User login
Clinical Alerts Predict Readmission
Rapid response systems (RRSs) have been developed to identify and treat deteriorating patients on general hospital units.[1] The most commonly proposed approach to the problem of identifying and stabilizing deteriorating hospitalized patients includes some combination of an early warning system to detect the deterioration and an RRS to deal with it. We previously demonstrated that a relatively simple hospital‐specific prediction model employing routine laboratory values and vital sign data is capable of predicting clinical deterioration, the need for intensive care unit (ICU) transfer, and hospital mortality in patients admitted to general medicine units.[2, 3, 4, 5, 6]
Hospital readmissions within 30 days of hospital discharge occur often and are difficult to predict. Starting in 2013, readmission penalties have been applied to specific conditions in the United States (acute myocardial infarction, heart failure, and pneumonia), with the expectation that additional conditions will be added to this group in years to come.[7, 8] Unfortunately, interventions developed to date have not been universally successful in preventing hospital readmissions for various medical conditions and patient types.[9] One potential explanation for this is the inability to reliably predict which patients are at risk for readmission to better target preventative interventions. Predictors of hospital readmission can be disease specific, such as the presence of multivessel disease in patients hospitalized with myocardial infarction,[10] or more general, such as lack of available medical follow‐up postdischarge.[11] Therefore, we performed a study to determine whether the occurrence of automated clinical deterioration alerts (CDAs) predicted 30‐day hospital readmission.
METHODS
Study Location
The study was conducted on 8 general medicine units of Barnes‐Jewish Hospital, a 1250‐bed academic medical center in St. Louis, Missouri (January 15, 2015December 12, 2015). Patient care on the inpatient medicine units is delivered by either attending hospitalist physicians or housestaff physicians under the supervision of an attending physician. The study was approved by the Washington University School of Medicine Human Studies Committee, and informed consent was waived.
Study Overview
We retrospectively evaluated all adult patients (aged >18 years) admitted through the emergency department or transferred directly to the general medicine units from other institutions. We excluded patients who died while hospitalized. All data were derived from the hospital informatics database provided by the Center for Clinical Excellence, BJC HealthCare.
Primary End Point
Readmission for any reason (ie, all‐cause readmission) to an acute care facility in the 30 days following discharge after the index hospitalization served as the primary end point. Barnes‐Jewish Hospital serves as the main teaching institution for BJC Healthcare, a large integrated healthcare system of both inpatient and outpatient care. The system includes a total of 12 hospitals and multiple community health locations in a compact geographic region surrounding and including St. Louis, Missouri, and we included readmission to any of these hospitals in our analysis. Persons treated within this healthcare system are, in nearly all cases, readmitted to 1 of the system's participating 12 hospitals. If a patient who receives healthcare in the system presents to a nonsystem hospital, he/she is often transferred back into the integrated system because of issues of insurance coverage. Patients with a 30‐day readmission were compared to those without a 30‐day readmission.
Variables
We recorded information regarding demographics, median income of the zip code of residence as a marker of socioeconomic status, admission to any BJC Healthcare facility within 6 months of the index admission, and comorbidities. To represent the global burden of comorbidities in each patient, we calculated their Charlson Comorbidity Index score.[12] Severity of illness was assessed using the All Patient RefinedDiagnosis Related Groups severity of illness score.
CDA Algorithm Overview
Details regarding the CDA model development and its implementation have been previously described in detail.[4, 5, 6] In brief, we applied logistic regression techniques to develop the CDA algorithm. Manually obtained vital signs, laboratory data, and pharmacy data inputted real time into the electronic medical record (EMR) were continuously assessed. The CDA algorithm searched for the 36 input variables (Table 1) as previously described from the EMR for all patients admitted to the 8 medicine units 24 hours per day and 7 days a week.[4, 5, 6] Values for every continuous parameter were scaled so that all measurements lay in the interval (0, 1) and were normalized by the minimum and maximum of the parameter. To capture the temporal effects in our data, we retain a sliding window of all the collected data points within the last 24 hours. We then subdivide these data into a series of n equally sized buckets (eg, 6 sequential buckets of 4 hours each). To capture variations within a bucket, we compute 3 values for each bucket: the minimum, maximum, and mean data points. Each of the resulting 3 n values are input to the logistic regression equation as separate variables.
| Age |
| Alanine aminotransferase |
| Alternative medicines |
| Anion gap |
| Anti‐infectives |
| Antineoplastics |
| Aspartate aminotransferase |
| Biologicals |
| Blood pressure, diastolic |
| Blood pressure, systolic |
| Calcium, serum |
| Calcium, serum, ionized |
| Cardiovascular agents |
| Central nervous system agents |
| Charlson Comorbidity Index |
| Coagulation modifiers |
| Estimated creatinine clearance |
| Gastrointestinal agents |
| Genitourinary tract agents |
| Hormones/hormone modifiers |
| Immunologic agents |
| Magnesium, serum |
| Metabolic agents |
| Miscellaneous agents |
| Nutritional products |
| Oxygen saturation, pulse oximetry |
| Phosphate, serum |
| Potassium, serum |
| Psychotherapeutic agents |
| Pulse |
| Radiologic agents |
| Respirations |
| Respiratory agents |
| Shock Index |
| Temperature |
| Topical agents |
The algorithm was first implemented in MATLAB (MathWorks, Natick, MA). For the purposes of training, we used a single 24‐hour window of data from each patient. The dataset's 36 input variables were divided into buckets and minimum/mean/maximum features wherever applicable, resulting in 398 variables. The first half of the original dataset was used to train the model. We then used the second half of the dataset as the validation dataset. We generated a predicted outcome for each case in the validation data, using the model parameter coefficients derived from the training data. We also employed bootstrap aggregation to improve classification accuracy and to address overfitting. We then applied various threshold cut points to convert these predictions into binary values and compared the results against the ICU transfer outcome. A threshold of 0.9760 for specificity was chosen to achieve a sensitivity of approximately 40%. These operating characteristics were chosen in turn to generate a manageable number of alerts per hospital nursing unit per day (estimated at 12 per nursing unit per day). At this cut point the C statistic was 0.8834, with an overall accuracy of 0.9292.[5] Patients with inputted data meeting the CDA threshold had a real‐time alert sent to the hospital rapid response team prompting a patient evaluation.
Statistical Analysis
The number of patients admitted to the 8 general medicine units of Barnes‐Jewish Hospital during the study period determined the sample size. Categorical variables were compared using 2 or Fisher exact test as appropriate. Continuous variables were compared using the Mann‐Whitney U test. All analyses were 2‐tailed, and a P value of <0.05 was assumed to represent statistical significance. We relied on logistic regression for identifying variables independently associated with 30‐day readmission. Based on univariate analysis, variables significant at P < 0.15 were entered into the model. To arrive at the most parsimonious model, we utilized a stepwise backward elimination approach. We evaluated collinearity with the variance inflation factor. We report adjusted odds ratios (ORs) and 95% confidence intervals (CIs) where appropriate. The model's goodness of fit was assessed via calculation of the Hosmer‐Lemeshow test. Receiver operating characteristic (ROC) curves were used to compare the predictive models for 30‐day readmission with or without the CDA variable. All statistical analyses were performed using SPSS (version 22.0; IBM, Armonk, NY).
RESULTS
The final cohort had 3015 patients with a mean age of 57.5 17.5 years and 47.8% males. The most common reasons for hospital admission were infection or sepsis syndrome including pneumonia and urinary tract infections (23.6%), congestive heart failure or other cardiac conditions (18.4%), respiratory distress including chronic obstructive pulmonary disease (16.2%), acute or chronic renal failure (9.7%), gastrointestinal disorders (8.4%), and diabetes mellitus management (7.4%). Overall, there were 567 (18.8%) patients who were readmitted within 30 days of their hospital discharge date.
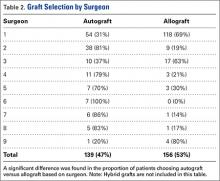
Table 2 shows the characteristics of patients readmitted within 30 days and of patients not requiring hospital readmission within 30 days. Patients requiring hospital readmission within 30 days were younger and had significantly more comorbidities as manifested by significantly greater Charlson scores and individual comorbidities including coronary artery disease, congestive heart disease, peripheral vascular disease, connective tissue disease, cirrhosis, diabetes mellitus with end‐organ complications, renal failure, and metastatic cancer. Patients with a 30‐day readmission had significantly longer duration of hospitalization, more emergency department visits in the 6 months prior to the index hospitalization, lower minimum hemoglobin measurements, higher minimum serum creatinine values, and were more likely to have Medicare or Medicaid insurance compared to patients without a 30‐day readmission.
| Variable | 30‐Day Readmission | P Value | |
|---|---|---|---|
| Yes (n = 567) | No (n = 2,448) | ||
| |||
| Age, y | 56.1 17.0 | 57.8 17.6 | 0.046 |
| Gender | |||
| Male | 252 (44.4) | 1,188 (48.5) | 0.079 |
| Female | 315 (55.6) | 1,260 (51.5) | |
| Race | |||
| Caucasian | 277 (48.9) | 1,234 (50.4) | 0.800 |
| African American | 257 (45.3) | 1,076 (44.0) | |
| Other | 33 (5.8) | 138 (5.6) | |
| Median income, dollars | 30,149 [25,23436,453] | 29,271 [24,83037,026] | 0.903 |
| BMI | 29.4 10.0 | 29.0 9.2 | 0.393 |
| APR‐DRG Severity of Illness Score | 2.6 0.4 | 2.5 0.5 | 0.152 |
| Charlson Comorbidity Index | 6 [39] | 5 [27] | <0.001 |
| ICU transfer during admission | 93 (16.4) | 410 (16.7) | 0.842 |
| Myocardial infarction | 83 (14.6) | 256 (10.5) | 0.005 |
| Congestive heart failure | 177 (31.2) | 540 (22.1) | <0.001 |
| Peripheral vascular disease | 76 (13.4) | 214 (8.7) | 0.001 |
| Cardiovascular disease | 69 (12.2) | 224 (9.2) | 0.029 |
| Dementia | 15 (2.6) | 80 (3.3) | 0.445 |
| Chronic obstructive pulmonary disease | 220 (38.8) | 855 (34.9) | 0.083 |
| Connective tissue disease | 45 (7.9) | 118 (4.8) | 0.003 |
| Peptic ulcer disease | 26 (4.6) | 111 (4.5) | 0.958 |
| Cirrhosis | 60 (10.6) | 141 (5.8) | <0.001 |
| Diabetes mellitus without end‐organ complications | 148 (26.1) | 625 (25.5) | 0.779 |
| Diabetes mellitus with end‐organ complications | 92 (16.2) | 197 (8.0) | <0.001 |
| Paralysis | 25 (4.4) | 77 (3.1) | 0.134 |
| Renal failure | 214 (37.7) | 620 (25.3) | <0.001 |
| Underlying malignancy | 85 (15.0) | 314 (12.8) | 0.171 |
| Metastatic cancer | 64 (11.3) | 163 (6.7) | <0.001 |
| Human immunodeficiency virus | 10 (1.8) | 47 (1.9) | 0.806 |
| Minimum hemoglobin, g/dL | 9.1 [7.411.4] | 10.7 [8.712.4] | <0.001 |
| Minimum creatinine, mg/dL | 1.12 [0.792.35] | 1.03 [0.791.63] | 0.006 |
| Length of stay, d | 3.8 [1.97.8] | 3.3 [1.85.9] | <0.001 |
| ED visit in the past year | 1 [03] | 0 [01] | <0.001 |
| Clinical deterioration alert triggered | 269 (47.4) | 872 (35.6%) | <0.001 |
| Insurance | |||
| Private | 111 (19.6) | 528 (21.6) | 0.020 |
| Medicare | 299 (52.7) | 1,217 (49.7) | |
| Medicaid | 129 (22.8) | 499 (20.4) | |
| Patient pay | 28 (4.9) | 204 (8.3) | |
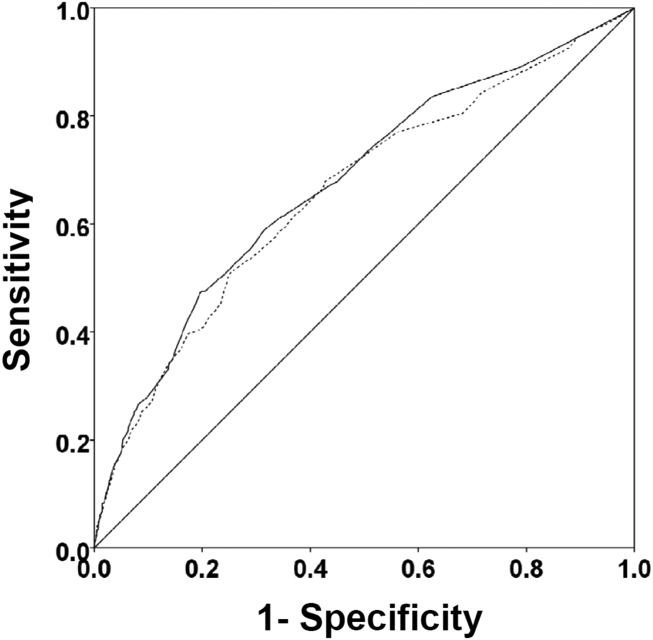
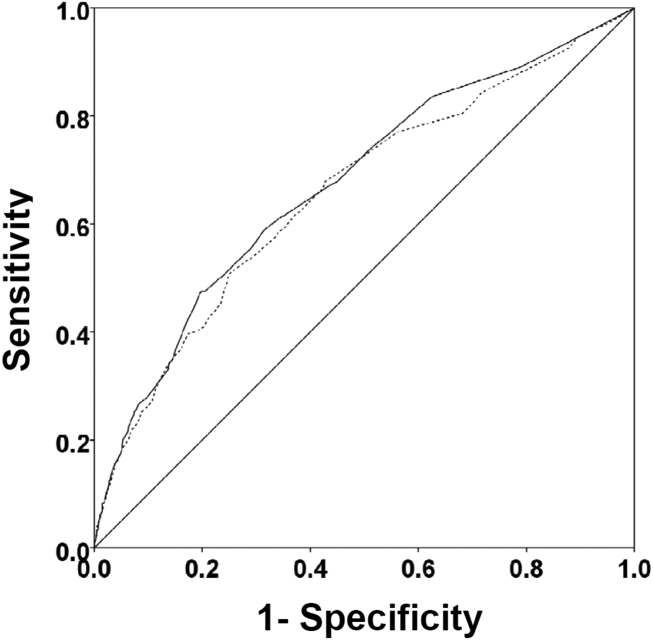
There were 1141 (34.4%) patients that triggered a CDA. Patients triggering a CDA were significantly more likely to have a 30‐day readmission compared to those who did not trigger a CDA (23.6% vs 15.9%; P < 0.001). Patients triggering a CDA were also significantly more likely to be readmitted within 60 days (31.7% vs 22.1%; P < 0.001) and 90 days (35.8% vs 26.2%; P < 0.001) compared to patients who did not trigger a CDA. Multiple logistic regression identified the triggering of a CDA to be independently associated with 30‐day readmission (OR: 1.40; 95% CI: 1.26‐1.55; P = 0.001) (Table 3). Other independent predictors of 30‐day readmission were: an emergency department visit in the previous 6 months, increasing age in 1‐year increments, presence of connective tissue disease, diabetes mellitus with end‐organ complications, chronic renal disease, cirrhosis, and metastatic cancer (Hosmer‐Lemeshow goodness of fit test, 0.363). Figure 1 reveals the ROC curves for the logistic regression model (Table 3) with and without the CDA variable. As the ROC curves document, the 2 models had similar sensitivity for the entire range of specificities. Reflecting this, the area under the ROC curve for the model inclusive of the CDA variable equaled 0.675 (95% CI: 0.649‐0.700), whereas the area under the ROC curve for the model excluding the CDA variable equaled 0.658 (95% CI: 0.632‐0.684).
| Variables | OR | 95% CI | P Value |
|---|---|---|---|
| |||
| Clinical deterioration alert | 1.40 | 1.261.55 | 0.001 |
| Age (1‐point increments) | 1.01 | 1.011.02 | 0.003 |
| Connective tissue disease | 1.63 | 1.341.98 | 0.012 |
| Cirrhosis | 1.25 | 1.171.33 | <0.001 |
| Diabetes mellitus with end‐organ complications | 1.23 | 1.131.33 | 0.010 |
| Chronic renal disease | 1.16 | 1.081.24 | 0.034 |
| Metastatic cancer | 1.12 | 1.081.17 | 0.002 |
| Emergency department visit in previous 6 months | 1.23 | 1.201.26 | <0.001 |
DISCUSSION
We demonstrated that the occurrence of an automated CDA is associated with increased risk for 30‐day hospital readmission. However, the addition of the CDA variable to the other variables identified to be independently associated with 30‐day readmission (Table 3) did not significantly add to the overall predictive accuracy of the derived logistic regression model. Other investigators have previously attempted to develop automated predictors of hospital readmission. Amarasingham et al. developed a real‐time electronic predictive model that identifies hospitalized heart failure patients at high risk for readmission or death from clinical and nonclinical risk factors present on admission.[13] Their electronic model demonstrated good discrimination for 30‐day mortality and readmission and performed as well, or better than, models developed by the Center for Medicaid and Medicare Services and the Acute Decompensated Heart Failure Registry. Similarly, Baillie et al. developed an automated prediction model that was effectively integrated into an existing EMR and identified patients on admission who were at risk for readmission within 30 days of discharge.[14] Our automated CDA differs from these previous risk predictors by surveying patients throughout their hospital stay as opposed to identifying risk for readmission at a single time point.
Several limitations of our study should be recognized. First, this was a noninterventional study aimed at examining the ability of CDAs to predict hospital readmission. Future studies are needed to assess whether the use of enhanced readmission prediction algorithms can be utilized to avert hospital readmissions. Second, the data derive from a single center, and this necessarily limits the generalizability of our findings. As such, our results may not reflect what one might see at other institutions. For example, Barnes‐Jewish Hospital has a regional referral pattern that includes community hospitals, regional long‐term acute care hospitals, nursing homes, and chronic wound, dialysis, and infusion clinics. This may explain, in part, the relatively high rate of hospital readmission observed in our cohort. Third, there is the possibility that CDAs were associated with readmission by chance given the number of potential predictor variables examined. The importance of CDAs as a determinant of rehospitalization requires confirmation in other independent populations. Fourth, it is likely that we did not capture all hospital readmissions, primarily those occurring outside of our hospital system. Therefore, we may have underestimated the actual rates of readmission for this cohort. Finally, we cannot be certain that all important predictors of hospital readmission were captured in this study.
The development of an accurate real‐time early warning system has the potential to identify patients at risk for various adverse outcomes including clinical deterioration, hospital death, and postdischarge readmission. By identifying patients at greatest risk for readmission, valuable healthcare resources can be better targeted to such populations. Our findings suggest that existing readmission predictors may suboptimally risk‐stratify patients, and it may be important to include additional clinical variables if pay for performance and other across‐institution comparisons are to be fair to institutions that care for more seriously ill patients. The variables identified as predictors of 30‐day hospital readmission in our study, with the exception of a CDA, are all readily identifiable clinical characteristics. The modest incremental value of a CDA to these clinical characteristics suggests that they would suffice for the identification of patients at high risk for hospital readmission. This is especially important for safety‐net institutions not routinely employing automated CDAs. These safety‐net hospitals provide a disproportionate level of care for patients who otherwise would have difficulty obtaining inpatient medical care and disproportionately carry the greatest burden of hospital readmissions.[15]
Disclosure
This study was funded in part by the Barnes‐Jewish Hospital Foundation and by grant number UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR or NIH.
- , , . Rapid‐response teams. N Engl J Med. 2011;365:139–146.
- , , , , , . Early prediction of septic shock in hospitalized patients. J Hosp Med. 2010;5:19–25.
- , , , et al. Implementation of a real‐time computerized sepsis alert in nonintensive care unit patients. Crit Care Med. 2011;39:469–473.
- , , , et al. Toward a two‐tier clinical warning system for hospitalized patients. AMIA Annu Symp Proc. 2011;2011:511–519.
- , , , et al. A trial of a real‐time alert for clinical deterioration in patients hospitalized on general medical wards. J Hosp Med. 2013;8:236–242.
- , , , et al. A randomized trial of real‐time automated clinical deterioration alerts sent to a rapid response team. J Hosp Med. 2014;9:424–429.
- , . Revisiting hospital readmissions JAMA. 2013;309:398–400.
- , , , . Adverse outcomes associated with delayed intensive care unit transfers in an integrated healthcare system. J Hosp Med. 2012;7:224–230.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011; 155:520–528.
- , , , et al. International variation in and factors associated with hospital readmission after myocardial infarction. JAMA. 2012;307:66–74.
- , , , , . Predictors of early readmission among patients 40 to 64 years of age hospitalized for chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11:685–694.
- , , , , , . Assessing illness severity: does clinical judgement work? J Chronic Dis. 1986;39:439–452.
- , , , et al. An automated model to identify heart failure patients at risk for 30‐day readmission or death using electronic medical record data. Med Care. 2010;48:981–988.
- , , , et al. The readmission risk flag: using the electronic health record to automatically identify patients at risk for 30‐day readmission. J Hosp Med. 2013;8:689–695.
- , , . The Medicare hospital readmissions reduction program: time for reform. JAMA. 2015;314:347–348.
Rapid response systems (RRSs) have been developed to identify and treat deteriorating patients on general hospital units.[1] The most commonly proposed approach to the problem of identifying and stabilizing deteriorating hospitalized patients includes some combination of an early warning system to detect the deterioration and an RRS to deal with it. We previously demonstrated that a relatively simple hospital‐specific prediction model employing routine laboratory values and vital sign data is capable of predicting clinical deterioration, the need for intensive care unit (ICU) transfer, and hospital mortality in patients admitted to general medicine units.[2, 3, 4, 5, 6]
Hospital readmissions within 30 days of hospital discharge occur often and are difficult to predict. Starting in 2013, readmission penalties have been applied to specific conditions in the United States (acute myocardial infarction, heart failure, and pneumonia), with the expectation that additional conditions will be added to this group in years to come.[7, 8] Unfortunately, interventions developed to date have not been universally successful in preventing hospital readmissions for various medical conditions and patient types.[9] One potential explanation for this is the inability to reliably predict which patients are at risk for readmission to better target preventative interventions. Predictors of hospital readmission can be disease specific, such as the presence of multivessel disease in patients hospitalized with myocardial infarction,[10] or more general, such as lack of available medical follow‐up postdischarge.[11] Therefore, we performed a study to determine whether the occurrence of automated clinical deterioration alerts (CDAs) predicted 30‐day hospital readmission.
METHODS
Study Location
The study was conducted on 8 general medicine units of Barnes‐Jewish Hospital, a 1250‐bed academic medical center in St. Louis, Missouri (January 15, 2015December 12, 2015). Patient care on the inpatient medicine units is delivered by either attending hospitalist physicians or housestaff physicians under the supervision of an attending physician. The study was approved by the Washington University School of Medicine Human Studies Committee, and informed consent was waived.
Study Overview
We retrospectively evaluated all adult patients (aged >18 years) admitted through the emergency department or transferred directly to the general medicine units from other institutions. We excluded patients who died while hospitalized. All data were derived from the hospital informatics database provided by the Center for Clinical Excellence, BJC HealthCare.
Primary End Point
Readmission for any reason (ie, all‐cause readmission) to an acute care facility in the 30 days following discharge after the index hospitalization served as the primary end point. Barnes‐Jewish Hospital serves as the main teaching institution for BJC Healthcare, a large integrated healthcare system of both inpatient and outpatient care. The system includes a total of 12 hospitals and multiple community health locations in a compact geographic region surrounding and including St. Louis, Missouri, and we included readmission to any of these hospitals in our analysis. Persons treated within this healthcare system are, in nearly all cases, readmitted to 1 of the system's participating 12 hospitals. If a patient who receives healthcare in the system presents to a nonsystem hospital, he/she is often transferred back into the integrated system because of issues of insurance coverage. Patients with a 30‐day readmission were compared to those without a 30‐day readmission.
Variables
We recorded information regarding demographics, median income of the zip code of residence as a marker of socioeconomic status, admission to any BJC Healthcare facility within 6 months of the index admission, and comorbidities. To represent the global burden of comorbidities in each patient, we calculated their Charlson Comorbidity Index score.[12] Severity of illness was assessed using the All Patient RefinedDiagnosis Related Groups severity of illness score.
CDA Algorithm Overview
Details regarding the CDA model development and its implementation have been previously described in detail.[4, 5, 6] In brief, we applied logistic regression techniques to develop the CDA algorithm. Manually obtained vital signs, laboratory data, and pharmacy data inputted real time into the electronic medical record (EMR) were continuously assessed. The CDA algorithm searched for the 36 input variables (Table 1) as previously described from the EMR for all patients admitted to the 8 medicine units 24 hours per day and 7 days a week.[4, 5, 6] Values for every continuous parameter were scaled so that all measurements lay in the interval (0, 1) and were normalized by the minimum and maximum of the parameter. To capture the temporal effects in our data, we retain a sliding window of all the collected data points within the last 24 hours. We then subdivide these data into a series of n equally sized buckets (eg, 6 sequential buckets of 4 hours each). To capture variations within a bucket, we compute 3 values for each bucket: the minimum, maximum, and mean data points. Each of the resulting 3 n values are input to the logistic regression equation as separate variables.
| Age |
| Alanine aminotransferase |
| Alternative medicines |
| Anion gap |
| Anti‐infectives |
| Antineoplastics |
| Aspartate aminotransferase |
| Biologicals |
| Blood pressure, diastolic |
| Blood pressure, systolic |
| Calcium, serum |
| Calcium, serum, ionized |
| Cardiovascular agents |
| Central nervous system agents |
| Charlson Comorbidity Index |
| Coagulation modifiers |
| Estimated creatinine clearance |
| Gastrointestinal agents |
| Genitourinary tract agents |
| Hormones/hormone modifiers |
| Immunologic agents |
| Magnesium, serum |
| Metabolic agents |
| Miscellaneous agents |
| Nutritional products |
| Oxygen saturation, pulse oximetry |
| Phosphate, serum |
| Potassium, serum |
| Psychotherapeutic agents |
| Pulse |
| Radiologic agents |
| Respirations |
| Respiratory agents |
| Shock Index |
| Temperature |
| Topical agents |
The algorithm was first implemented in MATLAB (MathWorks, Natick, MA). For the purposes of training, we used a single 24‐hour window of data from each patient. The dataset's 36 input variables were divided into buckets and minimum/mean/maximum features wherever applicable, resulting in 398 variables. The first half of the original dataset was used to train the model. We then used the second half of the dataset as the validation dataset. We generated a predicted outcome for each case in the validation data, using the model parameter coefficients derived from the training data. We also employed bootstrap aggregation to improve classification accuracy and to address overfitting. We then applied various threshold cut points to convert these predictions into binary values and compared the results against the ICU transfer outcome. A threshold of 0.9760 for specificity was chosen to achieve a sensitivity of approximately 40%. These operating characteristics were chosen in turn to generate a manageable number of alerts per hospital nursing unit per day (estimated at 12 per nursing unit per day). At this cut point the C statistic was 0.8834, with an overall accuracy of 0.9292.[5] Patients with inputted data meeting the CDA threshold had a real‐time alert sent to the hospital rapid response team prompting a patient evaluation.
Statistical Analysis
The number of patients admitted to the 8 general medicine units of Barnes‐Jewish Hospital during the study period determined the sample size. Categorical variables were compared using 2 or Fisher exact test as appropriate. Continuous variables were compared using the Mann‐Whitney U test. All analyses were 2‐tailed, and a P value of <0.05 was assumed to represent statistical significance. We relied on logistic regression for identifying variables independently associated with 30‐day readmission. Based on univariate analysis, variables significant at P < 0.15 were entered into the model. To arrive at the most parsimonious model, we utilized a stepwise backward elimination approach. We evaluated collinearity with the variance inflation factor. We report adjusted odds ratios (ORs) and 95% confidence intervals (CIs) where appropriate. The model's goodness of fit was assessed via calculation of the Hosmer‐Lemeshow test. Receiver operating characteristic (ROC) curves were used to compare the predictive models for 30‐day readmission with or without the CDA variable. All statistical analyses were performed using SPSS (version 22.0; IBM, Armonk, NY).
RESULTS
The final cohort had 3015 patients with a mean age of 57.5 17.5 years and 47.8% males. The most common reasons for hospital admission were infection or sepsis syndrome including pneumonia and urinary tract infections (23.6%), congestive heart failure or other cardiac conditions (18.4%), respiratory distress including chronic obstructive pulmonary disease (16.2%), acute or chronic renal failure (9.7%), gastrointestinal disorders (8.4%), and diabetes mellitus management (7.4%). Overall, there were 567 (18.8%) patients who were readmitted within 30 days of their hospital discharge date.
Table 2 shows the characteristics of patients readmitted within 30 days and of patients not requiring hospital readmission within 30 days. Patients requiring hospital readmission within 30 days were younger and had significantly more comorbidities as manifested by significantly greater Charlson scores and individual comorbidities including coronary artery disease, congestive heart disease, peripheral vascular disease, connective tissue disease, cirrhosis, diabetes mellitus with end‐organ complications, renal failure, and metastatic cancer. Patients with a 30‐day readmission had significantly longer duration of hospitalization, more emergency department visits in the 6 months prior to the index hospitalization, lower minimum hemoglobin measurements, higher minimum serum creatinine values, and were more likely to have Medicare or Medicaid insurance compared to patients without a 30‐day readmission.
| Variable | 30‐Day Readmission | P Value | |
|---|---|---|---|
| Yes (n = 567) | No (n = 2,448) | ||
| |||
| Age, y | 56.1 17.0 | 57.8 17.6 | 0.046 |
| Gender | |||
| Male | 252 (44.4) | 1,188 (48.5) | 0.079 |
| Female | 315 (55.6) | 1,260 (51.5) | |
| Race | |||
| Caucasian | 277 (48.9) | 1,234 (50.4) | 0.800 |
| African American | 257 (45.3) | 1,076 (44.0) | |
| Other | 33 (5.8) | 138 (5.6) | |
| Median income, dollars | 30,149 [25,23436,453] | 29,271 [24,83037,026] | 0.903 |
| BMI | 29.4 10.0 | 29.0 9.2 | 0.393 |
| APR‐DRG Severity of Illness Score | 2.6 0.4 | 2.5 0.5 | 0.152 |
| Charlson Comorbidity Index | 6 [39] | 5 [27] | <0.001 |
| ICU transfer during admission | 93 (16.4) | 410 (16.7) | 0.842 |
| Myocardial infarction | 83 (14.6) | 256 (10.5) | 0.005 |
| Congestive heart failure | 177 (31.2) | 540 (22.1) | <0.001 |
| Peripheral vascular disease | 76 (13.4) | 214 (8.7) | 0.001 |
| Cardiovascular disease | 69 (12.2) | 224 (9.2) | 0.029 |
| Dementia | 15 (2.6) | 80 (3.3) | 0.445 |
| Chronic obstructive pulmonary disease | 220 (38.8) | 855 (34.9) | 0.083 |
| Connective tissue disease | 45 (7.9) | 118 (4.8) | 0.003 |
| Peptic ulcer disease | 26 (4.6) | 111 (4.5) | 0.958 |
| Cirrhosis | 60 (10.6) | 141 (5.8) | <0.001 |
| Diabetes mellitus without end‐organ complications | 148 (26.1) | 625 (25.5) | 0.779 |
| Diabetes mellitus with end‐organ complications | 92 (16.2) | 197 (8.0) | <0.001 |
| Paralysis | 25 (4.4) | 77 (3.1) | 0.134 |
| Renal failure | 214 (37.7) | 620 (25.3) | <0.001 |
| Underlying malignancy | 85 (15.0) | 314 (12.8) | 0.171 |
| Metastatic cancer | 64 (11.3) | 163 (6.7) | <0.001 |
| Human immunodeficiency virus | 10 (1.8) | 47 (1.9) | 0.806 |
| Minimum hemoglobin, g/dL | 9.1 [7.411.4] | 10.7 [8.712.4] | <0.001 |
| Minimum creatinine, mg/dL | 1.12 [0.792.35] | 1.03 [0.791.63] | 0.006 |
| Length of stay, d | 3.8 [1.97.8] | 3.3 [1.85.9] | <0.001 |
| ED visit in the past year | 1 [03] | 0 [01] | <0.001 |
| Clinical deterioration alert triggered | 269 (47.4) | 872 (35.6%) | <0.001 |
| Insurance | |||
| Private | 111 (19.6) | 528 (21.6) | 0.020 |
| Medicare | 299 (52.7) | 1,217 (49.7) | |
| Medicaid | 129 (22.8) | 499 (20.4) | |
| Patient pay | 28 (4.9) | 204 (8.3) | |
There were 1141 (34.4%) patients that triggered a CDA. Patients triggering a CDA were significantly more likely to have a 30‐day readmission compared to those who did not trigger a CDA (23.6% vs 15.9%; P < 0.001). Patients triggering a CDA were also significantly more likely to be readmitted within 60 days (31.7% vs 22.1%; P < 0.001) and 90 days (35.8% vs 26.2%; P < 0.001) compared to patients who did not trigger a CDA. Multiple logistic regression identified the triggering of a CDA to be independently associated with 30‐day readmission (OR: 1.40; 95% CI: 1.26‐1.55; P = 0.001) (Table 3). Other independent predictors of 30‐day readmission were: an emergency department visit in the previous 6 months, increasing age in 1‐year increments, presence of connective tissue disease, diabetes mellitus with end‐organ complications, chronic renal disease, cirrhosis, and metastatic cancer (Hosmer‐Lemeshow goodness of fit test, 0.363). Figure 1 reveals the ROC curves for the logistic regression model (Table 3) with and without the CDA variable. As the ROC curves document, the 2 models had similar sensitivity for the entire range of specificities. Reflecting this, the area under the ROC curve for the model inclusive of the CDA variable equaled 0.675 (95% CI: 0.649‐0.700), whereas the area under the ROC curve for the model excluding the CDA variable equaled 0.658 (95% CI: 0.632‐0.684).
| Variables | OR | 95% CI | P Value |
|---|---|---|---|
| |||
| Clinical deterioration alert | 1.40 | 1.261.55 | 0.001 |
| Age (1‐point increments) | 1.01 | 1.011.02 | 0.003 |
| Connective tissue disease | 1.63 | 1.341.98 | 0.012 |
| Cirrhosis | 1.25 | 1.171.33 | <0.001 |
| Diabetes mellitus with end‐organ complications | 1.23 | 1.131.33 | 0.010 |
| Chronic renal disease | 1.16 | 1.081.24 | 0.034 |
| Metastatic cancer | 1.12 | 1.081.17 | 0.002 |
| Emergency department visit in previous 6 months | 1.23 | 1.201.26 | <0.001 |

DISCUSSION
We demonstrated that the occurrence of an automated CDA is associated with increased risk for 30‐day hospital readmission. However, the addition of the CDA variable to the other variables identified to be independently associated with 30‐day readmission (Table 3) did not significantly add to the overall predictive accuracy of the derived logistic regression model. Other investigators have previously attempted to develop automated predictors of hospital readmission. Amarasingham et al. developed a real‐time electronic predictive model that identifies hospitalized heart failure patients at high risk for readmission or death from clinical and nonclinical risk factors present on admission.[13] Their electronic model demonstrated good discrimination for 30‐day mortality and readmission and performed as well, or better than, models developed by the Center for Medicaid and Medicare Services and the Acute Decompensated Heart Failure Registry. Similarly, Baillie et al. developed an automated prediction model that was effectively integrated into an existing EMR and identified patients on admission who were at risk for readmission within 30 days of discharge.[14] Our automated CDA differs from these previous risk predictors by surveying patients throughout their hospital stay as opposed to identifying risk for readmission at a single time point.
Several limitations of our study should be recognized. First, this was a noninterventional study aimed at examining the ability of CDAs to predict hospital readmission. Future studies are needed to assess whether the use of enhanced readmission prediction algorithms can be utilized to avert hospital readmissions. Second, the data derive from a single center, and this necessarily limits the generalizability of our findings. As such, our results may not reflect what one might see at other institutions. For example, Barnes‐Jewish Hospital has a regional referral pattern that includes community hospitals, regional long‐term acute care hospitals, nursing homes, and chronic wound, dialysis, and infusion clinics. This may explain, in part, the relatively high rate of hospital readmission observed in our cohort. Third, there is the possibility that CDAs were associated with readmission by chance given the number of potential predictor variables examined. The importance of CDAs as a determinant of rehospitalization requires confirmation in other independent populations. Fourth, it is likely that we did not capture all hospital readmissions, primarily those occurring outside of our hospital system. Therefore, we may have underestimated the actual rates of readmission for this cohort. Finally, we cannot be certain that all important predictors of hospital readmission were captured in this study.
The development of an accurate real‐time early warning system has the potential to identify patients at risk for various adverse outcomes including clinical deterioration, hospital death, and postdischarge readmission. By identifying patients at greatest risk for readmission, valuable healthcare resources can be better targeted to such populations. Our findings suggest that existing readmission predictors may suboptimally risk‐stratify patients, and it may be important to include additional clinical variables if pay for performance and other across‐institution comparisons are to be fair to institutions that care for more seriously ill patients. The variables identified as predictors of 30‐day hospital readmission in our study, with the exception of a CDA, are all readily identifiable clinical characteristics. The modest incremental value of a CDA to these clinical characteristics suggests that they would suffice for the identification of patients at high risk for hospital readmission. This is especially important for safety‐net institutions not routinely employing automated CDAs. These safety‐net hospitals provide a disproportionate level of care for patients who otherwise would have difficulty obtaining inpatient medical care and disproportionately carry the greatest burden of hospital readmissions.[15]
Disclosure
This study was funded in part by the Barnes‐Jewish Hospital Foundation and by grant number UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR or NIH.
Rapid response systems (RRSs) have been developed to identify and treat deteriorating patients on general hospital units.[1] The most commonly proposed approach to the problem of identifying and stabilizing deteriorating hospitalized patients includes some combination of an early warning system to detect the deterioration and an RRS to deal with it. We previously demonstrated that a relatively simple hospital‐specific prediction model employing routine laboratory values and vital sign data is capable of predicting clinical deterioration, the need for intensive care unit (ICU) transfer, and hospital mortality in patients admitted to general medicine units.[2, 3, 4, 5, 6]
Hospital readmissions within 30 days of hospital discharge occur often and are difficult to predict. Starting in 2013, readmission penalties have been applied to specific conditions in the United States (acute myocardial infarction, heart failure, and pneumonia), with the expectation that additional conditions will be added to this group in years to come.[7, 8] Unfortunately, interventions developed to date have not been universally successful in preventing hospital readmissions for various medical conditions and patient types.[9] One potential explanation for this is the inability to reliably predict which patients are at risk for readmission to better target preventative interventions. Predictors of hospital readmission can be disease specific, such as the presence of multivessel disease in patients hospitalized with myocardial infarction,[10] or more general, such as lack of available medical follow‐up postdischarge.[11] Therefore, we performed a study to determine whether the occurrence of automated clinical deterioration alerts (CDAs) predicted 30‐day hospital readmission.
METHODS
Study Location
The study was conducted on 8 general medicine units of Barnes‐Jewish Hospital, a 1250‐bed academic medical center in St. Louis, Missouri (January 15, 2015December 12, 2015). Patient care on the inpatient medicine units is delivered by either attending hospitalist physicians or housestaff physicians under the supervision of an attending physician. The study was approved by the Washington University School of Medicine Human Studies Committee, and informed consent was waived.
Study Overview
We retrospectively evaluated all adult patients (aged >18 years) admitted through the emergency department or transferred directly to the general medicine units from other institutions. We excluded patients who died while hospitalized. All data were derived from the hospital informatics database provided by the Center for Clinical Excellence, BJC HealthCare.
Primary End Point
Readmission for any reason (ie, all‐cause readmission) to an acute care facility in the 30 days following discharge after the index hospitalization served as the primary end point. Barnes‐Jewish Hospital serves as the main teaching institution for BJC Healthcare, a large integrated healthcare system of both inpatient and outpatient care. The system includes a total of 12 hospitals and multiple community health locations in a compact geographic region surrounding and including St. Louis, Missouri, and we included readmission to any of these hospitals in our analysis. Persons treated within this healthcare system are, in nearly all cases, readmitted to 1 of the system's participating 12 hospitals. If a patient who receives healthcare in the system presents to a nonsystem hospital, he/she is often transferred back into the integrated system because of issues of insurance coverage. Patients with a 30‐day readmission were compared to those without a 30‐day readmission.
Variables
We recorded information regarding demographics, median income of the zip code of residence as a marker of socioeconomic status, admission to any BJC Healthcare facility within 6 months of the index admission, and comorbidities. To represent the global burden of comorbidities in each patient, we calculated their Charlson Comorbidity Index score.[12] Severity of illness was assessed using the All Patient RefinedDiagnosis Related Groups severity of illness score.
CDA Algorithm Overview
Details regarding the CDA model development and its implementation have been previously described in detail.[4, 5, 6] In brief, we applied logistic regression techniques to develop the CDA algorithm. Manually obtained vital signs, laboratory data, and pharmacy data inputted real time into the electronic medical record (EMR) were continuously assessed. The CDA algorithm searched for the 36 input variables (Table 1) as previously described from the EMR for all patients admitted to the 8 medicine units 24 hours per day and 7 days a week.[4, 5, 6] Values for every continuous parameter were scaled so that all measurements lay in the interval (0, 1) and were normalized by the minimum and maximum of the parameter. To capture the temporal effects in our data, we retain a sliding window of all the collected data points within the last 24 hours. We then subdivide these data into a series of n equally sized buckets (eg, 6 sequential buckets of 4 hours each). To capture variations within a bucket, we compute 3 values for each bucket: the minimum, maximum, and mean data points. Each of the resulting 3 n values are input to the logistic regression equation as separate variables.
| Age |
| Alanine aminotransferase |
| Alternative medicines |
| Anion gap |
| Anti‐infectives |
| Antineoplastics |
| Aspartate aminotransferase |
| Biologicals |
| Blood pressure, diastolic |
| Blood pressure, systolic |
| Calcium, serum |
| Calcium, serum, ionized |
| Cardiovascular agents |
| Central nervous system agents |
| Charlson Comorbidity Index |
| Coagulation modifiers |
| Estimated creatinine clearance |
| Gastrointestinal agents |
| Genitourinary tract agents |
| Hormones/hormone modifiers |
| Immunologic agents |
| Magnesium, serum |
| Metabolic agents |
| Miscellaneous agents |
| Nutritional products |
| Oxygen saturation, pulse oximetry |
| Phosphate, serum |
| Potassium, serum |
| Psychotherapeutic agents |
| Pulse |
| Radiologic agents |
| Respirations |
| Respiratory agents |
| Shock Index |
| Temperature |
| Topical agents |
The algorithm was first implemented in MATLAB (MathWorks, Natick, MA). For the purposes of training, we used a single 24‐hour window of data from each patient. The dataset's 36 input variables were divided into buckets and minimum/mean/maximum features wherever applicable, resulting in 398 variables. The first half of the original dataset was used to train the model. We then used the second half of the dataset as the validation dataset. We generated a predicted outcome for each case in the validation data, using the model parameter coefficients derived from the training data. We also employed bootstrap aggregation to improve classification accuracy and to address overfitting. We then applied various threshold cut points to convert these predictions into binary values and compared the results against the ICU transfer outcome. A threshold of 0.9760 for specificity was chosen to achieve a sensitivity of approximately 40%. These operating characteristics were chosen in turn to generate a manageable number of alerts per hospital nursing unit per day (estimated at 12 per nursing unit per day). At this cut point the C statistic was 0.8834, with an overall accuracy of 0.9292.[5] Patients with inputted data meeting the CDA threshold had a real‐time alert sent to the hospital rapid response team prompting a patient evaluation.
Statistical Analysis
The number of patients admitted to the 8 general medicine units of Barnes‐Jewish Hospital during the study period determined the sample size. Categorical variables were compared using 2 or Fisher exact test as appropriate. Continuous variables were compared using the Mann‐Whitney U test. All analyses were 2‐tailed, and a P value of <0.05 was assumed to represent statistical significance. We relied on logistic regression for identifying variables independently associated with 30‐day readmission. Based on univariate analysis, variables significant at P < 0.15 were entered into the model. To arrive at the most parsimonious model, we utilized a stepwise backward elimination approach. We evaluated collinearity with the variance inflation factor. We report adjusted odds ratios (ORs) and 95% confidence intervals (CIs) where appropriate. The model's goodness of fit was assessed via calculation of the Hosmer‐Lemeshow test. Receiver operating characteristic (ROC) curves were used to compare the predictive models for 30‐day readmission with or without the CDA variable. All statistical analyses were performed using SPSS (version 22.0; IBM, Armonk, NY).
RESULTS
The final cohort had 3015 patients with a mean age of 57.5 17.5 years and 47.8% males. The most common reasons for hospital admission were infection or sepsis syndrome including pneumonia and urinary tract infections (23.6%), congestive heart failure or other cardiac conditions (18.4%), respiratory distress including chronic obstructive pulmonary disease (16.2%), acute or chronic renal failure (9.7%), gastrointestinal disorders (8.4%), and diabetes mellitus management (7.4%). Overall, there were 567 (18.8%) patients who were readmitted within 30 days of their hospital discharge date.
Table 2 shows the characteristics of patients readmitted within 30 days and of patients not requiring hospital readmission within 30 days. Patients requiring hospital readmission within 30 days were younger and had significantly more comorbidities as manifested by significantly greater Charlson scores and individual comorbidities including coronary artery disease, congestive heart disease, peripheral vascular disease, connective tissue disease, cirrhosis, diabetes mellitus with end‐organ complications, renal failure, and metastatic cancer. Patients with a 30‐day readmission had significantly longer duration of hospitalization, more emergency department visits in the 6 months prior to the index hospitalization, lower minimum hemoglobin measurements, higher minimum serum creatinine values, and were more likely to have Medicare or Medicaid insurance compared to patients without a 30‐day readmission.
| Variable | 30‐Day Readmission | P Value | |
|---|---|---|---|
| Yes (n = 567) | No (n = 2,448) | ||
| |||
| Age, y | 56.1 17.0 | 57.8 17.6 | 0.046 |
| Gender | |||
| Male | 252 (44.4) | 1,188 (48.5) | 0.079 |
| Female | 315 (55.6) | 1,260 (51.5) | |
| Race | |||
| Caucasian | 277 (48.9) | 1,234 (50.4) | 0.800 |
| African American | 257 (45.3) | 1,076 (44.0) | |
| Other | 33 (5.8) | 138 (5.6) | |
| Median income, dollars | 30,149 [25,23436,453] | 29,271 [24,83037,026] | 0.903 |
| BMI | 29.4 10.0 | 29.0 9.2 | 0.393 |
| APR‐DRG Severity of Illness Score | 2.6 0.4 | 2.5 0.5 | 0.152 |
| Charlson Comorbidity Index | 6 [39] | 5 [27] | <0.001 |
| ICU transfer during admission | 93 (16.4) | 410 (16.7) | 0.842 |
| Myocardial infarction | 83 (14.6) | 256 (10.5) | 0.005 |
| Congestive heart failure | 177 (31.2) | 540 (22.1) | <0.001 |
| Peripheral vascular disease | 76 (13.4) | 214 (8.7) | 0.001 |
| Cardiovascular disease | 69 (12.2) | 224 (9.2) | 0.029 |
| Dementia | 15 (2.6) | 80 (3.3) | 0.445 |
| Chronic obstructive pulmonary disease | 220 (38.8) | 855 (34.9) | 0.083 |
| Connective tissue disease | 45 (7.9) | 118 (4.8) | 0.003 |
| Peptic ulcer disease | 26 (4.6) | 111 (4.5) | 0.958 |
| Cirrhosis | 60 (10.6) | 141 (5.8) | <0.001 |
| Diabetes mellitus without end‐organ complications | 148 (26.1) | 625 (25.5) | 0.779 |
| Diabetes mellitus with end‐organ complications | 92 (16.2) | 197 (8.0) | <0.001 |
| Paralysis | 25 (4.4) | 77 (3.1) | 0.134 |
| Renal failure | 214 (37.7) | 620 (25.3) | <0.001 |
| Underlying malignancy | 85 (15.0) | 314 (12.8) | 0.171 |
| Metastatic cancer | 64 (11.3) | 163 (6.7) | <0.001 |
| Human immunodeficiency virus | 10 (1.8) | 47 (1.9) | 0.806 |
| Minimum hemoglobin, g/dL | 9.1 [7.411.4] | 10.7 [8.712.4] | <0.001 |
| Minimum creatinine, mg/dL | 1.12 [0.792.35] | 1.03 [0.791.63] | 0.006 |
| Length of stay, d | 3.8 [1.97.8] | 3.3 [1.85.9] | <0.001 |
| ED visit in the past year | 1 [03] | 0 [01] | <0.001 |
| Clinical deterioration alert triggered | 269 (47.4) | 872 (35.6%) | <0.001 |
| Insurance | |||
| Private | 111 (19.6) | 528 (21.6) | 0.020 |
| Medicare | 299 (52.7) | 1,217 (49.7) | |
| Medicaid | 129 (22.8) | 499 (20.4) | |
| Patient pay | 28 (4.9) | 204 (8.3) | |
There were 1141 (34.4%) patients that triggered a CDA. Patients triggering a CDA were significantly more likely to have a 30‐day readmission compared to those who did not trigger a CDA (23.6% vs 15.9%; P < 0.001). Patients triggering a CDA were also significantly more likely to be readmitted within 60 days (31.7% vs 22.1%; P < 0.001) and 90 days (35.8% vs 26.2%; P < 0.001) compared to patients who did not trigger a CDA. Multiple logistic regression identified the triggering of a CDA to be independently associated with 30‐day readmission (OR: 1.40; 95% CI: 1.26‐1.55; P = 0.001) (Table 3). Other independent predictors of 30‐day readmission were: an emergency department visit in the previous 6 months, increasing age in 1‐year increments, presence of connective tissue disease, diabetes mellitus with end‐organ complications, chronic renal disease, cirrhosis, and metastatic cancer (Hosmer‐Lemeshow goodness of fit test, 0.363). Figure 1 reveals the ROC curves for the logistic regression model (Table 3) with and without the CDA variable. As the ROC curves document, the 2 models had similar sensitivity for the entire range of specificities. Reflecting this, the area under the ROC curve for the model inclusive of the CDA variable equaled 0.675 (95% CI: 0.649‐0.700), whereas the area under the ROC curve for the model excluding the CDA variable equaled 0.658 (95% CI: 0.632‐0.684).
| Variables | OR | 95% CI | P Value |
|---|---|---|---|
| |||
| Clinical deterioration alert | 1.40 | 1.261.55 | 0.001 |
| Age (1‐point increments) | 1.01 | 1.011.02 | 0.003 |
| Connective tissue disease | 1.63 | 1.341.98 | 0.012 |
| Cirrhosis | 1.25 | 1.171.33 | <0.001 |
| Diabetes mellitus with end‐organ complications | 1.23 | 1.131.33 | 0.010 |
| Chronic renal disease | 1.16 | 1.081.24 | 0.034 |
| Metastatic cancer | 1.12 | 1.081.17 | 0.002 |
| Emergency department visit in previous 6 months | 1.23 | 1.201.26 | <0.001 |

DISCUSSION
We demonstrated that the occurrence of an automated CDA is associated with increased risk for 30‐day hospital readmission. However, the addition of the CDA variable to the other variables identified to be independently associated with 30‐day readmission (Table 3) did not significantly add to the overall predictive accuracy of the derived logistic regression model. Other investigators have previously attempted to develop automated predictors of hospital readmission. Amarasingham et al. developed a real‐time electronic predictive model that identifies hospitalized heart failure patients at high risk for readmission or death from clinical and nonclinical risk factors present on admission.[13] Their electronic model demonstrated good discrimination for 30‐day mortality and readmission and performed as well, or better than, models developed by the Center for Medicaid and Medicare Services and the Acute Decompensated Heart Failure Registry. Similarly, Baillie et al. developed an automated prediction model that was effectively integrated into an existing EMR and identified patients on admission who were at risk for readmission within 30 days of discharge.[14] Our automated CDA differs from these previous risk predictors by surveying patients throughout their hospital stay as opposed to identifying risk for readmission at a single time point.
Several limitations of our study should be recognized. First, this was a noninterventional study aimed at examining the ability of CDAs to predict hospital readmission. Future studies are needed to assess whether the use of enhanced readmission prediction algorithms can be utilized to avert hospital readmissions. Second, the data derive from a single center, and this necessarily limits the generalizability of our findings. As such, our results may not reflect what one might see at other institutions. For example, Barnes‐Jewish Hospital has a regional referral pattern that includes community hospitals, regional long‐term acute care hospitals, nursing homes, and chronic wound, dialysis, and infusion clinics. This may explain, in part, the relatively high rate of hospital readmission observed in our cohort. Third, there is the possibility that CDAs were associated with readmission by chance given the number of potential predictor variables examined. The importance of CDAs as a determinant of rehospitalization requires confirmation in other independent populations. Fourth, it is likely that we did not capture all hospital readmissions, primarily those occurring outside of our hospital system. Therefore, we may have underestimated the actual rates of readmission for this cohort. Finally, we cannot be certain that all important predictors of hospital readmission were captured in this study.
The development of an accurate real‐time early warning system has the potential to identify patients at risk for various adverse outcomes including clinical deterioration, hospital death, and postdischarge readmission. By identifying patients at greatest risk for readmission, valuable healthcare resources can be better targeted to such populations. Our findings suggest that existing readmission predictors may suboptimally risk‐stratify patients, and it may be important to include additional clinical variables if pay for performance and other across‐institution comparisons are to be fair to institutions that care for more seriously ill patients. The variables identified as predictors of 30‐day hospital readmission in our study, with the exception of a CDA, are all readily identifiable clinical characteristics. The modest incremental value of a CDA to these clinical characteristics suggests that they would suffice for the identification of patients at high risk for hospital readmission. This is especially important for safety‐net institutions not routinely employing automated CDAs. These safety‐net hospitals provide a disproportionate level of care for patients who otherwise would have difficulty obtaining inpatient medical care and disproportionately carry the greatest burden of hospital readmissions.[15]
Disclosure
This study was funded in part by the Barnes‐Jewish Hospital Foundation and by grant number UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR or NIH.
- , , . Rapid‐response teams. N Engl J Med. 2011;365:139–146.
- , , , , , . Early prediction of septic shock in hospitalized patients. J Hosp Med. 2010;5:19–25.
- , , , et al. Implementation of a real‐time computerized sepsis alert in nonintensive care unit patients. Crit Care Med. 2011;39:469–473.
- , , , et al. Toward a two‐tier clinical warning system for hospitalized patients. AMIA Annu Symp Proc. 2011;2011:511–519.
- , , , et al. A trial of a real‐time alert for clinical deterioration in patients hospitalized on general medical wards. J Hosp Med. 2013;8:236–242.
- , , , et al. A randomized trial of real‐time automated clinical deterioration alerts sent to a rapid response team. J Hosp Med. 2014;9:424–429.
- , . Revisiting hospital readmissions JAMA. 2013;309:398–400.
- , , , . Adverse outcomes associated with delayed intensive care unit transfers in an integrated healthcare system. J Hosp Med. 2012;7:224–230.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011; 155:520–528.
- , , , et al. International variation in and factors associated with hospital readmission after myocardial infarction. JAMA. 2012;307:66–74.
- , , , , . Predictors of early readmission among patients 40 to 64 years of age hospitalized for chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11:685–694.
- , , , , , . Assessing illness severity: does clinical judgement work? J Chronic Dis. 1986;39:439–452.
- , , , et al. An automated model to identify heart failure patients at risk for 30‐day readmission or death using electronic medical record data. Med Care. 2010;48:981–988.
- , , , et al. The readmission risk flag: using the electronic health record to automatically identify patients at risk for 30‐day readmission. J Hosp Med. 2013;8:689–695.
- , , . The Medicare hospital readmissions reduction program: time for reform. JAMA. 2015;314:347–348.
- , , . Rapid‐response teams. N Engl J Med. 2011;365:139–146.
- , , , , , . Early prediction of septic shock in hospitalized patients. J Hosp Med. 2010;5:19–25.
- , , , et al. Implementation of a real‐time computerized sepsis alert in nonintensive care unit patients. Crit Care Med. 2011;39:469–473.
- , , , et al. Toward a two‐tier clinical warning system for hospitalized patients. AMIA Annu Symp Proc. 2011;2011:511–519.
- , , , et al. A trial of a real‐time alert for clinical deterioration in patients hospitalized on general medical wards. J Hosp Med. 2013;8:236–242.
- , , , et al. A randomized trial of real‐time automated clinical deterioration alerts sent to a rapid response team. J Hosp Med. 2014;9:424–429.
- , . Revisiting hospital readmissions JAMA. 2013;309:398–400.
- , , , . Adverse outcomes associated with delayed intensive care unit transfers in an integrated healthcare system. J Hosp Med. 2012;7:224–230.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011; 155:520–528.
- , , , et al. International variation in and factors associated with hospital readmission after myocardial infarction. JAMA. 2012;307:66–74.
- , , , , . Predictors of early readmission among patients 40 to 64 years of age hospitalized for chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11:685–694.
- , , , , , . Assessing illness severity: does clinical judgement work? J Chronic Dis. 1986;39:439–452.
- , , , et al. An automated model to identify heart failure patients at risk for 30‐day readmission or death using electronic medical record data. Med Care. 2010;48:981–988.
- , , , et al. The readmission risk flag: using the electronic health record to automatically identify patients at risk for 30‐day readmission. J Hosp Med. 2013;8:689–695.
- , , . The Medicare hospital readmissions reduction program: time for reform. JAMA. 2015;314:347–348.
MAGS Prevalence in Older Adults
Geriatric syndromes are common clinical conditions in older adults that do not fall into specific disease categories. Unlike the traditional definition of a syndrome, geriatric syndrome refers to a condition that is mediated by multiple shared underlying risk factors.[1, 2] Conditions commonly referred to as geriatric syndromes include delirium, cognitive impairment, falls, unintentional weight loss, depressive symptoms, and incontinence. Even though many perceive it as medical misnomer,[3] geriatric syndromes have been shown to negatively impact quality of life and activities of daily living in older adults.[2] They are also associated with adverse outcomes such as increased healthcare utilization, functional decline, and mortality, even after adjusting for age and disease severity.[4, 5, 6] Hospitalized older adults, including those discharged to skilled nursing facilities (SNFs)[7, 8] are particularly at high risk for new‐onset or exacerbation of geriatric syndromes and poor outcomes.[7, 9, 10] However, hospital providers seldom assess, manage, or document geriatric syndromes because they are often overshadowed by disease conditions that lead to an acute episode requiring hospitalization (e.g., heart disease).[11]
Pharmacotherapy is the cornerstone of hospital treatment, and it is well‐known that it affects multiple physiologic systems causing side effects apart from the condition they are approved to treat. Given that geriatric syndromes are a result of impairments in multiple organ systems, it is plausible that pharmacotherapy may initiate or worsen these syndromes.[12] Medication‐related problems in older adults are well known. Polypharmacy and adverse drug events (as a result of drug‐drug/disease interactions and changes in pharmacokinetics and pharmacodynamics) are prevalent in multimorbid elderly patients.[13, 14, 15, 16] The prescribing cascade[17] increases the medication burden and may be a contributing factor for geriatric syndromes in hospitalized patients.[18] For instance, laxatives may be prescribed to counteract constipation caused by anticholinergic drugs.
The American Geriatric Society (AGS) Beers list[19, 20] and similar criteria[21] provide excellent resources to identify medications with potentially harmful interactions or adverse effects in older adults. Although these lists include medicines associated with a specific geriatric syndrome, they were not developed to explicitly link medicines across multiple geriatric syndromes, regardless of indication or appropriateness. For example, medications that effect important geriatric syndromes like unintentional weight/appetite loss, depression, and urinary incontinence are not extensively covered. In addition, disease‐appropriate medications (eg, ‐blockers for systolic heart failure), that may be associated with a geriatric syndrome (eg, falls) are not included; however, they may be important to consider for a patient and clinician who are weighing the disease benefits compared to the geriatric syndrome‐related risks. Finally, the AGS 2015 Beers criteria panel mentions the limitation that many medication associations may be excluded because older adults are less represented in clinical trials.[20] Clinicians are currently limited in identifying medications potentially contributing to a broad set of geriatric syndromes in their patients without a specific list of medications associated with geriatric syndromes (MAGS).[20]
In response to this gap, identifying these medications is important and should be a starting point in efforts toward prevention and treatment of geriatric syndromes. The 2 main objectives of this study were to first identify medications that may meaningfully contribute to 6 geriatric syndromes and subsequently describe the frequency of these medications in a population transitioning from acute care to postacute care to highlight the need and potential impact of such a list.
METHODS
This study included 2 phases that aligned with our 2 primary objectives. Phase 1 involved identifying medications associated with 6 geriatric syndromes, and phase 2 included a cross‐sectional analysis of the prevalence of these medications in a sample of patients discharged to SNFs.
Phase 1: Development of the MAGS List
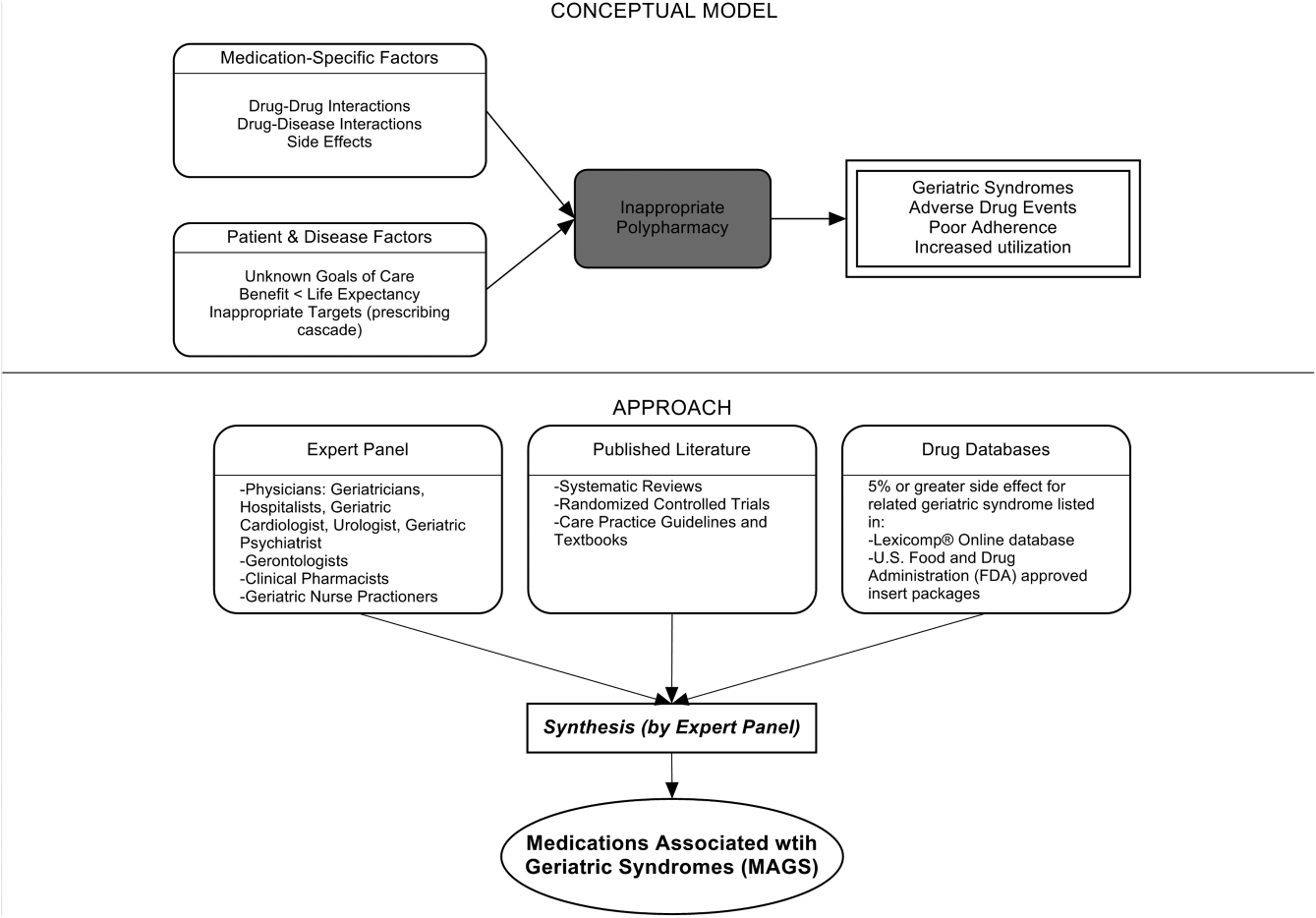
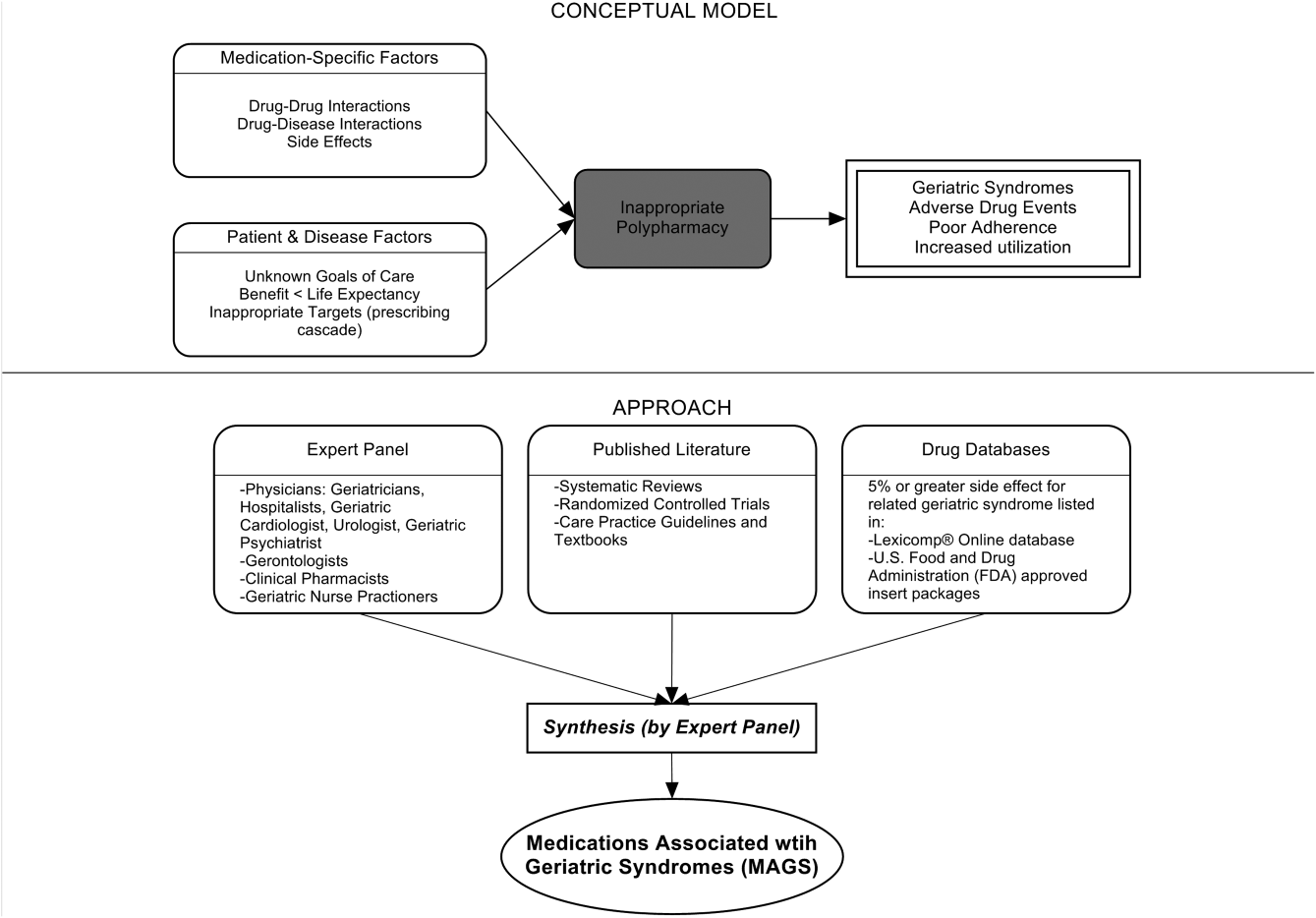
Figure 1 depicts the underlying conceptual model and approach that was used in phase 1. The interaction between the patient factors and medication leads to polypharmacy that contributes to geriatric syndromes and additional adverse outcomes. As a starting point for mitigating geriatric syndromes, we used an iterative analytical process to identify a list of medications associated with the following geriatric syndromes that were documented to be highly prevalent in patients discharged to SNFs: cognitive impairment, delirium, falls, unintentional weight and/or appetite loss, urinary incontinence, and depression.[8] To be inclusive and sensitive, our approach differed from traditional systematic reviews, and in fact was meant to bring together much of the established systematic literature about disparate geriatric syndromes in 1 place, because patients often do not experience a geriatric syndrome in isolation, but rather experience multiple geriatric syndromes.[8] The MAGS list had 3 main inclusion criteria (Figure 1): (1) evidence in the published literature (systematic reviews, cohort studies, randomized clinical trials) that the medication is related to the syndrome, (2) expert panel opinion, and (3) drug databases (Lexicomp Online database[22] and/or US Food and Drug Administration [FDA]approved package inserts).[23] We generated an initial list of medications based on these 3 main criteria to identify medications with significant associations to each geriatric syndrome. The list was further expanded and vetted using an iterative review of each medication list as it related to each geriatric syndrome through a series of group meetings focused around each geriatric syndrome. Following further discussion, we obtained agreement among all team members for medications included in the final list. For each geriatric syndrome, we excluded medications from consideration if they were used to treat the same geriatric syndrome (eg, ‐adrenergic blockers used to treat incontinence in men were listed as associated with incontinence only in women). We classified medications according to the Established Pharmacologic Class available at the FDA website. We also compared our final MAGS list with the 2015 AGS Beer's list[20] by identifying medications that were related to the 6 geriatric syndromes. This included Beers[20]‐cited rationale of anticholinergic, extrapyramidal symptoms, orthostatic hypotension (eg, falls), high‐risk adverse central nervous system effects, sedating, cognitive decline (eg, antipsychotics), delirium, falls, fractures, incontinence, and gastrointestinal (eg, nausea, vomiting). Specifically, we assessed whether the medications were included as inappropriate by the AGS Beers 2015[20] list and also whether they documented the syndrome association for that medication.
Phase 2: Prevalence of MAGS in Hospitalized Older Adults Discharged to SNFs
Sample
We next applied the MAGS list to a convenience sample of hospitalized patients discharged to SNFs to assess the prevalence of MAGS in this sample, and also to compare with the prevalence of Beers criteria[20] medications. Our sample was selected from data collected as part of a quality‐improvement project to reduce hospital readmissions in patients discharged to SNFs. The larger study enrolled a total 1093 medical and surgical patients who had Medicare insurance eligibility and were discharged from 1 large university hospital to 23 area SNFs from January 17, 2013 through July 31, 2014. The university institutional review board waived the requirement for written consent. For the purpose of this substudy. we selected the first 154 patients with complete chart abstraction (approximately 15% of the total) as a convenience sample.
Data Analysis
We applied descriptive statistics to summarize demographic and clinical characteristics of the convenience sample. To understand potential selection biases that could have resulted by the convenience sampling, we compared participant characteristics of the convenience sample (N = 154) with the characteristics of the remaining participants of the larger study (N = 939) using independent sample t tests and 2 tests for continuous and categorical measures, respectively. We applied the MAGS list and the AGS 2015 Beers criteria[20] for the sample of 154 and identified the medications associated with each of the 6 geriatric syndromes from the discharge medication lists completed by hospital clinical pharmacists. For each patient, we identified both scheduled and PRN (pro re nata, or as needed) medications associated with each geriatric syndrome. Thereafter, we determined whether the discharge list contained at least 1 medication associated with a geriatric syndrome per the MAGS list and the AGS Beers 2015 criteria,[20] and the percentage of overall medications that were part of the MAGS and Beers lists. Data were aggregated using means and standard deviations across syndromes (ie, number of discharge medications per syndrome per patient) along with the percentage of patients with 1 or more medications related to a specific syndrome and the percentage of medications that were MAGS. All analyses were performed using the SPSS statistical package (IBM SPSS Statistics for Windows, version 23.0; IBM, Armonk, NY).
RESULTS
Phase 1: MAGS List
The iterative process applied in this analysis generated a list of 513 medications associated with the 6 geriatric syndromes. The list of medications related to each syndrome and the corresponding rationale and relevant references for their inclusion is presented in the Supporting Information, Appendix 1, in the online version of this article. Table 1 summarizes these medications across 18 major drug categories. Antiepileptics were linked to all 6 geriatric syndromes, whereas antipsychotics, antidepressants, antiparkinsonism, and opioid agonists were associated with 5 syndromes. Ten of the 18 categories were associated with 3 geriatric syndromescognitive impairment, delirium, and falls. Four medication categories were associated with only 1 syndrome. Nonopioid/nonsteroidal anti‐inflammatory and/or analgesics and nonopioid cough suppressant and expectorant medications were associated with falls syndrome only. Hormone replacement medications were associated with depression only, and immunosuppressants were associated with unintentional weight and appetite loss only.
| Major Medication Category | Delirium | Cognitive Impairment | Falls | Unintentional Weight and Appetite Loss | Urinary Incontinence | Depression | Drug Class/Drug Within Each Category |
|---|---|---|---|---|---|---|---|
| |||||||
| Antipsychotics | ✓ | ✓ | ✓ | ✓ | Atypical and typical antipsychotics, buspirone | ||
| Antidepressants | ✓ | ✓ | ✓ | ✓ | ✓ | Tricyclic and tetracyclic antidepressants, serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitor, aminoketone | |
| Antiepileptics | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Antiepileptics, mood stabilizers, barbiturates |
| Antiparkinsonism | ✓ | ✓ | ✓ | ✓ | ✓ | Aromatic amino acid decarboxylation inhibitor and catechol‐o‐methyltransferase inhibitor, catecholamine‐depleting sympatholytic, catechol‐o‐methyltransferase inhibitor, dopaminergic agonist, ergot derivative, monoamine oxidase inhibitor, nonergot dopamine agonist, | |
| Benzodiazapines | ✓ | ✓ | ✓ | Benzodiazapines only | |||
| Nonbenzodiazepine hypnotics | ✓ | ✓ | ✓ | Benzodiazepine analogs, nonbenzodiazepine hypnotics, tranquilizers, ‐aminobutyric acid A receptor agonist | |||
| Opioid agonists | ✓ | ✓ | ✓ | ✓ | ✓ | Full or partial opioid agonists, opiates, opioids | |
| Nonopioid/nonsteroidal anti‐inflammatory and/or analgesics | ✓ | Nonopioid analgesics, NSAIDs, COX‐2 selective inhibitor NSAIDs | |||||
| Antihypertensives | ✓ | ✓ | ✓ | Calcium channel blocker, ‐adrenergic blocker, angiotensin‐converting enzyme inhibitor, angiotensin 2 receptor blocker, ‐adrenergic blocker, diuretics (loop, potassium sparing, thiazide), nitrate vasodilators, aldosterone blocker | |||
| Antiarrhythmic | ✓ | ✓ | ✓ | Antiarrhythmics, cardiac glycosides | |||
| Antidiabetics | ✓ | ✓ | Insulin and insulin analogs, sulfonylureas, ‐glucosidase inhibitor, amylin analog, biguanide, glinide, GLP‐1 receptor agonist, glucagon‐like peptide‐1 agonist | ||||
| Anticholinergics and/or antihistaminics | ✓ | ✓ | ✓ | ✓ | Anticholinergics, histamine receptor antagonists, muscarininc antagonists, combined anticholinergics, and histamine receptor antagonists | ||
| Antiemetics | ✓ | ✓ | ✓ | Antiemetics, dopaminergic antagonists, dopamine‐2 receptor antagonist | |||
| Hormone replacement | ✓ | Corticosteroids, progestin, estrogen, estrogen agonist/antagonist, gonadotropin releasing hormone receptor agonist | |||||
| Muscle relaxers | ✓ | ✓ | ✓ | ✓ | Muscle relaxers | ||
| Immunosuppressants | ✓ | Calcineurin inhibitor immunosuppressant, folate analog metabolic inhibitor, purine antimetabolite | |||||
| Nonopioid cough suppressants and expectorants | ✓ | Expectorant, non‐narcotic antitussive, ‐1 agonist, uncompetitive N‐methyl‐D‐aspartate receptor antagonist | |||||
| Antimicrobials | ✓ | ✓ | Macrolide, cephalosporin, penicillin class, rifamycin, non‐nucleoside analog reverse transcriptase inhibitor, influenza A M2 protein inhibitor | ||||
| Others | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ‐3‐adrenergic agonist, methylxanthine, cholinesterase inhibitor, interferon and , partial cholinergic nicotinic agonist, tyrosine hydroxylase, retinoid, serotonin‐1b and serotonin‐1d receptor agonist, stimulant laxative, vitamin K antagonist, platelet aggregation inhibitor |
Approximately 58% of the medications overlapped with the AGS 2015 Beer's Criteria[20] irrespective of whether the specific syndrome association was stated in the rationale.[20] Medications that overlapped were mostly in the delirium, cognitive impairment, and falls category with only a few overlaps in depression, unintentional weight loss, and urinary incontinence lists (see Supporting Information, Appendix 1, in the online version of this article).
Phase 2: Prevalence of MAGS
Among 154 participants, the mean age was 76.5 (10.6) years, 64.3% were female, 77.9% were white, and 96.1% non‐Hispanic. The median hospital length of stay was 6 days, with an interquartile range of 5 days. The orthopedic service discharged the highest proportion of patients (24%), followed by the geriatrics and internal medicine services, which each discharged 19.5% of the patients (Table 2). The remaining participants of the larger quality‐improvement project (N = 939) did not significantly differ on these demographic and clinical characteristics except for hospital length of stay, which was shorter in the sample analyzed (see Supporting Information, Appendix 2, in the online version of this article).
| Baseline Characteristics | Mean ( SD) or Percent (n) |
|---|---|
| |
| Age, y | 76.5 ( 10.6) |
| Sex | |
| Female | 64.3% (99) |
| Race | |
| White | 77.9% (126) |
| Black | 16.2% (25) |
| Unknown | 0.6% (1) |
| Declined | 0.6% (1) |
| Missing | 0.6% (1) |
| Ethnicity | |
| Non‐Hispanic | 96.1% (148) |
| Hispanic | 1.3% (2) |
| Unknown | 2.6% (4) |
| Hospital length of stay, d | 7.0 ( 4.2) |
| Hospital length of stay, d, median (IQR) | 6.0 (5.0) |
| No. of hospital discharge medications, count | 14.0 ( 4.7) |
| Discharge service | |
| Orthopedic service | 24.0% (37) |
| Geriatric service | 19.5% (30) |
| Internal medicine | 19.5% (30) |
| Other | 37.0% (57) |
Patients were discharged to SNFs with an average of 14.0 (4.7) medication orders. Overall, 43% (13%) of these discharge medication orders were MAGS. Every patient in the sample was ordered at least 1 medication associated with geriatric syndromes. Multiple MAGS were the norm, with an average of 5.9 (2.2) MAGS per patient. MAGS were also the norm, as 98.1% of the sample had medication orders associated with at least 2 different syndromes.
When the Beer's criteria[20] were applied to the medication orders (instead of the MAGS list), problematic medications appeared less common. Patients had an average of 3.04 (1.7) MAGS that were also listed on the AGS 2015 Beer's list,[20] representing an average of 22.3% of all discharge orders.
Table 3 illustrates the average number of medications per patient associated with each syndrome, and the percentage of patients (number in parentheses) discharged with at least 1 medication associated with each syndrome per the MAGS list and the Beers 2015 criteria.[20] For example, per the MAGS list, the syndrome most frequently associated with medications was falls, with patients discharged on an average of 5.5 (2.2) medications associated with falls, and 100% of the sample had at least 1 discharge medication associated with falls. Alternatively, the syndrome associated with the lowest frequency of medications was unintentional weight loss (with an average of 0.38 medications per patient), although 36% of these patients had more than 1 discharge medication associated with weight loss. As seen in Table 3, the mean and prevalence of 1 or more medications associated with each of the geriatric syndromes as identified by the Beers 2015 criteria[20] was lower than those identified by the MAGS list developed for this study.
| Geriatric Syndromes | Associated Medications per MAGS List | Associated Medications per AGS Beers 2015 Criteria | ||
|---|---|---|---|---|
| Mean SD | Percentage of Patients Receiving 1 Related Medication | Mean SD | Percentage of Patients Receiving 1 Related Medication | |
| ||||
| Cognitive impairment | 1.8 ( 1.2) | 84.4% (130) | 1.6 ( 1.2) | 78.6% (121) |
| Delirium | 1.4 ( 1.1) | 76.0% (117) | 1.3 ( 1.2) | 68.2% (105) |
| Falls | 5.5 ( 2.2) | 100% (154) | 2.6 ( 1.6) | 92.2% (142) |
| Unintentional weight and/or appetite loss | 0.4 ( 0.5) | 36.3% (56) | 0.1 ( 0.3) | 6.5% (10) |
| Urinary incontinence | 1.6 ( 1.0) | 85.7% (132) | 0.1 ( 0.2) | 5.8% (9) |
| Depression | 1.7 ( 1.0) | 90.9% (140) | 0.0 ( 0.0) | 0.0% (0) |
| All syndromes | 5.9 ( 2.2) | 100% (154) | 3.0 ( 1.7) | 95% (149) |
DISCUSSION
An iterative process of evidence review by a multidisciplinary panel resulted in a list of 513 medications associated with 6 common geriatric syndromes. This analysis demonstrated that hospitalized, older patients discharged to SNFs were frequently prescribed MAGS. The rate of prescribing ranged from 100% of patients with a medication associated with falls to 36% for unintentional weight loss. Moreover, an alarming 43% of all medications at hospital discharge were MAGS. For this vulnerable population, the combination of high prevalence of MAGS and high risk of geriatric syndromes emphasize a need to critically review the risks and benefits of MAGS throughout hospitalization and at the time of discharge.
A body of evidence demonstrates that many drugs in a typical older adult regimen have no specific clinical indication, are considered inappropriate, or have uncertain efficacy in the geriatric population.[24, 25, 26] This study builds on the foundational work described in landmark reviews such as the AGS Beers[20] and STOPP/START[21] (Screening Tool of Older Persons' Potentially Inappropriate Prescriptions/Screening Tool to Alert doctors to Right, i.e. appropriate indicated Treatment) criteria. Both of these tools, however, were specifically designed as screening tools to identify medications considered unsafe for older adults under most circumstances and within specific illness states.[19, 20, 21] They are most often utilized when starting a medication to avoid acute adverse events. In contrast, the MAGS list was developed to be inclusive of medications that are often appropriate for many medical diagnoses but may also contribute to underlying geriatric syndromes that are more chronic in nature. In addition, inclusion of such medicines increases the sensitivity of screening for medications that can be targeted through patient‐centered deprescribing efforts when clinically appropriate.
A major strength of this study is that we bring together evidence across a spectrum of geriatric syndromes commonly experienced by hospitalized elders. In addition to evaluating multiple syndromes, we applied multiple modalities; particularly the use of an iterative review process by a multidisciplinary team of experts and using Lexicomp and FDA insert packages for linking medications to specific geriatric conditions. The inclusion criteria were broadened beyond single sources of evidence in an effort to capture a comprehensive list of medications. As a result, the MAGS list can be implemented as a screening tool for deprescribing interventions and assessing medication appropriateness to address individual or clusters of geriatric syndromes within a patient.
In addition to expanding this knowledge base, clinical relevance of the MAGS list is highlighted by its application to a sample of hospitalized older adults discharged to SNFs, a cohort known to experience geriatric syndromes. In fact, 43% of patients' medications at hospital discharge were MAGS. Importantly, due to the cross‐sectional nature of this study, we cannot be certain if the medication caused or potentiated each of the geriatric syndromes. However, hospitals and SNFs are devoting major resources toward reduction of falls, avoidance of urinary catheter use, and reduction of preventable readmissions. These efforts can be complemented by considering the number of medications associated with falls, urinary incontinence, and overall MAGS burden. The striking prevalence of MAGS demonstrates a rigorous need to weigh the risks and benefits of these medications. Above all, the intent of this study is not to propose that any MAGS be reflexively stopped, but rather that the MAGS list should facilitate a holistic approach to care for the complex older adult. For example, standard therapies such as gabapentin may be appropriate for treating neuralgic pain but may also contribute to falls and urinary incontinence. Thus, alternative pain treatments could be selected in place of gabapentin for a 75‐year old patient who is experiencing recurrent falls and increasing incontinence. Therefore, the MAGS list enables a patient‐provider discussion wherein medications' therapeutic benefits can be weighed against risks posed by specific clusters of geriatric syndromes, potential impact on quality of life, and consistency with goals of care.
This study has some limitations. First, although we examined a broad number of geriatric syndromes, several other geriatric syndromes experienced by hospitalized older adults were not addressed including: fecal incontinence, insomnia, and functional impairment. These syndromes were intentionally excluded from the study a priori due to reasons of feasibility and scope. Second, unlike the Beer's 2015 criteria, the MAGS list does not sub‐classify associations of medications with geriatric syndromes for patients with specific diseases (eg, heart failure). In fact, our MAGS list included medications often indicated in treating these diagnoses. A clinician must work with the patient to weigh the disease‐specific benefits of some medications with the potential effect on geriatric syndrome symptoms and outcomes. Third, the instrument has a very high sensitivity, which was intended to generate an inclusive list of medications that enables providers to weigh risks of geriatric syndromes with the intended indication benefit. The objective is not to use this list as a reflexive tool but rather help clinicians identify a starting point to address geriatric syndromes in their patients to make patient‐centered medication decisions. Although the MAGS list is intentionally large (sensitive), the advent of advanced bioinformatics can enable MAGS to be assessed in the future for both clinical and research purposes. Fourth, FDA insert packages and Lexicomp databases report anything experienced by the patient while on the particular medication, but it might not necessarily imply a causative link. The high use of MAGS and the specific geriatric syndrome may coexist due to the high prevalence and interplay of multimorbidity, polypharmacy, and geriatric syndromes in this population. Last, the list was developed by expert panel members predominantly from a single institution, which may introduce bias. Despite these limitations, the prevalence of these medications in a sample of patients transitioning from acute to postacute care highlights the utility of the MAGS list in future clinical research and quality improvement endeavors.
In conclusion, the MAGS list provides a comprehensive and sensitive indicator of medications associated with any of 6 geriatric syndromes regardless of medication indication and appropriateness. The MAGS list provides an overall degree of medication burden with respect to geriatric syndromes and a foundation for future research to assess the relationship between the presence of geriatric syndromes and syndrome‐associated medications. The MAGS list is an important first step in summarizing the data that link medications to geriatric syndromes. Future studies are needed to broaden the analysis of MAGS for other common geriatric syndromes and to identify new and emerging medications not present during the time of this analysis. The MAGS list has the potential to facilitate deprescribing efforts needed to combat the epidemic of overprescribing that may be contributing to the burden of geriatric syndromes among older patients.
Acknowledgements
The authors thank Dr. Linda Beuscher, Dr. Patricia Blair Miller, Dr. Joseph Ouslander, Dr. William Stuart Reynolds, and Dr. Warren Taylor for providing their expertise and participating in the expert panel discussions that facilitated the development of the MAGS list. The authors also recognize the research support provided by Christopher Simon Coelho.
Disclosures: This research was supported by the Department of Health and Human Services, Centers for Medicare & Medicaid Services grant #1C1CMS331006 awarded to Principal Investigator, John F. Schnelle, PhD. Dr. Vasilevskis was supported by the National Institute on Aging of the National Institutes of Health award K23AG040157 and the Geriatric Research, Education and Clinical Center. Dr. Bell was supported by National Institute on Aging‐K award K23AG048347‐01A1. Dr. Mixon is supported by a Veterans Affairs Health Services Research & Development Career Development Award (12‐168). This research was also supported by the National Center for Advancing Translational Sciences Clinical and Translational Science award UL1TR000445. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the US Department of Health and Human Services or any of its agencies, the National Center for Advancing Translation Science, the National Institutes of Health, or the Department of Veterans Affairs. Each coauthor contributed significantly to the manuscript. Dr. Kripalani has received stock/stock options from Bioscape Digital, LLC. None of the other authors have significant conflicts of interest to report related to this project or the results reported within this article.
- , , , . Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–791.
- , , , . Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes. JAMA. 1995;273:1348–1353.
- , , , . Geriatric syndromes: medical misnomer or progress in geriatrics? Neth J Med. 2003;61:83–87.
- , , , et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one‐year survival and functional decline. PLoS One. 2011;6:e26951.
- , , , , . Geriatric conditions as predictors of increased number of hospital admissions and hospital bed days over one year: findings of a nationwide cohort of older adults from Taiwan. Arch Gerontol Geriatr. 2014;59:169–174.
- , , , , . Geriatric conditions and disability: the Health and Retirement Study. Ann Intern Med. 2007;147:156–164.
- , , , , , . A prospective cohort study of geriatric syndromes among older medical patients admitted to acute care hospitals. J Am Geriatr Soc. 2011;59:2001–2008.
- , , , et al. Geriatric syndromes in hospitalized older adults discharged to skilled nursing facilities. J Am Geriatr Soc. 2016;64(4):715–722.
- , , , et al. Discharge to a skilled nursing facility and subsequent clinical outcomes among older patients hospitalized for heart failure. Circ Heart Fail. 2011;4:293–300.
- . Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219–223.
- , , , , . Geriatric syndromes in elderly patients admitted to an inpatient cardiology ward. J Hosp Med. 2007;2:394–400.
- , , , . Effect of hospitalization on inappropriate prescribing in elderly Medicare beneficiaries. J Am Geriatr Soc. 2015;63:699–707.
- , , , et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community‐dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012;65:989–995.
- , , , , . Investigating polypharmacy and drug burden index in hospitalised older people. Intern Med J. 2013;43:912–918.
- , . Potentially harmful drug‐drug interactions in the elderly: a review. Am J Geriatr Pharmacother. 2011;9:364–377.
- , , , . Hospital admissions/visits associated with drug‐drug interactions: a systematic review and meta‐analysis. Pharmacoepidemiol Drug Saf. 2014;23:489–497.
- , . Optimising drug treatment for elderly people: the prescribing cascade. BMJ. 1997;315:1096–1099.
- , , , et al. Association between acute geriatric syndromes and medication‐related hospital admissions. Drugs Aging. 2012;29:691–699.
- American Geriatrics Society Beers Criteria Update Expert P. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616–631.
- By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227–2246.
- , . STOPP (Screening Tool of Older Persons' potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers' criteria. Age Ageing. 2008;37:673–679.
- , , , et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370:493–503.
- U.S. Food and Drug Administration. Drugs. Available at: http://www.fda.gov/Drugs/default.htm. Accessed May 15th, 2015.
- , , , et al. Inappropriate medication use among frail elderly inpatients. Ann Pharmacother. 2004;38:9–14.
- , , , et al. Inappropriate medications in elderly ICU survivors: where to intervene? Arch Intern Med. 2011;171:1032–1034.
- , , , et al. Appropriateness of medication prescribing in ambulatory elderly patients. J Am Geriatr Soc. 1994;42:1241–1247.
Geriatric syndromes are common clinical conditions in older adults that do not fall into specific disease categories. Unlike the traditional definition of a syndrome, geriatric syndrome refers to a condition that is mediated by multiple shared underlying risk factors.[1, 2] Conditions commonly referred to as geriatric syndromes include delirium, cognitive impairment, falls, unintentional weight loss, depressive symptoms, and incontinence. Even though many perceive it as medical misnomer,[3] geriatric syndromes have been shown to negatively impact quality of life and activities of daily living in older adults.[2] They are also associated with adverse outcomes such as increased healthcare utilization, functional decline, and mortality, even after adjusting for age and disease severity.[4, 5, 6] Hospitalized older adults, including those discharged to skilled nursing facilities (SNFs)[7, 8] are particularly at high risk for new‐onset or exacerbation of geriatric syndromes and poor outcomes.[7, 9, 10] However, hospital providers seldom assess, manage, or document geriatric syndromes because they are often overshadowed by disease conditions that lead to an acute episode requiring hospitalization (e.g., heart disease).[11]
Pharmacotherapy is the cornerstone of hospital treatment, and it is well‐known that it affects multiple physiologic systems causing side effects apart from the condition they are approved to treat. Given that geriatric syndromes are a result of impairments in multiple organ systems, it is plausible that pharmacotherapy may initiate or worsen these syndromes.[12] Medication‐related problems in older adults are well known. Polypharmacy and adverse drug events (as a result of drug‐drug/disease interactions and changes in pharmacokinetics and pharmacodynamics) are prevalent in multimorbid elderly patients.[13, 14, 15, 16] The prescribing cascade[17] increases the medication burden and may be a contributing factor for geriatric syndromes in hospitalized patients.[18] For instance, laxatives may be prescribed to counteract constipation caused by anticholinergic drugs.
The American Geriatric Society (AGS) Beers list[19, 20] and similar criteria[21] provide excellent resources to identify medications with potentially harmful interactions or adverse effects in older adults. Although these lists include medicines associated with a specific geriatric syndrome, they were not developed to explicitly link medicines across multiple geriatric syndromes, regardless of indication or appropriateness. For example, medications that effect important geriatric syndromes like unintentional weight/appetite loss, depression, and urinary incontinence are not extensively covered. In addition, disease‐appropriate medications (eg, ‐blockers for systolic heart failure), that may be associated with a geriatric syndrome (eg, falls) are not included; however, they may be important to consider for a patient and clinician who are weighing the disease benefits compared to the geriatric syndrome‐related risks. Finally, the AGS 2015 Beers criteria panel mentions the limitation that many medication associations may be excluded because older adults are less represented in clinical trials.[20] Clinicians are currently limited in identifying medications potentially contributing to a broad set of geriatric syndromes in their patients without a specific list of medications associated with geriatric syndromes (MAGS).[20]
In response to this gap, identifying these medications is important and should be a starting point in efforts toward prevention and treatment of geriatric syndromes. The 2 main objectives of this study were to first identify medications that may meaningfully contribute to 6 geriatric syndromes and subsequently describe the frequency of these medications in a population transitioning from acute care to postacute care to highlight the need and potential impact of such a list.
METHODS
This study included 2 phases that aligned with our 2 primary objectives. Phase 1 involved identifying medications associated with 6 geriatric syndromes, and phase 2 included a cross‐sectional analysis of the prevalence of these medications in a sample of patients discharged to SNFs.
Phase 1: Development of the MAGS List
Figure 1 depicts the underlying conceptual model and approach that was used in phase 1. The interaction between the patient factors and medication leads to polypharmacy that contributes to geriatric syndromes and additional adverse outcomes. As a starting point for mitigating geriatric syndromes, we used an iterative analytical process to identify a list of medications associated with the following geriatric syndromes that were documented to be highly prevalent in patients discharged to SNFs: cognitive impairment, delirium, falls, unintentional weight and/or appetite loss, urinary incontinence, and depression.[8] To be inclusive and sensitive, our approach differed from traditional systematic reviews, and in fact was meant to bring together much of the established systematic literature about disparate geriatric syndromes in 1 place, because patients often do not experience a geriatric syndrome in isolation, but rather experience multiple geriatric syndromes.[8] The MAGS list had 3 main inclusion criteria (Figure 1): (1) evidence in the published literature (systematic reviews, cohort studies, randomized clinical trials) that the medication is related to the syndrome, (2) expert panel opinion, and (3) drug databases (Lexicomp Online database[22] and/or US Food and Drug Administration [FDA]approved package inserts).[23] We generated an initial list of medications based on these 3 main criteria to identify medications with significant associations to each geriatric syndrome. The list was further expanded and vetted using an iterative review of each medication list as it related to each geriatric syndrome through a series of group meetings focused around each geriatric syndrome. Following further discussion, we obtained agreement among all team members for medications included in the final list. For each geriatric syndrome, we excluded medications from consideration if they were used to treat the same geriatric syndrome (eg, ‐adrenergic blockers used to treat incontinence in men were listed as associated with incontinence only in women). We classified medications according to the Established Pharmacologic Class available at the FDA website. We also compared our final MAGS list with the 2015 AGS Beer's list[20] by identifying medications that were related to the 6 geriatric syndromes. This included Beers[20]‐cited rationale of anticholinergic, extrapyramidal symptoms, orthostatic hypotension (eg, falls), high‐risk adverse central nervous system effects, sedating, cognitive decline (eg, antipsychotics), delirium, falls, fractures, incontinence, and gastrointestinal (eg, nausea, vomiting). Specifically, we assessed whether the medications were included as inappropriate by the AGS Beers 2015[20] list and also whether they documented the syndrome association for that medication.

Phase 2: Prevalence of MAGS in Hospitalized Older Adults Discharged to SNFs
Sample
We next applied the MAGS list to a convenience sample of hospitalized patients discharged to SNFs to assess the prevalence of MAGS in this sample, and also to compare with the prevalence of Beers criteria[20] medications. Our sample was selected from data collected as part of a quality‐improvement project to reduce hospital readmissions in patients discharged to SNFs. The larger study enrolled a total 1093 medical and surgical patients who had Medicare insurance eligibility and were discharged from 1 large university hospital to 23 area SNFs from January 17, 2013 through July 31, 2014. The university institutional review board waived the requirement for written consent. For the purpose of this substudy. we selected the first 154 patients with complete chart abstraction (approximately 15% of the total) as a convenience sample.
Data Analysis
We applied descriptive statistics to summarize demographic and clinical characteristics of the convenience sample. To understand potential selection biases that could have resulted by the convenience sampling, we compared participant characteristics of the convenience sample (N = 154) with the characteristics of the remaining participants of the larger study (N = 939) using independent sample t tests and 2 tests for continuous and categorical measures, respectively. We applied the MAGS list and the AGS 2015 Beers criteria[20] for the sample of 154 and identified the medications associated with each of the 6 geriatric syndromes from the discharge medication lists completed by hospital clinical pharmacists. For each patient, we identified both scheduled and PRN (pro re nata, or as needed) medications associated with each geriatric syndrome. Thereafter, we determined whether the discharge list contained at least 1 medication associated with a geriatric syndrome per the MAGS list and the AGS Beers 2015 criteria,[20] and the percentage of overall medications that were part of the MAGS and Beers lists. Data were aggregated using means and standard deviations across syndromes (ie, number of discharge medications per syndrome per patient) along with the percentage of patients with 1 or more medications related to a specific syndrome and the percentage of medications that were MAGS. All analyses were performed using the SPSS statistical package (IBM SPSS Statistics for Windows, version 23.0; IBM, Armonk, NY).
RESULTS
Phase 1: MAGS List
The iterative process applied in this analysis generated a list of 513 medications associated with the 6 geriatric syndromes. The list of medications related to each syndrome and the corresponding rationale and relevant references for their inclusion is presented in the Supporting Information, Appendix 1, in the online version of this article. Table 1 summarizes these medications across 18 major drug categories. Antiepileptics were linked to all 6 geriatric syndromes, whereas antipsychotics, antidepressants, antiparkinsonism, and opioid agonists were associated with 5 syndromes. Ten of the 18 categories were associated with 3 geriatric syndromescognitive impairment, delirium, and falls. Four medication categories were associated with only 1 syndrome. Nonopioid/nonsteroidal anti‐inflammatory and/or analgesics and nonopioid cough suppressant and expectorant medications were associated with falls syndrome only. Hormone replacement medications were associated with depression only, and immunosuppressants were associated with unintentional weight and appetite loss only.
| Major Medication Category | Delirium | Cognitive Impairment | Falls | Unintentional Weight and Appetite Loss | Urinary Incontinence | Depression | Drug Class/Drug Within Each Category |
|---|---|---|---|---|---|---|---|
| |||||||
| Antipsychotics | ✓ | ✓ | ✓ | ✓ | Atypical and typical antipsychotics, buspirone | ||
| Antidepressants | ✓ | ✓ | ✓ | ✓ | ✓ | Tricyclic and tetracyclic antidepressants, serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitor, aminoketone | |
| Antiepileptics | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Antiepileptics, mood stabilizers, barbiturates |
| Antiparkinsonism | ✓ | ✓ | ✓ | ✓ | ✓ | Aromatic amino acid decarboxylation inhibitor and catechol‐o‐methyltransferase inhibitor, catecholamine‐depleting sympatholytic, catechol‐o‐methyltransferase inhibitor, dopaminergic agonist, ergot derivative, monoamine oxidase inhibitor, nonergot dopamine agonist, | |
| Benzodiazapines | ✓ | ✓ | ✓ | Benzodiazapines only | |||
| Nonbenzodiazepine hypnotics | ✓ | ✓ | ✓ | Benzodiazepine analogs, nonbenzodiazepine hypnotics, tranquilizers, ‐aminobutyric acid A receptor agonist | |||
| Opioid agonists | ✓ | ✓ | ✓ | ✓ | ✓ | Full or partial opioid agonists, opiates, opioids | |
| Nonopioid/nonsteroidal anti‐inflammatory and/or analgesics | ✓ | Nonopioid analgesics, NSAIDs, COX‐2 selective inhibitor NSAIDs | |||||
| Antihypertensives | ✓ | ✓ | ✓ | Calcium channel blocker, ‐adrenergic blocker, angiotensin‐converting enzyme inhibitor, angiotensin 2 receptor blocker, ‐adrenergic blocker, diuretics (loop, potassium sparing, thiazide), nitrate vasodilators, aldosterone blocker | |||
| Antiarrhythmic | ✓ | ✓ | ✓ | Antiarrhythmics, cardiac glycosides | |||
| Antidiabetics | ✓ | ✓ | Insulin and insulin analogs, sulfonylureas, ‐glucosidase inhibitor, amylin analog, biguanide, glinide, GLP‐1 receptor agonist, glucagon‐like peptide‐1 agonist | ||||
| Anticholinergics and/or antihistaminics | ✓ | ✓ | ✓ | ✓ | Anticholinergics, histamine receptor antagonists, muscarininc antagonists, combined anticholinergics, and histamine receptor antagonists | ||
| Antiemetics | ✓ | ✓ | ✓ | Antiemetics, dopaminergic antagonists, dopamine‐2 receptor antagonist | |||
| Hormone replacement | ✓ | Corticosteroids, progestin, estrogen, estrogen agonist/antagonist, gonadotropin releasing hormone receptor agonist | |||||
| Muscle relaxers | ✓ | ✓ | ✓ | ✓ | Muscle relaxers | ||
| Immunosuppressants | ✓ | Calcineurin inhibitor immunosuppressant, folate analog metabolic inhibitor, purine antimetabolite | |||||
| Nonopioid cough suppressants and expectorants | ✓ | Expectorant, non‐narcotic antitussive, ‐1 agonist, uncompetitive N‐methyl‐D‐aspartate receptor antagonist | |||||
| Antimicrobials | ✓ | ✓ | Macrolide, cephalosporin, penicillin class, rifamycin, non‐nucleoside analog reverse transcriptase inhibitor, influenza A M2 protein inhibitor | ||||
| Others | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ‐3‐adrenergic agonist, methylxanthine, cholinesterase inhibitor, interferon and , partial cholinergic nicotinic agonist, tyrosine hydroxylase, retinoid, serotonin‐1b and serotonin‐1d receptor agonist, stimulant laxative, vitamin K antagonist, platelet aggregation inhibitor |
Approximately 58% of the medications overlapped with the AGS 2015 Beer's Criteria[20] irrespective of whether the specific syndrome association was stated in the rationale.[20] Medications that overlapped were mostly in the delirium, cognitive impairment, and falls category with only a few overlaps in depression, unintentional weight loss, and urinary incontinence lists (see Supporting Information, Appendix 1, in the online version of this article).
Phase 2: Prevalence of MAGS
Among 154 participants, the mean age was 76.5 (10.6) years, 64.3% were female, 77.9% were white, and 96.1% non‐Hispanic. The median hospital length of stay was 6 days, with an interquartile range of 5 days. The orthopedic service discharged the highest proportion of patients (24%), followed by the geriatrics and internal medicine services, which each discharged 19.5% of the patients (Table 2). The remaining participants of the larger quality‐improvement project (N = 939) did not significantly differ on these demographic and clinical characteristics except for hospital length of stay, which was shorter in the sample analyzed (see Supporting Information, Appendix 2, in the online version of this article).
| Baseline Characteristics | Mean ( SD) or Percent (n) |
|---|---|
| |
| Age, y | 76.5 ( 10.6) |
| Sex | |
| Female | 64.3% (99) |
| Race | |
| White | 77.9% (126) |
| Black | 16.2% (25) |
| Unknown | 0.6% (1) |
| Declined | 0.6% (1) |
| Missing | 0.6% (1) |
| Ethnicity | |
| Non‐Hispanic | 96.1% (148) |
| Hispanic | 1.3% (2) |
| Unknown | 2.6% (4) |
| Hospital length of stay, d | 7.0 ( 4.2) |
| Hospital length of stay, d, median (IQR) | 6.0 (5.0) |
| No. of hospital discharge medications, count | 14.0 ( 4.7) |
| Discharge service | |
| Orthopedic service | 24.0% (37) |
| Geriatric service | 19.5% (30) |
| Internal medicine | 19.5% (30) |
| Other | 37.0% (57) |
Patients were discharged to SNFs with an average of 14.0 (4.7) medication orders. Overall, 43% (13%) of these discharge medication orders were MAGS. Every patient in the sample was ordered at least 1 medication associated with geriatric syndromes. Multiple MAGS were the norm, with an average of 5.9 (2.2) MAGS per patient. MAGS were also the norm, as 98.1% of the sample had medication orders associated with at least 2 different syndromes.
When the Beer's criteria[20] were applied to the medication orders (instead of the MAGS list), problematic medications appeared less common. Patients had an average of 3.04 (1.7) MAGS that were also listed on the AGS 2015 Beer's list,[20] representing an average of 22.3% of all discharge orders.
Table 3 illustrates the average number of medications per patient associated with each syndrome, and the percentage of patients (number in parentheses) discharged with at least 1 medication associated with each syndrome per the MAGS list and the Beers 2015 criteria.[20] For example, per the MAGS list, the syndrome most frequently associated with medications was falls, with patients discharged on an average of 5.5 (2.2) medications associated with falls, and 100% of the sample had at least 1 discharge medication associated with falls. Alternatively, the syndrome associated with the lowest frequency of medications was unintentional weight loss (with an average of 0.38 medications per patient), although 36% of these patients had more than 1 discharge medication associated with weight loss. As seen in Table 3, the mean and prevalence of 1 or more medications associated with each of the geriatric syndromes as identified by the Beers 2015 criteria[20] was lower than those identified by the MAGS list developed for this study.
| Geriatric Syndromes | Associated Medications per MAGS List | Associated Medications per AGS Beers 2015 Criteria | ||
|---|---|---|---|---|
| Mean SD | Percentage of Patients Receiving 1 Related Medication | Mean SD | Percentage of Patients Receiving 1 Related Medication | |
| ||||
| Cognitive impairment | 1.8 ( 1.2) | 84.4% (130) | 1.6 ( 1.2) | 78.6% (121) |
| Delirium | 1.4 ( 1.1) | 76.0% (117) | 1.3 ( 1.2) | 68.2% (105) |
| Falls | 5.5 ( 2.2) | 100% (154) | 2.6 ( 1.6) | 92.2% (142) |
| Unintentional weight and/or appetite loss | 0.4 ( 0.5) | 36.3% (56) | 0.1 ( 0.3) | 6.5% (10) |
| Urinary incontinence | 1.6 ( 1.0) | 85.7% (132) | 0.1 ( 0.2) | 5.8% (9) |
| Depression | 1.7 ( 1.0) | 90.9% (140) | 0.0 ( 0.0) | 0.0% (0) |
| All syndromes | 5.9 ( 2.2) | 100% (154) | 3.0 ( 1.7) | 95% (149) |
DISCUSSION
An iterative process of evidence review by a multidisciplinary panel resulted in a list of 513 medications associated with 6 common geriatric syndromes. This analysis demonstrated that hospitalized, older patients discharged to SNFs were frequently prescribed MAGS. The rate of prescribing ranged from 100% of patients with a medication associated with falls to 36% for unintentional weight loss. Moreover, an alarming 43% of all medications at hospital discharge were MAGS. For this vulnerable population, the combination of high prevalence of MAGS and high risk of geriatric syndromes emphasize a need to critically review the risks and benefits of MAGS throughout hospitalization and at the time of discharge.
A body of evidence demonstrates that many drugs in a typical older adult regimen have no specific clinical indication, are considered inappropriate, or have uncertain efficacy in the geriatric population.[24, 25, 26] This study builds on the foundational work described in landmark reviews such as the AGS Beers[20] and STOPP/START[21] (Screening Tool of Older Persons' Potentially Inappropriate Prescriptions/Screening Tool to Alert doctors to Right, i.e. appropriate indicated Treatment) criteria. Both of these tools, however, were specifically designed as screening tools to identify medications considered unsafe for older adults under most circumstances and within specific illness states.[19, 20, 21] They are most often utilized when starting a medication to avoid acute adverse events. In contrast, the MAGS list was developed to be inclusive of medications that are often appropriate for many medical diagnoses but may also contribute to underlying geriatric syndromes that are more chronic in nature. In addition, inclusion of such medicines increases the sensitivity of screening for medications that can be targeted through patient‐centered deprescribing efforts when clinically appropriate.
A major strength of this study is that we bring together evidence across a spectrum of geriatric syndromes commonly experienced by hospitalized elders. In addition to evaluating multiple syndromes, we applied multiple modalities; particularly the use of an iterative review process by a multidisciplinary team of experts and using Lexicomp and FDA insert packages for linking medications to specific geriatric conditions. The inclusion criteria were broadened beyond single sources of evidence in an effort to capture a comprehensive list of medications. As a result, the MAGS list can be implemented as a screening tool for deprescribing interventions and assessing medication appropriateness to address individual or clusters of geriatric syndromes within a patient.
In addition to expanding this knowledge base, clinical relevance of the MAGS list is highlighted by its application to a sample of hospitalized older adults discharged to SNFs, a cohort known to experience geriatric syndromes. In fact, 43% of patients' medications at hospital discharge were MAGS. Importantly, due to the cross‐sectional nature of this study, we cannot be certain if the medication caused or potentiated each of the geriatric syndromes. However, hospitals and SNFs are devoting major resources toward reduction of falls, avoidance of urinary catheter use, and reduction of preventable readmissions. These efforts can be complemented by considering the number of medications associated with falls, urinary incontinence, and overall MAGS burden. The striking prevalence of MAGS demonstrates a rigorous need to weigh the risks and benefits of these medications. Above all, the intent of this study is not to propose that any MAGS be reflexively stopped, but rather that the MAGS list should facilitate a holistic approach to care for the complex older adult. For example, standard therapies such as gabapentin may be appropriate for treating neuralgic pain but may also contribute to falls and urinary incontinence. Thus, alternative pain treatments could be selected in place of gabapentin for a 75‐year old patient who is experiencing recurrent falls and increasing incontinence. Therefore, the MAGS list enables a patient‐provider discussion wherein medications' therapeutic benefits can be weighed against risks posed by specific clusters of geriatric syndromes, potential impact on quality of life, and consistency with goals of care.
This study has some limitations. First, although we examined a broad number of geriatric syndromes, several other geriatric syndromes experienced by hospitalized older adults were not addressed including: fecal incontinence, insomnia, and functional impairment. These syndromes were intentionally excluded from the study a priori due to reasons of feasibility and scope. Second, unlike the Beer's 2015 criteria, the MAGS list does not sub‐classify associations of medications with geriatric syndromes for patients with specific diseases (eg, heart failure). In fact, our MAGS list included medications often indicated in treating these diagnoses. A clinician must work with the patient to weigh the disease‐specific benefits of some medications with the potential effect on geriatric syndrome symptoms and outcomes. Third, the instrument has a very high sensitivity, which was intended to generate an inclusive list of medications that enables providers to weigh risks of geriatric syndromes with the intended indication benefit. The objective is not to use this list as a reflexive tool but rather help clinicians identify a starting point to address geriatric syndromes in their patients to make patient‐centered medication decisions. Although the MAGS list is intentionally large (sensitive), the advent of advanced bioinformatics can enable MAGS to be assessed in the future for both clinical and research purposes. Fourth, FDA insert packages and Lexicomp databases report anything experienced by the patient while on the particular medication, but it might not necessarily imply a causative link. The high use of MAGS and the specific geriatric syndrome may coexist due to the high prevalence and interplay of multimorbidity, polypharmacy, and geriatric syndromes in this population. Last, the list was developed by expert panel members predominantly from a single institution, which may introduce bias. Despite these limitations, the prevalence of these medications in a sample of patients transitioning from acute to postacute care highlights the utility of the MAGS list in future clinical research and quality improvement endeavors.
In conclusion, the MAGS list provides a comprehensive and sensitive indicator of medications associated with any of 6 geriatric syndromes regardless of medication indication and appropriateness. The MAGS list provides an overall degree of medication burden with respect to geriatric syndromes and a foundation for future research to assess the relationship between the presence of geriatric syndromes and syndrome‐associated medications. The MAGS list is an important first step in summarizing the data that link medications to geriatric syndromes. Future studies are needed to broaden the analysis of MAGS for other common geriatric syndromes and to identify new and emerging medications not present during the time of this analysis. The MAGS list has the potential to facilitate deprescribing efforts needed to combat the epidemic of overprescribing that may be contributing to the burden of geriatric syndromes among older patients.
Acknowledgements
The authors thank Dr. Linda Beuscher, Dr. Patricia Blair Miller, Dr. Joseph Ouslander, Dr. William Stuart Reynolds, and Dr. Warren Taylor for providing their expertise and participating in the expert panel discussions that facilitated the development of the MAGS list. The authors also recognize the research support provided by Christopher Simon Coelho.
Disclosures: This research was supported by the Department of Health and Human Services, Centers for Medicare & Medicaid Services grant #1C1CMS331006 awarded to Principal Investigator, John F. Schnelle, PhD. Dr. Vasilevskis was supported by the National Institute on Aging of the National Institutes of Health award K23AG040157 and the Geriatric Research, Education and Clinical Center. Dr. Bell was supported by National Institute on Aging‐K award K23AG048347‐01A1. Dr. Mixon is supported by a Veterans Affairs Health Services Research & Development Career Development Award (12‐168). This research was also supported by the National Center for Advancing Translational Sciences Clinical and Translational Science award UL1TR000445. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the US Department of Health and Human Services or any of its agencies, the National Center for Advancing Translation Science, the National Institutes of Health, or the Department of Veterans Affairs. Each coauthor contributed significantly to the manuscript. Dr. Kripalani has received stock/stock options from Bioscape Digital, LLC. None of the other authors have significant conflicts of interest to report related to this project or the results reported within this article.
Geriatric syndromes are common clinical conditions in older adults that do not fall into specific disease categories. Unlike the traditional definition of a syndrome, geriatric syndrome refers to a condition that is mediated by multiple shared underlying risk factors.[1, 2] Conditions commonly referred to as geriatric syndromes include delirium, cognitive impairment, falls, unintentional weight loss, depressive symptoms, and incontinence. Even though many perceive it as medical misnomer,[3] geriatric syndromes have been shown to negatively impact quality of life and activities of daily living in older adults.[2] They are also associated with adverse outcomes such as increased healthcare utilization, functional decline, and mortality, even after adjusting for age and disease severity.[4, 5, 6] Hospitalized older adults, including those discharged to skilled nursing facilities (SNFs)[7, 8] are particularly at high risk for new‐onset or exacerbation of geriatric syndromes and poor outcomes.[7, 9, 10] However, hospital providers seldom assess, manage, or document geriatric syndromes because they are often overshadowed by disease conditions that lead to an acute episode requiring hospitalization (e.g., heart disease).[11]
Pharmacotherapy is the cornerstone of hospital treatment, and it is well‐known that it affects multiple physiologic systems causing side effects apart from the condition they are approved to treat. Given that geriatric syndromes are a result of impairments in multiple organ systems, it is plausible that pharmacotherapy may initiate or worsen these syndromes.[12] Medication‐related problems in older adults are well known. Polypharmacy and adverse drug events (as a result of drug‐drug/disease interactions and changes in pharmacokinetics and pharmacodynamics) are prevalent in multimorbid elderly patients.[13, 14, 15, 16] The prescribing cascade[17] increases the medication burden and may be a contributing factor for geriatric syndromes in hospitalized patients.[18] For instance, laxatives may be prescribed to counteract constipation caused by anticholinergic drugs.
The American Geriatric Society (AGS) Beers list[19, 20] and similar criteria[21] provide excellent resources to identify medications with potentially harmful interactions or adverse effects in older adults. Although these lists include medicines associated with a specific geriatric syndrome, they were not developed to explicitly link medicines across multiple geriatric syndromes, regardless of indication or appropriateness. For example, medications that effect important geriatric syndromes like unintentional weight/appetite loss, depression, and urinary incontinence are not extensively covered. In addition, disease‐appropriate medications (eg, ‐blockers for systolic heart failure), that may be associated with a geriatric syndrome (eg, falls) are not included; however, they may be important to consider for a patient and clinician who are weighing the disease benefits compared to the geriatric syndrome‐related risks. Finally, the AGS 2015 Beers criteria panel mentions the limitation that many medication associations may be excluded because older adults are less represented in clinical trials.[20] Clinicians are currently limited in identifying medications potentially contributing to a broad set of geriatric syndromes in their patients without a specific list of medications associated with geriatric syndromes (MAGS).[20]
In response to this gap, identifying these medications is important and should be a starting point in efforts toward prevention and treatment of geriatric syndromes. The 2 main objectives of this study were to first identify medications that may meaningfully contribute to 6 geriatric syndromes and subsequently describe the frequency of these medications in a population transitioning from acute care to postacute care to highlight the need and potential impact of such a list.
METHODS
This study included 2 phases that aligned with our 2 primary objectives. Phase 1 involved identifying medications associated with 6 geriatric syndromes, and phase 2 included a cross‐sectional analysis of the prevalence of these medications in a sample of patients discharged to SNFs.
Phase 1: Development of the MAGS List
Figure 1 depicts the underlying conceptual model and approach that was used in phase 1. The interaction between the patient factors and medication leads to polypharmacy that contributes to geriatric syndromes and additional adverse outcomes. As a starting point for mitigating geriatric syndromes, we used an iterative analytical process to identify a list of medications associated with the following geriatric syndromes that were documented to be highly prevalent in patients discharged to SNFs: cognitive impairment, delirium, falls, unintentional weight and/or appetite loss, urinary incontinence, and depression.[8] To be inclusive and sensitive, our approach differed from traditional systematic reviews, and in fact was meant to bring together much of the established systematic literature about disparate geriatric syndromes in 1 place, because patients often do not experience a geriatric syndrome in isolation, but rather experience multiple geriatric syndromes.[8] The MAGS list had 3 main inclusion criteria (Figure 1): (1) evidence in the published literature (systematic reviews, cohort studies, randomized clinical trials) that the medication is related to the syndrome, (2) expert panel opinion, and (3) drug databases (Lexicomp Online database[22] and/or US Food and Drug Administration [FDA]approved package inserts).[23] We generated an initial list of medications based on these 3 main criteria to identify medications with significant associations to each geriatric syndrome. The list was further expanded and vetted using an iterative review of each medication list as it related to each geriatric syndrome through a series of group meetings focused around each geriatric syndrome. Following further discussion, we obtained agreement among all team members for medications included in the final list. For each geriatric syndrome, we excluded medications from consideration if they were used to treat the same geriatric syndrome (eg, ‐adrenergic blockers used to treat incontinence in men were listed as associated with incontinence only in women). We classified medications according to the Established Pharmacologic Class available at the FDA website. We also compared our final MAGS list with the 2015 AGS Beer's list[20] by identifying medications that were related to the 6 geriatric syndromes. This included Beers[20]‐cited rationale of anticholinergic, extrapyramidal symptoms, orthostatic hypotension (eg, falls), high‐risk adverse central nervous system effects, sedating, cognitive decline (eg, antipsychotics), delirium, falls, fractures, incontinence, and gastrointestinal (eg, nausea, vomiting). Specifically, we assessed whether the medications were included as inappropriate by the AGS Beers 2015[20] list and also whether they documented the syndrome association for that medication.

Phase 2: Prevalence of MAGS in Hospitalized Older Adults Discharged to SNFs
Sample
We next applied the MAGS list to a convenience sample of hospitalized patients discharged to SNFs to assess the prevalence of MAGS in this sample, and also to compare with the prevalence of Beers criteria[20] medications. Our sample was selected from data collected as part of a quality‐improvement project to reduce hospital readmissions in patients discharged to SNFs. The larger study enrolled a total 1093 medical and surgical patients who had Medicare insurance eligibility and were discharged from 1 large university hospital to 23 area SNFs from January 17, 2013 through July 31, 2014. The university institutional review board waived the requirement for written consent. For the purpose of this substudy. we selected the first 154 patients with complete chart abstraction (approximately 15% of the total) as a convenience sample.
Data Analysis
We applied descriptive statistics to summarize demographic and clinical characteristics of the convenience sample. To understand potential selection biases that could have resulted by the convenience sampling, we compared participant characteristics of the convenience sample (N = 154) with the characteristics of the remaining participants of the larger study (N = 939) using independent sample t tests and 2 tests for continuous and categorical measures, respectively. We applied the MAGS list and the AGS 2015 Beers criteria[20] for the sample of 154 and identified the medications associated with each of the 6 geriatric syndromes from the discharge medication lists completed by hospital clinical pharmacists. For each patient, we identified both scheduled and PRN (pro re nata, or as needed) medications associated with each geriatric syndrome. Thereafter, we determined whether the discharge list contained at least 1 medication associated with a geriatric syndrome per the MAGS list and the AGS Beers 2015 criteria,[20] and the percentage of overall medications that were part of the MAGS and Beers lists. Data were aggregated using means and standard deviations across syndromes (ie, number of discharge medications per syndrome per patient) along with the percentage of patients with 1 or more medications related to a specific syndrome and the percentage of medications that were MAGS. All analyses were performed using the SPSS statistical package (IBM SPSS Statistics for Windows, version 23.0; IBM, Armonk, NY).
RESULTS
Phase 1: MAGS List
The iterative process applied in this analysis generated a list of 513 medications associated with the 6 geriatric syndromes. The list of medications related to each syndrome and the corresponding rationale and relevant references for their inclusion is presented in the Supporting Information, Appendix 1, in the online version of this article. Table 1 summarizes these medications across 18 major drug categories. Antiepileptics were linked to all 6 geriatric syndromes, whereas antipsychotics, antidepressants, antiparkinsonism, and opioid agonists were associated with 5 syndromes. Ten of the 18 categories were associated with 3 geriatric syndromescognitive impairment, delirium, and falls. Four medication categories were associated with only 1 syndrome. Nonopioid/nonsteroidal anti‐inflammatory and/or analgesics and nonopioid cough suppressant and expectorant medications were associated with falls syndrome only. Hormone replacement medications were associated with depression only, and immunosuppressants were associated with unintentional weight and appetite loss only.
| Major Medication Category | Delirium | Cognitive Impairment | Falls | Unintentional Weight and Appetite Loss | Urinary Incontinence | Depression | Drug Class/Drug Within Each Category |
|---|---|---|---|---|---|---|---|
| |||||||
| Antipsychotics | ✓ | ✓ | ✓ | ✓ | Atypical and typical antipsychotics, buspirone | ||
| Antidepressants | ✓ | ✓ | ✓ | ✓ | ✓ | Tricyclic and tetracyclic antidepressants, serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitor, aminoketone | |
| Antiepileptics | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Antiepileptics, mood stabilizers, barbiturates |
| Antiparkinsonism | ✓ | ✓ | ✓ | ✓ | ✓ | Aromatic amino acid decarboxylation inhibitor and catechol‐o‐methyltransferase inhibitor, catecholamine‐depleting sympatholytic, catechol‐o‐methyltransferase inhibitor, dopaminergic agonist, ergot derivative, monoamine oxidase inhibitor, nonergot dopamine agonist, | |
| Benzodiazapines | ✓ | ✓ | ✓ | Benzodiazapines only | |||
| Nonbenzodiazepine hypnotics | ✓ | ✓ | ✓ | Benzodiazepine analogs, nonbenzodiazepine hypnotics, tranquilizers, ‐aminobutyric acid A receptor agonist | |||
| Opioid agonists | ✓ | ✓ | ✓ | ✓ | ✓ | Full or partial opioid agonists, opiates, opioids | |
| Nonopioid/nonsteroidal anti‐inflammatory and/or analgesics | ✓ | Nonopioid analgesics, NSAIDs, COX‐2 selective inhibitor NSAIDs | |||||
| Antihypertensives | ✓ | ✓ | ✓ | Calcium channel blocker, ‐adrenergic blocker, angiotensin‐converting enzyme inhibitor, angiotensin 2 receptor blocker, ‐adrenergic blocker, diuretics (loop, potassium sparing, thiazide), nitrate vasodilators, aldosterone blocker | |||
| Antiarrhythmic | ✓ | ✓ | ✓ | Antiarrhythmics, cardiac glycosides | |||
| Antidiabetics | ✓ | ✓ | Insulin and insulin analogs, sulfonylureas, ‐glucosidase inhibitor, amylin analog, biguanide, glinide, GLP‐1 receptor agonist, glucagon‐like peptide‐1 agonist | ||||
| Anticholinergics and/or antihistaminics | ✓ | ✓ | ✓ | ✓ | Anticholinergics, histamine receptor antagonists, muscarininc antagonists, combined anticholinergics, and histamine receptor antagonists | ||
| Antiemetics | ✓ | ✓ | ✓ | Antiemetics, dopaminergic antagonists, dopamine‐2 receptor antagonist | |||
| Hormone replacement | ✓ | Corticosteroids, progestin, estrogen, estrogen agonist/antagonist, gonadotropin releasing hormone receptor agonist | |||||
| Muscle relaxers | ✓ | ✓ | ✓ | ✓ | Muscle relaxers | ||
| Immunosuppressants | ✓ | Calcineurin inhibitor immunosuppressant, folate analog metabolic inhibitor, purine antimetabolite | |||||
| Nonopioid cough suppressants and expectorants | ✓ | Expectorant, non‐narcotic antitussive, ‐1 agonist, uncompetitive N‐methyl‐D‐aspartate receptor antagonist | |||||
| Antimicrobials | ✓ | ✓ | Macrolide, cephalosporin, penicillin class, rifamycin, non‐nucleoside analog reverse transcriptase inhibitor, influenza A M2 protein inhibitor | ||||
| Others | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ‐3‐adrenergic agonist, methylxanthine, cholinesterase inhibitor, interferon and , partial cholinergic nicotinic agonist, tyrosine hydroxylase, retinoid, serotonin‐1b and serotonin‐1d receptor agonist, stimulant laxative, vitamin K antagonist, platelet aggregation inhibitor |
Approximately 58% of the medications overlapped with the AGS 2015 Beer's Criteria[20] irrespective of whether the specific syndrome association was stated in the rationale.[20] Medications that overlapped were mostly in the delirium, cognitive impairment, and falls category with only a few overlaps in depression, unintentional weight loss, and urinary incontinence lists (see Supporting Information, Appendix 1, in the online version of this article).
Phase 2: Prevalence of MAGS
Among 154 participants, the mean age was 76.5 (10.6) years, 64.3% were female, 77.9% were white, and 96.1% non‐Hispanic. The median hospital length of stay was 6 days, with an interquartile range of 5 days. The orthopedic service discharged the highest proportion of patients (24%), followed by the geriatrics and internal medicine services, which each discharged 19.5% of the patients (Table 2). The remaining participants of the larger quality‐improvement project (N = 939) did not significantly differ on these demographic and clinical characteristics except for hospital length of stay, which was shorter in the sample analyzed (see Supporting Information, Appendix 2, in the online version of this article).
| Baseline Characteristics | Mean ( SD) or Percent (n) |
|---|---|
| |
| Age, y | 76.5 ( 10.6) |
| Sex | |
| Female | 64.3% (99) |
| Race | |
| White | 77.9% (126) |
| Black | 16.2% (25) |
| Unknown | 0.6% (1) |
| Declined | 0.6% (1) |
| Missing | 0.6% (1) |
| Ethnicity | |
| Non‐Hispanic | 96.1% (148) |
| Hispanic | 1.3% (2) |
| Unknown | 2.6% (4) |
| Hospital length of stay, d | 7.0 ( 4.2) |
| Hospital length of stay, d, median (IQR) | 6.0 (5.0) |
| No. of hospital discharge medications, count | 14.0 ( 4.7) |
| Discharge service | |
| Orthopedic service | 24.0% (37) |
| Geriatric service | 19.5% (30) |
| Internal medicine | 19.5% (30) |
| Other | 37.0% (57) |
Patients were discharged to SNFs with an average of 14.0 (4.7) medication orders. Overall, 43% (13%) of these discharge medication orders were MAGS. Every patient in the sample was ordered at least 1 medication associated with geriatric syndromes. Multiple MAGS were the norm, with an average of 5.9 (2.2) MAGS per patient. MAGS were also the norm, as 98.1% of the sample had medication orders associated with at least 2 different syndromes.
When the Beer's criteria[20] were applied to the medication orders (instead of the MAGS list), problematic medications appeared less common. Patients had an average of 3.04 (1.7) MAGS that were also listed on the AGS 2015 Beer's list,[20] representing an average of 22.3% of all discharge orders.
Table 3 illustrates the average number of medications per patient associated with each syndrome, and the percentage of patients (number in parentheses) discharged with at least 1 medication associated with each syndrome per the MAGS list and the Beers 2015 criteria.[20] For example, per the MAGS list, the syndrome most frequently associated with medications was falls, with patients discharged on an average of 5.5 (2.2) medications associated with falls, and 100% of the sample had at least 1 discharge medication associated with falls. Alternatively, the syndrome associated with the lowest frequency of medications was unintentional weight loss (with an average of 0.38 medications per patient), although 36% of these patients had more than 1 discharge medication associated with weight loss. As seen in Table 3, the mean and prevalence of 1 or more medications associated with each of the geriatric syndromes as identified by the Beers 2015 criteria[20] was lower than those identified by the MAGS list developed for this study.
| Geriatric Syndromes | Associated Medications per MAGS List | Associated Medications per AGS Beers 2015 Criteria | ||
|---|---|---|---|---|
| Mean SD | Percentage of Patients Receiving 1 Related Medication | Mean SD | Percentage of Patients Receiving 1 Related Medication | |
| ||||
| Cognitive impairment | 1.8 ( 1.2) | 84.4% (130) | 1.6 ( 1.2) | 78.6% (121) |
| Delirium | 1.4 ( 1.1) | 76.0% (117) | 1.3 ( 1.2) | 68.2% (105) |
| Falls | 5.5 ( 2.2) | 100% (154) | 2.6 ( 1.6) | 92.2% (142) |
| Unintentional weight and/or appetite loss | 0.4 ( 0.5) | 36.3% (56) | 0.1 ( 0.3) | 6.5% (10) |
| Urinary incontinence | 1.6 ( 1.0) | 85.7% (132) | 0.1 ( 0.2) | 5.8% (9) |
| Depression | 1.7 ( 1.0) | 90.9% (140) | 0.0 ( 0.0) | 0.0% (0) |
| All syndromes | 5.9 ( 2.2) | 100% (154) | 3.0 ( 1.7) | 95% (149) |
DISCUSSION
An iterative process of evidence review by a multidisciplinary panel resulted in a list of 513 medications associated with 6 common geriatric syndromes. This analysis demonstrated that hospitalized, older patients discharged to SNFs were frequently prescribed MAGS. The rate of prescribing ranged from 100% of patients with a medication associated with falls to 36% for unintentional weight loss. Moreover, an alarming 43% of all medications at hospital discharge were MAGS. For this vulnerable population, the combination of high prevalence of MAGS and high risk of geriatric syndromes emphasize a need to critically review the risks and benefits of MAGS throughout hospitalization and at the time of discharge.
A body of evidence demonstrates that many drugs in a typical older adult regimen have no specific clinical indication, are considered inappropriate, or have uncertain efficacy in the geriatric population.[24, 25, 26] This study builds on the foundational work described in landmark reviews such as the AGS Beers[20] and STOPP/START[21] (Screening Tool of Older Persons' Potentially Inappropriate Prescriptions/Screening Tool to Alert doctors to Right, i.e. appropriate indicated Treatment) criteria. Both of these tools, however, were specifically designed as screening tools to identify medications considered unsafe for older adults under most circumstances and within specific illness states.[19, 20, 21] They are most often utilized when starting a medication to avoid acute adverse events. In contrast, the MAGS list was developed to be inclusive of medications that are often appropriate for many medical diagnoses but may also contribute to underlying geriatric syndromes that are more chronic in nature. In addition, inclusion of such medicines increases the sensitivity of screening for medications that can be targeted through patient‐centered deprescribing efforts when clinically appropriate.
A major strength of this study is that we bring together evidence across a spectrum of geriatric syndromes commonly experienced by hospitalized elders. In addition to evaluating multiple syndromes, we applied multiple modalities; particularly the use of an iterative review process by a multidisciplinary team of experts and using Lexicomp and FDA insert packages for linking medications to specific geriatric conditions. The inclusion criteria were broadened beyond single sources of evidence in an effort to capture a comprehensive list of medications. As a result, the MAGS list can be implemented as a screening tool for deprescribing interventions and assessing medication appropriateness to address individual or clusters of geriatric syndromes within a patient.
In addition to expanding this knowledge base, clinical relevance of the MAGS list is highlighted by its application to a sample of hospitalized older adults discharged to SNFs, a cohort known to experience geriatric syndromes. In fact, 43% of patients' medications at hospital discharge were MAGS. Importantly, due to the cross‐sectional nature of this study, we cannot be certain if the medication caused or potentiated each of the geriatric syndromes. However, hospitals and SNFs are devoting major resources toward reduction of falls, avoidance of urinary catheter use, and reduction of preventable readmissions. These efforts can be complemented by considering the number of medications associated with falls, urinary incontinence, and overall MAGS burden. The striking prevalence of MAGS demonstrates a rigorous need to weigh the risks and benefits of these medications. Above all, the intent of this study is not to propose that any MAGS be reflexively stopped, but rather that the MAGS list should facilitate a holistic approach to care for the complex older adult. For example, standard therapies such as gabapentin may be appropriate for treating neuralgic pain but may also contribute to falls and urinary incontinence. Thus, alternative pain treatments could be selected in place of gabapentin for a 75‐year old patient who is experiencing recurrent falls and increasing incontinence. Therefore, the MAGS list enables a patient‐provider discussion wherein medications' therapeutic benefits can be weighed against risks posed by specific clusters of geriatric syndromes, potential impact on quality of life, and consistency with goals of care.
This study has some limitations. First, although we examined a broad number of geriatric syndromes, several other geriatric syndromes experienced by hospitalized older adults were not addressed including: fecal incontinence, insomnia, and functional impairment. These syndromes were intentionally excluded from the study a priori due to reasons of feasibility and scope. Second, unlike the Beer's 2015 criteria, the MAGS list does not sub‐classify associations of medications with geriatric syndromes for patients with specific diseases (eg, heart failure). In fact, our MAGS list included medications often indicated in treating these diagnoses. A clinician must work with the patient to weigh the disease‐specific benefits of some medications with the potential effect on geriatric syndrome symptoms and outcomes. Third, the instrument has a very high sensitivity, which was intended to generate an inclusive list of medications that enables providers to weigh risks of geriatric syndromes with the intended indication benefit. The objective is not to use this list as a reflexive tool but rather help clinicians identify a starting point to address geriatric syndromes in their patients to make patient‐centered medication decisions. Although the MAGS list is intentionally large (sensitive), the advent of advanced bioinformatics can enable MAGS to be assessed in the future for both clinical and research purposes. Fourth, FDA insert packages and Lexicomp databases report anything experienced by the patient while on the particular medication, but it might not necessarily imply a causative link. The high use of MAGS and the specific geriatric syndrome may coexist due to the high prevalence and interplay of multimorbidity, polypharmacy, and geriatric syndromes in this population. Last, the list was developed by expert panel members predominantly from a single institution, which may introduce bias. Despite these limitations, the prevalence of these medications in a sample of patients transitioning from acute to postacute care highlights the utility of the MAGS list in future clinical research and quality improvement endeavors.
In conclusion, the MAGS list provides a comprehensive and sensitive indicator of medications associated with any of 6 geriatric syndromes regardless of medication indication and appropriateness. The MAGS list provides an overall degree of medication burden with respect to geriatric syndromes and a foundation for future research to assess the relationship between the presence of geriatric syndromes and syndrome‐associated medications. The MAGS list is an important first step in summarizing the data that link medications to geriatric syndromes. Future studies are needed to broaden the analysis of MAGS for other common geriatric syndromes and to identify new and emerging medications not present during the time of this analysis. The MAGS list has the potential to facilitate deprescribing efforts needed to combat the epidemic of overprescribing that may be contributing to the burden of geriatric syndromes among older patients.
Acknowledgements
The authors thank Dr. Linda Beuscher, Dr. Patricia Blair Miller, Dr. Joseph Ouslander, Dr. William Stuart Reynolds, and Dr. Warren Taylor for providing their expertise and participating in the expert panel discussions that facilitated the development of the MAGS list. The authors also recognize the research support provided by Christopher Simon Coelho.
Disclosures: This research was supported by the Department of Health and Human Services, Centers for Medicare & Medicaid Services grant #1C1CMS331006 awarded to Principal Investigator, John F. Schnelle, PhD. Dr. Vasilevskis was supported by the National Institute on Aging of the National Institutes of Health award K23AG040157 and the Geriatric Research, Education and Clinical Center. Dr. Bell was supported by National Institute on Aging‐K award K23AG048347‐01A1. Dr. Mixon is supported by a Veterans Affairs Health Services Research & Development Career Development Award (12‐168). This research was also supported by the National Center for Advancing Translational Sciences Clinical and Translational Science award UL1TR000445. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the US Department of Health and Human Services or any of its agencies, the National Center for Advancing Translation Science, the National Institutes of Health, or the Department of Veterans Affairs. Each coauthor contributed significantly to the manuscript. Dr. Kripalani has received stock/stock options from Bioscape Digital, LLC. None of the other authors have significant conflicts of interest to report related to this project or the results reported within this article.
- , , , . Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–791.
- , , , . Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes. JAMA. 1995;273:1348–1353.
- , , , . Geriatric syndromes: medical misnomer or progress in geriatrics? Neth J Med. 2003;61:83–87.
- , , , et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one‐year survival and functional decline. PLoS One. 2011;6:e26951.
- , , , , . Geriatric conditions as predictors of increased number of hospital admissions and hospital bed days over one year: findings of a nationwide cohort of older adults from Taiwan. Arch Gerontol Geriatr. 2014;59:169–174.
- , , , , . Geriatric conditions and disability: the Health and Retirement Study. Ann Intern Med. 2007;147:156–164.
- , , , , , . A prospective cohort study of geriatric syndromes among older medical patients admitted to acute care hospitals. J Am Geriatr Soc. 2011;59:2001–2008.
- , , , et al. Geriatric syndromes in hospitalized older adults discharged to skilled nursing facilities. J Am Geriatr Soc. 2016;64(4):715–722.
- , , , et al. Discharge to a skilled nursing facility and subsequent clinical outcomes among older patients hospitalized for heart failure. Circ Heart Fail. 2011;4:293–300.
- . Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219–223.
- , , , , . Geriatric syndromes in elderly patients admitted to an inpatient cardiology ward. J Hosp Med. 2007;2:394–400.
- , , , . Effect of hospitalization on inappropriate prescribing in elderly Medicare beneficiaries. J Am Geriatr Soc. 2015;63:699–707.
- , , , et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community‐dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012;65:989–995.
- , , , , . Investigating polypharmacy and drug burden index in hospitalised older people. Intern Med J. 2013;43:912–918.
- , . Potentially harmful drug‐drug interactions in the elderly: a review. Am J Geriatr Pharmacother. 2011;9:364–377.
- , , , . Hospital admissions/visits associated with drug‐drug interactions: a systematic review and meta‐analysis. Pharmacoepidemiol Drug Saf. 2014;23:489–497.
- , . Optimising drug treatment for elderly people: the prescribing cascade. BMJ. 1997;315:1096–1099.
- , , , et al. Association between acute geriatric syndromes and medication‐related hospital admissions. Drugs Aging. 2012;29:691–699.
- American Geriatrics Society Beers Criteria Update Expert P. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616–631.
- By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227–2246.
- , . STOPP (Screening Tool of Older Persons' potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers' criteria. Age Ageing. 2008;37:673–679.
- , , , et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370:493–503.
- U.S. Food and Drug Administration. Drugs. Available at: http://www.fda.gov/Drugs/default.htm. Accessed May 15th, 2015.
- , , , et al. Inappropriate medication use among frail elderly inpatients. Ann Pharmacother. 2004;38:9–14.
- , , , et al. Inappropriate medications in elderly ICU survivors: where to intervene? Arch Intern Med. 2011;171:1032–1034.
- , , , et al. Appropriateness of medication prescribing in ambulatory elderly patients. J Am Geriatr Soc. 1994;42:1241–1247.
- , , , . Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–791.
- , , , . Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes. JAMA. 1995;273:1348–1353.
- , , , . Geriatric syndromes: medical misnomer or progress in geriatrics? Neth J Med. 2003;61:83–87.
- , , , et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one‐year survival and functional decline. PLoS One. 2011;6:e26951.
- , , , , . Geriatric conditions as predictors of increased number of hospital admissions and hospital bed days over one year: findings of a nationwide cohort of older adults from Taiwan. Arch Gerontol Geriatr. 2014;59:169–174.
- , , , , . Geriatric conditions and disability: the Health and Retirement Study. Ann Intern Med. 2007;147:156–164.
- , , , , , . A prospective cohort study of geriatric syndromes among older medical patients admitted to acute care hospitals. J Am Geriatr Soc. 2011;59:2001–2008.
- , , , et al. Geriatric syndromes in hospitalized older adults discharged to skilled nursing facilities. J Am Geriatr Soc. 2016;64(4):715–722.
- , , , et al. Discharge to a skilled nursing facility and subsequent clinical outcomes among older patients hospitalized for heart failure. Circ Heart Fail. 2011;4:293–300.
- . Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219–223.
- , , , , . Geriatric syndromes in elderly patients admitted to an inpatient cardiology ward. J Hosp Med. 2007;2:394–400.
- , , , . Effect of hospitalization on inappropriate prescribing in elderly Medicare beneficiaries. J Am Geriatr Soc. 2015;63:699–707.
- , , , et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community‐dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012;65:989–995.
- , , , , . Investigating polypharmacy and drug burden index in hospitalised older people. Intern Med J. 2013;43:912–918.
- , . Potentially harmful drug‐drug interactions in the elderly: a review. Am J Geriatr Pharmacother. 2011;9:364–377.
- , , , . Hospital admissions/visits associated with drug‐drug interactions: a systematic review and meta‐analysis. Pharmacoepidemiol Drug Saf. 2014;23:489–497.
- , . Optimising drug treatment for elderly people: the prescribing cascade. BMJ. 1997;315:1096–1099.
- , , , et al. Association between acute geriatric syndromes and medication‐related hospital admissions. Drugs Aging. 2012;29:691–699.
- American Geriatrics Society Beers Criteria Update Expert P. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616–631.
- By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227–2246.
- , . STOPP (Screening Tool of Older Persons' potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers' criteria. Age Ageing. 2008;37:673–679.
- , , , et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370:493–503.
- U.S. Food and Drug Administration. Drugs. Available at: http://www.fda.gov/Drugs/default.htm. Accessed May 15th, 2015.
- , , , et al. Inappropriate medication use among frail elderly inpatients. Ann Pharmacother. 2004;38:9–14.
- , , , et al. Inappropriate medications in elderly ICU survivors: where to intervene? Arch Intern Med. 2011;171:1032–1034.
- , , , et al. Appropriateness of medication prescribing in ambulatory elderly patients. J Am Geriatr Soc. 1994;42:1241–1247.
The Effect of Orthopedic Advertising and Self-Promotion on a Naïve Population
In 1975, the American Medical Association (AMA) lifted the professional ban on physician advertising after a successful Federal Trade Commission suit.1 Since then, there has been a marked increase in the number of physicians marketing themselves directly to patients and consumers. With the pervasive nature of the Internet, never before has it been so easy and inexpensive to effectively communicate with a targeted population of people and influence their behavior. Few would dispute the role of advertising on consumer choices when used to sell products and services, change behavior, and educate consumers across all types of industries and professions. Thus, it is reasonable to hypothesize that the nature and content of a surgeon’s web presence could significantly affect patients’ decision-making and their impression of the orthopedic surgery profession.
There is a lack of consensus among physician organizations regarding physician advertising. For example, the American Association of Physicians and Surgeons (AAPS) takes an ethical stand on physician self-promotion. Their position states “The physician should not solicit patients. Professional reputation is the major source of patient referrals. The physician should be circumspect and restrained in dealing with the communication media, always avoiding self-aggrandizement.2” In contrast, the AMA has a less defined stance on physician self-promotion. With the exception of conflicts of interest and privacy guidelines, the AMA has few recommendations regarding the content of physician websites. The organization’s position states “There are no restrictions on advertising by physicians except those that can be specifically justified to protect the public from deceptive practices. …Nothing in this opinion is intended to discourage or to limit advertising and representations which are not false or deceptive.3” This guideline emphasizes accuracy of health-related information, but does not limit physician self-promotion or self-aggrandizement. The American Academy of Orthopaedic Surgeons (AAOS) holds a similar position. In their position statement on advertising by orthopedic surgeons, they encourage advertising and competition as “ethical and acceptable” as long as they are representing services in a “clear and accurate manner.”4 Furthermore, the AAOS also states that “An orthopaedic surgeon shall not use photographs, images, endorsements and/or statements in a false or misleading manner that communicate a degree of relief, safety, effectiveness, or benefits from orthopaedic care that are not representative of results attained by that orthopaedic surgeon.”4 The surgeon is responsible for his/her advertising materials and the content and claims therein, and is generally policed by peers through a complaint process with the AAOS.
Notably, up to 75% of Americans use the Internet for health-related information and this number is likely to increase.5Patients who utilize the Internet must choose from a vast array of search results for medical information from credible resources. Which sources are to be believed and relied upon? This depends on the health literacy among the general population. Inadequate health literacy is defined as “limited ability to obtain, process, and understand basic health information and services needed to make appropriate health decisions and follow instructions for treatment.”6 Patients have different levels of health literacy often unknown to even the most well-intentioned healthcare professional. It is often difficult to provide appropriate and meaningful information at a level that is most beneficial to the patient. It is estimated that 89 million people in the US have insufficient health literacy to understand treatments or preventive care.7 Certainly, with this information in mind, the orthopedic surgeon must consider his/her audience, and the potential for a fiduciary responsibility when preparing Internet content.
A tangible example of marketing results is the increasing popularity of robotic surgery over the last decade.8 Hospitals routinely advertise the availability of robotic surgery at their institution through various means, including roadside billboards. Despite limited evidence supporting a benefit of robotic surgery beyond less expensive conventional laparoscopic surgery, patients are increasingly seeking robotic surgery.8 With society’s increasing infatuation with technology, this is likely based on the presumption that robotic surgery is better and safer than conventional methods. It is likely that marketing pressure is at least partly responsible for the widespread adoption of robotic-assisted surgery and words used in marketing highlighting novelty have an important influence on patient preference.8
Orthopedic surgery, with its large proportion of elective surgeries, offers a unique venue to study differences in patient perceptions. Preoperative evaluations in orthopedics are often performed after an assessment of a surgeon’s reputation, which offers the patient an ability to choose their surgeon within their community.
We pondered how different promotional styles would affect potential patients’ perceptions. Would people believe that a self-promoting physician was more competent? Could fellow doctors “see through” the self-promotion of their peers? Based on the premise that advertising and self-promotion are undertaken because they are effective, we hypothesized that nonphysician patients perceive self-promoting orthopedic surgeons more favorably compared to members of the medical community.
Although numerous anonymous physician review sites exist, our analysis focused on surgeon self-promotion through personal websites or web pages. Within these sources, there exists a wide array of information and methods that physicians utilize to present themselves. Some physicians merely post their educational background and qualifications. This appears most often when the physician is associated with an academic institution and their profile is part of an institution’s website. Others post extensive self-promoting statements about technical skill and innovations in clinical practice. They sometimes include information regarding charity donations, level of community involvement, and practice philosophy.
Materials and Methods
Categorization of Surgeon Websites and Ratings
Surgeon websites were selected from the 5 largest population centers in the United States. Analysis was undertaken to categorize the self-promotion content of each selected website using an objective scale to quantitatively assess the number of times that physicians referred to themselves in a positive manner. A thorough search of the literature did not reveal any validated questionnaire or assessment tool usable for this purpose. Five blinded raters were asked to count the number of positive self-directed remarks made by the author of each website. Websites were ranked based on the number of such statements. No rater was exposed to any styling or graphical information from any website. Only textual statements were used for the purposes of this study. All statements were printed on paper and evaluated without the use of a computer to prevent any searching or contamination of the subject or rater pool.
Websites were considered as self-promoting (using language that promotes the physician beyond the use of basic facts), or non-self-promoting(presenting little beyond basic biographical information) based on the presence of many (more than 5) or few (less than 5) self-promoting statements. The breakpoint of 5 self-promoting statements served to highlight a clear transition between the 2 general types of websites and provided a good demarcation between self-promoters and non-self-promoters. This distinction allowed for the choosing of contrasting websites, which could directly probe the question in our hypothesis about the effect of such websites on naïve or surgeon-peer respondents.
Each website was judged independently by 5 blinded raters. Inter-rater reliability scores were then calculated using Fleiss’ Kappa to assess reliability of the categorization of self-promoter or non-self-promoter. This value was calculated to be k = .80, 95% confidence interval (0.58-1.01), which is suggestive of a “substantial level of agreement.”9 Websites categorized as non-self-promoting contained a mean number of self-promoting statements of less than 2 (0-1.8) as judged by the 5 raters. By contrast, websites categorized as self-promoting had a mean number of self-promoting statements of 6.4 or higher (6.4-22.6). When the self-promoting websites and the non-self-promoting websites were compared, they were significantly different in the number of self-promoting statements t (43) = 7.90, P < .001, with self-promoting websites having significantly more self-promoting statements than non-self-promoting websites.
Surveys and Respondents
Next, a survey of 10 questions of interest was developed. A thorough literature search revealed no validated measure or survey to measure the effects of surgeon or physician self-promotion. We developed a 10-question survey to prove the impressions and allow for assessment of differences between respondent groups to measure the effect of promotion. The questions (see Appendix for survey questions) included a forced Likert rating system. Each response occurs and is presented on a scale from 0 to 3 (0 = Strongly Disagree, 1 = Disagree, 2 = Agree, and 3 = Strongly Agree).
Respondents were true volunteers recruited from 2 groups that were termed “surgeon-peers” and “naïve subjects.” Surgeon-peers were board-certified orthopedic surgeons (N = 21, all with medical doctorates). Demographic breakdown of the surgeon-peers revealed them to be reflective of the general population of orthopedic surgeons (71.4% male, 28.6% female, 90.2% Caucasian, 4.8% African American, and 4.8% Asian, all with professional degrees). Naïve subjects (N = 24, average age 41 years) were selected based on the criterion of having no affiliation with a healthcare system and no history of interaction with an orthopedic surgery or surgery in general. The demographic breakdown of naïve subjects was 45.8% male, 54.2% female, 79.1% Caucasian, 16.7% African American, and 4.2% Asian. Half of the naïve respondents had a Bachelor’s degree, 17% had a Master’s degree, 4% had a professional degree, and 29% had a high school diploma. No volunteer, in either group, received any form of inducement or reward for participation so as not to skew any responses in favor of physicians.
All participants were asked to read each surgeon’s statements and then complete a survey for each statement. Volunteers were not informed of a surgeon’s calculated level of self-promotion, and they were presented the survey questions in random order. Survey completion required unreimbursed time of approximately 1 to 2 hours.
Statistical Methods
The data compiled was then analyzed with SAS/STAT Software (SAS Institute Inc.) and a LR Type III analysis using the GENMOD procedure. The method of analysis and presentation of data focuses on the relationship between respondents perceptions between the surgeon-peer and naïve subject groups. The P values presented are the significance of the testing of interactions comparing the difference between surgeon-peers and naïve subjects, and the differences in their responses to each question for self-promoters and non-self-promoters. Surgeon-peers answer questions differently based on their assessment of a self-promoter or non-self-promoter website. It is this difference that is compared to the analogous difference for naïve subjects and statistically evaluated. The LR statistic for type III analysis tests if the differences are significantly different, ie, if the difference between the 2 subject groups is statistically significant. All statistical methods were performed by a qualified statistician who helped guide the design of this study.
Results
Each respondent was asked if they were aware that misinformation about doctors exists on the Internet. Half of the naïve subjects affirmed awareness of this whereas the other half were unaware. All surgeon-peers were aware of the presence of misinformation regarding physicians on the Internet.
The results of the comparisons are shown in the Table. The columns show the average response to each question for self-promoters and non-self-promoters grouped by either surgeon-peer or naïve subject. In judging the overall accuracy of statements made on the Internet, naïve subjects found no difference between self-promoters and non-self-promoters, whereas surgeon-peers judged the difference to be large and significant in favor of non-self-promoting surgeons. Surgeon-peers generally rated non-self-promoters with significantly more positive Likert scores, indicating improved “competence”, “excellence”, and “better quality of care” when compared to naïve respondents (Table). The direction and magnitude of the difference was also striking, with the naïve respondents favoring self-promoters on all of these questions. This held true for the choice of orthopedic surgeon, where naïve responders favored self-promoters and surgeon-peers favored non-self-promoters. Moreover, naïve subjects believed that self-promoters would be significantly more likely to help them in the event of a complication, whereas surgeon-peers believed the opposite. Even when the direction of difference was the same in both groups, statistically significant differences in the responses were evident, as was the case when respondents were asked “Did the surgeon inflate his/her technical skills?” or “Did the author of this statement seem arrogant?” Both groups favored self-promoters for these questions, but the differences were larger among surgeon-peers, indicating that naïve subjects were somewhat less sensitive to the differences between promoters and non-self-promoters. There was no difference between surgeon-peers and naïve subjects in their expectations of sanctions against self-promoters’ licenses when compared to non-self-promoters, which was the only question to fail to garner a significant difference between respondents.
Discussion
This study explores the differences in the perceptions of physician websites between board-certified orthopedic surgeons and naïve individuals. These websites contain varying amounts of information presented in numerous ways. While we did not poll the website authors regarding their intent, the purpose of a website seems naturally to communicate believable information to the public. The information provided ranges widely from basic facts regarding education and contact information to statements regarding technical skills, reputation, television appearances, and the friendly nature of the office staff.
Our results suggest that board-certified orthopedic surgeons, peers of the writers of these websites, tend to view self-promoting surgeons more negatively than do their nonphysician counterparts. These findings support our hypothesis that self-promoting surgeons are perceived more favorably by the naïve, nonphysician population.
At first glance, our results suggest that the mere absence of a surgeon from the medium may affect the patient’s choice, because 50% of our naïve respondents indicated that they would use the Internet to choose a doctor. Interestingly, both the surgeon-peer group and naïve subjects were equally aware that misinformation exists on the Internet. However, when reviewing the websites, naïve subjects were significantly more likely to view self-promoters as more competent, more excellent, and more likely to provide quality care, and were more likely to choose the self-promoter if they needed surgery compared to the surgeon-peer group. The naïve group viewed self-promoters as less likely to inflate their technical skills but more likely to be arrogant. They viewed self-promoters as more likely to help if things went wrong and more likely to make accurate statements compared to the surgeon-peer group. This suggests that patients with little experience are more likely to choose a self-promoting physician than one who does not self-promote for reasons that cannot be proven true or false in the confines of a website. Further study is needed to see if perceptions based on web content translate into actual changes in healthcare choices.
This study had several limitations. Though statistically sound, the sample size of 45 people was small and should likely be expanded in further investigations to allow for analysis of demographics and socioeconomic factors. The study focused only on the text content of websites and purposely removed the influences of the other potential content mentioned previously. While a biography serves as an introduction, further research is needed to determine how initial perceptions affect future perceptions throughout the course of the patient-physician relationship. The small number of Internet biographies used cannot represent the vast array of information that could be displayed in numerous ways, but was necessary given the length of time donated by each uncompensated subject (1-2 hours). To minimize complexity, we purposefully ignored websites in the middle, somewhere in the continuum between self-promoting and non-self-promoting. Instead we selected websites that would be stark in their self-promotion to allow for the assessment of our hypothesis. Finally, this study was not designed to address economic implications of promotional advertising. The goal of much advertising is to generate revenue, and in the case of orthopedic surgery, one goal is likely attracting more patients, but this effect is beyond the scope of the current study. Given the elective nature of many orthopedic surgery procedures, the effect of promotional websites on a person’s decision to have surgery or not is an important topic for future study.
Taken together, the data suggests a profound influence of the content of the Internet website in the impressions made on different groups of people. These facts, although profound in their influence and unregulated by the medical profession, present both great opportunities and liabilities. The opportunities arise from the professional community to help guide what surgeons do to generate interest on the Internet. The liabilities arise on consideration of the consequences of self-promotion in the setting of real world surgical complications.
1. Tomycz ND. A profession selling out: lamenting the paradigm shift in physician advertising. J Med Ethics. 2006;32(1):26-28.
2. The principles of medical ethics of the Association of American Physicians and Surgeons. Association of American Physicians and Surgeons Web site. http://www.aapsonline.org/index.php/principles_of_medical_ethics. Accessed September 20, 2013.
3. Opinion 5.027 – Use of health-related online sites. American Medical Association Web site. http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion5027.page. Accessed September 10, 2013.
4. Standards of professionalism. Advertising by orthopaedic surgeons. Adopted April 18, 2007. American Academy of Orthopaedic Surgeons Web site. http://www.aaos.org/cc_files/aaosorg/member/profcomp/advertisingbyos.pdf. Accessed May 6, 2016.
5. Mostaghimi A, Crotty BH, Landon BE. The availability and nature of physician information on the internet. J Gen Intern Med. 2010;25(11):1152-1156.
6. Ad Hoc Committee on Health Literacy for the Council on Scientific Affairs, American Medical Association. Health Literacy: Report of the Council on Scientific Affairs. JAMA. 1999;281(6):552-557. doi:10.1001/jama.281.6.552.
7. Leroy G, Endicott JE, Mouradi O, Kauchak D, Just ML. Improving perceived and actual text difficulty for health information consumers using semi-automated methods. AMIA Annu Symp Proc. 2012;2012:522–531.
8. Dixon PR, Grant RC, Urbach DR. The impact of promotional language on patient preference for innovative procedures. J Am Coll Surg. 2013;217(3):S100.
9. Landis JR, Koch GG. A one-way components of variance model for categorical data. Biometrics. 1977;33(4):671–679.
In 1975, the American Medical Association (AMA) lifted the professional ban on physician advertising after a successful Federal Trade Commission suit.1 Since then, there has been a marked increase in the number of physicians marketing themselves directly to patients and consumers. With the pervasive nature of the Internet, never before has it been so easy and inexpensive to effectively communicate with a targeted population of people and influence their behavior. Few would dispute the role of advertising on consumer choices when used to sell products and services, change behavior, and educate consumers across all types of industries and professions. Thus, it is reasonable to hypothesize that the nature and content of a surgeon’s web presence could significantly affect patients’ decision-making and their impression of the orthopedic surgery profession.
There is a lack of consensus among physician organizations regarding physician advertising. For example, the American Association of Physicians and Surgeons (AAPS) takes an ethical stand on physician self-promotion. Their position states “The physician should not solicit patients. Professional reputation is the major source of patient referrals. The physician should be circumspect and restrained in dealing with the communication media, always avoiding self-aggrandizement.2” In contrast, the AMA has a less defined stance on physician self-promotion. With the exception of conflicts of interest and privacy guidelines, the AMA has few recommendations regarding the content of physician websites. The organization’s position states “There are no restrictions on advertising by physicians except those that can be specifically justified to protect the public from deceptive practices. …Nothing in this opinion is intended to discourage or to limit advertising and representations which are not false or deceptive.3” This guideline emphasizes accuracy of health-related information, but does not limit physician self-promotion or self-aggrandizement. The American Academy of Orthopaedic Surgeons (AAOS) holds a similar position. In their position statement on advertising by orthopedic surgeons, they encourage advertising and competition as “ethical and acceptable” as long as they are representing services in a “clear and accurate manner.”4 Furthermore, the AAOS also states that “An orthopaedic surgeon shall not use photographs, images, endorsements and/or statements in a false or misleading manner that communicate a degree of relief, safety, effectiveness, or benefits from orthopaedic care that are not representative of results attained by that orthopaedic surgeon.”4 The surgeon is responsible for his/her advertising materials and the content and claims therein, and is generally policed by peers through a complaint process with the AAOS.
Notably, up to 75% of Americans use the Internet for health-related information and this number is likely to increase.5Patients who utilize the Internet must choose from a vast array of search results for medical information from credible resources. Which sources are to be believed and relied upon? This depends on the health literacy among the general population. Inadequate health literacy is defined as “limited ability to obtain, process, and understand basic health information and services needed to make appropriate health decisions and follow instructions for treatment.”6 Patients have different levels of health literacy often unknown to even the most well-intentioned healthcare professional. It is often difficult to provide appropriate and meaningful information at a level that is most beneficial to the patient. It is estimated that 89 million people in the US have insufficient health literacy to understand treatments or preventive care.7 Certainly, with this information in mind, the orthopedic surgeon must consider his/her audience, and the potential for a fiduciary responsibility when preparing Internet content.
A tangible example of marketing results is the increasing popularity of robotic surgery over the last decade.8 Hospitals routinely advertise the availability of robotic surgery at their institution through various means, including roadside billboards. Despite limited evidence supporting a benefit of robotic surgery beyond less expensive conventional laparoscopic surgery, patients are increasingly seeking robotic surgery.8 With society’s increasing infatuation with technology, this is likely based on the presumption that robotic surgery is better and safer than conventional methods. It is likely that marketing pressure is at least partly responsible for the widespread adoption of robotic-assisted surgery and words used in marketing highlighting novelty have an important influence on patient preference.8
Orthopedic surgery, with its large proportion of elective surgeries, offers a unique venue to study differences in patient perceptions. Preoperative evaluations in orthopedics are often performed after an assessment of a surgeon’s reputation, which offers the patient an ability to choose their surgeon within their community.
We pondered how different promotional styles would affect potential patients’ perceptions. Would people believe that a self-promoting physician was more competent? Could fellow doctors “see through” the self-promotion of their peers? Based on the premise that advertising and self-promotion are undertaken because they are effective, we hypothesized that nonphysician patients perceive self-promoting orthopedic surgeons more favorably compared to members of the medical community.
Although numerous anonymous physician review sites exist, our analysis focused on surgeon self-promotion through personal websites or web pages. Within these sources, there exists a wide array of information and methods that physicians utilize to present themselves. Some physicians merely post their educational background and qualifications. This appears most often when the physician is associated with an academic institution and their profile is part of an institution’s website. Others post extensive self-promoting statements about technical skill and innovations in clinical practice. They sometimes include information regarding charity donations, level of community involvement, and practice philosophy.
Materials and Methods
Categorization of Surgeon Websites and Ratings
Surgeon websites were selected from the 5 largest population centers in the United States. Analysis was undertaken to categorize the self-promotion content of each selected website using an objective scale to quantitatively assess the number of times that physicians referred to themselves in a positive manner. A thorough search of the literature did not reveal any validated questionnaire or assessment tool usable for this purpose. Five blinded raters were asked to count the number of positive self-directed remarks made by the author of each website. Websites were ranked based on the number of such statements. No rater was exposed to any styling or graphical information from any website. Only textual statements were used for the purposes of this study. All statements were printed on paper and evaluated without the use of a computer to prevent any searching or contamination of the subject or rater pool.
Websites were considered as self-promoting (using language that promotes the physician beyond the use of basic facts), or non-self-promoting(presenting little beyond basic biographical information) based on the presence of many (more than 5) or few (less than 5) self-promoting statements. The breakpoint of 5 self-promoting statements served to highlight a clear transition between the 2 general types of websites and provided a good demarcation between self-promoters and non-self-promoters. This distinction allowed for the choosing of contrasting websites, which could directly probe the question in our hypothesis about the effect of such websites on naïve or surgeon-peer respondents.
Each website was judged independently by 5 blinded raters. Inter-rater reliability scores were then calculated using Fleiss’ Kappa to assess reliability of the categorization of self-promoter or non-self-promoter. This value was calculated to be k = .80, 95% confidence interval (0.58-1.01), which is suggestive of a “substantial level of agreement.”9 Websites categorized as non-self-promoting contained a mean number of self-promoting statements of less than 2 (0-1.8) as judged by the 5 raters. By contrast, websites categorized as self-promoting had a mean number of self-promoting statements of 6.4 or higher (6.4-22.6). When the self-promoting websites and the non-self-promoting websites were compared, they were significantly different in the number of self-promoting statements t (43) = 7.90, P < .001, with self-promoting websites having significantly more self-promoting statements than non-self-promoting websites.
Surveys and Respondents
Next, a survey of 10 questions of interest was developed. A thorough literature search revealed no validated measure or survey to measure the effects of surgeon or physician self-promotion. We developed a 10-question survey to prove the impressions and allow for assessment of differences between respondent groups to measure the effect of promotion. The questions (see Appendix for survey questions) included a forced Likert rating system. Each response occurs and is presented on a scale from 0 to 3 (0 = Strongly Disagree, 1 = Disagree, 2 = Agree, and 3 = Strongly Agree).
Respondents were true volunteers recruited from 2 groups that were termed “surgeon-peers” and “naïve subjects.” Surgeon-peers were board-certified orthopedic surgeons (N = 21, all with medical doctorates). Demographic breakdown of the surgeon-peers revealed them to be reflective of the general population of orthopedic surgeons (71.4% male, 28.6% female, 90.2% Caucasian, 4.8% African American, and 4.8% Asian, all with professional degrees). Naïve subjects (N = 24, average age 41 years) were selected based on the criterion of having no affiliation with a healthcare system and no history of interaction with an orthopedic surgery or surgery in general. The demographic breakdown of naïve subjects was 45.8% male, 54.2% female, 79.1% Caucasian, 16.7% African American, and 4.2% Asian. Half of the naïve respondents had a Bachelor’s degree, 17% had a Master’s degree, 4% had a professional degree, and 29% had a high school diploma. No volunteer, in either group, received any form of inducement or reward for participation so as not to skew any responses in favor of physicians.
All participants were asked to read each surgeon’s statements and then complete a survey for each statement. Volunteers were not informed of a surgeon’s calculated level of self-promotion, and they were presented the survey questions in random order. Survey completion required unreimbursed time of approximately 1 to 2 hours.
Statistical Methods
The data compiled was then analyzed with SAS/STAT Software (SAS Institute Inc.) and a LR Type III analysis using the GENMOD procedure. The method of analysis and presentation of data focuses on the relationship between respondents perceptions between the surgeon-peer and naïve subject groups. The P values presented are the significance of the testing of interactions comparing the difference between surgeon-peers and naïve subjects, and the differences in their responses to each question for self-promoters and non-self-promoters. Surgeon-peers answer questions differently based on their assessment of a self-promoter or non-self-promoter website. It is this difference that is compared to the analogous difference for naïve subjects and statistically evaluated. The LR statistic for type III analysis tests if the differences are significantly different, ie, if the difference between the 2 subject groups is statistically significant. All statistical methods were performed by a qualified statistician who helped guide the design of this study.
Results
Each respondent was asked if they were aware that misinformation about doctors exists on the Internet. Half of the naïve subjects affirmed awareness of this whereas the other half were unaware. All surgeon-peers were aware of the presence of misinformation regarding physicians on the Internet.
The results of the comparisons are shown in the Table. The columns show the average response to each question for self-promoters and non-self-promoters grouped by either surgeon-peer or naïve subject. In judging the overall accuracy of statements made on the Internet, naïve subjects found no difference between self-promoters and non-self-promoters, whereas surgeon-peers judged the difference to be large and significant in favor of non-self-promoting surgeons. Surgeon-peers generally rated non-self-promoters with significantly more positive Likert scores, indicating improved “competence”, “excellence”, and “better quality of care” when compared to naïve respondents (Table). The direction and magnitude of the difference was also striking, with the naïve respondents favoring self-promoters on all of these questions. This held true for the choice of orthopedic surgeon, where naïve responders favored self-promoters and surgeon-peers favored non-self-promoters. Moreover, naïve subjects believed that self-promoters would be significantly more likely to help them in the event of a complication, whereas surgeon-peers believed the opposite. Even when the direction of difference was the same in both groups, statistically significant differences in the responses were evident, as was the case when respondents were asked “Did the surgeon inflate his/her technical skills?” or “Did the author of this statement seem arrogant?” Both groups favored self-promoters for these questions, but the differences were larger among surgeon-peers, indicating that naïve subjects were somewhat less sensitive to the differences between promoters and non-self-promoters. There was no difference between surgeon-peers and naïve subjects in their expectations of sanctions against self-promoters’ licenses when compared to non-self-promoters, which was the only question to fail to garner a significant difference between respondents.
Discussion
This study explores the differences in the perceptions of physician websites between board-certified orthopedic surgeons and naïve individuals. These websites contain varying amounts of information presented in numerous ways. While we did not poll the website authors regarding their intent, the purpose of a website seems naturally to communicate believable information to the public. The information provided ranges widely from basic facts regarding education and contact information to statements regarding technical skills, reputation, television appearances, and the friendly nature of the office staff.
Our results suggest that board-certified orthopedic surgeons, peers of the writers of these websites, tend to view self-promoting surgeons more negatively than do their nonphysician counterparts. These findings support our hypothesis that self-promoting surgeons are perceived more favorably by the naïve, nonphysician population.
At first glance, our results suggest that the mere absence of a surgeon from the medium may affect the patient’s choice, because 50% of our naïve respondents indicated that they would use the Internet to choose a doctor. Interestingly, both the surgeon-peer group and naïve subjects were equally aware that misinformation exists on the Internet. However, when reviewing the websites, naïve subjects were significantly more likely to view self-promoters as more competent, more excellent, and more likely to provide quality care, and were more likely to choose the self-promoter if they needed surgery compared to the surgeon-peer group. The naïve group viewed self-promoters as less likely to inflate their technical skills but more likely to be arrogant. They viewed self-promoters as more likely to help if things went wrong and more likely to make accurate statements compared to the surgeon-peer group. This suggests that patients with little experience are more likely to choose a self-promoting physician than one who does not self-promote for reasons that cannot be proven true or false in the confines of a website. Further study is needed to see if perceptions based on web content translate into actual changes in healthcare choices.
This study had several limitations. Though statistically sound, the sample size of 45 people was small and should likely be expanded in further investigations to allow for analysis of demographics and socioeconomic factors. The study focused only on the text content of websites and purposely removed the influences of the other potential content mentioned previously. While a biography serves as an introduction, further research is needed to determine how initial perceptions affect future perceptions throughout the course of the patient-physician relationship. The small number of Internet biographies used cannot represent the vast array of information that could be displayed in numerous ways, but was necessary given the length of time donated by each uncompensated subject (1-2 hours). To minimize complexity, we purposefully ignored websites in the middle, somewhere in the continuum between self-promoting and non-self-promoting. Instead we selected websites that would be stark in their self-promotion to allow for the assessment of our hypothesis. Finally, this study was not designed to address economic implications of promotional advertising. The goal of much advertising is to generate revenue, and in the case of orthopedic surgery, one goal is likely attracting more patients, but this effect is beyond the scope of the current study. Given the elective nature of many orthopedic surgery procedures, the effect of promotional websites on a person’s decision to have surgery or not is an important topic for future study.
Taken together, the data suggests a profound influence of the content of the Internet website in the impressions made on different groups of people. These facts, although profound in their influence and unregulated by the medical profession, present both great opportunities and liabilities. The opportunities arise from the professional community to help guide what surgeons do to generate interest on the Internet. The liabilities arise on consideration of the consequences of self-promotion in the setting of real world surgical complications.
In 1975, the American Medical Association (AMA) lifted the professional ban on physician advertising after a successful Federal Trade Commission suit.1 Since then, there has been a marked increase in the number of physicians marketing themselves directly to patients and consumers. With the pervasive nature of the Internet, never before has it been so easy and inexpensive to effectively communicate with a targeted population of people and influence their behavior. Few would dispute the role of advertising on consumer choices when used to sell products and services, change behavior, and educate consumers across all types of industries and professions. Thus, it is reasonable to hypothesize that the nature and content of a surgeon’s web presence could significantly affect patients’ decision-making and their impression of the orthopedic surgery profession.
There is a lack of consensus among physician organizations regarding physician advertising. For example, the American Association of Physicians and Surgeons (AAPS) takes an ethical stand on physician self-promotion. Their position states “The physician should not solicit patients. Professional reputation is the major source of patient referrals. The physician should be circumspect and restrained in dealing with the communication media, always avoiding self-aggrandizement.2” In contrast, the AMA has a less defined stance on physician self-promotion. With the exception of conflicts of interest and privacy guidelines, the AMA has few recommendations regarding the content of physician websites. The organization’s position states “There are no restrictions on advertising by physicians except those that can be specifically justified to protect the public from deceptive practices. …Nothing in this opinion is intended to discourage or to limit advertising and representations which are not false or deceptive.3” This guideline emphasizes accuracy of health-related information, but does not limit physician self-promotion or self-aggrandizement. The American Academy of Orthopaedic Surgeons (AAOS) holds a similar position. In their position statement on advertising by orthopedic surgeons, they encourage advertising and competition as “ethical and acceptable” as long as they are representing services in a “clear and accurate manner.”4 Furthermore, the AAOS also states that “An orthopaedic surgeon shall not use photographs, images, endorsements and/or statements in a false or misleading manner that communicate a degree of relief, safety, effectiveness, or benefits from orthopaedic care that are not representative of results attained by that orthopaedic surgeon.”4 The surgeon is responsible for his/her advertising materials and the content and claims therein, and is generally policed by peers through a complaint process with the AAOS.
Notably, up to 75% of Americans use the Internet for health-related information and this number is likely to increase.5Patients who utilize the Internet must choose from a vast array of search results for medical information from credible resources. Which sources are to be believed and relied upon? This depends on the health literacy among the general population. Inadequate health literacy is defined as “limited ability to obtain, process, and understand basic health information and services needed to make appropriate health decisions and follow instructions for treatment.”6 Patients have different levels of health literacy often unknown to even the most well-intentioned healthcare professional. It is often difficult to provide appropriate and meaningful information at a level that is most beneficial to the patient. It is estimated that 89 million people in the US have insufficient health literacy to understand treatments or preventive care.7 Certainly, with this information in mind, the orthopedic surgeon must consider his/her audience, and the potential for a fiduciary responsibility when preparing Internet content.
A tangible example of marketing results is the increasing popularity of robotic surgery over the last decade.8 Hospitals routinely advertise the availability of robotic surgery at their institution through various means, including roadside billboards. Despite limited evidence supporting a benefit of robotic surgery beyond less expensive conventional laparoscopic surgery, patients are increasingly seeking robotic surgery.8 With society’s increasing infatuation with technology, this is likely based on the presumption that robotic surgery is better and safer than conventional methods. It is likely that marketing pressure is at least partly responsible for the widespread adoption of robotic-assisted surgery and words used in marketing highlighting novelty have an important influence on patient preference.8
Orthopedic surgery, with its large proportion of elective surgeries, offers a unique venue to study differences in patient perceptions. Preoperative evaluations in orthopedics are often performed after an assessment of a surgeon’s reputation, which offers the patient an ability to choose their surgeon within their community.
We pondered how different promotional styles would affect potential patients’ perceptions. Would people believe that a self-promoting physician was more competent? Could fellow doctors “see through” the self-promotion of their peers? Based on the premise that advertising and self-promotion are undertaken because they are effective, we hypothesized that nonphysician patients perceive self-promoting orthopedic surgeons more favorably compared to members of the medical community.
Although numerous anonymous physician review sites exist, our analysis focused on surgeon self-promotion through personal websites or web pages. Within these sources, there exists a wide array of information and methods that physicians utilize to present themselves. Some physicians merely post their educational background and qualifications. This appears most often when the physician is associated with an academic institution and their profile is part of an institution’s website. Others post extensive self-promoting statements about technical skill and innovations in clinical practice. They sometimes include information regarding charity donations, level of community involvement, and practice philosophy.
Materials and Methods
Categorization of Surgeon Websites and Ratings
Surgeon websites were selected from the 5 largest population centers in the United States. Analysis was undertaken to categorize the self-promotion content of each selected website using an objective scale to quantitatively assess the number of times that physicians referred to themselves in a positive manner. A thorough search of the literature did not reveal any validated questionnaire or assessment tool usable for this purpose. Five blinded raters were asked to count the number of positive self-directed remarks made by the author of each website. Websites were ranked based on the number of such statements. No rater was exposed to any styling or graphical information from any website. Only textual statements were used for the purposes of this study. All statements were printed on paper and evaluated without the use of a computer to prevent any searching or contamination of the subject or rater pool.
Websites were considered as self-promoting (using language that promotes the physician beyond the use of basic facts), or non-self-promoting(presenting little beyond basic biographical information) based on the presence of many (more than 5) or few (less than 5) self-promoting statements. The breakpoint of 5 self-promoting statements served to highlight a clear transition between the 2 general types of websites and provided a good demarcation between self-promoters and non-self-promoters. This distinction allowed for the choosing of contrasting websites, which could directly probe the question in our hypothesis about the effect of such websites on naïve or surgeon-peer respondents.
Each website was judged independently by 5 blinded raters. Inter-rater reliability scores were then calculated using Fleiss’ Kappa to assess reliability of the categorization of self-promoter or non-self-promoter. This value was calculated to be k = .80, 95% confidence interval (0.58-1.01), which is suggestive of a “substantial level of agreement.”9 Websites categorized as non-self-promoting contained a mean number of self-promoting statements of less than 2 (0-1.8) as judged by the 5 raters. By contrast, websites categorized as self-promoting had a mean number of self-promoting statements of 6.4 or higher (6.4-22.6). When the self-promoting websites and the non-self-promoting websites were compared, they were significantly different in the number of self-promoting statements t (43) = 7.90, P < .001, with self-promoting websites having significantly more self-promoting statements than non-self-promoting websites.
Surveys and Respondents
Next, a survey of 10 questions of interest was developed. A thorough literature search revealed no validated measure or survey to measure the effects of surgeon or physician self-promotion. We developed a 10-question survey to prove the impressions and allow for assessment of differences between respondent groups to measure the effect of promotion. The questions (see Appendix for survey questions) included a forced Likert rating system. Each response occurs and is presented on a scale from 0 to 3 (0 = Strongly Disagree, 1 = Disagree, 2 = Agree, and 3 = Strongly Agree).
Respondents were true volunteers recruited from 2 groups that were termed “surgeon-peers” and “naïve subjects.” Surgeon-peers were board-certified orthopedic surgeons (N = 21, all with medical doctorates). Demographic breakdown of the surgeon-peers revealed them to be reflective of the general population of orthopedic surgeons (71.4% male, 28.6% female, 90.2% Caucasian, 4.8% African American, and 4.8% Asian, all with professional degrees). Naïve subjects (N = 24, average age 41 years) were selected based on the criterion of having no affiliation with a healthcare system and no history of interaction with an orthopedic surgery or surgery in general. The demographic breakdown of naïve subjects was 45.8% male, 54.2% female, 79.1% Caucasian, 16.7% African American, and 4.2% Asian. Half of the naïve respondents had a Bachelor’s degree, 17% had a Master’s degree, 4% had a professional degree, and 29% had a high school diploma. No volunteer, in either group, received any form of inducement or reward for participation so as not to skew any responses in favor of physicians.
All participants were asked to read each surgeon’s statements and then complete a survey for each statement. Volunteers were not informed of a surgeon’s calculated level of self-promotion, and they were presented the survey questions in random order. Survey completion required unreimbursed time of approximately 1 to 2 hours.
Statistical Methods
The data compiled was then analyzed with SAS/STAT Software (SAS Institute Inc.) and a LR Type III analysis using the GENMOD procedure. The method of analysis and presentation of data focuses on the relationship between respondents perceptions between the surgeon-peer and naïve subject groups. The P values presented are the significance of the testing of interactions comparing the difference between surgeon-peers and naïve subjects, and the differences in their responses to each question for self-promoters and non-self-promoters. Surgeon-peers answer questions differently based on their assessment of a self-promoter or non-self-promoter website. It is this difference that is compared to the analogous difference for naïve subjects and statistically evaluated. The LR statistic for type III analysis tests if the differences are significantly different, ie, if the difference between the 2 subject groups is statistically significant. All statistical methods were performed by a qualified statistician who helped guide the design of this study.
Results
Each respondent was asked if they were aware that misinformation about doctors exists on the Internet. Half of the naïve subjects affirmed awareness of this whereas the other half were unaware. All surgeon-peers were aware of the presence of misinformation regarding physicians on the Internet.
The results of the comparisons are shown in the Table. The columns show the average response to each question for self-promoters and non-self-promoters grouped by either surgeon-peer or naïve subject. In judging the overall accuracy of statements made on the Internet, naïve subjects found no difference between self-promoters and non-self-promoters, whereas surgeon-peers judged the difference to be large and significant in favor of non-self-promoting surgeons. Surgeon-peers generally rated non-self-promoters with significantly more positive Likert scores, indicating improved “competence”, “excellence”, and “better quality of care” when compared to naïve respondents (Table). The direction and magnitude of the difference was also striking, with the naïve respondents favoring self-promoters on all of these questions. This held true for the choice of orthopedic surgeon, where naïve responders favored self-promoters and surgeon-peers favored non-self-promoters. Moreover, naïve subjects believed that self-promoters would be significantly more likely to help them in the event of a complication, whereas surgeon-peers believed the opposite. Even when the direction of difference was the same in both groups, statistically significant differences in the responses were evident, as was the case when respondents were asked “Did the surgeon inflate his/her technical skills?” or “Did the author of this statement seem arrogant?” Both groups favored self-promoters for these questions, but the differences were larger among surgeon-peers, indicating that naïve subjects were somewhat less sensitive to the differences between promoters and non-self-promoters. There was no difference between surgeon-peers and naïve subjects in their expectations of sanctions against self-promoters’ licenses when compared to non-self-promoters, which was the only question to fail to garner a significant difference between respondents.
Discussion
This study explores the differences in the perceptions of physician websites between board-certified orthopedic surgeons and naïve individuals. These websites contain varying amounts of information presented in numerous ways. While we did not poll the website authors regarding their intent, the purpose of a website seems naturally to communicate believable information to the public. The information provided ranges widely from basic facts regarding education and contact information to statements regarding technical skills, reputation, television appearances, and the friendly nature of the office staff.
Our results suggest that board-certified orthopedic surgeons, peers of the writers of these websites, tend to view self-promoting surgeons more negatively than do their nonphysician counterparts. These findings support our hypothesis that self-promoting surgeons are perceived more favorably by the naïve, nonphysician population.
At first glance, our results suggest that the mere absence of a surgeon from the medium may affect the patient’s choice, because 50% of our naïve respondents indicated that they would use the Internet to choose a doctor. Interestingly, both the surgeon-peer group and naïve subjects were equally aware that misinformation exists on the Internet. However, when reviewing the websites, naïve subjects were significantly more likely to view self-promoters as more competent, more excellent, and more likely to provide quality care, and were more likely to choose the self-promoter if they needed surgery compared to the surgeon-peer group. The naïve group viewed self-promoters as less likely to inflate their technical skills but more likely to be arrogant. They viewed self-promoters as more likely to help if things went wrong and more likely to make accurate statements compared to the surgeon-peer group. This suggests that patients with little experience are more likely to choose a self-promoting physician than one who does not self-promote for reasons that cannot be proven true or false in the confines of a website. Further study is needed to see if perceptions based on web content translate into actual changes in healthcare choices.
This study had several limitations. Though statistically sound, the sample size of 45 people was small and should likely be expanded in further investigations to allow for analysis of demographics and socioeconomic factors. The study focused only on the text content of websites and purposely removed the influences of the other potential content mentioned previously. While a biography serves as an introduction, further research is needed to determine how initial perceptions affect future perceptions throughout the course of the patient-physician relationship. The small number of Internet biographies used cannot represent the vast array of information that could be displayed in numerous ways, but was necessary given the length of time donated by each uncompensated subject (1-2 hours). To minimize complexity, we purposefully ignored websites in the middle, somewhere in the continuum between self-promoting and non-self-promoting. Instead we selected websites that would be stark in their self-promotion to allow for the assessment of our hypothesis. Finally, this study was not designed to address economic implications of promotional advertising. The goal of much advertising is to generate revenue, and in the case of orthopedic surgery, one goal is likely attracting more patients, but this effect is beyond the scope of the current study. Given the elective nature of many orthopedic surgery procedures, the effect of promotional websites on a person’s decision to have surgery or not is an important topic for future study.
Taken together, the data suggests a profound influence of the content of the Internet website in the impressions made on different groups of people. These facts, although profound in their influence and unregulated by the medical profession, present both great opportunities and liabilities. The opportunities arise from the professional community to help guide what surgeons do to generate interest on the Internet. The liabilities arise on consideration of the consequences of self-promotion in the setting of real world surgical complications.
1. Tomycz ND. A profession selling out: lamenting the paradigm shift in physician advertising. J Med Ethics. 2006;32(1):26-28.
2. The principles of medical ethics of the Association of American Physicians and Surgeons. Association of American Physicians and Surgeons Web site. http://www.aapsonline.org/index.php/principles_of_medical_ethics. Accessed September 20, 2013.
3. Opinion 5.027 – Use of health-related online sites. American Medical Association Web site. http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion5027.page. Accessed September 10, 2013.
4. Standards of professionalism. Advertising by orthopaedic surgeons. Adopted April 18, 2007. American Academy of Orthopaedic Surgeons Web site. http://www.aaos.org/cc_files/aaosorg/member/profcomp/advertisingbyos.pdf. Accessed May 6, 2016.
5. Mostaghimi A, Crotty BH, Landon BE. The availability and nature of physician information on the internet. J Gen Intern Med. 2010;25(11):1152-1156.
6. Ad Hoc Committee on Health Literacy for the Council on Scientific Affairs, American Medical Association. Health Literacy: Report of the Council on Scientific Affairs. JAMA. 1999;281(6):552-557. doi:10.1001/jama.281.6.552.
7. Leroy G, Endicott JE, Mouradi O, Kauchak D, Just ML. Improving perceived and actual text difficulty for health information consumers using semi-automated methods. AMIA Annu Symp Proc. 2012;2012:522–531.
8. Dixon PR, Grant RC, Urbach DR. The impact of promotional language on patient preference for innovative procedures. J Am Coll Surg. 2013;217(3):S100.
9. Landis JR, Koch GG. A one-way components of variance model for categorical data. Biometrics. 1977;33(4):671–679.
1. Tomycz ND. A profession selling out: lamenting the paradigm shift in physician advertising. J Med Ethics. 2006;32(1):26-28.
2. The principles of medical ethics of the Association of American Physicians and Surgeons. Association of American Physicians and Surgeons Web site. http://www.aapsonline.org/index.php/principles_of_medical_ethics. Accessed September 20, 2013.
3. Opinion 5.027 – Use of health-related online sites. American Medical Association Web site. http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion5027.page. Accessed September 10, 2013.
4. Standards of professionalism. Advertising by orthopaedic surgeons. Adopted April 18, 2007. American Academy of Orthopaedic Surgeons Web site. http://www.aaos.org/cc_files/aaosorg/member/profcomp/advertisingbyos.pdf. Accessed May 6, 2016.
5. Mostaghimi A, Crotty BH, Landon BE. The availability and nature of physician information on the internet. J Gen Intern Med. 2010;25(11):1152-1156.
6. Ad Hoc Committee on Health Literacy for the Council on Scientific Affairs, American Medical Association. Health Literacy: Report of the Council on Scientific Affairs. JAMA. 1999;281(6):552-557. doi:10.1001/jama.281.6.552.
7. Leroy G, Endicott JE, Mouradi O, Kauchak D, Just ML. Improving perceived and actual text difficulty for health information consumers using semi-automated methods. AMIA Annu Symp Proc. 2012;2012:522–531.
8. Dixon PR, Grant RC, Urbach DR. The impact of promotional language on patient preference for innovative procedures. J Am Coll Surg. 2013;217(3):S100.
9. Landis JR, Koch GG. A one-way components of variance model for categorical data. Biometrics. 1977;33(4):671–679.
Mortality Risk and Patient Experience
Few today deny the importance of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey.[1, 2] The Centers for Medicare and Medicaid Services' (CMS) Value Based Purchasing incentive, sympathy for the ill, and relationships between the patient experience and quality of care provide sufficient justification.[3, 4] How to improve the experience scores is not well understood. The national scores have improved only modestly over the past 3 years.[5, 6]
Clinicians may not typically compartmentalize what they do to improve outcomes versus the patient experience. A possible source for new improvement strategies is to understand the types of patients in which both adverse outcomes and suboptimal experiences are likely to occur, then redesign the multidisciplinary care processes to address both concurrently.[7] Previous studies support the existence of a relationship between a higher mortality risk on admission and subsequent worse outcomes, as well as a relationship between worse outcomes and lower HCAHPS scores.[8, 9, 10, 11, 12, 13] We hypothesized the mortality risk on admission, patient experience, and outcomes might share a triad relationship (Figure 1). In this article we explore the third edge of this triangle, the association between the mortality risk on admission and the subsequent patient experience.
METHODS
We studied HCAHPS from 5 midwestern US hospitals having 113, 136, 304, 443, and 537 licensed beds, affiliated with the same multistate healthcare system. HCAHPS telephone surveys were administered via a vendor to a random sample of inpatients 18 years of age or older discharged from January 1, 2012 through June 30, 2014. Per CMS guidelines, surveyed patients must have been discharged alive after a hospital stay of at least 1 night.[14] Patients ineligible to be surveyed included those discharged to skilled nursing facilities or hospice care.[14] Because not all study hospitals provided obstetrical services, we restricted the analyses to medical and surgical respondents. With the permission of the local institutional review board, subjects' survey responses were linked confidentially to their clinical data.
We focused on the 8 dimensions of the care experience used in the CMS Value Based Purchasing program: communication with doctors, communication with nurses, responsiveness of hospital staff, pain management, communication about medicines, discharge information, hospital environment, and an overall rating of the hospital.[2] Following the scoring convention for publicly reported results, we dichotomized the 4‐level Likert scales into the most favorable response possible (always) versus all other responses.[15] Similarly we dichotomized the hospital rating scale at 9 and above for the most favorable response.
Our unit of analysis was an individual hospitalization. Our primary outcome of interest was whether or not the respondent provided the most favorable response for all questions answered within a given domain. For example, for the physician communication domain, the patient must have answered always to each of the 3 questions answered within the domain. This approach is appropriate for learning which patient‐level factors influence the survey responses, but differs from that used for the publically reported domain scores for which the relative performance of hospitals is the focus.[16] For the latter, the hospital was the unit of analysis, and the domain score was basically the average of the percentages of top box scores for the questions within a domain. For example, if 90% respondents from a hospital provided a top box response for courtesy, 80% for listening, and 70% for explanation, the hospital's physician communication score would be (90 + 80 + 70)/3 = 80%.[17]
Our primary explanatory variable was a binary high versus low mortality‐risk status of the respondent on admission based on age, gender, prior hospitalizations, clinical laboratory values, and diagnoses present on admission.[12] The calculated mortality risk was then dichotomized prior to the analysis at a probability of dying equal to 0.07 or higher. This corresponded roughly to the top quintile of predicted risk found in prior studies.[12, 13] During the study period, only 2 of the hospitals had the capability of generating mortality scores in real time, so for this study the mortality risk was calculated retrospectively, using information deemed present on admission.[12]
To estimate the sample size, we assumed that the high‐risk strata contained approximately 13% of respondents, and that the true percent of top box responses from patients in the lower‐risk stratum was approximately 80% for each domain. A meaningful difference in the proportion of most favorable responses was considered as an odds ratio (OR) of 0.75 for high risk versus low risk. A significance level of P < 0.003 was set to control study‐wide type I error due to multiple comparisons. We determined that for each dimension, approximately 8583 survey responses would be required for low‐risk patients and approximately 1116 responses for high‐risk patients to achieve 80% power under these assumptions. We were able to accrue the target number of surveys for all but 3 domains (pain management, communication about medicines, and hospital environment) because of data availability, and because patients are allowed to skip questions that do not apply. Univariate relationships were examined with 2, t test, and Fisher exact tests where indicated. Generalized linear mixed regression models with a logit link were fit to determine the association between patient mortality risk and the top box experience for each of the HCAHPS domains and for the overall rating. The patient's hospital was considered a random intercept to account for the patient‐hospital hierarchy and the unmeasured hospital‐specific practices. The multivariable models controlled for gender plus the HCAHPS patient‐mix adjustment variables of age, education, self‐rated health, language spoken at home, service line, and the number of days elapsed between the date of discharge and date of the survey.[18, 19, 20, 21] In keeping with the industry analyses, a second order interaction variable was included between surgery patients and age.[19] We considered the potential collinearity between the mortality risk status, age, and patient self‐reported health. We found the variance inflation factors were small, so we drew inference from the full multivariable model.
We also performed a post hoc sensitivity analysis to determine if our conclusions were biased due to missing patient responses for the risk‐adjustment variables. Accordingly, we imputed the response level most negatively associated with most HCAHPS domains as previously reported and reran the multivariable models.[19] We did not find a meaningful change in our conclusions (see Supporting Figure 1 in the online version of this article).
RESULTS
The hospitals discharged 152,333 patients during the study period, 39,905 of whom (26.2 %) had a predicted 30‐day mortality risk greater or equal to 0.07 (Table 1). Of the 36,280 high‐risk patients discharged alive, 5901 (16.3%) died in the ensuing 30 days, and 7951 (22%) were readmitted.
| Characteristic | Low‐Risk Stratum, No./Discharged (%) or Mean (SD) | High‐Risk Stratum, No./Discharged (%) or Mean (SD) | P Value* |
|---|---|---|---|
| |||
| Total discharges (row percent) | 112,428/152,333 (74) | 39,905/152,333 (26) | <0.001 |
| Total alive discharges (row percent) | 111,600/147,880 (75) | 36,280/147,880 (25) | <0.001 |
| No. of respondents (row percent) | 14,996/17,509 (86) | 2,513/17,509 (14) | |
| HCAHPS surveys completed | 14,996/111,600 (13) | 2,513/36,280 (7) | < 0.001 |
| Readmissions within 30 days (total discharges) | 12,311/112,428 (11) | 7,951/39,905 (20) | <0.001 |
| Readmissions within 30 days (alive discharges) | 12,311/111,600 (11) | 7,951/36,280 (22) | <0.001 |
| Readmissions within 30 days (respondents) | 1,220/14,996 (8) | 424/2,513 (17) | <0.001 |
| Mean predicted probability of 30‐day mortality (total discharges) | 0.022 (0.018) | 0.200 (0.151) | <0.001 |
| Mean predicted probability of 30‐day mortality (alive discharges) | 0.022 (0.018) | 0.187 (0.136) | <0.001 |
| Mean predicted probability of 30‐day mortality (respondents) | 0.020 (0.017) | 0.151 (0.098) | <0.001 |
| In‐hospital death (total discharges) | 828/112,428 (0.74) | 3,625/39,905 (9) | <0.001 |
| Mortality within 30 days (total discharges) | 2,455/112,428 (2) | 9,526/39,905 (24) | <0.001 |
| Mortality within 30 days (alive discharges) | 1,627/111,600 (1.5) | 5,901/36,280 (16) | <0.001 |
| Mortality within 30 days (respondents) | 9/14,996 (0.06) | 16/2,513 (0.64) | <0.001 |
| Female (total discharges) | 62,681/112,368 (56) | 21,058/39,897 (53) | <0.001 |
| Female (alive discharges) | 62,216/111,540 (56) | 19,164/36,272 (53) | <0.001 |
| Female (respondents) | 8,684/14,996 (58) | 1,318/2,513 (52) | <0.001 |
| Age (total discharges) | 61.3 (16.8) | 78.3 (12.5) | <0.001 |
| Age (alive discharges) | 61.2 (16.8) | 78.4 (12.5) | <0.001 |
| Age (respondents) | 63.1 (15.2) | 76.6 (11.5) | <0.001 |
| Highest education attained | |||
| 8th grade or less | 297/14,996 (2) | 98/2,513 (4) | |
| Some high school | 1,190/14,996 (8) | 267/2,513 (11) | |
| High school grad | 4,648/14,996 (31) | 930/2,513 (37) | <0.001 |
| Some college | 6,338/14,996 (42) | 768/2,513 (31) | |
| 4‐year college grad | 1,502/14,996 (10) | 183/2,513 (7) | |
| Missing response | 1,021/14,996 (7) | 267/2,513 (11) | |
| Language spoken at home | |||
| English | 13,763/14,996 (92) | 2,208/2,513 (88) | |
| Spanish | 56/14,996 (0.37) | 8/2,513 (0.32) | 0.47 |
| Chinese | 153/14,996 (1) | 31/2,513 (1) | |
| Missing response | 1,024/14,996 (7) | 266/2,513 (11) | |
| Self‐rated health | |||
| Excellent | 1,399/14,996 (9) | 114/2,513 (5) | |
| Very good | 3,916/14,996 (26) | 405/2,513 (16) | |
| Good | 4,861/14,996 (32) | 713/2,513 (28) | |
| Fair | 2,900/14,996 (19) | 652/2,513 (26) | <0.001 |
| Poor | 1,065/14,996 (7) | 396/2,513 (16) | |
| Missing response | 855/14,996 (6) | 233/2,513 (9) | |
| Length of hospitalization, d (respondents) | 3.5 (2.8) | 4.6 (3.6) | <0.001 |
| Consulting specialties (respondents) | 1.7 (1.0) | 2.2 (1.3) | <0.001 |
| Service line | |||
| Surgical | 6,380/14,996 (43) | 346/2,513 (14) | <0.001 |
| Medical | 8,616/14,996 (57) | 2,167/2,513 (86) | |
| HCAHPS | |||
| Domain 1: Communication With Doctors | 9,564/14,731 (65) | 1,339/2,462 (54) | <0.001 |
| Domain 2: Communication With Nurses | 10,097/14,991 (67) | 1,531/2,511 (61) | <0.001 |
| Domain 3: Responsiveness of Hospital Staff | 7,813/12,964 (60) | 1,158/2,277 (51) | <0.001 |
| Domain 4: Pain Management | 6,565/10,424 (63) | 786/1,328 (59) | 00.007 |
| Domain 5: Communication About Medicines | 3,769/8,088 (47) | 456/1,143 (40) | <0.001 |
| Domain 6: Discharge Information | 11,331/14,033 (81) | 1,767/2,230 (79) | 0.09 |
| Domain 7: Hospital Environment | 6,981/14,687 (48) | 1,093/2,451 (45) | 0.007 |
| Overall rating | 10,708/14,996 (71) | 1,695/2,513 (67) | <0.001 |
The high‐risk subset was under‐represented in those who completed the HCAHPS survey with 7% (2513/36,280) completing surveys compared to 13% of low‐risk patients (14,996/111,600) (P < 0.0001). Moreover, compared to high‐risk patients who were alive at discharge but did not complete surveys, high‐risk survey respondents were less likely to die within 30 days (16/2513 = 0.64% vs 5885/33,767 = 17.4%, P < 0.0001), and less likely to be readmitted (424/2513 = 16.9% vs 7527/33,767 = 22.3%, P < 0.0001).
On average, high‐risk respondents (compared to low risk) were slightly less likely to be female (52.4% vs 57.9%), less educated (30.6% with some college vs 42.3%), less likely to have been on a surgical service (13.8% vs 42.5%), and less likely to report good or better health (49.0% vs 68.0%, all P < 0.0001). High‐risk respondents were also older (76.6 vs 63.1 years), stayed in the hospital longer (4.6 vs 3.5 days), and received care from more specialties (2.2 vs 1.7 specialties) (all P < 0.0001). High‐risk respondents experienced more 30‐day readmissions (16.9% vs 8.1%) and deaths within 30 days (0.6 % vs 0.1 %, all P < 0.0001) than their low‐risk counterparts.
High‐risk respondents were less likely to provide the most favorable response (unadjusted) for all HCAHPS domains compared to low‐risk respondents, although the difference was not significant for discharge information (Table 1, Figure 2A). The gradient between high‐risk and low‐risk patients was seen for all domains within each hospital except for pain management, hospital environment, and overall rating (Figure 3).
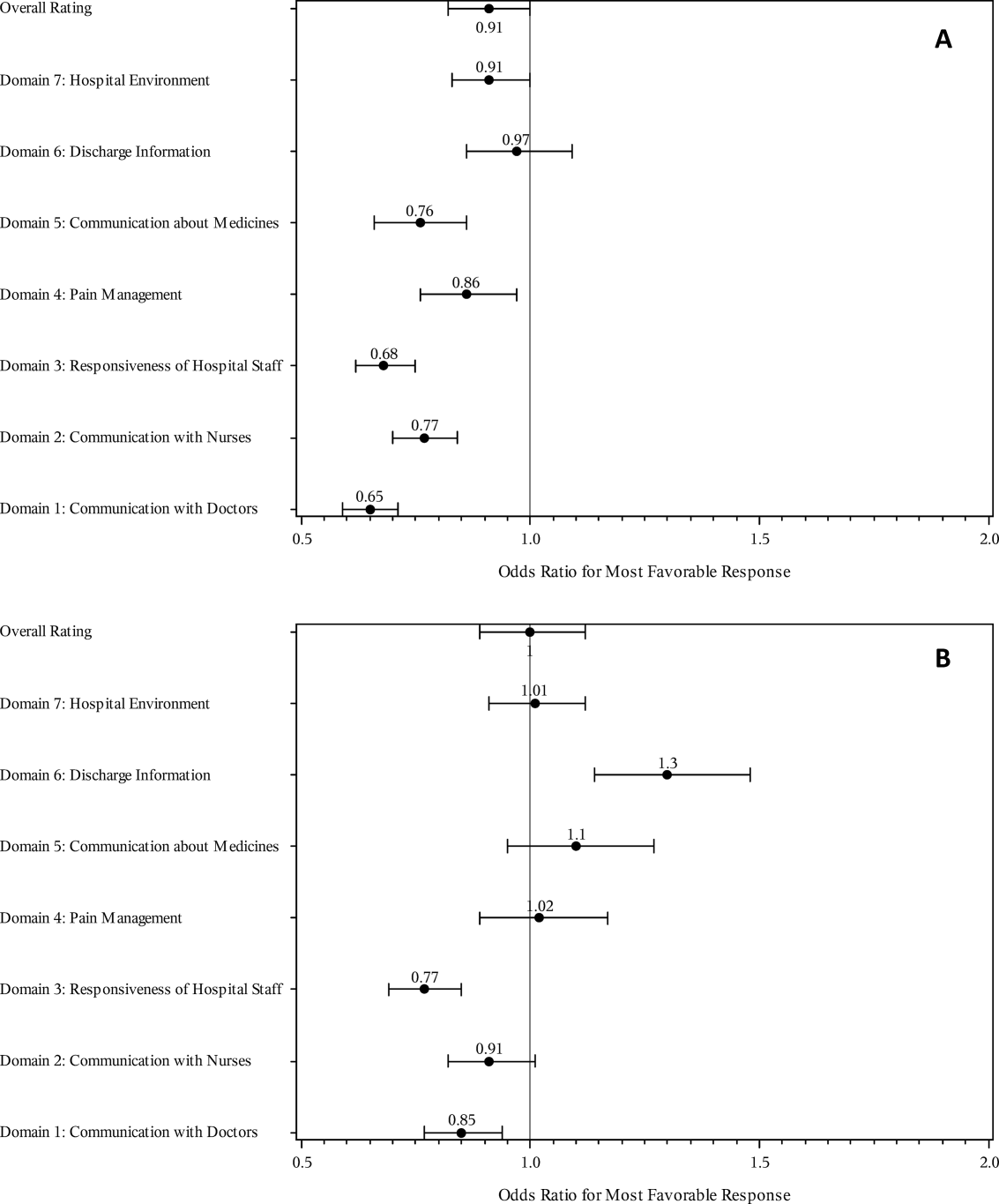
The multivariable regression models examined whether the mortality risk on admission simply represented older medical patients and/or those who considered themselves unhealthy (Figure 2B) (see Supporting Table 1 in the online version of this article). Accounting for hospital, age, gender, language, self‐reported health, educational level, service line, and days elapsed from discharge, respondents in the high‐mortality‐risk stratum were still less likely to report an always experience for doctor communication (OR: 0.85; 95% confidence interval [CI]: 0.77‐0.94) and responsiveness of hospital staff (OR: 0.77; 95% CI: 0.69‐0.85). Higher‐risk patients also tended to have less favorable experiences with nursing communication, although the CI crossed 1 (OR: 0.91; 95% CI: 0.82‐1.01). In contrast, higher‐risk patients were more likely to provide top box responses for having received discharge information (OR: 1.30; 95% CI: 1.14‐1.48). We did not find independent associations between mortality risk and the other domains when the patient risk‐adjustment factors were considered.[18, 19, 20, 21]
DISCUSSION
The high‐mortality‐risk stratum on admission contained a subset of patients who provided less favorable responses for almost all incentivized HCAHPS domains when other risk‐adjustment variables were not taken into consideration (Figure 2A). These univariate relationships weakened when we controlled for gender, the standard HCAHPS risk‐adjustment variables, and individual hospital influences (Figure 2B).[18, 19, 20, 21] After multivariable adjustment, survey respondents in the high‐risk category remained less likely to report their physicians always communicated well and to experience hospital staff responding quickly, but were more likely to report receiving discharge information. We did not find an independent association between the underlying mortality risk and the other incentivized HCAHPS domains after risk adjustment.
We are cautious with initial interpretations of our findings in light of the relatively small number of hospitals studied and the substantial survey response bias of healthier patients. Undoubtedly, the CMS exclusions of patients discharged to hospice or skilled nursing facilities provide a partial explanation for the selection bias, but the experience of those at high risk who did not complete surveys remains conjecture at this point.[14] Previous evidence suggests sicker patients and those with worse experiences are less likely to respond to the HCAHPS survey.[18, 22] On the other hand, it is possible that high‐risk nonrespondents who died could have received better communication and staff responsiveness.[23, 24] We were unable to find a previous, patient‐level study that explicitly tested the association between the admission mortality risk and the subsequent patient experience, yet our findings are consistent with a previous single‐site study of a surgical population showing lower overall ratings from patients with higher Injury Severity Scores.[25]
Our findings provide evidence of complex relationships among admission mortality risk, the 3 domains of the patient experience, and adverse outcomes, at least within the study hospitals (Figure 1). The developing field of palliative care has found very ill patients have special communication needs regarding goals of care, as well as physical symptoms, anxiety, and depression that might prompt more calls for help.[26] If these needs were more important for high‐risk compared to low‐risk patients, and were either not recognized or adequately addressed by the clinical teams at the study hospitals, then the high‐risk patients may have been less likely to perceive their physicians listened and explained things well, or that staff responded promptly to their requests for help.[27] On the other hand, the higher ratings for discharge information suggest the needs of the high‐risk patients were relatively easier to address by current practices at these hospitals. The lack of association between the mortality risk and the other HCAHPS domains may reflect the relatively stronger influence of age, gender, educational level, provider variability, and other unmeasured influences within the study sites, or that the level of patient need was similar among high‐risk and low‐risk patients within these domains.[27]
There are several possible confounders of our observed relationship between mortality risk and HCAHPS scores. The first category of confounders represents patient level variables that might impact the communication scores, some of which are part of the formula of our mortality prediction rule, for example, cognitive impairment and emergent admission.[18, 22, 27] The effect of the mortality risk could also be confounded by unmeasured patient‐level factors such as lower socioeconomic status.[28] A second category of confounders pertains to clinical outcomes and processes of care associated with serious illness irrespective of the risk of dying. More physicians involved in the care of the seriously ill (Table 1) may impact the communication scores, due to the larger opportunity for conflicting or confusing information presented to patients and their families.[29] The longer hospital stays, readmissions, and adverse events of the seriously ill may also underlie the apparent association between mortality risk and HCAHPS scores.[8, 9, 10]
Even if we do not understand precisely if and how the mortality risk might be associated with suboptimal physician communication and staff responsiveness, there may still be some value in considering how these possible relationships could be leveraged to improve patient care. We recall Berwick's insight that every system is perfectly designed to achieve the results it achieves.[7] We have previously argued for the use of mortality‐risk strata to initiate concurrent, multidisciplinary care processes to reduce adverse outcomes.[12, 13] Others have used risk‐based approaches for anticipating clinical deterioration of surgical patients, and determining the intensity of individualized case management services.[30, 31] In this framework, all patients receive a standard set of care processes, but higher‐risk patients receive additional efforts to promote better outcomes. An efficient extension of this approach is to assume patients at risk for adverse outcomes also have additional needs for communication, coordination of specialty care, and timely response to the call button. The admission mortality risk could be used as a determinant for the level of nurse staffing to reduce deaths plus shorten response time to the call button.[32, 33] Hospitalists and specialists could work together on a standard way to conference among themselves for high‐risk patients above that needed for less‐complex cases. Patients in the high‐risk strata could be screened early to see if they might benefit from the involvement of the palliative care team.[26]
Our study has limitations in addition to those already noted. First, our use of the top box as the formulation of the outcome of interest could be challenged. We chose this to be relevant to the Value‐Based Purchasing environment, but other formulations or use of other survey instruments may be needed to tease out the complex relationships we hypothesize. Next, we do not know the extent to which the patients and care processes reflected in our study represent other settings. The literature indicates some hospitals are more effective in providing care for certain subgroups of patients than for others, and that there is substantial regional variation in care intensity that is in turn associated with the patient experience.[29, 34] The mortality‐risk experience relationship for nonstudy hospitals could be weaker or stronger than what we found. Third, many hospitals may not have the capability to generate mortality scores on admission, although more hospitals may be able to do so in the future.[35] Explicit risk strata have the benefit of providing members of the multidisciplinary team with a quick preview of the clinical needs and prognoses of patients in much the way that the term baroque alerts the audience to the genre of music. Still, clinicians in any hospital could attempt to improve outcomes and experience through the use of informal risk assessment during interdisciplinary care rounds or by simply asking the team if they would be surprised if this patient died in the next year.[30, 36] Finally, we do not know if awareness of an experience risk will identify remediable practices that actually improve the experience. Clearly, future studies are needed to answer all of these concerns.
We have provided evidence that a group of patients who were at elevated risk for dying at the time of admission were more likely to have issues with physician communication and staff responsiveness than their lower‐risk counterparts. While we await future studies to confirm these findings, clinical teams can consider whether or not their patients' HCAHPS scores reflect how their system of care addresses the needs of these vulnerable people.
Acknowledgements
The authors thank Steven Lewis for assistance in the interpretation of the HCAHPS scores, Bonita Singal, MD, PhD, for initial statistical consultation, and Frank Smith, MD, for reviewing an earlier version of the manuscript. The authors acknowledge the input of the peer reviewers.
Disclosures: Dr. Cowen and Mr. Kabara had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: all authors. Acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: Dr. Cowen and Mr. Kabara. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Dr. Cowen and Mr. Kabara. Administrative, technical or material support: Ms. Czerwinski. Study supervision: Dr. Cowen and Ms. Czerwinski. Funding/support: internal. Conflicts of interest disclosures: no potential conflicts reported.
Disclosures
Dr. Cowen and Mr. Kabara had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: all authors. Acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: Dr. Cowen and Mr. Kabara. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Dr. Cowen and Mr. Kabara. Administrative, technical or material support: Ms. Czerwinski. Study supervision: Dr. Cowen and Ms. Czerwinski. Funding/support: internal. Conflicts of interest disclosures: no potential conflicts reported.
- , , , , . Measuring hospital care from the patients' perspective: an overview of the CAHPS hospital survey development process. Health Serv Res. 2005;40 (6 part 2):1977–1995.
- Centers for Medicare 79(163):49854–50449.
- , , , . The relationship between patients' perception of care and measures of hospital quality and safety. Health Serv Res. 2010;45(4):1024–1040.
- Centers for Medicare 312(7031):619–622.
- , , , , . Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41–48.
- , , , , , . Getting satisfaction: drivers of surgical Hospital Consumer Assessment of Health care Providers and Systems survey scores. J Surg Res. 2015;197(1):155–161.
- , , . Patient satisfaction and quality of surgical care in US hospitals. Ann Surg. 2015;261(1):2–8.
- , , . Is there a relationship between patient satisfaction and favorable outcomes? Ann Surg. 2014;260(4):592–598; discussion 598–600.
- , , , , . Mortality predictions on admission as a context for organizing care activities. J Hosp Med. 2013;8(5):229–235.
- , , , et al. Implementation of a mortality prediction rule for real‐time decision making: feasibility and validity. J Hosp Med. 2014;9(11):720–726.
- Centers for Medicare 40(6 pt 2):2078–2095.
- Centers for Medicare 44(2 pt 1):501–518.
- Patient‐mix coefficients for October 2015 (1Q14 through 4Q14 discharges) publicly reported HCAHPS Results. Available at: http://www.hcahpsonline.org/Files/October_2015_PMA_Web_Document_a.pdf. Published July 2, 2015. Accessed August 4, 2015.
- , , , , . Case‐mix adjustment of the CAHPS hospital survey. Health Serv Res. 2005;40(6):2162–2181.
- , , , et.al. Gender differences in patients' perceptions of inpatient care. Health Serv Res. 2012;47(4):1482–1501.
- , , , et al. Patterns of unit and item nonresponse in the CAHPS hospital survey. Health Serv Res. 2005;40(6 pt 2):2096–2119.
- , , , . The cost of satisfaction: a national study of patient satisfaction, health care utilization, expenditures, and mortality. Arch Intern Med. 2012;172(5):405–411.
- , , , et al. Care experiences of managed care Medicare enrollees near the end of life. J Am Geriatr Soc. 2013;61(3):407–412.
- , , , , . Measuring satisfaction: factors that drive hospital consumer assessment of healthcare providers and systems survey responses in a trauma and acute care surgery population. Am Surg. 2015;81(5):537–543.
- , . Palliative care for the seriously ill. N Engl J Med. 2015;373(8):747–755.
- , , , et.al. Components of care vary in importance for overall patient‐reported experience by type of hospitalization. Med Care. 2009;47(8):842–849.
- , , , et al. Socioeconomic status, structural and functional measures of social support, and mortality: the British Whitehall II cohort study, 1985–2009. Am J Epidemiol. 2012;175(12):1275–1283.
- , , , et al. Inpatient care intensity and patients' ratings of their hospital experiences. Health Aff (Millwood). 2009;28(1):103–112.
- , , , et al. A validated value‐based model to improve hospital‐wide perioperative outcomes. Ann Surg. 2010;252(3):486–498.
- , , , et al. Allocating scare resources in real‐time to reduce heart failure readmissions: a prospective, controlled study. BMJ Qual Saf. 2013;22(12):998–1005.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- , , , , , . Nurse staffing and inpatient hospital mortality. N Engl J Med. 2011;364(11):1037–1045.
- , , , et al. Do hospitals rank differently on HCAHPS for different patient subgroups? Med Care Res Rev. 2010;67(1):56–73.
- , , , , , . Risk‐adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232–239.
- , , , et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3(5):1379–1384.
Few today deny the importance of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey.[1, 2] The Centers for Medicare and Medicaid Services' (CMS) Value Based Purchasing incentive, sympathy for the ill, and relationships between the patient experience and quality of care provide sufficient justification.[3, 4] How to improve the experience scores is not well understood. The national scores have improved only modestly over the past 3 years.[5, 6]
Clinicians may not typically compartmentalize what they do to improve outcomes versus the patient experience. A possible source for new improvement strategies is to understand the types of patients in which both adverse outcomes and suboptimal experiences are likely to occur, then redesign the multidisciplinary care processes to address both concurrently.[7] Previous studies support the existence of a relationship between a higher mortality risk on admission and subsequent worse outcomes, as well as a relationship between worse outcomes and lower HCAHPS scores.[8, 9, 10, 11, 12, 13] We hypothesized the mortality risk on admission, patient experience, and outcomes might share a triad relationship (Figure 1). In this article we explore the third edge of this triangle, the association between the mortality risk on admission and the subsequent patient experience.

METHODS
We studied HCAHPS from 5 midwestern US hospitals having 113, 136, 304, 443, and 537 licensed beds, affiliated with the same multistate healthcare system. HCAHPS telephone surveys were administered via a vendor to a random sample of inpatients 18 years of age or older discharged from January 1, 2012 through June 30, 2014. Per CMS guidelines, surveyed patients must have been discharged alive after a hospital stay of at least 1 night.[14] Patients ineligible to be surveyed included those discharged to skilled nursing facilities or hospice care.[14] Because not all study hospitals provided obstetrical services, we restricted the analyses to medical and surgical respondents. With the permission of the local institutional review board, subjects' survey responses were linked confidentially to their clinical data.
We focused on the 8 dimensions of the care experience used in the CMS Value Based Purchasing program: communication with doctors, communication with nurses, responsiveness of hospital staff, pain management, communication about medicines, discharge information, hospital environment, and an overall rating of the hospital.[2] Following the scoring convention for publicly reported results, we dichotomized the 4‐level Likert scales into the most favorable response possible (always) versus all other responses.[15] Similarly we dichotomized the hospital rating scale at 9 and above for the most favorable response.
Our unit of analysis was an individual hospitalization. Our primary outcome of interest was whether or not the respondent provided the most favorable response for all questions answered within a given domain. For example, for the physician communication domain, the patient must have answered always to each of the 3 questions answered within the domain. This approach is appropriate for learning which patient‐level factors influence the survey responses, but differs from that used for the publically reported domain scores for which the relative performance of hospitals is the focus.[16] For the latter, the hospital was the unit of analysis, and the domain score was basically the average of the percentages of top box scores for the questions within a domain. For example, if 90% respondents from a hospital provided a top box response for courtesy, 80% for listening, and 70% for explanation, the hospital's physician communication score would be (90 + 80 + 70)/3 = 80%.[17]
Our primary explanatory variable was a binary high versus low mortality‐risk status of the respondent on admission based on age, gender, prior hospitalizations, clinical laboratory values, and diagnoses present on admission.[12] The calculated mortality risk was then dichotomized prior to the analysis at a probability of dying equal to 0.07 or higher. This corresponded roughly to the top quintile of predicted risk found in prior studies.[12, 13] During the study period, only 2 of the hospitals had the capability of generating mortality scores in real time, so for this study the mortality risk was calculated retrospectively, using information deemed present on admission.[12]
To estimate the sample size, we assumed that the high‐risk strata contained approximately 13% of respondents, and that the true percent of top box responses from patients in the lower‐risk stratum was approximately 80% for each domain. A meaningful difference in the proportion of most favorable responses was considered as an odds ratio (OR) of 0.75 for high risk versus low risk. A significance level of P < 0.003 was set to control study‐wide type I error due to multiple comparisons. We determined that for each dimension, approximately 8583 survey responses would be required for low‐risk patients and approximately 1116 responses for high‐risk patients to achieve 80% power under these assumptions. We were able to accrue the target number of surveys for all but 3 domains (pain management, communication about medicines, and hospital environment) because of data availability, and because patients are allowed to skip questions that do not apply. Univariate relationships were examined with 2, t test, and Fisher exact tests where indicated. Generalized linear mixed regression models with a logit link were fit to determine the association between patient mortality risk and the top box experience for each of the HCAHPS domains and for the overall rating. The patient's hospital was considered a random intercept to account for the patient‐hospital hierarchy and the unmeasured hospital‐specific practices. The multivariable models controlled for gender plus the HCAHPS patient‐mix adjustment variables of age, education, self‐rated health, language spoken at home, service line, and the number of days elapsed between the date of discharge and date of the survey.[18, 19, 20, 21] In keeping with the industry analyses, a second order interaction variable was included between surgery patients and age.[19] We considered the potential collinearity between the mortality risk status, age, and patient self‐reported health. We found the variance inflation factors were small, so we drew inference from the full multivariable model.
We also performed a post hoc sensitivity analysis to determine if our conclusions were biased due to missing patient responses for the risk‐adjustment variables. Accordingly, we imputed the response level most negatively associated with most HCAHPS domains as previously reported and reran the multivariable models.[19] We did not find a meaningful change in our conclusions (see Supporting Figure 1 in the online version of this article).
RESULTS
The hospitals discharged 152,333 patients during the study period, 39,905 of whom (26.2 %) had a predicted 30‐day mortality risk greater or equal to 0.07 (Table 1). Of the 36,280 high‐risk patients discharged alive, 5901 (16.3%) died in the ensuing 30 days, and 7951 (22%) were readmitted.
| Characteristic | Low‐Risk Stratum, No./Discharged (%) or Mean (SD) | High‐Risk Stratum, No./Discharged (%) or Mean (SD) | P Value* |
|---|---|---|---|
| |||
| Total discharges (row percent) | 112,428/152,333 (74) | 39,905/152,333 (26) | <0.001 |
| Total alive discharges (row percent) | 111,600/147,880 (75) | 36,280/147,880 (25) | <0.001 |
| No. of respondents (row percent) | 14,996/17,509 (86) | 2,513/17,509 (14) | |
| HCAHPS surveys completed | 14,996/111,600 (13) | 2,513/36,280 (7) | < 0.001 |
| Readmissions within 30 days (total discharges) | 12,311/112,428 (11) | 7,951/39,905 (20) | <0.001 |
| Readmissions within 30 days (alive discharges) | 12,311/111,600 (11) | 7,951/36,280 (22) | <0.001 |
| Readmissions within 30 days (respondents) | 1,220/14,996 (8) | 424/2,513 (17) | <0.001 |
| Mean predicted probability of 30‐day mortality (total discharges) | 0.022 (0.018) | 0.200 (0.151) | <0.001 |
| Mean predicted probability of 30‐day mortality (alive discharges) | 0.022 (0.018) | 0.187 (0.136) | <0.001 |
| Mean predicted probability of 30‐day mortality (respondents) | 0.020 (0.017) | 0.151 (0.098) | <0.001 |
| In‐hospital death (total discharges) | 828/112,428 (0.74) | 3,625/39,905 (9) | <0.001 |
| Mortality within 30 days (total discharges) | 2,455/112,428 (2) | 9,526/39,905 (24) | <0.001 |
| Mortality within 30 days (alive discharges) | 1,627/111,600 (1.5) | 5,901/36,280 (16) | <0.001 |
| Mortality within 30 days (respondents) | 9/14,996 (0.06) | 16/2,513 (0.64) | <0.001 |
| Female (total discharges) | 62,681/112,368 (56) | 21,058/39,897 (53) | <0.001 |
| Female (alive discharges) | 62,216/111,540 (56) | 19,164/36,272 (53) | <0.001 |
| Female (respondents) | 8,684/14,996 (58) | 1,318/2,513 (52) | <0.001 |
| Age (total discharges) | 61.3 (16.8) | 78.3 (12.5) | <0.001 |
| Age (alive discharges) | 61.2 (16.8) | 78.4 (12.5) | <0.001 |
| Age (respondents) | 63.1 (15.2) | 76.6 (11.5) | <0.001 |
| Highest education attained | |||
| 8th grade or less | 297/14,996 (2) | 98/2,513 (4) | |
| Some high school | 1,190/14,996 (8) | 267/2,513 (11) | |
| High school grad | 4,648/14,996 (31) | 930/2,513 (37) | <0.001 |
| Some college | 6,338/14,996 (42) | 768/2,513 (31) | |
| 4‐year college grad | 1,502/14,996 (10) | 183/2,513 (7) | |
| Missing response | 1,021/14,996 (7) | 267/2,513 (11) | |
| Language spoken at home | |||
| English | 13,763/14,996 (92) | 2,208/2,513 (88) | |
| Spanish | 56/14,996 (0.37) | 8/2,513 (0.32) | 0.47 |
| Chinese | 153/14,996 (1) | 31/2,513 (1) | |
| Missing response | 1,024/14,996 (7) | 266/2,513 (11) | |
| Self‐rated health | |||
| Excellent | 1,399/14,996 (9) | 114/2,513 (5) | |
| Very good | 3,916/14,996 (26) | 405/2,513 (16) | |
| Good | 4,861/14,996 (32) | 713/2,513 (28) | |
| Fair | 2,900/14,996 (19) | 652/2,513 (26) | <0.001 |
| Poor | 1,065/14,996 (7) | 396/2,513 (16) | |
| Missing response | 855/14,996 (6) | 233/2,513 (9) | |
| Length of hospitalization, d (respondents) | 3.5 (2.8) | 4.6 (3.6) | <0.001 |
| Consulting specialties (respondents) | 1.7 (1.0) | 2.2 (1.3) | <0.001 |
| Service line | |||
| Surgical | 6,380/14,996 (43) | 346/2,513 (14) | <0.001 |
| Medical | 8,616/14,996 (57) | 2,167/2,513 (86) | |
| HCAHPS | |||
| Domain 1: Communication With Doctors | 9,564/14,731 (65) | 1,339/2,462 (54) | <0.001 |
| Domain 2: Communication With Nurses | 10,097/14,991 (67) | 1,531/2,511 (61) | <0.001 |
| Domain 3: Responsiveness of Hospital Staff | 7,813/12,964 (60) | 1,158/2,277 (51) | <0.001 |
| Domain 4: Pain Management | 6,565/10,424 (63) | 786/1,328 (59) | 00.007 |
| Domain 5: Communication About Medicines | 3,769/8,088 (47) | 456/1,143 (40) | <0.001 |
| Domain 6: Discharge Information | 11,331/14,033 (81) | 1,767/2,230 (79) | 0.09 |
| Domain 7: Hospital Environment | 6,981/14,687 (48) | 1,093/2,451 (45) | 0.007 |
| Overall rating | 10,708/14,996 (71) | 1,695/2,513 (67) | <0.001 |
The high‐risk subset was under‐represented in those who completed the HCAHPS survey with 7% (2513/36,280) completing surveys compared to 13% of low‐risk patients (14,996/111,600) (P < 0.0001). Moreover, compared to high‐risk patients who were alive at discharge but did not complete surveys, high‐risk survey respondents were less likely to die within 30 days (16/2513 = 0.64% vs 5885/33,767 = 17.4%, P < 0.0001), and less likely to be readmitted (424/2513 = 16.9% vs 7527/33,767 = 22.3%, P < 0.0001).
On average, high‐risk respondents (compared to low risk) were slightly less likely to be female (52.4% vs 57.9%), less educated (30.6% with some college vs 42.3%), less likely to have been on a surgical service (13.8% vs 42.5%), and less likely to report good or better health (49.0% vs 68.0%, all P < 0.0001). High‐risk respondents were also older (76.6 vs 63.1 years), stayed in the hospital longer (4.6 vs 3.5 days), and received care from more specialties (2.2 vs 1.7 specialties) (all P < 0.0001). High‐risk respondents experienced more 30‐day readmissions (16.9% vs 8.1%) and deaths within 30 days (0.6 % vs 0.1 %, all P < 0.0001) than their low‐risk counterparts.
High‐risk respondents were less likely to provide the most favorable response (unadjusted) for all HCAHPS domains compared to low‐risk respondents, although the difference was not significant for discharge information (Table 1, Figure 2A). The gradient between high‐risk and low‐risk patients was seen for all domains within each hospital except for pain management, hospital environment, and overall rating (Figure 3).


The multivariable regression models examined whether the mortality risk on admission simply represented older medical patients and/or those who considered themselves unhealthy (Figure 2B) (see Supporting Table 1 in the online version of this article). Accounting for hospital, age, gender, language, self‐reported health, educational level, service line, and days elapsed from discharge, respondents in the high‐mortality‐risk stratum were still less likely to report an always experience for doctor communication (OR: 0.85; 95% confidence interval [CI]: 0.77‐0.94) and responsiveness of hospital staff (OR: 0.77; 95% CI: 0.69‐0.85). Higher‐risk patients also tended to have less favorable experiences with nursing communication, although the CI crossed 1 (OR: 0.91; 95% CI: 0.82‐1.01). In contrast, higher‐risk patients were more likely to provide top box responses for having received discharge information (OR: 1.30; 95% CI: 1.14‐1.48). We did not find independent associations between mortality risk and the other domains when the patient risk‐adjustment factors were considered.[18, 19, 20, 21]
DISCUSSION
The high‐mortality‐risk stratum on admission contained a subset of patients who provided less favorable responses for almost all incentivized HCAHPS domains when other risk‐adjustment variables were not taken into consideration (Figure 2A). These univariate relationships weakened when we controlled for gender, the standard HCAHPS risk‐adjustment variables, and individual hospital influences (Figure 2B).[18, 19, 20, 21] After multivariable adjustment, survey respondents in the high‐risk category remained less likely to report their physicians always communicated well and to experience hospital staff responding quickly, but were more likely to report receiving discharge information. We did not find an independent association between the underlying mortality risk and the other incentivized HCAHPS domains after risk adjustment.
We are cautious with initial interpretations of our findings in light of the relatively small number of hospitals studied and the substantial survey response bias of healthier patients. Undoubtedly, the CMS exclusions of patients discharged to hospice or skilled nursing facilities provide a partial explanation for the selection bias, but the experience of those at high risk who did not complete surveys remains conjecture at this point.[14] Previous evidence suggests sicker patients and those with worse experiences are less likely to respond to the HCAHPS survey.[18, 22] On the other hand, it is possible that high‐risk nonrespondents who died could have received better communication and staff responsiveness.[23, 24] We were unable to find a previous, patient‐level study that explicitly tested the association between the admission mortality risk and the subsequent patient experience, yet our findings are consistent with a previous single‐site study of a surgical population showing lower overall ratings from patients with higher Injury Severity Scores.[25]
Our findings provide evidence of complex relationships among admission mortality risk, the 3 domains of the patient experience, and adverse outcomes, at least within the study hospitals (Figure 1). The developing field of palliative care has found very ill patients have special communication needs regarding goals of care, as well as physical symptoms, anxiety, and depression that might prompt more calls for help.[26] If these needs were more important for high‐risk compared to low‐risk patients, and were either not recognized or adequately addressed by the clinical teams at the study hospitals, then the high‐risk patients may have been less likely to perceive their physicians listened and explained things well, or that staff responded promptly to their requests for help.[27] On the other hand, the higher ratings for discharge information suggest the needs of the high‐risk patients were relatively easier to address by current practices at these hospitals. The lack of association between the mortality risk and the other HCAHPS domains may reflect the relatively stronger influence of age, gender, educational level, provider variability, and other unmeasured influences within the study sites, or that the level of patient need was similar among high‐risk and low‐risk patients within these domains.[27]
There are several possible confounders of our observed relationship between mortality risk and HCAHPS scores. The first category of confounders represents patient level variables that might impact the communication scores, some of which are part of the formula of our mortality prediction rule, for example, cognitive impairment and emergent admission.[18, 22, 27] The effect of the mortality risk could also be confounded by unmeasured patient‐level factors such as lower socioeconomic status.[28] A second category of confounders pertains to clinical outcomes and processes of care associated with serious illness irrespective of the risk of dying. More physicians involved in the care of the seriously ill (Table 1) may impact the communication scores, due to the larger opportunity for conflicting or confusing information presented to patients and their families.[29] The longer hospital stays, readmissions, and adverse events of the seriously ill may also underlie the apparent association between mortality risk and HCAHPS scores.[8, 9, 10]
Even if we do not understand precisely if and how the mortality risk might be associated with suboptimal physician communication and staff responsiveness, there may still be some value in considering how these possible relationships could be leveraged to improve patient care. We recall Berwick's insight that every system is perfectly designed to achieve the results it achieves.[7] We have previously argued for the use of mortality‐risk strata to initiate concurrent, multidisciplinary care processes to reduce adverse outcomes.[12, 13] Others have used risk‐based approaches for anticipating clinical deterioration of surgical patients, and determining the intensity of individualized case management services.[30, 31] In this framework, all patients receive a standard set of care processes, but higher‐risk patients receive additional efforts to promote better outcomes. An efficient extension of this approach is to assume patients at risk for adverse outcomes also have additional needs for communication, coordination of specialty care, and timely response to the call button. The admission mortality risk could be used as a determinant for the level of nurse staffing to reduce deaths plus shorten response time to the call button.[32, 33] Hospitalists and specialists could work together on a standard way to conference among themselves for high‐risk patients above that needed for less‐complex cases. Patients in the high‐risk strata could be screened early to see if they might benefit from the involvement of the palliative care team.[26]
Our study has limitations in addition to those already noted. First, our use of the top box as the formulation of the outcome of interest could be challenged. We chose this to be relevant to the Value‐Based Purchasing environment, but other formulations or use of other survey instruments may be needed to tease out the complex relationships we hypothesize. Next, we do not know the extent to which the patients and care processes reflected in our study represent other settings. The literature indicates some hospitals are more effective in providing care for certain subgroups of patients than for others, and that there is substantial regional variation in care intensity that is in turn associated with the patient experience.[29, 34] The mortality‐risk experience relationship for nonstudy hospitals could be weaker or stronger than what we found. Third, many hospitals may not have the capability to generate mortality scores on admission, although more hospitals may be able to do so in the future.[35] Explicit risk strata have the benefit of providing members of the multidisciplinary team with a quick preview of the clinical needs and prognoses of patients in much the way that the term baroque alerts the audience to the genre of music. Still, clinicians in any hospital could attempt to improve outcomes and experience through the use of informal risk assessment during interdisciplinary care rounds or by simply asking the team if they would be surprised if this patient died in the next year.[30, 36] Finally, we do not know if awareness of an experience risk will identify remediable practices that actually improve the experience. Clearly, future studies are needed to answer all of these concerns.
We have provided evidence that a group of patients who were at elevated risk for dying at the time of admission were more likely to have issues with physician communication and staff responsiveness than their lower‐risk counterparts. While we await future studies to confirm these findings, clinical teams can consider whether or not their patients' HCAHPS scores reflect how their system of care addresses the needs of these vulnerable people.
Acknowledgements
The authors thank Steven Lewis for assistance in the interpretation of the HCAHPS scores, Bonita Singal, MD, PhD, for initial statistical consultation, and Frank Smith, MD, for reviewing an earlier version of the manuscript. The authors acknowledge the input of the peer reviewers.
Disclosures: Dr. Cowen and Mr. Kabara had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: all authors. Acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: Dr. Cowen and Mr. Kabara. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Dr. Cowen and Mr. Kabara. Administrative, technical or material support: Ms. Czerwinski. Study supervision: Dr. Cowen and Ms. Czerwinski. Funding/support: internal. Conflicts of interest disclosures: no potential conflicts reported.
Disclosures
Dr. Cowen and Mr. Kabara had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: all authors. Acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: Dr. Cowen and Mr. Kabara. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Dr. Cowen and Mr. Kabara. Administrative, technical or material support: Ms. Czerwinski. Study supervision: Dr. Cowen and Ms. Czerwinski. Funding/support: internal. Conflicts of interest disclosures: no potential conflicts reported.
Few today deny the importance of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey.[1, 2] The Centers for Medicare and Medicaid Services' (CMS) Value Based Purchasing incentive, sympathy for the ill, and relationships between the patient experience and quality of care provide sufficient justification.[3, 4] How to improve the experience scores is not well understood. The national scores have improved only modestly over the past 3 years.[5, 6]
Clinicians may not typically compartmentalize what they do to improve outcomes versus the patient experience. A possible source for new improvement strategies is to understand the types of patients in which both adverse outcomes and suboptimal experiences are likely to occur, then redesign the multidisciplinary care processes to address both concurrently.[7] Previous studies support the existence of a relationship between a higher mortality risk on admission and subsequent worse outcomes, as well as a relationship between worse outcomes and lower HCAHPS scores.[8, 9, 10, 11, 12, 13] We hypothesized the mortality risk on admission, patient experience, and outcomes might share a triad relationship (Figure 1). In this article we explore the third edge of this triangle, the association between the mortality risk on admission and the subsequent patient experience.

METHODS
We studied HCAHPS from 5 midwestern US hospitals having 113, 136, 304, 443, and 537 licensed beds, affiliated with the same multistate healthcare system. HCAHPS telephone surveys were administered via a vendor to a random sample of inpatients 18 years of age or older discharged from January 1, 2012 through June 30, 2014. Per CMS guidelines, surveyed patients must have been discharged alive after a hospital stay of at least 1 night.[14] Patients ineligible to be surveyed included those discharged to skilled nursing facilities or hospice care.[14] Because not all study hospitals provided obstetrical services, we restricted the analyses to medical and surgical respondents. With the permission of the local institutional review board, subjects' survey responses were linked confidentially to their clinical data.
We focused on the 8 dimensions of the care experience used in the CMS Value Based Purchasing program: communication with doctors, communication with nurses, responsiveness of hospital staff, pain management, communication about medicines, discharge information, hospital environment, and an overall rating of the hospital.[2] Following the scoring convention for publicly reported results, we dichotomized the 4‐level Likert scales into the most favorable response possible (always) versus all other responses.[15] Similarly we dichotomized the hospital rating scale at 9 and above for the most favorable response.
Our unit of analysis was an individual hospitalization. Our primary outcome of interest was whether or not the respondent provided the most favorable response for all questions answered within a given domain. For example, for the physician communication domain, the patient must have answered always to each of the 3 questions answered within the domain. This approach is appropriate for learning which patient‐level factors influence the survey responses, but differs from that used for the publically reported domain scores for which the relative performance of hospitals is the focus.[16] For the latter, the hospital was the unit of analysis, and the domain score was basically the average of the percentages of top box scores for the questions within a domain. For example, if 90% respondents from a hospital provided a top box response for courtesy, 80% for listening, and 70% for explanation, the hospital's physician communication score would be (90 + 80 + 70)/3 = 80%.[17]
Our primary explanatory variable was a binary high versus low mortality‐risk status of the respondent on admission based on age, gender, prior hospitalizations, clinical laboratory values, and diagnoses present on admission.[12] The calculated mortality risk was then dichotomized prior to the analysis at a probability of dying equal to 0.07 or higher. This corresponded roughly to the top quintile of predicted risk found in prior studies.[12, 13] During the study period, only 2 of the hospitals had the capability of generating mortality scores in real time, so for this study the mortality risk was calculated retrospectively, using information deemed present on admission.[12]
To estimate the sample size, we assumed that the high‐risk strata contained approximately 13% of respondents, and that the true percent of top box responses from patients in the lower‐risk stratum was approximately 80% for each domain. A meaningful difference in the proportion of most favorable responses was considered as an odds ratio (OR) of 0.75 for high risk versus low risk. A significance level of P < 0.003 was set to control study‐wide type I error due to multiple comparisons. We determined that for each dimension, approximately 8583 survey responses would be required for low‐risk patients and approximately 1116 responses for high‐risk patients to achieve 80% power under these assumptions. We were able to accrue the target number of surveys for all but 3 domains (pain management, communication about medicines, and hospital environment) because of data availability, and because patients are allowed to skip questions that do not apply. Univariate relationships were examined with 2, t test, and Fisher exact tests where indicated. Generalized linear mixed regression models with a logit link were fit to determine the association between patient mortality risk and the top box experience for each of the HCAHPS domains and for the overall rating. The patient's hospital was considered a random intercept to account for the patient‐hospital hierarchy and the unmeasured hospital‐specific practices. The multivariable models controlled for gender plus the HCAHPS patient‐mix adjustment variables of age, education, self‐rated health, language spoken at home, service line, and the number of days elapsed between the date of discharge and date of the survey.[18, 19, 20, 21] In keeping with the industry analyses, a second order interaction variable was included between surgery patients and age.[19] We considered the potential collinearity between the mortality risk status, age, and patient self‐reported health. We found the variance inflation factors were small, so we drew inference from the full multivariable model.
We also performed a post hoc sensitivity analysis to determine if our conclusions were biased due to missing patient responses for the risk‐adjustment variables. Accordingly, we imputed the response level most negatively associated with most HCAHPS domains as previously reported and reran the multivariable models.[19] We did not find a meaningful change in our conclusions (see Supporting Figure 1 in the online version of this article).
RESULTS
The hospitals discharged 152,333 patients during the study period, 39,905 of whom (26.2 %) had a predicted 30‐day mortality risk greater or equal to 0.07 (Table 1). Of the 36,280 high‐risk patients discharged alive, 5901 (16.3%) died in the ensuing 30 days, and 7951 (22%) were readmitted.
| Characteristic | Low‐Risk Stratum, No./Discharged (%) or Mean (SD) | High‐Risk Stratum, No./Discharged (%) or Mean (SD) | P Value* |
|---|---|---|---|
| |||
| Total discharges (row percent) | 112,428/152,333 (74) | 39,905/152,333 (26) | <0.001 |
| Total alive discharges (row percent) | 111,600/147,880 (75) | 36,280/147,880 (25) | <0.001 |
| No. of respondents (row percent) | 14,996/17,509 (86) | 2,513/17,509 (14) | |
| HCAHPS surveys completed | 14,996/111,600 (13) | 2,513/36,280 (7) | < 0.001 |
| Readmissions within 30 days (total discharges) | 12,311/112,428 (11) | 7,951/39,905 (20) | <0.001 |
| Readmissions within 30 days (alive discharges) | 12,311/111,600 (11) | 7,951/36,280 (22) | <0.001 |
| Readmissions within 30 days (respondents) | 1,220/14,996 (8) | 424/2,513 (17) | <0.001 |
| Mean predicted probability of 30‐day mortality (total discharges) | 0.022 (0.018) | 0.200 (0.151) | <0.001 |
| Mean predicted probability of 30‐day mortality (alive discharges) | 0.022 (0.018) | 0.187 (0.136) | <0.001 |
| Mean predicted probability of 30‐day mortality (respondents) | 0.020 (0.017) | 0.151 (0.098) | <0.001 |
| In‐hospital death (total discharges) | 828/112,428 (0.74) | 3,625/39,905 (9) | <0.001 |
| Mortality within 30 days (total discharges) | 2,455/112,428 (2) | 9,526/39,905 (24) | <0.001 |
| Mortality within 30 days (alive discharges) | 1,627/111,600 (1.5) | 5,901/36,280 (16) | <0.001 |
| Mortality within 30 days (respondents) | 9/14,996 (0.06) | 16/2,513 (0.64) | <0.001 |
| Female (total discharges) | 62,681/112,368 (56) | 21,058/39,897 (53) | <0.001 |
| Female (alive discharges) | 62,216/111,540 (56) | 19,164/36,272 (53) | <0.001 |
| Female (respondents) | 8,684/14,996 (58) | 1,318/2,513 (52) | <0.001 |
| Age (total discharges) | 61.3 (16.8) | 78.3 (12.5) | <0.001 |
| Age (alive discharges) | 61.2 (16.8) | 78.4 (12.5) | <0.001 |
| Age (respondents) | 63.1 (15.2) | 76.6 (11.5) | <0.001 |
| Highest education attained | |||
| 8th grade or less | 297/14,996 (2) | 98/2,513 (4) | |
| Some high school | 1,190/14,996 (8) | 267/2,513 (11) | |
| High school grad | 4,648/14,996 (31) | 930/2,513 (37) | <0.001 |
| Some college | 6,338/14,996 (42) | 768/2,513 (31) | |
| 4‐year college grad | 1,502/14,996 (10) | 183/2,513 (7) | |
| Missing response | 1,021/14,996 (7) | 267/2,513 (11) | |
| Language spoken at home | |||
| English | 13,763/14,996 (92) | 2,208/2,513 (88) | |
| Spanish | 56/14,996 (0.37) | 8/2,513 (0.32) | 0.47 |
| Chinese | 153/14,996 (1) | 31/2,513 (1) | |
| Missing response | 1,024/14,996 (7) | 266/2,513 (11) | |
| Self‐rated health | |||
| Excellent | 1,399/14,996 (9) | 114/2,513 (5) | |
| Very good | 3,916/14,996 (26) | 405/2,513 (16) | |
| Good | 4,861/14,996 (32) | 713/2,513 (28) | |
| Fair | 2,900/14,996 (19) | 652/2,513 (26) | <0.001 |
| Poor | 1,065/14,996 (7) | 396/2,513 (16) | |
| Missing response | 855/14,996 (6) | 233/2,513 (9) | |
| Length of hospitalization, d (respondents) | 3.5 (2.8) | 4.6 (3.6) | <0.001 |
| Consulting specialties (respondents) | 1.7 (1.0) | 2.2 (1.3) | <0.001 |
| Service line | |||
| Surgical | 6,380/14,996 (43) | 346/2,513 (14) | <0.001 |
| Medical | 8,616/14,996 (57) | 2,167/2,513 (86) | |
| HCAHPS | |||
| Domain 1: Communication With Doctors | 9,564/14,731 (65) | 1,339/2,462 (54) | <0.001 |
| Domain 2: Communication With Nurses | 10,097/14,991 (67) | 1,531/2,511 (61) | <0.001 |
| Domain 3: Responsiveness of Hospital Staff | 7,813/12,964 (60) | 1,158/2,277 (51) | <0.001 |
| Domain 4: Pain Management | 6,565/10,424 (63) | 786/1,328 (59) | 00.007 |
| Domain 5: Communication About Medicines | 3,769/8,088 (47) | 456/1,143 (40) | <0.001 |
| Domain 6: Discharge Information | 11,331/14,033 (81) | 1,767/2,230 (79) | 0.09 |
| Domain 7: Hospital Environment | 6,981/14,687 (48) | 1,093/2,451 (45) | 0.007 |
| Overall rating | 10,708/14,996 (71) | 1,695/2,513 (67) | <0.001 |
The high‐risk subset was under‐represented in those who completed the HCAHPS survey with 7% (2513/36,280) completing surveys compared to 13% of low‐risk patients (14,996/111,600) (P < 0.0001). Moreover, compared to high‐risk patients who were alive at discharge but did not complete surveys, high‐risk survey respondents were less likely to die within 30 days (16/2513 = 0.64% vs 5885/33,767 = 17.4%, P < 0.0001), and less likely to be readmitted (424/2513 = 16.9% vs 7527/33,767 = 22.3%, P < 0.0001).
On average, high‐risk respondents (compared to low risk) were slightly less likely to be female (52.4% vs 57.9%), less educated (30.6% with some college vs 42.3%), less likely to have been on a surgical service (13.8% vs 42.5%), and less likely to report good or better health (49.0% vs 68.0%, all P < 0.0001). High‐risk respondents were also older (76.6 vs 63.1 years), stayed in the hospital longer (4.6 vs 3.5 days), and received care from more specialties (2.2 vs 1.7 specialties) (all P < 0.0001). High‐risk respondents experienced more 30‐day readmissions (16.9% vs 8.1%) and deaths within 30 days (0.6 % vs 0.1 %, all P < 0.0001) than their low‐risk counterparts.
High‐risk respondents were less likely to provide the most favorable response (unadjusted) for all HCAHPS domains compared to low‐risk respondents, although the difference was not significant for discharge information (Table 1, Figure 2A). The gradient between high‐risk and low‐risk patients was seen for all domains within each hospital except for pain management, hospital environment, and overall rating (Figure 3).


The multivariable regression models examined whether the mortality risk on admission simply represented older medical patients and/or those who considered themselves unhealthy (Figure 2B) (see Supporting Table 1 in the online version of this article). Accounting for hospital, age, gender, language, self‐reported health, educational level, service line, and days elapsed from discharge, respondents in the high‐mortality‐risk stratum were still less likely to report an always experience for doctor communication (OR: 0.85; 95% confidence interval [CI]: 0.77‐0.94) and responsiveness of hospital staff (OR: 0.77; 95% CI: 0.69‐0.85). Higher‐risk patients also tended to have less favorable experiences with nursing communication, although the CI crossed 1 (OR: 0.91; 95% CI: 0.82‐1.01). In contrast, higher‐risk patients were more likely to provide top box responses for having received discharge information (OR: 1.30; 95% CI: 1.14‐1.48). We did not find independent associations between mortality risk and the other domains when the patient risk‐adjustment factors were considered.[18, 19, 20, 21]
DISCUSSION
The high‐mortality‐risk stratum on admission contained a subset of patients who provided less favorable responses for almost all incentivized HCAHPS domains when other risk‐adjustment variables were not taken into consideration (Figure 2A). These univariate relationships weakened when we controlled for gender, the standard HCAHPS risk‐adjustment variables, and individual hospital influences (Figure 2B).[18, 19, 20, 21] After multivariable adjustment, survey respondents in the high‐risk category remained less likely to report their physicians always communicated well and to experience hospital staff responding quickly, but were more likely to report receiving discharge information. We did not find an independent association between the underlying mortality risk and the other incentivized HCAHPS domains after risk adjustment.
We are cautious with initial interpretations of our findings in light of the relatively small number of hospitals studied and the substantial survey response bias of healthier patients. Undoubtedly, the CMS exclusions of patients discharged to hospice or skilled nursing facilities provide a partial explanation for the selection bias, but the experience of those at high risk who did not complete surveys remains conjecture at this point.[14] Previous evidence suggests sicker patients and those with worse experiences are less likely to respond to the HCAHPS survey.[18, 22] On the other hand, it is possible that high‐risk nonrespondents who died could have received better communication and staff responsiveness.[23, 24] We were unable to find a previous, patient‐level study that explicitly tested the association between the admission mortality risk and the subsequent patient experience, yet our findings are consistent with a previous single‐site study of a surgical population showing lower overall ratings from patients with higher Injury Severity Scores.[25]
Our findings provide evidence of complex relationships among admission mortality risk, the 3 domains of the patient experience, and adverse outcomes, at least within the study hospitals (Figure 1). The developing field of palliative care has found very ill patients have special communication needs regarding goals of care, as well as physical symptoms, anxiety, and depression that might prompt more calls for help.[26] If these needs were more important for high‐risk compared to low‐risk patients, and were either not recognized or adequately addressed by the clinical teams at the study hospitals, then the high‐risk patients may have been less likely to perceive their physicians listened and explained things well, or that staff responded promptly to their requests for help.[27] On the other hand, the higher ratings for discharge information suggest the needs of the high‐risk patients were relatively easier to address by current practices at these hospitals. The lack of association between the mortality risk and the other HCAHPS domains may reflect the relatively stronger influence of age, gender, educational level, provider variability, and other unmeasured influences within the study sites, or that the level of patient need was similar among high‐risk and low‐risk patients within these domains.[27]
There are several possible confounders of our observed relationship between mortality risk and HCAHPS scores. The first category of confounders represents patient level variables that might impact the communication scores, some of which are part of the formula of our mortality prediction rule, for example, cognitive impairment and emergent admission.[18, 22, 27] The effect of the mortality risk could also be confounded by unmeasured patient‐level factors such as lower socioeconomic status.[28] A second category of confounders pertains to clinical outcomes and processes of care associated with serious illness irrespective of the risk of dying. More physicians involved in the care of the seriously ill (Table 1) may impact the communication scores, due to the larger opportunity for conflicting or confusing information presented to patients and their families.[29] The longer hospital stays, readmissions, and adverse events of the seriously ill may also underlie the apparent association between mortality risk and HCAHPS scores.[8, 9, 10]
Even if we do not understand precisely if and how the mortality risk might be associated with suboptimal physician communication and staff responsiveness, there may still be some value in considering how these possible relationships could be leveraged to improve patient care. We recall Berwick's insight that every system is perfectly designed to achieve the results it achieves.[7] We have previously argued for the use of mortality‐risk strata to initiate concurrent, multidisciplinary care processes to reduce adverse outcomes.[12, 13] Others have used risk‐based approaches for anticipating clinical deterioration of surgical patients, and determining the intensity of individualized case management services.[30, 31] In this framework, all patients receive a standard set of care processes, but higher‐risk patients receive additional efforts to promote better outcomes. An efficient extension of this approach is to assume patients at risk for adverse outcomes also have additional needs for communication, coordination of specialty care, and timely response to the call button. The admission mortality risk could be used as a determinant for the level of nurse staffing to reduce deaths plus shorten response time to the call button.[32, 33] Hospitalists and specialists could work together on a standard way to conference among themselves for high‐risk patients above that needed for less‐complex cases. Patients in the high‐risk strata could be screened early to see if they might benefit from the involvement of the palliative care team.[26]
Our study has limitations in addition to those already noted. First, our use of the top box as the formulation of the outcome of interest could be challenged. We chose this to be relevant to the Value‐Based Purchasing environment, but other formulations or use of other survey instruments may be needed to tease out the complex relationships we hypothesize. Next, we do not know the extent to which the patients and care processes reflected in our study represent other settings. The literature indicates some hospitals are more effective in providing care for certain subgroups of patients than for others, and that there is substantial regional variation in care intensity that is in turn associated with the patient experience.[29, 34] The mortality‐risk experience relationship for nonstudy hospitals could be weaker or stronger than what we found. Third, many hospitals may not have the capability to generate mortality scores on admission, although more hospitals may be able to do so in the future.[35] Explicit risk strata have the benefit of providing members of the multidisciplinary team with a quick preview of the clinical needs and prognoses of patients in much the way that the term baroque alerts the audience to the genre of music. Still, clinicians in any hospital could attempt to improve outcomes and experience through the use of informal risk assessment during interdisciplinary care rounds or by simply asking the team if they would be surprised if this patient died in the next year.[30, 36] Finally, we do not know if awareness of an experience risk will identify remediable practices that actually improve the experience. Clearly, future studies are needed to answer all of these concerns.
We have provided evidence that a group of patients who were at elevated risk for dying at the time of admission were more likely to have issues with physician communication and staff responsiveness than their lower‐risk counterparts. While we await future studies to confirm these findings, clinical teams can consider whether or not their patients' HCAHPS scores reflect how their system of care addresses the needs of these vulnerable people.
Acknowledgements
The authors thank Steven Lewis for assistance in the interpretation of the HCAHPS scores, Bonita Singal, MD, PhD, for initial statistical consultation, and Frank Smith, MD, for reviewing an earlier version of the manuscript. The authors acknowledge the input of the peer reviewers.
Disclosures: Dr. Cowen and Mr. Kabara had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: all authors. Acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: Dr. Cowen and Mr. Kabara. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Dr. Cowen and Mr. Kabara. Administrative, technical or material support: Ms. Czerwinski. Study supervision: Dr. Cowen and Ms. Czerwinski. Funding/support: internal. Conflicts of interest disclosures: no potential conflicts reported.
Disclosures
Dr. Cowen and Mr. Kabara had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: all authors. Acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: Dr. Cowen and Mr. Kabara. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Dr. Cowen and Mr. Kabara. Administrative, technical or material support: Ms. Czerwinski. Study supervision: Dr. Cowen and Ms. Czerwinski. Funding/support: internal. Conflicts of interest disclosures: no potential conflicts reported.
- , , , , . Measuring hospital care from the patients' perspective: an overview of the CAHPS hospital survey development process. Health Serv Res. 2005;40 (6 part 2):1977–1995.
- Centers for Medicare 79(163):49854–50449.
- , , , . The relationship between patients' perception of care and measures of hospital quality and safety. Health Serv Res. 2010;45(4):1024–1040.
- Centers for Medicare 312(7031):619–622.
- , , , , . Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41–48.
- , , , , , . Getting satisfaction: drivers of surgical Hospital Consumer Assessment of Health care Providers and Systems survey scores. J Surg Res. 2015;197(1):155–161.
- , , . Patient satisfaction and quality of surgical care in US hospitals. Ann Surg. 2015;261(1):2–8.
- , , . Is there a relationship between patient satisfaction and favorable outcomes? Ann Surg. 2014;260(4):592–598; discussion 598–600.
- , , , , . Mortality predictions on admission as a context for organizing care activities. J Hosp Med. 2013;8(5):229–235.
- , , , et al. Implementation of a mortality prediction rule for real‐time decision making: feasibility and validity. J Hosp Med. 2014;9(11):720–726.
- Centers for Medicare 40(6 pt 2):2078–2095.
- Centers for Medicare 44(2 pt 1):501–518.
- Patient‐mix coefficients for October 2015 (1Q14 through 4Q14 discharges) publicly reported HCAHPS Results. Available at: http://www.hcahpsonline.org/Files/October_2015_PMA_Web_Document_a.pdf. Published July 2, 2015. Accessed August 4, 2015.
- , , , , . Case‐mix adjustment of the CAHPS hospital survey. Health Serv Res. 2005;40(6):2162–2181.
- , , , et.al. Gender differences in patients' perceptions of inpatient care. Health Serv Res. 2012;47(4):1482–1501.
- , , , et al. Patterns of unit and item nonresponse in the CAHPS hospital survey. Health Serv Res. 2005;40(6 pt 2):2096–2119.
- , , , . The cost of satisfaction: a national study of patient satisfaction, health care utilization, expenditures, and mortality. Arch Intern Med. 2012;172(5):405–411.
- , , , et al. Care experiences of managed care Medicare enrollees near the end of life. J Am Geriatr Soc. 2013;61(3):407–412.
- , , , , . Measuring satisfaction: factors that drive hospital consumer assessment of healthcare providers and systems survey responses in a trauma and acute care surgery population. Am Surg. 2015;81(5):537–543.
- , . Palliative care for the seriously ill. N Engl J Med. 2015;373(8):747–755.
- , , , et.al. Components of care vary in importance for overall patient‐reported experience by type of hospitalization. Med Care. 2009;47(8):842–849.
- , , , et al. Socioeconomic status, structural and functional measures of social support, and mortality: the British Whitehall II cohort study, 1985–2009. Am J Epidemiol. 2012;175(12):1275–1283.
- , , , et al. Inpatient care intensity and patients' ratings of their hospital experiences. Health Aff (Millwood). 2009;28(1):103–112.
- , , , et al. A validated value‐based model to improve hospital‐wide perioperative outcomes. Ann Surg. 2010;252(3):486–498.
- , , , et al. Allocating scare resources in real‐time to reduce heart failure readmissions: a prospective, controlled study. BMJ Qual Saf. 2013;22(12):998–1005.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- , , , , , . Nurse staffing and inpatient hospital mortality. N Engl J Med. 2011;364(11):1037–1045.
- , , , et al. Do hospitals rank differently on HCAHPS for different patient subgroups? Med Care Res Rev. 2010;67(1):56–73.
- , , , , , . Risk‐adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232–239.
- , , , et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3(5):1379–1384.
- , , , , . Measuring hospital care from the patients' perspective: an overview of the CAHPS hospital survey development process. Health Serv Res. 2005;40 (6 part 2):1977–1995.
- Centers for Medicare 79(163):49854–50449.
- , , , . The relationship between patients' perception of care and measures of hospital quality and safety. Health Serv Res. 2010;45(4):1024–1040.
- Centers for Medicare 312(7031):619–622.
- , , , , . Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41–48.
- , , , , , . Getting satisfaction: drivers of surgical Hospital Consumer Assessment of Health care Providers and Systems survey scores. J Surg Res. 2015;197(1):155–161.
- , , . Patient satisfaction and quality of surgical care in US hospitals. Ann Surg. 2015;261(1):2–8.
- , , . Is there a relationship between patient satisfaction and favorable outcomes? Ann Surg. 2014;260(4):592–598; discussion 598–600.
- , , , , . Mortality predictions on admission as a context for organizing care activities. J Hosp Med. 2013;8(5):229–235.
- , , , et al. Implementation of a mortality prediction rule for real‐time decision making: feasibility and validity. J Hosp Med. 2014;9(11):720–726.
- Centers for Medicare 40(6 pt 2):2078–2095.
- Centers for Medicare 44(2 pt 1):501–518.
- Patient‐mix coefficients for October 2015 (1Q14 through 4Q14 discharges) publicly reported HCAHPS Results. Available at: http://www.hcahpsonline.org/Files/October_2015_PMA_Web_Document_a.pdf. Published July 2, 2015. Accessed August 4, 2015.
- , , , , . Case‐mix adjustment of the CAHPS hospital survey. Health Serv Res. 2005;40(6):2162–2181.
- , , , et.al. Gender differences in patients' perceptions of inpatient care. Health Serv Res. 2012;47(4):1482–1501.
- , , , et al. Patterns of unit and item nonresponse in the CAHPS hospital survey. Health Serv Res. 2005;40(6 pt 2):2096–2119.
- , , , . The cost of satisfaction: a national study of patient satisfaction, health care utilization, expenditures, and mortality. Arch Intern Med. 2012;172(5):405–411.
- , , , et al. Care experiences of managed care Medicare enrollees near the end of life. J Am Geriatr Soc. 2013;61(3):407–412.
- , , , , . Measuring satisfaction: factors that drive hospital consumer assessment of healthcare providers and systems survey responses in a trauma and acute care surgery population. Am Surg. 2015;81(5):537–543.
- , . Palliative care for the seriously ill. N Engl J Med. 2015;373(8):747–755.
- , , , et.al. Components of care vary in importance for overall patient‐reported experience by type of hospitalization. Med Care. 2009;47(8):842–849.
- , , , et al. Socioeconomic status, structural and functional measures of social support, and mortality: the British Whitehall II cohort study, 1985–2009. Am J Epidemiol. 2012;175(12):1275–1283.
- , , , et al. Inpatient care intensity and patients' ratings of their hospital experiences. Health Aff (Millwood). 2009;28(1):103–112.
- , , , et al. A validated value‐based model to improve hospital‐wide perioperative outcomes. Ann Surg. 2010;252(3):486–498.
- , , , et al. Allocating scare resources in real‐time to reduce heart failure readmissions: a prospective, controlled study. BMJ Qual Saf. 2013;22(12):998–1005.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- , , , , , . Nurse staffing and inpatient hospital mortality. N Engl J Med. 2011;364(11):1037–1045.
- , , , et al. Do hospitals rank differently on HCAHPS for different patient subgroups? Med Care Res Rev. 2010;67(1):56–73.
- , , , , , . Risk‐adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232–239.
- , , , et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3(5):1379–1384.
Choosing a Graft for Anterior Cruciate Ligament Reconstruction: Surgeon Influence Reigns Supreme
Anterior cruciate ligament (ACL) injuries affect >175,000 people each year,1 with >100,000 Americans undergoing ACL reconstruction annually.2 Due to the high impact this injury has on the general population, and especially on athletes, it is important to determine the factors that influence a patient’s selection of a particular graft type. With increasing access to information and other outside influences, surgeons should attempt to provide as much objective information as possible in order to allow patients to make appropriate informed decisions regarding their graft choice for ACL surgery.
While autografts are used in >60% of primary ACL reconstructions, allografts are used in >80% of revision procedures.3 Both autografts and allografts offer advantages and disadvantages, and the advantages of each may depend on patient age, activity level, and occupation.4 For example, graft rerupture rates have been shown to be higher in patients with ACL allografts4, while kneeling pain has been shown to be worse in patients with bone-patellar tendon-bone (BPTB) autografts compared to hamstring autografts5 as well as BPTB allografts.4
Patient satisfaction rates are high for ACL autografts and allografts. Boonriong and Kietsiriroje6 have shown visual analog scale (VAS) patient satisfaction score averages to be 88 out of 100 for BPTB autografts and 93 out of 100 for hamstring tendon autografts. Fox and colleagues7 showed that 87% of patients were completely or mostly satisfied following revision ACL reconstruction with patellar tendon allograft. Cohen and colleagues8 evaluated 240 patients undergoing primary ACL reconstruction; 63.3% underwent ACL reconstruction with an allograft and 35.4% with an autograft. Of all patients enrolled in the study, 93% were satisfied with their graft choice, with 12.7% of patients opting to choose another graft if in the same situation again. Of those patients, 63.3% would have switched from an autograft to allograft. Although these numbers represent high patient satisfaction following a variety of ACL graft types, it is important to continue to identify graft selection factors in order to maximize patient outcomes.
The purposes of this prospective study were to assess patients’ knowledge of their graft type used for ACL reconstruction, to determine the most influential factors involved in graft selection, and to determine the level of satisfaction with the graft of choice at a minimum of 1-year follow-up. Based on a previous retrospective study,8 we hypothesized that physician recommendation would be the most influential factor in ACL graft selection. We also hypothesized that patients receiving an autograft would be more accurate in stating their graft harvest location compared to allograft patients.
Materials and Methods
We prospectively enrolled 304 patients who underwent primary ACL reconstruction from January 2008 to September 2013. Surgery was performed by 9 different surgeons within the same practice. All patients undergoing primary ACL reconstruction were eligible for the study.
All surgeons explained to each patient the pros and cons of each graft choice based upon peer-reviewed literature. Each patient was allowed to choose autograft or allograft, although most of the surgeons strongly encourage patients under age 25 years to choose autograft. One of the surgeons specifically encourages a patellar tendon autograft in patients under age 30 to 35 years, except for those patients with a narrow patellar tendon on magnetic resonance imaging, in which case he recommends a hamstring autograft. Another surgeon also specifically encourages patellar tendon autograft in patients under 35 years, except in skeletally immature patients, for whom he encourages hamstring autograft. However, none of the surgeons prohibited patients from choosing autograft or allograft, regardless of age.
The Institutional Review Board at our institution provided approval for this study. At the first postoperative follow-up appointment, each patient completed a questionnaire asking to select from a list the type (“your own” or “a cadaver”) and harvest site of the graft that was used for the surgery. Patients were also asked how they decided upon that graft type by ranking a list of 4 factors from 1 to 4. These included (1) physician recommendation, (2) family/friend’s recommendation, (3) coach’s recommendation, and (4) the media. Patients had the option of ranking more than one factor as most important in their decision. In addition, patients were asked to list any other factors that influenced their decision regarding graft type.
At a minimum of 1 year following surgery, patients completed the same questionnaire described above. In addition, patients were asked if they were satisfied with their graft and whether they would choose the same graft type if undergoing ACL reconstruction again. Patients who would have chosen a different graft were asked which graft they would have chosen and why. Any patient who experienced graft rupture prior to follow-up was included in the analysis.
Statistical Analysis
Chi square tests were used to compare dichotomous outcomes. A type I error of less than 5% (P < .05) was considered statistically significant.
Results
At least 1 year following ACL reconstruction, 213 of 304 patients (70%) successfully completed the same questionnaire as they did at their first postoperative follow-up appointment. The mean age of these patients at the time of surgery was 31.9 ± 11.0 years (range, 13.9 to 58.0 years). The mean follow-up time was 1.4 ± 0.4 years (range, 1.0 to 2.6 years), and 59% of these patients were male.
Autografts were used for 139 patients (139/304, 46%), allografts for 156 patients (156/304, 51%), and hybrid grafts for 9 patients (9/304, 3%). Overall, 77% of patients were accurate in stating the type of graft used for their ACL reconstruction, including 88% of autograft patients, 71% of allograft patients, and 11% of hybrid graft patients (Table 1). Patients who underwent reconstruction with an autograft were significantly more accurate in stating their graft type compared to patients with an allograft (P < .001). Graft type by surgeon is shown in Table 2. A statistically significant difference was found in the proportion of patients choosing autograft versus allograft based on surgeon (P < .0001).
When asked which type of graft was used for their surgery, 12 of 304 patients (4%) did not know their graft type or harvest location. Twenty-nine patients stated that their graft was an allograft but did not know the harvest location. Five patients stated that their graft was an autograft but did not know the harvest location. The 34 patients who classified their choice of graft but did not know the harvest site (11%) stated their surgeon never told them where their graft was from or they did not remember. A complete list of graft type responses is shown in Table 3.
Of the 29 patients who stated that their graft was an allograft but did not know the harvest location, 19 (66%) had a tibialis anterior allograft, 7 (24%) had a BPTB allograft, 2 (7%) had an Achilles tendon allograft, and 1 (3%) had a tibialis anterior autograft.
Physician recommendation was the most important decision-making factor listed for 82% of patients at their first postoperative appointment (Table 4). In addition to the 4 factors listed on our survey, patients were allowed to write in other factors involved in their decision. The most popular answers included recovery time, personal research on graft types, and prior personal experience with ACL reconstruction on the contralateral knee.
At the time of 1-year follow-up, 205 of 213 patients (96%) said they were satisfied with their graft choice (Table 5). All 4 unsatisfied autograft patients received a hamstring autograft, 3 of which were performed by the same surgeon. No significant difference was found in satisfaction rates between patients with autograft vs allograft (P = .87). There was a higher satisfaction rate among patients with a BPTB autograft compared to those with a hamstring autograft (P = .043). Of the unsatisfied patients, 3 patients stated that their graft had failed in the time prior to follow-up and 2 patients stated that they were having donor site pain following surgery with hamstring autograft and would consider an allograft if the reconstruction were repeated (Table 6). Two patients stated that they were unsatisfied with their graft but would need to do more research before deciding on a different graft type.
As shown in Tables 5 and 6, there is a discrepancy between the number of patients who were unsatisfied with their graft and the number of patients who stated that they would switch to a different graft type if they were to have ACL reconstruction again. A number of patients stated that they were satisfied with their graft, yet they would switch to a different graft. The main reasons for this related to issues from a hamstring autograft harvest site. One patient noted that although she was satisfied with her graft, she would switch after doing further research.
Discussion
Determining the decision-making factors for patients choosing between graft types for ACL reconstruction is important to ensure that patients can make a decision based on objective information. Several previous studies have evaluated patient selection of ACL grafts.8-10 All 3 of these studies showed that surgeon recommendation is the primary factor in a patient’s decision. Similar to previous studies, we also found that physician recommendation is the most influential factor involved in this decision.
At an average follow-up of 41 months, Cohen and colleagues8 found that 1.3% of patients did not know whether they received an autograft or allograft for their ACL reconstruction. Furthermore, 50.7% of patients stating they received an allograft in Cohen’s study8 were unsure of the harvest location. In our study, 4% of patients at their first postoperative visit did not know whether they had received an autograft or allograft and 10% of patients stating they received an allograft selected an unknown harvest site. In contrast, only 2% of autograft patients in our study were unsure of the harvest location at their first postoperative appointment. It is likely that, over time, patients with an allograft forget the harvest location, whereas autograft patients are more likely to remember the location of harvest. This is especially true in patients with anterior knee pain or hamstring pain following ACL reconstruction with a BPTB or hamstring tendon autograft, respectively.
In terms of patients’ knowledge of their graft type, we found an overall accuracy of 77%, with 88% of autograft patients, 71% of allograft patients, and 11% of hybrid graft patients remembering their graft type and harvest location. Although we do not believe it to be critical for patients to remember these details, we do believe that patients who do not know their graft type likely relied on the recommendation of their physician.
We found a significant difference in the proportion of patients choosing autograft vs allograft based on surgeon, despite these surgeons citing available data in the literature to each patient and ultimately allowing each patient to make his or her own decision. This is partly due to the low sample size of most of the surgeons involved. However, the main reason for this distortion is likely that different surgeons may highlight different aspects of the literature to “spin” patients towards one graft or another in certain cases.
Currently, there remains a lack of clarity in the literature on appropriate ACL graft choices for patients. With constant new findings being published on different aspects of various grafts, it is important for surgeons to remain up to date with the literature. Nevertheless, we believe that certain biases are inevitable among surgeons due to unique training experiences as well as experience with their own patients.
Cohen and colleagues8 found that only 7% of patients reported that their own personal research influenced their decision, and only 6.4% of patients reported the media as their primary decision-making factor. Cheung and colleagues9 conducted a retrospective study and found that more than half of patients did significant personal research prior to making a decision regarding their graft type. Most of this research was done using medical websites and literature. Koh and colleagues10 noted that >80% of patients consulted the internet for graft information before making a decision. Koh’s study10 was performed in Korea and therefore the high prevalence of internet use may be culturally-related.
Overall, quality of information for patients undergoing ACL reconstruction is mixed across the internet, with only 22.5% of top websites being affiliated with an academic institution and 35.5% of websites authored by private physicians or physician groups.11 Although a majority of internet websites offer discussion into the condition and surgical procedure of ACL reconstruction, less than half of these websites share the equally important information on the eligibility for surgery and concomitant complications following surgery.11In our study, only 39 patients (13%) listed the media as either the first (13, 4%) or second (26, 9%) most important factor in their graft decision. Clearly there is some discrepancy between studies regarding the influence of personal research and media. There are a few potential reasons for this. First, we did not explicitly ask patients if their own personal research had any influence on their graft decision. Rather, we asked patients to rank their decision-making factors, and few patients ranked the media as their first or second greatest influence. Second, the word “media” was used in our questionnaire rather than “online research” or “internet.” It may seem somewhat vague to patients what the word “media” really means in terms of their own research, whereas listing “online research” or “internet” as selection options may have influenced patient responses.
In our study, we asked patients for any additional factors that influenced their graft choice. Thirteen patients (4%) noted that “personal research” through internet, orthopaedic literature, and the media influenced their graft decision. This corroborates the idea that “media” may have seemed vague to some patients. Of these patients, 9 chose an autograft and 4 chose an allograft. The relative ease in accessing information regarding graft choice in ACL reconstruction should be noted. Numerous websites offer advice, graft options, and commentary from group practices and orthopaedic surgeons. Whether or not these sources provide reasonable support for one graft vs another graft remains to be answered. The physician should be responsible for providing the patient with this collected objective information.
In our study, 205 patients (96%) were satisfied with their graft choice at the time of follow-up, with 15 patients (7%) stating that they would have chosen a different graft type if they could redo the operation. Cheung and colleagues9 found a satisfaction rate of 87.4% at an average follow-up time of 19 months, with 4.6% stating they would have chosen a different graft type. Many factors can contribute to patient satisfaction after ACL reconstruction. Looking at patient variables such as age, demographics, occupation, activity level, surgical technique including tunnel placement and fixation, postoperative rehabilitation, and graft type may influence the success of the patient after ACL reconstruction.
The strengths of this study include the patient population size with 1-year follow-up as well as the prospective study design. In comparison to a previous retrospective study in 2009 by Cohen and colleagues8with a sample size of 240 patients, our study collected 213 patients with 70% follow-up at minimum 1 year. Collecting data prospectively ensures accurate representation of the factors influencing each patient’s graft selection, while follow-up data was useful for patient satisfaction.
The limitations of this study include the percentage of patients lost from follow-up as well as any bias generated from the organization of the questionnaire. Unfortunately, with a younger, transient population of patients undergoing ACL reconstruction in a major metropolitan area, a percentage of patients are lost to follow-up. Many attempts were made to locate these patients. Another potential limitation was the order of decision factors listed on the questionnaire. These factors were not ordered randomly on each survey, but were listed in the following order: (1) physician recommendation (2) family/friend’s recommendation (3) coach’s recommendation and (4) the media. This may have influenced patient responses. The organization of these factors in the questionnaire started with physician recommendation, which may have influenced the patient’s initial thought process of which factor had the greatest influence in their graft decision. In addition, for the surveys completed at least 1 year following surgery, some patients were contacted via e-mail and others via telephone. Thus, some patients may have changed their answers if they were able to see the questions rather than hearing the questions. We believe this is particularly true of the question regarding graft harvest site.
Our study indicates that the majority of patients undergoing ACL reconstruction are primarily influenced by the physician’s recommendation.
1. Madick S. Anterior cruciate ligament reconstruction of the knee. AORN J. 2011;93(2):210-222.
2. Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):661-681.
3. Paxton EW, Namba RS, Maletis GB, et al. A prospective study of 80,000 total joint and 5000 anterior cruciate ligament reconstruction procedures in a community-based registry in the United States. J Bone Joint Surg Am. 2010;92(suppl 2):117-132.
4. Kraeutler MJ, Bravman JT, McCarty EC. Bone-patellar tendon-bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: A meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
5. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
6. Boonriong T, Kietsiriroje N. Arthroscopically assisted anterior cruciate ligament reconstruction: comparison of bone-patellar tendon-bone versus hamstring tendon autograft. J Med Assoc Thai. 2004;87(9):1100-1107.
7. Fox JA, Pierce M, Bojchuk J, Hayden J, Bush-Joseph CA, Bach BR Jr. Revision anterior cruciate ligament reconstruction with nonirradiated fresh-frozen patellar tendon allograft. Arthroscopy. 2004;20(8):787-794.
8. Cohen SB, Yucha DT, Ciccotti MC, Goldstein DT, Ciccotti MA, Ciccotti MG. Factors affecting patient selection of graft type in anterior cruciate ligament reconstruction. Arthroscopy. 2009;25(9):1006-1010.
9. Cheung SC, Allen CR, Gallo RA, Ma CB, Feeley BT. Patients’ attitudes and factors in their selection of grafts for anterior cruciate ligament reconstruction. Knee. 2012;19(1):49-54.
10. Koh HS, In Y, Kong CG, Won HY, Kim KH, Lee JH. Factors affecting patients’ graft choice in anterior cruciate ligament reconstruction. Clin Orthop Surg. 2010;2(2):69-75.
11. Duncan IC, Kane PW, Lawson KA, Cohen SB, Ciccotti MG, Dodson CC. Evaluation of information available on the internet regarding anterior cruciate ligament reconstruction. Arthroscopy. 2013;29(6):1101-1107.
Anterior cruciate ligament (ACL) injuries affect >175,000 people each year,1 with >100,000 Americans undergoing ACL reconstruction annually.2 Due to the high impact this injury has on the general population, and especially on athletes, it is important to determine the factors that influence a patient’s selection of a particular graft type. With increasing access to information and other outside influences, surgeons should attempt to provide as much objective information as possible in order to allow patients to make appropriate informed decisions regarding their graft choice for ACL surgery.
While autografts are used in >60% of primary ACL reconstructions, allografts are used in >80% of revision procedures.3 Both autografts and allografts offer advantages and disadvantages, and the advantages of each may depend on patient age, activity level, and occupation.4 For example, graft rerupture rates have been shown to be higher in patients with ACL allografts4, while kneeling pain has been shown to be worse in patients with bone-patellar tendon-bone (BPTB) autografts compared to hamstring autografts5 as well as BPTB allografts.4
Patient satisfaction rates are high for ACL autografts and allografts. Boonriong and Kietsiriroje6 have shown visual analog scale (VAS) patient satisfaction score averages to be 88 out of 100 for BPTB autografts and 93 out of 100 for hamstring tendon autografts. Fox and colleagues7 showed that 87% of patients were completely or mostly satisfied following revision ACL reconstruction with patellar tendon allograft. Cohen and colleagues8 evaluated 240 patients undergoing primary ACL reconstruction; 63.3% underwent ACL reconstruction with an allograft and 35.4% with an autograft. Of all patients enrolled in the study, 93% were satisfied with their graft choice, with 12.7% of patients opting to choose another graft if in the same situation again. Of those patients, 63.3% would have switched from an autograft to allograft. Although these numbers represent high patient satisfaction following a variety of ACL graft types, it is important to continue to identify graft selection factors in order to maximize patient outcomes.
The purposes of this prospective study were to assess patients’ knowledge of their graft type used for ACL reconstruction, to determine the most influential factors involved in graft selection, and to determine the level of satisfaction with the graft of choice at a minimum of 1-year follow-up. Based on a previous retrospective study,8 we hypothesized that physician recommendation would be the most influential factor in ACL graft selection. We also hypothesized that patients receiving an autograft would be more accurate in stating their graft harvest location compared to allograft patients.
Materials and Methods
We prospectively enrolled 304 patients who underwent primary ACL reconstruction from January 2008 to September 2013. Surgery was performed by 9 different surgeons within the same practice. All patients undergoing primary ACL reconstruction were eligible for the study.
All surgeons explained to each patient the pros and cons of each graft choice based upon peer-reviewed literature. Each patient was allowed to choose autograft or allograft, although most of the surgeons strongly encourage patients under age 25 years to choose autograft. One of the surgeons specifically encourages a patellar tendon autograft in patients under age 30 to 35 years, except for those patients with a narrow patellar tendon on magnetic resonance imaging, in which case he recommends a hamstring autograft. Another surgeon also specifically encourages patellar tendon autograft in patients under 35 years, except in skeletally immature patients, for whom he encourages hamstring autograft. However, none of the surgeons prohibited patients from choosing autograft or allograft, regardless of age.
The Institutional Review Board at our institution provided approval for this study. At the first postoperative follow-up appointment, each patient completed a questionnaire asking to select from a list the type (“your own” or “a cadaver”) and harvest site of the graft that was used for the surgery. Patients were also asked how they decided upon that graft type by ranking a list of 4 factors from 1 to 4. These included (1) physician recommendation, (2) family/friend’s recommendation, (3) coach’s recommendation, and (4) the media. Patients had the option of ranking more than one factor as most important in their decision. In addition, patients were asked to list any other factors that influenced their decision regarding graft type.
At a minimum of 1 year following surgery, patients completed the same questionnaire described above. In addition, patients were asked if they were satisfied with their graft and whether they would choose the same graft type if undergoing ACL reconstruction again. Patients who would have chosen a different graft were asked which graft they would have chosen and why. Any patient who experienced graft rupture prior to follow-up was included in the analysis.
Statistical Analysis
Chi square tests were used to compare dichotomous outcomes. A type I error of less than 5% (P < .05) was considered statistically significant.
Results
At least 1 year following ACL reconstruction, 213 of 304 patients (70%) successfully completed the same questionnaire as they did at their first postoperative follow-up appointment. The mean age of these patients at the time of surgery was 31.9 ± 11.0 years (range, 13.9 to 58.0 years). The mean follow-up time was 1.4 ± 0.4 years (range, 1.0 to 2.6 years), and 59% of these patients were male.
Autografts were used for 139 patients (139/304, 46%), allografts for 156 patients (156/304, 51%), and hybrid grafts for 9 patients (9/304, 3%). Overall, 77% of patients were accurate in stating the type of graft used for their ACL reconstruction, including 88% of autograft patients, 71% of allograft patients, and 11% of hybrid graft patients (Table 1). Patients who underwent reconstruction with an autograft were significantly more accurate in stating their graft type compared to patients with an allograft (P < .001). Graft type by surgeon is shown in Table 2. A statistically significant difference was found in the proportion of patients choosing autograft versus allograft based on surgeon (P < .0001).
When asked which type of graft was used for their surgery, 12 of 304 patients (4%) did not know their graft type or harvest location. Twenty-nine patients stated that their graft was an allograft but did not know the harvest location. Five patients stated that their graft was an autograft but did not know the harvest location. The 34 patients who classified their choice of graft but did not know the harvest site (11%) stated their surgeon never told them where their graft was from or they did not remember. A complete list of graft type responses is shown in Table 3.
Of the 29 patients who stated that their graft was an allograft but did not know the harvest location, 19 (66%) had a tibialis anterior allograft, 7 (24%) had a BPTB allograft, 2 (7%) had an Achilles tendon allograft, and 1 (3%) had a tibialis anterior autograft.
Physician recommendation was the most important decision-making factor listed for 82% of patients at their first postoperative appointment (Table 4). In addition to the 4 factors listed on our survey, patients were allowed to write in other factors involved in their decision. The most popular answers included recovery time, personal research on graft types, and prior personal experience with ACL reconstruction on the contralateral knee.
At the time of 1-year follow-up, 205 of 213 patients (96%) said they were satisfied with their graft choice (Table 5). All 4 unsatisfied autograft patients received a hamstring autograft, 3 of which were performed by the same surgeon. No significant difference was found in satisfaction rates between patients with autograft vs allograft (P = .87). There was a higher satisfaction rate among patients with a BPTB autograft compared to those with a hamstring autograft (P = .043). Of the unsatisfied patients, 3 patients stated that their graft had failed in the time prior to follow-up and 2 patients stated that they were having donor site pain following surgery with hamstring autograft and would consider an allograft if the reconstruction were repeated (Table 6). Two patients stated that they were unsatisfied with their graft but would need to do more research before deciding on a different graft type.
As shown in Tables 5 and 6, there is a discrepancy between the number of patients who were unsatisfied with their graft and the number of patients who stated that they would switch to a different graft type if they were to have ACL reconstruction again. A number of patients stated that they were satisfied with their graft, yet they would switch to a different graft. The main reasons for this related to issues from a hamstring autograft harvest site. One patient noted that although she was satisfied with her graft, she would switch after doing further research.
Discussion
Determining the decision-making factors for patients choosing between graft types for ACL reconstruction is important to ensure that patients can make a decision based on objective information. Several previous studies have evaluated patient selection of ACL grafts.8-10 All 3 of these studies showed that surgeon recommendation is the primary factor in a patient’s decision. Similar to previous studies, we also found that physician recommendation is the most influential factor involved in this decision.
At an average follow-up of 41 months, Cohen and colleagues8 found that 1.3% of patients did not know whether they received an autograft or allograft for their ACL reconstruction. Furthermore, 50.7% of patients stating they received an allograft in Cohen’s study8 were unsure of the harvest location. In our study, 4% of patients at their first postoperative visit did not know whether they had received an autograft or allograft and 10% of patients stating they received an allograft selected an unknown harvest site. In contrast, only 2% of autograft patients in our study were unsure of the harvest location at their first postoperative appointment. It is likely that, over time, patients with an allograft forget the harvest location, whereas autograft patients are more likely to remember the location of harvest. This is especially true in patients with anterior knee pain or hamstring pain following ACL reconstruction with a BPTB or hamstring tendon autograft, respectively.
In terms of patients’ knowledge of their graft type, we found an overall accuracy of 77%, with 88% of autograft patients, 71% of allograft patients, and 11% of hybrid graft patients remembering their graft type and harvest location. Although we do not believe it to be critical for patients to remember these details, we do believe that patients who do not know their graft type likely relied on the recommendation of their physician.
We found a significant difference in the proportion of patients choosing autograft vs allograft based on surgeon, despite these surgeons citing available data in the literature to each patient and ultimately allowing each patient to make his or her own decision. This is partly due to the low sample size of most of the surgeons involved. However, the main reason for this distortion is likely that different surgeons may highlight different aspects of the literature to “spin” patients towards one graft or another in certain cases.
Currently, there remains a lack of clarity in the literature on appropriate ACL graft choices for patients. With constant new findings being published on different aspects of various grafts, it is important for surgeons to remain up to date with the literature. Nevertheless, we believe that certain biases are inevitable among surgeons due to unique training experiences as well as experience with their own patients.
Cohen and colleagues8 found that only 7% of patients reported that their own personal research influenced their decision, and only 6.4% of patients reported the media as their primary decision-making factor. Cheung and colleagues9 conducted a retrospective study and found that more than half of patients did significant personal research prior to making a decision regarding their graft type. Most of this research was done using medical websites and literature. Koh and colleagues10 noted that >80% of patients consulted the internet for graft information before making a decision. Koh’s study10 was performed in Korea and therefore the high prevalence of internet use may be culturally-related.
Overall, quality of information for patients undergoing ACL reconstruction is mixed across the internet, with only 22.5% of top websites being affiliated with an academic institution and 35.5% of websites authored by private physicians or physician groups.11 Although a majority of internet websites offer discussion into the condition and surgical procedure of ACL reconstruction, less than half of these websites share the equally important information on the eligibility for surgery and concomitant complications following surgery.11In our study, only 39 patients (13%) listed the media as either the first (13, 4%) or second (26, 9%) most important factor in their graft decision. Clearly there is some discrepancy between studies regarding the influence of personal research and media. There are a few potential reasons for this. First, we did not explicitly ask patients if their own personal research had any influence on their graft decision. Rather, we asked patients to rank their decision-making factors, and few patients ranked the media as their first or second greatest influence. Second, the word “media” was used in our questionnaire rather than “online research” or “internet.” It may seem somewhat vague to patients what the word “media” really means in terms of their own research, whereas listing “online research” or “internet” as selection options may have influenced patient responses.
In our study, we asked patients for any additional factors that influenced their graft choice. Thirteen patients (4%) noted that “personal research” through internet, orthopaedic literature, and the media influenced their graft decision. This corroborates the idea that “media” may have seemed vague to some patients. Of these patients, 9 chose an autograft and 4 chose an allograft. The relative ease in accessing information regarding graft choice in ACL reconstruction should be noted. Numerous websites offer advice, graft options, and commentary from group practices and orthopaedic surgeons. Whether or not these sources provide reasonable support for one graft vs another graft remains to be answered. The physician should be responsible for providing the patient with this collected objective information.
In our study, 205 patients (96%) were satisfied with their graft choice at the time of follow-up, with 15 patients (7%) stating that they would have chosen a different graft type if they could redo the operation. Cheung and colleagues9 found a satisfaction rate of 87.4% at an average follow-up time of 19 months, with 4.6% stating they would have chosen a different graft type. Many factors can contribute to patient satisfaction after ACL reconstruction. Looking at patient variables such as age, demographics, occupation, activity level, surgical technique including tunnel placement and fixation, postoperative rehabilitation, and graft type may influence the success of the patient after ACL reconstruction.
The strengths of this study include the patient population size with 1-year follow-up as well as the prospective study design. In comparison to a previous retrospective study in 2009 by Cohen and colleagues8with a sample size of 240 patients, our study collected 213 patients with 70% follow-up at minimum 1 year. Collecting data prospectively ensures accurate representation of the factors influencing each patient’s graft selection, while follow-up data was useful for patient satisfaction.
The limitations of this study include the percentage of patients lost from follow-up as well as any bias generated from the organization of the questionnaire. Unfortunately, with a younger, transient population of patients undergoing ACL reconstruction in a major metropolitan area, a percentage of patients are lost to follow-up. Many attempts were made to locate these patients. Another potential limitation was the order of decision factors listed on the questionnaire. These factors were not ordered randomly on each survey, but were listed in the following order: (1) physician recommendation (2) family/friend’s recommendation (3) coach’s recommendation and (4) the media. This may have influenced patient responses. The organization of these factors in the questionnaire started with physician recommendation, which may have influenced the patient’s initial thought process of which factor had the greatest influence in their graft decision. In addition, for the surveys completed at least 1 year following surgery, some patients were contacted via e-mail and others via telephone. Thus, some patients may have changed their answers if they were able to see the questions rather than hearing the questions. We believe this is particularly true of the question regarding graft harvest site.
Our study indicates that the majority of patients undergoing ACL reconstruction are primarily influenced by the physician’s recommendation.
Anterior cruciate ligament (ACL) injuries affect >175,000 people each year,1 with >100,000 Americans undergoing ACL reconstruction annually.2 Due to the high impact this injury has on the general population, and especially on athletes, it is important to determine the factors that influence a patient’s selection of a particular graft type. With increasing access to information and other outside influences, surgeons should attempt to provide as much objective information as possible in order to allow patients to make appropriate informed decisions regarding their graft choice for ACL surgery.
While autografts are used in >60% of primary ACL reconstructions, allografts are used in >80% of revision procedures.3 Both autografts and allografts offer advantages and disadvantages, and the advantages of each may depend on patient age, activity level, and occupation.4 For example, graft rerupture rates have been shown to be higher in patients with ACL allografts4, while kneeling pain has been shown to be worse in patients with bone-patellar tendon-bone (BPTB) autografts compared to hamstring autografts5 as well as BPTB allografts.4
Patient satisfaction rates are high for ACL autografts and allografts. Boonriong and Kietsiriroje6 have shown visual analog scale (VAS) patient satisfaction score averages to be 88 out of 100 for BPTB autografts and 93 out of 100 for hamstring tendon autografts. Fox and colleagues7 showed that 87% of patients were completely or mostly satisfied following revision ACL reconstruction with patellar tendon allograft. Cohen and colleagues8 evaluated 240 patients undergoing primary ACL reconstruction; 63.3% underwent ACL reconstruction with an allograft and 35.4% with an autograft. Of all patients enrolled in the study, 93% were satisfied with their graft choice, with 12.7% of patients opting to choose another graft if in the same situation again. Of those patients, 63.3% would have switched from an autograft to allograft. Although these numbers represent high patient satisfaction following a variety of ACL graft types, it is important to continue to identify graft selection factors in order to maximize patient outcomes.
The purposes of this prospective study were to assess patients’ knowledge of their graft type used for ACL reconstruction, to determine the most influential factors involved in graft selection, and to determine the level of satisfaction with the graft of choice at a minimum of 1-year follow-up. Based on a previous retrospective study,8 we hypothesized that physician recommendation would be the most influential factor in ACL graft selection. We also hypothesized that patients receiving an autograft would be more accurate in stating their graft harvest location compared to allograft patients.
Materials and Methods
We prospectively enrolled 304 patients who underwent primary ACL reconstruction from January 2008 to September 2013. Surgery was performed by 9 different surgeons within the same practice. All patients undergoing primary ACL reconstruction were eligible for the study.
All surgeons explained to each patient the pros and cons of each graft choice based upon peer-reviewed literature. Each patient was allowed to choose autograft or allograft, although most of the surgeons strongly encourage patients under age 25 years to choose autograft. One of the surgeons specifically encourages a patellar tendon autograft in patients under age 30 to 35 years, except for those patients with a narrow patellar tendon on magnetic resonance imaging, in which case he recommends a hamstring autograft. Another surgeon also specifically encourages patellar tendon autograft in patients under 35 years, except in skeletally immature patients, for whom he encourages hamstring autograft. However, none of the surgeons prohibited patients from choosing autograft or allograft, regardless of age.
The Institutional Review Board at our institution provided approval for this study. At the first postoperative follow-up appointment, each patient completed a questionnaire asking to select from a list the type (“your own” or “a cadaver”) and harvest site of the graft that was used for the surgery. Patients were also asked how they decided upon that graft type by ranking a list of 4 factors from 1 to 4. These included (1) physician recommendation, (2) family/friend’s recommendation, (3) coach’s recommendation, and (4) the media. Patients had the option of ranking more than one factor as most important in their decision. In addition, patients were asked to list any other factors that influenced their decision regarding graft type.
At a minimum of 1 year following surgery, patients completed the same questionnaire described above. In addition, patients were asked if they were satisfied with their graft and whether they would choose the same graft type if undergoing ACL reconstruction again. Patients who would have chosen a different graft were asked which graft they would have chosen and why. Any patient who experienced graft rupture prior to follow-up was included in the analysis.
Statistical Analysis
Chi square tests were used to compare dichotomous outcomes. A type I error of less than 5% (P < .05) was considered statistically significant.
Results
At least 1 year following ACL reconstruction, 213 of 304 patients (70%) successfully completed the same questionnaire as they did at their first postoperative follow-up appointment. The mean age of these patients at the time of surgery was 31.9 ± 11.0 years (range, 13.9 to 58.0 years). The mean follow-up time was 1.4 ± 0.4 years (range, 1.0 to 2.6 years), and 59% of these patients were male.
Autografts were used for 139 patients (139/304, 46%), allografts for 156 patients (156/304, 51%), and hybrid grafts for 9 patients (9/304, 3%). Overall, 77% of patients were accurate in stating the type of graft used for their ACL reconstruction, including 88% of autograft patients, 71% of allograft patients, and 11% of hybrid graft patients (Table 1). Patients who underwent reconstruction with an autograft were significantly more accurate in stating their graft type compared to patients with an allograft (P < .001). Graft type by surgeon is shown in Table 2. A statistically significant difference was found in the proportion of patients choosing autograft versus allograft based on surgeon (P < .0001).
When asked which type of graft was used for their surgery, 12 of 304 patients (4%) did not know their graft type or harvest location. Twenty-nine patients stated that their graft was an allograft but did not know the harvest location. Five patients stated that their graft was an autograft but did not know the harvest location. The 34 patients who classified their choice of graft but did not know the harvest site (11%) stated their surgeon never told them where their graft was from or they did not remember. A complete list of graft type responses is shown in Table 3.
Of the 29 patients who stated that their graft was an allograft but did not know the harvest location, 19 (66%) had a tibialis anterior allograft, 7 (24%) had a BPTB allograft, 2 (7%) had an Achilles tendon allograft, and 1 (3%) had a tibialis anterior autograft.
Physician recommendation was the most important decision-making factor listed for 82% of patients at their first postoperative appointment (Table 4). In addition to the 4 factors listed on our survey, patients were allowed to write in other factors involved in their decision. The most popular answers included recovery time, personal research on graft types, and prior personal experience with ACL reconstruction on the contralateral knee.
At the time of 1-year follow-up, 205 of 213 patients (96%) said they were satisfied with their graft choice (Table 5). All 4 unsatisfied autograft patients received a hamstring autograft, 3 of which were performed by the same surgeon. No significant difference was found in satisfaction rates between patients with autograft vs allograft (P = .87). There was a higher satisfaction rate among patients with a BPTB autograft compared to those with a hamstring autograft (P = .043). Of the unsatisfied patients, 3 patients stated that their graft had failed in the time prior to follow-up and 2 patients stated that they were having donor site pain following surgery with hamstring autograft and would consider an allograft if the reconstruction were repeated (Table 6). Two patients stated that they were unsatisfied with their graft but would need to do more research before deciding on a different graft type.
As shown in Tables 5 and 6, there is a discrepancy between the number of patients who were unsatisfied with their graft and the number of patients who stated that they would switch to a different graft type if they were to have ACL reconstruction again. A number of patients stated that they were satisfied with their graft, yet they would switch to a different graft. The main reasons for this related to issues from a hamstring autograft harvest site. One patient noted that although she was satisfied with her graft, she would switch after doing further research.
Discussion
Determining the decision-making factors for patients choosing between graft types for ACL reconstruction is important to ensure that patients can make a decision based on objective information. Several previous studies have evaluated patient selection of ACL grafts.8-10 All 3 of these studies showed that surgeon recommendation is the primary factor in a patient’s decision. Similar to previous studies, we also found that physician recommendation is the most influential factor involved in this decision.
At an average follow-up of 41 months, Cohen and colleagues8 found that 1.3% of patients did not know whether they received an autograft or allograft for their ACL reconstruction. Furthermore, 50.7% of patients stating they received an allograft in Cohen’s study8 were unsure of the harvest location. In our study, 4% of patients at their first postoperative visit did not know whether they had received an autograft or allograft and 10% of patients stating they received an allograft selected an unknown harvest site. In contrast, only 2% of autograft patients in our study were unsure of the harvest location at their first postoperative appointment. It is likely that, over time, patients with an allograft forget the harvest location, whereas autograft patients are more likely to remember the location of harvest. This is especially true in patients with anterior knee pain or hamstring pain following ACL reconstruction with a BPTB or hamstring tendon autograft, respectively.
In terms of patients’ knowledge of their graft type, we found an overall accuracy of 77%, with 88% of autograft patients, 71% of allograft patients, and 11% of hybrid graft patients remembering their graft type and harvest location. Although we do not believe it to be critical for patients to remember these details, we do believe that patients who do not know their graft type likely relied on the recommendation of their physician.
We found a significant difference in the proportion of patients choosing autograft vs allograft based on surgeon, despite these surgeons citing available data in the literature to each patient and ultimately allowing each patient to make his or her own decision. This is partly due to the low sample size of most of the surgeons involved. However, the main reason for this distortion is likely that different surgeons may highlight different aspects of the literature to “spin” patients towards one graft or another in certain cases.
Currently, there remains a lack of clarity in the literature on appropriate ACL graft choices for patients. With constant new findings being published on different aspects of various grafts, it is important for surgeons to remain up to date with the literature. Nevertheless, we believe that certain biases are inevitable among surgeons due to unique training experiences as well as experience with their own patients.
Cohen and colleagues8 found that only 7% of patients reported that their own personal research influenced their decision, and only 6.4% of patients reported the media as their primary decision-making factor. Cheung and colleagues9 conducted a retrospective study and found that more than half of patients did significant personal research prior to making a decision regarding their graft type. Most of this research was done using medical websites and literature. Koh and colleagues10 noted that >80% of patients consulted the internet for graft information before making a decision. Koh’s study10 was performed in Korea and therefore the high prevalence of internet use may be culturally-related.
Overall, quality of information for patients undergoing ACL reconstruction is mixed across the internet, with only 22.5% of top websites being affiliated with an academic institution and 35.5% of websites authored by private physicians or physician groups.11 Although a majority of internet websites offer discussion into the condition and surgical procedure of ACL reconstruction, less than half of these websites share the equally important information on the eligibility for surgery and concomitant complications following surgery.11In our study, only 39 patients (13%) listed the media as either the first (13, 4%) or second (26, 9%) most important factor in their graft decision. Clearly there is some discrepancy between studies regarding the influence of personal research and media. There are a few potential reasons for this. First, we did not explicitly ask patients if their own personal research had any influence on their graft decision. Rather, we asked patients to rank their decision-making factors, and few patients ranked the media as their first or second greatest influence. Second, the word “media” was used in our questionnaire rather than “online research” or “internet.” It may seem somewhat vague to patients what the word “media” really means in terms of their own research, whereas listing “online research” or “internet” as selection options may have influenced patient responses.
In our study, we asked patients for any additional factors that influenced their graft choice. Thirteen patients (4%) noted that “personal research” through internet, orthopaedic literature, and the media influenced their graft decision. This corroborates the idea that “media” may have seemed vague to some patients. Of these patients, 9 chose an autograft and 4 chose an allograft. The relative ease in accessing information regarding graft choice in ACL reconstruction should be noted. Numerous websites offer advice, graft options, and commentary from group practices and orthopaedic surgeons. Whether or not these sources provide reasonable support for one graft vs another graft remains to be answered. The physician should be responsible for providing the patient with this collected objective information.
In our study, 205 patients (96%) were satisfied with their graft choice at the time of follow-up, with 15 patients (7%) stating that they would have chosen a different graft type if they could redo the operation. Cheung and colleagues9 found a satisfaction rate of 87.4% at an average follow-up time of 19 months, with 4.6% stating they would have chosen a different graft type. Many factors can contribute to patient satisfaction after ACL reconstruction. Looking at patient variables such as age, demographics, occupation, activity level, surgical technique including tunnel placement and fixation, postoperative rehabilitation, and graft type may influence the success of the patient after ACL reconstruction.
The strengths of this study include the patient population size with 1-year follow-up as well as the prospective study design. In comparison to a previous retrospective study in 2009 by Cohen and colleagues8with a sample size of 240 patients, our study collected 213 patients with 70% follow-up at minimum 1 year. Collecting data prospectively ensures accurate representation of the factors influencing each patient’s graft selection, while follow-up data was useful for patient satisfaction.
The limitations of this study include the percentage of patients lost from follow-up as well as any bias generated from the organization of the questionnaire. Unfortunately, with a younger, transient population of patients undergoing ACL reconstruction in a major metropolitan area, a percentage of patients are lost to follow-up. Many attempts were made to locate these patients. Another potential limitation was the order of decision factors listed on the questionnaire. These factors were not ordered randomly on each survey, but were listed in the following order: (1) physician recommendation (2) family/friend’s recommendation (3) coach’s recommendation and (4) the media. This may have influenced patient responses. The organization of these factors in the questionnaire started with physician recommendation, which may have influenced the patient’s initial thought process of which factor had the greatest influence in their graft decision. In addition, for the surveys completed at least 1 year following surgery, some patients were contacted via e-mail and others via telephone. Thus, some patients may have changed their answers if they were able to see the questions rather than hearing the questions. We believe this is particularly true of the question regarding graft harvest site.
Our study indicates that the majority of patients undergoing ACL reconstruction are primarily influenced by the physician’s recommendation.
1. Madick S. Anterior cruciate ligament reconstruction of the knee. AORN J. 2011;93(2):210-222.
2. Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):661-681.
3. Paxton EW, Namba RS, Maletis GB, et al. A prospective study of 80,000 total joint and 5000 anterior cruciate ligament reconstruction procedures in a community-based registry in the United States. J Bone Joint Surg Am. 2010;92(suppl 2):117-132.
4. Kraeutler MJ, Bravman JT, McCarty EC. Bone-patellar tendon-bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: A meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
5. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
6. Boonriong T, Kietsiriroje N. Arthroscopically assisted anterior cruciate ligament reconstruction: comparison of bone-patellar tendon-bone versus hamstring tendon autograft. J Med Assoc Thai. 2004;87(9):1100-1107.
7. Fox JA, Pierce M, Bojchuk J, Hayden J, Bush-Joseph CA, Bach BR Jr. Revision anterior cruciate ligament reconstruction with nonirradiated fresh-frozen patellar tendon allograft. Arthroscopy. 2004;20(8):787-794.
8. Cohen SB, Yucha DT, Ciccotti MC, Goldstein DT, Ciccotti MA, Ciccotti MG. Factors affecting patient selection of graft type in anterior cruciate ligament reconstruction. Arthroscopy. 2009;25(9):1006-1010.
9. Cheung SC, Allen CR, Gallo RA, Ma CB, Feeley BT. Patients’ attitudes and factors in their selection of grafts for anterior cruciate ligament reconstruction. Knee. 2012;19(1):49-54.
10. Koh HS, In Y, Kong CG, Won HY, Kim KH, Lee JH. Factors affecting patients’ graft choice in anterior cruciate ligament reconstruction. Clin Orthop Surg. 2010;2(2):69-75.
11. Duncan IC, Kane PW, Lawson KA, Cohen SB, Ciccotti MG, Dodson CC. Evaluation of information available on the internet regarding anterior cruciate ligament reconstruction. Arthroscopy. 2013;29(6):1101-1107.
1. Madick S. Anterior cruciate ligament reconstruction of the knee. AORN J. 2011;93(2):210-222.
2. Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):661-681.
3. Paxton EW, Namba RS, Maletis GB, et al. A prospective study of 80,000 total joint and 5000 anterior cruciate ligament reconstruction procedures in a community-based registry in the United States. J Bone Joint Surg Am. 2010;92(suppl 2):117-132.
4. Kraeutler MJ, Bravman JT, McCarty EC. Bone-patellar tendon-bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: A meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
5. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
6. Boonriong T, Kietsiriroje N. Arthroscopically assisted anterior cruciate ligament reconstruction: comparison of bone-patellar tendon-bone versus hamstring tendon autograft. J Med Assoc Thai. 2004;87(9):1100-1107.
7. Fox JA, Pierce M, Bojchuk J, Hayden J, Bush-Joseph CA, Bach BR Jr. Revision anterior cruciate ligament reconstruction with nonirradiated fresh-frozen patellar tendon allograft. Arthroscopy. 2004;20(8):787-794.
8. Cohen SB, Yucha DT, Ciccotti MC, Goldstein DT, Ciccotti MA, Ciccotti MG. Factors affecting patient selection of graft type in anterior cruciate ligament reconstruction. Arthroscopy. 2009;25(9):1006-1010.
9. Cheung SC, Allen CR, Gallo RA, Ma CB, Feeley BT. Patients’ attitudes and factors in their selection of grafts for anterior cruciate ligament reconstruction. Knee. 2012;19(1):49-54.
10. Koh HS, In Y, Kong CG, Won HY, Kim KH, Lee JH. Factors affecting patients’ graft choice in anterior cruciate ligament reconstruction. Clin Orthop Surg. 2010;2(2):69-75.
11. Duncan IC, Kane PW, Lawson KA, Cohen SB, Ciccotti MG, Dodson CC. Evaluation of information available on the internet regarding anterior cruciate ligament reconstruction. Arthroscopy. 2013;29(6):1101-1107.
Actinic Keratosis as a Marker of Field Cancerization in Excision Specimens of Cutaneous Malignancies
The concept of field cancerization was first proposed in 1953 by Slaughter et al1 in their study of oral squamous carcinomas. Their findings of multifocal patches of premalignant disease, abnormal tissue surrounding tumors, multiple localized primary tumors, and tumor recurrence following surgical resection was suggestive of a field of dysplastic cells with malignant potential.1 Since then, modern molecular techniques have been used to establish a genetic basis for this model in many different types of cancer, including cutaneous malignancies.2-4 The field begins from a singular stem cell, which undergoes one or more genetic changes that allow for a growth advantage compared to surrounding cells. The stem cell then divides, forming a patch of clonal daughter cells that displace the surrounding normal epithelium. Growth of this patch eventually leads to a dysplastic field of monoclonal cells, which notably does not yet show invasive growth or metastatic behavior. Over time, continued carcinogenic exposure results in additional genetic alterations among different cells in the field, which leads to new subclonal proliferations that share common clonal origin but exhibit unique genetic changes. Eventually, transformative events may occur, resulting in cells with invasive and metastatic properties, thus forming a carcinoma.5
In the case of cutaneous malignancies, UV radiation in the form of UVA and UVB rays is the most common source of carcinogenesis. It is well established that UV radiation has numerous effects on the body, including but not limited to local and systemic immunosuppression, alteration of signal transduction pathways, and the development of mutations in DNA via direct damage by UVB or indirect damage by free radical formation with UVA.6,7 Normally, DNA is protected from UV radiation–induced genetic alteration by the p53 gene, TP53. As such, damage to this gene is highly associated with cancer induction. One study found that more than 90% of squamous cell carcinomas (SCCs) and more than 50% of basal cell carcinomas (BCCs) contain UV-like mutations in TP53.8 The concept of field cancerization suggests that because the skin surrounding cutaneous malignancies has been exposed to the same chronic UV light as the initial lesion, it is at an increased risk for genetic abnormalities and thus possible malignant transformation.
Actinic keratoses (AKs) are common neoplasms of the skin that generally are regarded as precancerous lesions or may be considered to be the earliest stage of SCC in situ.9 Actinic keratoses usually develop as a consequence of chronic exposure to UV radiation and often are clinically apparent as erythematous scaly papules in sun-exposed areas (Figure 1).10 They also are identified histologically as atypical keratinocytes along the basal layer of the epidermis with possible enlargement, hyperchromatic nuclei, lack of maturation, mitotic figures, inflammatory infiltrate, and/or hyperkeratosis.10 Furthermore, the genetic changes associated with AKs are well documented and are strongly associated with changes to p53.11 Given these characteristics, AKs serve as good markers of genetic damage with potential for malignancy. In this study, we used histologically identified AKs to assess the presence of field damage in the tissue immediately surrounding excision specimens of SCCs, BCCs, and malignant melanomas (MMs).
Methods
This study was approved by the Program for the Protection of Human Subjects at the Icahn School of Medicine at Mount Sinai (New York, New York) prior to initiation. All cutaneous specimens submitted to the dermatopathology service for consultation between April 2013 and June 2013 were reviewed for inclusion in this study. Data collection was extended for MMs to include all specimens from January 2013 to June 2013 given the limited number of cases in the original data collection period.
Initial screening for this study was done electronically and assessed for a diagnosis of SCC (Figure 2), BCC (Figure 3), or MM (Figure 4) as determined by a board-certified dermatopathologist (G.G.). The resulting pool of specimens was then screened to include only excision specimens and to exclude curettage specimens and superficial specimens that lacked dermis. In this study, we chose to look at reexcisions rather than initial biopsies so that there was a greater likelihood of having an intact epidermis surrounding a malignancy that could be assessed for the presence of AKs as markers for field cancerization. Specimens were examined in full via serial transverse cross-sections at 3-mm intervals. Additional step sections were obtained at smaller intervals when margins were close or unclear.
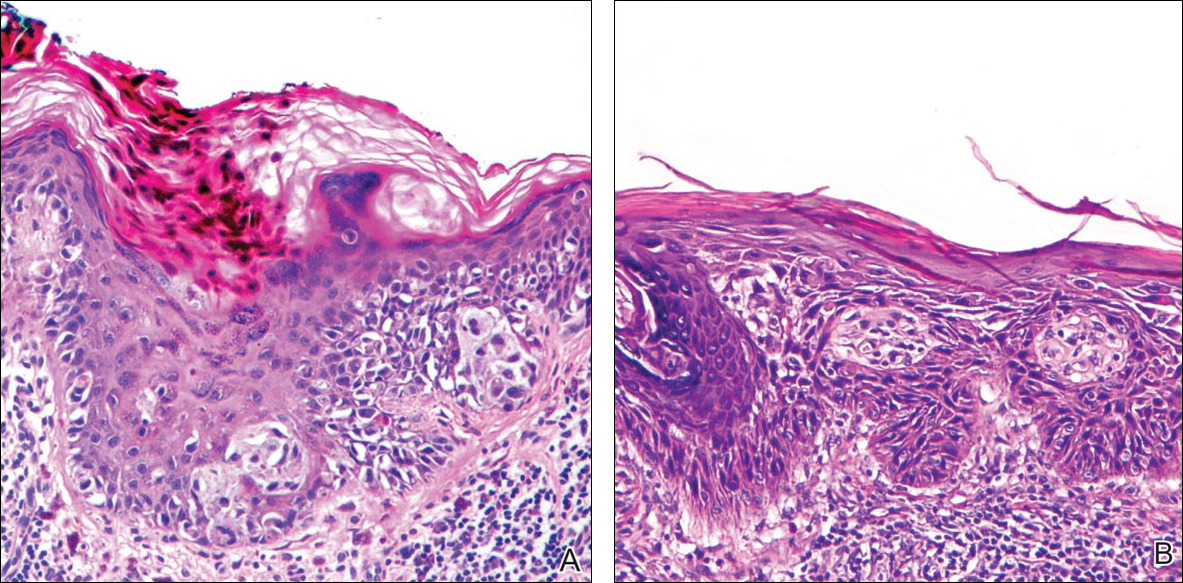
Selected cases were reassessed by a board-certified dermatopathologist (G.G.) to confirm the diagnosis and to assess for the presence of at least 1 AK within the specimen sample that was separated from the original malignancy by histologically normal-appearing cells. Samples were also assessed for the presence of an AK within 0.1 mm of the distal lateral margins of the tissue sample. Information regarding patient age, gender, lesion location, lesion type, and specimen size was collected for each sample. In accordance with institutional review board protocol, research data were collected without any protected health information. All analyses and results were deidentified and stored on a password-protected computer database. Statistical analysis was performed using SPSS software. When applicable, P<.05 was considered to indicate statistical significance.
Results
There were 205 cases that passed the initial screening filters, of which 56 were excluded due to the presence of curettage or lack of a sufficient tissue sample. Of the remaining 149 cases, the distribution by malignancy type was tabulated along with the percentage of observed AKs. If an AK was observed, the percentage that had an AK at the lateral margins (marginal AK) was determined (Table 1). A χ2 analysis determined that AKs were observed significantly more often in SCC specimens (57% [35/61]) than BCC (33% [21/64]) or malignant melanoma (25% [6/24]) specimens (P=.0125).
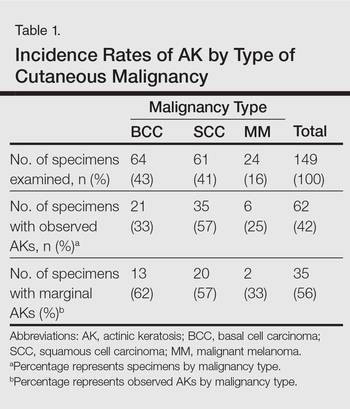
Statistics regarding patient age and gender as well as specimen size were stratified by malignancy type (Table 2). Using a receiver operating characteristic curve and the Youden index, an optimal cutoff of older than 67 years was determined to increase probability of observing an AK (P=.077) with sensitivity of 0.531 and specificity of 0.529. The distribution of specimen excision location for each malignancy type is shown in Table 3.
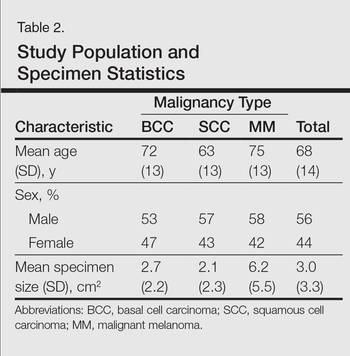
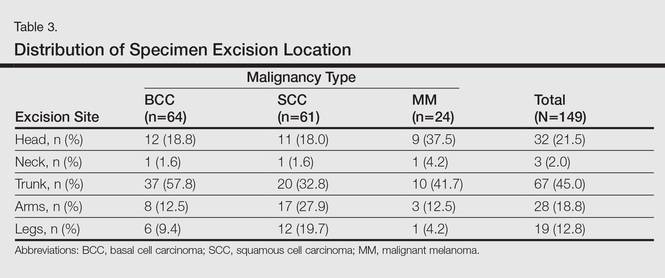
A multivariate analysis was performed to determine if the variables of patient age, gender, biopsy size, malignancy type (SCC, BCC, or MM), or cancer location (head, neck, trunk, arms, or legs) were independently useful in predicting whether an AK would be observed in the excision specimen. The significance of variables in the logistic regression model was assessed using a backward stepwise regression selection procedure entering variables if P<.15 and excluding variables if P>.25. Significant variables in predicting the occurrence of AK were SCC malignancy type (P=.007; odds ratio [OR], 2.61) and location on the head (P=.044; OR, 2.39) and arms (P=.042; OR, 2.55).
Comment
The χ2 analysis of our data showed that SCC specimens were significantly more likely to have an associated AK than either BCCs or MMs (P=.0125), which is not surprising given that AKs are considered by many to be early-stage SCCs.12 It is important to note, however, that BCCs and MMs both had nonnegligible rates of associated AKs. Although BCC and MM do not arise from the same background of genetic changes as SCC, this finding is noteworthy because it demonstrates definitive field damage with malignant potential in the area surrounding these cutaneous malignancies.
Our data also showed that there was a significantly greater association of AKs in malignancies located on the head (P=.044) and arms (P=.042), possibly because these 2 areas tend to be the most sun exposed and thus are more likely to have sustained field damage as evidenced by the higher percentage of AKs. A study by Jonason et al13 described a similar finding in which sun-exposed skin exhibited significantly more frequent (P=.04) and larger (P=.02) clonal patches of mutated p53 keratinocytes than sun-protected skin.
It is likely that the field damage surrounding the cutaneous lesions in our study is actually greater than what we reported because the AK was present at the margin of the excision specimens the majority of the time (56%), which suggests that there likely may have been more AKs found if a wider area surrounding the malignancy had been studied given that AKs often are at the periphery of the lesion and may be missed by a small excision. Fewer marginal AKs were observed with MM cases, possibly because the excision specimens were more than double the size of SCC or BCC excisions. Furthermore, there likely is to be more damage than what can be appreciated by visual changes alone.
Kanjilal et al14 used polymerase chain reaction and DNA sequencing to demonstrate numerous p53 mutations in nonmalignant-appearing skin surrounding BCCs and SCCs. Brennan et al15 found p53 mutations in surgical margins of excised SCCs considered to be tumor free by histopathologic analysis in more than half of the specimens studied. Notably, tumor recurrence was significantly more likely in areas where mutations were found and no tumor recurrence was seen in areas free of p53 mutations (P=.02).15 Tabor et al4 similarly found genetically altered fields in histologically clear surgical margins of SCCs but also showed that local tumor recurrence following excision had more molecular markers in common with the nonresected premalignant field than it did with the primary tumor. Thus, these studies provide a genetic basis for field damage that can exist even in histologically benign-appearing cells.
We believe our findings are clinically relevant, as they provide additional evidence for the theory of field cancerization as demonstrated by the nonnegligible rates of AKs and thus field damage with malignant potential in the skin immediately surrounding cutaneous malignancies. The limitations of our study, however, include a small sample size; no consideration of the effects of prior topical, field, or systemic treatments; and lack of a control group. Nevertheless, our findings emphasize the importance of assessing the extent of field damage when determining treatment strategies. Clinicians treating cutaneous malignancies should consider the need for field therapy, especially in sun-exposed regions, to avoid additional primary tumors.16 Further research is needed, however, to identify optimal methods for quantifying field damage clinically and determining the most effective treatment strategies.
- Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963-968.
- Braakhuis B, Tabor M, Kummer J, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Stern R, Bolshakov S, Nataraj A, et al. p53 Mutation in nonmelanoma skin cancers occurring in psoralen ultraviolet A-treated patients: evidence for heterogeneity and field cancerization. J Invest Dermatol. 2002;119:522-526.
- Tabor M, Brakenhoff R, van Houten VM, et al. Persistence of genetically altered fields in head and neck cancer patients: biological and clinical implications. Clin Cancer Res. 2001;7:1523-1532.
- Torezan L. Cutaneous field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol. 2013;88:775-786.
- Ullrich S, Kripke M, Ananthaswamy H. Mechanisms underlying UV-induced immune suppression: implications for sunscreen design. Exp Dermatol. 2002;11:1-4.
- de Gruijl FR. Photocarcinogenesis: UVA vs UVB. Methods Enzymol. 2000;319:359-366.
- Brash DE, Ziegler A, Jonason AS, et al. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J Investig Dermatol Symp Proc. 1996;1:136-142.
- Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol. 2006;155:9-22.
- Rossi R, Mori M, Lotti T. Actinic keratosis. Int J Dermatol. 2007;46:895-904.
- Ziegler A, Jonason AS, Leffel DJ, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773-776.
- Cockerell C. Histopathology of incipient intraepidermal squamous cell carcinoma (“actinic keratosis”). J Am Acad Dermatol. 2000;42:11-17.
- Jonason AS, Kunala S, Price GJ, et al. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci. 1996;93:14025-14029.
- Kanjilal S, Strom SS, Clayman GL, et al. p53 Mutations in nonmelanoma skin cancer of the head and neck: molecular evidence for field cancerization. Cancer Res. 1995;55:3604-3609.
- Brennan JA, Mao L, Hruban RH, et al. Molecular assessment of histopathological staging in squamous cell carcinoma of the head and neck. N Engl J Med. 1995;332:429-435.
- Braathen LR, Morton CA, Basset-Seguin N, et al. Photodynamic therapy for skin field cancerization: an international consensus. International Society for Photodynamic Therapy in Dermatology. J Eur Acad Dermatol Venereol. 2012;26:1063-1066.
The concept of field cancerization was first proposed in 1953 by Slaughter et al1 in their study of oral squamous carcinomas. Their findings of multifocal patches of premalignant disease, abnormal tissue surrounding tumors, multiple localized primary tumors, and tumor recurrence following surgical resection was suggestive of a field of dysplastic cells with malignant potential.1 Since then, modern molecular techniques have been used to establish a genetic basis for this model in many different types of cancer, including cutaneous malignancies.2-4 The field begins from a singular stem cell, which undergoes one or more genetic changes that allow for a growth advantage compared to surrounding cells. The stem cell then divides, forming a patch of clonal daughter cells that displace the surrounding normal epithelium. Growth of this patch eventually leads to a dysplastic field of monoclonal cells, which notably does not yet show invasive growth or metastatic behavior. Over time, continued carcinogenic exposure results in additional genetic alterations among different cells in the field, which leads to new subclonal proliferations that share common clonal origin but exhibit unique genetic changes. Eventually, transformative events may occur, resulting in cells with invasive and metastatic properties, thus forming a carcinoma.5
In the case of cutaneous malignancies, UV radiation in the form of UVA and UVB rays is the most common source of carcinogenesis. It is well established that UV radiation has numerous effects on the body, including but not limited to local and systemic immunosuppression, alteration of signal transduction pathways, and the development of mutations in DNA via direct damage by UVB or indirect damage by free radical formation with UVA.6,7 Normally, DNA is protected from UV radiation–induced genetic alteration by the p53 gene, TP53. As such, damage to this gene is highly associated with cancer induction. One study found that more than 90% of squamous cell carcinomas (SCCs) and more than 50% of basal cell carcinomas (BCCs) contain UV-like mutations in TP53.8 The concept of field cancerization suggests that because the skin surrounding cutaneous malignancies has been exposed to the same chronic UV light as the initial lesion, it is at an increased risk for genetic abnormalities and thus possible malignant transformation.
Actinic keratoses (AKs) are common neoplasms of the skin that generally are regarded as precancerous lesions or may be considered to be the earliest stage of SCC in situ.9 Actinic keratoses usually develop as a consequence of chronic exposure to UV radiation and often are clinically apparent as erythematous scaly papules in sun-exposed areas (Figure 1).10 They also are identified histologically as atypical keratinocytes along the basal layer of the epidermis with possible enlargement, hyperchromatic nuclei, lack of maturation, mitotic figures, inflammatory infiltrate, and/or hyperkeratosis.10 Furthermore, the genetic changes associated with AKs are well documented and are strongly associated with changes to p53.11 Given these characteristics, AKs serve as good markers of genetic damage with potential for malignancy. In this study, we used histologically identified AKs to assess the presence of field damage in the tissue immediately surrounding excision specimens of SCCs, BCCs, and malignant melanomas (MMs).

Methods
This study was approved by the Program for the Protection of Human Subjects at the Icahn School of Medicine at Mount Sinai (New York, New York) prior to initiation. All cutaneous specimens submitted to the dermatopathology service for consultation between April 2013 and June 2013 were reviewed for inclusion in this study. Data collection was extended for MMs to include all specimens from January 2013 to June 2013 given the limited number of cases in the original data collection period.
Initial screening for this study was done electronically and assessed for a diagnosis of SCC (Figure 2), BCC (Figure 3), or MM (Figure 4) as determined by a board-certified dermatopathologist (G.G.). The resulting pool of specimens was then screened to include only excision specimens and to exclude curettage specimens and superficial specimens that lacked dermis. In this study, we chose to look at reexcisions rather than initial biopsies so that there was a greater likelihood of having an intact epidermis surrounding a malignancy that could be assessed for the presence of AKs as markers for field cancerization. Specimens were examined in full via serial transverse cross-sections at 3-mm intervals. Additional step sections were obtained at smaller intervals when margins were close or unclear.



Selected cases were reassessed by a board-certified dermatopathologist (G.G.) to confirm the diagnosis and to assess for the presence of at least 1 AK within the specimen sample that was separated from the original malignancy by histologically normal-appearing cells. Samples were also assessed for the presence of an AK within 0.1 mm of the distal lateral margins of the tissue sample. Information regarding patient age, gender, lesion location, lesion type, and specimen size was collected for each sample. In accordance with institutional review board protocol, research data were collected without any protected health information. All analyses and results were deidentified and stored on a password-protected computer database. Statistical analysis was performed using SPSS software. When applicable, P<.05 was considered to indicate statistical significance.
Results
There were 205 cases that passed the initial screening filters, of which 56 were excluded due to the presence of curettage or lack of a sufficient tissue sample. Of the remaining 149 cases, the distribution by malignancy type was tabulated along with the percentage of observed AKs. If an AK was observed, the percentage that had an AK at the lateral margins (marginal AK) was determined (Table 1). A χ2 analysis determined that AKs were observed significantly more often in SCC specimens (57% [35/61]) than BCC (33% [21/64]) or malignant melanoma (25% [6/24]) specimens (P=.0125).

Statistics regarding patient age and gender as well as specimen size were stratified by malignancy type (Table 2). Using a receiver operating characteristic curve and the Youden index, an optimal cutoff of older than 67 years was determined to increase probability of observing an AK (P=.077) with sensitivity of 0.531 and specificity of 0.529. The distribution of specimen excision location for each malignancy type is shown in Table 3.


A multivariate analysis was performed to determine if the variables of patient age, gender, biopsy size, malignancy type (SCC, BCC, or MM), or cancer location (head, neck, trunk, arms, or legs) were independently useful in predicting whether an AK would be observed in the excision specimen. The significance of variables in the logistic regression model was assessed using a backward stepwise regression selection procedure entering variables if P<.15 and excluding variables if P>.25. Significant variables in predicting the occurrence of AK were SCC malignancy type (P=.007; odds ratio [OR], 2.61) and location on the head (P=.044; OR, 2.39) and arms (P=.042; OR, 2.55).
Comment
The χ2 analysis of our data showed that SCC specimens were significantly more likely to have an associated AK than either BCCs or MMs (P=.0125), which is not surprising given that AKs are considered by many to be early-stage SCCs.12 It is important to note, however, that BCCs and MMs both had nonnegligible rates of associated AKs. Although BCC and MM do not arise from the same background of genetic changes as SCC, this finding is noteworthy because it demonstrates definitive field damage with malignant potential in the area surrounding these cutaneous malignancies.
Our data also showed that there was a significantly greater association of AKs in malignancies located on the head (P=.044) and arms (P=.042), possibly because these 2 areas tend to be the most sun exposed and thus are more likely to have sustained field damage as evidenced by the higher percentage of AKs. A study by Jonason et al13 described a similar finding in which sun-exposed skin exhibited significantly more frequent (P=.04) and larger (P=.02) clonal patches of mutated p53 keratinocytes than sun-protected skin.
It is likely that the field damage surrounding the cutaneous lesions in our study is actually greater than what we reported because the AK was present at the margin of the excision specimens the majority of the time (56%), which suggests that there likely may have been more AKs found if a wider area surrounding the malignancy had been studied given that AKs often are at the periphery of the lesion and may be missed by a small excision. Fewer marginal AKs were observed with MM cases, possibly because the excision specimens were more than double the size of SCC or BCC excisions. Furthermore, there likely is to be more damage than what can be appreciated by visual changes alone.
Kanjilal et al14 used polymerase chain reaction and DNA sequencing to demonstrate numerous p53 mutations in nonmalignant-appearing skin surrounding BCCs and SCCs. Brennan et al15 found p53 mutations in surgical margins of excised SCCs considered to be tumor free by histopathologic analysis in more than half of the specimens studied. Notably, tumor recurrence was significantly more likely in areas where mutations were found and no tumor recurrence was seen in areas free of p53 mutations (P=.02).15 Tabor et al4 similarly found genetically altered fields in histologically clear surgical margins of SCCs but also showed that local tumor recurrence following excision had more molecular markers in common with the nonresected premalignant field than it did with the primary tumor. Thus, these studies provide a genetic basis for field damage that can exist even in histologically benign-appearing cells.
We believe our findings are clinically relevant, as they provide additional evidence for the theory of field cancerization as demonstrated by the nonnegligible rates of AKs and thus field damage with malignant potential in the skin immediately surrounding cutaneous malignancies. The limitations of our study, however, include a small sample size; no consideration of the effects of prior topical, field, or systemic treatments; and lack of a control group. Nevertheless, our findings emphasize the importance of assessing the extent of field damage when determining treatment strategies. Clinicians treating cutaneous malignancies should consider the need for field therapy, especially in sun-exposed regions, to avoid additional primary tumors.16 Further research is needed, however, to identify optimal methods for quantifying field damage clinically and determining the most effective treatment strategies.
The concept of field cancerization was first proposed in 1953 by Slaughter et al1 in their study of oral squamous carcinomas. Their findings of multifocal patches of premalignant disease, abnormal tissue surrounding tumors, multiple localized primary tumors, and tumor recurrence following surgical resection was suggestive of a field of dysplastic cells with malignant potential.1 Since then, modern molecular techniques have been used to establish a genetic basis for this model in many different types of cancer, including cutaneous malignancies.2-4 The field begins from a singular stem cell, which undergoes one or more genetic changes that allow for a growth advantage compared to surrounding cells. The stem cell then divides, forming a patch of clonal daughter cells that displace the surrounding normal epithelium. Growth of this patch eventually leads to a dysplastic field of monoclonal cells, which notably does not yet show invasive growth or metastatic behavior. Over time, continued carcinogenic exposure results in additional genetic alterations among different cells in the field, which leads to new subclonal proliferations that share common clonal origin but exhibit unique genetic changes. Eventually, transformative events may occur, resulting in cells with invasive and metastatic properties, thus forming a carcinoma.5
In the case of cutaneous malignancies, UV radiation in the form of UVA and UVB rays is the most common source of carcinogenesis. It is well established that UV radiation has numerous effects on the body, including but not limited to local and systemic immunosuppression, alteration of signal transduction pathways, and the development of mutations in DNA via direct damage by UVB or indirect damage by free radical formation with UVA.6,7 Normally, DNA is protected from UV radiation–induced genetic alteration by the p53 gene, TP53. As such, damage to this gene is highly associated with cancer induction. One study found that more than 90% of squamous cell carcinomas (SCCs) and more than 50% of basal cell carcinomas (BCCs) contain UV-like mutations in TP53.8 The concept of field cancerization suggests that because the skin surrounding cutaneous malignancies has been exposed to the same chronic UV light as the initial lesion, it is at an increased risk for genetic abnormalities and thus possible malignant transformation.
Actinic keratoses (AKs) are common neoplasms of the skin that generally are regarded as precancerous lesions or may be considered to be the earliest stage of SCC in situ.9 Actinic keratoses usually develop as a consequence of chronic exposure to UV radiation and often are clinically apparent as erythematous scaly papules in sun-exposed areas (Figure 1).10 They also are identified histologically as atypical keratinocytes along the basal layer of the epidermis with possible enlargement, hyperchromatic nuclei, lack of maturation, mitotic figures, inflammatory infiltrate, and/or hyperkeratosis.10 Furthermore, the genetic changes associated with AKs are well documented and are strongly associated with changes to p53.11 Given these characteristics, AKs serve as good markers of genetic damage with potential for malignancy. In this study, we used histologically identified AKs to assess the presence of field damage in the tissue immediately surrounding excision specimens of SCCs, BCCs, and malignant melanomas (MMs).

Methods
This study was approved by the Program for the Protection of Human Subjects at the Icahn School of Medicine at Mount Sinai (New York, New York) prior to initiation. All cutaneous specimens submitted to the dermatopathology service for consultation between April 2013 and June 2013 were reviewed for inclusion in this study. Data collection was extended for MMs to include all specimens from January 2013 to June 2013 given the limited number of cases in the original data collection period.
Initial screening for this study was done electronically and assessed for a diagnosis of SCC (Figure 2), BCC (Figure 3), or MM (Figure 4) as determined by a board-certified dermatopathologist (G.G.). The resulting pool of specimens was then screened to include only excision specimens and to exclude curettage specimens and superficial specimens that lacked dermis. In this study, we chose to look at reexcisions rather than initial biopsies so that there was a greater likelihood of having an intact epidermis surrounding a malignancy that could be assessed for the presence of AKs as markers for field cancerization. Specimens were examined in full via serial transverse cross-sections at 3-mm intervals. Additional step sections were obtained at smaller intervals when margins were close or unclear.



Selected cases were reassessed by a board-certified dermatopathologist (G.G.) to confirm the diagnosis and to assess for the presence of at least 1 AK within the specimen sample that was separated from the original malignancy by histologically normal-appearing cells. Samples were also assessed for the presence of an AK within 0.1 mm of the distal lateral margins of the tissue sample. Information regarding patient age, gender, lesion location, lesion type, and specimen size was collected for each sample. In accordance with institutional review board protocol, research data were collected without any protected health information. All analyses and results were deidentified and stored on a password-protected computer database. Statistical analysis was performed using SPSS software. When applicable, P<.05 was considered to indicate statistical significance.
Results
There were 205 cases that passed the initial screening filters, of which 56 were excluded due to the presence of curettage or lack of a sufficient tissue sample. Of the remaining 149 cases, the distribution by malignancy type was tabulated along with the percentage of observed AKs. If an AK was observed, the percentage that had an AK at the lateral margins (marginal AK) was determined (Table 1). A χ2 analysis determined that AKs were observed significantly more often in SCC specimens (57% [35/61]) than BCC (33% [21/64]) or malignant melanoma (25% [6/24]) specimens (P=.0125).

Statistics regarding patient age and gender as well as specimen size were stratified by malignancy type (Table 2). Using a receiver operating characteristic curve and the Youden index, an optimal cutoff of older than 67 years was determined to increase probability of observing an AK (P=.077) with sensitivity of 0.531 and specificity of 0.529. The distribution of specimen excision location for each malignancy type is shown in Table 3.


A multivariate analysis was performed to determine if the variables of patient age, gender, biopsy size, malignancy type (SCC, BCC, or MM), or cancer location (head, neck, trunk, arms, or legs) were independently useful in predicting whether an AK would be observed in the excision specimen. The significance of variables in the logistic regression model was assessed using a backward stepwise regression selection procedure entering variables if P<.15 and excluding variables if P>.25. Significant variables in predicting the occurrence of AK were SCC malignancy type (P=.007; odds ratio [OR], 2.61) and location on the head (P=.044; OR, 2.39) and arms (P=.042; OR, 2.55).
Comment
The χ2 analysis of our data showed that SCC specimens were significantly more likely to have an associated AK than either BCCs or MMs (P=.0125), which is not surprising given that AKs are considered by many to be early-stage SCCs.12 It is important to note, however, that BCCs and MMs both had nonnegligible rates of associated AKs. Although BCC and MM do not arise from the same background of genetic changes as SCC, this finding is noteworthy because it demonstrates definitive field damage with malignant potential in the area surrounding these cutaneous malignancies.
Our data also showed that there was a significantly greater association of AKs in malignancies located on the head (P=.044) and arms (P=.042), possibly because these 2 areas tend to be the most sun exposed and thus are more likely to have sustained field damage as evidenced by the higher percentage of AKs. A study by Jonason et al13 described a similar finding in which sun-exposed skin exhibited significantly more frequent (P=.04) and larger (P=.02) clonal patches of mutated p53 keratinocytes than sun-protected skin.
It is likely that the field damage surrounding the cutaneous lesions in our study is actually greater than what we reported because the AK was present at the margin of the excision specimens the majority of the time (56%), which suggests that there likely may have been more AKs found if a wider area surrounding the malignancy had been studied given that AKs often are at the periphery of the lesion and may be missed by a small excision. Fewer marginal AKs were observed with MM cases, possibly because the excision specimens were more than double the size of SCC or BCC excisions. Furthermore, there likely is to be more damage than what can be appreciated by visual changes alone.
Kanjilal et al14 used polymerase chain reaction and DNA sequencing to demonstrate numerous p53 mutations in nonmalignant-appearing skin surrounding BCCs and SCCs. Brennan et al15 found p53 mutations in surgical margins of excised SCCs considered to be tumor free by histopathologic analysis in more than half of the specimens studied. Notably, tumor recurrence was significantly more likely in areas where mutations were found and no tumor recurrence was seen in areas free of p53 mutations (P=.02).15 Tabor et al4 similarly found genetically altered fields in histologically clear surgical margins of SCCs but also showed that local tumor recurrence following excision had more molecular markers in common with the nonresected premalignant field than it did with the primary tumor. Thus, these studies provide a genetic basis for field damage that can exist even in histologically benign-appearing cells.
We believe our findings are clinically relevant, as they provide additional evidence for the theory of field cancerization as demonstrated by the nonnegligible rates of AKs and thus field damage with malignant potential in the skin immediately surrounding cutaneous malignancies. The limitations of our study, however, include a small sample size; no consideration of the effects of prior topical, field, or systemic treatments; and lack of a control group. Nevertheless, our findings emphasize the importance of assessing the extent of field damage when determining treatment strategies. Clinicians treating cutaneous malignancies should consider the need for field therapy, especially in sun-exposed regions, to avoid additional primary tumors.16 Further research is needed, however, to identify optimal methods for quantifying field damage clinically and determining the most effective treatment strategies.
- Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963-968.
- Braakhuis B, Tabor M, Kummer J, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Stern R, Bolshakov S, Nataraj A, et al. p53 Mutation in nonmelanoma skin cancers occurring in psoralen ultraviolet A-treated patients: evidence for heterogeneity and field cancerization. J Invest Dermatol. 2002;119:522-526.
- Tabor M, Brakenhoff R, van Houten VM, et al. Persistence of genetically altered fields in head and neck cancer patients: biological and clinical implications. Clin Cancer Res. 2001;7:1523-1532.
- Torezan L. Cutaneous field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol. 2013;88:775-786.
- Ullrich S, Kripke M, Ananthaswamy H. Mechanisms underlying UV-induced immune suppression: implications for sunscreen design. Exp Dermatol. 2002;11:1-4.
- de Gruijl FR. Photocarcinogenesis: UVA vs UVB. Methods Enzymol. 2000;319:359-366.
- Brash DE, Ziegler A, Jonason AS, et al. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J Investig Dermatol Symp Proc. 1996;1:136-142.
- Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol. 2006;155:9-22.
- Rossi R, Mori M, Lotti T. Actinic keratosis. Int J Dermatol. 2007;46:895-904.
- Ziegler A, Jonason AS, Leffel DJ, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773-776.
- Cockerell C. Histopathology of incipient intraepidermal squamous cell carcinoma (“actinic keratosis”). J Am Acad Dermatol. 2000;42:11-17.
- Jonason AS, Kunala S, Price GJ, et al. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci. 1996;93:14025-14029.
- Kanjilal S, Strom SS, Clayman GL, et al. p53 Mutations in nonmelanoma skin cancer of the head and neck: molecular evidence for field cancerization. Cancer Res. 1995;55:3604-3609.
- Brennan JA, Mao L, Hruban RH, et al. Molecular assessment of histopathological staging in squamous cell carcinoma of the head and neck. N Engl J Med. 1995;332:429-435.
- Braathen LR, Morton CA, Basset-Seguin N, et al. Photodynamic therapy for skin field cancerization: an international consensus. International Society for Photodynamic Therapy in Dermatology. J Eur Acad Dermatol Venereol. 2012;26:1063-1066.
- Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963-968.
- Braakhuis B, Tabor M, Kummer J, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Stern R, Bolshakov S, Nataraj A, et al. p53 Mutation in nonmelanoma skin cancers occurring in psoralen ultraviolet A-treated patients: evidence for heterogeneity and field cancerization. J Invest Dermatol. 2002;119:522-526.
- Tabor M, Brakenhoff R, van Houten VM, et al. Persistence of genetically altered fields in head and neck cancer patients: biological and clinical implications. Clin Cancer Res. 2001;7:1523-1532.
- Torezan L. Cutaneous field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol. 2013;88:775-786.
- Ullrich S, Kripke M, Ananthaswamy H. Mechanisms underlying UV-induced immune suppression: implications for sunscreen design. Exp Dermatol. 2002;11:1-4.
- de Gruijl FR. Photocarcinogenesis: UVA vs UVB. Methods Enzymol. 2000;319:359-366.
- Brash DE, Ziegler A, Jonason AS, et al. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J Investig Dermatol Symp Proc. 1996;1:136-142.
- Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol. 2006;155:9-22.
- Rossi R, Mori M, Lotti T. Actinic keratosis. Int J Dermatol. 2007;46:895-904.
- Ziegler A, Jonason AS, Leffel DJ, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773-776.
- Cockerell C. Histopathology of incipient intraepidermal squamous cell carcinoma (“actinic keratosis”). J Am Acad Dermatol. 2000;42:11-17.
- Jonason AS, Kunala S, Price GJ, et al. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci. 1996;93:14025-14029.
- Kanjilal S, Strom SS, Clayman GL, et al. p53 Mutations in nonmelanoma skin cancer of the head and neck: molecular evidence for field cancerization. Cancer Res. 1995;55:3604-3609.
- Brennan JA, Mao L, Hruban RH, et al. Molecular assessment of histopathological staging in squamous cell carcinoma of the head and neck. N Engl J Med. 1995;332:429-435.
- Braathen LR, Morton CA, Basset-Seguin N, et al. Photodynamic therapy for skin field cancerization: an international consensus. International Society for Photodynamic Therapy in Dermatology. J Eur Acad Dermatol Venereol. 2012;26:1063-1066.
Practice Points
- Clinically apparent and subclinical actinic keratoses usually are present in patients, a concept known as field cancerization, and it is important to treat both types of lesions.
- Actinic keratoses are present in the field of cutaneous malignancies, including basal cell carcinoma, squamous cell carcinoma, and melanoma.
Could a Specific Dietary Intake Be a Risk Factor for Cutaneous Melanoma?
The incidence of cutaneous melanoma (CM) has increased, warranting further study of new risk factors.1,2 Hereditary risk factors for CM include light-colored eyes; fair skin; light brown, blonde, or red hair; tendency to burn; high density of freckles; history of other types of skin cancer; high number of common, atypical, and/or congenital nevi; and family history of skin cancer, as well as risks related to the presence of CDKN2A, BRAF, and MC1R gene mutations. Environmental risk factors include UV exposure from sunlight or tanning beds, among others.3-5
Nutritional factors also have been suggested as possible modifiable risk factors for CM.6 Evidence from epidemiological studies show that diets rich in fruits and vegetables are associated with lower risks for several types of cancer.7,8 A growing number of studies have assessed the effects of diet and the intake of nutrients on the prevention of cancer, specifically the use of dietary supplements to protect the skin from the adverse effects of UV light.6
Preformed vitamin A (ie, retinol) is necessary for the regulation of cell differentiation and also can reduce the incidence of skin tumors in animals exposed to UV light. Certain carotenoids such as α-carotene and β-carotene are metabolized to retinol. These retinol precursors, along with antioxidant nutrients, are important components of fruits and vegetables and may account for the observed anticancer effects of these foods.8
The aim of this study was to assess the relationship between dietary intake and the risk for CM.
Methods
Participants
A case-control study was carried out between 2012 and 2013 at 3 reference centers in Porto Alegre, Brazil—Universidade Federal de Ciências da Saúde de Porto Alegre, Pontifícia Universidade Católica do Rio Grande do Sul, and Hospital de Clínicas de Porto Alegre—for the treatment of patients with CM. Enrolled patients were 18 years and older with a diagnosis of primary CM confirmed by histology. Controls were selected from patients at the same centers, and they were enrolled and matched by institution. Controls were frequency matched to cases by sex and age (+/– 5 years). Exclusion criteria for controls were those presenting with suspicious lesions and those needing radiation therapy or chemotherapy due to other diseases. The study was approved by the ethics committees of the participating centers and informed consent was obtained from all participants. A total of 191 participants (95 cases; 96 controls) were enrolled in the study.
Data Collection
After informed consent was obtained, participants were interviewed and were clinically examined by an experienced dermatologist (C.B.H. and M.M.S.). The questionnaire included sociodemographic variables, medical history, phenotypic characteristics (ie, Fitzpatrick skin type, skin/hair/eye color), family history of skin cancer, history of sunlight exposure, history of sunburns, use of artificial tanning, sunscreen use, and detailed dietary intake. Physical examination included the assessment of several melanocytic lesions (nevi, freckles/ephelides, lentigines, and café au lait spots), actinic keratoses, solar elastosis, and nonmelanocytic tumors following the International Agency for Research on Cancer (IARC) protocol.9
Using a food frequency questionnaire, participants were asked to report their usual frequency of consumption of each food from a list of 36 foods. The frequency of intake of all groups of food and beverages was defined according to the following scale: never, rarely (less than once monthly), once or twice weekly, 3 to 4 times weekly, 5 to 7 times weekly, and more than 7 times weekly. Combination of categories was based on the overall distribution among controls. Therefore, for some items such as mussels and fresh herbs, only 2 categories were used.
Statistical Analysis
A descriptive statistical analysis of the results was performed using SPSS version 20.0 with absolute and relative frequencies for the categorical variables, and mean, SD, and median for the continuous variables. The symmetry of distributions was investigated using the Kolmogorov-Smirnov test.
A t test for independent groups was applied for the continuous variables, while the Pearson χ2 test was used for the categorical variables. The Fisher exact test was used in situations in which at least 25% of the values of the cells presented an expected frequency of less than 5. Monte Carlo simulation was used when at least 1 variable had a polytomic characteristic. Odds ratio (OR) was used to estimate the strength of the association between exposures and outcome. An unconditional binary logistic regression was used to study the association between dietary variables and the risk for CM. To obtain unbiased estimates, multivariate analyses were performed controlling for 1 or more confounding variables. Using low exposure as a base category, the risks and 95% CIs were calculated for the high-exposure categories. Based on the results of bivariate analyses, variables with P≤.25 or lower were included in the models. The likelihood ratio test was used to decide which covariates should be maintained in the model. To test the goodness of fit of the models, the Hosmer-Lemeshow statistic was used.
Potential confounding factors considered in the logistic regression model were sex; age; education level; skin, hair, and eye color; Fitzpatrick skin type; presence of freckles, solar lentigines, and actinic keratosis; history of nonmelanoma skin cancer; number of melanocytic nevi; family history of skin cancer; sunburns in adulthood (≥6 episodes a year); occupational sun exposure; and history of sunscreen use in adulthood.
Results
A total of 191 participants were enrolled in the study (95 [49.7%] cases; 96 [50.3%] controls). Most participants were female (60.0% of cases; 59.4% of controls). The mean age (SD) of cases and controls was 56.8 (13.9) years and 56.5 (13.2) years, respectively. Mean body mass index (SD) did not differ between cases (27.2 [4.6]) and controls (28.2 [6.5]). Education levels of 8 years or less predominated in both groups (64.2% of cases; 57.3% of controls). No statistical difference was found for sex, age, education, or body mass index. The most frequent anatomic sites of CM were the trunk (54.7%) and arms (20.0%), and the most frequent histological type was superficial spreading (62.8%). The median Breslow thickness was 0.90 mm. Ulceration was observed in 20.9% of the cases, and 67% of participants with CM had a high mitotic rate (≥1 mitosis per square millimeter).
Phenotypic characteristics associated with an increased risk for melanoma were light brown hair (OR, 6.73; 95% CI, 3.30-14.2), blonde/red hair (OR, 21.7; 95% CI, 7.51-63.1), light-colored eyes (eg, blue, gray, green)(OR, 13.2; 95% CI, 6.13-28.7), light brown eyes (OR, 5.01; 95% CI, 2.24-11.5), and Fitzpatrick skin types I and II (OR, 7.37; 95% CI, 2.90-26.1). Family history of skin cancer was associated with an increased risk for CM (OR, 4.31; 95% CI, 1.86-10.7) as well as sunburns in adulthood (OR, 1.64; 95% CI, 1.17-1.99). Regular sunscreen use in adulthood had a 5-fold increased risk for CM compared to not using sunscreen regularly (OR, 5.6; 95% CI, 2.85-10.7). Regarding pigmented lesions, the presence of solar lentigines (OR, 4.8; 95% CI, 2.2-11.2), 60 or more nevi (OR, 5.4; 95% CI, 2.4-12.7), and freckles (OR, 3.7; 95% CI, 1.82-7.64) were all associated with an increased risk for CM. Solar elastosis (OR, 2.5; 95% CI, 1.08-5.85), actinic keratosis (OR, 9.1, 95% CI, 3.97-20.84), and occupational exposure to sun (OR, 2.57; 95% CI, 1.23-5.38) also were associated with an increased risk for melanoma.
The intake of most of the foods and beverages included in the study showed no association with CM. High frequency of butter intake (more than daily) was a protective factor for CM (OR, 0.33; 95% CI, 0.16-0.70) compared to low-frequency consumption (daily and less than daily). Consumption of mussels (OR, 0.53; 95% CI, 0.29-0.97) and oregano (OR, 0.28; 95% CI, 0.12-0.66) also were shown to be protective against CM (OR, 0.53; 95% CI, 0.29-0.97). Regarding beverages, those in the highest categories of consumption—liquor (OR, 2.12; 95% CI, 1.09-4.12) and spirits (OR, 2.23; 95% CI, 1.16-4.68)—were associated with an increased risk for CM.
To identify the relationship between CM and the consumption of some foods that were relevant on bivariate analysis, we performed a multivariate model. When adjustments were made, the association remained for butter (OR, 0.141; 95% CI, 0.032-0.613) and oregano (OR, 0.176; 95% CI, 0.042-0.735), while the risk associated with the consumption of both liquor (OR, 1.511; 95% CI, 0.39-5.90) and spirits (OR, 0.755; 95% CI, 0.130-4.393) disappeared (Table).
Comment
Observational studies show that diets rich in fruits and vegetables are associated with a lower risk for different types of cancers.7,8 According to some studies, more than 30% of cancers in adulthood could be prevented or delayed by appropriate dietary intake and physical activity.10 However, there are still limited data on some specific cancers such as CM.
Substantial differences in the incidence of CM among different populations have suggested that environmental factors may play an etiological role in the development of CM and diet could be one of the modifiable risk factors.11-13
Initially, we assessed the already known risk factors for CM, and results showed a significantly increased risk for participants with light brown, blonde, or red hair (P<.0001); light-colored and light brown eyes (P<.0001); Fitzpatrick skin types I and II (P<.0001); positive family history of skin cancer (P=.001); the presence of solar lentigines (P<.001), freckles (P<.001), and actinic keratosis (P<.0001); and high number of nevi (P<.0001). Sunburns in adulthood (P<.001) were associated with an increased risk for CM, and our findings are in agreement with the literature.12
Besides confirming the well-known risk factors for CM, our study also showed that some foods (eg, butter, oregano) may act as important protective factors in CM. It could be argued that the increased risks associated with the well-known risk factors (eg, Fitzpatrick skin type, number of sunburns) might not be as strong and/or could be modulated by dietary factors. To further elucidate this critical issue, we analyzed our data by examining the joint relationship between dietary consumption, individual characteristics, sun exposure, and melanoma. We conducted a multivariable analysis controlling for the well-known risk factors and our findings suggest that both butter and oregano, foods that are rich in vitamins A and D, are independent and protective risk factors for melanoma.
Vitamin A (retinol) is a fat-soluble, organic compound that cannot be synthesized by humans but is necessary for normal physiological function and therefore is classified as an essential nutrient. The main source of vitamin A in the human diet is from retinyl esters, mostly from animal products such as dairy products (eg, butter) as well as from plant-based, provitamin A carotenoids (α-carotene, β-carotene) that can be converted to retinol in the intestines.14
Some case-control studies have investigated the association of vitamin A intake and CM risk, reporting mixed findings. Naldi et al15 found a notable inverse association between vitamin A intake and CM risk. Le Marchand et al16 found no inverse association for carotenoids or retinol. Kirkpatrick et al17 found no evidence of a protective effect for vitamin A or carotenoids on CM. However, the Nurses’ Health Study and the Nurses’ Health Study II reported inverse associations between CM and retinol from foods and dietary supplements.8
Dairy products such as butter contain several components considered to be potentially anticarcinogenic, such as calcium, vitamin D, butyric acid, conjugated linoleic acid, sphingolipids, and probiotic bacteria. Some studies found an inverted association between melanoma and high intake of dairy products or other dietary sources of vitamin D, while some investigators showed no association.6,18
Fortes et al18 assessed the role of diet on CM and found no protective effects of butter intake against the development of melanoma; however, a protective effect was found for carrots, which are rich in provitamin A (β-carotene) and for the regular intake of herbs rich in polyphenols (eg, rosemary). In our study, we found a protective effect against CM for butter but not for other dairy products. These findings could be explained by the high content of vitamin A in butter in comparison to other dairy products. Habitual intake of oregano also was associated with a protective effect for CM. Oregano is rich in polyphenols such as carvacrol, thymol, and rosmarinic acid, which are known for their antioxidant capacities and the inhibition of cyclooxygenase.19-21 At experimental levels, both carvacrol and thymol have been shown to inhibit the growth of melanoma cells.19,20 Rosmarinic acid, contained by both rosemary and oregano, have been shown at experimental levels to have photoprotective effects against melanoma.21
The relationship between dietary and nutritional intake and CM has a great potential that should be further explored. Tong and Young22 showed that proanthocyanidins found in grape seeds, epigallocatechin-3-gallate, resveratrol, rosmarinic acid, lycopene, and fig latex have demonstrated clear anticancer effects toward melanoma.
The strength of this study is the high response rate of both cases and controls and the use of incidence melanoma cases that decrease recall bias. A limitation of our study is that food portions were based on average portion size for each food item and therefore it can capture habitual consumption but not calculate actual nutrient intake. Misclassification of dietary exposure also could be a problem. Part of this misclassification is a result of a food frequency questionnaire being an imperfect measure of dietary history; however, we evaluated the reproducibility of the food frequency questionnaire used in this case-control study. Overall, there was a fair to good reproducibility between answers in 2 different periods (12 months apart). For example, agreement for frequency of intake of fresh herbs, tomatoes, and butter were 90.8%, 83.1%, and 83.3%, respectively.
Our sample size had sufficient statistical power to detect the effects of diet on CM.
Conclusion
Our study indicates that butter and oregano intake seem to have a protective role against the development of CM. Further studies are needed to confirm these findings.
- Gilchrest B, Eller MS, Geller AC, et al. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341-1347.
- Lotti T, Bruscino N, Hercogova J, et al. Controversial issues on melanoma. Dermatol Ther. 2012;25:458-462.
- Ródenas JM, Delgado-Rodríguez M, Herranz MT, et al. Sun exposure, pigmentary traits, and risk of cutaneous malignant melanoma: a case-control study in a Mediterranean population. Cancer Causes Control. 1996;7:275-283.
- Autier P, Doré JF. Influence of sun exposures during childhood and during adulthood on melanoma risk. EEPIMEL and EORTC. Melanoma Cooperative Group. European Organization for research and treatment of cancer. Int J Cancer. 1998;77:533-537.
- Fortes C, Mastroeni S, Melchi F, et al. The association between residential pesticide use and cutaneous melanoma. Eur J Cancer. 2007;43:1066-1075.
- Jensen JD, Wing GJ, Dellavalle RP. Nutrition and melanoma prevention. Clin Dermatol. 2010;28:644-649.
- Millen AE, Tucker MA, Hartge P, et al. Diet and melanoma in a case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13:1042-1051.
- Feskanich D, Willett WC, Hunter DJ, et al. Dietary intakes of vitamins A, C, and E and risk of melanoma in two cohorts of women. Br J Cancer. 2003;88:1381-1387.
- English DR, Mac Lennan R, Rivers J, et al. Epidemiological studies of melanocytic naevi: protocol for identifying and recording naevi. International Agency for Research on Cancer (IARC) internal report. No. 90/002. Lyon, France: IARC; 1990.
- Cancer preventability statistics. World Cancer Research Fund website. http://www.wcrf-uk.org/uk/preventing-cancer/cancer-preventability-statistics. Accessed May 24, 2016.
- Gandini S, Raimondi S, Gnagnarella P, et al. Vitamin D and skin cancer: a meta-analysis. Eur J Cancer. 2009;45:634-641.
- Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: II. sun exposure. Eur J Cancer. 2005;41:45-60.
- Volkovova K, Bilanicova D, Bartonova A, et al. Associations between environmental factors and incidence of cutaneous melanoma. review. Environ Health. 2012;11(11, suppl 1):S12.
- Asgari MM, Brasky TM, White E. Association of vitamin A and carotenoid intake with melanoma risk in a large prospective cohort. J Invest Dermatol. 2012;132:1573-1582.
- Naldi L, Gallus S, Tavani A, et al. Risk of melanoma and vitamin A, coffee and alcohol: a case-control study from Italy. Eur J Cancer Prev. 2004;13:503-508.
- Le Marchand L, Saltzman BS, Hankin JH, et al. Sun exposure, diet, and melanoma in Hawaii Caucasians. Am J Epidemiol. 2006;164:232-245.
- Kirkpatrick CS, White E, Lee JA. Case-control study of malignant melanoma in Washington State. II. diet, alcohol, and obesity. Am J Epidemiol. 1994;139:869-880.
- Fortes C, Mastroeni S, Melchi F, et al. A protective effect of the Mediterranean diet for cutaneous melanoma. Int J Epidemiol. 2008;37:1018-1029.
- Landa P, Kokoska L, Pribylova M, et al. In vitro anti-inflammatory activity of carvacrol: inhibitory effect on COX-2 catalyzed prostaglandin E(2) biosynthesis. Arch Pharm Res. 2009;32:75-78.
- He L, Mo H, Hadisusilo S, et al. Isoprenoids suppress the growth of murine B16 melanomas in vitro and in vivo. J Nutr. 1997;127:668-674.
- Sánchez-Campillo M, Gabaldon JA, Castillo J, et al. Rosmarinic acid, a photo-protective agent against UV and other ionizing radiations. Food Chem Toxicol. 2009;47:386-392.
- Tong LX, Young LC. Nutrition: the future of melanoma prevention? J Am Acad Dermatol. 2014;71:151-160.
The incidence of cutaneous melanoma (CM) has increased, warranting further study of new risk factors.1,2 Hereditary risk factors for CM include light-colored eyes; fair skin; light brown, blonde, or red hair; tendency to burn; high density of freckles; history of other types of skin cancer; high number of common, atypical, and/or congenital nevi; and family history of skin cancer, as well as risks related to the presence of CDKN2A, BRAF, and MC1R gene mutations. Environmental risk factors include UV exposure from sunlight or tanning beds, among others.3-5
Nutritional factors also have been suggested as possible modifiable risk factors for CM.6 Evidence from epidemiological studies show that diets rich in fruits and vegetables are associated with lower risks for several types of cancer.7,8 A growing number of studies have assessed the effects of diet and the intake of nutrients on the prevention of cancer, specifically the use of dietary supplements to protect the skin from the adverse effects of UV light.6
Preformed vitamin A (ie, retinol) is necessary for the regulation of cell differentiation and also can reduce the incidence of skin tumors in animals exposed to UV light. Certain carotenoids such as α-carotene and β-carotene are metabolized to retinol. These retinol precursors, along with antioxidant nutrients, are important components of fruits and vegetables and may account for the observed anticancer effects of these foods.8
The aim of this study was to assess the relationship between dietary intake and the risk for CM.
Methods
Participants
A case-control study was carried out between 2012 and 2013 at 3 reference centers in Porto Alegre, Brazil—Universidade Federal de Ciências da Saúde de Porto Alegre, Pontifícia Universidade Católica do Rio Grande do Sul, and Hospital de Clínicas de Porto Alegre—for the treatment of patients with CM. Enrolled patients were 18 years and older with a diagnosis of primary CM confirmed by histology. Controls were selected from patients at the same centers, and they were enrolled and matched by institution. Controls were frequency matched to cases by sex and age (+/– 5 years). Exclusion criteria for controls were those presenting with suspicious lesions and those needing radiation therapy or chemotherapy due to other diseases. The study was approved by the ethics committees of the participating centers and informed consent was obtained from all participants. A total of 191 participants (95 cases; 96 controls) were enrolled in the study.
Data Collection
After informed consent was obtained, participants were interviewed and were clinically examined by an experienced dermatologist (C.B.H. and M.M.S.). The questionnaire included sociodemographic variables, medical history, phenotypic characteristics (ie, Fitzpatrick skin type, skin/hair/eye color), family history of skin cancer, history of sunlight exposure, history of sunburns, use of artificial tanning, sunscreen use, and detailed dietary intake. Physical examination included the assessment of several melanocytic lesions (nevi, freckles/ephelides, lentigines, and café au lait spots), actinic keratoses, solar elastosis, and nonmelanocytic tumors following the International Agency for Research on Cancer (IARC) protocol.9
Using a food frequency questionnaire, participants were asked to report their usual frequency of consumption of each food from a list of 36 foods. The frequency of intake of all groups of food and beverages was defined according to the following scale: never, rarely (less than once monthly), once or twice weekly, 3 to 4 times weekly, 5 to 7 times weekly, and more than 7 times weekly. Combination of categories was based on the overall distribution among controls. Therefore, for some items such as mussels and fresh herbs, only 2 categories were used.
Statistical Analysis
A descriptive statistical analysis of the results was performed using SPSS version 20.0 with absolute and relative frequencies for the categorical variables, and mean, SD, and median for the continuous variables. The symmetry of distributions was investigated using the Kolmogorov-Smirnov test.
A t test for independent groups was applied for the continuous variables, while the Pearson χ2 test was used for the categorical variables. The Fisher exact test was used in situations in which at least 25% of the values of the cells presented an expected frequency of less than 5. Monte Carlo simulation was used when at least 1 variable had a polytomic characteristic. Odds ratio (OR) was used to estimate the strength of the association between exposures and outcome. An unconditional binary logistic regression was used to study the association between dietary variables and the risk for CM. To obtain unbiased estimates, multivariate analyses were performed controlling for 1 or more confounding variables. Using low exposure as a base category, the risks and 95% CIs were calculated for the high-exposure categories. Based on the results of bivariate analyses, variables with P≤.25 or lower were included in the models. The likelihood ratio test was used to decide which covariates should be maintained in the model. To test the goodness of fit of the models, the Hosmer-Lemeshow statistic was used.
Potential confounding factors considered in the logistic regression model were sex; age; education level; skin, hair, and eye color; Fitzpatrick skin type; presence of freckles, solar lentigines, and actinic keratosis; history of nonmelanoma skin cancer; number of melanocytic nevi; family history of skin cancer; sunburns in adulthood (≥6 episodes a year); occupational sun exposure; and history of sunscreen use in adulthood.
Results
A total of 191 participants were enrolled in the study (95 [49.7%] cases; 96 [50.3%] controls). Most participants were female (60.0% of cases; 59.4% of controls). The mean age (SD) of cases and controls was 56.8 (13.9) years and 56.5 (13.2) years, respectively. Mean body mass index (SD) did not differ between cases (27.2 [4.6]) and controls (28.2 [6.5]). Education levels of 8 years or less predominated in both groups (64.2% of cases; 57.3% of controls). No statistical difference was found for sex, age, education, or body mass index. The most frequent anatomic sites of CM were the trunk (54.7%) and arms (20.0%), and the most frequent histological type was superficial spreading (62.8%). The median Breslow thickness was 0.90 mm. Ulceration was observed in 20.9% of the cases, and 67% of participants with CM had a high mitotic rate (≥1 mitosis per square millimeter).
Phenotypic characteristics associated with an increased risk for melanoma were light brown hair (OR, 6.73; 95% CI, 3.30-14.2), blonde/red hair (OR, 21.7; 95% CI, 7.51-63.1), light-colored eyes (eg, blue, gray, green)(OR, 13.2; 95% CI, 6.13-28.7), light brown eyes (OR, 5.01; 95% CI, 2.24-11.5), and Fitzpatrick skin types I and II (OR, 7.37; 95% CI, 2.90-26.1). Family history of skin cancer was associated with an increased risk for CM (OR, 4.31; 95% CI, 1.86-10.7) as well as sunburns in adulthood (OR, 1.64; 95% CI, 1.17-1.99). Regular sunscreen use in adulthood had a 5-fold increased risk for CM compared to not using sunscreen regularly (OR, 5.6; 95% CI, 2.85-10.7). Regarding pigmented lesions, the presence of solar lentigines (OR, 4.8; 95% CI, 2.2-11.2), 60 or more nevi (OR, 5.4; 95% CI, 2.4-12.7), and freckles (OR, 3.7; 95% CI, 1.82-7.64) were all associated with an increased risk for CM. Solar elastosis (OR, 2.5; 95% CI, 1.08-5.85), actinic keratosis (OR, 9.1, 95% CI, 3.97-20.84), and occupational exposure to sun (OR, 2.57; 95% CI, 1.23-5.38) also were associated with an increased risk for melanoma.
The intake of most of the foods and beverages included in the study showed no association with CM. High frequency of butter intake (more than daily) was a protective factor for CM (OR, 0.33; 95% CI, 0.16-0.70) compared to low-frequency consumption (daily and less than daily). Consumption of mussels (OR, 0.53; 95% CI, 0.29-0.97) and oregano (OR, 0.28; 95% CI, 0.12-0.66) also were shown to be protective against CM (OR, 0.53; 95% CI, 0.29-0.97). Regarding beverages, those in the highest categories of consumption—liquor (OR, 2.12; 95% CI, 1.09-4.12) and spirits (OR, 2.23; 95% CI, 1.16-4.68)—were associated with an increased risk for CM.
To identify the relationship between CM and the consumption of some foods that were relevant on bivariate analysis, we performed a multivariate model. When adjustments were made, the association remained for butter (OR, 0.141; 95% CI, 0.032-0.613) and oregano (OR, 0.176; 95% CI, 0.042-0.735), while the risk associated with the consumption of both liquor (OR, 1.511; 95% CI, 0.39-5.90) and spirits (OR, 0.755; 95% CI, 0.130-4.393) disappeared (Table).

Comment
Observational studies show that diets rich in fruits and vegetables are associated with a lower risk for different types of cancers.7,8 According to some studies, more than 30% of cancers in adulthood could be prevented or delayed by appropriate dietary intake and physical activity.10 However, there are still limited data on some specific cancers such as CM.
Substantial differences in the incidence of CM among different populations have suggested that environmental factors may play an etiological role in the development of CM and diet could be one of the modifiable risk factors.11-13
Initially, we assessed the already known risk factors for CM, and results showed a significantly increased risk for participants with light brown, blonde, or red hair (P<.0001); light-colored and light brown eyes (P<.0001); Fitzpatrick skin types I and II (P<.0001); positive family history of skin cancer (P=.001); the presence of solar lentigines (P<.001), freckles (P<.001), and actinic keratosis (P<.0001); and high number of nevi (P<.0001). Sunburns in adulthood (P<.001) were associated with an increased risk for CM, and our findings are in agreement with the literature.12
Besides confirming the well-known risk factors for CM, our study also showed that some foods (eg, butter, oregano) may act as important protective factors in CM. It could be argued that the increased risks associated with the well-known risk factors (eg, Fitzpatrick skin type, number of sunburns) might not be as strong and/or could be modulated by dietary factors. To further elucidate this critical issue, we analyzed our data by examining the joint relationship between dietary consumption, individual characteristics, sun exposure, and melanoma. We conducted a multivariable analysis controlling for the well-known risk factors and our findings suggest that both butter and oregano, foods that are rich in vitamins A and D, are independent and protective risk factors for melanoma.
Vitamin A (retinol) is a fat-soluble, organic compound that cannot be synthesized by humans but is necessary for normal physiological function and therefore is classified as an essential nutrient. The main source of vitamin A in the human diet is from retinyl esters, mostly from animal products such as dairy products (eg, butter) as well as from plant-based, provitamin A carotenoids (α-carotene, β-carotene) that can be converted to retinol in the intestines.14
Some case-control studies have investigated the association of vitamin A intake and CM risk, reporting mixed findings. Naldi et al15 found a notable inverse association between vitamin A intake and CM risk. Le Marchand et al16 found no inverse association for carotenoids or retinol. Kirkpatrick et al17 found no evidence of a protective effect for vitamin A or carotenoids on CM. However, the Nurses’ Health Study and the Nurses’ Health Study II reported inverse associations between CM and retinol from foods and dietary supplements.8
Dairy products such as butter contain several components considered to be potentially anticarcinogenic, such as calcium, vitamin D, butyric acid, conjugated linoleic acid, sphingolipids, and probiotic bacteria. Some studies found an inverted association between melanoma and high intake of dairy products or other dietary sources of vitamin D, while some investigators showed no association.6,18
Fortes et al18 assessed the role of diet on CM and found no protective effects of butter intake against the development of melanoma; however, a protective effect was found for carrots, which are rich in provitamin A (β-carotene) and for the regular intake of herbs rich in polyphenols (eg, rosemary). In our study, we found a protective effect against CM for butter but not for other dairy products. These findings could be explained by the high content of vitamin A in butter in comparison to other dairy products. Habitual intake of oregano also was associated with a protective effect for CM. Oregano is rich in polyphenols such as carvacrol, thymol, and rosmarinic acid, which are known for their antioxidant capacities and the inhibition of cyclooxygenase.19-21 At experimental levels, both carvacrol and thymol have been shown to inhibit the growth of melanoma cells.19,20 Rosmarinic acid, contained by both rosemary and oregano, have been shown at experimental levels to have photoprotective effects against melanoma.21
The relationship between dietary and nutritional intake and CM has a great potential that should be further explored. Tong and Young22 showed that proanthocyanidins found in grape seeds, epigallocatechin-3-gallate, resveratrol, rosmarinic acid, lycopene, and fig latex have demonstrated clear anticancer effects toward melanoma.
The strength of this study is the high response rate of both cases and controls and the use of incidence melanoma cases that decrease recall bias. A limitation of our study is that food portions were based on average portion size for each food item and therefore it can capture habitual consumption but not calculate actual nutrient intake. Misclassification of dietary exposure also could be a problem. Part of this misclassification is a result of a food frequency questionnaire being an imperfect measure of dietary history; however, we evaluated the reproducibility of the food frequency questionnaire used in this case-control study. Overall, there was a fair to good reproducibility between answers in 2 different periods (12 months apart). For example, agreement for frequency of intake of fresh herbs, tomatoes, and butter were 90.8%, 83.1%, and 83.3%, respectively.
Our sample size had sufficient statistical power to detect the effects of diet on CM.
Conclusion
Our study indicates that butter and oregano intake seem to have a protective role against the development of CM. Further studies are needed to confirm these findings.
The incidence of cutaneous melanoma (CM) has increased, warranting further study of new risk factors.1,2 Hereditary risk factors for CM include light-colored eyes; fair skin; light brown, blonde, or red hair; tendency to burn; high density of freckles; history of other types of skin cancer; high number of common, atypical, and/or congenital nevi; and family history of skin cancer, as well as risks related to the presence of CDKN2A, BRAF, and MC1R gene mutations. Environmental risk factors include UV exposure from sunlight or tanning beds, among others.3-5
Nutritional factors also have been suggested as possible modifiable risk factors for CM.6 Evidence from epidemiological studies show that diets rich in fruits and vegetables are associated with lower risks for several types of cancer.7,8 A growing number of studies have assessed the effects of diet and the intake of nutrients on the prevention of cancer, specifically the use of dietary supplements to protect the skin from the adverse effects of UV light.6
Preformed vitamin A (ie, retinol) is necessary for the regulation of cell differentiation and also can reduce the incidence of skin tumors in animals exposed to UV light. Certain carotenoids such as α-carotene and β-carotene are metabolized to retinol. These retinol precursors, along with antioxidant nutrients, are important components of fruits and vegetables and may account for the observed anticancer effects of these foods.8
The aim of this study was to assess the relationship between dietary intake and the risk for CM.
Methods
Participants
A case-control study was carried out between 2012 and 2013 at 3 reference centers in Porto Alegre, Brazil—Universidade Federal de Ciências da Saúde de Porto Alegre, Pontifícia Universidade Católica do Rio Grande do Sul, and Hospital de Clínicas de Porto Alegre—for the treatment of patients with CM. Enrolled patients were 18 years and older with a diagnosis of primary CM confirmed by histology. Controls were selected from patients at the same centers, and they were enrolled and matched by institution. Controls were frequency matched to cases by sex and age (+/– 5 years). Exclusion criteria for controls were those presenting with suspicious lesions and those needing radiation therapy or chemotherapy due to other diseases. The study was approved by the ethics committees of the participating centers and informed consent was obtained from all participants. A total of 191 participants (95 cases; 96 controls) were enrolled in the study.
Data Collection
After informed consent was obtained, participants were interviewed and were clinically examined by an experienced dermatologist (C.B.H. and M.M.S.). The questionnaire included sociodemographic variables, medical history, phenotypic characteristics (ie, Fitzpatrick skin type, skin/hair/eye color), family history of skin cancer, history of sunlight exposure, history of sunburns, use of artificial tanning, sunscreen use, and detailed dietary intake. Physical examination included the assessment of several melanocytic lesions (nevi, freckles/ephelides, lentigines, and café au lait spots), actinic keratoses, solar elastosis, and nonmelanocytic tumors following the International Agency for Research on Cancer (IARC) protocol.9
Using a food frequency questionnaire, participants were asked to report their usual frequency of consumption of each food from a list of 36 foods. The frequency of intake of all groups of food and beverages was defined according to the following scale: never, rarely (less than once monthly), once or twice weekly, 3 to 4 times weekly, 5 to 7 times weekly, and more than 7 times weekly. Combination of categories was based on the overall distribution among controls. Therefore, for some items such as mussels and fresh herbs, only 2 categories were used.
Statistical Analysis
A descriptive statistical analysis of the results was performed using SPSS version 20.0 with absolute and relative frequencies for the categorical variables, and mean, SD, and median for the continuous variables. The symmetry of distributions was investigated using the Kolmogorov-Smirnov test.
A t test for independent groups was applied for the continuous variables, while the Pearson χ2 test was used for the categorical variables. The Fisher exact test was used in situations in which at least 25% of the values of the cells presented an expected frequency of less than 5. Monte Carlo simulation was used when at least 1 variable had a polytomic characteristic. Odds ratio (OR) was used to estimate the strength of the association between exposures and outcome. An unconditional binary logistic regression was used to study the association between dietary variables and the risk for CM. To obtain unbiased estimates, multivariate analyses were performed controlling for 1 or more confounding variables. Using low exposure as a base category, the risks and 95% CIs were calculated for the high-exposure categories. Based on the results of bivariate analyses, variables with P≤.25 or lower were included in the models. The likelihood ratio test was used to decide which covariates should be maintained in the model. To test the goodness of fit of the models, the Hosmer-Lemeshow statistic was used.
Potential confounding factors considered in the logistic regression model were sex; age; education level; skin, hair, and eye color; Fitzpatrick skin type; presence of freckles, solar lentigines, and actinic keratosis; history of nonmelanoma skin cancer; number of melanocytic nevi; family history of skin cancer; sunburns in adulthood (≥6 episodes a year); occupational sun exposure; and history of sunscreen use in adulthood.
Results
A total of 191 participants were enrolled in the study (95 [49.7%] cases; 96 [50.3%] controls). Most participants were female (60.0% of cases; 59.4% of controls). The mean age (SD) of cases and controls was 56.8 (13.9) years and 56.5 (13.2) years, respectively. Mean body mass index (SD) did not differ between cases (27.2 [4.6]) and controls (28.2 [6.5]). Education levels of 8 years or less predominated in both groups (64.2% of cases; 57.3% of controls). No statistical difference was found for sex, age, education, or body mass index. The most frequent anatomic sites of CM were the trunk (54.7%) and arms (20.0%), and the most frequent histological type was superficial spreading (62.8%). The median Breslow thickness was 0.90 mm. Ulceration was observed in 20.9% of the cases, and 67% of participants with CM had a high mitotic rate (≥1 mitosis per square millimeter).
Phenotypic characteristics associated with an increased risk for melanoma were light brown hair (OR, 6.73; 95% CI, 3.30-14.2), blonde/red hair (OR, 21.7; 95% CI, 7.51-63.1), light-colored eyes (eg, blue, gray, green)(OR, 13.2; 95% CI, 6.13-28.7), light brown eyes (OR, 5.01; 95% CI, 2.24-11.5), and Fitzpatrick skin types I and II (OR, 7.37; 95% CI, 2.90-26.1). Family history of skin cancer was associated with an increased risk for CM (OR, 4.31; 95% CI, 1.86-10.7) as well as sunburns in adulthood (OR, 1.64; 95% CI, 1.17-1.99). Regular sunscreen use in adulthood had a 5-fold increased risk for CM compared to not using sunscreen regularly (OR, 5.6; 95% CI, 2.85-10.7). Regarding pigmented lesions, the presence of solar lentigines (OR, 4.8; 95% CI, 2.2-11.2), 60 or more nevi (OR, 5.4; 95% CI, 2.4-12.7), and freckles (OR, 3.7; 95% CI, 1.82-7.64) were all associated with an increased risk for CM. Solar elastosis (OR, 2.5; 95% CI, 1.08-5.85), actinic keratosis (OR, 9.1, 95% CI, 3.97-20.84), and occupational exposure to sun (OR, 2.57; 95% CI, 1.23-5.38) also were associated with an increased risk for melanoma.
The intake of most of the foods and beverages included in the study showed no association with CM. High frequency of butter intake (more than daily) was a protective factor for CM (OR, 0.33; 95% CI, 0.16-0.70) compared to low-frequency consumption (daily and less than daily). Consumption of mussels (OR, 0.53; 95% CI, 0.29-0.97) and oregano (OR, 0.28; 95% CI, 0.12-0.66) also were shown to be protective against CM (OR, 0.53; 95% CI, 0.29-0.97). Regarding beverages, those in the highest categories of consumption—liquor (OR, 2.12; 95% CI, 1.09-4.12) and spirits (OR, 2.23; 95% CI, 1.16-4.68)—were associated with an increased risk for CM.
To identify the relationship between CM and the consumption of some foods that were relevant on bivariate analysis, we performed a multivariate model. When adjustments were made, the association remained for butter (OR, 0.141; 95% CI, 0.032-0.613) and oregano (OR, 0.176; 95% CI, 0.042-0.735), while the risk associated with the consumption of both liquor (OR, 1.511; 95% CI, 0.39-5.90) and spirits (OR, 0.755; 95% CI, 0.130-4.393) disappeared (Table).

Comment
Observational studies show that diets rich in fruits and vegetables are associated with a lower risk for different types of cancers.7,8 According to some studies, more than 30% of cancers in adulthood could be prevented or delayed by appropriate dietary intake and physical activity.10 However, there are still limited data on some specific cancers such as CM.
Substantial differences in the incidence of CM among different populations have suggested that environmental factors may play an etiological role in the development of CM and diet could be one of the modifiable risk factors.11-13
Initially, we assessed the already known risk factors for CM, and results showed a significantly increased risk for participants with light brown, blonde, or red hair (P<.0001); light-colored and light brown eyes (P<.0001); Fitzpatrick skin types I and II (P<.0001); positive family history of skin cancer (P=.001); the presence of solar lentigines (P<.001), freckles (P<.001), and actinic keratosis (P<.0001); and high number of nevi (P<.0001). Sunburns in adulthood (P<.001) were associated with an increased risk for CM, and our findings are in agreement with the literature.12
Besides confirming the well-known risk factors for CM, our study also showed that some foods (eg, butter, oregano) may act as important protective factors in CM. It could be argued that the increased risks associated with the well-known risk factors (eg, Fitzpatrick skin type, number of sunburns) might not be as strong and/or could be modulated by dietary factors. To further elucidate this critical issue, we analyzed our data by examining the joint relationship between dietary consumption, individual characteristics, sun exposure, and melanoma. We conducted a multivariable analysis controlling for the well-known risk factors and our findings suggest that both butter and oregano, foods that are rich in vitamins A and D, are independent and protective risk factors for melanoma.
Vitamin A (retinol) is a fat-soluble, organic compound that cannot be synthesized by humans but is necessary for normal physiological function and therefore is classified as an essential nutrient. The main source of vitamin A in the human diet is from retinyl esters, mostly from animal products such as dairy products (eg, butter) as well as from plant-based, provitamin A carotenoids (α-carotene, β-carotene) that can be converted to retinol in the intestines.14
Some case-control studies have investigated the association of vitamin A intake and CM risk, reporting mixed findings. Naldi et al15 found a notable inverse association between vitamin A intake and CM risk. Le Marchand et al16 found no inverse association for carotenoids or retinol. Kirkpatrick et al17 found no evidence of a protective effect for vitamin A or carotenoids on CM. However, the Nurses’ Health Study and the Nurses’ Health Study II reported inverse associations between CM and retinol from foods and dietary supplements.8
Dairy products such as butter contain several components considered to be potentially anticarcinogenic, such as calcium, vitamin D, butyric acid, conjugated linoleic acid, sphingolipids, and probiotic bacteria. Some studies found an inverted association between melanoma and high intake of dairy products or other dietary sources of vitamin D, while some investigators showed no association.6,18
Fortes et al18 assessed the role of diet on CM and found no protective effects of butter intake against the development of melanoma; however, a protective effect was found for carrots, which are rich in provitamin A (β-carotene) and for the regular intake of herbs rich in polyphenols (eg, rosemary). In our study, we found a protective effect against CM for butter but not for other dairy products. These findings could be explained by the high content of vitamin A in butter in comparison to other dairy products. Habitual intake of oregano also was associated with a protective effect for CM. Oregano is rich in polyphenols such as carvacrol, thymol, and rosmarinic acid, which are known for their antioxidant capacities and the inhibition of cyclooxygenase.19-21 At experimental levels, both carvacrol and thymol have been shown to inhibit the growth of melanoma cells.19,20 Rosmarinic acid, contained by both rosemary and oregano, have been shown at experimental levels to have photoprotective effects against melanoma.21
The relationship between dietary and nutritional intake and CM has a great potential that should be further explored. Tong and Young22 showed that proanthocyanidins found in grape seeds, epigallocatechin-3-gallate, resveratrol, rosmarinic acid, lycopene, and fig latex have demonstrated clear anticancer effects toward melanoma.
The strength of this study is the high response rate of both cases and controls and the use of incidence melanoma cases that decrease recall bias. A limitation of our study is that food portions were based on average portion size for each food item and therefore it can capture habitual consumption but not calculate actual nutrient intake. Misclassification of dietary exposure also could be a problem. Part of this misclassification is a result of a food frequency questionnaire being an imperfect measure of dietary history; however, we evaluated the reproducibility of the food frequency questionnaire used in this case-control study. Overall, there was a fair to good reproducibility between answers in 2 different periods (12 months apart). For example, agreement for frequency of intake of fresh herbs, tomatoes, and butter were 90.8%, 83.1%, and 83.3%, respectively.
Our sample size had sufficient statistical power to detect the effects of diet on CM.
Conclusion
Our study indicates that butter and oregano intake seem to have a protective role against the development of CM. Further studies are needed to confirm these findings.
- Gilchrest B, Eller MS, Geller AC, et al. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341-1347.
- Lotti T, Bruscino N, Hercogova J, et al. Controversial issues on melanoma. Dermatol Ther. 2012;25:458-462.
- Ródenas JM, Delgado-Rodríguez M, Herranz MT, et al. Sun exposure, pigmentary traits, and risk of cutaneous malignant melanoma: a case-control study in a Mediterranean population. Cancer Causes Control. 1996;7:275-283.
- Autier P, Doré JF. Influence of sun exposures during childhood and during adulthood on melanoma risk. EEPIMEL and EORTC. Melanoma Cooperative Group. European Organization for research and treatment of cancer. Int J Cancer. 1998;77:533-537.
- Fortes C, Mastroeni S, Melchi F, et al. The association between residential pesticide use and cutaneous melanoma. Eur J Cancer. 2007;43:1066-1075.
- Jensen JD, Wing GJ, Dellavalle RP. Nutrition and melanoma prevention. Clin Dermatol. 2010;28:644-649.
- Millen AE, Tucker MA, Hartge P, et al. Diet and melanoma in a case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13:1042-1051.
- Feskanich D, Willett WC, Hunter DJ, et al. Dietary intakes of vitamins A, C, and E and risk of melanoma in two cohorts of women. Br J Cancer. 2003;88:1381-1387.
- English DR, Mac Lennan R, Rivers J, et al. Epidemiological studies of melanocytic naevi: protocol for identifying and recording naevi. International Agency for Research on Cancer (IARC) internal report. No. 90/002. Lyon, France: IARC; 1990.
- Cancer preventability statistics. World Cancer Research Fund website. http://www.wcrf-uk.org/uk/preventing-cancer/cancer-preventability-statistics. Accessed May 24, 2016.
- Gandini S, Raimondi S, Gnagnarella P, et al. Vitamin D and skin cancer: a meta-analysis. Eur J Cancer. 2009;45:634-641.
- Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: II. sun exposure. Eur J Cancer. 2005;41:45-60.
- Volkovova K, Bilanicova D, Bartonova A, et al. Associations between environmental factors and incidence of cutaneous melanoma. review. Environ Health. 2012;11(11, suppl 1):S12.
- Asgari MM, Brasky TM, White E. Association of vitamin A and carotenoid intake with melanoma risk in a large prospective cohort. J Invest Dermatol. 2012;132:1573-1582.
- Naldi L, Gallus S, Tavani A, et al. Risk of melanoma and vitamin A, coffee and alcohol: a case-control study from Italy. Eur J Cancer Prev. 2004;13:503-508.
- Le Marchand L, Saltzman BS, Hankin JH, et al. Sun exposure, diet, and melanoma in Hawaii Caucasians. Am J Epidemiol. 2006;164:232-245.
- Kirkpatrick CS, White E, Lee JA. Case-control study of malignant melanoma in Washington State. II. diet, alcohol, and obesity. Am J Epidemiol. 1994;139:869-880.
- Fortes C, Mastroeni S, Melchi F, et al. A protective effect of the Mediterranean diet for cutaneous melanoma. Int J Epidemiol. 2008;37:1018-1029.
- Landa P, Kokoska L, Pribylova M, et al. In vitro anti-inflammatory activity of carvacrol: inhibitory effect on COX-2 catalyzed prostaglandin E(2) biosynthesis. Arch Pharm Res. 2009;32:75-78.
- He L, Mo H, Hadisusilo S, et al. Isoprenoids suppress the growth of murine B16 melanomas in vitro and in vivo. J Nutr. 1997;127:668-674.
- Sánchez-Campillo M, Gabaldon JA, Castillo J, et al. Rosmarinic acid, a photo-protective agent against UV and other ionizing radiations. Food Chem Toxicol. 2009;47:386-392.
- Tong LX, Young LC. Nutrition: the future of melanoma prevention? J Am Acad Dermatol. 2014;71:151-160.
- Gilchrest B, Eller MS, Geller AC, et al. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341-1347.
- Lotti T, Bruscino N, Hercogova J, et al. Controversial issues on melanoma. Dermatol Ther. 2012;25:458-462.
- Ródenas JM, Delgado-Rodríguez M, Herranz MT, et al. Sun exposure, pigmentary traits, and risk of cutaneous malignant melanoma: a case-control study in a Mediterranean population. Cancer Causes Control. 1996;7:275-283.
- Autier P, Doré JF. Influence of sun exposures during childhood and during adulthood on melanoma risk. EEPIMEL and EORTC. Melanoma Cooperative Group. European Organization for research and treatment of cancer. Int J Cancer. 1998;77:533-537.
- Fortes C, Mastroeni S, Melchi F, et al. The association between residential pesticide use and cutaneous melanoma. Eur J Cancer. 2007;43:1066-1075.
- Jensen JD, Wing GJ, Dellavalle RP. Nutrition and melanoma prevention. Clin Dermatol. 2010;28:644-649.
- Millen AE, Tucker MA, Hartge P, et al. Diet and melanoma in a case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13:1042-1051.
- Feskanich D, Willett WC, Hunter DJ, et al. Dietary intakes of vitamins A, C, and E and risk of melanoma in two cohorts of women. Br J Cancer. 2003;88:1381-1387.
- English DR, Mac Lennan R, Rivers J, et al. Epidemiological studies of melanocytic naevi: protocol for identifying and recording naevi. International Agency for Research on Cancer (IARC) internal report. No. 90/002. Lyon, France: IARC; 1990.
- Cancer preventability statistics. World Cancer Research Fund website. http://www.wcrf-uk.org/uk/preventing-cancer/cancer-preventability-statistics. Accessed May 24, 2016.
- Gandini S, Raimondi S, Gnagnarella P, et al. Vitamin D and skin cancer: a meta-analysis. Eur J Cancer. 2009;45:634-641.
- Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: II. sun exposure. Eur J Cancer. 2005;41:45-60.
- Volkovova K, Bilanicova D, Bartonova A, et al. Associations between environmental factors and incidence of cutaneous melanoma. review. Environ Health. 2012;11(11, suppl 1):S12.
- Asgari MM, Brasky TM, White E. Association of vitamin A and carotenoid intake with melanoma risk in a large prospective cohort. J Invest Dermatol. 2012;132:1573-1582.
- Naldi L, Gallus S, Tavani A, et al. Risk of melanoma and vitamin A, coffee and alcohol: a case-control study from Italy. Eur J Cancer Prev. 2004;13:503-508.
- Le Marchand L, Saltzman BS, Hankin JH, et al. Sun exposure, diet, and melanoma in Hawaii Caucasians. Am J Epidemiol. 2006;164:232-245.
- Kirkpatrick CS, White E, Lee JA. Case-control study of malignant melanoma in Washington State. II. diet, alcohol, and obesity. Am J Epidemiol. 1994;139:869-880.
- Fortes C, Mastroeni S, Melchi F, et al. A protective effect of the Mediterranean diet for cutaneous melanoma. Int J Epidemiol. 2008;37:1018-1029.
- Landa P, Kokoska L, Pribylova M, et al. In vitro anti-inflammatory activity of carvacrol: inhibitory effect on COX-2 catalyzed prostaglandin E(2) biosynthesis. Arch Pharm Res. 2009;32:75-78.
- He L, Mo H, Hadisusilo S, et al. Isoprenoids suppress the growth of murine B16 melanomas in vitro and in vivo. J Nutr. 1997;127:668-674.
- Sánchez-Campillo M, Gabaldon JA, Castillo J, et al. Rosmarinic acid, a photo-protective agent against UV and other ionizing radiations. Food Chem Toxicol. 2009;47:386-392.
- Tong LX, Young LC. Nutrition: the future of melanoma prevention? J Am Acad Dermatol. 2014;71:151-160.
Practice Points
- Hereditary and environmental risk factors have been identified for cutaneous melanoma (CM). Nutritional factors have been suggested as possible modifiable risk factors.
- Foods rich in vitamins A and D may be protective risk factors for CM.
E-cigarettes: Who’s using them and why?
ABSTRACT
Background Electronic cigarettes (e-cigarettes) are often marketed as safe and effective aids for quitting cigarette smoking, but concerns remain that use of e-cigarettes might actually reduce the number of quit attempts. To address these issues, we characterized the utilization and demographic correlates of dual use of e-cigarettes and traditional cigarettes (referred to here as simply “cigarettes”) among smokers in a rural population of Illinois.
Methods The majority of survey participants were recruited from the 2014 Illinois State Fair and from another event—the Springfield Mile (a motorcycle racing event)—in Springfield, Ill. Survey questions explored participant demographics and cigarette and e-cigarette use history.
Results Of 201 total cigarette smokers, 79 smoked only tobacco cigarettes (smokers), while 122 also used e-cigarettes (dual users). Dual users did not differ significantly from smokers in gender, age, income, or education. Compared to smokers, dual users were more likely to smoke within 30 minutes of awakening (odds ratio [OR]=3.3; 95% confidence interval [CI], 1.8-6.3), but did not smoke more cigarettes per day or perceive a greater likelihood of quit success. Non-white dual users smoked fewer cigarettes per day than smokers. In addition, 79.5% of all dual users reported that they were using e-cigarettes to quit smoking or reduce the number of cigarettes smoked, and white respondents were 6 times more likely than non-whites to use e-cigarettes for ‘trying to quit smoking’ (OR=6.0; 95% CI, 1.1-32.9). Males and respondents with lower income were less likely to say they were using e-cigarettes to reduce the number of cigarettes smoked than females or participants with higher income (OR=0.2; 95% CI, 0.1-0.8 and OR=0.1; 95% CI, 0.0-0.5, respectively).
Conclusions E-cigarettes may significantly alter the landscape of nicotine physical dependence, and local influences likely are associated with use patterns. Future research should continue to examine whether dual use of traditional and electronic cigarettes impacts smoking cessation, and clinicians should be aware that local norms may create differences from national level data.
Approximately 21% of US adults use tobacco products at least occasionally.1 Although smoking prevalence has declined in recent years (from 21% in 2005 to 18% in 2013), it remains high among certain groups (eg, males and those with a high school education or less).2 As we know, the health burden of smoking—as a cause of death from cancer, pulmonary disease, and heart disease—is substantial,3,4 and rural areas experience a significantly higher prevalence of smoking compared to urban areas.2,5,6
However, it is unknown if the context and habits surrounding tobacco use in rural and/or Midwestern areas are similar to those of urban or nationally-representative populations. For example, while many urban residents may encounter a multitude of media messages encouraging smoking cessation resulting in less community acceptance of smoking, rural residents may be exposed to substantially fewer messages (eg, no city bus signs, billboards, subway posters, etc.) and the community may be more accommodating and tolerant of smoking.
Do e-cigarettes increase cigarette smoking?
Public health professionals are concerned about the increased use of e-cigarettes, particularly among young people, and whether this use increases the likelihood that individuals will start smoking tobacco cigarettes.7(Throughout this paper, we will use “cigarettes” and “smoking” to refer to the use of traditional tobacco cigarettes.) A recent study found that adolescents who used electronic nicotine delivery systems were twice as likely as non-users to have tried cigarettes in the past year.8
An onslaught of advertising. There are also concerns that e-cigarettes may serve to ‘renormalize’ nicotine addiction, in part through large-scale advertising, which was seen by nearly 70% of the participants in the 2014 National Youth Tobacco Survey.9 Largely as a result of that advertising, e-cigarette sales exceed $1.7 billion in the United States alone.10 With 15% of all US adults having ever tried electronic nicotine delivery systems and more than half (52%) of smokers having done so, questions regarding their health impact cannot be taken lightly.11
Do e-cigarettes help people quit smoking? E-cigarettes are often marketed as a safe and effective means for quitting cigarette smoking.12-14 (See "E-cigarettes: How "safe" are they?") Nearly two-thirds of physicians report being asked about e-cigarettes by their patients and approximately one-third of physicians recommend using them as a smoking cessation aid.15
Claims regarding the usefulness of e-cigarettes in smoking cessation, however, have not been substantiated by high-quality randomized controlled trials (RCTs). In fact, no RCTs have shown them to be safer or more effective than cessation treatments currently approved by the US Food and Drug Administration.16,17
Two studies reflect the conflicting data that are currently available. One small study found intensive e-cigarette users were 6 times more likely than non-users/triers to report successful smoking cessation.18 However, researchers surveying callers of a cigarette quit line found that smokers who used e-cigarettes (dual users) were less likely to quit smoking than non-users.19
The lack of good-quality data substantiates the concern that dual use might discourage quitting by normalizing cigarette use and reducing perceptions of harm.20,21 Dual use may also hamper smoking cessation efforts by increasing nicotine physical dependence and associated withdrawal symptoms when trying to quit.22 And finally, dual use may expose users to more carcinogens and toxins than those who use only one product, and the average number of cigarettes smoked per day may be significantly higher among dual users.23
Unique demographic factors at work? Finally, the social and community context within which smoking occurs, and the prevalence of smoking-associated demographic risk factors, may vary significantly between rural and urban areas and between seemingly similar rural areas.24-27 Few studies have examined differences in e-cigarette use between rural and urban areas. Those that have are contradictory, reporting that rural residents use e-cigarettes both more and less than their urban peers,28,29 but many of these studies were conducted outside the United States, where the context and norms associated with smoking and e-cigarette use likely vary.
For these reasons, we sought to examine e-cigarette use among residents of Illinois, the nation’s fifth largest state and one with a rural population exceeding 1.5 million.30 We compared dual users of e-cigarettes and cigarettes to smokers of cigarettes only in terms of demographic characteristics, nicotine physical dependence, and smoking cessation beliefs, and explored dual smokers' reasons for using both types of cigarettes.
MATERIALS AND METHODS
A survey was fielded during August and September 2014 in Springfield, Ill. To obtain responses, a booth was set up at both the Illinois State Fair and the Springfield Mile (a motorcycle racing event), and participants were recruited via direct solicitation by project staff. This was supplemented by an email invitation to all employees of the Southern Illinois University School of Medicine. The 2 venues and the email strategy were chosen because they draw from a large area of central and southern Illinois and were convenient to the location of the study team. Individuals were eligible to participate if they were ≥18 years of age and used any tobacco product or e-cigarettes. Survey elements were derived from 2 national surveys of health and behavior—the Minnesota Adult Tobacco Survey 201031 and the Brief Smoking Consequences Questionnaire-Adult.32
Survey questions assessed cigarette use, nicotine physical dependence, social norms, perceived risks and benefits, and smoking cessation beliefs and behaviors. Questions were slightly reworded to address not only the use of traditional cigarettes, but the use of e-cigarettes, as well. Ultimately, each participant answered a similarly-worded set of questions for both regular and e-cigarettes. Dual use of cigarettes and e-cigarettes was also assessed. Participants self-reported all data and survey responses on an electronic tablet and received a $10 (cash or gift card) incentive. This project was reviewed and approved by the Springfield Committee for Research Involving Human Subjects.
Stratification of results. Race was dichotomized into white and non-white. Education was stratified into 3 categories: up to and including high school graduation, some college but not a Bachelor’s degree, and Bachelor’s degree and above. Income was divided as being ≤$20,000 or >$20,000, and age was split into 2 groups by the median value. Analyses included descriptions of participant demographics, dual use status, measures of nicotine physical dependence, quit attempts, and e-cigarette use motivations. Bivariate relationships between dual use status and demographic characteristics, nicotine physical dependence, and smoking cessation beliefs were analyzed by chi-square (categorical variables) and ANOVA (continuous/Likert variables).
Multivariable logistic regression modeling of the demographic variables and dual use status (cigarette smoker only vs dual user) was performed to predict 3 factors: number of cigarettes smoked per day (≤10 vs 11+); time to first cigarette (≤30 vs 31+ minutes from waking); and perceived likelihood of quit attempt success (very/somewhat likely vs very/somewhat unlikely). Multivariable models examining the reasons for dual use included the demographic, nicotine physical dependence, and cessation belief items described previously.
RESULTS
Of 309 total survey participants (Fair=288; Race=12; Email=9), there were 235 current cigarette smokers consisting of 79 who smoked only cigarettes (smokers); 122 who used both cigarettes and e-cigarettes (dual users); and 34 former e-cigarette users. Only smokers and dual users were included in this analysis (N=201, although for the purposes of TABLE 1, N=200 or 199 because at least one participant did not provide answers to all of the questions). Approximately 51% of the smokers were male, 78% were white, 12% were 4-year college graduates, and 57% reported incomes >$20,000. The mean age was 37.7 years (SD=14.4); 50% of respondents were <35 years of age. Dual users did not vary significantly from smokers in terms of gender, age, education, or income (all P>.05). However, a greater proportion of whites vs non-whites were dual users (54.9% vs 42.3%; P=.035).
Click here to see an enlarged version of the table.
No big quit differences. Bivariate analyses revealed that dual users were no more likely than smokers to have attempted to quit smoking within the past year (X2=2.3; P=.14), consider quitting in the next one or 6 months (X2=1.1; P=.34), or differ in perceived likelihood of cessation success (X2=0.0; P=1.00). The proportion of dual users who smoked 11+ cigarettes per day did not differ from that of cigarette smokers for the group as a whole or when the group was stratified by gender, income, education, or age. However, among non-whites, dual users smoked fewer cigarettes than cigarette smokers (TABLE 1).
Predicting physical dependence. Significant differences also were observed regarding the timing of the first cigarette of the day, with dual users approximately 3 times more likely than smokers to smoke within 30 minutes of awakening (80% vs 54.4%; OR=3.3; 95% CI, 1.8-6.3), and this difference was upheld among males, females, whites, those with an income >$20,000, those with a high school education or less and those with some college education, and age >34 years. There was no association, however, between dual use and perceived likelihood of quit success.
We then performed multivariable logistic modeling on dual users to determine which variables might predict 3 measures of physical dependence: number of cigarettes smoked per day (≤10 vs 11+), time between waking and smoking the first cigarette of the day (≤30 vs 31+ minutes), and perceived likelihood of cessation success (TABLE 2). Male gender (OR=3.4; 95% CI, 1.8-6.5) and white race (OR=4.4; 95% CI, 1.9-10.1) were significant for predicting smoking 11+ cigarettes a day, while dual use status was insignificant (P=.104). Regarding time to first cigarette, only dual use was significant (OR=3.1; 95% CI, 1.6-5.9), with dual users approximately 3 times more likely than smokers to have their first cigarette within 30 minutes of waking. No variables were significant in predicting perceived likelihood of quit success.
Reasons for dual use. We examined reasons for dual use with the question: Do you use e-cigarettes to reduce your regular tobacco use? Here, 79.5% of smokers reported using e-cigarettes to quit smoking or reduce the number of cigarettes smoked.
A multivariable polynomial logistic regression that included only dual users was performed to examine which variables might predict use for tobacco cessation (“trying to quit smoking”) vs reduction in smoking intensity (“trying to reduce the number of regular cigarettes I smoke per day”) vs no change (“use the same amount of tobacco as always”) (TABLE 2). Whites were approximately 6 times more likely than non-whites to indicate they engage in dual use to try to quit smoking (OR=6.0; 95% CI, 1.1-32.9). Males and people with lower incomes were much less likely to indicate they engaged in dual use to try to reduce the number of regular cigarettes smoked than females or those with higher incomes (OR=0.2; 95% CI, 0.1-0.8 and OR=0.1, 95% CI, 0.0-0.5, respectively). No other demographic variables or measures of nicotine physical dependence were significantly different between dual users and smokers.
Click here to see an enlarged version of the table.
DISCUSSION
E-cigarettes are used by approximately half of smokers (52%), which is much higher than that reported by Delnevo, et al, in their analysis of the National Health Interview Study.33 There, prevalence of dual use of both cigarettes and e-cigarettes ranged from 3.4% to 12.7%. This substantial difference raises important questions regarding study population characterization. Were participants in our study representative of central Illinois, state fair attendees, or the agricultural profession? Further work to identify this group with an increased propensity for dual use will assist clinicians in developing appropriate intervention strategies.
Dual use in our study did not vary by many customary demographic variables. Nor was it associated with different rates of past or future quit attempts or perceived ability to successfully quit if quitting was attempted. These factors—high rates of dual use and insignificant effect on quit attempts—may have implications for local physicians counseling patients who smoke.
In our study, the majority of smokers already use e-cigarettes, and this does not seem to increase their ability/likelihood to quit smoking. Further, dual use did not seem to be associated with overall cigarette consumption; males and white participants smoked more cigarettes than females and non-whites. But dual use was associated with a measure of increased nicotine physical dependence (earlier first cigarette of the day). As a result, physicians may want to think twice before recommending e-cigarette use as a means of smoking cessation.
In addition to the high prevalence of e-cigarette use among smokers, a number of other interesting findings surfaced that run counter to some of the current literature. First, dual users are no more likely than smokers to have tried to quit in the past or to try to quit in the future.21,22,34 It could be that for the relatively small geographical area from which our participants were recruited (central Illinois; ~77% of participants from Sangamon County alone), the local context and culture of smoking differs from that associated with participants in other studies, who were mostly recruited from national and regional online surveys. However, there is no a priori reason to suspect Sangamon County is especially different, as it is quite similar to Illinois as a whole by many measures (eg, percentage rural: 14.1% vs 11.5%; percentage black (only): 12.4% vs 14.7%; education to at least a Bachelor’s degree: 33.0% vs 31.9%; and median household income: $55,565 vs $57,166).30
While we found that dual users did have one measure of increased nicotine physical dependence, the total number of cigarettes consumed per day was not significantly different from that of smokers.23-25 This is contrary to another study of nicotine physical dependence, but, unlike that study, we did not assess length of time of concurrent use.35 There is much uncertainty surrounding the issue of nicotine physical dependence and e-cigarette use, largely because the level of nicotine delivered by various e-products varies significantly.36
Cross-sectional nature, small sample size limit utility of data
There are significant limitations to this study, including the cross-sectional nature of the data, the small sample size, the use of self-report, and the limited scope of recruitment. The relatively small sample size limits our ability to observe small differences and effect sizes. However, small differences often lack practical significance. Finally, participation was limited to those attending a state fair or a local sporting event and those employed by a local medical school. Thus, the results may not be generalizable to populations outside central Illinois. On the other hand, the very low income sample recruited from the Midwestern US, which is underrepresented in prior e-cigarette research, might represent some of the strengths of this work.
Future investigations. Future studies should more closely examine e-cigarette use prevalence on smaller geographic scales and especially in rural areas where there is a paucity of research. As the majority of our respondents came from a single county in central Illinois, one has to ask the questions, “Is this a ‘hot spot’ for e-cigarette use?" And "Do other rural areas experience similar use?” It may be important to know if national surveys are sensitive enough to observe significant local variations. Research also should examine how e-cigarette use and the influence of local culture vary across wider areas.
Several specific areas of study would help to inform policy and intervention development. For example, is tobacco cigarette quit success impacted by concurrent e-cigarette use? While our study showed no difference in past or possible future quit attempts among dual users as compared with smokers, we did not assess actual quit success, and multiple participants in our study anecdotally described using e-cigarettes to successfully quit smoking.
In the end, the rapid increase in the use of e-cigarettes has the potential to significantly alter the landscape of nicotine physical dependence, and local culture and other influences are likely associated with use patterns.
CORRESPONDENCE
Wiley D. Jenkins, PhD, MPH, Science Director, Population Health Science Program, Southern Illinois University School of Medicine, 201 E. Madison St., Springfield, IL 62794-9664; wjenkins@siumed.edu.
1. Agaku IT, King BA, Husten CG, et al; Centers for Disease Control and Prevention (CDC). Tobacco product use among adults—United States, 2012-2013. MMWR Morb Mortal Wkly Rep. 2014;63:542-547.
2. Jamal A, Agaku IT, O’Connor E, et al. Current cigarette smoking among adults—United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2014;63:1108-1112.
3. Siegel RL, Jacobs EJ, Newton CC, et al. Deaths due to cigarette smoking for 12 smoking-related cancers in the United States. JAMA Intern Med. 2015;175:1574-1576.
4. US Department of Health and Human Services. Surgeon General. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. Available at: http://www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html. Accessed January 22, 2014.
5. Gamm LD, Hutchison LL, Dabney BJ, et al, eds. (2003). Rural Healthy People 2010: A companion document to Healthy People 2010. Volume 2. College Station, TX: The Texas A&M University System Health Science Center, School of Rural Public Health, Southwest Rural Health Research Center.
6. Doescher MP, Jackson JE, Jerant A, et al. Prevalence and trends in smoking: a national rural study. J Rural Health. 2006;22:112-118.
7. Bunnell RE, Agaku IT, Arrazola RA, et al. Intentions to smoke cigarettes among never-smoking US middle and high school electronic cigarette users: National Youth Tobacco Survey, 2011-2013. Nicotine Tob Res. 2015;17:228-235.
8. Cardenas VM, Evans VL, Balamurugan A, et al. Use of electronic nicotine delivery systems and recent initiation of smoking among US youth. Int J Public Health. 2016;61:237-241.
9. Auf R, Trepka MJ, Cano MA, et al. Electronic cigarettes: the renormalisation of nicotine use. BMJ. 2016;352:i425.
10. CNBC. E-cigarette sales are smoking hot, set to hit $1.7 billion. Available at: http://www.cnbc.com/id/100991511. Accessed April 5, 2016.
11. Weaver SR, Majeed BA, Pechacek TF, et al. Use of electronic nicotine delivery systems and other tobacco products among USA adults, 2014: results from a national survey. Int J Public Health. 2016;61:177-188.
12. Richardson A, Ganz O, Vallone D. Tobacco on the web: surveillance and characterisation of online tobacco and e-cigarette advertising. Tob Control. 2015;24:341-347.
13. Paek HJ, Kim S, Hove T, et al. Reduced harm or another gateway to smoking? source, message, and information characteristics of E-cigarette videos on YouTube. J Health Commun. 2014;19:545-560.
14. Kim AE, Arnold KY, Makarenko O. E-cigarette advertising expenditures in the U.S., 2011-2012. Am J Prev Med. 2014;46:409-412.
15. Steinberg MB, Giovenco DP, Delnevo CD. Patient-physician communication regarding electronic cigarettes. Prev Med Rep. 2015;2:96-98.
16. Gualano MR, Passi S, Bert F, et al. Electronic cigarettes: assessing the efficacy and the adverse effects through a systematic review of published studies. J Public Health (Oxf). 2015:37:488-497.
17. U.S. National Institutes of Health. ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/results?term=%22electronic+cigarette%22&Search=Search. Accessed July 10, 2015.
18. Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2015;17:127-133.
19. Vickerman KA, Carpenter KM, Altman T, et al. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787-1791.
20. Center for Disease Control and Prevention. Press Release February 28,2013. Available at: http://www.cdc.gov/media/releases/2013/p0228_electronic_cigarettes.html. Accessed July 8, 2015.
21. Pisinger C. Why public health people are more worried than excited over e-cigarettes. BMC Med. 2014;12:226.
22. Post A, Gilljam H, Rosendahl I, et al. Symptoms of nicotine dependence in a cohort of Swedish youths: a comparison between smokers, smokeless tobacco users and dual tobacco users. Addiction. 2010;105:740-746.
23. Mazurek JM, Syamlal G, King BA, et al; Division of Respiratory Disease Studies, National Institute for Occupational Safety and Health, CDC. Smokeless tobacco use among working adults—United States, 2005 and 2010. MMWR Morb Mortal Wkly Rep. 2014;63:477-482.
24. Hutcheson TD, Greiner KA, Ellerbeck EF, et al. Understanding smoking cessation in rural communities. J Rural Health. 2008;24:116-124.
25. McMillen R, Breen J, Cosby AG. Rural-urban differences in the social climate surrounding environmental tobacco smoke: a report from the 2002 Social Climate Survey of Tobacco Control. J Rural Health. 2004;20:7-16.
26. Butler KM, Rayens MK, Adkins S, et al. Culturally-specific smoking cessation outreach in a rural community. Public Health Nurs. 2014;31:44-54.
27. Butler KM, Hedgecock S, Record RA, et al. An evidence-based cessation strategy using rural smokers’ experiences with tobacco. Nurs Clin North Am. 2012;47:31-43.
28. Hamilton HA, Ferrence R, Boak A, et al. Ever use of nicotine and nonnicotine electronic cigarettes among high school students in Ontario, Canada. Nicotine Tob Res. 2015;17:1212-1218.
29. Goniewicz ML, Zielinska-Danch W. Electronic cigarette use among teenagers and young adults in Poland. Pediatrics. 2012;130:e879-e885.
30. US Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Available at: http://www.census.gov/geo/reference/ua/urban-rural-2010.html. Accessed March 13, 2016.
31. Minnesota Adult Tobacco Survey. Tobacco use in Minnesota: 1999-2014. Available at: http://www.mnadulttobaccosurvey.org/. Accessed April 27, 2016.
32. Rash CJ, Copeland AL. The Brief Smoking Consequences Questionnaire-Adult (BSCQ-A): development of a short form of the SCQ-A. Nicotine Tob Res. 2008;10:1633-1643.
33. Delnevo CD, Giovenco DP, Steinberg MB, et al. Patterns of electronic cigarette use among adults in the United States. Nicotine Tob Res. 2016;18:715-719.
34. Lee YO, Hebert CJ, Nonnemaker JM, et al. Multiple tobacco product use among adults in the United States: cigarettes, cigars, electronic cigarettes, hookah, smokeless tobacco, and snus. Prev Med. 2014;62:14-19.
35. Etter JF, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015;147:68-75.
36. Cobb CO, Hendricks PS, Eissenberg T. Electronic cigarettes and nicotine dependence: evolving products, evolving problems. BMC Med. 2015;13:119.
ABSTRACT
Background Electronic cigarettes (e-cigarettes) are often marketed as safe and effective aids for quitting cigarette smoking, but concerns remain that use of e-cigarettes might actually reduce the number of quit attempts. To address these issues, we characterized the utilization and demographic correlates of dual use of e-cigarettes and traditional cigarettes (referred to here as simply “cigarettes”) among smokers in a rural population of Illinois.
Methods The majority of survey participants were recruited from the 2014 Illinois State Fair and from another event—the Springfield Mile (a motorcycle racing event)—in Springfield, Ill. Survey questions explored participant demographics and cigarette and e-cigarette use history.
Results Of 201 total cigarette smokers, 79 smoked only tobacco cigarettes (smokers), while 122 also used e-cigarettes (dual users). Dual users did not differ significantly from smokers in gender, age, income, or education. Compared to smokers, dual users were more likely to smoke within 30 minutes of awakening (odds ratio [OR]=3.3; 95% confidence interval [CI], 1.8-6.3), but did not smoke more cigarettes per day or perceive a greater likelihood of quit success. Non-white dual users smoked fewer cigarettes per day than smokers. In addition, 79.5% of all dual users reported that they were using e-cigarettes to quit smoking or reduce the number of cigarettes smoked, and white respondents were 6 times more likely than non-whites to use e-cigarettes for ‘trying to quit smoking’ (OR=6.0; 95% CI, 1.1-32.9). Males and respondents with lower income were less likely to say they were using e-cigarettes to reduce the number of cigarettes smoked than females or participants with higher income (OR=0.2; 95% CI, 0.1-0.8 and OR=0.1; 95% CI, 0.0-0.5, respectively).
Conclusions E-cigarettes may significantly alter the landscape of nicotine physical dependence, and local influences likely are associated with use patterns. Future research should continue to examine whether dual use of traditional and electronic cigarettes impacts smoking cessation, and clinicians should be aware that local norms may create differences from national level data.
Approximately 21% of US adults use tobacco products at least occasionally.1 Although smoking prevalence has declined in recent years (from 21% in 2005 to 18% in 2013), it remains high among certain groups (eg, males and those with a high school education or less).2 As we know, the health burden of smoking—as a cause of death from cancer, pulmonary disease, and heart disease—is substantial,3,4 and rural areas experience a significantly higher prevalence of smoking compared to urban areas.2,5,6
However, it is unknown if the context and habits surrounding tobacco use in rural and/or Midwestern areas are similar to those of urban or nationally-representative populations. For example, while many urban residents may encounter a multitude of media messages encouraging smoking cessation resulting in less community acceptance of smoking, rural residents may be exposed to substantially fewer messages (eg, no city bus signs, billboards, subway posters, etc.) and the community may be more accommodating and tolerant of smoking.
Do e-cigarettes increase cigarette smoking?
Public health professionals are concerned about the increased use of e-cigarettes, particularly among young people, and whether this use increases the likelihood that individuals will start smoking tobacco cigarettes.7(Throughout this paper, we will use “cigarettes” and “smoking” to refer to the use of traditional tobacco cigarettes.) A recent study found that adolescents who used electronic nicotine delivery systems were twice as likely as non-users to have tried cigarettes in the past year.8
An onslaught of advertising. There are also concerns that e-cigarettes may serve to ‘renormalize’ nicotine addiction, in part through large-scale advertising, which was seen by nearly 70% of the participants in the 2014 National Youth Tobacco Survey.9 Largely as a result of that advertising, e-cigarette sales exceed $1.7 billion in the United States alone.10 With 15% of all US adults having ever tried electronic nicotine delivery systems and more than half (52%) of smokers having done so, questions regarding their health impact cannot be taken lightly.11
Do e-cigarettes help people quit smoking? E-cigarettes are often marketed as a safe and effective means for quitting cigarette smoking.12-14 (See "E-cigarettes: How "safe" are they?") Nearly two-thirds of physicians report being asked about e-cigarettes by their patients and approximately one-third of physicians recommend using them as a smoking cessation aid.15
Claims regarding the usefulness of e-cigarettes in smoking cessation, however, have not been substantiated by high-quality randomized controlled trials (RCTs). In fact, no RCTs have shown them to be safer or more effective than cessation treatments currently approved by the US Food and Drug Administration.16,17
Two studies reflect the conflicting data that are currently available. One small study found intensive e-cigarette users were 6 times more likely than non-users/triers to report successful smoking cessation.18 However, researchers surveying callers of a cigarette quit line found that smokers who used e-cigarettes (dual users) were less likely to quit smoking than non-users.19
The lack of good-quality data substantiates the concern that dual use might discourage quitting by normalizing cigarette use and reducing perceptions of harm.20,21 Dual use may also hamper smoking cessation efforts by increasing nicotine physical dependence and associated withdrawal symptoms when trying to quit.22 And finally, dual use may expose users to more carcinogens and toxins than those who use only one product, and the average number of cigarettes smoked per day may be significantly higher among dual users.23
Unique demographic factors at work? Finally, the social and community context within which smoking occurs, and the prevalence of smoking-associated demographic risk factors, may vary significantly between rural and urban areas and between seemingly similar rural areas.24-27 Few studies have examined differences in e-cigarette use between rural and urban areas. Those that have are contradictory, reporting that rural residents use e-cigarettes both more and less than their urban peers,28,29 but many of these studies were conducted outside the United States, where the context and norms associated with smoking and e-cigarette use likely vary.
For these reasons, we sought to examine e-cigarette use among residents of Illinois, the nation’s fifth largest state and one with a rural population exceeding 1.5 million.30 We compared dual users of e-cigarettes and cigarettes to smokers of cigarettes only in terms of demographic characteristics, nicotine physical dependence, and smoking cessation beliefs, and explored dual smokers' reasons for using both types of cigarettes.
MATERIALS AND METHODS
A survey was fielded during August and September 2014 in Springfield, Ill. To obtain responses, a booth was set up at both the Illinois State Fair and the Springfield Mile (a motorcycle racing event), and participants were recruited via direct solicitation by project staff. This was supplemented by an email invitation to all employees of the Southern Illinois University School of Medicine. The 2 venues and the email strategy were chosen because they draw from a large area of central and southern Illinois and were convenient to the location of the study team. Individuals were eligible to participate if they were ≥18 years of age and used any tobacco product or e-cigarettes. Survey elements were derived from 2 national surveys of health and behavior—the Minnesota Adult Tobacco Survey 201031 and the Brief Smoking Consequences Questionnaire-Adult.32
Survey questions assessed cigarette use, nicotine physical dependence, social norms, perceived risks and benefits, and smoking cessation beliefs and behaviors. Questions were slightly reworded to address not only the use of traditional cigarettes, but the use of e-cigarettes, as well. Ultimately, each participant answered a similarly-worded set of questions for both regular and e-cigarettes. Dual use of cigarettes and e-cigarettes was also assessed. Participants self-reported all data and survey responses on an electronic tablet and received a $10 (cash or gift card) incentive. This project was reviewed and approved by the Springfield Committee for Research Involving Human Subjects.
Stratification of results. Race was dichotomized into white and non-white. Education was stratified into 3 categories: up to and including high school graduation, some college but not a Bachelor’s degree, and Bachelor’s degree and above. Income was divided as being ≤$20,000 or >$20,000, and age was split into 2 groups by the median value. Analyses included descriptions of participant demographics, dual use status, measures of nicotine physical dependence, quit attempts, and e-cigarette use motivations. Bivariate relationships between dual use status and demographic characteristics, nicotine physical dependence, and smoking cessation beliefs were analyzed by chi-square (categorical variables) and ANOVA (continuous/Likert variables).
Multivariable logistic regression modeling of the demographic variables and dual use status (cigarette smoker only vs dual user) was performed to predict 3 factors: number of cigarettes smoked per day (≤10 vs 11+); time to first cigarette (≤30 vs 31+ minutes from waking); and perceived likelihood of quit attempt success (very/somewhat likely vs very/somewhat unlikely). Multivariable models examining the reasons for dual use included the demographic, nicotine physical dependence, and cessation belief items described previously.
RESULTS
Of 309 total survey participants (Fair=288; Race=12; Email=9), there were 235 current cigarette smokers consisting of 79 who smoked only cigarettes (smokers); 122 who used both cigarettes and e-cigarettes (dual users); and 34 former e-cigarette users. Only smokers and dual users were included in this analysis (N=201, although for the purposes of TABLE 1, N=200 or 199 because at least one participant did not provide answers to all of the questions). Approximately 51% of the smokers were male, 78% were white, 12% were 4-year college graduates, and 57% reported incomes >$20,000. The mean age was 37.7 years (SD=14.4); 50% of respondents were <35 years of age. Dual users did not vary significantly from smokers in terms of gender, age, education, or income (all P>.05). However, a greater proportion of whites vs non-whites were dual users (54.9% vs 42.3%; P=.035).
Click here to see an enlarged version of the table.
No big quit differences. Bivariate analyses revealed that dual users were no more likely than smokers to have attempted to quit smoking within the past year (X2=2.3; P=.14), consider quitting in the next one or 6 months (X2=1.1; P=.34), or differ in perceived likelihood of cessation success (X2=0.0; P=1.00). The proportion of dual users who smoked 11+ cigarettes per day did not differ from that of cigarette smokers for the group as a whole or when the group was stratified by gender, income, education, or age. However, among non-whites, dual users smoked fewer cigarettes than cigarette smokers (TABLE 1).
Predicting physical dependence. Significant differences also were observed regarding the timing of the first cigarette of the day, with dual users approximately 3 times more likely than smokers to smoke within 30 minutes of awakening (80% vs 54.4%; OR=3.3; 95% CI, 1.8-6.3), and this difference was upheld among males, females, whites, those with an income >$20,000, those with a high school education or less and those with some college education, and age >34 years. There was no association, however, between dual use and perceived likelihood of quit success.
We then performed multivariable logistic modeling on dual users to determine which variables might predict 3 measures of physical dependence: number of cigarettes smoked per day (≤10 vs 11+), time between waking and smoking the first cigarette of the day (≤30 vs 31+ minutes), and perceived likelihood of cessation success (TABLE 2). Male gender (OR=3.4; 95% CI, 1.8-6.5) and white race (OR=4.4; 95% CI, 1.9-10.1) were significant for predicting smoking 11+ cigarettes a day, while dual use status was insignificant (P=.104). Regarding time to first cigarette, only dual use was significant (OR=3.1; 95% CI, 1.6-5.9), with dual users approximately 3 times more likely than smokers to have their first cigarette within 30 minutes of waking. No variables were significant in predicting perceived likelihood of quit success.
Reasons for dual use. We examined reasons for dual use with the question: Do you use e-cigarettes to reduce your regular tobacco use? Here, 79.5% of smokers reported using e-cigarettes to quit smoking or reduce the number of cigarettes smoked.
A multivariable polynomial logistic regression that included only dual users was performed to examine which variables might predict use for tobacco cessation (“trying to quit smoking”) vs reduction in smoking intensity (“trying to reduce the number of regular cigarettes I smoke per day”) vs no change (“use the same amount of tobacco as always”) (TABLE 2). Whites were approximately 6 times more likely than non-whites to indicate they engage in dual use to try to quit smoking (OR=6.0; 95% CI, 1.1-32.9). Males and people with lower incomes were much less likely to indicate they engaged in dual use to try to reduce the number of regular cigarettes smoked than females or those with higher incomes (OR=0.2; 95% CI, 0.1-0.8 and OR=0.1, 95% CI, 0.0-0.5, respectively). No other demographic variables or measures of nicotine physical dependence were significantly different between dual users and smokers.
Click here to see an enlarged version of the table.
DISCUSSION
E-cigarettes are used by approximately half of smokers (52%), which is much higher than that reported by Delnevo, et al, in their analysis of the National Health Interview Study.33 There, prevalence of dual use of both cigarettes and e-cigarettes ranged from 3.4% to 12.7%. This substantial difference raises important questions regarding study population characterization. Were participants in our study representative of central Illinois, state fair attendees, or the agricultural profession? Further work to identify this group with an increased propensity for dual use will assist clinicians in developing appropriate intervention strategies.
Dual use in our study did not vary by many customary demographic variables. Nor was it associated with different rates of past or future quit attempts or perceived ability to successfully quit if quitting was attempted. These factors—high rates of dual use and insignificant effect on quit attempts—may have implications for local physicians counseling patients who smoke.
In our study, the majority of smokers already use e-cigarettes, and this does not seem to increase their ability/likelihood to quit smoking. Further, dual use did not seem to be associated with overall cigarette consumption; males and white participants smoked more cigarettes than females and non-whites. But dual use was associated with a measure of increased nicotine physical dependence (earlier first cigarette of the day). As a result, physicians may want to think twice before recommending e-cigarette use as a means of smoking cessation.
In addition to the high prevalence of e-cigarette use among smokers, a number of other interesting findings surfaced that run counter to some of the current literature. First, dual users are no more likely than smokers to have tried to quit in the past or to try to quit in the future.21,22,34 It could be that for the relatively small geographical area from which our participants were recruited (central Illinois; ~77% of participants from Sangamon County alone), the local context and culture of smoking differs from that associated with participants in other studies, who were mostly recruited from national and regional online surveys. However, there is no a priori reason to suspect Sangamon County is especially different, as it is quite similar to Illinois as a whole by many measures (eg, percentage rural: 14.1% vs 11.5%; percentage black (only): 12.4% vs 14.7%; education to at least a Bachelor’s degree: 33.0% vs 31.9%; and median household income: $55,565 vs $57,166).30
While we found that dual users did have one measure of increased nicotine physical dependence, the total number of cigarettes consumed per day was not significantly different from that of smokers.23-25 This is contrary to another study of nicotine physical dependence, but, unlike that study, we did not assess length of time of concurrent use.35 There is much uncertainty surrounding the issue of nicotine physical dependence and e-cigarette use, largely because the level of nicotine delivered by various e-products varies significantly.36
Cross-sectional nature, small sample size limit utility of data
There are significant limitations to this study, including the cross-sectional nature of the data, the small sample size, the use of self-report, and the limited scope of recruitment. The relatively small sample size limits our ability to observe small differences and effect sizes. However, small differences often lack practical significance. Finally, participation was limited to those attending a state fair or a local sporting event and those employed by a local medical school. Thus, the results may not be generalizable to populations outside central Illinois. On the other hand, the very low income sample recruited from the Midwestern US, which is underrepresented in prior e-cigarette research, might represent some of the strengths of this work.
Future investigations. Future studies should more closely examine e-cigarette use prevalence on smaller geographic scales and especially in rural areas where there is a paucity of research. As the majority of our respondents came from a single county in central Illinois, one has to ask the questions, “Is this a ‘hot spot’ for e-cigarette use?" And "Do other rural areas experience similar use?” It may be important to know if national surveys are sensitive enough to observe significant local variations. Research also should examine how e-cigarette use and the influence of local culture vary across wider areas.
Several specific areas of study would help to inform policy and intervention development. For example, is tobacco cigarette quit success impacted by concurrent e-cigarette use? While our study showed no difference in past or possible future quit attempts among dual users as compared with smokers, we did not assess actual quit success, and multiple participants in our study anecdotally described using e-cigarettes to successfully quit smoking.
In the end, the rapid increase in the use of e-cigarettes has the potential to significantly alter the landscape of nicotine physical dependence, and local culture and other influences are likely associated with use patterns.
CORRESPONDENCE
Wiley D. Jenkins, PhD, MPH, Science Director, Population Health Science Program, Southern Illinois University School of Medicine, 201 E. Madison St., Springfield, IL 62794-9664; wjenkins@siumed.edu.
ABSTRACT
Background Electronic cigarettes (e-cigarettes) are often marketed as safe and effective aids for quitting cigarette smoking, but concerns remain that use of e-cigarettes might actually reduce the number of quit attempts. To address these issues, we characterized the utilization and demographic correlates of dual use of e-cigarettes and traditional cigarettes (referred to here as simply “cigarettes”) among smokers in a rural population of Illinois.
Methods The majority of survey participants were recruited from the 2014 Illinois State Fair and from another event—the Springfield Mile (a motorcycle racing event)—in Springfield, Ill. Survey questions explored participant demographics and cigarette and e-cigarette use history.
Results Of 201 total cigarette smokers, 79 smoked only tobacco cigarettes (smokers), while 122 also used e-cigarettes (dual users). Dual users did not differ significantly from smokers in gender, age, income, or education. Compared to smokers, dual users were more likely to smoke within 30 minutes of awakening (odds ratio [OR]=3.3; 95% confidence interval [CI], 1.8-6.3), but did not smoke more cigarettes per day or perceive a greater likelihood of quit success. Non-white dual users smoked fewer cigarettes per day than smokers. In addition, 79.5% of all dual users reported that they were using e-cigarettes to quit smoking or reduce the number of cigarettes smoked, and white respondents were 6 times more likely than non-whites to use e-cigarettes for ‘trying to quit smoking’ (OR=6.0; 95% CI, 1.1-32.9). Males and respondents with lower income were less likely to say they were using e-cigarettes to reduce the number of cigarettes smoked than females or participants with higher income (OR=0.2; 95% CI, 0.1-0.8 and OR=0.1; 95% CI, 0.0-0.5, respectively).
Conclusions E-cigarettes may significantly alter the landscape of nicotine physical dependence, and local influences likely are associated with use patterns. Future research should continue to examine whether dual use of traditional and electronic cigarettes impacts smoking cessation, and clinicians should be aware that local norms may create differences from national level data.
Approximately 21% of US adults use tobacco products at least occasionally.1 Although smoking prevalence has declined in recent years (from 21% in 2005 to 18% in 2013), it remains high among certain groups (eg, males and those with a high school education or less).2 As we know, the health burden of smoking—as a cause of death from cancer, pulmonary disease, and heart disease—is substantial,3,4 and rural areas experience a significantly higher prevalence of smoking compared to urban areas.2,5,6
However, it is unknown if the context and habits surrounding tobacco use in rural and/or Midwestern areas are similar to those of urban or nationally-representative populations. For example, while many urban residents may encounter a multitude of media messages encouraging smoking cessation resulting in less community acceptance of smoking, rural residents may be exposed to substantially fewer messages (eg, no city bus signs, billboards, subway posters, etc.) and the community may be more accommodating and tolerant of smoking.
Do e-cigarettes increase cigarette smoking?
Public health professionals are concerned about the increased use of e-cigarettes, particularly among young people, and whether this use increases the likelihood that individuals will start smoking tobacco cigarettes.7(Throughout this paper, we will use “cigarettes” and “smoking” to refer to the use of traditional tobacco cigarettes.) A recent study found that adolescents who used electronic nicotine delivery systems were twice as likely as non-users to have tried cigarettes in the past year.8
An onslaught of advertising. There are also concerns that e-cigarettes may serve to ‘renormalize’ nicotine addiction, in part through large-scale advertising, which was seen by nearly 70% of the participants in the 2014 National Youth Tobacco Survey.9 Largely as a result of that advertising, e-cigarette sales exceed $1.7 billion in the United States alone.10 With 15% of all US adults having ever tried electronic nicotine delivery systems and more than half (52%) of smokers having done so, questions regarding their health impact cannot be taken lightly.11
Do e-cigarettes help people quit smoking? E-cigarettes are often marketed as a safe and effective means for quitting cigarette smoking.12-14 (See "E-cigarettes: How "safe" are they?") Nearly two-thirds of physicians report being asked about e-cigarettes by their patients and approximately one-third of physicians recommend using them as a smoking cessation aid.15
Claims regarding the usefulness of e-cigarettes in smoking cessation, however, have not been substantiated by high-quality randomized controlled trials (RCTs). In fact, no RCTs have shown them to be safer or more effective than cessation treatments currently approved by the US Food and Drug Administration.16,17
Two studies reflect the conflicting data that are currently available. One small study found intensive e-cigarette users were 6 times more likely than non-users/triers to report successful smoking cessation.18 However, researchers surveying callers of a cigarette quit line found that smokers who used e-cigarettes (dual users) were less likely to quit smoking than non-users.19
The lack of good-quality data substantiates the concern that dual use might discourage quitting by normalizing cigarette use and reducing perceptions of harm.20,21 Dual use may also hamper smoking cessation efforts by increasing nicotine physical dependence and associated withdrawal symptoms when trying to quit.22 And finally, dual use may expose users to more carcinogens and toxins than those who use only one product, and the average number of cigarettes smoked per day may be significantly higher among dual users.23
Unique demographic factors at work? Finally, the social and community context within which smoking occurs, and the prevalence of smoking-associated demographic risk factors, may vary significantly between rural and urban areas and between seemingly similar rural areas.24-27 Few studies have examined differences in e-cigarette use between rural and urban areas. Those that have are contradictory, reporting that rural residents use e-cigarettes both more and less than their urban peers,28,29 but many of these studies were conducted outside the United States, where the context and norms associated with smoking and e-cigarette use likely vary.
For these reasons, we sought to examine e-cigarette use among residents of Illinois, the nation’s fifth largest state and one with a rural population exceeding 1.5 million.30 We compared dual users of e-cigarettes and cigarettes to smokers of cigarettes only in terms of demographic characteristics, nicotine physical dependence, and smoking cessation beliefs, and explored dual smokers' reasons for using both types of cigarettes.
MATERIALS AND METHODS
A survey was fielded during August and September 2014 in Springfield, Ill. To obtain responses, a booth was set up at both the Illinois State Fair and the Springfield Mile (a motorcycle racing event), and participants were recruited via direct solicitation by project staff. This was supplemented by an email invitation to all employees of the Southern Illinois University School of Medicine. The 2 venues and the email strategy were chosen because they draw from a large area of central and southern Illinois and were convenient to the location of the study team. Individuals were eligible to participate if they were ≥18 years of age and used any tobacco product or e-cigarettes. Survey elements were derived from 2 national surveys of health and behavior—the Minnesota Adult Tobacco Survey 201031 and the Brief Smoking Consequences Questionnaire-Adult.32
Survey questions assessed cigarette use, nicotine physical dependence, social norms, perceived risks and benefits, and smoking cessation beliefs and behaviors. Questions were slightly reworded to address not only the use of traditional cigarettes, but the use of e-cigarettes, as well. Ultimately, each participant answered a similarly-worded set of questions for both regular and e-cigarettes. Dual use of cigarettes and e-cigarettes was also assessed. Participants self-reported all data and survey responses on an electronic tablet and received a $10 (cash or gift card) incentive. This project was reviewed and approved by the Springfield Committee for Research Involving Human Subjects.
Stratification of results. Race was dichotomized into white and non-white. Education was stratified into 3 categories: up to and including high school graduation, some college but not a Bachelor’s degree, and Bachelor’s degree and above. Income was divided as being ≤$20,000 or >$20,000, and age was split into 2 groups by the median value. Analyses included descriptions of participant demographics, dual use status, measures of nicotine physical dependence, quit attempts, and e-cigarette use motivations. Bivariate relationships between dual use status and demographic characteristics, nicotine physical dependence, and smoking cessation beliefs were analyzed by chi-square (categorical variables) and ANOVA (continuous/Likert variables).
Multivariable logistic regression modeling of the demographic variables and dual use status (cigarette smoker only vs dual user) was performed to predict 3 factors: number of cigarettes smoked per day (≤10 vs 11+); time to first cigarette (≤30 vs 31+ minutes from waking); and perceived likelihood of quit attempt success (very/somewhat likely vs very/somewhat unlikely). Multivariable models examining the reasons for dual use included the demographic, nicotine physical dependence, and cessation belief items described previously.
RESULTS
Of 309 total survey participants (Fair=288; Race=12; Email=9), there were 235 current cigarette smokers consisting of 79 who smoked only cigarettes (smokers); 122 who used both cigarettes and e-cigarettes (dual users); and 34 former e-cigarette users. Only smokers and dual users were included in this analysis (N=201, although for the purposes of TABLE 1, N=200 or 199 because at least one participant did not provide answers to all of the questions). Approximately 51% of the smokers were male, 78% were white, 12% were 4-year college graduates, and 57% reported incomes >$20,000. The mean age was 37.7 years (SD=14.4); 50% of respondents were <35 years of age. Dual users did not vary significantly from smokers in terms of gender, age, education, or income (all P>.05). However, a greater proportion of whites vs non-whites were dual users (54.9% vs 42.3%; P=.035).
Click here to see an enlarged version of the table.
No big quit differences. Bivariate analyses revealed that dual users were no more likely than smokers to have attempted to quit smoking within the past year (X2=2.3; P=.14), consider quitting in the next one or 6 months (X2=1.1; P=.34), or differ in perceived likelihood of cessation success (X2=0.0; P=1.00). The proportion of dual users who smoked 11+ cigarettes per day did not differ from that of cigarette smokers for the group as a whole or when the group was stratified by gender, income, education, or age. However, among non-whites, dual users smoked fewer cigarettes than cigarette smokers (TABLE 1).
Predicting physical dependence. Significant differences also were observed regarding the timing of the first cigarette of the day, with dual users approximately 3 times more likely than smokers to smoke within 30 minutes of awakening (80% vs 54.4%; OR=3.3; 95% CI, 1.8-6.3), and this difference was upheld among males, females, whites, those with an income >$20,000, those with a high school education or less and those with some college education, and age >34 years. There was no association, however, between dual use and perceived likelihood of quit success.
We then performed multivariable logistic modeling on dual users to determine which variables might predict 3 measures of physical dependence: number of cigarettes smoked per day (≤10 vs 11+), time between waking and smoking the first cigarette of the day (≤30 vs 31+ minutes), and perceived likelihood of cessation success (TABLE 2). Male gender (OR=3.4; 95% CI, 1.8-6.5) and white race (OR=4.4; 95% CI, 1.9-10.1) were significant for predicting smoking 11+ cigarettes a day, while dual use status was insignificant (P=.104). Regarding time to first cigarette, only dual use was significant (OR=3.1; 95% CI, 1.6-5.9), with dual users approximately 3 times more likely than smokers to have their first cigarette within 30 minutes of waking. No variables were significant in predicting perceived likelihood of quit success.
Reasons for dual use. We examined reasons for dual use with the question: Do you use e-cigarettes to reduce your regular tobacco use? Here, 79.5% of smokers reported using e-cigarettes to quit smoking or reduce the number of cigarettes smoked.
A multivariable polynomial logistic regression that included only dual users was performed to examine which variables might predict use for tobacco cessation (“trying to quit smoking”) vs reduction in smoking intensity (“trying to reduce the number of regular cigarettes I smoke per day”) vs no change (“use the same amount of tobacco as always”) (TABLE 2). Whites were approximately 6 times more likely than non-whites to indicate they engage in dual use to try to quit smoking (OR=6.0; 95% CI, 1.1-32.9). Males and people with lower incomes were much less likely to indicate they engaged in dual use to try to reduce the number of regular cigarettes smoked than females or those with higher incomes (OR=0.2; 95% CI, 0.1-0.8 and OR=0.1, 95% CI, 0.0-0.5, respectively). No other demographic variables or measures of nicotine physical dependence were significantly different between dual users and smokers.
Click here to see an enlarged version of the table.
DISCUSSION
E-cigarettes are used by approximately half of smokers (52%), which is much higher than that reported by Delnevo, et al, in their analysis of the National Health Interview Study.33 There, prevalence of dual use of both cigarettes and e-cigarettes ranged from 3.4% to 12.7%. This substantial difference raises important questions regarding study population characterization. Were participants in our study representative of central Illinois, state fair attendees, or the agricultural profession? Further work to identify this group with an increased propensity for dual use will assist clinicians in developing appropriate intervention strategies.
Dual use in our study did not vary by many customary demographic variables. Nor was it associated with different rates of past or future quit attempts or perceived ability to successfully quit if quitting was attempted. These factors—high rates of dual use and insignificant effect on quit attempts—may have implications for local physicians counseling patients who smoke.
In our study, the majority of smokers already use e-cigarettes, and this does not seem to increase their ability/likelihood to quit smoking. Further, dual use did not seem to be associated with overall cigarette consumption; males and white participants smoked more cigarettes than females and non-whites. But dual use was associated with a measure of increased nicotine physical dependence (earlier first cigarette of the day). As a result, physicians may want to think twice before recommending e-cigarette use as a means of smoking cessation.
In addition to the high prevalence of e-cigarette use among smokers, a number of other interesting findings surfaced that run counter to some of the current literature. First, dual users are no more likely than smokers to have tried to quit in the past or to try to quit in the future.21,22,34 It could be that for the relatively small geographical area from which our participants were recruited (central Illinois; ~77% of participants from Sangamon County alone), the local context and culture of smoking differs from that associated with participants in other studies, who were mostly recruited from national and regional online surveys. However, there is no a priori reason to suspect Sangamon County is especially different, as it is quite similar to Illinois as a whole by many measures (eg, percentage rural: 14.1% vs 11.5%; percentage black (only): 12.4% vs 14.7%; education to at least a Bachelor’s degree: 33.0% vs 31.9%; and median household income: $55,565 vs $57,166).30
While we found that dual users did have one measure of increased nicotine physical dependence, the total number of cigarettes consumed per day was not significantly different from that of smokers.23-25 This is contrary to another study of nicotine physical dependence, but, unlike that study, we did not assess length of time of concurrent use.35 There is much uncertainty surrounding the issue of nicotine physical dependence and e-cigarette use, largely because the level of nicotine delivered by various e-products varies significantly.36
Cross-sectional nature, small sample size limit utility of data
There are significant limitations to this study, including the cross-sectional nature of the data, the small sample size, the use of self-report, and the limited scope of recruitment. The relatively small sample size limits our ability to observe small differences and effect sizes. However, small differences often lack practical significance. Finally, participation was limited to those attending a state fair or a local sporting event and those employed by a local medical school. Thus, the results may not be generalizable to populations outside central Illinois. On the other hand, the very low income sample recruited from the Midwestern US, which is underrepresented in prior e-cigarette research, might represent some of the strengths of this work.
Future investigations. Future studies should more closely examine e-cigarette use prevalence on smaller geographic scales and especially in rural areas where there is a paucity of research. As the majority of our respondents came from a single county in central Illinois, one has to ask the questions, “Is this a ‘hot spot’ for e-cigarette use?" And "Do other rural areas experience similar use?” It may be important to know if national surveys are sensitive enough to observe significant local variations. Research also should examine how e-cigarette use and the influence of local culture vary across wider areas.
Several specific areas of study would help to inform policy and intervention development. For example, is tobacco cigarette quit success impacted by concurrent e-cigarette use? While our study showed no difference in past or possible future quit attempts among dual users as compared with smokers, we did not assess actual quit success, and multiple participants in our study anecdotally described using e-cigarettes to successfully quit smoking.
In the end, the rapid increase in the use of e-cigarettes has the potential to significantly alter the landscape of nicotine physical dependence, and local culture and other influences are likely associated with use patterns.
CORRESPONDENCE
Wiley D. Jenkins, PhD, MPH, Science Director, Population Health Science Program, Southern Illinois University School of Medicine, 201 E. Madison St., Springfield, IL 62794-9664; wjenkins@siumed.edu.
1. Agaku IT, King BA, Husten CG, et al; Centers for Disease Control and Prevention (CDC). Tobacco product use among adults—United States, 2012-2013. MMWR Morb Mortal Wkly Rep. 2014;63:542-547.
2. Jamal A, Agaku IT, O’Connor E, et al. Current cigarette smoking among adults—United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2014;63:1108-1112.
3. Siegel RL, Jacobs EJ, Newton CC, et al. Deaths due to cigarette smoking for 12 smoking-related cancers in the United States. JAMA Intern Med. 2015;175:1574-1576.
4. US Department of Health and Human Services. Surgeon General. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. Available at: http://www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html. Accessed January 22, 2014.
5. Gamm LD, Hutchison LL, Dabney BJ, et al, eds. (2003). Rural Healthy People 2010: A companion document to Healthy People 2010. Volume 2. College Station, TX: The Texas A&M University System Health Science Center, School of Rural Public Health, Southwest Rural Health Research Center.
6. Doescher MP, Jackson JE, Jerant A, et al. Prevalence and trends in smoking: a national rural study. J Rural Health. 2006;22:112-118.
7. Bunnell RE, Agaku IT, Arrazola RA, et al. Intentions to smoke cigarettes among never-smoking US middle and high school electronic cigarette users: National Youth Tobacco Survey, 2011-2013. Nicotine Tob Res. 2015;17:228-235.
8. Cardenas VM, Evans VL, Balamurugan A, et al. Use of electronic nicotine delivery systems and recent initiation of smoking among US youth. Int J Public Health. 2016;61:237-241.
9. Auf R, Trepka MJ, Cano MA, et al. Electronic cigarettes: the renormalisation of nicotine use. BMJ. 2016;352:i425.
10. CNBC. E-cigarette sales are smoking hot, set to hit $1.7 billion. Available at: http://www.cnbc.com/id/100991511. Accessed April 5, 2016.
11. Weaver SR, Majeed BA, Pechacek TF, et al. Use of electronic nicotine delivery systems and other tobacco products among USA adults, 2014: results from a national survey. Int J Public Health. 2016;61:177-188.
12. Richardson A, Ganz O, Vallone D. Tobacco on the web: surveillance and characterisation of online tobacco and e-cigarette advertising. Tob Control. 2015;24:341-347.
13. Paek HJ, Kim S, Hove T, et al. Reduced harm or another gateway to smoking? source, message, and information characteristics of E-cigarette videos on YouTube. J Health Commun. 2014;19:545-560.
14. Kim AE, Arnold KY, Makarenko O. E-cigarette advertising expenditures in the U.S., 2011-2012. Am J Prev Med. 2014;46:409-412.
15. Steinberg MB, Giovenco DP, Delnevo CD. Patient-physician communication regarding electronic cigarettes. Prev Med Rep. 2015;2:96-98.
16. Gualano MR, Passi S, Bert F, et al. Electronic cigarettes: assessing the efficacy and the adverse effects through a systematic review of published studies. J Public Health (Oxf). 2015:37:488-497.
17. U.S. National Institutes of Health. ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/results?term=%22electronic+cigarette%22&Search=Search. Accessed July 10, 2015.
18. Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2015;17:127-133.
19. Vickerman KA, Carpenter KM, Altman T, et al. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787-1791.
20. Center for Disease Control and Prevention. Press Release February 28,2013. Available at: http://www.cdc.gov/media/releases/2013/p0228_electronic_cigarettes.html. Accessed July 8, 2015.
21. Pisinger C. Why public health people are more worried than excited over e-cigarettes. BMC Med. 2014;12:226.
22. Post A, Gilljam H, Rosendahl I, et al. Symptoms of nicotine dependence in a cohort of Swedish youths: a comparison between smokers, smokeless tobacco users and dual tobacco users. Addiction. 2010;105:740-746.
23. Mazurek JM, Syamlal G, King BA, et al; Division of Respiratory Disease Studies, National Institute for Occupational Safety and Health, CDC. Smokeless tobacco use among working adults—United States, 2005 and 2010. MMWR Morb Mortal Wkly Rep. 2014;63:477-482.
24. Hutcheson TD, Greiner KA, Ellerbeck EF, et al. Understanding smoking cessation in rural communities. J Rural Health. 2008;24:116-124.
25. McMillen R, Breen J, Cosby AG. Rural-urban differences in the social climate surrounding environmental tobacco smoke: a report from the 2002 Social Climate Survey of Tobacco Control. J Rural Health. 2004;20:7-16.
26. Butler KM, Rayens MK, Adkins S, et al. Culturally-specific smoking cessation outreach in a rural community. Public Health Nurs. 2014;31:44-54.
27. Butler KM, Hedgecock S, Record RA, et al. An evidence-based cessation strategy using rural smokers’ experiences with tobacco. Nurs Clin North Am. 2012;47:31-43.
28. Hamilton HA, Ferrence R, Boak A, et al. Ever use of nicotine and nonnicotine electronic cigarettes among high school students in Ontario, Canada. Nicotine Tob Res. 2015;17:1212-1218.
29. Goniewicz ML, Zielinska-Danch W. Electronic cigarette use among teenagers and young adults in Poland. Pediatrics. 2012;130:e879-e885.
30. US Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Available at: http://www.census.gov/geo/reference/ua/urban-rural-2010.html. Accessed March 13, 2016.
31. Minnesota Adult Tobacco Survey. Tobacco use in Minnesota: 1999-2014. Available at: http://www.mnadulttobaccosurvey.org/. Accessed April 27, 2016.
32. Rash CJ, Copeland AL. The Brief Smoking Consequences Questionnaire-Adult (BSCQ-A): development of a short form of the SCQ-A. Nicotine Tob Res. 2008;10:1633-1643.
33. Delnevo CD, Giovenco DP, Steinberg MB, et al. Patterns of electronic cigarette use among adults in the United States. Nicotine Tob Res. 2016;18:715-719.
34. Lee YO, Hebert CJ, Nonnemaker JM, et al. Multiple tobacco product use among adults in the United States: cigarettes, cigars, electronic cigarettes, hookah, smokeless tobacco, and snus. Prev Med. 2014;62:14-19.
35. Etter JF, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015;147:68-75.
36. Cobb CO, Hendricks PS, Eissenberg T. Electronic cigarettes and nicotine dependence: evolving products, evolving problems. BMC Med. 2015;13:119.
1. Agaku IT, King BA, Husten CG, et al; Centers for Disease Control and Prevention (CDC). Tobacco product use among adults—United States, 2012-2013. MMWR Morb Mortal Wkly Rep. 2014;63:542-547.
2. Jamal A, Agaku IT, O’Connor E, et al. Current cigarette smoking among adults—United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2014;63:1108-1112.
3. Siegel RL, Jacobs EJ, Newton CC, et al. Deaths due to cigarette smoking for 12 smoking-related cancers in the United States. JAMA Intern Med. 2015;175:1574-1576.
4. US Department of Health and Human Services. Surgeon General. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. Available at: http://www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html. Accessed January 22, 2014.
5. Gamm LD, Hutchison LL, Dabney BJ, et al, eds. (2003). Rural Healthy People 2010: A companion document to Healthy People 2010. Volume 2. College Station, TX: The Texas A&M University System Health Science Center, School of Rural Public Health, Southwest Rural Health Research Center.
6. Doescher MP, Jackson JE, Jerant A, et al. Prevalence and trends in smoking: a national rural study. J Rural Health. 2006;22:112-118.
7. Bunnell RE, Agaku IT, Arrazola RA, et al. Intentions to smoke cigarettes among never-smoking US middle and high school electronic cigarette users: National Youth Tobacco Survey, 2011-2013. Nicotine Tob Res. 2015;17:228-235.
8. Cardenas VM, Evans VL, Balamurugan A, et al. Use of electronic nicotine delivery systems and recent initiation of smoking among US youth. Int J Public Health. 2016;61:237-241.
9. Auf R, Trepka MJ, Cano MA, et al. Electronic cigarettes: the renormalisation of nicotine use. BMJ. 2016;352:i425.
10. CNBC. E-cigarette sales are smoking hot, set to hit $1.7 billion. Available at: http://www.cnbc.com/id/100991511. Accessed April 5, 2016.
11. Weaver SR, Majeed BA, Pechacek TF, et al. Use of electronic nicotine delivery systems and other tobacco products among USA adults, 2014: results from a national survey. Int J Public Health. 2016;61:177-188.
12. Richardson A, Ganz O, Vallone D. Tobacco on the web: surveillance and characterisation of online tobacco and e-cigarette advertising. Tob Control. 2015;24:341-347.
13. Paek HJ, Kim S, Hove T, et al. Reduced harm or another gateway to smoking? source, message, and information characteristics of E-cigarette videos on YouTube. J Health Commun. 2014;19:545-560.
14. Kim AE, Arnold KY, Makarenko O. E-cigarette advertising expenditures in the U.S., 2011-2012. Am J Prev Med. 2014;46:409-412.
15. Steinberg MB, Giovenco DP, Delnevo CD. Patient-physician communication regarding electronic cigarettes. Prev Med Rep. 2015;2:96-98.
16. Gualano MR, Passi S, Bert F, et al. Electronic cigarettes: assessing the efficacy and the adverse effects through a systematic review of published studies. J Public Health (Oxf). 2015:37:488-497.
17. U.S. National Institutes of Health. ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/results?term=%22electronic+cigarette%22&Search=Search. Accessed July 10, 2015.
18. Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2015;17:127-133.
19. Vickerman KA, Carpenter KM, Altman T, et al. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787-1791.
20. Center for Disease Control and Prevention. Press Release February 28,2013. Available at: http://www.cdc.gov/media/releases/2013/p0228_electronic_cigarettes.html. Accessed July 8, 2015.
21. Pisinger C. Why public health people are more worried than excited over e-cigarettes. BMC Med. 2014;12:226.
22. Post A, Gilljam H, Rosendahl I, et al. Symptoms of nicotine dependence in a cohort of Swedish youths: a comparison between smokers, smokeless tobacco users and dual tobacco users. Addiction. 2010;105:740-746.
23. Mazurek JM, Syamlal G, King BA, et al; Division of Respiratory Disease Studies, National Institute for Occupational Safety and Health, CDC. Smokeless tobacco use among working adults—United States, 2005 and 2010. MMWR Morb Mortal Wkly Rep. 2014;63:477-482.
24. Hutcheson TD, Greiner KA, Ellerbeck EF, et al. Understanding smoking cessation in rural communities. J Rural Health. 2008;24:116-124.
25. McMillen R, Breen J, Cosby AG. Rural-urban differences in the social climate surrounding environmental tobacco smoke: a report from the 2002 Social Climate Survey of Tobacco Control. J Rural Health. 2004;20:7-16.
26. Butler KM, Rayens MK, Adkins S, et al. Culturally-specific smoking cessation outreach in a rural community. Public Health Nurs. 2014;31:44-54.
27. Butler KM, Hedgecock S, Record RA, et al. An evidence-based cessation strategy using rural smokers’ experiences with tobacco. Nurs Clin North Am. 2012;47:31-43.
28. Hamilton HA, Ferrence R, Boak A, et al. Ever use of nicotine and nonnicotine electronic cigarettes among high school students in Ontario, Canada. Nicotine Tob Res. 2015;17:1212-1218.
29. Goniewicz ML, Zielinska-Danch W. Electronic cigarette use among teenagers and young adults in Poland. Pediatrics. 2012;130:e879-e885.
30. US Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Available at: http://www.census.gov/geo/reference/ua/urban-rural-2010.html. Accessed March 13, 2016.
31. Minnesota Adult Tobacco Survey. Tobacco use in Minnesota: 1999-2014. Available at: http://www.mnadulttobaccosurvey.org/. Accessed April 27, 2016.
32. Rash CJ, Copeland AL. The Brief Smoking Consequences Questionnaire-Adult (BSCQ-A): development of a short form of the SCQ-A. Nicotine Tob Res. 2008;10:1633-1643.
33. Delnevo CD, Giovenco DP, Steinberg MB, et al. Patterns of electronic cigarette use among adults in the United States. Nicotine Tob Res. 2016;18:715-719.
34. Lee YO, Hebert CJ, Nonnemaker JM, et al. Multiple tobacco product use among adults in the United States: cigarettes, cigars, electronic cigarettes, hookah, smokeless tobacco, and snus. Prev Med. 2014;62:14-19.
35. Etter JF, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015;147:68-75.
36. Cobb CO, Hendricks PS, Eissenberg T. Electronic cigarettes and nicotine dependence: evolving products, evolving problems. BMC Med. 2015;13:119.
Hospice Care Insurance and Readmissions
Palliative care and hospice specialists consult on a variety of patients in the acute care setting that span all diagnoses and specialties. These include patients in the intensive care units, oncology units, as well as patients with end‐stage pulmonary, cardiac, and renal diseases. Discharge of these patients is often complicated by social issues, intensive personal care needs, and decreased functional status, as well as by the patient's insurance. Options for discharge disposition for patients accepting enrollment in hospice are often limited by financial constraints. Medicare pays for a set package of hospice benefits that do not include payment for room and board for hospice residential care and have a limited number of hours for a personal care attendant.[1] Hospice inpatient units are typically covered only for patients with acute care needs. Patients with secondary commercial insurance similarly find that custodial care benefits are often lacking, as most private and managed care plans mimic the Medicare hospice benefit.[2]
Palliative care inpatient consultation and palliative or hospice home care are associated with decreased 30‐day readmission rates.[3, 4, 5, 6] None of these studies, however, evaluated the effect of insurance status on readmission rates. Patients with dual coverage of Medicare and Medicaid are eligible for coverage of room and board (covered by Medicaid) in addition to the standard hospice benefit (covered by Medicare), and therefore have more options for discharge planning, including admission to a hospice residence, nursing home care with hospice services, or increased personal care attendant hours at home. Dual eligible patients (those with both Medicare and Medicaid) represent 20% of the Medicare population. They are generally poorer and with worse health status that those with Medicare alone; they have on average 25% more medical conditions than Medicare‐only patients.[7] Previous studies of readmissions and healthcare costs in the general population have found that dual eligible patients have higher rates of readmissions and higher overall healthcare costs compared with other groups.[7, 8, 9] However, these studies did not specifically look at patients near the end of life receiving hospice services. We hypothesize that dual eligible patients may actually have a lower rate of readmission at end of life compared with other groups, and that this effect may be partially mediated by discharge location (facility or home).
Previous studies have identified risk factors for 30‐day readmission to hospital, including living alone, insurance status, and poor or fair satisfaction with their primary care provider (PCP).[10] This study aims to evaluate, in the cohort of patients who have received a palliative care consultation during their hospital stay and who were discharged with hospice services, whether type of insurance is associated with risk of early readmission.
METHODS
Data were extracted from a replicate of Montefiore's Clinical Information System using healthcare surveillance software (Clinical Looking Glass; Emerging Health Information Technology, Yonkers, NY). We queried this database to find patients who received palliative care consultation from August 2010 to January 2014 at Montefiore Medical Center, an academic medical center in Bronx, NY, consisting of 3 general hospitals with 1491 beds. The medical center provides care to many underserved and minority patients and serves as the University Hospital of the Albert Einstein College of Medicine.
Inclusion Criteria
Patients who received a palliative care consultation were included if they were 65 years of age, nonpregnant, and admitted to the medical intensive care unit, any surgical intensive care unit, cardiac care unit, general medicine, surgery or surgical subspecialty service, family medicine, cardiology or oncology service, and discharged with hospice services.
Exclusion Criteria
Patients 65 years old and patients who died during the index admission were excluded, as were admissions to pediatrics, obstetrics, and psychology services, and uninsured patients.
The admission with the first palliative care consultation resulting in hospice referral was considered the index admission for these patients. Sociodemographic variables related to readmission such as age, race, gender, primary language, and socioeconomic status (SES) were examined.[11, 12, 13, 14, 15, 16] Clinical variables shown to be related to 30‐day readmissions in the literature including lab‐based acute physiology score (LAPS), blood urea nitrogen level (BUN), serum sodium level, serum albumin level, documentation of weight loss, and Charlson Comorbidity Index as well as its specific components were also extracted.[11, 13, 16, 17, 18, 19] Other variables related to the index admission such as length of stay for index admission, admission source on index admission (eg, from home, nursing home, other), and whether the primary care physician was listed in the chart were also examined.[11, 13, 16, 17, 19, 20] All of the variables were examined because they were hypothesized to be related to both insurance status and readmission. Markers of clinical severity, such as LAPS, BUN, hyponatremia, hypoalbuminemia, weight loss, and comorbidity could lead to readmission for symptom management or acute deterioration, and have been found be related to readmission in previous literature.
The predictor variable was insurance status at the time of index admission (dual eligible or all other). The main outcome variable was readmission to Montefiore Medical Center for any reason within 30 days of the index admission. Discharge location (hospice services in a facility vs home hospice) was examined as a potential mediator.
Statistical Analysis
Based on quality metrics available from our department, we expected to find at least 1000 patients 65 years of age seen by the palliative care consultation service with a discharge disposition including hospice services. This would give our study 85% power to detect a 10% difference in readmission rates between the 2 groups.
Patients were categorized as dual eligible if they were covered by Medicare and Medicaid only or if they were covered by Medicare, Medicaid, and private insurance. Controls were patients who were covered by Medicare only, Medicaid only, private insurance only, or Medicare and private insurance or Medicaid and private insurance. For the primary analysis, patients with and without dual eligibility were compared with respect to sociodemographic characteristics, healthcare process variables, and measures of comorbidity and illness severity using t tests for continuous variables and 2 tests for categorical variables. We used a 2 test to assess the univariable association between dual eligibility and 30‐day readmission. To address the question as to whether dual eligibility reduces the likelihood of a 30 day readmission, logistic regression was used to model 30‐day readmission by selecting from the covariates associated with the 30‐day readmissions at the 0.15 significance level. The Hosmer‐Lemeshow goodness of fit test was used to evaluate overall model performance.
For the secondary analysis, we assessed whether type and location of hospice services mediate the effect of insurance status on 30‐day readmissions using a Sobel‐Goodman test for mediation. Statistical analysis was conducted using statistical software (Stata statistical software, release 12; StataCorp, College Station, TX).
This research protocol was reviewed by the Montefiore Medical Center/Albert Einstein College of Medicine Institutional Review Board.
RESULTS
A total of 2755 inpatients were seen by the palliative care consultation service across the Montefiore Medical Center sites and discharged with hospice services. Of those, 1688 were dual eligible for Medicare and Medicaid, and 1067 were not. Specifically, 695 patients had Medicare only, 148 had private insurance only, 126 had Medicaid only, 78 had Medicare and private insurance, and 19 had Medicaid and private insurance. Univariable relationships between patient characteristics, insurance status, and readmission are shown in Table 1.
| Characteristic | Dual Eligible, N = 1,688 |
Not Dual Eligible, N = 1,067 |
P Value | 30‐Day Readmission | P Value | |
|---|---|---|---|---|---|---|
| Yes, N = 296 | No, N = 2,459 | |||||
| ||||||
| Sociodemographic | ||||||
| Age, y, mean SD | 81.6 9.0 | 79.4 8.9 | <0.05 | 77.8 8.8 | 81.1 9.0 | <0.05 |
| Female, n (%) | 1,092 (64.7) | 622 (58.3) | <0.05 | 171 (57.8) | 1,543 (62.7) | 0.095 |
| Has PCP, n (%) | 1,451 (86.0) | 951 (89.1) | <0.05 | 263 (88.9) | 2,139 (87.0) | NS |
| Speaks English, n (%) | 1,064 (63.0) | 728 (68.2) | <0.05 | 181 (61.1) | 1,611 (65.5) | 0.137 |
| SES, mean SD | 2.76 2.81 | 2.51 2.67 | <0.05 | 3.11 2.72 | 2.61 2.77 | <0.05 |
| Race/ethnicity | <0.05 | <0.05 | ||||
| Hispanic, n (%) | 587 (34.8) | 267 (31.3) | 100 (33.8) | 754 (30.7) | ||
| White, n (%) | 532 (31.5) | 290 (27.2) | 58 (19.6) | 764 (31.1) | ||
| Black, n (%) | 449 (26.6) | 420 (39.4) | 121 (40.9) | 748 (30.4) | ||
| Comorbidities, n (%) | ||||||
| Congestive heart failure | 555 (32.9) | 264 (24.7) | <0.05 | 104 (35.1) | 751 (30.5) | 0.106 |
| Cardiac valvular disease | 179 (10.6) | 76 (7.1) | <0.05 | 19 (6.4) | 227 (9.2) | 0.109 |
| Myocardial infarction | 165 (9.8) | 85 (8.0) | 0.11 | 31 (10.5) | 219 (8.9) | NS |
| Pulmonary disease | 480 (28.4) | 292 (27.4) | NS | 98 (33.1) | 674 (27.4) | 0.039 |
| Liver disease | 60 (3.6) | 54 (5.1) | 0.053 | 22 (7.4) | 92 (3.7) | <0.05 |
| Dementia | 135 (8.0) | 52 (4.9) | <0.05 | 11 (3.7) | 176 (7.2) | 0.026 |
| Diabetes, complicated | 125 (7.4) | 52 (4.9) | <0.05 | 15 (5.1) | 163 (6.6) | NS |
| Malignancy | 589 (34.9) | 499 (46.8) | <0.05 | 124 (41.9) | 921 (37.5) | 0.137 |
| Renal disease | 394 (23.3) | 225 (21.1) | NS | 72 (24.3) | 547 (22.2) | NS |
| Depression | 174 (10.3) | 85 (8.0) | <0.05 | 25 (8.4) | 234 (9.5) | NS |
| Peripheral vascular disease | 166 (9.8) | 72 (6.7) | <0.05 | 16 (5.4) | 222 (9.0) | 0.036 |
| Cerebrovascular disease | 282 (16.7) | 125 (11.7) | <0.05 | 33 (11.1) | 374 (15.2) | 0.063 |
| Clinical characteristics | ||||||
| LOS, mean SD | 10.9 9.93 | 10.6 9.61 | 0.19 | 9.3 8.0 | 10.9 10.0 | <0.05 |
| LAPS, mean SD | 38.4 27.9 | 34.6 26.9 | <0.05 | 33.8 25.2 | 37.3 27.8 | 0.039 |
| BUN, mean SD | 34.4 32.3 | 30.9 28.3 | <0.05 | 29.5 24.4 | 33.4 31.6 | 0.036 |
| Charlson score, mean SD | 4.62 3.37 | 5.28 3.56 | <0.05 | 5.1 3.5 | 4.8 3.5 | 0.152 |
In this sample, 9.2% of patients in the dual eligible group were readmitted within 30 days compared with 13.1% of others (2 = 10.3, P = 0.001). Of the total cohort, 1500 patients, including 862 dual eligible patients, were discharged to a facility, and 1255 patients, including 826 dual eligible patients, were discharged home. Dual eligible patients had a lower readmission rate compared with others in both settings (Figure 1). In univariable analysis, gender, age, hospital length of stay, race/ethnicity, SES, English as a primary language, LAPS, BUN, Charlson score, and comorbid peripheral vascular disease, cerebrovascular disease, heart disease, dementia, cancer, and liver disease were found to be related both to the predictor and the outcome variables and were included in the logistic regression model. While controlling for these variables, dual eligible patients had a lower odds of readmission within 30 days compared with others (odds ratio [OR]: 0.77; P = 0.041; 95% confidence interval [CI]: 0.59‐0.98) (Table 2). The Hosmer‐Lemeshow test was not significant, indicating that the overall model fit was good.
| Independent Variable | Odds Ratio | Standard Error | z Ratio | P Value |
|---|---|---|---|---|
| Dual eligibility | 0.77 | 0.10 | 2.05 | 0.041 |
| Gender | 1.16 | 0.15 | 1.17 | 0.244 |
| Age | 0.96 | 0.01 | 4.54 | 0.000 |
| Hospital length of stay | 0.97 | 0.01 | 3.33 | 0.001 |
| Black | 1.93 | 0.53 | 2.37 | 0.018 |
| White | 1.02 | 0.30 | 0.08 | 0.939 |
| Hispanic | 1.29 | 0.37 | 0.90 | 0.368 |
| Socioeconomic status | 0.96 | 0.02 | 1.63 | 0.103 |
| Primary language English | 0.81 | 0.12 | 1.43 | 0.154 |
| Peripheral vascular disease | 0.67 | 0.18 | 1.48 | 0.139 |
| Cerebrovascular disease | 0.86 | 0.17 | 0.73 | 0.465 |
| Dementia | 0.61 | 0.20 | 1.50 | 0.135 |
| Congestive heart failure | 1.75 | 0.26 | 3.83 | 0.000 |
| Cardiac valvular disease | 0.73 | 0.19 | 1.23 | 0.219 |
| Cancer | 0.92 | 0.15 | 0.51 | 0.608 |
| Liver disease | 1.80 | 0.47 | 2.25 | 0.024 |
| Lab‐based acute physiology score | 1.00 | 0.00 | 0.66 | 0.510 |
| Blood urea nitrogen | 1.00 | 0.00 | 1.29 | 0.197 |
| Charlson comorbidity score | 0.99 | 0.02 | 0.57 | 0.567 |
In the secondary analysis, we found that disposition (hospice services in a nursing home or hospice residence vs home hospice) partially mediates the relationship between insurance status and readmission, explaining 30% of the total effect (z = 5.06, P < 0.001). When accounting for disposition as a mediator, dual eligible patients still had a lower odds of readmission within 30 days compared with others, although the difference was no longer statistically significant (OR: 0.86; P = 0.24; 95% CI: 0.66‐ 1.11). Patients discharged with hospice services in a nursing home or hospice residence were less likely to be readmitted within 30 days (OR: 0.41; P < 0.001; 95% CI: 0.31‐0.54) (Table 3).
| Independent Variable | Odds Ratio | Standard Error | z Ratio | P Value |
|---|---|---|---|---|
| Dual eligibility | 0.86 | 0.11 | 1.17 | 0.244 |
| Discharge location | 0.40 | 0.59 | 6.22 | 0.000 |
| Gender | 1.17 | 0.16 | 1.22 | 0.223 |
| Age | 0.96 | 0.01 | 4.69 | 0.000 |
| Hospital length of stay | 0.98 | 0.01 | 2.57 | 0.010 |
| Black | 1.95 | 0.54 | 2.39 | 0.017 |
| White | 1.02 | 0.30 | 0.10 | 0.924 |
| Hispanic | 1.20 | 0.35 | 0.63 | 0.526 |
| Socioeconomic status | 0.96 | 0.02 | 1.51 | 0.132 |
| Primary language English | 0.78 | 0.11 | 1.69 | 0.090 |
| Peripheral vascular disease | 0.70 | 0.19 | 1.31 | 0.190 |
| Cerebrovascular disease | 0.89 | 0.18 | 0.56 | 0.579 |
| Dementia | 0.64 | 0.21 | 1.36 | 0.174 |
| Congestive heart failure | 1.75 | 0.26 | 3.80 | 0.000 |
| Cardiac valvular disease | 0.70 | 0.18 | 1.35 | 0.176 |
| Cancer | 0.91 | 0.15 | 0.59 | 0.552 |
| Liver disease | 1.75 | 0.46 | 2.12 | 0.034 |
| Lab‐based acute physiology score | 1.00 | 0.00 | 0.20 | 0.843 |
| Blood urea nitrogen | 1.00 | 0.00 | 1.10 | 0.270 |
| Charlson comorbidity score | 0.99 | 0.02 | 0.65 | 0.516 |
DISCUSSION
This study showed an association between dual coverage and lower odds of 30‐day readmission for patients discharged to hospice compared to all other insurance categories, excluding uninsured. This is the first study to date looking specifically at the association between insurance and readmission rates of patients discharged with hospice services. This association was attenuated, and no longer statistically significant, when accounting for discharge location.
These findings suggest that the added services available to patients enrolled in Medicare and Medicaid likely provide an enhanced level of postacute care. Patients with Medicaid have access to increased hours of personal care attendants as well as residential care, which often provides 24‐hour trained staff for rapid assessment of a change in clinical status and adjustment to therapeutic management. Combined with the Medicare hospice benefit, which provides better attention to symptom management, better supervision, and improved compliance with medications, as well as education of family and caregivers,[21, 22, 23] additional coverage with Medicaid is associated with a decrease in early readmission to the hospital.
It is often a financial hardship for family members or caregivers to take time off work to care for a dying patient. Without adequate postdischarge resources, the hospital to home transition will be ineffective, which has been shown to increase readmissions.[24] The option of increased attendant hours or residential care can have a positive impact on the financial and psychosocial stressors of caring for a family member at the end of life. Although we did not assess for this in our study, caregiver burnout often plays a role in emergency room visits and admissions of patients at the end of life.[25] The average age of the patients in our cohorts was 81 and 79 years; primary caregivers are often elderly with multiple medical conditions themselves and often struggle to provide the patient's care.[26, 27]
The main limitation of this study is that it is a retrospective observational study rather than a prospective randomized controlled trial. Many patients become dual eligible after requiring institutional custodial care, making the relationship between insurance status, discharge location, and readmissions complex and the causal relationship bidirectional. Patients discharged to hospice residence or to a nursing home with hospice services, who are more often dual eligible patients, are likely to receive more timely management of medical crises or changes in medical status, thus preventing readmission, whereas patients who receive home hospice with family providing the bulk of care may have a lower threshold for emergency room visits, possibly leading to greater incidence of readmission. Therefore, our results may be more a reflection of where the care is provided than what insurance the patient has. However, dual eligible patients discharged home also had a lower readmission rate compared with others, suggesting that insurance status has an independent association with readmission.
Unmeasured variables may explain the relationship between dual eligibility and 30‐day readmission rates. Some variables that we were not able to reliably measure in this study include functional status, number of hospitalizations in last year, patient educational level, patient self‐reported health status, quality of life, cognitive functioning, hearing or vision impairment, income, employment status, number of people in the home, and caregiver availability.[11, 12, 13, 19] However, omitting these variables from this study is more likely to bias our results toward the null, because these variables are likely related to dual eligibility and a higher, rather than lower, rate of readmission. We also did not measure whether participating decision makers were involved in the hospice admission or whether patients and families contacted their PCPs after discharge, variables found to be important in a previous pilot study.[5]
The generalizability of the results may be affected by the relative generosity of the New York State Medicaid benefits compared to many other states. New York State ranks third in the nation for eligibility and first for scope of services, including increased access to home‐ and community‐based services.[28] In addition, this study was a single‐center study in an urban, socioeconomically disadvantaged environment, explaining the higher rate of readmission compared to hospice patients nationally,[29] which is similar to other urban, academic medical centers.[5] For patients in our practice setting, the financial burden of paying privately for home care or residential custodial services is often prohibitive, which may not be the case in other settings.
Further research to identify whether discharge with hospice services mediates the relationship between insurance status and readmission could help confirm these findings. In addition, the relationship between caregiver burden and quality of life, and increased healthcare costs at the end of life should be explored. Overwhelming evidence suggests that being socioeconomically disadvantaged is a significant risk factor for early readmission, and enrolling these patients in Medicaid may modify this risk.[10, 30] Further research should explore whether policies that expand access to Medicaid or otherwise increase access to custodial care services can decrease burdensome hospital readmissions near the end of life.
Acknowledgements
The authors thank Galina Umanski for her technical support of this work.
Disclosure: This work was presented as a Power Point presentation on June 5, 2015 at the New York City Fellows' Palliative Care Research Day. The authors report no conflicts of interest.
- , . Hospice: comprehensive care at the end of life. Anest Clin N Am. 2006;24:181–204.
- . U.S. hospice benefits. J Pain Symptom Manage. 2009;38:105–109.
- , , , , . Inpatient palliative care consults and the probability of hospital readmission. Perm J. 2011;15:48–51.
- , , , . A hospice‐hospital partnership: reducing hospitalization costs and 30‐day readmissions among seriously ill adults. J Palliat Med. 2014;17:1005–1010.
- , , , , , . Rehospitalization of older adults discharged to home hospice care. J Palliat Med. 2014;17:841–844.
- , , , . Can palliative home care reduce 30‐day readmissions? Results of a propensity score matched cohort study. J Palliat Med. 2013;16:1290–1293.
- , , , , , . The Medicare Hospital Readmissions Reduction Program: potential unintended consequences for hospitals serving vulnerable populations. Health Serv Res. 2014;49:818–837.
- , , , . Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147:1219–1226.
- , , . Variations in 30‐day hospital readmission rates across primary care clinics within a tertiary referral center. J Hosp Med. 2014;9:688–694.
- , , , . Risk factors for early hospital readmission in low‐income elderly adults. J Am Geriatr Soc. 2014;62:489–494.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360:1418–1428.
- , , , , . Risk factors for 30‐day hospital readmission in patients >/=65 years of age. Proc (Bayl Univ Med Cent). 2008;21:363–372.
- , , , . Redefining readmission risk factors for general medicine patients. J Hosp Med. 2011;6:54–60.
- , , , . Racial/ethnic disparities in potentially preventable readmissions: the case of diabetes. Am J Public Health. 2005;95:1561–1567.
- , , . Thirty‐day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305:675–681.
- , , , et al. Factors associated with 30‐day readmission rates after percutaneous coronary intervention. Arch Intern Med. 2012;172:112–117.
- , , , . Risk factors for 30‐day readmission in general medical patients admitted from the emergency department: a single centre study. Intern Med J. 2012;42:677–682.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688–1698.
- , , , , , . Thirty‐day rehospitalizations after acute myocardial infarction: a cohort study. Ann Intern Med. 2012;157:11–18.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2009;25:211–219.
- , , , , , . An examination of adherence to pain medication plans in older cancer patients in hospice care. J Pain Symptom Manage. 2013;45:43–55.
- , , , et al. Hospice providers' key approaches to support informal caregivers in managing medications for patients in private residences. J Pain Symptom Manage. 2012;43:1060–1071.
- , . Hospice approach to palliative care, including Medicare hospice benefit. In: Yennurajalingam S, Bruera E, eds. Oxford American Handbook of Hospice and Palliative Medicine. New York, NY: Oxford University Press; 2011:229–239.
- , , . Predictors of thirty‐day readmission among hospitalized family medicine patients. J Am Board Fam Med. 2013;26:71–77.
- , , , , , . Emergency calls and need for emergency care in pateints looked after by a palliative care team: Retrospective interview study with bereaved relatives. BMC Palliat Care. 2008;7:11.
- , , , et al. Predictors of caregiver burden across the home‐based palliative care trajectory in Ontario, Canada [published online March 25, 2105]. Health Soc Care Community. doi: 10.1111/hsc.12219.
- , , , . Unique characteristics of informal hospice cancer caregiving. Support Care Cancer. 2015;23:2121–2128.
- , . Unsettling Scores: A Ranking of State Medicaid Programs. Washington, DC: Public Citizen Press; 2007.
- , , . 30‐day readmissions among seriously ill older adults. J Palliat Med. 2012;15:1–6.
- , . A path forward on Medicare readmissions. N Engl J Med. 2013;368:1175–1177.
Palliative care and hospice specialists consult on a variety of patients in the acute care setting that span all diagnoses and specialties. These include patients in the intensive care units, oncology units, as well as patients with end‐stage pulmonary, cardiac, and renal diseases. Discharge of these patients is often complicated by social issues, intensive personal care needs, and decreased functional status, as well as by the patient's insurance. Options for discharge disposition for patients accepting enrollment in hospice are often limited by financial constraints. Medicare pays for a set package of hospice benefits that do not include payment for room and board for hospice residential care and have a limited number of hours for a personal care attendant.[1] Hospice inpatient units are typically covered only for patients with acute care needs. Patients with secondary commercial insurance similarly find that custodial care benefits are often lacking, as most private and managed care plans mimic the Medicare hospice benefit.[2]
Palliative care inpatient consultation and palliative or hospice home care are associated with decreased 30‐day readmission rates.[3, 4, 5, 6] None of these studies, however, evaluated the effect of insurance status on readmission rates. Patients with dual coverage of Medicare and Medicaid are eligible for coverage of room and board (covered by Medicaid) in addition to the standard hospice benefit (covered by Medicare), and therefore have more options for discharge planning, including admission to a hospice residence, nursing home care with hospice services, or increased personal care attendant hours at home. Dual eligible patients (those with both Medicare and Medicaid) represent 20% of the Medicare population. They are generally poorer and with worse health status that those with Medicare alone; they have on average 25% more medical conditions than Medicare‐only patients.[7] Previous studies of readmissions and healthcare costs in the general population have found that dual eligible patients have higher rates of readmissions and higher overall healthcare costs compared with other groups.[7, 8, 9] However, these studies did not specifically look at patients near the end of life receiving hospice services. We hypothesize that dual eligible patients may actually have a lower rate of readmission at end of life compared with other groups, and that this effect may be partially mediated by discharge location (facility or home).
Previous studies have identified risk factors for 30‐day readmission to hospital, including living alone, insurance status, and poor or fair satisfaction with their primary care provider (PCP).[10] This study aims to evaluate, in the cohort of patients who have received a palliative care consultation during their hospital stay and who were discharged with hospice services, whether type of insurance is associated with risk of early readmission.
METHODS
Data were extracted from a replicate of Montefiore's Clinical Information System using healthcare surveillance software (Clinical Looking Glass; Emerging Health Information Technology, Yonkers, NY). We queried this database to find patients who received palliative care consultation from August 2010 to January 2014 at Montefiore Medical Center, an academic medical center in Bronx, NY, consisting of 3 general hospitals with 1491 beds. The medical center provides care to many underserved and minority patients and serves as the University Hospital of the Albert Einstein College of Medicine.
Inclusion Criteria
Patients who received a palliative care consultation were included if they were 65 years of age, nonpregnant, and admitted to the medical intensive care unit, any surgical intensive care unit, cardiac care unit, general medicine, surgery or surgical subspecialty service, family medicine, cardiology or oncology service, and discharged with hospice services.
Exclusion Criteria
Patients 65 years old and patients who died during the index admission were excluded, as were admissions to pediatrics, obstetrics, and psychology services, and uninsured patients.
The admission with the first palliative care consultation resulting in hospice referral was considered the index admission for these patients. Sociodemographic variables related to readmission such as age, race, gender, primary language, and socioeconomic status (SES) were examined.[11, 12, 13, 14, 15, 16] Clinical variables shown to be related to 30‐day readmissions in the literature including lab‐based acute physiology score (LAPS), blood urea nitrogen level (BUN), serum sodium level, serum albumin level, documentation of weight loss, and Charlson Comorbidity Index as well as its specific components were also extracted.[11, 13, 16, 17, 18, 19] Other variables related to the index admission such as length of stay for index admission, admission source on index admission (eg, from home, nursing home, other), and whether the primary care physician was listed in the chart were also examined.[11, 13, 16, 17, 19, 20] All of the variables were examined because they were hypothesized to be related to both insurance status and readmission. Markers of clinical severity, such as LAPS, BUN, hyponatremia, hypoalbuminemia, weight loss, and comorbidity could lead to readmission for symptom management or acute deterioration, and have been found be related to readmission in previous literature.
The predictor variable was insurance status at the time of index admission (dual eligible or all other). The main outcome variable was readmission to Montefiore Medical Center for any reason within 30 days of the index admission. Discharge location (hospice services in a facility vs home hospice) was examined as a potential mediator.
Statistical Analysis
Based on quality metrics available from our department, we expected to find at least 1000 patients 65 years of age seen by the palliative care consultation service with a discharge disposition including hospice services. This would give our study 85% power to detect a 10% difference in readmission rates between the 2 groups.
Patients were categorized as dual eligible if they were covered by Medicare and Medicaid only or if they were covered by Medicare, Medicaid, and private insurance. Controls were patients who were covered by Medicare only, Medicaid only, private insurance only, or Medicare and private insurance or Medicaid and private insurance. For the primary analysis, patients with and without dual eligibility were compared with respect to sociodemographic characteristics, healthcare process variables, and measures of comorbidity and illness severity using t tests for continuous variables and 2 tests for categorical variables. We used a 2 test to assess the univariable association between dual eligibility and 30‐day readmission. To address the question as to whether dual eligibility reduces the likelihood of a 30 day readmission, logistic regression was used to model 30‐day readmission by selecting from the covariates associated with the 30‐day readmissions at the 0.15 significance level. The Hosmer‐Lemeshow goodness of fit test was used to evaluate overall model performance.
For the secondary analysis, we assessed whether type and location of hospice services mediate the effect of insurance status on 30‐day readmissions using a Sobel‐Goodman test for mediation. Statistical analysis was conducted using statistical software (Stata statistical software, release 12; StataCorp, College Station, TX).
This research protocol was reviewed by the Montefiore Medical Center/Albert Einstein College of Medicine Institutional Review Board.
RESULTS
A total of 2755 inpatients were seen by the palliative care consultation service across the Montefiore Medical Center sites and discharged with hospice services. Of those, 1688 were dual eligible for Medicare and Medicaid, and 1067 were not. Specifically, 695 patients had Medicare only, 148 had private insurance only, 126 had Medicaid only, 78 had Medicare and private insurance, and 19 had Medicaid and private insurance. Univariable relationships between patient characteristics, insurance status, and readmission are shown in Table 1.
| Characteristic | Dual Eligible, N = 1,688 |
Not Dual Eligible, N = 1,067 |
P Value | 30‐Day Readmission | P Value | |
|---|---|---|---|---|---|---|
| Yes, N = 296 | No, N = 2,459 | |||||
| ||||||
| Sociodemographic | ||||||
| Age, y, mean SD | 81.6 9.0 | 79.4 8.9 | <0.05 | 77.8 8.8 | 81.1 9.0 | <0.05 |
| Female, n (%) | 1,092 (64.7) | 622 (58.3) | <0.05 | 171 (57.8) | 1,543 (62.7) | 0.095 |
| Has PCP, n (%) | 1,451 (86.0) | 951 (89.1) | <0.05 | 263 (88.9) | 2,139 (87.0) | NS |
| Speaks English, n (%) | 1,064 (63.0) | 728 (68.2) | <0.05 | 181 (61.1) | 1,611 (65.5) | 0.137 |
| SES, mean SD | 2.76 2.81 | 2.51 2.67 | <0.05 | 3.11 2.72 | 2.61 2.77 | <0.05 |
| Race/ethnicity | <0.05 | <0.05 | ||||
| Hispanic, n (%) | 587 (34.8) | 267 (31.3) | 100 (33.8) | 754 (30.7) | ||
| White, n (%) | 532 (31.5) | 290 (27.2) | 58 (19.6) | 764 (31.1) | ||
| Black, n (%) | 449 (26.6) | 420 (39.4) | 121 (40.9) | 748 (30.4) | ||
| Comorbidities, n (%) | ||||||
| Congestive heart failure | 555 (32.9) | 264 (24.7) | <0.05 | 104 (35.1) | 751 (30.5) | 0.106 |
| Cardiac valvular disease | 179 (10.6) | 76 (7.1) | <0.05 | 19 (6.4) | 227 (9.2) | 0.109 |
| Myocardial infarction | 165 (9.8) | 85 (8.0) | 0.11 | 31 (10.5) | 219 (8.9) | NS |
| Pulmonary disease | 480 (28.4) | 292 (27.4) | NS | 98 (33.1) | 674 (27.4) | 0.039 |
| Liver disease | 60 (3.6) | 54 (5.1) | 0.053 | 22 (7.4) | 92 (3.7) | <0.05 |
| Dementia | 135 (8.0) | 52 (4.9) | <0.05 | 11 (3.7) | 176 (7.2) | 0.026 |
| Diabetes, complicated | 125 (7.4) | 52 (4.9) | <0.05 | 15 (5.1) | 163 (6.6) | NS |
| Malignancy | 589 (34.9) | 499 (46.8) | <0.05 | 124 (41.9) | 921 (37.5) | 0.137 |
| Renal disease | 394 (23.3) | 225 (21.1) | NS | 72 (24.3) | 547 (22.2) | NS |
| Depression | 174 (10.3) | 85 (8.0) | <0.05 | 25 (8.4) | 234 (9.5) | NS |
| Peripheral vascular disease | 166 (9.8) | 72 (6.7) | <0.05 | 16 (5.4) | 222 (9.0) | 0.036 |
| Cerebrovascular disease | 282 (16.7) | 125 (11.7) | <0.05 | 33 (11.1) | 374 (15.2) | 0.063 |
| Clinical characteristics | ||||||
| LOS, mean SD | 10.9 9.93 | 10.6 9.61 | 0.19 | 9.3 8.0 | 10.9 10.0 | <0.05 |
| LAPS, mean SD | 38.4 27.9 | 34.6 26.9 | <0.05 | 33.8 25.2 | 37.3 27.8 | 0.039 |
| BUN, mean SD | 34.4 32.3 | 30.9 28.3 | <0.05 | 29.5 24.4 | 33.4 31.6 | 0.036 |
| Charlson score, mean SD | 4.62 3.37 | 5.28 3.56 | <0.05 | 5.1 3.5 | 4.8 3.5 | 0.152 |
In this sample, 9.2% of patients in the dual eligible group were readmitted within 30 days compared with 13.1% of others (2 = 10.3, P = 0.001). Of the total cohort, 1500 patients, including 862 dual eligible patients, were discharged to a facility, and 1255 patients, including 826 dual eligible patients, were discharged home. Dual eligible patients had a lower readmission rate compared with others in both settings (Figure 1). In univariable analysis, gender, age, hospital length of stay, race/ethnicity, SES, English as a primary language, LAPS, BUN, Charlson score, and comorbid peripheral vascular disease, cerebrovascular disease, heart disease, dementia, cancer, and liver disease were found to be related both to the predictor and the outcome variables and were included in the logistic regression model. While controlling for these variables, dual eligible patients had a lower odds of readmission within 30 days compared with others (odds ratio [OR]: 0.77; P = 0.041; 95% confidence interval [CI]: 0.59‐0.98) (Table 2). The Hosmer‐Lemeshow test was not significant, indicating that the overall model fit was good.
| Independent Variable | Odds Ratio | Standard Error | z Ratio | P Value |
|---|---|---|---|---|
| Dual eligibility | 0.77 | 0.10 | 2.05 | 0.041 |
| Gender | 1.16 | 0.15 | 1.17 | 0.244 |
| Age | 0.96 | 0.01 | 4.54 | 0.000 |
| Hospital length of stay | 0.97 | 0.01 | 3.33 | 0.001 |
| Black | 1.93 | 0.53 | 2.37 | 0.018 |
| White | 1.02 | 0.30 | 0.08 | 0.939 |
| Hispanic | 1.29 | 0.37 | 0.90 | 0.368 |
| Socioeconomic status | 0.96 | 0.02 | 1.63 | 0.103 |
| Primary language English | 0.81 | 0.12 | 1.43 | 0.154 |
| Peripheral vascular disease | 0.67 | 0.18 | 1.48 | 0.139 |
| Cerebrovascular disease | 0.86 | 0.17 | 0.73 | 0.465 |
| Dementia | 0.61 | 0.20 | 1.50 | 0.135 |
| Congestive heart failure | 1.75 | 0.26 | 3.83 | 0.000 |
| Cardiac valvular disease | 0.73 | 0.19 | 1.23 | 0.219 |
| Cancer | 0.92 | 0.15 | 0.51 | 0.608 |
| Liver disease | 1.80 | 0.47 | 2.25 | 0.024 |
| Lab‐based acute physiology score | 1.00 | 0.00 | 0.66 | 0.510 |
| Blood urea nitrogen | 1.00 | 0.00 | 1.29 | 0.197 |
| Charlson comorbidity score | 0.99 | 0.02 | 0.57 | 0.567 |

In the secondary analysis, we found that disposition (hospice services in a nursing home or hospice residence vs home hospice) partially mediates the relationship between insurance status and readmission, explaining 30% of the total effect (z = 5.06, P < 0.001). When accounting for disposition as a mediator, dual eligible patients still had a lower odds of readmission within 30 days compared with others, although the difference was no longer statistically significant (OR: 0.86; P = 0.24; 95% CI: 0.66‐ 1.11). Patients discharged with hospice services in a nursing home or hospice residence were less likely to be readmitted within 30 days (OR: 0.41; P < 0.001; 95% CI: 0.31‐0.54) (Table 3).
| Independent Variable | Odds Ratio | Standard Error | z Ratio | P Value |
|---|---|---|---|---|
| Dual eligibility | 0.86 | 0.11 | 1.17 | 0.244 |
| Discharge location | 0.40 | 0.59 | 6.22 | 0.000 |
| Gender | 1.17 | 0.16 | 1.22 | 0.223 |
| Age | 0.96 | 0.01 | 4.69 | 0.000 |
| Hospital length of stay | 0.98 | 0.01 | 2.57 | 0.010 |
| Black | 1.95 | 0.54 | 2.39 | 0.017 |
| White | 1.02 | 0.30 | 0.10 | 0.924 |
| Hispanic | 1.20 | 0.35 | 0.63 | 0.526 |
| Socioeconomic status | 0.96 | 0.02 | 1.51 | 0.132 |
| Primary language English | 0.78 | 0.11 | 1.69 | 0.090 |
| Peripheral vascular disease | 0.70 | 0.19 | 1.31 | 0.190 |
| Cerebrovascular disease | 0.89 | 0.18 | 0.56 | 0.579 |
| Dementia | 0.64 | 0.21 | 1.36 | 0.174 |
| Congestive heart failure | 1.75 | 0.26 | 3.80 | 0.000 |
| Cardiac valvular disease | 0.70 | 0.18 | 1.35 | 0.176 |
| Cancer | 0.91 | 0.15 | 0.59 | 0.552 |
| Liver disease | 1.75 | 0.46 | 2.12 | 0.034 |
| Lab‐based acute physiology score | 1.00 | 0.00 | 0.20 | 0.843 |
| Blood urea nitrogen | 1.00 | 0.00 | 1.10 | 0.270 |
| Charlson comorbidity score | 0.99 | 0.02 | 0.65 | 0.516 |
DISCUSSION
This study showed an association between dual coverage and lower odds of 30‐day readmission for patients discharged to hospice compared to all other insurance categories, excluding uninsured. This is the first study to date looking specifically at the association between insurance and readmission rates of patients discharged with hospice services. This association was attenuated, and no longer statistically significant, when accounting for discharge location.
These findings suggest that the added services available to patients enrolled in Medicare and Medicaid likely provide an enhanced level of postacute care. Patients with Medicaid have access to increased hours of personal care attendants as well as residential care, which often provides 24‐hour trained staff for rapid assessment of a change in clinical status and adjustment to therapeutic management. Combined with the Medicare hospice benefit, which provides better attention to symptom management, better supervision, and improved compliance with medications, as well as education of family and caregivers,[21, 22, 23] additional coverage with Medicaid is associated with a decrease in early readmission to the hospital.
It is often a financial hardship for family members or caregivers to take time off work to care for a dying patient. Without adequate postdischarge resources, the hospital to home transition will be ineffective, which has been shown to increase readmissions.[24] The option of increased attendant hours or residential care can have a positive impact on the financial and psychosocial stressors of caring for a family member at the end of life. Although we did not assess for this in our study, caregiver burnout often plays a role in emergency room visits and admissions of patients at the end of life.[25] The average age of the patients in our cohorts was 81 and 79 years; primary caregivers are often elderly with multiple medical conditions themselves and often struggle to provide the patient's care.[26, 27]
The main limitation of this study is that it is a retrospective observational study rather than a prospective randomized controlled trial. Many patients become dual eligible after requiring institutional custodial care, making the relationship between insurance status, discharge location, and readmissions complex and the causal relationship bidirectional. Patients discharged to hospice residence or to a nursing home with hospice services, who are more often dual eligible patients, are likely to receive more timely management of medical crises or changes in medical status, thus preventing readmission, whereas patients who receive home hospice with family providing the bulk of care may have a lower threshold for emergency room visits, possibly leading to greater incidence of readmission. Therefore, our results may be more a reflection of where the care is provided than what insurance the patient has. However, dual eligible patients discharged home also had a lower readmission rate compared with others, suggesting that insurance status has an independent association with readmission.
Unmeasured variables may explain the relationship between dual eligibility and 30‐day readmission rates. Some variables that we were not able to reliably measure in this study include functional status, number of hospitalizations in last year, patient educational level, patient self‐reported health status, quality of life, cognitive functioning, hearing or vision impairment, income, employment status, number of people in the home, and caregiver availability.[11, 12, 13, 19] However, omitting these variables from this study is more likely to bias our results toward the null, because these variables are likely related to dual eligibility and a higher, rather than lower, rate of readmission. We also did not measure whether participating decision makers were involved in the hospice admission or whether patients and families contacted their PCPs after discharge, variables found to be important in a previous pilot study.[5]
The generalizability of the results may be affected by the relative generosity of the New York State Medicaid benefits compared to many other states. New York State ranks third in the nation for eligibility and first for scope of services, including increased access to home‐ and community‐based services.[28] In addition, this study was a single‐center study in an urban, socioeconomically disadvantaged environment, explaining the higher rate of readmission compared to hospice patients nationally,[29] which is similar to other urban, academic medical centers.[5] For patients in our practice setting, the financial burden of paying privately for home care or residential custodial services is often prohibitive, which may not be the case in other settings.
Further research to identify whether discharge with hospice services mediates the relationship between insurance status and readmission could help confirm these findings. In addition, the relationship between caregiver burden and quality of life, and increased healthcare costs at the end of life should be explored. Overwhelming evidence suggests that being socioeconomically disadvantaged is a significant risk factor for early readmission, and enrolling these patients in Medicaid may modify this risk.[10, 30] Further research should explore whether policies that expand access to Medicaid or otherwise increase access to custodial care services can decrease burdensome hospital readmissions near the end of life.
Acknowledgements
The authors thank Galina Umanski for her technical support of this work.
Disclosure: This work was presented as a Power Point presentation on June 5, 2015 at the New York City Fellows' Palliative Care Research Day. The authors report no conflicts of interest.
Palliative care and hospice specialists consult on a variety of patients in the acute care setting that span all diagnoses and specialties. These include patients in the intensive care units, oncology units, as well as patients with end‐stage pulmonary, cardiac, and renal diseases. Discharge of these patients is often complicated by social issues, intensive personal care needs, and decreased functional status, as well as by the patient's insurance. Options for discharge disposition for patients accepting enrollment in hospice are often limited by financial constraints. Medicare pays for a set package of hospice benefits that do not include payment for room and board for hospice residential care and have a limited number of hours for a personal care attendant.[1] Hospice inpatient units are typically covered only for patients with acute care needs. Patients with secondary commercial insurance similarly find that custodial care benefits are often lacking, as most private and managed care plans mimic the Medicare hospice benefit.[2]
Palliative care inpatient consultation and palliative or hospice home care are associated with decreased 30‐day readmission rates.[3, 4, 5, 6] None of these studies, however, evaluated the effect of insurance status on readmission rates. Patients with dual coverage of Medicare and Medicaid are eligible for coverage of room and board (covered by Medicaid) in addition to the standard hospice benefit (covered by Medicare), and therefore have more options for discharge planning, including admission to a hospice residence, nursing home care with hospice services, or increased personal care attendant hours at home. Dual eligible patients (those with both Medicare and Medicaid) represent 20% of the Medicare population. They are generally poorer and with worse health status that those with Medicare alone; they have on average 25% more medical conditions than Medicare‐only patients.[7] Previous studies of readmissions and healthcare costs in the general population have found that dual eligible patients have higher rates of readmissions and higher overall healthcare costs compared with other groups.[7, 8, 9] However, these studies did not specifically look at patients near the end of life receiving hospice services. We hypothesize that dual eligible patients may actually have a lower rate of readmission at end of life compared with other groups, and that this effect may be partially mediated by discharge location (facility or home).
Previous studies have identified risk factors for 30‐day readmission to hospital, including living alone, insurance status, and poor or fair satisfaction with their primary care provider (PCP).[10] This study aims to evaluate, in the cohort of patients who have received a palliative care consultation during their hospital stay and who were discharged with hospice services, whether type of insurance is associated with risk of early readmission.
METHODS
Data were extracted from a replicate of Montefiore's Clinical Information System using healthcare surveillance software (Clinical Looking Glass; Emerging Health Information Technology, Yonkers, NY). We queried this database to find patients who received palliative care consultation from August 2010 to January 2014 at Montefiore Medical Center, an academic medical center in Bronx, NY, consisting of 3 general hospitals with 1491 beds. The medical center provides care to many underserved and minority patients and serves as the University Hospital of the Albert Einstein College of Medicine.
Inclusion Criteria
Patients who received a palliative care consultation were included if they were 65 years of age, nonpregnant, and admitted to the medical intensive care unit, any surgical intensive care unit, cardiac care unit, general medicine, surgery or surgical subspecialty service, family medicine, cardiology or oncology service, and discharged with hospice services.
Exclusion Criteria
Patients 65 years old and patients who died during the index admission were excluded, as were admissions to pediatrics, obstetrics, and psychology services, and uninsured patients.
The admission with the first palliative care consultation resulting in hospice referral was considered the index admission for these patients. Sociodemographic variables related to readmission such as age, race, gender, primary language, and socioeconomic status (SES) were examined.[11, 12, 13, 14, 15, 16] Clinical variables shown to be related to 30‐day readmissions in the literature including lab‐based acute physiology score (LAPS), blood urea nitrogen level (BUN), serum sodium level, serum albumin level, documentation of weight loss, and Charlson Comorbidity Index as well as its specific components were also extracted.[11, 13, 16, 17, 18, 19] Other variables related to the index admission such as length of stay for index admission, admission source on index admission (eg, from home, nursing home, other), and whether the primary care physician was listed in the chart were also examined.[11, 13, 16, 17, 19, 20] All of the variables were examined because they were hypothesized to be related to both insurance status and readmission. Markers of clinical severity, such as LAPS, BUN, hyponatremia, hypoalbuminemia, weight loss, and comorbidity could lead to readmission for symptom management or acute deterioration, and have been found be related to readmission in previous literature.
The predictor variable was insurance status at the time of index admission (dual eligible or all other). The main outcome variable was readmission to Montefiore Medical Center for any reason within 30 days of the index admission. Discharge location (hospice services in a facility vs home hospice) was examined as a potential mediator.
Statistical Analysis
Based on quality metrics available from our department, we expected to find at least 1000 patients 65 years of age seen by the palliative care consultation service with a discharge disposition including hospice services. This would give our study 85% power to detect a 10% difference in readmission rates between the 2 groups.
Patients were categorized as dual eligible if they were covered by Medicare and Medicaid only or if they were covered by Medicare, Medicaid, and private insurance. Controls were patients who were covered by Medicare only, Medicaid only, private insurance only, or Medicare and private insurance or Medicaid and private insurance. For the primary analysis, patients with and without dual eligibility were compared with respect to sociodemographic characteristics, healthcare process variables, and measures of comorbidity and illness severity using t tests for continuous variables and 2 tests for categorical variables. We used a 2 test to assess the univariable association between dual eligibility and 30‐day readmission. To address the question as to whether dual eligibility reduces the likelihood of a 30 day readmission, logistic regression was used to model 30‐day readmission by selecting from the covariates associated with the 30‐day readmissions at the 0.15 significance level. The Hosmer‐Lemeshow goodness of fit test was used to evaluate overall model performance.
For the secondary analysis, we assessed whether type and location of hospice services mediate the effect of insurance status on 30‐day readmissions using a Sobel‐Goodman test for mediation. Statistical analysis was conducted using statistical software (Stata statistical software, release 12; StataCorp, College Station, TX).
This research protocol was reviewed by the Montefiore Medical Center/Albert Einstein College of Medicine Institutional Review Board.
RESULTS
A total of 2755 inpatients were seen by the palliative care consultation service across the Montefiore Medical Center sites and discharged with hospice services. Of those, 1688 were dual eligible for Medicare and Medicaid, and 1067 were not. Specifically, 695 patients had Medicare only, 148 had private insurance only, 126 had Medicaid only, 78 had Medicare and private insurance, and 19 had Medicaid and private insurance. Univariable relationships between patient characteristics, insurance status, and readmission are shown in Table 1.
| Characteristic | Dual Eligible, N = 1,688 |
Not Dual Eligible, N = 1,067 |
P Value | 30‐Day Readmission | P Value | |
|---|---|---|---|---|---|---|
| Yes, N = 296 | No, N = 2,459 | |||||
| ||||||
| Sociodemographic | ||||||
| Age, y, mean SD | 81.6 9.0 | 79.4 8.9 | <0.05 | 77.8 8.8 | 81.1 9.0 | <0.05 |
| Female, n (%) | 1,092 (64.7) | 622 (58.3) | <0.05 | 171 (57.8) | 1,543 (62.7) | 0.095 |
| Has PCP, n (%) | 1,451 (86.0) | 951 (89.1) | <0.05 | 263 (88.9) | 2,139 (87.0) | NS |
| Speaks English, n (%) | 1,064 (63.0) | 728 (68.2) | <0.05 | 181 (61.1) | 1,611 (65.5) | 0.137 |
| SES, mean SD | 2.76 2.81 | 2.51 2.67 | <0.05 | 3.11 2.72 | 2.61 2.77 | <0.05 |
| Race/ethnicity | <0.05 | <0.05 | ||||
| Hispanic, n (%) | 587 (34.8) | 267 (31.3) | 100 (33.8) | 754 (30.7) | ||
| White, n (%) | 532 (31.5) | 290 (27.2) | 58 (19.6) | 764 (31.1) | ||
| Black, n (%) | 449 (26.6) | 420 (39.4) | 121 (40.9) | 748 (30.4) | ||
| Comorbidities, n (%) | ||||||
| Congestive heart failure | 555 (32.9) | 264 (24.7) | <0.05 | 104 (35.1) | 751 (30.5) | 0.106 |
| Cardiac valvular disease | 179 (10.6) | 76 (7.1) | <0.05 | 19 (6.4) | 227 (9.2) | 0.109 |
| Myocardial infarction | 165 (9.8) | 85 (8.0) | 0.11 | 31 (10.5) | 219 (8.9) | NS |
| Pulmonary disease | 480 (28.4) | 292 (27.4) | NS | 98 (33.1) | 674 (27.4) | 0.039 |
| Liver disease | 60 (3.6) | 54 (5.1) | 0.053 | 22 (7.4) | 92 (3.7) | <0.05 |
| Dementia | 135 (8.0) | 52 (4.9) | <0.05 | 11 (3.7) | 176 (7.2) | 0.026 |
| Diabetes, complicated | 125 (7.4) | 52 (4.9) | <0.05 | 15 (5.1) | 163 (6.6) | NS |
| Malignancy | 589 (34.9) | 499 (46.8) | <0.05 | 124 (41.9) | 921 (37.5) | 0.137 |
| Renal disease | 394 (23.3) | 225 (21.1) | NS | 72 (24.3) | 547 (22.2) | NS |
| Depression | 174 (10.3) | 85 (8.0) | <0.05 | 25 (8.4) | 234 (9.5) | NS |
| Peripheral vascular disease | 166 (9.8) | 72 (6.7) | <0.05 | 16 (5.4) | 222 (9.0) | 0.036 |
| Cerebrovascular disease | 282 (16.7) | 125 (11.7) | <0.05 | 33 (11.1) | 374 (15.2) | 0.063 |
| Clinical characteristics | ||||||
| LOS, mean SD | 10.9 9.93 | 10.6 9.61 | 0.19 | 9.3 8.0 | 10.9 10.0 | <0.05 |
| LAPS, mean SD | 38.4 27.9 | 34.6 26.9 | <0.05 | 33.8 25.2 | 37.3 27.8 | 0.039 |
| BUN, mean SD | 34.4 32.3 | 30.9 28.3 | <0.05 | 29.5 24.4 | 33.4 31.6 | 0.036 |
| Charlson score, mean SD | 4.62 3.37 | 5.28 3.56 | <0.05 | 5.1 3.5 | 4.8 3.5 | 0.152 |
In this sample, 9.2% of patients in the dual eligible group were readmitted within 30 days compared with 13.1% of others (2 = 10.3, P = 0.001). Of the total cohort, 1500 patients, including 862 dual eligible patients, were discharged to a facility, and 1255 patients, including 826 dual eligible patients, were discharged home. Dual eligible patients had a lower readmission rate compared with others in both settings (Figure 1). In univariable analysis, gender, age, hospital length of stay, race/ethnicity, SES, English as a primary language, LAPS, BUN, Charlson score, and comorbid peripheral vascular disease, cerebrovascular disease, heart disease, dementia, cancer, and liver disease were found to be related both to the predictor and the outcome variables and were included in the logistic regression model. While controlling for these variables, dual eligible patients had a lower odds of readmission within 30 days compared with others (odds ratio [OR]: 0.77; P = 0.041; 95% confidence interval [CI]: 0.59‐0.98) (Table 2). The Hosmer‐Lemeshow test was not significant, indicating that the overall model fit was good.
| Independent Variable | Odds Ratio | Standard Error | z Ratio | P Value |
|---|---|---|---|---|
| Dual eligibility | 0.77 | 0.10 | 2.05 | 0.041 |
| Gender | 1.16 | 0.15 | 1.17 | 0.244 |
| Age | 0.96 | 0.01 | 4.54 | 0.000 |
| Hospital length of stay | 0.97 | 0.01 | 3.33 | 0.001 |
| Black | 1.93 | 0.53 | 2.37 | 0.018 |
| White | 1.02 | 0.30 | 0.08 | 0.939 |
| Hispanic | 1.29 | 0.37 | 0.90 | 0.368 |
| Socioeconomic status | 0.96 | 0.02 | 1.63 | 0.103 |
| Primary language English | 0.81 | 0.12 | 1.43 | 0.154 |
| Peripheral vascular disease | 0.67 | 0.18 | 1.48 | 0.139 |
| Cerebrovascular disease | 0.86 | 0.17 | 0.73 | 0.465 |
| Dementia | 0.61 | 0.20 | 1.50 | 0.135 |
| Congestive heart failure | 1.75 | 0.26 | 3.83 | 0.000 |
| Cardiac valvular disease | 0.73 | 0.19 | 1.23 | 0.219 |
| Cancer | 0.92 | 0.15 | 0.51 | 0.608 |
| Liver disease | 1.80 | 0.47 | 2.25 | 0.024 |
| Lab‐based acute physiology score | 1.00 | 0.00 | 0.66 | 0.510 |
| Blood urea nitrogen | 1.00 | 0.00 | 1.29 | 0.197 |
| Charlson comorbidity score | 0.99 | 0.02 | 0.57 | 0.567 |

In the secondary analysis, we found that disposition (hospice services in a nursing home or hospice residence vs home hospice) partially mediates the relationship between insurance status and readmission, explaining 30% of the total effect (z = 5.06, P < 0.001). When accounting for disposition as a mediator, dual eligible patients still had a lower odds of readmission within 30 days compared with others, although the difference was no longer statistically significant (OR: 0.86; P = 0.24; 95% CI: 0.66‐ 1.11). Patients discharged with hospice services in a nursing home or hospice residence were less likely to be readmitted within 30 days (OR: 0.41; P < 0.001; 95% CI: 0.31‐0.54) (Table 3).
| Independent Variable | Odds Ratio | Standard Error | z Ratio | P Value |
|---|---|---|---|---|
| Dual eligibility | 0.86 | 0.11 | 1.17 | 0.244 |
| Discharge location | 0.40 | 0.59 | 6.22 | 0.000 |
| Gender | 1.17 | 0.16 | 1.22 | 0.223 |
| Age | 0.96 | 0.01 | 4.69 | 0.000 |
| Hospital length of stay | 0.98 | 0.01 | 2.57 | 0.010 |
| Black | 1.95 | 0.54 | 2.39 | 0.017 |
| White | 1.02 | 0.30 | 0.10 | 0.924 |
| Hispanic | 1.20 | 0.35 | 0.63 | 0.526 |
| Socioeconomic status | 0.96 | 0.02 | 1.51 | 0.132 |
| Primary language English | 0.78 | 0.11 | 1.69 | 0.090 |
| Peripheral vascular disease | 0.70 | 0.19 | 1.31 | 0.190 |
| Cerebrovascular disease | 0.89 | 0.18 | 0.56 | 0.579 |
| Dementia | 0.64 | 0.21 | 1.36 | 0.174 |
| Congestive heart failure | 1.75 | 0.26 | 3.80 | 0.000 |
| Cardiac valvular disease | 0.70 | 0.18 | 1.35 | 0.176 |
| Cancer | 0.91 | 0.15 | 0.59 | 0.552 |
| Liver disease | 1.75 | 0.46 | 2.12 | 0.034 |
| Lab‐based acute physiology score | 1.00 | 0.00 | 0.20 | 0.843 |
| Blood urea nitrogen | 1.00 | 0.00 | 1.10 | 0.270 |
| Charlson comorbidity score | 0.99 | 0.02 | 0.65 | 0.516 |
DISCUSSION
This study showed an association between dual coverage and lower odds of 30‐day readmission for patients discharged to hospice compared to all other insurance categories, excluding uninsured. This is the first study to date looking specifically at the association between insurance and readmission rates of patients discharged with hospice services. This association was attenuated, and no longer statistically significant, when accounting for discharge location.
These findings suggest that the added services available to patients enrolled in Medicare and Medicaid likely provide an enhanced level of postacute care. Patients with Medicaid have access to increased hours of personal care attendants as well as residential care, which often provides 24‐hour trained staff for rapid assessment of a change in clinical status and adjustment to therapeutic management. Combined with the Medicare hospice benefit, which provides better attention to symptom management, better supervision, and improved compliance with medications, as well as education of family and caregivers,[21, 22, 23] additional coverage with Medicaid is associated with a decrease in early readmission to the hospital.
It is often a financial hardship for family members or caregivers to take time off work to care for a dying patient. Without adequate postdischarge resources, the hospital to home transition will be ineffective, which has been shown to increase readmissions.[24] The option of increased attendant hours or residential care can have a positive impact on the financial and psychosocial stressors of caring for a family member at the end of life. Although we did not assess for this in our study, caregiver burnout often plays a role in emergency room visits and admissions of patients at the end of life.[25] The average age of the patients in our cohorts was 81 and 79 years; primary caregivers are often elderly with multiple medical conditions themselves and often struggle to provide the patient's care.[26, 27]
The main limitation of this study is that it is a retrospective observational study rather than a prospective randomized controlled trial. Many patients become dual eligible after requiring institutional custodial care, making the relationship between insurance status, discharge location, and readmissions complex and the causal relationship bidirectional. Patients discharged to hospice residence or to a nursing home with hospice services, who are more often dual eligible patients, are likely to receive more timely management of medical crises or changes in medical status, thus preventing readmission, whereas patients who receive home hospice with family providing the bulk of care may have a lower threshold for emergency room visits, possibly leading to greater incidence of readmission. Therefore, our results may be more a reflection of where the care is provided than what insurance the patient has. However, dual eligible patients discharged home also had a lower readmission rate compared with others, suggesting that insurance status has an independent association with readmission.
Unmeasured variables may explain the relationship between dual eligibility and 30‐day readmission rates. Some variables that we were not able to reliably measure in this study include functional status, number of hospitalizations in last year, patient educational level, patient self‐reported health status, quality of life, cognitive functioning, hearing or vision impairment, income, employment status, number of people in the home, and caregiver availability.[11, 12, 13, 19] However, omitting these variables from this study is more likely to bias our results toward the null, because these variables are likely related to dual eligibility and a higher, rather than lower, rate of readmission. We also did not measure whether participating decision makers were involved in the hospice admission or whether patients and families contacted their PCPs after discharge, variables found to be important in a previous pilot study.[5]
The generalizability of the results may be affected by the relative generosity of the New York State Medicaid benefits compared to many other states. New York State ranks third in the nation for eligibility and first for scope of services, including increased access to home‐ and community‐based services.[28] In addition, this study was a single‐center study in an urban, socioeconomically disadvantaged environment, explaining the higher rate of readmission compared to hospice patients nationally,[29] which is similar to other urban, academic medical centers.[5] For patients in our practice setting, the financial burden of paying privately for home care or residential custodial services is often prohibitive, which may not be the case in other settings.
Further research to identify whether discharge with hospice services mediates the relationship between insurance status and readmission could help confirm these findings. In addition, the relationship between caregiver burden and quality of life, and increased healthcare costs at the end of life should be explored. Overwhelming evidence suggests that being socioeconomically disadvantaged is a significant risk factor for early readmission, and enrolling these patients in Medicaid may modify this risk.[10, 30] Further research should explore whether policies that expand access to Medicaid or otherwise increase access to custodial care services can decrease burdensome hospital readmissions near the end of life.
Acknowledgements
The authors thank Galina Umanski for her technical support of this work.
Disclosure: This work was presented as a Power Point presentation on June 5, 2015 at the New York City Fellows' Palliative Care Research Day. The authors report no conflicts of interest.
- , . Hospice: comprehensive care at the end of life. Anest Clin N Am. 2006;24:181–204.
- . U.S. hospice benefits. J Pain Symptom Manage. 2009;38:105–109.
- , , , , . Inpatient palliative care consults and the probability of hospital readmission. Perm J. 2011;15:48–51.
- , , , . A hospice‐hospital partnership: reducing hospitalization costs and 30‐day readmissions among seriously ill adults. J Palliat Med. 2014;17:1005–1010.
- , , , , , . Rehospitalization of older adults discharged to home hospice care. J Palliat Med. 2014;17:841–844.
- , , , . Can palliative home care reduce 30‐day readmissions? Results of a propensity score matched cohort study. J Palliat Med. 2013;16:1290–1293.
- , , , , , . The Medicare Hospital Readmissions Reduction Program: potential unintended consequences for hospitals serving vulnerable populations. Health Serv Res. 2014;49:818–837.
- , , , . Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147:1219–1226.
- , , . Variations in 30‐day hospital readmission rates across primary care clinics within a tertiary referral center. J Hosp Med. 2014;9:688–694.
- , , , . Risk factors for early hospital readmission in low‐income elderly adults. J Am Geriatr Soc. 2014;62:489–494.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360:1418–1428.
- , , , , . Risk factors for 30‐day hospital readmission in patients >/=65 years of age. Proc (Bayl Univ Med Cent). 2008;21:363–372.
- , , , . Redefining readmission risk factors for general medicine patients. J Hosp Med. 2011;6:54–60.
- , , , . Racial/ethnic disparities in potentially preventable readmissions: the case of diabetes. Am J Public Health. 2005;95:1561–1567.
- , , . Thirty‐day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305:675–681.
- , , , et al. Factors associated with 30‐day readmission rates after percutaneous coronary intervention. Arch Intern Med. 2012;172:112–117.
- , , , . Risk factors for 30‐day readmission in general medical patients admitted from the emergency department: a single centre study. Intern Med J. 2012;42:677–682.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688–1698.
- , , , , , . Thirty‐day rehospitalizations after acute myocardial infarction: a cohort study. Ann Intern Med. 2012;157:11–18.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2009;25:211–219.
- , , , , , . An examination of adherence to pain medication plans in older cancer patients in hospice care. J Pain Symptom Manage. 2013;45:43–55.
- , , , et al. Hospice providers' key approaches to support informal caregivers in managing medications for patients in private residences. J Pain Symptom Manage. 2012;43:1060–1071.
- , . Hospice approach to palliative care, including Medicare hospice benefit. In: Yennurajalingam S, Bruera E, eds. Oxford American Handbook of Hospice and Palliative Medicine. New York, NY: Oxford University Press; 2011:229–239.
- , , . Predictors of thirty‐day readmission among hospitalized family medicine patients. J Am Board Fam Med. 2013;26:71–77.
- , , , , , . Emergency calls and need for emergency care in pateints looked after by a palliative care team: Retrospective interview study with bereaved relatives. BMC Palliat Care. 2008;7:11.
- , , , et al. Predictors of caregiver burden across the home‐based palliative care trajectory in Ontario, Canada [published online March 25, 2105]. Health Soc Care Community. doi: 10.1111/hsc.12219.
- , , , . Unique characteristics of informal hospice cancer caregiving. Support Care Cancer. 2015;23:2121–2128.
- , . Unsettling Scores: A Ranking of State Medicaid Programs. Washington, DC: Public Citizen Press; 2007.
- , , . 30‐day readmissions among seriously ill older adults. J Palliat Med. 2012;15:1–6.
- , . A path forward on Medicare readmissions. N Engl J Med. 2013;368:1175–1177.
- , . Hospice: comprehensive care at the end of life. Anest Clin N Am. 2006;24:181–204.
- . U.S. hospice benefits. J Pain Symptom Manage. 2009;38:105–109.
- , , , , . Inpatient palliative care consults and the probability of hospital readmission. Perm J. 2011;15:48–51.
- , , , . A hospice‐hospital partnership: reducing hospitalization costs and 30‐day readmissions among seriously ill adults. J Palliat Med. 2014;17:1005–1010.
- , , , , , . Rehospitalization of older adults discharged to home hospice care. J Palliat Med. 2014;17:841–844.
- , , , . Can palliative home care reduce 30‐day readmissions? Results of a propensity score matched cohort study. J Palliat Med. 2013;16:1290–1293.
- , , , , , . The Medicare Hospital Readmissions Reduction Program: potential unintended consequences for hospitals serving vulnerable populations. Health Serv Res. 2014;49:818–837.
- , , , . Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147:1219–1226.
- , , . Variations in 30‐day hospital readmission rates across primary care clinics within a tertiary referral center. J Hosp Med. 2014;9:688–694.
- , , , . Risk factors for early hospital readmission in low‐income elderly adults. J Am Geriatr Soc. 2014;62:489–494.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360:1418–1428.
- , , , , . Risk factors for 30‐day hospital readmission in patients >/=65 years of age. Proc (Bayl Univ Med Cent). 2008;21:363–372.
- , , , . Redefining readmission risk factors for general medicine patients. J Hosp Med. 2011;6:54–60.
- , , , . Racial/ethnic disparities in potentially preventable readmissions: the case of diabetes. Am J Public Health. 2005;95:1561–1567.
- , , . Thirty‐day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305:675–681.
- , , , et al. Factors associated with 30‐day readmission rates after percutaneous coronary intervention. Arch Intern Med. 2012;172:112–117.
- , , , . Risk factors for 30‐day readmission in general medical patients admitted from the emergency department: a single centre study. Intern Med J. 2012;42:677–682.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688–1698.
- , , , , , . Thirty‐day rehospitalizations after acute myocardial infarction: a cohort study. Ann Intern Med. 2012;157:11–18.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2009;25:211–219.
- , , , , , . An examination of adherence to pain medication plans in older cancer patients in hospice care. J Pain Symptom Manage. 2013;45:43–55.
- , , , et al. Hospice providers' key approaches to support informal caregivers in managing medications for patients in private residences. J Pain Symptom Manage. 2012;43:1060–1071.
- , . Hospice approach to palliative care, including Medicare hospice benefit. In: Yennurajalingam S, Bruera E, eds. Oxford American Handbook of Hospice and Palliative Medicine. New York, NY: Oxford University Press; 2011:229–239.
- , , . Predictors of thirty‐day readmission among hospitalized family medicine patients. J Am Board Fam Med. 2013;26:71–77.
- , , , , , . Emergency calls and need for emergency care in pateints looked after by a palliative care team: Retrospective interview study with bereaved relatives. BMC Palliat Care. 2008;7:11.
- , , , et al. Predictors of caregiver burden across the home‐based palliative care trajectory in Ontario, Canada [published online March 25, 2105]. Health Soc Care Community. doi: 10.1111/hsc.12219.
- , , , . Unique characteristics of informal hospice cancer caregiving. Support Care Cancer. 2015;23:2121–2128.
- , . Unsettling Scores: A Ranking of State Medicaid Programs. Washington, DC: Public Citizen Press; 2007.
- , , . 30‐day readmissions among seriously ill older adults. J Palliat Med. 2012;15:1–6.
- , . A path forward on Medicare readmissions. N Engl J Med. 2013;368:1175–1177.
SCHOLAR Project
The structure and function of academic hospital medicine programs (AHPs) has evolved significantly with the growth of hospital medicine.[1, 2, 3, 4] Many AHPs formed in response to regulatory and financial changes, which drove demand for increased trainee oversight, improved clinical efficiency, and growth in nonteaching services staffed by hospitalists. Differences in local organizational contexts and needs have contributed to great variability in AHP program design and operations. As AHPs have become more established, the need to engage academic hospitalists in scholarship and activities that support professional development and promotion has been recognized. Defining sustainable and successful positions for academic hospitalists is a priority called for by leaders in the field.[5, 6]
In this rapidly evolving context, AHPs have employed a variety of approaches to organizing clinical and academic faculty roles, without guiding evidence or consensus‐based performance benchmarks. A number of AHPs have achieved success along traditional academic metrics of research, scholarship, and education. Currently, it is not known whether specific approaches to AHP organization, structure, or definition of faculty roles are associated with achievement of more traditional markers of academic success.
The Academic Committee of the Society of Hospital Medicine (SHM), and the Academic Hospitalist Task Force of the Society of General Internal Medicine (SGIM) had separately initiated projects to explore characteristics associated with success in AHPs. In 2012, these organizations combined efforts to jointly develop and implement the SCHOLAR (SuCcessful HOspitaLists in Academics and Research) project. The goals were to identify successful AHPs using objective criteria, and to then study those groups in greater detail to generate insights that would be broadly relevant to the field. Efforts to clarify the factors within AHPs linked to success by traditional academic metrics will benefit hospitalists, their leaders, and key stakeholders striving to achieve optimal balance between clinical and academic roles. We describe the initial work of the SCHOLAR project, our definitions of academic success in AHPs, and the characteristics of a cohort of exemplary AHPs who achieved the highest levels on these metrics.
METHODS
Defining Success
The 11 members of the SCHOLAR project held a variety of clinical and academic roles within a geographically diverse group of AHPs. We sought to create a functional definition of success applicable to AHPs. As no gold standard currently exists, we used a consensus process among task force members to arrive at a definition that was quantifiable, feasible, and meaningful. The first step was brainstorming on conference calls held 1 to 2 times monthly over 4 months. Potential defining characteristics that emerged from these discussions related to research, teaching, and administrative activities. When potential characteristics were proposed, we considered how to operationalize each one. Each characteristic was discussed until there was consensus from the entire group. Those around education and administration were the most complex, as many roles are locally driven and defined, and challenging to quantify. For this reason, we focused on promotion as a more global approach to assessing academic hospitalist success in these areas. Although criteria for academic advancement also vary across institutions, we felt that promotion generally reflected having met some threshold of academic success. We also wanted to recognize that scholarship occurs outside the context of funded research. Ultimately, 3 key domains emerged: research grant funding, faculty promotion, and scholarship.
After these 3 domains were identified, the group sought to define quantitative metrics to assess performance. These discussions occurred on subsequent calls over a 4‐month period. Between calls, group members gathered additional information to facilitate assessment of the feasibility of proposed metrics, reporting on progress via email. Again, group consensus was sought for each metric considered. Data on grant funding and successful promotions were available from a previous survey conducted through the SHM in 2011. Leaders from 170 AHPs were contacted, with 50 providing complete responses to the 21‐item questionnaire (see Supporting Information, Appendix 1, in the online version of this article). Results of the survey, heretofore referred to as the Leaders of Academic Hospitalist Programs survey (LAHP‐50), have been described elsewhere.[7] For the purposes of this study, we used the self‐reported data about grant funding and promotions contained in the survey to reflect the current state of the field. Although the survey response rate was approximately 30%, the survey was not anonymous, and many reputationally prominent academic hospitalist programs were represented. For these reasons, the group members felt that the survey results were relevant for the purposes of assessing academic success.
In the LAHP‐50, funding was defined as principal investigator or coinvestigator roles on federally and nonfederally funded research, clinical trials, internal grants, and any other extramurally funded projects. Mean and median funding for the overall sample was calculated. Through a separate question, each program's total faculty full‐time equivalent (FTE) count was reported, allowing us to adjust for group size by assessing both total funding per group and funding/FTE for each responding AHP.
Promotions were defined by the self‐reported number of faculty at each of the following ranks: instructor, assistant professor, associate professor, full professor, and professor above scale/emeritus. In addition, a category of nonacademic track (eg, adjunct faculty, clinical associate) was included to capture hospitalists that did not fit into the traditional promotions categories. We did not distinguish between tenure‐track and nontenure‐track academic ranks. LAHP‐50 survey respondents reported the number of faculty in their group at each academic rank. Given that the majority of academic hospitalists hold a rank of assistant professor or lower,[6, 8, 9] and that the number of full professors was only 3% in the LAHP‐50 cohort, we combined the faculty at the associate and full professor ranks, defining successfully promoted faculty as the percent of hospitalists above the rank of assistant professor.
We created a new metric to assess scholarly output. We had considerable discussion of ways to assess the numbers of peer‐reviewed manuscripts generated by AHPs. However, the group had concerns about the feasibility of identification and attribution of authors to specific AHPs through literature searches. We considered examining only publications in the Journal of Hospital Medicine and the Journal of General Internal Medicine, but felt that this would exclude significant work published by hospitalists in fields of medical education or health services research that would more likely appear in alternate journals. Instead, we quantified scholarship based on the number of abstracts presented at national meetings. We focused on meetings of the SHM and SGIM as the primary professional societies representing hospital medicine. The group felt that even work published outside of the journals of our professional societies would likely be presented at those meetings. We used the following strategy: We reviewed research abstracts accepted for presentation as posters or oral abstracts at the 2010 and 2011 SHM national meetings, and research abstracts with a primary or secondary category of hospital medicine at the 2010 and 2011 SGIM national meetings. By including submissions at both SGIM and SHM meetings, we accounted for the fact that some programs may gravitate more to one society meeting or another. We did not include abstracts in the clinical vignettes or innovations categories. We tallied the number of abstracts by group affiliation of the authors for each of the 4 meetings above and created a cumulative total per group for the 2‐year period. Abstracts with authors from different AHPs were counted once for each individual group. Members of the study group reviewed abstracts from each of the meetings in pairs. Reviewers worked separately and compared tallies of results to ensure consistent tabulations. Internet searches were conducted to identify or confirm author affiliations if it was not apparent in the abstract author list. Abstract tallies were compiled without regard to whether programs had completed the LAHP‐50 survey; thus, we collected data on programs that did not respond to the LAHP‐50 survey.
Identification of the SCHOLAR Cohort
To identify our cohort of top‐performing AHPs, we combined the funding and promotions data from the LAHP‐50 sample with the abstract data. We limited our sample to adult hospital medicine groups to reduce heterogeneity. We created rank lists of programs in each category (grant funding, successful promotions, and scholarship), using data from the LAHP‐50 survey to rank programs on funding and promotions, and data from our abstract counts to rank on scholarship. We limited the top‐performing list in each category to 10 institutions as a cutoff. Because we set a threshold of at least $1 million in total funding, we identified only 9 top performing AHPs with regard to grant funding. We also calculated mean funding/FTE. We chose to rank programs only by funding/FTE rather than total funding per program to better account for group size. For successful promotions, we ranked programs by the percentage of senior faculty. For abstract counts, we included programs whose faculty presented abstracts at a minimum of 2 separate meetings, and ranked programs based on the total number of abstracts per group.
This process resulted in separate lists of top performing programs in each of the 3 domains we associated with academic success, arranged in descending order by grant dollars/FTE, percent of senior faculty, and abstract counts (Table 1). Seventeen different programs were represented across these 3 top 10 lists. One program appeared on all 3 lists, 8 programs appeared on 2 lists, and the remainder appeared on a single list (Table 2). Seven of these programs were identified solely based on abstract presentations, diversifying our top groups beyond only those who completed the LAHP‐50 survey. We considered all of these programs to represent high performance in academic hospital medicine. The group selected this inclusive approach because we recognized that any 1 metric was potentially limited, and we sought to identify diverse pathways to success.
| Funding | Promotions | Scholarship | |
|---|---|---|---|
| Grant $/FTE | Total Grant $ | Senior Faculty, No. (%) | Total Abstract Count |
| |||
| $1,409,090 | $15,500,000 | 3 (60%) | 23 |
| $1,000,000 | $9,000,000 | 3 (60%) | 21 |
| $750,000 | $8,000,000 | 4 (57%) | 20 |
| $478,609 | $6,700,535 | 9 (53%) | 15 |
| $347,826 | $3,000,000 | 8 (44%) | 11 |
| $86,956 | $3,000,000 | 14 (41%) | 11 |
| $66,666 | $2,000,000 | 17 (36%) | 10 |
| $46,153 | $1,500,000 | 9 (33%) | 10 |
| $38,461 | $1,000,000 | 2 (33%) | 9 |
| 4 (31%) | 9 | ||
| Selection Criteria for SCHOLAR Cohort | No. of Programs |
|---|---|
| |
| Abstracts, funding, and promotions | 1 |
| Abstracts plus promotions | 4 |
| Abstracts plus funding | 3 |
| Funding plus promotion | 1 |
| Funding only | 1 |
| Abstract only | 7 |
| Total | 17 |
| Top 10 abstract count | |
| 4 meetings | 2 |
| 3 meetings | 2 |
| 2 meetings | 6 |
The 17 unique adult AHPs appearing on at least 1 of the top 10 lists comprised the SCHOLAR cohort of programs that we studied in greater detail. Data reflecting program demographics were solicited directly from leaders of the AHPs identified in the SCHOLAR cohort, including size and age of program, reporting structure, number of faculty at various academic ranks (for programs that did not complete the LAHP‐50 survey), and number of faculty with fellowship training (defined as any postresidency fellowship program).
Subsequently, we performed comparative analyses between the programs in the SCHOLAR cohort to the general population of AHPs reflected by the LAHP‐50 sample. Because abstract presentations were not recorded in the original LAHP‐50 survey instrument, it was not possible to perform a benchmarking comparison for the scholarship domain.
Data Analysis
To measure the success of the SCHOLAR cohort we compared the grant funding and proportion of successfully promoted faculty at the SCHOLAR programs to those in the overall LAHP‐50 sample. Differences in mean and median grant funding were compared using t tests and Mann‐Whitney rank sum tests. Proportion of promoted faculty were compared using 2 tests. A 2‐tailed of 0.05 was used to test significance of differences.
RESULTS
Demographics
Among the AHPs in the SCHOLAR cohort, the mean program age was 13.2 years (range, 618 years), and the mean program size was 36 faculty (range, 1895; median, 28). On average, 15% of faculty members at SCHOLAR programs were fellowship trained (range, 0%37%). Reporting structure among the SCHOLAR programs was as follows: 53% were an independent division or section of the department of medicine; 29% were a section within general internal medicine, and 18% were an independent clinical group.
Grant Funding
Table 3 compares grant funding in the SCHOLAR programs to programs in the overall LAHP‐50 sample. Mean funding per group and mean funding per FTE were significantly higher in the SCHOLAR group than in the overall sample.
| Funding (Millions) | ||
|---|---|---|
| LAHP‐50 Overall Sample | SCHOLAR | |
| ||
| Median grant funding/AHP | 0.060 | 1.500* |
| Mean grant funding/AHP | 1.147 (015) | 3.984* (015) |
| Median grant funding/FTE | 0.004 | 0.038* |
| Mean grant funding/FTE | 0.095 (01.4) | 0.364* (01.4) |
Thirteen of the SCHOLAR programs were represented in the initial LAHP‐50, but 2 did not report a dollar amount for grants and contracts. Therefore, data for total grant funding were available for only 65% (11 of 17) of the programs in the SCHOLAR cohort. Of note, 28% of AHPs in the overall LAHP‐50 sample reported no external funding sources.
Faculty Promotion
Figure 1 demonstrates the proportion of faculty at various academic ranks. The percent of faculty above the rank of assistant professor in the SCHOLAR programs exceeded those in the overall LAHP‐50 by 5% (17.9% vs 12.8%, P = 0.01). Of note, 6% of the hospitalists at AHPs in the SCHOLAR programs were on nonfaculty tracks.
Scholarship
Mean abstract output over the 2‐year period measured was 10.8 (range, 323) in the SCHOLAR cohort. Because we did not collect these data for the LAHP‐50 group, comparative analyses were not possible.
DISCUSSION
Using a definition of academic success that incorporated metrics of grant funding, faculty promotion, and scholarly output, we identified a unique subset of successful AHPsthe SCHOLAR cohort. The programs represented in the SCHOLAR cohort were generally large and relatively mature. Despite this, the cohort consisted of mostly junior faculty, had a paucity of fellowship‐trained hospitalists, and not all reported grant funding.
Prior published work reported complementary findings.[6, 8, 9] A survey of 20 large, well‐established academic hospitalist programs in 2008 found that the majority of hospitalists were junior faculty with a limited publication portfolio. Of the 266 respondents in that study, 86% reported an academic rank at or below assistant professor; funding was not explored.[9] Our similar findings 4 years later add to this work by demonstrating trends over time, and suggest that progress toward creating successful pathways for academic advancement has been slow. In a 2012 survey of the SHM membership, 28% of hospitalists with academic appointments reported no current or future plans to engage in research.[8] These findings suggest that faculty in AHPs may define scholarship through nontraditional pathways, or in some cases choose not to pursue or prioritize scholarship altogether.
Our findings also add to the literature with regard to our assessment of funding, which was variable across the SCHOLAR group. The broad range of funding in the SCHOLAR programs for which we have data (grant dollars $0$15 million per program) suggests that opportunities to improve supported scholarship remain, even among a selected cohort of successful AHPs. The predominance of junior faculty in the SCHOLAR programs may be a reason for this variation. Junior faculty may be engaged in research with funding directed to senior mentors outside their AHP. Alternatively, they may pursue meaningful local hospital quality improvement or educational innovations not supported by external grants, or hold leadership roles in education, quality, or information technology that allow for advancement and promotion without external grant funding. As the scope and impact of these roles increases, senior leaders with alternate sources of support may rely less on research funds; this too may explain some of the differences. Our findings are congruent with results of a study that reviewed original research published by hospitalists, and concluded that the majority of hospitalist research was not externally funded.[8] Our approach for assessing grant funding by adjusting for FTE had the potential to inadvertently favor smaller well‐funded groups over larger ones; however, programs in our sample were similarly represented when ranked by funding/FTE or total grant dollars. As many successful AHPs do concentrate their research funding among a core of focused hospitalist researchers, our definition may not be the ideal metric for some programs.
We chose to define scholarship based on abstract output, rather than peer‐reviewed publications. Although this choice was necessary from a feasibility perspective, it may have excluded programs that prioritize peer‐reviewed publications over abstracts. Although we were unable to incorporate a search strategy to accurately and comprehensively track the publication output attributed specifically to hospitalist researchers and quantify it by program, others have since defined such an approach.[8] However, tracking abstracts theoretically allowed insights into a larger volume of innovative and creative work generated by top AHPs by potentially including work in the earlier stages of development.
We used a consensus‐based definition of success to define our SCHOLAR cohort. There are other ways to measure academic success, which if applied, may have yielded a different sample of programs. For example, over half of the original research articles published in the Journal of Hospital Medicine over a 7‐year span were generated from 5 academic centers.[8] This definition of success may be equally credible, though we note that 4 of these 5 programs were also included in the SCHOLAR cohort. We feel our broader approach was more reflective of the variety of pathways to success available to academic hospitalists. Before our metrics are applied as a benchmarking tool, however, they should ideally be combined with factors not measured in our study to ensure a more comprehensive or balanced reflection of academic success. Factors such as mentorship, level of hospitalist engagement,[10] prevalence of leadership opportunities, operational and fiscal infrastructure, and the impact of local quality, safety, and value efforts should be considered.
Comparison of successfully promoted faculty at AHPs across the country is inherently limited by the wide variation in promotion standards across different institutions; controlling for such differences was not possible with our methodology. For example, it appears that several programs with relatively few senior faculty may have met metrics leading to their inclusion in the SCHOLAR group because of their small program size. Future benchmarking efforts for promotion at AHPs should take scaling into account and consider both total number as well as percentage of senior faculty when evaluating success.
Our methodology has several limitations. Survey data were self‐reported and not independently validated, and as such are subject to recall and reporting biases. Response bias inherently excluded some AHPs that may have met our grant funding or promotions criteria had they participated in the initial LAHP‐50 survey, though we identified and included additional programs through our scholarship metric, increasing the representativeness of the SCHOLAR cohort. Given the dynamic nature of the field, the age of the data we relied upon for analysis limits the generalizability of our specific benchmarks to current practice. However, the development of academic success occurs over the long‐term, and published data on academic hospitalist productivity are consistent with this slower time course.[8] Despite these limitations, our data inform the general topic of gauging performance of AHPs, underscoring the challenges of developing and applying metrics of success, and highlight the variability of performance on selected metrics even among a relatively small group of 17 programs.
In conclusion, we have created a method to quantify academic success that may be useful to academic hospitalists and their group leaders as they set targets for improvement in the field. Even among our SCHOLAR cohort, room for ongoing improvement in development of funded scholarship and a core of senior faculty exists. Further investigation into the unique features of successful groups will offer insight to leaders in academic hospital medicine regarding infrastructure and processes that should be embraced to raise the bar for all AHPs. In addition, efforts to further define and validate nontraditional approaches to scholarship that allow for successful promotion at AHPs would be informative. We view our work less as a singular approach to benchmarking standards for AHPs, and more a call to action to continue efforts to balance scholarly activity and broad professional development of academic hospitalists with increasing clinical demands.
Acknowledgements
The authors thank all of the AHP leaders who participated in the SCHOLAR project. They also thank the Society of Hospital Medicine and Society of General Internal Medicine and the SHM Academic Committee and SGIM Academic Hospitalist Task Force for their support of this work.
Disclosures
The work reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, South Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. The authors report no conflicts of interest.
- , , , , . Characteristics of primary care providers who adopted the hospitalist model from 2001 to 2009. J Hosp Med. 2015;10(2):75–82.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , , , , . Updating threshold‐based identification of hospitalists in 2012 Medicare pay data. J Hosp Med. 2016;11(1):45–47.
- , , , . Use of hospitalists by Medicare beneficiaries: a national picture. Medicare Medicaid Res Rev. 2014;4(2).
- , , , , , . Challenges and opportunities in Academic Hospital Medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240–246.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- , , , , , . The structure of hospital medicine programs at academic medical centers [abstract]. J Hosp Med. 2012;7(suppl 2):s92.
- , , , , . Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148–154.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23–27.
- , , , et al. The key principles and characteristics of an effective hospital medicine group: an assessment guide for hospitals and hospitalists. J Hosp Med. 2014;9(2):123–128.
The structure and function of academic hospital medicine programs (AHPs) has evolved significantly with the growth of hospital medicine.[1, 2, 3, 4] Many AHPs formed in response to regulatory and financial changes, which drove demand for increased trainee oversight, improved clinical efficiency, and growth in nonteaching services staffed by hospitalists. Differences in local organizational contexts and needs have contributed to great variability in AHP program design and operations. As AHPs have become more established, the need to engage academic hospitalists in scholarship and activities that support professional development and promotion has been recognized. Defining sustainable and successful positions for academic hospitalists is a priority called for by leaders in the field.[5, 6]
In this rapidly evolving context, AHPs have employed a variety of approaches to organizing clinical and academic faculty roles, without guiding evidence or consensus‐based performance benchmarks. A number of AHPs have achieved success along traditional academic metrics of research, scholarship, and education. Currently, it is not known whether specific approaches to AHP organization, structure, or definition of faculty roles are associated with achievement of more traditional markers of academic success.
The Academic Committee of the Society of Hospital Medicine (SHM), and the Academic Hospitalist Task Force of the Society of General Internal Medicine (SGIM) had separately initiated projects to explore characteristics associated with success in AHPs. In 2012, these organizations combined efforts to jointly develop and implement the SCHOLAR (SuCcessful HOspitaLists in Academics and Research) project. The goals were to identify successful AHPs using objective criteria, and to then study those groups in greater detail to generate insights that would be broadly relevant to the field. Efforts to clarify the factors within AHPs linked to success by traditional academic metrics will benefit hospitalists, their leaders, and key stakeholders striving to achieve optimal balance between clinical and academic roles. We describe the initial work of the SCHOLAR project, our definitions of academic success in AHPs, and the characteristics of a cohort of exemplary AHPs who achieved the highest levels on these metrics.
METHODS
Defining Success
The 11 members of the SCHOLAR project held a variety of clinical and academic roles within a geographically diverse group of AHPs. We sought to create a functional definition of success applicable to AHPs. As no gold standard currently exists, we used a consensus process among task force members to arrive at a definition that was quantifiable, feasible, and meaningful. The first step was brainstorming on conference calls held 1 to 2 times monthly over 4 months. Potential defining characteristics that emerged from these discussions related to research, teaching, and administrative activities. When potential characteristics were proposed, we considered how to operationalize each one. Each characteristic was discussed until there was consensus from the entire group. Those around education and administration were the most complex, as many roles are locally driven and defined, and challenging to quantify. For this reason, we focused on promotion as a more global approach to assessing academic hospitalist success in these areas. Although criteria for academic advancement also vary across institutions, we felt that promotion generally reflected having met some threshold of academic success. We also wanted to recognize that scholarship occurs outside the context of funded research. Ultimately, 3 key domains emerged: research grant funding, faculty promotion, and scholarship.
After these 3 domains were identified, the group sought to define quantitative metrics to assess performance. These discussions occurred on subsequent calls over a 4‐month period. Between calls, group members gathered additional information to facilitate assessment of the feasibility of proposed metrics, reporting on progress via email. Again, group consensus was sought for each metric considered. Data on grant funding and successful promotions were available from a previous survey conducted through the SHM in 2011. Leaders from 170 AHPs were contacted, with 50 providing complete responses to the 21‐item questionnaire (see Supporting Information, Appendix 1, in the online version of this article). Results of the survey, heretofore referred to as the Leaders of Academic Hospitalist Programs survey (LAHP‐50), have been described elsewhere.[7] For the purposes of this study, we used the self‐reported data about grant funding and promotions contained in the survey to reflect the current state of the field. Although the survey response rate was approximately 30%, the survey was not anonymous, and many reputationally prominent academic hospitalist programs were represented. For these reasons, the group members felt that the survey results were relevant for the purposes of assessing academic success.
In the LAHP‐50, funding was defined as principal investigator or coinvestigator roles on federally and nonfederally funded research, clinical trials, internal grants, and any other extramurally funded projects. Mean and median funding for the overall sample was calculated. Through a separate question, each program's total faculty full‐time equivalent (FTE) count was reported, allowing us to adjust for group size by assessing both total funding per group and funding/FTE for each responding AHP.
Promotions were defined by the self‐reported number of faculty at each of the following ranks: instructor, assistant professor, associate professor, full professor, and professor above scale/emeritus. In addition, a category of nonacademic track (eg, adjunct faculty, clinical associate) was included to capture hospitalists that did not fit into the traditional promotions categories. We did not distinguish between tenure‐track and nontenure‐track academic ranks. LAHP‐50 survey respondents reported the number of faculty in their group at each academic rank. Given that the majority of academic hospitalists hold a rank of assistant professor or lower,[6, 8, 9] and that the number of full professors was only 3% in the LAHP‐50 cohort, we combined the faculty at the associate and full professor ranks, defining successfully promoted faculty as the percent of hospitalists above the rank of assistant professor.
We created a new metric to assess scholarly output. We had considerable discussion of ways to assess the numbers of peer‐reviewed manuscripts generated by AHPs. However, the group had concerns about the feasibility of identification and attribution of authors to specific AHPs through literature searches. We considered examining only publications in the Journal of Hospital Medicine and the Journal of General Internal Medicine, but felt that this would exclude significant work published by hospitalists in fields of medical education or health services research that would more likely appear in alternate journals. Instead, we quantified scholarship based on the number of abstracts presented at national meetings. We focused on meetings of the SHM and SGIM as the primary professional societies representing hospital medicine. The group felt that even work published outside of the journals of our professional societies would likely be presented at those meetings. We used the following strategy: We reviewed research abstracts accepted for presentation as posters or oral abstracts at the 2010 and 2011 SHM national meetings, and research abstracts with a primary or secondary category of hospital medicine at the 2010 and 2011 SGIM national meetings. By including submissions at both SGIM and SHM meetings, we accounted for the fact that some programs may gravitate more to one society meeting or another. We did not include abstracts in the clinical vignettes or innovations categories. We tallied the number of abstracts by group affiliation of the authors for each of the 4 meetings above and created a cumulative total per group for the 2‐year period. Abstracts with authors from different AHPs were counted once for each individual group. Members of the study group reviewed abstracts from each of the meetings in pairs. Reviewers worked separately and compared tallies of results to ensure consistent tabulations. Internet searches were conducted to identify or confirm author affiliations if it was not apparent in the abstract author list. Abstract tallies were compiled without regard to whether programs had completed the LAHP‐50 survey; thus, we collected data on programs that did not respond to the LAHP‐50 survey.
Identification of the SCHOLAR Cohort
To identify our cohort of top‐performing AHPs, we combined the funding and promotions data from the LAHP‐50 sample with the abstract data. We limited our sample to adult hospital medicine groups to reduce heterogeneity. We created rank lists of programs in each category (grant funding, successful promotions, and scholarship), using data from the LAHP‐50 survey to rank programs on funding and promotions, and data from our abstract counts to rank on scholarship. We limited the top‐performing list in each category to 10 institutions as a cutoff. Because we set a threshold of at least $1 million in total funding, we identified only 9 top performing AHPs with regard to grant funding. We also calculated mean funding/FTE. We chose to rank programs only by funding/FTE rather than total funding per program to better account for group size. For successful promotions, we ranked programs by the percentage of senior faculty. For abstract counts, we included programs whose faculty presented abstracts at a minimum of 2 separate meetings, and ranked programs based on the total number of abstracts per group.
This process resulted in separate lists of top performing programs in each of the 3 domains we associated with academic success, arranged in descending order by grant dollars/FTE, percent of senior faculty, and abstract counts (Table 1). Seventeen different programs were represented across these 3 top 10 lists. One program appeared on all 3 lists, 8 programs appeared on 2 lists, and the remainder appeared on a single list (Table 2). Seven of these programs were identified solely based on abstract presentations, diversifying our top groups beyond only those who completed the LAHP‐50 survey. We considered all of these programs to represent high performance in academic hospital medicine. The group selected this inclusive approach because we recognized that any 1 metric was potentially limited, and we sought to identify diverse pathways to success.
| Funding | Promotions | Scholarship | |
|---|---|---|---|
| Grant $/FTE | Total Grant $ | Senior Faculty, No. (%) | Total Abstract Count |
| |||
| $1,409,090 | $15,500,000 | 3 (60%) | 23 |
| $1,000,000 | $9,000,000 | 3 (60%) | 21 |
| $750,000 | $8,000,000 | 4 (57%) | 20 |
| $478,609 | $6,700,535 | 9 (53%) | 15 |
| $347,826 | $3,000,000 | 8 (44%) | 11 |
| $86,956 | $3,000,000 | 14 (41%) | 11 |
| $66,666 | $2,000,000 | 17 (36%) | 10 |
| $46,153 | $1,500,000 | 9 (33%) | 10 |
| $38,461 | $1,000,000 | 2 (33%) | 9 |
| 4 (31%) | 9 | ||
| Selection Criteria for SCHOLAR Cohort | No. of Programs |
|---|---|
| |
| Abstracts, funding, and promotions | 1 |
| Abstracts plus promotions | 4 |
| Abstracts plus funding | 3 |
| Funding plus promotion | 1 |
| Funding only | 1 |
| Abstract only | 7 |
| Total | 17 |
| Top 10 abstract count | |
| 4 meetings | 2 |
| 3 meetings | 2 |
| 2 meetings | 6 |
The 17 unique adult AHPs appearing on at least 1 of the top 10 lists comprised the SCHOLAR cohort of programs that we studied in greater detail. Data reflecting program demographics were solicited directly from leaders of the AHPs identified in the SCHOLAR cohort, including size and age of program, reporting structure, number of faculty at various academic ranks (for programs that did not complete the LAHP‐50 survey), and number of faculty with fellowship training (defined as any postresidency fellowship program).
Subsequently, we performed comparative analyses between the programs in the SCHOLAR cohort to the general population of AHPs reflected by the LAHP‐50 sample. Because abstract presentations were not recorded in the original LAHP‐50 survey instrument, it was not possible to perform a benchmarking comparison for the scholarship domain.
Data Analysis
To measure the success of the SCHOLAR cohort we compared the grant funding and proportion of successfully promoted faculty at the SCHOLAR programs to those in the overall LAHP‐50 sample. Differences in mean and median grant funding were compared using t tests and Mann‐Whitney rank sum tests. Proportion of promoted faculty were compared using 2 tests. A 2‐tailed of 0.05 was used to test significance of differences.
RESULTS
Demographics
Among the AHPs in the SCHOLAR cohort, the mean program age was 13.2 years (range, 618 years), and the mean program size was 36 faculty (range, 1895; median, 28). On average, 15% of faculty members at SCHOLAR programs were fellowship trained (range, 0%37%). Reporting structure among the SCHOLAR programs was as follows: 53% were an independent division or section of the department of medicine; 29% were a section within general internal medicine, and 18% were an independent clinical group.
Grant Funding
Table 3 compares grant funding in the SCHOLAR programs to programs in the overall LAHP‐50 sample. Mean funding per group and mean funding per FTE were significantly higher in the SCHOLAR group than in the overall sample.
| Funding (Millions) | ||
|---|---|---|
| LAHP‐50 Overall Sample | SCHOLAR | |
| ||
| Median grant funding/AHP | 0.060 | 1.500* |
| Mean grant funding/AHP | 1.147 (015) | 3.984* (015) |
| Median grant funding/FTE | 0.004 | 0.038* |
| Mean grant funding/FTE | 0.095 (01.4) | 0.364* (01.4) |
Thirteen of the SCHOLAR programs were represented in the initial LAHP‐50, but 2 did not report a dollar amount for grants and contracts. Therefore, data for total grant funding were available for only 65% (11 of 17) of the programs in the SCHOLAR cohort. Of note, 28% of AHPs in the overall LAHP‐50 sample reported no external funding sources.
Faculty Promotion
Figure 1 demonstrates the proportion of faculty at various academic ranks. The percent of faculty above the rank of assistant professor in the SCHOLAR programs exceeded those in the overall LAHP‐50 by 5% (17.9% vs 12.8%, P = 0.01). Of note, 6% of the hospitalists at AHPs in the SCHOLAR programs were on nonfaculty tracks.

Scholarship
Mean abstract output over the 2‐year period measured was 10.8 (range, 323) in the SCHOLAR cohort. Because we did not collect these data for the LAHP‐50 group, comparative analyses were not possible.
DISCUSSION
Using a definition of academic success that incorporated metrics of grant funding, faculty promotion, and scholarly output, we identified a unique subset of successful AHPsthe SCHOLAR cohort. The programs represented in the SCHOLAR cohort were generally large and relatively mature. Despite this, the cohort consisted of mostly junior faculty, had a paucity of fellowship‐trained hospitalists, and not all reported grant funding.
Prior published work reported complementary findings.[6, 8, 9] A survey of 20 large, well‐established academic hospitalist programs in 2008 found that the majority of hospitalists were junior faculty with a limited publication portfolio. Of the 266 respondents in that study, 86% reported an academic rank at or below assistant professor; funding was not explored.[9] Our similar findings 4 years later add to this work by demonstrating trends over time, and suggest that progress toward creating successful pathways for academic advancement has been slow. In a 2012 survey of the SHM membership, 28% of hospitalists with academic appointments reported no current or future plans to engage in research.[8] These findings suggest that faculty in AHPs may define scholarship through nontraditional pathways, or in some cases choose not to pursue or prioritize scholarship altogether.
Our findings also add to the literature with regard to our assessment of funding, which was variable across the SCHOLAR group. The broad range of funding in the SCHOLAR programs for which we have data (grant dollars $0$15 million per program) suggests that opportunities to improve supported scholarship remain, even among a selected cohort of successful AHPs. The predominance of junior faculty in the SCHOLAR programs may be a reason for this variation. Junior faculty may be engaged in research with funding directed to senior mentors outside their AHP. Alternatively, they may pursue meaningful local hospital quality improvement or educational innovations not supported by external grants, or hold leadership roles in education, quality, or information technology that allow for advancement and promotion without external grant funding. As the scope and impact of these roles increases, senior leaders with alternate sources of support may rely less on research funds; this too may explain some of the differences. Our findings are congruent with results of a study that reviewed original research published by hospitalists, and concluded that the majority of hospitalist research was not externally funded.[8] Our approach for assessing grant funding by adjusting for FTE had the potential to inadvertently favor smaller well‐funded groups over larger ones; however, programs in our sample were similarly represented when ranked by funding/FTE or total grant dollars. As many successful AHPs do concentrate their research funding among a core of focused hospitalist researchers, our definition may not be the ideal metric for some programs.
We chose to define scholarship based on abstract output, rather than peer‐reviewed publications. Although this choice was necessary from a feasibility perspective, it may have excluded programs that prioritize peer‐reviewed publications over abstracts. Although we were unable to incorporate a search strategy to accurately and comprehensively track the publication output attributed specifically to hospitalist researchers and quantify it by program, others have since defined such an approach.[8] However, tracking abstracts theoretically allowed insights into a larger volume of innovative and creative work generated by top AHPs by potentially including work in the earlier stages of development.
We used a consensus‐based definition of success to define our SCHOLAR cohort. There are other ways to measure academic success, which if applied, may have yielded a different sample of programs. For example, over half of the original research articles published in the Journal of Hospital Medicine over a 7‐year span were generated from 5 academic centers.[8] This definition of success may be equally credible, though we note that 4 of these 5 programs were also included in the SCHOLAR cohort. We feel our broader approach was more reflective of the variety of pathways to success available to academic hospitalists. Before our metrics are applied as a benchmarking tool, however, they should ideally be combined with factors not measured in our study to ensure a more comprehensive or balanced reflection of academic success. Factors such as mentorship, level of hospitalist engagement,[10] prevalence of leadership opportunities, operational and fiscal infrastructure, and the impact of local quality, safety, and value efforts should be considered.
Comparison of successfully promoted faculty at AHPs across the country is inherently limited by the wide variation in promotion standards across different institutions; controlling for such differences was not possible with our methodology. For example, it appears that several programs with relatively few senior faculty may have met metrics leading to their inclusion in the SCHOLAR group because of their small program size. Future benchmarking efforts for promotion at AHPs should take scaling into account and consider both total number as well as percentage of senior faculty when evaluating success.
Our methodology has several limitations. Survey data were self‐reported and not independently validated, and as such are subject to recall and reporting biases. Response bias inherently excluded some AHPs that may have met our grant funding or promotions criteria had they participated in the initial LAHP‐50 survey, though we identified and included additional programs through our scholarship metric, increasing the representativeness of the SCHOLAR cohort. Given the dynamic nature of the field, the age of the data we relied upon for analysis limits the generalizability of our specific benchmarks to current practice. However, the development of academic success occurs over the long‐term, and published data on academic hospitalist productivity are consistent with this slower time course.[8] Despite these limitations, our data inform the general topic of gauging performance of AHPs, underscoring the challenges of developing and applying metrics of success, and highlight the variability of performance on selected metrics even among a relatively small group of 17 programs.
In conclusion, we have created a method to quantify academic success that may be useful to academic hospitalists and their group leaders as they set targets for improvement in the field. Even among our SCHOLAR cohort, room for ongoing improvement in development of funded scholarship and a core of senior faculty exists. Further investigation into the unique features of successful groups will offer insight to leaders in academic hospital medicine regarding infrastructure and processes that should be embraced to raise the bar for all AHPs. In addition, efforts to further define and validate nontraditional approaches to scholarship that allow for successful promotion at AHPs would be informative. We view our work less as a singular approach to benchmarking standards for AHPs, and more a call to action to continue efforts to balance scholarly activity and broad professional development of academic hospitalists with increasing clinical demands.
Acknowledgements
The authors thank all of the AHP leaders who participated in the SCHOLAR project. They also thank the Society of Hospital Medicine and Society of General Internal Medicine and the SHM Academic Committee and SGIM Academic Hospitalist Task Force for their support of this work.
Disclosures
The work reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, South Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. The authors report no conflicts of interest.
The structure and function of academic hospital medicine programs (AHPs) has evolved significantly with the growth of hospital medicine.[1, 2, 3, 4] Many AHPs formed in response to regulatory and financial changes, which drove demand for increased trainee oversight, improved clinical efficiency, and growth in nonteaching services staffed by hospitalists. Differences in local organizational contexts and needs have contributed to great variability in AHP program design and operations. As AHPs have become more established, the need to engage academic hospitalists in scholarship and activities that support professional development and promotion has been recognized. Defining sustainable and successful positions for academic hospitalists is a priority called for by leaders in the field.[5, 6]
In this rapidly evolving context, AHPs have employed a variety of approaches to organizing clinical and academic faculty roles, without guiding evidence or consensus‐based performance benchmarks. A number of AHPs have achieved success along traditional academic metrics of research, scholarship, and education. Currently, it is not known whether specific approaches to AHP organization, structure, or definition of faculty roles are associated with achievement of more traditional markers of academic success.
The Academic Committee of the Society of Hospital Medicine (SHM), and the Academic Hospitalist Task Force of the Society of General Internal Medicine (SGIM) had separately initiated projects to explore characteristics associated with success in AHPs. In 2012, these organizations combined efforts to jointly develop and implement the SCHOLAR (SuCcessful HOspitaLists in Academics and Research) project. The goals were to identify successful AHPs using objective criteria, and to then study those groups in greater detail to generate insights that would be broadly relevant to the field. Efforts to clarify the factors within AHPs linked to success by traditional academic metrics will benefit hospitalists, their leaders, and key stakeholders striving to achieve optimal balance between clinical and academic roles. We describe the initial work of the SCHOLAR project, our definitions of academic success in AHPs, and the characteristics of a cohort of exemplary AHPs who achieved the highest levels on these metrics.
METHODS
Defining Success
The 11 members of the SCHOLAR project held a variety of clinical and academic roles within a geographically diverse group of AHPs. We sought to create a functional definition of success applicable to AHPs. As no gold standard currently exists, we used a consensus process among task force members to arrive at a definition that was quantifiable, feasible, and meaningful. The first step was brainstorming on conference calls held 1 to 2 times monthly over 4 months. Potential defining characteristics that emerged from these discussions related to research, teaching, and administrative activities. When potential characteristics were proposed, we considered how to operationalize each one. Each characteristic was discussed until there was consensus from the entire group. Those around education and administration were the most complex, as many roles are locally driven and defined, and challenging to quantify. For this reason, we focused on promotion as a more global approach to assessing academic hospitalist success in these areas. Although criteria for academic advancement also vary across institutions, we felt that promotion generally reflected having met some threshold of academic success. We also wanted to recognize that scholarship occurs outside the context of funded research. Ultimately, 3 key domains emerged: research grant funding, faculty promotion, and scholarship.
After these 3 domains were identified, the group sought to define quantitative metrics to assess performance. These discussions occurred on subsequent calls over a 4‐month period. Between calls, group members gathered additional information to facilitate assessment of the feasibility of proposed metrics, reporting on progress via email. Again, group consensus was sought for each metric considered. Data on grant funding and successful promotions were available from a previous survey conducted through the SHM in 2011. Leaders from 170 AHPs were contacted, with 50 providing complete responses to the 21‐item questionnaire (see Supporting Information, Appendix 1, in the online version of this article). Results of the survey, heretofore referred to as the Leaders of Academic Hospitalist Programs survey (LAHP‐50), have been described elsewhere.[7] For the purposes of this study, we used the self‐reported data about grant funding and promotions contained in the survey to reflect the current state of the field. Although the survey response rate was approximately 30%, the survey was not anonymous, and many reputationally prominent academic hospitalist programs were represented. For these reasons, the group members felt that the survey results were relevant for the purposes of assessing academic success.
In the LAHP‐50, funding was defined as principal investigator or coinvestigator roles on federally and nonfederally funded research, clinical trials, internal grants, and any other extramurally funded projects. Mean and median funding for the overall sample was calculated. Through a separate question, each program's total faculty full‐time equivalent (FTE) count was reported, allowing us to adjust for group size by assessing both total funding per group and funding/FTE for each responding AHP.
Promotions were defined by the self‐reported number of faculty at each of the following ranks: instructor, assistant professor, associate professor, full professor, and professor above scale/emeritus. In addition, a category of nonacademic track (eg, adjunct faculty, clinical associate) was included to capture hospitalists that did not fit into the traditional promotions categories. We did not distinguish between tenure‐track and nontenure‐track academic ranks. LAHP‐50 survey respondents reported the number of faculty in their group at each academic rank. Given that the majority of academic hospitalists hold a rank of assistant professor or lower,[6, 8, 9] and that the number of full professors was only 3% in the LAHP‐50 cohort, we combined the faculty at the associate and full professor ranks, defining successfully promoted faculty as the percent of hospitalists above the rank of assistant professor.
We created a new metric to assess scholarly output. We had considerable discussion of ways to assess the numbers of peer‐reviewed manuscripts generated by AHPs. However, the group had concerns about the feasibility of identification and attribution of authors to specific AHPs through literature searches. We considered examining only publications in the Journal of Hospital Medicine and the Journal of General Internal Medicine, but felt that this would exclude significant work published by hospitalists in fields of medical education or health services research that would more likely appear in alternate journals. Instead, we quantified scholarship based on the number of abstracts presented at national meetings. We focused on meetings of the SHM and SGIM as the primary professional societies representing hospital medicine. The group felt that even work published outside of the journals of our professional societies would likely be presented at those meetings. We used the following strategy: We reviewed research abstracts accepted for presentation as posters or oral abstracts at the 2010 and 2011 SHM national meetings, and research abstracts with a primary or secondary category of hospital medicine at the 2010 and 2011 SGIM national meetings. By including submissions at both SGIM and SHM meetings, we accounted for the fact that some programs may gravitate more to one society meeting or another. We did not include abstracts in the clinical vignettes or innovations categories. We tallied the number of abstracts by group affiliation of the authors for each of the 4 meetings above and created a cumulative total per group for the 2‐year period. Abstracts with authors from different AHPs were counted once for each individual group. Members of the study group reviewed abstracts from each of the meetings in pairs. Reviewers worked separately and compared tallies of results to ensure consistent tabulations. Internet searches were conducted to identify or confirm author affiliations if it was not apparent in the abstract author list. Abstract tallies were compiled without regard to whether programs had completed the LAHP‐50 survey; thus, we collected data on programs that did not respond to the LAHP‐50 survey.
Identification of the SCHOLAR Cohort
To identify our cohort of top‐performing AHPs, we combined the funding and promotions data from the LAHP‐50 sample with the abstract data. We limited our sample to adult hospital medicine groups to reduce heterogeneity. We created rank lists of programs in each category (grant funding, successful promotions, and scholarship), using data from the LAHP‐50 survey to rank programs on funding and promotions, and data from our abstract counts to rank on scholarship. We limited the top‐performing list in each category to 10 institutions as a cutoff. Because we set a threshold of at least $1 million in total funding, we identified only 9 top performing AHPs with regard to grant funding. We also calculated mean funding/FTE. We chose to rank programs only by funding/FTE rather than total funding per program to better account for group size. For successful promotions, we ranked programs by the percentage of senior faculty. For abstract counts, we included programs whose faculty presented abstracts at a minimum of 2 separate meetings, and ranked programs based on the total number of abstracts per group.
This process resulted in separate lists of top performing programs in each of the 3 domains we associated with academic success, arranged in descending order by grant dollars/FTE, percent of senior faculty, and abstract counts (Table 1). Seventeen different programs were represented across these 3 top 10 lists. One program appeared on all 3 lists, 8 programs appeared on 2 lists, and the remainder appeared on a single list (Table 2). Seven of these programs were identified solely based on abstract presentations, diversifying our top groups beyond only those who completed the LAHP‐50 survey. We considered all of these programs to represent high performance in academic hospital medicine. The group selected this inclusive approach because we recognized that any 1 metric was potentially limited, and we sought to identify diverse pathways to success.
| Funding | Promotions | Scholarship | |
|---|---|---|---|
| Grant $/FTE | Total Grant $ | Senior Faculty, No. (%) | Total Abstract Count |
| |||
| $1,409,090 | $15,500,000 | 3 (60%) | 23 |
| $1,000,000 | $9,000,000 | 3 (60%) | 21 |
| $750,000 | $8,000,000 | 4 (57%) | 20 |
| $478,609 | $6,700,535 | 9 (53%) | 15 |
| $347,826 | $3,000,000 | 8 (44%) | 11 |
| $86,956 | $3,000,000 | 14 (41%) | 11 |
| $66,666 | $2,000,000 | 17 (36%) | 10 |
| $46,153 | $1,500,000 | 9 (33%) | 10 |
| $38,461 | $1,000,000 | 2 (33%) | 9 |
| 4 (31%) | 9 | ||
| Selection Criteria for SCHOLAR Cohort | No. of Programs |
|---|---|
| |
| Abstracts, funding, and promotions | 1 |
| Abstracts plus promotions | 4 |
| Abstracts plus funding | 3 |
| Funding plus promotion | 1 |
| Funding only | 1 |
| Abstract only | 7 |
| Total | 17 |
| Top 10 abstract count | |
| 4 meetings | 2 |
| 3 meetings | 2 |
| 2 meetings | 6 |
The 17 unique adult AHPs appearing on at least 1 of the top 10 lists comprised the SCHOLAR cohort of programs that we studied in greater detail. Data reflecting program demographics were solicited directly from leaders of the AHPs identified in the SCHOLAR cohort, including size and age of program, reporting structure, number of faculty at various academic ranks (for programs that did not complete the LAHP‐50 survey), and number of faculty with fellowship training (defined as any postresidency fellowship program).
Subsequently, we performed comparative analyses between the programs in the SCHOLAR cohort to the general population of AHPs reflected by the LAHP‐50 sample. Because abstract presentations were not recorded in the original LAHP‐50 survey instrument, it was not possible to perform a benchmarking comparison for the scholarship domain.
Data Analysis
To measure the success of the SCHOLAR cohort we compared the grant funding and proportion of successfully promoted faculty at the SCHOLAR programs to those in the overall LAHP‐50 sample. Differences in mean and median grant funding were compared using t tests and Mann‐Whitney rank sum tests. Proportion of promoted faculty were compared using 2 tests. A 2‐tailed of 0.05 was used to test significance of differences.
RESULTS
Demographics
Among the AHPs in the SCHOLAR cohort, the mean program age was 13.2 years (range, 618 years), and the mean program size was 36 faculty (range, 1895; median, 28). On average, 15% of faculty members at SCHOLAR programs were fellowship trained (range, 0%37%). Reporting structure among the SCHOLAR programs was as follows: 53% were an independent division or section of the department of medicine; 29% were a section within general internal medicine, and 18% were an independent clinical group.
Grant Funding
Table 3 compares grant funding in the SCHOLAR programs to programs in the overall LAHP‐50 sample. Mean funding per group and mean funding per FTE were significantly higher in the SCHOLAR group than in the overall sample.
| Funding (Millions) | ||
|---|---|---|
| LAHP‐50 Overall Sample | SCHOLAR | |
| ||
| Median grant funding/AHP | 0.060 | 1.500* |
| Mean grant funding/AHP | 1.147 (015) | 3.984* (015) |
| Median grant funding/FTE | 0.004 | 0.038* |
| Mean grant funding/FTE | 0.095 (01.4) | 0.364* (01.4) |
Thirteen of the SCHOLAR programs were represented in the initial LAHP‐50, but 2 did not report a dollar amount for grants and contracts. Therefore, data for total grant funding were available for only 65% (11 of 17) of the programs in the SCHOLAR cohort. Of note, 28% of AHPs in the overall LAHP‐50 sample reported no external funding sources.
Faculty Promotion
Figure 1 demonstrates the proportion of faculty at various academic ranks. The percent of faculty above the rank of assistant professor in the SCHOLAR programs exceeded those in the overall LAHP‐50 by 5% (17.9% vs 12.8%, P = 0.01). Of note, 6% of the hospitalists at AHPs in the SCHOLAR programs were on nonfaculty tracks.

Scholarship
Mean abstract output over the 2‐year period measured was 10.8 (range, 323) in the SCHOLAR cohort. Because we did not collect these data for the LAHP‐50 group, comparative analyses were not possible.
DISCUSSION
Using a definition of academic success that incorporated metrics of grant funding, faculty promotion, and scholarly output, we identified a unique subset of successful AHPsthe SCHOLAR cohort. The programs represented in the SCHOLAR cohort were generally large and relatively mature. Despite this, the cohort consisted of mostly junior faculty, had a paucity of fellowship‐trained hospitalists, and not all reported grant funding.
Prior published work reported complementary findings.[6, 8, 9] A survey of 20 large, well‐established academic hospitalist programs in 2008 found that the majority of hospitalists were junior faculty with a limited publication portfolio. Of the 266 respondents in that study, 86% reported an academic rank at or below assistant professor; funding was not explored.[9] Our similar findings 4 years later add to this work by demonstrating trends over time, and suggest that progress toward creating successful pathways for academic advancement has been slow. In a 2012 survey of the SHM membership, 28% of hospitalists with academic appointments reported no current or future plans to engage in research.[8] These findings suggest that faculty in AHPs may define scholarship through nontraditional pathways, or in some cases choose not to pursue or prioritize scholarship altogether.
Our findings also add to the literature with regard to our assessment of funding, which was variable across the SCHOLAR group. The broad range of funding in the SCHOLAR programs for which we have data (grant dollars $0$15 million per program) suggests that opportunities to improve supported scholarship remain, even among a selected cohort of successful AHPs. The predominance of junior faculty in the SCHOLAR programs may be a reason for this variation. Junior faculty may be engaged in research with funding directed to senior mentors outside their AHP. Alternatively, they may pursue meaningful local hospital quality improvement or educational innovations not supported by external grants, or hold leadership roles in education, quality, or information technology that allow for advancement and promotion without external grant funding. As the scope and impact of these roles increases, senior leaders with alternate sources of support may rely less on research funds; this too may explain some of the differences. Our findings are congruent with results of a study that reviewed original research published by hospitalists, and concluded that the majority of hospitalist research was not externally funded.[8] Our approach for assessing grant funding by adjusting for FTE had the potential to inadvertently favor smaller well‐funded groups over larger ones; however, programs in our sample were similarly represented when ranked by funding/FTE or total grant dollars. As many successful AHPs do concentrate their research funding among a core of focused hospitalist researchers, our definition may not be the ideal metric for some programs.
We chose to define scholarship based on abstract output, rather than peer‐reviewed publications. Although this choice was necessary from a feasibility perspective, it may have excluded programs that prioritize peer‐reviewed publications over abstracts. Although we were unable to incorporate a search strategy to accurately and comprehensively track the publication output attributed specifically to hospitalist researchers and quantify it by program, others have since defined such an approach.[8] However, tracking abstracts theoretically allowed insights into a larger volume of innovative and creative work generated by top AHPs by potentially including work in the earlier stages of development.
We used a consensus‐based definition of success to define our SCHOLAR cohort. There are other ways to measure academic success, which if applied, may have yielded a different sample of programs. For example, over half of the original research articles published in the Journal of Hospital Medicine over a 7‐year span were generated from 5 academic centers.[8] This definition of success may be equally credible, though we note that 4 of these 5 programs were also included in the SCHOLAR cohort. We feel our broader approach was more reflective of the variety of pathways to success available to academic hospitalists. Before our metrics are applied as a benchmarking tool, however, they should ideally be combined with factors not measured in our study to ensure a more comprehensive or balanced reflection of academic success. Factors such as mentorship, level of hospitalist engagement,[10] prevalence of leadership opportunities, operational and fiscal infrastructure, and the impact of local quality, safety, and value efforts should be considered.
Comparison of successfully promoted faculty at AHPs across the country is inherently limited by the wide variation in promotion standards across different institutions; controlling for such differences was not possible with our methodology. For example, it appears that several programs with relatively few senior faculty may have met metrics leading to their inclusion in the SCHOLAR group because of their small program size. Future benchmarking efforts for promotion at AHPs should take scaling into account and consider both total number as well as percentage of senior faculty when evaluating success.
Our methodology has several limitations. Survey data were self‐reported and not independently validated, and as such are subject to recall and reporting biases. Response bias inherently excluded some AHPs that may have met our grant funding or promotions criteria had they participated in the initial LAHP‐50 survey, though we identified and included additional programs through our scholarship metric, increasing the representativeness of the SCHOLAR cohort. Given the dynamic nature of the field, the age of the data we relied upon for analysis limits the generalizability of our specific benchmarks to current practice. However, the development of academic success occurs over the long‐term, and published data on academic hospitalist productivity are consistent with this slower time course.[8] Despite these limitations, our data inform the general topic of gauging performance of AHPs, underscoring the challenges of developing and applying metrics of success, and highlight the variability of performance on selected metrics even among a relatively small group of 17 programs.
In conclusion, we have created a method to quantify academic success that may be useful to academic hospitalists and their group leaders as they set targets for improvement in the field. Even among our SCHOLAR cohort, room for ongoing improvement in development of funded scholarship and a core of senior faculty exists. Further investigation into the unique features of successful groups will offer insight to leaders in academic hospital medicine regarding infrastructure and processes that should be embraced to raise the bar for all AHPs. In addition, efforts to further define and validate nontraditional approaches to scholarship that allow for successful promotion at AHPs would be informative. We view our work less as a singular approach to benchmarking standards for AHPs, and more a call to action to continue efforts to balance scholarly activity and broad professional development of academic hospitalists with increasing clinical demands.
Acknowledgements
The authors thank all of the AHP leaders who participated in the SCHOLAR project. They also thank the Society of Hospital Medicine and Society of General Internal Medicine and the SHM Academic Committee and SGIM Academic Hospitalist Task Force for their support of this work.
Disclosures
The work reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, South Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. The authors report no conflicts of interest.
- , , , , . Characteristics of primary care providers who adopted the hospitalist model from 2001 to 2009. J Hosp Med. 2015;10(2):75–82.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , , , , . Updating threshold‐based identification of hospitalists in 2012 Medicare pay data. J Hosp Med. 2016;11(1):45–47.
- , , , . Use of hospitalists by Medicare beneficiaries: a national picture. Medicare Medicaid Res Rev. 2014;4(2).
- , , , , , . Challenges and opportunities in Academic Hospital Medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240–246.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- , , , , , . The structure of hospital medicine programs at academic medical centers [abstract]. J Hosp Med. 2012;7(suppl 2):s92.
- , , , , . Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148–154.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23–27.
- , , , et al. The key principles and characteristics of an effective hospital medicine group: an assessment guide for hospitals and hospitalists. J Hosp Med. 2014;9(2):123–128.
- , , , , . Characteristics of primary care providers who adopted the hospitalist model from 2001 to 2009. J Hosp Med. 2015;10(2):75–82.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , , , , . Updating threshold‐based identification of hospitalists in 2012 Medicare pay data. J Hosp Med. 2016;11(1):45–47.
- , , , . Use of hospitalists by Medicare beneficiaries: a national picture. Medicare Medicaid Res Rev. 2014;4(2).
- , , , , , . Challenges and opportunities in Academic Hospital Medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240–246.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- , , , , , . The structure of hospital medicine programs at academic medical centers [abstract]. J Hosp Med. 2012;7(suppl 2):s92.
- , , , , . Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148–154.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23–27.
- , , , et al. The key principles and characteristics of an effective hospital medicine group: an assessment guide for hospitals and hospitalists. J Hosp Med. 2014;9(2):123–128.