User login
STOP performing dilation and curettage for the evaluation of abnormal uterine bleeding
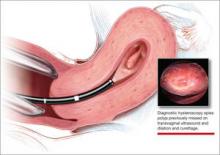
CASE: In-office hysteroscopy spies previously missed polyp
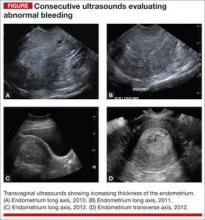
A 51-year-old woman with a history of breast cancer completed 5 years of tamoxifen. During her treatment she had a 3-year history of abnormal vaginal bleeding. Results from consecutive pelvic ultrasounds indicated that the patient had progressively thickening endometrium (from 1.4 cm to 2.5 cm to 4.7 cm). In-office biopsy was negative for endometrial pathology. An ultimate dilation and curettage (D&C) was performed with negative histologic diagnosis. The patient is seen in consultation, and the ultrasound images are reviewed (FIGURE).
These images show an increasing thickness of the ednometrium with definitive intracavitary pathology that was missed with the prevous clinical evaluation with enodmetrial biopsy and D&C. An in-office hysteroscopy is performed, and a large 5 x 4 x 7 cm cystic and fibrous polyp is identified with normal endometrium (VIDEO 1).
Hysteroscopy reveals massive polyp extending into a dilated lower uterine segment
Abnormal uterine bleeding
The evaluation of abnormal uterine bleeding. (AUB), as described in this clinical scenario, is quite common. As a consequence, many patients have a missed or inaccurate diagnosis and undergo unnecessary invasive procedures under general anesthesia.
AUB is one of the primary indications for a gynecologic consultation, accounting for approximately 33% of all gynecology visits, and for 69% of visits among postmenopausal women.1 Confirming the etiology and planning appropriate intervention is important in the clinical management of AUB because accurate diagnosis may result in avoiding major gynecologic surgery in favor of minimally invasive hysteroscopic management.
Diagnostic hysteroscopy is proven in AUB evaluation
Drawbacks of other diagnostic tools. It is generally accepted that the initial evaluation of women with AUB is performed with noninvasive transvaginal ultrasound (TVUS).1-3 As illustrated by the opening case, however, the accuracy of TVUS is limited in the diagnosis of focal endometrial lesions, and further investigation of the uterine cavity is warranted.
Evaluation of the uterine cavity with sonohysterography (SH)—vaginal ultrasound with the instillation of saline into the uterine cavity—is more accurate than TVUS alone. Yet, diagnostic hysteroscopy (DH) has proven to be superior to either modality for the accurate evaluation of intracavitary pathology.1-3
Evidence of DH superiority. Farquhar and colleagues reported results of a systematic review of studies published from 1980 to July 2001 that examined TVUS versus SH and DH for the investigation of AUB in premenopausal women. The researchers found that TVUS had a higher rate of false negatives for detecting intrauterine pathology, compared with SH and DH. They also found that DH was superior to SH in diagnosing submucous myomas.2
In 2010, results of a prospective comparison of TVUS, SH, and DH for detecting endometrial pathology, showed that DH had a significantly better diagnostic performance than SH and TVUS and that hysteroscopy was significantly more precise in the diagnosis of intracavitary masses.3
Again, in 2012, a prospective comparison of TVUS, SH, and DH in the diagnosis of AUB revealed that hysteroscopy provided the most accurate diagnosis.1
In a systematic review and meta-analysis, van Dongen found the accuracy of DH to be estimated at 96.9%.4
Hysteroscopy is also considered to be more comfortable for patients than SH.2
blinded sampling missed intracavitary lesions such as polyps and myomas that accounted for bleeding abnormalities.5
A procedure performed “blind” limits its usefulness in AUB evaluation
This statement is not a new realization. As far back as 1989, Loffer showed that blind D&C was less accurate for diagnosing AUB than was hysteroscopy with visually directed biopsy. The sensitivity of diagnosing the etiology of AUB with D&C was 65%, compared to a sensitivity of 98% with hysteroscopy with directed biopsy. Hysteroscopy was shown to be better because blinded sampling missed intracavitary lesions such as polyps and myomas that accounted for bleeding abnormalities.5
The limitations of D&C are evident in the opening case, as the D&C performed in the operating room under general anesthesia, without visualization of the uterine cavity, failed to identify the patient’s intrauterine pathology. D&C is seldom necessary to evaluate AUB and has significant surgical risks beyond general anesthesia, including cervical or uterine trauma that can occur with cervical dilation and instrumentation of the uterus.
As with D&C, endometrial biopsy has limitations in diagnosing abnormalities within the uterine cavity. In a 2008 prospective comparative study of hysteroscopy versus blind biopsy with a suction biopsy curette, Angioni and colleagues showed a significant difference in the capability of these two procedures to accurately diagnose the etiology of bleeding for menopausal women. Blinded biopsy had a sensitivity for diagnosing polyps, myomas, and hyperplasia of 11%, 13%, and 25%, respectively. The sensitivity of hysteroscopy to diagnose the same intracavitary pathology was 89%, 100%, and 74%, respectively.6
This does not mean, however, that the endometrial biopsy is not beneficial in the evaluation of AUB. Several authors recommend that in a clinically relevant situation, endometrial biopsy with a small suction curette should be performed concomitantly with hysteroscopy to improve the sensitivity of the overall evaluation with histology.7,8 And, as Loffer showed, the hysteroscopic evaluation with a visually directed biopsy is extremely accurate (VIDEO 2, VIDEO 3).5
The bottom line for D&C use in AUB evaluation
Sometimes a D&C is needed. For instance, when more tissue is needed for histologic evaluation than can be obtained with small suction curette at endometrial biopsy. However, there are several shortcomings of D&C for the evaluation of AUB:
- Most clinicians perform D&C in the operating room under general anesthesia.
- It is often done without concomitant hysteroscopy.
- There is significant potential to miss pathology, such as polyps or myomas.
- There is risk of uterine perforation with cervical dilation and uterine instrumentation.
- Hysteroscopy with visually directed biopsy provides a method that offers a more accurate diagnosis, and the procedure can be performed in the office.
In-office AUB evaluation using hysteroscopy is possible and advantageous
Hysteroscopy not only has increased accuracy for identifying the etiology of AUB, compared with D&C, but also offers the possibility of in-office use. Newer hysteroscopes with small diameters and decreasing costs of hysteroscopic equipment allow gynecologists to perform hysteroscopy economically and safely in the office.
Office evaluation of the uterine cavity and preoperative decision-making before a patient is taken to the operating room (OR) improve the likelihood that the appropriate procedure will be performed. They also provide an opportunity for the patient to see inside her own uterine cavity and for the surgeon to discuss management options with her (VIDEO 4: Diagnostic hysteroscopy with fundal myoma, VIDEO 5: Diagnostic hysteroscopy in a menopausal patient with atypical hyperplasia, VIDEO 6: Diagnostic hysteroscopy in a menopausal patient with polyps). If pathology is noted, there is a potential to treat abnormalities such as endometrial polyps at the same time, thus avoiding the OR altogether (VIDEO 7).
The small diameter of the hysteroscope allows evaluation in most menopausal and nulliparous patients comfortably without first having to dilate or soften the cervix. Paracervical placement of local anesthetic can be used as needed for patient comfort (VIDEO 8).9 A vaginoscopic approach will eliminate the discomfort of having to place a speculum (VIDEO 9).
It offers:
- a familiar and comfortable environment for the procedure
- saved time for patient and physician
- saved money for the patient with a large deductible or coinsurance
- no requirement for general anesthesia
- local anesthesia can be used but is not necessary
- immediate visual affirmation for the patient and physician
- a see and treat possibility
- possibility of preoperative decision-making
- saved trip to the OR if significant precancer or cancer is identified
- use in menopausal and nulliparous patients with no cervical preparation necessary when a small-diameter hysteroscope or flexible hysteroscope is used
- minimized discomfort from a speculum with the vaginoscopic approach for awake patients
- possibility of cervical access when needed (VIDEO 10).
My clinical recommendation
Use office hysteroscopy with endometrial biopsy as needed with the opportunity to perform a directed biopsy.
Coding for in-office hysteroscopy
Some procedures now can be performed in the office setting. Among these is operative hysteroscopy, for things such as abnormal uterine bleeding (AUB), foreign body removal, and tubal occlusion. When performing hysteroscopic evaluation of AUB, the Current Procedural Terminology (CPT) code 58558 (Hysteroscopy, surgical; with sampling (biopsy) of endometrium and/or polypectomy, with or without D&C) should be reported. This code is reported whether polyp(s) are removed or a sampling of the uterine lining or a full D&C is performed.
Under the Resource-Based Relative Value System (RBRVS), used by the majority of payers for reimbursement, there is a payment differential for site of service. In other words, when performed in the office setting, reimbursement will be higher than in the hospital setting to offset the increased practice expenses incurred. In the office setting, 58558 has 11.93 relative value units. In comparison, a D&C performed without hysteroscopy has 7.75 relative value units in the office setting. Keep in mind, however, that all supplies used in performing this procedure are included in the reimbursement amount.
Some payers will reimburse separately for administering a regional anesthetic, but a local anesthetic is considered integral to the procedure. Under CPT rules, you may bill separately for regional anesthesia. When performing office hysteroscopy, the most common regional anesthesia would be a paracervical nerve block (CPT code 66435—Injection, anesthetic agent; paracervical [uterine] nerve). Under CPT rules, coding should go in as 58558-47, 64435-51. The modifier -47 lets the payer know that the physician performed the regional block, and the modifier -51 identifies the regional block as a multiple procedure.
Medicare, however, will never reimburse separately for regional anesthesia performed by the operating physician, and because of this, Medicare’s Correct Coding Initiative (CCI) permanently bundles 64435 when billed with 58558. Medicare will not permit a modifier to be used to bypass this bundling edit, and separate payment is never allowed. If your payer has adopted this Medicare policy, separate payment will also not be made.
—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists
- Soguktas S, Cogendez E, Kayatas SE, Asoglu MR, Selcuk S, Ertekin A. Comparison of saline infusion sonohysterography and hysteroscopy in diagnosis of premenopausal women with abnormal uterine bleeding. Eur J Obstet Gynecol Repro Biol. 2012;161(1):66−70.
- Farquhar C, Ekeroma A, Furness S, Arroll B. A systematic review of transvaginal ultrasonography, sonohysterography and hysteroscopy for the investigation of abnormal uterine bleeding in premenopausal bleeding. Acta Obstet Gynecol Scand. 2003;82(6):493–504.
- Grimbizis GF, Tsolakidis D, Mikos T, et al. A prospective comparison of transvaginal ultrasound, saline infusion sonohysterography, and diagnostic hysteroscopy in the evaluation of endometrial pathology. Fertil Steril. 2010;94(7):2721−2725.
- van Dongen H, de Kroon CD, Jacobi CE, Trimbos JB, Jansen FW. Diagnostic hysteroscopy in abnormal uterine bleeding: a systematic review and meta-analysis. BJOG. 2007;114(6):664–75.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D & C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73(1):16–20.
- Angioni S, Loddo A, Milano F, Piras B, Minerba L, Melis GB. Detection of benign intracavitary lesions in postmenopausal women with abnormal uterine bleeding: a prospective comparative study on outpatient hysteroscopy and blind biopsy. J Minim Invasive Gynecol. 2008;15(1):87–91.
- Lo KY, Yuen PM. The role of outpatient diagnostic hysteroscopy in identifying anatomic pathology and histopathology in the endometrial cavity. J Am Assoc Gynecol Laparosc. 2000;7(3):381–385.
- Garuti G, Sambruni I, Colonneli M, Luerti M. Accuracy of hysteroscopy in predicting histopathology of endometrium in 1500 women. J Am Assoc Gynecol Laparosc. 2001;8(2):207–213.
- Munro MG, Brooks PG. Use of local anesthesia for office diagnostic and operative hysteroscopy. J Minim Invasive Gynecol. 2010;17(6):709–718.
CASE: In-office hysteroscopy spies previously missed polyp
A 51-year-old woman with a history of breast cancer completed 5 years of tamoxifen. During her treatment she had a 3-year history of abnormal vaginal bleeding. Results from consecutive pelvic ultrasounds indicated that the patient had progressively thickening endometrium (from 1.4 cm to 2.5 cm to 4.7 cm). In-office biopsy was negative for endometrial pathology. An ultimate dilation and curettage (D&C) was performed with negative histologic diagnosis. The patient is seen in consultation, and the ultrasound images are reviewed (FIGURE).
These images show an increasing thickness of the ednometrium with definitive intracavitary pathology that was missed with the prevous clinical evaluation with enodmetrial biopsy and D&C. An in-office hysteroscopy is performed, and a large 5 x 4 x 7 cm cystic and fibrous polyp is identified with normal endometrium (VIDEO 1).
Hysteroscopy reveals massive polyp extending into a dilated lower uterine segment
Abnormal uterine bleeding
The evaluation of abnormal uterine bleeding. (AUB), as described in this clinical scenario, is quite common. As a consequence, many patients have a missed or inaccurate diagnosis and undergo unnecessary invasive procedures under general anesthesia.
AUB is one of the primary indications for a gynecologic consultation, accounting for approximately 33% of all gynecology visits, and for 69% of visits among postmenopausal women.1 Confirming the etiology and planning appropriate intervention is important in the clinical management of AUB because accurate diagnosis may result in avoiding major gynecologic surgery in favor of minimally invasive hysteroscopic management.
Diagnostic hysteroscopy is proven in AUB evaluation
Drawbacks of other diagnostic tools. It is generally accepted that the initial evaluation of women with AUB is performed with noninvasive transvaginal ultrasound (TVUS).1-3 As illustrated by the opening case, however, the accuracy of TVUS is limited in the diagnosis of focal endometrial lesions, and further investigation of the uterine cavity is warranted.
Evaluation of the uterine cavity with sonohysterography (SH)—vaginal ultrasound with the instillation of saline into the uterine cavity—is more accurate than TVUS alone. Yet, diagnostic hysteroscopy (DH) has proven to be superior to either modality for the accurate evaluation of intracavitary pathology.1-3
Evidence of DH superiority. Farquhar and colleagues reported results of a systematic review of studies published from 1980 to July 2001 that examined TVUS versus SH and DH for the investigation of AUB in premenopausal women. The researchers found that TVUS had a higher rate of false negatives for detecting intrauterine pathology, compared with SH and DH. They also found that DH was superior to SH in diagnosing submucous myomas.2
In 2010, results of a prospective comparison of TVUS, SH, and DH for detecting endometrial pathology, showed that DH had a significantly better diagnostic performance than SH and TVUS and that hysteroscopy was significantly more precise in the diagnosis of intracavitary masses.3
Again, in 2012, a prospective comparison of TVUS, SH, and DH in the diagnosis of AUB revealed that hysteroscopy provided the most accurate diagnosis.1
In a systematic review and meta-analysis, van Dongen found the accuracy of DH to be estimated at 96.9%.4
Hysteroscopy is also considered to be more comfortable for patients than SH.2
blinded sampling missed intracavitary lesions such as polyps and myomas that accounted for bleeding abnormalities.5
A procedure performed “blind” limits its usefulness in AUB evaluation
This statement is not a new realization. As far back as 1989, Loffer showed that blind D&C was less accurate for diagnosing AUB than was hysteroscopy with visually directed biopsy. The sensitivity of diagnosing the etiology of AUB with D&C was 65%, compared to a sensitivity of 98% with hysteroscopy with directed biopsy. Hysteroscopy was shown to be better because blinded sampling missed intracavitary lesions such as polyps and myomas that accounted for bleeding abnormalities.5
The limitations of D&C are evident in the opening case, as the D&C performed in the operating room under general anesthesia, without visualization of the uterine cavity, failed to identify the patient’s intrauterine pathology. D&C is seldom necessary to evaluate AUB and has significant surgical risks beyond general anesthesia, including cervical or uterine trauma that can occur with cervical dilation and instrumentation of the uterus.
As with D&C, endometrial biopsy has limitations in diagnosing abnormalities within the uterine cavity. In a 2008 prospective comparative study of hysteroscopy versus blind biopsy with a suction biopsy curette, Angioni and colleagues showed a significant difference in the capability of these two procedures to accurately diagnose the etiology of bleeding for menopausal women. Blinded biopsy had a sensitivity for diagnosing polyps, myomas, and hyperplasia of 11%, 13%, and 25%, respectively. The sensitivity of hysteroscopy to diagnose the same intracavitary pathology was 89%, 100%, and 74%, respectively.6
This does not mean, however, that the endometrial biopsy is not beneficial in the evaluation of AUB. Several authors recommend that in a clinically relevant situation, endometrial biopsy with a small suction curette should be performed concomitantly with hysteroscopy to improve the sensitivity of the overall evaluation with histology.7,8 And, as Loffer showed, the hysteroscopic evaluation with a visually directed biopsy is extremely accurate (VIDEO 2, VIDEO 3).5
The bottom line for D&C use in AUB evaluation
Sometimes a D&C is needed. For instance, when more tissue is needed for histologic evaluation than can be obtained with small suction curette at endometrial biopsy. However, there are several shortcomings of D&C for the evaluation of AUB:
- Most clinicians perform D&C in the operating room under general anesthesia.
- It is often done without concomitant hysteroscopy.
- There is significant potential to miss pathology, such as polyps or myomas.
- There is risk of uterine perforation with cervical dilation and uterine instrumentation.
- Hysteroscopy with visually directed biopsy provides a method that offers a more accurate diagnosis, and the procedure can be performed in the office.
In-office AUB evaluation using hysteroscopy is possible and advantageous
Hysteroscopy not only has increased accuracy for identifying the etiology of AUB, compared with D&C, but also offers the possibility of in-office use. Newer hysteroscopes with small diameters and decreasing costs of hysteroscopic equipment allow gynecologists to perform hysteroscopy economically and safely in the office.
Office evaluation of the uterine cavity and preoperative decision-making before a patient is taken to the operating room (OR) improve the likelihood that the appropriate procedure will be performed. They also provide an opportunity for the patient to see inside her own uterine cavity and for the surgeon to discuss management options with her (VIDEO 4: Diagnostic hysteroscopy with fundal myoma, VIDEO 5: Diagnostic hysteroscopy in a menopausal patient with atypical hyperplasia, VIDEO 6: Diagnostic hysteroscopy in a menopausal patient with polyps). If pathology is noted, there is a potential to treat abnormalities such as endometrial polyps at the same time, thus avoiding the OR altogether (VIDEO 7).
The small diameter of the hysteroscope allows evaluation in most menopausal and nulliparous patients comfortably without first having to dilate or soften the cervix. Paracervical placement of local anesthetic can be used as needed for patient comfort (VIDEO 8).9 A vaginoscopic approach will eliminate the discomfort of having to place a speculum (VIDEO 9).
It offers:
- a familiar and comfortable environment for the procedure
- saved time for patient and physician
- saved money for the patient with a large deductible or coinsurance
- no requirement for general anesthesia
- local anesthesia can be used but is not necessary
- immediate visual affirmation for the patient and physician
- a see and treat possibility
- possibility of preoperative decision-making
- saved trip to the OR if significant precancer or cancer is identified
- use in menopausal and nulliparous patients with no cervical preparation necessary when a small-diameter hysteroscope or flexible hysteroscope is used
- minimized discomfort from a speculum with the vaginoscopic approach for awake patients
- possibility of cervical access when needed (VIDEO 10).
My clinical recommendation
Use office hysteroscopy with endometrial biopsy as needed with the opportunity to perform a directed biopsy.
Coding for in-office hysteroscopy
Some procedures now can be performed in the office setting. Among these is operative hysteroscopy, for things such as abnormal uterine bleeding (AUB), foreign body removal, and tubal occlusion. When performing hysteroscopic evaluation of AUB, the Current Procedural Terminology (CPT) code 58558 (Hysteroscopy, surgical; with sampling (biopsy) of endometrium and/or polypectomy, with or without D&C) should be reported. This code is reported whether polyp(s) are removed or a sampling of the uterine lining or a full D&C is performed.
Under the Resource-Based Relative Value System (RBRVS), used by the majority of payers for reimbursement, there is a payment differential for site of service. In other words, when performed in the office setting, reimbursement will be higher than in the hospital setting to offset the increased practice expenses incurred. In the office setting, 58558 has 11.93 relative value units. In comparison, a D&C performed without hysteroscopy has 7.75 relative value units in the office setting. Keep in mind, however, that all supplies used in performing this procedure are included in the reimbursement amount.
Some payers will reimburse separately for administering a regional anesthetic, but a local anesthetic is considered integral to the procedure. Under CPT rules, you may bill separately for regional anesthesia. When performing office hysteroscopy, the most common regional anesthesia would be a paracervical nerve block (CPT code 66435—Injection, anesthetic agent; paracervical [uterine] nerve). Under CPT rules, coding should go in as 58558-47, 64435-51. The modifier -47 lets the payer know that the physician performed the regional block, and the modifier -51 identifies the regional block as a multiple procedure.
Medicare, however, will never reimburse separately for regional anesthesia performed by the operating physician, and because of this, Medicare’s Correct Coding Initiative (CCI) permanently bundles 64435 when billed with 58558. Medicare will not permit a modifier to be used to bypass this bundling edit, and separate payment is never allowed. If your payer has adopted this Medicare policy, separate payment will also not be made.
—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists
CASE: In-office hysteroscopy spies previously missed polyp
A 51-year-old woman with a history of breast cancer completed 5 years of tamoxifen. During her treatment she had a 3-year history of abnormal vaginal bleeding. Results from consecutive pelvic ultrasounds indicated that the patient had progressively thickening endometrium (from 1.4 cm to 2.5 cm to 4.7 cm). In-office biopsy was negative for endometrial pathology. An ultimate dilation and curettage (D&C) was performed with negative histologic diagnosis. The patient is seen in consultation, and the ultrasound images are reviewed (FIGURE).
These images show an increasing thickness of the ednometrium with definitive intracavitary pathology that was missed with the prevous clinical evaluation with enodmetrial biopsy and D&C. An in-office hysteroscopy is performed, and a large 5 x 4 x 7 cm cystic and fibrous polyp is identified with normal endometrium (VIDEO 1).
Hysteroscopy reveals massive polyp extending into a dilated lower uterine segment
Abnormal uterine bleeding
The evaluation of abnormal uterine bleeding. (AUB), as described in this clinical scenario, is quite common. As a consequence, many patients have a missed or inaccurate diagnosis and undergo unnecessary invasive procedures under general anesthesia.
AUB is one of the primary indications for a gynecologic consultation, accounting for approximately 33% of all gynecology visits, and for 69% of visits among postmenopausal women.1 Confirming the etiology and planning appropriate intervention is important in the clinical management of AUB because accurate diagnosis may result in avoiding major gynecologic surgery in favor of minimally invasive hysteroscopic management.
Diagnostic hysteroscopy is proven in AUB evaluation
Drawbacks of other diagnostic tools. It is generally accepted that the initial evaluation of women with AUB is performed with noninvasive transvaginal ultrasound (TVUS).1-3 As illustrated by the opening case, however, the accuracy of TVUS is limited in the diagnosis of focal endometrial lesions, and further investigation of the uterine cavity is warranted.
Evaluation of the uterine cavity with sonohysterography (SH)—vaginal ultrasound with the instillation of saline into the uterine cavity—is more accurate than TVUS alone. Yet, diagnostic hysteroscopy (DH) has proven to be superior to either modality for the accurate evaluation of intracavitary pathology.1-3
Evidence of DH superiority. Farquhar and colleagues reported results of a systematic review of studies published from 1980 to July 2001 that examined TVUS versus SH and DH for the investigation of AUB in premenopausal women. The researchers found that TVUS had a higher rate of false negatives for detecting intrauterine pathology, compared with SH and DH. They also found that DH was superior to SH in diagnosing submucous myomas.2
In 2010, results of a prospective comparison of TVUS, SH, and DH for detecting endometrial pathology, showed that DH had a significantly better diagnostic performance than SH and TVUS and that hysteroscopy was significantly more precise in the diagnosis of intracavitary masses.3
Again, in 2012, a prospective comparison of TVUS, SH, and DH in the diagnosis of AUB revealed that hysteroscopy provided the most accurate diagnosis.1
In a systematic review and meta-analysis, van Dongen found the accuracy of DH to be estimated at 96.9%.4
Hysteroscopy is also considered to be more comfortable for patients than SH.2
blinded sampling missed intracavitary lesions such as polyps and myomas that accounted for bleeding abnormalities.5
A procedure performed “blind” limits its usefulness in AUB evaluation
This statement is not a new realization. As far back as 1989, Loffer showed that blind D&C was less accurate for diagnosing AUB than was hysteroscopy with visually directed biopsy. The sensitivity of diagnosing the etiology of AUB with D&C was 65%, compared to a sensitivity of 98% with hysteroscopy with directed biopsy. Hysteroscopy was shown to be better because blinded sampling missed intracavitary lesions such as polyps and myomas that accounted for bleeding abnormalities.5
The limitations of D&C are evident in the opening case, as the D&C performed in the operating room under general anesthesia, without visualization of the uterine cavity, failed to identify the patient’s intrauterine pathology. D&C is seldom necessary to evaluate AUB and has significant surgical risks beyond general anesthesia, including cervical or uterine trauma that can occur with cervical dilation and instrumentation of the uterus.
As with D&C, endometrial biopsy has limitations in diagnosing abnormalities within the uterine cavity. In a 2008 prospective comparative study of hysteroscopy versus blind biopsy with a suction biopsy curette, Angioni and colleagues showed a significant difference in the capability of these two procedures to accurately diagnose the etiology of bleeding for menopausal women. Blinded biopsy had a sensitivity for diagnosing polyps, myomas, and hyperplasia of 11%, 13%, and 25%, respectively. The sensitivity of hysteroscopy to diagnose the same intracavitary pathology was 89%, 100%, and 74%, respectively.6
This does not mean, however, that the endometrial biopsy is not beneficial in the evaluation of AUB. Several authors recommend that in a clinically relevant situation, endometrial biopsy with a small suction curette should be performed concomitantly with hysteroscopy to improve the sensitivity of the overall evaluation with histology.7,8 And, as Loffer showed, the hysteroscopic evaluation with a visually directed biopsy is extremely accurate (VIDEO 2, VIDEO 3).5
The bottom line for D&C use in AUB evaluation
Sometimes a D&C is needed. For instance, when more tissue is needed for histologic evaluation than can be obtained with small suction curette at endometrial biopsy. However, there are several shortcomings of D&C for the evaluation of AUB:
- Most clinicians perform D&C in the operating room under general anesthesia.
- It is often done without concomitant hysteroscopy.
- There is significant potential to miss pathology, such as polyps or myomas.
- There is risk of uterine perforation with cervical dilation and uterine instrumentation.
- Hysteroscopy with visually directed biopsy provides a method that offers a more accurate diagnosis, and the procedure can be performed in the office.
In-office AUB evaluation using hysteroscopy is possible and advantageous
Hysteroscopy not only has increased accuracy for identifying the etiology of AUB, compared with D&C, but also offers the possibility of in-office use. Newer hysteroscopes with small diameters and decreasing costs of hysteroscopic equipment allow gynecologists to perform hysteroscopy economically and safely in the office.
Office evaluation of the uterine cavity and preoperative decision-making before a patient is taken to the operating room (OR) improve the likelihood that the appropriate procedure will be performed. They also provide an opportunity for the patient to see inside her own uterine cavity and for the surgeon to discuss management options with her (VIDEO 4: Diagnostic hysteroscopy with fundal myoma, VIDEO 5: Diagnostic hysteroscopy in a menopausal patient with atypical hyperplasia, VIDEO 6: Diagnostic hysteroscopy in a menopausal patient with polyps). If pathology is noted, there is a potential to treat abnormalities such as endometrial polyps at the same time, thus avoiding the OR altogether (VIDEO 7).
The small diameter of the hysteroscope allows evaluation in most menopausal and nulliparous patients comfortably without first having to dilate or soften the cervix. Paracervical placement of local anesthetic can be used as needed for patient comfort (VIDEO 8).9 A vaginoscopic approach will eliminate the discomfort of having to place a speculum (VIDEO 9).
It offers:
- a familiar and comfortable environment for the procedure
- saved time for patient and physician
- saved money for the patient with a large deductible or coinsurance
- no requirement for general anesthesia
- local anesthesia can be used but is not necessary
- immediate visual affirmation for the patient and physician
- a see and treat possibility
- possibility of preoperative decision-making
- saved trip to the OR if significant precancer or cancer is identified
- use in menopausal and nulliparous patients with no cervical preparation necessary when a small-diameter hysteroscope or flexible hysteroscope is used
- minimized discomfort from a speculum with the vaginoscopic approach for awake patients
- possibility of cervical access when needed (VIDEO 10).
My clinical recommendation
Use office hysteroscopy with endometrial biopsy as needed with the opportunity to perform a directed biopsy.
Coding for in-office hysteroscopy
Some procedures now can be performed in the office setting. Among these is operative hysteroscopy, for things such as abnormal uterine bleeding (AUB), foreign body removal, and tubal occlusion. When performing hysteroscopic evaluation of AUB, the Current Procedural Terminology (CPT) code 58558 (Hysteroscopy, surgical; with sampling (biopsy) of endometrium and/or polypectomy, with or without D&C) should be reported. This code is reported whether polyp(s) are removed or a sampling of the uterine lining or a full D&C is performed.
Under the Resource-Based Relative Value System (RBRVS), used by the majority of payers for reimbursement, there is a payment differential for site of service. In other words, when performed in the office setting, reimbursement will be higher than in the hospital setting to offset the increased practice expenses incurred. In the office setting, 58558 has 11.93 relative value units. In comparison, a D&C performed without hysteroscopy has 7.75 relative value units in the office setting. Keep in mind, however, that all supplies used in performing this procedure are included in the reimbursement amount.
Some payers will reimburse separately for administering a regional anesthetic, but a local anesthetic is considered integral to the procedure. Under CPT rules, you may bill separately for regional anesthesia. When performing office hysteroscopy, the most common regional anesthesia would be a paracervical nerve block (CPT code 66435—Injection, anesthetic agent; paracervical [uterine] nerve). Under CPT rules, coding should go in as 58558-47, 64435-51. The modifier -47 lets the payer know that the physician performed the regional block, and the modifier -51 identifies the regional block as a multiple procedure.
Medicare, however, will never reimburse separately for regional anesthesia performed by the operating physician, and because of this, Medicare’s Correct Coding Initiative (CCI) permanently bundles 64435 when billed with 58558. Medicare will not permit a modifier to be used to bypass this bundling edit, and separate payment is never allowed. If your payer has adopted this Medicare policy, separate payment will also not be made.
—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists
- Soguktas S, Cogendez E, Kayatas SE, Asoglu MR, Selcuk S, Ertekin A. Comparison of saline infusion sonohysterography and hysteroscopy in diagnosis of premenopausal women with abnormal uterine bleeding. Eur J Obstet Gynecol Repro Biol. 2012;161(1):66−70.
- Farquhar C, Ekeroma A, Furness S, Arroll B. A systematic review of transvaginal ultrasonography, sonohysterography and hysteroscopy for the investigation of abnormal uterine bleeding in premenopausal bleeding. Acta Obstet Gynecol Scand. 2003;82(6):493–504.
- Grimbizis GF, Tsolakidis D, Mikos T, et al. A prospective comparison of transvaginal ultrasound, saline infusion sonohysterography, and diagnostic hysteroscopy in the evaluation of endometrial pathology. Fertil Steril. 2010;94(7):2721−2725.
- van Dongen H, de Kroon CD, Jacobi CE, Trimbos JB, Jansen FW. Diagnostic hysteroscopy in abnormal uterine bleeding: a systematic review and meta-analysis. BJOG. 2007;114(6):664–75.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D & C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73(1):16–20.
- Angioni S, Loddo A, Milano F, Piras B, Minerba L, Melis GB. Detection of benign intracavitary lesions in postmenopausal women with abnormal uterine bleeding: a prospective comparative study on outpatient hysteroscopy and blind biopsy. J Minim Invasive Gynecol. 2008;15(1):87–91.
- Lo KY, Yuen PM. The role of outpatient diagnostic hysteroscopy in identifying anatomic pathology and histopathology in the endometrial cavity. J Am Assoc Gynecol Laparosc. 2000;7(3):381–385.
- Garuti G, Sambruni I, Colonneli M, Luerti M. Accuracy of hysteroscopy in predicting histopathology of endometrium in 1500 women. J Am Assoc Gynecol Laparosc. 2001;8(2):207–213.
- Munro MG, Brooks PG. Use of local anesthesia for office diagnostic and operative hysteroscopy. J Minim Invasive Gynecol. 2010;17(6):709–718.
- Soguktas S, Cogendez E, Kayatas SE, Asoglu MR, Selcuk S, Ertekin A. Comparison of saline infusion sonohysterography and hysteroscopy in diagnosis of premenopausal women with abnormal uterine bleeding. Eur J Obstet Gynecol Repro Biol. 2012;161(1):66−70.
- Farquhar C, Ekeroma A, Furness S, Arroll B. A systematic review of transvaginal ultrasonography, sonohysterography and hysteroscopy for the investigation of abnormal uterine bleeding in premenopausal bleeding. Acta Obstet Gynecol Scand. 2003;82(6):493–504.
- Grimbizis GF, Tsolakidis D, Mikos T, et al. A prospective comparison of transvaginal ultrasound, saline infusion sonohysterography, and diagnostic hysteroscopy in the evaluation of endometrial pathology. Fertil Steril. 2010;94(7):2721−2725.
- van Dongen H, de Kroon CD, Jacobi CE, Trimbos JB, Jansen FW. Diagnostic hysteroscopy in abnormal uterine bleeding: a systematic review and meta-analysis. BJOG. 2007;114(6):664–75.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D & C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73(1):16–20.
- Angioni S, Loddo A, Milano F, Piras B, Minerba L, Melis GB. Detection of benign intracavitary lesions in postmenopausal women with abnormal uterine bleeding: a prospective comparative study on outpatient hysteroscopy and blind biopsy. J Minim Invasive Gynecol. 2008;15(1):87–91.
- Lo KY, Yuen PM. The role of outpatient diagnostic hysteroscopy in identifying anatomic pathology and histopathology in the endometrial cavity. J Am Assoc Gynecol Laparosc. 2000;7(3):381–385.
- Garuti G, Sambruni I, Colonneli M, Luerti M. Accuracy of hysteroscopy in predicting histopathology of endometrium in 1500 women. J Am Assoc Gynecol Laparosc. 2001;8(2):207–213.
- Munro MG, Brooks PG. Use of local anesthesia for office diagnostic and operative hysteroscopy. J Minim Invasive Gynecol. 2010;17(6):709–718.
Effective Clinical Documentation Can Influence Medicare Reimbursement
Back in the 1980s, I would go by medical records every day or two and find, on the front of the charts of my recently discharged patients, a form listing the diagnoses the hospital was billing to Medicare. Before the hospital could submit a patient’s bill, the attending physician was required to review the form and, by signing it, indicate agreement.
The requirement for this signature by the physician went away a long time ago and in my memory is one of the very few examples of reducing a doctor’s paperwork.
For my first few months in practice, I regularly would seek out the people who completed the form and explain they had misunderstood the patient’s clinical situation. “The main issue was a urinary tract infection,” I would say, “but you listed diabetes as the principal diagnosis.”
I don’t ever remember them changing anything based on my feedback. Instead, they explained to me that, for billing purposes, it was legitimate to list diabetes as the principal diagnosis because it had the additional benefit of resulting in a higher payment to the hospital than having “urinary tract infection” listed first.
Such was my introduction to the world of documentation and coding for hospital billing purposes and how it can sometimes differ significantly from the way a doctor sees the clinical picture. Things have evolved a lot since then, but the way doctors document medical conditions still has a huge influence on hospital reimbursement.
Hospital CDI Programs
About 80% of hospitals have formal clinical documentation improvement (CDI) programs to help ensure all clinical conditions are captured and described in the medical record in ways that are valuable for billing and other recordkeeping purposes. These programs might lead to you receive queries about your documentation. For example, you might be asked to clarify whether your patient’s pneumonia might be on the basis of aspiration.
Within SHM’s Code-H program, Dr. Richard Pinson, a former ED physician who now works with Houston-based HCQ Consulting, has a good presentation explaining these documentation issues. In it, he makes the point that, in addition to influencing how hospitals are paid, the way various conditions are documented also influences quality ratings.
Novel Approach
The most common approach to engaging hospitalists in CDI initiatives is to have them attend a presentation on the topic, then put in place documentation specialists who generate queries asking the doctor to clarify diagnoses when it might influence payment, severity of illness determination, etc. Dr. Kenji Asakura, a Seattle hospitalist, and Erik Ordal, MBA, have a company called ClinIntell that analyzes each hospitalist (or other specialty) group’s historical patient mix and trains them on the documentation issues that they see most often. The idea of this focused approach is to make “documentation queries” unnecessary, or at least much less necessary. The benefits of this approach are many, including reducing or eliminating the risk of “leading queries”—that is, queries that seem to encourage the doctor to document a diagnosis because it is an advantage to the hospital rather than a well-considered medical opinion. Leading queries can be regarded as fraudulent and can get a lot of people in trouble.
I asked Kenji and Erik if they could provide me with a list of common documentation issues that most hospitalists need to know more about. Table 1 is what they came up with. I hope it helps you and your practice.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM's "Best Practices in Managing a Hospital Medicine Program" course. Write to him at john.nelson@nelsonflores.com.
Back in the 1980s, I would go by medical records every day or two and find, on the front of the charts of my recently discharged patients, a form listing the diagnoses the hospital was billing to Medicare. Before the hospital could submit a patient’s bill, the attending physician was required to review the form and, by signing it, indicate agreement.
The requirement for this signature by the physician went away a long time ago and in my memory is one of the very few examples of reducing a doctor’s paperwork.
For my first few months in practice, I regularly would seek out the people who completed the form and explain they had misunderstood the patient’s clinical situation. “The main issue was a urinary tract infection,” I would say, “but you listed diabetes as the principal diagnosis.”
I don’t ever remember them changing anything based on my feedback. Instead, they explained to me that, for billing purposes, it was legitimate to list diabetes as the principal diagnosis because it had the additional benefit of resulting in a higher payment to the hospital than having “urinary tract infection” listed first.
Such was my introduction to the world of documentation and coding for hospital billing purposes and how it can sometimes differ significantly from the way a doctor sees the clinical picture. Things have evolved a lot since then, but the way doctors document medical conditions still has a huge influence on hospital reimbursement.
Hospital CDI Programs
About 80% of hospitals have formal clinical documentation improvement (CDI) programs to help ensure all clinical conditions are captured and described in the medical record in ways that are valuable for billing and other recordkeeping purposes. These programs might lead to you receive queries about your documentation. For example, you might be asked to clarify whether your patient’s pneumonia might be on the basis of aspiration.
Within SHM’s Code-H program, Dr. Richard Pinson, a former ED physician who now works with Houston-based HCQ Consulting, has a good presentation explaining these documentation issues. In it, he makes the point that, in addition to influencing how hospitals are paid, the way various conditions are documented also influences quality ratings.
Novel Approach
The most common approach to engaging hospitalists in CDI initiatives is to have them attend a presentation on the topic, then put in place documentation specialists who generate queries asking the doctor to clarify diagnoses when it might influence payment, severity of illness determination, etc. Dr. Kenji Asakura, a Seattle hospitalist, and Erik Ordal, MBA, have a company called ClinIntell that analyzes each hospitalist (or other specialty) group’s historical patient mix and trains them on the documentation issues that they see most often. The idea of this focused approach is to make “documentation queries” unnecessary, or at least much less necessary. The benefits of this approach are many, including reducing or eliminating the risk of “leading queries”—that is, queries that seem to encourage the doctor to document a diagnosis because it is an advantage to the hospital rather than a well-considered medical opinion. Leading queries can be regarded as fraudulent and can get a lot of people in trouble.
I asked Kenji and Erik if they could provide me with a list of common documentation issues that most hospitalists need to know more about. Table 1 is what they came up with. I hope it helps you and your practice.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM's "Best Practices in Managing a Hospital Medicine Program" course. Write to him at john.nelson@nelsonflores.com.
Back in the 1980s, I would go by medical records every day or two and find, on the front of the charts of my recently discharged patients, a form listing the diagnoses the hospital was billing to Medicare. Before the hospital could submit a patient’s bill, the attending physician was required to review the form and, by signing it, indicate agreement.
The requirement for this signature by the physician went away a long time ago and in my memory is one of the very few examples of reducing a doctor’s paperwork.
For my first few months in practice, I regularly would seek out the people who completed the form and explain they had misunderstood the patient’s clinical situation. “The main issue was a urinary tract infection,” I would say, “but you listed diabetes as the principal diagnosis.”
I don’t ever remember them changing anything based on my feedback. Instead, they explained to me that, for billing purposes, it was legitimate to list diabetes as the principal diagnosis because it had the additional benefit of resulting in a higher payment to the hospital than having “urinary tract infection” listed first.
Such was my introduction to the world of documentation and coding for hospital billing purposes and how it can sometimes differ significantly from the way a doctor sees the clinical picture. Things have evolved a lot since then, but the way doctors document medical conditions still has a huge influence on hospital reimbursement.
Hospital CDI Programs
About 80% of hospitals have formal clinical documentation improvement (CDI) programs to help ensure all clinical conditions are captured and described in the medical record in ways that are valuable for billing and other recordkeeping purposes. These programs might lead to you receive queries about your documentation. For example, you might be asked to clarify whether your patient’s pneumonia might be on the basis of aspiration.
Within SHM’s Code-H program, Dr. Richard Pinson, a former ED physician who now works with Houston-based HCQ Consulting, has a good presentation explaining these documentation issues. In it, he makes the point that, in addition to influencing how hospitals are paid, the way various conditions are documented also influences quality ratings.
Novel Approach
The most common approach to engaging hospitalists in CDI initiatives is to have them attend a presentation on the topic, then put in place documentation specialists who generate queries asking the doctor to clarify diagnoses when it might influence payment, severity of illness determination, etc. Dr. Kenji Asakura, a Seattle hospitalist, and Erik Ordal, MBA, have a company called ClinIntell that analyzes each hospitalist (or other specialty) group’s historical patient mix and trains them on the documentation issues that they see most often. The idea of this focused approach is to make “documentation queries” unnecessary, or at least much less necessary. The benefits of this approach are many, including reducing or eliminating the risk of “leading queries”—that is, queries that seem to encourage the doctor to document a diagnosis because it is an advantage to the hospital rather than a well-considered medical opinion. Leading queries can be regarded as fraudulent and can get a lot of people in trouble.
I asked Kenji and Erik if they could provide me with a list of common documentation issues that most hospitalists need to know more about. Table 1 is what they came up with. I hope it helps you and your practice.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM's "Best Practices in Managing a Hospital Medicine Program" course. Write to him at john.nelson@nelsonflores.com.
Coordinated Approach May Help in Caring for Hospitals’ Neediest Patients
To my way of thinking, a person’s diagnosis or pathophysiology is not as strong a predictor of needing inpatient hospital care as it might have been 10 or 20 years ago. Rather than the clinical diagnosis (e.g. pneumonia), it seems to me that frailty or social complexity often are the principal determinants of which patients are admitted to a hospital for medical conditions.
Some of these patients are admitted frequently but appear to realize little or no benefit from hospitalization. These patients typically have little or no social support, and they often have either significant mental health disorders or substance abuse, or both. Much has been written about these patients, and I recommend an article by Dr. Atul Gawande in the Jan. 24, 2011, issue of The New Yorker titled “The Hot Spotters: Can We Lower Medical Costs by Giving the Neediest Patients Better Care?”
The Agency for Healthcare Research and Quality’s “Statistical Brief 354” on how health-care expenditures are allocated across the population reported that 1% of the population accounted for more than 22% of health-care spending in 2008. One in 5 of those were in that category again in 2009. Some of these patients would benefit from care plans.
The Role of Care Plans
It seems that there may be few effective inpatient interventions that will benefit these patients. After all, they have chronic issues that require ongoing relationships with outpatient providers, something that many of these patients lack. But for some (most?) of these patients, it seems clear that frequent hospitalizations don’t help and sometimes just perpetuate or worsen the patient’s dependence on the hospital at a high financial cost to society—and significant frustration and burnout on the part of hospital caregivers, including hospitalists.
For most hospitals, this problem is significant enough to require some sort of coordinated approach to the care of the dozens of types of patients in this category. Implementing whatever plan of care seems appropriate to the caregivers during each admission is frustrating, ensures lots of variation in care, and makes it easier for manipulative patients to abuse the hospital resources and personnel.
A better approach is to follow the same plan of care from one hospital visit to the next. You already knew that. But developing a care plan to follow during each ED visit and admission is time-consuming and often fraught with uncertainty about where boundaries should be set. So if you’re like me, you might just try to guide the patient to discharge this time and hope that whoever sees the patient on the next admission will take the initiative to develop the care plan. The result is that few such plans are developed.
Your Hospital Needs a Care Plan
Relying on individual doctors or nurses to take the initiative to develop care plans will almost always mean few plans are developed, they will vary in their effectiveness, and other providers may not be aware a plan exists. This was the case at the hospital where I practice until I heard Dr. Rick Hilger, MD, SFHM, a hospitalist at Regions Hospital in Minneapolis, present on this topic at HM12 in San Diego.
Dr. Hilger led a multidisciplinary team to develop care plans (they call them “restriction care plans”) and found that they dramatically reduced the rate of hospital admissions and ED visits for these patients. Hearing about this experience served as a kick in the pants for me, so I did much the same thing at “my” hospital. We have now developed plans for more than 20 patients and found that they visit our ED and are admitted less often. And, anecdotally at least, hospitalists and other hospital staff find that the care plans reduce, at least a little, the stress of caring for these patients.
Unanswered Questions
Although it seems clear that care plans reduce visits to the hospital that develops them, I suspect that some of these patients aren’t consuming any fewer health-care resources. They may just seek care from a different hospital.
My home state of Washington is working to develop individual patient care plans available to all hospitals in the state. A system called the Emergency Department Information Exchange (EDIE) has been adopted by nearly all the hospitals in the state. It allows them to share information on ED visits and such things as care plans with one another. For example, through EDIE, each hospital could see the opiate dosing schedule and admission criteria agreed to by patient and primary-care physician.
So it seems that care plans and the technology to share them can make it more difficult for patients to harm themselves by visiting many hospitals to get excessive opiate prescriptions, for example. This should benefit the patient and lower ED and hospital expenditures for these patients. But we don’t know what portion of costs simply is shifted to other settings, so there is no easy way to know the net effect on health-care costs.
An important unanswered question is whether these care plans improve patient well-being. It seems clear they do in some cases, but it is hard to know whether some patients may be worse off because of the plan.
Conclusion
I think nearly every hospital would benefit from a care plan committee composed of at least one hospitalist, ED physician, a nursing representative, and potentially other disciplines (see “Care Plan Attributes,” above). Our committee includes our inpatient psychiatrist, a really valuable contributor.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at john.nelson@nelsonflores.com.
To my way of thinking, a person’s diagnosis or pathophysiology is not as strong a predictor of needing inpatient hospital care as it might have been 10 or 20 years ago. Rather than the clinical diagnosis (e.g. pneumonia), it seems to me that frailty or social complexity often are the principal determinants of which patients are admitted to a hospital for medical conditions.
Some of these patients are admitted frequently but appear to realize little or no benefit from hospitalization. These patients typically have little or no social support, and they often have either significant mental health disorders or substance abuse, or both. Much has been written about these patients, and I recommend an article by Dr. Atul Gawande in the Jan. 24, 2011, issue of The New Yorker titled “The Hot Spotters: Can We Lower Medical Costs by Giving the Neediest Patients Better Care?”
The Agency for Healthcare Research and Quality’s “Statistical Brief 354” on how health-care expenditures are allocated across the population reported that 1% of the population accounted for more than 22% of health-care spending in 2008. One in 5 of those were in that category again in 2009. Some of these patients would benefit from care plans.
The Role of Care Plans
It seems that there may be few effective inpatient interventions that will benefit these patients. After all, they have chronic issues that require ongoing relationships with outpatient providers, something that many of these patients lack. But for some (most?) of these patients, it seems clear that frequent hospitalizations don’t help and sometimes just perpetuate or worsen the patient’s dependence on the hospital at a high financial cost to society—and significant frustration and burnout on the part of hospital caregivers, including hospitalists.
For most hospitals, this problem is significant enough to require some sort of coordinated approach to the care of the dozens of types of patients in this category. Implementing whatever plan of care seems appropriate to the caregivers during each admission is frustrating, ensures lots of variation in care, and makes it easier for manipulative patients to abuse the hospital resources and personnel.
A better approach is to follow the same plan of care from one hospital visit to the next. You already knew that. But developing a care plan to follow during each ED visit and admission is time-consuming and often fraught with uncertainty about where boundaries should be set. So if you’re like me, you might just try to guide the patient to discharge this time and hope that whoever sees the patient on the next admission will take the initiative to develop the care plan. The result is that few such plans are developed.
Your Hospital Needs a Care Plan
Relying on individual doctors or nurses to take the initiative to develop care plans will almost always mean few plans are developed, they will vary in their effectiveness, and other providers may not be aware a plan exists. This was the case at the hospital where I practice until I heard Dr. Rick Hilger, MD, SFHM, a hospitalist at Regions Hospital in Minneapolis, present on this topic at HM12 in San Diego.
Dr. Hilger led a multidisciplinary team to develop care plans (they call them “restriction care plans”) and found that they dramatically reduced the rate of hospital admissions and ED visits for these patients. Hearing about this experience served as a kick in the pants for me, so I did much the same thing at “my” hospital. We have now developed plans for more than 20 patients and found that they visit our ED and are admitted less often. And, anecdotally at least, hospitalists and other hospital staff find that the care plans reduce, at least a little, the stress of caring for these patients.
Unanswered Questions
Although it seems clear that care plans reduce visits to the hospital that develops them, I suspect that some of these patients aren’t consuming any fewer health-care resources. They may just seek care from a different hospital.
My home state of Washington is working to develop individual patient care plans available to all hospitals in the state. A system called the Emergency Department Information Exchange (EDIE) has been adopted by nearly all the hospitals in the state. It allows them to share information on ED visits and such things as care plans with one another. For example, through EDIE, each hospital could see the opiate dosing schedule and admission criteria agreed to by patient and primary-care physician.
So it seems that care plans and the technology to share them can make it more difficult for patients to harm themselves by visiting many hospitals to get excessive opiate prescriptions, for example. This should benefit the patient and lower ED and hospital expenditures for these patients. But we don’t know what portion of costs simply is shifted to other settings, so there is no easy way to know the net effect on health-care costs.
An important unanswered question is whether these care plans improve patient well-being. It seems clear they do in some cases, but it is hard to know whether some patients may be worse off because of the plan.
Conclusion
I think nearly every hospital would benefit from a care plan committee composed of at least one hospitalist, ED physician, a nursing representative, and potentially other disciplines (see “Care Plan Attributes,” above). Our committee includes our inpatient psychiatrist, a really valuable contributor.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at john.nelson@nelsonflores.com.
To my way of thinking, a person’s diagnosis or pathophysiology is not as strong a predictor of needing inpatient hospital care as it might have been 10 or 20 years ago. Rather than the clinical diagnosis (e.g. pneumonia), it seems to me that frailty or social complexity often are the principal determinants of which patients are admitted to a hospital for medical conditions.
Some of these patients are admitted frequently but appear to realize little or no benefit from hospitalization. These patients typically have little or no social support, and they often have either significant mental health disorders or substance abuse, or both. Much has been written about these patients, and I recommend an article by Dr. Atul Gawande in the Jan. 24, 2011, issue of The New Yorker titled “The Hot Spotters: Can We Lower Medical Costs by Giving the Neediest Patients Better Care?”
The Agency for Healthcare Research and Quality’s “Statistical Brief 354” on how health-care expenditures are allocated across the population reported that 1% of the population accounted for more than 22% of health-care spending in 2008. One in 5 of those were in that category again in 2009. Some of these patients would benefit from care plans.
The Role of Care Plans
It seems that there may be few effective inpatient interventions that will benefit these patients. After all, they have chronic issues that require ongoing relationships with outpatient providers, something that many of these patients lack. But for some (most?) of these patients, it seems clear that frequent hospitalizations don’t help and sometimes just perpetuate or worsen the patient’s dependence on the hospital at a high financial cost to society—and significant frustration and burnout on the part of hospital caregivers, including hospitalists.
For most hospitals, this problem is significant enough to require some sort of coordinated approach to the care of the dozens of types of patients in this category. Implementing whatever plan of care seems appropriate to the caregivers during each admission is frustrating, ensures lots of variation in care, and makes it easier for manipulative patients to abuse the hospital resources and personnel.
A better approach is to follow the same plan of care from one hospital visit to the next. You already knew that. But developing a care plan to follow during each ED visit and admission is time-consuming and often fraught with uncertainty about where boundaries should be set. So if you’re like me, you might just try to guide the patient to discharge this time and hope that whoever sees the patient on the next admission will take the initiative to develop the care plan. The result is that few such plans are developed.
Your Hospital Needs a Care Plan
Relying on individual doctors or nurses to take the initiative to develop care plans will almost always mean few plans are developed, they will vary in their effectiveness, and other providers may not be aware a plan exists. This was the case at the hospital where I practice until I heard Dr. Rick Hilger, MD, SFHM, a hospitalist at Regions Hospital in Minneapolis, present on this topic at HM12 in San Diego.
Dr. Hilger led a multidisciplinary team to develop care plans (they call them “restriction care plans”) and found that they dramatically reduced the rate of hospital admissions and ED visits for these patients. Hearing about this experience served as a kick in the pants for me, so I did much the same thing at “my” hospital. We have now developed plans for more than 20 patients and found that they visit our ED and are admitted less often. And, anecdotally at least, hospitalists and other hospital staff find that the care plans reduce, at least a little, the stress of caring for these patients.
Unanswered Questions
Although it seems clear that care plans reduce visits to the hospital that develops them, I suspect that some of these patients aren’t consuming any fewer health-care resources. They may just seek care from a different hospital.
My home state of Washington is working to develop individual patient care plans available to all hospitals in the state. A system called the Emergency Department Information Exchange (EDIE) has been adopted by nearly all the hospitals in the state. It allows them to share information on ED visits and such things as care plans with one another. For example, through EDIE, each hospital could see the opiate dosing schedule and admission criteria agreed to by patient and primary-care physician.
So it seems that care plans and the technology to share them can make it more difficult for patients to harm themselves by visiting many hospitals to get excessive opiate prescriptions, for example. This should benefit the patient and lower ED and hospital expenditures for these patients. But we don’t know what portion of costs simply is shifted to other settings, so there is no easy way to know the net effect on health-care costs.
An important unanswered question is whether these care plans improve patient well-being. It seems clear they do in some cases, but it is hard to know whether some patients may be worse off because of the plan.
Conclusion
I think nearly every hospital would benefit from a care plan committee composed of at least one hospitalist, ED physician, a nursing representative, and potentially other disciplines (see “Care Plan Attributes,” above). Our committee includes our inpatient psychiatrist, a really valuable contributor.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at john.nelson@nelsonflores.com.
Communication Key to Peaceful Coexistence for Competing Hospital Medicine Groups
Experienced hospitalists and medical directors agree that the key to multiple hospitalist groups coexisting effectively under one roof—whether directly competing or not—is good communication. Effective communication can take time to build.
“Start by working together on something—anything, [such as] a hospital committee of some sort where there’s not likely to be much tension,” says hospitalist pioneer and practice consultant John Nelson, MD, MHM.
Dr. Nelson practices at Overlake Hospital in Bellevue, Wash., which has a hospitalist group employed by the hospital and another employed by Group Health Cooperative, a nonprofit health system in Washington state. It is important to put some trust in the trust bank, he says, “and that’s hard if you have no social connections at all. At my hospital, we enjoy each other’s company, we visit each other at lunch, and we even tried to have a journal club.” The two hospitalist groups work together on developing care protocols. Dr. Nelson says it also makes sense for the groups’ leaders to sit down together on a regular basis and have a venue for discussing important issues and solving problems that may arise.
Other suggestions for hospitalist groups working together under one roof include:
- Clearly define each group’s territory. The groups’ representatives can go out and try to persuade health plans or physician groups to shift their hospitalist allegiances, but there should be no “trolling” or “poaching” of patients going on inside the hospital’s walls. That will only confuse patients and disrupt the hospital’s larger service goals.
- Inform the ED and other key staff of your schedules. It’s important that everyone know exactly who is supposed to get which patients, and how these referrals get made. But recognize that mistakes happen and, hopefully, these will even out between the groups over time.
- Transparency, honesty, and even-handed treatment of all hospitalists can prevent resentment. Clearly defined guidelines and expectations are helpful. If the policy spells out transfers for an incorrectly referred patient, both sides should be accessible and cooperative with that process.
- Identify areas of common interest and agree to work together on these areas (i.e. competition-free zones). It might be possible, for example, for competing groups to take each others’ after-hours call on a rotating basis, with a firm commitment not to steal patients along the way.
- Spell out responsibilities in a way that everyone can agree is fair, such as alternating referrals or taking call on alternating days. For example, if subsidies are paid to more than one hospitalist group, is this done equitably, such as based on the number of hospitalist FTEs or shifts?
- Restrictive covenants and contractual noncompete clauses could become an issue in areas where multiple groups practice. Rather than using overly broad, blanket language, it could be clarified that such pacts apply only to the hospital where the physician currently works, and within a reasonable time frame. But everyone involved should be aware of what these covenants contain and, if they appear unreasonable, don’t sign them.
—Larry Beresford
Experienced hospitalists and medical directors agree that the key to multiple hospitalist groups coexisting effectively under one roof—whether directly competing or not—is good communication. Effective communication can take time to build.
“Start by working together on something—anything, [such as] a hospital committee of some sort where there’s not likely to be much tension,” says hospitalist pioneer and practice consultant John Nelson, MD, MHM.
Dr. Nelson practices at Overlake Hospital in Bellevue, Wash., which has a hospitalist group employed by the hospital and another employed by Group Health Cooperative, a nonprofit health system in Washington state. It is important to put some trust in the trust bank, he says, “and that’s hard if you have no social connections at all. At my hospital, we enjoy each other’s company, we visit each other at lunch, and we even tried to have a journal club.” The two hospitalist groups work together on developing care protocols. Dr. Nelson says it also makes sense for the groups’ leaders to sit down together on a regular basis and have a venue for discussing important issues and solving problems that may arise.
Other suggestions for hospitalist groups working together under one roof include:
- Clearly define each group’s territory. The groups’ representatives can go out and try to persuade health plans or physician groups to shift their hospitalist allegiances, but there should be no “trolling” or “poaching” of patients going on inside the hospital’s walls. That will only confuse patients and disrupt the hospital’s larger service goals.
- Inform the ED and other key staff of your schedules. It’s important that everyone know exactly who is supposed to get which patients, and how these referrals get made. But recognize that mistakes happen and, hopefully, these will even out between the groups over time.
- Transparency, honesty, and even-handed treatment of all hospitalists can prevent resentment. Clearly defined guidelines and expectations are helpful. If the policy spells out transfers for an incorrectly referred patient, both sides should be accessible and cooperative with that process.
- Identify areas of common interest and agree to work together on these areas (i.e. competition-free zones). It might be possible, for example, for competing groups to take each others’ after-hours call on a rotating basis, with a firm commitment not to steal patients along the way.
- Spell out responsibilities in a way that everyone can agree is fair, such as alternating referrals or taking call on alternating days. For example, if subsidies are paid to more than one hospitalist group, is this done equitably, such as based on the number of hospitalist FTEs or shifts?
- Restrictive covenants and contractual noncompete clauses could become an issue in areas where multiple groups practice. Rather than using overly broad, blanket language, it could be clarified that such pacts apply only to the hospital where the physician currently works, and within a reasonable time frame. But everyone involved should be aware of what these covenants contain and, if they appear unreasonable, don’t sign them.
—Larry Beresford
Experienced hospitalists and medical directors agree that the key to multiple hospitalist groups coexisting effectively under one roof—whether directly competing or not—is good communication. Effective communication can take time to build.
“Start by working together on something—anything, [such as] a hospital committee of some sort where there’s not likely to be much tension,” says hospitalist pioneer and practice consultant John Nelson, MD, MHM.
Dr. Nelson practices at Overlake Hospital in Bellevue, Wash., which has a hospitalist group employed by the hospital and another employed by Group Health Cooperative, a nonprofit health system in Washington state. It is important to put some trust in the trust bank, he says, “and that’s hard if you have no social connections at all. At my hospital, we enjoy each other’s company, we visit each other at lunch, and we even tried to have a journal club.” The two hospitalist groups work together on developing care protocols. Dr. Nelson says it also makes sense for the groups’ leaders to sit down together on a regular basis and have a venue for discussing important issues and solving problems that may arise.
Other suggestions for hospitalist groups working together under one roof include:
- Clearly define each group’s territory. The groups’ representatives can go out and try to persuade health plans or physician groups to shift their hospitalist allegiances, but there should be no “trolling” or “poaching” of patients going on inside the hospital’s walls. That will only confuse patients and disrupt the hospital’s larger service goals.
- Inform the ED and other key staff of your schedules. It’s important that everyone know exactly who is supposed to get which patients, and how these referrals get made. But recognize that mistakes happen and, hopefully, these will even out between the groups over time.
- Transparency, honesty, and even-handed treatment of all hospitalists can prevent resentment. Clearly defined guidelines and expectations are helpful. If the policy spells out transfers for an incorrectly referred patient, both sides should be accessible and cooperative with that process.
- Identify areas of common interest and agree to work together on these areas (i.e. competition-free zones). It might be possible, for example, for competing groups to take each others’ after-hours call on a rotating basis, with a firm commitment not to steal patients along the way.
- Spell out responsibilities in a way that everyone can agree is fair, such as alternating referrals or taking call on alternating days. For example, if subsidies are paid to more than one hospitalist group, is this done equitably, such as based on the number of hospitalist FTEs or shifts?
- Restrictive covenants and contractual noncompete clauses could become an issue in areas where multiple groups practice. Rather than using overly broad, blanket language, it could be clarified that such pacts apply only to the hospital where the physician currently works, and within a reasonable time frame. But everyone involved should be aware of what these covenants contain and, if they appear unreasonable, don’t sign them.
—Larry Beresford
A lifetime of service to women and their health—the career of Barbara S. Levy, MD

When Barbara S. Levy, MD, gave up her thriving private practice in Federal Way, Washington, last year to assume the newly created position of Vice President for Health Policy at the American College of Obstetricians and Gynecologists (ACOG), she set in motion a cascade of personal and professional changes.
On the personal front, besides the shift from clinical practice to health policy, the new post necessitated that she move from the West to the East Coast and endure temporary separation from her husband. But the opportunity to make a “real difference” in health policy made it worthwhile, she says.
On the professional front, the new position greatly broadened Dr. Levy’s opportunities to pursue a goal that has marked her entire career: doing “what’s best for our patients and their families.”
As Vice President for Health Policy, Dr. Levy:
- oversees ACOG’s legislative policy and regulatory affairs at the federal and state levels
- supervises ACOG’s Voluntary Review of Quality of Care, as well as the Safety Certification for Outpatient Practice Excellence for Women’s Health (SCOPE) program
- continues the College’s efforts on behalf of its Office of Global Women’s Health to promote national and international programs to improve women’s health worldwide.
“ACOG is doing so many exciting things in the global women’s health arena,” says Dr. Levy. “I am thrilled to be actively involved in these projects and to have the opportunity to expand ACOG’s leadership role in promoting quality health care for women.”
Another change necessitated by her post at ACOG: Dr. Levy has resigned her position on the OBG Management Board of Editors, a seat she has held since 1996.
In gratitude for her long tenure and the many milestones she helped bring about, the journal awarded her with lifetime achievement recognition at the 2013 ACOG annual clinical meeting for her service to OBG Management readers, women’s health, and the women of the United States.
A seasoned clinician who keeps the “big picture” in mind
Dr. Levy is not new to health policy. In addition to her private practice in Washington state, she helped design and served as medical director of the Women’s Health and Breast Center for the Franciscan Health System in Tacoma; she also served as medical director of women’s and children’s services for the health system. As a member of ACOG since 1984, Dr. Levy has served on several national committees and task forces. And she has worked hard as chair of the American Medical Association’s RVS Update Committee (RUC) to ensure that services in the women’s health arena are reimbursed as robustly as men’s healthcare services.
Dr. Levy has published or coauthored more than 65 studies and articles related to her main research interests, which include hysterectomy, endoscopic surgery, menopause and hormone therapy, osteoporosis, pelvic pain, surgical outcomes, female sexual function, and physician payment policy.
She is a member of the American Society for Reproductive Medicine and the American Urogynecologic Society and a past president of AAGL. She also has served as a consultant to and member of the US Food and Drug Administration’s ObGyn Devices Panel.
But it is her passion to enhance women’s health that drives her day to day.
“More and more of us are employed, and there are more rules than ever before,” she says. “When these rules limit our ability to provide the best care possible for our patients, we need to get a voice and do something about it.”
Choosing the rebel’s path
Dr. Levy graduated magna cum laude from Princeton University with a degree in psychology. She went on to earn her medical degree from the University of California, San Diego, followed by an internship and residency in obstetrics and gynecology at the University of Oregon Health Sciences Center (now the Oregon Health and Sciences University) in Portland.
Although Princeton graduates typically go on to medical school at Columbia or Yale, Dr. Levy made a very different choice.
“I’ve always been a rebel, so I didn’t want to follow the usual pathway,” she says.
At UC San Diego, Dr. Levy fell under the inspirational wing of Donna Brooks, MD, only the second female ObGyn in San Diego history.
“I was very lucky to have Donna as a mentor,” Dr. Levy says. “I would not have gone into obstetrics and gynecology without her.”
Two years into Dr. Levy’s residency at the University of Oregon, her husband’s career as a cardiac surgeon necessitated a move to the Seattle area. She finished her residency there, still under the auspices of the University of Oregon, with Leon Speroff, MD, as a mentor.
After residency, she was hired by the Mason Clinic in Seattle and began working with Glen E. Hayden, MD, and Richard Soderstrom, MD, who mentored her in vaginal surgery.
“It was a time when people coming out of residency went into practice with senior colleagues,” she explained. The senior physicians benefited from exposure to new ideas, and younger physicians gained from the senior physicians’ experience.
“In the end, under this arrangement, the patients benefited.”
Claiming a voice
Among the issues that concern Dr. Levy most are the rapid changes shaping medicine—not all of them beneficial.
“We’re spending too much money on health care and not getting value for what we’re spending,” she says. “It’s not sustainable.”
The move to ACOG provides her an opportunity to help frame how health care is delivered and, ultimately, to help slow the hemorrhage of healthcare dollars in unenriching ways.
“I couldn’t do that in a small practice,” she says. “In my view, the changes have to be geared around what’s best for the patient.”
She also strives to be a mentor to younger physicians, particularly women, in an era when red tape and time constraints significantly limit the altruism that was once the hallmark of the medical profession. She believes mentors remain important in medicine.
“Different people can help you at different times in your career,” she says, reflecting on her own experience and the doctors who shaped it.
“Senior physicians should take responsibility for guiding younger physicians when they come out of training,” she says. “They should graciously volunteer.”
Despite her many responsibilities, Dr. Levy finds time to connect with junior physicians. And despite her many accomplishments, she maintains a refreshing sense of modesty.
“I’m just an ordinary doc,” she says. “The only difference between me and everybody else out there is an intolerance when things aren’t right.”
“I speak out loudly.”
CLICK HERE to read insightful articles from Barbara S. Levy, MD, published in OBG Management in recent years.
We want to hear from you! Tell us what you think.

When Barbara S. Levy, MD, gave up her thriving private practice in Federal Way, Washington, last year to assume the newly created position of Vice President for Health Policy at the American College of Obstetricians and Gynecologists (ACOG), she set in motion a cascade of personal and professional changes.
On the personal front, besides the shift from clinical practice to health policy, the new post necessitated that she move from the West to the East Coast and endure temporary separation from her husband. But the opportunity to make a “real difference” in health policy made it worthwhile, she says.
On the professional front, the new position greatly broadened Dr. Levy’s opportunities to pursue a goal that has marked her entire career: doing “what’s best for our patients and their families.”
As Vice President for Health Policy, Dr. Levy:
- oversees ACOG’s legislative policy and regulatory affairs at the federal and state levels
- supervises ACOG’s Voluntary Review of Quality of Care, as well as the Safety Certification for Outpatient Practice Excellence for Women’s Health (SCOPE) program
- continues the College’s efforts on behalf of its Office of Global Women’s Health to promote national and international programs to improve women’s health worldwide.
“ACOG is doing so many exciting things in the global women’s health arena,” says Dr. Levy. “I am thrilled to be actively involved in these projects and to have the opportunity to expand ACOG’s leadership role in promoting quality health care for women.”
Another change necessitated by her post at ACOG: Dr. Levy has resigned her position on the OBG Management Board of Editors, a seat she has held since 1996.
In gratitude for her long tenure and the many milestones she helped bring about, the journal awarded her with lifetime achievement recognition at the 2013 ACOG annual clinical meeting for her service to OBG Management readers, women’s health, and the women of the United States.
A seasoned clinician who keeps the “big picture” in mind
Dr. Levy is not new to health policy. In addition to her private practice in Washington state, she helped design and served as medical director of the Women’s Health and Breast Center for the Franciscan Health System in Tacoma; she also served as medical director of women’s and children’s services for the health system. As a member of ACOG since 1984, Dr. Levy has served on several national committees and task forces. And she has worked hard as chair of the American Medical Association’s RVS Update Committee (RUC) to ensure that services in the women’s health arena are reimbursed as robustly as men’s healthcare services.
Dr. Levy has published or coauthored more than 65 studies and articles related to her main research interests, which include hysterectomy, endoscopic surgery, menopause and hormone therapy, osteoporosis, pelvic pain, surgical outcomes, female sexual function, and physician payment policy.
She is a member of the American Society for Reproductive Medicine and the American Urogynecologic Society and a past president of AAGL. She also has served as a consultant to and member of the US Food and Drug Administration’s ObGyn Devices Panel.
But it is her passion to enhance women’s health that drives her day to day.
“More and more of us are employed, and there are more rules than ever before,” she says. “When these rules limit our ability to provide the best care possible for our patients, we need to get a voice and do something about it.”
Choosing the rebel’s path
Dr. Levy graduated magna cum laude from Princeton University with a degree in psychology. She went on to earn her medical degree from the University of California, San Diego, followed by an internship and residency in obstetrics and gynecology at the University of Oregon Health Sciences Center (now the Oregon Health and Sciences University) in Portland.
Although Princeton graduates typically go on to medical school at Columbia or Yale, Dr. Levy made a very different choice.
“I’ve always been a rebel, so I didn’t want to follow the usual pathway,” she says.
At UC San Diego, Dr. Levy fell under the inspirational wing of Donna Brooks, MD, only the second female ObGyn in San Diego history.
“I was very lucky to have Donna as a mentor,” Dr. Levy says. “I would not have gone into obstetrics and gynecology without her.”
Two years into Dr. Levy’s residency at the University of Oregon, her husband’s career as a cardiac surgeon necessitated a move to the Seattle area. She finished her residency there, still under the auspices of the University of Oregon, with Leon Speroff, MD, as a mentor.
After residency, she was hired by the Mason Clinic in Seattle and began working with Glen E. Hayden, MD, and Richard Soderstrom, MD, who mentored her in vaginal surgery.
“It was a time when people coming out of residency went into practice with senior colleagues,” she explained. The senior physicians benefited from exposure to new ideas, and younger physicians gained from the senior physicians’ experience.
“In the end, under this arrangement, the patients benefited.”
Claiming a voice
Among the issues that concern Dr. Levy most are the rapid changes shaping medicine—not all of them beneficial.
“We’re spending too much money on health care and not getting value for what we’re spending,” she says. “It’s not sustainable.”
The move to ACOG provides her an opportunity to help frame how health care is delivered and, ultimately, to help slow the hemorrhage of healthcare dollars in unenriching ways.
“I couldn’t do that in a small practice,” she says. “In my view, the changes have to be geared around what’s best for the patient.”
She also strives to be a mentor to younger physicians, particularly women, in an era when red tape and time constraints significantly limit the altruism that was once the hallmark of the medical profession. She believes mentors remain important in medicine.
“Different people can help you at different times in your career,” she says, reflecting on her own experience and the doctors who shaped it.
“Senior physicians should take responsibility for guiding younger physicians when they come out of training,” she says. “They should graciously volunteer.”
Despite her many responsibilities, Dr. Levy finds time to connect with junior physicians. And despite her many accomplishments, she maintains a refreshing sense of modesty.
“I’m just an ordinary doc,” she says. “The only difference between me and everybody else out there is an intolerance when things aren’t right.”
“I speak out loudly.”
CLICK HERE to read insightful articles from Barbara S. Levy, MD, published in OBG Management in recent years.
We want to hear from you! Tell us what you think.

When Barbara S. Levy, MD, gave up her thriving private practice in Federal Way, Washington, last year to assume the newly created position of Vice President for Health Policy at the American College of Obstetricians and Gynecologists (ACOG), she set in motion a cascade of personal and professional changes.
On the personal front, besides the shift from clinical practice to health policy, the new post necessitated that she move from the West to the East Coast and endure temporary separation from her husband. But the opportunity to make a “real difference” in health policy made it worthwhile, she says.
On the professional front, the new position greatly broadened Dr. Levy’s opportunities to pursue a goal that has marked her entire career: doing “what’s best for our patients and their families.”
As Vice President for Health Policy, Dr. Levy:
- oversees ACOG’s legislative policy and regulatory affairs at the federal and state levels
- supervises ACOG’s Voluntary Review of Quality of Care, as well as the Safety Certification for Outpatient Practice Excellence for Women’s Health (SCOPE) program
- continues the College’s efforts on behalf of its Office of Global Women’s Health to promote national and international programs to improve women’s health worldwide.
“ACOG is doing so many exciting things in the global women’s health arena,” says Dr. Levy. “I am thrilled to be actively involved in these projects and to have the opportunity to expand ACOG’s leadership role in promoting quality health care for women.”
Another change necessitated by her post at ACOG: Dr. Levy has resigned her position on the OBG Management Board of Editors, a seat she has held since 1996.
In gratitude for her long tenure and the many milestones she helped bring about, the journal awarded her with lifetime achievement recognition at the 2013 ACOG annual clinical meeting for her service to OBG Management readers, women’s health, and the women of the United States.
A seasoned clinician who keeps the “big picture” in mind
Dr. Levy is not new to health policy. In addition to her private practice in Washington state, she helped design and served as medical director of the Women’s Health and Breast Center for the Franciscan Health System in Tacoma; she also served as medical director of women’s and children’s services for the health system. As a member of ACOG since 1984, Dr. Levy has served on several national committees and task forces. And she has worked hard as chair of the American Medical Association’s RVS Update Committee (RUC) to ensure that services in the women’s health arena are reimbursed as robustly as men’s healthcare services.
Dr. Levy has published or coauthored more than 65 studies and articles related to her main research interests, which include hysterectomy, endoscopic surgery, menopause and hormone therapy, osteoporosis, pelvic pain, surgical outcomes, female sexual function, and physician payment policy.
She is a member of the American Society for Reproductive Medicine and the American Urogynecologic Society and a past president of AAGL. She also has served as a consultant to and member of the US Food and Drug Administration’s ObGyn Devices Panel.
But it is her passion to enhance women’s health that drives her day to day.
“More and more of us are employed, and there are more rules than ever before,” she says. “When these rules limit our ability to provide the best care possible for our patients, we need to get a voice and do something about it.”
Choosing the rebel’s path
Dr. Levy graduated magna cum laude from Princeton University with a degree in psychology. She went on to earn her medical degree from the University of California, San Diego, followed by an internship and residency in obstetrics and gynecology at the University of Oregon Health Sciences Center (now the Oregon Health and Sciences University) in Portland.
Although Princeton graduates typically go on to medical school at Columbia or Yale, Dr. Levy made a very different choice.
“I’ve always been a rebel, so I didn’t want to follow the usual pathway,” she says.
At UC San Diego, Dr. Levy fell under the inspirational wing of Donna Brooks, MD, only the second female ObGyn in San Diego history.
“I was very lucky to have Donna as a mentor,” Dr. Levy says. “I would not have gone into obstetrics and gynecology without her.”
Two years into Dr. Levy’s residency at the University of Oregon, her husband’s career as a cardiac surgeon necessitated a move to the Seattle area. She finished her residency there, still under the auspices of the University of Oregon, with Leon Speroff, MD, as a mentor.
After residency, she was hired by the Mason Clinic in Seattle and began working with Glen E. Hayden, MD, and Richard Soderstrom, MD, who mentored her in vaginal surgery.
“It was a time when people coming out of residency went into practice with senior colleagues,” she explained. The senior physicians benefited from exposure to new ideas, and younger physicians gained from the senior physicians’ experience.
“In the end, under this arrangement, the patients benefited.”
Claiming a voice
Among the issues that concern Dr. Levy most are the rapid changes shaping medicine—not all of them beneficial.
“We’re spending too much money on health care and not getting value for what we’re spending,” she says. “It’s not sustainable.”
The move to ACOG provides her an opportunity to help frame how health care is delivered and, ultimately, to help slow the hemorrhage of healthcare dollars in unenriching ways.
“I couldn’t do that in a small practice,” she says. “In my view, the changes have to be geared around what’s best for the patient.”
She also strives to be a mentor to younger physicians, particularly women, in an era when red tape and time constraints significantly limit the altruism that was once the hallmark of the medical profession. She believes mentors remain important in medicine.
“Different people can help you at different times in your career,” she says, reflecting on her own experience and the doctors who shaped it.
“Senior physicians should take responsibility for guiding younger physicians when they come out of training,” she says. “They should graciously volunteer.”
Despite her many responsibilities, Dr. Levy finds time to connect with junior physicians. And despite her many accomplishments, she maintains a refreshing sense of modesty.
“I’m just an ordinary doc,” she says. “The only difference between me and everybody else out there is an intolerance when things aren’t right.”
“I speak out loudly.”
CLICK HERE to read insightful articles from Barbara S. Levy, MD, published in OBG Management in recent years.
We want to hear from you! Tell us what you think.
Does the addition of the Foley bulb to vaginal misoprostol for cervical ripening and labor induction reduce the time to delivery?
In this trial, women who had a singleton pregnancy at 35 weeks’ gestation or later were randomly allocated to the Foley bulb plus vaginal misoprostol (n = 56) or to vaginal misoprostol alone (n = 61) for cervical ripening and labor induction. All women had a vertex presentation, intact membranes, and an unfavorable cervix.
Women assigned to the combination group received vaginal misoprostol 25 μg every 4 hours and a Foley bulb inserted into the internal cervical os and filled with 60 mL of normal saline. Women assigned to vaginal misoprostol alone were given 25 μg every 4 hours. Intravenous oxytocin was given in each group when indicated, according to the discretion of the managing physician, at a rate of 2 mU/min, increasing by 2 mU every 20 min until regular uterine contractions occurred.
The primary outcome of the trial was the time from induction to delivery. Secondary outcomes were mode of delivery, tachysystole, and postpartum hemorrhage.
The median Bishop score was 3 in each group (range, 3–6).
Strengths and limitations of the trial
The strength of this study is its randomized design.
Among the limitations are its small sample size, which was inadequate to evaluate serious maternal and neonatal morbidities, and its lack of blinding, which may introduce bias among the managing physicians.
The primary outcome of induction-to-delivery time is not clinically important, particularly when multiparous women and those with a Bishop score above 5 are included, as they were in this study. Moreover, only women with a gestational age of at least 35 weeks were included, so the results do not apply to those with lower gestational ages.
Goal of induction is to achieve vaginal delivery within 24 hours
In the United States, at least one in every four pregnant women undergo induction of labor for any of a variety of obstetric, medical, and social indications.1 In nulliparous women who have an unfavorable cervix, induction of labor is associated with increased rates of prolonged labor, cesarean delivery, chorioamnionitis, and postpartum hemorrhage. The goal of induction of labor should be to achieve vaginal delivery within 24 hours and reduce the rate of cesarean delivery without increasing adverse maternal and neonatal outcomes.
Click here to access other Examining the Evidence articles on obstetrics.
Several methods are used for induction of labor with or without cervical ripening. They include the administration of oxytocin, oral or vaginal misoprostol in various doses, different preparations of prostaglandins, use of a Foley balloon filled with 30 to 100 mL of saline, or a combination of these methods. To date, none of these approaches has been shown to reduce the rate of cesarean delivery.
A similarly designed study produced very different findings. Data from multiple studies are mixed in regard to the induction-to-delivery time, rate of delivery within 24 and 48 hours, and side effects.1-6 These studies vary in inclusion criteria, method of induction or cervical ripening, dose of induction agent, Bishop score at randomization, primary outcomes, and sample size. A study from Brazil with a design similar to that of the study by Carbone and colleagues found a shorter mean time from induction to vaginal delivery in the vaginal misoprostol group, compared with the group allocated to the Foley bulb plus oxytocin. There were also more vaginal deliveries in the misoprostol group at 12 hours and 18 hours.6
Given the contradictory findings of Carbone and colleagues and an earlier trial of similar design,6 it is very unlikely that this trial is robust enough to change current clinical practice, which is highly variable. Among the methods for cervical ripening and labor induction in current use are oxytocin administration, oral or vaginal misoprostol, prostaglandin administration, the Foley bulb, vaginal dinoprostone, or a combination of methods.
We want to hear from you! Tell us what you think.
1. Hill JB, Thigpen BD, Bofill JA, et al. A randomized clinical trial comparing misoprostol versus cervical Foley plus oral misoprostol for cervical ripening and labor induction. Am J Perinatol. 2009;26(1):33-38.
2. Kehl S, Ehard A, Berlit S, et al. Combination of misoprostol and mechanical dilation for induction of labor: a randomized trial. Eur J Obstet Gynecol Reprod Biol. 2011;159(2):315-319.
3. Kashanian M, Akbarian AR, Fekrat M. Cervical ripening and induction of labor with intravaginal misoprostol and Foley catheter cervical traction. Int J Gynaecol Obstet. 2006;92(1):79-80.
4. Chung JH, Huang WH, Rumney PJ, et al. A prospective randomized controlled trial that compared misoprostol, Foley catheter and combination misoprostol-Foley catheter for labor induction. Am J Obstet Gynecol. 2003;189(4):1031-1035.
5. Rust OA, Greybush M, Atlas RO, et al. Preinduction cervical ripening: a randomized trial of intravaginal misoprostol alone vs a combination of trancervical Foley balloon and intravaginal misoprostol. J Reprod Med. 2001;46(10):899-904.
6. Moraes Filho OB, Albuquerque RM, Cecatti JG. A randomized controlled trial comparing vaginal misoprostol versus Foley catheter plus oxytocin for labor induction. Acta Obstet Gynecol. 2010;89(8):1045-1052.
In this trial, women who had a singleton pregnancy at 35 weeks’ gestation or later were randomly allocated to the Foley bulb plus vaginal misoprostol (n = 56) or to vaginal misoprostol alone (n = 61) for cervical ripening and labor induction. All women had a vertex presentation, intact membranes, and an unfavorable cervix.
Women assigned to the combination group received vaginal misoprostol 25 μg every 4 hours and a Foley bulb inserted into the internal cervical os and filled with 60 mL of normal saline. Women assigned to vaginal misoprostol alone were given 25 μg every 4 hours. Intravenous oxytocin was given in each group when indicated, according to the discretion of the managing physician, at a rate of 2 mU/min, increasing by 2 mU every 20 min until regular uterine contractions occurred.
The primary outcome of the trial was the time from induction to delivery. Secondary outcomes were mode of delivery, tachysystole, and postpartum hemorrhage.
The median Bishop score was 3 in each group (range, 3–6).
Strengths and limitations of the trial
The strength of this study is its randomized design.
Among the limitations are its small sample size, which was inadequate to evaluate serious maternal and neonatal morbidities, and its lack of blinding, which may introduce bias among the managing physicians.
The primary outcome of induction-to-delivery time is not clinically important, particularly when multiparous women and those with a Bishop score above 5 are included, as they were in this study. Moreover, only women with a gestational age of at least 35 weeks were included, so the results do not apply to those with lower gestational ages.
Goal of induction is to achieve vaginal delivery within 24 hours
In the United States, at least one in every four pregnant women undergo induction of labor for any of a variety of obstetric, medical, and social indications.1 In nulliparous women who have an unfavorable cervix, induction of labor is associated with increased rates of prolonged labor, cesarean delivery, chorioamnionitis, and postpartum hemorrhage. The goal of induction of labor should be to achieve vaginal delivery within 24 hours and reduce the rate of cesarean delivery without increasing adverse maternal and neonatal outcomes.
Click here to access other Examining the Evidence articles on obstetrics.
Several methods are used for induction of labor with or without cervical ripening. They include the administration of oxytocin, oral or vaginal misoprostol in various doses, different preparations of prostaglandins, use of a Foley balloon filled with 30 to 100 mL of saline, or a combination of these methods. To date, none of these approaches has been shown to reduce the rate of cesarean delivery.
A similarly designed study produced very different findings. Data from multiple studies are mixed in regard to the induction-to-delivery time, rate of delivery within 24 and 48 hours, and side effects.1-6 These studies vary in inclusion criteria, method of induction or cervical ripening, dose of induction agent, Bishop score at randomization, primary outcomes, and sample size. A study from Brazil with a design similar to that of the study by Carbone and colleagues found a shorter mean time from induction to vaginal delivery in the vaginal misoprostol group, compared with the group allocated to the Foley bulb plus oxytocin. There were also more vaginal deliveries in the misoprostol group at 12 hours and 18 hours.6
Given the contradictory findings of Carbone and colleagues and an earlier trial of similar design,6 it is very unlikely that this trial is robust enough to change current clinical practice, which is highly variable. Among the methods for cervical ripening and labor induction in current use are oxytocin administration, oral or vaginal misoprostol, prostaglandin administration, the Foley bulb, vaginal dinoprostone, or a combination of methods.
We want to hear from you! Tell us what you think.
In this trial, women who had a singleton pregnancy at 35 weeks’ gestation or later were randomly allocated to the Foley bulb plus vaginal misoprostol (n = 56) or to vaginal misoprostol alone (n = 61) for cervical ripening and labor induction. All women had a vertex presentation, intact membranes, and an unfavorable cervix.
Women assigned to the combination group received vaginal misoprostol 25 μg every 4 hours and a Foley bulb inserted into the internal cervical os and filled with 60 mL of normal saline. Women assigned to vaginal misoprostol alone were given 25 μg every 4 hours. Intravenous oxytocin was given in each group when indicated, according to the discretion of the managing physician, at a rate of 2 mU/min, increasing by 2 mU every 20 min until regular uterine contractions occurred.
The primary outcome of the trial was the time from induction to delivery. Secondary outcomes were mode of delivery, tachysystole, and postpartum hemorrhage.
The median Bishop score was 3 in each group (range, 3–6).
Strengths and limitations of the trial
The strength of this study is its randomized design.
Among the limitations are its small sample size, which was inadequate to evaluate serious maternal and neonatal morbidities, and its lack of blinding, which may introduce bias among the managing physicians.
The primary outcome of induction-to-delivery time is not clinically important, particularly when multiparous women and those with a Bishop score above 5 are included, as they were in this study. Moreover, only women with a gestational age of at least 35 weeks were included, so the results do not apply to those with lower gestational ages.
Goal of induction is to achieve vaginal delivery within 24 hours
In the United States, at least one in every four pregnant women undergo induction of labor for any of a variety of obstetric, medical, and social indications.1 In nulliparous women who have an unfavorable cervix, induction of labor is associated with increased rates of prolonged labor, cesarean delivery, chorioamnionitis, and postpartum hemorrhage. The goal of induction of labor should be to achieve vaginal delivery within 24 hours and reduce the rate of cesarean delivery without increasing adverse maternal and neonatal outcomes.
Click here to access other Examining the Evidence articles on obstetrics.
Several methods are used for induction of labor with or without cervical ripening. They include the administration of oxytocin, oral or vaginal misoprostol in various doses, different preparations of prostaglandins, use of a Foley balloon filled with 30 to 100 mL of saline, or a combination of these methods. To date, none of these approaches has been shown to reduce the rate of cesarean delivery.
A similarly designed study produced very different findings. Data from multiple studies are mixed in regard to the induction-to-delivery time, rate of delivery within 24 and 48 hours, and side effects.1-6 These studies vary in inclusion criteria, method of induction or cervical ripening, dose of induction agent, Bishop score at randomization, primary outcomes, and sample size. A study from Brazil with a design similar to that of the study by Carbone and colleagues found a shorter mean time from induction to vaginal delivery in the vaginal misoprostol group, compared with the group allocated to the Foley bulb plus oxytocin. There were also more vaginal deliveries in the misoprostol group at 12 hours and 18 hours.6
Given the contradictory findings of Carbone and colleagues and an earlier trial of similar design,6 it is very unlikely that this trial is robust enough to change current clinical practice, which is highly variable. Among the methods for cervical ripening and labor induction in current use are oxytocin administration, oral or vaginal misoprostol, prostaglandin administration, the Foley bulb, vaginal dinoprostone, or a combination of methods.
We want to hear from you! Tell us what you think.
1. Hill JB, Thigpen BD, Bofill JA, et al. A randomized clinical trial comparing misoprostol versus cervical Foley plus oral misoprostol for cervical ripening and labor induction. Am J Perinatol. 2009;26(1):33-38.
2. Kehl S, Ehard A, Berlit S, et al. Combination of misoprostol and mechanical dilation for induction of labor: a randomized trial. Eur J Obstet Gynecol Reprod Biol. 2011;159(2):315-319.
3. Kashanian M, Akbarian AR, Fekrat M. Cervical ripening and induction of labor with intravaginal misoprostol and Foley catheter cervical traction. Int J Gynaecol Obstet. 2006;92(1):79-80.
4. Chung JH, Huang WH, Rumney PJ, et al. A prospective randomized controlled trial that compared misoprostol, Foley catheter and combination misoprostol-Foley catheter for labor induction. Am J Obstet Gynecol. 2003;189(4):1031-1035.
5. Rust OA, Greybush M, Atlas RO, et al. Preinduction cervical ripening: a randomized trial of intravaginal misoprostol alone vs a combination of trancervical Foley balloon and intravaginal misoprostol. J Reprod Med. 2001;46(10):899-904.
6. Moraes Filho OB, Albuquerque RM, Cecatti JG. A randomized controlled trial comparing vaginal misoprostol versus Foley catheter plus oxytocin for labor induction. Acta Obstet Gynecol. 2010;89(8):1045-1052.
1. Hill JB, Thigpen BD, Bofill JA, et al. A randomized clinical trial comparing misoprostol versus cervical Foley plus oral misoprostol for cervical ripening and labor induction. Am J Perinatol. 2009;26(1):33-38.
2. Kehl S, Ehard A, Berlit S, et al. Combination of misoprostol and mechanical dilation for induction of labor: a randomized trial. Eur J Obstet Gynecol Reprod Biol. 2011;159(2):315-319.
3. Kashanian M, Akbarian AR, Fekrat M. Cervical ripening and induction of labor with intravaginal misoprostol and Foley catheter cervical traction. Int J Gynaecol Obstet. 2006;92(1):79-80.
4. Chung JH, Huang WH, Rumney PJ, et al. A prospective randomized controlled trial that compared misoprostol, Foley catheter and combination misoprostol-Foley catheter for labor induction. Am J Obstet Gynecol. 2003;189(4):1031-1035.
5. Rust OA, Greybush M, Atlas RO, et al. Preinduction cervical ripening: a randomized trial of intravaginal misoprostol alone vs a combination of trancervical Foley balloon and intravaginal misoprostol. J Reprod Med. 2001;46(10):899-904.
6. Moraes Filho OB, Albuquerque RM, Cecatti JG. A randomized controlled trial comparing vaginal misoprostol versus Foley catheter plus oxytocin for labor induction. Acta Obstet Gynecol. 2010;89(8):1045-1052.
Win Whitcomb: Front-Line Hospitalists Fight Against Health Care-Associated Infections (HAIs)
2013 marks a turning point in the way hospitals are held accountable for the prevention of healthcare-associated infections (HAIs). It has been known for some time that HAIs are a serious cause of morbidity, with 1 in 20 hospital patients in the U.S. acquiring one. That represents 1.7 million Americans and accounts for about 100,000 lives lost each year. On a personal note, my father died of an HAI after surgery in 2000.
Now, with the Affordable Care Act coming into full swing, hospitals must get serious about preventing HAIs. This presents a major opportunity for hospitalists. There are three ways that hospitals will be affected:
- Since 2008, hospitals have not been reimbursed at a higher rate for vascular catheter-associated infections, catheter-associated urinary tract infections (UTIs), or surgical-site infections when acquired in the hospital.
- Over the next few years, Medicare’s Hospital Value-Based Purchasing (HVBP) program will begin to pay hospitals more or less, depending on how they perform, on six HAIs.
- Beginning in October 2014, in a roll-up measure for hospital-acquired conditions (which include infections), the worst-performing quartile of U.S. hospitals will be penalized 1% of their Medicare inpatient payments (see Table 1, below).
There are six HAIs that will be increasingly tied to hospital reimbursement. Each can be partially or completely prevented based on sets of practices, or care bundles, that require teamwork both in the planning stages and at the bedside. And, of course, the single most important way to reduce the spread of HAIs is to clean your hands before and after each patient encounter.
Clostridium-Difficile-Associated Disease (CDAD)
It is likely that your hospital has some type of CDAD prevention program. Here are a few things to keep in mind for CDAD prevention:
- Avoid alcohol-based hand rubs, because they do not kill C. diff spores. Vigorous hand washing with soap and water is the best approach.
- Use clindamycin, fluoroquinolones, and third-generation cephalosporins judiciously, as their restriction has been associated with reduced rates of CDAD.
- Place patients with suspected or proven C. diff infection on contact precautions, including gloves and gowns.
Methicillin-Resistant Staphylococcus Aureus (MRSA)
This includes hospital-acquired MRSA bacteremia. This topic will be discussed in future columns. Approaches to prevention include hand hygiene, cohorting patients, effective environmental cleaning, and antibiotic stewardship.
Central-Line-Associated Bloodstream Infection (CLABSI)
Adherence to the central-line insertion bundle has been conclusively shown to prevent CLABSI. It will become a process measure for HVBP in the near future. Prevention measures include hand hygiene, maximal barrier precautions during insertion, skin antisepsis with chlorhexidine, avoidance of the femoral vein, and daily assessment for readiness to discontinue the central line (which should involve every hospitalist).
Catheter-Associated Urinary Tract Infection (CAUTI)
CAUTI has been mentioned frequently in this column, and for good reason: It is the most common HAI. Although the evidence supporting practices that prevent CAUTI is not as strong as for CLABSI, every institution should have a bundle of practices embedded in nurses’ and doctors’ workflow to prevent CAUTI (see “Quality Meets Finance,” January 2013, p. 31).
Surgical-Site Infection (SSI)
For the most part, SSI can be left to the surgeons and other operating room professionals. However, with increasing involvement of hospitalists in surgical cases, we must have an understanding of how SSIs are prevented. The World Health Organization surgical checklist (www.who.int/patientsafety/safesurgery) is a great starting point for any organization.
Ventilator-Associated Pneumonia (VAP)
For hospitalists who provide critical care, adherence to a VAP prevention bundle includes:
- Elevation of the head of the bed;
- Daily “sedation vacation” and readiness to extubate;
- Oral care with chlorhexidine; and
- Peptic ulcer disease and venous thromboembolism prophylaxis.
In 2009, the U.S. Department of Health and Human Services (HHS) launched an action plan to prevent HAIs. As part of this effort, the Agency for Health Research and Quality (AHRQ) created a comprehensive unit-based safety program (CUSP) aimed at preventing CLABSI and CAUTI. The effort also focuses on safety culture and teamwork. For those interested in participating, visit www.onthecuspstophai.org.
Another way to get involved is to work Partnership for Patients, a public-private partnership led by HHS (http://partnershipforpatients.cms.gov), if a team at your hospital is participating. The Partnership for Patients seeks to reduce harm, including HAIs, by 40% by the end of 2013 compared with a 2010 baseline.
Dr. Whitcomb is medical director of healthcare quality at Baystate Medical Center in Springfield, Mass. He is a co-founder and past president of SHM. Email him at wfwhit@comcast.net.
2013 marks a turning point in the way hospitals are held accountable for the prevention of healthcare-associated infections (HAIs). It has been known for some time that HAIs are a serious cause of morbidity, with 1 in 20 hospital patients in the U.S. acquiring one. That represents 1.7 million Americans and accounts for about 100,000 lives lost each year. On a personal note, my father died of an HAI after surgery in 2000.
Now, with the Affordable Care Act coming into full swing, hospitals must get serious about preventing HAIs. This presents a major opportunity for hospitalists. There are three ways that hospitals will be affected:
- Since 2008, hospitals have not been reimbursed at a higher rate for vascular catheter-associated infections, catheter-associated urinary tract infections (UTIs), or surgical-site infections when acquired in the hospital.
- Over the next few years, Medicare’s Hospital Value-Based Purchasing (HVBP) program will begin to pay hospitals more or less, depending on how they perform, on six HAIs.
- Beginning in October 2014, in a roll-up measure for hospital-acquired conditions (which include infections), the worst-performing quartile of U.S. hospitals will be penalized 1% of their Medicare inpatient payments (see Table 1, below).
There are six HAIs that will be increasingly tied to hospital reimbursement. Each can be partially or completely prevented based on sets of practices, or care bundles, that require teamwork both in the planning stages and at the bedside. And, of course, the single most important way to reduce the spread of HAIs is to clean your hands before and after each patient encounter.
Clostridium-Difficile-Associated Disease (CDAD)
It is likely that your hospital has some type of CDAD prevention program. Here are a few things to keep in mind for CDAD prevention:
- Avoid alcohol-based hand rubs, because they do not kill C. diff spores. Vigorous hand washing with soap and water is the best approach.
- Use clindamycin, fluoroquinolones, and third-generation cephalosporins judiciously, as their restriction has been associated with reduced rates of CDAD.
- Place patients with suspected or proven C. diff infection on contact precautions, including gloves and gowns.
Methicillin-Resistant Staphylococcus Aureus (MRSA)
This includes hospital-acquired MRSA bacteremia. This topic will be discussed in future columns. Approaches to prevention include hand hygiene, cohorting patients, effective environmental cleaning, and antibiotic stewardship.
Central-Line-Associated Bloodstream Infection (CLABSI)
Adherence to the central-line insertion bundle has been conclusively shown to prevent CLABSI. It will become a process measure for HVBP in the near future. Prevention measures include hand hygiene, maximal barrier precautions during insertion, skin antisepsis with chlorhexidine, avoidance of the femoral vein, and daily assessment for readiness to discontinue the central line (which should involve every hospitalist).
Catheter-Associated Urinary Tract Infection (CAUTI)
CAUTI has been mentioned frequently in this column, and for good reason: It is the most common HAI. Although the evidence supporting practices that prevent CAUTI is not as strong as for CLABSI, every institution should have a bundle of practices embedded in nurses’ and doctors’ workflow to prevent CAUTI (see “Quality Meets Finance,” January 2013, p. 31).
Surgical-Site Infection (SSI)
For the most part, SSI can be left to the surgeons and other operating room professionals. However, with increasing involvement of hospitalists in surgical cases, we must have an understanding of how SSIs are prevented. The World Health Organization surgical checklist (www.who.int/patientsafety/safesurgery) is a great starting point for any organization.
Ventilator-Associated Pneumonia (VAP)
For hospitalists who provide critical care, adherence to a VAP prevention bundle includes:
- Elevation of the head of the bed;
- Daily “sedation vacation” and readiness to extubate;
- Oral care with chlorhexidine; and
- Peptic ulcer disease and venous thromboembolism prophylaxis.
In 2009, the U.S. Department of Health and Human Services (HHS) launched an action plan to prevent HAIs. As part of this effort, the Agency for Health Research and Quality (AHRQ) created a comprehensive unit-based safety program (CUSP) aimed at preventing CLABSI and CAUTI. The effort also focuses on safety culture and teamwork. For those interested in participating, visit www.onthecuspstophai.org.
Another way to get involved is to work Partnership for Patients, a public-private partnership led by HHS (http://partnershipforpatients.cms.gov), if a team at your hospital is participating. The Partnership for Patients seeks to reduce harm, including HAIs, by 40% by the end of 2013 compared with a 2010 baseline.
Dr. Whitcomb is medical director of healthcare quality at Baystate Medical Center in Springfield, Mass. He is a co-founder and past president of SHM. Email him at wfwhit@comcast.net.
2013 marks a turning point in the way hospitals are held accountable for the prevention of healthcare-associated infections (HAIs). It has been known for some time that HAIs are a serious cause of morbidity, with 1 in 20 hospital patients in the U.S. acquiring one. That represents 1.7 million Americans and accounts for about 100,000 lives lost each year. On a personal note, my father died of an HAI after surgery in 2000.
Now, with the Affordable Care Act coming into full swing, hospitals must get serious about preventing HAIs. This presents a major opportunity for hospitalists. There are three ways that hospitals will be affected:
- Since 2008, hospitals have not been reimbursed at a higher rate for vascular catheter-associated infections, catheter-associated urinary tract infections (UTIs), or surgical-site infections when acquired in the hospital.
- Over the next few years, Medicare’s Hospital Value-Based Purchasing (HVBP) program will begin to pay hospitals more or less, depending on how they perform, on six HAIs.
- Beginning in October 2014, in a roll-up measure for hospital-acquired conditions (which include infections), the worst-performing quartile of U.S. hospitals will be penalized 1% of their Medicare inpatient payments (see Table 1, below).
There are six HAIs that will be increasingly tied to hospital reimbursement. Each can be partially or completely prevented based on sets of practices, or care bundles, that require teamwork both in the planning stages and at the bedside. And, of course, the single most important way to reduce the spread of HAIs is to clean your hands before and after each patient encounter.
Clostridium-Difficile-Associated Disease (CDAD)
It is likely that your hospital has some type of CDAD prevention program. Here are a few things to keep in mind for CDAD prevention:
- Avoid alcohol-based hand rubs, because they do not kill C. diff spores. Vigorous hand washing with soap and water is the best approach.
- Use clindamycin, fluoroquinolones, and third-generation cephalosporins judiciously, as their restriction has been associated with reduced rates of CDAD.
- Place patients with suspected or proven C. diff infection on contact precautions, including gloves and gowns.
Methicillin-Resistant Staphylococcus Aureus (MRSA)
This includes hospital-acquired MRSA bacteremia. This topic will be discussed in future columns. Approaches to prevention include hand hygiene, cohorting patients, effective environmental cleaning, and antibiotic stewardship.
Central-Line-Associated Bloodstream Infection (CLABSI)
Adherence to the central-line insertion bundle has been conclusively shown to prevent CLABSI. It will become a process measure for HVBP in the near future. Prevention measures include hand hygiene, maximal barrier precautions during insertion, skin antisepsis with chlorhexidine, avoidance of the femoral vein, and daily assessment for readiness to discontinue the central line (which should involve every hospitalist).
Catheter-Associated Urinary Tract Infection (CAUTI)
CAUTI has been mentioned frequently in this column, and for good reason: It is the most common HAI. Although the evidence supporting practices that prevent CAUTI is not as strong as for CLABSI, every institution should have a bundle of practices embedded in nurses’ and doctors’ workflow to prevent CAUTI (see “Quality Meets Finance,” January 2013, p. 31).
Surgical-Site Infection (SSI)
For the most part, SSI can be left to the surgeons and other operating room professionals. However, with increasing involvement of hospitalists in surgical cases, we must have an understanding of how SSIs are prevented. The World Health Organization surgical checklist (www.who.int/patientsafety/safesurgery) is a great starting point for any organization.
Ventilator-Associated Pneumonia (VAP)
For hospitalists who provide critical care, adherence to a VAP prevention bundle includes:
- Elevation of the head of the bed;
- Daily “sedation vacation” and readiness to extubate;
- Oral care with chlorhexidine; and
- Peptic ulcer disease and venous thromboembolism prophylaxis.
In 2009, the U.S. Department of Health and Human Services (HHS) launched an action plan to prevent HAIs. As part of this effort, the Agency for Health Research and Quality (AHRQ) created a comprehensive unit-based safety program (CUSP) aimed at preventing CLABSI and CAUTI. The effort also focuses on safety culture and teamwork. For those interested in participating, visit www.onthecuspstophai.org.
Another way to get involved is to work Partnership for Patients, a public-private partnership led by HHS (http://partnershipforpatients.cms.gov), if a team at your hospital is participating. The Partnership for Patients seeks to reduce harm, including HAIs, by 40% by the end of 2013 compared with a 2010 baseline.
Dr. Whitcomb is medical director of healthcare quality at Baystate Medical Center in Springfield, Mass. He is a co-founder and past president of SHM. Email him at wfwhit@comcast.net.
John Nelson: Excessive Workload a Concern for Many Hospitalists
“Forty percent of physicians reported that their typical inpatient census exceeded safe levels at least monthly.”1
This quote is taken from an article by Henry Michtalik and colleagues that appeared at the end of January this year in JAMA Internal Medicine. In 2010 the authors conducted an on-line survey asking hospitalists their perceptions of their workload. Respondents indicated that with concerning frequency a high workload prevented them from adequately discussing with patients treatment options or answering questions, delay admitting or discharging patients until the next day or shift, or in some other way risk patient safety or the overall quality of their work.
This alarming finding matches my anecdotal experience working with many different hospitalist groups around the country. I think few hospitalists were surprised by the survey’s findings. Excess hospitalist workloads are indeed a problem in some settings, and those who bear them are typically not shy about speaking out.
The demand for hospitalists has exceeded the supply of doctors available to do the work throughout the history of the field. Under the weight of stunningly rapid growth in referral volume, from about 1995 to 2002, it was reasonably common for the original doctors in a hospitalist practice to become overwhelmed and leave for other work after a year or two, sometimes resulting in the collapse of the practice. Most practices are no longer in such a rapid-growth phase, but for many of them, staffing has not yet caught up with workload. The result can be chronic excess work, and even if daily patient volume is not seen as being unsafe, the number of days or shifts worked might be excessive and lead to fatigue and poor performance.
Other Workload Data
The respondents to the Michtalik survey reported that regardless of any assistance, “they could safely see 15 patients per shift, if their effort was 100% clinical.” What we don’t know is how long their shifts were, whether they included things like ICU coverage, and how many shifts they work consecutively or in a year.
SHM’s 2012 State of Hospital Medicine report, which is based on 2011 data, provides additional context. It shows that hospitalists serving adult patients report a median 2,092 billed encounters annually (mean 2,245, standard deviation 1,161). They spread this work over a median 185 shifts (“work periods”) annually (mean 192). While there are lots of methodological problems in manipulating those numbers further, 2,092 encounters divided by 185 shifts yields 11.3 encounters per shift. These numbers exclude academicians who typically spend significant time in activities other than direct patient care, and I’m intentionally ignoring such issues as the night-shift doctor, who typically has low productivity, bringing down the average per full-time doctor in a practice.
The numbers from both surveys are sort of fuzzy because they aren’t audited or verified, but the 2012 State of Hospital Medicine data suggest that typical workloads aren’t too high in most practices, yet 40% of respondents in the Michtalik survey said they were high enough—unreasonably high—to risk quality and safety at least once a month.
One way to reconcile these findings is to take into account the standard deviation in daily volume in a single practice of about 30% to 40% on above or below the mean. If a hospitalist averages 14 encounters each day shift, then he should expect that the daily number might vary between about eight and 20. The Michtalik survey responses were likely reflecting the shifts on the high end.
Perspective
I wonder what a survey of physician workload opinions in other specialties would show, or what a survey of workers across all segments of the U.S. workforce in and out of healthcare would show. Of course, many or most jobs outside of healthcare don’t risk another’s health or well-being as significantly as ours do, but it would still be instructive to know how people in general think about the work they do.
I suspect a significant number of people across many different jobs feel like too much work is expected of them, and they can point to the ways their performance suffers as a result. It is difficult to know what portion of those who report too much work is just complaining versus a thoughtful self-reflection of the determinants of their performance. Lots of hospitalists do face worrisome high workloads, but some would probably still complain even with a much lower workload.
What Can Be Done?
For those practices facing remarkably high patient volumes, the solution is to make sure you’re recruiting additional doctors, and/or NPs/PAs, as fast as you can. But a portion of these practices must first convince their employers that more staff is needed. Some practices face a real uphill battle in getting the required additional funding, and the place to start is with a careful analysis of your current workload—based on hard numbers from your practice, not just anecdotes and estimates.
Don’t forget that some hospitalists put themselves in the position of having to manage high daily patient volumes by choosing a schedule of relatively few worked days annually. For example, a group working a seven-on/seven-off schedule that also has 14 shifts of time off means that each doctor will work only 168.5 shifts annually. Compressing a year’s worth of work into only 168 shifts means that each shift will be busy, and many will involve patient volumes that exceed what is seen as safe.
It could make more sense to titrate that same work volume over more annual shifts so that the average shift is less busy. I would love to see the Michtalik data segregated by those who work many shifts annually versus those who work few shifts. It is possible that those working more shifts have reported excessive workloads less often.
SHM has a role in influencing hospitalist workloads and promotes dissemination of data and opinions about it. At HM13 next month in Washington, D.C., I am leading a session titled “Hospitalist Workload: Is 15 the Right Number?” Although it won’t provide the “right” workload for all hospitalists, it will offer worthwhile data and food for thought.
It is much more difficult to do studies of how workload influences performance than something like effects of sleep deprivation on performance, so we may never get clear answers. You could take some consolation in the fact that successive surveys have shown little change or even modest decreases in annual patient encounters. But then again, maybe that hasn’t helped with excess work since providing hospital care gets harder and more complex every year.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM's "Best Practices in Managing a Hospital Medicine Program" course. Write to him at john.nelson@nelsonflores.com.
Reference
“Forty percent of physicians reported that their typical inpatient census exceeded safe levels at least monthly.”1
This quote is taken from an article by Henry Michtalik and colleagues that appeared at the end of January this year in JAMA Internal Medicine. In 2010 the authors conducted an on-line survey asking hospitalists their perceptions of their workload. Respondents indicated that with concerning frequency a high workload prevented them from adequately discussing with patients treatment options or answering questions, delay admitting or discharging patients until the next day or shift, or in some other way risk patient safety or the overall quality of their work.
This alarming finding matches my anecdotal experience working with many different hospitalist groups around the country. I think few hospitalists were surprised by the survey’s findings. Excess hospitalist workloads are indeed a problem in some settings, and those who bear them are typically not shy about speaking out.
The demand for hospitalists has exceeded the supply of doctors available to do the work throughout the history of the field. Under the weight of stunningly rapid growth in referral volume, from about 1995 to 2002, it was reasonably common for the original doctors in a hospitalist practice to become overwhelmed and leave for other work after a year or two, sometimes resulting in the collapse of the practice. Most practices are no longer in such a rapid-growth phase, but for many of them, staffing has not yet caught up with workload. The result can be chronic excess work, and even if daily patient volume is not seen as being unsafe, the number of days or shifts worked might be excessive and lead to fatigue and poor performance.
Other Workload Data
The respondents to the Michtalik survey reported that regardless of any assistance, “they could safely see 15 patients per shift, if their effort was 100% clinical.” What we don’t know is how long their shifts were, whether they included things like ICU coverage, and how many shifts they work consecutively or in a year.
SHM’s 2012 State of Hospital Medicine report, which is based on 2011 data, provides additional context. It shows that hospitalists serving adult patients report a median 2,092 billed encounters annually (mean 2,245, standard deviation 1,161). They spread this work over a median 185 shifts (“work periods”) annually (mean 192). While there are lots of methodological problems in manipulating those numbers further, 2,092 encounters divided by 185 shifts yields 11.3 encounters per shift. These numbers exclude academicians who typically spend significant time in activities other than direct patient care, and I’m intentionally ignoring such issues as the night-shift doctor, who typically has low productivity, bringing down the average per full-time doctor in a practice.
The numbers from both surveys are sort of fuzzy because they aren’t audited or verified, but the 2012 State of Hospital Medicine data suggest that typical workloads aren’t too high in most practices, yet 40% of respondents in the Michtalik survey said they were high enough—unreasonably high—to risk quality and safety at least once a month.
One way to reconcile these findings is to take into account the standard deviation in daily volume in a single practice of about 30% to 40% on above or below the mean. If a hospitalist averages 14 encounters each day shift, then he should expect that the daily number might vary between about eight and 20. The Michtalik survey responses were likely reflecting the shifts on the high end.
Perspective
I wonder what a survey of physician workload opinions in other specialties would show, or what a survey of workers across all segments of the U.S. workforce in and out of healthcare would show. Of course, many or most jobs outside of healthcare don’t risk another’s health or well-being as significantly as ours do, but it would still be instructive to know how people in general think about the work they do.
I suspect a significant number of people across many different jobs feel like too much work is expected of them, and they can point to the ways their performance suffers as a result. It is difficult to know what portion of those who report too much work is just complaining versus a thoughtful self-reflection of the determinants of their performance. Lots of hospitalists do face worrisome high workloads, but some would probably still complain even with a much lower workload.
What Can Be Done?
For those practices facing remarkably high patient volumes, the solution is to make sure you’re recruiting additional doctors, and/or NPs/PAs, as fast as you can. But a portion of these practices must first convince their employers that more staff is needed. Some practices face a real uphill battle in getting the required additional funding, and the place to start is with a careful analysis of your current workload—based on hard numbers from your practice, not just anecdotes and estimates.
Don’t forget that some hospitalists put themselves in the position of having to manage high daily patient volumes by choosing a schedule of relatively few worked days annually. For example, a group working a seven-on/seven-off schedule that also has 14 shifts of time off means that each doctor will work only 168.5 shifts annually. Compressing a year’s worth of work into only 168 shifts means that each shift will be busy, and many will involve patient volumes that exceed what is seen as safe.
It could make more sense to titrate that same work volume over more annual shifts so that the average shift is less busy. I would love to see the Michtalik data segregated by those who work many shifts annually versus those who work few shifts. It is possible that those working more shifts have reported excessive workloads less often.
SHM has a role in influencing hospitalist workloads and promotes dissemination of data and opinions about it. At HM13 next month in Washington, D.C., I am leading a session titled “Hospitalist Workload: Is 15 the Right Number?” Although it won’t provide the “right” workload for all hospitalists, it will offer worthwhile data and food for thought.
It is much more difficult to do studies of how workload influences performance than something like effects of sleep deprivation on performance, so we may never get clear answers. You could take some consolation in the fact that successive surveys have shown little change or even modest decreases in annual patient encounters. But then again, maybe that hasn’t helped with excess work since providing hospital care gets harder and more complex every year.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM's "Best Practices in Managing a Hospital Medicine Program" course. Write to him at john.nelson@nelsonflores.com.
Reference
“Forty percent of physicians reported that their typical inpatient census exceeded safe levels at least monthly.”1
This quote is taken from an article by Henry Michtalik and colleagues that appeared at the end of January this year in JAMA Internal Medicine. In 2010 the authors conducted an on-line survey asking hospitalists their perceptions of their workload. Respondents indicated that with concerning frequency a high workload prevented them from adequately discussing with patients treatment options or answering questions, delay admitting or discharging patients until the next day or shift, or in some other way risk patient safety or the overall quality of their work.
This alarming finding matches my anecdotal experience working with many different hospitalist groups around the country. I think few hospitalists were surprised by the survey’s findings. Excess hospitalist workloads are indeed a problem in some settings, and those who bear them are typically not shy about speaking out.
The demand for hospitalists has exceeded the supply of doctors available to do the work throughout the history of the field. Under the weight of stunningly rapid growth in referral volume, from about 1995 to 2002, it was reasonably common for the original doctors in a hospitalist practice to become overwhelmed and leave for other work after a year or two, sometimes resulting in the collapse of the practice. Most practices are no longer in such a rapid-growth phase, but for many of them, staffing has not yet caught up with workload. The result can be chronic excess work, and even if daily patient volume is not seen as being unsafe, the number of days or shifts worked might be excessive and lead to fatigue and poor performance.
Other Workload Data
The respondents to the Michtalik survey reported that regardless of any assistance, “they could safely see 15 patients per shift, if their effort was 100% clinical.” What we don’t know is how long their shifts were, whether they included things like ICU coverage, and how many shifts they work consecutively or in a year.
SHM’s 2012 State of Hospital Medicine report, which is based on 2011 data, provides additional context. It shows that hospitalists serving adult patients report a median 2,092 billed encounters annually (mean 2,245, standard deviation 1,161). They spread this work over a median 185 shifts (“work periods”) annually (mean 192). While there are lots of methodological problems in manipulating those numbers further, 2,092 encounters divided by 185 shifts yields 11.3 encounters per shift. These numbers exclude academicians who typically spend significant time in activities other than direct patient care, and I’m intentionally ignoring such issues as the night-shift doctor, who typically has low productivity, bringing down the average per full-time doctor in a practice.
The numbers from both surveys are sort of fuzzy because they aren’t audited or verified, but the 2012 State of Hospital Medicine data suggest that typical workloads aren’t too high in most practices, yet 40% of respondents in the Michtalik survey said they were high enough—unreasonably high—to risk quality and safety at least once a month.
One way to reconcile these findings is to take into account the standard deviation in daily volume in a single practice of about 30% to 40% on above or below the mean. If a hospitalist averages 14 encounters each day shift, then he should expect that the daily number might vary between about eight and 20. The Michtalik survey responses were likely reflecting the shifts on the high end.
Perspective
I wonder what a survey of physician workload opinions in other specialties would show, or what a survey of workers across all segments of the U.S. workforce in and out of healthcare would show. Of course, many or most jobs outside of healthcare don’t risk another’s health or well-being as significantly as ours do, but it would still be instructive to know how people in general think about the work they do.
I suspect a significant number of people across many different jobs feel like too much work is expected of them, and they can point to the ways their performance suffers as a result. It is difficult to know what portion of those who report too much work is just complaining versus a thoughtful self-reflection of the determinants of their performance. Lots of hospitalists do face worrisome high workloads, but some would probably still complain even with a much lower workload.
What Can Be Done?
For those practices facing remarkably high patient volumes, the solution is to make sure you’re recruiting additional doctors, and/or NPs/PAs, as fast as you can. But a portion of these practices must first convince their employers that more staff is needed. Some practices face a real uphill battle in getting the required additional funding, and the place to start is with a careful analysis of your current workload—based on hard numbers from your practice, not just anecdotes and estimates.
Don’t forget that some hospitalists put themselves in the position of having to manage high daily patient volumes by choosing a schedule of relatively few worked days annually. For example, a group working a seven-on/seven-off schedule that also has 14 shifts of time off means that each doctor will work only 168.5 shifts annually. Compressing a year’s worth of work into only 168 shifts means that each shift will be busy, and many will involve patient volumes that exceed what is seen as safe.
It could make more sense to titrate that same work volume over more annual shifts so that the average shift is less busy. I would love to see the Michtalik data segregated by those who work many shifts annually versus those who work few shifts. It is possible that those working more shifts have reported excessive workloads less often.
SHM has a role in influencing hospitalist workloads and promotes dissemination of data and opinions about it. At HM13 next month in Washington, D.C., I am leading a session titled “Hospitalist Workload: Is 15 the Right Number?” Although it won’t provide the “right” workload for all hospitalists, it will offer worthwhile data and food for thought.
It is much more difficult to do studies of how workload influences performance than something like effects of sleep deprivation on performance, so we may never get clear answers. You could take some consolation in the fact that successive surveys have shown little change or even modest decreases in annual patient encounters. But then again, maybe that hasn’t helped with excess work since providing hospital care gets harder and more complex every year.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM's "Best Practices in Managing a Hospital Medicine Program" course. Write to him at john.nelson@nelsonflores.com.
Reference
STOP using synthetic mesh for routine repair of pelvic organ prolapse
Have you read these recent articles in OBG Management about the surgical use of mesh? Click here to access the list.
CASE: Stage 2 prolapse to the hymenal ring
A 54-year-old Para 3 woman presents with stage 2 prolapse to the hymenal ring. The prolapse predominantly involves the anterior vagina, with her cervix prolapsing to 2 cm within the hymenal ring. The patient is bothered by the bulge and stress urinary leakage. She does not want to use a pessary and prefers to have definitive surgical correction, including hysterectomy.
The first surgeon she consulted recommended a vaginal hysterectomy, anterior colporrhaphy, anterior synthetic mesh vaginal colpopexy, and synthetic midurethral sling. The patient was concerned about mesh placement for prolapse and the sling after seeing ads on the Internet about vaginal mesh. She presents for a second opinion about surgical alternatives.
Stop routinely offering synthetic vaginal mesh for prolapse
Advantages to the use of synthetic vaginal mesh include improved subjective and objective cure rates for prolapse (especially for the anterior compartment) and fewer repeat surgeries for recurrent prolapse. However, disadvantages include:
- mesh exposure and extrusion through the vaginal epithelium
- overall higher reoperation for mesh-related complications and de novo stress urinary incontinence.1
Synthetic vaginal mesh should be reserved for special situations. Currently, experts agree that synthetic vaginal mesh is appropriate in cases of recurrent prolapse, advanced-stage prolapse, collagen deficiency, or in cases with relative contraindications to longer endoscopic or abdominal surgery, such as medical comorbidities or adhesions.2,3 Other indications for synthetic vaginal mesh include vaginal hysteropexy procedures.
Mesh is likely not necessary in:
- primary repairs
- prolapse < POPQ (pelvic organ prolapse quantification system) stage 2
- posterior prolapse
- patients with chronic pelvic pain.
Start offering, learning, and mastering native tissue repairs
For the patient in the opening case, who has symptomatic stage 2 uterovaginal prolapse and stress urinary incontinence, surgery, including a transvaginal hysterectomy, anterior colporrhaphy, uterosacral ligament suspension, and synthetic midurethral sling, is a reasonable alternative and has a high subjective and objective cure rate—81.2% rate for anterior prolapse and 98.3% rate for apical prolapse. Better outcomes are noted with stage 2 compared with stage 3 prolapse (92.4% vs 66.8%, respectively).4
Final note
If you are offering selective transvaginal synthetic mesh for prolapse repairs:
- Undergo training specific to each device.
- Track your outcomes—including objective, subjective, quality of life, and reoperation for complications and recurrence.
- Enroll in the national pelvic floor disorders registry, which is scheduled to debut in Fall 2013.
We want to hear from you! Tell us what you think.
1. Schmid C, Maher C, Feiner B, Baessler K, Glazener C. 2012 Cochrane Review: Surgical management of pelvic organ prolapse. Int Urogynecol J. 2012;23(suppl 2).-
2. Davila GW, Baessler K, Cosson M, Cardozo L. Selection of patients in whom vaginal graft use may be appropriate. Consensus of the 2nd IUGA Grafts Roundtable: optimizing safety and appropriateness of graft use in transvaginal pelvic reconstructive surgery. Int Urogynecol J. 2012;23(suppl 1):S7-S14.
3. Committee on Gynecologic Practice. American College of Obstetricians and Gynecologists American Urogynecologic Society. Committee Opinion No. 513: Vaginal placement of synthetic mesh for pelvic organ prolapse. Obstet Gynecol. 2011;118(6):1459-1464.
4. Margulies RU, Rogers MA, Morgan DM. Outcomes of transvaginal uterosacral ligament suspension: systematic review and metaanalysis. Am J Obstet Gynecol. 2010;202(2):124-134.
Have you read these recent articles in OBG Management about the surgical use of mesh? Click here to access the list.
CASE: Stage 2 prolapse to the hymenal ring
A 54-year-old Para 3 woman presents with stage 2 prolapse to the hymenal ring. The prolapse predominantly involves the anterior vagina, with her cervix prolapsing to 2 cm within the hymenal ring. The patient is bothered by the bulge and stress urinary leakage. She does not want to use a pessary and prefers to have definitive surgical correction, including hysterectomy.
The first surgeon she consulted recommended a vaginal hysterectomy, anterior colporrhaphy, anterior synthetic mesh vaginal colpopexy, and synthetic midurethral sling. The patient was concerned about mesh placement for prolapse and the sling after seeing ads on the Internet about vaginal mesh. She presents for a second opinion about surgical alternatives.
Stop routinely offering synthetic vaginal mesh for prolapse
Advantages to the use of synthetic vaginal mesh include improved subjective and objective cure rates for prolapse (especially for the anterior compartment) and fewer repeat surgeries for recurrent prolapse. However, disadvantages include:
- mesh exposure and extrusion through the vaginal epithelium
- overall higher reoperation for mesh-related complications and de novo stress urinary incontinence.1
Synthetic vaginal mesh should be reserved for special situations. Currently, experts agree that synthetic vaginal mesh is appropriate in cases of recurrent prolapse, advanced-stage prolapse, collagen deficiency, or in cases with relative contraindications to longer endoscopic or abdominal surgery, such as medical comorbidities or adhesions.2,3 Other indications for synthetic vaginal mesh include vaginal hysteropexy procedures.
Mesh is likely not necessary in:
- primary repairs
- prolapse < POPQ (pelvic organ prolapse quantification system) stage 2
- posterior prolapse
- patients with chronic pelvic pain.
Start offering, learning, and mastering native tissue repairs
For the patient in the opening case, who has symptomatic stage 2 uterovaginal prolapse and stress urinary incontinence, surgery, including a transvaginal hysterectomy, anterior colporrhaphy, uterosacral ligament suspension, and synthetic midurethral sling, is a reasonable alternative and has a high subjective and objective cure rate—81.2% rate for anterior prolapse and 98.3% rate for apical prolapse. Better outcomes are noted with stage 2 compared with stage 3 prolapse (92.4% vs 66.8%, respectively).4
Final note
If you are offering selective transvaginal synthetic mesh for prolapse repairs:
- Undergo training specific to each device.
- Track your outcomes—including objective, subjective, quality of life, and reoperation for complications and recurrence.
- Enroll in the national pelvic floor disorders registry, which is scheduled to debut in Fall 2013.
We want to hear from you! Tell us what you think.
Have you read these recent articles in OBG Management about the surgical use of mesh? Click here to access the list.
CASE: Stage 2 prolapse to the hymenal ring
A 54-year-old Para 3 woman presents with stage 2 prolapse to the hymenal ring. The prolapse predominantly involves the anterior vagina, with her cervix prolapsing to 2 cm within the hymenal ring. The patient is bothered by the bulge and stress urinary leakage. She does not want to use a pessary and prefers to have definitive surgical correction, including hysterectomy.
The first surgeon she consulted recommended a vaginal hysterectomy, anterior colporrhaphy, anterior synthetic mesh vaginal colpopexy, and synthetic midurethral sling. The patient was concerned about mesh placement for prolapse and the sling after seeing ads on the Internet about vaginal mesh. She presents for a second opinion about surgical alternatives.
Stop routinely offering synthetic vaginal mesh for prolapse
Advantages to the use of synthetic vaginal mesh include improved subjective and objective cure rates for prolapse (especially for the anterior compartment) and fewer repeat surgeries for recurrent prolapse. However, disadvantages include:
- mesh exposure and extrusion through the vaginal epithelium
- overall higher reoperation for mesh-related complications and de novo stress urinary incontinence.1
Synthetic vaginal mesh should be reserved for special situations. Currently, experts agree that synthetic vaginal mesh is appropriate in cases of recurrent prolapse, advanced-stage prolapse, collagen deficiency, or in cases with relative contraindications to longer endoscopic or abdominal surgery, such as medical comorbidities or adhesions.2,3 Other indications for synthetic vaginal mesh include vaginal hysteropexy procedures.
Mesh is likely not necessary in:
- primary repairs
- prolapse < POPQ (pelvic organ prolapse quantification system) stage 2
- posterior prolapse
- patients with chronic pelvic pain.
Start offering, learning, and mastering native tissue repairs
For the patient in the opening case, who has symptomatic stage 2 uterovaginal prolapse and stress urinary incontinence, surgery, including a transvaginal hysterectomy, anterior colporrhaphy, uterosacral ligament suspension, and synthetic midurethral sling, is a reasonable alternative and has a high subjective and objective cure rate—81.2% rate for anterior prolapse and 98.3% rate for apical prolapse. Better outcomes are noted with stage 2 compared with stage 3 prolapse (92.4% vs 66.8%, respectively).4
Final note
If you are offering selective transvaginal synthetic mesh for prolapse repairs:
- Undergo training specific to each device.
- Track your outcomes—including objective, subjective, quality of life, and reoperation for complications and recurrence.
- Enroll in the national pelvic floor disorders registry, which is scheduled to debut in Fall 2013.
We want to hear from you! Tell us what you think.
1. Schmid C, Maher C, Feiner B, Baessler K, Glazener C. 2012 Cochrane Review: Surgical management of pelvic organ prolapse. Int Urogynecol J. 2012;23(suppl 2).-
2. Davila GW, Baessler K, Cosson M, Cardozo L. Selection of patients in whom vaginal graft use may be appropriate. Consensus of the 2nd IUGA Grafts Roundtable: optimizing safety and appropriateness of graft use in transvaginal pelvic reconstructive surgery. Int Urogynecol J. 2012;23(suppl 1):S7-S14.
3. Committee on Gynecologic Practice. American College of Obstetricians and Gynecologists American Urogynecologic Society. Committee Opinion No. 513: Vaginal placement of synthetic mesh for pelvic organ prolapse. Obstet Gynecol. 2011;118(6):1459-1464.
4. Margulies RU, Rogers MA, Morgan DM. Outcomes of transvaginal uterosacral ligament suspension: systematic review and metaanalysis. Am J Obstet Gynecol. 2010;202(2):124-134.
1. Schmid C, Maher C, Feiner B, Baessler K, Glazener C. 2012 Cochrane Review: Surgical management of pelvic organ prolapse. Int Urogynecol J. 2012;23(suppl 2).-
2. Davila GW, Baessler K, Cosson M, Cardozo L. Selection of patients in whom vaginal graft use may be appropriate. Consensus of the 2nd IUGA Grafts Roundtable: optimizing safety and appropriateness of graft use in transvaginal pelvic reconstructive surgery. Int Urogynecol J. 2012;23(suppl 1):S7-S14.
3. Committee on Gynecologic Practice. American College of Obstetricians and Gynecologists American Urogynecologic Society. Committee Opinion No. 513: Vaginal placement of synthetic mesh for pelvic organ prolapse. Obstet Gynecol. 2011;118(6):1459-1464.
4. Margulies RU, Rogers MA, Morgan DM. Outcomes of transvaginal uterosacral ligament suspension: systematic review and metaanalysis. Am J Obstet Gynecol. 2010;202(2):124-134.
The robot is gaining ground in gynecologic surgery. Should you be using it?
The OBG Management expert panel
Arnold P. Advincula, MD, is Professor of Obstetrics and Gynecology at the University of Central Florida College of Medicine in Orlando, Florida. He is Medical Director of the Center for Specialized Gynecology at Florida Hospital Celebration Health and oversees its Fellowship Program in Minimally Invasive Surgery.
Cheryl B. Iglesia, MD, is Director of the Section of Female Pelvic Medicine and Reconstructive Surgery at MedStar Washington Hospital Center. She is also Associate Professor, Departments of Obstetrics and Gynecology and Urology, at Georgetown University School of Medicine in Washington, DC. She serves on the OBG Management Board of Editors.
Rosanne M. Kho, MD, is Associate Professor of Obstetrics and Gynecology at the Mayo Clinic in Scottsdale, Arizona.
Jamal Mourad, DO, practices Obstetrics and Gynecology at Southwest Women’s Care in Phoenix, Arizona.
Marie Fidela R. Paraiso, MD, is Professor of Surgery, Cleveland Clinic Lerner College of Medicine at Case Western Reserve University, and Head of the Center for Urogynecology and Reconstructive Pelvic Surgery of the Obstetrics, Gynecology, and Women’s Health Institute at the Cleveland Clinic in Ohio. Dr. Paraiso also holds a joint appointment in the Cleveland Clinic Glickman Urological and Kidney Institute.
Jason D. Wright, MD, is Levine Family Assistant Professor of Women’s Health and Florence Irving Assistant Professor of Obstetrics and Gynecology, Division of Gynecologic Oncology, at Columbia University College of Physicians and Surgeons in New York, New York.
Dr. Iglesia, Dr. Kho, Dr. Mourad, Dr. Paraiso, and Dr. Wright report no financial relationships relevant to this article. Dr. Advincula reports that he is a consultant to Blue Endo, Cooper Surgical, Covidien, Intuitive Surgical, and Surgiquest.
The publication of a large cohort study of hysterectomy for benign indications revived a debate over robotic assistance in gynecologic surgery.1 The study—by Jason D. Wright, MD, and colleagues—included 264,758 women who underwent hysterectomy for benign indications in 441 US hospitals from 2007 to 2010, and it produced some dramatic findings:
- The use of robotic assistance increased from 0.5% of all hysterectomies in 2007 to 9.5% in 2010
- Three years after the first robotic procedure in each hospital where robotics were used, robotic-assisted hysterectomy accounted for 22.4% of all hysterectomies
- Laparoscopic hysterectomy increased as well, from 24.3% of all hysterectomies in the first quarter of 2007 to 30.5% in the first quarter of 2010
- The rate of vaginal hysterectomy declined from 21.7% to 19.8% of all hysterectomies during the same time period
- Abdominal hysterectomy decreased from 53.6% to 43.1% of all hysterectomies
- Although robotic-assisted and laparoscopic hysterectomy had similar complication rates, transfusion requirements, and rates of discharge to a nursing facility, the robotic-assisted approach cost $2,189 more.1
An editorial accompanying this study opined that physicians and hospitals have a “duty” to make sure patients are aware not only of the benefits and risks of each surgical option but also of its financial cost.2 The editorialists suggested that cost should be taken into account by the surgeon, as well. When a more expensive treatment proves to be more effective than the conventional approach, there typically is little argument about paying the higher cost. When the new treatment or technology is equally effective, however, as is the case with robotic hysterectomy and the laparoscopic approach, the choice of treatment “should be more straightforward.”2 That is, the lower-cost treatment should be preferred, the editorialists concluded.
The study by Wright and colleagues and the accompanying editorial prompted some important questions:
- Should robotic assistance be offered for patients undergoing hysterectomy for benign indications when it produces outcomes equivalent to laparoscopic hysterectomy but costs one-third more?
- Is the robotic approach justified in other benign gynecologic surgical procedures?
To explore these questions, we invited a roster of experts in minimally invasive gynecologic surgery to share their perspective and experience, including the lead author of the article mentioned above, Jason D. Wright, MD. In this roundtable discussion, these experts discuss the robotic experience at their respective institutions, characterize the data to date, and offer valuable suggestions about whether and when to incorporate the robot into your surgical practice.
To read 9 recent articles from OBG Management on the pros and cons of robotic surgery, click here.
What is driving the demand?
OBG Management: How much of the demand for the robot do you think is patient-driven? Hospital-driven? Physician- or data-driven?
Cheryl B. Iglesia, MD: With any new technology, there is a honeymoon phase when providers, patients, and hospitals really tout innovation. With its superior optics and 3D vision, the robot certainly enjoyed an extended honeymoon, driven by innovation and a “cool factor.” However, as experience, comparative studies, and longer-term outcomes data become known, demand for new technology is tempered and refined. The choice of technology also has to include an acknowledgement of its cost-effectiveness—or lack of it.
Jamal Mourad, DO: I believe that the demand for minimally invasive procedures, including robotic-assisted surgeries, is multifaceted. The public has learned to have certain expectations about the care it receives. In addition, the media have incorporated a tremendous amount of information about minimally invasive surgery and robotic procedures into TV shows, newscasts, newspapers, and many other outlets. This information certainly stimulates, at the very least, curiosity on the part of the patient, which leads, in turn, to more inquiries about the robot during initial consultation with a surgeon.
Rosanne M. Kho, MD: Here at the Mayo Clinic in Arizona, we adopted use of the robot early. Following the lead of Javier Magrina, MD, we started with the Zeus system (Computer Motion) in 2003 and, subsequently, the da Vinci system (Intuitive Surgical) in 2004. At the time, not many data were available on the use of the robot in gynecology. We looked at the experience in urology and saw its applicability in gynecology. At our institution, therefore, our use of the robot was not driven by the market or data. It was primarily physician-driven and, fortunately, supported by our institution.
Jason D. Wright, MD: Demand for the robot stems from four different sources, in my opinion. Approximately 25% is patient-driven, 25% is surgeon-driven, 25% is hospital-driven, and let’s not forget industry—the makers of the robot—which accounts for 25% of demand. There is a delicate balance between all four buckets that is dynamic and always in flux. A shift too heavily in one direction can lead to problems, especially if variables such as proper infrastructure, outcomes data, or indications, just to name a few, are missing.
Marie Fidela R. Paraiso, MD: Many have criticized the marketing of robot-assisted surgery straight to the consumer. In fact, some investigators have found the claimed benefits of robotic surgery touted in marketing efforts by most hospitals to be unsubstantiated.3,4 That being said, hospitals market the robot to justify its cost and increase patient volume. And many surgeons who lack sufficient training or skill in advanced traditional laparoscopic surgery have embraced and marketed the robot platform as they have enhanced their surgical practices, converting open surgeries to minimally invasive procedures in the process. Patients are attracted to “new” procedures and equate them with “better” when they are choosing a physician or a procedure.
The demand for the robot is also physician driven. In my surgical armamentarium, I use this technology to do complex, multi-procedure pelvic floor repairs that require deep pelvic dissection and suturing or the addition of rectal prolapse procedures or retropubic space anti-incontinence procedures, with or without paravaginal repair.
OBG Management: What do the data reveal about robotic-assisted gynecologic surgery?
Marie Fidela R. Paraiso, MD: For benign conditions, data on robot-assisted laparoscopy are sparse and show no significant benefit, with higher cost and greater operative time than conventional laparoscopy in randomized trials.5,6 A Cochrane review and a large retrospective analysis support these findings.7,8 Another large retrospective study shows that robotic-assisted laparoscopy is associated with a shorter hospital stay, recovery time, postoperative time, and lower cost.9
Robotic surgery has proved to be beneficial in gynecologic oncology and has increased the use of minimally invasive access in gynecology, especially among novice or inexperienced minimally invasive surgeons. It also has helped some surgeons develop skills in advanced traditional laparoscopy.
Rosanne M. Kho, MD: Over the past 10 years in benign gynecology, retrospective comparative studies have found similar complication rates between the robotic approach and conventional laparoscopy. However, operative time and costs are higher in robotics.
One major advantage of the robot, demonstrated in multiple studies, is that the laparotomy rate for hysterectomy has declined in many centers and by as much as 14%, as Dr. Wright and his colleagues found, once the robotic platform becomes available.1
Are you using the robot more?
OBG Management: Has use of the robot increased at your institution?
Rosanne M. Kho, MD: With close to 10 years of experience with the robot, our utilization in gynecology is now stable—it’s neither increasing nor decreasing.
Cheryl B. Iglesia, MD: Yes, use of the robot at my institution has increased as more specialties have adapted the approach, including thoracic surgeons; ear, nose, and throat specialists; and colorectal surgeons. However, the majority of cases are either urologic or gynecologic in nature (urogynecology and gynecologic oncology).
Arnold P. Advincula, MD: This question is difficult to answer because, at Florida Hospital Celebration Health, we are a bit of an outlier. I function within a Global Robotics Institute that is dedicated to the safe and proper use of robotic technology by emphasizing optimal patient outcomes, teaching best practice techniques, and innovating future surgical platforms. Access is allowed for a limited number of surgeons in the various disciplines who are recognized pioneers and for high-volume surgeons with a proven track record of surgical outcomes. Our utilization across all disciplines, including gynecology, always has been high because of this infrastructure.
Jamal Mourad, DO: I have seen a steady, progressive growth in the number of robotic procedures performed at our institution. Initially, the urology department used our robotic equipment to perform prostatectomies. Shortly after that, quick acceptance by several departments, including gynecology, oncology, general surgery, colorectal surgery, and pediatric surgery, led to widespread use and acceptance.
OBG Management: Does increasing use of the robot make it more likely that it will be used in hysterectomy for benign indications?
Rosanne M. Kho, MD: Our primary approach to the simple hysterectomy for benign conditions (including nulliparous women, those with a uterus larger than 12 weeks’ size, and women who have undergone previous pelvic surgery) is still vaginal. Patients with pelvic pain, known endometriosis, and suspicious adnexal masses are approached laparoscopically or robotically.
Compared with conventional laparoscopy, we have found the robot particularly useful in obese patients and in cases requiring extensive dissection and suturing, such as in benign complex gynecology (involving endometriosis, ovarian remnant syndrome), urogynecology (for prolapse and fistulas), and all aspects of gynecologic oncology.
In our hands, the robot has been a facilitating tool, allowing us to perform complex procedures that would otherwise have been difficult to perform with conventional laparoscopy.
Which benign conditions are being addressed robotically?
OBG Management: What benign procedures in gynecologic surgery is the robot used for at your institution, Dr. Advincula?
Arnold P. Advincula, MD: It’s used for the entire gamut of benign procedures in gynecologic surgery, ranging from complex hysterectomy to reproductive surgical cases such as myomectomy and endometriosis resection to pelvic reconstructive surgery. Our success with such a broad range of applications is very much attributable to the infrastructure that we have in place that allows us to use the robot safely and efficiently.
![]()
OBG Management: What makes the robot so attractive?
Jamal Mourad, DO: Robotic technology allows for much-improved visualization, better dexterity and maneuverability, and near total control of the surgical field. I have found that the combination of these advantages permits predictable and reproducible procedures, less tissue trauma, less blood loss, a shorter hospital stay, and fewer conversions to laparotomy, even in very difficult and challenging situations, such as cases involving dense adhesions, a large uterus, or deep infiltrating endometriosis.
Cheryl B. Iglesia, MD: At my institution, the robot is used for sacrocolpopexy, some myomectomy and endometriosis cases (although haptic feedback for these tough endometriosis cases often makes laparoscopy more useful), and, rarely, fistulas (vesicouterine, ureterovaginal).
Jamal Mourad, DO: There are several experienced minimally invasive surgeons at my institution. In addition to hysterectomy, we perform sacrocolpopexy, myomectomy, and resection of severe endometriosis using the robot.
Marie Fidela R. Paraiso, MD: We use the robot for hysterectomy, myomectomy, sacrocolpopexy, Burch colposuspension, paravaginal repair, tubal reanastomosis, endometriosis resection, ureterolysis, and cerclage.
Jason D. Wright, MD: At my institution, robotic surgery for benign indications has been used predominately for hysterectomy and myomectomy, as well as sacrocolpopexy. Given the lack of data to guide implementation of robotic surgery in gynecology, it is difficult to determine which patients derive the most benefit from robotic-assisted procedures.
Should the robot be used for benign hysterectomy?
OBG Management: Do you believe use of the robot is justified in hysterectomy for benign indications?
Cheryl B. Iglesia, MD: No, I believe that most hysterectomies should be done vaginally. If, for some reason, the vaginal approach is not feasible, then laparoscopic hysterectomy is the next best choice and more cost-effective than robotic hysterectomy. Comparative studies and Dr. Wright’s JAMA article seem to concur.1 Open abdominal hysterectomy should be the last option.
Rosanne M. Kho, MD: I do believe that the robot is justified for use in hysterectomy for benign indications. It has provided many patients with the benefits of minimally invasive surgery, as studies have shown.1,9 In an ideal world, simple hysterectomies would be performed vaginally first and, as Dr. Iglesia noted, laparoscopically second. We do know, however, that not only are the learning curves for the vaginal and laparoscopic approaches steep, it is a challenge to teach these approaches effectively. The robotic platform has overcome these challenges with the 3D view, articulation of instruments, and a simulation and teaching console.
Marie Fidela R. Paraiso, MD: I agree with Dr. Iglesia. I do not think that use of the robot is justified for benign indications unless it is shown to be cost-effective and results in the same cure and complication rates, or if a surgeon does not have the skills or training in traditional laparoscopy and desires to offer his/her patients minimally invasive abdominal surgery. So far, two prospective trials have demonstrated that robotic-assisted hysterectomy for benign disease requires longer operative time and is, therefore, more costly in centers where there are surgeons who specialize in advanced laparoscopy.5,6 We still have not defined the subset of patients who would benefit from robotic-assisted laparoscopy if all things are equal.
Jason D. Wright, MD: I think we need more data on robotic surgery for benign gynecologic disease. To date, the majority of the data are retrospective, with most studies unable to demonstrate an advantage of robotic surgery over laparoscopy. These studies have shown that robotic-assisted surgery is substantially more costly, however. I think there are groups of patients who are likely to benefit from robotic surgery, but we need to better define this group of women.
Arnold P. Advincula, MD: I disagree. Despite some controversy, I believe that use of the robot is justified in hysterectomy for benign indications. In fact, it is in situations like the frozen pelvis from endometriosis, or the scarred anterior cul-de-sac from previous cesarean deliveries, that the robot adds value. Several studies speak to the feasibility, safety, and reproducibility of the robot under those circumstances.10,11 Of course, more recently, studies have challenged the use of robotics in benign gynecologic surgery, particularly hysterectomy. Those studies must be viewed with a critical eye. Individuals often can be swayed by the findings of randomized, controlled trials, but such studies are difficult to perform in surgery and there is really no way to be an expert in both conventional laparoscopy and robotic-assisted laparoscopy. As a surgical tool, a surgeon must commit to developing expertise with one or the other.
An often forgotten aspect that is critical to the success of both conventional laparoscopic surgeons and robotic surgeons is the presence of a well-run infrastructure and team to support the surgery. Without that, costs go up and patient outcomes go down for both approaches.
Jamal Mourad, DO: I agree. I believe there is a definitive justification for the use of the robot in benign gynecology. Most of the nearly 600,000 hysterectomies performed each year in the United States are still done by the open abdominal approach despite recognition that a minimally invasive approach (vaginal or laparoscopic) is the standard of care for benign hysterectomy. I incorporated robotic technology into a very busy laparoscopic practice in 2005. I continue to use laparoscopy as a very important tool in my armamentarium for minimally invasive surgery. As I mentioned earlier, robotic technology allows better visualization, dexterity, maneuverability, and control of the surgical field.
Ultimately, it is about taking care of our patients. Cost and efficiency are extremely important, but the patient is more important. The goal is excellence, not average care!
OBG Management: How would you advise clinicians about when to use the robot in gynecologic surgery?
Rosanne M. Kho, MD: Until the cost of robotic procedures declines (soon, I hope), clinicians need to be vigilant in the use of measures and techniques that help them remain efficient and work safely while performing robotic procedures. Such measures include training a dedicated robotic OR team (including a bedside assistant), optimizing trocar placement and docking time, reducing operative or console time, and minimizing the number of robotic and disposable laparoscopic instruments used per case. There are multiple excellent robotics courses that utilize simulation and cadaveric models that clinicians can make use of to advance their skills.
Cheryl B. Iglesia, MD: I would first advise surgeons to get appropriate training using modules, labs, or a robotic simulator—or all three—that is consistent with institutional and other guidelines.12 Second, get appropriate proctoring for your first few cases. Third, think vaginal first and laparoscopic second for straightforward hysterectomies. Robotic assistance has advantages if a lot of sewing is required (as in myomectomy or sacrocolpopexy) or a lot of dissection in small spaces is needed (such as in lymph node dissection in gynecologic oncology).
It is likely true that robotic training can be enabling technology and can improve a surgeon’s straight-stick laparoscopic skills. Mastering fundamentals of vaginal and laparoscopic surgery is the core to the foundation of gynecologic surgery. Robotic use can be narrowed to certain situations. I am not sure where single-port robotics will lead, but surgeons will need to assess that new technology as well. Most important, volume matters. High-volume surgeons and high-volume centers have the most experience with the fewest complications, as proven in multiple surgical subspecialties—not just gynecology.
Jason D. Wright, MD: I think gynecologic surgeons need to be aware of the lack of data for robotic gynecologic surgery and carefully choose which patients and procedures they utilize the robot for. Unfortunately, many of the claims of benefit of the robot are not supported by high-quality research.
Although the added costs of robotic surgery may not have an immediate impact, in the long term these costs will almost certainly be passed on not only to patients and hospitals but also to physicians.
Jamal Mourad, DO: Despite the clear advantages of laparoscopic hysterectomy, most surgeons still perform laparotomy as their preferred route for hysterectomy because of the complex skills needed to perform straight-stick laparoscopy. The FDA approved the da Vinci surgical system for gynecologic procedures in 2005. Since then, the number of minimally invasive hysterectomies has increased dramatically.13 The American College of Obstetricians and Gynecologists and AAGL recognize and endorse a minimally invasive approach (vaginal, laparoscopic) to the majority of hysterectomies.14 Despite this recognition, the total number of vaginal and laparoscopic hysterectomies has remained stagnant for the past 25 years. Since the introduction of the robotic platform into our specialty, the total number of laparotomies has decreased significantly, due in large part to acceptance of robotic-assisted procedures.
Arnold P. Advincula, MD: This question—how to advise surgeons—is complicated because it involves so many moving parts. The bottom line: As long as surgeons have the appropriate rationale and indications for its use, proper training with subsequent credentialing and privileging, and the infrastructure to allow for safe and efficient use of the technology with outcomes tracking, then I think the robot is justified for interested clinicians who truly believe it will enhance their performance and care of patients. If some pieces of this equation are missing, then I would caution surgeons about incorporating robotics into their surgical armamentarium. I feel very strongly that many of the issues we see today surrounding robotics are the result of disregarding these very important requirements for the adoption of technology in medicine. We have seen similar issues with transvaginal mesh. Let’s not let history repeat itself in the arena of robotics.
Jamal Mourad, DO: I agree. We need to do what is right. First, do no harm, then do what you would want done to you!
We want to hear from you! Tell us what you think.
1. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689-698.
2. Weissman JS, Zinner M. Comparative effectiveness research on robotic surgery. JAMA. 2013;309(7):721-722.
3. Schiavone MB, Kuo EC, Naumann RW, et al. The commercialization of robotic surgery: Unsubstantiated marketing of gynecologic surgery by hospitals. Am J Obstet Gynecol. 2012;207(3):174.e1-e7.
4. Kaunitz AM. Examining the Evidence. Are hospital claims about the robotic approach to gynecologic surgery based on reliable data—or mostly hype? OBG Manage. 2012;24(11):55-56.
5. Sarlos D, Kots L, Stevanovic N, et al. Robotic compared with conventional laparoscopic hysterectomy: A randomized controlled trial. Obstet Gynecol. 2012;120(3):604-611.
6. Paraiso MFR, Ridgeway B, Park AJ, et al. A randomized trial comparing conventional and robotic-assisted total laparoscopic hysterectomy [published online ahead of print February 8, 2013]. Am J Obstet Gynecol. doi:pii:S0002-9378(13)00144-0.
7. Paraiso MF, Jelovsek JE, Frick A, Chen GC, Barber MD. Laparoscopic compared with robotic sacrocolpopexy for vaginal prolapse: A randomized controlled trial. Obstet Gynecol. 2011;118(5):1105-1113.
8. Liu H, Lu D, Wang L, Shi G, Song H, Clark J. Robotic surgery for benign gynaecological disease. Cochrane Database Syst Rev. 2012;2:CD008978.-
9. Pasic R, Rizzo J, Fang H, Ross S, Moore M, Gunnarsson C. Comparing robot-assisted with conventional laparoscopic hysterectomy: Impact on cost and clinical outcomes. J Minim Invasive Gynecol. 2010;17(6):730-738.
10. Payne TN, Dauterive FR. A comparison of total laparoscopic hysterectomy to robotically assisted hysterectomy: Surgical outcomes in a community practice. J Minim Invasive Gynecol. 2008;15(3):286-291.
11. AAGL. Route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18(1):1-3.
12. American Urogynecologic Society. Guidelines for privileging and credentialing physicians for sacrocolpopexy for pelvic organ prolapse. Female Pelvic Med Reconstructive Surg. 2013;19(2):62-65.
13. Payne TN, Dauterive FR, Pitter MC, et al. Robotically assisted hysterectomy in patients with large uteri. Outcomes in five community practices. Obstet Gynecol. 2010;115(3):535-542.
14. Boggess JF, Gehrig PA, Cantrell L, et al. Perioperative outcomes of robotically assisted hysterectomy for benign cases with complex pathology. Obstet Gynecol. 2009;114(3):585-593.
The OBG Management expert panel
Arnold P. Advincula, MD, is Professor of Obstetrics and Gynecology at the University of Central Florida College of Medicine in Orlando, Florida. He is Medical Director of the Center for Specialized Gynecology at Florida Hospital Celebration Health and oversees its Fellowship Program in Minimally Invasive Surgery.
Cheryl B. Iglesia, MD, is Director of the Section of Female Pelvic Medicine and Reconstructive Surgery at MedStar Washington Hospital Center. She is also Associate Professor, Departments of Obstetrics and Gynecology and Urology, at Georgetown University School of Medicine in Washington, DC. She serves on the OBG Management Board of Editors.
Rosanne M. Kho, MD, is Associate Professor of Obstetrics and Gynecology at the Mayo Clinic in Scottsdale, Arizona.
Jamal Mourad, DO, practices Obstetrics and Gynecology at Southwest Women’s Care in Phoenix, Arizona.
Marie Fidela R. Paraiso, MD, is Professor of Surgery, Cleveland Clinic Lerner College of Medicine at Case Western Reserve University, and Head of the Center for Urogynecology and Reconstructive Pelvic Surgery of the Obstetrics, Gynecology, and Women’s Health Institute at the Cleveland Clinic in Ohio. Dr. Paraiso also holds a joint appointment in the Cleveland Clinic Glickman Urological and Kidney Institute.
Jason D. Wright, MD, is Levine Family Assistant Professor of Women’s Health and Florence Irving Assistant Professor of Obstetrics and Gynecology, Division of Gynecologic Oncology, at Columbia University College of Physicians and Surgeons in New York, New York.
Dr. Iglesia, Dr. Kho, Dr. Mourad, Dr. Paraiso, and Dr. Wright report no financial relationships relevant to this article. Dr. Advincula reports that he is a consultant to Blue Endo, Cooper Surgical, Covidien, Intuitive Surgical, and Surgiquest.
The publication of a large cohort study of hysterectomy for benign indications revived a debate over robotic assistance in gynecologic surgery.1 The study—by Jason D. Wright, MD, and colleagues—included 264,758 women who underwent hysterectomy for benign indications in 441 US hospitals from 2007 to 2010, and it produced some dramatic findings:
- The use of robotic assistance increased from 0.5% of all hysterectomies in 2007 to 9.5% in 2010
- Three years after the first robotic procedure in each hospital where robotics were used, robotic-assisted hysterectomy accounted for 22.4% of all hysterectomies
- Laparoscopic hysterectomy increased as well, from 24.3% of all hysterectomies in the first quarter of 2007 to 30.5% in the first quarter of 2010
- The rate of vaginal hysterectomy declined from 21.7% to 19.8% of all hysterectomies during the same time period
- Abdominal hysterectomy decreased from 53.6% to 43.1% of all hysterectomies
- Although robotic-assisted and laparoscopic hysterectomy had similar complication rates, transfusion requirements, and rates of discharge to a nursing facility, the robotic-assisted approach cost $2,189 more.1
An editorial accompanying this study opined that physicians and hospitals have a “duty” to make sure patients are aware not only of the benefits and risks of each surgical option but also of its financial cost.2 The editorialists suggested that cost should be taken into account by the surgeon, as well. When a more expensive treatment proves to be more effective than the conventional approach, there typically is little argument about paying the higher cost. When the new treatment or technology is equally effective, however, as is the case with robotic hysterectomy and the laparoscopic approach, the choice of treatment “should be more straightforward.”2 That is, the lower-cost treatment should be preferred, the editorialists concluded.
The study by Wright and colleagues and the accompanying editorial prompted some important questions:
- Should robotic assistance be offered for patients undergoing hysterectomy for benign indications when it produces outcomes equivalent to laparoscopic hysterectomy but costs one-third more?
- Is the robotic approach justified in other benign gynecologic surgical procedures?
To explore these questions, we invited a roster of experts in minimally invasive gynecologic surgery to share their perspective and experience, including the lead author of the article mentioned above, Jason D. Wright, MD. In this roundtable discussion, these experts discuss the robotic experience at their respective institutions, characterize the data to date, and offer valuable suggestions about whether and when to incorporate the robot into your surgical practice.
To read 9 recent articles from OBG Management on the pros and cons of robotic surgery, click here.
What is driving the demand?
OBG Management: How much of the demand for the robot do you think is patient-driven? Hospital-driven? Physician- or data-driven?
Cheryl B. Iglesia, MD: With any new technology, there is a honeymoon phase when providers, patients, and hospitals really tout innovation. With its superior optics and 3D vision, the robot certainly enjoyed an extended honeymoon, driven by innovation and a “cool factor.” However, as experience, comparative studies, and longer-term outcomes data become known, demand for new technology is tempered and refined. The choice of technology also has to include an acknowledgement of its cost-effectiveness—or lack of it.
Jamal Mourad, DO: I believe that the demand for minimally invasive procedures, including robotic-assisted surgeries, is multifaceted. The public has learned to have certain expectations about the care it receives. In addition, the media have incorporated a tremendous amount of information about minimally invasive surgery and robotic procedures into TV shows, newscasts, newspapers, and many other outlets. This information certainly stimulates, at the very least, curiosity on the part of the patient, which leads, in turn, to more inquiries about the robot during initial consultation with a surgeon.
Rosanne M. Kho, MD: Here at the Mayo Clinic in Arizona, we adopted use of the robot early. Following the lead of Javier Magrina, MD, we started with the Zeus system (Computer Motion) in 2003 and, subsequently, the da Vinci system (Intuitive Surgical) in 2004. At the time, not many data were available on the use of the robot in gynecology. We looked at the experience in urology and saw its applicability in gynecology. At our institution, therefore, our use of the robot was not driven by the market or data. It was primarily physician-driven and, fortunately, supported by our institution.
Jason D. Wright, MD: Demand for the robot stems from four different sources, in my opinion. Approximately 25% is patient-driven, 25% is surgeon-driven, 25% is hospital-driven, and let’s not forget industry—the makers of the robot—which accounts for 25% of demand. There is a delicate balance between all four buckets that is dynamic and always in flux. A shift too heavily in one direction can lead to problems, especially if variables such as proper infrastructure, outcomes data, or indications, just to name a few, are missing.
Marie Fidela R. Paraiso, MD: Many have criticized the marketing of robot-assisted surgery straight to the consumer. In fact, some investigators have found the claimed benefits of robotic surgery touted in marketing efforts by most hospitals to be unsubstantiated.3,4 That being said, hospitals market the robot to justify its cost and increase patient volume. And many surgeons who lack sufficient training or skill in advanced traditional laparoscopic surgery have embraced and marketed the robot platform as they have enhanced their surgical practices, converting open surgeries to minimally invasive procedures in the process. Patients are attracted to “new” procedures and equate them with “better” when they are choosing a physician or a procedure.
The demand for the robot is also physician driven. In my surgical armamentarium, I use this technology to do complex, multi-procedure pelvic floor repairs that require deep pelvic dissection and suturing or the addition of rectal prolapse procedures or retropubic space anti-incontinence procedures, with or without paravaginal repair.
OBG Management: What do the data reveal about robotic-assisted gynecologic surgery?
Marie Fidela R. Paraiso, MD: For benign conditions, data on robot-assisted laparoscopy are sparse and show no significant benefit, with higher cost and greater operative time than conventional laparoscopy in randomized trials.5,6 A Cochrane review and a large retrospective analysis support these findings.7,8 Another large retrospective study shows that robotic-assisted laparoscopy is associated with a shorter hospital stay, recovery time, postoperative time, and lower cost.9
Robotic surgery has proved to be beneficial in gynecologic oncology and has increased the use of minimally invasive access in gynecology, especially among novice or inexperienced minimally invasive surgeons. It also has helped some surgeons develop skills in advanced traditional laparoscopy.
Rosanne M. Kho, MD: Over the past 10 years in benign gynecology, retrospective comparative studies have found similar complication rates between the robotic approach and conventional laparoscopy. However, operative time and costs are higher in robotics.
One major advantage of the robot, demonstrated in multiple studies, is that the laparotomy rate for hysterectomy has declined in many centers and by as much as 14%, as Dr. Wright and his colleagues found, once the robotic platform becomes available.1

Are you using the robot more?
OBG Management: Has use of the robot increased at your institution?
Rosanne M. Kho, MD: With close to 10 years of experience with the robot, our utilization in gynecology is now stable—it’s neither increasing nor decreasing.
Cheryl B. Iglesia, MD: Yes, use of the robot at my institution has increased as more specialties have adapted the approach, including thoracic surgeons; ear, nose, and throat specialists; and colorectal surgeons. However, the majority of cases are either urologic or gynecologic in nature (urogynecology and gynecologic oncology).
Arnold P. Advincula, MD: This question is difficult to answer because, at Florida Hospital Celebration Health, we are a bit of an outlier. I function within a Global Robotics Institute that is dedicated to the safe and proper use of robotic technology by emphasizing optimal patient outcomes, teaching best practice techniques, and innovating future surgical platforms. Access is allowed for a limited number of surgeons in the various disciplines who are recognized pioneers and for high-volume surgeons with a proven track record of surgical outcomes. Our utilization across all disciplines, including gynecology, always has been high because of this infrastructure.
Jamal Mourad, DO: I have seen a steady, progressive growth in the number of robotic procedures performed at our institution. Initially, the urology department used our robotic equipment to perform prostatectomies. Shortly after that, quick acceptance by several departments, including gynecology, oncology, general surgery, colorectal surgery, and pediatric surgery, led to widespread use and acceptance.
OBG Management: Does increasing use of the robot make it more likely that it will be used in hysterectomy for benign indications?
Rosanne M. Kho, MD: Our primary approach to the simple hysterectomy for benign conditions (including nulliparous women, those with a uterus larger than 12 weeks’ size, and women who have undergone previous pelvic surgery) is still vaginal. Patients with pelvic pain, known endometriosis, and suspicious adnexal masses are approached laparoscopically or robotically.
Compared with conventional laparoscopy, we have found the robot particularly useful in obese patients and in cases requiring extensive dissection and suturing, such as in benign complex gynecology (involving endometriosis, ovarian remnant syndrome), urogynecology (for prolapse and fistulas), and all aspects of gynecologic oncology.
In our hands, the robot has been a facilitating tool, allowing us to perform complex procedures that would otherwise have been difficult to perform with conventional laparoscopy.
Which benign conditions are being addressed robotically?
OBG Management: What benign procedures in gynecologic surgery is the robot used for at your institution, Dr. Advincula?
Arnold P. Advincula, MD: It’s used for the entire gamut of benign procedures in gynecologic surgery, ranging from complex hysterectomy to reproductive surgical cases such as myomectomy and endometriosis resection to pelvic reconstructive surgery. Our success with such a broad range of applications is very much attributable to the infrastructure that we have in place that allows us to use the robot safely and efficiently.
![]()
OBG Management: What makes the robot so attractive?
Jamal Mourad, DO: Robotic technology allows for much-improved visualization, better dexterity and maneuverability, and near total control of the surgical field. I have found that the combination of these advantages permits predictable and reproducible procedures, less tissue trauma, less blood loss, a shorter hospital stay, and fewer conversions to laparotomy, even in very difficult and challenging situations, such as cases involving dense adhesions, a large uterus, or deep infiltrating endometriosis.
Cheryl B. Iglesia, MD: At my institution, the robot is used for sacrocolpopexy, some myomectomy and endometriosis cases (although haptic feedback for these tough endometriosis cases often makes laparoscopy more useful), and, rarely, fistulas (vesicouterine, ureterovaginal).
Jamal Mourad, DO: There are several experienced minimally invasive surgeons at my institution. In addition to hysterectomy, we perform sacrocolpopexy, myomectomy, and resection of severe endometriosis using the robot.
Marie Fidela R. Paraiso, MD: We use the robot for hysterectomy, myomectomy, sacrocolpopexy, Burch colposuspension, paravaginal repair, tubal reanastomosis, endometriosis resection, ureterolysis, and cerclage.
Jason D. Wright, MD: At my institution, robotic surgery for benign indications has been used predominately for hysterectomy and myomectomy, as well as sacrocolpopexy. Given the lack of data to guide implementation of robotic surgery in gynecology, it is difficult to determine which patients derive the most benefit from robotic-assisted procedures.
Should the robot be used for benign hysterectomy?
OBG Management: Do you believe use of the robot is justified in hysterectomy for benign indications?
Cheryl B. Iglesia, MD: No, I believe that most hysterectomies should be done vaginally. If, for some reason, the vaginal approach is not feasible, then laparoscopic hysterectomy is the next best choice and more cost-effective than robotic hysterectomy. Comparative studies and Dr. Wright’s JAMA article seem to concur.1 Open abdominal hysterectomy should be the last option.
Rosanne M. Kho, MD: I do believe that the robot is justified for use in hysterectomy for benign indications. It has provided many patients with the benefits of minimally invasive surgery, as studies have shown.1,9 In an ideal world, simple hysterectomies would be performed vaginally first and, as Dr. Iglesia noted, laparoscopically second. We do know, however, that not only are the learning curves for the vaginal and laparoscopic approaches steep, it is a challenge to teach these approaches effectively. The robotic platform has overcome these challenges with the 3D view, articulation of instruments, and a simulation and teaching console.
Marie Fidela R. Paraiso, MD: I agree with Dr. Iglesia. I do not think that use of the robot is justified for benign indications unless it is shown to be cost-effective and results in the same cure and complication rates, or if a surgeon does not have the skills or training in traditional laparoscopy and desires to offer his/her patients minimally invasive abdominal surgery. So far, two prospective trials have demonstrated that robotic-assisted hysterectomy for benign disease requires longer operative time and is, therefore, more costly in centers where there are surgeons who specialize in advanced laparoscopy.5,6 We still have not defined the subset of patients who would benefit from robotic-assisted laparoscopy if all things are equal.
Jason D. Wright, MD: I think we need more data on robotic surgery for benign gynecologic disease. To date, the majority of the data are retrospective, with most studies unable to demonstrate an advantage of robotic surgery over laparoscopy. These studies have shown that robotic-assisted surgery is substantially more costly, however. I think there are groups of patients who are likely to benefit from robotic surgery, but we need to better define this group of women.
Arnold P. Advincula, MD: I disagree. Despite some controversy, I believe that use of the robot is justified in hysterectomy for benign indications. In fact, it is in situations like the frozen pelvis from endometriosis, or the scarred anterior cul-de-sac from previous cesarean deliveries, that the robot adds value. Several studies speak to the feasibility, safety, and reproducibility of the robot under those circumstances.10,11 Of course, more recently, studies have challenged the use of robotics in benign gynecologic surgery, particularly hysterectomy. Those studies must be viewed with a critical eye. Individuals often can be swayed by the findings of randomized, controlled trials, but such studies are difficult to perform in surgery and there is really no way to be an expert in both conventional laparoscopy and robotic-assisted laparoscopy. As a surgical tool, a surgeon must commit to developing expertise with one or the other.
An often forgotten aspect that is critical to the success of both conventional laparoscopic surgeons and robotic surgeons is the presence of a well-run infrastructure and team to support the surgery. Without that, costs go up and patient outcomes go down for both approaches.
Jamal Mourad, DO: I agree. I believe there is a definitive justification for the use of the robot in benign gynecology. Most of the nearly 600,000 hysterectomies performed each year in the United States are still done by the open abdominal approach despite recognition that a minimally invasive approach (vaginal or laparoscopic) is the standard of care for benign hysterectomy. I incorporated robotic technology into a very busy laparoscopic practice in 2005. I continue to use laparoscopy as a very important tool in my armamentarium for minimally invasive surgery. As I mentioned earlier, robotic technology allows better visualization, dexterity, maneuverability, and control of the surgical field.
Ultimately, it is about taking care of our patients. Cost and efficiency are extremely important, but the patient is more important. The goal is excellence, not average care!
OBG Management: How would you advise clinicians about when to use the robot in gynecologic surgery?
Rosanne M. Kho, MD: Until the cost of robotic procedures declines (soon, I hope), clinicians need to be vigilant in the use of measures and techniques that help them remain efficient and work safely while performing robotic procedures. Such measures include training a dedicated robotic OR team (including a bedside assistant), optimizing trocar placement and docking time, reducing operative or console time, and minimizing the number of robotic and disposable laparoscopic instruments used per case. There are multiple excellent robotics courses that utilize simulation and cadaveric models that clinicians can make use of to advance their skills.
Cheryl B. Iglesia, MD: I would first advise surgeons to get appropriate training using modules, labs, or a robotic simulator—or all three—that is consistent with institutional and other guidelines.12 Second, get appropriate proctoring for your first few cases. Third, think vaginal first and laparoscopic second for straightforward hysterectomies. Robotic assistance has advantages if a lot of sewing is required (as in myomectomy or sacrocolpopexy) or a lot of dissection in small spaces is needed (such as in lymph node dissection in gynecologic oncology).
It is likely true that robotic training can be enabling technology and can improve a surgeon’s straight-stick laparoscopic skills. Mastering fundamentals of vaginal and laparoscopic surgery is the core to the foundation of gynecologic surgery. Robotic use can be narrowed to certain situations. I am not sure where single-port robotics will lead, but surgeons will need to assess that new technology as well. Most important, volume matters. High-volume surgeons and high-volume centers have the most experience with the fewest complications, as proven in multiple surgical subspecialties—not just gynecology.
Jason D. Wright, MD: I think gynecologic surgeons need to be aware of the lack of data for robotic gynecologic surgery and carefully choose which patients and procedures they utilize the robot for. Unfortunately, many of the claims of benefit of the robot are not supported by high-quality research.
Although the added costs of robotic surgery may not have an immediate impact, in the long term these costs will almost certainly be passed on not only to patients and hospitals but also to physicians.
Jamal Mourad, DO: Despite the clear advantages of laparoscopic hysterectomy, most surgeons still perform laparotomy as their preferred route for hysterectomy because of the complex skills needed to perform straight-stick laparoscopy. The FDA approved the da Vinci surgical system for gynecologic procedures in 2005. Since then, the number of minimally invasive hysterectomies has increased dramatically.13 The American College of Obstetricians and Gynecologists and AAGL recognize and endorse a minimally invasive approach (vaginal, laparoscopic) to the majority of hysterectomies.14 Despite this recognition, the total number of vaginal and laparoscopic hysterectomies has remained stagnant for the past 25 years. Since the introduction of the robotic platform into our specialty, the total number of laparotomies has decreased significantly, due in large part to acceptance of robotic-assisted procedures.
Arnold P. Advincula, MD: This question—how to advise surgeons—is complicated because it involves so many moving parts. The bottom line: As long as surgeons have the appropriate rationale and indications for its use, proper training with subsequent credentialing and privileging, and the infrastructure to allow for safe and efficient use of the technology with outcomes tracking, then I think the robot is justified for interested clinicians who truly believe it will enhance their performance and care of patients. If some pieces of this equation are missing, then I would caution surgeons about incorporating robotics into their surgical armamentarium. I feel very strongly that many of the issues we see today surrounding robotics are the result of disregarding these very important requirements for the adoption of technology in medicine. We have seen similar issues with transvaginal mesh. Let’s not let history repeat itself in the arena of robotics.
Jamal Mourad, DO: I agree. We need to do what is right. First, do no harm, then do what you would want done to you!
We want to hear from you! Tell us what you think.
The OBG Management expert panel
Arnold P. Advincula, MD, is Professor of Obstetrics and Gynecology at the University of Central Florida College of Medicine in Orlando, Florida. He is Medical Director of the Center for Specialized Gynecology at Florida Hospital Celebration Health and oversees its Fellowship Program in Minimally Invasive Surgery.
Cheryl B. Iglesia, MD, is Director of the Section of Female Pelvic Medicine and Reconstructive Surgery at MedStar Washington Hospital Center. She is also Associate Professor, Departments of Obstetrics and Gynecology and Urology, at Georgetown University School of Medicine in Washington, DC. She serves on the OBG Management Board of Editors.
Rosanne M. Kho, MD, is Associate Professor of Obstetrics and Gynecology at the Mayo Clinic in Scottsdale, Arizona.
Jamal Mourad, DO, practices Obstetrics and Gynecology at Southwest Women’s Care in Phoenix, Arizona.
Marie Fidela R. Paraiso, MD, is Professor of Surgery, Cleveland Clinic Lerner College of Medicine at Case Western Reserve University, and Head of the Center for Urogynecology and Reconstructive Pelvic Surgery of the Obstetrics, Gynecology, and Women’s Health Institute at the Cleveland Clinic in Ohio. Dr. Paraiso also holds a joint appointment in the Cleveland Clinic Glickman Urological and Kidney Institute.
Jason D. Wright, MD, is Levine Family Assistant Professor of Women’s Health and Florence Irving Assistant Professor of Obstetrics and Gynecology, Division of Gynecologic Oncology, at Columbia University College of Physicians and Surgeons in New York, New York.
Dr. Iglesia, Dr. Kho, Dr. Mourad, Dr. Paraiso, and Dr. Wright report no financial relationships relevant to this article. Dr. Advincula reports that he is a consultant to Blue Endo, Cooper Surgical, Covidien, Intuitive Surgical, and Surgiquest.
The publication of a large cohort study of hysterectomy for benign indications revived a debate over robotic assistance in gynecologic surgery.1 The study—by Jason D. Wright, MD, and colleagues—included 264,758 women who underwent hysterectomy for benign indications in 441 US hospitals from 2007 to 2010, and it produced some dramatic findings:
- The use of robotic assistance increased from 0.5% of all hysterectomies in 2007 to 9.5% in 2010
- Three years after the first robotic procedure in each hospital where robotics were used, robotic-assisted hysterectomy accounted for 22.4% of all hysterectomies
- Laparoscopic hysterectomy increased as well, from 24.3% of all hysterectomies in the first quarter of 2007 to 30.5% in the first quarter of 2010
- The rate of vaginal hysterectomy declined from 21.7% to 19.8% of all hysterectomies during the same time period
- Abdominal hysterectomy decreased from 53.6% to 43.1% of all hysterectomies
- Although robotic-assisted and laparoscopic hysterectomy had similar complication rates, transfusion requirements, and rates of discharge to a nursing facility, the robotic-assisted approach cost $2,189 more.1
An editorial accompanying this study opined that physicians and hospitals have a “duty” to make sure patients are aware not only of the benefits and risks of each surgical option but also of its financial cost.2 The editorialists suggested that cost should be taken into account by the surgeon, as well. When a more expensive treatment proves to be more effective than the conventional approach, there typically is little argument about paying the higher cost. When the new treatment or technology is equally effective, however, as is the case with robotic hysterectomy and the laparoscopic approach, the choice of treatment “should be more straightforward.”2 That is, the lower-cost treatment should be preferred, the editorialists concluded.
The study by Wright and colleagues and the accompanying editorial prompted some important questions:
- Should robotic assistance be offered for patients undergoing hysterectomy for benign indications when it produces outcomes equivalent to laparoscopic hysterectomy but costs one-third more?
- Is the robotic approach justified in other benign gynecologic surgical procedures?
To explore these questions, we invited a roster of experts in minimally invasive gynecologic surgery to share their perspective and experience, including the lead author of the article mentioned above, Jason D. Wright, MD. In this roundtable discussion, these experts discuss the robotic experience at their respective institutions, characterize the data to date, and offer valuable suggestions about whether and when to incorporate the robot into your surgical practice.
To read 9 recent articles from OBG Management on the pros and cons of robotic surgery, click here.
What is driving the demand?
OBG Management: How much of the demand for the robot do you think is patient-driven? Hospital-driven? Physician- or data-driven?
Cheryl B. Iglesia, MD: With any new technology, there is a honeymoon phase when providers, patients, and hospitals really tout innovation. With its superior optics and 3D vision, the robot certainly enjoyed an extended honeymoon, driven by innovation and a “cool factor.” However, as experience, comparative studies, and longer-term outcomes data become known, demand for new technology is tempered and refined. The choice of technology also has to include an acknowledgement of its cost-effectiveness—or lack of it.
Jamal Mourad, DO: I believe that the demand for minimally invasive procedures, including robotic-assisted surgeries, is multifaceted. The public has learned to have certain expectations about the care it receives. In addition, the media have incorporated a tremendous amount of information about minimally invasive surgery and robotic procedures into TV shows, newscasts, newspapers, and many other outlets. This information certainly stimulates, at the very least, curiosity on the part of the patient, which leads, in turn, to more inquiries about the robot during initial consultation with a surgeon.
Rosanne M. Kho, MD: Here at the Mayo Clinic in Arizona, we adopted use of the robot early. Following the lead of Javier Magrina, MD, we started with the Zeus system (Computer Motion) in 2003 and, subsequently, the da Vinci system (Intuitive Surgical) in 2004. At the time, not many data were available on the use of the robot in gynecology. We looked at the experience in urology and saw its applicability in gynecology. At our institution, therefore, our use of the robot was not driven by the market or data. It was primarily physician-driven and, fortunately, supported by our institution.
Jason D. Wright, MD: Demand for the robot stems from four different sources, in my opinion. Approximately 25% is patient-driven, 25% is surgeon-driven, 25% is hospital-driven, and let’s not forget industry—the makers of the robot—which accounts for 25% of demand. There is a delicate balance between all four buckets that is dynamic and always in flux. A shift too heavily in one direction can lead to problems, especially if variables such as proper infrastructure, outcomes data, or indications, just to name a few, are missing.
Marie Fidela R. Paraiso, MD: Many have criticized the marketing of robot-assisted surgery straight to the consumer. In fact, some investigators have found the claimed benefits of robotic surgery touted in marketing efforts by most hospitals to be unsubstantiated.3,4 That being said, hospitals market the robot to justify its cost and increase patient volume. And many surgeons who lack sufficient training or skill in advanced traditional laparoscopic surgery have embraced and marketed the robot platform as they have enhanced their surgical practices, converting open surgeries to minimally invasive procedures in the process. Patients are attracted to “new” procedures and equate them with “better” when they are choosing a physician or a procedure.
The demand for the robot is also physician driven. In my surgical armamentarium, I use this technology to do complex, multi-procedure pelvic floor repairs that require deep pelvic dissection and suturing or the addition of rectal prolapse procedures or retropubic space anti-incontinence procedures, with or without paravaginal repair.
OBG Management: What do the data reveal about robotic-assisted gynecologic surgery?
Marie Fidela R. Paraiso, MD: For benign conditions, data on robot-assisted laparoscopy are sparse and show no significant benefit, with higher cost and greater operative time than conventional laparoscopy in randomized trials.5,6 A Cochrane review and a large retrospective analysis support these findings.7,8 Another large retrospective study shows that robotic-assisted laparoscopy is associated with a shorter hospital stay, recovery time, postoperative time, and lower cost.9
Robotic surgery has proved to be beneficial in gynecologic oncology and has increased the use of minimally invasive access in gynecology, especially among novice or inexperienced minimally invasive surgeons. It also has helped some surgeons develop skills in advanced traditional laparoscopy.
Rosanne M. Kho, MD: Over the past 10 years in benign gynecology, retrospective comparative studies have found similar complication rates between the robotic approach and conventional laparoscopy. However, operative time and costs are higher in robotics.
One major advantage of the robot, demonstrated in multiple studies, is that the laparotomy rate for hysterectomy has declined in many centers and by as much as 14%, as Dr. Wright and his colleagues found, once the robotic platform becomes available.1

Are you using the robot more?
OBG Management: Has use of the robot increased at your institution?
Rosanne M. Kho, MD: With close to 10 years of experience with the robot, our utilization in gynecology is now stable—it’s neither increasing nor decreasing.
Cheryl B. Iglesia, MD: Yes, use of the robot at my institution has increased as more specialties have adapted the approach, including thoracic surgeons; ear, nose, and throat specialists; and colorectal surgeons. However, the majority of cases are either urologic or gynecologic in nature (urogynecology and gynecologic oncology).
Arnold P. Advincula, MD: This question is difficult to answer because, at Florida Hospital Celebration Health, we are a bit of an outlier. I function within a Global Robotics Institute that is dedicated to the safe and proper use of robotic technology by emphasizing optimal patient outcomes, teaching best practice techniques, and innovating future surgical platforms. Access is allowed for a limited number of surgeons in the various disciplines who are recognized pioneers and for high-volume surgeons with a proven track record of surgical outcomes. Our utilization across all disciplines, including gynecology, always has been high because of this infrastructure.
Jamal Mourad, DO: I have seen a steady, progressive growth in the number of robotic procedures performed at our institution. Initially, the urology department used our robotic equipment to perform prostatectomies. Shortly after that, quick acceptance by several departments, including gynecology, oncology, general surgery, colorectal surgery, and pediatric surgery, led to widespread use and acceptance.
OBG Management: Does increasing use of the robot make it more likely that it will be used in hysterectomy for benign indications?
Rosanne M. Kho, MD: Our primary approach to the simple hysterectomy for benign conditions (including nulliparous women, those with a uterus larger than 12 weeks’ size, and women who have undergone previous pelvic surgery) is still vaginal. Patients with pelvic pain, known endometriosis, and suspicious adnexal masses are approached laparoscopically or robotically.
Compared with conventional laparoscopy, we have found the robot particularly useful in obese patients and in cases requiring extensive dissection and suturing, such as in benign complex gynecology (involving endometriosis, ovarian remnant syndrome), urogynecology (for prolapse and fistulas), and all aspects of gynecologic oncology.
In our hands, the robot has been a facilitating tool, allowing us to perform complex procedures that would otherwise have been difficult to perform with conventional laparoscopy.
Which benign conditions are being addressed robotically?
OBG Management: What benign procedures in gynecologic surgery is the robot used for at your institution, Dr. Advincula?
Arnold P. Advincula, MD: It’s used for the entire gamut of benign procedures in gynecologic surgery, ranging from complex hysterectomy to reproductive surgical cases such as myomectomy and endometriosis resection to pelvic reconstructive surgery. Our success with such a broad range of applications is very much attributable to the infrastructure that we have in place that allows us to use the robot safely and efficiently.
![]()
OBG Management: What makes the robot so attractive?
Jamal Mourad, DO: Robotic technology allows for much-improved visualization, better dexterity and maneuverability, and near total control of the surgical field. I have found that the combination of these advantages permits predictable and reproducible procedures, less tissue trauma, less blood loss, a shorter hospital stay, and fewer conversions to laparotomy, even in very difficult and challenging situations, such as cases involving dense adhesions, a large uterus, or deep infiltrating endometriosis.
Cheryl B. Iglesia, MD: At my institution, the robot is used for sacrocolpopexy, some myomectomy and endometriosis cases (although haptic feedback for these tough endometriosis cases often makes laparoscopy more useful), and, rarely, fistulas (vesicouterine, ureterovaginal).
Jamal Mourad, DO: There are several experienced minimally invasive surgeons at my institution. In addition to hysterectomy, we perform sacrocolpopexy, myomectomy, and resection of severe endometriosis using the robot.
Marie Fidela R. Paraiso, MD: We use the robot for hysterectomy, myomectomy, sacrocolpopexy, Burch colposuspension, paravaginal repair, tubal reanastomosis, endometriosis resection, ureterolysis, and cerclage.
Jason D. Wright, MD: At my institution, robotic surgery for benign indications has been used predominately for hysterectomy and myomectomy, as well as sacrocolpopexy. Given the lack of data to guide implementation of robotic surgery in gynecology, it is difficult to determine which patients derive the most benefit from robotic-assisted procedures.
Should the robot be used for benign hysterectomy?
OBG Management: Do you believe use of the robot is justified in hysterectomy for benign indications?
Cheryl B. Iglesia, MD: No, I believe that most hysterectomies should be done vaginally. If, for some reason, the vaginal approach is not feasible, then laparoscopic hysterectomy is the next best choice and more cost-effective than robotic hysterectomy. Comparative studies and Dr. Wright’s JAMA article seem to concur.1 Open abdominal hysterectomy should be the last option.
Rosanne M. Kho, MD: I do believe that the robot is justified for use in hysterectomy for benign indications. It has provided many patients with the benefits of minimally invasive surgery, as studies have shown.1,9 In an ideal world, simple hysterectomies would be performed vaginally first and, as Dr. Iglesia noted, laparoscopically second. We do know, however, that not only are the learning curves for the vaginal and laparoscopic approaches steep, it is a challenge to teach these approaches effectively. The robotic platform has overcome these challenges with the 3D view, articulation of instruments, and a simulation and teaching console.
Marie Fidela R. Paraiso, MD: I agree with Dr. Iglesia. I do not think that use of the robot is justified for benign indications unless it is shown to be cost-effective and results in the same cure and complication rates, or if a surgeon does not have the skills or training in traditional laparoscopy and desires to offer his/her patients minimally invasive abdominal surgery. So far, two prospective trials have demonstrated that robotic-assisted hysterectomy for benign disease requires longer operative time and is, therefore, more costly in centers where there are surgeons who specialize in advanced laparoscopy.5,6 We still have not defined the subset of patients who would benefit from robotic-assisted laparoscopy if all things are equal.
Jason D. Wright, MD: I think we need more data on robotic surgery for benign gynecologic disease. To date, the majority of the data are retrospective, with most studies unable to demonstrate an advantage of robotic surgery over laparoscopy. These studies have shown that robotic-assisted surgery is substantially more costly, however. I think there are groups of patients who are likely to benefit from robotic surgery, but we need to better define this group of women.
Arnold P. Advincula, MD: I disagree. Despite some controversy, I believe that use of the robot is justified in hysterectomy for benign indications. In fact, it is in situations like the frozen pelvis from endometriosis, or the scarred anterior cul-de-sac from previous cesarean deliveries, that the robot adds value. Several studies speak to the feasibility, safety, and reproducibility of the robot under those circumstances.10,11 Of course, more recently, studies have challenged the use of robotics in benign gynecologic surgery, particularly hysterectomy. Those studies must be viewed with a critical eye. Individuals often can be swayed by the findings of randomized, controlled trials, but such studies are difficult to perform in surgery and there is really no way to be an expert in both conventional laparoscopy and robotic-assisted laparoscopy. As a surgical tool, a surgeon must commit to developing expertise with one or the other.
An often forgotten aspect that is critical to the success of both conventional laparoscopic surgeons and robotic surgeons is the presence of a well-run infrastructure and team to support the surgery. Without that, costs go up and patient outcomes go down for both approaches.
Jamal Mourad, DO: I agree. I believe there is a definitive justification for the use of the robot in benign gynecology. Most of the nearly 600,000 hysterectomies performed each year in the United States are still done by the open abdominal approach despite recognition that a minimally invasive approach (vaginal or laparoscopic) is the standard of care for benign hysterectomy. I incorporated robotic technology into a very busy laparoscopic practice in 2005. I continue to use laparoscopy as a very important tool in my armamentarium for minimally invasive surgery. As I mentioned earlier, robotic technology allows better visualization, dexterity, maneuverability, and control of the surgical field.
Ultimately, it is about taking care of our patients. Cost and efficiency are extremely important, but the patient is more important. The goal is excellence, not average care!
OBG Management: How would you advise clinicians about when to use the robot in gynecologic surgery?
Rosanne M. Kho, MD: Until the cost of robotic procedures declines (soon, I hope), clinicians need to be vigilant in the use of measures and techniques that help them remain efficient and work safely while performing robotic procedures. Such measures include training a dedicated robotic OR team (including a bedside assistant), optimizing trocar placement and docking time, reducing operative or console time, and minimizing the number of robotic and disposable laparoscopic instruments used per case. There are multiple excellent robotics courses that utilize simulation and cadaveric models that clinicians can make use of to advance their skills.
Cheryl B. Iglesia, MD: I would first advise surgeons to get appropriate training using modules, labs, or a robotic simulator—or all three—that is consistent with institutional and other guidelines.12 Second, get appropriate proctoring for your first few cases. Third, think vaginal first and laparoscopic second for straightforward hysterectomies. Robotic assistance has advantages if a lot of sewing is required (as in myomectomy or sacrocolpopexy) or a lot of dissection in small spaces is needed (such as in lymph node dissection in gynecologic oncology).
It is likely true that robotic training can be enabling technology and can improve a surgeon’s straight-stick laparoscopic skills. Mastering fundamentals of vaginal and laparoscopic surgery is the core to the foundation of gynecologic surgery. Robotic use can be narrowed to certain situations. I am not sure where single-port robotics will lead, but surgeons will need to assess that new technology as well. Most important, volume matters. High-volume surgeons and high-volume centers have the most experience with the fewest complications, as proven in multiple surgical subspecialties—not just gynecology.
Jason D. Wright, MD: I think gynecologic surgeons need to be aware of the lack of data for robotic gynecologic surgery and carefully choose which patients and procedures they utilize the robot for. Unfortunately, many of the claims of benefit of the robot are not supported by high-quality research.
Although the added costs of robotic surgery may not have an immediate impact, in the long term these costs will almost certainly be passed on not only to patients and hospitals but also to physicians.
Jamal Mourad, DO: Despite the clear advantages of laparoscopic hysterectomy, most surgeons still perform laparotomy as their preferred route for hysterectomy because of the complex skills needed to perform straight-stick laparoscopy. The FDA approved the da Vinci surgical system for gynecologic procedures in 2005. Since then, the number of minimally invasive hysterectomies has increased dramatically.13 The American College of Obstetricians and Gynecologists and AAGL recognize and endorse a minimally invasive approach (vaginal, laparoscopic) to the majority of hysterectomies.14 Despite this recognition, the total number of vaginal and laparoscopic hysterectomies has remained stagnant for the past 25 years. Since the introduction of the robotic platform into our specialty, the total number of laparotomies has decreased significantly, due in large part to acceptance of robotic-assisted procedures.
Arnold P. Advincula, MD: This question—how to advise surgeons—is complicated because it involves so many moving parts. The bottom line: As long as surgeons have the appropriate rationale and indications for its use, proper training with subsequent credentialing and privileging, and the infrastructure to allow for safe and efficient use of the technology with outcomes tracking, then I think the robot is justified for interested clinicians who truly believe it will enhance their performance and care of patients. If some pieces of this equation are missing, then I would caution surgeons about incorporating robotics into their surgical armamentarium. I feel very strongly that many of the issues we see today surrounding robotics are the result of disregarding these very important requirements for the adoption of technology in medicine. We have seen similar issues with transvaginal mesh. Let’s not let history repeat itself in the arena of robotics.
Jamal Mourad, DO: I agree. We need to do what is right. First, do no harm, then do what you would want done to you!
We want to hear from you! Tell us what you think.
1. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689-698.
2. Weissman JS, Zinner M. Comparative effectiveness research on robotic surgery. JAMA. 2013;309(7):721-722.
3. Schiavone MB, Kuo EC, Naumann RW, et al. The commercialization of robotic surgery: Unsubstantiated marketing of gynecologic surgery by hospitals. Am J Obstet Gynecol. 2012;207(3):174.e1-e7.
4. Kaunitz AM. Examining the Evidence. Are hospital claims about the robotic approach to gynecologic surgery based on reliable data—or mostly hype? OBG Manage. 2012;24(11):55-56.
5. Sarlos D, Kots L, Stevanovic N, et al. Robotic compared with conventional laparoscopic hysterectomy: A randomized controlled trial. Obstet Gynecol. 2012;120(3):604-611.
6. Paraiso MFR, Ridgeway B, Park AJ, et al. A randomized trial comparing conventional and robotic-assisted total laparoscopic hysterectomy [published online ahead of print February 8, 2013]. Am J Obstet Gynecol. doi:pii:S0002-9378(13)00144-0.
7. Paraiso MF, Jelovsek JE, Frick A, Chen GC, Barber MD. Laparoscopic compared with robotic sacrocolpopexy for vaginal prolapse: A randomized controlled trial. Obstet Gynecol. 2011;118(5):1105-1113.
8. Liu H, Lu D, Wang L, Shi G, Song H, Clark J. Robotic surgery for benign gynaecological disease. Cochrane Database Syst Rev. 2012;2:CD008978.-
9. Pasic R, Rizzo J, Fang H, Ross S, Moore M, Gunnarsson C. Comparing robot-assisted with conventional laparoscopic hysterectomy: Impact on cost and clinical outcomes. J Minim Invasive Gynecol. 2010;17(6):730-738.
10. Payne TN, Dauterive FR. A comparison of total laparoscopic hysterectomy to robotically assisted hysterectomy: Surgical outcomes in a community practice. J Minim Invasive Gynecol. 2008;15(3):286-291.
11. AAGL. Route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18(1):1-3.
12. American Urogynecologic Society. Guidelines for privileging and credentialing physicians for sacrocolpopexy for pelvic organ prolapse. Female Pelvic Med Reconstructive Surg. 2013;19(2):62-65.
13. Payne TN, Dauterive FR, Pitter MC, et al. Robotically assisted hysterectomy in patients with large uteri. Outcomes in five community practices. Obstet Gynecol. 2010;115(3):535-542.
14. Boggess JF, Gehrig PA, Cantrell L, et al. Perioperative outcomes of robotically assisted hysterectomy for benign cases with complex pathology. Obstet Gynecol. 2009;114(3):585-593.
1. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689-698.
2. Weissman JS, Zinner M. Comparative effectiveness research on robotic surgery. JAMA. 2013;309(7):721-722.
3. Schiavone MB, Kuo EC, Naumann RW, et al. The commercialization of robotic surgery: Unsubstantiated marketing of gynecologic surgery by hospitals. Am J Obstet Gynecol. 2012;207(3):174.e1-e7.
4. Kaunitz AM. Examining the Evidence. Are hospital claims about the robotic approach to gynecologic surgery based on reliable data—or mostly hype? OBG Manage. 2012;24(11):55-56.
5. Sarlos D, Kots L, Stevanovic N, et al. Robotic compared with conventional laparoscopic hysterectomy: A randomized controlled trial. Obstet Gynecol. 2012;120(3):604-611.
6. Paraiso MFR, Ridgeway B, Park AJ, et al. A randomized trial comparing conventional and robotic-assisted total laparoscopic hysterectomy [published online ahead of print February 8, 2013]. Am J Obstet Gynecol. doi:pii:S0002-9378(13)00144-0.
7. Paraiso MF, Jelovsek JE, Frick A, Chen GC, Barber MD. Laparoscopic compared with robotic sacrocolpopexy for vaginal prolapse: A randomized controlled trial. Obstet Gynecol. 2011;118(5):1105-1113.
8. Liu H, Lu D, Wang L, Shi G, Song H, Clark J. Robotic surgery for benign gynaecological disease. Cochrane Database Syst Rev. 2012;2:CD008978.-
9. Pasic R, Rizzo J, Fang H, Ross S, Moore M, Gunnarsson C. Comparing robot-assisted with conventional laparoscopic hysterectomy: Impact on cost and clinical outcomes. J Minim Invasive Gynecol. 2010;17(6):730-738.
10. Payne TN, Dauterive FR. A comparison of total laparoscopic hysterectomy to robotically assisted hysterectomy: Surgical outcomes in a community practice. J Minim Invasive Gynecol. 2008;15(3):286-291.
11. AAGL. Route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18(1):1-3.
12. American Urogynecologic Society. Guidelines for privileging and credentialing physicians for sacrocolpopexy for pelvic organ prolapse. Female Pelvic Med Reconstructive Surg. 2013;19(2):62-65.
13. Payne TN, Dauterive FR, Pitter MC, et al. Robotically assisted hysterectomy in patients with large uteri. Outcomes in five community practices. Obstet Gynecol. 2010;115(3):535-542.
14. Boggess JF, Gehrig PA, Cantrell L, et al. Perioperative outcomes of robotically assisted hysterectomy for benign cases with complex pathology. Obstet Gynecol. 2009;114(3):585-593.