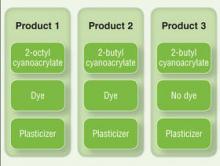
User login
Wearable Health Device Dermatitis: A Case of Acrylate-Related Contact Allergy
Mobile health devices enable patients and clinicians to monitor the type, quantity, and quality of everyday activities and hold the promise of improving patient health and health care practices.1 In 2013, 75% of surveyed consumers in the United States owned a fitness technology product, either a dedicated fitness device, application, or portable blood pressure monitor.2 Ownership of dedicated wearable fitness devices among consumers in the United States increased from 3% in 2012 to 9% in 2013. The immense popularity of wearable fitness devices is evident in the trajectory of their reported sales, which increased from $43 million in 2009 to $854 million in 2013.2 Recognizing that “widespread adoption and use of mobile technologies is opening new and innovative ways to improve health,”3 the US Food and Drug Administration (FDA) ruled that “[technologies] that can pose a greater risk to patients will require FDA review.” One popular class of mobile technologies—activity and sleep sensors—falls outside the FDA’s regulatory guidance. To enable continuous monitoring, these sensors often are embedded into wearable devices.
Reports in the media have documented skin rashes arising in conjunction with use of one type of device,4 which may be related to nickel contact allergy, and the manufacturer has reported that the metal housing consists of surgical stainless steel that is known to contain nickel. We report a complication related to continuous use of an unregulated, commercially available, watchlike wearable sensor that was linked not to nickel but to an acrylate-containing component.
Case Report
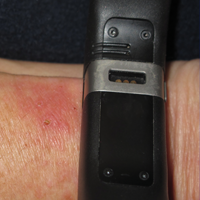
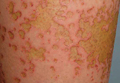
An otherwise healthy 52-year-old woman with no history of contact allergy presented with an intensely itchy eruption involving the left wrist arising 4 days after continuous use of a new watchlike wearable fitness sensor. By day 11, the eruption evolved into a well-demarcated, erythematous, scaly plaque at the location where the device’s rechargeable battery metal housing came into contact with skin (Figure 1).
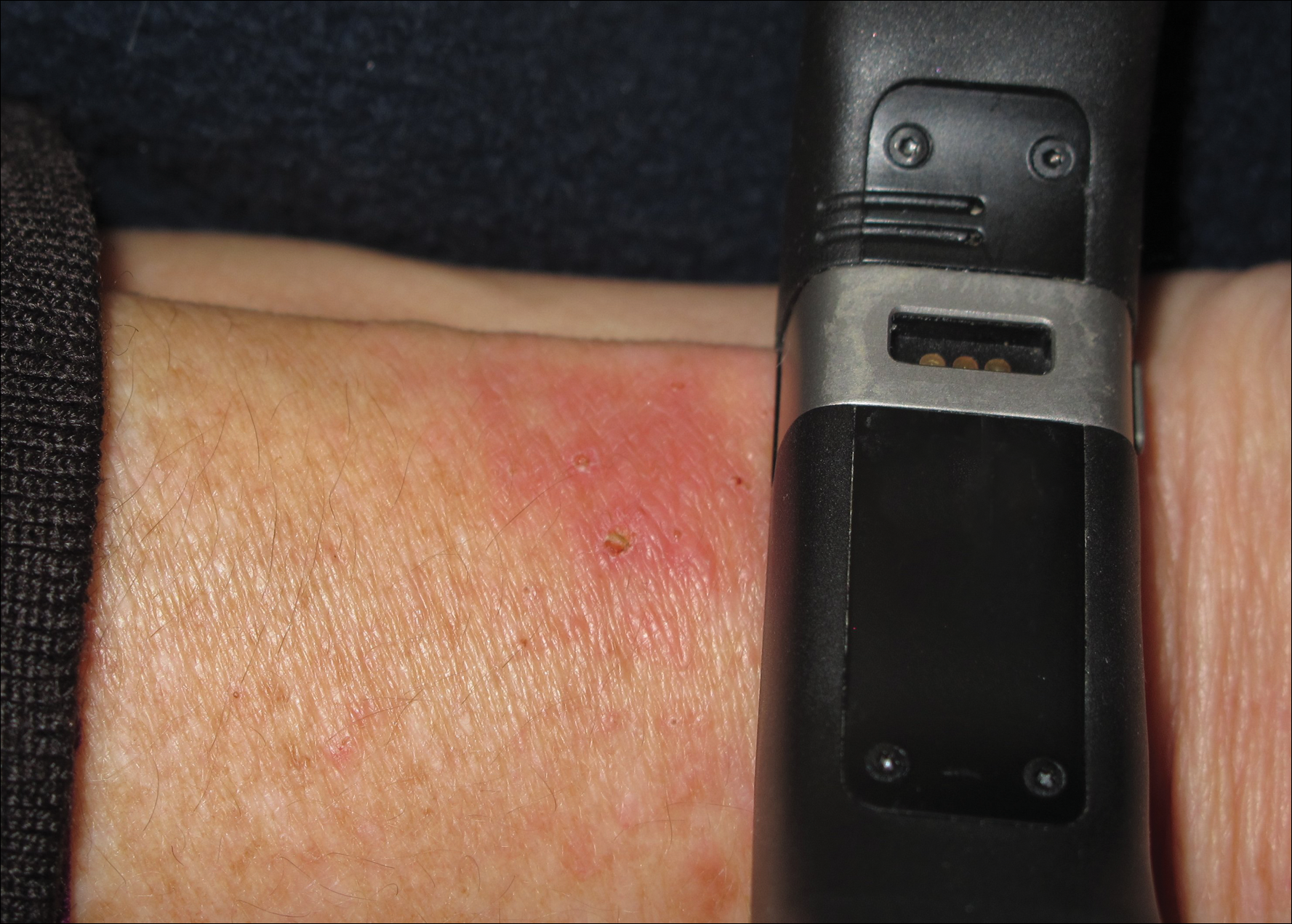
Dimethylglyoxime testing of the metal housing and clips was negative, but testing of contacts within the housing was positive for nickel (Figure 2). Epicutaneous patch testing of the patient using a modified North American Contact Dermatitis Group patch test series (Table) demonstrated no reaction to nickel, instead showing a strong positive (2+) reaction at 48 and 72 hours to methyl methacrylate 2% and a positive (1+) reaction at 96 hours to ethyl acrylate 0.1% (Figure 3).


Comment
Acrylates are used as adhesives to bond metal to plastic and as part of lithium ion polymer batteries, presumably similar to the one used in this device.5 Our patient had a history of using acrylic nail polish, which may have been a source of prior sensitization. Exposure to sweat or other moisture could theoretically dissolve such a water-soluble polymer,6 allowing for skin contact. Other acrylate polymers have been reported to break down slowly in contact with water, leading to contact sensitization to the monomer.7 The manufacturer of the device was contacted for additional information but declined to provide specific details regarding the device’s composition (personal communication, January 2014).
Although not considered toxic,8 acrylate was named Allergen of the Year in 2012 by the American Contact Dermatitis Society.9-11 Nickel might be a source of allergy for some other patients who wear mobile health devices, but we concluded that this particular patient developed allergic contact dermatitis from prolonged exposure to low levels of methyl methacrylate or another acrylate due to gradual breakdown of the acrylate polymer used in the rechargeable battery housing for this wearable health device.
Given the FDA’s tailored risk approach to regulation, many wearable sensors that may contain potential contact allergens such as nickel and acrylates do not fall under the FDA regulatory framework. This case should alert physicians to the lack of regulatory oversight for many mobile technologies. They should consider a screening history for contact allergens before recommending wearable sensors and broader testing for contact allergens should exposed patients develop reactions. Future wearable sensor materials and designs should minimize exposure to allergens given prolonged contact with continuous use. In the absence of regulation, manufacturers of these devices should consider due care testing prior to commercialization.
Acknowledgment
We are indebted to Alexander S. Rattner, PhD (State College, Pennsylvania), who provided his engineering expertise and insight during conversations with the authors.
- Dobkin BH, Dorsch A. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25:788-798.
- Consumer interest in purchasing wearable fitness devices in 2014 quadruples, according to CEA Study [press release]. Arlington, VA: Consumer Electronics Association; December 11, 2013.
- US Food and Drug Administration. Mobile medical applications. http://www.fda.gov/medicaldevices/digitalhealth/mobilemedicalapplications/default.htm. Updated September 22, 2015. Accessed July 26, 2017.
- Northrup L. Fitbit Force is an amazing device, except for my contact dermatitis. Consumerist website. http://consumerist.com/2014/01/13/fitbit-force-is-an-amazing-device-except-for-my-contact-dermatitis/. Published January 13, 2014. Accessed January 12, 2017.
- Stern B. Inside Fitbit Force. Adafruit website. http://learn.adafruit.com/fitbit-force-teardown/inside-fitbit-force. Published December 11, 2013. Updated May 4, 2015. Accessed January 12, 2017.
- Pemberton MA, Lohmann BS. Risk assessment of residual monomer migrating from acrylic polymers and causing allergic contact dermatitis during normal handling and use. Regul Toxicol Pharmacol. 2014;69:467-475.
- Guin JD, Baas K, Nelson-Adesokan P. Contact sensitization to cyanoacrylate adhesive as a cause of severe onychodystrophy. Int J Dermatol. 1998;37:31-36.
- Zondlo Fiume M. Final report on the safety assessment of Acrylates Copolymer and 33 related cosmetic ingredients. Int J Toxicol. 2002;21(suppl 3):1-50.
- Sasseville D. Acrylates. Dermatitis. 2012;23:3-5.
- Bowen C, Bidinger J, Hivnor C, et al. Allergic contact dermatitis to 2-octyl cyanoacrylate. Cutis. 2014;94:183-186.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review [published online July 11, 2016]. Contact Dermatitis. 2016;75:157-164.
Mobile health devices enable patients and clinicians to monitor the type, quantity, and quality of everyday activities and hold the promise of improving patient health and health care practices.1 In 2013, 75% of surveyed consumers in the United States owned a fitness technology product, either a dedicated fitness device, application, or portable blood pressure monitor.2 Ownership of dedicated wearable fitness devices among consumers in the United States increased from 3% in 2012 to 9% in 2013. The immense popularity of wearable fitness devices is evident in the trajectory of their reported sales, which increased from $43 million in 2009 to $854 million in 2013.2 Recognizing that “widespread adoption and use of mobile technologies is opening new and innovative ways to improve health,”3 the US Food and Drug Administration (FDA) ruled that “[technologies] that can pose a greater risk to patients will require FDA review.” One popular class of mobile technologies—activity and sleep sensors—falls outside the FDA’s regulatory guidance. To enable continuous monitoring, these sensors often are embedded into wearable devices.
Reports in the media have documented skin rashes arising in conjunction with use of one type of device,4 which may be related to nickel contact allergy, and the manufacturer has reported that the metal housing consists of surgical stainless steel that is known to contain nickel. We report a complication related to continuous use of an unregulated, commercially available, watchlike wearable sensor that was linked not to nickel but to an acrylate-containing component.
Case Report
An otherwise healthy 52-year-old woman with no history of contact allergy presented with an intensely itchy eruption involving the left wrist arising 4 days after continuous use of a new watchlike wearable fitness sensor. By day 11, the eruption evolved into a well-demarcated, erythematous, scaly plaque at the location where the device’s rechargeable battery metal housing came into contact with skin (Figure 1).

Dimethylglyoxime testing of the metal housing and clips was negative, but testing of contacts within the housing was positive for nickel (Figure 2). Epicutaneous patch testing of the patient using a modified North American Contact Dermatitis Group patch test series (Table) demonstrated no reaction to nickel, instead showing a strong positive (2+) reaction at 48 and 72 hours to methyl methacrylate 2% and a positive (1+) reaction at 96 hours to ethyl acrylate 0.1% (Figure 3).


Comment
Acrylates are used as adhesives to bond metal to plastic and as part of lithium ion polymer batteries, presumably similar to the one used in this device.5 Our patient had a history of using acrylic nail polish, which may have been a source of prior sensitization. Exposure to sweat or other moisture could theoretically dissolve such a water-soluble polymer,6 allowing for skin contact. Other acrylate polymers have been reported to break down slowly in contact with water, leading to contact sensitization to the monomer.7 The manufacturer of the device was contacted for additional information but declined to provide specific details regarding the device’s composition (personal communication, January 2014).
Although not considered toxic,8 acrylate was named Allergen of the Year in 2012 by the American Contact Dermatitis Society.9-11 Nickel might be a source of allergy for some other patients who wear mobile health devices, but we concluded that this particular patient developed allergic contact dermatitis from prolonged exposure to low levels of methyl methacrylate or another acrylate due to gradual breakdown of the acrylate polymer used in the rechargeable battery housing for this wearable health device.
Given the FDA’s tailored risk approach to regulation, many wearable sensors that may contain potential contact allergens such as nickel and acrylates do not fall under the FDA regulatory framework. This case should alert physicians to the lack of regulatory oversight for many mobile technologies. They should consider a screening history for contact allergens before recommending wearable sensors and broader testing for contact allergens should exposed patients develop reactions. Future wearable sensor materials and designs should minimize exposure to allergens given prolonged contact with continuous use. In the absence of regulation, manufacturers of these devices should consider due care testing prior to commercialization.
Acknowledgment
We are indebted to Alexander S. Rattner, PhD (State College, Pennsylvania), who provided his engineering expertise and insight during conversations with the authors.
Mobile health devices enable patients and clinicians to monitor the type, quantity, and quality of everyday activities and hold the promise of improving patient health and health care practices.1 In 2013, 75% of surveyed consumers in the United States owned a fitness technology product, either a dedicated fitness device, application, or portable blood pressure monitor.2 Ownership of dedicated wearable fitness devices among consumers in the United States increased from 3% in 2012 to 9% in 2013. The immense popularity of wearable fitness devices is evident in the trajectory of their reported sales, which increased from $43 million in 2009 to $854 million in 2013.2 Recognizing that “widespread adoption and use of mobile technologies is opening new and innovative ways to improve health,”3 the US Food and Drug Administration (FDA) ruled that “[technologies] that can pose a greater risk to patients will require FDA review.” One popular class of mobile technologies—activity and sleep sensors—falls outside the FDA’s regulatory guidance. To enable continuous monitoring, these sensors often are embedded into wearable devices.
Reports in the media have documented skin rashes arising in conjunction with use of one type of device,4 which may be related to nickel contact allergy, and the manufacturer has reported that the metal housing consists of surgical stainless steel that is known to contain nickel. We report a complication related to continuous use of an unregulated, commercially available, watchlike wearable sensor that was linked not to nickel but to an acrylate-containing component.
Case Report
An otherwise healthy 52-year-old woman with no history of contact allergy presented with an intensely itchy eruption involving the left wrist arising 4 days after continuous use of a new watchlike wearable fitness sensor. By day 11, the eruption evolved into a well-demarcated, erythematous, scaly plaque at the location where the device’s rechargeable battery metal housing came into contact with skin (Figure 1).

Dimethylglyoxime testing of the metal housing and clips was negative, but testing of contacts within the housing was positive for nickel (Figure 2). Epicutaneous patch testing of the patient using a modified North American Contact Dermatitis Group patch test series (Table) demonstrated no reaction to nickel, instead showing a strong positive (2+) reaction at 48 and 72 hours to methyl methacrylate 2% and a positive (1+) reaction at 96 hours to ethyl acrylate 0.1% (Figure 3).


Comment
Acrylates are used as adhesives to bond metal to plastic and as part of lithium ion polymer batteries, presumably similar to the one used in this device.5 Our patient had a history of using acrylic nail polish, which may have been a source of prior sensitization. Exposure to sweat or other moisture could theoretically dissolve such a water-soluble polymer,6 allowing for skin contact. Other acrylate polymers have been reported to break down slowly in contact with water, leading to contact sensitization to the monomer.7 The manufacturer of the device was contacted for additional information but declined to provide specific details regarding the device’s composition (personal communication, January 2014).
Although not considered toxic,8 acrylate was named Allergen of the Year in 2012 by the American Contact Dermatitis Society.9-11 Nickel might be a source of allergy for some other patients who wear mobile health devices, but we concluded that this particular patient developed allergic contact dermatitis from prolonged exposure to low levels of methyl methacrylate or another acrylate due to gradual breakdown of the acrylate polymer used in the rechargeable battery housing for this wearable health device.
Given the FDA’s tailored risk approach to regulation, many wearable sensors that may contain potential contact allergens such as nickel and acrylates do not fall under the FDA regulatory framework. This case should alert physicians to the lack of regulatory oversight for many mobile technologies. They should consider a screening history for contact allergens before recommending wearable sensors and broader testing for contact allergens should exposed patients develop reactions. Future wearable sensor materials and designs should minimize exposure to allergens given prolonged contact with continuous use. In the absence of regulation, manufacturers of these devices should consider due care testing prior to commercialization.
Acknowledgment
We are indebted to Alexander S. Rattner, PhD (State College, Pennsylvania), who provided his engineering expertise and insight during conversations with the authors.
- Dobkin BH, Dorsch A. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25:788-798.
- Consumer interest in purchasing wearable fitness devices in 2014 quadruples, according to CEA Study [press release]. Arlington, VA: Consumer Electronics Association; December 11, 2013.
- US Food and Drug Administration. Mobile medical applications. http://www.fda.gov/medicaldevices/digitalhealth/mobilemedicalapplications/default.htm. Updated September 22, 2015. Accessed July 26, 2017.
- Northrup L. Fitbit Force is an amazing device, except for my contact dermatitis. Consumerist website. http://consumerist.com/2014/01/13/fitbit-force-is-an-amazing-device-except-for-my-contact-dermatitis/. Published January 13, 2014. Accessed January 12, 2017.
- Stern B. Inside Fitbit Force. Adafruit website. http://learn.adafruit.com/fitbit-force-teardown/inside-fitbit-force. Published December 11, 2013. Updated May 4, 2015. Accessed January 12, 2017.
- Pemberton MA, Lohmann BS. Risk assessment of residual monomer migrating from acrylic polymers and causing allergic contact dermatitis during normal handling and use. Regul Toxicol Pharmacol. 2014;69:467-475.
- Guin JD, Baas K, Nelson-Adesokan P. Contact sensitization to cyanoacrylate adhesive as a cause of severe onychodystrophy. Int J Dermatol. 1998;37:31-36.
- Zondlo Fiume M. Final report on the safety assessment of Acrylates Copolymer and 33 related cosmetic ingredients. Int J Toxicol. 2002;21(suppl 3):1-50.
- Sasseville D. Acrylates. Dermatitis. 2012;23:3-5.
- Bowen C, Bidinger J, Hivnor C, et al. Allergic contact dermatitis to 2-octyl cyanoacrylate. Cutis. 2014;94:183-186.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review [published online July 11, 2016]. Contact Dermatitis. 2016;75:157-164.
- Dobkin BH, Dorsch A. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25:788-798.
- Consumer interest in purchasing wearable fitness devices in 2014 quadruples, according to CEA Study [press release]. Arlington, VA: Consumer Electronics Association; December 11, 2013.
- US Food and Drug Administration. Mobile medical applications. http://www.fda.gov/medicaldevices/digitalhealth/mobilemedicalapplications/default.htm. Updated September 22, 2015. Accessed July 26, 2017.
- Northrup L. Fitbit Force is an amazing device, except for my contact dermatitis. Consumerist website. http://consumerist.com/2014/01/13/fitbit-force-is-an-amazing-device-except-for-my-contact-dermatitis/. Published January 13, 2014. Accessed January 12, 2017.
- Stern B. Inside Fitbit Force. Adafruit website. http://learn.adafruit.com/fitbit-force-teardown/inside-fitbit-force. Published December 11, 2013. Updated May 4, 2015. Accessed January 12, 2017.
- Pemberton MA, Lohmann BS. Risk assessment of residual monomer migrating from acrylic polymers and causing allergic contact dermatitis during normal handling and use. Regul Toxicol Pharmacol. 2014;69:467-475.
- Guin JD, Baas K, Nelson-Adesokan P. Contact sensitization to cyanoacrylate adhesive as a cause of severe onychodystrophy. Int J Dermatol. 1998;37:31-36.
- Zondlo Fiume M. Final report on the safety assessment of Acrylates Copolymer and 33 related cosmetic ingredients. Int J Toxicol. 2002;21(suppl 3):1-50.
- Sasseville D. Acrylates. Dermatitis. 2012;23:3-5.
- Bowen C, Bidinger J, Hivnor C, et al. Allergic contact dermatitis to 2-octyl cyanoacrylate. Cutis. 2014;94:183-186.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review [published online July 11, 2016]. Contact Dermatitis. 2016;75:157-164.
Practice Points
- Mobile wearable health devices are likely to become an important potential source of contact sensitization as their use increases given their often prolonged contact time with the skin.
- Mobile wearable health devices may pose a risk for allergic contact dermatitis as a result of a variety of components that come into contact with the skin, including but not limited to metals, rubber components, adhesives, and dyes.
Allergic Reaction to Vanadium Causes a Diffuse Eczematous Eruption and Titanium Alloy Orthopedic Implant Failure
Metal allergy in patients with orthopedic implants can cause serious problems including dermatitis and implant failure.1 As life expectancy increases, the general population ages, and more metallic orthopedic implants are placed,2 allergy to these implants is expected to be a problem of greater significance. Uncertainty remains regarding best practice for patients with suspected metal implant allergy.1 The major questions are: Who should be tested? When should they be tested? What are the optimal tests to diagnose metal allergy?3-8
We report the case of a patient with vanadium allergy who developed a diffuse eczematous dermatitis and implant failure after receiving a vanadium-containing titanium alloy orthopedic implant in the left foot. This case is remarkable because hypersensitivity reactions to titanium-based hardware are rare, as they traditionally have not been thought to provoke allergic reactions.9
Case Report
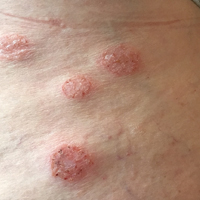
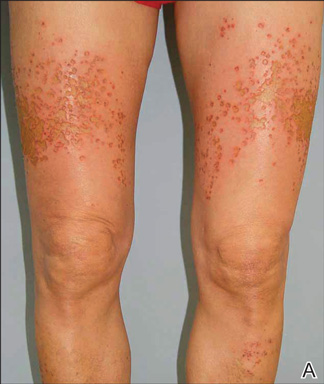
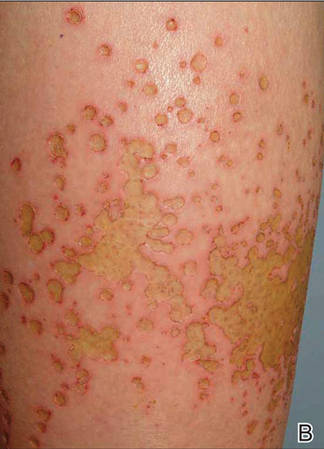
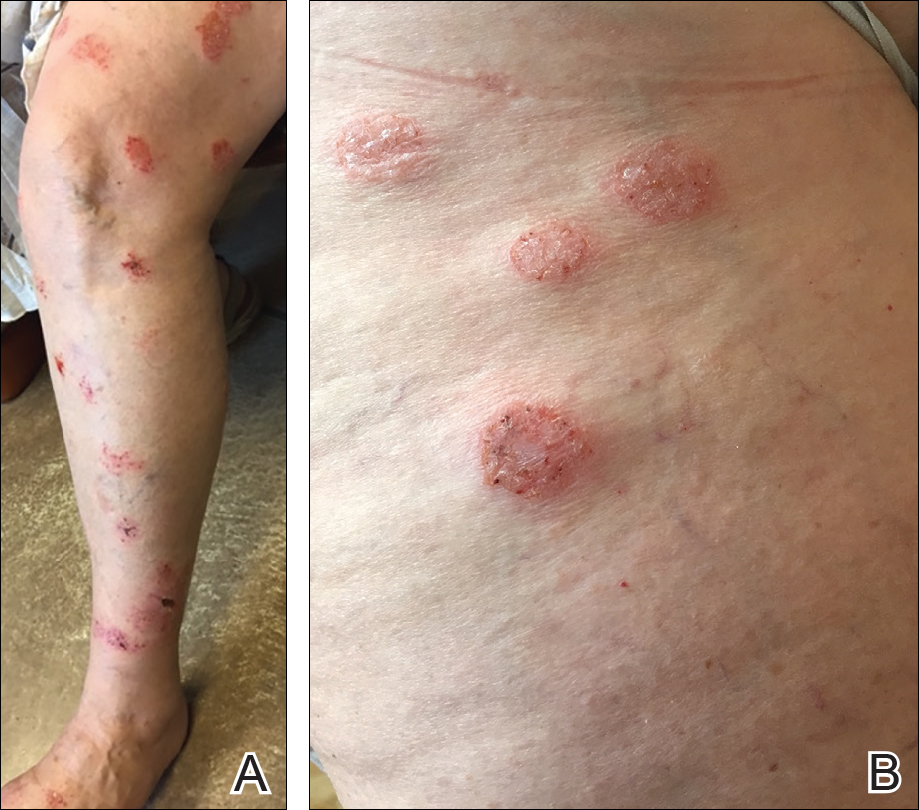
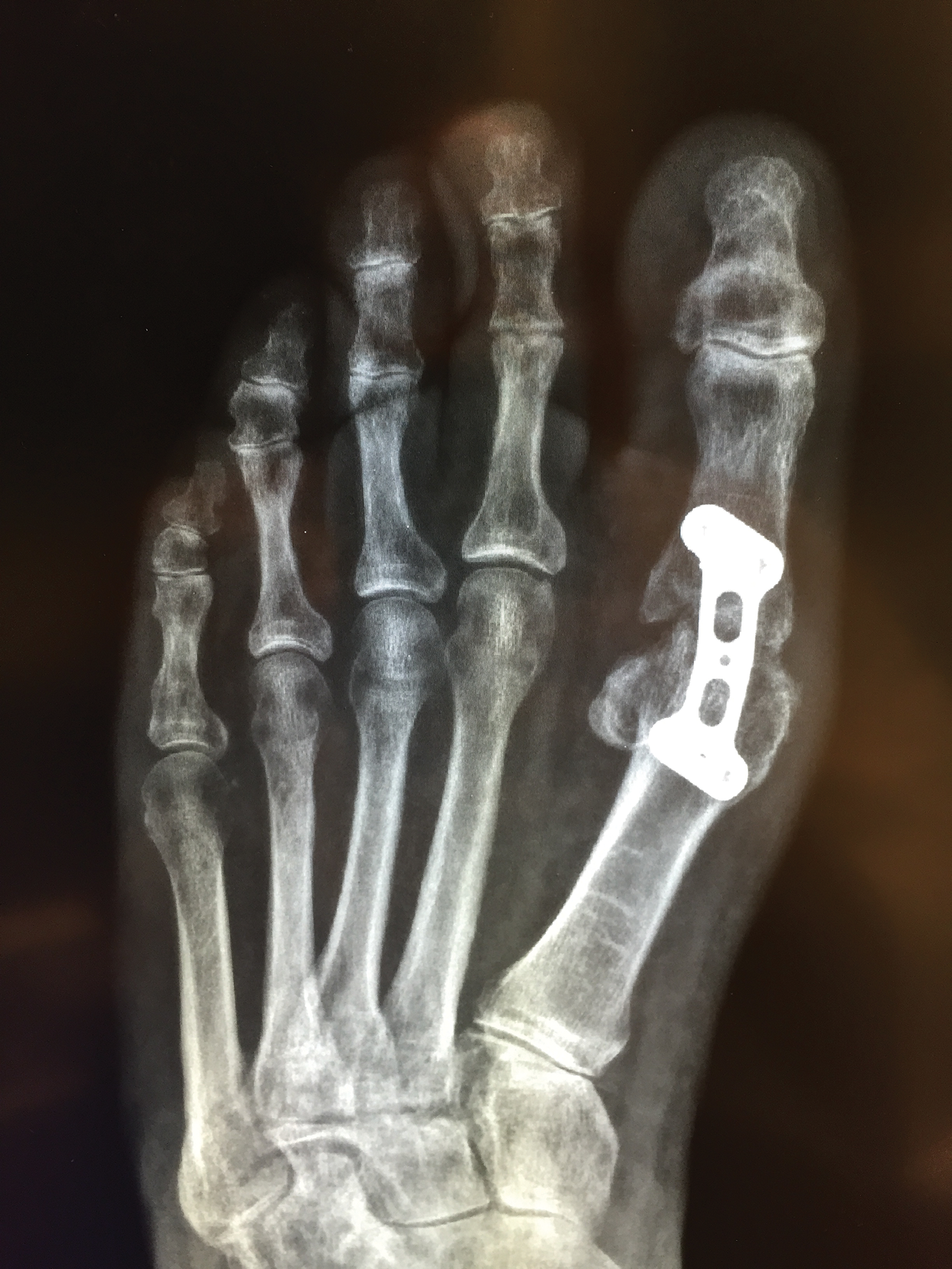
A 62-year-old woman who was otherwise healthy presented with an eruption of more than 80 pruritic, nummular, eczematous plaques on the arms, legs, back, and buttocks of 3 weeks’ duration (Figure 1). She had a history of allergy to metal used in costume jewelry. Six weeks prior, the patient underwent implantation of a titanium alloy plate in the left foot for surgical repair of painful deforming osteoarthritis. A radiograph of the foot showed appropriate placement. According to the manufacturer, the plate was composed of the compound Ti6Al4V, which contained 90% titanium, 6% aluminum, and 4% vanadium. The lesions developed on the skin close to but not directly over the surgical site.
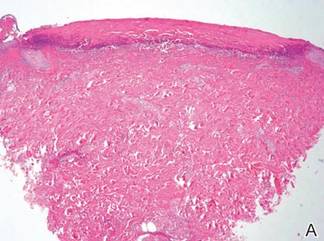
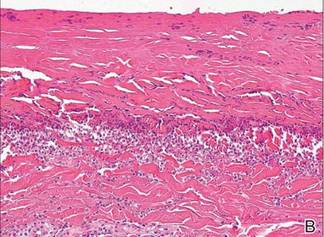
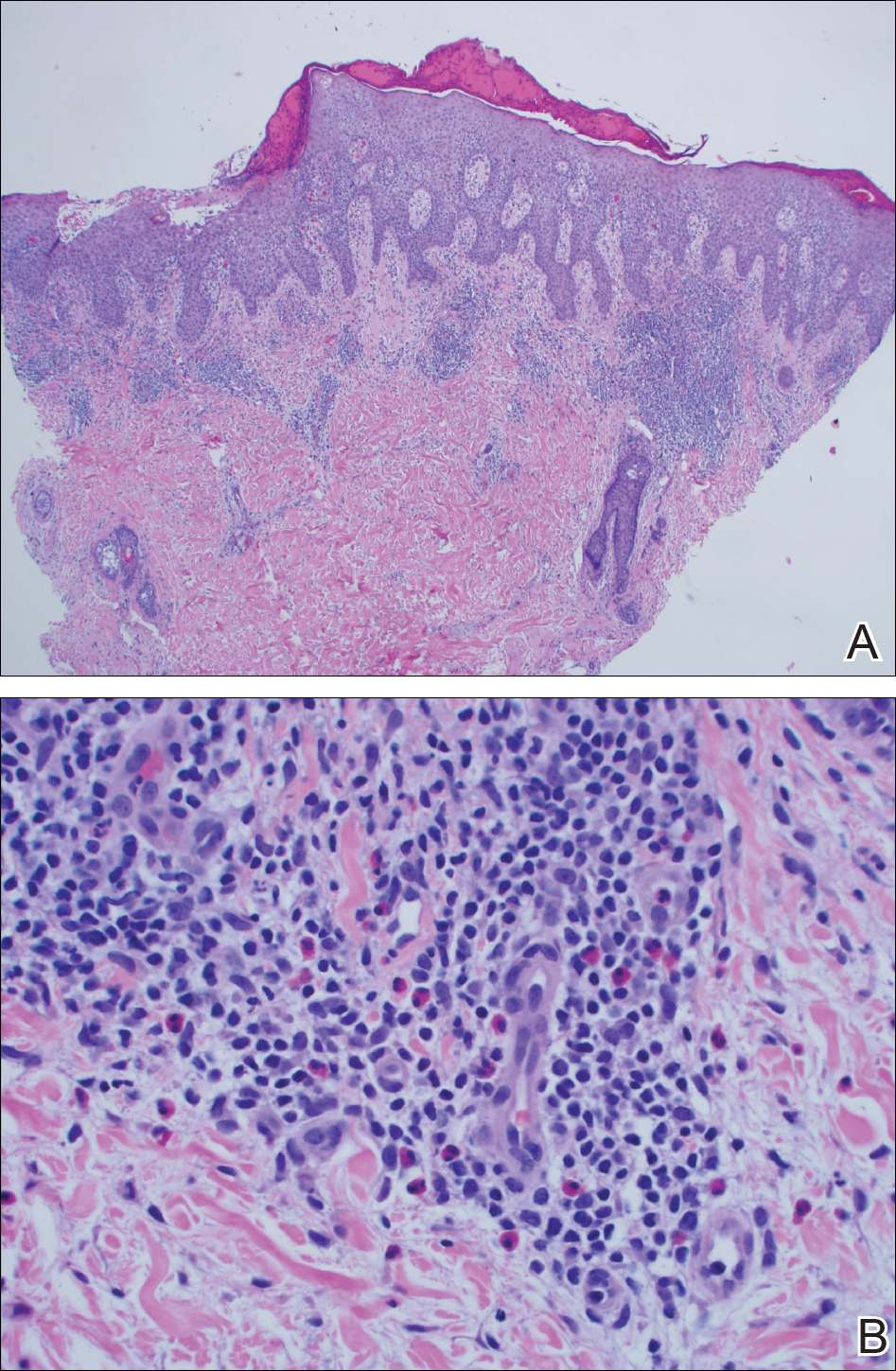
A punch biopsy of one of the lesions on the shoulder showed lymphoeosinophilic spongiosis consistent with a delayed hypersensitivity reaction (Figure 2). There was mild clinical improvement of the eruption with topical steroids. A course of prednisone for systemic effect resulted in clearing of the eruption, but it promptly recurred on cessation of the steroids. The patient was then patch tested using the North American 80 Comprehensive Series, with an additional 59 common textile, shampoo, fragrance, and several metal allergens, all of which were negative.
The patient had persistent pain and swelling at the surgical site, and radiographs taken postoperatively at 6 months showed implant failure (Figure 3). The hardware was surgically removed 8 months after implantation (Figure 4) and the plate and screws were submitted to the Institute for Mineral Resources Geosciences LA-ICP-MS Facility and the Lunar and Planetary Laboratory at the University of Arizona (Tucson, Arizona) for analysis. The skin lesions began to improve days after the hardware was removed and the eruption cleared over the following 3 weeks with no additional treatment.

After the hardware was removed, it was analyzed to determine the elemental composition of the plate and screws, and the patient was then patch tested with the major metal components of the implant: aluminum chloride hexahydrate 2.0% pet, elemental titanium 10.0% pet, titanium dioxide 10.0% pet, titanium (III) nitride 5.0% pet, titanium (III) oxalate decahydrate 5.0% pet, elemental vanadium 5.0% pet, and vanadium (III) chloride 1.0% pet. She demonstrated a 1+ reaction (erythema and induration) to vanadium trichloride at 72 and 96 hours.
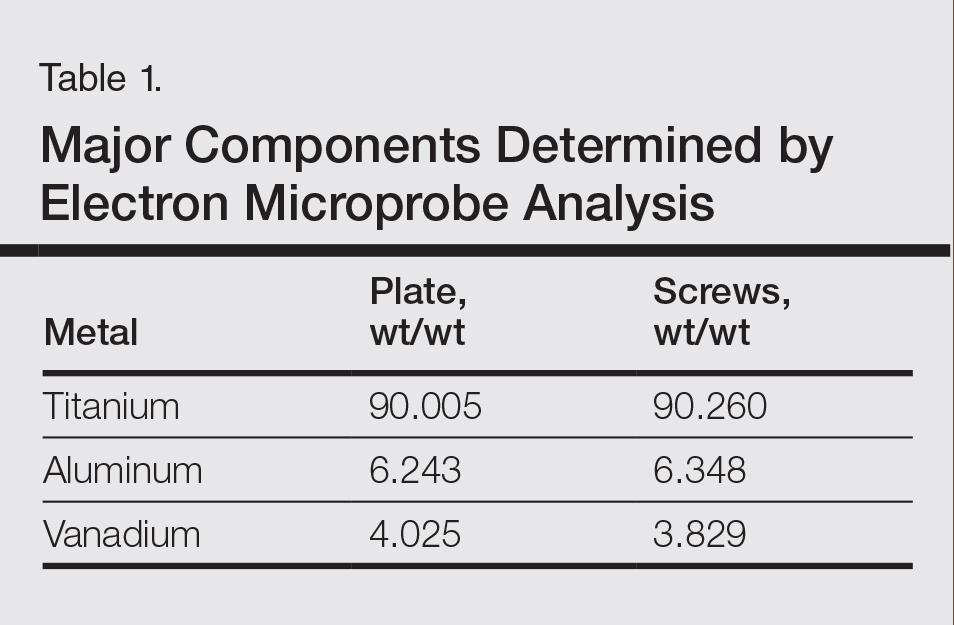
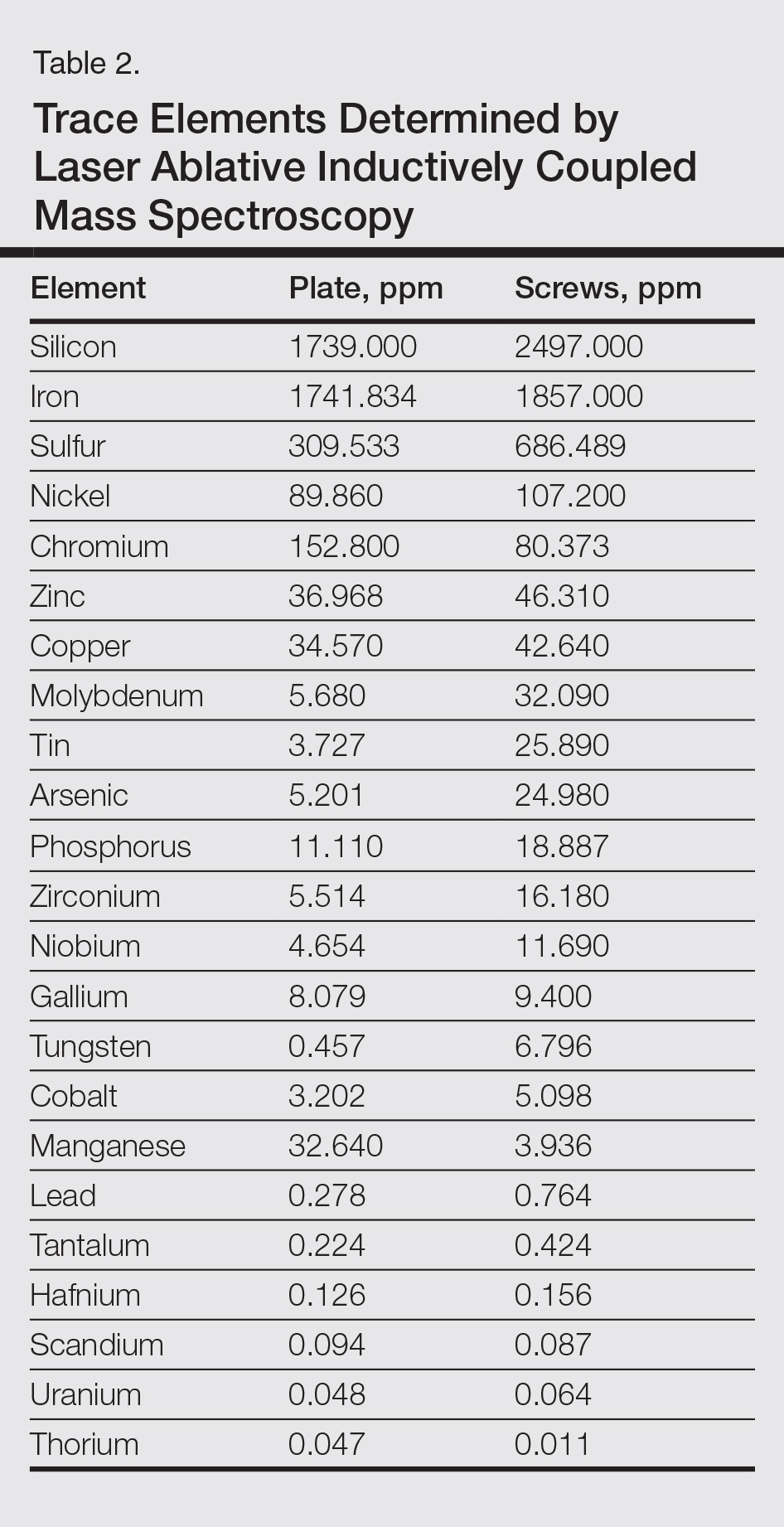
The plate and screws removed from the patient were sterilized and submitted for analysis. Electron microprobe analysis confirmed that the major elemental composition of the plate and screws essentially matched the manufacturer’s listing (Table 1). The trace elements were determined using laser ablative inductively coupled mass spectroscopy, which demonstrated that the screws were of different metal composition from the plate (Table 2). Electron microprobe analysis also was used to determine the microstructure of the plate and screws. The plate had 2 distinct phases consisting of a titanium-aluminum phase and a vanadium phase, whereas the screw was much more homogeneous. Basic electrochemical studies were performed in a salt solution replicating the tissue of the foot. These studies showed that galvanic corrosion could have occurred between the plate and screws due to the differences of composition.
Comment
Titanium is an attractive metal to use in orthopedic implants. It has a high strength-to-weight ratio, a low modulus of elasticity, and good resistance to corrosion. Titanium can be categorized as either commercially pure titanium (cp-Ti) or a titanium alloy. Colloquially, both cp-Ti and titanium alloys are often referred to simply as titanium, but the distinction is important when it comes to medical implants and devices. Commercially pure titanium is more than 99% pure titanium, but up to 1% of its volume can be comprised of impurities.10 In titanium alloys, the alloy elements are intentionally added to create a material with optimal properties. The 2 most common types of titanium that are used for orthopedic implants are cp-Ti and Ti6Al4V, a titanium alloy containing approximately 90% titanium, 6% aluminum, and 4% vanadium. Similar to cp-Ti, titanium alloys also can contain impurities such as aluminum, beryllium, cobalt, chromium, iron, nickel, and palladium, among many others. Although these impurities often are considered negligible from a metallurgy perspective, as they do not change the properties of the material, these trace elements may be present in large enough quantities to cause hypersensitivity reactions.11
Several weeks after implantation of a titanium alloy metal plate in the left foot, a widespread eczematous eruption developed in our patient who had no prior skin disease. The eruption was steroid responsive but did not clear until the plate was removed. Detailed metallurgy analysis confirmed that vanadium was present and was not homogeneously distributed in the plate. The plate also was different in composition from the screws. Additional studies showed that galvanic corrosion between the plate and the chemically different screws might have contributed to the release of vanadium in the tissue.
Vanadium is known to be allergenic, especially in the presence of implant failure.12,13 In our patient, patch testing with more than 100 allergens was negative, except for vanadium trichloride 1%. Our patient’s presentation strongly suggested that she developed a vanadium allergy manifesting as systemic allergic contact dermatitis. She demonstrated no history of skin disease, a widespread eczematous eruption after exposure, histology consistent with systemic contact allergy, a positive patch test to vanadium, and clearance of the eruption on removal of the antigen, which have been proposed as objective criteria that support a diagnosis of metal implant allergy.14 She refused our suggestion to reimplant a portion of the remaining plate under the skin without screws and monitor for recurrence of the eruption. She did not have a lesion overlying the surgical site, but she did develop lesions near the surgical scar. The literature indicates that cutaneous manifestations of allergy to metallic implants can be both localized and generalized.14
Although reports are rare, other researchers have found vanadium allergy in patients with metal orthopedic implants.5,12,13,15 The scarcity of literature on vanadium allergy seems to suggest that it is a rare entity, but we believe that it may be more common. Vanadium allergy may be underdiagnosed because it is not a standard patch test allergen. Furthermore, many of those who do choose to test for it use what we believe to be ineffective formulas of vanadium when patch testing patients. Our patient demonstrated a positive patch test reaction only to vanadium trichloride and not to pure vanadium, which is consistent with the small number of other studies that investigated vanadium allergy.5,12,13,15 We believe that vanadium trichloride is more water soluble than elemental vanadium,16 and thus more likely to identify true vanadium allergy than other test materials.
Although reports of vanadium allergy in patients with metal implants are rare in the medical literature, the material science literature clearly states that vanadium is toxic and that vanadium-containing implants are problematic.17-20 It has been shown that although Ti6Al4V implants are considered highly resistant to corrosion, they will slowly and continuously corrode in a physiologic environment and release titanium, aluminum, and vanadium ions, both systemically and into the peri-implant space.11 To address these problems with vanadium, vanadium-free titanium alloys such as Ti6Al7Nb have specifically been developed for medical use to address the problems caused by vanadium. Ti6Al7Nb contains 7% niobium rather than vanadium and appears to have some improved qualities in surgical implants.17
There is still a great deal of uncertainty around metal implant allergy. Allergy to metal implants can be difficult to diagnose for several reasons. Some metals are not conducive to patch testing because of their low bioavailability. Additionally, we lack validated and standardized patch test formulas for metals that can be diagnosed by patch testing. Furthermore, there is uncertainty about what to do after allergy to a metal implant is diagnosed; in some cases (eg, with more extensive procedures such as total joint replacements), removal or replacement of the implant may be associated with increased risk of further complications.6,21
Conclusion
We suggest that manufacturers consider vanadium-free alloys such as Ti7Al6Nb, which contains niobium instead of vanadium, in their surgical implants,22 and if surgeons have a choice, they should consider using titanium implants with niobium rather than vanadium.10 We suggest that clinicians consider vanadium allergy in patients with Ti6Al4V surgical implants and signs of a hypersensitivity reaction, and include vanadium trichloride 1% when patch testing.
Acknowledgment
The authors would like to thank Nicholas R. Krasnow, PhD (Tucson, Arizona), for his invaluable help coordinating, performing, and interpreting the metal analyses.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
- Thyssen JP, Johansen JD, Menné T, et al. Hypersensitivity reactions from metallic implants: a future challenge that needs to be addressed. Br J Dermatol. 2010;162:235-236.
- Aquino M, Mucci T. Systemic contact dermatitis and allergy to biomedical devices. Curr Allergy Asthma Rep. 2013;13:518-527.
- Krecisz B, Kiec-Swierczynska M, Chomiczewska-Skora D. Allergy to orthopedic metal implants—a prospective study. Int J Occup Med Environ Health. 2012;25:463-469.
- Atanaskova Mesinkovska N, Tellez A, Molina L, et al. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-693.
- Frigerio E, Pigatto PD, Guzzi G, et al. Metal sensitivity in patients with orthopaedic implants: a prospective study. Contact Dermatitis. 2011;64:273-279.
- Amini M, Mayes WH, Tzeng TH, et al. Evaluation and management of metal hypersensitivity in total joint arthroplasty: a systematic review. J Long Term Eff Med Implants. 2014;24:25-36.
- Thomas P, Bandl WD, Maier S, et al. Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T-cell hyperresponsiveness in vitro: case report and review of the literature. Contact Dermatitis. 2006;55:199-202.
- Wood MM, Warshaw EM. Hypersensitivity reactions to titanium: diagnosis and management. Dermatitis. 2015;26:7-25.
- Cadosch D, Chan E, Gautschi OP, et al. Metal is not inert: role of metal ions released by biocorrosion in aseptic loosening—current concepts. J Biomed Mater Res A. 2009;91:1252-1262.
- Granchi D, Cenni E, Trisolino G, et al. Sensitivity to implant materials in patients undergoing total hip replacement. J Biomed Mater Res B Appl Biomater. 2006;77:257-264.
- Granchi D, Cenni E, Tigani D, et al. Sensitivity to implant materials in patients with total knee arthroplasties. Biomaterials. 2008;29:1494-1500.
- Thyssen JP, Menné T, Schalock PC, et al. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164:473-478.
- Kręcisz B, Kieć-Świerczyńska M, Bąkowicz-Mitura K. Allergy to metals as a cause of orthopedic implant failure. Int J Occup Med Environ Health. 2006;19:178-180.
- Costigan M, Cary R, Dobson S. Vanadium Pentoxide and Other Inorganic Vanadium Compounds. Geneva, Switzerland: World Health Organization; 2001.
- Challa VS, Mali S, Misra RD. Reduced toxicity and superior cellular response of preosteoblasts to Ti-6Al-7Nb alloy and comparison with Ti-6Al-4V. J Biomed Mater Res A. 2013;101:2083-2089.
- Okazaki Y, Rao S, Ito Y, et al. Corrosion resistance, mechanical properties, corrosion fatigue strength and cytocompatibility of new Ti alloys without Al and V. Biomaterials. 1998;19:1197-1215.
- Paszenda Z, Walke W, Jadacka S. Electrochemical investigations of Ti6Al4V and Ti6Al7Nb alloys used on implants in bone surgery. J Achievements Materials Manufacturing Eng. 2010;38:24-32.
- Wang K. The use of titanium for medical applications in the USA. Materials Sci Eng A. 1996:134-137.
- Haseeb M, Butt MF, Altaf T, et al. Indications of implant removal: a study of 83 cases. Int J Health Sci (Qassim). 2017;11:1-7.
- Geetha M, Singh AK, Asokamani R, et al. Ti based biomaterials, the ultimate choice for orthopaedic implants—a review. Progress Materials Sci. 2009;54:397-425.
Metal allergy in patients with orthopedic implants can cause serious problems including dermatitis and implant failure.1 As life expectancy increases, the general population ages, and more metallic orthopedic implants are placed,2 allergy to these implants is expected to be a problem of greater significance. Uncertainty remains regarding best practice for patients with suspected metal implant allergy.1 The major questions are: Who should be tested? When should they be tested? What are the optimal tests to diagnose metal allergy?3-8
We report the case of a patient with vanadium allergy who developed a diffuse eczematous dermatitis and implant failure after receiving a vanadium-containing titanium alloy orthopedic implant in the left foot. This case is remarkable because hypersensitivity reactions to titanium-based hardware are rare, as they traditionally have not been thought to provoke allergic reactions.9
Case Report
A 62-year-old woman who was otherwise healthy presented with an eruption of more than 80 pruritic, nummular, eczematous plaques on the arms, legs, back, and buttocks of 3 weeks’ duration (Figure 1). She had a history of allergy to metal used in costume jewelry. Six weeks prior, the patient underwent implantation of a titanium alloy plate in the left foot for surgical repair of painful deforming osteoarthritis. A radiograph of the foot showed appropriate placement. According to the manufacturer, the plate was composed of the compound Ti6Al4V, which contained 90% titanium, 6% aluminum, and 4% vanadium. The lesions developed on the skin close to but not directly over the surgical site.

A punch biopsy of one of the lesions on the shoulder showed lymphoeosinophilic spongiosis consistent with a delayed hypersensitivity reaction (Figure 2). There was mild clinical improvement of the eruption with topical steroids. A course of prednisone for systemic effect resulted in clearing of the eruption, but it promptly recurred on cessation of the steroids. The patient was then patch tested using the North American 80 Comprehensive Series, with an additional 59 common textile, shampoo, fragrance, and several metal allergens, all of which were negative.

The patient had persistent pain and swelling at the surgical site, and radiographs taken postoperatively at 6 months showed implant failure (Figure 3). The hardware was surgically removed 8 months after implantation (Figure 4) and the plate and screws were submitted to the Institute for Mineral Resources Geosciences LA-ICP-MS Facility and the Lunar and Planetary Laboratory at the University of Arizona (Tucson, Arizona) for analysis. The skin lesions began to improve days after the hardware was removed and the eruption cleared over the following 3 weeks with no additional treatment.


After the hardware was removed, it was analyzed to determine the elemental composition of the plate and screws, and the patient was then patch tested with the major metal components of the implant: aluminum chloride hexahydrate 2.0% pet, elemental titanium 10.0% pet, titanium dioxide 10.0% pet, titanium (III) nitride 5.0% pet, titanium (III) oxalate decahydrate 5.0% pet, elemental vanadium 5.0% pet, and vanadium (III) chloride 1.0% pet. She demonstrated a 1+ reaction (erythema and induration) to vanadium trichloride at 72 and 96 hours.
The plate and screws removed from the patient were sterilized and submitted for analysis. Electron microprobe analysis confirmed that the major elemental composition of the plate and screws essentially matched the manufacturer’s listing (Table 1). The trace elements were determined using laser ablative inductively coupled mass spectroscopy, which demonstrated that the screws were of different metal composition from the plate (Table 2). Electron microprobe analysis also was used to determine the microstructure of the plate and screws. The plate had 2 distinct phases consisting of a titanium-aluminum phase and a vanadium phase, whereas the screw was much more homogeneous. Basic electrochemical studies were performed in a salt solution replicating the tissue of the foot. These studies showed that galvanic corrosion could have occurred between the plate and screws due to the differences of composition.
Comment
Titanium is an attractive metal to use in orthopedic implants. It has a high strength-to-weight ratio, a low modulus of elasticity, and good resistance to corrosion. Titanium can be categorized as either commercially pure titanium (cp-Ti) or a titanium alloy. Colloquially, both cp-Ti and titanium alloys are often referred to simply as titanium, but the distinction is important when it comes to medical implants and devices. Commercially pure titanium is more than 99% pure titanium, but up to 1% of its volume can be comprised of impurities.10 In titanium alloys, the alloy elements are intentionally added to create a material with optimal properties. The 2 most common types of titanium that are used for orthopedic implants are cp-Ti and Ti6Al4V, a titanium alloy containing approximately 90% titanium, 6% aluminum, and 4% vanadium. Similar to cp-Ti, titanium alloys also can contain impurities such as aluminum, beryllium, cobalt, chromium, iron, nickel, and palladium, among many others. Although these impurities often are considered negligible from a metallurgy perspective, as they do not change the properties of the material, these trace elements may be present in large enough quantities to cause hypersensitivity reactions.11
Several weeks after implantation of a titanium alloy metal plate in the left foot, a widespread eczematous eruption developed in our patient who had no prior skin disease. The eruption was steroid responsive but did not clear until the plate was removed. Detailed metallurgy analysis confirmed that vanadium was present and was not homogeneously distributed in the plate. The plate also was different in composition from the screws. Additional studies showed that galvanic corrosion between the plate and the chemically different screws might have contributed to the release of vanadium in the tissue.
Vanadium is known to be allergenic, especially in the presence of implant failure.12,13 In our patient, patch testing with more than 100 allergens was negative, except for vanadium trichloride 1%. Our patient’s presentation strongly suggested that she developed a vanadium allergy manifesting as systemic allergic contact dermatitis. She demonstrated no history of skin disease, a widespread eczematous eruption after exposure, histology consistent with systemic contact allergy, a positive patch test to vanadium, and clearance of the eruption on removal of the antigen, which have been proposed as objective criteria that support a diagnosis of metal implant allergy.14 She refused our suggestion to reimplant a portion of the remaining plate under the skin without screws and monitor for recurrence of the eruption. She did not have a lesion overlying the surgical site, but she did develop lesions near the surgical scar. The literature indicates that cutaneous manifestations of allergy to metallic implants can be both localized and generalized.14
Although reports are rare, other researchers have found vanadium allergy in patients with metal orthopedic implants.5,12,13,15 The scarcity of literature on vanadium allergy seems to suggest that it is a rare entity, but we believe that it may be more common. Vanadium allergy may be underdiagnosed because it is not a standard patch test allergen. Furthermore, many of those who do choose to test for it use what we believe to be ineffective formulas of vanadium when patch testing patients. Our patient demonstrated a positive patch test reaction only to vanadium trichloride and not to pure vanadium, which is consistent with the small number of other studies that investigated vanadium allergy.5,12,13,15 We believe that vanadium trichloride is more water soluble than elemental vanadium,16 and thus more likely to identify true vanadium allergy than other test materials.
Although reports of vanadium allergy in patients with metal implants are rare in the medical literature, the material science literature clearly states that vanadium is toxic and that vanadium-containing implants are problematic.17-20 It has been shown that although Ti6Al4V implants are considered highly resistant to corrosion, they will slowly and continuously corrode in a physiologic environment and release titanium, aluminum, and vanadium ions, both systemically and into the peri-implant space.11 To address these problems with vanadium, vanadium-free titanium alloys such as Ti6Al7Nb have specifically been developed for medical use to address the problems caused by vanadium. Ti6Al7Nb contains 7% niobium rather than vanadium and appears to have some improved qualities in surgical implants.17
There is still a great deal of uncertainty around metal implant allergy. Allergy to metal implants can be difficult to diagnose for several reasons. Some metals are not conducive to patch testing because of their low bioavailability. Additionally, we lack validated and standardized patch test formulas for metals that can be diagnosed by patch testing. Furthermore, there is uncertainty about what to do after allergy to a metal implant is diagnosed; in some cases (eg, with more extensive procedures such as total joint replacements), removal or replacement of the implant may be associated with increased risk of further complications.6,21
Conclusion
We suggest that manufacturers consider vanadium-free alloys such as Ti7Al6Nb, which contains niobium instead of vanadium, in their surgical implants,22 and if surgeons have a choice, they should consider using titanium implants with niobium rather than vanadium.10 We suggest that clinicians consider vanadium allergy in patients with Ti6Al4V surgical implants and signs of a hypersensitivity reaction, and include vanadium trichloride 1% when patch testing.
Acknowledgment
The authors would like to thank Nicholas R. Krasnow, PhD (Tucson, Arizona), for his invaluable help coordinating, performing, and interpreting the metal analyses.
Metal allergy in patients with orthopedic implants can cause serious problems including dermatitis and implant failure.1 As life expectancy increases, the general population ages, and more metallic orthopedic implants are placed,2 allergy to these implants is expected to be a problem of greater significance. Uncertainty remains regarding best practice for patients with suspected metal implant allergy.1 The major questions are: Who should be tested? When should they be tested? What are the optimal tests to diagnose metal allergy?3-8
We report the case of a patient with vanadium allergy who developed a diffuse eczematous dermatitis and implant failure after receiving a vanadium-containing titanium alloy orthopedic implant in the left foot. This case is remarkable because hypersensitivity reactions to titanium-based hardware are rare, as they traditionally have not been thought to provoke allergic reactions.9
Case Report
A 62-year-old woman who was otherwise healthy presented with an eruption of more than 80 pruritic, nummular, eczematous plaques on the arms, legs, back, and buttocks of 3 weeks’ duration (Figure 1). She had a history of allergy to metal used in costume jewelry. Six weeks prior, the patient underwent implantation of a titanium alloy plate in the left foot for surgical repair of painful deforming osteoarthritis. A radiograph of the foot showed appropriate placement. According to the manufacturer, the plate was composed of the compound Ti6Al4V, which contained 90% titanium, 6% aluminum, and 4% vanadium. The lesions developed on the skin close to but not directly over the surgical site.

A punch biopsy of one of the lesions on the shoulder showed lymphoeosinophilic spongiosis consistent with a delayed hypersensitivity reaction (Figure 2). There was mild clinical improvement of the eruption with topical steroids. A course of prednisone for systemic effect resulted in clearing of the eruption, but it promptly recurred on cessation of the steroids. The patient was then patch tested using the North American 80 Comprehensive Series, with an additional 59 common textile, shampoo, fragrance, and several metal allergens, all of which were negative.

The patient had persistent pain and swelling at the surgical site, and radiographs taken postoperatively at 6 months showed implant failure (Figure 3). The hardware was surgically removed 8 months after implantation (Figure 4) and the plate and screws were submitted to the Institute for Mineral Resources Geosciences LA-ICP-MS Facility and the Lunar and Planetary Laboratory at the University of Arizona (Tucson, Arizona) for analysis. The skin lesions began to improve days after the hardware was removed and the eruption cleared over the following 3 weeks with no additional treatment.


After the hardware was removed, it was analyzed to determine the elemental composition of the plate and screws, and the patient was then patch tested with the major metal components of the implant: aluminum chloride hexahydrate 2.0% pet, elemental titanium 10.0% pet, titanium dioxide 10.0% pet, titanium (III) nitride 5.0% pet, titanium (III) oxalate decahydrate 5.0% pet, elemental vanadium 5.0% pet, and vanadium (III) chloride 1.0% pet. She demonstrated a 1+ reaction (erythema and induration) to vanadium trichloride at 72 and 96 hours.
The plate and screws removed from the patient were sterilized and submitted for analysis. Electron microprobe analysis confirmed that the major elemental composition of the plate and screws essentially matched the manufacturer’s listing (Table 1). The trace elements were determined using laser ablative inductively coupled mass spectroscopy, which demonstrated that the screws were of different metal composition from the plate (Table 2). Electron microprobe analysis also was used to determine the microstructure of the plate and screws. The plate had 2 distinct phases consisting of a titanium-aluminum phase and a vanadium phase, whereas the screw was much more homogeneous. Basic electrochemical studies were performed in a salt solution replicating the tissue of the foot. These studies showed that galvanic corrosion could have occurred between the plate and screws due to the differences of composition.
Comment
Titanium is an attractive metal to use in orthopedic implants. It has a high strength-to-weight ratio, a low modulus of elasticity, and good resistance to corrosion. Titanium can be categorized as either commercially pure titanium (cp-Ti) or a titanium alloy. Colloquially, both cp-Ti and titanium alloys are often referred to simply as titanium, but the distinction is important when it comes to medical implants and devices. Commercially pure titanium is more than 99% pure titanium, but up to 1% of its volume can be comprised of impurities.10 In titanium alloys, the alloy elements are intentionally added to create a material with optimal properties. The 2 most common types of titanium that are used for orthopedic implants are cp-Ti and Ti6Al4V, a titanium alloy containing approximately 90% titanium, 6% aluminum, and 4% vanadium. Similar to cp-Ti, titanium alloys also can contain impurities such as aluminum, beryllium, cobalt, chromium, iron, nickel, and palladium, among many others. Although these impurities often are considered negligible from a metallurgy perspective, as they do not change the properties of the material, these trace elements may be present in large enough quantities to cause hypersensitivity reactions.11
Several weeks after implantation of a titanium alloy metal plate in the left foot, a widespread eczematous eruption developed in our patient who had no prior skin disease. The eruption was steroid responsive but did not clear until the plate was removed. Detailed metallurgy analysis confirmed that vanadium was present and was not homogeneously distributed in the plate. The plate also was different in composition from the screws. Additional studies showed that galvanic corrosion between the plate and the chemically different screws might have contributed to the release of vanadium in the tissue.
Vanadium is known to be allergenic, especially in the presence of implant failure.12,13 In our patient, patch testing with more than 100 allergens was negative, except for vanadium trichloride 1%. Our patient’s presentation strongly suggested that she developed a vanadium allergy manifesting as systemic allergic contact dermatitis. She demonstrated no history of skin disease, a widespread eczematous eruption after exposure, histology consistent with systemic contact allergy, a positive patch test to vanadium, and clearance of the eruption on removal of the antigen, which have been proposed as objective criteria that support a diagnosis of metal implant allergy.14 She refused our suggestion to reimplant a portion of the remaining plate under the skin without screws and monitor for recurrence of the eruption. She did not have a lesion overlying the surgical site, but she did develop lesions near the surgical scar. The literature indicates that cutaneous manifestations of allergy to metallic implants can be both localized and generalized.14
Although reports are rare, other researchers have found vanadium allergy in patients with metal orthopedic implants.5,12,13,15 The scarcity of literature on vanadium allergy seems to suggest that it is a rare entity, but we believe that it may be more common. Vanadium allergy may be underdiagnosed because it is not a standard patch test allergen. Furthermore, many of those who do choose to test for it use what we believe to be ineffective formulas of vanadium when patch testing patients. Our patient demonstrated a positive patch test reaction only to vanadium trichloride and not to pure vanadium, which is consistent with the small number of other studies that investigated vanadium allergy.5,12,13,15 We believe that vanadium trichloride is more water soluble than elemental vanadium,16 and thus more likely to identify true vanadium allergy than other test materials.
Although reports of vanadium allergy in patients with metal implants are rare in the medical literature, the material science literature clearly states that vanadium is toxic and that vanadium-containing implants are problematic.17-20 It has been shown that although Ti6Al4V implants are considered highly resistant to corrosion, they will slowly and continuously corrode in a physiologic environment and release titanium, aluminum, and vanadium ions, both systemically and into the peri-implant space.11 To address these problems with vanadium, vanadium-free titanium alloys such as Ti6Al7Nb have specifically been developed for medical use to address the problems caused by vanadium. Ti6Al7Nb contains 7% niobium rather than vanadium and appears to have some improved qualities in surgical implants.17
There is still a great deal of uncertainty around metal implant allergy. Allergy to metal implants can be difficult to diagnose for several reasons. Some metals are not conducive to patch testing because of their low bioavailability. Additionally, we lack validated and standardized patch test formulas for metals that can be diagnosed by patch testing. Furthermore, there is uncertainty about what to do after allergy to a metal implant is diagnosed; in some cases (eg, with more extensive procedures such as total joint replacements), removal or replacement of the implant may be associated with increased risk of further complications.6,21
Conclusion
We suggest that manufacturers consider vanadium-free alloys such as Ti7Al6Nb, which contains niobium instead of vanadium, in their surgical implants,22 and if surgeons have a choice, they should consider using titanium implants with niobium rather than vanadium.10 We suggest that clinicians consider vanadium allergy in patients with Ti6Al4V surgical implants and signs of a hypersensitivity reaction, and include vanadium trichloride 1% when patch testing.
Acknowledgment
The authors would like to thank Nicholas R. Krasnow, PhD (Tucson, Arizona), for his invaluable help coordinating, performing, and interpreting the metal analyses.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
- Thyssen JP, Johansen JD, Menné T, et al. Hypersensitivity reactions from metallic implants: a future challenge that needs to be addressed. Br J Dermatol. 2010;162:235-236.
- Aquino M, Mucci T. Systemic contact dermatitis and allergy to biomedical devices. Curr Allergy Asthma Rep. 2013;13:518-527.
- Krecisz B, Kiec-Swierczynska M, Chomiczewska-Skora D. Allergy to orthopedic metal implants—a prospective study. Int J Occup Med Environ Health. 2012;25:463-469.
- Atanaskova Mesinkovska N, Tellez A, Molina L, et al. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-693.
- Frigerio E, Pigatto PD, Guzzi G, et al. Metal sensitivity in patients with orthopaedic implants: a prospective study. Contact Dermatitis. 2011;64:273-279.
- Amini M, Mayes WH, Tzeng TH, et al. Evaluation and management of metal hypersensitivity in total joint arthroplasty: a systematic review. J Long Term Eff Med Implants. 2014;24:25-36.
- Thomas P, Bandl WD, Maier S, et al. Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T-cell hyperresponsiveness in vitro: case report and review of the literature. Contact Dermatitis. 2006;55:199-202.
- Wood MM, Warshaw EM. Hypersensitivity reactions to titanium: diagnosis and management. Dermatitis. 2015;26:7-25.
- Cadosch D, Chan E, Gautschi OP, et al. Metal is not inert: role of metal ions released by biocorrosion in aseptic loosening—current concepts. J Biomed Mater Res A. 2009;91:1252-1262.
- Granchi D, Cenni E, Trisolino G, et al. Sensitivity to implant materials in patients undergoing total hip replacement. J Biomed Mater Res B Appl Biomater. 2006;77:257-264.
- Granchi D, Cenni E, Tigani D, et al. Sensitivity to implant materials in patients with total knee arthroplasties. Biomaterials. 2008;29:1494-1500.
- Thyssen JP, Menné T, Schalock PC, et al. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164:473-478.
- Kręcisz B, Kieć-Świerczyńska M, Bąkowicz-Mitura K. Allergy to metals as a cause of orthopedic implant failure. Int J Occup Med Environ Health. 2006;19:178-180.
- Costigan M, Cary R, Dobson S. Vanadium Pentoxide and Other Inorganic Vanadium Compounds. Geneva, Switzerland: World Health Organization; 2001.
- Challa VS, Mali S, Misra RD. Reduced toxicity and superior cellular response of preosteoblasts to Ti-6Al-7Nb alloy and comparison with Ti-6Al-4V. J Biomed Mater Res A. 2013;101:2083-2089.
- Okazaki Y, Rao S, Ito Y, et al. Corrosion resistance, mechanical properties, corrosion fatigue strength and cytocompatibility of new Ti alloys without Al and V. Biomaterials. 1998;19:1197-1215.
- Paszenda Z, Walke W, Jadacka S. Electrochemical investigations of Ti6Al4V and Ti6Al7Nb alloys used on implants in bone surgery. J Achievements Materials Manufacturing Eng. 2010;38:24-32.
- Wang K. The use of titanium for medical applications in the USA. Materials Sci Eng A. 1996:134-137.
- Haseeb M, Butt MF, Altaf T, et al. Indications of implant removal: a study of 83 cases. Int J Health Sci (Qassim). 2017;11:1-7.
- Geetha M, Singh AK, Asokamani R, et al. Ti based biomaterials, the ultimate choice for orthopaedic implants—a review. Progress Materials Sci. 2009;54:397-425.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
- Thyssen JP, Johansen JD, Menné T, et al. Hypersensitivity reactions from metallic implants: a future challenge that needs to be addressed. Br J Dermatol. 2010;162:235-236.
- Aquino M, Mucci T. Systemic contact dermatitis and allergy to biomedical devices. Curr Allergy Asthma Rep. 2013;13:518-527.
- Krecisz B, Kiec-Swierczynska M, Chomiczewska-Skora D. Allergy to orthopedic metal implants—a prospective study. Int J Occup Med Environ Health. 2012;25:463-469.
- Atanaskova Mesinkovska N, Tellez A, Molina L, et al. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-693.
- Frigerio E, Pigatto PD, Guzzi G, et al. Metal sensitivity in patients with orthopaedic implants: a prospective study. Contact Dermatitis. 2011;64:273-279.
- Amini M, Mayes WH, Tzeng TH, et al. Evaluation and management of metal hypersensitivity in total joint arthroplasty: a systematic review. J Long Term Eff Med Implants. 2014;24:25-36.
- Thomas P, Bandl WD, Maier S, et al. Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T-cell hyperresponsiveness in vitro: case report and review of the literature. Contact Dermatitis. 2006;55:199-202.
- Wood MM, Warshaw EM. Hypersensitivity reactions to titanium: diagnosis and management. Dermatitis. 2015;26:7-25.
- Cadosch D, Chan E, Gautschi OP, et al. Metal is not inert: role of metal ions released by biocorrosion in aseptic loosening—current concepts. J Biomed Mater Res A. 2009;91:1252-1262.
- Granchi D, Cenni E, Trisolino G, et al. Sensitivity to implant materials in patients undergoing total hip replacement. J Biomed Mater Res B Appl Biomater. 2006;77:257-264.
- Granchi D, Cenni E, Tigani D, et al. Sensitivity to implant materials in patients with total knee arthroplasties. Biomaterials. 2008;29:1494-1500.
- Thyssen JP, Menné T, Schalock PC, et al. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164:473-478.
- Kręcisz B, Kieć-Świerczyńska M, Bąkowicz-Mitura K. Allergy to metals as a cause of orthopedic implant failure. Int J Occup Med Environ Health. 2006;19:178-180.
- Costigan M, Cary R, Dobson S. Vanadium Pentoxide and Other Inorganic Vanadium Compounds. Geneva, Switzerland: World Health Organization; 2001.
- Challa VS, Mali S, Misra RD. Reduced toxicity and superior cellular response of preosteoblasts to Ti-6Al-7Nb alloy and comparison with Ti-6Al-4V. J Biomed Mater Res A. 2013;101:2083-2089.
- Okazaki Y, Rao S, Ito Y, et al. Corrosion resistance, mechanical properties, corrosion fatigue strength and cytocompatibility of new Ti alloys without Al and V. Biomaterials. 1998;19:1197-1215.
- Paszenda Z, Walke W, Jadacka S. Electrochemical investigations of Ti6Al4V and Ti6Al7Nb alloys used on implants in bone surgery. J Achievements Materials Manufacturing Eng. 2010;38:24-32.
- Wang K. The use of titanium for medical applications in the USA. Materials Sci Eng A. 1996:134-137.
- Haseeb M, Butt MF, Altaf T, et al. Indications of implant removal: a study of 83 cases. Int J Health Sci (Qassim). 2017;11:1-7.
- Geetha M, Singh AK, Asokamani R, et al. Ti based biomaterials, the ultimate choice for orthopaedic implants—a review. Progress Materials Sci. 2009;54:397-425.
Practice Points
- Vanadium may be an underrecognized allergen in patients with metal implants.
- Consider vanadium allergy in those with surgical implants and signs of hypersensitivity reaction.
- Test for allergy with vanadium trichloride.
- Niobium is an alternative for implants in vanadium-allergic patients.
Concomitant Sensitization to Inhaled Budesonide and Oral Nystatin Presenting as Allergic Contact Stomatitis and Systemic Allergic Contact Dermatitis
The development of concomitant allergic reactions to multiple drugs is uncommon. Dermatitis induced by topical or inhaled corticosteroids (eg, budesonide) is rare,1 and allergic reactions associated with oral nystatin, a macrolide antifungal drug, also are unusual.2 We present the case of concomitant sensitization to inhaled budesonide and oral nystatin presenting as allergic contact stomatitis and systemic allergic contact dermatitis. Concomitant allergic reactions to these treatments are rare and may result in diagnostic challenges for the physician.
Case Report
A 66-year-old woman presented to the Allergy Department for evaluation of painful erosions on the oral mucosa that had developed 72 hours after she started treatment with inhaled budesonide (400 mcg every 12 hours) prescribed by her general practitioner for a nonproductive cough. Budesonide inhalation was discontinued due to suspected oral candidiasis and treatment with oral nystatin (500,000 IU every 8 hours) was started, but the erosions did not resolve. After 2 days of treatment with oral nystatin, the patient presented with erythematous macules on the abdomen and thighs as well as a larger erythematous and edematous lesion with papules and vesicles on the hypothenar eminence of the right hand. Nystatin was discontinued and the lesions turned desquamative and healed spontaneously 7 days later. The oral lesions resolved after 15 days with no further treatment.
Patch testing was conducted using a commercially standard series of contact allergens, all of which showed negative results at 48 and 96 hours except for budesonide and triamcinolone, which led to the diagnosis of allergic contact stomatitis from the inhaled budesonide. Patch testing with other corticosteroids was negative. Challenge tests with alternative corticosteroids (ie, oral methylprednisolone, parenteral betamethasone, topical mometasone furoate, inhaled fluticasone) were negative.
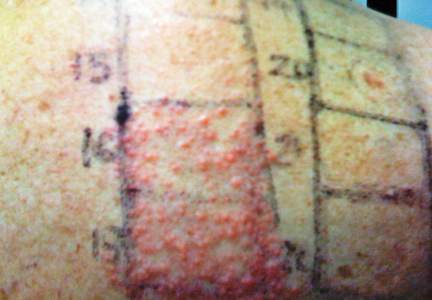
In order to rule out involvement of oral nystatin, a single-blind, placebo-controlled oral challenge test was performed. Eight hours after taking oral nystatin (500,000 IU), erythematous macules developed on the patient’s abdomen along with an erythematous, 3×4-cm lesion with papules on the hypothenar eminence of the right hand that was similar in appearance to the original presentation. The lesion on the hand was biopsied and histologic examination revealed spongiosis, edema of the superficial dermis, perivascular lymphocytic infiltrates, and extravasated erythrocytes with no vasculitis. Further patch testing subsequently was conducted with antifungal and antibiotic macrolides in different vehicles (ie, petrolatum, water, polyethylene glycol), as well as with excipients of the oral nystatin formulation that had been tested (Figure). Patch testing was positive with nystatin 10% in petrolatum and nystatin 30,000 IU and 90,000 IU in polyethylene glycol. Testing also were conducted in 7 healthy volunteers to rule out an irritant reaction and showed negative results. Finally, challenge tests conducted in our patient with another antifungal macrolide (parenteral amphotericin B) and antibiotic macrolides (oral clarithromycin, erythromycin, and azithromycin) were negative.
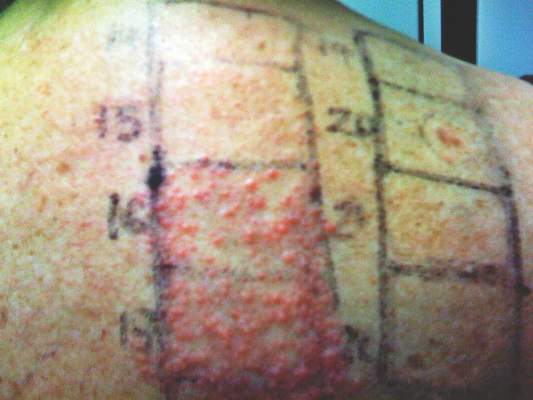
Patch and challenge test results along with the histologic findings led to diagnosis of concomitant systemic allergic contact dermatitis from oral nystatin.
Comment
Our patient presented with 2 unusual delayed hypersensitivity reactions that occurred in the same medical episode: allergic contact stomatitis from inhaled budesonide and systemic allergic contact dermatitis from oral nystatin. It is noteworthy that, despite the poor intestinal absorption of nystatin, systemic contact dermatitis to this drug has been previously described.3 Patch testing with macrolides proved useful for diagnosis in our patient, and based on the results we concluded that polyethylene glycol seemed to be the optimal vehicle for patch testing macrolide drugs versus water or petrolatum, as has been previously suggested.4
When a diagnosis of drug allergy is established, it is important to rule out cross-reactivity with other similar drugs by assessing if they produce the same reaction despite differences in chemical structure. Possible cross-reactivity of nystatin with other macrolides (validated on patch testing) has been reported but the tolerability was not evaluated.5 Our patient showed good tolerability to other macrolide drugs, both antibiotics and antifungals. Therefore, nystatin does not seem to cross-react with other structurally related drugs belonging to the macrolide group based on our results.
Corticosteroid allergies are more common than those associated with macrolides, especially contact dermatitis. Nonhalogenated corticosteroids (eg, hydrocortisone, budesonide) are most frequently associated with allergic reactions,6 and patch testing remains the diagnostic method of choice for the detection of delayed hypersensitivity to corticosteroids. In Europe, standard series include budesonide and tixocortol pivalate, and in the United States they include hydrocortisone 17–butyrate, triamcinolone acetonide, and clobetasol 17–propionate.6
To assess cross-reactivity among topical corticosteroids, patch testing with other steroids should be performed. In 1989, Coopman et al7 established a classification system for corticosteroids based on molecular structure, thus dividing them into 4 empirical groups: group A, hydrocortisone type; group B, acetonide type; group C, betamethasone type; and group D, ester type. The investigators hypothesized that allergic contact reactions occurred more frequently with corticosteroids belonging to the same group, while cross-reactions were uncommon between groups; however, cross-reactivity is known to occur among corticosteroids belonging to different groups in standard clinical practice, which conflicts with this claim.
Due to distinctively different behaviors among certain compounds in group D, Matura et al8 proposed subdividing the ester steroids into 2 groups: group D1, containing C16 methyl substitution and halogenation on the B ring, and group D2, comprising the labile ester steroids that lack both substitutions. A modified classification system including these subdivided groups is presented in the Table.8
In recent years, new corticosteroid drugs such as deflazacort, fluticasone propionate, and mometasone furoate have been developed, but classification of these agents has been difficult due to differences in their chemical structure, although mometasone furoate and fluticasone propionate have been included in group D1.9 Futhermore, the structural differences of these new steroids may mean less cross-reactivity with other steroids, which would facilitate their use in patients who are allergic to classic steroids. However, cross-reactivity between mometasone furoate and corticosteroids belonging to group B has already been described,10 which may restrict its use in patients who are allergic to other corticosteroids.
The classification of corticosteroids can provide useful information about cross-reactivity, which may help physicians in choosing an alternative drug in patients with an allergy to topical corticosteroids, but this advice about cross-reactivity does not seem to apply to systemic allergic dermatitis or immediate-type reactions to corticosteroids.11 Therefore, in these types of reactions, an individualized evaluation of the sensitization profile is needed, performing wider studies with alternative corticosteroids by skin tests with late readings and challenge tests.
It is important to emphasize that hypersensitivity to corticosteroids should always be considered in the differential diagnosis along with oral candidiasis when oropharyngeal symptoms appear during inhaled corticosteroid along with oral candidiasis. We recommend that all drugs involved in a presumed allergic reaction must be systematically evaluated because an unexpected concomitant sensitization to multiple drugs could be present.
- English JS. Corticosteroid-induced contact dermatitis: a pragmatic approach. Clin Exp Dermatol. 2000;25:261-264.
- Martínez FV, Muñoz Pamplona MP, García EC, et al. Delayed hypersensitivity to oral nystatin. Contact Dermatitis. 2007;57:200-201.
- Quirce S, Parra F, Lázaro M, et al. Generalized dermatitis due to oral nystatin. Contact Dermatitis. 1991;25:197-198.
- de Groot AC, Conemans JM. Nystatin allergy: petrolatum is not the optimal vehicle for patch testing. Dermatol Clin. 1990;8:153-155.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60.
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727.
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34.
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704.
- Baeck M, Chamelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modeling tools. Allergy. 2011;66:1367-1374.
- Seyfarth F, Elsner P, Tittelbach J, et al. Contact allergy to mometasone furoate with cross-reactivity to group B corticosteroids. Contact Dermatitis. 2008;58:180-181.
- Torres MJ, Canto G. Hypersensitivity reactions to corticosteroids. Curr Opin Allergy Clin Immunol. 2010;10:273-279.
The development of concomitant allergic reactions to multiple drugs is uncommon. Dermatitis induced by topical or inhaled corticosteroids (eg, budesonide) is rare,1 and allergic reactions associated with oral nystatin, a macrolide antifungal drug, also are unusual.2 We present the case of concomitant sensitization to inhaled budesonide and oral nystatin presenting as allergic contact stomatitis and systemic allergic contact dermatitis. Concomitant allergic reactions to these treatments are rare and may result in diagnostic challenges for the physician.
Case Report
A 66-year-old woman presented to the Allergy Department for evaluation of painful erosions on the oral mucosa that had developed 72 hours after she started treatment with inhaled budesonide (400 mcg every 12 hours) prescribed by her general practitioner for a nonproductive cough. Budesonide inhalation was discontinued due to suspected oral candidiasis and treatment with oral nystatin (500,000 IU every 8 hours) was started, but the erosions did not resolve. After 2 days of treatment with oral nystatin, the patient presented with erythematous macules on the abdomen and thighs as well as a larger erythematous and edematous lesion with papules and vesicles on the hypothenar eminence of the right hand. Nystatin was discontinued and the lesions turned desquamative and healed spontaneously 7 days later. The oral lesions resolved after 15 days with no further treatment.
Patch testing was conducted using a commercially standard series of contact allergens, all of which showed negative results at 48 and 96 hours except for budesonide and triamcinolone, which led to the diagnosis of allergic contact stomatitis from the inhaled budesonide. Patch testing with other corticosteroids was negative. Challenge tests with alternative corticosteroids (ie, oral methylprednisolone, parenteral betamethasone, topical mometasone furoate, inhaled fluticasone) were negative.
In order to rule out involvement of oral nystatin, a single-blind, placebo-controlled oral challenge test was performed. Eight hours after taking oral nystatin (500,000 IU), erythematous macules developed on the patient’s abdomen along with an erythematous, 3×4-cm lesion with papules on the hypothenar eminence of the right hand that was similar in appearance to the original presentation. The lesion on the hand was biopsied and histologic examination revealed spongiosis, edema of the superficial dermis, perivascular lymphocytic infiltrates, and extravasated erythrocytes with no vasculitis. Further patch testing subsequently was conducted with antifungal and antibiotic macrolides in different vehicles (ie, petrolatum, water, polyethylene glycol), as well as with excipients of the oral nystatin formulation that had been tested (Figure). Patch testing was positive with nystatin 10% in petrolatum and nystatin 30,000 IU and 90,000 IU in polyethylene glycol. Testing also were conducted in 7 healthy volunteers to rule out an irritant reaction and showed negative results. Finally, challenge tests conducted in our patient with another antifungal macrolide (parenteral amphotericin B) and antibiotic macrolides (oral clarithromycin, erythromycin, and azithromycin) were negative.

Patch and challenge test results along with the histologic findings led to diagnosis of concomitant systemic allergic contact dermatitis from oral nystatin.
Comment
Our patient presented with 2 unusual delayed hypersensitivity reactions that occurred in the same medical episode: allergic contact stomatitis from inhaled budesonide and systemic allergic contact dermatitis from oral nystatin. It is noteworthy that, despite the poor intestinal absorption of nystatin, systemic contact dermatitis to this drug has been previously described.3 Patch testing with macrolides proved useful for diagnosis in our patient, and based on the results we concluded that polyethylene glycol seemed to be the optimal vehicle for patch testing macrolide drugs versus water or petrolatum, as has been previously suggested.4
When a diagnosis of drug allergy is established, it is important to rule out cross-reactivity with other similar drugs by assessing if they produce the same reaction despite differences in chemical structure. Possible cross-reactivity of nystatin with other macrolides (validated on patch testing) has been reported but the tolerability was not evaluated.5 Our patient showed good tolerability to other macrolide drugs, both antibiotics and antifungals. Therefore, nystatin does not seem to cross-react with other structurally related drugs belonging to the macrolide group based on our results.
Corticosteroid allergies are more common than those associated with macrolides, especially contact dermatitis. Nonhalogenated corticosteroids (eg, hydrocortisone, budesonide) are most frequently associated with allergic reactions,6 and patch testing remains the diagnostic method of choice for the detection of delayed hypersensitivity to corticosteroids. In Europe, standard series include budesonide and tixocortol pivalate, and in the United States they include hydrocortisone 17–butyrate, triamcinolone acetonide, and clobetasol 17–propionate.6
To assess cross-reactivity among topical corticosteroids, patch testing with other steroids should be performed. In 1989, Coopman et al7 established a classification system for corticosteroids based on molecular structure, thus dividing them into 4 empirical groups: group A, hydrocortisone type; group B, acetonide type; group C, betamethasone type; and group D, ester type. The investigators hypothesized that allergic contact reactions occurred more frequently with corticosteroids belonging to the same group, while cross-reactions were uncommon between groups; however, cross-reactivity is known to occur among corticosteroids belonging to different groups in standard clinical practice, which conflicts with this claim.
Due to distinctively different behaviors among certain compounds in group D, Matura et al8 proposed subdividing the ester steroids into 2 groups: group D1, containing C16 methyl substitution and halogenation on the B ring, and group D2, comprising the labile ester steroids that lack both substitutions. A modified classification system including these subdivided groups is presented in the Table.8
In recent years, new corticosteroid drugs such as deflazacort, fluticasone propionate, and mometasone furoate have been developed, but classification of these agents has been difficult due to differences in their chemical structure, although mometasone furoate and fluticasone propionate have been included in group D1.9 Futhermore, the structural differences of these new steroids may mean less cross-reactivity with other steroids, which would facilitate their use in patients who are allergic to classic steroids. However, cross-reactivity between mometasone furoate and corticosteroids belonging to group B has already been described,10 which may restrict its use in patients who are allergic to other corticosteroids.
The classification of corticosteroids can provide useful information about cross-reactivity, which may help physicians in choosing an alternative drug in patients with an allergy to topical corticosteroids, but this advice about cross-reactivity does not seem to apply to systemic allergic dermatitis or immediate-type reactions to corticosteroids.11 Therefore, in these types of reactions, an individualized evaluation of the sensitization profile is needed, performing wider studies with alternative corticosteroids by skin tests with late readings and challenge tests.
It is important to emphasize that hypersensitivity to corticosteroids should always be considered in the differential diagnosis along with oral candidiasis when oropharyngeal symptoms appear during inhaled corticosteroid along with oral candidiasis. We recommend that all drugs involved in a presumed allergic reaction must be systematically evaluated because an unexpected concomitant sensitization to multiple drugs could be present.
The development of concomitant allergic reactions to multiple drugs is uncommon. Dermatitis induced by topical or inhaled corticosteroids (eg, budesonide) is rare,1 and allergic reactions associated with oral nystatin, a macrolide antifungal drug, also are unusual.2 We present the case of concomitant sensitization to inhaled budesonide and oral nystatin presenting as allergic contact stomatitis and systemic allergic contact dermatitis. Concomitant allergic reactions to these treatments are rare and may result in diagnostic challenges for the physician.
Case Report
A 66-year-old woman presented to the Allergy Department for evaluation of painful erosions on the oral mucosa that had developed 72 hours after she started treatment with inhaled budesonide (400 mcg every 12 hours) prescribed by her general practitioner for a nonproductive cough. Budesonide inhalation was discontinued due to suspected oral candidiasis and treatment with oral nystatin (500,000 IU every 8 hours) was started, but the erosions did not resolve. After 2 days of treatment with oral nystatin, the patient presented with erythematous macules on the abdomen and thighs as well as a larger erythematous and edematous lesion with papules and vesicles on the hypothenar eminence of the right hand. Nystatin was discontinued and the lesions turned desquamative and healed spontaneously 7 days later. The oral lesions resolved after 15 days with no further treatment.
Patch testing was conducted using a commercially standard series of contact allergens, all of which showed negative results at 48 and 96 hours except for budesonide and triamcinolone, which led to the diagnosis of allergic contact stomatitis from the inhaled budesonide. Patch testing with other corticosteroids was negative. Challenge tests with alternative corticosteroids (ie, oral methylprednisolone, parenteral betamethasone, topical mometasone furoate, inhaled fluticasone) were negative.
In order to rule out involvement of oral nystatin, a single-blind, placebo-controlled oral challenge test was performed. Eight hours after taking oral nystatin (500,000 IU), erythematous macules developed on the patient’s abdomen along with an erythematous, 3×4-cm lesion with papules on the hypothenar eminence of the right hand that was similar in appearance to the original presentation. The lesion on the hand was biopsied and histologic examination revealed spongiosis, edema of the superficial dermis, perivascular lymphocytic infiltrates, and extravasated erythrocytes with no vasculitis. Further patch testing subsequently was conducted with antifungal and antibiotic macrolides in different vehicles (ie, petrolatum, water, polyethylene glycol), as well as with excipients of the oral nystatin formulation that had been tested (Figure). Patch testing was positive with nystatin 10% in petrolatum and nystatin 30,000 IU and 90,000 IU in polyethylene glycol. Testing also were conducted in 7 healthy volunteers to rule out an irritant reaction and showed negative results. Finally, challenge tests conducted in our patient with another antifungal macrolide (parenteral amphotericin B) and antibiotic macrolides (oral clarithromycin, erythromycin, and azithromycin) were negative.

Patch and challenge test results along with the histologic findings led to diagnosis of concomitant systemic allergic contact dermatitis from oral nystatin.
Comment
Our patient presented with 2 unusual delayed hypersensitivity reactions that occurred in the same medical episode: allergic contact stomatitis from inhaled budesonide and systemic allergic contact dermatitis from oral nystatin. It is noteworthy that, despite the poor intestinal absorption of nystatin, systemic contact dermatitis to this drug has been previously described.3 Patch testing with macrolides proved useful for diagnosis in our patient, and based on the results we concluded that polyethylene glycol seemed to be the optimal vehicle for patch testing macrolide drugs versus water or petrolatum, as has been previously suggested.4
When a diagnosis of drug allergy is established, it is important to rule out cross-reactivity with other similar drugs by assessing if they produce the same reaction despite differences in chemical structure. Possible cross-reactivity of nystatin with other macrolides (validated on patch testing) has been reported but the tolerability was not evaluated.5 Our patient showed good tolerability to other macrolide drugs, both antibiotics and antifungals. Therefore, nystatin does not seem to cross-react with other structurally related drugs belonging to the macrolide group based on our results.
Corticosteroid allergies are more common than those associated with macrolides, especially contact dermatitis. Nonhalogenated corticosteroids (eg, hydrocortisone, budesonide) are most frequently associated with allergic reactions,6 and patch testing remains the diagnostic method of choice for the detection of delayed hypersensitivity to corticosteroids. In Europe, standard series include budesonide and tixocortol pivalate, and in the United States they include hydrocortisone 17–butyrate, triamcinolone acetonide, and clobetasol 17–propionate.6
To assess cross-reactivity among topical corticosteroids, patch testing with other steroids should be performed. In 1989, Coopman et al7 established a classification system for corticosteroids based on molecular structure, thus dividing them into 4 empirical groups: group A, hydrocortisone type; group B, acetonide type; group C, betamethasone type; and group D, ester type. The investigators hypothesized that allergic contact reactions occurred more frequently with corticosteroids belonging to the same group, while cross-reactions were uncommon between groups; however, cross-reactivity is known to occur among corticosteroids belonging to different groups in standard clinical practice, which conflicts with this claim.
Due to distinctively different behaviors among certain compounds in group D, Matura et al8 proposed subdividing the ester steroids into 2 groups: group D1, containing C16 methyl substitution and halogenation on the B ring, and group D2, comprising the labile ester steroids that lack both substitutions. A modified classification system including these subdivided groups is presented in the Table.8
In recent years, new corticosteroid drugs such as deflazacort, fluticasone propionate, and mometasone furoate have been developed, but classification of these agents has been difficult due to differences in their chemical structure, although mometasone furoate and fluticasone propionate have been included in group D1.9 Futhermore, the structural differences of these new steroids may mean less cross-reactivity with other steroids, which would facilitate their use in patients who are allergic to classic steroids. However, cross-reactivity between mometasone furoate and corticosteroids belonging to group B has already been described,10 which may restrict its use in patients who are allergic to other corticosteroids.
The classification of corticosteroids can provide useful information about cross-reactivity, which may help physicians in choosing an alternative drug in patients with an allergy to topical corticosteroids, but this advice about cross-reactivity does not seem to apply to systemic allergic dermatitis or immediate-type reactions to corticosteroids.11 Therefore, in these types of reactions, an individualized evaluation of the sensitization profile is needed, performing wider studies with alternative corticosteroids by skin tests with late readings and challenge tests.
It is important to emphasize that hypersensitivity to corticosteroids should always be considered in the differential diagnosis along with oral candidiasis when oropharyngeal symptoms appear during inhaled corticosteroid along with oral candidiasis. We recommend that all drugs involved in a presumed allergic reaction must be systematically evaluated because an unexpected concomitant sensitization to multiple drugs could be present.
- English JS. Corticosteroid-induced contact dermatitis: a pragmatic approach. Clin Exp Dermatol. 2000;25:261-264.
- Martínez FV, Muñoz Pamplona MP, García EC, et al. Delayed hypersensitivity to oral nystatin. Contact Dermatitis. 2007;57:200-201.
- Quirce S, Parra F, Lázaro M, et al. Generalized dermatitis due to oral nystatin. Contact Dermatitis. 1991;25:197-198.
- de Groot AC, Conemans JM. Nystatin allergy: petrolatum is not the optimal vehicle for patch testing. Dermatol Clin. 1990;8:153-155.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60.
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727.
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34.
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704.
- Baeck M, Chamelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modeling tools. Allergy. 2011;66:1367-1374.
- Seyfarth F, Elsner P, Tittelbach J, et al. Contact allergy to mometasone furoate with cross-reactivity to group B corticosteroids. Contact Dermatitis. 2008;58:180-181.
- Torres MJ, Canto G. Hypersensitivity reactions to corticosteroids. Curr Opin Allergy Clin Immunol. 2010;10:273-279.
- English JS. Corticosteroid-induced contact dermatitis: a pragmatic approach. Clin Exp Dermatol. 2000;25:261-264.
- Martínez FV, Muñoz Pamplona MP, García EC, et al. Delayed hypersensitivity to oral nystatin. Contact Dermatitis. 2007;57:200-201.
- Quirce S, Parra F, Lázaro M, et al. Generalized dermatitis due to oral nystatin. Contact Dermatitis. 1991;25:197-198.
- de Groot AC, Conemans JM. Nystatin allergy: petrolatum is not the optimal vehicle for patch testing. Dermatol Clin. 1990;8:153-155.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60.
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727.
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34.
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704.
- Baeck M, Chamelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modeling tools. Allergy. 2011;66:1367-1374.
- Seyfarth F, Elsner P, Tittelbach J, et al. Contact allergy to mometasone furoate with cross-reactivity to group B corticosteroids. Contact Dermatitis. 2008;58:180-181.
- Torres MJ, Canto G. Hypersensitivity reactions to corticosteroids. Curr Opin Allergy Clin Immunol. 2010;10:273-279.
Practice Points
- When lesions develop in the oral cavity during treatment with inhaled corticosteroids, delayed contact allergy should be considered in the differential diagnosis along with fungal infection.
- Although it generally is not considered to be allergenic due to its poor intestinal absorption, oral nystatin may induce systemic allergic disorders.
- All drugs involved in a presumed allergic reaction must be evaluated since concomitant sensitization to multiple drugs could be present. Patch and challenge testing should be conducted to diagnose allergic contact dermatitis and assess drug cross-reactivity.
Differentiation of Latex Allergy From Irritant Contact Dermatitis
Latex allergy is an all-encompassing term used to describe hypersensitivity reactions to products containing natural rubber latex from the Hevea brasiliensis tree and affects approximately 1% to 2% of the general population.1 Although latex gloves are the most widely known culprits, several other commonly used products can contain natural rubber latex, including adhesive tape, balloons, condoms, rubber bands, paint, tourniquets, electrode pads, and Foley catheters.2 The term latex allergy often is used as a general diagnosis, but there are in fact 3 distinct mechanisms by which individuals may develop an adverse reaction to latex-containing products: irritant contact dermatitis, allergic contact dermatitis (type IV hypersensitivity) and true latex allergy (type I hypersensitivity).
Irritant Contact Dermatitis
Irritant contact dermatitis, a nonimmunologic reaction, occurs due to mechanical factors (eg, friction) or contact with chemicals, which can have irritating and dehydrating effects. Individuals with irritant contact dermatitis do not have true latex allergy and will not necessarily develop a reaction to products containing natural rubber latex. Incorrectly attributing these irritant contact dermatitis reactions to latex allergy and simply advising patients to avoid all latex products (eg, use nitrile gloves rather than latex gloves) will not address the underlying problem. Rather, these patients must be informed that the dermatitis is a result of a disruption to the natural, protective skin barrier and not an allergic reaction.
Allergic Contact Dermatitis
Allergic contact dermatitis to rubber is caused by a type IV (delayed) hypersensitivity reaction and is the result of exposure to the accelerators present in rubber products in sensitive individuals. Individuals experiencing this type of reaction typically develop localized erythema, pruritus, and urticarial lesions 48 hours after exposure.3 Incorrectly labeling this problem as latex allergy and recommending nonlatex rubber substitutes (eg, hypoallergenic gloves) likely will not be effective, as these nonlatex replacement products contain the same accelerators as do latex gloves.
True Latex Allergy
The most severe form of latex allergy, often referred to as true latex allergy, is caused by a type I (immediate) hypersensitivity reaction mediated by immunoglobulin E (IgE) antibodies. Individuals experiencing this type of reaction have a systemic response to latex proteins that may result in fulminant anaphylaxis. Individuals with true latex allergy must absolutely avoid latex products, and substituting nonlatex products is the most effective approach.
Latex Reactions in Medical Practice
The varying propensity of certain populations to develop latex allergy has been well documented; for example, the prevalence of hypersensitivity in patients with spina bifida ranges from 20% to 65%, figures that are much higher than those reported in the general population.3 This hypersensitivity in patients with spina bifida most likely results from repeated exposure to latex products during corrective surgeries and diagnostic procedures early in life. Atopic individuals, such as those with allergic rhinitis, eczema, and asthma, have a 4-fold increased risk for developing latex allergy compared to nonatopic individuals.4 The risk of latex allergy among health care workers is increased due to increased exposure to rubber products. One study found that the risk of latex sensitization among health care workers exposed to products containing latex was 4.3%, while the risk in the general population was only 1.37%.1 Those at highest risk for sensitization include dental assistants, operating room personnel, hospital housekeeping staff, and paramedics or emergency medical technicians.3 However, sensitization documented on laboratory assessment does not reliably correlate with symptomatic allergy, as many patients with a positive IgE test do not show clinical symptoms. Schmid et al4 demonstrated that a 1.3% prevalence of clinically symptomatic latex allergy among health care workers may approximate the prevalence of latex allergy in the general population. In a study by Brown et al,5 although 12.5% of anesthesiologists were found to be sensitized to latex, only 2.4% had clinically symptomatic allergic reactions.
Testing for Latex Allergy
Several diagnostic tests are available to establish a diagnosis of type I sensitization or true latex allergy. Skin prick testing is an in vivo assay and is the gold standard for diagnosing IgE-mediated type I hypersensitivity to latex. The test involves pricking the skin of the forearm and applying a commercial extract of nonammoniated latex to monitor for development of a wheal within several minutes. The skin prick test should be performed in a health care setting equipped with oxygen, epinephrine, and latex-free resuscitation equipment in case of anaphylaxis following exposure. Although latex skin prick testing is the gold standard, it is rarely performed in the United States because there is no US Food and Drug Administration–approved natural rubber latex reagent.3 Consequently, physicians who wish to perform skin prick testing for latex allergy are forced to develop improvised reagents from the H brasiliensis tree itself or from highly allergenic latex gloves. Standardized latex allergens are commercially available in Europe.
The most noninvasive method of latex allergy testing is an in vitro assay for latex-specific IgE antibodies, which can be detected by either a radioallergosorbent test (RAST) or enzyme-linked immunosorbent assay (ELISA). The presence of antilatex IgE antibodies confirms sensitization but does not necessarily mean the patient will develop a symptomatic reaction following exposure. Due to the unavailability of a standardized reagent for the skin prick test in the United States, evaluation of latex-specific serum IgE levels may be the best alternative. While the skin prick test has the highest sensitivity, the sensitivity and specificity of latex-specific serum IgE testing are 50% to 90% and 80% to 87%, respectively.6
The wear test (also known as the use or glove provocation test), can be used to diagnose clinically symptomatic latex allergy when there is a discrepancy between the patient’s clinical history and results from skin prick or serum IgE antibody testing. To perform the wear test, place a natural rubber latex glove on one of the patient’s fingers for 15 minutes and monitor the area for development of urticaria. If there is no evidence of allergic reaction within 15 minutes, place the glove on the whole hand for an additional 15 minutes. The patient is said to be nonreactive if a latex glove can be placed on the entire hand for 15 minutes without evidence of reaction.3
Lastly, patch testing can differentiate between irritant contact and allergic contact (type IV hypersensitivity) dermatitis. Apply a small amount of each substance of interest onto a separate disc and place the discs in direct contact with the skin using hypoallergenic tape. With type IV latex hypersensitivity, the skin underneath the disc will become erythematous with developing papulovesicles, starting between 2 and 5 days after exposure. The Figure outlines the differentiation of true latex allergy from irritant and allergic contact dermatitis and identifies methods for making these diagnoses.
General Medical Protocol With Latex Reactions
To reduce the incidence of latex allergic reactions among health care workers and patients, Kumar2 recommends putting a protocol in place to document steps in preventing, diagnosing, and treating latex allergy. This protocol includes employee and patient education about the risks for developing latex allergy and the signs and symptoms of a reaction; available diagnostic testing; and alternative products (eg, hypoallergenic gloves) that are available to individuals with a known or suspected allergy. At-risk health care workers who have not been sensitized should be advised to avoid latex-containing products.3 Routine questioning and diagnostic testing may be necessary as part of every preoperative assessment, as there have been reported cases of anaphylaxis in patients with undocumented allergies.7 Anaphylaxis caused by latex allergy is the second leading cause of perioperative anaphylaxis, accounting for as many as 20% of cases.8 With the use of preventative measures and early identification of at-risk patients, the incidence of latex-related anaphylaxis is decreasing.8 Ascertaining valuable information about the patient’s medical history, such as known allergies to foods that have cross-reactivity to latex (eg, bananas, mango, kiwi, avocado), is one simple way of identifying a patient who should be tested for possible underlying latex allergy.8 Total avoidance of latex-containing products (eg, in the workplace) can further reduce the incidence of allergic reactions by decreasing primary sensitization and risk of exposure.
Conclusion
Patients claiming to be allergic to latex without documentation should be tested. The diagnostic testing available in the United States includes patch testing, wear (or glove provocation) testing, or assessment of IgE antibody titer. Accurate differentiation among irritant contact dermatitis, allergic contact dermatitis, and true latex allergy is paramount for properly educating patients and effectively treating these conditions. Additionally, distinguishing patients with true latex allergy from those who have been misdiagnosed can save resources and reduce health care costs.
- Bousquet J, Flahault A, Vandenplas O, et al. Natural rubber latex allergy among health care workers: a systematic review of the evidence. J Allergy Clin Immunol. 2006;118:447-454.
- Kumar RP. Latex allergy in clinical practice. Indian J Dermatol. 2012;57:66-70.
- Taylor JS, Erkek E. Latex allergy: diagnosis and management. Dermatol Ther. 2004;17:289-301.
- Schmid K, Christoph Broding H, Niklas D, et al. Latex sensitization in dental students using powder-free gloves low in latex protein: a cross-sectional study. Contact Dermatitis. 2002;47:103-108.
- Brown RH, Schauble JF, Hamilton RG. Prevalence of latex allergy among anesthesiologists: identification of sensitized but asymptomatic individuals. Anesthesiology. 1998;89:292-299.
- Pollart SM, Warniment C, Mori T. Latex allergy. Am Fam Physician. 2009;80:1413-1418.
- Duger C, Kol IO, Kaygusuz K, et al. A perioperative anaphylactic reaction caused by latex in a patient with no history of allergy. Anaesth Pain Intensive Care. 2012;16:71-73.
- Hepner DL, Castells MC. Anaphylaxis during the perioperative period. Anesth Analg. 2003;97:1381-1395.
Latex allergy is an all-encompassing term used to describe hypersensitivity reactions to products containing natural rubber latex from the Hevea brasiliensis tree and affects approximately 1% to 2% of the general population.1 Although latex gloves are the most widely known culprits, several other commonly used products can contain natural rubber latex, including adhesive tape, balloons, condoms, rubber bands, paint, tourniquets, electrode pads, and Foley catheters.2 The term latex allergy often is used as a general diagnosis, but there are in fact 3 distinct mechanisms by which individuals may develop an adverse reaction to latex-containing products: irritant contact dermatitis, allergic contact dermatitis (type IV hypersensitivity) and true latex allergy (type I hypersensitivity).
Irritant Contact Dermatitis
Irritant contact dermatitis, a nonimmunologic reaction, occurs due to mechanical factors (eg, friction) or contact with chemicals, which can have irritating and dehydrating effects. Individuals with irritant contact dermatitis do not have true latex allergy and will not necessarily develop a reaction to products containing natural rubber latex. Incorrectly attributing these irritant contact dermatitis reactions to latex allergy and simply advising patients to avoid all latex products (eg, use nitrile gloves rather than latex gloves) will not address the underlying problem. Rather, these patients must be informed that the dermatitis is a result of a disruption to the natural, protective skin barrier and not an allergic reaction.
Allergic Contact Dermatitis
Allergic contact dermatitis to rubber is caused by a type IV (delayed) hypersensitivity reaction and is the result of exposure to the accelerators present in rubber products in sensitive individuals. Individuals experiencing this type of reaction typically develop localized erythema, pruritus, and urticarial lesions 48 hours after exposure.3 Incorrectly labeling this problem as latex allergy and recommending nonlatex rubber substitutes (eg, hypoallergenic gloves) likely will not be effective, as these nonlatex replacement products contain the same accelerators as do latex gloves.
True Latex Allergy
The most severe form of latex allergy, often referred to as true latex allergy, is caused by a type I (immediate) hypersensitivity reaction mediated by immunoglobulin E (IgE) antibodies. Individuals experiencing this type of reaction have a systemic response to latex proteins that may result in fulminant anaphylaxis. Individuals with true latex allergy must absolutely avoid latex products, and substituting nonlatex products is the most effective approach.
Latex Reactions in Medical Practice
The varying propensity of certain populations to develop latex allergy has been well documented; for example, the prevalence of hypersensitivity in patients with spina bifida ranges from 20% to 65%, figures that are much higher than those reported in the general population.3 This hypersensitivity in patients with spina bifida most likely results from repeated exposure to latex products during corrective surgeries and diagnostic procedures early in life. Atopic individuals, such as those with allergic rhinitis, eczema, and asthma, have a 4-fold increased risk for developing latex allergy compared to nonatopic individuals.4 The risk of latex allergy among health care workers is increased due to increased exposure to rubber products. One study found that the risk of latex sensitization among health care workers exposed to products containing latex was 4.3%, while the risk in the general population was only 1.37%.1 Those at highest risk for sensitization include dental assistants, operating room personnel, hospital housekeeping staff, and paramedics or emergency medical technicians.3 However, sensitization documented on laboratory assessment does not reliably correlate with symptomatic allergy, as many patients with a positive IgE test do not show clinical symptoms. Schmid et al4 demonstrated that a 1.3% prevalence of clinically symptomatic latex allergy among health care workers may approximate the prevalence of latex allergy in the general population. In a study by Brown et al,5 although 12.5% of anesthesiologists were found to be sensitized to latex, only 2.4% had clinically symptomatic allergic reactions.
Testing for Latex Allergy
Several diagnostic tests are available to establish a diagnosis of type I sensitization or true latex allergy. Skin prick testing is an in vivo assay and is the gold standard for diagnosing IgE-mediated type I hypersensitivity to latex. The test involves pricking the skin of the forearm and applying a commercial extract of nonammoniated latex to monitor for development of a wheal within several minutes. The skin prick test should be performed in a health care setting equipped with oxygen, epinephrine, and latex-free resuscitation equipment in case of anaphylaxis following exposure. Although latex skin prick testing is the gold standard, it is rarely performed in the United States because there is no US Food and Drug Administration–approved natural rubber latex reagent.3 Consequently, physicians who wish to perform skin prick testing for latex allergy are forced to develop improvised reagents from the H brasiliensis tree itself or from highly allergenic latex gloves. Standardized latex allergens are commercially available in Europe.
The most noninvasive method of latex allergy testing is an in vitro assay for latex-specific IgE antibodies, which can be detected by either a radioallergosorbent test (RAST) or enzyme-linked immunosorbent assay (ELISA). The presence of antilatex IgE antibodies confirms sensitization but does not necessarily mean the patient will develop a symptomatic reaction following exposure. Due to the unavailability of a standardized reagent for the skin prick test in the United States, evaluation of latex-specific serum IgE levels may be the best alternative. While the skin prick test has the highest sensitivity, the sensitivity and specificity of latex-specific serum IgE testing are 50% to 90% and 80% to 87%, respectively.6
The wear test (also known as the use or glove provocation test), can be used to diagnose clinically symptomatic latex allergy when there is a discrepancy between the patient’s clinical history and results from skin prick or serum IgE antibody testing. To perform the wear test, place a natural rubber latex glove on one of the patient’s fingers for 15 minutes and monitor the area for development of urticaria. If there is no evidence of allergic reaction within 15 minutes, place the glove on the whole hand for an additional 15 minutes. The patient is said to be nonreactive if a latex glove can be placed on the entire hand for 15 minutes without evidence of reaction.3
Lastly, patch testing can differentiate between irritant contact and allergic contact (type IV hypersensitivity) dermatitis. Apply a small amount of each substance of interest onto a separate disc and place the discs in direct contact with the skin using hypoallergenic tape. With type IV latex hypersensitivity, the skin underneath the disc will become erythematous with developing papulovesicles, starting between 2 and 5 days after exposure. The Figure outlines the differentiation of true latex allergy from irritant and allergic contact dermatitis and identifies methods for making these diagnoses.
General Medical Protocol With Latex Reactions
To reduce the incidence of latex allergic reactions among health care workers and patients, Kumar2 recommends putting a protocol in place to document steps in preventing, diagnosing, and treating latex allergy. This protocol includes employee and patient education about the risks for developing latex allergy and the signs and symptoms of a reaction; available diagnostic testing; and alternative products (eg, hypoallergenic gloves) that are available to individuals with a known or suspected allergy. At-risk health care workers who have not been sensitized should be advised to avoid latex-containing products.3 Routine questioning and diagnostic testing may be necessary as part of every preoperative assessment, as there have been reported cases of anaphylaxis in patients with undocumented allergies.7 Anaphylaxis caused by latex allergy is the second leading cause of perioperative anaphylaxis, accounting for as many as 20% of cases.8 With the use of preventative measures and early identification of at-risk patients, the incidence of latex-related anaphylaxis is decreasing.8 Ascertaining valuable information about the patient’s medical history, such as known allergies to foods that have cross-reactivity to latex (eg, bananas, mango, kiwi, avocado), is one simple way of identifying a patient who should be tested for possible underlying latex allergy.8 Total avoidance of latex-containing products (eg, in the workplace) can further reduce the incidence of allergic reactions by decreasing primary sensitization and risk of exposure.
Conclusion
Patients claiming to be allergic to latex without documentation should be tested. The diagnostic testing available in the United States includes patch testing, wear (or glove provocation) testing, or assessment of IgE antibody titer. Accurate differentiation among irritant contact dermatitis, allergic contact dermatitis, and true latex allergy is paramount for properly educating patients and effectively treating these conditions. Additionally, distinguishing patients with true latex allergy from those who have been misdiagnosed can save resources and reduce health care costs.
Latex allergy is an all-encompassing term used to describe hypersensitivity reactions to products containing natural rubber latex from the Hevea brasiliensis tree and affects approximately 1% to 2% of the general population.1 Although latex gloves are the most widely known culprits, several other commonly used products can contain natural rubber latex, including adhesive tape, balloons, condoms, rubber bands, paint, tourniquets, electrode pads, and Foley catheters.2 The term latex allergy often is used as a general diagnosis, but there are in fact 3 distinct mechanisms by which individuals may develop an adverse reaction to latex-containing products: irritant contact dermatitis, allergic contact dermatitis (type IV hypersensitivity) and true latex allergy (type I hypersensitivity).
Irritant Contact Dermatitis
Irritant contact dermatitis, a nonimmunologic reaction, occurs due to mechanical factors (eg, friction) or contact with chemicals, which can have irritating and dehydrating effects. Individuals with irritant contact dermatitis do not have true latex allergy and will not necessarily develop a reaction to products containing natural rubber latex. Incorrectly attributing these irritant contact dermatitis reactions to latex allergy and simply advising patients to avoid all latex products (eg, use nitrile gloves rather than latex gloves) will not address the underlying problem. Rather, these patients must be informed that the dermatitis is a result of a disruption to the natural, protective skin barrier and not an allergic reaction.
Allergic Contact Dermatitis
Allergic contact dermatitis to rubber is caused by a type IV (delayed) hypersensitivity reaction and is the result of exposure to the accelerators present in rubber products in sensitive individuals. Individuals experiencing this type of reaction typically develop localized erythema, pruritus, and urticarial lesions 48 hours after exposure.3 Incorrectly labeling this problem as latex allergy and recommending nonlatex rubber substitutes (eg, hypoallergenic gloves) likely will not be effective, as these nonlatex replacement products contain the same accelerators as do latex gloves.
True Latex Allergy
The most severe form of latex allergy, often referred to as true latex allergy, is caused by a type I (immediate) hypersensitivity reaction mediated by immunoglobulin E (IgE) antibodies. Individuals experiencing this type of reaction have a systemic response to latex proteins that may result in fulminant anaphylaxis. Individuals with true latex allergy must absolutely avoid latex products, and substituting nonlatex products is the most effective approach.
Latex Reactions in Medical Practice
The varying propensity of certain populations to develop latex allergy has been well documented; for example, the prevalence of hypersensitivity in patients with spina bifida ranges from 20% to 65%, figures that are much higher than those reported in the general population.3 This hypersensitivity in patients with spina bifida most likely results from repeated exposure to latex products during corrective surgeries and diagnostic procedures early in life. Atopic individuals, such as those with allergic rhinitis, eczema, and asthma, have a 4-fold increased risk for developing latex allergy compared to nonatopic individuals.4 The risk of latex allergy among health care workers is increased due to increased exposure to rubber products. One study found that the risk of latex sensitization among health care workers exposed to products containing latex was 4.3%, while the risk in the general population was only 1.37%.1 Those at highest risk for sensitization include dental assistants, operating room personnel, hospital housekeeping staff, and paramedics or emergency medical technicians.3 However, sensitization documented on laboratory assessment does not reliably correlate with symptomatic allergy, as many patients with a positive IgE test do not show clinical symptoms. Schmid et al4 demonstrated that a 1.3% prevalence of clinically symptomatic latex allergy among health care workers may approximate the prevalence of latex allergy in the general population. In a study by Brown et al,5 although 12.5% of anesthesiologists were found to be sensitized to latex, only 2.4% had clinically symptomatic allergic reactions.
Testing for Latex Allergy
Several diagnostic tests are available to establish a diagnosis of type I sensitization or true latex allergy. Skin prick testing is an in vivo assay and is the gold standard for diagnosing IgE-mediated type I hypersensitivity to latex. The test involves pricking the skin of the forearm and applying a commercial extract of nonammoniated latex to monitor for development of a wheal within several minutes. The skin prick test should be performed in a health care setting equipped with oxygen, epinephrine, and latex-free resuscitation equipment in case of anaphylaxis following exposure. Although latex skin prick testing is the gold standard, it is rarely performed in the United States because there is no US Food and Drug Administration–approved natural rubber latex reagent.3 Consequently, physicians who wish to perform skin prick testing for latex allergy are forced to develop improvised reagents from the H brasiliensis tree itself or from highly allergenic latex gloves. Standardized latex allergens are commercially available in Europe.
The most noninvasive method of latex allergy testing is an in vitro assay for latex-specific IgE antibodies, which can be detected by either a radioallergosorbent test (RAST) or enzyme-linked immunosorbent assay (ELISA). The presence of antilatex IgE antibodies confirms sensitization but does not necessarily mean the patient will develop a symptomatic reaction following exposure. Due to the unavailability of a standardized reagent for the skin prick test in the United States, evaluation of latex-specific serum IgE levels may be the best alternative. While the skin prick test has the highest sensitivity, the sensitivity and specificity of latex-specific serum IgE testing are 50% to 90% and 80% to 87%, respectively.6
The wear test (also known as the use or glove provocation test), can be used to diagnose clinically symptomatic latex allergy when there is a discrepancy between the patient’s clinical history and results from skin prick or serum IgE antibody testing. To perform the wear test, place a natural rubber latex glove on one of the patient’s fingers for 15 minutes and monitor the area for development of urticaria. If there is no evidence of allergic reaction within 15 minutes, place the glove on the whole hand for an additional 15 minutes. The patient is said to be nonreactive if a latex glove can be placed on the entire hand for 15 minutes without evidence of reaction.3
Lastly, patch testing can differentiate between irritant contact and allergic contact (type IV hypersensitivity) dermatitis. Apply a small amount of each substance of interest onto a separate disc and place the discs in direct contact with the skin using hypoallergenic tape. With type IV latex hypersensitivity, the skin underneath the disc will become erythematous with developing papulovesicles, starting between 2 and 5 days after exposure. The Figure outlines the differentiation of true latex allergy from irritant and allergic contact dermatitis and identifies methods for making these diagnoses.
General Medical Protocol With Latex Reactions
To reduce the incidence of latex allergic reactions among health care workers and patients, Kumar2 recommends putting a protocol in place to document steps in preventing, diagnosing, and treating latex allergy. This protocol includes employee and patient education about the risks for developing latex allergy and the signs and symptoms of a reaction; available diagnostic testing; and alternative products (eg, hypoallergenic gloves) that are available to individuals with a known or suspected allergy. At-risk health care workers who have not been sensitized should be advised to avoid latex-containing products.3 Routine questioning and diagnostic testing may be necessary as part of every preoperative assessment, as there have been reported cases of anaphylaxis in patients with undocumented allergies.7 Anaphylaxis caused by latex allergy is the second leading cause of perioperative anaphylaxis, accounting for as many as 20% of cases.8 With the use of preventative measures and early identification of at-risk patients, the incidence of latex-related anaphylaxis is decreasing.8 Ascertaining valuable information about the patient’s medical history, such as known allergies to foods that have cross-reactivity to latex (eg, bananas, mango, kiwi, avocado), is one simple way of identifying a patient who should be tested for possible underlying latex allergy.8 Total avoidance of latex-containing products (eg, in the workplace) can further reduce the incidence of allergic reactions by decreasing primary sensitization and risk of exposure.
Conclusion
Patients claiming to be allergic to latex without documentation should be tested. The diagnostic testing available in the United States includes patch testing, wear (or glove provocation) testing, or assessment of IgE antibody titer. Accurate differentiation among irritant contact dermatitis, allergic contact dermatitis, and true latex allergy is paramount for properly educating patients and effectively treating these conditions. Additionally, distinguishing patients with true latex allergy from those who have been misdiagnosed can save resources and reduce health care costs.
- Bousquet J, Flahault A, Vandenplas O, et al. Natural rubber latex allergy among health care workers: a systematic review of the evidence. J Allergy Clin Immunol. 2006;118:447-454.
- Kumar RP. Latex allergy in clinical practice. Indian J Dermatol. 2012;57:66-70.
- Taylor JS, Erkek E. Latex allergy: diagnosis and management. Dermatol Ther. 2004;17:289-301.
- Schmid K, Christoph Broding H, Niklas D, et al. Latex sensitization in dental students using powder-free gloves low in latex protein: a cross-sectional study. Contact Dermatitis. 2002;47:103-108.
- Brown RH, Schauble JF, Hamilton RG. Prevalence of latex allergy among anesthesiologists: identification of sensitized but asymptomatic individuals. Anesthesiology. 1998;89:292-299.
- Pollart SM, Warniment C, Mori T. Latex allergy. Am Fam Physician. 2009;80:1413-1418.
- Duger C, Kol IO, Kaygusuz K, et al. A perioperative anaphylactic reaction caused by latex in a patient with no history of allergy. Anaesth Pain Intensive Care. 2012;16:71-73.
- Hepner DL, Castells MC. Anaphylaxis during the perioperative period. Anesth Analg. 2003;97:1381-1395.
- Bousquet J, Flahault A, Vandenplas O, et al. Natural rubber latex allergy among health care workers: a systematic review of the evidence. J Allergy Clin Immunol. 2006;118:447-454.
- Kumar RP. Latex allergy in clinical practice. Indian J Dermatol. 2012;57:66-70.
- Taylor JS, Erkek E. Latex allergy: diagnosis and management. Dermatol Ther. 2004;17:289-301.
- Schmid K, Christoph Broding H, Niklas D, et al. Latex sensitization in dental students using powder-free gloves low in latex protein: a cross-sectional study. Contact Dermatitis. 2002;47:103-108.
- Brown RH, Schauble JF, Hamilton RG. Prevalence of latex allergy among anesthesiologists: identification of sensitized but asymptomatic individuals. Anesthesiology. 1998;89:292-299.
- Pollart SM, Warniment C, Mori T. Latex allergy. Am Fam Physician. 2009;80:1413-1418.
- Duger C, Kol IO, Kaygusuz K, et al. A perioperative anaphylactic reaction caused by latex in a patient with no history of allergy. Anaesth Pain Intensive Care. 2012;16:71-73.
- Hepner DL, Castells MC. Anaphylaxis during the perioperative period. Anesth Analg. 2003;97:1381-1395.
Practice Points
- The term latex allergy often is used as a general diagnosis to describe 3 types of reactions to natural rubber latex, including irritant contact dermatitis, allergic contact dermatitis (type IV hypersensitivity reaction), and true latex allergy (type I hypersensitivity reaction).
- The latex skin prick test is considered the gold standard for diagnosis of true latex allergy, but this method is not available in the United States. In vitro assay for latex-specific immunoglobulin E antibodies is the best alternative.
Black Salve and Bloodroot Extract in Dermatologic Conditions
Black salve is composed of various ingredients, many of which are inert; however, some black salves contain escharotics, the 2 most common are zinc chloride and bloodroot (Sanguinaria canadensis) extract. In high doses, such as those contained in most black salve products, these corrosive agents can indiscriminately damage both healthy and diseased tissue.1 Nevertheless, many black salve products currently are advertised as safe and natural methods for curing skin cancer2-4 or treating a variety of other skin conditions (eg, moles, warts, skin tags, boils, abscesses, bee stings, other minor wounds)1,5 and even nondermatologic conditions such as a sore throat.6 Despite the information and testimonials that are widely available on the Internet, black salve use has not been validated by rigorous studies. Black salve is not regulated by the US Food and Drug Administration, resulting in poor quality control and inconsistent user instructions. We report the case of application of black salve to a biopsy site of a compound nevus with moderate atypia that resulted in the formation of a dermatitis plaque with subsequent scarring and basal layer pigmentation.
Case Report
A 35-year-old woman with a family history of melanoma presented for follow-up of a compound nevus with moderate atypia on the right anterior thigh that had been biopsied 6 months prior. Complete excision of the lesion was recommended at the initial presentation but was not performed due to scheduling conflicts. The patient reported applying black salve to the biopsy site and also to the left thigh 3 months later. There was no reaction on the left thigh after one 24-hour application of black salve, but an area around the biopsy site on the right thigh became thickened and irritated with superficial erosion of the skin following 2 applications of black salve, each of 24 hours’ duration. Physical examination revealed a granulomatous plaque at the biopsy site that was approximately 5 cm in diameter (Figure 1A). One year later the lesion had completely healed (Figure 1B) and a biopsy revealed scarring with basal layer pigmentation (Figure 2).
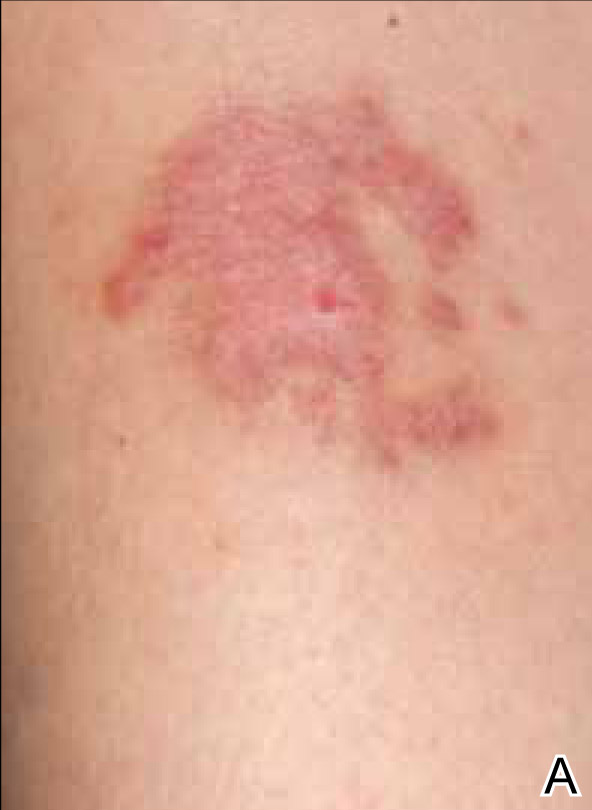 | 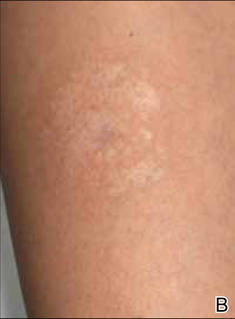 | 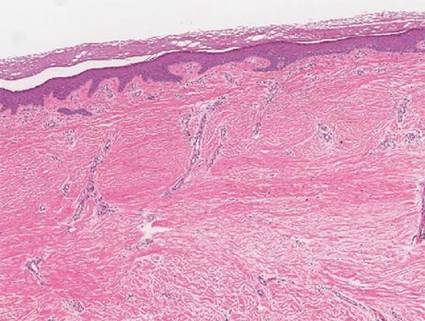 | |||
| Figure 1. A 5-cm granulomatous reaction surrounding a biopsy site on the right anterior thigh 3 months after application of black salve (A). One year later, the lesion had completely healed (B). | Figure 2. A biopsy one year following application of black salve demonstrated scarring with basal layer pigmentation (H&E, original magnification ×4). | ||||
Comment
A Web search using the term black salve yields a large number of products labeled as skin cancer salves, many showing glowing reviews and some being sold by major US retailers. The ingredients in black salves often vary in the innocuous substances they contain, but most products include the escharotics zinc chloride and bloodroot extract, which is derived from the plant S canadensis.1,3 For example, the ingredients of one popular black salve product include zinc chloride, chaparral (active ingredient is nordihydroguaiaretic acid), graviola leaf extract, oleander leaf extract, bloodroot extract, and glycerine,7 while another product includes bloodroot extract, zinc chloride, chaparral, cayenne pepper, red clover, birch bark, dimethyl sulfoxide, and burdock root.4
Bloodroot extract’s antimicrobial, anti-inflammatory, antioxidant, and immunomodulatory effects derive from its benzylisoquinoline alkaloids including sanguinarine, allocryptopine, berberine, coptisine, protopine, and stylopine.3,8 Bloodroot extract possesses some degree of tumoricidal potency, with one study finding that it selectively targets cancer cells.9 However, this differential response is seen only at low doses and not at the high concentrations contained in most black salve products.1 According to fluorometric assays, sanguinarine is not selective for tumor cells and therefore damages healthy tissue in addition to the unwanted lesions.6,10,11 The US Food and Drug Administration includes black salve products on its list of fake cancer cures that consumers should avoid.12 Reports of extensive damage from black salve use include skin ulceration2,10 and complete loss of a naris1 and nasal ala.5 Our case suggests the possible association between black salve use and an irritant reaction and erosion of the skin.
Furthermore, reliance on black salve alone in the treatment of skin cancer poses the threat of recurrence or metastasis of cancer because there is no way to know if the salve completely removed the cancer without a biopsy. Self-treatment can delay more effective therapy and may require further treatments.
Black salve should be subject to standarddrug regulations and its use discouraged by dermatologists due to the associated harmful effects and the availability of safer treatments. To better treat and inform their patients, dermatologists should be aware that patients may be attracted to alternative treatments such as black salves.
1. Eastman KL, McFarland LV, Raugi GJ. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289.
2. Eastman KL, McFarland LV, Raugi GJ. Buyer beware: a black salve caution. J Am Acad Dermatol. 2011;65:e154-e155.
3. Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80.
4. McDaniel S, Goldman GD. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
5. Payne CE. ‘Black Salve’ and melanomas [published online ahead of print August 11, 2010]. J Plast Reconstr Aesthet Surg. 2011;64:422.
6. Cienki JJ, Zaret L. An Internet misadventure: bloodroot salve toxicity. J Altern Complement Med. 2010;16:1125-1127.
7. Cansema and escharotics. Alpha Omega Labs Web site. http://www.altcancer.com/faqcan.htm. Accessed May 6, 2015.
8. Vlachojannis C, Magora F, Chrubasik S. Rise and fall of oral health products with Canadian bloodroot extract. Phytother Res. 2012;26:1423-1426.
9. Ahmad N, Gupta S, Husain MM, et al. Differential antiproliferative and apoptotic response of sanguinarine for cancer cells versus normal cells. Clin Cancer Res. 2000;6:1524-1528.
10. Saltzberg F, Barron G, Fenske N. Deforming self-treatment with herbal “black salve.” Dermatol Surg. 2009;35:1152-1154.
11. Debiton E, Madelmont JC, Legault J, et al. Sanguinarine-induced apoptosis is associated with an early and severe cellular glutathione depletion. Cancer Chemother Pharmacol. 2003;51:474-482.
12. 187 fake cancer “cures” consumers should avoid. US Food and Drug Administration Web site. http://www.fda.gov/Drugs/GuidanceCompliance RegulatoryInformation/EnforcementActivitiesbyFDA/ucm171057.htm. Updated July 9, 2009. Accessed May 6, 2015.
Black salve is composed of various ingredients, many of which are inert; however, some black salves contain escharotics, the 2 most common are zinc chloride and bloodroot (Sanguinaria canadensis) extract. In high doses, such as those contained in most black salve products, these corrosive agents can indiscriminately damage both healthy and diseased tissue.1 Nevertheless, many black salve products currently are advertised as safe and natural methods for curing skin cancer2-4 or treating a variety of other skin conditions (eg, moles, warts, skin tags, boils, abscesses, bee stings, other minor wounds)1,5 and even nondermatologic conditions such as a sore throat.6 Despite the information and testimonials that are widely available on the Internet, black salve use has not been validated by rigorous studies. Black salve is not regulated by the US Food and Drug Administration, resulting in poor quality control and inconsistent user instructions. We report the case of application of black salve to a biopsy site of a compound nevus with moderate atypia that resulted in the formation of a dermatitis plaque with subsequent scarring and basal layer pigmentation.
Case Report
A 35-year-old woman with a family history of melanoma presented for follow-up of a compound nevus with moderate atypia on the right anterior thigh that had been biopsied 6 months prior. Complete excision of the lesion was recommended at the initial presentation but was not performed due to scheduling conflicts. The patient reported applying black salve to the biopsy site and also to the left thigh 3 months later. There was no reaction on the left thigh after one 24-hour application of black salve, but an area around the biopsy site on the right thigh became thickened and irritated with superficial erosion of the skin following 2 applications of black salve, each of 24 hours’ duration. Physical examination revealed a granulomatous plaque at the biopsy site that was approximately 5 cm in diameter (Figure 1A). One year later the lesion had completely healed (Figure 1B) and a biopsy revealed scarring with basal layer pigmentation (Figure 2).
 |  |  | |||
| Figure 1. A 5-cm granulomatous reaction surrounding a biopsy site on the right anterior thigh 3 months after application of black salve (A). One year later, the lesion had completely healed (B). | Figure 2. A biopsy one year following application of black salve demonstrated scarring with basal layer pigmentation (H&E, original magnification ×4). | ||||
Comment
A Web search using the term black salve yields a large number of products labeled as skin cancer salves, many showing glowing reviews and some being sold by major US retailers. The ingredients in black salves often vary in the innocuous substances they contain, but most products include the escharotics zinc chloride and bloodroot extract, which is derived from the plant S canadensis.1,3 For example, the ingredients of one popular black salve product include zinc chloride, chaparral (active ingredient is nordihydroguaiaretic acid), graviola leaf extract, oleander leaf extract, bloodroot extract, and glycerine,7 while another product includes bloodroot extract, zinc chloride, chaparral, cayenne pepper, red clover, birch bark, dimethyl sulfoxide, and burdock root.4
Bloodroot extract’s antimicrobial, anti-inflammatory, antioxidant, and immunomodulatory effects derive from its benzylisoquinoline alkaloids including sanguinarine, allocryptopine, berberine, coptisine, protopine, and stylopine.3,8 Bloodroot extract possesses some degree of tumoricidal potency, with one study finding that it selectively targets cancer cells.9 However, this differential response is seen only at low doses and not at the high concentrations contained in most black salve products.1 According to fluorometric assays, sanguinarine is not selective for tumor cells and therefore damages healthy tissue in addition to the unwanted lesions.6,10,11 The US Food and Drug Administration includes black salve products on its list of fake cancer cures that consumers should avoid.12 Reports of extensive damage from black salve use include skin ulceration2,10 and complete loss of a naris1 and nasal ala.5 Our case suggests the possible association between black salve use and an irritant reaction and erosion of the skin.
Furthermore, reliance on black salve alone in the treatment of skin cancer poses the threat of recurrence or metastasis of cancer because there is no way to know if the salve completely removed the cancer without a biopsy. Self-treatment can delay more effective therapy and may require further treatments.
Black salve should be subject to standarddrug regulations and its use discouraged by dermatologists due to the associated harmful effects and the availability of safer treatments. To better treat and inform their patients, dermatologists should be aware that patients may be attracted to alternative treatments such as black salves.
Black salve is composed of various ingredients, many of which are inert; however, some black salves contain escharotics, the 2 most common are zinc chloride and bloodroot (Sanguinaria canadensis) extract. In high doses, such as those contained in most black salve products, these corrosive agents can indiscriminately damage both healthy and diseased tissue.1 Nevertheless, many black salve products currently are advertised as safe and natural methods for curing skin cancer2-4 or treating a variety of other skin conditions (eg, moles, warts, skin tags, boils, abscesses, bee stings, other minor wounds)1,5 and even nondermatologic conditions such as a sore throat.6 Despite the information and testimonials that are widely available on the Internet, black salve use has not been validated by rigorous studies. Black salve is not regulated by the US Food and Drug Administration, resulting in poor quality control and inconsistent user instructions. We report the case of application of black salve to a biopsy site of a compound nevus with moderate atypia that resulted in the formation of a dermatitis plaque with subsequent scarring and basal layer pigmentation.
Case Report
A 35-year-old woman with a family history of melanoma presented for follow-up of a compound nevus with moderate atypia on the right anterior thigh that had been biopsied 6 months prior. Complete excision of the lesion was recommended at the initial presentation but was not performed due to scheduling conflicts. The patient reported applying black salve to the biopsy site and also to the left thigh 3 months later. There was no reaction on the left thigh after one 24-hour application of black salve, but an area around the biopsy site on the right thigh became thickened and irritated with superficial erosion of the skin following 2 applications of black salve, each of 24 hours’ duration. Physical examination revealed a granulomatous plaque at the biopsy site that was approximately 5 cm in diameter (Figure 1A). One year later the lesion had completely healed (Figure 1B) and a biopsy revealed scarring with basal layer pigmentation (Figure 2).
 |  |  | |||
| Figure 1. A 5-cm granulomatous reaction surrounding a biopsy site on the right anterior thigh 3 months after application of black salve (A). One year later, the lesion had completely healed (B). | Figure 2. A biopsy one year following application of black salve demonstrated scarring with basal layer pigmentation (H&E, original magnification ×4). | ||||
Comment
A Web search using the term black salve yields a large number of products labeled as skin cancer salves, many showing glowing reviews and some being sold by major US retailers. The ingredients in black salves often vary in the innocuous substances they contain, but most products include the escharotics zinc chloride and bloodroot extract, which is derived from the plant S canadensis.1,3 For example, the ingredients of one popular black salve product include zinc chloride, chaparral (active ingredient is nordihydroguaiaretic acid), graviola leaf extract, oleander leaf extract, bloodroot extract, and glycerine,7 while another product includes bloodroot extract, zinc chloride, chaparral, cayenne pepper, red clover, birch bark, dimethyl sulfoxide, and burdock root.4
Bloodroot extract’s antimicrobial, anti-inflammatory, antioxidant, and immunomodulatory effects derive from its benzylisoquinoline alkaloids including sanguinarine, allocryptopine, berberine, coptisine, protopine, and stylopine.3,8 Bloodroot extract possesses some degree of tumoricidal potency, with one study finding that it selectively targets cancer cells.9 However, this differential response is seen only at low doses and not at the high concentrations contained in most black salve products.1 According to fluorometric assays, sanguinarine is not selective for tumor cells and therefore damages healthy tissue in addition to the unwanted lesions.6,10,11 The US Food and Drug Administration includes black salve products on its list of fake cancer cures that consumers should avoid.12 Reports of extensive damage from black salve use include skin ulceration2,10 and complete loss of a naris1 and nasal ala.5 Our case suggests the possible association between black salve use and an irritant reaction and erosion of the skin.
Furthermore, reliance on black salve alone in the treatment of skin cancer poses the threat of recurrence or metastasis of cancer because there is no way to know if the salve completely removed the cancer without a biopsy. Self-treatment can delay more effective therapy and may require further treatments.
Black salve should be subject to standarddrug regulations and its use discouraged by dermatologists due to the associated harmful effects and the availability of safer treatments. To better treat and inform their patients, dermatologists should be aware that patients may be attracted to alternative treatments such as black salves.
1. Eastman KL, McFarland LV, Raugi GJ. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289.
2. Eastman KL, McFarland LV, Raugi GJ. Buyer beware: a black salve caution. J Am Acad Dermatol. 2011;65:e154-e155.
3. Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80.
4. McDaniel S, Goldman GD. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
5. Payne CE. ‘Black Salve’ and melanomas [published online ahead of print August 11, 2010]. J Plast Reconstr Aesthet Surg. 2011;64:422.
6. Cienki JJ, Zaret L. An Internet misadventure: bloodroot salve toxicity. J Altern Complement Med. 2010;16:1125-1127.
7. Cansema and escharotics. Alpha Omega Labs Web site. http://www.altcancer.com/faqcan.htm. Accessed May 6, 2015.
8. Vlachojannis C, Magora F, Chrubasik S. Rise and fall of oral health products with Canadian bloodroot extract. Phytother Res. 2012;26:1423-1426.
9. Ahmad N, Gupta S, Husain MM, et al. Differential antiproliferative and apoptotic response of sanguinarine for cancer cells versus normal cells. Clin Cancer Res. 2000;6:1524-1528.
10. Saltzberg F, Barron G, Fenske N. Deforming self-treatment with herbal “black salve.” Dermatol Surg. 2009;35:1152-1154.
11. Debiton E, Madelmont JC, Legault J, et al. Sanguinarine-induced apoptosis is associated with an early and severe cellular glutathione depletion. Cancer Chemother Pharmacol. 2003;51:474-482.
12. 187 fake cancer “cures” consumers should avoid. US Food and Drug Administration Web site. http://www.fda.gov/Drugs/GuidanceCompliance RegulatoryInformation/EnforcementActivitiesbyFDA/ucm171057.htm. Updated July 9, 2009. Accessed May 6, 2015.
1. Eastman KL, McFarland LV, Raugi GJ. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289.
2. Eastman KL, McFarland LV, Raugi GJ. Buyer beware: a black salve caution. J Am Acad Dermatol. 2011;65:e154-e155.
3. Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80.
4. McDaniel S, Goldman GD. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
5. Payne CE. ‘Black Salve’ and melanomas [published online ahead of print August 11, 2010]. J Plast Reconstr Aesthet Surg. 2011;64:422.
6. Cienki JJ, Zaret L. An Internet misadventure: bloodroot salve toxicity. J Altern Complement Med. 2010;16:1125-1127.
7. Cansema and escharotics. Alpha Omega Labs Web site. http://www.altcancer.com/faqcan.htm. Accessed May 6, 2015.
8. Vlachojannis C, Magora F, Chrubasik S. Rise and fall of oral health products with Canadian bloodroot extract. Phytother Res. 2012;26:1423-1426.
9. Ahmad N, Gupta S, Husain MM, et al. Differential antiproliferative and apoptotic response of sanguinarine for cancer cells versus normal cells. Clin Cancer Res. 2000;6:1524-1528.
10. Saltzberg F, Barron G, Fenske N. Deforming self-treatment with herbal “black salve.” Dermatol Surg. 2009;35:1152-1154.
11. Debiton E, Madelmont JC, Legault J, et al. Sanguinarine-induced apoptosis is associated with an early and severe cellular glutathione depletion. Cancer Chemother Pharmacol. 2003;51:474-482.
12. 187 fake cancer “cures” consumers should avoid. US Food and Drug Administration Web site. http://www.fda.gov/Drugs/GuidanceCompliance RegulatoryInformation/EnforcementActivitiesbyFDA/ucm171057.htm. Updated July 9, 2009. Accessed May 6, 2015.
Practice Points
- Clinicians should be aware that black salve containing bloodroot extract is a popular alternative treatment used to cure a variety of skin ailments.
- Black salve containing bloodroot extract is not selective for tumor cells. Various case reports have shown that black salve can result in extensive tissue damage and recurrence or metastasis of skin cancer.
- Damage to healthy tissue can occur with as few as 2 applications of black salve.
Sulfur Spring Dermatitis
Sulfur spring dermatitis is characterized by multiple punched-out erosions and pits. In prior case reports, patients often presented with painful swollen lesions that developed within 24 hours of bathing in hot sulfur springs.1 Because spa therapy and thermal spring baths are common in modern society, dermatologists should be aware of sulfur spring dermatitis as a potential adverse effect.
Case Report
A healthy 65-year-old man presented with painful skin lesions on the legs that developed after bathing for 25 minutes in a hot sulfur spring 1 day prior. The patient had no history of dermatologic disease. He reported a 10-year history of bathing in a hot sulfur spring for 20 minutes every 3 days in the winter. This time, he bathed 5 minutes longer than usual. No skin condition was noted prior to bathing, but he reported feeling a tickling sensation and scratching the legs while he was immersed in the water. One hour after bathing, he noted confluent, punched-out, round ulcers with peripheral erythema on the thighs and shins (Figure 1).
|
|
A skin biopsy revealed sharply demarcated, homogeneous coagulation necrosis of the epidermis. Many neutrophils were present under the necrosis (Figure 2). Periodic acid–Schiff and acid-fast stains were negative for infectious organisms, and a skin tissue culture yielded negative results. Intensive wound care was started with nitrofurazone ointment 0.2%. The ulcers healed gradually in the following months with scar formation and hyperpigmentation.
Comment
Thermal sulfur baths are a form of balneotherapy promoted in many cultures for improvement of skin conditions; however, certain uncommon skin problems may occur after bathing in hot sulfur springs.2 In particular, sulfur spring dermatitis is a potential adverse effect.
Thermal sulfur water is known to exert anti-inflammatory, keratoplastic, and antipruriginous effects. As a result, it often is used in many cultures as an alternative treatment of various skin conditions.2-4 Moreover, thermal sulfur baths are popular in northeastern Asian countries for their effects on mental health.5 Hot springs in northern Taiwan, which contain large amounts of hydrogen sulfide, sulfate, and sulfur differ from other thermal springs in that they are rather acidic in nature and release geothermal energy from volcanic activity.6 In addition to hot sulfur springs, there are neutral salt and CO2 springs in Taiwan.5 However, spring dermatitis has only been associated with bathing in hot sulfur springs due to high concentrations of hydrogen sulfide that break down keratin and cause dissolution of the stratum corneum.7
The incidence of sulfur spring dermatitis is unknown. Although the largest known case series reported 44 cases occurring within a decade in Taiwan,1 it is rarely seen in our daily practice. Previously reported cases of sulfur spring dermatitis noted clinical findings of swelling of the affected area followed by punched-out erosions with surrounding erythema. Most lesions gradually healed with dry brownish crusts. A patch test with sulfur spring water and sulfur compounds showed negative results; therefore, the mechanism is unlikely to be allergic reaction.1 The clinical differential diagnosis includes factitious ulcers as well as viral and fungal infections. A tissue culture should be performed to exclude infectious conditions.
This characteristic skin disease does not present in all individuals after bathing in hot sulfur springs. Lesions may present anywhere on the body with a predilection for skin folds, including the penis and scrotum. Preexisting skin conditions such as pruritus and xerosis are considered to be contributing factors. The possible etiology of sulfur spring dermatitis may be acid irritation from the unstable amount of soluble sulfur in the water, which is enhanced by the heat.1 In our patient, no prior skin disease was noted, but he scratched the skin on the thighs while bathing, which may have contributed to the development of lesions in this area rather than in the skin folds.
The skin biopsy specimen demonstrated epidermal coagulation necrosis, mild superficial dermal damage, and preservation of the pilosebaceous appendages. The ulcers were painful during healing and resolved with scarring and hyperpigmentation. The histopathologic findings and clinical course in our patient were similar to cases of superficial second-degree burns.8 It is possible that the keratoplastic effect of sulfur at high concentrations along with thermal water caused the skin condition.
Conclusion
Individuals who engage in thermal sulfur baths should be aware of potential adverse effects such as sulfur spring dermatitis, especially those with preexisting skin disorders.
1. Sun CC, Sue MS. Sulfur spring dermatitis. Contact Dermatitis. 1995;32:31-34.
2. Matz H, Orion E, Wolf R. Balneotherapy in dermatology. Dermatol Ther. 2003;16:132-140.
3. Leslie KS, Millington GW, Levell NJ. Sulphur and skin: from Satan to Saddam! J Cosmet Dermatol. 2004;3:94-98.
4. Millikan LE. Unapproved treatments or indications in dermatology: physical therapy including balneotherapy. Clin Dermatol. 2000;18:125-129.
5. Nirei H, Furuno K, Kusuda T. Medical geology in Japan. In: Selinus O, Finkelman RB, Centeno JA, eds. Medical Geology: A Regional Synthesis. New York, NY: Springer; 2010:329-354.
6. Liu CM, Song SR, Chen YL, et al. Characteristics and origins of hot springs in the Tatun Volcano Group in northern Taiwan. Terr Atmos Ocean Sci. 2011;22:475-489.
7. Lin AN, Reimer RJ, Carter DM. Sulfur revisited. J Am Acad Dermatol. 1988;18:553-558.
8. Weedon D. Reaction to physical agents. In: Weedon D. Weedon’s Skin Pathology. 3rd ed. London, England: Churchill Livingstone, Elsevier Health; 2010:525-540.
Sulfur spring dermatitis is characterized by multiple punched-out erosions and pits. In prior case reports, patients often presented with painful swollen lesions that developed within 24 hours of bathing in hot sulfur springs.1 Because spa therapy and thermal spring baths are common in modern society, dermatologists should be aware of sulfur spring dermatitis as a potential adverse effect.
Case Report
A healthy 65-year-old man presented with painful skin lesions on the legs that developed after bathing for 25 minutes in a hot sulfur spring 1 day prior. The patient had no history of dermatologic disease. He reported a 10-year history of bathing in a hot sulfur spring for 20 minutes every 3 days in the winter. This time, he bathed 5 minutes longer than usual. No skin condition was noted prior to bathing, but he reported feeling a tickling sensation and scratching the legs while he was immersed in the water. One hour after bathing, he noted confluent, punched-out, round ulcers with peripheral erythema on the thighs and shins (Figure 1).
|
|
A skin biopsy revealed sharply demarcated, homogeneous coagulation necrosis of the epidermis. Many neutrophils were present under the necrosis (Figure 2). Periodic acid–Schiff and acid-fast stains were negative for infectious organisms, and a skin tissue culture yielded negative results. Intensive wound care was started with nitrofurazone ointment 0.2%. The ulcers healed gradually in the following months with scar formation and hyperpigmentation.
Comment
Thermal sulfur baths are a form of balneotherapy promoted in many cultures for improvement of skin conditions; however, certain uncommon skin problems may occur after bathing in hot sulfur springs.2 In particular, sulfur spring dermatitis is a potential adverse effect.
Thermal sulfur water is known to exert anti-inflammatory, keratoplastic, and antipruriginous effects. As a result, it often is used in many cultures as an alternative treatment of various skin conditions.2-4 Moreover, thermal sulfur baths are popular in northeastern Asian countries for their effects on mental health.5 Hot springs in northern Taiwan, which contain large amounts of hydrogen sulfide, sulfate, and sulfur differ from other thermal springs in that they are rather acidic in nature and release geothermal energy from volcanic activity.6 In addition to hot sulfur springs, there are neutral salt and CO2 springs in Taiwan.5 However, spring dermatitis has only been associated with bathing in hot sulfur springs due to high concentrations of hydrogen sulfide that break down keratin and cause dissolution of the stratum corneum.7
The incidence of sulfur spring dermatitis is unknown. Although the largest known case series reported 44 cases occurring within a decade in Taiwan,1 it is rarely seen in our daily practice. Previously reported cases of sulfur spring dermatitis noted clinical findings of swelling of the affected area followed by punched-out erosions with surrounding erythema. Most lesions gradually healed with dry brownish crusts. A patch test with sulfur spring water and sulfur compounds showed negative results; therefore, the mechanism is unlikely to be allergic reaction.1 The clinical differential diagnosis includes factitious ulcers as well as viral and fungal infections. A tissue culture should be performed to exclude infectious conditions.
This characteristic skin disease does not present in all individuals after bathing in hot sulfur springs. Lesions may present anywhere on the body with a predilection for skin folds, including the penis and scrotum. Preexisting skin conditions such as pruritus and xerosis are considered to be contributing factors. The possible etiology of sulfur spring dermatitis may be acid irritation from the unstable amount of soluble sulfur in the water, which is enhanced by the heat.1 In our patient, no prior skin disease was noted, but he scratched the skin on the thighs while bathing, which may have contributed to the development of lesions in this area rather than in the skin folds.
The skin biopsy specimen demonstrated epidermal coagulation necrosis, mild superficial dermal damage, and preservation of the pilosebaceous appendages. The ulcers were painful during healing and resolved with scarring and hyperpigmentation. The histopathologic findings and clinical course in our patient were similar to cases of superficial second-degree burns.8 It is possible that the keratoplastic effect of sulfur at high concentrations along with thermal water caused the skin condition.
Conclusion
Individuals who engage in thermal sulfur baths should be aware of potential adverse effects such as sulfur spring dermatitis, especially those with preexisting skin disorders.
Sulfur spring dermatitis is characterized by multiple punched-out erosions and pits. In prior case reports, patients often presented with painful swollen lesions that developed within 24 hours of bathing in hot sulfur springs.1 Because spa therapy and thermal spring baths are common in modern society, dermatologists should be aware of sulfur spring dermatitis as a potential adverse effect.
Case Report
A healthy 65-year-old man presented with painful skin lesions on the legs that developed after bathing for 25 minutes in a hot sulfur spring 1 day prior. The patient had no history of dermatologic disease. He reported a 10-year history of bathing in a hot sulfur spring for 20 minutes every 3 days in the winter. This time, he bathed 5 minutes longer than usual. No skin condition was noted prior to bathing, but he reported feeling a tickling sensation and scratching the legs while he was immersed in the water. One hour after bathing, he noted confluent, punched-out, round ulcers with peripheral erythema on the thighs and shins (Figure 1).
|
|
A skin biopsy revealed sharply demarcated, homogeneous coagulation necrosis of the epidermis. Many neutrophils were present under the necrosis (Figure 2). Periodic acid–Schiff and acid-fast stains were negative for infectious organisms, and a skin tissue culture yielded negative results. Intensive wound care was started with nitrofurazone ointment 0.2%. The ulcers healed gradually in the following months with scar formation and hyperpigmentation.
Comment
Thermal sulfur baths are a form of balneotherapy promoted in many cultures for improvement of skin conditions; however, certain uncommon skin problems may occur after bathing in hot sulfur springs.2 In particular, sulfur spring dermatitis is a potential adverse effect.
Thermal sulfur water is known to exert anti-inflammatory, keratoplastic, and antipruriginous effects. As a result, it often is used in many cultures as an alternative treatment of various skin conditions.2-4 Moreover, thermal sulfur baths are popular in northeastern Asian countries for their effects on mental health.5 Hot springs in northern Taiwan, which contain large amounts of hydrogen sulfide, sulfate, and sulfur differ from other thermal springs in that they are rather acidic in nature and release geothermal energy from volcanic activity.6 In addition to hot sulfur springs, there are neutral salt and CO2 springs in Taiwan.5 However, spring dermatitis has only been associated with bathing in hot sulfur springs due to high concentrations of hydrogen sulfide that break down keratin and cause dissolution of the stratum corneum.7
The incidence of sulfur spring dermatitis is unknown. Although the largest known case series reported 44 cases occurring within a decade in Taiwan,1 it is rarely seen in our daily practice. Previously reported cases of sulfur spring dermatitis noted clinical findings of swelling of the affected area followed by punched-out erosions with surrounding erythema. Most lesions gradually healed with dry brownish crusts. A patch test with sulfur spring water and sulfur compounds showed negative results; therefore, the mechanism is unlikely to be allergic reaction.1 The clinical differential diagnosis includes factitious ulcers as well as viral and fungal infections. A tissue culture should be performed to exclude infectious conditions.
This characteristic skin disease does not present in all individuals after bathing in hot sulfur springs. Lesions may present anywhere on the body with a predilection for skin folds, including the penis and scrotum. Preexisting skin conditions such as pruritus and xerosis are considered to be contributing factors. The possible etiology of sulfur spring dermatitis may be acid irritation from the unstable amount of soluble sulfur in the water, which is enhanced by the heat.1 In our patient, no prior skin disease was noted, but he scratched the skin on the thighs while bathing, which may have contributed to the development of lesions in this area rather than in the skin folds.
The skin biopsy specimen demonstrated epidermal coagulation necrosis, mild superficial dermal damage, and preservation of the pilosebaceous appendages. The ulcers were painful during healing and resolved with scarring and hyperpigmentation. The histopathologic findings and clinical course in our patient were similar to cases of superficial second-degree burns.8 It is possible that the keratoplastic effect of sulfur at high concentrations along with thermal water caused the skin condition.
Conclusion
Individuals who engage in thermal sulfur baths should be aware of potential adverse effects such as sulfur spring dermatitis, especially those with preexisting skin disorders.
1. Sun CC, Sue MS. Sulfur spring dermatitis. Contact Dermatitis. 1995;32:31-34.
2. Matz H, Orion E, Wolf R. Balneotherapy in dermatology. Dermatol Ther. 2003;16:132-140.
3. Leslie KS, Millington GW, Levell NJ. Sulphur and skin: from Satan to Saddam! J Cosmet Dermatol. 2004;3:94-98.
4. Millikan LE. Unapproved treatments or indications in dermatology: physical therapy including balneotherapy. Clin Dermatol. 2000;18:125-129.
5. Nirei H, Furuno K, Kusuda T. Medical geology in Japan. In: Selinus O, Finkelman RB, Centeno JA, eds. Medical Geology: A Regional Synthesis. New York, NY: Springer; 2010:329-354.
6. Liu CM, Song SR, Chen YL, et al. Characteristics and origins of hot springs in the Tatun Volcano Group in northern Taiwan. Terr Atmos Ocean Sci. 2011;22:475-489.
7. Lin AN, Reimer RJ, Carter DM. Sulfur revisited. J Am Acad Dermatol. 1988;18:553-558.
8. Weedon D. Reaction to physical agents. In: Weedon D. Weedon’s Skin Pathology. 3rd ed. London, England: Churchill Livingstone, Elsevier Health; 2010:525-540.
1. Sun CC, Sue MS. Sulfur spring dermatitis. Contact Dermatitis. 1995;32:31-34.
2. Matz H, Orion E, Wolf R. Balneotherapy in dermatology. Dermatol Ther. 2003;16:132-140.
3. Leslie KS, Millington GW, Levell NJ. Sulphur and skin: from Satan to Saddam! J Cosmet Dermatol. 2004;3:94-98.
4. Millikan LE. Unapproved treatments or indications in dermatology: physical therapy including balneotherapy. Clin Dermatol. 2000;18:125-129.
5. Nirei H, Furuno K, Kusuda T. Medical geology in Japan. In: Selinus O, Finkelman RB, Centeno JA, eds. Medical Geology: A Regional Synthesis. New York, NY: Springer; 2010:329-354.
6. Liu CM, Song SR, Chen YL, et al. Characteristics and origins of hot springs in the Tatun Volcano Group in northern Taiwan. Terr Atmos Ocean Sci. 2011;22:475-489.
7. Lin AN, Reimer RJ, Carter DM. Sulfur revisited. J Am Acad Dermatol. 1988;18:553-558.
8. Weedon D. Reaction to physical agents. In: Weedon D. Weedon’s Skin Pathology. 3rd ed. London, England: Churchill Livingstone, Elsevier Health; 2010:525-540.
Practice Points
- The clinical findings of sulfur spring dermatitis are similar to those of a superficial second-degree burn.
- Careful evaluation of the patient’s clinical history and recognition of characteristic findings are important for correct diagnosis.
- Patients with preexisting skin disorders who engage in thermal sulfur baths should be aware of the potential adverse effect of sulfur spring dermatitis.
Allergic Contact Dermatitis to 2-Octyl Cyanoacrylate
Cyanoacrylates are widely used in adhesive products, with applications ranging from household products to nail and beauty salons and even dentistry. A topical skin adhesive containing 2-octyl cyanoacrylate was approved in 1998 for topical application for closure of skin edges of wounds from surgical incisions.1 Usually cyanoacrylates are not strong sensitizers, and despite their extensive use, there have been relatively few reports of associated allergic contact dermatitis (ACD).2-5 We report 4 cases of ACD to 2-octyl cyanoacrylate used in postsurgical wound closures as confirmed by patch tests.
Case Reports
Patient 1
A 33-year-old woman presented with an intensely pruritic peri-incisional rash on the lower back and right buttock of 1 week’s duration. The eruption started roughly 1 week following surgical implantation of a spinal cord stimulator for treatment of chronic back pain. Both incisions made during the implantation were closed with 2-octyl cyanoacrylate. The patient denied any prior exposure to topical skin adhesives or any history of contact dermatitis to nickel or other materials. The patient did not dress the wounds and did not apply topical agents to the area.
Physical examination revealed 6- to 8-cm linear surgical scars on the midline lumbar back and superior right buttock with surrounding excoriated erythematous papules coalescing into plaques consistent with acute eczematous dermatitis (Figure 1). Similar papules and plaques were scattered across the abdomen and chest. She was given triamcinolone acetonide ointment 0.1% twice daily and hydroxyzine pamoate 25 mg 3 times daily for itching. The surgical wounds healed within 2 weeks of presentation with postinflammatory hyperpigmentation surrounding the scars.
 | 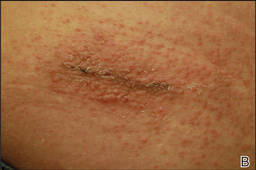 |
| Figure 1. Surgical scars with surrounding excoriated erythematous papules coalescing into plaques on the midline lumbar back (A) and superior right buttock (B). | |
Six weeks later she underwent patch testing to confirm the diagnosis. She was screened using the North American Contact Dermatitis Group standard 65-allergen series and a miscellaneous tray including hardware obtained from the spinal cord stimulator device manufacturer. A use test to 2-octyl cyanoacrylate also was performed. At 96 hours, true positives included cinnamic aldehyde (1+), nickel (1+), bacitracin (1+), fragrance mix (2+), disperse blue dyes 106 and 124 (2+), and 2-octyl cyanoacrylate (3+)(1+=weak positive; 2+=strong positive; 3+=extreme reaction). There was no response to any components of the device. The pattern of dermatitis and positive patch-test results strongly supported the diagnosis of ACD to 2-octyl cyanoacrylate.
Patients 2, 3, and 4
Three patients—a 65-year-old woman, a 35-year-old woman, and a 44-year-old woman—presented to us with eczematous dermatitis at laparoscopic portal sites that were closed with 2-octyl cyanoacrylate (Figures 2 and 3). They presented approximately 1 week following laparoscopic Nissen fundoplication, laparoscopic left hepatectomy, and laparoscopic cholecystectomy, respectively. None of these 3 patients had been using any topical medications. All of them had a positive reaction (2+) to 2-octyl cyanoacrylate on use testing. Interestingly, use tests for 2 other cyanoacrylates containing 2-butyl cyanoacrylate were negative in 2 patients.
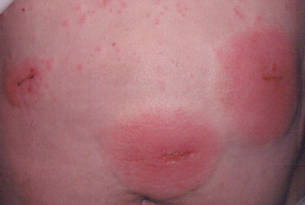 |
| Figure 2. Acute eczematous plaques at wound closures. |
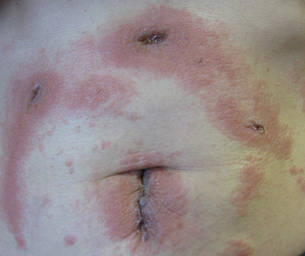 |
| Figure 3. Coalescing acute eczematous plaques focused at wound closures. |
Although patient 1 reported no prior exposure to 2-octyl cyanoacrylate, these 3 additional patients reported prior exposure with no reaction. Other possible contact allergens associated with wound closure included iodine, topical antibiotics, and dressing tape.
Comment
Contact allergies to acrylates are not uncommon. In a series of 275 patients, Kanerva et al6 found that 17.5% of patients had an allergic reaction to at least 1 acrylate or methacrylate. In the same series, no allergic reactions to cyanoacrylates were noted.6 The role of methacrylates in the development of occupational ACD and irritant dermatitis has been well characterized among dentists, orthopedic surgeons, beauticians, and industrial workers who are commonly exposed to these agents.7-12 Partially because of their longer carbon chains, cyanoacrylates have reduced toxicity and improved bonding strength as well as flexibility. Given their availability and the ease and speed of their use, skin adhesives have become widely used in the closure of surgical wounds.13-16
Postoperative contact dermatitis is problematic, as patients are exposed to many potential allergens during surgery. In our clinical practice, the most common allergens causing ACD associated with surgery are iodine, topical antibiotics (ie, bacitracin, neomycin), tape adhesives, suture materials, and less commonly surgical hardware. Although they are rarely reported, contact allergies to skin adhesives such as cyanoacrylates are of particular importance because they may complicate surgical wounds, leading to dehiscence, infection, and scarring, among other complications. In our patients, there were no adverse outcomes in wound healing with the exception of postinflammatory hyperpigmentation.
Under ideal conditions, 2-octyl cyanoacrylate generally is not a strong sensitizer; however, application to open wounds or thinner skin such as the eyelids may permit exposure of antigen-presenting cells to cyanoacrylate monomers, thereby initiating sensitization. Postsurgical occlusive dressings, which often are left in place for 7 to 14 days, also may contribute to sensitization. The role of the degradation of skin adhesive products in the development of contact dermatitis is unknown.
Management of ACD from skin adhesives should involve the immediate removal of any remaining adhesive. One manufacturer recommends removal of the product using acetone or petroleum jelly.1 In our experience, rubbing the adhesive with 2×2-in gauze pads or using forceps have been successful methods for removal. The use of petroleum jelly prior to rubbing with gauze also can aid in removal of the adhesive. Warm water soaks and soap also may be helpful but are not expected to immediately loosen the bond. A mid-potency steroid ointment such as triamcinolone may be effective in treating dermatitis, though the use of higher-potency steroids such as clobetasol may be needed for severe reactions.1,2
As members of the cyano group, cyanoacrylates are highly reactive molecules that polymerize and rapidly bind to the stratum corneum when they come in contact with traces of water. During polymerization, the individual constituents or monomer cyanoacrylate molecules are joined into a polymer chain, which should be trapped by keratinocytes and not reach immunomodulators2,10; however, as postulated during the first report of contact dermatitis, an arid environment could delay polymerization and increase the risk of sensitization.2 The first report was made in Las Vegas, Nevada,2 and our cases presented in San Antonio, Texas.
There currently are 2 main cutaneous adhesives containing cyanoacrylate on the market, including 2-octyl cyanoacrylate and 2-butyl cyanoacrylate. These products are known by various trade names and differ primarily in the length of the carbon chain in the cyanoacrylate. A dye is added to allow better visibility of the glue during application, and a plasticizer increases viscosity and accelerates polymerization. The 2 most widely used products contain the same dye (D&C Violet No. 2) and similar but proprietary plasticizers.
Although plasticizers and dyes may be potential contact allergens, we postulated that the cyanoacrylate was the responsible sensitizer in our cases. Because the individual ingredients were not readily available for use testing, we devised a logical method to attempt to determine the specific component of the skin adhesive that was responsible for contact sensitization (Figure 4). Patients 3 and 4 in our series were tested using this method and were found to be sensitive to the product containing 2-octyl cyanoacrylate but not the products containing 2-butyl cyanoacrylate.
Conclusion
Given the many advantages of cyanoacrylates, it is likely that their use in skin adhesive products will continue to increase. Our 4 patients may represent a rise in the incidence of ACD associated with increased use of skin adhesives, but it is important to look critically at this agent when patients present with postoperative pruritus in the absence of topical bacitracin or neomycin use and surgical dressing irritation. By using the technique we described, it is possible to identify the component responsible for the reaction; however, in the future, the exact mechanisms of sensitization and the specific components should be further elucidated by researchers working in conjunction with the manufacturers. Use testing on abraded skin and/or under occlusive dressings more closely mimics the initial exposure and may have a role in determining true allergy.
1. Dermabond Advanced [package insert]. San Lorenzo, PR: Ethicon, LLC; 2013.
2. Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octyl cyanoacrylate. Arch Dermatol. 2008;144:814-815.
3. Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
4. El-Dars LD, Chaudhury W, Hughes TM, et al. Allergic contact dermatitis to Dermabond after orthopaedic joint replacement. Contact Dermatitis. 2010;62:315-317.
5. Howard BK, Hudkins ML. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
6. Kanerva L, Jolanki R, Estlander T. 10 years of patch testing with the (meth)acrylate series. Contact Dermatitis. 1997;37:255-258.
7. Belsito DV. Contact dermatitis to ethyl-cyanoacrylate-containing glue. Contact Dermatitis. 1987;17:234-236.
8. Leggat PA, Kedjarune U, Smith DR. Toxicity of cyanoacrylate adhesives and their occupational impacts for dental staff. Ind Health. 2004;42:207-211.
9. Conde-Salazar L, Rojo S, Guimaraens D. Occupational allergic contact dermatitis from cyanoacrylate. Am J Contact Dermat. 1998;9:188-189.
10. Aalto-Korte K, Alanko K, Kuuliala O, et al. Occupational methacrylate and acrylate allergy from glues. Contact Dermatitis. 2008;58:340-346.
11. Tomb RR, Lepoittevin JP, Durepaire F, et al. Ectopic contact dermatitis from ethyl cyanoacrylate instant adhesives. Contact Dermatitis. 1993;28:206-208.
12. Dragu A, Unglaub F, Schwarz S, et al. Foreign body reaction after usage of tissue adhesives for skin closure: a case report and review of the literature. Arch Orthop Trauma Surg. 2009;129:167-169.
13. Eaglstein WH, Sullivan T. Cyanoacrylates for skin closure. Dermatol Clin. 2005;23:193-198.
14. Singer AJ, Quinn JV, Hollander JE. The cyanoacrylate topical skin adhesives. Am J Emerg Med. 2008;26:490-496.
15. Singer AJ, Thode HC Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg. 2004;187:238-248.
16. Calnan CD. Cyanoacrylate dermatitis. Contact Dermatitis. 1979;5:165-167.
Cyanoacrylates are widely used in adhesive products, with applications ranging from household products to nail and beauty salons and even dentistry. A topical skin adhesive containing 2-octyl cyanoacrylate was approved in 1998 for topical application for closure of skin edges of wounds from surgical incisions.1 Usually cyanoacrylates are not strong sensitizers, and despite their extensive use, there have been relatively few reports of associated allergic contact dermatitis (ACD).2-5 We report 4 cases of ACD to 2-octyl cyanoacrylate used in postsurgical wound closures as confirmed by patch tests.
Case Reports
Patient 1
A 33-year-old woman presented with an intensely pruritic peri-incisional rash on the lower back and right buttock of 1 week’s duration. The eruption started roughly 1 week following surgical implantation of a spinal cord stimulator for treatment of chronic back pain. Both incisions made during the implantation were closed with 2-octyl cyanoacrylate. The patient denied any prior exposure to topical skin adhesives or any history of contact dermatitis to nickel or other materials. The patient did not dress the wounds and did not apply topical agents to the area.
Physical examination revealed 6- to 8-cm linear surgical scars on the midline lumbar back and superior right buttock with surrounding excoriated erythematous papules coalescing into plaques consistent with acute eczematous dermatitis (Figure 1). Similar papules and plaques were scattered across the abdomen and chest. She was given triamcinolone acetonide ointment 0.1% twice daily and hydroxyzine pamoate 25 mg 3 times daily for itching. The surgical wounds healed within 2 weeks of presentation with postinflammatory hyperpigmentation surrounding the scars.
 |  |
| Figure 1. Surgical scars with surrounding excoriated erythematous papules coalescing into plaques on the midline lumbar back (A) and superior right buttock (B). | |
Six weeks later she underwent patch testing to confirm the diagnosis. She was screened using the North American Contact Dermatitis Group standard 65-allergen series and a miscellaneous tray including hardware obtained from the spinal cord stimulator device manufacturer. A use test to 2-octyl cyanoacrylate also was performed. At 96 hours, true positives included cinnamic aldehyde (1+), nickel (1+), bacitracin (1+), fragrance mix (2+), disperse blue dyes 106 and 124 (2+), and 2-octyl cyanoacrylate (3+)(1+=weak positive; 2+=strong positive; 3+=extreme reaction). There was no response to any components of the device. The pattern of dermatitis and positive patch-test results strongly supported the diagnosis of ACD to 2-octyl cyanoacrylate.
Patients 2, 3, and 4
Three patients—a 65-year-old woman, a 35-year-old woman, and a 44-year-old woman—presented to us with eczematous dermatitis at laparoscopic portal sites that were closed with 2-octyl cyanoacrylate (Figures 2 and 3). They presented approximately 1 week following laparoscopic Nissen fundoplication, laparoscopic left hepatectomy, and laparoscopic cholecystectomy, respectively. None of these 3 patients had been using any topical medications. All of them had a positive reaction (2+) to 2-octyl cyanoacrylate on use testing. Interestingly, use tests for 2 other cyanoacrylates containing 2-butyl cyanoacrylate were negative in 2 patients.
 |
| Figure 2. Acute eczematous plaques at wound closures. |
 |
| Figure 3. Coalescing acute eczematous plaques focused at wound closures. |
Although patient 1 reported no prior exposure to 2-octyl cyanoacrylate, these 3 additional patients reported prior exposure with no reaction. Other possible contact allergens associated with wound closure included iodine, topical antibiotics, and dressing tape.
Comment
Contact allergies to acrylates are not uncommon. In a series of 275 patients, Kanerva et al6 found that 17.5% of patients had an allergic reaction to at least 1 acrylate or methacrylate. In the same series, no allergic reactions to cyanoacrylates were noted.6 The role of methacrylates in the development of occupational ACD and irritant dermatitis has been well characterized among dentists, orthopedic surgeons, beauticians, and industrial workers who are commonly exposed to these agents.7-12 Partially because of their longer carbon chains, cyanoacrylates have reduced toxicity and improved bonding strength as well as flexibility. Given their availability and the ease and speed of their use, skin adhesives have become widely used in the closure of surgical wounds.13-16
Postoperative contact dermatitis is problematic, as patients are exposed to many potential allergens during surgery. In our clinical practice, the most common allergens causing ACD associated with surgery are iodine, topical antibiotics (ie, bacitracin, neomycin), tape adhesives, suture materials, and less commonly surgical hardware. Although they are rarely reported, contact allergies to skin adhesives such as cyanoacrylates are of particular importance because they may complicate surgical wounds, leading to dehiscence, infection, and scarring, among other complications. In our patients, there were no adverse outcomes in wound healing with the exception of postinflammatory hyperpigmentation.
Under ideal conditions, 2-octyl cyanoacrylate generally is not a strong sensitizer; however, application to open wounds or thinner skin such as the eyelids may permit exposure of antigen-presenting cells to cyanoacrylate monomers, thereby initiating sensitization. Postsurgical occlusive dressings, which often are left in place for 7 to 14 days, also may contribute to sensitization. The role of the degradation of skin adhesive products in the development of contact dermatitis is unknown.
Management of ACD from skin adhesives should involve the immediate removal of any remaining adhesive. One manufacturer recommends removal of the product using acetone or petroleum jelly.1 In our experience, rubbing the adhesive with 2×2-in gauze pads or using forceps have been successful methods for removal. The use of petroleum jelly prior to rubbing with gauze also can aid in removal of the adhesive. Warm water soaks and soap also may be helpful but are not expected to immediately loosen the bond. A mid-potency steroid ointment such as triamcinolone may be effective in treating dermatitis, though the use of higher-potency steroids such as clobetasol may be needed for severe reactions.1,2
As members of the cyano group, cyanoacrylates are highly reactive molecules that polymerize and rapidly bind to the stratum corneum when they come in contact with traces of water. During polymerization, the individual constituents or monomer cyanoacrylate molecules are joined into a polymer chain, which should be trapped by keratinocytes and not reach immunomodulators2,10; however, as postulated during the first report of contact dermatitis, an arid environment could delay polymerization and increase the risk of sensitization.2 The first report was made in Las Vegas, Nevada,2 and our cases presented in San Antonio, Texas.
There currently are 2 main cutaneous adhesives containing cyanoacrylate on the market, including 2-octyl cyanoacrylate and 2-butyl cyanoacrylate. These products are known by various trade names and differ primarily in the length of the carbon chain in the cyanoacrylate. A dye is added to allow better visibility of the glue during application, and a plasticizer increases viscosity and accelerates polymerization. The 2 most widely used products contain the same dye (D&C Violet No. 2) and similar but proprietary plasticizers.
Although plasticizers and dyes may be potential contact allergens, we postulated that the cyanoacrylate was the responsible sensitizer in our cases. Because the individual ingredients were not readily available for use testing, we devised a logical method to attempt to determine the specific component of the skin adhesive that was responsible for contact sensitization (Figure 4). Patients 3 and 4 in our series were tested using this method and were found to be sensitive to the product containing 2-octyl cyanoacrylate but not the products containing 2-butyl cyanoacrylate.
Conclusion
Given the many advantages of cyanoacrylates, it is likely that their use in skin adhesive products will continue to increase. Our 4 patients may represent a rise in the incidence of ACD associated with increased use of skin adhesives, but it is important to look critically at this agent when patients present with postoperative pruritus in the absence of topical bacitracin or neomycin use and surgical dressing irritation. By using the technique we described, it is possible to identify the component responsible for the reaction; however, in the future, the exact mechanisms of sensitization and the specific components should be further elucidated by researchers working in conjunction with the manufacturers. Use testing on abraded skin and/or under occlusive dressings more closely mimics the initial exposure and may have a role in determining true allergy.
Cyanoacrylates are widely used in adhesive products, with applications ranging from household products to nail and beauty salons and even dentistry. A topical skin adhesive containing 2-octyl cyanoacrylate was approved in 1998 for topical application for closure of skin edges of wounds from surgical incisions.1 Usually cyanoacrylates are not strong sensitizers, and despite their extensive use, there have been relatively few reports of associated allergic contact dermatitis (ACD).2-5 We report 4 cases of ACD to 2-octyl cyanoacrylate used in postsurgical wound closures as confirmed by patch tests.
Case Reports
Patient 1
A 33-year-old woman presented with an intensely pruritic peri-incisional rash on the lower back and right buttock of 1 week’s duration. The eruption started roughly 1 week following surgical implantation of a spinal cord stimulator for treatment of chronic back pain. Both incisions made during the implantation were closed with 2-octyl cyanoacrylate. The patient denied any prior exposure to topical skin adhesives or any history of contact dermatitis to nickel or other materials. The patient did not dress the wounds and did not apply topical agents to the area.
Physical examination revealed 6- to 8-cm linear surgical scars on the midline lumbar back and superior right buttock with surrounding excoriated erythematous papules coalescing into plaques consistent with acute eczematous dermatitis (Figure 1). Similar papules and plaques were scattered across the abdomen and chest. She was given triamcinolone acetonide ointment 0.1% twice daily and hydroxyzine pamoate 25 mg 3 times daily for itching. The surgical wounds healed within 2 weeks of presentation with postinflammatory hyperpigmentation surrounding the scars.
 |  |
| Figure 1. Surgical scars with surrounding excoriated erythematous papules coalescing into plaques on the midline lumbar back (A) and superior right buttock (B). | |
Six weeks later she underwent patch testing to confirm the diagnosis. She was screened using the North American Contact Dermatitis Group standard 65-allergen series and a miscellaneous tray including hardware obtained from the spinal cord stimulator device manufacturer. A use test to 2-octyl cyanoacrylate also was performed. At 96 hours, true positives included cinnamic aldehyde (1+), nickel (1+), bacitracin (1+), fragrance mix (2+), disperse blue dyes 106 and 124 (2+), and 2-octyl cyanoacrylate (3+)(1+=weak positive; 2+=strong positive; 3+=extreme reaction). There was no response to any components of the device. The pattern of dermatitis and positive patch-test results strongly supported the diagnosis of ACD to 2-octyl cyanoacrylate.
Patients 2, 3, and 4
Three patients—a 65-year-old woman, a 35-year-old woman, and a 44-year-old woman—presented to us with eczematous dermatitis at laparoscopic portal sites that were closed with 2-octyl cyanoacrylate (Figures 2 and 3). They presented approximately 1 week following laparoscopic Nissen fundoplication, laparoscopic left hepatectomy, and laparoscopic cholecystectomy, respectively. None of these 3 patients had been using any topical medications. All of them had a positive reaction (2+) to 2-octyl cyanoacrylate on use testing. Interestingly, use tests for 2 other cyanoacrylates containing 2-butyl cyanoacrylate were negative in 2 patients.
 |
| Figure 2. Acute eczematous plaques at wound closures. |
 |
| Figure 3. Coalescing acute eczematous plaques focused at wound closures. |
Although patient 1 reported no prior exposure to 2-octyl cyanoacrylate, these 3 additional patients reported prior exposure with no reaction. Other possible contact allergens associated with wound closure included iodine, topical antibiotics, and dressing tape.
Comment
Contact allergies to acrylates are not uncommon. In a series of 275 patients, Kanerva et al6 found that 17.5% of patients had an allergic reaction to at least 1 acrylate or methacrylate. In the same series, no allergic reactions to cyanoacrylates were noted.6 The role of methacrylates in the development of occupational ACD and irritant dermatitis has been well characterized among dentists, orthopedic surgeons, beauticians, and industrial workers who are commonly exposed to these agents.7-12 Partially because of their longer carbon chains, cyanoacrylates have reduced toxicity and improved bonding strength as well as flexibility. Given their availability and the ease and speed of their use, skin adhesives have become widely used in the closure of surgical wounds.13-16
Postoperative contact dermatitis is problematic, as patients are exposed to many potential allergens during surgery. In our clinical practice, the most common allergens causing ACD associated with surgery are iodine, topical antibiotics (ie, bacitracin, neomycin), tape adhesives, suture materials, and less commonly surgical hardware. Although they are rarely reported, contact allergies to skin adhesives such as cyanoacrylates are of particular importance because they may complicate surgical wounds, leading to dehiscence, infection, and scarring, among other complications. In our patients, there were no adverse outcomes in wound healing with the exception of postinflammatory hyperpigmentation.
Under ideal conditions, 2-octyl cyanoacrylate generally is not a strong sensitizer; however, application to open wounds or thinner skin such as the eyelids may permit exposure of antigen-presenting cells to cyanoacrylate monomers, thereby initiating sensitization. Postsurgical occlusive dressings, which often are left in place for 7 to 14 days, also may contribute to sensitization. The role of the degradation of skin adhesive products in the development of contact dermatitis is unknown.
Management of ACD from skin adhesives should involve the immediate removal of any remaining adhesive. One manufacturer recommends removal of the product using acetone or petroleum jelly.1 In our experience, rubbing the adhesive with 2×2-in gauze pads or using forceps have been successful methods for removal. The use of petroleum jelly prior to rubbing with gauze also can aid in removal of the adhesive. Warm water soaks and soap also may be helpful but are not expected to immediately loosen the bond. A mid-potency steroid ointment such as triamcinolone may be effective in treating dermatitis, though the use of higher-potency steroids such as clobetasol may be needed for severe reactions.1,2
As members of the cyano group, cyanoacrylates are highly reactive molecules that polymerize and rapidly bind to the stratum corneum when they come in contact with traces of water. During polymerization, the individual constituents or monomer cyanoacrylate molecules are joined into a polymer chain, which should be trapped by keratinocytes and not reach immunomodulators2,10; however, as postulated during the first report of contact dermatitis, an arid environment could delay polymerization and increase the risk of sensitization.2 The first report was made in Las Vegas, Nevada,2 and our cases presented in San Antonio, Texas.
There currently are 2 main cutaneous adhesives containing cyanoacrylate on the market, including 2-octyl cyanoacrylate and 2-butyl cyanoacrylate. These products are known by various trade names and differ primarily in the length of the carbon chain in the cyanoacrylate. A dye is added to allow better visibility of the glue during application, and a plasticizer increases viscosity and accelerates polymerization. The 2 most widely used products contain the same dye (D&C Violet No. 2) and similar but proprietary plasticizers.
Although plasticizers and dyes may be potential contact allergens, we postulated that the cyanoacrylate was the responsible sensitizer in our cases. Because the individual ingredients were not readily available for use testing, we devised a logical method to attempt to determine the specific component of the skin adhesive that was responsible for contact sensitization (Figure 4). Patients 3 and 4 in our series were tested using this method and were found to be sensitive to the product containing 2-octyl cyanoacrylate but not the products containing 2-butyl cyanoacrylate.
Conclusion
Given the many advantages of cyanoacrylates, it is likely that their use in skin adhesive products will continue to increase. Our 4 patients may represent a rise in the incidence of ACD associated with increased use of skin adhesives, but it is important to look critically at this agent when patients present with postoperative pruritus in the absence of topical bacitracin or neomycin use and surgical dressing irritation. By using the technique we described, it is possible to identify the component responsible for the reaction; however, in the future, the exact mechanisms of sensitization and the specific components should be further elucidated by researchers working in conjunction with the manufacturers. Use testing on abraded skin and/or under occlusive dressings more closely mimics the initial exposure and may have a role in determining true allergy.
1. Dermabond Advanced [package insert]. San Lorenzo, PR: Ethicon, LLC; 2013.
2. Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octyl cyanoacrylate. Arch Dermatol. 2008;144:814-815.
3. Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
4. El-Dars LD, Chaudhury W, Hughes TM, et al. Allergic contact dermatitis to Dermabond after orthopaedic joint replacement. Contact Dermatitis. 2010;62:315-317.
5. Howard BK, Hudkins ML. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
6. Kanerva L, Jolanki R, Estlander T. 10 years of patch testing with the (meth)acrylate series. Contact Dermatitis. 1997;37:255-258.
7. Belsito DV. Contact dermatitis to ethyl-cyanoacrylate-containing glue. Contact Dermatitis. 1987;17:234-236.
8. Leggat PA, Kedjarune U, Smith DR. Toxicity of cyanoacrylate adhesives and their occupational impacts for dental staff. Ind Health. 2004;42:207-211.
9. Conde-Salazar L, Rojo S, Guimaraens D. Occupational allergic contact dermatitis from cyanoacrylate. Am J Contact Dermat. 1998;9:188-189.
10. Aalto-Korte K, Alanko K, Kuuliala O, et al. Occupational methacrylate and acrylate allergy from glues. Contact Dermatitis. 2008;58:340-346.
11. Tomb RR, Lepoittevin JP, Durepaire F, et al. Ectopic contact dermatitis from ethyl cyanoacrylate instant adhesives. Contact Dermatitis. 1993;28:206-208.
12. Dragu A, Unglaub F, Schwarz S, et al. Foreign body reaction after usage of tissue adhesives for skin closure: a case report and review of the literature. Arch Orthop Trauma Surg. 2009;129:167-169.
13. Eaglstein WH, Sullivan T. Cyanoacrylates for skin closure. Dermatol Clin. 2005;23:193-198.
14. Singer AJ, Quinn JV, Hollander JE. The cyanoacrylate topical skin adhesives. Am J Emerg Med. 2008;26:490-496.
15. Singer AJ, Thode HC Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg. 2004;187:238-248.
16. Calnan CD. Cyanoacrylate dermatitis. Contact Dermatitis. 1979;5:165-167.
1. Dermabond Advanced [package insert]. San Lorenzo, PR: Ethicon, LLC; 2013.
2. Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octyl cyanoacrylate. Arch Dermatol. 2008;144:814-815.
3. Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
4. El-Dars LD, Chaudhury W, Hughes TM, et al. Allergic contact dermatitis to Dermabond after orthopaedic joint replacement. Contact Dermatitis. 2010;62:315-317.
5. Howard BK, Hudkins ML. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
6. Kanerva L, Jolanki R, Estlander T. 10 years of patch testing with the (meth)acrylate series. Contact Dermatitis. 1997;37:255-258.
7. Belsito DV. Contact dermatitis to ethyl-cyanoacrylate-containing glue. Contact Dermatitis. 1987;17:234-236.
8. Leggat PA, Kedjarune U, Smith DR. Toxicity of cyanoacrylate adhesives and their occupational impacts for dental staff. Ind Health. 2004;42:207-211.
9. Conde-Salazar L, Rojo S, Guimaraens D. Occupational allergic contact dermatitis from cyanoacrylate. Am J Contact Dermat. 1998;9:188-189.
10. Aalto-Korte K, Alanko K, Kuuliala O, et al. Occupational methacrylate and acrylate allergy from glues. Contact Dermatitis. 2008;58:340-346.
11. Tomb RR, Lepoittevin JP, Durepaire F, et al. Ectopic contact dermatitis from ethyl cyanoacrylate instant adhesives. Contact Dermatitis. 1993;28:206-208.
12. Dragu A, Unglaub F, Schwarz S, et al. Foreign body reaction after usage of tissue adhesives for skin closure: a case report and review of the literature. Arch Orthop Trauma Surg. 2009;129:167-169.
13. Eaglstein WH, Sullivan T. Cyanoacrylates for skin closure. Dermatol Clin. 2005;23:193-198.
14. Singer AJ, Quinn JV, Hollander JE. The cyanoacrylate topical skin adhesives. Am J Emerg Med. 2008;26:490-496.
15. Singer AJ, Thode HC Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg. 2004;187:238-248.
16. Calnan CD. Cyanoacrylate dermatitis. Contact Dermatitis. 1979;5:165-167.
Practice Points
- It is important for physicians to recognize that skin adhesives are a potential source of allergic contact dermatitis (ACD) in a postsurgical setting.
- There are 3 primary components of skin adhesives that are potential contactants, including a cyanoacrylate, a plasticizer, and a dye.
- Treatment of ACD to skin adhesives is straightforward, including removal of any remaining adhesive and applying topical steroids.
Allergic Contact Dermatitis From Ketoconazole
Case Report
A 65-year-old man presented to the dermatology department for treatment of a scaly rash on the face and scalp. A diagnosis of seborrheic dermatitis was made, and he was prescribed ketoconazole cream 2% and shampoo 2%. Two days later, the patient presented to the emergency department for facial swelling and pruritus, which began 1 day after he began using the ketoconazole cream and shampoo. He reported itching and burning on the face that began within several hours of application followed by progressive facial edema. The patient denied shortness of breath or swelling of the tongue. Physical examination revealed mild facial induration with erythematous plaques on the bilateral cheeks, forehead, and eyelids. The patient was instructed to stop using the ketoconazole cream and shampoo. Within several days of discontinuing use of the ketoconazole products, the dermatitis resolved following treatment with oral diphenhydramine and topical desonide.
Review of the patient’s medical record revealed several likely relevant incidences of undiagnosed recurrent dermatitis. Approximately 2 years earlier, the patient had called his primary care provider to report pain, burning, redness, and itching in the right buttock area following use of ketoconazole cream that the physician had prescribed. Allergic contact dermatitis also had been documented in the patient’s dermatology problem list approximately 1.5 years prior to the current presentation, though a likely causative agent was not listed. Approximately 3 months prior to the current presentation, the patient presented with lower leg rash and edema with documentation of possible allergic reaction to ketoconazole cream.
The patient was patch tested several weeks after discontinuation of the ketoconazole products using the 2012 North American Contact Dermatitis Group series (70 allergens), a supplemental series (36 allergens), an antifungal series (10 allergens), and personal products including ketoconazole cream and shampoo (diluted 1:100). Clinically relevant reactions at 72 hours included an extreme reaction (+++) to the patient’s personal ketoconazole cream 2% (E. Fougera & Co)(Figure 1), and strong reactions (++) to purified ketoconazole 5% in petrolatum and ketoconazole cream 2% (E. Fougera & Co) in an antifungal series (Figure 2). A doubtful reaction to methyl methacrylate was not deemed clinically relevant. No reactions were noted to terbinafine cream 1%, clotrimazole cream 1%, nystatin cream, nystatin ointment, econazole nitrate cream 1%, miconazole nitrate cream 2%, tolnaftate cream 1%, or purified clotrimazole 1% in petrolatum.
 |
Figure 1. Reading at 72 hours of patient’s personal products (ketoconazole cream 2% and ketoconazole shampoo 2%). |
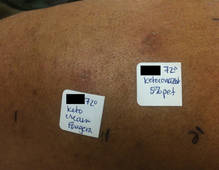 |
Figure 2. Reading at 72 hours of an antifungal series (ketoconazole cream 2% and purified ketocona-zole 5% in petrolatum). |
Comment
Ketoconazole is a widely used antifungal but rarely is reported as a cause of allergic contact dermatitis. Allergies to inactive ingredients, especially vehicles and preservatives, are more common than allergies to ketoconazole itself. In our patient, allergy to inactive ingredients was ruled out by negative reactions to individual constituents and/or negative reactions to other products containing those ingredients. A literature review via Ovid using the search terms ketoconazole, allergic contact dermatitis, and allergy found 4 reports involving 9 documented patients with type IV hypersensitivity to ketoconazole,1-4 and 1 report of 2 patients who developed anaphylaxis from oral ketoconazole.1 Of the 9 dermatitis cases, 3 patients had positive patch tests to only ketoconazole with no reactions to other imidazoles.2,3 Monoallergy to clotrimazole also has been reported.5 A study by Dooms-Goossens et al4 showed that ketoconazole ranked seventh of 11 imidazole derivatives in its frequency to cause allergic contact dermatitis and did not demonstrate statistically significant cross-reactivity with other imidazoles; cross-reactivity usually occurred with miconazole and sulconazole.
Conclusion
This case of contact dermatitis to ketoconazole demonstrates the importance of patch testing with personal products as well as the unpredictability of cross-reactions within the imidazole class of antifungals.
Acknowledgment
This material is the result of work supported with resources and the use of facilities at the Minneapolis Veterans Affairs Health Care System.
1. Garcia-Bravo B, Mazuecos J, Rodriguez-Pichardo A, et al. Hypersensitivity to ketoconazole preparations: study of 4 cases. Contact Dermatitis. 1989;21:346-348.
2. Valsecchi R, Pansera B, di Landro A, et al. Contact dermatitis from ketoconazole. Contact Dermatitis. 1993;29:162.
3. Santucci B, Cannistraci C, Cristaudo A, et al. Contact dermatitis from ketoconazole cream. Contact Dermatitis. 1992;27:274-275.
4. Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77.
5. Pullen SK, Warshaw EM. Vulvar allergic contact dermatitis from clotrimazole. Dermatitis. 2010;21:59-60.
Case Report
A 65-year-old man presented to the dermatology department for treatment of a scaly rash on the face and scalp. A diagnosis of seborrheic dermatitis was made, and he was prescribed ketoconazole cream 2% and shampoo 2%. Two days later, the patient presented to the emergency department for facial swelling and pruritus, which began 1 day after he began using the ketoconazole cream and shampoo. He reported itching and burning on the face that began within several hours of application followed by progressive facial edema. The patient denied shortness of breath or swelling of the tongue. Physical examination revealed mild facial induration with erythematous plaques on the bilateral cheeks, forehead, and eyelids. The patient was instructed to stop using the ketoconazole cream and shampoo. Within several days of discontinuing use of the ketoconazole products, the dermatitis resolved following treatment with oral diphenhydramine and topical desonide.
Review of the patient’s medical record revealed several likely relevant incidences of undiagnosed recurrent dermatitis. Approximately 2 years earlier, the patient had called his primary care provider to report pain, burning, redness, and itching in the right buttock area following use of ketoconazole cream that the physician had prescribed. Allergic contact dermatitis also had been documented in the patient’s dermatology problem list approximately 1.5 years prior to the current presentation, though a likely causative agent was not listed. Approximately 3 months prior to the current presentation, the patient presented with lower leg rash and edema with documentation of possible allergic reaction to ketoconazole cream.
The patient was patch tested several weeks after discontinuation of the ketoconazole products using the 2012 North American Contact Dermatitis Group series (70 allergens), a supplemental series (36 allergens), an antifungal series (10 allergens), and personal products including ketoconazole cream and shampoo (diluted 1:100). Clinically relevant reactions at 72 hours included an extreme reaction (+++) to the patient’s personal ketoconazole cream 2% (E. Fougera & Co)(Figure 1), and strong reactions (++) to purified ketoconazole 5% in petrolatum and ketoconazole cream 2% (E. Fougera & Co) in an antifungal series (Figure 2). A doubtful reaction to methyl methacrylate was not deemed clinically relevant. No reactions were noted to terbinafine cream 1%, clotrimazole cream 1%, nystatin cream, nystatin ointment, econazole nitrate cream 1%, miconazole nitrate cream 2%, tolnaftate cream 1%, or purified clotrimazole 1% in petrolatum.
 |
Figure 1. Reading at 72 hours of patient’s personal products (ketoconazole cream 2% and ketoconazole shampoo 2%). |
 |
Figure 2. Reading at 72 hours of an antifungal series (ketoconazole cream 2% and purified ketocona-zole 5% in petrolatum). |
Comment
Ketoconazole is a widely used antifungal but rarely is reported as a cause of allergic contact dermatitis. Allergies to inactive ingredients, especially vehicles and preservatives, are more common than allergies to ketoconazole itself. In our patient, allergy to inactive ingredients was ruled out by negative reactions to individual constituents and/or negative reactions to other products containing those ingredients. A literature review via Ovid using the search terms ketoconazole, allergic contact dermatitis, and allergy found 4 reports involving 9 documented patients with type IV hypersensitivity to ketoconazole,1-4 and 1 report of 2 patients who developed anaphylaxis from oral ketoconazole.1 Of the 9 dermatitis cases, 3 patients had positive patch tests to only ketoconazole with no reactions to other imidazoles.2,3 Monoallergy to clotrimazole also has been reported.5 A study by Dooms-Goossens et al4 showed that ketoconazole ranked seventh of 11 imidazole derivatives in its frequency to cause allergic contact dermatitis and did not demonstrate statistically significant cross-reactivity with other imidazoles; cross-reactivity usually occurred with miconazole and sulconazole.
Conclusion
This case of contact dermatitis to ketoconazole demonstrates the importance of patch testing with personal products as well as the unpredictability of cross-reactions within the imidazole class of antifungals.
Acknowledgment
This material is the result of work supported with resources and the use of facilities at the Minneapolis Veterans Affairs Health Care System.
Case Report
A 65-year-old man presented to the dermatology department for treatment of a scaly rash on the face and scalp. A diagnosis of seborrheic dermatitis was made, and he was prescribed ketoconazole cream 2% and shampoo 2%. Two days later, the patient presented to the emergency department for facial swelling and pruritus, which began 1 day after he began using the ketoconazole cream and shampoo. He reported itching and burning on the face that began within several hours of application followed by progressive facial edema. The patient denied shortness of breath or swelling of the tongue. Physical examination revealed mild facial induration with erythematous plaques on the bilateral cheeks, forehead, and eyelids. The patient was instructed to stop using the ketoconazole cream and shampoo. Within several days of discontinuing use of the ketoconazole products, the dermatitis resolved following treatment with oral diphenhydramine and topical desonide.
Review of the patient’s medical record revealed several likely relevant incidences of undiagnosed recurrent dermatitis. Approximately 2 years earlier, the patient had called his primary care provider to report pain, burning, redness, and itching in the right buttock area following use of ketoconazole cream that the physician had prescribed. Allergic contact dermatitis also had been documented in the patient’s dermatology problem list approximately 1.5 years prior to the current presentation, though a likely causative agent was not listed. Approximately 3 months prior to the current presentation, the patient presented with lower leg rash and edema with documentation of possible allergic reaction to ketoconazole cream.
The patient was patch tested several weeks after discontinuation of the ketoconazole products using the 2012 North American Contact Dermatitis Group series (70 allergens), a supplemental series (36 allergens), an antifungal series (10 allergens), and personal products including ketoconazole cream and shampoo (diluted 1:100). Clinically relevant reactions at 72 hours included an extreme reaction (+++) to the patient’s personal ketoconazole cream 2% (E. Fougera & Co)(Figure 1), and strong reactions (++) to purified ketoconazole 5% in petrolatum and ketoconazole cream 2% (E. Fougera & Co) in an antifungal series (Figure 2). A doubtful reaction to methyl methacrylate was not deemed clinically relevant. No reactions were noted to terbinafine cream 1%, clotrimazole cream 1%, nystatin cream, nystatin ointment, econazole nitrate cream 1%, miconazole nitrate cream 2%, tolnaftate cream 1%, or purified clotrimazole 1% in petrolatum.
 |
Figure 1. Reading at 72 hours of patient’s personal products (ketoconazole cream 2% and ketoconazole shampoo 2%). |
 |
Figure 2. Reading at 72 hours of an antifungal series (ketoconazole cream 2% and purified ketocona-zole 5% in petrolatum). |
Comment
Ketoconazole is a widely used antifungal but rarely is reported as a cause of allergic contact dermatitis. Allergies to inactive ingredients, especially vehicles and preservatives, are more common than allergies to ketoconazole itself. In our patient, allergy to inactive ingredients was ruled out by negative reactions to individual constituents and/or negative reactions to other products containing those ingredients. A literature review via Ovid using the search terms ketoconazole, allergic contact dermatitis, and allergy found 4 reports involving 9 documented patients with type IV hypersensitivity to ketoconazole,1-4 and 1 report of 2 patients who developed anaphylaxis from oral ketoconazole.1 Of the 9 dermatitis cases, 3 patients had positive patch tests to only ketoconazole with no reactions to other imidazoles.2,3 Monoallergy to clotrimazole also has been reported.5 A study by Dooms-Goossens et al4 showed that ketoconazole ranked seventh of 11 imidazole derivatives in its frequency to cause allergic contact dermatitis and did not demonstrate statistically significant cross-reactivity with other imidazoles; cross-reactivity usually occurred with miconazole and sulconazole.
Conclusion
This case of contact dermatitis to ketoconazole demonstrates the importance of patch testing with personal products as well as the unpredictability of cross-reactions within the imidazole class of antifungals.
Acknowledgment
This material is the result of work supported with resources and the use of facilities at the Minneapolis Veterans Affairs Health Care System.
1. Garcia-Bravo B, Mazuecos J, Rodriguez-Pichardo A, et al. Hypersensitivity to ketoconazole preparations: study of 4 cases. Contact Dermatitis. 1989;21:346-348.
2. Valsecchi R, Pansera B, di Landro A, et al. Contact dermatitis from ketoconazole. Contact Dermatitis. 1993;29:162.
3. Santucci B, Cannistraci C, Cristaudo A, et al. Contact dermatitis from ketoconazole cream. Contact Dermatitis. 1992;27:274-275.
4. Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77.
5. Pullen SK, Warshaw EM. Vulvar allergic contact dermatitis from clotrimazole. Dermatitis. 2010;21:59-60.
1. Garcia-Bravo B, Mazuecos J, Rodriguez-Pichardo A, et al. Hypersensitivity to ketoconazole preparations: study of 4 cases. Contact Dermatitis. 1989;21:346-348.
2. Valsecchi R, Pansera B, di Landro A, et al. Contact dermatitis from ketoconazole. Contact Dermatitis. 1993;29:162.
3. Santucci B, Cannistraci C, Cristaudo A, et al. Contact dermatitis from ketoconazole cream. Contact Dermatitis. 1992;27:274-275.
4. Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77.
5. Pullen SK, Warshaw EM. Vulvar allergic contact dermatitis from clotrimazole. Dermatitis. 2010;21:59-60.
- Contact allergy to topical ketoconazole is rare and its cross-reactivity with other imidazole antifungals is unpredictable.
- Patch testing to personal products often is important for detecting rare allergies.