User login
What’s Eating You? Rhipicephalus Ticks Revisited
Characteristics
Rhipicephalus ticks belong to the Ixodidae family of hard-bodied ticks. They are large and teardrop shaped with an inornate scutum (hard dorsal plate) and relatively short mouthparts attached at a hexagonal basis capitulum (base of the head to which mouthparts are attached)(Figure).1 Widely spaced eyes and festoons also are present. The first pair of coxae—attachment base for the first pair of legs—are characteristically bifid; males have a pair of sclerotized adanal plates on the ventral surface adjacent to the anus as well as accessory adanal shields.2Rhipicephalus (formerly Boophilus) microplus (the so-called cattle tick) is a newly added species; it lacks posterior festoons, and the anal groove is absent.3

Almost all Rhipicephalus ticks, except for R microplus, are 3-host ticks in which a single blood meal is consumed from a vertebrate host at each active life stage—larva, nymph, and adult—to complete development.4,5 In contrast to most ixodid ticks, which are exophilic (living outside of human habitation), the Rhipicephalus sanguineus sensu lato species (the brown dog tick) is highly endophilic (adapted to indoor living) and often can be found hidden in cracks and crevices of walls in homes and peridomestic structures.6 It is predominately monotropic (all developmental stages feed on the same host species) and has a strong host preference for dogs, though it occasionally feeds on other hosts (eg, humans).7 Although most common in tropical and subtropical climates, they can be found anywhere there are dogs due to their ability to colonize indoor dwellings.8 In contrast, R microplus ticks have a predilection for cattle and livestock rather than humans, posing a notable concern to livestock worldwide. Infestation results in transmission of disease-causing pathogens, such as Babesia and Anaplasma species, which costs the cattle industry billions of dollars annually.9
Clinical Manifestations and Treatment
Tick bites usually manifest as intensely pruritic, erythematous papules at the site of tick attachment due to a local type IV hypersensitivity reaction to antigens in the tick’s saliva. This reaction can be long-lasting. In addition to pruritic papules following a bite, an attached tick can be mistaken for a skin neoplasm or nevus. Given that ticks are small, especially during the larval stage, dermoscopy may be helpful in making a diagnosis.10 Symptomatic relief usually can be achieved with topical antipruritics or oral antihistamines.
Of public health concern, brown dog ticks are important vectors of Rickettsia rickettsii (the causative organism of Rocky Mountain spotted fever [RMSF]) in the Western hemisphere, and Rickettsia conorii (the causative organism of Mediterranean spotted fever [MSF][also known as Boutonneuse fever]) in the Eastern hemisphere.11 Bites by ticks carrying rickettsial disease classically manifest with early symptoms of fever, headache, and myalgia, followed by a rash or by a localized eschar or tache noire (a black, necrotic, scabbed lesion) that represents direct endothelial invasion and vascular damage by Rickettsia.12 Rocky Mountain spotted fever and MSF are more prevalent during summer, likely due, in part, to the combination of increased outdoor activity and a higher rate of tick-questing (host-seeking) behavior in warmer climates.4,7
Rocky Mountain Spotted Fever—Dermacentor variabilis is the primary vector of RMSF in the southeastern United States; Dermacentor andersoni is the major vector of RMSF in Rocky Mountain states. Rhipicephalus sanguineus sensu lato is an important vector of RMSF in the southwestern United States, Mexico, and Central America.11,13
Early symptoms of RMSF are nonspecific and can include fever, headache, arthralgia, myalgia, and malaise. Gastrointestinal tract symptoms (eg, nausea, vomiting, anorexia) may occur; notable abdominal pain occurs in some patients, particularly children. A characteristic petechial rash occurs in as many as 90% of patients, typically at the third to fifth day of illness, and classically begins on the wrists and ankles, with progression to the palms and soles before spreading centripetally to the arms, legs, and trunk.14 An eschar at the inoculation site is uncommon in RMSF; when present, it is more suggestive of MSF.15
The classic triad of fever, headache, and rash is present in 3% of patients during the first 3 days after a tick bite and in 60% to 70% within 2 weeks.16 A rash often is absent when patients first seek medical attention and may not develop (absent in 9% to 12% of cases; so-called spotless RMSF). Therefore, absence of rash should not be a reason to withhold treatment.16 Empiric treatment with doxycycline should be started promptly for all suspected cases of RMSF because of the rapid progression of disease and an increased risk for morbidity and mortality with delayed diagnosis.
Patients do not become antibody positive until 7 to 10 days after symptoms begin; therefore, treatment should not be delayed while awaiting serologic test results. The case fatality rate in the United States is estimated to be 5% to 10% overall and as high as 40% to 50% among patients who are not treated until day 8 or 9 of illness.17
Cutaneous complications include skin necrosis and gangrene due to continuous tissue damage in severe cases.16 Severe infection also may manifest with signs of multiorgan system damage, including altered mental status, cerebral edema, meningismus, transient deafness, myocarditis, pulmonary hemorrhage and edema, conjunctivitis, retinal abnormalities, and acute renal failure.14,16 Risk factors for more severe illness include delayed treatment, age 40 years or older or younger than 10 years, and underlying medical conditions such as alcoholic liver disease and glucose-6-phosphate dehydrogenase deficiency. However, even some healthy young patients die of this disease.17
Mediterranean Spotted Fever—Rhipicephalus sanguineus sensu lato is the primary vector of MSF, which is prevalent in areas adjacent to the Mediterranean Sea, including southern Europe, Africa, and Central Asia; Sicily is the most highly affected region.18 Findings with MSF are nearly identical to those of RMSF, except that tache noire is more common, present in as many as 70% of cases at the site of the inoculating tick bite, and MSF typically follows a less severe clinical course.12 Similar to other rickettsial diseases, the pathogenesis of MSF involves direct injury to vascular endothelial cells, causing a vasculitis that is responsible for the clinical abnormalities observed.
Patients with severe MSF experience complications similar to severe RMSF, including neurologic manifestations and multiorgan damage.18 Risk factors include advanced age, immunocompromised state, cardiac disease, chronic alcoholism, diabetes mellitus, glucose-6-phosphate dehydrogenase deficiency, respiratory insufficiency, and delayed treatment.18
Treatment—For all spotted fever group rickettsial infections, doxycycline is the treatment of choice for all patients, including children and pregnant women. Treatment should be started without delay; recommended dosages are 100 mg twice daily for children weighing more than 45 kg and adults, and 2.2 mg/kg twice daily for children weighing 45 kg or less.12
Rhipicephalus tick bites rarely can result in paralysis; however, Dermacentor ticks are responsible for most cases of tick-related paralysis in North America. Other pathogens proven or reputed to be transmitted by Rhipicephalus sanguineus sensu lato with zoonotic potential include but are not limited to Rickettsia massiliae, Coxiella burnetti, Anaplasma platys, Leishmania infantum, and Crimean-Congo hemorrhagic fever virus (Nairovirus).19
Environmental Treatment and Prevention
The most effective way to prevent tick-borne illness is avoidance of tick bites. Primary prevention methods include vector control, use of repellents (eg, N,N-diethyl-meta-toluamide [DEET]), picaridin, permethrin), avoidance of areas with a high tick burden, use of protective clothing, and detection and removal of ticks as soon as possible.
Environmental and veterinary controls also are important methods of tick-bite prevention. A veterinarian can recommend a variety of agents for dogs and cats that prevent attachment of ticks. Environmental controls include synthetic or natural product-based chemical acaricides and nonchemical methods, such as landscape management (eg, sealing cracks and crevices in homes and controlling tall grasses, weeds, and leaf debris) to minimize potential tick habitat.20 Secondary prevention includes antibiotics for prophylaxis or for treatment of tick-borne disease, when indicated.
Numerous tick repellents are available commercially; others are being studied. DEET, the most widely used topical repellent, has a broad spectrum of activity against many tick species.21 In addition, DEET has a well-known safety and toxicity profile, with rare adverse effects, and is safe for use in pregnant women and children older than 2 years. Alternative repellents, such as those containing picaridin, ethyl butylacetylaminopropionate (IR3535 [Merck]), oil of lemon eucalyptus, and 2-undecanone can be effective; some show efficacy comparable to that of DEET.22 Permethrin, a synthetic pyrethroid, is a highly efficacious tick repellent and insecticide, especially when used in conjunction with a topical repellent such as DEET. Unlike topically applied repellents, permethrin spray is applied to fabric (eg, clothing, shoes, bed nets, camping gear), not to skin.
Indiscriminate use of acaricides worldwide has led to increasing selection of acaricide resistance in Rhipicephalus tick species, which is especially true with the use of acaricides in controlling R microplus livestock infestations; several tick populations now show resistance to all major classes of these compounds.23-25 For that reason, there has been an increasing effort to develop new chemical and nonchemical approaches to tick control that are more environmentally sustainable and strategies to minimize development and progression of resistance such as rotation of acaricides; reducing the frequency of their application; use of pesticide mixtures, synergists, or both; and increasing use of nonacaricidal methods of control.26
Prompt removal of ticks is important for preventing the transmission of tick-borne disease. Proper removal involves rubbing the tick in a circular motion with a moist gauze pad or using fine-tipped tweezers to grasp the tick as close to the skin surface as possible and pulling upward with a steady pressure.17,27 It is important not to jerk, twist, squeeze, smash, or burn the tick, as this can result in insufficient removal of mouthparts or spread contaminated tick fluids to mucous membranes, increasing the risk for infection. Application of petroleum jelly or nail polish to aid in tick removal have not been shown to be effective and are not recommended.16,28
- Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152:173-185. doi:10.1016/j.vetpar.2007.12.030
- Madder M, Fourie JJ, Schetters TPM. Arachnida, Metastigmata, Ixodidae (except Ixodes holocyclus). In: Marchiondo AA, Cruthers LR, Fourie JJ, eds. Parasiticide Screening: In Vitro and In Vivo Tests With Relevant Parasite Rearing and Host Infection/Infestation Methods. Volume 1. Elsevier Academic Press; 2019:19-20.
- Burger TD, Shao R, Barker SC. Phylogenetic analysis of mitochondrial genome sequences indicates that the cattle tick, Rhipicephalus (Boophilus) microplus, contains a cryptic species. Mol Phylogenet Evol. 2014;76:241-253. doi:10.1016/j.ympev.2014.03.017
- Gray J, Dantas-Torres F, Estrada-Peña A, et al. Systematics and ecology of the brown dog tick, Rhipicephalus sanguineus. Ticks Tick Borne Dis. 2013;4:171-180. doi:10.1016/j.ttbdis.2012.12.003
- Tian Y, Lord CC, Kaufman PE. Brown dog tick, Rhipicephalus Sanguineus Latrielle (Arachnida: Acari: Ixodidae): EENY-221/IN378. EDIS. March 26, 2020. Accessed January 3, 2024. https://doi.org/10.32473/edis-in378-2020
- Saleh MN, Allen KE, Lineberry MW, et al. Ticks infesting dogs and cats in North America: biology, geographic distribution, and pathogen transmission. Vet Parasitol. 2021;294:109392. doi:10.1016/j.vetpar.2021.109392
- Dantas-Torres F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors. 2010;3:26. doi:10.1186/1756-3305-3-26
- Dryden MW, Payne PA. Biology and control of ticks infesting dogs and cats in North America. Vet Ther. 2004;5:139-154.
- Nyangiwe N, Yawa M, Muchenje V. Driving forces for changes in geographic range of cattle ticks (Acari: Ixodidae) in Africa: a Review. S Afr J Anim Sci. 2018;48:829. doi:10.4314/sajas.v48i5.4
- Ramot Y, Zlotogorski A, Mumcuoglu KY. Brown dog tick (Rhipicephalus sanguineus) infestation of the penis detected by dermoscopy. Int J Dermatol. 2012;51:1402-1403. doi:10.1111/j.1365-4632.2010.04756.x
- Tucker NSG, Weeks ENI, Beati L, et al. Prevalence and distribution of pathogen infection and permethrin resistance in tropical and temperate populations of Rhipicephalus sanguineus s.l. collected worldwide. Med Vet Entomol. 2021;35:147-157. doi:10.1111/mve.12479
- McClain MT, Sexton DJ, Hall KK, eds. Other spotted fever group rickettsial infections. UpToDate. Updated October 10, 2022. Accessed January 3, 2024. https://www.uptodate.com/contents/other-spotted-fever-group-rickettsial-infections
- Ribeiro CM, Carvalho JLB, Bastos PAS, et al. Prevalence of Rickettsia rickettsii in ticks: systematic review and meta-analysis. Vector Borne Zoonotic Dis. 2021;21:557-565. doi:10.1089/vbz.2021.0004
- Pace EJ, O’Reilly M. Tickborne diseases: diagnosis and management. Am Fam Physician. 2020;101:530-540.
- Patterson JW. Weedon’s Skin Pathology. 5th ed. Elsevier; 2020.
- Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis. 2007;7:724-732. doi:10.1016/S1473-3099(07)70261-X
- Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep. 2016;65:1-44. doi:10.15585/mmwr.rr6502a1
- Rossio R, Conalbi V, Castagna V, et al. Mediterranean spotted fever and hearing impairment: a rare complication. Int J Infect Dis. 2015;35:34-36. doi:10.1016/j.ijid.2015.04.005
- Dantas-Torres F, Otranto D. Further thoughts on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Vet Parasitol. 2015;208:9-13. doi:10.1016/j.vetpar.2014.12.014
- Eisen RJ, Kugeler KJ, Eisen L, et al. Tick-borne zoonoses in the United States: persistent and emerging threats to human health. ILAR J. 2017;58:319-335. doi:10.1093/ilar/ilx005
- Nguyen QD, Vu MN, Hebert AA. Insect repellents: an updated review for the clinician. J Am Acad Dermatol. 2018;88:123-130. doi:10.1016/j.jaad.2018.10.053
- Pages F, Dautel H, Duvallet G, et al. Tick repellents for human use: prevention of tick bites and tick-borne diseases. Vector Borne Zoonotic Dis. 2014;14:85-93. doi:10.1089/vbz.2013.1410
- Rodriguez-Vivas RI, Alonso-Díaz MA, et al. Prevalence and potential risk factors for organophosphate and pyrethroid resistance in Boophilus microplus ticks on cattle ranches from the State of Yucatan, Mexico. Vet Parasitol. 2006;136:335-342. doi:10.1016/j.vetpar.2005.05.069
- Rodríguez-Vivas RI, Rodríguez-Arevalo F, Alonso-Díaz MA, et al. Prevalence and potential risk factors for amitraz resistance in Boophilus microplus ticks in cattle farms in the State of Yucatan, Mexico. Prev Vet Med. 2006;75:280-286. doi:10.1016/j.prevetmed.2006.04.001
- Perez-Cogollo LC, Rodriguez-Vivas RI, Ramirez-Cruz GT, et al. First report of the cattle tick Rhipicephalus microplus resistant to ivermectin in Mexico. Vet Parasitol. 2010;168:165-169. doi:10.1016/j.vetpar.2009.10.021
- Rodriguez-Vivas RI, Jonsson NN, Bhushan C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol Res.2018;117:3-29. doi:10.1007/s00436-017-5677-6
- Centers for Disease Control and Prevention. Tick removal. Updated May 13, 2022. Accessed January 3, 2024. https://www.cdc.gov/ticks/removing_a_tick.html
- Diaz JH. Chemical and plant-based insect repellents: efficacy, safety, and toxicity. Wilderness Environ Med. 2016;27:153-163. doi:10.1016/j.wem.2015.11.007
Characteristics
Rhipicephalus ticks belong to the Ixodidae family of hard-bodied ticks. They are large and teardrop shaped with an inornate scutum (hard dorsal plate) and relatively short mouthparts attached at a hexagonal basis capitulum (base of the head to which mouthparts are attached)(Figure).1 Widely spaced eyes and festoons also are present. The first pair of coxae—attachment base for the first pair of legs—are characteristically bifid; males have a pair of sclerotized adanal plates on the ventral surface adjacent to the anus as well as accessory adanal shields.2Rhipicephalus (formerly Boophilus) microplus (the so-called cattle tick) is a newly added species; it lacks posterior festoons, and the anal groove is absent.3

Almost all Rhipicephalus ticks, except for R microplus, are 3-host ticks in which a single blood meal is consumed from a vertebrate host at each active life stage—larva, nymph, and adult—to complete development.4,5 In contrast to most ixodid ticks, which are exophilic (living outside of human habitation), the Rhipicephalus sanguineus sensu lato species (the brown dog tick) is highly endophilic (adapted to indoor living) and often can be found hidden in cracks and crevices of walls in homes and peridomestic structures.6 It is predominately monotropic (all developmental stages feed on the same host species) and has a strong host preference for dogs, though it occasionally feeds on other hosts (eg, humans).7 Although most common in tropical and subtropical climates, they can be found anywhere there are dogs due to their ability to colonize indoor dwellings.8 In contrast, R microplus ticks have a predilection for cattle and livestock rather than humans, posing a notable concern to livestock worldwide. Infestation results in transmission of disease-causing pathogens, such as Babesia and Anaplasma species, which costs the cattle industry billions of dollars annually.9
Clinical Manifestations and Treatment
Tick bites usually manifest as intensely pruritic, erythematous papules at the site of tick attachment due to a local type IV hypersensitivity reaction to antigens in the tick’s saliva. This reaction can be long-lasting. In addition to pruritic papules following a bite, an attached tick can be mistaken for a skin neoplasm or nevus. Given that ticks are small, especially during the larval stage, dermoscopy may be helpful in making a diagnosis.10 Symptomatic relief usually can be achieved with topical antipruritics or oral antihistamines.
Of public health concern, brown dog ticks are important vectors of Rickettsia rickettsii (the causative organism of Rocky Mountain spotted fever [RMSF]) in the Western hemisphere, and Rickettsia conorii (the causative organism of Mediterranean spotted fever [MSF][also known as Boutonneuse fever]) in the Eastern hemisphere.11 Bites by ticks carrying rickettsial disease classically manifest with early symptoms of fever, headache, and myalgia, followed by a rash or by a localized eschar or tache noire (a black, necrotic, scabbed lesion) that represents direct endothelial invasion and vascular damage by Rickettsia.12 Rocky Mountain spotted fever and MSF are more prevalent during summer, likely due, in part, to the combination of increased outdoor activity and a higher rate of tick-questing (host-seeking) behavior in warmer climates.4,7
Rocky Mountain Spotted Fever—Dermacentor variabilis is the primary vector of RMSF in the southeastern United States; Dermacentor andersoni is the major vector of RMSF in Rocky Mountain states. Rhipicephalus sanguineus sensu lato is an important vector of RMSF in the southwestern United States, Mexico, and Central America.11,13
Early symptoms of RMSF are nonspecific and can include fever, headache, arthralgia, myalgia, and malaise. Gastrointestinal tract symptoms (eg, nausea, vomiting, anorexia) may occur; notable abdominal pain occurs in some patients, particularly children. A characteristic petechial rash occurs in as many as 90% of patients, typically at the third to fifth day of illness, and classically begins on the wrists and ankles, with progression to the palms and soles before spreading centripetally to the arms, legs, and trunk.14 An eschar at the inoculation site is uncommon in RMSF; when present, it is more suggestive of MSF.15
The classic triad of fever, headache, and rash is present in 3% of patients during the first 3 days after a tick bite and in 60% to 70% within 2 weeks.16 A rash often is absent when patients first seek medical attention and may not develop (absent in 9% to 12% of cases; so-called spotless RMSF). Therefore, absence of rash should not be a reason to withhold treatment.16 Empiric treatment with doxycycline should be started promptly for all suspected cases of RMSF because of the rapid progression of disease and an increased risk for morbidity and mortality with delayed diagnosis.
Patients do not become antibody positive until 7 to 10 days after symptoms begin; therefore, treatment should not be delayed while awaiting serologic test results. The case fatality rate in the United States is estimated to be 5% to 10% overall and as high as 40% to 50% among patients who are not treated until day 8 or 9 of illness.17
Cutaneous complications include skin necrosis and gangrene due to continuous tissue damage in severe cases.16 Severe infection also may manifest with signs of multiorgan system damage, including altered mental status, cerebral edema, meningismus, transient deafness, myocarditis, pulmonary hemorrhage and edema, conjunctivitis, retinal abnormalities, and acute renal failure.14,16 Risk factors for more severe illness include delayed treatment, age 40 years or older or younger than 10 years, and underlying medical conditions such as alcoholic liver disease and glucose-6-phosphate dehydrogenase deficiency. However, even some healthy young patients die of this disease.17
Mediterranean Spotted Fever—Rhipicephalus sanguineus sensu lato is the primary vector of MSF, which is prevalent in areas adjacent to the Mediterranean Sea, including southern Europe, Africa, and Central Asia; Sicily is the most highly affected region.18 Findings with MSF are nearly identical to those of RMSF, except that tache noire is more common, present in as many as 70% of cases at the site of the inoculating tick bite, and MSF typically follows a less severe clinical course.12 Similar to other rickettsial diseases, the pathogenesis of MSF involves direct injury to vascular endothelial cells, causing a vasculitis that is responsible for the clinical abnormalities observed.
Patients with severe MSF experience complications similar to severe RMSF, including neurologic manifestations and multiorgan damage.18 Risk factors include advanced age, immunocompromised state, cardiac disease, chronic alcoholism, diabetes mellitus, glucose-6-phosphate dehydrogenase deficiency, respiratory insufficiency, and delayed treatment.18
Treatment—For all spotted fever group rickettsial infections, doxycycline is the treatment of choice for all patients, including children and pregnant women. Treatment should be started without delay; recommended dosages are 100 mg twice daily for children weighing more than 45 kg and adults, and 2.2 mg/kg twice daily for children weighing 45 kg or less.12
Rhipicephalus tick bites rarely can result in paralysis; however, Dermacentor ticks are responsible for most cases of tick-related paralysis in North America. Other pathogens proven or reputed to be transmitted by Rhipicephalus sanguineus sensu lato with zoonotic potential include but are not limited to Rickettsia massiliae, Coxiella burnetti, Anaplasma platys, Leishmania infantum, and Crimean-Congo hemorrhagic fever virus (Nairovirus).19
Environmental Treatment and Prevention
The most effective way to prevent tick-borne illness is avoidance of tick bites. Primary prevention methods include vector control, use of repellents (eg, N,N-diethyl-meta-toluamide [DEET]), picaridin, permethrin), avoidance of areas with a high tick burden, use of protective clothing, and detection and removal of ticks as soon as possible.
Environmental and veterinary controls also are important methods of tick-bite prevention. A veterinarian can recommend a variety of agents for dogs and cats that prevent attachment of ticks. Environmental controls include synthetic or natural product-based chemical acaricides and nonchemical methods, such as landscape management (eg, sealing cracks and crevices in homes and controlling tall grasses, weeds, and leaf debris) to minimize potential tick habitat.20 Secondary prevention includes antibiotics for prophylaxis or for treatment of tick-borne disease, when indicated.
Numerous tick repellents are available commercially; others are being studied. DEET, the most widely used topical repellent, has a broad spectrum of activity against many tick species.21 In addition, DEET has a well-known safety and toxicity profile, with rare adverse effects, and is safe for use in pregnant women and children older than 2 years. Alternative repellents, such as those containing picaridin, ethyl butylacetylaminopropionate (IR3535 [Merck]), oil of lemon eucalyptus, and 2-undecanone can be effective; some show efficacy comparable to that of DEET.22 Permethrin, a synthetic pyrethroid, is a highly efficacious tick repellent and insecticide, especially when used in conjunction with a topical repellent such as DEET. Unlike topically applied repellents, permethrin spray is applied to fabric (eg, clothing, shoes, bed nets, camping gear), not to skin.
Indiscriminate use of acaricides worldwide has led to increasing selection of acaricide resistance in Rhipicephalus tick species, which is especially true with the use of acaricides in controlling R microplus livestock infestations; several tick populations now show resistance to all major classes of these compounds.23-25 For that reason, there has been an increasing effort to develop new chemical and nonchemical approaches to tick control that are more environmentally sustainable and strategies to minimize development and progression of resistance such as rotation of acaricides; reducing the frequency of their application; use of pesticide mixtures, synergists, or both; and increasing use of nonacaricidal methods of control.26
Prompt removal of ticks is important for preventing the transmission of tick-borne disease. Proper removal involves rubbing the tick in a circular motion with a moist gauze pad or using fine-tipped tweezers to grasp the tick as close to the skin surface as possible and pulling upward with a steady pressure.17,27 It is important not to jerk, twist, squeeze, smash, or burn the tick, as this can result in insufficient removal of mouthparts or spread contaminated tick fluids to mucous membranes, increasing the risk for infection. Application of petroleum jelly or nail polish to aid in tick removal have not been shown to be effective and are not recommended.16,28
Characteristics
Rhipicephalus ticks belong to the Ixodidae family of hard-bodied ticks. They are large and teardrop shaped with an inornate scutum (hard dorsal plate) and relatively short mouthparts attached at a hexagonal basis capitulum (base of the head to which mouthparts are attached)(Figure).1 Widely spaced eyes and festoons also are present. The first pair of coxae—attachment base for the first pair of legs—are characteristically bifid; males have a pair of sclerotized adanal plates on the ventral surface adjacent to the anus as well as accessory adanal shields.2Rhipicephalus (formerly Boophilus) microplus (the so-called cattle tick) is a newly added species; it lacks posterior festoons, and the anal groove is absent.3

Almost all Rhipicephalus ticks, except for R microplus, are 3-host ticks in which a single blood meal is consumed from a vertebrate host at each active life stage—larva, nymph, and adult—to complete development.4,5 In contrast to most ixodid ticks, which are exophilic (living outside of human habitation), the Rhipicephalus sanguineus sensu lato species (the brown dog tick) is highly endophilic (adapted to indoor living) and often can be found hidden in cracks and crevices of walls in homes and peridomestic structures.6 It is predominately monotropic (all developmental stages feed on the same host species) and has a strong host preference for dogs, though it occasionally feeds on other hosts (eg, humans).7 Although most common in tropical and subtropical climates, they can be found anywhere there are dogs due to their ability to colonize indoor dwellings.8 In contrast, R microplus ticks have a predilection for cattle and livestock rather than humans, posing a notable concern to livestock worldwide. Infestation results in transmission of disease-causing pathogens, such as Babesia and Anaplasma species, which costs the cattle industry billions of dollars annually.9
Clinical Manifestations and Treatment
Tick bites usually manifest as intensely pruritic, erythematous papules at the site of tick attachment due to a local type IV hypersensitivity reaction to antigens in the tick’s saliva. This reaction can be long-lasting. In addition to pruritic papules following a bite, an attached tick can be mistaken for a skin neoplasm or nevus. Given that ticks are small, especially during the larval stage, dermoscopy may be helpful in making a diagnosis.10 Symptomatic relief usually can be achieved with topical antipruritics or oral antihistamines.
Of public health concern, brown dog ticks are important vectors of Rickettsia rickettsii (the causative organism of Rocky Mountain spotted fever [RMSF]) in the Western hemisphere, and Rickettsia conorii (the causative organism of Mediterranean spotted fever [MSF][also known as Boutonneuse fever]) in the Eastern hemisphere.11 Bites by ticks carrying rickettsial disease classically manifest with early symptoms of fever, headache, and myalgia, followed by a rash or by a localized eschar or tache noire (a black, necrotic, scabbed lesion) that represents direct endothelial invasion and vascular damage by Rickettsia.12 Rocky Mountain spotted fever and MSF are more prevalent during summer, likely due, in part, to the combination of increased outdoor activity and a higher rate of tick-questing (host-seeking) behavior in warmer climates.4,7
Rocky Mountain Spotted Fever—Dermacentor variabilis is the primary vector of RMSF in the southeastern United States; Dermacentor andersoni is the major vector of RMSF in Rocky Mountain states. Rhipicephalus sanguineus sensu lato is an important vector of RMSF in the southwestern United States, Mexico, and Central America.11,13
Early symptoms of RMSF are nonspecific and can include fever, headache, arthralgia, myalgia, and malaise. Gastrointestinal tract symptoms (eg, nausea, vomiting, anorexia) may occur; notable abdominal pain occurs in some patients, particularly children. A characteristic petechial rash occurs in as many as 90% of patients, typically at the third to fifth day of illness, and classically begins on the wrists and ankles, with progression to the palms and soles before spreading centripetally to the arms, legs, and trunk.14 An eschar at the inoculation site is uncommon in RMSF; when present, it is more suggestive of MSF.15
The classic triad of fever, headache, and rash is present in 3% of patients during the first 3 days after a tick bite and in 60% to 70% within 2 weeks.16 A rash often is absent when patients first seek medical attention and may not develop (absent in 9% to 12% of cases; so-called spotless RMSF). Therefore, absence of rash should not be a reason to withhold treatment.16 Empiric treatment with doxycycline should be started promptly for all suspected cases of RMSF because of the rapid progression of disease and an increased risk for morbidity and mortality with delayed diagnosis.
Patients do not become antibody positive until 7 to 10 days after symptoms begin; therefore, treatment should not be delayed while awaiting serologic test results. The case fatality rate in the United States is estimated to be 5% to 10% overall and as high as 40% to 50% among patients who are not treated until day 8 or 9 of illness.17
Cutaneous complications include skin necrosis and gangrene due to continuous tissue damage in severe cases.16 Severe infection also may manifest with signs of multiorgan system damage, including altered mental status, cerebral edema, meningismus, transient deafness, myocarditis, pulmonary hemorrhage and edema, conjunctivitis, retinal abnormalities, and acute renal failure.14,16 Risk factors for more severe illness include delayed treatment, age 40 years or older or younger than 10 years, and underlying medical conditions such as alcoholic liver disease and glucose-6-phosphate dehydrogenase deficiency. However, even some healthy young patients die of this disease.17
Mediterranean Spotted Fever—Rhipicephalus sanguineus sensu lato is the primary vector of MSF, which is prevalent in areas adjacent to the Mediterranean Sea, including southern Europe, Africa, and Central Asia; Sicily is the most highly affected region.18 Findings with MSF are nearly identical to those of RMSF, except that tache noire is more common, present in as many as 70% of cases at the site of the inoculating tick bite, and MSF typically follows a less severe clinical course.12 Similar to other rickettsial diseases, the pathogenesis of MSF involves direct injury to vascular endothelial cells, causing a vasculitis that is responsible for the clinical abnormalities observed.
Patients with severe MSF experience complications similar to severe RMSF, including neurologic manifestations and multiorgan damage.18 Risk factors include advanced age, immunocompromised state, cardiac disease, chronic alcoholism, diabetes mellitus, glucose-6-phosphate dehydrogenase deficiency, respiratory insufficiency, and delayed treatment.18
Treatment—For all spotted fever group rickettsial infections, doxycycline is the treatment of choice for all patients, including children and pregnant women. Treatment should be started without delay; recommended dosages are 100 mg twice daily for children weighing more than 45 kg and adults, and 2.2 mg/kg twice daily for children weighing 45 kg or less.12
Rhipicephalus tick bites rarely can result in paralysis; however, Dermacentor ticks are responsible for most cases of tick-related paralysis in North America. Other pathogens proven or reputed to be transmitted by Rhipicephalus sanguineus sensu lato with zoonotic potential include but are not limited to Rickettsia massiliae, Coxiella burnetti, Anaplasma platys, Leishmania infantum, and Crimean-Congo hemorrhagic fever virus (Nairovirus).19
Environmental Treatment and Prevention
The most effective way to prevent tick-borne illness is avoidance of tick bites. Primary prevention methods include vector control, use of repellents (eg, N,N-diethyl-meta-toluamide [DEET]), picaridin, permethrin), avoidance of areas with a high tick burden, use of protective clothing, and detection and removal of ticks as soon as possible.
Environmental and veterinary controls also are important methods of tick-bite prevention. A veterinarian can recommend a variety of agents for dogs and cats that prevent attachment of ticks. Environmental controls include synthetic or natural product-based chemical acaricides and nonchemical methods, such as landscape management (eg, sealing cracks and crevices in homes and controlling tall grasses, weeds, and leaf debris) to minimize potential tick habitat.20 Secondary prevention includes antibiotics for prophylaxis or for treatment of tick-borne disease, when indicated.
Numerous tick repellents are available commercially; others are being studied. DEET, the most widely used topical repellent, has a broad spectrum of activity against many tick species.21 In addition, DEET has a well-known safety and toxicity profile, with rare adverse effects, and is safe for use in pregnant women and children older than 2 years. Alternative repellents, such as those containing picaridin, ethyl butylacetylaminopropionate (IR3535 [Merck]), oil of lemon eucalyptus, and 2-undecanone can be effective; some show efficacy comparable to that of DEET.22 Permethrin, a synthetic pyrethroid, is a highly efficacious tick repellent and insecticide, especially when used in conjunction with a topical repellent such as DEET. Unlike topically applied repellents, permethrin spray is applied to fabric (eg, clothing, shoes, bed nets, camping gear), not to skin.
Indiscriminate use of acaricides worldwide has led to increasing selection of acaricide resistance in Rhipicephalus tick species, which is especially true with the use of acaricides in controlling R microplus livestock infestations; several tick populations now show resistance to all major classes of these compounds.23-25 For that reason, there has been an increasing effort to develop new chemical and nonchemical approaches to tick control that are more environmentally sustainable and strategies to minimize development and progression of resistance such as rotation of acaricides; reducing the frequency of their application; use of pesticide mixtures, synergists, or both; and increasing use of nonacaricidal methods of control.26
Prompt removal of ticks is important for preventing the transmission of tick-borne disease. Proper removal involves rubbing the tick in a circular motion with a moist gauze pad or using fine-tipped tweezers to grasp the tick as close to the skin surface as possible and pulling upward with a steady pressure.17,27 It is important not to jerk, twist, squeeze, smash, or burn the tick, as this can result in insufficient removal of mouthparts or spread contaminated tick fluids to mucous membranes, increasing the risk for infection. Application of petroleum jelly or nail polish to aid in tick removal have not been shown to be effective and are not recommended.16,28
- Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152:173-185. doi:10.1016/j.vetpar.2007.12.030
- Madder M, Fourie JJ, Schetters TPM. Arachnida, Metastigmata, Ixodidae (except Ixodes holocyclus). In: Marchiondo AA, Cruthers LR, Fourie JJ, eds. Parasiticide Screening: In Vitro and In Vivo Tests With Relevant Parasite Rearing and Host Infection/Infestation Methods. Volume 1. Elsevier Academic Press; 2019:19-20.
- Burger TD, Shao R, Barker SC. Phylogenetic analysis of mitochondrial genome sequences indicates that the cattle tick, Rhipicephalus (Boophilus) microplus, contains a cryptic species. Mol Phylogenet Evol. 2014;76:241-253. doi:10.1016/j.ympev.2014.03.017
- Gray J, Dantas-Torres F, Estrada-Peña A, et al. Systematics and ecology of the brown dog tick, Rhipicephalus sanguineus. Ticks Tick Borne Dis. 2013;4:171-180. doi:10.1016/j.ttbdis.2012.12.003
- Tian Y, Lord CC, Kaufman PE. Brown dog tick, Rhipicephalus Sanguineus Latrielle (Arachnida: Acari: Ixodidae): EENY-221/IN378. EDIS. March 26, 2020. Accessed January 3, 2024. https://doi.org/10.32473/edis-in378-2020
- Saleh MN, Allen KE, Lineberry MW, et al. Ticks infesting dogs and cats in North America: biology, geographic distribution, and pathogen transmission. Vet Parasitol. 2021;294:109392. doi:10.1016/j.vetpar.2021.109392
- Dantas-Torres F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors. 2010;3:26. doi:10.1186/1756-3305-3-26
- Dryden MW, Payne PA. Biology and control of ticks infesting dogs and cats in North America. Vet Ther. 2004;5:139-154.
- Nyangiwe N, Yawa M, Muchenje V. Driving forces for changes in geographic range of cattle ticks (Acari: Ixodidae) in Africa: a Review. S Afr J Anim Sci. 2018;48:829. doi:10.4314/sajas.v48i5.4
- Ramot Y, Zlotogorski A, Mumcuoglu KY. Brown dog tick (Rhipicephalus sanguineus) infestation of the penis detected by dermoscopy. Int J Dermatol. 2012;51:1402-1403. doi:10.1111/j.1365-4632.2010.04756.x
- Tucker NSG, Weeks ENI, Beati L, et al. Prevalence and distribution of pathogen infection and permethrin resistance in tropical and temperate populations of Rhipicephalus sanguineus s.l. collected worldwide. Med Vet Entomol. 2021;35:147-157. doi:10.1111/mve.12479
- McClain MT, Sexton DJ, Hall KK, eds. Other spotted fever group rickettsial infections. UpToDate. Updated October 10, 2022. Accessed January 3, 2024. https://www.uptodate.com/contents/other-spotted-fever-group-rickettsial-infections
- Ribeiro CM, Carvalho JLB, Bastos PAS, et al. Prevalence of Rickettsia rickettsii in ticks: systematic review and meta-analysis. Vector Borne Zoonotic Dis. 2021;21:557-565. doi:10.1089/vbz.2021.0004
- Pace EJ, O’Reilly M. Tickborne diseases: diagnosis and management. Am Fam Physician. 2020;101:530-540.
- Patterson JW. Weedon’s Skin Pathology. 5th ed. Elsevier; 2020.
- Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis. 2007;7:724-732. doi:10.1016/S1473-3099(07)70261-X
- Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep. 2016;65:1-44. doi:10.15585/mmwr.rr6502a1
- Rossio R, Conalbi V, Castagna V, et al. Mediterranean spotted fever and hearing impairment: a rare complication. Int J Infect Dis. 2015;35:34-36. doi:10.1016/j.ijid.2015.04.005
- Dantas-Torres F, Otranto D. Further thoughts on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Vet Parasitol. 2015;208:9-13. doi:10.1016/j.vetpar.2014.12.014
- Eisen RJ, Kugeler KJ, Eisen L, et al. Tick-borne zoonoses in the United States: persistent and emerging threats to human health. ILAR J. 2017;58:319-335. doi:10.1093/ilar/ilx005
- Nguyen QD, Vu MN, Hebert AA. Insect repellents: an updated review for the clinician. J Am Acad Dermatol. 2018;88:123-130. doi:10.1016/j.jaad.2018.10.053
- Pages F, Dautel H, Duvallet G, et al. Tick repellents for human use: prevention of tick bites and tick-borne diseases. Vector Borne Zoonotic Dis. 2014;14:85-93. doi:10.1089/vbz.2013.1410
- Rodriguez-Vivas RI, Alonso-Díaz MA, et al. Prevalence and potential risk factors for organophosphate and pyrethroid resistance in Boophilus microplus ticks on cattle ranches from the State of Yucatan, Mexico. Vet Parasitol. 2006;136:335-342. doi:10.1016/j.vetpar.2005.05.069
- Rodríguez-Vivas RI, Rodríguez-Arevalo F, Alonso-Díaz MA, et al. Prevalence and potential risk factors for amitraz resistance in Boophilus microplus ticks in cattle farms in the State of Yucatan, Mexico. Prev Vet Med. 2006;75:280-286. doi:10.1016/j.prevetmed.2006.04.001
- Perez-Cogollo LC, Rodriguez-Vivas RI, Ramirez-Cruz GT, et al. First report of the cattle tick Rhipicephalus microplus resistant to ivermectin in Mexico. Vet Parasitol. 2010;168:165-169. doi:10.1016/j.vetpar.2009.10.021
- Rodriguez-Vivas RI, Jonsson NN, Bhushan C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol Res.2018;117:3-29. doi:10.1007/s00436-017-5677-6
- Centers for Disease Control and Prevention. Tick removal. Updated May 13, 2022. Accessed January 3, 2024. https://www.cdc.gov/ticks/removing_a_tick.html
- Diaz JH. Chemical and plant-based insect repellents: efficacy, safety, and toxicity. Wilderness Environ Med. 2016;27:153-163. doi:10.1016/j.wem.2015.11.007
- Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152:173-185. doi:10.1016/j.vetpar.2007.12.030
- Madder M, Fourie JJ, Schetters TPM. Arachnida, Metastigmata, Ixodidae (except Ixodes holocyclus). In: Marchiondo AA, Cruthers LR, Fourie JJ, eds. Parasiticide Screening: In Vitro and In Vivo Tests With Relevant Parasite Rearing and Host Infection/Infestation Methods. Volume 1. Elsevier Academic Press; 2019:19-20.
- Burger TD, Shao R, Barker SC. Phylogenetic analysis of mitochondrial genome sequences indicates that the cattle tick, Rhipicephalus (Boophilus) microplus, contains a cryptic species. Mol Phylogenet Evol. 2014;76:241-253. doi:10.1016/j.ympev.2014.03.017
- Gray J, Dantas-Torres F, Estrada-Peña A, et al. Systematics and ecology of the brown dog tick, Rhipicephalus sanguineus. Ticks Tick Borne Dis. 2013;4:171-180. doi:10.1016/j.ttbdis.2012.12.003
- Tian Y, Lord CC, Kaufman PE. Brown dog tick, Rhipicephalus Sanguineus Latrielle (Arachnida: Acari: Ixodidae): EENY-221/IN378. EDIS. March 26, 2020. Accessed January 3, 2024. https://doi.org/10.32473/edis-in378-2020
- Saleh MN, Allen KE, Lineberry MW, et al. Ticks infesting dogs and cats in North America: biology, geographic distribution, and pathogen transmission. Vet Parasitol. 2021;294:109392. doi:10.1016/j.vetpar.2021.109392
- Dantas-Torres F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors. 2010;3:26. doi:10.1186/1756-3305-3-26
- Dryden MW, Payne PA. Biology and control of ticks infesting dogs and cats in North America. Vet Ther. 2004;5:139-154.
- Nyangiwe N, Yawa M, Muchenje V. Driving forces for changes in geographic range of cattle ticks (Acari: Ixodidae) in Africa: a Review. S Afr J Anim Sci. 2018;48:829. doi:10.4314/sajas.v48i5.4
- Ramot Y, Zlotogorski A, Mumcuoglu KY. Brown dog tick (Rhipicephalus sanguineus) infestation of the penis detected by dermoscopy. Int J Dermatol. 2012;51:1402-1403. doi:10.1111/j.1365-4632.2010.04756.x
- Tucker NSG, Weeks ENI, Beati L, et al. Prevalence and distribution of pathogen infection and permethrin resistance in tropical and temperate populations of Rhipicephalus sanguineus s.l. collected worldwide. Med Vet Entomol. 2021;35:147-157. doi:10.1111/mve.12479
- McClain MT, Sexton DJ, Hall KK, eds. Other spotted fever group rickettsial infections. UpToDate. Updated October 10, 2022. Accessed January 3, 2024. https://www.uptodate.com/contents/other-spotted-fever-group-rickettsial-infections
- Ribeiro CM, Carvalho JLB, Bastos PAS, et al. Prevalence of Rickettsia rickettsii in ticks: systematic review and meta-analysis. Vector Borne Zoonotic Dis. 2021;21:557-565. doi:10.1089/vbz.2021.0004
- Pace EJ, O’Reilly M. Tickborne diseases: diagnosis and management. Am Fam Physician. 2020;101:530-540.
- Patterson JW. Weedon’s Skin Pathology. 5th ed. Elsevier; 2020.
- Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis. 2007;7:724-732. doi:10.1016/S1473-3099(07)70261-X
- Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep. 2016;65:1-44. doi:10.15585/mmwr.rr6502a1
- Rossio R, Conalbi V, Castagna V, et al. Mediterranean spotted fever and hearing impairment: a rare complication. Int J Infect Dis. 2015;35:34-36. doi:10.1016/j.ijid.2015.04.005
- Dantas-Torres F, Otranto D. Further thoughts on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Vet Parasitol. 2015;208:9-13. doi:10.1016/j.vetpar.2014.12.014
- Eisen RJ, Kugeler KJ, Eisen L, et al. Tick-borne zoonoses in the United States: persistent and emerging threats to human health. ILAR J. 2017;58:319-335. doi:10.1093/ilar/ilx005
- Nguyen QD, Vu MN, Hebert AA. Insect repellents: an updated review for the clinician. J Am Acad Dermatol. 2018;88:123-130. doi:10.1016/j.jaad.2018.10.053
- Pages F, Dautel H, Duvallet G, et al. Tick repellents for human use: prevention of tick bites and tick-borne diseases. Vector Borne Zoonotic Dis. 2014;14:85-93. doi:10.1089/vbz.2013.1410
- Rodriguez-Vivas RI, Alonso-Díaz MA, et al. Prevalence and potential risk factors for organophosphate and pyrethroid resistance in Boophilus microplus ticks on cattle ranches from the State of Yucatan, Mexico. Vet Parasitol. 2006;136:335-342. doi:10.1016/j.vetpar.2005.05.069
- Rodríguez-Vivas RI, Rodríguez-Arevalo F, Alonso-Díaz MA, et al. Prevalence and potential risk factors for amitraz resistance in Boophilus microplus ticks in cattle farms in the State of Yucatan, Mexico. Prev Vet Med. 2006;75:280-286. doi:10.1016/j.prevetmed.2006.04.001
- Perez-Cogollo LC, Rodriguez-Vivas RI, Ramirez-Cruz GT, et al. First report of the cattle tick Rhipicephalus microplus resistant to ivermectin in Mexico. Vet Parasitol. 2010;168:165-169. doi:10.1016/j.vetpar.2009.10.021
- Rodriguez-Vivas RI, Jonsson NN, Bhushan C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol Res.2018;117:3-29. doi:10.1007/s00436-017-5677-6
- Centers for Disease Control and Prevention. Tick removal. Updated May 13, 2022. Accessed January 3, 2024. https://www.cdc.gov/ticks/removing_a_tick.html
- Diaz JH. Chemical and plant-based insect repellents: efficacy, safety, and toxicity. Wilderness Environ Med. 2016;27:153-163. doi:10.1016/j.wem.2015.11.007
PRACTICE POINTS
- Rhipicephalus ticks are vectors of a variety of diseases, including the rickettsial diseases Rocky Mountain spotted fever and Mediterranean spotted fever.
- Presenting symptoms of a tick bite include intensely pruritic, erythematous papules and nodules at the site of tick attachment.
- If rickettsial disease is suspected, treatment with doxycycline should be initiated immediately; do not delay treatment to await results of confirmatory tests or because of the absence of a rash.
- Primary methods of prevention of tick-borne disease include repellents, protective clothing, vector control, and prompt removal of the tick.
Botanical Briefs: Neem Oil (Azadirachta indica)
Commonly known as neem or nimba, Azadirachta indica traditionally has been used as an oil or poultice to lighten skin pigment and reduce joint inflammation. Neem is a drought-resistant evergreen tree with thin serrated leaves, white fragrant flowers, and olivelike fruit (Figure 1). This plant is indigenous to India but also is readily found within tropical and semitropical environments throughout the Middle East, Southeast Asia, North Africa, and Australia.

Traditional Uses
For more than 4000 years, neem leaves, bark, fruit, and seeds have been used in food, insecticide, and herbal medicine cross-culturally in Indian Ayurvedic medicine and across Southeast Asia, particularly in Cambodia, Laos, Thailand, Myanmar, and Vietnam.1-3 Because of its many essential nutrients—oleic acid, palmitic acid, stearic acid, linoleic acid, behenic acid, arachidic acid, and palmitoleic acid—and readily available nature, some ethnic groups include neem in their diet.4 Neem commonly is used as a seasoning in soups and rice, eaten as a cooked vegetable, infused into teas and tonics, and pickled with other spices.5
All parts of the neem tree—both externally and internally—have been utilized in traditional medicine for the treatment of various diseases and ailments. The flowers have been used to treat eye diseases and dyspepsia, the fruit has been employed as an anthelmintic, the seeds and leaves have been used for malaria treatment and insecticide, the stem bark has been used for the treatment of diarrhea, and the root bark has been used for skin diseases and inflammation.6 Neem oil is a yellow-brown bitter substance that often is utilized to treat skin diseases such as psoriasis, eczema, fungal infections, and abscesses.
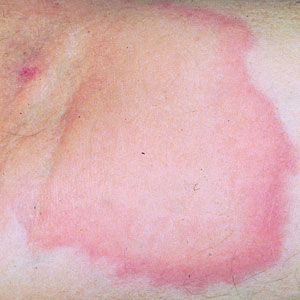
Case Report—A 77-year-old man presented with a diffuse rash across the lower back. He reported that he had been using topical neem oil to alleviate lower back pain and arthritis for the last 6 months with noted relief and improvement of back pain. After roughly 3 to 4 months of using neem oil, he noted a rash on the lower back, bilateral flanks, and buttocks (Figure 2). The rash was asymptomatic, and he denied any pruritus, scaling, pain, or burning. The patient was referred to dermatology and received a diagnosis of chemical leukoderma secondary to contact with A indica. The patient was advised to stop using the topical neem oil, and the rash was simply monitored, as it was asymptomatic.
Bioactivity
Research has elucidated multiple bioactivity mechanisms of neem, including melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.1,7-9 Literature on the diverse phytochemical components of A indica indicate high levels of limonoids, flavonoids, and triterpenoids that are responsible for much of its antioxidant, anti-inflammatory, and insecticide properties.1,10
Melanogenesis-Inhibitory Activity—To date, neem has been added to a number of cosmetic products used in Ayurvedic medicine. One study of isolated compounds in A indica showed superior inhibitory activities against melanogenesis with minimal toxicity to cells (86.5%–105.1% cell viability). Western blot analysis of samples extracted and isolated from neem root and bark showed melanogenesis-inhibitory activities in B16 melanoma cells through the inhibition of microphthalmia-associated transcription factor expression and decreased expression of tyrosinase, as well as tyrosinase-related proteins 1 and 2, which are largely responsible for melanin synthesis.11 In another study, A indica flowers and their extracted constituents—6-deacetylnimbin and kaempferide—suggest melanogenesis-inhibitory activities in B16 melanoma cells with little to no toxicity to the cells (81.0%–111.7% cell viability).1 In an evaluationof A indica seed extracts, some of the isolated limonoids and diterpenoids exhibited a marked melanogenesis-inhibitory effect (74%–91% reduction of melanin content) with no toxicity to the cell.5 All of these studies indicate that active compounds in neem root, bark, flowers, and seeds may be potential skin-lightening agents.
Toxicity Against Pests—Neem seeds have phytochemicals that convey some insecticidal properties. The seeds often are ground into a powder, combined with water, and sprayed onto crops to act as an insecticide. As a natural method of nonpesticidal management, A indica acts as an antifeedant, insect repellent, and egg-laying deterrent that protects crops from damage. Studies of A indica have noted effective nonpesticidal management against arthropod pests such as armyworm, termites, and the oriental fruit fly.7,12,13
Antimalarial Activity—One study indicated that nimbolide, a limonoid from the neem plant, demonstrated antimalarial activity against Plasmodium falciparum. In separate cultures of asexual parasites and mature gametocytes, parasite numbers were less than 50% of the number in control cultures (8.0% vs 8.5% parasitemia, respectively).14 Thus, the lower parasite numbers indicated by this study highlight the antimalarial utility of nimbolide and neem oil.
Antioxidant and Anti-inflammatory Activity—Neem bark has been reported to have considerable antioxidant activity due to its high phenolic content.1,15 One study showed that azadirachtin and nimbolide in neem exhibited concentration-dependent antiradical scavenging activity and antioxidant properties.16
The anti-inflammatory potential for neem may occur via the inhibition of the nuclear factor-κB signaling pathway, which is linked to cancer, inflammation, and apoptosis.17 It also has been observed that nimbidin within neem extracts—such as leaves, bark, and seed extract—suppresses the function of macrophages and neutrophils relevant to inflammation.16 Another study indicated neem’s anti-inflammatory activity due to the regulation of proinflammatory enzymes such as cyclooxygenase and lipoxygenase.18
Safety, Toxicity, and Risks
Ingestion—Although neem is safe to use in the general population, neem oil poisoning has been reported, particularly in young children. Ingesting large quantities of neem has resulted in vomiting, hepatic toxicity, metabolic acidosis, late neurologic sequelae, and encephalopathy in young children.19 The diagnosis of neem oil poisoning is based on patient history, clinical examination, and imaging findings. Poisoning can manifest as drowsiness, tachypnea, and generalized seizures.20
Topical Application—Topical use of neem appears to be safe if the substance is diluted with other ingredients. However, direct application to the skin is not advised, as it may cause leukoderma and could induce allergic contact dermatitis and other allergic reactions.4
Final Thoughts
The use of neem extract for disease prevention and treatment has been prevalent around the world since ancient times. Neem has been documented to possess melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity by means of tyrosinase inhibition, phytochemical production, limonoid expression, and nuclear factor-κB regulation, respectively. However, topical use of neem may trigger a cutaneous response, highlighting the importance of considering a diagnosis of neem oil–induced chemical leukoderma when patients present with a hypopigmented rash and relevant history.
- Kitdamrongtham W, Ishii K, Ebina K, et al. Limonoids and flavonoids from the flowers of Azadirachta indica var. siamensis, and their melanogenesis-inhibitory and cytotoxic activities. Chem Biodivers. 2014;11:73-84. doi:10.1002/cbdv.201300266
- Singh A, Srivastava PS, Lakshmikumaran M. Comparison of AFLP and SAMPL markers for assessment of intra-population genetic variation in Azadirachta indica A. Juss. Plant Sci. 2002;162:17-25. doi:10.1016/S0168-9452(01)00503-9
- Pandey G, Verma K, Singh M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta Indica (neem) leaves. Int J Pharm Pharmaceut Sci. 2014;6:444-447.
- Romita P, Calogiuri G, Bellino M, et al. Allergic contact dermatitis caused by neem oil: an underrated allergen. Contact Dermatitis. 2019;81:133-134. doi:10.1111/cod. 13256
- Akihisa T, Noto T, Takahashi A, et al. Melanogenesis inhibitory, anti-inflammatory, and chemopreventive effects of limonoids from the seeds of Azadirachta indica A. Juss. (neem). J Oleo Sci. 2009;58:581-594.
- Subapriya R, Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anticancer Agents. 2005;5:149-156. doi:10.2174/1568011053174828
- Areekul S, Sinchaisri P, Tigvatananon S. Effect of Thai plant extracts on the Oriental fruit fly. I: toxicity test. Agriculture and Natural Resources. 1987;21:395-407.
- Rochanakij S, Thebtaranonth Y, Yenjai C, et al. Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture. Southeast Asian J Trop Med Public Health. 1985;16:66-72.
- Sithisarn P, Supabphol R, Gritsanapan W. Antioxidant activity of Siamese neem tree (VP1209). J Ethnopharmacol. 2005;99:109-112. doi:10.1016/j.jep.2005.02.008
- Yin F, Lei XX, Cheng L, et al. Isolation and structure identification of the compounds from the seeds and leaves of Azadirachta indica A. Juss. J China Pharmaceut University. 2005;36:10-12.
- Su S, Cheng J, Zhang C, et al. Melanogenesis-inhibitory activities of limonoids and tricyclic diterpenoids from Azadirachta indica. Bioorganic Chemistry. 2020;100:103941. doi:j.bioorg.2020.103941
- Tulashie SK, Adjei F, Abraham J, et al. Potential of neem extracts as natural insecticide against fall armyworm (Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Case Stud Chem Environ Eng. 2021;4:100130. doi:10.1016/j.cscee.2021.100130
- Yashroy RC, Gupta PK. Neem-seed oil inhibits growth of termite surface-tunnels. Indian J Toxicol. 2000;7:49-50.
- Udeinya JI, Shu EN, Quakyi I, et al. An antimalarial neem leaf extract has both schizonticidal and gametocytocidal activities. Am J Therapeutics. 2008;15:108-110. doi:10.1097/MJT.0b013e31804c6d1d
- Bindurani R, Kumar K. Evaluation of antioxidant activity of hydro distilled extracts of leaf, heart wood and flower of Azadirachta indica. Int J Pharm Sci Rev Res. 2013;20:222.
- Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment [published online March 1, 2016]. Evid Based Complement Alternat Med. doi:10.1155/2016/7382506
- Schumacher M, Cerella C, Reuter S, et al. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011;6:149-160. doi:10.1007/s12263-010-0194-6
- Kaur G, Sarwar Alam M, Athar M. Nimbidin suppresses functions of macrophages and neutrophils: relevance to its anti-inflammatory mechanisms. Phytotherapy Res. 2004;18:419-424. doi:10.1002/ptr.1474
- Dhongade RK, Kavade SG, Damle RS. Neem oil poisoning. Indian Pediatr. 2008;45:56-57.
- Bhaskar MV, Pramod SJ, Jeevika MU, et al. MR imaging findings of neem oil poisoning. Am J Neuroradiol. 2010;31:E60-E61. doi:10.3174/ajnr.A2146
Commonly known as neem or nimba, Azadirachta indica traditionally has been used as an oil or poultice to lighten skin pigment and reduce joint inflammation. Neem is a drought-resistant evergreen tree with thin serrated leaves, white fragrant flowers, and olivelike fruit (Figure 1). This plant is indigenous to India but also is readily found within tropical and semitropical environments throughout the Middle East, Southeast Asia, North Africa, and Australia.

Traditional Uses
For more than 4000 years, neem leaves, bark, fruit, and seeds have been used in food, insecticide, and herbal medicine cross-culturally in Indian Ayurvedic medicine and across Southeast Asia, particularly in Cambodia, Laos, Thailand, Myanmar, and Vietnam.1-3 Because of its many essential nutrients—oleic acid, palmitic acid, stearic acid, linoleic acid, behenic acid, arachidic acid, and palmitoleic acid—and readily available nature, some ethnic groups include neem in their diet.4 Neem commonly is used as a seasoning in soups and rice, eaten as a cooked vegetable, infused into teas and tonics, and pickled with other spices.5
All parts of the neem tree—both externally and internally—have been utilized in traditional medicine for the treatment of various diseases and ailments. The flowers have been used to treat eye diseases and dyspepsia, the fruit has been employed as an anthelmintic, the seeds and leaves have been used for malaria treatment and insecticide, the stem bark has been used for the treatment of diarrhea, and the root bark has been used for skin diseases and inflammation.6 Neem oil is a yellow-brown bitter substance that often is utilized to treat skin diseases such as psoriasis, eczema, fungal infections, and abscesses.
Case Report—A 77-year-old man presented with a diffuse rash across the lower back. He reported that he had been using topical neem oil to alleviate lower back pain and arthritis for the last 6 months with noted relief and improvement of back pain. After roughly 3 to 4 months of using neem oil, he noted a rash on the lower back, bilateral flanks, and buttocks (Figure 2). The rash was asymptomatic, and he denied any pruritus, scaling, pain, or burning. The patient was referred to dermatology and received a diagnosis of chemical leukoderma secondary to contact with A indica. The patient was advised to stop using the topical neem oil, and the rash was simply monitored, as it was asymptomatic.

Bioactivity
Research has elucidated multiple bioactivity mechanisms of neem, including melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.1,7-9 Literature on the diverse phytochemical components of A indica indicate high levels of limonoids, flavonoids, and triterpenoids that are responsible for much of its antioxidant, anti-inflammatory, and insecticide properties.1,10
Melanogenesis-Inhibitory Activity—To date, neem has been added to a number of cosmetic products used in Ayurvedic medicine. One study of isolated compounds in A indica showed superior inhibitory activities against melanogenesis with minimal toxicity to cells (86.5%–105.1% cell viability). Western blot analysis of samples extracted and isolated from neem root and bark showed melanogenesis-inhibitory activities in B16 melanoma cells through the inhibition of microphthalmia-associated transcription factor expression and decreased expression of tyrosinase, as well as tyrosinase-related proteins 1 and 2, which are largely responsible for melanin synthesis.11 In another study, A indica flowers and their extracted constituents—6-deacetylnimbin and kaempferide—suggest melanogenesis-inhibitory activities in B16 melanoma cells with little to no toxicity to the cells (81.0%–111.7% cell viability).1 In an evaluationof A indica seed extracts, some of the isolated limonoids and diterpenoids exhibited a marked melanogenesis-inhibitory effect (74%–91% reduction of melanin content) with no toxicity to the cell.5 All of these studies indicate that active compounds in neem root, bark, flowers, and seeds may be potential skin-lightening agents.
Toxicity Against Pests—Neem seeds have phytochemicals that convey some insecticidal properties. The seeds often are ground into a powder, combined with water, and sprayed onto crops to act as an insecticide. As a natural method of nonpesticidal management, A indica acts as an antifeedant, insect repellent, and egg-laying deterrent that protects crops from damage. Studies of A indica have noted effective nonpesticidal management against arthropod pests such as armyworm, termites, and the oriental fruit fly.7,12,13
Antimalarial Activity—One study indicated that nimbolide, a limonoid from the neem plant, demonstrated antimalarial activity against Plasmodium falciparum. In separate cultures of asexual parasites and mature gametocytes, parasite numbers were less than 50% of the number in control cultures (8.0% vs 8.5% parasitemia, respectively).14 Thus, the lower parasite numbers indicated by this study highlight the antimalarial utility of nimbolide and neem oil.
Antioxidant and Anti-inflammatory Activity—Neem bark has been reported to have considerable antioxidant activity due to its high phenolic content.1,15 One study showed that azadirachtin and nimbolide in neem exhibited concentration-dependent antiradical scavenging activity and antioxidant properties.16
The anti-inflammatory potential for neem may occur via the inhibition of the nuclear factor-κB signaling pathway, which is linked to cancer, inflammation, and apoptosis.17 It also has been observed that nimbidin within neem extracts—such as leaves, bark, and seed extract—suppresses the function of macrophages and neutrophils relevant to inflammation.16 Another study indicated neem’s anti-inflammatory activity due to the regulation of proinflammatory enzymes such as cyclooxygenase and lipoxygenase.18
Safety, Toxicity, and Risks
Ingestion—Although neem is safe to use in the general population, neem oil poisoning has been reported, particularly in young children. Ingesting large quantities of neem has resulted in vomiting, hepatic toxicity, metabolic acidosis, late neurologic sequelae, and encephalopathy in young children.19 The diagnosis of neem oil poisoning is based on patient history, clinical examination, and imaging findings. Poisoning can manifest as drowsiness, tachypnea, and generalized seizures.20
Topical Application—Topical use of neem appears to be safe if the substance is diluted with other ingredients. However, direct application to the skin is not advised, as it may cause leukoderma and could induce allergic contact dermatitis and other allergic reactions.4
Final Thoughts
The use of neem extract for disease prevention and treatment has been prevalent around the world since ancient times. Neem has been documented to possess melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity by means of tyrosinase inhibition, phytochemical production, limonoid expression, and nuclear factor-κB regulation, respectively. However, topical use of neem may trigger a cutaneous response, highlighting the importance of considering a diagnosis of neem oil–induced chemical leukoderma when patients present with a hypopigmented rash and relevant history.
Commonly known as neem or nimba, Azadirachta indica traditionally has been used as an oil or poultice to lighten skin pigment and reduce joint inflammation. Neem is a drought-resistant evergreen tree with thin serrated leaves, white fragrant flowers, and olivelike fruit (Figure 1). This plant is indigenous to India but also is readily found within tropical and semitropical environments throughout the Middle East, Southeast Asia, North Africa, and Australia.

Traditional Uses
For more than 4000 years, neem leaves, bark, fruit, and seeds have been used in food, insecticide, and herbal medicine cross-culturally in Indian Ayurvedic medicine and across Southeast Asia, particularly in Cambodia, Laos, Thailand, Myanmar, and Vietnam.1-3 Because of its many essential nutrients—oleic acid, palmitic acid, stearic acid, linoleic acid, behenic acid, arachidic acid, and palmitoleic acid—and readily available nature, some ethnic groups include neem in their diet.4 Neem commonly is used as a seasoning in soups and rice, eaten as a cooked vegetable, infused into teas and tonics, and pickled with other spices.5
All parts of the neem tree—both externally and internally—have been utilized in traditional medicine for the treatment of various diseases and ailments. The flowers have been used to treat eye diseases and dyspepsia, the fruit has been employed as an anthelmintic, the seeds and leaves have been used for malaria treatment and insecticide, the stem bark has been used for the treatment of diarrhea, and the root bark has been used for skin diseases and inflammation.6 Neem oil is a yellow-brown bitter substance that often is utilized to treat skin diseases such as psoriasis, eczema, fungal infections, and abscesses.
Case Report—A 77-year-old man presented with a diffuse rash across the lower back. He reported that he had been using topical neem oil to alleviate lower back pain and arthritis for the last 6 months with noted relief and improvement of back pain. After roughly 3 to 4 months of using neem oil, he noted a rash on the lower back, bilateral flanks, and buttocks (Figure 2). The rash was asymptomatic, and he denied any pruritus, scaling, pain, or burning. The patient was referred to dermatology and received a diagnosis of chemical leukoderma secondary to contact with A indica. The patient was advised to stop using the topical neem oil, and the rash was simply monitored, as it was asymptomatic.

Bioactivity
Research has elucidated multiple bioactivity mechanisms of neem, including melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.1,7-9 Literature on the diverse phytochemical components of A indica indicate high levels of limonoids, flavonoids, and triterpenoids that are responsible for much of its antioxidant, anti-inflammatory, and insecticide properties.1,10
Melanogenesis-Inhibitory Activity—To date, neem has been added to a number of cosmetic products used in Ayurvedic medicine. One study of isolated compounds in A indica showed superior inhibitory activities against melanogenesis with minimal toxicity to cells (86.5%–105.1% cell viability). Western blot analysis of samples extracted and isolated from neem root and bark showed melanogenesis-inhibitory activities in B16 melanoma cells through the inhibition of microphthalmia-associated transcription factor expression and decreased expression of tyrosinase, as well as tyrosinase-related proteins 1 and 2, which are largely responsible for melanin synthesis.11 In another study, A indica flowers and their extracted constituents—6-deacetylnimbin and kaempferide—suggest melanogenesis-inhibitory activities in B16 melanoma cells with little to no toxicity to the cells (81.0%–111.7% cell viability).1 In an evaluationof A indica seed extracts, some of the isolated limonoids and diterpenoids exhibited a marked melanogenesis-inhibitory effect (74%–91% reduction of melanin content) with no toxicity to the cell.5 All of these studies indicate that active compounds in neem root, bark, flowers, and seeds may be potential skin-lightening agents.
Toxicity Against Pests—Neem seeds have phytochemicals that convey some insecticidal properties. The seeds often are ground into a powder, combined with water, and sprayed onto crops to act as an insecticide. As a natural method of nonpesticidal management, A indica acts as an antifeedant, insect repellent, and egg-laying deterrent that protects crops from damage. Studies of A indica have noted effective nonpesticidal management against arthropod pests such as armyworm, termites, and the oriental fruit fly.7,12,13
Antimalarial Activity—One study indicated that nimbolide, a limonoid from the neem plant, demonstrated antimalarial activity against Plasmodium falciparum. In separate cultures of asexual parasites and mature gametocytes, parasite numbers were less than 50% of the number in control cultures (8.0% vs 8.5% parasitemia, respectively).14 Thus, the lower parasite numbers indicated by this study highlight the antimalarial utility of nimbolide and neem oil.
Antioxidant and Anti-inflammatory Activity—Neem bark has been reported to have considerable antioxidant activity due to its high phenolic content.1,15 One study showed that azadirachtin and nimbolide in neem exhibited concentration-dependent antiradical scavenging activity and antioxidant properties.16
The anti-inflammatory potential for neem may occur via the inhibition of the nuclear factor-κB signaling pathway, which is linked to cancer, inflammation, and apoptosis.17 It also has been observed that nimbidin within neem extracts—such as leaves, bark, and seed extract—suppresses the function of macrophages and neutrophils relevant to inflammation.16 Another study indicated neem’s anti-inflammatory activity due to the regulation of proinflammatory enzymes such as cyclooxygenase and lipoxygenase.18
Safety, Toxicity, and Risks
Ingestion—Although neem is safe to use in the general population, neem oil poisoning has been reported, particularly in young children. Ingesting large quantities of neem has resulted in vomiting, hepatic toxicity, metabolic acidosis, late neurologic sequelae, and encephalopathy in young children.19 The diagnosis of neem oil poisoning is based on patient history, clinical examination, and imaging findings. Poisoning can manifest as drowsiness, tachypnea, and generalized seizures.20
Topical Application—Topical use of neem appears to be safe if the substance is diluted with other ingredients. However, direct application to the skin is not advised, as it may cause leukoderma and could induce allergic contact dermatitis and other allergic reactions.4
Final Thoughts
The use of neem extract for disease prevention and treatment has been prevalent around the world since ancient times. Neem has been documented to possess melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity by means of tyrosinase inhibition, phytochemical production, limonoid expression, and nuclear factor-κB regulation, respectively. However, topical use of neem may trigger a cutaneous response, highlighting the importance of considering a diagnosis of neem oil–induced chemical leukoderma when patients present with a hypopigmented rash and relevant history.
- Kitdamrongtham W, Ishii K, Ebina K, et al. Limonoids and flavonoids from the flowers of Azadirachta indica var. siamensis, and their melanogenesis-inhibitory and cytotoxic activities. Chem Biodivers. 2014;11:73-84. doi:10.1002/cbdv.201300266
- Singh A, Srivastava PS, Lakshmikumaran M. Comparison of AFLP and SAMPL markers for assessment of intra-population genetic variation in Azadirachta indica A. Juss. Plant Sci. 2002;162:17-25. doi:10.1016/S0168-9452(01)00503-9
- Pandey G, Verma K, Singh M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta Indica (neem) leaves. Int J Pharm Pharmaceut Sci. 2014;6:444-447.
- Romita P, Calogiuri G, Bellino M, et al. Allergic contact dermatitis caused by neem oil: an underrated allergen. Contact Dermatitis. 2019;81:133-134. doi:10.1111/cod. 13256
- Akihisa T, Noto T, Takahashi A, et al. Melanogenesis inhibitory, anti-inflammatory, and chemopreventive effects of limonoids from the seeds of Azadirachta indica A. Juss. (neem). J Oleo Sci. 2009;58:581-594.
- Subapriya R, Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anticancer Agents. 2005;5:149-156. doi:10.2174/1568011053174828
- Areekul S, Sinchaisri P, Tigvatananon S. Effect of Thai plant extracts on the Oriental fruit fly. I: toxicity test. Agriculture and Natural Resources. 1987;21:395-407.
- Rochanakij S, Thebtaranonth Y, Yenjai C, et al. Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture. Southeast Asian J Trop Med Public Health. 1985;16:66-72.
- Sithisarn P, Supabphol R, Gritsanapan W. Antioxidant activity of Siamese neem tree (VP1209). J Ethnopharmacol. 2005;99:109-112. doi:10.1016/j.jep.2005.02.008
- Yin F, Lei XX, Cheng L, et al. Isolation and structure identification of the compounds from the seeds and leaves of Azadirachta indica A. Juss. J China Pharmaceut University. 2005;36:10-12.
- Su S, Cheng J, Zhang C, et al. Melanogenesis-inhibitory activities of limonoids and tricyclic diterpenoids from Azadirachta indica. Bioorganic Chemistry. 2020;100:103941. doi:j.bioorg.2020.103941
- Tulashie SK, Adjei F, Abraham J, et al. Potential of neem extracts as natural insecticide against fall armyworm (Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Case Stud Chem Environ Eng. 2021;4:100130. doi:10.1016/j.cscee.2021.100130
- Yashroy RC, Gupta PK. Neem-seed oil inhibits growth of termite surface-tunnels. Indian J Toxicol. 2000;7:49-50.
- Udeinya JI, Shu EN, Quakyi I, et al. An antimalarial neem leaf extract has both schizonticidal and gametocytocidal activities. Am J Therapeutics. 2008;15:108-110. doi:10.1097/MJT.0b013e31804c6d1d
- Bindurani R, Kumar K. Evaluation of antioxidant activity of hydro distilled extracts of leaf, heart wood and flower of Azadirachta indica. Int J Pharm Sci Rev Res. 2013;20:222.
- Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment [published online March 1, 2016]. Evid Based Complement Alternat Med. doi:10.1155/2016/7382506
- Schumacher M, Cerella C, Reuter S, et al. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011;6:149-160. doi:10.1007/s12263-010-0194-6
- Kaur G, Sarwar Alam M, Athar M. Nimbidin suppresses functions of macrophages and neutrophils: relevance to its anti-inflammatory mechanisms. Phytotherapy Res. 2004;18:419-424. doi:10.1002/ptr.1474
- Dhongade RK, Kavade SG, Damle RS. Neem oil poisoning. Indian Pediatr. 2008;45:56-57.
- Bhaskar MV, Pramod SJ, Jeevika MU, et al. MR imaging findings of neem oil poisoning. Am J Neuroradiol. 2010;31:E60-E61. doi:10.3174/ajnr.A2146
- Kitdamrongtham W, Ishii K, Ebina K, et al. Limonoids and flavonoids from the flowers of Azadirachta indica var. siamensis, and their melanogenesis-inhibitory and cytotoxic activities. Chem Biodivers. 2014;11:73-84. doi:10.1002/cbdv.201300266
- Singh A, Srivastava PS, Lakshmikumaran M. Comparison of AFLP and SAMPL markers for assessment of intra-population genetic variation in Azadirachta indica A. Juss. Plant Sci. 2002;162:17-25. doi:10.1016/S0168-9452(01)00503-9
- Pandey G, Verma K, Singh M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta Indica (neem) leaves. Int J Pharm Pharmaceut Sci. 2014;6:444-447.
- Romita P, Calogiuri G, Bellino M, et al. Allergic contact dermatitis caused by neem oil: an underrated allergen. Contact Dermatitis. 2019;81:133-134. doi:10.1111/cod. 13256
- Akihisa T, Noto T, Takahashi A, et al. Melanogenesis inhibitory, anti-inflammatory, and chemopreventive effects of limonoids from the seeds of Azadirachta indica A. Juss. (neem). J Oleo Sci. 2009;58:581-594.
- Subapriya R, Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anticancer Agents. 2005;5:149-156. doi:10.2174/1568011053174828
- Areekul S, Sinchaisri P, Tigvatananon S. Effect of Thai plant extracts on the Oriental fruit fly. I: toxicity test. Agriculture and Natural Resources. 1987;21:395-407.
- Rochanakij S, Thebtaranonth Y, Yenjai C, et al. Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture. Southeast Asian J Trop Med Public Health. 1985;16:66-72.
- Sithisarn P, Supabphol R, Gritsanapan W. Antioxidant activity of Siamese neem tree (VP1209). J Ethnopharmacol. 2005;99:109-112. doi:10.1016/j.jep.2005.02.008
- Yin F, Lei XX, Cheng L, et al. Isolation and structure identification of the compounds from the seeds and leaves of Azadirachta indica A. Juss. J China Pharmaceut University. 2005;36:10-12.
- Su S, Cheng J, Zhang C, et al. Melanogenesis-inhibitory activities of limonoids and tricyclic diterpenoids from Azadirachta indica. Bioorganic Chemistry. 2020;100:103941. doi:j.bioorg.2020.103941
- Tulashie SK, Adjei F, Abraham J, et al. Potential of neem extracts as natural insecticide against fall armyworm (Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Case Stud Chem Environ Eng. 2021;4:100130. doi:10.1016/j.cscee.2021.100130
- Yashroy RC, Gupta PK. Neem-seed oil inhibits growth of termite surface-tunnels. Indian J Toxicol. 2000;7:49-50.
- Udeinya JI, Shu EN, Quakyi I, et al. An antimalarial neem leaf extract has both schizonticidal and gametocytocidal activities. Am J Therapeutics. 2008;15:108-110. doi:10.1097/MJT.0b013e31804c6d1d
- Bindurani R, Kumar K. Evaluation of antioxidant activity of hydro distilled extracts of leaf, heart wood and flower of Azadirachta indica. Int J Pharm Sci Rev Res. 2013;20:222.
- Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment [published online March 1, 2016]. Evid Based Complement Alternat Med. doi:10.1155/2016/7382506
- Schumacher M, Cerella C, Reuter S, et al. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011;6:149-160. doi:10.1007/s12263-010-0194-6
- Kaur G, Sarwar Alam M, Athar M. Nimbidin suppresses functions of macrophages and neutrophils: relevance to its anti-inflammatory mechanisms. Phytotherapy Res. 2004;18:419-424. doi:10.1002/ptr.1474
- Dhongade RK, Kavade SG, Damle RS. Neem oil poisoning. Indian Pediatr. 2008;45:56-57.
- Bhaskar MV, Pramod SJ, Jeevika MU, et al. MR imaging findings of neem oil poisoning. Am J Neuroradiol. 2010;31:E60-E61. doi:10.3174/ajnr.A2146
Practice Points
- Neem is a traditional herb with various bioactivities, such as melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.
- Neem should be used with caution as a remedy because of its skin-lightening properties, which are attributed to melanogenesis-inhibitory activity via tyrosinase inhibition.
- Chemical leukoderma should be included in the differential diagnosis when a patient presents with a hypopigmented rash after topical use of neem products.
Botanical Briefs: Contact Dermatitis Induced by Western Poison Ivy (Toxicodendron rydbergii)
Clinical Importance
Western poison ivy (Toxicodendron rydbergii) is responsible for many of the cases of Toxicodendron contact dermatitis (TCD) reported in the western and northern United States. Toxicodendron plants cause more cases of allergic contact dermatitis (ACD) in North America than any other allergen1; 9 million Americans present to physician offices and 1.6 million present to emergency departments annually for ACD, emphasizing the notable medical burden of this condition.2,3 Exposure to urushiol, a plant resin containing potent allergens, precipitates this form of ACD.
An estimated 50% to 75% of adults in the United States demonstrate clinical sensitivity and exhibit ACD following contact with T rydbergii.4 Campers, hikers, firefighters, and forest workers often risk increased exposure through physical contact or aerosolized allergens in smoke. According to the Centers for Disease Control and Prevention, the incidence of visits to US emergency departments for TCD nearly doubled from 2002 to 2012,5 which may be explained by atmospheric CO2 levels that both promote increased growth of Toxicodendron species and augment their toxicity.6
Cutaneous Manifestations
The clinical presentation of T rydbergii contact dermatitis is similar to other allergenic members of the Toxicodendron genus. Patients sensitive to urushiol typically develop a pruritic erythematous rash within 1 to 2 days of exposure (range, 5 hours to 15 days).7 Erythematous and edematous streaks initially manifest on the extremities and often progress to bullae and oozing papulovesicles. In early disease, patients also may display black lesions on or near the rash8 (so-called black-dot dermatitis) caused by oxidized urushiol deposited on the skin—an uncommon yet classic presentation of TCD. Generally, symptoms resolve without complications and with few sequalae, though hyperpigmentation or a secondary infection can develop on or near affected areas.9,10
Taxonomy
The Toxicodendron genus belongs to the Anacardiaceae family, which includes pistachios, mangos, and cashews, and causes more cases of ACD than every other plant combined.4 (Shelled pistachios and cashews do not possess cross-reacting allergens and should not worry consumers; mango skin does contain urushiol.)
Toxicodendron (formerly part of the Rhus genus) includes several species of poison oak, poison ivy, and poison sumac and can be found in shrubs (T rydbergii and Toxicodendron diversilobum), vines (Toxicodendron radicans and Toxicodendron pubescens), and trees (Toxicodendron vernix). In addition, Toxicodendron taxa can hybridize with other taxa in close geographic proximity to form morphologic intermediates. Some individual plants have features of multiple species.11
Etymology
The common name of T rydbergii—western poison ivy—misleads the public; the plant contains no poison that can cause death and does not grow as ivy by wrapping around trees, as T radicans and English ivy (Hedera helix) do. Its formal genus, Toxicodendron, means “poison tree” in Greek and was given its generic name by the English botanist Phillip Miller in 1768,12 which caused the renaming of Rhus rydbergii as T rydbergii. The species name honors Per Axel Rydberg, a 19th and 20th century Swedish-American botanist.
Distribution
Toxicodendron rydbergii grows in California and other states in the western half of the United States as well as the states bordering Canada and Mexico. In Canada, it reigns as the most dominant form of poison ivy.13 Hikers and campers find T rydbergii in a variety of areas, including roadsides, river bottoms, sandy shores, talus slopes, precipices, and floodplains.11 This taxon grows under a variety of conditions and in distinct regions, and it thrives in both full sun or shade.
Identifying Features
Toxicodendron rydbergii turns red earlier than most plants; early red summer leaves should serve as a warning sign to hikers from a distance (Figure 1). It displays trifoliate ovate leaves (ie, each leaf contains 3 leaflets) on a dwarf nonclimbing shrub (Figure 2). Although the plant shares common features with its cousin T radicans (eastern poison ivy), T rydbergii is easily distinguished by its thicker stems, absence of aerial rootlets (abundant in T radicans), and short (approximately 1 meter) height.4

Curly hairs occupy the underside of T rydbergii leaflets and along the midrib; leaflet margins appear lobed or rounded. Lenticels appear as small holes in the bark that turn gray in the cold and become brighter come spring.13

The plant bears glabrous long petioles (leaf stems) and densely grouped clusters of yellow flowers. In autumn, the globose fruit—formed in clusters between each twig and leaf petiole (known as an axillary position)—change from yellow-green to tan (Figure 3). When urushiol exudes from damaged leaflets or other plant parts, it oxidizes on exposure to air and creates hardened black deposits on the plant. Even when grown in garden pots, T rydbergii maintains its distinguishing features.11

Dermatitis-Inducing Plant Parts
All parts of T rydbergii including leaves, stems, roots, and fruit contain the allergenic sap throughout the year.14 A person must damage or bruise the plant for urushiol to be released and produce its allergenic effects; softly brushing against undamaged plants typically does not induce dermatitis.4
Pathophysiology of Urushiol
Urushiol, a pale yellow, oily mixture of organic compounds conserved throughout all Toxicodendron species, contains highly allergenic alkyl catechols. These catechols possess hydroxyl groups at positions 1 and 2 on a benzene ring; the hydrocarbon side chain of poison ivies (typically 15–carbon atoms long) attaches at position 3.15 The catechols and the aliphatic side chain contribute to the plant’s antigenic and dermatitis-inducing properties.16
The high lipophilicity of urushiol allows for rapid and unforgiving absorption into the skin, notwithstanding attempts to wash it off. Upon direct contact, catechols of urushiol penetrate the epidermis and become oxidized to quinone intermediates that bind to antigen-presenting cells in the epidermis and dermis. Epidermal Langerhans cells and dermal macrophages internalize and present the antigen to CD4+ T cells in nearby lymph nodes. This sequence results in production of inflammatory mediators, clonal expansion of T-effector and T-memory cells specific to the allergenic catechols, and an ensuing cytotoxic response against epidermal cells and the dermal vasculature. Keratinocytes and monocytes mediate the inflammatory response by releasing other cytokines.4,17
Sensitization to urushiol generally occurs at 8 to 14 years of age; therefore, infants have lower susceptibility to dermatitis upon contact with T rydbergii.18 Most animals do not experience sensitization upon contact; in fact, birds and forest animals consume the urushiol-rich fruit of T rydbergii without harm.3
Prevention and Treatment
Toxicodendron dermatitis typically lasts 1 to 3 weeks but can remain for as long as 6 weeks without treatment.19 Recognition and physical avoidance of the plant provides the most promising preventive strategy. Immediate rinsing with soap and water can prevent TCD by breaking down urushiol and its allergenic components; however, this is an option for only a short time, as the skin absorbs 50% of urushiol within 10 minutes after contact.20 Nevertheless, patients must seize the earliest opportunity to wash off the affected area and remove any residual urushiol. Patients must be cautious when removing and washing clothing to prevent further contact.
Most health care providers treat TCD with a corticosteroid to reduce inflammation and intense pruritus. A high-potency topical corticosteroid (eg, clobetasol) may prove effective in providing early therapeutic relief in mild disease.21 A short course of a systemic steroid quickly and effectively quenches intense itching and should not be limited to what the clinician considers severe disease. Do not underestimate the patient’s symptoms with this eruption.
Prednisone dosing begins at 1 mg/kg daily and is then tapered slowly over 2 weeks (no shorter a time) for an optimal treatment course of 15 days.22 Prescribing an inadequate dosage and course of a corticosteroid leaves the patient susceptible to rebound dermatitis—and loss of trust in their provider.
Intramuscular injection of the long-acting corticosteroid triamcinolone acetonide with rapid-onset betamethasone provides rapid relief and fewer adverse effects than an oral corticosteroid.22 Despite the long-standing use of sedating oral antihistamines by clinicians, these drugs provide no benefit for pruritus or sleep because the histamine does not cause the itching of TCD, and antihistamines disrupt normal sleep architecture.23-25
Patients can consider several over-the-counter products that have varying degrees of efficacy.4,26 The few products for which prospective studies support their use include Tecnu (Tec Laboraties Inc), Zanfel (RhusTox), and the well-known soaps Dial (Henkel Corporation) and Goop (Critzas Industries, Inc).27,28
Aside from treating the direct effects of TCD, clinicians also must take note of any look for signs of secondary infection and occasionally should consider supplementing treatment with an antibiotic.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed December 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK557866/
- The Lewin Group. The Burden of Skin Diseases 2005. Society for Investigative Dermatology and American Academy of Dermatology Association; 2005:37-40. Accessed December 26, 2023. https://www.lewin.com/content/dam/Lewin/Resources/Site_Sections/Publications/april2005skindisease.pdf
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 Suppl 1):S29-S34.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fretwell S. Poison ivy cases on the rise. The State. Updated May 15,2017. Accessed December 26, 2023. https://www.thestate.com/news/local/article150403932.html
- Mohan JE, Ziska LH, Schlesinger WH, et al. Biomass and toxicity responses of poison ivy (Toxicodendron radicans) to elevated atmospheric CO2. Proc Natl Acad Sci U S A. 2006;103:9086-9089. doi:10.1073/pnas.0602392103
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249. doi:10.1067/mjd.2001.114295
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Gillis WT. The systematics and ecology of poison-ivy and the poison-oaks (Toxicodendron, Anacardiaceae). Rhodora. 1971;73:370-443.
- Reveal JL. Typification of six Philip Miller names of temperate North American Toxicodendron (Anacardiaceae) with proposals (999-1000) to reject T. crenatum and T. volubile. TAXON. 1991;40:333-335. doi:10.2307/1222994
- Guin JD, Gillis WT, Beaman JH. Recognizing the Toxicodendrons (poison ivy, poison oak, and poison sumac). J Am Acad Dermatol. 1981;4:99-114. doi:10.1016/s0190-9622(81)70014-8
- Lee NP, Arriola ER. Poison ivy, oak, and sumac dermatitis. West J Med. 1999;171:354-355.
- Marks JG Jr, Anderson BE, DeLeo VA, eds. Contact and Occupational Dermatology. Jaypee Brothers Medical Publishers Ltd; 2016.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Kalish RS. Recent developments in the pathogenesis of allergic contact dermatitis. Arch Dermatol. 1991;127:1558-1563.
- Fisher AA, Mitchell J. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. 4th ed. Williams & Wilkins; 1995:461-523.
- Labib A, Yosipovitch G. Itchy Toxicodendron plant dermatitis. Allergies. 2022;2:16-22. doi:10.3390/allergies2010002
- Fisher AA. Poison ivy/oak dermatitis part I: prevention—soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated October 16, 2023. Accessed December 26, 2023. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis
- Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135:1522-1525. doi:10.1001/archderm.135.12.1522
- He A, Feldman SR, Fleischer AB Jr. An assessment of the use of antihistamines in the management of atopic dermatitis. J Am Acad Dermatol. 2018;79:92-96. doi:10.1016/j.jaad.2017.12.077
- van Zuuren EJ, Apfelbacher CJ, Fedorowicz Z, et al. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst Rev. 2014;3:25. doi:10.1186/2046-4053-3-25
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2019;81:E25. doi:10.1016/j.jaad.2017.12.081
- Stibich AS, Yagan M, Sharma V, et al. Cost-effective post-exposure prevention of poison ivy dermatitis. Int J Dermatol. 2000;39:515-518. doi:10.1046/j.1365-4362.2000.00003.x
- Davila A, Laurora M, Fulton J, et al. A new topical agent, Zanfel, ameliorates urushiol-induced Toxicodendron allergic contact dermatitis [abstract]. Ann Emerg Med. 2003;42:S98.
Clinical Importance
Western poison ivy (Toxicodendron rydbergii) is responsible for many of the cases of Toxicodendron contact dermatitis (TCD) reported in the western and northern United States. Toxicodendron plants cause more cases of allergic contact dermatitis (ACD) in North America than any other allergen1; 9 million Americans present to physician offices and 1.6 million present to emergency departments annually for ACD, emphasizing the notable medical burden of this condition.2,3 Exposure to urushiol, a plant resin containing potent allergens, precipitates this form of ACD.
An estimated 50% to 75% of adults in the United States demonstrate clinical sensitivity and exhibit ACD following contact with T rydbergii.4 Campers, hikers, firefighters, and forest workers often risk increased exposure through physical contact or aerosolized allergens in smoke. According to the Centers for Disease Control and Prevention, the incidence of visits to US emergency departments for TCD nearly doubled from 2002 to 2012,5 which may be explained by atmospheric CO2 levels that both promote increased growth of Toxicodendron species and augment their toxicity.6
Cutaneous Manifestations
The clinical presentation of T rydbergii contact dermatitis is similar to other allergenic members of the Toxicodendron genus. Patients sensitive to urushiol typically develop a pruritic erythematous rash within 1 to 2 days of exposure (range, 5 hours to 15 days).7 Erythematous and edematous streaks initially manifest on the extremities and often progress to bullae and oozing papulovesicles. In early disease, patients also may display black lesions on or near the rash8 (so-called black-dot dermatitis) caused by oxidized urushiol deposited on the skin—an uncommon yet classic presentation of TCD. Generally, symptoms resolve without complications and with few sequalae, though hyperpigmentation or a secondary infection can develop on or near affected areas.9,10
Taxonomy
The Toxicodendron genus belongs to the Anacardiaceae family, which includes pistachios, mangos, and cashews, and causes more cases of ACD than every other plant combined.4 (Shelled pistachios and cashews do not possess cross-reacting allergens and should not worry consumers; mango skin does contain urushiol.)
Toxicodendron (formerly part of the Rhus genus) includes several species of poison oak, poison ivy, and poison sumac and can be found in shrubs (T rydbergii and Toxicodendron diversilobum), vines (Toxicodendron radicans and Toxicodendron pubescens), and trees (Toxicodendron vernix). In addition, Toxicodendron taxa can hybridize with other taxa in close geographic proximity to form morphologic intermediates. Some individual plants have features of multiple species.11
Etymology
The common name of T rydbergii—western poison ivy—misleads the public; the plant contains no poison that can cause death and does not grow as ivy by wrapping around trees, as T radicans and English ivy (Hedera helix) do. Its formal genus, Toxicodendron, means “poison tree” in Greek and was given its generic name by the English botanist Phillip Miller in 1768,12 which caused the renaming of Rhus rydbergii as T rydbergii. The species name honors Per Axel Rydberg, a 19th and 20th century Swedish-American botanist.
Distribution
Toxicodendron rydbergii grows in California and other states in the western half of the United States as well as the states bordering Canada and Mexico. In Canada, it reigns as the most dominant form of poison ivy.13 Hikers and campers find T rydbergii in a variety of areas, including roadsides, river bottoms, sandy shores, talus slopes, precipices, and floodplains.11 This taxon grows under a variety of conditions and in distinct regions, and it thrives in both full sun or shade.
Identifying Features
Toxicodendron rydbergii turns red earlier than most plants; early red summer leaves should serve as a warning sign to hikers from a distance (Figure 1). It displays trifoliate ovate leaves (ie, each leaf contains 3 leaflets) on a dwarf nonclimbing shrub (Figure 2). Although the plant shares common features with its cousin T radicans (eastern poison ivy), T rydbergii is easily distinguished by its thicker stems, absence of aerial rootlets (abundant in T radicans), and short (approximately 1 meter) height.4

Curly hairs occupy the underside of T rydbergii leaflets and along the midrib; leaflet margins appear lobed or rounded. Lenticels appear as small holes in the bark that turn gray in the cold and become brighter come spring.13

The plant bears glabrous long petioles (leaf stems) and densely grouped clusters of yellow flowers. In autumn, the globose fruit—formed in clusters between each twig and leaf petiole (known as an axillary position)—change from yellow-green to tan (Figure 3). When urushiol exudes from damaged leaflets or other plant parts, it oxidizes on exposure to air and creates hardened black deposits on the plant. Even when grown in garden pots, T rydbergii maintains its distinguishing features.11

Dermatitis-Inducing Plant Parts
All parts of T rydbergii including leaves, stems, roots, and fruit contain the allergenic sap throughout the year.14 A person must damage or bruise the plant for urushiol to be released and produce its allergenic effects; softly brushing against undamaged plants typically does not induce dermatitis.4
Pathophysiology of Urushiol
Urushiol, a pale yellow, oily mixture of organic compounds conserved throughout all Toxicodendron species, contains highly allergenic alkyl catechols. These catechols possess hydroxyl groups at positions 1 and 2 on a benzene ring; the hydrocarbon side chain of poison ivies (typically 15–carbon atoms long) attaches at position 3.15 The catechols and the aliphatic side chain contribute to the plant’s antigenic and dermatitis-inducing properties.16
The high lipophilicity of urushiol allows for rapid and unforgiving absorption into the skin, notwithstanding attempts to wash it off. Upon direct contact, catechols of urushiol penetrate the epidermis and become oxidized to quinone intermediates that bind to antigen-presenting cells in the epidermis and dermis. Epidermal Langerhans cells and dermal macrophages internalize and present the antigen to CD4+ T cells in nearby lymph nodes. This sequence results in production of inflammatory mediators, clonal expansion of T-effector and T-memory cells specific to the allergenic catechols, and an ensuing cytotoxic response against epidermal cells and the dermal vasculature. Keratinocytes and monocytes mediate the inflammatory response by releasing other cytokines.4,17
Sensitization to urushiol generally occurs at 8 to 14 years of age; therefore, infants have lower susceptibility to dermatitis upon contact with T rydbergii.18 Most animals do not experience sensitization upon contact; in fact, birds and forest animals consume the urushiol-rich fruit of T rydbergii without harm.3
Prevention and Treatment
Toxicodendron dermatitis typically lasts 1 to 3 weeks but can remain for as long as 6 weeks without treatment.19 Recognition and physical avoidance of the plant provides the most promising preventive strategy. Immediate rinsing with soap and water can prevent TCD by breaking down urushiol and its allergenic components; however, this is an option for only a short time, as the skin absorbs 50% of urushiol within 10 minutes after contact.20 Nevertheless, patients must seize the earliest opportunity to wash off the affected area and remove any residual urushiol. Patients must be cautious when removing and washing clothing to prevent further contact.
Most health care providers treat TCD with a corticosteroid to reduce inflammation and intense pruritus. A high-potency topical corticosteroid (eg, clobetasol) may prove effective in providing early therapeutic relief in mild disease.21 A short course of a systemic steroid quickly and effectively quenches intense itching and should not be limited to what the clinician considers severe disease. Do not underestimate the patient’s symptoms with this eruption.
Prednisone dosing begins at 1 mg/kg daily and is then tapered slowly over 2 weeks (no shorter a time) for an optimal treatment course of 15 days.22 Prescribing an inadequate dosage and course of a corticosteroid leaves the patient susceptible to rebound dermatitis—and loss of trust in their provider.
Intramuscular injection of the long-acting corticosteroid triamcinolone acetonide with rapid-onset betamethasone provides rapid relief and fewer adverse effects than an oral corticosteroid.22 Despite the long-standing use of sedating oral antihistamines by clinicians, these drugs provide no benefit for pruritus or sleep because the histamine does not cause the itching of TCD, and antihistamines disrupt normal sleep architecture.23-25
Patients can consider several over-the-counter products that have varying degrees of efficacy.4,26 The few products for which prospective studies support their use include Tecnu (Tec Laboraties Inc), Zanfel (RhusTox), and the well-known soaps Dial (Henkel Corporation) and Goop (Critzas Industries, Inc).27,28
Aside from treating the direct effects of TCD, clinicians also must take note of any look for signs of secondary infection and occasionally should consider supplementing treatment with an antibiotic.
Clinical Importance
Western poison ivy (Toxicodendron rydbergii) is responsible for many of the cases of Toxicodendron contact dermatitis (TCD) reported in the western and northern United States. Toxicodendron plants cause more cases of allergic contact dermatitis (ACD) in North America than any other allergen1; 9 million Americans present to physician offices and 1.6 million present to emergency departments annually for ACD, emphasizing the notable medical burden of this condition.2,3 Exposure to urushiol, a plant resin containing potent allergens, precipitates this form of ACD.
An estimated 50% to 75% of adults in the United States demonstrate clinical sensitivity and exhibit ACD following contact with T rydbergii.4 Campers, hikers, firefighters, and forest workers often risk increased exposure through physical contact or aerosolized allergens in smoke. According to the Centers for Disease Control and Prevention, the incidence of visits to US emergency departments for TCD nearly doubled from 2002 to 2012,5 which may be explained by atmospheric CO2 levels that both promote increased growth of Toxicodendron species and augment their toxicity.6
Cutaneous Manifestations
The clinical presentation of T rydbergii contact dermatitis is similar to other allergenic members of the Toxicodendron genus. Patients sensitive to urushiol typically develop a pruritic erythematous rash within 1 to 2 days of exposure (range, 5 hours to 15 days).7 Erythematous and edematous streaks initially manifest on the extremities and often progress to bullae and oozing papulovesicles. In early disease, patients also may display black lesions on or near the rash8 (so-called black-dot dermatitis) caused by oxidized urushiol deposited on the skin—an uncommon yet classic presentation of TCD. Generally, symptoms resolve without complications and with few sequalae, though hyperpigmentation or a secondary infection can develop on or near affected areas.9,10
Taxonomy
The Toxicodendron genus belongs to the Anacardiaceae family, which includes pistachios, mangos, and cashews, and causes more cases of ACD than every other plant combined.4 (Shelled pistachios and cashews do not possess cross-reacting allergens and should not worry consumers; mango skin does contain urushiol.)
Toxicodendron (formerly part of the Rhus genus) includes several species of poison oak, poison ivy, and poison sumac and can be found in shrubs (T rydbergii and Toxicodendron diversilobum), vines (Toxicodendron radicans and Toxicodendron pubescens), and trees (Toxicodendron vernix). In addition, Toxicodendron taxa can hybridize with other taxa in close geographic proximity to form morphologic intermediates. Some individual plants have features of multiple species.11
Etymology
The common name of T rydbergii—western poison ivy—misleads the public; the plant contains no poison that can cause death and does not grow as ivy by wrapping around trees, as T radicans and English ivy (Hedera helix) do. Its formal genus, Toxicodendron, means “poison tree” in Greek and was given its generic name by the English botanist Phillip Miller in 1768,12 which caused the renaming of Rhus rydbergii as T rydbergii. The species name honors Per Axel Rydberg, a 19th and 20th century Swedish-American botanist.
Distribution
Toxicodendron rydbergii grows in California and other states in the western half of the United States as well as the states bordering Canada and Mexico. In Canada, it reigns as the most dominant form of poison ivy.13 Hikers and campers find T rydbergii in a variety of areas, including roadsides, river bottoms, sandy shores, talus slopes, precipices, and floodplains.11 This taxon grows under a variety of conditions and in distinct regions, and it thrives in both full sun or shade.
Identifying Features
Toxicodendron rydbergii turns red earlier than most plants; early red summer leaves should serve as a warning sign to hikers from a distance (Figure 1). It displays trifoliate ovate leaves (ie, each leaf contains 3 leaflets) on a dwarf nonclimbing shrub (Figure 2). Although the plant shares common features with its cousin T radicans (eastern poison ivy), T rydbergii is easily distinguished by its thicker stems, absence of aerial rootlets (abundant in T radicans), and short (approximately 1 meter) height.4

Curly hairs occupy the underside of T rydbergii leaflets and along the midrib; leaflet margins appear lobed or rounded. Lenticels appear as small holes in the bark that turn gray in the cold and become brighter come spring.13

The plant bears glabrous long petioles (leaf stems) and densely grouped clusters of yellow flowers. In autumn, the globose fruit—formed in clusters between each twig and leaf petiole (known as an axillary position)—change from yellow-green to tan (Figure 3). When urushiol exudes from damaged leaflets or other plant parts, it oxidizes on exposure to air and creates hardened black deposits on the plant. Even when grown in garden pots, T rydbergii maintains its distinguishing features.11

Dermatitis-Inducing Plant Parts
All parts of T rydbergii including leaves, stems, roots, and fruit contain the allergenic sap throughout the year.14 A person must damage or bruise the plant for urushiol to be released and produce its allergenic effects; softly brushing against undamaged plants typically does not induce dermatitis.4
Pathophysiology of Urushiol
Urushiol, a pale yellow, oily mixture of organic compounds conserved throughout all Toxicodendron species, contains highly allergenic alkyl catechols. These catechols possess hydroxyl groups at positions 1 and 2 on a benzene ring; the hydrocarbon side chain of poison ivies (typically 15–carbon atoms long) attaches at position 3.15 The catechols and the aliphatic side chain contribute to the plant’s antigenic and dermatitis-inducing properties.16
The high lipophilicity of urushiol allows for rapid and unforgiving absorption into the skin, notwithstanding attempts to wash it off. Upon direct contact, catechols of urushiol penetrate the epidermis and become oxidized to quinone intermediates that bind to antigen-presenting cells in the epidermis and dermis. Epidermal Langerhans cells and dermal macrophages internalize and present the antigen to CD4+ T cells in nearby lymph nodes. This sequence results in production of inflammatory mediators, clonal expansion of T-effector and T-memory cells specific to the allergenic catechols, and an ensuing cytotoxic response against epidermal cells and the dermal vasculature. Keratinocytes and monocytes mediate the inflammatory response by releasing other cytokines.4,17
Sensitization to urushiol generally occurs at 8 to 14 years of age; therefore, infants have lower susceptibility to dermatitis upon contact with T rydbergii.18 Most animals do not experience sensitization upon contact; in fact, birds and forest animals consume the urushiol-rich fruit of T rydbergii without harm.3
Prevention and Treatment
Toxicodendron dermatitis typically lasts 1 to 3 weeks but can remain for as long as 6 weeks without treatment.19 Recognition and physical avoidance of the plant provides the most promising preventive strategy. Immediate rinsing with soap and water can prevent TCD by breaking down urushiol and its allergenic components; however, this is an option for only a short time, as the skin absorbs 50% of urushiol within 10 minutes after contact.20 Nevertheless, patients must seize the earliest opportunity to wash off the affected area and remove any residual urushiol. Patients must be cautious when removing and washing clothing to prevent further contact.
Most health care providers treat TCD with a corticosteroid to reduce inflammation and intense pruritus. A high-potency topical corticosteroid (eg, clobetasol) may prove effective in providing early therapeutic relief in mild disease.21 A short course of a systemic steroid quickly and effectively quenches intense itching and should not be limited to what the clinician considers severe disease. Do not underestimate the patient’s symptoms with this eruption.
Prednisone dosing begins at 1 mg/kg daily and is then tapered slowly over 2 weeks (no shorter a time) for an optimal treatment course of 15 days.22 Prescribing an inadequate dosage and course of a corticosteroid leaves the patient susceptible to rebound dermatitis—and loss of trust in their provider.
Intramuscular injection of the long-acting corticosteroid triamcinolone acetonide with rapid-onset betamethasone provides rapid relief and fewer adverse effects than an oral corticosteroid.22 Despite the long-standing use of sedating oral antihistamines by clinicians, these drugs provide no benefit for pruritus or sleep because the histamine does not cause the itching of TCD, and antihistamines disrupt normal sleep architecture.23-25
Patients can consider several over-the-counter products that have varying degrees of efficacy.4,26 The few products for which prospective studies support their use include Tecnu (Tec Laboraties Inc), Zanfel (RhusTox), and the well-known soaps Dial (Henkel Corporation) and Goop (Critzas Industries, Inc).27,28
Aside from treating the direct effects of TCD, clinicians also must take note of any look for signs of secondary infection and occasionally should consider supplementing treatment with an antibiotic.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed December 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK557866/
- The Lewin Group. The Burden of Skin Diseases 2005. Society for Investigative Dermatology and American Academy of Dermatology Association; 2005:37-40. Accessed December 26, 2023. https://www.lewin.com/content/dam/Lewin/Resources/Site_Sections/Publications/april2005skindisease.pdf
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 Suppl 1):S29-S34.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fretwell S. Poison ivy cases on the rise. The State. Updated May 15,2017. Accessed December 26, 2023. https://www.thestate.com/news/local/article150403932.html
- Mohan JE, Ziska LH, Schlesinger WH, et al. Biomass and toxicity responses of poison ivy (Toxicodendron radicans) to elevated atmospheric CO2. Proc Natl Acad Sci U S A. 2006;103:9086-9089. doi:10.1073/pnas.0602392103
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249. doi:10.1067/mjd.2001.114295
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Gillis WT. The systematics and ecology of poison-ivy and the poison-oaks (Toxicodendron, Anacardiaceae). Rhodora. 1971;73:370-443.
- Reveal JL. Typification of six Philip Miller names of temperate North American Toxicodendron (Anacardiaceae) with proposals (999-1000) to reject T. crenatum and T. volubile. TAXON. 1991;40:333-335. doi:10.2307/1222994
- Guin JD, Gillis WT, Beaman JH. Recognizing the Toxicodendrons (poison ivy, poison oak, and poison sumac). J Am Acad Dermatol. 1981;4:99-114. doi:10.1016/s0190-9622(81)70014-8
- Lee NP, Arriola ER. Poison ivy, oak, and sumac dermatitis. West J Med. 1999;171:354-355.
- Marks JG Jr, Anderson BE, DeLeo VA, eds. Contact and Occupational Dermatology. Jaypee Brothers Medical Publishers Ltd; 2016.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Kalish RS. Recent developments in the pathogenesis of allergic contact dermatitis. Arch Dermatol. 1991;127:1558-1563.
- Fisher AA, Mitchell J. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. 4th ed. Williams & Wilkins; 1995:461-523.
- Labib A, Yosipovitch G. Itchy Toxicodendron plant dermatitis. Allergies. 2022;2:16-22. doi:10.3390/allergies2010002
- Fisher AA. Poison ivy/oak dermatitis part I: prevention—soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated October 16, 2023. Accessed December 26, 2023. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis
- Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135:1522-1525. doi:10.1001/archderm.135.12.1522
- He A, Feldman SR, Fleischer AB Jr. An assessment of the use of antihistamines in the management of atopic dermatitis. J Am Acad Dermatol. 2018;79:92-96. doi:10.1016/j.jaad.2017.12.077
- van Zuuren EJ, Apfelbacher CJ, Fedorowicz Z, et al. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst Rev. 2014;3:25. doi:10.1186/2046-4053-3-25
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2019;81:E25. doi:10.1016/j.jaad.2017.12.081
- Stibich AS, Yagan M, Sharma V, et al. Cost-effective post-exposure prevention of poison ivy dermatitis. Int J Dermatol. 2000;39:515-518. doi:10.1046/j.1365-4362.2000.00003.x
- Davila A, Laurora M, Fulton J, et al. A new topical agent, Zanfel, ameliorates urushiol-induced Toxicodendron allergic contact dermatitis [abstract]. Ann Emerg Med. 2003;42:S98.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed December 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK557866/
- The Lewin Group. The Burden of Skin Diseases 2005. Society for Investigative Dermatology and American Academy of Dermatology Association; 2005:37-40. Accessed December 26, 2023. https://www.lewin.com/content/dam/Lewin/Resources/Site_Sections/Publications/april2005skindisease.pdf
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 Suppl 1):S29-S34.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fretwell S. Poison ivy cases on the rise. The State. Updated May 15,2017. Accessed December 26, 2023. https://www.thestate.com/news/local/article150403932.html
- Mohan JE, Ziska LH, Schlesinger WH, et al. Biomass and toxicity responses of poison ivy (Toxicodendron radicans) to elevated atmospheric CO2. Proc Natl Acad Sci U S A. 2006;103:9086-9089. doi:10.1073/pnas.0602392103
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249. doi:10.1067/mjd.2001.114295
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Gillis WT. The systematics and ecology of poison-ivy and the poison-oaks (Toxicodendron, Anacardiaceae). Rhodora. 1971;73:370-443.
- Reveal JL. Typification of six Philip Miller names of temperate North American Toxicodendron (Anacardiaceae) with proposals (999-1000) to reject T. crenatum and T. volubile. TAXON. 1991;40:333-335. doi:10.2307/1222994
- Guin JD, Gillis WT, Beaman JH. Recognizing the Toxicodendrons (poison ivy, poison oak, and poison sumac). J Am Acad Dermatol. 1981;4:99-114. doi:10.1016/s0190-9622(81)70014-8
- Lee NP, Arriola ER. Poison ivy, oak, and sumac dermatitis. West J Med. 1999;171:354-355.
- Marks JG Jr, Anderson BE, DeLeo VA, eds. Contact and Occupational Dermatology. Jaypee Brothers Medical Publishers Ltd; 2016.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Kalish RS. Recent developments in the pathogenesis of allergic contact dermatitis. Arch Dermatol. 1991;127:1558-1563.
- Fisher AA, Mitchell J. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. 4th ed. Williams & Wilkins; 1995:461-523.
- Labib A, Yosipovitch G. Itchy Toxicodendron plant dermatitis. Allergies. 2022;2:16-22. doi:10.3390/allergies2010002
- Fisher AA. Poison ivy/oak dermatitis part I: prevention—soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated October 16, 2023. Accessed December 26, 2023. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis
- Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135:1522-1525. doi:10.1001/archderm.135.12.1522
- He A, Feldman SR, Fleischer AB Jr. An assessment of the use of antihistamines in the management of atopic dermatitis. J Am Acad Dermatol. 2018;79:92-96. doi:10.1016/j.jaad.2017.12.077
- van Zuuren EJ, Apfelbacher CJ, Fedorowicz Z, et al. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst Rev. 2014;3:25. doi:10.1186/2046-4053-3-25
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2019;81:E25. doi:10.1016/j.jaad.2017.12.081
- Stibich AS, Yagan M, Sharma V, et al. Cost-effective post-exposure prevention of poison ivy dermatitis. Int J Dermatol. 2000;39:515-518. doi:10.1046/j.1365-4362.2000.00003.x
- Davila A, Laurora M, Fulton J, et al. A new topical agent, Zanfel, ameliorates urushiol-induced Toxicodendron allergic contact dermatitis [abstract]. Ann Emerg Med. 2003;42:S98.
PRACTICE POINTS
- Western poison ivy (Toxicodendron rydbergii) accounts for many of the cases of Toxicodendron contact dermatitis (TCD) in the western and northern United States. Individuals in these regions should be educated on how to identify T rydbergii to avoid TCD.
- Dermatologists should include TCD in the differential diagnosis when a patient presents with an erythematous pruritic rash in a linear pattern with sharp borders.
- Most patients who experience intense itching and pain from TCD benefit greatly from prompt treatment with an oral or intramuscular corticosteroid. Topical steroids rarely provide relief; oral antihistamines provide no benefit.
What’s Eating You? Update on the Sticktight Flea (Echidnophaga gallinacea)
Fleas (order Siphonaptera) are vectors for various diseases, such as plague (as carriers of Yersinia pestis) and rickettsial infections.1-4 The sticktight flea (Echidnophaga gallinacea) commonly is seen on birds and mammals, including ground squirrels, dogs, cats, and rodents, and can attach to its host for days at a time by burrowing its head into the skin. Similar to other fleas, the sticktight flea needs a blood supply to reproduce.5 Therefore, it is important to study the sticktight flea, its habitat, and infection patterns to improve public health and prevent infestation.
Identification
Echidnophaga gallinacea is named for the female flea’s behavior—it “sticks tight” to the surface of the host by embedding its head into the skin for days at a time.5 The sticktight flea and the rat flea (Xenopsylla cheopis) can be differentiated by the sticktight’s reduced thorax and lack of a pleural rod (the vertical ridge that divides the mesosternum above the second pair of legs)(Figure, A and B). The sticktight flea can be differentiated from the dog flea (Ctenocephalides canis) and the cat flea (Ctenocephalides felis) by its lack of genal ctenidia (horizontal combs in the mustache area) and pronotal ctenidia (vertical combs behind the head)(Figure, B and C).6,7 Other defining features of E gallinacea include 2 pairs of large postantennal setae (hairs) on its anteriorly flattened head; a C-shaped reproductive organ known as the spermatheca; and broad maxillary lacinia (Figure, C).8
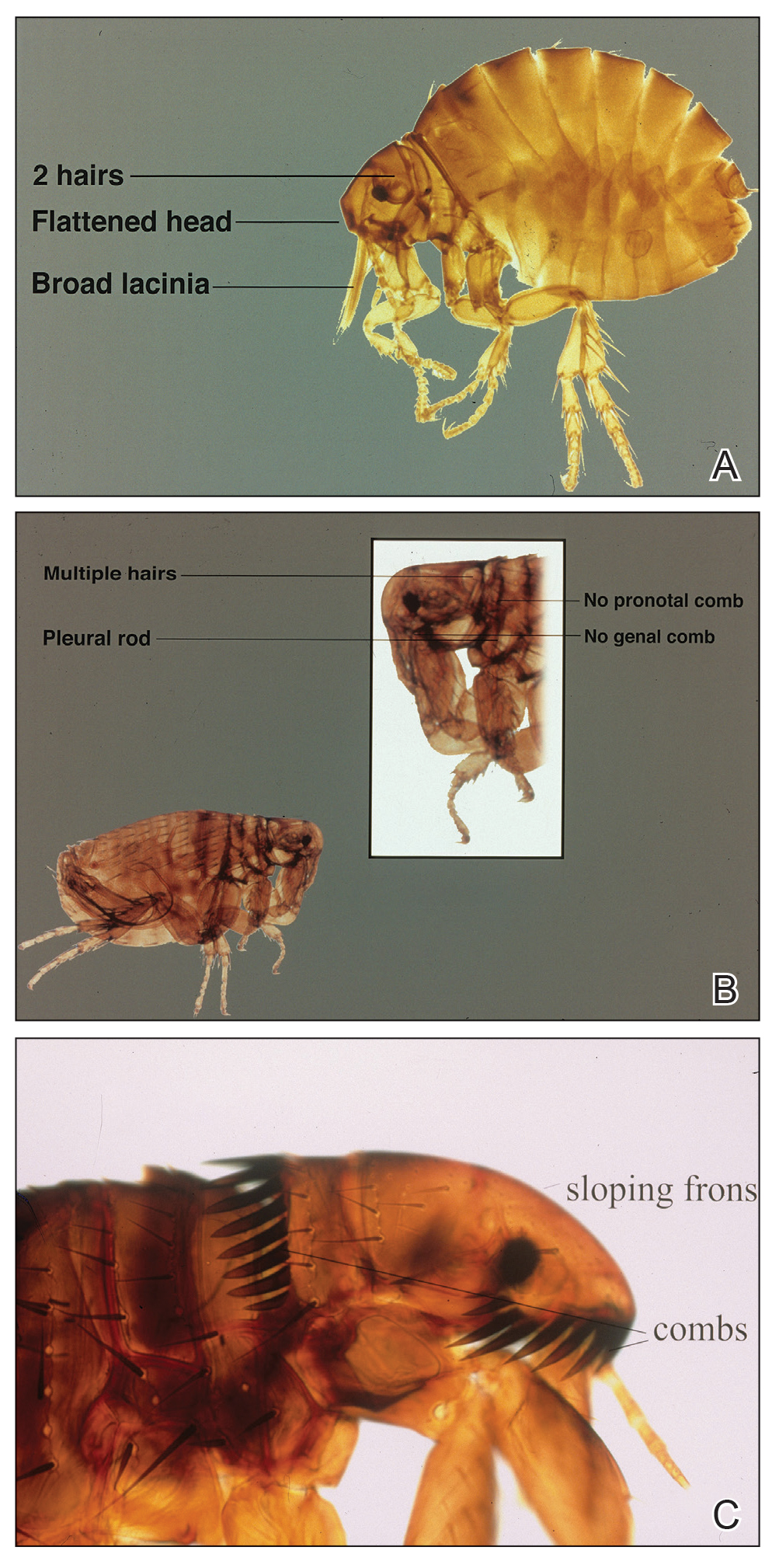
Habitat, Seasonality, and Behavior
Echidnophaga gallinacea commonly infests the comb, wattles, and surrounding ears of chickens; the flea also has been found on dogs, cats, rodents, and other species of birds.9 The sticktight flea is more prevalent in summer and autumn, which may explain its predominance in warmer climates, including California, Florida, Mexico, Egypt, Africa, and Iran.1,9-11
When a female sticktight flea begins to feed, it stays on the host for days at a time, waiting for a male.5 The female deposits its fertilized eggs in nests on the host or in lesions caused by infestation. Eventually, eggs hatch and fall into soil, where they lay dormant or grow to adulthood.5
Cutaneous Reaction to Infestation
Flea bites cause a hypersensitivity reaction, with pruritic pustules and erythematous papules that have a central punctum.12 In a reported case in Los Angeles, California, a female sticktight flea buried itself into the cheek of a young boy for more than 12 hours. The lesion was not marked by surrounding erythema, tenderness, pruritus, or swelling; however, several days after the flea was removed, erythema developed at the site then spontaneously resolved.7 In a study of dogs that were infested with E gallinacea, the flea never disengaged to attach to a human; when the flea was deliberately placed on a human, it fed and left hastily.11
Management
Because E gallinacea burrows its head into the skin, the best removal method is applying slow gentle traction under sterile conditions to ensure removal of mouthparts.7 An oral antihistamine can be administered or a topical antihistamine or corticosteroid can be applied to the affected area.12 Flea infestation should be treated with an insecticide. Affected animals should be treated by a veterinarian using a pesticide, such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, or pyriproxyfen.13
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123. doi:10.1111/j.1948-7134.2011.00148.x
- Jiang J, Maina AN, Knobel DL, et al. Molecular detection of Rickettsia felis and Candidatus Rickettsia asemboensis in fleas from human habitats, Asembo, Kenya. Vector Borne Zoonotic Dis. 2013;13:550-558. doi:10.1089/vbz.2012.1123
- López-Pérez AM, Chaves A, Sánchez-Montes S, et al. Diversity of rickettsiae in domestic, synanthropic, and sylvatic mammals and their ectoparasites in a spotted fever-epidemic region at the western US-Mexico border. Transbound Emerg Dis. 2022;69:609-622. doi:10.1111/tbed.14027
- Ehlers J, Krüger A, Rakotondranary SJ, et al. Molecular detection of Rickettsia spp., Borrelia spp., Bartonella spp. and Yersinia pestis in ectoparasites of endemic and domestic animals in southwest Madagascar. Acta Trop. 2020;205:105339. doi:10.1016/j.actatropica.2020.105339
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948. doi:10.1645/GE-769R.1
- Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676. doi:10.1016/j.ijid.2009.11.011
- Linardi PM, Santos JLC. Ctenocephalides felis felis vs. Ctenocephalides canis (Siphonaptera: Pulicidae): some issues in correctly identify these species. Rev Bras Parasitol Vet. 2012;21:345-354. doi:10.1590/s1984-29612012000400002
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4. https://doi.org/10.5070/D36vb8p1b1
- Mirzaei M, Ghashghaei O, Yakhchali M. Prevalence of ectoparasites of indigenous chickens from Dalahu region, Kermanshah province, Iran. Turkiye Parazitol Derg. 2016;40:13-16. doi:10.5152/tpd.2016.4185
- Farid DS, Sallam NH, Eldein AMS, et al. Cross-sectional seasonal prevalence and relative risk of ectoparasitic infestations of rodents in North Sinai, Egypt. Vet World. 2021;14:2996-3006. doi:10.14202/vetworld.2021.2996-3006
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140. doi:10.1016/0304-4017(87)90031-8
- Anderson J, Paterek E. Flea bites. StatPearls [Internet]. StatPearls Publishing; 2023. Updated August 8, 2023. Accessed November 27, 2023. https://www.ncbi.nlm.nih.gov/books/NBK541118/
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596. doi:10.1638/2007-0062.1
Fleas (order Siphonaptera) are vectors for various diseases, such as plague (as carriers of Yersinia pestis) and rickettsial infections.1-4 The sticktight flea (Echidnophaga gallinacea) commonly is seen on birds and mammals, including ground squirrels, dogs, cats, and rodents, and can attach to its host for days at a time by burrowing its head into the skin. Similar to other fleas, the sticktight flea needs a blood supply to reproduce.5 Therefore, it is important to study the sticktight flea, its habitat, and infection patterns to improve public health and prevent infestation.
Identification
Echidnophaga gallinacea is named for the female flea’s behavior—it “sticks tight” to the surface of the host by embedding its head into the skin for days at a time.5 The sticktight flea and the rat flea (Xenopsylla cheopis) can be differentiated by the sticktight’s reduced thorax and lack of a pleural rod (the vertical ridge that divides the mesosternum above the second pair of legs)(Figure, A and B). The sticktight flea can be differentiated from the dog flea (Ctenocephalides canis) and the cat flea (Ctenocephalides felis) by its lack of genal ctenidia (horizontal combs in the mustache area) and pronotal ctenidia (vertical combs behind the head)(Figure, B and C).6,7 Other defining features of E gallinacea include 2 pairs of large postantennal setae (hairs) on its anteriorly flattened head; a C-shaped reproductive organ known as the spermatheca; and broad maxillary lacinia (Figure, C).8

Habitat, Seasonality, and Behavior
Echidnophaga gallinacea commonly infests the comb, wattles, and surrounding ears of chickens; the flea also has been found on dogs, cats, rodents, and other species of birds.9 The sticktight flea is more prevalent in summer and autumn, which may explain its predominance in warmer climates, including California, Florida, Mexico, Egypt, Africa, and Iran.1,9-11
When a female sticktight flea begins to feed, it stays on the host for days at a time, waiting for a male.5 The female deposits its fertilized eggs in nests on the host or in lesions caused by infestation. Eventually, eggs hatch and fall into soil, where they lay dormant or grow to adulthood.5
Cutaneous Reaction to Infestation
Flea bites cause a hypersensitivity reaction, with pruritic pustules and erythematous papules that have a central punctum.12 In a reported case in Los Angeles, California, a female sticktight flea buried itself into the cheek of a young boy for more than 12 hours. The lesion was not marked by surrounding erythema, tenderness, pruritus, or swelling; however, several days after the flea was removed, erythema developed at the site then spontaneously resolved.7 In a study of dogs that were infested with E gallinacea, the flea never disengaged to attach to a human; when the flea was deliberately placed on a human, it fed and left hastily.11
Management
Because E gallinacea burrows its head into the skin, the best removal method is applying slow gentle traction under sterile conditions to ensure removal of mouthparts.7 An oral antihistamine can be administered or a topical antihistamine or corticosteroid can be applied to the affected area.12 Flea infestation should be treated with an insecticide. Affected animals should be treated by a veterinarian using a pesticide, such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, or pyriproxyfen.13
Fleas (order Siphonaptera) are vectors for various diseases, such as plague (as carriers of Yersinia pestis) and rickettsial infections.1-4 The sticktight flea (Echidnophaga gallinacea) commonly is seen on birds and mammals, including ground squirrels, dogs, cats, and rodents, and can attach to its host for days at a time by burrowing its head into the skin. Similar to other fleas, the sticktight flea needs a blood supply to reproduce.5 Therefore, it is important to study the sticktight flea, its habitat, and infection patterns to improve public health and prevent infestation.
Identification
Echidnophaga gallinacea is named for the female flea’s behavior—it “sticks tight” to the surface of the host by embedding its head into the skin for days at a time.5 The sticktight flea and the rat flea (Xenopsylla cheopis) can be differentiated by the sticktight’s reduced thorax and lack of a pleural rod (the vertical ridge that divides the mesosternum above the second pair of legs)(Figure, A and B). The sticktight flea can be differentiated from the dog flea (Ctenocephalides canis) and the cat flea (Ctenocephalides felis) by its lack of genal ctenidia (horizontal combs in the mustache area) and pronotal ctenidia (vertical combs behind the head)(Figure, B and C).6,7 Other defining features of E gallinacea include 2 pairs of large postantennal setae (hairs) on its anteriorly flattened head; a C-shaped reproductive organ known as the spermatheca; and broad maxillary lacinia (Figure, C).8

Habitat, Seasonality, and Behavior
Echidnophaga gallinacea commonly infests the comb, wattles, and surrounding ears of chickens; the flea also has been found on dogs, cats, rodents, and other species of birds.9 The sticktight flea is more prevalent in summer and autumn, which may explain its predominance in warmer climates, including California, Florida, Mexico, Egypt, Africa, and Iran.1,9-11
When a female sticktight flea begins to feed, it stays on the host for days at a time, waiting for a male.5 The female deposits its fertilized eggs in nests on the host or in lesions caused by infestation. Eventually, eggs hatch and fall into soil, where they lay dormant or grow to adulthood.5
Cutaneous Reaction to Infestation
Flea bites cause a hypersensitivity reaction, with pruritic pustules and erythematous papules that have a central punctum.12 In a reported case in Los Angeles, California, a female sticktight flea buried itself into the cheek of a young boy for more than 12 hours. The lesion was not marked by surrounding erythema, tenderness, pruritus, or swelling; however, several days after the flea was removed, erythema developed at the site then spontaneously resolved.7 In a study of dogs that were infested with E gallinacea, the flea never disengaged to attach to a human; when the flea was deliberately placed on a human, it fed and left hastily.11
Management
Because E gallinacea burrows its head into the skin, the best removal method is applying slow gentle traction under sterile conditions to ensure removal of mouthparts.7 An oral antihistamine can be administered or a topical antihistamine or corticosteroid can be applied to the affected area.12 Flea infestation should be treated with an insecticide. Affected animals should be treated by a veterinarian using a pesticide, such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, or pyriproxyfen.13
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123. doi:10.1111/j.1948-7134.2011.00148.x
- Jiang J, Maina AN, Knobel DL, et al. Molecular detection of Rickettsia felis and Candidatus Rickettsia asemboensis in fleas from human habitats, Asembo, Kenya. Vector Borne Zoonotic Dis. 2013;13:550-558. doi:10.1089/vbz.2012.1123
- López-Pérez AM, Chaves A, Sánchez-Montes S, et al. Diversity of rickettsiae in domestic, synanthropic, and sylvatic mammals and their ectoparasites in a spotted fever-epidemic region at the western US-Mexico border. Transbound Emerg Dis. 2022;69:609-622. doi:10.1111/tbed.14027
- Ehlers J, Krüger A, Rakotondranary SJ, et al. Molecular detection of Rickettsia spp., Borrelia spp., Bartonella spp. and Yersinia pestis in ectoparasites of endemic and domestic animals in southwest Madagascar. Acta Trop. 2020;205:105339. doi:10.1016/j.actatropica.2020.105339
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948. doi:10.1645/GE-769R.1
- Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676. doi:10.1016/j.ijid.2009.11.011
- Linardi PM, Santos JLC. Ctenocephalides felis felis vs. Ctenocephalides canis (Siphonaptera: Pulicidae): some issues in correctly identify these species. Rev Bras Parasitol Vet. 2012;21:345-354. doi:10.1590/s1984-29612012000400002
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4. https://doi.org/10.5070/D36vb8p1b1
- Mirzaei M, Ghashghaei O, Yakhchali M. Prevalence of ectoparasites of indigenous chickens from Dalahu region, Kermanshah province, Iran. Turkiye Parazitol Derg. 2016;40:13-16. doi:10.5152/tpd.2016.4185
- Farid DS, Sallam NH, Eldein AMS, et al. Cross-sectional seasonal prevalence and relative risk of ectoparasitic infestations of rodents in North Sinai, Egypt. Vet World. 2021;14:2996-3006. doi:10.14202/vetworld.2021.2996-3006
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140. doi:10.1016/0304-4017(87)90031-8
- Anderson J, Paterek E. Flea bites. StatPearls [Internet]. StatPearls Publishing; 2023. Updated August 8, 2023. Accessed November 27, 2023. https://www.ncbi.nlm.nih.gov/books/NBK541118/
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596. doi:10.1638/2007-0062.1
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123. doi:10.1111/j.1948-7134.2011.00148.x
- Jiang J, Maina AN, Knobel DL, et al. Molecular detection of Rickettsia felis and Candidatus Rickettsia asemboensis in fleas from human habitats, Asembo, Kenya. Vector Borne Zoonotic Dis. 2013;13:550-558. doi:10.1089/vbz.2012.1123
- López-Pérez AM, Chaves A, Sánchez-Montes S, et al. Diversity of rickettsiae in domestic, synanthropic, and sylvatic mammals and their ectoparasites in a spotted fever-epidemic region at the western US-Mexico border. Transbound Emerg Dis. 2022;69:609-622. doi:10.1111/tbed.14027
- Ehlers J, Krüger A, Rakotondranary SJ, et al. Molecular detection of Rickettsia spp., Borrelia spp., Bartonella spp. and Yersinia pestis in ectoparasites of endemic and domestic animals in southwest Madagascar. Acta Trop. 2020;205:105339. doi:10.1016/j.actatropica.2020.105339
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948. doi:10.1645/GE-769R.1
- Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676. doi:10.1016/j.ijid.2009.11.011
- Linardi PM, Santos JLC. Ctenocephalides felis felis vs. Ctenocephalides canis (Siphonaptera: Pulicidae): some issues in correctly identify these species. Rev Bras Parasitol Vet. 2012;21:345-354. doi:10.1590/s1984-29612012000400002
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4. https://doi.org/10.5070/D36vb8p1b1
- Mirzaei M, Ghashghaei O, Yakhchali M. Prevalence of ectoparasites of indigenous chickens from Dalahu region, Kermanshah province, Iran. Turkiye Parazitol Derg. 2016;40:13-16. doi:10.5152/tpd.2016.4185
- Farid DS, Sallam NH, Eldein AMS, et al. Cross-sectional seasonal prevalence and relative risk of ectoparasitic infestations of rodents in North Sinai, Egypt. Vet World. 2021;14:2996-3006. doi:10.14202/vetworld.2021.2996-3006
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140. doi:10.1016/0304-4017(87)90031-8
- Anderson J, Paterek E. Flea bites. StatPearls [Internet]. StatPearls Publishing; 2023. Updated August 8, 2023. Accessed November 27, 2023. https://www.ncbi.nlm.nih.gov/books/NBK541118/
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596. doi:10.1638/2007-0062.1
Practice Points
- The sticktight flea (Echidnophaga gallinacea) attaches to its host by embedding its head in the skin for days at a time.
- Unlike other fleas that bite and run, the sticktight flea can be identified dermoscopically.
- The sticktight flea serves as a vector for plague as a carrier of Yersinia pestis, rickettsial infections, and other diseases.
Botanical Briefs: Australian Stinging Tree (Dendrocnide moroides)
Clinical Importance
Dendrocnide moroides is arguably the most brutal of stinging plants, even leading to death in dogs, horses, and humans in rare cases.1-3 Commonly called gympie-gympie (based on its discovery by gold miners near the town of Gympie in Queensland, Australia), D moroides also has been referred to as the mulberrylike stinging tree or stinger.2,4-6
Family and Nomenclature
The Australian stinging tree belongs to the family Urticaceae (known as the nettle family) within the order Rosales.1,2,3,5 Urticaceae is derived from the Latin term urere (to burn)—an apt description of the clinical experience of patients with D moroides–induced urticaria.
Urticaceae includes 54 genera, comprising herbs, shrubs, small trees, and vines found predominantly in tropical regions. Dendrocnide comprises approximately 40 species, all commonly known in Australia as stinging trees.2,7,8
Distribution
Dendrocnide moroides is found in the rainforests of Australia and Southeast Asia.2 Because the plant has a strong need for sunlight and wind protection, it typically is found in light-filled gaps within the rainforest, in moist ravines, along the edges of creeks, and on land bordering the rainforest.3,6
Appearance
Although D moroides is referred to as a tree, it is an understory shrub that typically grows to 3 m, with heart-shaped, serrated, dark green leaves that are 50-cm wide (Figure 1).6 The leaves are produced consistently through the year, with variable growth depending on the season.9

The plant is covered in what appears to be soft downy fur made up of trichomes (or plant hairs).1,6 The density of the hairs on leaves decreases as they age.2,9 The fruit, which is actually edible (if one is careful to avoid hairs), appears similar to red to dark purple raspberries growing on long stems.5,6
Cutaneous Manifestations
Symptoms of contact with the stems and leaves of D moroides range from slight irritation to serious neurologic disorders, including neuropathy. The severity of the reaction depends on the person, how much skin was contacted, and how one came into contact with the plant.1,5 Upon touch, there is an immediate reaction, with burning, urticaria, and edema. Pain increases, peaking 30 minutes later; then the pain slowly subsides.1 Tachycardia and throbbing regional lymphadenopathy can occur for 1 to 4 hours.1,6
Cutaneous Findings—Examination reveals immediate piloerection, erythema due to arteriolar dilation, and local swelling.2 These findings may disappear after 1 hour or last as long as 24 hours.1 Although objective signs may fade, subjective pain, pruritus, and burning can persist for months.3
Dermatitis-Inducing Plant Parts
After contact with the stems or leaves, the sharp trichomes become embedded in the skin, making them difficult to remove.1 The toxins are contained in siliceous hairs that the human body cannot break down.3 Symptoms can be experienced for as long as 1 year after contact, especially when the skin is pressed firmly or washed with hot or cold water.3,6 Because the plant’s hairs are shed continuously, being in close proximity to D moroides for longer than 20 minutes can lead to extreme sneezing, nosebleeds, and major respiratory damage from inhaling hairs.1,6,9
The stinging hairs of D moroides differ from irritant hairs on other plants because they contain physiologically active substances. Stinging hairs are classified as either a hypodermic syringe, which expels liquid only, or as a tragia-type syringe, in which liquid and sharp crystals are injected.
The Australian stinging tree falls into the first of these 2 groups (Figure 2)1; the sharp tip of the hair breaks on contact, leading to expulsion of the toxin into skin.1,4 The hairs function as a defense against mammalian herbivores but typically have no impact on pests.1 Nocturnal beetles and on occasion possums and red-legged pademelons dare to eat D moroides.3,6
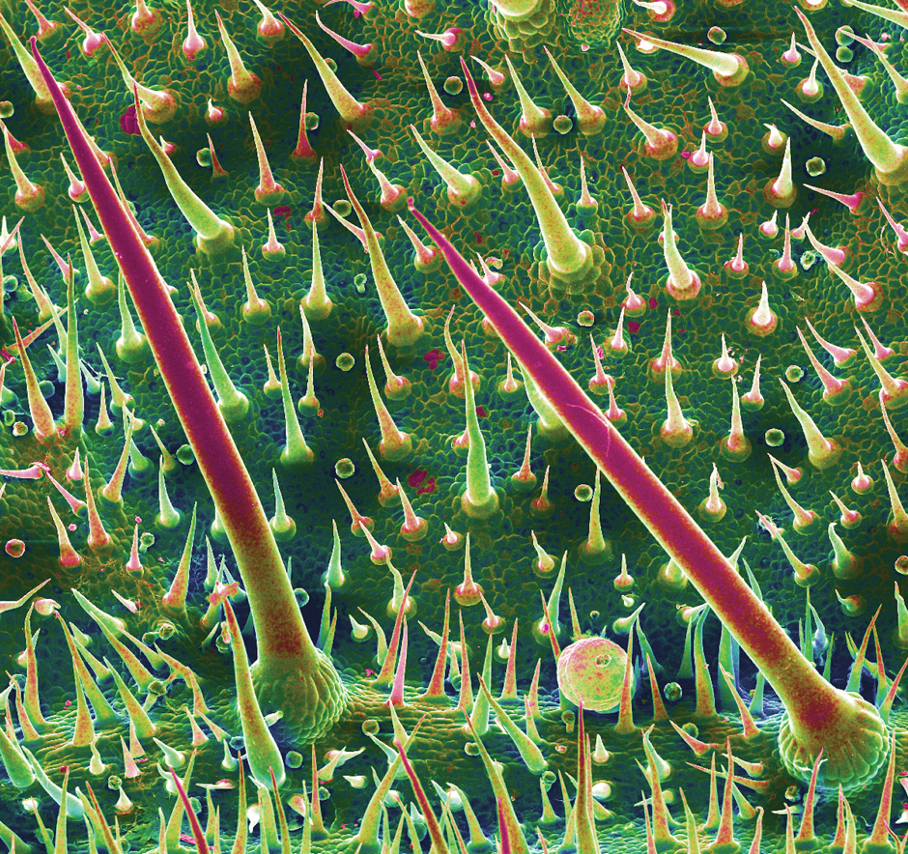
The Irritant
Initially, formic acid was proposed as the irritant chemical in D moroides1; other candidates have included neurotransmitters, such as histamine, acetylcholine, and serotonin, as well as inorganic ions, such as potassium. These compounds may play a role but none explain the persistent sensory effects and years-long stable nature of the toxin.1,4
The most likely culprit irritant is a member of a newly discovered family of neurotoxins, the gympietides. These knot-shaped chemicals, found in D moroides and some spider venoms, have the ability to activate voltage-gated sodium channels of cutaneous neurons and cause local cutaneous vasodilation by stimulating neurotransmitter release.4 These neurotoxins not only generate pain but also suppress the mechanism used to interrupt those pain signals.10 Synthesized gympietides can replicate the effects of natural contact, indicating that they are the primary active toxins. These toxins are ultrastable, thus producing lasting effects.1
Although much is understood about the evolution and distribution of D moroides and the ecological role that it plays, there is still more to learn about the plant’s toxicology.
Prevention and Treatment
Prevention—Dendrocnide moroides dermatitis is best prevented by avoiding contact with the plant and related species, as well as wearing upper body clothing with long sleeves, pants, and boots, though plant hairs can still penetrate garments and sting.2,3
Therapy—There is no reversal therapy of D moroides dermatitis but symptoms can be managed.4 For pain, analgesics, such as opioids, have been used; on occasion, however, pain is so intense that even morphine does not help.4,10
Systemic or topical corticosteroids are the main therapy for many forms of plant-induced dermatitis because they are able to decrease cytokine production and stop lymphocyte production. Adding an oral antihistamine can alleviate histamine-mediated pruritus but not pruritus that is mediated by other chemicals.11
Other methods of relieving symptoms of D moroides dermatitis have been proposed or reported anecdotally. Diluted hydrochloric acid can be applied to the skin to denature remaining toxin.4 The sap of Alocasia brisbanensis (the cunjevoi plant) can be rubbed on affected areas to provide a cooling effect, but do not allow A brisbanensis sap to enter the mouth, as it contains calcium oxalate, a toxic irritant found in dumb cane (Dieffenbachia species). The roots of the Australian stinging tree also can be ground and made into a paste, which is applied to the skin.3 However, given the stability of the toxin, we do not recommend these remedies.
Instead, heavy-duty masking tape or hot wax can be applied to remove plant hairs from the skin. The most successful method of removing plant hair is hair removal wax strips, which are considered an essential component of a first aid kit where D moroides is found.3
- Ensikat H-J, Wessely H, Engeser M, et al. Distribution, ecology, chemistry and toxicology of plant stinging hairs. Toxins (Basel). 2021;13:141. doi:10.3390/toxins13020141
- Schmitt C, Parola P, de Haro L. Painful sting after exposure to Dendrocnide sp: two case reports. Wilderness Environ Med. 2013;24:471-473. doi:10.1016/j.wem.2013.03.021
- Hurley M. Selective stingers. ECOS. 2000;105:18-23. Accessed October 13, 2023. https://www.writingclearscience.com.au/wp-content/uploads/2015/06/stingers.pdf
- Gilding EK, Jami S, Deuis JR, et al. Neurotoxic peptides from the venom of the giant Australian stinging tree. Sci Adv. 2020;6:eabb8828. doi:10.1126/sciadv.abb8828
- Dendrocnide moroides. James Cook University Australia website. Accessed Accessed October 13, 2023. https://www.jcu.edu.au/discover-nature-at-jcu/plants/plants-by-scientific-name2/dendrocnide-moroides
- Hurley M. ‘The worst kind of pain you can imagine’—what it’s like to be stung by a stinging tree. The Conversation. September 28, 2018. Accessed October 13, 2023. https://theconversation.com/the-worst-kind-of-pain-you-can-imagine-what-its-like-to-be-stung-by-a-stinging-tree-103220
- Urticaceae: plant family. Britannica [Internet]. Accessed October 13, 2023. https://www.britannica.com/plant/Urticaceae
- Stinging trees (genus Dendrocnide). iNaturalist.ca [Internet]. Accessed October 13, 2023. https://inaturalist.ca/taxa/129502-Dendrocnide
- Hurley M. Growth dynamics and leaf quality of the stinging trees Dendrocnide moroides and Dendrocnide cordifolia (family Urticaceae) in Australian tropical rainforest: implications for herbivores. Aust J Bot. 2000;48:191-201. doi:10.1071/BT98006
- How the giant stinging tree of Australia can inflict months of agony. Nature. September 17, 2020. Accessed October 13, 2023. https://www.nature.com/articles/d41586-020-02668-9
- Chang Y-T, Shen J-J, Wong W-R, et al. Alternative therapy for autosensitization dermatitis. Chang Gung Med J. 2009;32:668-673.
Clinical Importance
Dendrocnide moroides is arguably the most brutal of stinging plants, even leading to death in dogs, horses, and humans in rare cases.1-3 Commonly called gympie-gympie (based on its discovery by gold miners near the town of Gympie in Queensland, Australia), D moroides also has been referred to as the mulberrylike stinging tree or stinger.2,4-6
Family and Nomenclature
The Australian stinging tree belongs to the family Urticaceae (known as the nettle family) within the order Rosales.1,2,3,5 Urticaceae is derived from the Latin term urere (to burn)—an apt description of the clinical experience of patients with D moroides–induced urticaria.
Urticaceae includes 54 genera, comprising herbs, shrubs, small trees, and vines found predominantly in tropical regions. Dendrocnide comprises approximately 40 species, all commonly known in Australia as stinging trees.2,7,8
Distribution
Dendrocnide moroides is found in the rainforests of Australia and Southeast Asia.2 Because the plant has a strong need for sunlight and wind protection, it typically is found in light-filled gaps within the rainforest, in moist ravines, along the edges of creeks, and on land bordering the rainforest.3,6
Appearance
Although D moroides is referred to as a tree, it is an understory shrub that typically grows to 3 m, with heart-shaped, serrated, dark green leaves that are 50-cm wide (Figure 1).6 The leaves are produced consistently through the year, with variable growth depending on the season.9

The plant is covered in what appears to be soft downy fur made up of trichomes (or plant hairs).1,6 The density of the hairs on leaves decreases as they age.2,9 The fruit, which is actually edible (if one is careful to avoid hairs), appears similar to red to dark purple raspberries growing on long stems.5,6
Cutaneous Manifestations
Symptoms of contact with the stems and leaves of D moroides range from slight irritation to serious neurologic disorders, including neuropathy. The severity of the reaction depends on the person, how much skin was contacted, and how one came into contact with the plant.1,5 Upon touch, there is an immediate reaction, with burning, urticaria, and edema. Pain increases, peaking 30 minutes later; then the pain slowly subsides.1 Tachycardia and throbbing regional lymphadenopathy can occur for 1 to 4 hours.1,6
Cutaneous Findings—Examination reveals immediate piloerection, erythema due to arteriolar dilation, and local swelling.2 These findings may disappear after 1 hour or last as long as 24 hours.1 Although objective signs may fade, subjective pain, pruritus, and burning can persist for months.3
Dermatitis-Inducing Plant Parts
After contact with the stems or leaves, the sharp trichomes become embedded in the skin, making them difficult to remove.1 The toxins are contained in siliceous hairs that the human body cannot break down.3 Symptoms can be experienced for as long as 1 year after contact, especially when the skin is pressed firmly or washed with hot or cold water.3,6 Because the plant’s hairs are shed continuously, being in close proximity to D moroides for longer than 20 minutes can lead to extreme sneezing, nosebleeds, and major respiratory damage from inhaling hairs.1,6,9
The stinging hairs of D moroides differ from irritant hairs on other plants because they contain physiologically active substances. Stinging hairs are classified as either a hypodermic syringe, which expels liquid only, or as a tragia-type syringe, in which liquid and sharp crystals are injected.
The Australian stinging tree falls into the first of these 2 groups (Figure 2)1; the sharp tip of the hair breaks on contact, leading to expulsion of the toxin into skin.1,4 The hairs function as a defense against mammalian herbivores but typically have no impact on pests.1 Nocturnal beetles and on occasion possums and red-legged pademelons dare to eat D moroides.3,6

The Irritant
Initially, formic acid was proposed as the irritant chemical in D moroides1; other candidates have included neurotransmitters, such as histamine, acetylcholine, and serotonin, as well as inorganic ions, such as potassium. These compounds may play a role but none explain the persistent sensory effects and years-long stable nature of the toxin.1,4
The most likely culprit irritant is a member of a newly discovered family of neurotoxins, the gympietides. These knot-shaped chemicals, found in D moroides and some spider venoms, have the ability to activate voltage-gated sodium channels of cutaneous neurons and cause local cutaneous vasodilation by stimulating neurotransmitter release.4 These neurotoxins not only generate pain but also suppress the mechanism used to interrupt those pain signals.10 Synthesized gympietides can replicate the effects of natural contact, indicating that they are the primary active toxins. These toxins are ultrastable, thus producing lasting effects.1
Although much is understood about the evolution and distribution of D moroides and the ecological role that it plays, there is still more to learn about the plant’s toxicology.
Prevention and Treatment
Prevention—Dendrocnide moroides dermatitis is best prevented by avoiding contact with the plant and related species, as well as wearing upper body clothing with long sleeves, pants, and boots, though plant hairs can still penetrate garments and sting.2,3
Therapy—There is no reversal therapy of D moroides dermatitis but symptoms can be managed.4 For pain, analgesics, such as opioids, have been used; on occasion, however, pain is so intense that even morphine does not help.4,10
Systemic or topical corticosteroids are the main therapy for many forms of plant-induced dermatitis because they are able to decrease cytokine production and stop lymphocyte production. Adding an oral antihistamine can alleviate histamine-mediated pruritus but not pruritus that is mediated by other chemicals.11
Other methods of relieving symptoms of D moroides dermatitis have been proposed or reported anecdotally. Diluted hydrochloric acid can be applied to the skin to denature remaining toxin.4 The sap of Alocasia brisbanensis (the cunjevoi plant) can be rubbed on affected areas to provide a cooling effect, but do not allow A brisbanensis sap to enter the mouth, as it contains calcium oxalate, a toxic irritant found in dumb cane (Dieffenbachia species). The roots of the Australian stinging tree also can be ground and made into a paste, which is applied to the skin.3 However, given the stability of the toxin, we do not recommend these remedies.
Instead, heavy-duty masking tape or hot wax can be applied to remove plant hairs from the skin. The most successful method of removing plant hair is hair removal wax strips, which are considered an essential component of a first aid kit where D moroides is found.3
Clinical Importance
Dendrocnide moroides is arguably the most brutal of stinging plants, even leading to death in dogs, horses, and humans in rare cases.1-3 Commonly called gympie-gympie (based on its discovery by gold miners near the town of Gympie in Queensland, Australia), D moroides also has been referred to as the mulberrylike stinging tree or stinger.2,4-6
Family and Nomenclature
The Australian stinging tree belongs to the family Urticaceae (known as the nettle family) within the order Rosales.1,2,3,5 Urticaceae is derived from the Latin term urere (to burn)—an apt description of the clinical experience of patients with D moroides–induced urticaria.
Urticaceae includes 54 genera, comprising herbs, shrubs, small trees, and vines found predominantly in tropical regions. Dendrocnide comprises approximately 40 species, all commonly known in Australia as stinging trees.2,7,8
Distribution
Dendrocnide moroides is found in the rainforests of Australia and Southeast Asia.2 Because the plant has a strong need for sunlight and wind protection, it typically is found in light-filled gaps within the rainforest, in moist ravines, along the edges of creeks, and on land bordering the rainforest.3,6
Appearance
Although D moroides is referred to as a tree, it is an understory shrub that typically grows to 3 m, with heart-shaped, serrated, dark green leaves that are 50-cm wide (Figure 1).6 The leaves are produced consistently through the year, with variable growth depending on the season.9

The plant is covered in what appears to be soft downy fur made up of trichomes (or plant hairs).1,6 The density of the hairs on leaves decreases as they age.2,9 The fruit, which is actually edible (if one is careful to avoid hairs), appears similar to red to dark purple raspberries growing on long stems.5,6
Cutaneous Manifestations
Symptoms of contact with the stems and leaves of D moroides range from slight irritation to serious neurologic disorders, including neuropathy. The severity of the reaction depends on the person, how much skin was contacted, and how one came into contact with the plant.1,5 Upon touch, there is an immediate reaction, with burning, urticaria, and edema. Pain increases, peaking 30 minutes later; then the pain slowly subsides.1 Tachycardia and throbbing regional lymphadenopathy can occur for 1 to 4 hours.1,6
Cutaneous Findings—Examination reveals immediate piloerection, erythema due to arteriolar dilation, and local swelling.2 These findings may disappear after 1 hour or last as long as 24 hours.1 Although objective signs may fade, subjective pain, pruritus, and burning can persist for months.3
Dermatitis-Inducing Plant Parts
After contact with the stems or leaves, the sharp trichomes become embedded in the skin, making them difficult to remove.1 The toxins are contained in siliceous hairs that the human body cannot break down.3 Symptoms can be experienced for as long as 1 year after contact, especially when the skin is pressed firmly or washed with hot or cold water.3,6 Because the plant’s hairs are shed continuously, being in close proximity to D moroides for longer than 20 minutes can lead to extreme sneezing, nosebleeds, and major respiratory damage from inhaling hairs.1,6,9
The stinging hairs of D moroides differ from irritant hairs on other plants because they contain physiologically active substances. Stinging hairs are classified as either a hypodermic syringe, which expels liquid only, or as a tragia-type syringe, in which liquid and sharp crystals are injected.
The Australian stinging tree falls into the first of these 2 groups (Figure 2)1; the sharp tip of the hair breaks on contact, leading to expulsion of the toxin into skin.1,4 The hairs function as a defense against mammalian herbivores but typically have no impact on pests.1 Nocturnal beetles and on occasion possums and red-legged pademelons dare to eat D moroides.3,6

The Irritant
Initially, formic acid was proposed as the irritant chemical in D moroides1; other candidates have included neurotransmitters, such as histamine, acetylcholine, and serotonin, as well as inorganic ions, such as potassium. These compounds may play a role but none explain the persistent sensory effects and years-long stable nature of the toxin.1,4
The most likely culprit irritant is a member of a newly discovered family of neurotoxins, the gympietides. These knot-shaped chemicals, found in D moroides and some spider venoms, have the ability to activate voltage-gated sodium channels of cutaneous neurons and cause local cutaneous vasodilation by stimulating neurotransmitter release.4 These neurotoxins not only generate pain but also suppress the mechanism used to interrupt those pain signals.10 Synthesized gympietides can replicate the effects of natural contact, indicating that they are the primary active toxins. These toxins are ultrastable, thus producing lasting effects.1
Although much is understood about the evolution and distribution of D moroides and the ecological role that it plays, there is still more to learn about the plant’s toxicology.
Prevention and Treatment
Prevention—Dendrocnide moroides dermatitis is best prevented by avoiding contact with the plant and related species, as well as wearing upper body clothing with long sleeves, pants, and boots, though plant hairs can still penetrate garments and sting.2,3
Therapy—There is no reversal therapy of D moroides dermatitis but symptoms can be managed.4 For pain, analgesics, such as opioids, have been used; on occasion, however, pain is so intense that even morphine does not help.4,10
Systemic or topical corticosteroids are the main therapy for many forms of plant-induced dermatitis because they are able to decrease cytokine production and stop lymphocyte production. Adding an oral antihistamine can alleviate histamine-mediated pruritus but not pruritus that is mediated by other chemicals.11
Other methods of relieving symptoms of D moroides dermatitis have been proposed or reported anecdotally. Diluted hydrochloric acid can be applied to the skin to denature remaining toxin.4 The sap of Alocasia brisbanensis (the cunjevoi plant) can be rubbed on affected areas to provide a cooling effect, but do not allow A brisbanensis sap to enter the mouth, as it contains calcium oxalate, a toxic irritant found in dumb cane (Dieffenbachia species). The roots of the Australian stinging tree also can be ground and made into a paste, which is applied to the skin.3 However, given the stability of the toxin, we do not recommend these remedies.
Instead, heavy-duty masking tape or hot wax can be applied to remove plant hairs from the skin. The most successful method of removing plant hair is hair removal wax strips, which are considered an essential component of a first aid kit where D moroides is found.3
- Ensikat H-J, Wessely H, Engeser M, et al. Distribution, ecology, chemistry and toxicology of plant stinging hairs. Toxins (Basel). 2021;13:141. doi:10.3390/toxins13020141
- Schmitt C, Parola P, de Haro L. Painful sting after exposure to Dendrocnide sp: two case reports. Wilderness Environ Med. 2013;24:471-473. doi:10.1016/j.wem.2013.03.021
- Hurley M. Selective stingers. ECOS. 2000;105:18-23. Accessed October 13, 2023. https://www.writingclearscience.com.au/wp-content/uploads/2015/06/stingers.pdf
- Gilding EK, Jami S, Deuis JR, et al. Neurotoxic peptides from the venom of the giant Australian stinging tree. Sci Adv. 2020;6:eabb8828. doi:10.1126/sciadv.abb8828
- Dendrocnide moroides. James Cook University Australia website. Accessed Accessed October 13, 2023. https://www.jcu.edu.au/discover-nature-at-jcu/plants/plants-by-scientific-name2/dendrocnide-moroides
- Hurley M. ‘The worst kind of pain you can imagine’—what it’s like to be stung by a stinging tree. The Conversation. September 28, 2018. Accessed October 13, 2023. https://theconversation.com/the-worst-kind-of-pain-you-can-imagine-what-its-like-to-be-stung-by-a-stinging-tree-103220
- Urticaceae: plant family. Britannica [Internet]. Accessed October 13, 2023. https://www.britannica.com/plant/Urticaceae
- Stinging trees (genus Dendrocnide). iNaturalist.ca [Internet]. Accessed October 13, 2023. https://inaturalist.ca/taxa/129502-Dendrocnide
- Hurley M. Growth dynamics and leaf quality of the stinging trees Dendrocnide moroides and Dendrocnide cordifolia (family Urticaceae) in Australian tropical rainforest: implications for herbivores. Aust J Bot. 2000;48:191-201. doi:10.1071/BT98006
- How the giant stinging tree of Australia can inflict months of agony. Nature. September 17, 2020. Accessed October 13, 2023. https://www.nature.com/articles/d41586-020-02668-9
- Chang Y-T, Shen J-J, Wong W-R, et al. Alternative therapy for autosensitization dermatitis. Chang Gung Med J. 2009;32:668-673.
- Ensikat H-J, Wessely H, Engeser M, et al. Distribution, ecology, chemistry and toxicology of plant stinging hairs. Toxins (Basel). 2021;13:141. doi:10.3390/toxins13020141
- Schmitt C, Parola P, de Haro L. Painful sting after exposure to Dendrocnide sp: two case reports. Wilderness Environ Med. 2013;24:471-473. doi:10.1016/j.wem.2013.03.021
- Hurley M. Selective stingers. ECOS. 2000;105:18-23. Accessed October 13, 2023. https://www.writingclearscience.com.au/wp-content/uploads/2015/06/stingers.pdf
- Gilding EK, Jami S, Deuis JR, et al. Neurotoxic peptides from the venom of the giant Australian stinging tree. Sci Adv. 2020;6:eabb8828. doi:10.1126/sciadv.abb8828
- Dendrocnide moroides. James Cook University Australia website. Accessed Accessed October 13, 2023. https://www.jcu.edu.au/discover-nature-at-jcu/plants/plants-by-scientific-name2/dendrocnide-moroides
- Hurley M. ‘The worst kind of pain you can imagine’—what it’s like to be stung by a stinging tree. The Conversation. September 28, 2018. Accessed October 13, 2023. https://theconversation.com/the-worst-kind-of-pain-you-can-imagine-what-its-like-to-be-stung-by-a-stinging-tree-103220
- Urticaceae: plant family. Britannica [Internet]. Accessed October 13, 2023. https://www.britannica.com/plant/Urticaceae
- Stinging trees (genus Dendrocnide). iNaturalist.ca [Internet]. Accessed October 13, 2023. https://inaturalist.ca/taxa/129502-Dendrocnide
- Hurley M. Growth dynamics and leaf quality of the stinging trees Dendrocnide moroides and Dendrocnide cordifolia (family Urticaceae) in Australian tropical rainforest: implications for herbivores. Aust J Bot. 2000;48:191-201. doi:10.1071/BT98006
- How the giant stinging tree of Australia can inflict months of agony. Nature. September 17, 2020. Accessed October 13, 2023. https://www.nature.com/articles/d41586-020-02668-9
- Chang Y-T, Shen J-J, Wong W-R, et al. Alternative therapy for autosensitization dermatitis. Chang Gung Med J. 2009;32:668-673.
Practice Points
- Dendrocnide moroides is arguably the most brutal of stinging plants, even leading to death in dogs, horses, and humans in rare cases.
- Clinical observations after contact reveal immediate piloerection and local swelling, which may disappear after 1 hour or last as long as 24 hours, but subjective pain, pruritus, and burning can persist for months.
- The most successful method of removing plant hair is hair removal wax strips, which are considered an essential component of a first aid kit where D moroides is found.
What’s Eating You? Phlebotomine Sandflies and Leishmania Parasites
The genus Leishmania comprises protozoan parasites that cause approximately 2 million new cases of leishmaniasis each year across 98 countries.1 These protozoa are obligate intracellular parasites of phlebotomine sandfly species that transmit leishmaniasis and result in a considerable parasitic cause of fatalities globally, second only to malaria.2,3
Phlebotomine sandflies primarily live in tropical and subtropical regions and function as vectors for many pathogens in addition to Leishmania species, such as Bartonella species and arboviruses.3 In 2004, it was noted that the majority of leishmaniasis cases affected developing countries: 90% of visceral leishmaniasis cases occurred in Bangladesh, India, Nepal, Sudan, and Brazil, and 90% of cutaneous leishmaniasis cases occurred in Afghanistan, Algeria, Brazil, Iran, Peru, Saudi Arabia, and Syria.4 Of note, with recent environmental changes, phlebotomine sandflies have gradually migrated to more northerly latitudes, extending into Europe.5
Twenty Leishmania species and 30 sandfly species have been identified as causes of leishmaniasis.4Leishmania infection occurs when an infected sandfly bites a mammalian host and transmits the parasite’s flagellated form, known as a promastigote. Host inflammatory cells, such as monocytes and dendritic cells, phagocytize parasites that enter the skin. The interaction between parasites and dendritic cells become an important factor in the outcome of Leishmania infection in the host because dendritic cells promote development of CD4 and CD8 T lymphocytes with specificity to target Leishmania parasites and protect the host.1
The number of cases of leishmaniasis has increased worldwide, most likely due to changes in the environment and human behaviors such as urbanization, the creation of new settlements, and migration from rural to urban areas.3,5 Important risk factors in individual patients include malnutrition; low-quality housing and sanitation; a history of migration or travel; and immunosuppression, such as that caused by HIV co-infection.2,5
Case Report
An otherwise healthy 25-year-old Bangladeshi man presented to our community hospital for evaluation of a painful leg ulcer of 1 month’s duration. The patient had migrated from Bangladesh to Panama, then to Costa Rica, followed by Guatemala, Honduras, Mexico, and, last, Texas. In Texas, he was identified by the US Immigration and Customs Enforcement, transported to a detention facility, and transferred to this hospital shortly afterward.
The patient reported that, during his extensive migration, he had lived in the jungle and reported what he described as mosquito bites on the legs. He subsequently developed a 3-cm ulcerated and crusted plaque with rolled borders on the right medial ankle (Figure 1). In addition, he had a palpable nodular cord on the medial leg from the ankle lesion to the mid thigh that was consistent with lymphocutaneous spread. Ultrasonography was negative for deep-vein thrombosis.
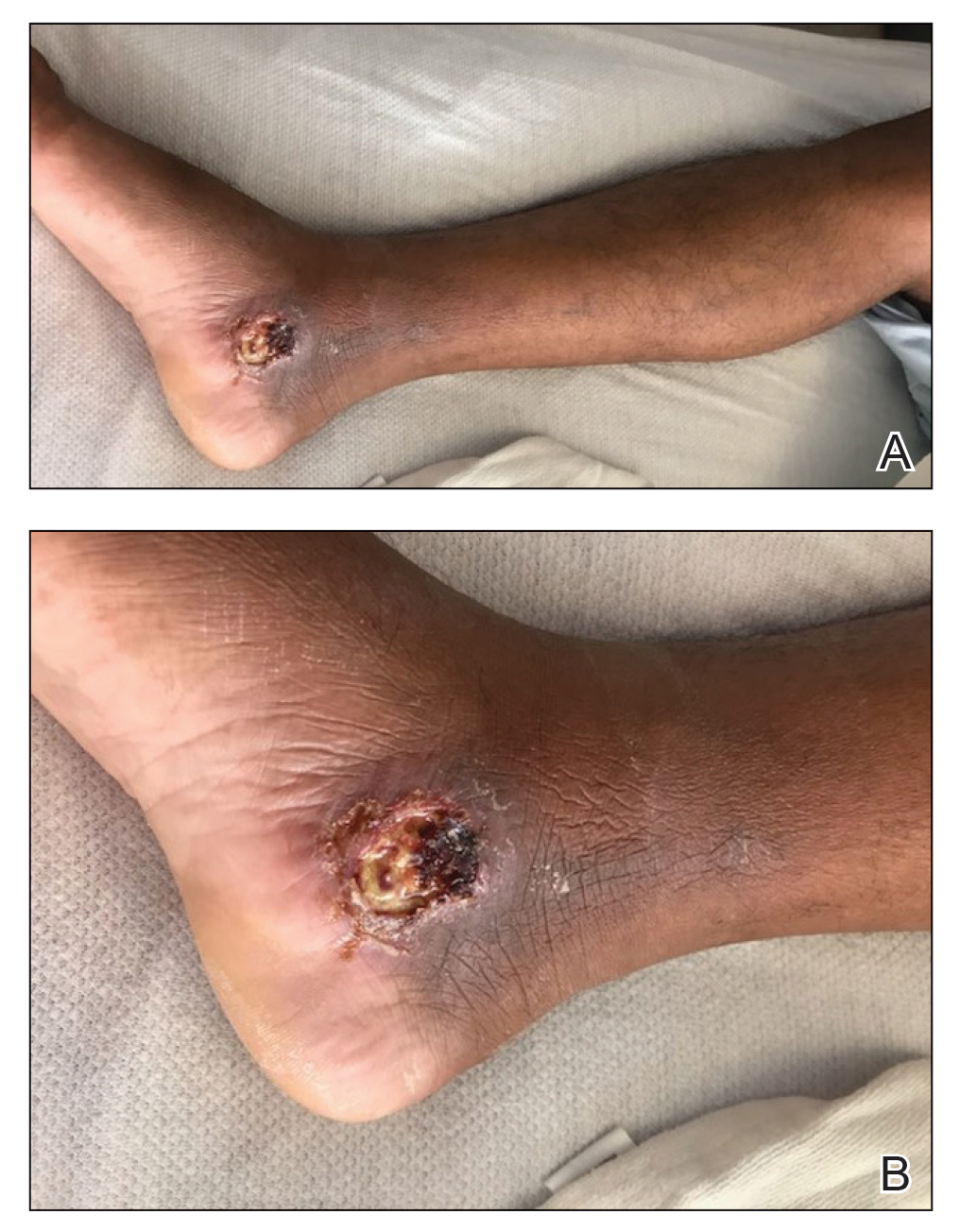
Because the patient’s recent migration from Central America was highly concerning for microbial infection, vancomycin and piperacillin-tazobactam were started empirically on admission. A punch biopsy from the right medial ankle was nondiagnostic, showing acute and chronic necrotizing inflammation along with numerous epithelioid histiocytes with a vaguely granulomatous appearance (Figure 2). A specimen from the right medial ankle that had already been taken by an astute border patrol medical provider was sent to the Centers for Disease Control and Prevention (CDC) for polymerase chain reaction analysis following admission and was found to be positive for Leishmania panamensis.
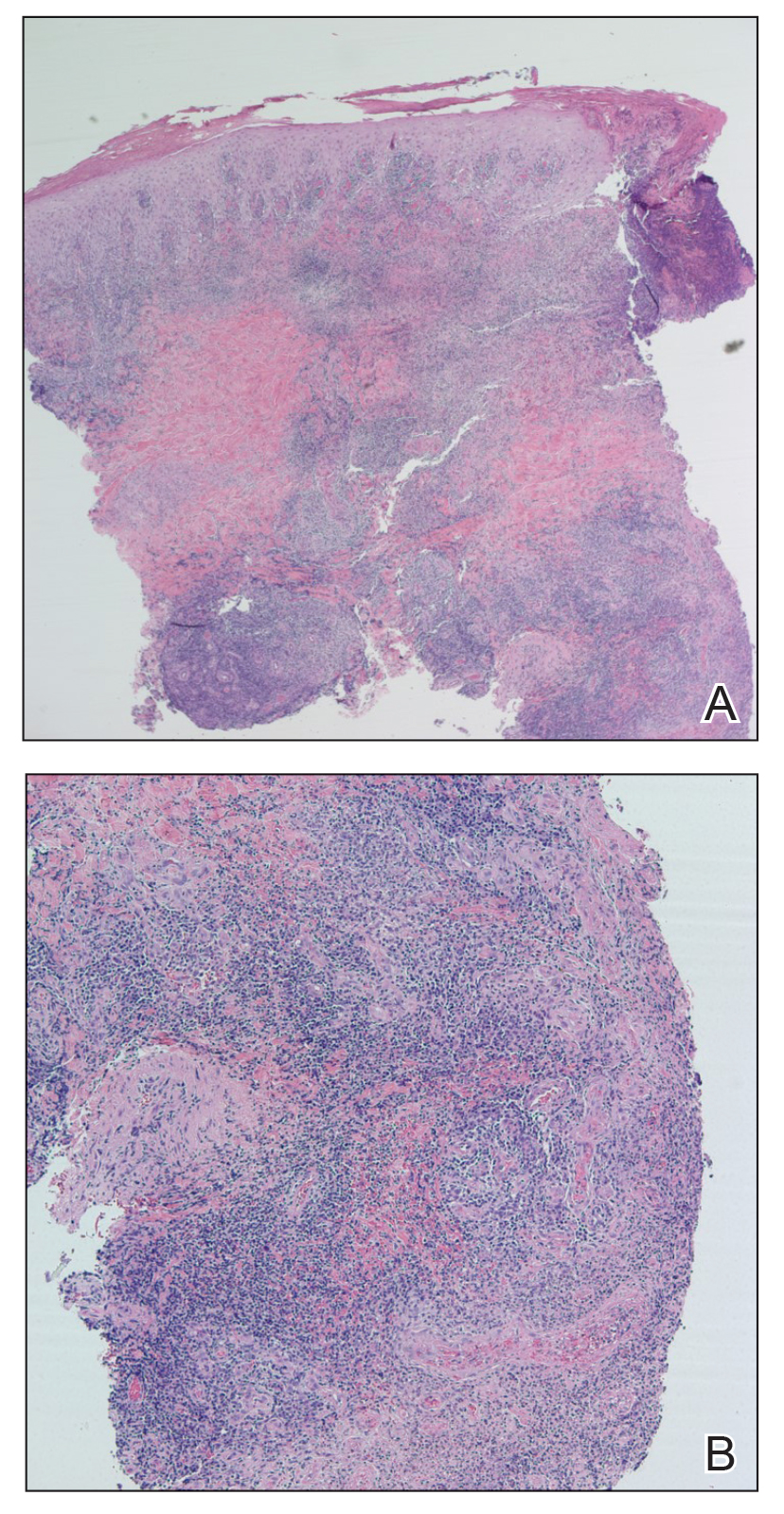
Given the concern for mucocutaneous leishmaniasis with this particular species, otolaryngology was consulted; however, the patient did not demonstrate mucocutaneous disease. Because of the elevated risk for persistent disease with L panamensis, systemic therapy was indicated and administered: IV amphotericin B 200 mg on days 1 through 5 and again on day 10. Improvement in the ulcer was seen after the 10-day regimen was completed.
Comment
Leishmaniasis can be broadly classified by geographic region or clinical presentation. Under the geographic region system, leishmaniasis can be categorized as Old World or New World. Old World leishmaniasis primarily is transmitted by Phlebotomus sandflies and carries the parasites Leishmania major and Leishmania tropica, among others. New World leishmaniasis is caused by Lutzomyia sandflies, which carry Leishmania mexicana, Leishmania braziliensis, Leishmania amazonensis, and others.6
Our patient presented with cutaneous leishmaniasis, one of 4 primary clinical disease forms of leishmaniasis; the other 3 forms under this classification system are diffuse cutaneous, mucocutaneous, and visceral leishmaniasis, also known as kala-azar.3,6 Cutaneous leishmaniasis is limited to the skin, particularly the face and extremities. This form is more common with Old World vectors, with most cases occurring in Peru, Brazil, and the Middle East. In Old World cutaneous leishmaniasis, the disease begins with a solitary nodule at the site of the bite that ulcerates and can continue to spread in a sporotrichoid pattern. This cutaneous form tends to heal slowly over months to years with residual scarring. New World cutaneous leishmaniasis can present with a variety of clinical manifestations, including ulcerative, sarcoidlike, miliary, and nodular lesions.6,7
The diffuse form of cutaneous leishmaniasis begins in a similar manner to the Old World cutaneous form: a single nodule spreads widely over the body, especially the nose, and covers the patient’s skin with keloidal or verrucous lesions that do not ulcerate. These nodules contain large groupings of Leishmania-filled foamy macrophages. Often, patients with diffuse cutaneous leishmaniasis are immunosuppressed and are unable to develop an immune response to leishmanin and other skin antigens.6,7
Mucocutaneous leishmaniasis predominantly is caused by the New World species L braziliensis but also has been attributed to L amazonensis, L panamensis, and L guyanensis. This form manifests as mucosal lesions that can develop simultaneously with cutaneous lesions but more commonly appear months to years after resolution of the skin infection. Patients often present with ulceration of the lip, nose, and oropharynx, and destruction of the nasopharynx can result in severe consequences such as obstruction of the airway and perforation of the nasal septum (also known as espundia).6,7
The most severe presentation of leishmaniasis is the visceral form (kala-azar), which presents with parasitic infection of the liver, spleen, and bone marrow. Most commonly caused by Leishmania donovani, Leishmania infantum, and Leishmania chagasi, this form has a long incubation period spanning months to years before presenting with diarrhea, hepatomegaly, splenomegaly, darkening of the skin (in Hindi, kala-azar means “black fever”), pancytopenia, lymphadenopathy, nephritis, and intestinal hemorrhage, among other severe manifestations. Visceral leishmaniasis has a poor prognosis: patients succumb to disease within 2 years if not treated.6,7
Diagnosis—Diagnosing leishmaniasis starts with a complete personal and medical history, paying close attention to travel and exposures. Diagnosis is most successfully performed by polymerase chain reaction analysis, which is both highly sensitive and specific but also can be determined by culture using Novy-McNeal-Nicolle medium or by light microscopy. Histologic findings include the marquee sign, which describes an array of amastigotes (promastigotes that have developed into the intracellular tissue-stage form) with kinetoplasts surrounding the periphery of parasitized histiocytes. Giemsa staining can be helpful in identifying organisms.2,6,7
The diagnosis in our case was challenging, as none of the above findings were seen in our patient. The specimen taken by the border patrol medical provider was negative on Gram, Giemsa, and Grocott-Gömöri methenamine silver staining; no amastigotes were identified. Another diagnostic modality (not performed in our patient) is the Montenegro delayed skin-reaction test, which often is positive in patients with cutaneous leishmaniasis but also yields a positive result in patients who have been cured of Leishmania infection.6
An important consideration in the diagnostic workup of leishmaniasis is that collaboration with the CDC can be helpful, such as in our case, as they provide clear guidance for specimen collection and processing.2
Treatment—Treating leishmaniasis is challenging and complex. Even the initial decision to treat depends on several factors, including the form of infection. Most visceral and mucocutaneous infections should be treated due to both the lack of self-resolution of these forms and the higher risk for a potentially life-threatening disease course; in contrast, cutaneous forms require further consideration before initiating treatment. Some indicators for treating cutaneous leishmaniasis include widespread infection, intention to decrease scarring, and lesions with the potential to cause further complications (eg, on the face or ears or close to joints).6-8
The treatment of choice for cutaneous and mucocutaneous leishmaniasis is pentavalent antimony; however, this drug can only be obtained in the United States for investigational use, requiring approval by the CDC. A 20-day intravenous or intramuscular course of 20 mg/kg per day typically is used for cutaneous cases; a 28-day course typically is used for mucosal forms.
Amphotericin B is not only the treatment of choice for visceral leishmaniasis but also is an important alternative therapy for patients with mucosal leishmaniasis or who are co-infected with HIV. Patients with visceral infection also should receive supportive care for any concomitant afflictions, such as malnutrition or other infections. Although different regimens have been described, the US Food and Drug Administration has created outlines of specific intravenous infusion schedules for liposomal amphotericin B in immunocompetent and immunosuppressed patients.8 Liposomal amphotericin B also has a more favorable toxicity profile than conventional amphotericin B deoxycholate, which is otherwise effective in combating visceral leishmaniasis.6-8
Other treatments that have been attempted include pentamidine, miltefosine, thermotherapy, oral itraconazole and fluconazole, rifampicin, metronidazole and cotrimoxazole, dapsone, photodynamic therapy, thermotherapy, topical paromomycin formulations, intralesional pentavalent antimony, and laser cryotherapy. Notable among these other agents is miltefosine, a US Food and Drug Administration–approved oral medication for adults and adolescents (used off-label for patients younger than 12 years) with cutaneous leishmaniasis caused by L braziliensis, L panamensis, or L guyanensis. Other oral options mentioned include the so-called azole antifungal medications, which historically have produced variable results. From the CDC’s reports, ketoconazole was moderately effective in Guatemala and Panama,8 whereas itraconazole did not demonstrate efficacy in Colombia, and the efficacy of fluconazole was inconsistent in different countries.8 When considering one of the local (as opposed to oral and parenteral) therapies mentioned, the extent of cutaneous findings as well as the risk of mucosal spread should be factored in.6-8
Understandably, a number of considerations can come into play in determining the appropriate treatment modality, including body region affected, clinical form, severity, and Leishmania species.6-8 Our case is of particular interest because it demonstrates the complexities behind the diagnosis and treatment of cutaneous leishmaniasis, with careful consideration geared toward the species; for example, because our patient was infected with L panamensis, which is known to cause mucocutaneous disease, the infectious disease service decided to pursue systemic therapy with amphotericin B rather than topical treatment.
Prevention—Vector control is the primary means of preventing leishmaniasis under 2 umbrellas: environmental management and synthetic insecticides. The goal of environmental management is to eliminate the phlebotomine sandfly habitat; this was the primary method of vector control until 1940. Until that time, tree stumps were removed, indoor cracks and crevices were filled to prevent sandfly emergence, and areas around animal shelters were cleaned. These methods were highly dependent on community awareness and involvement; today, they can be combined with synthetic insecticides to offer maximum protection.
Synthetic insecticides include indoor sprays, treated nets, repellents, and impregnated dog collars, all of which control sandflies. However, the use of these insecticides in endemic areas, such as India, has driven development of insecticide resistance in many sandfly vector species.3
As of 2020, 5 vaccines against Leishmania have been created. Two are approved–one in Brazil and one in Uzbekistan–for human use as immunotherapy, while the other 3 have been developed to immunize dogs in Brazil. However, the effectiveness of these vaccines is under debate. First, one of the vaccines used as immunotherapy for cutaneous leishmaniasis must be used in combination with conventional chemotherapy; second, long-term effects of the canine vaccine are unknown.1 A preventive vaccine for humans is under development.1,3
Final Thoughts
Leishmaniasis remains a notable parasitic disease that is increasing in prevalence worldwide. Clinicians should be aware of this disease because early detection and treatment are essential to control infection.3 Health care providers in the United States should be especially aware of this condition among patients who have a history of travel or migration; those in Texas should recognize the current endemic status of leishmaniasis there.4,6
- Coutinho De Oliveira B, Duthie MS, Alves Pereira VR. Vaccines for leishmaniasis and the implications of their development for American tegumentary leishmaniasis. Hum Vaccin Immunother. 2020;16:919-930. doi:10.1080/21645515.2019.1678998
- Chan CX, Simmons BJ, Call JE, et al. Cutaneous leishmaniasis successfully treated with miltefosine. Cutis. 2020;106:206-209. doi:10.12788/cutis.0086
- Balaska S, Fotakis EA, Chaskopoulou A, et al. Chemical control and insecticide resistance status of sand fly vectors worldwide. PLoS Negl Trop Dis. 2021;15:E0009586. doi:10.1371/journal.pntd.0009586
- Desjeux P. Leishmaniasis. Nat Rev Microbiol. 2004;2:692. doi:10.1038/nrmicro981
- Michelutti A, Toniolo F, Bertola M, et al. Occurrence of Phlebotomine sand flies (Diptera: Psychodidae) in the northeastern plain of Italy. Parasit Vectors. 2021;14:164. doi:10.1186/s13071-021-04652-2
- Alkihan A, Hocker TLH. Infectious diseases: parasites and other creatures: protozoa. In: Alikhan A, Hocker TLH, eds. Review of Dermatology. Elsevier; 2024:329-331.
- Dinulos JGH. Infestations and bites. In: Habif TP, ed. Clinical Dermatology. Elsevier; 2016:630-634.
- Centers for Disease Control and Prevention. Leishmaniasis: resources for health professionals. US Department of Health and Human Services. March 20, 2023. Accessed October 5, 2023. https://www.cdc.gov/parasites/leishmaniasis/health_professionals/index.html#:~:text=Liposomal%20amphotericin%20B%20is%20FDA,treatment%20of%20choice%20for%20U.S
The genus Leishmania comprises protozoan parasites that cause approximately 2 million new cases of leishmaniasis each year across 98 countries.1 These protozoa are obligate intracellular parasites of phlebotomine sandfly species that transmit leishmaniasis and result in a considerable parasitic cause of fatalities globally, second only to malaria.2,3
Phlebotomine sandflies primarily live in tropical and subtropical regions and function as vectors for many pathogens in addition to Leishmania species, such as Bartonella species and arboviruses.3 In 2004, it was noted that the majority of leishmaniasis cases affected developing countries: 90% of visceral leishmaniasis cases occurred in Bangladesh, India, Nepal, Sudan, and Brazil, and 90% of cutaneous leishmaniasis cases occurred in Afghanistan, Algeria, Brazil, Iran, Peru, Saudi Arabia, and Syria.4 Of note, with recent environmental changes, phlebotomine sandflies have gradually migrated to more northerly latitudes, extending into Europe.5
Twenty Leishmania species and 30 sandfly species have been identified as causes of leishmaniasis.4Leishmania infection occurs when an infected sandfly bites a mammalian host and transmits the parasite’s flagellated form, known as a promastigote. Host inflammatory cells, such as monocytes and dendritic cells, phagocytize parasites that enter the skin. The interaction between parasites and dendritic cells become an important factor in the outcome of Leishmania infection in the host because dendritic cells promote development of CD4 and CD8 T lymphocytes with specificity to target Leishmania parasites and protect the host.1
The number of cases of leishmaniasis has increased worldwide, most likely due to changes in the environment and human behaviors such as urbanization, the creation of new settlements, and migration from rural to urban areas.3,5 Important risk factors in individual patients include malnutrition; low-quality housing and sanitation; a history of migration or travel; and immunosuppression, such as that caused by HIV co-infection.2,5
Case Report
An otherwise healthy 25-year-old Bangladeshi man presented to our community hospital for evaluation of a painful leg ulcer of 1 month’s duration. The patient had migrated from Bangladesh to Panama, then to Costa Rica, followed by Guatemala, Honduras, Mexico, and, last, Texas. In Texas, he was identified by the US Immigration and Customs Enforcement, transported to a detention facility, and transferred to this hospital shortly afterward.
The patient reported that, during his extensive migration, he had lived in the jungle and reported what he described as mosquito bites on the legs. He subsequently developed a 3-cm ulcerated and crusted plaque with rolled borders on the right medial ankle (Figure 1). In addition, he had a palpable nodular cord on the medial leg from the ankle lesion to the mid thigh that was consistent with lymphocutaneous spread. Ultrasonography was negative for deep-vein thrombosis.

Because the patient’s recent migration from Central America was highly concerning for microbial infection, vancomycin and piperacillin-tazobactam were started empirically on admission. A punch biopsy from the right medial ankle was nondiagnostic, showing acute and chronic necrotizing inflammation along with numerous epithelioid histiocytes with a vaguely granulomatous appearance (Figure 2). A specimen from the right medial ankle that had already been taken by an astute border patrol medical provider was sent to the Centers for Disease Control and Prevention (CDC) for polymerase chain reaction analysis following admission and was found to be positive for Leishmania panamensis.

Given the concern for mucocutaneous leishmaniasis with this particular species, otolaryngology was consulted; however, the patient did not demonstrate mucocutaneous disease. Because of the elevated risk for persistent disease with L panamensis, systemic therapy was indicated and administered: IV amphotericin B 200 mg on days 1 through 5 and again on day 10. Improvement in the ulcer was seen after the 10-day regimen was completed.
Comment
Leishmaniasis can be broadly classified by geographic region or clinical presentation. Under the geographic region system, leishmaniasis can be categorized as Old World or New World. Old World leishmaniasis primarily is transmitted by Phlebotomus sandflies and carries the parasites Leishmania major and Leishmania tropica, among others. New World leishmaniasis is caused by Lutzomyia sandflies, which carry Leishmania mexicana, Leishmania braziliensis, Leishmania amazonensis, and others.6
Our patient presented with cutaneous leishmaniasis, one of 4 primary clinical disease forms of leishmaniasis; the other 3 forms under this classification system are diffuse cutaneous, mucocutaneous, and visceral leishmaniasis, also known as kala-azar.3,6 Cutaneous leishmaniasis is limited to the skin, particularly the face and extremities. This form is more common with Old World vectors, with most cases occurring in Peru, Brazil, and the Middle East. In Old World cutaneous leishmaniasis, the disease begins with a solitary nodule at the site of the bite that ulcerates and can continue to spread in a sporotrichoid pattern. This cutaneous form tends to heal slowly over months to years with residual scarring. New World cutaneous leishmaniasis can present with a variety of clinical manifestations, including ulcerative, sarcoidlike, miliary, and nodular lesions.6,7
The diffuse form of cutaneous leishmaniasis begins in a similar manner to the Old World cutaneous form: a single nodule spreads widely over the body, especially the nose, and covers the patient’s skin with keloidal or verrucous lesions that do not ulcerate. These nodules contain large groupings of Leishmania-filled foamy macrophages. Often, patients with diffuse cutaneous leishmaniasis are immunosuppressed and are unable to develop an immune response to leishmanin and other skin antigens.6,7
Mucocutaneous leishmaniasis predominantly is caused by the New World species L braziliensis but also has been attributed to L amazonensis, L panamensis, and L guyanensis. This form manifests as mucosal lesions that can develop simultaneously with cutaneous lesions but more commonly appear months to years after resolution of the skin infection. Patients often present with ulceration of the lip, nose, and oropharynx, and destruction of the nasopharynx can result in severe consequences such as obstruction of the airway and perforation of the nasal septum (also known as espundia).6,7
The most severe presentation of leishmaniasis is the visceral form (kala-azar), which presents with parasitic infection of the liver, spleen, and bone marrow. Most commonly caused by Leishmania donovani, Leishmania infantum, and Leishmania chagasi, this form has a long incubation period spanning months to years before presenting with diarrhea, hepatomegaly, splenomegaly, darkening of the skin (in Hindi, kala-azar means “black fever”), pancytopenia, lymphadenopathy, nephritis, and intestinal hemorrhage, among other severe manifestations. Visceral leishmaniasis has a poor prognosis: patients succumb to disease within 2 years if not treated.6,7
Diagnosis—Diagnosing leishmaniasis starts with a complete personal and medical history, paying close attention to travel and exposures. Diagnosis is most successfully performed by polymerase chain reaction analysis, which is both highly sensitive and specific but also can be determined by culture using Novy-McNeal-Nicolle medium or by light microscopy. Histologic findings include the marquee sign, which describes an array of amastigotes (promastigotes that have developed into the intracellular tissue-stage form) with kinetoplasts surrounding the periphery of parasitized histiocytes. Giemsa staining can be helpful in identifying organisms.2,6,7
The diagnosis in our case was challenging, as none of the above findings were seen in our patient. The specimen taken by the border patrol medical provider was negative on Gram, Giemsa, and Grocott-Gömöri methenamine silver staining; no amastigotes were identified. Another diagnostic modality (not performed in our patient) is the Montenegro delayed skin-reaction test, which often is positive in patients with cutaneous leishmaniasis but also yields a positive result in patients who have been cured of Leishmania infection.6
An important consideration in the diagnostic workup of leishmaniasis is that collaboration with the CDC can be helpful, such as in our case, as they provide clear guidance for specimen collection and processing.2
Treatment—Treating leishmaniasis is challenging and complex. Even the initial decision to treat depends on several factors, including the form of infection. Most visceral and mucocutaneous infections should be treated due to both the lack of self-resolution of these forms and the higher risk for a potentially life-threatening disease course; in contrast, cutaneous forms require further consideration before initiating treatment. Some indicators for treating cutaneous leishmaniasis include widespread infection, intention to decrease scarring, and lesions with the potential to cause further complications (eg, on the face or ears or close to joints).6-8
The treatment of choice for cutaneous and mucocutaneous leishmaniasis is pentavalent antimony; however, this drug can only be obtained in the United States for investigational use, requiring approval by the CDC. A 20-day intravenous or intramuscular course of 20 mg/kg per day typically is used for cutaneous cases; a 28-day course typically is used for mucosal forms.
Amphotericin B is not only the treatment of choice for visceral leishmaniasis but also is an important alternative therapy for patients with mucosal leishmaniasis or who are co-infected with HIV. Patients with visceral infection also should receive supportive care for any concomitant afflictions, such as malnutrition or other infections. Although different regimens have been described, the US Food and Drug Administration has created outlines of specific intravenous infusion schedules for liposomal amphotericin B in immunocompetent and immunosuppressed patients.8 Liposomal amphotericin B also has a more favorable toxicity profile than conventional amphotericin B deoxycholate, which is otherwise effective in combating visceral leishmaniasis.6-8
Other treatments that have been attempted include pentamidine, miltefosine, thermotherapy, oral itraconazole and fluconazole, rifampicin, metronidazole and cotrimoxazole, dapsone, photodynamic therapy, thermotherapy, topical paromomycin formulations, intralesional pentavalent antimony, and laser cryotherapy. Notable among these other agents is miltefosine, a US Food and Drug Administration–approved oral medication for adults and adolescents (used off-label for patients younger than 12 years) with cutaneous leishmaniasis caused by L braziliensis, L panamensis, or L guyanensis. Other oral options mentioned include the so-called azole antifungal medications, which historically have produced variable results. From the CDC’s reports, ketoconazole was moderately effective in Guatemala and Panama,8 whereas itraconazole did not demonstrate efficacy in Colombia, and the efficacy of fluconazole was inconsistent in different countries.8 When considering one of the local (as opposed to oral and parenteral) therapies mentioned, the extent of cutaneous findings as well as the risk of mucosal spread should be factored in.6-8
Understandably, a number of considerations can come into play in determining the appropriate treatment modality, including body region affected, clinical form, severity, and Leishmania species.6-8 Our case is of particular interest because it demonstrates the complexities behind the diagnosis and treatment of cutaneous leishmaniasis, with careful consideration geared toward the species; for example, because our patient was infected with L panamensis, which is known to cause mucocutaneous disease, the infectious disease service decided to pursue systemic therapy with amphotericin B rather than topical treatment.
Prevention—Vector control is the primary means of preventing leishmaniasis under 2 umbrellas: environmental management and synthetic insecticides. The goal of environmental management is to eliminate the phlebotomine sandfly habitat; this was the primary method of vector control until 1940. Until that time, tree stumps were removed, indoor cracks and crevices were filled to prevent sandfly emergence, and areas around animal shelters were cleaned. These methods were highly dependent on community awareness and involvement; today, they can be combined with synthetic insecticides to offer maximum protection.
Synthetic insecticides include indoor sprays, treated nets, repellents, and impregnated dog collars, all of which control sandflies. However, the use of these insecticides in endemic areas, such as India, has driven development of insecticide resistance in many sandfly vector species.3
As of 2020, 5 vaccines against Leishmania have been created. Two are approved–one in Brazil and one in Uzbekistan–for human use as immunotherapy, while the other 3 have been developed to immunize dogs in Brazil. However, the effectiveness of these vaccines is under debate. First, one of the vaccines used as immunotherapy for cutaneous leishmaniasis must be used in combination with conventional chemotherapy; second, long-term effects of the canine vaccine are unknown.1 A preventive vaccine for humans is under development.1,3
Final Thoughts
Leishmaniasis remains a notable parasitic disease that is increasing in prevalence worldwide. Clinicians should be aware of this disease because early detection and treatment are essential to control infection.3 Health care providers in the United States should be especially aware of this condition among patients who have a history of travel or migration; those in Texas should recognize the current endemic status of leishmaniasis there.4,6
The genus Leishmania comprises protozoan parasites that cause approximately 2 million new cases of leishmaniasis each year across 98 countries.1 These protozoa are obligate intracellular parasites of phlebotomine sandfly species that transmit leishmaniasis and result in a considerable parasitic cause of fatalities globally, second only to malaria.2,3
Phlebotomine sandflies primarily live in tropical and subtropical regions and function as vectors for many pathogens in addition to Leishmania species, such as Bartonella species and arboviruses.3 In 2004, it was noted that the majority of leishmaniasis cases affected developing countries: 90% of visceral leishmaniasis cases occurred in Bangladesh, India, Nepal, Sudan, and Brazil, and 90% of cutaneous leishmaniasis cases occurred in Afghanistan, Algeria, Brazil, Iran, Peru, Saudi Arabia, and Syria.4 Of note, with recent environmental changes, phlebotomine sandflies have gradually migrated to more northerly latitudes, extending into Europe.5
Twenty Leishmania species and 30 sandfly species have been identified as causes of leishmaniasis.4Leishmania infection occurs when an infected sandfly bites a mammalian host and transmits the parasite’s flagellated form, known as a promastigote. Host inflammatory cells, such as monocytes and dendritic cells, phagocytize parasites that enter the skin. The interaction between parasites and dendritic cells become an important factor in the outcome of Leishmania infection in the host because dendritic cells promote development of CD4 and CD8 T lymphocytes with specificity to target Leishmania parasites and protect the host.1
The number of cases of leishmaniasis has increased worldwide, most likely due to changes in the environment and human behaviors such as urbanization, the creation of new settlements, and migration from rural to urban areas.3,5 Important risk factors in individual patients include malnutrition; low-quality housing and sanitation; a history of migration or travel; and immunosuppression, such as that caused by HIV co-infection.2,5
Case Report
An otherwise healthy 25-year-old Bangladeshi man presented to our community hospital for evaluation of a painful leg ulcer of 1 month’s duration. The patient had migrated from Bangladesh to Panama, then to Costa Rica, followed by Guatemala, Honduras, Mexico, and, last, Texas. In Texas, he was identified by the US Immigration and Customs Enforcement, transported to a detention facility, and transferred to this hospital shortly afterward.
The patient reported that, during his extensive migration, he had lived in the jungle and reported what he described as mosquito bites on the legs. He subsequently developed a 3-cm ulcerated and crusted plaque with rolled borders on the right medial ankle (Figure 1). In addition, he had a palpable nodular cord on the medial leg from the ankle lesion to the mid thigh that was consistent with lymphocutaneous spread. Ultrasonography was negative for deep-vein thrombosis.

Because the patient’s recent migration from Central America was highly concerning for microbial infection, vancomycin and piperacillin-tazobactam were started empirically on admission. A punch biopsy from the right medial ankle was nondiagnostic, showing acute and chronic necrotizing inflammation along with numerous epithelioid histiocytes with a vaguely granulomatous appearance (Figure 2). A specimen from the right medial ankle that had already been taken by an astute border patrol medical provider was sent to the Centers for Disease Control and Prevention (CDC) for polymerase chain reaction analysis following admission and was found to be positive for Leishmania panamensis.

Given the concern for mucocutaneous leishmaniasis with this particular species, otolaryngology was consulted; however, the patient did not demonstrate mucocutaneous disease. Because of the elevated risk for persistent disease with L panamensis, systemic therapy was indicated and administered: IV amphotericin B 200 mg on days 1 through 5 and again on day 10. Improvement in the ulcer was seen after the 10-day regimen was completed.
Comment
Leishmaniasis can be broadly classified by geographic region or clinical presentation. Under the geographic region system, leishmaniasis can be categorized as Old World or New World. Old World leishmaniasis primarily is transmitted by Phlebotomus sandflies and carries the parasites Leishmania major and Leishmania tropica, among others. New World leishmaniasis is caused by Lutzomyia sandflies, which carry Leishmania mexicana, Leishmania braziliensis, Leishmania amazonensis, and others.6
Our patient presented with cutaneous leishmaniasis, one of 4 primary clinical disease forms of leishmaniasis; the other 3 forms under this classification system are diffuse cutaneous, mucocutaneous, and visceral leishmaniasis, also known as kala-azar.3,6 Cutaneous leishmaniasis is limited to the skin, particularly the face and extremities. This form is more common with Old World vectors, with most cases occurring in Peru, Brazil, and the Middle East. In Old World cutaneous leishmaniasis, the disease begins with a solitary nodule at the site of the bite that ulcerates and can continue to spread in a sporotrichoid pattern. This cutaneous form tends to heal slowly over months to years with residual scarring. New World cutaneous leishmaniasis can present with a variety of clinical manifestations, including ulcerative, sarcoidlike, miliary, and nodular lesions.6,7
The diffuse form of cutaneous leishmaniasis begins in a similar manner to the Old World cutaneous form: a single nodule spreads widely over the body, especially the nose, and covers the patient’s skin with keloidal or verrucous lesions that do not ulcerate. These nodules contain large groupings of Leishmania-filled foamy macrophages. Often, patients with diffuse cutaneous leishmaniasis are immunosuppressed and are unable to develop an immune response to leishmanin and other skin antigens.6,7
Mucocutaneous leishmaniasis predominantly is caused by the New World species L braziliensis but also has been attributed to L amazonensis, L panamensis, and L guyanensis. This form manifests as mucosal lesions that can develop simultaneously with cutaneous lesions but more commonly appear months to years after resolution of the skin infection. Patients often present with ulceration of the lip, nose, and oropharynx, and destruction of the nasopharynx can result in severe consequences such as obstruction of the airway and perforation of the nasal septum (also known as espundia).6,7
The most severe presentation of leishmaniasis is the visceral form (kala-azar), which presents with parasitic infection of the liver, spleen, and bone marrow. Most commonly caused by Leishmania donovani, Leishmania infantum, and Leishmania chagasi, this form has a long incubation period spanning months to years before presenting with diarrhea, hepatomegaly, splenomegaly, darkening of the skin (in Hindi, kala-azar means “black fever”), pancytopenia, lymphadenopathy, nephritis, and intestinal hemorrhage, among other severe manifestations. Visceral leishmaniasis has a poor prognosis: patients succumb to disease within 2 years if not treated.6,7
Diagnosis—Diagnosing leishmaniasis starts with a complete personal and medical history, paying close attention to travel and exposures. Diagnosis is most successfully performed by polymerase chain reaction analysis, which is both highly sensitive and specific but also can be determined by culture using Novy-McNeal-Nicolle medium or by light microscopy. Histologic findings include the marquee sign, which describes an array of amastigotes (promastigotes that have developed into the intracellular tissue-stage form) with kinetoplasts surrounding the periphery of parasitized histiocytes. Giemsa staining can be helpful in identifying organisms.2,6,7
The diagnosis in our case was challenging, as none of the above findings were seen in our patient. The specimen taken by the border patrol medical provider was negative on Gram, Giemsa, and Grocott-Gömöri methenamine silver staining; no amastigotes were identified. Another diagnostic modality (not performed in our patient) is the Montenegro delayed skin-reaction test, which often is positive in patients with cutaneous leishmaniasis but also yields a positive result in patients who have been cured of Leishmania infection.6
An important consideration in the diagnostic workup of leishmaniasis is that collaboration with the CDC can be helpful, such as in our case, as they provide clear guidance for specimen collection and processing.2
Treatment—Treating leishmaniasis is challenging and complex. Even the initial decision to treat depends on several factors, including the form of infection. Most visceral and mucocutaneous infections should be treated due to both the lack of self-resolution of these forms and the higher risk for a potentially life-threatening disease course; in contrast, cutaneous forms require further consideration before initiating treatment. Some indicators for treating cutaneous leishmaniasis include widespread infection, intention to decrease scarring, and lesions with the potential to cause further complications (eg, on the face or ears or close to joints).6-8
The treatment of choice for cutaneous and mucocutaneous leishmaniasis is pentavalent antimony; however, this drug can only be obtained in the United States for investigational use, requiring approval by the CDC. A 20-day intravenous or intramuscular course of 20 mg/kg per day typically is used for cutaneous cases; a 28-day course typically is used for mucosal forms.
Amphotericin B is not only the treatment of choice for visceral leishmaniasis but also is an important alternative therapy for patients with mucosal leishmaniasis or who are co-infected with HIV. Patients with visceral infection also should receive supportive care for any concomitant afflictions, such as malnutrition or other infections. Although different regimens have been described, the US Food and Drug Administration has created outlines of specific intravenous infusion schedules for liposomal amphotericin B in immunocompetent and immunosuppressed patients.8 Liposomal amphotericin B also has a more favorable toxicity profile than conventional amphotericin B deoxycholate, which is otherwise effective in combating visceral leishmaniasis.6-8
Other treatments that have been attempted include pentamidine, miltefosine, thermotherapy, oral itraconazole and fluconazole, rifampicin, metronidazole and cotrimoxazole, dapsone, photodynamic therapy, thermotherapy, topical paromomycin formulations, intralesional pentavalent antimony, and laser cryotherapy. Notable among these other agents is miltefosine, a US Food and Drug Administration–approved oral medication for adults and adolescents (used off-label for patients younger than 12 years) with cutaneous leishmaniasis caused by L braziliensis, L panamensis, or L guyanensis. Other oral options mentioned include the so-called azole antifungal medications, which historically have produced variable results. From the CDC’s reports, ketoconazole was moderately effective in Guatemala and Panama,8 whereas itraconazole did not demonstrate efficacy in Colombia, and the efficacy of fluconazole was inconsistent in different countries.8 When considering one of the local (as opposed to oral and parenteral) therapies mentioned, the extent of cutaneous findings as well as the risk of mucosal spread should be factored in.6-8
Understandably, a number of considerations can come into play in determining the appropriate treatment modality, including body region affected, clinical form, severity, and Leishmania species.6-8 Our case is of particular interest because it demonstrates the complexities behind the diagnosis and treatment of cutaneous leishmaniasis, with careful consideration geared toward the species; for example, because our patient was infected with L panamensis, which is known to cause mucocutaneous disease, the infectious disease service decided to pursue systemic therapy with amphotericin B rather than topical treatment.
Prevention—Vector control is the primary means of preventing leishmaniasis under 2 umbrellas: environmental management and synthetic insecticides. The goal of environmental management is to eliminate the phlebotomine sandfly habitat; this was the primary method of vector control until 1940. Until that time, tree stumps were removed, indoor cracks and crevices were filled to prevent sandfly emergence, and areas around animal shelters were cleaned. These methods were highly dependent on community awareness and involvement; today, they can be combined with synthetic insecticides to offer maximum protection.
Synthetic insecticides include indoor sprays, treated nets, repellents, and impregnated dog collars, all of which control sandflies. However, the use of these insecticides in endemic areas, such as India, has driven development of insecticide resistance in many sandfly vector species.3
As of 2020, 5 vaccines against Leishmania have been created. Two are approved–one in Brazil and one in Uzbekistan–for human use as immunotherapy, while the other 3 have been developed to immunize dogs in Brazil. However, the effectiveness of these vaccines is under debate. First, one of the vaccines used as immunotherapy for cutaneous leishmaniasis must be used in combination with conventional chemotherapy; second, long-term effects of the canine vaccine are unknown.1 A preventive vaccine for humans is under development.1,3
Final Thoughts
Leishmaniasis remains a notable parasitic disease that is increasing in prevalence worldwide. Clinicians should be aware of this disease because early detection and treatment are essential to control infection.3 Health care providers in the United States should be especially aware of this condition among patients who have a history of travel or migration; those in Texas should recognize the current endemic status of leishmaniasis there.4,6
- Coutinho De Oliveira B, Duthie MS, Alves Pereira VR. Vaccines for leishmaniasis and the implications of their development for American tegumentary leishmaniasis. Hum Vaccin Immunother. 2020;16:919-930. doi:10.1080/21645515.2019.1678998
- Chan CX, Simmons BJ, Call JE, et al. Cutaneous leishmaniasis successfully treated with miltefosine. Cutis. 2020;106:206-209. doi:10.12788/cutis.0086
- Balaska S, Fotakis EA, Chaskopoulou A, et al. Chemical control and insecticide resistance status of sand fly vectors worldwide. PLoS Negl Trop Dis. 2021;15:E0009586. doi:10.1371/journal.pntd.0009586
- Desjeux P. Leishmaniasis. Nat Rev Microbiol. 2004;2:692. doi:10.1038/nrmicro981
- Michelutti A, Toniolo F, Bertola M, et al. Occurrence of Phlebotomine sand flies (Diptera: Psychodidae) in the northeastern plain of Italy. Parasit Vectors. 2021;14:164. doi:10.1186/s13071-021-04652-2
- Alkihan A, Hocker TLH. Infectious diseases: parasites and other creatures: protozoa. In: Alikhan A, Hocker TLH, eds. Review of Dermatology. Elsevier; 2024:329-331.
- Dinulos JGH. Infestations and bites. In: Habif TP, ed. Clinical Dermatology. Elsevier; 2016:630-634.
- Centers for Disease Control and Prevention. Leishmaniasis: resources for health professionals. US Department of Health and Human Services. March 20, 2023. Accessed October 5, 2023. https://www.cdc.gov/parasites/leishmaniasis/health_professionals/index.html#:~:text=Liposomal%20amphotericin%20B%20is%20FDA,treatment%20of%20choice%20for%20U.S
- Coutinho De Oliveira B, Duthie MS, Alves Pereira VR. Vaccines for leishmaniasis and the implications of their development for American tegumentary leishmaniasis. Hum Vaccin Immunother. 2020;16:919-930. doi:10.1080/21645515.2019.1678998
- Chan CX, Simmons BJ, Call JE, et al. Cutaneous leishmaniasis successfully treated with miltefosine. Cutis. 2020;106:206-209. doi:10.12788/cutis.0086
- Balaska S, Fotakis EA, Chaskopoulou A, et al. Chemical control and insecticide resistance status of sand fly vectors worldwide. PLoS Negl Trop Dis. 2021;15:E0009586. doi:10.1371/journal.pntd.0009586
- Desjeux P. Leishmaniasis. Nat Rev Microbiol. 2004;2:692. doi:10.1038/nrmicro981
- Michelutti A, Toniolo F, Bertola M, et al. Occurrence of Phlebotomine sand flies (Diptera: Psychodidae) in the northeastern plain of Italy. Parasit Vectors. 2021;14:164. doi:10.1186/s13071-021-04652-2
- Alkihan A, Hocker TLH. Infectious diseases: parasites and other creatures: protozoa. In: Alikhan A, Hocker TLH, eds. Review of Dermatology. Elsevier; 2024:329-331.
- Dinulos JGH. Infestations and bites. In: Habif TP, ed. Clinical Dermatology. Elsevier; 2016:630-634.
- Centers for Disease Control and Prevention. Leishmaniasis: resources for health professionals. US Department of Health and Human Services. March 20, 2023. Accessed October 5, 2023. https://www.cdc.gov/parasites/leishmaniasis/health_professionals/index.html#:~:text=Liposomal%20amphotericin%20B%20is%20FDA,treatment%20of%20choice%20for%20U.S
Practice Points
- The Phlebotomus and Lutzomyia genera of sandflies are vectors of Leishmania parasites, which can result in an array of clinical findings associated with leishmaniasis.
- Treatment options for leishmaniasis differ based on whether the infection is considered uncomplicated or complicated, which depends on the species of Leishmania; the number, size, and location of the lesion(s); and host immune status.
- All US practitioners should be aware of this pathogen, especially with regard to patients who have a history of travel to other countries. Health care professionals in states such as Texas and Oklahoma should be especially cognizant because these constitute one of the few areas in the United States where locally acquired cases of leishmaniasis have been reported.
What’s Eating You? Noble False Widow Spider (Steatoda nobilis)
Incidence and Characteristics
The noble false widow spider (Steatoda nobilis) is one of the world’s most invasive spider species, having spread across the globe from Madeira and the Canary Islands into the North Atlantic.1,2Steatoda comprise multiple species of false widow spiders, named for their resemblance to black widow spiders (Latrodectus). The noble false widow spider is the dominant species in buildings in southern Ireland and Great Britain, with a population surge in 2018 that caused multiple temporary school closures in London, England, for fumigation.3 The noble false widow spider was first documented in the United States in Ventura County, California, in 2011, with numerous specimens found in urban areas (eg, in parks, underneath garbage cans) closer to the coastline as well as farther inland. The species may have been introduced to this area by way of Port Hueneme, a city in California with a US naval base with routes to various other military bases in Western Europe.4 Given its already rapid expansion outside of the United States with a concurrent rise in bite reports, dermatologists should be familiar with these invasive and potentially dangerous arachnids.
The spread of noble false widow spiders is assisted by their wide range of temperature tolerance and ability to survive for months with little food and no water. They can live for several years, with one report of a noble false widow spider living up to 7 years.5 These spiders are found inside homes and buildings year-round, and they prefer to build their webs in an elevated position such as the top corner of a room. Steatoda weave tangle webs with crisscrossing threads that often have a denser middle section.5
Noble false widow spiders are sexually dimorphic, with males typically no larger than 1-cm long and females up to 1.4-cm long. They have a dark brown to black thorax and brown abdomen with red-brown legs. Males have brighter cream-colored abdominal markings than females, who lack markings altogether on their distinctive globular abdomen (Figure). The abdominal markings are known to resemble a skull or house.

Although noble false widow spiders are not exclusively synanthropic, they can be found in any crevice in homes or other structures where there are humans such as office buildings.5-7 Up until the last 20 years, reports of bites from noble false widow spiders worldwide were few and far between. In Great Britain, the spiders were first considered to be common in the 1980s, with recent evidence of an urban population boom in the last 5 to 10 years that has coincided with an increase in bite reports.5,8,9
Clinical Significance
Most bites occur in a defensive manner, such as when humans perform activities that disturb the hiding space, cause vibrations in the web, or compress the body of the arachnid. Most envenomations in Great Britain occur while the individual is in bed, though they also may occur during other activities that disturb the spider, such as moving boxes or putting on a pair of pants.5 Occupational exposure to noble false widow spiders may soon be a concern for those involved in construction, carpentry, cleaning, and decorating given their recent invasive spread into the United States.
The venom from these spiders is neurotoxic and cytotoxic, causing moderate to intense pain that may resemble a wasp sting. The incidence of steatodism—which can include symptoms of pain in addition to fever, hypotension, headache, lethargy, nausea, localized diaphoresis, abdominal pain, paresthesias, and malaise—is unknown but reportedly rare.5,10 There are considerable similarities between Steatoda and true black widow spider venom, which explains the symptom overlap with latrodectism. There are reports of severe debilitation lasting weeks due to pain and decreased affected limb movement after bites from noble false widow spiders.10-12
Nearly all noble false widow spider bite reports describe immediate pain upon bite/envenomation, which is unlike the delayed pain from a black widow spider bite (after 10 minutes or more).6,13,14 Erythema and swelling occur around a pale raised site of envenomation lasting up to 72 hours. The bite site may be highly tender and blister or ulcerate, with reports of cellulitis and local skin necrosis.7,15 Pruritus during this period can be intense, and excoriation increases the risk for complications such as infection. Reports of anaphylaxis following a noble false widow spider bite are rare.5,16 The incidence of bites may be underreported due to the lack of proper identification of the responsible arachnid for those who do not seek care or require hospitalization, though this is not unique to Steatoda.
There are reports of secondary infection after bites and even cases of limb amputation, septicemia, and death.14,17 However, it is unknown if noble false widow spiders are vectors for bacteria transmitted during envenomation, and infection likely is secondary to scratching or inadequate wound care.18,19 Potentially pathogenic bacteria have been isolated from the body surfaces of the noble false widow spider, including Pseudomonas putida, Staphylococcus capitis, and Staphylococcus epidermidis.20 Fortunately, most captured cases (ie, events in which the biting arachnid was properly identified) report symptoms ranging from mild to moderate in severity without the need for hospitalization. A series of 24 reports revealed that all individuals experienced sharp pain upon the initial bite followed by erythema, and 18 of them experienced considerable swelling of the area soon thereafter. One individual experienced temporary paralysis of the affected limb, and 3 individuals experienced hypotension or hypertension in addition to fever, skin necrosis, or cellulitis.14
Treatment
The envenomation site should be washed with antibacterial soap and warm water and should be kept clean to prevent infection. There is no evidence that tight pressure bandaging of these bite sites will restrict venom flow; because it may worsen pain in the area, pressure bandaging is not recommended. When possible, the arachnid should be collected for identification. Supportive care is warranted for symptoms of pain, erythema, and swelling, with the use of cool compresses, oral pain relievers (eg, nonsteroidal anti-inflammatory drugs, acetaminophen), topical anesthetic (eg, lidocaine), or antihistamines as needed.
Urgent care is warranted for patients who experience severe symptoms of steatodism such as hypertension, lymphadenopathy, paresthesia, or limb paralysis. Limited reports show onset of this distress typically within an hour of envenomation. Treatments analogous to those for latrodectism including muscle relaxers and pain medications have demonstrated rapid attenuation of symptoms upon intramuscular administration of antivenom made from Latrodectus species.21-23
Signs of infection warrant bacterial culture with antibiotic susceptibilities to ensure adequate treatment.20 Infections from spider bites can present a few days to a week following envenomation. Symptoms may include spreading redness or an enlarging wound site, pus formation, worsening or unrelenting pain after 24 hours, fevers, flulike symptoms, and muscle cramps.
Final Thoughts
- Kulczycki A, Legittimo C, Simeon E, et al. New records of Steatoda nobilis (Thorell, 1875) (Araneae, Theridiidae), an introduced species on the Italian mainland and in Sardinia. Bull Br Arachnological Soc. 2012;15:269-272.
- Bauer T, Feldmeier S, Krehenwinkel H, et al. Steatoda nobilis, a false widow on the rise: a synthesis of past and current distribution trends. NeoBiota. 2019; 42:19. doi:10.3897/neobiota.42.31582
- Murphy A. Web of cries: false widow spider infestation fears forceeleventh school in London to close as outbreak spreads. The Sun.October 19, 2018. Accessed September 21, 2023. https://www.thesun.co.uk/news/7534016/false-widow-spider-infestation-fears-force-eleventh-londonschool-closing
- Vetter R, Rust M. A large European combfoot spider, Steatoda nobilis (Thorell 1875)(Araneae: Theridiidae), newly established in Ventura County, California. The Pan-Pacific Entomologist. 2012;88:92-97.
- Hambler C. The ‘noble false widow’ spider Steatoda nobilis is an emerging public health and ecological threat. OSF Preprints. Preprint posted online October 15, 2019. doi:10.31219/osf.io/axbd4
- Dunbar J, Schulte J, Lyons K, et al. New Irish record for Steatoda triangulosa (Walckenaer, 1802), and new county records for Steatoda nobilis (Thorell, 1875), Steatoda bipunctata (Linnaeus, 1758) and Steatoda grossa (C.L. Koch, 1838). Ir Naturalists J. 2018;36:39-43.
- Duon M, Dunbar J, Afoullouss S, et al. Occurrence, reproductive rate and identification of the non-native noble false widow spider Steatoda nobilis (Thorell, 1875) in Ireland. Biol Environment: Proc Royal Ir Acad. 2017;117B:77-89. doi:10.3318/bioe.2017.11
- Burrows T. Great bitten: Britain’s spider bite capital revealed as Essex with 450 attacks—find out where your town ranks. The Sun. Published April 3, 2019. Accessed September 14, 2023. https://www.thesun.co.uk/news/8782355/britains-spider-bite-capital-revealed-as-essex-with-450- attacks-find-out-where-your-town-ranks/
- Wathen T. Essex is the UK capital for spider bites—and the amount is terrifying. Essex News. April 4, 2019. Accessed September 21, 2023. https://www.essexlive.news/news/essex-news/essex-uk-capital-spider-bites- 2720935
- Dunbar J, Afoullouss S, Sulpice R, et al. Envenomation by the noble false widow spider Steatoda nobilis (Thorell, 1875)—five new cases of steatodism from Ireland and Great Britain. Clin Toxicol (Phila). 2018;56:433-435. doi:10.1080/15563650.2017.1393084
- Dunbar J, Fort A, Redureau D, et al. Venomics approach reveals a high proportion of Latrodectus-like toxins in the venom of the noble false widow spider Steatoda nobilis. Toxins. 2020;12:402.
- Warrell D, Shaheen J, Hillyard P, et al. Neurotoxic envenoming by an immigrant spider (Steatoda nobilis) in southern England. Toxicon. 1991;29:1263-1265.
- Zhou H, Xu K, Zheng PY, et. al. Clinical characteristics of patients with black widow spider bites: a report of 59 patients and single-center experience. World J Emerg Med. 2021;12:317-320. doi:10.5847/wjem.j.1920-8642.2021.04.011
- Dunbar J, Vitkauskaite A, O’Keeffe D, et. al. Bites by the noble false widow spider Steatoda nobilis can induce Latrodectus-like symptoms and vector-borne bacterial infections with implications for public health: a case series. Clin Toxicol (Phila). 2022;60:59-70. doi:10.1080/15563650.2021.1928165
- Dunbar J, Sulpice R, Dugon M. The kiss of (cell) death: can venom-induced immune response contribute to dermal necrosis following arthropod envenomations? Clin Toxicol. 2019;57:677-685. doi:10.1080/15563650.2019.1578367
- Magee J. Bite ‘nightmare’: close encounter with a false widow. The Bournemouth Echo. September 7, 2009. Accessed September 21, 2023. http://www.bournemouthecho.co.uk/news/4582887.Bite____nightmare_____close_encounter_with_a_false_widow_spider/
- Marsh H. Woman nearly loses hand after bite from false widow. Daily Echo. April 17, 2012. Accessed September 21, 2023. https://www.bournemouthecho.co.uk/news/9652335.woman-nearly-loses-hand-after-bite-from-false-widow-spider/
- Stuber N, Nentwig W. How informative are case studies of spider bites in the medical literature? Toxicon. 2016;114:40-44. doi:10.1016/j.toxicon.2016.02.023
- Vetter R, Swanson D, Weinstein S, et. al. Do spiders vector bacteria during bites? the evidence indicates otherwise. Toxicon. 2015;93:171-174. doi:10.1016/j.toxicon.2014.11.229
- Dunbar J, Khan N, Abberton C, et al. Synanthropic spiders, including the global invasive noble false widow Steatoda nobilis, are reservoirs for medically important and antibiotic resistant bacteria. Sci Rep. 2020;10:20916. doi:10.1038/s41598-020-77839-9
- Atakuziev BU, Wright CE, Graudins A, et al. Efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of clinical envenomation by the cupboard spider Steatoda capensis (Theridiidae). Toxicon. 2014;86:68-78. doi:10.1016/j.toxicon.2014.04.011
- Graudins A, Gunja N, Broady KW, et al. Clinical and in vitro evidence for the efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of envenomation by a cupboard spider (Steatoda grossa). Toxicon. 2002;40:767-775. doi:10.1016/S0041-0101(01)00280-X.
- South M, Wirth P, Winkel KD. Redback spider antivenom used to treat envenomation by a juvenile Steatoda spider. Med J Aust. 1998;169:642-642. doi:10.5694/j.1326-5377.1998.tb123445.x
Incidence and Characteristics
The noble false widow spider (Steatoda nobilis) is one of the world’s most invasive spider species, having spread across the globe from Madeira and the Canary Islands into the North Atlantic.1,2Steatoda comprise multiple species of false widow spiders, named for their resemblance to black widow spiders (Latrodectus). The noble false widow spider is the dominant species in buildings in southern Ireland and Great Britain, with a population surge in 2018 that caused multiple temporary school closures in London, England, for fumigation.3 The noble false widow spider was first documented in the United States in Ventura County, California, in 2011, with numerous specimens found in urban areas (eg, in parks, underneath garbage cans) closer to the coastline as well as farther inland. The species may have been introduced to this area by way of Port Hueneme, a city in California with a US naval base with routes to various other military bases in Western Europe.4 Given its already rapid expansion outside of the United States with a concurrent rise in bite reports, dermatologists should be familiar with these invasive and potentially dangerous arachnids.
The spread of noble false widow spiders is assisted by their wide range of temperature tolerance and ability to survive for months with little food and no water. They can live for several years, with one report of a noble false widow spider living up to 7 years.5 These spiders are found inside homes and buildings year-round, and they prefer to build their webs in an elevated position such as the top corner of a room. Steatoda weave tangle webs with crisscrossing threads that often have a denser middle section.5
Noble false widow spiders are sexually dimorphic, with males typically no larger than 1-cm long and females up to 1.4-cm long. They have a dark brown to black thorax and brown abdomen with red-brown legs. Males have brighter cream-colored abdominal markings than females, who lack markings altogether on their distinctive globular abdomen (Figure). The abdominal markings are known to resemble a skull or house.

Although noble false widow spiders are not exclusively synanthropic, they can be found in any crevice in homes or other structures where there are humans such as office buildings.5-7 Up until the last 20 years, reports of bites from noble false widow spiders worldwide were few and far between. In Great Britain, the spiders were first considered to be common in the 1980s, with recent evidence of an urban population boom in the last 5 to 10 years that has coincided with an increase in bite reports.5,8,9
Clinical Significance
Most bites occur in a defensive manner, such as when humans perform activities that disturb the hiding space, cause vibrations in the web, or compress the body of the arachnid. Most envenomations in Great Britain occur while the individual is in bed, though they also may occur during other activities that disturb the spider, such as moving boxes or putting on a pair of pants.5 Occupational exposure to noble false widow spiders may soon be a concern for those involved in construction, carpentry, cleaning, and decorating given their recent invasive spread into the United States.
The venom from these spiders is neurotoxic and cytotoxic, causing moderate to intense pain that may resemble a wasp sting. The incidence of steatodism—which can include symptoms of pain in addition to fever, hypotension, headache, lethargy, nausea, localized diaphoresis, abdominal pain, paresthesias, and malaise—is unknown but reportedly rare.5,10 There are considerable similarities between Steatoda and true black widow spider venom, which explains the symptom overlap with latrodectism. There are reports of severe debilitation lasting weeks due to pain and decreased affected limb movement after bites from noble false widow spiders.10-12
Nearly all noble false widow spider bite reports describe immediate pain upon bite/envenomation, which is unlike the delayed pain from a black widow spider bite (after 10 minutes or more).6,13,14 Erythema and swelling occur around a pale raised site of envenomation lasting up to 72 hours. The bite site may be highly tender and blister or ulcerate, with reports of cellulitis and local skin necrosis.7,15 Pruritus during this period can be intense, and excoriation increases the risk for complications such as infection. Reports of anaphylaxis following a noble false widow spider bite are rare.5,16 The incidence of bites may be underreported due to the lack of proper identification of the responsible arachnid for those who do not seek care or require hospitalization, though this is not unique to Steatoda.
There are reports of secondary infection after bites and even cases of limb amputation, septicemia, and death.14,17 However, it is unknown if noble false widow spiders are vectors for bacteria transmitted during envenomation, and infection likely is secondary to scratching or inadequate wound care.18,19 Potentially pathogenic bacteria have been isolated from the body surfaces of the noble false widow spider, including Pseudomonas putida, Staphylococcus capitis, and Staphylococcus epidermidis.20 Fortunately, most captured cases (ie, events in which the biting arachnid was properly identified) report symptoms ranging from mild to moderate in severity without the need for hospitalization. A series of 24 reports revealed that all individuals experienced sharp pain upon the initial bite followed by erythema, and 18 of them experienced considerable swelling of the area soon thereafter. One individual experienced temporary paralysis of the affected limb, and 3 individuals experienced hypotension or hypertension in addition to fever, skin necrosis, or cellulitis.14
Treatment
The envenomation site should be washed with antibacterial soap and warm water and should be kept clean to prevent infection. There is no evidence that tight pressure bandaging of these bite sites will restrict venom flow; because it may worsen pain in the area, pressure bandaging is not recommended. When possible, the arachnid should be collected for identification. Supportive care is warranted for symptoms of pain, erythema, and swelling, with the use of cool compresses, oral pain relievers (eg, nonsteroidal anti-inflammatory drugs, acetaminophen), topical anesthetic (eg, lidocaine), or antihistamines as needed.
Urgent care is warranted for patients who experience severe symptoms of steatodism such as hypertension, lymphadenopathy, paresthesia, or limb paralysis. Limited reports show onset of this distress typically within an hour of envenomation. Treatments analogous to those for latrodectism including muscle relaxers and pain medications have demonstrated rapid attenuation of symptoms upon intramuscular administration of antivenom made from Latrodectus species.21-23
Signs of infection warrant bacterial culture with antibiotic susceptibilities to ensure adequate treatment.20 Infections from spider bites can present a few days to a week following envenomation. Symptoms may include spreading redness or an enlarging wound site, pus formation, worsening or unrelenting pain after 24 hours, fevers, flulike symptoms, and muscle cramps.
Final Thoughts
Incidence and Characteristics
The noble false widow spider (Steatoda nobilis) is one of the world’s most invasive spider species, having spread across the globe from Madeira and the Canary Islands into the North Atlantic.1,2Steatoda comprise multiple species of false widow spiders, named for their resemblance to black widow spiders (Latrodectus). The noble false widow spider is the dominant species in buildings in southern Ireland and Great Britain, with a population surge in 2018 that caused multiple temporary school closures in London, England, for fumigation.3 The noble false widow spider was first documented in the United States in Ventura County, California, in 2011, with numerous specimens found in urban areas (eg, in parks, underneath garbage cans) closer to the coastline as well as farther inland. The species may have been introduced to this area by way of Port Hueneme, a city in California with a US naval base with routes to various other military bases in Western Europe.4 Given its already rapid expansion outside of the United States with a concurrent rise in bite reports, dermatologists should be familiar with these invasive and potentially dangerous arachnids.
The spread of noble false widow spiders is assisted by their wide range of temperature tolerance and ability to survive for months with little food and no water. They can live for several years, with one report of a noble false widow spider living up to 7 years.5 These spiders are found inside homes and buildings year-round, and they prefer to build their webs in an elevated position such as the top corner of a room. Steatoda weave tangle webs with crisscrossing threads that often have a denser middle section.5
Noble false widow spiders are sexually dimorphic, with males typically no larger than 1-cm long and females up to 1.4-cm long. They have a dark brown to black thorax and brown abdomen with red-brown legs. Males have brighter cream-colored abdominal markings than females, who lack markings altogether on their distinctive globular abdomen (Figure). The abdominal markings are known to resemble a skull or house.

Although noble false widow spiders are not exclusively synanthropic, they can be found in any crevice in homes or other structures where there are humans such as office buildings.5-7 Up until the last 20 years, reports of bites from noble false widow spiders worldwide were few and far between. In Great Britain, the spiders were first considered to be common in the 1980s, with recent evidence of an urban population boom in the last 5 to 10 years that has coincided with an increase in bite reports.5,8,9
Clinical Significance
Most bites occur in a defensive manner, such as when humans perform activities that disturb the hiding space, cause vibrations in the web, or compress the body of the arachnid. Most envenomations in Great Britain occur while the individual is in bed, though they also may occur during other activities that disturb the spider, such as moving boxes or putting on a pair of pants.5 Occupational exposure to noble false widow spiders may soon be a concern for those involved in construction, carpentry, cleaning, and decorating given their recent invasive spread into the United States.
The venom from these spiders is neurotoxic and cytotoxic, causing moderate to intense pain that may resemble a wasp sting. The incidence of steatodism—which can include symptoms of pain in addition to fever, hypotension, headache, lethargy, nausea, localized diaphoresis, abdominal pain, paresthesias, and malaise—is unknown but reportedly rare.5,10 There are considerable similarities between Steatoda and true black widow spider venom, which explains the symptom overlap with latrodectism. There are reports of severe debilitation lasting weeks due to pain and decreased affected limb movement after bites from noble false widow spiders.10-12
Nearly all noble false widow spider bite reports describe immediate pain upon bite/envenomation, which is unlike the delayed pain from a black widow spider bite (after 10 minutes or more).6,13,14 Erythema and swelling occur around a pale raised site of envenomation lasting up to 72 hours. The bite site may be highly tender and blister or ulcerate, with reports of cellulitis and local skin necrosis.7,15 Pruritus during this period can be intense, and excoriation increases the risk for complications such as infection. Reports of anaphylaxis following a noble false widow spider bite are rare.5,16 The incidence of bites may be underreported due to the lack of proper identification of the responsible arachnid for those who do not seek care or require hospitalization, though this is not unique to Steatoda.
There are reports of secondary infection after bites and even cases of limb amputation, septicemia, and death.14,17 However, it is unknown if noble false widow spiders are vectors for bacteria transmitted during envenomation, and infection likely is secondary to scratching or inadequate wound care.18,19 Potentially pathogenic bacteria have been isolated from the body surfaces of the noble false widow spider, including Pseudomonas putida, Staphylococcus capitis, and Staphylococcus epidermidis.20 Fortunately, most captured cases (ie, events in which the biting arachnid was properly identified) report symptoms ranging from mild to moderate in severity without the need for hospitalization. A series of 24 reports revealed that all individuals experienced sharp pain upon the initial bite followed by erythema, and 18 of them experienced considerable swelling of the area soon thereafter. One individual experienced temporary paralysis of the affected limb, and 3 individuals experienced hypotension or hypertension in addition to fever, skin necrosis, or cellulitis.14
Treatment
The envenomation site should be washed with antibacterial soap and warm water and should be kept clean to prevent infection. There is no evidence that tight pressure bandaging of these bite sites will restrict venom flow; because it may worsen pain in the area, pressure bandaging is not recommended. When possible, the arachnid should be collected for identification. Supportive care is warranted for symptoms of pain, erythema, and swelling, with the use of cool compresses, oral pain relievers (eg, nonsteroidal anti-inflammatory drugs, acetaminophen), topical anesthetic (eg, lidocaine), or antihistamines as needed.
Urgent care is warranted for patients who experience severe symptoms of steatodism such as hypertension, lymphadenopathy, paresthesia, or limb paralysis. Limited reports show onset of this distress typically within an hour of envenomation. Treatments analogous to those for latrodectism including muscle relaxers and pain medications have demonstrated rapid attenuation of symptoms upon intramuscular administration of antivenom made from Latrodectus species.21-23
Signs of infection warrant bacterial culture with antibiotic susceptibilities to ensure adequate treatment.20 Infections from spider bites can present a few days to a week following envenomation. Symptoms may include spreading redness or an enlarging wound site, pus formation, worsening or unrelenting pain after 24 hours, fevers, flulike symptoms, and muscle cramps.
Final Thoughts
- Kulczycki A, Legittimo C, Simeon E, et al. New records of Steatoda nobilis (Thorell, 1875) (Araneae, Theridiidae), an introduced species on the Italian mainland and in Sardinia. Bull Br Arachnological Soc. 2012;15:269-272.
- Bauer T, Feldmeier S, Krehenwinkel H, et al. Steatoda nobilis, a false widow on the rise: a synthesis of past and current distribution trends. NeoBiota. 2019; 42:19. doi:10.3897/neobiota.42.31582
- Murphy A. Web of cries: false widow spider infestation fears forceeleventh school in London to close as outbreak spreads. The Sun.October 19, 2018. Accessed September 21, 2023. https://www.thesun.co.uk/news/7534016/false-widow-spider-infestation-fears-force-eleventh-londonschool-closing
- Vetter R, Rust M. A large European combfoot spider, Steatoda nobilis (Thorell 1875)(Araneae: Theridiidae), newly established in Ventura County, California. The Pan-Pacific Entomologist. 2012;88:92-97.
- Hambler C. The ‘noble false widow’ spider Steatoda nobilis is an emerging public health and ecological threat. OSF Preprints. Preprint posted online October 15, 2019. doi:10.31219/osf.io/axbd4
- Dunbar J, Schulte J, Lyons K, et al. New Irish record for Steatoda triangulosa (Walckenaer, 1802), and new county records for Steatoda nobilis (Thorell, 1875), Steatoda bipunctata (Linnaeus, 1758) and Steatoda grossa (C.L. Koch, 1838). Ir Naturalists J. 2018;36:39-43.
- Duon M, Dunbar J, Afoullouss S, et al. Occurrence, reproductive rate and identification of the non-native noble false widow spider Steatoda nobilis (Thorell, 1875) in Ireland. Biol Environment: Proc Royal Ir Acad. 2017;117B:77-89. doi:10.3318/bioe.2017.11
- Burrows T. Great bitten: Britain’s spider bite capital revealed as Essex with 450 attacks—find out where your town ranks. The Sun. Published April 3, 2019. Accessed September 14, 2023. https://www.thesun.co.uk/news/8782355/britains-spider-bite-capital-revealed-as-essex-with-450- attacks-find-out-where-your-town-ranks/
- Wathen T. Essex is the UK capital for spider bites—and the amount is terrifying. Essex News. April 4, 2019. Accessed September 21, 2023. https://www.essexlive.news/news/essex-news/essex-uk-capital-spider-bites- 2720935
- Dunbar J, Afoullouss S, Sulpice R, et al. Envenomation by the noble false widow spider Steatoda nobilis (Thorell, 1875)—five new cases of steatodism from Ireland and Great Britain. Clin Toxicol (Phila). 2018;56:433-435. doi:10.1080/15563650.2017.1393084
- Dunbar J, Fort A, Redureau D, et al. Venomics approach reveals a high proportion of Latrodectus-like toxins in the venom of the noble false widow spider Steatoda nobilis. Toxins. 2020;12:402.
- Warrell D, Shaheen J, Hillyard P, et al. Neurotoxic envenoming by an immigrant spider (Steatoda nobilis) in southern England. Toxicon. 1991;29:1263-1265.
- Zhou H, Xu K, Zheng PY, et. al. Clinical characteristics of patients with black widow spider bites: a report of 59 patients and single-center experience. World J Emerg Med. 2021;12:317-320. doi:10.5847/wjem.j.1920-8642.2021.04.011
- Dunbar J, Vitkauskaite A, O’Keeffe D, et. al. Bites by the noble false widow spider Steatoda nobilis can induce Latrodectus-like symptoms and vector-borne bacterial infections with implications for public health: a case series. Clin Toxicol (Phila). 2022;60:59-70. doi:10.1080/15563650.2021.1928165
- Dunbar J, Sulpice R, Dugon M. The kiss of (cell) death: can venom-induced immune response contribute to dermal necrosis following arthropod envenomations? Clin Toxicol. 2019;57:677-685. doi:10.1080/15563650.2019.1578367
- Magee J. Bite ‘nightmare’: close encounter with a false widow. The Bournemouth Echo. September 7, 2009. Accessed September 21, 2023. http://www.bournemouthecho.co.uk/news/4582887.Bite____nightmare_____close_encounter_with_a_false_widow_spider/
- Marsh H. Woman nearly loses hand after bite from false widow. Daily Echo. April 17, 2012. Accessed September 21, 2023. https://www.bournemouthecho.co.uk/news/9652335.woman-nearly-loses-hand-after-bite-from-false-widow-spider/
- Stuber N, Nentwig W. How informative are case studies of spider bites in the medical literature? Toxicon. 2016;114:40-44. doi:10.1016/j.toxicon.2016.02.023
- Vetter R, Swanson D, Weinstein S, et. al. Do spiders vector bacteria during bites? the evidence indicates otherwise. Toxicon. 2015;93:171-174. doi:10.1016/j.toxicon.2014.11.229
- Dunbar J, Khan N, Abberton C, et al. Synanthropic spiders, including the global invasive noble false widow Steatoda nobilis, are reservoirs for medically important and antibiotic resistant bacteria. Sci Rep. 2020;10:20916. doi:10.1038/s41598-020-77839-9
- Atakuziev BU, Wright CE, Graudins A, et al. Efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of clinical envenomation by the cupboard spider Steatoda capensis (Theridiidae). Toxicon. 2014;86:68-78. doi:10.1016/j.toxicon.2014.04.011
- Graudins A, Gunja N, Broady KW, et al. Clinical and in vitro evidence for the efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of envenomation by a cupboard spider (Steatoda grossa). Toxicon. 2002;40:767-775. doi:10.1016/S0041-0101(01)00280-X.
- South M, Wirth P, Winkel KD. Redback spider antivenom used to treat envenomation by a juvenile Steatoda spider. Med J Aust. 1998;169:642-642. doi:10.5694/j.1326-5377.1998.tb123445.x
- Kulczycki A, Legittimo C, Simeon E, et al. New records of Steatoda nobilis (Thorell, 1875) (Araneae, Theridiidae), an introduced species on the Italian mainland and in Sardinia. Bull Br Arachnological Soc. 2012;15:269-272.
- Bauer T, Feldmeier S, Krehenwinkel H, et al. Steatoda nobilis, a false widow on the rise: a synthesis of past and current distribution trends. NeoBiota. 2019; 42:19. doi:10.3897/neobiota.42.31582
- Murphy A. Web of cries: false widow spider infestation fears forceeleventh school in London to close as outbreak spreads. The Sun.October 19, 2018. Accessed September 21, 2023. https://www.thesun.co.uk/news/7534016/false-widow-spider-infestation-fears-force-eleventh-londonschool-closing
- Vetter R, Rust M. A large European combfoot spider, Steatoda nobilis (Thorell 1875)(Araneae: Theridiidae), newly established in Ventura County, California. The Pan-Pacific Entomologist. 2012;88:92-97.
- Hambler C. The ‘noble false widow’ spider Steatoda nobilis is an emerging public health and ecological threat. OSF Preprints. Preprint posted online October 15, 2019. doi:10.31219/osf.io/axbd4
- Dunbar J, Schulte J, Lyons K, et al. New Irish record for Steatoda triangulosa (Walckenaer, 1802), and new county records for Steatoda nobilis (Thorell, 1875), Steatoda bipunctata (Linnaeus, 1758) and Steatoda grossa (C.L. Koch, 1838). Ir Naturalists J. 2018;36:39-43.
- Duon M, Dunbar J, Afoullouss S, et al. Occurrence, reproductive rate and identification of the non-native noble false widow spider Steatoda nobilis (Thorell, 1875) in Ireland. Biol Environment: Proc Royal Ir Acad. 2017;117B:77-89. doi:10.3318/bioe.2017.11
- Burrows T. Great bitten: Britain’s spider bite capital revealed as Essex with 450 attacks—find out where your town ranks. The Sun. Published April 3, 2019. Accessed September 14, 2023. https://www.thesun.co.uk/news/8782355/britains-spider-bite-capital-revealed-as-essex-with-450- attacks-find-out-where-your-town-ranks/
- Wathen T. Essex is the UK capital for spider bites—and the amount is terrifying. Essex News. April 4, 2019. Accessed September 21, 2023. https://www.essexlive.news/news/essex-news/essex-uk-capital-spider-bites- 2720935
- Dunbar J, Afoullouss S, Sulpice R, et al. Envenomation by the noble false widow spider Steatoda nobilis (Thorell, 1875)—five new cases of steatodism from Ireland and Great Britain. Clin Toxicol (Phila). 2018;56:433-435. doi:10.1080/15563650.2017.1393084
- Dunbar J, Fort A, Redureau D, et al. Venomics approach reveals a high proportion of Latrodectus-like toxins in the venom of the noble false widow spider Steatoda nobilis. Toxins. 2020;12:402.
- Warrell D, Shaheen J, Hillyard P, et al. Neurotoxic envenoming by an immigrant spider (Steatoda nobilis) in southern England. Toxicon. 1991;29:1263-1265.
- Zhou H, Xu K, Zheng PY, et. al. Clinical characteristics of patients with black widow spider bites: a report of 59 patients and single-center experience. World J Emerg Med. 2021;12:317-320. doi:10.5847/wjem.j.1920-8642.2021.04.011
- Dunbar J, Vitkauskaite A, O’Keeffe D, et. al. Bites by the noble false widow spider Steatoda nobilis can induce Latrodectus-like symptoms and vector-borne bacterial infections with implications for public health: a case series. Clin Toxicol (Phila). 2022;60:59-70. doi:10.1080/15563650.2021.1928165
- Dunbar J, Sulpice R, Dugon M. The kiss of (cell) death: can venom-induced immune response contribute to dermal necrosis following arthropod envenomations? Clin Toxicol. 2019;57:677-685. doi:10.1080/15563650.2019.1578367
- Magee J. Bite ‘nightmare’: close encounter with a false widow. The Bournemouth Echo. September 7, 2009. Accessed September 21, 2023. http://www.bournemouthecho.co.uk/news/4582887.Bite____nightmare_____close_encounter_with_a_false_widow_spider/
- Marsh H. Woman nearly loses hand after bite from false widow. Daily Echo. April 17, 2012. Accessed September 21, 2023. https://www.bournemouthecho.co.uk/news/9652335.woman-nearly-loses-hand-after-bite-from-false-widow-spider/
- Stuber N, Nentwig W. How informative are case studies of spider bites in the medical literature? Toxicon. 2016;114:40-44. doi:10.1016/j.toxicon.2016.02.023
- Vetter R, Swanson D, Weinstein S, et. al. Do spiders vector bacteria during bites? the evidence indicates otherwise. Toxicon. 2015;93:171-174. doi:10.1016/j.toxicon.2014.11.229
- Dunbar J, Khan N, Abberton C, et al. Synanthropic spiders, including the global invasive noble false widow Steatoda nobilis, are reservoirs for medically important and antibiotic resistant bacteria. Sci Rep. 2020;10:20916. doi:10.1038/s41598-020-77839-9
- Atakuziev BU, Wright CE, Graudins A, et al. Efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of clinical envenomation by the cupboard spider Steatoda capensis (Theridiidae). Toxicon. 2014;86:68-78. doi:10.1016/j.toxicon.2014.04.011
- Graudins A, Gunja N, Broady KW, et al. Clinical and in vitro evidence for the efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of envenomation by a cupboard spider (Steatoda grossa). Toxicon. 2002;40:767-775. doi:10.1016/S0041-0101(01)00280-X.
- South M, Wirth P, Winkel KD. Redback spider antivenom used to treat envenomation by a juvenile Steatoda spider. Med J Aust. 1998;169:642-642. doi:10.5694/j.1326-5377.1998.tb123445.x
PRACTICE POINTS
- With evidence of a recent population boom of noble false widow spiders in Europe and spread to California, dermatologists should be aware of these spiders and their bites.
- Symptoms of Steatoda bites (steatodism) include immediate pain followed by intense pruritus, swelling, erythema, and possibly systemic symptoms such as fever. Secondary infections such as cellulitis and septicemia are risks.
- The envenomation site should be kept clean to prevent secondary infection, and medical care should be sought when there is evidence of ulceration or cellulitis.
What’s Eating You? Tropical Rat Mite (Ornithonyssus bacoti)
The tropical rat mite (Ornithonyssus bacoti) belongs to the family Macronyssidae. Theses mites are commonly mistaken for red bird mites or Nordic bird mites because they belong to the same family and have similar characteristics.1 Although O bacoti is called the tropical rat mite, it also can be found in moderate climates.2,3
Characteristics
The life cycle of a tropical rat mite lasts 11 to 13 days and includes 5 stages: egg, larva, protonymph, deutonymph, and adult.1,2 The length of the mite (0.3–0.7 mm) varies with the stage of development.1 Adults can reach 0.75 to 1.40 mm, with females larger than males and possibly visible with the naked eye.1,2
Two or 3 days after a blood meal, the female mite lays approximately 100 eggs in its nest but not on the surface of a host. The eggs hatch into larvae after 1 to 4 days and go on to complete their life cyle.1 During developmental stages, mites occupy their hosts for blood meals. Mites search for their hosts at night and prefer wild or pet rodents for blood meals but are not host specific and can be found on many mammals including birds, cats, racoons, and squirrels.4
Although tropical rat mites prefer rodent hosts, they can infest humans when their preferred host is unavailable. In the United States, the first case of human dermatitis due to a tropical rate mite occurred in 1923. In Europe, rat mite dermatitis was first reported in a human in 1931, possibly due to contamination of sailing vessels.4
Infestation and Transmission
Tropical rat mites prefer wild and pet rodents as hosts because the mites are able to feed on their blood over long periods.4 During the day, the mite spends most of its time hiding in dark dry spaces; it is most active during the night, traveling to find a host for meals.3-5 If a preferred host is not present, the mite may choose to infest a human.5
Human infestation occurs most often upon close bodily contact with an infected animal or pet rodent that was sold without parasites having been eliminated.3-5 Mites are able to survive without a host for as long as 6 months; they may travel after a meal.1,2 Therefore, individuals who do not have a pet rodent can be infested if an infected wild rodent has infested their living space.1,3-5
Clinical Presentation of Infestation
Patients infested with tropical rat mites present with pruritic cutaneous lesions, most often on unclothed parts of the body that are easily exposed to mites; lesions rarely occur on the scalp.5 People of any age or gender can be infested. Rat mite bites can present as single or grouped, pruritic, erythematous papules ranging in size from 4 to 10 mm in diameter.5-7 Excoriations may be present due to excessive scratching. Although rare, vesicles or nodules have been reported.5,7
Diagnosis of the underlying cause of the cutaneous manifestations often is difficult because mites are not visible during the day, as they are less active then.2 Lesions often are misdiagnosed as an allergic response, a bacterial infection, or various forms of dermatitis.1 A parasitic cause often is not considered unless the physician or patient detects a mite or many trials of therapies fail to provide relief.1,3-5 Eliciting a thorough history may disclose that the patient has had close contact with rodents or lives in a community center, shelter, or shared space. If any of the patient’s close contacts have a similar presentation, infestation with mites should be considered.
Treatment and Prevention
Patients should be educated about treatment options and measures that need to be taken to prevent reinfection. It has been reported that tropical rat mites can survive without a blood meal for as long as 6 months; therefore, meticulous inspection and decontamination of all living spaces is required.1,4 Once identified, physicians may prescribe an antiparasitic such as permethrin or pyriproxyfen to prevent further infestation and eliminate mites on the host.5 Lindane and benzyl benzoate previously were reported to be effective but should be prescribed only in correctly diagnosed cases due to the potential adverse effects of both therapies.4,7-10 For effective treatment, physicians should thoroughly review the proper application of topical treatments with patients. Topical creams should be massaged into the skin from the head to the soles of the feet, covering all creases of the skin and between the fingers and toes. Antiparasitic creams should be left on the skin for 8 to 14 hours, and all members of the household should be examined and treated, if necessary, by a physician. A thorough bath removes tropical rat mites, but preventive measures should be taken to prevent reinfestation.4 Antihistamines or glucocorticoids also can be used as symptomatic treatment.6,8
Avoiding Reinfestation—Preventive measures should be taken to prevent reinfestation, including evaluation by an exterminator for any wild rodents to remove nests and treat the living space with an acaricide.5 Insecticides administered by exterminators, including malathion, methyl carbamate, and lindane, also have been reported to be effective for preventing reinfestation.5,7-9 A veterinarian should be consulted if the patient owns any pets to ensure proper identification of any potential tropical rat mites and treatments that may be necessary for any household pets.1
Case Report
A 68-year-old man presented to the dermatology outpatient clinic with diffuse pruritus of the skin and scalp. He reported no other symptoms and had never had a total-body skin examination. His primary care physician recently prescribed a dose pack of methylprednisolone 4-mg tablets, which relieved the symptoms except for a mild scalp itch. His wife did not experience itching, and he denied noticing mites or fleas on his pet dog. Physical examination did not reveal any contributory findings, such as erythema or rash. Ketoconazole shampoo 2% and fluocinolone solution 0.01% were prescribed for scalp pruritus; however, he could not afford those medications and therefore did not take them.
Two weeks later, the patient presented with diffuse itching that involved the scalp, trunk, and extremities. He denied groin pruritus. He reported that the itching was worse at night. His wife continued to be asymptomatic. The patient reported that his health screening was up-to-date, and he had no interval health changes. A complete blood cell count, thyroid studies, and a comprehensive metabolic panel performed recently were within reference range. He denied recent travel or taking new medications. Physical examination revealed a somewhat linear distribution of erythematous urticarial papules on the right side of the abdomen. Red dermatographic excoriations were noted on the back. No burrows were visualized. He was given intramuscular triamcinolone 60 mg and was advised to have his house evaluated for bed bugs and his pet dog evaluated by a veterinarian for mites. During the triamcinolone injection, the medical assistant observed a 1- to 2-mm red insect, which fell into his clothing and could not be further evaluated.
After 1 month, the patient had no improvement of the pruritus; instead, it became worse. During this time, his wife developed intermittent urticarial-like eruptions. He was taking oral diphenhydramine nightly and applying triamcinolone cream 0.5% that he had at home from an earlier skin problem as needed. Physical examination findings correlated with worsening symptoms. He had multiple erythematous urticarial papules—many of which were excoriated—across the chest, abdomen, buttocks, and back. The arms had multiple excoriations. The urticarial papules coalesced in the anterior axillary folds, yet no burrows were visualized. In the left anterior axillary fold adjacent to one of the urticarial papules, a 1-mm mobile mite was identified on dermoscopy. Further evaluation by microscopy showed morphologic characteristics of a tropical rat mite (Figure). The patient admitted that his house had a mouse infestation that he was struggling to eliminate. Permethrin cream 5% was prescribed. Because the patient could not afford the prescription, he was advised to use the triamcinolone cream 0.5%and oral diphenhydramine that he had at home nightly for symptomatic relief. He was advised to hire an exterminator to eradicate the mouse and mite infestation to prevent reinfestation.

Identification of Rate Mite Dermatitis
The characteristics of tropical rat mite dermatitis can be confused with many other conditions, such as infection. Even when a mite is identified, it can be difficult to classify it as a tropical rat mite. To confirm the diagnosis of tropical rat mite dermatitis, the parasite needs to be identified. Skin scrapings can be collected from pruritic lesions and examined microscopically in the hope of revealing the rat mites. The recommendation is to collect skin scrapings from the dorsal aspect of the hands or from the neck.5 Patients may report finding mites in their living space or on their bedding or clothing.
Although the tropical rat mite was reported as a vector for endemic typhus between humans, no other cases of transmission between humans have been reported since.11,12 Studies reporting non–human subject research and case reports have shown that O bacoti is a vector for Rickettsia akari, Coxiella burnetii, Francisella tularensis, Yersinia pestis, Eastern equine encephalitis virus (Alphavirus), Enterovirus (Picornaviridae), Langat virus (Flavivirus), and Hantaan orthohantavirus.5,11-17 However, no cases of these infectious diseases being transmitted naturally have been reported.5
Confirmation of O bacoti as a vector for human pathogens is difficult because it relies on identification of the mite in the clinic.5 The epidemiologic importance of the mite in transmitting infectious disease is unknown; reports of human cases of mite infestation are rare. We present this information to increase awareness and help dermatologists and other health care providers identify O bacoti.
- Beck W, Fölster-Holst R. Tropical rat mites (Ornithonyssus bacoti)—serious ectoparasites. J Dtsch Dermatol Ges. 2009;7:667-670. doi:10.1111/j.1610-0387.2009.07140.x
- Baumstark J, Beck W, Hofmann H. Outbreak of tropical rat mite (Ornithonyssus bacoti) dermatitis in a home for disabled persons. Dermatology. 2007;215:66-68. doi:10.1159/000102037
- Beck W. Occurrence of a house-infesting tropical rat mite (Ornithonyssus bacoti) on murides and human beings. Travel Med Infect Dis. 2008;6:245-249. doi:10.1016/j.tmaid.2008.01.002
- Beck W. Tropical rat mites as newly emerging disease pathogens in rodents and man. Trav Med Infect Dis. 2007;5:403. doi:10.1016/j.tmaid.2007.09.016
- Engel PM, Welzel J, Maass M, et al. Tropical rat mite dermatitis: case report and review. Clin Infect Dis. 1998;27:1465-1469. doi:10.1086/515016
- Hetherington GW, Holder WR, Smith EB. Rat mite dermatitis. JAMA. 1971;215:1499-1500.
- Fox JG. Outbreak of tropical rat mite dermatitis in laboratory personnel. Arch Dermatol. 1982;118:676-678. doi:10.1001/archderm.1982.01650210056019
- Fishman HC. Rat mite dermatitis. Cutis. 1988;42:414-416.
- Ram SM, Satija KC, Kaushik RK. Ornithonyssus bacoti infestation in laboratory personnel and veterinary students. Int J Zoonoses. 1986;13:138-140.
- Brown S, Becher J, Brady W. Treatment of ectoparasitic infections: review of the English-language literature, 1982-1992. Clin Infect Dis. 1995;20(suppl 1):S104-S109. doi:10.1093/clinids/20.supplement_1.s104
- Reeves WK, Loftis AD, Szumlas DE, et al. Rickettsial pathogens in the tropical rat mite Ornithonyssus bacoti (Acari: Macronyssidae) from Egyptian rats (Rattus spp.). Exp Appl Acarol. 2007;41:101-107. doi:10.1007/s10493-006-9040-3
- Philip CB, Hughes LE. The tropical rat mite; Liponyssus bacoti, as an experimental vector of rickettsialpox. Am J Trop Med Hyg. 1948;28:697-705. doi:10.4269/ajtmh.1948.s1-28.697
- Zemskaia AA, Pchelkina AA. Experimental infection of ticks Dermanyssus gallinae Redi Bdellonyssus bacoti Hirst with Q fever. Dokl Akad Nauk SSSR. 1955;101:391-392.
- Hopla CE. Experimental transmission of tularemia by the tropical rat mite. Am J Trop Med Hyg. 1951;31:768-783. doi:10.4269/ajtmh.1951.s1-31.768
- Clark GM, Lutz AE, Fadnessl. Observations on the ability of Haemogamasus liponyssoides Ewing and Ornithonyssus bacoti (Hirst) (Acarina, Gamasina) to retain eastern equine encephalitis virus: preliminary report. Am J Trop Med Hyg. 1966;15:107-112. doi:10.4269/ajtmh.1966.15.107
- Schwab M, Allen R, Sulkin SE. The tropical rat mite (Liponyssus bacoti) as an experimental vector of Coxsackie virus. Am J Trop Med Hyg. 1952;1:982-986. doi:10.4269/ajtmh.1952.1.982
- Durden LA, Turell MJ. Inefficient mechanical transmission of Langat (tick-borne encephalitis virus complex) virus by blood-feeding mites (Acari) to laboratory mice. J Med Entomol. 1993;30:639-641. doi:10.1093/jmedent/30.3.639
The tropical rat mite (Ornithonyssus bacoti) belongs to the family Macronyssidae. Theses mites are commonly mistaken for red bird mites or Nordic bird mites because they belong to the same family and have similar characteristics.1 Although O bacoti is called the tropical rat mite, it also can be found in moderate climates.2,3
Characteristics
The life cycle of a tropical rat mite lasts 11 to 13 days and includes 5 stages: egg, larva, protonymph, deutonymph, and adult.1,2 The length of the mite (0.3–0.7 mm) varies with the stage of development.1 Adults can reach 0.75 to 1.40 mm, with females larger than males and possibly visible with the naked eye.1,2
Two or 3 days after a blood meal, the female mite lays approximately 100 eggs in its nest but not on the surface of a host. The eggs hatch into larvae after 1 to 4 days and go on to complete their life cyle.1 During developmental stages, mites occupy their hosts for blood meals. Mites search for their hosts at night and prefer wild or pet rodents for blood meals but are not host specific and can be found on many mammals including birds, cats, racoons, and squirrels.4
Although tropical rat mites prefer rodent hosts, they can infest humans when their preferred host is unavailable. In the United States, the first case of human dermatitis due to a tropical rate mite occurred in 1923. In Europe, rat mite dermatitis was first reported in a human in 1931, possibly due to contamination of sailing vessels.4
Infestation and Transmission
Tropical rat mites prefer wild and pet rodents as hosts because the mites are able to feed on their blood over long periods.4 During the day, the mite spends most of its time hiding in dark dry spaces; it is most active during the night, traveling to find a host for meals.3-5 If a preferred host is not present, the mite may choose to infest a human.5
Human infestation occurs most often upon close bodily contact with an infected animal or pet rodent that was sold without parasites having been eliminated.3-5 Mites are able to survive without a host for as long as 6 months; they may travel after a meal.1,2 Therefore, individuals who do not have a pet rodent can be infested if an infected wild rodent has infested their living space.1,3-5
Clinical Presentation of Infestation
Patients infested with tropical rat mites present with pruritic cutaneous lesions, most often on unclothed parts of the body that are easily exposed to mites; lesions rarely occur on the scalp.5 People of any age or gender can be infested. Rat mite bites can present as single or grouped, pruritic, erythematous papules ranging in size from 4 to 10 mm in diameter.5-7 Excoriations may be present due to excessive scratching. Although rare, vesicles or nodules have been reported.5,7
Diagnosis of the underlying cause of the cutaneous manifestations often is difficult because mites are not visible during the day, as they are less active then.2 Lesions often are misdiagnosed as an allergic response, a bacterial infection, or various forms of dermatitis.1 A parasitic cause often is not considered unless the physician or patient detects a mite or many trials of therapies fail to provide relief.1,3-5 Eliciting a thorough history may disclose that the patient has had close contact with rodents or lives in a community center, shelter, or shared space. If any of the patient’s close contacts have a similar presentation, infestation with mites should be considered.
Treatment and Prevention
Patients should be educated about treatment options and measures that need to be taken to prevent reinfection. It has been reported that tropical rat mites can survive without a blood meal for as long as 6 months; therefore, meticulous inspection and decontamination of all living spaces is required.1,4 Once identified, physicians may prescribe an antiparasitic such as permethrin or pyriproxyfen to prevent further infestation and eliminate mites on the host.5 Lindane and benzyl benzoate previously were reported to be effective but should be prescribed only in correctly diagnosed cases due to the potential adverse effects of both therapies.4,7-10 For effective treatment, physicians should thoroughly review the proper application of topical treatments with patients. Topical creams should be massaged into the skin from the head to the soles of the feet, covering all creases of the skin and between the fingers and toes. Antiparasitic creams should be left on the skin for 8 to 14 hours, and all members of the household should be examined and treated, if necessary, by a physician. A thorough bath removes tropical rat mites, but preventive measures should be taken to prevent reinfestation.4 Antihistamines or glucocorticoids also can be used as symptomatic treatment.6,8
Avoiding Reinfestation—Preventive measures should be taken to prevent reinfestation, including evaluation by an exterminator for any wild rodents to remove nests and treat the living space with an acaricide.5 Insecticides administered by exterminators, including malathion, methyl carbamate, and lindane, also have been reported to be effective for preventing reinfestation.5,7-9 A veterinarian should be consulted if the patient owns any pets to ensure proper identification of any potential tropical rat mites and treatments that may be necessary for any household pets.1
Case Report
A 68-year-old man presented to the dermatology outpatient clinic with diffuse pruritus of the skin and scalp. He reported no other symptoms and had never had a total-body skin examination. His primary care physician recently prescribed a dose pack of methylprednisolone 4-mg tablets, which relieved the symptoms except for a mild scalp itch. His wife did not experience itching, and he denied noticing mites or fleas on his pet dog. Physical examination did not reveal any contributory findings, such as erythema or rash. Ketoconazole shampoo 2% and fluocinolone solution 0.01% were prescribed for scalp pruritus; however, he could not afford those medications and therefore did not take them.
Two weeks later, the patient presented with diffuse itching that involved the scalp, trunk, and extremities. He denied groin pruritus. He reported that the itching was worse at night. His wife continued to be asymptomatic. The patient reported that his health screening was up-to-date, and he had no interval health changes. A complete blood cell count, thyroid studies, and a comprehensive metabolic panel performed recently were within reference range. He denied recent travel or taking new medications. Physical examination revealed a somewhat linear distribution of erythematous urticarial papules on the right side of the abdomen. Red dermatographic excoriations were noted on the back. No burrows were visualized. He was given intramuscular triamcinolone 60 mg and was advised to have his house evaluated for bed bugs and his pet dog evaluated by a veterinarian for mites. During the triamcinolone injection, the medical assistant observed a 1- to 2-mm red insect, which fell into his clothing and could not be further evaluated.
After 1 month, the patient had no improvement of the pruritus; instead, it became worse. During this time, his wife developed intermittent urticarial-like eruptions. He was taking oral diphenhydramine nightly and applying triamcinolone cream 0.5% that he had at home from an earlier skin problem as needed. Physical examination findings correlated with worsening symptoms. He had multiple erythematous urticarial papules—many of which were excoriated—across the chest, abdomen, buttocks, and back. The arms had multiple excoriations. The urticarial papules coalesced in the anterior axillary folds, yet no burrows were visualized. In the left anterior axillary fold adjacent to one of the urticarial papules, a 1-mm mobile mite was identified on dermoscopy. Further evaluation by microscopy showed morphologic characteristics of a tropical rat mite (Figure). The patient admitted that his house had a mouse infestation that he was struggling to eliminate. Permethrin cream 5% was prescribed. Because the patient could not afford the prescription, he was advised to use the triamcinolone cream 0.5%and oral diphenhydramine that he had at home nightly for symptomatic relief. He was advised to hire an exterminator to eradicate the mouse and mite infestation to prevent reinfestation.

Identification of Rate Mite Dermatitis
The characteristics of tropical rat mite dermatitis can be confused with many other conditions, such as infection. Even when a mite is identified, it can be difficult to classify it as a tropical rat mite. To confirm the diagnosis of tropical rat mite dermatitis, the parasite needs to be identified. Skin scrapings can be collected from pruritic lesions and examined microscopically in the hope of revealing the rat mites. The recommendation is to collect skin scrapings from the dorsal aspect of the hands or from the neck.5 Patients may report finding mites in their living space or on their bedding or clothing.
Although the tropical rat mite was reported as a vector for endemic typhus between humans, no other cases of transmission between humans have been reported since.11,12 Studies reporting non–human subject research and case reports have shown that O bacoti is a vector for Rickettsia akari, Coxiella burnetii, Francisella tularensis, Yersinia pestis, Eastern equine encephalitis virus (Alphavirus), Enterovirus (Picornaviridae), Langat virus (Flavivirus), and Hantaan orthohantavirus.5,11-17 However, no cases of these infectious diseases being transmitted naturally have been reported.5
Confirmation of O bacoti as a vector for human pathogens is difficult because it relies on identification of the mite in the clinic.5 The epidemiologic importance of the mite in transmitting infectious disease is unknown; reports of human cases of mite infestation are rare. We present this information to increase awareness and help dermatologists and other health care providers identify O bacoti.
The tropical rat mite (Ornithonyssus bacoti) belongs to the family Macronyssidae. Theses mites are commonly mistaken for red bird mites or Nordic bird mites because they belong to the same family and have similar characteristics.1 Although O bacoti is called the tropical rat mite, it also can be found in moderate climates.2,3
Characteristics
The life cycle of a tropical rat mite lasts 11 to 13 days and includes 5 stages: egg, larva, protonymph, deutonymph, and adult.1,2 The length of the mite (0.3–0.7 mm) varies with the stage of development.1 Adults can reach 0.75 to 1.40 mm, with females larger than males and possibly visible with the naked eye.1,2
Two or 3 days after a blood meal, the female mite lays approximately 100 eggs in its nest but not on the surface of a host. The eggs hatch into larvae after 1 to 4 days and go on to complete their life cyle.1 During developmental stages, mites occupy their hosts for blood meals. Mites search for their hosts at night and prefer wild or pet rodents for blood meals but are not host specific and can be found on many mammals including birds, cats, racoons, and squirrels.4
Although tropical rat mites prefer rodent hosts, they can infest humans when their preferred host is unavailable. In the United States, the first case of human dermatitis due to a tropical rate mite occurred in 1923. In Europe, rat mite dermatitis was first reported in a human in 1931, possibly due to contamination of sailing vessels.4
Infestation and Transmission
Tropical rat mites prefer wild and pet rodents as hosts because the mites are able to feed on their blood over long periods.4 During the day, the mite spends most of its time hiding in dark dry spaces; it is most active during the night, traveling to find a host for meals.3-5 If a preferred host is not present, the mite may choose to infest a human.5
Human infestation occurs most often upon close bodily contact with an infected animal or pet rodent that was sold without parasites having been eliminated.3-5 Mites are able to survive without a host for as long as 6 months; they may travel after a meal.1,2 Therefore, individuals who do not have a pet rodent can be infested if an infected wild rodent has infested their living space.1,3-5
Clinical Presentation of Infestation
Patients infested with tropical rat mites present with pruritic cutaneous lesions, most often on unclothed parts of the body that are easily exposed to mites; lesions rarely occur on the scalp.5 People of any age or gender can be infested. Rat mite bites can present as single or grouped, pruritic, erythematous papules ranging in size from 4 to 10 mm in diameter.5-7 Excoriations may be present due to excessive scratching. Although rare, vesicles or nodules have been reported.5,7
Diagnosis of the underlying cause of the cutaneous manifestations often is difficult because mites are not visible during the day, as they are less active then.2 Lesions often are misdiagnosed as an allergic response, a bacterial infection, or various forms of dermatitis.1 A parasitic cause often is not considered unless the physician or patient detects a mite or many trials of therapies fail to provide relief.1,3-5 Eliciting a thorough history may disclose that the patient has had close contact with rodents or lives in a community center, shelter, or shared space. If any of the patient’s close contacts have a similar presentation, infestation with mites should be considered.
Treatment and Prevention
Patients should be educated about treatment options and measures that need to be taken to prevent reinfection. It has been reported that tropical rat mites can survive without a blood meal for as long as 6 months; therefore, meticulous inspection and decontamination of all living spaces is required.1,4 Once identified, physicians may prescribe an antiparasitic such as permethrin or pyriproxyfen to prevent further infestation and eliminate mites on the host.5 Lindane and benzyl benzoate previously were reported to be effective but should be prescribed only in correctly diagnosed cases due to the potential adverse effects of both therapies.4,7-10 For effective treatment, physicians should thoroughly review the proper application of topical treatments with patients. Topical creams should be massaged into the skin from the head to the soles of the feet, covering all creases of the skin and between the fingers and toes. Antiparasitic creams should be left on the skin for 8 to 14 hours, and all members of the household should be examined and treated, if necessary, by a physician. A thorough bath removes tropical rat mites, but preventive measures should be taken to prevent reinfestation.4 Antihistamines or glucocorticoids also can be used as symptomatic treatment.6,8
Avoiding Reinfestation—Preventive measures should be taken to prevent reinfestation, including evaluation by an exterminator for any wild rodents to remove nests and treat the living space with an acaricide.5 Insecticides administered by exterminators, including malathion, methyl carbamate, and lindane, also have been reported to be effective for preventing reinfestation.5,7-9 A veterinarian should be consulted if the patient owns any pets to ensure proper identification of any potential tropical rat mites and treatments that may be necessary for any household pets.1
Case Report
A 68-year-old man presented to the dermatology outpatient clinic with diffuse pruritus of the skin and scalp. He reported no other symptoms and had never had a total-body skin examination. His primary care physician recently prescribed a dose pack of methylprednisolone 4-mg tablets, which relieved the symptoms except for a mild scalp itch. His wife did not experience itching, and he denied noticing mites or fleas on his pet dog. Physical examination did not reveal any contributory findings, such as erythema or rash. Ketoconazole shampoo 2% and fluocinolone solution 0.01% were prescribed for scalp pruritus; however, he could not afford those medications and therefore did not take them.
Two weeks later, the patient presented with diffuse itching that involved the scalp, trunk, and extremities. He denied groin pruritus. He reported that the itching was worse at night. His wife continued to be asymptomatic. The patient reported that his health screening was up-to-date, and he had no interval health changes. A complete blood cell count, thyroid studies, and a comprehensive metabolic panel performed recently were within reference range. He denied recent travel or taking new medications. Physical examination revealed a somewhat linear distribution of erythematous urticarial papules on the right side of the abdomen. Red dermatographic excoriations were noted on the back. No burrows were visualized. He was given intramuscular triamcinolone 60 mg and was advised to have his house evaluated for bed bugs and his pet dog evaluated by a veterinarian for mites. During the triamcinolone injection, the medical assistant observed a 1- to 2-mm red insect, which fell into his clothing and could not be further evaluated.
After 1 month, the patient had no improvement of the pruritus; instead, it became worse. During this time, his wife developed intermittent urticarial-like eruptions. He was taking oral diphenhydramine nightly and applying triamcinolone cream 0.5% that he had at home from an earlier skin problem as needed. Physical examination findings correlated with worsening symptoms. He had multiple erythematous urticarial papules—many of which were excoriated—across the chest, abdomen, buttocks, and back. The arms had multiple excoriations. The urticarial papules coalesced in the anterior axillary folds, yet no burrows were visualized. In the left anterior axillary fold adjacent to one of the urticarial papules, a 1-mm mobile mite was identified on dermoscopy. Further evaluation by microscopy showed morphologic characteristics of a tropical rat mite (Figure). The patient admitted that his house had a mouse infestation that he was struggling to eliminate. Permethrin cream 5% was prescribed. Because the patient could not afford the prescription, he was advised to use the triamcinolone cream 0.5%and oral diphenhydramine that he had at home nightly for symptomatic relief. He was advised to hire an exterminator to eradicate the mouse and mite infestation to prevent reinfestation.

Identification of Rate Mite Dermatitis
The characteristics of tropical rat mite dermatitis can be confused with many other conditions, such as infection. Even when a mite is identified, it can be difficult to classify it as a tropical rat mite. To confirm the diagnosis of tropical rat mite dermatitis, the parasite needs to be identified. Skin scrapings can be collected from pruritic lesions and examined microscopically in the hope of revealing the rat mites. The recommendation is to collect skin scrapings from the dorsal aspect of the hands or from the neck.5 Patients may report finding mites in their living space or on their bedding or clothing.
Although the tropical rat mite was reported as a vector for endemic typhus between humans, no other cases of transmission between humans have been reported since.11,12 Studies reporting non–human subject research and case reports have shown that O bacoti is a vector for Rickettsia akari, Coxiella burnetii, Francisella tularensis, Yersinia pestis, Eastern equine encephalitis virus (Alphavirus), Enterovirus (Picornaviridae), Langat virus (Flavivirus), and Hantaan orthohantavirus.5,11-17 However, no cases of these infectious diseases being transmitted naturally have been reported.5
Confirmation of O bacoti as a vector for human pathogens is difficult because it relies on identification of the mite in the clinic.5 The epidemiologic importance of the mite in transmitting infectious disease is unknown; reports of human cases of mite infestation are rare. We present this information to increase awareness and help dermatologists and other health care providers identify O bacoti.
- Beck W, Fölster-Holst R. Tropical rat mites (Ornithonyssus bacoti)—serious ectoparasites. J Dtsch Dermatol Ges. 2009;7:667-670. doi:10.1111/j.1610-0387.2009.07140.x
- Baumstark J, Beck W, Hofmann H. Outbreak of tropical rat mite (Ornithonyssus bacoti) dermatitis in a home for disabled persons. Dermatology. 2007;215:66-68. doi:10.1159/000102037
- Beck W. Occurrence of a house-infesting tropical rat mite (Ornithonyssus bacoti) on murides and human beings. Travel Med Infect Dis. 2008;6:245-249. doi:10.1016/j.tmaid.2008.01.002
- Beck W. Tropical rat mites as newly emerging disease pathogens in rodents and man. Trav Med Infect Dis. 2007;5:403. doi:10.1016/j.tmaid.2007.09.016
- Engel PM, Welzel J, Maass M, et al. Tropical rat mite dermatitis: case report and review. Clin Infect Dis. 1998;27:1465-1469. doi:10.1086/515016
- Hetherington GW, Holder WR, Smith EB. Rat mite dermatitis. JAMA. 1971;215:1499-1500.
- Fox JG. Outbreak of tropical rat mite dermatitis in laboratory personnel. Arch Dermatol. 1982;118:676-678. doi:10.1001/archderm.1982.01650210056019
- Fishman HC. Rat mite dermatitis. Cutis. 1988;42:414-416.
- Ram SM, Satija KC, Kaushik RK. Ornithonyssus bacoti infestation in laboratory personnel and veterinary students. Int J Zoonoses. 1986;13:138-140.
- Brown S, Becher J, Brady W. Treatment of ectoparasitic infections: review of the English-language literature, 1982-1992. Clin Infect Dis. 1995;20(suppl 1):S104-S109. doi:10.1093/clinids/20.supplement_1.s104
- Reeves WK, Loftis AD, Szumlas DE, et al. Rickettsial pathogens in the tropical rat mite Ornithonyssus bacoti (Acari: Macronyssidae) from Egyptian rats (Rattus spp.). Exp Appl Acarol. 2007;41:101-107. doi:10.1007/s10493-006-9040-3
- Philip CB, Hughes LE. The tropical rat mite; Liponyssus bacoti, as an experimental vector of rickettsialpox. Am J Trop Med Hyg. 1948;28:697-705. doi:10.4269/ajtmh.1948.s1-28.697
- Zemskaia AA, Pchelkina AA. Experimental infection of ticks Dermanyssus gallinae Redi Bdellonyssus bacoti Hirst with Q fever. Dokl Akad Nauk SSSR. 1955;101:391-392.
- Hopla CE. Experimental transmission of tularemia by the tropical rat mite. Am J Trop Med Hyg. 1951;31:768-783. doi:10.4269/ajtmh.1951.s1-31.768
- Clark GM, Lutz AE, Fadnessl. Observations on the ability of Haemogamasus liponyssoides Ewing and Ornithonyssus bacoti (Hirst) (Acarina, Gamasina) to retain eastern equine encephalitis virus: preliminary report. Am J Trop Med Hyg. 1966;15:107-112. doi:10.4269/ajtmh.1966.15.107
- Schwab M, Allen R, Sulkin SE. The tropical rat mite (Liponyssus bacoti) as an experimental vector of Coxsackie virus. Am J Trop Med Hyg. 1952;1:982-986. doi:10.4269/ajtmh.1952.1.982
- Durden LA, Turell MJ. Inefficient mechanical transmission of Langat (tick-borne encephalitis virus complex) virus by blood-feeding mites (Acari) to laboratory mice. J Med Entomol. 1993;30:639-641. doi:10.1093/jmedent/30.3.639
- Beck W, Fölster-Holst R. Tropical rat mites (Ornithonyssus bacoti)—serious ectoparasites. J Dtsch Dermatol Ges. 2009;7:667-670. doi:10.1111/j.1610-0387.2009.07140.x
- Baumstark J, Beck W, Hofmann H. Outbreak of tropical rat mite (Ornithonyssus bacoti) dermatitis in a home for disabled persons. Dermatology. 2007;215:66-68. doi:10.1159/000102037
- Beck W. Occurrence of a house-infesting tropical rat mite (Ornithonyssus bacoti) on murides and human beings. Travel Med Infect Dis. 2008;6:245-249. doi:10.1016/j.tmaid.2008.01.002
- Beck W. Tropical rat mites as newly emerging disease pathogens in rodents and man. Trav Med Infect Dis. 2007;5:403. doi:10.1016/j.tmaid.2007.09.016
- Engel PM, Welzel J, Maass M, et al. Tropical rat mite dermatitis: case report and review. Clin Infect Dis. 1998;27:1465-1469. doi:10.1086/515016
- Hetherington GW, Holder WR, Smith EB. Rat mite dermatitis. JAMA. 1971;215:1499-1500.
- Fox JG. Outbreak of tropical rat mite dermatitis in laboratory personnel. Arch Dermatol. 1982;118:676-678. doi:10.1001/archderm.1982.01650210056019
- Fishman HC. Rat mite dermatitis. Cutis. 1988;42:414-416.
- Ram SM, Satija KC, Kaushik RK. Ornithonyssus bacoti infestation in laboratory personnel and veterinary students. Int J Zoonoses. 1986;13:138-140.
- Brown S, Becher J, Brady W. Treatment of ectoparasitic infections: review of the English-language literature, 1982-1992. Clin Infect Dis. 1995;20(suppl 1):S104-S109. doi:10.1093/clinids/20.supplement_1.s104
- Reeves WK, Loftis AD, Szumlas DE, et al. Rickettsial pathogens in the tropical rat mite Ornithonyssus bacoti (Acari: Macronyssidae) from Egyptian rats (Rattus spp.). Exp Appl Acarol. 2007;41:101-107. doi:10.1007/s10493-006-9040-3
- Philip CB, Hughes LE. The tropical rat mite; Liponyssus bacoti, as an experimental vector of rickettsialpox. Am J Trop Med Hyg. 1948;28:697-705. doi:10.4269/ajtmh.1948.s1-28.697
- Zemskaia AA, Pchelkina AA. Experimental infection of ticks Dermanyssus gallinae Redi Bdellonyssus bacoti Hirst with Q fever. Dokl Akad Nauk SSSR. 1955;101:391-392.
- Hopla CE. Experimental transmission of tularemia by the tropical rat mite. Am J Trop Med Hyg. 1951;31:768-783. doi:10.4269/ajtmh.1951.s1-31.768
- Clark GM, Lutz AE, Fadnessl. Observations on the ability of Haemogamasus liponyssoides Ewing and Ornithonyssus bacoti (Hirst) (Acarina, Gamasina) to retain eastern equine encephalitis virus: preliminary report. Am J Trop Med Hyg. 1966;15:107-112. doi:10.4269/ajtmh.1966.15.107
- Schwab M, Allen R, Sulkin SE. The tropical rat mite (Liponyssus bacoti) as an experimental vector of Coxsackie virus. Am J Trop Med Hyg. 1952;1:982-986. doi:10.4269/ajtmh.1952.1.982
- Durden LA, Turell MJ. Inefficient mechanical transmission of Langat (tick-borne encephalitis virus complex) virus by blood-feeding mites (Acari) to laboratory mice. J Med Entomol. 1993;30:639-641. doi:10.1093/jmedent/30.3.639
Practice Points
- The tropical rat mite (Ornithonyssus bacoti) can infest humans who make bodily contact with a rodent, reside in living spaces infested with rodents, or own any pets.
- Patients infested with rat mites may present with pruritic, erythematous, cutaneous lesions with secondary excoriations that can be mistaken for an infection or dermatitis.
- The recommended treatment of rate mite infestation includes antiparasitic medications such as permethrin or pyriproxyfen. Preventive measures include proper disinfestation of living spaces.
What’s Eating You? Triatoma and Arilus cristatus Bugs
Classification
Triatomine bugs (Triatoma) and the wheel bug (Arilus cristatus) are part of the family Reduviidae (order Hemiptera, a name that describes the sucking proboscis on the front of the insect’s head).1,2 Both arthropods are found in multiple countries and are especially common in warmer areas, including in the United States, where they can be seen from Texas to California.3,4 Because blood-feeding triatomines need a blood meal to survive while laying eggs and then throughout their 5 developmental nymph stages to undergo molting, they feed on mammals, such as opossums, raccoons, pack rats, and armadillos, whereas wheel bugs mainly prey on soft-bodied insects.1,4-6
Triatoma bugs seek cutaneous blood vessels using thermosensors in their antennae to locate blood flow under the skin for feeding. After inserting the proboscis, they release nitric oxide and an anticoagulant that allows for continuous blood flow while feeding.7 It has been reported that triatomine bugs are not able to bite through clothing, instead seeking exposed skin, particularly near mucous membranes, such as the hands, arms, feet, head, and trunk. The name kissing bug for triatomines was coined because bites near the mouth are common.6 The bite typically is painless and occurs mainly at night when the insect is most active. After obtaining a blood meal, triatomine bugs seek shelter and hide in mud and daub structures, cracks, crevices, and furniture.1,8
Unlike Triatoma species, A cristatus does not require a blood meal for development and survival, leading it to prey on soft-bodied insects. Piercing prey with the proboscis, wheel bugs inject a toxin to digest the contents and suck the digested contents through this apparatus.4 Because the wheel bug does not require a blood meal, it typically bites a human only for defense if it feels threatened. Unlike the painless bite of a triatomine bug, the bite of A cristatus is extremely painful; it has been described as the worst arthropod bite—worse than a hornet’s sting. The pain from the bite is caused by the toxin being injected into the skin; possible retention of the proboscis makes the pain worse.4,9 In addition, when A cristatus is disturbed, it exudes pungent material from a pair of bright orange subrectal glands while stridulating to repulse predators.9
Although Triatoma species and A cristatus have separate roles in nature and vastly different impacts on health, they often are mistaken for the same arthropod when seen in nature. Features that members of Reduviidae share include large bodies (relative to their overall length); long thin legs; a narrow head; wings; and a long sucking proboscis on the front of the head.10

Characteristics that differentiate Triatoma and A cristatus species include size, color, and distinctive markings. Most triatomine bugs are 12- to 36-mm long; are dark brown or black; and have what are called tiger-stripe orange markings on the peripheral two-thirds of the body (Figure 1).11 In contrast, wheel bugs commonly are bigger—measuring longer than 1.25 inches—and gray, with a cogwheel-like structure on the thorax (Figure 2).10

Dermatologic Presentation and Clinical Symptoms
The area of involved skin on patients presenting with Triatoma or A cristatus bites may resemble other insect bites. Many bites from Triatoma bugs and A cristatus initially present as an erythematous, raised, pruritic papule with a central punctum that is visible because of the involvement of the proboscis. However, other presentations of bites from both arthropods have been reported4,6,7: grouped vesicles on an erythematous base; indurated, giant, urticarial-type wheels measuring 10 to 15 mm in diameter; and hemorrhagic bullous nodules (Figure 3). Associated lymphangitis or lymphadenitis is typical of the latter 2 variations.6 These variations in presentation can be mistaken for other causes of similarly presenting lesions, such as shingles or spider bites, leading to delayed or missed diagnosis.
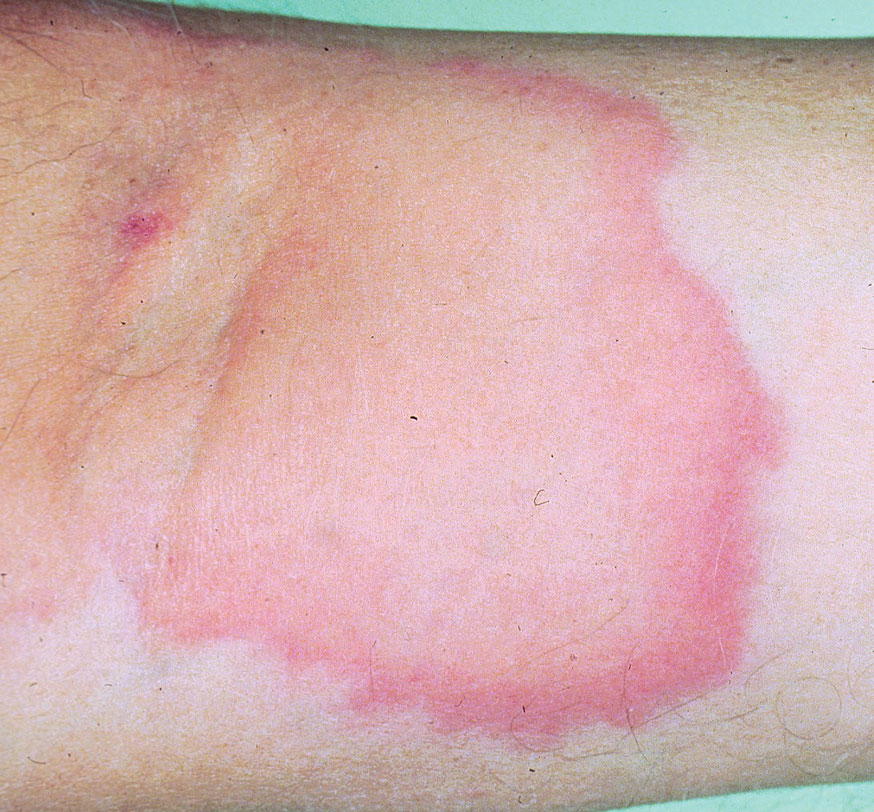
Patients may present with a single bite or multiple bites due to the feeding pattern of Triatoma bugs; if the host moves or disrupts its feeding, the arthropod takes multiple bites to finish feeding.8 In comparison, 4 common variations of wheel bug bites have been reported: (1) a painful bite without complications; (2) a cutaneous horn and papilloma at the site of toxin injection; (3) a necrotic ulcer around the central punctum caused by injected toxin; and (4) an abscess under the central punctum due to secondary infection.4
Anaphylaxis—Although the bites of Triatoma and A cristatus present differently, both can cause anaphylaxis. Triatoma is implicated more often than A cristatus as the cause of anaphylaxis.12 In fact, Triatoma bites are among the more common causes of anaphylaxis from bug bites, with multiple cases of these reactions reported in the literature.12,13
Symptoms of Triatoma anaphylaxis include acute-onset urticarial rash, flushing, dyspnea, wheezing, nausea, vomiting, and localized edema.2 The cause of anaphylaxis is proteins in Triatoma saliva, including 20-kDa procalin, which incites the systemic reaction. Other potential causes of anaphylaxis include serine protease, which has similarities to salivary protein and desmoglein in humans.11
The degree of reaction to a bite depends on the patient's sensitization to antigenic proteins in each insect’s saliva.4,6 Patients who have a bite from a triatomine bug are at risk for subsequent bites, as household infestation is likely due to the pliability of the insect, allowing it to hide in small spaces unnoticed.8 In the case of a bite from Triatoma or A cristatus, sensitization may lead to severe and worsening reactions with subsequent bites, which ultimately can result in life-threatening anaphylaxis.1,6
Treatment and Prevention
Treatment of Triatoma and A cristatus bites depends on the severity of the patient’s reaction to the bite. A local reaction to a bite from either insect can be treated supportively with local corticosteroids and antihistamines.3 If the patient is sensitized to proteins associated with a bite, standard anaphylaxis treatment such as epinephrine and intravenous antihistamines may be indicated.14 Secondary infection can be treated with antibiotics; a formed abscess might need to be drained or debrided.15
There’s No Place Like Home—Because Triatoma bugs have a pliable exoskeleton and can squeeze into small spaces, they commonly infest dwellings where they find multiple attractants: light, heat, carbon dioxide, and lactic acid.8 The more household occupants (including pets), the higher the levels of carbon dioxide and lactic acid, thus the greater the attraction. Infestation of a home can lead to the spread of diseases harbored by Triatoma, including Chagas disease, which is caused by the parasite Trypanosoma cruzi.5
Preventive measures can be taken to reduce the risk and extent of home infestation by Triatoma bugs, including insecticides, a solid foundation, window screens, air conditioning, sealing of cracks and crevices, outdoor light management, and removal of clutter throughout the house.8 Because Triatoma bugs cannot bite through clothing, protective clothing and bug repellent on exposed skin can be employed. Another degree of protection is offered by pest management, especially control of rodents by removing food, water, and nests in areas where triatomine bugs feed off of that population.8,14
Unlike triatomine bugs, wheel bugs tend not to invade houses; therefore, these preventive measures are unnecessary. If a wheel bug is identified, do not engage the arthropod due to the defensive nature of its attack.4,9 Such deliberate avoidance should ensure protection from the wheel bug’s painful bite.
Medical Complications
Although triatomine bugs and wheel bugs are in the same taxonomic family, subsequent infection is unique to Triatoma bugs because they need a blood meal to survive. Because Triatoma bugs feed on mammals, they present an increased opportunity for transmitting the causative agents of infection from hosts on which they have fed.12 The principal parasite transmitted by triatomines is T cruzi, which causes Chagas disease and lives in the gastrointestinal (GI) tract of the insect.5 When a triatomine bug seeks out a mucosal surface to bite, including the mouth, it defecates and urinates during or shortly after feeding, leading to contamination of the initial wound or mucosal surfaces. In addition, Triatoma bugs are vectors for transmission of Serratia marcescans, Bartonella henselae, and Mycobacterium leprae.16
Chagas Disease—This infection has 3 stages: acute, intermediate, and chronic.5 The acute stage can present with symptoms of conjunctivitis, fever, lymphadenopathy, hepatosplenomegaly, and anemia. The intermediate stage typically is asymptomatic. The chronic stage usually involves the heart and GI tract and causes cardiac aneurysms, cardiomegaly, megacolon, and megaesophagus. Initial symptoms can be a localized nodule (chagoma) at the inoculation site, fever, fatigue, lymphadenopathy, and hepatosplenomegaly.2 Unilateral palpebral edema with associated lymphadenopathy (Romaña sign) also can be seen—not to be confused with bilateral swelling in an acute reaction to an insect bite. Romaña sign is pathognomonic of T cruzi infection; bilateral palpebral swelling is typical of an allergic reaction.12
Identification of a triatomine bite is the first step in diagnosing Chagas disease, which can be life-threatening. Among chronic carriers of Chagas disease, 30% develop GI and cardiac symptoms, of which 20% to 30% develop cardiomyopathy, with serious symptoms that present 10 to 20 years after the asymptomatic intermediate phase.2
Chagas disease is endemic to Central and South America but is also seen in North America; 28,000 new cases are reported annually in South America and North America combined. Human migration from endemic areas and from rural to urban areas has promoted the spread of Chagas disease.2 However, patients in the United States have a relatively low risk for Chagas disease, largely because of the quality of housing construction and use of insecticides.
Treatment options for Chagas disease include nifurtimox and benznidazole. Without treatment, the host immune response typically controls acute replication of the parasite but will lead to a chronic state, ultimately involving the heart and GI tract.5
- Vetter R. Kissing bugs (Triatoma) and the skin. Dermatol Online J. 2001;7:6.
- Zemore ZM, Wills BK. Kissing bug bite. StatPearls [Internet]. StatPearlsPublishing; 2023.
- Edwards L, Lynch PJ. Anaphylactic reaction to kissing bug bites. Ariz Med. 1984;41:159-161.
- Smith FD, Miller NG, Carnazzo SJ, et al. Insect bite by Arilus cristatus, a North American reduviid. AMA Arch Derm. 1958;77:324-330. doi:10.1001/archderm.1958.01560030070011
- Nguyen T, Waseem M. Chagas disease. StatPearls [Internet]. StatPearls Publishing; 2022.
- Shields TL, Walsh EN. Kissing bug bite. AMA Arch Derm. 1956;74:14-21. doi:10.1001/archderm.1956.01550070016004
- Beatty NL, Klotz SA. The midnight bite! a kissing bug nightmare. Am J Med. 2018;131:E43-E44. doi:10.1016/j.amjmed.2017.10.013
- Klotz SA, Smith SL, Schmidt JO. Kissing bug intrusions into homes in the Southwest United States. Insects. 2021;12:654. doi:10.3390/insects12070654
- Aldrich JR, Chauhan KR, Zhang A, et al. Exocrine secretions of wheel bugs (Heteroptera: Reduviidae: Arilus spp.): clarification and chemistry. Z Naturforsch C J Biosci. 2013;68:522-526.
- Boggs J. They’re wheel bugs, NOT kissing bugs. Buckeye Yard and Garden onLine [Internet]. September 17, 2020. Accessed May 25, 2023. https://bygl.osu.edu/node/1688
- Weber RW. Allergen of the month—assassin bug. Ann Allergy Asthma Immunol. 2015;115:A9.
- Klotz JH, Dorn PL, Logan JL, et al. “Kissing bugs”: potential disease vectors and cause of anaphylaxis. Clin Infect Dis 2010;50:1629-1634. doi:10.1086/652769
- Anderson C, Belnap C. The kiss of death: a rare case of anaphylaxis to the bite of the “red margined kissing bug”. Hawaii J Med Public Health. 2015;74(9 suppl 2):33-35.
- Moffitt JE, Venarske D, Goddard J, et al. Allergic reactions to Triatoma bites. Ann Allergy Asthma Immunol. 2003;91:122-128. doi:10.1016/s1081-1206(10)62165-5
- Burnett JW, Calton GJ, Morgan RJ. Triatoma: the “kissing bug”. Cutis. 1987;39:399.
- Vieira CB, Praça YR, Bentes K, et al. Triatomines: Trypanosomatids, bacteria, and viruses potential vectors? Front Cell Infect Microbiol. 2018;8:405. doi:10.3389/fcimb.2018.00405
Classification
Triatomine bugs (Triatoma) and the wheel bug (Arilus cristatus) are part of the family Reduviidae (order Hemiptera, a name that describes the sucking proboscis on the front of the insect’s head).1,2 Both arthropods are found in multiple countries and are especially common in warmer areas, including in the United States, where they can be seen from Texas to California.3,4 Because blood-feeding triatomines need a blood meal to survive while laying eggs and then throughout their 5 developmental nymph stages to undergo molting, they feed on mammals, such as opossums, raccoons, pack rats, and armadillos, whereas wheel bugs mainly prey on soft-bodied insects.1,4-6
Triatoma bugs seek cutaneous blood vessels using thermosensors in their antennae to locate blood flow under the skin for feeding. After inserting the proboscis, they release nitric oxide and an anticoagulant that allows for continuous blood flow while feeding.7 It has been reported that triatomine bugs are not able to bite through clothing, instead seeking exposed skin, particularly near mucous membranes, such as the hands, arms, feet, head, and trunk. The name kissing bug for triatomines was coined because bites near the mouth are common.6 The bite typically is painless and occurs mainly at night when the insect is most active. After obtaining a blood meal, triatomine bugs seek shelter and hide in mud and daub structures, cracks, crevices, and furniture.1,8
Unlike Triatoma species, A cristatus does not require a blood meal for development and survival, leading it to prey on soft-bodied insects. Piercing prey with the proboscis, wheel bugs inject a toxin to digest the contents and suck the digested contents through this apparatus.4 Because the wheel bug does not require a blood meal, it typically bites a human only for defense if it feels threatened. Unlike the painless bite of a triatomine bug, the bite of A cristatus is extremely painful; it has been described as the worst arthropod bite—worse than a hornet’s sting. The pain from the bite is caused by the toxin being injected into the skin; possible retention of the proboscis makes the pain worse.4,9 In addition, when A cristatus is disturbed, it exudes pungent material from a pair of bright orange subrectal glands while stridulating to repulse predators.9
Although Triatoma species and A cristatus have separate roles in nature and vastly different impacts on health, they often are mistaken for the same arthropod when seen in nature. Features that members of Reduviidae share include large bodies (relative to their overall length); long thin legs; a narrow head; wings; and a long sucking proboscis on the front of the head.10

Characteristics that differentiate Triatoma and A cristatus species include size, color, and distinctive markings. Most triatomine bugs are 12- to 36-mm long; are dark brown or black; and have what are called tiger-stripe orange markings on the peripheral two-thirds of the body (Figure 1).11 In contrast, wheel bugs commonly are bigger—measuring longer than 1.25 inches—and gray, with a cogwheel-like structure on the thorax (Figure 2).10

Dermatologic Presentation and Clinical Symptoms
The area of involved skin on patients presenting with Triatoma or A cristatus bites may resemble other insect bites. Many bites from Triatoma bugs and A cristatus initially present as an erythematous, raised, pruritic papule with a central punctum that is visible because of the involvement of the proboscis. However, other presentations of bites from both arthropods have been reported4,6,7: grouped vesicles on an erythematous base; indurated, giant, urticarial-type wheels measuring 10 to 15 mm in diameter; and hemorrhagic bullous nodules (Figure 3). Associated lymphangitis or lymphadenitis is typical of the latter 2 variations.6 These variations in presentation can be mistaken for other causes of similarly presenting lesions, such as shingles or spider bites, leading to delayed or missed diagnosis.

Patients may present with a single bite or multiple bites due to the feeding pattern of Triatoma bugs; if the host moves or disrupts its feeding, the arthropod takes multiple bites to finish feeding.8 In comparison, 4 common variations of wheel bug bites have been reported: (1) a painful bite without complications; (2) a cutaneous horn and papilloma at the site of toxin injection; (3) a necrotic ulcer around the central punctum caused by injected toxin; and (4) an abscess under the central punctum due to secondary infection.4
Anaphylaxis—Although the bites of Triatoma and A cristatus present differently, both can cause anaphylaxis. Triatoma is implicated more often than A cristatus as the cause of anaphylaxis.12 In fact, Triatoma bites are among the more common causes of anaphylaxis from bug bites, with multiple cases of these reactions reported in the literature.12,13
Symptoms of Triatoma anaphylaxis include acute-onset urticarial rash, flushing, dyspnea, wheezing, nausea, vomiting, and localized edema.2 The cause of anaphylaxis is proteins in Triatoma saliva, including 20-kDa procalin, which incites the systemic reaction. Other potential causes of anaphylaxis include serine protease, which has similarities to salivary protein and desmoglein in humans.11
The degree of reaction to a bite depends on the patient's sensitization to antigenic proteins in each insect’s saliva.4,6 Patients who have a bite from a triatomine bug are at risk for subsequent bites, as household infestation is likely due to the pliability of the insect, allowing it to hide in small spaces unnoticed.8 In the case of a bite from Triatoma or A cristatus, sensitization may lead to severe and worsening reactions with subsequent bites, which ultimately can result in life-threatening anaphylaxis.1,6
Treatment and Prevention
Treatment of Triatoma and A cristatus bites depends on the severity of the patient’s reaction to the bite. A local reaction to a bite from either insect can be treated supportively with local corticosteroids and antihistamines.3 If the patient is sensitized to proteins associated with a bite, standard anaphylaxis treatment such as epinephrine and intravenous antihistamines may be indicated.14 Secondary infection can be treated with antibiotics; a formed abscess might need to be drained or debrided.15
There’s No Place Like Home—Because Triatoma bugs have a pliable exoskeleton and can squeeze into small spaces, they commonly infest dwellings where they find multiple attractants: light, heat, carbon dioxide, and lactic acid.8 The more household occupants (including pets), the higher the levels of carbon dioxide and lactic acid, thus the greater the attraction. Infestation of a home can lead to the spread of diseases harbored by Triatoma, including Chagas disease, which is caused by the parasite Trypanosoma cruzi.5
Preventive measures can be taken to reduce the risk and extent of home infestation by Triatoma bugs, including insecticides, a solid foundation, window screens, air conditioning, sealing of cracks and crevices, outdoor light management, and removal of clutter throughout the house.8 Because Triatoma bugs cannot bite through clothing, protective clothing and bug repellent on exposed skin can be employed. Another degree of protection is offered by pest management, especially control of rodents by removing food, water, and nests in areas where triatomine bugs feed off of that population.8,14
Unlike triatomine bugs, wheel bugs tend not to invade houses; therefore, these preventive measures are unnecessary. If a wheel bug is identified, do not engage the arthropod due to the defensive nature of its attack.4,9 Such deliberate avoidance should ensure protection from the wheel bug’s painful bite.
Medical Complications
Although triatomine bugs and wheel bugs are in the same taxonomic family, subsequent infection is unique to Triatoma bugs because they need a blood meal to survive. Because Triatoma bugs feed on mammals, they present an increased opportunity for transmitting the causative agents of infection from hosts on which they have fed.12 The principal parasite transmitted by triatomines is T cruzi, which causes Chagas disease and lives in the gastrointestinal (GI) tract of the insect.5 When a triatomine bug seeks out a mucosal surface to bite, including the mouth, it defecates and urinates during or shortly after feeding, leading to contamination of the initial wound or mucosal surfaces. In addition, Triatoma bugs are vectors for transmission of Serratia marcescans, Bartonella henselae, and Mycobacterium leprae.16
Chagas Disease—This infection has 3 stages: acute, intermediate, and chronic.5 The acute stage can present with symptoms of conjunctivitis, fever, lymphadenopathy, hepatosplenomegaly, and anemia. The intermediate stage typically is asymptomatic. The chronic stage usually involves the heart and GI tract and causes cardiac aneurysms, cardiomegaly, megacolon, and megaesophagus. Initial symptoms can be a localized nodule (chagoma) at the inoculation site, fever, fatigue, lymphadenopathy, and hepatosplenomegaly.2 Unilateral palpebral edema with associated lymphadenopathy (Romaña sign) also can be seen—not to be confused with bilateral swelling in an acute reaction to an insect bite. Romaña sign is pathognomonic of T cruzi infection; bilateral palpebral swelling is typical of an allergic reaction.12
Identification of a triatomine bite is the first step in diagnosing Chagas disease, which can be life-threatening. Among chronic carriers of Chagas disease, 30% develop GI and cardiac symptoms, of which 20% to 30% develop cardiomyopathy, with serious symptoms that present 10 to 20 years after the asymptomatic intermediate phase.2
Chagas disease is endemic to Central and South America but is also seen in North America; 28,000 new cases are reported annually in South America and North America combined. Human migration from endemic areas and from rural to urban areas has promoted the spread of Chagas disease.2 However, patients in the United States have a relatively low risk for Chagas disease, largely because of the quality of housing construction and use of insecticides.
Treatment options for Chagas disease include nifurtimox and benznidazole. Without treatment, the host immune response typically controls acute replication of the parasite but will lead to a chronic state, ultimately involving the heart and GI tract.5
Classification
Triatomine bugs (Triatoma) and the wheel bug (Arilus cristatus) are part of the family Reduviidae (order Hemiptera, a name that describes the sucking proboscis on the front of the insect’s head).1,2 Both arthropods are found in multiple countries and are especially common in warmer areas, including in the United States, where they can be seen from Texas to California.3,4 Because blood-feeding triatomines need a blood meal to survive while laying eggs and then throughout their 5 developmental nymph stages to undergo molting, they feed on mammals, such as opossums, raccoons, pack rats, and armadillos, whereas wheel bugs mainly prey on soft-bodied insects.1,4-6
Triatoma bugs seek cutaneous blood vessels using thermosensors in their antennae to locate blood flow under the skin for feeding. After inserting the proboscis, they release nitric oxide and an anticoagulant that allows for continuous blood flow while feeding.7 It has been reported that triatomine bugs are not able to bite through clothing, instead seeking exposed skin, particularly near mucous membranes, such as the hands, arms, feet, head, and trunk. The name kissing bug for triatomines was coined because bites near the mouth are common.6 The bite typically is painless and occurs mainly at night when the insect is most active. After obtaining a blood meal, triatomine bugs seek shelter and hide in mud and daub structures, cracks, crevices, and furniture.1,8
Unlike Triatoma species, A cristatus does not require a blood meal for development and survival, leading it to prey on soft-bodied insects. Piercing prey with the proboscis, wheel bugs inject a toxin to digest the contents and suck the digested contents through this apparatus.4 Because the wheel bug does not require a blood meal, it typically bites a human only for defense if it feels threatened. Unlike the painless bite of a triatomine bug, the bite of A cristatus is extremely painful; it has been described as the worst arthropod bite—worse than a hornet’s sting. The pain from the bite is caused by the toxin being injected into the skin; possible retention of the proboscis makes the pain worse.4,9 In addition, when A cristatus is disturbed, it exudes pungent material from a pair of bright orange subrectal glands while stridulating to repulse predators.9
Although Triatoma species and A cristatus have separate roles in nature and vastly different impacts on health, they often are mistaken for the same arthropod when seen in nature. Features that members of Reduviidae share include large bodies (relative to their overall length); long thin legs; a narrow head; wings; and a long sucking proboscis on the front of the head.10

Characteristics that differentiate Triatoma and A cristatus species include size, color, and distinctive markings. Most triatomine bugs are 12- to 36-mm long; are dark brown or black; and have what are called tiger-stripe orange markings on the peripheral two-thirds of the body (Figure 1).11 In contrast, wheel bugs commonly are bigger—measuring longer than 1.25 inches—and gray, with a cogwheel-like structure on the thorax (Figure 2).10

Dermatologic Presentation and Clinical Symptoms
The area of involved skin on patients presenting with Triatoma or A cristatus bites may resemble other insect bites. Many bites from Triatoma bugs and A cristatus initially present as an erythematous, raised, pruritic papule with a central punctum that is visible because of the involvement of the proboscis. However, other presentations of bites from both arthropods have been reported4,6,7: grouped vesicles on an erythematous base; indurated, giant, urticarial-type wheels measuring 10 to 15 mm in diameter; and hemorrhagic bullous nodules (Figure 3). Associated lymphangitis or lymphadenitis is typical of the latter 2 variations.6 These variations in presentation can be mistaken for other causes of similarly presenting lesions, such as shingles or spider bites, leading to delayed or missed diagnosis.

Patients may present with a single bite or multiple bites due to the feeding pattern of Triatoma bugs; if the host moves or disrupts its feeding, the arthropod takes multiple bites to finish feeding.8 In comparison, 4 common variations of wheel bug bites have been reported: (1) a painful bite without complications; (2) a cutaneous horn and papilloma at the site of toxin injection; (3) a necrotic ulcer around the central punctum caused by injected toxin; and (4) an abscess under the central punctum due to secondary infection.4
Anaphylaxis—Although the bites of Triatoma and A cristatus present differently, both can cause anaphylaxis. Triatoma is implicated more often than A cristatus as the cause of anaphylaxis.12 In fact, Triatoma bites are among the more common causes of anaphylaxis from bug bites, with multiple cases of these reactions reported in the literature.12,13
Symptoms of Triatoma anaphylaxis include acute-onset urticarial rash, flushing, dyspnea, wheezing, nausea, vomiting, and localized edema.2 The cause of anaphylaxis is proteins in Triatoma saliva, including 20-kDa procalin, which incites the systemic reaction. Other potential causes of anaphylaxis include serine protease, which has similarities to salivary protein and desmoglein in humans.11
The degree of reaction to a bite depends on the patient's sensitization to antigenic proteins in each insect’s saliva.4,6 Patients who have a bite from a triatomine bug are at risk for subsequent bites, as household infestation is likely due to the pliability of the insect, allowing it to hide in small spaces unnoticed.8 In the case of a bite from Triatoma or A cristatus, sensitization may lead to severe and worsening reactions with subsequent bites, which ultimately can result in life-threatening anaphylaxis.1,6
Treatment and Prevention
Treatment of Triatoma and A cristatus bites depends on the severity of the patient’s reaction to the bite. A local reaction to a bite from either insect can be treated supportively with local corticosteroids and antihistamines.3 If the patient is sensitized to proteins associated with a bite, standard anaphylaxis treatment such as epinephrine and intravenous antihistamines may be indicated.14 Secondary infection can be treated with antibiotics; a formed abscess might need to be drained or debrided.15
There’s No Place Like Home—Because Triatoma bugs have a pliable exoskeleton and can squeeze into small spaces, they commonly infest dwellings where they find multiple attractants: light, heat, carbon dioxide, and lactic acid.8 The more household occupants (including pets), the higher the levels of carbon dioxide and lactic acid, thus the greater the attraction. Infestation of a home can lead to the spread of diseases harbored by Triatoma, including Chagas disease, which is caused by the parasite Trypanosoma cruzi.5
Preventive measures can be taken to reduce the risk and extent of home infestation by Triatoma bugs, including insecticides, a solid foundation, window screens, air conditioning, sealing of cracks and crevices, outdoor light management, and removal of clutter throughout the house.8 Because Triatoma bugs cannot bite through clothing, protective clothing and bug repellent on exposed skin can be employed. Another degree of protection is offered by pest management, especially control of rodents by removing food, water, and nests in areas where triatomine bugs feed off of that population.8,14
Unlike triatomine bugs, wheel bugs tend not to invade houses; therefore, these preventive measures are unnecessary. If a wheel bug is identified, do not engage the arthropod due to the defensive nature of its attack.4,9 Such deliberate avoidance should ensure protection from the wheel bug’s painful bite.
Medical Complications
Although triatomine bugs and wheel bugs are in the same taxonomic family, subsequent infection is unique to Triatoma bugs because they need a blood meal to survive. Because Triatoma bugs feed on mammals, they present an increased opportunity for transmitting the causative agents of infection from hosts on which they have fed.12 The principal parasite transmitted by triatomines is T cruzi, which causes Chagas disease and lives in the gastrointestinal (GI) tract of the insect.5 When a triatomine bug seeks out a mucosal surface to bite, including the mouth, it defecates and urinates during or shortly after feeding, leading to contamination of the initial wound or mucosal surfaces. In addition, Triatoma bugs are vectors for transmission of Serratia marcescans, Bartonella henselae, and Mycobacterium leprae.16
Chagas Disease—This infection has 3 stages: acute, intermediate, and chronic.5 The acute stage can present with symptoms of conjunctivitis, fever, lymphadenopathy, hepatosplenomegaly, and anemia. The intermediate stage typically is asymptomatic. The chronic stage usually involves the heart and GI tract and causes cardiac aneurysms, cardiomegaly, megacolon, and megaesophagus. Initial symptoms can be a localized nodule (chagoma) at the inoculation site, fever, fatigue, lymphadenopathy, and hepatosplenomegaly.2 Unilateral palpebral edema with associated lymphadenopathy (Romaña sign) also can be seen—not to be confused with bilateral swelling in an acute reaction to an insect bite. Romaña sign is pathognomonic of T cruzi infection; bilateral palpebral swelling is typical of an allergic reaction.12
Identification of a triatomine bite is the first step in diagnosing Chagas disease, which can be life-threatening. Among chronic carriers of Chagas disease, 30% develop GI and cardiac symptoms, of which 20% to 30% develop cardiomyopathy, with serious symptoms that present 10 to 20 years after the asymptomatic intermediate phase.2
Chagas disease is endemic to Central and South America but is also seen in North America; 28,000 new cases are reported annually in South America and North America combined. Human migration from endemic areas and from rural to urban areas has promoted the spread of Chagas disease.2 However, patients in the United States have a relatively low risk for Chagas disease, largely because of the quality of housing construction and use of insecticides.
Treatment options for Chagas disease include nifurtimox and benznidazole. Without treatment, the host immune response typically controls acute replication of the parasite but will lead to a chronic state, ultimately involving the heart and GI tract.5
- Vetter R. Kissing bugs (Triatoma) and the skin. Dermatol Online J. 2001;7:6.
- Zemore ZM, Wills BK. Kissing bug bite. StatPearls [Internet]. StatPearlsPublishing; 2023.
- Edwards L, Lynch PJ. Anaphylactic reaction to kissing bug bites. Ariz Med. 1984;41:159-161.
- Smith FD, Miller NG, Carnazzo SJ, et al. Insect bite by Arilus cristatus, a North American reduviid. AMA Arch Derm. 1958;77:324-330. doi:10.1001/archderm.1958.01560030070011
- Nguyen T, Waseem M. Chagas disease. StatPearls [Internet]. StatPearls Publishing; 2022.
- Shields TL, Walsh EN. Kissing bug bite. AMA Arch Derm. 1956;74:14-21. doi:10.1001/archderm.1956.01550070016004
- Beatty NL, Klotz SA. The midnight bite! a kissing bug nightmare. Am J Med. 2018;131:E43-E44. doi:10.1016/j.amjmed.2017.10.013
- Klotz SA, Smith SL, Schmidt JO. Kissing bug intrusions into homes in the Southwest United States. Insects. 2021;12:654. doi:10.3390/insects12070654
- Aldrich JR, Chauhan KR, Zhang A, et al. Exocrine secretions of wheel bugs (Heteroptera: Reduviidae: Arilus spp.): clarification and chemistry. Z Naturforsch C J Biosci. 2013;68:522-526.
- Boggs J. They’re wheel bugs, NOT kissing bugs. Buckeye Yard and Garden onLine [Internet]. September 17, 2020. Accessed May 25, 2023. https://bygl.osu.edu/node/1688
- Weber RW. Allergen of the month—assassin bug. Ann Allergy Asthma Immunol. 2015;115:A9.
- Klotz JH, Dorn PL, Logan JL, et al. “Kissing bugs”: potential disease vectors and cause of anaphylaxis. Clin Infect Dis 2010;50:1629-1634. doi:10.1086/652769
- Anderson C, Belnap C. The kiss of death: a rare case of anaphylaxis to the bite of the “red margined kissing bug”. Hawaii J Med Public Health. 2015;74(9 suppl 2):33-35.
- Moffitt JE, Venarske D, Goddard J, et al. Allergic reactions to Triatoma bites. Ann Allergy Asthma Immunol. 2003;91:122-128. doi:10.1016/s1081-1206(10)62165-5
- Burnett JW, Calton GJ, Morgan RJ. Triatoma: the “kissing bug”. Cutis. 1987;39:399.
- Vieira CB, Praça YR, Bentes K, et al. Triatomines: Trypanosomatids, bacteria, and viruses potential vectors? Front Cell Infect Microbiol. 2018;8:405. doi:10.3389/fcimb.2018.00405
- Vetter R. Kissing bugs (Triatoma) and the skin. Dermatol Online J. 2001;7:6.
- Zemore ZM, Wills BK. Kissing bug bite. StatPearls [Internet]. StatPearlsPublishing; 2023.
- Edwards L, Lynch PJ. Anaphylactic reaction to kissing bug bites. Ariz Med. 1984;41:159-161.
- Smith FD, Miller NG, Carnazzo SJ, et al. Insect bite by Arilus cristatus, a North American reduviid. AMA Arch Derm. 1958;77:324-330. doi:10.1001/archderm.1958.01560030070011
- Nguyen T, Waseem M. Chagas disease. StatPearls [Internet]. StatPearls Publishing; 2022.
- Shields TL, Walsh EN. Kissing bug bite. AMA Arch Derm. 1956;74:14-21. doi:10.1001/archderm.1956.01550070016004
- Beatty NL, Klotz SA. The midnight bite! a kissing bug nightmare. Am J Med. 2018;131:E43-E44. doi:10.1016/j.amjmed.2017.10.013
- Klotz SA, Smith SL, Schmidt JO. Kissing bug intrusions into homes in the Southwest United States. Insects. 2021;12:654. doi:10.3390/insects12070654
- Aldrich JR, Chauhan KR, Zhang A, et al. Exocrine secretions of wheel bugs (Heteroptera: Reduviidae: Arilus spp.): clarification and chemistry. Z Naturforsch C J Biosci. 2013;68:522-526.
- Boggs J. They’re wheel bugs, NOT kissing bugs. Buckeye Yard and Garden onLine [Internet]. September 17, 2020. Accessed May 25, 2023. https://bygl.osu.edu/node/1688
- Weber RW. Allergen of the month—assassin bug. Ann Allergy Asthma Immunol. 2015;115:A9.
- Klotz JH, Dorn PL, Logan JL, et al. “Kissing bugs”: potential disease vectors and cause of anaphylaxis. Clin Infect Dis 2010;50:1629-1634. doi:10.1086/652769
- Anderson C, Belnap C. The kiss of death: a rare case of anaphylaxis to the bite of the “red margined kissing bug”. Hawaii J Med Public Health. 2015;74(9 suppl 2):33-35.
- Moffitt JE, Venarske D, Goddard J, et al. Allergic reactions to Triatoma bites. Ann Allergy Asthma Immunol. 2003;91:122-128. doi:10.1016/s1081-1206(10)62165-5
- Burnett JW, Calton GJ, Morgan RJ. Triatoma: the “kissing bug”. Cutis. 1987;39:399.
- Vieira CB, Praça YR, Bentes K, et al. Triatomines: Trypanosomatids, bacteria, and viruses potential vectors? Front Cell Infect Microbiol. 2018;8:405. doi:10.3389/fcimb.2018.00405
Practice Points
- Triatomine bugs (Triatoma) and the wheel bug (Arilus cristatus) are found throughout North America with a concentration in southern regions.
- Bites of triatomine bugs can cause anaphylaxis; prevention of bites to diminish household infestation is important because sensitization can result in increased severity of anaphylaxis upon subsequent exposure.
- Chagas disease—caused by transmission of the parasite Trypanosoma cruzi—can be a complication of a Triatoma bite in endemic areas; treatments include nifurtimox and benznidazole.
- Left undiagnosed and untreated, Chagas disease can have long-lasting implications for cardiac and gastrointestinal pathology.
Botanical Briefs: Handling the Heat From Capsicum Peppers
Cutaneous Manifestations
Capsicum peppers are used worldwide in preparing spicy dishes. Their active ingredient—capsaicin—is used as a topical medicine to treat localized pain. Capsicum peppers can cause irritant contact dermatitis with symptoms of erythema, cutaneous burning, and itch.1
Irritant contact dermatitis is a common occupational skin disorder. Many cooks have experienced the sting of a chili pepper after contact with the hands or eyes. Cases of chronic exposure to Capsicum peppers with persistent burning and pain have been called Hunan hand syndrome.2Capsicum peppers also have induced allergic contact dermatitis in a food production worker.3
Capsicum peppers also are used in pepper spray, tear gas, and animal repellents because of their stinging properties. These agents usually cause cutaneous tingling and burning that soon resolves; however, a review of 31 studies showed that crowd-control methods with Capsicum-containing tear gas and pepper spray can cause moderate to severe skin damage such as a persistent skin rash or erythema, or even first-, second-, or third-degree burns.4
Topical application of capsaicin isolate is meant to cause burning and deplete local neuropeptides, with a cutaneous reaction that ranges from mild to intolerable.5,6 Capsaicin also is found in other products. In one published case report, a 3-year-old boy broke out in facial urticaria when his mother kissed him on the cheek after she applied lip plumper containing capsaicin to her lips.7 Dermatologists should consider capsaicin an active ingredient that can irritate the skin in the garden, in the kitchen, and in topical products.
Obtaining Relief
Capsaicin-induced dermatitis can be relieved by washing the area with soap, detergent, baking soda, or oily compounds that act as solvents for the nonpolar capsaicin.8 Application of ice water or a high-potency topical steroid also may help. If the reaction is severe and persistent, a continuous stellate ganglion block may alleviate the pain of capsaicin-induced contact dermatitis.9
Identifying Features and Plant Facts
The Capsicum genus includes chili peppers, paprika, and red peppers. Capsicum peppers are native to tropical regions of the Americas (Figure). The use of Capsicum peppers in food can be traced to Indigenous peoples of Mexico as early as 7000

Capsicum belongs to the family Solanaceae, which includes tobacco, tomatoes, potatoes, and nightshade plants. There are many varieties of peppers in the Capsicum genus, with 5 domesticated species: Capsicum annuum, Capsicum baccatum, Capsicum chinense, Capsicum frutescens, and Capsicum pubescens. These include bell, poblano, cayenne, tabasco, habanero, and ají peppers, among others. Capsicum species grow as a shrub with flowers that rotate to stellate corollas and rounded berries of different sizes and colors.12 Capsaicin and other alkaloids are concentrated in the fruit; therefore, Capsicum dermatitis is most commonly induced by contact with the flesh of peppers.
Irritant Chemicals
Capsaicin (8-methyl-6-nonanoyl vanillylamide) is a nonpolar phenol, which is why washing skin that has come in contact with capsaicin with water or vinegar alone is insufficient to solubilize it.13 Capsaicin binds to the transient receptor potential vanilloid 1 (TRPV1), a calcium channel on neurons that opens in response to heat. When bound, the channel opens at a lower temperature threshold and depolarizes nerve endings, leading to vasodilation and activation of sensory nerves.14 Substance P is released and the individual experiences a painful burning sensation. When purified capsaicin is frequently applied at an appropriate dose, synthesis of substance P is diminished, resulting in reduced local pain overall.15
Capsaicin does not affect neurons without TRPV1, and administration of capsaicin is not painful if given with anesthesia. An inappropriately high dose of capsaicin destroys cells in the epidermal barrier, resulting in water loss and inducing release of vasoactive peptides and inflammatory cytokines.1 Careful handling of Capsicum peppers and capsaicin products can reduce the risk for irritation.
Medicinal Use
On-/Off-Label and Potential Uses—Capsaicin is US Food and Drug Administration approved for use in arthritis and musculoskeletal pain. It also is used to treat diabetic neuropathy,5 postherpetic neuralgia,6 psoriasis,16 and other conditions. Studies have shown that capsaicin might be useful in treating trigeminal neuralgia,17 fibromyalgia,18 migraines,14 cluster headaches,9 and HIV-associated distal sensory neuropathy.5
Delivery of Capsaicin—Capsaicin preferentially acts on C-fibers, which transmit dull, aching, chronic pain.19 The compound is available as a cream, lotion, and large bandage (for the lower back), as well as low- and high-dose patches. Capsaicin creams, lotions, and the low-dose patch are uncomfortable and must be applied for 4 to 6 weeks to take effect, which may impact patient adherence. The high-dose patch, which requires administration under local anesthesia by a health care worker, brings pain relief with a single use and improves adherence.11 Synthetic TRPV1-agonist injectables based on capsaicin have undergone clinical trials for localized pain (eg, postoperative musculoskeletal pain); many patients experience pain relief, though benefit fades over weeks to months.20,21
Use in Traditional Medicine—Capsicum peppers have been used to aid digestion and promote healing in gastrointestinal conditions, such as dyspepsia.22 The peppers are a source of important vitamins and minerals, including vitamins A, C, and E; many of the B complex vitamins; and magnesium, calcium, and iron.23
Use as Cancer Therapy—Studies of the use of capsaicin in treating cancer have produced controversial results. In cell and animal models, capsaicin induces apoptosis through downregulation of the Bcl-2 protein; upregulation of oxidative stress, tribbles-related protein 3 (TRIB3), and caspase-3; and other pathways.19,24-26 On the other hand, consumption of Capsicum peppers has been associated with cancer of the stomach and gallbladder.27 Capsaicin might have anticarcinogenic properties, but its mechanism of action varies, depending on variables not fully understood.
Final Thoughts
Capsaicin is a neuropeptide-active compound found in Capsicum peppers that has many promising applications for use. However, dermatologists should be aware of the possibility of a skin reaction to this compound from handling peppers and using topical medicines. Exposure to capsaicin can cause irritant contact dermatitis that may require clinical care.
- Otang WM, Grierson DS, Afolayan AJ. A survey of plants responsible for causing irritant contact dermatitis in the Amathole district, Eastern Cape, South Africa. J Ethnopharmacol. 2014;157:274-284. doi:10.1016/j.jep.2014.10.002
- Weinberg RB. Hunan hand. N Engl J Med. 1981;305:1020.
- Lambrecht C, Goossens A. Occupational allergic contact dermatitis caused by capsicum. Contact Dermatitis. 2015;72:252-253. doi:10.1111/cod.12345
- Haar RJ, Iacopino V, Ranadive N, et al. Health impacts of chemical irritants used for crowd control: a systematic review of the injuries and deaths caused by tear gas and pepper spray. BMC Public Health. 2017;17:831. doi:10.1186/s12889-017-4814-6
- Simpson DM, Robinson-Papp J, Van J, et al. Capsaicin 8% patch in painful diabetic peripheral neuropathy: a randomized, double-blind, placebo-controlled study. J Pain. 2017;18:42-53. doi:10.1016/j.jpain.2016.09.008
- Yong YL, Tan LT-H, Ming LC, et al. The effectiveness and safety of topical capsaicin in postherpetic neuralgia: a systematic review and meta-analysis. Front Pharmacol. 2016;7:538. doi:10.3389/fphar.2016.00538
- Firoz EF, Levin JM, Hartman RD, et al. Lip plumper contact urticaria. J Am Acad Dermatol. 2009;60:861-863. doi:10.1016/j.jaad.2008.09.028
- Jones LA, Tandberg D, Troutman WG. Household treatment for “chile burns” of the hands. J Toxicol Clin Toxicol. 1987;25:483-491. doi:10.3109/15563658708992651
- Saxena AK, Mandhyan R. Multimodal approach for the management of Hunan hand syndrome: a case report. Pain Pract. 2013;13:227-230. doi:10.1111/j.1533-2500.2012.00567.x
- Cordell GA, Araujo OE. Capsaicin: identification, nomenclature, and pharmacotherapy. Ann Pharmacother. 1993;27:330-336. doi:10.1177/106002809302700316
- Baranidharan G, Das S, Bhaskar A. A review of the high-concentration capsaicin patch and experience in its use in the management of neuropathic pain. Ther Adv Neurol Disord. 2013;6:287-297. doi:10.1177/1756285613496862
- Carrizo García C, Barfuss MHJ, Sehr EM, et al. Phylogenetic relationships, diversification and expansion of chili peppers (Capsicum, Solanaceae). Ann Bot. 2016;118:35-51. doi:10.1093/aob/mcw079
- Basharat S, Gilani SA, Iftikhar F, et al. Capsaicin: plants of the genus Capsicum and positive effect of Oriental spice on skin health. Skin Pharmacol Physiol. 2020;33:331-341. doi:10.1159/000512196
- Hopps JJ, Dunn WR, Randall MD. Vasorelaxation to capsaicin and its effects on calcium influx in arteries. Eur J Pharmacol. 2012;681:88-93. doi:10.1016/j.ejphar.2012.02.019
- Burks TF, Buck SH, Miller MS. Mechanisms of depletion of substance P by capsaicin. Fed Proc. 1985;44:2531-2534.
- Ellis CN, Berberian B, Sulica VI, et al. A double-blind evaluation of topical capsaicin in pruritic psoriasis. J Am Acad Dermatol. 1993;29:438-442. doi:10.1016/0190-9622(93)70208-b
- Fusco BM, Alessandri M. Analgesic effect of capsaicin in idiopathic trigeminal neuralgia. Anesth Analg. 1992;74:375-377. doi:10.1213/00000539-199203000-00011
- Casanueva B, Rodero B, Quintial C, et al. Short-term efficacy of topical capsaicin therapy in severely affected fibromyalgia patients. Rheumatol Int. 2013;33:2665-2670. doi:10.1007/s00296-012-2490-5
- Bley K, Boorman G, Mohammad B, et al. A comprehensive review of the carcinogenic and anticarcinogenic potential of capsaicin. Toxicol Pathol. 2012;40:847-873. doi:10.1177/0192623312444471
- Jones IA, Togashi R, Wilson ML, et al. Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol. 2019;15:77-90. doi:10.1038/s41584-018-0123-4
- Campbell JN, Stevens R, Hanson P, et al. Injectable capsaicin for the management of pain due to osteoarthritis. Molecules. 2021;26:778.
- Maji AK, Banerji P. Phytochemistry and gastrointestinal benefits of the medicinal spice, Capsicum annum L. (chilli): a review. J Complement Integr Med. 2016;13:97-122. doi:10.1515jcim-2015-0037
- Baenas N, Belovié M, Ilie N, et al. Industrial use of pepper (Capsicum annum L.) derived products: technological benefits and biological advantages. Food Chem. 2019;274:872-885. doi:10.1016/j.foodchem.2018.09.047
- Lin RJ, Wu IJ, Hong JY, et al. Capsaicin-induced TRIB3 upregulation promotes apoptosis in cancer cells. Cancer Manag Res. 2018;10:4237-4248. doi:10.2147/CMAR.S162383
- Jung MY, Kang HJ, Moon A. Capsaicin-induced apoptosis in SK-Hep-1 hepatocarcinoma cells involves Bcl-2 downregulation and caspase-3 activation. Cancer Lett. 2001;165:139-145. doi:10.1016/s0304-3835(01)00426-8
- Ito K, Nakazato T, Yamato K, et al. Induction of apoptosis in leukemic cells by homovanillic acid derivative, capsaicin, through oxidative stress: implication of phosphorylation of p53 at Ser-15 residue by reactive oxygen species. Cancer Res. 2004;64:1071-1078. doi:10.1158/0008-5472.can-03-1670
- Báez S, Tsuchiya Y, Calvo A, et al. Genetic variants involved in gallstone formation and capsaicin metabolism, and the risk of gallbladder cancer in Chilean women. World J Gastroenterol. 2010;16:372-378. doi:10.3748/wjg.v16.i3.372
Cutaneous Manifestations
Capsicum peppers are used worldwide in preparing spicy dishes. Their active ingredient—capsaicin—is used as a topical medicine to treat localized pain. Capsicum peppers can cause irritant contact dermatitis with symptoms of erythema, cutaneous burning, and itch.1
Irritant contact dermatitis is a common occupational skin disorder. Many cooks have experienced the sting of a chili pepper after contact with the hands or eyes. Cases of chronic exposure to Capsicum peppers with persistent burning and pain have been called Hunan hand syndrome.2Capsicum peppers also have induced allergic contact dermatitis in a food production worker.3
Capsicum peppers also are used in pepper spray, tear gas, and animal repellents because of their stinging properties. These agents usually cause cutaneous tingling and burning that soon resolves; however, a review of 31 studies showed that crowd-control methods with Capsicum-containing tear gas and pepper spray can cause moderate to severe skin damage such as a persistent skin rash or erythema, or even first-, second-, or third-degree burns.4
Topical application of capsaicin isolate is meant to cause burning and deplete local neuropeptides, with a cutaneous reaction that ranges from mild to intolerable.5,6 Capsaicin also is found in other products. In one published case report, a 3-year-old boy broke out in facial urticaria when his mother kissed him on the cheek after she applied lip plumper containing capsaicin to her lips.7 Dermatologists should consider capsaicin an active ingredient that can irritate the skin in the garden, in the kitchen, and in topical products.
Obtaining Relief
Capsaicin-induced dermatitis can be relieved by washing the area with soap, detergent, baking soda, or oily compounds that act as solvents for the nonpolar capsaicin.8 Application of ice water or a high-potency topical steroid also may help. If the reaction is severe and persistent, a continuous stellate ganglion block may alleviate the pain of capsaicin-induced contact dermatitis.9
Identifying Features and Plant Facts
The Capsicum genus includes chili peppers, paprika, and red peppers. Capsicum peppers are native to tropical regions of the Americas (Figure). The use of Capsicum peppers in food can be traced to Indigenous peoples of Mexico as early as 7000

Capsicum belongs to the family Solanaceae, which includes tobacco, tomatoes, potatoes, and nightshade plants. There are many varieties of peppers in the Capsicum genus, with 5 domesticated species: Capsicum annuum, Capsicum baccatum, Capsicum chinense, Capsicum frutescens, and Capsicum pubescens. These include bell, poblano, cayenne, tabasco, habanero, and ají peppers, among others. Capsicum species grow as a shrub with flowers that rotate to stellate corollas and rounded berries of different sizes and colors.12 Capsaicin and other alkaloids are concentrated in the fruit; therefore, Capsicum dermatitis is most commonly induced by contact with the flesh of peppers.
Irritant Chemicals
Capsaicin (8-methyl-6-nonanoyl vanillylamide) is a nonpolar phenol, which is why washing skin that has come in contact with capsaicin with water or vinegar alone is insufficient to solubilize it.13 Capsaicin binds to the transient receptor potential vanilloid 1 (TRPV1), a calcium channel on neurons that opens in response to heat. When bound, the channel opens at a lower temperature threshold and depolarizes nerve endings, leading to vasodilation and activation of sensory nerves.14 Substance P is released and the individual experiences a painful burning sensation. When purified capsaicin is frequently applied at an appropriate dose, synthesis of substance P is diminished, resulting in reduced local pain overall.15
Capsaicin does not affect neurons without TRPV1, and administration of capsaicin is not painful if given with anesthesia. An inappropriately high dose of capsaicin destroys cells in the epidermal barrier, resulting in water loss and inducing release of vasoactive peptides and inflammatory cytokines.1 Careful handling of Capsicum peppers and capsaicin products can reduce the risk for irritation.
Medicinal Use
On-/Off-Label and Potential Uses—Capsaicin is US Food and Drug Administration approved for use in arthritis and musculoskeletal pain. It also is used to treat diabetic neuropathy,5 postherpetic neuralgia,6 psoriasis,16 and other conditions. Studies have shown that capsaicin might be useful in treating trigeminal neuralgia,17 fibromyalgia,18 migraines,14 cluster headaches,9 and HIV-associated distal sensory neuropathy.5
Delivery of Capsaicin—Capsaicin preferentially acts on C-fibers, which transmit dull, aching, chronic pain.19 The compound is available as a cream, lotion, and large bandage (for the lower back), as well as low- and high-dose patches. Capsaicin creams, lotions, and the low-dose patch are uncomfortable and must be applied for 4 to 6 weeks to take effect, which may impact patient adherence. The high-dose patch, which requires administration under local anesthesia by a health care worker, brings pain relief with a single use and improves adherence.11 Synthetic TRPV1-agonist injectables based on capsaicin have undergone clinical trials for localized pain (eg, postoperative musculoskeletal pain); many patients experience pain relief, though benefit fades over weeks to months.20,21
Use in Traditional Medicine—Capsicum peppers have been used to aid digestion and promote healing in gastrointestinal conditions, such as dyspepsia.22 The peppers are a source of important vitamins and minerals, including vitamins A, C, and E; many of the B complex vitamins; and magnesium, calcium, and iron.23
Use as Cancer Therapy—Studies of the use of capsaicin in treating cancer have produced controversial results. In cell and animal models, capsaicin induces apoptosis through downregulation of the Bcl-2 protein; upregulation of oxidative stress, tribbles-related protein 3 (TRIB3), and caspase-3; and other pathways.19,24-26 On the other hand, consumption of Capsicum peppers has been associated with cancer of the stomach and gallbladder.27 Capsaicin might have anticarcinogenic properties, but its mechanism of action varies, depending on variables not fully understood.
Final Thoughts
Capsaicin is a neuropeptide-active compound found in Capsicum peppers that has many promising applications for use. However, dermatologists should be aware of the possibility of a skin reaction to this compound from handling peppers and using topical medicines. Exposure to capsaicin can cause irritant contact dermatitis that may require clinical care.
Cutaneous Manifestations
Capsicum peppers are used worldwide in preparing spicy dishes. Their active ingredient—capsaicin—is used as a topical medicine to treat localized pain. Capsicum peppers can cause irritant contact dermatitis with symptoms of erythema, cutaneous burning, and itch.1
Irritant contact dermatitis is a common occupational skin disorder. Many cooks have experienced the sting of a chili pepper after contact with the hands or eyes. Cases of chronic exposure to Capsicum peppers with persistent burning and pain have been called Hunan hand syndrome.2Capsicum peppers also have induced allergic contact dermatitis in a food production worker.3
Capsicum peppers also are used in pepper spray, tear gas, and animal repellents because of their stinging properties. These agents usually cause cutaneous tingling and burning that soon resolves; however, a review of 31 studies showed that crowd-control methods with Capsicum-containing tear gas and pepper spray can cause moderate to severe skin damage such as a persistent skin rash or erythema, or even first-, second-, or third-degree burns.4
Topical application of capsaicin isolate is meant to cause burning and deplete local neuropeptides, with a cutaneous reaction that ranges from mild to intolerable.5,6 Capsaicin also is found in other products. In one published case report, a 3-year-old boy broke out in facial urticaria when his mother kissed him on the cheek after she applied lip plumper containing capsaicin to her lips.7 Dermatologists should consider capsaicin an active ingredient that can irritate the skin in the garden, in the kitchen, and in topical products.
Obtaining Relief
Capsaicin-induced dermatitis can be relieved by washing the area with soap, detergent, baking soda, or oily compounds that act as solvents for the nonpolar capsaicin.8 Application of ice water or a high-potency topical steroid also may help. If the reaction is severe and persistent, a continuous stellate ganglion block may alleviate the pain of capsaicin-induced contact dermatitis.9
Identifying Features and Plant Facts
The Capsicum genus includes chili peppers, paprika, and red peppers. Capsicum peppers are native to tropical regions of the Americas (Figure). The use of Capsicum peppers in food can be traced to Indigenous peoples of Mexico as early as 7000

Capsicum belongs to the family Solanaceae, which includes tobacco, tomatoes, potatoes, and nightshade plants. There are many varieties of peppers in the Capsicum genus, with 5 domesticated species: Capsicum annuum, Capsicum baccatum, Capsicum chinense, Capsicum frutescens, and Capsicum pubescens. These include bell, poblano, cayenne, tabasco, habanero, and ají peppers, among others. Capsicum species grow as a shrub with flowers that rotate to stellate corollas and rounded berries of different sizes and colors.12 Capsaicin and other alkaloids are concentrated in the fruit; therefore, Capsicum dermatitis is most commonly induced by contact with the flesh of peppers.
Irritant Chemicals
Capsaicin (8-methyl-6-nonanoyl vanillylamide) is a nonpolar phenol, which is why washing skin that has come in contact with capsaicin with water or vinegar alone is insufficient to solubilize it.13 Capsaicin binds to the transient receptor potential vanilloid 1 (TRPV1), a calcium channel on neurons that opens in response to heat. When bound, the channel opens at a lower temperature threshold and depolarizes nerve endings, leading to vasodilation and activation of sensory nerves.14 Substance P is released and the individual experiences a painful burning sensation. When purified capsaicin is frequently applied at an appropriate dose, synthesis of substance P is diminished, resulting in reduced local pain overall.15
Capsaicin does not affect neurons without TRPV1, and administration of capsaicin is not painful if given with anesthesia. An inappropriately high dose of capsaicin destroys cells in the epidermal barrier, resulting in water loss and inducing release of vasoactive peptides and inflammatory cytokines.1 Careful handling of Capsicum peppers and capsaicin products can reduce the risk for irritation.
Medicinal Use
On-/Off-Label and Potential Uses—Capsaicin is US Food and Drug Administration approved for use in arthritis and musculoskeletal pain. It also is used to treat diabetic neuropathy,5 postherpetic neuralgia,6 psoriasis,16 and other conditions. Studies have shown that capsaicin might be useful in treating trigeminal neuralgia,17 fibromyalgia,18 migraines,14 cluster headaches,9 and HIV-associated distal sensory neuropathy.5
Delivery of Capsaicin—Capsaicin preferentially acts on C-fibers, which transmit dull, aching, chronic pain.19 The compound is available as a cream, lotion, and large bandage (for the lower back), as well as low- and high-dose patches. Capsaicin creams, lotions, and the low-dose patch are uncomfortable and must be applied for 4 to 6 weeks to take effect, which may impact patient adherence. The high-dose patch, which requires administration under local anesthesia by a health care worker, brings pain relief with a single use and improves adherence.11 Synthetic TRPV1-agonist injectables based on capsaicin have undergone clinical trials for localized pain (eg, postoperative musculoskeletal pain); many patients experience pain relief, though benefit fades over weeks to months.20,21
Use in Traditional Medicine—Capsicum peppers have been used to aid digestion and promote healing in gastrointestinal conditions, such as dyspepsia.22 The peppers are a source of important vitamins and minerals, including vitamins A, C, and E; many of the B complex vitamins; and magnesium, calcium, and iron.23
Use as Cancer Therapy—Studies of the use of capsaicin in treating cancer have produced controversial results. In cell and animal models, capsaicin induces apoptosis through downregulation of the Bcl-2 protein; upregulation of oxidative stress, tribbles-related protein 3 (TRIB3), and caspase-3; and other pathways.19,24-26 On the other hand, consumption of Capsicum peppers has been associated with cancer of the stomach and gallbladder.27 Capsaicin might have anticarcinogenic properties, but its mechanism of action varies, depending on variables not fully understood.
Final Thoughts
Capsaicin is a neuropeptide-active compound found in Capsicum peppers that has many promising applications for use. However, dermatologists should be aware of the possibility of a skin reaction to this compound from handling peppers and using topical medicines. Exposure to capsaicin can cause irritant contact dermatitis that may require clinical care.
- Otang WM, Grierson DS, Afolayan AJ. A survey of plants responsible for causing irritant contact dermatitis in the Amathole district, Eastern Cape, South Africa. J Ethnopharmacol. 2014;157:274-284. doi:10.1016/j.jep.2014.10.002
- Weinberg RB. Hunan hand. N Engl J Med. 1981;305:1020.
- Lambrecht C, Goossens A. Occupational allergic contact dermatitis caused by capsicum. Contact Dermatitis. 2015;72:252-253. doi:10.1111/cod.12345
- Haar RJ, Iacopino V, Ranadive N, et al. Health impacts of chemical irritants used for crowd control: a systematic review of the injuries and deaths caused by tear gas and pepper spray. BMC Public Health. 2017;17:831. doi:10.1186/s12889-017-4814-6
- Simpson DM, Robinson-Papp J, Van J, et al. Capsaicin 8% patch in painful diabetic peripheral neuropathy: a randomized, double-blind, placebo-controlled study. J Pain. 2017;18:42-53. doi:10.1016/j.jpain.2016.09.008
- Yong YL, Tan LT-H, Ming LC, et al. The effectiveness and safety of topical capsaicin in postherpetic neuralgia: a systematic review and meta-analysis. Front Pharmacol. 2016;7:538. doi:10.3389/fphar.2016.00538
- Firoz EF, Levin JM, Hartman RD, et al. Lip plumper contact urticaria. J Am Acad Dermatol. 2009;60:861-863. doi:10.1016/j.jaad.2008.09.028
- Jones LA, Tandberg D, Troutman WG. Household treatment for “chile burns” of the hands. J Toxicol Clin Toxicol. 1987;25:483-491. doi:10.3109/15563658708992651
- Saxena AK, Mandhyan R. Multimodal approach for the management of Hunan hand syndrome: a case report. Pain Pract. 2013;13:227-230. doi:10.1111/j.1533-2500.2012.00567.x
- Cordell GA, Araujo OE. Capsaicin: identification, nomenclature, and pharmacotherapy. Ann Pharmacother. 1993;27:330-336. doi:10.1177/106002809302700316
- Baranidharan G, Das S, Bhaskar A. A review of the high-concentration capsaicin patch and experience in its use in the management of neuropathic pain. Ther Adv Neurol Disord. 2013;6:287-297. doi:10.1177/1756285613496862
- Carrizo García C, Barfuss MHJ, Sehr EM, et al. Phylogenetic relationships, diversification and expansion of chili peppers (Capsicum, Solanaceae). Ann Bot. 2016;118:35-51. doi:10.1093/aob/mcw079
- Basharat S, Gilani SA, Iftikhar F, et al. Capsaicin: plants of the genus Capsicum and positive effect of Oriental spice on skin health. Skin Pharmacol Physiol. 2020;33:331-341. doi:10.1159/000512196
- Hopps JJ, Dunn WR, Randall MD. Vasorelaxation to capsaicin and its effects on calcium influx in arteries. Eur J Pharmacol. 2012;681:88-93. doi:10.1016/j.ejphar.2012.02.019
- Burks TF, Buck SH, Miller MS. Mechanisms of depletion of substance P by capsaicin. Fed Proc. 1985;44:2531-2534.
- Ellis CN, Berberian B, Sulica VI, et al. A double-blind evaluation of topical capsaicin in pruritic psoriasis. J Am Acad Dermatol. 1993;29:438-442. doi:10.1016/0190-9622(93)70208-b
- Fusco BM, Alessandri M. Analgesic effect of capsaicin in idiopathic trigeminal neuralgia. Anesth Analg. 1992;74:375-377. doi:10.1213/00000539-199203000-00011
- Casanueva B, Rodero B, Quintial C, et al. Short-term efficacy of topical capsaicin therapy in severely affected fibromyalgia patients. Rheumatol Int. 2013;33:2665-2670. doi:10.1007/s00296-012-2490-5
- Bley K, Boorman G, Mohammad B, et al. A comprehensive review of the carcinogenic and anticarcinogenic potential of capsaicin. Toxicol Pathol. 2012;40:847-873. doi:10.1177/0192623312444471
- Jones IA, Togashi R, Wilson ML, et al. Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol. 2019;15:77-90. doi:10.1038/s41584-018-0123-4
- Campbell JN, Stevens R, Hanson P, et al. Injectable capsaicin for the management of pain due to osteoarthritis. Molecules. 2021;26:778.
- Maji AK, Banerji P. Phytochemistry and gastrointestinal benefits of the medicinal spice, Capsicum annum L. (chilli): a review. J Complement Integr Med. 2016;13:97-122. doi:10.1515jcim-2015-0037
- Baenas N, Belovié M, Ilie N, et al. Industrial use of pepper (Capsicum annum L.) derived products: technological benefits and biological advantages. Food Chem. 2019;274:872-885. doi:10.1016/j.foodchem.2018.09.047
- Lin RJ, Wu IJ, Hong JY, et al. Capsaicin-induced TRIB3 upregulation promotes apoptosis in cancer cells. Cancer Manag Res. 2018;10:4237-4248. doi:10.2147/CMAR.S162383
- Jung MY, Kang HJ, Moon A. Capsaicin-induced apoptosis in SK-Hep-1 hepatocarcinoma cells involves Bcl-2 downregulation and caspase-3 activation. Cancer Lett. 2001;165:139-145. doi:10.1016/s0304-3835(01)00426-8
- Ito K, Nakazato T, Yamato K, et al. Induction of apoptosis in leukemic cells by homovanillic acid derivative, capsaicin, through oxidative stress: implication of phosphorylation of p53 at Ser-15 residue by reactive oxygen species. Cancer Res. 2004;64:1071-1078. doi:10.1158/0008-5472.can-03-1670
- Báez S, Tsuchiya Y, Calvo A, et al. Genetic variants involved in gallstone formation and capsaicin metabolism, and the risk of gallbladder cancer in Chilean women. World J Gastroenterol. 2010;16:372-378. doi:10.3748/wjg.v16.i3.372
- Otang WM, Grierson DS, Afolayan AJ. A survey of plants responsible for causing irritant contact dermatitis in the Amathole district, Eastern Cape, South Africa. J Ethnopharmacol. 2014;157:274-284. doi:10.1016/j.jep.2014.10.002
- Weinberg RB. Hunan hand. N Engl J Med. 1981;305:1020.
- Lambrecht C, Goossens A. Occupational allergic contact dermatitis caused by capsicum. Contact Dermatitis. 2015;72:252-253. doi:10.1111/cod.12345
- Haar RJ, Iacopino V, Ranadive N, et al. Health impacts of chemical irritants used for crowd control: a systematic review of the injuries and deaths caused by tear gas and pepper spray. BMC Public Health. 2017;17:831. doi:10.1186/s12889-017-4814-6
- Simpson DM, Robinson-Papp J, Van J, et al. Capsaicin 8% patch in painful diabetic peripheral neuropathy: a randomized, double-blind, placebo-controlled study. J Pain. 2017;18:42-53. doi:10.1016/j.jpain.2016.09.008
- Yong YL, Tan LT-H, Ming LC, et al. The effectiveness and safety of topical capsaicin in postherpetic neuralgia: a systematic review and meta-analysis. Front Pharmacol. 2016;7:538. doi:10.3389/fphar.2016.00538
- Firoz EF, Levin JM, Hartman RD, et al. Lip plumper contact urticaria. J Am Acad Dermatol. 2009;60:861-863. doi:10.1016/j.jaad.2008.09.028
- Jones LA, Tandberg D, Troutman WG. Household treatment for “chile burns” of the hands. J Toxicol Clin Toxicol. 1987;25:483-491. doi:10.3109/15563658708992651
- Saxena AK, Mandhyan R. Multimodal approach for the management of Hunan hand syndrome: a case report. Pain Pract. 2013;13:227-230. doi:10.1111/j.1533-2500.2012.00567.x
- Cordell GA, Araujo OE. Capsaicin: identification, nomenclature, and pharmacotherapy. Ann Pharmacother. 1993;27:330-336. doi:10.1177/106002809302700316
- Baranidharan G, Das S, Bhaskar A. A review of the high-concentration capsaicin patch and experience in its use in the management of neuropathic pain. Ther Adv Neurol Disord. 2013;6:287-297. doi:10.1177/1756285613496862
- Carrizo García C, Barfuss MHJ, Sehr EM, et al. Phylogenetic relationships, diversification and expansion of chili peppers (Capsicum, Solanaceae). Ann Bot. 2016;118:35-51. doi:10.1093/aob/mcw079
- Basharat S, Gilani SA, Iftikhar F, et al. Capsaicin: plants of the genus Capsicum and positive effect of Oriental spice on skin health. Skin Pharmacol Physiol. 2020;33:331-341. doi:10.1159/000512196
- Hopps JJ, Dunn WR, Randall MD. Vasorelaxation to capsaicin and its effects on calcium influx in arteries. Eur J Pharmacol. 2012;681:88-93. doi:10.1016/j.ejphar.2012.02.019
- Burks TF, Buck SH, Miller MS. Mechanisms of depletion of substance P by capsaicin. Fed Proc. 1985;44:2531-2534.
- Ellis CN, Berberian B, Sulica VI, et al. A double-blind evaluation of topical capsaicin in pruritic psoriasis. J Am Acad Dermatol. 1993;29:438-442. doi:10.1016/0190-9622(93)70208-b
- Fusco BM, Alessandri M. Analgesic effect of capsaicin in idiopathic trigeminal neuralgia. Anesth Analg. 1992;74:375-377. doi:10.1213/00000539-199203000-00011
- Casanueva B, Rodero B, Quintial C, et al. Short-term efficacy of topical capsaicin therapy in severely affected fibromyalgia patients. Rheumatol Int. 2013;33:2665-2670. doi:10.1007/s00296-012-2490-5
- Bley K, Boorman G, Mohammad B, et al. A comprehensive review of the carcinogenic and anticarcinogenic potential of capsaicin. Toxicol Pathol. 2012;40:847-873. doi:10.1177/0192623312444471
- Jones IA, Togashi R, Wilson ML, et al. Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol. 2019;15:77-90. doi:10.1038/s41584-018-0123-4
- Campbell JN, Stevens R, Hanson P, et al. Injectable capsaicin for the management of pain due to osteoarthritis. Molecules. 2021;26:778.
- Maji AK, Banerji P. Phytochemistry and gastrointestinal benefits of the medicinal spice, Capsicum annum L. (chilli): a review. J Complement Integr Med. 2016;13:97-122. doi:10.1515jcim-2015-0037
- Baenas N, Belovié M, Ilie N, et al. Industrial use of pepper (Capsicum annum L.) derived products: technological benefits and biological advantages. Food Chem. 2019;274:872-885. doi:10.1016/j.foodchem.2018.09.047
- Lin RJ, Wu IJ, Hong JY, et al. Capsaicin-induced TRIB3 upregulation promotes apoptosis in cancer cells. Cancer Manag Res. 2018;10:4237-4248. doi:10.2147/CMAR.S162383
- Jung MY, Kang HJ, Moon A. Capsaicin-induced apoptosis in SK-Hep-1 hepatocarcinoma cells involves Bcl-2 downregulation and caspase-3 activation. Cancer Lett. 2001;165:139-145. doi:10.1016/s0304-3835(01)00426-8
- Ito K, Nakazato T, Yamato K, et al. Induction of apoptosis in leukemic cells by homovanillic acid derivative, capsaicin, through oxidative stress: implication of phosphorylation of p53 at Ser-15 residue by reactive oxygen species. Cancer Res. 2004;64:1071-1078. doi:10.1158/0008-5472.can-03-1670
- Báez S, Tsuchiya Y, Calvo A, et al. Genetic variants involved in gallstone formation and capsaicin metabolism, and the risk of gallbladder cancer in Chilean women. World J Gastroenterol. 2010;16:372-378. doi:10.3748/wjg.v16.i3.372
Practice Points
- Capsicum peppers—used worldwide in food preparation, pepper spray, and cosmetic products—can cause irritant dermatitis from the active ingredient capsaicin.
- Capsaicin, which is isolated as a medication to treat musculoskeletal pain, postherpetic neuralgia, and more, can cause a mild local skin reaction.