User login
Navigating Moonlighting Opportunities During Dermatology Training
Navigating Moonlighting Opportunities During Dermatology Training
Residents and fellows in training have to navigate time management to balance reading, hands-on training, family responsibilities, exercise, diet, and sleep requirements. In addition, they grapple with the stress of financial commitments for food, housing, clothing, family members, transportation, and student loans. A brilliant friend of mine once said that she struggled throughout residency and her early career to find balance until it finally occurred to her that, while balance was aspirational, resilience was key. All that said, residents in training may find it appealing to earn a little extra money and gain additional clinical experience through moonlighting. This article discusses some key considerations when embarking on such a decision, including the effects of moonlighting on other commitments and some logistical factors to consider.
Will Moonlighting Adversely Affect My Other Commitments?
Residency and fellowship are precious opportunities to gain medical knowledge, hone your ability to make diagnoses through complex pattern recognition, and refine the necessary surgical and interpersonal skills to carry you through a successful career. Dermatology encompasses a vast array of conditions related only by their manifestation in skin. Dermatology residents and fellows may spend fewer sleepless hours on call, but the reading requirements are massive. Our treatment armamentarium has expanded rapidly with highly effective treatments for chronic conditions that have a dramatic impact on quality of life. With so many effective agents available, the choice often relates as much to comorbidities as to disease severity and location. There is so much to learn.
While making a full commitment to acquiring the skills of an expert clinician, it is important for residents to remain aware of those who depend on you—in particular, the fleeting time you have with your growing children. They grow up fast, and your interactions with them determine who they will grow up to be. In the past, salt, silk, gold, and jewels were the world’s greatest luxuries. Now, it’s time—time with family, time for self-care, time to reflect, and time to rest and renew. Be careful how you squander time in exchange for material possessions.
What Logistical Factors Should You Consider When Embarking on Moonlighting?
There are clearly stated policies from the Accreditation Council for Graduate Medical Education for when moonlighting can occur during training.1 It should not occur during typical residency or fellowship work hours, and the individual must be in good standing academically and progressing well on their journey to becoming a competent dermatologist. They must also have the appropriate skills to practice in the field of medicine chosen for moonlighting.
Moonlighting opportunities may exist in the form of emergency department or “quick clinic” coverage, especially for the evaluation and treatment of acute minor illnesses. Fellows who have completed a dermatology residency may supervise dermatology residents in afterhours or weekend clinics, offering enhanced opportunities for autonomy, additional clinical experience, and some welcome cash. To make such clinics viable, the office space must be available; the building must be open; and the costs of the space, scheduling, reception, and security services must be covered as well as nursing support (which should be voluntary and likely will require overtime pay scales). After all of these—as well as supplies—have been paid for, what is left is what is available to distribute as pay for service. Working through these factors provides valuable experience in resource management and helps prepare trainees for the economic realities of private practice. Large organizations may be able to provide the space and support, but all of that needs to be paid for through the proceeds that come from the patient care provided. No-show rates often are quite high for after-hours and weekend clinics, but the expenses for those unfilled appointment slots remain and must be paid in full. Be sure the demand exists and that you plan appropriately with strategic overbooking based on historical data on patient mix, procedural needs, and no-show rates.
My department has supported resident and fellow requests for moonlighting opportunities in the past. The most successful model was to have a limited number of early morning appointment slots prior to the start of morning didactics. Security typically already exists, rooms are available, and patients can be seen and still get to work or get their kids to school. No-show rates remained very low for morning appointments, and strategic overbooking was unnecessary.
In contrast, evening and weekend clinics start out strong with high patient satisfaction and deteriorate fairly quickly with accelerating no-show rates. People are busy at the end of the day, and unforeseen circumstances often affect their ability to keep an appointment. Weekends are precious; potential patients may be less schedule minded in the evenings and on weekends, and the residents and fellows themselves often find it stressful to commit to giving up a chunk of weekend time on a scheduled basis.
Before you commit to a moonlighting job, be sure to weigh all of the above factors and be sure the juice is worth the squeeze.
Final Thoughts
Moonlighting opportunities are a way to acquire both clinical and management skills and can provide a welcome extra bit of cash to ease financial burdens, but these benefits should be balanced with other time commitments and overall quality of life. Time is precious—choose wisely and be sure you spend it well.
- Accreditation Council for Graduate Medical Education. Common Program Requirements (Residency). Updated September 17, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2023v3.pdf
Residents and fellows in training have to navigate time management to balance reading, hands-on training, family responsibilities, exercise, diet, and sleep requirements. In addition, they grapple with the stress of financial commitments for food, housing, clothing, family members, transportation, and student loans. A brilliant friend of mine once said that she struggled throughout residency and her early career to find balance until it finally occurred to her that, while balance was aspirational, resilience was key. All that said, residents in training may find it appealing to earn a little extra money and gain additional clinical experience through moonlighting. This article discusses some key considerations when embarking on such a decision, including the effects of moonlighting on other commitments and some logistical factors to consider.
Will Moonlighting Adversely Affect My Other Commitments?
Residency and fellowship are precious opportunities to gain medical knowledge, hone your ability to make diagnoses through complex pattern recognition, and refine the necessary surgical and interpersonal skills to carry you through a successful career. Dermatology encompasses a vast array of conditions related only by their manifestation in skin. Dermatology residents and fellows may spend fewer sleepless hours on call, but the reading requirements are massive. Our treatment armamentarium has expanded rapidly with highly effective treatments for chronic conditions that have a dramatic impact on quality of life. With so many effective agents available, the choice often relates as much to comorbidities as to disease severity and location. There is so much to learn.
While making a full commitment to acquiring the skills of an expert clinician, it is important for residents to remain aware of those who depend on you—in particular, the fleeting time you have with your growing children. They grow up fast, and your interactions with them determine who they will grow up to be. In the past, salt, silk, gold, and jewels were the world’s greatest luxuries. Now, it’s time—time with family, time for self-care, time to reflect, and time to rest and renew. Be careful how you squander time in exchange for material possessions.
What Logistical Factors Should You Consider When Embarking on Moonlighting?
There are clearly stated policies from the Accreditation Council for Graduate Medical Education for when moonlighting can occur during training.1 It should not occur during typical residency or fellowship work hours, and the individual must be in good standing academically and progressing well on their journey to becoming a competent dermatologist. They must also have the appropriate skills to practice in the field of medicine chosen for moonlighting.
Moonlighting opportunities may exist in the form of emergency department or “quick clinic” coverage, especially for the evaluation and treatment of acute minor illnesses. Fellows who have completed a dermatology residency may supervise dermatology residents in afterhours or weekend clinics, offering enhanced opportunities for autonomy, additional clinical experience, and some welcome cash. To make such clinics viable, the office space must be available; the building must be open; and the costs of the space, scheduling, reception, and security services must be covered as well as nursing support (which should be voluntary and likely will require overtime pay scales). After all of these—as well as supplies—have been paid for, what is left is what is available to distribute as pay for service. Working through these factors provides valuable experience in resource management and helps prepare trainees for the economic realities of private practice. Large organizations may be able to provide the space and support, but all of that needs to be paid for through the proceeds that come from the patient care provided. No-show rates often are quite high for after-hours and weekend clinics, but the expenses for those unfilled appointment slots remain and must be paid in full. Be sure the demand exists and that you plan appropriately with strategic overbooking based on historical data on patient mix, procedural needs, and no-show rates.
My department has supported resident and fellow requests for moonlighting opportunities in the past. The most successful model was to have a limited number of early morning appointment slots prior to the start of morning didactics. Security typically already exists, rooms are available, and patients can be seen and still get to work or get their kids to school. No-show rates remained very low for morning appointments, and strategic overbooking was unnecessary.
In contrast, evening and weekend clinics start out strong with high patient satisfaction and deteriorate fairly quickly with accelerating no-show rates. People are busy at the end of the day, and unforeseen circumstances often affect their ability to keep an appointment. Weekends are precious; potential patients may be less schedule minded in the evenings and on weekends, and the residents and fellows themselves often find it stressful to commit to giving up a chunk of weekend time on a scheduled basis.
Before you commit to a moonlighting job, be sure to weigh all of the above factors and be sure the juice is worth the squeeze.
Final Thoughts
Moonlighting opportunities are a way to acquire both clinical and management skills and can provide a welcome extra bit of cash to ease financial burdens, but these benefits should be balanced with other time commitments and overall quality of life. Time is precious—choose wisely and be sure you spend it well.
Residents and fellows in training have to navigate time management to balance reading, hands-on training, family responsibilities, exercise, diet, and sleep requirements. In addition, they grapple with the stress of financial commitments for food, housing, clothing, family members, transportation, and student loans. A brilliant friend of mine once said that she struggled throughout residency and her early career to find balance until it finally occurred to her that, while balance was aspirational, resilience was key. All that said, residents in training may find it appealing to earn a little extra money and gain additional clinical experience through moonlighting. This article discusses some key considerations when embarking on such a decision, including the effects of moonlighting on other commitments and some logistical factors to consider.
Will Moonlighting Adversely Affect My Other Commitments?
Residency and fellowship are precious opportunities to gain medical knowledge, hone your ability to make diagnoses through complex pattern recognition, and refine the necessary surgical and interpersonal skills to carry you through a successful career. Dermatology encompasses a vast array of conditions related only by their manifestation in skin. Dermatology residents and fellows may spend fewer sleepless hours on call, but the reading requirements are massive. Our treatment armamentarium has expanded rapidly with highly effective treatments for chronic conditions that have a dramatic impact on quality of life. With so many effective agents available, the choice often relates as much to comorbidities as to disease severity and location. There is so much to learn.
While making a full commitment to acquiring the skills of an expert clinician, it is important for residents to remain aware of those who depend on you—in particular, the fleeting time you have with your growing children. They grow up fast, and your interactions with them determine who they will grow up to be. In the past, salt, silk, gold, and jewels were the world’s greatest luxuries. Now, it’s time—time with family, time for self-care, time to reflect, and time to rest and renew. Be careful how you squander time in exchange for material possessions.
What Logistical Factors Should You Consider When Embarking on Moonlighting?
There are clearly stated policies from the Accreditation Council for Graduate Medical Education for when moonlighting can occur during training.1 It should not occur during typical residency or fellowship work hours, and the individual must be in good standing academically and progressing well on their journey to becoming a competent dermatologist. They must also have the appropriate skills to practice in the field of medicine chosen for moonlighting.
Moonlighting opportunities may exist in the form of emergency department or “quick clinic” coverage, especially for the evaluation and treatment of acute minor illnesses. Fellows who have completed a dermatology residency may supervise dermatology residents in afterhours or weekend clinics, offering enhanced opportunities for autonomy, additional clinical experience, and some welcome cash. To make such clinics viable, the office space must be available; the building must be open; and the costs of the space, scheduling, reception, and security services must be covered as well as nursing support (which should be voluntary and likely will require overtime pay scales). After all of these—as well as supplies—have been paid for, what is left is what is available to distribute as pay for service. Working through these factors provides valuable experience in resource management and helps prepare trainees for the economic realities of private practice. Large organizations may be able to provide the space and support, but all of that needs to be paid for through the proceeds that come from the patient care provided. No-show rates often are quite high for after-hours and weekend clinics, but the expenses for those unfilled appointment slots remain and must be paid in full. Be sure the demand exists and that you plan appropriately with strategic overbooking based on historical data on patient mix, procedural needs, and no-show rates.
My department has supported resident and fellow requests for moonlighting opportunities in the past. The most successful model was to have a limited number of early morning appointment slots prior to the start of morning didactics. Security typically already exists, rooms are available, and patients can be seen and still get to work or get their kids to school. No-show rates remained very low for morning appointments, and strategic overbooking was unnecessary.
In contrast, evening and weekend clinics start out strong with high patient satisfaction and deteriorate fairly quickly with accelerating no-show rates. People are busy at the end of the day, and unforeseen circumstances often affect their ability to keep an appointment. Weekends are precious; potential patients may be less schedule minded in the evenings and on weekends, and the residents and fellows themselves often find it stressful to commit to giving up a chunk of weekend time on a scheduled basis.
Before you commit to a moonlighting job, be sure to weigh all of the above factors and be sure the juice is worth the squeeze.
Final Thoughts
Moonlighting opportunities are a way to acquire both clinical and management skills and can provide a welcome extra bit of cash to ease financial burdens, but these benefits should be balanced with other time commitments and overall quality of life. Time is precious—choose wisely and be sure you spend it well.
- Accreditation Council for Graduate Medical Education. Common Program Requirements (Residency). Updated September 17, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2023v3.pdf
- Accreditation Council for Graduate Medical Education. Common Program Requirements (Residency). Updated September 17, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2023v3.pdf
Navigating Moonlighting Opportunities During Dermatology Training
Navigating Moonlighting Opportunities During Dermatology Training
PRACTICE POINTS
- Dermatology training demands extensive study and hands-on skill development, which need to be balanced with family time, finances, and self-care.
- Before moonlighting, ensure it will not compromise your family’s quality of life or your core residency/fellowship commitments and that your program’s policies permit it.
- Carefully assess logistics to determine if an afterhours or weekend clinic can be a financially viable moonlighting opportunity.
Immune Responses and Health Disparities Warrant Scabies Vaccine Development
Immune Responses and Health Disparities Warrant Scabies Vaccine Development
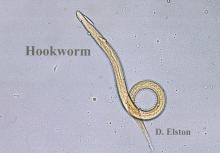
The scabies mite, originally known as Acarus scabiei,1 now is considered an arthropod of the class Arachnida, order Astigmata, and family Sarcoptidae.2 Scabies mites are able to adhere to the surface of human skin.3 The mites burrow and lay eggs in the top layer of the epidermis; most patients have 10 to 15 mites.3 The patient’s immune system incites an allergic reaction to the mite protein and feces in the skin, causing itching and rash.4
Scabies is common in indigenous populations and in low-income areas of developing countries.5 It is most prevalent in Africa, South America, Australia, and Southeast Asia, in part due to poverty, poor nutritional status, homelessness, and inadequate hygiene.2 In 2009, the World Health Organization declared scabies a neglected skin disease2; however, in 2010, 1.5 million disability adjusted life-years were attributed to scabies,6 and it is estimated that 200 million people worldwide have scabies at any given time. Children and elderly individuals in resource-poor communities are the most at risk. In fact, 5% to 50% of children in low-income areas have scabies.4
The purpose of this article is to provide background on scabies and its effect on the human immune system. We also discuss manipulation of the immune response for the purposes of creating a potential scabies vaccine.
Life Cycle and Transmission
The life cycle of Sarcoptes scabiei consists of 4 stages. The first is the egg. As female scabies mites burrow under the skin, they lay 2 to 3 ovular eggs per day.3 The second stage is the larva. When the egg hatches, the larva has 3 pairs of legs and travels to the surface of the skin where it burrows into the stratum corneum, creating short, nearly invisible burrows called molting pouches. After 3 to 4 days, the larva molts into a nymph, which is the third stage. The nymph has 4 pairs of legs and will continue to grow before molting into an adult, which is the fourth stage. Both the larva and nymph may be found in hair follicles or molting pouches. The fourth stage is the adult, which is round and saclike and does not have eyes. Adult females are 0.30 mm to 0.45 mm long and 0.25 mm to 0.35 mm wide, which is half the size of adult males.3 On warm skin, the female mite can crawl at a rate of 2.5 cm per minute.7
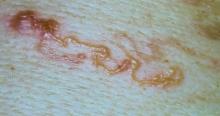
Scabies mites mate via an active male penetrating the molting pouch of a female. This only occurs once but leaves the female fertile for the rest of her life. Once a female is pregnant, she leaves her molting pouch and travels along the surface of the skin looking for a place to make her permanent burrow.3 The most common sites for scabies burrows are the axillae, umbilicus, interdigital spaces, beltline, buttocks, flexor surfaces of the wrists, female nipples, and male penile shaft.5 Once she finds an acceptable location, the female scabies mite will create a serpentine burrow and lay her eggs. Once she burrows, she will stay there and continue to lay eggs for the rest of her life, lengthening the burrow as needed.3 Female mites lay their eggs in the superficial epidermis, and the eggs take approximately 2 to 3 weeks to hatch. Female mites die 30 to 60 days later.2
Scabies infestations typically spread via the transfer of pregnant adult females during skin-to-skin contact, but they also can spread via fomites.3 During all stages of their life cycle, scabies mites can secrete enzymes that allow them to penetrate the intact epidermis in less than 30 minutes; in fact, an otherwise healthy patient with scabies must have 15 to 20 minutes of close skin-to-skin contact with an infected individual for the disease to be transmitted.7 Because scabies mites can survive for more than 3 days outside the human body, it is thought that fomites also may be involved in transmission. Scabies mites also have been collected from clothing, bedding, and furniture, which further supports the idea that fomites are involved in disease transmission.7
Clinical Manifestation of Scabies
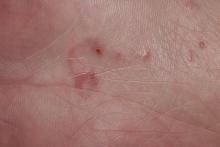
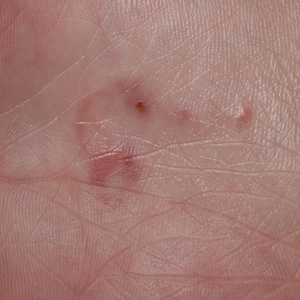
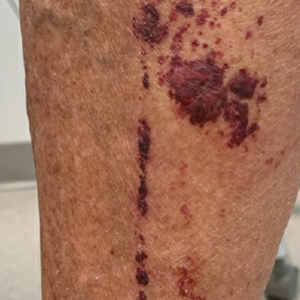
Scabies symptoms include severe pruritus as well as linear burrows and vesicles in the interdigital spaces on the hands, wrists, arms and legs, and lower abdomen. Infants and young children also can develop a rash on the palms, soles, ankles, and scalp. Men can develop inflammatory scabies nodules on the penis and scrotum, while women can develop these nodules on the nipple.4 Type I and type IV hypersensitivity reactions contribute to the rash and itching associated with scabies infestation via host allergic and inflammatory reactions to the mites and their byproducts. Patients with scabies typically are infested with fewer than 15 mites,6 but just a few can cause substantial pruritus and scratching, leading to hyperkeratosis.8
Additionally, when patients with scabies scratch the skin, they become vulnerable to bacterial infections.4 Scabies lesions can be coinfected with group A streptococci and Staphylococcus aureus,8 potentially leading to abscesses and septicemia. These secondary infections also can cause renal and cardiac complications; in fact, in tropical areas, scabies infections are considered a risk factor for kidney disease and rheumatic heart disease.4
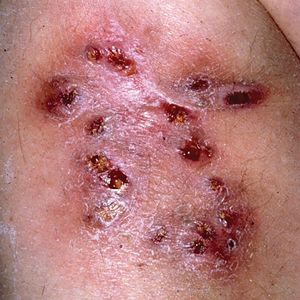
The 2 main forms of scabies infestations are ordinary and crusted. The most common form is ordinary scabies, which typically manifests with fewer than 15 mites per patient; crusted scabies (CS) is the more rare and extreme form.6 Cases of CS present with thousands to millions of mites per patient, leading to more widespread and severe symptoms.4 Because of the large increase in the number of mites, CS is more contagious than ordinary scabies.6
Patients with CS typically present with hyperkeratotic skin disease, as evidenced by thick scaly crusts with large numbers of mites, which can lead to permanent skin disfiguration. Patients with CS also can develop deep fissuring of the crusts, within which other microbes can gain entry to the body and lead to secondary infection and possibly sepsis and death. Also, because of the increased number of mites as well as the crusted skin, patients with CS are contagious for longer. As it is more difficult to eradicate, reinfestation is common with CS.6
Patients with compromised immune systems are predisposed to CS. Specifically, patients with HIV or human T-lymphotropic virus 1 or those undergoing organ transplantation are thought to be the most at risk for CS.6 Crusted scabies also has been identified in large numbers in patients with Down syndrome and in Aboriginal Australians; however, the reasoning for this is poorly understood.6
Immune Response
The inflammatory reaction associated with scabies infestations occurs 4 to 6 weeks after initial exposure. It is hypothesized that scabies can alter parts of the host immune system, which contributes to the delayed onset of symptoms. Scabies mites also produce inactivated protease paralogues and serpins, which help to protect the mites from the host immune system by inhibiting the complement system.6
The complement system is part of the innate immune response and is the first line of defense against pathogens. Specifically with scabies infestations, C3 and C4 complement components have been found in skin lesions.6 C3a and C4a fragments cause local inflammation, while C3a and C5a activate mast cells to release histamine and tumor necrosis factor (TNF) α, further amplifying the inflammatory response; however, CS lesions show low C3 and C4, which can indicate immunodeficiency in patients with CS. It also can be due to the sheer number of mites in a CS infection causing the host immune system to be overloaded.6
Innate effector immune cells also are an important part of the innate immune response to scabies; for example, eosinophilia is seen in scabies infections. Specifically, in CS, eosinophils help modulate and sustain the T-helper (Th) 2 inflammatory response. One cytokine secreted by Th2 cells is IL-5, which is closely associated with the attraction, maturation, and survival of eosinophils.6 Eosinophils also can influence the Th1 inflammatory response in that they produce IL-12, interferon (IFN) γ, and several Toll-like receptors. Furthermore, eosinophilic expression of IL-2 can lead to expansion of regulatory T cells, while eosinophilic expression of IL-10 and transforming growth factor (TGF) Β also can suppress local inflammation by influencing regulatory T cells.6
Additionally, mast cells and basophils are important in the IgE-mediated allergic reaction as well as the host immune response to parasites. When activated, basophils and mast cells produce TNF-α, IL-6, Il-4, IL-5, and IL-13, which contribute to the Th2 inflammatory response; however, the role of mast cells and basophils in scabies infections still is poorly understood.6
Macrophages, neutrophils, and dendritic cells (DCs) contribute to phagocytosis, antigen presentation, and differentiation of T cells, which also contribute to the inflammatory and allergic reactions associated with parasitic infections.6 Macrophages have been found in low numbers in scabies infestation, possibly due to immune-modulating molecules secreted by scabies mites. Early in an infestation, the mites secrete immune-modulating molecules, which inhibit macrophage migration to the site of inflammation, allowing the mites to grow.6 Neutrophils and DCs also are involved in the host immune response to scabies. Neutrophils are the predominant inflammatory cell infiltrate in scabies lesions. The scabies protein SMSB4 inhibits neutrophil opsonization and phagocytosis, thus suppressing bacterial killing.6 Some of the first antigen-presenting cells encountered by the antigen are DCs. They are involved in preparing the antigens for presentation to effector T cells, which leads to T-cell differentiation and activation.6
Cytokines are another important factor in the innate immune response. The host immune response to ordinary scabies is Th1-cell mediated, during which CD4+ and CD8+ T cells secrete IFN-γ, TNF-α, and IL-2.6 Therefore, IFN- γ and TNF-α are elevated in the serum of patients with ordinary scabies. Conversely, the host immune response to CS is Th2-cell mediated. T-helper 2 cells are needed in IgE-mediated hypersensitivity reactions, and they secrete IL-4, IL-5, and IL-13. In the serum of patients with CS, IL-l4, IL-5, and IL-13 are elevated while IFN-γ is decreased.6 Additionally, IL-6, TGF-Β, IL-23, IL-1Β, or IL-18 can induce Th17 cells to generate and secrete IL-17, which enhances the inflammatory response by inducing further expression of TNF-α, IL-1Β, IL-6, keratinocytes, and fibroblasts. T-helper 17 and IL-17 also are involved in psoriasis and atopic dermatitis, as well as Leishmania major and Schistosoma japonicum.6
Regulatory T cells Tregs secrete TGF-Β and IL-10, which suppress pathologic inflammation, and IL-10 is substantially reduced in patients with CS compared to those with ordinary scabies and uninfected control patients. Additionally, IL-10 can inhibit the synthesis of TNF-γ and IFN-α. Reduced IL-10 expression can lead to proliferation of IL-17 secretion, resulting in a regulatory T cell/Th17 dysfunctional immune response.6
Immunoglobulins are antibodies that are involved in the host’s adaptive immune response. The first antibody to appear in response to an antigen is IgM, and IgM bound to scabies antigens is present in 74%6 of patients with ordinary scabies. Because IgM is the first antibody to appear in response to a scabies infection, detection of serum IgM may allow for earlier detection of scabies; however, IgM has a high cross-reactivity between scabies mites and dust mites, which can hinder scabies diagnosis via IgM detection.6
Both patients with ordinary scabies and CS also show an increased circulatory IgG concentration compared to control groups; patients with CS have higher concentrations. Increased IgG also can be in part due to concurrent bacterial infections.6 When IgG or IgM antibodies bind to a pathogen, they activate the complement cascade, which further enhances the activity of these antibodies.9
Additionally, IgA is important in mucosal immune function. In both patients with ordinary scabies and CS, there is increased IgA binding to recombinant scabies mite antigens.6 Sarcoptes scabiei proteases that are localized in the mite’s gut and scybala suggest their involvement in mite digestion and burrowing. The increased secretion of these proteases into the host skin may contribute to the increased IgA,9 and these increased IgA levels have been shown to be positively correlated with severity of scabies infection.6
Also essential in allergic and parasitic inflammation, IgE is observed at higher levels in secondary infections of scabies compared to primary infections.6 Additionally, T-cell infiltrates are implicated in adaptive immune response to scabies. CD4+ T cells are the most prevalent T cells in ordinary scabies skin lesions; however, CD4+ T cells are minimal and CD8+ T cells are elevated in CS skin lesions. The increased CD8+ T cells may cause apoptosis of keratinocytes, leading to epidermal hyperproliferation. The apoptotic keratinocytes can secrete cytokines, which can lead to tissue damage.6 These T cells also may be involved in the failure of the skin’s immune system to mount an effective response to the parasite infestation, leading to uncontrolled parasitic growth. Because patients with AIDS who are infected with scabies mites often develop CS, it is also thought that CD4+ T cells are essential in the immune response to scabies.6
Diagnosis and Current Treatment Options
Current diagnosis of scabies is based on mites, eggs, and fecal matter from the host’s skin. Dermoscopy and fluorescent dermoscopy can be helpful in identifying the mites, eggs, and feces on the patient’s skin. Scabies treatment sometimes may be based solely on symptoms without any positive tests.8
Acaricides are the current method of treatment for scabies infestations.5 Acaricides can be expensive and toxic to the environment and food sources,10 and some agents have been associated with neurotoxicity5 in children or the development of certain cancers.11 Although topical acaricides are the standard form of treatment, oral ivermectin also can be used. Ivermectin is not associated with selective fetal toxicity, but there are limited safety data in pregnant women and in children weighing less than 15 kg (33 lb). Additionally, because symptoms typically are not present during an early infection, treating everyone in the household and those who had close contact with the patient can help prevent reinfection.4
Although these drugs have been shown to be effective at treating scabies, scabies mites are becoming increasingly resistant to acaricides.5 There are 4 main proposed mechanisms for why this occurs.12 The first is through voltage-gated sodium channels, which are involved in the normal functioning of neurons and myocytes. Permethrin, a type of acaricide, binds to voltage-gated sodium channels when it is in an open or active state and prevents it from closing. This creates repetitive neuron firing and hyperactivity, which ultimately kills the scabies mite. Some mites have mutated to close this channel, which reduces the binding potential of permethrin. Glutathione S-transferase is another mechanism of resistance. It catalyzes a bond that tags drugs for elimination. Increased activity or expressivity of glutathione S-transferase by scabies mites can lead to drug resistance.12 Adenosine triphosphate– binding cassette (ABC) transporters also may contribute to this resistance. The ABC transporters use adenosine triphosphate to facilitate the import or export of molecules. Scabies mites express a protein called the multidrug-resistant protein, which is an ABC transporter that is associated with drug resistance and is present in scabies mites.12 Lastly, ligand-gated chloride channels have been implicated in scabies resistance to acaricides. Ligand-gated chloride channels also are important in normal functioning of neurons and myocytes. Some antiparasitic drugs act on these channels, leading to a continuous influx of chloride, but some scabies mites have mutated this pathway.12
Pesticides and the Risk for Cancer
Pesticides commonly are used to treat scabies; however, a link between pesticide exposure and leukemia and lymphoma has been seen through epidemiologic studies, and there also is increasing biological evidence to suggest this.11 For example, the pesticide permethrin, which works by paralyzing the nervous system of insects,13 has been associated with an increased risk for leukemia and lymphoma in humans. Permethrin is a pyrethroid and, compared to control patients, children with leukemia had higher levels of pyrethroid metabolites in their blood.14 Numerical and structural chromosomal aberrations that give rise to gene fusions are the most common abnormalities seen in leukemia, and permethrin has been shown to induce DNA breaks, chromosome aberrations, and sister chromatid exchanges.14 Permethrin also has been associated with an increased risk for multiple myeloma.13
Furthermore, in utero exposure to pesticides has been associated with an increased risk for childhood leukemia.15 Pesticide exposure shortly before conception, during pregnancy, and after birth is associated with an increased risk for acute lymphocytic leukemia.16 In fact, the children of mothers who were exposed to pesticides 3 months before conception have been found to be at least twice as likely to be diagnosed with acute lymphocytic leukemia within the first year of life compared with children whose mothers were not exposed to pesticides.17 It is hypothesized that permethrin can cross the placenta and alter the hematopoietic precursor cells in the fetus, resulting in leukemogenesis.18 Pyrethroid metabolites also have been detected in umbilical cord blood samples and breast milk.15
In contrast to the research demonstrating a link between permethrin and cancer, other studies have found no association between permethrin19 and leukemia20; non-Hodgkin lymphoma19; or cancers of the colon, rectum, pancreas, lungs, skin, female breast, prostate, and urinary bladder.20 Because of conflicting research on the link between permethrin and cancer, more research is needed.,20
Importance of a Scabies Vaccine
Because scabies mites are developing increasing treatment resistance, more radical approaches such as vaccines are becoming important. While a scabies vaccine is still aspirational, animals that have been infected for a second time with scabies demonstrate a milder response to the second infection compared to the first infection, which could mean there is a potential for disease prevention through a vaccine.21 While educating patients and physicians, reporting cases of infection, and improving drug supply and access can help decrease scabies infestations, these are costly and difficult to implement. Scabies already is most prevalent in low-income areas, so costly interventions are even less feasible. An effective, one-dose vaccine would cost less than these efforts and therefore could be implemented more easily.9
In older adults, scabies more often manifests atypically and is more likely to progress to CS. Aged care centers are prone to institutional outbreaks, even in developed countries, so a vaccine also would greatly help this population. Additionally, the number of children attending day care centers, which also are prone to scabies outbreaks, is increasing. When a child contracts scabies, all close contacts need to be treated, so a preventive vaccine can be useful.9
One potential candidate for a scabies vaccine is total mite extract. Studies show that rabbits immunized with a total mite extract induce antibodies to more antigens than rabbits naturally infested with scabies mites; however, the mites cannot be cultured in vitro, which makes obtaining a large amount of their total extract difficult. Therefore, recombinant vaccines also have been proposed, as they are more easily available.22 One recombinant vaccine candidate is recombinant S scabiei serpin (rSs-serpin). Immunization with rSs-serpin has strong immunogenicity and produced immune protection in rabbits.22
Two other recombinant vaccine candidates are the rSs chitinaselike protein (CLP) 12 and the rSsCLP5. Chitinaselike proteins are very similar to chitinases; however, they are unable to degrade chitin. They are involved in immune reactions to infections, and CLPs from scabies mites have been shown to induce the host immune response.22 For example, in a particular rabbit study, rSsCLP5 demonstrated high immunoreactivity and immunogenicity. In fact, after exposure to S scabiei, 74.3% of rabbits who were vaccinated with rSsCLP5 had no detectable lesions.5 Also, after immunization with rSsCLP5 and rSsCLP12, there were increased levels of specific IgG and IgE antibodies produced and decreased numbers of infesting mites.22 Weight loss also is associated with severe scabies infection. Rabbits vaccinated with rSsCLP5 and exposed to the parasite gained weight, indicating protection via rSsCLP5. Even rabbits who did develop symptoms of scabies after immunization with rSsCLP5 and exposure to S scabiei showed less serious manifestations.5
A combination vaccine cocktail of rSs-serpin, rSsCLP12, and rSsCLP5 also has been proposed by Shen et al.22 Four test groups and a control group (n=12 per group) were included in a vaccine trial. Between 83.33% and 91.67% of rabbits vaccinated with this mixed recombinant cocktail vaccine had no detectable skin lesions from scabies. After immunization with the cocktail vaccine, the specific serum IgG and IgE antibodies also increased. For both IgG and IgE, increased levels were first detected at 1 week postimmunization and peaked at 2 weeks postimmunization.22 A multiepitope vaccine derived from these 3 recombinant proteins also was explored by Shen et al22; fewer rabbits vaccinated with it had no detectable scabies skin lesions compared to those treated with the vaccine cocktail. Although the multiepitope vaccine yielded less immume protection, it was associated with a slower disease course and milder symptoms compared with no vaccination.22
Two more proposed scabies recombinant vaccine candidates are derived from the antigens Ssag1 and Ssag2; however, rabbits vaccinated with Ssag1 or Ssag2 showed no immune protection or mite burden reduction.22 The lack of protection could be due to denaturation or degradation of the protective antigens. It also can be due to the low abundance of these antigens, meaning they may not be vital for the mite’s survival—survival—a potential avenue for future research. The antigens also could have lost their native structure and immunogenic properties during the purification and production process. Therefore, more research is needed to investigate how to purify these vaccines to keep the peptides more structurally similar to their native makeups.10 More research also is needed to better understand the antigen or antigens and their mechanisms that elicit a protective immune response.9
Final Thoughts
Scabies causes severe pruritus in mild cases but also can lead to severe disfigurement, sepsis, and even death. Scabies infestations are seen disproportionately more often in low-income and resource-poor communities, and the current treatment options are less accessible to these populations. Scabies infestations induce a complex immune response that involves multiple aspects of both the innate and adaptive immune systems and can be targeted to create a scabies vaccine. Development of a scabies vaccine is crucial considering the growing resistance to current standard treatments. Acaricides potentially are associated with an increased risk for malignancy, which further amplifies the need for a scabies vaccine. There currently are multiple promising scabies vaccine candidates; however, more research is needed to better understand the host’s immune response to scabies as well as how to more accurately and efficiently produce the vaccine. The development of a safe, effective, economical vaccine that can be mass distributed would be beneficial in the treatment of scabies, especially in resource-poor communities.
- Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10:297. doi:10.1186/s13071-017-2234-1
- Murray RL, Crane JS. Scabies. In: StatPearls. StatPearls Publishing. Updated July 31, 2023.
- Centers for Disease Control and Prevention. CDC—scabies—biology. November 2, 2010. https://www.cdc.gov/dpdx/scabies/index.html
- World Health Organization. Scabies. May 31, 2023. Accessed May 8, 2025. https://www.who.int/news-room/fact-sheets/detail/scabies
- Shen N, Zhang H, Ren Y, et al. A chitinase-like protein from Sarcoptes scabiei as a candidate anti-mite vaccine that contributes to immune protection in rabbits. Parasit Vectors. 2018;11:599. doi:10.1186/s13071- 018-3184-y
- Bhat SA, Mounsey KE, Liu X, et al. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasit Vectors. 2017;10:385. doi:10.1186/s13071-017-2320-4
- Hicks MI, Elston DM. Scabies. Dermatolog Ther. 2009;22:279-292. doi:10.1111/j.1529-8019.2009.01243.x
- Morgan MS, Arlian LG, Rider SD, et al. A proteomic analysis of Sarcoptes scabiei (acari: Sarcoptidae). J Med Entomol. 2016;53:553-561. doi:10.1093/jme/tjv247
- Liu X, Walton S, Mounsey K. Vaccine against scabies: necessity and possibility. Parasitology. 2014;141:725-732. doi:10.1017 /s0031182013002047
- Casais R, Granda V, Balseiro A, et al. Vaccination of rabbits with immunodominant antigens from Sarcoptes scabiei induced high levels of humoral responses and pro-inflammatory cytokines but confers limited protection. Parasit Vectors. 2016;9:435. doi:10.1186 /s13071-016-1717-9?
- Navarrete-Meneses MP, Pedraza-Meléndez AI, Salas-Labadía C, et al. Low concentrations of permethrin and malathion induce numerical and structural abnormalities in KMT2A and IGH genes in vitro. J Appl Toxicol. 2018;38:1262-1270. doi:10.1002/jat.3638
- Khalil S, Abbas O, Kibbi AG, et al. Scabies in the age of increasing drug resistance. PLoS Negl Trop Dis. 2017;11:E0005920. doi:10.1371 /journal.pntd.0005920
- Rusiecki JA, Patel R, Koutros S, et al. Cancer incidence among pesticide applicators exposed to permethrin in the Agricultural Health Study. Environ Health Perspect. 2009;117:581-586. doi:10.1289 /ehp.11318
- Navarrete-Meneses MP, Salas-Labadía C, Sanabrais-Jiménez M, et al. Exposure to the insecticides permethrin and malathion induces leukemia and lymphoma-associated gene aberrations in vitro. Toxicol In Vitro. 2017;44:17-26. doi:10.1016/j.tiv.2017.06.013
- Navarrete-Meneses MDP, Pérez-Vera P. Pyrethroid pesticide exposure and hematological cancer: epidemiological, biological and molecular evidence. Rev Environ Health. 2019;34:197-210. doi:10.1515 /reveh-2018-0070
- Madrigal JM, Jones RR, Gunier RB, et al. Residential exposure to carbamate, organophosphate, and pyrethroid insecticides in house dust and risk of childhood acute lymphoblastic leukemia. Environ Res. 2021;201:111501. doi:10.1016/j.envres.2021.111501
- Ferreira JD, Couto AC, Pombo-de-Oliveira MS, et al. In utero pesticide exposure and leukemia in Brazilian children <2 years of age. Environ Health Perspect. 2013;121:269-275. doi:10.1289/ehp.1103942
- Borkhardt A, Wilda M, Fuchs U, et al. Congenital leukaemia after heavy abuse of permethrin during pregnancy. Arch Dis Child Fetal Neonatal Ed. 2003;88:F436-F437. doi:10.1136/fn.88.5.f436
- De Roos AJ, Schinasi LH, Miligi L, et al. Occupational insecticide exposure and risk of non]Hodgkin lymphoma: a pooled case]control study from the InterLymph consortium. Int J Cancer. 2021;149:1768-1786. doi:10.1002/ijc.33740
- Boffett, P, Desai V. Exposure to permethrin and cancer risk: a systematic review. Crit Rev Toxicol. 2018;48:433-442. doi:10.1080/1040 8444.2018.1439449
- Adji A, Rumokoy LJM, Salaki CL. Scabies vaccine as a new breakthrough for the challenge of acaricides resistance. Adv Biolog Sci Res. 2020;8:208-213. doi:10.2991/absr.k.200513.036
- Shen N, Wei W, Chen Y, et al. Vaccination with a cocktail vaccine elicits significant protection against Sarcoptes scabiei in rabbits, whereas the multi-epitope vaccine offers limited protection. Exp Parasitol. 2023;245:108442. doi:10.1016/j.exppara.2022.108442
The scabies mite, originally known as Acarus scabiei,1 now is considered an arthropod of the class Arachnida, order Astigmata, and family Sarcoptidae.2 Scabies mites are able to adhere to the surface of human skin.3 The mites burrow and lay eggs in the top layer of the epidermis; most patients have 10 to 15 mites.3 The patient’s immune system incites an allergic reaction to the mite protein and feces in the skin, causing itching and rash.4
Scabies is common in indigenous populations and in low-income areas of developing countries.5 It is most prevalent in Africa, South America, Australia, and Southeast Asia, in part due to poverty, poor nutritional status, homelessness, and inadequate hygiene.2 In 2009, the World Health Organization declared scabies a neglected skin disease2; however, in 2010, 1.5 million disability adjusted life-years were attributed to scabies,6 and it is estimated that 200 million people worldwide have scabies at any given time. Children and elderly individuals in resource-poor communities are the most at risk. In fact, 5% to 50% of children in low-income areas have scabies.4
The purpose of this article is to provide background on scabies and its effect on the human immune system. We also discuss manipulation of the immune response for the purposes of creating a potential scabies vaccine.
Life Cycle and Transmission
The life cycle of Sarcoptes scabiei consists of 4 stages. The first is the egg. As female scabies mites burrow under the skin, they lay 2 to 3 ovular eggs per day.3 The second stage is the larva. When the egg hatches, the larva has 3 pairs of legs and travels to the surface of the skin where it burrows into the stratum corneum, creating short, nearly invisible burrows called molting pouches. After 3 to 4 days, the larva molts into a nymph, which is the third stage. The nymph has 4 pairs of legs and will continue to grow before molting into an adult, which is the fourth stage. Both the larva and nymph may be found in hair follicles or molting pouches. The fourth stage is the adult, which is round and saclike and does not have eyes. Adult females are 0.30 mm to 0.45 mm long and 0.25 mm to 0.35 mm wide, which is half the size of adult males.3 On warm skin, the female mite can crawl at a rate of 2.5 cm per minute.7
Scabies mites mate via an active male penetrating the molting pouch of a female. This only occurs once but leaves the female fertile for the rest of her life. Once a female is pregnant, she leaves her molting pouch and travels along the surface of the skin looking for a place to make her permanent burrow.3 The most common sites for scabies burrows are the axillae, umbilicus, interdigital spaces, beltline, buttocks, flexor surfaces of the wrists, female nipples, and male penile shaft.5 Once she finds an acceptable location, the female scabies mite will create a serpentine burrow and lay her eggs. Once she burrows, she will stay there and continue to lay eggs for the rest of her life, lengthening the burrow as needed.3 Female mites lay their eggs in the superficial epidermis, and the eggs take approximately 2 to 3 weeks to hatch. Female mites die 30 to 60 days later.2
Scabies infestations typically spread via the transfer of pregnant adult females during skin-to-skin contact, but they also can spread via fomites.3 During all stages of their life cycle, scabies mites can secrete enzymes that allow them to penetrate the intact epidermis in less than 30 minutes; in fact, an otherwise healthy patient with scabies must have 15 to 20 minutes of close skin-to-skin contact with an infected individual for the disease to be transmitted.7 Because scabies mites can survive for more than 3 days outside the human body, it is thought that fomites also may be involved in transmission. Scabies mites also have been collected from clothing, bedding, and furniture, which further supports the idea that fomites are involved in disease transmission.7
Clinical Manifestation of Scabies
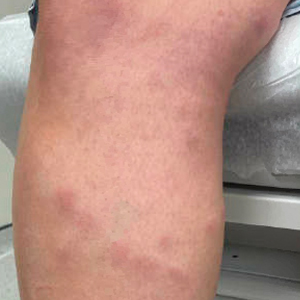
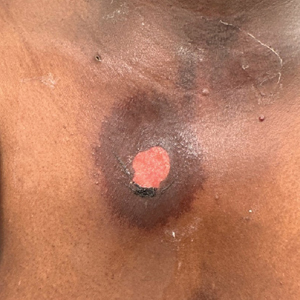
Scabies symptoms include severe pruritus as well as linear burrows and vesicles in the interdigital spaces on the hands, wrists, arms and legs, and lower abdomen. Infants and young children also can develop a rash on the palms, soles, ankles, and scalp. Men can develop inflammatory scabies nodules on the penis and scrotum, while women can develop these nodules on the nipple.4 Type I and type IV hypersensitivity reactions contribute to the rash and itching associated with scabies infestation via host allergic and inflammatory reactions to the mites and their byproducts. Patients with scabies typically are infested with fewer than 15 mites,6 but just a few can cause substantial pruritus and scratching, leading to hyperkeratosis.8
Additionally, when patients with scabies scratch the skin, they become vulnerable to bacterial infections.4 Scabies lesions can be coinfected with group A streptococci and Staphylococcus aureus,8 potentially leading to abscesses and septicemia. These secondary infections also can cause renal and cardiac complications; in fact, in tropical areas, scabies infections are considered a risk factor for kidney disease and rheumatic heart disease.4
The 2 main forms of scabies infestations are ordinary and crusted. The most common form is ordinary scabies, which typically manifests with fewer than 15 mites per patient; crusted scabies (CS) is the more rare and extreme form.6 Cases of CS present with thousands to millions of mites per patient, leading to more widespread and severe symptoms.4 Because of the large increase in the number of mites, CS is more contagious than ordinary scabies.6
Patients with CS typically present with hyperkeratotic skin disease, as evidenced by thick scaly crusts with large numbers of mites, which can lead to permanent skin disfiguration. Patients with CS also can develop deep fissuring of the crusts, within which other microbes can gain entry to the body and lead to secondary infection and possibly sepsis and death. Also, because of the increased number of mites as well as the crusted skin, patients with CS are contagious for longer. As it is more difficult to eradicate, reinfestation is common with CS.6
Patients with compromised immune systems are predisposed to CS. Specifically, patients with HIV or human T-lymphotropic virus 1 or those undergoing organ transplantation are thought to be the most at risk for CS.6 Crusted scabies also has been identified in large numbers in patients with Down syndrome and in Aboriginal Australians; however, the reasoning for this is poorly understood.6
Immune Response
The inflammatory reaction associated with scabies infestations occurs 4 to 6 weeks after initial exposure. It is hypothesized that scabies can alter parts of the host immune system, which contributes to the delayed onset of symptoms. Scabies mites also produce inactivated protease paralogues and serpins, which help to protect the mites from the host immune system by inhibiting the complement system.6
The complement system is part of the innate immune response and is the first line of defense against pathogens. Specifically with scabies infestations, C3 and C4 complement components have been found in skin lesions.6 C3a and C4a fragments cause local inflammation, while C3a and C5a activate mast cells to release histamine and tumor necrosis factor (TNF) α, further amplifying the inflammatory response; however, CS lesions show low C3 and C4, which can indicate immunodeficiency in patients with CS. It also can be due to the sheer number of mites in a CS infection causing the host immune system to be overloaded.6
Innate effector immune cells also are an important part of the innate immune response to scabies; for example, eosinophilia is seen in scabies infections. Specifically, in CS, eosinophils help modulate and sustain the T-helper (Th) 2 inflammatory response. One cytokine secreted by Th2 cells is IL-5, which is closely associated with the attraction, maturation, and survival of eosinophils.6 Eosinophils also can influence the Th1 inflammatory response in that they produce IL-12, interferon (IFN) γ, and several Toll-like receptors. Furthermore, eosinophilic expression of IL-2 can lead to expansion of regulatory T cells, while eosinophilic expression of IL-10 and transforming growth factor (TGF) Β also can suppress local inflammation by influencing regulatory T cells.6
Additionally, mast cells and basophils are important in the IgE-mediated allergic reaction as well as the host immune response to parasites. When activated, basophils and mast cells produce TNF-α, IL-6, Il-4, IL-5, and IL-13, which contribute to the Th2 inflammatory response; however, the role of mast cells and basophils in scabies infections still is poorly understood.6
Macrophages, neutrophils, and dendritic cells (DCs) contribute to phagocytosis, antigen presentation, and differentiation of T cells, which also contribute to the inflammatory and allergic reactions associated with parasitic infections.6 Macrophages have been found in low numbers in scabies infestation, possibly due to immune-modulating molecules secreted by scabies mites. Early in an infestation, the mites secrete immune-modulating molecules, which inhibit macrophage migration to the site of inflammation, allowing the mites to grow.6 Neutrophils and DCs also are involved in the host immune response to scabies. Neutrophils are the predominant inflammatory cell infiltrate in scabies lesions. The scabies protein SMSB4 inhibits neutrophil opsonization and phagocytosis, thus suppressing bacterial killing.6 Some of the first antigen-presenting cells encountered by the antigen are DCs. They are involved in preparing the antigens for presentation to effector T cells, which leads to T-cell differentiation and activation.6
Cytokines are another important factor in the innate immune response. The host immune response to ordinary scabies is Th1-cell mediated, during which CD4+ and CD8+ T cells secrete IFN-γ, TNF-α, and IL-2.6 Therefore, IFN- γ and TNF-α are elevated in the serum of patients with ordinary scabies. Conversely, the host immune response to CS is Th2-cell mediated. T-helper 2 cells are needed in IgE-mediated hypersensitivity reactions, and they secrete IL-4, IL-5, and IL-13. In the serum of patients with CS, IL-l4, IL-5, and IL-13 are elevated while IFN-γ is decreased.6 Additionally, IL-6, TGF-Β, IL-23, IL-1Β, or IL-18 can induce Th17 cells to generate and secrete IL-17, which enhances the inflammatory response by inducing further expression of TNF-α, IL-1Β, IL-6, keratinocytes, and fibroblasts. T-helper 17 and IL-17 also are involved in psoriasis and atopic dermatitis, as well as Leishmania major and Schistosoma japonicum.6
Regulatory T cells Tregs secrete TGF-Β and IL-10, which suppress pathologic inflammation, and IL-10 is substantially reduced in patients with CS compared to those with ordinary scabies and uninfected control patients. Additionally, IL-10 can inhibit the synthesis of TNF-γ and IFN-α. Reduced IL-10 expression can lead to proliferation of IL-17 secretion, resulting in a regulatory T cell/Th17 dysfunctional immune response.6
Immunoglobulins are antibodies that are involved in the host’s adaptive immune response. The first antibody to appear in response to an antigen is IgM, and IgM bound to scabies antigens is present in 74%6 of patients with ordinary scabies. Because IgM is the first antibody to appear in response to a scabies infection, detection of serum IgM may allow for earlier detection of scabies; however, IgM has a high cross-reactivity between scabies mites and dust mites, which can hinder scabies diagnosis via IgM detection.6
Both patients with ordinary scabies and CS also show an increased circulatory IgG concentration compared to control groups; patients with CS have higher concentrations. Increased IgG also can be in part due to concurrent bacterial infections.6 When IgG or IgM antibodies bind to a pathogen, they activate the complement cascade, which further enhances the activity of these antibodies.9
Additionally, IgA is important in mucosal immune function. In both patients with ordinary scabies and CS, there is increased IgA binding to recombinant scabies mite antigens.6 Sarcoptes scabiei proteases that are localized in the mite’s gut and scybala suggest their involvement in mite digestion and burrowing. The increased secretion of these proteases into the host skin may contribute to the increased IgA,9 and these increased IgA levels have been shown to be positively correlated with severity of scabies infection.6
Also essential in allergic and parasitic inflammation, IgE is observed at higher levels in secondary infections of scabies compared to primary infections.6 Additionally, T-cell infiltrates are implicated in adaptive immune response to scabies. CD4+ T cells are the most prevalent T cells in ordinary scabies skin lesions; however, CD4+ T cells are minimal and CD8+ T cells are elevated in CS skin lesions. The increased CD8+ T cells may cause apoptosis of keratinocytes, leading to epidermal hyperproliferation. The apoptotic keratinocytes can secrete cytokines, which can lead to tissue damage.6 These T cells also may be involved in the failure of the skin’s immune system to mount an effective response to the parasite infestation, leading to uncontrolled parasitic growth. Because patients with AIDS who are infected with scabies mites often develop CS, it is also thought that CD4+ T cells are essential in the immune response to scabies.6
Diagnosis and Current Treatment Options
Current diagnosis of scabies is based on mites, eggs, and fecal matter from the host’s skin. Dermoscopy and fluorescent dermoscopy can be helpful in identifying the mites, eggs, and feces on the patient’s skin. Scabies treatment sometimes may be based solely on symptoms without any positive tests.8
Acaricides are the current method of treatment for scabies infestations.5 Acaricides can be expensive and toxic to the environment and food sources,10 and some agents have been associated with neurotoxicity5 in children or the development of certain cancers.11 Although topical acaricides are the standard form of treatment, oral ivermectin also can be used. Ivermectin is not associated with selective fetal toxicity, but there are limited safety data in pregnant women and in children weighing less than 15 kg (33 lb). Additionally, because symptoms typically are not present during an early infection, treating everyone in the household and those who had close contact with the patient can help prevent reinfection.4
Although these drugs have been shown to be effective at treating scabies, scabies mites are becoming increasingly resistant to acaricides.5 There are 4 main proposed mechanisms for why this occurs.12 The first is through voltage-gated sodium channels, which are involved in the normal functioning of neurons and myocytes. Permethrin, a type of acaricide, binds to voltage-gated sodium channels when it is in an open or active state and prevents it from closing. This creates repetitive neuron firing and hyperactivity, which ultimately kills the scabies mite. Some mites have mutated to close this channel, which reduces the binding potential of permethrin. Glutathione S-transferase is another mechanism of resistance. It catalyzes a bond that tags drugs for elimination. Increased activity or expressivity of glutathione S-transferase by scabies mites can lead to drug resistance.12 Adenosine triphosphate– binding cassette (ABC) transporters also may contribute to this resistance. The ABC transporters use adenosine triphosphate to facilitate the import or export of molecules. Scabies mites express a protein called the multidrug-resistant protein, which is an ABC transporter that is associated with drug resistance and is present in scabies mites.12 Lastly, ligand-gated chloride channels have been implicated in scabies resistance to acaricides. Ligand-gated chloride channels also are important in normal functioning of neurons and myocytes. Some antiparasitic drugs act on these channels, leading to a continuous influx of chloride, but some scabies mites have mutated this pathway.12
Pesticides and the Risk for Cancer
Pesticides commonly are used to treat scabies; however, a link between pesticide exposure and leukemia and lymphoma has been seen through epidemiologic studies, and there also is increasing biological evidence to suggest this.11 For example, the pesticide permethrin, which works by paralyzing the nervous system of insects,13 has been associated with an increased risk for leukemia and lymphoma in humans. Permethrin is a pyrethroid and, compared to control patients, children with leukemia had higher levels of pyrethroid metabolites in their blood.14 Numerical and structural chromosomal aberrations that give rise to gene fusions are the most common abnormalities seen in leukemia, and permethrin has been shown to induce DNA breaks, chromosome aberrations, and sister chromatid exchanges.14 Permethrin also has been associated with an increased risk for multiple myeloma.13
Furthermore, in utero exposure to pesticides has been associated with an increased risk for childhood leukemia.15 Pesticide exposure shortly before conception, during pregnancy, and after birth is associated with an increased risk for acute lymphocytic leukemia.16 In fact, the children of mothers who were exposed to pesticides 3 months before conception have been found to be at least twice as likely to be diagnosed with acute lymphocytic leukemia within the first year of life compared with children whose mothers were not exposed to pesticides.17 It is hypothesized that permethrin can cross the placenta and alter the hematopoietic precursor cells in the fetus, resulting in leukemogenesis.18 Pyrethroid metabolites also have been detected in umbilical cord blood samples and breast milk.15
In contrast to the research demonstrating a link between permethrin and cancer, other studies have found no association between permethrin19 and leukemia20; non-Hodgkin lymphoma19; or cancers of the colon, rectum, pancreas, lungs, skin, female breast, prostate, and urinary bladder.20 Because of conflicting research on the link between permethrin and cancer, more research is needed.,20
Importance of a Scabies Vaccine
Because scabies mites are developing increasing treatment resistance, more radical approaches such as vaccines are becoming important. While a scabies vaccine is still aspirational, animals that have been infected for a second time with scabies demonstrate a milder response to the second infection compared to the first infection, which could mean there is a potential for disease prevention through a vaccine.21 While educating patients and physicians, reporting cases of infection, and improving drug supply and access can help decrease scabies infestations, these are costly and difficult to implement. Scabies already is most prevalent in low-income areas, so costly interventions are even less feasible. An effective, one-dose vaccine would cost less than these efforts and therefore could be implemented more easily.9
In older adults, scabies more often manifests atypically and is more likely to progress to CS. Aged care centers are prone to institutional outbreaks, even in developed countries, so a vaccine also would greatly help this population. Additionally, the number of children attending day care centers, which also are prone to scabies outbreaks, is increasing. When a child contracts scabies, all close contacts need to be treated, so a preventive vaccine can be useful.9
One potential candidate for a scabies vaccine is total mite extract. Studies show that rabbits immunized with a total mite extract induce antibodies to more antigens than rabbits naturally infested with scabies mites; however, the mites cannot be cultured in vitro, which makes obtaining a large amount of their total extract difficult. Therefore, recombinant vaccines also have been proposed, as they are more easily available.22 One recombinant vaccine candidate is recombinant S scabiei serpin (rSs-serpin). Immunization with rSs-serpin has strong immunogenicity and produced immune protection in rabbits.22
Two other recombinant vaccine candidates are the rSs chitinaselike protein (CLP) 12 and the rSsCLP5. Chitinaselike proteins are very similar to chitinases; however, they are unable to degrade chitin. They are involved in immune reactions to infections, and CLPs from scabies mites have been shown to induce the host immune response.22 For example, in a particular rabbit study, rSsCLP5 demonstrated high immunoreactivity and immunogenicity. In fact, after exposure to S scabiei, 74.3% of rabbits who were vaccinated with rSsCLP5 had no detectable lesions.5 Also, after immunization with rSsCLP5 and rSsCLP12, there were increased levels of specific IgG and IgE antibodies produced and decreased numbers of infesting mites.22 Weight loss also is associated with severe scabies infection. Rabbits vaccinated with rSsCLP5 and exposed to the parasite gained weight, indicating protection via rSsCLP5. Even rabbits who did develop symptoms of scabies after immunization with rSsCLP5 and exposure to S scabiei showed less serious manifestations.5
A combination vaccine cocktail of rSs-serpin, rSsCLP12, and rSsCLP5 also has been proposed by Shen et al.22 Four test groups and a control group (n=12 per group) were included in a vaccine trial. Between 83.33% and 91.67% of rabbits vaccinated with this mixed recombinant cocktail vaccine had no detectable skin lesions from scabies. After immunization with the cocktail vaccine, the specific serum IgG and IgE antibodies also increased. For both IgG and IgE, increased levels were first detected at 1 week postimmunization and peaked at 2 weeks postimmunization.22 A multiepitope vaccine derived from these 3 recombinant proteins also was explored by Shen et al22; fewer rabbits vaccinated with it had no detectable scabies skin lesions compared to those treated with the vaccine cocktail. Although the multiepitope vaccine yielded less immume protection, it was associated with a slower disease course and milder symptoms compared with no vaccination.22
Two more proposed scabies recombinant vaccine candidates are derived from the antigens Ssag1 and Ssag2; however, rabbits vaccinated with Ssag1 or Ssag2 showed no immune protection or mite burden reduction.22 The lack of protection could be due to denaturation or degradation of the protective antigens. It also can be due to the low abundance of these antigens, meaning they may not be vital for the mite’s survival—survival—a potential avenue for future research. The antigens also could have lost their native structure and immunogenic properties during the purification and production process. Therefore, more research is needed to investigate how to purify these vaccines to keep the peptides more structurally similar to their native makeups.10 More research also is needed to better understand the antigen or antigens and their mechanisms that elicit a protective immune response.9
Final Thoughts
Scabies causes severe pruritus in mild cases but also can lead to severe disfigurement, sepsis, and even death. Scabies infestations are seen disproportionately more often in low-income and resource-poor communities, and the current treatment options are less accessible to these populations. Scabies infestations induce a complex immune response that involves multiple aspects of both the innate and adaptive immune systems and can be targeted to create a scabies vaccine. Development of a scabies vaccine is crucial considering the growing resistance to current standard treatments. Acaricides potentially are associated with an increased risk for malignancy, which further amplifies the need for a scabies vaccine. There currently are multiple promising scabies vaccine candidates; however, more research is needed to better understand the host’s immune response to scabies as well as how to more accurately and efficiently produce the vaccine. The development of a safe, effective, economical vaccine that can be mass distributed would be beneficial in the treatment of scabies, especially in resource-poor communities.
The scabies mite, originally known as Acarus scabiei,1 now is considered an arthropod of the class Arachnida, order Astigmata, and family Sarcoptidae.2 Scabies mites are able to adhere to the surface of human skin.3 The mites burrow and lay eggs in the top layer of the epidermis; most patients have 10 to 15 mites.3 The patient’s immune system incites an allergic reaction to the mite protein and feces in the skin, causing itching and rash.4
Scabies is common in indigenous populations and in low-income areas of developing countries.5 It is most prevalent in Africa, South America, Australia, and Southeast Asia, in part due to poverty, poor nutritional status, homelessness, and inadequate hygiene.2 In 2009, the World Health Organization declared scabies a neglected skin disease2; however, in 2010, 1.5 million disability adjusted life-years were attributed to scabies,6 and it is estimated that 200 million people worldwide have scabies at any given time. Children and elderly individuals in resource-poor communities are the most at risk. In fact, 5% to 50% of children in low-income areas have scabies.4
The purpose of this article is to provide background on scabies and its effect on the human immune system. We also discuss manipulation of the immune response for the purposes of creating a potential scabies vaccine.
Life Cycle and Transmission
The life cycle of Sarcoptes scabiei consists of 4 stages. The first is the egg. As female scabies mites burrow under the skin, they lay 2 to 3 ovular eggs per day.3 The second stage is the larva. When the egg hatches, the larva has 3 pairs of legs and travels to the surface of the skin where it burrows into the stratum corneum, creating short, nearly invisible burrows called molting pouches. After 3 to 4 days, the larva molts into a nymph, which is the third stage. The nymph has 4 pairs of legs and will continue to grow before molting into an adult, which is the fourth stage. Both the larva and nymph may be found in hair follicles or molting pouches. The fourth stage is the adult, which is round and saclike and does not have eyes. Adult females are 0.30 mm to 0.45 mm long and 0.25 mm to 0.35 mm wide, which is half the size of adult males.3 On warm skin, the female mite can crawl at a rate of 2.5 cm per minute.7
Scabies mites mate via an active male penetrating the molting pouch of a female. This only occurs once but leaves the female fertile for the rest of her life. Once a female is pregnant, she leaves her molting pouch and travels along the surface of the skin looking for a place to make her permanent burrow.3 The most common sites for scabies burrows are the axillae, umbilicus, interdigital spaces, beltline, buttocks, flexor surfaces of the wrists, female nipples, and male penile shaft.5 Once she finds an acceptable location, the female scabies mite will create a serpentine burrow and lay her eggs. Once she burrows, she will stay there and continue to lay eggs for the rest of her life, lengthening the burrow as needed.3 Female mites lay their eggs in the superficial epidermis, and the eggs take approximately 2 to 3 weeks to hatch. Female mites die 30 to 60 days later.2
Scabies infestations typically spread via the transfer of pregnant adult females during skin-to-skin contact, but they also can spread via fomites.3 During all stages of their life cycle, scabies mites can secrete enzymes that allow them to penetrate the intact epidermis in less than 30 minutes; in fact, an otherwise healthy patient with scabies must have 15 to 20 minutes of close skin-to-skin contact with an infected individual for the disease to be transmitted.7 Because scabies mites can survive for more than 3 days outside the human body, it is thought that fomites also may be involved in transmission. Scabies mites also have been collected from clothing, bedding, and furniture, which further supports the idea that fomites are involved in disease transmission.7
Clinical Manifestation of Scabies
Scabies symptoms include severe pruritus as well as linear burrows and vesicles in the interdigital spaces on the hands, wrists, arms and legs, and lower abdomen. Infants and young children also can develop a rash on the palms, soles, ankles, and scalp. Men can develop inflammatory scabies nodules on the penis and scrotum, while women can develop these nodules on the nipple.4 Type I and type IV hypersensitivity reactions contribute to the rash and itching associated with scabies infestation via host allergic and inflammatory reactions to the mites and their byproducts. Patients with scabies typically are infested with fewer than 15 mites,6 but just a few can cause substantial pruritus and scratching, leading to hyperkeratosis.8
Additionally, when patients with scabies scratch the skin, they become vulnerable to bacterial infections.4 Scabies lesions can be coinfected with group A streptococci and Staphylococcus aureus,8 potentially leading to abscesses and septicemia. These secondary infections also can cause renal and cardiac complications; in fact, in tropical areas, scabies infections are considered a risk factor for kidney disease and rheumatic heart disease.4
The 2 main forms of scabies infestations are ordinary and crusted. The most common form is ordinary scabies, which typically manifests with fewer than 15 mites per patient; crusted scabies (CS) is the more rare and extreme form.6 Cases of CS present with thousands to millions of mites per patient, leading to more widespread and severe symptoms.4 Because of the large increase in the number of mites, CS is more contagious than ordinary scabies.6
Patients with CS typically present with hyperkeratotic skin disease, as evidenced by thick scaly crusts with large numbers of mites, which can lead to permanent skin disfiguration. Patients with CS also can develop deep fissuring of the crusts, within which other microbes can gain entry to the body and lead to secondary infection and possibly sepsis and death. Also, because of the increased number of mites as well as the crusted skin, patients with CS are contagious for longer. As it is more difficult to eradicate, reinfestation is common with CS.6
Patients with compromised immune systems are predisposed to CS. Specifically, patients with HIV or human T-lymphotropic virus 1 or those undergoing organ transplantation are thought to be the most at risk for CS.6 Crusted scabies also has been identified in large numbers in patients with Down syndrome and in Aboriginal Australians; however, the reasoning for this is poorly understood.6
Immune Response
The inflammatory reaction associated with scabies infestations occurs 4 to 6 weeks after initial exposure. It is hypothesized that scabies can alter parts of the host immune system, which contributes to the delayed onset of symptoms. Scabies mites also produce inactivated protease paralogues and serpins, which help to protect the mites from the host immune system by inhibiting the complement system.6
The complement system is part of the innate immune response and is the first line of defense against pathogens. Specifically with scabies infestations, C3 and C4 complement components have been found in skin lesions.6 C3a and C4a fragments cause local inflammation, while C3a and C5a activate mast cells to release histamine and tumor necrosis factor (TNF) α, further amplifying the inflammatory response; however, CS lesions show low C3 and C4, which can indicate immunodeficiency in patients with CS. It also can be due to the sheer number of mites in a CS infection causing the host immune system to be overloaded.6
Innate effector immune cells also are an important part of the innate immune response to scabies; for example, eosinophilia is seen in scabies infections. Specifically, in CS, eosinophils help modulate and sustain the T-helper (Th) 2 inflammatory response. One cytokine secreted by Th2 cells is IL-5, which is closely associated with the attraction, maturation, and survival of eosinophils.6 Eosinophils also can influence the Th1 inflammatory response in that they produce IL-12, interferon (IFN) γ, and several Toll-like receptors. Furthermore, eosinophilic expression of IL-2 can lead to expansion of regulatory T cells, while eosinophilic expression of IL-10 and transforming growth factor (TGF) Β also can suppress local inflammation by influencing regulatory T cells.6
Additionally, mast cells and basophils are important in the IgE-mediated allergic reaction as well as the host immune response to parasites. When activated, basophils and mast cells produce TNF-α, IL-6, Il-4, IL-5, and IL-13, which contribute to the Th2 inflammatory response; however, the role of mast cells and basophils in scabies infections still is poorly understood.6
Macrophages, neutrophils, and dendritic cells (DCs) contribute to phagocytosis, antigen presentation, and differentiation of T cells, which also contribute to the inflammatory and allergic reactions associated with parasitic infections.6 Macrophages have been found in low numbers in scabies infestation, possibly due to immune-modulating molecules secreted by scabies mites. Early in an infestation, the mites secrete immune-modulating molecules, which inhibit macrophage migration to the site of inflammation, allowing the mites to grow.6 Neutrophils and DCs also are involved in the host immune response to scabies. Neutrophils are the predominant inflammatory cell infiltrate in scabies lesions. The scabies protein SMSB4 inhibits neutrophil opsonization and phagocytosis, thus suppressing bacterial killing.6 Some of the first antigen-presenting cells encountered by the antigen are DCs. They are involved in preparing the antigens for presentation to effector T cells, which leads to T-cell differentiation and activation.6
Cytokines are another important factor in the innate immune response. The host immune response to ordinary scabies is Th1-cell mediated, during which CD4+ and CD8+ T cells secrete IFN-γ, TNF-α, and IL-2.6 Therefore, IFN- γ and TNF-α are elevated in the serum of patients with ordinary scabies. Conversely, the host immune response to CS is Th2-cell mediated. T-helper 2 cells are needed in IgE-mediated hypersensitivity reactions, and they secrete IL-4, IL-5, and IL-13. In the serum of patients with CS, IL-l4, IL-5, and IL-13 are elevated while IFN-γ is decreased.6 Additionally, IL-6, TGF-Β, IL-23, IL-1Β, or IL-18 can induce Th17 cells to generate and secrete IL-17, which enhances the inflammatory response by inducing further expression of TNF-α, IL-1Β, IL-6, keratinocytes, and fibroblasts. T-helper 17 and IL-17 also are involved in psoriasis and atopic dermatitis, as well as Leishmania major and Schistosoma japonicum.6
Regulatory T cells Tregs secrete TGF-Β and IL-10, which suppress pathologic inflammation, and IL-10 is substantially reduced in patients with CS compared to those with ordinary scabies and uninfected control patients. Additionally, IL-10 can inhibit the synthesis of TNF-γ and IFN-α. Reduced IL-10 expression can lead to proliferation of IL-17 secretion, resulting in a regulatory T cell/Th17 dysfunctional immune response.6
Immunoglobulins are antibodies that are involved in the host’s adaptive immune response. The first antibody to appear in response to an antigen is IgM, and IgM bound to scabies antigens is present in 74%6 of patients with ordinary scabies. Because IgM is the first antibody to appear in response to a scabies infection, detection of serum IgM may allow for earlier detection of scabies; however, IgM has a high cross-reactivity between scabies mites and dust mites, which can hinder scabies diagnosis via IgM detection.6
Both patients with ordinary scabies and CS also show an increased circulatory IgG concentration compared to control groups; patients with CS have higher concentrations. Increased IgG also can be in part due to concurrent bacterial infections.6 When IgG or IgM antibodies bind to a pathogen, they activate the complement cascade, which further enhances the activity of these antibodies.9
Additionally, IgA is important in mucosal immune function. In both patients with ordinary scabies and CS, there is increased IgA binding to recombinant scabies mite antigens.6 Sarcoptes scabiei proteases that are localized in the mite’s gut and scybala suggest their involvement in mite digestion and burrowing. The increased secretion of these proteases into the host skin may contribute to the increased IgA,9 and these increased IgA levels have been shown to be positively correlated with severity of scabies infection.6
Also essential in allergic and parasitic inflammation, IgE is observed at higher levels in secondary infections of scabies compared to primary infections.6 Additionally, T-cell infiltrates are implicated in adaptive immune response to scabies. CD4+ T cells are the most prevalent T cells in ordinary scabies skin lesions; however, CD4+ T cells are minimal and CD8+ T cells are elevated in CS skin lesions. The increased CD8+ T cells may cause apoptosis of keratinocytes, leading to epidermal hyperproliferation. The apoptotic keratinocytes can secrete cytokines, which can lead to tissue damage.6 These T cells also may be involved in the failure of the skin’s immune system to mount an effective response to the parasite infestation, leading to uncontrolled parasitic growth. Because patients with AIDS who are infected with scabies mites often develop CS, it is also thought that CD4+ T cells are essential in the immune response to scabies.6
Diagnosis and Current Treatment Options
Current diagnosis of scabies is based on mites, eggs, and fecal matter from the host’s skin. Dermoscopy and fluorescent dermoscopy can be helpful in identifying the mites, eggs, and feces on the patient’s skin. Scabies treatment sometimes may be based solely on symptoms without any positive tests.8
Acaricides are the current method of treatment for scabies infestations.5 Acaricides can be expensive and toxic to the environment and food sources,10 and some agents have been associated with neurotoxicity5 in children or the development of certain cancers.11 Although topical acaricides are the standard form of treatment, oral ivermectin also can be used. Ivermectin is not associated with selective fetal toxicity, but there are limited safety data in pregnant women and in children weighing less than 15 kg (33 lb). Additionally, because symptoms typically are not present during an early infection, treating everyone in the household and those who had close contact with the patient can help prevent reinfection.4
Although these drugs have been shown to be effective at treating scabies, scabies mites are becoming increasingly resistant to acaricides.5 There are 4 main proposed mechanisms for why this occurs.12 The first is through voltage-gated sodium channels, which are involved in the normal functioning of neurons and myocytes. Permethrin, a type of acaricide, binds to voltage-gated sodium channels when it is in an open or active state and prevents it from closing. This creates repetitive neuron firing and hyperactivity, which ultimately kills the scabies mite. Some mites have mutated to close this channel, which reduces the binding potential of permethrin. Glutathione S-transferase is another mechanism of resistance. It catalyzes a bond that tags drugs for elimination. Increased activity or expressivity of glutathione S-transferase by scabies mites can lead to drug resistance.12 Adenosine triphosphate– binding cassette (ABC) transporters also may contribute to this resistance. The ABC transporters use adenosine triphosphate to facilitate the import or export of molecules. Scabies mites express a protein called the multidrug-resistant protein, which is an ABC transporter that is associated with drug resistance and is present in scabies mites.12 Lastly, ligand-gated chloride channels have been implicated in scabies resistance to acaricides. Ligand-gated chloride channels also are important in normal functioning of neurons and myocytes. Some antiparasitic drugs act on these channels, leading to a continuous influx of chloride, but some scabies mites have mutated this pathway.12
Pesticides and the Risk for Cancer
Pesticides commonly are used to treat scabies; however, a link between pesticide exposure and leukemia and lymphoma has been seen through epidemiologic studies, and there also is increasing biological evidence to suggest this.11 For example, the pesticide permethrin, which works by paralyzing the nervous system of insects,13 has been associated with an increased risk for leukemia and lymphoma in humans. Permethrin is a pyrethroid and, compared to control patients, children with leukemia had higher levels of pyrethroid metabolites in their blood.14 Numerical and structural chromosomal aberrations that give rise to gene fusions are the most common abnormalities seen in leukemia, and permethrin has been shown to induce DNA breaks, chromosome aberrations, and sister chromatid exchanges.14 Permethrin also has been associated with an increased risk for multiple myeloma.13
Furthermore, in utero exposure to pesticides has been associated with an increased risk for childhood leukemia.15 Pesticide exposure shortly before conception, during pregnancy, and after birth is associated with an increased risk for acute lymphocytic leukemia.16 In fact, the children of mothers who were exposed to pesticides 3 months before conception have been found to be at least twice as likely to be diagnosed with acute lymphocytic leukemia within the first year of life compared with children whose mothers were not exposed to pesticides.17 It is hypothesized that permethrin can cross the placenta and alter the hematopoietic precursor cells in the fetus, resulting in leukemogenesis.18 Pyrethroid metabolites also have been detected in umbilical cord blood samples and breast milk.15
In contrast to the research demonstrating a link between permethrin and cancer, other studies have found no association between permethrin19 and leukemia20; non-Hodgkin lymphoma19; or cancers of the colon, rectum, pancreas, lungs, skin, female breast, prostate, and urinary bladder.20 Because of conflicting research on the link between permethrin and cancer, more research is needed.,20
Importance of a Scabies Vaccine
Because scabies mites are developing increasing treatment resistance, more radical approaches such as vaccines are becoming important. While a scabies vaccine is still aspirational, animals that have been infected for a second time with scabies demonstrate a milder response to the second infection compared to the first infection, which could mean there is a potential for disease prevention through a vaccine.21 While educating patients and physicians, reporting cases of infection, and improving drug supply and access can help decrease scabies infestations, these are costly and difficult to implement. Scabies already is most prevalent in low-income areas, so costly interventions are even less feasible. An effective, one-dose vaccine would cost less than these efforts and therefore could be implemented more easily.9
In older adults, scabies more often manifests atypically and is more likely to progress to CS. Aged care centers are prone to institutional outbreaks, even in developed countries, so a vaccine also would greatly help this population. Additionally, the number of children attending day care centers, which also are prone to scabies outbreaks, is increasing. When a child contracts scabies, all close contacts need to be treated, so a preventive vaccine can be useful.9
One potential candidate for a scabies vaccine is total mite extract. Studies show that rabbits immunized with a total mite extract induce antibodies to more antigens than rabbits naturally infested with scabies mites; however, the mites cannot be cultured in vitro, which makes obtaining a large amount of their total extract difficult. Therefore, recombinant vaccines also have been proposed, as they are more easily available.22 One recombinant vaccine candidate is recombinant S scabiei serpin (rSs-serpin). Immunization with rSs-serpin has strong immunogenicity and produced immune protection in rabbits.22
Two other recombinant vaccine candidates are the rSs chitinaselike protein (CLP) 12 and the rSsCLP5. Chitinaselike proteins are very similar to chitinases; however, they are unable to degrade chitin. They are involved in immune reactions to infections, and CLPs from scabies mites have been shown to induce the host immune response.22 For example, in a particular rabbit study, rSsCLP5 demonstrated high immunoreactivity and immunogenicity. In fact, after exposure to S scabiei, 74.3% of rabbits who were vaccinated with rSsCLP5 had no detectable lesions.5 Also, after immunization with rSsCLP5 and rSsCLP12, there were increased levels of specific IgG and IgE antibodies produced and decreased numbers of infesting mites.22 Weight loss also is associated with severe scabies infection. Rabbits vaccinated with rSsCLP5 and exposed to the parasite gained weight, indicating protection via rSsCLP5. Even rabbits who did develop symptoms of scabies after immunization with rSsCLP5 and exposure to S scabiei showed less serious manifestations.5
A combination vaccine cocktail of rSs-serpin, rSsCLP12, and rSsCLP5 also has been proposed by Shen et al.22 Four test groups and a control group (n=12 per group) were included in a vaccine trial. Between 83.33% and 91.67% of rabbits vaccinated with this mixed recombinant cocktail vaccine had no detectable skin lesions from scabies. After immunization with the cocktail vaccine, the specific serum IgG and IgE antibodies also increased. For both IgG and IgE, increased levels were first detected at 1 week postimmunization and peaked at 2 weeks postimmunization.22 A multiepitope vaccine derived from these 3 recombinant proteins also was explored by Shen et al22; fewer rabbits vaccinated with it had no detectable scabies skin lesions compared to those treated with the vaccine cocktail. Although the multiepitope vaccine yielded less immume protection, it was associated with a slower disease course and milder symptoms compared with no vaccination.22
Two more proposed scabies recombinant vaccine candidates are derived from the antigens Ssag1 and Ssag2; however, rabbits vaccinated with Ssag1 or Ssag2 showed no immune protection or mite burden reduction.22 The lack of protection could be due to denaturation or degradation of the protective antigens. It also can be due to the low abundance of these antigens, meaning they may not be vital for the mite’s survival—survival—a potential avenue for future research. The antigens also could have lost their native structure and immunogenic properties during the purification and production process. Therefore, more research is needed to investigate how to purify these vaccines to keep the peptides more structurally similar to their native makeups.10 More research also is needed to better understand the antigen or antigens and their mechanisms that elicit a protective immune response.9
Final Thoughts
Scabies causes severe pruritus in mild cases but also can lead to severe disfigurement, sepsis, and even death. Scabies infestations are seen disproportionately more often in low-income and resource-poor communities, and the current treatment options are less accessible to these populations. Scabies infestations induce a complex immune response that involves multiple aspects of both the innate and adaptive immune systems and can be targeted to create a scabies vaccine. Development of a scabies vaccine is crucial considering the growing resistance to current standard treatments. Acaricides potentially are associated with an increased risk for malignancy, which further amplifies the need for a scabies vaccine. There currently are multiple promising scabies vaccine candidates; however, more research is needed to better understand the host’s immune response to scabies as well as how to more accurately and efficiently produce the vaccine. The development of a safe, effective, economical vaccine that can be mass distributed would be beneficial in the treatment of scabies, especially in resource-poor communities.
- Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10:297. doi:10.1186/s13071-017-2234-1
- Murray RL, Crane JS. Scabies. In: StatPearls. StatPearls Publishing. Updated July 31, 2023.
- Centers for Disease Control and Prevention. CDC—scabies—biology. November 2, 2010. https://www.cdc.gov/dpdx/scabies/index.html
- World Health Organization. Scabies. May 31, 2023. Accessed May 8, 2025. https://www.who.int/news-room/fact-sheets/detail/scabies
- Shen N, Zhang H, Ren Y, et al. A chitinase-like protein from Sarcoptes scabiei as a candidate anti-mite vaccine that contributes to immune protection in rabbits. Parasit Vectors. 2018;11:599. doi:10.1186/s13071- 018-3184-y
- Bhat SA, Mounsey KE, Liu X, et al. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasit Vectors. 2017;10:385. doi:10.1186/s13071-017-2320-4
- Hicks MI, Elston DM. Scabies. Dermatolog Ther. 2009;22:279-292. doi:10.1111/j.1529-8019.2009.01243.x
- Morgan MS, Arlian LG, Rider SD, et al. A proteomic analysis of Sarcoptes scabiei (acari: Sarcoptidae). J Med Entomol. 2016;53:553-561. doi:10.1093/jme/tjv247
- Liu X, Walton S, Mounsey K. Vaccine against scabies: necessity and possibility. Parasitology. 2014;141:725-732. doi:10.1017 /s0031182013002047
- Casais R, Granda V, Balseiro A, et al. Vaccination of rabbits with immunodominant antigens from Sarcoptes scabiei induced high levels of humoral responses and pro-inflammatory cytokines but confers limited protection. Parasit Vectors. 2016;9:435. doi:10.1186 /s13071-016-1717-9?
- Navarrete-Meneses MP, Pedraza-Meléndez AI, Salas-Labadía C, et al. Low concentrations of permethrin and malathion induce numerical and structural abnormalities in KMT2A and IGH genes in vitro. J Appl Toxicol. 2018;38:1262-1270. doi:10.1002/jat.3638
- Khalil S, Abbas O, Kibbi AG, et al. Scabies in the age of increasing drug resistance. PLoS Negl Trop Dis. 2017;11:E0005920. doi:10.1371 /journal.pntd.0005920
- Rusiecki JA, Patel R, Koutros S, et al. Cancer incidence among pesticide applicators exposed to permethrin in the Agricultural Health Study. Environ Health Perspect. 2009;117:581-586. doi:10.1289 /ehp.11318
- Navarrete-Meneses MP, Salas-Labadía C, Sanabrais-Jiménez M, et al. Exposure to the insecticides permethrin and malathion induces leukemia and lymphoma-associated gene aberrations in vitro. Toxicol In Vitro. 2017;44:17-26. doi:10.1016/j.tiv.2017.06.013
- Navarrete-Meneses MDP, Pérez-Vera P. Pyrethroid pesticide exposure and hematological cancer: epidemiological, biological and molecular evidence. Rev Environ Health. 2019;34:197-210. doi:10.1515 /reveh-2018-0070
- Madrigal JM, Jones RR, Gunier RB, et al. Residential exposure to carbamate, organophosphate, and pyrethroid insecticides in house dust and risk of childhood acute lymphoblastic leukemia. Environ Res. 2021;201:111501. doi:10.1016/j.envres.2021.111501
- Ferreira JD, Couto AC, Pombo-de-Oliveira MS, et al. In utero pesticide exposure and leukemia in Brazilian children <2 years of age. Environ Health Perspect. 2013;121:269-275. doi:10.1289/ehp.1103942
- Borkhardt A, Wilda M, Fuchs U, et al. Congenital leukaemia after heavy abuse of permethrin during pregnancy. Arch Dis Child Fetal Neonatal Ed. 2003;88:F436-F437. doi:10.1136/fn.88.5.f436
- De Roos AJ, Schinasi LH, Miligi L, et al. Occupational insecticide exposure and risk of non]Hodgkin lymphoma: a pooled case]control study from the InterLymph consortium. Int J Cancer. 2021;149:1768-1786. doi:10.1002/ijc.33740
- Boffett, P, Desai V. Exposure to permethrin and cancer risk: a systematic review. Crit Rev Toxicol. 2018;48:433-442. doi:10.1080/1040 8444.2018.1439449
- Adji A, Rumokoy LJM, Salaki CL. Scabies vaccine as a new breakthrough for the challenge of acaricides resistance. Adv Biolog Sci Res. 2020;8:208-213. doi:10.2991/absr.k.200513.036
- Shen N, Wei W, Chen Y, et al. Vaccination with a cocktail vaccine elicits significant protection against Sarcoptes scabiei in rabbits, whereas the multi-epitope vaccine offers limited protection. Exp Parasitol. 2023;245:108442. doi:10.1016/j.exppara.2022.108442
- Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10:297. doi:10.1186/s13071-017-2234-1
- Murray RL, Crane JS. Scabies. In: StatPearls. StatPearls Publishing. Updated July 31, 2023.
- Centers for Disease Control and Prevention. CDC—scabies—biology. November 2, 2010. https://www.cdc.gov/dpdx/scabies/index.html
- World Health Organization. Scabies. May 31, 2023. Accessed May 8, 2025. https://www.who.int/news-room/fact-sheets/detail/scabies
- Shen N, Zhang H, Ren Y, et al. A chitinase-like protein from Sarcoptes scabiei as a candidate anti-mite vaccine that contributes to immune protection in rabbits. Parasit Vectors. 2018;11:599. doi:10.1186/s13071- 018-3184-y
- Bhat SA, Mounsey KE, Liu X, et al. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasit Vectors. 2017;10:385. doi:10.1186/s13071-017-2320-4
- Hicks MI, Elston DM. Scabies. Dermatolog Ther. 2009;22:279-292. doi:10.1111/j.1529-8019.2009.01243.x
- Morgan MS, Arlian LG, Rider SD, et al. A proteomic analysis of Sarcoptes scabiei (acari: Sarcoptidae). J Med Entomol. 2016;53:553-561. doi:10.1093/jme/tjv247
- Liu X, Walton S, Mounsey K. Vaccine against scabies: necessity and possibility. Parasitology. 2014;141:725-732. doi:10.1017 /s0031182013002047
- Casais R, Granda V, Balseiro A, et al. Vaccination of rabbits with immunodominant antigens from Sarcoptes scabiei induced high levels of humoral responses and pro-inflammatory cytokines but confers limited protection. Parasit Vectors. 2016;9:435. doi:10.1186 /s13071-016-1717-9?
- Navarrete-Meneses MP, Pedraza-Meléndez AI, Salas-Labadía C, et al. Low concentrations of permethrin and malathion induce numerical and structural abnormalities in KMT2A and IGH genes in vitro. J Appl Toxicol. 2018;38:1262-1270. doi:10.1002/jat.3638
- Khalil S, Abbas O, Kibbi AG, et al. Scabies in the age of increasing drug resistance. PLoS Negl Trop Dis. 2017;11:E0005920. doi:10.1371 /journal.pntd.0005920
- Rusiecki JA, Patel R, Koutros S, et al. Cancer incidence among pesticide applicators exposed to permethrin in the Agricultural Health Study. Environ Health Perspect. 2009;117:581-586. doi:10.1289 /ehp.11318
- Navarrete-Meneses MP, Salas-Labadía C, Sanabrais-Jiménez M, et al. Exposure to the insecticides permethrin and malathion induces leukemia and lymphoma-associated gene aberrations in vitro. Toxicol In Vitro. 2017;44:17-26. doi:10.1016/j.tiv.2017.06.013
- Navarrete-Meneses MDP, Pérez-Vera P. Pyrethroid pesticide exposure and hematological cancer: epidemiological, biological and molecular evidence. Rev Environ Health. 2019;34:197-210. doi:10.1515 /reveh-2018-0070
- Madrigal JM, Jones RR, Gunier RB, et al. Residential exposure to carbamate, organophosphate, and pyrethroid insecticides in house dust and risk of childhood acute lymphoblastic leukemia. Environ Res. 2021;201:111501. doi:10.1016/j.envres.2021.111501
- Ferreira JD, Couto AC, Pombo-de-Oliveira MS, et al. In utero pesticide exposure and leukemia in Brazilian children <2 years of age. Environ Health Perspect. 2013;121:269-275. doi:10.1289/ehp.1103942
- Borkhardt A, Wilda M, Fuchs U, et al. Congenital leukaemia after heavy abuse of permethrin during pregnancy. Arch Dis Child Fetal Neonatal Ed. 2003;88:F436-F437. doi:10.1136/fn.88.5.f436
- De Roos AJ, Schinasi LH, Miligi L, et al. Occupational insecticide exposure and risk of non]Hodgkin lymphoma: a pooled case]control study from the InterLymph consortium. Int J Cancer. 2021;149:1768-1786. doi:10.1002/ijc.33740
- Boffett, P, Desai V. Exposure to permethrin and cancer risk: a systematic review. Crit Rev Toxicol. 2018;48:433-442. doi:10.1080/1040 8444.2018.1439449
- Adji A, Rumokoy LJM, Salaki CL. Scabies vaccine as a new breakthrough for the challenge of acaricides resistance. Adv Biolog Sci Res. 2020;8:208-213. doi:10.2991/absr.k.200513.036
- Shen N, Wei W, Chen Y, et al. Vaccination with a cocktail vaccine elicits significant protection against Sarcoptes scabiei in rabbits, whereas the multi-epitope vaccine offers limited protection. Exp Parasitol. 2023;245:108442. doi:10.1016/j.exppara.2022.108442
Immune Responses and Health Disparities Warrant Scabies Vaccine Development
Immune Responses and Health Disparities Warrant Scabies Vaccine Development
PRACTICE POINTS
- Dermatologists should be aware of the impact scabies has on patients, especially on those in lower socioeconomic groups.
- Physicians and patients should be educated on scabies prevention and treatment to help decrease the spread of scabies infections.
What’s Eating You? Hookworm and Cutaneous Larva Migrans
What’s Eating You? Hookworm and
Cutaneous Larva Migrans
It is estimated that the prevalence of human hookworm infection is approximately 450 million individuals worldwide, representing a substantial global disease burden.1 The annual global public health burden ranges from approximately 2 million to 4 million disability-adjusted life-years and $10 billion to $140 billion in hookwormrelated costs.2 In this article, we discuss the lifecycle, transmission, and disease burden of cutaneous larva migrans (CLM) as well as prevention and treatment strategies.
Background
The Ancylostomatidae nematode family comprises at least 68 known species of hookworm that infect more than 110 different species of mammals.3 Many of these parasites are able to infect more than 1 primary host species, but from a disease perspective they can be classified as either anthropophilic, with humans as the intended host, or zoonotic, with humans as an incidental host. It is important to make this distinction because, though the lifecycles and biology of hookworm species generally are similar, the manifestations of incidental human infection from zoonotic hookworms are different from those of anthropophilic hookworms. Of the anthropophilic species, Necator americanus and Ancylostoma duodenale predominate. In the instance of zoonotic hookworm, dog-infecting A caninum and cat- and doginfecting A braziliense and Uncinaria stenocephala are common causes of incidental human disease.3
The life cycle of Ancylostomatidae organisms is astounding. Through millions of years of co-evolution with mammals,4 these parasitic worms have developed perhaps one of the most circuitous paths to propagate themselves in the natural world. Hookworms start their arduous journey as eggs deposited in soil, sand, and ground vegetation from the feces of infected animals.5 Approximately 1 day after the eggs are deposited, they hatch and begin the larval stage, during which they become infective 1 to 5 weeks later. At this point, the larvae become sensitive to their environment, responding to rising temperatures, increasing carbon dioxide levels, and vibrations in the soil—all of which suggest the presence of a potential host and contribute to a concordant increase in undulatory movement of the larvae.5,6 Here, the most vulnerable tissues include the uncovered soles, palms, and buttocks of host mammals that come into contact with contaminated soil. In an undulating fashion and guided by temperature cues, the larvae locate the skin of the host and utilize a mixture of enzymes including hyaluronidases, metalloprotease, and other proteases to penetrate the epidermis.7 Anthropophilic hookworms such as N americanus and A duodenale will enter the circulatory system; from there, the hookworms migrate through the right-sided cardiopulmonary circuit and eventually ascend into the pulmonary vasculature.8 They then penetrate the lung capillary beds and parenchyma to reach the alveoli, ascend the respiratory tree, and, with the help of the mucociliary escalator, reach the esophagus, where they are swallowed by the host. In the gastrointestinal tract, adult hookworms consume host blood, mate, and lay eggs over a period of approximately 1 to 3 years if left untreated.9 Eggs are laid into the lower gastrointestinal tract, and the journey begins again in feces contacting ground or soil.
Geographic Distribution
Hookworms are found in almost all regions of the world, with species-specific distributions that highlight tropical and subtropical regions. Necator americanus and A duodenale are the most common hookworm species, with the former found predominantly in Southeast Asia and Latin America and the latter in Asia-Pacific regions.10 The highest prevalence of hookworms is in Southeast Asia followed by Sub-Saharan Africa, and the unique climate and soil composition of a region help determine the best environments for specific species of hookworm to thrive.11 In addition, socioeconomics and social determinants of health play a big role in the spread of hookworms, as hygiene practices (eg, wearing clean shoes and clothing, bathing), infrastructure (eg, clean water and streets), and anthelmintic campaigns help reduce transmission.12 Soil-transmitted helminths were once endemic to the southeastern United States, with some reports of approximately 40% of individuals infected in the south in the early 1900s.13 Anthelmintic campaigns such as water, sanitation, and hygiene programs as well as deworming of humans and livestock have proven effective in reducing the prevalence of helminth disease in industrialized nations.13,14 However, zoonotic infections remain a problem in these regions, and in some parts of the United States more than 40% of sampled cats and dogs harbored species such as A braziliense.15
Clinical Manifestation
Initial hookworm infection often goes unnoticed because symptoms can range in severity, but it is characterized by transient ground itch—a local pruritic, erythematous, and papular eruption that develops in response to epidermal penetration.16 Because the larvae must traverse the host from skin to target organs for reproduction over several weeks, iron-deficiency anemia will manifest much later than signs of the initial penetration. In the case of incidental infection from zoonotic Ancylostomatidae organisms, the misguided larvae result in CLM, an often intensely pruritic skin condition that will self-resolve in 2 to 8 weeks with eventual death of the larvae.5
Diagnosis and Pathology of Disease
Zoonotic Hookworm—The major presenting sign of zoonotic hookworm infection is CLM. The diagnosis of CLM usually is made clinically, as the larvae themselves are 0.5 mm thick to 10 mm long (Figure 1) and usually extend several centimeters beyond the dermal lesion, with dermoscopy having limited utility.17 Patients may begin to experience itching as little as 1 hour after hookworm penetration of the skin.18 Once in contact with the skin, the hookworms’ hyaluronidases and proteases are capable of breaking through the epidermis, but zoonotic hookworms typically are unable to penetrate the basal layer of the human epidermis and remain entombed between the stratum granulosum and stratum corneum. With the exception of rare cases of direct or indirect pulmonary involvement resulting in Löffler syndrome,19 the larvae will die within weeks to months, and symptoms will subsequently resolve.
Although the infection generally is self-limiting, the dermatologic manifestations of CLM can be severe and warrant intervention. The lesions start as small reddish papules at the site of penetration (Figure 2), then the hallmark elevated, migrating, serpiginous, urticarial rash develops (Figure 3). Cutaneous larva migrans generally manifests unilaterally and is both erythematous and intensely pruritic. As the larvae migrate, they leave behind 1- to 5-cm tunneled creeping eruptions in their wake. The lesions, which can manifest with pain or be painless, may develop eczematous, bullous, follicular, or impetiginized appearances.20 Atypical manifestations include folliculitis and urticarial plaques.17
cutaneous larval migrans on the palm.
Anthropophilic Hookworm—The lifecycles of N americanus and A duodenale are completed in human infection. Dermatologic manifestations are transient with the development of ground itch at the site of epidermal penetration. The hookworms employ collagenases that allow penetration of the basal layer of the skin, and eosinophilia develops as the parasites travel from the skin to the small intestine. Once attached to the gastrointestinal lumen, blood meals and proteolytic enzymes result in iron-deficiency anemia in the host and may lead to weakness, fatigue, and low birth weights in pregnant patients. With prolonged infection or heavy parasitic burden, patients can develop hypoproteinemia, anasarca, and yellowing of the skin known as chlorosis.11 A clinical diagnosis can be made by examining patient stool samples for eggs, and definitive characterization can be made using molecular tools such as polymerase chain reaction.21,22
Common to hookworm infections is the immune reaction, which promotes inflammation with localized eosinophilia and mastocytosis.11 In a clinical biopsy specimen of gut—usually obtained through esophagogastroduodenoscopy— T-helper (Th) 2–type immune (IL-4, IL-5, IL-9 and IL-13), regulatory Th10 (IL-10 and transcription growth factor β), and some evidence of Th1 (interferon gamma and IL-2) cytokines are present, but little evidence of Th17-type immune response was found.23 It is believed that in zoonotic infections, antiparasitic IgE from basophils are somewhat successful at trapping the helminths in the epidermis, but in the anthropophilic species, IgE and Th2 responses are ineffective at clearing the parasite from the gut, and the defeated immune system transitions to a host-tolerance approach of limiting infection.11 It is now believed that this natural armistice can be manipulated into a potential therapy against autoimmune and inflammatory conditions. Intentional infection with zoonotic whipworm or hookworm has been proposed as a mechanism of switching Th1 and Th2 responses to host-tolerant mechanisms in conditions such as Crohn disease and celiac disease,24 and it has even been hypothesized that prior hookworm infection may reduce the chance of developing allergic conditions such as eczema.25
Treatment and Prevention
The World Health Organization and Centers for Disease Control and Prevention recommend a single oral dose of 400 mg albendazole for adults or 10 to 15 mg/kg in children for CLM. A single dose of ivermectin at 12 mg in adults or 150 μg/kg in children can be used as an alternative where albendazole is not available.11 Topical applications of thiabendazole 10% to 15% under occlusion or 3 times daily for 15 days without occlusion also can manage CLM, and pruritus can be treated with topical corticosteroids for symptomatic relief. Oral albendazole 400 mg twice daily or mebendazole 100 mg twice daily for 3 days or a single 500-mg dose, as well as 11 mg/kg (up to a maximum of 1 g) oral pyrantel pamoate once daily for 3 days can be used to treat intestinal hookworm infection, though it should be avoided in pregnancy. Iron deficiency should be managed with supplementation.11
Prevention of hookworm infection is focused around 2 broad public health efforts: mass drug administration programs and the water, sanitation, and hygiene program. In mass drug administration, treatments such as benzimidazoles are given in mass to communities affected by endemic hookworm as a single dose to reduce the burden of disease. Together, these strategies effectively eliminated hookworms in many developed nations, but areas of resurgence are beginning to surface worldwide. With changes in climate, emerging drug resistance, and socioeconomic disparities, particularly affecting the southeast, a resurgence of hookworm has occurred in the United States.26 One recent study demonstrated that almost one-third (19/55) of children sampled in an impoverished area of rural Alabama had hookworm eggs in their stool.27 Furthermore, pets serve not only as zoonotic reservoirs for CLM recurrence but also as vehicles for the evolution of drug-resistant strains, leading some to call for a ban of animals from beaches and playgrounds as well as tightly controlled veterinary programs.5,28 Ubiquitous benzimidazole use in livestock has led to bendazole-resistant strains, and it is likely that with continued and poorly adherent drug use, more zoonotic and anthropophilic drug-resistant strains of hookworm will emerge.29,30
Conclusion
The burden of hookworm infection and CLM is substantial in parts of the United States. Dermatologists play a critical role in the recognition and management of hookworm infection for both treatment of affected patients and the subsequent prevention of its spread. As drug-resistant strains evolve, clinicians, public health officials, and scientists need to continue to work together to prevent and treat hookworm infection.
- Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211-1259.
- Bartsch SM, Hotez PJ, Asti L, et al. The global economic and health burden of human hookworm infection. PLoS Negl Trop Dis. 2016;10:E0004922.
- Seguel M, Gottdenker N. The diversity and impact of hookworm infections in wildlife. Int J Parasitol Parasites Wildl. 2017;6:177-194.
- Adams BJ, Peat SM, Dillman AR. Phylogeny and evolution. In: Entomopathogenic Nematodes: Systematics, Phylogeny and Bacterial Symbionts. Brill; 2010:693-733.
- Heukelbach J, Feldmeier H. Epidemiological and clinical characteristics of hookworm-related cutaneous larva migrans. Lancet Infect Dis. 2008;8:302-309.
- Haas W, Haberl B, Idris I, et al. Infective larvae of the human hookworms Necator americanus and Ancylostoma duodenale differ in their orientation behaviour when crawling on surfaces. Parasitol Res. 2005;95:25-29.
- Hotez P, Narasimhan S, Haggerty J, et al. Hyaluronidase from infective Ancylostoma hookworm larvae and its possible function as a virulence factor in tissue invasion and in cutaneous larva migrans. Infect Immun. 1992;60:1018-1023.
- Brooker S, Bethony J, Hotez PJ. Human hookworm infection in the 21st century. Adv Parasitol. 2004;58:197-288.
- Hoagland K, Schad G. Necator americanus and Ancylostoma duodenale: life history parameters and epidemiological implications of two sympatric hookworms of humans. Exp Parasitol. 1978;44:36-49.
- Clements ACA, Alene KA. Global distribution of human hookworm species and differences in their morbidity effects: a systematic review. Lancet Microbe. 2022;3:E72-E79.
- Loukas A, Hotez PJ, Diemert D, et al. Hookworm infection. Nat Rev Dis Primers. 2016;2:1-18.
- Gazzinelli A, Correa-Oliveira R, Yang GJ, et al. A research agenda for helminth diseases of humans: social ecology, environmental determinants, and health systems. PLoS Negl Trop Dis. 2012;6:E1603.
- Starr MC, Montgomery SP. Soil-transmitted helminthiasis in the United States: a systematic review—1940-2010. Am J Trop Med Hyg. 2011;85:680-684.
- Strunz EC, Addiss DG, Stocks ME, et al. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and metaanalysis. PLoS Med. 2014;11:E1001620.
- Liotta JL, Youn H, Aksel S, et al. Prevalence of Ancylostoma braziliense in dogs from Alachua and Marion Counties, Florida, United States. J Parasitol. 2012;98:1039-1040.
- Hotez PJ, Brooker S, Bethony JM, et al. Hookworm infection. N Engl J Med. 2004;351:799-807.
- Prickett KA, Ferringer TC. What’s eating you? cutaneous larva migrans. Cutis. 2015;95:126-128.
- Feldmeier H, Schuster A. Mini review: hookworm-related cutaneous larva migrans. Eur J Clin Microbiol Infect Dis. 2012;31:915-918.
- Tan SK, Liu TT. Cutaneous larva migrans complicated by Löffler syndrome. Arch Dermatol. 2010;146:210-212.
- Eksomtramage T, Aiempanakit K. Bullous and pustular cutaneous larva migrans: two case reports and a literature review. IDCases. 2018;12:130-132.
- Utzinger J, Rinaldi L, Lohourignon LK, et al. FLOTAC: a new sensitive technique for the diagnosis of hookworm infections in humans. Trans R Soc Trop Med Hyg. 2008;102:84-90.
- Chidambaram M, Parija SC, Toi PC, et al. Evaluation of the utility of conventional polymerase chain reaction for detection and species differentiation in human hookworm infections. Trop Parasitol. 2017;7:111-116.
- Gaze S, McSorley HJ, Daveson J, et al. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Pathog. 2012;8:E1002520.
- Croese J, O’Neil J, Masson J, et al. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors. Gut. 2006;55:136-137.
- Mpairwe H, Amoah AS. Parasites and allergy: observations from Africa. Parasite Immunol. 2019;41:E12589.
- Albonico M, Savioli L. Hookworm: a neglected resurgent infection. Editorial. BMJ. 2017;359:j4813.
- McKenna ML, McAtee S, Bryan PE, et al. Human intestinal parasite burden and poor sanitation in rural Alabama. Am J Trop Med Hyg. 2017;97:1623-1628.
- Traversa D. Pet roundworms and hookworms: a continuing need for global worming. Parasit Vectors. 2012;5:1-19.
- Geerts S, Gryseels B. Drug resistance in human helminths: current situation and lessons from livestock. Clin Microbiol Rev. 2000;13:207-222.
- Jimenez Castro PD, Howell SB, Schaefer JJ, et al. Multiple drug resistance in the canine hookworm Ancylostoma caninum: an emerging threat? Parasit Vectors. 2019;12:1-15.
It is estimated that the prevalence of human hookworm infection is approximately 450 million individuals worldwide, representing a substantial global disease burden.1 The annual global public health burden ranges from approximately 2 million to 4 million disability-adjusted life-years and $10 billion to $140 billion in hookwormrelated costs.2 In this article, we discuss the lifecycle, transmission, and disease burden of cutaneous larva migrans (CLM) as well as prevention and treatment strategies.
Background
The Ancylostomatidae nematode family comprises at least 68 known species of hookworm that infect more than 110 different species of mammals.3 Many of these parasites are able to infect more than 1 primary host species, but from a disease perspective they can be classified as either anthropophilic, with humans as the intended host, or zoonotic, with humans as an incidental host. It is important to make this distinction because, though the lifecycles and biology of hookworm species generally are similar, the manifestations of incidental human infection from zoonotic hookworms are different from those of anthropophilic hookworms. Of the anthropophilic species, Necator americanus and Ancylostoma duodenale predominate. In the instance of zoonotic hookworm, dog-infecting A caninum and cat- and doginfecting A braziliense and Uncinaria stenocephala are common causes of incidental human disease.3
The life cycle of Ancylostomatidae organisms is astounding. Through millions of years of co-evolution with mammals,4 these parasitic worms have developed perhaps one of the most circuitous paths to propagate themselves in the natural world. Hookworms start their arduous journey as eggs deposited in soil, sand, and ground vegetation from the feces of infected animals.5 Approximately 1 day after the eggs are deposited, they hatch and begin the larval stage, during which they become infective 1 to 5 weeks later. At this point, the larvae become sensitive to their environment, responding to rising temperatures, increasing carbon dioxide levels, and vibrations in the soil—all of which suggest the presence of a potential host and contribute to a concordant increase in undulatory movement of the larvae.5,6 Here, the most vulnerable tissues include the uncovered soles, palms, and buttocks of host mammals that come into contact with contaminated soil. In an undulating fashion and guided by temperature cues, the larvae locate the skin of the host and utilize a mixture of enzymes including hyaluronidases, metalloprotease, and other proteases to penetrate the epidermis.7 Anthropophilic hookworms such as N americanus and A duodenale will enter the circulatory system; from there, the hookworms migrate through the right-sided cardiopulmonary circuit and eventually ascend into the pulmonary vasculature.8 They then penetrate the lung capillary beds and parenchyma to reach the alveoli, ascend the respiratory tree, and, with the help of the mucociliary escalator, reach the esophagus, where they are swallowed by the host. In the gastrointestinal tract, adult hookworms consume host blood, mate, and lay eggs over a period of approximately 1 to 3 years if left untreated.9 Eggs are laid into the lower gastrointestinal tract, and the journey begins again in feces contacting ground or soil.
Geographic Distribution
Hookworms are found in almost all regions of the world, with species-specific distributions that highlight tropical and subtropical regions. Necator americanus and A duodenale are the most common hookworm species, with the former found predominantly in Southeast Asia and Latin America and the latter in Asia-Pacific regions.10 The highest prevalence of hookworms is in Southeast Asia followed by Sub-Saharan Africa, and the unique climate and soil composition of a region help determine the best environments for specific species of hookworm to thrive.11 In addition, socioeconomics and social determinants of health play a big role in the spread of hookworms, as hygiene practices (eg, wearing clean shoes and clothing, bathing), infrastructure (eg, clean water and streets), and anthelmintic campaigns help reduce transmission.12 Soil-transmitted helminths were once endemic to the southeastern United States, with some reports of approximately 40% of individuals infected in the south in the early 1900s.13 Anthelmintic campaigns such as water, sanitation, and hygiene programs as well as deworming of humans and livestock have proven effective in reducing the prevalence of helminth disease in industrialized nations.13,14 However, zoonotic infections remain a problem in these regions, and in some parts of the United States more than 40% of sampled cats and dogs harbored species such as A braziliense.15
Clinical Manifestation
Initial hookworm infection often goes unnoticed because symptoms can range in severity, but it is characterized by transient ground itch—a local pruritic, erythematous, and papular eruption that develops in response to epidermal penetration.16 Because the larvae must traverse the host from skin to target organs for reproduction over several weeks, iron-deficiency anemia will manifest much later than signs of the initial penetration. In the case of incidental infection from zoonotic Ancylostomatidae organisms, the misguided larvae result in CLM, an often intensely pruritic skin condition that will self-resolve in 2 to 8 weeks with eventual death of the larvae.5
Diagnosis and Pathology of Disease
Zoonotic Hookworm—The major presenting sign of zoonotic hookworm infection is CLM. The diagnosis of CLM usually is made clinically, as the larvae themselves are 0.5 mm thick to 10 mm long (Figure 1) and usually extend several centimeters beyond the dermal lesion, with dermoscopy having limited utility.17 Patients may begin to experience itching as little as 1 hour after hookworm penetration of the skin.18 Once in contact with the skin, the hookworms’ hyaluronidases and proteases are capable of breaking through the epidermis, but zoonotic hookworms typically are unable to penetrate the basal layer of the human epidermis and remain entombed between the stratum granulosum and stratum corneum. With the exception of rare cases of direct or indirect pulmonary involvement resulting in Löffler syndrome,19 the larvae will die within weeks to months, and symptoms will subsequently resolve.
Although the infection generally is self-limiting, the dermatologic manifestations of CLM can be severe and warrant intervention. The lesions start as small reddish papules at the site of penetration (Figure 2), then the hallmark elevated, migrating, serpiginous, urticarial rash develops (Figure 3). Cutaneous larva migrans generally manifests unilaterally and is both erythematous and intensely pruritic. As the larvae migrate, they leave behind 1- to 5-cm tunneled creeping eruptions in their wake. The lesions, which can manifest with pain or be painless, may develop eczematous, bullous, follicular, or impetiginized appearances.20 Atypical manifestations include folliculitis and urticarial plaques.17
cutaneous larval migrans on the palm.
Anthropophilic Hookworm—The lifecycles of N americanus and A duodenale are completed in human infection. Dermatologic manifestations are transient with the development of ground itch at the site of epidermal penetration. The hookworms employ collagenases that allow penetration of the basal layer of the skin, and eosinophilia develops as the parasites travel from the skin to the small intestine. Once attached to the gastrointestinal lumen, blood meals and proteolytic enzymes result in iron-deficiency anemia in the host and may lead to weakness, fatigue, and low birth weights in pregnant patients. With prolonged infection or heavy parasitic burden, patients can develop hypoproteinemia, anasarca, and yellowing of the skin known as chlorosis.11 A clinical diagnosis can be made by examining patient stool samples for eggs, and definitive characterization can be made using molecular tools such as polymerase chain reaction.21,22
Common to hookworm infections is the immune reaction, which promotes inflammation with localized eosinophilia and mastocytosis.11 In a clinical biopsy specimen of gut—usually obtained through esophagogastroduodenoscopy— T-helper (Th) 2–type immune (IL-4, IL-5, IL-9 and IL-13), regulatory Th10 (IL-10 and transcription growth factor β), and some evidence of Th1 (interferon gamma and IL-2) cytokines are present, but little evidence of Th17-type immune response was found.23 It is believed that in zoonotic infections, antiparasitic IgE from basophils are somewhat successful at trapping the helminths in the epidermis, but in the anthropophilic species, IgE and Th2 responses are ineffective at clearing the parasite from the gut, and the defeated immune system transitions to a host-tolerance approach of limiting infection.11 It is now believed that this natural armistice can be manipulated into a potential therapy against autoimmune and inflammatory conditions. Intentional infection with zoonotic whipworm or hookworm has been proposed as a mechanism of switching Th1 and Th2 responses to host-tolerant mechanisms in conditions such as Crohn disease and celiac disease,24 and it has even been hypothesized that prior hookworm infection may reduce the chance of developing allergic conditions such as eczema.25
Treatment and Prevention
The World Health Organization and Centers for Disease Control and Prevention recommend a single oral dose of 400 mg albendazole for adults or 10 to 15 mg/kg in children for CLM. A single dose of ivermectin at 12 mg in adults or 150 μg/kg in children can be used as an alternative where albendazole is not available.11 Topical applications of thiabendazole 10% to 15% under occlusion or 3 times daily for 15 days without occlusion also can manage CLM, and pruritus can be treated with topical corticosteroids for symptomatic relief. Oral albendazole 400 mg twice daily or mebendazole 100 mg twice daily for 3 days or a single 500-mg dose, as well as 11 mg/kg (up to a maximum of 1 g) oral pyrantel pamoate once daily for 3 days can be used to treat intestinal hookworm infection, though it should be avoided in pregnancy. Iron deficiency should be managed with supplementation.11
Prevention of hookworm infection is focused around 2 broad public health efforts: mass drug administration programs and the water, sanitation, and hygiene program. In mass drug administration, treatments such as benzimidazoles are given in mass to communities affected by endemic hookworm as a single dose to reduce the burden of disease. Together, these strategies effectively eliminated hookworms in many developed nations, but areas of resurgence are beginning to surface worldwide. With changes in climate, emerging drug resistance, and socioeconomic disparities, particularly affecting the southeast, a resurgence of hookworm has occurred in the United States.26 One recent study demonstrated that almost one-third (19/55) of children sampled in an impoverished area of rural Alabama had hookworm eggs in their stool.27 Furthermore, pets serve not only as zoonotic reservoirs for CLM recurrence but also as vehicles for the evolution of drug-resistant strains, leading some to call for a ban of animals from beaches and playgrounds as well as tightly controlled veterinary programs.5,28 Ubiquitous benzimidazole use in livestock has led to bendazole-resistant strains, and it is likely that with continued and poorly adherent drug use, more zoonotic and anthropophilic drug-resistant strains of hookworm will emerge.29,30
Conclusion
The burden of hookworm infection and CLM is substantial in parts of the United States. Dermatologists play a critical role in the recognition and management of hookworm infection for both treatment of affected patients and the subsequent prevention of its spread. As drug-resistant strains evolve, clinicians, public health officials, and scientists need to continue to work together to prevent and treat hookworm infection.
It is estimated that the prevalence of human hookworm infection is approximately 450 million individuals worldwide, representing a substantial global disease burden.1 The annual global public health burden ranges from approximately 2 million to 4 million disability-adjusted life-years and $10 billion to $140 billion in hookwormrelated costs.2 In this article, we discuss the lifecycle, transmission, and disease burden of cutaneous larva migrans (CLM) as well as prevention and treatment strategies.
Background
The Ancylostomatidae nematode family comprises at least 68 known species of hookworm that infect more than 110 different species of mammals.3 Many of these parasites are able to infect more than 1 primary host species, but from a disease perspective they can be classified as either anthropophilic, with humans as the intended host, or zoonotic, with humans as an incidental host. It is important to make this distinction because, though the lifecycles and biology of hookworm species generally are similar, the manifestations of incidental human infection from zoonotic hookworms are different from those of anthropophilic hookworms. Of the anthropophilic species, Necator americanus and Ancylostoma duodenale predominate. In the instance of zoonotic hookworm, dog-infecting A caninum and cat- and doginfecting A braziliense and Uncinaria stenocephala are common causes of incidental human disease.3
The life cycle of Ancylostomatidae organisms is astounding. Through millions of years of co-evolution with mammals,4 these parasitic worms have developed perhaps one of the most circuitous paths to propagate themselves in the natural world. Hookworms start their arduous journey as eggs deposited in soil, sand, and ground vegetation from the feces of infected animals.5 Approximately 1 day after the eggs are deposited, they hatch and begin the larval stage, during which they become infective 1 to 5 weeks later. At this point, the larvae become sensitive to their environment, responding to rising temperatures, increasing carbon dioxide levels, and vibrations in the soil—all of which suggest the presence of a potential host and contribute to a concordant increase in undulatory movement of the larvae.5,6 Here, the most vulnerable tissues include the uncovered soles, palms, and buttocks of host mammals that come into contact with contaminated soil. In an undulating fashion and guided by temperature cues, the larvae locate the skin of the host and utilize a mixture of enzymes including hyaluronidases, metalloprotease, and other proteases to penetrate the epidermis.7 Anthropophilic hookworms such as N americanus and A duodenale will enter the circulatory system; from there, the hookworms migrate through the right-sided cardiopulmonary circuit and eventually ascend into the pulmonary vasculature.8 They then penetrate the lung capillary beds and parenchyma to reach the alveoli, ascend the respiratory tree, and, with the help of the mucociliary escalator, reach the esophagus, where they are swallowed by the host. In the gastrointestinal tract, adult hookworms consume host blood, mate, and lay eggs over a period of approximately 1 to 3 years if left untreated.9 Eggs are laid into the lower gastrointestinal tract, and the journey begins again in feces contacting ground or soil.
Geographic Distribution
Hookworms are found in almost all regions of the world, with species-specific distributions that highlight tropical and subtropical regions. Necator americanus and A duodenale are the most common hookworm species, with the former found predominantly in Southeast Asia and Latin America and the latter in Asia-Pacific regions.10 The highest prevalence of hookworms is in Southeast Asia followed by Sub-Saharan Africa, and the unique climate and soil composition of a region help determine the best environments for specific species of hookworm to thrive.11 In addition, socioeconomics and social determinants of health play a big role in the spread of hookworms, as hygiene practices (eg, wearing clean shoes and clothing, bathing), infrastructure (eg, clean water and streets), and anthelmintic campaigns help reduce transmission.12 Soil-transmitted helminths were once endemic to the southeastern United States, with some reports of approximately 40% of individuals infected in the south in the early 1900s.13 Anthelmintic campaigns such as water, sanitation, and hygiene programs as well as deworming of humans and livestock have proven effective in reducing the prevalence of helminth disease in industrialized nations.13,14 However, zoonotic infections remain a problem in these regions, and in some parts of the United States more than 40% of sampled cats and dogs harbored species such as A braziliense.15
Clinical Manifestation
Initial hookworm infection often goes unnoticed because symptoms can range in severity, but it is characterized by transient ground itch—a local pruritic, erythematous, and papular eruption that develops in response to epidermal penetration.16 Because the larvae must traverse the host from skin to target organs for reproduction over several weeks, iron-deficiency anemia will manifest much later than signs of the initial penetration. In the case of incidental infection from zoonotic Ancylostomatidae organisms, the misguided larvae result in CLM, an often intensely pruritic skin condition that will self-resolve in 2 to 8 weeks with eventual death of the larvae.5
Diagnosis and Pathology of Disease
Zoonotic Hookworm—The major presenting sign of zoonotic hookworm infection is CLM. The diagnosis of CLM usually is made clinically, as the larvae themselves are 0.5 mm thick to 10 mm long (Figure 1) and usually extend several centimeters beyond the dermal lesion, with dermoscopy having limited utility.17 Patients may begin to experience itching as little as 1 hour after hookworm penetration of the skin.18 Once in contact with the skin, the hookworms’ hyaluronidases and proteases are capable of breaking through the epidermis, but zoonotic hookworms typically are unable to penetrate the basal layer of the human epidermis and remain entombed between the stratum granulosum and stratum corneum. With the exception of rare cases of direct or indirect pulmonary involvement resulting in Löffler syndrome,19 the larvae will die within weeks to months, and symptoms will subsequently resolve.
Although the infection generally is self-limiting, the dermatologic manifestations of CLM can be severe and warrant intervention. The lesions start as small reddish papules at the site of penetration (Figure 2), then the hallmark elevated, migrating, serpiginous, urticarial rash develops (Figure 3). Cutaneous larva migrans generally manifests unilaterally and is both erythematous and intensely pruritic. As the larvae migrate, they leave behind 1- to 5-cm tunneled creeping eruptions in their wake. The lesions, which can manifest with pain or be painless, may develop eczematous, bullous, follicular, or impetiginized appearances.20 Atypical manifestations include folliculitis and urticarial plaques.17
cutaneous larval migrans on the palm.
Anthropophilic Hookworm—The lifecycles of N americanus and A duodenale are completed in human infection. Dermatologic manifestations are transient with the development of ground itch at the site of epidermal penetration. The hookworms employ collagenases that allow penetration of the basal layer of the skin, and eosinophilia develops as the parasites travel from the skin to the small intestine. Once attached to the gastrointestinal lumen, blood meals and proteolytic enzymes result in iron-deficiency anemia in the host and may lead to weakness, fatigue, and low birth weights in pregnant patients. With prolonged infection or heavy parasitic burden, patients can develop hypoproteinemia, anasarca, and yellowing of the skin known as chlorosis.11 A clinical diagnosis can be made by examining patient stool samples for eggs, and definitive characterization can be made using molecular tools such as polymerase chain reaction.21,22
Common to hookworm infections is the immune reaction, which promotes inflammation with localized eosinophilia and mastocytosis.11 In a clinical biopsy specimen of gut—usually obtained through esophagogastroduodenoscopy— T-helper (Th) 2–type immune (IL-4, IL-5, IL-9 and IL-13), regulatory Th10 (IL-10 and transcription growth factor β), and some evidence of Th1 (interferon gamma and IL-2) cytokines are present, but little evidence of Th17-type immune response was found.23 It is believed that in zoonotic infections, antiparasitic IgE from basophils are somewhat successful at trapping the helminths in the epidermis, but in the anthropophilic species, IgE and Th2 responses are ineffective at clearing the parasite from the gut, and the defeated immune system transitions to a host-tolerance approach of limiting infection.11 It is now believed that this natural armistice can be manipulated into a potential therapy against autoimmune and inflammatory conditions. Intentional infection with zoonotic whipworm or hookworm has been proposed as a mechanism of switching Th1 and Th2 responses to host-tolerant mechanisms in conditions such as Crohn disease and celiac disease,24 and it has even been hypothesized that prior hookworm infection may reduce the chance of developing allergic conditions such as eczema.25
Treatment and Prevention
The World Health Organization and Centers for Disease Control and Prevention recommend a single oral dose of 400 mg albendazole for adults or 10 to 15 mg/kg in children for CLM. A single dose of ivermectin at 12 mg in adults or 150 μg/kg in children can be used as an alternative where albendazole is not available.11 Topical applications of thiabendazole 10% to 15% under occlusion or 3 times daily for 15 days without occlusion also can manage CLM, and pruritus can be treated with topical corticosteroids for symptomatic relief. Oral albendazole 400 mg twice daily or mebendazole 100 mg twice daily for 3 days or a single 500-mg dose, as well as 11 mg/kg (up to a maximum of 1 g) oral pyrantel pamoate once daily for 3 days can be used to treat intestinal hookworm infection, though it should be avoided in pregnancy. Iron deficiency should be managed with supplementation.11
Prevention of hookworm infection is focused around 2 broad public health efforts: mass drug administration programs and the water, sanitation, and hygiene program. In mass drug administration, treatments such as benzimidazoles are given in mass to communities affected by endemic hookworm as a single dose to reduce the burden of disease. Together, these strategies effectively eliminated hookworms in many developed nations, but areas of resurgence are beginning to surface worldwide. With changes in climate, emerging drug resistance, and socioeconomic disparities, particularly affecting the southeast, a resurgence of hookworm has occurred in the United States.26 One recent study demonstrated that almost one-third (19/55) of children sampled in an impoverished area of rural Alabama had hookworm eggs in their stool.27 Furthermore, pets serve not only as zoonotic reservoirs for CLM recurrence but also as vehicles for the evolution of drug-resistant strains, leading some to call for a ban of animals from beaches and playgrounds as well as tightly controlled veterinary programs.5,28 Ubiquitous benzimidazole use in livestock has led to bendazole-resistant strains, and it is likely that with continued and poorly adherent drug use, more zoonotic and anthropophilic drug-resistant strains of hookworm will emerge.29,30
Conclusion
The burden of hookworm infection and CLM is substantial in parts of the United States. Dermatologists play a critical role in the recognition and management of hookworm infection for both treatment of affected patients and the subsequent prevention of its spread. As drug-resistant strains evolve, clinicians, public health officials, and scientists need to continue to work together to prevent and treat hookworm infection.
- Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211-1259.
- Bartsch SM, Hotez PJ, Asti L, et al. The global economic and health burden of human hookworm infection. PLoS Negl Trop Dis. 2016;10:E0004922.
- Seguel M, Gottdenker N. The diversity and impact of hookworm infections in wildlife. Int J Parasitol Parasites Wildl. 2017;6:177-194.
- Adams BJ, Peat SM, Dillman AR. Phylogeny and evolution. In: Entomopathogenic Nematodes: Systematics, Phylogeny and Bacterial Symbionts. Brill; 2010:693-733.
- Heukelbach J, Feldmeier H. Epidemiological and clinical characteristics of hookworm-related cutaneous larva migrans. Lancet Infect Dis. 2008;8:302-309.
- Haas W, Haberl B, Idris I, et al. Infective larvae of the human hookworms Necator americanus and Ancylostoma duodenale differ in their orientation behaviour when crawling on surfaces. Parasitol Res. 2005;95:25-29.
- Hotez P, Narasimhan S, Haggerty J, et al. Hyaluronidase from infective Ancylostoma hookworm larvae and its possible function as a virulence factor in tissue invasion and in cutaneous larva migrans. Infect Immun. 1992;60:1018-1023.
- Brooker S, Bethony J, Hotez PJ. Human hookworm infection in the 21st century. Adv Parasitol. 2004;58:197-288.
- Hoagland K, Schad G. Necator americanus and Ancylostoma duodenale: life history parameters and epidemiological implications of two sympatric hookworms of humans. Exp Parasitol. 1978;44:36-49.
- Clements ACA, Alene KA. Global distribution of human hookworm species and differences in their morbidity effects: a systematic review. Lancet Microbe. 2022;3:E72-E79.
- Loukas A, Hotez PJ, Diemert D, et al. Hookworm infection. Nat Rev Dis Primers. 2016;2:1-18.
- Gazzinelli A, Correa-Oliveira R, Yang GJ, et al. A research agenda for helminth diseases of humans: social ecology, environmental determinants, and health systems. PLoS Negl Trop Dis. 2012;6:E1603.
- Starr MC, Montgomery SP. Soil-transmitted helminthiasis in the United States: a systematic review—1940-2010. Am J Trop Med Hyg. 2011;85:680-684.
- Strunz EC, Addiss DG, Stocks ME, et al. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and metaanalysis. PLoS Med. 2014;11:E1001620.
- Liotta JL, Youn H, Aksel S, et al. Prevalence of Ancylostoma braziliense in dogs from Alachua and Marion Counties, Florida, United States. J Parasitol. 2012;98:1039-1040.
- Hotez PJ, Brooker S, Bethony JM, et al. Hookworm infection. N Engl J Med. 2004;351:799-807.
- Prickett KA, Ferringer TC. What’s eating you? cutaneous larva migrans. Cutis. 2015;95:126-128.
- Feldmeier H, Schuster A. Mini review: hookworm-related cutaneous larva migrans. Eur J Clin Microbiol Infect Dis. 2012;31:915-918.
- Tan SK, Liu TT. Cutaneous larva migrans complicated by Löffler syndrome. Arch Dermatol. 2010;146:210-212.
- Eksomtramage T, Aiempanakit K. Bullous and pustular cutaneous larva migrans: two case reports and a literature review. IDCases. 2018;12:130-132.
- Utzinger J, Rinaldi L, Lohourignon LK, et al. FLOTAC: a new sensitive technique for the diagnosis of hookworm infections in humans. Trans R Soc Trop Med Hyg. 2008;102:84-90.
- Chidambaram M, Parija SC, Toi PC, et al. Evaluation of the utility of conventional polymerase chain reaction for detection and species differentiation in human hookworm infections. Trop Parasitol. 2017;7:111-116.
- Gaze S, McSorley HJ, Daveson J, et al. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Pathog. 2012;8:E1002520.
- Croese J, O’Neil J, Masson J, et al. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors. Gut. 2006;55:136-137.
- Mpairwe H, Amoah AS. Parasites and allergy: observations from Africa. Parasite Immunol. 2019;41:E12589.
- Albonico M, Savioli L. Hookworm: a neglected resurgent infection. Editorial. BMJ. 2017;359:j4813.
- McKenna ML, McAtee S, Bryan PE, et al. Human intestinal parasite burden and poor sanitation in rural Alabama. Am J Trop Med Hyg. 2017;97:1623-1628.
- Traversa D. Pet roundworms and hookworms: a continuing need for global worming. Parasit Vectors. 2012;5:1-19.
- Geerts S, Gryseels B. Drug resistance in human helminths: current situation and lessons from livestock. Clin Microbiol Rev. 2000;13:207-222.
- Jimenez Castro PD, Howell SB, Schaefer JJ, et al. Multiple drug resistance in the canine hookworm Ancylostoma caninum: an emerging threat? Parasit Vectors. 2019;12:1-15.
- Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211-1259.
- Bartsch SM, Hotez PJ, Asti L, et al. The global economic and health burden of human hookworm infection. PLoS Negl Trop Dis. 2016;10:E0004922.
- Seguel M, Gottdenker N. The diversity and impact of hookworm infections in wildlife. Int J Parasitol Parasites Wildl. 2017;6:177-194.
- Adams BJ, Peat SM, Dillman AR. Phylogeny and evolution. In: Entomopathogenic Nematodes: Systematics, Phylogeny and Bacterial Symbionts. Brill; 2010:693-733.
- Heukelbach J, Feldmeier H. Epidemiological and clinical characteristics of hookworm-related cutaneous larva migrans. Lancet Infect Dis. 2008;8:302-309.
- Haas W, Haberl B, Idris I, et al. Infective larvae of the human hookworms Necator americanus and Ancylostoma duodenale differ in their orientation behaviour when crawling on surfaces. Parasitol Res. 2005;95:25-29.
- Hotez P, Narasimhan S, Haggerty J, et al. Hyaluronidase from infective Ancylostoma hookworm larvae and its possible function as a virulence factor in tissue invasion and in cutaneous larva migrans. Infect Immun. 1992;60:1018-1023.
- Brooker S, Bethony J, Hotez PJ. Human hookworm infection in the 21st century. Adv Parasitol. 2004;58:197-288.
- Hoagland K, Schad G. Necator americanus and Ancylostoma duodenale: life history parameters and epidemiological implications of two sympatric hookworms of humans. Exp Parasitol. 1978;44:36-49.
- Clements ACA, Alene KA. Global distribution of human hookworm species and differences in their morbidity effects: a systematic review. Lancet Microbe. 2022;3:E72-E79.
- Loukas A, Hotez PJ, Diemert D, et al. Hookworm infection. Nat Rev Dis Primers. 2016;2:1-18.
- Gazzinelli A, Correa-Oliveira R, Yang GJ, et al. A research agenda for helminth diseases of humans: social ecology, environmental determinants, and health systems. PLoS Negl Trop Dis. 2012;6:E1603.
- Starr MC, Montgomery SP. Soil-transmitted helminthiasis in the United States: a systematic review—1940-2010. Am J Trop Med Hyg. 2011;85:680-684.
- Strunz EC, Addiss DG, Stocks ME, et al. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and metaanalysis. PLoS Med. 2014;11:E1001620.
- Liotta JL, Youn H, Aksel S, et al. Prevalence of Ancylostoma braziliense in dogs from Alachua and Marion Counties, Florida, United States. J Parasitol. 2012;98:1039-1040.
- Hotez PJ, Brooker S, Bethony JM, et al. Hookworm infection. N Engl J Med. 2004;351:799-807.
- Prickett KA, Ferringer TC. What’s eating you? cutaneous larva migrans. Cutis. 2015;95:126-128.
- Feldmeier H, Schuster A. Mini review: hookworm-related cutaneous larva migrans. Eur J Clin Microbiol Infect Dis. 2012;31:915-918.
- Tan SK, Liu TT. Cutaneous larva migrans complicated by Löffler syndrome. Arch Dermatol. 2010;146:210-212.
- Eksomtramage T, Aiempanakit K. Bullous and pustular cutaneous larva migrans: two case reports and a literature review. IDCases. 2018;12:130-132.
- Utzinger J, Rinaldi L, Lohourignon LK, et al. FLOTAC: a new sensitive technique for the diagnosis of hookworm infections in humans. Trans R Soc Trop Med Hyg. 2008;102:84-90.
- Chidambaram M, Parija SC, Toi PC, et al. Evaluation of the utility of conventional polymerase chain reaction for detection and species differentiation in human hookworm infections. Trop Parasitol. 2017;7:111-116.
- Gaze S, McSorley HJ, Daveson J, et al. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Pathog. 2012;8:E1002520.
- Croese J, O’Neil J, Masson J, et al. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors. Gut. 2006;55:136-137.
- Mpairwe H, Amoah AS. Parasites and allergy: observations from Africa. Parasite Immunol. 2019;41:E12589.
- Albonico M, Savioli L. Hookworm: a neglected resurgent infection. Editorial. BMJ. 2017;359:j4813.
- McKenna ML, McAtee S, Bryan PE, et al. Human intestinal parasite burden and poor sanitation in rural Alabama. Am J Trop Med Hyg. 2017;97:1623-1628.
- Traversa D. Pet roundworms and hookworms: a continuing need for global worming. Parasit Vectors. 2012;5:1-19.
- Geerts S, Gryseels B. Drug resistance in human helminths: current situation and lessons from livestock. Clin Microbiol Rev. 2000;13:207-222.
- Jimenez Castro PD, Howell SB, Schaefer JJ, et al. Multiple drug resistance in the canine hookworm Ancylostoma caninum: an emerging threat? Parasit Vectors. 2019;12:1-15.
What’s Eating You? Hookworm and
Cutaneous Larva Migrans
What’s Eating You? Hookworm and
Cutaneous Larva Migrans
PRACTICE POINTS
- Anthropophilic hookworm infection should be considered with evidence of either transient ground itch or iron-deficient anemia in individuals who go barefoot, permitting ground-to-skin transmission.
- Zoonotic hookworm infection manifests as cutaneous larva migrans, an elevated serpiginous rash that, while usually self-resolving, can be intensely pruritic and should be treated accordingly.
- Considered a neglected tropical disease, hookworm infection still represents an enormous global disease burden. In addition to ongoing afflicted regions, hookworms are making a resurgence in developed nations, and drug-resistant strains have evolved.
Asteraceae Dermatitis: Everyday Plants With Allergenic Potential
The Asteraceae (formerly Compositae) family of plants is derived from the ancient Greek word aster, meaning “star,” referring to the starlike arrangement of flower petals around a central disc known as a capitulum. What initially appears as a single flower is actually a composite of several smaller flowers, hence the former name Compositae.1 Well-known members of the Asteraceae family include ornamental annuals (eg, sunflowers, marigolds, cosmos), herbaceous perennials (eg, chrysanthemums, dandelions), vegetables (eg, lettuce, chicory, artichokes), herbs (eg, chamomile, tarragon), and weeds (eg, ragweed, horseweed, capeweed)(Figure 1).2

There are more than 25,000 species of Asteraceae plants that thrive in a wide range of climates worldwide. Cases of Asteraceae-induced skin reactions have been reported in North America, Europe, Asia, and Australia.3 Members of the Asteraceae family are ubiquitous in gardens, along roadsides, and in the wilderness. Occupational exposure commonly affects gardeners, florists, farmers, and forestry workers through either direct contact with plants or via airborne pollen. Furthermore, plants of the Asteraceae family are used in various products, including pediculicides (eg, insect repellents), cosmetics (eg, eye creams, body washes), and food products (eg, cooking oils, sweetening agents, coffee substitutes, herbal teas).4-6 These plants have substantial allergic potential, resulting in numerous cutaneous reactions.
Allergic Potential
Asteraceae plants can elicit both immediate and delayed hypersensitivity reactions (HSRs); for instance, exposure to ragweed pollen may cause an IgE-mediated type 1 HSR manifesting as allergic rhinitis or a type IV HSR manifesting as airborne allergic contact dermatitis.7,8 The main contact allergens present in Asteraceae plants are sesquiterpene lactones, which are found in the leaves, stems, flowers, and pollen.9-11 Sesquiterpene lactones consist of an α-methyl group attached to a lactone ring combined with a sesquiterpene.12 Patch testing can be used to diagnose Asteraceae allergy; however, the results are not consistently reliable because there is no perfect screening allergen. Patch test preparations commonly used to detect Asteraceae allergy include Compositae mix (consisting of Anthemis nobilis extract, Chamomilla recutita extract, Achillea millefolium extract, Tanacetum vulgare extract, Arnica montana extract, and parthenolide) and sesquiterpene lactone mix (consisting of alantolactone, dehydrocostus lactone, and costunolide). In North America, the prevalence of positive patch tests to Compositae mix and sesquiterpene lactone mix is approximately 2% and 0.5%, respectively.13 When patch testing is performed, both Compositae mix and sesquiterpene lactone mix should be utilized to minimize the risk of missing Asteraceae allergy, as sesquiterpene lactone mix alone does not detect all Compositae-sensitized patients. Additionally, it may be necessary to test supplemental Asteraceae allergens, including preparations from specific plants to which the patient has been exposed. Exposure to Asteraceae-containing cosmetic products may lead to dermatitis, though this is highly dependent on the particular plant species involved. For instance, the prevalence of sensitization is high in arnica (tincture) and elecampane but low with more commonly used species such as German chamomile.14
Cutaneous Manifestations
Asteraceae dermatitis, which also is known as Australian bush dermatitis, weed dermatitis, and chrysanthemum dermatitis,2 can manifest on any area of the body that directly contacts the plant or is exposed to the pollen. Asteraceae dermatitis historically was reported in older adults with a recent history of plant exposure.6,15 However, recent data have shown a female preponderance and a younger mean age of onset (46–49 years).16
There are multiple distinct clinical manifestations of Asteraceae dermatitis. The most common cutaneous finding is localized vesicular or eczematous patches on the hands or wrists. Other variations include eczematous rashes on the exposed skin of the hands, arms, face, and neck; generalized eczema; and isolated facial eczema.16,17 These variations can be attributed to contact dermatitis caused by airborne pollen, which may mimic photodermatitis. However, airborne Asteraceae dermatitis can be distinguished clinically from photodermatitis by the involvement of sun-protected areas such as the skinfolds of the eyelids, retroauricular sulci, and nasolabial folds (Figure 2).2,9 In rare cases, systemic allergic contact dermatitis can occur if the Asteraceae allergen is ingested.2,18
Other diagnostic clues include dermatitis that flares during the summer, at the peak of the growing season, with remission in the cooler months. Potential risk factors include a childhood history of atopic dermatitis and allergic rhinitis.16 With prolonged exposure, patients may develop chronic actinic dermatitis, an immunologically mediated photodermatosis characterized by lichenified and pruritic eczematous plaques located predominantly on sun-exposed areas with notable sparing of the skin folds.19 The association between Asteraceae dermatitis and chronic actinic dermatitis is highly variable, with some studies reporting a 25% correlation and others finding a stronger association of up to 80%.2,15,20 Asteraceae allergy appears to be a relatively uncommon cause of photoallergy in North America. In one recent study, 16% (3/19) of patients with chronic actinic dermatitis had positive patch or photopatch tests to sesquiterpene lactone mix, but in another large study of photopatch testing it was reported to be a rare photoallergen.21,22
Parthenium dermatitis is an allergic contact dermatitis caused by exposure to Parthenium hysterophorus, a weed of the Asteraceae family that is responsible for 30% of cases of contact dermatitis in India.23,24 Unlike the more classic manifestation of Asteraceae dermatitis, which primarily affects the upper extremities in cases from North America and Europe, Parthenium dermatitis typically occurs in an airborne pattern distribution.24
Management
While complete avoidance of Asteraceae plants is ideal, it often is unrealistic due to their abundance in nature. Therefore, minimizing exposure to the causative plants is recommended. Primary preventive measures such as wearing protective gloves and clothing and applying bentonite clay prior to exposure should be taken when working outdoors. Promptly showering after contact with plants also can reduce the risk for Asteraceae dermatitis.
Symptomatic treatment is appropriate for mild cases and includes topical corticosteroids and calcineurin inhibitors. For severe cases, systemic corticosteroids may be needed for acute flares, with azathioprine, mycophenolate, cyclosporine, or methotrexate available for recalcitrant disease. Verma et al25 found that treatment with azathioprine for 6 months resulted in greater than 60% clearance in all 12 patients, with a majority achieving 80% to 100% clearance. Methotrexate has been used at doses of 15 mg once weekly.26 Narrowband UVB and psoralen plus UVA have been effective in extensive cases; however, care should be exercised in patients with photosensitive dermatitis, who instead should practice strict photoprotection.27-29 Lakshmi et al30 reported the use of cyclosporine during the acute phase of Asteraceae dermatitis at a dose of 2.5 mg/kg daily for 4 to 8 weeks. There have been several case reports of dupilumab treating allergic contact dermatitis; however, there have been 3 cases of patients with atopic dermatitis developing Asteraceae dermatitis while taking dupilumab.31,32 Recently, oral Janus kinase inhibitors have shown success in treating refractory cases of airborne Asteraceae dermatitis.33,34 Further research is needed to determine the safety and efficacy of dupilumab and Janus kinase inhibitors for treatment of Asteraceae dermatitis.
Final Thoughts
The Asteraceae plant family is vast and diverse, with more than 200 species reported to cause allergic contact dermatitis.12 Common modes of contact include gardening, occupational exposure, airborne pollen, and use of pediculicides and cosmetics that contain components of Asteraceae plants. Educating patients on how to minimize contact with Asteraceae plants is the most effective management strategy; topical agents and oral immunosuppressives can be used for symptomatic treatment.
- Morhardt S, Morhardt E. California Desert Flowers: An Introduction to Families, Genera, and Species. University of California Press; 2004.
- Gordon LA. Compositae dermatitis. Australas J Dermatol. 1999;40:123-130. doi:10.1046/j.1440-0960.1999.00341.x
- Denisow-Pietrzyk M, Pietrzyk Ł, Denisow B. Asteraceae species as potential environmental factors of allergy. Environ Sci Pollut Res Int. 2019;26:6290-6300. doi:10.1007/s11356-019-04146-w
- Paulsen E, Chistensen LP, Andersen KE. Cosmetics and herbal remedies with Compositae plant extracts—are they tolerated by Compositae-allergic patients? Contact Dermatitis. 2008;58:15-23. doi:10.1111/j.1600-0536.2007.01250.x
- Burry JN, Reid JG, Kirk J. Australian bush dermatitis. Contact Dermatitis. 1975;1:263-264. doi:10.1111/j.1600-0536.1975.tb05422.x
- Punchihewa N, Palmer A, Nixon R. Allergic contact dermatitis to Compositae: an Australian case series. Contact Dermatitis. 2022;87:356-362. doi:10.1111/cod.14162
- Chen KW, Marusciac L, Tamas PT, et al. Ragweed pollen allergy: burden, characteristics, and management of an imported allergen source in Europe. Int Arch Allergy Immunol. 2018;176:163-180. doi:10.1159/000487997
- Schloemer JA, Zirwas MJ, Burkhart CG. Airborne contact dermatitis: common causes in the USA. Int J Dermatol. 2015;54:271-274. doi:10.1111/ijd.12692
- Arlette J, Mitchell JC. Compositae dermatitis. current aspects. Contact Dermatitis. 1981;7:129-136. doi:10.1111/j.1600-0536.1981.tb04584.x
- Mitchell JC, Dupuis G. Allergic contact dermatitis from sesquiterpenoids of the Compositae family of plants. Br J Dermatol. 1971;84:139-150. doi:10.1111/j.1365-2133.1971.tb06857.x
- Salapovic H, Geier J, Reznicek G. Quantification of Sesquiterpene lactones in Asteraceae plant extracts: evaluation of their allergenic potential. Sci Pharm. 2013;81:807-818. doi:10.3797/scipharm.1306-17
- Paulsen E. Compositae dermatitis: a survey. Contact Dermatitis. 1992;26:76-86. doi:10.1111/j.1600-0536.1992.tb00888.x. Published correction appears in Contact Dermatitis. 1992;27:208.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Paulsen E. Contact sensitization from Compositae-containing herbal remedies and cosmetics. Contact Dermatitis. 2002;47:189-198. doi:10.1034/j.1600-0536.2002.470401.x
- Frain-Bell W, Johnson BE. Contact allergic sensitivity to plants and the photosensitivity dermatitis and actinic reticuloid syndrome. Br J Dermatol. 1979;101:503-512.
- Paulsen E, Andersen KE. Clinical patterns of Compositae dermatitis in Danish monosensitized patients. Contact Dermatitis. 2018;78:185-193. doi:10.1111/cod.12916
- Jovanovic´ M, Poljacki M. Compositae dermatitis. Med Pregl. 2003;56:43-49. doi:10.2298/mpns0302043j
- Krook G. Occupational dermatitis from Lactuca sativa (lettuce) and Cichorium (endive). simultaneous occurrence of immediate and delayed allergy as a cause of contact dermatitis. Contact Dermatitis. 1977;3:27-36. doi:10.1111/j.1600-0536.1977.tb03583.x
- Paek SY, Lim HW. Chronic actinic dermatitis. Dermatol Clin. 2014;32:355-361, viii-ix. doi:10.1016/j.det.2014.03.007
- du P Menagé H, Hawk JL, White IR. Sesquiterpene lactone mix contact sensitivity and its relationship to chronic actinic dermatitis: a follow-up study. Contact Dermatitis. 1998;39:119-122. doi:10.1111/j.1600-0536.1998.tb05859.x
- Wang CX, Belsito DV. Chronic actinic dermatitis revisited. Dermatitis. 2020;31:68-74. doi:10.1097/DER.0000000000000531
- DeLeo VA, Adler BL, Warshaw EM, et al. Photopatch test results of the North American contact dermatitis group, 1999-2009. Photodermatol Photoimmunol Photomed. 2022;38:288-291. doi:10.1111/phpp.12742
- McGovern TW, LaWarre S. Botanical briefs: the scourge of India—Parthenium hysterophorus L. Cutis. 2001;67:27-34. Published correction appears in Cutis. 2001;67:154.
- Sharma VK, Verma P, Maharaja K. Parthenium dermatitis. Photochem Photobiol Sci. 2013;12:85-94. doi:10.1039/c2pp25186h
- Verma KK, Bansal A, Sethuraman G. Parthenium dermatitis treated with azathioprine weekly pulse doses. Indian J Dermatol Venereol Leprol. 2006;72:24-27. doi:10.4103/0378-6323.19713
- Sharma VK, Bhat R, Sethuraman G, et al. Treatment of Parthenium dermatitis with methotrexate. Contact Dermatitis. 2007;57:118-119. doi:10.1111/j.1600-0536.2006.00950.x
- Burke DA, Corey G, Storrs FJ. Psoralen plus UVA protocol for Compositae photosensitivity. Am J Contact Dermat. 1996;7:171-176.
- Lovell CR. Allergic contact dermatitis due to plants. In: Plants and the Skin. Blackwell Scientific Publications; 1993:96-254.
- Dogra S, Parsad D, Handa S. Narrowband ultraviolet B in airborne contact dermatitis: a ray of hope! Br J Dermatol. 2004;150:373-374. doi:10.1111/j.1365-2133.2004.05724.x
- Lakshmi C, Srinivas CR, Jayaraman A. Ciclosporin in Parthenium dermatitis—a report of 2 cases. Contact Dermatitis. 2008;59:245-248. doi:10.1111/j.1600-0536.2007.01208.x
- Hendricks AJ, Yosipovitch G, Shi VY. Dupilumab use in dermatologic conditions beyond atopic dermatitis—a systematic review. J Dermatolog Treat. 2021;32:19-28. doi:10.1080/09546634.2019.1689227
- Napolitano M, Fabbrocini G, Patruno C. Allergic contact dermatitis to Compositae: a possible cause of dupilumab-associated facial and neck dermatitis in atopic dermatitis patients? Contact Dermatitis. 2021;85:473-474. doi:10.1111/cod.13898
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis. Contact Dermatitis. 2023;88:150-152. doi:10.1111/cod.14234
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544. doi:10.1111/cod.14204
The Asteraceae (formerly Compositae) family of plants is derived from the ancient Greek word aster, meaning “star,” referring to the starlike arrangement of flower petals around a central disc known as a capitulum. What initially appears as a single flower is actually a composite of several smaller flowers, hence the former name Compositae.1 Well-known members of the Asteraceae family include ornamental annuals (eg, sunflowers, marigolds, cosmos), herbaceous perennials (eg, chrysanthemums, dandelions), vegetables (eg, lettuce, chicory, artichokes), herbs (eg, chamomile, tarragon), and weeds (eg, ragweed, horseweed, capeweed)(Figure 1).2

There are more than 25,000 species of Asteraceae plants that thrive in a wide range of climates worldwide. Cases of Asteraceae-induced skin reactions have been reported in North America, Europe, Asia, and Australia.3 Members of the Asteraceae family are ubiquitous in gardens, along roadsides, and in the wilderness. Occupational exposure commonly affects gardeners, florists, farmers, and forestry workers through either direct contact with plants or via airborne pollen. Furthermore, plants of the Asteraceae family are used in various products, including pediculicides (eg, insect repellents), cosmetics (eg, eye creams, body washes), and food products (eg, cooking oils, sweetening agents, coffee substitutes, herbal teas).4-6 These plants have substantial allergic potential, resulting in numerous cutaneous reactions.
Allergic Potential
Asteraceae plants can elicit both immediate and delayed hypersensitivity reactions (HSRs); for instance, exposure to ragweed pollen may cause an IgE-mediated type 1 HSR manifesting as allergic rhinitis or a type IV HSR manifesting as airborne allergic contact dermatitis.7,8 The main contact allergens present in Asteraceae plants are sesquiterpene lactones, which are found in the leaves, stems, flowers, and pollen.9-11 Sesquiterpene lactones consist of an α-methyl group attached to a lactone ring combined with a sesquiterpene.12 Patch testing can be used to diagnose Asteraceae allergy; however, the results are not consistently reliable because there is no perfect screening allergen. Patch test preparations commonly used to detect Asteraceae allergy include Compositae mix (consisting of Anthemis nobilis extract, Chamomilla recutita extract, Achillea millefolium extract, Tanacetum vulgare extract, Arnica montana extract, and parthenolide) and sesquiterpene lactone mix (consisting of alantolactone, dehydrocostus lactone, and costunolide). In North America, the prevalence of positive patch tests to Compositae mix and sesquiterpene lactone mix is approximately 2% and 0.5%, respectively.13 When patch testing is performed, both Compositae mix and sesquiterpene lactone mix should be utilized to minimize the risk of missing Asteraceae allergy, as sesquiterpene lactone mix alone does not detect all Compositae-sensitized patients. Additionally, it may be necessary to test supplemental Asteraceae allergens, including preparations from specific plants to which the patient has been exposed. Exposure to Asteraceae-containing cosmetic products may lead to dermatitis, though this is highly dependent on the particular plant species involved. For instance, the prevalence of sensitization is high in arnica (tincture) and elecampane but low with more commonly used species such as German chamomile.14
Cutaneous Manifestations
Asteraceae dermatitis, which also is known as Australian bush dermatitis, weed dermatitis, and chrysanthemum dermatitis,2 can manifest on any area of the body that directly contacts the plant or is exposed to the pollen. Asteraceae dermatitis historically was reported in older adults with a recent history of plant exposure.6,15 However, recent data have shown a female preponderance and a younger mean age of onset (46–49 years).16
There are multiple distinct clinical manifestations of Asteraceae dermatitis. The most common cutaneous finding is localized vesicular or eczematous patches on the hands or wrists. Other variations include eczematous rashes on the exposed skin of the hands, arms, face, and neck; generalized eczema; and isolated facial eczema.16,17 These variations can be attributed to contact dermatitis caused by airborne pollen, which may mimic photodermatitis. However, airborne Asteraceae dermatitis can be distinguished clinically from photodermatitis by the involvement of sun-protected areas such as the skinfolds of the eyelids, retroauricular sulci, and nasolabial folds (Figure 2).2,9 In rare cases, systemic allergic contact dermatitis can occur if the Asteraceae allergen is ingested.2,18

Other diagnostic clues include dermatitis that flares during the summer, at the peak of the growing season, with remission in the cooler months. Potential risk factors include a childhood history of atopic dermatitis and allergic rhinitis.16 With prolonged exposure, patients may develop chronic actinic dermatitis, an immunologically mediated photodermatosis characterized by lichenified and pruritic eczematous plaques located predominantly on sun-exposed areas with notable sparing of the skin folds.19 The association between Asteraceae dermatitis and chronic actinic dermatitis is highly variable, with some studies reporting a 25% correlation and others finding a stronger association of up to 80%.2,15,20 Asteraceae allergy appears to be a relatively uncommon cause of photoallergy in North America. In one recent study, 16% (3/19) of patients with chronic actinic dermatitis had positive patch or photopatch tests to sesquiterpene lactone mix, but in another large study of photopatch testing it was reported to be a rare photoallergen.21,22
Parthenium dermatitis is an allergic contact dermatitis caused by exposure to Parthenium hysterophorus, a weed of the Asteraceae family that is responsible for 30% of cases of contact dermatitis in India.23,24 Unlike the more classic manifestation of Asteraceae dermatitis, which primarily affects the upper extremities in cases from North America and Europe, Parthenium dermatitis typically occurs in an airborne pattern distribution.24
Management
While complete avoidance of Asteraceae plants is ideal, it often is unrealistic due to their abundance in nature. Therefore, minimizing exposure to the causative plants is recommended. Primary preventive measures such as wearing protective gloves and clothing and applying bentonite clay prior to exposure should be taken when working outdoors. Promptly showering after contact with plants also can reduce the risk for Asteraceae dermatitis.
Symptomatic treatment is appropriate for mild cases and includes topical corticosteroids and calcineurin inhibitors. For severe cases, systemic corticosteroids may be needed for acute flares, with azathioprine, mycophenolate, cyclosporine, or methotrexate available for recalcitrant disease. Verma et al25 found that treatment with azathioprine for 6 months resulted in greater than 60% clearance in all 12 patients, with a majority achieving 80% to 100% clearance. Methotrexate has been used at doses of 15 mg once weekly.26 Narrowband UVB and psoralen plus UVA have been effective in extensive cases; however, care should be exercised in patients with photosensitive dermatitis, who instead should practice strict photoprotection.27-29 Lakshmi et al30 reported the use of cyclosporine during the acute phase of Asteraceae dermatitis at a dose of 2.5 mg/kg daily for 4 to 8 weeks. There have been several case reports of dupilumab treating allergic contact dermatitis; however, there have been 3 cases of patients with atopic dermatitis developing Asteraceae dermatitis while taking dupilumab.31,32 Recently, oral Janus kinase inhibitors have shown success in treating refractory cases of airborne Asteraceae dermatitis.33,34 Further research is needed to determine the safety and efficacy of dupilumab and Janus kinase inhibitors for treatment of Asteraceae dermatitis.
Final Thoughts
The Asteraceae plant family is vast and diverse, with more than 200 species reported to cause allergic contact dermatitis.12 Common modes of contact include gardening, occupational exposure, airborne pollen, and use of pediculicides and cosmetics that contain components of Asteraceae plants. Educating patients on how to minimize contact with Asteraceae plants is the most effective management strategy; topical agents and oral immunosuppressives can be used for symptomatic treatment.
The Asteraceae (formerly Compositae) family of plants is derived from the ancient Greek word aster, meaning “star,” referring to the starlike arrangement of flower petals around a central disc known as a capitulum. What initially appears as a single flower is actually a composite of several smaller flowers, hence the former name Compositae.1 Well-known members of the Asteraceae family include ornamental annuals (eg, sunflowers, marigolds, cosmos), herbaceous perennials (eg, chrysanthemums, dandelions), vegetables (eg, lettuce, chicory, artichokes), herbs (eg, chamomile, tarragon), and weeds (eg, ragweed, horseweed, capeweed)(Figure 1).2

There are more than 25,000 species of Asteraceae plants that thrive in a wide range of climates worldwide. Cases of Asteraceae-induced skin reactions have been reported in North America, Europe, Asia, and Australia.3 Members of the Asteraceae family are ubiquitous in gardens, along roadsides, and in the wilderness. Occupational exposure commonly affects gardeners, florists, farmers, and forestry workers through either direct contact with plants or via airborne pollen. Furthermore, plants of the Asteraceae family are used in various products, including pediculicides (eg, insect repellents), cosmetics (eg, eye creams, body washes), and food products (eg, cooking oils, sweetening agents, coffee substitutes, herbal teas).4-6 These plants have substantial allergic potential, resulting in numerous cutaneous reactions.
Allergic Potential
Asteraceae plants can elicit both immediate and delayed hypersensitivity reactions (HSRs); for instance, exposure to ragweed pollen may cause an IgE-mediated type 1 HSR manifesting as allergic rhinitis or a type IV HSR manifesting as airborne allergic contact dermatitis.7,8 The main contact allergens present in Asteraceae plants are sesquiterpene lactones, which are found in the leaves, stems, flowers, and pollen.9-11 Sesquiterpene lactones consist of an α-methyl group attached to a lactone ring combined with a sesquiterpene.12 Patch testing can be used to diagnose Asteraceae allergy; however, the results are not consistently reliable because there is no perfect screening allergen. Patch test preparations commonly used to detect Asteraceae allergy include Compositae mix (consisting of Anthemis nobilis extract, Chamomilla recutita extract, Achillea millefolium extract, Tanacetum vulgare extract, Arnica montana extract, and parthenolide) and sesquiterpene lactone mix (consisting of alantolactone, dehydrocostus lactone, and costunolide). In North America, the prevalence of positive patch tests to Compositae mix and sesquiterpene lactone mix is approximately 2% and 0.5%, respectively.13 When patch testing is performed, both Compositae mix and sesquiterpene lactone mix should be utilized to minimize the risk of missing Asteraceae allergy, as sesquiterpene lactone mix alone does not detect all Compositae-sensitized patients. Additionally, it may be necessary to test supplemental Asteraceae allergens, including preparations from specific plants to which the patient has been exposed. Exposure to Asteraceae-containing cosmetic products may lead to dermatitis, though this is highly dependent on the particular plant species involved. For instance, the prevalence of sensitization is high in arnica (tincture) and elecampane but low with more commonly used species such as German chamomile.14
Cutaneous Manifestations
Asteraceae dermatitis, which also is known as Australian bush dermatitis, weed dermatitis, and chrysanthemum dermatitis,2 can manifest on any area of the body that directly contacts the plant or is exposed to the pollen. Asteraceae dermatitis historically was reported in older adults with a recent history of plant exposure.6,15 However, recent data have shown a female preponderance and a younger mean age of onset (46–49 years).16
There are multiple distinct clinical manifestations of Asteraceae dermatitis. The most common cutaneous finding is localized vesicular or eczematous patches on the hands or wrists. Other variations include eczematous rashes on the exposed skin of the hands, arms, face, and neck; generalized eczema; and isolated facial eczema.16,17 These variations can be attributed to contact dermatitis caused by airborne pollen, which may mimic photodermatitis. However, airborne Asteraceae dermatitis can be distinguished clinically from photodermatitis by the involvement of sun-protected areas such as the skinfolds of the eyelids, retroauricular sulci, and nasolabial folds (Figure 2).2,9 In rare cases, systemic allergic contact dermatitis can occur if the Asteraceae allergen is ingested.2,18

Other diagnostic clues include dermatitis that flares during the summer, at the peak of the growing season, with remission in the cooler months. Potential risk factors include a childhood history of atopic dermatitis and allergic rhinitis.16 With prolonged exposure, patients may develop chronic actinic dermatitis, an immunologically mediated photodermatosis characterized by lichenified and pruritic eczematous plaques located predominantly on sun-exposed areas with notable sparing of the skin folds.19 The association between Asteraceae dermatitis and chronic actinic dermatitis is highly variable, with some studies reporting a 25% correlation and others finding a stronger association of up to 80%.2,15,20 Asteraceae allergy appears to be a relatively uncommon cause of photoallergy in North America. In one recent study, 16% (3/19) of patients with chronic actinic dermatitis had positive patch or photopatch tests to sesquiterpene lactone mix, but in another large study of photopatch testing it was reported to be a rare photoallergen.21,22
Parthenium dermatitis is an allergic contact dermatitis caused by exposure to Parthenium hysterophorus, a weed of the Asteraceae family that is responsible for 30% of cases of contact dermatitis in India.23,24 Unlike the more classic manifestation of Asteraceae dermatitis, which primarily affects the upper extremities in cases from North America and Europe, Parthenium dermatitis typically occurs in an airborne pattern distribution.24
Management
While complete avoidance of Asteraceae plants is ideal, it often is unrealistic due to their abundance in nature. Therefore, minimizing exposure to the causative plants is recommended. Primary preventive measures such as wearing protective gloves and clothing and applying bentonite clay prior to exposure should be taken when working outdoors. Promptly showering after contact with plants also can reduce the risk for Asteraceae dermatitis.
Symptomatic treatment is appropriate for mild cases and includes topical corticosteroids and calcineurin inhibitors. For severe cases, systemic corticosteroids may be needed for acute flares, with azathioprine, mycophenolate, cyclosporine, or methotrexate available for recalcitrant disease. Verma et al25 found that treatment with azathioprine for 6 months resulted in greater than 60% clearance in all 12 patients, with a majority achieving 80% to 100% clearance. Methotrexate has been used at doses of 15 mg once weekly.26 Narrowband UVB and psoralen plus UVA have been effective in extensive cases; however, care should be exercised in patients with photosensitive dermatitis, who instead should practice strict photoprotection.27-29 Lakshmi et al30 reported the use of cyclosporine during the acute phase of Asteraceae dermatitis at a dose of 2.5 mg/kg daily for 4 to 8 weeks. There have been several case reports of dupilumab treating allergic contact dermatitis; however, there have been 3 cases of patients with atopic dermatitis developing Asteraceae dermatitis while taking dupilumab.31,32 Recently, oral Janus kinase inhibitors have shown success in treating refractory cases of airborne Asteraceae dermatitis.33,34 Further research is needed to determine the safety and efficacy of dupilumab and Janus kinase inhibitors for treatment of Asteraceae dermatitis.
Final Thoughts
The Asteraceae plant family is vast and diverse, with more than 200 species reported to cause allergic contact dermatitis.12 Common modes of contact include gardening, occupational exposure, airborne pollen, and use of pediculicides and cosmetics that contain components of Asteraceae plants. Educating patients on how to minimize contact with Asteraceae plants is the most effective management strategy; topical agents and oral immunosuppressives can be used for symptomatic treatment.
- Morhardt S, Morhardt E. California Desert Flowers: An Introduction to Families, Genera, and Species. University of California Press; 2004.
- Gordon LA. Compositae dermatitis. Australas J Dermatol. 1999;40:123-130. doi:10.1046/j.1440-0960.1999.00341.x
- Denisow-Pietrzyk M, Pietrzyk Ł, Denisow B. Asteraceae species as potential environmental factors of allergy. Environ Sci Pollut Res Int. 2019;26:6290-6300. doi:10.1007/s11356-019-04146-w
- Paulsen E, Chistensen LP, Andersen KE. Cosmetics and herbal remedies with Compositae plant extracts—are they tolerated by Compositae-allergic patients? Contact Dermatitis. 2008;58:15-23. doi:10.1111/j.1600-0536.2007.01250.x
- Burry JN, Reid JG, Kirk J. Australian bush dermatitis. Contact Dermatitis. 1975;1:263-264. doi:10.1111/j.1600-0536.1975.tb05422.x
- Punchihewa N, Palmer A, Nixon R. Allergic contact dermatitis to Compositae: an Australian case series. Contact Dermatitis. 2022;87:356-362. doi:10.1111/cod.14162
- Chen KW, Marusciac L, Tamas PT, et al. Ragweed pollen allergy: burden, characteristics, and management of an imported allergen source in Europe. Int Arch Allergy Immunol. 2018;176:163-180. doi:10.1159/000487997
- Schloemer JA, Zirwas MJ, Burkhart CG. Airborne contact dermatitis: common causes in the USA. Int J Dermatol. 2015;54:271-274. doi:10.1111/ijd.12692
- Arlette J, Mitchell JC. Compositae dermatitis. current aspects. Contact Dermatitis. 1981;7:129-136. doi:10.1111/j.1600-0536.1981.tb04584.x
- Mitchell JC, Dupuis G. Allergic contact dermatitis from sesquiterpenoids of the Compositae family of plants. Br J Dermatol. 1971;84:139-150. doi:10.1111/j.1365-2133.1971.tb06857.x
- Salapovic H, Geier J, Reznicek G. Quantification of Sesquiterpene lactones in Asteraceae plant extracts: evaluation of their allergenic potential. Sci Pharm. 2013;81:807-818. doi:10.3797/scipharm.1306-17
- Paulsen E. Compositae dermatitis: a survey. Contact Dermatitis. 1992;26:76-86. doi:10.1111/j.1600-0536.1992.tb00888.x. Published correction appears in Contact Dermatitis. 1992;27:208.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Paulsen E. Contact sensitization from Compositae-containing herbal remedies and cosmetics. Contact Dermatitis. 2002;47:189-198. doi:10.1034/j.1600-0536.2002.470401.x
- Frain-Bell W, Johnson BE. Contact allergic sensitivity to plants and the photosensitivity dermatitis and actinic reticuloid syndrome. Br J Dermatol. 1979;101:503-512.
- Paulsen E, Andersen KE. Clinical patterns of Compositae dermatitis in Danish monosensitized patients. Contact Dermatitis. 2018;78:185-193. doi:10.1111/cod.12916
- Jovanovic´ M, Poljacki M. Compositae dermatitis. Med Pregl. 2003;56:43-49. doi:10.2298/mpns0302043j
- Krook G. Occupational dermatitis from Lactuca sativa (lettuce) and Cichorium (endive). simultaneous occurrence of immediate and delayed allergy as a cause of contact dermatitis. Contact Dermatitis. 1977;3:27-36. doi:10.1111/j.1600-0536.1977.tb03583.x
- Paek SY, Lim HW. Chronic actinic dermatitis. Dermatol Clin. 2014;32:355-361, viii-ix. doi:10.1016/j.det.2014.03.007
- du P Menagé H, Hawk JL, White IR. Sesquiterpene lactone mix contact sensitivity and its relationship to chronic actinic dermatitis: a follow-up study. Contact Dermatitis. 1998;39:119-122. doi:10.1111/j.1600-0536.1998.tb05859.x
- Wang CX, Belsito DV. Chronic actinic dermatitis revisited. Dermatitis. 2020;31:68-74. doi:10.1097/DER.0000000000000531
- DeLeo VA, Adler BL, Warshaw EM, et al. Photopatch test results of the North American contact dermatitis group, 1999-2009. Photodermatol Photoimmunol Photomed. 2022;38:288-291. doi:10.1111/phpp.12742
- McGovern TW, LaWarre S. Botanical briefs: the scourge of India—Parthenium hysterophorus L. Cutis. 2001;67:27-34. Published correction appears in Cutis. 2001;67:154.
- Sharma VK, Verma P, Maharaja K. Parthenium dermatitis. Photochem Photobiol Sci. 2013;12:85-94. doi:10.1039/c2pp25186h
- Verma KK, Bansal A, Sethuraman G. Parthenium dermatitis treated with azathioprine weekly pulse doses. Indian J Dermatol Venereol Leprol. 2006;72:24-27. doi:10.4103/0378-6323.19713
- Sharma VK, Bhat R, Sethuraman G, et al. Treatment of Parthenium dermatitis with methotrexate. Contact Dermatitis. 2007;57:118-119. doi:10.1111/j.1600-0536.2006.00950.x
- Burke DA, Corey G, Storrs FJ. Psoralen plus UVA protocol for Compositae photosensitivity. Am J Contact Dermat. 1996;7:171-176.
- Lovell CR. Allergic contact dermatitis due to plants. In: Plants and the Skin. Blackwell Scientific Publications; 1993:96-254.
- Dogra S, Parsad D, Handa S. Narrowband ultraviolet B in airborne contact dermatitis: a ray of hope! Br J Dermatol. 2004;150:373-374. doi:10.1111/j.1365-2133.2004.05724.x
- Lakshmi C, Srinivas CR, Jayaraman A. Ciclosporin in Parthenium dermatitis—a report of 2 cases. Contact Dermatitis. 2008;59:245-248. doi:10.1111/j.1600-0536.2007.01208.x
- Hendricks AJ, Yosipovitch G, Shi VY. Dupilumab use in dermatologic conditions beyond atopic dermatitis—a systematic review. J Dermatolog Treat. 2021;32:19-28. doi:10.1080/09546634.2019.1689227
- Napolitano M, Fabbrocini G, Patruno C. Allergic contact dermatitis to Compositae: a possible cause of dupilumab-associated facial and neck dermatitis in atopic dermatitis patients? Contact Dermatitis. 2021;85:473-474. doi:10.1111/cod.13898
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis. Contact Dermatitis. 2023;88:150-152. doi:10.1111/cod.14234
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544. doi:10.1111/cod.14204
- Morhardt S, Morhardt E. California Desert Flowers: An Introduction to Families, Genera, and Species. University of California Press; 2004.
- Gordon LA. Compositae dermatitis. Australas J Dermatol. 1999;40:123-130. doi:10.1046/j.1440-0960.1999.00341.x
- Denisow-Pietrzyk M, Pietrzyk Ł, Denisow B. Asteraceae species as potential environmental factors of allergy. Environ Sci Pollut Res Int. 2019;26:6290-6300. doi:10.1007/s11356-019-04146-w
- Paulsen E, Chistensen LP, Andersen KE. Cosmetics and herbal remedies with Compositae plant extracts—are they tolerated by Compositae-allergic patients? Contact Dermatitis. 2008;58:15-23. doi:10.1111/j.1600-0536.2007.01250.x
- Burry JN, Reid JG, Kirk J. Australian bush dermatitis. Contact Dermatitis. 1975;1:263-264. doi:10.1111/j.1600-0536.1975.tb05422.x
- Punchihewa N, Palmer A, Nixon R. Allergic contact dermatitis to Compositae: an Australian case series. Contact Dermatitis. 2022;87:356-362. doi:10.1111/cod.14162
- Chen KW, Marusciac L, Tamas PT, et al. Ragweed pollen allergy: burden, characteristics, and management of an imported allergen source in Europe. Int Arch Allergy Immunol. 2018;176:163-180. doi:10.1159/000487997
- Schloemer JA, Zirwas MJ, Burkhart CG. Airborne contact dermatitis: common causes in the USA. Int J Dermatol. 2015;54:271-274. doi:10.1111/ijd.12692
- Arlette J, Mitchell JC. Compositae dermatitis. current aspects. Contact Dermatitis. 1981;7:129-136. doi:10.1111/j.1600-0536.1981.tb04584.x
- Mitchell JC, Dupuis G. Allergic contact dermatitis from sesquiterpenoids of the Compositae family of plants. Br J Dermatol. 1971;84:139-150. doi:10.1111/j.1365-2133.1971.tb06857.x
- Salapovic H, Geier J, Reznicek G. Quantification of Sesquiterpene lactones in Asteraceae plant extracts: evaluation of their allergenic potential. Sci Pharm. 2013;81:807-818. doi:10.3797/scipharm.1306-17
- Paulsen E. Compositae dermatitis: a survey. Contact Dermatitis. 1992;26:76-86. doi:10.1111/j.1600-0536.1992.tb00888.x. Published correction appears in Contact Dermatitis. 1992;27:208.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Paulsen E. Contact sensitization from Compositae-containing herbal remedies and cosmetics. Contact Dermatitis. 2002;47:189-198. doi:10.1034/j.1600-0536.2002.470401.x
- Frain-Bell W, Johnson BE. Contact allergic sensitivity to plants and the photosensitivity dermatitis and actinic reticuloid syndrome. Br J Dermatol. 1979;101:503-512.
- Paulsen E, Andersen KE. Clinical patterns of Compositae dermatitis in Danish monosensitized patients. Contact Dermatitis. 2018;78:185-193. doi:10.1111/cod.12916
- Jovanovic´ M, Poljacki M. Compositae dermatitis. Med Pregl. 2003;56:43-49. doi:10.2298/mpns0302043j
- Krook G. Occupational dermatitis from Lactuca sativa (lettuce) and Cichorium (endive). simultaneous occurrence of immediate and delayed allergy as a cause of contact dermatitis. Contact Dermatitis. 1977;3:27-36. doi:10.1111/j.1600-0536.1977.tb03583.x
- Paek SY, Lim HW. Chronic actinic dermatitis. Dermatol Clin. 2014;32:355-361, viii-ix. doi:10.1016/j.det.2014.03.007
- du P Menagé H, Hawk JL, White IR. Sesquiterpene lactone mix contact sensitivity and its relationship to chronic actinic dermatitis: a follow-up study. Contact Dermatitis. 1998;39:119-122. doi:10.1111/j.1600-0536.1998.tb05859.x
- Wang CX, Belsito DV. Chronic actinic dermatitis revisited. Dermatitis. 2020;31:68-74. doi:10.1097/DER.0000000000000531
- DeLeo VA, Adler BL, Warshaw EM, et al. Photopatch test results of the North American contact dermatitis group, 1999-2009. Photodermatol Photoimmunol Photomed. 2022;38:288-291. doi:10.1111/phpp.12742
- McGovern TW, LaWarre S. Botanical briefs: the scourge of India—Parthenium hysterophorus L. Cutis. 2001;67:27-34. Published correction appears in Cutis. 2001;67:154.
- Sharma VK, Verma P, Maharaja K. Parthenium dermatitis. Photochem Photobiol Sci. 2013;12:85-94. doi:10.1039/c2pp25186h
- Verma KK, Bansal A, Sethuraman G. Parthenium dermatitis treated with azathioprine weekly pulse doses. Indian J Dermatol Venereol Leprol. 2006;72:24-27. doi:10.4103/0378-6323.19713
- Sharma VK, Bhat R, Sethuraman G, et al. Treatment of Parthenium dermatitis with methotrexate. Contact Dermatitis. 2007;57:118-119. doi:10.1111/j.1600-0536.2006.00950.x
- Burke DA, Corey G, Storrs FJ. Psoralen plus UVA protocol for Compositae photosensitivity. Am J Contact Dermat. 1996;7:171-176.
- Lovell CR. Allergic contact dermatitis due to plants. In: Plants and the Skin. Blackwell Scientific Publications; 1993:96-254.
- Dogra S, Parsad D, Handa S. Narrowband ultraviolet B in airborne contact dermatitis: a ray of hope! Br J Dermatol. 2004;150:373-374. doi:10.1111/j.1365-2133.2004.05724.x
- Lakshmi C, Srinivas CR, Jayaraman A. Ciclosporin in Parthenium dermatitis—a report of 2 cases. Contact Dermatitis. 2008;59:245-248. doi:10.1111/j.1600-0536.2007.01208.x
- Hendricks AJ, Yosipovitch G, Shi VY. Dupilumab use in dermatologic conditions beyond atopic dermatitis—a systematic review. J Dermatolog Treat. 2021;32:19-28. doi:10.1080/09546634.2019.1689227
- Napolitano M, Fabbrocini G, Patruno C. Allergic contact dermatitis to Compositae: a possible cause of dupilumab-associated facial and neck dermatitis in atopic dermatitis patients? Contact Dermatitis. 2021;85:473-474. doi:10.1111/cod.13898
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis. Contact Dermatitis. 2023;88:150-152. doi:10.1111/cod.14234
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544. doi:10.1111/cod.14204
Practice Points
- Asteraceae dermatitis can occur from direct contact with plants of the Asteraceae family; through airborne pollen; or from exposure to topical medications, cooking products, and cosmetics.
- Patient education on primary prevention, especially protective clothing, is crucial, as these plants are ubiquitous outdoors and have diverse phenotypes.
- Management of mild Asteraceae dermatitis consists primarily of topical corticosteroids and calcineurin inhibitors, while systemic corticosteroids and other immunosuppressive agents are utilized for severe or recalcitrant cases.
Purpuric Lesions on the Leg
THE DIAGNOSIS: Dengue Hemorrhagic Fever
The retiform purpura observed in our patient was suggestive of a vasculitic, thrombotic, or embolic etiology. Dengue IgM serologic testing performed based on her extensive travel history and recent return from a dengue-endemic area was positive, indicating acute infection. A clinical diagnosis of dengue hemorrhagic fever (DHF) was made based on the hemorrhagic appearance of the lesion. Histopathology revealed leukocytoclastic vasculitis (Figure). Anti–double-stranded DNA, antideoxyribonuclease, C3 and C4, CH50 (total hemolytic complement), antineutrophil cytoplasmic antibodies, HIV, and hepatitis B virus tests were normal. Direct immunofluorescence was negative.
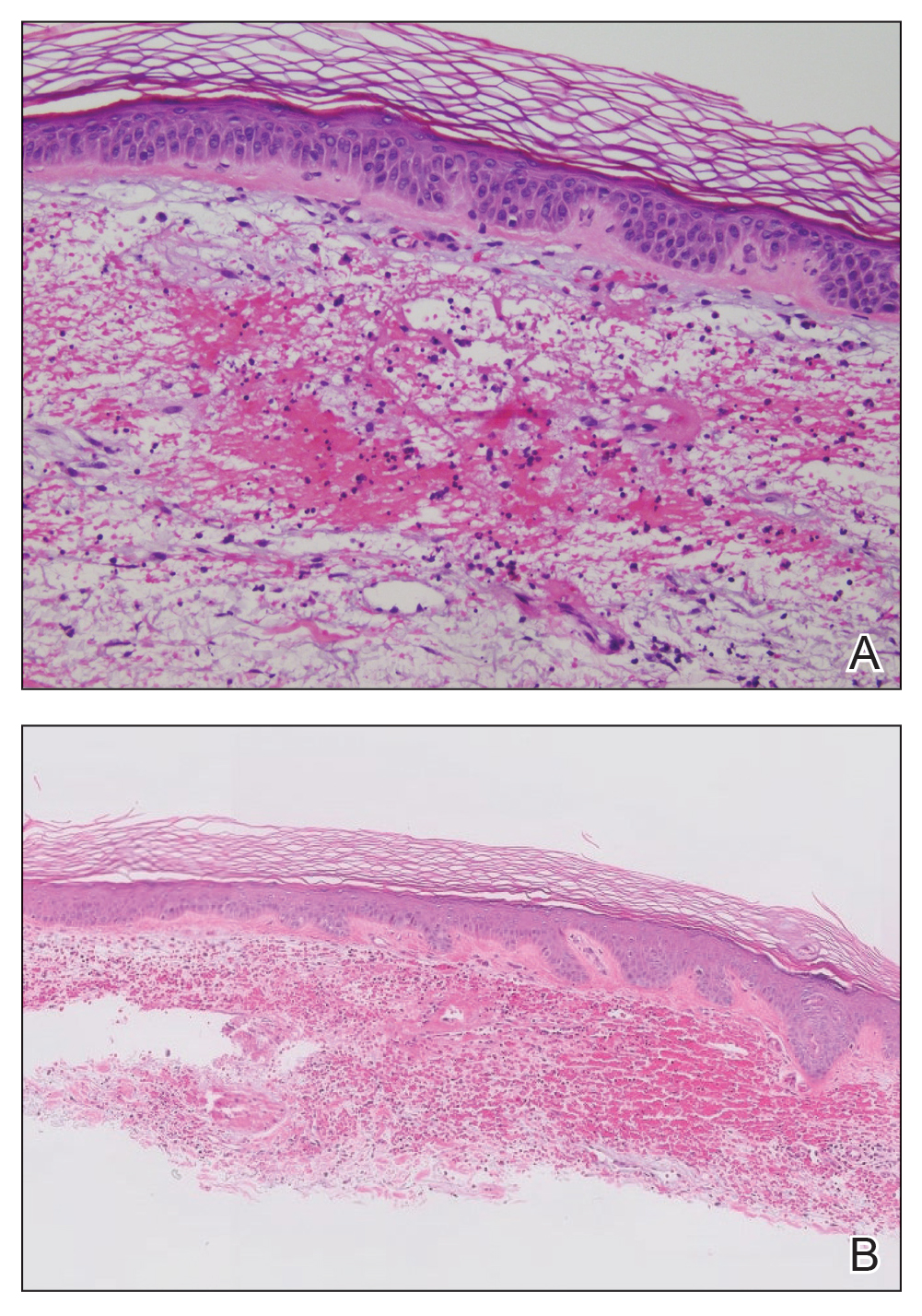
Dengue virus is a single-stranded RNA virus transmitted by Aedes aegypti and Aedes albopictus mosquitoes and is one of the most prevalent arthropod-borne viruses affecting humans today.1,2 Infection with the dengue virus generally is seen in travelers visiting tropical regions of Africa, Mexico, South America, South and Central Asia, Southeast Asia, and the Caribbean.1 The Table shows the global distribution of dengue serotypes from 2000 to 2014.3,4 There are 4 serotypes of the dengue virus: DENV-1 to DENV-4. Infection with 1 strain elicits longlasting immunity to that strain, but subsequent infection with another strain can result in severe DHF due to antibody cross-reaction.1
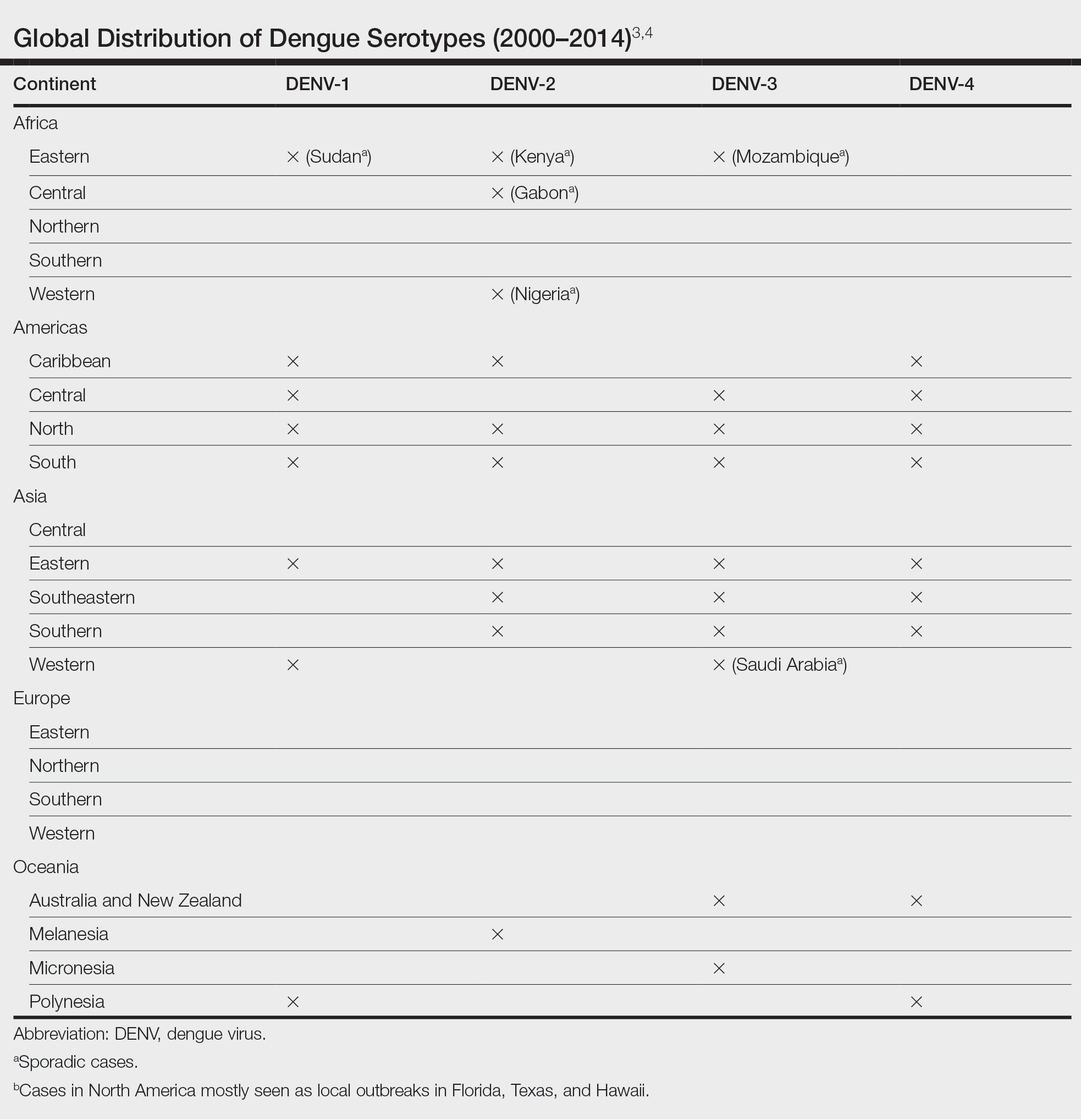
Dengue virus infection ranges from mildly symptomatic to a spectrum of increasingly severe conditions that comprise dengue fever (DF) and DHF, as well as dengue shock syndrome and brain stem hemorrhage, which may be fatal.2,5 Dengue fever manifests as severe myalgia, fever, headache (usually retro-orbital), arthralgia, erythema, and rubelliform exanthema.6 The frequency of skin eruptions in patients with DF varies with the virus strain and outbreaks.7 The lesions initially develop with the onset of fever and manifest as flushing or erythematous mottling of the face, neck, and chest areas.1,7 The morbilliform eruption develops 2 to 6 days after the onset of the fever, beginning on the trunk and spreading to the face and extremities.1,7 The rash may become confluent with characteristic sparing of small round areas of normal skin described as white islands in a sea of red.2 Verrucous papules on the ears also have been described and may resemble those seen in Cowden syndrome. In patients with prior infection with a different strain of the virus, hemorrhagic lesions may develop, including characteristic retiform purpura, a positive tourniquet test, and the appearance of petechiae on the lower legs. Pruritus and desquamation, especially on the palms and soles, may follow the termination of the eruption.7
The differential diagnosis of DF includes measles, rubella, enteroviruses, and influenza. Chikungunya and West Nile viruses in Asia and Africa and the O’nyong-nyong virus in Africa are also arboviruses that cause a clinical picture similar to DF but not DHF. Other diagnostic considerations include phases of scarlet fever, typhoid, malaria, leptospirosis, hepatitis A, and trypanosomal and rickettsial diseases.7 The differential diagnosis of DHF includes antineutrophil cytoplasmic antibody–associated vasculitis, rheumatoid vasculitis, and bacterial septic vasculitis.
Acute clinical diagnosis of DF can be challenging because of the nonspecific symptoms that can be seen in almost every infectious disease. Clinical presentation assessment should be confirmed with laboratory testing.6 Dengue virus infection usually is confirmed by the identification of viral genomic RNA, antigens, or the antibodies it elicits. Enzyme-linked immunosorbent assay–based serologic tests are cost-effective and easy to perform.5 IgM antibodies usually show cross-reactivity with platelets, but the antibody levels are not positively correlated with the severity of DF.8 Primary infection with the dengue virus is characterized by the elevation of specific IgM levels that usually occurs 3 to 5 days after symptom onset and persists during the postfebrile stage (up to 30 to 60 days). In secondary infections, the IgM levels usually rise more slowly and reach a lower level than in primary infections.9 For both primary and secondary infections, testing IgM levels after the febrile stage may be helpful with the laboratory diagnosis.
Currently, there is no antiviral drug available for dengue. Treatment of dengue infection is symptomatic and supportive.2
Dengue hemorrhagic fever is indicated by a rising hematocrit (≥20%) and a falling platelet count (>100,000/mm3) accompanying clinical signs of hemorrhage. Treatment includes intravenous fluid replacement and careful clinical monitoring of hematocrit levels, platelet count, vitals, urine output, and other signs of shock.5 For patients with a history of dengue infection, travel to areas with other serotypes is not recommended.
If any travel to a high-risk area is planned, countryspecific travel recommendations and warnings should be reviewed from the Centers for Disease Control and Prevention’s website (https://wwwnc.cdc.gov/travel/notices/level1/dengue-global). Use of an Environmental Protection Agency–registered insect repellent to avoid mosquito bites and acetaminophen for managing symptoms is advised. During travel, staying in places with window and door screens and using a bed net during sleep are suggested. Long-sleeved shirts and long pants also are preferred. Travelers should see a health care provider if they have symptoms of dengue.10
African tick bite fever (ATBF) is caused by Rickettsia africae transmitted by Amblyomma ticks. Skin findings in ATBF include erythematous, firm, tender papules with central eschars consistent with the feeding patterns of ticks.11 Histopathology of ATBF usually includes fibrinoid necrosis of vessels in the dermis with a perivascular inflammatory infiltrate and coagulation necrosis of the surrounding dermis consistent with eschar formation.12 The lack of an eschar weighs against this diagnosis.
African trypanosomiasis (also known as sleeping sickness) is caused by protozoa transmitted by the tsetse fly. A chancrelike, circumscribed, rubbery, indurated red or violaceous nodule measuring 2 to 5 cm in diameter often develops as the earliest cutaneous sign of the disease.13 Nonspecific histopathologic findings, such as infiltration of lymphocytes and macrophages and proliferation of endothelial cells and fibroblasts, may be observed.14 Extravascular parasites have been noted in skin biopsies.15 In later stages, skin lesions called trypanids may be observed as macular, papular, annular, targetoid, purpuric, and erythematous lesions, and histopathologic findings consistent with vasculitis also may be seen.13
Chikungunya virus infection is an acute-onset, mosquito-borne viral disease. Skin manifestations may start with nonspecific, generalized, morbilliform, maculopapular rashes coinciding with fever, which also may be seen initially with DHF. Skin hyperpigmentation, mostly centrofacial and involving the nose (chik sign); purpuric and ecchymotic lesions over the trunk and flexors of limbs in adults, often surmounted by subepidermal bullae and lesions resembling toxic epidermal necrolysis; and nonhealing ulcers in the genital and groin areas are common skin manifestations of chikungunya infection.16 Intraepithelial splitting with acantholysis and perivascular lymphohistiocytic infiltration may be observed in the histopathology of blistering lesions, which are not consistent with DHF.17
Zika virus infection is caused by an arbovirus within the Flaviviridae family, which also includes the dengue virus. Initial mucocutaneous findings of the Zika virus include nonspecific diffuse maculopapular eruptions. The eruption generally spares the palms and soles; however, various manifestations including involvement of the palms and soles have been reported.18 The morbilliform eruption begins on the face and extends to the trunk and extremities. Mild hemorrhagic manifestations, including petechiae and bleeding gums, may be observed. Distinguishing between dengue and Zika virus infection relies on the severity of symptoms and laboratory tests, including polymerase chain reaction or IgM antibody testing.19 The other conditions listed do not produce hemorrhagic fever.
- Pincus LB, Grossman ME, Fox LP. The exanthem of dengue fever: clinical features of two US tourists traveling abroad. J Am Acad Dermatol. 2008;58:308-316. doi:10.1016/j.jaad.2007.08.042
- Radakovic-Fijan S, Graninger W, Müller C, et al. Dengue hemorrhagic fever in a British travel guide. J Am Acad Dermatol. 2002;46:430-433. doi:10.1067/mjd.2002.111904
- Yamashita A, Sakamoto T, Sekizuka T, et al. DGV: dengue genographic viewer. Front Microbiol. 2016;7:875. doi:10.3389/fmicb.2016.00875
- Centers for Disease and Prevention. Dengue in the US states and territories. Updated October 7, 2020. Accessed September 30, 2024. https://www.cdc.gov/dengue/data-research/facts-stats/?CDC_AAref_Val=https://www.cdc.gov/dengue/areaswithrisk/in-the-us.html
- Khetarpal N, Khanna I. Dengue fever: causes, complications, and vaccine strategies. J Immunol Res. 2016;2016:6803098. doi:10.1155/2016/6803098
- Muller DA, Depelsenaire AC, Young PR. Clinical and laboratory diagnosis of dengue virus infection. J Infect Dis. 2017;215(suppl 2):S89-S95. doi:10.1093/infdis/jiw649
- Waterman SH, Gubler DJ. Dengue fever. Clin Dermatol. 1989;7:117-122. doi:10.1016/0738-081x(89)90034-5
- Lin CF, Lei HY, Liu CC, et al. Generation of IgM anti-platelet autoantibody in dengue patients. J Med Virol. 2001;63:143-149. doi:10.1002/1096- 9071(20000201)63:2<143::AID-JMV1009>3.0.CO;2-L
- Tripathi NK, Shrivastava A, Dash PK, et al. Detection of dengue virus. Methods Mol Biol. 2011;665:51-64. doi:10.1007/978-1-60761-817-1_4
- Centers for Disease Control and Prevention. Plan for travel. Accessed September 30, 2024. https://wwwnc.cdc.gov/travel
- Mack I, Ritz N. African tick-bite fever. N Engl J Med. 2019;380:960. doi:10.1056/NEJMicm1810093
- Lepidi H, Fournier PE, Raoult D. Histologic features and immunodetection of African tick-bite fever eschar. Emerg Infect Dis. 2006;12:1332- 1337. doi:10.3201/eid1209.051540
- McGovern TW, Williams W, Fitzpatrick JE, et al. Cutaneous manifestations of African trypanosomiasis. Arch Dermatol. 1995;131:1178-1182.
- Kristensson K, Bentivoglio M. Pathology of African trypanosomiasis. In: Dumas M, Bouteille B, Buguet A, eds. Progress in Human African Trypanosomiasis, Sleeping Sickness. Springer; 1999:157-181.
- Capewell P, Cren-Travaillé C, Marchesi F, et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. Elife. 2016;5:e17716. doi:10.7554/eLife.17716
- Singal A. Chikungunya and skin: current perspective. Indian Dermatol Online J. 2017;8:307-309. doi:10.4103/idoj.IDOJ_93_17
- Robin S, Ramful D, Zettor J, et al. Severe bullous skin lesions associated with chikungunya virus infection in small infants. Eur J Pediatr. 2009;169:67-72. doi:10.1007/s00431-009-0986-0
- Hussain A, Ali F, Latiwesh OB, et al. A comprehensive review of the manifestations and pathogenesis of Zika virus in neonates and adults. Cureus. 2018;10:E3290. doi:10.7759/cureus.3290
- Farahnik B, Beroukhim K, Blattner CM, et al. Cutaneous manifestations of the Zika virus. J Am Acad Dermatol. 2016;74:1286-1287. doi:10.1016/j.jaad.2016.02.1232
THE DIAGNOSIS: Dengue Hemorrhagic Fever
The retiform purpura observed in our patient was suggestive of a vasculitic, thrombotic, or embolic etiology. Dengue IgM serologic testing performed based on her extensive travel history and recent return from a dengue-endemic area was positive, indicating acute infection. A clinical diagnosis of dengue hemorrhagic fever (DHF) was made based on the hemorrhagic appearance of the lesion. Histopathology revealed leukocytoclastic vasculitis (Figure). Anti–double-stranded DNA, antideoxyribonuclease, C3 and C4, CH50 (total hemolytic complement), antineutrophil cytoplasmic antibodies, HIV, and hepatitis B virus tests were normal. Direct immunofluorescence was negative.

Dengue virus is a single-stranded RNA virus transmitted by Aedes aegypti and Aedes albopictus mosquitoes and is one of the most prevalent arthropod-borne viruses affecting humans today.1,2 Infection with the dengue virus generally is seen in travelers visiting tropical regions of Africa, Mexico, South America, South and Central Asia, Southeast Asia, and the Caribbean.1 The Table shows the global distribution of dengue serotypes from 2000 to 2014.3,4 There are 4 serotypes of the dengue virus: DENV-1 to DENV-4. Infection with 1 strain elicits longlasting immunity to that strain, but subsequent infection with another strain can result in severe DHF due to antibody cross-reaction.1

Dengue virus infection ranges from mildly symptomatic to a spectrum of increasingly severe conditions that comprise dengue fever (DF) and DHF, as well as dengue shock syndrome and brain stem hemorrhage, which may be fatal.2,5 Dengue fever manifests as severe myalgia, fever, headache (usually retro-orbital), arthralgia, erythema, and rubelliform exanthema.6 The frequency of skin eruptions in patients with DF varies with the virus strain and outbreaks.7 The lesions initially develop with the onset of fever and manifest as flushing or erythematous mottling of the face, neck, and chest areas.1,7 The morbilliform eruption develops 2 to 6 days after the onset of the fever, beginning on the trunk and spreading to the face and extremities.1,7 The rash may become confluent with characteristic sparing of small round areas of normal skin described as white islands in a sea of red.2 Verrucous papules on the ears also have been described and may resemble those seen in Cowden syndrome. In patients with prior infection with a different strain of the virus, hemorrhagic lesions may develop, including characteristic retiform purpura, a positive tourniquet test, and the appearance of petechiae on the lower legs. Pruritus and desquamation, especially on the palms and soles, may follow the termination of the eruption.7
The differential diagnosis of DF includes measles, rubella, enteroviruses, and influenza. Chikungunya and West Nile viruses in Asia and Africa and the O’nyong-nyong virus in Africa are also arboviruses that cause a clinical picture similar to DF but not DHF. Other diagnostic considerations include phases of scarlet fever, typhoid, malaria, leptospirosis, hepatitis A, and trypanosomal and rickettsial diseases.7 The differential diagnosis of DHF includes antineutrophil cytoplasmic antibody–associated vasculitis, rheumatoid vasculitis, and bacterial septic vasculitis.
Acute clinical diagnosis of DF can be challenging because of the nonspecific symptoms that can be seen in almost every infectious disease. Clinical presentation assessment should be confirmed with laboratory testing.6 Dengue virus infection usually is confirmed by the identification of viral genomic RNA, antigens, or the antibodies it elicits. Enzyme-linked immunosorbent assay–based serologic tests are cost-effective and easy to perform.5 IgM antibodies usually show cross-reactivity with platelets, but the antibody levels are not positively correlated with the severity of DF.8 Primary infection with the dengue virus is characterized by the elevation of specific IgM levels that usually occurs 3 to 5 days after symptom onset and persists during the postfebrile stage (up to 30 to 60 days). In secondary infections, the IgM levels usually rise more slowly and reach a lower level than in primary infections.9 For both primary and secondary infections, testing IgM levels after the febrile stage may be helpful with the laboratory diagnosis.
Currently, there is no antiviral drug available for dengue. Treatment of dengue infection is symptomatic and supportive.2
Dengue hemorrhagic fever is indicated by a rising hematocrit (≥20%) and a falling platelet count (>100,000/mm3) accompanying clinical signs of hemorrhage. Treatment includes intravenous fluid replacement and careful clinical monitoring of hematocrit levels, platelet count, vitals, urine output, and other signs of shock.5 For patients with a history of dengue infection, travel to areas with other serotypes is not recommended.
If any travel to a high-risk area is planned, countryspecific travel recommendations and warnings should be reviewed from the Centers for Disease Control and Prevention’s website (https://wwwnc.cdc.gov/travel/notices/level1/dengue-global). Use of an Environmental Protection Agency–registered insect repellent to avoid mosquito bites and acetaminophen for managing symptoms is advised. During travel, staying in places with window and door screens and using a bed net during sleep are suggested. Long-sleeved shirts and long pants also are preferred. Travelers should see a health care provider if they have symptoms of dengue.10
African tick bite fever (ATBF) is caused by Rickettsia africae transmitted by Amblyomma ticks. Skin findings in ATBF include erythematous, firm, tender papules with central eschars consistent with the feeding patterns of ticks.11 Histopathology of ATBF usually includes fibrinoid necrosis of vessels in the dermis with a perivascular inflammatory infiltrate and coagulation necrosis of the surrounding dermis consistent with eschar formation.12 The lack of an eschar weighs against this diagnosis.
African trypanosomiasis (also known as sleeping sickness) is caused by protozoa transmitted by the tsetse fly. A chancrelike, circumscribed, rubbery, indurated red or violaceous nodule measuring 2 to 5 cm in diameter often develops as the earliest cutaneous sign of the disease.13 Nonspecific histopathologic findings, such as infiltration of lymphocytes and macrophages and proliferation of endothelial cells and fibroblasts, may be observed.14 Extravascular parasites have been noted in skin biopsies.15 In later stages, skin lesions called trypanids may be observed as macular, papular, annular, targetoid, purpuric, and erythematous lesions, and histopathologic findings consistent with vasculitis also may be seen.13
Chikungunya virus infection is an acute-onset, mosquito-borne viral disease. Skin manifestations may start with nonspecific, generalized, morbilliform, maculopapular rashes coinciding with fever, which also may be seen initially with DHF. Skin hyperpigmentation, mostly centrofacial and involving the nose (chik sign); purpuric and ecchymotic lesions over the trunk and flexors of limbs in adults, often surmounted by subepidermal bullae and lesions resembling toxic epidermal necrolysis; and nonhealing ulcers in the genital and groin areas are common skin manifestations of chikungunya infection.16 Intraepithelial splitting with acantholysis and perivascular lymphohistiocytic infiltration may be observed in the histopathology of blistering lesions, which are not consistent with DHF.17
Zika virus infection is caused by an arbovirus within the Flaviviridae family, which also includes the dengue virus. Initial mucocutaneous findings of the Zika virus include nonspecific diffuse maculopapular eruptions. The eruption generally spares the palms and soles; however, various manifestations including involvement of the palms and soles have been reported.18 The morbilliform eruption begins on the face and extends to the trunk and extremities. Mild hemorrhagic manifestations, including petechiae and bleeding gums, may be observed. Distinguishing between dengue and Zika virus infection relies on the severity of symptoms and laboratory tests, including polymerase chain reaction or IgM antibody testing.19 The other conditions listed do not produce hemorrhagic fever.
THE DIAGNOSIS: Dengue Hemorrhagic Fever
The retiform purpura observed in our patient was suggestive of a vasculitic, thrombotic, or embolic etiology. Dengue IgM serologic testing performed based on her extensive travel history and recent return from a dengue-endemic area was positive, indicating acute infection. A clinical diagnosis of dengue hemorrhagic fever (DHF) was made based on the hemorrhagic appearance of the lesion. Histopathology revealed leukocytoclastic vasculitis (Figure). Anti–double-stranded DNA, antideoxyribonuclease, C3 and C4, CH50 (total hemolytic complement), antineutrophil cytoplasmic antibodies, HIV, and hepatitis B virus tests were normal. Direct immunofluorescence was negative.

Dengue virus is a single-stranded RNA virus transmitted by Aedes aegypti and Aedes albopictus mosquitoes and is one of the most prevalent arthropod-borne viruses affecting humans today.1,2 Infection with the dengue virus generally is seen in travelers visiting tropical regions of Africa, Mexico, South America, South and Central Asia, Southeast Asia, and the Caribbean.1 The Table shows the global distribution of dengue serotypes from 2000 to 2014.3,4 There are 4 serotypes of the dengue virus: DENV-1 to DENV-4. Infection with 1 strain elicits longlasting immunity to that strain, but subsequent infection with another strain can result in severe DHF due to antibody cross-reaction.1

Dengue virus infection ranges from mildly symptomatic to a spectrum of increasingly severe conditions that comprise dengue fever (DF) and DHF, as well as dengue shock syndrome and brain stem hemorrhage, which may be fatal.2,5 Dengue fever manifests as severe myalgia, fever, headache (usually retro-orbital), arthralgia, erythema, and rubelliform exanthema.6 The frequency of skin eruptions in patients with DF varies with the virus strain and outbreaks.7 The lesions initially develop with the onset of fever and manifest as flushing or erythematous mottling of the face, neck, and chest areas.1,7 The morbilliform eruption develops 2 to 6 days after the onset of the fever, beginning on the trunk and spreading to the face and extremities.1,7 The rash may become confluent with characteristic sparing of small round areas of normal skin described as white islands in a sea of red.2 Verrucous papules on the ears also have been described and may resemble those seen in Cowden syndrome. In patients with prior infection with a different strain of the virus, hemorrhagic lesions may develop, including characteristic retiform purpura, a positive tourniquet test, and the appearance of petechiae on the lower legs. Pruritus and desquamation, especially on the palms and soles, may follow the termination of the eruption.7
The differential diagnosis of DF includes measles, rubella, enteroviruses, and influenza. Chikungunya and West Nile viruses in Asia and Africa and the O’nyong-nyong virus in Africa are also arboviruses that cause a clinical picture similar to DF but not DHF. Other diagnostic considerations include phases of scarlet fever, typhoid, malaria, leptospirosis, hepatitis A, and trypanosomal and rickettsial diseases.7 The differential diagnosis of DHF includes antineutrophil cytoplasmic antibody–associated vasculitis, rheumatoid vasculitis, and bacterial septic vasculitis.
Acute clinical diagnosis of DF can be challenging because of the nonspecific symptoms that can be seen in almost every infectious disease. Clinical presentation assessment should be confirmed with laboratory testing.6 Dengue virus infection usually is confirmed by the identification of viral genomic RNA, antigens, or the antibodies it elicits. Enzyme-linked immunosorbent assay–based serologic tests are cost-effective and easy to perform.5 IgM antibodies usually show cross-reactivity with platelets, but the antibody levels are not positively correlated with the severity of DF.8 Primary infection with the dengue virus is characterized by the elevation of specific IgM levels that usually occurs 3 to 5 days after symptom onset and persists during the postfebrile stage (up to 30 to 60 days). In secondary infections, the IgM levels usually rise more slowly and reach a lower level than in primary infections.9 For both primary and secondary infections, testing IgM levels after the febrile stage may be helpful with the laboratory diagnosis.
Currently, there is no antiviral drug available for dengue. Treatment of dengue infection is symptomatic and supportive.2
Dengue hemorrhagic fever is indicated by a rising hematocrit (≥20%) and a falling platelet count (>100,000/mm3) accompanying clinical signs of hemorrhage. Treatment includes intravenous fluid replacement and careful clinical monitoring of hematocrit levels, platelet count, vitals, urine output, and other signs of shock.5 For patients with a history of dengue infection, travel to areas with other serotypes is not recommended.
If any travel to a high-risk area is planned, countryspecific travel recommendations and warnings should be reviewed from the Centers for Disease Control and Prevention’s website (https://wwwnc.cdc.gov/travel/notices/level1/dengue-global). Use of an Environmental Protection Agency–registered insect repellent to avoid mosquito bites and acetaminophen for managing symptoms is advised. During travel, staying in places with window and door screens and using a bed net during sleep are suggested. Long-sleeved shirts and long pants also are preferred. Travelers should see a health care provider if they have symptoms of dengue.10
African tick bite fever (ATBF) is caused by Rickettsia africae transmitted by Amblyomma ticks. Skin findings in ATBF include erythematous, firm, tender papules with central eschars consistent with the feeding patterns of ticks.11 Histopathology of ATBF usually includes fibrinoid necrosis of vessels in the dermis with a perivascular inflammatory infiltrate and coagulation necrosis of the surrounding dermis consistent with eschar formation.12 The lack of an eschar weighs against this diagnosis.
African trypanosomiasis (also known as sleeping sickness) is caused by protozoa transmitted by the tsetse fly. A chancrelike, circumscribed, rubbery, indurated red or violaceous nodule measuring 2 to 5 cm in diameter often develops as the earliest cutaneous sign of the disease.13 Nonspecific histopathologic findings, such as infiltration of lymphocytes and macrophages and proliferation of endothelial cells and fibroblasts, may be observed.14 Extravascular parasites have been noted in skin biopsies.15 In later stages, skin lesions called trypanids may be observed as macular, papular, annular, targetoid, purpuric, and erythematous lesions, and histopathologic findings consistent with vasculitis also may be seen.13
Chikungunya virus infection is an acute-onset, mosquito-borne viral disease. Skin manifestations may start with nonspecific, generalized, morbilliform, maculopapular rashes coinciding with fever, which also may be seen initially with DHF. Skin hyperpigmentation, mostly centrofacial and involving the nose (chik sign); purpuric and ecchymotic lesions over the trunk and flexors of limbs in adults, often surmounted by subepidermal bullae and lesions resembling toxic epidermal necrolysis; and nonhealing ulcers in the genital and groin areas are common skin manifestations of chikungunya infection.16 Intraepithelial splitting with acantholysis and perivascular lymphohistiocytic infiltration may be observed in the histopathology of blistering lesions, which are not consistent with DHF.17
Zika virus infection is caused by an arbovirus within the Flaviviridae family, which also includes the dengue virus. Initial mucocutaneous findings of the Zika virus include nonspecific diffuse maculopapular eruptions. The eruption generally spares the palms and soles; however, various manifestations including involvement of the palms and soles have been reported.18 The morbilliform eruption begins on the face and extends to the trunk and extremities. Mild hemorrhagic manifestations, including petechiae and bleeding gums, may be observed. Distinguishing between dengue and Zika virus infection relies on the severity of symptoms and laboratory tests, including polymerase chain reaction or IgM antibody testing.19 The other conditions listed do not produce hemorrhagic fever.
- Pincus LB, Grossman ME, Fox LP. The exanthem of dengue fever: clinical features of two US tourists traveling abroad. J Am Acad Dermatol. 2008;58:308-316. doi:10.1016/j.jaad.2007.08.042
- Radakovic-Fijan S, Graninger W, Müller C, et al. Dengue hemorrhagic fever in a British travel guide. J Am Acad Dermatol. 2002;46:430-433. doi:10.1067/mjd.2002.111904
- Yamashita A, Sakamoto T, Sekizuka T, et al. DGV: dengue genographic viewer. Front Microbiol. 2016;7:875. doi:10.3389/fmicb.2016.00875
- Centers for Disease and Prevention. Dengue in the US states and territories. Updated October 7, 2020. Accessed September 30, 2024. https://www.cdc.gov/dengue/data-research/facts-stats/?CDC_AAref_Val=https://www.cdc.gov/dengue/areaswithrisk/in-the-us.html
- Khetarpal N, Khanna I. Dengue fever: causes, complications, and vaccine strategies. J Immunol Res. 2016;2016:6803098. doi:10.1155/2016/6803098
- Muller DA, Depelsenaire AC, Young PR. Clinical and laboratory diagnosis of dengue virus infection. J Infect Dis. 2017;215(suppl 2):S89-S95. doi:10.1093/infdis/jiw649
- Waterman SH, Gubler DJ. Dengue fever. Clin Dermatol. 1989;7:117-122. doi:10.1016/0738-081x(89)90034-5
- Lin CF, Lei HY, Liu CC, et al. Generation of IgM anti-platelet autoantibody in dengue patients. J Med Virol. 2001;63:143-149. doi:10.1002/1096- 9071(20000201)63:2<143::AID-JMV1009>3.0.CO;2-L
- Tripathi NK, Shrivastava A, Dash PK, et al. Detection of dengue virus. Methods Mol Biol. 2011;665:51-64. doi:10.1007/978-1-60761-817-1_4
- Centers for Disease Control and Prevention. Plan for travel. Accessed September 30, 2024. https://wwwnc.cdc.gov/travel
- Mack I, Ritz N. African tick-bite fever. N Engl J Med. 2019;380:960. doi:10.1056/NEJMicm1810093
- Lepidi H, Fournier PE, Raoult D. Histologic features and immunodetection of African tick-bite fever eschar. Emerg Infect Dis. 2006;12:1332- 1337. doi:10.3201/eid1209.051540
- McGovern TW, Williams W, Fitzpatrick JE, et al. Cutaneous manifestations of African trypanosomiasis. Arch Dermatol. 1995;131:1178-1182.
- Kristensson K, Bentivoglio M. Pathology of African trypanosomiasis. In: Dumas M, Bouteille B, Buguet A, eds. Progress in Human African Trypanosomiasis, Sleeping Sickness. Springer; 1999:157-181.
- Capewell P, Cren-Travaillé C, Marchesi F, et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. Elife. 2016;5:e17716. doi:10.7554/eLife.17716
- Singal A. Chikungunya and skin: current perspective. Indian Dermatol Online J. 2017;8:307-309. doi:10.4103/idoj.IDOJ_93_17
- Robin S, Ramful D, Zettor J, et al. Severe bullous skin lesions associated with chikungunya virus infection in small infants. Eur J Pediatr. 2009;169:67-72. doi:10.1007/s00431-009-0986-0
- Hussain A, Ali F, Latiwesh OB, et al. A comprehensive review of the manifestations and pathogenesis of Zika virus in neonates and adults. Cureus. 2018;10:E3290. doi:10.7759/cureus.3290
- Farahnik B, Beroukhim K, Blattner CM, et al. Cutaneous manifestations of the Zika virus. J Am Acad Dermatol. 2016;74:1286-1287. doi:10.1016/j.jaad.2016.02.1232
- Pincus LB, Grossman ME, Fox LP. The exanthem of dengue fever: clinical features of two US tourists traveling abroad. J Am Acad Dermatol. 2008;58:308-316. doi:10.1016/j.jaad.2007.08.042
- Radakovic-Fijan S, Graninger W, Müller C, et al. Dengue hemorrhagic fever in a British travel guide. J Am Acad Dermatol. 2002;46:430-433. doi:10.1067/mjd.2002.111904
- Yamashita A, Sakamoto T, Sekizuka T, et al. DGV: dengue genographic viewer. Front Microbiol. 2016;7:875. doi:10.3389/fmicb.2016.00875
- Centers for Disease and Prevention. Dengue in the US states and territories. Updated October 7, 2020. Accessed September 30, 2024. https://www.cdc.gov/dengue/data-research/facts-stats/?CDC_AAref_Val=https://www.cdc.gov/dengue/areaswithrisk/in-the-us.html
- Khetarpal N, Khanna I. Dengue fever: causes, complications, and vaccine strategies. J Immunol Res. 2016;2016:6803098. doi:10.1155/2016/6803098
- Muller DA, Depelsenaire AC, Young PR. Clinical and laboratory diagnosis of dengue virus infection. J Infect Dis. 2017;215(suppl 2):S89-S95. doi:10.1093/infdis/jiw649
- Waterman SH, Gubler DJ. Dengue fever. Clin Dermatol. 1989;7:117-122. doi:10.1016/0738-081x(89)90034-5
- Lin CF, Lei HY, Liu CC, et al. Generation of IgM anti-platelet autoantibody in dengue patients. J Med Virol. 2001;63:143-149. doi:10.1002/1096- 9071(20000201)63:2<143::AID-JMV1009>3.0.CO;2-L
- Tripathi NK, Shrivastava A, Dash PK, et al. Detection of dengue virus. Methods Mol Biol. 2011;665:51-64. doi:10.1007/978-1-60761-817-1_4
- Centers for Disease Control and Prevention. Plan for travel. Accessed September 30, 2024. https://wwwnc.cdc.gov/travel
- Mack I, Ritz N. African tick-bite fever. N Engl J Med. 2019;380:960. doi:10.1056/NEJMicm1810093
- Lepidi H, Fournier PE, Raoult D. Histologic features and immunodetection of African tick-bite fever eschar. Emerg Infect Dis. 2006;12:1332- 1337. doi:10.3201/eid1209.051540
- McGovern TW, Williams W, Fitzpatrick JE, et al. Cutaneous manifestations of African trypanosomiasis. Arch Dermatol. 1995;131:1178-1182.
- Kristensson K, Bentivoglio M. Pathology of African trypanosomiasis. In: Dumas M, Bouteille B, Buguet A, eds. Progress in Human African Trypanosomiasis, Sleeping Sickness. Springer; 1999:157-181.
- Capewell P, Cren-Travaillé C, Marchesi F, et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. Elife. 2016;5:e17716. doi:10.7554/eLife.17716
- Singal A. Chikungunya and skin: current perspective. Indian Dermatol Online J. 2017;8:307-309. doi:10.4103/idoj.IDOJ_93_17
- Robin S, Ramful D, Zettor J, et al. Severe bullous skin lesions associated with chikungunya virus infection in small infants. Eur J Pediatr. 2009;169:67-72. doi:10.1007/s00431-009-0986-0
- Hussain A, Ali F, Latiwesh OB, et al. A comprehensive review of the manifestations and pathogenesis of Zika virus in neonates and adults. Cureus. 2018;10:E3290. doi:10.7759/cureus.3290
- Farahnik B, Beroukhim K, Blattner CM, et al. Cutaneous manifestations of the Zika virus. J Am Acad Dermatol. 2016;74:1286-1287. doi:10.1016/j.jaad.2016.02.1232
A 74-year-old woman who frequently traveled abroad presented to the dermatology department with retiform purpura of the lower leg along with gastrointestinal cramps, fatigue, and myalgia. The patient reported that the symptoms had started 10 days after returning from a recent trip to Africa.
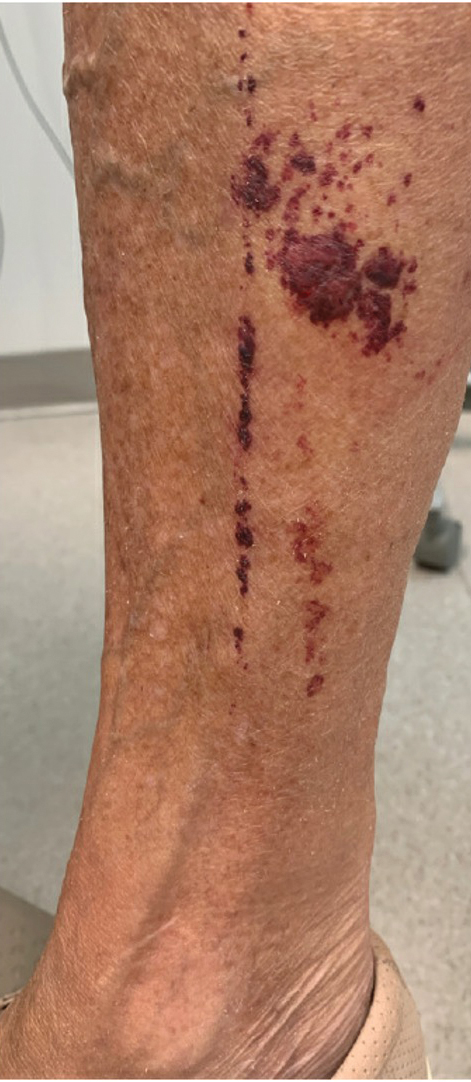
Multiple Draining Sinus Tracts on the Thigh
The Diagnosis: Mycobacterial Infection
An injury sustained in a wet environment that results in chronic indolent abscesses, nodules, or draining sinus tracts suggests a mycobacterial infection. In our patient, a culture revealed MycobacteriuM fortuitum, which is classified in the rapid grower nontuberculous mycobacteria (NTM) group, along with Mycobacterium chelonae and Mycobacterium abscessus.1 The patient’s history of skin injury while cutting wet grass and the common presence of M fortuitum in the environment suggested that the organism entered the wound. The patient healed completely following surgical excision and a 2-month course of clarithromycin 1 g daily and rifampin 600 mg daily.
MycobacteriuM fortuitum was first isolated from an amphibian source in 1905 and later identified in a human with cutaneous infection in 1938. It commonly is found in soil and water.2 Skin and soft-tissue infections with M fortuitum usually are acquired from direct entry of the organism through a damaged skin barrier from trauma, medical injection, surgery, or tattoo placement.2,3
Skin lesions caused by NTM often are nonspecific and can mimic a variety of other dermatologic conditions, making clinical diagnosis challenging. As such, cutaneous manifestations of M fortuitum infection can include recurrent cutaneous abscesses, nodular lesions, chronic discharging sinuses, cellulitis, and surgical site infections.4 Although cutaneous infection with M fortuitum classically manifests with a single subcutaneous nodule at the site of trauma or surgery,5 it also can manifest as multiple draining sinus tracts, as seen in our patient. Hence, the diagnosis and treatment of cutaneous NTM infection is challenging, especially when M fortuitum skin manifestations can take up to 4 to 6 weeks to develop after inoculation. Diagnosis often requires a detailed patient history, tissue cultures, and histopathology.5
In recent years, rapid detection with polymerase chain reaction (PCR) techniques has been employed more widely. Notably, a molecular system based on multiplex real-time PCR with high-resolution melting was shown to have a sensitivity of up to 54% for distinguishing M fortuitum from other NTM.6 More recently, a 2-step real-time PCR method has demonstrated diagnostic sensitivity and specificity for differentiating NTM from Mycobacterium tuberculosis infections and identifying the causative NTM agent.7
Compared to immunocompetent individuals, those who are immunocompromised are more susceptible to less pathogenic strains of NTM, which can cause dissemination and lead to tenosynovitis, myositis, osteomyelitis, and septic arthritis.8-12 Nonetheless, cases of infections with NTM—including M fortuitum—are becoming harder to treat. Several single nucleotide polymorphisms and point mutations have been demonstrated in the ribosomal RNA methylase gene erm(39) related to clarithromycin resistance and in the rrl gene related to linezolid resistance.13 Due to increasing inducible resistance to common classes of antibiotics, such as macrolides and linezolid, treatment of M fortuitum requires multidrug regimens.13,14 Drug susceptibility testing also may be required, as M fortuitum has shown low resistance to tigecycline, tetracycline, cefmetazole, imipenem, and aminoglycosides (eg, amikacin, tobramycin, neomycin, gentamycin). Surgery is an important adjunctive tool in treating M fortuitum infections; patients with a single lesion are more likely to undergo surgical treatment alone or in combination with antibiotic therapy.15 More recently, antimicrobial photodynamic therapy has been explored as an alternative to eliminate NTM, including M fortuitum.16
The differential diagnosis for skin lesions manifesting with draining fistulae and sinus tracts includes conditions with infectious (cellulitis and chromomycosis) and inflammatory (pyoderma gangrenosum [PG] and hidradenitis suppurativa [HS]) causes.
Cellulitis is a common infection of the skin and subcutaneous tissue that predominantly is caused by gram-positive organisms such as β-hemolytic streptococci.17 Clinical manifestations include acute skin erythema, swelling, tenderness, and warmth. The legs are the most common sites of infection, but any area of the skin can be involved.17 Cellulitis comprises 10% of all infectious disease hospitalizations and up to 11% of all dermatologic admissions.18,19 It frequently is misdiagnosed, perhaps due to the lack of a reliable confirmatory laboratory test or imaging study, in addition to the plethora of diseases that mimic cellulitis, such as stasis dermatitis, lipodermatosclerosis, contact dermatitis, lymphedema, eosinophilic cellulitis, and papular urticaria.20,21 The consequences of misdiagnosis include but are not limited to unnecessary hospitalizations, inappropriate antibiotic use, and delayed management of the disease; thus, there is an urgent need for a reliable standard test to confirm the diagnosis, especially among nonspecialist physicians. 20 Most patients with uncomplicated cellulitis can be treated with empiric oral antibiotics that target β-hemolytic streptococci (ie, penicillin V potassium, amoxicillin).17 Methicillin-resistant Staphylococcus aureus coverage generally is unnecessary for nonpurulent cellulitis, but clinicians can consider adding amoxicillin-clavulanate, dicloxacillin, and cephalexin to the regimen. For purulent cellulitis, incision and drainage should be performed. In severe cases that manifest with sepsis, altered mental status, or hemodynamic instability, inpatient management is required.17
Chromomycosis (also known as chromoblastomycosis) is a chronic, indolent, granulomatous, suppurative mycosis of the skin and subcutaneous tissue22 that is caused by traumatic inoculation of various fungi of the order Chaetothyriales and family Herpotrichiellaceae, which are present in soil, plants, and decomposing wood. Chromomycosis is prevalent in tropical and subtropical regions.23,24 Clinically, it manifests as oligosymptomatic or asymptomatic lesions around an infection site that can manifest as papules with centrifugal growth evolving into nodular, verrucous, plaque, tumoral, or atrophic forms.22 Diagnosis is made with direct microscopy using potassium hydroxide, which reveals muriform bodies. Fungal culture in Sabouraud agar also can be used to isolate the causative pathogen.22 Unfortunately, chromomycosis is difficult to treat, with low cure rates and high relapse rates. Antifungal agents combined with surgery, cryotherapy, or thermotherapy often are used, with cure rates ranging from 15% to 80%.22,25
Pyoderma gangrenosum is a reactive noninfectious inflammatory dermatosis associated with inflammatory bowel disease and rheumatoid arthritis. The exact etiology is not clearly understood, but it generally is considered an autoinflammatory disorder.26 The most common form—classical PG—occurs in approximately 85% of cases and manifests as a painful erythematous lesion that progresses to a blistered or necrotic ulcer. It primarily affects the lower legs but can occur in other body sites.27 The diagnosis is based on clinical symptoms after excluding other similar conditions; histopathology of biopsied wound tissues often are required for confirmation. Treatment of PG starts with fast-acting immunosuppressive drugs (corticosteroids and/or cyclosporine) followed by slowacting immunosuppressive drugs (biologics).26
Hidradenitis suppurativa is a chronic recurrent disease of the hair follicle unit that develops after puberty.28 Clinically, HS manifests with painful nodules, abscesses, chronically draining fistulas, and scarring in areas of the body rich in apocrine glands.29,30 Treatment of HS is challenging due to its diverse clinical manifestations and unclear etiology. Topical therapy, systemic treatments, biologic agents, surgery, and light therapy have shown variable results.28,31
- Franco-Paredes C, Marcos LA, Henao-Martínez AF, et al. Cutaneous mycobacterial infections. Clin Microbiol Rev. 2018;32: E00069-18. doi:10.1128/CMR.00069-18
- Brown TH. The rapidly growing mycobacteria—MycobacteriuM fortuitum and Mycobacterium chelonae. Infect Control. 1985;6:283-238. doi:10.1017/s0195941700061762
- Hooper J; Beltrami EJ; Santoro F; et al. Remember the fite: a case of cutaneous MycobacteriuM fortuitum infection. Am J Dermatopathol. 2023;45:214-215. doi:10.1097/DAD.0000000000002336
- Franco-Paredes C, Chastain DB, Allen L, et al. Overview of cutaneous mycobacterial infections. Curr Trop Med Rep. 2018;5:228-232. doi:10.1007/s40475-018-0161-7
- Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33:563-77. doi:10.1016/j.det.2015.03.017
- Peixoto ADS, Montenegro LML, Lima AS, et al. Identification of nontuberculous mycobacteria species by multiplex real-time PCR with high-resolution melting. Rev Soc Bras Med Trop. 2020;53:E20200211. doi:10.1590/0037-8682-0211-2020
- Park J, Kwak N, Chae JC, et al. A two-step real-time PCR method to identify Mycobacterium tuberculosis infections and six dominant nontuberculous mycobacterial infections from clinical specimens. Microbiol Spectr. 2023:E0160623. doi:10.1128/spectrum.01606-23
- Fowler J, Mahlen SD. Localized cutaneous infections in immunocompetent individuals due to rapidly growing mycobacteria. Arch Pathol Lab Med. 2014;138:1106-1109. doi:10.5858/arpa.2012-0203-RS
- Gardini G, Gregori N, Matteelli A, et al. Mycobacterial skin infection. Curr Opin Infect Dis. 2022;35:79-87. doi:10.1097/QCO.0000000000000820
- Wang SH, Pancholi P. Mycobacterial skin and soft tissue infection. Curr Infect Dis Rep. 2014;16:438. doi:10.1007/s11908-014-0438-5
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Mougari F, Guglielmetti L, Raskine L, et al. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Expert Rev Anti Infect Ther. 2016;14:1139-1154. doi:10.1080/14787210.201 6.1238304
- Tu HZ, Lee HS, Chen YS, et al. High rates of antimicrobial resistance in rapidly growing mycobacterial infections in Taiwan. Pathogens. 2022;11:969. doi:10.3390/pathogens11090969
- Hashemzadeh M, Zadegan Dezfuli AA, Khosravi AD, et al. F requency of mutations in erm(39) related to clarithromycin resistance and in rrl related to linezolid resistance in clinical isolates of MycobacteriuM fortuitum in Iran. Acta Microbiol Immunol Hung. 2023;70:167-176. doi:10.1556/030.2023.02020
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292. doi:10.1001/archderm.142.10.1287
- Miretti M, Juri L, Peralta A, et al. Photoinactivation of non-tuberculous mycobacteria using Zn-phthalocyanine loaded into liposomes. Tuberculosis (Edinb). 2022;136:102247. doi:10.1016/j.tube.2022.102247
- Bystritsky RJ. Cellulitis. Infect Dis Clin North Am. 2021;35:49-60. doi:10.1016/j.idc.2020.10.002
- Christensen K, Holman R, Steiner C, et al. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025-1035. doi:10.1086/605562
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483. doi:10.1016/J.JAAD.2020.07.055
- Cutler TS, Jannat-Khah DP, Kam B, et al. Prevalence of misdiagnosis of cellulitis: a systematic review and meta-analysis. J Hosp Med. 2023;18:254-261. doi:10.1002/jhm.12977
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012;79:547-52. doi:10.3949/ccjm.79a.11121
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- McGinnis MR. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol. 1983;8:1-16.
- Rubin HA, Bruce S, Rosen T, et al. Evidence for percutaneous inoculation as the mode of transmission for chromoblastomycosis. J Am Acad Dermatol. 1991;25:951-954.
- Bonifaz A, Paredes-Solís V, Saúl A. Treating chromoblastomycosis with systemic antifungals. Expert Opin Pharmacother. 2004;5:247-254.
- Maverakis E, Marzano AV, Le ST, et al. Pyoderma gangrenosum. Nat Rev Dis Primers. 2020;6:81. doi:10.1038/s41572-020-0213-x
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224-228. doi:10.7861/clinmedicine.19-3-224
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Daxhelet M, Suppa M, White J, et al. Proposed definitions of typical lesions in hidradenitis suppurativa. Dermatology. 2020;236:431-438. doi:10.1159/000507348
- Amat-Samaranch V, Agut-Busquet E, Vilarrasa E, et al. New perspectives on the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis. 2021;12:20406223211055920. doi:10.1177/20406223211055920
The Diagnosis: Mycobacterial Infection
An injury sustained in a wet environment that results in chronic indolent abscesses, nodules, or draining sinus tracts suggests a mycobacterial infection. In our patient, a culture revealed MycobacteriuM fortuitum, which is classified in the rapid grower nontuberculous mycobacteria (NTM) group, along with Mycobacterium chelonae and Mycobacterium abscessus.1 The patient’s history of skin injury while cutting wet grass and the common presence of M fortuitum in the environment suggested that the organism entered the wound. The patient healed completely following surgical excision and a 2-month course of clarithromycin 1 g daily and rifampin 600 mg daily.
MycobacteriuM fortuitum was first isolated from an amphibian source in 1905 and later identified in a human with cutaneous infection in 1938. It commonly is found in soil and water.2 Skin and soft-tissue infections with M fortuitum usually are acquired from direct entry of the organism through a damaged skin barrier from trauma, medical injection, surgery, or tattoo placement.2,3
Skin lesions caused by NTM often are nonspecific and can mimic a variety of other dermatologic conditions, making clinical diagnosis challenging. As such, cutaneous manifestations of M fortuitum infection can include recurrent cutaneous abscesses, nodular lesions, chronic discharging sinuses, cellulitis, and surgical site infections.4 Although cutaneous infection with M fortuitum classically manifests with a single subcutaneous nodule at the site of trauma or surgery,5 it also can manifest as multiple draining sinus tracts, as seen in our patient. Hence, the diagnosis and treatment of cutaneous NTM infection is challenging, especially when M fortuitum skin manifestations can take up to 4 to 6 weeks to develop after inoculation. Diagnosis often requires a detailed patient history, tissue cultures, and histopathology.5
In recent years, rapid detection with polymerase chain reaction (PCR) techniques has been employed more widely. Notably, a molecular system based on multiplex real-time PCR with high-resolution melting was shown to have a sensitivity of up to 54% for distinguishing M fortuitum from other NTM.6 More recently, a 2-step real-time PCR method has demonstrated diagnostic sensitivity and specificity for differentiating NTM from Mycobacterium tuberculosis infections and identifying the causative NTM agent.7
Compared to immunocompetent individuals, those who are immunocompromised are more susceptible to less pathogenic strains of NTM, which can cause dissemination and lead to tenosynovitis, myositis, osteomyelitis, and septic arthritis.8-12 Nonetheless, cases of infections with NTM—including M fortuitum—are becoming harder to treat. Several single nucleotide polymorphisms and point mutations have been demonstrated in the ribosomal RNA methylase gene erm(39) related to clarithromycin resistance and in the rrl gene related to linezolid resistance.13 Due to increasing inducible resistance to common classes of antibiotics, such as macrolides and linezolid, treatment of M fortuitum requires multidrug regimens.13,14 Drug susceptibility testing also may be required, as M fortuitum has shown low resistance to tigecycline, tetracycline, cefmetazole, imipenem, and aminoglycosides (eg, amikacin, tobramycin, neomycin, gentamycin). Surgery is an important adjunctive tool in treating M fortuitum infections; patients with a single lesion are more likely to undergo surgical treatment alone or in combination with antibiotic therapy.15 More recently, antimicrobial photodynamic therapy has been explored as an alternative to eliminate NTM, including M fortuitum.16
The differential diagnosis for skin lesions manifesting with draining fistulae and sinus tracts includes conditions with infectious (cellulitis and chromomycosis) and inflammatory (pyoderma gangrenosum [PG] and hidradenitis suppurativa [HS]) causes.
Cellulitis is a common infection of the skin and subcutaneous tissue that predominantly is caused by gram-positive organisms such as β-hemolytic streptococci.17 Clinical manifestations include acute skin erythema, swelling, tenderness, and warmth. The legs are the most common sites of infection, but any area of the skin can be involved.17 Cellulitis comprises 10% of all infectious disease hospitalizations and up to 11% of all dermatologic admissions.18,19 It frequently is misdiagnosed, perhaps due to the lack of a reliable confirmatory laboratory test or imaging study, in addition to the plethora of diseases that mimic cellulitis, such as stasis dermatitis, lipodermatosclerosis, contact dermatitis, lymphedema, eosinophilic cellulitis, and papular urticaria.20,21 The consequences of misdiagnosis include but are not limited to unnecessary hospitalizations, inappropriate antibiotic use, and delayed management of the disease; thus, there is an urgent need for a reliable standard test to confirm the diagnosis, especially among nonspecialist physicians. 20 Most patients with uncomplicated cellulitis can be treated with empiric oral antibiotics that target β-hemolytic streptococci (ie, penicillin V potassium, amoxicillin).17 Methicillin-resistant Staphylococcus aureus coverage generally is unnecessary for nonpurulent cellulitis, but clinicians can consider adding amoxicillin-clavulanate, dicloxacillin, and cephalexin to the regimen. For purulent cellulitis, incision and drainage should be performed. In severe cases that manifest with sepsis, altered mental status, or hemodynamic instability, inpatient management is required.17
Chromomycosis (also known as chromoblastomycosis) is a chronic, indolent, granulomatous, suppurative mycosis of the skin and subcutaneous tissue22 that is caused by traumatic inoculation of various fungi of the order Chaetothyriales and family Herpotrichiellaceae, which are present in soil, plants, and decomposing wood. Chromomycosis is prevalent in tropical and subtropical regions.23,24 Clinically, it manifests as oligosymptomatic or asymptomatic lesions around an infection site that can manifest as papules with centrifugal growth evolving into nodular, verrucous, plaque, tumoral, or atrophic forms.22 Diagnosis is made with direct microscopy using potassium hydroxide, which reveals muriform bodies. Fungal culture in Sabouraud agar also can be used to isolate the causative pathogen.22 Unfortunately, chromomycosis is difficult to treat, with low cure rates and high relapse rates. Antifungal agents combined with surgery, cryotherapy, or thermotherapy often are used, with cure rates ranging from 15% to 80%.22,25
Pyoderma gangrenosum is a reactive noninfectious inflammatory dermatosis associated with inflammatory bowel disease and rheumatoid arthritis. The exact etiology is not clearly understood, but it generally is considered an autoinflammatory disorder.26 The most common form—classical PG—occurs in approximately 85% of cases and manifests as a painful erythematous lesion that progresses to a blistered or necrotic ulcer. It primarily affects the lower legs but can occur in other body sites.27 The diagnosis is based on clinical symptoms after excluding other similar conditions; histopathology of biopsied wound tissues often are required for confirmation. Treatment of PG starts with fast-acting immunosuppressive drugs (corticosteroids and/or cyclosporine) followed by slowacting immunosuppressive drugs (biologics).26
Hidradenitis suppurativa is a chronic recurrent disease of the hair follicle unit that develops after puberty.28 Clinically, HS manifests with painful nodules, abscesses, chronically draining fistulas, and scarring in areas of the body rich in apocrine glands.29,30 Treatment of HS is challenging due to its diverse clinical manifestations and unclear etiology. Topical therapy, systemic treatments, biologic agents, surgery, and light therapy have shown variable results.28,31
The Diagnosis: Mycobacterial Infection
An injury sustained in a wet environment that results in chronic indolent abscesses, nodules, or draining sinus tracts suggests a mycobacterial infection. In our patient, a culture revealed MycobacteriuM fortuitum, which is classified in the rapid grower nontuberculous mycobacteria (NTM) group, along with Mycobacterium chelonae and Mycobacterium abscessus.1 The patient’s history of skin injury while cutting wet grass and the common presence of M fortuitum in the environment suggested that the organism entered the wound. The patient healed completely following surgical excision and a 2-month course of clarithromycin 1 g daily and rifampin 600 mg daily.
MycobacteriuM fortuitum was first isolated from an amphibian source in 1905 and later identified in a human with cutaneous infection in 1938. It commonly is found in soil and water.2 Skin and soft-tissue infections with M fortuitum usually are acquired from direct entry of the organism through a damaged skin barrier from trauma, medical injection, surgery, or tattoo placement.2,3
Skin lesions caused by NTM often are nonspecific and can mimic a variety of other dermatologic conditions, making clinical diagnosis challenging. As such, cutaneous manifestations of M fortuitum infection can include recurrent cutaneous abscesses, nodular lesions, chronic discharging sinuses, cellulitis, and surgical site infections.4 Although cutaneous infection with M fortuitum classically manifests with a single subcutaneous nodule at the site of trauma or surgery,5 it also can manifest as multiple draining sinus tracts, as seen in our patient. Hence, the diagnosis and treatment of cutaneous NTM infection is challenging, especially when M fortuitum skin manifestations can take up to 4 to 6 weeks to develop after inoculation. Diagnosis often requires a detailed patient history, tissue cultures, and histopathology.5
In recent years, rapid detection with polymerase chain reaction (PCR) techniques has been employed more widely. Notably, a molecular system based on multiplex real-time PCR with high-resolution melting was shown to have a sensitivity of up to 54% for distinguishing M fortuitum from other NTM.6 More recently, a 2-step real-time PCR method has demonstrated diagnostic sensitivity and specificity for differentiating NTM from Mycobacterium tuberculosis infections and identifying the causative NTM agent.7
Compared to immunocompetent individuals, those who are immunocompromised are more susceptible to less pathogenic strains of NTM, which can cause dissemination and lead to tenosynovitis, myositis, osteomyelitis, and septic arthritis.8-12 Nonetheless, cases of infections with NTM—including M fortuitum—are becoming harder to treat. Several single nucleotide polymorphisms and point mutations have been demonstrated in the ribosomal RNA methylase gene erm(39) related to clarithromycin resistance and in the rrl gene related to linezolid resistance.13 Due to increasing inducible resistance to common classes of antibiotics, such as macrolides and linezolid, treatment of M fortuitum requires multidrug regimens.13,14 Drug susceptibility testing also may be required, as M fortuitum has shown low resistance to tigecycline, tetracycline, cefmetazole, imipenem, and aminoglycosides (eg, amikacin, tobramycin, neomycin, gentamycin). Surgery is an important adjunctive tool in treating M fortuitum infections; patients with a single lesion are more likely to undergo surgical treatment alone or in combination with antibiotic therapy.15 More recently, antimicrobial photodynamic therapy has been explored as an alternative to eliminate NTM, including M fortuitum.16
The differential diagnosis for skin lesions manifesting with draining fistulae and sinus tracts includes conditions with infectious (cellulitis and chromomycosis) and inflammatory (pyoderma gangrenosum [PG] and hidradenitis suppurativa [HS]) causes.
Cellulitis is a common infection of the skin and subcutaneous tissue that predominantly is caused by gram-positive organisms such as β-hemolytic streptococci.17 Clinical manifestations include acute skin erythema, swelling, tenderness, and warmth. The legs are the most common sites of infection, but any area of the skin can be involved.17 Cellulitis comprises 10% of all infectious disease hospitalizations and up to 11% of all dermatologic admissions.18,19 It frequently is misdiagnosed, perhaps due to the lack of a reliable confirmatory laboratory test or imaging study, in addition to the plethora of diseases that mimic cellulitis, such as stasis dermatitis, lipodermatosclerosis, contact dermatitis, lymphedema, eosinophilic cellulitis, and papular urticaria.20,21 The consequences of misdiagnosis include but are not limited to unnecessary hospitalizations, inappropriate antibiotic use, and delayed management of the disease; thus, there is an urgent need for a reliable standard test to confirm the diagnosis, especially among nonspecialist physicians. 20 Most patients with uncomplicated cellulitis can be treated with empiric oral antibiotics that target β-hemolytic streptococci (ie, penicillin V potassium, amoxicillin).17 Methicillin-resistant Staphylococcus aureus coverage generally is unnecessary for nonpurulent cellulitis, but clinicians can consider adding amoxicillin-clavulanate, dicloxacillin, and cephalexin to the regimen. For purulent cellulitis, incision and drainage should be performed. In severe cases that manifest with sepsis, altered mental status, or hemodynamic instability, inpatient management is required.17
Chromomycosis (also known as chromoblastomycosis) is a chronic, indolent, granulomatous, suppurative mycosis of the skin and subcutaneous tissue22 that is caused by traumatic inoculation of various fungi of the order Chaetothyriales and family Herpotrichiellaceae, which are present in soil, plants, and decomposing wood. Chromomycosis is prevalent in tropical and subtropical regions.23,24 Clinically, it manifests as oligosymptomatic or asymptomatic lesions around an infection site that can manifest as papules with centrifugal growth evolving into nodular, verrucous, plaque, tumoral, or atrophic forms.22 Diagnosis is made with direct microscopy using potassium hydroxide, which reveals muriform bodies. Fungal culture in Sabouraud agar also can be used to isolate the causative pathogen.22 Unfortunately, chromomycosis is difficult to treat, with low cure rates and high relapse rates. Antifungal agents combined with surgery, cryotherapy, or thermotherapy often are used, with cure rates ranging from 15% to 80%.22,25
Pyoderma gangrenosum is a reactive noninfectious inflammatory dermatosis associated with inflammatory bowel disease and rheumatoid arthritis. The exact etiology is not clearly understood, but it generally is considered an autoinflammatory disorder.26 The most common form—classical PG—occurs in approximately 85% of cases and manifests as a painful erythematous lesion that progresses to a blistered or necrotic ulcer. It primarily affects the lower legs but can occur in other body sites.27 The diagnosis is based on clinical symptoms after excluding other similar conditions; histopathology of biopsied wound tissues often are required for confirmation. Treatment of PG starts with fast-acting immunosuppressive drugs (corticosteroids and/or cyclosporine) followed by slowacting immunosuppressive drugs (biologics).26
Hidradenitis suppurativa is a chronic recurrent disease of the hair follicle unit that develops after puberty.28 Clinically, HS manifests with painful nodules, abscesses, chronically draining fistulas, and scarring in areas of the body rich in apocrine glands.29,30 Treatment of HS is challenging due to its diverse clinical manifestations and unclear etiology. Topical therapy, systemic treatments, biologic agents, surgery, and light therapy have shown variable results.28,31
- Franco-Paredes C, Marcos LA, Henao-Martínez AF, et al. Cutaneous mycobacterial infections. Clin Microbiol Rev. 2018;32: E00069-18. doi:10.1128/CMR.00069-18
- Brown TH. The rapidly growing mycobacteria—MycobacteriuM fortuitum and Mycobacterium chelonae. Infect Control. 1985;6:283-238. doi:10.1017/s0195941700061762
- Hooper J; Beltrami EJ; Santoro F; et al. Remember the fite: a case of cutaneous MycobacteriuM fortuitum infection. Am J Dermatopathol. 2023;45:214-215. doi:10.1097/DAD.0000000000002336
- Franco-Paredes C, Chastain DB, Allen L, et al. Overview of cutaneous mycobacterial infections. Curr Trop Med Rep. 2018;5:228-232. doi:10.1007/s40475-018-0161-7
- Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33:563-77. doi:10.1016/j.det.2015.03.017
- Peixoto ADS, Montenegro LML, Lima AS, et al. Identification of nontuberculous mycobacteria species by multiplex real-time PCR with high-resolution melting. Rev Soc Bras Med Trop. 2020;53:E20200211. doi:10.1590/0037-8682-0211-2020
- Park J, Kwak N, Chae JC, et al. A two-step real-time PCR method to identify Mycobacterium tuberculosis infections and six dominant nontuberculous mycobacterial infections from clinical specimens. Microbiol Spectr. 2023:E0160623. doi:10.1128/spectrum.01606-23
- Fowler J, Mahlen SD. Localized cutaneous infections in immunocompetent individuals due to rapidly growing mycobacteria. Arch Pathol Lab Med. 2014;138:1106-1109. doi:10.5858/arpa.2012-0203-RS
- Gardini G, Gregori N, Matteelli A, et al. Mycobacterial skin infection. Curr Opin Infect Dis. 2022;35:79-87. doi:10.1097/QCO.0000000000000820
- Wang SH, Pancholi P. Mycobacterial skin and soft tissue infection. Curr Infect Dis Rep. 2014;16:438. doi:10.1007/s11908-014-0438-5
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Mougari F, Guglielmetti L, Raskine L, et al. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Expert Rev Anti Infect Ther. 2016;14:1139-1154. doi:10.1080/14787210.201 6.1238304
- Tu HZ, Lee HS, Chen YS, et al. High rates of antimicrobial resistance in rapidly growing mycobacterial infections in Taiwan. Pathogens. 2022;11:969. doi:10.3390/pathogens11090969
- Hashemzadeh M, Zadegan Dezfuli AA, Khosravi AD, et al. F requency of mutations in erm(39) related to clarithromycin resistance and in rrl related to linezolid resistance in clinical isolates of MycobacteriuM fortuitum in Iran. Acta Microbiol Immunol Hung. 2023;70:167-176. doi:10.1556/030.2023.02020
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292. doi:10.1001/archderm.142.10.1287
- Miretti M, Juri L, Peralta A, et al. Photoinactivation of non-tuberculous mycobacteria using Zn-phthalocyanine loaded into liposomes. Tuberculosis (Edinb). 2022;136:102247. doi:10.1016/j.tube.2022.102247
- Bystritsky RJ. Cellulitis. Infect Dis Clin North Am. 2021;35:49-60. doi:10.1016/j.idc.2020.10.002
- Christensen K, Holman R, Steiner C, et al. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025-1035. doi:10.1086/605562
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483. doi:10.1016/J.JAAD.2020.07.055
- Cutler TS, Jannat-Khah DP, Kam B, et al. Prevalence of misdiagnosis of cellulitis: a systematic review and meta-analysis. J Hosp Med. 2023;18:254-261. doi:10.1002/jhm.12977
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012;79:547-52. doi:10.3949/ccjm.79a.11121
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- McGinnis MR. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol. 1983;8:1-16.
- Rubin HA, Bruce S, Rosen T, et al. Evidence for percutaneous inoculation as the mode of transmission for chromoblastomycosis. J Am Acad Dermatol. 1991;25:951-954.
- Bonifaz A, Paredes-Solís V, Saúl A. Treating chromoblastomycosis with systemic antifungals. Expert Opin Pharmacother. 2004;5:247-254.
- Maverakis E, Marzano AV, Le ST, et al. Pyoderma gangrenosum. Nat Rev Dis Primers. 2020;6:81. doi:10.1038/s41572-020-0213-x
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224-228. doi:10.7861/clinmedicine.19-3-224
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Daxhelet M, Suppa M, White J, et al. Proposed definitions of typical lesions in hidradenitis suppurativa. Dermatology. 2020;236:431-438. doi:10.1159/000507348
- Amat-Samaranch V, Agut-Busquet E, Vilarrasa E, et al. New perspectives on the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis. 2021;12:20406223211055920. doi:10.1177/20406223211055920
- Franco-Paredes C, Marcos LA, Henao-Martínez AF, et al. Cutaneous mycobacterial infections. Clin Microbiol Rev. 2018;32: E00069-18. doi:10.1128/CMR.00069-18
- Brown TH. The rapidly growing mycobacteria—MycobacteriuM fortuitum and Mycobacterium chelonae. Infect Control. 1985;6:283-238. doi:10.1017/s0195941700061762
- Hooper J; Beltrami EJ; Santoro F; et al. Remember the fite: a case of cutaneous MycobacteriuM fortuitum infection. Am J Dermatopathol. 2023;45:214-215. doi:10.1097/DAD.0000000000002336
- Franco-Paredes C, Chastain DB, Allen L, et al. Overview of cutaneous mycobacterial infections. Curr Trop Med Rep. 2018;5:228-232. doi:10.1007/s40475-018-0161-7
- Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33:563-77. doi:10.1016/j.det.2015.03.017
- Peixoto ADS, Montenegro LML, Lima AS, et al. Identification of nontuberculous mycobacteria species by multiplex real-time PCR with high-resolution melting. Rev Soc Bras Med Trop. 2020;53:E20200211. doi:10.1590/0037-8682-0211-2020
- Park J, Kwak N, Chae JC, et al. A two-step real-time PCR method to identify Mycobacterium tuberculosis infections and six dominant nontuberculous mycobacterial infections from clinical specimens. Microbiol Spectr. 2023:E0160623. doi:10.1128/spectrum.01606-23
- Fowler J, Mahlen SD. Localized cutaneous infections in immunocompetent individuals due to rapidly growing mycobacteria. Arch Pathol Lab Med. 2014;138:1106-1109. doi:10.5858/arpa.2012-0203-RS
- Gardini G, Gregori N, Matteelli A, et al. Mycobacterial skin infection. Curr Opin Infect Dis. 2022;35:79-87. doi:10.1097/QCO.0000000000000820
- Wang SH, Pancholi P. Mycobacterial skin and soft tissue infection. Curr Infect Dis Rep. 2014;16:438. doi:10.1007/s11908-014-0438-5
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Mougari F, Guglielmetti L, Raskine L, et al. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Expert Rev Anti Infect Ther. 2016;14:1139-1154. doi:10.1080/14787210.201 6.1238304
- Tu HZ, Lee HS, Chen YS, et al. High rates of antimicrobial resistance in rapidly growing mycobacterial infections in Taiwan. Pathogens. 2022;11:969. doi:10.3390/pathogens11090969
- Hashemzadeh M, Zadegan Dezfuli AA, Khosravi AD, et al. F requency of mutations in erm(39) related to clarithromycin resistance and in rrl related to linezolid resistance in clinical isolates of MycobacteriuM fortuitum in Iran. Acta Microbiol Immunol Hung. 2023;70:167-176. doi:10.1556/030.2023.02020
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292. doi:10.1001/archderm.142.10.1287
- Miretti M, Juri L, Peralta A, et al. Photoinactivation of non-tuberculous mycobacteria using Zn-phthalocyanine loaded into liposomes. Tuberculosis (Edinb). 2022;136:102247. doi:10.1016/j.tube.2022.102247
- Bystritsky RJ. Cellulitis. Infect Dis Clin North Am. 2021;35:49-60. doi:10.1016/j.idc.2020.10.002
- Christensen K, Holman R, Steiner C, et al. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025-1035. doi:10.1086/605562
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483. doi:10.1016/J.JAAD.2020.07.055
- Cutler TS, Jannat-Khah DP, Kam B, et al. Prevalence of misdiagnosis of cellulitis: a systematic review and meta-analysis. J Hosp Med. 2023;18:254-261. doi:10.1002/jhm.12977
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012;79:547-52. doi:10.3949/ccjm.79a.11121
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- McGinnis MR. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol. 1983;8:1-16.
- Rubin HA, Bruce S, Rosen T, et al. Evidence for percutaneous inoculation as the mode of transmission for chromoblastomycosis. J Am Acad Dermatol. 1991;25:951-954.
- Bonifaz A, Paredes-Solís V, Saúl A. Treating chromoblastomycosis with systemic antifungals. Expert Opin Pharmacother. 2004;5:247-254.
- Maverakis E, Marzano AV, Le ST, et al. Pyoderma gangrenosum. Nat Rev Dis Primers. 2020;6:81. doi:10.1038/s41572-020-0213-x
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224-228. doi:10.7861/clinmedicine.19-3-224
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Daxhelet M, Suppa M, White J, et al. Proposed definitions of typical lesions in hidradenitis suppurativa. Dermatology. 2020;236:431-438. doi:10.1159/000507348
- Amat-Samaranch V, Agut-Busquet E, Vilarrasa E, et al. New perspectives on the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis. 2021;12:20406223211055920. doi:10.1177/20406223211055920
A 40-year-old woman presented with multiple draining sinus tracts on the right thigh following an injury sustained weeks earlier while mowing wet grass.
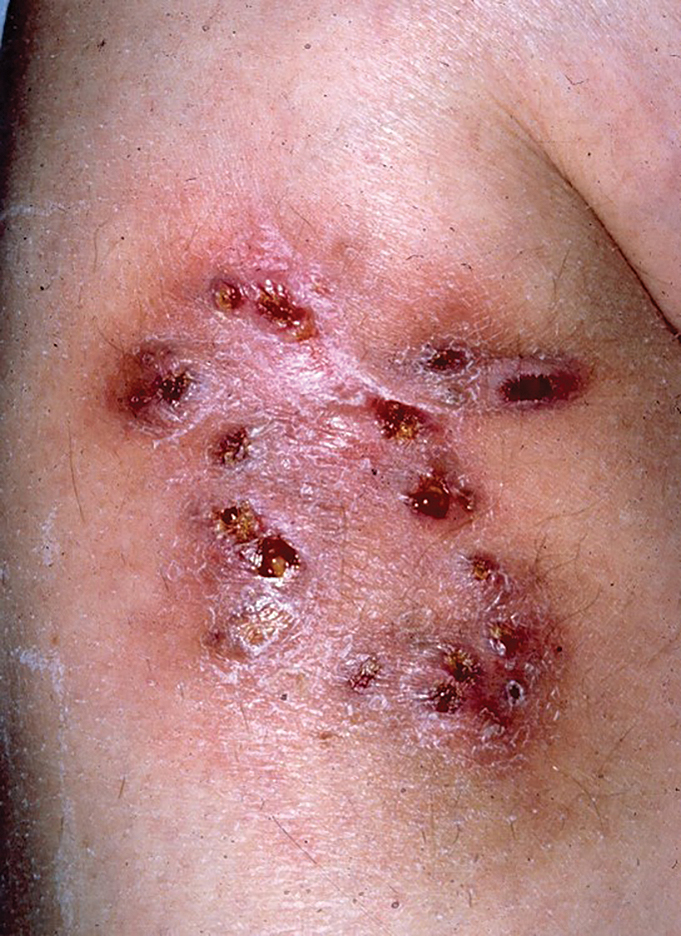
Erythema Nodosum Triggered by a Bite From a Copperhead Snake
The clinical manifestations of snakebites vary based on the species of snake, bite location, and amount and strength of the venom injected. Locally acting toxins in snake venom predominantly consist of enzymes, such as phospholipase A2, that cause local tissue destruction and can result in pain, swelling, blistering, ecchymosis, and tissue necrosis at the site of the bite within hours to days after the bite.1 Systemically acting toxins can target a wide variety of tissues and cause severe systemic complications including paralysis, rhabdomyolysis secondary to muscle damage, coagulopathy, sepsis, and cardiorespiratory failure.2
Although pain and swelling following snakebites typically resolve by 1 month after envenomation, copperhead snakes—a type of pit viper—may cause residual symptoms of pain and swelling lasting for a year or more.3 Additional cutaneous manifestations of copperhead snakebites include wound infections at the bite site, such as cellulitis and necrotizing fasciitis. More devastating complications that have been described following snake envenomation include tissue injury of an entire extremity and development of compartment syndrome, which requires urgent fasciotomy to prevent potential loss of the affected limb.4
Physicians should be aware of the potential complications of snakebites to properly manage and counsel their patients. We describe a 42-year-old woman with tender, erythematous, subcutaneous nodules persisting for 4 months following a copperhead snakebite. A biopsy confirmed the diagnosis of snakebite-associated erythema nodosum (EN).
Case Report
A 42-year-old woman presented to our clinic with progressive tender, pruritic, deep-seated, erythematous nodules in multiple locations on the legs after sustaining a bite by a copperhead snake on the left foot 4 months prior. The lesions tended to fluctuate in intensity. In the days following the bite, she initially developed painful red bumps on the left foot just proximal to the bite site with associated pain and swelling extending up to just below the left knee. She reported no other notable symptoms such as fever, arthralgia, fatigue, or gastrointestinal tract symptoms. Physical examination revealed bilateral pitting edema, which was worse in the left leg, along with multiple deep, palpable, tender subcutaneous nodules with erythematous surface change (Figure 1).
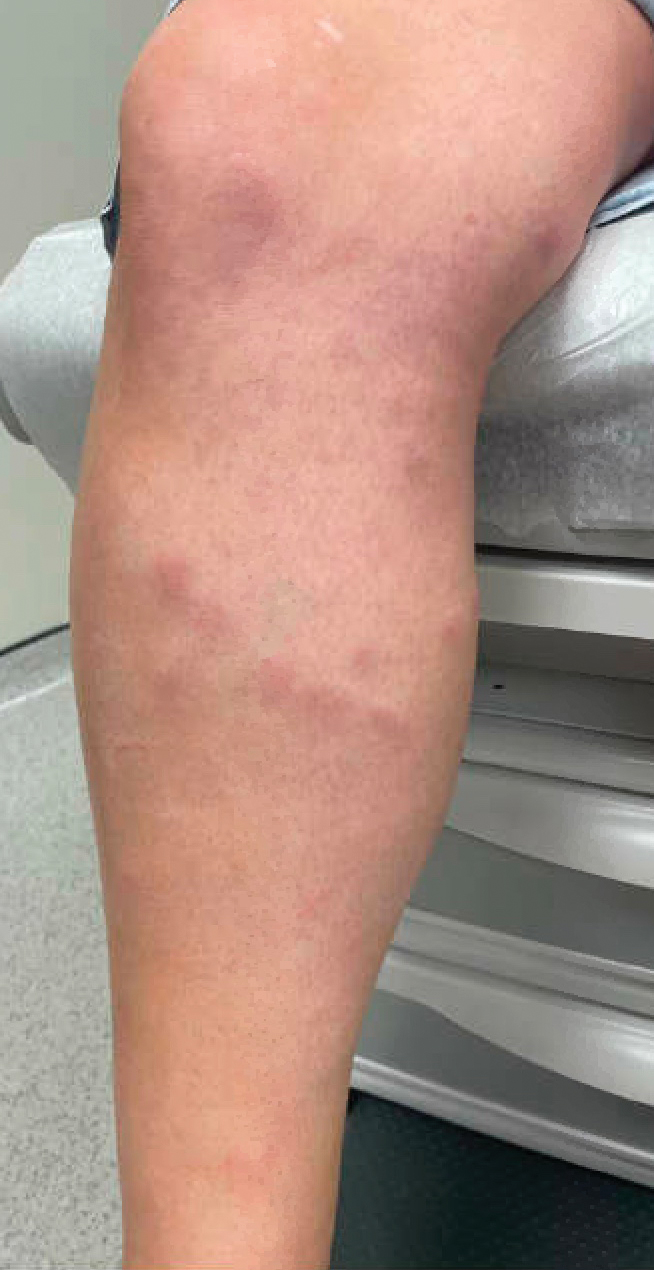
Workup performed by an outside provider over the previous month included 2 venous duplex ultrasounds of the left leg, which showed no signs of deep vein thrombosis. Additionally, the patient underwent lateral and anteroposterior radiographs of the left foot, tibia, and fibula, which showed no evidence of fracture.
Given the morphology and distribution of the lesions (Figure 2), EN was strongly favored as the cause of the symptoms, and a biopsy confirmed the diagnosis. All immunohistochemical stains including auramine-rhodamine for acid-fast bacilli, Grocott-Gomori methenamine silver for fungal organisms, and Brown and Brenn were negative. Given the waxing and waning course of the lesions, which suggested an active neutrophilic rather than purely chronic granulomatous phase of EN, the patient was treated with colchicine 0.6 mg twice daily for 1 month.
Causes of EN and Clinical Manifestations
Erythema nodosum is a common form of septal panniculitis that can be precipitated by inflammatory conditions, infection, or medications (commonly oral contraceptive pills) but often is idiopathic.5 The acute phase is neutrophilic, with evolution over time to a granulomatous phase. Common etiologies include sarcoidosis; inflammatory bowel disease; and bacterial or fungal infections such as Streptococcus (especially common in children), histoplasmosis, and coccidioidomycosis. The patient was otherwise healthy and was not taking any medications that are known triggers of EN. A PubMed search of articles indexed for MEDLINE in the English-language literature using the terms copperhead snake bite, erythema nodosum snake, and copperhead snake erythema nodosum revealed no reports of EN following a bite from a copperhead snake; however, in one case, an adder bite led to erysipelas, likely due to disturbed blood and lymphatic flow, which then triggered EN.6 Additionally, EN has been reported as a delayed reaction to jellyfish stings.7
Clinical features of EN include the development of tender, erythematous, subcutaneous nodules and plaques most frequently over the pretibial region. Lesions typically evolve from raised, deep-seated nodules into flat indurated plaques over a span of weeks. Occasionally, there is a slight prodromal phase marked by nonspecific symptoms such as fever and arthralgia lasting for 3 to 6 days. Erythema nodosum typically results in spontaneous resolution after 4 to 8 weeks, and management involves treatment of any underlying condition with symptomatic care. Interestingly, our patient experienced persistent symptoms over the course of 4 months, with development of new nodular lesions throughout this time period. The most frequently used drugs for the management of symptomatic EN include nonsteroidal anti-inflammatory drugs, colchicine, and potassium iodide.8 A characteristic histologic finding of the granulomatous phase is the Miescher radial granuloma, which is a septal collection of histiocytes surrounding a cleft.9
Snakebite Reactions
Snakebites can result in a wide range of local and systemic manifestations, as snake venom may contain 20 or more toxins.10 Local complications of pit viper bites include pain, swelling, and fang marks; when examining fang marks, the presence of 2 distinct puncture wounds often indicates envenomation with a poisonous snake, whereas nonvenomous snakebites often result in smaller puncture wounds arranged in an arc. Following bites, pain can develop immediately and spread proximally up the affected limb, which occurred in our patient in the days following the bite. Intense local reactions can occur, as bites often result in intense edema of the affected limb spreading to the trunk in the days to weeks after the bite, occasionally accompanied by regional lymphadenopathy. Some bites can result in local necrosis and secondary bacterial infection caused by organisms in the oral cavity of the culprit snake.
Although they were not present in our patient, snakebites can result in a wide range of systemic toxicities ranging from clotting defects and hemolysis to neurotoxicity, myotoxicity, and nephrotoxicity.10 In severe cases, snake venom can result in disseminated intravascular coagulation, sepsis, and cardiorespiratory collapse.
The eastern copperhead (Agkistrodon contortrix) is a species of venomous snake that is endemic to eastern North America. Copperheads are members of the subfamily Crotalinae in the family Viperidae.11 Reported reactions to copperhead bites include cellulitis, necrotizing fasciitis, compartment syndrome, and tissue necrosis of an entire affected extremity.12,13 Our patient displayed no systemic symptoms to suggest envenomation.
Management of Snakebites
Treatment of snakebites varies based on the constellation and severity of symptoms as well as how recently the envenomation occurred. In urgent cases, antivenom may be administered to prevent further toxicity. In cases of progressive compartment syndrome, emergent surgical procedures such as fasciotomy or amputation are required to prevent further complications. When a superimposed bacterial infection is suspected, broad-spectrum antibiotics are required. Because our patient presented 4 months following the initial bite with isolated cutaneous manifestations, she was treated symptomatically with colchicine for EN.1,2
Final Thoughts
Our patient presented with EN following a bite from a copperhead snake. Physicians should be aware of possible etiologies of EN to evaluate patients who present with new-onset tender subcutaneous nodules. Additionally, physicians should be aware of venomous snakes endemic to their region and also understand the various complications that can result following a snakebite, with the potential for lingering cutaneous manifestations weeks to months following the initial bite.
- Warrell DA. Snake bite. Lancet. 2010;375:77-88. doi:10.1016/S0140-6736(09)61754-2
- White J. Overview of venomous snakes of the world. In: Dart RC, eds. Medical Toxicology. 3rd ed. Lippincott, Williams, & Wilkins; 2004:1543
- Spiller HA, Bosse GM. Prospective study of morbidity associated with snakebite envenomation. J Toxicol Clin Toxicol. 2003;41:125-130. doi:10.1081/clt-120019127
- Scharman EJ, Noffsinger VD. Copperhead snakebites: clinical severity of local effects. Ann Emerg Med. 2001;38:55-61. doi:10.1067/mem.2001.116148
- Hafsi W, Badri T. Erythema nodosum. In: StatPearls. StatPearls Publishing; November 28, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470369/
- Nowowiejska J, Baran A, Flisiak I. Rare coexistence of unilateral erythema nodosum with erysipelas in the area of previous adder bite. Przegl Epidemiol. 2020;74:355-361. doi:10.32394/pe.74.28
- Auerbach PS, Hays JT. Erythema nodosum following a jellyfish sting. J Emerg Med. 1987;5:487-491. doi:10.1016/0736-4679(87)90211-3
- Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327. doi:10.1111/j.1529-8019.2010.01332.x
- Sánchez Yus E, Sanz Vico MD, de Diego V. Miescher’s radial granuloma. a characteristic marker of erythema nodosum. Am J Dermatopathol. 1989;11:434-442. doi:10.1097/00000372-198910000-00005
- Mehta SR, Sashindran VK. Clinical features and management of snake bite. Med J Armed Forces India. 2002;58:247-249. doi:10.1016/S0377-1237(02)80140-X
- Brys AK, Gandolfi BM, Levinson H, et al. Copperhead envenomation resulting in a rare case of hand compartment syndrome and subsequent fasciotomy. Plast Reconstr Surg Glob Open. 2015;3:E396. doi:10.1097/GOX.0000000000000367
- Clark RF, Selden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993;11:583-586. doi:10.1016/0736-4679(93)90313-v
- Buchanan JT, Thurman J. Crotalidae envenomation. In: StatPearls. StatPearls Publishing; October 3, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551615/
The clinical manifestations of snakebites vary based on the species of snake, bite location, and amount and strength of the venom injected. Locally acting toxins in snake venom predominantly consist of enzymes, such as phospholipase A2, that cause local tissue destruction and can result in pain, swelling, blistering, ecchymosis, and tissue necrosis at the site of the bite within hours to days after the bite.1 Systemically acting toxins can target a wide variety of tissues and cause severe systemic complications including paralysis, rhabdomyolysis secondary to muscle damage, coagulopathy, sepsis, and cardiorespiratory failure.2
Although pain and swelling following snakebites typically resolve by 1 month after envenomation, copperhead snakes—a type of pit viper—may cause residual symptoms of pain and swelling lasting for a year or more.3 Additional cutaneous manifestations of copperhead snakebites include wound infections at the bite site, such as cellulitis and necrotizing fasciitis. More devastating complications that have been described following snake envenomation include tissue injury of an entire extremity and development of compartment syndrome, which requires urgent fasciotomy to prevent potential loss of the affected limb.4
Physicians should be aware of the potential complications of snakebites to properly manage and counsel their patients. We describe a 42-year-old woman with tender, erythematous, subcutaneous nodules persisting for 4 months following a copperhead snakebite. A biopsy confirmed the diagnosis of snakebite-associated erythema nodosum (EN).
Case Report
A 42-year-old woman presented to our clinic with progressive tender, pruritic, deep-seated, erythematous nodules in multiple locations on the legs after sustaining a bite by a copperhead snake on the left foot 4 months prior. The lesions tended to fluctuate in intensity. In the days following the bite, she initially developed painful red bumps on the left foot just proximal to the bite site with associated pain and swelling extending up to just below the left knee. She reported no other notable symptoms such as fever, arthralgia, fatigue, or gastrointestinal tract symptoms. Physical examination revealed bilateral pitting edema, which was worse in the left leg, along with multiple deep, palpable, tender subcutaneous nodules with erythematous surface change (Figure 1).

Workup performed by an outside provider over the previous month included 2 venous duplex ultrasounds of the left leg, which showed no signs of deep vein thrombosis. Additionally, the patient underwent lateral and anteroposterior radiographs of the left foot, tibia, and fibula, which showed no evidence of fracture.
Given the morphology and distribution of the lesions (Figure 2), EN was strongly favored as the cause of the symptoms, and a biopsy confirmed the diagnosis. All immunohistochemical stains including auramine-rhodamine for acid-fast bacilli, Grocott-Gomori methenamine silver for fungal organisms, and Brown and Brenn were negative. Given the waxing and waning course of the lesions, which suggested an active neutrophilic rather than purely chronic granulomatous phase of EN, the patient was treated with colchicine 0.6 mg twice daily for 1 month.

Causes of EN and Clinical Manifestations
Erythema nodosum is a common form of septal panniculitis that can be precipitated by inflammatory conditions, infection, or medications (commonly oral contraceptive pills) but often is idiopathic.5 The acute phase is neutrophilic, with evolution over time to a granulomatous phase. Common etiologies include sarcoidosis; inflammatory bowel disease; and bacterial or fungal infections such as Streptococcus (especially common in children), histoplasmosis, and coccidioidomycosis. The patient was otherwise healthy and was not taking any medications that are known triggers of EN. A PubMed search of articles indexed for MEDLINE in the English-language literature using the terms copperhead snake bite, erythema nodosum snake, and copperhead snake erythema nodosum revealed no reports of EN following a bite from a copperhead snake; however, in one case, an adder bite led to erysipelas, likely due to disturbed blood and lymphatic flow, which then triggered EN.6 Additionally, EN has been reported as a delayed reaction to jellyfish stings.7
Clinical features of EN include the development of tender, erythematous, subcutaneous nodules and plaques most frequently over the pretibial region. Lesions typically evolve from raised, deep-seated nodules into flat indurated plaques over a span of weeks. Occasionally, there is a slight prodromal phase marked by nonspecific symptoms such as fever and arthralgia lasting for 3 to 6 days. Erythema nodosum typically results in spontaneous resolution after 4 to 8 weeks, and management involves treatment of any underlying condition with symptomatic care. Interestingly, our patient experienced persistent symptoms over the course of 4 months, with development of new nodular lesions throughout this time period. The most frequently used drugs for the management of symptomatic EN include nonsteroidal anti-inflammatory drugs, colchicine, and potassium iodide.8 A characteristic histologic finding of the granulomatous phase is the Miescher radial granuloma, which is a septal collection of histiocytes surrounding a cleft.9
Snakebite Reactions
Snakebites can result in a wide range of local and systemic manifestations, as snake venom may contain 20 or more toxins.10 Local complications of pit viper bites include pain, swelling, and fang marks; when examining fang marks, the presence of 2 distinct puncture wounds often indicates envenomation with a poisonous snake, whereas nonvenomous snakebites often result in smaller puncture wounds arranged in an arc. Following bites, pain can develop immediately and spread proximally up the affected limb, which occurred in our patient in the days following the bite. Intense local reactions can occur, as bites often result in intense edema of the affected limb spreading to the trunk in the days to weeks after the bite, occasionally accompanied by regional lymphadenopathy. Some bites can result in local necrosis and secondary bacterial infection caused by organisms in the oral cavity of the culprit snake.
Although they were not present in our patient, snakebites can result in a wide range of systemic toxicities ranging from clotting defects and hemolysis to neurotoxicity, myotoxicity, and nephrotoxicity.10 In severe cases, snake venom can result in disseminated intravascular coagulation, sepsis, and cardiorespiratory collapse.
The eastern copperhead (Agkistrodon contortrix) is a species of venomous snake that is endemic to eastern North America. Copperheads are members of the subfamily Crotalinae in the family Viperidae.11 Reported reactions to copperhead bites include cellulitis, necrotizing fasciitis, compartment syndrome, and tissue necrosis of an entire affected extremity.12,13 Our patient displayed no systemic symptoms to suggest envenomation.
Management of Snakebites
Treatment of snakebites varies based on the constellation and severity of symptoms as well as how recently the envenomation occurred. In urgent cases, antivenom may be administered to prevent further toxicity. In cases of progressive compartment syndrome, emergent surgical procedures such as fasciotomy or amputation are required to prevent further complications. When a superimposed bacterial infection is suspected, broad-spectrum antibiotics are required. Because our patient presented 4 months following the initial bite with isolated cutaneous manifestations, she was treated symptomatically with colchicine for EN.1,2
Final Thoughts
Our patient presented with EN following a bite from a copperhead snake. Physicians should be aware of possible etiologies of EN to evaluate patients who present with new-onset tender subcutaneous nodules. Additionally, physicians should be aware of venomous snakes endemic to their region and also understand the various complications that can result following a snakebite, with the potential for lingering cutaneous manifestations weeks to months following the initial bite.
The clinical manifestations of snakebites vary based on the species of snake, bite location, and amount and strength of the venom injected. Locally acting toxins in snake venom predominantly consist of enzymes, such as phospholipase A2, that cause local tissue destruction and can result in pain, swelling, blistering, ecchymosis, and tissue necrosis at the site of the bite within hours to days after the bite.1 Systemically acting toxins can target a wide variety of tissues and cause severe systemic complications including paralysis, rhabdomyolysis secondary to muscle damage, coagulopathy, sepsis, and cardiorespiratory failure.2
Although pain and swelling following snakebites typically resolve by 1 month after envenomation, copperhead snakes—a type of pit viper—may cause residual symptoms of pain and swelling lasting for a year or more.3 Additional cutaneous manifestations of copperhead snakebites include wound infections at the bite site, such as cellulitis and necrotizing fasciitis. More devastating complications that have been described following snake envenomation include tissue injury of an entire extremity and development of compartment syndrome, which requires urgent fasciotomy to prevent potential loss of the affected limb.4
Physicians should be aware of the potential complications of snakebites to properly manage and counsel their patients. We describe a 42-year-old woman with tender, erythematous, subcutaneous nodules persisting for 4 months following a copperhead snakebite. A biopsy confirmed the diagnosis of snakebite-associated erythema nodosum (EN).
Case Report
A 42-year-old woman presented to our clinic with progressive tender, pruritic, deep-seated, erythematous nodules in multiple locations on the legs after sustaining a bite by a copperhead snake on the left foot 4 months prior. The lesions tended to fluctuate in intensity. In the days following the bite, she initially developed painful red bumps on the left foot just proximal to the bite site with associated pain and swelling extending up to just below the left knee. She reported no other notable symptoms such as fever, arthralgia, fatigue, or gastrointestinal tract symptoms. Physical examination revealed bilateral pitting edema, which was worse in the left leg, along with multiple deep, palpable, tender subcutaneous nodules with erythematous surface change (Figure 1).

Workup performed by an outside provider over the previous month included 2 venous duplex ultrasounds of the left leg, which showed no signs of deep vein thrombosis. Additionally, the patient underwent lateral and anteroposterior radiographs of the left foot, tibia, and fibula, which showed no evidence of fracture.
Given the morphology and distribution of the lesions (Figure 2), EN was strongly favored as the cause of the symptoms, and a biopsy confirmed the diagnosis. All immunohistochemical stains including auramine-rhodamine for acid-fast bacilli, Grocott-Gomori methenamine silver for fungal organisms, and Brown and Brenn were negative. Given the waxing and waning course of the lesions, which suggested an active neutrophilic rather than purely chronic granulomatous phase of EN, the patient was treated with colchicine 0.6 mg twice daily for 1 month.

Causes of EN and Clinical Manifestations
Erythema nodosum is a common form of septal panniculitis that can be precipitated by inflammatory conditions, infection, or medications (commonly oral contraceptive pills) but often is idiopathic.5 The acute phase is neutrophilic, with evolution over time to a granulomatous phase. Common etiologies include sarcoidosis; inflammatory bowel disease; and bacterial or fungal infections such as Streptococcus (especially common in children), histoplasmosis, and coccidioidomycosis. The patient was otherwise healthy and was not taking any medications that are known triggers of EN. A PubMed search of articles indexed for MEDLINE in the English-language literature using the terms copperhead snake bite, erythema nodosum snake, and copperhead snake erythema nodosum revealed no reports of EN following a bite from a copperhead snake; however, in one case, an adder bite led to erysipelas, likely due to disturbed blood and lymphatic flow, which then triggered EN.6 Additionally, EN has been reported as a delayed reaction to jellyfish stings.7
Clinical features of EN include the development of tender, erythematous, subcutaneous nodules and plaques most frequently over the pretibial region. Lesions typically evolve from raised, deep-seated nodules into flat indurated plaques over a span of weeks. Occasionally, there is a slight prodromal phase marked by nonspecific symptoms such as fever and arthralgia lasting for 3 to 6 days. Erythema nodosum typically results in spontaneous resolution after 4 to 8 weeks, and management involves treatment of any underlying condition with symptomatic care. Interestingly, our patient experienced persistent symptoms over the course of 4 months, with development of new nodular lesions throughout this time period. The most frequently used drugs for the management of symptomatic EN include nonsteroidal anti-inflammatory drugs, colchicine, and potassium iodide.8 A characteristic histologic finding of the granulomatous phase is the Miescher radial granuloma, which is a septal collection of histiocytes surrounding a cleft.9
Snakebite Reactions
Snakebites can result in a wide range of local and systemic manifestations, as snake venom may contain 20 or more toxins.10 Local complications of pit viper bites include pain, swelling, and fang marks; when examining fang marks, the presence of 2 distinct puncture wounds often indicates envenomation with a poisonous snake, whereas nonvenomous snakebites often result in smaller puncture wounds arranged in an arc. Following bites, pain can develop immediately and spread proximally up the affected limb, which occurred in our patient in the days following the bite. Intense local reactions can occur, as bites often result in intense edema of the affected limb spreading to the trunk in the days to weeks after the bite, occasionally accompanied by regional lymphadenopathy. Some bites can result in local necrosis and secondary bacterial infection caused by organisms in the oral cavity of the culprit snake.
Although they were not present in our patient, snakebites can result in a wide range of systemic toxicities ranging from clotting defects and hemolysis to neurotoxicity, myotoxicity, and nephrotoxicity.10 In severe cases, snake venom can result in disseminated intravascular coagulation, sepsis, and cardiorespiratory collapse.
The eastern copperhead (Agkistrodon contortrix) is a species of venomous snake that is endemic to eastern North America. Copperheads are members of the subfamily Crotalinae in the family Viperidae.11 Reported reactions to copperhead bites include cellulitis, necrotizing fasciitis, compartment syndrome, and tissue necrosis of an entire affected extremity.12,13 Our patient displayed no systemic symptoms to suggest envenomation.
Management of Snakebites
Treatment of snakebites varies based on the constellation and severity of symptoms as well as how recently the envenomation occurred. In urgent cases, antivenom may be administered to prevent further toxicity. In cases of progressive compartment syndrome, emergent surgical procedures such as fasciotomy or amputation are required to prevent further complications. When a superimposed bacterial infection is suspected, broad-spectrum antibiotics are required. Because our patient presented 4 months following the initial bite with isolated cutaneous manifestations, she was treated symptomatically with colchicine for EN.1,2
Final Thoughts
Our patient presented with EN following a bite from a copperhead snake. Physicians should be aware of possible etiologies of EN to evaluate patients who present with new-onset tender subcutaneous nodules. Additionally, physicians should be aware of venomous snakes endemic to their region and also understand the various complications that can result following a snakebite, with the potential for lingering cutaneous manifestations weeks to months following the initial bite.
- Warrell DA. Snake bite. Lancet. 2010;375:77-88. doi:10.1016/S0140-6736(09)61754-2
- White J. Overview of venomous snakes of the world. In: Dart RC, eds. Medical Toxicology. 3rd ed. Lippincott, Williams, & Wilkins; 2004:1543
- Spiller HA, Bosse GM. Prospective study of morbidity associated with snakebite envenomation. J Toxicol Clin Toxicol. 2003;41:125-130. doi:10.1081/clt-120019127
- Scharman EJ, Noffsinger VD. Copperhead snakebites: clinical severity of local effects. Ann Emerg Med. 2001;38:55-61. doi:10.1067/mem.2001.116148
- Hafsi W, Badri T. Erythema nodosum. In: StatPearls. StatPearls Publishing; November 28, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470369/
- Nowowiejska J, Baran A, Flisiak I. Rare coexistence of unilateral erythema nodosum with erysipelas in the area of previous adder bite. Przegl Epidemiol. 2020;74:355-361. doi:10.32394/pe.74.28
- Auerbach PS, Hays JT. Erythema nodosum following a jellyfish sting. J Emerg Med. 1987;5:487-491. doi:10.1016/0736-4679(87)90211-3
- Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327. doi:10.1111/j.1529-8019.2010.01332.x
- Sánchez Yus E, Sanz Vico MD, de Diego V. Miescher’s radial granuloma. a characteristic marker of erythema nodosum. Am J Dermatopathol. 1989;11:434-442. doi:10.1097/00000372-198910000-00005
- Mehta SR, Sashindran VK. Clinical features and management of snake bite. Med J Armed Forces India. 2002;58:247-249. doi:10.1016/S0377-1237(02)80140-X
- Brys AK, Gandolfi BM, Levinson H, et al. Copperhead envenomation resulting in a rare case of hand compartment syndrome and subsequent fasciotomy. Plast Reconstr Surg Glob Open. 2015;3:E396. doi:10.1097/GOX.0000000000000367
- Clark RF, Selden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993;11:583-586. doi:10.1016/0736-4679(93)90313-v
- Buchanan JT, Thurman J. Crotalidae envenomation. In: StatPearls. StatPearls Publishing; October 3, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551615/
- Warrell DA. Snake bite. Lancet. 2010;375:77-88. doi:10.1016/S0140-6736(09)61754-2
- White J. Overview of venomous snakes of the world. In: Dart RC, eds. Medical Toxicology. 3rd ed. Lippincott, Williams, & Wilkins; 2004:1543
- Spiller HA, Bosse GM. Prospective study of morbidity associated with snakebite envenomation. J Toxicol Clin Toxicol. 2003;41:125-130. doi:10.1081/clt-120019127
- Scharman EJ, Noffsinger VD. Copperhead snakebites: clinical severity of local effects. Ann Emerg Med. 2001;38:55-61. doi:10.1067/mem.2001.116148
- Hafsi W, Badri T. Erythema nodosum. In: StatPearls. StatPearls Publishing; November 28, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470369/
- Nowowiejska J, Baran A, Flisiak I. Rare coexistence of unilateral erythema nodosum with erysipelas in the area of previous adder bite. Przegl Epidemiol. 2020;74:355-361. doi:10.32394/pe.74.28
- Auerbach PS, Hays JT. Erythema nodosum following a jellyfish sting. J Emerg Med. 1987;5:487-491. doi:10.1016/0736-4679(87)90211-3
- Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327. doi:10.1111/j.1529-8019.2010.01332.x
- Sánchez Yus E, Sanz Vico MD, de Diego V. Miescher’s radial granuloma. a characteristic marker of erythema nodosum. Am J Dermatopathol. 1989;11:434-442. doi:10.1097/00000372-198910000-00005
- Mehta SR, Sashindran VK. Clinical features and management of snake bite. Med J Armed Forces India. 2002;58:247-249. doi:10.1016/S0377-1237(02)80140-X
- Brys AK, Gandolfi BM, Levinson H, et al. Copperhead envenomation resulting in a rare case of hand compartment syndrome and subsequent fasciotomy. Plast Reconstr Surg Glob Open. 2015;3:E396. doi:10.1097/GOX.0000000000000367
- Clark RF, Selden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993;11:583-586. doi:10.1016/0736-4679(93)90313-v
- Buchanan JT, Thurman J. Crotalidae envenomation. In: StatPearls. StatPearls Publishing; October 3, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551615/
Practice Points
- Erythema nodosum (EN) can occur following snakebites from pit vipers such as the eastern copperhead.
- The acute phase of EN is neutrophilic and responds to colchicine. The chronic phase of EN is granulomatous and responds best to rest and elevation as well as nonsteroidal anti-inflammatory drugs and iodides.
Generalized Fixed Drug Eruptions Require Urgent Care: A Case Series
Recognizing cutaneous drug eruptions is important for treatment and prevention of recurrence. Fixed drug eruptions (FDEs) typically are harmless but can have major negative cosmetic consequences for patients. In its more severe forms, patients are at risk for widespread epithelial necrosis with accompanying complications. We report 1 patient with generalized FDE and 2 with generalized bullous FDE. We also discuss the recognition and treatment of the condition. Two patients previously had been diagnosed with systemic lupus erythematosus (SLE).
Case Series
Patient 1—A 60-year-old woman presented to dermatology with a rash on the trunk and groin folds of 4 days’ duration. She had a history of SLE and cutaneous lupus treated with hydroxychloroquine 200 mg twice daily and topical corticosteroids. She had started sulfamethoxazole-trimethoprim for a urinary tract infection with a rash appearing 1 day later. She reported burning skin pain with progression to blisters that “sloughed” off. She denied any known history of allergy to sulfa drugs. Prior to evaluation by dermatology, she visited an urgent care facility and was prescribed hydroxyzine and intramuscular corticosteroids. At presentation to dermatology 3 days after taking sulfamethoxazole-trimethoprim, she had annular flaccid bullae and superficial erosions with dusky borders on the right posterior thigh, right side of the chest, left inframammary fold, and right inguinal fold (Figure 1). She had no ocular, oral, or vaginal erosions. A diagnosis of generalized bullous FDE was favored over erythema multiforme or Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN). Shave biopsies from lesions on the right posterior thigh and right inguinal fold demonstrated interface dermatitis with epidermal necrosis, pigment incontinence, and numerous eosinophils. Direct immunofluorescence of the perilesional skin was negative for immunoprotein deposition. These findings were consistent with the clinical impression of generalized bullous FDE. Prior to receiving the histopathology report, the patient was initiated on a regimen of cyclosporine 5 mg/kg/d in the setting of normal renal function and followed until the eruption resolved completely. Cyclosporine was tapered at 2 weeks and discontinued at 3 weeks.
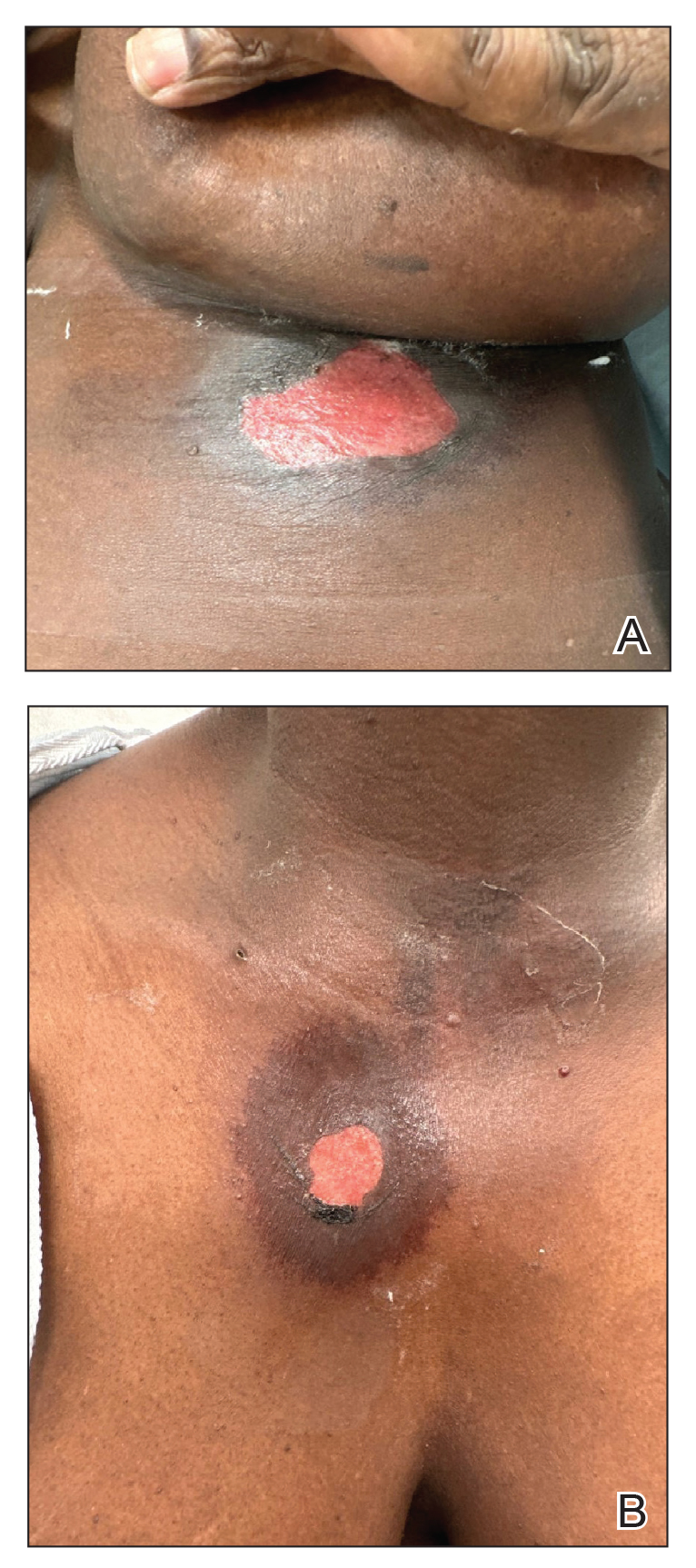
Patient 2—A 32-year-old woman presented for follow-up management of discoid lupus erythematosus. She had a history of systemic and cutaneous lupus, juvenile rheumatoid arthritis, and mixed connective tissue disease managed with prednisone, hydroxychloroquine, azathioprine, and belimumab. Physical examination revealed scarring alopecia with dyspigmentation and active inflammation consistent with uncontrolled cutaneous lupus. However, she also had oval-shaped hyperpigmented patches over the left breast, clavicle, and anterior chest consistent with a generalized FDE (Figure 2). The patient did not recall a history of similar lesions and could not identify a possible trigger. She was counseled on possible culprits and advised to avoid unnecessary medications. She had an unremarkable clinical course; therefore, no further intervention was necessary.
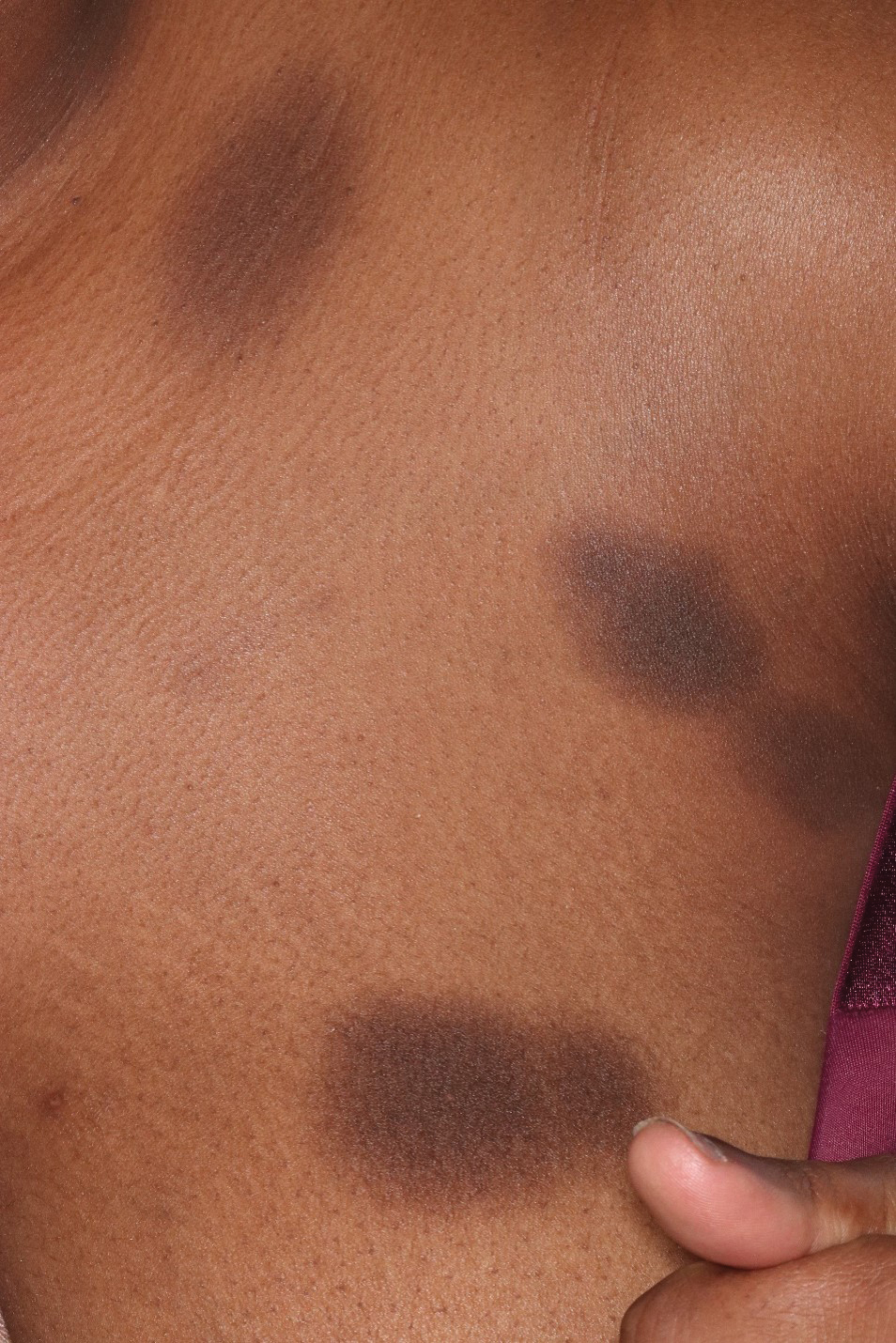
Patient 3—A 33-year-old man presented to the emergency department with a painful rash on the chest and back of 2 days’ duration that began 1 hour after taking naproxen (dosage unknown) for back pain. He had no notable medical history. The patient stated that the rash had slowly worsened and started to develop blisters. He visited an urgent care facility 1 day prior to the current presentation and was started on a 5-day course of prednisone 40 mg daily; the first 2 doses did not help. He denied any mucosal involvement apart from a tender lesion on the penis. He reported a history of an allergic reaction to penicillin. Physical examination revealed extensive dusky violaceous annular plaques with erythematous borders across the anterior and posterior trunk (Figure 3). Multiple flaccid bullae developed within these plaques, involving 15% of the body surface area. He was diagnosed with generalized bullous FDE based on the clinical history and histopathology. He was admitted to the burn intensive care unit and treated with cyclosporine 3 mg/kg/d with subsequent resolution of the eruption.
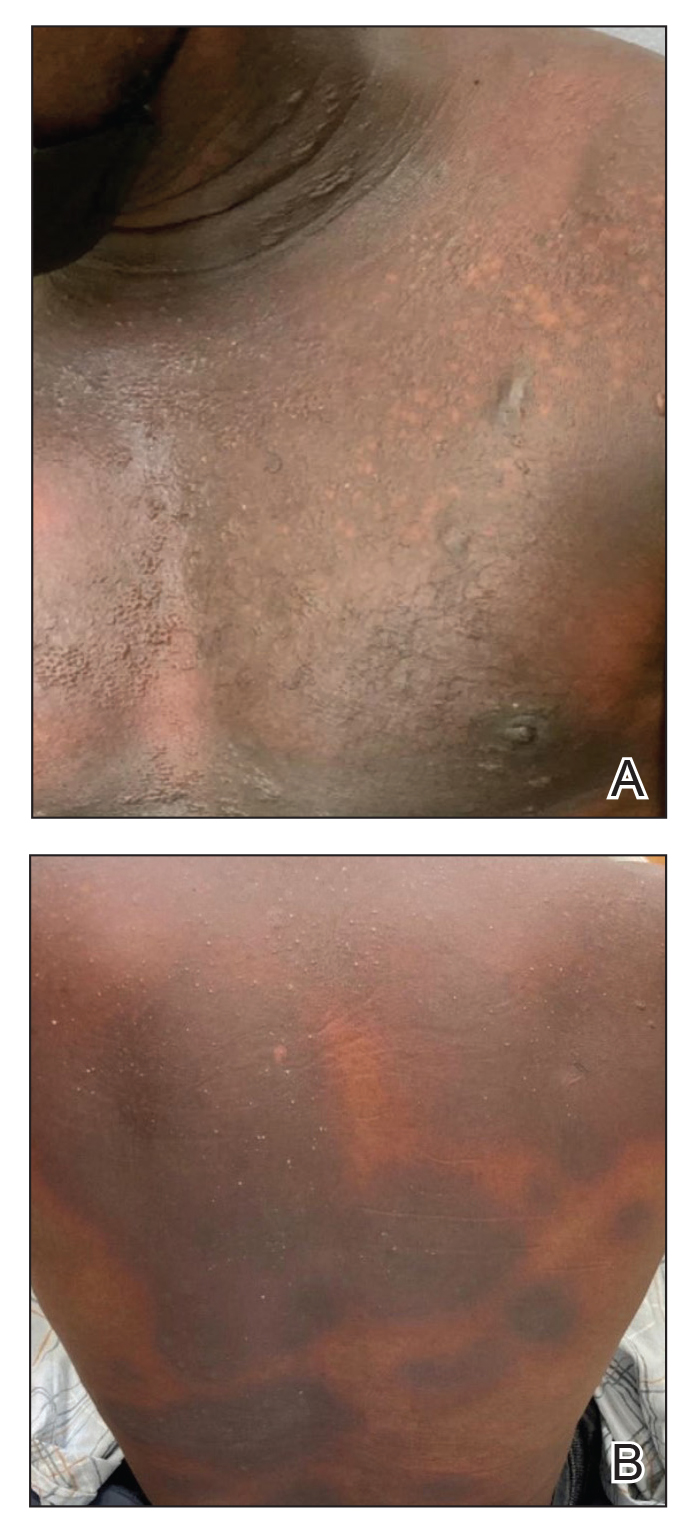
Comment
Presentation of FDEs—A fixed drug eruption manifests with 1 or more well-demarcated, red or violaceous, annular patches that resolve with postinflammatory hyperpigmentation; it occasionally may manifest with bullae. Initial eruptions may occur up to 2 weeks following medication exposure, but recurrent eruptions usually happen within minutes to hours later. They often are in the same location as prior lesions. A fixed drug eruption can be solitary, scattered, or generalized; a generalized FDE typically demonstrates multiple bilateral lesions that may itch, burn, or cause no symptoms. Patients can experience an FDE at any age, though the median age is reported as 35 to 60 years of age.1 A fixed drug eruption usually occurs after ingestion of oral medications, though there have been a few reports with iodinated contrast.2 Well-known culprits include antibiotics (eg, sulfamethoxazole-trimethoprim, tetracyclines, penicillins/cephalosporins, quinolones, dapsone), nonsteroidal anti-inflammatory drugs, acetaminophen (eg, paracetamol), barbiturates, antimalarials, and anticonvulsants. It also can occur with vaccines or with certain foods (fixed food eruption).3,4 Clinicians may try an oral drug challenge to identify the cause of an FDE, but in patients with a history of a generalized FDE, the risk for developing an increasingly severe reaction with repeated exposure to the medication is too high.5
Histopathology—Patch testing at the site of prior eruption with suspected drug culprits may be useful.6 Histopathology of FDE typically demonstrates vacuolar changes at the dermoepidermal junction with a lichenoid lymphocytic infiltrate. Early lesions often show a predominance of eosinophils. Subepidermal clefting is a feature of the bullous variant. In an active lesion, there are large numbers of CD8+ T lymphocytes expressing natural killer cell–associated molecules.7 The pathologic mechanism is not well understood, though it has been hypothesized that memory CD8+ cells are maintained in specific regions of the epidermis by IL-15 produced in the microenvironment and are activated upon rechallenge.7Considerations in Generalized Bullous FDE—Generalized FDE is defined in the literature as an FDE with involvement of 3 of 6 body areas: head, neck, trunk, upper limbs, lower limbs, and genital area. It may cover more or less than 10% of the body surface area.8-10 Although an isolated FDE frequently is asymptomatic and may not be cause for alarm, recurring drug eruptions increase the risk for development of generalized bullous FDE. Generalized bullous FDE is a rare subset. It is frequently misdiagnosed, and data on its incidence are uncertain.11 Of note, several pathologies causing bullous lesions may be in the differential diagnosis, including bullous pemphigoid; pemphigus vulgaris; bullous SLE; or bullae from cutaneous lupus, staphylococcal scalded skin syndrome, erythema multiforme, or SJS/TEN.12 When matched for body surface area involvement with SJS/TEN, generalized bullous FDE shares nearly identical mortality rates10; therefore, these patients should be treated with the same level of urgency and admitted to a critical care or burn unit, as they are at serious risk for infection and other complications.13
Clinical history and presentation along with histopathologic findings help to narrow down the differential diagnosis. Clinically, generalized bullous FDE does not affect the surrounding skin and manifests sooner after drug exposure (1–24 hours) with less mucosal involvement than SJS/TEN.9 Additionally, SJS/TEN patients frequently have generalized malaise and/or fever, while generalized bullous FDE patients do not. Finally, patients with generalized bullous FDE may report a history of a cutaneous eruption similar in morphology or in the same location.
Histopathologically, generalized bullous FDE may be similar to FDE with the addition of a subepidermal blister. Generalized bullous FDE patients have greater eosinophil infiltration and dermal melanophages than patients with SJS/TEN.9 Cellular infiltrates in generalized bullous FDE include more dermal CD41 cells, such as Foxp31 regulatory T cells; fewer intraepidermal CD561 cells; and fewer intraepidermal cells with granulysin.9 Occasionally, generalized bullous FDE causes full-thickness necrosis. In those cases, generalized bullous FDE cannot reliably be distinguished from other conditions with epidermal necrolysis on histopathology.13
FDE Diagnostics—A cytotoxin produced by
Management—Avoidance of the inciting drug often is sufficient for patients with an FDE, as demonstrated in patient 2 in our case series. Clinicians also should counsel patients on avoidance of potential cross-reacting drugs. Symptomatic treatment for itch or pain is appropriate and may include antihistamines or topical steroids. Nonsteroidal anti-inflammatory drugs may exacerbate or be causative of FDE. For generalized bullous FDE, cyclosporine is favored in the literature15,16 and was used to successfully treat both patients 1 and 3 in our case series. A short course of systemic corticosteroids or intravenous immunoglobulin also may be considered. Mild cases of generalized bullous FDE may be treated with close outpatient follow-up (patient 1), while severe cases require inpatient or even critical care monitoring with aggressive medical management to prevent the progression of skin desquamation (patient 3). Patients with severe oral lesions may require inpatient support for fluid maintenance.
Lupus History—Two patients in our case series had a history of lupus. Lupus itself can cause primary bullous lesions. Similar to FDE, bullous SLE can involve sun-exposed and nonexposed areas of the skin as well as the mucous membranes with a predilection for the lower vermilion lip.17 In bullous SLE, tense subepidermal blisters with a neutrophil-rich infiltrate form due to circulating antibodies to type VII collagen. These blisters have an erythematous or urticated base, most commonly on the face, upper trunk, and proximal extremities.18 In both SLE with skin manifestations and lupus limited to the skin, bullae may form due to extensive vacuolar degeneration. Similar to TEN, they can form rapidly in a widespread distribution.17 However, there is limited mucosal involvement, no clear drug association, and a better prognosis. Bullae caused by lupus will frequently demonstrate deposition of immunoproteins IgG, IgM, IgA, and complement component 3 at the basement membrane zone in perilesional skin on direct immunofluorescence. However, negative direct immunofluorescence does not rule out lupus.12 At the same time, patients with lupus frequently have comorbidities requiring multiple medications; the need for these medications may predispose patients to higher rates of cutaneous drug eruptions.19 To our knowledge, there is no known association between FDE and lupus.
Conclusion
Patients with acute eruptions following the initiation of a new prescription or over-the-counter medication require urgent evaluation. Generalized bullous FDE requires timely diagnosis and intervention. Patients with lupus have an increased risk for cutaneous drug eruptions due to polypharmacy. Further investigation is necessary to determine if there is a pathophysiologic mechanism responsible for the development of FDE in lupus patients.
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925.
- Gavin M, Sharp L, Walker K, et al. Contrast-induced generalized bullous fixed drug eruption resembling Stevens-Johnson syndrome. Proc (Bayl Univ Med Cent). 2019;32:601-602.
- Kabir S, Feit EJ, Heilman ER. Generalized fixed drug eruption following Pfizer-BioNtech COVID-19 vaccination. Clin Case Rep. 2022;10:E6684.
- Choi S, Kim SH, Hwang JH, et al. Rapidly progressing generalized bullous fixed drug eruption after the first dose of COVID-19 messenger RNA vaccination. J Dermatol. 2023;50:1190-1193.
- Mahboob A, Haroon TS. Drugs causing fixed eruptions: a study of 450 cases. Int J Dermatol. 1998;37:833-838.
- Shiohara T. Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol. 2009;9:316-321.
- Mizukawa Y, Yamazaki Y, Shiohara T. In vivo dynamics of intraepidermal CD8+ T cells and CD4+ T cells during the evolution of fixed drug eruption. Br J Dermatol. 2008;158:1230-1238.
- Lee CH, Chen YC, Cho YT, et al. Fixed-drug eruption: a retrospective study in a single referral center in northern Taiwan. Dermatologica Sinica. 2012;30:11-15.
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548.
- Lipowicz S, Sekula P, Ingen-Housz-Oro S, et al. Prognosis of generalized bullous fixed drug eruption: comparison with Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2013;168:726-732.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ranario JS, Smith JL. Bullous lesions in a patient with systemic lupus erythematosus. J Clin Aesthet Dermatol. 2014;7:44-49.
- Perron E, Viarnaud A, Marciano L, et al. Clinical and histological features of fixed drug eruption: a single-centre series of 73 cases with comparison between bullous and non-bullous forms. Eur J Dermatol. 2021;31:372-380.
- Chen CB, Kuo KL, Wang CW, et al. Detecting lesional granulysin levels for rapid diagnosis of cytotoxic T lymphocyte-mediated bullous skin disorders. J Allergy Clin Immunol Pract. 2021;9:1327-1337.e3.
- Beniwal R, Gupta LK, Khare AK, et al. Cyclosporine in generalized bullous-fixed drug eruption. Indian J Dermatol. 2018;63:432-433.
- Vargas Mora P, García S, Valenzuela F, et al. Generalized bullous fixed drug eruption successfully treated with cyclosporine. Dermatol Ther. 2020;33:E13492.
- Montagnon CM, Tolkachjov SN, Murrell DF, et al. Subepithelial autoimmune blistering dermatoses: clinical features and diagnosis. J Am Acad Dermatol. 2021;85:1-14.
- Sebaratnam DF, Murrell DF. Bullous systemic lupus erythematosus. Dermatol Clin. 2011;29:649-653.
- Zonzits E, Aberer W, Tappeiner G. Drug eruptions from mesna. After cyclophosphamide treatment of patients with systemic lupus erythematosus and dermatomyositis. Arch Dermatol. 1992;128:80-82.
Recognizing cutaneous drug eruptions is important for treatment and prevention of recurrence. Fixed drug eruptions (FDEs) typically are harmless but can have major negative cosmetic consequences for patients. In its more severe forms, patients are at risk for widespread epithelial necrosis with accompanying complications. We report 1 patient with generalized FDE and 2 with generalized bullous FDE. We also discuss the recognition and treatment of the condition. Two patients previously had been diagnosed with systemic lupus erythematosus (SLE).
Case Series
Patient 1—A 60-year-old woman presented to dermatology with a rash on the trunk and groin folds of 4 days’ duration. She had a history of SLE and cutaneous lupus treated with hydroxychloroquine 200 mg twice daily and topical corticosteroids. She had started sulfamethoxazole-trimethoprim for a urinary tract infection with a rash appearing 1 day later. She reported burning skin pain with progression to blisters that “sloughed” off. She denied any known history of allergy to sulfa drugs. Prior to evaluation by dermatology, she visited an urgent care facility and was prescribed hydroxyzine and intramuscular corticosteroids. At presentation to dermatology 3 days after taking sulfamethoxazole-trimethoprim, she had annular flaccid bullae and superficial erosions with dusky borders on the right posterior thigh, right side of the chest, left inframammary fold, and right inguinal fold (Figure 1). She had no ocular, oral, or vaginal erosions. A diagnosis of generalized bullous FDE was favored over erythema multiforme or Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN). Shave biopsies from lesions on the right posterior thigh and right inguinal fold demonstrated interface dermatitis with epidermal necrosis, pigment incontinence, and numerous eosinophils. Direct immunofluorescence of the perilesional skin was negative for immunoprotein deposition. These findings were consistent with the clinical impression of generalized bullous FDE. Prior to receiving the histopathology report, the patient was initiated on a regimen of cyclosporine 5 mg/kg/d in the setting of normal renal function and followed until the eruption resolved completely. Cyclosporine was tapered at 2 weeks and discontinued at 3 weeks.

Patient 2—A 32-year-old woman presented for follow-up management of discoid lupus erythematosus. She had a history of systemic and cutaneous lupus, juvenile rheumatoid arthritis, and mixed connective tissue disease managed with prednisone, hydroxychloroquine, azathioprine, and belimumab. Physical examination revealed scarring alopecia with dyspigmentation and active inflammation consistent with uncontrolled cutaneous lupus. However, she also had oval-shaped hyperpigmented patches over the left breast, clavicle, and anterior chest consistent with a generalized FDE (Figure 2). The patient did not recall a history of similar lesions and could not identify a possible trigger. She was counseled on possible culprits and advised to avoid unnecessary medications. She had an unremarkable clinical course; therefore, no further intervention was necessary.

Patient 3—A 33-year-old man presented to the emergency department with a painful rash on the chest and back of 2 days’ duration that began 1 hour after taking naproxen (dosage unknown) for back pain. He had no notable medical history. The patient stated that the rash had slowly worsened and started to develop blisters. He visited an urgent care facility 1 day prior to the current presentation and was started on a 5-day course of prednisone 40 mg daily; the first 2 doses did not help. He denied any mucosal involvement apart from a tender lesion on the penis. He reported a history of an allergic reaction to penicillin. Physical examination revealed extensive dusky violaceous annular plaques with erythematous borders across the anterior and posterior trunk (Figure 3). Multiple flaccid bullae developed within these plaques, involving 15% of the body surface area. He was diagnosed with generalized bullous FDE based on the clinical history and histopathology. He was admitted to the burn intensive care unit and treated with cyclosporine 3 mg/kg/d with subsequent resolution of the eruption.

Comment
Presentation of FDEs—A fixed drug eruption manifests with 1 or more well-demarcated, red or violaceous, annular patches that resolve with postinflammatory hyperpigmentation; it occasionally may manifest with bullae. Initial eruptions may occur up to 2 weeks following medication exposure, but recurrent eruptions usually happen within minutes to hours later. They often are in the same location as prior lesions. A fixed drug eruption can be solitary, scattered, or generalized; a generalized FDE typically demonstrates multiple bilateral lesions that may itch, burn, or cause no symptoms. Patients can experience an FDE at any age, though the median age is reported as 35 to 60 years of age.1 A fixed drug eruption usually occurs after ingestion of oral medications, though there have been a few reports with iodinated contrast.2 Well-known culprits include antibiotics (eg, sulfamethoxazole-trimethoprim, tetracyclines, penicillins/cephalosporins, quinolones, dapsone), nonsteroidal anti-inflammatory drugs, acetaminophen (eg, paracetamol), barbiturates, antimalarials, and anticonvulsants. It also can occur with vaccines or with certain foods (fixed food eruption).3,4 Clinicians may try an oral drug challenge to identify the cause of an FDE, but in patients with a history of a generalized FDE, the risk for developing an increasingly severe reaction with repeated exposure to the medication is too high.5
Histopathology—Patch testing at the site of prior eruption with suspected drug culprits may be useful.6 Histopathology of FDE typically demonstrates vacuolar changes at the dermoepidermal junction with a lichenoid lymphocytic infiltrate. Early lesions often show a predominance of eosinophils. Subepidermal clefting is a feature of the bullous variant. In an active lesion, there are large numbers of CD8+ T lymphocytes expressing natural killer cell–associated molecules.7 The pathologic mechanism is not well understood, though it has been hypothesized that memory CD8+ cells are maintained in specific regions of the epidermis by IL-15 produced in the microenvironment and are activated upon rechallenge.7Considerations in Generalized Bullous FDE—Generalized FDE is defined in the literature as an FDE with involvement of 3 of 6 body areas: head, neck, trunk, upper limbs, lower limbs, and genital area. It may cover more or less than 10% of the body surface area.8-10 Although an isolated FDE frequently is asymptomatic and may not be cause for alarm, recurring drug eruptions increase the risk for development of generalized bullous FDE. Generalized bullous FDE is a rare subset. It is frequently misdiagnosed, and data on its incidence are uncertain.11 Of note, several pathologies causing bullous lesions may be in the differential diagnosis, including bullous pemphigoid; pemphigus vulgaris; bullous SLE; or bullae from cutaneous lupus, staphylococcal scalded skin syndrome, erythema multiforme, or SJS/TEN.12 When matched for body surface area involvement with SJS/TEN, generalized bullous FDE shares nearly identical mortality rates10; therefore, these patients should be treated with the same level of urgency and admitted to a critical care or burn unit, as they are at serious risk for infection and other complications.13
Clinical history and presentation along with histopathologic findings help to narrow down the differential diagnosis. Clinically, generalized bullous FDE does not affect the surrounding skin and manifests sooner after drug exposure (1–24 hours) with less mucosal involvement than SJS/TEN.9 Additionally, SJS/TEN patients frequently have generalized malaise and/or fever, while generalized bullous FDE patients do not. Finally, patients with generalized bullous FDE may report a history of a cutaneous eruption similar in morphology or in the same location.
Histopathologically, generalized bullous FDE may be similar to FDE with the addition of a subepidermal blister. Generalized bullous FDE patients have greater eosinophil infiltration and dermal melanophages than patients with SJS/TEN.9 Cellular infiltrates in generalized bullous FDE include more dermal CD41 cells, such as Foxp31 regulatory T cells; fewer intraepidermal CD561 cells; and fewer intraepidermal cells with granulysin.9 Occasionally, generalized bullous FDE causes full-thickness necrosis. In those cases, generalized bullous FDE cannot reliably be distinguished from other conditions with epidermal necrolysis on histopathology.13
FDE Diagnostics—A cytotoxin produced by
Management—Avoidance of the inciting drug often is sufficient for patients with an FDE, as demonstrated in patient 2 in our case series. Clinicians also should counsel patients on avoidance of potential cross-reacting drugs. Symptomatic treatment for itch or pain is appropriate and may include antihistamines or topical steroids. Nonsteroidal anti-inflammatory drugs may exacerbate or be causative of FDE. For generalized bullous FDE, cyclosporine is favored in the literature15,16 and was used to successfully treat both patients 1 and 3 in our case series. A short course of systemic corticosteroids or intravenous immunoglobulin also may be considered. Mild cases of generalized bullous FDE may be treated with close outpatient follow-up (patient 1), while severe cases require inpatient or even critical care monitoring with aggressive medical management to prevent the progression of skin desquamation (patient 3). Patients with severe oral lesions may require inpatient support for fluid maintenance.
Lupus History—Two patients in our case series had a history of lupus. Lupus itself can cause primary bullous lesions. Similar to FDE, bullous SLE can involve sun-exposed and nonexposed areas of the skin as well as the mucous membranes with a predilection for the lower vermilion lip.17 In bullous SLE, tense subepidermal blisters with a neutrophil-rich infiltrate form due to circulating antibodies to type VII collagen. These blisters have an erythematous or urticated base, most commonly on the face, upper trunk, and proximal extremities.18 In both SLE with skin manifestations and lupus limited to the skin, bullae may form due to extensive vacuolar degeneration. Similar to TEN, they can form rapidly in a widespread distribution.17 However, there is limited mucosal involvement, no clear drug association, and a better prognosis. Bullae caused by lupus will frequently demonstrate deposition of immunoproteins IgG, IgM, IgA, and complement component 3 at the basement membrane zone in perilesional skin on direct immunofluorescence. However, negative direct immunofluorescence does not rule out lupus.12 At the same time, patients with lupus frequently have comorbidities requiring multiple medications; the need for these medications may predispose patients to higher rates of cutaneous drug eruptions.19 To our knowledge, there is no known association between FDE and lupus.
Conclusion
Patients with acute eruptions following the initiation of a new prescription or over-the-counter medication require urgent evaluation. Generalized bullous FDE requires timely diagnosis and intervention. Patients with lupus have an increased risk for cutaneous drug eruptions due to polypharmacy. Further investigation is necessary to determine if there is a pathophysiologic mechanism responsible for the development of FDE in lupus patients.
Recognizing cutaneous drug eruptions is important for treatment and prevention of recurrence. Fixed drug eruptions (FDEs) typically are harmless but can have major negative cosmetic consequences for patients. In its more severe forms, patients are at risk for widespread epithelial necrosis with accompanying complications. We report 1 patient with generalized FDE and 2 with generalized bullous FDE. We also discuss the recognition and treatment of the condition. Two patients previously had been diagnosed with systemic lupus erythematosus (SLE).
Case Series
Patient 1—A 60-year-old woman presented to dermatology with a rash on the trunk and groin folds of 4 days’ duration. She had a history of SLE and cutaneous lupus treated with hydroxychloroquine 200 mg twice daily and topical corticosteroids. She had started sulfamethoxazole-trimethoprim for a urinary tract infection with a rash appearing 1 day later. She reported burning skin pain with progression to blisters that “sloughed” off. She denied any known history of allergy to sulfa drugs. Prior to evaluation by dermatology, she visited an urgent care facility and was prescribed hydroxyzine and intramuscular corticosteroids. At presentation to dermatology 3 days after taking sulfamethoxazole-trimethoprim, she had annular flaccid bullae and superficial erosions with dusky borders on the right posterior thigh, right side of the chest, left inframammary fold, and right inguinal fold (Figure 1). She had no ocular, oral, or vaginal erosions. A diagnosis of generalized bullous FDE was favored over erythema multiforme or Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN). Shave biopsies from lesions on the right posterior thigh and right inguinal fold demonstrated interface dermatitis with epidermal necrosis, pigment incontinence, and numerous eosinophils. Direct immunofluorescence of the perilesional skin was negative for immunoprotein deposition. These findings were consistent with the clinical impression of generalized bullous FDE. Prior to receiving the histopathology report, the patient was initiated on a regimen of cyclosporine 5 mg/kg/d in the setting of normal renal function and followed until the eruption resolved completely. Cyclosporine was tapered at 2 weeks and discontinued at 3 weeks.

Patient 2—A 32-year-old woman presented for follow-up management of discoid lupus erythematosus. She had a history of systemic and cutaneous lupus, juvenile rheumatoid arthritis, and mixed connective tissue disease managed with prednisone, hydroxychloroquine, azathioprine, and belimumab. Physical examination revealed scarring alopecia with dyspigmentation and active inflammation consistent with uncontrolled cutaneous lupus. However, she also had oval-shaped hyperpigmented patches over the left breast, clavicle, and anterior chest consistent with a generalized FDE (Figure 2). The patient did not recall a history of similar lesions and could not identify a possible trigger. She was counseled on possible culprits and advised to avoid unnecessary medications. She had an unremarkable clinical course; therefore, no further intervention was necessary.

Patient 3—A 33-year-old man presented to the emergency department with a painful rash on the chest and back of 2 days’ duration that began 1 hour after taking naproxen (dosage unknown) for back pain. He had no notable medical history. The patient stated that the rash had slowly worsened and started to develop blisters. He visited an urgent care facility 1 day prior to the current presentation and was started on a 5-day course of prednisone 40 mg daily; the first 2 doses did not help. He denied any mucosal involvement apart from a tender lesion on the penis. He reported a history of an allergic reaction to penicillin. Physical examination revealed extensive dusky violaceous annular plaques with erythematous borders across the anterior and posterior trunk (Figure 3). Multiple flaccid bullae developed within these plaques, involving 15% of the body surface area. He was diagnosed with generalized bullous FDE based on the clinical history and histopathology. He was admitted to the burn intensive care unit and treated with cyclosporine 3 mg/kg/d with subsequent resolution of the eruption.

Comment
Presentation of FDEs—A fixed drug eruption manifests with 1 or more well-demarcated, red or violaceous, annular patches that resolve with postinflammatory hyperpigmentation; it occasionally may manifest with bullae. Initial eruptions may occur up to 2 weeks following medication exposure, but recurrent eruptions usually happen within minutes to hours later. They often are in the same location as prior lesions. A fixed drug eruption can be solitary, scattered, or generalized; a generalized FDE typically demonstrates multiple bilateral lesions that may itch, burn, or cause no symptoms. Patients can experience an FDE at any age, though the median age is reported as 35 to 60 years of age.1 A fixed drug eruption usually occurs after ingestion of oral medications, though there have been a few reports with iodinated contrast.2 Well-known culprits include antibiotics (eg, sulfamethoxazole-trimethoprim, tetracyclines, penicillins/cephalosporins, quinolones, dapsone), nonsteroidal anti-inflammatory drugs, acetaminophen (eg, paracetamol), barbiturates, antimalarials, and anticonvulsants. It also can occur with vaccines or with certain foods (fixed food eruption).3,4 Clinicians may try an oral drug challenge to identify the cause of an FDE, but in patients with a history of a generalized FDE, the risk for developing an increasingly severe reaction with repeated exposure to the medication is too high.5
Histopathology—Patch testing at the site of prior eruption with suspected drug culprits may be useful.6 Histopathology of FDE typically demonstrates vacuolar changes at the dermoepidermal junction with a lichenoid lymphocytic infiltrate. Early lesions often show a predominance of eosinophils. Subepidermal clefting is a feature of the bullous variant. In an active lesion, there are large numbers of CD8+ T lymphocytes expressing natural killer cell–associated molecules.7 The pathologic mechanism is not well understood, though it has been hypothesized that memory CD8+ cells are maintained in specific regions of the epidermis by IL-15 produced in the microenvironment and are activated upon rechallenge.7Considerations in Generalized Bullous FDE—Generalized FDE is defined in the literature as an FDE with involvement of 3 of 6 body areas: head, neck, trunk, upper limbs, lower limbs, and genital area. It may cover more or less than 10% of the body surface area.8-10 Although an isolated FDE frequently is asymptomatic and may not be cause for alarm, recurring drug eruptions increase the risk for development of generalized bullous FDE. Generalized bullous FDE is a rare subset. It is frequently misdiagnosed, and data on its incidence are uncertain.11 Of note, several pathologies causing bullous lesions may be in the differential diagnosis, including bullous pemphigoid; pemphigus vulgaris; bullous SLE; or bullae from cutaneous lupus, staphylococcal scalded skin syndrome, erythema multiforme, or SJS/TEN.12 When matched for body surface area involvement with SJS/TEN, generalized bullous FDE shares nearly identical mortality rates10; therefore, these patients should be treated with the same level of urgency and admitted to a critical care or burn unit, as they are at serious risk for infection and other complications.13
Clinical history and presentation along with histopathologic findings help to narrow down the differential diagnosis. Clinically, generalized bullous FDE does not affect the surrounding skin and manifests sooner after drug exposure (1–24 hours) with less mucosal involvement than SJS/TEN.9 Additionally, SJS/TEN patients frequently have generalized malaise and/or fever, while generalized bullous FDE patients do not. Finally, patients with generalized bullous FDE may report a history of a cutaneous eruption similar in morphology or in the same location.
Histopathologically, generalized bullous FDE may be similar to FDE with the addition of a subepidermal blister. Generalized bullous FDE patients have greater eosinophil infiltration and dermal melanophages than patients with SJS/TEN.9 Cellular infiltrates in generalized bullous FDE include more dermal CD41 cells, such as Foxp31 regulatory T cells; fewer intraepidermal CD561 cells; and fewer intraepidermal cells with granulysin.9 Occasionally, generalized bullous FDE causes full-thickness necrosis. In those cases, generalized bullous FDE cannot reliably be distinguished from other conditions with epidermal necrolysis on histopathology.13
FDE Diagnostics—A cytotoxin produced by
Management—Avoidance of the inciting drug often is sufficient for patients with an FDE, as demonstrated in patient 2 in our case series. Clinicians also should counsel patients on avoidance of potential cross-reacting drugs. Symptomatic treatment for itch or pain is appropriate and may include antihistamines or topical steroids. Nonsteroidal anti-inflammatory drugs may exacerbate or be causative of FDE. For generalized bullous FDE, cyclosporine is favored in the literature15,16 and was used to successfully treat both patients 1 and 3 in our case series. A short course of systemic corticosteroids or intravenous immunoglobulin also may be considered. Mild cases of generalized bullous FDE may be treated with close outpatient follow-up (patient 1), while severe cases require inpatient or even critical care monitoring with aggressive medical management to prevent the progression of skin desquamation (patient 3). Patients with severe oral lesions may require inpatient support for fluid maintenance.
Lupus History—Two patients in our case series had a history of lupus. Lupus itself can cause primary bullous lesions. Similar to FDE, bullous SLE can involve sun-exposed and nonexposed areas of the skin as well as the mucous membranes with a predilection for the lower vermilion lip.17 In bullous SLE, tense subepidermal blisters with a neutrophil-rich infiltrate form due to circulating antibodies to type VII collagen. These blisters have an erythematous or urticated base, most commonly on the face, upper trunk, and proximal extremities.18 In both SLE with skin manifestations and lupus limited to the skin, bullae may form due to extensive vacuolar degeneration. Similar to TEN, they can form rapidly in a widespread distribution.17 However, there is limited mucosal involvement, no clear drug association, and a better prognosis. Bullae caused by lupus will frequently demonstrate deposition of immunoproteins IgG, IgM, IgA, and complement component 3 at the basement membrane zone in perilesional skin on direct immunofluorescence. However, negative direct immunofluorescence does not rule out lupus.12 At the same time, patients with lupus frequently have comorbidities requiring multiple medications; the need for these medications may predispose patients to higher rates of cutaneous drug eruptions.19 To our knowledge, there is no known association between FDE and lupus.
Conclusion
Patients with acute eruptions following the initiation of a new prescription or over-the-counter medication require urgent evaluation. Generalized bullous FDE requires timely diagnosis and intervention. Patients with lupus have an increased risk for cutaneous drug eruptions due to polypharmacy. Further investigation is necessary to determine if there is a pathophysiologic mechanism responsible for the development of FDE in lupus patients.
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925.
- Gavin M, Sharp L, Walker K, et al. Contrast-induced generalized bullous fixed drug eruption resembling Stevens-Johnson syndrome. Proc (Bayl Univ Med Cent). 2019;32:601-602.
- Kabir S, Feit EJ, Heilman ER. Generalized fixed drug eruption following Pfizer-BioNtech COVID-19 vaccination. Clin Case Rep. 2022;10:E6684.
- Choi S, Kim SH, Hwang JH, et al. Rapidly progressing generalized bullous fixed drug eruption after the first dose of COVID-19 messenger RNA vaccination. J Dermatol. 2023;50:1190-1193.
- Mahboob A, Haroon TS. Drugs causing fixed eruptions: a study of 450 cases. Int J Dermatol. 1998;37:833-838.
- Shiohara T. Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol. 2009;9:316-321.
- Mizukawa Y, Yamazaki Y, Shiohara T. In vivo dynamics of intraepidermal CD8+ T cells and CD4+ T cells during the evolution of fixed drug eruption. Br J Dermatol. 2008;158:1230-1238.
- Lee CH, Chen YC, Cho YT, et al. Fixed-drug eruption: a retrospective study in a single referral center in northern Taiwan. Dermatologica Sinica. 2012;30:11-15.
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548.
- Lipowicz S, Sekula P, Ingen-Housz-Oro S, et al. Prognosis of generalized bullous fixed drug eruption: comparison with Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2013;168:726-732.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ranario JS, Smith JL. Bullous lesions in a patient with systemic lupus erythematosus. J Clin Aesthet Dermatol. 2014;7:44-49.
- Perron E, Viarnaud A, Marciano L, et al. Clinical and histological features of fixed drug eruption: a single-centre series of 73 cases with comparison between bullous and non-bullous forms. Eur J Dermatol. 2021;31:372-380.
- Chen CB, Kuo KL, Wang CW, et al. Detecting lesional granulysin levels for rapid diagnosis of cytotoxic T lymphocyte-mediated bullous skin disorders. J Allergy Clin Immunol Pract. 2021;9:1327-1337.e3.
- Beniwal R, Gupta LK, Khare AK, et al. Cyclosporine in generalized bullous-fixed drug eruption. Indian J Dermatol. 2018;63:432-433.
- Vargas Mora P, García S, Valenzuela F, et al. Generalized bullous fixed drug eruption successfully treated with cyclosporine. Dermatol Ther. 2020;33:E13492.
- Montagnon CM, Tolkachjov SN, Murrell DF, et al. Subepithelial autoimmune blistering dermatoses: clinical features and diagnosis. J Am Acad Dermatol. 2021;85:1-14.
- Sebaratnam DF, Murrell DF. Bullous systemic lupus erythematosus. Dermatol Clin. 2011;29:649-653.
- Zonzits E, Aberer W, Tappeiner G. Drug eruptions from mesna. After cyclophosphamide treatment of patients with systemic lupus erythematosus and dermatomyositis. Arch Dermatol. 1992;128:80-82.
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925.
- Gavin M, Sharp L, Walker K, et al. Contrast-induced generalized bullous fixed drug eruption resembling Stevens-Johnson syndrome. Proc (Bayl Univ Med Cent). 2019;32:601-602.
- Kabir S, Feit EJ, Heilman ER. Generalized fixed drug eruption following Pfizer-BioNtech COVID-19 vaccination. Clin Case Rep. 2022;10:E6684.
- Choi S, Kim SH, Hwang JH, et al. Rapidly progressing generalized bullous fixed drug eruption after the first dose of COVID-19 messenger RNA vaccination. J Dermatol. 2023;50:1190-1193.
- Mahboob A, Haroon TS. Drugs causing fixed eruptions: a study of 450 cases. Int J Dermatol. 1998;37:833-838.
- Shiohara T. Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol. 2009;9:316-321.
- Mizukawa Y, Yamazaki Y, Shiohara T. In vivo dynamics of intraepidermal CD8+ T cells and CD4+ T cells during the evolution of fixed drug eruption. Br J Dermatol. 2008;158:1230-1238.
- Lee CH, Chen YC, Cho YT, et al. Fixed-drug eruption: a retrospective study in a single referral center in northern Taiwan. Dermatologica Sinica. 2012;30:11-15.
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548.
- Lipowicz S, Sekula P, Ingen-Housz-Oro S, et al. Prognosis of generalized bullous fixed drug eruption: comparison with Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2013;168:726-732.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ranario JS, Smith JL. Bullous lesions in a patient with systemic lupus erythematosus. J Clin Aesthet Dermatol. 2014;7:44-49.
- Perron E, Viarnaud A, Marciano L, et al. Clinical and histological features of fixed drug eruption: a single-centre series of 73 cases with comparison between bullous and non-bullous forms. Eur J Dermatol. 2021;31:372-380.
- Chen CB, Kuo KL, Wang CW, et al. Detecting lesional granulysin levels for rapid diagnosis of cytotoxic T lymphocyte-mediated bullous skin disorders. J Allergy Clin Immunol Pract. 2021;9:1327-1337.e3.
- Beniwal R, Gupta LK, Khare AK, et al. Cyclosporine in generalized bullous-fixed drug eruption. Indian J Dermatol. 2018;63:432-433.
- Vargas Mora P, García S, Valenzuela F, et al. Generalized bullous fixed drug eruption successfully treated with cyclosporine. Dermatol Ther. 2020;33:E13492.
- Montagnon CM, Tolkachjov SN, Murrell DF, et al. Subepithelial autoimmune blistering dermatoses: clinical features and diagnosis. J Am Acad Dermatol. 2021;85:1-14.
- Sebaratnam DF, Murrell DF. Bullous systemic lupus erythematosus. Dermatol Clin. 2011;29:649-653.
- Zonzits E, Aberer W, Tappeiner G. Drug eruptions from mesna. After cyclophosphamide treatment of patients with systemic lupus erythematosus and dermatomyositis. Arch Dermatol. 1992;128:80-82.
Practice Points
- Although localized fixed drug eruption (FDE) is a relatively benign diagnosis, generalized bullous FDE requires urgent management and may necessitate intensive burn care.
- Patients with lupus are at increased risk for drug eruptions due to polypharmacy, and there is a wide differential for bullous eruptions in these patients.
Reticulated Brownish Erythema on the Lower Back
The Diagnosis: Erythema Ab Igne
Based on the patient's long-standing history of back pain treated with heating pads as well as the normal laboratory findings and skin examination, a diagnosis of erythema ab igne (EAI) was made.
Erythema ab igne presents as reticulated brownish erythema or hyperpigmentation on sites exposed to prolonged use of heat sources such as heating pads, laptops, and space heaters. Erythema ab igne most commonly affects the lower back, thighs, or legs1-6; however, EAI can appear on atypical sites such as the forehead and eyebrows due to newer technology (eg, virtual reality headsets).7 The level of heat required for EAI to occur is below the threshold for thermal burns (<45 °C [113 °F]).1 Erythema ab igne can occur at any age, and woman are more commonly affected than men.8 The pathophysiology currently is unknown; however, recurrent and prolonged heat exposure may damage superficial vessels. As a result, hemosiderin accumulates in the skin, and hyperpigmentation subsequently occurs.9
The diagnosis of EAI is clinical, and early stages of the rash present as blanching reticulated erythema in areas associated with heat exposure. If the offending source of heat is not removed, EAI can progress to nonblanching, fixed, hyperpigmented plaques with skin atrophy, bullae, or hyperkeratosis. Patients often are asymptomatic; however, mild burning may occur.2 Histopathology reveals cellular atypia, epidermal atrophy, dilation of dermal blood vessels, a minute inflammatory infiltrate, and keratinocyte apoptosis.10 Skin biopsy may be necessary in cases of suspected malignancy due to chronic heat exposure. Lesions that ulcerate or evolve should raise suspicion for malignancy.11 Squamous cell carcinoma is the most common malignancy associated with EAI; other malignancies that may manifest include basal cell carcinoma, Merkel cell carcinoma, or cutaneous marginal zone lymphoma.2,12-14
Erythema ab igne often is mistaken for livedo reticularis, which appears more erythematous without hyperpigmentation or epidermal changes and may be associated with a pathologic state.15 The differential diagnosis in our patient, who was in her 40s with a history of fatigue and joint pain, included livedo reticularis associated with lupus; however, the history of heating pad use, normal laboratory findings, and presence of epidermal changes suggested EAI. Lupus typically affects the hand and knee joints.16 Additionally, livedo reticularis more commonly appears on the legs.15
Other differentials for EAI include livedo racemosa, cutaneous T-cell lymphoma, and cutis marmorata. Livedo racemosa presents with broken rings of erythema in young to middle-aged women and primarily affects the trunk and proximal limbs. It is associated with an underlying condition such as polyarteritis nodosa and less commonly with lupus erythematosus with antiphospholipid or Sneddon syndrome.15,17 Cutaneous T-cell lymphoma typically manifests with poikilodermatous patches larger than the palm, especially in covered areas of skin.18 Cutis marmorata is transient and temperature dependent.9
The key intervention for EAI is removal of the offending heat source.2 Patients should be counseled that the erythema and hyperpigmentation may take months to years to resolve. Topical hydroquinone or tretinoin may be used in cases of persistent hyperpigmentation.19 Patients who continue to use heating pads for long-standing pain should be advised to limit their use to short intervals without occlusion. If malignancy is a concern, a biopsy should be performed.20
- Wipf AJ, Brown MR. Malignant transformation of erythema ab igne. JAAD Case Rep. 2022;26:85-87. doi:10.1016/j.jdcr.2022.06.018
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Patel DP. The evolving nomenclature of erythema ab igne-redness from fire. JAMA Dermatol. 2017;153:685. doi:10.1001/jamadermatol.2017.2021
- Arnold AW, Itin PH. Laptop computer-induced erythema ab igne in a child and review of the literature. Pediatrics. 2010;126:E1227-E1230. doi:10.1542/peds.2010-1390
- Riahi RR, Cohen PR. Laptop-induced erythema ab igne: report and review of literature. Dermatol Online J. 2012;18:5.
- Haleem Z, Philip J, Muhammad S. Erythema ab igne: a rare presentation of toasted skin syndrome with the use of a space heater. Cureus. 2021;13:e13401. doi:10.7759/cureus.13401
- Moreau T, Benzaquen M, Gueissaz F. Erythema ab igne after using a virtual reality headset: a new phenomenon to know. J Eur Acad Dermatol Venereol. 2022;36:E932-E933. doi:10.1111/jdv.18371
- Ozturk M, An I. Clinical features and etiology of patients with erythema ab igne: a retrospective multicenter study. J Cosmet Dermatol. 2020;19:1774-1779. doi:10.1111/jocd.13210
- Gmuca S, Yu J, Weiss PF, et al. Erythema ab igne in an adolescent with chronic pain: an alarming cutaneous eruption from heat exposure. Pediatr Emerg Care. 2020;36:E236-E238. doi:10.1097 /PEC.0000000000001460
- Wells A, Desai A, Rudnick EW, et al. Erythema ab igne with features resembling keratosis lichenoides chronica. J Cutan Pathol. 2021;48:151-153. doi:10.1111/cup.13885
- Milchak M, Smucker J, Chung CG, et al. Erythema ab igne due to heating pad use: a case report and review of clinical presentation, prevention, and complications. Case Rep Med. 2016;2016:1862480. doi:10.1155/2016/1862480
- Daneshvar E, Seraji S, Kamyab-Hesari K, et al. Basal cell carcinoma associated with erythema ab igne. Dermatol Online J. 2020;26:13030 /qt3kz985b4.
- Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1988;124:110-113.
- Wharton J, Roffwarg D, Miller J, et al. Cutaneous marginal zone lymphoma arising in the setting of erythema ab igne. J Am Acad Dermatol. 2010;62:1080-1081. doi:10.1016/j.jaad.2009.08.005
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103 /2229-5178.164493
- Grossman JM. Lupus arthritis. Best Pract Res Clin Rheumatol. 2009;23:495-506. doi:10.1016/j.berh.2009.04.003
- Aria AB, Chen L, Silapunt S. Erythema ab igne from heating pad use: a report of three clinical cases and a differential diagnosis. Cureus. 2018;10:E2635. doi:10.7759/cureus.2635
- Wilcox RA. Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol. 2017;92:1085-1102. doi:10.1002/ajh.24876
- Pennitz A, Kinberger M, Avila Valle G, et al. Self-applied topical interventions for melasma: a systematic review and meta-analysis of data from randomized, investigator-blinded clinical trials. Br J Dermatol. 2022;187:309-317.
- Sahl WJ, Taira JW. Erythema ab igne: treatment with 5-fluorouracil cream. J Am Acad Dermatol. 1992;27:109-110.
The Diagnosis: Erythema Ab Igne
Based on the patient's long-standing history of back pain treated with heating pads as well as the normal laboratory findings and skin examination, a diagnosis of erythema ab igne (EAI) was made.
Erythema ab igne presents as reticulated brownish erythema or hyperpigmentation on sites exposed to prolonged use of heat sources such as heating pads, laptops, and space heaters. Erythema ab igne most commonly affects the lower back, thighs, or legs1-6; however, EAI can appear on atypical sites such as the forehead and eyebrows due to newer technology (eg, virtual reality headsets).7 The level of heat required for EAI to occur is below the threshold for thermal burns (<45 °C [113 °F]).1 Erythema ab igne can occur at any age, and woman are more commonly affected than men.8 The pathophysiology currently is unknown; however, recurrent and prolonged heat exposure may damage superficial vessels. As a result, hemosiderin accumulates in the skin, and hyperpigmentation subsequently occurs.9
The diagnosis of EAI is clinical, and early stages of the rash present as blanching reticulated erythema in areas associated with heat exposure. If the offending source of heat is not removed, EAI can progress to nonblanching, fixed, hyperpigmented plaques with skin atrophy, bullae, or hyperkeratosis. Patients often are asymptomatic; however, mild burning may occur.2 Histopathology reveals cellular atypia, epidermal atrophy, dilation of dermal blood vessels, a minute inflammatory infiltrate, and keratinocyte apoptosis.10 Skin biopsy may be necessary in cases of suspected malignancy due to chronic heat exposure. Lesions that ulcerate or evolve should raise suspicion for malignancy.11 Squamous cell carcinoma is the most common malignancy associated with EAI; other malignancies that may manifest include basal cell carcinoma, Merkel cell carcinoma, or cutaneous marginal zone lymphoma.2,12-14
Erythema ab igne often is mistaken for livedo reticularis, which appears more erythematous without hyperpigmentation or epidermal changes and may be associated with a pathologic state.15 The differential diagnosis in our patient, who was in her 40s with a history of fatigue and joint pain, included livedo reticularis associated with lupus; however, the history of heating pad use, normal laboratory findings, and presence of epidermal changes suggested EAI. Lupus typically affects the hand and knee joints.16 Additionally, livedo reticularis more commonly appears on the legs.15
Other differentials for EAI include livedo racemosa, cutaneous T-cell lymphoma, and cutis marmorata. Livedo racemosa presents with broken rings of erythema in young to middle-aged women and primarily affects the trunk and proximal limbs. It is associated with an underlying condition such as polyarteritis nodosa and less commonly with lupus erythematosus with antiphospholipid or Sneddon syndrome.15,17 Cutaneous T-cell lymphoma typically manifests with poikilodermatous patches larger than the palm, especially in covered areas of skin.18 Cutis marmorata is transient and temperature dependent.9
The key intervention for EAI is removal of the offending heat source.2 Patients should be counseled that the erythema and hyperpigmentation may take months to years to resolve. Topical hydroquinone or tretinoin may be used in cases of persistent hyperpigmentation.19 Patients who continue to use heating pads for long-standing pain should be advised to limit their use to short intervals without occlusion. If malignancy is a concern, a biopsy should be performed.20
The Diagnosis: Erythema Ab Igne
Based on the patient's long-standing history of back pain treated with heating pads as well as the normal laboratory findings and skin examination, a diagnosis of erythema ab igne (EAI) was made.
Erythema ab igne presents as reticulated brownish erythema or hyperpigmentation on sites exposed to prolonged use of heat sources such as heating pads, laptops, and space heaters. Erythema ab igne most commonly affects the lower back, thighs, or legs1-6; however, EAI can appear on atypical sites such as the forehead and eyebrows due to newer technology (eg, virtual reality headsets).7 The level of heat required for EAI to occur is below the threshold for thermal burns (<45 °C [113 °F]).1 Erythema ab igne can occur at any age, and woman are more commonly affected than men.8 The pathophysiology currently is unknown; however, recurrent and prolonged heat exposure may damage superficial vessels. As a result, hemosiderin accumulates in the skin, and hyperpigmentation subsequently occurs.9
The diagnosis of EAI is clinical, and early stages of the rash present as blanching reticulated erythema in areas associated with heat exposure. If the offending source of heat is not removed, EAI can progress to nonblanching, fixed, hyperpigmented plaques with skin atrophy, bullae, or hyperkeratosis. Patients often are asymptomatic; however, mild burning may occur.2 Histopathology reveals cellular atypia, epidermal atrophy, dilation of dermal blood vessels, a minute inflammatory infiltrate, and keratinocyte apoptosis.10 Skin biopsy may be necessary in cases of suspected malignancy due to chronic heat exposure. Lesions that ulcerate or evolve should raise suspicion for malignancy.11 Squamous cell carcinoma is the most common malignancy associated with EAI; other malignancies that may manifest include basal cell carcinoma, Merkel cell carcinoma, or cutaneous marginal zone lymphoma.2,12-14
Erythema ab igne often is mistaken for livedo reticularis, which appears more erythematous without hyperpigmentation or epidermal changes and may be associated with a pathologic state.15 The differential diagnosis in our patient, who was in her 40s with a history of fatigue and joint pain, included livedo reticularis associated with lupus; however, the history of heating pad use, normal laboratory findings, and presence of epidermal changes suggested EAI. Lupus typically affects the hand and knee joints.16 Additionally, livedo reticularis more commonly appears on the legs.15
Other differentials for EAI include livedo racemosa, cutaneous T-cell lymphoma, and cutis marmorata. Livedo racemosa presents with broken rings of erythema in young to middle-aged women and primarily affects the trunk and proximal limbs. It is associated with an underlying condition such as polyarteritis nodosa and less commonly with lupus erythematosus with antiphospholipid or Sneddon syndrome.15,17 Cutaneous T-cell lymphoma typically manifests with poikilodermatous patches larger than the palm, especially in covered areas of skin.18 Cutis marmorata is transient and temperature dependent.9
The key intervention for EAI is removal of the offending heat source.2 Patients should be counseled that the erythema and hyperpigmentation may take months to years to resolve. Topical hydroquinone or tretinoin may be used in cases of persistent hyperpigmentation.19 Patients who continue to use heating pads for long-standing pain should be advised to limit their use to short intervals without occlusion. If malignancy is a concern, a biopsy should be performed.20
- Wipf AJ, Brown MR. Malignant transformation of erythema ab igne. JAAD Case Rep. 2022;26:85-87. doi:10.1016/j.jdcr.2022.06.018
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Patel DP. The evolving nomenclature of erythema ab igne-redness from fire. JAMA Dermatol. 2017;153:685. doi:10.1001/jamadermatol.2017.2021
- Arnold AW, Itin PH. Laptop computer-induced erythema ab igne in a child and review of the literature. Pediatrics. 2010;126:E1227-E1230. doi:10.1542/peds.2010-1390
- Riahi RR, Cohen PR. Laptop-induced erythema ab igne: report and review of literature. Dermatol Online J. 2012;18:5.
- Haleem Z, Philip J, Muhammad S. Erythema ab igne: a rare presentation of toasted skin syndrome with the use of a space heater. Cureus. 2021;13:e13401. doi:10.7759/cureus.13401
- Moreau T, Benzaquen M, Gueissaz F. Erythema ab igne after using a virtual reality headset: a new phenomenon to know. J Eur Acad Dermatol Venereol. 2022;36:E932-E933. doi:10.1111/jdv.18371
- Ozturk M, An I. Clinical features and etiology of patients with erythema ab igne: a retrospective multicenter study. J Cosmet Dermatol. 2020;19:1774-1779. doi:10.1111/jocd.13210
- Gmuca S, Yu J, Weiss PF, et al. Erythema ab igne in an adolescent with chronic pain: an alarming cutaneous eruption from heat exposure. Pediatr Emerg Care. 2020;36:E236-E238. doi:10.1097 /PEC.0000000000001460
- Wells A, Desai A, Rudnick EW, et al. Erythema ab igne with features resembling keratosis lichenoides chronica. J Cutan Pathol. 2021;48:151-153. doi:10.1111/cup.13885
- Milchak M, Smucker J, Chung CG, et al. Erythema ab igne due to heating pad use: a case report and review of clinical presentation, prevention, and complications. Case Rep Med. 2016;2016:1862480. doi:10.1155/2016/1862480
- Daneshvar E, Seraji S, Kamyab-Hesari K, et al. Basal cell carcinoma associated with erythema ab igne. Dermatol Online J. 2020;26:13030 /qt3kz985b4.
- Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1988;124:110-113.
- Wharton J, Roffwarg D, Miller J, et al. Cutaneous marginal zone lymphoma arising in the setting of erythema ab igne. J Am Acad Dermatol. 2010;62:1080-1081. doi:10.1016/j.jaad.2009.08.005
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103 /2229-5178.164493
- Grossman JM. Lupus arthritis. Best Pract Res Clin Rheumatol. 2009;23:495-506. doi:10.1016/j.berh.2009.04.003
- Aria AB, Chen L, Silapunt S. Erythema ab igne from heating pad use: a report of three clinical cases and a differential diagnosis. Cureus. 2018;10:E2635. doi:10.7759/cureus.2635
- Wilcox RA. Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol. 2017;92:1085-1102. doi:10.1002/ajh.24876
- Pennitz A, Kinberger M, Avila Valle G, et al. Self-applied topical interventions for melasma: a systematic review and meta-analysis of data from randomized, investigator-blinded clinical trials. Br J Dermatol. 2022;187:309-317.
- Sahl WJ, Taira JW. Erythema ab igne: treatment with 5-fluorouracil cream. J Am Acad Dermatol. 1992;27:109-110.
- Wipf AJ, Brown MR. Malignant transformation of erythema ab igne. JAAD Case Rep. 2022;26:85-87. doi:10.1016/j.jdcr.2022.06.018
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Patel DP. The evolving nomenclature of erythema ab igne-redness from fire. JAMA Dermatol. 2017;153:685. doi:10.1001/jamadermatol.2017.2021
- Arnold AW, Itin PH. Laptop computer-induced erythema ab igne in a child and review of the literature. Pediatrics. 2010;126:E1227-E1230. doi:10.1542/peds.2010-1390
- Riahi RR, Cohen PR. Laptop-induced erythema ab igne: report and review of literature. Dermatol Online J. 2012;18:5.
- Haleem Z, Philip J, Muhammad S. Erythema ab igne: a rare presentation of toasted skin syndrome with the use of a space heater. Cureus. 2021;13:e13401. doi:10.7759/cureus.13401
- Moreau T, Benzaquen M, Gueissaz F. Erythema ab igne after using a virtual reality headset: a new phenomenon to know. J Eur Acad Dermatol Venereol. 2022;36:E932-E933. doi:10.1111/jdv.18371
- Ozturk M, An I. Clinical features and etiology of patients with erythema ab igne: a retrospective multicenter study. J Cosmet Dermatol. 2020;19:1774-1779. doi:10.1111/jocd.13210
- Gmuca S, Yu J, Weiss PF, et al. Erythema ab igne in an adolescent with chronic pain: an alarming cutaneous eruption from heat exposure. Pediatr Emerg Care. 2020;36:E236-E238. doi:10.1097 /PEC.0000000000001460
- Wells A, Desai A, Rudnick EW, et al. Erythema ab igne with features resembling keratosis lichenoides chronica. J Cutan Pathol. 2021;48:151-153. doi:10.1111/cup.13885
- Milchak M, Smucker J, Chung CG, et al. Erythema ab igne due to heating pad use: a case report and review of clinical presentation, prevention, and complications. Case Rep Med. 2016;2016:1862480. doi:10.1155/2016/1862480
- Daneshvar E, Seraji S, Kamyab-Hesari K, et al. Basal cell carcinoma associated with erythema ab igne. Dermatol Online J. 2020;26:13030 /qt3kz985b4.
- Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1988;124:110-113.
- Wharton J, Roffwarg D, Miller J, et al. Cutaneous marginal zone lymphoma arising in the setting of erythema ab igne. J Am Acad Dermatol. 2010;62:1080-1081. doi:10.1016/j.jaad.2009.08.005
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103 /2229-5178.164493
- Grossman JM. Lupus arthritis. Best Pract Res Clin Rheumatol. 2009;23:495-506. doi:10.1016/j.berh.2009.04.003
- Aria AB, Chen L, Silapunt S. Erythema ab igne from heating pad use: a report of three clinical cases and a differential diagnosis. Cureus. 2018;10:E2635. doi:10.7759/cureus.2635
- Wilcox RA. Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol. 2017;92:1085-1102. doi:10.1002/ajh.24876
- Pennitz A, Kinberger M, Avila Valle G, et al. Self-applied topical interventions for melasma: a systematic review and meta-analysis of data from randomized, investigator-blinded clinical trials. Br J Dermatol. 2022;187:309-317.
- Sahl WJ, Taira JW. Erythema ab igne: treatment with 5-fluorouracil cream. J Am Acad Dermatol. 1992;27:109-110.
A 42-year-old woman presented with an asymptomatic, erythematous, lacelike rash on the lower back of 8 months’ duration that was first noticed by her husband. The patient had a long-standing history of chronic fatigue and lower back pain treated with acetaminophen, diclofenac gel, and heating pads. Physical examination revealed reticulated brownish erythema confined to the lower back. Laboratory findings were unremarkable.
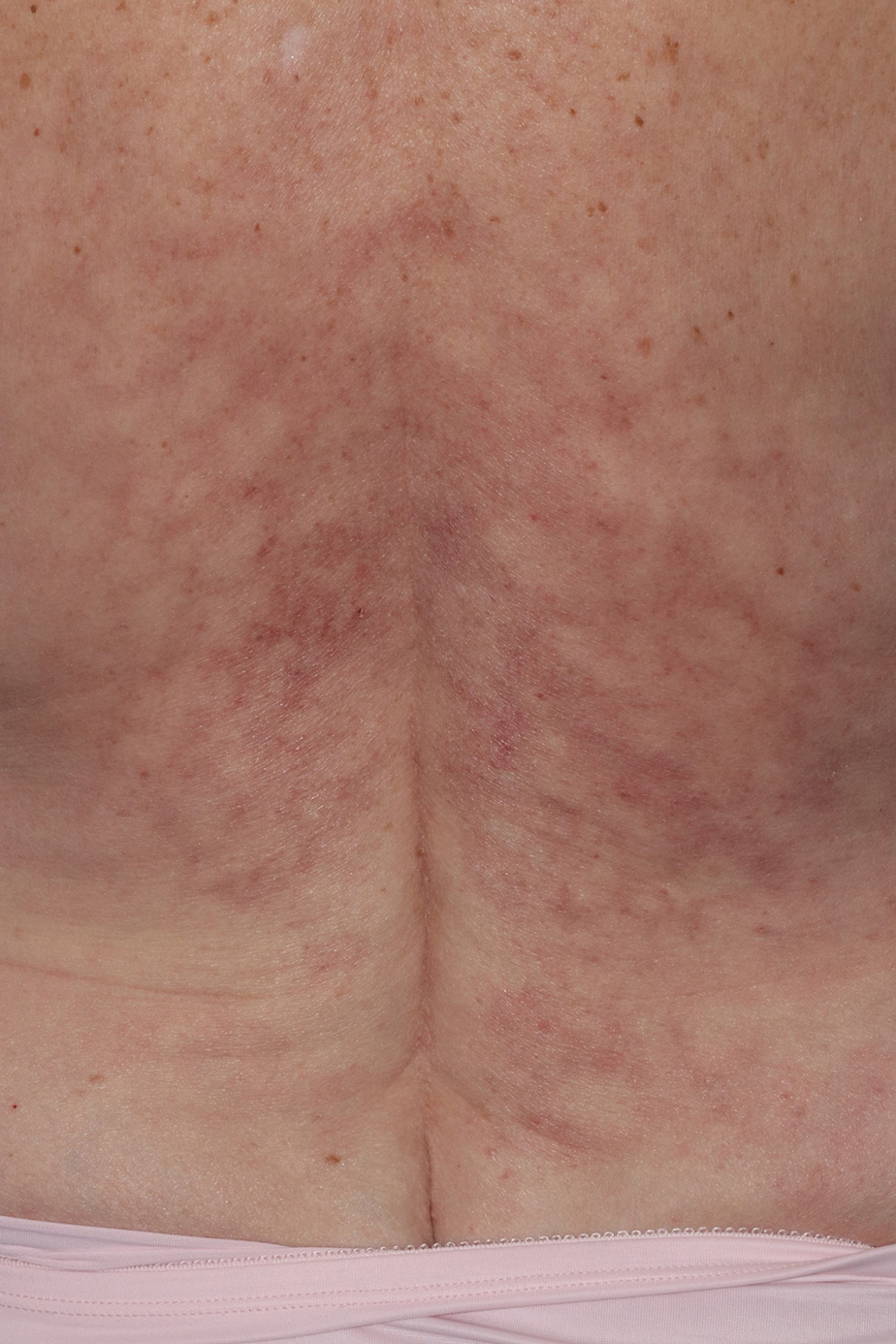
Aquatic Antagonists: Dermatologic Injuries From Sea Urchins (Echinoidea)
Sea urchins—members of the phylum Echinodermata and the class Echinoidea—are spiny marine invertebrates. Their consumption of fleshy algae makes them essential players in maintaining reef ecosystems.1,2 Echinoids, a class that includes heart urchins and sand dollars, are ubiquitous in benthic marine environments, both free floating and rock boring, and inhabit a wide range of latitudes spanning from polar oceans to warm seas.3 Despite their immobility and nonaggression, sea urchin puncture wounds are common among divers, snorkelers, swimmers, surfers, and fishers who accidentally come into contact with their sharp spines. Although the epidemiology of sea urchin exposure and injury is difficult to assess, the American Association of Poison Control Centers’ most recent annual report in 2022 documents approximately 1426 annual aquatic bites and/or envenomations.4
Sea Urchin Morphology and Toxicity
Echinoderms (a term of Greek origin meaning spiny skin) share a radially symmetric calcium carbonate skeleton (termed stereom) that is supported by collagenous ligaments.1 Sea urchins possess spines composed of calcite crystals, which radiate from their body and play a role in locomotion and defense against predators—namely sea otters, starfish/sea stars, wolf eels, and triggerfish, among others (Figure).5 These brittle spines can easily penetrate human skin and subsequently break off the sea urchin body. Most species of sea urchins possess solid spines, but a small percentage (80 of approximately 700 extant species) have hollow spines containing various toxic substances.6 Penetration and systemic absorption of the toxins within these spines can generate severe systemic responses.
The venomous flower urchin (Toxopneustes pileolus), found in the Indian and Pacific oceans, is one of the more common species known to produce a systemic reaction involving neuromuscular blockage.7-9 The most common species harvested off the Pacific coast of the United States—Strongylocentrotus purpuratus (purple sea urchin) and Strongylocentrotus franciscanus (red sea urchins)—are not inherently venomous.8

Both the sea urchin body and spines are covered in a unique epithelium thought to be responsible for the majority of their proinflammatory and pronociceptive properties. Epithelial compounds identified include serotonin, histamines, steroids, glycosides, hemolysins, proteases, and bradykininlike and cholinergic substances.5,7 Additionally, certain sea urchin species possess 3-pronged pincerlike organs at the base of spines called pedicellariae, which are used in feeding.10 Skin penetration by the pedicellariae is especially dangerous, as they tightly adhere to wounds and contain venom-producing organs that allow them to continue injecting toxins after their detachment from the sea urchin body.11
Presentation and Diagnosis of Sea Urchin Injuries
Sea urchin injuries have a wide range of manifestations depending on the number of spines involved, the presence of venom, the depth and location of spine penetration, the duration of spine retention in the skin, and the time before treatment initiation. The most common site of sea urchin injury unsurprisingly is the lower extremities and feet, often in the context of divers and swimmers walking across the sea floor. The hands are another frequently injured site, along with the legs, arms, back, scalp, and even oral mucosa.11
Although clinical history and presentation frequently reveal the mechanism of aquatic injury, patients often are unsure of the agent to which they were exposed and may be unaware of retained foreign bodies. Dermoscopy can distinguish the distinct lines radiating from the core of sea urchin spines from other foreign bodies lodged within the skin.6 It also can be used to locate spines for removal or for their analysis following punch biopsy.6,12 The radiopaque nature of sea urchin spines makes radiography and magnetic resonance imaging useful tools in assessment of periarticular soft-tissue damage and spine removal.8,11,13 Ultrasonography can reveal spines that no longer appear on radiography due to absorption by human tissue.14
Immediate Dermatologic Effects
Sea urchin injuries can be broadly categorized into immediate and delayed reactions. Immediate manifestations of contact with sea urchin spines include localized pain, bleeding, erythema, myalgia, and edema at the site of injury that can last from a few hours to 1 week without proper wound care and spine removal.5 Systemic symptoms ranging from dizziness, lightheadedness, paresthesia, aphonia, paralysis, coma, and death generally are only seen following injuries from venomous species, attachment of pedicellariae, injuries involving neurovascular structures, or penetration by more than 15 spines.7,11
Initial treatment includes soaking the wound in hot water (113 °F [45 °C]) for 30 to 90 minutes and subsequently removing spines and pedicellariae to prevent development of delayed reactions.5,15,16 The compounds in the sea urchin epithelium are heat labile and will be inactivated upon soaking in hot water.16 Extraction of spines can be difficult, as they are brittle and easily break in the skin. Successful removal has been reported using forceps and a hypodermic needle as well as excision; both approaches may require local anesthesia.8,17 Another technique involves freezing the localized area with liquid nitrogen to allow easier removal upon skin blistering.18 Punch biopsy also has been utilized as an effective means of ensuring all spiny fragments are removed.9,19,20 These spines often cause black or purple tattoolike staining at the puncture site, which can persist for a few days after spine extraction.8 Ablation using the erbium-doped:YAG laser may be helpful for removal of associated pigment.21,22
Delayed Dermatologic Effects
Delayed reactions to sea urchin injuries often are attributable to prolonged retention of spines in the skin. Granulomatous reactions typically manifest 2 weeks after injury as firm nonsuppurative nodules with central umbilication and a hyperkeratotic surface.7 These nodules may or may not be painful. Histopathology most often reveals foreign body and sarcoidal-type granulomatous reactions. However, tuberculoid, necrobiotic, and suppurative granulomas also may develop.13 Other microscopic features include inflammatory reactions, suppurative dermatitis, focal necrosis, and microabscesses.23 Wounds with progression to granulomatous disease often require surgical debridement.
Other more serious sequalae can result from involvement of joint capsules, especially in the hands and feet. Sea urchin injury involving joint spaces should be treated aggressively, as progression to inflammatory or infectious synovitis and tenosynovitis can cause irreversible loss of joint function. Inflammatory synovitis occurs 1 to 2 months on average after injury following a period of minimal symptoms and begins as a gradual increase in joint swelling and decrease in range of motion.8 Infectious tenosynovitis manifests quite similarly. Although suppurative etiologies generally progress with a more acute onset, certain infectious organisms (eg, Mycobacterium) take on an indolent course and should not be overlooked as a cause of delayed symptoms.8 The Kavanel cardinal signs are a sensitive tool used in the diagnosis of infectious flexor sheath tenosynovitis.8,24 If suspicion for joint infection is high, emergency referral should be made for debridement and culture-guided antibiotic therapy. Left untreated, infectious tenosynovitis can result in tendon necrosis or rupture, digit necrosis, and systemic infection.24 Patients with joint involvement should be referred to specialty care (eg, hand surgeon), as they often require synovectomy and surgical removal of foreign material.8
From 1 month to 1 year after injury, prolonged granulomatous synovitis of the hand may eventually lead to joint destruction known as “sea urchin arthritis.” These patients present with decreased range of motion and numerous nodules on the hand with a hyperkeratotic surface. Radiography reveals joint space narrowing, osteolysis, subchondral sclerosis, and periosteal reaction. Synovectomy and debridement are necessary to prevent irreversible joint damage or the need for arthrodesis and bone grafting.24
Other Treatment Considerations
Other important considerations in the care of sea urchin spine injuries include assessment of tetanus immunization status and administration of necessary prophylaxis as soon as possible, even in delayed presentations (Table).16,25 Cultures should be taken only if infection is suspected. Prophylactic antibiotics are not recommended unless the patient is immunocompromised or otherwise has impaired wound healing. If a patient presents with systemic symptoms, they should be referred to an emergency care facility for further management.
Final Thoughts
Sea urchin injuries can lead to serious complications if not diagnosed quickly and treated properly. Retention of sea urchin spines in the deep tissues and joint spaces may lead to granulomas, inflammatory and infectious tenosynovitis (including mycobacterial infection), and sea urchin arthritis requiring surgical debridement and possible irreversible joint damage, up to a year after initial injury. Patients should be educated on the possibility of developing these delayed reactions and instructed to seek immediate care. Joint deformities, range-of-motion deficits, and involvement of neurovascular structures should be considered emergent and referred for proper management. Shoes and diving gear offer some protection but are easily penetrable by sharp sea urchin spines. Preventive focus should be aimed at educating patients and providers on the importance of prompt spine removal upon injury. Although dermatologic and systemic manifestations vary widely, a thorough history, physical examination, and appropriate use of imaging modalities can facilitate accurate diagnosis and guide treatment.
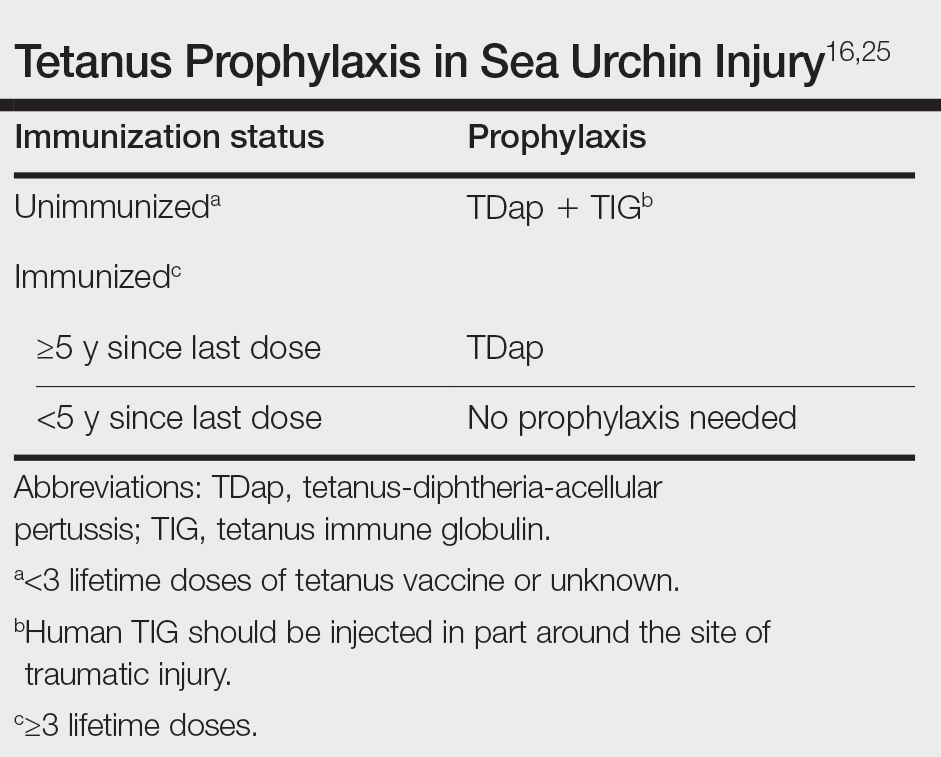
- Amemiya CT, Miyake T, Rast JP. Echinoderms. Curr Biol. 2005;15:R944-R946. doi:10.1016/j.cub.2005.11.026
- Koch NM, Coppard SE, Lessios HA, et al. A phylogenomic resolution of the sea urchin tree of life. BMC Evol Biol. 2018;18:189. doi:10.1186/s12862-018-1300-4
- Amir Y, Insler M, Giller A, et al. Senescence and longevity of sea urchins. Genes (Basel). 2020;11:573. doi:10.3390/genes11050573
- Gummin DD, Mowry JB, Beuhler MC, et al. 2022 Annual Report of the National Poison Data System® (NPDS) from America's Poison Centers®: 40th annual report. Clin Toxicol (Phila). 2023;61:717-939. doi:10.1080/15563650.2023.2268981
- Gelman Y, Kong EL, Murphy-Lavoie HM. Sea urchin toxicity. In: StatPearls [Internet]. StatPearls Publishing; 2021.
- Suarez-Conde MF, Vallone MG, González VM, et al. Sea urchin skin lesions: a case report. Dermatol Pract Concept. 2021;11:E2021009. doi:10.5826/dpc.1102a09
- Al-Kathiri L, Al-Najjar T, Sulaiman I. Sea urchin granuloma of the hands: a case report. Oman Med J. 2019;34:350-353. doi:10.5001/omj.2019.68
- Dahl WJ, Jebson P, Louis DS. Sea urchin injuries to the hand: a case report and review of the literature. Iowa Orthop J. 2010;30:153-156.
- Hatakeyama T, Ichise A, Unno H, et al. Carbohydrate recognition by the rhamnose-binding lectin SUL-I with a novel three-domain structure isolated from the venom of globiferous pedicellariae of the flower sea urchin Toxopneustes pileolus. Protein Sci. 2017;26:1574-1583. doi:10.1002/pro.3185
- Balhara KS, Stolbach A. Marine envenomations. Emerg Med Clin North Am. 2014;32:223-243. doi:10.1016/j.emc.2013.09.009
- Schwartz Z, Cohen M, Lipner SR. Sea urchin injuries: a review and clinical approach algorithm. J Dermatolog Treat. 2021;32:150-156. doi:10.1080/09546634.2019.1638884
- Park SJ, Park JW, Choi SY, et al. Use of dermoscopy after punch removal of a veiled sea urchin spine. Dermatol Ther. 2021;34:E14947. doi:10.1111/dth.14947
- Wada T, Soma T, Gaman K, et al. Sea urchin spine arthritis of the hand. J Hand Surg Am. 2008;33:398-401. doi:10.1016/j.jhsa.2007.11.016
- Groleau S, Chhem RK, Younge D, et al. Ultrasonography of foreign-body tenosynovitis. Can Assoc Radiol J. 1992;43:454-456.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337. doi:10.1016/j.emc.2016.12.004
- Noonburg GE. Management of extremity trauma and related infections occurring in the aquatic environment. J Am Acad Orthop Surg. 2005;13:243-253. doi:10.5435/00124635-200507000-00004
- Haddad Junior V. Observation of initial clinical manifestations and repercussions from the treatment of 314 human injuries caused by black sea urchins (Echinometra lucunter) on the southeastern Brazilian coast. Rev Soc Bras Med Trop. 2012;45:390-392. doi:10.1590/s0037-86822012000300021
- Gargus MD, Morohashi DK. A sea-urchin spine chilling remedy. N Engl J Med. 2012;367:1867-1868. doi:10.1056/NEJMc1209382
- Sjøberg T, de Weerd L. The usefulness of a skin biopsy punch to remove sea urchin spines. ANZ J Surg. 2010;80:383. doi:10.1111/j.1445-2197.2010.05296.x
- Cardenas-de la Garza JA, Cuellar-Barboza A, Ancer-Arellano J, et al. Classic dermatological tools: foreign body removal with punch biopsy.J Am Acad Dermatol. 2019;81:E93-E94. doi:10.1016/j.jaad.2018.10.038
- Gungor S, Tarikçi N, Gokdemir G. Removal of sea urchin spines using erbium-doped yttrium aluminum garnet ablation. Dermatol Surg. 2012;38:508-510. doi:10.1111/j.1524-4725.2011.02259.x
- Böer A, Ochsendorf FR, Beier C, et al. Effective removal of sea-urchin spines by erbium:YAG laser ablation. Br J Dermatol. 2001;145:169-170. doi:10.1046/j.1365-2133.2001.04306.x
- De La Torre C, Toribio J. Sea-urchin granuloma: histologic profile. a pathologic study of 50 biopsies. J Cutan Pathol. 2001;28:223-228. doi:10.1034/j.1600-0560.2001.028005223.x
- Yi A, Kennedy C, Chia B, et al. Radiographic soft tissue thickness differentiating pyogenic flexor tenosynovitis from other finger infections. J Hand Surg Am. 2019;44:394-399. doi:10.1016/j.jhsa.2019.01.013
- Callison C, Nguyen H. Tetanus prophylaxis. In: StatPearls [Internet]. StatPearls Publishing; 2022.
Sea urchins—members of the phylum Echinodermata and the class Echinoidea—are spiny marine invertebrates. Their consumption of fleshy algae makes them essential players in maintaining reef ecosystems.1,2 Echinoids, a class that includes heart urchins and sand dollars, are ubiquitous in benthic marine environments, both free floating and rock boring, and inhabit a wide range of latitudes spanning from polar oceans to warm seas.3 Despite their immobility and nonaggression, sea urchin puncture wounds are common among divers, snorkelers, swimmers, surfers, and fishers who accidentally come into contact with their sharp spines. Although the epidemiology of sea urchin exposure and injury is difficult to assess, the American Association of Poison Control Centers’ most recent annual report in 2022 documents approximately 1426 annual aquatic bites and/or envenomations.4
Sea Urchin Morphology and Toxicity
Echinoderms (a term of Greek origin meaning spiny skin) share a radially symmetric calcium carbonate skeleton (termed stereom) that is supported by collagenous ligaments.1 Sea urchins possess spines composed of calcite crystals, which radiate from their body and play a role in locomotion and defense against predators—namely sea otters, starfish/sea stars, wolf eels, and triggerfish, among others (Figure).5 These brittle spines can easily penetrate human skin and subsequently break off the sea urchin body. Most species of sea urchins possess solid spines, but a small percentage (80 of approximately 700 extant species) have hollow spines containing various toxic substances.6 Penetration and systemic absorption of the toxins within these spines can generate severe systemic responses.
The venomous flower urchin (Toxopneustes pileolus), found in the Indian and Pacific oceans, is one of the more common species known to produce a systemic reaction involving neuromuscular blockage.7-9 The most common species harvested off the Pacific coast of the United States—Strongylocentrotus purpuratus (purple sea urchin) and Strongylocentrotus franciscanus (red sea urchins)—are not inherently venomous.8

Both the sea urchin body and spines are covered in a unique epithelium thought to be responsible for the majority of their proinflammatory and pronociceptive properties. Epithelial compounds identified include serotonin, histamines, steroids, glycosides, hemolysins, proteases, and bradykininlike and cholinergic substances.5,7 Additionally, certain sea urchin species possess 3-pronged pincerlike organs at the base of spines called pedicellariae, which are used in feeding.10 Skin penetration by the pedicellariae is especially dangerous, as they tightly adhere to wounds and contain venom-producing organs that allow them to continue injecting toxins after their detachment from the sea urchin body.11
Presentation and Diagnosis of Sea Urchin Injuries
Sea urchin injuries have a wide range of manifestations depending on the number of spines involved, the presence of venom, the depth and location of spine penetration, the duration of spine retention in the skin, and the time before treatment initiation. The most common site of sea urchin injury unsurprisingly is the lower extremities and feet, often in the context of divers and swimmers walking across the sea floor. The hands are another frequently injured site, along with the legs, arms, back, scalp, and even oral mucosa.11
Although clinical history and presentation frequently reveal the mechanism of aquatic injury, patients often are unsure of the agent to which they were exposed and may be unaware of retained foreign bodies. Dermoscopy can distinguish the distinct lines radiating from the core of sea urchin spines from other foreign bodies lodged within the skin.6 It also can be used to locate spines for removal or for their analysis following punch biopsy.6,12 The radiopaque nature of sea urchin spines makes radiography and magnetic resonance imaging useful tools in assessment of periarticular soft-tissue damage and spine removal.8,11,13 Ultrasonography can reveal spines that no longer appear on radiography due to absorption by human tissue.14
Immediate Dermatologic Effects
Sea urchin injuries can be broadly categorized into immediate and delayed reactions. Immediate manifestations of contact with sea urchin spines include localized pain, bleeding, erythema, myalgia, and edema at the site of injury that can last from a few hours to 1 week without proper wound care and spine removal.5 Systemic symptoms ranging from dizziness, lightheadedness, paresthesia, aphonia, paralysis, coma, and death generally are only seen following injuries from venomous species, attachment of pedicellariae, injuries involving neurovascular structures, or penetration by more than 15 spines.7,11
Initial treatment includes soaking the wound in hot water (113 °F [45 °C]) for 30 to 90 minutes and subsequently removing spines and pedicellariae to prevent development of delayed reactions.5,15,16 The compounds in the sea urchin epithelium are heat labile and will be inactivated upon soaking in hot water.16 Extraction of spines can be difficult, as they are brittle and easily break in the skin. Successful removal has been reported using forceps and a hypodermic needle as well as excision; both approaches may require local anesthesia.8,17 Another technique involves freezing the localized area with liquid nitrogen to allow easier removal upon skin blistering.18 Punch biopsy also has been utilized as an effective means of ensuring all spiny fragments are removed.9,19,20 These spines often cause black or purple tattoolike staining at the puncture site, which can persist for a few days after spine extraction.8 Ablation using the erbium-doped:YAG laser may be helpful for removal of associated pigment.21,22
Delayed Dermatologic Effects
Delayed reactions to sea urchin injuries often are attributable to prolonged retention of spines in the skin. Granulomatous reactions typically manifest 2 weeks after injury as firm nonsuppurative nodules with central umbilication and a hyperkeratotic surface.7 These nodules may or may not be painful. Histopathology most often reveals foreign body and sarcoidal-type granulomatous reactions. However, tuberculoid, necrobiotic, and suppurative granulomas also may develop.13 Other microscopic features include inflammatory reactions, suppurative dermatitis, focal necrosis, and microabscesses.23 Wounds with progression to granulomatous disease often require surgical debridement.
Other more serious sequalae can result from involvement of joint capsules, especially in the hands and feet. Sea urchin injury involving joint spaces should be treated aggressively, as progression to inflammatory or infectious synovitis and tenosynovitis can cause irreversible loss of joint function. Inflammatory synovitis occurs 1 to 2 months on average after injury following a period of minimal symptoms and begins as a gradual increase in joint swelling and decrease in range of motion.8 Infectious tenosynovitis manifests quite similarly. Although suppurative etiologies generally progress with a more acute onset, certain infectious organisms (eg, Mycobacterium) take on an indolent course and should not be overlooked as a cause of delayed symptoms.8 The Kavanel cardinal signs are a sensitive tool used in the diagnosis of infectious flexor sheath tenosynovitis.8,24 If suspicion for joint infection is high, emergency referral should be made for debridement and culture-guided antibiotic therapy. Left untreated, infectious tenosynovitis can result in tendon necrosis or rupture, digit necrosis, and systemic infection.24 Patients with joint involvement should be referred to specialty care (eg, hand surgeon), as they often require synovectomy and surgical removal of foreign material.8
From 1 month to 1 year after injury, prolonged granulomatous synovitis of the hand may eventually lead to joint destruction known as “sea urchin arthritis.” These patients present with decreased range of motion and numerous nodules on the hand with a hyperkeratotic surface. Radiography reveals joint space narrowing, osteolysis, subchondral sclerosis, and periosteal reaction. Synovectomy and debridement are necessary to prevent irreversible joint damage or the need for arthrodesis and bone grafting.24
Other Treatment Considerations
Other important considerations in the care of sea urchin spine injuries include assessment of tetanus immunization status and administration of necessary prophylaxis as soon as possible, even in delayed presentations (Table).16,25 Cultures should be taken only if infection is suspected. Prophylactic antibiotics are not recommended unless the patient is immunocompromised or otherwise has impaired wound healing. If a patient presents with systemic symptoms, they should be referred to an emergency care facility for further management.
Final Thoughts
Sea urchin injuries can lead to serious complications if not diagnosed quickly and treated properly. Retention of sea urchin spines in the deep tissues and joint spaces may lead to granulomas, inflammatory and infectious tenosynovitis (including mycobacterial infection), and sea urchin arthritis requiring surgical debridement and possible irreversible joint damage, up to a year after initial injury. Patients should be educated on the possibility of developing these delayed reactions and instructed to seek immediate care. Joint deformities, range-of-motion deficits, and involvement of neurovascular structures should be considered emergent and referred for proper management. Shoes and diving gear offer some protection but are easily penetrable by sharp sea urchin spines. Preventive focus should be aimed at educating patients and providers on the importance of prompt spine removal upon injury. Although dermatologic and systemic manifestations vary widely, a thorough history, physical examination, and appropriate use of imaging modalities can facilitate accurate diagnosis and guide treatment.

Sea urchins—members of the phylum Echinodermata and the class Echinoidea—are spiny marine invertebrates. Their consumption of fleshy algae makes them essential players in maintaining reef ecosystems.1,2 Echinoids, a class that includes heart urchins and sand dollars, are ubiquitous in benthic marine environments, both free floating and rock boring, and inhabit a wide range of latitudes spanning from polar oceans to warm seas.3 Despite their immobility and nonaggression, sea urchin puncture wounds are common among divers, snorkelers, swimmers, surfers, and fishers who accidentally come into contact with their sharp spines. Although the epidemiology of sea urchin exposure and injury is difficult to assess, the American Association of Poison Control Centers’ most recent annual report in 2022 documents approximately 1426 annual aquatic bites and/or envenomations.4
Sea Urchin Morphology and Toxicity
Echinoderms (a term of Greek origin meaning spiny skin) share a radially symmetric calcium carbonate skeleton (termed stereom) that is supported by collagenous ligaments.1 Sea urchins possess spines composed of calcite crystals, which radiate from their body and play a role in locomotion and defense against predators—namely sea otters, starfish/sea stars, wolf eels, and triggerfish, among others (Figure).5 These brittle spines can easily penetrate human skin and subsequently break off the sea urchin body. Most species of sea urchins possess solid spines, but a small percentage (80 of approximately 700 extant species) have hollow spines containing various toxic substances.6 Penetration and systemic absorption of the toxins within these spines can generate severe systemic responses.
The venomous flower urchin (Toxopneustes pileolus), found in the Indian and Pacific oceans, is one of the more common species known to produce a systemic reaction involving neuromuscular blockage.7-9 The most common species harvested off the Pacific coast of the United States—Strongylocentrotus purpuratus (purple sea urchin) and Strongylocentrotus franciscanus (red sea urchins)—are not inherently venomous.8

Both the sea urchin body and spines are covered in a unique epithelium thought to be responsible for the majority of their proinflammatory and pronociceptive properties. Epithelial compounds identified include serotonin, histamines, steroids, glycosides, hemolysins, proteases, and bradykininlike and cholinergic substances.5,7 Additionally, certain sea urchin species possess 3-pronged pincerlike organs at the base of spines called pedicellariae, which are used in feeding.10 Skin penetration by the pedicellariae is especially dangerous, as they tightly adhere to wounds and contain venom-producing organs that allow them to continue injecting toxins after their detachment from the sea urchin body.11
Presentation and Diagnosis of Sea Urchin Injuries
Sea urchin injuries have a wide range of manifestations depending on the number of spines involved, the presence of venom, the depth and location of spine penetration, the duration of spine retention in the skin, and the time before treatment initiation. The most common site of sea urchin injury unsurprisingly is the lower extremities and feet, often in the context of divers and swimmers walking across the sea floor. The hands are another frequently injured site, along with the legs, arms, back, scalp, and even oral mucosa.11
Although clinical history and presentation frequently reveal the mechanism of aquatic injury, patients often are unsure of the agent to which they were exposed and may be unaware of retained foreign bodies. Dermoscopy can distinguish the distinct lines radiating from the core of sea urchin spines from other foreign bodies lodged within the skin.6 It also can be used to locate spines for removal or for their analysis following punch biopsy.6,12 The radiopaque nature of sea urchin spines makes radiography and magnetic resonance imaging useful tools in assessment of periarticular soft-tissue damage and spine removal.8,11,13 Ultrasonography can reveal spines that no longer appear on radiography due to absorption by human tissue.14
Immediate Dermatologic Effects
Sea urchin injuries can be broadly categorized into immediate and delayed reactions. Immediate manifestations of contact with sea urchin spines include localized pain, bleeding, erythema, myalgia, and edema at the site of injury that can last from a few hours to 1 week without proper wound care and spine removal.5 Systemic symptoms ranging from dizziness, lightheadedness, paresthesia, aphonia, paralysis, coma, and death generally are only seen following injuries from venomous species, attachment of pedicellariae, injuries involving neurovascular structures, or penetration by more than 15 spines.7,11
Initial treatment includes soaking the wound in hot water (113 °F [45 °C]) for 30 to 90 minutes and subsequently removing spines and pedicellariae to prevent development of delayed reactions.5,15,16 The compounds in the sea urchin epithelium are heat labile and will be inactivated upon soaking in hot water.16 Extraction of spines can be difficult, as they are brittle and easily break in the skin. Successful removal has been reported using forceps and a hypodermic needle as well as excision; both approaches may require local anesthesia.8,17 Another technique involves freezing the localized area with liquid nitrogen to allow easier removal upon skin blistering.18 Punch biopsy also has been utilized as an effective means of ensuring all spiny fragments are removed.9,19,20 These spines often cause black or purple tattoolike staining at the puncture site, which can persist for a few days after spine extraction.8 Ablation using the erbium-doped:YAG laser may be helpful for removal of associated pigment.21,22
Delayed Dermatologic Effects
Delayed reactions to sea urchin injuries often are attributable to prolonged retention of spines in the skin. Granulomatous reactions typically manifest 2 weeks after injury as firm nonsuppurative nodules with central umbilication and a hyperkeratotic surface.7 These nodules may or may not be painful. Histopathology most often reveals foreign body and sarcoidal-type granulomatous reactions. However, tuberculoid, necrobiotic, and suppurative granulomas also may develop.13 Other microscopic features include inflammatory reactions, suppurative dermatitis, focal necrosis, and microabscesses.23 Wounds with progression to granulomatous disease often require surgical debridement.
Other more serious sequalae can result from involvement of joint capsules, especially in the hands and feet. Sea urchin injury involving joint spaces should be treated aggressively, as progression to inflammatory or infectious synovitis and tenosynovitis can cause irreversible loss of joint function. Inflammatory synovitis occurs 1 to 2 months on average after injury following a period of minimal symptoms and begins as a gradual increase in joint swelling and decrease in range of motion.8 Infectious tenosynovitis manifests quite similarly. Although suppurative etiologies generally progress with a more acute onset, certain infectious organisms (eg, Mycobacterium) take on an indolent course and should not be overlooked as a cause of delayed symptoms.8 The Kavanel cardinal signs are a sensitive tool used in the diagnosis of infectious flexor sheath tenosynovitis.8,24 If suspicion for joint infection is high, emergency referral should be made for debridement and culture-guided antibiotic therapy. Left untreated, infectious tenosynovitis can result in tendon necrosis or rupture, digit necrosis, and systemic infection.24 Patients with joint involvement should be referred to specialty care (eg, hand surgeon), as they often require synovectomy and surgical removal of foreign material.8
From 1 month to 1 year after injury, prolonged granulomatous synovitis of the hand may eventually lead to joint destruction known as “sea urchin arthritis.” These patients present with decreased range of motion and numerous nodules on the hand with a hyperkeratotic surface. Radiography reveals joint space narrowing, osteolysis, subchondral sclerosis, and periosteal reaction. Synovectomy and debridement are necessary to prevent irreversible joint damage or the need for arthrodesis and bone grafting.24
Other Treatment Considerations
Other important considerations in the care of sea urchin spine injuries include assessment of tetanus immunization status and administration of necessary prophylaxis as soon as possible, even in delayed presentations (Table).16,25 Cultures should be taken only if infection is suspected. Prophylactic antibiotics are not recommended unless the patient is immunocompromised or otherwise has impaired wound healing. If a patient presents with systemic symptoms, they should be referred to an emergency care facility for further management.
Final Thoughts
Sea urchin injuries can lead to serious complications if not diagnosed quickly and treated properly. Retention of sea urchin spines in the deep tissues and joint spaces may lead to granulomas, inflammatory and infectious tenosynovitis (including mycobacterial infection), and sea urchin arthritis requiring surgical debridement and possible irreversible joint damage, up to a year after initial injury. Patients should be educated on the possibility of developing these delayed reactions and instructed to seek immediate care. Joint deformities, range-of-motion deficits, and involvement of neurovascular structures should be considered emergent and referred for proper management. Shoes and diving gear offer some protection but are easily penetrable by sharp sea urchin spines. Preventive focus should be aimed at educating patients and providers on the importance of prompt spine removal upon injury. Although dermatologic and systemic manifestations vary widely, a thorough history, physical examination, and appropriate use of imaging modalities can facilitate accurate diagnosis and guide treatment.

- Amemiya CT, Miyake T, Rast JP. Echinoderms. Curr Biol. 2005;15:R944-R946. doi:10.1016/j.cub.2005.11.026
- Koch NM, Coppard SE, Lessios HA, et al. A phylogenomic resolution of the sea urchin tree of life. BMC Evol Biol. 2018;18:189. doi:10.1186/s12862-018-1300-4
- Amir Y, Insler M, Giller A, et al. Senescence and longevity of sea urchins. Genes (Basel). 2020;11:573. doi:10.3390/genes11050573
- Gummin DD, Mowry JB, Beuhler MC, et al. 2022 Annual Report of the National Poison Data System® (NPDS) from America's Poison Centers®: 40th annual report. Clin Toxicol (Phila). 2023;61:717-939. doi:10.1080/15563650.2023.2268981
- Gelman Y, Kong EL, Murphy-Lavoie HM. Sea urchin toxicity. In: StatPearls [Internet]. StatPearls Publishing; 2021.
- Suarez-Conde MF, Vallone MG, González VM, et al. Sea urchin skin lesions: a case report. Dermatol Pract Concept. 2021;11:E2021009. doi:10.5826/dpc.1102a09
- Al-Kathiri L, Al-Najjar T, Sulaiman I. Sea urchin granuloma of the hands: a case report. Oman Med J. 2019;34:350-353. doi:10.5001/omj.2019.68
- Dahl WJ, Jebson P, Louis DS. Sea urchin injuries to the hand: a case report and review of the literature. Iowa Orthop J. 2010;30:153-156.
- Hatakeyama T, Ichise A, Unno H, et al. Carbohydrate recognition by the rhamnose-binding lectin SUL-I with a novel three-domain structure isolated from the venom of globiferous pedicellariae of the flower sea urchin Toxopneustes pileolus. Protein Sci. 2017;26:1574-1583. doi:10.1002/pro.3185
- Balhara KS, Stolbach A. Marine envenomations. Emerg Med Clin North Am. 2014;32:223-243. doi:10.1016/j.emc.2013.09.009
- Schwartz Z, Cohen M, Lipner SR. Sea urchin injuries: a review and clinical approach algorithm. J Dermatolog Treat. 2021;32:150-156. doi:10.1080/09546634.2019.1638884
- Park SJ, Park JW, Choi SY, et al. Use of dermoscopy after punch removal of a veiled sea urchin spine. Dermatol Ther. 2021;34:E14947. doi:10.1111/dth.14947
- Wada T, Soma T, Gaman K, et al. Sea urchin spine arthritis of the hand. J Hand Surg Am. 2008;33:398-401. doi:10.1016/j.jhsa.2007.11.016
- Groleau S, Chhem RK, Younge D, et al. Ultrasonography of foreign-body tenosynovitis. Can Assoc Radiol J. 1992;43:454-456.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337. doi:10.1016/j.emc.2016.12.004
- Noonburg GE. Management of extremity trauma and related infections occurring in the aquatic environment. J Am Acad Orthop Surg. 2005;13:243-253. doi:10.5435/00124635-200507000-00004
- Haddad Junior V. Observation of initial clinical manifestations and repercussions from the treatment of 314 human injuries caused by black sea urchins (Echinometra lucunter) on the southeastern Brazilian coast. Rev Soc Bras Med Trop. 2012;45:390-392. doi:10.1590/s0037-86822012000300021
- Gargus MD, Morohashi DK. A sea-urchin spine chilling remedy. N Engl J Med. 2012;367:1867-1868. doi:10.1056/NEJMc1209382
- Sjøberg T, de Weerd L. The usefulness of a skin biopsy punch to remove sea urchin spines. ANZ J Surg. 2010;80:383. doi:10.1111/j.1445-2197.2010.05296.x
- Cardenas-de la Garza JA, Cuellar-Barboza A, Ancer-Arellano J, et al. Classic dermatological tools: foreign body removal with punch biopsy.J Am Acad Dermatol. 2019;81:E93-E94. doi:10.1016/j.jaad.2018.10.038
- Gungor S, Tarikçi N, Gokdemir G. Removal of sea urchin spines using erbium-doped yttrium aluminum garnet ablation. Dermatol Surg. 2012;38:508-510. doi:10.1111/j.1524-4725.2011.02259.x
- Böer A, Ochsendorf FR, Beier C, et al. Effective removal of sea-urchin spines by erbium:YAG laser ablation. Br J Dermatol. 2001;145:169-170. doi:10.1046/j.1365-2133.2001.04306.x
- De La Torre C, Toribio J. Sea-urchin granuloma: histologic profile. a pathologic study of 50 biopsies. J Cutan Pathol. 2001;28:223-228. doi:10.1034/j.1600-0560.2001.028005223.x
- Yi A, Kennedy C, Chia B, et al. Radiographic soft tissue thickness differentiating pyogenic flexor tenosynovitis from other finger infections. J Hand Surg Am. 2019;44:394-399. doi:10.1016/j.jhsa.2019.01.013
- Callison C, Nguyen H. Tetanus prophylaxis. In: StatPearls [Internet]. StatPearls Publishing; 2022.
- Amemiya CT, Miyake T, Rast JP. Echinoderms. Curr Biol. 2005;15:R944-R946. doi:10.1016/j.cub.2005.11.026
- Koch NM, Coppard SE, Lessios HA, et al. A phylogenomic resolution of the sea urchin tree of life. BMC Evol Biol. 2018;18:189. doi:10.1186/s12862-018-1300-4
- Amir Y, Insler M, Giller A, et al. Senescence and longevity of sea urchins. Genes (Basel). 2020;11:573. doi:10.3390/genes11050573
- Gummin DD, Mowry JB, Beuhler MC, et al. 2022 Annual Report of the National Poison Data System® (NPDS) from America's Poison Centers®: 40th annual report. Clin Toxicol (Phila). 2023;61:717-939. doi:10.1080/15563650.2023.2268981
- Gelman Y, Kong EL, Murphy-Lavoie HM. Sea urchin toxicity. In: StatPearls [Internet]. StatPearls Publishing; 2021.
- Suarez-Conde MF, Vallone MG, González VM, et al. Sea urchin skin lesions: a case report. Dermatol Pract Concept. 2021;11:E2021009. doi:10.5826/dpc.1102a09
- Al-Kathiri L, Al-Najjar T, Sulaiman I. Sea urchin granuloma of the hands: a case report. Oman Med J. 2019;34:350-353. doi:10.5001/omj.2019.68
- Dahl WJ, Jebson P, Louis DS. Sea urchin injuries to the hand: a case report and review of the literature. Iowa Orthop J. 2010;30:153-156.
- Hatakeyama T, Ichise A, Unno H, et al. Carbohydrate recognition by the rhamnose-binding lectin SUL-I with a novel three-domain structure isolated from the venom of globiferous pedicellariae of the flower sea urchin Toxopneustes pileolus. Protein Sci. 2017;26:1574-1583. doi:10.1002/pro.3185
- Balhara KS, Stolbach A. Marine envenomations. Emerg Med Clin North Am. 2014;32:223-243. doi:10.1016/j.emc.2013.09.009
- Schwartz Z, Cohen M, Lipner SR. Sea urchin injuries: a review and clinical approach algorithm. J Dermatolog Treat. 2021;32:150-156. doi:10.1080/09546634.2019.1638884
- Park SJ, Park JW, Choi SY, et al. Use of dermoscopy after punch removal of a veiled sea urchin spine. Dermatol Ther. 2021;34:E14947. doi:10.1111/dth.14947
- Wada T, Soma T, Gaman K, et al. Sea urchin spine arthritis of the hand. J Hand Surg Am. 2008;33:398-401. doi:10.1016/j.jhsa.2007.11.016
- Groleau S, Chhem RK, Younge D, et al. Ultrasonography of foreign-body tenosynovitis. Can Assoc Radiol J. 1992;43:454-456.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337. doi:10.1016/j.emc.2016.12.004
- Noonburg GE. Management of extremity trauma and related infections occurring in the aquatic environment. J Am Acad Orthop Surg. 2005;13:243-253. doi:10.5435/00124635-200507000-00004
- Haddad Junior V. Observation of initial clinical manifestations and repercussions from the treatment of 314 human injuries caused by black sea urchins (Echinometra lucunter) on the southeastern Brazilian coast. Rev Soc Bras Med Trop. 2012;45:390-392. doi:10.1590/s0037-86822012000300021
- Gargus MD, Morohashi DK. A sea-urchin spine chilling remedy. N Engl J Med. 2012;367:1867-1868. doi:10.1056/NEJMc1209382
- Sjøberg T, de Weerd L. The usefulness of a skin biopsy punch to remove sea urchin spines. ANZ J Surg. 2010;80:383. doi:10.1111/j.1445-2197.2010.05296.x
- Cardenas-de la Garza JA, Cuellar-Barboza A, Ancer-Arellano J, et al. Classic dermatological tools: foreign body removal with punch biopsy.J Am Acad Dermatol. 2019;81:E93-E94. doi:10.1016/j.jaad.2018.10.038
- Gungor S, Tarikçi N, Gokdemir G. Removal of sea urchin spines using erbium-doped yttrium aluminum garnet ablation. Dermatol Surg. 2012;38:508-510. doi:10.1111/j.1524-4725.2011.02259.x
- Böer A, Ochsendorf FR, Beier C, et al. Effective removal of sea-urchin spines by erbium:YAG laser ablation. Br J Dermatol. 2001;145:169-170. doi:10.1046/j.1365-2133.2001.04306.x
- De La Torre C, Toribio J. Sea-urchin granuloma: histologic profile. a pathologic study of 50 biopsies. J Cutan Pathol. 2001;28:223-228. doi:10.1034/j.1600-0560.2001.028005223.x
- Yi A, Kennedy C, Chia B, et al. Radiographic soft tissue thickness differentiating pyogenic flexor tenosynovitis from other finger infections. J Hand Surg Am. 2019;44:394-399. doi:10.1016/j.jhsa.2019.01.013
- Callison C, Nguyen H. Tetanus prophylaxis. In: StatPearls [Internet]. StatPearls Publishing; 2022.
Practice Points
- Sea urchin spines easily become embedded in human skin upon contact and cause localized pain, edema, and black or purple pinpoint markings.
- Immediate treatment includes soaking in hot water (113 12°F [45 12°C]) for 30 to 90 minutes to inactivate proinflammatory compounds, followed by extraction of the spines.
- Successful methods of spine removal include the use of forceps and a hypodermic needle, as well as excision, liquid nitrogen, and punch biopsy.
- Prompt removal of the spines can reduce the incidence of delayed granulomatous reactions, synovitis, and sea urchin arthritis.