User login
Imaging Evaluation of Superior Labral Anteroposterior (SLAP) Tears
Superior labral anteroposterior (SLAP) tears are common labral injuries. They occur at the attachment of the long head of the biceps tendon on the superior glenoid and extend anterior and/or posterior to the biceps anchor. The mechanism of action for SLAP tears is traction on the superior labrum by the long head of the biceps tendon, resulting in “peeling” of the labrum off the glenoid. Such forces may result from repetitive overhead arm motion (pitching) or from direct trauma.
Clinical diagnosis is challenging with SLAP tears, as they often present with nonspecific shoulder pain and may not be associated with an acute injury. A further complication is that they are often associated with other shoulder pathology, such as rotator cuff tears.1 As physical examination is typically nonspecific, proper diagnostic imaging is essential for diagnosis.
We prefer to assess potential SLAP tears with magnetic resonance arthrography (MRA).2 Dilute (1:200) gadolinium contrast material (12-15 mL) is introduced into the glenohumeral joint under sonographic or fluoroscopic guidance. Capsular distention by dilute intra-articular contrast enables superior imaging resolution of the labroligamentous complex. We think the increase in diagnostic confidence enabled by direct arthrography outweighs the additional invasiveness and cost associated with MRA relative to noncontrast magnetic resonance imaging (MRI).
The MRA protocol differs from our routine noncontrast shoulder imaging. We perform a fat-saturated coronal oblique T1 sequence that maximizes the conspicuity of intra-articular contrast in the plane that optimally visualizes the superior labrum. Three planes of intermediate-weighted fast spin echo not only contrast the high-signal intra-articular fluid with the low-signal fibrocartilaginous labrum and the stratified intermediate signal of glenoid articular cartilage, but they also allow optimal assessment of the rotator cuff. In addition, we perform a fat-saturated coronal T2 sequence that highlights all fluid signal structures as well as edema.
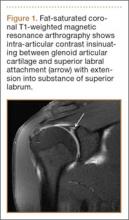
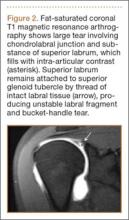
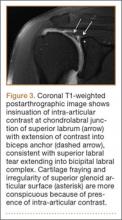
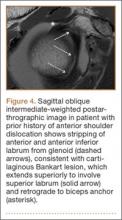
SLAP tears appear on MRA as the insinuation of intra-articular contrast between the articular cartilage and the attachment of the superior labrum,3 within the substance of the labrum, or as detachment of the labrum from the glenoid rim4 (Figure 1). Findings can range from labral fraying to complete detachment with displacement. Tears can extend into other quadrants of the labrum, extend from a Bankart lesion, or involve the biceps tendon and/or the glenohumeral ligaments (Figures 2–4). Up to 10 types of SLAP tears have been described on arthroscopy. This classification scheme, however, is seldom helpful in the interpretation of SLAP tears on MRI. More important in guiding treatment is having a detailed description of the tear, including location, extent, and morphology, along with associated abnormalities.
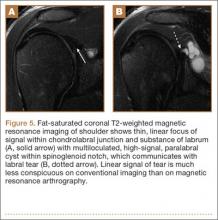
Several findings can aid in the diagnosis of SLAP tears. Normal anatomical variants of the anterior-superior labrum do not extend posterior to the biceps anchor—an important finding for discerning normal morphologic variants from tears. Therefore, high signal within the posterior third of the superior labrum or extension of high signal laterally within the labrum and away from the glenoid suggests a SLAP tear.5 A paralabral cyst is almost always associated with a labral tear,1 so signal abnormality of the superior labrum with a paralabral cyst suggests a SLAP tear (Figure 5).
MRA is not the only method for diagnosing SLAP tears. Standard 3-Tesla MRI had 83% sensitivity and 99% specificity for diagnosing SLAP tears in a recent study, though MRA had 98% sensitivity and 99% specificity—a statistically significant sensitivity difference.6 In another study, computed tomography arthrography (CTA) had 95% sensitivity and 88% specificity for diagnosing recurrent SLAP tears after surgery.7 CTA is associated with ionizing radiation and is limited in its assessment of other structures that may show concomitant abnormalities, such as the articular cartilage and the rotator cuff. Indirect MRA, wherein magnetic resonance sequences are obtained after intravenous injection of gadolinium contrast and exercise of the affected shoulder, had a high sensitivity of detection of labral tears of all types.8
MRA is most sensitive and specific for diagnosing SLAP tears; 3-Tesla MRI, indirect MRA, and CTA are useful alternative modalities for cases in which MRA cannot be performed.
1. Chang D, Mohana-Borges A, Borso M, Chung CB. SLAP lesions: anatomy, clinical presentation, MR imaging diagnosis and characterization. Eur J Radiol. 2008;68(1):72-87.
2. Jee WH, McCauley TR, Katz LD, Matheny JM, Ruwe PA, Daigneault JP. Superior labral anterior posterior (SLAP) lesions of the glenoid labrum: reliability and accuracy of MR arthrography for diagnosis. Radiology. 2001;218(1):127-132.
3. Fitzpatrick D, Walz DM. Shoulder MR imaging normal variants and imaging artifacts. Magn Reson Imaging Clin N Am. 2010;18(4):615-632.
4. Bencardino JT, Beltran J, Rosenberg ZS, et al. Superior labrum anterior-posterior lesions: diagnosis with MR arthrography of the shoulder. Radiology. 2000;214(1):267-271.
5. Tuite MJ, Cirillo RL, De Smet AA, Orwin JF. Superior labrum anterior-posterior (SLAP) tears: evaluation of three MR signs on T2-weighted images. Radiology. 2000;215(3):841-845.
6. Magee T. 3-T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192(1):86-92.
7. De Filippo M, Araoz PA, Pogliacomi F, et al. Recurrent superior labral anterior-to-posterior tears after surgery: detection and grading with CT arthrography. Radiology. 2009;252(3):781-788.
8. Fallahi F, Green N, Gadde S, Jeavons L, Armstrong P, Jonker L. Indirect magnetic resonance arthrography of the shoulder; a reliable diagnostic tool for investigation of suspected labral pathology. Skeletal Radiol. 2013;42(9):1225-1233.
Superior labral anteroposterior (SLAP) tears are common labral injuries. They occur at the attachment of the long head of the biceps tendon on the superior glenoid and extend anterior and/or posterior to the biceps anchor. The mechanism of action for SLAP tears is traction on the superior labrum by the long head of the biceps tendon, resulting in “peeling” of the labrum off the glenoid. Such forces may result from repetitive overhead arm motion (pitching) or from direct trauma.
Clinical diagnosis is challenging with SLAP tears, as they often present with nonspecific shoulder pain and may not be associated with an acute injury. A further complication is that they are often associated with other shoulder pathology, such as rotator cuff tears.1 As physical examination is typically nonspecific, proper diagnostic imaging is essential for diagnosis.
We prefer to assess potential SLAP tears with magnetic resonance arthrography (MRA).2 Dilute (1:200) gadolinium contrast material (12-15 mL) is introduced into the glenohumeral joint under sonographic or fluoroscopic guidance. Capsular distention by dilute intra-articular contrast enables superior imaging resolution of the labroligamentous complex. We think the increase in diagnostic confidence enabled by direct arthrography outweighs the additional invasiveness and cost associated with MRA relative to noncontrast magnetic resonance imaging (MRI).
The MRA protocol differs from our routine noncontrast shoulder imaging. We perform a fat-saturated coronal oblique T1 sequence that maximizes the conspicuity of intra-articular contrast in the plane that optimally visualizes the superior labrum. Three planes of intermediate-weighted fast spin echo not only contrast the high-signal intra-articular fluid with the low-signal fibrocartilaginous labrum and the stratified intermediate signal of glenoid articular cartilage, but they also allow optimal assessment of the rotator cuff. In addition, we perform a fat-saturated coronal T2 sequence that highlights all fluid signal structures as well as edema.
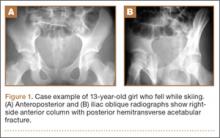
SLAP tears appear on MRA as the insinuation of intra-articular contrast between the articular cartilage and the attachment of the superior labrum,3 within the substance of the labrum, or as detachment of the labrum from the glenoid rim4 (Figure 1). Findings can range from labral fraying to complete detachment with displacement. Tears can extend into other quadrants of the labrum, extend from a Bankart lesion, or involve the biceps tendon and/or the glenohumeral ligaments (Figures 2–4). Up to 10 types of SLAP tears have been described on arthroscopy. This classification scheme, however, is seldom helpful in the interpretation of SLAP tears on MRI. More important in guiding treatment is having a detailed description of the tear, including location, extent, and morphology, along with associated abnormalities.
Several findings can aid in the diagnosis of SLAP tears. Normal anatomical variants of the anterior-superior labrum do not extend posterior to the biceps anchor—an important finding for discerning normal morphologic variants from tears. Therefore, high signal within the posterior third of the superior labrum or extension of high signal laterally within the labrum and away from the glenoid suggests a SLAP tear.5 A paralabral cyst is almost always associated with a labral tear,1 so signal abnormality of the superior labrum with a paralabral cyst suggests a SLAP tear (Figure 5).
MRA is not the only method for diagnosing SLAP tears. Standard 3-Tesla MRI had 83% sensitivity and 99% specificity for diagnosing SLAP tears in a recent study, though MRA had 98% sensitivity and 99% specificity—a statistically significant sensitivity difference.6 In another study, computed tomography arthrography (CTA) had 95% sensitivity and 88% specificity for diagnosing recurrent SLAP tears after surgery.7 CTA is associated with ionizing radiation and is limited in its assessment of other structures that may show concomitant abnormalities, such as the articular cartilage and the rotator cuff. Indirect MRA, wherein magnetic resonance sequences are obtained after intravenous injection of gadolinium contrast and exercise of the affected shoulder, had a high sensitivity of detection of labral tears of all types.8
MRA is most sensitive and specific for diagnosing SLAP tears; 3-Tesla MRI, indirect MRA, and CTA are useful alternative modalities for cases in which MRA cannot be performed.
Superior labral anteroposterior (SLAP) tears are common labral injuries. They occur at the attachment of the long head of the biceps tendon on the superior glenoid and extend anterior and/or posterior to the biceps anchor. The mechanism of action for SLAP tears is traction on the superior labrum by the long head of the biceps tendon, resulting in “peeling” of the labrum off the glenoid. Such forces may result from repetitive overhead arm motion (pitching) or from direct trauma.
Clinical diagnosis is challenging with SLAP tears, as they often present with nonspecific shoulder pain and may not be associated with an acute injury. A further complication is that they are often associated with other shoulder pathology, such as rotator cuff tears.1 As physical examination is typically nonspecific, proper diagnostic imaging is essential for diagnosis.
We prefer to assess potential SLAP tears with magnetic resonance arthrography (MRA).2 Dilute (1:200) gadolinium contrast material (12-15 mL) is introduced into the glenohumeral joint under sonographic or fluoroscopic guidance. Capsular distention by dilute intra-articular contrast enables superior imaging resolution of the labroligamentous complex. We think the increase in diagnostic confidence enabled by direct arthrography outweighs the additional invasiveness and cost associated with MRA relative to noncontrast magnetic resonance imaging (MRI).
The MRA protocol differs from our routine noncontrast shoulder imaging. We perform a fat-saturated coronal oblique T1 sequence that maximizes the conspicuity of intra-articular contrast in the plane that optimally visualizes the superior labrum. Three planes of intermediate-weighted fast spin echo not only contrast the high-signal intra-articular fluid with the low-signal fibrocartilaginous labrum and the stratified intermediate signal of glenoid articular cartilage, but they also allow optimal assessment of the rotator cuff. In addition, we perform a fat-saturated coronal T2 sequence that highlights all fluid signal structures as well as edema.
SLAP tears appear on MRA as the insinuation of intra-articular contrast between the articular cartilage and the attachment of the superior labrum,3 within the substance of the labrum, or as detachment of the labrum from the glenoid rim4 (Figure 1). Findings can range from labral fraying to complete detachment with displacement. Tears can extend into other quadrants of the labrum, extend from a Bankart lesion, or involve the biceps tendon and/or the glenohumeral ligaments (Figures 2–4). Up to 10 types of SLAP tears have been described on arthroscopy. This classification scheme, however, is seldom helpful in the interpretation of SLAP tears on MRI. More important in guiding treatment is having a detailed description of the tear, including location, extent, and morphology, along with associated abnormalities.
Several findings can aid in the diagnosis of SLAP tears. Normal anatomical variants of the anterior-superior labrum do not extend posterior to the biceps anchor—an important finding for discerning normal morphologic variants from tears. Therefore, high signal within the posterior third of the superior labrum or extension of high signal laterally within the labrum and away from the glenoid suggests a SLAP tear.5 A paralabral cyst is almost always associated with a labral tear,1 so signal abnormality of the superior labrum with a paralabral cyst suggests a SLAP tear (Figure 5).
MRA is not the only method for diagnosing SLAP tears. Standard 3-Tesla MRI had 83% sensitivity and 99% specificity for diagnosing SLAP tears in a recent study, though MRA had 98% sensitivity and 99% specificity—a statistically significant sensitivity difference.6 In another study, computed tomography arthrography (CTA) had 95% sensitivity and 88% specificity for diagnosing recurrent SLAP tears after surgery.7 CTA is associated with ionizing radiation and is limited in its assessment of other structures that may show concomitant abnormalities, such as the articular cartilage and the rotator cuff. Indirect MRA, wherein magnetic resonance sequences are obtained after intravenous injection of gadolinium contrast and exercise of the affected shoulder, had a high sensitivity of detection of labral tears of all types.8
MRA is most sensitive and specific for diagnosing SLAP tears; 3-Tesla MRI, indirect MRA, and CTA are useful alternative modalities for cases in which MRA cannot be performed.
1. Chang D, Mohana-Borges A, Borso M, Chung CB. SLAP lesions: anatomy, clinical presentation, MR imaging diagnosis and characterization. Eur J Radiol. 2008;68(1):72-87.
2. Jee WH, McCauley TR, Katz LD, Matheny JM, Ruwe PA, Daigneault JP. Superior labral anterior posterior (SLAP) lesions of the glenoid labrum: reliability and accuracy of MR arthrography for diagnosis. Radiology. 2001;218(1):127-132.
3. Fitzpatrick D, Walz DM. Shoulder MR imaging normal variants and imaging artifacts. Magn Reson Imaging Clin N Am. 2010;18(4):615-632.
4. Bencardino JT, Beltran J, Rosenberg ZS, et al. Superior labrum anterior-posterior lesions: diagnosis with MR arthrography of the shoulder. Radiology. 2000;214(1):267-271.
5. Tuite MJ, Cirillo RL, De Smet AA, Orwin JF. Superior labrum anterior-posterior (SLAP) tears: evaluation of three MR signs on T2-weighted images. Radiology. 2000;215(3):841-845.
6. Magee T. 3-T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192(1):86-92.
7. De Filippo M, Araoz PA, Pogliacomi F, et al. Recurrent superior labral anterior-to-posterior tears after surgery: detection and grading with CT arthrography. Radiology. 2009;252(3):781-788.
8. Fallahi F, Green N, Gadde S, Jeavons L, Armstrong P, Jonker L. Indirect magnetic resonance arthrography of the shoulder; a reliable diagnostic tool for investigation of suspected labral pathology. Skeletal Radiol. 2013;42(9):1225-1233.
1. Chang D, Mohana-Borges A, Borso M, Chung CB. SLAP lesions: anatomy, clinical presentation, MR imaging diagnosis and characterization. Eur J Radiol. 2008;68(1):72-87.
2. Jee WH, McCauley TR, Katz LD, Matheny JM, Ruwe PA, Daigneault JP. Superior labral anterior posterior (SLAP) lesions of the glenoid labrum: reliability and accuracy of MR arthrography for diagnosis. Radiology. 2001;218(1):127-132.
3. Fitzpatrick D, Walz DM. Shoulder MR imaging normal variants and imaging artifacts. Magn Reson Imaging Clin N Am. 2010;18(4):615-632.
4. Bencardino JT, Beltran J, Rosenberg ZS, et al. Superior labrum anterior-posterior lesions: diagnosis with MR arthrography of the shoulder. Radiology. 2000;214(1):267-271.
5. Tuite MJ, Cirillo RL, De Smet AA, Orwin JF. Superior labrum anterior-posterior (SLAP) tears: evaluation of three MR signs on T2-weighted images. Radiology. 2000;215(3):841-845.
6. Magee T. 3-T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192(1):86-92.
7. De Filippo M, Araoz PA, Pogliacomi F, et al. Recurrent superior labral anterior-to-posterior tears after surgery: detection and grading with CT arthrography. Radiology. 2009;252(3):781-788.
8. Fallahi F, Green N, Gadde S, Jeavons L, Armstrong P, Jonker L. Indirect magnetic resonance arthrography of the shoulder; a reliable diagnostic tool for investigation of suspected labral pathology. Skeletal Radiol. 2013;42(9):1225-1233.
Measurement of Resource Utilization for Total and Reverse Shoulder Arthroplasty
As total health care costs reach almost $3 trillion per year—capturing more than 17% of the total US gross domestic product—payers are searching for more effective ways to limit health care spending.1,2 One increasingly discussed plan is payment bundling.3 This one-lump-sum payment model arose as a result of rapid year-on-year increases in total reimbursements under the current, fee-for-service model. The Centers for Medicare & Medicaid Services hypothesized that a single all-inclusive payment for a procedure or set of services would incentivize improvements in patient-centered care and disincentivize cost-shifting behaviors.4 Bundled reimbursement is becoming increasingly common in orthopedic practice. With the recent introduction of the Bundled Payment for Care Improvement Initiative, several orthopedic practices around the United States are already actively engaged in creating models for bundled payment for common elective procedures and for associated services provided up to 90 days after surgery.3,5
Bundled payment increases the burden on the provider to understand the cost of care provided during a care cycle. However, not only has the current system blinded physicians to the cost of care, but current antitrust legislation has made discussions of pricing with colleagues (so-called price collusion) illegal and subject to fines of up to $1 million per person and $100 million per organization,6 therefore limiting orthopedic physician involvement.
Given these legal constraints, instead of measuring direct costs of goods, we developed a “grocery list” approach in which direct comparisons are made of resources (goods and services) used and delivered during the entire 90-day cycle of care for patients who undergo anatomical total shoulder arthroplasty (TSA) or reverse shoulder arthroplasty (RSA). We used one surgeon’s practice experience as a model for measuring other orthopedic surgeons’ resource utilization, based on their electronic medical records (EMR) system data. By capturing the costs of the components of resource utilization rather than just the final cost of care, we can assess, compare, understand, endorse, and address these driving factors.
1. The significance of resource utilization
To maximize the efficiency of their practices, high-volume shoulder surgeons have introduced standardization to health care delivery.7 Identifying specific efficiencies makes uniform acceptance of beneficial practice patterns possible.
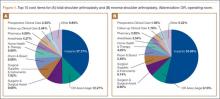
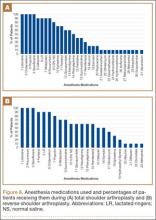
To facilitate comparison of goods and services used during an episode of surgical care, Virani and colleagues8,9 studied the costs of TSA and RSA and calculated the top 10 driving cost factors for these procedures (Figure 1). Their systematic analysis provided a framework for a common method of communication, allowing an orthopedic surgeon to gain a more complete understanding of the resources used during a particular operative procedure in his or her practice, and allowing several physicians to compare and contrast the resources collectively used for a single procedure, facilitating an understanding of different practice patterns within a local community. At a societal level, these data can be collected to help guide overall recommendations.
2. How we defined utilization
To define the resources used, we had to decide which procedure components cost the most. Virani and colleagues8,9 found that the top 10 cost drivers accounted for 93.11% and 94.77% of the total cost of the TSA and RSA care cycles, respectively (Figure 1). For each cost driver, information on resources used (goods, services, overhead) was collected on 2 forms, the Hospital Utilization Form (7 hospital-based items) and the Clinical Utilization Form (3 non-hospital-based items). To make hospital data easy to compile, we piloted use of a “smart form” in the EpicCare EMR system to isolate and auto-populate specific data fields.
3. EMR data collection
With EMR becoming mandatory for all public and private health care providers starting in 2014, utilization data are now included in a single unified system. Working with our in-house information technology department, we developed an algorithm to populate this information in a separate, easy-to-follow hospital utilization form. This form can be adopted by other institutions. Although EpicCare EMR is used by 52% of hospitals and at our institution, the data points required to make the same measurements are generalizable and exist in other EMRs.
Smartlinks, a tool in this EMR, allows utilization data to be quickly retrieved from different locations in a medical record and allows a form to be electronically completed in seconds. Data can be retrieved for any patient in the EMR system, regardless of when that patient’s hospital stay occurred. We populated data from surgeries performed 2 years before the start of this project.
4. What we can learn from these data
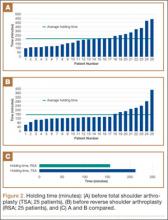
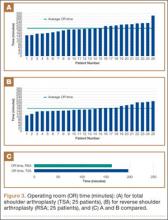
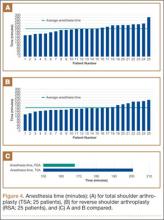
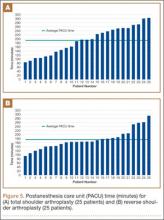
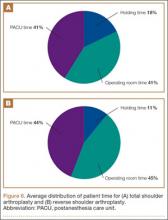
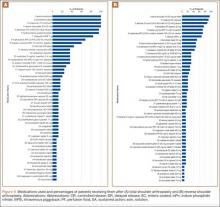
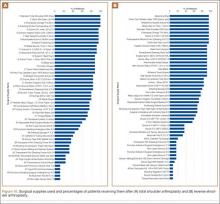
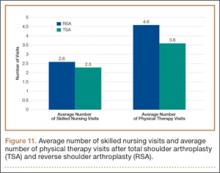
Data from a pilot study of 25 patients who underwent primary anatomical TSA for osteoarthritis and 25 patients who underwent primary RSA for massive rotator cuff tear allowed us to generate graphical representations of a single surgeon’s practice patterns that most affected the cost of care. Time in holding, time in the operating room, time in the postanesthesia care unit, and percentage of patients receiving different medications were recorded for each procedure (Figures 2–11). The study did not capture the wide variances in practice patterns in shoulder arthroplasty, and therefore other surgeons’ resource utilization may differ from ours. However, replicating this methodology at other institutions will produce a more robust data set from which conclusions about resource utilization and, indirectly, cost of care can be made.
5. Future possibilities
By using existing EMR tools to better understand resource utilization, orthopedic surgeons can play a constructive role in the dialogue on health care costs and new reimbursement models. The data presented here are not meant to be interpreted as hard and fast numbers on global resource utilization, but instead we intend to establish a model for collecting data on resource utilization. Resource utilization begins the dialogue that allows orthopedic surgeons and specialty societies to speak a common language without discussing actual cost numbers, which is discouraged under antitrust regulation. The data presented will allow comparisons to be made between surgeons in all practice settings to highlight areas of inconsistency in order to further improve patient care. Although this work involved only 50 patients undergoing only 2 types of surgeries, the resource-capturing methodology can be expanded to include more procedures and orthopedic practices. As all hospitals are now required to have EMRs, the metrics tracked in this work can be found on any patient medical record and auto-populated using our open-source utilization forms. Starting this data collection at your hospital may require no more than a conversation with the informatics department, as the metrics can for the most part be populated into a database on surgeon request.
As orthopedic surgeons return to the economic health care discussion, this information could prove essential in helping the individual surgeon and the orthopedic community justify the cost of care as well as fully understand the cost drivers for musculoskeletal care.
Click here to read the commentary on this article by Peter D. McCann, MD
1. National health expenditures 2013 highlights. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/downloads/highlights.pdf. Accessed September 14, 2015.
2. Wilson KB. Health care costs 101: slow growth persists. California HealthCare Foundation website. http://www.chcf.org/publications/2014/07/health-care-costs-101. Published July 2014. Accessed August 24, 2015.
3. Froimson MI, Rana A, White RE Jr, et al. Bundled Payments for Care Improvement Initiative: the next evolution of payment formulations: AAHKS Bundled Payment Task Force. J Arthroplasty. 2013;28(8 suppl):157-165.
4. Morley M, Bogasky S, Gage B, Flood S, Ingber MJ. Medicare post-acute care episodes and payment bundling. Medicare Medicaid Res Rev. 2014;4(1).
5. Teusink MJ, Virani NA, Polikandriotis JA, Frankle MA. Cost analysis in shoulder arthroplasty surgery. Adv Orthop. 2012;2012:692869.
6. Fassbender E, Pandya S. Legislation focuses on AAOS priorities. American Academy of Orthopaedic Surgeons website. http://www.aaos.org/news/aaosnow/may14/advocacy2.asp. AAOS Now. Published May 2014. Accessed August 24, 2015.
7. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
8. Virani NA, Williams CD, Clark R, Polikandriotis J, Downes KL, Frankle MA. Preparing for the bundled-payment initiative: the cost and clinical outcomes of reverse shoulder arthroplasty for the surgical treatment of advanced rotator cuff deficiency at an average 4-year follow-up. J Shoulder Elbow Surg. 2013;22(12):1612-1622.
9. Virani NA, Williams CD, Clark R, Polikandriotis J, Downes KL, Frankle MA. Preparing for the bundled-payment initiative: the cost and clinical outcomes of total shoulder arthroplasty for the surgical treatment of glenohumeral arthritis at an average 4-year follow-up. J Shoulder Elbow Surg. 2013;22(12):1601-1611.
As total health care costs reach almost $3 trillion per year—capturing more than 17% of the total US gross domestic product—payers are searching for more effective ways to limit health care spending.1,2 One increasingly discussed plan is payment bundling.3 This one-lump-sum payment model arose as a result of rapid year-on-year increases in total reimbursements under the current, fee-for-service model. The Centers for Medicare & Medicaid Services hypothesized that a single all-inclusive payment for a procedure or set of services would incentivize improvements in patient-centered care and disincentivize cost-shifting behaviors.4 Bundled reimbursement is becoming increasingly common in orthopedic practice. With the recent introduction of the Bundled Payment for Care Improvement Initiative, several orthopedic practices around the United States are already actively engaged in creating models for bundled payment for common elective procedures and for associated services provided up to 90 days after surgery.3,5
Bundled payment increases the burden on the provider to understand the cost of care provided during a care cycle. However, not only has the current system blinded physicians to the cost of care, but current antitrust legislation has made discussions of pricing with colleagues (so-called price collusion) illegal and subject to fines of up to $1 million per person and $100 million per organization,6 therefore limiting orthopedic physician involvement.
Given these legal constraints, instead of measuring direct costs of goods, we developed a “grocery list” approach in which direct comparisons are made of resources (goods and services) used and delivered during the entire 90-day cycle of care for patients who undergo anatomical total shoulder arthroplasty (TSA) or reverse shoulder arthroplasty (RSA). We used one surgeon’s practice experience as a model for measuring other orthopedic surgeons’ resource utilization, based on their electronic medical records (EMR) system data. By capturing the costs of the components of resource utilization rather than just the final cost of care, we can assess, compare, understand, endorse, and address these driving factors.
1. The significance of resource utilization
To maximize the efficiency of their practices, high-volume shoulder surgeons have introduced standardization to health care delivery.7 Identifying specific efficiencies makes uniform acceptance of beneficial practice patterns possible.
To facilitate comparison of goods and services used during an episode of surgical care, Virani and colleagues8,9 studied the costs of TSA and RSA and calculated the top 10 driving cost factors for these procedures (Figure 1). Their systematic analysis provided a framework for a common method of communication, allowing an orthopedic surgeon to gain a more complete understanding of the resources used during a particular operative procedure in his or her practice, and allowing several physicians to compare and contrast the resources collectively used for a single procedure, facilitating an understanding of different practice patterns within a local community. At a societal level, these data can be collected to help guide overall recommendations.
2. How we defined utilization
To define the resources used, we had to decide which procedure components cost the most. Virani and colleagues8,9 found that the top 10 cost drivers accounted for 93.11% and 94.77% of the total cost of the TSA and RSA care cycles, respectively (Figure 1). For each cost driver, information on resources used (goods, services, overhead) was collected on 2 forms, the Hospital Utilization Form (7 hospital-based items) and the Clinical Utilization Form (3 non-hospital-based items). To make hospital data easy to compile, we piloted use of a “smart form” in the EpicCare EMR system to isolate and auto-populate specific data fields.
3. EMR data collection
With EMR becoming mandatory for all public and private health care providers starting in 2014, utilization data are now included in a single unified system. Working with our in-house information technology department, we developed an algorithm to populate this information in a separate, easy-to-follow hospital utilization form. This form can be adopted by other institutions. Although EpicCare EMR is used by 52% of hospitals and at our institution, the data points required to make the same measurements are generalizable and exist in other EMRs.
Smartlinks, a tool in this EMR, allows utilization data to be quickly retrieved from different locations in a medical record and allows a form to be electronically completed in seconds. Data can be retrieved for any patient in the EMR system, regardless of when that patient’s hospital stay occurred. We populated data from surgeries performed 2 years before the start of this project.
4. What we can learn from these data
Data from a pilot study of 25 patients who underwent primary anatomical TSA for osteoarthritis and 25 patients who underwent primary RSA for massive rotator cuff tear allowed us to generate graphical representations of a single surgeon’s practice patterns that most affected the cost of care. Time in holding, time in the operating room, time in the postanesthesia care unit, and percentage of patients receiving different medications were recorded for each procedure (Figures 2–11). The study did not capture the wide variances in practice patterns in shoulder arthroplasty, and therefore other surgeons’ resource utilization may differ from ours. However, replicating this methodology at other institutions will produce a more robust data set from which conclusions about resource utilization and, indirectly, cost of care can be made.
5. Future possibilities
By using existing EMR tools to better understand resource utilization, orthopedic surgeons can play a constructive role in the dialogue on health care costs and new reimbursement models. The data presented here are not meant to be interpreted as hard and fast numbers on global resource utilization, but instead we intend to establish a model for collecting data on resource utilization. Resource utilization begins the dialogue that allows orthopedic surgeons and specialty societies to speak a common language without discussing actual cost numbers, which is discouraged under antitrust regulation. The data presented will allow comparisons to be made between surgeons in all practice settings to highlight areas of inconsistency in order to further improve patient care. Although this work involved only 50 patients undergoing only 2 types of surgeries, the resource-capturing methodology can be expanded to include more procedures and orthopedic practices. As all hospitals are now required to have EMRs, the metrics tracked in this work can be found on any patient medical record and auto-populated using our open-source utilization forms. Starting this data collection at your hospital may require no more than a conversation with the informatics department, as the metrics can for the most part be populated into a database on surgeon request.
As orthopedic surgeons return to the economic health care discussion, this information could prove essential in helping the individual surgeon and the orthopedic community justify the cost of care as well as fully understand the cost drivers for musculoskeletal care.
Click here to read the commentary on this article by Peter D. McCann, MD
As total health care costs reach almost $3 trillion per year—capturing more than 17% of the total US gross domestic product—payers are searching for more effective ways to limit health care spending.1,2 One increasingly discussed plan is payment bundling.3 This one-lump-sum payment model arose as a result of rapid year-on-year increases in total reimbursements under the current, fee-for-service model. The Centers for Medicare & Medicaid Services hypothesized that a single all-inclusive payment for a procedure or set of services would incentivize improvements in patient-centered care and disincentivize cost-shifting behaviors.4 Bundled reimbursement is becoming increasingly common in orthopedic practice. With the recent introduction of the Bundled Payment for Care Improvement Initiative, several orthopedic practices around the United States are already actively engaged in creating models for bundled payment for common elective procedures and for associated services provided up to 90 days after surgery.3,5
Bundled payment increases the burden on the provider to understand the cost of care provided during a care cycle. However, not only has the current system blinded physicians to the cost of care, but current antitrust legislation has made discussions of pricing with colleagues (so-called price collusion) illegal and subject to fines of up to $1 million per person and $100 million per organization,6 therefore limiting orthopedic physician involvement.
Given these legal constraints, instead of measuring direct costs of goods, we developed a “grocery list” approach in which direct comparisons are made of resources (goods and services) used and delivered during the entire 90-day cycle of care for patients who undergo anatomical total shoulder arthroplasty (TSA) or reverse shoulder arthroplasty (RSA). We used one surgeon’s practice experience as a model for measuring other orthopedic surgeons’ resource utilization, based on their electronic medical records (EMR) system data. By capturing the costs of the components of resource utilization rather than just the final cost of care, we can assess, compare, understand, endorse, and address these driving factors.
1. The significance of resource utilization
To maximize the efficiency of their practices, high-volume shoulder surgeons have introduced standardization to health care delivery.7 Identifying specific efficiencies makes uniform acceptance of beneficial practice patterns possible.
To facilitate comparison of goods and services used during an episode of surgical care, Virani and colleagues8,9 studied the costs of TSA and RSA and calculated the top 10 driving cost factors for these procedures (Figure 1). Their systematic analysis provided a framework for a common method of communication, allowing an orthopedic surgeon to gain a more complete understanding of the resources used during a particular operative procedure in his or her practice, and allowing several physicians to compare and contrast the resources collectively used for a single procedure, facilitating an understanding of different practice patterns within a local community. At a societal level, these data can be collected to help guide overall recommendations.
2. How we defined utilization
To define the resources used, we had to decide which procedure components cost the most. Virani and colleagues8,9 found that the top 10 cost drivers accounted for 93.11% and 94.77% of the total cost of the TSA and RSA care cycles, respectively (Figure 1). For each cost driver, information on resources used (goods, services, overhead) was collected on 2 forms, the Hospital Utilization Form (7 hospital-based items) and the Clinical Utilization Form (3 non-hospital-based items). To make hospital data easy to compile, we piloted use of a “smart form” in the EpicCare EMR system to isolate and auto-populate specific data fields.
3. EMR data collection
With EMR becoming mandatory for all public and private health care providers starting in 2014, utilization data are now included in a single unified system. Working with our in-house information technology department, we developed an algorithm to populate this information in a separate, easy-to-follow hospital utilization form. This form can be adopted by other institutions. Although EpicCare EMR is used by 52% of hospitals and at our institution, the data points required to make the same measurements are generalizable and exist in other EMRs.
Smartlinks, a tool in this EMR, allows utilization data to be quickly retrieved from different locations in a medical record and allows a form to be electronically completed in seconds. Data can be retrieved for any patient in the EMR system, regardless of when that patient’s hospital stay occurred. We populated data from surgeries performed 2 years before the start of this project.
4. What we can learn from these data
Data from a pilot study of 25 patients who underwent primary anatomical TSA for osteoarthritis and 25 patients who underwent primary RSA for massive rotator cuff tear allowed us to generate graphical representations of a single surgeon’s practice patterns that most affected the cost of care. Time in holding, time in the operating room, time in the postanesthesia care unit, and percentage of patients receiving different medications were recorded for each procedure (Figures 2–11). The study did not capture the wide variances in practice patterns in shoulder arthroplasty, and therefore other surgeons’ resource utilization may differ from ours. However, replicating this methodology at other institutions will produce a more robust data set from which conclusions about resource utilization and, indirectly, cost of care can be made.
5. Future possibilities
By using existing EMR tools to better understand resource utilization, orthopedic surgeons can play a constructive role in the dialogue on health care costs and new reimbursement models. The data presented here are not meant to be interpreted as hard and fast numbers on global resource utilization, but instead we intend to establish a model for collecting data on resource utilization. Resource utilization begins the dialogue that allows orthopedic surgeons and specialty societies to speak a common language without discussing actual cost numbers, which is discouraged under antitrust regulation. The data presented will allow comparisons to be made between surgeons in all practice settings to highlight areas of inconsistency in order to further improve patient care. Although this work involved only 50 patients undergoing only 2 types of surgeries, the resource-capturing methodology can be expanded to include more procedures and orthopedic practices. As all hospitals are now required to have EMRs, the metrics tracked in this work can be found on any patient medical record and auto-populated using our open-source utilization forms. Starting this data collection at your hospital may require no more than a conversation with the informatics department, as the metrics can for the most part be populated into a database on surgeon request.
As orthopedic surgeons return to the economic health care discussion, this information could prove essential in helping the individual surgeon and the orthopedic community justify the cost of care as well as fully understand the cost drivers for musculoskeletal care.
Click here to read the commentary on this article by Peter D. McCann, MD
1. National health expenditures 2013 highlights. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/downloads/highlights.pdf. Accessed September 14, 2015.
2. Wilson KB. Health care costs 101: slow growth persists. California HealthCare Foundation website. http://www.chcf.org/publications/2014/07/health-care-costs-101. Published July 2014. Accessed August 24, 2015.
3. Froimson MI, Rana A, White RE Jr, et al. Bundled Payments for Care Improvement Initiative: the next evolution of payment formulations: AAHKS Bundled Payment Task Force. J Arthroplasty. 2013;28(8 suppl):157-165.
4. Morley M, Bogasky S, Gage B, Flood S, Ingber MJ. Medicare post-acute care episodes and payment bundling. Medicare Medicaid Res Rev. 2014;4(1).
5. Teusink MJ, Virani NA, Polikandriotis JA, Frankle MA. Cost analysis in shoulder arthroplasty surgery. Adv Orthop. 2012;2012:692869.
6. Fassbender E, Pandya S. Legislation focuses on AAOS priorities. American Academy of Orthopaedic Surgeons website. http://www.aaos.org/news/aaosnow/may14/advocacy2.asp. AAOS Now. Published May 2014. Accessed August 24, 2015.
7. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
8. Virani NA, Williams CD, Clark R, Polikandriotis J, Downes KL, Frankle MA. Preparing for the bundled-payment initiative: the cost and clinical outcomes of reverse shoulder arthroplasty for the surgical treatment of advanced rotator cuff deficiency at an average 4-year follow-up. J Shoulder Elbow Surg. 2013;22(12):1612-1622.
9. Virani NA, Williams CD, Clark R, Polikandriotis J, Downes KL, Frankle MA. Preparing for the bundled-payment initiative: the cost and clinical outcomes of total shoulder arthroplasty for the surgical treatment of glenohumeral arthritis at an average 4-year follow-up. J Shoulder Elbow Surg. 2013;22(12):1601-1611.
1. National health expenditures 2013 highlights. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/downloads/highlights.pdf. Accessed September 14, 2015.
2. Wilson KB. Health care costs 101: slow growth persists. California HealthCare Foundation website. http://www.chcf.org/publications/2014/07/health-care-costs-101. Published July 2014. Accessed August 24, 2015.
3. Froimson MI, Rana A, White RE Jr, et al. Bundled Payments for Care Improvement Initiative: the next evolution of payment formulations: AAHKS Bundled Payment Task Force. J Arthroplasty. 2013;28(8 suppl):157-165.
4. Morley M, Bogasky S, Gage B, Flood S, Ingber MJ. Medicare post-acute care episodes and payment bundling. Medicare Medicaid Res Rev. 2014;4(1).
5. Teusink MJ, Virani NA, Polikandriotis JA, Frankle MA. Cost analysis in shoulder arthroplasty surgery. Adv Orthop. 2012;2012:692869.
6. Fassbender E, Pandya S. Legislation focuses on AAOS priorities. American Academy of Orthopaedic Surgeons website. http://www.aaos.org/news/aaosnow/may14/advocacy2.asp. AAOS Now. Published May 2014. Accessed August 24, 2015.
7. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
8. Virani NA, Williams CD, Clark R, Polikandriotis J, Downes KL, Frankle MA. Preparing for the bundled-payment initiative: the cost and clinical outcomes of reverse shoulder arthroplasty for the surgical treatment of advanced rotator cuff deficiency at an average 4-year follow-up. J Shoulder Elbow Surg. 2013;22(12):1612-1622.
9. Virani NA, Williams CD, Clark R, Polikandriotis J, Downes KL, Frankle MA. Preparing for the bundled-payment initiative: the cost and clinical outcomes of total shoulder arthroplasty for the surgical treatment of glenohumeral arthritis at an average 4-year follow-up. J Shoulder Elbow Surg. 2013;22(12):1601-1611.
Technique of Open Reduction and Internal Fixation of Comminuted Proximal Humerus Fractures With Allograft Femoral Head Metaphyseal Reconstruction
Proximal humerus fractures are exceedingly common and account for almost 5% of all fractures. As osteoporosis is a risk factor for these fractures, their incidence rises with patient age.1
In 1970, Neer2 described these type of fractures and classified them as having 2, 3, or 4 parts based on the amount of angulation and displacement of the humeral head and the greater and lesser tuberosities with respect to the shaft.
Three- and 4-part proximal humerus fractures can be treated either nonoperatively, or surgically with closed reduction and percutaneous fixation, intramedullary fixation, open reduction and internal fixation (ORIF), or arthroplasty. There remains controversy over the best treatment, but a key component of any surgical treatment is anatomical reduction, stable fixation, and then healing of the tuberosities. A current common form of treatment is augmentation with an allograft fibula placed in the medullary canal. Although not formally reported, anecdotal evidence demonstrates that revision to arthroplasty is very difficult in the setting of an ingrown graft in the medullary canal of the humerus.
In this article, we present a novel technique of using allograft femoral head to reconstruct the metaphysis in ORIF of comminuted proximal humerus fractures.
Technique
Presented in Figure 1 are preoperative images of a representative displaced 4-part proximal humerus fracture treated surgically using the technique described here. General anesthesia is used. After intubation on the operating table, the patient is placed in the beach-chair position with about 75° of hip flexion. All bony prominences are padded, and the head and trunk are well secured. A pneumatic arm positioner is used to alleviate the need for an assistant to manipulate the arm. An image intensifier is used before preparing to verify that appropriate images of the proximal humerus can be obtained. Once adequate images are confirmed, the floor can be marked at the position of the fluoroscopic unit’s wheels to allow easy reproduction of images once the arm is prepared and draped. The intensifier is then removed from the field, the shoulder is prepared and draped in usual fashion, and prophylactic antibiotics are administered.
A deltopectoral incision is used, and sharp dissection is made through the subcutaneous tissue to raise full-thickness subcutaneous flaps on each side. The deltopectoral interval is sharply dissected while protecting the cephalic vein. Subdeltoid adhesions are then released. Palpation of the axillary nerve in the quadrilateral space to identify its location is helpful to avoid injury during the procedure.
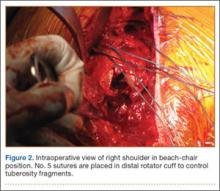
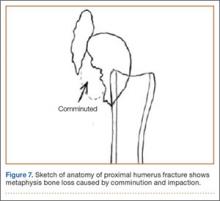
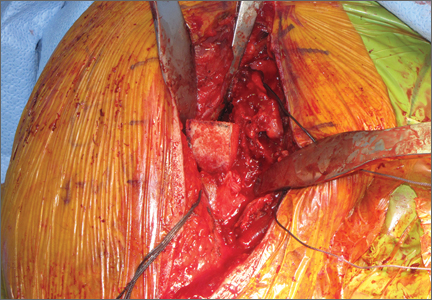
The fracture is then identified, and No. 5 permanent suture is placed through the posterior and superior rotator cuff and through the subscapularis insertion (Figure 2). The tuberosities are freed from the humeral head sharply. A blunt elevator is then used to gently elevate the humeral head upward, with care taken to avoid comminuting the metaphyseal bone while levering. Reduction is achieved by manipulating the sutures and levering the head with the elevator while placing the arm in extension and posterior translation. Fluoroscopic images are used to verify correct anatomical alignment. Generally, the metaphysis demonstrates comminution and impaction, with poor bone quality necessitating use of bone graft.
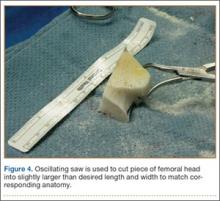
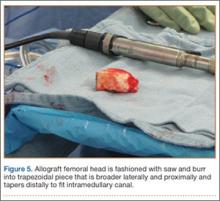
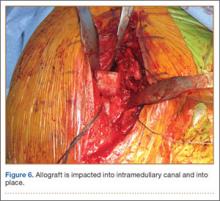
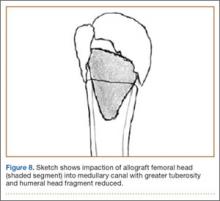
A frozen allograft femoral head is then obtained and split into 2 equal pieces using a saw (Figures 3–5). One piece is fashioned with a saw and a burr into a trapezoid such that the proximal portion is wider, and the distal, tapered portion is sized to fit the canal. The broad, proximal portion of the graft will serve as a pedestal to reduce the head to the shaft. Measuring the internal diameter of the humeral canal can be useful in estimating the necessary dimensions of the distal portion of the allograft. The graft often needs several small adjustments that necessitate attempting to place it in the intramedullary canal and then trimming as necessary to ensure proper fit distally within the shaft. For this reason, it is beneficial to perform the graft preparation near the surgical field. Once completed, the distal portion is then impacted into the humeral canal (Figure 6). Because of this impaction, there is no possibility for subsidence or pistoning of the graft within the canal, which can occur with a fibular graft. The humeral head is reduced onto the shaft with the already placed sutures; this is achieved by abducting the shoulder. The image intensifier is then used to confirm appropriate alignment and positioning of the fragments, making sure that both neck–shaft angle and medial calcar alignment have been restored (Figures 7, 8).
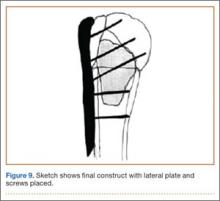
An appropriately sized proximal humerus plate is then selected based on the location of the fracture line. We have used standard lateral proximal humerus locking plates as well as laterality-specific anterolateral proximal humerus plates and found that both are suitable for incorporation of the screws through the graft and into the head. The plate is positioned on the humerus, and a guide pin is placed by hand through the proximal-most hole so that the appropriate height of the plate can be verified on fluoroscopy. The first screw is then a nonlocking bicortical screw placed through the oval hole in the shaft of the plate to allow further fine manipulation of the plate more proximally or distally as needed. The final height is confirmed, and the screw is firmly tightened (Figure 9). The locking-screw guide is fixed to the proximal portion of the plate, and 2 locking screws are then placed into the head. The arm is then rotated to an anteroposterior view by placing the arm in external rotation and neutral flexion and is then abducted and internally rotated to recreate a lateral view to perform final verification of the position of the plate on orthogonal images. If the surgeon is satisfied with the position of the plate, another nonlocking screw is placed distally, and then the proximal holes are used to place locking screws as needed. If the surgeon is not satisfied, the 2 proximal screws can be removed and the plate repositioned.
After each screw is placed, fluoroscopy is used to ensure there has been no breach of the articular surface. The number of proximal screws placed depends on fracture configuration and surgeon preference.
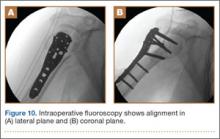
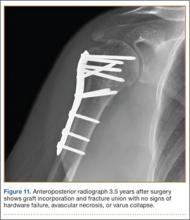
The sutures through the rotator cuff are then fixed to the plate, securing the tuberosities. Final intraoperative radiographs are used to confirm reduction, alignment, and final position of hardware (Figure 10). After copious irrigation, a surgical drain is placed as needed, and the wound is closed in layered fashion. Three years after surgery, follow-up examination revealed no radiographic change in alignment, no necrosis, and no varus collapse (Figure 11), and the patient was pain-free during activities.
Discussion
Surgical treatment of comminuted proximal humerus fractures usually consists of some type of plate fixation with screw fixation of the shaft, screws or smooth pegs to support the chondral surfaces, and screw fixation or suture cerclage of the tuberosities.
Fixed-angle locking-plate-and-screw constructs increased the biomechanical stability and pullout strength of proximal humerus plates.3,4 Nevertheless, avascular necrosis, malunion, and nonunion are still known complications of proximal humerus fractures, especially those with comminution, with up to 14% of patients still experiencing loss of fixation.5
For this reason, several authors have proposed using allograft bone and/or augmentation with calcium-containing cement to supplement fixation and provide an endosteal form of support for the head and tuberosities to decrease the risk for varus collapse. Osteobiologics (eg, calcium phosphate or sulfate cement) have been shown to decrease the risk for loss of reduction of proximal humerus fractures and decrease the risk for intra-articular screw penetration.6,7 Many calcium phosphate cements are commercially available. Cost and availability are 2 reasons that these supplements are not more widely used. Cancellous chips have also been used to aid in the reduction of proximal humerus fractures.8 No randomized study has been conducted to show a clinical advantage of this technique, though retrospective studies have shown that it is not as advantageous as using calcium phosphate cement with respect to loss of reduction or screw penetration.6 Certainly, cancellous chips are easily available in most hospitals and are less expensive than some alternatives. A recent review of these techniques in osteoporotic proximal humerus fractures found no clear indication for using one of these supplements over another.9
However, some fracture patterns require a structural graft to reduce the tuberosities and head component. Although described more than 30 years ago as a treatment for nonunions with an intramedullary “peg” of iliac crest graft,10 the graft most commonly reported today is allograft fibula.11-15 This technique consists of preparing the humeral shaft and often the fractured head segment with reaming to create a channel to receive the graft. Even with use of a small fibula, it is often time-consuming to use a saw, rasp, or burr to size the fibular segment to fit the medullary canal of the humerus. Once in place, the graft provides a strut on which the head fragment can be reduced and around which the tuberosities can be reduced. Although this technique is successful clinically and is biomechanically superior to plate-only constructs,16,17 concerns remain.
One such concern is keeping this graft in routine supply at most hospitals. Supply and pricing from vendors can differ significantly between hospitals, and a surgeon may need to request grafts in advance, which makes their use nonviable in a trauma case. Certain grafts are often kept in routine supply based on their overall utilization. At our institution, allograft femoral heads meet this criterion and are routinely stocked.
Of more importance are the ramifications of these procedures for future revision surgeries. The need for arthroplasty revision is common after ORIF of a proximal humerus fracture.18
Arthroplasty revision is an already challenging procedure that becomes more complex with the need to remove 6 to 8 cm of ingrown endosteal bone from a shell of outer osteoporotic cortical bone. Our experience with these complex revisions provided the impetus to search for an alternate graft type that still provides a strut for reducing the head and tuberosities but limits the amount of endosteal bone that would need to be removed in arthroplasty revision in order to place a stemmed component into the humeral canal.
Some currently available arthroplasty fracture systems modify the previous anatomy of the stem to provide a more anatomical platform to reduce the tuberosities to a broader metaphyseal construct that incorporates bone grafting to assist with healing.
Because of these concerns and factors, we adapted our technique to create an individual-specific pedestal with allograft femoral head that can be anatomically matched to each patient. This provides a strut to reduce the head and tuberosity fragments but still limits the amount of allograft bone needed to seat into the existing canal. The geometry of the allograft can also be customized to the fracture, with most 3- and 4-part fractures needing a trapezoidal strut that resembles the metaphyseal portion of a fracture-specific shoulder arthroplasty implant.
We have used this technique for comminuted 3- and 4-part fractures of the proximal humerus in 14 cases with at least 2-year follow-up and in several more cases that have not reached 2-year follow-up. All cases have gone on to radiographic union; none have had to be revised either with revision ORIF or to an arthroplasty. Formal measurements of final postoperative range of motion have not been tabulated in all cases, as some cases have been lost to follow-up after radiographic union was achieved. Medium- and long-term results are not yet available, but no short-term complications have been noted.
Disadvantages of this technique are that, while an individualized graft is created, proper shaping still takes time, and a moderate amount of the femoral head is not used. However, we have found that, if a graft is inadvertently undersized, there is still ample femoral head remaining to create another sized graft. Other disadvantages are the added cost and the (rare) risk of disease transmission, which come with use of any allograft, but the technique is used instead of another type of allograft, so these disadvantages are largely equivalent. At our hospital, differences in cost and availability between femoral head or fibular allografts are negligible.
This procedure, which is easily performed in a short amount of time, allows a stable base of bone graft to be used as an aid in the anatomical reduction of proximal humerus fractures, without the need for reaming and preparation of the medullary canal and without further increasing the difficulty associated with a future revision procedure.
1. Barrett JA, Baron JA, Karagas MR, Beach ML. Fracture risk in the U.S. Medicare population. J Clin Epidemiol. 1999;52(3):243-249.
2. Neer CS 2nd. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
3. Liew AS, Johnson JA, Patterson SD, King GJ, Chess DG. Effect of screw placement on fixation in the humeral head. J Shoulder Elbow Surg. 2000;9(5):423-426.
4. Weinstein DM, Bratton DR, Ciccone WJ 2nd, Elias JJ. Locking plates improve torsional resistance in the stabilization of three-part proximal humeral fractures. J Shoulder Elbow Surg. 2006;15(2):239-243.
5. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
6. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction-internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012;21(6):741-748.
7. Gradl G, Knobe M, Stoffel M, Prescher A, Dirrichs T, Pape HC. Biomechanical evaluation of locking plate fixation of proximal humeral fractures augmented with calcium phosphate cement. J Orthop Trauma. 2013;27(7):399-404.
8. Ong CC, Kwon YW, Walsh M, Davidovitch R, Zuckerman JD, Egol KA. Outcomes of open reduction and internal fixation of proximal humerus fractures managed with locking plates. Am J Orthop. 2012;41(9):407-412.
9. Namdari S, Voleti PB, Mehta S. Evaluation of the osteoporotic proximal humeral fracture and strategies for structural augmentation during surgical treatment. J Shoulder Elbow Surg. 2012;21(12):1787-1795.
10. Scheck M. Surgical treatment of nonunions of the surgical neck of the humerus. Clin Orthop Relat Res. 1982;(167):255-259.
11. Hettrich CM, Neviaser A, Beamer BS, Paul O, Helfet DL, Lorich DG. Locked plating of the proximal humerus using an endosteal implant. J Orthop Trauma. 2012;26(4):212-215.
12. Neviaser AS, Hettrich CM, Beamer BS, Dines JS, Lorich DG. Endosteal strut augment reduces complications associated with proximal humeral locking plates. Clin Orthop Relat Res. 2011;469(12):3300-3306.
13. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J Orthop Trauma. 2008;22(3):195-200.
14. Matassi F, Angeloni R, Carulli C, et al. Locking plate and fibular allograft augmentation in unstable fractures of proximal humerus. Injury. 2012;43(11):1939-1942.
15. Little MT, Berkes MB, Schottel PC, et al. The impact of preoperative coronal plane deformity on proximal humerus fixation with endosteal augmentation. J Orthop Trauma. 2014;28(6):338-347.
16. Mathison C, Chaudhary R, Beaupre L, Reynolds M, Adeeb S, Bouliane M. Biomechanical analysis of proximal humeral fixation using locking plate fixation with an intramedullary fibular allograft. Clin Biomech. 2010;25(7):642-646.
17. Chow RM, Begum F, Beaupre LA, Carey JP, Adeeb S, Bouliane MJ. Proximal humeral fracture fixation: locking plate construct +/- intramedullary fibular allograft. J Shoulder Elbow Surg. 2012;21(7):894-901.
18. Jost B, Spross C, Grehn H, Gerber C. Locking plate fixation of fractures of the proximal humerus: analysis of complications, revision strategies and outcome. J Shoulder Elbow Surg. 2013;22(4):542-549.
Proximal humerus fractures are exceedingly common and account for almost 5% of all fractures. As osteoporosis is a risk factor for these fractures, their incidence rises with patient age.1
In 1970, Neer2 described these type of fractures and classified them as having 2, 3, or 4 parts based on the amount of angulation and displacement of the humeral head and the greater and lesser tuberosities with respect to the shaft.
Three- and 4-part proximal humerus fractures can be treated either nonoperatively, or surgically with closed reduction and percutaneous fixation, intramedullary fixation, open reduction and internal fixation (ORIF), or arthroplasty. There remains controversy over the best treatment, but a key component of any surgical treatment is anatomical reduction, stable fixation, and then healing of the tuberosities. A current common form of treatment is augmentation with an allograft fibula placed in the medullary canal. Although not formally reported, anecdotal evidence demonstrates that revision to arthroplasty is very difficult in the setting of an ingrown graft in the medullary canal of the humerus.
In this article, we present a novel technique of using allograft femoral head to reconstruct the metaphysis in ORIF of comminuted proximal humerus fractures.
Technique
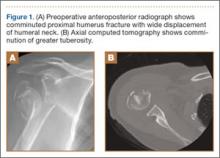
Presented in Figure 1 are preoperative images of a representative displaced 4-part proximal humerus fracture treated surgically using the technique described here. General anesthesia is used. After intubation on the operating table, the patient is placed in the beach-chair position with about 75° of hip flexion. All bony prominences are padded, and the head and trunk are well secured. A pneumatic arm positioner is used to alleviate the need for an assistant to manipulate the arm. An image intensifier is used before preparing to verify that appropriate images of the proximal humerus can be obtained. Once adequate images are confirmed, the floor can be marked at the position of the fluoroscopic unit’s wheels to allow easy reproduction of images once the arm is prepared and draped. The intensifier is then removed from the field, the shoulder is prepared and draped in usual fashion, and prophylactic antibiotics are administered.
A deltopectoral incision is used, and sharp dissection is made through the subcutaneous tissue to raise full-thickness subcutaneous flaps on each side. The deltopectoral interval is sharply dissected while protecting the cephalic vein. Subdeltoid adhesions are then released. Palpation of the axillary nerve in the quadrilateral space to identify its location is helpful to avoid injury during the procedure.
The fracture is then identified, and No. 5 permanent suture is placed through the posterior and superior rotator cuff and through the subscapularis insertion (Figure 2). The tuberosities are freed from the humeral head sharply. A blunt elevator is then used to gently elevate the humeral head upward, with care taken to avoid comminuting the metaphyseal bone while levering. Reduction is achieved by manipulating the sutures and levering the head with the elevator while placing the arm in extension and posterior translation. Fluoroscopic images are used to verify correct anatomical alignment. Generally, the metaphysis demonstrates comminution and impaction, with poor bone quality necessitating use of bone graft.
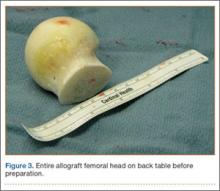
A frozen allograft femoral head is then obtained and split into 2 equal pieces using a saw (Figures 3–5). One piece is fashioned with a saw and a burr into a trapezoid such that the proximal portion is wider, and the distal, tapered portion is sized to fit the canal. The broad, proximal portion of the graft will serve as a pedestal to reduce the head to the shaft. Measuring the internal diameter of the humeral canal can be useful in estimating the necessary dimensions of the distal portion of the allograft. The graft often needs several small adjustments that necessitate attempting to place it in the intramedullary canal and then trimming as necessary to ensure proper fit distally within the shaft. For this reason, it is beneficial to perform the graft preparation near the surgical field. Once completed, the distal portion is then impacted into the humeral canal (Figure 6). Because of this impaction, there is no possibility for subsidence or pistoning of the graft within the canal, which can occur with a fibular graft. The humeral head is reduced onto the shaft with the already placed sutures; this is achieved by abducting the shoulder. The image intensifier is then used to confirm appropriate alignment and positioning of the fragments, making sure that both neck–shaft angle and medial calcar alignment have been restored (Figures 7, 8).
An appropriately sized proximal humerus plate is then selected based on the location of the fracture line. We have used standard lateral proximal humerus locking plates as well as laterality-specific anterolateral proximal humerus plates and found that both are suitable for incorporation of the screws through the graft and into the head. The plate is positioned on the humerus, and a guide pin is placed by hand through the proximal-most hole so that the appropriate height of the plate can be verified on fluoroscopy. The first screw is then a nonlocking bicortical screw placed through the oval hole in the shaft of the plate to allow further fine manipulation of the plate more proximally or distally as needed. The final height is confirmed, and the screw is firmly tightened (Figure 9). The locking-screw guide is fixed to the proximal portion of the plate, and 2 locking screws are then placed into the head. The arm is then rotated to an anteroposterior view by placing the arm in external rotation and neutral flexion and is then abducted and internally rotated to recreate a lateral view to perform final verification of the position of the plate on orthogonal images. If the surgeon is satisfied with the position of the plate, another nonlocking screw is placed distally, and then the proximal holes are used to place locking screws as needed. If the surgeon is not satisfied, the 2 proximal screws can be removed and the plate repositioned.
After each screw is placed, fluoroscopy is used to ensure there has been no breach of the articular surface. The number of proximal screws placed depends on fracture configuration and surgeon preference.
The sutures through the rotator cuff are then fixed to the plate, securing the tuberosities. Final intraoperative radiographs are used to confirm reduction, alignment, and final position of hardware (Figure 10). After copious irrigation, a surgical drain is placed as needed, and the wound is closed in layered fashion. Three years after surgery, follow-up examination revealed no radiographic change in alignment, no necrosis, and no varus collapse (Figure 11), and the patient was pain-free during activities.
Discussion
Surgical treatment of comminuted proximal humerus fractures usually consists of some type of plate fixation with screw fixation of the shaft, screws or smooth pegs to support the chondral surfaces, and screw fixation or suture cerclage of the tuberosities.
Fixed-angle locking-plate-and-screw constructs increased the biomechanical stability and pullout strength of proximal humerus plates.3,4 Nevertheless, avascular necrosis, malunion, and nonunion are still known complications of proximal humerus fractures, especially those with comminution, with up to 14% of patients still experiencing loss of fixation.5
For this reason, several authors have proposed using allograft bone and/or augmentation with calcium-containing cement to supplement fixation and provide an endosteal form of support for the head and tuberosities to decrease the risk for varus collapse. Osteobiologics (eg, calcium phosphate or sulfate cement) have been shown to decrease the risk for loss of reduction of proximal humerus fractures and decrease the risk for intra-articular screw penetration.6,7 Many calcium phosphate cements are commercially available. Cost and availability are 2 reasons that these supplements are not more widely used. Cancellous chips have also been used to aid in the reduction of proximal humerus fractures.8 No randomized study has been conducted to show a clinical advantage of this technique, though retrospective studies have shown that it is not as advantageous as using calcium phosphate cement with respect to loss of reduction or screw penetration.6 Certainly, cancellous chips are easily available in most hospitals and are less expensive than some alternatives. A recent review of these techniques in osteoporotic proximal humerus fractures found no clear indication for using one of these supplements over another.9
However, some fracture patterns require a structural graft to reduce the tuberosities and head component. Although described more than 30 years ago as a treatment for nonunions with an intramedullary “peg” of iliac crest graft,10 the graft most commonly reported today is allograft fibula.11-15 This technique consists of preparing the humeral shaft and often the fractured head segment with reaming to create a channel to receive the graft. Even with use of a small fibula, it is often time-consuming to use a saw, rasp, or burr to size the fibular segment to fit the medullary canal of the humerus. Once in place, the graft provides a strut on which the head fragment can be reduced and around which the tuberosities can be reduced. Although this technique is successful clinically and is biomechanically superior to plate-only constructs,16,17 concerns remain.
One such concern is keeping this graft in routine supply at most hospitals. Supply and pricing from vendors can differ significantly between hospitals, and a surgeon may need to request grafts in advance, which makes their use nonviable in a trauma case. Certain grafts are often kept in routine supply based on their overall utilization. At our institution, allograft femoral heads meet this criterion and are routinely stocked.
Of more importance are the ramifications of these procedures for future revision surgeries. The need for arthroplasty revision is common after ORIF of a proximal humerus fracture.18
Arthroplasty revision is an already challenging procedure that becomes more complex with the need to remove 6 to 8 cm of ingrown endosteal bone from a shell of outer osteoporotic cortical bone. Our experience with these complex revisions provided the impetus to search for an alternate graft type that still provides a strut for reducing the head and tuberosities but limits the amount of endosteal bone that would need to be removed in arthroplasty revision in order to place a stemmed component into the humeral canal.
Some currently available arthroplasty fracture systems modify the previous anatomy of the stem to provide a more anatomical platform to reduce the tuberosities to a broader metaphyseal construct that incorporates bone grafting to assist with healing.
Because of these concerns and factors, we adapted our technique to create an individual-specific pedestal with allograft femoral head that can be anatomically matched to each patient. This provides a strut to reduce the head and tuberosity fragments but still limits the amount of allograft bone needed to seat into the existing canal. The geometry of the allograft can also be customized to the fracture, with most 3- and 4-part fractures needing a trapezoidal strut that resembles the metaphyseal portion of a fracture-specific shoulder arthroplasty implant.
We have used this technique for comminuted 3- and 4-part fractures of the proximal humerus in 14 cases with at least 2-year follow-up and in several more cases that have not reached 2-year follow-up. All cases have gone on to radiographic union; none have had to be revised either with revision ORIF or to an arthroplasty. Formal measurements of final postoperative range of motion have not been tabulated in all cases, as some cases have been lost to follow-up after radiographic union was achieved. Medium- and long-term results are not yet available, but no short-term complications have been noted.
Disadvantages of this technique are that, while an individualized graft is created, proper shaping still takes time, and a moderate amount of the femoral head is not used. However, we have found that, if a graft is inadvertently undersized, there is still ample femoral head remaining to create another sized graft. Other disadvantages are the added cost and the (rare) risk of disease transmission, which come with use of any allograft, but the technique is used instead of another type of allograft, so these disadvantages are largely equivalent. At our hospital, differences in cost and availability between femoral head or fibular allografts are negligible.
This procedure, which is easily performed in a short amount of time, allows a stable base of bone graft to be used as an aid in the anatomical reduction of proximal humerus fractures, without the need for reaming and preparation of the medullary canal and without further increasing the difficulty associated with a future revision procedure.
Proximal humerus fractures are exceedingly common and account for almost 5% of all fractures. As osteoporosis is a risk factor for these fractures, their incidence rises with patient age.1
In 1970, Neer2 described these type of fractures and classified them as having 2, 3, or 4 parts based on the amount of angulation and displacement of the humeral head and the greater and lesser tuberosities with respect to the shaft.
Three- and 4-part proximal humerus fractures can be treated either nonoperatively, or surgically with closed reduction and percutaneous fixation, intramedullary fixation, open reduction and internal fixation (ORIF), or arthroplasty. There remains controversy over the best treatment, but a key component of any surgical treatment is anatomical reduction, stable fixation, and then healing of the tuberosities. A current common form of treatment is augmentation with an allograft fibula placed in the medullary canal. Although not formally reported, anecdotal evidence demonstrates that revision to arthroplasty is very difficult in the setting of an ingrown graft in the medullary canal of the humerus.
In this article, we present a novel technique of using allograft femoral head to reconstruct the metaphysis in ORIF of comminuted proximal humerus fractures.
Technique
Presented in Figure 1 are preoperative images of a representative displaced 4-part proximal humerus fracture treated surgically using the technique described here. General anesthesia is used. After intubation on the operating table, the patient is placed in the beach-chair position with about 75° of hip flexion. All bony prominences are padded, and the head and trunk are well secured. A pneumatic arm positioner is used to alleviate the need for an assistant to manipulate the arm. An image intensifier is used before preparing to verify that appropriate images of the proximal humerus can be obtained. Once adequate images are confirmed, the floor can be marked at the position of the fluoroscopic unit’s wheels to allow easy reproduction of images once the arm is prepared and draped. The intensifier is then removed from the field, the shoulder is prepared and draped in usual fashion, and prophylactic antibiotics are administered.
A deltopectoral incision is used, and sharp dissection is made through the subcutaneous tissue to raise full-thickness subcutaneous flaps on each side. The deltopectoral interval is sharply dissected while protecting the cephalic vein. Subdeltoid adhesions are then released. Palpation of the axillary nerve in the quadrilateral space to identify its location is helpful to avoid injury during the procedure.
The fracture is then identified, and No. 5 permanent suture is placed through the posterior and superior rotator cuff and through the subscapularis insertion (Figure 2). The tuberosities are freed from the humeral head sharply. A blunt elevator is then used to gently elevate the humeral head upward, with care taken to avoid comminuting the metaphyseal bone while levering. Reduction is achieved by manipulating the sutures and levering the head with the elevator while placing the arm in extension and posterior translation. Fluoroscopic images are used to verify correct anatomical alignment. Generally, the metaphysis demonstrates comminution and impaction, with poor bone quality necessitating use of bone graft.
A frozen allograft femoral head is then obtained and split into 2 equal pieces using a saw (Figures 3–5). One piece is fashioned with a saw and a burr into a trapezoid such that the proximal portion is wider, and the distal, tapered portion is sized to fit the canal. The broad, proximal portion of the graft will serve as a pedestal to reduce the head to the shaft. Measuring the internal diameter of the humeral canal can be useful in estimating the necessary dimensions of the distal portion of the allograft. The graft often needs several small adjustments that necessitate attempting to place it in the intramedullary canal and then trimming as necessary to ensure proper fit distally within the shaft. For this reason, it is beneficial to perform the graft preparation near the surgical field. Once completed, the distal portion is then impacted into the humeral canal (Figure 6). Because of this impaction, there is no possibility for subsidence or pistoning of the graft within the canal, which can occur with a fibular graft. The humeral head is reduced onto the shaft with the already placed sutures; this is achieved by abducting the shoulder. The image intensifier is then used to confirm appropriate alignment and positioning of the fragments, making sure that both neck–shaft angle and medial calcar alignment have been restored (Figures 7, 8).
An appropriately sized proximal humerus plate is then selected based on the location of the fracture line. We have used standard lateral proximal humerus locking plates as well as laterality-specific anterolateral proximal humerus plates and found that both are suitable for incorporation of the screws through the graft and into the head. The plate is positioned on the humerus, and a guide pin is placed by hand through the proximal-most hole so that the appropriate height of the plate can be verified on fluoroscopy. The first screw is then a nonlocking bicortical screw placed through the oval hole in the shaft of the plate to allow further fine manipulation of the plate more proximally or distally as needed. The final height is confirmed, and the screw is firmly tightened (Figure 9). The locking-screw guide is fixed to the proximal portion of the plate, and 2 locking screws are then placed into the head. The arm is then rotated to an anteroposterior view by placing the arm in external rotation and neutral flexion and is then abducted and internally rotated to recreate a lateral view to perform final verification of the position of the plate on orthogonal images. If the surgeon is satisfied with the position of the plate, another nonlocking screw is placed distally, and then the proximal holes are used to place locking screws as needed. If the surgeon is not satisfied, the 2 proximal screws can be removed and the plate repositioned.
After each screw is placed, fluoroscopy is used to ensure there has been no breach of the articular surface. The number of proximal screws placed depends on fracture configuration and surgeon preference.
The sutures through the rotator cuff are then fixed to the plate, securing the tuberosities. Final intraoperative radiographs are used to confirm reduction, alignment, and final position of hardware (Figure 10). After copious irrigation, a surgical drain is placed as needed, and the wound is closed in layered fashion. Three years after surgery, follow-up examination revealed no radiographic change in alignment, no necrosis, and no varus collapse (Figure 11), and the patient was pain-free during activities.
Discussion
Surgical treatment of comminuted proximal humerus fractures usually consists of some type of plate fixation with screw fixation of the shaft, screws or smooth pegs to support the chondral surfaces, and screw fixation or suture cerclage of the tuberosities.
Fixed-angle locking-plate-and-screw constructs increased the biomechanical stability and pullout strength of proximal humerus plates.3,4 Nevertheless, avascular necrosis, malunion, and nonunion are still known complications of proximal humerus fractures, especially those with comminution, with up to 14% of patients still experiencing loss of fixation.5
For this reason, several authors have proposed using allograft bone and/or augmentation with calcium-containing cement to supplement fixation and provide an endosteal form of support for the head and tuberosities to decrease the risk for varus collapse. Osteobiologics (eg, calcium phosphate or sulfate cement) have been shown to decrease the risk for loss of reduction of proximal humerus fractures and decrease the risk for intra-articular screw penetration.6,7 Many calcium phosphate cements are commercially available. Cost and availability are 2 reasons that these supplements are not more widely used. Cancellous chips have also been used to aid in the reduction of proximal humerus fractures.8 No randomized study has been conducted to show a clinical advantage of this technique, though retrospective studies have shown that it is not as advantageous as using calcium phosphate cement with respect to loss of reduction or screw penetration.6 Certainly, cancellous chips are easily available in most hospitals and are less expensive than some alternatives. A recent review of these techniques in osteoporotic proximal humerus fractures found no clear indication for using one of these supplements over another.9
However, some fracture patterns require a structural graft to reduce the tuberosities and head component. Although described more than 30 years ago as a treatment for nonunions with an intramedullary “peg” of iliac crest graft,10 the graft most commonly reported today is allograft fibula.11-15 This technique consists of preparing the humeral shaft and often the fractured head segment with reaming to create a channel to receive the graft. Even with use of a small fibula, it is often time-consuming to use a saw, rasp, or burr to size the fibular segment to fit the medullary canal of the humerus. Once in place, the graft provides a strut on which the head fragment can be reduced and around which the tuberosities can be reduced. Although this technique is successful clinically and is biomechanically superior to plate-only constructs,16,17 concerns remain.
One such concern is keeping this graft in routine supply at most hospitals. Supply and pricing from vendors can differ significantly between hospitals, and a surgeon may need to request grafts in advance, which makes their use nonviable in a trauma case. Certain grafts are often kept in routine supply based on their overall utilization. At our institution, allograft femoral heads meet this criterion and are routinely stocked.
Of more importance are the ramifications of these procedures for future revision surgeries. The need for arthroplasty revision is common after ORIF of a proximal humerus fracture.18
Arthroplasty revision is an already challenging procedure that becomes more complex with the need to remove 6 to 8 cm of ingrown endosteal bone from a shell of outer osteoporotic cortical bone. Our experience with these complex revisions provided the impetus to search for an alternate graft type that still provides a strut for reducing the head and tuberosities but limits the amount of endosteal bone that would need to be removed in arthroplasty revision in order to place a stemmed component into the humeral canal.
Some currently available arthroplasty fracture systems modify the previous anatomy of the stem to provide a more anatomical platform to reduce the tuberosities to a broader metaphyseal construct that incorporates bone grafting to assist with healing.
Because of these concerns and factors, we adapted our technique to create an individual-specific pedestal with allograft femoral head that can be anatomically matched to each patient. This provides a strut to reduce the head and tuberosity fragments but still limits the amount of allograft bone needed to seat into the existing canal. The geometry of the allograft can also be customized to the fracture, with most 3- and 4-part fractures needing a trapezoidal strut that resembles the metaphyseal portion of a fracture-specific shoulder arthroplasty implant.
We have used this technique for comminuted 3- and 4-part fractures of the proximal humerus in 14 cases with at least 2-year follow-up and in several more cases that have not reached 2-year follow-up. All cases have gone on to radiographic union; none have had to be revised either with revision ORIF or to an arthroplasty. Formal measurements of final postoperative range of motion have not been tabulated in all cases, as some cases have been lost to follow-up after radiographic union was achieved. Medium- and long-term results are not yet available, but no short-term complications have been noted.
Disadvantages of this technique are that, while an individualized graft is created, proper shaping still takes time, and a moderate amount of the femoral head is not used. However, we have found that, if a graft is inadvertently undersized, there is still ample femoral head remaining to create another sized graft. Other disadvantages are the added cost and the (rare) risk of disease transmission, which come with use of any allograft, but the technique is used instead of another type of allograft, so these disadvantages are largely equivalent. At our hospital, differences in cost and availability between femoral head or fibular allografts are negligible.
This procedure, which is easily performed in a short amount of time, allows a stable base of bone graft to be used as an aid in the anatomical reduction of proximal humerus fractures, without the need for reaming and preparation of the medullary canal and without further increasing the difficulty associated with a future revision procedure.
1. Barrett JA, Baron JA, Karagas MR, Beach ML. Fracture risk in the U.S. Medicare population. J Clin Epidemiol. 1999;52(3):243-249.
2. Neer CS 2nd. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
3. Liew AS, Johnson JA, Patterson SD, King GJ, Chess DG. Effect of screw placement on fixation in the humeral head. J Shoulder Elbow Surg. 2000;9(5):423-426.
4. Weinstein DM, Bratton DR, Ciccone WJ 2nd, Elias JJ. Locking plates improve torsional resistance in the stabilization of three-part proximal humeral fractures. J Shoulder Elbow Surg. 2006;15(2):239-243.
5. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
6. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction-internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012;21(6):741-748.
7. Gradl G, Knobe M, Stoffel M, Prescher A, Dirrichs T, Pape HC. Biomechanical evaluation of locking plate fixation of proximal humeral fractures augmented with calcium phosphate cement. J Orthop Trauma. 2013;27(7):399-404.
8. Ong CC, Kwon YW, Walsh M, Davidovitch R, Zuckerman JD, Egol KA. Outcomes of open reduction and internal fixation of proximal humerus fractures managed with locking plates. Am J Orthop. 2012;41(9):407-412.
9. Namdari S, Voleti PB, Mehta S. Evaluation of the osteoporotic proximal humeral fracture and strategies for structural augmentation during surgical treatment. J Shoulder Elbow Surg. 2012;21(12):1787-1795.
10. Scheck M. Surgical treatment of nonunions of the surgical neck of the humerus. Clin Orthop Relat Res. 1982;(167):255-259.
11. Hettrich CM, Neviaser A, Beamer BS, Paul O, Helfet DL, Lorich DG. Locked plating of the proximal humerus using an endosteal implant. J Orthop Trauma. 2012;26(4):212-215.
12. Neviaser AS, Hettrich CM, Beamer BS, Dines JS, Lorich DG. Endosteal strut augment reduces complications associated with proximal humeral locking plates. Clin Orthop Relat Res. 2011;469(12):3300-3306.
13. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J Orthop Trauma. 2008;22(3):195-200.
14. Matassi F, Angeloni R, Carulli C, et al. Locking plate and fibular allograft augmentation in unstable fractures of proximal humerus. Injury. 2012;43(11):1939-1942.
15. Little MT, Berkes MB, Schottel PC, et al. The impact of preoperative coronal plane deformity on proximal humerus fixation with endosteal augmentation. J Orthop Trauma. 2014;28(6):338-347.
16. Mathison C, Chaudhary R, Beaupre L, Reynolds M, Adeeb S, Bouliane M. Biomechanical analysis of proximal humeral fixation using locking plate fixation with an intramedullary fibular allograft. Clin Biomech. 2010;25(7):642-646.
17. Chow RM, Begum F, Beaupre LA, Carey JP, Adeeb S, Bouliane MJ. Proximal humeral fracture fixation: locking plate construct +/- intramedullary fibular allograft. J Shoulder Elbow Surg. 2012;21(7):894-901.
18. Jost B, Spross C, Grehn H, Gerber C. Locking plate fixation of fractures of the proximal humerus: analysis of complications, revision strategies and outcome. J Shoulder Elbow Surg. 2013;22(4):542-549.
1. Barrett JA, Baron JA, Karagas MR, Beach ML. Fracture risk in the U.S. Medicare population. J Clin Epidemiol. 1999;52(3):243-249.
2. Neer CS 2nd. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
3. Liew AS, Johnson JA, Patterson SD, King GJ, Chess DG. Effect of screw placement on fixation in the humeral head. J Shoulder Elbow Surg. 2000;9(5):423-426.
4. Weinstein DM, Bratton DR, Ciccone WJ 2nd, Elias JJ. Locking plates improve torsional resistance in the stabilization of three-part proximal humeral fractures. J Shoulder Elbow Surg. 2006;15(2):239-243.
5. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
6. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction-internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012;21(6):741-748.
7. Gradl G, Knobe M, Stoffel M, Prescher A, Dirrichs T, Pape HC. Biomechanical evaluation of locking plate fixation of proximal humeral fractures augmented with calcium phosphate cement. J Orthop Trauma. 2013;27(7):399-404.
8. Ong CC, Kwon YW, Walsh M, Davidovitch R, Zuckerman JD, Egol KA. Outcomes of open reduction and internal fixation of proximal humerus fractures managed with locking plates. Am J Orthop. 2012;41(9):407-412.
9. Namdari S, Voleti PB, Mehta S. Evaluation of the osteoporotic proximal humeral fracture and strategies for structural augmentation during surgical treatment. J Shoulder Elbow Surg. 2012;21(12):1787-1795.
10. Scheck M. Surgical treatment of nonunions of the surgical neck of the humerus. Clin Orthop Relat Res. 1982;(167):255-259.
11. Hettrich CM, Neviaser A, Beamer BS, Paul O, Helfet DL, Lorich DG. Locked plating of the proximal humerus using an endosteal implant. J Orthop Trauma. 2012;26(4):212-215.
12. Neviaser AS, Hettrich CM, Beamer BS, Dines JS, Lorich DG. Endosteal strut augment reduces complications associated with proximal humeral locking plates. Clin Orthop Relat Res. 2011;469(12):3300-3306.
13. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J Orthop Trauma. 2008;22(3):195-200.
14. Matassi F, Angeloni R, Carulli C, et al. Locking plate and fibular allograft augmentation in unstable fractures of proximal humerus. Injury. 2012;43(11):1939-1942.
15. Little MT, Berkes MB, Schottel PC, et al. The impact of preoperative coronal plane deformity on proximal humerus fixation with endosteal augmentation. J Orthop Trauma. 2014;28(6):338-347.
16. Mathison C, Chaudhary R, Beaupre L, Reynolds M, Adeeb S, Bouliane M. Biomechanical analysis of proximal humeral fixation using locking plate fixation with an intramedullary fibular allograft. Clin Biomech. 2010;25(7):642-646.
17. Chow RM, Begum F, Beaupre LA, Carey JP, Adeeb S, Bouliane MJ. Proximal humeral fracture fixation: locking plate construct +/- intramedullary fibular allograft. J Shoulder Elbow Surg. 2012;21(7):894-901.
18. Jost B, Spross C, Grehn H, Gerber C. Locking plate fixation of fractures of the proximal humerus: analysis of complications, revision strategies and outcome. J Shoulder Elbow Surg. 2013;22(4):542-549.
Treatment of Acetabular Fractures in Adolescents
In children, pelvic fractures are uncommon, with an incidence ranging from 1% to 4.6% of all pediatric fractures,1-4 and acetabular fractures make up only 0.8% to 15% of pelvic fractures.1,3,5,6 Acetabular fractures are so uncommon in children partly because of the cartilaginous nature of the immature acetabulum. The increased cartilage volume relative to adults provides greater capacity for energy absorption, resulting in greater elastic and plastic deformation before fracture occurrence. More force is therefore required to cause a fracture, and associated visceral injuries, head injuries, and long-bone fractures are common.3,7,8
The impact of acetabular fractures on adolescents warrants special attention because any resulting disability will affect them during their most productive years. Both avascular necrosis (AVN) and degenerative arthritis are particularly devastating complications in this age group. Complications such as premature physeal closure9-15 are unique to adolescents, and there is little information available on how injury in older children affects growth in this area.
There have been very few studies of the outcomes of these injuries in children. Mostly, there have been case reports and small series primarily dealing with nonoperative management of acetabular fractures in adolescents.3,10,11,16-20 By contrast, operative treatment of acetabular fractures in adults has been well described, and outcomes widely reported. As a result, much of our knowledge about managing these injuries is extrapolated from the adult literature. Although treatment of acetabular fractures in adults has evolved substantially, treatment of these injuries in adolescents remains primarily nonoperative. We conducted a study to evaluate outcomes of treatment of adolescent acetabular fractures.
Patients and Methods
After obtaining institutional review board approval for this study, we retrospectively reviewed the cases of all adolescent patients admitted with a diagnosis of acetabular fracture to 2 academic institutions between 1991 and 2003. Thirty-eight patients (28 males, 10 females) were identified. Mean age at time of injury was 15 years (range, 11-18 years). Mean follow-up was 3.2 years (range, 5-180 months).
Data on fracture types, treatment methods, associated injuries, complications, union rates, pain, and return to normal activities were collected. Acetabular fractures were classified according to the system of Letournel and Judet.21 There were 20 elementary and 18 associated fractures.
Of the 38 patients, 30 sustained high-energy trauma in motor vehicle accidents (25) or in falls from significant heights (5). The other 8 patients injured themselves playing sports (4 had severe traumatic brain injury, 2 had labial wounds, and 2 had injuries involving the abdominal viscera). Twelve patients had associated pelvic ring injuries, 18 had femoral head dislocations, 2 had femoral head fractures, and 13 had evidence of impaction injury to the femoral head articular cartilage. Twelve patients had marginal impaction of the acetabular wall. Fifteen patients had open triradiate physes at time of injury (Table 1).
Thirty-seven of the 38 patients were treated with open reduction and internal fixation (ORIF) by an experienced orthopedic trauma surgeon; 1 patient with a stable posterior wall fracture was treated nonoperatively. Surgical indications were articular displacement of more than 1 mm, hip joint instability, irreducible hip dislocation, and intra-articular fracture fragments. In the 37 surgically treated cases, the approaches used were Kocher-Langenbeck (22), ilioinguinal (8), combined Kocher-Langenbeck/ilioinguinal (5), and triradiate (2).
Immediate postoperative radiographs were evaluated by 3 orthopedic surgeons blinded to the patients’ clinical outcomes. Displacement was evaluated on anteroposterior (AP) and Judet views of the pelvis, as described by Matta,22 and reductions were classified as anatomical (0-1 mm of displacement), imperfect (>1 to 3 mm), poor (>3 mm), or surgical secondary congruence (Table 2).
Results
Thirty-seven patients underwent acetabular fracture ORIF. Immediate postoperative radiographs showed 30 anatomical reductions and 7 imperfect reductions. One patient had surgical secondary congruence and developed AVN of the hip. We could not identify an association between the quality of the reduction and the outcome with respect to pain or return to activity. However, no patient had a poor reduction. An illustrative case is presented in Figures 1 to 4.
All acetabular fractures united within 4.5 months (range, 3.0-8.0 months) after the index procedure. Early postoperative complications included 3 cases of meralgia paresthetica and 13 cases of abductor weakness. Meralgia paresthetica resolved spontaneously in all 3 patients. Of the 13 patients with abductor weakness, 11 improved with physical therapy, 1 was limited by the head injury, and 1 subsequently underwent hip fusion. One patient had a deep vein thrombosis (DVT) that was identified before surgery and managed with warfarin.
Other complications included 1 case of deep infection of the surgical wound. This infection presented 4 months after surgery and was treated with débridement, hardware removal, and a 3-month course of antibiotics. Two patients who sustained hip dislocations at time of injury developed AVN of the femoral head. Both developed osteoarthritis, and 1 underwent hip fusion. Eight patients developed heterotopic ossification on the side of the acetabular fracture; 4 of them underwent surgical excision. Four patients required a separate operation for hardware removal. Four patients with triradiate cartilage involvement went on to premature closure. No patient had any leg-length discrepancy or dysplasia at time of follow-up.
Thirty-four of the 38 patients returned to their regular activities. For these patients, mean time to return to full activity was 7.0 months (range, 3-30 months); there was no difference in mean time to return to full activity between skeletally mature and skeletally immature patients (6.6 vs 7.4 months; P = .57). Of the other 4 patients, 1 had permanent cognitive and physical disability with an ataxic gait as a result of a traumatic brain injury, 2 were limited by AVN (1 underwent hip fusion), and 1 was limited by an ipsilateral knee injury.
Of the 38 patients, 29 were pain-free; 6 had occasional, intermittent mild pain that did not limit their activities; and 3 had severe, activity-limiting pain. Of the 6 patients with mild pain, 2 had femoral impaction injuries, and 4 had marginal impaction injuries. Of the 3 patients with severe pain, 2 developed femoral head AVN, and 1 had multiple ipsilateral extremity injuries involving the femur, knee, and tibia.
Discussion
The traditional treatment for acetabular fractures in children has been nonoperative,8,10 and there are few specific treatment guidelines.13 Recent recommendations are nonoperative treatment for minimally displaced fractures (<1 mm) and acetabular fracture ORIF for fractures displaced more than 2 mm.11 No clear consensus exists on management for fractures displaced 1 to 2 mm. Few studies have investigated the outcomes of operative management of these fractures in the pediatric or adolescent population.
In our series of adolescent acetabular fractures, we examined unions, complications, and return to activity. Of 38 patients with acetabular fractures, 37 were treated with ORIF. Anatomical reduction was achieved in the majority of patients. Posterior wall fractures were by far the most common fracture type, which is consistent with previous reports.10,11 All acetabular fractures united, and most patients were pain-free at latest follow-up. There was a low incidence of major complications in our patient population. One major complication was a DVT in a 14-year-old boy who was in a motor vehicle accident and sustained a T-type fracture of the right acetabulum with contralateral femoral shaft and ankle fractures. The DVT was in the right internal iliac and common femoral veins and was diagnosed on magnetic resonance venography. The patient was treated with warfarin for 3 months without incident.
Two patients developed AVN of the femoral head. One of these patients was an 11-year-old girl who was in a motor vehicle accident and sustained a T-type fracture with marginal impaction of the posterior wall, posterior hip dislocation, and a pelvic ring injury. She was treated with ORIF through combined Kocher-Langenbeck/ilioinguinal approaches. By 4 months after surgery, the acetabular fracture was united. Nine months after surgery, she still had pain (activity-limiting) and a 35° flexion contracture of the hip, and she was ambulating with a cane. The diagnosis was AVN of the hip. The patient underwent hip fusion 1 year after surgery.
The second patient with femoral head AVN was a 12-year-old boy who fell while skiing and sustained a fracture of the posterior wall and a hip dislocation with impaction of the femoral head. Initial treatment at an outside institution consisted of open reduction of the hip and excision of a “loose body” from the joint. Eight weeks after surgery, the patient continued to have pain and was referred to our institution. A second operation was performed. Findings included a defect involving 40% of the posterior wall, and signs that the posterior wall had been excised during the initial operation. The patient eventually developed AVN of the hip. This patient was also diagnosed with a deep wound infection 4 months after surgery. He presented with pain and a fluid collection around the hip. The infection was not confirmed through fluid culture, and, as he eventually developed AVN of the hip, his symptoms may have been the result of chondrolysis or AVN rather than infection.
There were no cases of nonunion or malunion, leg-length discrepancy, or permanent sciatic nerve palsy. Although there were a few cases of premature closure of the triradiate cartilage, no acetabular dysplasia was seen at latest follow-up, likely because of the relative maturity of our pediatric group (age range, 11-18 years). Age at time of injury is thought to be the most important factor influencing growth and development of the acetabulum.9,13 In addition, previous studies have demonstrated a tendency toward acetabular fractures in patients with mature triradiate cartilage—versus pelvic ring injuries in patients with immature triradiate cartilage.8,11 This may also account for the older age of our study group.
Minor complications (eg, meralgia paresthetica) resolved spontaneously. The most common complications were abductor weakness and heterotopic ossification. In only 4 cases was a secondary procedure for excision of the heterotopic bone required. Abductor weakness, more commonly associated with a Kocher-Langenbeck approach to the hip, resolved with therapy in almost all cases. Only 4 of our patients required removal of hardware from the acetabulum.
Although the majority of acetabular fractures resulted from high-energy trauma, 8 cases were sports-related. Six of these involved posterior wall fractures, suggesting the injury resulted from a fall on flexed knee and hip. This was not known to be a common mechanism of injury in this age group.3,7 An additional concern was how to size the posterior wall fragment when not ossified. At one center, preoperative magnetic resonance imaging (MRI) was effectively used to size the osteochondral posterior wall fragment as standard protocol for patients with posterior wall fractures in this age group—resulting in better decisions regarding the need for ORIF. At the other institution, preoperative MRI was not performed routinely for this subset of patients.
Thirty-four of our 38 patients returned to their normal activities. Of the other 4 patients, 1 was permanently disabled secondary to traumatic brain injury, 1 had other ipsilateral extremity injuries that limited his mobility, and 2 developed AVN of the femoral head. Both patients with AVN had hip dislocations. Four of the 6 patients who were symptomatic during activity sustained impaction injuries of the femoral head or posterior wall. This suggests that poorer outcomes may be associated with dislocation or with articular injuries—similar to what has been reported in the adult literature.
This study had several limitations. First, it was a retrospective case series, so there was no control group for comparison. Second, the relatively short follow-up did not allow evaluation of the incidence of degenerative arthritis secondary to articular injury, the symptoms of which may develop 1 to 2 decades after injury.13 This phenomenon was well described by Letournel and Judet21 in the adult population, and there is no reason to presume the adolescent population is any different. Third, our sample was small and unlikely to represent a uniform sampling of the general pediatric population. Fourth, it was not possible to draw detailed conclusions about the outcome of ORIF for a particular type of acetabular fracture. Fifth, we did not see as many of the associated visceral injuries that are so prevalent in the literature. This may reflect improvement in safety specifications for automobiles, or our group may not have had the most severe or high-energy injuries. Here our population sample may have skewed our results, leading to better than expected outcomes.
One last study limitation, a major one, was the age of our population, 11 to 18 years, which makes it difficult to extrapolate results to the entire pediatric population. On one hand, a more immature skeleton has a higher chance of remodeling and is more forgiving of deformities and small amounts of displacement. On the other hand, injury and premature triradiate cartilage fusion in a younger patient can lead to significant deformity and acetabular dysplasia.9 Whether ORIF of these fractures would alter the outcome of an injury to the triradiate cartilage is yet to be determined.
Conclusion
In agreement with earlier studies,10,11,15,18 the good outcomes in our series correlated with congruence of reduction. Outcome predictors such as dislocation, femoral head injury, and marginal impaction are similar to those described in the adult literature. Although our study did not have a nonoperative group for comparison, the favorable outcomes of ORIF of acetabular fractures suggest that a more aggressive approach to treatment should be considered. Given the added benefits of early, pain-free mobilization, we think that only stable, undisplaced fractures (<1 mm) should be managed nonoperatively. In the adolescent population, we recommend ORIF for optimal management of unstable acetabular fractures, fractures with any hip subluxation, and fractures displaced more than 1 mm.
1. Canale ST, Beaty JH. Fractures of the pelvis. In: Beaty JH, Kassler JR, eds. Rockwood and Wilkin’s Fractures in Children. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:883-991.
2. Demetriades D, Karaiskakis M, Velmahos GC, Alo K, Murray J, Chan L. Pelvic fractures in pediatric and adult trauma patients: are they different injuries? J Trauma. 2003;54(6):1146-1151.
3. Grisoni N, Connor S, Marsh E, Thompson GH, Cooperman DR, Blakemore LC. Pelvic fractures in a pediatric level I trauma center. J Orthop Trauma. 2002;16(7):458-463.
4. Ismail N, Bellemare JF, Mollitt DL, Di Scala C, Koeppel B, Tepas JJ. Death from pelvic fracture: children are different. J Pediatr Surg. 1996;31(1):82-85.
5. Schlickwei W, Keck T. Pelvic and acetabular fractures in childhood. Injury. 2005;36(suppl 1):A57-A63.
6. Swiontkowski MF. Fractures and dislocations about the hip and pelvis. In: Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. Philadelphia, PA: Saunders; 2003:371-406.
7. Silber JS, Flynn JM, Koffler KM, Dormans JP, Drummond DS. Analysis of the cause, classification, and associated injuries of 166 consecutive pediatric pelvic fractures. J Pediatr Orthop. 2001;21(4):446-450.
8. Silber JS, Flynn JM. Changing patterns of pediatric pelvic fractures with skeletal maturation: implications for classification and management. J Pediatr Orthop. 2002;22(1):22-26.
9. Bucholz RW, Ezaki M, Ogden JA. Injury to the acetabular triradiate physeal cartilage. J Bone Joint Surg Am. 1982;64(4):600-609.
10. Heeg M, Klasen HJ, Visser JD. Acetabular fractures in children and adolescents. J Bone Joint Surg Br. 1989;71(3):418-421.
11. Heeg M, de Ridder VA, Tornetta P, de Lange S, Klasen HJ. Acetabular fractures in children and adolescents. Clin Orthop Relat Res. 2000;(376):80-86.
12. Heeg M, Visser JD, Oostvogel HJ. Injuries of the acetabular triradiate cartilage and sacroiliac joint. J Bone Joint Surg Br. 1988;70(1):34-37.
13. Liporace FA, Ong B, Mohaideen A, Ong A, Koval KJ. Development and injury of the triradiate cartilage with its effects on acetabular development: review of the literature. J Trauma. 2003;54(6):1245-1249.
14. Rodrigues KF. Injury of the acetabular epiphysis. Injury. 1973;4(3):258-260.
15. Trousdale RT, Ganz R. Posttraumatic acetabular dysplasia. Clin Orthop Relat Res. 1994;(305):124-132.
16. Brooks E, Rosman M. Central fracture-dislocation of the hip in a child. J Trauma. 1988;28(11):1590-1592.
17. Habacker TA, Heinrich SD, Dehne R. Fracture of the superior pelvic quadrant in a child. J Pediatr Orthop. 1995;15(1):69-72.
18. Karunakar MA, Goulet JA, Mueller KL, Bedi A, Le TT. Operative treatment of unstable pediatric pelvis and acetabular fractures. J Pediatr Orthop. 2005;25(1):34-38.
19. Rieger H, Brug E. Fractures of the pelvis in children. Clin Orthop Relat Res. 1997;(336);226-239.
20. Torode I, Zieg D. Pelvic fractures in children. J Pediatr Orthop. 1985;5(1):76-84.
21. Letournel E, Judet R. Fractures of the Acetabulum. 2nd ed. New York, NY: Springer-Verlag; 1993.
22. Matta JM. Fractures of the acetabulum: accuracy of reduction and clinical results in patients managed operatively within three weeks of the injury. J Bone Joint Surg Am. 1996;78(11):1632-1645.
In children, pelvic fractures are uncommon, with an incidence ranging from 1% to 4.6% of all pediatric fractures,1-4 and acetabular fractures make up only 0.8% to 15% of pelvic fractures.1,3,5,6 Acetabular fractures are so uncommon in children partly because of the cartilaginous nature of the immature acetabulum. The increased cartilage volume relative to adults provides greater capacity for energy absorption, resulting in greater elastic and plastic deformation before fracture occurrence. More force is therefore required to cause a fracture, and associated visceral injuries, head injuries, and long-bone fractures are common.3,7,8
The impact of acetabular fractures on adolescents warrants special attention because any resulting disability will affect them during their most productive years. Both avascular necrosis (AVN) and degenerative arthritis are particularly devastating complications in this age group. Complications such as premature physeal closure9-15 are unique to adolescents, and there is little information available on how injury in older children affects growth in this area.
There have been very few studies of the outcomes of these injuries in children. Mostly, there have been case reports and small series primarily dealing with nonoperative management of acetabular fractures in adolescents.3,10,11,16-20 By contrast, operative treatment of acetabular fractures in adults has been well described, and outcomes widely reported. As a result, much of our knowledge about managing these injuries is extrapolated from the adult literature. Although treatment of acetabular fractures in adults has evolved substantially, treatment of these injuries in adolescents remains primarily nonoperative. We conducted a study to evaluate outcomes of treatment of adolescent acetabular fractures.
Patients and Methods
After obtaining institutional review board approval for this study, we retrospectively reviewed the cases of all adolescent patients admitted with a diagnosis of acetabular fracture to 2 academic institutions between 1991 and 2003. Thirty-eight patients (28 males, 10 females) were identified. Mean age at time of injury was 15 years (range, 11-18 years). Mean follow-up was 3.2 years (range, 5-180 months).
Data on fracture types, treatment methods, associated injuries, complications, union rates, pain, and return to normal activities were collected. Acetabular fractures were classified according to the system of Letournel and Judet.21 There were 20 elementary and 18 associated fractures.
Of the 38 patients, 30 sustained high-energy trauma in motor vehicle accidents (25) or in falls from significant heights (5). The other 8 patients injured themselves playing sports (4 had severe traumatic brain injury, 2 had labial wounds, and 2 had injuries involving the abdominal viscera). Twelve patients had associated pelvic ring injuries, 18 had femoral head dislocations, 2 had femoral head fractures, and 13 had evidence of impaction injury to the femoral head articular cartilage. Twelve patients had marginal impaction of the acetabular wall. Fifteen patients had open triradiate physes at time of injury (Table 1).
Thirty-seven of the 38 patients were treated with open reduction and internal fixation (ORIF) by an experienced orthopedic trauma surgeon; 1 patient with a stable posterior wall fracture was treated nonoperatively. Surgical indications were articular displacement of more than 1 mm, hip joint instability, irreducible hip dislocation, and intra-articular fracture fragments. In the 37 surgically treated cases, the approaches used were Kocher-Langenbeck (22), ilioinguinal (8), combined Kocher-Langenbeck/ilioinguinal (5), and triradiate (2).
Immediate postoperative radiographs were evaluated by 3 orthopedic surgeons blinded to the patients’ clinical outcomes. Displacement was evaluated on anteroposterior (AP) and Judet views of the pelvis, as described by Matta,22 and reductions were classified as anatomical (0-1 mm of displacement), imperfect (>1 to 3 mm), poor (>3 mm), or surgical secondary congruence (Table 2).
Results
Thirty-seven patients underwent acetabular fracture ORIF. Immediate postoperative radiographs showed 30 anatomical reductions and 7 imperfect reductions. One patient had surgical secondary congruence and developed AVN of the hip. We could not identify an association between the quality of the reduction and the outcome with respect to pain or return to activity. However, no patient had a poor reduction. An illustrative case is presented in Figures 1 to 4.
All acetabular fractures united within 4.5 months (range, 3.0-8.0 months) after the index procedure. Early postoperative complications included 3 cases of meralgia paresthetica and 13 cases of abductor weakness. Meralgia paresthetica resolved spontaneously in all 3 patients. Of the 13 patients with abductor weakness, 11 improved with physical therapy, 1 was limited by the head injury, and 1 subsequently underwent hip fusion. One patient had a deep vein thrombosis (DVT) that was identified before surgery and managed with warfarin.
Other complications included 1 case of deep infection of the surgical wound. This infection presented 4 months after surgery and was treated with débridement, hardware removal, and a 3-month course of antibiotics. Two patients who sustained hip dislocations at time of injury developed AVN of the femoral head. Both developed osteoarthritis, and 1 underwent hip fusion. Eight patients developed heterotopic ossification on the side of the acetabular fracture; 4 of them underwent surgical excision. Four patients required a separate operation for hardware removal. Four patients with triradiate cartilage involvement went on to premature closure. No patient had any leg-length discrepancy or dysplasia at time of follow-up.
Thirty-four of the 38 patients returned to their regular activities. For these patients, mean time to return to full activity was 7.0 months (range, 3-30 months); there was no difference in mean time to return to full activity between skeletally mature and skeletally immature patients (6.6 vs 7.4 months; P = .57). Of the other 4 patients, 1 had permanent cognitive and physical disability with an ataxic gait as a result of a traumatic brain injury, 2 were limited by AVN (1 underwent hip fusion), and 1 was limited by an ipsilateral knee injury.
Of the 38 patients, 29 were pain-free; 6 had occasional, intermittent mild pain that did not limit their activities; and 3 had severe, activity-limiting pain. Of the 6 patients with mild pain, 2 had femoral impaction injuries, and 4 had marginal impaction injuries. Of the 3 patients with severe pain, 2 developed femoral head AVN, and 1 had multiple ipsilateral extremity injuries involving the femur, knee, and tibia.
Discussion
The traditional treatment for acetabular fractures in children has been nonoperative,8,10 and there are few specific treatment guidelines.13 Recent recommendations are nonoperative treatment for minimally displaced fractures (<1 mm) and acetabular fracture ORIF for fractures displaced more than 2 mm.11 No clear consensus exists on management for fractures displaced 1 to 2 mm. Few studies have investigated the outcomes of operative management of these fractures in the pediatric or adolescent population.
In our series of adolescent acetabular fractures, we examined unions, complications, and return to activity. Of 38 patients with acetabular fractures, 37 were treated with ORIF. Anatomical reduction was achieved in the majority of patients. Posterior wall fractures were by far the most common fracture type, which is consistent with previous reports.10,11 All acetabular fractures united, and most patients were pain-free at latest follow-up. There was a low incidence of major complications in our patient population. One major complication was a DVT in a 14-year-old boy who was in a motor vehicle accident and sustained a T-type fracture of the right acetabulum with contralateral femoral shaft and ankle fractures. The DVT was in the right internal iliac and common femoral veins and was diagnosed on magnetic resonance venography. The patient was treated with warfarin for 3 months without incident.
Two patients developed AVN of the femoral head. One of these patients was an 11-year-old girl who was in a motor vehicle accident and sustained a T-type fracture with marginal impaction of the posterior wall, posterior hip dislocation, and a pelvic ring injury. She was treated with ORIF through combined Kocher-Langenbeck/ilioinguinal approaches. By 4 months after surgery, the acetabular fracture was united. Nine months after surgery, she still had pain (activity-limiting) and a 35° flexion contracture of the hip, and she was ambulating with a cane. The diagnosis was AVN of the hip. The patient underwent hip fusion 1 year after surgery.
The second patient with femoral head AVN was a 12-year-old boy who fell while skiing and sustained a fracture of the posterior wall and a hip dislocation with impaction of the femoral head. Initial treatment at an outside institution consisted of open reduction of the hip and excision of a “loose body” from the joint. Eight weeks after surgery, the patient continued to have pain and was referred to our institution. A second operation was performed. Findings included a defect involving 40% of the posterior wall, and signs that the posterior wall had been excised during the initial operation. The patient eventually developed AVN of the hip. This patient was also diagnosed with a deep wound infection 4 months after surgery. He presented with pain and a fluid collection around the hip. The infection was not confirmed through fluid culture, and, as he eventually developed AVN of the hip, his symptoms may have been the result of chondrolysis or AVN rather than infection.
There were no cases of nonunion or malunion, leg-length discrepancy, or permanent sciatic nerve palsy. Although there were a few cases of premature closure of the triradiate cartilage, no acetabular dysplasia was seen at latest follow-up, likely because of the relative maturity of our pediatric group (age range, 11-18 years). Age at time of injury is thought to be the most important factor influencing growth and development of the acetabulum.9,13 In addition, previous studies have demonstrated a tendency toward acetabular fractures in patients with mature triradiate cartilage—versus pelvic ring injuries in patients with immature triradiate cartilage.8,11 This may also account for the older age of our study group.
Minor complications (eg, meralgia paresthetica) resolved spontaneously. The most common complications were abductor weakness and heterotopic ossification. In only 4 cases was a secondary procedure for excision of the heterotopic bone required. Abductor weakness, more commonly associated with a Kocher-Langenbeck approach to the hip, resolved with therapy in almost all cases. Only 4 of our patients required removal of hardware from the acetabulum.
Although the majority of acetabular fractures resulted from high-energy trauma, 8 cases were sports-related. Six of these involved posterior wall fractures, suggesting the injury resulted from a fall on flexed knee and hip. This was not known to be a common mechanism of injury in this age group.3,7 An additional concern was how to size the posterior wall fragment when not ossified. At one center, preoperative magnetic resonance imaging (MRI) was effectively used to size the osteochondral posterior wall fragment as standard protocol for patients with posterior wall fractures in this age group—resulting in better decisions regarding the need for ORIF. At the other institution, preoperative MRI was not performed routinely for this subset of patients.
Thirty-four of our 38 patients returned to their normal activities. Of the other 4 patients, 1 was permanently disabled secondary to traumatic brain injury, 1 had other ipsilateral extremity injuries that limited his mobility, and 2 developed AVN of the femoral head. Both patients with AVN had hip dislocations. Four of the 6 patients who were symptomatic during activity sustained impaction injuries of the femoral head or posterior wall. This suggests that poorer outcomes may be associated with dislocation or with articular injuries—similar to what has been reported in the adult literature.
This study had several limitations. First, it was a retrospective case series, so there was no control group for comparison. Second, the relatively short follow-up did not allow evaluation of the incidence of degenerative arthritis secondary to articular injury, the symptoms of which may develop 1 to 2 decades after injury.13 This phenomenon was well described by Letournel and Judet21 in the adult population, and there is no reason to presume the adolescent population is any different. Third, our sample was small and unlikely to represent a uniform sampling of the general pediatric population. Fourth, it was not possible to draw detailed conclusions about the outcome of ORIF for a particular type of acetabular fracture. Fifth, we did not see as many of the associated visceral injuries that are so prevalent in the literature. This may reflect improvement in safety specifications for automobiles, or our group may not have had the most severe or high-energy injuries. Here our population sample may have skewed our results, leading to better than expected outcomes.
One last study limitation, a major one, was the age of our population, 11 to 18 years, which makes it difficult to extrapolate results to the entire pediatric population. On one hand, a more immature skeleton has a higher chance of remodeling and is more forgiving of deformities and small amounts of displacement. On the other hand, injury and premature triradiate cartilage fusion in a younger patient can lead to significant deformity and acetabular dysplasia.9 Whether ORIF of these fractures would alter the outcome of an injury to the triradiate cartilage is yet to be determined.
Conclusion
In agreement with earlier studies,10,11,15,18 the good outcomes in our series correlated with congruence of reduction. Outcome predictors such as dislocation, femoral head injury, and marginal impaction are similar to those described in the adult literature. Although our study did not have a nonoperative group for comparison, the favorable outcomes of ORIF of acetabular fractures suggest that a more aggressive approach to treatment should be considered. Given the added benefits of early, pain-free mobilization, we think that only stable, undisplaced fractures (<1 mm) should be managed nonoperatively. In the adolescent population, we recommend ORIF for optimal management of unstable acetabular fractures, fractures with any hip subluxation, and fractures displaced more than 1 mm.
In children, pelvic fractures are uncommon, with an incidence ranging from 1% to 4.6% of all pediatric fractures,1-4 and acetabular fractures make up only 0.8% to 15% of pelvic fractures.1,3,5,6 Acetabular fractures are so uncommon in children partly because of the cartilaginous nature of the immature acetabulum. The increased cartilage volume relative to adults provides greater capacity for energy absorption, resulting in greater elastic and plastic deformation before fracture occurrence. More force is therefore required to cause a fracture, and associated visceral injuries, head injuries, and long-bone fractures are common.3,7,8
The impact of acetabular fractures on adolescents warrants special attention because any resulting disability will affect them during their most productive years. Both avascular necrosis (AVN) and degenerative arthritis are particularly devastating complications in this age group. Complications such as premature physeal closure9-15 are unique to adolescents, and there is little information available on how injury in older children affects growth in this area.
There have been very few studies of the outcomes of these injuries in children. Mostly, there have been case reports and small series primarily dealing with nonoperative management of acetabular fractures in adolescents.3,10,11,16-20 By contrast, operative treatment of acetabular fractures in adults has been well described, and outcomes widely reported. As a result, much of our knowledge about managing these injuries is extrapolated from the adult literature. Although treatment of acetabular fractures in adults has evolved substantially, treatment of these injuries in adolescents remains primarily nonoperative. We conducted a study to evaluate outcomes of treatment of adolescent acetabular fractures.
Patients and Methods
After obtaining institutional review board approval for this study, we retrospectively reviewed the cases of all adolescent patients admitted with a diagnosis of acetabular fracture to 2 academic institutions between 1991 and 2003. Thirty-eight patients (28 males, 10 females) were identified. Mean age at time of injury was 15 years (range, 11-18 years). Mean follow-up was 3.2 years (range, 5-180 months).
Data on fracture types, treatment methods, associated injuries, complications, union rates, pain, and return to normal activities were collected. Acetabular fractures were classified according to the system of Letournel and Judet.21 There were 20 elementary and 18 associated fractures.
Of the 38 patients, 30 sustained high-energy trauma in motor vehicle accidents (25) or in falls from significant heights (5). The other 8 patients injured themselves playing sports (4 had severe traumatic brain injury, 2 had labial wounds, and 2 had injuries involving the abdominal viscera). Twelve patients had associated pelvic ring injuries, 18 had femoral head dislocations, 2 had femoral head fractures, and 13 had evidence of impaction injury to the femoral head articular cartilage. Twelve patients had marginal impaction of the acetabular wall. Fifteen patients had open triradiate physes at time of injury (Table 1).
Thirty-seven of the 38 patients were treated with open reduction and internal fixation (ORIF) by an experienced orthopedic trauma surgeon; 1 patient with a stable posterior wall fracture was treated nonoperatively. Surgical indications were articular displacement of more than 1 mm, hip joint instability, irreducible hip dislocation, and intra-articular fracture fragments. In the 37 surgically treated cases, the approaches used were Kocher-Langenbeck (22), ilioinguinal (8), combined Kocher-Langenbeck/ilioinguinal (5), and triradiate (2).
Immediate postoperative radiographs were evaluated by 3 orthopedic surgeons blinded to the patients’ clinical outcomes. Displacement was evaluated on anteroposterior (AP) and Judet views of the pelvis, as described by Matta,22 and reductions were classified as anatomical (0-1 mm of displacement), imperfect (>1 to 3 mm), poor (>3 mm), or surgical secondary congruence (Table 2).
Results
Thirty-seven patients underwent acetabular fracture ORIF. Immediate postoperative radiographs showed 30 anatomical reductions and 7 imperfect reductions. One patient had surgical secondary congruence and developed AVN of the hip. We could not identify an association between the quality of the reduction and the outcome with respect to pain or return to activity. However, no patient had a poor reduction. An illustrative case is presented in Figures 1 to 4.
All acetabular fractures united within 4.5 months (range, 3.0-8.0 months) after the index procedure. Early postoperative complications included 3 cases of meralgia paresthetica and 13 cases of abductor weakness. Meralgia paresthetica resolved spontaneously in all 3 patients. Of the 13 patients with abductor weakness, 11 improved with physical therapy, 1 was limited by the head injury, and 1 subsequently underwent hip fusion. One patient had a deep vein thrombosis (DVT) that was identified before surgery and managed with warfarin.
Other complications included 1 case of deep infection of the surgical wound. This infection presented 4 months after surgery and was treated with débridement, hardware removal, and a 3-month course of antibiotics. Two patients who sustained hip dislocations at time of injury developed AVN of the femoral head. Both developed osteoarthritis, and 1 underwent hip fusion. Eight patients developed heterotopic ossification on the side of the acetabular fracture; 4 of them underwent surgical excision. Four patients required a separate operation for hardware removal. Four patients with triradiate cartilage involvement went on to premature closure. No patient had any leg-length discrepancy or dysplasia at time of follow-up.
Thirty-four of the 38 patients returned to their regular activities. For these patients, mean time to return to full activity was 7.0 months (range, 3-30 months); there was no difference in mean time to return to full activity between skeletally mature and skeletally immature patients (6.6 vs 7.4 months; P = .57). Of the other 4 patients, 1 had permanent cognitive and physical disability with an ataxic gait as a result of a traumatic brain injury, 2 were limited by AVN (1 underwent hip fusion), and 1 was limited by an ipsilateral knee injury.
Of the 38 patients, 29 were pain-free; 6 had occasional, intermittent mild pain that did not limit their activities; and 3 had severe, activity-limiting pain. Of the 6 patients with mild pain, 2 had femoral impaction injuries, and 4 had marginal impaction injuries. Of the 3 patients with severe pain, 2 developed femoral head AVN, and 1 had multiple ipsilateral extremity injuries involving the femur, knee, and tibia.
Discussion
The traditional treatment for acetabular fractures in children has been nonoperative,8,10 and there are few specific treatment guidelines.13 Recent recommendations are nonoperative treatment for minimally displaced fractures (<1 mm) and acetabular fracture ORIF for fractures displaced more than 2 mm.11 No clear consensus exists on management for fractures displaced 1 to 2 mm. Few studies have investigated the outcomes of operative management of these fractures in the pediatric or adolescent population.
In our series of adolescent acetabular fractures, we examined unions, complications, and return to activity. Of 38 patients with acetabular fractures, 37 were treated with ORIF. Anatomical reduction was achieved in the majority of patients. Posterior wall fractures were by far the most common fracture type, which is consistent with previous reports.10,11 All acetabular fractures united, and most patients were pain-free at latest follow-up. There was a low incidence of major complications in our patient population. One major complication was a DVT in a 14-year-old boy who was in a motor vehicle accident and sustained a T-type fracture of the right acetabulum with contralateral femoral shaft and ankle fractures. The DVT was in the right internal iliac and common femoral veins and was diagnosed on magnetic resonance venography. The patient was treated with warfarin for 3 months without incident.
Two patients developed AVN of the femoral head. One of these patients was an 11-year-old girl who was in a motor vehicle accident and sustained a T-type fracture with marginal impaction of the posterior wall, posterior hip dislocation, and a pelvic ring injury. She was treated with ORIF through combined Kocher-Langenbeck/ilioinguinal approaches. By 4 months after surgery, the acetabular fracture was united. Nine months after surgery, she still had pain (activity-limiting) and a 35° flexion contracture of the hip, and she was ambulating with a cane. The diagnosis was AVN of the hip. The patient underwent hip fusion 1 year after surgery.
The second patient with femoral head AVN was a 12-year-old boy who fell while skiing and sustained a fracture of the posterior wall and a hip dislocation with impaction of the femoral head. Initial treatment at an outside institution consisted of open reduction of the hip and excision of a “loose body” from the joint. Eight weeks after surgery, the patient continued to have pain and was referred to our institution. A second operation was performed. Findings included a defect involving 40% of the posterior wall, and signs that the posterior wall had been excised during the initial operation. The patient eventually developed AVN of the hip. This patient was also diagnosed with a deep wound infection 4 months after surgery. He presented with pain and a fluid collection around the hip. The infection was not confirmed through fluid culture, and, as he eventually developed AVN of the hip, his symptoms may have been the result of chondrolysis or AVN rather than infection.
There were no cases of nonunion or malunion, leg-length discrepancy, or permanent sciatic nerve palsy. Although there were a few cases of premature closure of the triradiate cartilage, no acetabular dysplasia was seen at latest follow-up, likely because of the relative maturity of our pediatric group (age range, 11-18 years). Age at time of injury is thought to be the most important factor influencing growth and development of the acetabulum.9,13 In addition, previous studies have demonstrated a tendency toward acetabular fractures in patients with mature triradiate cartilage—versus pelvic ring injuries in patients with immature triradiate cartilage.8,11 This may also account for the older age of our study group.
Minor complications (eg, meralgia paresthetica) resolved spontaneously. The most common complications were abductor weakness and heterotopic ossification. In only 4 cases was a secondary procedure for excision of the heterotopic bone required. Abductor weakness, more commonly associated with a Kocher-Langenbeck approach to the hip, resolved with therapy in almost all cases. Only 4 of our patients required removal of hardware from the acetabulum.
Although the majority of acetabular fractures resulted from high-energy trauma, 8 cases were sports-related. Six of these involved posterior wall fractures, suggesting the injury resulted from a fall on flexed knee and hip. This was not known to be a common mechanism of injury in this age group.3,7 An additional concern was how to size the posterior wall fragment when not ossified. At one center, preoperative magnetic resonance imaging (MRI) was effectively used to size the osteochondral posterior wall fragment as standard protocol for patients with posterior wall fractures in this age group—resulting in better decisions regarding the need for ORIF. At the other institution, preoperative MRI was not performed routinely for this subset of patients.
Thirty-four of our 38 patients returned to their normal activities. Of the other 4 patients, 1 was permanently disabled secondary to traumatic brain injury, 1 had other ipsilateral extremity injuries that limited his mobility, and 2 developed AVN of the femoral head. Both patients with AVN had hip dislocations. Four of the 6 patients who were symptomatic during activity sustained impaction injuries of the femoral head or posterior wall. This suggests that poorer outcomes may be associated with dislocation or with articular injuries—similar to what has been reported in the adult literature.
This study had several limitations. First, it was a retrospective case series, so there was no control group for comparison. Second, the relatively short follow-up did not allow evaluation of the incidence of degenerative arthritis secondary to articular injury, the symptoms of which may develop 1 to 2 decades after injury.13 This phenomenon was well described by Letournel and Judet21 in the adult population, and there is no reason to presume the adolescent population is any different. Third, our sample was small and unlikely to represent a uniform sampling of the general pediatric population. Fourth, it was not possible to draw detailed conclusions about the outcome of ORIF for a particular type of acetabular fracture. Fifth, we did not see as many of the associated visceral injuries that are so prevalent in the literature. This may reflect improvement in safety specifications for automobiles, or our group may not have had the most severe or high-energy injuries. Here our population sample may have skewed our results, leading to better than expected outcomes.
One last study limitation, a major one, was the age of our population, 11 to 18 years, which makes it difficult to extrapolate results to the entire pediatric population. On one hand, a more immature skeleton has a higher chance of remodeling and is more forgiving of deformities and small amounts of displacement. On the other hand, injury and premature triradiate cartilage fusion in a younger patient can lead to significant deformity and acetabular dysplasia.9 Whether ORIF of these fractures would alter the outcome of an injury to the triradiate cartilage is yet to be determined.
Conclusion
In agreement with earlier studies,10,11,15,18 the good outcomes in our series correlated with congruence of reduction. Outcome predictors such as dislocation, femoral head injury, and marginal impaction are similar to those described in the adult literature. Although our study did not have a nonoperative group for comparison, the favorable outcomes of ORIF of acetabular fractures suggest that a more aggressive approach to treatment should be considered. Given the added benefits of early, pain-free mobilization, we think that only stable, undisplaced fractures (<1 mm) should be managed nonoperatively. In the adolescent population, we recommend ORIF for optimal management of unstable acetabular fractures, fractures with any hip subluxation, and fractures displaced more than 1 mm.
1. Canale ST, Beaty JH. Fractures of the pelvis. In: Beaty JH, Kassler JR, eds. Rockwood and Wilkin’s Fractures in Children. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:883-991.
2. Demetriades D, Karaiskakis M, Velmahos GC, Alo K, Murray J, Chan L. Pelvic fractures in pediatric and adult trauma patients: are they different injuries? J Trauma. 2003;54(6):1146-1151.
3. Grisoni N, Connor S, Marsh E, Thompson GH, Cooperman DR, Blakemore LC. Pelvic fractures in a pediatric level I trauma center. J Orthop Trauma. 2002;16(7):458-463.
4. Ismail N, Bellemare JF, Mollitt DL, Di Scala C, Koeppel B, Tepas JJ. Death from pelvic fracture: children are different. J Pediatr Surg. 1996;31(1):82-85.
5. Schlickwei W, Keck T. Pelvic and acetabular fractures in childhood. Injury. 2005;36(suppl 1):A57-A63.
6. Swiontkowski MF. Fractures and dislocations about the hip and pelvis. In: Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. Philadelphia, PA: Saunders; 2003:371-406.
7. Silber JS, Flynn JM, Koffler KM, Dormans JP, Drummond DS. Analysis of the cause, classification, and associated injuries of 166 consecutive pediatric pelvic fractures. J Pediatr Orthop. 2001;21(4):446-450.
8. Silber JS, Flynn JM. Changing patterns of pediatric pelvic fractures with skeletal maturation: implications for classification and management. J Pediatr Orthop. 2002;22(1):22-26.
9. Bucholz RW, Ezaki M, Ogden JA. Injury to the acetabular triradiate physeal cartilage. J Bone Joint Surg Am. 1982;64(4):600-609.
10. Heeg M, Klasen HJ, Visser JD. Acetabular fractures in children and adolescents. J Bone Joint Surg Br. 1989;71(3):418-421.
11. Heeg M, de Ridder VA, Tornetta P, de Lange S, Klasen HJ. Acetabular fractures in children and adolescents. Clin Orthop Relat Res. 2000;(376):80-86.
12. Heeg M, Visser JD, Oostvogel HJ. Injuries of the acetabular triradiate cartilage and sacroiliac joint. J Bone Joint Surg Br. 1988;70(1):34-37.
13. Liporace FA, Ong B, Mohaideen A, Ong A, Koval KJ. Development and injury of the triradiate cartilage with its effects on acetabular development: review of the literature. J Trauma. 2003;54(6):1245-1249.
14. Rodrigues KF. Injury of the acetabular epiphysis. Injury. 1973;4(3):258-260.
15. Trousdale RT, Ganz R. Posttraumatic acetabular dysplasia. Clin Orthop Relat Res. 1994;(305):124-132.
16. Brooks E, Rosman M. Central fracture-dislocation of the hip in a child. J Trauma. 1988;28(11):1590-1592.
17. Habacker TA, Heinrich SD, Dehne R. Fracture of the superior pelvic quadrant in a child. J Pediatr Orthop. 1995;15(1):69-72.
18. Karunakar MA, Goulet JA, Mueller KL, Bedi A, Le TT. Operative treatment of unstable pediatric pelvis and acetabular fractures. J Pediatr Orthop. 2005;25(1):34-38.
19. Rieger H, Brug E. Fractures of the pelvis in children. Clin Orthop Relat Res. 1997;(336);226-239.
20. Torode I, Zieg D. Pelvic fractures in children. J Pediatr Orthop. 1985;5(1):76-84.
21. Letournel E, Judet R. Fractures of the Acetabulum. 2nd ed. New York, NY: Springer-Verlag; 1993.
22. Matta JM. Fractures of the acetabulum: accuracy of reduction and clinical results in patients managed operatively within three weeks of the injury. J Bone Joint Surg Am. 1996;78(11):1632-1645.
1. Canale ST, Beaty JH. Fractures of the pelvis. In: Beaty JH, Kassler JR, eds. Rockwood and Wilkin’s Fractures in Children. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:883-991.
2. Demetriades D, Karaiskakis M, Velmahos GC, Alo K, Murray J, Chan L. Pelvic fractures in pediatric and adult trauma patients: are they different injuries? J Trauma. 2003;54(6):1146-1151.
3. Grisoni N, Connor S, Marsh E, Thompson GH, Cooperman DR, Blakemore LC. Pelvic fractures in a pediatric level I trauma center. J Orthop Trauma. 2002;16(7):458-463.
4. Ismail N, Bellemare JF, Mollitt DL, Di Scala C, Koeppel B, Tepas JJ. Death from pelvic fracture: children are different. J Pediatr Surg. 1996;31(1):82-85.
5. Schlickwei W, Keck T. Pelvic and acetabular fractures in childhood. Injury. 2005;36(suppl 1):A57-A63.
6. Swiontkowski MF. Fractures and dislocations about the hip and pelvis. In: Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. Philadelphia, PA: Saunders; 2003:371-406.
7. Silber JS, Flynn JM, Koffler KM, Dormans JP, Drummond DS. Analysis of the cause, classification, and associated injuries of 166 consecutive pediatric pelvic fractures. J Pediatr Orthop. 2001;21(4):446-450.
8. Silber JS, Flynn JM. Changing patterns of pediatric pelvic fractures with skeletal maturation: implications for classification and management. J Pediatr Orthop. 2002;22(1):22-26.
9. Bucholz RW, Ezaki M, Ogden JA. Injury to the acetabular triradiate physeal cartilage. J Bone Joint Surg Am. 1982;64(4):600-609.
10. Heeg M, Klasen HJ, Visser JD. Acetabular fractures in children and adolescents. J Bone Joint Surg Br. 1989;71(3):418-421.
11. Heeg M, de Ridder VA, Tornetta P, de Lange S, Klasen HJ. Acetabular fractures in children and adolescents. Clin Orthop Relat Res. 2000;(376):80-86.
12. Heeg M, Visser JD, Oostvogel HJ. Injuries of the acetabular triradiate cartilage and sacroiliac joint. J Bone Joint Surg Br. 1988;70(1):34-37.
13. Liporace FA, Ong B, Mohaideen A, Ong A, Koval KJ. Development and injury of the triradiate cartilage with its effects on acetabular development: review of the literature. J Trauma. 2003;54(6):1245-1249.
14. Rodrigues KF. Injury of the acetabular epiphysis. Injury. 1973;4(3):258-260.
15. Trousdale RT, Ganz R. Posttraumatic acetabular dysplasia. Clin Orthop Relat Res. 1994;(305):124-132.
16. Brooks E, Rosman M. Central fracture-dislocation of the hip in a child. J Trauma. 1988;28(11):1590-1592.
17. Habacker TA, Heinrich SD, Dehne R. Fracture of the superior pelvic quadrant in a child. J Pediatr Orthop. 1995;15(1):69-72.
18. Karunakar MA, Goulet JA, Mueller KL, Bedi A, Le TT. Operative treatment of unstable pediatric pelvis and acetabular fractures. J Pediatr Orthop. 2005;25(1):34-38.
19. Rieger H, Brug E. Fractures of the pelvis in children. Clin Orthop Relat Res. 1997;(336);226-239.
20. Torode I, Zieg D. Pelvic fractures in children. J Pediatr Orthop. 1985;5(1):76-84.
21. Letournel E, Judet R. Fractures of the Acetabulum. 2nd ed. New York, NY: Springer-Verlag; 1993.
22. Matta JM. Fractures of the acetabulum: accuracy of reduction and clinical results in patients managed operatively within three weeks of the injury. J Bone Joint Surg Am. 1996;78(11):1632-1645.
Hip Fracture and the Weekend Effect: Does Weekend Admission Affect Patient Outcomes?
Weekend admission has been hypothesized to be a risk factor for increased patient mortality and complications during hospital stays—commonly referred to as the weekend effect.1 Reduced hospital staffing on weekends, particularly of senior-level physicians and ancillary nursing services, may affect the quality of diagnosis and management for patients admitted for traumatic and emergent conditions. Investigators have found increased mortality in weekend admissions for stroke,2 subdural hematoma,3 gastrointestinal bleeding,4,5 atrial fibrillation,6 and pulmonary embolism.7 Investigators have not found increased mortality in weekend admissions for hip fracture, though the majority of the data was derived from European patient populations, which may be subject to management and staffing strategies different from those for US patients.8-10 Furthermore, data on this topic in US patients are limited to a multispecialty study of 50 different admission diagnoses, which used 1 year of data from a single US state.1
We conducted a study to comprehensively assess the effect of weekend admission on adverse outcomes during hospital stays. The literature suggests that surgery for hip fracture can be delayed up to 48 hours without significant additional risk of death,11-13 allowing orthopedic departments to stabilize routine hip fracture admissions on weekends and operate whenever limited surgical teams become available. Surgical delay has not been thoroughly analyzed by day of admission among US patients,14 but the combined potential of more conservative preoperative management and the availability of fewer senior physicians and ancillary providers may result in worse outcomes for weekend versus weekday admissions.
Materials and Methods
Study Population
Part of the Healthcare Cost and Utilization Project, the Nationwide Inpatient Sample (NIS) provides a 20% representative sample of annual US hospital admissions.15 For these admissions, the NIS includes data related to demographic and clinical variables, such as International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes, as well as descriptive variables for the hospitals where the patients were admitted. The NIS is publicly available to researchers. As its health information is deidentified, we did not have to obtain institutional review board approval for this study.
Ascertainment of Cases
Our initial study population, drawn from the period 1998–2010, consisted of 821,531 patients with a principal ICD-9-CM diagnosis of femoral neck fracture (820.0-820.9). To best capture the typical presentation of hip fracture, we excluded:
- Patients with open femoral neck fractures (820.1, 820.3, 820.9).
- Patients who did not have open reduction and internal fixation (ORIF) (79.35), hemiarthroplasty (81.52), closed reduction and internal fixation (CRIF) (79.15), internal fixation (78.55), or total hip arthroplasty (THA) (81.51) as their primary surgical procedure.
- Patients admitted from sources other than the emergency department.
- Patients who underwent surgery before admission.
- Patients whose admission type was not classified as emergency or urgent.
Ascertainment of Covariates
For all patients, we extracted data on exposure of interest, day of admission (weekend or weekday), and demographic variables including age, sex, race (white, black, Hispanic, other, missing), and insurance (Medicare, Medicaid, private, other). We used the Elixhauser method to determine 30 different comorbidities from ICD-9-CM diagnosis coding16 and sorted patients by total number of comorbidities (0, 1, 2, 3 or 4, ≥5). As has been done before,17 we excluded blood loss anemia, coagulopathy, and fluid and electrolyte disorders from this comorbidity calculation, as these conditions can be secondary to trauma. We also extracted data on the admission itself, including hospital region (Northeast, Midwest, South, West), hospital bed size (small, medium, large), hospital teaching status (nonteaching, teaching), and hospital location (rural, urban). We used diagnosis codes to categorize fracture location as “not otherwise specified” (820.8), intracapsular (820.0), or extracapsular (820.2).
Because of low frequencies, we collapsed 2 race designations (Native American, Asian or Pacific Islander) into the “other race” category and 2 insurance designations (self-pay, no charge) into the “other insurance” category. For a substantial number of patients, race information was missing, so we included “missing” as its own category in analyses. Patients who were missing data on day of admission, age, sex, insurance, or hospital characteristics were excluded from our final cohort, as missing frequencies for each variable were small.
Ascertainment of Outcomes
For all patients, we extracted data on death status at discharge and length of hospital stay. We log-transformed length of stay because of its right skew, assigning the value of 12 hours to patients admitted and discharged the same day. Perioperative complications were calculated using ICD-9-CM codes as defined by a recent study of orthopedics-related complications by Lin and colleagues.18 There were 14 possible complications, including acute renal failure (584.5-9), tachycardia (427), wound hemorrhage (719.15, 998.31-2), wound disruption (998.3, 998.31-2), wound infection (682.6, 686.9, 891, 891.1-2, 894, 894.1-2, 998.5, 998.51, 998.6, 998.83, 998.59), deep vein thrombosis (453.4, 453.41-2, 453.9), acute myocardial infarction (410, 410.01, 410.11, 410.2, 410.21, 410.3, 410.31, 410.4, 410.41, 410.5, 410.51, 410.6, 410.9, 410.91, 997.1), pneumonia (480-480.9, 481, 482-482.9, 483, 483.1, 483.8, 484, 484.1, 484.3, 484.5-8, 485, 486, 487, 507), pulmonary embolism (415.11, 415.19), sepsis (995.91-2), stroke (997.02), urinary tract infection (599, 997.5), implant infection (996.66-7, 996.69), and incision and débridement (86.04, 86.09, 86.22, 86.28, 86.3). In our statistical analyses, we examined both the risk of having a complicated admission (≥1 perioperative complication) and the risk of having each specific complication.
Statistical Analysis
To assess similarity between weekend and weekday admissions, we used the Fisher exact test and χ2P values. Logistic regression was used to calculate the odds ratios (ORs) of mortality and perioperative complications for weekend versus weekday admissions. Linear regression was used to calculate parameter estimates for length of hospital stay for weekend versus weekday admissions. We interpreted parameter estimates as percentage differences using the formula 100(eb–1), where b is the estimated standardized regression coefficient of a log-transformed outcome variable.19 All regression models were controlled for age, sex, race, insurance, number of comorbidities, fracture location, hospital region, hospital bed size, hospital teaching status, and hospital location. We also stratified our study population by surgical delay in hours (<24, 24-48, 49-72, 73-120, ≥121) and by surgery performed (ORIF, hemiarthroplasty, CRIF, internal fixation only, THA, multiple procedures) to examine the effect of weekend admission on mortality, perioperative complications, and length of stay within each stratum. We did not control for these variables in our regression models because they were potential mediators of mortality, complications, and length of stay. All statistical analyses in this study were performed using SAS Version 9.1 (SAS Institute), and P < .05 was interpreted as statistically significant.
Results
After exclusions, our study population consisted of 96,892 weekend admissions and 248,097 weekday admissions. Among all admissions, mean age was 79.3 years (range, 0-113 years), with patients primarily being female and white, paying with Medicare, and having 1 to 4 comorbidities. Admissions were primarily for extracapsular femoral neck fractures and occurred most often in the South region, in hospitals with large beds, in nonteaching hospitals, and in urban locations. Table 1 lists details of baseline characteristics for weekend and weekday admissions.
Hospital stay details, including surgical delay and procedure performed, were examined for weekend and weekday admissions. Mean delay to surgery was 31.0 hours for weekend admissions and 30.2 hours for weekday admissions (P < .0001). The difference was driven by a higher proportion of weekend admissions in which surgery was performed 24 to 120 hours after admission. Patients admitted on the weekend also underwent more ORIF procedures and fewer hemiarthroplasties. Table 2 is a full list of hospital stay characteristics.
In regression analyses, weekend OR of mortality was 0.94 (95% CI, 0.89-0.99), weekend OR of having at least 1 complication was 1.00 (95% CI, 0.98-1.02), and weekend mean hospital stay was 3.74% shorter (95% CI, 3.40-4.08) in comparison with weekday figures. Within our models, risk of mortality and complications and mean length of stay increased as the number of patient comorbidities increased. Table 3 lists selected results from our regression models. Comprehensive tables for each outcome’s model are presented in Appendices 1 to 3.
In our analyses of specific complications, there were no significant associations between weekend admissions and risk of acute renal failure, wound hemorrhage, wound disruption, wound infection, deep vein thrombosis, myocardial infarction, pneumonia, pulmonary embolism, sepsis, urinary tract infection, implant infection, or incision and débridement. In addition, we found a lower risk of tachycardia (OR, 0.90; 95% CI, 0.82-1.00) and a higher risk (P < .10) of stroke (OR, 1.16; 95% CI, 0.99-1.35). Table 4 is a full list of the specific complications and their risks for weekend versus weekday admissions.
According to stratified analyses involving surgical delay, weekend admissions in which patients had surgery the same day as admission had decreased risk of mortality (OR, 0.81; 95% CI, 0.72-0.91) and perioperative complications (OR, 0.96; 95% CI, 0.92-0.99). In addition, hospital stay was shorter for weekend admissions with surgical delay of less than 24 hours (4.89% shorter; 95% CI, 4.22-5.55), 24 to 48 hours (5.93% shorter; 95% CI, 5.51-6.35), and 49 to 72 hours (3.50% shorter; 95% CI, 2.80-4.20). When admissions were stratified by procedure performed, patients who were admitted on the weekend and underwent ORIF, hemiarthroplasty, CRIF, internal fixation only, and THA had shorter stays than patients admitted on weekdays. For all surgeries performed, the risk of both mortality and complications did not significantly differ by day of admission. Table 5 lists the comprehensive results of all our stratified analyses.
Discussion
In this large, multiyear analysis of patients admitted for hip fracture in the United States, risk of mortality was slightly lower for weekend versus weekday admissions, hospital stay was significantly shorter, and risk of perioperative complications was not significantly different between admission types. In secondary analyses, shorter hospital stay was limited to patients who were admitted on weekends and underwent surgery within 48 hours. Our results therefore suggest that the weekend effect does not apply to hip fracture patients in the United States.
Our results are largely consistent with the literature on the topic.11-14 An Australian study of 4183 patients with acute hip fracture found no significant difference in 2- or 30-day mortality among weekend and weekday admissions.11 Similarly, 2 Danish studies did not find a difference in hospital-stay or 30-day mortality between weekend and weekday admissions among samples of 600 and 38,020 patients with hip fracture, respectively.12,13 In US patients, a cross-specialty study that included hip fractures did not find a difference in hospital-stay mortality among 22,001 admissions in the state of California in 1998.14 Our analysis significantly extended the findings of these studies by using comprehensive admission data from 46 US states over a 13-year period (1998–2010) and by examining outcomes other than mortality, including perioperative complications and length of hospital stay.
Our study had several limitations. First, the clinical data on fracture diagnoses and surgical procedures were based on ICD-9-CM codes, limiting our ability to account for the full details of fracture severity and subsequent management. Second, our analyses were limited to outcomes during the hospital stay, and we could not examine the effect of weekend admission on readmission and long-term mortality. Third, because of the dichotomization of admission day in the NIS database, we could not selectively examine the effect of Friday, Saturday, or Sunday admission on our outcomes. Fourth, we excluded admissions that were missing demographic and clinical data, potentially creating a complete-case bias. However, these exclusions were needed to accurately capture the common presentation of acute hip fracture, and there is no reason to believe that differences in record coding were nonrandom. Last, our study was observational, and we cannot rule out the effect of residual confounding on our results.
Our results failed to show a weekend effect on mortality, perioperative complications, or length of hospital stay in US patients with hip fracture. The reason for this, as suggested before,12 may be that hip fractures are becoming easier to diagnose. Furthermore, the observation that hospital stay was shorter for weekend admissions suggests that, despite decreased staffing of nursing and rehabilitation services, the lower volume of elective surgeries on weekends may actually increase staff availability to hip fracture patients.
1. Cram P, Hillis SL, Barnett M, Rosenthal GE. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am J Med. 2004;117(3):151-157.
2. Saposnik G, Baibergenova A, Bayer N, Hachinski V. Weekends: a dangerous time for having a stroke? Stroke. 2007;38(4):1211-1215.
3. Busl KM, Prabhakaran S. Predictors of mortality in nontraumatic subdural hematoma. J Neurosurg. 2013;119(5):1296-1301.
4. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol. 2009;7(3):296e1-302e1.
5. Shaheen AA, Kaplan GG, Myers RP. Weekend versus weekday admission and mortality from gastrointestinal hemorrhage caused by peptic ulcer disease. Clin Gastroenterol Hepatol. 2009;7(3):303-310.
6. Deshmukh A, Pant S, Kumar G, Bursac Z, Paydak H, Mehta JL. Comparison of outcomes of weekend versus weekday admissions for atrial fibrillation. Am J Cardiol. 2012;110(2):208-211.
7. Aujesky D, Jiménez D, Mor MK, Geng M, Fine MJ, Ibrahim SA. Weekend versus weekday admission and mortality after acute pulmonary embolism. Circulation. 2009;119(7):962-968.
8. Clarke MS, Wills RA, Bowman RV, et al. Exploratory study of the ‘weekend effect’ for acute medical admissions to public hospitals in Queensland, Australia. Intern Med J. 2010;40(11):777-783.
9. Daugaard CL, Jørgensen HL, Riis T, Lauritzen JB, Duus BR, Van der mark S. Is mortality after hip fracture associated with surgical delay or admission during weekends and public holidays? A retrospective study of 38,020 patients. Acta Orthop. 2012;83(6):609-613.
10. Foss NB, Kehlet H. Short-term mortality in hip fracture patients admitted during weekends and holidays. Br J Anaesth. 2006;96(4):450-4514.
11. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis and meta-regression. Can J Anaesth. 2008;55(3):146-154.
12. Zuckerman JD, Skovron ML, Koval KJ, Aharonoff G, Frankel VH. Postoperative complications and mortality associated with operative delay in older patients who have a fracture of the hip. J Bone Joint Surg Am. 1995;77(10):1551-1556.
13. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927.
14. Ho V, Hamilton BH, Roos LL. Multiple approaches to assessing the effects of delays for hip fracture patients in the United States and Canada. Health Serv Res. 2000;34(7):1499-1518.
15. Steiner C, Elixhauser A, Schnaier J. The Healthcare Cost and Utilization Project: an overview. Eff Clin Pract. 2002;5(3):143-151.
16. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27.
17. Brasel KJ, Guse CE, Layde P, Weigelt JA. Rib fractures: relationship with pneumonia and mortality. Crit Care Med. 2006;34(6):1642-1646.
18. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
19. Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. 2nd ed. New York, NY: Springer-Verlag; 2012. Statistics for Biology and Health.
Weekend admission has been hypothesized to be a risk factor for increased patient mortality and complications during hospital stays—commonly referred to as the weekend effect.1 Reduced hospital staffing on weekends, particularly of senior-level physicians and ancillary nursing services, may affect the quality of diagnosis and management for patients admitted for traumatic and emergent conditions. Investigators have found increased mortality in weekend admissions for stroke,2 subdural hematoma,3 gastrointestinal bleeding,4,5 atrial fibrillation,6 and pulmonary embolism.7 Investigators have not found increased mortality in weekend admissions for hip fracture, though the majority of the data was derived from European patient populations, which may be subject to management and staffing strategies different from those for US patients.8-10 Furthermore, data on this topic in US patients are limited to a multispecialty study of 50 different admission diagnoses, which used 1 year of data from a single US state.1
We conducted a study to comprehensively assess the effect of weekend admission on adverse outcomes during hospital stays. The literature suggests that surgery for hip fracture can be delayed up to 48 hours without significant additional risk of death,11-13 allowing orthopedic departments to stabilize routine hip fracture admissions on weekends and operate whenever limited surgical teams become available. Surgical delay has not been thoroughly analyzed by day of admission among US patients,14 but the combined potential of more conservative preoperative management and the availability of fewer senior physicians and ancillary providers may result in worse outcomes for weekend versus weekday admissions.
Materials and Methods
Study Population
Part of the Healthcare Cost and Utilization Project, the Nationwide Inpatient Sample (NIS) provides a 20% representative sample of annual US hospital admissions.15 For these admissions, the NIS includes data related to demographic and clinical variables, such as International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes, as well as descriptive variables for the hospitals where the patients were admitted. The NIS is publicly available to researchers. As its health information is deidentified, we did not have to obtain institutional review board approval for this study.
Ascertainment of Cases
Our initial study population, drawn from the period 1998–2010, consisted of 821,531 patients with a principal ICD-9-CM diagnosis of femoral neck fracture (820.0-820.9). To best capture the typical presentation of hip fracture, we excluded:
- Patients with open femoral neck fractures (820.1, 820.3, 820.9).
- Patients who did not have open reduction and internal fixation (ORIF) (79.35), hemiarthroplasty (81.52), closed reduction and internal fixation (CRIF) (79.15), internal fixation (78.55), or total hip arthroplasty (THA) (81.51) as their primary surgical procedure.
- Patients admitted from sources other than the emergency department.
- Patients who underwent surgery before admission.
- Patients whose admission type was not classified as emergency or urgent.
Ascertainment of Covariates
For all patients, we extracted data on exposure of interest, day of admission (weekend or weekday), and demographic variables including age, sex, race (white, black, Hispanic, other, missing), and insurance (Medicare, Medicaid, private, other). We used the Elixhauser method to determine 30 different comorbidities from ICD-9-CM diagnosis coding16 and sorted patients by total number of comorbidities (0, 1, 2, 3 or 4, ≥5). As has been done before,17 we excluded blood loss anemia, coagulopathy, and fluid and electrolyte disorders from this comorbidity calculation, as these conditions can be secondary to trauma. We also extracted data on the admission itself, including hospital region (Northeast, Midwest, South, West), hospital bed size (small, medium, large), hospital teaching status (nonteaching, teaching), and hospital location (rural, urban). We used diagnosis codes to categorize fracture location as “not otherwise specified” (820.8), intracapsular (820.0), or extracapsular (820.2).
Because of low frequencies, we collapsed 2 race designations (Native American, Asian or Pacific Islander) into the “other race” category and 2 insurance designations (self-pay, no charge) into the “other insurance” category. For a substantial number of patients, race information was missing, so we included “missing” as its own category in analyses. Patients who were missing data on day of admission, age, sex, insurance, or hospital characteristics were excluded from our final cohort, as missing frequencies for each variable were small.
Ascertainment of Outcomes
For all patients, we extracted data on death status at discharge and length of hospital stay. We log-transformed length of stay because of its right skew, assigning the value of 12 hours to patients admitted and discharged the same day. Perioperative complications were calculated using ICD-9-CM codes as defined by a recent study of orthopedics-related complications by Lin and colleagues.18 There were 14 possible complications, including acute renal failure (584.5-9), tachycardia (427), wound hemorrhage (719.15, 998.31-2), wound disruption (998.3, 998.31-2), wound infection (682.6, 686.9, 891, 891.1-2, 894, 894.1-2, 998.5, 998.51, 998.6, 998.83, 998.59), deep vein thrombosis (453.4, 453.41-2, 453.9), acute myocardial infarction (410, 410.01, 410.11, 410.2, 410.21, 410.3, 410.31, 410.4, 410.41, 410.5, 410.51, 410.6, 410.9, 410.91, 997.1), pneumonia (480-480.9, 481, 482-482.9, 483, 483.1, 483.8, 484, 484.1, 484.3, 484.5-8, 485, 486, 487, 507), pulmonary embolism (415.11, 415.19), sepsis (995.91-2), stroke (997.02), urinary tract infection (599, 997.5), implant infection (996.66-7, 996.69), and incision and débridement (86.04, 86.09, 86.22, 86.28, 86.3). In our statistical analyses, we examined both the risk of having a complicated admission (≥1 perioperative complication) and the risk of having each specific complication.
Statistical Analysis
To assess similarity between weekend and weekday admissions, we used the Fisher exact test and χ2P values. Logistic regression was used to calculate the odds ratios (ORs) of mortality and perioperative complications for weekend versus weekday admissions. Linear regression was used to calculate parameter estimates for length of hospital stay for weekend versus weekday admissions. We interpreted parameter estimates as percentage differences using the formula 100(eb–1), where b is the estimated standardized regression coefficient of a log-transformed outcome variable.19 All regression models were controlled for age, sex, race, insurance, number of comorbidities, fracture location, hospital region, hospital bed size, hospital teaching status, and hospital location. We also stratified our study population by surgical delay in hours (<24, 24-48, 49-72, 73-120, ≥121) and by surgery performed (ORIF, hemiarthroplasty, CRIF, internal fixation only, THA, multiple procedures) to examine the effect of weekend admission on mortality, perioperative complications, and length of stay within each stratum. We did not control for these variables in our regression models because they were potential mediators of mortality, complications, and length of stay. All statistical analyses in this study were performed using SAS Version 9.1 (SAS Institute), and P < .05 was interpreted as statistically significant.
Results
After exclusions, our study population consisted of 96,892 weekend admissions and 248,097 weekday admissions. Among all admissions, mean age was 79.3 years (range, 0-113 years), with patients primarily being female and white, paying with Medicare, and having 1 to 4 comorbidities. Admissions were primarily for extracapsular femoral neck fractures and occurred most often in the South region, in hospitals with large beds, in nonteaching hospitals, and in urban locations. Table 1 lists details of baseline characteristics for weekend and weekday admissions.
Hospital stay details, including surgical delay and procedure performed, were examined for weekend and weekday admissions. Mean delay to surgery was 31.0 hours for weekend admissions and 30.2 hours for weekday admissions (P < .0001). The difference was driven by a higher proportion of weekend admissions in which surgery was performed 24 to 120 hours after admission. Patients admitted on the weekend also underwent more ORIF procedures and fewer hemiarthroplasties. Table 2 is a full list of hospital stay characteristics.
In regression analyses, weekend OR of mortality was 0.94 (95% CI, 0.89-0.99), weekend OR of having at least 1 complication was 1.00 (95% CI, 0.98-1.02), and weekend mean hospital stay was 3.74% shorter (95% CI, 3.40-4.08) in comparison with weekday figures. Within our models, risk of mortality and complications and mean length of stay increased as the number of patient comorbidities increased. Table 3 lists selected results from our regression models. Comprehensive tables for each outcome’s model are presented in Appendices 1 to 3.
In our analyses of specific complications, there were no significant associations between weekend admissions and risk of acute renal failure, wound hemorrhage, wound disruption, wound infection, deep vein thrombosis, myocardial infarction, pneumonia, pulmonary embolism, sepsis, urinary tract infection, implant infection, or incision and débridement. In addition, we found a lower risk of tachycardia (OR, 0.90; 95% CI, 0.82-1.00) and a higher risk (P < .10) of stroke (OR, 1.16; 95% CI, 0.99-1.35). Table 4 is a full list of the specific complications and their risks for weekend versus weekday admissions.
According to stratified analyses involving surgical delay, weekend admissions in which patients had surgery the same day as admission had decreased risk of mortality (OR, 0.81; 95% CI, 0.72-0.91) and perioperative complications (OR, 0.96; 95% CI, 0.92-0.99). In addition, hospital stay was shorter for weekend admissions with surgical delay of less than 24 hours (4.89% shorter; 95% CI, 4.22-5.55), 24 to 48 hours (5.93% shorter; 95% CI, 5.51-6.35), and 49 to 72 hours (3.50% shorter; 95% CI, 2.80-4.20). When admissions were stratified by procedure performed, patients who were admitted on the weekend and underwent ORIF, hemiarthroplasty, CRIF, internal fixation only, and THA had shorter stays than patients admitted on weekdays. For all surgeries performed, the risk of both mortality and complications did not significantly differ by day of admission. Table 5 lists the comprehensive results of all our stratified analyses.
Discussion
In this large, multiyear analysis of patients admitted for hip fracture in the United States, risk of mortality was slightly lower for weekend versus weekday admissions, hospital stay was significantly shorter, and risk of perioperative complications was not significantly different between admission types. In secondary analyses, shorter hospital stay was limited to patients who were admitted on weekends and underwent surgery within 48 hours. Our results therefore suggest that the weekend effect does not apply to hip fracture patients in the United States.
Our results are largely consistent with the literature on the topic.11-14 An Australian study of 4183 patients with acute hip fracture found no significant difference in 2- or 30-day mortality among weekend and weekday admissions.11 Similarly, 2 Danish studies did not find a difference in hospital-stay or 30-day mortality between weekend and weekday admissions among samples of 600 and 38,020 patients with hip fracture, respectively.12,13 In US patients, a cross-specialty study that included hip fractures did not find a difference in hospital-stay mortality among 22,001 admissions in the state of California in 1998.14 Our analysis significantly extended the findings of these studies by using comprehensive admission data from 46 US states over a 13-year period (1998–2010) and by examining outcomes other than mortality, including perioperative complications and length of hospital stay.
Our study had several limitations. First, the clinical data on fracture diagnoses and surgical procedures were based on ICD-9-CM codes, limiting our ability to account for the full details of fracture severity and subsequent management. Second, our analyses were limited to outcomes during the hospital stay, and we could not examine the effect of weekend admission on readmission and long-term mortality. Third, because of the dichotomization of admission day in the NIS database, we could not selectively examine the effect of Friday, Saturday, or Sunday admission on our outcomes. Fourth, we excluded admissions that were missing demographic and clinical data, potentially creating a complete-case bias. However, these exclusions were needed to accurately capture the common presentation of acute hip fracture, and there is no reason to believe that differences in record coding were nonrandom. Last, our study was observational, and we cannot rule out the effect of residual confounding on our results.
Our results failed to show a weekend effect on mortality, perioperative complications, or length of hospital stay in US patients with hip fracture. The reason for this, as suggested before,12 may be that hip fractures are becoming easier to diagnose. Furthermore, the observation that hospital stay was shorter for weekend admissions suggests that, despite decreased staffing of nursing and rehabilitation services, the lower volume of elective surgeries on weekends may actually increase staff availability to hip fracture patients.
Weekend admission has been hypothesized to be a risk factor for increased patient mortality and complications during hospital stays—commonly referred to as the weekend effect.1 Reduced hospital staffing on weekends, particularly of senior-level physicians and ancillary nursing services, may affect the quality of diagnosis and management for patients admitted for traumatic and emergent conditions. Investigators have found increased mortality in weekend admissions for stroke,2 subdural hematoma,3 gastrointestinal bleeding,4,5 atrial fibrillation,6 and pulmonary embolism.7 Investigators have not found increased mortality in weekend admissions for hip fracture, though the majority of the data was derived from European patient populations, which may be subject to management and staffing strategies different from those for US patients.8-10 Furthermore, data on this topic in US patients are limited to a multispecialty study of 50 different admission diagnoses, which used 1 year of data from a single US state.1
We conducted a study to comprehensively assess the effect of weekend admission on adverse outcomes during hospital stays. The literature suggests that surgery for hip fracture can be delayed up to 48 hours without significant additional risk of death,11-13 allowing orthopedic departments to stabilize routine hip fracture admissions on weekends and operate whenever limited surgical teams become available. Surgical delay has not been thoroughly analyzed by day of admission among US patients,14 but the combined potential of more conservative preoperative management and the availability of fewer senior physicians and ancillary providers may result in worse outcomes for weekend versus weekday admissions.
Materials and Methods
Study Population
Part of the Healthcare Cost and Utilization Project, the Nationwide Inpatient Sample (NIS) provides a 20% representative sample of annual US hospital admissions.15 For these admissions, the NIS includes data related to demographic and clinical variables, such as International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes, as well as descriptive variables for the hospitals where the patients were admitted. The NIS is publicly available to researchers. As its health information is deidentified, we did not have to obtain institutional review board approval for this study.
Ascertainment of Cases
Our initial study population, drawn from the period 1998–2010, consisted of 821,531 patients with a principal ICD-9-CM diagnosis of femoral neck fracture (820.0-820.9). To best capture the typical presentation of hip fracture, we excluded:
- Patients with open femoral neck fractures (820.1, 820.3, 820.9).
- Patients who did not have open reduction and internal fixation (ORIF) (79.35), hemiarthroplasty (81.52), closed reduction and internal fixation (CRIF) (79.15), internal fixation (78.55), or total hip arthroplasty (THA) (81.51) as their primary surgical procedure.
- Patients admitted from sources other than the emergency department.
- Patients who underwent surgery before admission.
- Patients whose admission type was not classified as emergency or urgent.
Ascertainment of Covariates
For all patients, we extracted data on exposure of interest, day of admission (weekend or weekday), and demographic variables including age, sex, race (white, black, Hispanic, other, missing), and insurance (Medicare, Medicaid, private, other). We used the Elixhauser method to determine 30 different comorbidities from ICD-9-CM diagnosis coding16 and sorted patients by total number of comorbidities (0, 1, 2, 3 or 4, ≥5). As has been done before,17 we excluded blood loss anemia, coagulopathy, and fluid and electrolyte disorders from this comorbidity calculation, as these conditions can be secondary to trauma. We also extracted data on the admission itself, including hospital region (Northeast, Midwest, South, West), hospital bed size (small, medium, large), hospital teaching status (nonteaching, teaching), and hospital location (rural, urban). We used diagnosis codes to categorize fracture location as “not otherwise specified” (820.8), intracapsular (820.0), or extracapsular (820.2).
Because of low frequencies, we collapsed 2 race designations (Native American, Asian or Pacific Islander) into the “other race” category and 2 insurance designations (self-pay, no charge) into the “other insurance” category. For a substantial number of patients, race information was missing, so we included “missing” as its own category in analyses. Patients who were missing data on day of admission, age, sex, insurance, or hospital characteristics were excluded from our final cohort, as missing frequencies for each variable were small.
Ascertainment of Outcomes
For all patients, we extracted data on death status at discharge and length of hospital stay. We log-transformed length of stay because of its right skew, assigning the value of 12 hours to patients admitted and discharged the same day. Perioperative complications were calculated using ICD-9-CM codes as defined by a recent study of orthopedics-related complications by Lin and colleagues.18 There were 14 possible complications, including acute renal failure (584.5-9), tachycardia (427), wound hemorrhage (719.15, 998.31-2), wound disruption (998.3, 998.31-2), wound infection (682.6, 686.9, 891, 891.1-2, 894, 894.1-2, 998.5, 998.51, 998.6, 998.83, 998.59), deep vein thrombosis (453.4, 453.41-2, 453.9), acute myocardial infarction (410, 410.01, 410.11, 410.2, 410.21, 410.3, 410.31, 410.4, 410.41, 410.5, 410.51, 410.6, 410.9, 410.91, 997.1), pneumonia (480-480.9, 481, 482-482.9, 483, 483.1, 483.8, 484, 484.1, 484.3, 484.5-8, 485, 486, 487, 507), pulmonary embolism (415.11, 415.19), sepsis (995.91-2), stroke (997.02), urinary tract infection (599, 997.5), implant infection (996.66-7, 996.69), and incision and débridement (86.04, 86.09, 86.22, 86.28, 86.3). In our statistical analyses, we examined both the risk of having a complicated admission (≥1 perioperative complication) and the risk of having each specific complication.
Statistical Analysis
To assess similarity between weekend and weekday admissions, we used the Fisher exact test and χ2P values. Logistic regression was used to calculate the odds ratios (ORs) of mortality and perioperative complications for weekend versus weekday admissions. Linear regression was used to calculate parameter estimates for length of hospital stay for weekend versus weekday admissions. We interpreted parameter estimates as percentage differences using the formula 100(eb–1), where b is the estimated standardized regression coefficient of a log-transformed outcome variable.19 All regression models were controlled for age, sex, race, insurance, number of comorbidities, fracture location, hospital region, hospital bed size, hospital teaching status, and hospital location. We also stratified our study population by surgical delay in hours (<24, 24-48, 49-72, 73-120, ≥121) and by surgery performed (ORIF, hemiarthroplasty, CRIF, internal fixation only, THA, multiple procedures) to examine the effect of weekend admission on mortality, perioperative complications, and length of stay within each stratum. We did not control for these variables in our regression models because they were potential mediators of mortality, complications, and length of stay. All statistical analyses in this study were performed using SAS Version 9.1 (SAS Institute), and P < .05 was interpreted as statistically significant.
Results
After exclusions, our study population consisted of 96,892 weekend admissions and 248,097 weekday admissions. Among all admissions, mean age was 79.3 years (range, 0-113 years), with patients primarily being female and white, paying with Medicare, and having 1 to 4 comorbidities. Admissions were primarily for extracapsular femoral neck fractures and occurred most often in the South region, in hospitals with large beds, in nonteaching hospitals, and in urban locations. Table 1 lists details of baseline characteristics for weekend and weekday admissions.
Hospital stay details, including surgical delay and procedure performed, were examined for weekend and weekday admissions. Mean delay to surgery was 31.0 hours for weekend admissions and 30.2 hours for weekday admissions (P < .0001). The difference was driven by a higher proportion of weekend admissions in which surgery was performed 24 to 120 hours after admission. Patients admitted on the weekend also underwent more ORIF procedures and fewer hemiarthroplasties. Table 2 is a full list of hospital stay characteristics.
In regression analyses, weekend OR of mortality was 0.94 (95% CI, 0.89-0.99), weekend OR of having at least 1 complication was 1.00 (95% CI, 0.98-1.02), and weekend mean hospital stay was 3.74% shorter (95% CI, 3.40-4.08) in comparison with weekday figures. Within our models, risk of mortality and complications and mean length of stay increased as the number of patient comorbidities increased. Table 3 lists selected results from our regression models. Comprehensive tables for each outcome’s model are presented in Appendices 1 to 3.
In our analyses of specific complications, there were no significant associations between weekend admissions and risk of acute renal failure, wound hemorrhage, wound disruption, wound infection, deep vein thrombosis, myocardial infarction, pneumonia, pulmonary embolism, sepsis, urinary tract infection, implant infection, or incision and débridement. In addition, we found a lower risk of tachycardia (OR, 0.90; 95% CI, 0.82-1.00) and a higher risk (P < .10) of stroke (OR, 1.16; 95% CI, 0.99-1.35). Table 4 is a full list of the specific complications and their risks for weekend versus weekday admissions.
According to stratified analyses involving surgical delay, weekend admissions in which patients had surgery the same day as admission had decreased risk of mortality (OR, 0.81; 95% CI, 0.72-0.91) and perioperative complications (OR, 0.96; 95% CI, 0.92-0.99). In addition, hospital stay was shorter for weekend admissions with surgical delay of less than 24 hours (4.89% shorter; 95% CI, 4.22-5.55), 24 to 48 hours (5.93% shorter; 95% CI, 5.51-6.35), and 49 to 72 hours (3.50% shorter; 95% CI, 2.80-4.20). When admissions were stratified by procedure performed, patients who were admitted on the weekend and underwent ORIF, hemiarthroplasty, CRIF, internal fixation only, and THA had shorter stays than patients admitted on weekdays. For all surgeries performed, the risk of both mortality and complications did not significantly differ by day of admission. Table 5 lists the comprehensive results of all our stratified analyses.
Discussion
In this large, multiyear analysis of patients admitted for hip fracture in the United States, risk of mortality was slightly lower for weekend versus weekday admissions, hospital stay was significantly shorter, and risk of perioperative complications was not significantly different between admission types. In secondary analyses, shorter hospital stay was limited to patients who were admitted on weekends and underwent surgery within 48 hours. Our results therefore suggest that the weekend effect does not apply to hip fracture patients in the United States.
Our results are largely consistent with the literature on the topic.11-14 An Australian study of 4183 patients with acute hip fracture found no significant difference in 2- or 30-day mortality among weekend and weekday admissions.11 Similarly, 2 Danish studies did not find a difference in hospital-stay or 30-day mortality between weekend and weekday admissions among samples of 600 and 38,020 patients with hip fracture, respectively.12,13 In US patients, a cross-specialty study that included hip fractures did not find a difference in hospital-stay mortality among 22,001 admissions in the state of California in 1998.14 Our analysis significantly extended the findings of these studies by using comprehensive admission data from 46 US states over a 13-year period (1998–2010) and by examining outcomes other than mortality, including perioperative complications and length of hospital stay.
Our study had several limitations. First, the clinical data on fracture diagnoses and surgical procedures were based on ICD-9-CM codes, limiting our ability to account for the full details of fracture severity and subsequent management. Second, our analyses were limited to outcomes during the hospital stay, and we could not examine the effect of weekend admission on readmission and long-term mortality. Third, because of the dichotomization of admission day in the NIS database, we could not selectively examine the effect of Friday, Saturday, or Sunday admission on our outcomes. Fourth, we excluded admissions that were missing demographic and clinical data, potentially creating a complete-case bias. However, these exclusions were needed to accurately capture the common presentation of acute hip fracture, and there is no reason to believe that differences in record coding were nonrandom. Last, our study was observational, and we cannot rule out the effect of residual confounding on our results.
Our results failed to show a weekend effect on mortality, perioperative complications, or length of hospital stay in US patients with hip fracture. The reason for this, as suggested before,12 may be that hip fractures are becoming easier to diagnose. Furthermore, the observation that hospital stay was shorter for weekend admissions suggests that, despite decreased staffing of nursing and rehabilitation services, the lower volume of elective surgeries on weekends may actually increase staff availability to hip fracture patients.
1. Cram P, Hillis SL, Barnett M, Rosenthal GE. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am J Med. 2004;117(3):151-157.
2. Saposnik G, Baibergenova A, Bayer N, Hachinski V. Weekends: a dangerous time for having a stroke? Stroke. 2007;38(4):1211-1215.
3. Busl KM, Prabhakaran S. Predictors of mortality in nontraumatic subdural hematoma. J Neurosurg. 2013;119(5):1296-1301.
4. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol. 2009;7(3):296e1-302e1.
5. Shaheen AA, Kaplan GG, Myers RP. Weekend versus weekday admission and mortality from gastrointestinal hemorrhage caused by peptic ulcer disease. Clin Gastroenterol Hepatol. 2009;7(3):303-310.
6. Deshmukh A, Pant S, Kumar G, Bursac Z, Paydak H, Mehta JL. Comparison of outcomes of weekend versus weekday admissions for atrial fibrillation. Am J Cardiol. 2012;110(2):208-211.
7. Aujesky D, Jiménez D, Mor MK, Geng M, Fine MJ, Ibrahim SA. Weekend versus weekday admission and mortality after acute pulmonary embolism. Circulation. 2009;119(7):962-968.
8. Clarke MS, Wills RA, Bowman RV, et al. Exploratory study of the ‘weekend effect’ for acute medical admissions to public hospitals in Queensland, Australia. Intern Med J. 2010;40(11):777-783.
9. Daugaard CL, Jørgensen HL, Riis T, Lauritzen JB, Duus BR, Van der mark S. Is mortality after hip fracture associated with surgical delay or admission during weekends and public holidays? A retrospective study of 38,020 patients. Acta Orthop. 2012;83(6):609-613.
10. Foss NB, Kehlet H. Short-term mortality in hip fracture patients admitted during weekends and holidays. Br J Anaesth. 2006;96(4):450-4514.
11. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis and meta-regression. Can J Anaesth. 2008;55(3):146-154.
12. Zuckerman JD, Skovron ML, Koval KJ, Aharonoff G, Frankel VH. Postoperative complications and mortality associated with operative delay in older patients who have a fracture of the hip. J Bone Joint Surg Am. 1995;77(10):1551-1556.
13. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927.
14. Ho V, Hamilton BH, Roos LL. Multiple approaches to assessing the effects of delays for hip fracture patients in the United States and Canada. Health Serv Res. 2000;34(7):1499-1518.
15. Steiner C, Elixhauser A, Schnaier J. The Healthcare Cost and Utilization Project: an overview. Eff Clin Pract. 2002;5(3):143-151.
16. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27.
17. Brasel KJ, Guse CE, Layde P, Weigelt JA. Rib fractures: relationship with pneumonia and mortality. Crit Care Med. 2006;34(6):1642-1646.
18. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
19. Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. 2nd ed. New York, NY: Springer-Verlag; 2012. Statistics for Biology and Health.
1. Cram P, Hillis SL, Barnett M, Rosenthal GE. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am J Med. 2004;117(3):151-157.
2. Saposnik G, Baibergenova A, Bayer N, Hachinski V. Weekends: a dangerous time for having a stroke? Stroke. 2007;38(4):1211-1215.
3. Busl KM, Prabhakaran S. Predictors of mortality in nontraumatic subdural hematoma. J Neurosurg. 2013;119(5):1296-1301.
4. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol. 2009;7(3):296e1-302e1.
5. Shaheen AA, Kaplan GG, Myers RP. Weekend versus weekday admission and mortality from gastrointestinal hemorrhage caused by peptic ulcer disease. Clin Gastroenterol Hepatol. 2009;7(3):303-310.
6. Deshmukh A, Pant S, Kumar G, Bursac Z, Paydak H, Mehta JL. Comparison of outcomes of weekend versus weekday admissions for atrial fibrillation. Am J Cardiol. 2012;110(2):208-211.
7. Aujesky D, Jiménez D, Mor MK, Geng M, Fine MJ, Ibrahim SA. Weekend versus weekday admission and mortality after acute pulmonary embolism. Circulation. 2009;119(7):962-968.
8. Clarke MS, Wills RA, Bowman RV, et al. Exploratory study of the ‘weekend effect’ for acute medical admissions to public hospitals in Queensland, Australia. Intern Med J. 2010;40(11):777-783.
9. Daugaard CL, Jørgensen HL, Riis T, Lauritzen JB, Duus BR, Van der mark S. Is mortality after hip fracture associated with surgical delay or admission during weekends and public holidays? A retrospective study of 38,020 patients. Acta Orthop. 2012;83(6):609-613.
10. Foss NB, Kehlet H. Short-term mortality in hip fracture patients admitted during weekends and holidays. Br J Anaesth. 2006;96(4):450-4514.
11. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis and meta-regression. Can J Anaesth. 2008;55(3):146-154.
12. Zuckerman JD, Skovron ML, Koval KJ, Aharonoff G, Frankel VH. Postoperative complications and mortality associated with operative delay in older patients who have a fracture of the hip. J Bone Joint Surg Am. 1995;77(10):1551-1556.
13. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927.
14. Ho V, Hamilton BH, Roos LL. Multiple approaches to assessing the effects of delays for hip fracture patients in the United States and Canada. Health Serv Res. 2000;34(7):1499-1518.
15. Steiner C, Elixhauser A, Schnaier J. The Healthcare Cost and Utilization Project: an overview. Eff Clin Pract. 2002;5(3):143-151.
16. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27.
17. Brasel KJ, Guse CE, Layde P, Weigelt JA. Rib fractures: relationship with pneumonia and mortality. Crit Care Med. 2006;34(6):1642-1646.
18. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
19. Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. 2nd ed. New York, NY: Springer-Verlag; 2012. Statistics for Biology and Health.
Taxonomy of Seven‐Day Readmissions
Unplanned hospital readmissions are regarded as a core measure of quality of care and may comprise a large avoidable cause of healthcare expenditures.[1, 2, 3, 4, 5] An estimated 20% of Medicare patients who are discharged from a hospital are readmitted within 30 days.[1, 6] This has led the Centers for Medicare & Medicaid Services and other payers to reduce reimbursements for unplanned 30‐day hospital readmissions.
Efforts to decrease readmission rates have been hampered by ineffective risk prediction models, and strategies to reduce readmissions have found limited success.[7] Understanding the mechanism of readmissions is necessary for accurate prediction and prevention. This can be achieved only through analysis of patient data and medical narratives obtained from patient interviews or detailed chart reviews.[8] Studies attempting to identify mechanisms of readmission using narrative chart reviews have been limited by small sample size, highly selected patient samples, and poor interobserver agreement.[8, 9, 10]
Our objective in this study was to identify specific mechanisms and risk factors of unplanned readmissions from the medicine service of a large urban hospital by reviewing medical charts for each case. Given the inverse relationship between time since discharge from the initial admission and the probability of an avoidable readmission,[8] we focused our review on 7‐day readmissions.
METHODS
Setting
The study took place within Bellevue Hospital Center, an 800‐bed teaching hospital that serves a culturally and racially diverse inner‐city population in New York City. Bellevue is 1 of 11 acute‐care facilities managed by Health and Hospitals Corporation. The Bellevue inpatient medicine service is staffed by board‐certified general internists (180 beds), oncologists (20 beds), and pulmonologists (20 beds), who function as hospitalists in supervision of housestaff and physicians. Their efforts are supported by case managers and social workers who meet every weekday with physicians and nurses to plan discharges as multidisciplinary teams. Weekend support is minimal, consisting of an on‐call social worker to assist with urgent matters only. Upon discharge, patients are referred directly to 1 or more of Bellevue's outpatient clinics or to their own primary care providers outside Bellevue. There is a single electronic medical record for Bellevue, which spans the full range of care provided in the outpatient clinics, emergency department, and inpatient service.
Patients
Eligible patients were discharged from the Bellevue medical service between July 1, 2010 and July 1, 2011, and readmitted to any service at Bellevue within 7 days. During the study period, there were 8421 discharges. Discharges included transfers to other hospitals or rehabilitation centers, and excluded patients who died during hospitalization. Of these, 6781 were not readmitted, 1581 were readmitted within 30 days (18.8%), and 549 were readmitted within 7 days (6.5%). From the latter group, 20 consecutive cases were excluded after use in an exploratory pilot study, 84 consecutive cases were excluded after use in a formal pilot study, leaving 445 cases, from which 400 cases were randomly selected via terminal digit of the medical record number. We selected 400 chart reviews as a reasonable sample size to provide a 95% confidence interval, with a margin of error less than 4.9% for any of the proportions of the 5 readmission categories. Of these, 65 were determined to be planned readmissions (eg, for elective chemotherapy). The remaining 335 unplanned 7‐day readmissions served as the subjects of this review. The study was approved by the institutional review board of New York University School of Medicine.
Reviewers
Three of the authors of this paper (Drs. Janjigian, Bails, and Link) were actively practicing board‐certified internal medicine physicians with 7, 19, and 26 years, respectively, of postresidency clinical experience during the review period of this study. Every case was reviewed by 2 investigators. One author (Drs. Janjigian) reviewed readmissions from the first 6 months of the calendar year, the second (Dr. Bails) reviewed readmissions from the last 6 months, and the third (Dr. Link) reviewed all 335 readmissions.
Data Collection
Using the electronic medical record, each readmission was reviewed with the intent to identify the sequence of events leading up to the readmission, most commonly achieved by analyzing the discharge summary from the initial admission and the admission note from the second admission. Further chart review was completed as necessary to establish the clearest narrative and to classify the readmission into 1 of 5 categories based on the cause. Narratives are defined here as the sequence of events leading to the readmission as determined by chart review and not by patient interviews. Narratives were recorded for each case to assist with understanding how each author determined the classification, and were used when disagreements required group consensus. Time spent on individual chart reviews varied widely, from 1 to 30 minutes, depending on the complexity of each case. For example, an against medical advice (AMA) discharge could be immediately identified in the medical record, whereas a determination that an incomplete workup was conducted would require reviewing the admission note from the readmission, the discharge summary from the index admission, review of progress and consult notes, and even vital signs, labs, and radiology.
An algorithm for classifying contributory causes of readmission into 1 of 5 categories was created from narratives compiled from a pilot of 84, 7‐day readmissions to Bellevue during the previous year. Six readmitted patients were interviewed by a study author during this pilot phase. These narratives were determined by consensus of the authors to provide no additional relevant information from that obtained through chart review alone. The 5 categories are identified in Figure 1 as follows:
- Second admission was not medically necessary.
- Second admission followed an elopement (patient left without knowledge of the hospital staff) or discharge AMA during the first admission.
- Second admission was caused by a deficiency in the discharge process of the first admission, attributable to the hospital system or providers.
- Second admission was caused by a factor attributable to the patient including substance use or nonadherence to the treatment plan from the first admission.
- Second admission was related to a complication of the primary disease or its treatment or an unrelated condition that could not reasonably have been predicted or prevented by a competent physician meeting the standard of care.
Categories 3, 4, and 5 were further divided into more specific subcategories as shown in Figure 1.

Each readmission was assigned a single category from the algorithm using a stepwise process in which a higher‐order cause excluded consideration of a downstream category. For example, if the second admission was not medically necessary (category 1), an incorrect decision to readmit the patient was considered the primary cause of the readmission, and no consideration was given to categories 2 through 5. In this manner, each patient was assigned to a single category. We considered readmissions attributable to provider error (categories 1 and 3) to be avoidable. Examples of readmissions in each category with narratives are shown in the Supporting Information, Appendix 1, in the online version of this article. Discrepancies in classification were resolved by consensus of all authors.
Statistical Analysis
Unweighted kappa values were measured to assess agreement between authors in the assignment of the major category among the 5 choices in the algorithm. [2] tests were used to compare categorical variables between 2 groups (readmitted vs not readmitted) or between several groups (5 categories of readmissions), whereas Kruskal‐Wallis tests were used for continuous variables.
Only the first readmission was used in analysis of patient characteristics when multiple readmissions occurred for an individual patient. Unique patients were used for analysis of nonreadmitted patients. The generalized estimating equation method was used to adjust for correlations between multiple readmissions within patients.
RESULTS
During this period, 270 patients accounted for 335 readmissions. Characteristics of patients readmitted within 7 days are shown in Table 1 and compared with those of patients who were not readmitted during the same study period. Patients who were readmitted were more likely to have had a longer length of stay during the first admission.
| Characteristic | Not Readmitted, n=6,781 | Readmitted, n=270 | P Value |
|---|---|---|---|
| |||
| Male gender (% of category) | 4,224 (62.3%) | 180 (66.7%) | 0.15 |
| Mean age, y (SD) | 56.1 (16.3) | 55.1 (16.3) | 0.65 |
| Median initial LOS [interquartile range] | 3 [2, 6] | 4 [2, 9] | 0.002 |
| Mean days between admissions (SD) | NA | 3.8 (2.1) | NA |
| AMA discharge (% of category) | 413 (6.1%) | 20 (7.4%) | 0.38 |
Results of categorization of readmission are shown in Table 2. Readmissions related to the discharge process (category 3) were further divided into subcategories (Table 3). Category 5 (unpredictable/unpreventable complication of primary diagnosis or unrelated event) constituted the highest percentage of readmissions at 46%, followed by category 4 (patient behavior) at 19%, category 3 (discharge process deficiency) at 17%, category 2 (AMA) at 12%, and category 1 (unnecessary admission) at 7%. Readmissions designated as preventable (categories 1 and 3) accounted for 24% of all readmissions. Readmissions due to patient factors (categories 2 and 4) accounted for 31% of all readmissions. Notably, 21% of all readmissions were due to patients who eloped or left AMA during the first discharge or who returned because of substance abuse during the interim (categories 2 and 4a). Among the preventable readmissions, the most commonly designated cause of readmission was a perceived premature discharge (category 3b2), accounting for 6% of all readmissions.
| Category 1: Second Admission Not Medically Necessary | Category 2: First Admission AMA | Category 3: Deficiency in the Discharge Process | Category 4: Patient Behavior | Category 5: Unpredictable Complication of Primary or Alternate Diagnosis | P Value* | |
|---|---|---|---|---|---|---|
| ||||||
| Total (%) | 22 (6.6%) | 39 (11.6%) | 56 (16.7%) | 63 (18.8%) | 155 (46.3%) | |
| Male (%) | 11 (50.0%) | 29 (74.4%) | 38 (67.9%) | 54 (85.7%) | 91 (58.7%) | 0.005 |
| Mean age, y (SD) | 61.8 (13.7) | 48.1 (13.2) | 58.6 (14.4) | 53.3 (11.8) | 55.1 (17.7) | 0.004 |
| Median LOS [IQR] | 2.5 [2.0, 7.0] | 2.0 [1.0, 6.0] | 5.0 [2.0, 8.5] | 4.0 [2.0, 6.0] | 5.0 [2.0, 10.0] | 0.03 |
| Mean days between admissions (SD) | 3.8 (2.2) | 3.1 (2.2) | 3.3 (2.0) | 3.8 (2.1) | 4.1 (2.1) | 0.27 |
| Category | Description | No. | % of Total |
|---|---|---|---|
| 3a1 | Overdosing of a prescribed medication | 3 | 0.9 |
| 3a2 | Underdosing of a prescribed medication | 5 | 1.5 |
| 3a3 | Adverse medication effect | 2 | 0.6 |
| 3b1 | Inadequate functional status | 3 | 0.9 |
| 3b2 | Premature discharge | 20 | 6.0 |
| 3c1 | Patient unable to fill prescriptions | 9 | 2.7 |
| 3c2 | Follow‐up arrangements inadequate | 6 | 1.8 |
| 3c3 | Discharge setting not appropriate | 5 | 1.5 |
| 3c4 | Inadequate communication of plan to receiving facility | 2 | 0.6 |
| 3c5 | Other | 1 | 0.3 |
| 4a | Patient behaviorsubstance use | 30 | 9 |
| 4b | Patient behavioradherence to discharge plan | 30 | 9 |
| 4c | Patient behaviorrefusal of discharge plan | 3 | 0.9 |
| 5a | Disease complication | 103 | 30.7 |
| 5b | Unrelated condition | 52 | 15.5 |
Variance was statistically significant across major categories for gender, mean age, and median length of stay. The interobserver level of agreement across the 5 major categories was substantial among both pairs of reviewers (Table 4).
| Pair of Reviewers | No. of Readmissions Reviewed | No. of Agreements (%) | Unweighted Kappa |
|---|---|---|---|
| Dr. LinkDr. Bails | 135 | 113 (83.7) | 0.78 |
| Dr. LinkDr. Janjigian | 200 | 163 (81.5) | 0.72 |
The 46 patients who had more than 1, 7‐day readmission during this study period were responsible for 106 readmissions. The majority of this group were readmitted twice (78%), with a range of 2 to 5 readmissions. Within this group, 24% were considered preventable readmissions (8 from category 1, 17 from category 3), and 76% were considered not preventable (10 from category 2, 27 from category 4, and 44 from category 5).
DISCUSSION
The purpose of this retrospective review was to identify causes of unplanned 7‐day readmissions after discharge from the medical service of a large urban teaching hospital. Rather than focus on risk factors for readmissions, which other studies have done, we reviewed charts of readmitted patients using a novel categorization algorithm to group patients into common mechanisms that elucidate why a particular patient was readmitted. By examining the chart in detail, we were able to identify etiologies of readmission that are potentially avoidable.
Some authors have questioned the use of readmissions as a measurement of the quality of care a hospital provides due to the high proportion of unavoidable readmissions in a given sample.[8, 10] We hoped to identify systems errors that could be targets of quality improvement initiatives, and therefore chose to focus entirely on 7‐day readmissions as these have been shown to be more preventable than 30‐day readmissions.[8] We had the ability to review any aspect of the medical chart (eg, vitals or labs on discharge, any clinical note), which provided the highest probability of discovering a systems error. Despite these efforts to identify preventable errors, we identified the most common mechanism of readmission as an unpredictable or unpreventable event related to the primary diagnosis or its treatment from the initial admission (category 5a, 30.7% of total readmissions). Review of examples from this category elucidates how an unpredictable readmission could occur within such a short time frame (see Supporting Information, Appendix 1, in the online version of this article). The 7‐day window precluded identification of clinic access barriers, thereby eliminating from analysis 1 mechanism for preventable readmissions.
Nonetheless, our study demonstrates room for improvement in provider behavior and hospital systems related to the discharge process. Nearly a quarter of all readmissions and the majority of preventable readmissions were related to systems issues, such as timing and coordination of the first discharge, and lack of medical necessity for the second admission (see Supporting Information, Appendix 1, in the online version of this article). Prior studies found that shorter length of stay was associated with increased preventable readmissions, a finding that our study does not support.[10, 11] We suspect that patients in this group had longer lengths of stay during the index hospitalization due to complexity of medical illness, limited social support network, or lack of insurance, among other factors, that exposed flaws in systems processes and provider judgment. The mechanisms of readmission related to discharge planning that we identified in this study, including comprehensiveness of care, coordination of care, and medication administration, all represent potential opportunities for intervention.
Of note, there was a high percentage of readmissions attributable to patient behaviors, such as AMA discharges, substance abuse following discharge, and nonadherence to the treatment plan. These factors are likely over‐represented in the Bellevue patient population compared to that of private hospital settings and no doubt exacerbate the readmission rates in urban hospitals treating patients with a high degree of social and behavioral health needs. Although patient‐related factors such as AMA discharges and substance abuse are potentially addressable, our reviewers felt that these were not preventable based on current knowledge and standards of care.
Studies that have attempted to classify readmissions as potentially avoidable have not shown good interobserver agreement when more than 1 reviewer was involved.[9, 10] Additionally, there is not a validated tool available to classify types of readmissions. By using a pilot sample of 84 cases to develop the model, confirming the accuracy of the chart by personally interviewing a sample of readmitted patients for comparison, and by employing experienced inpatient attending physicians to perform the reviews, we were able to develop an algorithm that achieved substantial reliability in assigning each readmission into 1 of 5 distinct categories.
Our literature search revealed only a single study that attempted to classify readmissions in a similar manner. Readmissions within 6 months at 9 Veterans Affairs hospitals were classified into causal categories of systems, provider, and patient etiology.[9] Overall, 34% of readmissions were deemed to be preventable compared to 24% in our study. Most readmissions (68%) were due to a worsening of a clinical condition, 4.5% were attributed to the admitting provider having too low a threshold to justify admission, and 2.7% were due to the patient not abstaining from drugs or alcohol. Though the study design and patient population differed from our own, the similarities in methods and results lend validity to the results and conclusions of our study.
Another limitation of our study is that readmissions to other hospitals were not included. In this respect, our estimate of the rate of readmission was an understatement of the true value. Nonetheless, the categorization of causes for readmission was not likely to be affected by the site of the second admission. Another limitation of this study was the small number of subjects reviewed relative to other studies that analyzed demographics and risk factors in large databases of readmissions.[12] However, the depth of the present review provides an understanding of the sequence of events leading to the readmission and permits development of strategies to prevent their occurrence.
We identified mechanisms of readmissions that can lay the groundwork for future interventions and safely reduce readmissions rates at little cost. To reduce admissions that may not be medically necessary, the narratives presented in the supplementary appendix suggest that improvement in communication between the admitting provider for the readmission and a provider familiar with the patient could have led to avoidance of the readmission. Similarly, enhanced communication to receiving nursing facilities would decrease the chances of the patient being immediately sent back as occurred multiple times in our cohort. Formal mandatory assessment of functional status for vulnerable patients would identify patients who may not be fully ready for discharge.
In conclusion, we found through detailed chart review of patients readmitted within 7 days to an urban teaching hospital that the majority of readmissions were not avoidable and were due to unpredictable complications of the primary diagnosis from the index hospitalization or a condition unrelated to the initial stay. This conclusion, in concurrence with those of other studies,[8, 10] questions the value of a readmission as a valid metric of quality though supports further improvements in hospital systems to reduce preventable readmissions.
Disclosure
Nothing to report.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , . The rate and cost of hospital readmissions for preventable conditions. Med Care Res Rev. 2004;61(2):225–240.
- Centers for Medicare and Medicaid Services. 9th scope of work version #080108‐0. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/QualityImprovementOrgs/downloads/9thSOWBaseContract_C_08‐01‐2008_2_.pdf. Accessed April 10, 2014.
- , . Hospital readmissions in the Medicare population. N Engl J Med. 1984;311(21):1349–1353.
- U.S. Department of Health 155(8):520–528.
- , , , et al. Incidence of potentially avoidable urgent readmissions and their relation to all‐cause urgent readmissions. CMAJ. 2011;183(14):E1067–E1072.
- , , , et al. Classifying general medicine readmissions. Are they preventable? Veterans Affairs Cooperative Studies in Health Services Group on Primary Care and Hospital Readmissions. J Gen Intern Med. 1996;11(10):597–607.
- , , , . Hospitalists assess the causes of early hospital readmissions. J Hosp Med. 2011;6(7):383–388.
- , , . Early readmissions to the department of medicine as a screening tool for monitoring quality of care problems. Medicine. 2008;87(5):294–300.
- , , , et al. Incidence and predictors of all and preventable adverse drug reactions in frail elderly persons after hospital stay. J Gerontol A Biol Sci Med Sci. 2006;61(5):511–515.
Unplanned hospital readmissions are regarded as a core measure of quality of care and may comprise a large avoidable cause of healthcare expenditures.[1, 2, 3, 4, 5] An estimated 20% of Medicare patients who are discharged from a hospital are readmitted within 30 days.[1, 6] This has led the Centers for Medicare & Medicaid Services and other payers to reduce reimbursements for unplanned 30‐day hospital readmissions.
Efforts to decrease readmission rates have been hampered by ineffective risk prediction models, and strategies to reduce readmissions have found limited success.[7] Understanding the mechanism of readmissions is necessary for accurate prediction and prevention. This can be achieved only through analysis of patient data and medical narratives obtained from patient interviews or detailed chart reviews.[8] Studies attempting to identify mechanisms of readmission using narrative chart reviews have been limited by small sample size, highly selected patient samples, and poor interobserver agreement.[8, 9, 10]
Our objective in this study was to identify specific mechanisms and risk factors of unplanned readmissions from the medicine service of a large urban hospital by reviewing medical charts for each case. Given the inverse relationship between time since discharge from the initial admission and the probability of an avoidable readmission,[8] we focused our review on 7‐day readmissions.
METHODS
Setting
The study took place within Bellevue Hospital Center, an 800‐bed teaching hospital that serves a culturally and racially diverse inner‐city population in New York City. Bellevue is 1 of 11 acute‐care facilities managed by Health and Hospitals Corporation. The Bellevue inpatient medicine service is staffed by board‐certified general internists (180 beds), oncologists (20 beds), and pulmonologists (20 beds), who function as hospitalists in supervision of housestaff and physicians. Their efforts are supported by case managers and social workers who meet every weekday with physicians and nurses to plan discharges as multidisciplinary teams. Weekend support is minimal, consisting of an on‐call social worker to assist with urgent matters only. Upon discharge, patients are referred directly to 1 or more of Bellevue's outpatient clinics or to their own primary care providers outside Bellevue. There is a single electronic medical record for Bellevue, which spans the full range of care provided in the outpatient clinics, emergency department, and inpatient service.
Patients
Eligible patients were discharged from the Bellevue medical service between July 1, 2010 and July 1, 2011, and readmitted to any service at Bellevue within 7 days. During the study period, there were 8421 discharges. Discharges included transfers to other hospitals or rehabilitation centers, and excluded patients who died during hospitalization. Of these, 6781 were not readmitted, 1581 were readmitted within 30 days (18.8%), and 549 were readmitted within 7 days (6.5%). From the latter group, 20 consecutive cases were excluded after use in an exploratory pilot study, 84 consecutive cases were excluded after use in a formal pilot study, leaving 445 cases, from which 400 cases were randomly selected via terminal digit of the medical record number. We selected 400 chart reviews as a reasonable sample size to provide a 95% confidence interval, with a margin of error less than 4.9% for any of the proportions of the 5 readmission categories. Of these, 65 were determined to be planned readmissions (eg, for elective chemotherapy). The remaining 335 unplanned 7‐day readmissions served as the subjects of this review. The study was approved by the institutional review board of New York University School of Medicine.
Reviewers
Three of the authors of this paper (Drs. Janjigian, Bails, and Link) were actively practicing board‐certified internal medicine physicians with 7, 19, and 26 years, respectively, of postresidency clinical experience during the review period of this study. Every case was reviewed by 2 investigators. One author (Drs. Janjigian) reviewed readmissions from the first 6 months of the calendar year, the second (Dr. Bails) reviewed readmissions from the last 6 months, and the third (Dr. Link) reviewed all 335 readmissions.
Data Collection
Using the electronic medical record, each readmission was reviewed with the intent to identify the sequence of events leading up to the readmission, most commonly achieved by analyzing the discharge summary from the initial admission and the admission note from the second admission. Further chart review was completed as necessary to establish the clearest narrative and to classify the readmission into 1 of 5 categories based on the cause. Narratives are defined here as the sequence of events leading to the readmission as determined by chart review and not by patient interviews. Narratives were recorded for each case to assist with understanding how each author determined the classification, and were used when disagreements required group consensus. Time spent on individual chart reviews varied widely, from 1 to 30 minutes, depending on the complexity of each case. For example, an against medical advice (AMA) discharge could be immediately identified in the medical record, whereas a determination that an incomplete workup was conducted would require reviewing the admission note from the readmission, the discharge summary from the index admission, review of progress and consult notes, and even vital signs, labs, and radiology.
An algorithm for classifying contributory causes of readmission into 1 of 5 categories was created from narratives compiled from a pilot of 84, 7‐day readmissions to Bellevue during the previous year. Six readmitted patients were interviewed by a study author during this pilot phase. These narratives were determined by consensus of the authors to provide no additional relevant information from that obtained through chart review alone. The 5 categories are identified in Figure 1 as follows:
- Second admission was not medically necessary.
- Second admission followed an elopement (patient left without knowledge of the hospital staff) or discharge AMA during the first admission.
- Second admission was caused by a deficiency in the discharge process of the first admission, attributable to the hospital system or providers.
- Second admission was caused by a factor attributable to the patient including substance use or nonadherence to the treatment plan from the first admission.
- Second admission was related to a complication of the primary disease or its treatment or an unrelated condition that could not reasonably have been predicted or prevented by a competent physician meeting the standard of care.
Categories 3, 4, and 5 were further divided into more specific subcategories as shown in Figure 1.

Each readmission was assigned a single category from the algorithm using a stepwise process in which a higher‐order cause excluded consideration of a downstream category. For example, if the second admission was not medically necessary (category 1), an incorrect decision to readmit the patient was considered the primary cause of the readmission, and no consideration was given to categories 2 through 5. In this manner, each patient was assigned to a single category. We considered readmissions attributable to provider error (categories 1 and 3) to be avoidable. Examples of readmissions in each category with narratives are shown in the Supporting Information, Appendix 1, in the online version of this article. Discrepancies in classification were resolved by consensus of all authors.
Statistical Analysis
Unweighted kappa values were measured to assess agreement between authors in the assignment of the major category among the 5 choices in the algorithm. [2] tests were used to compare categorical variables between 2 groups (readmitted vs not readmitted) or between several groups (5 categories of readmissions), whereas Kruskal‐Wallis tests were used for continuous variables.
Only the first readmission was used in analysis of patient characteristics when multiple readmissions occurred for an individual patient. Unique patients were used for analysis of nonreadmitted patients. The generalized estimating equation method was used to adjust for correlations between multiple readmissions within patients.
RESULTS
During this period, 270 patients accounted for 335 readmissions. Characteristics of patients readmitted within 7 days are shown in Table 1 and compared with those of patients who were not readmitted during the same study period. Patients who were readmitted were more likely to have had a longer length of stay during the first admission.
| Characteristic | Not Readmitted, n=6,781 | Readmitted, n=270 | P Value |
|---|---|---|---|
| |||
| Male gender (% of category) | 4,224 (62.3%) | 180 (66.7%) | 0.15 |
| Mean age, y (SD) | 56.1 (16.3) | 55.1 (16.3) | 0.65 |
| Median initial LOS [interquartile range] | 3 [2, 6] | 4 [2, 9] | 0.002 |
| Mean days between admissions (SD) | NA | 3.8 (2.1) | NA |
| AMA discharge (% of category) | 413 (6.1%) | 20 (7.4%) | 0.38 |
Results of categorization of readmission are shown in Table 2. Readmissions related to the discharge process (category 3) were further divided into subcategories (Table 3). Category 5 (unpredictable/unpreventable complication of primary diagnosis or unrelated event) constituted the highest percentage of readmissions at 46%, followed by category 4 (patient behavior) at 19%, category 3 (discharge process deficiency) at 17%, category 2 (AMA) at 12%, and category 1 (unnecessary admission) at 7%. Readmissions designated as preventable (categories 1 and 3) accounted for 24% of all readmissions. Readmissions due to patient factors (categories 2 and 4) accounted for 31% of all readmissions. Notably, 21% of all readmissions were due to patients who eloped or left AMA during the first discharge or who returned because of substance abuse during the interim (categories 2 and 4a). Among the preventable readmissions, the most commonly designated cause of readmission was a perceived premature discharge (category 3b2), accounting for 6% of all readmissions.
| Category 1: Second Admission Not Medically Necessary | Category 2: First Admission AMA | Category 3: Deficiency in the Discharge Process | Category 4: Patient Behavior | Category 5: Unpredictable Complication of Primary or Alternate Diagnosis | P Value* | |
|---|---|---|---|---|---|---|
| ||||||
| Total (%) | 22 (6.6%) | 39 (11.6%) | 56 (16.7%) | 63 (18.8%) | 155 (46.3%) | |
| Male (%) | 11 (50.0%) | 29 (74.4%) | 38 (67.9%) | 54 (85.7%) | 91 (58.7%) | 0.005 |
| Mean age, y (SD) | 61.8 (13.7) | 48.1 (13.2) | 58.6 (14.4) | 53.3 (11.8) | 55.1 (17.7) | 0.004 |
| Median LOS [IQR] | 2.5 [2.0, 7.0] | 2.0 [1.0, 6.0] | 5.0 [2.0, 8.5] | 4.0 [2.0, 6.0] | 5.0 [2.0, 10.0] | 0.03 |
| Mean days between admissions (SD) | 3.8 (2.2) | 3.1 (2.2) | 3.3 (2.0) | 3.8 (2.1) | 4.1 (2.1) | 0.27 |
| Category | Description | No. | % of Total |
|---|---|---|---|
| 3a1 | Overdosing of a prescribed medication | 3 | 0.9 |
| 3a2 | Underdosing of a prescribed medication | 5 | 1.5 |
| 3a3 | Adverse medication effect | 2 | 0.6 |
| 3b1 | Inadequate functional status | 3 | 0.9 |
| 3b2 | Premature discharge | 20 | 6.0 |
| 3c1 | Patient unable to fill prescriptions | 9 | 2.7 |
| 3c2 | Follow‐up arrangements inadequate | 6 | 1.8 |
| 3c3 | Discharge setting not appropriate | 5 | 1.5 |
| 3c4 | Inadequate communication of plan to receiving facility | 2 | 0.6 |
| 3c5 | Other | 1 | 0.3 |
| 4a | Patient behaviorsubstance use | 30 | 9 |
| 4b | Patient behavioradherence to discharge plan | 30 | 9 |
| 4c | Patient behaviorrefusal of discharge plan | 3 | 0.9 |
| 5a | Disease complication | 103 | 30.7 |
| 5b | Unrelated condition | 52 | 15.5 |
Variance was statistically significant across major categories for gender, mean age, and median length of stay. The interobserver level of agreement across the 5 major categories was substantial among both pairs of reviewers (Table 4).
| Pair of Reviewers | No. of Readmissions Reviewed | No. of Agreements (%) | Unweighted Kappa |
|---|---|---|---|
| Dr. LinkDr. Bails | 135 | 113 (83.7) | 0.78 |
| Dr. LinkDr. Janjigian | 200 | 163 (81.5) | 0.72 |
The 46 patients who had more than 1, 7‐day readmission during this study period were responsible for 106 readmissions. The majority of this group were readmitted twice (78%), with a range of 2 to 5 readmissions. Within this group, 24% were considered preventable readmissions (8 from category 1, 17 from category 3), and 76% were considered not preventable (10 from category 2, 27 from category 4, and 44 from category 5).
DISCUSSION
The purpose of this retrospective review was to identify causes of unplanned 7‐day readmissions after discharge from the medical service of a large urban teaching hospital. Rather than focus on risk factors for readmissions, which other studies have done, we reviewed charts of readmitted patients using a novel categorization algorithm to group patients into common mechanisms that elucidate why a particular patient was readmitted. By examining the chart in detail, we were able to identify etiologies of readmission that are potentially avoidable.
Some authors have questioned the use of readmissions as a measurement of the quality of care a hospital provides due to the high proportion of unavoidable readmissions in a given sample.[8, 10] We hoped to identify systems errors that could be targets of quality improvement initiatives, and therefore chose to focus entirely on 7‐day readmissions as these have been shown to be more preventable than 30‐day readmissions.[8] We had the ability to review any aspect of the medical chart (eg, vitals or labs on discharge, any clinical note), which provided the highest probability of discovering a systems error. Despite these efforts to identify preventable errors, we identified the most common mechanism of readmission as an unpredictable or unpreventable event related to the primary diagnosis or its treatment from the initial admission (category 5a, 30.7% of total readmissions). Review of examples from this category elucidates how an unpredictable readmission could occur within such a short time frame (see Supporting Information, Appendix 1, in the online version of this article). The 7‐day window precluded identification of clinic access barriers, thereby eliminating from analysis 1 mechanism for preventable readmissions.
Nonetheless, our study demonstrates room for improvement in provider behavior and hospital systems related to the discharge process. Nearly a quarter of all readmissions and the majority of preventable readmissions were related to systems issues, such as timing and coordination of the first discharge, and lack of medical necessity for the second admission (see Supporting Information, Appendix 1, in the online version of this article). Prior studies found that shorter length of stay was associated with increased preventable readmissions, a finding that our study does not support.[10, 11] We suspect that patients in this group had longer lengths of stay during the index hospitalization due to complexity of medical illness, limited social support network, or lack of insurance, among other factors, that exposed flaws in systems processes and provider judgment. The mechanisms of readmission related to discharge planning that we identified in this study, including comprehensiveness of care, coordination of care, and medication administration, all represent potential opportunities for intervention.
Of note, there was a high percentage of readmissions attributable to patient behaviors, such as AMA discharges, substance abuse following discharge, and nonadherence to the treatment plan. These factors are likely over‐represented in the Bellevue patient population compared to that of private hospital settings and no doubt exacerbate the readmission rates in urban hospitals treating patients with a high degree of social and behavioral health needs. Although patient‐related factors such as AMA discharges and substance abuse are potentially addressable, our reviewers felt that these were not preventable based on current knowledge and standards of care.
Studies that have attempted to classify readmissions as potentially avoidable have not shown good interobserver agreement when more than 1 reviewer was involved.[9, 10] Additionally, there is not a validated tool available to classify types of readmissions. By using a pilot sample of 84 cases to develop the model, confirming the accuracy of the chart by personally interviewing a sample of readmitted patients for comparison, and by employing experienced inpatient attending physicians to perform the reviews, we were able to develop an algorithm that achieved substantial reliability in assigning each readmission into 1 of 5 distinct categories.
Our literature search revealed only a single study that attempted to classify readmissions in a similar manner. Readmissions within 6 months at 9 Veterans Affairs hospitals were classified into causal categories of systems, provider, and patient etiology.[9] Overall, 34% of readmissions were deemed to be preventable compared to 24% in our study. Most readmissions (68%) were due to a worsening of a clinical condition, 4.5% were attributed to the admitting provider having too low a threshold to justify admission, and 2.7% were due to the patient not abstaining from drugs or alcohol. Though the study design and patient population differed from our own, the similarities in methods and results lend validity to the results and conclusions of our study.
Another limitation of our study is that readmissions to other hospitals were not included. In this respect, our estimate of the rate of readmission was an understatement of the true value. Nonetheless, the categorization of causes for readmission was not likely to be affected by the site of the second admission. Another limitation of this study was the small number of subjects reviewed relative to other studies that analyzed demographics and risk factors in large databases of readmissions.[12] However, the depth of the present review provides an understanding of the sequence of events leading to the readmission and permits development of strategies to prevent their occurrence.
We identified mechanisms of readmissions that can lay the groundwork for future interventions and safely reduce readmissions rates at little cost. To reduce admissions that may not be medically necessary, the narratives presented in the supplementary appendix suggest that improvement in communication between the admitting provider for the readmission and a provider familiar with the patient could have led to avoidance of the readmission. Similarly, enhanced communication to receiving nursing facilities would decrease the chances of the patient being immediately sent back as occurred multiple times in our cohort. Formal mandatory assessment of functional status for vulnerable patients would identify patients who may not be fully ready for discharge.
In conclusion, we found through detailed chart review of patients readmitted within 7 days to an urban teaching hospital that the majority of readmissions were not avoidable and were due to unpredictable complications of the primary diagnosis from the index hospitalization or a condition unrelated to the initial stay. This conclusion, in concurrence with those of other studies,[8, 10] questions the value of a readmission as a valid metric of quality though supports further improvements in hospital systems to reduce preventable readmissions.
Disclosure
Nothing to report.
Unplanned hospital readmissions are regarded as a core measure of quality of care and may comprise a large avoidable cause of healthcare expenditures.[1, 2, 3, 4, 5] An estimated 20% of Medicare patients who are discharged from a hospital are readmitted within 30 days.[1, 6] This has led the Centers for Medicare & Medicaid Services and other payers to reduce reimbursements for unplanned 30‐day hospital readmissions.
Efforts to decrease readmission rates have been hampered by ineffective risk prediction models, and strategies to reduce readmissions have found limited success.[7] Understanding the mechanism of readmissions is necessary for accurate prediction and prevention. This can be achieved only through analysis of patient data and medical narratives obtained from patient interviews or detailed chart reviews.[8] Studies attempting to identify mechanisms of readmission using narrative chart reviews have been limited by small sample size, highly selected patient samples, and poor interobserver agreement.[8, 9, 10]
Our objective in this study was to identify specific mechanisms and risk factors of unplanned readmissions from the medicine service of a large urban hospital by reviewing medical charts for each case. Given the inverse relationship between time since discharge from the initial admission and the probability of an avoidable readmission,[8] we focused our review on 7‐day readmissions.
METHODS
Setting
The study took place within Bellevue Hospital Center, an 800‐bed teaching hospital that serves a culturally and racially diverse inner‐city population in New York City. Bellevue is 1 of 11 acute‐care facilities managed by Health and Hospitals Corporation. The Bellevue inpatient medicine service is staffed by board‐certified general internists (180 beds), oncologists (20 beds), and pulmonologists (20 beds), who function as hospitalists in supervision of housestaff and physicians. Their efforts are supported by case managers and social workers who meet every weekday with physicians and nurses to plan discharges as multidisciplinary teams. Weekend support is minimal, consisting of an on‐call social worker to assist with urgent matters only. Upon discharge, patients are referred directly to 1 or more of Bellevue's outpatient clinics or to their own primary care providers outside Bellevue. There is a single electronic medical record for Bellevue, which spans the full range of care provided in the outpatient clinics, emergency department, and inpatient service.
Patients
Eligible patients were discharged from the Bellevue medical service between July 1, 2010 and July 1, 2011, and readmitted to any service at Bellevue within 7 days. During the study period, there were 8421 discharges. Discharges included transfers to other hospitals or rehabilitation centers, and excluded patients who died during hospitalization. Of these, 6781 were not readmitted, 1581 were readmitted within 30 days (18.8%), and 549 were readmitted within 7 days (6.5%). From the latter group, 20 consecutive cases were excluded after use in an exploratory pilot study, 84 consecutive cases were excluded after use in a formal pilot study, leaving 445 cases, from which 400 cases were randomly selected via terminal digit of the medical record number. We selected 400 chart reviews as a reasonable sample size to provide a 95% confidence interval, with a margin of error less than 4.9% for any of the proportions of the 5 readmission categories. Of these, 65 were determined to be planned readmissions (eg, for elective chemotherapy). The remaining 335 unplanned 7‐day readmissions served as the subjects of this review. The study was approved by the institutional review board of New York University School of Medicine.
Reviewers
Three of the authors of this paper (Drs. Janjigian, Bails, and Link) were actively practicing board‐certified internal medicine physicians with 7, 19, and 26 years, respectively, of postresidency clinical experience during the review period of this study. Every case was reviewed by 2 investigators. One author (Drs. Janjigian) reviewed readmissions from the first 6 months of the calendar year, the second (Dr. Bails) reviewed readmissions from the last 6 months, and the third (Dr. Link) reviewed all 335 readmissions.
Data Collection
Using the electronic medical record, each readmission was reviewed with the intent to identify the sequence of events leading up to the readmission, most commonly achieved by analyzing the discharge summary from the initial admission and the admission note from the second admission. Further chart review was completed as necessary to establish the clearest narrative and to classify the readmission into 1 of 5 categories based on the cause. Narratives are defined here as the sequence of events leading to the readmission as determined by chart review and not by patient interviews. Narratives were recorded for each case to assist with understanding how each author determined the classification, and were used when disagreements required group consensus. Time spent on individual chart reviews varied widely, from 1 to 30 minutes, depending on the complexity of each case. For example, an against medical advice (AMA) discharge could be immediately identified in the medical record, whereas a determination that an incomplete workup was conducted would require reviewing the admission note from the readmission, the discharge summary from the index admission, review of progress and consult notes, and even vital signs, labs, and radiology.
An algorithm for classifying contributory causes of readmission into 1 of 5 categories was created from narratives compiled from a pilot of 84, 7‐day readmissions to Bellevue during the previous year. Six readmitted patients were interviewed by a study author during this pilot phase. These narratives were determined by consensus of the authors to provide no additional relevant information from that obtained through chart review alone. The 5 categories are identified in Figure 1 as follows:
- Second admission was not medically necessary.
- Second admission followed an elopement (patient left without knowledge of the hospital staff) or discharge AMA during the first admission.
- Second admission was caused by a deficiency in the discharge process of the first admission, attributable to the hospital system or providers.
- Second admission was caused by a factor attributable to the patient including substance use or nonadherence to the treatment plan from the first admission.
- Second admission was related to a complication of the primary disease or its treatment or an unrelated condition that could not reasonably have been predicted or prevented by a competent physician meeting the standard of care.
Categories 3, 4, and 5 were further divided into more specific subcategories as shown in Figure 1.

Each readmission was assigned a single category from the algorithm using a stepwise process in which a higher‐order cause excluded consideration of a downstream category. For example, if the second admission was not medically necessary (category 1), an incorrect decision to readmit the patient was considered the primary cause of the readmission, and no consideration was given to categories 2 through 5. In this manner, each patient was assigned to a single category. We considered readmissions attributable to provider error (categories 1 and 3) to be avoidable. Examples of readmissions in each category with narratives are shown in the Supporting Information, Appendix 1, in the online version of this article. Discrepancies in classification were resolved by consensus of all authors.
Statistical Analysis
Unweighted kappa values were measured to assess agreement between authors in the assignment of the major category among the 5 choices in the algorithm. [2] tests were used to compare categorical variables between 2 groups (readmitted vs not readmitted) or between several groups (5 categories of readmissions), whereas Kruskal‐Wallis tests were used for continuous variables.
Only the first readmission was used in analysis of patient characteristics when multiple readmissions occurred for an individual patient. Unique patients were used for analysis of nonreadmitted patients. The generalized estimating equation method was used to adjust for correlations between multiple readmissions within patients.
RESULTS
During this period, 270 patients accounted for 335 readmissions. Characteristics of patients readmitted within 7 days are shown in Table 1 and compared with those of patients who were not readmitted during the same study period. Patients who were readmitted were more likely to have had a longer length of stay during the first admission.
| Characteristic | Not Readmitted, n=6,781 | Readmitted, n=270 | P Value |
|---|---|---|---|
| |||
| Male gender (% of category) | 4,224 (62.3%) | 180 (66.7%) | 0.15 |
| Mean age, y (SD) | 56.1 (16.3) | 55.1 (16.3) | 0.65 |
| Median initial LOS [interquartile range] | 3 [2, 6] | 4 [2, 9] | 0.002 |
| Mean days between admissions (SD) | NA | 3.8 (2.1) | NA |
| AMA discharge (% of category) | 413 (6.1%) | 20 (7.4%) | 0.38 |
Results of categorization of readmission are shown in Table 2. Readmissions related to the discharge process (category 3) were further divided into subcategories (Table 3). Category 5 (unpredictable/unpreventable complication of primary diagnosis or unrelated event) constituted the highest percentage of readmissions at 46%, followed by category 4 (patient behavior) at 19%, category 3 (discharge process deficiency) at 17%, category 2 (AMA) at 12%, and category 1 (unnecessary admission) at 7%. Readmissions designated as preventable (categories 1 and 3) accounted for 24% of all readmissions. Readmissions due to patient factors (categories 2 and 4) accounted for 31% of all readmissions. Notably, 21% of all readmissions were due to patients who eloped or left AMA during the first discharge or who returned because of substance abuse during the interim (categories 2 and 4a). Among the preventable readmissions, the most commonly designated cause of readmission was a perceived premature discharge (category 3b2), accounting for 6% of all readmissions.
| Category 1: Second Admission Not Medically Necessary | Category 2: First Admission AMA | Category 3: Deficiency in the Discharge Process | Category 4: Patient Behavior | Category 5: Unpredictable Complication of Primary or Alternate Diagnosis | P Value* | |
|---|---|---|---|---|---|---|
| ||||||
| Total (%) | 22 (6.6%) | 39 (11.6%) | 56 (16.7%) | 63 (18.8%) | 155 (46.3%) | |
| Male (%) | 11 (50.0%) | 29 (74.4%) | 38 (67.9%) | 54 (85.7%) | 91 (58.7%) | 0.005 |
| Mean age, y (SD) | 61.8 (13.7) | 48.1 (13.2) | 58.6 (14.4) | 53.3 (11.8) | 55.1 (17.7) | 0.004 |
| Median LOS [IQR] | 2.5 [2.0, 7.0] | 2.0 [1.0, 6.0] | 5.0 [2.0, 8.5] | 4.0 [2.0, 6.0] | 5.0 [2.0, 10.0] | 0.03 |
| Mean days between admissions (SD) | 3.8 (2.2) | 3.1 (2.2) | 3.3 (2.0) | 3.8 (2.1) | 4.1 (2.1) | 0.27 |
| Category | Description | No. | % of Total |
|---|---|---|---|
| 3a1 | Overdosing of a prescribed medication | 3 | 0.9 |
| 3a2 | Underdosing of a prescribed medication | 5 | 1.5 |
| 3a3 | Adverse medication effect | 2 | 0.6 |
| 3b1 | Inadequate functional status | 3 | 0.9 |
| 3b2 | Premature discharge | 20 | 6.0 |
| 3c1 | Patient unable to fill prescriptions | 9 | 2.7 |
| 3c2 | Follow‐up arrangements inadequate | 6 | 1.8 |
| 3c3 | Discharge setting not appropriate | 5 | 1.5 |
| 3c4 | Inadequate communication of plan to receiving facility | 2 | 0.6 |
| 3c5 | Other | 1 | 0.3 |
| 4a | Patient behaviorsubstance use | 30 | 9 |
| 4b | Patient behavioradherence to discharge plan | 30 | 9 |
| 4c | Patient behaviorrefusal of discharge plan | 3 | 0.9 |
| 5a | Disease complication | 103 | 30.7 |
| 5b | Unrelated condition | 52 | 15.5 |
Variance was statistically significant across major categories for gender, mean age, and median length of stay. The interobserver level of agreement across the 5 major categories was substantial among both pairs of reviewers (Table 4).
| Pair of Reviewers | No. of Readmissions Reviewed | No. of Agreements (%) | Unweighted Kappa |
|---|---|---|---|
| Dr. LinkDr. Bails | 135 | 113 (83.7) | 0.78 |
| Dr. LinkDr. Janjigian | 200 | 163 (81.5) | 0.72 |
The 46 patients who had more than 1, 7‐day readmission during this study period were responsible for 106 readmissions. The majority of this group were readmitted twice (78%), with a range of 2 to 5 readmissions. Within this group, 24% were considered preventable readmissions (8 from category 1, 17 from category 3), and 76% were considered not preventable (10 from category 2, 27 from category 4, and 44 from category 5).
DISCUSSION
The purpose of this retrospective review was to identify causes of unplanned 7‐day readmissions after discharge from the medical service of a large urban teaching hospital. Rather than focus on risk factors for readmissions, which other studies have done, we reviewed charts of readmitted patients using a novel categorization algorithm to group patients into common mechanisms that elucidate why a particular patient was readmitted. By examining the chart in detail, we were able to identify etiologies of readmission that are potentially avoidable.
Some authors have questioned the use of readmissions as a measurement of the quality of care a hospital provides due to the high proportion of unavoidable readmissions in a given sample.[8, 10] We hoped to identify systems errors that could be targets of quality improvement initiatives, and therefore chose to focus entirely on 7‐day readmissions as these have been shown to be more preventable than 30‐day readmissions.[8] We had the ability to review any aspect of the medical chart (eg, vitals or labs on discharge, any clinical note), which provided the highest probability of discovering a systems error. Despite these efforts to identify preventable errors, we identified the most common mechanism of readmission as an unpredictable or unpreventable event related to the primary diagnosis or its treatment from the initial admission (category 5a, 30.7% of total readmissions). Review of examples from this category elucidates how an unpredictable readmission could occur within such a short time frame (see Supporting Information, Appendix 1, in the online version of this article). The 7‐day window precluded identification of clinic access barriers, thereby eliminating from analysis 1 mechanism for preventable readmissions.
Nonetheless, our study demonstrates room for improvement in provider behavior and hospital systems related to the discharge process. Nearly a quarter of all readmissions and the majority of preventable readmissions were related to systems issues, such as timing and coordination of the first discharge, and lack of medical necessity for the second admission (see Supporting Information, Appendix 1, in the online version of this article). Prior studies found that shorter length of stay was associated with increased preventable readmissions, a finding that our study does not support.[10, 11] We suspect that patients in this group had longer lengths of stay during the index hospitalization due to complexity of medical illness, limited social support network, or lack of insurance, among other factors, that exposed flaws in systems processes and provider judgment. The mechanisms of readmission related to discharge planning that we identified in this study, including comprehensiveness of care, coordination of care, and medication administration, all represent potential opportunities for intervention.
Of note, there was a high percentage of readmissions attributable to patient behaviors, such as AMA discharges, substance abuse following discharge, and nonadherence to the treatment plan. These factors are likely over‐represented in the Bellevue patient population compared to that of private hospital settings and no doubt exacerbate the readmission rates in urban hospitals treating patients with a high degree of social and behavioral health needs. Although patient‐related factors such as AMA discharges and substance abuse are potentially addressable, our reviewers felt that these were not preventable based on current knowledge and standards of care.
Studies that have attempted to classify readmissions as potentially avoidable have not shown good interobserver agreement when more than 1 reviewer was involved.[9, 10] Additionally, there is not a validated tool available to classify types of readmissions. By using a pilot sample of 84 cases to develop the model, confirming the accuracy of the chart by personally interviewing a sample of readmitted patients for comparison, and by employing experienced inpatient attending physicians to perform the reviews, we were able to develop an algorithm that achieved substantial reliability in assigning each readmission into 1 of 5 distinct categories.
Our literature search revealed only a single study that attempted to classify readmissions in a similar manner. Readmissions within 6 months at 9 Veterans Affairs hospitals were classified into causal categories of systems, provider, and patient etiology.[9] Overall, 34% of readmissions were deemed to be preventable compared to 24% in our study. Most readmissions (68%) were due to a worsening of a clinical condition, 4.5% were attributed to the admitting provider having too low a threshold to justify admission, and 2.7% were due to the patient not abstaining from drugs or alcohol. Though the study design and patient population differed from our own, the similarities in methods and results lend validity to the results and conclusions of our study.
Another limitation of our study is that readmissions to other hospitals were not included. In this respect, our estimate of the rate of readmission was an understatement of the true value. Nonetheless, the categorization of causes for readmission was not likely to be affected by the site of the second admission. Another limitation of this study was the small number of subjects reviewed relative to other studies that analyzed demographics and risk factors in large databases of readmissions.[12] However, the depth of the present review provides an understanding of the sequence of events leading to the readmission and permits development of strategies to prevent their occurrence.
We identified mechanisms of readmissions that can lay the groundwork for future interventions and safely reduce readmissions rates at little cost. To reduce admissions that may not be medically necessary, the narratives presented in the supplementary appendix suggest that improvement in communication between the admitting provider for the readmission and a provider familiar with the patient could have led to avoidance of the readmission. Similarly, enhanced communication to receiving nursing facilities would decrease the chances of the patient being immediately sent back as occurred multiple times in our cohort. Formal mandatory assessment of functional status for vulnerable patients would identify patients who may not be fully ready for discharge.
In conclusion, we found through detailed chart review of patients readmitted within 7 days to an urban teaching hospital that the majority of readmissions were not avoidable and were due to unpredictable complications of the primary diagnosis from the index hospitalization or a condition unrelated to the initial stay. This conclusion, in concurrence with those of other studies,[8, 10] questions the value of a readmission as a valid metric of quality though supports further improvements in hospital systems to reduce preventable readmissions.
Disclosure
Nothing to report.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , . The rate and cost of hospital readmissions for preventable conditions. Med Care Res Rev. 2004;61(2):225–240.
- Centers for Medicare and Medicaid Services. 9th scope of work version #080108‐0. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/QualityImprovementOrgs/downloads/9thSOWBaseContract_C_08‐01‐2008_2_.pdf. Accessed April 10, 2014.
- , . Hospital readmissions in the Medicare population. N Engl J Med. 1984;311(21):1349–1353.
- U.S. Department of Health 155(8):520–528.
- , , , et al. Incidence of potentially avoidable urgent readmissions and their relation to all‐cause urgent readmissions. CMAJ. 2011;183(14):E1067–E1072.
- , , , et al. Classifying general medicine readmissions. Are they preventable? Veterans Affairs Cooperative Studies in Health Services Group on Primary Care and Hospital Readmissions. J Gen Intern Med. 1996;11(10):597–607.
- , , , . Hospitalists assess the causes of early hospital readmissions. J Hosp Med. 2011;6(7):383–388.
- , , . Early readmissions to the department of medicine as a screening tool for monitoring quality of care problems. Medicine. 2008;87(5):294–300.
- , , , et al. Incidence and predictors of all and preventable adverse drug reactions in frail elderly persons after hospital stay. J Gerontol A Biol Sci Med Sci. 2006;61(5):511–515.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , . The rate and cost of hospital readmissions for preventable conditions. Med Care Res Rev. 2004;61(2):225–240.
- Centers for Medicare and Medicaid Services. 9th scope of work version #080108‐0. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/QualityImprovementOrgs/downloads/9thSOWBaseContract_C_08‐01‐2008_2_.pdf. Accessed April 10, 2014.
- , . Hospital readmissions in the Medicare population. N Engl J Med. 1984;311(21):1349–1353.
- U.S. Department of Health 155(8):520–528.
- , , , et al. Incidence of potentially avoidable urgent readmissions and their relation to all‐cause urgent readmissions. CMAJ. 2011;183(14):E1067–E1072.
- , , , et al. Classifying general medicine readmissions. Are they preventable? Veterans Affairs Cooperative Studies in Health Services Group on Primary Care and Hospital Readmissions. J Gen Intern Med. 1996;11(10):597–607.
- , , , . Hospitalists assess the causes of early hospital readmissions. J Hosp Med. 2011;6(7):383–388.
- , , . Early readmissions to the department of medicine as a screening tool for monitoring quality of care problems. Medicine. 2008;87(5):294–300.
- , , , et al. Incidence and predictors of all and preventable adverse drug reactions in frail elderly persons after hospital stay. J Gerontol A Biol Sci Med Sci. 2006;61(5):511–515.
© 2015 Society of Hospital Medicine
TEE Impact on Managing Stroke Patients
Specific transesophageal echocardiography (TEE) findings associated with stroke include cardiac thrombi (particularly left atrial appendage [LAA]), left atrial spontaneous echo contrast, interatrial septal anomalies (particularly patent foramen ovale [PFO]), and atheromatous disease of the aorta. In younger patients (aged <50 years) with stroke of uncertain etiology, TEE is often recommended because of reported higher yield than transthoracic echocardiogram (TTE), particularly in detecting PFO or atrial septal aneurysm (ASA).[1]
Aside from oral anticoagulation in patients with an intracardiac thrombus, current guidelines and scientific evidence do not support specific therapeutic interventions for the other TEE findings. For example, the most effective therapy for stroke prevention with findings of aortic arch plaque remains uncertain. In addition, the very rare patient presenting with stroke from a cardiac tumor, which is generally visible on TTE, might benefit from surgical removal.[2]
We sought to examine the benefit of performing TEE after a normal TTE in patients over age 50 years admitted with a stroke of uncertain etiology. We hypothesized that there would be minimal change in management based on TEE findings after a normal TTE in older patients hospitalized with an unexplained stroke.
METHODS
Over a 4‐year period from 2009 to 2012, all patients over the age of 50 years admitted to our community‐based teaching hospital with a primary diagnosis of ischemic stroke were identified and retrospectively screened by review of our institutional echocardiography database during this time period. Stroke diagnosis had to be confirmed with acute or subacute ischemia on brain magnetic resonance imaging. Patients with an indication for anticoagulation or who had a known history of atrial fibrillation or flutter were excluded. Patients were monitored with continuous telemetry during hospital admission and were also excluded if they developed atrial fibrillation or flutter after admission. Additionally, patients were excluded if a neurologist‐directed evaluation revealed another etiology for the stroke.
A TTE acquired in all patients was performed according to Intersocietal Commission for the Accreditation of Echocardiography Laboratories standards and included 2‐dimensional, color Doppler, continuous wave, and pulse wave data. Images were obtained in the parasternal long and short axis, apical 4‐chamber, 2‐chamber, and long axis views. An abnormal TTE was defined as a study with a prosthetic valve, abnormal left ventricular (LV) systolic function, an intracardiac mass, intracardiac shunt, or severe valvular heart disease, as these significant findings may explain stroke.
Standardized TEE images were obtained with midesophageal 4‐chamber, mitral commissural, 2‐chamber, long axis, ascending aorta long axis, aortic valve short axis, right ventricular inflow‐outflow, and bicaval views. Detailed multiplanar evaluation of the LAA was performed. If no interatrial shunt was visualized with color flow Doppler in the bicaval view, agitated intravenous saline was administered for further evaluation. Additional standard images were obtained of the descending aorta and aortic arch in the short and long axis. Transgastric images were obtained when feasible or necessary.
The study was submitted to our institutional review board. As no patient identifiers were stored, and we used previously existing data from an institutional echocardiography database to conduct the study, it was determined to be exempt.
Statistical analysis was performed by recording the prevalence of each potential cardiac source of embolism.
RESULTS
Of the 853 consecutive patients screened, 456 were excluded because of atrial fibrillation, atrial flutter, or another etiology of stroke. An additional 134 patients were excluded with an abnormal TTE or if a TEE was not performed. The remaining 263 patients were analyzed based on TEE findings (Figure 1).
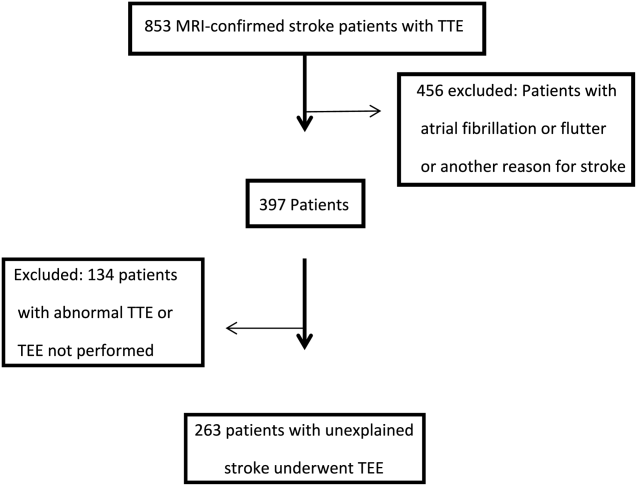
The mean age was 66.7 years (range, 5091 years), and 42.5% were female. A possible etiology of stroke (Table 1) discovered included complex plaque of the ascending aorta or arch 44/263 (16.7%), PFO 18/263 (6.8%), atrial septal aneurysm 25/263 (9.5%), and both ASA and PFO in 11/263 (4.2%), and spontaneous contrast was seen in the left atrium or LAA in 13/263 (4.9%) patients. One patient had a thrombus in the LAA for which anticoagulation was prescribed. No other intracardiac masses were identified.
| Potential Source | No. (%) |
|---|---|
| |
| Atrial septal aneurysm | 25 (5.3%) |
| Patent foramen ovale | 18 (2.7%) |
| Atrial septal aneurysm and patent foramen ovale | 11 (4.2%) |
| Complex aortic plaque | 44 (16.7%) |
| Spontaneous contrast | 13 (4.9%) |
| Left atrial appendage thrombus* | 1 (0.4%) |
| Total | 112 (42.6%) |
Overall, 42.6% of patients had a TEE finding which could explain the etiology of stroke or transient ischemic attack (TIA), but only 1 patient (0.4%) had a finding that changed therapy. Follow‐up was available at 6 months for 85 patients, and 13 (15%) of these patients had been discovered to develop atrial fibrillation in the interim.
DISCUSSION
Our study retrospectively analyzed the utility of TEE in patients over age 50 years admitted with ischemic stroke without a clear etiology. We found that TEE provides significant incremental diagnostic benefit as compared to TTE in identifying a possible etiology of stroke in these patients. This is consistent with prior studies showing a high diagnostic yield of TEE in patient with ischemic stroke of uncertain etiology.[3] However, in our study, based on current guidelines, virtually none of these findings directly altered patient management.
The 2014 guidelines for secondary stroke prevention recommend antiplatelet and statin therapy (in addition to lifestyle modification, smoking cessation, and blood glucose and blood pressure control) as a standard medical regimen in patients with stroke or TIA of uncertain etiology. The finding of aortic arch atheroma does not warrant supplementary treatment in addition to an antiplatelet and statin according to current guidelines. Atherosclerosis of the aortic arch is an important source of cerebral embolism, particularly in cases where plaque is >4 mm in size.[4] A recent study by Amarenco et al., comparing efficacy of combined antiplatelet therapy (clopidogrel and aspirin) to warfarin in recurrent stroke prevention in patients with >4 mm aortic arch plaque, showed nonsignificant reduction in rate of recurrent stroke with dual antiplatelet therapy.[5] However, optimal therapy for these patients still remains uncertain beyond standard stroke‐prevention treatment. Although there are emerging data on therapeutic options in patients with complex atheroma, there is currently no specific guideline‐recommended therapy or consensus among stroke neurologists. Potentially, if an individual practitioner had a strong feeling on therapeutic modifications based on the presence of complex aortic arch atheroma, the TEE would have value to their patient. However, in our study, which had a prevalence of 16.8% of complex plaque of the ascending aorta or arch, there were no therapeutic changes based on this finding. This reinforces the limited value of this test that we observed in our study population.
Anticoagulation has not been shown to be superior to aspirin in patients with PFO (with or without ASA), and recent studies showed no benefit of procedural PFO closure compared to best medical management for stroke prevention (Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment [RESPECT], Evaluation of the STARFlex Septal Closure System in Patients with a Stroke and/or Transient Ischemic Attack due to Presumed Paradoxical Embolism through a Patent Foramen Ovale [CLOSURE I]).[6, 7] However, a patient with a PFO and deep vein thrombosis would benefit from anticoagulation and consideration of PFO closure.[8] This rare entity could be excluded with a simple lower extremity duplex without the need for a TEE, which does come with a small risk of complications related to anesthesia and local oropharyngeal trauma as well as discomfort to the patient and increased cost. Spontaneous echo contrast is not an independent indication for anticoagulation. If spontaneous contrast were associated with mitral stenosis and an embolic event, then anticoagulation would be indicated.[9] Mitral stenosis is easily diagnosed with TTE.
LAA or left atrial thrombus is the predominant finding exclusive to TEE that would change management for secondary stroke prevention, specifically anticoagulation. Fifteen studies representing over 3000 patients in a 2014 meta‐analysis reported the prevalence of left atrial or LAA thrombus in patients aged 55 years with a cryptogenic stroke to be 4%, with a range in the studies of 0% to 21.2%.[3] The wide range of prevalence of this finding is likely related to the prevalence of known atrial arrhythmias or structural heart disease in the population of patients included in the analysis. Left atrial or LAA thrombus in the absence of systolic dysfunction, severe valve disease, or known atrial fibrillation is exceedingly uncommon (0.3%).[10] It is likely that the few patients with left atrial or LAA thrombus without 1 of these conditions probably has undiagnosed paroxysmal atrial fibrillation. In previous studies that showed a high prevalence of left atrial or LAA thrombus, there was no mention of the presence or absence of LV dysfunction or severe valve disease in patients with left atrial or LAA thrombus. Additionally, these studies only required a 12‐lead electrocardiogram or did not specify the presence or duration of continuous rhythm monitoring.[11, 12, 13, 14] Several of the studies with high incidence of left atrial or LAA thrombus specifically stated that some of these patients were known to have atrial fibrillation.[11, 13]
Approximately 8% of patients admitted with stroke are found to have atrial fibrillation only after admission with continuous electrocardiogram monitoring. The detection rate is nearly half if monitoring is limited to 24 hours instead of several days. Overall, detection rates of atrial fibrillation following stroke are relatively low during initial hospitalization.[15] More intense monitoring for atrial fibrillation in patients with a stroke of uncertain etiology with the use of a subcutaneous implantable cardiac monitor increases the detection rate to 12.4% at 1 year, and increases with longer monitoring time.[16] Therefore, identification of older stroke patients without significant stroke risk factors may be candidates for longer‐term cardiac monitoring to increase yield for detection of atrial fibrillation. Currently, continuous electrocardiographic monitoring of patients for the duration of their hospitalization and up to 30 days afterward is recommended.[8]
Our study differs from prior studies that showed a much higher prevalence of LAA or left atrial thrombus in 2 important ways. Patients with severe valve disease or LV dysfunction were excluded on the basis of TTE. Additionally, our patients underwent continuous electrocardiographic monitoring for the duration of their hospitalization and were excluded with a prior history or newly discovered atrial fibrillation or flutter. Our intention was to examine the value of adding TEE when no other etiology of stroke was identified. Value can be defined as healthcare outcomes achieved per dollar spent. Our study was not designed to look at long‐term outcomes; rather, we used immediate change in patient management as a surrogate.
There are several limitations to our study that must be noted. This was a single‐center study potentially creating a bias as less stringent selection of patients undergoing TEE may be the practice at other institutions. This analysis was retrospective; therefore, there may have been bias as to which patients were selected to undergo TEE. Additionally, stroke subtype was not specified, and the pretest probability of a cardioembolic source differs based on subtype. Last, we focused this study on immediate changes in clinical management prompted by TEE results, and did not assess patient perceptions of TEE value related to enhanced knowledge about the etiology of their stroke; this area represents an opportunity for further research.
CONCLUSIONS
TEE provides a substantial increase in possible explanation of stroke etiology in patients over age 50 years admitted with a stroke of uncertain cause and a normal TTE. However, there is minimal incremental value in regard to change in therapeutic management in these patients. In a time of increased focus on providing cost effective healthcare, our findings suggest that the need for TEE in this stroke population should be more closely examined.
Disclosure: Nothing to report.
- , , . Influence of transesophageal echocardiogram on therapy and prognosis in young patients with TIA or ischemic stroke. Neth Heart J. 2009;17:373–377.
- , , , et al. Diagnosis of Heart Tumors by Transesophageal Echocardiography: a multicentre study in 154 patients. Eur Heart J. 1993;14:1223–1228.
- , , , , , . Transesophageal echocardiography in patients with cryptogenic ischemic stroke: a systematic review. Am Heart J. 2014;168:706–712.
- , , . Protruding atheromas in the thoracic aorta and systemic embolization. Ann Intern Med. 1991;115:423–427.
- , , , et al.; The Aortic Arch Related Cerebral Hazard Trial Investigators. Clopidogrel plus aspirin versus warfarin in patients with stroke and aortic arch plaques. Stroke. 2014;45:1248–1257.
- , , , et al.; RESPECT Investigators. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092–1100.
- , , , et al.; CLOSURE I Investigators. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–999.
- , , , et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236.
- , , , et al. 2014 AHA/ACA guideline for the management of patients with valvular heart disease. J Am Coll Cardiol. 2014; 63:e57–e185.
- , , , . Clinical and echocardiographic characteristics of patients with left atrial thrombus and sinus rhythm: experience in 20 643 consecutive transesophageal echocardiographic examinations. Circulation. 2002;105(1):27–31.
- , , , et al. Usefulness of transesophageal echocardiography in unexplained cerebral ischemia. Am J Cardiol. 1993;72:1448–1452.
- , , , , , . Transesophageal echocardiography in patients with recent stroke and normal carotid arteries. Am J Cardiol. 2001;88:820–823.
- , , , et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke. 2006;37:2531–2534.
- , , , , , . Age‐dependent prevalence of cardioembolic sources detected by TEE: diagnostic and therapeutic implications. Echocardiography. 1997;14:597–606.
- , , , et al. Continuous stroke unit electrocardiographic monitoring versus 24‐hour Holter electrocardiography for detection of paroxysmal atrial fibrillation after stroke. Stroke. 2012;43:2689–2694.
- , , , et al.; CRYSTAL AF Investigators. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478–2486.
Specific transesophageal echocardiography (TEE) findings associated with stroke include cardiac thrombi (particularly left atrial appendage [LAA]), left atrial spontaneous echo contrast, interatrial septal anomalies (particularly patent foramen ovale [PFO]), and atheromatous disease of the aorta. In younger patients (aged <50 years) with stroke of uncertain etiology, TEE is often recommended because of reported higher yield than transthoracic echocardiogram (TTE), particularly in detecting PFO or atrial septal aneurysm (ASA).[1]
Aside from oral anticoagulation in patients with an intracardiac thrombus, current guidelines and scientific evidence do not support specific therapeutic interventions for the other TEE findings. For example, the most effective therapy for stroke prevention with findings of aortic arch plaque remains uncertain. In addition, the very rare patient presenting with stroke from a cardiac tumor, which is generally visible on TTE, might benefit from surgical removal.[2]
We sought to examine the benefit of performing TEE after a normal TTE in patients over age 50 years admitted with a stroke of uncertain etiology. We hypothesized that there would be minimal change in management based on TEE findings after a normal TTE in older patients hospitalized with an unexplained stroke.
METHODS
Over a 4‐year period from 2009 to 2012, all patients over the age of 50 years admitted to our community‐based teaching hospital with a primary diagnosis of ischemic stroke were identified and retrospectively screened by review of our institutional echocardiography database during this time period. Stroke diagnosis had to be confirmed with acute or subacute ischemia on brain magnetic resonance imaging. Patients with an indication for anticoagulation or who had a known history of atrial fibrillation or flutter were excluded. Patients were monitored with continuous telemetry during hospital admission and were also excluded if they developed atrial fibrillation or flutter after admission. Additionally, patients were excluded if a neurologist‐directed evaluation revealed another etiology for the stroke.
A TTE acquired in all patients was performed according to Intersocietal Commission for the Accreditation of Echocardiography Laboratories standards and included 2‐dimensional, color Doppler, continuous wave, and pulse wave data. Images were obtained in the parasternal long and short axis, apical 4‐chamber, 2‐chamber, and long axis views. An abnormal TTE was defined as a study with a prosthetic valve, abnormal left ventricular (LV) systolic function, an intracardiac mass, intracardiac shunt, or severe valvular heart disease, as these significant findings may explain stroke.
Standardized TEE images were obtained with midesophageal 4‐chamber, mitral commissural, 2‐chamber, long axis, ascending aorta long axis, aortic valve short axis, right ventricular inflow‐outflow, and bicaval views. Detailed multiplanar evaluation of the LAA was performed. If no interatrial shunt was visualized with color flow Doppler in the bicaval view, agitated intravenous saline was administered for further evaluation. Additional standard images were obtained of the descending aorta and aortic arch in the short and long axis. Transgastric images were obtained when feasible or necessary.
The study was submitted to our institutional review board. As no patient identifiers were stored, and we used previously existing data from an institutional echocardiography database to conduct the study, it was determined to be exempt.
Statistical analysis was performed by recording the prevalence of each potential cardiac source of embolism.
RESULTS
Of the 853 consecutive patients screened, 456 were excluded because of atrial fibrillation, atrial flutter, or another etiology of stroke. An additional 134 patients were excluded with an abnormal TTE or if a TEE was not performed. The remaining 263 patients were analyzed based on TEE findings (Figure 1).

The mean age was 66.7 years (range, 5091 years), and 42.5% were female. A possible etiology of stroke (Table 1) discovered included complex plaque of the ascending aorta or arch 44/263 (16.7%), PFO 18/263 (6.8%), atrial septal aneurysm 25/263 (9.5%), and both ASA and PFO in 11/263 (4.2%), and spontaneous contrast was seen in the left atrium or LAA in 13/263 (4.9%) patients. One patient had a thrombus in the LAA for which anticoagulation was prescribed. No other intracardiac masses were identified.
| Potential Source | No. (%) |
|---|---|
| |
| Atrial septal aneurysm | 25 (5.3%) |
| Patent foramen ovale | 18 (2.7%) |
| Atrial septal aneurysm and patent foramen ovale | 11 (4.2%) |
| Complex aortic plaque | 44 (16.7%) |
| Spontaneous contrast | 13 (4.9%) |
| Left atrial appendage thrombus* | 1 (0.4%) |
| Total | 112 (42.6%) |
Overall, 42.6% of patients had a TEE finding which could explain the etiology of stroke or transient ischemic attack (TIA), but only 1 patient (0.4%) had a finding that changed therapy. Follow‐up was available at 6 months for 85 patients, and 13 (15%) of these patients had been discovered to develop atrial fibrillation in the interim.
DISCUSSION
Our study retrospectively analyzed the utility of TEE in patients over age 50 years admitted with ischemic stroke without a clear etiology. We found that TEE provides significant incremental diagnostic benefit as compared to TTE in identifying a possible etiology of stroke in these patients. This is consistent with prior studies showing a high diagnostic yield of TEE in patient with ischemic stroke of uncertain etiology.[3] However, in our study, based on current guidelines, virtually none of these findings directly altered patient management.
The 2014 guidelines for secondary stroke prevention recommend antiplatelet and statin therapy (in addition to lifestyle modification, smoking cessation, and blood glucose and blood pressure control) as a standard medical regimen in patients with stroke or TIA of uncertain etiology. The finding of aortic arch atheroma does not warrant supplementary treatment in addition to an antiplatelet and statin according to current guidelines. Atherosclerosis of the aortic arch is an important source of cerebral embolism, particularly in cases where plaque is >4 mm in size.[4] A recent study by Amarenco et al., comparing efficacy of combined antiplatelet therapy (clopidogrel and aspirin) to warfarin in recurrent stroke prevention in patients with >4 mm aortic arch plaque, showed nonsignificant reduction in rate of recurrent stroke with dual antiplatelet therapy.[5] However, optimal therapy for these patients still remains uncertain beyond standard stroke‐prevention treatment. Although there are emerging data on therapeutic options in patients with complex atheroma, there is currently no specific guideline‐recommended therapy or consensus among stroke neurologists. Potentially, if an individual practitioner had a strong feeling on therapeutic modifications based on the presence of complex aortic arch atheroma, the TEE would have value to their patient. However, in our study, which had a prevalence of 16.8% of complex plaque of the ascending aorta or arch, there were no therapeutic changes based on this finding. This reinforces the limited value of this test that we observed in our study population.
Anticoagulation has not been shown to be superior to aspirin in patients with PFO (with or without ASA), and recent studies showed no benefit of procedural PFO closure compared to best medical management for stroke prevention (Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment [RESPECT], Evaluation of the STARFlex Septal Closure System in Patients with a Stroke and/or Transient Ischemic Attack due to Presumed Paradoxical Embolism through a Patent Foramen Ovale [CLOSURE I]).[6, 7] However, a patient with a PFO and deep vein thrombosis would benefit from anticoagulation and consideration of PFO closure.[8] This rare entity could be excluded with a simple lower extremity duplex without the need for a TEE, which does come with a small risk of complications related to anesthesia and local oropharyngeal trauma as well as discomfort to the patient and increased cost. Spontaneous echo contrast is not an independent indication for anticoagulation. If spontaneous contrast were associated with mitral stenosis and an embolic event, then anticoagulation would be indicated.[9] Mitral stenosis is easily diagnosed with TTE.
LAA or left atrial thrombus is the predominant finding exclusive to TEE that would change management for secondary stroke prevention, specifically anticoagulation. Fifteen studies representing over 3000 patients in a 2014 meta‐analysis reported the prevalence of left atrial or LAA thrombus in patients aged 55 years with a cryptogenic stroke to be 4%, with a range in the studies of 0% to 21.2%.[3] The wide range of prevalence of this finding is likely related to the prevalence of known atrial arrhythmias or structural heart disease in the population of patients included in the analysis. Left atrial or LAA thrombus in the absence of systolic dysfunction, severe valve disease, or known atrial fibrillation is exceedingly uncommon (0.3%).[10] It is likely that the few patients with left atrial or LAA thrombus without 1 of these conditions probably has undiagnosed paroxysmal atrial fibrillation. In previous studies that showed a high prevalence of left atrial or LAA thrombus, there was no mention of the presence or absence of LV dysfunction or severe valve disease in patients with left atrial or LAA thrombus. Additionally, these studies only required a 12‐lead electrocardiogram or did not specify the presence or duration of continuous rhythm monitoring.[11, 12, 13, 14] Several of the studies with high incidence of left atrial or LAA thrombus specifically stated that some of these patients were known to have atrial fibrillation.[11, 13]
Approximately 8% of patients admitted with stroke are found to have atrial fibrillation only after admission with continuous electrocardiogram monitoring. The detection rate is nearly half if monitoring is limited to 24 hours instead of several days. Overall, detection rates of atrial fibrillation following stroke are relatively low during initial hospitalization.[15] More intense monitoring for atrial fibrillation in patients with a stroke of uncertain etiology with the use of a subcutaneous implantable cardiac monitor increases the detection rate to 12.4% at 1 year, and increases with longer monitoring time.[16] Therefore, identification of older stroke patients without significant stroke risk factors may be candidates for longer‐term cardiac monitoring to increase yield for detection of atrial fibrillation. Currently, continuous electrocardiographic monitoring of patients for the duration of their hospitalization and up to 30 days afterward is recommended.[8]
Our study differs from prior studies that showed a much higher prevalence of LAA or left atrial thrombus in 2 important ways. Patients with severe valve disease or LV dysfunction were excluded on the basis of TTE. Additionally, our patients underwent continuous electrocardiographic monitoring for the duration of their hospitalization and were excluded with a prior history or newly discovered atrial fibrillation or flutter. Our intention was to examine the value of adding TEE when no other etiology of stroke was identified. Value can be defined as healthcare outcomes achieved per dollar spent. Our study was not designed to look at long‐term outcomes; rather, we used immediate change in patient management as a surrogate.
There are several limitations to our study that must be noted. This was a single‐center study potentially creating a bias as less stringent selection of patients undergoing TEE may be the practice at other institutions. This analysis was retrospective; therefore, there may have been bias as to which patients were selected to undergo TEE. Additionally, stroke subtype was not specified, and the pretest probability of a cardioembolic source differs based on subtype. Last, we focused this study on immediate changes in clinical management prompted by TEE results, and did not assess patient perceptions of TEE value related to enhanced knowledge about the etiology of their stroke; this area represents an opportunity for further research.
CONCLUSIONS
TEE provides a substantial increase in possible explanation of stroke etiology in patients over age 50 years admitted with a stroke of uncertain cause and a normal TTE. However, there is minimal incremental value in regard to change in therapeutic management in these patients. In a time of increased focus on providing cost effective healthcare, our findings suggest that the need for TEE in this stroke population should be more closely examined.
Disclosure: Nothing to report.
Specific transesophageal echocardiography (TEE) findings associated with stroke include cardiac thrombi (particularly left atrial appendage [LAA]), left atrial spontaneous echo contrast, interatrial septal anomalies (particularly patent foramen ovale [PFO]), and atheromatous disease of the aorta. In younger patients (aged <50 years) with stroke of uncertain etiology, TEE is often recommended because of reported higher yield than transthoracic echocardiogram (TTE), particularly in detecting PFO or atrial septal aneurysm (ASA).[1]
Aside from oral anticoagulation in patients with an intracardiac thrombus, current guidelines and scientific evidence do not support specific therapeutic interventions for the other TEE findings. For example, the most effective therapy for stroke prevention with findings of aortic arch plaque remains uncertain. In addition, the very rare patient presenting with stroke from a cardiac tumor, which is generally visible on TTE, might benefit from surgical removal.[2]
We sought to examine the benefit of performing TEE after a normal TTE in patients over age 50 years admitted with a stroke of uncertain etiology. We hypothesized that there would be minimal change in management based on TEE findings after a normal TTE in older patients hospitalized with an unexplained stroke.
METHODS
Over a 4‐year period from 2009 to 2012, all patients over the age of 50 years admitted to our community‐based teaching hospital with a primary diagnosis of ischemic stroke were identified and retrospectively screened by review of our institutional echocardiography database during this time period. Stroke diagnosis had to be confirmed with acute or subacute ischemia on brain magnetic resonance imaging. Patients with an indication for anticoagulation or who had a known history of atrial fibrillation or flutter were excluded. Patients were monitored with continuous telemetry during hospital admission and were also excluded if they developed atrial fibrillation or flutter after admission. Additionally, patients were excluded if a neurologist‐directed evaluation revealed another etiology for the stroke.
A TTE acquired in all patients was performed according to Intersocietal Commission for the Accreditation of Echocardiography Laboratories standards and included 2‐dimensional, color Doppler, continuous wave, and pulse wave data. Images were obtained in the parasternal long and short axis, apical 4‐chamber, 2‐chamber, and long axis views. An abnormal TTE was defined as a study with a prosthetic valve, abnormal left ventricular (LV) systolic function, an intracardiac mass, intracardiac shunt, or severe valvular heart disease, as these significant findings may explain stroke.
Standardized TEE images were obtained with midesophageal 4‐chamber, mitral commissural, 2‐chamber, long axis, ascending aorta long axis, aortic valve short axis, right ventricular inflow‐outflow, and bicaval views. Detailed multiplanar evaluation of the LAA was performed. If no interatrial shunt was visualized with color flow Doppler in the bicaval view, agitated intravenous saline was administered for further evaluation. Additional standard images were obtained of the descending aorta and aortic arch in the short and long axis. Transgastric images were obtained when feasible or necessary.
The study was submitted to our institutional review board. As no patient identifiers were stored, and we used previously existing data from an institutional echocardiography database to conduct the study, it was determined to be exempt.
Statistical analysis was performed by recording the prevalence of each potential cardiac source of embolism.
RESULTS
Of the 853 consecutive patients screened, 456 were excluded because of atrial fibrillation, atrial flutter, or another etiology of stroke. An additional 134 patients were excluded with an abnormal TTE or if a TEE was not performed. The remaining 263 patients were analyzed based on TEE findings (Figure 1).

The mean age was 66.7 years (range, 5091 years), and 42.5% were female. A possible etiology of stroke (Table 1) discovered included complex plaque of the ascending aorta or arch 44/263 (16.7%), PFO 18/263 (6.8%), atrial septal aneurysm 25/263 (9.5%), and both ASA and PFO in 11/263 (4.2%), and spontaneous contrast was seen in the left atrium or LAA in 13/263 (4.9%) patients. One patient had a thrombus in the LAA for which anticoagulation was prescribed. No other intracardiac masses were identified.
| Potential Source | No. (%) |
|---|---|
| |
| Atrial septal aneurysm | 25 (5.3%) |
| Patent foramen ovale | 18 (2.7%) |
| Atrial septal aneurysm and patent foramen ovale | 11 (4.2%) |
| Complex aortic plaque | 44 (16.7%) |
| Spontaneous contrast | 13 (4.9%) |
| Left atrial appendage thrombus* | 1 (0.4%) |
| Total | 112 (42.6%) |
Overall, 42.6% of patients had a TEE finding which could explain the etiology of stroke or transient ischemic attack (TIA), but only 1 patient (0.4%) had a finding that changed therapy. Follow‐up was available at 6 months for 85 patients, and 13 (15%) of these patients had been discovered to develop atrial fibrillation in the interim.
DISCUSSION
Our study retrospectively analyzed the utility of TEE in patients over age 50 years admitted with ischemic stroke without a clear etiology. We found that TEE provides significant incremental diagnostic benefit as compared to TTE in identifying a possible etiology of stroke in these patients. This is consistent with prior studies showing a high diagnostic yield of TEE in patient with ischemic stroke of uncertain etiology.[3] However, in our study, based on current guidelines, virtually none of these findings directly altered patient management.
The 2014 guidelines for secondary stroke prevention recommend antiplatelet and statin therapy (in addition to lifestyle modification, smoking cessation, and blood glucose and blood pressure control) as a standard medical regimen in patients with stroke or TIA of uncertain etiology. The finding of aortic arch atheroma does not warrant supplementary treatment in addition to an antiplatelet and statin according to current guidelines. Atherosclerosis of the aortic arch is an important source of cerebral embolism, particularly in cases where plaque is >4 mm in size.[4] A recent study by Amarenco et al., comparing efficacy of combined antiplatelet therapy (clopidogrel and aspirin) to warfarin in recurrent stroke prevention in patients with >4 mm aortic arch plaque, showed nonsignificant reduction in rate of recurrent stroke with dual antiplatelet therapy.[5] However, optimal therapy for these patients still remains uncertain beyond standard stroke‐prevention treatment. Although there are emerging data on therapeutic options in patients with complex atheroma, there is currently no specific guideline‐recommended therapy or consensus among stroke neurologists. Potentially, if an individual practitioner had a strong feeling on therapeutic modifications based on the presence of complex aortic arch atheroma, the TEE would have value to their patient. However, in our study, which had a prevalence of 16.8% of complex plaque of the ascending aorta or arch, there were no therapeutic changes based on this finding. This reinforces the limited value of this test that we observed in our study population.
Anticoagulation has not been shown to be superior to aspirin in patients with PFO (with or without ASA), and recent studies showed no benefit of procedural PFO closure compared to best medical management for stroke prevention (Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment [RESPECT], Evaluation of the STARFlex Septal Closure System in Patients with a Stroke and/or Transient Ischemic Attack due to Presumed Paradoxical Embolism through a Patent Foramen Ovale [CLOSURE I]).[6, 7] However, a patient with a PFO and deep vein thrombosis would benefit from anticoagulation and consideration of PFO closure.[8] This rare entity could be excluded with a simple lower extremity duplex without the need for a TEE, which does come with a small risk of complications related to anesthesia and local oropharyngeal trauma as well as discomfort to the patient and increased cost. Spontaneous echo contrast is not an independent indication for anticoagulation. If spontaneous contrast were associated with mitral stenosis and an embolic event, then anticoagulation would be indicated.[9] Mitral stenosis is easily diagnosed with TTE.
LAA or left atrial thrombus is the predominant finding exclusive to TEE that would change management for secondary stroke prevention, specifically anticoagulation. Fifteen studies representing over 3000 patients in a 2014 meta‐analysis reported the prevalence of left atrial or LAA thrombus in patients aged 55 years with a cryptogenic stroke to be 4%, with a range in the studies of 0% to 21.2%.[3] The wide range of prevalence of this finding is likely related to the prevalence of known atrial arrhythmias or structural heart disease in the population of patients included in the analysis. Left atrial or LAA thrombus in the absence of systolic dysfunction, severe valve disease, or known atrial fibrillation is exceedingly uncommon (0.3%).[10] It is likely that the few patients with left atrial or LAA thrombus without 1 of these conditions probably has undiagnosed paroxysmal atrial fibrillation. In previous studies that showed a high prevalence of left atrial or LAA thrombus, there was no mention of the presence or absence of LV dysfunction or severe valve disease in patients with left atrial or LAA thrombus. Additionally, these studies only required a 12‐lead electrocardiogram or did not specify the presence or duration of continuous rhythm monitoring.[11, 12, 13, 14] Several of the studies with high incidence of left atrial or LAA thrombus specifically stated that some of these patients were known to have atrial fibrillation.[11, 13]
Approximately 8% of patients admitted with stroke are found to have atrial fibrillation only after admission with continuous electrocardiogram monitoring. The detection rate is nearly half if monitoring is limited to 24 hours instead of several days. Overall, detection rates of atrial fibrillation following stroke are relatively low during initial hospitalization.[15] More intense monitoring for atrial fibrillation in patients with a stroke of uncertain etiology with the use of a subcutaneous implantable cardiac monitor increases the detection rate to 12.4% at 1 year, and increases with longer monitoring time.[16] Therefore, identification of older stroke patients without significant stroke risk factors may be candidates for longer‐term cardiac monitoring to increase yield for detection of atrial fibrillation. Currently, continuous electrocardiographic monitoring of patients for the duration of their hospitalization and up to 30 days afterward is recommended.[8]
Our study differs from prior studies that showed a much higher prevalence of LAA or left atrial thrombus in 2 important ways. Patients with severe valve disease or LV dysfunction were excluded on the basis of TTE. Additionally, our patients underwent continuous electrocardiographic monitoring for the duration of their hospitalization and were excluded with a prior history or newly discovered atrial fibrillation or flutter. Our intention was to examine the value of adding TEE when no other etiology of stroke was identified. Value can be defined as healthcare outcomes achieved per dollar spent. Our study was not designed to look at long‐term outcomes; rather, we used immediate change in patient management as a surrogate.
There are several limitations to our study that must be noted. This was a single‐center study potentially creating a bias as less stringent selection of patients undergoing TEE may be the practice at other institutions. This analysis was retrospective; therefore, there may have been bias as to which patients were selected to undergo TEE. Additionally, stroke subtype was not specified, and the pretest probability of a cardioembolic source differs based on subtype. Last, we focused this study on immediate changes in clinical management prompted by TEE results, and did not assess patient perceptions of TEE value related to enhanced knowledge about the etiology of their stroke; this area represents an opportunity for further research.
CONCLUSIONS
TEE provides a substantial increase in possible explanation of stroke etiology in patients over age 50 years admitted with a stroke of uncertain cause and a normal TTE. However, there is minimal incremental value in regard to change in therapeutic management in these patients. In a time of increased focus on providing cost effective healthcare, our findings suggest that the need for TEE in this stroke population should be more closely examined.
Disclosure: Nothing to report.
- , , . Influence of transesophageal echocardiogram on therapy and prognosis in young patients with TIA or ischemic stroke. Neth Heart J. 2009;17:373–377.
- , , , et al. Diagnosis of Heart Tumors by Transesophageal Echocardiography: a multicentre study in 154 patients. Eur Heart J. 1993;14:1223–1228.
- , , , , , . Transesophageal echocardiography in patients with cryptogenic ischemic stroke: a systematic review. Am Heart J. 2014;168:706–712.
- , , . Protruding atheromas in the thoracic aorta and systemic embolization. Ann Intern Med. 1991;115:423–427.
- , , , et al.; The Aortic Arch Related Cerebral Hazard Trial Investigators. Clopidogrel plus aspirin versus warfarin in patients with stroke and aortic arch plaques. Stroke. 2014;45:1248–1257.
- , , , et al.; RESPECT Investigators. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092–1100.
- , , , et al.; CLOSURE I Investigators. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–999.
- , , , et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236.
- , , , et al. 2014 AHA/ACA guideline for the management of patients with valvular heart disease. J Am Coll Cardiol. 2014; 63:e57–e185.
- , , , . Clinical and echocardiographic characteristics of patients with left atrial thrombus and sinus rhythm: experience in 20 643 consecutive transesophageal echocardiographic examinations. Circulation. 2002;105(1):27–31.
- , , , et al. Usefulness of transesophageal echocardiography in unexplained cerebral ischemia. Am J Cardiol. 1993;72:1448–1452.
- , , , , , . Transesophageal echocardiography in patients with recent stroke and normal carotid arteries. Am J Cardiol. 2001;88:820–823.
- , , , et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke. 2006;37:2531–2534.
- , , , , , . Age‐dependent prevalence of cardioembolic sources detected by TEE: diagnostic and therapeutic implications. Echocardiography. 1997;14:597–606.
- , , , et al. Continuous stroke unit electrocardiographic monitoring versus 24‐hour Holter electrocardiography for detection of paroxysmal atrial fibrillation after stroke. Stroke. 2012;43:2689–2694.
- , , , et al.; CRYSTAL AF Investigators. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478–2486.
- , , . Influence of transesophageal echocardiogram on therapy and prognosis in young patients with TIA or ischemic stroke. Neth Heart J. 2009;17:373–377.
- , , , et al. Diagnosis of Heart Tumors by Transesophageal Echocardiography: a multicentre study in 154 patients. Eur Heart J. 1993;14:1223–1228.
- , , , , , . Transesophageal echocardiography in patients with cryptogenic ischemic stroke: a systematic review. Am Heart J. 2014;168:706–712.
- , , . Protruding atheromas in the thoracic aorta and systemic embolization. Ann Intern Med. 1991;115:423–427.
- , , , et al.; The Aortic Arch Related Cerebral Hazard Trial Investigators. Clopidogrel plus aspirin versus warfarin in patients with stroke and aortic arch plaques. Stroke. 2014;45:1248–1257.
- , , , et al.; RESPECT Investigators. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092–1100.
- , , , et al.; CLOSURE I Investigators. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–999.
- , , , et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236.
- , , , et al. 2014 AHA/ACA guideline for the management of patients with valvular heart disease. J Am Coll Cardiol. 2014; 63:e57–e185.
- , , , . Clinical and echocardiographic characteristics of patients with left atrial thrombus and sinus rhythm: experience in 20 643 consecutive transesophageal echocardiographic examinations. Circulation. 2002;105(1):27–31.
- , , , et al. Usefulness of transesophageal echocardiography in unexplained cerebral ischemia. Am J Cardiol. 1993;72:1448–1452.
- , , , , , . Transesophageal echocardiography in patients with recent stroke and normal carotid arteries. Am J Cardiol. 2001;88:820–823.
- , , , et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke. 2006;37:2531–2534.
- , , , , , . Age‐dependent prevalence of cardioembolic sources detected by TEE: diagnostic and therapeutic implications. Echocardiography. 1997;14:597–606.
- , , , et al. Continuous stroke unit electrocardiographic monitoring versus 24‐hour Holter electrocardiography for detection of paroxysmal atrial fibrillation after stroke. Stroke. 2012;43:2689–2694.
- , , , et al.; CRYSTAL AF Investigators. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478–2486.
© 2015 Society of Hospital Medicine
Patients with
Staphylococcus aureus is one the most common pathogens isolated in nosocomial and community‐onset bloodstream infections (BSI) in the United States.[1, 2] S aureus bacteremia (SAB) has been reported in the literature to have substantial morbidity and mortality, with rates ranging between 15% and 60% worldwide.[3, 4, 5, 6] In the United States, patients with infections due to S aureus have on average 3 times the length of hospital stay than inpatients without these infections (14.3 days vs 4.5 days; P<0.01).[7] Healthcare costs are negatively impacted by these infections. In a recent meta‐analysis, Zimlichman et al.[8] reported that central‐line BSI (CLABSI) and surgical‐site infection (SSI) caused by methicillin‐resistant S aureus (MRSA) resulted in the highest estimated costs associated with hospital‐acquired infections in the United States ($58,614 [95% CI: $16,760‐$174,755] for CLABSI and $42,300 [95% CI: $4,005‐$82,670] for SSIs).
Appropriate management of SAB includes not only selecting the correct antimicrobial based on susceptibilities but also timely control of the source of infection, appropriate use of ancillary studies when indicated, and pharmacokinetic and pharmacodynamic therapeutic monitoring of antimicrobial therapy when vancomycin is used.[9] Consultation with an infectious diseases (ID) specialist has been associated with increased compliance with evidence‐based strategies in the management of SAB,[10, 11, 12, 13, 14] such as appropriate antibiotic choice, optimized duration of treatment, removal of the source of infection, and better use of cardiac echocardiography, resulting in improved outcomes.[13, 14]
Some, but not all, institutions have adopted bundles,[14] mandatory ID consultation[10] or daily prospective audit and feedback review[15] as part of antimicrobial stewardship program (ASP) interventions aiming to optimize the management of SABs. As part of our ASP quality improvement activities we performed the present study to determine our institutional rate of clinical failure in the treatment of SAB, to identify current practice patterns in the delivery of processes of care, and evaluate their association with clinical outcomes of hospitalized patients with SAB to identify future areas of improvement.
METHODS
A retrospective cohort study was performed at a 1558 licensed‐bed tertiary teaching hospital in Miami, Florida. All hospitalized patients 18 years of age or older with at least 1 positive blood culture with MRSA or methicillin‐susceptible S aureus (MSSA) between January 1, 2012 and April 30, 2013 were included. Patients were identified from the electronic microbiology laboratory database. For the purposes of this study, only the first episode of SAB was included in the analysis. Patients were excluded if aged younger than 18 years or if SAB was detected in an outpatient setting. The primary outcome was clinical failure, defined as a composite endpoint of in‐hospital mortality or persistent bacteremia; persistent bacteremia was defined as bacteremia for 7 or more days after the first positive blood culture. S aureus isolates were identified by standard methods.[16] Species identification was performed by latex agglutination. Antimicrobial susceptibility testing was performed using an automated system (Vitek 2; bioMerieux, Durham, NC) according to standard guidelines.
Data collected included baseline demographics, comorbidities, and treating healthcare provider's service; provider's service was categorized into 1 of 5 groups: internal medicine (academic), internal medicine (hospitalist), surgery, trauma, or neurosurgery. Duration of bacteremia was recorded and defined as the time between first positive and first negative blood culture. The time of first positive culture was defined as the date in which the culture was obtained. Patients who failed to have at least 1 follow‐up blood culture were not counted toward the main outcome. Additionally, presence of a foreign body (cardiac device, orthopedic prosthesis, tunneled catheter, nontunneled catheter) and presumed source of infection as documented in the electronic medical record by the treating service was also collected. Infections were considered community associated when onset of bacteremia occurred within the first 72 hours of admission, and hospital associated if onset of bacteremia occurred after 72 hours of admission.
Based on current practice guidelines,[9] the variables considered processes of care were the time to obtain the first follow‐up blood culture, time from first positive blood culture to initiation of appropriate antibiotic therapy (defined as a loading dose of vancomycin of 15 mg/kg, or a ‐lactam if the organism was susceptible), time to obtain the first vancomycin trough (when indicated), time from first positive blood culture to consultation with ID specialist, appropriate antibiotic de‐escalation (vancomycin to ‐lactam antibiotic if the organism was susceptible and the patient had no allergies or contraindications), and obtaining an echocardiographic study (transthoracic echocardiogram or transesophageal echocardiogram).
Statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC). Differences in proportions were analyzed with 2 or Fisher exact test, accordingly. Differences in means among continuous variables were evaluated using independent samples of paired samples t tests as appropriate for the analysis. Continuous variables were dichotomized using a clinically established cutoff to determine relative risk (RR). A univariate analysis of risk factors associated with clinical failure was performed. Multivariable analyses were performed using logistic regression. Models were created using the backward stepwise approach and included all variables found to be statistically significant at less than 0.05 level in the univariate model and those of clinical significance. The study was reviewed and approved by the institutional review boards at the University of Miami and Jackson Memorial Hospital.
RESULTS
During the study period, 241 patients with a first episode of SAB were identified. MRSA and MSSA were isolated in 124 (51.4%) and 117 (48.5%) patients, respectively. Demographic and clinical characteristics of the study population based on isolate are summarized in Table 1. One hundred seventy‐nine (74.3%) patients were under the care of internal medicine services. There was no association between treating service (medical vs surgical) and clinical failure.
| Variable | MRSA, N= 124 (%) | MSSA, N= 117(%) | Overall, N=241 |
|---|---|---|---|
| |||
| Demographics | |||
| Age, y (mean) | 53.915.57 | 53.915.22 | 53.915.3 |
| Age greater than 60 years | 41 (33.1) | 39 (33.3) | 80 (33.2) |
| Male sex | 80 (64.5) | 80 (68.4) | 160 (66.4) |
| White race | 63 (50.8) | 69 (59) | 132 (54.8) |
| Comorbidities | |||
| Diabetes mellitus | 35 (28.2) | 40 (34.2) | 75 (30.7) |
| Hypertension | 56 (45.2) | 40 (34.2) | 96 (39.8) |
| CHF | 6 (4.8) | 9 (7.7) | 15 (6.2) |
| CVD | 8 (6.4) | 6 (5.1) | 14 (5.8) |
| Chronic pulmonary disease | 14 (11.3) | 14 (12) | 28 (11.6) |
| Malignancy | 9 (7.3) | 19 (16.2) | 28 (11.6) |
| Active chemotherapy | 5 (4) | 10 (8.5) | 15 (6.2) |
| HIV | 27 (21.8) | 17 (14.5) | 44 (18.2) |
| Cirrhosis | 6 (4.8) | 8 (6.8) | 14 (5.8) |
| Hepatitis C infection | 7 (5.6) | 11 (9.4) | 18 (7.5) |
| Acute kidney injury | 88 (71) | 80 (68.4) | 168 (69.7) |
| Chronic kidney disease | 29 (23.4) | 24 (20.5) | 53 (22) |
| End‐stage renal disease | 25 (20.2) | 22 (18.8) | 47 (19.5) |
| Connective tissue disease | 3 (2.4) | 3 (2.6) | 6 (2.5) |
| Alcohol abuse | 3 (2.4) | 1 (0.8) | 4 (1.7) |
| IVDU | 4 (3.2) | 5 (4.3) | 9 (3.7) |
| Hemiplegia | 4 (3.2) | 0 | 4 (1.7) |
| Chronic osteomyelitis | 4 (3.2) | 0 | 4 (1.7) |
| History of transplant | 7 (5.6) | 0 | 7 (2.9) |
| Surgery during current admission | 29 (23.4) | 46 (39.3) | 75 (31.1) |
| Surgery during the previous 30 days | 31 (25) | 36 (30.8) | 67 (25.3) |
| Treating service | |||
| Medical service | 89 (71.8) | 90 (76.9) | 179 (74.3) |
| Surgical service | 21 (16.9) | 16 (13.7) | 37 (15.3) |
| Other | 7 (5.6) | 11 (9.4) | 18 (7.5) |
| Presence of foreign body | |||
| PICC line | 24 (19.3) | 34 (29.1) | 58 (24.1) |
| Tunneled CVC | 24 (19.3) | 15 (12.8) | 39 (16.2) |
| Nontunneled CVC | 13 (10.5) | 28 (23.9) | 41 (17) |
| AV fistula | 3 (2.4) | 7 (6) | 10 (4.1) |
| Cardiac device | 8 (6.4) | 9 (7.7) | 17 (7) |
| Other | 4 (3.2) | 11 (9.4) | 15 (6.2) |
| Source of infection | |||
| CLABSI | 32 (25.8) | 21 (17.9) | 53 (22) |
| SSTI | 24 (19.3) | 20 (17.1) | 44 (18.2) |
| Endocarditis | 10 (8.1) | 7 (6) | 17 (7) |
| Thrombophlebitis | 2 (1.6) | 2 (1.7) | 4 (1.7) |
| Prostatic abscess | 3 (2.4) | 1 (0.8) | 4 (1.7) |
| Paravertebral abscess | 2 (1.6) | 2 (1.7) | 4 (1.7) |
| Mediastinal abscess | 2 (1.6) | 1 (0.8) | 3 (1.2) |
| CAP | 4 (3.2) | 4 (3.4) | 8 (3.3) |
| VAP | 3 (2.4) | 2 (1.7) | 5 (2.1) |
| Surgical site infection | 2 (1.6) | 1 (0.8) | 3 (1.2) |
| Ventriculostomy | 0 | 1 (0.8) | 1 (0.4) |
| Bone or joint infection | 2 (1.6) | 3 (2.6) | 5 (2.1) |
| Unknown | 38 (30.6) | 52 (44.4) | 90 (37.3) |
| Onset | |||
| Community onset* | 77 (62.1) | 77 (65.8) | 154 (63.9) |
| Hospital onset | 47 (37.9) | 40 (34.2) | 87 (36.1) |
The onset of infection occurred in the community in 77 (62.1%) patients with MRSA and in 77 (65.8%) patients with MSSA. The documented source of bacteremia was unknown in 30% of patients with MRSA and 44% of those with MSSA BSI. When ID specialists were consulted, patients were more likely to have a source of infection identified (RR: 1.5; 95% confidence interval [CI]: 1.2‐1.8; P<0.0001). The most commonly documented sources of infection were CLABSI, which occurred in 32 (25.8%) patients with MRSA and 21 (17.9%) patients with MSSA, followed by skin and soft tissue infections in 24 (19.3%) patients with MRSA BSI and 20 (17.1%) patients with MSSA BSI. All patients with CLABSI had documentation of catheter removal.
Clinical failure (defined as in‐hospital mortality or persistent bacteremia) occurred in 78 (32.4%) patients. Of these, 50 (20.7%) represented in‐hospital mortality, and 31 (12.9%) had persistent bacteremia. Table 2 summarizes the demographic and clinical characteristics associated with clinical failure. In the univariate analysis, the variables statistically significantly associated with clinical failure were: age greater than 60 years (RR: 1.4; 95% CI: 1.1‐1.8; P=0.001), bacteremia due to MRSA (RR: 1.7; 95% CI: 1.1‐2.5; P=0.008), white race (RR: 0.7; 95% CI: 0.6‐1; P=0.03), acute kidney injury during admission (RR: 2.2; 95% CI: 1.3‐3.7; P=0.004), presence of nontunneled central venous catheters at the onset of bacteremia (RR: 1.9; 95% CI: 1.3‐2.7; P=0.004), and endocarditis (RR: 2.9; 95% CI: 2.1‐3.9; P<0.0001). In the multivariable analysis, age greater than 60 years and endocarditis were found to be independent risk factors for the development of clinical failure.
| Variable | Clinical Failure, N=78 (%) | No Clinical Failure, N=163 (%) | Unadjusted RR (CI) | P Value* | Adjusted OR (CI) | P Value* |
|---|---|---|---|---|---|---|
| ||||||
| Demographics | ||||||
| Age >60 years | 37 (47.4) | 43 (26.4) | 1.4 (1.1‐1.8) | 0.001 | 2.4 (1.2‐4.5) | 0.008 |
| Male | 46 (60) | 114 (69.9) | 0.7 (0.5‐1.04) | 0.09 | ||
| White race | 35 (44.9) | 97 (59.5) | 0.7 (0.6‐1) | 0.03 | 0.5 (0.3‐1.02) | 0.058 |
| Isolate | ||||||
| MRSA | 50 (64.1) | 74 (45.4) | 1.7 (1.1‐2.5) | 0.008 | 1.8 (0.6‐5.2) | 0.3 |
| MSSA | 28 (35.9) | 89 (54.6) | 0.6 (0.4‐0.9) | 0.008 | ||
| Comorbidities | ||||||
| Diabetes mellitus | 21 (26.9) | 54 (33.1) | 0.8 (0.5‐1.2) | 0.34 | ||
| Cirrhosis | 6 (7.7) | 8 (4.9) | 1.3 (0.7‐2.5) | 0.35 | ||
| Acute kidney injury | 65 (83.3) | 103 (63.2) | 2.2 (1.3‐3.7) | 0.004 | 1.6 (0.5‐5.4) | 0.43 |
| Chronic kidney disease | 12 (15.4) | 41 (25.1) | 0.6 (0.4‐1.1) | 0.11 | ||
| End‐stage renal disease | 15 (19.2) | 32 (19.6) | 1 (0.6‐1.5) | 0.94 | ||
| IVDU | 3 (3.8) | 6 (3.7) | 1.03 (0.4‐2.6) | 1 | ||
| Treating service | ||||||
| Medical | 61 (78.2) | 118 (72.4) | 1.3 (0.7‐2.6) | 0.33 | ||
| Surgical | 11 (14.1) | 67 (41.1) | 1 (0.9‐1.1) | 0.71 | ||
| Presence of foreign body | ||||||
| Cardiac device | 6 (7.7) | 11 (6.7) | 1.1 (0.6‐2.1) | 0.78 | ||
| PICC line | 20 (25.6) | 38 (23.3) | 1.1 (0.7‐1.6) | 0.69 | ||
| Nontunneled CVC | 22 (28.2) | 19 (11.7) | 1.9 (1.3‐2.7) | 0.004 | 3.6 (0.7‐17.7) | 0.11 |
| Tunneled CVC | 15 (19.2) | 24 (14.7) | 1.2 (0.8‐1.9) | 0.36 | ||
| AV fistula | 0 | 10 (6.1) | 0.1 (0.09‐2) | 0.15 | ||
| Other | 4 (5.1) | 11 (6.7) | 0.8 (0.3‐1.9) | 0.64 | ||
| Onset | ||||||
| Community onset | 46 (59) | 108 (66.3) | 0.8 (0.6‐1.2) | 0.27 | ||
| Hospital onset | 32 (41) | 55 (33.7) | 1.2 (0.8‐1.8) | 0.27 | ||
| Source | ||||||
| CLABSI | 15 (19.2) | 38 (23.3) | 0.8 (0.5‐1.4) | 0.48 | ||
| SSTI | 12 (15.4) | 32 (19.6) | 0.8 (0.5‐1.4) | 0.44 | ||
| Endocarditis | 14 (17.9) | 3 (1.8) | 2.9 (2.1‐3.9) | <0.0001 | 9.4 (2.2‐1.1) | 0.003 |
| Thrombophlebitis | 0 | 4 (2.4) | 0.3 (0.02‐4.2) | 0.37 | ||
| Prostatic abscess | 1 (1.3) | 3 (1.8) | 0.8 (0.1‐4.2) | 0.76 | ||
| Paravertebral abscess | 0 | 4 (2.4) | 0.3 (0.02‐4.2) | 0.37 | ||
| Mediastinal abscess | 1 (1.3) | 2 (1.2) | 1.03 (0.2‐5.1) | 0.97 | ||
| CAP | 4 (5.1) | 4 (2.4) | 1.5 (0.8‐3.2) | 0.21 | ||
| VAP | 2 (2.6) | 3 (1.8) | 1.2 (0.4‐3.7) | 0.7 | ||
| Surgical site infection | 1 (1.3) | 2 (1.2) | 1.03 (0.2‐5.2) | 0.97 | ||
| Ventriculostomy | 0 | 1 (0.6) | 0.8 (0.1‐8.5) | 0.82 | ||
| Bone or joint infection | 1 (1.3) | 4 (2.4) | 0.6 (0.1‐3.6) | 0.59 | ||
| Unknown | 27 (34.6) | 63 (38.6) | 0.9 (0.6‐1.3) | 0.55 | ||
Performance of Process of Care and Association With Outcomes
The analysis of the performance of the processes of care and outcomes is shown in Table 3. After adjusting for relevant clinical and demographic characteristics, and those with a level of significance of <0.05, obtaining follow‐up blood cultures more than 4 days after the onset of bacteremia independently increased the risk of clinical failure (RR: 6.5; 95% CI: 2.1‐20.5; P=0.001). When consultation with an ID specialist was obtained within the first 6 days from onset of bacteremia, the risk of clinical failure was 0.3 (95% CI: 0.1‐0.9; P=0.03); however, consultation with an ID specialist overall was not associated with clinical failure (RR: 1; 95% CI: 0.7‐1.4; P=0.98).
| Variable | Clinical Failure, n=78 (%) | No Clinical Failure, n=163 (%) | Unadjusted RR (CI) | P Value* | Adjusted OR (CI) | P Value* |
|---|---|---|---|---|---|---|
| ||||||
| Timing of follow‐up blood culture, n=200 | ||||||
| Less than 2 days | 30 (19.2) | 87 (53.4) | 0.7 (0.5‐0.9) | 0.01 | 1.2 (0.5‐2.9) | 0.60 |
| 24 days (ref) | 16 (20.5) | 39 (23.9) | 0.9 (0.8‐1.1) | 0.53 | ||
| More than 4 days | 19 (24.3) | 9 (5.5) | 1.3 (1.1‐1.5) | <0.0001 | 6.6 (2.1‐20.5) | 0.001 |
| Early antibiotic therapy, n=232 | 66 (84.6) | 132 (81) | 1.2 (0.7‐2.3) | 0.45 | ||
| Monitoring of vancomycin levels, n=156 | 37 (20.8) | 97 (59.5) | 0.8 (0.6‐1.03) | 0.09 | ||
| Therapy with ‐lactam, n=103‖ | 7 (8.8) | 49 (30.1) | 0.4 (0.2‐0.8) | 0.01 | 0.1 (0.04‐0.5) | 0.002 |
| Consultation with ID specialist, n=241 | 31 (39.7) | 66 (40.5) | 1 (0.7‐1.4) | 0.98 | ||
| Early consultation with ID specialist, n=97# | 19 (24.3) | 56 (34.3) | 0.5 (0.3‐0.8) | 0.006 | 0.3 (0.1‐0.9) | 0.03 |
| Echocardiography, n=241 | 45 (57.7) | 96 (58.9) | 1 (0.7‐1.4) | 0.86 | ||
| Early echocardiography, n=141** | 35 (44.9) | 91 (55.8) | 0.7 (0.5‐1.07) | 0.11 | ||
A comparison of the average number of days to performance of processes of care is presented in Table 4. Patients with clinical failure had significantly greater elapsed time from the first positive blood culture to the first follow‐up blood culture as compared to those who did not have clinical failure (mean 2.321.3 days vs 3.883.37; P<0.0001). Forty‐one patients (17.1%) failed to have at least 1 follow‐up blood culture.
| Process of Care | Clinical Failure | No Clinical Failure | P Value* |
|---|---|---|---|
| |||
| First follow‐up blood culture, n=200 | 3.883.37 | 2.321.3 | <0.0001 |
| Consultation with infectious diseases, n=97 | 6.96.55 | 4.354.34 | 0.06 |
| First antibiotic dose, n=232 | 0.431.05 | 0.57 1.11 | 0.63 |
| First dose of ‐lactam, n=56 | 4.41.6 | 3.51.4 | 0.1 |
| First vancomycin trough, n=156 | 2.632.04 | 2.552.02 | 0.81 |
| Echocardiography, n=141 | 3.421.74 | 3.312.05 | 0.47 |
Among patients with clinical failure, an ID specialist was consulted at a mean time of 7 days from the onset of bacteremia, compared to patients with no clinical failure in whom a consult was obtained at a mean of 4 days (P=0.06) (Table 4). Overall, ID specialists were only consulted in 97/241 (40.2%) episodes.
Echocardiographic studies were performed in 141/241 (58.5)% of episodes, and they were more likely to be obtained when an ID specialist was consulted (RR: 1.7; 95% CI: 1.4‐2.1; P<0.0001). Lack of performance of these studies was not associated with clinical failure (Table 3).
Antibiotic Administration and De‐escalation of Therapy
There were no significant differences in the average time from the first positive blood culture to the administration of antibiotics between patients who had clinical failure and those who did not (0.571.11 vs 0.431.05; P=0.63) (Table 4).
Patients with MSSA BSI and no documented penicillin allergy were treated with ‐lactam or cephalosporin antibiotics in 56/103(54.3%) episodes. Patients were 2.5 times more likely to receive ‐lactam antibiotics when an ID specialist was consulted (95% CI: 1.8‐3.5; P<0.0001). Among patients with MSSA BSI, treatment with ‐lactams was an independent predictor of decreased risk of clinical failure (RR: 0.2; 95% CI: 0.07‐0.9; P=0.005) (Table 3).
DISCUSSION
Our study showed a significant rate of morbidity associated with S aureus bacteremia and identified processes of care in the management of SAB that impact patient outcomes.
Our results show that early consultation with an ID specialist was associated with a decreased risk of developing clinical failure, increased likelihood of identification of a source of infection, and positively impacted administration of appropriate antibiotic therapy, especially in cases of MSSA BSI, with overall improvement in patient outcomes. However, consultation with an ID specialist was only obtained in 40.2% of our cases, which is consistent with published data.[10, 11, 12, 13] Consultation with an ID specialist itself did not impact clinical failure, but rather timeliness in obtaining expert guidance was associated with better outcomes. As shown in previous studies,[10, 11, 12, 13, 14] compliance with the standards of care and patient prognosis are improved when ID specialists are involved in the management of SAB. Our study reiterates that early consultation with an ID specialist has a positive outcome in patient care, as opposed to delaying consultation once the patient has persistent bacteremia for more than 7 days. This association could be explained by considering that the majority of the standards of care are time sensitive, which include: obtaining surveillance blood cultures 48 to 96 hours after initial detection[10] or initiating therapy,[11, 14] removal of foci of infection,[10, 11, 12, 14] use of parenteral ‐lactams for the treatment of MSSA,[10, 11, 13, 14] performing echocardiography when clinically indicated,[10, 11, 13, 14] and appropriate duration of therapy.[10, 13, 14] Importantly, studies have shown that when ID specialists' recommendations are followed, patients are more likely to be cured,[10, 11, 13] and are less likely to relapse.[10, 11, 12] Given the complexities of treating patients with SAB and high rates of clinical failures, routine guidance could be beneficial to healthcare providers as part of a multidisciplinary structured strategy that is set in motion the moment a patient with SAB is identified by the microbiology laboratory. The processes of care outlined in this this study can serve as quality of care indicators and be integrated into a structured strategy to optimize the management of SAB.
Regarding optimal timing for follow‐up blood cultures, our results show that delays in obtaining follow‐up blood cultures (more than 4 days from onset of bacteremia) was independently associated with increased risk of clinical failure. Timely follow‐up blood cultures have been previously identified as quality of care indicators.[10, 11, 13, 14] Compliance with obtaining follow‐up blood cultures improves when this step is integrated into a bundle of care.[14]
Antimicrobial therapy was promptly initiated in the majority of the patients in our study. However, areas for improvement were identified. Vancomycin was the empirical therapy of choice in most of the cases, but an appropriate dose was only received by 65% of the patients, and vancomycin levels after the fourth dose were obtained in 85.9% of instances when indicated. Although in our cohort these results were not significantly associated with clinical failure, previous studies have described attainment of a target therapeutic vancomycin trough (1520 mg/dL) as a factor for treatment success.[17, 18] This problem could be addressed through physician education on therapeutic drug monitoring,[19] as well as through an ASP intervention, which have successfully led efforts to improve vancomycin utilization and dosing.[20] Among patients with MSSA BSI, therapy with ‐lactams was associated with improved outcomes, and was more likely to be administered when an ID specialist was consulted. This is in accordance with previous studies that have shown that higher rates of appropriate antimicrobial therapy are achieved when ID specialists are involved in management of SAB.[10, 11, 13, 14] The use of ‐lactams for treatment of MSSA BSI has been consistently associated with lower SAB‐related mortality and relapse.[21, 22, 23, 24, 25, 26]
Echocardiographic studies were obtained in only half of the patients in our cohort, and they were twice more likely to be obtained when an ID specialist was consulted. Although we did not evaluate the appropriateness of the echocardiographic study, the increased proportion of studies performed when ID specialists were consulted could indicate a more in‐depth evaluation of the case. Moreover, in our cohort, when ID specialists where involved in direct patient care, a source of infection was more likely to be identified. This is in accordance with previous studies proposing that because evaluation by ID specialists are more detailed, they lead to increased use in ancillary studies and recognition of complicated cases.[10, 12]
Limitations of this study include its retrospective design and the fact that it was performed in a single institution. The source of infection was defined as documented by treating providers and not by independent diagnostic criteria. Antibiotic use was collected throughout duration of admission, and was not followed after patients were discharged, as these data were not available on the electronic medical record for all patients. Deaths that may have occurred after hospital discharge were not included. We did not account for elevated vancomycin minimum inhibitory concentration as a risk factor for the main outcome, and adjustment of vancomycin based on serum levels was not factored in. Acute kidney injury was accounted for anytime during hospitalization, but not in relation to antimicrobial administration. Despite the limitations, our study has strengths that make our results generalizable. Although our institution is a single medical center, it serves a large and diverse population as reflected in our cases. Even though this is a retrospective cohort study, the use of a centralized electronic medical record allowed us to identify each aspect of the management of SAB, as implemented by different treating services (medical and surgical), as continuous variables (days) rather than only in a dichotomous fashion. Moreover, by being a community teaching hospital, we were able to explore aspects of the practice of physicians in training versus practicing clinicians. These results could be extrapolated to other healthcare facilities aiming to improve the management of SAB.
CONCLUSIONS
Our results suggest that obtaining timely follow‐up blood cultures, use of ‐lactams in patients with MSSA BSI, and early consultation with infectious diseases are the processes of care that could serve as quality and patient‐safety indicators for the management of SAB. These results contribute to a growing body of evidence supporting the implementation of structured processes of care to optimize the management and clinical outcomes of hospitalized patients with SAB.
Disclosure: Nothing to report.
- , , , , , . Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–317.
- , , , . Laboratory‐based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann Clin Microbiol Antimicrob. 2006;5:2.
- , , , , , . The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005;26(2):166–174.
- , , , . Staphylococcal bacteremia and altered host resistance. Ann Intern Medicine. 1968;69(5):859–873.
- , , , , , . Comparison of mortality associated with methicillin‐resistant and methicillin‐susceptible Staphylococcus aureus bacteremia: a meta‐analysis. Clin Infect Dis. 2003;36(1):53–59.
- . Unfavourable prognostic factors in Staphylococcus aureus septicemia and endocarditis. Scand J Infect Dis. 1985;17(2):179–187.
- , , , et al. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample Database. Arch Intern Med. 2005;165(15):1756–1761.
- , , , et al. Health care–associated infections: a meta‐analysis of costs and financial impact on the us health care system. JAMA Intern Med. 2013;173(22):2039–2046.
- , , , et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin‐resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–e55.
- , , , , . Impact of routine infectious diseases service consultation on the evaluation, management, and outcomes of Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46(7):1000–1008.
- , , , et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis. 1998;27(3):478–486.
- , , , . Infectious disease consultation for Staphylococcus aureus bacteremia improves patient management and outcomes. Infect Dis Clin Pract (Baltim Md). 2012;20(4):261–267.
- , , , , . The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med. 2010;123(7):631–637.
- , , , et al. Impact of an evidence‐based bundle intervention in the quality‐of‐care management and outcome of Staphylococcus aureus bacteremia. Clin Infect Dis. 2013;57(9):1225–1233.
- , , , et al. Comparison of prior authorization and prospective audit with feedback for antimicrobial stewardship. Infect Control Hosp Epidemiol. 2014;35(9):1092–1099.
- Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty‐First Informational Supplement. Wayne, PA: Clinical Laboratory Standards Institute; 2011.
- , , , . Impact of vancomycin exposure on outcomes in patients with methicillin‐resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011;52(8):975–981.
- , , , , . High‐dose vancomycin therapy for methicillin‐resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166(19):2138–2144.
- , , , . Strategies for physician education in therapeutic drug monitoring. Clin Chem. 1998;44(2):401–407.
- , . Impact of antimicrobial stewardship program on vancomycin use in a pediatric teaching hospital. Pediatr Infect Dis J. 2010;29(8):707–711.
- , . Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother. 1990;34(6):1227–1231.
- , , , et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine. 2003;82(5):322–332.
- , , , . Impact of empirical‐therapy selection on outcomes of intravenous drug users with infective endocarditis caused by methicillin‐susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51(10):3731–3733.
- , , , et al. Use of vancomycin or first‐generation cephalosporins for the treatment of hemodialysis‐dependent patients with methicillin‐susceptible Staphylococcus aureus bacteremia. Clin Infect Dis. 2007;44(2):190–196.
- , , , et al. Outcome of vancomycin treatment in patients with methicillin‐susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2008;52(1):192–197.
- , , , et al. Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin‐susceptible Staphylococcus aureus bacteremia. BMC Infect Dis. 2011;11:279.
Staphylococcus aureus is one the most common pathogens isolated in nosocomial and community‐onset bloodstream infections (BSI) in the United States.[1, 2] S aureus bacteremia (SAB) has been reported in the literature to have substantial morbidity and mortality, with rates ranging between 15% and 60% worldwide.[3, 4, 5, 6] In the United States, patients with infections due to S aureus have on average 3 times the length of hospital stay than inpatients without these infections (14.3 days vs 4.5 days; P<0.01).[7] Healthcare costs are negatively impacted by these infections. In a recent meta‐analysis, Zimlichman et al.[8] reported that central‐line BSI (CLABSI) and surgical‐site infection (SSI) caused by methicillin‐resistant S aureus (MRSA) resulted in the highest estimated costs associated with hospital‐acquired infections in the United States ($58,614 [95% CI: $16,760‐$174,755] for CLABSI and $42,300 [95% CI: $4,005‐$82,670] for SSIs).
Appropriate management of SAB includes not only selecting the correct antimicrobial based on susceptibilities but also timely control of the source of infection, appropriate use of ancillary studies when indicated, and pharmacokinetic and pharmacodynamic therapeutic monitoring of antimicrobial therapy when vancomycin is used.[9] Consultation with an infectious diseases (ID) specialist has been associated with increased compliance with evidence‐based strategies in the management of SAB,[10, 11, 12, 13, 14] such as appropriate antibiotic choice, optimized duration of treatment, removal of the source of infection, and better use of cardiac echocardiography, resulting in improved outcomes.[13, 14]
Some, but not all, institutions have adopted bundles,[14] mandatory ID consultation[10] or daily prospective audit and feedback review[15] as part of antimicrobial stewardship program (ASP) interventions aiming to optimize the management of SABs. As part of our ASP quality improvement activities we performed the present study to determine our institutional rate of clinical failure in the treatment of SAB, to identify current practice patterns in the delivery of processes of care, and evaluate their association with clinical outcomes of hospitalized patients with SAB to identify future areas of improvement.
METHODS
A retrospective cohort study was performed at a 1558 licensed‐bed tertiary teaching hospital in Miami, Florida. All hospitalized patients 18 years of age or older with at least 1 positive blood culture with MRSA or methicillin‐susceptible S aureus (MSSA) between January 1, 2012 and April 30, 2013 were included. Patients were identified from the electronic microbiology laboratory database. For the purposes of this study, only the first episode of SAB was included in the analysis. Patients were excluded if aged younger than 18 years or if SAB was detected in an outpatient setting. The primary outcome was clinical failure, defined as a composite endpoint of in‐hospital mortality or persistent bacteremia; persistent bacteremia was defined as bacteremia for 7 or more days after the first positive blood culture. S aureus isolates were identified by standard methods.[16] Species identification was performed by latex agglutination. Antimicrobial susceptibility testing was performed using an automated system (Vitek 2; bioMerieux, Durham, NC) according to standard guidelines.
Data collected included baseline demographics, comorbidities, and treating healthcare provider's service; provider's service was categorized into 1 of 5 groups: internal medicine (academic), internal medicine (hospitalist), surgery, trauma, or neurosurgery. Duration of bacteremia was recorded and defined as the time between first positive and first negative blood culture. The time of first positive culture was defined as the date in which the culture was obtained. Patients who failed to have at least 1 follow‐up blood culture were not counted toward the main outcome. Additionally, presence of a foreign body (cardiac device, orthopedic prosthesis, tunneled catheter, nontunneled catheter) and presumed source of infection as documented in the electronic medical record by the treating service was also collected. Infections were considered community associated when onset of bacteremia occurred within the first 72 hours of admission, and hospital associated if onset of bacteremia occurred after 72 hours of admission.
Based on current practice guidelines,[9] the variables considered processes of care were the time to obtain the first follow‐up blood culture, time from first positive blood culture to initiation of appropriate antibiotic therapy (defined as a loading dose of vancomycin of 15 mg/kg, or a ‐lactam if the organism was susceptible), time to obtain the first vancomycin trough (when indicated), time from first positive blood culture to consultation with ID specialist, appropriate antibiotic de‐escalation (vancomycin to ‐lactam antibiotic if the organism was susceptible and the patient had no allergies or contraindications), and obtaining an echocardiographic study (transthoracic echocardiogram or transesophageal echocardiogram).
Statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC). Differences in proportions were analyzed with 2 or Fisher exact test, accordingly. Differences in means among continuous variables were evaluated using independent samples of paired samples t tests as appropriate for the analysis. Continuous variables were dichotomized using a clinically established cutoff to determine relative risk (RR). A univariate analysis of risk factors associated with clinical failure was performed. Multivariable analyses were performed using logistic regression. Models were created using the backward stepwise approach and included all variables found to be statistically significant at less than 0.05 level in the univariate model and those of clinical significance. The study was reviewed and approved by the institutional review boards at the University of Miami and Jackson Memorial Hospital.
RESULTS
During the study period, 241 patients with a first episode of SAB were identified. MRSA and MSSA were isolated in 124 (51.4%) and 117 (48.5%) patients, respectively. Demographic and clinical characteristics of the study population based on isolate are summarized in Table 1. One hundred seventy‐nine (74.3%) patients were under the care of internal medicine services. There was no association between treating service (medical vs surgical) and clinical failure.
| Variable | MRSA, N= 124 (%) | MSSA, N= 117(%) | Overall, N=241 |
|---|---|---|---|
| |||
| Demographics | |||
| Age, y (mean) | 53.915.57 | 53.915.22 | 53.915.3 |
| Age greater than 60 years | 41 (33.1) | 39 (33.3) | 80 (33.2) |
| Male sex | 80 (64.5) | 80 (68.4) | 160 (66.4) |
| White race | 63 (50.8) | 69 (59) | 132 (54.8) |
| Comorbidities | |||
| Diabetes mellitus | 35 (28.2) | 40 (34.2) | 75 (30.7) |
| Hypertension | 56 (45.2) | 40 (34.2) | 96 (39.8) |
| CHF | 6 (4.8) | 9 (7.7) | 15 (6.2) |
| CVD | 8 (6.4) | 6 (5.1) | 14 (5.8) |
| Chronic pulmonary disease | 14 (11.3) | 14 (12) | 28 (11.6) |
| Malignancy | 9 (7.3) | 19 (16.2) | 28 (11.6) |
| Active chemotherapy | 5 (4) | 10 (8.5) | 15 (6.2) |
| HIV | 27 (21.8) | 17 (14.5) | 44 (18.2) |
| Cirrhosis | 6 (4.8) | 8 (6.8) | 14 (5.8) |
| Hepatitis C infection | 7 (5.6) | 11 (9.4) | 18 (7.5) |
| Acute kidney injury | 88 (71) | 80 (68.4) | 168 (69.7) |
| Chronic kidney disease | 29 (23.4) | 24 (20.5) | 53 (22) |
| End‐stage renal disease | 25 (20.2) | 22 (18.8) | 47 (19.5) |
| Connective tissue disease | 3 (2.4) | 3 (2.6) | 6 (2.5) |
| Alcohol abuse | 3 (2.4) | 1 (0.8) | 4 (1.7) |
| IVDU | 4 (3.2) | 5 (4.3) | 9 (3.7) |
| Hemiplegia | 4 (3.2) | 0 | 4 (1.7) |
| Chronic osteomyelitis | 4 (3.2) | 0 | 4 (1.7) |
| History of transplant | 7 (5.6) | 0 | 7 (2.9) |
| Surgery during current admission | 29 (23.4) | 46 (39.3) | 75 (31.1) |
| Surgery during the previous 30 days | 31 (25) | 36 (30.8) | 67 (25.3) |
| Treating service | |||
| Medical service | 89 (71.8) | 90 (76.9) | 179 (74.3) |
| Surgical service | 21 (16.9) | 16 (13.7) | 37 (15.3) |
| Other | 7 (5.6) | 11 (9.4) | 18 (7.5) |
| Presence of foreign body | |||
| PICC line | 24 (19.3) | 34 (29.1) | 58 (24.1) |
| Tunneled CVC | 24 (19.3) | 15 (12.8) | 39 (16.2) |
| Nontunneled CVC | 13 (10.5) | 28 (23.9) | 41 (17) |
| AV fistula | 3 (2.4) | 7 (6) | 10 (4.1) |
| Cardiac device | 8 (6.4) | 9 (7.7) | 17 (7) |
| Other | 4 (3.2) | 11 (9.4) | 15 (6.2) |
| Source of infection | |||
| CLABSI | 32 (25.8) | 21 (17.9) | 53 (22) |
| SSTI | 24 (19.3) | 20 (17.1) | 44 (18.2) |
| Endocarditis | 10 (8.1) | 7 (6) | 17 (7) |
| Thrombophlebitis | 2 (1.6) | 2 (1.7) | 4 (1.7) |
| Prostatic abscess | 3 (2.4) | 1 (0.8) | 4 (1.7) |
| Paravertebral abscess | 2 (1.6) | 2 (1.7) | 4 (1.7) |
| Mediastinal abscess | 2 (1.6) | 1 (0.8) | 3 (1.2) |
| CAP | 4 (3.2) | 4 (3.4) | 8 (3.3) |
| VAP | 3 (2.4) | 2 (1.7) | 5 (2.1) |
| Surgical site infection | 2 (1.6) | 1 (0.8) | 3 (1.2) |
| Ventriculostomy | 0 | 1 (0.8) | 1 (0.4) |
| Bone or joint infection | 2 (1.6) | 3 (2.6) | 5 (2.1) |
| Unknown | 38 (30.6) | 52 (44.4) | 90 (37.3) |
| Onset | |||
| Community onset* | 77 (62.1) | 77 (65.8) | 154 (63.9) |
| Hospital onset | 47 (37.9) | 40 (34.2) | 87 (36.1) |
The onset of infection occurred in the community in 77 (62.1%) patients with MRSA and in 77 (65.8%) patients with MSSA. The documented source of bacteremia was unknown in 30% of patients with MRSA and 44% of those with MSSA BSI. When ID specialists were consulted, patients were more likely to have a source of infection identified (RR: 1.5; 95% confidence interval [CI]: 1.2‐1.8; P<0.0001). The most commonly documented sources of infection were CLABSI, which occurred in 32 (25.8%) patients with MRSA and 21 (17.9%) patients with MSSA, followed by skin and soft tissue infections in 24 (19.3%) patients with MRSA BSI and 20 (17.1%) patients with MSSA BSI. All patients with CLABSI had documentation of catheter removal.
Clinical failure (defined as in‐hospital mortality or persistent bacteremia) occurred in 78 (32.4%) patients. Of these, 50 (20.7%) represented in‐hospital mortality, and 31 (12.9%) had persistent bacteremia. Table 2 summarizes the demographic and clinical characteristics associated with clinical failure. In the univariate analysis, the variables statistically significantly associated with clinical failure were: age greater than 60 years (RR: 1.4; 95% CI: 1.1‐1.8; P=0.001), bacteremia due to MRSA (RR: 1.7; 95% CI: 1.1‐2.5; P=0.008), white race (RR: 0.7; 95% CI: 0.6‐1; P=0.03), acute kidney injury during admission (RR: 2.2; 95% CI: 1.3‐3.7; P=0.004), presence of nontunneled central venous catheters at the onset of bacteremia (RR: 1.9; 95% CI: 1.3‐2.7; P=0.004), and endocarditis (RR: 2.9; 95% CI: 2.1‐3.9; P<0.0001). In the multivariable analysis, age greater than 60 years and endocarditis were found to be independent risk factors for the development of clinical failure.
| Variable | Clinical Failure, N=78 (%) | No Clinical Failure, N=163 (%) | Unadjusted RR (CI) | P Value* | Adjusted OR (CI) | P Value* |
|---|---|---|---|---|---|---|
| ||||||
| Demographics | ||||||
| Age >60 years | 37 (47.4) | 43 (26.4) | 1.4 (1.1‐1.8) | 0.001 | 2.4 (1.2‐4.5) | 0.008 |
| Male | 46 (60) | 114 (69.9) | 0.7 (0.5‐1.04) | 0.09 | ||
| White race | 35 (44.9) | 97 (59.5) | 0.7 (0.6‐1) | 0.03 | 0.5 (0.3‐1.02) | 0.058 |
| Isolate | ||||||
| MRSA | 50 (64.1) | 74 (45.4) | 1.7 (1.1‐2.5) | 0.008 | 1.8 (0.6‐5.2) | 0.3 |
| MSSA | 28 (35.9) | 89 (54.6) | 0.6 (0.4‐0.9) | 0.008 | ||
| Comorbidities | ||||||
| Diabetes mellitus | 21 (26.9) | 54 (33.1) | 0.8 (0.5‐1.2) | 0.34 | ||
| Cirrhosis | 6 (7.7) | 8 (4.9) | 1.3 (0.7‐2.5) | 0.35 | ||
| Acute kidney injury | 65 (83.3) | 103 (63.2) | 2.2 (1.3‐3.7) | 0.004 | 1.6 (0.5‐5.4) | 0.43 |
| Chronic kidney disease | 12 (15.4) | 41 (25.1) | 0.6 (0.4‐1.1) | 0.11 | ||
| End‐stage renal disease | 15 (19.2) | 32 (19.6) | 1 (0.6‐1.5) | 0.94 | ||
| IVDU | 3 (3.8) | 6 (3.7) | 1.03 (0.4‐2.6) | 1 | ||
| Treating service | ||||||
| Medical | 61 (78.2) | 118 (72.4) | 1.3 (0.7‐2.6) | 0.33 | ||
| Surgical | 11 (14.1) | 67 (41.1) | 1 (0.9‐1.1) | 0.71 | ||
| Presence of foreign body | ||||||
| Cardiac device | 6 (7.7) | 11 (6.7) | 1.1 (0.6‐2.1) | 0.78 | ||
| PICC line | 20 (25.6) | 38 (23.3) | 1.1 (0.7‐1.6) | 0.69 | ||
| Nontunneled CVC | 22 (28.2) | 19 (11.7) | 1.9 (1.3‐2.7) | 0.004 | 3.6 (0.7‐17.7) | 0.11 |
| Tunneled CVC | 15 (19.2) | 24 (14.7) | 1.2 (0.8‐1.9) | 0.36 | ||
| AV fistula | 0 | 10 (6.1) | 0.1 (0.09‐2) | 0.15 | ||
| Other | 4 (5.1) | 11 (6.7) | 0.8 (0.3‐1.9) | 0.64 | ||
| Onset | ||||||
| Community onset | 46 (59) | 108 (66.3) | 0.8 (0.6‐1.2) | 0.27 | ||
| Hospital onset | 32 (41) | 55 (33.7) | 1.2 (0.8‐1.8) | 0.27 | ||
| Source | ||||||
| CLABSI | 15 (19.2) | 38 (23.3) | 0.8 (0.5‐1.4) | 0.48 | ||
| SSTI | 12 (15.4) | 32 (19.6) | 0.8 (0.5‐1.4) | 0.44 | ||
| Endocarditis | 14 (17.9) | 3 (1.8) | 2.9 (2.1‐3.9) | <0.0001 | 9.4 (2.2‐1.1) | 0.003 |
| Thrombophlebitis | 0 | 4 (2.4) | 0.3 (0.02‐4.2) | 0.37 | ||
| Prostatic abscess | 1 (1.3) | 3 (1.8) | 0.8 (0.1‐4.2) | 0.76 | ||
| Paravertebral abscess | 0 | 4 (2.4) | 0.3 (0.02‐4.2) | 0.37 | ||
| Mediastinal abscess | 1 (1.3) | 2 (1.2) | 1.03 (0.2‐5.1) | 0.97 | ||
| CAP | 4 (5.1) | 4 (2.4) | 1.5 (0.8‐3.2) | 0.21 | ||
| VAP | 2 (2.6) | 3 (1.8) | 1.2 (0.4‐3.7) | 0.7 | ||
| Surgical site infection | 1 (1.3) | 2 (1.2) | 1.03 (0.2‐5.2) | 0.97 | ||
| Ventriculostomy | 0 | 1 (0.6) | 0.8 (0.1‐8.5) | 0.82 | ||
| Bone or joint infection | 1 (1.3) | 4 (2.4) | 0.6 (0.1‐3.6) | 0.59 | ||
| Unknown | 27 (34.6) | 63 (38.6) | 0.9 (0.6‐1.3) | 0.55 | ||
Performance of Process of Care and Association With Outcomes
The analysis of the performance of the processes of care and outcomes is shown in Table 3. After adjusting for relevant clinical and demographic characteristics, and those with a level of significance of <0.05, obtaining follow‐up blood cultures more than 4 days after the onset of bacteremia independently increased the risk of clinical failure (RR: 6.5; 95% CI: 2.1‐20.5; P=0.001). When consultation with an ID specialist was obtained within the first 6 days from onset of bacteremia, the risk of clinical failure was 0.3 (95% CI: 0.1‐0.9; P=0.03); however, consultation with an ID specialist overall was not associated with clinical failure (RR: 1; 95% CI: 0.7‐1.4; P=0.98).
| Variable | Clinical Failure, n=78 (%) | No Clinical Failure, n=163 (%) | Unadjusted RR (CI) | P Value* | Adjusted OR (CI) | P Value* |
|---|---|---|---|---|---|---|
| ||||||
| Timing of follow‐up blood culture, n=200 | ||||||
| Less than 2 days | 30 (19.2) | 87 (53.4) | 0.7 (0.5‐0.9) | 0.01 | 1.2 (0.5‐2.9) | 0.60 |
| 24 days (ref) | 16 (20.5) | 39 (23.9) | 0.9 (0.8‐1.1) | 0.53 | ||
| More than 4 days | 19 (24.3) | 9 (5.5) | 1.3 (1.1‐1.5) | <0.0001 | 6.6 (2.1‐20.5) | 0.001 |
| Early antibiotic therapy, n=232 | 66 (84.6) | 132 (81) | 1.2 (0.7‐2.3) | 0.45 | ||
| Monitoring of vancomycin levels, n=156 | 37 (20.8) | 97 (59.5) | 0.8 (0.6‐1.03) | 0.09 | ||
| Therapy with ‐lactam, n=103‖ | 7 (8.8) | 49 (30.1) | 0.4 (0.2‐0.8) | 0.01 | 0.1 (0.04‐0.5) | 0.002 |
| Consultation with ID specialist, n=241 | 31 (39.7) | 66 (40.5) | 1 (0.7‐1.4) | 0.98 | ||
| Early consultation with ID specialist, n=97# | 19 (24.3) | 56 (34.3) | 0.5 (0.3‐0.8) | 0.006 | 0.3 (0.1‐0.9) | 0.03 |
| Echocardiography, n=241 | 45 (57.7) | 96 (58.9) | 1 (0.7‐1.4) | 0.86 | ||
| Early echocardiography, n=141** | 35 (44.9) | 91 (55.8) | 0.7 (0.5‐1.07) | 0.11 | ||
A comparison of the average number of days to performance of processes of care is presented in Table 4. Patients with clinical failure had significantly greater elapsed time from the first positive blood culture to the first follow‐up blood culture as compared to those who did not have clinical failure (mean 2.321.3 days vs 3.883.37; P<0.0001). Forty‐one patients (17.1%) failed to have at least 1 follow‐up blood culture.
| Process of Care | Clinical Failure | No Clinical Failure | P Value* |
|---|---|---|---|
| |||
| First follow‐up blood culture, n=200 | 3.883.37 | 2.321.3 | <0.0001 |
| Consultation with infectious diseases, n=97 | 6.96.55 | 4.354.34 | 0.06 |
| First antibiotic dose, n=232 | 0.431.05 | 0.57 1.11 | 0.63 |
| First dose of ‐lactam, n=56 | 4.41.6 | 3.51.4 | 0.1 |
| First vancomycin trough, n=156 | 2.632.04 | 2.552.02 | 0.81 |
| Echocardiography, n=141 | 3.421.74 | 3.312.05 | 0.47 |
Among patients with clinical failure, an ID specialist was consulted at a mean time of 7 days from the onset of bacteremia, compared to patients with no clinical failure in whom a consult was obtained at a mean of 4 days (P=0.06) (Table 4). Overall, ID specialists were only consulted in 97/241 (40.2%) episodes.
Echocardiographic studies were performed in 141/241 (58.5)% of episodes, and they were more likely to be obtained when an ID specialist was consulted (RR: 1.7; 95% CI: 1.4‐2.1; P<0.0001). Lack of performance of these studies was not associated with clinical failure (Table 3).
Antibiotic Administration and De‐escalation of Therapy
There were no significant differences in the average time from the first positive blood culture to the administration of antibiotics between patients who had clinical failure and those who did not (0.571.11 vs 0.431.05; P=0.63) (Table 4).
Patients with MSSA BSI and no documented penicillin allergy were treated with ‐lactam or cephalosporin antibiotics in 56/103(54.3%) episodes. Patients were 2.5 times more likely to receive ‐lactam antibiotics when an ID specialist was consulted (95% CI: 1.8‐3.5; P<0.0001). Among patients with MSSA BSI, treatment with ‐lactams was an independent predictor of decreased risk of clinical failure (RR: 0.2; 95% CI: 0.07‐0.9; P=0.005) (Table 3).
DISCUSSION
Our study showed a significant rate of morbidity associated with S aureus bacteremia and identified processes of care in the management of SAB that impact patient outcomes.
Our results show that early consultation with an ID specialist was associated with a decreased risk of developing clinical failure, increased likelihood of identification of a source of infection, and positively impacted administration of appropriate antibiotic therapy, especially in cases of MSSA BSI, with overall improvement in patient outcomes. However, consultation with an ID specialist was only obtained in 40.2% of our cases, which is consistent with published data.[10, 11, 12, 13] Consultation with an ID specialist itself did not impact clinical failure, but rather timeliness in obtaining expert guidance was associated with better outcomes. As shown in previous studies,[10, 11, 12, 13, 14] compliance with the standards of care and patient prognosis are improved when ID specialists are involved in the management of SAB. Our study reiterates that early consultation with an ID specialist has a positive outcome in patient care, as opposed to delaying consultation once the patient has persistent bacteremia for more than 7 days. This association could be explained by considering that the majority of the standards of care are time sensitive, which include: obtaining surveillance blood cultures 48 to 96 hours after initial detection[10] or initiating therapy,[11, 14] removal of foci of infection,[10, 11, 12, 14] use of parenteral ‐lactams for the treatment of MSSA,[10, 11, 13, 14] performing echocardiography when clinically indicated,[10, 11, 13, 14] and appropriate duration of therapy.[10, 13, 14] Importantly, studies have shown that when ID specialists' recommendations are followed, patients are more likely to be cured,[10, 11, 13] and are less likely to relapse.[10, 11, 12] Given the complexities of treating patients with SAB and high rates of clinical failures, routine guidance could be beneficial to healthcare providers as part of a multidisciplinary structured strategy that is set in motion the moment a patient with SAB is identified by the microbiology laboratory. The processes of care outlined in this this study can serve as quality of care indicators and be integrated into a structured strategy to optimize the management of SAB.
Regarding optimal timing for follow‐up blood cultures, our results show that delays in obtaining follow‐up blood cultures (more than 4 days from onset of bacteremia) was independently associated with increased risk of clinical failure. Timely follow‐up blood cultures have been previously identified as quality of care indicators.[10, 11, 13, 14] Compliance with obtaining follow‐up blood cultures improves when this step is integrated into a bundle of care.[14]
Antimicrobial therapy was promptly initiated in the majority of the patients in our study. However, areas for improvement were identified. Vancomycin was the empirical therapy of choice in most of the cases, but an appropriate dose was only received by 65% of the patients, and vancomycin levels after the fourth dose were obtained in 85.9% of instances when indicated. Although in our cohort these results were not significantly associated with clinical failure, previous studies have described attainment of a target therapeutic vancomycin trough (1520 mg/dL) as a factor for treatment success.[17, 18] This problem could be addressed through physician education on therapeutic drug monitoring,[19] as well as through an ASP intervention, which have successfully led efforts to improve vancomycin utilization and dosing.[20] Among patients with MSSA BSI, therapy with ‐lactams was associated with improved outcomes, and was more likely to be administered when an ID specialist was consulted. This is in accordance with previous studies that have shown that higher rates of appropriate antimicrobial therapy are achieved when ID specialists are involved in management of SAB.[10, 11, 13, 14] The use of ‐lactams for treatment of MSSA BSI has been consistently associated with lower SAB‐related mortality and relapse.[21, 22, 23, 24, 25, 26]
Echocardiographic studies were obtained in only half of the patients in our cohort, and they were twice more likely to be obtained when an ID specialist was consulted. Although we did not evaluate the appropriateness of the echocardiographic study, the increased proportion of studies performed when ID specialists were consulted could indicate a more in‐depth evaluation of the case. Moreover, in our cohort, when ID specialists where involved in direct patient care, a source of infection was more likely to be identified. This is in accordance with previous studies proposing that because evaluation by ID specialists are more detailed, they lead to increased use in ancillary studies and recognition of complicated cases.[10, 12]
Limitations of this study include its retrospective design and the fact that it was performed in a single institution. The source of infection was defined as documented by treating providers and not by independent diagnostic criteria. Antibiotic use was collected throughout duration of admission, and was not followed after patients were discharged, as these data were not available on the electronic medical record for all patients. Deaths that may have occurred after hospital discharge were not included. We did not account for elevated vancomycin minimum inhibitory concentration as a risk factor for the main outcome, and adjustment of vancomycin based on serum levels was not factored in. Acute kidney injury was accounted for anytime during hospitalization, but not in relation to antimicrobial administration. Despite the limitations, our study has strengths that make our results generalizable. Although our institution is a single medical center, it serves a large and diverse population as reflected in our cases. Even though this is a retrospective cohort study, the use of a centralized electronic medical record allowed us to identify each aspect of the management of SAB, as implemented by different treating services (medical and surgical), as continuous variables (days) rather than only in a dichotomous fashion. Moreover, by being a community teaching hospital, we were able to explore aspects of the practice of physicians in training versus practicing clinicians. These results could be extrapolated to other healthcare facilities aiming to improve the management of SAB.
CONCLUSIONS
Our results suggest that obtaining timely follow‐up blood cultures, use of ‐lactams in patients with MSSA BSI, and early consultation with infectious diseases are the processes of care that could serve as quality and patient‐safety indicators for the management of SAB. These results contribute to a growing body of evidence supporting the implementation of structured processes of care to optimize the management and clinical outcomes of hospitalized patients with SAB.
Disclosure: Nothing to report.
Staphylococcus aureus is one the most common pathogens isolated in nosocomial and community‐onset bloodstream infections (BSI) in the United States.[1, 2] S aureus bacteremia (SAB) has been reported in the literature to have substantial morbidity and mortality, with rates ranging between 15% and 60% worldwide.[3, 4, 5, 6] In the United States, patients with infections due to S aureus have on average 3 times the length of hospital stay than inpatients without these infections (14.3 days vs 4.5 days; P<0.01).[7] Healthcare costs are negatively impacted by these infections. In a recent meta‐analysis, Zimlichman et al.[8] reported that central‐line BSI (CLABSI) and surgical‐site infection (SSI) caused by methicillin‐resistant S aureus (MRSA) resulted in the highest estimated costs associated with hospital‐acquired infections in the United States ($58,614 [95% CI: $16,760‐$174,755] for CLABSI and $42,300 [95% CI: $4,005‐$82,670] for SSIs).
Appropriate management of SAB includes not only selecting the correct antimicrobial based on susceptibilities but also timely control of the source of infection, appropriate use of ancillary studies when indicated, and pharmacokinetic and pharmacodynamic therapeutic monitoring of antimicrobial therapy when vancomycin is used.[9] Consultation with an infectious diseases (ID) specialist has been associated with increased compliance with evidence‐based strategies in the management of SAB,[10, 11, 12, 13, 14] such as appropriate antibiotic choice, optimized duration of treatment, removal of the source of infection, and better use of cardiac echocardiography, resulting in improved outcomes.[13, 14]
Some, but not all, institutions have adopted bundles,[14] mandatory ID consultation[10] or daily prospective audit and feedback review[15] as part of antimicrobial stewardship program (ASP) interventions aiming to optimize the management of SABs. As part of our ASP quality improvement activities we performed the present study to determine our institutional rate of clinical failure in the treatment of SAB, to identify current practice patterns in the delivery of processes of care, and evaluate their association with clinical outcomes of hospitalized patients with SAB to identify future areas of improvement.
METHODS
A retrospective cohort study was performed at a 1558 licensed‐bed tertiary teaching hospital in Miami, Florida. All hospitalized patients 18 years of age or older with at least 1 positive blood culture with MRSA or methicillin‐susceptible S aureus (MSSA) between January 1, 2012 and April 30, 2013 were included. Patients were identified from the electronic microbiology laboratory database. For the purposes of this study, only the first episode of SAB was included in the analysis. Patients were excluded if aged younger than 18 years or if SAB was detected in an outpatient setting. The primary outcome was clinical failure, defined as a composite endpoint of in‐hospital mortality or persistent bacteremia; persistent bacteremia was defined as bacteremia for 7 or more days after the first positive blood culture. S aureus isolates were identified by standard methods.[16] Species identification was performed by latex agglutination. Antimicrobial susceptibility testing was performed using an automated system (Vitek 2; bioMerieux, Durham, NC) according to standard guidelines.
Data collected included baseline demographics, comorbidities, and treating healthcare provider's service; provider's service was categorized into 1 of 5 groups: internal medicine (academic), internal medicine (hospitalist), surgery, trauma, or neurosurgery. Duration of bacteremia was recorded and defined as the time between first positive and first negative blood culture. The time of first positive culture was defined as the date in which the culture was obtained. Patients who failed to have at least 1 follow‐up blood culture were not counted toward the main outcome. Additionally, presence of a foreign body (cardiac device, orthopedic prosthesis, tunneled catheter, nontunneled catheter) and presumed source of infection as documented in the electronic medical record by the treating service was also collected. Infections were considered community associated when onset of bacteremia occurred within the first 72 hours of admission, and hospital associated if onset of bacteremia occurred after 72 hours of admission.
Based on current practice guidelines,[9] the variables considered processes of care were the time to obtain the first follow‐up blood culture, time from first positive blood culture to initiation of appropriate antibiotic therapy (defined as a loading dose of vancomycin of 15 mg/kg, or a ‐lactam if the organism was susceptible), time to obtain the first vancomycin trough (when indicated), time from first positive blood culture to consultation with ID specialist, appropriate antibiotic de‐escalation (vancomycin to ‐lactam antibiotic if the organism was susceptible and the patient had no allergies or contraindications), and obtaining an echocardiographic study (transthoracic echocardiogram or transesophageal echocardiogram).
Statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC). Differences in proportions were analyzed with 2 or Fisher exact test, accordingly. Differences in means among continuous variables were evaluated using independent samples of paired samples t tests as appropriate for the analysis. Continuous variables were dichotomized using a clinically established cutoff to determine relative risk (RR). A univariate analysis of risk factors associated with clinical failure was performed. Multivariable analyses were performed using logistic regression. Models were created using the backward stepwise approach and included all variables found to be statistically significant at less than 0.05 level in the univariate model and those of clinical significance. The study was reviewed and approved by the institutional review boards at the University of Miami and Jackson Memorial Hospital.
RESULTS
During the study period, 241 patients with a first episode of SAB were identified. MRSA and MSSA were isolated in 124 (51.4%) and 117 (48.5%) patients, respectively. Demographic and clinical characteristics of the study population based on isolate are summarized in Table 1. One hundred seventy‐nine (74.3%) patients were under the care of internal medicine services. There was no association between treating service (medical vs surgical) and clinical failure.
| Variable | MRSA, N= 124 (%) | MSSA, N= 117(%) | Overall, N=241 |
|---|---|---|---|
| |||
| Demographics | |||
| Age, y (mean) | 53.915.57 | 53.915.22 | 53.915.3 |
| Age greater than 60 years | 41 (33.1) | 39 (33.3) | 80 (33.2) |
| Male sex | 80 (64.5) | 80 (68.4) | 160 (66.4) |
| White race | 63 (50.8) | 69 (59) | 132 (54.8) |
| Comorbidities | |||
| Diabetes mellitus | 35 (28.2) | 40 (34.2) | 75 (30.7) |
| Hypertension | 56 (45.2) | 40 (34.2) | 96 (39.8) |
| CHF | 6 (4.8) | 9 (7.7) | 15 (6.2) |
| CVD | 8 (6.4) | 6 (5.1) | 14 (5.8) |
| Chronic pulmonary disease | 14 (11.3) | 14 (12) | 28 (11.6) |
| Malignancy | 9 (7.3) | 19 (16.2) | 28 (11.6) |
| Active chemotherapy | 5 (4) | 10 (8.5) | 15 (6.2) |
| HIV | 27 (21.8) | 17 (14.5) | 44 (18.2) |
| Cirrhosis | 6 (4.8) | 8 (6.8) | 14 (5.8) |
| Hepatitis C infection | 7 (5.6) | 11 (9.4) | 18 (7.5) |
| Acute kidney injury | 88 (71) | 80 (68.4) | 168 (69.7) |
| Chronic kidney disease | 29 (23.4) | 24 (20.5) | 53 (22) |
| End‐stage renal disease | 25 (20.2) | 22 (18.8) | 47 (19.5) |
| Connective tissue disease | 3 (2.4) | 3 (2.6) | 6 (2.5) |
| Alcohol abuse | 3 (2.4) | 1 (0.8) | 4 (1.7) |
| IVDU | 4 (3.2) | 5 (4.3) | 9 (3.7) |
| Hemiplegia | 4 (3.2) | 0 | 4 (1.7) |
| Chronic osteomyelitis | 4 (3.2) | 0 | 4 (1.7) |
| History of transplant | 7 (5.6) | 0 | 7 (2.9) |
| Surgery during current admission | 29 (23.4) | 46 (39.3) | 75 (31.1) |
| Surgery during the previous 30 days | 31 (25) | 36 (30.8) | 67 (25.3) |
| Treating service | |||
| Medical service | 89 (71.8) | 90 (76.9) | 179 (74.3) |
| Surgical service | 21 (16.9) | 16 (13.7) | 37 (15.3) |
| Other | 7 (5.6) | 11 (9.4) | 18 (7.5) |
| Presence of foreign body | |||
| PICC line | 24 (19.3) | 34 (29.1) | 58 (24.1) |
| Tunneled CVC | 24 (19.3) | 15 (12.8) | 39 (16.2) |
| Nontunneled CVC | 13 (10.5) | 28 (23.9) | 41 (17) |
| AV fistula | 3 (2.4) | 7 (6) | 10 (4.1) |
| Cardiac device | 8 (6.4) | 9 (7.7) | 17 (7) |
| Other | 4 (3.2) | 11 (9.4) | 15 (6.2) |
| Source of infection | |||
| CLABSI | 32 (25.8) | 21 (17.9) | 53 (22) |
| SSTI | 24 (19.3) | 20 (17.1) | 44 (18.2) |
| Endocarditis | 10 (8.1) | 7 (6) | 17 (7) |
| Thrombophlebitis | 2 (1.6) | 2 (1.7) | 4 (1.7) |
| Prostatic abscess | 3 (2.4) | 1 (0.8) | 4 (1.7) |
| Paravertebral abscess | 2 (1.6) | 2 (1.7) | 4 (1.7) |
| Mediastinal abscess | 2 (1.6) | 1 (0.8) | 3 (1.2) |
| CAP | 4 (3.2) | 4 (3.4) | 8 (3.3) |
| VAP | 3 (2.4) | 2 (1.7) | 5 (2.1) |
| Surgical site infection | 2 (1.6) | 1 (0.8) | 3 (1.2) |
| Ventriculostomy | 0 | 1 (0.8) | 1 (0.4) |
| Bone or joint infection | 2 (1.6) | 3 (2.6) | 5 (2.1) |
| Unknown | 38 (30.6) | 52 (44.4) | 90 (37.3) |
| Onset | |||
| Community onset* | 77 (62.1) | 77 (65.8) | 154 (63.9) |
| Hospital onset | 47 (37.9) | 40 (34.2) | 87 (36.1) |
The onset of infection occurred in the community in 77 (62.1%) patients with MRSA and in 77 (65.8%) patients with MSSA. The documented source of bacteremia was unknown in 30% of patients with MRSA and 44% of those with MSSA BSI. When ID specialists were consulted, patients were more likely to have a source of infection identified (RR: 1.5; 95% confidence interval [CI]: 1.2‐1.8; P<0.0001). The most commonly documented sources of infection were CLABSI, which occurred in 32 (25.8%) patients with MRSA and 21 (17.9%) patients with MSSA, followed by skin and soft tissue infections in 24 (19.3%) patients with MRSA BSI and 20 (17.1%) patients with MSSA BSI. All patients with CLABSI had documentation of catheter removal.
Clinical failure (defined as in‐hospital mortality or persistent bacteremia) occurred in 78 (32.4%) patients. Of these, 50 (20.7%) represented in‐hospital mortality, and 31 (12.9%) had persistent bacteremia. Table 2 summarizes the demographic and clinical characteristics associated with clinical failure. In the univariate analysis, the variables statistically significantly associated with clinical failure were: age greater than 60 years (RR: 1.4; 95% CI: 1.1‐1.8; P=0.001), bacteremia due to MRSA (RR: 1.7; 95% CI: 1.1‐2.5; P=0.008), white race (RR: 0.7; 95% CI: 0.6‐1; P=0.03), acute kidney injury during admission (RR: 2.2; 95% CI: 1.3‐3.7; P=0.004), presence of nontunneled central venous catheters at the onset of bacteremia (RR: 1.9; 95% CI: 1.3‐2.7; P=0.004), and endocarditis (RR: 2.9; 95% CI: 2.1‐3.9; P<0.0001). In the multivariable analysis, age greater than 60 years and endocarditis were found to be independent risk factors for the development of clinical failure.
| Variable | Clinical Failure, N=78 (%) | No Clinical Failure, N=163 (%) | Unadjusted RR (CI) | P Value* | Adjusted OR (CI) | P Value* |
|---|---|---|---|---|---|---|
| ||||||
| Demographics | ||||||
| Age >60 years | 37 (47.4) | 43 (26.4) | 1.4 (1.1‐1.8) | 0.001 | 2.4 (1.2‐4.5) | 0.008 |
| Male | 46 (60) | 114 (69.9) | 0.7 (0.5‐1.04) | 0.09 | ||
| White race | 35 (44.9) | 97 (59.5) | 0.7 (0.6‐1) | 0.03 | 0.5 (0.3‐1.02) | 0.058 |
| Isolate | ||||||
| MRSA | 50 (64.1) | 74 (45.4) | 1.7 (1.1‐2.5) | 0.008 | 1.8 (0.6‐5.2) | 0.3 |
| MSSA | 28 (35.9) | 89 (54.6) | 0.6 (0.4‐0.9) | 0.008 | ||
| Comorbidities | ||||||
| Diabetes mellitus | 21 (26.9) | 54 (33.1) | 0.8 (0.5‐1.2) | 0.34 | ||
| Cirrhosis | 6 (7.7) | 8 (4.9) | 1.3 (0.7‐2.5) | 0.35 | ||
| Acute kidney injury | 65 (83.3) | 103 (63.2) | 2.2 (1.3‐3.7) | 0.004 | 1.6 (0.5‐5.4) | 0.43 |
| Chronic kidney disease | 12 (15.4) | 41 (25.1) | 0.6 (0.4‐1.1) | 0.11 | ||
| End‐stage renal disease | 15 (19.2) | 32 (19.6) | 1 (0.6‐1.5) | 0.94 | ||
| IVDU | 3 (3.8) | 6 (3.7) | 1.03 (0.4‐2.6) | 1 | ||
| Treating service | ||||||
| Medical | 61 (78.2) | 118 (72.4) | 1.3 (0.7‐2.6) | 0.33 | ||
| Surgical | 11 (14.1) | 67 (41.1) | 1 (0.9‐1.1) | 0.71 | ||
| Presence of foreign body | ||||||
| Cardiac device | 6 (7.7) | 11 (6.7) | 1.1 (0.6‐2.1) | 0.78 | ||
| PICC line | 20 (25.6) | 38 (23.3) | 1.1 (0.7‐1.6) | 0.69 | ||
| Nontunneled CVC | 22 (28.2) | 19 (11.7) | 1.9 (1.3‐2.7) | 0.004 | 3.6 (0.7‐17.7) | 0.11 |
| Tunneled CVC | 15 (19.2) | 24 (14.7) | 1.2 (0.8‐1.9) | 0.36 | ||
| AV fistula | 0 | 10 (6.1) | 0.1 (0.09‐2) | 0.15 | ||
| Other | 4 (5.1) | 11 (6.7) | 0.8 (0.3‐1.9) | 0.64 | ||
| Onset | ||||||
| Community onset | 46 (59) | 108 (66.3) | 0.8 (0.6‐1.2) | 0.27 | ||
| Hospital onset | 32 (41) | 55 (33.7) | 1.2 (0.8‐1.8) | 0.27 | ||
| Source | ||||||
| CLABSI | 15 (19.2) | 38 (23.3) | 0.8 (0.5‐1.4) | 0.48 | ||
| SSTI | 12 (15.4) | 32 (19.6) | 0.8 (0.5‐1.4) | 0.44 | ||
| Endocarditis | 14 (17.9) | 3 (1.8) | 2.9 (2.1‐3.9) | <0.0001 | 9.4 (2.2‐1.1) | 0.003 |
| Thrombophlebitis | 0 | 4 (2.4) | 0.3 (0.02‐4.2) | 0.37 | ||
| Prostatic abscess | 1 (1.3) | 3 (1.8) | 0.8 (0.1‐4.2) | 0.76 | ||
| Paravertebral abscess | 0 | 4 (2.4) | 0.3 (0.02‐4.2) | 0.37 | ||
| Mediastinal abscess | 1 (1.3) | 2 (1.2) | 1.03 (0.2‐5.1) | 0.97 | ||
| CAP | 4 (5.1) | 4 (2.4) | 1.5 (0.8‐3.2) | 0.21 | ||
| VAP | 2 (2.6) | 3 (1.8) | 1.2 (0.4‐3.7) | 0.7 | ||
| Surgical site infection | 1 (1.3) | 2 (1.2) | 1.03 (0.2‐5.2) | 0.97 | ||
| Ventriculostomy | 0 | 1 (0.6) | 0.8 (0.1‐8.5) | 0.82 | ||
| Bone or joint infection | 1 (1.3) | 4 (2.4) | 0.6 (0.1‐3.6) | 0.59 | ||
| Unknown | 27 (34.6) | 63 (38.6) | 0.9 (0.6‐1.3) | 0.55 | ||
Performance of Process of Care and Association With Outcomes
The analysis of the performance of the processes of care and outcomes is shown in Table 3. After adjusting for relevant clinical and demographic characteristics, and those with a level of significance of <0.05, obtaining follow‐up blood cultures more than 4 days after the onset of bacteremia independently increased the risk of clinical failure (RR: 6.5; 95% CI: 2.1‐20.5; P=0.001). When consultation with an ID specialist was obtained within the first 6 days from onset of bacteremia, the risk of clinical failure was 0.3 (95% CI: 0.1‐0.9; P=0.03); however, consultation with an ID specialist overall was not associated with clinical failure (RR: 1; 95% CI: 0.7‐1.4; P=0.98).
| Variable | Clinical Failure, n=78 (%) | No Clinical Failure, n=163 (%) | Unadjusted RR (CI) | P Value* | Adjusted OR (CI) | P Value* |
|---|---|---|---|---|---|---|
| ||||||
| Timing of follow‐up blood culture, n=200 | ||||||
| Less than 2 days | 30 (19.2) | 87 (53.4) | 0.7 (0.5‐0.9) | 0.01 | 1.2 (0.5‐2.9) | 0.60 |
| 24 days (ref) | 16 (20.5) | 39 (23.9) | 0.9 (0.8‐1.1) | 0.53 | ||
| More than 4 days | 19 (24.3) | 9 (5.5) | 1.3 (1.1‐1.5) | <0.0001 | 6.6 (2.1‐20.5) | 0.001 |
| Early antibiotic therapy, n=232 | 66 (84.6) | 132 (81) | 1.2 (0.7‐2.3) | 0.45 | ||
| Monitoring of vancomycin levels, n=156 | 37 (20.8) | 97 (59.5) | 0.8 (0.6‐1.03) | 0.09 | ||
| Therapy with ‐lactam, n=103‖ | 7 (8.8) | 49 (30.1) | 0.4 (0.2‐0.8) | 0.01 | 0.1 (0.04‐0.5) | 0.002 |
| Consultation with ID specialist, n=241 | 31 (39.7) | 66 (40.5) | 1 (0.7‐1.4) | 0.98 | ||
| Early consultation with ID specialist, n=97# | 19 (24.3) | 56 (34.3) | 0.5 (0.3‐0.8) | 0.006 | 0.3 (0.1‐0.9) | 0.03 |
| Echocardiography, n=241 | 45 (57.7) | 96 (58.9) | 1 (0.7‐1.4) | 0.86 | ||
| Early echocardiography, n=141** | 35 (44.9) | 91 (55.8) | 0.7 (0.5‐1.07) | 0.11 | ||
A comparison of the average number of days to performance of processes of care is presented in Table 4. Patients with clinical failure had significantly greater elapsed time from the first positive blood culture to the first follow‐up blood culture as compared to those who did not have clinical failure (mean 2.321.3 days vs 3.883.37; P<0.0001). Forty‐one patients (17.1%) failed to have at least 1 follow‐up blood culture.
| Process of Care | Clinical Failure | No Clinical Failure | P Value* |
|---|---|---|---|
| |||
| First follow‐up blood culture, n=200 | 3.883.37 | 2.321.3 | <0.0001 |
| Consultation with infectious diseases, n=97 | 6.96.55 | 4.354.34 | 0.06 |
| First antibiotic dose, n=232 | 0.431.05 | 0.57 1.11 | 0.63 |
| First dose of ‐lactam, n=56 | 4.41.6 | 3.51.4 | 0.1 |
| First vancomycin trough, n=156 | 2.632.04 | 2.552.02 | 0.81 |
| Echocardiography, n=141 | 3.421.74 | 3.312.05 | 0.47 |
Among patients with clinical failure, an ID specialist was consulted at a mean time of 7 days from the onset of bacteremia, compared to patients with no clinical failure in whom a consult was obtained at a mean of 4 days (P=0.06) (Table 4). Overall, ID specialists were only consulted in 97/241 (40.2%) episodes.
Echocardiographic studies were performed in 141/241 (58.5)% of episodes, and they were more likely to be obtained when an ID specialist was consulted (RR: 1.7; 95% CI: 1.4‐2.1; P<0.0001). Lack of performance of these studies was not associated with clinical failure (Table 3).
Antibiotic Administration and De‐escalation of Therapy
There were no significant differences in the average time from the first positive blood culture to the administration of antibiotics between patients who had clinical failure and those who did not (0.571.11 vs 0.431.05; P=0.63) (Table 4).
Patients with MSSA BSI and no documented penicillin allergy were treated with ‐lactam or cephalosporin antibiotics in 56/103(54.3%) episodes. Patients were 2.5 times more likely to receive ‐lactam antibiotics when an ID specialist was consulted (95% CI: 1.8‐3.5; P<0.0001). Among patients with MSSA BSI, treatment with ‐lactams was an independent predictor of decreased risk of clinical failure (RR: 0.2; 95% CI: 0.07‐0.9; P=0.005) (Table 3).
DISCUSSION
Our study showed a significant rate of morbidity associated with S aureus bacteremia and identified processes of care in the management of SAB that impact patient outcomes.
Our results show that early consultation with an ID specialist was associated with a decreased risk of developing clinical failure, increased likelihood of identification of a source of infection, and positively impacted administration of appropriate antibiotic therapy, especially in cases of MSSA BSI, with overall improvement in patient outcomes. However, consultation with an ID specialist was only obtained in 40.2% of our cases, which is consistent with published data.[10, 11, 12, 13] Consultation with an ID specialist itself did not impact clinical failure, but rather timeliness in obtaining expert guidance was associated with better outcomes. As shown in previous studies,[10, 11, 12, 13, 14] compliance with the standards of care and patient prognosis are improved when ID specialists are involved in the management of SAB. Our study reiterates that early consultation with an ID specialist has a positive outcome in patient care, as opposed to delaying consultation once the patient has persistent bacteremia for more than 7 days. This association could be explained by considering that the majority of the standards of care are time sensitive, which include: obtaining surveillance blood cultures 48 to 96 hours after initial detection[10] or initiating therapy,[11, 14] removal of foci of infection,[10, 11, 12, 14] use of parenteral ‐lactams for the treatment of MSSA,[10, 11, 13, 14] performing echocardiography when clinically indicated,[10, 11, 13, 14] and appropriate duration of therapy.[10, 13, 14] Importantly, studies have shown that when ID specialists' recommendations are followed, patients are more likely to be cured,[10, 11, 13] and are less likely to relapse.[10, 11, 12] Given the complexities of treating patients with SAB and high rates of clinical failures, routine guidance could be beneficial to healthcare providers as part of a multidisciplinary structured strategy that is set in motion the moment a patient with SAB is identified by the microbiology laboratory. The processes of care outlined in this this study can serve as quality of care indicators and be integrated into a structured strategy to optimize the management of SAB.
Regarding optimal timing for follow‐up blood cultures, our results show that delays in obtaining follow‐up blood cultures (more than 4 days from onset of bacteremia) was independently associated with increased risk of clinical failure. Timely follow‐up blood cultures have been previously identified as quality of care indicators.[10, 11, 13, 14] Compliance with obtaining follow‐up blood cultures improves when this step is integrated into a bundle of care.[14]
Antimicrobial therapy was promptly initiated in the majority of the patients in our study. However, areas for improvement were identified. Vancomycin was the empirical therapy of choice in most of the cases, but an appropriate dose was only received by 65% of the patients, and vancomycin levels after the fourth dose were obtained in 85.9% of instances when indicated. Although in our cohort these results were not significantly associated with clinical failure, previous studies have described attainment of a target therapeutic vancomycin trough (1520 mg/dL) as a factor for treatment success.[17, 18] This problem could be addressed through physician education on therapeutic drug monitoring,[19] as well as through an ASP intervention, which have successfully led efforts to improve vancomycin utilization and dosing.[20] Among patients with MSSA BSI, therapy with ‐lactams was associated with improved outcomes, and was more likely to be administered when an ID specialist was consulted. This is in accordance with previous studies that have shown that higher rates of appropriate antimicrobial therapy are achieved when ID specialists are involved in management of SAB.[10, 11, 13, 14] The use of ‐lactams for treatment of MSSA BSI has been consistently associated with lower SAB‐related mortality and relapse.[21, 22, 23, 24, 25, 26]
Echocardiographic studies were obtained in only half of the patients in our cohort, and they were twice more likely to be obtained when an ID specialist was consulted. Although we did not evaluate the appropriateness of the echocardiographic study, the increased proportion of studies performed when ID specialists were consulted could indicate a more in‐depth evaluation of the case. Moreover, in our cohort, when ID specialists where involved in direct patient care, a source of infection was more likely to be identified. This is in accordance with previous studies proposing that because evaluation by ID specialists are more detailed, they lead to increased use in ancillary studies and recognition of complicated cases.[10, 12]
Limitations of this study include its retrospective design and the fact that it was performed in a single institution. The source of infection was defined as documented by treating providers and not by independent diagnostic criteria. Antibiotic use was collected throughout duration of admission, and was not followed after patients were discharged, as these data were not available on the electronic medical record for all patients. Deaths that may have occurred after hospital discharge were not included. We did not account for elevated vancomycin minimum inhibitory concentration as a risk factor for the main outcome, and adjustment of vancomycin based on serum levels was not factored in. Acute kidney injury was accounted for anytime during hospitalization, but not in relation to antimicrobial administration. Despite the limitations, our study has strengths that make our results generalizable. Although our institution is a single medical center, it serves a large and diverse population as reflected in our cases. Even though this is a retrospective cohort study, the use of a centralized electronic medical record allowed us to identify each aspect of the management of SAB, as implemented by different treating services (medical and surgical), as continuous variables (days) rather than only in a dichotomous fashion. Moreover, by being a community teaching hospital, we were able to explore aspects of the practice of physicians in training versus practicing clinicians. These results could be extrapolated to other healthcare facilities aiming to improve the management of SAB.
CONCLUSIONS
Our results suggest that obtaining timely follow‐up blood cultures, use of ‐lactams in patients with MSSA BSI, and early consultation with infectious diseases are the processes of care that could serve as quality and patient‐safety indicators for the management of SAB. These results contribute to a growing body of evidence supporting the implementation of structured processes of care to optimize the management and clinical outcomes of hospitalized patients with SAB.
Disclosure: Nothing to report.
- , , , , , . Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–317.
- , , , . Laboratory‐based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann Clin Microbiol Antimicrob. 2006;5:2.
- , , , , , . The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005;26(2):166–174.
- , , , . Staphylococcal bacteremia and altered host resistance. Ann Intern Medicine. 1968;69(5):859–873.
- , , , , , . Comparison of mortality associated with methicillin‐resistant and methicillin‐susceptible Staphylococcus aureus bacteremia: a meta‐analysis. Clin Infect Dis. 2003;36(1):53–59.
- . Unfavourable prognostic factors in Staphylococcus aureus septicemia and endocarditis. Scand J Infect Dis. 1985;17(2):179–187.
- , , , et al. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample Database. Arch Intern Med. 2005;165(15):1756–1761.
- , , , et al. Health care–associated infections: a meta‐analysis of costs and financial impact on the us health care system. JAMA Intern Med. 2013;173(22):2039–2046.
- , , , et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin‐resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–e55.
- , , , , . Impact of routine infectious diseases service consultation on the evaluation, management, and outcomes of Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46(7):1000–1008.
- , , , et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis. 1998;27(3):478–486.
- , , , . Infectious disease consultation for Staphylococcus aureus bacteremia improves patient management and outcomes. Infect Dis Clin Pract (Baltim Md). 2012;20(4):261–267.
- , , , , . The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med. 2010;123(7):631–637.
- , , , et al. Impact of an evidence‐based bundle intervention in the quality‐of‐care management and outcome of Staphylococcus aureus bacteremia. Clin Infect Dis. 2013;57(9):1225–1233.
- , , , et al. Comparison of prior authorization and prospective audit with feedback for antimicrobial stewardship. Infect Control Hosp Epidemiol. 2014;35(9):1092–1099.
- Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty‐First Informational Supplement. Wayne, PA: Clinical Laboratory Standards Institute; 2011.
- , , , . Impact of vancomycin exposure on outcomes in patients with methicillin‐resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011;52(8):975–981.
- , , , , . High‐dose vancomycin therapy for methicillin‐resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166(19):2138–2144.
- , , , . Strategies for physician education in therapeutic drug monitoring. Clin Chem. 1998;44(2):401–407.
- , . Impact of antimicrobial stewardship program on vancomycin use in a pediatric teaching hospital. Pediatr Infect Dis J. 2010;29(8):707–711.
- , . Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother. 1990;34(6):1227–1231.
- , , , et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine. 2003;82(5):322–332.
- , , , . Impact of empirical‐therapy selection on outcomes of intravenous drug users with infective endocarditis caused by methicillin‐susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51(10):3731–3733.
- , , , et al. Use of vancomycin or first‐generation cephalosporins for the treatment of hemodialysis‐dependent patients with methicillin‐susceptible Staphylococcus aureus bacteremia. Clin Infect Dis. 2007;44(2):190–196.
- , , , et al. Outcome of vancomycin treatment in patients with methicillin‐susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2008;52(1):192–197.
- , , , et al. Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin‐susceptible Staphylococcus aureus bacteremia. BMC Infect Dis. 2011;11:279.
- , , , , , . Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–317.
- , , , . Laboratory‐based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann Clin Microbiol Antimicrob. 2006;5:2.
- , , , , , . The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005;26(2):166–174.
- , , , . Staphylococcal bacteremia and altered host resistance. Ann Intern Medicine. 1968;69(5):859–873.
- , , , , , . Comparison of mortality associated with methicillin‐resistant and methicillin‐susceptible Staphylococcus aureus bacteremia: a meta‐analysis. Clin Infect Dis. 2003;36(1):53–59.
- . Unfavourable prognostic factors in Staphylococcus aureus septicemia and endocarditis. Scand J Infect Dis. 1985;17(2):179–187.
- , , , et al. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample Database. Arch Intern Med. 2005;165(15):1756–1761.
- , , , et al. Health care–associated infections: a meta‐analysis of costs and financial impact on the us health care system. JAMA Intern Med. 2013;173(22):2039–2046.
- , , , et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin‐resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–e55.
- , , , , . Impact of routine infectious diseases service consultation on the evaluation, management, and outcomes of Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46(7):1000–1008.
- , , , et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis. 1998;27(3):478–486.
- , , , . Infectious disease consultation for Staphylococcus aureus bacteremia improves patient management and outcomes. Infect Dis Clin Pract (Baltim Md). 2012;20(4):261–267.
- , , , , . The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med. 2010;123(7):631–637.
- , , , et al. Impact of an evidence‐based bundle intervention in the quality‐of‐care management and outcome of Staphylococcus aureus bacteremia. Clin Infect Dis. 2013;57(9):1225–1233.
- , , , et al. Comparison of prior authorization and prospective audit with feedback for antimicrobial stewardship. Infect Control Hosp Epidemiol. 2014;35(9):1092–1099.
- Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty‐First Informational Supplement. Wayne, PA: Clinical Laboratory Standards Institute; 2011.
- , , , . Impact of vancomycin exposure on outcomes in patients with methicillin‐resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011;52(8):975–981.
- , , , , . High‐dose vancomycin therapy for methicillin‐resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166(19):2138–2144.
- , , , . Strategies for physician education in therapeutic drug monitoring. Clin Chem. 1998;44(2):401–407.
- , . Impact of antimicrobial stewardship program on vancomycin use in a pediatric teaching hospital. Pediatr Infect Dis J. 2010;29(8):707–711.
- , . Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother. 1990;34(6):1227–1231.
- , , , et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine. 2003;82(5):322–332.
- , , , . Impact of empirical‐therapy selection on outcomes of intravenous drug users with infective endocarditis caused by methicillin‐susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51(10):3731–3733.
- , , , et al. Use of vancomycin or first‐generation cephalosporins for the treatment of hemodialysis‐dependent patients with methicillin‐susceptible Staphylococcus aureus bacteremia. Clin Infect Dis. 2007;44(2):190–196.
- , , , et al. Outcome of vancomycin treatment in patients with methicillin‐susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2008;52(1):192–197.
- , , , et al. Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin‐susceptible Staphylococcus aureus bacteremia. BMC Infect Dis. 2011;11:279.
© 2015 Society of Hospital Medicine
Measuring Patient Experiences
The hospitalized patient experience has become an area of increased focus for hospitals given the recent coupling of patient satisfaction to reimbursement rates for Medicare patients.[1] Although patient experiences are multifactorial, 1 component is the relationship that hospitalized patients develop with their inpatient physicians. In recognition of the importance of this relationship, several organizations including the Society of Hospital Medicine, Society of General Internal Medicine, American College of Physicians, the American College of Emergency Physicians, and the Accreditation Council for Graduate Medical Education have recommended that patients know and understand who is guiding their care at all times during their hospitalization.[2, 3] Unfortunately, previous studies have shown that hospitalized patients often lack the ability to identify[4, 5] and understand their course of care.[6, 7] This may be due to numerous clinical factors including lack of a prior relationship, rapid pace of clinical care, and the frequent transitions of care found in both hospitalists and general medicine teaching services.[5, 8, 9] Regardless of the cause, one could hypothesize that patients who are unable to identify or understand the role of their physician may be less informed about their hospitalization, which may lead to further confusion, dissatisfaction, and ultimately a poor experience.
Given the proliferation of nonteaching hospitalist services in teaching hospitals, it is important to understand if patient experiences differ between general medicine teaching and hospitalist services. Several reasons could explain why patient experiences may vary on these services. For example, patients on a hospitalist service will likely interact with a single physician caretaker, which may give a feeling of more personalized care. In contrast, patients on general medicine teaching services are cared for by larger teams of residents under the supervision of an attending physician. Residents are also subjected to duty‐hour restrictions, clinic responsibilities, and other educational requirements that may impede the continuity of care for hospitalized patients.[10, 11, 12] Although 1 study has shown that hospitalist‐intensive hospitals perform better on patient satisfaction measures,[13] no study to date has compared patient‐reported experiences on general medicine teaching and nonteaching hospitalist services. This study aimed to evaluate the hospitalized patient experience on both teaching and nonteaching hospitalist services by assessing several patient‐reported measures of their experience, namely their confidence in their ability to identify their physician(s), understand their roles, and their rating of both the coordination and overall care.
METHODS
Study Design
We performed a retrospective cohort analysis at the University of Chicago Medical Center between July 2007 and June 2013. Data were acquired as part of the Hospitalist Project, an ongoing study that is used to evaluate the impact of hospitalists, and now serves as infrastructure to continue research related to hospital care at University of Chicago.[14] Patients were cared for by either the general medicine teaching service or the nonteaching hospitalist service. General medicine teaching services were composed of an attending physician who rotates for 2 weeks at a time, a second‐ or third‐year medicine resident, 1 to 2 medicine interns, and 1 to 2 medical students.[15] The attending physician assigned to the patient's hospitalization was the attending listed on the first day of hospitalization, regardless of the length of hospitalization. Nonteaching hospitalist services consisted of a single hospitalist who worked 7‐day shifts, and were assisted by a nurse practitioner/physician's assistant (NPA). The majority of attendings on the hospitalist service were less than 5 years out of residency. Both services admitted 7 days a week, with patients initially admitted to the general medicine teaching service until resident caps were met, after which all subsequent admissions were admitted to the hospitalist service. In addition, the hospitalist service is also responsible for specific patient subpopulations, such as lung and renal transplants, and oncologic patients who have previously established care with our institution.
Data Collection
During a 30‐day posthospitalization follow‐up questionnaire, patients were surveyed regarding their confidence in their ability to identify and understand the roles of their physician(s) and their perceptions of the overall coordination of care and their overall care, using a 5‐point Likert scale (1 = poor understanding to 5 = excellent understanding). Questions related to satisfaction with care and coordination were derived from the Picker‐Commonwealth Survey, a previously validated survey meant to evaluate patient‐centered care.[16] Patients were also asked to report their race, level of education, comorbid diseases, and whether they had any prior hospitalizations within 1 year. Chart review was performed to obtain patient age, gender, and hospital length of stay (LOS), and calculated Charlson Comorbidity Index (CCI).[17] Patients with missing data or responses to survey questions were excluded from final analysis. The University of Chicago Institutional Review Board approved the study protocol, and all patients provided written consented prior to participation.
Data Analysis
After initial analysis noted that outcomes were skewed, the decision was made to dichotomize the data and use logistic rather than linear regression models. Patient responses to the follow‐up phone questionnaire were dichotomized to reflect the top 2 categories (excellent and very good). Pearson 2 analysis was used to assess for any differences in demographic characteristics, disease severity, and measures of patient experience between the 2 services. To assess if service type was associated with differences in our 4 measures of patient experience, we created a 3‐level mixed‐effects logistic regression using a logit function while controlling for age, gender, race, CCI, LOS, previous hospitalizations within 1 year, level of education, and academic year. These models studied the longitudinal association between teaching service and the 4 outcome measures, while also controlling for the cluster effect of time nested within individual patients who were clustered within physicians. The model included random intercepts at both the patient and physician level and also included a random effect of service (teaching vs nonteaching) at the patient level. A Hausman test was used to determine if these random‐effects models improved fit over a fixed‐effects model, and the intraclass correlations were compared using likelihood ratio tests to determine the appropriateness of a 3‐level versus 2‐level model. Data management and 2 analyses were performed using Stata version 13.0 (StataCorp, College Station, TX), and mixed‐effects regression models were done in SuperMix (Scientific Software International, Skokie, IL).
RESULTS
In total, 14,855 patients were enrolled during their hospitalization with 57% and 61% completing the 30‐day follow‐up survey on the hospitalist and general medicine teaching service, respectively. In total, 4131 (69%) and 4322 (48%) of the hospitalist and general medicine services, respectively, either did not answer all survey questions, or were missing basic demographic data, and thus were excluded. Data from 4591 patients on the general medicine teaching (52% of those enrolled at hospitalization) and 1811 on the hospitalist service (31% of those enrolled at hospitalization) were used for final analysis (Figure 1). Respondents were predominantly female (61% and 56%), African American (75% and 63%), with a mean age of 56.2 (19.4) and 57.1 (16.1) years, for the general medicine teaching and hospitalist services, respectively. A majority of patients (71% and 66%) had a CCI of 0 to 3 on both services. There were differences in self‐reported comorbidities between the 2 groups, with hospitalist services having a higher prevalence of cancer (20% vs 7%), renal disease (25% vs 18%), and liver disease (23% vs 7%). Patients on the hospitalist service had a longer mean LOS (5.5 vs 4.8 days), a greater percentage of a hospitalization within 1 year (58% vs 52%), and a larger proportion who were admitted in 2011 to 2013 compared to 2007 to 2010 (75% vs 39%), when compared to the general medicine teaching services. Median LOS and interquartile ranges were similar between both groups. Although most baseline demographics were statistically different between the 2 groups (Table 1), these differences were likely clinically insignificant. Compared to those who responded to the follow‐up survey, nonresponders were more likely to be African American (73% and 64%, P < 0.001) and female (60% and 56%, P < 0.01). The nonresponders were more likely to be hospitalized in the past 1 year (62% and 53%, P < 0.001) and have a lower CCI (CCI 03 [75% and 80%, P < 0.001]) compared to responders. Demographics between responders and nonresponders were also statistically different from one another.
| Variable | General Medicine Teaching | Nonteaching Hospitalist | P Value |
|---|---|---|---|
| |||
| Total (n) | 4,591 | 1,811 | <0.001 |
| Attending classification, hospitalist, n (%) | 1,147 (25) | 1,811 (100) | |
| Response rate, % | 61 | 57 | <0.01 |
| Age, y, mean SD | 56.2 19.4 | 57.1 16.1 | <0.01 |
| Gender, n (%) | <0.01 | ||
| Male | 1,796 (39) | 805 (44) | |
| Female | 2,795 (61) | 1,004 (56) | |
| Race, n (%) | <0.01 | ||
| African American | 3,440 (75) | 1,092 (63) | |
| White | 900 (20) | 571 (32) | |
| Asian/Pacific | 38 (1) | 17 (1) | |
| Other | 20 (1) | 10 (1) | |
| Unknown | 134 (3) | 52 (3) | |
| Charlson Comorbidity Index, n (%) | <0.001 | ||
| 0 | 1,635 (36) | 532 (29) | |
| 12 | 1,590 (35) | 675 (37) | |
| 39 | 1,366 (30) | 602 (33) | |
| Self‐reported comorbidities | |||
| Anemia/sickle cell disease | 1,201 (26) | 408 (23) | 0.003 |
| Asthma/COPD | 1,251 (28) | 432 (24) | 0.006 |
| Cancer* | 300 (7) | 371 (20) | <0.001 |
| Depression | 1,035 (23) | 411 (23) | 0.887 |
| Diabetes | 1,381 (30) | 584 (32) | 0.087 |
| Gastrointestinal | 1,140 (25) | 485 (27) | 0.104 |
| Cardiac | 1,336 (29) | 520 (29) | 0.770 |
| Hypertension | 2,566 (56) | 1,042 (58) | 0.222 |
| HIV/AIDS | 151 (3) | 40 (2) | 0.022 |
| Kidney disease | 828 (18) | 459 (25) | <0.001 |
| Liver disease | 313 (7) | 417 (23) | <0.001 |
| Stroke | 543 (12) | 201 (11) | 0.417 |
| Education level | 0.066 | ||
| High school | 2,248 (49) | 832 (46) | |
| Junior college/college | 1,878 (41) | 781 (43) | |
| Postgraduate | 388 (8) | 173 (10) | |
| Don't know | 77 (2) | 23 (1) | |
| Academic year, n (%) | <0.001 | ||
| July 2007 June 2008 | 938 (20) | 90 (5) | |
| July 2008 June 2009 | 702 (15) | 148 (8) | |
| July 2009 June 2010 | 576(13) | 85 (5) | |
| July 2010 June 2011 | 602 (13) | 138 (8) | |
| July 2011 June 2012 | 769 (17) | 574 (32) | |
| July 2012 June 2013 | 1,004 (22) | 774 (43) | |
| Length of stay, d, mean SD | 4.8 7.3 | 5.5 6.4 | <0.01 |
| Prior hospitalization (within 1 year), yes, n (%) | 2,379 (52) | 1,039 (58) | <0.01 |
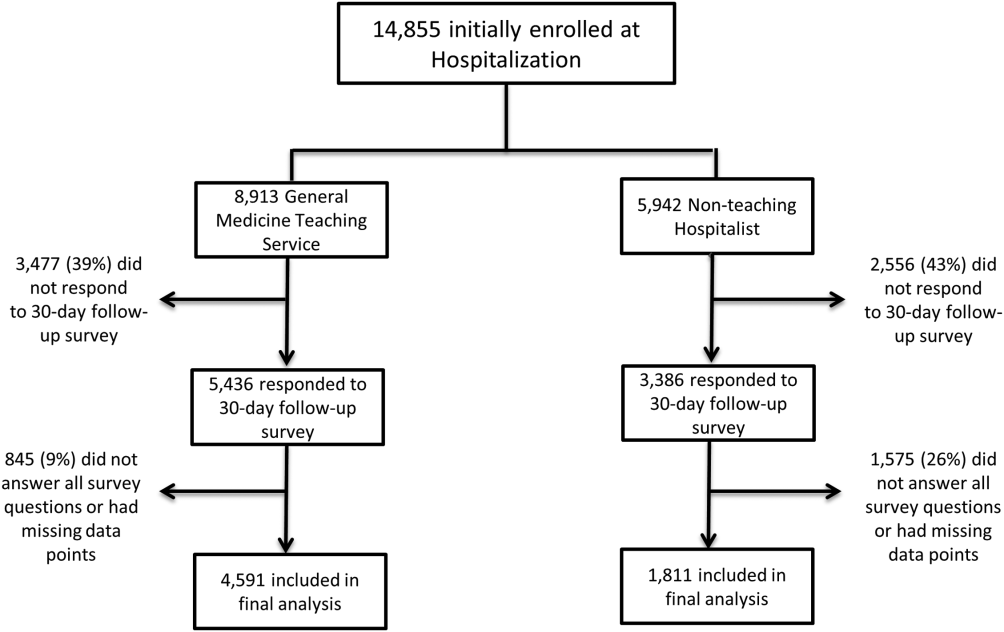
Unadjusted results revealed that patients on the hospitalist service were more confident in their abilities to identify their physician(s) (50% vs 45%, P < 0.001), perceived greater ability in understanding the role of their physician(s) (54% vs 50%, P < 0.001), and reported greater satisfaction with coordination and teamwork (68% vs 64%, P = 0.006) and with overall care (73% vs 67%, P < 0.001) (Figure 2).
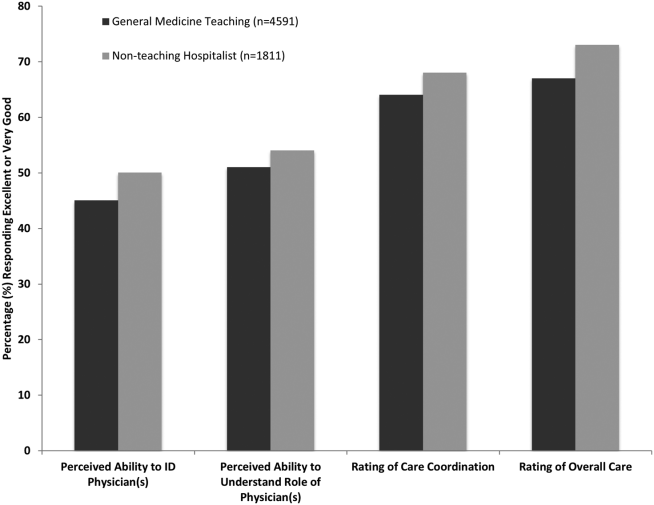
From the mixed‐effects regression models it was discovered that admission to the hospitalist service was associated with a higher odds ratio (OR) of reporting overall care as excellent or very good (OR: 1.33; 95% confidence interval [CI]: 1.15‐1.47). There was no difference between services in patients' ability to identify their physician(s) (OR: 0.89; 95% CI: 0.61‐1.11), in patients reporting a better understanding of the role of their physician(s) (OR: 1.09; 95% CI: 0.94‐1.23), or in their rating of overall coordination and teamwork (OR: 0.71; 95% CI: 0.42‐1.89).
A subgroup analysis was performed on the 25% of hospitalist attendings in the general medicine teaching service comparing this cohort to the hospitalist services, and it was found that patients perceived better overall care on the hospitalist service (OR: 1.17; 95% CI: 1.01‐ 1.31) than on the general medicine service (Table 2). All other domains in the subgroup analysis were not statistically significant. Finally, an ordinal logistic regression was performed for each of these outcomes, but it did not show any major differences compared to the logistic regression of dichotomous outcomes.
| Domains in Patient Experience* | Odds Ratio (95% CI) | P Value |
|---|---|---|
| ||
| How would you rate your ability to identify the physicians and trainees on your general medicine team during the hospitalization? | ||
| Model 1 | 0.89 (0.611.11) | 0.32 |
| Model 2 | 0.98 (0.671.22) | 0.86 |
| How would you rate your understanding of the roles of the physicians and trainees on your general medicine team? | ||
| Model 1 | 1.09 (0.941.23) | 0.25 |
| Model 2 | 1.19 (0.981.36) | 0.08 |
| How would you rate the overall coordination and teamwork among the doctors and nurses who care for you during your hospital stay? | ||
| Model 1 | 0.71 (0.421.89) | 0.18 |
| Model 2 | 0.82 (0.651.20) | 0.23 |
| Overall, how would you rate the care you received at the hospital? | ||
| Model 1 | 1.33 (1.151.47) | 0.001 |
| Model 2 | 1.17 (1.011.31) | 0.04 |
DISCUSSION
This study is the first to directly compare measures of patient experience on hospitalist and general medicine teaching services in a large, multiyear comparison across multiple domains. In adjusted analysis, we found that patients on nonteaching hospitalist services rated their overall care better than those on general medicine teaching services, whereas no differences in patients' ability to identify their physician(s), understand their role in their care, or rating of coordination of care were found. Although the magnitude of the differences in rating of overall care may appear small, it remains noteworthy because of the recent focus on patient experience at the reimbursement level, where small differences in performance can lead to large changes in payment. Because of the observational design of this study, it is important to consider mechanisms that could account for our findings.
The first are the structural differences between the 2 services. Our subgroup analysis comparing patients rating of overall care on a general medicine service with a hospitalist attending to a pure hospitalist cohort found a significant difference between the groups, indicating that the structural differences between the 2 groups may be a significant contributor to patient satisfaction ratings. Under the care of a hospitalist service, a patient would only interact with a single physician on a daily basis, possibly leading to a more meaningful relationship and improved communication between patient and provider. Alternatively, while on a general medicine teaching service, patients would likely interact with multiple physicians, as a result making their confidence in their ability to identify and perception at understanding physicians' roles more challenging.[18] This dilemma is further compounded by duty hour restrictions, which have subsequently led to increased fragmentation in housestaff scheduling. The patient experience on the general medicine teaching service may be further complicated by recent data that show residents spend a minority of time in direct patient care,[19, 20] which could additionally contribute to patients' inability to understand who their physicians are and to the decreased satisfaction with their care. This combination of structural complexity, duty hour reform, and reduced direct patient interaction would likely decrease the chance a patient will interact with the same resident on a consistent basis,[5, 21] thus making the ability to truly understand who their caretakers are, and the role they play, more difficult.
Another contributing factor could be the use of NPAs on our hospitalist service. Given that these providers often see the patient on a more continual basis, hospitalized patients' exposure to a single, continuous caretaker may be a factor in our findings.[22] Furthermore, with studies showing that hospitalists also spend a small fraction of their day in direct patient care,[23, 24, 25] the use of NPAs may allow our hospitalists to spend greater amounts of time with their patients, thus improving patients' rating of their overall care and influencing their perceived ability to understand their physician's role.
Although there was no difference between general medicine teaching and hospitalist services with respect to patient understanding of their roles, our data suggest that both groups would benefit from interventions to target this area. Focused attempts at improving patient's ability to identify and explain the roles of their inpatient physician(s) have been performed. For example, previous studies have attempted to improve a patient's ability to identify their physician through physician facecards[8, 9] or the use of other simple interventions (ie, bedside whiteboards).[4, 26] Results from such interventions are mixed, as they have demonstrated the capacity to improve patients' ability to identify who their physician is, whereas few have shown any appreciable improvement in patient satisfaction.[26]
Although our findings suggest that structural differences in team composition may be a possible explanation, it is also important to consider how the quality of care a patient receives affects their experience. For instance, hospitalists have been shown to produce moderate improvements in patient‐centered outcomes such as 30‐day readmission[27] and hospital length of stay[14, 28, 29, 30, 31] when compared to other care providers, which in turn could be reflected in the patient's perception of their overall care. In a large national study of acute care hospitals using the Hospital Consumer Assessment of Healthcare Providers and Systems survey, Chen and colleagues found that for most measures of patient satisfaction, hospitals with greater use of hospitalist care were associated with better patient‐centered care.[13] These outcomes were in part driven by patient‐centered domains such as discharge planning, pain control, and medication management. It is possible that patients are sensitive to the improved outcomes that are associated with hospitalist services, and reflect this in their measures of patient satisfaction.
Last, because this is an observational study and not a randomized trial, it is possible that the clinical differences in the patients cared for by these services could have led to our findings. Although the clinical significance of the differences in patient demographics were small, patients seen on the hospitalist service were more likely to be older white males, with a slightly longer LOS, greater comorbidities, and more hospitalizations in the previous year than those seen on the general medicine teaching service. Additionally, our hospitalist service frequently cares for highly specific subpopulations (ie, liver and renal transplant patients, and oncology patients), which could have influenced our results. For example, transplant patients who may be very grateful for their second chance, are preferentially admitted to the hospitalist service, which could have biased our results in favor of hospitalists.[32] Unfortunately, we were unable to control for all such factors.
Although we hope that multivariable analysis can adjust for many of these differences, we are not able to account for possible unmeasured confounders such as time of day of admission, health literacy, personality differences, physician turnover, or nursing and other ancillary care that could contribute to these findings. In addition to its observational study design, our study has several other limitations. First, our study was performed at a single institution, thus limiting its generalizability. Second, as a retrospective study based on observational data, no definitive conclusions regarding causality can be made. Third, although our response rate was low, it is comparable to other studies that have examined underserved populations.[33, 34] Fourth, because our survey was performed 30 days after hospitalization, this may impart imprecision on our outcomes measures. Finally, we were not able to mitigate selection bias through imputation for missing data .
All together, given the small absolute differences between the groups in patients' ratings of their overall care compared to large differences in possible confounders, these findings call for further exploration into the significance and possible mechanisms of these outcomes. Our study raises the potential possibility that the structural component of a care team may play a role in overall patient satisfaction. If this is the case, future studies of team structure could help inform how best to optimize this component for the patient experience. On the other hand, if process differences are to explain our findings, it is important to distill the types of processes hospitalists are using to improve the patient experience and potentially export this to resident services.
Finally, if similar results were found in other institutions, these findings could have implications on how hospitals respond to new payment models that are linked to patient‐experience measures. For example, the Hospital Value‐Based Purchasing Program currently links the Centers for Medicare and Medicaid Services payments to a set of quality measures that consist of (1) clinical processes of care (70%) and (2) the patient experience (30%).[1] Given this linkage, any small changes in the domain of patient satisfaction could have large payment implications on a national level.
CONCLUSION
In summary, in this large‐scale multiyear study, patients cared for by a nonteaching hospitalist service reported greater satisfaction with their overall care than patients cared for by a general medicine teaching service. This difference could be mediated by the structural differences between these 2 services. As hospitals seek to optimize patient experiences in an era where reimbursement models are now being linked to patient‐experience measures, future work should focus on further understanding the mechanisms for these findings.
Disclosures
Financial support for this work was provided by the Robert Wood Johnson Investigator Program (RWJF Grant ID 63910 PI Meltzer), a Midcareer Career Development Award from the National Institute of Aging (1 K24 AG031326‐01, PI Meltzer), and a Clinical and Translational Science Award (NIH/NCATS 2UL1TR000430‐08, PI Solway, Meltzer Core Leader). The authors report no conflicts of interest.
- Hospital Consumer Assessment of Healthcare Providers and Systems. HCAHPS fact sheet. CAHPS hospital survey August 2013. Available at: http://www.hcahpsonline.org/files/August_2013_HCAHPS_Fact_Sheet3.pdf. Accessed February 2, 2015.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364–370.
- Accreditation Council for Graduate Medical Education. Common program requirements. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/CPRs2013.pdf. Accessed January 15, 2015.
- , , . Increasing a patient's ability to identify his or her attending physician using a patient room display. Arch Intern Med. 2010;170(12):1084–1085.
- , , , , , . Ability of hospitalized patients to identify their in‐hospital physicians. Arch Intern Med. 2009;169(2):199–201.
- , , , et al. Hospitalized patients' understanding of their plan of care. Mayo Clin Proc. 2010;85(1):47–52.
- , , , et al. Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan. Arch Intern Med. 1997;157(9):1026–1030.
- , , , et al. Improving inpatients' identification of their doctors: use of FACE cards. Jt Comm J Qual Patient Saf. 2009;35(12):613–619.
- , , , , , . The impact of facecards on patients' knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9(3):137–141.
- , , . Restructuring an inpatient resident service to improve outcomes for residents, students, and patients. Acad Med. 2011;86(12):1500–1507.
- , , . Residency training in the modern era: the pipe dream of less time to learn more, care better, and be more professional. Arch Intern Med. 2005;165(22):2561–2562.
- , , , , . Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , , , . Hospitalist staffing and patient satisfaction in the national Medicare population. J Hosp Med. 2013;8(3):126–131.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , , , , . The Effects of on‐duty napping on intern sleep time and fatigue. Ann Intern Med. 2006;144(11):792–798.
- , , , et al. Patients evaluate their hospital care: a national survey. Health Aff (Millwood). 1991;10(4):254–267.
- , , , . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Agency for Healthcare Research and Quality. Welcome to HCUPnet. Available at: http://hcupnet.ahrq.gov/HCUPnet.jsp?Id=F70FC59C286BADCB371(4):293–295.
- , , , et al. In the wake of the 2003 and 2011 duty hours regulations, how do internal medicine interns spend their time? J Gen Intern Med. 2013;28(8):1042–1047.
- , , , , , . The composition of intern work while on call. J Gen Intern Med. 2012;27(11):1432–1437.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- , , , et al. The impact of hospitalist discontinuity on hospital cost, readmissions, and patient satisfaction. J Gen Intern Med. 2014;29(7):1004–1008.
- , , , , . Hospitalist time usage and cyclicality: opportunities to improve efficiency. J Hosp Med. 2010;5(6):329–334.
- , , , et al. Where did the day go?—a time‐motion study of hospitalists. J Hosp Med. 2010;5(6):323–328.
- , , . How hospitalists spend their time: insights on efficiency and safety. J Hosp Med. 2006;1(2):88–93.
- , , . Patient satisfaction associated with correct identification of physician's photographs. Mayo Clin Proc. 2001;76(6):604–608.
- , , , . Comparing patient outcomes of academician‐preceptors, hospitalist‐preceptors, and hospitalists on internal medicine services in an academic medical center. J Gen Intern Med. 2014;29(12):1672–1678.
- , , , . Comparison of processes and outcomes of pneumonia care between hospitalists and community‐based primary care physicians. Mayo Clin Proc. 2002;77(10):1053–1058.
- , , , , , . Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357(25):2589–2600.
- . A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3):248–254.
- , . Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9(1):58.
- , . Patients' experiences of everyday life after lung transplantation. J Clin Nurs. 2009;18(24):3472–3479.
- , , , et al. Optimal design features for surveying low‐income populations. J Health Care Poor Underserved. 2005;16(4):677–690.
The hospitalized patient experience has become an area of increased focus for hospitals given the recent coupling of patient satisfaction to reimbursement rates for Medicare patients.[1] Although patient experiences are multifactorial, 1 component is the relationship that hospitalized patients develop with their inpatient physicians. In recognition of the importance of this relationship, several organizations including the Society of Hospital Medicine, Society of General Internal Medicine, American College of Physicians, the American College of Emergency Physicians, and the Accreditation Council for Graduate Medical Education have recommended that patients know and understand who is guiding their care at all times during their hospitalization.[2, 3] Unfortunately, previous studies have shown that hospitalized patients often lack the ability to identify[4, 5] and understand their course of care.[6, 7] This may be due to numerous clinical factors including lack of a prior relationship, rapid pace of clinical care, and the frequent transitions of care found in both hospitalists and general medicine teaching services.[5, 8, 9] Regardless of the cause, one could hypothesize that patients who are unable to identify or understand the role of their physician may be less informed about their hospitalization, which may lead to further confusion, dissatisfaction, and ultimately a poor experience.
Given the proliferation of nonteaching hospitalist services in teaching hospitals, it is important to understand if patient experiences differ between general medicine teaching and hospitalist services. Several reasons could explain why patient experiences may vary on these services. For example, patients on a hospitalist service will likely interact with a single physician caretaker, which may give a feeling of more personalized care. In contrast, patients on general medicine teaching services are cared for by larger teams of residents under the supervision of an attending physician. Residents are also subjected to duty‐hour restrictions, clinic responsibilities, and other educational requirements that may impede the continuity of care for hospitalized patients.[10, 11, 12] Although 1 study has shown that hospitalist‐intensive hospitals perform better on patient satisfaction measures,[13] no study to date has compared patient‐reported experiences on general medicine teaching and nonteaching hospitalist services. This study aimed to evaluate the hospitalized patient experience on both teaching and nonteaching hospitalist services by assessing several patient‐reported measures of their experience, namely their confidence in their ability to identify their physician(s), understand their roles, and their rating of both the coordination and overall care.
METHODS
Study Design
We performed a retrospective cohort analysis at the University of Chicago Medical Center between July 2007 and June 2013. Data were acquired as part of the Hospitalist Project, an ongoing study that is used to evaluate the impact of hospitalists, and now serves as infrastructure to continue research related to hospital care at University of Chicago.[14] Patients were cared for by either the general medicine teaching service or the nonteaching hospitalist service. General medicine teaching services were composed of an attending physician who rotates for 2 weeks at a time, a second‐ or third‐year medicine resident, 1 to 2 medicine interns, and 1 to 2 medical students.[15] The attending physician assigned to the patient's hospitalization was the attending listed on the first day of hospitalization, regardless of the length of hospitalization. Nonteaching hospitalist services consisted of a single hospitalist who worked 7‐day shifts, and were assisted by a nurse practitioner/physician's assistant (NPA). The majority of attendings on the hospitalist service were less than 5 years out of residency. Both services admitted 7 days a week, with patients initially admitted to the general medicine teaching service until resident caps were met, after which all subsequent admissions were admitted to the hospitalist service. In addition, the hospitalist service is also responsible for specific patient subpopulations, such as lung and renal transplants, and oncologic patients who have previously established care with our institution.
Data Collection
During a 30‐day posthospitalization follow‐up questionnaire, patients were surveyed regarding their confidence in their ability to identify and understand the roles of their physician(s) and their perceptions of the overall coordination of care and their overall care, using a 5‐point Likert scale (1 = poor understanding to 5 = excellent understanding). Questions related to satisfaction with care and coordination were derived from the Picker‐Commonwealth Survey, a previously validated survey meant to evaluate patient‐centered care.[16] Patients were also asked to report their race, level of education, comorbid diseases, and whether they had any prior hospitalizations within 1 year. Chart review was performed to obtain patient age, gender, and hospital length of stay (LOS), and calculated Charlson Comorbidity Index (CCI).[17] Patients with missing data or responses to survey questions were excluded from final analysis. The University of Chicago Institutional Review Board approved the study protocol, and all patients provided written consented prior to participation.
Data Analysis
After initial analysis noted that outcomes were skewed, the decision was made to dichotomize the data and use logistic rather than linear regression models. Patient responses to the follow‐up phone questionnaire were dichotomized to reflect the top 2 categories (excellent and very good). Pearson 2 analysis was used to assess for any differences in demographic characteristics, disease severity, and measures of patient experience between the 2 services. To assess if service type was associated with differences in our 4 measures of patient experience, we created a 3‐level mixed‐effects logistic regression using a logit function while controlling for age, gender, race, CCI, LOS, previous hospitalizations within 1 year, level of education, and academic year. These models studied the longitudinal association between teaching service and the 4 outcome measures, while also controlling for the cluster effect of time nested within individual patients who were clustered within physicians. The model included random intercepts at both the patient and physician level and also included a random effect of service (teaching vs nonteaching) at the patient level. A Hausman test was used to determine if these random‐effects models improved fit over a fixed‐effects model, and the intraclass correlations were compared using likelihood ratio tests to determine the appropriateness of a 3‐level versus 2‐level model. Data management and 2 analyses were performed using Stata version 13.0 (StataCorp, College Station, TX), and mixed‐effects regression models were done in SuperMix (Scientific Software International, Skokie, IL).
RESULTS
In total, 14,855 patients were enrolled during their hospitalization with 57% and 61% completing the 30‐day follow‐up survey on the hospitalist and general medicine teaching service, respectively. In total, 4131 (69%) and 4322 (48%) of the hospitalist and general medicine services, respectively, either did not answer all survey questions, or were missing basic demographic data, and thus were excluded. Data from 4591 patients on the general medicine teaching (52% of those enrolled at hospitalization) and 1811 on the hospitalist service (31% of those enrolled at hospitalization) were used for final analysis (Figure 1). Respondents were predominantly female (61% and 56%), African American (75% and 63%), with a mean age of 56.2 (19.4) and 57.1 (16.1) years, for the general medicine teaching and hospitalist services, respectively. A majority of patients (71% and 66%) had a CCI of 0 to 3 on both services. There were differences in self‐reported comorbidities between the 2 groups, with hospitalist services having a higher prevalence of cancer (20% vs 7%), renal disease (25% vs 18%), and liver disease (23% vs 7%). Patients on the hospitalist service had a longer mean LOS (5.5 vs 4.8 days), a greater percentage of a hospitalization within 1 year (58% vs 52%), and a larger proportion who were admitted in 2011 to 2013 compared to 2007 to 2010 (75% vs 39%), when compared to the general medicine teaching services. Median LOS and interquartile ranges were similar between both groups. Although most baseline demographics were statistically different between the 2 groups (Table 1), these differences were likely clinically insignificant. Compared to those who responded to the follow‐up survey, nonresponders were more likely to be African American (73% and 64%, P < 0.001) and female (60% and 56%, P < 0.01). The nonresponders were more likely to be hospitalized in the past 1 year (62% and 53%, P < 0.001) and have a lower CCI (CCI 03 [75% and 80%, P < 0.001]) compared to responders. Demographics between responders and nonresponders were also statistically different from one another.
| Variable | General Medicine Teaching | Nonteaching Hospitalist | P Value |
|---|---|---|---|
| |||
| Total (n) | 4,591 | 1,811 | <0.001 |
| Attending classification, hospitalist, n (%) | 1,147 (25) | 1,811 (100) | |
| Response rate, % | 61 | 57 | <0.01 |
| Age, y, mean SD | 56.2 19.4 | 57.1 16.1 | <0.01 |
| Gender, n (%) | <0.01 | ||
| Male | 1,796 (39) | 805 (44) | |
| Female | 2,795 (61) | 1,004 (56) | |
| Race, n (%) | <0.01 | ||
| African American | 3,440 (75) | 1,092 (63) | |
| White | 900 (20) | 571 (32) | |
| Asian/Pacific | 38 (1) | 17 (1) | |
| Other | 20 (1) | 10 (1) | |
| Unknown | 134 (3) | 52 (3) | |
| Charlson Comorbidity Index, n (%) | <0.001 | ||
| 0 | 1,635 (36) | 532 (29) | |
| 12 | 1,590 (35) | 675 (37) | |
| 39 | 1,366 (30) | 602 (33) | |
| Self‐reported comorbidities | |||
| Anemia/sickle cell disease | 1,201 (26) | 408 (23) | 0.003 |
| Asthma/COPD | 1,251 (28) | 432 (24) | 0.006 |
| Cancer* | 300 (7) | 371 (20) | <0.001 |
| Depression | 1,035 (23) | 411 (23) | 0.887 |
| Diabetes | 1,381 (30) | 584 (32) | 0.087 |
| Gastrointestinal | 1,140 (25) | 485 (27) | 0.104 |
| Cardiac | 1,336 (29) | 520 (29) | 0.770 |
| Hypertension | 2,566 (56) | 1,042 (58) | 0.222 |
| HIV/AIDS | 151 (3) | 40 (2) | 0.022 |
| Kidney disease | 828 (18) | 459 (25) | <0.001 |
| Liver disease | 313 (7) | 417 (23) | <0.001 |
| Stroke | 543 (12) | 201 (11) | 0.417 |
| Education level | 0.066 | ||
| High school | 2,248 (49) | 832 (46) | |
| Junior college/college | 1,878 (41) | 781 (43) | |
| Postgraduate | 388 (8) | 173 (10) | |
| Don't know | 77 (2) | 23 (1) | |
| Academic year, n (%) | <0.001 | ||
| July 2007 June 2008 | 938 (20) | 90 (5) | |
| July 2008 June 2009 | 702 (15) | 148 (8) | |
| July 2009 June 2010 | 576(13) | 85 (5) | |
| July 2010 June 2011 | 602 (13) | 138 (8) | |
| July 2011 June 2012 | 769 (17) | 574 (32) | |
| July 2012 June 2013 | 1,004 (22) | 774 (43) | |
| Length of stay, d, mean SD | 4.8 7.3 | 5.5 6.4 | <0.01 |
| Prior hospitalization (within 1 year), yes, n (%) | 2,379 (52) | 1,039 (58) | <0.01 |

Unadjusted results revealed that patients on the hospitalist service were more confident in their abilities to identify their physician(s) (50% vs 45%, P < 0.001), perceived greater ability in understanding the role of their physician(s) (54% vs 50%, P < 0.001), and reported greater satisfaction with coordination and teamwork (68% vs 64%, P = 0.006) and with overall care (73% vs 67%, P < 0.001) (Figure 2).

From the mixed‐effects regression models it was discovered that admission to the hospitalist service was associated with a higher odds ratio (OR) of reporting overall care as excellent or very good (OR: 1.33; 95% confidence interval [CI]: 1.15‐1.47). There was no difference between services in patients' ability to identify their physician(s) (OR: 0.89; 95% CI: 0.61‐1.11), in patients reporting a better understanding of the role of their physician(s) (OR: 1.09; 95% CI: 0.94‐1.23), or in their rating of overall coordination and teamwork (OR: 0.71; 95% CI: 0.42‐1.89).
A subgroup analysis was performed on the 25% of hospitalist attendings in the general medicine teaching service comparing this cohort to the hospitalist services, and it was found that patients perceived better overall care on the hospitalist service (OR: 1.17; 95% CI: 1.01‐ 1.31) than on the general medicine service (Table 2). All other domains in the subgroup analysis were not statistically significant. Finally, an ordinal logistic regression was performed for each of these outcomes, but it did not show any major differences compared to the logistic regression of dichotomous outcomes.
| Domains in Patient Experience* | Odds Ratio (95% CI) | P Value |
|---|---|---|
| ||
| How would you rate your ability to identify the physicians and trainees on your general medicine team during the hospitalization? | ||
| Model 1 | 0.89 (0.611.11) | 0.32 |
| Model 2 | 0.98 (0.671.22) | 0.86 |
| How would you rate your understanding of the roles of the physicians and trainees on your general medicine team? | ||
| Model 1 | 1.09 (0.941.23) | 0.25 |
| Model 2 | 1.19 (0.981.36) | 0.08 |
| How would you rate the overall coordination and teamwork among the doctors and nurses who care for you during your hospital stay? | ||
| Model 1 | 0.71 (0.421.89) | 0.18 |
| Model 2 | 0.82 (0.651.20) | 0.23 |
| Overall, how would you rate the care you received at the hospital? | ||
| Model 1 | 1.33 (1.151.47) | 0.001 |
| Model 2 | 1.17 (1.011.31) | 0.04 |
DISCUSSION
This study is the first to directly compare measures of patient experience on hospitalist and general medicine teaching services in a large, multiyear comparison across multiple domains. In adjusted analysis, we found that patients on nonteaching hospitalist services rated their overall care better than those on general medicine teaching services, whereas no differences in patients' ability to identify their physician(s), understand their role in their care, or rating of coordination of care were found. Although the magnitude of the differences in rating of overall care may appear small, it remains noteworthy because of the recent focus on patient experience at the reimbursement level, where small differences in performance can lead to large changes in payment. Because of the observational design of this study, it is important to consider mechanisms that could account for our findings.
The first are the structural differences between the 2 services. Our subgroup analysis comparing patients rating of overall care on a general medicine service with a hospitalist attending to a pure hospitalist cohort found a significant difference between the groups, indicating that the structural differences between the 2 groups may be a significant contributor to patient satisfaction ratings. Under the care of a hospitalist service, a patient would only interact with a single physician on a daily basis, possibly leading to a more meaningful relationship and improved communication between patient and provider. Alternatively, while on a general medicine teaching service, patients would likely interact with multiple physicians, as a result making their confidence in their ability to identify and perception at understanding physicians' roles more challenging.[18] This dilemma is further compounded by duty hour restrictions, which have subsequently led to increased fragmentation in housestaff scheduling. The patient experience on the general medicine teaching service may be further complicated by recent data that show residents spend a minority of time in direct patient care,[19, 20] which could additionally contribute to patients' inability to understand who their physicians are and to the decreased satisfaction with their care. This combination of structural complexity, duty hour reform, and reduced direct patient interaction would likely decrease the chance a patient will interact with the same resident on a consistent basis,[5, 21] thus making the ability to truly understand who their caretakers are, and the role they play, more difficult.
Another contributing factor could be the use of NPAs on our hospitalist service. Given that these providers often see the patient on a more continual basis, hospitalized patients' exposure to a single, continuous caretaker may be a factor in our findings.[22] Furthermore, with studies showing that hospitalists also spend a small fraction of their day in direct patient care,[23, 24, 25] the use of NPAs may allow our hospitalists to spend greater amounts of time with their patients, thus improving patients' rating of their overall care and influencing their perceived ability to understand their physician's role.
Although there was no difference between general medicine teaching and hospitalist services with respect to patient understanding of their roles, our data suggest that both groups would benefit from interventions to target this area. Focused attempts at improving patient's ability to identify and explain the roles of their inpatient physician(s) have been performed. For example, previous studies have attempted to improve a patient's ability to identify their physician through physician facecards[8, 9] or the use of other simple interventions (ie, bedside whiteboards).[4, 26] Results from such interventions are mixed, as they have demonstrated the capacity to improve patients' ability to identify who their physician is, whereas few have shown any appreciable improvement in patient satisfaction.[26]
Although our findings suggest that structural differences in team composition may be a possible explanation, it is also important to consider how the quality of care a patient receives affects their experience. For instance, hospitalists have been shown to produce moderate improvements in patient‐centered outcomes such as 30‐day readmission[27] and hospital length of stay[14, 28, 29, 30, 31] when compared to other care providers, which in turn could be reflected in the patient's perception of their overall care. In a large national study of acute care hospitals using the Hospital Consumer Assessment of Healthcare Providers and Systems survey, Chen and colleagues found that for most measures of patient satisfaction, hospitals with greater use of hospitalist care were associated with better patient‐centered care.[13] These outcomes were in part driven by patient‐centered domains such as discharge planning, pain control, and medication management. It is possible that patients are sensitive to the improved outcomes that are associated with hospitalist services, and reflect this in their measures of patient satisfaction.
Last, because this is an observational study and not a randomized trial, it is possible that the clinical differences in the patients cared for by these services could have led to our findings. Although the clinical significance of the differences in patient demographics were small, patients seen on the hospitalist service were more likely to be older white males, with a slightly longer LOS, greater comorbidities, and more hospitalizations in the previous year than those seen on the general medicine teaching service. Additionally, our hospitalist service frequently cares for highly specific subpopulations (ie, liver and renal transplant patients, and oncology patients), which could have influenced our results. For example, transplant patients who may be very grateful for their second chance, are preferentially admitted to the hospitalist service, which could have biased our results in favor of hospitalists.[32] Unfortunately, we were unable to control for all such factors.
Although we hope that multivariable analysis can adjust for many of these differences, we are not able to account for possible unmeasured confounders such as time of day of admission, health literacy, personality differences, physician turnover, or nursing and other ancillary care that could contribute to these findings. In addition to its observational study design, our study has several other limitations. First, our study was performed at a single institution, thus limiting its generalizability. Second, as a retrospective study based on observational data, no definitive conclusions regarding causality can be made. Third, although our response rate was low, it is comparable to other studies that have examined underserved populations.[33, 34] Fourth, because our survey was performed 30 days after hospitalization, this may impart imprecision on our outcomes measures. Finally, we were not able to mitigate selection bias through imputation for missing data .
All together, given the small absolute differences between the groups in patients' ratings of their overall care compared to large differences in possible confounders, these findings call for further exploration into the significance and possible mechanisms of these outcomes. Our study raises the potential possibility that the structural component of a care team may play a role in overall patient satisfaction. If this is the case, future studies of team structure could help inform how best to optimize this component for the patient experience. On the other hand, if process differences are to explain our findings, it is important to distill the types of processes hospitalists are using to improve the patient experience and potentially export this to resident services.
Finally, if similar results were found in other institutions, these findings could have implications on how hospitals respond to new payment models that are linked to patient‐experience measures. For example, the Hospital Value‐Based Purchasing Program currently links the Centers for Medicare and Medicaid Services payments to a set of quality measures that consist of (1) clinical processes of care (70%) and (2) the patient experience (30%).[1] Given this linkage, any small changes in the domain of patient satisfaction could have large payment implications on a national level.
CONCLUSION
In summary, in this large‐scale multiyear study, patients cared for by a nonteaching hospitalist service reported greater satisfaction with their overall care than patients cared for by a general medicine teaching service. This difference could be mediated by the structural differences between these 2 services. As hospitals seek to optimize patient experiences in an era where reimbursement models are now being linked to patient‐experience measures, future work should focus on further understanding the mechanisms for these findings.
Disclosures
Financial support for this work was provided by the Robert Wood Johnson Investigator Program (RWJF Grant ID 63910 PI Meltzer), a Midcareer Career Development Award from the National Institute of Aging (1 K24 AG031326‐01, PI Meltzer), and a Clinical and Translational Science Award (NIH/NCATS 2UL1TR000430‐08, PI Solway, Meltzer Core Leader). The authors report no conflicts of interest.
The hospitalized patient experience has become an area of increased focus for hospitals given the recent coupling of patient satisfaction to reimbursement rates for Medicare patients.[1] Although patient experiences are multifactorial, 1 component is the relationship that hospitalized patients develop with their inpatient physicians. In recognition of the importance of this relationship, several organizations including the Society of Hospital Medicine, Society of General Internal Medicine, American College of Physicians, the American College of Emergency Physicians, and the Accreditation Council for Graduate Medical Education have recommended that patients know and understand who is guiding their care at all times during their hospitalization.[2, 3] Unfortunately, previous studies have shown that hospitalized patients often lack the ability to identify[4, 5] and understand their course of care.[6, 7] This may be due to numerous clinical factors including lack of a prior relationship, rapid pace of clinical care, and the frequent transitions of care found in both hospitalists and general medicine teaching services.[5, 8, 9] Regardless of the cause, one could hypothesize that patients who are unable to identify or understand the role of their physician may be less informed about their hospitalization, which may lead to further confusion, dissatisfaction, and ultimately a poor experience.
Given the proliferation of nonteaching hospitalist services in teaching hospitals, it is important to understand if patient experiences differ between general medicine teaching and hospitalist services. Several reasons could explain why patient experiences may vary on these services. For example, patients on a hospitalist service will likely interact with a single physician caretaker, which may give a feeling of more personalized care. In contrast, patients on general medicine teaching services are cared for by larger teams of residents under the supervision of an attending physician. Residents are also subjected to duty‐hour restrictions, clinic responsibilities, and other educational requirements that may impede the continuity of care for hospitalized patients.[10, 11, 12] Although 1 study has shown that hospitalist‐intensive hospitals perform better on patient satisfaction measures,[13] no study to date has compared patient‐reported experiences on general medicine teaching and nonteaching hospitalist services. This study aimed to evaluate the hospitalized patient experience on both teaching and nonteaching hospitalist services by assessing several patient‐reported measures of their experience, namely their confidence in their ability to identify their physician(s), understand their roles, and their rating of both the coordination and overall care.
METHODS
Study Design
We performed a retrospective cohort analysis at the University of Chicago Medical Center between July 2007 and June 2013. Data were acquired as part of the Hospitalist Project, an ongoing study that is used to evaluate the impact of hospitalists, and now serves as infrastructure to continue research related to hospital care at University of Chicago.[14] Patients were cared for by either the general medicine teaching service or the nonteaching hospitalist service. General medicine teaching services were composed of an attending physician who rotates for 2 weeks at a time, a second‐ or third‐year medicine resident, 1 to 2 medicine interns, and 1 to 2 medical students.[15] The attending physician assigned to the patient's hospitalization was the attending listed on the first day of hospitalization, regardless of the length of hospitalization. Nonteaching hospitalist services consisted of a single hospitalist who worked 7‐day shifts, and were assisted by a nurse practitioner/physician's assistant (NPA). The majority of attendings on the hospitalist service were less than 5 years out of residency. Both services admitted 7 days a week, with patients initially admitted to the general medicine teaching service until resident caps were met, after which all subsequent admissions were admitted to the hospitalist service. In addition, the hospitalist service is also responsible for specific patient subpopulations, such as lung and renal transplants, and oncologic patients who have previously established care with our institution.
Data Collection
During a 30‐day posthospitalization follow‐up questionnaire, patients were surveyed regarding their confidence in their ability to identify and understand the roles of their physician(s) and their perceptions of the overall coordination of care and their overall care, using a 5‐point Likert scale (1 = poor understanding to 5 = excellent understanding). Questions related to satisfaction with care and coordination were derived from the Picker‐Commonwealth Survey, a previously validated survey meant to evaluate patient‐centered care.[16] Patients were also asked to report their race, level of education, comorbid diseases, and whether they had any prior hospitalizations within 1 year. Chart review was performed to obtain patient age, gender, and hospital length of stay (LOS), and calculated Charlson Comorbidity Index (CCI).[17] Patients with missing data or responses to survey questions were excluded from final analysis. The University of Chicago Institutional Review Board approved the study protocol, and all patients provided written consented prior to participation.
Data Analysis
After initial analysis noted that outcomes were skewed, the decision was made to dichotomize the data and use logistic rather than linear regression models. Patient responses to the follow‐up phone questionnaire were dichotomized to reflect the top 2 categories (excellent and very good). Pearson 2 analysis was used to assess for any differences in demographic characteristics, disease severity, and measures of patient experience between the 2 services. To assess if service type was associated with differences in our 4 measures of patient experience, we created a 3‐level mixed‐effects logistic regression using a logit function while controlling for age, gender, race, CCI, LOS, previous hospitalizations within 1 year, level of education, and academic year. These models studied the longitudinal association between teaching service and the 4 outcome measures, while also controlling for the cluster effect of time nested within individual patients who were clustered within physicians. The model included random intercepts at both the patient and physician level and also included a random effect of service (teaching vs nonteaching) at the patient level. A Hausman test was used to determine if these random‐effects models improved fit over a fixed‐effects model, and the intraclass correlations were compared using likelihood ratio tests to determine the appropriateness of a 3‐level versus 2‐level model. Data management and 2 analyses were performed using Stata version 13.0 (StataCorp, College Station, TX), and mixed‐effects regression models were done in SuperMix (Scientific Software International, Skokie, IL).
RESULTS
In total, 14,855 patients were enrolled during their hospitalization with 57% and 61% completing the 30‐day follow‐up survey on the hospitalist and general medicine teaching service, respectively. In total, 4131 (69%) and 4322 (48%) of the hospitalist and general medicine services, respectively, either did not answer all survey questions, or were missing basic demographic data, and thus were excluded. Data from 4591 patients on the general medicine teaching (52% of those enrolled at hospitalization) and 1811 on the hospitalist service (31% of those enrolled at hospitalization) were used for final analysis (Figure 1). Respondents were predominantly female (61% and 56%), African American (75% and 63%), with a mean age of 56.2 (19.4) and 57.1 (16.1) years, for the general medicine teaching and hospitalist services, respectively. A majority of patients (71% and 66%) had a CCI of 0 to 3 on both services. There were differences in self‐reported comorbidities between the 2 groups, with hospitalist services having a higher prevalence of cancer (20% vs 7%), renal disease (25% vs 18%), and liver disease (23% vs 7%). Patients on the hospitalist service had a longer mean LOS (5.5 vs 4.8 days), a greater percentage of a hospitalization within 1 year (58% vs 52%), and a larger proportion who were admitted in 2011 to 2013 compared to 2007 to 2010 (75% vs 39%), when compared to the general medicine teaching services. Median LOS and interquartile ranges were similar between both groups. Although most baseline demographics were statistically different between the 2 groups (Table 1), these differences were likely clinically insignificant. Compared to those who responded to the follow‐up survey, nonresponders were more likely to be African American (73% and 64%, P < 0.001) and female (60% and 56%, P < 0.01). The nonresponders were more likely to be hospitalized in the past 1 year (62% and 53%, P < 0.001) and have a lower CCI (CCI 03 [75% and 80%, P < 0.001]) compared to responders. Demographics between responders and nonresponders were also statistically different from one another.
| Variable | General Medicine Teaching | Nonteaching Hospitalist | P Value |
|---|---|---|---|
| |||
| Total (n) | 4,591 | 1,811 | <0.001 |
| Attending classification, hospitalist, n (%) | 1,147 (25) | 1,811 (100) | |
| Response rate, % | 61 | 57 | <0.01 |
| Age, y, mean SD | 56.2 19.4 | 57.1 16.1 | <0.01 |
| Gender, n (%) | <0.01 | ||
| Male | 1,796 (39) | 805 (44) | |
| Female | 2,795 (61) | 1,004 (56) | |
| Race, n (%) | <0.01 | ||
| African American | 3,440 (75) | 1,092 (63) | |
| White | 900 (20) | 571 (32) | |
| Asian/Pacific | 38 (1) | 17 (1) | |
| Other | 20 (1) | 10 (1) | |
| Unknown | 134 (3) | 52 (3) | |
| Charlson Comorbidity Index, n (%) | <0.001 | ||
| 0 | 1,635 (36) | 532 (29) | |
| 12 | 1,590 (35) | 675 (37) | |
| 39 | 1,366 (30) | 602 (33) | |
| Self‐reported comorbidities | |||
| Anemia/sickle cell disease | 1,201 (26) | 408 (23) | 0.003 |
| Asthma/COPD | 1,251 (28) | 432 (24) | 0.006 |
| Cancer* | 300 (7) | 371 (20) | <0.001 |
| Depression | 1,035 (23) | 411 (23) | 0.887 |
| Diabetes | 1,381 (30) | 584 (32) | 0.087 |
| Gastrointestinal | 1,140 (25) | 485 (27) | 0.104 |
| Cardiac | 1,336 (29) | 520 (29) | 0.770 |
| Hypertension | 2,566 (56) | 1,042 (58) | 0.222 |
| HIV/AIDS | 151 (3) | 40 (2) | 0.022 |
| Kidney disease | 828 (18) | 459 (25) | <0.001 |
| Liver disease | 313 (7) | 417 (23) | <0.001 |
| Stroke | 543 (12) | 201 (11) | 0.417 |
| Education level | 0.066 | ||
| High school | 2,248 (49) | 832 (46) | |
| Junior college/college | 1,878 (41) | 781 (43) | |
| Postgraduate | 388 (8) | 173 (10) | |
| Don't know | 77 (2) | 23 (1) | |
| Academic year, n (%) | <0.001 | ||
| July 2007 June 2008 | 938 (20) | 90 (5) | |
| July 2008 June 2009 | 702 (15) | 148 (8) | |
| July 2009 June 2010 | 576(13) | 85 (5) | |
| July 2010 June 2011 | 602 (13) | 138 (8) | |
| July 2011 June 2012 | 769 (17) | 574 (32) | |
| July 2012 June 2013 | 1,004 (22) | 774 (43) | |
| Length of stay, d, mean SD | 4.8 7.3 | 5.5 6.4 | <0.01 |
| Prior hospitalization (within 1 year), yes, n (%) | 2,379 (52) | 1,039 (58) | <0.01 |

Unadjusted results revealed that patients on the hospitalist service were more confident in their abilities to identify their physician(s) (50% vs 45%, P < 0.001), perceived greater ability in understanding the role of their physician(s) (54% vs 50%, P < 0.001), and reported greater satisfaction with coordination and teamwork (68% vs 64%, P = 0.006) and with overall care (73% vs 67%, P < 0.001) (Figure 2).

From the mixed‐effects regression models it was discovered that admission to the hospitalist service was associated with a higher odds ratio (OR) of reporting overall care as excellent or very good (OR: 1.33; 95% confidence interval [CI]: 1.15‐1.47). There was no difference between services in patients' ability to identify their physician(s) (OR: 0.89; 95% CI: 0.61‐1.11), in patients reporting a better understanding of the role of their physician(s) (OR: 1.09; 95% CI: 0.94‐1.23), or in their rating of overall coordination and teamwork (OR: 0.71; 95% CI: 0.42‐1.89).
A subgroup analysis was performed on the 25% of hospitalist attendings in the general medicine teaching service comparing this cohort to the hospitalist services, and it was found that patients perceived better overall care on the hospitalist service (OR: 1.17; 95% CI: 1.01‐ 1.31) than on the general medicine service (Table 2). All other domains in the subgroup analysis were not statistically significant. Finally, an ordinal logistic regression was performed for each of these outcomes, but it did not show any major differences compared to the logistic regression of dichotomous outcomes.
| Domains in Patient Experience* | Odds Ratio (95% CI) | P Value |
|---|---|---|
| ||
| How would you rate your ability to identify the physicians and trainees on your general medicine team during the hospitalization? | ||
| Model 1 | 0.89 (0.611.11) | 0.32 |
| Model 2 | 0.98 (0.671.22) | 0.86 |
| How would you rate your understanding of the roles of the physicians and trainees on your general medicine team? | ||
| Model 1 | 1.09 (0.941.23) | 0.25 |
| Model 2 | 1.19 (0.981.36) | 0.08 |
| How would you rate the overall coordination and teamwork among the doctors and nurses who care for you during your hospital stay? | ||
| Model 1 | 0.71 (0.421.89) | 0.18 |
| Model 2 | 0.82 (0.651.20) | 0.23 |
| Overall, how would you rate the care you received at the hospital? | ||
| Model 1 | 1.33 (1.151.47) | 0.001 |
| Model 2 | 1.17 (1.011.31) | 0.04 |
DISCUSSION
This study is the first to directly compare measures of patient experience on hospitalist and general medicine teaching services in a large, multiyear comparison across multiple domains. In adjusted analysis, we found that patients on nonteaching hospitalist services rated their overall care better than those on general medicine teaching services, whereas no differences in patients' ability to identify their physician(s), understand their role in their care, or rating of coordination of care were found. Although the magnitude of the differences in rating of overall care may appear small, it remains noteworthy because of the recent focus on patient experience at the reimbursement level, where small differences in performance can lead to large changes in payment. Because of the observational design of this study, it is important to consider mechanisms that could account for our findings.
The first are the structural differences between the 2 services. Our subgroup analysis comparing patients rating of overall care on a general medicine service with a hospitalist attending to a pure hospitalist cohort found a significant difference between the groups, indicating that the structural differences between the 2 groups may be a significant contributor to patient satisfaction ratings. Under the care of a hospitalist service, a patient would only interact with a single physician on a daily basis, possibly leading to a more meaningful relationship and improved communication between patient and provider. Alternatively, while on a general medicine teaching service, patients would likely interact with multiple physicians, as a result making their confidence in their ability to identify and perception at understanding physicians' roles more challenging.[18] This dilemma is further compounded by duty hour restrictions, which have subsequently led to increased fragmentation in housestaff scheduling. The patient experience on the general medicine teaching service may be further complicated by recent data that show residents spend a minority of time in direct patient care,[19, 20] which could additionally contribute to patients' inability to understand who their physicians are and to the decreased satisfaction with their care. This combination of structural complexity, duty hour reform, and reduced direct patient interaction would likely decrease the chance a patient will interact with the same resident on a consistent basis,[5, 21] thus making the ability to truly understand who their caretakers are, and the role they play, more difficult.
Another contributing factor could be the use of NPAs on our hospitalist service. Given that these providers often see the patient on a more continual basis, hospitalized patients' exposure to a single, continuous caretaker may be a factor in our findings.[22] Furthermore, with studies showing that hospitalists also spend a small fraction of their day in direct patient care,[23, 24, 25] the use of NPAs may allow our hospitalists to spend greater amounts of time with their patients, thus improving patients' rating of their overall care and influencing their perceived ability to understand their physician's role.
Although there was no difference between general medicine teaching and hospitalist services with respect to patient understanding of their roles, our data suggest that both groups would benefit from interventions to target this area. Focused attempts at improving patient's ability to identify and explain the roles of their inpatient physician(s) have been performed. For example, previous studies have attempted to improve a patient's ability to identify their physician through physician facecards[8, 9] or the use of other simple interventions (ie, bedside whiteboards).[4, 26] Results from such interventions are mixed, as they have demonstrated the capacity to improve patients' ability to identify who their physician is, whereas few have shown any appreciable improvement in patient satisfaction.[26]
Although our findings suggest that structural differences in team composition may be a possible explanation, it is also important to consider how the quality of care a patient receives affects their experience. For instance, hospitalists have been shown to produce moderate improvements in patient‐centered outcomes such as 30‐day readmission[27] and hospital length of stay[14, 28, 29, 30, 31] when compared to other care providers, which in turn could be reflected in the patient's perception of their overall care. In a large national study of acute care hospitals using the Hospital Consumer Assessment of Healthcare Providers and Systems survey, Chen and colleagues found that for most measures of patient satisfaction, hospitals with greater use of hospitalist care were associated with better patient‐centered care.[13] These outcomes were in part driven by patient‐centered domains such as discharge planning, pain control, and medication management. It is possible that patients are sensitive to the improved outcomes that are associated with hospitalist services, and reflect this in their measures of patient satisfaction.
Last, because this is an observational study and not a randomized trial, it is possible that the clinical differences in the patients cared for by these services could have led to our findings. Although the clinical significance of the differences in patient demographics were small, patients seen on the hospitalist service were more likely to be older white males, with a slightly longer LOS, greater comorbidities, and more hospitalizations in the previous year than those seen on the general medicine teaching service. Additionally, our hospitalist service frequently cares for highly specific subpopulations (ie, liver and renal transplant patients, and oncology patients), which could have influenced our results. For example, transplant patients who may be very grateful for their second chance, are preferentially admitted to the hospitalist service, which could have biased our results in favor of hospitalists.[32] Unfortunately, we were unable to control for all such factors.
Although we hope that multivariable analysis can adjust for many of these differences, we are not able to account for possible unmeasured confounders such as time of day of admission, health literacy, personality differences, physician turnover, or nursing and other ancillary care that could contribute to these findings. In addition to its observational study design, our study has several other limitations. First, our study was performed at a single institution, thus limiting its generalizability. Second, as a retrospective study based on observational data, no definitive conclusions regarding causality can be made. Third, although our response rate was low, it is comparable to other studies that have examined underserved populations.[33, 34] Fourth, because our survey was performed 30 days after hospitalization, this may impart imprecision on our outcomes measures. Finally, we were not able to mitigate selection bias through imputation for missing data .
All together, given the small absolute differences between the groups in patients' ratings of their overall care compared to large differences in possible confounders, these findings call for further exploration into the significance and possible mechanisms of these outcomes. Our study raises the potential possibility that the structural component of a care team may play a role in overall patient satisfaction. If this is the case, future studies of team structure could help inform how best to optimize this component for the patient experience. On the other hand, if process differences are to explain our findings, it is important to distill the types of processes hospitalists are using to improve the patient experience and potentially export this to resident services.
Finally, if similar results were found in other institutions, these findings could have implications on how hospitals respond to new payment models that are linked to patient‐experience measures. For example, the Hospital Value‐Based Purchasing Program currently links the Centers for Medicare and Medicaid Services payments to a set of quality measures that consist of (1) clinical processes of care (70%) and (2) the patient experience (30%).[1] Given this linkage, any small changes in the domain of patient satisfaction could have large payment implications on a national level.
CONCLUSION
In summary, in this large‐scale multiyear study, patients cared for by a nonteaching hospitalist service reported greater satisfaction with their overall care than patients cared for by a general medicine teaching service. This difference could be mediated by the structural differences between these 2 services. As hospitals seek to optimize patient experiences in an era where reimbursement models are now being linked to patient‐experience measures, future work should focus on further understanding the mechanisms for these findings.
Disclosures
Financial support for this work was provided by the Robert Wood Johnson Investigator Program (RWJF Grant ID 63910 PI Meltzer), a Midcareer Career Development Award from the National Institute of Aging (1 K24 AG031326‐01, PI Meltzer), and a Clinical and Translational Science Award (NIH/NCATS 2UL1TR000430‐08, PI Solway, Meltzer Core Leader). The authors report no conflicts of interest.
- Hospital Consumer Assessment of Healthcare Providers and Systems. HCAHPS fact sheet. CAHPS hospital survey August 2013. Available at: http://www.hcahpsonline.org/files/August_2013_HCAHPS_Fact_Sheet3.pdf. Accessed February 2, 2015.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364–370.
- Accreditation Council for Graduate Medical Education. Common program requirements. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/CPRs2013.pdf. Accessed January 15, 2015.
- , , . Increasing a patient's ability to identify his or her attending physician using a patient room display. Arch Intern Med. 2010;170(12):1084–1085.
- , , , , , . Ability of hospitalized patients to identify their in‐hospital physicians. Arch Intern Med. 2009;169(2):199–201.
- , , , et al. Hospitalized patients' understanding of their plan of care. Mayo Clin Proc. 2010;85(1):47–52.
- , , , et al. Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan. Arch Intern Med. 1997;157(9):1026–1030.
- , , , et al. Improving inpatients' identification of their doctors: use of FACE cards. Jt Comm J Qual Patient Saf. 2009;35(12):613–619.
- , , , , , . The impact of facecards on patients' knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9(3):137–141.
- , , . Restructuring an inpatient resident service to improve outcomes for residents, students, and patients. Acad Med. 2011;86(12):1500–1507.
- , , . Residency training in the modern era: the pipe dream of less time to learn more, care better, and be more professional. Arch Intern Med. 2005;165(22):2561–2562.
- , , , , . Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , , , . Hospitalist staffing and patient satisfaction in the national Medicare population. J Hosp Med. 2013;8(3):126–131.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , , , , . The Effects of on‐duty napping on intern sleep time and fatigue. Ann Intern Med. 2006;144(11):792–798.
- , , , et al. Patients evaluate their hospital care: a national survey. Health Aff (Millwood). 1991;10(4):254–267.
- , , , . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Agency for Healthcare Research and Quality. Welcome to HCUPnet. Available at: http://hcupnet.ahrq.gov/HCUPnet.jsp?Id=F70FC59C286BADCB371(4):293–295.
- , , , et al. In the wake of the 2003 and 2011 duty hours regulations, how do internal medicine interns spend their time? J Gen Intern Med. 2013;28(8):1042–1047.
- , , , , , . The composition of intern work while on call. J Gen Intern Med. 2012;27(11):1432–1437.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- , , , et al. The impact of hospitalist discontinuity on hospital cost, readmissions, and patient satisfaction. J Gen Intern Med. 2014;29(7):1004–1008.
- , , , , . Hospitalist time usage and cyclicality: opportunities to improve efficiency. J Hosp Med. 2010;5(6):329–334.
- , , , et al. Where did the day go?—a time‐motion study of hospitalists. J Hosp Med. 2010;5(6):323–328.
- , , . How hospitalists spend their time: insights on efficiency and safety. J Hosp Med. 2006;1(2):88–93.
- , , . Patient satisfaction associated with correct identification of physician's photographs. Mayo Clin Proc. 2001;76(6):604–608.
- , , , . Comparing patient outcomes of academician‐preceptors, hospitalist‐preceptors, and hospitalists on internal medicine services in an academic medical center. J Gen Intern Med. 2014;29(12):1672–1678.
- , , , . Comparison of processes and outcomes of pneumonia care between hospitalists and community‐based primary care physicians. Mayo Clin Proc. 2002;77(10):1053–1058.
- , , , , , . Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357(25):2589–2600.
- . A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3):248–254.
- , . Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9(1):58.
- , . Patients' experiences of everyday life after lung transplantation. J Clin Nurs. 2009;18(24):3472–3479.
- , , , et al. Optimal design features for surveying low‐income populations. J Health Care Poor Underserved. 2005;16(4):677–690.
- Hospital Consumer Assessment of Healthcare Providers and Systems. HCAHPS fact sheet. CAHPS hospital survey August 2013. Available at: http://www.hcahpsonline.org/files/August_2013_HCAHPS_Fact_Sheet3.pdf. Accessed February 2, 2015.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364–370.
- Accreditation Council for Graduate Medical Education. Common program requirements. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/CPRs2013.pdf. Accessed January 15, 2015.
- , , . Increasing a patient's ability to identify his or her attending physician using a patient room display. Arch Intern Med. 2010;170(12):1084–1085.
- , , , , , . Ability of hospitalized patients to identify their in‐hospital physicians. Arch Intern Med. 2009;169(2):199–201.
- , , , et al. Hospitalized patients' understanding of their plan of care. Mayo Clin Proc. 2010;85(1):47–52.
- , , , et al. Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan. Arch Intern Med. 1997;157(9):1026–1030.
- , , , et al. Improving inpatients' identification of their doctors: use of FACE cards. Jt Comm J Qual Patient Saf. 2009;35(12):613–619.
- , , , , , . The impact of facecards on patients' knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9(3):137–141.
- , , . Restructuring an inpatient resident service to improve outcomes for residents, students, and patients. Acad Med. 2011;86(12):1500–1507.
- , , . Residency training in the modern era: the pipe dream of less time to learn more, care better, and be more professional. Arch Intern Med. 2005;165(22):2561–2562.
- , , , , . Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , , , . Hospitalist staffing and patient satisfaction in the national Medicare population. J Hosp Med. 2013;8(3):126–131.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , , , , . The Effects of on‐duty napping on intern sleep time and fatigue. Ann Intern Med. 2006;144(11):792–798.
- , , , et al. Patients evaluate their hospital care: a national survey. Health Aff (Millwood). 1991;10(4):254–267.
- , , , . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Agency for Healthcare Research and Quality. Welcome to HCUPnet. Available at: http://hcupnet.ahrq.gov/HCUPnet.jsp?Id=F70FC59C286BADCB371(4):293–295.
- , , , et al. In the wake of the 2003 and 2011 duty hours regulations, how do internal medicine interns spend their time? J Gen Intern Med. 2013;28(8):1042–1047.
- , , , , , . The composition of intern work while on call. J Gen Intern Med. 2012;27(11):1432–1437.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- , , , et al. The impact of hospitalist discontinuity on hospital cost, readmissions, and patient satisfaction. J Gen Intern Med. 2014;29(7):1004–1008.
- , , , , . Hospitalist time usage and cyclicality: opportunities to improve efficiency. J Hosp Med. 2010;5(6):329–334.
- , , , et al. Where did the day go?—a time‐motion study of hospitalists. J Hosp Med. 2010;5(6):323–328.
- , , . How hospitalists spend their time: insights on efficiency and safety. J Hosp Med. 2006;1(2):88–93.
- , , . Patient satisfaction associated with correct identification of physician's photographs. Mayo Clin Proc. 2001;76(6):604–608.
- , , , . Comparing patient outcomes of academician‐preceptors, hospitalist‐preceptors, and hospitalists on internal medicine services in an academic medical center. J Gen Intern Med. 2014;29(12):1672–1678.
- , , , . Comparison of processes and outcomes of pneumonia care between hospitalists and community‐based primary care physicians. Mayo Clin Proc. 2002;77(10):1053–1058.
- , , , , , . Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357(25):2589–2600.
- . A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3):248–254.
- , . Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9(1):58.
- , . Patients' experiences of everyday life after lung transplantation. J Clin Nurs. 2009;18(24):3472–3479.
- , , , et al. Optimal design features for surveying low‐income populations. J Health Care Poor Underserved. 2005;16(4):677–690.
© 2015 Society of Hospital Medicine
Ultrabrief Cognitive Screening Outcomes
Hospitalization is a critical time for older patients with cognitive impairment. Past research has found that hospitalized older adults with cognitive dysfunction have more rapid cognitive decline, increased morbidity and mortality, and higher costs of healthcare utilization.[1, 2, 3] Those with preexisting cognitive dysfunction, such as dementia, are most susceptible to the negative impacts of hospitalization.[4, 5, 6, 7, 8] Identification of cognitive deficits upon admission is important for risk stratification of patients and prevention of negative hospital health events.
Frontline healthcare providers are underequipped to detect acute cognitive dysfunction.[9, 10] Current practice and research for the detection of cognitive dysfunction in the acute care setting utilizes instruments that require training[11] and are relatively lengthy (>5 minutes).[12] Although these cognitive screening tests are accurate and reliable, the time requirement is not feasible in a fast‐paced clinical setting. A possible alternative is the use of ultra‐brief cognitive screening instruments (<1 minute) that have the potential to identify those individuals requiring additional evaluation and follow‐up. These brief instruments are composed of screening tools that emphasize core features of acute cognitive dysfunction such as level of arousal or attention.[13, 14, 15, 16] Arousal, the ability to respond to or interact with the environment,[15] is an important component of cognition because it is generally preserved in chronic cognitive disorders (eg, dementia). Thus, an alteration in arousal may be a harbinger of more acute impairment[17] in need of evaluation, and in these lowered states of arousal it may be difficult to test for attention.[18] Attention is a broadly defined cognitive domain indicating focus.[19] Older adults, regardless of preexisting cognitive dysfunction, warrant additional cognitive testing if levels of arousal or attention are altered[20, 21] due to the significant relationship to delirium, which is associated with adverse events in this population. Recent research has demonstrated that these brief cognitive screening instruments provide information about the risk for delirium and are a strong test for clinical characteristics of delirium.[16, 21]
The purpose of this analysis was to demonstrate the clinical outcomes of poor performance on ultrabrief assessments arousal and attention by frontline staff using a quality improvement database. Specific objectives include determining (1) the association of poor performance on brief cognitive assessments and hospital outcomes and (2) the inter‐relationship between alterations in the levels of arousal and attention on in‐hospital and discharge outcomes.
METHODS
Setting and Study Design
This is a secondary analysis of data collected from a quality improvement program for delirium risk modification.[22] This program collected data from October 2010 until September 2012 at a Veterans Affairs (VA) tertiary referral center for the New England region. Patients aged 60 years or older and admitted to medical wards were screened upon admission or transfer to VA Boston Healthcare System and provided appropriate interventions to modify delirium risk. Excluded were individuals admitted as observational status, or those readmitted within 30 days of initial screening, and those screened more than 72 hours after admission. Age and sex were abstracted from the medical record. Outcome data were collected from the medical record for the purpose of operating and sustaining the program. In a previous article, the length of stay (LOS) outcome was reported in a subset of this population.[23] The analysis presented here includes the full cohort, presents the interaction with month of the year backward (MOYB), and provides additional outcomes not included in the other article. The VA institutional review board (IRB) reviewed and approved the secondary data analysis of the quality improvement project.
Measures
Brief Cognitive Screening
The baseline assessments of levels of arousal and attention were collected within 72 hours of admission to identify delirium risk. Trained study staff, not involved in the clinical care of patients, administered these assessments as part of the quality improvement project. It is estimated that these assessments took less than 1 minute to complete per individual, but actual administration time was not measured. Assessments were documented within the electronic health record as part of a delirium risk stratification system.
Arousal
The arousal level assessment was the modified Richmond Agitation and Sedation Scale (mRASS). The mRASS is a brief, reliable, observational tool used to determine arousal level.[15, 17] It is a text modification of the RASS[17] for less acutely ill patients, capturing hyperactive and hypoactive altered levels of arousal. The mRASS asks an open‐ended question followed by observation for 10 seconds and completion of a 5 to+4 rating scale. Alert and calm (score=0) is considered normal, with positive numbers related to an increased level of arousal and attention, whereas negative numbers denote decreased levels. For the analyses, an mRASS of 0 is utilized as the reference. Categories were collapsed into 2 and 2 due to few patients on the extremes of the mRASS.
Attention
The MOYB is a brief measure of attention that is included in several instruments for delirium.[19, 24, 25] For this study, the patient was asked to recite the 12 months backward beginning with December. A correct score was given if the individual was able to recite all 12 months to January without any error. An incorrect score was given if any mistake was made. Scoring for the MOYB is not standardized by age, preexisting medical diagnosis, or any other rational.[26] Others have used July or June as a cutoff for a correct score on the MOYB,[21, 25] but a more conservative score of correct to January was used in this study, which has been previously used.[26, 27, 28, 29, 30] A score of not completed was given when the patient was unable to participate or declined to complete the assessment. For the analysis, a correct score on the MOYB is the referent group.
Outcomes
In‐hospital outcomes included (1) restraint use and (2) in‐hospital mortality. Physical restraint use was identified by focused medical record review and identification of required restraint documentation, which, by center policy requires daily review and documentation. Any restraint use during the hospitalization was included.
Discharge outcomes included (1) LOS, (2) discharge other than a location to home, and (3) variable direct costs. LOS was calculated from date of admission until date of discharge. Discharge disposition was identified in the electronic medical record discharge documentation and categorized into discharge to the prehospital residence (home) or not. Hospital variable direct costs were collected from the VA decision support system,[31] a centrally maintained administrative database. The VA decision support system is challenged with accounting for costs of a single‐day admission and patients who are hospitalized from VA long‐term care. To address the missing data from these cases, multiple imputations (n=20) of the missing data were performed.[32] Sensitivity analyses were performed to determine the impact of the imputation and the cost analysis strategy (see Supporting Information, Appendix 1, in the online version of this article).
Statistical Analyses
For this analysis, outcomes are reported at each level of performance on the mRASS (1 to1) and MOYB (correct, incorrect, not completed). For each analysis, the performance with a mean and standard deviation (SD) are reported for continuous outcomes and a percentage for dichotomous outcomes. For dichotomous outcomes, including restraint use, in‐hospital mortality, and discharge disposition, a risk ratio (RR) with 95% confidence interval (CI) is presented. The median is presented for the cost data because variable direct cost is highly skewed. For LOS and cost outcomes, unadjusted incident rate ratio (IRR) from a Poisson regression relative to the referent is presented to compare the categories. A Poisson regression was selected because LOS (a count of days) and variable direct costs (a count of dollars) are highly skewed. The output of Poisson regression produces an IRR and 95% CI relative to the referent group. The Poisson regression could not be adjusted because the quality improvement nature of these data limited the number of covariates collected. Sensitivity analyses did not identify significant interactions of age and sex (results not shown).
MOYB was also compared by level of arousal (mRASS=0 vs all others). Due to the relatively few patients with positive mRASS, it was compressed into a category of abnormal mRASS relative to alert and calm. Similar to the previous analyses, Poisson regression was performed to calculate the IRR (95% CI) relative to correct MOYB for the continuous variables. An RR was calculated for the dichotomous variables. All statistical analyses were performed using Stata version 11.0 (StataCorp, College Station, TX).
RESULTS
Population Description
Over the 2‐year project timeline, a total of 3232 unique individual records were analyzed (Table 1). Patients admitted and screened within the prior 30 days (n=501) and patients screened more than 3 days after admission (n=664) were not included in the analysis. Older adults were on average 74.7 years old (SD=9.8), and 98.2% were male, consistent with the veteran population. Altered level of arousal, as defined by an abnormal mRASS score, was found in 15.3% of the population. Average LOS was 5.2 days (SD=5.6), restraint use occurred in 5.5% during the hospital stay, patients were likely to be discharged home (71.7%), and a small portion died during hospitalization (1.3%). Mean variable direct costs were $11,084 with expected variability (SD=$15,682, median $6,614). Patients who died during the hospital stay had significantly longer LOS (mean 16.8 [SD=12.5] vs 5.1 [SD=5.4] days, P<0.001) and higher variable direct costs ($43,879 [SD=$37,334] vs $12,544 [SD=$16,802], P<0.001), justifying their removal from these analyses.
| Characteristic | Result, N=3,232, Mean (SD) or % (n) |
|---|---|
| |
| Age, y | 74.7 (9.8) |
| Male | 98.2 (3,174) |
| mRASS | |
| 2 | 2.0% (64) |
| 1 | 8.5% (273) |
| 0 | 84.7% (2,737) |
| 1 | 4.0% (131) |
| 2 | 0.8% (27) |
| MOYB | |
| Correct | 48.8% (1,578) |
| Incorrect | 45.1% (1,457) |
| Not completed | 6.1% (197) |
| Restraint use | 5.5% (177) |
| In‐hospital mortality | 1.3% (41) |
| Length of stay, da | 5.1 (5.4) |
| Discharge other than homea | 71.7% (2,292) |
| Variable direct hospital cost, $a | 11,084 (15,682) |
| Median cost, $ | 6,614 |
Impact of Altered Level of Arousal on Outcomes
There is an association between a deviation from a normal level of arousal (mRASS not equal to 0) and worsening outcomes (Table 2). Relative to a normal level of arousal (4.9SD 5.2 days), decreased level of arousal (negative mRASS), and increased arousal (positive mRASS) resulted in longer LOS (6.0SD 5.6 days, 5.7SD 6.8 days, respectively). Similarly, increased or decreased arousal was associated with heightened risk of restraints and less frequent discharge to home. In‐hospital mortality and hospital variable direct costs were significantly higher in those with decreased levels of arousal (IRR: 2.8, 95% CI: 1.36.0; IRR: 1.10, 95% CI: 0.951.26, respectively). The pattern does not hold for increased arousal with respect to in‐hospital mortality and variable direct hospital cost outcomes. The unadjusted analysis found that, relative to normal arousal, there is a significant change in outcomes with decreased levels of arousal. Increased arousal is associated with worsened IRR in LOS, restraint use, and discharge home, but not in‐hospital mortality and variable direct cost.
| mRASS Alert and Calm, n=2,737 | mRASS Negative, n=337 | mRASS Positive, n=158 | ||||
|---|---|---|---|---|---|---|
| Value | IRR/RR (95%CI) | Value | IRR/RR (95% CI) | Value | IRR/RR (95% CI) | |
| ||||||
| Restraint use % (n) | 4.2% (114) | Referent | 10.4% (35) | 2.49 (1.743.57) | 17.7% (28) | 4.25 (2.916.23) |
| In‐hospital mortality % (n) | 1.0% (26) | Referent | 2.7% (9) | 2.81 (1.335.95) | 1.3% (2) | 1.33 (0.325.56) |
| Length of stay, d (SD)a | 4.9 (5.2) | Referent | 6.0 (5.6) | 1.24 (1.181.30) | 5.7 (6.8) | 1.17 (1.091.25) |
| Discharge other than home, % (n)a | 24.9% (675) | Referent | 46.7% (153) | 1.87 (1.642.14) | 48.1% (75) | 1.93 (1.612.30) |
| Variable direct cost, $ (SD)a, b | 10,581 (14,928) | Referent | 11,604 (13,852) | 1.10 (0.951.26) | 10,640 (10,771) | 1.01 (0.851.19) |
| Median cost, $ | 6,318 | 7,738 | 7,858 | |||
Impact of Altered Attention on Outcomes
Patients who completed the MOYB incorrectly had increased restraint use (RR: 2.11, 95% CI 1.443.11) and LOS (IRR: 1.06, 95% CI: 1.021.10), but no difference in in‐hospital mortality, discharge home (RR: 0.78, 95% CI: 0.750.82), and variable direct costs, relative to those who completed the MOYB correctly (Table 3). Importantly, patients who did not complete the MOYB assessment had a 2‐fold increase in restraint use (RR: 2.05, 95% CI: 0.944.50), in‐hospital mortality was nearly 6‐fold higher (RR: 6.36, 95% CI: 2.1618.69), longer LOS (IRR: 1.12, 95% CI: 1.031.21), and returned home less frequently (RR: 1.77, 95% CI: 1.262.48).
| mRASS Normal | mRASS Abnormal | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MOYB Correct (n=1,431) | MOYB Incorrect (n=1,181) | MOYB Incomplete (n=125) | MOYB Correct (n=147) | MOYB Incorrect (n=276) | MOYB Incomplete (n=72) | |||||||
| Value | IRR/RR (95% CI) | Value | IRR/RR (95% CI) | Value | IRR/RR (95% CI) | Value | IRR/RR (95% CI) | Value | IRR/RR (95% CI) | Value | IRR/RR (95% CI) | |
| ||||||||||||
| Restraint use, % (n) | 2.7% (39) | Referent | 5.8% (68) | 2.11 (1.44‐3.11) | 5.6% (7) | 2.05 (0.94‐4.50) | 2.7% (4) | 1.00 (0.36‐2.75) | 13.8% (38) | 5.05 (3.29‐7.75) | 29.2% (21) | 10.70 (6.66‐17.20) |
| In‐hospital mortality, % (n) | 0.6% (9) | Referent | 1.0% (12) | 1.62 (0.68‐ 3.82) | 4.0% (5) | 6.36 (2.16‐18.69) | 1.4% (2) | 2.16 (0.47‐9.92) | 2.2% (6) | 3.46 (1.24‐9.63) | 4.2% (3) | 6.63 (1.83‐23.95) |
| Length of stay, d (SD)a | 4.7 (5.4) | Referent | 5.0 (5.1) | 1.06 (1.02‐1.10) | 5.3 (5.0) | 1.12 (1.03‐1.21) | 5.4 (6.0) | 1.13 (1.05‐1.22) | 5.9 (4.4) | 1.23 (1.17‐1.30) | 7.5 (10.0) | 1.55 (1.44‐1.73) |
| Discharge other than home, % (n)a | 17.9% (255) | Referent | 32.7% (382) | 1.82 (1.56‐ 2.14) | 31.7% (38) | 1.77 (1.26‐2.48) | 29.7% (43) | 1.65 (1.20‐2.28) | 53.3% (144) | 2.97 (2.42‐3.64) | 59.4% (41) | 3.31 (2.38‐4.61) |
| Variable direct cost, $ (SD)a, b | 10,609 (16,154) | Referent | 10,482 (13,495) | 0.99 (0.89‐1.10) | 11,213 (12,994) | 1.06 (0.85‐1.32) | 12,010 (15,636) | 1.13 (0.90‐1.42) | 10,776 (10,680) | 1.02 (0.88‐1.17) | 11,815 (14,604) | 1.11 (0.82‐1.51) |
| Median cost, $ | 6,338 | 6,248 | 6,630 | 7,023 | 8,093 | 8,180 | ||||||
Inter‐relationship of Altered Level of Arousal and Attention on Outcomes
The inter‐relationship of altered level of arousal and attention is presented in Table 3. Of patients with a normal mRASS, 52% had correct MOYB. The percentage of correct MOYB declined with the level of arousal, such that 38% had normal MOYB and a mRASS of 1 and 9% had normal MOYB with mRASS of 2. In general, in‐hospital outcomes (restraints and mortality) are associated with MOYB performance, and discharge outcomes (LOS, discharge location, and variable direct costs) are associated with mRASS. Those patients who did not complete the MOYB demonstrated worse outcomes, regardless of mRASS performance, including a 6‐fold increase in mortality and significant increases in LOS and discharge location.
DISCUSSION
Impaired performance on a one‐time assessment of arousal or attention during hospitalization demonstrated a relationship with in‐hospital and discharge outcomes. Relative to normal levels of arousal and attention, alterations in attention, level of arousal, or both were associated with progressively negative consequences. Combined with the prognostic value, the administration of ultra‐brief cognitive screening measures may have value in the identification of patients who would benefit from additional screening, supporting prior work in this area.[23] The brevity of the assessments enhances clinical utility and implementation potential.
Cognitive function during hospitalization has been associated with many negative outcomes including delirium, falls, pressure ulcers, and functional decline.[3, 33, 34, 35, 36, 37] The findings of this analysis are consistent with previous studies and provide important clinical implications. First, prior work in cognitive screening has focused on more time‐consuming instruments.[12] By focusing on brief instruments, particularly those under 1 minute that do not require paper or props, a user‐friendly tool that is associated with health outcomes is provided.
In addition, this analysis demonstrates that each assessment, when used individually, has some prognostic significance associated with the identification of delirium or other types of cognitive impairment. When used alone, abnormal scores on the mRASS or MOYB may be indicative of individuals requiring further cognitive assessment, supporting previous research.[16, 23] Individuals with abnormal scores on both the mRASS and MOYB identify a high‐risk group in need of further clinical assessment for delirium (Figure 1). Neither of these assessments are meant to be used as the only means to diagnosis delirium, but together they identify key clinical characteristics of delirium (arousal and attention).[16, 18, 21] Considering the significant negative consequences associated with delirium, it is not surprising that tools identifying core features of delirium, such as those presented here, would also be associated with in‐hospital and discharge outcomes.
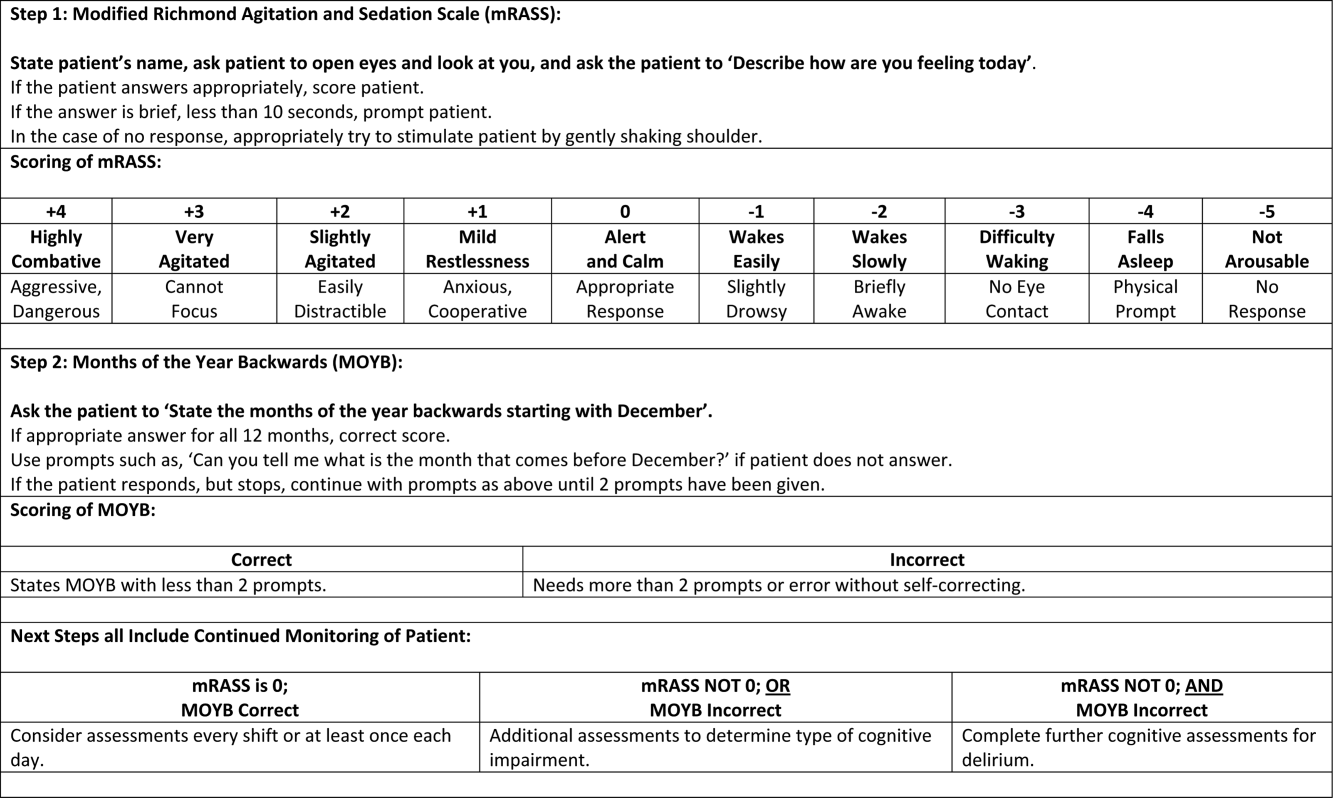
The quality improvement design of this project allowed the recording of outcomes in those who were unable or refused to complete the screening. This may be a potentially high‐risk group who would otherwise go unnoticed. A recent editorial from the American and European Delirium Societies highlights that individuals who are unable or refuse to complete testing due to impaired arousal are neglected in the most recent American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition definition of delirium.[18] Further work to identify and intervene on behalf of individuals who are unable to complete testing will aid in understanding arousal and its relationship to delirium and other disorders.
This analysis provides additional insight in the selection of measures of arousal and attention. Level of arousal is a complex concept that involves components of awareness and alertness, including external stimuli and self‐awareness.[38, 39, 40] As an ultra‐brief measure of arousal level, the mRASS incorporates both external stimuli (asking an open‐ended question) and self‐awareness (describing current state) to determine basic cognitive function. Attention can be defined as the selection of stimuli for further cognitive processing.[40] Attention is an umbrella term referring to many cognitive processes, ranging from sustained attention and working memory to executive function such as set shifting and multitasking. Ultra‐brief measures of attention, such as MOYB, are basic tasks of sustained attention with components of working memory.[19] An alteration in attention may be indicative of a more significant global change in cognition[41] beyond basic cognitive function assessed by administration of the mRASS, such as delirium.[42] The relationship between level of arousal and attention is complex, and arguments have been made that one has to have a certain level of arousal to attend to a stimuli, whereas others have found that one has to have a certain level of attention.[18, 39, 40] Administration of both the mRASS and MOYB is a useful bedside tool for clinicians to examine both basic cognitive function and more complex tasks of attention.
The quality improvement nature of this work has limitations and strengths that deserve mention. The significant strength of this work is the robust sample size. Also, trained staff not involved in the direct clinical care of patients administered the cognitive screens, suggesting that nonclinically trained personnel could be utilized for risk assessment. The major limitation is the restricted amount of covariate data that were collected. Data for this project were collected to operationalize and demonstrate the impact and business case of a delirium risk modification program,[17] limiting the ability to perform adjustment for other covariates such as comorbidity and reason for admission. Also, due to the nature of this project, a diagnosis of delirium was not determined. A limitation of excluding in‐hospital deaths from the cost analysis was that some individuals at high risk died early, thus costing less overall. Generalizability is limited by an over‐representation of males within a single setting. Further use and understanding of mRASS and MOYB in other population is warranted and welcomed. Use of MOYB is also a limitation considering that scores are not standardized across patients or settings.[26] Data regarding administration time of either of these tools were not collected; therefore, determining that these are ultra‐brief assessments (<1 minute) is based on estimates. As such, these measures should not be the sole source of information for clinical evaluation and diagnosis.
CONCLUSION
This work found that impaired performance on brief cognitive assessments of arousal and attention in hospitalized patients were associated with restraint use, in‐hospital mortality, longer LOS, less discharge home, and hospital costs. Routine screening of older patients with brief, user‐friendly cognitive assessments upon admission can identify those who would benefit from additional assessment and intervention to alleviate individual and economic burdens.
Acknowledgements
The authors are indebted to the veterans who participated in their delirium and fall reduction programs. The authors are thankful for the guidance of the VA Boston Healthcare System Delirium Task Force and Patient Safety Officers for continued collaboration to improve outcomes for the veterans they serve.
Disclosures: Dr. Yevchak and Ms. Doherty contributed equally to this article and agreed to share first authorship. This material is based upon work supported by the Department of Veterans Affairs Office of Patient Safety Delirium Patient Safety Center of Inquiry and a Geriatrics and Extended Care T21 Alternative to Non‐institutional Long Term Care award. Archambault, Doherty, Fonda, Kelly, and Rudolph are employees of the US government. Dr. Rudolph also received support from a VA Career Development Award. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government. The authors report no conflicts of interest.
- , , , , , . Delirium superimposed on dementia predicts 12‐month survival in elderly patients discharged from a postacute rehabilitation facility. J Gerontol A Biol Sci Med Sci. 2007;62(11):1306–1309.
- , , , . Delirium superimposed on dementia is associated with prolonged length of stay and poor outcomes in hospitalized older adults. J Hosp Med. 2013;8(9):500–505.
- , , , et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med. 2010;5(2):69–75.
- , , , et al. Association between endothelial dysfunction and acute brain dysfunction during critical illness. Anesthesiology. 2013;118(3):631–639.
- , , , et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72(18):1570–1575.
- , , , et al. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Intern Med. 2012;156(12):848–856.
- , , , et al. Delirium: an independent predictor of functional decline after cardiac surgery. J Am Geriatr Soc. 2010;58(4):643–649.
- , . The importance of delirium: economic and societal costs. J Am Geriatr Soc. 2011;59:S241–S243.
- , , , , , . Managing delirium in the acute care setting: a pilot focus group study. Int J Older People Nurs. 2012;7(2):152–162.
- , , , et al. Barriers and facilitators to implementing delirium rounds in a clinical trial across three diverse hospital settings. Clin Nurs Res. 2014;23(2):201–215.
- , , , , , . Validation of the confusion assessment method in the palliative care setting. Palliat Med. 2009;23(1):40–45.
- , , , . Does this patient have delirium? Value of bedside instruments. JAMA. 2010;304(7):779–786.
- , , , et al. Three core domains of delirium validated using exploratory and confirmatory factor analyses. Psychosomatics. 2013;54(3):227–238.
- , , . A neurologist's approach to delirium: diagnosis and management of toxic metabolic encephalopathies. Eur J Intern Med. 2014;25(2):112–116.
- , , ; the VADWG. Serial administration of a modified Richmond Agitation and Sedation Scale for delirium screening. J Hosp Med. 2012;7(5):450–453.
- . The diagnostic performance of the Richmond Agitation Sedation Scale for detecting delirium in older emergency department patients. Acad Emerg Med. 2015;22(7):878–882.
- , , , et al. The Richmond Agitation Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344.
- European Delirium Association, American Delirium Society. The DSM‐5 criteria, level of arousal and delirium diagnosis: inclusiveness is safer. BMC Med. 2014;12:141.
- , , , , , . Pay attention! The critical importance of assessing attention in older adults with dementia. J Gerontol Nurs. 2012;38(11):23–27.
- , , . Delirium: a disorder of consciousness? Med Hypotheses. 2013;80(4):399–404.
- , , , et al. Attention! A good bedside test for delirium? J Neurol Neurosurg Psychiatry. 2014;85(10):1122–1131.
- , , . A delirium risk modification program is associated with hospital outcomes. J Am Med Dir Assoc. 2014;15(12):11.
- , , , et al. Impaired arousal in older adults is associated with prolonged hospital stay and discharge to skilled nursing facility. J Am Med Dir Assoc. 2015;16(7):586–589.
- , , , et al. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing. 2014;43(4):496–502.
- , , , , , . Reliability of a structured assessment for nonclinicians to detect delirium among new admissions to postacute care. J Am Med Dir Assoc. 2006;7(7):412–415.
- , , , . Reciting the months of the year backwards: what is a ‘normal’ score? Age Ageing. 2015;44(3):537–538.
- , , . A Delirium risk modification program is associated with hospital outcomes. J Am Med Dir Assoc. 2014;15(12):957.e957–957.e911.
- , , , et al. 3D‐CAM: derivation and validation of a 3‐minute diagnostic interview for CAM‐defined delirium: a cross‐sectional diagnostic test study. Ann Intern Med. 2014;161(8):554–561.
- , , , , , . Reliability of a structured assessment for non‐clinicians to detect delirium among new admissions to post‐acute care. J Am Med Dir Assoc. 2006;7:412–415.
- , , , et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119(2):229–236.
- , . Use of the Decision Support System for VA cost‐effectiveness research. Med Care. 1999;37(4 suppl Va):AS63–AS70.
- , , , , . Cost analysis in the Department of Veterans Affairs: consensus and future directions. Med Care. 1999;37(4 Suppl Va):AS3‐AS8.
- , , . Delirium: a symptom of how hospital care is failing older persons and a window to improve quality of hospital care. Am J Med. 1999;106(5):565–573.
- , , , et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676.
- , , , . Iatrogenic causes of falls in hospitalised elderly patients: a case‐control study. Postgrad Med J. 2002;78(922):487–489.
- , , , , . A controlled quality improvement trial to reduce the use of physical restraints in older hospitalized adults. J Am Geriatr Soc. 2014;62(3):541–545.
- , , , . Evaluation of the mobile acute care of the elderly (mace) service. JAMA Intern Med. 2013;173(11):990–996.
- , . Conscience and consciousness: a definition. J Med Life. 2014;7(1):104–108.
- , , , et al. Consciousness in humans and non‐human animals: recent advances and future directions. Front Psychol. 2013;4:625.
- . Interdependence of attention and consciousness. In: Rahul B, Bikas KC, eds. Progress in Brain Research. Vol. 168. New York, NY: Elsevier; 2007:65–75.
- , , . Relationship between cognitive and non‐cognitive symptoms of delirium. Asian J Psychiatr. 2013;6(2):106–112.
- , , , , , . Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948.
Hospitalization is a critical time for older patients with cognitive impairment. Past research has found that hospitalized older adults with cognitive dysfunction have more rapid cognitive decline, increased morbidity and mortality, and higher costs of healthcare utilization.[1, 2, 3] Those with preexisting cognitive dysfunction, such as dementia, are most susceptible to the negative impacts of hospitalization.[4, 5, 6, 7, 8] Identification of cognitive deficits upon admission is important for risk stratification of patients and prevention of negative hospital health events.
Frontline healthcare providers are underequipped to detect acute cognitive dysfunction.[9, 10] Current practice and research for the detection of cognitive dysfunction in the acute care setting utilizes instruments that require training[11] and are relatively lengthy (>5 minutes).[12] Although these cognitive screening tests are accurate and reliable, the time requirement is not feasible in a fast‐paced clinical setting. A possible alternative is the use of ultra‐brief cognitive screening instruments (<1 minute) that have the potential to identify those individuals requiring additional evaluation and follow‐up. These brief instruments are composed of screening tools that emphasize core features of acute cognitive dysfunction such as level of arousal or attention.[13, 14, 15, 16] Arousal, the ability to respond to or interact with the environment,[15] is an important component of cognition because it is generally preserved in chronic cognitive disorders (eg, dementia). Thus, an alteration in arousal may be a harbinger of more acute impairment[17] in need of evaluation, and in these lowered states of arousal it may be difficult to test for attention.[18] Attention is a broadly defined cognitive domain indicating focus.[19] Older adults, regardless of preexisting cognitive dysfunction, warrant additional cognitive testing if levels of arousal or attention are altered[20, 21] due to the significant relationship to delirium, which is associated with adverse events in this population. Recent research has demonstrated that these brief cognitive screening instruments provide information about the risk for delirium and are a strong test for clinical characteristics of delirium.[16, 21]
The purpose of this analysis was to demonstrate the clinical outcomes of poor performance on ultrabrief assessments arousal and attention by frontline staff using a quality improvement database. Specific objectives include determining (1) the association of poor performance on brief cognitive assessments and hospital outcomes and (2) the inter‐relationship between alterations in the levels of arousal and attention on in‐hospital and discharge outcomes.
METHODS
Setting and Study Design
This is a secondary analysis of data collected from a quality improvement program for delirium risk modification.[22] This program collected data from October 2010 until September 2012 at a Veterans Affairs (VA) tertiary referral center for the New England region. Patients aged 60 years or older and admitted to medical wards were screened upon admission or transfer to VA Boston Healthcare System and provided appropriate interventions to modify delirium risk. Excluded were individuals admitted as observational status, or those readmitted within 30 days of initial screening, and those screened more than 72 hours after admission. Age and sex were abstracted from the medical record. Outcome data were collected from the medical record for the purpose of operating and sustaining the program. In a previous article, the length of stay (LOS) outcome was reported in a subset of this population.[23] The analysis presented here includes the full cohort, presents the interaction with month of the year backward (MOYB), and provides additional outcomes not included in the other article. The VA institutional review board (IRB) reviewed and approved the secondary data analysis of the quality improvement project.
Measures
Brief Cognitive Screening
The baseline assessments of levels of arousal and attention were collected within 72 hours of admission to identify delirium risk. Trained study staff, not involved in the clinical care of patients, administered these assessments as part of the quality improvement project. It is estimated that these assessments took less than 1 minute to complete per individual, but actual administration time was not measured. Assessments were documented within the electronic health record as part of a delirium risk stratification system.
Arousal
The arousal level assessment was the modified Richmond Agitation and Sedation Scale (mRASS). The mRASS is a brief, reliable, observational tool used to determine arousal level.[15, 17] It is a text modification of the RASS[17] for less acutely ill patients, capturing hyperactive and hypoactive altered levels of arousal. The mRASS asks an open‐ended question followed by observation for 10 seconds and completion of a 5 to+4 rating scale. Alert and calm (score=0) is considered normal, with positive numbers related to an increased level of arousal and attention, whereas negative numbers denote decreased levels. For the analyses, an mRASS of 0 is utilized as the reference. Categories were collapsed into 2 and 2 due to few patients on the extremes of the mRASS.
Attention
The MOYB is a brief measure of attention that is included in several instruments for delirium.[19, 24, 25] For this study, the patient was asked to recite the 12 months backward beginning with December. A correct score was given if the individual was able to recite all 12 months to January without any error. An incorrect score was given if any mistake was made. Scoring for the MOYB is not standardized by age, preexisting medical diagnosis, or any other rational.[26] Others have used July or June as a cutoff for a correct score on the MOYB,[21, 25] but a more conservative score of correct to January was used in this study, which has been previously used.[26, 27, 28, 29, 30] A score of not completed was given when the patient was unable to participate or declined to complete the assessment. For the analysis, a correct score on the MOYB is the referent group.
Outcomes
In‐hospital outcomes included (1) restraint use and (2) in‐hospital mortality. Physical restraint use was identified by focused medical record review and identification of required restraint documentation, which, by center policy requires daily review and documentation. Any restraint use during the hospitalization was included.
Discharge outcomes included (1) LOS, (2) discharge other than a location to home, and (3) variable direct costs. LOS was calculated from date of admission until date of discharge. Discharge disposition was identified in the electronic medical record discharge documentation and categorized into discharge to the prehospital residence (home) or not. Hospital variable direct costs were collected from the VA decision support system,[31] a centrally maintained administrative database. The VA decision support system is challenged with accounting for costs of a single‐day admission and patients who are hospitalized from VA long‐term care. To address the missing data from these cases, multiple imputations (n=20) of the missing data were performed.[32] Sensitivity analyses were performed to determine the impact of the imputation and the cost analysis strategy (see Supporting Information, Appendix 1, in the online version of this article).
Statistical Analyses
For this analysis, outcomes are reported at each level of performance on the mRASS (1 to1) and MOYB (correct, incorrect, not completed). For each analysis, the performance with a mean and standard deviation (SD) are reported for continuous outcomes and a percentage for dichotomous outcomes. For dichotomous outcomes, including restraint use, in‐hospital mortality, and discharge disposition, a risk ratio (RR) with 95% confidence interval (CI) is presented. The median is presented for the cost data because variable direct cost is highly skewed. For LOS and cost outcomes, unadjusted incident rate ratio (IRR) from a Poisson regression relative to the referent is presented to compare the categories. A Poisson regression was selected because LOS (a count of days) and variable direct costs (a count of dollars) are highly skewed. The output of Poisson regression produces an IRR and 95% CI relative to the referent group. The Poisson regression could not be adjusted because the quality improvement nature of these data limited the number of covariates collected. Sensitivity analyses did not identify significant interactions of age and sex (results not shown).
MOYB was also compared by level of arousal (mRASS=0 vs all others). Due to the relatively few patients with positive mRASS, it was compressed into a category of abnormal mRASS relative to alert and calm. Similar to the previous analyses, Poisson regression was performed to calculate the IRR (95% CI) relative to correct MOYB for the continuous variables. An RR was calculated for the dichotomous variables. All statistical analyses were performed using Stata version 11.0 (StataCorp, College Station, TX).
RESULTS
Population Description
Over the 2‐year project timeline, a total of 3232 unique individual records were analyzed (Table 1). Patients admitted and screened within the prior 30 days (n=501) and patients screened more than 3 days after admission (n=664) were not included in the analysis. Older adults were on average 74.7 years old (SD=9.8), and 98.2% were male, consistent with the veteran population. Altered level of arousal, as defined by an abnormal mRASS score, was found in 15.3% of the population. Average LOS was 5.2 days (SD=5.6), restraint use occurred in 5.5% during the hospital stay, patients were likely to be discharged home (71.7%), and a small portion died during hospitalization (1.3%). Mean variable direct costs were $11,084 with expected variability (SD=$15,682, median $6,614). Patients who died during the hospital stay had significantly longer LOS (mean 16.8 [SD=12.5] vs 5.1 [SD=5.4] days, P<0.001) and higher variable direct costs ($43,879 [SD=$37,334] vs $12,544 [SD=$16,802], P<0.001), justifying their removal from these analyses.
| Characteristic | Result, N=3,232, Mean (SD) or % (n) |
|---|---|
| |
| Age, y | 74.7 (9.8) |
| Male | 98.2 (3,174) |
| mRASS | |
| 2 | 2.0% (64) |
| 1 | 8.5% (273) |
| 0 | 84.7% (2,737) |
| 1 | 4.0% (131) |
| 2 | 0.8% (27) |
| MOYB | |
| Correct | 48.8% (1,578) |
| Incorrect | 45.1% (1,457) |
| Not completed | 6.1% (197) |
| Restraint use | 5.5% (177) |
| In‐hospital mortality | 1.3% (41) |
| Length of stay, da | 5.1 (5.4) |
| Discharge other than homea | 71.7% (2,292) |
| Variable direct hospital cost, $a | 11,084 (15,682) |
| Median cost, $ | 6,614 |
Impact of Altered Level of Arousal on Outcomes
There is an association between a deviation from a normal level of arousal (mRASS not equal to 0) and worsening outcomes (Table 2). Relative to a normal level of arousal (4.9SD 5.2 days), decreased level of arousal (negative mRASS), and increased arousal (positive mRASS) resulted in longer LOS (6.0SD 5.6 days, 5.7SD 6.8 days, respectively). Similarly, increased or decreased arousal was associated with heightened risk of restraints and less frequent discharge to home. In‐hospital mortality and hospital variable direct costs were significantly higher in those with decreased levels of arousal (IRR: 2.8, 95% CI: 1.36.0; IRR: 1.10, 95% CI: 0.951.26, respectively). The pattern does not hold for increased arousal with respect to in‐hospital mortality and variable direct hospital cost outcomes. The unadjusted analysis found that, relative to normal arousal, there is a significant change in outcomes with decreased levels of arousal. Increased arousal is associated with worsened IRR in LOS, restraint use, and discharge home, but not in‐hospital mortality and variable direct cost.
| mRASS Alert and Calm, n=2,737 | mRASS Negative, n=337 | mRASS Positive, n=158 | ||||
|---|---|---|---|---|---|---|
| Value | IRR/RR (95%CI) | Value | IRR/RR (95% CI) | Value | IRR/RR (95% CI) | |
| ||||||
| Restraint use % (n) | 4.2% (114) | Referent | 10.4% (35) | 2.49 (1.743.57) | 17.7% (28) | 4.25 (2.916.23) |
| In‐hospital mortality % (n) | 1.0% (26) | Referent | 2.7% (9) | 2.81 (1.335.95) | 1.3% (2) | 1.33 (0.325.56) |
| Length of stay, d (SD)a | 4.9 (5.2) | Referent | 6.0 (5.6) | 1.24 (1.181.30) | 5.7 (6.8) | 1.17 (1.091.25) |
| Discharge other than home, % (n)a | 24.9% (675) | Referent | 46.7% (153) | 1.87 (1.642.14) | 48.1% (75) | 1.93 (1.612.30) |
| Variable direct cost, $ (SD)a, b | 10,581 (14,928) | Referent | 11,604 (13,852) | 1.10 (0.951.26) | 10,640 (10,771) | 1.01 (0.851.19) |
| Median cost, $ | 6,318 | 7,738 | 7,858 | |||
Impact of Altered Attention on Outcomes
Patients who completed the MOYB incorrectly had increased restraint use (RR: 2.11, 95% CI 1.443.11) and LOS (IRR: 1.06, 95% CI: 1.021.10), but no difference in in‐hospital mortality, discharge home (RR: 0.78, 95% CI: 0.750.82), and variable direct costs, relative to those who completed the MOYB correctly (Table 3). Importantly, patients who did not complete the MOYB assessment had a 2‐fold increase in restraint use (RR: 2.05, 95% CI: 0.944.50), in‐hospital mortality was nearly 6‐fold higher (RR: 6.36, 95% CI: 2.1618.69), longer LOS (IRR: 1.12, 95% CI: 1.031.21), and returned home less frequently (RR: 1.77, 95% CI: 1.262.48).
| mRASS Normal | mRASS Abnormal | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MOYB Correct (n=1,431) | MOYB Incorrect (n=1,181) | MOYB Incomplete (n=125) | MOYB Correct (n=147) | MOYB Incorrect (n=276) | MOYB Incomplete (n=72) | |||||||
| Value | IRR/RR (95% CI) | Value | IRR/RR (95% CI) | Value | IRR/RR (95% CI) | Value | IRR/RR (95% CI) | Value | IRR/RR (95% CI) | Value | IRR/RR (95% CI) | |
| ||||||||||||
| Restraint use, % (n) | 2.7% (39) | Referent | 5.8% (68) | 2.11 (1.44‐3.11) | 5.6% (7) | 2.05 (0.94‐4.50) | 2.7% (4) | 1.00 (0.36‐2.75) | 13.8% (38) | 5.05 (3.29‐7.75) | 29.2% (21) | 10.70 (6.66‐17.20) |
| In‐hospital mortality, % (n) | 0.6% (9) | Referent | 1.0% (12) | 1.62 (0.68‐ 3.82) | 4.0% (5) | 6.36 (2.16‐18.69) | 1.4% (2) | 2.16 (0.47‐9.92) | 2.2% (6) | 3.46 (1.24‐9.63) | 4.2% (3) | 6.63 (1.83‐23.95) |
| Length of stay, d (SD)a | 4.7 (5.4) | Referent | 5.0 (5.1) | 1.06 (1.02‐1.10) | 5.3 (5.0) | 1.12 (1.03‐1.21) | 5.4 (6.0) | 1.13 (1.05‐1.22) | 5.9 (4.4) | 1.23 (1.17‐1.30) | 7.5 (10.0) | 1.55 (1.44‐1.73) |
| Discharge other than home, % (n)a | 17.9% (255) | Referent | 32.7% (382) | 1.82 (1.56‐ 2.14) | 31.7% (38) | 1.77 (1.26‐2.48) | 29.7% (43) | 1.65 (1.20‐2.28) | 53.3% (144) | 2.97 (2.42‐3.64) | 59.4% (41) | 3.31 (2.38‐4.61) |
| Variable direct cost, $ (SD)a, b | 10,609 (16,154) | Referent | 10,482 (13,495) | 0.99 (0.89‐1.10) | 11,213 (12,994) | 1.06 (0.85‐1.32) | 12,010 (15,636) | 1.13 (0.90‐1.42) | 10,776 (10,680) | 1.02 (0.88‐1.17) | 11,815 (14,604) | 1.11 (0.82‐1.51) |
| Median cost, $ | 6,338 | 6,248 | 6,630 | 7,023 | 8,093 | 8,180 | ||||||
Inter‐relationship of Altered Level of Arousal and Attention on Outcomes
The inter‐relationship of altered level of arousal and attention is presented in Table 3. Of patients with a normal mRASS, 52% had correct MOYB. The percentage of correct MOYB declined with the level of arousal, such that 38% had normal MOYB and a mRASS of 1 and 9% had normal MOYB with mRASS of 2. In general, in‐hospital outcomes (restraints and mortality) are associated with MOYB performance, and discharge outcomes (LOS, discharge location, and variable direct costs) are associated with mRASS. Those patients who did not complete the MOYB demonstrated worse outcomes, regardless of mRASS performance, including a 6‐fold increase in mortality and significant increases in LOS and discharge location.
DISCUSSION
Impaired performance on a one‐time assessment of arousal or attention during hospitalization demonstrated a relationship with in‐hospital and discharge outcomes. Relative to normal levels of arousal and attention, alterations in attention, level of arousal, or both were associated with progressively negative consequences. Combined with the prognostic value, the administration of ultra‐brief cognitive screening measures may have value in the identification of patients who would benefit from additional screening, supporting prior work in this area.[23] The brevity of the assessments enhances clinical utility and implementation potential.
Cognitive function during hospitalization has been associated with many negative outcomes including delirium, falls, pressure ulcers, and functional decline.[3, 33, 34, 35, 36, 37] The findings of this analysis are consistent with previous studies and provide important clinical implications. First, prior work in cognitive screening has focused on more time‐consuming instruments.[12] By focusing on brief instruments, particularly those under 1 minute that do not require paper or props, a user‐friendly tool that is associated with health outcomes is provided.
In addition, this analysis demonstrates that each assessment, when used individually, has some prognostic significance associated with the identification of delirium or other types of cognitive impairment. When used alone, abnormal scores on the mRASS or MOYB may be indicative of individuals requiring further cognitive assessment, supporting previous research.[16, 23] Individuals with abnormal scores on both the mRASS and MOYB identify a high‐risk group in need of further clinical assessment for delirium (Figure 1). Neither of these assessments are meant to be used as the only means to diagnosis delirium, but together they identify key clinical characteristics of delirium (arousal and attention).[16, 18, 21] Considering the significant negative consequences associated with delirium, it is not surprising that tools identifying core features of delirium, such as those presented here, would also be associated with in‐hospital and discharge outcomes.

The quality improvement design of this project allowed the recording of outcomes in those who were unable or refused to complete the screening. This may be a potentially high‐risk group who would otherwise go unnoticed. A recent editorial from the American and European Delirium Societies highlights that individuals who are unable or refuse to complete testing due to impaired arousal are neglected in the most recent American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition definition of delirium.[18] Further work to identify and intervene on behalf of individuals who are unable to complete testing will aid in understanding arousal and its relationship to delirium and other disorders.
This analysis provides additional insight in the selection of measures of arousal and attention. Level of arousal is a complex concept that involves components of awareness and alertness, including external stimuli and self‐awareness.[38, 39, 40] As an ultra‐brief measure of arousal level, the mRASS incorporates both external stimuli (asking an open‐ended question) and self‐awareness (describing current state) to determine basic cognitive function. Attention can be defined as the selection of stimuli for further cognitive processing.[40] Attention is an umbrella term referring to many cognitive processes, ranging from sustained attention and working memory to executive function such as set shifting and multitasking. Ultra‐brief measures of attention, such as MOYB, are basic tasks of sustained attention with components of working memory.[19] An alteration in attention may be indicative of a more significant global change in cognition[41] beyond basic cognitive function assessed by administration of the mRASS, such as delirium.[42] The relationship between level of arousal and attention is complex, and arguments have been made that one has to have a certain level of arousal to attend to a stimuli, whereas others have found that one has to have a certain level of attention.[18, 39, 40] Administration of both the mRASS and MOYB is a useful bedside tool for clinicians to examine both basic cognitive function and more complex tasks of attention.
The quality improvement nature of this work has limitations and strengths that deserve mention. The significant strength of this work is the robust sample size. Also, trained staff not involved in the direct clinical care of patients administered the cognitive screens, suggesting that nonclinically trained personnel could be utilized for risk assessment. The major limitation is the restricted amount of covariate data that were collected. Data for this project were collected to operationalize and demonstrate the impact and business case of a delirium risk modification program,[17] limiting the ability to perform adjustment for other covariates such as comorbidity and reason for admission. Also, due to the nature of this project, a diagnosis of delirium was not determined. A limitation of excluding in‐hospital deaths from the cost analysis was that some individuals at high risk died early, thus costing less overall. Generalizability is limited by an over‐representation of males within a single setting. Further use and understanding of mRASS and MOYB in other population is warranted and welcomed. Use of MOYB is also a limitation considering that scores are not standardized across patients or settings.[26] Data regarding administration time of either of these tools were not collected; therefore, determining that these are ultra‐brief assessments (<1 minute) is based on estimates. As such, these measures should not be the sole source of information for clinical evaluation and diagnosis.
CONCLUSION
This work found that impaired performance on brief cognitive assessments of arousal and attention in hospitalized patients were associated with restraint use, in‐hospital mortality, longer LOS, less discharge home, and hospital costs. Routine screening of older patients with brief, user‐friendly cognitive assessments upon admission can identify those who would benefit from additional assessment and intervention to alleviate individual and economic burdens.
Acknowledgements
The authors are indebted to the veterans who participated in their delirium and fall reduction programs. The authors are thankful for the guidance of the VA Boston Healthcare System Delirium Task Force and Patient Safety Officers for continued collaboration to improve outcomes for the veterans they serve.
Disclosures: Dr. Yevchak and Ms. Doherty contributed equally to this article and agreed to share first authorship. This material is based upon work supported by the Department of Veterans Affairs Office of Patient Safety Delirium Patient Safety Center of Inquiry and a Geriatrics and Extended Care T21 Alternative to Non‐institutional Long Term Care award. Archambault, Doherty, Fonda, Kelly, and Rudolph are employees of the US government. Dr. Rudolph also received support from a VA Career Development Award. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government. The authors report no conflicts of interest.
Hospitalization is a critical time for older patients with cognitive impairment. Past research has found that hospitalized older adults with cognitive dysfunction have more rapid cognitive decline, increased morbidity and mortality, and higher costs of healthcare utilization.[1, 2, 3] Those with preexisting cognitive dysfunction, such as dementia, are most susceptible to the negative impacts of hospitalization.[4, 5, 6, 7, 8] Identification of cognitive deficits upon admission is important for risk stratification of patients and prevention of negative hospital health events.
Frontline healthcare providers are underequipped to detect acute cognitive dysfunction.[9, 10] Current practice and research for the detection of cognitive dysfunction in the acute care setting utilizes instruments that require training[11] and are relatively lengthy (>5 minutes).[12] Although these cognitive screening tests are accurate and reliable, the time requirement is not feasible in a fast‐paced clinical setting. A possible alternative is the use of ultra‐brief cognitive screening instruments (<1 minute) that have the potential to identify those individuals requiring additional evaluation and follow‐up. These brief instruments are composed of screening tools that emphasize core features of acute cognitive dysfunction such as level of arousal or attention.[13, 14, 15, 16] Arousal, the ability to respond to or interact with the environment,[15] is an important component of cognition because it is generally preserved in chronic cognitive disorders (eg, dementia). Thus, an alteration in arousal may be a harbinger of more acute impairment[17] in need of evaluation, and in these lowered states of arousal it may be difficult to test for attention.[18] Attention is a broadly defined cognitive domain indicating focus.[19] Older adults, regardless of preexisting cognitive dysfunction, warrant additional cognitive testing if levels of arousal or attention are altered[20, 21] due to the significant relationship to delirium, which is associated with adverse events in this population. Recent research has demonstrated that these brief cognitive screening instruments provide information about the risk for delirium and are a strong test for clinical characteristics of delirium.[16, 21]
The purpose of this analysis was to demonstrate the clinical outcomes of poor performance on ultrabrief assessments arousal and attention by frontline staff using a quality improvement database. Specific objectives include determining (1) the association of poor performance on brief cognitive assessments and hospital outcomes and (2) the inter‐relationship between alterations in the levels of arousal and attention on in‐hospital and discharge outcomes.
METHODS
Setting and Study Design
This is a secondary analysis of data collected from a quality improvement program for delirium risk modification.[22] This program collected data from October 2010 until September 2012 at a Veterans Affairs (VA) tertiary referral center for the New England region. Patients aged 60 years or older and admitted to medical wards were screened upon admission or transfer to VA Boston Healthcare System and provided appropriate interventions to modify delirium risk. Excluded were individuals admitted as observational status, or those readmitted within 30 days of initial screening, and those screened more than 72 hours after admission. Age and sex were abstracted from the medical record. Outcome data were collected from the medical record for the purpose of operating and sustaining the program. In a previous article, the length of stay (LOS) outcome was reported in a subset of this population.[23] The analysis presented here includes the full cohort, presents the interaction with month of the year backward (MOYB), and provides additional outcomes not included in the other article. The VA institutional review board (IRB) reviewed and approved the secondary data analysis of the quality improvement project.
Measures
Brief Cognitive Screening
The baseline assessments of levels of arousal and attention were collected within 72 hours of admission to identify delirium risk. Trained study staff, not involved in the clinical care of patients, administered these assessments as part of the quality improvement project. It is estimated that these assessments took less than 1 minute to complete per individual, but actual administration time was not measured. Assessments were documented within the electronic health record as part of a delirium risk stratification system.
Arousal
The arousal level assessment was the modified Richmond Agitation and Sedation Scale (mRASS). The mRASS is a brief, reliable, observational tool used to determine arousal level.[15, 17] It is a text modification of the RASS[17] for less acutely ill patients, capturing hyperactive and hypoactive altered levels of arousal. The mRASS asks an open‐ended question followed by observation for 10 seconds and completion of a 5 to+4 rating scale. Alert and calm (score=0) is considered normal, with positive numbers related to an increased level of arousal and attention, whereas negative numbers denote decreased levels. For the analyses, an mRASS of 0 is utilized as the reference. Categories were collapsed into 2 and 2 due to few patients on the extremes of the mRASS.
Attention
The MOYB is a brief measure of attention that is included in several instruments for delirium.[19, 24, 25] For this study, the patient was asked to recite the 12 months backward beginning with December. A correct score was given if the individual was able to recite all 12 months to January without any error. An incorrect score was given if any mistake was made. Scoring for the MOYB is not standardized by age, preexisting medical diagnosis, or any other rational.[26] Others have used July or June as a cutoff for a correct score on the MOYB,[21, 25] but a more conservative score of correct to January was used in this study, which has been previously used.[26, 27, 28, 29, 30] A score of not completed was given when the patient was unable to participate or declined to complete the assessment. For the analysis, a correct score on the MOYB is the referent group.
Outcomes
In‐hospital outcomes included (1) restraint use and (2) in‐hospital mortality. Physical restraint use was identified by focused medical record review and identification of required restraint documentation, which, by center policy requires daily review and documentation. Any restraint use during the hospitalization was included.
Discharge outcomes included (1) LOS, (2) discharge other than a location to home, and (3) variable direct costs. LOS was calculated from date of admission until date of discharge. Discharge disposition was identified in the electronic medical record discharge documentation and categorized into discharge to the prehospital residence (home) or not. Hospital variable direct costs were collected from the VA decision support system,[31] a centrally maintained administrative database. The VA decision support system is challenged with accounting for costs of a single‐day admission and patients who are hospitalized from VA long‐term care. To address the missing data from these cases, multiple imputations (n=20) of the missing data were performed.[32] Sensitivity analyses were performed to determine the impact of the imputation and the cost analysis strategy (see Supporting Information, Appendix 1, in the online version of this article).
Statistical Analyses
For this analysis, outcomes are reported at each level of performance on the mRASS (1 to1) and MOYB (correct, incorrect, not completed). For each analysis, the performance with a mean and standard deviation (SD) are reported for continuous outcomes and a percentage for dichotomous outcomes. For dichotomous outcomes, including restraint use, in‐hospital mortality, and discharge disposition, a risk ratio (RR) with 95% confidence interval (CI) is presented. The median is presented for the cost data because variable direct cost is highly skewed. For LOS and cost outcomes, unadjusted incident rate ratio (IRR) from a Poisson regression relative to the referent is presented to compare the categories. A Poisson regression was selected because LOS (a count of days) and variable direct costs (a count of dollars) are highly skewed. The output of Poisson regression produces an IRR and 95% CI relative to the referent group. The Poisson regression could not be adjusted because the quality improvement nature of these data limited the number of covariates collected. Sensitivity analyses did not identify significant interactions of age and sex (results not shown).
MOYB was also compared by level of arousal (mRASS=0 vs all others). Due to the relatively few patients with positive mRASS, it was compressed into a category of abnormal mRASS relative to alert and calm. Similar to the previous analyses, Poisson regression was performed to calculate the IRR (95% CI) relative to correct MOYB for the continuous variables. An RR was calculated for the dichotomous variables. All statistical analyses were performed using Stata version 11.0 (StataCorp, College Station, TX).
RESULTS
Population Description
Over the 2‐year project timeline, a total of 3232 unique individual records were analyzed (Table 1). Patients admitted and screened within the prior 30 days (n=501) and patients screened more than 3 days after admission (n=664) were not included in the analysis. Older adults were on average 74.7 years old (SD=9.8), and 98.2% were male, consistent with the veteran population. Altered level of arousal, as defined by an abnormal mRASS score, was found in 15.3% of the population. Average LOS was 5.2 days (SD=5.6), restraint use occurred in 5.5% during the hospital stay, patients were likely to be discharged home (71.7%), and a small portion died during hospitalization (1.3%). Mean variable direct costs were $11,084 with expected variability (SD=$15,682, median $6,614). Patients who died during the hospital stay had significantly longer LOS (mean 16.8 [SD=12.5] vs 5.1 [SD=5.4] days, P<0.001) and higher variable direct costs ($43,879 [SD=$37,334] vs $12,544 [SD=$16,802], P<0.001), justifying their removal from these analyses.
| Characteristic | Result, N=3,232, Mean (SD) or % (n) |
|---|---|
| |
| Age, y | 74.7 (9.8) |
| Male | 98.2 (3,174) |
| mRASS | |
| 2 | 2.0% (64) |
| 1 | 8.5% (273) |
| 0 | 84.7% (2,737) |
| 1 | 4.0% (131) |
| 2 | 0.8% (27) |
| MOYB | |
| Correct | 48.8% (1,578) |
| Incorrect | 45.1% (1,457) |
| Not completed | 6.1% (197) |
| Restraint use | 5.5% (177) |
| In‐hospital mortality | 1.3% (41) |
| Length of stay, da | 5.1 (5.4) |
| Discharge other than homea | 71.7% (2,292) |
| Variable direct hospital cost, $a | 11,084 (15,682) |
| Median cost, $ | 6,614 |
Impact of Altered Level of Arousal on Outcomes
There is an association between a deviation from a normal level of arousal (mRASS not equal to 0) and worsening outcomes (Table 2). Relative to a normal level of arousal (4.9SD 5.2 days), decreased level of arousal (negative mRASS), and increased arousal (positive mRASS) resulted in longer LOS (6.0SD 5.6 days, 5.7SD 6.8 days, respectively). Similarly, increased or decreased arousal was associated with heightened risk of restraints and less frequent discharge to home. In‐hospital mortality and hospital variable direct costs were significantly higher in those with decreased levels of arousal (IRR: 2.8, 95% CI: 1.36.0; IRR: 1.10, 95% CI: 0.951.26, respectively). The pattern does not hold for increased arousal with respect to in‐hospital mortality and variable direct hospital cost outcomes. The unadjusted analysis found that, relative to normal arousal, there is a significant change in outcomes with decreased levels of arousal. Increased arousal is associated with worsened IRR in LOS, restraint use, and discharge home, but not in‐hospital mortality and variable direct cost.
| mRASS Alert and Calm, n=2,737 | mRASS Negative, n=337 | mRASS Positive, n=158 | ||||
|---|---|---|---|---|---|---|
| Value | IRR/RR (95%CI) | Value | IRR/RR (95% CI) | Value | IRR/RR (95% CI) | |
| ||||||
| Restraint use % (n) | 4.2% (114) | Referent | 10.4% (35) | 2.49 (1.743.57) | 17.7% (28) | 4.25 (2.916.23) |
| In‐hospital mortality % (n) | 1.0% (26) | Referent | 2.7% (9) | 2.81 (1.335.95) | 1.3% (2) | 1.33 (0.325.56) |
| Length of stay, d (SD)a | 4.9 (5.2) | Referent | 6.0 (5.6) | 1.24 (1.181.30) | 5.7 (6.8) | 1.17 (1.091.25) |
| Discharge other than home, % (n)a | 24.9% (675) | Referent | 46.7% (153) | 1.87 (1.642.14) | 48.1% (75) | 1.93 (1.612.30) |
| Variable direct cost, $ (SD)a, b | 10,581 (14,928) | Referent | 11,604 (13,852) | 1.10 (0.951.26) | 10,640 (10,771) | 1.01 (0.851.19) |
| Median cost, $ | 6,318 | 7,738 | 7,858 | |||
Impact of Altered Attention on Outcomes
Patients who completed the MOYB incorrectly had increased restraint use (RR: 2.11, 95% CI 1.443.11) and LOS (IRR: 1.06, 95% CI: 1.021.10), but no difference in in‐hospital mortality, discharge home (RR: 0.78, 95% CI: 0.750.82), and variable direct costs, relative to those who completed the MOYB correctly (Table 3). Importantly, patients who did not complete the MOYB assessment had a 2‐fold increase in restraint use (RR: 2.05, 95% CI: 0.944.50), in‐hospital mortality was nearly 6‐fold higher (RR: 6.36, 95% CI: 2.1618.69), longer LOS (IRR: 1.12, 95% CI: 1.031.21), and returned home less frequently (RR: 1.77, 95% CI: 1.262.48).
| mRASS Normal | mRASS Abnormal | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MOYB Correct (n=1,431) | MOYB Incorrect (n=1,181) | MOYB Incomplete (n=125) | MOYB Correct (n=147) | MOYB Incorrect (n=276) | MOYB Incomplete (n=72) | |||||||
| Value | IRR/RR (95% CI) | Value | IRR/RR (95% CI) | Value | IRR/RR (95% CI) | Value | IRR/RR (95% CI) | Value | IRR/RR (95% CI) | Value | IRR/RR (95% CI) | |
| ||||||||||||
| Restraint use, % (n) | 2.7% (39) | Referent | 5.8% (68) | 2.11 (1.44‐3.11) | 5.6% (7) | 2.05 (0.94‐4.50) | 2.7% (4) | 1.00 (0.36‐2.75) | 13.8% (38) | 5.05 (3.29‐7.75) | 29.2% (21) | 10.70 (6.66‐17.20) |
| In‐hospital mortality, % (n) | 0.6% (9) | Referent | 1.0% (12) | 1.62 (0.68‐ 3.82) | 4.0% (5) | 6.36 (2.16‐18.69) | 1.4% (2) | 2.16 (0.47‐9.92) | 2.2% (6) | 3.46 (1.24‐9.63) | 4.2% (3) | 6.63 (1.83‐23.95) |
| Length of stay, d (SD)a | 4.7 (5.4) | Referent | 5.0 (5.1) | 1.06 (1.02‐1.10) | 5.3 (5.0) | 1.12 (1.03‐1.21) | 5.4 (6.0) | 1.13 (1.05‐1.22) | 5.9 (4.4) | 1.23 (1.17‐1.30) | 7.5 (10.0) | 1.55 (1.44‐1.73) |
| Discharge other than home, % (n)a | 17.9% (255) | Referent | 32.7% (382) | 1.82 (1.56‐ 2.14) | 31.7% (38) | 1.77 (1.26‐2.48) | 29.7% (43) | 1.65 (1.20‐2.28) | 53.3% (144) | 2.97 (2.42‐3.64) | 59.4% (41) | 3.31 (2.38‐4.61) |
| Variable direct cost, $ (SD)a, b | 10,609 (16,154) | Referent | 10,482 (13,495) | 0.99 (0.89‐1.10) | 11,213 (12,994) | 1.06 (0.85‐1.32) | 12,010 (15,636) | 1.13 (0.90‐1.42) | 10,776 (10,680) | 1.02 (0.88‐1.17) | 11,815 (14,604) | 1.11 (0.82‐1.51) |
| Median cost, $ | 6,338 | 6,248 | 6,630 | 7,023 | 8,093 | 8,180 | ||||||
Inter‐relationship of Altered Level of Arousal and Attention on Outcomes
The inter‐relationship of altered level of arousal and attention is presented in Table 3. Of patients with a normal mRASS, 52% had correct MOYB. The percentage of correct MOYB declined with the level of arousal, such that 38% had normal MOYB and a mRASS of 1 and 9% had normal MOYB with mRASS of 2. In general, in‐hospital outcomes (restraints and mortality) are associated with MOYB performance, and discharge outcomes (LOS, discharge location, and variable direct costs) are associated with mRASS. Those patients who did not complete the MOYB demonstrated worse outcomes, regardless of mRASS performance, including a 6‐fold increase in mortality and significant increases in LOS and discharge location.
DISCUSSION
Impaired performance on a one‐time assessment of arousal or attention during hospitalization demonstrated a relationship with in‐hospital and discharge outcomes. Relative to normal levels of arousal and attention, alterations in attention, level of arousal, or both were associated with progressively negative consequences. Combined with the prognostic value, the administration of ultra‐brief cognitive screening measures may have value in the identification of patients who would benefit from additional screening, supporting prior work in this area.[23] The brevity of the assessments enhances clinical utility and implementation potential.
Cognitive function during hospitalization has been associated with many negative outcomes including delirium, falls, pressure ulcers, and functional decline.[3, 33, 34, 35, 36, 37] The findings of this analysis are consistent with previous studies and provide important clinical implications. First, prior work in cognitive screening has focused on more time‐consuming instruments.[12] By focusing on brief instruments, particularly those under 1 minute that do not require paper or props, a user‐friendly tool that is associated with health outcomes is provided.
In addition, this analysis demonstrates that each assessment, when used individually, has some prognostic significance associated with the identification of delirium or other types of cognitive impairment. When used alone, abnormal scores on the mRASS or MOYB may be indicative of individuals requiring further cognitive assessment, supporting previous research.[16, 23] Individuals with abnormal scores on both the mRASS and MOYB identify a high‐risk group in need of further clinical assessment for delirium (Figure 1). Neither of these assessments are meant to be used as the only means to diagnosis delirium, but together they identify key clinical characteristics of delirium (arousal and attention).[16, 18, 21] Considering the significant negative consequences associated with delirium, it is not surprising that tools identifying core features of delirium, such as those presented here, would also be associated with in‐hospital and discharge outcomes.

The quality improvement design of this project allowed the recording of outcomes in those who were unable or refused to complete the screening. This may be a potentially high‐risk group who would otherwise go unnoticed. A recent editorial from the American and European Delirium Societies highlights that individuals who are unable or refuse to complete testing due to impaired arousal are neglected in the most recent American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition definition of delirium.[18] Further work to identify and intervene on behalf of individuals who are unable to complete testing will aid in understanding arousal and its relationship to delirium and other disorders.
This analysis provides additional insight in the selection of measures of arousal and attention. Level of arousal is a complex concept that involves components of awareness and alertness, including external stimuli and self‐awareness.[38, 39, 40] As an ultra‐brief measure of arousal level, the mRASS incorporates both external stimuli (asking an open‐ended question) and self‐awareness (describing current state) to determine basic cognitive function. Attention can be defined as the selection of stimuli for further cognitive processing.[40] Attention is an umbrella term referring to many cognitive processes, ranging from sustained attention and working memory to executive function such as set shifting and multitasking. Ultra‐brief measures of attention, such as MOYB, are basic tasks of sustained attention with components of working memory.[19] An alteration in attention may be indicative of a more significant global change in cognition[41] beyond basic cognitive function assessed by administration of the mRASS, such as delirium.[42] The relationship between level of arousal and attention is complex, and arguments have been made that one has to have a certain level of arousal to attend to a stimuli, whereas others have found that one has to have a certain level of attention.[18, 39, 40] Administration of both the mRASS and MOYB is a useful bedside tool for clinicians to examine both basic cognitive function and more complex tasks of attention.
The quality improvement nature of this work has limitations and strengths that deserve mention. The significant strength of this work is the robust sample size. Also, trained staff not involved in the direct clinical care of patients administered the cognitive screens, suggesting that nonclinically trained personnel could be utilized for risk assessment. The major limitation is the restricted amount of covariate data that were collected. Data for this project were collected to operationalize and demonstrate the impact and business case of a delirium risk modification program,[17] limiting the ability to perform adjustment for other covariates such as comorbidity and reason for admission. Also, due to the nature of this project, a diagnosis of delirium was not determined. A limitation of excluding in‐hospital deaths from the cost analysis was that some individuals at high risk died early, thus costing less overall. Generalizability is limited by an over‐representation of males within a single setting. Further use and understanding of mRASS and MOYB in other population is warranted and welcomed. Use of MOYB is also a limitation considering that scores are not standardized across patients or settings.[26] Data regarding administration time of either of these tools were not collected; therefore, determining that these are ultra‐brief assessments (<1 minute) is based on estimates. As such, these measures should not be the sole source of information for clinical evaluation and diagnosis.
CONCLUSION
This work found that impaired performance on brief cognitive assessments of arousal and attention in hospitalized patients were associated with restraint use, in‐hospital mortality, longer LOS, less discharge home, and hospital costs. Routine screening of older patients with brief, user‐friendly cognitive assessments upon admission can identify those who would benefit from additional assessment and intervention to alleviate individual and economic burdens.
Acknowledgements
The authors are indebted to the veterans who participated in their delirium and fall reduction programs. The authors are thankful for the guidance of the VA Boston Healthcare System Delirium Task Force and Patient Safety Officers for continued collaboration to improve outcomes for the veterans they serve.
Disclosures: Dr. Yevchak and Ms. Doherty contributed equally to this article and agreed to share first authorship. This material is based upon work supported by the Department of Veterans Affairs Office of Patient Safety Delirium Patient Safety Center of Inquiry and a Geriatrics and Extended Care T21 Alternative to Non‐institutional Long Term Care award. Archambault, Doherty, Fonda, Kelly, and Rudolph are employees of the US government. Dr. Rudolph also received support from a VA Career Development Award. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government. The authors report no conflicts of interest.
- , , , , , . Delirium superimposed on dementia predicts 12‐month survival in elderly patients discharged from a postacute rehabilitation facility. J Gerontol A Biol Sci Med Sci. 2007;62(11):1306–1309.
- , , , . Delirium superimposed on dementia is associated with prolonged length of stay and poor outcomes in hospitalized older adults. J Hosp Med. 2013;8(9):500–505.
- , , , et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med. 2010;5(2):69–75.
- , , , et al. Association between endothelial dysfunction and acute brain dysfunction during critical illness. Anesthesiology. 2013;118(3):631–639.
- , , , et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72(18):1570–1575.
- , , , et al. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Intern Med. 2012;156(12):848–856.
- , , , et al. Delirium: an independent predictor of functional decline after cardiac surgery. J Am Geriatr Soc. 2010;58(4):643–649.
- , . The importance of delirium: economic and societal costs. J Am Geriatr Soc. 2011;59:S241–S243.
- , , , , , . Managing delirium in the acute care setting: a pilot focus group study. Int J Older People Nurs. 2012;7(2):152–162.
- , , , et al. Barriers and facilitators to implementing delirium rounds in a clinical trial across three diverse hospital settings. Clin Nurs Res. 2014;23(2):201–215.
- , , , , , . Validation of the confusion assessment method in the palliative care setting. Palliat Med. 2009;23(1):40–45.
- , , , . Does this patient have delirium? Value of bedside instruments. JAMA. 2010;304(7):779–786.
- , , , et al. Three core domains of delirium validated using exploratory and confirmatory factor analyses. Psychosomatics. 2013;54(3):227–238.
- , , . A neurologist's approach to delirium: diagnosis and management of toxic metabolic encephalopathies. Eur J Intern Med. 2014;25(2):112–116.
- , , ; the VADWG. Serial administration of a modified Richmond Agitation and Sedation Scale for delirium screening. J Hosp Med. 2012;7(5):450–453.
- . The diagnostic performance of the Richmond Agitation Sedation Scale for detecting delirium in older emergency department patients. Acad Emerg Med. 2015;22(7):878–882.
- , , , et al. The Richmond Agitation Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344.
- European Delirium Association, American Delirium Society. The DSM‐5 criteria, level of arousal and delirium diagnosis: inclusiveness is safer. BMC Med. 2014;12:141.
- , , , , , . Pay attention! The critical importance of assessing attention in older adults with dementia. J Gerontol Nurs. 2012;38(11):23–27.
- , , . Delirium: a disorder of consciousness? Med Hypotheses. 2013;80(4):399–404.
- , , , et al. Attention! A good bedside test for delirium? J Neurol Neurosurg Psychiatry. 2014;85(10):1122–1131.
- , , . A delirium risk modification program is associated with hospital outcomes. J Am Med Dir Assoc. 2014;15(12):11.
- , , , et al. Impaired arousal in older adults is associated with prolonged hospital stay and discharge to skilled nursing facility. J Am Med Dir Assoc. 2015;16(7):586–589.
- , , , et al. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing. 2014;43(4):496–502.
- , , , , , . Reliability of a structured assessment for nonclinicians to detect delirium among new admissions to postacute care. J Am Med Dir Assoc. 2006;7(7):412–415.
- , , , . Reciting the months of the year backwards: what is a ‘normal’ score? Age Ageing. 2015;44(3):537–538.
- , , . A Delirium risk modification program is associated with hospital outcomes. J Am Med Dir Assoc. 2014;15(12):957.e957–957.e911.
- , , , et al. 3D‐CAM: derivation and validation of a 3‐minute diagnostic interview for CAM‐defined delirium: a cross‐sectional diagnostic test study. Ann Intern Med. 2014;161(8):554–561.
- , , , , , . Reliability of a structured assessment for non‐clinicians to detect delirium among new admissions to post‐acute care. J Am Med Dir Assoc. 2006;7:412–415.
- , , , et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119(2):229–236.
- , . Use of the Decision Support System for VA cost‐effectiveness research. Med Care. 1999;37(4 suppl Va):AS63–AS70.
- , , , , . Cost analysis in the Department of Veterans Affairs: consensus and future directions. Med Care. 1999;37(4 Suppl Va):AS3‐AS8.
- , , . Delirium: a symptom of how hospital care is failing older persons and a window to improve quality of hospital care. Am J Med. 1999;106(5):565–573.
- , , , et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676.
- , , , . Iatrogenic causes of falls in hospitalised elderly patients: a case‐control study. Postgrad Med J. 2002;78(922):487–489.
- , , , , . A controlled quality improvement trial to reduce the use of physical restraints in older hospitalized adults. J Am Geriatr Soc. 2014;62(3):541–545.
- , , , . Evaluation of the mobile acute care of the elderly (mace) service. JAMA Intern Med. 2013;173(11):990–996.
- , . Conscience and consciousness: a definition. J Med Life. 2014;7(1):104–108.
- , , , et al. Consciousness in humans and non‐human animals: recent advances and future directions. Front Psychol. 2013;4:625.
- . Interdependence of attention and consciousness. In: Rahul B, Bikas KC, eds. Progress in Brain Research. Vol. 168. New York, NY: Elsevier; 2007:65–75.
- , , . Relationship between cognitive and non‐cognitive symptoms of delirium. Asian J Psychiatr. 2013;6(2):106–112.
- , , , , , . Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948.
- , , , , , . Delirium superimposed on dementia predicts 12‐month survival in elderly patients discharged from a postacute rehabilitation facility. J Gerontol A Biol Sci Med Sci. 2007;62(11):1306–1309.
- , , , . Delirium superimposed on dementia is associated with prolonged length of stay and poor outcomes in hospitalized older adults. J Hosp Med. 2013;8(9):500–505.
- , , , et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med. 2010;5(2):69–75.
- , , , et al. Association between endothelial dysfunction and acute brain dysfunction during critical illness. Anesthesiology. 2013;118(3):631–639.
- , , , et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72(18):1570–1575.
- , , , et al. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Intern Med. 2012;156(12):848–856.
- , , , et al. Delirium: an independent predictor of functional decline after cardiac surgery. J Am Geriatr Soc. 2010;58(4):643–649.
- , . The importance of delirium: economic and societal costs. J Am Geriatr Soc. 2011;59:S241–S243.
- , , , , , . Managing delirium in the acute care setting: a pilot focus group study. Int J Older People Nurs. 2012;7(2):152–162.
- , , , et al. Barriers and facilitators to implementing delirium rounds in a clinical trial across three diverse hospital settings. Clin Nurs Res. 2014;23(2):201–215.
- , , , , , . Validation of the confusion assessment method in the palliative care setting. Palliat Med. 2009;23(1):40–45.
- , , , . Does this patient have delirium? Value of bedside instruments. JAMA. 2010;304(7):779–786.
- , , , et al. Three core domains of delirium validated using exploratory and confirmatory factor analyses. Psychosomatics. 2013;54(3):227–238.
- , , . A neurologist's approach to delirium: diagnosis and management of toxic metabolic encephalopathies. Eur J Intern Med. 2014;25(2):112–116.
- , , ; the VADWG. Serial administration of a modified Richmond Agitation and Sedation Scale for delirium screening. J Hosp Med. 2012;7(5):450–453.
- . The diagnostic performance of the Richmond Agitation Sedation Scale for detecting delirium in older emergency department patients. Acad Emerg Med. 2015;22(7):878–882.
- , , , et al. The Richmond Agitation Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344.
- European Delirium Association, American Delirium Society. The DSM‐5 criteria, level of arousal and delirium diagnosis: inclusiveness is safer. BMC Med. 2014;12:141.
- , , , , , . Pay attention! The critical importance of assessing attention in older adults with dementia. J Gerontol Nurs. 2012;38(11):23–27.
- , , . Delirium: a disorder of consciousness? Med Hypotheses. 2013;80(4):399–404.
- , , , et al. Attention! A good bedside test for delirium? J Neurol Neurosurg Psychiatry. 2014;85(10):1122–1131.
- , , . A delirium risk modification program is associated with hospital outcomes. J Am Med Dir Assoc. 2014;15(12):11.
- , , , et al. Impaired arousal in older adults is associated with prolonged hospital stay and discharge to skilled nursing facility. J Am Med Dir Assoc. 2015;16(7):586–589.
- , , , et al. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing. 2014;43(4):496–502.
- , , , , , . Reliability of a structured assessment for nonclinicians to detect delirium among new admissions to postacute care. J Am Med Dir Assoc. 2006;7(7):412–415.
- , , , . Reciting the months of the year backwards: what is a ‘normal’ score? Age Ageing. 2015;44(3):537–538.
- , , . A Delirium risk modification program is associated with hospital outcomes. J Am Med Dir Assoc. 2014;15(12):957.e957–957.e911.
- , , , et al. 3D‐CAM: derivation and validation of a 3‐minute diagnostic interview for CAM‐defined delirium: a cross‐sectional diagnostic test study. Ann Intern Med. 2014;161(8):554–561.
- , , , , , . Reliability of a structured assessment for non‐clinicians to detect delirium among new admissions to post‐acute care. J Am Med Dir Assoc. 2006;7:412–415.
- , , , et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119(2):229–236.
- , . Use of the Decision Support System for VA cost‐effectiveness research. Med Care. 1999;37(4 suppl Va):AS63–AS70.
- , , , , . Cost analysis in the Department of Veterans Affairs: consensus and future directions. Med Care. 1999;37(4 Suppl Va):AS3‐AS8.
- , , . Delirium: a symptom of how hospital care is failing older persons and a window to improve quality of hospital care. Am J Med. 1999;106(5):565–573.
- , , , et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676.
- , , , . Iatrogenic causes of falls in hospitalised elderly patients: a case‐control study. Postgrad Med J. 2002;78(922):487–489.
- , , , , . A controlled quality improvement trial to reduce the use of physical restraints in older hospitalized adults. J Am Geriatr Soc. 2014;62(3):541–545.
- , , , . Evaluation of the mobile acute care of the elderly (mace) service. JAMA Intern Med. 2013;173(11):990–996.
- , . Conscience and consciousness: a definition. J Med Life. 2014;7(1):104–108.
- , , , et al. Consciousness in humans and non‐human animals: recent advances and future directions. Front Psychol. 2013;4:625.
- . Interdependence of attention and consciousness. In: Rahul B, Bikas KC, eds. Progress in Brain Research. Vol. 168. New York, NY: Elsevier; 2007:65–75.
- , , . Relationship between cognitive and non‐cognitive symptoms of delirium. Asian J Psychiatr. 2013;6(2):106–112.
- , , , , , . Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948.
© 2015 Society of Hospital Medicine