User login
Maximizing Efficiency in the Operating Room for Total Joint Arthroplasty
Developing a high-efficiency operating room (OR) is both a challenging and rewarding goal for any healthcare system. The OR is traditionally a high-cost/high-revenue environment1 and operative efficacy has been correlated with low complication rates and surgical success.2 An efficient OR is one that maximizes utilization while providing safe, reproducible, cost-effective, high-quality care. Total joint arthroplasty (TJA) has occupied the center stage for OR efficiency research, in part due to increasing demands from our aging population3 and economic pressures related to high implant costs, decreased reimbursement, and competition for market shares when OR time and space are limited.
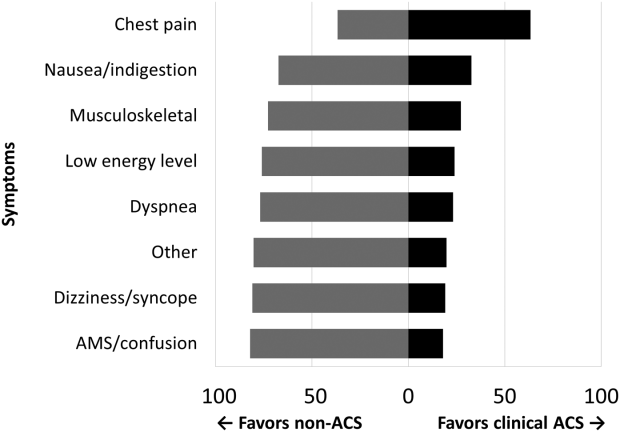
A PubMed search on OR efficiency in TJA shows a disproportionately high focus on surgical technique, such as use of patient-specific instrumentation (PSI), computer-assisted surgery (CAS), minimally invasive surgery, and closure with barbed suture. In a retrospective review of 352 TKA patients who had PSI vs conventional instrumentation, DeHaan and colleagues4 found that PSI was associated with significantly decreased operative and room turnover times (20.4 minutes and 6.4 minutes, respectively). In another prospective multicenter study, Mont and colleagues5 showed a reduction in surgical time by 8.90 min for navigated total knee arthroplasty (TKA) performed with single-use instruments, cutting blocks, and trials. Other investigators compared PSI to CAS in TKA and found PSI to be 1.45 times more profitable than CAS, with 3 PSI cases performed in an 8-hour OR day compared to 2 CAS cases.6
There is no question that improved surgical technique can enhance OR efficiency. However, this model, while promising, is difficult to implement on a wide scale due to surgeon preferences, vendor limitations, and added costs related to the advanced preoperative imaging studies, manufacturing of the custom guides, and maintenance of navigation equipment. In addition, while interventions such as the use of barbed suture have the potential for speeding closure time, the time saved (4.7 minutes in one randomized trial)7 may not be enough to affect major utilization differences per OR per day. These technologies are also frequently employed by high-volume surgeons with high-volume teams and institutions.
Ideally, we need investment in the human capital and a collective change in work cultures to produce high-quality, well-choreographed, easily reproducible routines. An efficient OR requires the synchronous involvement of a large team of individuals, including hospital administrators, surgery schedulers, surgeons, anesthesiologists, preoperative holding area staff, OR nurses, surgical attendants, sterile processing personnel, and recovery room nurses. Case schedulers should match allocated block time with time required for surgery based on the historical performance of the individual surgeon, preferably scheduling similar cases on the same day. Preoperative work-up and medical clearance should be completed prior to scheduling to avoid last-minute cancellations. Patient reminders and accommodations for those traveling from long distances can further minimize late arrivals. Prompt initiation of the perioperative clinical pathway upon a patient’s check-in is important. The surgical site should be marked and the anesthesia plan confirmed upon arrival in the preoperative holding area. Necessary products need to be ready and/or administrated in time for transfer to the OR. These include prophylactic antibiotics, coagulation factors (eg, tranexamic acid), and blood products as indicated. Spinal anesthesia, regional nerve blocks, and intravenous (IV) lines should be completed before transfer to the OR. A “block room” close to the OR can allow concurrent induction of anesthesia and has been shown to increase the number of surgical cases performed during a regular workday.8 Hair clipping within the surgical site and pre-scrubbing of the operative extremity should also be performed prior to transfer to the OR in order to minimize micro-organisms and dispersal of loose hair onto the sterile field.
Upon arrival of the patient to the OR, instrument tables based on the surgeon preference cards should be opened, instrument count and implant templating completed, necessary imaging displayed, and OR staff ready with specific responsibilities assigned to each member. Small and colleagues9 showed that using dedicated orthopedic staff familiar with the surgical routine decreased operative time by 19 minutes per procedure, or 1.25 hours for a surgeon performing 4 primary TJAs per day. Practices such as routine placement of a urinary catheter should be seriously scrutinized. In a randomized prospective study of patients undergoing total hip arthroplasty under spinal anesthesia, Miller and colleagues10 found no benefit for indwelling catheters in preventing urinary retention. In another randomized prospective study, Huang and colleagues11 found the prevalence of urinary tract infections was significantly higher in TJA patients who received indwelling urinary catheters.
A scrub nurse familiar with the instruments, their assembly, and the sequence of events can ensure efficient surgical flow. The scrub nurse needs to anticipate missing or defective tools and call for them, ideally before the incision is made. Direct comparison studies are needed to assess the efficacy of routine intraoperative imaging vs commercially available universal cup alignment guides or clinical examinations in determining acceptable component positioning and limb length. Following component implantation and before wound closure, the circulating nurse should initiate the process of acquisition of a recovery room bed, make sure dressing supplies and necessary equipment are available, and call for surgical attendants. Lack of surgical attendants, delayed transfer from the OR table to hospital bed, and prolonged acquisition of a recovery room bed have been identified as major OR inefficiencies in a retrospective study by Attarian and colleagues.12
In summary, time is the OR’s most valuable resource.13 We believe that a consistent, almost automated attitude to the above procedures decreases variability and improves efficiency. By providing clear communication of the surgical needs with the team, having consistent anesthesia and nursing staff, implementing consistent perioperative protocols, and insuring that all necessary instruments and modalities are available prior to starting the procedure, we were able to sustainably increase OR throughput in a large teaching hospital.9,14 This process, however, requires constant review to identify and eliminate new gaps, with each member of the team sharing a frank desire to improve. In this regard, hospital administrators share the duty to facilitate the implementation of any necessary changes, allocation of needed resources, and rewarding good effort, which could ultimately increase staff satisfaction and retention. Because efficiency is the ratio of benefits (eg, revenue, safety, etc.) to investment (eg, implant costs, wages, etc.), raises the question: what would be the effect of transitioning from hourly-wage to a salary-based system for key support staff? Unlike hourly-wage personnel, who have no incentive for productivity, a salaried employee assigned to a high-efficiency OR will inherently strive for improvement, employing higher organizational skills to accomplish a common goal. To our knowledge, there is no published data on this topic.
1. Krupka DC, Sandberg WS. Operating room design and its impact on operating room economics. Curr Opin Anaesthesiol. 2006;19(2):185-191.
2. Scott WN, Booth RE Jr, Dalury DF, Healy WL, Lonner JH. Efficiency and economics in joint arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 5:33-36.
3. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
4. DeHaan AM, Adams JR, DeHart ML, Huff TW. Patient-specific versus conventional instrumentation for total knee arthroplasty: peri-operative and cost differences. J Arthroplasty. 2014;29(11):2065-2069.
5. Mont MA, McElroy MJ, Johnson AJ, Pivec R; Single-Use Multicenter Trial Group Writing Group. Single-use instruments, cutting blocks, and trials increase efficiency in the operating room during total knee arthroplasty: a prospective comparison of navigated and non-navigated cases. J Arthroplasty. 2013;28(7):1135-1140.
6. Lionberger DR, Crocker CL, Chen V. Patient specific instrumentation. J Arthroplasty. 2014;29(9):1699-1704.
7. Sah AP. Is there an advantage to knotless barbed suture in TKA wound closure? A randomized trial in simultaneous bilateral TKAs. Clin Orthop Relat Res. 2015;473(6):2019-2027.
8. Torkki PM, Marjamaa RA, Torkki MI, Kallio PE, Kirvelä OA. Use of anesthesia induction rooms can increase the number of urgent orthopedic cases completed within 7 hours. Anesthesiology. 2005;103(2):401-405.
9. Small TJ, Gad BV, Klika AK, Mounir-Soliman LS, Gerritsen RL, Barsoum WK. Dedicated orthopedic operating room unit improves operating room efficiency. J Arthroplasty. 2013;28(7):1066-1071.e2.
10. Miller AG, McKenzie J, Greenky M, et al. Spinal anesthesia: should everyone receive a urinary catheter?: a randomized, prospective study of patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2013;95(16):1498-1503.
11. Huang Z, Ma J, Shen B, Pei F. General anesthesia: to catheterize or not? A prospective randomized controlled study of patients undergoing total knee arthroplasty. J Arthroplasty. 2015;30(3):502-506.
12. Attarian DE, Wahl JE, Wellman SS, Bolognesi MP. Developing a high-efficiency operating room for total joint arthroplasty in an academic setting. Clin Orthop Relat Res. 2013;471(6):1832-1836.
13. Gamble M. 6 cornerstones of operating room efficiency: best practices for each. Becker’s Hospital Review Web site. http://www.beckershospitalreview.com/or-efficiencies/6-cornerstones-of-operating-room-efficiency-best-practices-for-each.html. Updated January 18, 2013. Accessed September 3, 2015.
14. Smith MP, Sandberg WS, Foss J, et al. High-throughput operating room system for joint arthroplasties durably outperforms routine processes. Anesthesiology. 2008;109(1):25-35.
Developing a high-efficiency operating room (OR) is both a challenging and rewarding goal for any healthcare system. The OR is traditionally a high-cost/high-revenue environment1 and operative efficacy has been correlated with low complication rates and surgical success.2 An efficient OR is one that maximizes utilization while providing safe, reproducible, cost-effective, high-quality care. Total joint arthroplasty (TJA) has occupied the center stage for OR efficiency research, in part due to increasing demands from our aging population3 and economic pressures related to high implant costs, decreased reimbursement, and competition for market shares when OR time and space are limited.
A PubMed search on OR efficiency in TJA shows a disproportionately high focus on surgical technique, such as use of patient-specific instrumentation (PSI), computer-assisted surgery (CAS), minimally invasive surgery, and closure with barbed suture. In a retrospective review of 352 TKA patients who had PSI vs conventional instrumentation, DeHaan and colleagues4 found that PSI was associated with significantly decreased operative and room turnover times (20.4 minutes and 6.4 minutes, respectively). In another prospective multicenter study, Mont and colleagues5 showed a reduction in surgical time by 8.90 min for navigated total knee arthroplasty (TKA) performed with single-use instruments, cutting blocks, and trials. Other investigators compared PSI to CAS in TKA and found PSI to be 1.45 times more profitable than CAS, with 3 PSI cases performed in an 8-hour OR day compared to 2 CAS cases.6
There is no question that improved surgical technique can enhance OR efficiency. However, this model, while promising, is difficult to implement on a wide scale due to surgeon preferences, vendor limitations, and added costs related to the advanced preoperative imaging studies, manufacturing of the custom guides, and maintenance of navigation equipment. In addition, while interventions such as the use of barbed suture have the potential for speeding closure time, the time saved (4.7 minutes in one randomized trial)7 may not be enough to affect major utilization differences per OR per day. These technologies are also frequently employed by high-volume surgeons with high-volume teams and institutions.
Ideally, we need investment in the human capital and a collective change in work cultures to produce high-quality, well-choreographed, easily reproducible routines. An efficient OR requires the synchronous involvement of a large team of individuals, including hospital administrators, surgery schedulers, surgeons, anesthesiologists, preoperative holding area staff, OR nurses, surgical attendants, sterile processing personnel, and recovery room nurses. Case schedulers should match allocated block time with time required for surgery based on the historical performance of the individual surgeon, preferably scheduling similar cases on the same day. Preoperative work-up and medical clearance should be completed prior to scheduling to avoid last-minute cancellations. Patient reminders and accommodations for those traveling from long distances can further minimize late arrivals. Prompt initiation of the perioperative clinical pathway upon a patient’s check-in is important. The surgical site should be marked and the anesthesia plan confirmed upon arrival in the preoperative holding area. Necessary products need to be ready and/or administrated in time for transfer to the OR. These include prophylactic antibiotics, coagulation factors (eg, tranexamic acid), and blood products as indicated. Spinal anesthesia, regional nerve blocks, and intravenous (IV) lines should be completed before transfer to the OR. A “block room” close to the OR can allow concurrent induction of anesthesia and has been shown to increase the number of surgical cases performed during a regular workday.8 Hair clipping within the surgical site and pre-scrubbing of the operative extremity should also be performed prior to transfer to the OR in order to minimize micro-organisms and dispersal of loose hair onto the sterile field.
Upon arrival of the patient to the OR, instrument tables based on the surgeon preference cards should be opened, instrument count and implant templating completed, necessary imaging displayed, and OR staff ready with specific responsibilities assigned to each member. Small and colleagues9 showed that using dedicated orthopedic staff familiar with the surgical routine decreased operative time by 19 minutes per procedure, or 1.25 hours for a surgeon performing 4 primary TJAs per day. Practices such as routine placement of a urinary catheter should be seriously scrutinized. In a randomized prospective study of patients undergoing total hip arthroplasty under spinal anesthesia, Miller and colleagues10 found no benefit for indwelling catheters in preventing urinary retention. In another randomized prospective study, Huang and colleagues11 found the prevalence of urinary tract infections was significantly higher in TJA patients who received indwelling urinary catheters.
A scrub nurse familiar with the instruments, their assembly, and the sequence of events can ensure efficient surgical flow. The scrub nurse needs to anticipate missing or defective tools and call for them, ideally before the incision is made. Direct comparison studies are needed to assess the efficacy of routine intraoperative imaging vs commercially available universal cup alignment guides or clinical examinations in determining acceptable component positioning and limb length. Following component implantation and before wound closure, the circulating nurse should initiate the process of acquisition of a recovery room bed, make sure dressing supplies and necessary equipment are available, and call for surgical attendants. Lack of surgical attendants, delayed transfer from the OR table to hospital bed, and prolonged acquisition of a recovery room bed have been identified as major OR inefficiencies in a retrospective study by Attarian and colleagues.12
In summary, time is the OR’s most valuable resource.13 We believe that a consistent, almost automated attitude to the above procedures decreases variability and improves efficiency. By providing clear communication of the surgical needs with the team, having consistent anesthesia and nursing staff, implementing consistent perioperative protocols, and insuring that all necessary instruments and modalities are available prior to starting the procedure, we were able to sustainably increase OR throughput in a large teaching hospital.9,14 This process, however, requires constant review to identify and eliminate new gaps, with each member of the team sharing a frank desire to improve. In this regard, hospital administrators share the duty to facilitate the implementation of any necessary changes, allocation of needed resources, and rewarding good effort, which could ultimately increase staff satisfaction and retention. Because efficiency is the ratio of benefits (eg, revenue, safety, etc.) to investment (eg, implant costs, wages, etc.), raises the question: what would be the effect of transitioning from hourly-wage to a salary-based system for key support staff? Unlike hourly-wage personnel, who have no incentive for productivity, a salaried employee assigned to a high-efficiency OR will inherently strive for improvement, employing higher organizational skills to accomplish a common goal. To our knowledge, there is no published data on this topic.
Developing a high-efficiency operating room (OR) is both a challenging and rewarding goal for any healthcare system. The OR is traditionally a high-cost/high-revenue environment1 and operative efficacy has been correlated with low complication rates and surgical success.2 An efficient OR is one that maximizes utilization while providing safe, reproducible, cost-effective, high-quality care. Total joint arthroplasty (TJA) has occupied the center stage for OR efficiency research, in part due to increasing demands from our aging population3 and economic pressures related to high implant costs, decreased reimbursement, and competition for market shares when OR time and space are limited.
A PubMed search on OR efficiency in TJA shows a disproportionately high focus on surgical technique, such as use of patient-specific instrumentation (PSI), computer-assisted surgery (CAS), minimally invasive surgery, and closure with barbed suture. In a retrospective review of 352 TKA patients who had PSI vs conventional instrumentation, DeHaan and colleagues4 found that PSI was associated with significantly decreased operative and room turnover times (20.4 minutes and 6.4 minutes, respectively). In another prospective multicenter study, Mont and colleagues5 showed a reduction in surgical time by 8.90 min for navigated total knee arthroplasty (TKA) performed with single-use instruments, cutting blocks, and trials. Other investigators compared PSI to CAS in TKA and found PSI to be 1.45 times more profitable than CAS, with 3 PSI cases performed in an 8-hour OR day compared to 2 CAS cases.6
There is no question that improved surgical technique can enhance OR efficiency. However, this model, while promising, is difficult to implement on a wide scale due to surgeon preferences, vendor limitations, and added costs related to the advanced preoperative imaging studies, manufacturing of the custom guides, and maintenance of navigation equipment. In addition, while interventions such as the use of barbed suture have the potential for speeding closure time, the time saved (4.7 minutes in one randomized trial)7 may not be enough to affect major utilization differences per OR per day. These technologies are also frequently employed by high-volume surgeons with high-volume teams and institutions.
Ideally, we need investment in the human capital and a collective change in work cultures to produce high-quality, well-choreographed, easily reproducible routines. An efficient OR requires the synchronous involvement of a large team of individuals, including hospital administrators, surgery schedulers, surgeons, anesthesiologists, preoperative holding area staff, OR nurses, surgical attendants, sterile processing personnel, and recovery room nurses. Case schedulers should match allocated block time with time required for surgery based on the historical performance of the individual surgeon, preferably scheduling similar cases on the same day. Preoperative work-up and medical clearance should be completed prior to scheduling to avoid last-minute cancellations. Patient reminders and accommodations for those traveling from long distances can further minimize late arrivals. Prompt initiation of the perioperative clinical pathway upon a patient’s check-in is important. The surgical site should be marked and the anesthesia plan confirmed upon arrival in the preoperative holding area. Necessary products need to be ready and/or administrated in time for transfer to the OR. These include prophylactic antibiotics, coagulation factors (eg, tranexamic acid), and blood products as indicated. Spinal anesthesia, regional nerve blocks, and intravenous (IV) lines should be completed before transfer to the OR. A “block room” close to the OR can allow concurrent induction of anesthesia and has been shown to increase the number of surgical cases performed during a regular workday.8 Hair clipping within the surgical site and pre-scrubbing of the operative extremity should also be performed prior to transfer to the OR in order to minimize micro-organisms and dispersal of loose hair onto the sterile field.
Upon arrival of the patient to the OR, instrument tables based on the surgeon preference cards should be opened, instrument count and implant templating completed, necessary imaging displayed, and OR staff ready with specific responsibilities assigned to each member. Small and colleagues9 showed that using dedicated orthopedic staff familiar with the surgical routine decreased operative time by 19 minutes per procedure, or 1.25 hours for a surgeon performing 4 primary TJAs per day. Practices such as routine placement of a urinary catheter should be seriously scrutinized. In a randomized prospective study of patients undergoing total hip arthroplasty under spinal anesthesia, Miller and colleagues10 found no benefit for indwelling catheters in preventing urinary retention. In another randomized prospective study, Huang and colleagues11 found the prevalence of urinary tract infections was significantly higher in TJA patients who received indwelling urinary catheters.
A scrub nurse familiar with the instruments, their assembly, and the sequence of events can ensure efficient surgical flow. The scrub nurse needs to anticipate missing or defective tools and call for them, ideally before the incision is made. Direct comparison studies are needed to assess the efficacy of routine intraoperative imaging vs commercially available universal cup alignment guides or clinical examinations in determining acceptable component positioning and limb length. Following component implantation and before wound closure, the circulating nurse should initiate the process of acquisition of a recovery room bed, make sure dressing supplies and necessary equipment are available, and call for surgical attendants. Lack of surgical attendants, delayed transfer from the OR table to hospital bed, and prolonged acquisition of a recovery room bed have been identified as major OR inefficiencies in a retrospective study by Attarian and colleagues.12
In summary, time is the OR’s most valuable resource.13 We believe that a consistent, almost automated attitude to the above procedures decreases variability and improves efficiency. By providing clear communication of the surgical needs with the team, having consistent anesthesia and nursing staff, implementing consistent perioperative protocols, and insuring that all necessary instruments and modalities are available prior to starting the procedure, we were able to sustainably increase OR throughput in a large teaching hospital.9,14 This process, however, requires constant review to identify and eliminate new gaps, with each member of the team sharing a frank desire to improve. In this regard, hospital administrators share the duty to facilitate the implementation of any necessary changes, allocation of needed resources, and rewarding good effort, which could ultimately increase staff satisfaction and retention. Because efficiency is the ratio of benefits (eg, revenue, safety, etc.) to investment (eg, implant costs, wages, etc.), raises the question: what would be the effect of transitioning from hourly-wage to a salary-based system for key support staff? Unlike hourly-wage personnel, who have no incentive for productivity, a salaried employee assigned to a high-efficiency OR will inherently strive for improvement, employing higher organizational skills to accomplish a common goal. To our knowledge, there is no published data on this topic.
1. Krupka DC, Sandberg WS. Operating room design and its impact on operating room economics. Curr Opin Anaesthesiol. 2006;19(2):185-191.
2. Scott WN, Booth RE Jr, Dalury DF, Healy WL, Lonner JH. Efficiency and economics in joint arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 5:33-36.
3. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
4. DeHaan AM, Adams JR, DeHart ML, Huff TW. Patient-specific versus conventional instrumentation for total knee arthroplasty: peri-operative and cost differences. J Arthroplasty. 2014;29(11):2065-2069.
5. Mont MA, McElroy MJ, Johnson AJ, Pivec R; Single-Use Multicenter Trial Group Writing Group. Single-use instruments, cutting blocks, and trials increase efficiency in the operating room during total knee arthroplasty: a prospective comparison of navigated and non-navigated cases. J Arthroplasty. 2013;28(7):1135-1140.
6. Lionberger DR, Crocker CL, Chen V. Patient specific instrumentation. J Arthroplasty. 2014;29(9):1699-1704.
7. Sah AP. Is there an advantage to knotless barbed suture in TKA wound closure? A randomized trial in simultaneous bilateral TKAs. Clin Orthop Relat Res. 2015;473(6):2019-2027.
8. Torkki PM, Marjamaa RA, Torkki MI, Kallio PE, Kirvelä OA. Use of anesthesia induction rooms can increase the number of urgent orthopedic cases completed within 7 hours. Anesthesiology. 2005;103(2):401-405.
9. Small TJ, Gad BV, Klika AK, Mounir-Soliman LS, Gerritsen RL, Barsoum WK. Dedicated orthopedic operating room unit improves operating room efficiency. J Arthroplasty. 2013;28(7):1066-1071.e2.
10. Miller AG, McKenzie J, Greenky M, et al. Spinal anesthesia: should everyone receive a urinary catheter?: a randomized, prospective study of patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2013;95(16):1498-1503.
11. Huang Z, Ma J, Shen B, Pei F. General anesthesia: to catheterize or not? A prospective randomized controlled study of patients undergoing total knee arthroplasty. J Arthroplasty. 2015;30(3):502-506.
12. Attarian DE, Wahl JE, Wellman SS, Bolognesi MP. Developing a high-efficiency operating room for total joint arthroplasty in an academic setting. Clin Orthop Relat Res. 2013;471(6):1832-1836.
13. Gamble M. 6 cornerstones of operating room efficiency: best practices for each. Becker’s Hospital Review Web site. http://www.beckershospitalreview.com/or-efficiencies/6-cornerstones-of-operating-room-efficiency-best-practices-for-each.html. Updated January 18, 2013. Accessed September 3, 2015.
14. Smith MP, Sandberg WS, Foss J, et al. High-throughput operating room system for joint arthroplasties durably outperforms routine processes. Anesthesiology. 2008;109(1):25-35.
1. Krupka DC, Sandberg WS. Operating room design and its impact on operating room economics. Curr Opin Anaesthesiol. 2006;19(2):185-191.
2. Scott WN, Booth RE Jr, Dalury DF, Healy WL, Lonner JH. Efficiency and economics in joint arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 5:33-36.
3. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
4. DeHaan AM, Adams JR, DeHart ML, Huff TW. Patient-specific versus conventional instrumentation for total knee arthroplasty: peri-operative and cost differences. J Arthroplasty. 2014;29(11):2065-2069.
5. Mont MA, McElroy MJ, Johnson AJ, Pivec R; Single-Use Multicenter Trial Group Writing Group. Single-use instruments, cutting blocks, and trials increase efficiency in the operating room during total knee arthroplasty: a prospective comparison of navigated and non-navigated cases. J Arthroplasty. 2013;28(7):1135-1140.
6. Lionberger DR, Crocker CL, Chen V. Patient specific instrumentation. J Arthroplasty. 2014;29(9):1699-1704.
7. Sah AP. Is there an advantage to knotless barbed suture in TKA wound closure? A randomized trial in simultaneous bilateral TKAs. Clin Orthop Relat Res. 2015;473(6):2019-2027.
8. Torkki PM, Marjamaa RA, Torkki MI, Kallio PE, Kirvelä OA. Use of anesthesia induction rooms can increase the number of urgent orthopedic cases completed within 7 hours. Anesthesiology. 2005;103(2):401-405.
9. Small TJ, Gad BV, Klika AK, Mounir-Soliman LS, Gerritsen RL, Barsoum WK. Dedicated orthopedic operating room unit improves operating room efficiency. J Arthroplasty. 2013;28(7):1066-1071.e2.
10. Miller AG, McKenzie J, Greenky M, et al. Spinal anesthesia: should everyone receive a urinary catheter?: a randomized, prospective study of patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2013;95(16):1498-1503.
11. Huang Z, Ma J, Shen B, Pei F. General anesthesia: to catheterize or not? A prospective randomized controlled study of patients undergoing total knee arthroplasty. J Arthroplasty. 2015;30(3):502-506.
12. Attarian DE, Wahl JE, Wellman SS, Bolognesi MP. Developing a high-efficiency operating room for total joint arthroplasty in an academic setting. Clin Orthop Relat Res. 2013;471(6):1832-1836.
13. Gamble M. 6 cornerstones of operating room efficiency: best practices for each. Becker’s Hospital Review Web site. http://www.beckershospitalreview.com/or-efficiencies/6-cornerstones-of-operating-room-efficiency-best-practices-for-each.html. Updated January 18, 2013. Accessed September 3, 2015.
14. Smith MP, Sandberg WS, Foss J, et al. High-throughput operating room system for joint arthroplasties durably outperforms routine processes. Anesthesiology. 2008;109(1):25-35.
Biomechanical Evaluation of All-Polyethylene Pegged Bony Ingrowth Glenoid Fixation Techniques on Implant Micromotion
Since Neer and colleagues1 first reported in 1982, glenoid loosening persists as a common cause of anatomic total shoulder arthroplasty (TSA) failure.1-4 Currently, cemented, all-polyethylene glenoid components are the gold standard, and minimum clinical survival of 10 to 15 years is expected.3,5 Several clinical studies5-9 and in vitro biomechanical studies10 have suggested an advantage of pegged over keeled glenoid components, but glenoid component loosening remains a frequent complication,11 with the cement–implant interface suggested as the weak link of fixation.10,12 In addition to mechanical loosening, poor cement penetration and heat-induced necrosis have been postulated as contributing to glenoid component loosening.13,14
Because of these potential complications, there is a growing consideration to minimize or abandon cement fixation and rely on biological fixation to polyethylene for long-term component stability.15 A newer pegged glenoid component design consists of traditional, peripherally located pegs designed for cement fixation as well as a central, uncemented, fluted, interference-fit peg that allows for bony ingrowth. Short-term clinical studies have shown that bony ingrowth into the space between the flutes can be achieved with a hybrid cementation technique and that, when that occurs, excellent outcomes are likely.13,16-19 The immediate in vivo stability of this implant design upon initial implantation, before the cement has cured, has prompted some surgeons to consider implanting the device without cement. In a recent series in which this implant design was used without cement, clinical and radiographic results were promising.15
Despite the widespread clinical use, little biomechanical work has been done to characterize initial fixation of all-polyethylene pegged glenoid implants. We conducted a study to compare glenoid micromotion in an all-polyethylene, centrally fluted pegged glenoid component as a function of 3 fixation techniques: cementless interference-fit fixation, hybrid partial cementation based on manufacturer recommendations, and full cementation to simulate a gold-standard, traditional, cemented, pegged design.
Materials and MethodsBiomechanical Testing
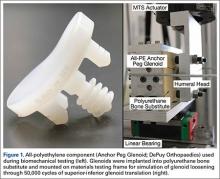
The biomechanical testing methodology used in this study was based on previous studies20-23 and on ASTM standard F2028-1224 using polyurethane bone substitute 0.24 g/cm3 (Pacific Research Laboratories) with ultimate strength of 4.9 MPa and compressive modulus of 123 MPa for component implantation. This material was selected because its mechanical properties are similar to those of cancellous glenoid bone in primary shoulder arthroplasty,25 and it minimizes variability with use of cadaveric specimens. Components were mounted on an MTS 858 Mini-Bionix II materials testing frame (Figure 1). A static compressive load of 756 N (170 lb) was applied via a mass-pulley system simulating the joint compressive force the shoulder is likely to experience during higher load activities.24,26 The glenoid component was positioned on a linear bearing to allow for joint compression.
Test Groups and Cement Fixation Techniques
All-polyethylene pegged glenoid components (Anchor Peg Glenoid, size 44; DePuy Orthopaedics) were used for biomechanical testing (Figure 1). Polyurethane blocks were reamed with a size 44 reamer until the superior-inferior distance reached 33 mm, ensuring complete seating of implant. Three fixation-technique groups were formed: interference-fit, hybrid cement, and fully cemented. Interference-fit fixation was done without polymethylmethacrylate (PMMA) cement. In hybrid fixation, 2 cm3 of PMMA (SpeedSet Cement, Stryker Orthopaedics) was injected (using a catheter tip syringe) into the peripheral peg holes and manually pressurized; the central peg was press-fit into polyurethane bone substitute. In the fully cemented group, both peripheral and central peg holes received PMMA; the peripheral peg holes were cemented as in hybrid fixation, and the central peg hole was injected with 3 cm3 of PMMA, which was then manually pressurized. The humeral head component (Global Advantage, 44×18 mm; DePuy Synthes) was mounted on the test frame actuator and centrally located within the glenoid at the start of the test.
Determination of Humeral Head Translation via Subluxation Testing
Humeral head subluxation distance, simulating a humeral head rim loading event, was calculated on the basis of preliminary tests outlined in the ASTM standard.24 Three glenoids (1 per fixation technique) were mounted on the test frame with a humeral head positioned centrally within the glenoid. After the joint compressive force was applied, the humeral head was translated along the true superior axis of the glenoid at a rate of 50 mm/min. Testing software was used to record humeral head displacement and load data at a frequency of 100 Hz. Humeral head subluxation displacement was determined at the end of the linear region of the force versus displacement response. This distance, averaged from the 3 subluxation tests, was used as the subluxation distance during cyclic testing.
Determination of Glenoid Component Motion via Cyclic Testing
After subluxation displacement was determined, glenoid components were mounted on the test frame (5 per fixation technique) and subjected to 50,000 cycles of humeral head translation at a frequency of 2 Hz. Amplitude of the humeral head displacement against the glenoid component followed a sinusoidal pattern with maxima and minima represented by the subluxation displacement (positive and negative, respectively). Glenoid edge compression/distraction of the superior edge and glenoid inferior/superior translation were monitored with 2 variable resistance reluctance transducers (Microminiature DVRT; 4.5-µm resolution; MicroStrain) secured to the glenoid component and testing fixture.
Microminiature DVRT measurements of glenoid motion were taken for 5 consecutive cycles at cycles 1, 20, 100, 500, 1000, 5000, 10,000, 15,000, 20,000, 30,000, 40,000, and 50,000. Distraction-compression displacement and superior-inferior translation measurements were recorded relative to the glenoid position with the humeral head at the neutral position at a given cycle. Final glenoid micromotion data were calculated from the mean of consecutive cycles at each cycle time point.
Statistical Analysis
Glenoid motion results are reported as means and standard deviations. Comparisons with 2 factors of fixation technique and number of cycles for glenoid distraction, glenoid compression, and absolute glenoid translation were characterized with 2-way analysis of variance (SigmaPlot Version 11.0; Systat Software), with the Holm-Šídák test used for post hoc determination of significant relationships.
Results
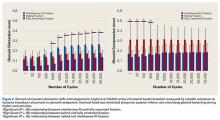
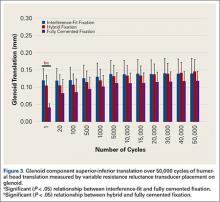
Under subluxation testing, the humeral head translation distance at the end of the linear region was determined to be 0.50 mm. Subsequently for cyclic testing, the humeral head was then translated 0.50 mm from the neutral position of the humeral head along both the superior and inferior axes of the glenoid. All glenoids successfully completed the entire 50,000 cycles of testing. For the glenoid component, Figure 2 depicts distraction and compression, and Figure 3 depicts superior-inferior translation.
Glenoid Component Distraction
Overall, mean (SD) glenoid distraction was significantly higher for interference-fit fixation, 0.21 (0.10) mm, than for hybrid cement fixation, 0.16 (0.05) mm (P < .001), and fully cemented fixation, 0.09 (0.07) mm (P < .001). It was also significantly higher for hybrid fixation than fully cemented fixation (P < .001). From cycle 1000 to cycle 50,000, distraction was significantly higher for interference-fit fixation than for fully cemented fixation at each time point (P < .05).
Glenoid Component Compression
Mean (SD) compression was significantly higher for hybrid cement fixation, 0.31 (0.13) mm, than for interference-fit fixation, 0.17 (0.07) mm (P < .001), and fully cemented fixation, 0.17 (0.08) mm (P < .001). No significant difference was found between interference-fit and fully cemented fixation (P = .793) (Figure 2). At cycles 1, 20, 100, and 500, compression was significantly higher for hybrid fixation than for fully cemented fixation (P < .05). In addition, at cycle 500, it was significantly higher for hybrid fixation than for interference-fit fixation (P < .05).
Glenoid Component Translation
Mean (SD) glenoid translation was significantly lower for fully cemented fixation, 0.10 (0.04) mm, than for interference-fit fixation, 0.13 (0.04) mm (P < .001), and hybrid cement fixation, 0.13 (0.03) mm (P < .001), with all time points considered. There was no significant difference between interference-fit and hybrid fixation (P = .343). Initial translation at cycle 1 was significantly higher for interference-fit and hybid fixation than for fully cemented fixation.
Discussion
Despite advances in glenoid component design, glenoid loosening remains the most common cause of anatomical TSA failure. Recent implants have been designed to take advantage of an all-polyethylene component while providing long-lasting fixation through bony ingrowth into a central peg. In a study of the hybrid cementation technique drescribed here, Groh17 found no glenoid loosening or radiolucent lines but discovered fingerlike projections of bone between the flanges of the implant in 24 (29%) of 83 cases. Churchill and colleagues16 also reported bony ingrowth into the central peg in 15 (75%) of 20 patients. Furthermore, Arnold and colleagues13 reported complete bony ingrowth (6/6 inter-fin compartments) in 23 (71%) of 35 shoulders at a mean of 43 months. Wirth and colleagues19 reported increased radiodensity between the flanges of the central peg in 30 of 44 cases (68%) and osteolysis around the central peg in 3 of 44 cases (7%) at 3 years.
There are also reports of successful bony ingrowth associated with all-polyethylene components implanted without cement. In a canine study using an early ingrowth implant design, Wirth and colleagues27 showed that, though initial fixation was superior with a cemented, keeled implant, pullout strength of the uncemented, pegged implant improved over time and eventually far surpassed that of the cemented, keeled implant owing to both the loosening of the cemented component and the bony ingrowth into the central peg component. Furthermore, Anglin and colleagues10 confirmed that component micromotion was lower with pegged glenoid components than with keeled components in a biomechanical model. De Wilde and colleagues15 recently reported on a series of uncemented, central fluted peg glenoids implanted in 34 patients followed clinically and with computed tomography for a minimum of 24 months. The investigators found bony ingrowth into the central peg in 27 (79%) of 34 patients and no signs of loosening in 30 (88%) of 34 patients. Incomplete lucencies around 1 or 2 peripheral pegs were found in 2 (6%) of 34 patients, and complete lucencies around 2 or more peripheral pegs were found in 2 (6%) of 34 patients. However, there was no statistical difference in clinical outcome between patients with and without loosening.
With this type of implant, initial fixation that provides stability while minimizing micromotion under biomechanical loading likely is crucial for attaining bony growth within the central peg flanges. To our knowledge, this is the first biomechanical study to compare micromotion using 3 different fixation methods with a central fluted peg glenoid component design. Of all these fixation methods, fully cemented fixation yielded the most stable glenoid throughout testing with respect to the evaluated parameters. However, this method is not necessarily clinically applicable, as a fully cemented glenoid would inhibit any bony growth within the central flange, which is necessary for long-term biological fixation. Our data showed that, though glenoid distraction was significantly lower with hybrid cement fixation, this fixation method exhibited significantly higher glenoid compression. In addition, there were no significant differences between glenoid components with hybrid fixation and glenoid components with interference-fit fixation with respect to component translation in the superior-inferior direction. These findings may indicate that initial fixation is not significantly improved by the addition of cement to the peripheral pegs in a glenoid component with a central fluted peg design.
The interference fit of the central peg is primarily responsible for conferring long-term implant stability,13,27 which is ultimately achieved by bony formation between the flutes of the peg. Other authors have reported that, for bony ingrowth to occur, micromotion between the bone–implant interface must not exceed 20 to 150 µm.28-30 Other than for interference-fit distraction at more than 1000 cycles and hybrid cement fixation compression at each time point throughout testing, our data fall within the reported upper limits of micromotion to support bony ingrowth. Increased micromotion in the interference-fit fixation group is seen at later time points and may be caused by the inability to simulate the potential fixation gained from bony ingrowth allowed with this surgical technique. Research is needed to further explain this increase in distraction.
Results from this study must be interpreted with caution because of limitations of the in vitro testing methodology. This biomechanical model using bone substitute characterizes glenoid fixation at time zero, directly after implantation, followed by repetitive cyclic loading simulating 5 years of implant service. This differs from the clinical scenario in which the shoulder undergoes postoperative immobilization or protected motion during which the early phases of bony remodeling are likely occurring. Furthermore, simulation of 5 years of implant service may not be necessary for an implant that is expected to achieve ultimate fixation by bony ingrowth within the first several months after implantation. Use of this implant without cement is classified off-label, and surgeons should take this into consideration during implantation. Last, this study could not simulate the effect of bony ingrowth on fixation, though our experimental technique of cementing the central peg may be a gross approximation of a fully ingrown central peg and its expected rigid fixation.
Fully cemented fixation of a polyethylene glenoid is superior to hybrid cement fixation and interference-fit fixation with respect to early glenoid micromotion. However, the long-term stability of a fully cemented polyethylene glenoid component remains a clinical concern, as fixation is achieved by bony ingrowth around the central fluted peg of the implant. In this study, interference-fit and hybrid fixation had equivocal component micromotion in biomechanical testing. Our findings suggest that cementation of the peripheral pegs confers no additional initial stability over an uncemented interference-fit technique in a biomechanical model. More research is needed to further evaluate interference-fit fixation as a viable option for implantation of a central fluted, all-polyethylene glenoid component.
1. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
2. Sperling JW, Cofield RH, O’Driscoll SW, Torchia ME, Rowland CM. Radiographic assessment of ingrowth total shoulder arthroplasty. J Shoulder Elbow Surg. 2000;9(6):507-513.
3. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
4. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
5. Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):859-863.
6. Edwards TB, Labriola JE, Stanley RJ, O’Connor DP, Elkousy HA, Gartsman GM. Radiographic comparison of pegged and keeled glenoid components using modern cementing techniques: a prospective randomized study. J Shoulder Elbow Surg. 2010;19(2):251-257.
7. Gartsman GM, Elkousy HA, Warnock KM, Edwards TB, O’Connor DP. Radiographic comparison of pegged and keeled glenoid components. J Shoulder Elbow Surg. 2005;14(3):252-257.
8. Klepps S, Chiang AS, Miller S, Jiang CY, Hazrati Y, Flatow EL. Incidence of early radiolucent glenoid lines in patients having total shoulder replacements. Clin Orthop Relat Res. 2005;(435):118-125.
9. Lazarus MD, Jensen KL, Southworth C, Matsen FA 3rd. The radiographic evaluation of keeled and pegged glenoid component insertion. J Bone Joint Surg Am. 2002;84(7):1174-1182.
10. Anglin C, Wyss UP, Nyffeler RW, Gerber C. Loosening performance of cemented glenoid prosthesis design pairs. Clin Biomech. 2001;16(2):144-150.
11. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
12. Gregory T, Hansen U, Taillieu F, et al. Glenoid loosening after total shoulder arthroplasty: an in vitro CT-scan study. J Orthop Res. 2009;27(12):1589-1595.
13. Arnold RM, High RR, Grosshans KT, Walker CW, Fehringer EV. Bone presence between the central peg’s radial fins of a partially cemented pegged all poly glenoid component suggest few radiolucencies. J Shoulder Elbow Surg. 2011;20(2):315-321.
14. Churchill RS, Boorman RS, Fehringer EV, Matsen FA 3rd. Glenoid cementing may generate sufficient heat to endanger the surrounding bone. Clin Orthop Relat Res. 2004;(419):76-79.
15. De Wilde L, Dayerizadeh N, De Neve F, Basamania C, Van Tongel A. Fully uncemented glenoid component in total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(10):e1-e7.
16. Churchill RS, Zellmer C, Zimmers HJ, Ruggero R. Clinical and radiographic analysis of a partially cemented glenoid implant: five-year minimum follow-up. J Shoulder Elbow Surg. 2010;19(7):1091-1097.
17. Groh GI. Survival and radiographic analysis of a glenoid component with a cementless fluted central peg. J Shoulder Elbow Surg. 2010;19(8):1265-1268.
18. Vidil A, Valenti P, Guichoux F, Barthas JH. CT scan evaluation of glenoid component fixation: a prospective study of 27 minimally cemented shoulder arthroplasties. Eur J Orthop Surg Traumatol. 2012;23(5):521-525.
19. Wirth MA, Loredo R, Garcia G, Rockwood CA Jr, Southworth C, Iannotti JP. Total shoulder arthroplasty with an all-polyethylene pegged bone-ingrowth glenoid component: a clinical and radiographic outcome study. J Bone Joint Surg Am. 2012;94(3):260-267.
20. Anglin C, Wyss UP, Pichora DR. Mechanical testing of shoulder prostheses and recommendations for glenoid design. J Shoulder Elbow Surg. 2000;9(4):323-331.
21. Hoenig MP, Loeffler B, Brown S, et al. Reverse glenoid component fixation: is a posterior screw necessary? J Shoulder Elbow Surg. 2010;19(4):544-549.
22. Sarah J, Sanjay G, Sanjay S, et al. Failure mechanism of the all-polyethylene glenoid implant. J Biomech. 2010;43(4):714-719.
23. Suárez DR, Nerkens W, Valstar ER, Rozing PM, van Keulen F. Interface micromotions increase with less-conforming cementless glenoid components. J Shoulder Elbow Surg. 2012;21(4):474-482.
24. ASTM International. Standard Test Methods for Dynamic Evaluation of Glenoid Loosening or Disassociation. West Conshocken, PA: ASTM International; 2012. ASTM F2028-08.
25. Anglin C, Tolhurst P, Wyss UP, Pichora DR. Glenoid cancellous bone strength and modulus. J Biomech. 1999;32(10):1091-1097.
26. Anglin C, Wyss U, Pichora D. Glenohumeral contact forces. Proc Inst Mech Eng H. 2000;214(6):637-644.
27. Wirth MA, Korvick DL, Basamania CJ, Toro F, Aufdemorte TB, Rockwood CA Jr. Radiologic, mechanical, and histologic evaluation of 2 glenoid prosthesis designs in a canine model. J Shoulder Elbow Surg. 2001;10(2):140-148.
28. Pilliar RM, Lee JM, Maniatopoulos C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin Orthop Relat Res. 1986;(208):108-113.
29. Ramamurti BS, Orr TE, Bragdon CR, Lowenstein JD, Jasty M, Harris WH. Factors influencing stability at the interface between a porous surface and cancellous bone: a finite element analysis of a canine in vivo micromotion experiment. J Biomed Mater Res. 1997;36(2):274-280.
30. Şahin S, Cehreli MC, Yalçın E. The influence of functional forces on the biomechanics of implant-supported prostheses—a review. J Dent. 2002;30(7-8):271-282.
Since Neer and colleagues1 first reported in 1982, glenoid loosening persists as a common cause of anatomic total shoulder arthroplasty (TSA) failure.1-4 Currently, cemented, all-polyethylene glenoid components are the gold standard, and minimum clinical survival of 10 to 15 years is expected.3,5 Several clinical studies5-9 and in vitro biomechanical studies10 have suggested an advantage of pegged over keeled glenoid components, but glenoid component loosening remains a frequent complication,11 with the cement–implant interface suggested as the weak link of fixation.10,12 In addition to mechanical loosening, poor cement penetration and heat-induced necrosis have been postulated as contributing to glenoid component loosening.13,14
Because of these potential complications, there is a growing consideration to minimize or abandon cement fixation and rely on biological fixation to polyethylene for long-term component stability.15 A newer pegged glenoid component design consists of traditional, peripherally located pegs designed for cement fixation as well as a central, uncemented, fluted, interference-fit peg that allows for bony ingrowth. Short-term clinical studies have shown that bony ingrowth into the space between the flutes can be achieved with a hybrid cementation technique and that, when that occurs, excellent outcomes are likely.13,16-19 The immediate in vivo stability of this implant design upon initial implantation, before the cement has cured, has prompted some surgeons to consider implanting the device without cement. In a recent series in which this implant design was used without cement, clinical and radiographic results were promising.15
Despite the widespread clinical use, little biomechanical work has been done to characterize initial fixation of all-polyethylene pegged glenoid implants. We conducted a study to compare glenoid micromotion in an all-polyethylene, centrally fluted pegged glenoid component as a function of 3 fixation techniques: cementless interference-fit fixation, hybrid partial cementation based on manufacturer recommendations, and full cementation to simulate a gold-standard, traditional, cemented, pegged design.
Materials and MethodsBiomechanical Testing
The biomechanical testing methodology used in this study was based on previous studies20-23 and on ASTM standard F2028-1224 using polyurethane bone substitute 0.24 g/cm3 (Pacific Research Laboratories) with ultimate strength of 4.9 MPa and compressive modulus of 123 MPa for component implantation. This material was selected because its mechanical properties are similar to those of cancellous glenoid bone in primary shoulder arthroplasty,25 and it minimizes variability with use of cadaveric specimens. Components were mounted on an MTS 858 Mini-Bionix II materials testing frame (Figure 1). A static compressive load of 756 N (170 lb) was applied via a mass-pulley system simulating the joint compressive force the shoulder is likely to experience during higher load activities.24,26 The glenoid component was positioned on a linear bearing to allow for joint compression.
Test Groups and Cement Fixation Techniques
All-polyethylene pegged glenoid components (Anchor Peg Glenoid, size 44; DePuy Orthopaedics) were used for biomechanical testing (Figure 1). Polyurethane blocks were reamed with a size 44 reamer until the superior-inferior distance reached 33 mm, ensuring complete seating of implant. Three fixation-technique groups were formed: interference-fit, hybrid cement, and fully cemented. Interference-fit fixation was done without polymethylmethacrylate (PMMA) cement. In hybrid fixation, 2 cm3 of PMMA (SpeedSet Cement, Stryker Orthopaedics) was injected (using a catheter tip syringe) into the peripheral peg holes and manually pressurized; the central peg was press-fit into polyurethane bone substitute. In the fully cemented group, both peripheral and central peg holes received PMMA; the peripheral peg holes were cemented as in hybrid fixation, and the central peg hole was injected with 3 cm3 of PMMA, which was then manually pressurized. The humeral head component (Global Advantage, 44×18 mm; DePuy Synthes) was mounted on the test frame actuator and centrally located within the glenoid at the start of the test.
Determination of Humeral Head Translation via Subluxation Testing
Humeral head subluxation distance, simulating a humeral head rim loading event, was calculated on the basis of preliminary tests outlined in the ASTM standard.24 Three glenoids (1 per fixation technique) were mounted on the test frame with a humeral head positioned centrally within the glenoid. After the joint compressive force was applied, the humeral head was translated along the true superior axis of the glenoid at a rate of 50 mm/min. Testing software was used to record humeral head displacement and load data at a frequency of 100 Hz. Humeral head subluxation displacement was determined at the end of the linear region of the force versus displacement response. This distance, averaged from the 3 subluxation tests, was used as the subluxation distance during cyclic testing.
Determination of Glenoid Component Motion via Cyclic Testing
After subluxation displacement was determined, glenoid components were mounted on the test frame (5 per fixation technique) and subjected to 50,000 cycles of humeral head translation at a frequency of 2 Hz. Amplitude of the humeral head displacement against the glenoid component followed a sinusoidal pattern with maxima and minima represented by the subluxation displacement (positive and negative, respectively). Glenoid edge compression/distraction of the superior edge and glenoid inferior/superior translation were monitored with 2 variable resistance reluctance transducers (Microminiature DVRT; 4.5-µm resolution; MicroStrain) secured to the glenoid component and testing fixture.
Microminiature DVRT measurements of glenoid motion were taken for 5 consecutive cycles at cycles 1, 20, 100, 500, 1000, 5000, 10,000, 15,000, 20,000, 30,000, 40,000, and 50,000. Distraction-compression displacement and superior-inferior translation measurements were recorded relative to the glenoid position with the humeral head at the neutral position at a given cycle. Final glenoid micromotion data were calculated from the mean of consecutive cycles at each cycle time point.
Statistical Analysis
Glenoid motion results are reported as means and standard deviations. Comparisons with 2 factors of fixation technique and number of cycles for glenoid distraction, glenoid compression, and absolute glenoid translation were characterized with 2-way analysis of variance (SigmaPlot Version 11.0; Systat Software), with the Holm-Šídák test used for post hoc determination of significant relationships.
Results
Under subluxation testing, the humeral head translation distance at the end of the linear region was determined to be 0.50 mm. Subsequently for cyclic testing, the humeral head was then translated 0.50 mm from the neutral position of the humeral head along both the superior and inferior axes of the glenoid. All glenoids successfully completed the entire 50,000 cycles of testing. For the glenoid component, Figure 2 depicts distraction and compression, and Figure 3 depicts superior-inferior translation.
Glenoid Component Distraction
Overall, mean (SD) glenoid distraction was significantly higher for interference-fit fixation, 0.21 (0.10) mm, than for hybrid cement fixation, 0.16 (0.05) mm (P < .001), and fully cemented fixation, 0.09 (0.07) mm (P < .001). It was also significantly higher for hybrid fixation than fully cemented fixation (P < .001). From cycle 1000 to cycle 50,000, distraction was significantly higher for interference-fit fixation than for fully cemented fixation at each time point (P < .05).
Glenoid Component Compression
Mean (SD) compression was significantly higher for hybrid cement fixation, 0.31 (0.13) mm, than for interference-fit fixation, 0.17 (0.07) mm (P < .001), and fully cemented fixation, 0.17 (0.08) mm (P < .001). No significant difference was found between interference-fit and fully cemented fixation (P = .793) (Figure 2). At cycles 1, 20, 100, and 500, compression was significantly higher for hybrid fixation than for fully cemented fixation (P < .05). In addition, at cycle 500, it was significantly higher for hybrid fixation than for interference-fit fixation (P < .05).
Glenoid Component Translation
Mean (SD) glenoid translation was significantly lower for fully cemented fixation, 0.10 (0.04) mm, than for interference-fit fixation, 0.13 (0.04) mm (P < .001), and hybrid cement fixation, 0.13 (0.03) mm (P < .001), with all time points considered. There was no significant difference between interference-fit and hybrid fixation (P = .343). Initial translation at cycle 1 was significantly higher for interference-fit and hybid fixation than for fully cemented fixation.
Discussion
Despite advances in glenoid component design, glenoid loosening remains the most common cause of anatomical TSA failure. Recent implants have been designed to take advantage of an all-polyethylene component while providing long-lasting fixation through bony ingrowth into a central peg. In a study of the hybrid cementation technique drescribed here, Groh17 found no glenoid loosening or radiolucent lines but discovered fingerlike projections of bone between the flanges of the implant in 24 (29%) of 83 cases. Churchill and colleagues16 also reported bony ingrowth into the central peg in 15 (75%) of 20 patients. Furthermore, Arnold and colleagues13 reported complete bony ingrowth (6/6 inter-fin compartments) in 23 (71%) of 35 shoulders at a mean of 43 months. Wirth and colleagues19 reported increased radiodensity between the flanges of the central peg in 30 of 44 cases (68%) and osteolysis around the central peg in 3 of 44 cases (7%) at 3 years.
There are also reports of successful bony ingrowth associated with all-polyethylene components implanted without cement. In a canine study using an early ingrowth implant design, Wirth and colleagues27 showed that, though initial fixation was superior with a cemented, keeled implant, pullout strength of the uncemented, pegged implant improved over time and eventually far surpassed that of the cemented, keeled implant owing to both the loosening of the cemented component and the bony ingrowth into the central peg component. Furthermore, Anglin and colleagues10 confirmed that component micromotion was lower with pegged glenoid components than with keeled components in a biomechanical model. De Wilde and colleagues15 recently reported on a series of uncemented, central fluted peg glenoids implanted in 34 patients followed clinically and with computed tomography for a minimum of 24 months. The investigators found bony ingrowth into the central peg in 27 (79%) of 34 patients and no signs of loosening in 30 (88%) of 34 patients. Incomplete lucencies around 1 or 2 peripheral pegs were found in 2 (6%) of 34 patients, and complete lucencies around 2 or more peripheral pegs were found in 2 (6%) of 34 patients. However, there was no statistical difference in clinical outcome between patients with and without loosening.
With this type of implant, initial fixation that provides stability while minimizing micromotion under biomechanical loading likely is crucial for attaining bony growth within the central peg flanges. To our knowledge, this is the first biomechanical study to compare micromotion using 3 different fixation methods with a central fluted peg glenoid component design. Of all these fixation methods, fully cemented fixation yielded the most stable glenoid throughout testing with respect to the evaluated parameters. However, this method is not necessarily clinically applicable, as a fully cemented glenoid would inhibit any bony growth within the central flange, which is necessary for long-term biological fixation. Our data showed that, though glenoid distraction was significantly lower with hybrid cement fixation, this fixation method exhibited significantly higher glenoid compression. In addition, there were no significant differences between glenoid components with hybrid fixation and glenoid components with interference-fit fixation with respect to component translation in the superior-inferior direction. These findings may indicate that initial fixation is not significantly improved by the addition of cement to the peripheral pegs in a glenoid component with a central fluted peg design.
The interference fit of the central peg is primarily responsible for conferring long-term implant stability,13,27 which is ultimately achieved by bony formation between the flutes of the peg. Other authors have reported that, for bony ingrowth to occur, micromotion between the bone–implant interface must not exceed 20 to 150 µm.28-30 Other than for interference-fit distraction at more than 1000 cycles and hybrid cement fixation compression at each time point throughout testing, our data fall within the reported upper limits of micromotion to support bony ingrowth. Increased micromotion in the interference-fit fixation group is seen at later time points and may be caused by the inability to simulate the potential fixation gained from bony ingrowth allowed with this surgical technique. Research is needed to further explain this increase in distraction.
Results from this study must be interpreted with caution because of limitations of the in vitro testing methodology. This biomechanical model using bone substitute characterizes glenoid fixation at time zero, directly after implantation, followed by repetitive cyclic loading simulating 5 years of implant service. This differs from the clinical scenario in which the shoulder undergoes postoperative immobilization or protected motion during which the early phases of bony remodeling are likely occurring. Furthermore, simulation of 5 years of implant service may not be necessary for an implant that is expected to achieve ultimate fixation by bony ingrowth within the first several months after implantation. Use of this implant without cement is classified off-label, and surgeons should take this into consideration during implantation. Last, this study could not simulate the effect of bony ingrowth on fixation, though our experimental technique of cementing the central peg may be a gross approximation of a fully ingrown central peg and its expected rigid fixation.
Fully cemented fixation of a polyethylene glenoid is superior to hybrid cement fixation and interference-fit fixation with respect to early glenoid micromotion. However, the long-term stability of a fully cemented polyethylene glenoid component remains a clinical concern, as fixation is achieved by bony ingrowth around the central fluted peg of the implant. In this study, interference-fit and hybrid fixation had equivocal component micromotion in biomechanical testing. Our findings suggest that cementation of the peripheral pegs confers no additional initial stability over an uncemented interference-fit technique in a biomechanical model. More research is needed to further evaluate interference-fit fixation as a viable option for implantation of a central fluted, all-polyethylene glenoid component.
Since Neer and colleagues1 first reported in 1982, glenoid loosening persists as a common cause of anatomic total shoulder arthroplasty (TSA) failure.1-4 Currently, cemented, all-polyethylene glenoid components are the gold standard, and minimum clinical survival of 10 to 15 years is expected.3,5 Several clinical studies5-9 and in vitro biomechanical studies10 have suggested an advantage of pegged over keeled glenoid components, but glenoid component loosening remains a frequent complication,11 with the cement–implant interface suggested as the weak link of fixation.10,12 In addition to mechanical loosening, poor cement penetration and heat-induced necrosis have been postulated as contributing to glenoid component loosening.13,14
Because of these potential complications, there is a growing consideration to minimize or abandon cement fixation and rely on biological fixation to polyethylene for long-term component stability.15 A newer pegged glenoid component design consists of traditional, peripherally located pegs designed for cement fixation as well as a central, uncemented, fluted, interference-fit peg that allows for bony ingrowth. Short-term clinical studies have shown that bony ingrowth into the space between the flutes can be achieved with a hybrid cementation technique and that, when that occurs, excellent outcomes are likely.13,16-19 The immediate in vivo stability of this implant design upon initial implantation, before the cement has cured, has prompted some surgeons to consider implanting the device without cement. In a recent series in which this implant design was used without cement, clinical and radiographic results were promising.15
Despite the widespread clinical use, little biomechanical work has been done to characterize initial fixation of all-polyethylene pegged glenoid implants. We conducted a study to compare glenoid micromotion in an all-polyethylene, centrally fluted pegged glenoid component as a function of 3 fixation techniques: cementless interference-fit fixation, hybrid partial cementation based on manufacturer recommendations, and full cementation to simulate a gold-standard, traditional, cemented, pegged design.
Materials and MethodsBiomechanical Testing
The biomechanical testing methodology used in this study was based on previous studies20-23 and on ASTM standard F2028-1224 using polyurethane bone substitute 0.24 g/cm3 (Pacific Research Laboratories) with ultimate strength of 4.9 MPa and compressive modulus of 123 MPa for component implantation. This material was selected because its mechanical properties are similar to those of cancellous glenoid bone in primary shoulder arthroplasty,25 and it minimizes variability with use of cadaveric specimens. Components were mounted on an MTS 858 Mini-Bionix II materials testing frame (Figure 1). A static compressive load of 756 N (170 lb) was applied via a mass-pulley system simulating the joint compressive force the shoulder is likely to experience during higher load activities.24,26 The glenoid component was positioned on a linear bearing to allow for joint compression.
Test Groups and Cement Fixation Techniques
All-polyethylene pegged glenoid components (Anchor Peg Glenoid, size 44; DePuy Orthopaedics) were used for biomechanical testing (Figure 1). Polyurethane blocks were reamed with a size 44 reamer until the superior-inferior distance reached 33 mm, ensuring complete seating of implant. Three fixation-technique groups were formed: interference-fit, hybrid cement, and fully cemented. Interference-fit fixation was done without polymethylmethacrylate (PMMA) cement. In hybrid fixation, 2 cm3 of PMMA (SpeedSet Cement, Stryker Orthopaedics) was injected (using a catheter tip syringe) into the peripheral peg holes and manually pressurized; the central peg was press-fit into polyurethane bone substitute. In the fully cemented group, both peripheral and central peg holes received PMMA; the peripheral peg holes were cemented as in hybrid fixation, and the central peg hole was injected with 3 cm3 of PMMA, which was then manually pressurized. The humeral head component (Global Advantage, 44×18 mm; DePuy Synthes) was mounted on the test frame actuator and centrally located within the glenoid at the start of the test.
Determination of Humeral Head Translation via Subluxation Testing
Humeral head subluxation distance, simulating a humeral head rim loading event, was calculated on the basis of preliminary tests outlined in the ASTM standard.24 Three glenoids (1 per fixation technique) were mounted on the test frame with a humeral head positioned centrally within the glenoid. After the joint compressive force was applied, the humeral head was translated along the true superior axis of the glenoid at a rate of 50 mm/min. Testing software was used to record humeral head displacement and load data at a frequency of 100 Hz. Humeral head subluxation displacement was determined at the end of the linear region of the force versus displacement response. This distance, averaged from the 3 subluxation tests, was used as the subluxation distance during cyclic testing.
Determination of Glenoid Component Motion via Cyclic Testing
After subluxation displacement was determined, glenoid components were mounted on the test frame (5 per fixation technique) and subjected to 50,000 cycles of humeral head translation at a frequency of 2 Hz. Amplitude of the humeral head displacement against the glenoid component followed a sinusoidal pattern with maxima and minima represented by the subluxation displacement (positive and negative, respectively). Glenoid edge compression/distraction of the superior edge and glenoid inferior/superior translation were monitored with 2 variable resistance reluctance transducers (Microminiature DVRT; 4.5-µm resolution; MicroStrain) secured to the glenoid component and testing fixture.
Microminiature DVRT measurements of glenoid motion were taken for 5 consecutive cycles at cycles 1, 20, 100, 500, 1000, 5000, 10,000, 15,000, 20,000, 30,000, 40,000, and 50,000. Distraction-compression displacement and superior-inferior translation measurements were recorded relative to the glenoid position with the humeral head at the neutral position at a given cycle. Final glenoid micromotion data were calculated from the mean of consecutive cycles at each cycle time point.
Statistical Analysis
Glenoid motion results are reported as means and standard deviations. Comparisons with 2 factors of fixation technique and number of cycles for glenoid distraction, glenoid compression, and absolute glenoid translation were characterized with 2-way analysis of variance (SigmaPlot Version 11.0; Systat Software), with the Holm-Šídák test used for post hoc determination of significant relationships.
Results
Under subluxation testing, the humeral head translation distance at the end of the linear region was determined to be 0.50 mm. Subsequently for cyclic testing, the humeral head was then translated 0.50 mm from the neutral position of the humeral head along both the superior and inferior axes of the glenoid. All glenoids successfully completed the entire 50,000 cycles of testing. For the glenoid component, Figure 2 depicts distraction and compression, and Figure 3 depicts superior-inferior translation.
Glenoid Component Distraction
Overall, mean (SD) glenoid distraction was significantly higher for interference-fit fixation, 0.21 (0.10) mm, than for hybrid cement fixation, 0.16 (0.05) mm (P < .001), and fully cemented fixation, 0.09 (0.07) mm (P < .001). It was also significantly higher for hybrid fixation than fully cemented fixation (P < .001). From cycle 1000 to cycle 50,000, distraction was significantly higher for interference-fit fixation than for fully cemented fixation at each time point (P < .05).
Glenoid Component Compression
Mean (SD) compression was significantly higher for hybrid cement fixation, 0.31 (0.13) mm, than for interference-fit fixation, 0.17 (0.07) mm (P < .001), and fully cemented fixation, 0.17 (0.08) mm (P < .001). No significant difference was found between interference-fit and fully cemented fixation (P = .793) (Figure 2). At cycles 1, 20, 100, and 500, compression was significantly higher for hybrid fixation than for fully cemented fixation (P < .05). In addition, at cycle 500, it was significantly higher for hybrid fixation than for interference-fit fixation (P < .05).
Glenoid Component Translation
Mean (SD) glenoid translation was significantly lower for fully cemented fixation, 0.10 (0.04) mm, than for interference-fit fixation, 0.13 (0.04) mm (P < .001), and hybrid cement fixation, 0.13 (0.03) mm (P < .001), with all time points considered. There was no significant difference between interference-fit and hybrid fixation (P = .343). Initial translation at cycle 1 was significantly higher for interference-fit and hybid fixation than for fully cemented fixation.
Discussion
Despite advances in glenoid component design, glenoid loosening remains the most common cause of anatomical TSA failure. Recent implants have been designed to take advantage of an all-polyethylene component while providing long-lasting fixation through bony ingrowth into a central peg. In a study of the hybrid cementation technique drescribed here, Groh17 found no glenoid loosening or radiolucent lines but discovered fingerlike projections of bone between the flanges of the implant in 24 (29%) of 83 cases. Churchill and colleagues16 also reported bony ingrowth into the central peg in 15 (75%) of 20 patients. Furthermore, Arnold and colleagues13 reported complete bony ingrowth (6/6 inter-fin compartments) in 23 (71%) of 35 shoulders at a mean of 43 months. Wirth and colleagues19 reported increased radiodensity between the flanges of the central peg in 30 of 44 cases (68%) and osteolysis around the central peg in 3 of 44 cases (7%) at 3 years.
There are also reports of successful bony ingrowth associated with all-polyethylene components implanted without cement. In a canine study using an early ingrowth implant design, Wirth and colleagues27 showed that, though initial fixation was superior with a cemented, keeled implant, pullout strength of the uncemented, pegged implant improved over time and eventually far surpassed that of the cemented, keeled implant owing to both the loosening of the cemented component and the bony ingrowth into the central peg component. Furthermore, Anglin and colleagues10 confirmed that component micromotion was lower with pegged glenoid components than with keeled components in a biomechanical model. De Wilde and colleagues15 recently reported on a series of uncemented, central fluted peg glenoids implanted in 34 patients followed clinically and with computed tomography for a minimum of 24 months. The investigators found bony ingrowth into the central peg in 27 (79%) of 34 patients and no signs of loosening in 30 (88%) of 34 patients. Incomplete lucencies around 1 or 2 peripheral pegs were found in 2 (6%) of 34 patients, and complete lucencies around 2 or more peripheral pegs were found in 2 (6%) of 34 patients. However, there was no statistical difference in clinical outcome between patients with and without loosening.
With this type of implant, initial fixation that provides stability while minimizing micromotion under biomechanical loading likely is crucial for attaining bony growth within the central peg flanges. To our knowledge, this is the first biomechanical study to compare micromotion using 3 different fixation methods with a central fluted peg glenoid component design. Of all these fixation methods, fully cemented fixation yielded the most stable glenoid throughout testing with respect to the evaluated parameters. However, this method is not necessarily clinically applicable, as a fully cemented glenoid would inhibit any bony growth within the central flange, which is necessary for long-term biological fixation. Our data showed that, though glenoid distraction was significantly lower with hybrid cement fixation, this fixation method exhibited significantly higher glenoid compression. In addition, there were no significant differences between glenoid components with hybrid fixation and glenoid components with interference-fit fixation with respect to component translation in the superior-inferior direction. These findings may indicate that initial fixation is not significantly improved by the addition of cement to the peripheral pegs in a glenoid component with a central fluted peg design.
The interference fit of the central peg is primarily responsible for conferring long-term implant stability,13,27 which is ultimately achieved by bony formation between the flutes of the peg. Other authors have reported that, for bony ingrowth to occur, micromotion between the bone–implant interface must not exceed 20 to 150 µm.28-30 Other than for interference-fit distraction at more than 1000 cycles and hybrid cement fixation compression at each time point throughout testing, our data fall within the reported upper limits of micromotion to support bony ingrowth. Increased micromotion in the interference-fit fixation group is seen at later time points and may be caused by the inability to simulate the potential fixation gained from bony ingrowth allowed with this surgical technique. Research is needed to further explain this increase in distraction.
Results from this study must be interpreted with caution because of limitations of the in vitro testing methodology. This biomechanical model using bone substitute characterizes glenoid fixation at time zero, directly after implantation, followed by repetitive cyclic loading simulating 5 years of implant service. This differs from the clinical scenario in which the shoulder undergoes postoperative immobilization or protected motion during which the early phases of bony remodeling are likely occurring. Furthermore, simulation of 5 years of implant service may not be necessary for an implant that is expected to achieve ultimate fixation by bony ingrowth within the first several months after implantation. Use of this implant without cement is classified off-label, and surgeons should take this into consideration during implantation. Last, this study could not simulate the effect of bony ingrowth on fixation, though our experimental technique of cementing the central peg may be a gross approximation of a fully ingrown central peg and its expected rigid fixation.
Fully cemented fixation of a polyethylene glenoid is superior to hybrid cement fixation and interference-fit fixation with respect to early glenoid micromotion. However, the long-term stability of a fully cemented polyethylene glenoid component remains a clinical concern, as fixation is achieved by bony ingrowth around the central fluted peg of the implant. In this study, interference-fit and hybrid fixation had equivocal component micromotion in biomechanical testing. Our findings suggest that cementation of the peripheral pegs confers no additional initial stability over an uncemented interference-fit technique in a biomechanical model. More research is needed to further evaluate interference-fit fixation as a viable option for implantation of a central fluted, all-polyethylene glenoid component.
1. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
2. Sperling JW, Cofield RH, O’Driscoll SW, Torchia ME, Rowland CM. Radiographic assessment of ingrowth total shoulder arthroplasty. J Shoulder Elbow Surg. 2000;9(6):507-513.
3. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
4. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
5. Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):859-863.
6. Edwards TB, Labriola JE, Stanley RJ, O’Connor DP, Elkousy HA, Gartsman GM. Radiographic comparison of pegged and keeled glenoid components using modern cementing techniques: a prospective randomized study. J Shoulder Elbow Surg. 2010;19(2):251-257.
7. Gartsman GM, Elkousy HA, Warnock KM, Edwards TB, O’Connor DP. Radiographic comparison of pegged and keeled glenoid components. J Shoulder Elbow Surg. 2005;14(3):252-257.
8. Klepps S, Chiang AS, Miller S, Jiang CY, Hazrati Y, Flatow EL. Incidence of early radiolucent glenoid lines in patients having total shoulder replacements. Clin Orthop Relat Res. 2005;(435):118-125.
9. Lazarus MD, Jensen KL, Southworth C, Matsen FA 3rd. The radiographic evaluation of keeled and pegged glenoid component insertion. J Bone Joint Surg Am. 2002;84(7):1174-1182.
10. Anglin C, Wyss UP, Nyffeler RW, Gerber C. Loosening performance of cemented glenoid prosthesis design pairs. Clin Biomech. 2001;16(2):144-150.
11. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
12. Gregory T, Hansen U, Taillieu F, et al. Glenoid loosening after total shoulder arthroplasty: an in vitro CT-scan study. J Orthop Res. 2009;27(12):1589-1595.
13. Arnold RM, High RR, Grosshans KT, Walker CW, Fehringer EV. Bone presence between the central peg’s radial fins of a partially cemented pegged all poly glenoid component suggest few radiolucencies. J Shoulder Elbow Surg. 2011;20(2):315-321.
14. Churchill RS, Boorman RS, Fehringer EV, Matsen FA 3rd. Glenoid cementing may generate sufficient heat to endanger the surrounding bone. Clin Orthop Relat Res. 2004;(419):76-79.
15. De Wilde L, Dayerizadeh N, De Neve F, Basamania C, Van Tongel A. Fully uncemented glenoid component in total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(10):e1-e7.
16. Churchill RS, Zellmer C, Zimmers HJ, Ruggero R. Clinical and radiographic analysis of a partially cemented glenoid implant: five-year minimum follow-up. J Shoulder Elbow Surg. 2010;19(7):1091-1097.
17. Groh GI. Survival and radiographic analysis of a glenoid component with a cementless fluted central peg. J Shoulder Elbow Surg. 2010;19(8):1265-1268.
18. Vidil A, Valenti P, Guichoux F, Barthas JH. CT scan evaluation of glenoid component fixation: a prospective study of 27 minimally cemented shoulder arthroplasties. Eur J Orthop Surg Traumatol. 2012;23(5):521-525.
19. Wirth MA, Loredo R, Garcia G, Rockwood CA Jr, Southworth C, Iannotti JP. Total shoulder arthroplasty with an all-polyethylene pegged bone-ingrowth glenoid component: a clinical and radiographic outcome study. J Bone Joint Surg Am. 2012;94(3):260-267.
20. Anglin C, Wyss UP, Pichora DR. Mechanical testing of shoulder prostheses and recommendations for glenoid design. J Shoulder Elbow Surg. 2000;9(4):323-331.
21. Hoenig MP, Loeffler B, Brown S, et al. Reverse glenoid component fixation: is a posterior screw necessary? J Shoulder Elbow Surg. 2010;19(4):544-549.
22. Sarah J, Sanjay G, Sanjay S, et al. Failure mechanism of the all-polyethylene glenoid implant. J Biomech. 2010;43(4):714-719.
23. Suárez DR, Nerkens W, Valstar ER, Rozing PM, van Keulen F. Interface micromotions increase with less-conforming cementless glenoid components. J Shoulder Elbow Surg. 2012;21(4):474-482.
24. ASTM International. Standard Test Methods for Dynamic Evaluation of Glenoid Loosening or Disassociation. West Conshocken, PA: ASTM International; 2012. ASTM F2028-08.
25. Anglin C, Tolhurst P, Wyss UP, Pichora DR. Glenoid cancellous bone strength and modulus. J Biomech. 1999;32(10):1091-1097.
26. Anglin C, Wyss U, Pichora D. Glenohumeral contact forces. Proc Inst Mech Eng H. 2000;214(6):637-644.
27. Wirth MA, Korvick DL, Basamania CJ, Toro F, Aufdemorte TB, Rockwood CA Jr. Radiologic, mechanical, and histologic evaluation of 2 glenoid prosthesis designs in a canine model. J Shoulder Elbow Surg. 2001;10(2):140-148.
28. Pilliar RM, Lee JM, Maniatopoulos C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin Orthop Relat Res. 1986;(208):108-113.
29. Ramamurti BS, Orr TE, Bragdon CR, Lowenstein JD, Jasty M, Harris WH. Factors influencing stability at the interface between a porous surface and cancellous bone: a finite element analysis of a canine in vivo micromotion experiment. J Biomed Mater Res. 1997;36(2):274-280.
30. Şahin S, Cehreli MC, Yalçın E. The influence of functional forces on the biomechanics of implant-supported prostheses—a review. J Dent. 2002;30(7-8):271-282.
1. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
2. Sperling JW, Cofield RH, O’Driscoll SW, Torchia ME, Rowland CM. Radiographic assessment of ingrowth total shoulder arthroplasty. J Shoulder Elbow Surg. 2000;9(6):507-513.
3. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
4. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
5. Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):859-863.
6. Edwards TB, Labriola JE, Stanley RJ, O’Connor DP, Elkousy HA, Gartsman GM. Radiographic comparison of pegged and keeled glenoid components using modern cementing techniques: a prospective randomized study. J Shoulder Elbow Surg. 2010;19(2):251-257.
7. Gartsman GM, Elkousy HA, Warnock KM, Edwards TB, O’Connor DP. Radiographic comparison of pegged and keeled glenoid components. J Shoulder Elbow Surg. 2005;14(3):252-257.
8. Klepps S, Chiang AS, Miller S, Jiang CY, Hazrati Y, Flatow EL. Incidence of early radiolucent glenoid lines in patients having total shoulder replacements. Clin Orthop Relat Res. 2005;(435):118-125.
9. Lazarus MD, Jensen KL, Southworth C, Matsen FA 3rd. The radiographic evaluation of keeled and pegged glenoid component insertion. J Bone Joint Surg Am. 2002;84(7):1174-1182.
10. Anglin C, Wyss UP, Nyffeler RW, Gerber C. Loosening performance of cemented glenoid prosthesis design pairs. Clin Biomech. 2001;16(2):144-150.
11. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
12. Gregory T, Hansen U, Taillieu F, et al. Glenoid loosening after total shoulder arthroplasty: an in vitro CT-scan study. J Orthop Res. 2009;27(12):1589-1595.
13. Arnold RM, High RR, Grosshans KT, Walker CW, Fehringer EV. Bone presence between the central peg’s radial fins of a partially cemented pegged all poly glenoid component suggest few radiolucencies. J Shoulder Elbow Surg. 2011;20(2):315-321.
14. Churchill RS, Boorman RS, Fehringer EV, Matsen FA 3rd. Glenoid cementing may generate sufficient heat to endanger the surrounding bone. Clin Orthop Relat Res. 2004;(419):76-79.
15. De Wilde L, Dayerizadeh N, De Neve F, Basamania C, Van Tongel A. Fully uncemented glenoid component in total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(10):e1-e7.
16. Churchill RS, Zellmer C, Zimmers HJ, Ruggero R. Clinical and radiographic analysis of a partially cemented glenoid implant: five-year minimum follow-up. J Shoulder Elbow Surg. 2010;19(7):1091-1097.
17. Groh GI. Survival and radiographic analysis of a glenoid component with a cementless fluted central peg. J Shoulder Elbow Surg. 2010;19(8):1265-1268.
18. Vidil A, Valenti P, Guichoux F, Barthas JH. CT scan evaluation of glenoid component fixation: a prospective study of 27 minimally cemented shoulder arthroplasties. Eur J Orthop Surg Traumatol. 2012;23(5):521-525.
19. Wirth MA, Loredo R, Garcia G, Rockwood CA Jr, Southworth C, Iannotti JP. Total shoulder arthroplasty with an all-polyethylene pegged bone-ingrowth glenoid component: a clinical and radiographic outcome study. J Bone Joint Surg Am. 2012;94(3):260-267.
20. Anglin C, Wyss UP, Pichora DR. Mechanical testing of shoulder prostheses and recommendations for glenoid design. J Shoulder Elbow Surg. 2000;9(4):323-331.
21. Hoenig MP, Loeffler B, Brown S, et al. Reverse glenoid component fixation: is a posterior screw necessary? J Shoulder Elbow Surg. 2010;19(4):544-549.
22. Sarah J, Sanjay G, Sanjay S, et al. Failure mechanism of the all-polyethylene glenoid implant. J Biomech. 2010;43(4):714-719.
23. Suárez DR, Nerkens W, Valstar ER, Rozing PM, van Keulen F. Interface micromotions increase with less-conforming cementless glenoid components. J Shoulder Elbow Surg. 2012;21(4):474-482.
24. ASTM International. Standard Test Methods for Dynamic Evaluation of Glenoid Loosening or Disassociation. West Conshocken, PA: ASTM International; 2012. ASTM F2028-08.
25. Anglin C, Tolhurst P, Wyss UP, Pichora DR. Glenoid cancellous bone strength and modulus. J Biomech. 1999;32(10):1091-1097.
26. Anglin C, Wyss U, Pichora D. Glenohumeral contact forces. Proc Inst Mech Eng H. 2000;214(6):637-644.
27. Wirth MA, Korvick DL, Basamania CJ, Toro F, Aufdemorte TB, Rockwood CA Jr. Radiologic, mechanical, and histologic evaluation of 2 glenoid prosthesis designs in a canine model. J Shoulder Elbow Surg. 2001;10(2):140-148.
28. Pilliar RM, Lee JM, Maniatopoulos C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin Orthop Relat Res. 1986;(208):108-113.
29. Ramamurti BS, Orr TE, Bragdon CR, Lowenstein JD, Jasty M, Harris WH. Factors influencing stability at the interface between a porous surface and cancellous bone: a finite element analysis of a canine in vivo micromotion experiment. J Biomed Mater Res. 1997;36(2):274-280.
30. Şahin S, Cehreli MC, Yalçın E. The influence of functional forces on the biomechanics of implant-supported prostheses—a review. J Dent. 2002;30(7-8):271-282.
Promoting Scholarship for Hospitalists
Academic hospital medicine is a fast‐growing specialty and has a strong emphasis on high‐value care, efficiency, and quality improvement (QI).[1] Developing scholarly work in these areas and describing findings in peer‐reviewed publications can help disseminate ideas and innovations more widely. In addition, success in academic medicine, at least in part, continues to be measured by traditional academic benchmarks, including the production of scholarly publications, conference presentations, and abstracts.[2]
Hospital medicine, however, faces challenges in providing an academic environment conducive to fostering scholarly work. As a relatively young specialty, there may be a dearth of senior mentors and experienced researchers; lack of structured mentorship can be associated with failure to produce publications or lead national teaching sessions.[3] Relatively few hospitalists undergo fellowships or other specialized training that provides a clinical research background, and internal medicine residency programs rarely provide the comprehensive research skill set required to design, implement, or disseminate academic work.[4, 5, 6] Finally, heavy clinical responsibilities may hinder efforts to conduct and sustain research.
A works‐in‐progress (WIP) session, commonly employed in clinical research groups, can provide a forum to discuss and receive feedback on evolving projects and can foster mentorship, motivation, and training.[7] Although a WIP session may stimulate discussion and advance project ideas, academic hospitalist groups do not commonly employ this model, and it is not known if a regularly scheduled WIP session can provide the mentorship, training, and motivation necessary to assist junior faculty in advancing scholarly project to completion.[8] In this article, we describe how we developed a regular WIP series to promote scholarship activities within our rapidly growing, primarily clinically focused Division of Hospital Medicine (DHM) at the University of California, San Francisco (UCSF), and the results of a survey of WIP participants. We hope that our experience can help illustrate key features of such a model, as well as describe inherent challenges and lessons learned to help promote successful academic efforts at other institutions.
METHODS
Program Setting
During years 2010 to 2013, the time period captured by our survey, the DHM at UCSF grew from 37 to 46 full‐time hospitalists, with 76% primarily clinical faculty (nonresearchers) and 24% primarily clinician‐investigators (researchers), defined as individuals having completed a 2‐year clinical research fellowship and/or dedicating 70% time in their faculty position to clinical research. In addition, there were between 1 and 3 hospitalist fellows per year. In 2012, a PhD researcher joined the division to support research and academic activities within the division as well as to pursue an independent research career.
Program Description
The DHM WIP, named the Incubator, was initially developed in 2007 when researchers recognized the need and desire for a forum where scholarly projects could be reviewed and evaluated. In the first year, the Incubator was primarily utilized by junior research‐trained mentees applying for National Institutes of Health career development awards. However, it soon became clear that nonresearch trained junior fellow and faculty members were pursuing scholarly projects needing additional guidance and input. In particular, the Incubator became frequently utilized by academic hospital medicine fellows and resident trainees pursuing QI and education projects. Over time, more DHM faculty, and junior faculty in particular, began to present their projects and receive structured feedback from researchers as well as other senior members of the group.
Incubator is structured as a 50‐minute session held from 1:10 to 2:00 pm on Thursdays in a DHM conference room. The time was selected because it did not conflict with other divisional conferences and to reserve mornings for clinical responsibilities. Incubator is held on most weeks of the year except for holidays or when there is no scheduled presenter. Presenting at Incubator is voluntary, and presenters sign up for open spots in advance with the upcoming presenter schedule sent out to the division in advance of the conference. Incubator is also used as a forum to provide feedback on anticipated abstract submissions for professional society meetings. For the purposes of the survey described in this article, we did not include Incubator sessions on reviewing abstracts/posters. Trainees and hospitalists present a broad range of projects at any stage of preparation. These include project ideas, grant applications, manuscripts, abstracts, and oral presentations at any stage of completion for feedback. Our mission was to create a forum where researchers, clinicians, and educators meet to provide the tools and guidance necessary to promote scholarly projects across the range of the division's activities by connecting individuals with complementary skills and interests and providing necessary mentorship and peer support. We have defined scholarship broadly, including evaluation of QI, global health, or other health system innovations, as well as advancements in medical education and traditional clinical research.
All faculty are invited to Incubator, and attendees include senior and junior faculty, researchers in the division, fellows, and occasionally residents and medical students. One week prior to the session, an administrative assistant solicits project information, including any related materials and questions the presenter may have for the group using a prespecified template, and emails this information to division members for review. In addition, the same materials are also printed prior to Incubator for any attendees who may not have reviewed the material in advance. Also, prior to the session, a physician is specified to serve as moderator of the discussion, and another physician is assigned the role of primary reviewer to provide the initial specific feedback and recommendations. The role of the moderator is to manage the discussion and keep the focus on time, and is assigned to a researcher or senior clinical faculty member. The role of primary reviewer is assigned to provide more junior faculty (both researchers and nonresearchers) the opportunity to practice their editing and critiquing skills by providing the initial feedback. Presenters and moderators receive worksheets outlining the structure of Incubator and their respective roles (see Supporting Information, Appendix 1, in the online version of this article).
Incubator begins with the presenter providing a brief synopsis of their project and their specific goals and objectives for the session. The moderator then leads the discussion and guides the format, often starting with any questions the group may have for the presenter followed by the specific feedback from the primary reviewer. The primary reviewer, having reviewed the materials in advance of the session, answers the prespecified questions as listed by the presenter, occasionally providing additional targeted feedback. The session is then opened to the rest of the group for feedback and suggestions. Meanwhile, the presenter is encouraged to wait until the end of the hour to summarize their take on the feedback and what their initial thoughts on the next to do items would be (Table 1).
| Presenter | Administrative assistant |
| 2‐ to 3‐sentence summary of career focus | Schedule session and conference room |
| Distribute short set of materials in advance | Collect presenters' materials in advance |
| Summarize feedback at end of session | Prepare materials for Incubator |
| Brainstorm on next steps at end of session | Monitor attendance and topics of presentation |
| Primary reviewer | Moderator |
| Junior faculty (24 years) | Senior or research faculty |
| Provide brief overview of project | Keep session on time |
| Reiterate key questions | Give additional input |
| Provide 2 major, 3 minor suggestions | Summarize comments from group at the end |
| Constructive, outside the box feedback | Allow last 10 minutes for presenter to discuss plans |
Program Evaluation
Survey Respondents and Process
We retrospectively surveyed the lead presenter for each Incubator session held between May 2010 through November 2013. Surveys were administered through the Research Electronic Data Capture application (REDCap).[9] Participants who were lead presenters at Incubator for more than 1 Incubator session completed a survey for each individual presentation. Therefore, some presenters completed more than 1 survey. The presenters included resident physicians, hospital medicine fellows, junior faculty, and researchers. We defined researchers as hospitalists who had completed a 2‐year research fellowship and/or devoted at least 70% time in their faculty position to research.
Survey Development and Domains
We developed a survey questionnaire using the Kirkpatrick 4‐level model to evaluate the educational experience of the primary presenters and to determine how the session impacted their progress on the project, with each model component graded according to a Likert scale.[10] The 4 major components of the model are: (1) Reaction: participants' estimates of satisfaction with Incubator; (2) Learning: extent of knowledge acquisition achieved at Incubator; (3) Behavior: extent to which learning has been applied or transfer of skills through participation in Incubator; and (4) Results: results of the project, wider changes in organizational scholarship as impacted by Incubator.
We also collected information on the presenter's status at time of presentation including career paths (researcher or nonresearcher), their job description (faculty, fellow, resident), and the total number of years on faculty (if applicable). Hospitalists in their first 2 years on the faculty were considered junior physicians. We also collected information on the number of times they had presented at the Incubator sessions and stage of progress of the project, whether in the early, mid, or late phase at the time of presentation. Early phase was defined as presenting an initial project idea or brainstorming possible project options and/or directions. Mid phase was defined as presenting initial results, data, and initial drafts prior to completion of analysis. Late phase was defined as presenting a project nearing completion such as a written abstract, oral presentation, paper, or grant application. Respondents were also asked to identify the main focus of their projects, selecting the categories based on the interests of the division, including medical education, clinical research, QI, high‐value care, and global health.
Survey Data Analysis
We converted Likert scale data into dichotomous variables, with paring of positive responses versus the negative options. We summarized survey responses using descriptive statistics and determined if there were any differences in responses between career researchers and nonresearchers using 2 tests. All analysis was performed using StataSE version 13.1 (StataCorp, College Station, TX).
RESULTS
Survey Respondent Characteristics
We received 51 completed surveys from presenters at an Incubator session, for a total survey response rate of 70%. Of the 51 presentations, 26 (51%) of the projects were led by physicians in training or junior faculty, and 35 (69%) of the presenters were nonresearchers.
Project Characteristics
The most frequently presented topic areas were QI (N = 20), clinical research (N = 14), medical education (N = 6), and global health (N = 6). Whereas researchers were more likely to present clinical research topics and grant applications, nonresearchers more often presented on QI or medical education projects (Table 2). Projects were presented at all stages of development, with the middle stage, where presenters presented initial results, being the most common phase.
| All | Nonresearcher, No. (%) | Researcher, No. (%) | P Value | |
|---|---|---|---|---|
| ||||
| Total | 51 | 35 | 16 | |
| Trainee or junior faculty | 19 (54%) | 7 (44%) | 0.49 | |
| Topic of project | 0.02 | |||
| Quality improvement | 20 (39%) | 15 (43%) | 5 (31%) | |
| Clinical research | 14 (27%) | 8 (23%) | 6 (38%) | |
| Medical education | 6 (12%) | 5 (14%) | 1 (6%) | |
| Health technology | 4 (8%) | 0 (0%) | 4 (25%) | |
| High‐value care | 1 (2%) | 1 (3%) | 0 (0%) | |
| Global health | 6 (12%) | 6 (12%) | 0 (0%) | |
| Stage of project | 0.31 | |||
| Early* | 12 (23%) | 7 (20%) | 5 (31%) | |
| Middle | 24 (47%) | 19 (54%) | 5 (31%) | |
| Late | 15 (29%) | 9 (26%) | 6 (38%) | |
Impact of Incubator
The reaction to the session was very positive, with 100% of respondents recommending Incubator to others (Table 3), and 35% reported learning as a result of the session. Twenty‐three (45%) of respondents reported that the session helped reframe the project idea and changed the study design, and 20 (39%) reported improved written or oral presentation style. A majority (45, 88%) reported that Incubator was valuable in advancing the project to completion.
| All | Nonresearcher, No. (%) | Researcher, No. (%) | P Value | |
|---|---|---|---|---|
| ||||
| Trainee or junior faculty | 51 | 35 (69%) | 16 (31%) | 0.49 |
| Reaction | ||||
| Satisfied with their WIP session | 50 (98%) | 35 (100%) | 15 (94%) | 0.25 |
| Would recommend WIP to others | 51 (100%) | 35 (100%) | 16 (100%) | 1.00 |
| Any of the above | 35 (100%) | 16 (100%) | 1.00 | |
| Learning | ||||
| Advanced research methodology | 18 (35%) | 12 (34%) | 6 (38%) | 0.82 |
| Advanced knowledge in the area | 9 (18%) | 5 (14%) | 4 (25%) | 0.35 |
| Any of the above | 14 (40%) | 9 (56%) | 0.28 | |
| Behavior | ||||
| Current project | ||||
| Reframed project idea | 23 (45%) | 15 (43%) | 8 (50%) | 0.63 |
| Changed study design or methodology | 23 (45%) | 16 (46%) | 7 (44%) | 0.9 |
| Improved written or oral presentation style | 20 (39%) | 15 (43%) | 5 (31%) | 0.43 |
| Future projects | ||||
| Changed approach to future projects | 19 (37%) | 17 (49%) | 2 (13%) | 0.01 |
| Any of the above | 34 (97%) | 14 (88%) | 0.17 | |
| Results | ||||
| Valuable in advancing project to completion | 45 (88%) | 31 (89%) | 14 (88%) | 0.18 |
| Provided mentoring and peer support | 29 (57%) | 24 (69%) | 5 (31%) | 0.01 |
| Connected individuals with similar results | 13 (13%) | 9 (26%) | 4 (25%) | 0.96 |
| Any of the above | 34 (97%) | 14 (88%) | 0.17 | |
Survey results of researchers compared to nonresearchers were similar overall, although nonresearchers were more likely to report changes in behavior and in improved mentoring as a result of presenting at Incubator. Notably, 17 (49%) of nonresearchers reported that Incubator changed their approach to future projects as opposed to only 2 (13%) researchers (P = 0.01). In addition, 24 (69%) nonresearchers reported value in mentorship and peer support compared to 5 (31%) researchers (P = 0.01). A reasonably large proportion of projects originally presented during the Incubator sessions became published articles at the time of survey completion (N = 19, 37%) or were publications in progress (N = 14, 27%). For all remaining items, there were no statistically significant differences in the survey responses among junior faculty/trainees (N = 26) compared to nonjunior faculty (N = 25) presenters (P > 0.05).
Attendance at Incubator During the Study Period
Attendance at Incubator was open and voluntary for all DHM faculty, fellows, and collaborating UCSF trainees. From July 2012, when we began tracking attendance, through the end of the survey period in November 2013, the average number of attendees for each session was 10.7 (standard deviation [SD] 3.8). On average, 50% (SD 16%) of attendees at Incubator were career researchers.
DISCUSSION
The results of this program evaluation suggest that a WIP session employed by an academic division of hospital medicine, consisting of a weekly moderated session, can help advance scholarly work. Our evaluation found that presenters, both researchers and nonresearchers, favorably viewed the regular WIP sessions and reported that feedback in the Incubator helped them advance their project to completion. Importantly, nonresearch‐focused faculty and fellows reported the biggest gains in learning from presenting at Incubator. Whereas half the Incubator attendees were career researchers, consistent with the observation that researchers within the division were most committed to attending Incubator regularly, 69% of the presenters were nonresearchers, demonstrating strong participation among both researchers and nonresearchers within the division.
WIP sessions, though informal, are interactive, inspire critical self‐reflection, and encourage physicians to act on generated ideas, as evidenced by the change in behavior of the participants after the session. These sessions allow for transformative learning by encouraging physicians to be open to alternative viewpoints and engage in discourse, boosting learning beyond just content knowledge. Prior assessments of WIP seminars similarly found high satisfaction with these formats.[11]
Although we cannot identify specifically which features made Incubator effective, we believe that our WIP had some characteristics that may have contributed to its success and may aid in implementation at other institutions: holding the session regularly, voluntary participation, distributing the materials and questions for the group in advance, and designating a moderator for the session in advance to facilitate discussion.
A potential strength of the Incubator is that both researchers and nonresearchers attend. We hypothesize that combining these groups provides improved mentorship and learning for nonresearchers, in particular. In addition, it creates a mutually beneficial environment where each group is able to witness the diversity of projects within the division and learn to provide focused, constructive feedback on the presented work. Not only did this create a transparent environment with better understanding of divisional activities, but also fostered collaboration among hospitalists with similar interests and complementary skills.
Challenges, Setbacks, Updated Approaches
The creation of a successful Incubator session, however, was not without its challenges. At initial inception, the WIP was attended primarily by researchers and had low overall attendance. Members of the division who were primarily clinicians initially perceived the conferences as largely inapplicable to their career objectives and had competing demands from patient care, educational, or administrative responsibilities. However, over time and with encouragement from divisional leaders and service line directors, increasing numbers of hospitalists began to participate in Incubator. The timing of Incubator during afternoons after the Department of Medicine Grand Rounds was chosen specifically to allow clinicians to complete their responsibilities, including morning rounds and teaching, to allow better attendance.
In addition, the results of our survey informed changes to the structure of Incubator. The efficacy of assigning a primary reviewer for each session was not clear, so this component was eventually dropped. The finding that nonresearchers in particular reported a benefit from mentoring and peer‐support at Incubator led to the implementation of querying the presenter for a wish list of faculty attendees at their Incubator session. We then sent a special invitation to those faculty members thought to have special insights on the project. This gave junior faculty the opportunity to present their projects to more senior faculty members within their areas of research, as well as to receive focused expert feedback.
Finally, we have initiated special Incubator sessions focused more on didactics to teach the process of writing manuscripts and brainstorming workshop ideas for national meetings.
Limitations
Our study has limitations. It is a single‐center study based on a small overall sample size, and it is not certain whether a similar innovation would have comparable effects at another institution. In addition, generalizability of our results may be limited for hospital medicine groups without a robust research program. We did not have a control group nor do we know whether participants would have been equally successful without Incubator. We also were unable to assess how Incubator affected long‐term outcomes such as promotion and overall publication record, as we do not have detailed data on productivity prior to the survey period. Finally, we are unable to quantify the effect of Incubator on scholarly success in the division. Although the numbers of published articles and grant funding has increased since the Incubator began (data not shown), the division also grew both in number of research‐focused and nonresearch‐focused faculty, and this study does not account for other temporal changes that may have contributed to improvements in the scholarly output of the division.
CONCLUSIONS
In summary, the Incubator has been a successful program that fostered progress on scholarly projects within a largely clinically focused DHM. Given the importance of scholarship in academic hospital medicine, a WIP session such as the one we describe is a valuable way to support and mentor junior hospitalists and nonresearchers.
Acknowledgements
The authors extend special thanks to Oralia Schatzman, divisional administrative assistant, who organized and arranged the Incubator sessions and recorded attendance, and to Katherine Li, who collected data on numbers of faculty within the division over the duration of the study.
Disclosures: Dr. Hemali Patel, Dr. Margaret Fang, and Mr. James Harrison report no conflicts of interest. At the time the research was conducted, Dr. Kangelaris was supported by the National Heart, Lung, and Blood Institute (1K23HL116800‐01). Dr. Auerbach was supported by the National Heart, Lung, and Blood Institute (K24 K24HL098372).
- , , , . Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161–166.
- , , , , . Tried and true: a survey of successfully promoted academic hospitalists. J Hosp Med. 2011;6(7):411–415.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23–27.
- , , , , , . The PRIME curriculum. J Gen Intern Med. 2006;21(5):506–509.
- , , , . Hospital medicine fellowships: works in progress. Am J Med. 2006;119(1):72.e1–7.
- , , . Instituting systems‐based practice and practice‐based learning and improvement: a curriculum of inquiry. Med Educ Online. 2013;18:21612.
- , , , et al. A physician peer support writing group. Fam Med. 2003;35(3):195–201.
- , , , . Research in progress conference for hospitalists provides valuable peer mentoring. J Hosp Med. 2011;6(1):43–46.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- , . Evaluating Training Programs: The Four Levels. 3rd ed. San Francisco, CA: Berrett‐Koehler; 2006.
- , , . Works‐in‐progress: guiding junior scientists through career development applications. J Cancer Educ. 2008;23(3):142–148.
Academic hospital medicine is a fast‐growing specialty and has a strong emphasis on high‐value care, efficiency, and quality improvement (QI).[1] Developing scholarly work in these areas and describing findings in peer‐reviewed publications can help disseminate ideas and innovations more widely. In addition, success in academic medicine, at least in part, continues to be measured by traditional academic benchmarks, including the production of scholarly publications, conference presentations, and abstracts.[2]
Hospital medicine, however, faces challenges in providing an academic environment conducive to fostering scholarly work. As a relatively young specialty, there may be a dearth of senior mentors and experienced researchers; lack of structured mentorship can be associated with failure to produce publications or lead national teaching sessions.[3] Relatively few hospitalists undergo fellowships or other specialized training that provides a clinical research background, and internal medicine residency programs rarely provide the comprehensive research skill set required to design, implement, or disseminate academic work.[4, 5, 6] Finally, heavy clinical responsibilities may hinder efforts to conduct and sustain research.
A works‐in‐progress (WIP) session, commonly employed in clinical research groups, can provide a forum to discuss and receive feedback on evolving projects and can foster mentorship, motivation, and training.[7] Although a WIP session may stimulate discussion and advance project ideas, academic hospitalist groups do not commonly employ this model, and it is not known if a regularly scheduled WIP session can provide the mentorship, training, and motivation necessary to assist junior faculty in advancing scholarly project to completion.[8] In this article, we describe how we developed a regular WIP series to promote scholarship activities within our rapidly growing, primarily clinically focused Division of Hospital Medicine (DHM) at the University of California, San Francisco (UCSF), and the results of a survey of WIP participants. We hope that our experience can help illustrate key features of such a model, as well as describe inherent challenges and lessons learned to help promote successful academic efforts at other institutions.
METHODS
Program Setting
During years 2010 to 2013, the time period captured by our survey, the DHM at UCSF grew from 37 to 46 full‐time hospitalists, with 76% primarily clinical faculty (nonresearchers) and 24% primarily clinician‐investigators (researchers), defined as individuals having completed a 2‐year clinical research fellowship and/or dedicating 70% time in their faculty position to clinical research. In addition, there were between 1 and 3 hospitalist fellows per year. In 2012, a PhD researcher joined the division to support research and academic activities within the division as well as to pursue an independent research career.
Program Description
The DHM WIP, named the Incubator, was initially developed in 2007 when researchers recognized the need and desire for a forum where scholarly projects could be reviewed and evaluated. In the first year, the Incubator was primarily utilized by junior research‐trained mentees applying for National Institutes of Health career development awards. However, it soon became clear that nonresearch trained junior fellow and faculty members were pursuing scholarly projects needing additional guidance and input. In particular, the Incubator became frequently utilized by academic hospital medicine fellows and resident trainees pursuing QI and education projects. Over time, more DHM faculty, and junior faculty in particular, began to present their projects and receive structured feedback from researchers as well as other senior members of the group.
Incubator is structured as a 50‐minute session held from 1:10 to 2:00 pm on Thursdays in a DHM conference room. The time was selected because it did not conflict with other divisional conferences and to reserve mornings for clinical responsibilities. Incubator is held on most weeks of the year except for holidays or when there is no scheduled presenter. Presenting at Incubator is voluntary, and presenters sign up for open spots in advance with the upcoming presenter schedule sent out to the division in advance of the conference. Incubator is also used as a forum to provide feedback on anticipated abstract submissions for professional society meetings. For the purposes of the survey described in this article, we did not include Incubator sessions on reviewing abstracts/posters. Trainees and hospitalists present a broad range of projects at any stage of preparation. These include project ideas, grant applications, manuscripts, abstracts, and oral presentations at any stage of completion for feedback. Our mission was to create a forum where researchers, clinicians, and educators meet to provide the tools and guidance necessary to promote scholarly projects across the range of the division's activities by connecting individuals with complementary skills and interests and providing necessary mentorship and peer support. We have defined scholarship broadly, including evaluation of QI, global health, or other health system innovations, as well as advancements in medical education and traditional clinical research.
All faculty are invited to Incubator, and attendees include senior and junior faculty, researchers in the division, fellows, and occasionally residents and medical students. One week prior to the session, an administrative assistant solicits project information, including any related materials and questions the presenter may have for the group using a prespecified template, and emails this information to division members for review. In addition, the same materials are also printed prior to Incubator for any attendees who may not have reviewed the material in advance. Also, prior to the session, a physician is specified to serve as moderator of the discussion, and another physician is assigned the role of primary reviewer to provide the initial specific feedback and recommendations. The role of the moderator is to manage the discussion and keep the focus on time, and is assigned to a researcher or senior clinical faculty member. The role of primary reviewer is assigned to provide more junior faculty (both researchers and nonresearchers) the opportunity to practice their editing and critiquing skills by providing the initial feedback. Presenters and moderators receive worksheets outlining the structure of Incubator and their respective roles (see Supporting Information, Appendix 1, in the online version of this article).
Incubator begins with the presenter providing a brief synopsis of their project and their specific goals and objectives for the session. The moderator then leads the discussion and guides the format, often starting with any questions the group may have for the presenter followed by the specific feedback from the primary reviewer. The primary reviewer, having reviewed the materials in advance of the session, answers the prespecified questions as listed by the presenter, occasionally providing additional targeted feedback. The session is then opened to the rest of the group for feedback and suggestions. Meanwhile, the presenter is encouraged to wait until the end of the hour to summarize their take on the feedback and what their initial thoughts on the next to do items would be (Table 1).
| Presenter | Administrative assistant |
| 2‐ to 3‐sentence summary of career focus | Schedule session and conference room |
| Distribute short set of materials in advance | Collect presenters' materials in advance |
| Summarize feedback at end of session | Prepare materials for Incubator |
| Brainstorm on next steps at end of session | Monitor attendance and topics of presentation |
| Primary reviewer | Moderator |
| Junior faculty (24 years) | Senior or research faculty |
| Provide brief overview of project | Keep session on time |
| Reiterate key questions | Give additional input |
| Provide 2 major, 3 minor suggestions | Summarize comments from group at the end |
| Constructive, outside the box feedback | Allow last 10 minutes for presenter to discuss plans |
Program Evaluation
Survey Respondents and Process
We retrospectively surveyed the lead presenter for each Incubator session held between May 2010 through November 2013. Surveys were administered through the Research Electronic Data Capture application (REDCap).[9] Participants who were lead presenters at Incubator for more than 1 Incubator session completed a survey for each individual presentation. Therefore, some presenters completed more than 1 survey. The presenters included resident physicians, hospital medicine fellows, junior faculty, and researchers. We defined researchers as hospitalists who had completed a 2‐year research fellowship and/or devoted at least 70% time in their faculty position to research.
Survey Development and Domains
We developed a survey questionnaire using the Kirkpatrick 4‐level model to evaluate the educational experience of the primary presenters and to determine how the session impacted their progress on the project, with each model component graded according to a Likert scale.[10] The 4 major components of the model are: (1) Reaction: participants' estimates of satisfaction with Incubator; (2) Learning: extent of knowledge acquisition achieved at Incubator; (3) Behavior: extent to which learning has been applied or transfer of skills through participation in Incubator; and (4) Results: results of the project, wider changes in organizational scholarship as impacted by Incubator.
We also collected information on the presenter's status at time of presentation including career paths (researcher or nonresearcher), their job description (faculty, fellow, resident), and the total number of years on faculty (if applicable). Hospitalists in their first 2 years on the faculty were considered junior physicians. We also collected information on the number of times they had presented at the Incubator sessions and stage of progress of the project, whether in the early, mid, or late phase at the time of presentation. Early phase was defined as presenting an initial project idea or brainstorming possible project options and/or directions. Mid phase was defined as presenting initial results, data, and initial drafts prior to completion of analysis. Late phase was defined as presenting a project nearing completion such as a written abstract, oral presentation, paper, or grant application. Respondents were also asked to identify the main focus of their projects, selecting the categories based on the interests of the division, including medical education, clinical research, QI, high‐value care, and global health.
Survey Data Analysis
We converted Likert scale data into dichotomous variables, with paring of positive responses versus the negative options. We summarized survey responses using descriptive statistics and determined if there were any differences in responses between career researchers and nonresearchers using 2 tests. All analysis was performed using StataSE version 13.1 (StataCorp, College Station, TX).
RESULTS
Survey Respondent Characteristics
We received 51 completed surveys from presenters at an Incubator session, for a total survey response rate of 70%. Of the 51 presentations, 26 (51%) of the projects were led by physicians in training or junior faculty, and 35 (69%) of the presenters were nonresearchers.
Project Characteristics
The most frequently presented topic areas were QI (N = 20), clinical research (N = 14), medical education (N = 6), and global health (N = 6). Whereas researchers were more likely to present clinical research topics and grant applications, nonresearchers more often presented on QI or medical education projects (Table 2). Projects were presented at all stages of development, with the middle stage, where presenters presented initial results, being the most common phase.
| All | Nonresearcher, No. (%) | Researcher, No. (%) | P Value | |
|---|---|---|---|---|
| ||||
| Total | 51 | 35 | 16 | |
| Trainee or junior faculty | 19 (54%) | 7 (44%) | 0.49 | |
| Topic of project | 0.02 | |||
| Quality improvement | 20 (39%) | 15 (43%) | 5 (31%) | |
| Clinical research | 14 (27%) | 8 (23%) | 6 (38%) | |
| Medical education | 6 (12%) | 5 (14%) | 1 (6%) | |
| Health technology | 4 (8%) | 0 (0%) | 4 (25%) | |
| High‐value care | 1 (2%) | 1 (3%) | 0 (0%) | |
| Global health | 6 (12%) | 6 (12%) | 0 (0%) | |
| Stage of project | 0.31 | |||
| Early* | 12 (23%) | 7 (20%) | 5 (31%) | |
| Middle | 24 (47%) | 19 (54%) | 5 (31%) | |
| Late | 15 (29%) | 9 (26%) | 6 (38%) | |
Impact of Incubator
The reaction to the session was very positive, with 100% of respondents recommending Incubator to others (Table 3), and 35% reported learning as a result of the session. Twenty‐three (45%) of respondents reported that the session helped reframe the project idea and changed the study design, and 20 (39%) reported improved written or oral presentation style. A majority (45, 88%) reported that Incubator was valuable in advancing the project to completion.
| All | Nonresearcher, No. (%) | Researcher, No. (%) | P Value | |
|---|---|---|---|---|
| ||||
| Trainee or junior faculty | 51 | 35 (69%) | 16 (31%) | 0.49 |
| Reaction | ||||
| Satisfied with their WIP session | 50 (98%) | 35 (100%) | 15 (94%) | 0.25 |
| Would recommend WIP to others | 51 (100%) | 35 (100%) | 16 (100%) | 1.00 |
| Any of the above | 35 (100%) | 16 (100%) | 1.00 | |
| Learning | ||||
| Advanced research methodology | 18 (35%) | 12 (34%) | 6 (38%) | 0.82 |
| Advanced knowledge in the area | 9 (18%) | 5 (14%) | 4 (25%) | 0.35 |
| Any of the above | 14 (40%) | 9 (56%) | 0.28 | |
| Behavior | ||||
| Current project | ||||
| Reframed project idea | 23 (45%) | 15 (43%) | 8 (50%) | 0.63 |
| Changed study design or methodology | 23 (45%) | 16 (46%) | 7 (44%) | 0.9 |
| Improved written or oral presentation style | 20 (39%) | 15 (43%) | 5 (31%) | 0.43 |
| Future projects | ||||
| Changed approach to future projects | 19 (37%) | 17 (49%) | 2 (13%) | 0.01 |
| Any of the above | 34 (97%) | 14 (88%) | 0.17 | |
| Results | ||||
| Valuable in advancing project to completion | 45 (88%) | 31 (89%) | 14 (88%) | 0.18 |
| Provided mentoring and peer support | 29 (57%) | 24 (69%) | 5 (31%) | 0.01 |
| Connected individuals with similar results | 13 (13%) | 9 (26%) | 4 (25%) | 0.96 |
| Any of the above | 34 (97%) | 14 (88%) | 0.17 | |
Survey results of researchers compared to nonresearchers were similar overall, although nonresearchers were more likely to report changes in behavior and in improved mentoring as a result of presenting at Incubator. Notably, 17 (49%) of nonresearchers reported that Incubator changed their approach to future projects as opposed to only 2 (13%) researchers (P = 0.01). In addition, 24 (69%) nonresearchers reported value in mentorship and peer support compared to 5 (31%) researchers (P = 0.01). A reasonably large proportion of projects originally presented during the Incubator sessions became published articles at the time of survey completion (N = 19, 37%) or were publications in progress (N = 14, 27%). For all remaining items, there were no statistically significant differences in the survey responses among junior faculty/trainees (N = 26) compared to nonjunior faculty (N = 25) presenters (P > 0.05).
Attendance at Incubator During the Study Period
Attendance at Incubator was open and voluntary for all DHM faculty, fellows, and collaborating UCSF trainees. From July 2012, when we began tracking attendance, through the end of the survey period in November 2013, the average number of attendees for each session was 10.7 (standard deviation [SD] 3.8). On average, 50% (SD 16%) of attendees at Incubator were career researchers.
DISCUSSION
The results of this program evaluation suggest that a WIP session employed by an academic division of hospital medicine, consisting of a weekly moderated session, can help advance scholarly work. Our evaluation found that presenters, both researchers and nonresearchers, favorably viewed the regular WIP sessions and reported that feedback in the Incubator helped them advance their project to completion. Importantly, nonresearch‐focused faculty and fellows reported the biggest gains in learning from presenting at Incubator. Whereas half the Incubator attendees were career researchers, consistent with the observation that researchers within the division were most committed to attending Incubator regularly, 69% of the presenters were nonresearchers, demonstrating strong participation among both researchers and nonresearchers within the division.
WIP sessions, though informal, are interactive, inspire critical self‐reflection, and encourage physicians to act on generated ideas, as evidenced by the change in behavior of the participants after the session. These sessions allow for transformative learning by encouraging physicians to be open to alternative viewpoints and engage in discourse, boosting learning beyond just content knowledge. Prior assessments of WIP seminars similarly found high satisfaction with these formats.[11]
Although we cannot identify specifically which features made Incubator effective, we believe that our WIP had some characteristics that may have contributed to its success and may aid in implementation at other institutions: holding the session regularly, voluntary participation, distributing the materials and questions for the group in advance, and designating a moderator for the session in advance to facilitate discussion.
A potential strength of the Incubator is that both researchers and nonresearchers attend. We hypothesize that combining these groups provides improved mentorship and learning for nonresearchers, in particular. In addition, it creates a mutually beneficial environment where each group is able to witness the diversity of projects within the division and learn to provide focused, constructive feedback on the presented work. Not only did this create a transparent environment with better understanding of divisional activities, but also fostered collaboration among hospitalists with similar interests and complementary skills.
Challenges, Setbacks, Updated Approaches
The creation of a successful Incubator session, however, was not without its challenges. At initial inception, the WIP was attended primarily by researchers and had low overall attendance. Members of the division who were primarily clinicians initially perceived the conferences as largely inapplicable to their career objectives and had competing demands from patient care, educational, or administrative responsibilities. However, over time and with encouragement from divisional leaders and service line directors, increasing numbers of hospitalists began to participate in Incubator. The timing of Incubator during afternoons after the Department of Medicine Grand Rounds was chosen specifically to allow clinicians to complete their responsibilities, including morning rounds and teaching, to allow better attendance.
In addition, the results of our survey informed changes to the structure of Incubator. The efficacy of assigning a primary reviewer for each session was not clear, so this component was eventually dropped. The finding that nonresearchers in particular reported a benefit from mentoring and peer‐support at Incubator led to the implementation of querying the presenter for a wish list of faculty attendees at their Incubator session. We then sent a special invitation to those faculty members thought to have special insights on the project. This gave junior faculty the opportunity to present their projects to more senior faculty members within their areas of research, as well as to receive focused expert feedback.
Finally, we have initiated special Incubator sessions focused more on didactics to teach the process of writing manuscripts and brainstorming workshop ideas for national meetings.
Limitations
Our study has limitations. It is a single‐center study based on a small overall sample size, and it is not certain whether a similar innovation would have comparable effects at another institution. In addition, generalizability of our results may be limited for hospital medicine groups without a robust research program. We did not have a control group nor do we know whether participants would have been equally successful without Incubator. We also were unable to assess how Incubator affected long‐term outcomes such as promotion and overall publication record, as we do not have detailed data on productivity prior to the survey period. Finally, we are unable to quantify the effect of Incubator on scholarly success in the division. Although the numbers of published articles and grant funding has increased since the Incubator began (data not shown), the division also grew both in number of research‐focused and nonresearch‐focused faculty, and this study does not account for other temporal changes that may have contributed to improvements in the scholarly output of the division.
CONCLUSIONS
In summary, the Incubator has been a successful program that fostered progress on scholarly projects within a largely clinically focused DHM. Given the importance of scholarship in academic hospital medicine, a WIP session such as the one we describe is a valuable way to support and mentor junior hospitalists and nonresearchers.
Acknowledgements
The authors extend special thanks to Oralia Schatzman, divisional administrative assistant, who organized and arranged the Incubator sessions and recorded attendance, and to Katherine Li, who collected data on numbers of faculty within the division over the duration of the study.
Disclosures: Dr. Hemali Patel, Dr. Margaret Fang, and Mr. James Harrison report no conflicts of interest. At the time the research was conducted, Dr. Kangelaris was supported by the National Heart, Lung, and Blood Institute (1K23HL116800‐01). Dr. Auerbach was supported by the National Heart, Lung, and Blood Institute (K24 K24HL098372).
Academic hospital medicine is a fast‐growing specialty and has a strong emphasis on high‐value care, efficiency, and quality improvement (QI).[1] Developing scholarly work in these areas and describing findings in peer‐reviewed publications can help disseminate ideas and innovations more widely. In addition, success in academic medicine, at least in part, continues to be measured by traditional academic benchmarks, including the production of scholarly publications, conference presentations, and abstracts.[2]
Hospital medicine, however, faces challenges in providing an academic environment conducive to fostering scholarly work. As a relatively young specialty, there may be a dearth of senior mentors and experienced researchers; lack of structured mentorship can be associated with failure to produce publications or lead national teaching sessions.[3] Relatively few hospitalists undergo fellowships or other specialized training that provides a clinical research background, and internal medicine residency programs rarely provide the comprehensive research skill set required to design, implement, or disseminate academic work.[4, 5, 6] Finally, heavy clinical responsibilities may hinder efforts to conduct and sustain research.
A works‐in‐progress (WIP) session, commonly employed in clinical research groups, can provide a forum to discuss and receive feedback on evolving projects and can foster mentorship, motivation, and training.[7] Although a WIP session may stimulate discussion and advance project ideas, academic hospitalist groups do not commonly employ this model, and it is not known if a regularly scheduled WIP session can provide the mentorship, training, and motivation necessary to assist junior faculty in advancing scholarly project to completion.[8] In this article, we describe how we developed a regular WIP series to promote scholarship activities within our rapidly growing, primarily clinically focused Division of Hospital Medicine (DHM) at the University of California, San Francisco (UCSF), and the results of a survey of WIP participants. We hope that our experience can help illustrate key features of such a model, as well as describe inherent challenges and lessons learned to help promote successful academic efforts at other institutions.
METHODS
Program Setting
During years 2010 to 2013, the time period captured by our survey, the DHM at UCSF grew from 37 to 46 full‐time hospitalists, with 76% primarily clinical faculty (nonresearchers) and 24% primarily clinician‐investigators (researchers), defined as individuals having completed a 2‐year clinical research fellowship and/or dedicating 70% time in their faculty position to clinical research. In addition, there were between 1 and 3 hospitalist fellows per year. In 2012, a PhD researcher joined the division to support research and academic activities within the division as well as to pursue an independent research career.
Program Description
The DHM WIP, named the Incubator, was initially developed in 2007 when researchers recognized the need and desire for a forum where scholarly projects could be reviewed and evaluated. In the first year, the Incubator was primarily utilized by junior research‐trained mentees applying for National Institutes of Health career development awards. However, it soon became clear that nonresearch trained junior fellow and faculty members were pursuing scholarly projects needing additional guidance and input. In particular, the Incubator became frequently utilized by academic hospital medicine fellows and resident trainees pursuing QI and education projects. Over time, more DHM faculty, and junior faculty in particular, began to present their projects and receive structured feedback from researchers as well as other senior members of the group.
Incubator is structured as a 50‐minute session held from 1:10 to 2:00 pm on Thursdays in a DHM conference room. The time was selected because it did not conflict with other divisional conferences and to reserve mornings for clinical responsibilities. Incubator is held on most weeks of the year except for holidays or when there is no scheduled presenter. Presenting at Incubator is voluntary, and presenters sign up for open spots in advance with the upcoming presenter schedule sent out to the division in advance of the conference. Incubator is also used as a forum to provide feedback on anticipated abstract submissions for professional society meetings. For the purposes of the survey described in this article, we did not include Incubator sessions on reviewing abstracts/posters. Trainees and hospitalists present a broad range of projects at any stage of preparation. These include project ideas, grant applications, manuscripts, abstracts, and oral presentations at any stage of completion for feedback. Our mission was to create a forum where researchers, clinicians, and educators meet to provide the tools and guidance necessary to promote scholarly projects across the range of the division's activities by connecting individuals with complementary skills and interests and providing necessary mentorship and peer support. We have defined scholarship broadly, including evaluation of QI, global health, or other health system innovations, as well as advancements in medical education and traditional clinical research.
All faculty are invited to Incubator, and attendees include senior and junior faculty, researchers in the division, fellows, and occasionally residents and medical students. One week prior to the session, an administrative assistant solicits project information, including any related materials and questions the presenter may have for the group using a prespecified template, and emails this information to division members for review. In addition, the same materials are also printed prior to Incubator for any attendees who may not have reviewed the material in advance. Also, prior to the session, a physician is specified to serve as moderator of the discussion, and another physician is assigned the role of primary reviewer to provide the initial specific feedback and recommendations. The role of the moderator is to manage the discussion and keep the focus on time, and is assigned to a researcher or senior clinical faculty member. The role of primary reviewer is assigned to provide more junior faculty (both researchers and nonresearchers) the opportunity to practice their editing and critiquing skills by providing the initial feedback. Presenters and moderators receive worksheets outlining the structure of Incubator and their respective roles (see Supporting Information, Appendix 1, in the online version of this article).
Incubator begins with the presenter providing a brief synopsis of their project and their specific goals and objectives for the session. The moderator then leads the discussion and guides the format, often starting with any questions the group may have for the presenter followed by the specific feedback from the primary reviewer. The primary reviewer, having reviewed the materials in advance of the session, answers the prespecified questions as listed by the presenter, occasionally providing additional targeted feedback. The session is then opened to the rest of the group for feedback and suggestions. Meanwhile, the presenter is encouraged to wait until the end of the hour to summarize their take on the feedback and what their initial thoughts on the next to do items would be (Table 1).
| Presenter | Administrative assistant |
| 2‐ to 3‐sentence summary of career focus | Schedule session and conference room |
| Distribute short set of materials in advance | Collect presenters' materials in advance |
| Summarize feedback at end of session | Prepare materials for Incubator |
| Brainstorm on next steps at end of session | Monitor attendance and topics of presentation |
| Primary reviewer | Moderator |
| Junior faculty (24 years) | Senior or research faculty |
| Provide brief overview of project | Keep session on time |
| Reiterate key questions | Give additional input |
| Provide 2 major, 3 minor suggestions | Summarize comments from group at the end |
| Constructive, outside the box feedback | Allow last 10 minutes for presenter to discuss plans |
Program Evaluation
Survey Respondents and Process
We retrospectively surveyed the lead presenter for each Incubator session held between May 2010 through November 2013. Surveys were administered through the Research Electronic Data Capture application (REDCap).[9] Participants who were lead presenters at Incubator for more than 1 Incubator session completed a survey for each individual presentation. Therefore, some presenters completed more than 1 survey. The presenters included resident physicians, hospital medicine fellows, junior faculty, and researchers. We defined researchers as hospitalists who had completed a 2‐year research fellowship and/or devoted at least 70% time in their faculty position to research.
Survey Development and Domains
We developed a survey questionnaire using the Kirkpatrick 4‐level model to evaluate the educational experience of the primary presenters and to determine how the session impacted their progress on the project, with each model component graded according to a Likert scale.[10] The 4 major components of the model are: (1) Reaction: participants' estimates of satisfaction with Incubator; (2) Learning: extent of knowledge acquisition achieved at Incubator; (3) Behavior: extent to which learning has been applied or transfer of skills through participation in Incubator; and (4) Results: results of the project, wider changes in organizational scholarship as impacted by Incubator.
We also collected information on the presenter's status at time of presentation including career paths (researcher or nonresearcher), their job description (faculty, fellow, resident), and the total number of years on faculty (if applicable). Hospitalists in their first 2 years on the faculty were considered junior physicians. We also collected information on the number of times they had presented at the Incubator sessions and stage of progress of the project, whether in the early, mid, or late phase at the time of presentation. Early phase was defined as presenting an initial project idea or brainstorming possible project options and/or directions. Mid phase was defined as presenting initial results, data, and initial drafts prior to completion of analysis. Late phase was defined as presenting a project nearing completion such as a written abstract, oral presentation, paper, or grant application. Respondents were also asked to identify the main focus of their projects, selecting the categories based on the interests of the division, including medical education, clinical research, QI, high‐value care, and global health.
Survey Data Analysis
We converted Likert scale data into dichotomous variables, with paring of positive responses versus the negative options. We summarized survey responses using descriptive statistics and determined if there were any differences in responses between career researchers and nonresearchers using 2 tests. All analysis was performed using StataSE version 13.1 (StataCorp, College Station, TX).
RESULTS
Survey Respondent Characteristics
We received 51 completed surveys from presenters at an Incubator session, for a total survey response rate of 70%. Of the 51 presentations, 26 (51%) of the projects were led by physicians in training or junior faculty, and 35 (69%) of the presenters were nonresearchers.
Project Characteristics
The most frequently presented topic areas were QI (N = 20), clinical research (N = 14), medical education (N = 6), and global health (N = 6). Whereas researchers were more likely to present clinical research topics and grant applications, nonresearchers more often presented on QI or medical education projects (Table 2). Projects were presented at all stages of development, with the middle stage, where presenters presented initial results, being the most common phase.
| All | Nonresearcher, No. (%) | Researcher, No. (%) | P Value | |
|---|---|---|---|---|
| ||||
| Total | 51 | 35 | 16 | |
| Trainee or junior faculty | 19 (54%) | 7 (44%) | 0.49 | |
| Topic of project | 0.02 | |||
| Quality improvement | 20 (39%) | 15 (43%) | 5 (31%) | |
| Clinical research | 14 (27%) | 8 (23%) | 6 (38%) | |
| Medical education | 6 (12%) | 5 (14%) | 1 (6%) | |
| Health technology | 4 (8%) | 0 (0%) | 4 (25%) | |
| High‐value care | 1 (2%) | 1 (3%) | 0 (0%) | |
| Global health | 6 (12%) | 6 (12%) | 0 (0%) | |
| Stage of project | 0.31 | |||
| Early* | 12 (23%) | 7 (20%) | 5 (31%) | |
| Middle | 24 (47%) | 19 (54%) | 5 (31%) | |
| Late | 15 (29%) | 9 (26%) | 6 (38%) | |
Impact of Incubator
The reaction to the session was very positive, with 100% of respondents recommending Incubator to others (Table 3), and 35% reported learning as a result of the session. Twenty‐three (45%) of respondents reported that the session helped reframe the project idea and changed the study design, and 20 (39%) reported improved written or oral presentation style. A majority (45, 88%) reported that Incubator was valuable in advancing the project to completion.
| All | Nonresearcher, No. (%) | Researcher, No. (%) | P Value | |
|---|---|---|---|---|
| ||||
| Trainee or junior faculty | 51 | 35 (69%) | 16 (31%) | 0.49 |
| Reaction | ||||
| Satisfied with their WIP session | 50 (98%) | 35 (100%) | 15 (94%) | 0.25 |
| Would recommend WIP to others | 51 (100%) | 35 (100%) | 16 (100%) | 1.00 |
| Any of the above | 35 (100%) | 16 (100%) | 1.00 | |
| Learning | ||||
| Advanced research methodology | 18 (35%) | 12 (34%) | 6 (38%) | 0.82 |
| Advanced knowledge in the area | 9 (18%) | 5 (14%) | 4 (25%) | 0.35 |
| Any of the above | 14 (40%) | 9 (56%) | 0.28 | |
| Behavior | ||||
| Current project | ||||
| Reframed project idea | 23 (45%) | 15 (43%) | 8 (50%) | 0.63 |
| Changed study design or methodology | 23 (45%) | 16 (46%) | 7 (44%) | 0.9 |
| Improved written or oral presentation style | 20 (39%) | 15 (43%) | 5 (31%) | 0.43 |
| Future projects | ||||
| Changed approach to future projects | 19 (37%) | 17 (49%) | 2 (13%) | 0.01 |
| Any of the above | 34 (97%) | 14 (88%) | 0.17 | |
| Results | ||||
| Valuable in advancing project to completion | 45 (88%) | 31 (89%) | 14 (88%) | 0.18 |
| Provided mentoring and peer support | 29 (57%) | 24 (69%) | 5 (31%) | 0.01 |
| Connected individuals with similar results | 13 (13%) | 9 (26%) | 4 (25%) | 0.96 |
| Any of the above | 34 (97%) | 14 (88%) | 0.17 | |
Survey results of researchers compared to nonresearchers were similar overall, although nonresearchers were more likely to report changes in behavior and in improved mentoring as a result of presenting at Incubator. Notably, 17 (49%) of nonresearchers reported that Incubator changed their approach to future projects as opposed to only 2 (13%) researchers (P = 0.01). In addition, 24 (69%) nonresearchers reported value in mentorship and peer support compared to 5 (31%) researchers (P = 0.01). A reasonably large proportion of projects originally presented during the Incubator sessions became published articles at the time of survey completion (N = 19, 37%) or were publications in progress (N = 14, 27%). For all remaining items, there were no statistically significant differences in the survey responses among junior faculty/trainees (N = 26) compared to nonjunior faculty (N = 25) presenters (P > 0.05).
Attendance at Incubator During the Study Period
Attendance at Incubator was open and voluntary for all DHM faculty, fellows, and collaborating UCSF trainees. From July 2012, when we began tracking attendance, through the end of the survey period in November 2013, the average number of attendees for each session was 10.7 (standard deviation [SD] 3.8). On average, 50% (SD 16%) of attendees at Incubator were career researchers.
DISCUSSION
The results of this program evaluation suggest that a WIP session employed by an academic division of hospital medicine, consisting of a weekly moderated session, can help advance scholarly work. Our evaluation found that presenters, both researchers and nonresearchers, favorably viewed the regular WIP sessions and reported that feedback in the Incubator helped them advance their project to completion. Importantly, nonresearch‐focused faculty and fellows reported the biggest gains in learning from presenting at Incubator. Whereas half the Incubator attendees were career researchers, consistent with the observation that researchers within the division were most committed to attending Incubator regularly, 69% of the presenters were nonresearchers, demonstrating strong participation among both researchers and nonresearchers within the division.
WIP sessions, though informal, are interactive, inspire critical self‐reflection, and encourage physicians to act on generated ideas, as evidenced by the change in behavior of the participants after the session. These sessions allow for transformative learning by encouraging physicians to be open to alternative viewpoints and engage in discourse, boosting learning beyond just content knowledge. Prior assessments of WIP seminars similarly found high satisfaction with these formats.[11]
Although we cannot identify specifically which features made Incubator effective, we believe that our WIP had some characteristics that may have contributed to its success and may aid in implementation at other institutions: holding the session regularly, voluntary participation, distributing the materials and questions for the group in advance, and designating a moderator for the session in advance to facilitate discussion.
A potential strength of the Incubator is that both researchers and nonresearchers attend. We hypothesize that combining these groups provides improved mentorship and learning for nonresearchers, in particular. In addition, it creates a mutually beneficial environment where each group is able to witness the diversity of projects within the division and learn to provide focused, constructive feedback on the presented work. Not only did this create a transparent environment with better understanding of divisional activities, but also fostered collaboration among hospitalists with similar interests and complementary skills.
Challenges, Setbacks, Updated Approaches
The creation of a successful Incubator session, however, was not without its challenges. At initial inception, the WIP was attended primarily by researchers and had low overall attendance. Members of the division who were primarily clinicians initially perceived the conferences as largely inapplicable to their career objectives and had competing demands from patient care, educational, or administrative responsibilities. However, over time and with encouragement from divisional leaders and service line directors, increasing numbers of hospitalists began to participate in Incubator. The timing of Incubator during afternoons after the Department of Medicine Grand Rounds was chosen specifically to allow clinicians to complete their responsibilities, including morning rounds and teaching, to allow better attendance.
In addition, the results of our survey informed changes to the structure of Incubator. The efficacy of assigning a primary reviewer for each session was not clear, so this component was eventually dropped. The finding that nonresearchers in particular reported a benefit from mentoring and peer‐support at Incubator led to the implementation of querying the presenter for a wish list of faculty attendees at their Incubator session. We then sent a special invitation to those faculty members thought to have special insights on the project. This gave junior faculty the opportunity to present their projects to more senior faculty members within their areas of research, as well as to receive focused expert feedback.
Finally, we have initiated special Incubator sessions focused more on didactics to teach the process of writing manuscripts and brainstorming workshop ideas for national meetings.
Limitations
Our study has limitations. It is a single‐center study based on a small overall sample size, and it is not certain whether a similar innovation would have comparable effects at another institution. In addition, generalizability of our results may be limited for hospital medicine groups without a robust research program. We did not have a control group nor do we know whether participants would have been equally successful without Incubator. We also were unable to assess how Incubator affected long‐term outcomes such as promotion and overall publication record, as we do not have detailed data on productivity prior to the survey period. Finally, we are unable to quantify the effect of Incubator on scholarly success in the division. Although the numbers of published articles and grant funding has increased since the Incubator began (data not shown), the division also grew both in number of research‐focused and nonresearch‐focused faculty, and this study does not account for other temporal changes that may have contributed to improvements in the scholarly output of the division.
CONCLUSIONS
In summary, the Incubator has been a successful program that fostered progress on scholarly projects within a largely clinically focused DHM. Given the importance of scholarship in academic hospital medicine, a WIP session such as the one we describe is a valuable way to support and mentor junior hospitalists and nonresearchers.
Acknowledgements
The authors extend special thanks to Oralia Schatzman, divisional administrative assistant, who organized and arranged the Incubator sessions and recorded attendance, and to Katherine Li, who collected data on numbers of faculty within the division over the duration of the study.
Disclosures: Dr. Hemali Patel, Dr. Margaret Fang, and Mr. James Harrison report no conflicts of interest. At the time the research was conducted, Dr. Kangelaris was supported by the National Heart, Lung, and Blood Institute (1K23HL116800‐01). Dr. Auerbach was supported by the National Heart, Lung, and Blood Institute (K24 K24HL098372).
- , , , . Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161–166.
- , , , , . Tried and true: a survey of successfully promoted academic hospitalists. J Hosp Med. 2011;6(7):411–415.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23–27.
- , , , , , . The PRIME curriculum. J Gen Intern Med. 2006;21(5):506–509.
- , , , . Hospital medicine fellowships: works in progress. Am J Med. 2006;119(1):72.e1–7.
- , , . Instituting systems‐based practice and practice‐based learning and improvement: a curriculum of inquiry. Med Educ Online. 2013;18:21612.
- , , , et al. A physician peer support writing group. Fam Med. 2003;35(3):195–201.
- , , , . Research in progress conference for hospitalists provides valuable peer mentoring. J Hosp Med. 2011;6(1):43–46.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- , . Evaluating Training Programs: The Four Levels. 3rd ed. San Francisco, CA: Berrett‐Koehler; 2006.
- , , . Works‐in‐progress: guiding junior scientists through career development applications. J Cancer Educ. 2008;23(3):142–148.
- , , , . Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161–166.
- , , , , . Tried and true: a survey of successfully promoted academic hospitalists. J Hosp Med. 2011;6(7):411–415.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23–27.
- , , , , , . The PRIME curriculum. J Gen Intern Med. 2006;21(5):506–509.
- , , , . Hospital medicine fellowships: works in progress. Am J Med. 2006;119(1):72.e1–7.
- , , . Instituting systems‐based practice and practice‐based learning and improvement: a curriculum of inquiry. Med Educ Online. 2013;18:21612.
- , , , et al. A physician peer support writing group. Fam Med. 2003;35(3):195–201.
- , , , . Research in progress conference for hospitalists provides valuable peer mentoring. J Hosp Med. 2011;6(1):43–46.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- , . Evaluating Training Programs: The Four Levels. 3rd ed. San Francisco, CA: Berrett‐Koehler; 2006.
- , , . Works‐in‐progress: guiding junior scientists through career development applications. J Cancer Educ. 2008;23(3):142–148.
© 2016 Society of Hospital Medicine
Assessment of Personal Medical History Knowledge in Adolescents with Sickle Cell Disease: A Pilot Study
From the Departments of Psychology (Ms. Zhao, Drs. Russell, Wesley, and Porter) and Hematology (Mss. Johnson and Pullen, Dr. Hankins), St. Jude Children’s Research Hospital, Memphis, TN.
Abstract
- Background: Children with sickle cell disease (SCD) are surviving into adulthood. Mastery of disease knowledge may facilitate treatment continuity in adult care.
- Objective: To assess the accuracy and extent of medical history knowledge among adolescents with SCD through the use of a personal health record (PHR) form.
- Methods: 68 adolescent patients with SCD (52.9% male; mean age, 16.8 years; 100% African American) completed a PHR listing significant prior medical events (eg, disease complications, diagnostic evaluations, treatments). Responses were compared against participants’ electronic medical record. An agreement percentage was calculated to determine accuracy of knowledge.
- Results: Most adolescents correctly reported their sickle cell genotype (100%), usage of penicillin (97.1%), prior hospitalizations (96.5%), history of prior blood transfusions (93.8%), usage of hydroxyurea (88.2%), and allergies (85.2%). Fewer adolescents accurately reported usage of opioids (52.9%), prior acute chest syndrome events (50.9%), baseline hemoglobin (41.8%), and hepatitis (43.3%), pneumovax (30.2%), and menactra (14.5%) vaccinations.
- Conclusion: Adolescents are aware of most but not all aspects of their medical history. The present findings can inform areas of knowledge deficits. Future targeted interventions for transition education and preparation may be tailored based on individual disease knowledge.
Sickle cell disease (SCD) is a genetic disorder characterized by abnormal sickle hemoglobin resulting in chronic hemolytic anemia and vaso-occlusion [1]. More than 95% of children with SCD in the United States survive into adulthood; however, young adults (YAs) are at risk for mortality shortly after transfer to adult health care [2–5]. Specifically, YAs with SCD (ages 18 to 30) have increased hospital utilization, emergency department visits, and mortality compared to other age-groups [4–7]. During this critical period, transition preparation that includes improving disease literacy and ensuring medical history knowledge may be necessary for optimal outcomes.
In the extant YA literature, significant gaps in medical history knowledge during the transition period were observed in pediatric cancer and inflammatory bowel disease patients [8,9]. YAs often require multidisciplinary management of their chronic disease complications [10]. Therefore, possessing comprehensive knowledge of personal health history may facilitate communication with different adult care providers and promote continuity of care. In the SCD transition literature, transition readiness measures have been developed to assess several aspects of knowledge, including medical and disease knowledge; however, these measures are primarily self-reported perceptions of knowledge and do not evaluate the accuracy of knowledge [11,12]. The current pilot study addresses this gap with the aim of assessing medical history knowledge accuracy in adolescents with SCD.
Methods
Participants
From March 2011 to January 2014, adolescents (aged 15–18 years) with SCD (any genotype) were approached during their regular health maintenance visits by hematology social workers. They were invited to complete the Personal Health Record (PHR) as an implementation effort of transition preparation within our pediatric SCD program.
Personal Health Record
The PHR was developed through literature review and discussions with area adult hematologists. The form was modeled after first visit intake forms used in adult hematology clinics. It was reviewed by the hematology medical team and the institution’s patient education committee. Prior to implementation, the form was piloted to obtain patient feedback on format and content. The PHR consists of 33 questions with 168 possible items/data points covering 12 domains: personal information (eg, contact information, SCD genotype), health provider information, personal health history (ie, health diagnoses), blood transfusion history, sickle cell pain events, hospitalization history in the previous year, diagnostic testing history (eg, laboratory tests), current medications, immunizations, advance directives, resource information (eg, disability benefits), and activities of daily living. Some questions required patients to check “Yes” or “No” (eg, “Have you been hospitalized in the past year? Have you received flu vaccine?”) while some required a written response (eg, “What medicines do you currently take?”).
Adolescents were instructed to complete the PHR independently without the help of their caregivers. After completing the form, the social worker reviewed the answers and/or asked participants’ perspectives about communicating health information to providers. A copy of the completed PHR was provided to the adolescent to promote continued education regarding medical history knowledge. The retrospective review of the PHR answers and participants’ characteristics was approved by the institutional review board with a waiver of consent from participants.
Statistical Methods
PHR answers were compared with each individual’s electronic medical record (EMR) for accuracy of responses. PHR responses were considered accurate only if they matched the information in the EMR. PHR items absent in the EMR were not coded (inability to verify the accuracy of responses) to capture the most accurate depiction of adolescents’ medical history knowledge. Coding was checked by at least 2 coders for response accuracy. Due to lack of EMR information for certain items, we could not verify the accuracy of many PHR items. Therefore, only items with at least 75% of data verified (across all patients who completed the PHR) were included in subsequent analyses.
Using SPSS (version 18), an agreement percentage was calculated for each patient across verifiable items and used as the primary outcome measure of knowledge accuracy. We used t tests to investigate gender or genotype differences in medical history accuracy. To examine genotype differences, we stratified the sample by SCD genotype: HbSS/Sβ0 thalassemia and HbSC/Sβ+ thalassemia [13].
Results
Patient Characteristics
Knowledge Accuracy Among Adolescents with SCD
Seventeen items in 6 PHR domains had the highest number of data points (at least 75% verified), and therefore were the only items that could be analyzed. Analyzed items included information about sickle cell genotype, eye doctor care, comorbid health issues (eg, asthma), allergies, hospitalizations, surgeries, transfusions, acute chest syndrome (ACS) episodes, eye problems, baseline hemoglobin level, and vaccination history as well as adolescents’ knowledge of current medications, including hydroxyurea, penicillin, and opioid pain medications.
Gender was not significantly associated with overall accuracy (P = 0.36). A significant difference was found in sickle genotype such that individuals with HbSC/Sβ+ thalassemia genotype (mean number of items, 8.23; SD = 1.70) were more accurate reporters of their medical history than those with HbSS/Sβ0 thalassemia genotype (mean number of items, 7.14; SD = 1.75; t(65) = –2.59, P = 0.01). Specifically, those with HbSS/Sβ0 thalassemia genotype were significantly less accurate reporters of vaccination history (meningococcus t(60) = 3.55, P = 0.001; pneumococcus t(60) = 2.46, P = 0.02; hepatitis t(64) = 2.18, P = 0.03, eye problems t(62) = 3.62; P = 0.001, and surgical history t(62) = 2.14, P = 0.04).
Discussion
In the present study, we utilized the PHR to assess the accuracy of medical history knowledge of adolescents with SCD preparing to transition to adult care. Most adolescents were accurate reporters of important disease-relevant information (eg, genotype, transfusion history, hydroxyurea use), which may be a result of these topics being frequently discussed or recently encountered. For example, 97% of adolescents accurately reported penicillin use which may be related to our program’s emphasis on infection prevention education. However, disease knowledge of immunization history, prior ACS events, and opioid medication use might have been more difficult to recall due to the long interval from their occurrence until the completion of the PHR. Further, frequent changes in opioid medication use may have impacted the accuracy of adolescents’ answers with EMR data.
Individuals with HbSC/Sβ+ thalassemia genotype were more accurate reporters of their medical history, but the magnitude of difference was not large. These individuals tend to have fewer health issues and therefore less health information to recall, leading to higher accuracy. Furthermore, evidence demonstrates that individuals with HbSS/Sβ0 thalassemia genotype are at greater risk for cerebrovascular events and subsequent cognitive deficits [14], leading to more memory deficits and difficulty understanding and retaining health information [15]. The results suggest that patient health literacy, or an individual’s capacity to understand basic health information [16], may be a mediating factor in assessing for transition readiness. This is especially important given SCD risk for cognitive deficits [17].
Only 17 PHR items were analyzed due to conservative selection of items. Thus the present findings are not representative of the entire medical history. Additionally, the accuracy of medical history knowledge results may be limited by conservatism with abstracting information from the EMR (PHR information was considered accurate if it matched the information found in their EMR). Finally, we did not systematically assess the feasibility and utility of the PHR; ongoing participant feedback would aid in improving the PHR tool and implementation. It would be important to validate the PHR in a larger sample. However, our study is the first to our knowledge to systematically evaluate medical history knowledge among youth with SCD.
Conclusion and Practice Implications
The present study demonstrates that use of the PHR during regular health maintenance visits can help identify gaps in knowledge among adolescents with SCD who are approaching transfer to adult care. Sufficient knowledge of one’s medical history is an important aspect in transition preparation as it can facilitate the communication of medical information, thereby ensuring continuity of care [18,19]. The PHR could be used to teach medical history knowledge, assess a patient’s level of transition readiness at different time points, and identify areas for further targeted intervention. Knowledge tools, such as the PHR, can be investigated prospectively to assess the association of disease literacy and clinical outcomes, serving as a possible predictive instrument for transition health outcomes.
Corresponding author: Jerlym S. Porter, PhD, MPH, St. Jude Children’s Research Hospital, Dept. of Psychology, 262 Danny Thomas Pl., Mail Stop 740, Memphis, TN 38105, jerlym.porter@stjude.org.
Funding/support: This work was supported in part by HRSA grant 6 U1EMC19331-03-02 (PI: Hankins).
Financial disclosures: None.
Author contributions: conception and design, MJ, AP, KMW, JSH, JSP; analysis and interpretation of data, MSZ, KMR, JSP; drafting of article, MSZ, JSP; critical revision of the article, MSZ, MJ, AP, KMW, JSH, JSP; provision of study materials or patients, MJ, AP; statistical expertise, KMR; obtaining of funding, JSH; collection and assembly of data, MSZ, MJ, AP, KMR, KMW.
1. Quinn CT. Sickle cell disease in childhood: from newborn screening through transition to adult medical care. Pediatr Clin North Am 2013;60:1363–81.
2. Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med 2010;38:S512–21.
3. Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999-2009). Pediatr Blood Cancer 2013;60:1482–6.
4. de Montalembert M, Guitton C. Transition from paediatric to adult care for patients with sickle cell disease. Br J Haematol 2014;164:630–5.
5. Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood 2010;115:3447–52.
6. Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA 2010;303:1288–94.
7. Lanzkron S, Carroll CP, Haywood Jr C. Mortality rates and age at death from sickle cell disease: U.S., 1979-2005. Public Health Rep 2013;128:110–6.
8. Kadan-Lottick NS, Robison LL, Gurney JG, et al. Childhood cancer survivors' knowledge about their past diagnosis and treatment: Childhood Cancer Survivor Study. JAMA 2002:287:1832–9.
9. Hait EJ, Barendse RM, Arnold JH, et al. Transition of adolescents with inflammatory bowel disease from pediatric to adult care: a survey of adult gastroenterologists. J Pediatr Gastroenterol Nutr 2009;48:61–5.
10. Kennedy A, Sawyer S. Transition from pediatric to adult services: are we getting it right? Curr Opin Pediatr 2008;20:403–9.
11. Sobota A, Akinlonu A, Champigny M, et al. Self-reported transition readiness among young adults with sickle cell disease. J Pediatr Hematol Oncol 2014;36:389–94.
12. Treadwell M, Johnson S, Sisler I, et al. Development of a sickle cell disease readiness for transition assessment. Int J Adolesc Med Health 2016;28:193–201.
13. Dampier C, Ely B, Brodecki D, et al. Pain characteristics and age-related pain trajectories in infants and young children with sickle cell disease. Pediatr Blood Cancer 2014;61:291–6.
14. Venkataraman A, Adams RJ. Neurologic complications of sickle cell disease. Handb Clin Neurol 2014;120:1015–25.
15. Porter JS, Matthews CS, Carroll YM, et al. Genetic education and sickle cell disease: feasibility and efficacy of a program tailored to adolescents. J Pediatr Hematol Oncol 2014;36:572–7.
16. Centers for Disease Control and Prevention. Health literacy. 2015. Accessed 26 Oct 2015 at www.cdc.gov/healthliteracy/index.html.
17. Armstrong FD, Thompson Jr RJ, Wang W, et al. Cognitive functioning and brain magnetic resonance imaging in children with sickle cell disease. Neuropsychology Committee of the Cooperative Study of Sickle Cell Disease. Pediatrics 1996;97:864–70.
18. Kanter J, Kruse-Jarres R. Management of sickle cell disease from childhood through adulthood. Blood Rev 2013;27:279–87.
19. Treadwell M, Telfair J, Gibson RW, et al. Transition from pediatric to adult care in sickle cell disease: establishing evidence-based practice and directions for research. Am J Hematol 2011;86:116–2.
From the Departments of Psychology (Ms. Zhao, Drs. Russell, Wesley, and Porter) and Hematology (Mss. Johnson and Pullen, Dr. Hankins), St. Jude Children’s Research Hospital, Memphis, TN.
Abstract
- Background: Children with sickle cell disease (SCD) are surviving into adulthood. Mastery of disease knowledge may facilitate treatment continuity in adult care.
- Objective: To assess the accuracy and extent of medical history knowledge among adolescents with SCD through the use of a personal health record (PHR) form.
- Methods: 68 adolescent patients with SCD (52.9% male; mean age, 16.8 years; 100% African American) completed a PHR listing significant prior medical events (eg, disease complications, diagnostic evaluations, treatments). Responses were compared against participants’ electronic medical record. An agreement percentage was calculated to determine accuracy of knowledge.
- Results: Most adolescents correctly reported their sickle cell genotype (100%), usage of penicillin (97.1%), prior hospitalizations (96.5%), history of prior blood transfusions (93.8%), usage of hydroxyurea (88.2%), and allergies (85.2%). Fewer adolescents accurately reported usage of opioids (52.9%), prior acute chest syndrome events (50.9%), baseline hemoglobin (41.8%), and hepatitis (43.3%), pneumovax (30.2%), and menactra (14.5%) vaccinations.
- Conclusion: Adolescents are aware of most but not all aspects of their medical history. The present findings can inform areas of knowledge deficits. Future targeted interventions for transition education and preparation may be tailored based on individual disease knowledge.
Sickle cell disease (SCD) is a genetic disorder characterized by abnormal sickle hemoglobin resulting in chronic hemolytic anemia and vaso-occlusion [1]. More than 95% of children with SCD in the United States survive into adulthood; however, young adults (YAs) are at risk for mortality shortly after transfer to adult health care [2–5]. Specifically, YAs with SCD (ages 18 to 30) have increased hospital utilization, emergency department visits, and mortality compared to other age-groups [4–7]. During this critical period, transition preparation that includes improving disease literacy and ensuring medical history knowledge may be necessary for optimal outcomes.
In the extant YA literature, significant gaps in medical history knowledge during the transition period were observed in pediatric cancer and inflammatory bowel disease patients [8,9]. YAs often require multidisciplinary management of their chronic disease complications [10]. Therefore, possessing comprehensive knowledge of personal health history may facilitate communication with different adult care providers and promote continuity of care. In the SCD transition literature, transition readiness measures have been developed to assess several aspects of knowledge, including medical and disease knowledge; however, these measures are primarily self-reported perceptions of knowledge and do not evaluate the accuracy of knowledge [11,12]. The current pilot study addresses this gap with the aim of assessing medical history knowledge accuracy in adolescents with SCD.
Methods
Participants
From March 2011 to January 2014, adolescents (aged 15–18 years) with SCD (any genotype) were approached during their regular health maintenance visits by hematology social workers. They were invited to complete the Personal Health Record (PHR) as an implementation effort of transition preparation within our pediatric SCD program.
Personal Health Record
The PHR was developed through literature review and discussions with area adult hematologists. The form was modeled after first visit intake forms used in adult hematology clinics. It was reviewed by the hematology medical team and the institution’s patient education committee. Prior to implementation, the form was piloted to obtain patient feedback on format and content. The PHR consists of 33 questions with 168 possible items/data points covering 12 domains: personal information (eg, contact information, SCD genotype), health provider information, personal health history (ie, health diagnoses), blood transfusion history, sickle cell pain events, hospitalization history in the previous year, diagnostic testing history (eg, laboratory tests), current medications, immunizations, advance directives, resource information (eg, disability benefits), and activities of daily living. Some questions required patients to check “Yes” or “No” (eg, “Have you been hospitalized in the past year? Have you received flu vaccine?”) while some required a written response (eg, “What medicines do you currently take?”).
Adolescents were instructed to complete the PHR independently without the help of their caregivers. After completing the form, the social worker reviewed the answers and/or asked participants’ perspectives about communicating health information to providers. A copy of the completed PHR was provided to the adolescent to promote continued education regarding medical history knowledge. The retrospective review of the PHR answers and participants’ characteristics was approved by the institutional review board with a waiver of consent from participants.
Statistical Methods
PHR answers were compared with each individual’s electronic medical record (EMR) for accuracy of responses. PHR responses were considered accurate only if they matched the information in the EMR. PHR items absent in the EMR were not coded (inability to verify the accuracy of responses) to capture the most accurate depiction of adolescents’ medical history knowledge. Coding was checked by at least 2 coders for response accuracy. Due to lack of EMR information for certain items, we could not verify the accuracy of many PHR items. Therefore, only items with at least 75% of data verified (across all patients who completed the PHR) were included in subsequent analyses.
Using SPSS (version 18), an agreement percentage was calculated for each patient across verifiable items and used as the primary outcome measure of knowledge accuracy. We used t tests to investigate gender or genotype differences in medical history accuracy. To examine genotype differences, we stratified the sample by SCD genotype: HbSS/Sβ0 thalassemia and HbSC/Sβ+ thalassemia [13].
Results
Patient Characteristics
Knowledge Accuracy Among Adolescents with SCD
Seventeen items in 6 PHR domains had the highest number of data points (at least 75% verified), and therefore were the only items that could be analyzed. Analyzed items included information about sickle cell genotype, eye doctor care, comorbid health issues (eg, asthma), allergies, hospitalizations, surgeries, transfusions, acute chest syndrome (ACS) episodes, eye problems, baseline hemoglobin level, and vaccination history as well as adolescents’ knowledge of current medications, including hydroxyurea, penicillin, and opioid pain medications.
Gender was not significantly associated with overall accuracy (P = 0.36). A significant difference was found in sickle genotype such that individuals with HbSC/Sβ+ thalassemia genotype (mean number of items, 8.23; SD = 1.70) were more accurate reporters of their medical history than those with HbSS/Sβ0 thalassemia genotype (mean number of items, 7.14; SD = 1.75; t(65) = –2.59, P = 0.01). Specifically, those with HbSS/Sβ0 thalassemia genotype were significantly less accurate reporters of vaccination history (meningococcus t(60) = 3.55, P = 0.001; pneumococcus t(60) = 2.46, P = 0.02; hepatitis t(64) = 2.18, P = 0.03, eye problems t(62) = 3.62; P = 0.001, and surgical history t(62) = 2.14, P = 0.04).
Discussion
In the present study, we utilized the PHR to assess the accuracy of medical history knowledge of adolescents with SCD preparing to transition to adult care. Most adolescents were accurate reporters of important disease-relevant information (eg, genotype, transfusion history, hydroxyurea use), which may be a result of these topics being frequently discussed or recently encountered. For example, 97% of adolescents accurately reported penicillin use which may be related to our program’s emphasis on infection prevention education. However, disease knowledge of immunization history, prior ACS events, and opioid medication use might have been more difficult to recall due to the long interval from their occurrence until the completion of the PHR. Further, frequent changes in opioid medication use may have impacted the accuracy of adolescents’ answers with EMR data.
Individuals with HbSC/Sβ+ thalassemia genotype were more accurate reporters of their medical history, but the magnitude of difference was not large. These individuals tend to have fewer health issues and therefore less health information to recall, leading to higher accuracy. Furthermore, evidence demonstrates that individuals with HbSS/Sβ0 thalassemia genotype are at greater risk for cerebrovascular events and subsequent cognitive deficits [14], leading to more memory deficits and difficulty understanding and retaining health information [15]. The results suggest that patient health literacy, or an individual’s capacity to understand basic health information [16], may be a mediating factor in assessing for transition readiness. This is especially important given SCD risk for cognitive deficits [17].
Only 17 PHR items were analyzed due to conservative selection of items. Thus the present findings are not representative of the entire medical history. Additionally, the accuracy of medical history knowledge results may be limited by conservatism with abstracting information from the EMR (PHR information was considered accurate if it matched the information found in their EMR). Finally, we did not systematically assess the feasibility and utility of the PHR; ongoing participant feedback would aid in improving the PHR tool and implementation. It would be important to validate the PHR in a larger sample. However, our study is the first to our knowledge to systematically evaluate medical history knowledge among youth with SCD.
Conclusion and Practice Implications
The present study demonstrates that use of the PHR during regular health maintenance visits can help identify gaps in knowledge among adolescents with SCD who are approaching transfer to adult care. Sufficient knowledge of one’s medical history is an important aspect in transition preparation as it can facilitate the communication of medical information, thereby ensuring continuity of care [18,19]. The PHR could be used to teach medical history knowledge, assess a patient’s level of transition readiness at different time points, and identify areas for further targeted intervention. Knowledge tools, such as the PHR, can be investigated prospectively to assess the association of disease literacy and clinical outcomes, serving as a possible predictive instrument for transition health outcomes.
Corresponding author: Jerlym S. Porter, PhD, MPH, St. Jude Children’s Research Hospital, Dept. of Psychology, 262 Danny Thomas Pl., Mail Stop 740, Memphis, TN 38105, jerlym.porter@stjude.org.
Funding/support: This work was supported in part by HRSA grant 6 U1EMC19331-03-02 (PI: Hankins).
Financial disclosures: None.
Author contributions: conception and design, MJ, AP, KMW, JSH, JSP; analysis and interpretation of data, MSZ, KMR, JSP; drafting of article, MSZ, JSP; critical revision of the article, MSZ, MJ, AP, KMW, JSH, JSP; provision of study materials or patients, MJ, AP; statistical expertise, KMR; obtaining of funding, JSH; collection and assembly of data, MSZ, MJ, AP, KMR, KMW.
From the Departments of Psychology (Ms. Zhao, Drs. Russell, Wesley, and Porter) and Hematology (Mss. Johnson and Pullen, Dr. Hankins), St. Jude Children’s Research Hospital, Memphis, TN.
Abstract
- Background: Children with sickle cell disease (SCD) are surviving into adulthood. Mastery of disease knowledge may facilitate treatment continuity in adult care.
- Objective: To assess the accuracy and extent of medical history knowledge among adolescents with SCD through the use of a personal health record (PHR) form.
- Methods: 68 adolescent patients with SCD (52.9% male; mean age, 16.8 years; 100% African American) completed a PHR listing significant prior medical events (eg, disease complications, diagnostic evaluations, treatments). Responses were compared against participants’ electronic medical record. An agreement percentage was calculated to determine accuracy of knowledge.
- Results: Most adolescents correctly reported their sickle cell genotype (100%), usage of penicillin (97.1%), prior hospitalizations (96.5%), history of prior blood transfusions (93.8%), usage of hydroxyurea (88.2%), and allergies (85.2%). Fewer adolescents accurately reported usage of opioids (52.9%), prior acute chest syndrome events (50.9%), baseline hemoglobin (41.8%), and hepatitis (43.3%), pneumovax (30.2%), and menactra (14.5%) vaccinations.
- Conclusion: Adolescents are aware of most but not all aspects of their medical history. The present findings can inform areas of knowledge deficits. Future targeted interventions for transition education and preparation may be tailored based on individual disease knowledge.
Sickle cell disease (SCD) is a genetic disorder characterized by abnormal sickle hemoglobin resulting in chronic hemolytic anemia and vaso-occlusion [1]. More than 95% of children with SCD in the United States survive into adulthood; however, young adults (YAs) are at risk for mortality shortly after transfer to adult health care [2–5]. Specifically, YAs with SCD (ages 18 to 30) have increased hospital utilization, emergency department visits, and mortality compared to other age-groups [4–7]. During this critical period, transition preparation that includes improving disease literacy and ensuring medical history knowledge may be necessary for optimal outcomes.
In the extant YA literature, significant gaps in medical history knowledge during the transition period were observed in pediatric cancer and inflammatory bowel disease patients [8,9]. YAs often require multidisciplinary management of their chronic disease complications [10]. Therefore, possessing comprehensive knowledge of personal health history may facilitate communication with different adult care providers and promote continuity of care. In the SCD transition literature, transition readiness measures have been developed to assess several aspects of knowledge, including medical and disease knowledge; however, these measures are primarily self-reported perceptions of knowledge and do not evaluate the accuracy of knowledge [11,12]. The current pilot study addresses this gap with the aim of assessing medical history knowledge accuracy in adolescents with SCD.
Methods
Participants
From March 2011 to January 2014, adolescents (aged 15–18 years) with SCD (any genotype) were approached during their regular health maintenance visits by hematology social workers. They were invited to complete the Personal Health Record (PHR) as an implementation effort of transition preparation within our pediatric SCD program.
Personal Health Record
The PHR was developed through literature review and discussions with area adult hematologists. The form was modeled after first visit intake forms used in adult hematology clinics. It was reviewed by the hematology medical team and the institution’s patient education committee. Prior to implementation, the form was piloted to obtain patient feedback on format and content. The PHR consists of 33 questions with 168 possible items/data points covering 12 domains: personal information (eg, contact information, SCD genotype), health provider information, personal health history (ie, health diagnoses), blood transfusion history, sickle cell pain events, hospitalization history in the previous year, diagnostic testing history (eg, laboratory tests), current medications, immunizations, advance directives, resource information (eg, disability benefits), and activities of daily living. Some questions required patients to check “Yes” or “No” (eg, “Have you been hospitalized in the past year? Have you received flu vaccine?”) while some required a written response (eg, “What medicines do you currently take?”).
Adolescents were instructed to complete the PHR independently without the help of their caregivers. After completing the form, the social worker reviewed the answers and/or asked participants’ perspectives about communicating health information to providers. A copy of the completed PHR was provided to the adolescent to promote continued education regarding medical history knowledge. The retrospective review of the PHR answers and participants’ characteristics was approved by the institutional review board with a waiver of consent from participants.
Statistical Methods
PHR answers were compared with each individual’s electronic medical record (EMR) for accuracy of responses. PHR responses were considered accurate only if they matched the information in the EMR. PHR items absent in the EMR were not coded (inability to verify the accuracy of responses) to capture the most accurate depiction of adolescents’ medical history knowledge. Coding was checked by at least 2 coders for response accuracy. Due to lack of EMR information for certain items, we could not verify the accuracy of many PHR items. Therefore, only items with at least 75% of data verified (across all patients who completed the PHR) were included in subsequent analyses.
Using SPSS (version 18), an agreement percentage was calculated for each patient across verifiable items and used as the primary outcome measure of knowledge accuracy. We used t tests to investigate gender or genotype differences in medical history accuracy. To examine genotype differences, we stratified the sample by SCD genotype: HbSS/Sβ0 thalassemia and HbSC/Sβ+ thalassemia [13].
Results
Patient Characteristics
Knowledge Accuracy Among Adolescents with SCD
Seventeen items in 6 PHR domains had the highest number of data points (at least 75% verified), and therefore were the only items that could be analyzed. Analyzed items included information about sickle cell genotype, eye doctor care, comorbid health issues (eg, asthma), allergies, hospitalizations, surgeries, transfusions, acute chest syndrome (ACS) episodes, eye problems, baseline hemoglobin level, and vaccination history as well as adolescents’ knowledge of current medications, including hydroxyurea, penicillin, and opioid pain medications.
Gender was not significantly associated with overall accuracy (P = 0.36). A significant difference was found in sickle genotype such that individuals with HbSC/Sβ+ thalassemia genotype (mean number of items, 8.23; SD = 1.70) were more accurate reporters of their medical history than those with HbSS/Sβ0 thalassemia genotype (mean number of items, 7.14; SD = 1.75; t(65) = –2.59, P = 0.01). Specifically, those with HbSS/Sβ0 thalassemia genotype were significantly less accurate reporters of vaccination history (meningococcus t(60) = 3.55, P = 0.001; pneumococcus t(60) = 2.46, P = 0.02; hepatitis t(64) = 2.18, P = 0.03, eye problems t(62) = 3.62; P = 0.001, and surgical history t(62) = 2.14, P = 0.04).
Discussion
In the present study, we utilized the PHR to assess the accuracy of medical history knowledge of adolescents with SCD preparing to transition to adult care. Most adolescents were accurate reporters of important disease-relevant information (eg, genotype, transfusion history, hydroxyurea use), which may be a result of these topics being frequently discussed or recently encountered. For example, 97% of adolescents accurately reported penicillin use which may be related to our program’s emphasis on infection prevention education. However, disease knowledge of immunization history, prior ACS events, and opioid medication use might have been more difficult to recall due to the long interval from their occurrence until the completion of the PHR. Further, frequent changes in opioid medication use may have impacted the accuracy of adolescents’ answers with EMR data.
Individuals with HbSC/Sβ+ thalassemia genotype were more accurate reporters of their medical history, but the magnitude of difference was not large. These individuals tend to have fewer health issues and therefore less health information to recall, leading to higher accuracy. Furthermore, evidence demonstrates that individuals with HbSS/Sβ0 thalassemia genotype are at greater risk for cerebrovascular events and subsequent cognitive deficits [14], leading to more memory deficits and difficulty understanding and retaining health information [15]. The results suggest that patient health literacy, or an individual’s capacity to understand basic health information [16], may be a mediating factor in assessing for transition readiness. This is especially important given SCD risk for cognitive deficits [17].
Only 17 PHR items were analyzed due to conservative selection of items. Thus the present findings are not representative of the entire medical history. Additionally, the accuracy of medical history knowledge results may be limited by conservatism with abstracting information from the EMR (PHR information was considered accurate if it matched the information found in their EMR). Finally, we did not systematically assess the feasibility and utility of the PHR; ongoing participant feedback would aid in improving the PHR tool and implementation. It would be important to validate the PHR in a larger sample. However, our study is the first to our knowledge to systematically evaluate medical history knowledge among youth with SCD.
Conclusion and Practice Implications
The present study demonstrates that use of the PHR during regular health maintenance visits can help identify gaps in knowledge among adolescents with SCD who are approaching transfer to adult care. Sufficient knowledge of one’s medical history is an important aspect in transition preparation as it can facilitate the communication of medical information, thereby ensuring continuity of care [18,19]. The PHR could be used to teach medical history knowledge, assess a patient’s level of transition readiness at different time points, and identify areas for further targeted intervention. Knowledge tools, such as the PHR, can be investigated prospectively to assess the association of disease literacy and clinical outcomes, serving as a possible predictive instrument for transition health outcomes.
Corresponding author: Jerlym S. Porter, PhD, MPH, St. Jude Children’s Research Hospital, Dept. of Psychology, 262 Danny Thomas Pl., Mail Stop 740, Memphis, TN 38105, jerlym.porter@stjude.org.
Funding/support: This work was supported in part by HRSA grant 6 U1EMC19331-03-02 (PI: Hankins).
Financial disclosures: None.
Author contributions: conception and design, MJ, AP, KMW, JSH, JSP; analysis and interpretation of data, MSZ, KMR, JSP; drafting of article, MSZ, JSP; critical revision of the article, MSZ, MJ, AP, KMW, JSH, JSP; provision of study materials or patients, MJ, AP; statistical expertise, KMR; obtaining of funding, JSH; collection and assembly of data, MSZ, MJ, AP, KMR, KMW.
1. Quinn CT. Sickle cell disease in childhood: from newborn screening through transition to adult medical care. Pediatr Clin North Am 2013;60:1363–81.
2. Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med 2010;38:S512–21.
3. Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999-2009). Pediatr Blood Cancer 2013;60:1482–6.
4. de Montalembert M, Guitton C. Transition from paediatric to adult care for patients with sickle cell disease. Br J Haematol 2014;164:630–5.
5. Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood 2010;115:3447–52.
6. Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA 2010;303:1288–94.
7. Lanzkron S, Carroll CP, Haywood Jr C. Mortality rates and age at death from sickle cell disease: U.S., 1979-2005. Public Health Rep 2013;128:110–6.
8. Kadan-Lottick NS, Robison LL, Gurney JG, et al. Childhood cancer survivors' knowledge about their past diagnosis and treatment: Childhood Cancer Survivor Study. JAMA 2002:287:1832–9.
9. Hait EJ, Barendse RM, Arnold JH, et al. Transition of adolescents with inflammatory bowel disease from pediatric to adult care: a survey of adult gastroenterologists. J Pediatr Gastroenterol Nutr 2009;48:61–5.
10. Kennedy A, Sawyer S. Transition from pediatric to adult services: are we getting it right? Curr Opin Pediatr 2008;20:403–9.
11. Sobota A, Akinlonu A, Champigny M, et al. Self-reported transition readiness among young adults with sickle cell disease. J Pediatr Hematol Oncol 2014;36:389–94.
12. Treadwell M, Johnson S, Sisler I, et al. Development of a sickle cell disease readiness for transition assessment. Int J Adolesc Med Health 2016;28:193–201.
13. Dampier C, Ely B, Brodecki D, et al. Pain characteristics and age-related pain trajectories in infants and young children with sickle cell disease. Pediatr Blood Cancer 2014;61:291–6.
14. Venkataraman A, Adams RJ. Neurologic complications of sickle cell disease. Handb Clin Neurol 2014;120:1015–25.
15. Porter JS, Matthews CS, Carroll YM, et al. Genetic education and sickle cell disease: feasibility and efficacy of a program tailored to adolescents. J Pediatr Hematol Oncol 2014;36:572–7.
16. Centers for Disease Control and Prevention. Health literacy. 2015. Accessed 26 Oct 2015 at www.cdc.gov/healthliteracy/index.html.
17. Armstrong FD, Thompson Jr RJ, Wang W, et al. Cognitive functioning and brain magnetic resonance imaging in children with sickle cell disease. Neuropsychology Committee of the Cooperative Study of Sickle Cell Disease. Pediatrics 1996;97:864–70.
18. Kanter J, Kruse-Jarres R. Management of sickle cell disease from childhood through adulthood. Blood Rev 2013;27:279–87.
19. Treadwell M, Telfair J, Gibson RW, et al. Transition from pediatric to adult care in sickle cell disease: establishing evidence-based practice and directions for research. Am J Hematol 2011;86:116–2.
1. Quinn CT. Sickle cell disease in childhood: from newborn screening through transition to adult medical care. Pediatr Clin North Am 2013;60:1363–81.
2. Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med 2010;38:S512–21.
3. Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999-2009). Pediatr Blood Cancer 2013;60:1482–6.
4. de Montalembert M, Guitton C. Transition from paediatric to adult care for patients with sickle cell disease. Br J Haematol 2014;164:630–5.
5. Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood 2010;115:3447–52.
6. Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA 2010;303:1288–94.
7. Lanzkron S, Carroll CP, Haywood Jr C. Mortality rates and age at death from sickle cell disease: U.S., 1979-2005. Public Health Rep 2013;128:110–6.
8. Kadan-Lottick NS, Robison LL, Gurney JG, et al. Childhood cancer survivors' knowledge about their past diagnosis and treatment: Childhood Cancer Survivor Study. JAMA 2002:287:1832–9.
9. Hait EJ, Barendse RM, Arnold JH, et al. Transition of adolescents with inflammatory bowel disease from pediatric to adult care: a survey of adult gastroenterologists. J Pediatr Gastroenterol Nutr 2009;48:61–5.
10. Kennedy A, Sawyer S. Transition from pediatric to adult services: are we getting it right? Curr Opin Pediatr 2008;20:403–9.
11. Sobota A, Akinlonu A, Champigny M, et al. Self-reported transition readiness among young adults with sickle cell disease. J Pediatr Hematol Oncol 2014;36:389–94.
12. Treadwell M, Johnson S, Sisler I, et al. Development of a sickle cell disease readiness for transition assessment. Int J Adolesc Med Health 2016;28:193–201.
13. Dampier C, Ely B, Brodecki D, et al. Pain characteristics and age-related pain trajectories in infants and young children with sickle cell disease. Pediatr Blood Cancer 2014;61:291–6.
14. Venkataraman A, Adams RJ. Neurologic complications of sickle cell disease. Handb Clin Neurol 2014;120:1015–25.
15. Porter JS, Matthews CS, Carroll YM, et al. Genetic education and sickle cell disease: feasibility and efficacy of a program tailored to adolescents. J Pediatr Hematol Oncol 2014;36:572–7.
16. Centers for Disease Control and Prevention. Health literacy. 2015. Accessed 26 Oct 2015 at www.cdc.gov/healthliteracy/index.html.
17. Armstrong FD, Thompson Jr RJ, Wang W, et al. Cognitive functioning and brain magnetic resonance imaging in children with sickle cell disease. Neuropsychology Committee of the Cooperative Study of Sickle Cell Disease. Pediatrics 1996;97:864–70.
18. Kanter J, Kruse-Jarres R. Management of sickle cell disease from childhood through adulthood. Blood Rev 2013;27:279–87.
19. Treadwell M, Telfair J, Gibson RW, et al. Transition from pediatric to adult care in sickle cell disease: establishing evidence-based practice and directions for research. Am J Hematol 2011;86:116–2.
Shoulder Arthroplasty: Disposition and Perioperative Outcomes in Patients With and Without Rheumatoid Arthritis
Shoulder arthroplasty (SA), including total SA (TSA) and reverse TSA, is an effective surgical treatment for fracture and primary or secondary degenerative disease of the shoulder.1 Over the past few decades, use of SA has increased dramatically, from about 5000 cases in 1990 to 7000 in 2000 and more than 26,000 in 2008.1,2
Complications associated with SA generally are classified as perioperative (occurring during the operative index) or long-term (postdischarge).3 Long-term complications include implant loosening, instability, revision, infection, rotator cuff tear, neural injury, and deltoid detachment.1,4,5 Perioperative complications, which are less commonly reported, include intraoperative fracture, infection, neural injury, venous thromboembolic events (VTEs, including pulmonary embolism [PE] and deep vein thrombosis [DVT]), transfusion, and death.3,6-10
SA is an attractive treatment option for patients with rheumatoid arthritis (RA), as the effects of pain on these patients are greater in the shoulder joint than in any other joint.11 Patients with RA pose unique orthopedic surgical challenges, including any combination of decreased bone mineralization, poor capsular tissue integrity, and osteonecrosis.3,12 In addition, RA patients may be taking immunosuppressive medications that have severe side effects, and they may require multiple surgeries.12,13 These factors predispose patients with RA to complications that include infection and wound dehiscence.3,5,12-14
The complex nature of RA has prompted investigators to examine outcome measures in this patient group. Hambright and colleagues3 used the Nationwide Inpatient Sample (NIS) to examine perioperative outcomes in RA patients who underwent TSA between 1988 and 2005.3 They found that TSA patients with RA had shorter and less costly hospital stays and were more likely to have a routine discharge.3 Using the same patient population drawn from the period 2006–2011, we conducted a study to determine if this unexpected trend persists as the number of TSAs and quality of postoperative care continue to increase. Given the potential for anemia of chronic disease and the systemic inflammatory nature of RA, we hypothesized that the perioperative complication profile of RA patients would be worse than that of non-RA patients.
Materials and Methods
NIS data were acquired for the period 2006–2011. The NIS is the largest publicly available all-payer inpatient database, with a random 20% sample of about 1000 US hospitals accounting for 7 to 8 million inpatient stays. The database supplies weights used to estimate national totals, at about 35 million inpatient visits per year. NIS inpatient data are limited to the operative index. Postdischarge information is not available. The NIS is managed by the Healthcare Cost and Utilization Project, which is sponsored by the Agency for Healthcare Research and Quality. The quality of NIS data is assessed and validated by an independent contractor. NIS data have been widely used to examine perioperative outcomes.15-17
NIS data cover patient and hospital demographics, hospital length of stay (LOS), discharge status, payer information, charges, and perioperative outcomes and procedure/diagnosis codes (ICD-9; International Classification of Diseases, Ninth Revision18).
As our Institutional Review Board (IRB) reviewed the database and determined the project was not human subject research, IRB involvement was not required. This study paralleled successful efforts with similar RA and non-RA patients who had shoulder and elbow surgery.3,19 SA patients were identified by ICD-9 procedure code 81.80, but this code does not specify whether the prosthesis was unconstrained, semiconstrained, or constrained. ICD-9 coding also does not specify whether the TSA was traditional or reverse. Patients with RA were identified by ICD-9 diagnosis codes 714.0, 714.1, and 714.2. Patients without one of these codes were placed in the non-RA cohort. Patients with codes associated with pathologic fractures secondary to metastatic cancer or bone malignant neoplasm as a secondary or primary diagnosis and patients who had revision surgery indicated by code 81.83 were excluded, as they have a disproportionately higher comorbidity burden.
After each cohort was defined, demographic data (age, sex, race, income quartile based on ZIP postal code) were compared, as were data on primary payer, hospital demographics, LOS (≤5 days, defined as perioperative index), discharge type, inflation-adjusted charges in 2014 dollars based on the Consumer Price Indexes (http://www.bls.gov/cpi/), and mortality. Perioperative complications—respiratory, gastrointestinal, genitourinary, accidental puncture/laceration, central nervous system, wound dehiscence, device-related (including embolism, fibrosis, hemorrhage, pain, stenosis, or thrombus caused by any device, implant, or graft), cardiac, hematoma/seroma, acute respiratory distress syndrome, postoperative shock, VTE, postoperative infection complications, and intraoperative transfusions—were considered using ICD-9 codes (996.X-999.X and 99.X, respectively).20 Although commonly used to determine perioperative comorbidity burden using ICD-9 coding, the modified Charlson index was not considered because RA is a component of the index and would therefore bias the variable.3,21
Statistical analyses, including χ2 tests and 2-sample t tests, were performed for categorical and continuous variables, respectively. P < .05 was considered significant. Fisher exact test was used for cohorts with fewer than 5 occurrences. Multivariate logistic regression models were then calculated to determine the effect of RA on different outcomes and complications, with age, race, sex, hospital region, hospital type, number of hospital beds, primary payer, and hospital ownership as covariates. Statistical analyses were performed using the R statistical programming language.22
Results
Of the 34,970 patients who underwent SA between 2006 and 2011, 1674 (4.8%) had a diagnosis of RA and 33,296 (95.2%) did not. On average, patients with RA tended to be younger than patients without RA (66.4 vs 69.1 years; P < .001), and a larger percentage of RA patients were female (75.5% vs 54.4%; P < .001). Compared with non-RA patients, RA patients comprised a different ethnic group and had a different expected primary payer (P < .001). SA patients with and without RA did not differ in income quartile based on ZIP code, total number of hospital beds, hospital region, or hospital teaching status (P = .34, .78, .59, and .82, respectively) (Table 1).
LOS was significantly (P < .001) statistically longer for RA patients (2.196 days) than for non-RA patients (2.085 days). RA patients were significantly less likely to be discharged home (63.0% vs 67.6%; P < .001). (Routine discharge was defined as discharge home, whereas nonroutine discharge was defined as discharge to a short-term hospital, skilled nursing facility, intermediate care, another type of facility, home health care, against medical advice, or death.) In addition, inflation-adjusted charges associated with SA were significantly higher (P = .018) for RA patients ($54,284) than for non-RA patients ($52,663) (Table 1).
Regarding the rates of complications that occurred during the perioperative index, there were no significant differences between RA and non-RA cohorts. These complications included respiratory, gastrointestinal, genitourinary, accidental puncture/laceration, central nervous system, wound dehiscence, device-related, cardiac, hematoma/seroma, acute respiratory distress syndrome, postoperative shock, VTE, and postoperative infection (Table 2). In addition, there was no significant difference in mortality between the groups (P = .48).
In TSA, blood transfusions were more likely (P < .001) to be given to RA patients (9.00%) than to non-RA patients (6.16%). Multivariate regression analyses were performed with age, race, sex, hospital region, hospital type, number of hospital beds, primary payer, and hospital ownership as covariates. These analyses revealed that transfusion (P < .001), discharge type (P = .002), total inflation-adjusted charges (P < .001), and LOS (P = .047) remained significant (Table 3).
Discussion
Large national databases like NIS allow study of uncommon medical occurrences and help delineate risks and trends that otherwise might be indeterminable. Although it has been suggested that patients with RA may have poorer long-term outcomes after SA, the perioperative risk profile indicates that TSA is well tolerated in RA patients during the operative index.3,23-25
The data on this study’s 34,970 patients, drawn from the period 2006–2011, demonstrated no significant differences in safety profile with respect to the 14 perioperative complications and outcomes examined, except blood transfusion rate. Rates of postoperative infection (RA, 0.24%; non-RA, 0.14%; P = .303), VTE (RA, 0.30%; non-RA, 0.25%; P = .905), and transfusion (RA, 9.00%; non-RA, 6.16%; P < .001) are of particular interest because of the severity of these situations.
Postoperative infection is a potentially serious complication and often occurs secondary to diabetes, RA, lupus erythematosus, prior surgery, or a nosocomial or remote source.1 The often costly treatment options include antibiotic suppression, irrigation and debridement with implant retention, 1-stage exchange with antibiotic-impregnated cement fixation, staged reimplantation, resection arthroplasty, arthrodesis, and amputation.1 The overall 0.14% infection rate determined in this study is lower than the 0.7% reported for SA patients in the literature.1 Given the nature of the NIS database, this rate underestimates the true postoperative infection rate, as any infection that occurred after the perioperative period is not captured.26 The present study’s perioperative infection rates (RA, 0.24%; non-RA, 0.14%) for the period 2006–2011 are comparable to the rates (RA, 0.17%; non-RA, 0.24%) reported by Hambright and colleagues3 for the same patient population over the preceding, 18-year period (1988–2005) and similarly do not significantly differ between groups. Although infection is uncommon in the immediate perioperative period, the ICD-9 codes used refer specifically to infection resulting from surgery and do not represent concomitant infection.
VTEs, which include PEs and DVTs, are rare but potentially life-threatening surgical complications.27,28 Mechanical prophylaxis and chemical prophylaxis have been recommended for major orthopedic surgery, particularly lower extremity surgery, such as total hip arthroplasty (THA) and total knee arthroplasty (TKA).28,29 In the present study, VTE rates were low, 0.30% (RA) and 0.25% (non-RA), and not significantly different in bivariate or multivariate analyses. These rates are comparable to those found in other national-database SA studies.28 VTEs that occur outside the index hospital admission are not captured in this database. Therefore, the rates in the present study may be lower than the true incidence after SA. Mortality secondary to VTE usually occurs within 24 hours but may occur up to 90 days after surgery. DVT rates, on the other hand, are difficult to evaluate because of differences in screening practices.27,28,30,31
That RA patients were more likely than non-RA patients to receive perioperative blood transfusions supports prior findings that SA patients with RA were more likely than SA patients with osteoarthritis (OA) to receive perioperative blood transfusions.8 RA patients have been shown to have high rates of anemia of chronic disease, ranging from 22% to 77%.32 During joint replacement, these patients often require transfusions.32,33 However, these findings differ from prior findings of no differences between RA and non-RA patients in the same patient population during the period 1988–2005.3 This difference may be a product of the constantly changing transfusion guidelines and increased use; transfusion rates increased 140% between 1997 and 2007, making transfusions the fastest growing common procedure in the United States during that time.34 There was no difference between RA and non-RA patients in household income (as determined by ZIP code analysis), number of hospital beds, hospital region, or hospital teaching status. Compared with non-RA patients, RA patients were more likely to be younger, female, and of a difference race and to have a different expected primary payer (P < .001).These findings are consistent with previous findings in the literature.3 In the present SA study, however, RA patients were more likely than non-RA patients to have longer LOS, higher inflation-adjusted hospital charges, and nonroutine discharge. These findings deviate from those of the study covering the preceding 18 years (1988–2005).3 Despite the findings of a changing environment of care for RA patients, by Hambright and colleagues3 and Weiss and colleagues,35 the trend appears to have shifted. Both groups had shorter average LOS than either group from the preceding 18 years.3 Although statistically significant in bivariate analysis, the difference in LOS between the 2 groups differed by an average of 0.11 day (2 hours 24 minutes) and was not clinically relevant.
In addition, the higher charges for patients with RA represent a deviation from the preceding 18 years.3 Other studies have also shown that RA is associated with increased cost in TSA.36 Patients with RA often have rotator cuff pathology, indicating reverse SA may be used more frequently.37,38 The increased implant cost associated with reverse SA may account for the increased costs in RA patients.39 As mentioned, TSA type is not captured in the NIS database. In addition, that RA patients were less likely than non-RA patients to have routine discharge may indicate RA cases are more complex because of their complications.1,5,14,40 A recent study of complications in RA patients (1163 who underwent THA, 2692 who underwent TKA) found that THA patients with RA were significantly more likely than THA patients with OA to dislocate, and TKA patients with RA were significantly more likely than TKA patients with OA to develop an infection after surgery.41 Postoperative dislocation has been shown to increase hospital costs in other orthopedic procedures.42 Also, during TSA, patients with RA are more likely than patients with OA to receive intraoperative blood transfusions.8 These complications—combined with the fact that RA is a chronic, progressive, systemic inflammatory disease that can affect soft tissue and blood vessel wall healing and is associated with medications having potential side effects—could contribute to the apparent increased hospital charges and LOS.3,12,13,43 Factors that include surgeon preference, impact of primary payer, and hospital practice may also affect final charges. Total charges in the NIS database include administrative fees, hospital costs, device-related costs, operating room costs, and ancillary staff costs. Total charges do not include professional fees and differ from the total cost that represents the amount reimbursed by the payer. Charges tend to correlate with but overestimate the total costs.44
This study had several important limitations. As mentioned, only events that occur during the operative admission are captured in the NIS database, and thus postoperative complications or serious adverse events that lead to readmission cannot be identified. In addition, outpatient TSAs are not captured in the NIS database, and thus inclusion of only inpatient procedures yields higher average LOS and total charges.45 Given the limited granularity of ICD-9 coding, this study could not determine RA severity, estimated blood loss, length of surgery, complication severity, type of TSA procedure/prosthesis, or cause of death. Although commonly used to determine comorbidity burden, the modified Charlson index could not be used, and therefore could not be entered as a covariate in multivariate analysis. Furthermore, the NIS database does not include imaging or patient-reported outcomes information, such as improvements in pain or function, which are of crucial importance in considering surgery.
Conclusion
Our findings corroborated findings that the demographics and the perioperative safety profile for TSA were similar for patients with and without RA. The risk for complications or death in the perioperative period was low. Compared with non-RA patients, RA patients had significantly higher charges and longer LOS and were less likely to be discharged home after surgery. The 0.11-day difference in LOS, though statistically significant, was not clinically relevant. These findings differ from those for the preceding, 18-year period (1988–2005). Future research should focus on the causes of these changes.
1. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
3. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
4. van de Sande MA, Brand R, Rozing PM. Indications, complications, and results of shoulder arthroplasty. Scand J Rheumatol. 2006;35(6):426-434.
5. Wirth MA, Rockwood CA Jr. Complications of shoulder arthroplasty. Clin Orthop Relat Res. 1994;(307):47-69.
6. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):
1915-1923.
7. Sperling JW, Kozak TK, Hanssen AD, Cofield RH. Infection after shoulder arthroplasty. Clin Orthop Relat Res. 2001;(382):206-216.
8. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
9. Kumar S, Sperling JW, Haidukewych GH, Cofield RH. Periprosthetic humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am. 2004;86(4):680-689.
10. Sperling JW, Cofield RH. Pulmonary embolism following shoulder arthroplasty. J Bone Joint Surg Am. 2002;84(11):1939-1941.
11. Tanaka E, Saito A, Kamitsuji S, et al. Impact of shoulder, elbow, and knee joint involvement on assessment of rheumatoid arthritis using the American College of Rheumatology core data set. Arthritis Rheum. 2005;53(6):864-871.
12. Nassar J, Cracchiolo A 3rd. Complications in surgery of the foot and ankle in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2001;(391):140-152.
13. den Broeder AA, Creemers MC, Fransen J, et al. Risk factors for surgical site infections and other complications in elective surgery in patients with rheumatoid arthritis with special attention for anti-tumor necrosis factor: a large retrospective study. J Rheumatol. 2007;34(4):689-695.
14. Sanchez-Sotelo J. (i) Shoulder arthroplasty for osteoarthritis and rheumatoid arthritis. Curr Orthop. 2007;21(6):405-414.
15. Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project (HCUP). Overview of the National (Nationwide) Inpatient Sample (NIS). 2012. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed February 3, 2015.
16. Hervey SL, Purves HR, Guller U, Toth AP, Vail TP, Pietrobon R. Provider volume of total knee arthroplasties and patient outcomes in the HCUP-Nationwide Inpatient Sample. J Bone Joint Surg Am. 2003;85(9):1775-1783.
17. Noskin GA, Rubin RJ, Schentag JJ, et al. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample database. Arch Intern Med. 2005;165(15):1756-1761.
18. World Health Organization. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Geneva, Switzerland: World Health Organization; 2008.
19. Cook C, Hawkins R, Aldridge JM 3rd, Tolan S, Krupp R, Bolognesi M. Comparison of perioperative complications in patients with and without rheumatoid arthritis who receive total elbow replacement. J Shoulder Elbow Surg. 2009;18(1):21-26.
20. Goz V, Weinreb JH, McCarthy I, Schwab F, Lafage V, Errico TJ. Perioperative complications and mortality after spinal fusions: analysis of trends and risk factors. Spine. 2013;38(22):1970-1976.
21. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
22. R: a language and environment for statistical computing [computer program]. Vienna, Austria: Foundation for Statistical Computing; 2012.
23. Cuomo F, Greller MJ, Zuckerman JD. The rheumatoid shoulder. Rheum Dis Clin North Am. 1998;24(1):67-82.
24. Kelly IG, Foster RS, Fisher WD. Neer total shoulder replacement in rheumatoid arthritis. J Bone Joint Surg Br. 1987;69(5):723-726.
25. Donigan JA, Frisella WA, Haase D, Dolan L, Wolf B. Pre-operative and intra-operative factors related to shoulder arthroplasty outcomes. Iowa Orthop J. 2009;29:60-66.
26. Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479.
27. Willis AA, Warren RF, Craig EV, et al. Deep vein thrombosis after reconstructive shoulder arthroplasty: a prospective observational study. J Shoulder Elbow Surg. 2009;18(1):100-106.
28. Jameson SS, James P, Howcroft DW, et al. Venous thromboembolic events are rare after shoulder surgery: analysis of a national database. J Shoulder Elbow Surg. 2011;20(5):
764-770.
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest J. 2012;141(2 suppl):e278S-e325S.
30. White CB, Sperling JW, Cofield RH, Rowland CM. Ninety-day mortality after shoulder arthroplasty. J Arthroplasty. 2003;18(7):886-888.
31. Lussana F, Squizzato A, Permunian ET, Cattaneo M. A systematic review on the effect of aspirin in the prevention of post-operative arterial thrombosis in patients undergoing total hip and total knee arthroplasty. Thromb Res. 2014;134(3):599-603.
32. Wilson A, Yu H, Goodnough LT, Nissenson AR. Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature. Am J Med. 2004;116(7):50-57.
33. Mercuriali F, Gualtieri G, Sinigaglia L, et al. Use of recombinant human erythropoietin to assist autologous blood donation by anemic rheumatoid arthritis patients undergoing major orthopedic surgery. Transfusion. 1994;34(6):501-506.
34. Shander A, Gross I, Hill S, et al. A new perspective on best transfusion practices. Blood Transfus. 2013;11(2):193-202.
35. Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatology. 2008;47(4):491-494.
36. Davis DE, Paxton ES, Maltenfort M, Abboud J. Factors affecting hospital charges after total shoulder arthroplasty: an evaluation of the national inpatient sample database.
J Shoulder Elbow Surg. 2014;23(12):1860-1866.
37. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
38. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
39. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288.
40. Garner RW, Mowat AG, Hazleman BL. Wound healing after operations of patients with rheumatoid arthritis. J Bone Joint Surg Br. 1973;55(1):134-144.
41. Ravi B, Croxford R, Hollands S, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263.
42. Sanchez-Sotelo J, Haidukewych GJ, Boberg CJ. Hospital cost of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88(2):290-294.
43. Ward MM. Decreases in rates of hospitalizations for manifestations of severe rheumatoid arthritis, 1983-2001. Arthritis Rheum. 2004;50(4):1122-1131.
44. Goz V, Weinreb JH, Schwab F, Lafage V, Errico TJ. Comparison of complications, costs, and length of stay of three different lumbar interbody fusion techniques: an analysis of the Nationwide Inpatient Sample database. Spine J. 2014;14(9):2019-2027.
45. Goz V, Errico TJ, Weinreb JH, et al. Vertebroplasty and kyphoplasty: national outcomes and trends in utilization from 2005 through 2010. Spine J. 2015;15(5):959-965.
Shoulder arthroplasty (SA), including total SA (TSA) and reverse TSA, is an effective surgical treatment for fracture and primary or secondary degenerative disease of the shoulder.1 Over the past few decades, use of SA has increased dramatically, from about 5000 cases in 1990 to 7000 in 2000 and more than 26,000 in 2008.1,2
Complications associated with SA generally are classified as perioperative (occurring during the operative index) or long-term (postdischarge).3 Long-term complications include implant loosening, instability, revision, infection, rotator cuff tear, neural injury, and deltoid detachment.1,4,5 Perioperative complications, which are less commonly reported, include intraoperative fracture, infection, neural injury, venous thromboembolic events (VTEs, including pulmonary embolism [PE] and deep vein thrombosis [DVT]), transfusion, and death.3,6-10
SA is an attractive treatment option for patients with rheumatoid arthritis (RA), as the effects of pain on these patients are greater in the shoulder joint than in any other joint.11 Patients with RA pose unique orthopedic surgical challenges, including any combination of decreased bone mineralization, poor capsular tissue integrity, and osteonecrosis.3,12 In addition, RA patients may be taking immunosuppressive medications that have severe side effects, and they may require multiple surgeries.12,13 These factors predispose patients with RA to complications that include infection and wound dehiscence.3,5,12-14
The complex nature of RA has prompted investigators to examine outcome measures in this patient group. Hambright and colleagues3 used the Nationwide Inpatient Sample (NIS) to examine perioperative outcomes in RA patients who underwent TSA between 1988 and 2005.3 They found that TSA patients with RA had shorter and less costly hospital stays and were more likely to have a routine discharge.3 Using the same patient population drawn from the period 2006–2011, we conducted a study to determine if this unexpected trend persists as the number of TSAs and quality of postoperative care continue to increase. Given the potential for anemia of chronic disease and the systemic inflammatory nature of RA, we hypothesized that the perioperative complication profile of RA patients would be worse than that of non-RA patients.
Materials and Methods
NIS data were acquired for the period 2006–2011. The NIS is the largest publicly available all-payer inpatient database, with a random 20% sample of about 1000 US hospitals accounting for 7 to 8 million inpatient stays. The database supplies weights used to estimate national totals, at about 35 million inpatient visits per year. NIS inpatient data are limited to the operative index. Postdischarge information is not available. The NIS is managed by the Healthcare Cost and Utilization Project, which is sponsored by the Agency for Healthcare Research and Quality. The quality of NIS data is assessed and validated by an independent contractor. NIS data have been widely used to examine perioperative outcomes.15-17
NIS data cover patient and hospital demographics, hospital length of stay (LOS), discharge status, payer information, charges, and perioperative outcomes and procedure/diagnosis codes (ICD-9; International Classification of Diseases, Ninth Revision18).
As our Institutional Review Board (IRB) reviewed the database and determined the project was not human subject research, IRB involvement was not required. This study paralleled successful efforts with similar RA and non-RA patients who had shoulder and elbow surgery.3,19 SA patients were identified by ICD-9 procedure code 81.80, but this code does not specify whether the prosthesis was unconstrained, semiconstrained, or constrained. ICD-9 coding also does not specify whether the TSA was traditional or reverse. Patients with RA were identified by ICD-9 diagnosis codes 714.0, 714.1, and 714.2. Patients without one of these codes were placed in the non-RA cohort. Patients with codes associated with pathologic fractures secondary to metastatic cancer or bone malignant neoplasm as a secondary or primary diagnosis and patients who had revision surgery indicated by code 81.83 were excluded, as they have a disproportionately higher comorbidity burden.
After each cohort was defined, demographic data (age, sex, race, income quartile based on ZIP postal code) were compared, as were data on primary payer, hospital demographics, LOS (≤5 days, defined as perioperative index), discharge type, inflation-adjusted charges in 2014 dollars based on the Consumer Price Indexes (http://www.bls.gov/cpi/), and mortality. Perioperative complications—respiratory, gastrointestinal, genitourinary, accidental puncture/laceration, central nervous system, wound dehiscence, device-related (including embolism, fibrosis, hemorrhage, pain, stenosis, or thrombus caused by any device, implant, or graft), cardiac, hematoma/seroma, acute respiratory distress syndrome, postoperative shock, VTE, postoperative infection complications, and intraoperative transfusions—were considered using ICD-9 codes (996.X-999.X and 99.X, respectively).20 Although commonly used to determine perioperative comorbidity burden using ICD-9 coding, the modified Charlson index was not considered because RA is a component of the index and would therefore bias the variable.3,21
Statistical analyses, including χ2 tests and 2-sample t tests, were performed for categorical and continuous variables, respectively. P < .05 was considered significant. Fisher exact test was used for cohorts with fewer than 5 occurrences. Multivariate logistic regression models were then calculated to determine the effect of RA on different outcomes and complications, with age, race, sex, hospital region, hospital type, number of hospital beds, primary payer, and hospital ownership as covariates. Statistical analyses were performed using the R statistical programming language.22
Results
Of the 34,970 patients who underwent SA between 2006 and 2011, 1674 (4.8%) had a diagnosis of RA and 33,296 (95.2%) did not. On average, patients with RA tended to be younger than patients without RA (66.4 vs 69.1 years; P < .001), and a larger percentage of RA patients were female (75.5% vs 54.4%; P < .001). Compared with non-RA patients, RA patients comprised a different ethnic group and had a different expected primary payer (P < .001). SA patients with and without RA did not differ in income quartile based on ZIP code, total number of hospital beds, hospital region, or hospital teaching status (P = .34, .78, .59, and .82, respectively) (Table 1).
LOS was significantly (P < .001) statistically longer for RA patients (2.196 days) than for non-RA patients (2.085 days). RA patients were significantly less likely to be discharged home (63.0% vs 67.6%; P < .001). (Routine discharge was defined as discharge home, whereas nonroutine discharge was defined as discharge to a short-term hospital, skilled nursing facility, intermediate care, another type of facility, home health care, against medical advice, or death.) In addition, inflation-adjusted charges associated with SA were significantly higher (P = .018) for RA patients ($54,284) than for non-RA patients ($52,663) (Table 1).
Regarding the rates of complications that occurred during the perioperative index, there were no significant differences between RA and non-RA cohorts. These complications included respiratory, gastrointestinal, genitourinary, accidental puncture/laceration, central nervous system, wound dehiscence, device-related, cardiac, hematoma/seroma, acute respiratory distress syndrome, postoperative shock, VTE, and postoperative infection (Table 2). In addition, there was no significant difference in mortality between the groups (P = .48).
In TSA, blood transfusions were more likely (P < .001) to be given to RA patients (9.00%) than to non-RA patients (6.16%). Multivariate regression analyses were performed with age, race, sex, hospital region, hospital type, number of hospital beds, primary payer, and hospital ownership as covariates. These analyses revealed that transfusion (P < .001), discharge type (P = .002), total inflation-adjusted charges (P < .001), and LOS (P = .047) remained significant (Table 3).
Discussion
Large national databases like NIS allow study of uncommon medical occurrences and help delineate risks and trends that otherwise might be indeterminable. Although it has been suggested that patients with RA may have poorer long-term outcomes after SA, the perioperative risk profile indicates that TSA is well tolerated in RA patients during the operative index.3,23-25
The data on this study’s 34,970 patients, drawn from the period 2006–2011, demonstrated no significant differences in safety profile with respect to the 14 perioperative complications and outcomes examined, except blood transfusion rate. Rates of postoperative infection (RA, 0.24%; non-RA, 0.14%; P = .303), VTE (RA, 0.30%; non-RA, 0.25%; P = .905), and transfusion (RA, 9.00%; non-RA, 6.16%; P < .001) are of particular interest because of the severity of these situations.
Postoperative infection is a potentially serious complication and often occurs secondary to diabetes, RA, lupus erythematosus, prior surgery, or a nosocomial or remote source.1 The often costly treatment options include antibiotic suppression, irrigation and debridement with implant retention, 1-stage exchange with antibiotic-impregnated cement fixation, staged reimplantation, resection arthroplasty, arthrodesis, and amputation.1 The overall 0.14% infection rate determined in this study is lower than the 0.7% reported for SA patients in the literature.1 Given the nature of the NIS database, this rate underestimates the true postoperative infection rate, as any infection that occurred after the perioperative period is not captured.26 The present study’s perioperative infection rates (RA, 0.24%; non-RA, 0.14%) for the period 2006–2011 are comparable to the rates (RA, 0.17%; non-RA, 0.24%) reported by Hambright and colleagues3 for the same patient population over the preceding, 18-year period (1988–2005) and similarly do not significantly differ between groups. Although infection is uncommon in the immediate perioperative period, the ICD-9 codes used refer specifically to infection resulting from surgery and do not represent concomitant infection.
VTEs, which include PEs and DVTs, are rare but potentially life-threatening surgical complications.27,28 Mechanical prophylaxis and chemical prophylaxis have been recommended for major orthopedic surgery, particularly lower extremity surgery, such as total hip arthroplasty (THA) and total knee arthroplasty (TKA).28,29 In the present study, VTE rates were low, 0.30% (RA) and 0.25% (non-RA), and not significantly different in bivariate or multivariate analyses. These rates are comparable to those found in other national-database SA studies.28 VTEs that occur outside the index hospital admission are not captured in this database. Therefore, the rates in the present study may be lower than the true incidence after SA. Mortality secondary to VTE usually occurs within 24 hours but may occur up to 90 days after surgery. DVT rates, on the other hand, are difficult to evaluate because of differences in screening practices.27,28,30,31
That RA patients were more likely than non-RA patients to receive perioperative blood transfusions supports prior findings that SA patients with RA were more likely than SA patients with osteoarthritis (OA) to receive perioperative blood transfusions.8 RA patients have been shown to have high rates of anemia of chronic disease, ranging from 22% to 77%.32 During joint replacement, these patients often require transfusions.32,33 However, these findings differ from prior findings of no differences between RA and non-RA patients in the same patient population during the period 1988–2005.3 This difference may be a product of the constantly changing transfusion guidelines and increased use; transfusion rates increased 140% between 1997 and 2007, making transfusions the fastest growing common procedure in the United States during that time.34 There was no difference between RA and non-RA patients in household income (as determined by ZIP code analysis), number of hospital beds, hospital region, or hospital teaching status. Compared with non-RA patients, RA patients were more likely to be younger, female, and of a difference race and to have a different expected primary payer (P < .001).These findings are consistent with previous findings in the literature.3 In the present SA study, however, RA patients were more likely than non-RA patients to have longer LOS, higher inflation-adjusted hospital charges, and nonroutine discharge. These findings deviate from those of the study covering the preceding 18 years (1988–2005).3 Despite the findings of a changing environment of care for RA patients, by Hambright and colleagues3 and Weiss and colleagues,35 the trend appears to have shifted. Both groups had shorter average LOS than either group from the preceding 18 years.3 Although statistically significant in bivariate analysis, the difference in LOS between the 2 groups differed by an average of 0.11 day (2 hours 24 minutes) and was not clinically relevant.
In addition, the higher charges for patients with RA represent a deviation from the preceding 18 years.3 Other studies have also shown that RA is associated with increased cost in TSA.36 Patients with RA often have rotator cuff pathology, indicating reverse SA may be used more frequently.37,38 The increased implant cost associated with reverse SA may account for the increased costs in RA patients.39 As mentioned, TSA type is not captured in the NIS database. In addition, that RA patients were less likely than non-RA patients to have routine discharge may indicate RA cases are more complex because of their complications.1,5,14,40 A recent study of complications in RA patients (1163 who underwent THA, 2692 who underwent TKA) found that THA patients with RA were significantly more likely than THA patients with OA to dislocate, and TKA patients with RA were significantly more likely than TKA patients with OA to develop an infection after surgery.41 Postoperative dislocation has been shown to increase hospital costs in other orthopedic procedures.42 Also, during TSA, patients with RA are more likely than patients with OA to receive intraoperative blood transfusions.8 These complications—combined with the fact that RA is a chronic, progressive, systemic inflammatory disease that can affect soft tissue and blood vessel wall healing and is associated with medications having potential side effects—could contribute to the apparent increased hospital charges and LOS.3,12,13,43 Factors that include surgeon preference, impact of primary payer, and hospital practice may also affect final charges. Total charges in the NIS database include administrative fees, hospital costs, device-related costs, operating room costs, and ancillary staff costs. Total charges do not include professional fees and differ from the total cost that represents the amount reimbursed by the payer. Charges tend to correlate with but overestimate the total costs.44
This study had several important limitations. As mentioned, only events that occur during the operative admission are captured in the NIS database, and thus postoperative complications or serious adverse events that lead to readmission cannot be identified. In addition, outpatient TSAs are not captured in the NIS database, and thus inclusion of only inpatient procedures yields higher average LOS and total charges.45 Given the limited granularity of ICD-9 coding, this study could not determine RA severity, estimated blood loss, length of surgery, complication severity, type of TSA procedure/prosthesis, or cause of death. Although commonly used to determine comorbidity burden, the modified Charlson index could not be used, and therefore could not be entered as a covariate in multivariate analysis. Furthermore, the NIS database does not include imaging or patient-reported outcomes information, such as improvements in pain or function, which are of crucial importance in considering surgery.
Conclusion
Our findings corroborated findings that the demographics and the perioperative safety profile for TSA were similar for patients with and without RA. The risk for complications or death in the perioperative period was low. Compared with non-RA patients, RA patients had significantly higher charges and longer LOS and were less likely to be discharged home after surgery. The 0.11-day difference in LOS, though statistically significant, was not clinically relevant. These findings differ from those for the preceding, 18-year period (1988–2005). Future research should focus on the causes of these changes.
Shoulder arthroplasty (SA), including total SA (TSA) and reverse TSA, is an effective surgical treatment for fracture and primary or secondary degenerative disease of the shoulder.1 Over the past few decades, use of SA has increased dramatically, from about 5000 cases in 1990 to 7000 in 2000 and more than 26,000 in 2008.1,2
Complications associated with SA generally are classified as perioperative (occurring during the operative index) or long-term (postdischarge).3 Long-term complications include implant loosening, instability, revision, infection, rotator cuff tear, neural injury, and deltoid detachment.1,4,5 Perioperative complications, which are less commonly reported, include intraoperative fracture, infection, neural injury, venous thromboembolic events (VTEs, including pulmonary embolism [PE] and deep vein thrombosis [DVT]), transfusion, and death.3,6-10
SA is an attractive treatment option for patients with rheumatoid arthritis (RA), as the effects of pain on these patients are greater in the shoulder joint than in any other joint.11 Patients with RA pose unique orthopedic surgical challenges, including any combination of decreased bone mineralization, poor capsular tissue integrity, and osteonecrosis.3,12 In addition, RA patients may be taking immunosuppressive medications that have severe side effects, and they may require multiple surgeries.12,13 These factors predispose patients with RA to complications that include infection and wound dehiscence.3,5,12-14
The complex nature of RA has prompted investigators to examine outcome measures in this patient group. Hambright and colleagues3 used the Nationwide Inpatient Sample (NIS) to examine perioperative outcomes in RA patients who underwent TSA between 1988 and 2005.3 They found that TSA patients with RA had shorter and less costly hospital stays and were more likely to have a routine discharge.3 Using the same patient population drawn from the period 2006–2011, we conducted a study to determine if this unexpected trend persists as the number of TSAs and quality of postoperative care continue to increase. Given the potential for anemia of chronic disease and the systemic inflammatory nature of RA, we hypothesized that the perioperative complication profile of RA patients would be worse than that of non-RA patients.
Materials and Methods
NIS data were acquired for the period 2006–2011. The NIS is the largest publicly available all-payer inpatient database, with a random 20% sample of about 1000 US hospitals accounting for 7 to 8 million inpatient stays. The database supplies weights used to estimate national totals, at about 35 million inpatient visits per year. NIS inpatient data are limited to the operative index. Postdischarge information is not available. The NIS is managed by the Healthcare Cost and Utilization Project, which is sponsored by the Agency for Healthcare Research and Quality. The quality of NIS data is assessed and validated by an independent contractor. NIS data have been widely used to examine perioperative outcomes.15-17
NIS data cover patient and hospital demographics, hospital length of stay (LOS), discharge status, payer information, charges, and perioperative outcomes and procedure/diagnosis codes (ICD-9; International Classification of Diseases, Ninth Revision18).
As our Institutional Review Board (IRB) reviewed the database and determined the project was not human subject research, IRB involvement was not required. This study paralleled successful efforts with similar RA and non-RA patients who had shoulder and elbow surgery.3,19 SA patients were identified by ICD-9 procedure code 81.80, but this code does not specify whether the prosthesis was unconstrained, semiconstrained, or constrained. ICD-9 coding also does not specify whether the TSA was traditional or reverse. Patients with RA were identified by ICD-9 diagnosis codes 714.0, 714.1, and 714.2. Patients without one of these codes were placed in the non-RA cohort. Patients with codes associated with pathologic fractures secondary to metastatic cancer or bone malignant neoplasm as a secondary or primary diagnosis and patients who had revision surgery indicated by code 81.83 were excluded, as they have a disproportionately higher comorbidity burden.
After each cohort was defined, demographic data (age, sex, race, income quartile based on ZIP postal code) were compared, as were data on primary payer, hospital demographics, LOS (≤5 days, defined as perioperative index), discharge type, inflation-adjusted charges in 2014 dollars based on the Consumer Price Indexes (http://www.bls.gov/cpi/), and mortality. Perioperative complications—respiratory, gastrointestinal, genitourinary, accidental puncture/laceration, central nervous system, wound dehiscence, device-related (including embolism, fibrosis, hemorrhage, pain, stenosis, or thrombus caused by any device, implant, or graft), cardiac, hematoma/seroma, acute respiratory distress syndrome, postoperative shock, VTE, postoperative infection complications, and intraoperative transfusions—were considered using ICD-9 codes (996.X-999.X and 99.X, respectively).20 Although commonly used to determine perioperative comorbidity burden using ICD-9 coding, the modified Charlson index was not considered because RA is a component of the index and would therefore bias the variable.3,21
Statistical analyses, including χ2 tests and 2-sample t tests, were performed for categorical and continuous variables, respectively. P < .05 was considered significant. Fisher exact test was used for cohorts with fewer than 5 occurrences. Multivariate logistic regression models were then calculated to determine the effect of RA on different outcomes and complications, with age, race, sex, hospital region, hospital type, number of hospital beds, primary payer, and hospital ownership as covariates. Statistical analyses were performed using the R statistical programming language.22
Results
Of the 34,970 patients who underwent SA between 2006 and 2011, 1674 (4.8%) had a diagnosis of RA and 33,296 (95.2%) did not. On average, patients with RA tended to be younger than patients without RA (66.4 vs 69.1 years; P < .001), and a larger percentage of RA patients were female (75.5% vs 54.4%; P < .001). Compared with non-RA patients, RA patients comprised a different ethnic group and had a different expected primary payer (P < .001). SA patients with and without RA did not differ in income quartile based on ZIP code, total number of hospital beds, hospital region, or hospital teaching status (P = .34, .78, .59, and .82, respectively) (Table 1).
LOS was significantly (P < .001) statistically longer for RA patients (2.196 days) than for non-RA patients (2.085 days). RA patients were significantly less likely to be discharged home (63.0% vs 67.6%; P < .001). (Routine discharge was defined as discharge home, whereas nonroutine discharge was defined as discharge to a short-term hospital, skilled nursing facility, intermediate care, another type of facility, home health care, against medical advice, or death.) In addition, inflation-adjusted charges associated with SA were significantly higher (P = .018) for RA patients ($54,284) than for non-RA patients ($52,663) (Table 1).
Regarding the rates of complications that occurred during the perioperative index, there were no significant differences between RA and non-RA cohorts. These complications included respiratory, gastrointestinal, genitourinary, accidental puncture/laceration, central nervous system, wound dehiscence, device-related, cardiac, hematoma/seroma, acute respiratory distress syndrome, postoperative shock, VTE, and postoperative infection (Table 2). In addition, there was no significant difference in mortality between the groups (P = .48).
In TSA, blood transfusions were more likely (P < .001) to be given to RA patients (9.00%) than to non-RA patients (6.16%). Multivariate regression analyses were performed with age, race, sex, hospital region, hospital type, number of hospital beds, primary payer, and hospital ownership as covariates. These analyses revealed that transfusion (P < .001), discharge type (P = .002), total inflation-adjusted charges (P < .001), and LOS (P = .047) remained significant (Table 3).
Discussion
Large national databases like NIS allow study of uncommon medical occurrences and help delineate risks and trends that otherwise might be indeterminable. Although it has been suggested that patients with RA may have poorer long-term outcomes after SA, the perioperative risk profile indicates that TSA is well tolerated in RA patients during the operative index.3,23-25
The data on this study’s 34,970 patients, drawn from the period 2006–2011, demonstrated no significant differences in safety profile with respect to the 14 perioperative complications and outcomes examined, except blood transfusion rate. Rates of postoperative infection (RA, 0.24%; non-RA, 0.14%; P = .303), VTE (RA, 0.30%; non-RA, 0.25%; P = .905), and transfusion (RA, 9.00%; non-RA, 6.16%; P < .001) are of particular interest because of the severity of these situations.
Postoperative infection is a potentially serious complication and often occurs secondary to diabetes, RA, lupus erythematosus, prior surgery, or a nosocomial or remote source.1 The often costly treatment options include antibiotic suppression, irrigation and debridement with implant retention, 1-stage exchange with antibiotic-impregnated cement fixation, staged reimplantation, resection arthroplasty, arthrodesis, and amputation.1 The overall 0.14% infection rate determined in this study is lower than the 0.7% reported for SA patients in the literature.1 Given the nature of the NIS database, this rate underestimates the true postoperative infection rate, as any infection that occurred after the perioperative period is not captured.26 The present study’s perioperative infection rates (RA, 0.24%; non-RA, 0.14%) for the period 2006–2011 are comparable to the rates (RA, 0.17%; non-RA, 0.24%) reported by Hambright and colleagues3 for the same patient population over the preceding, 18-year period (1988–2005) and similarly do not significantly differ between groups. Although infection is uncommon in the immediate perioperative period, the ICD-9 codes used refer specifically to infection resulting from surgery and do not represent concomitant infection.
VTEs, which include PEs and DVTs, are rare but potentially life-threatening surgical complications.27,28 Mechanical prophylaxis and chemical prophylaxis have been recommended for major orthopedic surgery, particularly lower extremity surgery, such as total hip arthroplasty (THA) and total knee arthroplasty (TKA).28,29 In the present study, VTE rates were low, 0.30% (RA) and 0.25% (non-RA), and not significantly different in bivariate or multivariate analyses. These rates are comparable to those found in other national-database SA studies.28 VTEs that occur outside the index hospital admission are not captured in this database. Therefore, the rates in the present study may be lower than the true incidence after SA. Mortality secondary to VTE usually occurs within 24 hours but may occur up to 90 days after surgery. DVT rates, on the other hand, are difficult to evaluate because of differences in screening practices.27,28,30,31
That RA patients were more likely than non-RA patients to receive perioperative blood transfusions supports prior findings that SA patients with RA were more likely than SA patients with osteoarthritis (OA) to receive perioperative blood transfusions.8 RA patients have been shown to have high rates of anemia of chronic disease, ranging from 22% to 77%.32 During joint replacement, these patients often require transfusions.32,33 However, these findings differ from prior findings of no differences between RA and non-RA patients in the same patient population during the period 1988–2005.3 This difference may be a product of the constantly changing transfusion guidelines and increased use; transfusion rates increased 140% between 1997 and 2007, making transfusions the fastest growing common procedure in the United States during that time.34 There was no difference between RA and non-RA patients in household income (as determined by ZIP code analysis), number of hospital beds, hospital region, or hospital teaching status. Compared with non-RA patients, RA patients were more likely to be younger, female, and of a difference race and to have a different expected primary payer (P < .001).These findings are consistent with previous findings in the literature.3 In the present SA study, however, RA patients were more likely than non-RA patients to have longer LOS, higher inflation-adjusted hospital charges, and nonroutine discharge. These findings deviate from those of the study covering the preceding 18 years (1988–2005).3 Despite the findings of a changing environment of care for RA patients, by Hambright and colleagues3 and Weiss and colleagues,35 the trend appears to have shifted. Both groups had shorter average LOS than either group from the preceding 18 years.3 Although statistically significant in bivariate analysis, the difference in LOS between the 2 groups differed by an average of 0.11 day (2 hours 24 minutes) and was not clinically relevant.
In addition, the higher charges for patients with RA represent a deviation from the preceding 18 years.3 Other studies have also shown that RA is associated with increased cost in TSA.36 Patients with RA often have rotator cuff pathology, indicating reverse SA may be used more frequently.37,38 The increased implant cost associated with reverse SA may account for the increased costs in RA patients.39 As mentioned, TSA type is not captured in the NIS database. In addition, that RA patients were less likely than non-RA patients to have routine discharge may indicate RA cases are more complex because of their complications.1,5,14,40 A recent study of complications in RA patients (1163 who underwent THA, 2692 who underwent TKA) found that THA patients with RA were significantly more likely than THA patients with OA to dislocate, and TKA patients with RA were significantly more likely than TKA patients with OA to develop an infection after surgery.41 Postoperative dislocation has been shown to increase hospital costs in other orthopedic procedures.42 Also, during TSA, patients with RA are more likely than patients with OA to receive intraoperative blood transfusions.8 These complications—combined with the fact that RA is a chronic, progressive, systemic inflammatory disease that can affect soft tissue and blood vessel wall healing and is associated with medications having potential side effects—could contribute to the apparent increased hospital charges and LOS.3,12,13,43 Factors that include surgeon preference, impact of primary payer, and hospital practice may also affect final charges. Total charges in the NIS database include administrative fees, hospital costs, device-related costs, operating room costs, and ancillary staff costs. Total charges do not include professional fees and differ from the total cost that represents the amount reimbursed by the payer. Charges tend to correlate with but overestimate the total costs.44
This study had several important limitations. As mentioned, only events that occur during the operative admission are captured in the NIS database, and thus postoperative complications or serious adverse events that lead to readmission cannot be identified. In addition, outpatient TSAs are not captured in the NIS database, and thus inclusion of only inpatient procedures yields higher average LOS and total charges.45 Given the limited granularity of ICD-9 coding, this study could not determine RA severity, estimated blood loss, length of surgery, complication severity, type of TSA procedure/prosthesis, or cause of death. Although commonly used to determine comorbidity burden, the modified Charlson index could not be used, and therefore could not be entered as a covariate in multivariate analysis. Furthermore, the NIS database does not include imaging or patient-reported outcomes information, such as improvements in pain or function, which are of crucial importance in considering surgery.
Conclusion
Our findings corroborated findings that the demographics and the perioperative safety profile for TSA were similar for patients with and without RA. The risk for complications or death in the perioperative period was low. Compared with non-RA patients, RA patients had significantly higher charges and longer LOS and were less likely to be discharged home after surgery. The 0.11-day difference in LOS, though statistically significant, was not clinically relevant. These findings differ from those for the preceding, 18-year period (1988–2005). Future research should focus on the causes of these changes.
1. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
3. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
4. van de Sande MA, Brand R, Rozing PM. Indications, complications, and results of shoulder arthroplasty. Scand J Rheumatol. 2006;35(6):426-434.
5. Wirth MA, Rockwood CA Jr. Complications of shoulder arthroplasty. Clin Orthop Relat Res. 1994;(307):47-69.
6. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):
1915-1923.
7. Sperling JW, Kozak TK, Hanssen AD, Cofield RH. Infection after shoulder arthroplasty. Clin Orthop Relat Res. 2001;(382):206-216.
8. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
9. Kumar S, Sperling JW, Haidukewych GH, Cofield RH. Periprosthetic humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am. 2004;86(4):680-689.
10. Sperling JW, Cofield RH. Pulmonary embolism following shoulder arthroplasty. J Bone Joint Surg Am. 2002;84(11):1939-1941.
11. Tanaka E, Saito A, Kamitsuji S, et al. Impact of shoulder, elbow, and knee joint involvement on assessment of rheumatoid arthritis using the American College of Rheumatology core data set. Arthritis Rheum. 2005;53(6):864-871.
12. Nassar J, Cracchiolo A 3rd. Complications in surgery of the foot and ankle in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2001;(391):140-152.
13. den Broeder AA, Creemers MC, Fransen J, et al. Risk factors for surgical site infections and other complications in elective surgery in patients with rheumatoid arthritis with special attention for anti-tumor necrosis factor: a large retrospective study. J Rheumatol. 2007;34(4):689-695.
14. Sanchez-Sotelo J. (i) Shoulder arthroplasty for osteoarthritis and rheumatoid arthritis. Curr Orthop. 2007;21(6):405-414.
15. Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project (HCUP). Overview of the National (Nationwide) Inpatient Sample (NIS). 2012. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed February 3, 2015.
16. Hervey SL, Purves HR, Guller U, Toth AP, Vail TP, Pietrobon R. Provider volume of total knee arthroplasties and patient outcomes in the HCUP-Nationwide Inpatient Sample. J Bone Joint Surg Am. 2003;85(9):1775-1783.
17. Noskin GA, Rubin RJ, Schentag JJ, et al. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample database. Arch Intern Med. 2005;165(15):1756-1761.
18. World Health Organization. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Geneva, Switzerland: World Health Organization; 2008.
19. Cook C, Hawkins R, Aldridge JM 3rd, Tolan S, Krupp R, Bolognesi M. Comparison of perioperative complications in patients with and without rheumatoid arthritis who receive total elbow replacement. J Shoulder Elbow Surg. 2009;18(1):21-26.
20. Goz V, Weinreb JH, McCarthy I, Schwab F, Lafage V, Errico TJ. Perioperative complications and mortality after spinal fusions: analysis of trends and risk factors. Spine. 2013;38(22):1970-1976.
21. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
22. R: a language and environment for statistical computing [computer program]. Vienna, Austria: Foundation for Statistical Computing; 2012.
23. Cuomo F, Greller MJ, Zuckerman JD. The rheumatoid shoulder. Rheum Dis Clin North Am. 1998;24(1):67-82.
24. Kelly IG, Foster RS, Fisher WD. Neer total shoulder replacement in rheumatoid arthritis. J Bone Joint Surg Br. 1987;69(5):723-726.
25. Donigan JA, Frisella WA, Haase D, Dolan L, Wolf B. Pre-operative and intra-operative factors related to shoulder arthroplasty outcomes. Iowa Orthop J. 2009;29:60-66.
26. Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479.
27. Willis AA, Warren RF, Craig EV, et al. Deep vein thrombosis after reconstructive shoulder arthroplasty: a prospective observational study. J Shoulder Elbow Surg. 2009;18(1):100-106.
28. Jameson SS, James P, Howcroft DW, et al. Venous thromboembolic events are rare after shoulder surgery: analysis of a national database. J Shoulder Elbow Surg. 2011;20(5):
764-770.
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest J. 2012;141(2 suppl):e278S-e325S.
30. White CB, Sperling JW, Cofield RH, Rowland CM. Ninety-day mortality after shoulder arthroplasty. J Arthroplasty. 2003;18(7):886-888.
31. Lussana F, Squizzato A, Permunian ET, Cattaneo M. A systematic review on the effect of aspirin in the prevention of post-operative arterial thrombosis in patients undergoing total hip and total knee arthroplasty. Thromb Res. 2014;134(3):599-603.
32. Wilson A, Yu H, Goodnough LT, Nissenson AR. Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature. Am J Med. 2004;116(7):50-57.
33. Mercuriali F, Gualtieri G, Sinigaglia L, et al. Use of recombinant human erythropoietin to assist autologous blood donation by anemic rheumatoid arthritis patients undergoing major orthopedic surgery. Transfusion. 1994;34(6):501-506.
34. Shander A, Gross I, Hill S, et al. A new perspective on best transfusion practices. Blood Transfus. 2013;11(2):193-202.
35. Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatology. 2008;47(4):491-494.
36. Davis DE, Paxton ES, Maltenfort M, Abboud J. Factors affecting hospital charges after total shoulder arthroplasty: an evaluation of the national inpatient sample database.
J Shoulder Elbow Surg. 2014;23(12):1860-1866.
37. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
38. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
39. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288.
40. Garner RW, Mowat AG, Hazleman BL. Wound healing after operations of patients with rheumatoid arthritis. J Bone Joint Surg Br. 1973;55(1):134-144.
41. Ravi B, Croxford R, Hollands S, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263.
42. Sanchez-Sotelo J, Haidukewych GJ, Boberg CJ. Hospital cost of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88(2):290-294.
43. Ward MM. Decreases in rates of hospitalizations for manifestations of severe rheumatoid arthritis, 1983-2001. Arthritis Rheum. 2004;50(4):1122-1131.
44. Goz V, Weinreb JH, Schwab F, Lafage V, Errico TJ. Comparison of complications, costs, and length of stay of three different lumbar interbody fusion techniques: an analysis of the Nationwide Inpatient Sample database. Spine J. 2014;14(9):2019-2027.
45. Goz V, Errico TJ, Weinreb JH, et al. Vertebroplasty and kyphoplasty: national outcomes and trends in utilization from 2005 through 2010. Spine J. 2015;15(5):959-965.
1. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
3. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
4. van de Sande MA, Brand R, Rozing PM. Indications, complications, and results of shoulder arthroplasty. Scand J Rheumatol. 2006;35(6):426-434.
5. Wirth MA, Rockwood CA Jr. Complications of shoulder arthroplasty. Clin Orthop Relat Res. 1994;(307):47-69.
6. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):
1915-1923.
7. Sperling JW, Kozak TK, Hanssen AD, Cofield RH. Infection after shoulder arthroplasty. Clin Orthop Relat Res. 2001;(382):206-216.
8. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
9. Kumar S, Sperling JW, Haidukewych GH, Cofield RH. Periprosthetic humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am. 2004;86(4):680-689.
10. Sperling JW, Cofield RH. Pulmonary embolism following shoulder arthroplasty. J Bone Joint Surg Am. 2002;84(11):1939-1941.
11. Tanaka E, Saito A, Kamitsuji S, et al. Impact of shoulder, elbow, and knee joint involvement on assessment of rheumatoid arthritis using the American College of Rheumatology core data set. Arthritis Rheum. 2005;53(6):864-871.
12. Nassar J, Cracchiolo A 3rd. Complications in surgery of the foot and ankle in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2001;(391):140-152.
13. den Broeder AA, Creemers MC, Fransen J, et al. Risk factors for surgical site infections and other complications in elective surgery in patients with rheumatoid arthritis with special attention for anti-tumor necrosis factor: a large retrospective study. J Rheumatol. 2007;34(4):689-695.
14. Sanchez-Sotelo J. (i) Shoulder arthroplasty for osteoarthritis and rheumatoid arthritis. Curr Orthop. 2007;21(6):405-414.
15. Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project (HCUP). Overview of the National (Nationwide) Inpatient Sample (NIS). 2012. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed February 3, 2015.
16. Hervey SL, Purves HR, Guller U, Toth AP, Vail TP, Pietrobon R. Provider volume of total knee arthroplasties and patient outcomes in the HCUP-Nationwide Inpatient Sample. J Bone Joint Surg Am. 2003;85(9):1775-1783.
17. Noskin GA, Rubin RJ, Schentag JJ, et al. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample database. Arch Intern Med. 2005;165(15):1756-1761.
18. World Health Organization. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Geneva, Switzerland: World Health Organization; 2008.
19. Cook C, Hawkins R, Aldridge JM 3rd, Tolan S, Krupp R, Bolognesi M. Comparison of perioperative complications in patients with and without rheumatoid arthritis who receive total elbow replacement. J Shoulder Elbow Surg. 2009;18(1):21-26.
20. Goz V, Weinreb JH, McCarthy I, Schwab F, Lafage V, Errico TJ. Perioperative complications and mortality after spinal fusions: analysis of trends and risk factors. Spine. 2013;38(22):1970-1976.
21. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
22. R: a language and environment for statistical computing [computer program]. Vienna, Austria: Foundation for Statistical Computing; 2012.
23. Cuomo F, Greller MJ, Zuckerman JD. The rheumatoid shoulder. Rheum Dis Clin North Am. 1998;24(1):67-82.
24. Kelly IG, Foster RS, Fisher WD. Neer total shoulder replacement in rheumatoid arthritis. J Bone Joint Surg Br. 1987;69(5):723-726.
25. Donigan JA, Frisella WA, Haase D, Dolan L, Wolf B. Pre-operative and intra-operative factors related to shoulder arthroplasty outcomes. Iowa Orthop J. 2009;29:60-66.
26. Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479.
27. Willis AA, Warren RF, Craig EV, et al. Deep vein thrombosis after reconstructive shoulder arthroplasty: a prospective observational study. J Shoulder Elbow Surg. 2009;18(1):100-106.
28. Jameson SS, James P, Howcroft DW, et al. Venous thromboembolic events are rare after shoulder surgery: analysis of a national database. J Shoulder Elbow Surg. 2011;20(5):
764-770.
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest J. 2012;141(2 suppl):e278S-e325S.
30. White CB, Sperling JW, Cofield RH, Rowland CM. Ninety-day mortality after shoulder arthroplasty. J Arthroplasty. 2003;18(7):886-888.
31. Lussana F, Squizzato A, Permunian ET, Cattaneo M. A systematic review on the effect of aspirin in the prevention of post-operative arterial thrombosis in patients undergoing total hip and total knee arthroplasty. Thromb Res. 2014;134(3):599-603.
32. Wilson A, Yu H, Goodnough LT, Nissenson AR. Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature. Am J Med. 2004;116(7):50-57.
33. Mercuriali F, Gualtieri G, Sinigaglia L, et al. Use of recombinant human erythropoietin to assist autologous blood donation by anemic rheumatoid arthritis patients undergoing major orthopedic surgery. Transfusion. 1994;34(6):501-506.
34. Shander A, Gross I, Hill S, et al. A new perspective on best transfusion practices. Blood Transfus. 2013;11(2):193-202.
35. Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatology. 2008;47(4):491-494.
36. Davis DE, Paxton ES, Maltenfort M, Abboud J. Factors affecting hospital charges after total shoulder arthroplasty: an evaluation of the national inpatient sample database.
J Shoulder Elbow Surg. 2014;23(12):1860-1866.
37. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
38. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
39. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288.
40. Garner RW, Mowat AG, Hazleman BL. Wound healing after operations of patients with rheumatoid arthritis. J Bone Joint Surg Br. 1973;55(1):134-144.
41. Ravi B, Croxford R, Hollands S, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263.
42. Sanchez-Sotelo J, Haidukewych GJ, Boberg CJ. Hospital cost of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88(2):290-294.
43. Ward MM. Decreases in rates of hospitalizations for manifestations of severe rheumatoid arthritis, 1983-2001. Arthritis Rheum. 2004;50(4):1122-1131.
44. Goz V, Weinreb JH, Schwab F, Lafage V, Errico TJ. Comparison of complications, costs, and length of stay of three different lumbar interbody fusion techniques: an analysis of the Nationwide Inpatient Sample database. Spine J. 2014;14(9):2019-2027.
45. Goz V, Errico TJ, Weinreb JH, et al. Vertebroplasty and kyphoplasty: national outcomes and trends in utilization from 2005 through 2010. Spine J. 2015;15(5):959-965.
Resource Utilization and Satisfaction
The patient experience has become increasingly important to healthcare in the United States. It is now a metric used commonly to determine physician compensation and accounts for nearly 30% of the Centers for Medicare and Medicaid Services' (CMS) Value‐Based Purchasing (VBP) reimbursement for fiscal years 2015 and 2016.[1, 2]
In April 2015, CMS added a 5‐star patient experience score to its Hospital Compare website in an attempt to address the Affordable Care Act's call for transparent and easily understandable public reporting.[3] A hospital's principal score is the Summary Star Rating, which is based on responses to the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey. The formulas used to calculate Summary Star Ratings have been reported by CMS.[4]
Studies published over the past decade suggest that gender, age, education level, length of hospital stay, travel distance, and other factors may influence patient satisfaction.[5, 6, 7, 8] One study utilizing a national dataset suggested that higher patient satisfaction was associated with greater inpatient healthcare utilization and higher healthcare expenditures.[9] It is therefore possible that emphasizing patient experience scores could adversely impact healthcare resource utilization. However, positive patient experience may also be an important independent dimension of quality for patients and correlate with improved clinical outcomes.[10]
We know of no literature describing patient factors associated with the Summary Star Rating. Given that this rating is now used as a standard metric by which patient experience can be compared across more than 3,500 hospitals,[11] data describing the association between patient‐level factors and the Summary Star Rating may provide hospitals with an opportunity to target improvement efforts. We aimed to determine the degree to which resource utilization is associated with a satisfaction score based on the Summary Star Rating methodology.
METHODS
The study was conducted at the University of Rochester Medical Center (URMC), an 830‐bed tertiary care center in upstate New York. This was a retrospective review of all HCAHPS surveys returned to URMC over a 27‐month period from January 1, 2012 to April 1, 2014. URMC follows the standard CMS process for determining which patients receive surveys as follows. During the study timeframe, HCAHPS surveys were mailed to patients 18 years of age and older who had an inpatient stay spanning at least 1 midnight. Surveys were mailed within 5 days of discharge, and were generally returned within 6 weeks. URMC did not utilize telephone or email surveys during the study period. Surveys were not sent to patients who (1) were transferred to another facility, (2) were discharged to hospice, (3) died during the hospitalization, (4) received psychiatric or rehabilitative services during the hospitalization, (5) had an international address, and/or (6) were prisoners.
The survey vendor (Press Ganey, South Bend, IN) for URMC provided raw data for returned surveys with patient answers to questions. Administrative and billing databases were used to add demographic and clinical data for the corresponding hospitalization to the dataset. These data included age, gender, payer status (public, private, self, charity), length of stay, number of attendings who saw the patient (based on encounters documented in the electronic medical record (EMR)), all discharge International Classification of Diseases, 9th Revision (ICD‐9) diagnoses for the hospitalization, total charges for the hospitalization, and intensive care unit (ICU) utilization as evidenced by a documented encounter with a member of the Division of Critical Care/Pulmonary Medicine.
CMS analyzes surveys within 1 of 3 clinical service categories (medical, surgical, or obstetrics/gynecology) based on the discharging service. To parallel this approach, each returned survey was placed into 1 of these categories based on the clinical service of the discharging physician. Patients placed in the obstetrics/gynecology category (n = 1317, 13%) will be analyzed in a future analysis given inherent differences in patient characteristics that require evaluation of other variables.
Approximations of CMS Summary Star Rating
The HCAHPS survey is a multiple‐choice questionnaire that includes several domains of patient satisfaction. Respondents are asked to rate areas of satisfaction with their hospital experience on a Likert scale. CMS uses a weighted average of Likert responses to a subset of HCAHPS questions to calculate a hospital's raw score in 11 domains, as well as an overall raw summary score. CMS then adjusts each raw score for differences between hospitals (eg, clustering, improvement over time, method of survey) to determine a hospital's star rating in each domain and an overall Summary Star Rating (the Summary Star Rating is the primary factor by which consumers can compare hospitals).[4] Because our data were from a single hospital system, the between‐hospital scoring adjustments utilized by CMS were not applicable. Instead, we calculated the raw scores exactly as CMS does prior to the adjustments. Thus, our scores reflect the scores that CMS would have given URMC during the study period prior to standardized adjustments; we refer to this as the raw satisfaction rating (RSR). We calculated an RSR for every eligible survey. The RSR was calculated as a continuous variable from 0 (lowest) to 1 (highest). Detailed explanation of our RSR calculation is available in the Supporting Information in the online version of this article.
Statistical Analysis
All analyses were performed in aggregate and by service (medical vs surgical). Categorical variables were summarized using frequencies with percentages. Comparisons across levels of categorical variables were performed with the 2 test. We report bivariate associations between the independent variables and RSRs in the top decile using unadjusted odds ratios (ORs) with 95% confidence intervals (CIs). Similarly, multivariable logistic regression was used for adjusted analyses. For the variables of severity of illness and resource intensity, the group with the lowest illness severity and lowest resource use served as the reference groups. We modeled patients without an ICU encounter and with an ICU encounter separately.
Charges, number of unique attendings encountered, and lengths of stay were highly correlated, and likely various measures of the same underlying construct of resource intensity, and therefore could not be entered into our models simultaneously. We combined these into a resource intensity score using factor analysis with a varimax rotation, and extracted factor scores for a single factor (supported by a scree plot). We then placed patients into 4 groups based on the distribution of the factor scores: low (<25th percentile), moderate (25th50th percentile), major (50th75th percentile), and extreme (>75th percentile).
We used the Charlson‐Deyo comorbidity score as our disease severity index.[12] The index uses ICD‐9 diagnoses with points assigned for the impact of each diagnosis on morbidity and the points summed to an overall score. This provides a measure of disease severity for a patient based on the number of diagnoses and relative mortality of the individual diagnoses. Scores were categorized as 0 (representing no major illness burden), 1 to 3, 4 to 6, and >6.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), and P values <0.05 were considered statistically significant. This study was approved by the institutional review board at the University of Rochester Medical Center.
RESULTS
Our initial search identified 10,007 returned surveys (29% of eligible patients returned surveys during the study period). Of these, 5059 (51%) were categorized as medical, 3630 (36%) as surgical, and 1317 (13%) as obstetrics/gynecology. One survey did not have the service of the discharging physician recorded and was excluded. Cohort demographics and relationship to RSRs in the top decile for the 8689 medical and surgical patients can be found in Table 1. The most common discharge diagnosis‐related groups (DRGs) for medical patients were 247, percutaneous cardiovascular procedure with drug‐eluding stent without major complications or comorbidities (MCC) (3.8%); 871, septicemia or severe sepsis without mechanical ventilation >96 hours with MCC (2.7%); and 392, esophagitis, gastroenteritis, and miscellaneous digestive disorders with MCC (2.3%). The most common DRGs for surgical patients were 460, spinal fusion except cervical without MCC (3.5%); 328, stomach, esophageal and duodenal procedure without complication or comorbidities or MCC (3.3%); and 491, back and neck procedure excluding spinal fusion without complication or comorbidities or MCC (3.1%).
| Overall | Medical | Surgical | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | <90th | Top Decile | P | Total | <90th | Top Decile | P | Total | <90th | Top Decile | P | |
| ||||||||||||
| Overall | 8,689 | 7,789 (90) | 900 (10) | 5,059 | 4,646 (92) | 413 (8) | 3,630 | 3,143 (87) | 487 (13) | |||
| Age, y | ||||||||||||
| <30 | 419 (5) | 371 (89) | 48 (12) | <0.001 | 218 (4) | 208 (95) | 10 (5) | <0.001 | 201 (6) | 163 (81) | 38 (19) | <0.001 |
| 3049 | 1,029 (12) | 902 (88) | 127 (12) | 533 (11) | 482 (90) | 51 (10) | 496 (14) | 420 (85) | 76 (15) | |||
| 5069 | 3,911 (45) | 3,450 (88) | 461 (12) | 2,136 (42) | 1,930 (90) | 206 (10) | 1,775 (49) | 1,520 (86) | 255 (14) | |||
| >69 | 3,330 (38) | 3,066 (92) | 264 (8) | 2,172 (43) | 2,026 (93) | 146 (7) | 1,158 (32) | 1,040 (90) | 118 (10) | |||
| Gender | ||||||||||||
| Male | 4,640 (53) | 4,142 (89) | 498 (11) | 0.220 | 2,596 (51) | 2,379 (92) | 217 (8) | 0.602 | 2,044 (56) | 1,763 (86) | 281 (14) | 0.506 |
| Female | 4,049 (47) | 3,647 (90) | 402 (10) | 2,463 (49) | 2,267 (92) | 196 (8) | 1,586 (44) | 1,380 (87) | 206 (13) | |||
| ICU encounter | ||||||||||||
| No | 7,122 (82) | 6,441 (90) | 681 (10) | <0.001 | 4,547 (90) | 4,193 (92) | 354 (8) | <0.001 | 2,575 (71) | 2,248 (87) | 327 (13) | 0.048 |
| Yes | 1,567 (18) | 1,348 (86) | 219 (14) | 512 (10) | 453 (89) | 59 (12) | 1,055 (29) | 895 (85) | 160 (15) | |||
| Payer | ||||||||||||
| Public | 5,564 (64) | 5,036 (91) | 528 (10) | <0.001 | 3,424 (68) | 3,161 (92) | 263 (8) | 0.163 | 2,140 (59) | 1,875 (88) | 265 (12) | 0.148 |
| Private | 3,064 (35) | 2,702 (88) | 362 (12) | 1,603 (32) | 1,458 (91) | 145 (9) | 1,461 (40) | 1,244 (85) | 217 (15) | |||
| Charity | 45 (1) | 37 (82) | 8 (18) | 25 (1) | 21 (84) | 4 (16) | 20 (1) | 16 (80) | 4 (20) | |||
| Self | 16 (0) | 14 (88) | 2 (13) | 7 (0) | 6 (86) | 1 (14) | 9 (0) | 8 (89) | 1 (11) | |||
| Length of stay, d | ||||||||||||
| <3 | 3,156 (36) | 2,930 (93) | 226 (7) | <0.001 | 1,961 (39) | 1,865 (95) | 96 (5) | <0.001 | 1,195 (33) | 1,065 (89) | 130 (11) | <0.001 |
| 36 | 3,330 (38) | 2,959 (89) | 371 (11) | 1,867 (37) | 1,702 (91) | 165 (9) | 1,463 (40) | 1,257 (86) | 206 (14) | |||
| >6 | 2,203 (25) | 1,900 (86) | 303 (14) | 1,231 (24) | 1,079 (88) | 152 (12) | 972 (27) | 821 (85) | 151 (16) | |||
| No. of attendings | ||||||||||||
| <4 | 3,959 (46) | 3,615 (91) | 344 (9) | <0.001 | 2,307 (46) | 2,160 (94) | 147 (6) | <0.001 | 1,652 (46) | 1,455 (88) | 197 (12) | 0.052 |
| 46 | 3,067 (35) | 2,711 (88) | 356 (12) | 1,836 (36) | 1,663 (91) | 173 (9) | 1,231 (34) | 1,048 (85) | 183 (15) | |||
| >6 | 1,663 (19) | 1,463 (88) | 200 (12) | 916 (18) | 823 (90) | 93 (10) | 747 (21) | 640 (86) | 107 (14) | |||
| Severity index* | ||||||||||||
| 0 (lowest) | 2,812 (32) | 2,505 (89) | 307 (11) | 0.272 | 1,273 (25) | 1,185 (93) | 88 (7) | 0.045 | 1,539 (42) | 1,320 (86) | 219 (14) | 0.261 |
| 13 | 4,253 (49) | 3,827 (90) | 426 (10) | 2,604 (52) | 2,395 (92) | 209 (8) | 1,649 (45) | 1,432 (87) | 217 (13) | |||
| 46 | 1163 (13) | 1,052 (91) | 111 (10) | 849 (17) | 770 (91) | 79 (9) | 314 (9) | 282 (90) | 32 (10) | |||
| >6 (highest) | 461 (5) | 405 (88) | 56 (12) | 333 (7) | 296 (89) | 37 (11) | 128 (4) | 109 (85) | 19 (15) | |||
| Charges, | ||||||||||||
| Low | 1,820 (21) | 1,707 (94) | 113 (6) | <0.001 | 1,426 (28) | 1,357 (95) | 69 (5) | <0.001 | 394 (11) | 350 (89) | 44 (11) | 0.007 |
| Medium | 5,094 (59) | 4,581 (90) | 513 (10) | 2,807 (56) | 2,582 (92) | 225 (8) | 2,287 (63) | 1,999 (87) | 288 (13) | |||
| High | 1,775 (20) | 1,501 (85) | 274 (15) | 826 (16) | 707 (86) | 119 (14) | 949 (26) | 794 (84) | 155 (16) | |||
Unadjusted analysis of medical and surgical patients identified significant associations of several variables with a top decile RSR (Table 2). Patients with longer lengths of stay (OR: 2.07, 95% CI: 1.72‐2.48), more attendings (OR: 1.44, 95% CI: 1.19‐1.73), and higher hospital charges (OR: 2.76, 95% CI: 2.19‐3.47) were more likely to report an RSR in the top decile. Patients without an ICU encounter (OR: 0.65, 95% CI: 0.55‐0.77) and on a medical service (OR: 0.57, 95% CI: 0.5‐ 0.66) were less likely to report an RSR in the top decile. Several associations were identified in only the medical or surgical cohorts. In the medical cohort, patients with the highest illness severity index (OR: 1.68, 95% CI: 1.12‐ 2.52) and with 7 different attending physicians (OR: 1.66, 95% CI: 1.27‐2.18) were more likely to report RSRs in the top decile. In the surgical cohort, patients <30 years of age (OR: 2.05, 95% CI 1.38‐3.07) were more likely to report an RSR in the top decile than patients >69 years of age. Insurance payer category and gender were not significantly associated with top decile RSRs.
| Overall | Medical | Surgical | ||||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | |
| ||||||
| Age, y | ||||||
| <30 | 1.5 (1.082.08) | 0.014 | 0.67 (0.351.29) | 0.227 | 2.05 (1.383.07) | <0.001 |
| 3049 | 1.64 (1.312.05) | <.001 | 1.47 (1.052.05) | 0.024 | 1.59 (1.172.17) | 0.003 |
| 5069 | 1.55 (1.321.82) | <.001 | 1.48 (1.191.85) | 0.001 | 1.48 (1.171.86) | 0.001 |
| >69 | Ref | Ref | Ref | |||
| Gender | ||||||
| Male | 1.09 (0.951.25) | 0.220 | 1.06 (0.861.29) | 0.602 | 1.07 (0.881.3) | 0.506 |
| Female | Ref | Ref | Ref | |||
| ICU encounter | ||||||
| No | 0.65 (0.550.77) | <0.001 | 0.65 (0.480.87) | 0.004 | 0.81 (0.661) | 0.048 |
| Yes | Ref | Ref | Ref | |||
| Payer | ||||||
| Public | 0.73 (0.173.24) | 0.683 | 0.5 (0.064.16) | 0.521 | 1.13 (0.149.08) | 0.908 |
| Private | 0.94 (0.214.14) | 0.933 | 0.6 (0.074.99) | 0.634 | 1.4 (0.1711.21) | 0.754 |
| Charity | 1.51 (0.298.02) | 0.626 | 1.14 (0.1112.25) | 0.912 | 2 (0.1920.97) | 0.563 |
| Self | Ref | Ref | Ref | |||
| Length of stay, d | ||||||
| <3 | Ref | Ref | Ref | |||
| 36 | 1.63 (1.371.93) | <0.001 | 1.88 (1.452.44) | <0.001 | 1.34 (1.061.7) | 0.014 |
| >6 | 2.07 (1.722.48) | <0.001 | 2.74 (2.13.57) | <0.001 | 1.51 (1.171.94) | 0.001 |
| No. of attendings | ||||||
| <4 | Ref | Ref | Ref | |||
| 46 | 1.38 (1.181.61) | <0.001 | 1.53 (1.221.92) | <0.001 | 1.29 (1.041.6) | 0.021 |
| >6 | 1.44 (1.191.73) | <0.001 | 1.66 (1.272.18) | <0.001 | 1.23 (0.961.59) | 0.102 |
| Severity index* | ||||||
| 0 (lowest) | Ref | Ref | Ref | |||
| 13 | 0.91 (0.781.06) | 0.224 | 1.18 (0.911.52) | 0.221 | 0.91 (0.751.12) | 0.380 |
| 46 | 0.86 (0.681.08) | 0.200 | 1.38 (1.011.9) | 0.046 | 0.68 (0.461.01) | 0.058 |
| >6 (highest) | 1.13 (0.831.53) | 0.436 | 1.68 (1.122.52) | 0.012 | 1.05 (0.631.75) | 0.849 |
| Charges | ||||||
| Low | Ref | Ref | Ref | |||
| Medium | 1.69 (1.372.09) | <0.001 | 1.71 (1.32.26) | <0.001 | 1.15 (0.821.61) | 0.428 |
| High | 2.76 (2.193.47) | <0.001 | 3.31 (2.434.51) | <0.001 | 1.55 (1.092.22) | 0.016 |
| Service | ||||||
| Medical | 0.57 (0.50.66) | <0.001 | ||||
| Surgical | Ref | |||||
Multivariable modeling (Table 3) for all patients without an ICU encounter suggested that (1) patients aged <30 years, 30 to 49 years, and 50 to 69 years were more likely to report top decile RSRs when compared to patients 70 years and older (OR: 1.61, 95% CI: 1.09‐2.36; OR: 1.44, 95% CI: 1.08‐1.93; and OR: 1.39, 95% CI: 1.13‐1.71, respectively) and (2), when compared to patients with extreme resource intensity scores, patients with higher resource intensity scores were more likely to report top decile RSRs (moderate [OR: 1.42, 95% CI: 1.11‐1.83], major [OR: 1.56, 95% CI: 1.22‐2.01], and extreme [OR: 2.29, 95% CI: 1.8‐2.92]. These results were relatively consistent within medical and surgical subgroups (Table 3).
| Overall | Medical | Surgical | ||||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | |
| ||||||
| Age, y | ||||||
| <30 | 1.61 (1.092.36) | 0.016 | 0.82 (0.41.7) | 0.596 | 2.31 (1.393.82) | 0.001 |
| 3049 | 1.44 (1.081.93) | 0.014 | 1.55 (1.032.32) | 0.034 | 1.41 (0.912.17) | 0.120 |
| 5069 | 1.39 (1.131.71) | 0.002 | 1.44 (1.11.88) | 0.008 | 1.39 (11.93) | 0.049 |
| >69 | Ref | Ref | Ref | |||
| Sex | ||||||
| Male | 1 (0.851.17) | 0.964 | 1 (0.81.25) | 0.975 | 0.99 (0.791.26) | 0.965 |
| Female | Ref | Ref | Ref | |||
| Payer | ||||||
| Public | 0.62 (0.142.8) | 0.531 | 0.42 (0.053.67) | 0.432 | 1.03 (0.128.59) | 0.978 |
| Private | 0.67 (0.153.02) | 0.599 | 0.42 (0.053.67) | 0.434 | 1.17 (0.149.69) | 0.884 |
| Charity | 1.54 (0.288.41) | 0.620 | 1 (0.0911.13) | 0.999 | 2.56 (0.2328.25) | 0.444 |
| Self | Ref | Ref | Ref | |||
| Severity index | ||||||
| 0 (lowest) | Ref | Ref | Ref | |||
| 13 | 1.07 (0.891.29) | 0.485 | 1.18 (0.881.58) | 0.267 | 1 (0.781.29) | 0.986 |
| 46 | 1.14 (0.861.51) | 0.377 | 1.42 (0.992.04) | 0.056 | 0.6 (0.331.1) | 0.100 |
| >6 (highest) | 1.31 (0.911.9) | 0.150 | 1.47 (0.932.33) | 0.097 | 1.1 (0.542.21) | 0.795 |
| Resource intensity score | ||||||
| Low | Ref | Ref | Ref | |||
| Moderate | 1.42 (1.111.83) | 0.006 | 1.6 (1.112.3) | 0.011 | 0.94 (0.661.34) | 0.722 |
| Major | 1.56 (1.222.01) | 0.001 | 1.69 (1.182.43) | 0.004 | 1.28 (0.911.8) | 0.151 |
| Extreme | 2.29 (1.82.92) | <0.001 | 2.72 (1.943.82) | <0.001 | 1.63 (1.172.26) | 0.004 |
| Service | ||||||
| Medical | 0.59 (0.50.69) | <0.001 | ||||
| Surgical | Ref | |||||
In those with at least 1 ICU attending encounter (see Supporting Table 1 in the online version of this article), no variables demonstrated significant association with top decile RSRs in the overall group or in the medical subgroup. For surgical patients with at least 1 ICU attending encounter (see Supporting Table 1 in the online version of this article), patients aged 30 to 49 and 50 to 69 years were more likely to provide top decile RSRs (OR: 1.93, 95% CI: 1.08‐3.46 and OR: 1.65, 95% CI 1.07‐2.53, respectively). Resource intensity was not significantly associated with top decile RSRs.
DISCUSSION
Our analysis suggests that, for patients on the general care floors, resource utilization is associated with the RSR and, therefore, potentially the CMS Summary Star Rating. Adjusting for severity of illness, patients with higher resource utilization were more likely to report top decile RSRs.
Prior data regarding utilization and satisfaction are mixed. In a 2‐year, prospective, national examination, patients in the highest quartile of patient satisfaction had increased healthcare and prescription drug expenditures as well as increased rates of hospitalization when compared with patients in the lowest quartile of patient satisfaction.[9] However, a recent national study of surgical administrative databases suggested hospitals with high patient satisfaction provided more efficient care.[13]
One reason for the conflicting data may be that large, national evaluations are unable to control for between‐hospital confounders (ie, hospital quality of care). By capturing all eligible returned surveys at 1 institution, our design allowed us to collect granular data. We found that in 1 hospital setting, patient population, facilities, and food services, patients receiving more clinical resources generally assigned higher ratings than patients receiving less.
It is possible that utilization is a proxy for serious illness, and that patients with serious illness receive more attention during hospitalization and are more satisfied when discharged in a good state of health. However, we did adjust for severity of illness in our model using the Charlson‐Deyo index and we suggest that, other factors being equal, hospitals with higher per‐patient expenditures may be assigned higher Summary Star Ratings.
CMS has recently implemented a number of metrics designed to decrease healthcare costs by improving quality, safety, and efficiency. Concurrently, CMS has also prioritized patient experience. The Summary Star Rating was created to provide healthcare consumers with an easy way to compare the patient experience between hospitals[4]; however, our data suggest that this metric may be at odds with inpatient cost savings and efficiency metrics.
Per‐patient spending becomes particularly salient when considering that in fiscal year 2016, CMS' hospital VBP reimbursement will include 2 metrics: an efficiency outcome measure labeled Medicare spending per beneficiary, and a patient experience outcome measure based on HCAHPS survey dimensions.[2] Together, these 2 metrics will comprise nearly half of the total VBP performance score used to determine reimbursement. Although our data suggest that these 2 VBP metrics may be correlated, it should be noted that we measured inpatient hospital charges, whereas the CMS efficiency outcome measure includes costs for episode of care spanning 3 days prior to hospitalization to 30 days after hospitalization.
Patient expectations likely play a role in satisfaction.[14, 15, 16] In an outpatient setting, physician fulfillment of patient requests has been associated with positive patient evaluations of care.[17] However, patients appear to value education, shared decision making, and provider empathy more than testing and intervention.[14, 18, 19, 20, 21, 22, 23] Perhaps, in the absence of the former attributes, patients use additional resource expenditure as a proxy.
It is not clear that higher resource expenditure improves outcomes. A landmark study of nearly 1 million Medicare enrollees by Fisher et al. suggests that, although Medicare patients in higher‐spending regions receive more care than those in lower‐spending regions, this does not result in better health outcomes, specifically with regard to mortality.[24, 25] Patients who live in areas of high hospital capacity use the hospital more frequently than do patients in areas of low hospital capacity, but this does not appear to result in improved mortality rates.[26] In fact, physicians in areas of high healthcare capacity report more difficulty maintaining high‐quality patient relationships and feel less able to provide high‐quality care than physicians in lower‐capacity areas.[27]
We hypothesize the cause of the association between resource utilization and patient satisfaction could be that patients (1) perceive that a doctor who allows them to stay longer in the hospital or who performs additional testing cares more about their well‐being and (2) that these patients feel more strongly that their concerns are being heard and addressed by their physicians. A systematic review of primary care patients identified many studies that found a positive association between meeting patient expectations and satisfaction with care, but also suggested that although patients frequently expect information, physicians misperceive this as an expectation of specific action.[28] A separate systematic review found that patient education in the form of decision aides can help patients develop more reasonable expectations and reduce utilization of certain discretionary procedures such as elective surgeries and prostate‐specific antigen testing.[29]
We did not specifically address clinical outcomes in our analysis because the clinical outcomes on which CMS currently adjusts VBP reimbursement focus on 30‐day mortality for specific diagnoses, nosocomial infections, and iatrogenic events.[30] Our data include only returned surveys from living patients, and it is likely that 30‐day mortality was similar throughout all subsets of patients. Additionally, the nosocomial and iatrogenic outcome measures used by CMS are sufficiently rare on the general floors and are unlikely to have significantly influenced our results.[31]
Our study has several strengths. Nearly all medical and surgical patient surveys returned during the study period were included, and therefore our calculations are likely to accurately reflect the Summary Star Rating that would have been assigned for the period. Second, the large sample size helps attenuate potential differences in commonly used outcome metrics. Third, by adjusting for a variety of demographic and clinical variables, we were able to decrease the likelihood of unidentified confounders.
Notably, we identified 38 (0.4%) surveys returned for patients under 18 years of age at admission. These surveys were included in our analysis because, to the best of our knowledge, they would have existed in the pool of surveys CMS could have used to assign a Summary Star Rating.
Our study also has limitations. First, geographically diverse data are needed to ensure generalizability. Second, we used the Charlson‐Deyo Comorbidity Index to describe the degree of illness for each patient. This index represents a patient's total illness burden but may not describe the relative severity of the patient's current illness relative to another patient. Third, we selected variables we felt were most likely to be associated with patient experience, but unidentified confounding remains possible. Fourth, attendings caring for ICU patients fall within the Division of Critical Care/Pulmonary Medicine. Therefore, we may have inadvertently placed patients into the ICU cohort who received a pulmonary/critical care consult on the general floors. Fifth, our data describe associations only for patients who returned surveys. Although there may be inherent biases in patients who return surveys, HCAHPS survey responses are used by CMS to determine a hospital's overall satisfaction score.
CONCLUSION
For patients who return HCAHPS surveys, resource utilization may be positively associated with a hospital's Summary Star Rating. These data suggest that hospitals with higher per‐patient expenditures may receive higher Summary Star Ratings, which could result in hospitals with higher per‐patient resource utilization appearing more attractive to healthcare consumers. Future studies should attempt to confirm our findings at other institutions and to determine causative factors.
Acknowledgements
The authors thank Jason Machan, PhD (Department of Orthopedics and Surgery, Warren Alpert Medical School, Brown University, Providence, Rhode Island) for his help with study design, and Ms. Brenda Foster (data analyst, University of Rochester Medical Center, Rochester, NY) for her help with data collection.
Disclosures: Nothing to report.
- , , . Redesigning physician compensation and improving ED performance. Healthc Financ Manage. 2011;65(6):114–117.
- QualityNet. Available at: https://www.qualitynet.org/dcs/ContentServer?c=Page97(13):1041–1048.
- , , , . Factors determining inpatient satisfaction with care. Soc Sci Med. 2002;54(4):493–504.
- , , , , . Patient satisfaction revisited: a multilevel approach. Soc Sci Med. 2009;69(1):68–75.
- , , , et al. Predictors of patient satisfaction with hospital health care. BMC Health Serv Res. 2006;6:102.
- , , , . The cost of satisfaction: a national study of patient satisfaction, health care utilization, expenditures, and mortality. Arch Intern Med. 2012;172(5):405–411.
- , , , , . Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41–48.
- Becker's Infection Control and Clinical Quality. Star Ratings go live on Hospital Compare: how many hospitals got 5 stars? Available at: http://www.beckershospitalreview.com/quality/star‐ratings‐go‐live‐on‐hospital‐compare‐how‐many‐hospitals‐got‐5‐stars.html. Published April 16, 2015. Accessed October 5, 2015.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- , , . Patient satisfaction and quality of surgical care in US hospitals. Ann Surg. 2015;261(1):2–8.
- , , , , . Should health care providers be accountable for patients' care experiences? J Gen Intern Med. 2015;30(2):253–256.
- , , , , . Unmet expectations for care and the patient‐physician relationship. J Gen Intern Med. 2002;17(11):817–824.
- , , , et al. Do unmet expectations for specific tests, referrals, and new medications reduce patients' satisfaction? J Gen Intern Med. 2004;19(11):1080–1087.
- , , , , , . Request fulfillment in office practice: antecedents and relationship to outcomes. Med Care. 2002;40(1):38–51.
- , , , et al. Factors associated with patient satisfaction with care among dermatological outpatients. Br J Dermatol. 2001;145(4):617–623.
- , , , . Patient expectations of emergency department care: phase II—a cross‐sectional survey. CJEM. 2006;8(3):148–157.
- , , , , . Patients' perspectives on ideal physician behaviors. Mayo Clin Proc. 2006;81(3):338–344.
- , . What do people want from their health care? A qualitative study. J Participat Med. 2015;18:e10.
- , , . Evaluations of care by adults following a denial of an advertisement‐related prescription drug request: the role of expectations, symptom severity, and physician communication style. Soc Sci Med. 2006;62(4):888–899.
- , , , , , . Getting to “no”: strategies primary care physicians use to deny patient requests. Arch Intern Med. 2010;170(4):381–388.
- , , , , , . The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003;138(4):273–287.
- , , , , , . The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med. 2003;138(4):288–298.
- , , , et al. Associations among hospital capacity, utilization, and mortality of US Medicare beneficiaries, controlling for sociodemographic factors. Health Serv Res. 2000;34(6):1351–1362.
- , , , . Regional variations in health care intensity and physician perceptions of quality of care. Ann Intern Med. 2006;144(9):641–649.
- , , . Visit‐specific expectations and patient‐centered outcomes: a literature review. Arch Fam Med. 2000;9(10):1148–1155.
- , , , et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431.
- Centers for Medicare and Medicaid Services. Hospital Compare. Outcome domain. Available at: https://www.medicare.gov/hospitalcompare/data/outcome‐domain.html. Accessed October 5, 2015.
- Centers for Disease Control and Prevention. 2013 national and state healthcare‐associated infections progress report. Available at: www.cdc.gov/hai/progress‐report/index.html. Accessed October 5, 2015.
The patient experience has become increasingly important to healthcare in the United States. It is now a metric used commonly to determine physician compensation and accounts for nearly 30% of the Centers for Medicare and Medicaid Services' (CMS) Value‐Based Purchasing (VBP) reimbursement for fiscal years 2015 and 2016.[1, 2]
In April 2015, CMS added a 5‐star patient experience score to its Hospital Compare website in an attempt to address the Affordable Care Act's call for transparent and easily understandable public reporting.[3] A hospital's principal score is the Summary Star Rating, which is based on responses to the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey. The formulas used to calculate Summary Star Ratings have been reported by CMS.[4]
Studies published over the past decade suggest that gender, age, education level, length of hospital stay, travel distance, and other factors may influence patient satisfaction.[5, 6, 7, 8] One study utilizing a national dataset suggested that higher patient satisfaction was associated with greater inpatient healthcare utilization and higher healthcare expenditures.[9] It is therefore possible that emphasizing patient experience scores could adversely impact healthcare resource utilization. However, positive patient experience may also be an important independent dimension of quality for patients and correlate with improved clinical outcomes.[10]
We know of no literature describing patient factors associated with the Summary Star Rating. Given that this rating is now used as a standard metric by which patient experience can be compared across more than 3,500 hospitals,[11] data describing the association between patient‐level factors and the Summary Star Rating may provide hospitals with an opportunity to target improvement efforts. We aimed to determine the degree to which resource utilization is associated with a satisfaction score based on the Summary Star Rating methodology.
METHODS
The study was conducted at the University of Rochester Medical Center (URMC), an 830‐bed tertiary care center in upstate New York. This was a retrospective review of all HCAHPS surveys returned to URMC over a 27‐month period from January 1, 2012 to April 1, 2014. URMC follows the standard CMS process for determining which patients receive surveys as follows. During the study timeframe, HCAHPS surveys were mailed to patients 18 years of age and older who had an inpatient stay spanning at least 1 midnight. Surveys were mailed within 5 days of discharge, and were generally returned within 6 weeks. URMC did not utilize telephone or email surveys during the study period. Surveys were not sent to patients who (1) were transferred to another facility, (2) were discharged to hospice, (3) died during the hospitalization, (4) received psychiatric or rehabilitative services during the hospitalization, (5) had an international address, and/or (6) were prisoners.
The survey vendor (Press Ganey, South Bend, IN) for URMC provided raw data for returned surveys with patient answers to questions. Administrative and billing databases were used to add demographic and clinical data for the corresponding hospitalization to the dataset. These data included age, gender, payer status (public, private, self, charity), length of stay, number of attendings who saw the patient (based on encounters documented in the electronic medical record (EMR)), all discharge International Classification of Diseases, 9th Revision (ICD‐9) diagnoses for the hospitalization, total charges for the hospitalization, and intensive care unit (ICU) utilization as evidenced by a documented encounter with a member of the Division of Critical Care/Pulmonary Medicine.
CMS analyzes surveys within 1 of 3 clinical service categories (medical, surgical, or obstetrics/gynecology) based on the discharging service. To parallel this approach, each returned survey was placed into 1 of these categories based on the clinical service of the discharging physician. Patients placed in the obstetrics/gynecology category (n = 1317, 13%) will be analyzed in a future analysis given inherent differences in patient characteristics that require evaluation of other variables.
Approximations of CMS Summary Star Rating
The HCAHPS survey is a multiple‐choice questionnaire that includes several domains of patient satisfaction. Respondents are asked to rate areas of satisfaction with their hospital experience on a Likert scale. CMS uses a weighted average of Likert responses to a subset of HCAHPS questions to calculate a hospital's raw score in 11 domains, as well as an overall raw summary score. CMS then adjusts each raw score for differences between hospitals (eg, clustering, improvement over time, method of survey) to determine a hospital's star rating in each domain and an overall Summary Star Rating (the Summary Star Rating is the primary factor by which consumers can compare hospitals).[4] Because our data were from a single hospital system, the between‐hospital scoring adjustments utilized by CMS were not applicable. Instead, we calculated the raw scores exactly as CMS does prior to the adjustments. Thus, our scores reflect the scores that CMS would have given URMC during the study period prior to standardized adjustments; we refer to this as the raw satisfaction rating (RSR). We calculated an RSR for every eligible survey. The RSR was calculated as a continuous variable from 0 (lowest) to 1 (highest). Detailed explanation of our RSR calculation is available in the Supporting Information in the online version of this article.
Statistical Analysis
All analyses were performed in aggregate and by service (medical vs surgical). Categorical variables were summarized using frequencies with percentages. Comparisons across levels of categorical variables were performed with the 2 test. We report bivariate associations between the independent variables and RSRs in the top decile using unadjusted odds ratios (ORs) with 95% confidence intervals (CIs). Similarly, multivariable logistic regression was used for adjusted analyses. For the variables of severity of illness and resource intensity, the group with the lowest illness severity and lowest resource use served as the reference groups. We modeled patients without an ICU encounter and with an ICU encounter separately.
Charges, number of unique attendings encountered, and lengths of stay were highly correlated, and likely various measures of the same underlying construct of resource intensity, and therefore could not be entered into our models simultaneously. We combined these into a resource intensity score using factor analysis with a varimax rotation, and extracted factor scores for a single factor (supported by a scree plot). We then placed patients into 4 groups based on the distribution of the factor scores: low (<25th percentile), moderate (25th50th percentile), major (50th75th percentile), and extreme (>75th percentile).
We used the Charlson‐Deyo comorbidity score as our disease severity index.[12] The index uses ICD‐9 diagnoses with points assigned for the impact of each diagnosis on morbidity and the points summed to an overall score. This provides a measure of disease severity for a patient based on the number of diagnoses and relative mortality of the individual diagnoses. Scores were categorized as 0 (representing no major illness burden), 1 to 3, 4 to 6, and >6.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), and P values <0.05 were considered statistically significant. This study was approved by the institutional review board at the University of Rochester Medical Center.
RESULTS
Our initial search identified 10,007 returned surveys (29% of eligible patients returned surveys during the study period). Of these, 5059 (51%) were categorized as medical, 3630 (36%) as surgical, and 1317 (13%) as obstetrics/gynecology. One survey did not have the service of the discharging physician recorded and was excluded. Cohort demographics and relationship to RSRs in the top decile for the 8689 medical and surgical patients can be found in Table 1. The most common discharge diagnosis‐related groups (DRGs) for medical patients were 247, percutaneous cardiovascular procedure with drug‐eluding stent without major complications or comorbidities (MCC) (3.8%); 871, septicemia or severe sepsis without mechanical ventilation >96 hours with MCC (2.7%); and 392, esophagitis, gastroenteritis, and miscellaneous digestive disorders with MCC (2.3%). The most common DRGs for surgical patients were 460, spinal fusion except cervical without MCC (3.5%); 328, stomach, esophageal and duodenal procedure without complication or comorbidities or MCC (3.3%); and 491, back and neck procedure excluding spinal fusion without complication or comorbidities or MCC (3.1%).
| Overall | Medical | Surgical | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | <90th | Top Decile | P | Total | <90th | Top Decile | P | Total | <90th | Top Decile | P | |
| ||||||||||||
| Overall | 8,689 | 7,789 (90) | 900 (10) | 5,059 | 4,646 (92) | 413 (8) | 3,630 | 3,143 (87) | 487 (13) | |||
| Age, y | ||||||||||||
| <30 | 419 (5) | 371 (89) | 48 (12) | <0.001 | 218 (4) | 208 (95) | 10 (5) | <0.001 | 201 (6) | 163 (81) | 38 (19) | <0.001 |
| 3049 | 1,029 (12) | 902 (88) | 127 (12) | 533 (11) | 482 (90) | 51 (10) | 496 (14) | 420 (85) | 76 (15) | |||
| 5069 | 3,911 (45) | 3,450 (88) | 461 (12) | 2,136 (42) | 1,930 (90) | 206 (10) | 1,775 (49) | 1,520 (86) | 255 (14) | |||
| >69 | 3,330 (38) | 3,066 (92) | 264 (8) | 2,172 (43) | 2,026 (93) | 146 (7) | 1,158 (32) | 1,040 (90) | 118 (10) | |||
| Gender | ||||||||||||
| Male | 4,640 (53) | 4,142 (89) | 498 (11) | 0.220 | 2,596 (51) | 2,379 (92) | 217 (8) | 0.602 | 2,044 (56) | 1,763 (86) | 281 (14) | 0.506 |
| Female | 4,049 (47) | 3,647 (90) | 402 (10) | 2,463 (49) | 2,267 (92) | 196 (8) | 1,586 (44) | 1,380 (87) | 206 (13) | |||
| ICU encounter | ||||||||||||
| No | 7,122 (82) | 6,441 (90) | 681 (10) | <0.001 | 4,547 (90) | 4,193 (92) | 354 (8) | <0.001 | 2,575 (71) | 2,248 (87) | 327 (13) | 0.048 |
| Yes | 1,567 (18) | 1,348 (86) | 219 (14) | 512 (10) | 453 (89) | 59 (12) | 1,055 (29) | 895 (85) | 160 (15) | |||
| Payer | ||||||||||||
| Public | 5,564 (64) | 5,036 (91) | 528 (10) | <0.001 | 3,424 (68) | 3,161 (92) | 263 (8) | 0.163 | 2,140 (59) | 1,875 (88) | 265 (12) | 0.148 |
| Private | 3,064 (35) | 2,702 (88) | 362 (12) | 1,603 (32) | 1,458 (91) | 145 (9) | 1,461 (40) | 1,244 (85) | 217 (15) | |||
| Charity | 45 (1) | 37 (82) | 8 (18) | 25 (1) | 21 (84) | 4 (16) | 20 (1) | 16 (80) | 4 (20) | |||
| Self | 16 (0) | 14 (88) | 2 (13) | 7 (0) | 6 (86) | 1 (14) | 9 (0) | 8 (89) | 1 (11) | |||
| Length of stay, d | ||||||||||||
| <3 | 3,156 (36) | 2,930 (93) | 226 (7) | <0.001 | 1,961 (39) | 1,865 (95) | 96 (5) | <0.001 | 1,195 (33) | 1,065 (89) | 130 (11) | <0.001 |
| 36 | 3,330 (38) | 2,959 (89) | 371 (11) | 1,867 (37) | 1,702 (91) | 165 (9) | 1,463 (40) | 1,257 (86) | 206 (14) | |||
| >6 | 2,203 (25) | 1,900 (86) | 303 (14) | 1,231 (24) | 1,079 (88) | 152 (12) | 972 (27) | 821 (85) | 151 (16) | |||
| No. of attendings | ||||||||||||
| <4 | 3,959 (46) | 3,615 (91) | 344 (9) | <0.001 | 2,307 (46) | 2,160 (94) | 147 (6) | <0.001 | 1,652 (46) | 1,455 (88) | 197 (12) | 0.052 |
| 46 | 3,067 (35) | 2,711 (88) | 356 (12) | 1,836 (36) | 1,663 (91) | 173 (9) | 1,231 (34) | 1,048 (85) | 183 (15) | |||
| >6 | 1,663 (19) | 1,463 (88) | 200 (12) | 916 (18) | 823 (90) | 93 (10) | 747 (21) | 640 (86) | 107 (14) | |||
| Severity index* | ||||||||||||
| 0 (lowest) | 2,812 (32) | 2,505 (89) | 307 (11) | 0.272 | 1,273 (25) | 1,185 (93) | 88 (7) | 0.045 | 1,539 (42) | 1,320 (86) | 219 (14) | 0.261 |
| 13 | 4,253 (49) | 3,827 (90) | 426 (10) | 2,604 (52) | 2,395 (92) | 209 (8) | 1,649 (45) | 1,432 (87) | 217 (13) | |||
| 46 | 1163 (13) | 1,052 (91) | 111 (10) | 849 (17) | 770 (91) | 79 (9) | 314 (9) | 282 (90) | 32 (10) | |||
| >6 (highest) | 461 (5) | 405 (88) | 56 (12) | 333 (7) | 296 (89) | 37 (11) | 128 (4) | 109 (85) | 19 (15) | |||
| Charges, | ||||||||||||
| Low | 1,820 (21) | 1,707 (94) | 113 (6) | <0.001 | 1,426 (28) | 1,357 (95) | 69 (5) | <0.001 | 394 (11) | 350 (89) | 44 (11) | 0.007 |
| Medium | 5,094 (59) | 4,581 (90) | 513 (10) | 2,807 (56) | 2,582 (92) | 225 (8) | 2,287 (63) | 1,999 (87) | 288 (13) | |||
| High | 1,775 (20) | 1,501 (85) | 274 (15) | 826 (16) | 707 (86) | 119 (14) | 949 (26) | 794 (84) | 155 (16) | |||
Unadjusted analysis of medical and surgical patients identified significant associations of several variables with a top decile RSR (Table 2). Patients with longer lengths of stay (OR: 2.07, 95% CI: 1.72‐2.48), more attendings (OR: 1.44, 95% CI: 1.19‐1.73), and higher hospital charges (OR: 2.76, 95% CI: 2.19‐3.47) were more likely to report an RSR in the top decile. Patients without an ICU encounter (OR: 0.65, 95% CI: 0.55‐0.77) and on a medical service (OR: 0.57, 95% CI: 0.5‐ 0.66) were less likely to report an RSR in the top decile. Several associations were identified in only the medical or surgical cohorts. In the medical cohort, patients with the highest illness severity index (OR: 1.68, 95% CI: 1.12‐ 2.52) and with 7 different attending physicians (OR: 1.66, 95% CI: 1.27‐2.18) were more likely to report RSRs in the top decile. In the surgical cohort, patients <30 years of age (OR: 2.05, 95% CI 1.38‐3.07) were more likely to report an RSR in the top decile than patients >69 years of age. Insurance payer category and gender were not significantly associated with top decile RSRs.
| Overall | Medical | Surgical | ||||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | |
| ||||||
| Age, y | ||||||
| <30 | 1.5 (1.082.08) | 0.014 | 0.67 (0.351.29) | 0.227 | 2.05 (1.383.07) | <0.001 |
| 3049 | 1.64 (1.312.05) | <.001 | 1.47 (1.052.05) | 0.024 | 1.59 (1.172.17) | 0.003 |
| 5069 | 1.55 (1.321.82) | <.001 | 1.48 (1.191.85) | 0.001 | 1.48 (1.171.86) | 0.001 |
| >69 | Ref | Ref | Ref | |||
| Gender | ||||||
| Male | 1.09 (0.951.25) | 0.220 | 1.06 (0.861.29) | 0.602 | 1.07 (0.881.3) | 0.506 |
| Female | Ref | Ref | Ref | |||
| ICU encounter | ||||||
| No | 0.65 (0.550.77) | <0.001 | 0.65 (0.480.87) | 0.004 | 0.81 (0.661) | 0.048 |
| Yes | Ref | Ref | Ref | |||
| Payer | ||||||
| Public | 0.73 (0.173.24) | 0.683 | 0.5 (0.064.16) | 0.521 | 1.13 (0.149.08) | 0.908 |
| Private | 0.94 (0.214.14) | 0.933 | 0.6 (0.074.99) | 0.634 | 1.4 (0.1711.21) | 0.754 |
| Charity | 1.51 (0.298.02) | 0.626 | 1.14 (0.1112.25) | 0.912 | 2 (0.1920.97) | 0.563 |
| Self | Ref | Ref | Ref | |||
| Length of stay, d | ||||||
| <3 | Ref | Ref | Ref | |||
| 36 | 1.63 (1.371.93) | <0.001 | 1.88 (1.452.44) | <0.001 | 1.34 (1.061.7) | 0.014 |
| >6 | 2.07 (1.722.48) | <0.001 | 2.74 (2.13.57) | <0.001 | 1.51 (1.171.94) | 0.001 |
| No. of attendings | ||||||
| <4 | Ref | Ref | Ref | |||
| 46 | 1.38 (1.181.61) | <0.001 | 1.53 (1.221.92) | <0.001 | 1.29 (1.041.6) | 0.021 |
| >6 | 1.44 (1.191.73) | <0.001 | 1.66 (1.272.18) | <0.001 | 1.23 (0.961.59) | 0.102 |
| Severity index* | ||||||
| 0 (lowest) | Ref | Ref | Ref | |||
| 13 | 0.91 (0.781.06) | 0.224 | 1.18 (0.911.52) | 0.221 | 0.91 (0.751.12) | 0.380 |
| 46 | 0.86 (0.681.08) | 0.200 | 1.38 (1.011.9) | 0.046 | 0.68 (0.461.01) | 0.058 |
| >6 (highest) | 1.13 (0.831.53) | 0.436 | 1.68 (1.122.52) | 0.012 | 1.05 (0.631.75) | 0.849 |
| Charges | ||||||
| Low | Ref | Ref | Ref | |||
| Medium | 1.69 (1.372.09) | <0.001 | 1.71 (1.32.26) | <0.001 | 1.15 (0.821.61) | 0.428 |
| High | 2.76 (2.193.47) | <0.001 | 3.31 (2.434.51) | <0.001 | 1.55 (1.092.22) | 0.016 |
| Service | ||||||
| Medical | 0.57 (0.50.66) | <0.001 | ||||
| Surgical | Ref | |||||
Multivariable modeling (Table 3) for all patients without an ICU encounter suggested that (1) patients aged <30 years, 30 to 49 years, and 50 to 69 years were more likely to report top decile RSRs when compared to patients 70 years and older (OR: 1.61, 95% CI: 1.09‐2.36; OR: 1.44, 95% CI: 1.08‐1.93; and OR: 1.39, 95% CI: 1.13‐1.71, respectively) and (2), when compared to patients with extreme resource intensity scores, patients with higher resource intensity scores were more likely to report top decile RSRs (moderate [OR: 1.42, 95% CI: 1.11‐1.83], major [OR: 1.56, 95% CI: 1.22‐2.01], and extreme [OR: 2.29, 95% CI: 1.8‐2.92]. These results were relatively consistent within medical and surgical subgroups (Table 3).
| Overall | Medical | Surgical | ||||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | |
| ||||||
| Age, y | ||||||
| <30 | 1.61 (1.092.36) | 0.016 | 0.82 (0.41.7) | 0.596 | 2.31 (1.393.82) | 0.001 |
| 3049 | 1.44 (1.081.93) | 0.014 | 1.55 (1.032.32) | 0.034 | 1.41 (0.912.17) | 0.120 |
| 5069 | 1.39 (1.131.71) | 0.002 | 1.44 (1.11.88) | 0.008 | 1.39 (11.93) | 0.049 |
| >69 | Ref | Ref | Ref | |||
| Sex | ||||||
| Male | 1 (0.851.17) | 0.964 | 1 (0.81.25) | 0.975 | 0.99 (0.791.26) | 0.965 |
| Female | Ref | Ref | Ref | |||
| Payer | ||||||
| Public | 0.62 (0.142.8) | 0.531 | 0.42 (0.053.67) | 0.432 | 1.03 (0.128.59) | 0.978 |
| Private | 0.67 (0.153.02) | 0.599 | 0.42 (0.053.67) | 0.434 | 1.17 (0.149.69) | 0.884 |
| Charity | 1.54 (0.288.41) | 0.620 | 1 (0.0911.13) | 0.999 | 2.56 (0.2328.25) | 0.444 |
| Self | Ref | Ref | Ref | |||
| Severity index | ||||||
| 0 (lowest) | Ref | Ref | Ref | |||
| 13 | 1.07 (0.891.29) | 0.485 | 1.18 (0.881.58) | 0.267 | 1 (0.781.29) | 0.986 |
| 46 | 1.14 (0.861.51) | 0.377 | 1.42 (0.992.04) | 0.056 | 0.6 (0.331.1) | 0.100 |
| >6 (highest) | 1.31 (0.911.9) | 0.150 | 1.47 (0.932.33) | 0.097 | 1.1 (0.542.21) | 0.795 |
| Resource intensity score | ||||||
| Low | Ref | Ref | Ref | |||
| Moderate | 1.42 (1.111.83) | 0.006 | 1.6 (1.112.3) | 0.011 | 0.94 (0.661.34) | 0.722 |
| Major | 1.56 (1.222.01) | 0.001 | 1.69 (1.182.43) | 0.004 | 1.28 (0.911.8) | 0.151 |
| Extreme | 2.29 (1.82.92) | <0.001 | 2.72 (1.943.82) | <0.001 | 1.63 (1.172.26) | 0.004 |
| Service | ||||||
| Medical | 0.59 (0.50.69) | <0.001 | ||||
| Surgical | Ref | |||||
In those with at least 1 ICU attending encounter (see Supporting Table 1 in the online version of this article), no variables demonstrated significant association with top decile RSRs in the overall group or in the medical subgroup. For surgical patients with at least 1 ICU attending encounter (see Supporting Table 1 in the online version of this article), patients aged 30 to 49 and 50 to 69 years were more likely to provide top decile RSRs (OR: 1.93, 95% CI: 1.08‐3.46 and OR: 1.65, 95% CI 1.07‐2.53, respectively). Resource intensity was not significantly associated with top decile RSRs.
DISCUSSION
Our analysis suggests that, for patients on the general care floors, resource utilization is associated with the RSR and, therefore, potentially the CMS Summary Star Rating. Adjusting for severity of illness, patients with higher resource utilization were more likely to report top decile RSRs.
Prior data regarding utilization and satisfaction are mixed. In a 2‐year, prospective, national examination, patients in the highest quartile of patient satisfaction had increased healthcare and prescription drug expenditures as well as increased rates of hospitalization when compared with patients in the lowest quartile of patient satisfaction.[9] However, a recent national study of surgical administrative databases suggested hospitals with high patient satisfaction provided more efficient care.[13]
One reason for the conflicting data may be that large, national evaluations are unable to control for between‐hospital confounders (ie, hospital quality of care). By capturing all eligible returned surveys at 1 institution, our design allowed us to collect granular data. We found that in 1 hospital setting, patient population, facilities, and food services, patients receiving more clinical resources generally assigned higher ratings than patients receiving less.
It is possible that utilization is a proxy for serious illness, and that patients with serious illness receive more attention during hospitalization and are more satisfied when discharged in a good state of health. However, we did adjust for severity of illness in our model using the Charlson‐Deyo index and we suggest that, other factors being equal, hospitals with higher per‐patient expenditures may be assigned higher Summary Star Ratings.
CMS has recently implemented a number of metrics designed to decrease healthcare costs by improving quality, safety, and efficiency. Concurrently, CMS has also prioritized patient experience. The Summary Star Rating was created to provide healthcare consumers with an easy way to compare the patient experience between hospitals[4]; however, our data suggest that this metric may be at odds with inpatient cost savings and efficiency metrics.
Per‐patient spending becomes particularly salient when considering that in fiscal year 2016, CMS' hospital VBP reimbursement will include 2 metrics: an efficiency outcome measure labeled Medicare spending per beneficiary, and a patient experience outcome measure based on HCAHPS survey dimensions.[2] Together, these 2 metrics will comprise nearly half of the total VBP performance score used to determine reimbursement. Although our data suggest that these 2 VBP metrics may be correlated, it should be noted that we measured inpatient hospital charges, whereas the CMS efficiency outcome measure includes costs for episode of care spanning 3 days prior to hospitalization to 30 days after hospitalization.
Patient expectations likely play a role in satisfaction.[14, 15, 16] In an outpatient setting, physician fulfillment of patient requests has been associated with positive patient evaluations of care.[17] However, patients appear to value education, shared decision making, and provider empathy more than testing and intervention.[14, 18, 19, 20, 21, 22, 23] Perhaps, in the absence of the former attributes, patients use additional resource expenditure as a proxy.
It is not clear that higher resource expenditure improves outcomes. A landmark study of nearly 1 million Medicare enrollees by Fisher et al. suggests that, although Medicare patients in higher‐spending regions receive more care than those in lower‐spending regions, this does not result in better health outcomes, specifically with regard to mortality.[24, 25] Patients who live in areas of high hospital capacity use the hospital more frequently than do patients in areas of low hospital capacity, but this does not appear to result in improved mortality rates.[26] In fact, physicians in areas of high healthcare capacity report more difficulty maintaining high‐quality patient relationships and feel less able to provide high‐quality care than physicians in lower‐capacity areas.[27]
We hypothesize the cause of the association between resource utilization and patient satisfaction could be that patients (1) perceive that a doctor who allows them to stay longer in the hospital or who performs additional testing cares more about their well‐being and (2) that these patients feel more strongly that their concerns are being heard and addressed by their physicians. A systematic review of primary care patients identified many studies that found a positive association between meeting patient expectations and satisfaction with care, but also suggested that although patients frequently expect information, physicians misperceive this as an expectation of specific action.[28] A separate systematic review found that patient education in the form of decision aides can help patients develop more reasonable expectations and reduce utilization of certain discretionary procedures such as elective surgeries and prostate‐specific antigen testing.[29]
We did not specifically address clinical outcomes in our analysis because the clinical outcomes on which CMS currently adjusts VBP reimbursement focus on 30‐day mortality for specific diagnoses, nosocomial infections, and iatrogenic events.[30] Our data include only returned surveys from living patients, and it is likely that 30‐day mortality was similar throughout all subsets of patients. Additionally, the nosocomial and iatrogenic outcome measures used by CMS are sufficiently rare on the general floors and are unlikely to have significantly influenced our results.[31]
Our study has several strengths. Nearly all medical and surgical patient surveys returned during the study period were included, and therefore our calculations are likely to accurately reflect the Summary Star Rating that would have been assigned for the period. Second, the large sample size helps attenuate potential differences in commonly used outcome metrics. Third, by adjusting for a variety of demographic and clinical variables, we were able to decrease the likelihood of unidentified confounders.
Notably, we identified 38 (0.4%) surveys returned for patients under 18 years of age at admission. These surveys were included in our analysis because, to the best of our knowledge, they would have existed in the pool of surveys CMS could have used to assign a Summary Star Rating.
Our study also has limitations. First, geographically diverse data are needed to ensure generalizability. Second, we used the Charlson‐Deyo Comorbidity Index to describe the degree of illness for each patient. This index represents a patient's total illness burden but may not describe the relative severity of the patient's current illness relative to another patient. Third, we selected variables we felt were most likely to be associated with patient experience, but unidentified confounding remains possible. Fourth, attendings caring for ICU patients fall within the Division of Critical Care/Pulmonary Medicine. Therefore, we may have inadvertently placed patients into the ICU cohort who received a pulmonary/critical care consult on the general floors. Fifth, our data describe associations only for patients who returned surveys. Although there may be inherent biases in patients who return surveys, HCAHPS survey responses are used by CMS to determine a hospital's overall satisfaction score.
CONCLUSION
For patients who return HCAHPS surveys, resource utilization may be positively associated with a hospital's Summary Star Rating. These data suggest that hospitals with higher per‐patient expenditures may receive higher Summary Star Ratings, which could result in hospitals with higher per‐patient resource utilization appearing more attractive to healthcare consumers. Future studies should attempt to confirm our findings at other institutions and to determine causative factors.
Acknowledgements
The authors thank Jason Machan, PhD (Department of Orthopedics and Surgery, Warren Alpert Medical School, Brown University, Providence, Rhode Island) for his help with study design, and Ms. Brenda Foster (data analyst, University of Rochester Medical Center, Rochester, NY) for her help with data collection.
Disclosures: Nothing to report.
The patient experience has become increasingly important to healthcare in the United States. It is now a metric used commonly to determine physician compensation and accounts for nearly 30% of the Centers for Medicare and Medicaid Services' (CMS) Value‐Based Purchasing (VBP) reimbursement for fiscal years 2015 and 2016.[1, 2]
In April 2015, CMS added a 5‐star patient experience score to its Hospital Compare website in an attempt to address the Affordable Care Act's call for transparent and easily understandable public reporting.[3] A hospital's principal score is the Summary Star Rating, which is based on responses to the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey. The formulas used to calculate Summary Star Ratings have been reported by CMS.[4]
Studies published over the past decade suggest that gender, age, education level, length of hospital stay, travel distance, and other factors may influence patient satisfaction.[5, 6, 7, 8] One study utilizing a national dataset suggested that higher patient satisfaction was associated with greater inpatient healthcare utilization and higher healthcare expenditures.[9] It is therefore possible that emphasizing patient experience scores could adversely impact healthcare resource utilization. However, positive patient experience may also be an important independent dimension of quality for patients and correlate with improved clinical outcomes.[10]
We know of no literature describing patient factors associated with the Summary Star Rating. Given that this rating is now used as a standard metric by which patient experience can be compared across more than 3,500 hospitals,[11] data describing the association between patient‐level factors and the Summary Star Rating may provide hospitals with an opportunity to target improvement efforts. We aimed to determine the degree to which resource utilization is associated with a satisfaction score based on the Summary Star Rating methodology.
METHODS
The study was conducted at the University of Rochester Medical Center (URMC), an 830‐bed tertiary care center in upstate New York. This was a retrospective review of all HCAHPS surveys returned to URMC over a 27‐month period from January 1, 2012 to April 1, 2014. URMC follows the standard CMS process for determining which patients receive surveys as follows. During the study timeframe, HCAHPS surveys were mailed to patients 18 years of age and older who had an inpatient stay spanning at least 1 midnight. Surveys were mailed within 5 days of discharge, and were generally returned within 6 weeks. URMC did not utilize telephone or email surveys during the study period. Surveys were not sent to patients who (1) were transferred to another facility, (2) were discharged to hospice, (3) died during the hospitalization, (4) received psychiatric or rehabilitative services during the hospitalization, (5) had an international address, and/or (6) were prisoners.
The survey vendor (Press Ganey, South Bend, IN) for URMC provided raw data for returned surveys with patient answers to questions. Administrative and billing databases were used to add demographic and clinical data for the corresponding hospitalization to the dataset. These data included age, gender, payer status (public, private, self, charity), length of stay, number of attendings who saw the patient (based on encounters documented in the electronic medical record (EMR)), all discharge International Classification of Diseases, 9th Revision (ICD‐9) diagnoses for the hospitalization, total charges for the hospitalization, and intensive care unit (ICU) utilization as evidenced by a documented encounter with a member of the Division of Critical Care/Pulmonary Medicine.
CMS analyzes surveys within 1 of 3 clinical service categories (medical, surgical, or obstetrics/gynecology) based on the discharging service. To parallel this approach, each returned survey was placed into 1 of these categories based on the clinical service of the discharging physician. Patients placed in the obstetrics/gynecology category (n = 1317, 13%) will be analyzed in a future analysis given inherent differences in patient characteristics that require evaluation of other variables.
Approximations of CMS Summary Star Rating
The HCAHPS survey is a multiple‐choice questionnaire that includes several domains of patient satisfaction. Respondents are asked to rate areas of satisfaction with their hospital experience on a Likert scale. CMS uses a weighted average of Likert responses to a subset of HCAHPS questions to calculate a hospital's raw score in 11 domains, as well as an overall raw summary score. CMS then adjusts each raw score for differences between hospitals (eg, clustering, improvement over time, method of survey) to determine a hospital's star rating in each domain and an overall Summary Star Rating (the Summary Star Rating is the primary factor by which consumers can compare hospitals).[4] Because our data were from a single hospital system, the between‐hospital scoring adjustments utilized by CMS were not applicable. Instead, we calculated the raw scores exactly as CMS does prior to the adjustments. Thus, our scores reflect the scores that CMS would have given URMC during the study period prior to standardized adjustments; we refer to this as the raw satisfaction rating (RSR). We calculated an RSR for every eligible survey. The RSR was calculated as a continuous variable from 0 (lowest) to 1 (highest). Detailed explanation of our RSR calculation is available in the Supporting Information in the online version of this article.
Statistical Analysis
All analyses were performed in aggregate and by service (medical vs surgical). Categorical variables were summarized using frequencies with percentages. Comparisons across levels of categorical variables were performed with the 2 test. We report bivariate associations between the independent variables and RSRs in the top decile using unadjusted odds ratios (ORs) with 95% confidence intervals (CIs). Similarly, multivariable logistic regression was used for adjusted analyses. For the variables of severity of illness and resource intensity, the group with the lowest illness severity and lowest resource use served as the reference groups. We modeled patients without an ICU encounter and with an ICU encounter separately.
Charges, number of unique attendings encountered, and lengths of stay were highly correlated, and likely various measures of the same underlying construct of resource intensity, and therefore could not be entered into our models simultaneously. We combined these into a resource intensity score using factor analysis with a varimax rotation, and extracted factor scores for a single factor (supported by a scree plot). We then placed patients into 4 groups based on the distribution of the factor scores: low (<25th percentile), moderate (25th50th percentile), major (50th75th percentile), and extreme (>75th percentile).
We used the Charlson‐Deyo comorbidity score as our disease severity index.[12] The index uses ICD‐9 diagnoses with points assigned for the impact of each diagnosis on morbidity and the points summed to an overall score. This provides a measure of disease severity for a patient based on the number of diagnoses and relative mortality of the individual diagnoses. Scores were categorized as 0 (representing no major illness burden), 1 to 3, 4 to 6, and >6.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), and P values <0.05 were considered statistically significant. This study was approved by the institutional review board at the University of Rochester Medical Center.
RESULTS
Our initial search identified 10,007 returned surveys (29% of eligible patients returned surveys during the study period). Of these, 5059 (51%) were categorized as medical, 3630 (36%) as surgical, and 1317 (13%) as obstetrics/gynecology. One survey did not have the service of the discharging physician recorded and was excluded. Cohort demographics and relationship to RSRs in the top decile for the 8689 medical and surgical patients can be found in Table 1. The most common discharge diagnosis‐related groups (DRGs) for medical patients were 247, percutaneous cardiovascular procedure with drug‐eluding stent without major complications or comorbidities (MCC) (3.8%); 871, septicemia or severe sepsis without mechanical ventilation >96 hours with MCC (2.7%); and 392, esophagitis, gastroenteritis, and miscellaneous digestive disorders with MCC (2.3%). The most common DRGs for surgical patients were 460, spinal fusion except cervical without MCC (3.5%); 328, stomach, esophageal and duodenal procedure without complication or comorbidities or MCC (3.3%); and 491, back and neck procedure excluding spinal fusion without complication or comorbidities or MCC (3.1%).
| Overall | Medical | Surgical | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | <90th | Top Decile | P | Total | <90th | Top Decile | P | Total | <90th | Top Decile | P | |
| ||||||||||||
| Overall | 8,689 | 7,789 (90) | 900 (10) | 5,059 | 4,646 (92) | 413 (8) | 3,630 | 3,143 (87) | 487 (13) | |||
| Age, y | ||||||||||||
| <30 | 419 (5) | 371 (89) | 48 (12) | <0.001 | 218 (4) | 208 (95) | 10 (5) | <0.001 | 201 (6) | 163 (81) | 38 (19) | <0.001 |
| 3049 | 1,029 (12) | 902 (88) | 127 (12) | 533 (11) | 482 (90) | 51 (10) | 496 (14) | 420 (85) | 76 (15) | |||
| 5069 | 3,911 (45) | 3,450 (88) | 461 (12) | 2,136 (42) | 1,930 (90) | 206 (10) | 1,775 (49) | 1,520 (86) | 255 (14) | |||
| >69 | 3,330 (38) | 3,066 (92) | 264 (8) | 2,172 (43) | 2,026 (93) | 146 (7) | 1,158 (32) | 1,040 (90) | 118 (10) | |||
| Gender | ||||||||||||
| Male | 4,640 (53) | 4,142 (89) | 498 (11) | 0.220 | 2,596 (51) | 2,379 (92) | 217 (8) | 0.602 | 2,044 (56) | 1,763 (86) | 281 (14) | 0.506 |
| Female | 4,049 (47) | 3,647 (90) | 402 (10) | 2,463 (49) | 2,267 (92) | 196 (8) | 1,586 (44) | 1,380 (87) | 206 (13) | |||
| ICU encounter | ||||||||||||
| No | 7,122 (82) | 6,441 (90) | 681 (10) | <0.001 | 4,547 (90) | 4,193 (92) | 354 (8) | <0.001 | 2,575 (71) | 2,248 (87) | 327 (13) | 0.048 |
| Yes | 1,567 (18) | 1,348 (86) | 219 (14) | 512 (10) | 453 (89) | 59 (12) | 1,055 (29) | 895 (85) | 160 (15) | |||
| Payer | ||||||||||||
| Public | 5,564 (64) | 5,036 (91) | 528 (10) | <0.001 | 3,424 (68) | 3,161 (92) | 263 (8) | 0.163 | 2,140 (59) | 1,875 (88) | 265 (12) | 0.148 |
| Private | 3,064 (35) | 2,702 (88) | 362 (12) | 1,603 (32) | 1,458 (91) | 145 (9) | 1,461 (40) | 1,244 (85) | 217 (15) | |||
| Charity | 45 (1) | 37 (82) | 8 (18) | 25 (1) | 21 (84) | 4 (16) | 20 (1) | 16 (80) | 4 (20) | |||
| Self | 16 (0) | 14 (88) | 2 (13) | 7 (0) | 6 (86) | 1 (14) | 9 (0) | 8 (89) | 1 (11) | |||
| Length of stay, d | ||||||||||||
| <3 | 3,156 (36) | 2,930 (93) | 226 (7) | <0.001 | 1,961 (39) | 1,865 (95) | 96 (5) | <0.001 | 1,195 (33) | 1,065 (89) | 130 (11) | <0.001 |
| 36 | 3,330 (38) | 2,959 (89) | 371 (11) | 1,867 (37) | 1,702 (91) | 165 (9) | 1,463 (40) | 1,257 (86) | 206 (14) | |||
| >6 | 2,203 (25) | 1,900 (86) | 303 (14) | 1,231 (24) | 1,079 (88) | 152 (12) | 972 (27) | 821 (85) | 151 (16) | |||
| No. of attendings | ||||||||||||
| <4 | 3,959 (46) | 3,615 (91) | 344 (9) | <0.001 | 2,307 (46) | 2,160 (94) | 147 (6) | <0.001 | 1,652 (46) | 1,455 (88) | 197 (12) | 0.052 |
| 46 | 3,067 (35) | 2,711 (88) | 356 (12) | 1,836 (36) | 1,663 (91) | 173 (9) | 1,231 (34) | 1,048 (85) | 183 (15) | |||
| >6 | 1,663 (19) | 1,463 (88) | 200 (12) | 916 (18) | 823 (90) | 93 (10) | 747 (21) | 640 (86) | 107 (14) | |||
| Severity index* | ||||||||||||
| 0 (lowest) | 2,812 (32) | 2,505 (89) | 307 (11) | 0.272 | 1,273 (25) | 1,185 (93) | 88 (7) | 0.045 | 1,539 (42) | 1,320 (86) | 219 (14) | 0.261 |
| 13 | 4,253 (49) | 3,827 (90) | 426 (10) | 2,604 (52) | 2,395 (92) | 209 (8) | 1,649 (45) | 1,432 (87) | 217 (13) | |||
| 46 | 1163 (13) | 1,052 (91) | 111 (10) | 849 (17) | 770 (91) | 79 (9) | 314 (9) | 282 (90) | 32 (10) | |||
| >6 (highest) | 461 (5) | 405 (88) | 56 (12) | 333 (7) | 296 (89) | 37 (11) | 128 (4) | 109 (85) | 19 (15) | |||
| Charges, | ||||||||||||
| Low | 1,820 (21) | 1,707 (94) | 113 (6) | <0.001 | 1,426 (28) | 1,357 (95) | 69 (5) | <0.001 | 394 (11) | 350 (89) | 44 (11) | 0.007 |
| Medium | 5,094 (59) | 4,581 (90) | 513 (10) | 2,807 (56) | 2,582 (92) | 225 (8) | 2,287 (63) | 1,999 (87) | 288 (13) | |||
| High | 1,775 (20) | 1,501 (85) | 274 (15) | 826 (16) | 707 (86) | 119 (14) | 949 (26) | 794 (84) | 155 (16) | |||
Unadjusted analysis of medical and surgical patients identified significant associations of several variables with a top decile RSR (Table 2). Patients with longer lengths of stay (OR: 2.07, 95% CI: 1.72‐2.48), more attendings (OR: 1.44, 95% CI: 1.19‐1.73), and higher hospital charges (OR: 2.76, 95% CI: 2.19‐3.47) were more likely to report an RSR in the top decile. Patients without an ICU encounter (OR: 0.65, 95% CI: 0.55‐0.77) and on a medical service (OR: 0.57, 95% CI: 0.5‐ 0.66) were less likely to report an RSR in the top decile. Several associations were identified in only the medical or surgical cohorts. In the medical cohort, patients with the highest illness severity index (OR: 1.68, 95% CI: 1.12‐ 2.52) and with 7 different attending physicians (OR: 1.66, 95% CI: 1.27‐2.18) were more likely to report RSRs in the top decile. In the surgical cohort, patients <30 years of age (OR: 2.05, 95% CI 1.38‐3.07) were more likely to report an RSR in the top decile than patients >69 years of age. Insurance payer category and gender were not significantly associated with top decile RSRs.
| Overall | Medical | Surgical | ||||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | |
| ||||||
| Age, y | ||||||
| <30 | 1.5 (1.082.08) | 0.014 | 0.67 (0.351.29) | 0.227 | 2.05 (1.383.07) | <0.001 |
| 3049 | 1.64 (1.312.05) | <.001 | 1.47 (1.052.05) | 0.024 | 1.59 (1.172.17) | 0.003 |
| 5069 | 1.55 (1.321.82) | <.001 | 1.48 (1.191.85) | 0.001 | 1.48 (1.171.86) | 0.001 |
| >69 | Ref | Ref | Ref | |||
| Gender | ||||||
| Male | 1.09 (0.951.25) | 0.220 | 1.06 (0.861.29) | 0.602 | 1.07 (0.881.3) | 0.506 |
| Female | Ref | Ref | Ref | |||
| ICU encounter | ||||||
| No | 0.65 (0.550.77) | <0.001 | 0.65 (0.480.87) | 0.004 | 0.81 (0.661) | 0.048 |
| Yes | Ref | Ref | Ref | |||
| Payer | ||||||
| Public | 0.73 (0.173.24) | 0.683 | 0.5 (0.064.16) | 0.521 | 1.13 (0.149.08) | 0.908 |
| Private | 0.94 (0.214.14) | 0.933 | 0.6 (0.074.99) | 0.634 | 1.4 (0.1711.21) | 0.754 |
| Charity | 1.51 (0.298.02) | 0.626 | 1.14 (0.1112.25) | 0.912 | 2 (0.1920.97) | 0.563 |
| Self | Ref | Ref | Ref | |||
| Length of stay, d | ||||||
| <3 | Ref | Ref | Ref | |||
| 36 | 1.63 (1.371.93) | <0.001 | 1.88 (1.452.44) | <0.001 | 1.34 (1.061.7) | 0.014 |
| >6 | 2.07 (1.722.48) | <0.001 | 2.74 (2.13.57) | <0.001 | 1.51 (1.171.94) | 0.001 |
| No. of attendings | ||||||
| <4 | Ref | Ref | Ref | |||
| 46 | 1.38 (1.181.61) | <0.001 | 1.53 (1.221.92) | <0.001 | 1.29 (1.041.6) | 0.021 |
| >6 | 1.44 (1.191.73) | <0.001 | 1.66 (1.272.18) | <0.001 | 1.23 (0.961.59) | 0.102 |
| Severity index* | ||||||
| 0 (lowest) | Ref | Ref | Ref | |||
| 13 | 0.91 (0.781.06) | 0.224 | 1.18 (0.911.52) | 0.221 | 0.91 (0.751.12) | 0.380 |
| 46 | 0.86 (0.681.08) | 0.200 | 1.38 (1.011.9) | 0.046 | 0.68 (0.461.01) | 0.058 |
| >6 (highest) | 1.13 (0.831.53) | 0.436 | 1.68 (1.122.52) | 0.012 | 1.05 (0.631.75) | 0.849 |
| Charges | ||||||
| Low | Ref | Ref | Ref | |||
| Medium | 1.69 (1.372.09) | <0.001 | 1.71 (1.32.26) | <0.001 | 1.15 (0.821.61) | 0.428 |
| High | 2.76 (2.193.47) | <0.001 | 3.31 (2.434.51) | <0.001 | 1.55 (1.092.22) | 0.016 |
| Service | ||||||
| Medical | 0.57 (0.50.66) | <0.001 | ||||
| Surgical | Ref | |||||
Multivariable modeling (Table 3) for all patients without an ICU encounter suggested that (1) patients aged <30 years, 30 to 49 years, and 50 to 69 years were more likely to report top decile RSRs when compared to patients 70 years and older (OR: 1.61, 95% CI: 1.09‐2.36; OR: 1.44, 95% CI: 1.08‐1.93; and OR: 1.39, 95% CI: 1.13‐1.71, respectively) and (2), when compared to patients with extreme resource intensity scores, patients with higher resource intensity scores were more likely to report top decile RSRs (moderate [OR: 1.42, 95% CI: 1.11‐1.83], major [OR: 1.56, 95% CI: 1.22‐2.01], and extreme [OR: 2.29, 95% CI: 1.8‐2.92]. These results were relatively consistent within medical and surgical subgroups (Table 3).
| Overall | Medical | Surgical | ||||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | |
| ||||||
| Age, y | ||||||
| <30 | 1.61 (1.092.36) | 0.016 | 0.82 (0.41.7) | 0.596 | 2.31 (1.393.82) | 0.001 |
| 3049 | 1.44 (1.081.93) | 0.014 | 1.55 (1.032.32) | 0.034 | 1.41 (0.912.17) | 0.120 |
| 5069 | 1.39 (1.131.71) | 0.002 | 1.44 (1.11.88) | 0.008 | 1.39 (11.93) | 0.049 |
| >69 | Ref | Ref | Ref | |||
| Sex | ||||||
| Male | 1 (0.851.17) | 0.964 | 1 (0.81.25) | 0.975 | 0.99 (0.791.26) | 0.965 |
| Female | Ref | Ref | Ref | |||
| Payer | ||||||
| Public | 0.62 (0.142.8) | 0.531 | 0.42 (0.053.67) | 0.432 | 1.03 (0.128.59) | 0.978 |
| Private | 0.67 (0.153.02) | 0.599 | 0.42 (0.053.67) | 0.434 | 1.17 (0.149.69) | 0.884 |
| Charity | 1.54 (0.288.41) | 0.620 | 1 (0.0911.13) | 0.999 | 2.56 (0.2328.25) | 0.444 |
| Self | Ref | Ref | Ref | |||
| Severity index | ||||||
| 0 (lowest) | Ref | Ref | Ref | |||
| 13 | 1.07 (0.891.29) | 0.485 | 1.18 (0.881.58) | 0.267 | 1 (0.781.29) | 0.986 |
| 46 | 1.14 (0.861.51) | 0.377 | 1.42 (0.992.04) | 0.056 | 0.6 (0.331.1) | 0.100 |
| >6 (highest) | 1.31 (0.911.9) | 0.150 | 1.47 (0.932.33) | 0.097 | 1.1 (0.542.21) | 0.795 |
| Resource intensity score | ||||||
| Low | Ref | Ref | Ref | |||
| Moderate | 1.42 (1.111.83) | 0.006 | 1.6 (1.112.3) | 0.011 | 0.94 (0.661.34) | 0.722 |
| Major | 1.56 (1.222.01) | 0.001 | 1.69 (1.182.43) | 0.004 | 1.28 (0.911.8) | 0.151 |
| Extreme | 2.29 (1.82.92) | <0.001 | 2.72 (1.943.82) | <0.001 | 1.63 (1.172.26) | 0.004 |
| Service | ||||||
| Medical | 0.59 (0.50.69) | <0.001 | ||||
| Surgical | Ref | |||||
In those with at least 1 ICU attending encounter (see Supporting Table 1 in the online version of this article), no variables demonstrated significant association with top decile RSRs in the overall group or in the medical subgroup. For surgical patients with at least 1 ICU attending encounter (see Supporting Table 1 in the online version of this article), patients aged 30 to 49 and 50 to 69 years were more likely to provide top decile RSRs (OR: 1.93, 95% CI: 1.08‐3.46 and OR: 1.65, 95% CI 1.07‐2.53, respectively). Resource intensity was not significantly associated with top decile RSRs.
DISCUSSION
Our analysis suggests that, for patients on the general care floors, resource utilization is associated with the RSR and, therefore, potentially the CMS Summary Star Rating. Adjusting for severity of illness, patients with higher resource utilization were more likely to report top decile RSRs.
Prior data regarding utilization and satisfaction are mixed. In a 2‐year, prospective, national examination, patients in the highest quartile of patient satisfaction had increased healthcare and prescription drug expenditures as well as increased rates of hospitalization when compared with patients in the lowest quartile of patient satisfaction.[9] However, a recent national study of surgical administrative databases suggested hospitals with high patient satisfaction provided more efficient care.[13]
One reason for the conflicting data may be that large, national evaluations are unable to control for between‐hospital confounders (ie, hospital quality of care). By capturing all eligible returned surveys at 1 institution, our design allowed us to collect granular data. We found that in 1 hospital setting, patient population, facilities, and food services, patients receiving more clinical resources generally assigned higher ratings than patients receiving less.
It is possible that utilization is a proxy for serious illness, and that patients with serious illness receive more attention during hospitalization and are more satisfied when discharged in a good state of health. However, we did adjust for severity of illness in our model using the Charlson‐Deyo index and we suggest that, other factors being equal, hospitals with higher per‐patient expenditures may be assigned higher Summary Star Ratings.
CMS has recently implemented a number of metrics designed to decrease healthcare costs by improving quality, safety, and efficiency. Concurrently, CMS has also prioritized patient experience. The Summary Star Rating was created to provide healthcare consumers with an easy way to compare the patient experience between hospitals[4]; however, our data suggest that this metric may be at odds with inpatient cost savings and efficiency metrics.
Per‐patient spending becomes particularly salient when considering that in fiscal year 2016, CMS' hospital VBP reimbursement will include 2 metrics: an efficiency outcome measure labeled Medicare spending per beneficiary, and a patient experience outcome measure based on HCAHPS survey dimensions.[2] Together, these 2 metrics will comprise nearly half of the total VBP performance score used to determine reimbursement. Although our data suggest that these 2 VBP metrics may be correlated, it should be noted that we measured inpatient hospital charges, whereas the CMS efficiency outcome measure includes costs for episode of care spanning 3 days prior to hospitalization to 30 days after hospitalization.
Patient expectations likely play a role in satisfaction.[14, 15, 16] In an outpatient setting, physician fulfillment of patient requests has been associated with positive patient evaluations of care.[17] However, patients appear to value education, shared decision making, and provider empathy more than testing and intervention.[14, 18, 19, 20, 21, 22, 23] Perhaps, in the absence of the former attributes, patients use additional resource expenditure as a proxy.
It is not clear that higher resource expenditure improves outcomes. A landmark study of nearly 1 million Medicare enrollees by Fisher et al. suggests that, although Medicare patients in higher‐spending regions receive more care than those in lower‐spending regions, this does not result in better health outcomes, specifically with regard to mortality.[24, 25] Patients who live in areas of high hospital capacity use the hospital more frequently than do patients in areas of low hospital capacity, but this does not appear to result in improved mortality rates.[26] In fact, physicians in areas of high healthcare capacity report more difficulty maintaining high‐quality patient relationships and feel less able to provide high‐quality care than physicians in lower‐capacity areas.[27]
We hypothesize the cause of the association between resource utilization and patient satisfaction could be that patients (1) perceive that a doctor who allows them to stay longer in the hospital or who performs additional testing cares more about their well‐being and (2) that these patients feel more strongly that their concerns are being heard and addressed by their physicians. A systematic review of primary care patients identified many studies that found a positive association between meeting patient expectations and satisfaction with care, but also suggested that although patients frequently expect information, physicians misperceive this as an expectation of specific action.[28] A separate systematic review found that patient education in the form of decision aides can help patients develop more reasonable expectations and reduce utilization of certain discretionary procedures such as elective surgeries and prostate‐specific antigen testing.[29]
We did not specifically address clinical outcomes in our analysis because the clinical outcomes on which CMS currently adjusts VBP reimbursement focus on 30‐day mortality for specific diagnoses, nosocomial infections, and iatrogenic events.[30] Our data include only returned surveys from living patients, and it is likely that 30‐day mortality was similar throughout all subsets of patients. Additionally, the nosocomial and iatrogenic outcome measures used by CMS are sufficiently rare on the general floors and are unlikely to have significantly influenced our results.[31]
Our study has several strengths. Nearly all medical and surgical patient surveys returned during the study period were included, and therefore our calculations are likely to accurately reflect the Summary Star Rating that would have been assigned for the period. Second, the large sample size helps attenuate potential differences in commonly used outcome metrics. Third, by adjusting for a variety of demographic and clinical variables, we were able to decrease the likelihood of unidentified confounders.
Notably, we identified 38 (0.4%) surveys returned for patients under 18 years of age at admission. These surveys were included in our analysis because, to the best of our knowledge, they would have existed in the pool of surveys CMS could have used to assign a Summary Star Rating.
Our study also has limitations. First, geographically diverse data are needed to ensure generalizability. Second, we used the Charlson‐Deyo Comorbidity Index to describe the degree of illness for each patient. This index represents a patient's total illness burden but may not describe the relative severity of the patient's current illness relative to another patient. Third, we selected variables we felt were most likely to be associated with patient experience, but unidentified confounding remains possible. Fourth, attendings caring for ICU patients fall within the Division of Critical Care/Pulmonary Medicine. Therefore, we may have inadvertently placed patients into the ICU cohort who received a pulmonary/critical care consult on the general floors. Fifth, our data describe associations only for patients who returned surveys. Although there may be inherent biases in patients who return surveys, HCAHPS survey responses are used by CMS to determine a hospital's overall satisfaction score.
CONCLUSION
For patients who return HCAHPS surveys, resource utilization may be positively associated with a hospital's Summary Star Rating. These data suggest that hospitals with higher per‐patient expenditures may receive higher Summary Star Ratings, which could result in hospitals with higher per‐patient resource utilization appearing more attractive to healthcare consumers. Future studies should attempt to confirm our findings at other institutions and to determine causative factors.
Acknowledgements
The authors thank Jason Machan, PhD (Department of Orthopedics and Surgery, Warren Alpert Medical School, Brown University, Providence, Rhode Island) for his help with study design, and Ms. Brenda Foster (data analyst, University of Rochester Medical Center, Rochester, NY) for her help with data collection.
Disclosures: Nothing to report.
- , , . Redesigning physician compensation and improving ED performance. Healthc Financ Manage. 2011;65(6):114–117.
- QualityNet. Available at: https://www.qualitynet.org/dcs/ContentServer?c=Page97(13):1041–1048.
- , , , . Factors determining inpatient satisfaction with care. Soc Sci Med. 2002;54(4):493–504.
- , , , , . Patient satisfaction revisited: a multilevel approach. Soc Sci Med. 2009;69(1):68–75.
- , , , et al. Predictors of patient satisfaction with hospital health care. BMC Health Serv Res. 2006;6:102.
- , , , . The cost of satisfaction: a national study of patient satisfaction, health care utilization, expenditures, and mortality. Arch Intern Med. 2012;172(5):405–411.
- , , , , . Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41–48.
- Becker's Infection Control and Clinical Quality. Star Ratings go live on Hospital Compare: how many hospitals got 5 stars? Available at: http://www.beckershospitalreview.com/quality/star‐ratings‐go‐live‐on‐hospital‐compare‐how‐many‐hospitals‐got‐5‐stars.html. Published April 16, 2015. Accessed October 5, 2015.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- , , . Patient satisfaction and quality of surgical care in US hospitals. Ann Surg. 2015;261(1):2–8.
- , , , , . Should health care providers be accountable for patients' care experiences? J Gen Intern Med. 2015;30(2):253–256.
- , , , , . Unmet expectations for care and the patient‐physician relationship. J Gen Intern Med. 2002;17(11):817–824.
- , , , et al. Do unmet expectations for specific tests, referrals, and new medications reduce patients' satisfaction? J Gen Intern Med. 2004;19(11):1080–1087.
- , , , , , . Request fulfillment in office practice: antecedents and relationship to outcomes. Med Care. 2002;40(1):38–51.
- , , , et al. Factors associated with patient satisfaction with care among dermatological outpatients. Br J Dermatol. 2001;145(4):617–623.
- , , , . Patient expectations of emergency department care: phase II—a cross‐sectional survey. CJEM. 2006;8(3):148–157.
- , , , , . Patients' perspectives on ideal physician behaviors. Mayo Clin Proc. 2006;81(3):338–344.
- , . What do people want from their health care? A qualitative study. J Participat Med. 2015;18:e10.
- , , . Evaluations of care by adults following a denial of an advertisement‐related prescription drug request: the role of expectations, symptom severity, and physician communication style. Soc Sci Med. 2006;62(4):888–899.
- , , , , , . Getting to “no”: strategies primary care physicians use to deny patient requests. Arch Intern Med. 2010;170(4):381–388.
- , , , , , . The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003;138(4):273–287.
- , , , , , . The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med. 2003;138(4):288–298.
- , , , et al. Associations among hospital capacity, utilization, and mortality of US Medicare beneficiaries, controlling for sociodemographic factors. Health Serv Res. 2000;34(6):1351–1362.
- , , , . Regional variations in health care intensity and physician perceptions of quality of care. Ann Intern Med. 2006;144(9):641–649.
- , , . Visit‐specific expectations and patient‐centered outcomes: a literature review. Arch Fam Med. 2000;9(10):1148–1155.
- , , , et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431.
- Centers for Medicare and Medicaid Services. Hospital Compare. Outcome domain. Available at: https://www.medicare.gov/hospitalcompare/data/outcome‐domain.html. Accessed October 5, 2015.
- Centers for Disease Control and Prevention. 2013 national and state healthcare‐associated infections progress report. Available at: www.cdc.gov/hai/progress‐report/index.html. Accessed October 5, 2015.
- , , . Redesigning physician compensation and improving ED performance. Healthc Financ Manage. 2011;65(6):114–117.
- QualityNet. Available at: https://www.qualitynet.org/dcs/ContentServer?c=Page97(13):1041–1048.
- , , , . Factors determining inpatient satisfaction with care. Soc Sci Med. 2002;54(4):493–504.
- , , , , . Patient satisfaction revisited: a multilevel approach. Soc Sci Med. 2009;69(1):68–75.
- , , , et al. Predictors of patient satisfaction with hospital health care. BMC Health Serv Res. 2006;6:102.
- , , , . The cost of satisfaction: a national study of patient satisfaction, health care utilization, expenditures, and mortality. Arch Intern Med. 2012;172(5):405–411.
- , , , , . Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41–48.
- Becker's Infection Control and Clinical Quality. Star Ratings go live on Hospital Compare: how many hospitals got 5 stars? Available at: http://www.beckershospitalreview.com/quality/star‐ratings‐go‐live‐on‐hospital‐compare‐how‐many‐hospitals‐got‐5‐stars.html. Published April 16, 2015. Accessed October 5, 2015.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- , , . Patient satisfaction and quality of surgical care in US hospitals. Ann Surg. 2015;261(1):2–8.
- , , , , . Should health care providers be accountable for patients' care experiences? J Gen Intern Med. 2015;30(2):253–256.
- , , , , . Unmet expectations for care and the patient‐physician relationship. J Gen Intern Med. 2002;17(11):817–824.
- , , , et al. Do unmet expectations for specific tests, referrals, and new medications reduce patients' satisfaction? J Gen Intern Med. 2004;19(11):1080–1087.
- , , , , , . Request fulfillment in office practice: antecedents and relationship to outcomes. Med Care. 2002;40(1):38–51.
- , , , et al. Factors associated with patient satisfaction with care among dermatological outpatients. Br J Dermatol. 2001;145(4):617–623.
- , , , . Patient expectations of emergency department care: phase II—a cross‐sectional survey. CJEM. 2006;8(3):148–157.
- , , , , . Patients' perspectives on ideal physician behaviors. Mayo Clin Proc. 2006;81(3):338–344.
- , . What do people want from their health care? A qualitative study. J Participat Med. 2015;18:e10.
- , , . Evaluations of care by adults following a denial of an advertisement‐related prescription drug request: the role of expectations, symptom severity, and physician communication style. Soc Sci Med. 2006;62(4):888–899.
- , , , , , . Getting to “no”: strategies primary care physicians use to deny patient requests. Arch Intern Med. 2010;170(4):381–388.
- , , , , , . The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003;138(4):273–287.
- , , , , , . The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med. 2003;138(4):288–298.
- , , , et al. Associations among hospital capacity, utilization, and mortality of US Medicare beneficiaries, controlling for sociodemographic factors. Health Serv Res. 2000;34(6):1351–1362.
- , , , . Regional variations in health care intensity and physician perceptions of quality of care. Ann Intern Med. 2006;144(9):641–649.
- , , . Visit‐specific expectations and patient‐centered outcomes: a literature review. Arch Fam Med. 2000;9(10):1148–1155.
- , , , et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431.
- Centers for Medicare and Medicaid Services. Hospital Compare. Outcome domain. Available at: https://www.medicare.gov/hospitalcompare/data/outcome‐domain.html. Accessed October 5, 2015.
- Centers for Disease Control and Prevention. 2013 national and state healthcare‐associated infections progress report. Available at: www.cdc.gov/hai/progress‐report/index.html. Accessed October 5, 2015.
Skin Lesions in Patients Treated With Imatinib Mesylate: A 5-Year Prospective Study
Imatinib mesylate (IM) represents the first-line treatment of chronic myeloid leukemia (CML) and gastrointestinal stromal tumors (GISTs). Its pharmacological activity is related to a specific action on several tyrosine kinases in different tumors, including Bcr-Abl in CML, c-Kit (CD117) in GIST, and platelet-derived growth factor receptor in dermatofibrosarcoma protuberans.1,2
Imatinib mesylate has been shown to improve progression-free survival and overall survival2; however, it also has several side effects. Among the adverse effects (AEs), less than 10% are nonhematologic, such as nausea, vomiting, diarrhea, muscle cramps, and cutaneous reactions.3,4
We followed patients who were treated with IM for 5 years to identify AEs of therapy.
Methods
The aim of this prospective study was to identify and collect data regarding IM cutaneous side effects so that clinicians can detect AEs early and differentiate them from AEs caused by other medications. All patients were subjected to a median of 5 years’ follow-up. We included all the patients treated with IM and excluded patients who had a history of eczematous dermatitis, psoriasis, renal impairment, or dyshidrosis palmoplantar. Before starting IM, all patients presented for a dermatologic visit. They were subsequently evaluated every 3 months.
The incidence rate was defined as the ratio of patients with cutaneous side effects and the total patients treated with IM. Furthermore, we calculated the ratio between each class of patient with a specific cutaneous manifestation and the entire cohort of patients with cutaneous side effects related to IM.
When necessary, microbiological, serological, and histopathological analyses were performed.
Results
In 60 months, we followed 220 patients treated with IM. Among them, 55 (25%) developed cutaneous side effects (35 males; 20 females). The incidence rate of the patients with cutaneous side effects was 1:4. The median age of the entire cohort was 52.5 years. Fifty patients were being treated for CML and 5 for GISTs. All patients received IM at a dosage of 400 mg daily.
The following skin diseases were observed in patients treated with IM (Table): 19 patients with maculopapular rash with pruritus (no maculopapular rash without pruritus was detected), 7 patients with eczematous dermatitis such as stasis dermatitis and seborrheic dermatitis, 6 patients with onychodystrophy melanonychia (Figure 1), 5 patients with psoriasis, 5 patients with skin cancers including basal cell carcinoma (BCC)(Figure 2), 3 patients with periorbital edema (Figure 3), 3 patients with mycosis, 3 patients with dermatofibromas, 2 patients with dyshidrosis palmoplantar, 1 patient with pityriasis rosea–like eruption (Figure 4), and 1 patient with actinic keratoses on the face. No hypopigmentation or hyperpigmentation, excluding the individual case of melanonychia, was observed.
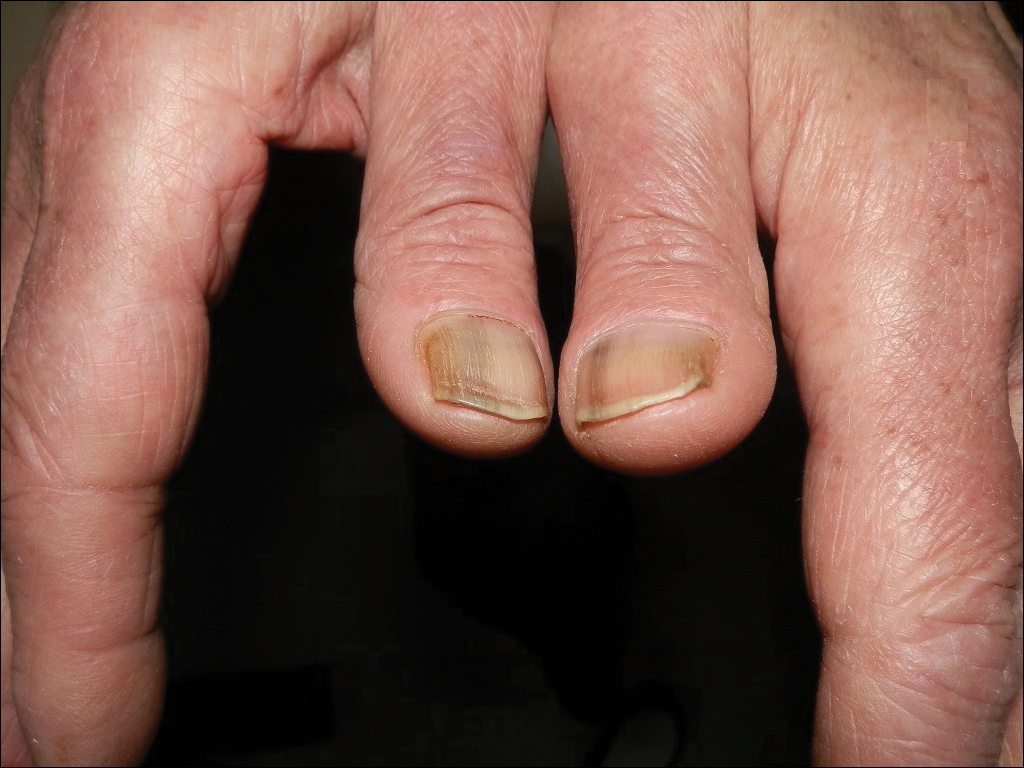
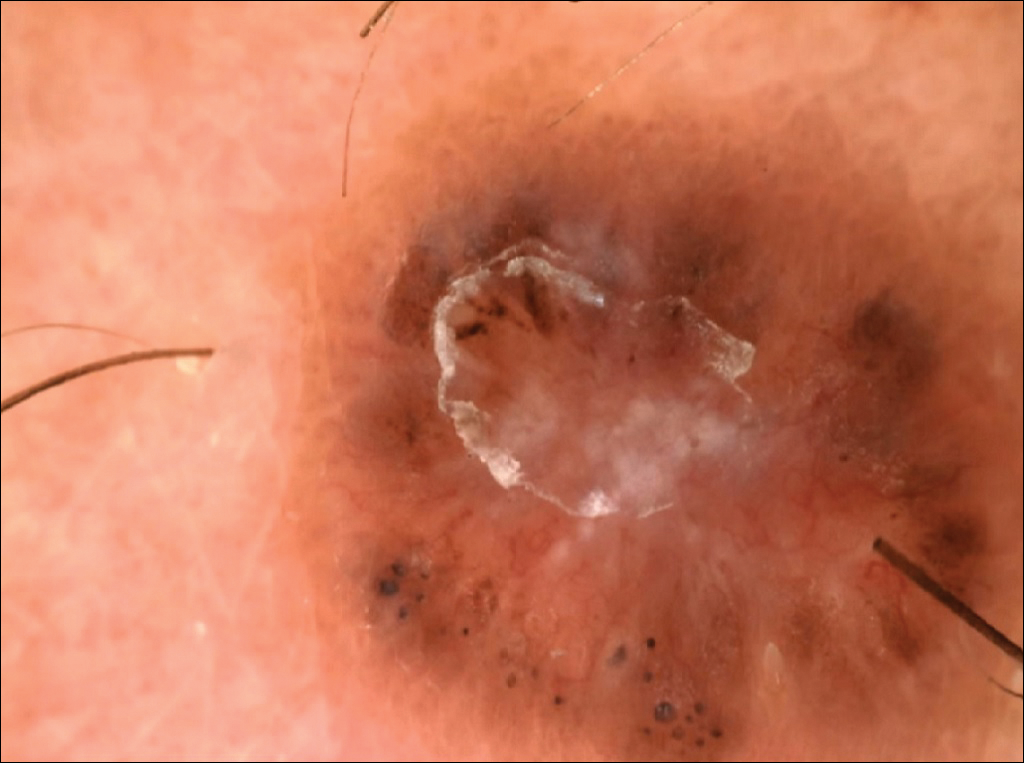
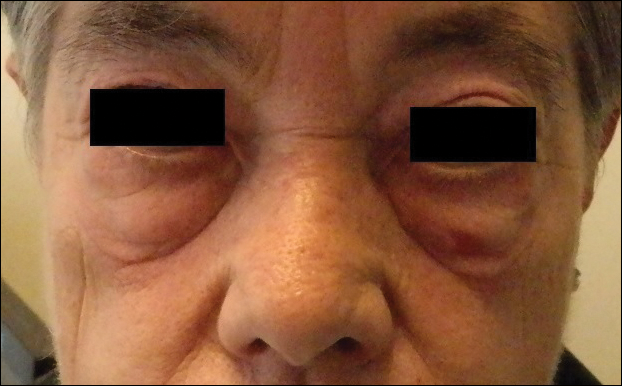
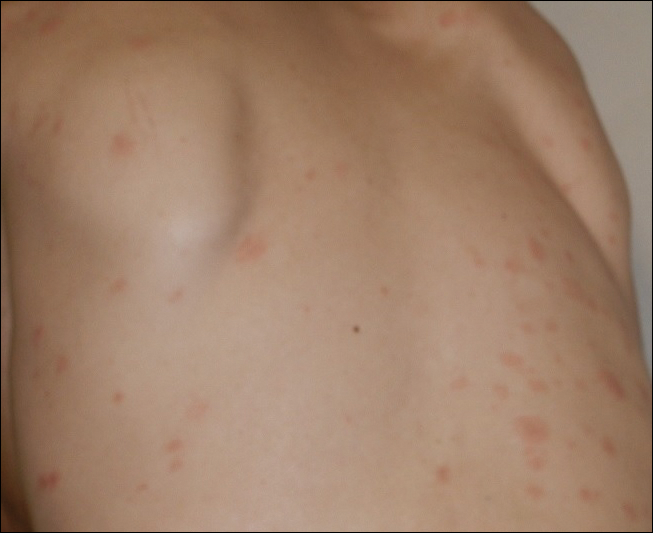
All cutaneous diseases reported in this study appeared after IM therapy (median, 3.8 months). The median time to onset for each cutaneous disorder is reported in the Table. During the first dermatologic visit before starting IM therapy, none of the patients showed any of these cutaneous diseases.
The adverse cutaneous reactions were treated with appropriate drugs. Generally, eczematous dermatitis was treated using topical steroids, emollients, and oral antihistamines. In patients with maculopapular rash with pruritus, oral corticosteroids (eg, betamethasone 3 mg daily or prednisolone 1 mg/kg) in association with antihistamine was necessary. Psoriasis was completely improved with topical betamethasone 0.5 mg and calcipotriol 50 µg. Skin cancers were treated with surgical excision with histologic examination. All treatments are outlined in the Table.
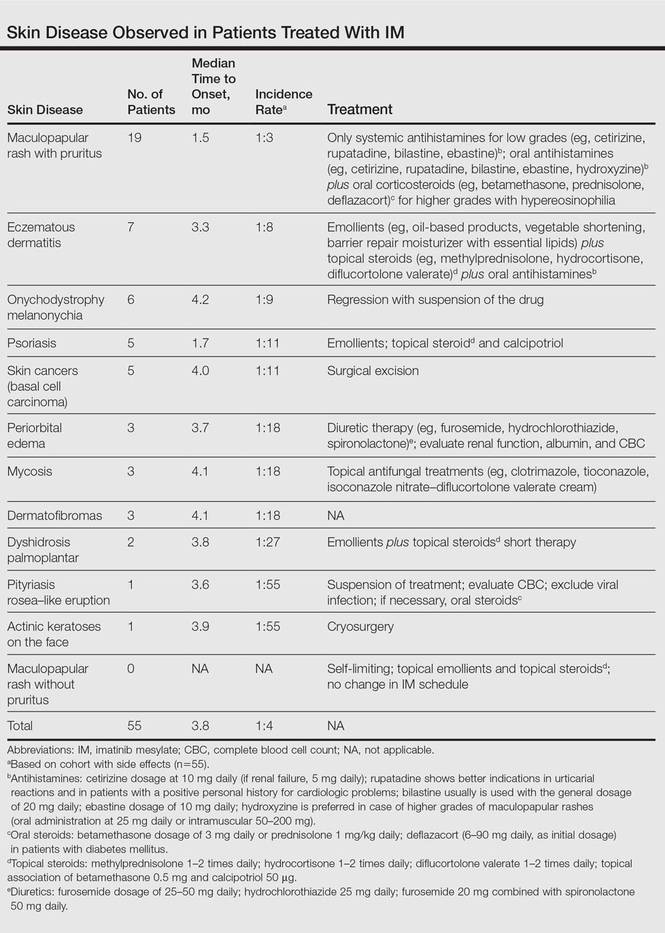
Imatinib mesylate therapy was suspended in 2 patients with maculopapular rash with moderate to severe pruritus; however, despite the temporary suspension of the drug and the appropriate therapies (corticosteroids and antihistamines), cutaneous side effects reappeared 7 to 10 days after therapy resumed. Therefore, the treatment was permanently suspended in these 2 cases and IM was replaced with erlotinib, a second-generation Bcr-Abl tyrosine kinase inhibitor.
Comment
The introduction of IM for the treatment of GIST and CML has changed the history of these diseases. The drug typically is well tolerated and few patients have reported severe AEs. Mild skin reactions are relatively frequent, ranging from 7% to 21% of patients treated.3 In our case, the percentage was relatively higher (25%), likely because of close monitoring of patients, with an increase in the incidence rate.
Imatinib mesylate cutaneous reactions are dose dependent.4 Indeed, in all our cases, the cutaneous reactions arose with an IM dosage of 400 mg daily, which is compatible with the definition of dose-independent cutaneous AEs.
The most common cutaneous AEs reported in the literature were swelling/edema and maculopapular rash. Swelling is the most common AE described during therapy with IM with an incidence of 63% to 84%.5 Swelling often involves the periorbital area and occurs approximately 6 weeks after starting IM. Although its pathogenesis is uncertain, it has been shown that IM blocks the platelet-derived growth factor receptor expressed on blood vessels that regulates the transportation transcapillary. The inhibition of this receptor can lead to increased pore pressure, resulting in edema and erythema. Maculopapular eruptions (50% of cases) often affect the trunk and the limbs and are accompanied by pruritus. Commonly, these rashes arise after 9 weeks of IM therapy. These eruptions are self-limiting and only topical emollients and steroids are required, without any change in IM schedule. To treat maculopapular eruptions with pruritus, oral steroids and antihistamines may be helpful, without suspending IM treatment. When grade 2 or 3 pruriginous maculopapular eruptions arise, the suspension of IM combined with steroids and antihistamines may be necessary. When the readministration of IM is required, it is mandatory to start IM at a lower dose (50–100 mg/d), administering prednisolone 0.5 to 1.0 mg/kg daily. Then, the steroid gradually can be tapered.6 Critical cutaneous AEs that are resistant to supportive measures warrant suspension of IM therapy. However, the incidence of this event is small (<1% of all patients).7
Regarding severe cutaneous AEs from IM therapy, Hsiao et al8 reported the case of Stevens-Johnson syndrome. In this case, IM was immediately stopped and systemic steroids were started. Rarely, erythroderma (grade 4 toxicity) can develop for which a prompt and perpetual suspension of IM is necessary and supportive care therapy with oral and topical steroids is recommended.9
Hyperpigmentation induced by IM, mostly in patients with Fitzpatrick skin types V to VI and with a general prevalence of 16% to 40% in treated patients, often is related to a mutation of c-Kit or other kinases that are activated rather than inhibited by the drug, resulting in overstimulation of melanogenesis.10 The prevalence of Fitzpatrick skin types I to III determined the absence of pigmentation changes in our cohort, excluding melanonychia. Hyperpigmentation was observed in the skin as well as the appendages such as nails, resulting in melanonychia (Figure 1). However, Brazzelli et al11 reported hypopigmentation in 5 white patients treated with IM; furthermore, they found a direct correlation between hypopigmentation and development of skin cancers in these patients. The susceptibility to develop skin cancers may persist, even without a clear manifestation of hypopigmentation, as reported in the current analysis. We documented BCC in 5 patients, 1 patient developed actinic keratoses, and 3 patients developed dermatofibromas. However, these neoplasms probably were not provoked by IM. On the contrary, we did not note squamous cell carcinoma, which was reported by Baskaynak et al12 in 2 CML patients treated with IM.
The administration of IM can be associated with exacerbation of psoriasis. Paradoxically, in genetically predisposed individuals, tumor necrosis factor α (TNF-α) antagonists, such as IM, seem to induce psoriasis, producing IFN-α rather than TNF-α and increasing inflammation.13 In fact, some research shows induction of psoriasis by anti–TNF-α drugs.14-16 Two cases of IM associated with psoriasis have been reported, and both cases represented an exacerbation of previously diagnosed psoriasis.13,17 On the contrary, in our analysis we reported 5 cases of psoriasis vulgaris induced by IM administration. Our patients developed cutaneous psoriatic lesions approximately 1.7 months after the start of IM therapy.
The pityriasis rosea–like eruption (Figure 4) presented as nonpruritic, erythematous, scaly patches on the trunk and extremities, and arose 3.6 months after the start of treatment. This particular cutaneous AE is rare. In 3 case reports, the IM dosage also was 400 mg daily.18-20 The pathophysiology of this rare skin reaction stems from the pharmacological effect of IM rather than a hypersensitivity reaction.18
Deininger et al7 reported that patients with a high basophil count (>20%) rarely show urticarial eruptions after IM due to histamine release from basophils. Premedication with an antihistamine was helpful and the urticarial eruption resolved after normalization in basophil count.7
Given the importance of IM for patients who have limited therapeutic alternatives for their disease and the ability to safely treat the cutaneous AEs, as demonstrated in our analysis, the suspension of IM for dermatological complications is necessary only in rare cases, as shown by the low number of patients (n=2) who had to discontinue therapy. The cutaneous AEs should be diagnosed and treated early with less impact on chemotherapy treatments. The administration of IM should involve a coordinated effort among oncologists and dermatologists to prevent important complications.
- Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
- Scheinfeld N. Imatinib mesylate and dermatology part 2: a review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol. 2006;5:228-231.
- Breccia M, Carmosimo I, Russo E, et al. Early and tardive skin adverse events in chronic myeloid leukaemia patients treated with imatinib. Eur J Haematol. 2005;74:121-123.
- Ugurel S, Hildebrand R, Dippel E, et al. Dose dependent severe cutaneous reactions to imatinib. Br J Cancer. 2003;88:1157-1159.
- Valeyrie L, Bastuji-Garin S, Revuz J, et al. Adverse cutaneous reactions to imatinib (STI571) in Philadelphia chromosome-positive leukaemias: a prospective study of 54 patients. J Am Acad Dermatol. 2003;48:201-206.
- Scott LC, White JD, Reid R, et al. Management of skin toxicity related to the use of imatinibnmesylate (STI571, GlivecTM) for advanced stage gastrointestinal stromal tumors. Sarcoma. 2005;9:157-160.
- Deininger MW, O’Brien SG, Ford JM, et al. Practical management of patients with chronic myeloid leukemia receiving imatinib. J Clin Oncol. 2003;21:1637-1647.
- Hsiao LT, Chung HM, Lin JT, et al. Stevens-Johnson syndrome after treatment with STI571: a case report. Br J Haematol. 2002;117:620-622.
- Sehgal VN, Srivastava G, Sardana K. Erythroderma/exfoliative dermatitis: a synopsis. Int J Dermatol. 2004;43:39-47.
- Pietras K, Pahler J, Bergers G, et al. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19.
- Brazzelli V, Prestinari F, Barbagallo T, et al. A long-term time course of colorimetric assessment of the effects of imatinib mesylate on skin pigmentation: a study of five patients. J Eur Acad Dermatol Venerol. 2007;21:384-387.
- Baskaynak G, Kreuzer KA, Schwarz M, et al. Squamous cutaneous epithelial cell carcinoma in two CML patients with progressive disease under imatinib treatment. Eur J Haematol. 2003;70:231-234.
- Cheng H, Geist DE, Piperdi M, et al. Management of imatinib-related exacerbation of psoriasis in a patient with a gastrointestinal stromal tumor. Australas J Dermatol. 2009;50:41-43.
- Faillace C, Duarte GV, Cunha RS, et al. Severe infliximab-induced psoriasis treated with adalimumab switching. Int J Dermatol. 2013;52:234-238.
- Iborra M, Beltrán B, Bastida G, et al. Infliximab and adalimumab-induced psoriasis in Crohn’s disease: a aradoxical side effect. J Crohns Colitis. 2011;5:157-161.
- Fernandes IC, Torres T, Sanches M, et al. Psoriasis induced by infliximab. Acta Med Port. 2011;24:709-712.
- Woo SM, Huh CH, Park KC, et al. Exacerbation of psoriasis in a chronic myelogenous leukemia patient treated with imatinib. J Dermatol. 2007;34:724-726.
- Brazzelli V, Prestinari F, Roveda E, et al. Pytiriasis rosea-like eruption during treatment with imatinib mesylate. description of 3 cases. J Am Acad Dermatol. 2005;53:240-243.
- Konstantapoulos K, Papadogianni A, Dimopoulou M, et al. Pytriasis rosea associated with imatinib (STI571, Gleevec). Dermatology. 2002;205:172-173.
- Cho AY, Kim DH, Im M, et al. Pityriasis rosealike drug eruption induced by imatinib mesylate (Gleevec). Ann Dermatol. 2011;23(suppl 3):360-363.
Imatinib mesylate (IM) represents the first-line treatment of chronic myeloid leukemia (CML) and gastrointestinal stromal tumors (GISTs). Its pharmacological activity is related to a specific action on several tyrosine kinases in different tumors, including Bcr-Abl in CML, c-Kit (CD117) in GIST, and platelet-derived growth factor receptor in dermatofibrosarcoma protuberans.1,2
Imatinib mesylate has been shown to improve progression-free survival and overall survival2; however, it also has several side effects. Among the adverse effects (AEs), less than 10% are nonhematologic, such as nausea, vomiting, diarrhea, muscle cramps, and cutaneous reactions.3,4
We followed patients who were treated with IM for 5 years to identify AEs of therapy.
Methods
The aim of this prospective study was to identify and collect data regarding IM cutaneous side effects so that clinicians can detect AEs early and differentiate them from AEs caused by other medications. All patients were subjected to a median of 5 years’ follow-up. We included all the patients treated with IM and excluded patients who had a history of eczematous dermatitis, psoriasis, renal impairment, or dyshidrosis palmoplantar. Before starting IM, all patients presented for a dermatologic visit. They were subsequently evaluated every 3 months.
The incidence rate was defined as the ratio of patients with cutaneous side effects and the total patients treated with IM. Furthermore, we calculated the ratio between each class of patient with a specific cutaneous manifestation and the entire cohort of patients with cutaneous side effects related to IM.
When necessary, microbiological, serological, and histopathological analyses were performed.
Results
In 60 months, we followed 220 patients treated with IM. Among them, 55 (25%) developed cutaneous side effects (35 males; 20 females). The incidence rate of the patients with cutaneous side effects was 1:4. The median age of the entire cohort was 52.5 years. Fifty patients were being treated for CML and 5 for GISTs. All patients received IM at a dosage of 400 mg daily.
The following skin diseases were observed in patients treated with IM (Table): 19 patients with maculopapular rash with pruritus (no maculopapular rash without pruritus was detected), 7 patients with eczematous dermatitis such as stasis dermatitis and seborrheic dermatitis, 6 patients with onychodystrophy melanonychia (Figure 1), 5 patients with psoriasis, 5 patients with skin cancers including basal cell carcinoma (BCC)(Figure 2), 3 patients with periorbital edema (Figure 3), 3 patients with mycosis, 3 patients with dermatofibromas, 2 patients with dyshidrosis palmoplantar, 1 patient with pityriasis rosea–like eruption (Figure 4), and 1 patient with actinic keratoses on the face. No hypopigmentation or hyperpigmentation, excluding the individual case of melanonychia, was observed.




All cutaneous diseases reported in this study appeared after IM therapy (median, 3.8 months). The median time to onset for each cutaneous disorder is reported in the Table. During the first dermatologic visit before starting IM therapy, none of the patients showed any of these cutaneous diseases.
The adverse cutaneous reactions were treated with appropriate drugs. Generally, eczematous dermatitis was treated using topical steroids, emollients, and oral antihistamines. In patients with maculopapular rash with pruritus, oral corticosteroids (eg, betamethasone 3 mg daily or prednisolone 1 mg/kg) in association with antihistamine was necessary. Psoriasis was completely improved with topical betamethasone 0.5 mg and calcipotriol 50 µg. Skin cancers were treated with surgical excision with histologic examination. All treatments are outlined in the Table.

Imatinib mesylate therapy was suspended in 2 patients with maculopapular rash with moderate to severe pruritus; however, despite the temporary suspension of the drug and the appropriate therapies (corticosteroids and antihistamines), cutaneous side effects reappeared 7 to 10 days after therapy resumed. Therefore, the treatment was permanently suspended in these 2 cases and IM was replaced with erlotinib, a second-generation Bcr-Abl tyrosine kinase inhibitor.
Comment
The introduction of IM for the treatment of GIST and CML has changed the history of these diseases. The drug typically is well tolerated and few patients have reported severe AEs. Mild skin reactions are relatively frequent, ranging from 7% to 21% of patients treated.3 In our case, the percentage was relatively higher (25%), likely because of close monitoring of patients, with an increase in the incidence rate.
Imatinib mesylate cutaneous reactions are dose dependent.4 Indeed, in all our cases, the cutaneous reactions arose with an IM dosage of 400 mg daily, which is compatible with the definition of dose-independent cutaneous AEs.
The most common cutaneous AEs reported in the literature were swelling/edema and maculopapular rash. Swelling is the most common AE described during therapy with IM with an incidence of 63% to 84%.5 Swelling often involves the periorbital area and occurs approximately 6 weeks after starting IM. Although its pathogenesis is uncertain, it has been shown that IM blocks the platelet-derived growth factor receptor expressed on blood vessels that regulates the transportation transcapillary. The inhibition of this receptor can lead to increased pore pressure, resulting in edema and erythema. Maculopapular eruptions (50% of cases) often affect the trunk and the limbs and are accompanied by pruritus. Commonly, these rashes arise after 9 weeks of IM therapy. These eruptions are self-limiting and only topical emollients and steroids are required, without any change in IM schedule. To treat maculopapular eruptions with pruritus, oral steroids and antihistamines may be helpful, without suspending IM treatment. When grade 2 or 3 pruriginous maculopapular eruptions arise, the suspension of IM combined with steroids and antihistamines may be necessary. When the readministration of IM is required, it is mandatory to start IM at a lower dose (50–100 mg/d), administering prednisolone 0.5 to 1.0 mg/kg daily. Then, the steroid gradually can be tapered.6 Critical cutaneous AEs that are resistant to supportive measures warrant suspension of IM therapy. However, the incidence of this event is small (<1% of all patients).7
Regarding severe cutaneous AEs from IM therapy, Hsiao et al8 reported the case of Stevens-Johnson syndrome. In this case, IM was immediately stopped and systemic steroids were started. Rarely, erythroderma (grade 4 toxicity) can develop for which a prompt and perpetual suspension of IM is necessary and supportive care therapy with oral and topical steroids is recommended.9
Hyperpigmentation induced by IM, mostly in patients with Fitzpatrick skin types V to VI and with a general prevalence of 16% to 40% in treated patients, often is related to a mutation of c-Kit or other kinases that are activated rather than inhibited by the drug, resulting in overstimulation of melanogenesis.10 The prevalence of Fitzpatrick skin types I to III determined the absence of pigmentation changes in our cohort, excluding melanonychia. Hyperpigmentation was observed in the skin as well as the appendages such as nails, resulting in melanonychia (Figure 1). However, Brazzelli et al11 reported hypopigmentation in 5 white patients treated with IM; furthermore, they found a direct correlation between hypopigmentation and development of skin cancers in these patients. The susceptibility to develop skin cancers may persist, even without a clear manifestation of hypopigmentation, as reported in the current analysis. We documented BCC in 5 patients, 1 patient developed actinic keratoses, and 3 patients developed dermatofibromas. However, these neoplasms probably were not provoked by IM. On the contrary, we did not note squamous cell carcinoma, which was reported by Baskaynak et al12 in 2 CML patients treated with IM.
The administration of IM can be associated with exacerbation of psoriasis. Paradoxically, in genetically predisposed individuals, tumor necrosis factor α (TNF-α) antagonists, such as IM, seem to induce psoriasis, producing IFN-α rather than TNF-α and increasing inflammation.13 In fact, some research shows induction of psoriasis by anti–TNF-α drugs.14-16 Two cases of IM associated with psoriasis have been reported, and both cases represented an exacerbation of previously diagnosed psoriasis.13,17 On the contrary, in our analysis we reported 5 cases of psoriasis vulgaris induced by IM administration. Our patients developed cutaneous psoriatic lesions approximately 1.7 months after the start of IM therapy.
The pityriasis rosea–like eruption (Figure 4) presented as nonpruritic, erythematous, scaly patches on the trunk and extremities, and arose 3.6 months after the start of treatment. This particular cutaneous AE is rare. In 3 case reports, the IM dosage also was 400 mg daily.18-20 The pathophysiology of this rare skin reaction stems from the pharmacological effect of IM rather than a hypersensitivity reaction.18
Deininger et al7 reported that patients with a high basophil count (>20%) rarely show urticarial eruptions after IM due to histamine release from basophils. Premedication with an antihistamine was helpful and the urticarial eruption resolved after normalization in basophil count.7
Given the importance of IM for patients who have limited therapeutic alternatives for their disease and the ability to safely treat the cutaneous AEs, as demonstrated in our analysis, the suspension of IM for dermatological complications is necessary only in rare cases, as shown by the low number of patients (n=2) who had to discontinue therapy. The cutaneous AEs should be diagnosed and treated early with less impact on chemotherapy treatments. The administration of IM should involve a coordinated effort among oncologists and dermatologists to prevent important complications.
Imatinib mesylate (IM) represents the first-line treatment of chronic myeloid leukemia (CML) and gastrointestinal stromal tumors (GISTs). Its pharmacological activity is related to a specific action on several tyrosine kinases in different tumors, including Bcr-Abl in CML, c-Kit (CD117) in GIST, and platelet-derived growth factor receptor in dermatofibrosarcoma protuberans.1,2
Imatinib mesylate has been shown to improve progression-free survival and overall survival2; however, it also has several side effects. Among the adverse effects (AEs), less than 10% are nonhematologic, such as nausea, vomiting, diarrhea, muscle cramps, and cutaneous reactions.3,4
We followed patients who were treated with IM for 5 years to identify AEs of therapy.
Methods
The aim of this prospective study was to identify and collect data regarding IM cutaneous side effects so that clinicians can detect AEs early and differentiate them from AEs caused by other medications. All patients were subjected to a median of 5 years’ follow-up. We included all the patients treated with IM and excluded patients who had a history of eczematous dermatitis, psoriasis, renal impairment, or dyshidrosis palmoplantar. Before starting IM, all patients presented for a dermatologic visit. They were subsequently evaluated every 3 months.
The incidence rate was defined as the ratio of patients with cutaneous side effects and the total patients treated with IM. Furthermore, we calculated the ratio between each class of patient with a specific cutaneous manifestation and the entire cohort of patients with cutaneous side effects related to IM.
When necessary, microbiological, serological, and histopathological analyses were performed.
Results
In 60 months, we followed 220 patients treated with IM. Among them, 55 (25%) developed cutaneous side effects (35 males; 20 females). The incidence rate of the patients with cutaneous side effects was 1:4. The median age of the entire cohort was 52.5 years. Fifty patients were being treated for CML and 5 for GISTs. All patients received IM at a dosage of 400 mg daily.
The following skin diseases were observed in patients treated with IM (Table): 19 patients with maculopapular rash with pruritus (no maculopapular rash without pruritus was detected), 7 patients with eczematous dermatitis such as stasis dermatitis and seborrheic dermatitis, 6 patients with onychodystrophy melanonychia (Figure 1), 5 patients with psoriasis, 5 patients with skin cancers including basal cell carcinoma (BCC)(Figure 2), 3 patients with periorbital edema (Figure 3), 3 patients with mycosis, 3 patients with dermatofibromas, 2 patients with dyshidrosis palmoplantar, 1 patient with pityriasis rosea–like eruption (Figure 4), and 1 patient with actinic keratoses on the face. No hypopigmentation or hyperpigmentation, excluding the individual case of melanonychia, was observed.




All cutaneous diseases reported in this study appeared after IM therapy (median, 3.8 months). The median time to onset for each cutaneous disorder is reported in the Table. During the first dermatologic visit before starting IM therapy, none of the patients showed any of these cutaneous diseases.
The adverse cutaneous reactions were treated with appropriate drugs. Generally, eczematous dermatitis was treated using topical steroids, emollients, and oral antihistamines. In patients with maculopapular rash with pruritus, oral corticosteroids (eg, betamethasone 3 mg daily or prednisolone 1 mg/kg) in association with antihistamine was necessary. Psoriasis was completely improved with topical betamethasone 0.5 mg and calcipotriol 50 µg. Skin cancers were treated with surgical excision with histologic examination. All treatments are outlined in the Table.

Imatinib mesylate therapy was suspended in 2 patients with maculopapular rash with moderate to severe pruritus; however, despite the temporary suspension of the drug and the appropriate therapies (corticosteroids and antihistamines), cutaneous side effects reappeared 7 to 10 days after therapy resumed. Therefore, the treatment was permanently suspended in these 2 cases and IM was replaced with erlotinib, a second-generation Bcr-Abl tyrosine kinase inhibitor.
Comment
The introduction of IM for the treatment of GIST and CML has changed the history of these diseases. The drug typically is well tolerated and few patients have reported severe AEs. Mild skin reactions are relatively frequent, ranging from 7% to 21% of patients treated.3 In our case, the percentage was relatively higher (25%), likely because of close monitoring of patients, with an increase in the incidence rate.
Imatinib mesylate cutaneous reactions are dose dependent.4 Indeed, in all our cases, the cutaneous reactions arose with an IM dosage of 400 mg daily, which is compatible with the definition of dose-independent cutaneous AEs.
The most common cutaneous AEs reported in the literature were swelling/edema and maculopapular rash. Swelling is the most common AE described during therapy with IM with an incidence of 63% to 84%.5 Swelling often involves the periorbital area and occurs approximately 6 weeks after starting IM. Although its pathogenesis is uncertain, it has been shown that IM blocks the platelet-derived growth factor receptor expressed on blood vessels that regulates the transportation transcapillary. The inhibition of this receptor can lead to increased pore pressure, resulting in edema and erythema. Maculopapular eruptions (50% of cases) often affect the trunk and the limbs and are accompanied by pruritus. Commonly, these rashes arise after 9 weeks of IM therapy. These eruptions are self-limiting and only topical emollients and steroids are required, without any change in IM schedule. To treat maculopapular eruptions with pruritus, oral steroids and antihistamines may be helpful, without suspending IM treatment. When grade 2 or 3 pruriginous maculopapular eruptions arise, the suspension of IM combined with steroids and antihistamines may be necessary. When the readministration of IM is required, it is mandatory to start IM at a lower dose (50–100 mg/d), administering prednisolone 0.5 to 1.0 mg/kg daily. Then, the steroid gradually can be tapered.6 Critical cutaneous AEs that are resistant to supportive measures warrant suspension of IM therapy. However, the incidence of this event is small (<1% of all patients).7
Regarding severe cutaneous AEs from IM therapy, Hsiao et al8 reported the case of Stevens-Johnson syndrome. In this case, IM was immediately stopped and systemic steroids were started. Rarely, erythroderma (grade 4 toxicity) can develop for which a prompt and perpetual suspension of IM is necessary and supportive care therapy with oral and topical steroids is recommended.9
Hyperpigmentation induced by IM, mostly in patients with Fitzpatrick skin types V to VI and with a general prevalence of 16% to 40% in treated patients, often is related to a mutation of c-Kit or other kinases that are activated rather than inhibited by the drug, resulting in overstimulation of melanogenesis.10 The prevalence of Fitzpatrick skin types I to III determined the absence of pigmentation changes in our cohort, excluding melanonychia. Hyperpigmentation was observed in the skin as well as the appendages such as nails, resulting in melanonychia (Figure 1). However, Brazzelli et al11 reported hypopigmentation in 5 white patients treated with IM; furthermore, they found a direct correlation between hypopigmentation and development of skin cancers in these patients. The susceptibility to develop skin cancers may persist, even without a clear manifestation of hypopigmentation, as reported in the current analysis. We documented BCC in 5 patients, 1 patient developed actinic keratoses, and 3 patients developed dermatofibromas. However, these neoplasms probably were not provoked by IM. On the contrary, we did not note squamous cell carcinoma, which was reported by Baskaynak et al12 in 2 CML patients treated with IM.
The administration of IM can be associated with exacerbation of psoriasis. Paradoxically, in genetically predisposed individuals, tumor necrosis factor α (TNF-α) antagonists, such as IM, seem to induce psoriasis, producing IFN-α rather than TNF-α and increasing inflammation.13 In fact, some research shows induction of psoriasis by anti–TNF-α drugs.14-16 Two cases of IM associated with psoriasis have been reported, and both cases represented an exacerbation of previously diagnosed psoriasis.13,17 On the contrary, in our analysis we reported 5 cases of psoriasis vulgaris induced by IM administration. Our patients developed cutaneous psoriatic lesions approximately 1.7 months after the start of IM therapy.
The pityriasis rosea–like eruption (Figure 4) presented as nonpruritic, erythematous, scaly patches on the trunk and extremities, and arose 3.6 months after the start of treatment. This particular cutaneous AE is rare. In 3 case reports, the IM dosage also was 400 mg daily.18-20 The pathophysiology of this rare skin reaction stems from the pharmacological effect of IM rather than a hypersensitivity reaction.18
Deininger et al7 reported that patients with a high basophil count (>20%) rarely show urticarial eruptions after IM due to histamine release from basophils. Premedication with an antihistamine was helpful and the urticarial eruption resolved after normalization in basophil count.7
Given the importance of IM for patients who have limited therapeutic alternatives for their disease and the ability to safely treat the cutaneous AEs, as demonstrated in our analysis, the suspension of IM for dermatological complications is necessary only in rare cases, as shown by the low number of patients (n=2) who had to discontinue therapy. The cutaneous AEs should be diagnosed and treated early with less impact on chemotherapy treatments. The administration of IM should involve a coordinated effort among oncologists and dermatologists to prevent important complications.
- Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
- Scheinfeld N. Imatinib mesylate and dermatology part 2: a review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol. 2006;5:228-231.
- Breccia M, Carmosimo I, Russo E, et al. Early and tardive skin adverse events in chronic myeloid leukaemia patients treated with imatinib. Eur J Haematol. 2005;74:121-123.
- Ugurel S, Hildebrand R, Dippel E, et al. Dose dependent severe cutaneous reactions to imatinib. Br J Cancer. 2003;88:1157-1159.
- Valeyrie L, Bastuji-Garin S, Revuz J, et al. Adverse cutaneous reactions to imatinib (STI571) in Philadelphia chromosome-positive leukaemias: a prospective study of 54 patients. J Am Acad Dermatol. 2003;48:201-206.
- Scott LC, White JD, Reid R, et al. Management of skin toxicity related to the use of imatinibnmesylate (STI571, GlivecTM) for advanced stage gastrointestinal stromal tumors. Sarcoma. 2005;9:157-160.
- Deininger MW, O’Brien SG, Ford JM, et al. Practical management of patients with chronic myeloid leukemia receiving imatinib. J Clin Oncol. 2003;21:1637-1647.
- Hsiao LT, Chung HM, Lin JT, et al. Stevens-Johnson syndrome after treatment with STI571: a case report. Br J Haematol. 2002;117:620-622.
- Sehgal VN, Srivastava G, Sardana K. Erythroderma/exfoliative dermatitis: a synopsis. Int J Dermatol. 2004;43:39-47.
- Pietras K, Pahler J, Bergers G, et al. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19.
- Brazzelli V, Prestinari F, Barbagallo T, et al. A long-term time course of colorimetric assessment of the effects of imatinib mesylate on skin pigmentation: a study of five patients. J Eur Acad Dermatol Venerol. 2007;21:384-387.
- Baskaynak G, Kreuzer KA, Schwarz M, et al. Squamous cutaneous epithelial cell carcinoma in two CML patients with progressive disease under imatinib treatment. Eur J Haematol. 2003;70:231-234.
- Cheng H, Geist DE, Piperdi M, et al. Management of imatinib-related exacerbation of psoriasis in a patient with a gastrointestinal stromal tumor. Australas J Dermatol. 2009;50:41-43.
- Faillace C, Duarte GV, Cunha RS, et al. Severe infliximab-induced psoriasis treated with adalimumab switching. Int J Dermatol. 2013;52:234-238.
- Iborra M, Beltrán B, Bastida G, et al. Infliximab and adalimumab-induced psoriasis in Crohn’s disease: a aradoxical side effect. J Crohns Colitis. 2011;5:157-161.
- Fernandes IC, Torres T, Sanches M, et al. Psoriasis induced by infliximab. Acta Med Port. 2011;24:709-712.
- Woo SM, Huh CH, Park KC, et al. Exacerbation of psoriasis in a chronic myelogenous leukemia patient treated with imatinib. J Dermatol. 2007;34:724-726.
- Brazzelli V, Prestinari F, Roveda E, et al. Pytiriasis rosea-like eruption during treatment with imatinib mesylate. description of 3 cases. J Am Acad Dermatol. 2005;53:240-243.
- Konstantapoulos K, Papadogianni A, Dimopoulou M, et al. Pytriasis rosea associated with imatinib (STI571, Gleevec). Dermatology. 2002;205:172-173.
- Cho AY, Kim DH, Im M, et al. Pityriasis rosealike drug eruption induced by imatinib mesylate (Gleevec). Ann Dermatol. 2011;23(suppl 3):360-363.
- Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
- Scheinfeld N. Imatinib mesylate and dermatology part 2: a review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol. 2006;5:228-231.
- Breccia M, Carmosimo I, Russo E, et al. Early and tardive skin adverse events in chronic myeloid leukaemia patients treated with imatinib. Eur J Haematol. 2005;74:121-123.
- Ugurel S, Hildebrand R, Dippel E, et al. Dose dependent severe cutaneous reactions to imatinib. Br J Cancer. 2003;88:1157-1159.
- Valeyrie L, Bastuji-Garin S, Revuz J, et al. Adverse cutaneous reactions to imatinib (STI571) in Philadelphia chromosome-positive leukaemias: a prospective study of 54 patients. J Am Acad Dermatol. 2003;48:201-206.
- Scott LC, White JD, Reid R, et al. Management of skin toxicity related to the use of imatinibnmesylate (STI571, GlivecTM) for advanced stage gastrointestinal stromal tumors. Sarcoma. 2005;9:157-160.
- Deininger MW, O’Brien SG, Ford JM, et al. Practical management of patients with chronic myeloid leukemia receiving imatinib. J Clin Oncol. 2003;21:1637-1647.
- Hsiao LT, Chung HM, Lin JT, et al. Stevens-Johnson syndrome after treatment with STI571: a case report. Br J Haematol. 2002;117:620-622.
- Sehgal VN, Srivastava G, Sardana K. Erythroderma/exfoliative dermatitis: a synopsis. Int J Dermatol. 2004;43:39-47.
- Pietras K, Pahler J, Bergers G, et al. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19.
- Brazzelli V, Prestinari F, Barbagallo T, et al. A long-term time course of colorimetric assessment of the effects of imatinib mesylate on skin pigmentation: a study of five patients. J Eur Acad Dermatol Venerol. 2007;21:384-387.
- Baskaynak G, Kreuzer KA, Schwarz M, et al. Squamous cutaneous epithelial cell carcinoma in two CML patients with progressive disease under imatinib treatment. Eur J Haematol. 2003;70:231-234.
- Cheng H, Geist DE, Piperdi M, et al. Management of imatinib-related exacerbation of psoriasis in a patient with a gastrointestinal stromal tumor. Australas J Dermatol. 2009;50:41-43.
- Faillace C, Duarte GV, Cunha RS, et al. Severe infliximab-induced psoriasis treated with adalimumab switching. Int J Dermatol. 2013;52:234-238.
- Iborra M, Beltrán B, Bastida G, et al. Infliximab and adalimumab-induced psoriasis in Crohn’s disease: a aradoxical side effect. J Crohns Colitis. 2011;5:157-161.
- Fernandes IC, Torres T, Sanches M, et al. Psoriasis induced by infliximab. Acta Med Port. 2011;24:709-712.
- Woo SM, Huh CH, Park KC, et al. Exacerbation of psoriasis in a chronic myelogenous leukemia patient treated with imatinib. J Dermatol. 2007;34:724-726.
- Brazzelli V, Prestinari F, Roveda E, et al. Pytiriasis rosea-like eruption during treatment with imatinib mesylate. description of 3 cases. J Am Acad Dermatol. 2005;53:240-243.
- Konstantapoulos K, Papadogianni A, Dimopoulou M, et al. Pytriasis rosea associated with imatinib (STI571, Gleevec). Dermatology. 2002;205:172-173.
- Cho AY, Kim DH, Im M, et al. Pityriasis rosealike drug eruption induced by imatinib mesylate (Gleevec). Ann Dermatol. 2011;23(suppl 3):360-363.
Practice Points
- The most common cutaneous adverse reactions from imatinib mesylate (IM) are swelling and edema.
- Maculopapular rash with pruritus is one of the most common side effects from IM and can be effectively treated with oral or systemic antihistamines.
- The onset of periorbital edema requires a complete evaluation of renal function.
Linea Aspera as Rotational Landmark for Tumor Endoprostheses: A Computed Tomography Study
The distal or proximal femur with tumor endoprosthesis is commonly replaced after segmental resections for bone tumors, complex trauma, or revision arthroplasty. In conventional joint replacements, correct rotational alignment of the component is referenced off anatomical landmarks in the proximal or distal femur. After tumor resection, however, these landmarks are often not available for rotational orientation. There are no reports of studies validating a particular method of establishing rotation in these cases.
To establish a guide for rotational alignment of tumor endoprostheses, we set out to define the natural location of the linea aspera (LA) based on axial computed tomography (CT) scans. The LA is often the most outstanding visible bony landmark on a cross-section of the femur during surgery, and it would be helpful to know its normal orientation in relation to the true anteroposterior (AP) axis of the femur and to the femoral version. We wanted to answer these 5 questions:
1. Is the prominence of the LA easily identifiable on cross-section at different levels of the femoral shaft?
2. Does an axis passing through the LA correspond to the AP axis of the femur?
3. If not, is this axis offset internally or externally and by how much?
4. Is this offset constant at all levels of the femoral shaft?
5. How does the LA axis relate to the femoral neck axis at these levels?
The answers determine if the LA can be reliably used for rotational alignment of tumor endoprostheses.
Materials and Methods
After this study received Institutional Review Board approval, we retrospectively reviewed whole-body fluorine-18-deoxyglucose (FDG) positron emission tomography–computed tomography (PET-CT) studies performed in our hospital between 2003 and 2006 to identify those with full-length bilateral femur CT scans. These scans were available on the hospital’s computerized picture archiving system (General Electric). Patients could be included in the study as long as they were at least 18 years old at time of scan and did not have any pathology that deformed the femur, broke a cortex, or otherwise caused any gross asymmetry of the femur. Of the 72 patients with full-length femur CT scans, 3 were excluded: 1 with a congenital hip dysplasia, 1 with an old, malunited femoral fracture, and 1 who was 15 years old at time of scan.
Axial Slice Selection
For each patient, scout AP films were used to measure femoral shaft length from the top of the greater trochanter to the end of the lateral femoral condyle. The levels of the proximal third, midshaft, and distal third were then calculated based on this length. The LA was studied on the axial slices nearest these levels. Next, we scrolled through the scans to identify an axial slice that best showed the femoral neck axis. The literature on CT measurement of femoral anteversion is varied. Some articles describe a technique that uses 2 superimposed axial slices, and others describe a single axial slice.1-3 We used 1 axial slice to draw the femoral neck axis because our computer software could not superimpose 2 images on 1 screen and because the CT scans were not made under specific protocols to measure anteversion but rather were part of a cancer staging work-up. Axial cuts were made at 5-mm intervals, and not all scans included a single slice capturing the head, neck, and greater trochanter. Therefore, we used a (previously described) method in which the femoral neck axis is drawn on a slice that most captured the femoral neck, usually toward its base.4 Last, in order to draw the posterior condyle (PC) axis, we selected an axial slice that showed the posterior-most aspects of the femoral condyles at the intercondylar notch.
Determining Anteroposterior and Posterior Condyle Axes of Femur
As we made all measurements for each femur off a single CT scan, we were able to use a straight horizontal line—drawn on-screen with a software tool—as a reference for measuring rotation. On a distal femur cut, the PC axis is drawn by connecting the posterior-most points of both condyles. The software calculates the angle formed—the PC angle (Figure 1). This angle, the degree to which the PC axis deviates from a straight horizontal line on-screen, can be used to account for gross rotation of the limb on comparison of images. The AP axis of the femur is the axis perpendicular to the PC axis. As such, the PC angle can also be used to determine degree of deviation of the AP axis from a straight vertical line on-screen. The AP axis was used when calculating the LA axis at the various levels of the femur (Figure 2).
Femoral Version
We used the software tool to draw the femoral neck axis. From the end of this line, a straight horizontal line is drawn on-screen (Figure 3). The software calculates the angle formed—the femoral neck axis angle. We assigned a positive value for a femoral head that pointed anteriorly on the image and a negative value for a head that pointed posteriorly. Adjusting for external rotation of the limb involved calculating the femoral version by subtracting the PC angle from the neck axis angle; adjusting for internal rotation involved adding these 2 angles.
Linea Aspera Morphology
After viewing the first 20 CT scans, we identified 3 types of LA morphology. Type I presents as a thickening on the posterior cortex with a sharp apex; type II presents as a flat-faced but distinct ridge of bone between the medial and lateral lips; and in type III there is no distinct cortical thickening with blunted medial and lateral lips; the latter is always more prominent.
Linea Aspera Axis Offset
From the most posterior point of the LA, a line drawn forward bisecting the femoral canal defined the LA axis. In type I morphology, the posterior-most point was the apex; in type II, the middle of flat posterior surface was used as the starting point; in type III, the lateral lip was used, as it was sharper than the medial lip. This line is again referenced with a straight horizontal line across the image. The PC angle is then added to account for limb rotation, and the result is the LA angle. As the AP axis is perpendicular to the PC axis, the LA angle is subtracted from 90°; the difference represents the amount of offset of the LA axis from the AP axis. By convention, we assigned this a positive value for an LA lateral to the midpoint of the femur and a negative value for an LA medial to the midpoint (Figure 4).
Linea Aspera Axis and Femoral Neck Axis
The angle between the LA axis and the PC axis was measured. The femoral version angle was subtracted from that angle to obtain the arc between the LA axis and the femoral neck axis.
Statistical Analyses
All analyses were performed with SAS 9.1 (SAS Institute). All tests were 2-sided and conducted at the .05 significance level. No adjustments were made for multiple testing. Statistical analysis was performed with nonparametric tests and without making assumptions about the distribution of the study population. Univariate analyses were performed to test for significant side-to-side differences in femoral length, femoral version angle, and LA torsion angles at each level. A multivariate analysis was performed to test for interactions between sex, side, and level. In all analyses, P < .05 was used as the cutoff value for statistical significance.
Results
Femoral lengths varied by side and sex. The left side was longer than the right by a mean of 1.3 mm (P = .008). With multivariate analysis taking into account sex and age (cumulated per decade), there was still a significant effect of side on femoral length. Sex also had a significant effect on femoral length, with females’ femurs shorter by 21.7 mm (standard error, 5.0 mm). Mean (SD) anteversion of the femoral neck was 7.9° (12.7°) on the left and 13.3° (13.0°) on the right; the difference between sides was significant (P < .001). In a multivariate analysis performed to identify potential predictors of femoral version, side still had a significant (P < .001) independent effect; sex and age did not have an effect.
LA morphology varied according to femoral shaft level (Table 1). The morphology was type I in 75% of patients at the distal femur and 74% of patients at the midshaft femur, while only 53% of patients had a type I morphology at the proximal femur. The proportion of type III morphology was larger in the proximal femur (41%) than in the other locations.
The LA axis of the femur did not correspond exactly to the AP axis at all femoral levels. At the distal femur, mean (SD) lateral offset of the LA axis was 5.5° (7.5°) on the left and 8.3° (8.9°) on the right. At the midshaft, mean (SD) medial offset of the LA axis was 3.1° (8.4°) on the left and 1.2° (7.9°) on the right. At the proximal femur, mean (SD) lateral offset of the LA axis was 5.4° (9.2°) on the left and 6.2° (8.3°) on the right. The side-to-side differences were statistically significant for the distal femur and midshaft but not the proximal femur. Table 2 lists the 95% confidence intervals for the mean values. As the range of differences was small (0.7°-2.8°), and the differences may not be clinically detected on gross inspection during surgery, we pooled both sides’ values to arrive at a single mean for each level. The LA axis was offset a mean (SD) of 6.9° (8.3°) laterally at the distal femur, 2.2° (8.2°) medially at the midshaft, and 5.8° (8.6°) laterally at the proximal femur. Figure 5 shows the frequency of distribution of LA axis offset.Offset of the LA axis from the AP axis of the femur was significantly (P < .001) different for each femoral level, even when a multivariate analysis was performed to determine the effect of sex, age, or side. Age and sex had no significant effect on mean offset of LA axis from AP axis.
We compared the mean arc between femoral neck axis and LA axis after referencing both off the PC axis. At the distal femur, mean (SD) arc between these 2 axes was 76.6° (13.1°) on the left and 68.3° (13.6°) on the right (mean difference, 8.3°); at the midshaft, mean (SD) arc was 85.2° (13.5°) on the left and 77.9° (13.1°) on the right (mean difference, 7.4°); at the proximal femur, mean (SD) arc was 76.7° (11.9°) on the left and 70.5° (12.8°) on the right (mean difference, 6.2°). The side-to-side differences were statistically significant (P < .001) for all locations.
In multivariate analysis, sex and age did not have an effect on mean arc between the 2 axes. Side and femoral level, however, had a significant effect (P < .001).
Discussion
In total hip arthroplasty, the goal is to restore femoral anteversion, usually referenced to the remaining femoral neck segment.3 In total knee arthroplasty (TKA), proper rotation preserves normal patellofemoral tracking.5 Various landmarks are used, such as the PCs or the epicondyles. After tumor resections, these landmarks are often lost.6 However, there are no reports of studies validating a particular method of achieving proper rotational orientation of tumor endoprostheses, though several methods are being used. One method involves inserting 2 drill bits before osteotomy—one proximal to the intended level of resection on the anterior femur, and the other on the anterior tibial shaft. The straight line formed can establish a plane of rotation (and length), which the surgeon must aim to restore when the components are placed. This method is useful for distal femur resections but not proximal femur resections. Another method, based on the LA’s anatomical position on the posterior aspect of the femur,4 uses the prominence of the LA to align the prosthesis. With this method, the LA is assumed to be directly posterior (6 o’clock) on the femur. However, this assumption has not been confirmed by any study. A third method, described by Heck and Carnesale,5 involves marking the anterior aspect of the femur after resection and aligning the components to it. The authors cautioned against using the LA as a landmark, saying that its course is highly variable.
The LA is a narrow, elevated length of bone, with medial and lateral lips, that serves as an attachment site for muscles in the posterior thigh. Proximally, the LA presents with lateral, medial, and intermediate lips. In the midshaft, it is often elevated by an underlying bony ridge or pilaster complex. Distally, it diverges into 2 ridges that form the triangular popliteal surface.1,7 For the LA to be a reliable landmark, first it must be clearly identifiable on viewing a femoral cross-section. The LA that presents with type I or II morphology is distinctly identifiable, and an axis from its apex and bisecting the canal can easily be constructed. In our study, the LA presented with type I or II morphology in 82% of distal femoral sections and 99% of midshaft femoral sections. Therefore, the LA is a conspicuous landmark at these levels. In the proximal femur, 59% had type I or II morphology. Type III morphology could be identified on cross-sections by the persisting prominence of the lateral lip. However, it may be difficult to appreciate the LA with this morphology at surgery.
Once the LA is identified, its normal cross-sectional position must be defined. One way to do this is to establish the relationship of its axis (LA axis) to the true AP axis. Based on mean values, the LA axis is laterally offset 7º at the distal third of the femur, medially offset 2º at the midshaft, and laterally offset 6º at the proximal third. Therefore, for ideal placement with the LA used for orientation, the component must be internally rotated 7º relative to the LA for femoral resection at the distal third, externally rotated 2º for resection at the midshaft, and internally rotated 6º for resection at the proximal third. Studies have demonstrated that joint contact forces and mechanical alignment of the lower limb can be altered with as little as 5º of femoral malrotation.8,9 Although such a small degree of malrotation is often asymptomatic, it can have long-term effects on soft-tissue tension and patellar tracking.10,11 Rotating-platform mobile-bearing TKA designs can compensate for femoral malrotation, but they may have little to no effect on patellar tracking.12 Therefore, we think aligning the components as near as possible to their natural orientation can prove beneficial in long-term patient management.
Another way of defining the normal cross-sectional position of the LA is to relate it to the femoral neck axis. We measured the difference between these 2 axes. Mean differences were 72º (distal femur), 81.5º (midshaft), and 73.5º (proximal third). Mean arc differences at all levels were larger on the left side—a reflection of the femoral neck being less anteverted on that side in our measurements. Standard deviations were smaller for measurements of LA axis offset from AP axis (range, 7.5°-9.2°) than for measurements of arc between LA axis and femoral neck axis (range, 11.9°-13.6°). This finding indicates there is less variation in the former method, making it preferable for defining the cross-sectional position of the LA.
It has been said that the course of the LA is variable, and our data provide confirmation. The LA does not lie directly posterior (6 o’clock), and it does not trace a straight longitudinal course along the posterior femur, as demonstrated by the different LA axis offsets at 3 levels. However, we may still use it as a landmark if we remain aware how much the LA is offset from the AP axis at each femoral level. Figures 6A-6D, which show CT scans of a patient who underwent distal femoral resection and replacement with an endoprosthesis, illustrate how the LA axis was measured before surgery and how proper prosthesis placement was confirmed after surgery.
In hip arthroplasty, restoration of normal femoral version is the reference for endoprosthetic placement. The literature on “normal” femoral anteversion varies with the method used. In a review of studies on CT-measured adult femoral version, reported values ranged from 6.3° to 40°.2 Mean femoral version in our study ranged from 8° to 13°. Orthopedics textbooks generally put the value at 10° to 15º, and this seems to be the range that surgeons target.6 However, we found a statistically significant mean side-to-side difference of 5.4°. This finding is possibly explained by our large sample—it was larger than the samples used in other studies of CT-measured femoral version. Other studies have found mean side-to-side differences of up to 4.0º.5 Another explanation for our finding is that the studies may differ methodologically. The studies that established values for femoral anteversion were based on CT protocols—thinner slices (1-5 mm), use of foot holders to standardize limb rotation, use of 2 axial cuts in proximal femur to establish femoral neck axis2,13—designed specifically for this measurement. As the CT scans reviewed in our study are not designed for this purpose, errors in femoral version measurement may have been introduced, which may also explain why there is larger variation in measurements of the arc between the LA axis and the femoral neck axis.
Conclusion
The LA does not lie directly on the posterior surface of the femur. It deviates 6.9° laterally at the distal femur, 2.2° medially at the midshaft, and 6.9° laterally at the proximal third. As the LA is an easily identifiable structure on cross-sections of the femoral shaft at the midshaft and distal third of the femur, it may be useful as a rotational landmark for resections at these levels if these deviations are considered during tumor endoprosthetic replacements.
1. Desai SC, Willson S. Radiology of the linea aspera. Australas Radiol. 1985;29(3):273-274.
2. Kuo TY, Skedros JG, Bloebaum RD. Measurement of femoral anteversion by biplane radiography and computed tomography imaging: comparison with an anatomic reference. Invest Radiol. 2003;38(4):221-229.
3. Wines AP, McNicol D. Computed tomography measurement of the accuracy of component version in total hip arthroplasty. J Arthroplasty. 2006;21(5):696-701.
4. Gray H. Anatomy of the Human Body. Philadelphia, PA: Lea & Febiger; 1918.
5. Heck RK, Carnesale PG. General principles of tumors. In: Canale ST, ed. Campbell’s Operative Orthopaedics. Vol 1. 10th ed. St. Louis, MO: Mosby; 2003:733-791.
6. Katz, MA, Beck TD, Silber JS, Seldes RM, Lotke PA. Determining femoral rotational alignment in total knee arthroplasty: reliability of techniques. J Arthroplasty. 2001;16(3):301-305.
7. Pitt MJ. Radiology of the femoral linea aspera–pilaster complex: the track sign. Radiology. 1982;142(1):66.
8. Bretin P, O’Loughlin PF, Suero EM, et al. Influence of femoral malrotation on knee joint alignment and intra-articular contact pressures. Arch Orthop Trauma Surg. 2011;131(8):1115-1120.
9. Zihlmann MS, Stacoff A, Romero J, Quervain IK, Stüssi E. Biomechanical background and clinical observations of rotational malalignment in TKA: literature review and consequences. Clin Biomech. 2005;20(7):661-668.
10. Ghosh KM, Merican AM, Iranpour F, Deehan DJ, Amis AA. The effect of femoral component rotation on the extensor retinaculum of the knee. J Orthop Res. 2010;28(9):1136-1141.
11. Verlinden C, Uvin P, Labey L, Luyckx JP, Bellemans J, Vandenneucker H. The influence of malrotation of the femoral component in total knee replacement on the mechanics of patellofemoral contact during gait: an in vitro biomechanical study. J Bone Joint Surg Br. 2010;92(5):737-742.
12. Kessler O, Patil S, Colwell CW Jr, D’Lima DD. The effect of femoral component malrotation on patellar biomechanics. J Biomech. 2008;41(16):3332-3339.
13. Strecker W, Keppler P, Gebhard F, Kinzl L. Length and torsion of the lower limb. J Bone Joint Surg Br. 1997;79(6):1019-1023.
The distal or proximal femur with tumor endoprosthesis is commonly replaced after segmental resections for bone tumors, complex trauma, or revision arthroplasty. In conventional joint replacements, correct rotational alignment of the component is referenced off anatomical landmarks in the proximal or distal femur. After tumor resection, however, these landmarks are often not available for rotational orientation. There are no reports of studies validating a particular method of establishing rotation in these cases.
To establish a guide for rotational alignment of tumor endoprostheses, we set out to define the natural location of the linea aspera (LA) based on axial computed tomography (CT) scans. The LA is often the most outstanding visible bony landmark on a cross-section of the femur during surgery, and it would be helpful to know its normal orientation in relation to the true anteroposterior (AP) axis of the femur and to the femoral version. We wanted to answer these 5 questions:
1. Is the prominence of the LA easily identifiable on cross-section at different levels of the femoral shaft?
2. Does an axis passing through the LA correspond to the AP axis of the femur?
3. If not, is this axis offset internally or externally and by how much?
4. Is this offset constant at all levels of the femoral shaft?
5. How does the LA axis relate to the femoral neck axis at these levels?
The answers determine if the LA can be reliably used for rotational alignment of tumor endoprostheses.
Materials and Methods
After this study received Institutional Review Board approval, we retrospectively reviewed whole-body fluorine-18-deoxyglucose (FDG) positron emission tomography–computed tomography (PET-CT) studies performed in our hospital between 2003 and 2006 to identify those with full-length bilateral femur CT scans. These scans were available on the hospital’s computerized picture archiving system (General Electric). Patients could be included in the study as long as they were at least 18 years old at time of scan and did not have any pathology that deformed the femur, broke a cortex, or otherwise caused any gross asymmetry of the femur. Of the 72 patients with full-length femur CT scans, 3 were excluded: 1 with a congenital hip dysplasia, 1 with an old, malunited femoral fracture, and 1 who was 15 years old at time of scan.
Axial Slice Selection
For each patient, scout AP films were used to measure femoral shaft length from the top of the greater trochanter to the end of the lateral femoral condyle. The levels of the proximal third, midshaft, and distal third were then calculated based on this length. The LA was studied on the axial slices nearest these levels. Next, we scrolled through the scans to identify an axial slice that best showed the femoral neck axis. The literature on CT measurement of femoral anteversion is varied. Some articles describe a technique that uses 2 superimposed axial slices, and others describe a single axial slice.1-3 We used 1 axial slice to draw the femoral neck axis because our computer software could not superimpose 2 images on 1 screen and because the CT scans were not made under specific protocols to measure anteversion but rather were part of a cancer staging work-up. Axial cuts were made at 5-mm intervals, and not all scans included a single slice capturing the head, neck, and greater trochanter. Therefore, we used a (previously described) method in which the femoral neck axis is drawn on a slice that most captured the femoral neck, usually toward its base.4 Last, in order to draw the posterior condyle (PC) axis, we selected an axial slice that showed the posterior-most aspects of the femoral condyles at the intercondylar notch.
Determining Anteroposterior and Posterior Condyle Axes of Femur
As we made all measurements for each femur off a single CT scan, we were able to use a straight horizontal line—drawn on-screen with a software tool—as a reference for measuring rotation. On a distal femur cut, the PC axis is drawn by connecting the posterior-most points of both condyles. The software calculates the angle formed—the PC angle (Figure 1). This angle, the degree to which the PC axis deviates from a straight horizontal line on-screen, can be used to account for gross rotation of the limb on comparison of images. The AP axis of the femur is the axis perpendicular to the PC axis. As such, the PC angle can also be used to determine degree of deviation of the AP axis from a straight vertical line on-screen. The AP axis was used when calculating the LA axis at the various levels of the femur (Figure 2).
Femoral Version
We used the software tool to draw the femoral neck axis. From the end of this line, a straight horizontal line is drawn on-screen (Figure 3). The software calculates the angle formed—the femoral neck axis angle. We assigned a positive value for a femoral head that pointed anteriorly on the image and a negative value for a head that pointed posteriorly. Adjusting for external rotation of the limb involved calculating the femoral version by subtracting the PC angle from the neck axis angle; adjusting for internal rotation involved adding these 2 angles.
Linea Aspera Morphology
After viewing the first 20 CT scans, we identified 3 types of LA morphology. Type I presents as a thickening on the posterior cortex with a sharp apex; type II presents as a flat-faced but distinct ridge of bone between the medial and lateral lips; and in type III there is no distinct cortical thickening with blunted medial and lateral lips; the latter is always more prominent.
Linea Aspera Axis Offset
From the most posterior point of the LA, a line drawn forward bisecting the femoral canal defined the LA axis. In type I morphology, the posterior-most point was the apex; in type II, the middle of flat posterior surface was used as the starting point; in type III, the lateral lip was used, as it was sharper than the medial lip. This line is again referenced with a straight horizontal line across the image. The PC angle is then added to account for limb rotation, and the result is the LA angle. As the AP axis is perpendicular to the PC axis, the LA angle is subtracted from 90°; the difference represents the amount of offset of the LA axis from the AP axis. By convention, we assigned this a positive value for an LA lateral to the midpoint of the femur and a negative value for an LA medial to the midpoint (Figure 4).
Linea Aspera Axis and Femoral Neck Axis
The angle between the LA axis and the PC axis was measured. The femoral version angle was subtracted from that angle to obtain the arc between the LA axis and the femoral neck axis.
Statistical Analyses
All analyses were performed with SAS 9.1 (SAS Institute). All tests were 2-sided and conducted at the .05 significance level. No adjustments were made for multiple testing. Statistical analysis was performed with nonparametric tests and without making assumptions about the distribution of the study population. Univariate analyses were performed to test for significant side-to-side differences in femoral length, femoral version angle, and LA torsion angles at each level. A multivariate analysis was performed to test for interactions between sex, side, and level. In all analyses, P < .05 was used as the cutoff value for statistical significance.
Results
Femoral lengths varied by side and sex. The left side was longer than the right by a mean of 1.3 mm (P = .008). With multivariate analysis taking into account sex and age (cumulated per decade), there was still a significant effect of side on femoral length. Sex also had a significant effect on femoral length, with females’ femurs shorter by 21.7 mm (standard error, 5.0 mm). Mean (SD) anteversion of the femoral neck was 7.9° (12.7°) on the left and 13.3° (13.0°) on the right; the difference between sides was significant (P < .001). In a multivariate analysis performed to identify potential predictors of femoral version, side still had a significant (P < .001) independent effect; sex and age did not have an effect.
LA morphology varied according to femoral shaft level (Table 1). The morphology was type I in 75% of patients at the distal femur and 74% of patients at the midshaft femur, while only 53% of patients had a type I morphology at the proximal femur. The proportion of type III morphology was larger in the proximal femur (41%) than in the other locations.
The LA axis of the femur did not correspond exactly to the AP axis at all femoral levels. At the distal femur, mean (SD) lateral offset of the LA axis was 5.5° (7.5°) on the left and 8.3° (8.9°) on the right. At the midshaft, mean (SD) medial offset of the LA axis was 3.1° (8.4°) on the left and 1.2° (7.9°) on the right. At the proximal femur, mean (SD) lateral offset of the LA axis was 5.4° (9.2°) on the left and 6.2° (8.3°) on the right. The side-to-side differences were statistically significant for the distal femur and midshaft but not the proximal femur. Table 2 lists the 95% confidence intervals for the mean values. As the range of differences was small (0.7°-2.8°), and the differences may not be clinically detected on gross inspection during surgery, we pooled both sides’ values to arrive at a single mean for each level. The LA axis was offset a mean (SD) of 6.9° (8.3°) laterally at the distal femur, 2.2° (8.2°) medially at the midshaft, and 5.8° (8.6°) laterally at the proximal femur. Figure 5 shows the frequency of distribution of LA axis offset.Offset of the LA axis from the AP axis of the femur was significantly (P < .001) different for each femoral level, even when a multivariate analysis was performed to determine the effect of sex, age, or side. Age and sex had no significant effect on mean offset of LA axis from AP axis.
We compared the mean arc between femoral neck axis and LA axis after referencing both off the PC axis. At the distal femur, mean (SD) arc between these 2 axes was 76.6° (13.1°) on the left and 68.3° (13.6°) on the right (mean difference, 8.3°); at the midshaft, mean (SD) arc was 85.2° (13.5°) on the left and 77.9° (13.1°) on the right (mean difference, 7.4°); at the proximal femur, mean (SD) arc was 76.7° (11.9°) on the left and 70.5° (12.8°) on the right (mean difference, 6.2°). The side-to-side differences were statistically significant (P < .001) for all locations.
In multivariate analysis, sex and age did not have an effect on mean arc between the 2 axes. Side and femoral level, however, had a significant effect (P < .001).
Discussion
In total hip arthroplasty, the goal is to restore femoral anteversion, usually referenced to the remaining femoral neck segment.3 In total knee arthroplasty (TKA), proper rotation preserves normal patellofemoral tracking.5 Various landmarks are used, such as the PCs or the epicondyles. After tumor resections, these landmarks are often lost.6 However, there are no reports of studies validating a particular method of achieving proper rotational orientation of tumor endoprostheses, though several methods are being used. One method involves inserting 2 drill bits before osteotomy—one proximal to the intended level of resection on the anterior femur, and the other on the anterior tibial shaft. The straight line formed can establish a plane of rotation (and length), which the surgeon must aim to restore when the components are placed. This method is useful for distal femur resections but not proximal femur resections. Another method, based on the LA’s anatomical position on the posterior aspect of the femur,4 uses the prominence of the LA to align the prosthesis. With this method, the LA is assumed to be directly posterior (6 o’clock) on the femur. However, this assumption has not been confirmed by any study. A third method, described by Heck and Carnesale,5 involves marking the anterior aspect of the femur after resection and aligning the components to it. The authors cautioned against using the LA as a landmark, saying that its course is highly variable.
The LA is a narrow, elevated length of bone, with medial and lateral lips, that serves as an attachment site for muscles in the posterior thigh. Proximally, the LA presents with lateral, medial, and intermediate lips. In the midshaft, it is often elevated by an underlying bony ridge or pilaster complex. Distally, it diverges into 2 ridges that form the triangular popliteal surface.1,7 For the LA to be a reliable landmark, first it must be clearly identifiable on viewing a femoral cross-section. The LA that presents with type I or II morphology is distinctly identifiable, and an axis from its apex and bisecting the canal can easily be constructed. In our study, the LA presented with type I or II morphology in 82% of distal femoral sections and 99% of midshaft femoral sections. Therefore, the LA is a conspicuous landmark at these levels. In the proximal femur, 59% had type I or II morphology. Type III morphology could be identified on cross-sections by the persisting prominence of the lateral lip. However, it may be difficult to appreciate the LA with this morphology at surgery.
Once the LA is identified, its normal cross-sectional position must be defined. One way to do this is to establish the relationship of its axis (LA axis) to the true AP axis. Based on mean values, the LA axis is laterally offset 7º at the distal third of the femur, medially offset 2º at the midshaft, and laterally offset 6º at the proximal third. Therefore, for ideal placement with the LA used for orientation, the component must be internally rotated 7º relative to the LA for femoral resection at the distal third, externally rotated 2º for resection at the midshaft, and internally rotated 6º for resection at the proximal third. Studies have demonstrated that joint contact forces and mechanical alignment of the lower limb can be altered with as little as 5º of femoral malrotation.8,9 Although such a small degree of malrotation is often asymptomatic, it can have long-term effects on soft-tissue tension and patellar tracking.10,11 Rotating-platform mobile-bearing TKA designs can compensate for femoral malrotation, but they may have little to no effect on patellar tracking.12 Therefore, we think aligning the components as near as possible to their natural orientation can prove beneficial in long-term patient management.
Another way of defining the normal cross-sectional position of the LA is to relate it to the femoral neck axis. We measured the difference between these 2 axes. Mean differences were 72º (distal femur), 81.5º (midshaft), and 73.5º (proximal third). Mean arc differences at all levels were larger on the left side—a reflection of the femoral neck being less anteverted on that side in our measurements. Standard deviations were smaller for measurements of LA axis offset from AP axis (range, 7.5°-9.2°) than for measurements of arc between LA axis and femoral neck axis (range, 11.9°-13.6°). This finding indicates there is less variation in the former method, making it preferable for defining the cross-sectional position of the LA.
It has been said that the course of the LA is variable, and our data provide confirmation. The LA does not lie directly posterior (6 o’clock), and it does not trace a straight longitudinal course along the posterior femur, as demonstrated by the different LA axis offsets at 3 levels. However, we may still use it as a landmark if we remain aware how much the LA is offset from the AP axis at each femoral level. Figures 6A-6D, which show CT scans of a patient who underwent distal femoral resection and replacement with an endoprosthesis, illustrate how the LA axis was measured before surgery and how proper prosthesis placement was confirmed after surgery.
In hip arthroplasty, restoration of normal femoral version is the reference for endoprosthetic placement. The literature on “normal” femoral anteversion varies with the method used. In a review of studies on CT-measured adult femoral version, reported values ranged from 6.3° to 40°.2 Mean femoral version in our study ranged from 8° to 13°. Orthopedics textbooks generally put the value at 10° to 15º, and this seems to be the range that surgeons target.6 However, we found a statistically significant mean side-to-side difference of 5.4°. This finding is possibly explained by our large sample—it was larger than the samples used in other studies of CT-measured femoral version. Other studies have found mean side-to-side differences of up to 4.0º.5 Another explanation for our finding is that the studies may differ methodologically. The studies that established values for femoral anteversion were based on CT protocols—thinner slices (1-5 mm), use of foot holders to standardize limb rotation, use of 2 axial cuts in proximal femur to establish femoral neck axis2,13—designed specifically for this measurement. As the CT scans reviewed in our study are not designed for this purpose, errors in femoral version measurement may have been introduced, which may also explain why there is larger variation in measurements of the arc between the LA axis and the femoral neck axis.
Conclusion
The LA does not lie directly on the posterior surface of the femur. It deviates 6.9° laterally at the distal femur, 2.2° medially at the midshaft, and 6.9° laterally at the proximal third. As the LA is an easily identifiable structure on cross-sections of the femoral shaft at the midshaft and distal third of the femur, it may be useful as a rotational landmark for resections at these levels if these deviations are considered during tumor endoprosthetic replacements.
The distal or proximal femur with tumor endoprosthesis is commonly replaced after segmental resections for bone tumors, complex trauma, or revision arthroplasty. In conventional joint replacements, correct rotational alignment of the component is referenced off anatomical landmarks in the proximal or distal femur. After tumor resection, however, these landmarks are often not available for rotational orientation. There are no reports of studies validating a particular method of establishing rotation in these cases.
To establish a guide for rotational alignment of tumor endoprostheses, we set out to define the natural location of the linea aspera (LA) based on axial computed tomography (CT) scans. The LA is often the most outstanding visible bony landmark on a cross-section of the femur during surgery, and it would be helpful to know its normal orientation in relation to the true anteroposterior (AP) axis of the femur and to the femoral version. We wanted to answer these 5 questions:
1. Is the prominence of the LA easily identifiable on cross-section at different levels of the femoral shaft?
2. Does an axis passing through the LA correspond to the AP axis of the femur?
3. If not, is this axis offset internally or externally and by how much?
4. Is this offset constant at all levels of the femoral shaft?
5. How does the LA axis relate to the femoral neck axis at these levels?
The answers determine if the LA can be reliably used for rotational alignment of tumor endoprostheses.
Materials and Methods
After this study received Institutional Review Board approval, we retrospectively reviewed whole-body fluorine-18-deoxyglucose (FDG) positron emission tomography–computed tomography (PET-CT) studies performed in our hospital between 2003 and 2006 to identify those with full-length bilateral femur CT scans. These scans were available on the hospital’s computerized picture archiving system (General Electric). Patients could be included in the study as long as they were at least 18 years old at time of scan and did not have any pathology that deformed the femur, broke a cortex, or otherwise caused any gross asymmetry of the femur. Of the 72 patients with full-length femur CT scans, 3 were excluded: 1 with a congenital hip dysplasia, 1 with an old, malunited femoral fracture, and 1 who was 15 years old at time of scan.
Axial Slice Selection
For each patient, scout AP films were used to measure femoral shaft length from the top of the greater trochanter to the end of the lateral femoral condyle. The levels of the proximal third, midshaft, and distal third were then calculated based on this length. The LA was studied on the axial slices nearest these levels. Next, we scrolled through the scans to identify an axial slice that best showed the femoral neck axis. The literature on CT measurement of femoral anteversion is varied. Some articles describe a technique that uses 2 superimposed axial slices, and others describe a single axial slice.1-3 We used 1 axial slice to draw the femoral neck axis because our computer software could not superimpose 2 images on 1 screen and because the CT scans were not made under specific protocols to measure anteversion but rather were part of a cancer staging work-up. Axial cuts were made at 5-mm intervals, and not all scans included a single slice capturing the head, neck, and greater trochanter. Therefore, we used a (previously described) method in which the femoral neck axis is drawn on a slice that most captured the femoral neck, usually toward its base.4 Last, in order to draw the posterior condyle (PC) axis, we selected an axial slice that showed the posterior-most aspects of the femoral condyles at the intercondylar notch.
Determining Anteroposterior and Posterior Condyle Axes of Femur
As we made all measurements for each femur off a single CT scan, we were able to use a straight horizontal line—drawn on-screen with a software tool—as a reference for measuring rotation. On a distal femur cut, the PC axis is drawn by connecting the posterior-most points of both condyles. The software calculates the angle formed—the PC angle (Figure 1). This angle, the degree to which the PC axis deviates from a straight horizontal line on-screen, can be used to account for gross rotation of the limb on comparison of images. The AP axis of the femur is the axis perpendicular to the PC axis. As such, the PC angle can also be used to determine degree of deviation of the AP axis from a straight vertical line on-screen. The AP axis was used when calculating the LA axis at the various levels of the femur (Figure 2).
Femoral Version
We used the software tool to draw the femoral neck axis. From the end of this line, a straight horizontal line is drawn on-screen (Figure 3). The software calculates the angle formed—the femoral neck axis angle. We assigned a positive value for a femoral head that pointed anteriorly on the image and a negative value for a head that pointed posteriorly. Adjusting for external rotation of the limb involved calculating the femoral version by subtracting the PC angle from the neck axis angle; adjusting for internal rotation involved adding these 2 angles.
Linea Aspera Morphology
After viewing the first 20 CT scans, we identified 3 types of LA morphology. Type I presents as a thickening on the posterior cortex with a sharp apex; type II presents as a flat-faced but distinct ridge of bone between the medial and lateral lips; and in type III there is no distinct cortical thickening with blunted medial and lateral lips; the latter is always more prominent.
Linea Aspera Axis Offset
From the most posterior point of the LA, a line drawn forward bisecting the femoral canal defined the LA axis. In type I morphology, the posterior-most point was the apex; in type II, the middle of flat posterior surface was used as the starting point; in type III, the lateral lip was used, as it was sharper than the medial lip. This line is again referenced with a straight horizontal line across the image. The PC angle is then added to account for limb rotation, and the result is the LA angle. As the AP axis is perpendicular to the PC axis, the LA angle is subtracted from 90°; the difference represents the amount of offset of the LA axis from the AP axis. By convention, we assigned this a positive value for an LA lateral to the midpoint of the femur and a negative value for an LA medial to the midpoint (Figure 4).
Linea Aspera Axis and Femoral Neck Axis
The angle between the LA axis and the PC axis was measured. The femoral version angle was subtracted from that angle to obtain the arc between the LA axis and the femoral neck axis.
Statistical Analyses
All analyses were performed with SAS 9.1 (SAS Institute). All tests were 2-sided and conducted at the .05 significance level. No adjustments were made for multiple testing. Statistical analysis was performed with nonparametric tests and without making assumptions about the distribution of the study population. Univariate analyses were performed to test for significant side-to-side differences in femoral length, femoral version angle, and LA torsion angles at each level. A multivariate analysis was performed to test for interactions between sex, side, and level. In all analyses, P < .05 was used as the cutoff value for statistical significance.
Results
Femoral lengths varied by side and sex. The left side was longer than the right by a mean of 1.3 mm (P = .008). With multivariate analysis taking into account sex and age (cumulated per decade), there was still a significant effect of side on femoral length. Sex also had a significant effect on femoral length, with females’ femurs shorter by 21.7 mm (standard error, 5.0 mm). Mean (SD) anteversion of the femoral neck was 7.9° (12.7°) on the left and 13.3° (13.0°) on the right; the difference between sides was significant (P < .001). In a multivariate analysis performed to identify potential predictors of femoral version, side still had a significant (P < .001) independent effect; sex and age did not have an effect.
LA morphology varied according to femoral shaft level (Table 1). The morphology was type I in 75% of patients at the distal femur and 74% of patients at the midshaft femur, while only 53% of patients had a type I morphology at the proximal femur. The proportion of type III morphology was larger in the proximal femur (41%) than in the other locations.
The LA axis of the femur did not correspond exactly to the AP axis at all femoral levels. At the distal femur, mean (SD) lateral offset of the LA axis was 5.5° (7.5°) on the left and 8.3° (8.9°) on the right. At the midshaft, mean (SD) medial offset of the LA axis was 3.1° (8.4°) on the left and 1.2° (7.9°) on the right. At the proximal femur, mean (SD) lateral offset of the LA axis was 5.4° (9.2°) on the left and 6.2° (8.3°) on the right. The side-to-side differences were statistically significant for the distal femur and midshaft but not the proximal femur. Table 2 lists the 95% confidence intervals for the mean values. As the range of differences was small (0.7°-2.8°), and the differences may not be clinically detected on gross inspection during surgery, we pooled both sides’ values to arrive at a single mean for each level. The LA axis was offset a mean (SD) of 6.9° (8.3°) laterally at the distal femur, 2.2° (8.2°) medially at the midshaft, and 5.8° (8.6°) laterally at the proximal femur. Figure 5 shows the frequency of distribution of LA axis offset.Offset of the LA axis from the AP axis of the femur was significantly (P < .001) different for each femoral level, even when a multivariate analysis was performed to determine the effect of sex, age, or side. Age and sex had no significant effect on mean offset of LA axis from AP axis.
We compared the mean arc between femoral neck axis and LA axis after referencing both off the PC axis. At the distal femur, mean (SD) arc between these 2 axes was 76.6° (13.1°) on the left and 68.3° (13.6°) on the right (mean difference, 8.3°); at the midshaft, mean (SD) arc was 85.2° (13.5°) on the left and 77.9° (13.1°) on the right (mean difference, 7.4°); at the proximal femur, mean (SD) arc was 76.7° (11.9°) on the left and 70.5° (12.8°) on the right (mean difference, 6.2°). The side-to-side differences were statistically significant (P < .001) for all locations.
In multivariate analysis, sex and age did not have an effect on mean arc between the 2 axes. Side and femoral level, however, had a significant effect (P < .001).
Discussion
In total hip arthroplasty, the goal is to restore femoral anteversion, usually referenced to the remaining femoral neck segment.3 In total knee arthroplasty (TKA), proper rotation preserves normal patellofemoral tracking.5 Various landmarks are used, such as the PCs or the epicondyles. After tumor resections, these landmarks are often lost.6 However, there are no reports of studies validating a particular method of achieving proper rotational orientation of tumor endoprostheses, though several methods are being used. One method involves inserting 2 drill bits before osteotomy—one proximal to the intended level of resection on the anterior femur, and the other on the anterior tibial shaft. The straight line formed can establish a plane of rotation (and length), which the surgeon must aim to restore when the components are placed. This method is useful for distal femur resections but not proximal femur resections. Another method, based on the LA’s anatomical position on the posterior aspect of the femur,4 uses the prominence of the LA to align the prosthesis. With this method, the LA is assumed to be directly posterior (6 o’clock) on the femur. However, this assumption has not been confirmed by any study. A third method, described by Heck and Carnesale,5 involves marking the anterior aspect of the femur after resection and aligning the components to it. The authors cautioned against using the LA as a landmark, saying that its course is highly variable.
The LA is a narrow, elevated length of bone, with medial and lateral lips, that serves as an attachment site for muscles in the posterior thigh. Proximally, the LA presents with lateral, medial, and intermediate lips. In the midshaft, it is often elevated by an underlying bony ridge or pilaster complex. Distally, it diverges into 2 ridges that form the triangular popliteal surface.1,7 For the LA to be a reliable landmark, first it must be clearly identifiable on viewing a femoral cross-section. The LA that presents with type I or II morphology is distinctly identifiable, and an axis from its apex and bisecting the canal can easily be constructed. In our study, the LA presented with type I or II morphology in 82% of distal femoral sections and 99% of midshaft femoral sections. Therefore, the LA is a conspicuous landmark at these levels. In the proximal femur, 59% had type I or II morphology. Type III morphology could be identified on cross-sections by the persisting prominence of the lateral lip. However, it may be difficult to appreciate the LA with this morphology at surgery.
Once the LA is identified, its normal cross-sectional position must be defined. One way to do this is to establish the relationship of its axis (LA axis) to the true AP axis. Based on mean values, the LA axis is laterally offset 7º at the distal third of the femur, medially offset 2º at the midshaft, and laterally offset 6º at the proximal third. Therefore, for ideal placement with the LA used for orientation, the component must be internally rotated 7º relative to the LA for femoral resection at the distal third, externally rotated 2º for resection at the midshaft, and internally rotated 6º for resection at the proximal third. Studies have demonstrated that joint contact forces and mechanical alignment of the lower limb can be altered with as little as 5º of femoral malrotation.8,9 Although such a small degree of malrotation is often asymptomatic, it can have long-term effects on soft-tissue tension and patellar tracking.10,11 Rotating-platform mobile-bearing TKA designs can compensate for femoral malrotation, but they may have little to no effect on patellar tracking.12 Therefore, we think aligning the components as near as possible to their natural orientation can prove beneficial in long-term patient management.
Another way of defining the normal cross-sectional position of the LA is to relate it to the femoral neck axis. We measured the difference between these 2 axes. Mean differences were 72º (distal femur), 81.5º (midshaft), and 73.5º (proximal third). Mean arc differences at all levels were larger on the left side—a reflection of the femoral neck being less anteverted on that side in our measurements. Standard deviations were smaller for measurements of LA axis offset from AP axis (range, 7.5°-9.2°) than for measurements of arc between LA axis and femoral neck axis (range, 11.9°-13.6°). This finding indicates there is less variation in the former method, making it preferable for defining the cross-sectional position of the LA.
It has been said that the course of the LA is variable, and our data provide confirmation. The LA does not lie directly posterior (6 o’clock), and it does not trace a straight longitudinal course along the posterior femur, as demonstrated by the different LA axis offsets at 3 levels. However, we may still use it as a landmark if we remain aware how much the LA is offset from the AP axis at each femoral level. Figures 6A-6D, which show CT scans of a patient who underwent distal femoral resection and replacement with an endoprosthesis, illustrate how the LA axis was measured before surgery and how proper prosthesis placement was confirmed after surgery.
In hip arthroplasty, restoration of normal femoral version is the reference for endoprosthetic placement. The literature on “normal” femoral anteversion varies with the method used. In a review of studies on CT-measured adult femoral version, reported values ranged from 6.3° to 40°.2 Mean femoral version in our study ranged from 8° to 13°. Orthopedics textbooks generally put the value at 10° to 15º, and this seems to be the range that surgeons target.6 However, we found a statistically significant mean side-to-side difference of 5.4°. This finding is possibly explained by our large sample—it was larger than the samples used in other studies of CT-measured femoral version. Other studies have found mean side-to-side differences of up to 4.0º.5 Another explanation for our finding is that the studies may differ methodologically. The studies that established values for femoral anteversion were based on CT protocols—thinner slices (1-5 mm), use of foot holders to standardize limb rotation, use of 2 axial cuts in proximal femur to establish femoral neck axis2,13—designed specifically for this measurement. As the CT scans reviewed in our study are not designed for this purpose, errors in femoral version measurement may have been introduced, which may also explain why there is larger variation in measurements of the arc between the LA axis and the femoral neck axis.
Conclusion
The LA does not lie directly on the posterior surface of the femur. It deviates 6.9° laterally at the distal femur, 2.2° medially at the midshaft, and 6.9° laterally at the proximal third. As the LA is an easily identifiable structure on cross-sections of the femoral shaft at the midshaft and distal third of the femur, it may be useful as a rotational landmark for resections at these levels if these deviations are considered during tumor endoprosthetic replacements.
1. Desai SC, Willson S. Radiology of the linea aspera. Australas Radiol. 1985;29(3):273-274.
2. Kuo TY, Skedros JG, Bloebaum RD. Measurement of femoral anteversion by biplane radiography and computed tomography imaging: comparison with an anatomic reference. Invest Radiol. 2003;38(4):221-229.
3. Wines AP, McNicol D. Computed tomography measurement of the accuracy of component version in total hip arthroplasty. J Arthroplasty. 2006;21(5):696-701.
4. Gray H. Anatomy of the Human Body. Philadelphia, PA: Lea & Febiger; 1918.
5. Heck RK, Carnesale PG. General principles of tumors. In: Canale ST, ed. Campbell’s Operative Orthopaedics. Vol 1. 10th ed. St. Louis, MO: Mosby; 2003:733-791.
6. Katz, MA, Beck TD, Silber JS, Seldes RM, Lotke PA. Determining femoral rotational alignment in total knee arthroplasty: reliability of techniques. J Arthroplasty. 2001;16(3):301-305.
7. Pitt MJ. Radiology of the femoral linea aspera–pilaster complex: the track sign. Radiology. 1982;142(1):66.
8. Bretin P, O’Loughlin PF, Suero EM, et al. Influence of femoral malrotation on knee joint alignment and intra-articular contact pressures. Arch Orthop Trauma Surg. 2011;131(8):1115-1120.
9. Zihlmann MS, Stacoff A, Romero J, Quervain IK, Stüssi E. Biomechanical background and clinical observations of rotational malalignment in TKA: literature review and consequences. Clin Biomech. 2005;20(7):661-668.
10. Ghosh KM, Merican AM, Iranpour F, Deehan DJ, Amis AA. The effect of femoral component rotation on the extensor retinaculum of the knee. J Orthop Res. 2010;28(9):1136-1141.
11. Verlinden C, Uvin P, Labey L, Luyckx JP, Bellemans J, Vandenneucker H. The influence of malrotation of the femoral component in total knee replacement on the mechanics of patellofemoral contact during gait: an in vitro biomechanical study. J Bone Joint Surg Br. 2010;92(5):737-742.
12. Kessler O, Patil S, Colwell CW Jr, D’Lima DD. The effect of femoral component malrotation on patellar biomechanics. J Biomech. 2008;41(16):3332-3339.
13. Strecker W, Keppler P, Gebhard F, Kinzl L. Length and torsion of the lower limb. J Bone Joint Surg Br. 1997;79(6):1019-1023.
1. Desai SC, Willson S. Radiology of the linea aspera. Australas Radiol. 1985;29(3):273-274.
2. Kuo TY, Skedros JG, Bloebaum RD. Measurement of femoral anteversion by biplane radiography and computed tomography imaging: comparison with an anatomic reference. Invest Radiol. 2003;38(4):221-229.
3. Wines AP, McNicol D. Computed tomography measurement of the accuracy of component version in total hip arthroplasty. J Arthroplasty. 2006;21(5):696-701.
4. Gray H. Anatomy of the Human Body. Philadelphia, PA: Lea & Febiger; 1918.
5. Heck RK, Carnesale PG. General principles of tumors. In: Canale ST, ed. Campbell’s Operative Orthopaedics. Vol 1. 10th ed. St. Louis, MO: Mosby; 2003:733-791.
6. Katz, MA, Beck TD, Silber JS, Seldes RM, Lotke PA. Determining femoral rotational alignment in total knee arthroplasty: reliability of techniques. J Arthroplasty. 2001;16(3):301-305.
7. Pitt MJ. Radiology of the femoral linea aspera–pilaster complex: the track sign. Radiology. 1982;142(1):66.
8. Bretin P, O’Loughlin PF, Suero EM, et al. Influence of femoral malrotation on knee joint alignment and intra-articular contact pressures. Arch Orthop Trauma Surg. 2011;131(8):1115-1120.
9. Zihlmann MS, Stacoff A, Romero J, Quervain IK, Stüssi E. Biomechanical background and clinical observations of rotational malalignment in TKA: literature review and consequences. Clin Biomech. 2005;20(7):661-668.
10. Ghosh KM, Merican AM, Iranpour F, Deehan DJ, Amis AA. The effect of femoral component rotation on the extensor retinaculum of the knee. J Orthop Res. 2010;28(9):1136-1141.
11. Verlinden C, Uvin P, Labey L, Luyckx JP, Bellemans J, Vandenneucker H. The influence of malrotation of the femoral component in total knee replacement on the mechanics of patellofemoral contact during gait: an in vitro biomechanical study. J Bone Joint Surg Br. 2010;92(5):737-742.
12. Kessler O, Patil S, Colwell CW Jr, D’Lima DD. The effect of femoral component malrotation on patellar biomechanics. J Biomech. 2008;41(16):3332-3339.
13. Strecker W, Keppler P, Gebhard F, Kinzl L. Length and torsion of the lower limb. J Bone Joint Surg Br. 1997;79(6):1019-1023.
IVC and Mortality in ADHF
Heart failure costs the United States an excess of $30 billion annually, and costs are projected to increase to nearly $70 billion by 2030.[1] Heart failure accounts for over 1 million hospitalizations and is the leading cause of hospitalization in patients >65 years of age.[2] After hospitalization, approximately 50% of patients are readmitted within 6 months of hospital discharge.[3] Mortality rates from heart failure have improved but remain high.[4] Approximately 50% of patients diagnosed with heart failure die within 5 years, and the overall 1‐year mortality rate is 30%.[1]
Prognostic markers and scoring systems for acute decompensated heart failure (ADHF) continue to emerge, but few bedside tools are available to clinicians. Age, brain natriuretic peptide, and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) levels have been shown to correlate with postdischarge rates of readmission and mortality.[5] A study evaluating the prognostic value of a bedside inferior vena cava (IVC) ultrasound exam demonstrated that lack of improvement in IVC distention from admission to discharge was associated with higher 30‐day readmission rates.[6] Two studies using data from comprehensive transthoracic echocardiograms in heart failure patients demonstrated that a dilated, noncollapsible IVC is associated with higher risk of mortality; however, it is well recognized that obtaining comprehensive transthoracic echocardiograms in all patients hospitalized with heart failure is neither cost‐effective nor practical.[7]
In recent years, multiple studies have emerged demonstrating that noncardiologists can perform focused cardiac ultrasound exams with high reproducibility and accuracy to guide management of patients with ADHF.[8, 9, 10, 11, 12, 13, 14] However, it is unknown whether IVC characteristics from a focused cardiac ultrasound exam performed by a noncardiologist can predict mortality of patients hospitalized with ADHF. The aim of this study was to assess whether a hospitalist‐performed focused ultrasound exam to measure the IVC diameter at admission and discharge can predict mortality in a general medicine ward population hospitalized with ADHF.
METHODS
Study Design
A prospective, observational study of patients admitted to a general medicine ward with ADHF between January 2012 and March 2013 was performed using convenience sampling. The setting was a 247‐bed, university‐affiliated hospital in Madrid, Spain. Inclusion criteria were adult patients admitted with a primary diagnosis of ADHF per the European Society of Cardiology (ESC) criteria.[15] Exclusion criteria were admission to the intensive care unit for mechanical ventilation, need for chronic hemodialysis, or a noncardiac terminal illness with a life expectancy of less than 3 months. All patients provided written informed consent prior to enrollment. This study complies with the Declaration of Helsinki and was approved by the local ethics committee.
The primary outcome was all‐cause mortality at 90 days after hospitalization. The secondary outcomes were hospital readmission at 90 and 180 days, and mortality at 180 days. Patients were prospectively followed up at 30, 60, 90, and 180 days after discharge by telephone interview or by review of the patient's electronic health record. Patients who died within 90 days of discharge were categorized as nonsurvivors, whereas those alive at 90 days were categorized as survivors.
The following data were recorded on admission: age, gender, blood pressure, heart rate, functional class per New York Heart Association (NYHA) classification, comorbidities (hypertension, diabetes mellitus, atrial fibrillation, chronic obstructive pulmonary disease), primary etiology of heart failure, medications, electrocardiogram, NT‐terminal pro‐BNP, hemoglobin, albumin, creatinine, sodium, measurement of performance of activities of daily living (modified Barthel index), and comorbidity score (age‐adjusted Charlson score). A research coordinator interviewed subjects to gather data to calculate a modified Barthel index.[16] Age‐adjusted Charlson comorbidity scores were calculated using age and diagnoses per International Classification of Diseases, Ninth Revision coding.[17]
IVC Measurement
An internal medicine hospitalist with expertise in point‐of‐care ultrasonography (G.G.C.) performed all focused cardiac ultrasound exams to measure the IVC diameter and collapsibility at the time of admission and discharge. This physician was not involved in the inpatient medical management of study subjects. A second physician (N.J.S.) randomly reviewed 10% of the IVC images for quality assurance. Admission IVC measurements were acquired within 24 hours of arrival to the emergency department after the on‐call medical team was contacted to admit the patient. Measurement of the IVC maximum (IVCmax) and IVC minimum (IVCmin) diameters was obtained just distal to the hepatic veinIVC junction, or 2 cm from the IVCright atrial junction using a long‐axis view of the IVC. Measurement of the IVC diameter was consistent with the technique recommended by the American Society of Echocardiography and European Society of Echocardiography guidelines.[18, 19] The IVC collapsibility index (IVCCI) was calculated as (IVCmaxIVCmin)/IVCmax per guidelines.[18] Focused cardiac ultrasound exams were performed using a General Electric Logiq E device (GE Healthcare, Little Chalfont, United Kingdom) with a 3.5 MHz curvilinear transducer. Inpatient medical management by the primary medical team was guided by protocols from the ESC guidelines on the treatment of ADHF.[15] A comprehensive transthoracic echocardiogram (TTE) was performed on all study subjects by the echocardiography laboratory within 24 hours of hospitalization as part of the study protocol. One of 3 senior cardiologists read all comprehensive TTEs. NT‐proBNP was measured on admission and discharge by electrochemiluminescence.
Statistical Analysis
We calculated the required sample size based on published mortality and readmission rates. For our primary outcome of 90‐day mortality, we calculated a required sample size of 64 to achieve 80% power based on 90‐day and 1‐year mortality rates of 21% and 33%, respectively, among Spanish elderly patients (age 70 years) hospitalized with ADHF.[20] For our secondary outcome of 90‐day readmissions, we calculated a sample size of 28 based on a 41% readmission rate.[21] Therefore, our target subject enrollment was at least 70 patients to achieve a power of 80%.
Statistical analyses were performed using SPSS 17.0 statistical package (SPSS Inc., Chicago, IL). Subject characteristics that were categorical variables (demographics and comorbidities) were summarized as counts and percentages. Continuous variables, including IVC measurements, were summarized as means with standard deviations. Differences between categorical variables were analyzed using the Fisher exact test. Survival curves with log‐rank statistics were used to perform survival analysis. The nonparametric Mann‐Whitney U test was used to assess associations between the change in IVCCI, and readmissions and mortality at 90 and 180 days. Predictors of readmission and death were evaluated using a multivariate Cox proportional hazards regression analysis. Given the limited number of primary outcome events, we used age, IVC diameter, and log NT‐proBNP in the multivariate regression analysis based on past studies showing prognostic significance of these variables.[6, 22, 23, 24, 25, 26, 27, 28] Optimal cutoff values for IVC diameter for death and readmission prediction were determined by constructing receiver operating characteristic (ROC) curves and calculating the area under the curve (AUC) for different IVC diameters. NT‐proBNP values were log‐transformed to minimize skewing as reported in previous studies.[29]
RESULTS
Patient Characteristics
Ninety‐seven patients admitted with ADHF were recruited for the study. Optimal acoustic windows to measure the IVC diameter were acquired in 90 patients (93%). Because measurement of discharge IVC diameter was required to calculate the change from admission to discharge, 8 patients who died during initial hospitalization were excluded from the final data analysis. An additional two patients were excluded due to missing discharge NT‐proBNP measurement or missing comprehensive echocardiogram data. The study cohort from whom data were analyzed included 80 of 97 total patients (82%).
Baseline demographic, clinical, laboratory, and comprehensive echocardiographic characteristics of nonsurvivors and survivors at 90 days are demonstrated in Table 1. Eleven patients (13.7%) died during the first 90 days postdischarge, and all deaths were due to cardiovascular complications. Nonsurvivors were older (86 vs 76 years; P = 0.02), less independent in performance of their activities of daily living (Barthel index of 58.1 vs 81.9; P = 0.01), and were more likely to have advanced heart failure with an NYHA functional class of III or IV (72% vs 33%; P = 0.016). Atrial fibrillation (90% vs 55%; P = 0.008) and lower systolic blood pressure (127 mm Hg vs 147 mm Hg; P = 0.01) were more common in nonsurvivors than survivors, and fewer nonsurvivors were taking a ‐blocker (18% vs 59%; P = 0.01). Baseline comprehensive echocardiographic findings were similar between the survivors and nonsurvivors, except left atrial diameter was larger in nonsurvivors versus survivors (54 mm vs 49 mm; P = 0.04).
| Total Cohort, n = 80 | Nonsurvivors, n = 11 | Survivors, n = 69 | P Value | |
|---|---|---|---|---|
| ||||
| Demographics | ||||
| Age, y* | 78 (13) | 86 (7) | 76 (14) | 0.02 |
| Men, n (%) | 34 (42) | 3 (27) | 26 (38) | 0.3 |
| Vital signs* | ||||
| Heart rate, beats/min | 94 (23) | 99 (26) | 95 (23) | 0.5 |
| SBP, mm Hg | 141 (27) | 127 (22) | 147 (25) | 0.01 |
| Comorbidities, n (%) | ||||
| Hypertension | 72 (90) | 10 (91) | 54 (78) | 0.3 |
| Diabetes mellitus | 35 (44) | 3 (27) | 26 (38) | 0.3 |
| Atrial fibrillation | 48 (60) | 10 (90) | 38 (55) | 0.008 |
| COPD | 22 (27) | 3 (27) | 16 (23) | 0.5 |
| Etiology of heart failure | ||||
| Ischemic | 20 (25) | 1 (9) | 16 (23) | 0.1 |
| Hypertensive | 22 (27) | 2 (18) | 18 (26) | 0.4 |
| Valvulopathy | 29 (36) | 7 (64) | 19 (27) | 0.07 |
| Other | 18 (22) | 1 (9) | 16 (23) | 0.09 |
| NYHA IIIIV | 38 (47) | 8 (72) | 23 (33) | 0.016 |
| Charlson score* | 7.5 (2) | 9.0 (3) | 7.1 (2) | 0.02 |
| Barthel index* | 76 (31) | 58 (37) | 81.9 (28) | 0.01 |
| Medications | ||||
| ‐blocker | 44 (55) | 2 (18) | 41 (59) | 0.01 |
| ACE inhibitor/ARB | 48 (60) | 3 (27) | 35 (51) | 0.1 |
| Loop diuretic | 78 (97) | 10 (91) | 67 (97) | 0.9 |
| Aldosterone antagonist | 31 (39) | 4 (36) | 21 (30) | 0.4 |
| Lab results* | ||||
| Sodium, mmol/L | 137 (4.8) | 138 (6) | 139 (4) | 0.6 |
| Creatinine, umol/L | 1.24 (0.4) | 1.40 (0.5) | 1.17 (0.4) | 0.1 |
| eGFR, mL/min | 57.8 (20) | 51.2 (20) | 60.2 (19) | 0.1 |
| Albumin, g/L | 3.4 (0.4) | 3.3 (0.38) | 3.5 (0.41) | 0.1 |
| Hemoglobin, g/dL | 12.0 (2) | 10.9 (1.8) | 12.5 (2.0) | 0.01 |
| Echo parameters* | ||||
| LVEF, % | 52.1 (15) | 51.9 (17) | 51.6 (15) | 0.9 |
| LA diameter, mm | 50.1 (10) | 54 (11) | 49 (11) | 0.04 |
| RVDD, mm | 32.0 (11) | 34 (10) | 31 (11) | 0.2 |
| TAPSE, mm | 18.5 (7) | 17.4 (4) | 18.8 (7) | 0.6 |
| PASP, mm Hg | 51.2 (16) | 53.9 (17) | 50.2 (17) | 0.2 |
| Admission* | ||||
| NT‐proBNP, pg/mL | 8,816 (14,260) | 9,413 (5,703) | 8,762 (15,368) | 0.81 |
| Log NT‐proBNP | 3.66 (0.50) | 3.88 (0.31 | 3.62 (0.52) | 0.11 |
| IVCmax, cm | 2.12 (0.59) | 2.39 (0.37) | 2.06 (0.59) | 0.02 |
| IVCmin, cm | 1.63 (0.69) | 1.82 (0.66) | 1.56 (0.67) | 0.25 |
| IVCCI, % | 25.7 (0.16) | 25.9 (17.0) | 26.2 (16.0) | 0.95 |
| Discharge* | ||||
| NT‐proBNP, pg/mL | 3,132 (3,093) | 4,693 (4,383) | 2,909 (2,847) | 0.08 |
| Log NT‐proBNP | 3.27 (0.49) | 3.51 (0.37) | 3.23 (0.50) | 0.08 |
| IVCmax, cm | 1.87 (0.68) | 1.97 (0.54) | 1.81 (0.66) | 0.45 |
| IVCmin, cm | 1.33 (0.75) | 1.40 (0.65) | 1.27 (0.71) | 0.56 |
| IVCCI, % | 33.1 (0.20) | 32.0 (21.0) | 34.2 (19.0) | 0.74 |
From admission to discharge, the total study cohort demonstrated a highly statistically significant reduction in NT‐proBNP (8816 vs 3093; P < 0.001), log NT‐proBNP (3.66 vs 3.27; P < 0.001), IVCmax (2.12 vs 1.87; P < 0.001), IVCmin (1.63 vs 1.33; P < 0.001), and IVCCI (25.7% vs 33.1%; P < 0.001). The admission and discharge NT‐proBNP and IVC characteristics of the survivors and nonsurvivors are displayed in Table 2. The only statistically significant difference between nonsurvivors and survivors was the admission IVCmax (2.39 vs 2.06; P = 0.02). There was not a statistically significant difference in the discharge IVCmax between nonsurvivors and survivors.
| Admission | Discharge | Difference (DischargeAdmission) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Nonsurvivors | Survivors | P Value | Nonsurvivors | Survivors | P Value | Nonsurvivors | Survivors | P Value | |
| |||||||||
| NT‐proBNP, pg/mL | 9,413 (5,703) | 8,762 (15,368) | 0.81 | 4,693 (4,383) | 2,909 (2,847) | 0.08 | 3,717 5,043 | 5,026 11,507 | 0.7 |
| Log NT‐proBNP | 3.88 0.31 | 3.62 0.52 | 0.11 | 3.51 0.37 | 3.23 0.50 | 0.08 | 0.29 0.36 | 0.38 0.37 | 0.4 |
| IVCmax, cm | 2.39 0.37 | 2.06 0.59 | 0.02 | 1.97 0.54 | 1.81 0.66 | 0.45 | 0.39 0.56 | 0.25 0.51 | 0.4 |
| IVCmin, cm | 1.82 0.66 | 1.56 0.67 | 0.25 | 1.40 0.65 | 1.27 0.71 | 0.56 | 0.37 0.52 | 0.30 0.64 | 0.7 |
| IVCCI, % | 25.9 17.0 | 26.2 16.0 | 0.95 | 32.0 21.0 | 34.2 19.0 | 0.74 | 3.7 7.9 | 8.3 22 | 0.5 |
Outcomes
For the primary outcome of 90‐day mortality, the ROC curves showed a similar AUC for the admission IVCmax diameter (AUC: 0.69; 95% confidence interval [CI]: 0.53‐0.85), log NT‐proBNP at discharge (AUC: 0.67; 95% CI: 0.49‐0.85), and log NT‐proBNP at admission (AUC: 0.69; 95% CI: 0.52‐0.85). The optimal cutoff value for the admission IVCmax diameter to predict mortality was 1.9 cm (sensitivity 100%, specificity 38%) based on the ROC curves (see Supporting Information, Appendices 1 and 2, in the online version of this article). An admission IVCmax diameter 1.9 cm was associated with a higher mortality rate at 90 days (25.4% vs 3.4%; P = 0.009) and 180 days (29.3% vs 3.4%; P = 0.003). The Cox survival curves showed significantly lower survival rates in patients with an admission IVCmax diameter 1.9 cm (74.1 vs 96.7%; P = 0.012) (Figures 1 and 2). Based on the multivariate Cox proportional hazards regression analysis with age, IVCmax diameter, and log NT‐proBNP at admission, the admission IVCmax diameter and age were independent predictors of 90‐ and 180‐day mortality. The hazard ratios for death by age, admission IVCmax diameter, and log NT‐proBNP are shown in Table 3.
| Endpoint | Variable | HR (95% CI) | P Value |
|---|---|---|---|
| |||
| 90‐day mortality | Age | 1.14 (1.031.26) | 0.009 |
| IVC diameter at admission | 5.88 (1.2128.1) | 0.025 | |
| Log NT‐proBNP at admission | 1.00 (1.001.00) | 0.910 | |
| 90‐day readmission | Age | 1.06 (1.001.12) | 0.025 |
| IVC diameter at admission | 3.20 (1.248.21) | 0.016 | |
| Log NT‐proBNP at discharge | 1.00 (1.001.00) | 0.910 | |
| 180‐day mortality | Age | 1.12 (1.031.22) | 0.007 |
| IVC diameter at admission | 4.77 (1.2118.7) | 0.025 | |
| Log NT‐proBNP at admission | 1.00 (1.001.00) | 0.610 | |
| 180‐day readmission | Age | 1.06 (1.011.11) | 0.009 |
| IVC diameter at admission | 2.56 (1.145.74) | 0.022 | |
| Log NT‐proBNP at discharge | 1.00 (1.001.00) | 0.610 | |
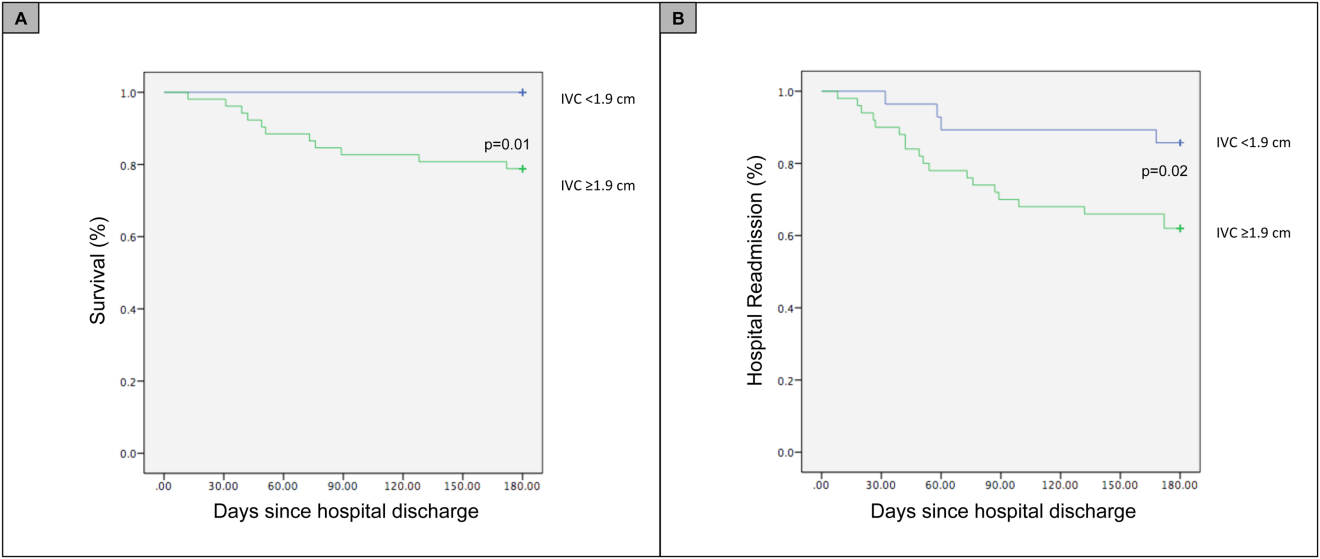
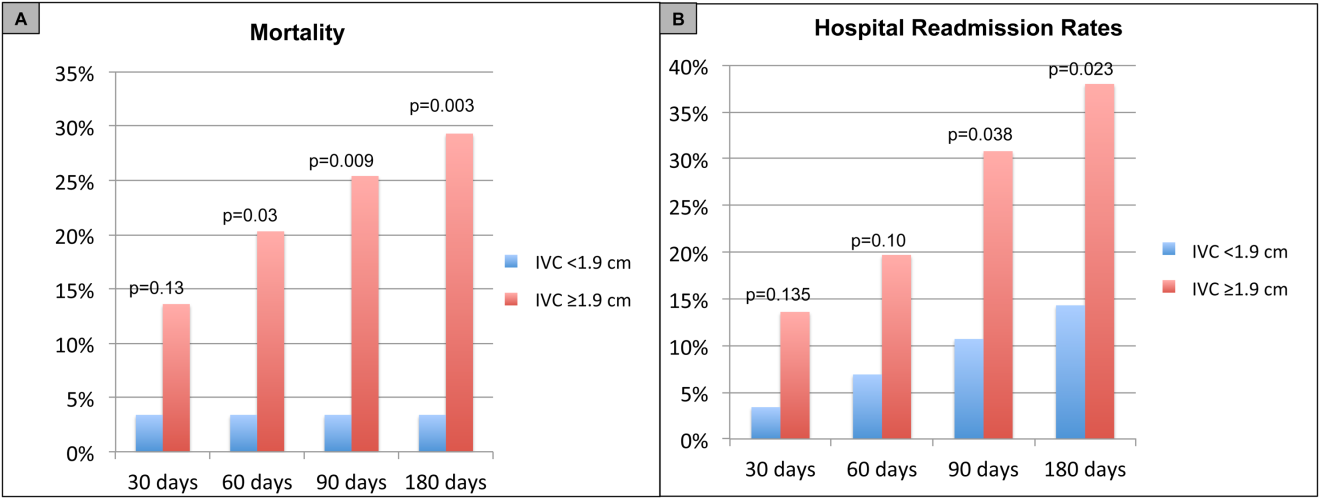
For the secondary outcome of 90‐day readmissions, 19 patients (24%) were readmitted, and the mean index admission IVCmax diameter was significantly greater in patients who were readmitted (2.36 vs 1.98 cm; P = 0.04). The ROC curves for readmission at 90 days showed that an index admission IVCmax diameter of 1.9 cm had the greatest AUC (0.61; 95% CI: 0.49‐0.74). The optimal cutoff value of an index admission IVCmax to predict readmission was also 1.9 cm (sensitivity 94%, specificity 42%) (see Supporting Information, Appendices 1 and 2, in the online version of this article). The Cox survival analysis showed that patients with an index admission IVCmax diameter 1.9 cm had a higher readmission rate at 90 days (30.8% vs 10.7%; P = 0.04) and 180 days (38.0 vs 14.3%; P = 0.02) (Figures 1 and 2). Using a multivariate Cox proportional regression analysis, the hazard ratios for the variables of age, admission IVCmax diameter, and log NT‐proBNP are shown in Table 3.
DISCUSSION
Our study found that a dilated IVC at admission is associated with a poor prognosis after hospitalization for ADHF. Patients with a dilated IVC 1.9 cm at admission had higher mortality and readmission rates at 90 and 180 days postdischarge.
The effect of a dilated IVC on mortality may be mediated through unrecognized right ventricular disease with or without significant pulmonary hypertension, supporting the notion that right heart function is an important determinant of prognosis in patients with ADHF.[30, 31] Similar to elevated jugular venous distension, bedside ultrasound examination of the IVC diameter can serve as a rapid and noninvasive measurement of right atrial pressure.[32] Elevated right atrial pressure is most often due to elevated left ventricular filling pressure transmitted via the pulmonary vasculature, but it is important to note that right‐ and left‐sided cardiac pressures are often discordant in heart failure patients.[33, 34]
Few studies have evaluated the prognostic value of IVC diameter and collapsibility in patients with heart failure. Nath et al.[24] evaluated the prognostic value of IVC diameter in stable veterans referred for outpatient echocardiography. Patients with a dilated IVC >2 cm that did not collapse with inspiration had higher 90‐day and 1‐year mortality rates. A subsequent study by Pellicori et al.[22] investigated the relationship between IVC diameter and other prognostic markers in stable cardiac patients. Pellicori et al. demonstrated that IVC diameter and serum NT‐proBNP levels were independent predictors of a composite endpoint of cardiovascular death or heart failure hospitalization at 1 year.[22] Most recently, Lee et al.[23] evaluated whether a dilated IVC in patients with a history of advanced systolic heart failure with a reduced ejection fraction of 30% and repeated hospitalizations (2) predicted worsening renal failure and adverse cardiovascular outcomes (death or hospitalization for ADHF). The study concluded that age, IVC diameter >2.1 cm, and worsening renal failure predicted cardiovascular death or hospitalization for ADHF.[23]
Our study demonstrated that an admission IVCmax 1.9 cm in hospitalized ADHF patients predicted higher postdischarge mortality at 90 and 180 days. Our findings are consistent with the above‐mentioned studies with a few important differences. First, all of our patients were hospitalized with acute decompensated heart failure. Nath et al. and Pellicori et al. evaluated stable ambulatory patients seen in an echocardiography lab and cardiology clinic, respectively. Only 12.1% of patients in the Nath study had a history of heart failure, and none were reported to have ADHF. More importantly, our study improves our understanding of patients with heart failure with a preserved ejection fraction, an important gap in the literature. The mean ejection fraction of patients in our study was 52% consistent with heart failure with preserved ejection fraction, whereas patients in the Pellicori et al. and Lee et al. studies had heart failure with reduced (42%) or severely reduced (30%) ejection fraction, respectively. We did not anticipate finding heart failure with preserved ejection fraction in the majority of patients, but our study's findings will add to our understanding of this increasingly common type of heart failure.
Compared to previous studies that utilized a registered diagnostic cardiac sonographer to obtain a comprehensive TTE to prognosticate patients, our study utilized point‐of‐care ultrasonography. Nath et al. commented that obtaining a comprehensive echocardiogram on every patient with ADHF is unlikely to be cost‐effective or feasible. Our study utilized a more realistic approach with a frontline internal medicinetrained hospitalist acquiring and interpreting images of the IVC at the bedside using a basic portable ultrasound machine.
Our study did not show that plasma natriuretic peptides levels are predictive of death or readmission after hospitalization for ADHF as shown in previous studies.[22, 35, 36] The small sample size, relatively low event rate, or predominance of heart failure with preserved ejection fraction may explain this inconsistency with prior studies.
Previous studies have reported hospital readmission rates for ADHF of 30% to 44% after 1 to 6 months.[6, 37] Goonewardena et al. showed a 41.3% readmission rate at 30 days in patients with severely reduced left ventricular ejection fraction (mean 29%), and readmitted patients had an IVCmax diameter >2 cm and an IVC collapsibility <50% on admission and discharge.[6] Carbone et al. demonstrated absence of improvement in the minimum IVC diameter from admission to discharge using hand‐carried ultrasound in patients with ischemic heart disease (ejection fraction 33%) predicted readmission at 60 days.[38] Hospital readmission rates in our study are consistent with these previously published studies. We found readmission rates for patients with ADHF and an admission IVCmax 1.9 cm to be 30.8% and 38.0% after 90 and 180 days, respectively.
Important limitations of our study are the small sample size and single institution setting. A larger sample size may have demonstrated that change in IVC diameter and NT‐proBNP levels from admission to discharge to be predictive of mortality or readmission. Further, we found an IVCmax diameter 1.9 cm to be the optimal cutoff to predict mortality, which is less than an IVCmax diameter >2.0 cm reported in other studies. The relatively smaller IVC diameter in Spanish heart failure patients may be explained by the lower body mass index of this population. An IVCmax diameter 1.9 cm was found to be the optimal cutoff to predict an elevated right atrial pressure >10 mm Hg in a study of Japanese cardiac patients with a relatively lower body mass index.[39] Another limitation is the timing of the admission IVC measurement within the first 24 hours of arrival to the hospital rather than immediately upon arrival to the emergency department. We were not able to control for interventions given in the emergency department prior to the measurement of the admission IVC, including doses of diuretics. Further, unlike the comprehensive TTEs in the United States, TTEs in Spain do not routinely include an assessment of the IVC. Therefore, we were not able to compare our bedside IVC measurements to those from a comprehensive TTE. An important limitation of our regression analysis is the inclusion of only 3 variables. The selection of variables (age, NT‐proBNP, and IVC diameter) was based on prior studies demonstrating their prognostic value.[6, 22, 25] Due to the low event rate (n = 11), we could not include in the regression model other variables that differed significantly between nonsurvivors and survivors, including NYHA class, presence of atrial fibrillation, and use of ‐blockers.
Perhaps in a larger study population the admission IVCmax diameter may not be as predictive of 90‐day mortality as other variables. The findings of our exploratory analysis should be confirmed in a future study with a larger sample size.
The clinical implications of our study are 3‐fold. First, our study demonstrates that IVC images acquired by a hospitalist at the bedside using a portable ultrasound machine can be used to predict postdischarge mortality and readmission of patients with ADHF. Second, the predominant type of heart failure in our study was heart failure with preserved ejection fraction. Currently, approximately 50% of patients hospitalized with ADHF have heart failure with preserved ejection fraction.[40] Our study adds to the understanding of prognosis of these patients whose heart failure pathophysiology is not well understood. Finally, palliative care services are underutilized in patients with advanced heart failure.[41, 42] IVC measurements and other prognostic markers in heart failure may guide discussions about goals of care with patients and families, and facilitate timely referrals for palliative care services.
CONCLUSIONS
Point‐of‐care ultrasound evaluation of IVC diameter at the time of admission can be used to prognosticate patients hospitalized with acute decompensated heart failure. An admission IVCmax diameter 1.9 cm is associated with a higher rate of 90‐day and 180‐day readmission and mortality after hospitalization. Future studies should evaluate the combination of IVC characteristics with other markers of severity of illness to prognosticate patients with heart failure.
Disclosures
This study was supported by a grant from the Madrid‐Castilla la Mancha Society of Internal Medicine. Dr. Restrepo is partially supported by award number K23HL096054 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. The authors report no conflicts of interest.
- , , , et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322.
- , , . Hospitalization for congestive heart failure: United States, 2000–2010. NCHS Data Brief. 2012(108):1–8.
- , . Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126(4):501–506.
- , , , et al. Generalizability and longitudinal outcomes of a national heart failure clinical registry: Comparison of Acute Decompensated Heart Failure National Registry (ADHERE) and non‐ADHERE Medicare beneficiaries. Am Heart J. 2010;160(5):885–892.
- , , , et al. Prognostic markers of acute decompensated heart failure: the emerging roles of cardiac biomarkers and prognostic scores. Arch Cardiovasc Dis. 2015;108(1):64–74.
- , , , et al. Comparison of hand‐carried ultrasound assessment of the inferior vena cava and N‐terminal pro‐brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging. 2008;1(5):595–601.
- , , , , . Echocardiography in acute heart failure: current perspectives. J Card Fail. 2016;22(1):82–94.
- , , , , . Usefulness of a hand‐held ultrasound device for bedside examination of left ventricular function. Am J Cardiol. 2002;90(9):1038–1039.
- , , , , , . Feasibility of point‐of‐care echocardiography by internal medicine house staff. Am Heart J. 2004;147(3):476–481.
- , , , , , . The use of small personal ultrasound devices by internists without formal training in echocardiography. Eur J Echocardiogr. 2003;4(2):141–147.
- , , , et al. Diagnostic accuracy of hospitalist‐performed hand‐carried ultrasound echocardiography after a brief training program. J Hosp Med. 2009;4(6):340–349.
- , , , . Point‐of‐care multi‐organ ultrasound improves diagnostic accuracy in adults presenting to the emergency department with acute dyspnea. West J Emerg Med. 2016;17(1):46–53.
- , , , , , . Acute heart failure: the role of focused emergency cardiopulmonary ultrasound in identification and early management. Eur J Heart Fail. 2015;17(12):1223–1227.
- , , , et al. Hand‐carried echocardiography by hospitalists: a randomized trial. Am J Med. 2011;124(8):766–774.
- , , , et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail. 2008;10(10):933–989.
- , , , , , . Adaptation of the modified Barthel Index for use in physical medicine and rehabilitation in Turkey. Scand J Rehabil Med. 2000;32(2):87–92.
- , , , . Complications, comorbidities, and mortality: improving classification and prediction. Health Serv Res. 1997;32(2):229–238; discussion 239–242.
- , , , et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713; quiz 786–688.
- , , , et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463.
- , , , , . Mortality and functional evolution at one year after hospital admission due to heart failure (HF) in elderly patients. Arch Gerontol Geriatr. 2012;54(1):261–265.
- , , , et al. Early and long‐term outcomes of heart failure in elderly persons, 2001–2005. Arch Intern Med. 2008;168(22):2481–2488.
- , , , et al. IVC diameter in patients with chronic heart failure: relationships and prognostic significance. JACC Cardiovasc Imaging. 2013;6(1):16–28.
- , , , et al. Prognostic significance of dilated inferior vena cava in advanced decompensated heart failure. Int J Cardiovasc Imaging. 2014;30(7):1289–1295.
- , , . A dilated inferior vena cava is a marker of poor survival. Am Heart J. 2006;151(3):730–735.
- , , , et al. Predischarge B‐type natriuretic peptide assay for identifying patients at high risk of re‐admission after decompensated heart failure. J Am Coll Cardiol. 2004;43(4):635–641.
- , , , et al. A rapid bedside test for B‐type peptide predicts treatment outcomes in patients admitted for decompensated heart failure: a pilot study. J Am Coll Cardiol. 2001;37(2):386–391.
- , , , , , . N‐terminal‐pro‐brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110(15):2168–2174.
- , , , , , . Lowered B‐type natriuretic peptide in response to levosimendan or dobutamine treatment is associated with improved survival in patients with severe acutely decompensated heart failure. J Am Coll Cardiol. 2009;53(25):2343–2348.
- , , , . Long‐term clinical variation of NT‐proBNP in stable chronic heart failure patients. Eur Heart J. 2007;28(2):177–182.
- , , , et al. Right atrial volume index in chronic systolic heart failure and prognosis. JACC Cardiovasc Imaging. 2009;2(5):527–534.
- , , , et al. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol. 2012;59(3):222–231.
- , , , et al. A comparison by medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure. Am J Cardiol. 2007;99(11):1614–1616.
- , , . Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66(4):493–496.
- , , , , , . Relationship between right and left‐sided filling pressures in 1000 patients with advanced heart failure. J Heart Lung Transplant. 1999;18(11):1126–1132.
- , , , et al. Admission B‐type natriuretic peptide levels and in‐hospital mortality in acute decompensated heart failure. J Am Coll Cardiol. 2007;49(19):1943–1950.
- , , , et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10(9):824–839.
- , , , et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157(1):99–104.
- , , , et al. Inferior vena cava parameters predict re‐admission in ischaemic heart failure. Eur J Clin Invest. 2014;44(4):341–349.
- , , , et al. Estimation of right atrial pressure on inferior vena cava ultrasound in Asian patients. Circ J. 2014;78(4):962–966.
- , , , , . Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47(1):76–84.
- , , , , . Palliative care referral among patients hospitalized with advanced heart failure. J Palliat Med. 2014;17(10):1115–1120.
- , , . Engaging heart failure clinicians to increase palliative care referrals: overcoming barriers, improving techniques. J Palliat Med. 2014;17(7):753–760.
Heart failure costs the United States an excess of $30 billion annually, and costs are projected to increase to nearly $70 billion by 2030.[1] Heart failure accounts for over 1 million hospitalizations and is the leading cause of hospitalization in patients >65 years of age.[2] After hospitalization, approximately 50% of patients are readmitted within 6 months of hospital discharge.[3] Mortality rates from heart failure have improved but remain high.[4] Approximately 50% of patients diagnosed with heart failure die within 5 years, and the overall 1‐year mortality rate is 30%.[1]
Prognostic markers and scoring systems for acute decompensated heart failure (ADHF) continue to emerge, but few bedside tools are available to clinicians. Age, brain natriuretic peptide, and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) levels have been shown to correlate with postdischarge rates of readmission and mortality.[5] A study evaluating the prognostic value of a bedside inferior vena cava (IVC) ultrasound exam demonstrated that lack of improvement in IVC distention from admission to discharge was associated with higher 30‐day readmission rates.[6] Two studies using data from comprehensive transthoracic echocardiograms in heart failure patients demonstrated that a dilated, noncollapsible IVC is associated with higher risk of mortality; however, it is well recognized that obtaining comprehensive transthoracic echocardiograms in all patients hospitalized with heart failure is neither cost‐effective nor practical.[7]
In recent years, multiple studies have emerged demonstrating that noncardiologists can perform focused cardiac ultrasound exams with high reproducibility and accuracy to guide management of patients with ADHF.[8, 9, 10, 11, 12, 13, 14] However, it is unknown whether IVC characteristics from a focused cardiac ultrasound exam performed by a noncardiologist can predict mortality of patients hospitalized with ADHF. The aim of this study was to assess whether a hospitalist‐performed focused ultrasound exam to measure the IVC diameter at admission and discharge can predict mortality in a general medicine ward population hospitalized with ADHF.
METHODS
Study Design
A prospective, observational study of patients admitted to a general medicine ward with ADHF between January 2012 and March 2013 was performed using convenience sampling. The setting was a 247‐bed, university‐affiliated hospital in Madrid, Spain. Inclusion criteria were adult patients admitted with a primary diagnosis of ADHF per the European Society of Cardiology (ESC) criteria.[15] Exclusion criteria were admission to the intensive care unit for mechanical ventilation, need for chronic hemodialysis, or a noncardiac terminal illness with a life expectancy of less than 3 months. All patients provided written informed consent prior to enrollment. This study complies with the Declaration of Helsinki and was approved by the local ethics committee.
The primary outcome was all‐cause mortality at 90 days after hospitalization. The secondary outcomes were hospital readmission at 90 and 180 days, and mortality at 180 days. Patients were prospectively followed up at 30, 60, 90, and 180 days after discharge by telephone interview or by review of the patient's electronic health record. Patients who died within 90 days of discharge were categorized as nonsurvivors, whereas those alive at 90 days were categorized as survivors.
The following data were recorded on admission: age, gender, blood pressure, heart rate, functional class per New York Heart Association (NYHA) classification, comorbidities (hypertension, diabetes mellitus, atrial fibrillation, chronic obstructive pulmonary disease), primary etiology of heart failure, medications, electrocardiogram, NT‐terminal pro‐BNP, hemoglobin, albumin, creatinine, sodium, measurement of performance of activities of daily living (modified Barthel index), and comorbidity score (age‐adjusted Charlson score). A research coordinator interviewed subjects to gather data to calculate a modified Barthel index.[16] Age‐adjusted Charlson comorbidity scores were calculated using age and diagnoses per International Classification of Diseases, Ninth Revision coding.[17]
IVC Measurement
An internal medicine hospitalist with expertise in point‐of‐care ultrasonography (G.G.C.) performed all focused cardiac ultrasound exams to measure the IVC diameter and collapsibility at the time of admission and discharge. This physician was not involved in the inpatient medical management of study subjects. A second physician (N.J.S.) randomly reviewed 10% of the IVC images for quality assurance. Admission IVC measurements were acquired within 24 hours of arrival to the emergency department after the on‐call medical team was contacted to admit the patient. Measurement of the IVC maximum (IVCmax) and IVC minimum (IVCmin) diameters was obtained just distal to the hepatic veinIVC junction, or 2 cm from the IVCright atrial junction using a long‐axis view of the IVC. Measurement of the IVC diameter was consistent with the technique recommended by the American Society of Echocardiography and European Society of Echocardiography guidelines.[18, 19] The IVC collapsibility index (IVCCI) was calculated as (IVCmaxIVCmin)/IVCmax per guidelines.[18] Focused cardiac ultrasound exams were performed using a General Electric Logiq E device (GE Healthcare, Little Chalfont, United Kingdom) with a 3.5 MHz curvilinear transducer. Inpatient medical management by the primary medical team was guided by protocols from the ESC guidelines on the treatment of ADHF.[15] A comprehensive transthoracic echocardiogram (TTE) was performed on all study subjects by the echocardiography laboratory within 24 hours of hospitalization as part of the study protocol. One of 3 senior cardiologists read all comprehensive TTEs. NT‐proBNP was measured on admission and discharge by electrochemiluminescence.
Statistical Analysis
We calculated the required sample size based on published mortality and readmission rates. For our primary outcome of 90‐day mortality, we calculated a required sample size of 64 to achieve 80% power based on 90‐day and 1‐year mortality rates of 21% and 33%, respectively, among Spanish elderly patients (age 70 years) hospitalized with ADHF.[20] For our secondary outcome of 90‐day readmissions, we calculated a sample size of 28 based on a 41% readmission rate.[21] Therefore, our target subject enrollment was at least 70 patients to achieve a power of 80%.
Statistical analyses were performed using SPSS 17.0 statistical package (SPSS Inc., Chicago, IL). Subject characteristics that were categorical variables (demographics and comorbidities) were summarized as counts and percentages. Continuous variables, including IVC measurements, were summarized as means with standard deviations. Differences between categorical variables were analyzed using the Fisher exact test. Survival curves with log‐rank statistics were used to perform survival analysis. The nonparametric Mann‐Whitney U test was used to assess associations between the change in IVCCI, and readmissions and mortality at 90 and 180 days. Predictors of readmission and death were evaluated using a multivariate Cox proportional hazards regression analysis. Given the limited number of primary outcome events, we used age, IVC diameter, and log NT‐proBNP in the multivariate regression analysis based on past studies showing prognostic significance of these variables.[6, 22, 23, 24, 25, 26, 27, 28] Optimal cutoff values for IVC diameter for death and readmission prediction were determined by constructing receiver operating characteristic (ROC) curves and calculating the area under the curve (AUC) for different IVC diameters. NT‐proBNP values were log‐transformed to minimize skewing as reported in previous studies.[29]
RESULTS
Patient Characteristics
Ninety‐seven patients admitted with ADHF were recruited for the study. Optimal acoustic windows to measure the IVC diameter were acquired in 90 patients (93%). Because measurement of discharge IVC diameter was required to calculate the change from admission to discharge, 8 patients who died during initial hospitalization were excluded from the final data analysis. An additional two patients were excluded due to missing discharge NT‐proBNP measurement or missing comprehensive echocardiogram data. The study cohort from whom data were analyzed included 80 of 97 total patients (82%).
Baseline demographic, clinical, laboratory, and comprehensive echocardiographic characteristics of nonsurvivors and survivors at 90 days are demonstrated in Table 1. Eleven patients (13.7%) died during the first 90 days postdischarge, and all deaths were due to cardiovascular complications. Nonsurvivors were older (86 vs 76 years; P = 0.02), less independent in performance of their activities of daily living (Barthel index of 58.1 vs 81.9; P = 0.01), and were more likely to have advanced heart failure with an NYHA functional class of III or IV (72% vs 33%; P = 0.016). Atrial fibrillation (90% vs 55%; P = 0.008) and lower systolic blood pressure (127 mm Hg vs 147 mm Hg; P = 0.01) were more common in nonsurvivors than survivors, and fewer nonsurvivors were taking a ‐blocker (18% vs 59%; P = 0.01). Baseline comprehensive echocardiographic findings were similar between the survivors and nonsurvivors, except left atrial diameter was larger in nonsurvivors versus survivors (54 mm vs 49 mm; P = 0.04).
| Total Cohort, n = 80 | Nonsurvivors, n = 11 | Survivors, n = 69 | P Value | |
|---|---|---|---|---|
| ||||
| Demographics | ||||
| Age, y* | 78 (13) | 86 (7) | 76 (14) | 0.02 |
| Men, n (%) | 34 (42) | 3 (27) | 26 (38) | 0.3 |
| Vital signs* | ||||
| Heart rate, beats/min | 94 (23) | 99 (26) | 95 (23) | 0.5 |
| SBP, mm Hg | 141 (27) | 127 (22) | 147 (25) | 0.01 |
| Comorbidities, n (%) | ||||
| Hypertension | 72 (90) | 10 (91) | 54 (78) | 0.3 |
| Diabetes mellitus | 35 (44) | 3 (27) | 26 (38) | 0.3 |
| Atrial fibrillation | 48 (60) | 10 (90) | 38 (55) | 0.008 |
| COPD | 22 (27) | 3 (27) | 16 (23) | 0.5 |
| Etiology of heart failure | ||||
| Ischemic | 20 (25) | 1 (9) | 16 (23) | 0.1 |
| Hypertensive | 22 (27) | 2 (18) | 18 (26) | 0.4 |
| Valvulopathy | 29 (36) | 7 (64) | 19 (27) | 0.07 |
| Other | 18 (22) | 1 (9) | 16 (23) | 0.09 |
| NYHA IIIIV | 38 (47) | 8 (72) | 23 (33) | 0.016 |
| Charlson score* | 7.5 (2) | 9.0 (3) | 7.1 (2) | 0.02 |
| Barthel index* | 76 (31) | 58 (37) | 81.9 (28) | 0.01 |
| Medications | ||||
| ‐blocker | 44 (55) | 2 (18) | 41 (59) | 0.01 |
| ACE inhibitor/ARB | 48 (60) | 3 (27) | 35 (51) | 0.1 |
| Loop diuretic | 78 (97) | 10 (91) | 67 (97) | 0.9 |
| Aldosterone antagonist | 31 (39) | 4 (36) | 21 (30) | 0.4 |
| Lab results* | ||||
| Sodium, mmol/L | 137 (4.8) | 138 (6) | 139 (4) | 0.6 |
| Creatinine, umol/L | 1.24 (0.4) | 1.40 (0.5) | 1.17 (0.4) | 0.1 |
| eGFR, mL/min | 57.8 (20) | 51.2 (20) | 60.2 (19) | 0.1 |
| Albumin, g/L | 3.4 (0.4) | 3.3 (0.38) | 3.5 (0.41) | 0.1 |
| Hemoglobin, g/dL | 12.0 (2) | 10.9 (1.8) | 12.5 (2.0) | 0.01 |
| Echo parameters* | ||||
| LVEF, % | 52.1 (15) | 51.9 (17) | 51.6 (15) | 0.9 |
| LA diameter, mm | 50.1 (10) | 54 (11) | 49 (11) | 0.04 |
| RVDD, mm | 32.0 (11) | 34 (10) | 31 (11) | 0.2 |
| TAPSE, mm | 18.5 (7) | 17.4 (4) | 18.8 (7) | 0.6 |
| PASP, mm Hg | 51.2 (16) | 53.9 (17) | 50.2 (17) | 0.2 |
| Admission* | ||||
| NT‐proBNP, pg/mL | 8,816 (14,260) | 9,413 (5,703) | 8,762 (15,368) | 0.81 |
| Log NT‐proBNP | 3.66 (0.50) | 3.88 (0.31 | 3.62 (0.52) | 0.11 |
| IVCmax, cm | 2.12 (0.59) | 2.39 (0.37) | 2.06 (0.59) | 0.02 |
| IVCmin, cm | 1.63 (0.69) | 1.82 (0.66) | 1.56 (0.67) | 0.25 |
| IVCCI, % | 25.7 (0.16) | 25.9 (17.0) | 26.2 (16.0) | 0.95 |
| Discharge* | ||||
| NT‐proBNP, pg/mL | 3,132 (3,093) | 4,693 (4,383) | 2,909 (2,847) | 0.08 |
| Log NT‐proBNP | 3.27 (0.49) | 3.51 (0.37) | 3.23 (0.50) | 0.08 |
| IVCmax, cm | 1.87 (0.68) | 1.97 (0.54) | 1.81 (0.66) | 0.45 |
| IVCmin, cm | 1.33 (0.75) | 1.40 (0.65) | 1.27 (0.71) | 0.56 |
| IVCCI, % | 33.1 (0.20) | 32.0 (21.0) | 34.2 (19.0) | 0.74 |
From admission to discharge, the total study cohort demonstrated a highly statistically significant reduction in NT‐proBNP (8816 vs 3093; P < 0.001), log NT‐proBNP (3.66 vs 3.27; P < 0.001), IVCmax (2.12 vs 1.87; P < 0.001), IVCmin (1.63 vs 1.33; P < 0.001), and IVCCI (25.7% vs 33.1%; P < 0.001). The admission and discharge NT‐proBNP and IVC characteristics of the survivors and nonsurvivors are displayed in Table 2. The only statistically significant difference between nonsurvivors and survivors was the admission IVCmax (2.39 vs 2.06; P = 0.02). There was not a statistically significant difference in the discharge IVCmax between nonsurvivors and survivors.
| Admission | Discharge | Difference (DischargeAdmission) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Nonsurvivors | Survivors | P Value | Nonsurvivors | Survivors | P Value | Nonsurvivors | Survivors | P Value | |
| |||||||||
| NT‐proBNP, pg/mL | 9,413 (5,703) | 8,762 (15,368) | 0.81 | 4,693 (4,383) | 2,909 (2,847) | 0.08 | 3,717 5,043 | 5,026 11,507 | 0.7 |
| Log NT‐proBNP | 3.88 0.31 | 3.62 0.52 | 0.11 | 3.51 0.37 | 3.23 0.50 | 0.08 | 0.29 0.36 | 0.38 0.37 | 0.4 |
| IVCmax, cm | 2.39 0.37 | 2.06 0.59 | 0.02 | 1.97 0.54 | 1.81 0.66 | 0.45 | 0.39 0.56 | 0.25 0.51 | 0.4 |
| IVCmin, cm | 1.82 0.66 | 1.56 0.67 | 0.25 | 1.40 0.65 | 1.27 0.71 | 0.56 | 0.37 0.52 | 0.30 0.64 | 0.7 |
| IVCCI, % | 25.9 17.0 | 26.2 16.0 | 0.95 | 32.0 21.0 | 34.2 19.0 | 0.74 | 3.7 7.9 | 8.3 22 | 0.5 |
Outcomes
For the primary outcome of 90‐day mortality, the ROC curves showed a similar AUC for the admission IVCmax diameter (AUC: 0.69; 95% confidence interval [CI]: 0.53‐0.85), log NT‐proBNP at discharge (AUC: 0.67; 95% CI: 0.49‐0.85), and log NT‐proBNP at admission (AUC: 0.69; 95% CI: 0.52‐0.85). The optimal cutoff value for the admission IVCmax diameter to predict mortality was 1.9 cm (sensitivity 100%, specificity 38%) based on the ROC curves (see Supporting Information, Appendices 1 and 2, in the online version of this article). An admission IVCmax diameter 1.9 cm was associated with a higher mortality rate at 90 days (25.4% vs 3.4%; P = 0.009) and 180 days (29.3% vs 3.4%; P = 0.003). The Cox survival curves showed significantly lower survival rates in patients with an admission IVCmax diameter 1.9 cm (74.1 vs 96.7%; P = 0.012) (Figures 1 and 2). Based on the multivariate Cox proportional hazards regression analysis with age, IVCmax diameter, and log NT‐proBNP at admission, the admission IVCmax diameter and age were independent predictors of 90‐ and 180‐day mortality. The hazard ratios for death by age, admission IVCmax diameter, and log NT‐proBNP are shown in Table 3.
| Endpoint | Variable | HR (95% CI) | P Value |
|---|---|---|---|
| |||
| 90‐day mortality | Age | 1.14 (1.031.26) | 0.009 |
| IVC diameter at admission | 5.88 (1.2128.1) | 0.025 | |
| Log NT‐proBNP at admission | 1.00 (1.001.00) | 0.910 | |
| 90‐day readmission | Age | 1.06 (1.001.12) | 0.025 |
| IVC diameter at admission | 3.20 (1.248.21) | 0.016 | |
| Log NT‐proBNP at discharge | 1.00 (1.001.00) | 0.910 | |
| 180‐day mortality | Age | 1.12 (1.031.22) | 0.007 |
| IVC diameter at admission | 4.77 (1.2118.7) | 0.025 | |
| Log NT‐proBNP at admission | 1.00 (1.001.00) | 0.610 | |
| 180‐day readmission | Age | 1.06 (1.011.11) | 0.009 |
| IVC diameter at admission | 2.56 (1.145.74) | 0.022 | |
| Log NT‐proBNP at discharge | 1.00 (1.001.00) | 0.610 | |


For the secondary outcome of 90‐day readmissions, 19 patients (24%) were readmitted, and the mean index admission IVCmax diameter was significantly greater in patients who were readmitted (2.36 vs 1.98 cm; P = 0.04). The ROC curves for readmission at 90 days showed that an index admission IVCmax diameter of 1.9 cm had the greatest AUC (0.61; 95% CI: 0.49‐0.74). The optimal cutoff value of an index admission IVCmax to predict readmission was also 1.9 cm (sensitivity 94%, specificity 42%) (see Supporting Information, Appendices 1 and 2, in the online version of this article). The Cox survival analysis showed that patients with an index admission IVCmax diameter 1.9 cm had a higher readmission rate at 90 days (30.8% vs 10.7%; P = 0.04) and 180 days (38.0 vs 14.3%; P = 0.02) (Figures 1 and 2). Using a multivariate Cox proportional regression analysis, the hazard ratios for the variables of age, admission IVCmax diameter, and log NT‐proBNP are shown in Table 3.
DISCUSSION
Our study found that a dilated IVC at admission is associated with a poor prognosis after hospitalization for ADHF. Patients with a dilated IVC 1.9 cm at admission had higher mortality and readmission rates at 90 and 180 days postdischarge.
The effect of a dilated IVC on mortality may be mediated through unrecognized right ventricular disease with or without significant pulmonary hypertension, supporting the notion that right heart function is an important determinant of prognosis in patients with ADHF.[30, 31] Similar to elevated jugular venous distension, bedside ultrasound examination of the IVC diameter can serve as a rapid and noninvasive measurement of right atrial pressure.[32] Elevated right atrial pressure is most often due to elevated left ventricular filling pressure transmitted via the pulmonary vasculature, but it is important to note that right‐ and left‐sided cardiac pressures are often discordant in heart failure patients.[33, 34]
Few studies have evaluated the prognostic value of IVC diameter and collapsibility in patients with heart failure. Nath et al.[24] evaluated the prognostic value of IVC diameter in stable veterans referred for outpatient echocardiography. Patients with a dilated IVC >2 cm that did not collapse with inspiration had higher 90‐day and 1‐year mortality rates. A subsequent study by Pellicori et al.[22] investigated the relationship between IVC diameter and other prognostic markers in stable cardiac patients. Pellicori et al. demonstrated that IVC diameter and serum NT‐proBNP levels were independent predictors of a composite endpoint of cardiovascular death or heart failure hospitalization at 1 year.[22] Most recently, Lee et al.[23] evaluated whether a dilated IVC in patients with a history of advanced systolic heart failure with a reduced ejection fraction of 30% and repeated hospitalizations (2) predicted worsening renal failure and adverse cardiovascular outcomes (death or hospitalization for ADHF). The study concluded that age, IVC diameter >2.1 cm, and worsening renal failure predicted cardiovascular death or hospitalization for ADHF.[23]
Our study demonstrated that an admission IVCmax 1.9 cm in hospitalized ADHF patients predicted higher postdischarge mortality at 90 and 180 days. Our findings are consistent with the above‐mentioned studies with a few important differences. First, all of our patients were hospitalized with acute decompensated heart failure. Nath et al. and Pellicori et al. evaluated stable ambulatory patients seen in an echocardiography lab and cardiology clinic, respectively. Only 12.1% of patients in the Nath study had a history of heart failure, and none were reported to have ADHF. More importantly, our study improves our understanding of patients with heart failure with a preserved ejection fraction, an important gap in the literature. The mean ejection fraction of patients in our study was 52% consistent with heart failure with preserved ejection fraction, whereas patients in the Pellicori et al. and Lee et al. studies had heart failure with reduced (42%) or severely reduced (30%) ejection fraction, respectively. We did not anticipate finding heart failure with preserved ejection fraction in the majority of patients, but our study's findings will add to our understanding of this increasingly common type of heart failure.
Compared to previous studies that utilized a registered diagnostic cardiac sonographer to obtain a comprehensive TTE to prognosticate patients, our study utilized point‐of‐care ultrasonography. Nath et al. commented that obtaining a comprehensive echocardiogram on every patient with ADHF is unlikely to be cost‐effective or feasible. Our study utilized a more realistic approach with a frontline internal medicinetrained hospitalist acquiring and interpreting images of the IVC at the bedside using a basic portable ultrasound machine.
Our study did not show that plasma natriuretic peptides levels are predictive of death or readmission after hospitalization for ADHF as shown in previous studies.[22, 35, 36] The small sample size, relatively low event rate, or predominance of heart failure with preserved ejection fraction may explain this inconsistency with prior studies.
Previous studies have reported hospital readmission rates for ADHF of 30% to 44% after 1 to 6 months.[6, 37] Goonewardena et al. showed a 41.3% readmission rate at 30 days in patients with severely reduced left ventricular ejection fraction (mean 29%), and readmitted patients had an IVCmax diameter >2 cm and an IVC collapsibility <50% on admission and discharge.[6] Carbone et al. demonstrated absence of improvement in the minimum IVC diameter from admission to discharge using hand‐carried ultrasound in patients with ischemic heart disease (ejection fraction 33%) predicted readmission at 60 days.[38] Hospital readmission rates in our study are consistent with these previously published studies. We found readmission rates for patients with ADHF and an admission IVCmax 1.9 cm to be 30.8% and 38.0% after 90 and 180 days, respectively.
Important limitations of our study are the small sample size and single institution setting. A larger sample size may have demonstrated that change in IVC diameter and NT‐proBNP levels from admission to discharge to be predictive of mortality or readmission. Further, we found an IVCmax diameter 1.9 cm to be the optimal cutoff to predict mortality, which is less than an IVCmax diameter >2.0 cm reported in other studies. The relatively smaller IVC diameter in Spanish heart failure patients may be explained by the lower body mass index of this population. An IVCmax diameter 1.9 cm was found to be the optimal cutoff to predict an elevated right atrial pressure >10 mm Hg in a study of Japanese cardiac patients with a relatively lower body mass index.[39] Another limitation is the timing of the admission IVC measurement within the first 24 hours of arrival to the hospital rather than immediately upon arrival to the emergency department. We were not able to control for interventions given in the emergency department prior to the measurement of the admission IVC, including doses of diuretics. Further, unlike the comprehensive TTEs in the United States, TTEs in Spain do not routinely include an assessment of the IVC. Therefore, we were not able to compare our bedside IVC measurements to those from a comprehensive TTE. An important limitation of our regression analysis is the inclusion of only 3 variables. The selection of variables (age, NT‐proBNP, and IVC diameter) was based on prior studies demonstrating their prognostic value.[6, 22, 25] Due to the low event rate (n = 11), we could not include in the regression model other variables that differed significantly between nonsurvivors and survivors, including NYHA class, presence of atrial fibrillation, and use of ‐blockers.
Perhaps in a larger study population the admission IVCmax diameter may not be as predictive of 90‐day mortality as other variables. The findings of our exploratory analysis should be confirmed in a future study with a larger sample size.
The clinical implications of our study are 3‐fold. First, our study demonstrates that IVC images acquired by a hospitalist at the bedside using a portable ultrasound machine can be used to predict postdischarge mortality and readmission of patients with ADHF. Second, the predominant type of heart failure in our study was heart failure with preserved ejection fraction. Currently, approximately 50% of patients hospitalized with ADHF have heart failure with preserved ejection fraction.[40] Our study adds to the understanding of prognosis of these patients whose heart failure pathophysiology is not well understood. Finally, palliative care services are underutilized in patients with advanced heart failure.[41, 42] IVC measurements and other prognostic markers in heart failure may guide discussions about goals of care with patients and families, and facilitate timely referrals for palliative care services.
CONCLUSIONS
Point‐of‐care ultrasound evaluation of IVC diameter at the time of admission can be used to prognosticate patients hospitalized with acute decompensated heart failure. An admission IVCmax diameter 1.9 cm is associated with a higher rate of 90‐day and 180‐day readmission and mortality after hospitalization. Future studies should evaluate the combination of IVC characteristics with other markers of severity of illness to prognosticate patients with heart failure.
Disclosures
This study was supported by a grant from the Madrid‐Castilla la Mancha Society of Internal Medicine. Dr. Restrepo is partially supported by award number K23HL096054 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. The authors report no conflicts of interest.
Heart failure costs the United States an excess of $30 billion annually, and costs are projected to increase to nearly $70 billion by 2030.[1] Heart failure accounts for over 1 million hospitalizations and is the leading cause of hospitalization in patients >65 years of age.[2] After hospitalization, approximately 50% of patients are readmitted within 6 months of hospital discharge.[3] Mortality rates from heart failure have improved but remain high.[4] Approximately 50% of patients diagnosed with heart failure die within 5 years, and the overall 1‐year mortality rate is 30%.[1]
Prognostic markers and scoring systems for acute decompensated heart failure (ADHF) continue to emerge, but few bedside tools are available to clinicians. Age, brain natriuretic peptide, and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) levels have been shown to correlate with postdischarge rates of readmission and mortality.[5] A study evaluating the prognostic value of a bedside inferior vena cava (IVC) ultrasound exam demonstrated that lack of improvement in IVC distention from admission to discharge was associated with higher 30‐day readmission rates.[6] Two studies using data from comprehensive transthoracic echocardiograms in heart failure patients demonstrated that a dilated, noncollapsible IVC is associated with higher risk of mortality; however, it is well recognized that obtaining comprehensive transthoracic echocardiograms in all patients hospitalized with heart failure is neither cost‐effective nor practical.[7]
In recent years, multiple studies have emerged demonstrating that noncardiologists can perform focused cardiac ultrasound exams with high reproducibility and accuracy to guide management of patients with ADHF.[8, 9, 10, 11, 12, 13, 14] However, it is unknown whether IVC characteristics from a focused cardiac ultrasound exam performed by a noncardiologist can predict mortality of patients hospitalized with ADHF. The aim of this study was to assess whether a hospitalist‐performed focused ultrasound exam to measure the IVC diameter at admission and discharge can predict mortality in a general medicine ward population hospitalized with ADHF.
METHODS
Study Design
A prospective, observational study of patients admitted to a general medicine ward with ADHF between January 2012 and March 2013 was performed using convenience sampling. The setting was a 247‐bed, university‐affiliated hospital in Madrid, Spain. Inclusion criteria were adult patients admitted with a primary diagnosis of ADHF per the European Society of Cardiology (ESC) criteria.[15] Exclusion criteria were admission to the intensive care unit for mechanical ventilation, need for chronic hemodialysis, or a noncardiac terminal illness with a life expectancy of less than 3 months. All patients provided written informed consent prior to enrollment. This study complies with the Declaration of Helsinki and was approved by the local ethics committee.
The primary outcome was all‐cause mortality at 90 days after hospitalization. The secondary outcomes were hospital readmission at 90 and 180 days, and mortality at 180 days. Patients were prospectively followed up at 30, 60, 90, and 180 days after discharge by telephone interview or by review of the patient's electronic health record. Patients who died within 90 days of discharge were categorized as nonsurvivors, whereas those alive at 90 days were categorized as survivors.
The following data were recorded on admission: age, gender, blood pressure, heart rate, functional class per New York Heart Association (NYHA) classification, comorbidities (hypertension, diabetes mellitus, atrial fibrillation, chronic obstructive pulmonary disease), primary etiology of heart failure, medications, electrocardiogram, NT‐terminal pro‐BNP, hemoglobin, albumin, creatinine, sodium, measurement of performance of activities of daily living (modified Barthel index), and comorbidity score (age‐adjusted Charlson score). A research coordinator interviewed subjects to gather data to calculate a modified Barthel index.[16] Age‐adjusted Charlson comorbidity scores were calculated using age and diagnoses per International Classification of Diseases, Ninth Revision coding.[17]
IVC Measurement
An internal medicine hospitalist with expertise in point‐of‐care ultrasonography (G.G.C.) performed all focused cardiac ultrasound exams to measure the IVC diameter and collapsibility at the time of admission and discharge. This physician was not involved in the inpatient medical management of study subjects. A second physician (N.J.S.) randomly reviewed 10% of the IVC images for quality assurance. Admission IVC measurements were acquired within 24 hours of arrival to the emergency department after the on‐call medical team was contacted to admit the patient. Measurement of the IVC maximum (IVCmax) and IVC minimum (IVCmin) diameters was obtained just distal to the hepatic veinIVC junction, or 2 cm from the IVCright atrial junction using a long‐axis view of the IVC. Measurement of the IVC diameter was consistent with the technique recommended by the American Society of Echocardiography and European Society of Echocardiography guidelines.[18, 19] The IVC collapsibility index (IVCCI) was calculated as (IVCmaxIVCmin)/IVCmax per guidelines.[18] Focused cardiac ultrasound exams were performed using a General Electric Logiq E device (GE Healthcare, Little Chalfont, United Kingdom) with a 3.5 MHz curvilinear transducer. Inpatient medical management by the primary medical team was guided by protocols from the ESC guidelines on the treatment of ADHF.[15] A comprehensive transthoracic echocardiogram (TTE) was performed on all study subjects by the echocardiography laboratory within 24 hours of hospitalization as part of the study protocol. One of 3 senior cardiologists read all comprehensive TTEs. NT‐proBNP was measured on admission and discharge by electrochemiluminescence.
Statistical Analysis
We calculated the required sample size based on published mortality and readmission rates. For our primary outcome of 90‐day mortality, we calculated a required sample size of 64 to achieve 80% power based on 90‐day and 1‐year mortality rates of 21% and 33%, respectively, among Spanish elderly patients (age 70 years) hospitalized with ADHF.[20] For our secondary outcome of 90‐day readmissions, we calculated a sample size of 28 based on a 41% readmission rate.[21] Therefore, our target subject enrollment was at least 70 patients to achieve a power of 80%.
Statistical analyses were performed using SPSS 17.0 statistical package (SPSS Inc., Chicago, IL). Subject characteristics that were categorical variables (demographics and comorbidities) were summarized as counts and percentages. Continuous variables, including IVC measurements, were summarized as means with standard deviations. Differences between categorical variables were analyzed using the Fisher exact test. Survival curves with log‐rank statistics were used to perform survival analysis. The nonparametric Mann‐Whitney U test was used to assess associations between the change in IVCCI, and readmissions and mortality at 90 and 180 days. Predictors of readmission and death were evaluated using a multivariate Cox proportional hazards regression analysis. Given the limited number of primary outcome events, we used age, IVC diameter, and log NT‐proBNP in the multivariate regression analysis based on past studies showing prognostic significance of these variables.[6, 22, 23, 24, 25, 26, 27, 28] Optimal cutoff values for IVC diameter for death and readmission prediction were determined by constructing receiver operating characteristic (ROC) curves and calculating the area under the curve (AUC) for different IVC diameters. NT‐proBNP values were log‐transformed to minimize skewing as reported in previous studies.[29]
RESULTS
Patient Characteristics
Ninety‐seven patients admitted with ADHF were recruited for the study. Optimal acoustic windows to measure the IVC diameter were acquired in 90 patients (93%). Because measurement of discharge IVC diameter was required to calculate the change from admission to discharge, 8 patients who died during initial hospitalization were excluded from the final data analysis. An additional two patients were excluded due to missing discharge NT‐proBNP measurement or missing comprehensive echocardiogram data. The study cohort from whom data were analyzed included 80 of 97 total patients (82%).
Baseline demographic, clinical, laboratory, and comprehensive echocardiographic characteristics of nonsurvivors and survivors at 90 days are demonstrated in Table 1. Eleven patients (13.7%) died during the first 90 days postdischarge, and all deaths were due to cardiovascular complications. Nonsurvivors were older (86 vs 76 years; P = 0.02), less independent in performance of their activities of daily living (Barthel index of 58.1 vs 81.9; P = 0.01), and were more likely to have advanced heart failure with an NYHA functional class of III or IV (72% vs 33%; P = 0.016). Atrial fibrillation (90% vs 55%; P = 0.008) and lower systolic blood pressure (127 mm Hg vs 147 mm Hg; P = 0.01) were more common in nonsurvivors than survivors, and fewer nonsurvivors were taking a ‐blocker (18% vs 59%; P = 0.01). Baseline comprehensive echocardiographic findings were similar between the survivors and nonsurvivors, except left atrial diameter was larger in nonsurvivors versus survivors (54 mm vs 49 mm; P = 0.04).
| Total Cohort, n = 80 | Nonsurvivors, n = 11 | Survivors, n = 69 | P Value | |
|---|---|---|---|---|
| ||||
| Demographics | ||||
| Age, y* | 78 (13) | 86 (7) | 76 (14) | 0.02 |
| Men, n (%) | 34 (42) | 3 (27) | 26 (38) | 0.3 |
| Vital signs* | ||||
| Heart rate, beats/min | 94 (23) | 99 (26) | 95 (23) | 0.5 |
| SBP, mm Hg | 141 (27) | 127 (22) | 147 (25) | 0.01 |
| Comorbidities, n (%) | ||||
| Hypertension | 72 (90) | 10 (91) | 54 (78) | 0.3 |
| Diabetes mellitus | 35 (44) | 3 (27) | 26 (38) | 0.3 |
| Atrial fibrillation | 48 (60) | 10 (90) | 38 (55) | 0.008 |
| COPD | 22 (27) | 3 (27) | 16 (23) | 0.5 |
| Etiology of heart failure | ||||
| Ischemic | 20 (25) | 1 (9) | 16 (23) | 0.1 |
| Hypertensive | 22 (27) | 2 (18) | 18 (26) | 0.4 |
| Valvulopathy | 29 (36) | 7 (64) | 19 (27) | 0.07 |
| Other | 18 (22) | 1 (9) | 16 (23) | 0.09 |
| NYHA IIIIV | 38 (47) | 8 (72) | 23 (33) | 0.016 |
| Charlson score* | 7.5 (2) | 9.0 (3) | 7.1 (2) | 0.02 |
| Barthel index* | 76 (31) | 58 (37) | 81.9 (28) | 0.01 |
| Medications | ||||
| ‐blocker | 44 (55) | 2 (18) | 41 (59) | 0.01 |
| ACE inhibitor/ARB | 48 (60) | 3 (27) | 35 (51) | 0.1 |
| Loop diuretic | 78 (97) | 10 (91) | 67 (97) | 0.9 |
| Aldosterone antagonist | 31 (39) | 4 (36) | 21 (30) | 0.4 |
| Lab results* | ||||
| Sodium, mmol/L | 137 (4.8) | 138 (6) | 139 (4) | 0.6 |
| Creatinine, umol/L | 1.24 (0.4) | 1.40 (0.5) | 1.17 (0.4) | 0.1 |
| eGFR, mL/min | 57.8 (20) | 51.2 (20) | 60.2 (19) | 0.1 |
| Albumin, g/L | 3.4 (0.4) | 3.3 (0.38) | 3.5 (0.41) | 0.1 |
| Hemoglobin, g/dL | 12.0 (2) | 10.9 (1.8) | 12.5 (2.0) | 0.01 |
| Echo parameters* | ||||
| LVEF, % | 52.1 (15) | 51.9 (17) | 51.6 (15) | 0.9 |
| LA diameter, mm | 50.1 (10) | 54 (11) | 49 (11) | 0.04 |
| RVDD, mm | 32.0 (11) | 34 (10) | 31 (11) | 0.2 |
| TAPSE, mm | 18.5 (7) | 17.4 (4) | 18.8 (7) | 0.6 |
| PASP, mm Hg | 51.2 (16) | 53.9 (17) | 50.2 (17) | 0.2 |
| Admission* | ||||
| NT‐proBNP, pg/mL | 8,816 (14,260) | 9,413 (5,703) | 8,762 (15,368) | 0.81 |
| Log NT‐proBNP | 3.66 (0.50) | 3.88 (0.31 | 3.62 (0.52) | 0.11 |
| IVCmax, cm | 2.12 (0.59) | 2.39 (0.37) | 2.06 (0.59) | 0.02 |
| IVCmin, cm | 1.63 (0.69) | 1.82 (0.66) | 1.56 (0.67) | 0.25 |
| IVCCI, % | 25.7 (0.16) | 25.9 (17.0) | 26.2 (16.0) | 0.95 |
| Discharge* | ||||
| NT‐proBNP, pg/mL | 3,132 (3,093) | 4,693 (4,383) | 2,909 (2,847) | 0.08 |
| Log NT‐proBNP | 3.27 (0.49) | 3.51 (0.37) | 3.23 (0.50) | 0.08 |
| IVCmax, cm | 1.87 (0.68) | 1.97 (0.54) | 1.81 (0.66) | 0.45 |
| IVCmin, cm | 1.33 (0.75) | 1.40 (0.65) | 1.27 (0.71) | 0.56 |
| IVCCI, % | 33.1 (0.20) | 32.0 (21.0) | 34.2 (19.0) | 0.74 |
From admission to discharge, the total study cohort demonstrated a highly statistically significant reduction in NT‐proBNP (8816 vs 3093; P < 0.001), log NT‐proBNP (3.66 vs 3.27; P < 0.001), IVCmax (2.12 vs 1.87; P < 0.001), IVCmin (1.63 vs 1.33; P < 0.001), and IVCCI (25.7% vs 33.1%; P < 0.001). The admission and discharge NT‐proBNP and IVC characteristics of the survivors and nonsurvivors are displayed in Table 2. The only statistically significant difference between nonsurvivors and survivors was the admission IVCmax (2.39 vs 2.06; P = 0.02). There was not a statistically significant difference in the discharge IVCmax between nonsurvivors and survivors.
| Admission | Discharge | Difference (DischargeAdmission) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Nonsurvivors | Survivors | P Value | Nonsurvivors | Survivors | P Value | Nonsurvivors | Survivors | P Value | |
| |||||||||
| NT‐proBNP, pg/mL | 9,413 (5,703) | 8,762 (15,368) | 0.81 | 4,693 (4,383) | 2,909 (2,847) | 0.08 | 3,717 5,043 | 5,026 11,507 | 0.7 |
| Log NT‐proBNP | 3.88 0.31 | 3.62 0.52 | 0.11 | 3.51 0.37 | 3.23 0.50 | 0.08 | 0.29 0.36 | 0.38 0.37 | 0.4 |
| IVCmax, cm | 2.39 0.37 | 2.06 0.59 | 0.02 | 1.97 0.54 | 1.81 0.66 | 0.45 | 0.39 0.56 | 0.25 0.51 | 0.4 |
| IVCmin, cm | 1.82 0.66 | 1.56 0.67 | 0.25 | 1.40 0.65 | 1.27 0.71 | 0.56 | 0.37 0.52 | 0.30 0.64 | 0.7 |
| IVCCI, % | 25.9 17.0 | 26.2 16.0 | 0.95 | 32.0 21.0 | 34.2 19.0 | 0.74 | 3.7 7.9 | 8.3 22 | 0.5 |
Outcomes
For the primary outcome of 90‐day mortality, the ROC curves showed a similar AUC for the admission IVCmax diameter (AUC: 0.69; 95% confidence interval [CI]: 0.53‐0.85), log NT‐proBNP at discharge (AUC: 0.67; 95% CI: 0.49‐0.85), and log NT‐proBNP at admission (AUC: 0.69; 95% CI: 0.52‐0.85). The optimal cutoff value for the admission IVCmax diameter to predict mortality was 1.9 cm (sensitivity 100%, specificity 38%) based on the ROC curves (see Supporting Information, Appendices 1 and 2, in the online version of this article). An admission IVCmax diameter 1.9 cm was associated with a higher mortality rate at 90 days (25.4% vs 3.4%; P = 0.009) and 180 days (29.3% vs 3.4%; P = 0.003). The Cox survival curves showed significantly lower survival rates in patients with an admission IVCmax diameter 1.9 cm (74.1 vs 96.7%; P = 0.012) (Figures 1 and 2). Based on the multivariate Cox proportional hazards regression analysis with age, IVCmax diameter, and log NT‐proBNP at admission, the admission IVCmax diameter and age were independent predictors of 90‐ and 180‐day mortality. The hazard ratios for death by age, admission IVCmax diameter, and log NT‐proBNP are shown in Table 3.
| Endpoint | Variable | HR (95% CI) | P Value |
|---|---|---|---|
| |||
| 90‐day mortality | Age | 1.14 (1.031.26) | 0.009 |
| IVC diameter at admission | 5.88 (1.2128.1) | 0.025 | |
| Log NT‐proBNP at admission | 1.00 (1.001.00) | 0.910 | |
| 90‐day readmission | Age | 1.06 (1.001.12) | 0.025 |
| IVC diameter at admission | 3.20 (1.248.21) | 0.016 | |
| Log NT‐proBNP at discharge | 1.00 (1.001.00) | 0.910 | |
| 180‐day mortality | Age | 1.12 (1.031.22) | 0.007 |
| IVC diameter at admission | 4.77 (1.2118.7) | 0.025 | |
| Log NT‐proBNP at admission | 1.00 (1.001.00) | 0.610 | |
| 180‐day readmission | Age | 1.06 (1.011.11) | 0.009 |
| IVC diameter at admission | 2.56 (1.145.74) | 0.022 | |
| Log NT‐proBNP at discharge | 1.00 (1.001.00) | 0.610 | |


For the secondary outcome of 90‐day readmissions, 19 patients (24%) were readmitted, and the mean index admission IVCmax diameter was significantly greater in patients who were readmitted (2.36 vs 1.98 cm; P = 0.04). The ROC curves for readmission at 90 days showed that an index admission IVCmax diameter of 1.9 cm had the greatest AUC (0.61; 95% CI: 0.49‐0.74). The optimal cutoff value of an index admission IVCmax to predict readmission was also 1.9 cm (sensitivity 94%, specificity 42%) (see Supporting Information, Appendices 1 and 2, in the online version of this article). The Cox survival analysis showed that patients with an index admission IVCmax diameter 1.9 cm had a higher readmission rate at 90 days (30.8% vs 10.7%; P = 0.04) and 180 days (38.0 vs 14.3%; P = 0.02) (Figures 1 and 2). Using a multivariate Cox proportional regression analysis, the hazard ratios for the variables of age, admission IVCmax diameter, and log NT‐proBNP are shown in Table 3.
DISCUSSION
Our study found that a dilated IVC at admission is associated with a poor prognosis after hospitalization for ADHF. Patients with a dilated IVC 1.9 cm at admission had higher mortality and readmission rates at 90 and 180 days postdischarge.
The effect of a dilated IVC on mortality may be mediated through unrecognized right ventricular disease with or without significant pulmonary hypertension, supporting the notion that right heart function is an important determinant of prognosis in patients with ADHF.[30, 31] Similar to elevated jugular venous distension, bedside ultrasound examination of the IVC diameter can serve as a rapid and noninvasive measurement of right atrial pressure.[32] Elevated right atrial pressure is most often due to elevated left ventricular filling pressure transmitted via the pulmonary vasculature, but it is important to note that right‐ and left‐sided cardiac pressures are often discordant in heart failure patients.[33, 34]
Few studies have evaluated the prognostic value of IVC diameter and collapsibility in patients with heart failure. Nath et al.[24] evaluated the prognostic value of IVC diameter in stable veterans referred for outpatient echocardiography. Patients with a dilated IVC >2 cm that did not collapse with inspiration had higher 90‐day and 1‐year mortality rates. A subsequent study by Pellicori et al.[22] investigated the relationship between IVC diameter and other prognostic markers in stable cardiac patients. Pellicori et al. demonstrated that IVC diameter and serum NT‐proBNP levels were independent predictors of a composite endpoint of cardiovascular death or heart failure hospitalization at 1 year.[22] Most recently, Lee et al.[23] evaluated whether a dilated IVC in patients with a history of advanced systolic heart failure with a reduced ejection fraction of 30% and repeated hospitalizations (2) predicted worsening renal failure and adverse cardiovascular outcomes (death or hospitalization for ADHF). The study concluded that age, IVC diameter >2.1 cm, and worsening renal failure predicted cardiovascular death or hospitalization for ADHF.[23]
Our study demonstrated that an admission IVCmax 1.9 cm in hospitalized ADHF patients predicted higher postdischarge mortality at 90 and 180 days. Our findings are consistent with the above‐mentioned studies with a few important differences. First, all of our patients were hospitalized with acute decompensated heart failure. Nath et al. and Pellicori et al. evaluated stable ambulatory patients seen in an echocardiography lab and cardiology clinic, respectively. Only 12.1% of patients in the Nath study had a history of heart failure, and none were reported to have ADHF. More importantly, our study improves our understanding of patients with heart failure with a preserved ejection fraction, an important gap in the literature. The mean ejection fraction of patients in our study was 52% consistent with heart failure with preserved ejection fraction, whereas patients in the Pellicori et al. and Lee et al. studies had heart failure with reduced (42%) or severely reduced (30%) ejection fraction, respectively. We did not anticipate finding heart failure with preserved ejection fraction in the majority of patients, but our study's findings will add to our understanding of this increasingly common type of heart failure.
Compared to previous studies that utilized a registered diagnostic cardiac sonographer to obtain a comprehensive TTE to prognosticate patients, our study utilized point‐of‐care ultrasonography. Nath et al. commented that obtaining a comprehensive echocardiogram on every patient with ADHF is unlikely to be cost‐effective or feasible. Our study utilized a more realistic approach with a frontline internal medicinetrained hospitalist acquiring and interpreting images of the IVC at the bedside using a basic portable ultrasound machine.
Our study did not show that plasma natriuretic peptides levels are predictive of death or readmission after hospitalization for ADHF as shown in previous studies.[22, 35, 36] The small sample size, relatively low event rate, or predominance of heart failure with preserved ejection fraction may explain this inconsistency with prior studies.
Previous studies have reported hospital readmission rates for ADHF of 30% to 44% after 1 to 6 months.[6, 37] Goonewardena et al. showed a 41.3% readmission rate at 30 days in patients with severely reduced left ventricular ejection fraction (mean 29%), and readmitted patients had an IVCmax diameter >2 cm and an IVC collapsibility <50% on admission and discharge.[6] Carbone et al. demonstrated absence of improvement in the minimum IVC diameter from admission to discharge using hand‐carried ultrasound in patients with ischemic heart disease (ejection fraction 33%) predicted readmission at 60 days.[38] Hospital readmission rates in our study are consistent with these previously published studies. We found readmission rates for patients with ADHF and an admission IVCmax 1.9 cm to be 30.8% and 38.0% after 90 and 180 days, respectively.
Important limitations of our study are the small sample size and single institution setting. A larger sample size may have demonstrated that change in IVC diameter and NT‐proBNP levels from admission to discharge to be predictive of mortality or readmission. Further, we found an IVCmax diameter 1.9 cm to be the optimal cutoff to predict mortality, which is less than an IVCmax diameter >2.0 cm reported in other studies. The relatively smaller IVC diameter in Spanish heart failure patients may be explained by the lower body mass index of this population. An IVCmax diameter 1.9 cm was found to be the optimal cutoff to predict an elevated right atrial pressure >10 mm Hg in a study of Japanese cardiac patients with a relatively lower body mass index.[39] Another limitation is the timing of the admission IVC measurement within the first 24 hours of arrival to the hospital rather than immediately upon arrival to the emergency department. We were not able to control for interventions given in the emergency department prior to the measurement of the admission IVC, including doses of diuretics. Further, unlike the comprehensive TTEs in the United States, TTEs in Spain do not routinely include an assessment of the IVC. Therefore, we were not able to compare our bedside IVC measurements to those from a comprehensive TTE. An important limitation of our regression analysis is the inclusion of only 3 variables. The selection of variables (age, NT‐proBNP, and IVC diameter) was based on prior studies demonstrating their prognostic value.[6, 22, 25] Due to the low event rate (n = 11), we could not include in the regression model other variables that differed significantly between nonsurvivors and survivors, including NYHA class, presence of atrial fibrillation, and use of ‐blockers.
Perhaps in a larger study population the admission IVCmax diameter may not be as predictive of 90‐day mortality as other variables. The findings of our exploratory analysis should be confirmed in a future study with a larger sample size.
The clinical implications of our study are 3‐fold. First, our study demonstrates that IVC images acquired by a hospitalist at the bedside using a portable ultrasound machine can be used to predict postdischarge mortality and readmission of patients with ADHF. Second, the predominant type of heart failure in our study was heart failure with preserved ejection fraction. Currently, approximately 50% of patients hospitalized with ADHF have heart failure with preserved ejection fraction.[40] Our study adds to the understanding of prognosis of these patients whose heart failure pathophysiology is not well understood. Finally, palliative care services are underutilized in patients with advanced heart failure.[41, 42] IVC measurements and other prognostic markers in heart failure may guide discussions about goals of care with patients and families, and facilitate timely referrals for palliative care services.
CONCLUSIONS
Point‐of‐care ultrasound evaluation of IVC diameter at the time of admission can be used to prognosticate patients hospitalized with acute decompensated heart failure. An admission IVCmax diameter 1.9 cm is associated with a higher rate of 90‐day and 180‐day readmission and mortality after hospitalization. Future studies should evaluate the combination of IVC characteristics with other markers of severity of illness to prognosticate patients with heart failure.
Disclosures
This study was supported by a grant from the Madrid‐Castilla la Mancha Society of Internal Medicine. Dr. Restrepo is partially supported by award number K23HL096054 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. The authors report no conflicts of interest.
- , , , et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322.
- , , . Hospitalization for congestive heart failure: United States, 2000–2010. NCHS Data Brief. 2012(108):1–8.
- , . Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126(4):501–506.
- , , , et al. Generalizability and longitudinal outcomes of a national heart failure clinical registry: Comparison of Acute Decompensated Heart Failure National Registry (ADHERE) and non‐ADHERE Medicare beneficiaries. Am Heart J. 2010;160(5):885–892.
- , , , et al. Prognostic markers of acute decompensated heart failure: the emerging roles of cardiac biomarkers and prognostic scores. Arch Cardiovasc Dis. 2015;108(1):64–74.
- , , , et al. Comparison of hand‐carried ultrasound assessment of the inferior vena cava and N‐terminal pro‐brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging. 2008;1(5):595–601.
- , , , , . Echocardiography in acute heart failure: current perspectives. J Card Fail. 2016;22(1):82–94.
- , , , , . Usefulness of a hand‐held ultrasound device for bedside examination of left ventricular function. Am J Cardiol. 2002;90(9):1038–1039.
- , , , , , . Feasibility of point‐of‐care echocardiography by internal medicine house staff. Am Heart J. 2004;147(3):476–481.
- , , , , , . The use of small personal ultrasound devices by internists without formal training in echocardiography. Eur J Echocardiogr. 2003;4(2):141–147.
- , , , et al. Diagnostic accuracy of hospitalist‐performed hand‐carried ultrasound echocardiography after a brief training program. J Hosp Med. 2009;4(6):340–349.
- , , , . Point‐of‐care multi‐organ ultrasound improves diagnostic accuracy in adults presenting to the emergency department with acute dyspnea. West J Emerg Med. 2016;17(1):46–53.
- , , , , , . Acute heart failure: the role of focused emergency cardiopulmonary ultrasound in identification and early management. Eur J Heart Fail. 2015;17(12):1223–1227.
- , , , et al. Hand‐carried echocardiography by hospitalists: a randomized trial. Am J Med. 2011;124(8):766–774.
- , , , et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail. 2008;10(10):933–989.
- , , , , , . Adaptation of the modified Barthel Index for use in physical medicine and rehabilitation in Turkey. Scand J Rehabil Med. 2000;32(2):87–92.
- , , , . Complications, comorbidities, and mortality: improving classification and prediction. Health Serv Res. 1997;32(2):229–238; discussion 239–242.
- , , , et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713; quiz 786–688.
- , , , et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463.
- , , , , . Mortality and functional evolution at one year after hospital admission due to heart failure (HF) in elderly patients. Arch Gerontol Geriatr. 2012;54(1):261–265.
- , , , et al. Early and long‐term outcomes of heart failure in elderly persons, 2001–2005. Arch Intern Med. 2008;168(22):2481–2488.
- , , , et al. IVC diameter in patients with chronic heart failure: relationships and prognostic significance. JACC Cardiovasc Imaging. 2013;6(1):16–28.
- , , , et al. Prognostic significance of dilated inferior vena cava in advanced decompensated heart failure. Int J Cardiovasc Imaging. 2014;30(7):1289–1295.
- , , . A dilated inferior vena cava is a marker of poor survival. Am Heart J. 2006;151(3):730–735.
- , , , et al. Predischarge B‐type natriuretic peptide assay for identifying patients at high risk of re‐admission after decompensated heart failure. J Am Coll Cardiol. 2004;43(4):635–641.
- , , , et al. A rapid bedside test for B‐type peptide predicts treatment outcomes in patients admitted for decompensated heart failure: a pilot study. J Am Coll Cardiol. 2001;37(2):386–391.
- , , , , , . N‐terminal‐pro‐brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110(15):2168–2174.
- , , , , , . Lowered B‐type natriuretic peptide in response to levosimendan or dobutamine treatment is associated with improved survival in patients with severe acutely decompensated heart failure. J Am Coll Cardiol. 2009;53(25):2343–2348.
- , , , . Long‐term clinical variation of NT‐proBNP in stable chronic heart failure patients. Eur Heart J. 2007;28(2):177–182.
- , , , et al. Right atrial volume index in chronic systolic heart failure and prognosis. JACC Cardiovasc Imaging. 2009;2(5):527–534.
- , , , et al. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol. 2012;59(3):222–231.
- , , , et al. A comparison by medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure. Am J Cardiol. 2007;99(11):1614–1616.
- , , . Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66(4):493–496.
- , , , , , . Relationship between right and left‐sided filling pressures in 1000 patients with advanced heart failure. J Heart Lung Transplant. 1999;18(11):1126–1132.
- , , , et al. Admission B‐type natriuretic peptide levels and in‐hospital mortality in acute decompensated heart failure. J Am Coll Cardiol. 2007;49(19):1943–1950.
- , , , et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10(9):824–839.
- , , , et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157(1):99–104.
- , , , et al. Inferior vena cava parameters predict re‐admission in ischaemic heart failure. Eur J Clin Invest. 2014;44(4):341–349.
- , , , et al. Estimation of right atrial pressure on inferior vena cava ultrasound in Asian patients. Circ J. 2014;78(4):962–966.
- , , , , . Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47(1):76–84.
- , , , , . Palliative care referral among patients hospitalized with advanced heart failure. J Palliat Med. 2014;17(10):1115–1120.
- , , . Engaging heart failure clinicians to increase palliative care referrals: overcoming barriers, improving techniques. J Palliat Med. 2014;17(7):753–760.
- , , , et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322.
- , , . Hospitalization for congestive heart failure: United States, 2000–2010. NCHS Data Brief. 2012(108):1–8.
- , . Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126(4):501–506.
- , , , et al. Generalizability and longitudinal outcomes of a national heart failure clinical registry: Comparison of Acute Decompensated Heart Failure National Registry (ADHERE) and non‐ADHERE Medicare beneficiaries. Am Heart J. 2010;160(5):885–892.
- , , , et al. Prognostic markers of acute decompensated heart failure: the emerging roles of cardiac biomarkers and prognostic scores. Arch Cardiovasc Dis. 2015;108(1):64–74.
- , , , et al. Comparison of hand‐carried ultrasound assessment of the inferior vena cava and N‐terminal pro‐brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging. 2008;1(5):595–601.
- , , , , . Echocardiography in acute heart failure: current perspectives. J Card Fail. 2016;22(1):82–94.
- , , , , . Usefulness of a hand‐held ultrasound device for bedside examination of left ventricular function. Am J Cardiol. 2002;90(9):1038–1039.
- , , , , , . Feasibility of point‐of‐care echocardiography by internal medicine house staff. Am Heart J. 2004;147(3):476–481.
- , , , , , . The use of small personal ultrasound devices by internists without formal training in echocardiography. Eur J Echocardiogr. 2003;4(2):141–147.
- , , , et al. Diagnostic accuracy of hospitalist‐performed hand‐carried ultrasound echocardiography after a brief training program. J Hosp Med. 2009;4(6):340–349.
- , , , . Point‐of‐care multi‐organ ultrasound improves diagnostic accuracy in adults presenting to the emergency department with acute dyspnea. West J Emerg Med. 2016;17(1):46–53.
- , , , , , . Acute heart failure: the role of focused emergency cardiopulmonary ultrasound in identification and early management. Eur J Heart Fail. 2015;17(12):1223–1227.
- , , , et al. Hand‐carried echocardiography by hospitalists: a randomized trial. Am J Med. 2011;124(8):766–774.
- , , , et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail. 2008;10(10):933–989.
- , , , , , . Adaptation of the modified Barthel Index for use in physical medicine and rehabilitation in Turkey. Scand J Rehabil Med. 2000;32(2):87–92.
- , , , . Complications, comorbidities, and mortality: improving classification and prediction. Health Serv Res. 1997;32(2):229–238; discussion 239–242.
- , , , et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713; quiz 786–688.
- , , , et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463.
- , , , , . Mortality and functional evolution at one year after hospital admission due to heart failure (HF) in elderly patients. Arch Gerontol Geriatr. 2012;54(1):261–265.
- , , , et al. Early and long‐term outcomes of heart failure in elderly persons, 2001–2005. Arch Intern Med. 2008;168(22):2481–2488.
- , , , et al. IVC diameter in patients with chronic heart failure: relationships and prognostic significance. JACC Cardiovasc Imaging. 2013;6(1):16–28.
- , , , et al. Prognostic significance of dilated inferior vena cava in advanced decompensated heart failure. Int J Cardiovasc Imaging. 2014;30(7):1289–1295.
- , , . A dilated inferior vena cava is a marker of poor survival. Am Heart J. 2006;151(3):730–735.
- , , , et al. Predischarge B‐type natriuretic peptide assay for identifying patients at high risk of re‐admission after decompensated heart failure. J Am Coll Cardiol. 2004;43(4):635–641.
- , , , et al. A rapid bedside test for B‐type peptide predicts treatment outcomes in patients admitted for decompensated heart failure: a pilot study. J Am Coll Cardiol. 2001;37(2):386–391.
- , , , , , . N‐terminal‐pro‐brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110(15):2168–2174.
- , , , , , . Lowered B‐type natriuretic peptide in response to levosimendan or dobutamine treatment is associated with improved survival in patients with severe acutely decompensated heart failure. J Am Coll Cardiol. 2009;53(25):2343–2348.
- , , , . Long‐term clinical variation of NT‐proBNP in stable chronic heart failure patients. Eur Heart J. 2007;28(2):177–182.
- , , , et al. Right atrial volume index in chronic systolic heart failure and prognosis. JACC Cardiovasc Imaging. 2009;2(5):527–534.
- , , , et al. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol. 2012;59(3):222–231.
- , , , et al. A comparison by medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure. Am J Cardiol. 2007;99(11):1614–1616.
- , , . Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66(4):493–496.
- , , , , , . Relationship between right and left‐sided filling pressures in 1000 patients with advanced heart failure. J Heart Lung Transplant. 1999;18(11):1126–1132.
- , , , et al. Admission B‐type natriuretic peptide levels and in‐hospital mortality in acute decompensated heart failure. J Am Coll Cardiol. 2007;49(19):1943–1950.
- , , , et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10(9):824–839.
- , , , et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157(1):99–104.
- , , , et al. Inferior vena cava parameters predict re‐admission in ischaemic heart failure. Eur J Clin Invest. 2014;44(4):341–349.
- , , , et al. Estimation of right atrial pressure on inferior vena cava ultrasound in Asian patients. Circ J. 2014;78(4):962–966.
- , , , , . Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47(1):76–84.
- , , , , . Palliative care referral among patients hospitalized with advanced heart failure. J Palliat Med. 2014;17(10):1115–1120.
- , , . Engaging heart failure clinicians to increase palliative care referrals: overcoming barriers, improving techniques. J Palliat Med. 2014;17(7):753–760.
Mortality Due to Elevated Troponin
Acute coronary syndromes (ACS) are potentially lethal and present with a wide variety of symptoms. As such, physicians frequently order cardiac biomarkers, such as cardiac troponin, for patients with acute complaints. Elevated troponin is associated with higher risk of mortality regardless of the causes, which can be myriad, both chronic and acute.[1] Among patients with an elevated troponin, distinguishing ACS from non‐ACS can be challenging.
Making the distinction between ACS and non‐ACS troponin elevation is crucial because the underlying pathophysiology and subsequent management strategies are markedly different.[2] According to evidence‐based practice guidelines, ACS is managed with antiplatelet drugs, statins, and percutaneous coronary intervention, improving clinical outcomes.[3] In contrast, care for patients with non‐ACS troponin elevations is usually supportive, with a focus on the underlying conditions. The lack of specific treatment options for such patients is concerning given that several series have suggested that non‐ACS troponin patients may have a higher mortality risk than ACS patients.[4, 5, 6] Non‐ACS troponin elevation can be the result of a multitude of conditions.[7, 8] What remains unclear at this point is whether the excess mortality observed with non‐ACS troponin elevation is due to myocardial damage or to the underlying conditions that predispose to troponin release.
Using data from a quality improvement (QI) project collected at our Veterans Affairs (VA) medical center, we investigated the mortality risk associated with ACS and non‐ACS troponin elevation including an analysis of factors associated with mortality. We hypothesized that non‐ACS troponin elevation will have a higher mortality risk than troponin elevation due to ACS, and that important contributors to this relationship could be identified to provide direction for future investigation directed at modifying this mortality risk.
METHODS
We analyzed data that were prospectively collected for a quality initiative between 2006 and 2007. The project was a collaborative endeavor between cardiology, hospital medicine, and emergency medicine with the process goal of better identifying patients with ACS to hopefully improve outcomes. The QI team was consulted in real time to assist with treatment recommendations; no retrospective decisions were made regarding whether or not ACS was present. As the goal of the project was to improve cardiovascular outcomes, consultative advice was freely provided, and no physicians or teams were subject to any adverse repercussions for their diagnoses or management decisions.
A cardiologist‐led team was created to improve quality of care for myocardial infarction patients by evaluating all patients at our facility with an elevated troponin. On a daily basis, a specialist clinical coordinator (nurse practitioner or physician assistant) received a list of all patients with elevated troponin from the chemistry lab. The coordinator reviewed the patients' medical records with a cardiologist. A positive troponin was defined as a troponin T level of greater than 0.03 ng/mL (99th percentile at our facility). Each attending cardiologist prospectively determined if troponin elevation was related to clinical findings consistent with an ACS based on review of the patients' symptoms (duration, quality, severity, chronicity, and alleviating/aggravating factors), medical history, and noninvasive cardiac testing including electrocardiograms, cardiac biomarkers, and any other available imaging tests.
We have previously demonstrated that the cardiologists at our facility have a similar rate of diagnosing ACS.[9] All cardiologists at our facility maintain current American Board of Internal Medicine certification in cardiovascular disease and have academic appointments at the University of Florida College of Medicine. All patients were followed prospectively, and data on their medical history, acute evaluation, and outcomes were tracked in an electronic database. Given the higher risk of mortality with ST‐elevation myocardial infarction, such patients were excluded from this investigation. By definition, patients with unstable angina do not have elevated biomarkers and thus would not have been included in the database to begin with. Prospectively recorded data elements included: age, gender, chief complaint, tobacco use, presence of hypertension, hyperlipidemia, prior coronary disease, chronic kidney disease, diabetes mellitus, cardiac troponin values, serum creatinine, electrocardiogram (ECG) variables, Thrombolysis in Myocardial Infarction (TIMI) score, and if the patient was placed under hospice care or an active do‐not‐resuscitate (DNR) order. Additional data elements gathered at a later date included maximum temperature, white blood cell count, N‐terminal pro‐brain natriuretic peptide (NT‐proBNP), administration of advanced cardiac life support (ACLS), and admission to an intensive care unit (ICU). All consecutive patients with elevated troponin were included in the database; if patients were included more than once, we used their index evaluation only. All patients with troponin elevation after revascularization (percutaneous coronary intervention or coronary bypass surgery) were excluded. Our investigational design was reviewed by our institutional review board, who waived the requirement for formal written informed consent and approved use of data from this QI project for research purposes.
We focused this investigation on an analysis of all‐cause mortality in February 2014. We analyzed mortality at 30 days, 1 year, and 6 years. As secondary outcomes we analyzed the likelihood of the patients' chief complaint for the diagnosis of ACS and evaluated predictors of mortality based on Cox proportional hazard modeling. Mortality within the VA system is reliably tracked and compares favorably to the Social Security National Death Index Master File for accuracy.[10, 11]
Categorical variables were compared by 2 test. The Student t test was used to compare normally distributed continuous variables, and nonparametric tests were used for non‐normal distributions as appropriate. Mortality data at 30 days, 1 year, and 6 years were compared by log‐rank test and Kaplan‐Meier graphs. A formal power analysis was not performed; the entire available population was included. A Cox proportional hazard model was created to estimate mortality risk at each time point. Variables included in our Cox regression model were age, gender, history of coronary artery disease (CAD), hypertension, diabetes mellitus or hyperlipidemia, ACS diagnosis, dynamic ECG changes, TIMI risk score, initial troponin level, creatinine level at time of initial troponin (per mg/dL), presence of fever, maximum white blood cell count, NT‐proBNP level (per 1000 pg/mL), if ACLS was performed, if the patient was under hospice care, if there was a DNR order, and if they required ICU admission. This model was also constructed independently for the ACS and non‐ACS cohorts for mortality at 1 year. A forward stepwise model was used. Statistical results were considered significant at P < 0.05. Statistical analyses were performed using SPSS version 21 (IBM, Armonk, NY).
RESULTS
Among the 761 patients, 502 (66.0%) were classified as non‐ACS and 259 (34.0%) as ACS (Table 1). The mean age was higher in the non‐ACS group (71 years vs 69 years in the ACS group, P = 0.006). Hypertension, diabetes mellitus, and prior CAD were frequent in both groups and not significantly different. Median initial troponin T was higher in the ACS group (0.12 ng/mL vs 0.06 ng/mL, P < 0.001) as were the frequency of a TIMI risk score >2 (92.5% vs 74.3%, P < 0.001) and new ECG changes (29.7% vs 8.2%, P < 0.001). Hospice, DNR orders, and administration of ACLS were not different between groups; however, admission to the ICU was more frequent in the ACS group (44.8% vs 31.9%, P < 0.001). Chest pain was the symptom with the highest positive predictive value for the diagnosis of ACS (63.3%), whereas the least predictive was altered mental status or confusion (18.0%) (Figure 1).
| Non‐ACS, N = 502 | ACS, N = 259 | P Value | |
|---|---|---|---|
| |||
| Baseline characteristics, n (%) | |||
| Age, y | 71 11 | 69 11 | 0.006 |
| Female | 6 (1.2%) | 1 (0.4%) | 0.27 |
| Coronary artery disease | 244 (48.6%) | 141 (54.4%) | 0.13 |
| Hypertension | 381 (75.9%) | 203 (78.4%) | 0.44 |
| Diabetes mellitus | 220 (43.8%) | 119 (45.9%) | 0.58 |
| Hyperlipidemia | 268 (53.4%) | 170 (65.6%) | 0.001 |
| Current smoker | 24 (4.8%) | 49 (18.9%) | <0.001 |
| Clinical presentation | |||
| Initial troponin T, ng/mL, median [IQR] | 0.06 [0.040.11] | 0.12 [0.050.32] | <0.001 |
| White cell count, 109/L, median [IQR] | 10 [8.014.0] | 11 [8.015.0] | 0.005 |
| NT‐proBNP, pg/mL, median [IQR] | 3,531 [1,20110,519] | 1,932 [3199,100] | 0.001 |
| Creatinine, mg/dL, median [IQR] | 1.6 [1.12.4] | 1.1 [0.91.5] | <0.001 |
| New ECG changes, no. (%) | 41 (8.2%) | 77 (29.7%) | <0.001 |
| TIMI score over 2, no. (%) | 365 (74.3%) | 235 (92.5%) | <0.001 |
| Fever (over 100.4 F), no. (%) | 75 (15.0%) | 38 (14.7%) | 0.91 |
| Hospice, no. (%) | 8 (1.6%) | 5 (1.9%) | 0.73 |
| Do not resuscitate, no. (%) | 62 (12.4%) | 30 (11.6%) | 0.76 |
| Intensive care admission, no. (%) | 160 (31.9%) | 116 (44.8%) | <0.001 |
| ACLS administered, no. (%) | 38 (7.6%) | 17 (6.6%) | 0.6 |
| Outcomes, no. (%) | |||
| Death, 30 days | 67 (13.3%) | 30 (11.6%) | 0.49 |
| Death, 1 year | 211 (42.0%) | 75 (29.0%) | <0.001 |
| Death, 6 years | 390 (77.7%) | 152 (58.7%) | <0.001 |
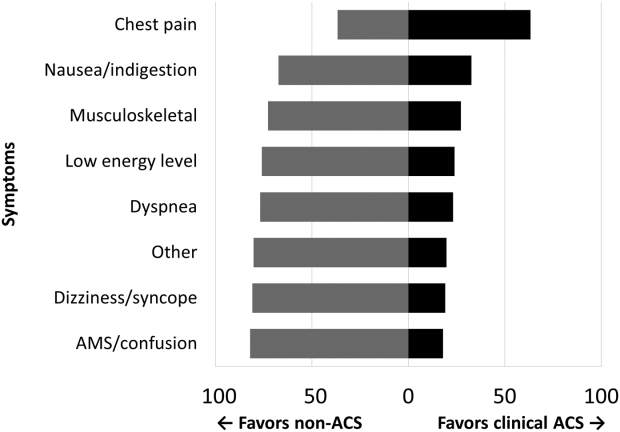
Mortality at 30 days was not different between the 2 groups, but mortality was higher for the non‐ACS cohort at 1 year and at 6 years (Table 1). Kaplan‐Meier curves demonstrate that mortality for the 2 cohorts begins to diverge between 30 and 60 days until approximately 2 years when the curves again are parallel (Figure 2).
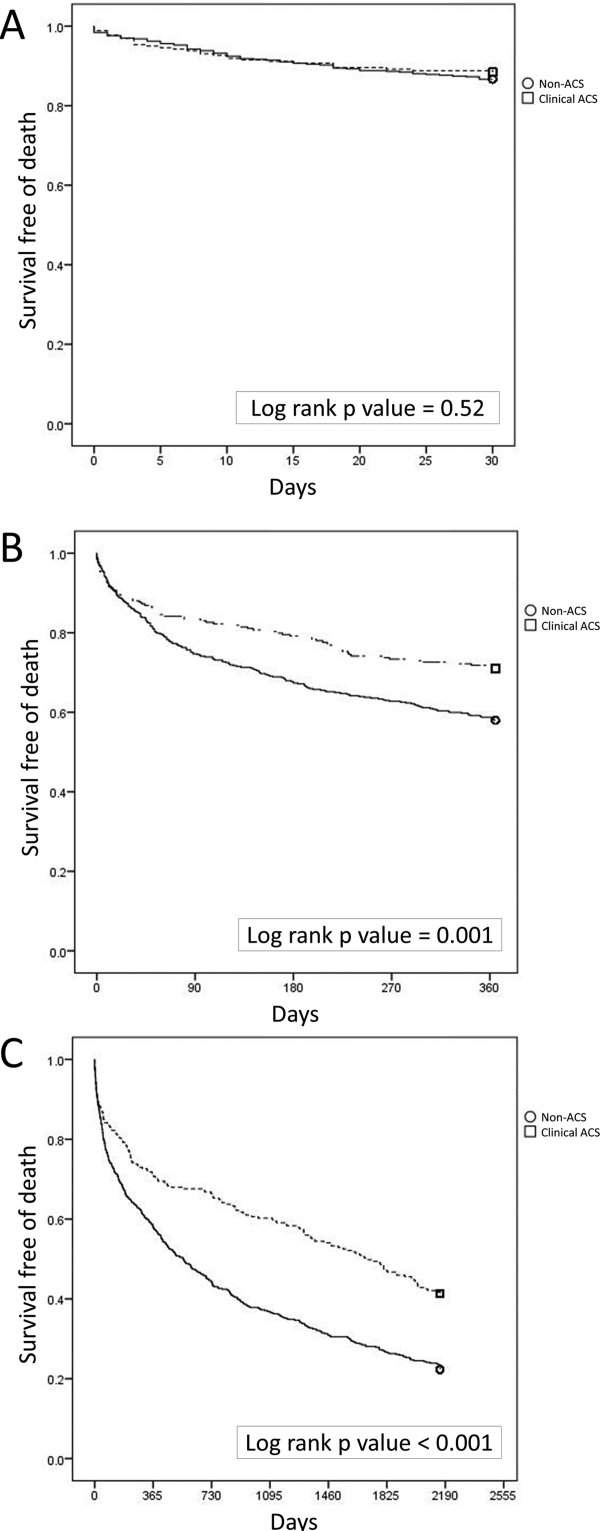
In Cox proportional hazards models, 5 factors were associated with higher mortality at 30 days, 1 year, and at 6 years: age, hospice, DNR order, need for ACLS, and admission to the ICU (Table 2). Additionally, at 1 and 6 years, NT‐proBNP and non‐ACS were associated with higher mortality. At 6 years, creatinine was an additional significant factor. We separated the ACS and non‐ACS cohorts and performed the same model for 1‐year mortality (Table 3). The models yielded similar factors associated with higher mortality: hospice, DNR order, need for ACLS, age, and NT‐proBNP, with ICU admission being significant only in the non‐ACS cohort.
| P Value | Hazard Ratio | 95% CI | |
|---|---|---|---|
| |||
| 30 days | |||
| Intensive care unit admission | <0.0001 | 2.18 | 1.283.72 |
| Hospice | <0.0001 | 4.67 | 1.9111.40 |
| Do not resuscitate | <0.0001 | 3.19 | 1.945.24 |
| ACLS performed | <0.0001 | 10.17 | 6.0317.17 |
| Age, per year | <0.0001 | 1.04 | 1.021.06 |
| 1 year | |||
| Intensive care unit admission | <0.0001 | 1.66 | 1.262.20 |
| Hospice | <0.0001 | 4.98 | 2.699.21 |
| Do not resuscitate | <0.0001 | 2.52 | 1.833.47 |
| Non‐ACS | <0.0001 | 1.57 | 1.192.08 |
| ACLS performed | <0.0001 | 6.03 | 4.178.72 |
| Age, per year | <0.0001 | 1.03 | 1.021.04 |
| NT‐proBNP, per 1,000 pg/mL | <0.0001 | 1.02 | 1.011.03 |
| Extended follow‐up | |||
| Intensive care unit admission | <0.0001 | 1.35 | 1.111.65 |
| Hospice | <0.0001 | 3.81 | 2.136.81 |
| Do not resuscitate | <0.0001 | 2.11 | 1.622.74 |
| Non‐ACS | <0.0001 | 1.53 | 1.251.88 |
| ACLS performed | <0.0001 | 4.19 | 3.015.84 |
| Age, per year | <0.0001 | 1.03 | 1.031.04 |
| Creatinine, per mg/dL | 0.02 | 1.06 | 1.011.12 |
| NT‐proBNP, per 1,000 pg/mL | <0.0001 | 1.02 | 1.021.03 |
| P Value | Hazard Ratio | 95% CI | |
|---|---|---|---|
| |||
| Non‐ACS | |||
| Intensive care unit admission | <0.0001 | 1.86 | 1.352.58 |
| Hospice | <0.0001 | 7.55 | 3.5715.93 |
| Do not resuscitate | <0.0001 | 2.33 | 1.603.41 |
| ACLS performed | <0.0001 | 4.42 | 2.836.92 |
| Age, per year | <0.0001 | 1.03 | 1.011.04 |
| NT‐proBNP, per 1,000 pg/mL | 0.002 | 1.02 | 1.011.03 |
| Clinical ACS | |||
| Hospice | 0.036 | 3.17 | 1.089.32 |
| Do not resuscitate | 0.003 | 2.49 | 1.364.55 |
| ACLS performed | <0.0001 | 12.04 | 6.3322.91 |
| Age, per year | <0.0001 | 1.05 | 1.021.07 |
| NT‐proBNP, per 1,000 pg/mL | 0.001 | 1.04 | 1.011.06 |
DISCUSSION
Our findings confirm the important, but perhaps not well‐recognized, fact that an elevated troponin without ACS is associated with higher mortality than with ACS. This has been previously observed in veteran and nonveteran populations.[4, 6, 8, 12] The novel finding from our investigation is that mortality risk with troponin elevation is most strongly associated with unmodifiable clinical factors that are plausible explanations of risk. Furthermore, the distribution of these factors between our 2 cohorts does not sufficiently explain the difference in risk between ACS and non‐ACS patients.
At each time point we evaluated, ICU admission and need for ACLS were associated with mortality. These are indicators of a severely ill population and are not surprising to find associated with mortality. Many hospitals have instituted some form of pre‐code approach or rapid response team to identify patients before they need ACLS. These efforts, although well meaning, have not yielded convincing results of effectiveness.[13] Hospice and DNR patients were also, not surprisingly, associated with higher mortality. Although these factors were statistically significant, the low prevalence suggests that they are not clinically impactful on the primary questions of the investigation. These factors can be altered but are not intended as modifiable as they reflect the wishes of patients and their decision makers. The distribution of the factors in our model, however, did not adequately explain the higher risk of death with non‐ACS troponin elevation. For example, ACLS administration, hospice care, and DNR orders were strong predictors but were similar between the groups. ICU admission was actually more common with ACS patients, despite strong association with mortality. Age and NT‐proBNP were associated with mortality and higher in the non‐ACS group; however the magnitude of hazard was less than for the other factors. These findings lead us back to the possible explanation that non‐ACS troponin elevation stands as an independent risk factor, and that ACS patients have a distinct advantage in the myriad treatments available. If ACS patients were misdiagnosed as non‐ACS and failed to receive appropriate treatments, that might have contributed to higher mortality; however, we consider that unlikely given that the goal of the QI project was to minimize missed ACS diagnoses.
The overall mortality risk in our study was high: 12.7% at 30 days and 37.6% at 1 year. This reflects the high‐risk population with elevated troponin seen at our facility with ages nearly 70 years and high prevalence of multiple cardiovascular risk factors. Despite a high event rate, many clinically relevant risk factors were not retained in our Cox hazard model. Among sepsis patients, elevation in troponin is associated with mortality[14]; however, in our population neither fever or white blood cell count were significant mortality factors. The relationship between chronic kidney disease and troponin is complex. Renal dysfunction may result in troponin elevation and troponin elevation is a predictor of risk within kidney disease patients.[15] In our study, we did not evaluate chronic kidney disease as a predictor, instead opting to use the serum creatinine. This was not associated with mortality except at the 6‐year time point.
The TIMI score was not associated with mortality in either the overall population or the ACS cohort. The proportion of patients in our cohort with TIMI score under 3 was 16.5% as compared with 21.6% in the original derivation study.[16] The limited data on the prognostic value of the TIMI score within a veteran population suggest a modest predictive capacity.[17] Our data raises the possibility that TIMI is not an optimal choice; however, our analysis only includes all‐cause mortality, different from the original intended use of TIMI, predicting a variety of major cardiac events.
Our data confirm that ACS can be detected in a wide range of clinical presentations. Within our population of troponin positive patients, those with chest pain were most likely to be diagnosed with ACS, although one‐third of chest pain patients were felt to have a non‐ACS diagnosis. On the opposite end of the spectrum, an elevation in troponin with altered mental status or confusion was rarely diagnosed as ACSonly 18% of the time. Many symptoms were poor predictors of ACS; however, none were low enough to disregard. Our data would suggest that most patients with elevated troponin warrant evaluation by a cardiovascular expert.
Our study population came from a single VA hospital that is comprised of elderly and predominantly male patients limiting applicability to other populations. Despite this, other investigations in younger populations and with a higher proportion of women have found similar mortality trends.[4, 8, 12] We did not have sufficient data to determine the cause of death or to further classify as cardiac versus noncardiac; knowledge of the cause of the specific death may better inform future investigations into this important clinical question. Our investigation did not use a standardized definition to determine ACS, a notable limitation that could introduce bias or variation in care. Because all determinations about ACS were made prospectively as part of a QI project, we have little reason to suspect any systematic bias to the determination of ACS. With regard to variation in care, we have previously presented data demonstrating consistent rates of ACS diagnosis across the physicians at our facility.
Based on our investigation and others on this topic, non‐ACS troponin elevation is a common, high‐risk clinical scenario. In our cohort, non‐ACS troponin elevation is about twice as frequent as ACS, and the problem is likely to grow dramatically within the next few years as ultrasensitive troponin assays are eventually approved for use in the United States. These assays are much more sensitive than the current assays, and may make it challenging to distinguish between someone with an acute supply/demand mismatch from someone with an elevated troponin due to chronic, but stable, illness such as CAD, heart failure, or diabetes. Non‐ACS troponin elevation remains poorly understood, with no viable treatment options other than addressing the pathophysiology resulting in the troponin elevation. Due to the heterogeneity of the diagnoses and pathophysiological conditions that result in elevated troponin, a unifying treatment is not likely feasible.
In conclusion, in this elderly, male veteran population, the mortality impact associated with a cardiac troponin elevation was not limited to ACS, as mortality was high among those without ACS. Factors independently associated with this non‐ACS mortality risk were plausible, but did not elucidate the reasons why non‐ACS troponin elevation carries a higher risk. Attempting to better understand the biological basis for the troponin elevation in these non‐ACS patients is a critical unmet need.
Disclosure
Nothing to report.
- , , , , . Prognostic significance of elevated troponin in non‐cardiac hospitalized patients: a systematic review and meta‐analysis. Ann Med. 2014;46:653–663.
- , , , , , ; Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–1598.
- , , , et al.; ACC/AHA Task Force Members; Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. 2014 AHA/ACC guideline for the management of patients with non‐st‐elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–2394.
- , , , , , . Acute coronary syndrome vs nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med. 2007;167:276–281.
- , , , , , , TOTAL‐AMI study group. Type 2 myocardial infarction in clinical practice. Heart. 2015;101:101–106.
- , , , et al. Outcomes of hospitalized patients with non‐acute coronary syndrome and elevated cardiac troponin level. Am J Med. 2011;124:630–635.
- , , , et al. Classification of myocardial infarction: frequency and features of type 2 myocardial infarction. Am J Med. 2013;126:789–797.
- , , , , . Cardiac troponin I elevation in hospitalized patients without acute coronary syndromes. Am J Cardiol. 2008;101:1384–1388.
- , , , . Inter‐provider variation in diagnoses and cardiac catheterization use (abstract). Cardiology. 2014;128:346.
- , , , . A primer and comparative review of major us mortality databases. Ann Epidemiol. 2002;12:462–468.
- , , . Assessment of vital status in department of veterans affairs national databases. Comparison with state death certificates. Ann Epidemiol. 2001;11:286–291.
- , , , , , . Raised cardiac troponin T levels in patients without acute coronary syndrome. Postgrad Med J. 2007;83:200–205.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170:18–26.
- , , , , . Prognostic value of troponins in sepsis: a meta‐analysis. Intensive Care Med. 2013;39:1181–1189.
- , , , et al. Prognostic value of cardiac troponin in patients with chronic kidney disease without suspected acute coronary syndrome: a systematic review and meta‐analysis. Ann Intern Med. 2014;161:491–501.
- , , , et al. The TIMI risk score for unstable angina/non‐ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842.
- , , , , , . Usefulness of the TIMI risk score in predicting both short‐ and long‐term outcomes in the Veterans Affairs non‐Q‐wave myocardial infarction strategies in‐hospital (VANQWISH) trial. Am J Cardiol. 2002;90:922–926.
Acute coronary syndromes (ACS) are potentially lethal and present with a wide variety of symptoms. As such, physicians frequently order cardiac biomarkers, such as cardiac troponin, for patients with acute complaints. Elevated troponin is associated with higher risk of mortality regardless of the causes, which can be myriad, both chronic and acute.[1] Among patients with an elevated troponin, distinguishing ACS from non‐ACS can be challenging.
Making the distinction between ACS and non‐ACS troponin elevation is crucial because the underlying pathophysiology and subsequent management strategies are markedly different.[2] According to evidence‐based practice guidelines, ACS is managed with antiplatelet drugs, statins, and percutaneous coronary intervention, improving clinical outcomes.[3] In contrast, care for patients with non‐ACS troponin elevations is usually supportive, with a focus on the underlying conditions. The lack of specific treatment options for such patients is concerning given that several series have suggested that non‐ACS troponin patients may have a higher mortality risk than ACS patients.[4, 5, 6] Non‐ACS troponin elevation can be the result of a multitude of conditions.[7, 8] What remains unclear at this point is whether the excess mortality observed with non‐ACS troponin elevation is due to myocardial damage or to the underlying conditions that predispose to troponin release.
Using data from a quality improvement (QI) project collected at our Veterans Affairs (VA) medical center, we investigated the mortality risk associated with ACS and non‐ACS troponin elevation including an analysis of factors associated with mortality. We hypothesized that non‐ACS troponin elevation will have a higher mortality risk than troponin elevation due to ACS, and that important contributors to this relationship could be identified to provide direction for future investigation directed at modifying this mortality risk.
METHODS
We analyzed data that were prospectively collected for a quality initiative between 2006 and 2007. The project was a collaborative endeavor between cardiology, hospital medicine, and emergency medicine with the process goal of better identifying patients with ACS to hopefully improve outcomes. The QI team was consulted in real time to assist with treatment recommendations; no retrospective decisions were made regarding whether or not ACS was present. As the goal of the project was to improve cardiovascular outcomes, consultative advice was freely provided, and no physicians or teams were subject to any adverse repercussions for their diagnoses or management decisions.
A cardiologist‐led team was created to improve quality of care for myocardial infarction patients by evaluating all patients at our facility with an elevated troponin. On a daily basis, a specialist clinical coordinator (nurse practitioner or physician assistant) received a list of all patients with elevated troponin from the chemistry lab. The coordinator reviewed the patients' medical records with a cardiologist. A positive troponin was defined as a troponin T level of greater than 0.03 ng/mL (99th percentile at our facility). Each attending cardiologist prospectively determined if troponin elevation was related to clinical findings consistent with an ACS based on review of the patients' symptoms (duration, quality, severity, chronicity, and alleviating/aggravating factors), medical history, and noninvasive cardiac testing including electrocardiograms, cardiac biomarkers, and any other available imaging tests.
We have previously demonstrated that the cardiologists at our facility have a similar rate of diagnosing ACS.[9] All cardiologists at our facility maintain current American Board of Internal Medicine certification in cardiovascular disease and have academic appointments at the University of Florida College of Medicine. All patients were followed prospectively, and data on their medical history, acute evaluation, and outcomes were tracked in an electronic database. Given the higher risk of mortality with ST‐elevation myocardial infarction, such patients were excluded from this investigation. By definition, patients with unstable angina do not have elevated biomarkers and thus would not have been included in the database to begin with. Prospectively recorded data elements included: age, gender, chief complaint, tobacco use, presence of hypertension, hyperlipidemia, prior coronary disease, chronic kidney disease, diabetes mellitus, cardiac troponin values, serum creatinine, electrocardiogram (ECG) variables, Thrombolysis in Myocardial Infarction (TIMI) score, and if the patient was placed under hospice care or an active do‐not‐resuscitate (DNR) order. Additional data elements gathered at a later date included maximum temperature, white blood cell count, N‐terminal pro‐brain natriuretic peptide (NT‐proBNP), administration of advanced cardiac life support (ACLS), and admission to an intensive care unit (ICU). All consecutive patients with elevated troponin were included in the database; if patients were included more than once, we used their index evaluation only. All patients with troponin elevation after revascularization (percutaneous coronary intervention or coronary bypass surgery) were excluded. Our investigational design was reviewed by our institutional review board, who waived the requirement for formal written informed consent and approved use of data from this QI project for research purposes.
We focused this investigation on an analysis of all‐cause mortality in February 2014. We analyzed mortality at 30 days, 1 year, and 6 years. As secondary outcomes we analyzed the likelihood of the patients' chief complaint for the diagnosis of ACS and evaluated predictors of mortality based on Cox proportional hazard modeling. Mortality within the VA system is reliably tracked and compares favorably to the Social Security National Death Index Master File for accuracy.[10, 11]
Categorical variables were compared by 2 test. The Student t test was used to compare normally distributed continuous variables, and nonparametric tests were used for non‐normal distributions as appropriate. Mortality data at 30 days, 1 year, and 6 years were compared by log‐rank test and Kaplan‐Meier graphs. A formal power analysis was not performed; the entire available population was included. A Cox proportional hazard model was created to estimate mortality risk at each time point. Variables included in our Cox regression model were age, gender, history of coronary artery disease (CAD), hypertension, diabetes mellitus or hyperlipidemia, ACS diagnosis, dynamic ECG changes, TIMI risk score, initial troponin level, creatinine level at time of initial troponin (per mg/dL), presence of fever, maximum white blood cell count, NT‐proBNP level (per 1000 pg/mL), if ACLS was performed, if the patient was under hospice care, if there was a DNR order, and if they required ICU admission. This model was also constructed independently for the ACS and non‐ACS cohorts for mortality at 1 year. A forward stepwise model was used. Statistical results were considered significant at P < 0.05. Statistical analyses were performed using SPSS version 21 (IBM, Armonk, NY).
RESULTS
Among the 761 patients, 502 (66.0%) were classified as non‐ACS and 259 (34.0%) as ACS (Table 1). The mean age was higher in the non‐ACS group (71 years vs 69 years in the ACS group, P = 0.006). Hypertension, diabetes mellitus, and prior CAD were frequent in both groups and not significantly different. Median initial troponin T was higher in the ACS group (0.12 ng/mL vs 0.06 ng/mL, P < 0.001) as were the frequency of a TIMI risk score >2 (92.5% vs 74.3%, P < 0.001) and new ECG changes (29.7% vs 8.2%, P < 0.001). Hospice, DNR orders, and administration of ACLS were not different between groups; however, admission to the ICU was more frequent in the ACS group (44.8% vs 31.9%, P < 0.001). Chest pain was the symptom with the highest positive predictive value for the diagnosis of ACS (63.3%), whereas the least predictive was altered mental status or confusion (18.0%) (Figure 1).
| Non‐ACS, N = 502 | ACS, N = 259 | P Value | |
|---|---|---|---|
| |||
| Baseline characteristics, n (%) | |||
| Age, y | 71 11 | 69 11 | 0.006 |
| Female | 6 (1.2%) | 1 (0.4%) | 0.27 |
| Coronary artery disease | 244 (48.6%) | 141 (54.4%) | 0.13 |
| Hypertension | 381 (75.9%) | 203 (78.4%) | 0.44 |
| Diabetes mellitus | 220 (43.8%) | 119 (45.9%) | 0.58 |
| Hyperlipidemia | 268 (53.4%) | 170 (65.6%) | 0.001 |
| Current smoker | 24 (4.8%) | 49 (18.9%) | <0.001 |
| Clinical presentation | |||
| Initial troponin T, ng/mL, median [IQR] | 0.06 [0.040.11] | 0.12 [0.050.32] | <0.001 |
| White cell count, 109/L, median [IQR] | 10 [8.014.0] | 11 [8.015.0] | 0.005 |
| NT‐proBNP, pg/mL, median [IQR] | 3,531 [1,20110,519] | 1,932 [3199,100] | 0.001 |
| Creatinine, mg/dL, median [IQR] | 1.6 [1.12.4] | 1.1 [0.91.5] | <0.001 |
| New ECG changes, no. (%) | 41 (8.2%) | 77 (29.7%) | <0.001 |
| TIMI score over 2, no. (%) | 365 (74.3%) | 235 (92.5%) | <0.001 |
| Fever (over 100.4 F), no. (%) | 75 (15.0%) | 38 (14.7%) | 0.91 |
| Hospice, no. (%) | 8 (1.6%) | 5 (1.9%) | 0.73 |
| Do not resuscitate, no. (%) | 62 (12.4%) | 30 (11.6%) | 0.76 |
| Intensive care admission, no. (%) | 160 (31.9%) | 116 (44.8%) | <0.001 |
| ACLS administered, no. (%) | 38 (7.6%) | 17 (6.6%) | 0.6 |
| Outcomes, no. (%) | |||
| Death, 30 days | 67 (13.3%) | 30 (11.6%) | 0.49 |
| Death, 1 year | 211 (42.0%) | 75 (29.0%) | <0.001 |
| Death, 6 years | 390 (77.7%) | 152 (58.7%) | <0.001 |

Mortality at 30 days was not different between the 2 groups, but mortality was higher for the non‐ACS cohort at 1 year and at 6 years (Table 1). Kaplan‐Meier curves demonstrate that mortality for the 2 cohorts begins to diverge between 30 and 60 days until approximately 2 years when the curves again are parallel (Figure 2).

In Cox proportional hazards models, 5 factors were associated with higher mortality at 30 days, 1 year, and at 6 years: age, hospice, DNR order, need for ACLS, and admission to the ICU (Table 2). Additionally, at 1 and 6 years, NT‐proBNP and non‐ACS were associated with higher mortality. At 6 years, creatinine was an additional significant factor. We separated the ACS and non‐ACS cohorts and performed the same model for 1‐year mortality (Table 3). The models yielded similar factors associated with higher mortality: hospice, DNR order, need for ACLS, age, and NT‐proBNP, with ICU admission being significant only in the non‐ACS cohort.
| P Value | Hazard Ratio | 95% CI | |
|---|---|---|---|
| |||
| 30 days | |||
| Intensive care unit admission | <0.0001 | 2.18 | 1.283.72 |
| Hospice | <0.0001 | 4.67 | 1.9111.40 |
| Do not resuscitate | <0.0001 | 3.19 | 1.945.24 |
| ACLS performed | <0.0001 | 10.17 | 6.0317.17 |
| Age, per year | <0.0001 | 1.04 | 1.021.06 |
| 1 year | |||
| Intensive care unit admission | <0.0001 | 1.66 | 1.262.20 |
| Hospice | <0.0001 | 4.98 | 2.699.21 |
| Do not resuscitate | <0.0001 | 2.52 | 1.833.47 |
| Non‐ACS | <0.0001 | 1.57 | 1.192.08 |
| ACLS performed | <0.0001 | 6.03 | 4.178.72 |
| Age, per year | <0.0001 | 1.03 | 1.021.04 |
| NT‐proBNP, per 1,000 pg/mL | <0.0001 | 1.02 | 1.011.03 |
| Extended follow‐up | |||
| Intensive care unit admission | <0.0001 | 1.35 | 1.111.65 |
| Hospice | <0.0001 | 3.81 | 2.136.81 |
| Do not resuscitate | <0.0001 | 2.11 | 1.622.74 |
| Non‐ACS | <0.0001 | 1.53 | 1.251.88 |
| ACLS performed | <0.0001 | 4.19 | 3.015.84 |
| Age, per year | <0.0001 | 1.03 | 1.031.04 |
| Creatinine, per mg/dL | 0.02 | 1.06 | 1.011.12 |
| NT‐proBNP, per 1,000 pg/mL | <0.0001 | 1.02 | 1.021.03 |
| P Value | Hazard Ratio | 95% CI | |
|---|---|---|---|
| |||
| Non‐ACS | |||
| Intensive care unit admission | <0.0001 | 1.86 | 1.352.58 |
| Hospice | <0.0001 | 7.55 | 3.5715.93 |
| Do not resuscitate | <0.0001 | 2.33 | 1.603.41 |
| ACLS performed | <0.0001 | 4.42 | 2.836.92 |
| Age, per year | <0.0001 | 1.03 | 1.011.04 |
| NT‐proBNP, per 1,000 pg/mL | 0.002 | 1.02 | 1.011.03 |
| Clinical ACS | |||
| Hospice | 0.036 | 3.17 | 1.089.32 |
| Do not resuscitate | 0.003 | 2.49 | 1.364.55 |
| ACLS performed | <0.0001 | 12.04 | 6.3322.91 |
| Age, per year | <0.0001 | 1.05 | 1.021.07 |
| NT‐proBNP, per 1,000 pg/mL | 0.001 | 1.04 | 1.011.06 |
DISCUSSION
Our findings confirm the important, but perhaps not well‐recognized, fact that an elevated troponin without ACS is associated with higher mortality than with ACS. This has been previously observed in veteran and nonveteran populations.[4, 6, 8, 12] The novel finding from our investigation is that mortality risk with troponin elevation is most strongly associated with unmodifiable clinical factors that are plausible explanations of risk. Furthermore, the distribution of these factors between our 2 cohorts does not sufficiently explain the difference in risk between ACS and non‐ACS patients.
At each time point we evaluated, ICU admission and need for ACLS were associated with mortality. These are indicators of a severely ill population and are not surprising to find associated with mortality. Many hospitals have instituted some form of pre‐code approach or rapid response team to identify patients before they need ACLS. These efforts, although well meaning, have not yielded convincing results of effectiveness.[13] Hospice and DNR patients were also, not surprisingly, associated with higher mortality. Although these factors were statistically significant, the low prevalence suggests that they are not clinically impactful on the primary questions of the investigation. These factors can be altered but are not intended as modifiable as they reflect the wishes of patients and their decision makers. The distribution of the factors in our model, however, did not adequately explain the higher risk of death with non‐ACS troponin elevation. For example, ACLS administration, hospice care, and DNR orders were strong predictors but were similar between the groups. ICU admission was actually more common with ACS patients, despite strong association with mortality. Age and NT‐proBNP were associated with mortality and higher in the non‐ACS group; however the magnitude of hazard was less than for the other factors. These findings lead us back to the possible explanation that non‐ACS troponin elevation stands as an independent risk factor, and that ACS patients have a distinct advantage in the myriad treatments available. If ACS patients were misdiagnosed as non‐ACS and failed to receive appropriate treatments, that might have contributed to higher mortality; however, we consider that unlikely given that the goal of the QI project was to minimize missed ACS diagnoses.
The overall mortality risk in our study was high: 12.7% at 30 days and 37.6% at 1 year. This reflects the high‐risk population with elevated troponin seen at our facility with ages nearly 70 years and high prevalence of multiple cardiovascular risk factors. Despite a high event rate, many clinically relevant risk factors were not retained in our Cox hazard model. Among sepsis patients, elevation in troponin is associated with mortality[14]; however, in our population neither fever or white blood cell count were significant mortality factors. The relationship between chronic kidney disease and troponin is complex. Renal dysfunction may result in troponin elevation and troponin elevation is a predictor of risk within kidney disease patients.[15] In our study, we did not evaluate chronic kidney disease as a predictor, instead opting to use the serum creatinine. This was not associated with mortality except at the 6‐year time point.
The TIMI score was not associated with mortality in either the overall population or the ACS cohort. The proportion of patients in our cohort with TIMI score under 3 was 16.5% as compared with 21.6% in the original derivation study.[16] The limited data on the prognostic value of the TIMI score within a veteran population suggest a modest predictive capacity.[17] Our data raises the possibility that TIMI is not an optimal choice; however, our analysis only includes all‐cause mortality, different from the original intended use of TIMI, predicting a variety of major cardiac events.
Our data confirm that ACS can be detected in a wide range of clinical presentations. Within our population of troponin positive patients, those with chest pain were most likely to be diagnosed with ACS, although one‐third of chest pain patients were felt to have a non‐ACS diagnosis. On the opposite end of the spectrum, an elevation in troponin with altered mental status or confusion was rarely diagnosed as ACSonly 18% of the time. Many symptoms were poor predictors of ACS; however, none were low enough to disregard. Our data would suggest that most patients with elevated troponin warrant evaluation by a cardiovascular expert.
Our study population came from a single VA hospital that is comprised of elderly and predominantly male patients limiting applicability to other populations. Despite this, other investigations in younger populations and with a higher proportion of women have found similar mortality trends.[4, 8, 12] We did not have sufficient data to determine the cause of death or to further classify as cardiac versus noncardiac; knowledge of the cause of the specific death may better inform future investigations into this important clinical question. Our investigation did not use a standardized definition to determine ACS, a notable limitation that could introduce bias or variation in care. Because all determinations about ACS were made prospectively as part of a QI project, we have little reason to suspect any systematic bias to the determination of ACS. With regard to variation in care, we have previously presented data demonstrating consistent rates of ACS diagnosis across the physicians at our facility.
Based on our investigation and others on this topic, non‐ACS troponin elevation is a common, high‐risk clinical scenario. In our cohort, non‐ACS troponin elevation is about twice as frequent as ACS, and the problem is likely to grow dramatically within the next few years as ultrasensitive troponin assays are eventually approved for use in the United States. These assays are much more sensitive than the current assays, and may make it challenging to distinguish between someone with an acute supply/demand mismatch from someone with an elevated troponin due to chronic, but stable, illness such as CAD, heart failure, or diabetes. Non‐ACS troponin elevation remains poorly understood, with no viable treatment options other than addressing the pathophysiology resulting in the troponin elevation. Due to the heterogeneity of the diagnoses and pathophysiological conditions that result in elevated troponin, a unifying treatment is not likely feasible.
In conclusion, in this elderly, male veteran population, the mortality impact associated with a cardiac troponin elevation was not limited to ACS, as mortality was high among those without ACS. Factors independently associated with this non‐ACS mortality risk were plausible, but did not elucidate the reasons why non‐ACS troponin elevation carries a higher risk. Attempting to better understand the biological basis for the troponin elevation in these non‐ACS patients is a critical unmet need.
Disclosure
Nothing to report.
Acute coronary syndromes (ACS) are potentially lethal and present with a wide variety of symptoms. As such, physicians frequently order cardiac biomarkers, such as cardiac troponin, for patients with acute complaints. Elevated troponin is associated with higher risk of mortality regardless of the causes, which can be myriad, both chronic and acute.[1] Among patients with an elevated troponin, distinguishing ACS from non‐ACS can be challenging.
Making the distinction between ACS and non‐ACS troponin elevation is crucial because the underlying pathophysiology and subsequent management strategies are markedly different.[2] According to evidence‐based practice guidelines, ACS is managed with antiplatelet drugs, statins, and percutaneous coronary intervention, improving clinical outcomes.[3] In contrast, care for patients with non‐ACS troponin elevations is usually supportive, with a focus on the underlying conditions. The lack of specific treatment options for such patients is concerning given that several series have suggested that non‐ACS troponin patients may have a higher mortality risk than ACS patients.[4, 5, 6] Non‐ACS troponin elevation can be the result of a multitude of conditions.[7, 8] What remains unclear at this point is whether the excess mortality observed with non‐ACS troponin elevation is due to myocardial damage or to the underlying conditions that predispose to troponin release.
Using data from a quality improvement (QI) project collected at our Veterans Affairs (VA) medical center, we investigated the mortality risk associated with ACS and non‐ACS troponin elevation including an analysis of factors associated with mortality. We hypothesized that non‐ACS troponin elevation will have a higher mortality risk than troponin elevation due to ACS, and that important contributors to this relationship could be identified to provide direction for future investigation directed at modifying this mortality risk.
METHODS
We analyzed data that were prospectively collected for a quality initiative between 2006 and 2007. The project was a collaborative endeavor between cardiology, hospital medicine, and emergency medicine with the process goal of better identifying patients with ACS to hopefully improve outcomes. The QI team was consulted in real time to assist with treatment recommendations; no retrospective decisions were made regarding whether or not ACS was present. As the goal of the project was to improve cardiovascular outcomes, consultative advice was freely provided, and no physicians or teams were subject to any adverse repercussions for their diagnoses or management decisions.
A cardiologist‐led team was created to improve quality of care for myocardial infarction patients by evaluating all patients at our facility with an elevated troponin. On a daily basis, a specialist clinical coordinator (nurse practitioner or physician assistant) received a list of all patients with elevated troponin from the chemistry lab. The coordinator reviewed the patients' medical records with a cardiologist. A positive troponin was defined as a troponin T level of greater than 0.03 ng/mL (99th percentile at our facility). Each attending cardiologist prospectively determined if troponin elevation was related to clinical findings consistent with an ACS based on review of the patients' symptoms (duration, quality, severity, chronicity, and alleviating/aggravating factors), medical history, and noninvasive cardiac testing including electrocardiograms, cardiac biomarkers, and any other available imaging tests.
We have previously demonstrated that the cardiologists at our facility have a similar rate of diagnosing ACS.[9] All cardiologists at our facility maintain current American Board of Internal Medicine certification in cardiovascular disease and have academic appointments at the University of Florida College of Medicine. All patients were followed prospectively, and data on their medical history, acute evaluation, and outcomes were tracked in an electronic database. Given the higher risk of mortality with ST‐elevation myocardial infarction, such patients were excluded from this investigation. By definition, patients with unstable angina do not have elevated biomarkers and thus would not have been included in the database to begin with. Prospectively recorded data elements included: age, gender, chief complaint, tobacco use, presence of hypertension, hyperlipidemia, prior coronary disease, chronic kidney disease, diabetes mellitus, cardiac troponin values, serum creatinine, electrocardiogram (ECG) variables, Thrombolysis in Myocardial Infarction (TIMI) score, and if the patient was placed under hospice care or an active do‐not‐resuscitate (DNR) order. Additional data elements gathered at a later date included maximum temperature, white blood cell count, N‐terminal pro‐brain natriuretic peptide (NT‐proBNP), administration of advanced cardiac life support (ACLS), and admission to an intensive care unit (ICU). All consecutive patients with elevated troponin were included in the database; if patients were included more than once, we used their index evaluation only. All patients with troponin elevation after revascularization (percutaneous coronary intervention or coronary bypass surgery) were excluded. Our investigational design was reviewed by our institutional review board, who waived the requirement for formal written informed consent and approved use of data from this QI project for research purposes.
We focused this investigation on an analysis of all‐cause mortality in February 2014. We analyzed mortality at 30 days, 1 year, and 6 years. As secondary outcomes we analyzed the likelihood of the patients' chief complaint for the diagnosis of ACS and evaluated predictors of mortality based on Cox proportional hazard modeling. Mortality within the VA system is reliably tracked and compares favorably to the Social Security National Death Index Master File for accuracy.[10, 11]
Categorical variables were compared by 2 test. The Student t test was used to compare normally distributed continuous variables, and nonparametric tests were used for non‐normal distributions as appropriate. Mortality data at 30 days, 1 year, and 6 years were compared by log‐rank test and Kaplan‐Meier graphs. A formal power analysis was not performed; the entire available population was included. A Cox proportional hazard model was created to estimate mortality risk at each time point. Variables included in our Cox regression model were age, gender, history of coronary artery disease (CAD), hypertension, diabetes mellitus or hyperlipidemia, ACS diagnosis, dynamic ECG changes, TIMI risk score, initial troponin level, creatinine level at time of initial troponin (per mg/dL), presence of fever, maximum white blood cell count, NT‐proBNP level (per 1000 pg/mL), if ACLS was performed, if the patient was under hospice care, if there was a DNR order, and if they required ICU admission. This model was also constructed independently for the ACS and non‐ACS cohorts for mortality at 1 year. A forward stepwise model was used. Statistical results were considered significant at P < 0.05. Statistical analyses were performed using SPSS version 21 (IBM, Armonk, NY).
RESULTS
Among the 761 patients, 502 (66.0%) were classified as non‐ACS and 259 (34.0%) as ACS (Table 1). The mean age was higher in the non‐ACS group (71 years vs 69 years in the ACS group, P = 0.006). Hypertension, diabetes mellitus, and prior CAD were frequent in both groups and not significantly different. Median initial troponin T was higher in the ACS group (0.12 ng/mL vs 0.06 ng/mL, P < 0.001) as were the frequency of a TIMI risk score >2 (92.5% vs 74.3%, P < 0.001) and new ECG changes (29.7% vs 8.2%, P < 0.001). Hospice, DNR orders, and administration of ACLS were not different between groups; however, admission to the ICU was more frequent in the ACS group (44.8% vs 31.9%, P < 0.001). Chest pain was the symptom with the highest positive predictive value for the diagnosis of ACS (63.3%), whereas the least predictive was altered mental status or confusion (18.0%) (Figure 1).
| Non‐ACS, N = 502 | ACS, N = 259 | P Value | |
|---|---|---|---|
| |||
| Baseline characteristics, n (%) | |||
| Age, y | 71 11 | 69 11 | 0.006 |
| Female | 6 (1.2%) | 1 (0.4%) | 0.27 |
| Coronary artery disease | 244 (48.6%) | 141 (54.4%) | 0.13 |
| Hypertension | 381 (75.9%) | 203 (78.4%) | 0.44 |
| Diabetes mellitus | 220 (43.8%) | 119 (45.9%) | 0.58 |
| Hyperlipidemia | 268 (53.4%) | 170 (65.6%) | 0.001 |
| Current smoker | 24 (4.8%) | 49 (18.9%) | <0.001 |
| Clinical presentation | |||
| Initial troponin T, ng/mL, median [IQR] | 0.06 [0.040.11] | 0.12 [0.050.32] | <0.001 |
| White cell count, 109/L, median [IQR] | 10 [8.014.0] | 11 [8.015.0] | 0.005 |
| NT‐proBNP, pg/mL, median [IQR] | 3,531 [1,20110,519] | 1,932 [3199,100] | 0.001 |
| Creatinine, mg/dL, median [IQR] | 1.6 [1.12.4] | 1.1 [0.91.5] | <0.001 |
| New ECG changes, no. (%) | 41 (8.2%) | 77 (29.7%) | <0.001 |
| TIMI score over 2, no. (%) | 365 (74.3%) | 235 (92.5%) | <0.001 |
| Fever (over 100.4 F), no. (%) | 75 (15.0%) | 38 (14.7%) | 0.91 |
| Hospice, no. (%) | 8 (1.6%) | 5 (1.9%) | 0.73 |
| Do not resuscitate, no. (%) | 62 (12.4%) | 30 (11.6%) | 0.76 |
| Intensive care admission, no. (%) | 160 (31.9%) | 116 (44.8%) | <0.001 |
| ACLS administered, no. (%) | 38 (7.6%) | 17 (6.6%) | 0.6 |
| Outcomes, no. (%) | |||
| Death, 30 days | 67 (13.3%) | 30 (11.6%) | 0.49 |
| Death, 1 year | 211 (42.0%) | 75 (29.0%) | <0.001 |
| Death, 6 years | 390 (77.7%) | 152 (58.7%) | <0.001 |

Mortality at 30 days was not different between the 2 groups, but mortality was higher for the non‐ACS cohort at 1 year and at 6 years (Table 1). Kaplan‐Meier curves demonstrate that mortality for the 2 cohorts begins to diverge between 30 and 60 days until approximately 2 years when the curves again are parallel (Figure 2).

In Cox proportional hazards models, 5 factors were associated with higher mortality at 30 days, 1 year, and at 6 years: age, hospice, DNR order, need for ACLS, and admission to the ICU (Table 2). Additionally, at 1 and 6 years, NT‐proBNP and non‐ACS were associated with higher mortality. At 6 years, creatinine was an additional significant factor. We separated the ACS and non‐ACS cohorts and performed the same model for 1‐year mortality (Table 3). The models yielded similar factors associated with higher mortality: hospice, DNR order, need for ACLS, age, and NT‐proBNP, with ICU admission being significant only in the non‐ACS cohort.
| P Value | Hazard Ratio | 95% CI | |
|---|---|---|---|
| |||
| 30 days | |||
| Intensive care unit admission | <0.0001 | 2.18 | 1.283.72 |
| Hospice | <0.0001 | 4.67 | 1.9111.40 |
| Do not resuscitate | <0.0001 | 3.19 | 1.945.24 |
| ACLS performed | <0.0001 | 10.17 | 6.0317.17 |
| Age, per year | <0.0001 | 1.04 | 1.021.06 |
| 1 year | |||
| Intensive care unit admission | <0.0001 | 1.66 | 1.262.20 |
| Hospice | <0.0001 | 4.98 | 2.699.21 |
| Do not resuscitate | <0.0001 | 2.52 | 1.833.47 |
| Non‐ACS | <0.0001 | 1.57 | 1.192.08 |
| ACLS performed | <0.0001 | 6.03 | 4.178.72 |
| Age, per year | <0.0001 | 1.03 | 1.021.04 |
| NT‐proBNP, per 1,000 pg/mL | <0.0001 | 1.02 | 1.011.03 |
| Extended follow‐up | |||
| Intensive care unit admission | <0.0001 | 1.35 | 1.111.65 |
| Hospice | <0.0001 | 3.81 | 2.136.81 |
| Do not resuscitate | <0.0001 | 2.11 | 1.622.74 |
| Non‐ACS | <0.0001 | 1.53 | 1.251.88 |
| ACLS performed | <0.0001 | 4.19 | 3.015.84 |
| Age, per year | <0.0001 | 1.03 | 1.031.04 |
| Creatinine, per mg/dL | 0.02 | 1.06 | 1.011.12 |
| NT‐proBNP, per 1,000 pg/mL | <0.0001 | 1.02 | 1.021.03 |
| P Value | Hazard Ratio | 95% CI | |
|---|---|---|---|
| |||
| Non‐ACS | |||
| Intensive care unit admission | <0.0001 | 1.86 | 1.352.58 |
| Hospice | <0.0001 | 7.55 | 3.5715.93 |
| Do not resuscitate | <0.0001 | 2.33 | 1.603.41 |
| ACLS performed | <0.0001 | 4.42 | 2.836.92 |
| Age, per year | <0.0001 | 1.03 | 1.011.04 |
| NT‐proBNP, per 1,000 pg/mL | 0.002 | 1.02 | 1.011.03 |
| Clinical ACS | |||
| Hospice | 0.036 | 3.17 | 1.089.32 |
| Do not resuscitate | 0.003 | 2.49 | 1.364.55 |
| ACLS performed | <0.0001 | 12.04 | 6.3322.91 |
| Age, per year | <0.0001 | 1.05 | 1.021.07 |
| NT‐proBNP, per 1,000 pg/mL | 0.001 | 1.04 | 1.011.06 |
DISCUSSION
Our findings confirm the important, but perhaps not well‐recognized, fact that an elevated troponin without ACS is associated with higher mortality than with ACS. This has been previously observed in veteran and nonveteran populations.[4, 6, 8, 12] The novel finding from our investigation is that mortality risk with troponin elevation is most strongly associated with unmodifiable clinical factors that are plausible explanations of risk. Furthermore, the distribution of these factors between our 2 cohorts does not sufficiently explain the difference in risk between ACS and non‐ACS patients.
At each time point we evaluated, ICU admission and need for ACLS were associated with mortality. These are indicators of a severely ill population and are not surprising to find associated with mortality. Many hospitals have instituted some form of pre‐code approach or rapid response team to identify patients before they need ACLS. These efforts, although well meaning, have not yielded convincing results of effectiveness.[13] Hospice and DNR patients were also, not surprisingly, associated with higher mortality. Although these factors were statistically significant, the low prevalence suggests that they are not clinically impactful on the primary questions of the investigation. These factors can be altered but are not intended as modifiable as they reflect the wishes of patients and their decision makers. The distribution of the factors in our model, however, did not adequately explain the higher risk of death with non‐ACS troponin elevation. For example, ACLS administration, hospice care, and DNR orders were strong predictors but were similar between the groups. ICU admission was actually more common with ACS patients, despite strong association with mortality. Age and NT‐proBNP were associated with mortality and higher in the non‐ACS group; however the magnitude of hazard was less than for the other factors. These findings lead us back to the possible explanation that non‐ACS troponin elevation stands as an independent risk factor, and that ACS patients have a distinct advantage in the myriad treatments available. If ACS patients were misdiagnosed as non‐ACS and failed to receive appropriate treatments, that might have contributed to higher mortality; however, we consider that unlikely given that the goal of the QI project was to minimize missed ACS diagnoses.
The overall mortality risk in our study was high: 12.7% at 30 days and 37.6% at 1 year. This reflects the high‐risk population with elevated troponin seen at our facility with ages nearly 70 years and high prevalence of multiple cardiovascular risk factors. Despite a high event rate, many clinically relevant risk factors were not retained in our Cox hazard model. Among sepsis patients, elevation in troponin is associated with mortality[14]; however, in our population neither fever or white blood cell count were significant mortality factors. The relationship between chronic kidney disease and troponin is complex. Renal dysfunction may result in troponin elevation and troponin elevation is a predictor of risk within kidney disease patients.[15] In our study, we did not evaluate chronic kidney disease as a predictor, instead opting to use the serum creatinine. This was not associated with mortality except at the 6‐year time point.
The TIMI score was not associated with mortality in either the overall population or the ACS cohort. The proportion of patients in our cohort with TIMI score under 3 was 16.5% as compared with 21.6% in the original derivation study.[16] The limited data on the prognostic value of the TIMI score within a veteran population suggest a modest predictive capacity.[17] Our data raises the possibility that TIMI is not an optimal choice; however, our analysis only includes all‐cause mortality, different from the original intended use of TIMI, predicting a variety of major cardiac events.
Our data confirm that ACS can be detected in a wide range of clinical presentations. Within our population of troponin positive patients, those with chest pain were most likely to be diagnosed with ACS, although one‐third of chest pain patients were felt to have a non‐ACS diagnosis. On the opposite end of the spectrum, an elevation in troponin with altered mental status or confusion was rarely diagnosed as ACSonly 18% of the time. Many symptoms were poor predictors of ACS; however, none were low enough to disregard. Our data would suggest that most patients with elevated troponin warrant evaluation by a cardiovascular expert.
Our study population came from a single VA hospital that is comprised of elderly and predominantly male patients limiting applicability to other populations. Despite this, other investigations in younger populations and with a higher proportion of women have found similar mortality trends.[4, 8, 12] We did not have sufficient data to determine the cause of death or to further classify as cardiac versus noncardiac; knowledge of the cause of the specific death may better inform future investigations into this important clinical question. Our investigation did not use a standardized definition to determine ACS, a notable limitation that could introduce bias or variation in care. Because all determinations about ACS were made prospectively as part of a QI project, we have little reason to suspect any systematic bias to the determination of ACS. With regard to variation in care, we have previously presented data demonstrating consistent rates of ACS diagnosis across the physicians at our facility.
Based on our investigation and others on this topic, non‐ACS troponin elevation is a common, high‐risk clinical scenario. In our cohort, non‐ACS troponin elevation is about twice as frequent as ACS, and the problem is likely to grow dramatically within the next few years as ultrasensitive troponin assays are eventually approved for use in the United States. These assays are much more sensitive than the current assays, and may make it challenging to distinguish between someone with an acute supply/demand mismatch from someone with an elevated troponin due to chronic, but stable, illness such as CAD, heart failure, or diabetes. Non‐ACS troponin elevation remains poorly understood, with no viable treatment options other than addressing the pathophysiology resulting in the troponin elevation. Due to the heterogeneity of the diagnoses and pathophysiological conditions that result in elevated troponin, a unifying treatment is not likely feasible.
In conclusion, in this elderly, male veteran population, the mortality impact associated with a cardiac troponin elevation was not limited to ACS, as mortality was high among those without ACS. Factors independently associated with this non‐ACS mortality risk were plausible, but did not elucidate the reasons why non‐ACS troponin elevation carries a higher risk. Attempting to better understand the biological basis for the troponin elevation in these non‐ACS patients is a critical unmet need.
Disclosure
Nothing to report.
- , , , , . Prognostic significance of elevated troponin in non‐cardiac hospitalized patients: a systematic review and meta‐analysis. Ann Med. 2014;46:653–663.
- , , , , , ; Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–1598.
- , , , et al.; ACC/AHA Task Force Members; Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. 2014 AHA/ACC guideline for the management of patients with non‐st‐elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–2394.
- , , , , , . Acute coronary syndrome vs nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med. 2007;167:276–281.
- , , , , , , TOTAL‐AMI study group. Type 2 myocardial infarction in clinical practice. Heart. 2015;101:101–106.
- , , , et al. Outcomes of hospitalized patients with non‐acute coronary syndrome and elevated cardiac troponin level. Am J Med. 2011;124:630–635.
- , , , et al. Classification of myocardial infarction: frequency and features of type 2 myocardial infarction. Am J Med. 2013;126:789–797.
- , , , , . Cardiac troponin I elevation in hospitalized patients without acute coronary syndromes. Am J Cardiol. 2008;101:1384–1388.
- , , , . Inter‐provider variation in diagnoses and cardiac catheterization use (abstract). Cardiology. 2014;128:346.
- , , , . A primer and comparative review of major us mortality databases. Ann Epidemiol. 2002;12:462–468.
- , , . Assessment of vital status in department of veterans affairs national databases. Comparison with state death certificates. Ann Epidemiol. 2001;11:286–291.
- , , , , , . Raised cardiac troponin T levels in patients without acute coronary syndrome. Postgrad Med J. 2007;83:200–205.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170:18–26.
- , , , , . Prognostic value of troponins in sepsis: a meta‐analysis. Intensive Care Med. 2013;39:1181–1189.
- , , , et al. Prognostic value of cardiac troponin in patients with chronic kidney disease without suspected acute coronary syndrome: a systematic review and meta‐analysis. Ann Intern Med. 2014;161:491–501.
- , , , et al. The TIMI risk score for unstable angina/non‐ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842.
- , , , , , . Usefulness of the TIMI risk score in predicting both short‐ and long‐term outcomes in the Veterans Affairs non‐Q‐wave myocardial infarction strategies in‐hospital (VANQWISH) trial. Am J Cardiol. 2002;90:922–926.
- , , , , . Prognostic significance of elevated troponin in non‐cardiac hospitalized patients: a systematic review and meta‐analysis. Ann Med. 2014;46:653–663.
- , , , , , ; Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–1598.
- , , , et al.; ACC/AHA Task Force Members; Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. 2014 AHA/ACC guideline for the management of patients with non‐st‐elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–2394.
- , , , , , . Acute coronary syndrome vs nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med. 2007;167:276–281.
- , , , , , , TOTAL‐AMI study group. Type 2 myocardial infarction in clinical practice. Heart. 2015;101:101–106.
- , , , et al. Outcomes of hospitalized patients with non‐acute coronary syndrome and elevated cardiac troponin level. Am J Med. 2011;124:630–635.
- , , , et al. Classification of myocardial infarction: frequency and features of type 2 myocardial infarction. Am J Med. 2013;126:789–797.
- , , , , . Cardiac troponin I elevation in hospitalized patients without acute coronary syndromes. Am J Cardiol. 2008;101:1384–1388.
- , , , . Inter‐provider variation in diagnoses and cardiac catheterization use (abstract). Cardiology. 2014;128:346.
- , , , . A primer and comparative review of major us mortality databases. Ann Epidemiol. 2002;12:462–468.
- , , . Assessment of vital status in department of veterans affairs national databases. Comparison with state death certificates. Ann Epidemiol. 2001;11:286–291.
- , , , , , . Raised cardiac troponin T levels in patients without acute coronary syndrome. Postgrad Med J. 2007;83:200–205.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170:18–26.
- , , , , . Prognostic value of troponins in sepsis: a meta‐analysis. Intensive Care Med. 2013;39:1181–1189.
- , , , et al. Prognostic value of cardiac troponin in patients with chronic kidney disease without suspected acute coronary syndrome: a systematic review and meta‐analysis. Ann Intern Med. 2014;161:491–501.
- , , , et al. The TIMI risk score for unstable angina/non‐ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842.
- , , , , , . Usefulness of the TIMI risk score in predicting both short‐ and long‐term outcomes in the Veterans Affairs non‐Q‐wave myocardial infarction strategies in‐hospital (VANQWISH) trial. Am J Cardiol. 2002;90:922–926.