar to acetaminophen (NNT, 4-16),18 but with a greater downside.
Minor AEs. Four placebo-controlled trials reported on minor AEs.19-22 Those most commonly reported by patients taking tramadol were nausea, vomiting, dizziness, constipation, somnolence, tiredness, and headache.
Overall, 39% of those who received tramadol experienced minor AEs, compared with 18% of patients receiving placebo—an NNH of 5.10 Thus, tramadol’s NNH for minor AEs is equivalent to its NNT for pain relief. In active-controlled studies, there was a higher risk of minor AEs in those receiving tramadol compared with diclofenac or dextropropoxyphene, but a lower risk compared with those taking pentazocine.10
Major AEs. An analysis of the placebo-controlled trials revealed that 21% of those who received tramadol had major AEs—defined as an event that resulted in cessation of treatment—compared with 8% of those taking placebo. By this measure, the NNH was 8: One in 8 patients stopped taking tramadol because of a major AE.10
Among the active-controlled trials, participants taking tramadol were more likely to report a major AE compared with those receiving either diclofenac or dextropropoxyphene (NNH=5), but less likely compared with patients taking pentazocine. In a trial that compared tramadol alone with paracetamol, 2 out of 10 in the tramadol group discontinued treatment; none in the paracetamol group did.13
TABLE 1
Tramadol and other opioids for OA pain: NNT and NNH
| Treatment | NNT | NNH |
|---|---|---|
| Tramadol10 | 6 | 5 |
| Opioids (overall)11 | 25 | 12 |
| NNH, number needed to harm; NNT, number needed to treat; OA, osteoarthritis. | ||
Post-review RCTs provide further evidence
We identified 4 double-blind RCTs of tramadol for the treatment of OA that were of at least 6 weeks’ duration,19-22 published after the 2006 review. The results of these studies (TABLE 2) were broadly consistent with those of the systematic review. Two of the 4 studies had active controls, with one comparing tramadol with diclofenac19 and the other with celecoxib.21 Tramadol and diclofenac were found to be equally effective; celecoxib appeared to be superior in terms of pain relief, global improvement, and physical function, but no statistical comparisons were reported.
TABLE 2
Tramadol for OA: Post-review RCTs are consistent with meta-analysis
| Study duration (N) | Intervention groups | Primary outcome measures | Improvement in | Adverse effects | ||
|---|---|---|---|---|---|---|
| Pain | Global assessment | Function | ||||
| Gana*20 12 wk (1020) | Tramadol ER Placebo | WOMAC OA index (pain and physical function subscales) 100-mm VAS: Subject global disease | Treatment groups, 35% Placebo, 25% | Treatment groups, 32%-36% Placebo, 24% | Treatment groups, 31%-33% Placebo, 22% | ≥1 AE Withdrawals due to AEs |
| Delemos*21 12 wk (1001) | Tramadol ER Placebo | WOMAC OA index (pain and physical function subscales) 100-mm VAS: Subject global disease | Tramadol, 27%-39% Celecoxib, 45% Placebo, 32% | Tramadol, 28%-40% Celecoxib, 44% Placebo, 30% | Tramadol, 26%-35% Celecoxib, 43% Placebo, 28% | ≥1 AE Withdrawals due to AEs |
| Burch†22 12 wk (646) | Tramadol (Contramid OAD) 100 mg titrating to 300 mg Placebo | Pain intensity (11-point numerical scale) Physician/patient global impressions of change (7-point scale) | Treatment group, 40% Placebo, 33% | Treatment group, 80% Placebo, 69% | NA | AEs Placebo: Nausea, 5.6%; constipation, 4.2%; dizziness/vertigo, 3.7%; somnolence, 3.7% Withdrawals due to AEs |
| Beaulieu*19 6 wk (128) | Tramadol CR 200 mg titrating to 400 mg Diclofenac SR 75 mg titrating to 150 mg | WOMAC OA index (pain and physical function subscales) 100-mm VAS: Pain intensity Subject global disease Physician/patient global impressions of change (7-point scale) | Both groups, ~29% | Tramadol, 67%‡ Diclofenac, 54%‡ | Tramadol, 29%‡ Diclofenac, 29%‡ | Withdrawals due to AEs Tramadol, 16% Diclofenac, 15% |
| *Hip or knee osteoarthritis. †Knee osteoarthritis. ‡Not statistically significant. AEs, adverse events; CR, controlled release; ER, extended release; NA, not assessed; OA, osteoarthritis; OAD, once a day; RCTs, randomized controlled trials; SR, sustained release; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities. | ||||||
Oral and transdermal opioids: Pain relief but high risk
Among the patients with OA of the hip or knee—the study population for the review of oral and transdermal opioids—all the opioids tested were more effective than placebo. The benefits, however, were small to moderate, and were off set by large increases in the risk of AEs and a high dropout rate.
Four of the 10 trials reported the number of patients experiencing any AE: 23% of those taking opioids vs 15% of patients on placebo.11 This represents an NNH of 12 (TABLE 1). All 10 trials reported the number of patients who withdrew due to AEs. Those receiving opioids were 4 times as likely to withdraw due to AEs, compared with those taking placebo. The NNH to cause one additional withdrawal was 19 (95% CI, 13-29).
Bottom line
The data highlight both the limited role of opioids (including tramadol) in OA treatment and—when they are being considered for this patient population—the importance of making patients aware that the risks may outweigh the benefits. Used judiciously and with adequate patient counseling, tramadol may be an option when COX-2-specific inhibitors and NSAIDs fail or cannot be tolerated. Although the small-to-moderate benefits of non-tramadol opioids are generally outweighed by large increases in the risk of AEs, their use may be considered for severe OA pain if tramadol is ineffective or causes intolerable AEs.
CORRESPONDENCE
Faline Howes, BMedSci, MBBS, MPH, FRACGP, Menzies Research Institute Tasmania, Private Bag 23, University of Tasmania, Hobart, Tasmania, Australia 7001; Faline.Howes@ utas.edu.au
1. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
2. Bitton R. The economic burden of osteoarthritis. Am J Manag Care. 2009;15(suppl):S230-S235.
3. Altman RD, Hochberg MC, Moskowitz RW, et al. Recommendations for the medical management of osteoarthritis of the hip and knee. Arthritis Rheum. 2000;43:1905-1915.
4. Gibson TP. Pharmacokinetics, efficacy, and safety of analgesia with a focus on tramadol HCl. Am J Med. 1996;101(suppl 1A):47S-53S.
5. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613-2620.
6. Warner M, Chen LH, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999-2006. NCHS data brief, no 22. Hyattsville, MD: National Center for Health Statistics; 2009.
7. Von Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind? Pain. 2004;109:207-209.
8. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137-167.
9. Pletcher MJ, Kertesz SG, Kohn MA, et al. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299:70-78.
10. Cepeda MS, Camargo F, Zea C, et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2006;(3):CD005522.-
11. Nuesch E, Rutjes AW, Husni E, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. 2009;(4):CD003115.-
12. Ehrich EW, Davies GM, Watson DJ, et al. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol. 2000;27:2635-2641.
13. Bianchi M, Broggini M, Balzarini P, et al. Effects of tramadol on synovial fluid concentrations of substance P and interleukin-6 in patients with knee osteoarthritis: comparison with paracetamol. Int Immunopharm. 2003;3:1901-1908.
14. Wilder-Smith C, Hill L, Spargo K, et al. Treatment of severe pain from osteoarthritis with slow-release tramadol or dihydrocodeine in combination with NSAIDs: a randomised study comparing analgesia, antinociception and gastrointestinal effects. Pain. 2001;91:23-31.
15. Jensen E, Ginsberg F. Tramadol versus dextropropoxyphene in the treatment of osteoarthritis: a short-term double-blind study. Drug Invest. 1994;8:211-218.
16. Bird H, Hill J, Stratford M, et al. A double-blind cross-over study comparing the analgesic efficacy of tramadol with pentazocine in patients with osteoarthritis. J Drug Dev Clin Pract. 1995;7:181-188.
17. Pavelka K, Peliskova Z, Stehlikova H, et al. Intraindividual differences in pain relief and functional improvement in osteoarthritis with diclofenac or tramadol. Clin Drug Invest. 1998;16:421-429.
18. Townheed TE, Maxwell L, Judd MG, et al. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1):CD004257.-
19. Beaulieu AD, Peloso PM, Haraoui B, et al. Once-daily, controlled-release tramadol and sustained-release diclofenac relieve chronic pain due to osteoarthritis: a randomized controlled trial. Pain Res Manag. 2008;13:103-110.
20. Gana TJ, Pascual ML, Fleming RR, et al. Extended-release tramadol in the treatment of osteoarthritis: a multicenter, randomized, double-blind, placebo-controlled clinical trial. Curr Med Res Opin. 2006;22:1391-1401.
21. Delemos BP, Xiang J, Benson C, et al. Tramadol hydrochloride extended-release once-daily in the treatment of osteoarthritis of the knee and/or hip: a double-blind, randomized, dose-ranging trial. Am J Ther. 2010 Mar 3 [Epub ahead of print].
22. Burch F, Fishman R, Messina N, et al. A comparison of the analgesic efficacy of Tramadol Contramid OAD versus placebo in patients with pain due to osteoarthritis. J Pain Symptom Manage. 2007;34:328-338.
Osteoarthritis (OA) affects nearly 27 million Americans, or about 12% of US adults.1 As the average age of the population increases, the prevalence and burden of this debilitating disorder continue to rise.2
The American College of Rheumatology (ACR)’s guidelines for the medical management of OA of the hip and knee, last updated in 2000,3 focus on controlling pain and improving function and health-related quality of life while minimizing the toxic effects of therapy. The guidelines recommend tramadol—an atypical opioid with 2 distinct mechanisms of action4—for moderate-to-severe pain in OA patients who either have contraindications to COX-2 inhibitors and non steroidal anti-inflammatory drugs (NSAIDs) or have failed to respond to previous oral therapy. Patients with severe pain who don’t respond to or are unable to tolerate tramadol may be candidates for more traditional opioid therapy, the guidelines indicate.3
In recent years, however, the use (and abuse) of opioids has skyrocketed. Between 1997 and 2007, US per capita retail purchases of hydrocodone and oxycodone increased 4-fold and 9-fold, respectively.5 In a similar time frame (1996-2006), the number of deaths from opioid overdose more than tripled, going from 4000 to 13,800 annually.6 Not surprisingly, the use of narcotics for noncancer pain remains controversial.7,8 But inadequately treated pain continues to be a serious public health problem, as well.9
This is the third in a series of articles based on the findings of the Cochrane Musculoskeletal Group (CMSG). One of the largest groups in the Cochrane Collaboration, the CMSG synthesizes the results of clinical trials to determine whether interventions for the prevention, treatment, and rehabilitation of musculoskeletal disorders are safe and effective. In this installment, the reviewers use detailed analysis, as well as a case study, to bring their findings to the attention of family physicians in a practical, clinically relevant context.
In 2006 and 2009, respectively, the Cochrane Collaboration published systematic reviews of tramadol (for OA in any joint)10 and other oral and transdermal opioids (for OA of the hip or knee).11 The reviewers’ findings, presented here along with data from more recent trials, can help ensure that you prescribe opioids for patients with OA only when their use is clinically appropriate and evidence-based. We’ve also included a case study (see page 211), so you can assess your knowledge and clinical skills.
CASE Carol J, an active 72-year-old, was diagnosed with OA in her right hip 5 years ago. Now she reports that the pain is getting progressively worse, making it harder and harder to turn over in bed at night or get in and out of the car. The pain is particularly bad at night, Carol says, and she’s had interrupted sleep for months. The patient has taken acetaminophen for the pain since her OA diagnosis, but now finds the analgesic is ineffective, even at the maximum dose of 4 g per day.
Carol has hypertension, which was difficult to manage until she began taking a combination ACE inhibitor/diuretic. She also has moderate renal impairment and mild chronic obstructive pulmonary disease, which limits her exercise tolerance. Nonetheless, she continues to smoke. The patient lives with and cares for her husband, who has Alzheimer’s disease, and worries about her ability to continue to care for him.
What are her treatment options?
Full-dose acetaminophen is no longer helping Carol, and NSAIDs are contraindicated because she takes an ACE inhibitor/diuretic and has moderate renal impairment. Increasing exercise will be a challenge. You strongly encourage her to stop smoking, emphasizing that this is particularly important to reduce the risk involved with any future joint replacement surgery.
Oral dosing options for the patient include:
- prescribing tramadol, starting with a low-dose immediate-release formulation taken one hour before bedtime (The controlled-release formulation is not advisable, given her age and renal function.) or
- adding a traditional opioid, eg, codeine 30 to 60 mg every 6 hours as needed, to her regular acetaminophen regimen.
Codeine and hydrocodone are available in combination preparations with acetaminophen, which may be convenient for some patients. However, hydrocodone was not one of the opioids tested in the trials included in the Cochrane reviews, and evidence of its use in OA is lacking.
Intra-articular corticosteroid injection, performed under imaging guidance, is another option for Carol. You explain that although there have been no studies of intra-articular corticosteroid injections for OA of the hip, these are used occasionally and may provide short-term symptom relief.7
You emphasize that surgery is likely to give her the best long-term outcome. In view of the patient’s circumstances and the need to care for her husband, however, you prescribe tramadol 50 mg at night. (Because of Carol’s age, renal impairment, and the possible adverse effects, it’s wise to start with a low dose and titrate upwards.) You warn her of the risks associated with opioids and advise her to alert your office staff if she experiences any adverse effects.
Before the patient leaves, you arrange an orthopedic consult and schedule a return visit for the following week. At your urging, she agrees to look into respite options for her husband.
Tramadol produces modest results—or none at all
The tramadol review10 included 11 randomized controlled trials (RCTs) with a total of 1019 participants who took tramadol or tramadol/acetaminophen (paracetamol) and 920 controls. In 6 of the 11 studies, the controls received placebo; the remaining 5 trials used “active controls,” with the control group for each RCT receiving a different analgesic. (To learn more about the methodology, see “How the reviews were conducted”.)
Placebo-controlled trials. Compared with patients on placebo, those receiving tramadol had an average absolute reduction in pain of 8.5 mm on a 0-100 mm visual analog scale (VAS) (95% confidence interval [CI], -12.05 to -4.9). That small benefit, however, did not reach the level defined as the minimal perceptible clinical improvement—a reduction of 9.7 mm on Western Ontario and McMaster Universities (WOMAC)’s OA pain subscale.12
Active-controlled trials. In the 5 RCTs comparing tramadol with another active agent, tramadol proved to be no better than the control drug. In fact, in a study of tramadol vs acetaminophen, 500 mg acetaminophen 3 times a day provided more pain relief than 50 mg tramadol 3 times a day.13 Although this was a small (N=20), short-term (7-day) study, this finding is notable because participants took less than the usual acetaminophen dose of 1 g up to 4 times a day.
Nor was tramadol superior to the agents it was compared with in the 4 other active-controlled trials—dihydrocodeine,14 dextropropoxyphene,15 pentazocine,16 and diclofenac17—in reducing pain intensity. It is important to keep in mind, however, that in each of these studies, both the quantity and quality of the evidence was limited. (Two studies did not use numerical scales,14,16 for example; all had methodological issues; and none lasted longer than 28 days.)
The Cochrane Musculoskeletal Group conducted a review of tramadol and a review of other oral opioids and transdermal fentanyl for the treatment of osteoarthritis (OA). Both reviews featured pain, function, and safety as primary outcomes. The tramadol review included randomized controlled trials (RCTs) for OA in any joint, while the oral and transdermal opioid review included randomized and quasi-randomized trials of treatment for OA of the hip or knee. Other parameters follow:
The tramadol review included 11 RCTs, with a total of 1019 participants receiving either tramadol alone or tramadol/acetaminophen (paracetamol) and 920 controls. In 6 of the 11 studies, the controls received placebo; the remaining 5 studies featured “active control.” That is, the control groups received acetaminophen 500 mg 3 times daily, diclofenac (25-50 mg up to 3 times daily on demand), dihydrocodeine 60 mg twice daily, dextropropoxyphene 100 mg 3 times daily, or pentazocine 50 mg 4 times per day. Because each of these agents was used in only one trial, the reviewers could not reach definitive conclusions about tramadol’s performance relative to other medications. The average number of participants in the tramadol and control groups was 91 and 80, respectively. The average length of follow-up was 35 days.
The 11 RCTs included in this review used a variety of pain scales to assess the results of tramadol, active control medications, and placebo. For comparative purposes, the reviewers pooled the results from studies that used numerical scales (0 to 100 and 0 to 10) to assess pain intensity. As a reference, we have used 9.7 and 9.3, respectively, determined by other researchers to be the minimal perceptible clinical improvements on the Western Ontario and McMaster Universities (WOMAC) pain and physical function 0-100 mm visual analog scales.12
The review of oral and transdermal opioids included 10 studies, with a total of 1541 patients receiving opioids and 727 receiving placebo.17 There were 3 trials of codeine (in 2 of the 3, a simple analgesic [acetaminophen 3000 mg/d or ibuprofen 1200 mg/d] was co-administered to both the treatment and control groups); other opioids included in the trials were oxycodone (4 trials), oxymorphone (2 trials), morphine (1 trial), and transdermal fentanyl (1 trial).
A modest boost in well-being
The reviewers measured function in 2 ways, focusing on both global improvement and improvement in physical function.
Global assessment. For the global assessment, the reviewers defined a treatment response as achieving at least a moderate improvement. By that standard, tramadol may improve overall well-being more than placebo. In the placebo-controlled trials, the number needed to treat (NNT) to elicit one treatment response was 6.
Three of the trials with active controls included global/functional assessments, and the results—bearing in mind the reduced quality and quantity of the evidence—were mixed. In a comparison of tramadol with dextropropoxyphene, tramadol increased the likelihood of moderate improvement by 38% (relative risk, 1.38 (95% CI, 1.15-1.67).10 In a trial of tramadol vs pentazocine, tramadol was more effective in reducing the duration of morning stiffness (by about 10 minutes), but not its severity. Tramadol was comparable with pentazocine in the 7 other measures of OA and function.16 In the tramadol-diclofenac study, both drugs were equally effective.17
Physical function. Four of the 6 placebo-controlled tramadol studies included in the Cochrane review used the WOMAC Index score, which included the physical function subscale. The tramadol group had a larger reduction in the score than the placebo group, by 0.34 mm (95% CI, -0.49 to -0.19). While this was equivalent to an 8.5% relative reduction in mean baseline score, it is still small compared with the minimal perceptible clinical improvement level of 9.3 mm on a 0-100 scale needed for the WOMAC physical function subscale. A similar improvement was reported for those taking tramadol compared with diclofenac—the only one of the active-controlled studies to report on physical function.17
Other opioids relieve pain, improve function—but how much?
The review of oral and transdermal opioids for OA11 encompassed 10 trials, with a total of 1541 patients receiving opioids and 727 on placebo. The opioids used in the trials were codeine, oxycodone, oxymorphone, morphine, and transdermal fentanyl. (For more details, see “How the reviews were conducted”.)
Pain. The trials included in the review used a variety of scales to measure pain, so the reviewers gauged results by the proportion of patients responding to treatment. Response was defined as a 50% improvement in pain score.
In the overall analysis, 35% of patients taking opioids responded to treatment, vs 31% of those on placebo—or 4 more patients in 100. That represents an NNT of 25. (A subgroup analysis did not demonstrate any significant differences in effect size among the opioids tested. In addition, the effect size was similar regardless of the potency of the opioid or the administration route.)
Function. Seven of the 10 trials (1794 participants, including both the treatment groups and controls) used validated function scores to measure physical function after 4 weeks of treatment. Here, too, the reviewers defined a treatment response as a 50% improvement in score.
Their finding? Opioids had a greater effect on function compared with placebo, equaling 0.7 on a WOMAC disability scale of 1 to 10. This means that about 3 more patients in 100 responded to treatment with opioids vs placebo—an NNT of 30.
But what about safety?
Opioids, including tramadol, are associated with adverse events (AEs), which may be minor or major. To determine when, or whether, the benefits outweigh the risks for treating patients with OA, both reviews reported on AEs and the number of participants who stopped taking the drug because of AEs.
AEs limit tramadol’s usefulness
While tramadol was more effective than placebo at reducing pain intensity, relieving symptoms, and improving function, the benefits were small—with an overall NNT of 6 (TABLE 1). This is similar to acetaminophen (NNT, 4-16),18 but with a greater downside.
Minor AEs. Four placebo-controlled trials reported on minor AEs.19-22 Those most commonly reported by patients taking tramadol were nausea, vomiting, dizziness, constipation, somnolence, tiredness, and headache.
Overall, 39% of those who received tramadol experienced minor AEs, compared with 18% of patients receiving placebo—an NNH of 5.10 Thus, tramadol’s NNH for minor AEs is equivalent to its NNT for pain relief. In active-controlled studies, there was a higher risk of minor AEs in those receiving tramadol compared with diclofenac or dextropropoxyphene, but a lower risk compared with those taking pentazocine.10
Major AEs. An analysis of the placebo-controlled trials revealed that 21% of those who received tramadol had major AEs—defined as an event that resulted in cessation of treatment—compared with 8% of those taking placebo. By this measure, the NNH was 8: One in 8 patients stopped taking tramadol because of a major AE.10
Among the active-controlled trials, participants taking tramadol were more likely to report a major AE compared with those receiving either diclofenac or dextropropoxyphene (NNH=5), but less likely compared with patients taking pentazocine. In a trial that compared tramadol alone with paracetamol, 2 out of 10 in the tramadol group discontinued treatment; none in the paracetamol group did.13
TABLE 1
Tramadol and other opioids for OA pain: NNT and NNH
| Treatment | NNT | NNH |
|---|---|---|
| Tramadol10 | 6 | 5 |
| Opioids (overall)11 | 25 | 12 |
| NNH, number needed to harm; NNT, number needed to treat; OA, osteoarthritis. | ||
Post-review RCTs provide further evidence
We identified 4 double-blind RCTs of tramadol for the treatment of OA that were of at least 6 weeks’ duration,19-22 published after the 2006 review. The results of these studies (TABLE 2) were broadly consistent with those of the systematic review. Two of the 4 studies had active controls, with one comparing tramadol with diclofenac19 and the other with celecoxib.21 Tramadol and diclofenac were found to be equally effective; celecoxib appeared to be superior in terms of pain relief, global improvement, and physical function, but no statistical comparisons were reported.
TABLE 2
Tramadol for OA: Post-review RCTs are consistent with meta-analysis
| Study duration (N) | Intervention groups | Primary outcome measures | Improvement in | Adverse effects | ||
|---|---|---|---|---|---|---|
| Pain | Global assessment | Function | ||||
| Gana*20 12 wk (1020) | Tramadol ER Placebo | WOMAC OA index (pain and physical function subscales) 100-mm VAS: Subject global disease | Treatment groups, 35% Placebo, 25% | Treatment groups, 32%-36% Placebo, 24% | Treatment groups, 31%-33% Placebo, 22% | ≥1 AE Withdrawals due to AEs |
| Delemos*21 12 wk (1001) | Tramadol ER Placebo | WOMAC OA index (pain and physical function subscales) 100-mm VAS: Subject global disease | Tramadol, 27%-39% Celecoxib, 45% Placebo, 32% | Tramadol, 28%-40% Celecoxib, 44% Placebo, 30% | Tramadol, 26%-35% Celecoxib, 43% Placebo, 28% | ≥1 AE Withdrawals due to AEs |
| Burch†22 12 wk (646) | Tramadol (Contramid OAD) 100 mg titrating to 300 mg Placebo | Pain intensity (11-point numerical scale) Physician/patient global impressions of change (7-point scale) | Treatment group, 40% Placebo, 33% | Treatment group, 80% Placebo, 69% | NA | AEs Placebo: Nausea, 5.6%; constipation, 4.2%; dizziness/vertigo, 3.7%; somnolence, 3.7% Withdrawals due to AEs |
| Beaulieu*19 6 wk (128) | Tramadol CR 200 mg titrating to 400 mg Diclofenac SR 75 mg titrating to 150 mg | WOMAC OA index (pain and physical function subscales) 100-mm VAS: Pain intensity Subject global disease Physician/patient global impressions of change (7-point scale) | Both groups, ~29% | Tramadol, 67%‡ Diclofenac, 54%‡ | Tramadol, 29%‡ Diclofenac, 29%‡ | Withdrawals due to AEs Tramadol, 16% Diclofenac, 15% |
| *Hip or knee osteoarthritis. †Knee osteoarthritis. ‡Not statistically significant. AEs, adverse events; CR, controlled release; ER, extended release; NA, not assessed; OA, osteoarthritis; OAD, once a day; RCTs, randomized controlled trials; SR, sustained release; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities. | ||||||
Oral and transdermal opioids: Pain relief but high risk
Among the patients with OA of the hip or knee—the study population for the review of oral and transdermal opioids—all the opioids tested were more effective than placebo. The benefits, however, were small to moderate, and were off set by large increases in the risk of AEs and a high dropout rate.
Four of the 10 trials reported the number of patients experiencing any AE: 23% of those taking opioids vs 15% of patients on placebo.11 This represents an NNH of 12 (TABLE 1). All 10 trials reported the number of patients who withdrew due to AEs. Those receiving opioids were 4 times as likely to withdraw due to AEs, compared with those taking placebo. The NNH to cause one additional withdrawal was 19 (95% CI, 13-29).
Bottom line
The data highlight both the limited role of opioids (including tramadol) in OA treatment and—when they are being considered for this patient population—the importance of making patients aware that the risks may outweigh the benefits. Used judiciously and with adequate patient counseling, tramadol may be an option when COX-2-specific inhibitors and NSAIDs fail or cannot be tolerated. Although the small-to-moderate benefits of non-tramadol opioids are generally outweighed by large increases in the risk of AEs, their use may be considered for severe OA pain if tramadol is ineffective or causes intolerable AEs.
CORRESPONDENCE
Faline Howes, BMedSci, MBBS, MPH, FRACGP, Menzies Research Institute Tasmania, Private Bag 23, University of Tasmania, Hobart, Tasmania, Australia 7001; Faline.Howes@ utas.edu.au
Osteoarthritis (OA) affects nearly 27 million Americans, or about 12% of US adults.1 As the average age of the population increases, the prevalence and burden of this debilitating disorder continue to rise.2
The American College of Rheumatology (ACR)’s guidelines for the medical management of OA of the hip and knee, last updated in 2000,3 focus on controlling pain and improving function and health-related quality of life while minimizing the toxic effects of therapy. The guidelines recommend tramadol—an atypical opioid with 2 distinct mechanisms of action4—for moderate-to-severe pain in OA patients who either have contraindications to COX-2 inhibitors and non steroidal anti-inflammatory drugs (NSAIDs) or have failed to respond to previous oral therapy. Patients with severe pain who don’t respond to or are unable to tolerate tramadol may be candidates for more traditional opioid therapy, the guidelines indicate.3
In recent years, however, the use (and abuse) of opioids has skyrocketed. Between 1997 and 2007, US per capita retail purchases of hydrocodone and oxycodone increased 4-fold and 9-fold, respectively.5 In a similar time frame (1996-2006), the number of deaths from opioid overdose more than tripled, going from 4000 to 13,800 annually.6 Not surprisingly, the use of narcotics for noncancer pain remains controversial.7,8 But inadequately treated pain continues to be a serious public health problem, as well.9
This is the third in a series of articles based on the findings of the Cochrane Musculoskeletal Group (CMSG). One of the largest groups in the Cochrane Collaboration, the CMSG synthesizes the results of clinical trials to determine whether interventions for the prevention, treatment, and rehabilitation of musculoskeletal disorders are safe and effective. In this installment, the reviewers use detailed analysis, as well as a case study, to bring their findings to the attention of family physicians in a practical, clinically relevant context.
In 2006 and 2009, respectively, the Cochrane Collaboration published systematic reviews of tramadol (for OA in any joint)10 and other oral and transdermal opioids (for OA of the hip or knee).11 The reviewers’ findings, presented here along with data from more recent trials, can help ensure that you prescribe opioids for patients with OA only when their use is clinically appropriate and evidence-based. We’ve also included a case study (see page 211), so you can assess your knowledge and clinical skills.
CASE Carol J, an active 72-year-old, was diagnosed with OA in her right hip 5 years ago. Now she reports that the pain is getting progressively worse, making it harder and harder to turn over in bed at night or get in and out of the car. The pain is particularly bad at night, Carol says, and she’s had interrupted sleep for months. The patient has taken acetaminophen for the pain since her OA diagnosis, but now finds the analgesic is ineffective, even at the maximum dose of 4 g per day.
Carol has hypertension, which was difficult to manage until she began taking a combination ACE inhibitor/diuretic. She also has moderate renal impairment and mild chronic obstructive pulmonary disease, which limits her exercise tolerance. Nonetheless, she continues to smoke. The patient lives with and cares for her husband, who has Alzheimer’s disease, and worries about her ability to continue to care for him.
What are her treatment options?
Full-dose acetaminophen is no longer helping Carol, and NSAIDs are contraindicated because she takes an ACE inhibitor/diuretic and has moderate renal impairment. Increasing exercise will be a challenge. You strongly encourage her to stop smoking, emphasizing that this is particularly important to reduce the risk involved with any future joint replacement surgery.
Oral dosing options for the patient include:
- prescribing tramadol, starting with a low-dose immediate-release formulation taken one hour before bedtime (The controlled-release formulation is not advisable, given her age and renal function.) or
- adding a traditional opioid, eg, codeine 30 to 60 mg every 6 hours as needed, to her regular acetaminophen regimen.
Codeine and hydrocodone are available in combination preparations with acetaminophen, which may be convenient for some patients. However, hydrocodone was not one of the opioids tested in the trials included in the Cochrane reviews, and evidence of its use in OA is lacking.
Intra-articular corticosteroid injection, performed under imaging guidance, is another option for Carol. You explain that although there have been no studies of intra-articular corticosteroid injections for OA of the hip, these are used occasionally and may provide short-term symptom relief.7
You emphasize that surgery is likely to give her the best long-term outcome. In view of the patient’s circumstances and the need to care for her husband, however, you prescribe tramadol 50 mg at night. (Because of Carol’s age, renal impairment, and the possible adverse effects, it’s wise to start with a low dose and titrate upwards.) You warn her of the risks associated with opioids and advise her to alert your office staff if she experiences any adverse effects.
Before the patient leaves, you arrange an orthopedic consult and schedule a return visit for the following week. At your urging, she agrees to look into respite options for her husband.
Tramadol produces modest results—or none at all
The tramadol review10 included 11 randomized controlled trials (RCTs) with a total of 1019 participants who took tramadol or tramadol/acetaminophen (paracetamol) and 920 controls. In 6 of the 11 studies, the controls received placebo; the remaining 5 trials used “active controls,” with the control group for each RCT receiving a different analgesic. (To learn more about the methodology, see “How the reviews were conducted”.)
Placebo-controlled trials. Compared with patients on placebo, those receiving tramadol had an average absolute reduction in pain of 8.5 mm on a 0-100 mm visual analog scale (VAS) (95% confidence interval [CI], -12.05 to -4.9). That small benefit, however, did not reach the level defined as the minimal perceptible clinical improvement—a reduction of 9.7 mm on Western Ontario and McMaster Universities (WOMAC)’s OA pain subscale.12
Active-controlled trials. In the 5 RCTs comparing tramadol with another active agent, tramadol proved to be no better than the control drug. In fact, in a study of tramadol vs acetaminophen, 500 mg acetaminophen 3 times a day provided more pain relief than 50 mg tramadol 3 times a day.13 Although this was a small (N=20), short-term (7-day) study, this finding is notable because participants took less than the usual acetaminophen dose of 1 g up to 4 times a day.
Nor was tramadol superior to the agents it was compared with in the 4 other active-controlled trials—dihydrocodeine,14 dextropropoxyphene,15 pentazocine,16 and diclofenac17—in reducing pain intensity. It is important to keep in mind, however, that in each of these studies, both the quantity and quality of the evidence was limited. (Two studies did not use numerical scales,14,16 for example; all had methodological issues; and none lasted longer than 28 days.)
The Cochrane Musculoskeletal Group conducted a review of tramadol and a review of other oral opioids and transdermal fentanyl for the treatment of osteoarthritis (OA). Both reviews featured pain, function, and safety as primary outcomes. The tramadol review included randomized controlled trials (RCTs) for OA in any joint, while the oral and transdermal opioid review included randomized and quasi-randomized trials of treatment for OA of the hip or knee. Other parameters follow:
The tramadol review included 11 RCTs, with a total of 1019 participants receiving either tramadol alone or tramadol/acetaminophen (paracetamol) and 920 controls. In 6 of the 11 studies, the controls received placebo; the remaining 5 studies featured “active control.” That is, the control groups received acetaminophen 500 mg 3 times daily, diclofenac (25-50 mg up to 3 times daily on demand), dihydrocodeine 60 mg twice daily, dextropropoxyphene 100 mg 3 times daily, or pentazocine 50 mg 4 times per day. Because each of these agents was used in only one trial, the reviewers could not reach definitive conclusions about tramadol’s performance relative to other medications. The average number of participants in the tramadol and control groups was 91 and 80, respectively. The average length of follow-up was 35 days.
The 11 RCTs included in this review used a variety of pain scales to assess the results of tramadol, active control medications, and placebo. For comparative purposes, the reviewers pooled the results from studies that used numerical scales (0 to 100 and 0 to 10) to assess pain intensity. As a reference, we have used 9.7 and 9.3, respectively, determined by other researchers to be the minimal perceptible clinical improvements on the Western Ontario and McMaster Universities (WOMAC) pain and physical function 0-100 mm visual analog scales.12
The review of oral and transdermal opioids included 10 studies, with a total of 1541 patients receiving opioids and 727 receiving placebo.17 There were 3 trials of codeine (in 2 of the 3, a simple analgesic [acetaminophen 3000 mg/d or ibuprofen 1200 mg/d] was co-administered to both the treatment and control groups); other opioids included in the trials were oxycodone (4 trials), oxymorphone (2 trials), morphine (1 trial), and transdermal fentanyl (1 trial).
A modest boost in well-being
The reviewers measured function in 2 ways, focusing on both global improvement and improvement in physical function.
Global assessment. For the global assessment, the reviewers defined a treatment response as achieving at least a moderate improvement. By that standard, tramadol may improve overall well-being more than placebo. In the placebo-controlled trials, the number needed to treat (NNT) to elicit one treatment response was 6.
Three of the trials with active controls included global/functional assessments, and the results—bearing in mind the reduced quality and quantity of the evidence—were mixed. In a comparison of tramadol with dextropropoxyphene, tramadol increased the likelihood of moderate improvement by 38% (relative risk, 1.38 (95% CI, 1.15-1.67).10 In a trial of tramadol vs pentazocine, tramadol was more effective in reducing the duration of morning stiffness (by about 10 minutes), but not its severity. Tramadol was comparable with pentazocine in the 7 other measures of OA and function.16 In the tramadol-diclofenac study, both drugs were equally effective.17
Physical function. Four of the 6 placebo-controlled tramadol studies included in the Cochrane review used the WOMAC Index score, which included the physical function subscale. The tramadol group had a larger reduction in the score than the placebo group, by 0.34 mm (95% CI, -0.49 to -0.19). While this was equivalent to an 8.5% relative reduction in mean baseline score, it is still small compared with the minimal perceptible clinical improvement level of 9.3 mm on a 0-100 scale needed for the WOMAC physical function subscale. A similar improvement was reported for those taking tramadol compared with diclofenac—the only one of the active-controlled studies to report on physical function.17
Other opioids relieve pain, improve function—but how much?
The review of oral and transdermal opioids for OA11 encompassed 10 trials, with a total of 1541 patients receiving opioids and 727 on placebo. The opioids used in the trials were codeine, oxycodone, oxymorphone, morphine, and transdermal fentanyl. (For more details, see “How the reviews were conducted”.)
Pain. The trials included in the review used a variety of scales to measure pain, so the reviewers gauged results by the proportion of patients responding to treatment. Response was defined as a 50% improvement in pain score.
In the overall analysis, 35% of patients taking opioids responded to treatment, vs 31% of those on placebo—or 4 more patients in 100. That represents an NNT of 25. (A subgroup analysis did not demonstrate any significant differences in effect size among the opioids tested. In addition, the effect size was similar regardless of the potency of the opioid or the administration route.)
Function. Seven of the 10 trials (1794 participants, including both the treatment groups and controls) used validated function scores to measure physical function after 4 weeks of treatment. Here, too, the reviewers defined a treatment response as a 50% improvement in score.
Their finding? Opioids had a greater effect on function compared with placebo, equaling 0.7 on a WOMAC disability scale of 1 to 10. This means that about 3 more patients in 100 responded to treatment with opioids vs placebo—an NNT of 30.
But what about safety?
Opioids, including tramadol, are associated with adverse events (AEs), which may be minor or major. To determine when, or whether, the benefits outweigh the risks for treating patients with OA, both reviews reported on AEs and the number of participants who stopped taking the drug because of AEs.
AEs limit tramadol’s usefulness
While tramadol was more effective than placebo at reducing pain intensity, relieving symptoms, and improving function, the benefits were small—with an overall NNT of 6 (TABLE 1). This is similar to acetaminophen (NNT, 4-16),18 but with a greater downside.
Minor AEs. Four placebo-controlled trials reported on minor AEs.19-22 Those most commonly reported by patients taking tramadol were nausea, vomiting, dizziness, constipation, somnolence, tiredness, and headache.
Overall, 39% of those who received tramadol experienced minor AEs, compared with 18% of patients receiving placebo—an NNH of 5.10 Thus, tramadol’s NNH for minor AEs is equivalent to its NNT for pain relief. In active-controlled studies, there was a higher risk of minor AEs in those receiving tramadol compared with diclofenac or dextropropoxyphene, but a lower risk compared with those taking pentazocine.10
Major AEs. An analysis of the placebo-controlled trials revealed that 21% of those who received tramadol had major AEs—defined as an event that resulted in cessation of treatment—compared with 8% of those taking placebo. By this measure, the NNH was 8: One in 8 patients stopped taking tramadol because of a major AE.10
Among the active-controlled trials, participants taking tramadol were more likely to report a major AE compared with those receiving either diclofenac or dextropropoxyphene (NNH=5), but less likely compared with patients taking pentazocine. In a trial that compared tramadol alone with paracetamol, 2 out of 10 in the tramadol group discontinued treatment; none in the paracetamol group did.13
TABLE 1
Tramadol and other opioids for OA pain: NNT and NNH
| Treatment | NNT | NNH |
|---|---|---|
| Tramadol10 | 6 | 5 |
| Opioids (overall)11 | 25 | 12 |
| NNH, number needed to harm; NNT, number needed to treat; OA, osteoarthritis. | ||
Post-review RCTs provide further evidence
We identified 4 double-blind RCTs of tramadol for the treatment of OA that were of at least 6 weeks’ duration,19-22 published after the 2006 review. The results of these studies (TABLE 2) were broadly consistent with those of the systematic review. Two of the 4 studies had active controls, with one comparing tramadol with diclofenac19 and the other with celecoxib.21 Tramadol and diclofenac were found to be equally effective; celecoxib appeared to be superior in terms of pain relief, global improvement, and physical function, but no statistical comparisons were reported.
TABLE 2
Tramadol for OA: Post-review RCTs are consistent with meta-analysis
| Study duration (N) | Intervention groups | Primary outcome measures | Improvement in | Adverse effects | ||
|---|---|---|---|---|---|---|
| Pain | Global assessment | Function | ||||
| Gana*20 12 wk (1020) | Tramadol ER Placebo | WOMAC OA index (pain and physical function subscales) 100-mm VAS: Subject global disease | Treatment groups, 35% Placebo, 25% | Treatment groups, 32%-36% Placebo, 24% | Treatment groups, 31%-33% Placebo, 22% | ≥1 AE Withdrawals due to AEs |
| Delemos*21 12 wk (1001) | Tramadol ER Placebo | WOMAC OA index (pain and physical function subscales) 100-mm VAS: Subject global disease | Tramadol, 27%-39% Celecoxib, 45% Placebo, 32% | Tramadol, 28%-40% Celecoxib, 44% Placebo, 30% | Tramadol, 26%-35% Celecoxib, 43% Placebo, 28% | ≥1 AE Withdrawals due to AEs |
| Burch†22 12 wk (646) | Tramadol (Contramid OAD) 100 mg titrating to 300 mg Placebo | Pain intensity (11-point numerical scale) Physician/patient global impressions of change (7-point scale) | Treatment group, 40% Placebo, 33% | Treatment group, 80% Placebo, 69% | NA | AEs Placebo: Nausea, 5.6%; constipation, 4.2%; dizziness/vertigo, 3.7%; somnolence, 3.7% Withdrawals due to AEs |
| Beaulieu*19 6 wk (128) | Tramadol CR 200 mg titrating to 400 mg Diclofenac SR 75 mg titrating to 150 mg | WOMAC OA index (pain and physical function subscales) 100-mm VAS: Pain intensity Subject global disease Physician/patient global impressions of change (7-point scale) | Both groups, ~29% | Tramadol, 67%‡ Diclofenac, 54%‡ | Tramadol, 29%‡ Diclofenac, 29%‡ | Withdrawals due to AEs Tramadol, 16% Diclofenac, 15% |
| *Hip or knee osteoarthritis. †Knee osteoarthritis. ‡Not statistically significant. AEs, adverse events; CR, controlled release; ER, extended release; NA, not assessed; OA, osteoarthritis; OAD, once a day; RCTs, randomized controlled trials; SR, sustained release; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities. | ||||||
Oral and transdermal opioids: Pain relief but high risk
Among the patients with OA of the hip or knee—the study population for the review of oral and transdermal opioids—all the opioids tested were more effective than placebo. The benefits, however, were small to moderate, and were off set by large increases in the risk of AEs and a high dropout rate.
Four of the 10 trials reported the number of patients experiencing any AE: 23% of those taking opioids vs 15% of patients on placebo.11 This represents an NNH of 12 (TABLE 1). All 10 trials reported the number of patients who withdrew due to AEs. Those receiving opioids were 4 times as likely to withdraw due to AEs, compared with those taking placebo. The NNH to cause one additional withdrawal was 19 (95% CI, 13-29).
Bottom line
The data highlight both the limited role of opioids (including tramadol) in OA treatment and—when they are being considered for this patient population—the importance of making patients aware that the risks may outweigh the benefits. Used judiciously and with adequate patient counseling, tramadol may be an option when COX-2-specific inhibitors and NSAIDs fail or cannot be tolerated. Although the small-to-moderate benefits of non-tramadol opioids are generally outweighed by large increases in the risk of AEs, their use may be considered for severe OA pain if tramadol is ineffective or causes intolerable AEs.
CORRESPONDENCE
Faline Howes, BMedSci, MBBS, MPH, FRACGP, Menzies Research Institute Tasmania, Private Bag 23, University of Tasmania, Hobart, Tasmania, Australia 7001; Faline.Howes@ utas.edu.au
1. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
2. Bitton R. The economic burden of osteoarthritis. Am J Manag Care. 2009;15(suppl):S230-S235.
3. Altman RD, Hochberg MC, Moskowitz RW, et al. Recommendations for the medical management of osteoarthritis of the hip and knee. Arthritis Rheum. 2000;43:1905-1915.
4. Gibson TP. Pharmacokinetics, efficacy, and safety of analgesia with a focus on tramadol HCl. Am J Med. 1996;101(suppl 1A):47S-53S.
5. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613-2620.
6. Warner M, Chen LH, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999-2006. NCHS data brief, no 22. Hyattsville, MD: National Center for Health Statistics; 2009.
7. Von Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind? Pain. 2004;109:207-209.
8. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137-167.
9. Pletcher MJ, Kertesz SG, Kohn MA, et al. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299:70-78.
10. Cepeda MS, Camargo F, Zea C, et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2006;(3):CD005522.-
11. Nuesch E, Rutjes AW, Husni E, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. 2009;(4):CD003115.-
12. Ehrich EW, Davies GM, Watson DJ, et al. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol. 2000;27:2635-2641.
13. Bianchi M, Broggini M, Balzarini P, et al. Effects of tramadol on synovial fluid concentrations of substance P and interleukin-6 in patients with knee osteoarthritis: comparison with paracetamol. Int Immunopharm. 2003;3:1901-1908.
14. Wilder-Smith C, Hill L, Spargo K, et al. Treatment of severe pain from osteoarthritis with slow-release tramadol or dihydrocodeine in combination with NSAIDs: a randomised study comparing analgesia, antinociception and gastrointestinal effects. Pain. 2001;91:23-31.
15. Jensen E, Ginsberg F. Tramadol versus dextropropoxyphene in the treatment of osteoarthritis: a short-term double-blind study. Drug Invest. 1994;8:211-218.
16. Bird H, Hill J, Stratford M, et al. A double-blind cross-over study comparing the analgesic efficacy of tramadol with pentazocine in patients with osteoarthritis. J Drug Dev Clin Pract. 1995;7:181-188.
17. Pavelka K, Peliskova Z, Stehlikova H, et al. Intraindividual differences in pain relief and functional improvement in osteoarthritis with diclofenac or tramadol. Clin Drug Invest. 1998;16:421-429.
18. Townheed TE, Maxwell L, Judd MG, et al. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1):CD004257.-
19. Beaulieu AD, Peloso PM, Haraoui B, et al. Once-daily, controlled-release tramadol and sustained-release diclofenac relieve chronic pain due to osteoarthritis: a randomized controlled trial. Pain Res Manag. 2008;13:103-110.
20. Gana TJ, Pascual ML, Fleming RR, et al. Extended-release tramadol in the treatment of osteoarthritis: a multicenter, randomized, double-blind, placebo-controlled clinical trial. Curr Med Res Opin. 2006;22:1391-1401.
21. Delemos BP, Xiang J, Benson C, et al. Tramadol hydrochloride extended-release once-daily in the treatment of osteoarthritis of the knee and/or hip: a double-blind, randomized, dose-ranging trial. Am J Ther. 2010 Mar 3 [Epub ahead of print].
22. Burch F, Fishman R, Messina N, et al. A comparison of the analgesic efficacy of Tramadol Contramid OAD versus placebo in patients with pain due to osteoarthritis. J Pain Symptom Manage. 2007;34:328-338.
1. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
2. Bitton R. The economic burden of osteoarthritis. Am J Manag Care. 2009;15(suppl):S230-S235.
3. Altman RD, Hochberg MC, Moskowitz RW, et al. Recommendations for the medical management of osteoarthritis of the hip and knee. Arthritis Rheum. 2000;43:1905-1915.
4. Gibson TP. Pharmacokinetics, efficacy, and safety of analgesia with a focus on tramadol HCl. Am J Med. 1996;101(suppl 1A):47S-53S.
5. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613-2620.
6. Warner M, Chen LH, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999-2006. NCHS data brief, no 22. Hyattsville, MD: National Center for Health Statistics; 2009.
7. Von Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind? Pain. 2004;109:207-209.
8. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137-167.
9. Pletcher MJ, Kertesz SG, Kohn MA, et al. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299:70-78.
10. Cepeda MS, Camargo F, Zea C, et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2006;(3):CD005522.-
11. Nuesch E, Rutjes AW, Husni E, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. 2009;(4):CD003115.-
12. Ehrich EW, Davies GM, Watson DJ, et al. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol. 2000;27:2635-2641.
13. Bianchi M, Broggini M, Balzarini P, et al. Effects of tramadol on synovial fluid concentrations of substance P and interleukin-6 in patients with knee osteoarthritis: comparison with paracetamol. Int Immunopharm. 2003;3:1901-1908.
14. Wilder-Smith C, Hill L, Spargo K, et al. Treatment of severe pain from osteoarthritis with slow-release tramadol or dihydrocodeine in combination with NSAIDs: a randomised study comparing analgesia, antinociception and gastrointestinal effects. Pain. 2001;91:23-31.
15. Jensen E, Ginsberg F. Tramadol versus dextropropoxyphene in the treatment of osteoarthritis: a short-term double-blind study. Drug Invest. 1994;8:211-218.
16. Bird H, Hill J, Stratford M, et al. A double-blind cross-over study comparing the analgesic efficacy of tramadol with pentazocine in patients with osteoarthritis. J Drug Dev Clin Pract. 1995;7:181-188.
17. Pavelka K, Peliskova Z, Stehlikova H, et al. Intraindividual differences in pain relief and functional improvement in osteoarthritis with diclofenac or tramadol. Clin Drug Invest. 1998;16:421-429.
18. Townheed TE, Maxwell L, Judd MG, et al. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1):CD004257.-
19. Beaulieu AD, Peloso PM, Haraoui B, et al. Once-daily, controlled-release tramadol and sustained-release diclofenac relieve chronic pain due to osteoarthritis: a randomized controlled trial. Pain Res Manag. 2008;13:103-110.
20. Gana TJ, Pascual ML, Fleming RR, et al. Extended-release tramadol in the treatment of osteoarthritis: a multicenter, randomized, double-blind, placebo-controlled clinical trial. Curr Med Res Opin. 2006;22:1391-1401.
21. Delemos BP, Xiang J, Benson C, et al. Tramadol hydrochloride extended-release once-daily in the treatment of osteoarthritis of the knee and/or hip: a double-blind, randomized, dose-ranging trial. Am J Ther. 2010 Mar 3 [Epub ahead of print].
22. Burch F, Fishman R, Messina N, et al. A comparison of the analgesic efficacy of Tramadol Contramid OAD versus placebo in patients with pain due to osteoarthritis. J Pain Symptom Manage. 2007;34:328-338.
Limp in children: Differentiating benign from dire causes
• Use radiographs to identify bone changes from disease (as well as fracture) when evaluating a limp. C
• Consider growth plate injuries as well as toddler’s fracture; both may be radiographically occult and require immobilization for treatment. C
• Consider child abuse if the patient has an isolated mid-shaft tibial fracture. C
• Assess for fever, elevated sedimentation rate, elevated C-reactive protein, and leukocytosis when radiographs are unrevealing or when a patient has systemic symptoms associated with limp. These factors are predictors of septic arthritis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A mother brings her 4-year-old son to the office because he has been limping. She isn’t aware of a specific trauma. But the boy and his twin brother, while recovering from “colds,” were rough-housing in their room when this son complained of pain. He is afebrile and points to his knee as the area of pain.
Although limping in children is common—the incidence is roughly 2 per 10001—it is never normal. It indicates pain, weakness, or structural abnormality.2 Most cases result from trauma.1 Limp usually resolves with little intervention and no sequelae. However, the differential diagnosis is broad and daunting (TABLE 1), and some causes of limp are associated with significant morbidity.
TABLE 1
Possible causes of limp in a child1-3,17
| Traumatic/mechanical Fractures, stress fractures Muscle injuries Sprains/strains Contusions Developmental dysplasia of the hip Slipped capital femoral epiphysis Tarsal coalition Child abuse Overuse injuries Leg length discrepancy |
| Infectious Septic arthritis Osteomyelitis Lyme disease Psoas abscess Diskitis |
| Inflammatory Transient synovitis Juvenile rheumatoid arthritis Ankylosing spondylitis Reiter syndrome Lupus |
| Vascular Legg-Calve-Perthes disease Osteonecrosis Hemoglobinopathies (sickle cell disease) |
| Neoplastic Leukemia, lymphoma Malignant/lytic tumors (Ewing sarcoma, osteogenic sarcoma, etc.) |
| Metabolic Rickets Hyperparathyroidism |
| Neuromuscular Muscular dystrophy Cerebral palsy Peripheral neuropathy |
Helpful tips for your initial assessment
Many textbook authors have described some causes of limp as “painless.” However, truly painless limp is rare, seldom acute, and usually the result of mechanical or neuromuscular disorders.1 A more likely explanation for acute “painless” limp is that a young child with pain is unable to express pain or accurately identify its location. Further, the child may instinctively avoid painful positions or movements and, thus, may present only with decreased movement of an extremity or refusal to bear weight.3
With a child who has knee pain, remember the pediatrics maxim: “Knee pain equals hip pain,”3 underscoring the diagnostic difficulty with limp.
Also bear in mind that children of different ages tend to have different etiologies of limp (TABLE 2). For example, septic arthritis, osteomyelitis, and transient synovitis occur more commonly in children under 10 years. Legg-Calve-Perthes disease and leukemia are more common in children between the ages of 4 and 10. Slipped capital femoral epiphysis (SCFE) is more common in boys over the age of 11.
TABLE 2
Common causes of limp according to child’s age1
| < 3 years | 3-10 years | 11-18 years |
|---|---|---|
| Foreign body | Legg-Calve-Perthes disease | Juvenile arthritis |
| Osteomyelitis | Osteomyelitis | Slipped capital femoral epiphysis |
| Septic arthritis | Septic arthritis | Trauma (physeal fracture) |
| Toddler’s fracture | Transient synovitis | Tumor |
| Transient synovitis | Trauma (physeal fracture) | |
| Tumor | Tumor |
Fracture
Fracture is a possibility across all age ranges, necessitating radiographs if suspected. Beyond detecting fractures, x-ray films can identify bony changes associated with disease (eg, Legg-Calve-Perthes disease, SCFE). Radiographs can also identify a clinically significant joint effusion at the hip.4 However, x-ray results may be falsely negative for some fracture types.
Salter-Harris Type I fractures are transverse fractures through the growth plate with epiphyseal separation from the metaphysis.5 Typical findings are a history of trauma and point tenderness over the epiphyseal plate. Type I fractures are radiographically occult, making the injury easy to mistake as a sprain. Nonetheless, growth plate injuries are common in children, requiring immobilization.
Toddler’s fracture was first described as a spiral, oblique undisplaced fracture of the distal tibial shaft in children from 9 months to 3 years of age.6 It results from a rotational or twisting force through the tibia while the leg rotates internally on a planted foot.7,8 This is the most common tibial fracture in infants and young children.9 The incidence has been reported as 0.6 to 2.5 per 1000 pediatric visits.10 Accurate diagnosis is important because current treatment recommendations suggest a long leg cast for 3 to 5 weeks, followed by a short leg cast for a total of 6 weeks.11
Despite being the most common tibial fracture, toddler’s fracture is easily missed. Initial radiographs are only 53% sensitive.7,10 This implies that nearly 50% of children with tibial fracture will have an initially negative x-ray result. However, nearly 94% of children with a confirmed toddler’s fracture have been unable to bear weight.12 Evidence suggests that despite negative radiographs, patients with point tenderness over the tibia and an inability to bear weight should be treated for presumed toddler’s fracture.12
Another confusing aspect of toddler’s fracture is that the causative injury is often considered insignificant by parents—eg, tripping, falling from a modest height, or a twisting motion.7,8 These events may occur countless times during the average day of a toddler. Often parents do not witness the injury and are unable to describe the mechanism of injury.7
When to suspect child abuse. When a child presents with fracture after an unwitnessed trauma and the story does not match the injury pattern, consider child abuse. With tibial fractures, the location of the fracture can help distinguish a result of abuse from a toddler’s fracture. Toddler’s fracture is classically described as a distal tibial fracture. In contrast, a midshaft tibial fracture often suggests child abuse.8,13 In a small retrospective study of 37 children diagnosed with toddler’s fracture, 4 midshaft tibial fractures were found.8 Child abuse was confirmed in 2 of these cases.8 However, other authors, including Dr. Dunbar in his sentinel article,6 assert that toddler’s fracture may occasionally extend into the midshaft of the tibia. Consequently, a midshaft tibial fracture is not pathognomonic for child abuse. But the diagnosis should be considered. Perform a careful examination for other signs of abuse or neglect, and do not hesitate to report suspected child abuse to the proper local and state authorities.14
Transient synovitis vs septic arthritis
A child who limps or refuses to bear weight on a limb often has associated symptoms of acute illness. In these cases, or when radiographs have ruled out apparent abnormalities such as Legg-Calve-Perthes disease, SCFE, and fracture, consider septic arthritis or transient synovitis (FIGURE). Both may present with limp and fever as well as pain, decreased range of motion, bone tenderness, swelling, and warmth.15
Transient synovitis is the most common cause of hip pain in children up to 10 years of age, with a 3% risk of occurrence through childhood.16,17 Its cause is unclear, but many experts have proposed a viral agent.17 Transient synovitis universally resolves without sequelae in 1 to 2 weeks. Therefore, prescribe rest and nonsteroidal anti-inflammatory drugs (NSAIDs) for symptomatic relief, and reassure parents.16
Septic arthritis, although often similar in presentation to transient synovitis, requires hospitalization, operative drainage, and parenteral antibiotics.18 A delay in diagnosis is associated with poor outcome, including osteonecrosis, growth arrest, permanent loss of joint function, and sepsis.3,18
Several studies have shown children with septic arthritis usually appear more acutely ill than those with transient synovitis.4,18-21 They are described as toxic-appearing, and have leukocytosis, a high erythrocyte sedimentation rate (ESR), and a high fever.19 However, no single marker or specific laboratory value consistently identifies septic arthritis. Many studies have been performed in an effort to identify a collection of factors, or an algorithm, that can predict the probability of septic arthritis.
Fever, an elevated ESR, and leukocytosis are independent multivariate clinical predictors for septic arthritis. The prediction algorithm published by Jung et al is the only study to have included C-reactive protein (CRP) as a predictive factor,4 which happens to be an excellent independent predictor of septic arthritis. Specifically, with a normal CRP <1 mg/dL, the probability of a patientnot having septic arthritis is 87%.22
While no predictive algorithm has been conclusively validated, the fact that the same clinical and laboratory predictors are consistently identified can be useful. Simply, if a patient presents with joint pain and 2 or more of the 4 predictors, septic arthritis must be fully evaluated. The presence of 2 of 4 predictors suggests a risk of septic arthritis between 10% and 40%.4,18,20 A single predictor is associated with a risk of 1% to 10%.4,18,20 Yet, you must interpret these clinical predictors in light of the full clinical picture, as septic arthritis is still possible in patients with only 1 predictor. Such possibilities require cautious management and close follow-up.
With 2 of 4 predictors present, suspect septic arthritis and order an ultrasound of the affected joint. If effusion is present, aspirate the joint. Some authors suggest that all patients with hip pain should undergo ultrasound, and that those with a joint effusion should undergo aspiration.15 However, joint aspiration, particularly of the hip, can be associated with multiple complications and should be avoided if possible.22 Effusion is also possible with transient synovitis and noninfectious causes of joint pain, but the aspirate will have a negative culture and normal gram stain findings. Ultrasound has been shown to be 100% accurate in predicting the presence of effusion.23
FIGURE
Diagnostic algorithm for pediatric limp3,4,6,8-12,15
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; MRI, magnetic resonance imaging; NSAIDs, nonsteroidal anti-inflammatory drugs; WBCs, white blood cells.
How the opening case resolved
The boy avoided weight-bearing on the affected leg, but had no focal bone tenderness. Moving the hip, but not the knee, reproduced pain. Radiographs were negative for fracture or changes typical of Legg-Calve-Perthes disease. He was afebrile in the office, but the mother described a fever at home. The child appeared ill, but stable. We decided to obtain a blood sample.
Results for CRP, ESR, and white blood cell count were normal. With this information, we reassured the mother that the diagnosis was likely transient synovitis. We advised a weight-appropriate dose of ibuprofen and scheduled a follow-up appointment for 2 days later.
CORRESPONDENCE John Whiteside, MD, St. Mary’s Family Medicine Residency, 1160 Patterson Road, Grand Junction, CO 81506; john.whiteside@stmarygj.org
1. Abbassian A. The limping child: a clinical approach to diagnosis. Br J Hosp Med. 2007;68:246-250.
2. Leung AK, Lemay JF. The limping child. J Ped Health Care. 2004;18:219-223.
3. Frick SL. Evaluation of the child who has hip pain. Orthop Clin North Am. 2006;37:133-140.
4. Jung ST, Rowe SM, Moon ES, et al. Significance of laboratory and radiologic findings for differentiating between septic arthritis and transient synovitis of the hip. J Pediatr Orthop. 2003;23:368-372.
5. Brown JH, DeLuca SA. Growth plate injuries: Salter-Harris classification. Am Fam Physician. 1992;46:1180-1184.
6. Dunbar JS, Owen HF, Nogrady MB, et al. Obscure tibial fracture of infants–the toddler’s fracture. J Can Assoc Radiol. 1964;15:136-144.
7. Miller JH, Sanderson RA. Scintigraphy of toddler’s fracture. J Nucl Med. 1988;29:2001-2003.
8. Tenenbein M, Reed MH, Black GB. The toddler’s fracture revisited. Am J Emerg Med. 1990;8:208-211.
9. Tschoepe EJ, John SD, Swischuk LE. Tibial fractures in infants and children: emphasis on subtle injuries. Emerg Radiol. 1998;5:245-252.
10. Clancy J, Pieterse J, Roberston P, et al. Toddler’s fracture. J Accid Emerg Med. 1996;13:366-367.
11. Wheeless CR. Cast treatment of tibial fractures. In:Wheeless’ Textbook of Orthopaedics. 2011. Available at:http://www.wheelessonline.com/ortho/cast_treatment_of_tibial_fractures. Accessed March 11, 2011.
12. Halsey MF, Finzel KC, Carrion WV, et al. Toddler’s fracture: presumptive diagnosis and treatment. J Pediatr Orthop. 2001;21:152-156.
13. Mellick LB, Milker L, Egsieker E. Childhood accidental spiral tibial (CAST) fractures. Ped Emerg Care. 1999;15:307-309.
14. Jenny C. Committee on Child Abuse and Neglect. Evaluating infants and young children with multiple fractures. Pediatrics. 2006;118:1299-1303.
15. Dabney KW, Lipton G. Evaluation of limp in children. Curr Opin Pediatr. 1995;7:88-94.
16. Sherry DD. Limb pain in childhood. Pediatr Rev. 1990;12:39-46.
17. Do TT. Transient synovitis as a cause of painful limps in children. Curr Opin Pediatr. 2000;12:48-51.
18. Kocher MS, Zurakowski D, Kasser JR. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 1999;81:1662-1670.
19. Luhmann SJ, Jones A, Schoolman M, et al. Differentiation between septic arthritis and transient synovitis of the hip in children with clinical prediction algorithms. J Bone Joint Surg Am. 2004;86-A:956-962.
20. Kocher MS, Mandiga R, Zurakowski D, et al. Validation of a clinical prediction rule for the differentiation between septic arthritis and transient synovitis of the hip in children. J Bone Joint Surg Am. 2004;86-A:1629-1635.
21. Delaney RA, Lenehan B, O’Sullivan L, et al. The limping child: an algorithm to outrule musculoskeletal sepsis. Ir J Med Sci. 2007;176:181-187.
22. Levine MJ, McGuire KJ, McGowan KL, et al. Assessment of the test characteristics of C-reactive protein for septic arthritis in children. J Pediatr Orthop. 2003;23:373-377.
23. Alexander JE, Seibert JJ, Glasier CM, et al. High-resolution hip ultrasound in the limping child. J Clin Ultrasound. 1989;17:19-24.
• Use radiographs to identify bone changes from disease (as well as fracture) when evaluating a limp. C
• Consider growth plate injuries as well as toddler’s fracture; both may be radiographically occult and require immobilization for treatment. C
• Consider child abuse if the patient has an isolated mid-shaft tibial fracture. C
• Assess for fever, elevated sedimentation rate, elevated C-reactive protein, and leukocytosis when radiographs are unrevealing or when a patient has systemic symptoms associated with limp. These factors are predictors of septic arthritis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A mother brings her 4-year-old son to the office because he has been limping. She isn’t aware of a specific trauma. But the boy and his twin brother, while recovering from “colds,” were rough-housing in their room when this son complained of pain. He is afebrile and points to his knee as the area of pain.
Although limping in children is common—the incidence is roughly 2 per 10001—it is never normal. It indicates pain, weakness, or structural abnormality.2 Most cases result from trauma.1 Limp usually resolves with little intervention and no sequelae. However, the differential diagnosis is broad and daunting (TABLE 1), and some causes of limp are associated with significant morbidity.
TABLE 1
Possible causes of limp in a child1-3,17
| Traumatic/mechanical Fractures, stress fractures Muscle injuries Sprains/strains Contusions Developmental dysplasia of the hip Slipped capital femoral epiphysis Tarsal coalition Child abuse Overuse injuries Leg length discrepancy |
| Infectious Septic arthritis Osteomyelitis Lyme disease Psoas abscess Diskitis |
| Inflammatory Transient synovitis Juvenile rheumatoid arthritis Ankylosing spondylitis Reiter syndrome Lupus |
| Vascular Legg-Calve-Perthes disease Osteonecrosis Hemoglobinopathies (sickle cell disease) |
| Neoplastic Leukemia, lymphoma Malignant/lytic tumors (Ewing sarcoma, osteogenic sarcoma, etc.) |
| Metabolic Rickets Hyperparathyroidism |
| Neuromuscular Muscular dystrophy Cerebral palsy Peripheral neuropathy |
Helpful tips for your initial assessment
Many textbook authors have described some causes of limp as “painless.” However, truly painless limp is rare, seldom acute, and usually the result of mechanical or neuromuscular disorders.1 A more likely explanation for acute “painless” limp is that a young child with pain is unable to express pain or accurately identify its location. Further, the child may instinctively avoid painful positions or movements and, thus, may present only with decreased movement of an extremity or refusal to bear weight.3
With a child who has knee pain, remember the pediatrics maxim: “Knee pain equals hip pain,”3 underscoring the diagnostic difficulty with limp.
Also bear in mind that children of different ages tend to have different etiologies of limp (TABLE 2). For example, septic arthritis, osteomyelitis, and transient synovitis occur more commonly in children under 10 years. Legg-Calve-Perthes disease and leukemia are more common in children between the ages of 4 and 10. Slipped capital femoral epiphysis (SCFE) is more common in boys over the age of 11.
TABLE 2
Common causes of limp according to child’s age1
| < 3 years | 3-10 years | 11-18 years |
|---|---|---|
| Foreign body | Legg-Calve-Perthes disease | Juvenile arthritis |
| Osteomyelitis | Osteomyelitis | Slipped capital femoral epiphysis |
| Septic arthritis | Septic arthritis | Trauma (physeal fracture) |
| Toddler’s fracture | Transient synovitis | Tumor |
| Transient synovitis | Trauma (physeal fracture) | |
| Tumor | Tumor |
Fracture
Fracture is a possibility across all age ranges, necessitating radiographs if suspected. Beyond detecting fractures, x-ray films can identify bony changes associated with disease (eg, Legg-Calve-Perthes disease, SCFE). Radiographs can also identify a clinically significant joint effusion at the hip.4 However, x-ray results may be falsely negative for some fracture types.
Salter-Harris Type I fractures are transverse fractures through the growth plate with epiphyseal separation from the metaphysis.5 Typical findings are a history of trauma and point tenderness over the epiphyseal plate. Type I fractures are radiographically occult, making the injury easy to mistake as a sprain. Nonetheless, growth plate injuries are common in children, requiring immobilization.
Toddler’s fracture was first described as a spiral, oblique undisplaced fracture of the distal tibial shaft in children from 9 months to 3 years of age.6 It results from a rotational or twisting force through the tibia while the leg rotates internally on a planted foot.7,8 This is the most common tibial fracture in infants and young children.9 The incidence has been reported as 0.6 to 2.5 per 1000 pediatric visits.10 Accurate diagnosis is important because current treatment recommendations suggest a long leg cast for 3 to 5 weeks, followed by a short leg cast for a total of 6 weeks.11
Despite being the most common tibial fracture, toddler’s fracture is easily missed. Initial radiographs are only 53% sensitive.7,10 This implies that nearly 50% of children with tibial fracture will have an initially negative x-ray result. However, nearly 94% of children with a confirmed toddler’s fracture have been unable to bear weight.12 Evidence suggests that despite negative radiographs, patients with point tenderness over the tibia and an inability to bear weight should be treated for presumed toddler’s fracture.12
Another confusing aspect of toddler’s fracture is that the causative injury is often considered insignificant by parents—eg, tripping, falling from a modest height, or a twisting motion.7,8 These events may occur countless times during the average day of a toddler. Often parents do not witness the injury and are unable to describe the mechanism of injury.7
When to suspect child abuse. When a child presents with fracture after an unwitnessed trauma and the story does not match the injury pattern, consider child abuse. With tibial fractures, the location of the fracture can help distinguish a result of abuse from a toddler’s fracture. Toddler’s fracture is classically described as a distal tibial fracture. In contrast, a midshaft tibial fracture often suggests child abuse.8,13 In a small retrospective study of 37 children diagnosed with toddler’s fracture, 4 midshaft tibial fractures were found.8 Child abuse was confirmed in 2 of these cases.8 However, other authors, including Dr. Dunbar in his sentinel article,6 assert that toddler’s fracture may occasionally extend into the midshaft of the tibia. Consequently, a midshaft tibial fracture is not pathognomonic for child abuse. But the diagnosis should be considered. Perform a careful examination for other signs of abuse or neglect, and do not hesitate to report suspected child abuse to the proper local and state authorities.14
Transient synovitis vs septic arthritis
A child who limps or refuses to bear weight on a limb often has associated symptoms of acute illness. In these cases, or when radiographs have ruled out apparent abnormalities such as Legg-Calve-Perthes disease, SCFE, and fracture, consider septic arthritis or transient synovitis (FIGURE). Both may present with limp and fever as well as pain, decreased range of motion, bone tenderness, swelling, and warmth.15
Transient synovitis is the most common cause of hip pain in children up to 10 years of age, with a 3% risk of occurrence through childhood.16,17 Its cause is unclear, but many experts have proposed a viral agent.17 Transient synovitis universally resolves without sequelae in 1 to 2 weeks. Therefore, prescribe rest and nonsteroidal anti-inflammatory drugs (NSAIDs) for symptomatic relief, and reassure parents.16
Septic arthritis, although often similar in presentation to transient synovitis, requires hospitalization, operative drainage, and parenteral antibiotics.18 A delay in diagnosis is associated with poor outcome, including osteonecrosis, growth arrest, permanent loss of joint function, and sepsis.3,18
Several studies have shown children with septic arthritis usually appear more acutely ill than those with transient synovitis.4,18-21 They are described as toxic-appearing, and have leukocytosis, a high erythrocyte sedimentation rate (ESR), and a high fever.19 However, no single marker or specific laboratory value consistently identifies septic arthritis. Many studies have been performed in an effort to identify a collection of factors, or an algorithm, that can predict the probability of septic arthritis.
Fever, an elevated ESR, and leukocytosis are independent multivariate clinical predictors for septic arthritis. The prediction algorithm published by Jung et al is the only study to have included C-reactive protein (CRP) as a predictive factor,4 which happens to be an excellent independent predictor of septic arthritis. Specifically, with a normal CRP <1 mg/dL, the probability of a patientnot having septic arthritis is 87%.22
While no predictive algorithm has been conclusively validated, the fact that the same clinical and laboratory predictors are consistently identified can be useful. Simply, if a patient presents with joint pain and 2 or more of the 4 predictors, septic arthritis must be fully evaluated. The presence of 2 of 4 predictors suggests a risk of septic arthritis between 10% and 40%.4,18,20 A single predictor is associated with a risk of 1% to 10%.4,18,20 Yet, you must interpret these clinical predictors in light of the full clinical picture, as septic arthritis is still possible in patients with only 1 predictor. Such possibilities require cautious management and close follow-up.
With 2 of 4 predictors present, suspect septic arthritis and order an ultrasound of the affected joint. If effusion is present, aspirate the joint. Some authors suggest that all patients with hip pain should undergo ultrasound, and that those with a joint effusion should undergo aspiration.15 However, joint aspiration, particularly of the hip, can be associated with multiple complications and should be avoided if possible.22 Effusion is also possible with transient synovitis and noninfectious causes of joint pain, but the aspirate will have a negative culture and normal gram stain findings. Ultrasound has been shown to be 100% accurate in predicting the presence of effusion.23
FIGURE
Diagnostic algorithm for pediatric limp3,4,6,8-12,15
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; MRI, magnetic resonance imaging; NSAIDs, nonsteroidal anti-inflammatory drugs; WBCs, white blood cells.
How the opening case resolved
The boy avoided weight-bearing on the affected leg, but had no focal bone tenderness. Moving the hip, but not the knee, reproduced pain. Radiographs were negative for fracture or changes typical of Legg-Calve-Perthes disease. He was afebrile in the office, but the mother described a fever at home. The child appeared ill, but stable. We decided to obtain a blood sample.
Results for CRP, ESR, and white blood cell count were normal. With this information, we reassured the mother that the diagnosis was likely transient synovitis. We advised a weight-appropriate dose of ibuprofen and scheduled a follow-up appointment for 2 days later.
CORRESPONDENCE John Whiteside, MD, St. Mary’s Family Medicine Residency, 1160 Patterson Road, Grand Junction, CO 81506; john.whiteside@stmarygj.org
• Use radiographs to identify bone changes from disease (as well as fracture) when evaluating a limp. C
• Consider growth plate injuries as well as toddler’s fracture; both may be radiographically occult and require immobilization for treatment. C
• Consider child abuse if the patient has an isolated mid-shaft tibial fracture. C
• Assess for fever, elevated sedimentation rate, elevated C-reactive protein, and leukocytosis when radiographs are unrevealing or when a patient has systemic symptoms associated with limp. These factors are predictors of septic arthritis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A mother brings her 4-year-old son to the office because he has been limping. She isn’t aware of a specific trauma. But the boy and his twin brother, while recovering from “colds,” were rough-housing in their room when this son complained of pain. He is afebrile and points to his knee as the area of pain.
Although limping in children is common—the incidence is roughly 2 per 10001—it is never normal. It indicates pain, weakness, or structural abnormality.2 Most cases result from trauma.1 Limp usually resolves with little intervention and no sequelae. However, the differential diagnosis is broad and daunting (TABLE 1), and some causes of limp are associated with significant morbidity.
TABLE 1
Possible causes of limp in a child1-3,17
| Traumatic/mechanical Fractures, stress fractures Muscle injuries Sprains/strains Contusions Developmental dysplasia of the hip Slipped capital femoral epiphysis Tarsal coalition Child abuse Overuse injuries Leg length discrepancy |
| Infectious Septic arthritis Osteomyelitis Lyme disease Psoas abscess Diskitis |
| Inflammatory Transient synovitis Juvenile rheumatoid arthritis Ankylosing spondylitis Reiter syndrome Lupus |
| Vascular Legg-Calve-Perthes disease Osteonecrosis Hemoglobinopathies (sickle cell disease) |
| Neoplastic Leukemia, lymphoma Malignant/lytic tumors (Ewing sarcoma, osteogenic sarcoma, etc.) |
| Metabolic Rickets Hyperparathyroidism |
| Neuromuscular Muscular dystrophy Cerebral palsy Peripheral neuropathy |
Helpful tips for your initial assessment
Many textbook authors have described some causes of limp as “painless.” However, truly painless limp is rare, seldom acute, and usually the result of mechanical or neuromuscular disorders.1 A more likely explanation for acute “painless” limp is that a young child with pain is unable to express pain or accurately identify its location. Further, the child may instinctively avoid painful positions or movements and, thus, may present only with decreased movement of an extremity or refusal to bear weight.3
With a child who has knee pain, remember the pediatrics maxim: “Knee pain equals hip pain,”3 underscoring the diagnostic difficulty with limp.
Also bear in mind that children of different ages tend to have different etiologies of limp (TABLE 2). For example, septic arthritis, osteomyelitis, and transient synovitis occur more commonly in children under 10 years. Legg-Calve-Perthes disease and leukemia are more common in children between the ages of 4 and 10. Slipped capital femoral epiphysis (SCFE) is more common in boys over the age of 11.
TABLE 2
Common causes of limp according to child’s age1
| < 3 years | 3-10 years | 11-18 years |
|---|---|---|
| Foreign body | Legg-Calve-Perthes disease | Juvenile arthritis |
| Osteomyelitis | Osteomyelitis | Slipped capital femoral epiphysis |
| Septic arthritis | Septic arthritis | Trauma (physeal fracture) |
| Toddler’s fracture | Transient synovitis | Tumor |
| Transient synovitis | Trauma (physeal fracture) | |
| Tumor | Tumor |
Fracture
Fracture is a possibility across all age ranges, necessitating radiographs if suspected. Beyond detecting fractures, x-ray films can identify bony changes associated with disease (eg, Legg-Calve-Perthes disease, SCFE). Radiographs can also identify a clinically significant joint effusion at the hip.4 However, x-ray results may be falsely negative for some fracture types.
Salter-Harris Type I fractures are transverse fractures through the growth plate with epiphyseal separation from the metaphysis.5 Typical findings are a history of trauma and point tenderness over the epiphyseal plate. Type I fractures are radiographically occult, making the injury easy to mistake as a sprain. Nonetheless, growth plate injuries are common in children, requiring immobilization.
Toddler’s fracture was first described as a spiral, oblique undisplaced fracture of the distal tibial shaft in children from 9 months to 3 years of age.6 It results from a rotational or twisting force through the tibia while the leg rotates internally on a planted foot.7,8 This is the most common tibial fracture in infants and young children.9 The incidence has been reported as 0.6 to 2.5 per 1000 pediatric visits.10 Accurate diagnosis is important because current treatment recommendations suggest a long leg cast for 3 to 5 weeks, followed by a short leg cast for a total of 6 weeks.11
Despite being the most common tibial fracture, toddler’s fracture is easily missed. Initial radiographs are only 53% sensitive.7,10 This implies that nearly 50% of children with tibial fracture will have an initially negative x-ray result. However, nearly 94% of children with a confirmed toddler’s fracture have been unable to bear weight.12 Evidence suggests that despite negative radiographs, patients with point tenderness over the tibia and an inability to bear weight should be treated for presumed toddler’s fracture.12
Another confusing aspect of toddler’s fracture is that the causative injury is often considered insignificant by parents—eg, tripping, falling from a modest height, or a twisting motion.7,8 These events may occur countless times during the average day of a toddler. Often parents do not witness the injury and are unable to describe the mechanism of injury.7
When to suspect child abuse. When a child presents with fracture after an unwitnessed trauma and the story does not match the injury pattern, consider child abuse. With tibial fractures, the location of the fracture can help distinguish a result of abuse from a toddler’s fracture. Toddler’s fracture is classically described as a distal tibial fracture. In contrast, a midshaft tibial fracture often suggests child abuse.8,13 In a small retrospective study of 37 children diagnosed with toddler’s fracture, 4 midshaft tibial fractures were found.8 Child abuse was confirmed in 2 of these cases.8 However, other authors, including Dr. Dunbar in his sentinel article,6 assert that toddler’s fracture may occasionally extend into the midshaft of the tibia. Consequently, a midshaft tibial fracture is not pathognomonic for child abuse. But the diagnosis should be considered. Perform a careful examination for other signs of abuse or neglect, and do not hesitate to report suspected child abuse to the proper local and state authorities.14
Transient synovitis vs septic arthritis
A child who limps or refuses to bear weight on a limb often has associated symptoms of acute illness. In these cases, or when radiographs have ruled out apparent abnormalities such as Legg-Calve-Perthes disease, SCFE, and fracture, consider septic arthritis or transient synovitis (FIGURE). Both may present with limp and fever as well as pain, decreased range of motion, bone tenderness, swelling, and warmth.15
Transient synovitis is the most common cause of hip pain in children up to 10 years of age, with a 3% risk of occurrence through childhood.16,17 Its cause is unclear, but many experts have proposed a viral agent.17 Transient synovitis universally resolves without sequelae in 1 to 2 weeks. Therefore, prescribe rest and nonsteroidal anti-inflammatory drugs (NSAIDs) for symptomatic relief, and reassure parents.16
Septic arthritis, although often similar in presentation to transient synovitis, requires hospitalization, operative drainage, and parenteral antibiotics.18 A delay in diagnosis is associated with poor outcome, including osteonecrosis, growth arrest, permanent loss of joint function, and sepsis.3,18
Several studies have shown children with septic arthritis usually appear more acutely ill than those with transient synovitis.4,18-21 They are described as toxic-appearing, and have leukocytosis, a high erythrocyte sedimentation rate (ESR), and a high fever.19 However, no single marker or specific laboratory value consistently identifies septic arthritis. Many studies have been performed in an effort to identify a collection of factors, or an algorithm, that can predict the probability of septic arthritis.
Fever, an elevated ESR, and leukocytosis are independent multivariate clinical predictors for septic arthritis. The prediction algorithm published by Jung et al is the only study to have included C-reactive protein (CRP) as a predictive factor,4 which happens to be an excellent independent predictor of septic arthritis. Specifically, with a normal CRP <1 mg/dL, the probability of a patientnot having septic arthritis is 87%.22
While no predictive algorithm has been conclusively validated, the fact that the same clinical and laboratory predictors are consistently identified can be useful. Simply, if a patient presents with joint pain and 2 or more of the 4 predictors, septic arthritis must be fully evaluated. The presence of 2 of 4 predictors suggests a risk of septic arthritis between 10% and 40%.4,18,20 A single predictor is associated with a risk of 1% to 10%.4,18,20 Yet, you must interpret these clinical predictors in light of the full clinical picture, as septic arthritis is still possible in patients with only 1 predictor. Such possibilities require cautious management and close follow-up.
With 2 of 4 predictors present, suspect septic arthritis and order an ultrasound of the affected joint. If effusion is present, aspirate the joint. Some authors suggest that all patients with hip pain should undergo ultrasound, and that those with a joint effusion should undergo aspiration.15 However, joint aspiration, particularly of the hip, can be associated with multiple complications and should be avoided if possible.22 Effusion is also possible with transient synovitis and noninfectious causes of joint pain, but the aspirate will have a negative culture and normal gram stain findings. Ultrasound has been shown to be 100% accurate in predicting the presence of effusion.23
FIGURE
Diagnostic algorithm for pediatric limp3,4,6,8-12,15
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; MRI, magnetic resonance imaging; NSAIDs, nonsteroidal anti-inflammatory drugs; WBCs, white blood cells.
How the opening case resolved
The boy avoided weight-bearing on the affected leg, but had no focal bone tenderness. Moving the hip, but not the knee, reproduced pain. Radiographs were negative for fracture or changes typical of Legg-Calve-Perthes disease. He was afebrile in the office, but the mother described a fever at home. The child appeared ill, but stable. We decided to obtain a blood sample.
Results for CRP, ESR, and white blood cell count were normal. With this information, we reassured the mother that the diagnosis was likely transient synovitis. We advised a weight-appropriate dose of ibuprofen and scheduled a follow-up appointment for 2 days later.
CORRESPONDENCE John Whiteside, MD, St. Mary’s Family Medicine Residency, 1160 Patterson Road, Grand Junction, CO 81506; john.whiteside@stmarygj.org
1. Abbassian A. The limping child: a clinical approach to diagnosis. Br J Hosp Med. 2007;68:246-250.
2. Leung AK, Lemay JF. The limping child. J Ped Health Care. 2004;18:219-223.
3. Frick SL. Evaluation of the child who has hip pain. Orthop Clin North Am. 2006;37:133-140.
4. Jung ST, Rowe SM, Moon ES, et al. Significance of laboratory and radiologic findings for differentiating between septic arthritis and transient synovitis of the hip. J Pediatr Orthop. 2003;23:368-372.
5. Brown JH, DeLuca SA. Growth plate injuries: Salter-Harris classification. Am Fam Physician. 1992;46:1180-1184.
6. Dunbar JS, Owen HF, Nogrady MB, et al. Obscure tibial fracture of infants–the toddler’s fracture. J Can Assoc Radiol. 1964;15:136-144.
7. Miller JH, Sanderson RA. Scintigraphy of toddler’s fracture. J Nucl Med. 1988;29:2001-2003.
8. Tenenbein M, Reed MH, Black GB. The toddler’s fracture revisited. Am J Emerg Med. 1990;8:208-211.
9. Tschoepe EJ, John SD, Swischuk LE. Tibial fractures in infants and children: emphasis on subtle injuries. Emerg Radiol. 1998;5:245-252.
10. Clancy J, Pieterse J, Roberston P, et al. Toddler’s fracture. J Accid Emerg Med. 1996;13:366-367.
11. Wheeless CR. Cast treatment of tibial fractures. In:Wheeless’ Textbook of Orthopaedics. 2011. Available at:http://www.wheelessonline.com/ortho/cast_treatment_of_tibial_fractures. Accessed March 11, 2011.
12. Halsey MF, Finzel KC, Carrion WV, et al. Toddler’s fracture: presumptive diagnosis and treatment. J Pediatr Orthop. 2001;21:152-156.
13. Mellick LB, Milker L, Egsieker E. Childhood accidental spiral tibial (CAST) fractures. Ped Emerg Care. 1999;15:307-309.
14. Jenny C. Committee on Child Abuse and Neglect. Evaluating infants and young children with multiple fractures. Pediatrics. 2006;118:1299-1303.
15. Dabney KW, Lipton G. Evaluation of limp in children. Curr Opin Pediatr. 1995;7:88-94.
16. Sherry DD. Limb pain in childhood. Pediatr Rev. 1990;12:39-46.
17. Do TT. Transient synovitis as a cause of painful limps in children. Curr Opin Pediatr. 2000;12:48-51.
18. Kocher MS, Zurakowski D, Kasser JR. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 1999;81:1662-1670.
19. Luhmann SJ, Jones A, Schoolman M, et al. Differentiation between septic arthritis and transient synovitis of the hip in children with clinical prediction algorithms. J Bone Joint Surg Am. 2004;86-A:956-962.
20. Kocher MS, Mandiga R, Zurakowski D, et al. Validation of a clinical prediction rule for the differentiation between septic arthritis and transient synovitis of the hip in children. J Bone Joint Surg Am. 2004;86-A:1629-1635.
21. Delaney RA, Lenehan B, O’Sullivan L, et al. The limping child: an algorithm to outrule musculoskeletal sepsis. Ir J Med Sci. 2007;176:181-187.
22. Levine MJ, McGuire KJ, McGowan KL, et al. Assessment of the test characteristics of C-reactive protein for septic arthritis in children. J Pediatr Orthop. 2003;23:373-377.
23. Alexander JE, Seibert JJ, Glasier CM, et al. High-resolution hip ultrasound in the limping child. J Clin Ultrasound. 1989;17:19-24.
1. Abbassian A. The limping child: a clinical approach to diagnosis. Br J Hosp Med. 2007;68:246-250.
2. Leung AK, Lemay JF. The limping child. J Ped Health Care. 2004;18:219-223.
3. Frick SL. Evaluation of the child who has hip pain. Orthop Clin North Am. 2006;37:133-140.
4. Jung ST, Rowe SM, Moon ES, et al. Significance of laboratory and radiologic findings for differentiating between septic arthritis and transient synovitis of the hip. J Pediatr Orthop. 2003;23:368-372.
5. Brown JH, DeLuca SA. Growth plate injuries: Salter-Harris classification. Am Fam Physician. 1992;46:1180-1184.
6. Dunbar JS, Owen HF, Nogrady MB, et al. Obscure tibial fracture of infants–the toddler’s fracture. J Can Assoc Radiol. 1964;15:136-144.
7. Miller JH, Sanderson RA. Scintigraphy of toddler’s fracture. J Nucl Med. 1988;29:2001-2003.
8. Tenenbein M, Reed MH, Black GB. The toddler’s fracture revisited. Am J Emerg Med. 1990;8:208-211.
9. Tschoepe EJ, John SD, Swischuk LE. Tibial fractures in infants and children: emphasis on subtle injuries. Emerg Radiol. 1998;5:245-252.
10. Clancy J, Pieterse J, Roberston P, et al. Toddler’s fracture. J Accid Emerg Med. 1996;13:366-367.
11. Wheeless CR. Cast treatment of tibial fractures. In:Wheeless’ Textbook of Orthopaedics. 2011. Available at:http://www.wheelessonline.com/ortho/cast_treatment_of_tibial_fractures. Accessed March 11, 2011.
12. Halsey MF, Finzel KC, Carrion WV, et al. Toddler’s fracture: presumptive diagnosis and treatment. J Pediatr Orthop. 2001;21:152-156.
13. Mellick LB, Milker L, Egsieker E. Childhood accidental spiral tibial (CAST) fractures. Ped Emerg Care. 1999;15:307-309.
14. Jenny C. Committee on Child Abuse and Neglect. Evaluating infants and young children with multiple fractures. Pediatrics. 2006;118:1299-1303.
15. Dabney KW, Lipton G. Evaluation of limp in children. Curr Opin Pediatr. 1995;7:88-94.
16. Sherry DD. Limb pain in childhood. Pediatr Rev. 1990;12:39-46.
17. Do TT. Transient synovitis as a cause of painful limps in children. Curr Opin Pediatr. 2000;12:48-51.
18. Kocher MS, Zurakowski D, Kasser JR. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 1999;81:1662-1670.
19. Luhmann SJ, Jones A, Schoolman M, et al. Differentiation between septic arthritis and transient synovitis of the hip in children with clinical prediction algorithms. J Bone Joint Surg Am. 2004;86-A:956-962.
20. Kocher MS, Mandiga R, Zurakowski D, et al. Validation of a clinical prediction rule for the differentiation between septic arthritis and transient synovitis of the hip in children. J Bone Joint Surg Am. 2004;86-A:1629-1635.
21. Delaney RA, Lenehan B, O’Sullivan L, et al. The limping child: an algorithm to outrule musculoskeletal sepsis. Ir J Med Sci. 2007;176:181-187.
22. Levine MJ, McGuire KJ, McGowan KL, et al. Assessment of the test characteristics of C-reactive protein for septic arthritis in children. J Pediatr Orthop. 2003;23:373-377.
23. Alexander JE, Seibert JJ, Glasier CM, et al. High-resolution hip ultrasound in the limping child. J Clin Ultrasound. 1989;17:19-24.