User login
Smartphones: Dermatologic Impact of the Digital Age
Over the last decade, the use of mobile phones has changed drastically with the advent of more technologically advanced smartphones.1 Mobile phones are no longer used primarily as devices for talking but rather for text messaging, reading the news, drafting emails, browsing websites, and connecting with others on social media. Considering the increased utility and popularity of social media along with the greater reliance on smartphones, individuals in the United States and worldwide are undoubtedly spending more time on their handheld devices.2 With the increase in use and overuse of smartphones, many aspects of society and health are likely affected. Many celebrities who frequently post on social media platforms also have alluded to or directly discussed changes in their dermatologic health secondary to their increased use of smartphones.3 Numerous studies have investigated the positive and negative effects of smartphone use on various musculoskeletal conditions of the upper extremities4,5 and the social effects of smartphone use on behavior and child development.6,7 Lee et al8 studied the effects of smartphone use on upper extremity muscle pain and activity in relation to 1- or 2-handed operation. In this study, Lee et al8 measured the muscle activity and tenderness in 10 women aged 20 to 22 years after a series of timed periods of smartphone use. They concluded that smartphone use resulted in greater muscle activity and tenderness, especially in 1-handed use compared to 2-handed use.8 Inal et al9 investigated smartphone overuse effects on hand strength and function in 102 college students and discovered that smartphone overuse was correlated with decreased pinch strength, increased median nerve cross-sectional area, and pain in the first digits.9
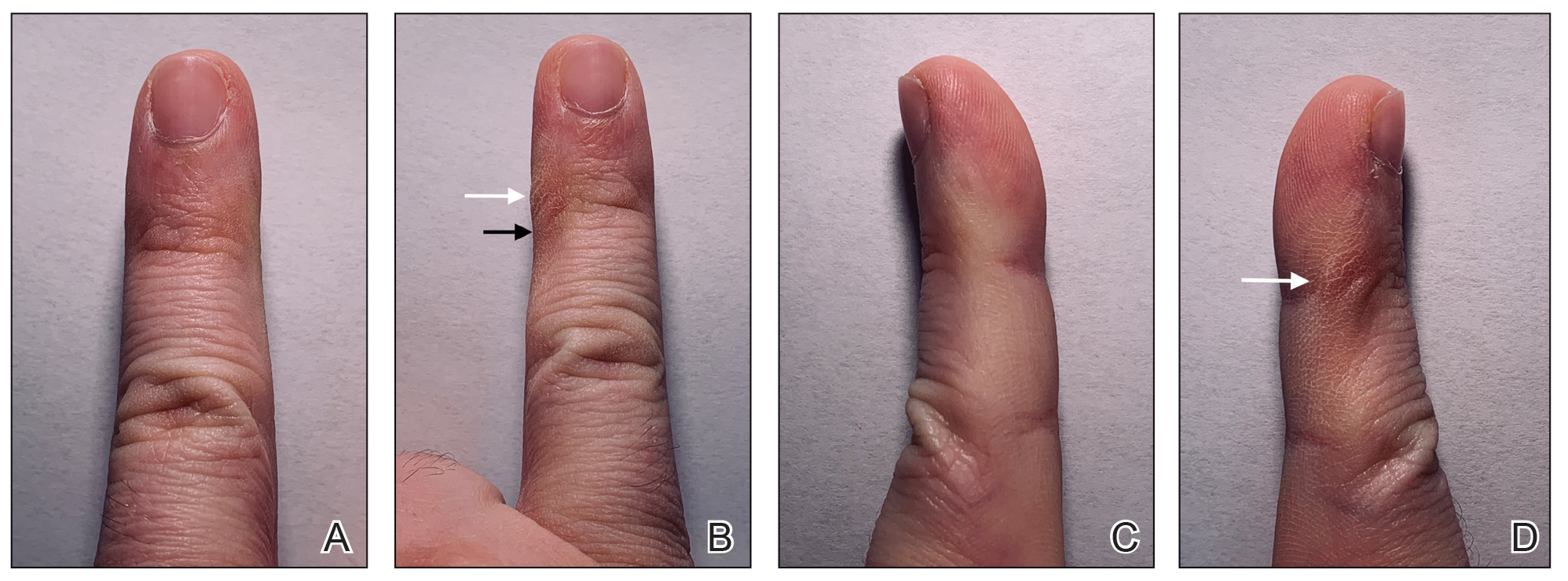
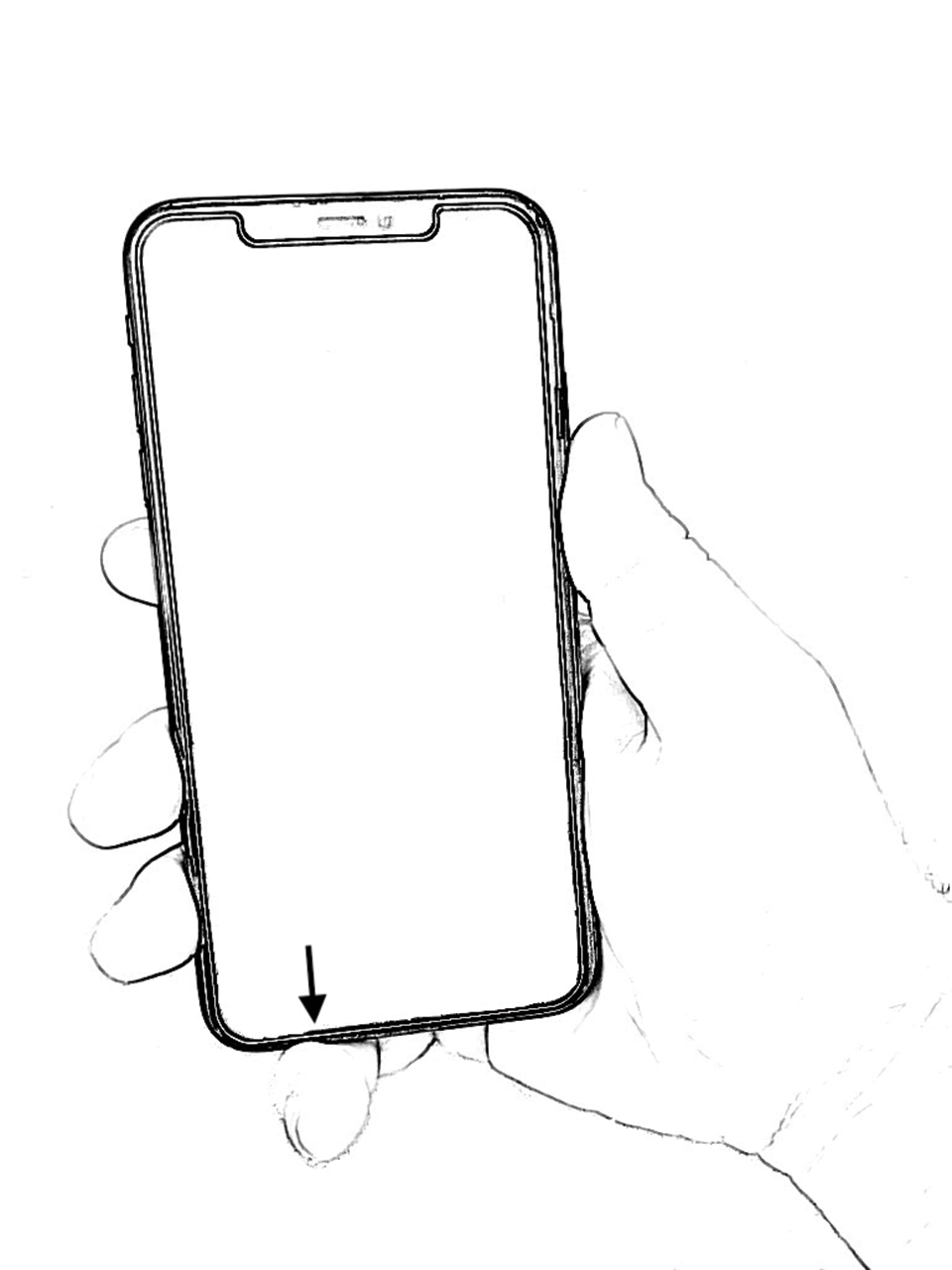
However, few articles have been published investigating skin changes to the digits in relation to smartphone use (Figure 1). In a PubMed search of articles indexed for MEDLINE using the terms smartphone, phone, cell phone, electronic device, handheld device, fifth digit, or skin changes, the authors were unable to find any studies in the literature that involved smartphone use and skin changes to the digits. Based on informal clinical observation and personal experiences, we hypothesized that changes to the fifth digit, likely due to holding a smartphone, would be prevalent and would correlate with amount of time spent on smartphones per day (Figure 2). We also were interested in investigating any other potential correlations with changes to the fifth digit, such as type of smartphone used.
Methods
The study used a cross-sectional design. From September 2018 to December 2018, 374 individuals 18 years or older were recruited to complete a 5-minute anonymous survey online. Using email referrals and social media, participants were presented with a link to a Google survey that only allowed 1 submission per account. On the first page of the survey, participants were presented with a letter explaining that completion of the survey was entirely voluntary, participants were free to withdraw from the study at any time, and participants were providing consent in completing the survey. The protocol was determined to be exempt by the institutional review board at Nova Southeastern University (Fort Lauderdale, Florida) in September 2018.
Survey Design
A 20-item survey was designed to measure the amount of time spent using smartphones per day, classify the type of phone used, and quantify skin changes noticed by each respondent. Demographic information for each respondent also was gathered using the survey. The survey was pilot tested to ensure that respondents were able to understand the items.
One item asked if respondents owned a handheld smartphone. Two items assessed how much time was spent on smartphones per day (ie, <1 hour, 1–2 hours, 2–3 hours, 3–4 hours, 4–5 hours, >5 hours) and the type of smartphone used (ie, Apple iPhone, Samsung Galaxy, Google Pixel, Huawei, LG, other). Six items assessed skin changes to the digits, namely the fifth digit (eg, Do you notice any changes to your fifth digit [pinky finger] that would likely be contributed to how you hold your smartphone, such as divot, callus, bruise, wound, misalignment, bend?). Eleven items were used to collect basic demographic information, including age, sex, legal marital status, ethnicity, race, annual household income, highest-earned educational degree, current employment status, health insurance status, and state of residence.
Statistical Analysis
All data were analyzed using IBM SPSS Statistics 23. The association between changes to the fifth digit and time spent on the phone, hand dominance, and socioeconomic factors (ie, age,
Results
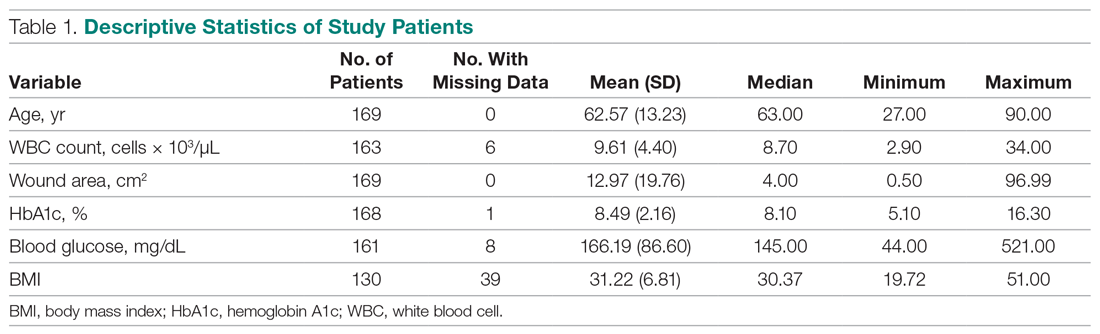
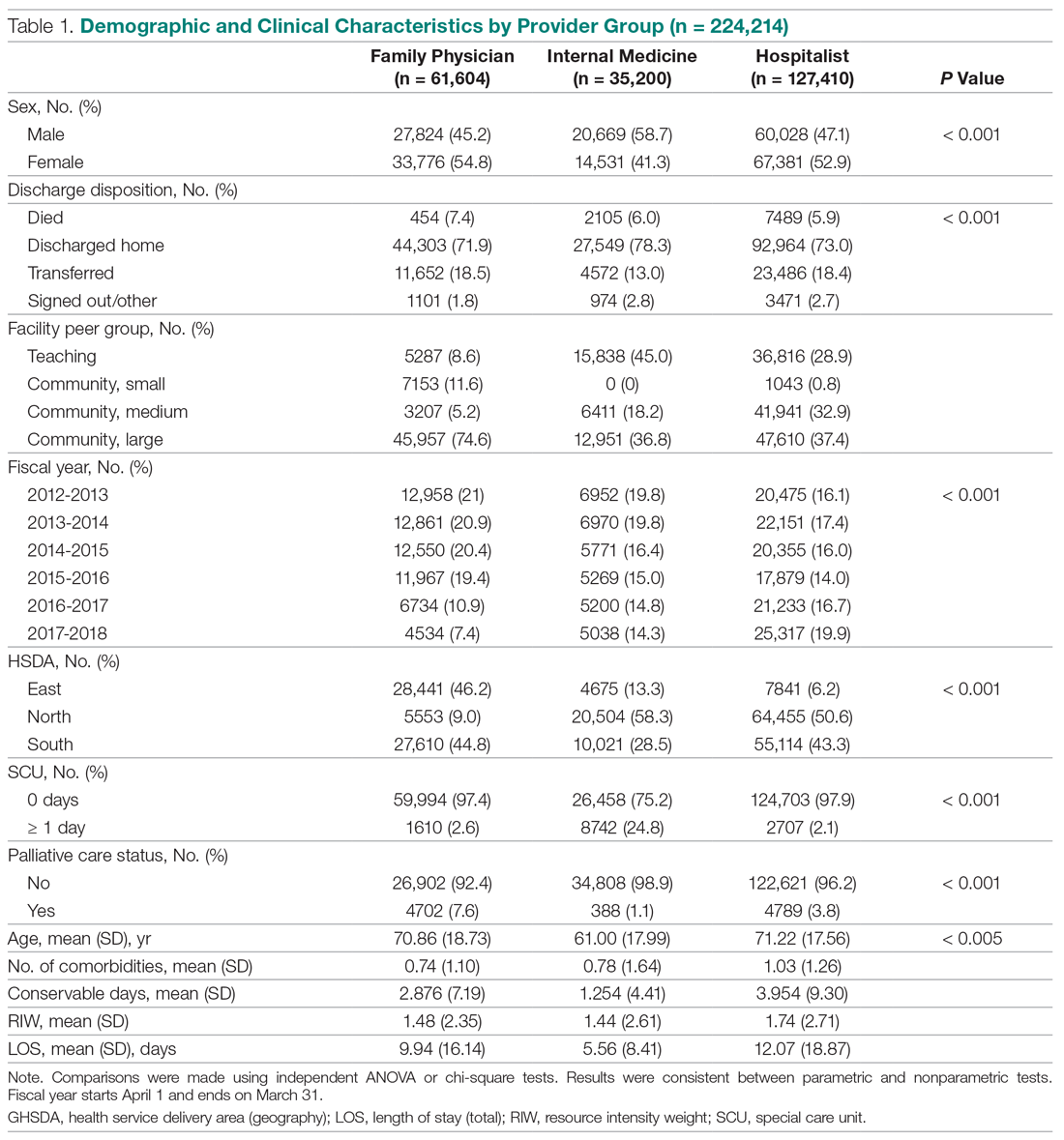
The mean age of the 374 respondents was 33.8 years (range, 18–72 years). One hundred nine respondents were men (29.1%), 262 were women (70.1%), and 3 did not specify (0.8%). Two hundred thirty-four respondents (62.6%) were single, 271 (72.5%) were white, 171 (45.7%) had a bachelor’s degree, and174 (46.5%) were employed full time. Annual household income was normally distributed among the respondents, with 28 (7.5%) earning less than $10,000 per year, 130 (34.8%) earning $10,000 to$49,999 per year, 136 (36.4%) earning $50,000 to $99,999 per year, 52 (13.9%) earning $100,000 to$149,999 per year, and 28 (7.5%) earning more than $150,000 per year. The demographic characteristics of the respondents are presented in Table 1.
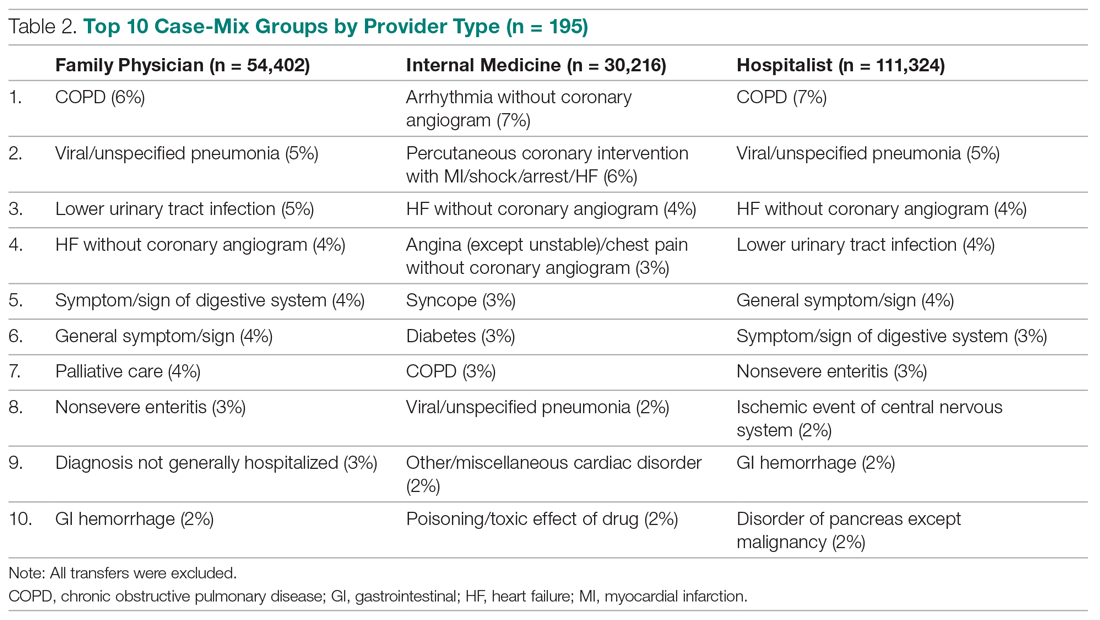
Eighty-five (22.7%) respondents admitted to changes to the fifth digit that they associated with holding a smartphone, whereas 289 (77.3%) reported no changes. When asked about the average amount of time spent on their smartphone per day, 17 (4.5%) respondents answered less than 1 hour, 70 (18.7%) answered 1 to 2 hours, 69 (18.4%) answered 2 to 3 hours, 77 (20.6%) answered 3 to 4 hours, 57 (15.2%) answered 4 to 5 hours, and 84 (22.5%) answered more than 5 hours. One hundred ninety-nine (53.2%) respondents indicated they used an Apple iPhone, 95 (25.4%) used a Samsung Galaxy phone, 9 (2.4%) used a Google Pixel phone, 3 (0.8%) used a Huawei phone, 23 (6.1%) used an LG phone, and 45 (12.0%) used another type of smartphone. The characteristics of smartphone use as reported by the respondents are presented in Table 2.
Comment
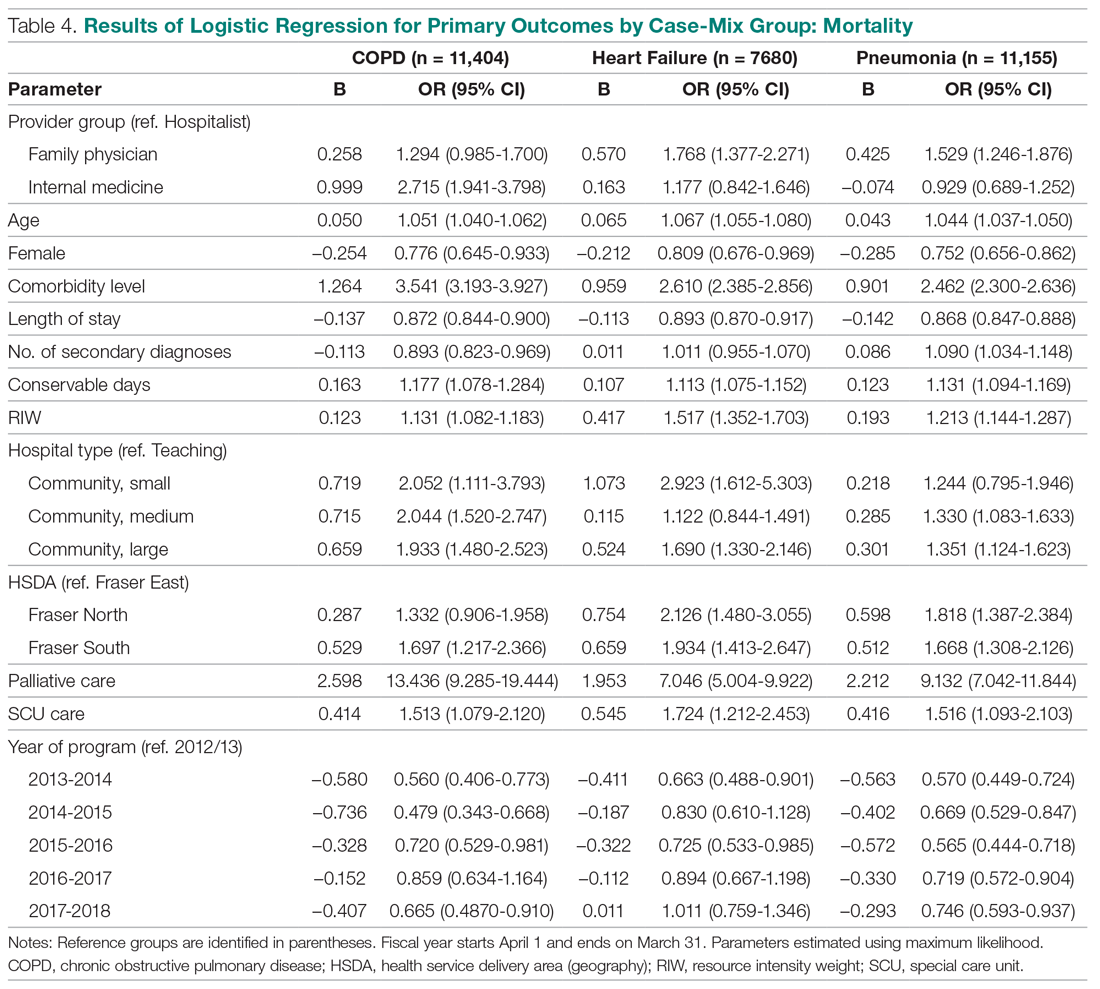
Consistent with our hypothesis, changes to the fifth digit were prevalent in the surveyed population, with 85 (22.7%) respondents admitting to changes to their fifth digit from holding a smartphone. The changes to the fifth digit were described as 1 or more of the following: divot (impression), callus (skin thickening), bruise, wound, misalignment, or bending. Most respondents who noted skin changes on the survey endorsed changes consistent with calluses and/or divots. These changes can be described as scaly, lichenified, well-demarcated papules or plaques with variable overlying hyperpigmentation and surrounding erythema. In cases with resulting chronic indentations of the skin, one also would observe localized sclerosis, atrophy, and/or induration of the area, which we found to be less prevalent than expected considering the popularity and notable reliance on smartphones.2
The most commonly reported chronic skin changes to the fifth digit are similar to those of lichen simplex chronicus and/or exogenous lobular panniculitis, which can be both symptomatically and cosmetically troubling for a patient. Functional impairment in movement of the fifth digit may result from the overlying lichenification and induration, as well as from lipoatrophy of the underlying traumatized subcutaneous fat, especially if the affected area is overlying the proximal interphalangeal joint of the fifth digit. These resulting alterations in the skin of the fifth digit also may be cosmetically displeasing to the patient.
On histology, we would expect similar changes to that of lichen simplex chronicus—compact hyperkeratosis and hypergranulosis—and/or an exogenous lobular panniculitis. Lobular panniculitis demonstrates necrosis of the fat lobule; vacuolated spaces; and lipomembranous changes such as fatty cystic degeneration with feathery eosinophilic material in an arabesque pattern, which has been described as frost on a windowpane, or a ferning pattern at the edge of the lipid vacuole.10
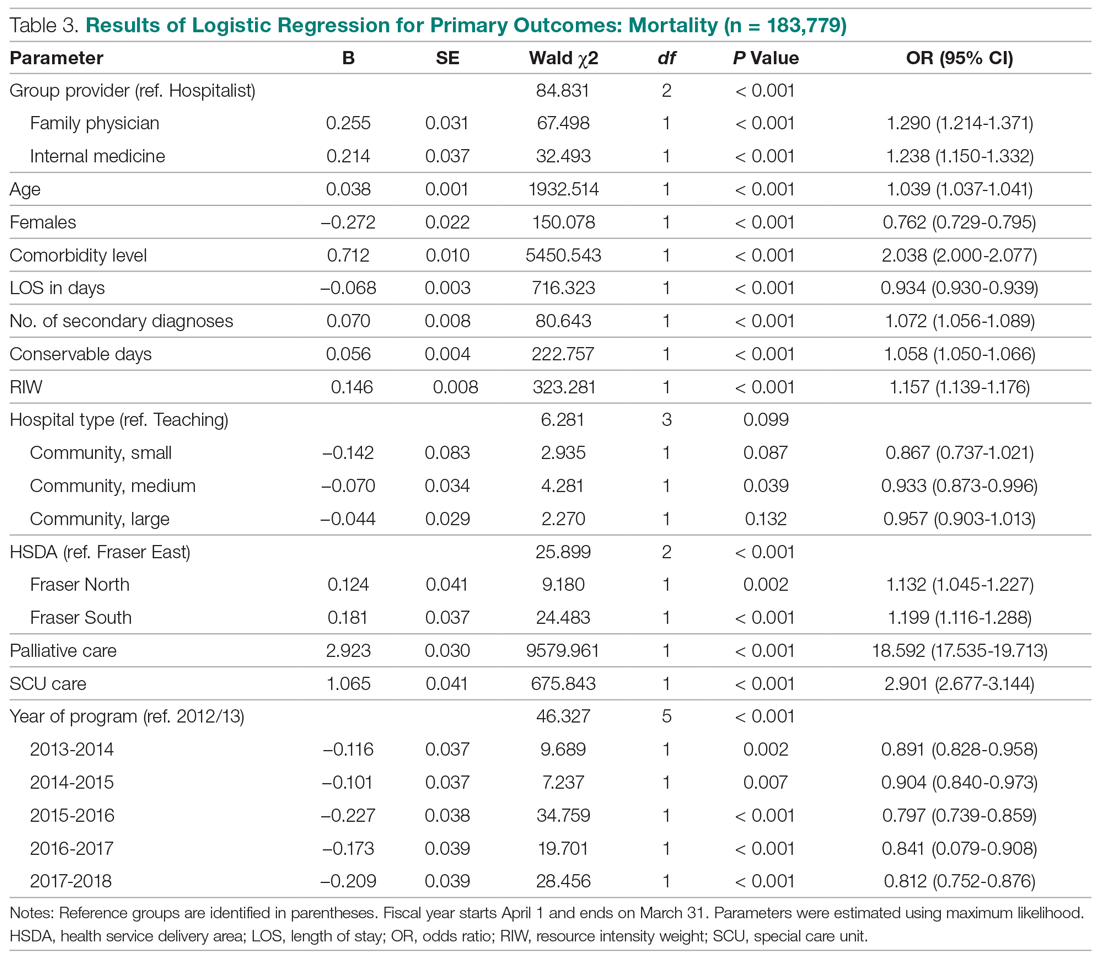
We also were correct in our hypothesis that prevalence of changes to the fifth digit correlate with amount of time spent on smartphones per day. Bivariate and multivariate logistic regression analysis showed that a change to the fifth digit was not significantly associated with hand dominance or socioeconomic factors (ie, age, sex, legal marital status, ethnicity, race, annual household income, highest-earned educational degree, current employment status, health insurance status, and state of residence). Controlling for all other factors, the only factor that significantly increased the odds of experiencing a change to the fifth digit was the amount of time spent on the phone per day. The respondents who spent more than 5 hours per day on their phones had 5-times greater odds of experiencing a change to their fifth digit compared with respondents who spent less than 1 hour per day on their phones (P=.045).
Although no other correlations with changes to the fifth digit, such as type of smartphone used, were found in our study, future studies should continue to investigate other potential factors that play a role in smartphone use changing the appearance and function of the digits. Our lack of significant correlations with changes to the fifth digit could be attributed to a small sample size and other possible factors, such as the frequent design changes of smartphones by manufacturers. Our study also is limited by the possibility of other factors contributing to these observed skin changes. Although we have anecdotally observed these skin changes and have hypothesized that smartphones are the culprit, other causes, such as holding certain tools, could lead to these skin changes. In addition, there are many different ways to hold a smartphone, and certain hand positionings may be more or less prone to skin changes described in our study. Various accessories, such as cases and gripping devices, also may change the way smartphones are held and would skew the results of our survey. Future studies could examine different ways smartphones are held, how various accessories affect these skin changes, and the size or model of phones that make these skin changes more or less prevalent.
Conclusion
Our study is an initial step in uncovering a possible phenomenon of smartphone use affecting the digits, namely the fifth digit. Our findings demonstrate that the amount of time spent on the phone per day significantly increases the odds of experiencing a change to the fifth digit. We expect these potential skin changes as well as other musculoskeletal changes to increase in prevalence as daily smartphone use continues to increase. With the lack of studies investigating skin changes to the digits in relation to smartphone use, future studies are needed to verify our results and confirm the presence of this issue.
- Ko PH, Hwang YH, Liang HW. Influence of smartphone use styles on typing performance and biomechanical exposure. Ergonomics. 2015;59:821-828.
- Chang J, Choi B, Tjolleng A, et al. Effects of button position on a soft keyboard: muscle activity, touch time, and discomfort in two-thumb text entry. Appl Ergon. 2017;60:282-292.
- Park JH, Christman MP, Linos E, et al. Dermatology on Instagram: an analysis of hashtags. J Drugs Dermatol. 2018;17:482-484.
- Algar L, Valdes K. Using smartphone applications as hand therapy interventions. J Hand Ther. 2014;27:254-257.
- Megna, M, Gisonni P, Napolitano M, et al. The effect of smartphone addiction on hand joints in psoriatic patients: an ultrasound-based study. J Eur Acad Dermatol Venereol. 2017;32:73-78.
- Christensen MA, Bettencourt L, Kaye L, et al. Direct measurements of smartphone screen-time: relationships with demographics and sleep. PLoS One. 2016;11:E0165331.
- Lemola S, Perkinson-Gloor N, Brand S, et al. Adolescents’ electronic media use at night, sleep disturbance, and depressive symptoms in the smartphone age. J Youth Adolesc. 2014;44:405-418.
- Lee M, Hong Y, Lee S, et al. The effects of smartphone use on upper extremity muscle activity and pain threshold. J Phys Ther Sci. 2015;27:1743-1745.
- Inal EE, Demirci K, Çetintürk A, et al. Effects of smartphone overuse on hand function, pinch strength, and the median nerve. Muscle Nerve. 2015;52:183-188.
- Elston D, Ferringer T, Ko C, et al. Dermatopathology. 3rd ed. New York, NY: Elsevier Health Sciences; 2018.
Over the last decade, the use of mobile phones has changed drastically with the advent of more technologically advanced smartphones.1 Mobile phones are no longer used primarily as devices for talking but rather for text messaging, reading the news, drafting emails, browsing websites, and connecting with others on social media. Considering the increased utility and popularity of social media along with the greater reliance on smartphones, individuals in the United States and worldwide are undoubtedly spending more time on their handheld devices.2 With the increase in use and overuse of smartphones, many aspects of society and health are likely affected. Many celebrities who frequently post on social media platforms also have alluded to or directly discussed changes in their dermatologic health secondary to their increased use of smartphones.3 Numerous studies have investigated the positive and negative effects of smartphone use on various musculoskeletal conditions of the upper extremities4,5 and the social effects of smartphone use on behavior and child development.6,7 Lee et al8 studied the effects of smartphone use on upper extremity muscle pain and activity in relation to 1- or 2-handed operation. In this study, Lee et al8 measured the muscle activity and tenderness in 10 women aged 20 to 22 years after a series of timed periods of smartphone use. They concluded that smartphone use resulted in greater muscle activity and tenderness, especially in 1-handed use compared to 2-handed use.8 Inal et al9 investigated smartphone overuse effects on hand strength and function in 102 college students and discovered that smartphone overuse was correlated with decreased pinch strength, increased median nerve cross-sectional area, and pain in the first digits.9
However, few articles have been published investigating skin changes to the digits in relation to smartphone use (Figure 1). In a PubMed search of articles indexed for MEDLINE using the terms smartphone, phone, cell phone, electronic device, handheld device, fifth digit, or skin changes, the authors were unable to find any studies in the literature that involved smartphone use and skin changes to the digits. Based on informal clinical observation and personal experiences, we hypothesized that changes to the fifth digit, likely due to holding a smartphone, would be prevalent and would correlate with amount of time spent on smartphones per day (Figure 2). We also were interested in investigating any other potential correlations with changes to the fifth digit, such as type of smartphone used.
Methods
The study used a cross-sectional design. From September 2018 to December 2018, 374 individuals 18 years or older were recruited to complete a 5-minute anonymous survey online. Using email referrals and social media, participants were presented with a link to a Google survey that only allowed 1 submission per account. On the first page of the survey, participants were presented with a letter explaining that completion of the survey was entirely voluntary, participants were free to withdraw from the study at any time, and participants were providing consent in completing the survey. The protocol was determined to be exempt by the institutional review board at Nova Southeastern University (Fort Lauderdale, Florida) in September 2018.
Survey Design
A 20-item survey was designed to measure the amount of time spent using smartphones per day, classify the type of phone used, and quantify skin changes noticed by each respondent. Demographic information for each respondent also was gathered using the survey. The survey was pilot tested to ensure that respondents were able to understand the items.
One item asked if respondents owned a handheld smartphone. Two items assessed how much time was spent on smartphones per day (ie, <1 hour, 1–2 hours, 2–3 hours, 3–4 hours, 4–5 hours, >5 hours) and the type of smartphone used (ie, Apple iPhone, Samsung Galaxy, Google Pixel, Huawei, LG, other). Six items assessed skin changes to the digits, namely the fifth digit (eg, Do you notice any changes to your fifth digit [pinky finger] that would likely be contributed to how you hold your smartphone, such as divot, callus, bruise, wound, misalignment, bend?). Eleven items were used to collect basic demographic information, including age, sex, legal marital status, ethnicity, race, annual household income, highest-earned educational degree, current employment status, health insurance status, and state of residence.
Statistical Analysis
All data were analyzed using IBM SPSS Statistics 23. The association between changes to the fifth digit and time spent on the phone, hand dominance, and socioeconomic factors (ie, age,
Results
The mean age of the 374 respondents was 33.8 years (range, 18–72 years). One hundred nine respondents were men (29.1%), 262 were women (70.1%), and 3 did not specify (0.8%). Two hundred thirty-four respondents (62.6%) were single, 271 (72.5%) were white, 171 (45.7%) had a bachelor’s degree, and174 (46.5%) were employed full time. Annual household income was normally distributed among the respondents, with 28 (7.5%) earning less than $10,000 per year, 130 (34.8%) earning $10,000 to$49,999 per year, 136 (36.4%) earning $50,000 to $99,999 per year, 52 (13.9%) earning $100,000 to$149,999 per year, and 28 (7.5%) earning more than $150,000 per year. The demographic characteristics of the respondents are presented in Table 1.
Eighty-five (22.7%) respondents admitted to changes to the fifth digit that they associated with holding a smartphone, whereas 289 (77.3%) reported no changes. When asked about the average amount of time spent on their smartphone per day, 17 (4.5%) respondents answered less than 1 hour, 70 (18.7%) answered 1 to 2 hours, 69 (18.4%) answered 2 to 3 hours, 77 (20.6%) answered 3 to 4 hours, 57 (15.2%) answered 4 to 5 hours, and 84 (22.5%) answered more than 5 hours. One hundred ninety-nine (53.2%) respondents indicated they used an Apple iPhone, 95 (25.4%) used a Samsung Galaxy phone, 9 (2.4%) used a Google Pixel phone, 3 (0.8%) used a Huawei phone, 23 (6.1%) used an LG phone, and 45 (12.0%) used another type of smartphone. The characteristics of smartphone use as reported by the respondents are presented in Table 2.
Comment
Consistent with our hypothesis, changes to the fifth digit were prevalent in the surveyed population, with 85 (22.7%) respondents admitting to changes to their fifth digit from holding a smartphone. The changes to the fifth digit were described as 1 or more of the following: divot (impression), callus (skin thickening), bruise, wound, misalignment, or bending. Most respondents who noted skin changes on the survey endorsed changes consistent with calluses and/or divots. These changes can be described as scaly, lichenified, well-demarcated papules or plaques with variable overlying hyperpigmentation and surrounding erythema. In cases with resulting chronic indentations of the skin, one also would observe localized sclerosis, atrophy, and/or induration of the area, which we found to be less prevalent than expected considering the popularity and notable reliance on smartphones.2
The most commonly reported chronic skin changes to the fifth digit are similar to those of lichen simplex chronicus and/or exogenous lobular panniculitis, which can be both symptomatically and cosmetically troubling for a patient. Functional impairment in movement of the fifth digit may result from the overlying lichenification and induration, as well as from lipoatrophy of the underlying traumatized subcutaneous fat, especially if the affected area is overlying the proximal interphalangeal joint of the fifth digit. These resulting alterations in the skin of the fifth digit also may be cosmetically displeasing to the patient.
On histology, we would expect similar changes to that of lichen simplex chronicus—compact hyperkeratosis and hypergranulosis—and/or an exogenous lobular panniculitis. Lobular panniculitis demonstrates necrosis of the fat lobule; vacuolated spaces; and lipomembranous changes such as fatty cystic degeneration with feathery eosinophilic material in an arabesque pattern, which has been described as frost on a windowpane, or a ferning pattern at the edge of the lipid vacuole.10
We also were correct in our hypothesis that prevalence of changes to the fifth digit correlate with amount of time spent on smartphones per day. Bivariate and multivariate logistic regression analysis showed that a change to the fifth digit was not significantly associated with hand dominance or socioeconomic factors (ie, age, sex, legal marital status, ethnicity, race, annual household income, highest-earned educational degree, current employment status, health insurance status, and state of residence). Controlling for all other factors, the only factor that significantly increased the odds of experiencing a change to the fifth digit was the amount of time spent on the phone per day. The respondents who spent more than 5 hours per day on their phones had 5-times greater odds of experiencing a change to their fifth digit compared with respondents who spent less than 1 hour per day on their phones (P=.045).
Although no other correlations with changes to the fifth digit, such as type of smartphone used, were found in our study, future studies should continue to investigate other potential factors that play a role in smartphone use changing the appearance and function of the digits. Our lack of significant correlations with changes to the fifth digit could be attributed to a small sample size and other possible factors, such as the frequent design changes of smartphones by manufacturers. Our study also is limited by the possibility of other factors contributing to these observed skin changes. Although we have anecdotally observed these skin changes and have hypothesized that smartphones are the culprit, other causes, such as holding certain tools, could lead to these skin changes. In addition, there are many different ways to hold a smartphone, and certain hand positionings may be more or less prone to skin changes described in our study. Various accessories, such as cases and gripping devices, also may change the way smartphones are held and would skew the results of our survey. Future studies could examine different ways smartphones are held, how various accessories affect these skin changes, and the size or model of phones that make these skin changes more or less prevalent.
Conclusion
Our study is an initial step in uncovering a possible phenomenon of smartphone use affecting the digits, namely the fifth digit. Our findings demonstrate that the amount of time spent on the phone per day significantly increases the odds of experiencing a change to the fifth digit. We expect these potential skin changes as well as other musculoskeletal changes to increase in prevalence as daily smartphone use continues to increase. With the lack of studies investigating skin changes to the digits in relation to smartphone use, future studies are needed to verify our results and confirm the presence of this issue.
Over the last decade, the use of mobile phones has changed drastically with the advent of more technologically advanced smartphones.1 Mobile phones are no longer used primarily as devices for talking but rather for text messaging, reading the news, drafting emails, browsing websites, and connecting with others on social media. Considering the increased utility and popularity of social media along with the greater reliance on smartphones, individuals in the United States and worldwide are undoubtedly spending more time on their handheld devices.2 With the increase in use and overuse of smartphones, many aspects of society and health are likely affected. Many celebrities who frequently post on social media platforms also have alluded to or directly discussed changes in their dermatologic health secondary to their increased use of smartphones.3 Numerous studies have investigated the positive and negative effects of smartphone use on various musculoskeletal conditions of the upper extremities4,5 and the social effects of smartphone use on behavior and child development.6,7 Lee et al8 studied the effects of smartphone use on upper extremity muscle pain and activity in relation to 1- or 2-handed operation. In this study, Lee et al8 measured the muscle activity and tenderness in 10 women aged 20 to 22 years after a series of timed periods of smartphone use. They concluded that smartphone use resulted in greater muscle activity and tenderness, especially in 1-handed use compared to 2-handed use.8 Inal et al9 investigated smartphone overuse effects on hand strength and function in 102 college students and discovered that smartphone overuse was correlated with decreased pinch strength, increased median nerve cross-sectional area, and pain in the first digits.9
However, few articles have been published investigating skin changes to the digits in relation to smartphone use (Figure 1). In a PubMed search of articles indexed for MEDLINE using the terms smartphone, phone, cell phone, electronic device, handheld device, fifth digit, or skin changes, the authors were unable to find any studies in the literature that involved smartphone use and skin changes to the digits. Based on informal clinical observation and personal experiences, we hypothesized that changes to the fifth digit, likely due to holding a smartphone, would be prevalent and would correlate with amount of time spent on smartphones per day (Figure 2). We also were interested in investigating any other potential correlations with changes to the fifth digit, such as type of smartphone used.
Methods
The study used a cross-sectional design. From September 2018 to December 2018, 374 individuals 18 years or older were recruited to complete a 5-minute anonymous survey online. Using email referrals and social media, participants were presented with a link to a Google survey that only allowed 1 submission per account. On the first page of the survey, participants were presented with a letter explaining that completion of the survey was entirely voluntary, participants were free to withdraw from the study at any time, and participants were providing consent in completing the survey. The protocol was determined to be exempt by the institutional review board at Nova Southeastern University (Fort Lauderdale, Florida) in September 2018.
Survey Design
A 20-item survey was designed to measure the amount of time spent using smartphones per day, classify the type of phone used, and quantify skin changes noticed by each respondent. Demographic information for each respondent also was gathered using the survey. The survey was pilot tested to ensure that respondents were able to understand the items.
One item asked if respondents owned a handheld smartphone. Two items assessed how much time was spent on smartphones per day (ie, <1 hour, 1–2 hours, 2–3 hours, 3–4 hours, 4–5 hours, >5 hours) and the type of smartphone used (ie, Apple iPhone, Samsung Galaxy, Google Pixel, Huawei, LG, other). Six items assessed skin changes to the digits, namely the fifth digit (eg, Do you notice any changes to your fifth digit [pinky finger] that would likely be contributed to how you hold your smartphone, such as divot, callus, bruise, wound, misalignment, bend?). Eleven items were used to collect basic demographic information, including age, sex, legal marital status, ethnicity, race, annual household income, highest-earned educational degree, current employment status, health insurance status, and state of residence.
Statistical Analysis
All data were analyzed using IBM SPSS Statistics 23. The association between changes to the fifth digit and time spent on the phone, hand dominance, and socioeconomic factors (ie, age,
Results
The mean age of the 374 respondents was 33.8 years (range, 18–72 years). One hundred nine respondents were men (29.1%), 262 were women (70.1%), and 3 did not specify (0.8%). Two hundred thirty-four respondents (62.6%) were single, 271 (72.5%) were white, 171 (45.7%) had a bachelor’s degree, and174 (46.5%) were employed full time. Annual household income was normally distributed among the respondents, with 28 (7.5%) earning less than $10,000 per year, 130 (34.8%) earning $10,000 to$49,999 per year, 136 (36.4%) earning $50,000 to $99,999 per year, 52 (13.9%) earning $100,000 to$149,999 per year, and 28 (7.5%) earning more than $150,000 per year. The demographic characteristics of the respondents are presented in Table 1.
Eighty-five (22.7%) respondents admitted to changes to the fifth digit that they associated with holding a smartphone, whereas 289 (77.3%) reported no changes. When asked about the average amount of time spent on their smartphone per day, 17 (4.5%) respondents answered less than 1 hour, 70 (18.7%) answered 1 to 2 hours, 69 (18.4%) answered 2 to 3 hours, 77 (20.6%) answered 3 to 4 hours, 57 (15.2%) answered 4 to 5 hours, and 84 (22.5%) answered more than 5 hours. One hundred ninety-nine (53.2%) respondents indicated they used an Apple iPhone, 95 (25.4%) used a Samsung Galaxy phone, 9 (2.4%) used a Google Pixel phone, 3 (0.8%) used a Huawei phone, 23 (6.1%) used an LG phone, and 45 (12.0%) used another type of smartphone. The characteristics of smartphone use as reported by the respondents are presented in Table 2.
Comment
Consistent with our hypothesis, changes to the fifth digit were prevalent in the surveyed population, with 85 (22.7%) respondents admitting to changes to their fifth digit from holding a smartphone. The changes to the fifth digit were described as 1 or more of the following: divot (impression), callus (skin thickening), bruise, wound, misalignment, or bending. Most respondents who noted skin changes on the survey endorsed changes consistent with calluses and/or divots. These changes can be described as scaly, lichenified, well-demarcated papules or plaques with variable overlying hyperpigmentation and surrounding erythema. In cases with resulting chronic indentations of the skin, one also would observe localized sclerosis, atrophy, and/or induration of the area, which we found to be less prevalent than expected considering the popularity and notable reliance on smartphones.2
The most commonly reported chronic skin changes to the fifth digit are similar to those of lichen simplex chronicus and/or exogenous lobular panniculitis, which can be both symptomatically and cosmetically troubling for a patient. Functional impairment in movement of the fifth digit may result from the overlying lichenification and induration, as well as from lipoatrophy of the underlying traumatized subcutaneous fat, especially if the affected area is overlying the proximal interphalangeal joint of the fifth digit. These resulting alterations in the skin of the fifth digit also may be cosmetically displeasing to the patient.
On histology, we would expect similar changes to that of lichen simplex chronicus—compact hyperkeratosis and hypergranulosis—and/or an exogenous lobular panniculitis. Lobular panniculitis demonstrates necrosis of the fat lobule; vacuolated spaces; and lipomembranous changes such as fatty cystic degeneration with feathery eosinophilic material in an arabesque pattern, which has been described as frost on a windowpane, or a ferning pattern at the edge of the lipid vacuole.10
We also were correct in our hypothesis that prevalence of changes to the fifth digit correlate with amount of time spent on smartphones per day. Bivariate and multivariate logistic regression analysis showed that a change to the fifth digit was not significantly associated with hand dominance or socioeconomic factors (ie, age, sex, legal marital status, ethnicity, race, annual household income, highest-earned educational degree, current employment status, health insurance status, and state of residence). Controlling for all other factors, the only factor that significantly increased the odds of experiencing a change to the fifth digit was the amount of time spent on the phone per day. The respondents who spent more than 5 hours per day on their phones had 5-times greater odds of experiencing a change to their fifth digit compared with respondents who spent less than 1 hour per day on their phones (P=.045).
Although no other correlations with changes to the fifth digit, such as type of smartphone used, were found in our study, future studies should continue to investigate other potential factors that play a role in smartphone use changing the appearance and function of the digits. Our lack of significant correlations with changes to the fifth digit could be attributed to a small sample size and other possible factors, such as the frequent design changes of smartphones by manufacturers. Our study also is limited by the possibility of other factors contributing to these observed skin changes. Although we have anecdotally observed these skin changes and have hypothesized that smartphones are the culprit, other causes, such as holding certain tools, could lead to these skin changes. In addition, there are many different ways to hold a smartphone, and certain hand positionings may be more or less prone to skin changes described in our study. Various accessories, such as cases and gripping devices, also may change the way smartphones are held and would skew the results of our survey. Future studies could examine different ways smartphones are held, how various accessories affect these skin changes, and the size or model of phones that make these skin changes more or less prevalent.
Conclusion
Our study is an initial step in uncovering a possible phenomenon of smartphone use affecting the digits, namely the fifth digit. Our findings demonstrate that the amount of time spent on the phone per day significantly increases the odds of experiencing a change to the fifth digit. We expect these potential skin changes as well as other musculoskeletal changes to increase in prevalence as daily smartphone use continues to increase. With the lack of studies investigating skin changes to the digits in relation to smartphone use, future studies are needed to verify our results and confirm the presence of this issue.
- Ko PH, Hwang YH, Liang HW. Influence of smartphone use styles on typing performance and biomechanical exposure. Ergonomics. 2015;59:821-828.
- Chang J, Choi B, Tjolleng A, et al. Effects of button position on a soft keyboard: muscle activity, touch time, and discomfort in two-thumb text entry. Appl Ergon. 2017;60:282-292.
- Park JH, Christman MP, Linos E, et al. Dermatology on Instagram: an analysis of hashtags. J Drugs Dermatol. 2018;17:482-484.
- Algar L, Valdes K. Using smartphone applications as hand therapy interventions. J Hand Ther. 2014;27:254-257.
- Megna, M, Gisonni P, Napolitano M, et al. The effect of smartphone addiction on hand joints in psoriatic patients: an ultrasound-based study. J Eur Acad Dermatol Venereol. 2017;32:73-78.
- Christensen MA, Bettencourt L, Kaye L, et al. Direct measurements of smartphone screen-time: relationships with demographics and sleep. PLoS One. 2016;11:E0165331.
- Lemola S, Perkinson-Gloor N, Brand S, et al. Adolescents’ electronic media use at night, sleep disturbance, and depressive symptoms in the smartphone age. J Youth Adolesc. 2014;44:405-418.
- Lee M, Hong Y, Lee S, et al. The effects of smartphone use on upper extremity muscle activity and pain threshold. J Phys Ther Sci. 2015;27:1743-1745.
- Inal EE, Demirci K, Çetintürk A, et al. Effects of smartphone overuse on hand function, pinch strength, and the median nerve. Muscle Nerve. 2015;52:183-188.
- Elston D, Ferringer T, Ko C, et al. Dermatopathology. 3rd ed. New York, NY: Elsevier Health Sciences; 2018.
- Ko PH, Hwang YH, Liang HW. Influence of smartphone use styles on typing performance and biomechanical exposure. Ergonomics. 2015;59:821-828.
- Chang J, Choi B, Tjolleng A, et al. Effects of button position on a soft keyboard: muscle activity, touch time, and discomfort in two-thumb text entry. Appl Ergon. 2017;60:282-292.
- Park JH, Christman MP, Linos E, et al. Dermatology on Instagram: an analysis of hashtags. J Drugs Dermatol. 2018;17:482-484.
- Algar L, Valdes K. Using smartphone applications as hand therapy interventions. J Hand Ther. 2014;27:254-257.
- Megna, M, Gisonni P, Napolitano M, et al. The effect of smartphone addiction on hand joints in psoriatic patients: an ultrasound-based study. J Eur Acad Dermatol Venereol. 2017;32:73-78.
- Christensen MA, Bettencourt L, Kaye L, et al. Direct measurements of smartphone screen-time: relationships with demographics and sleep. PLoS One. 2016;11:E0165331.
- Lemola S, Perkinson-Gloor N, Brand S, et al. Adolescents’ electronic media use at night, sleep disturbance, and depressive symptoms in the smartphone age. J Youth Adolesc. 2014;44:405-418.
- Lee M, Hong Y, Lee S, et al. The effects of smartphone use on upper extremity muscle activity and pain threshold. J Phys Ther Sci. 2015;27:1743-1745.
- Inal EE, Demirci K, Çetintürk A, et al. Effects of smartphone overuse on hand function, pinch strength, and the median nerve. Muscle Nerve. 2015;52:183-188.
- Elston D, Ferringer T, Ko C, et al. Dermatopathology. 3rd ed. New York, NY: Elsevier Health Sciences; 2018.
Practice Points
- The amount of time spent on a smartphone was found to directly correlate with skin changes to the fifth digit.
- Skin changes to the fifth digit were mostly reported to be divots (impressions) or calluses.
Factors Associated With Lower-Extremity Amputation in Patients With Diabetic Foot Ulcers
From Northwell Health System, Lake Success, NY.
Abstract
- Objective: To explore factors associated with lower-extremity amputation (LEA) in patients with diabetic foot ulcers using data from the Online Wound Electronic Medical Record Database.
- Design: Retrospective analysis of medical records.
- Setting and participants: Data from 169 individuals with previously diagnosed diabetes mellitus who received wound care for a 6-month period within a span of 2 years was analyzed. A baseline evaluation was obtained and wound(s) were treated, managed, and monitored.
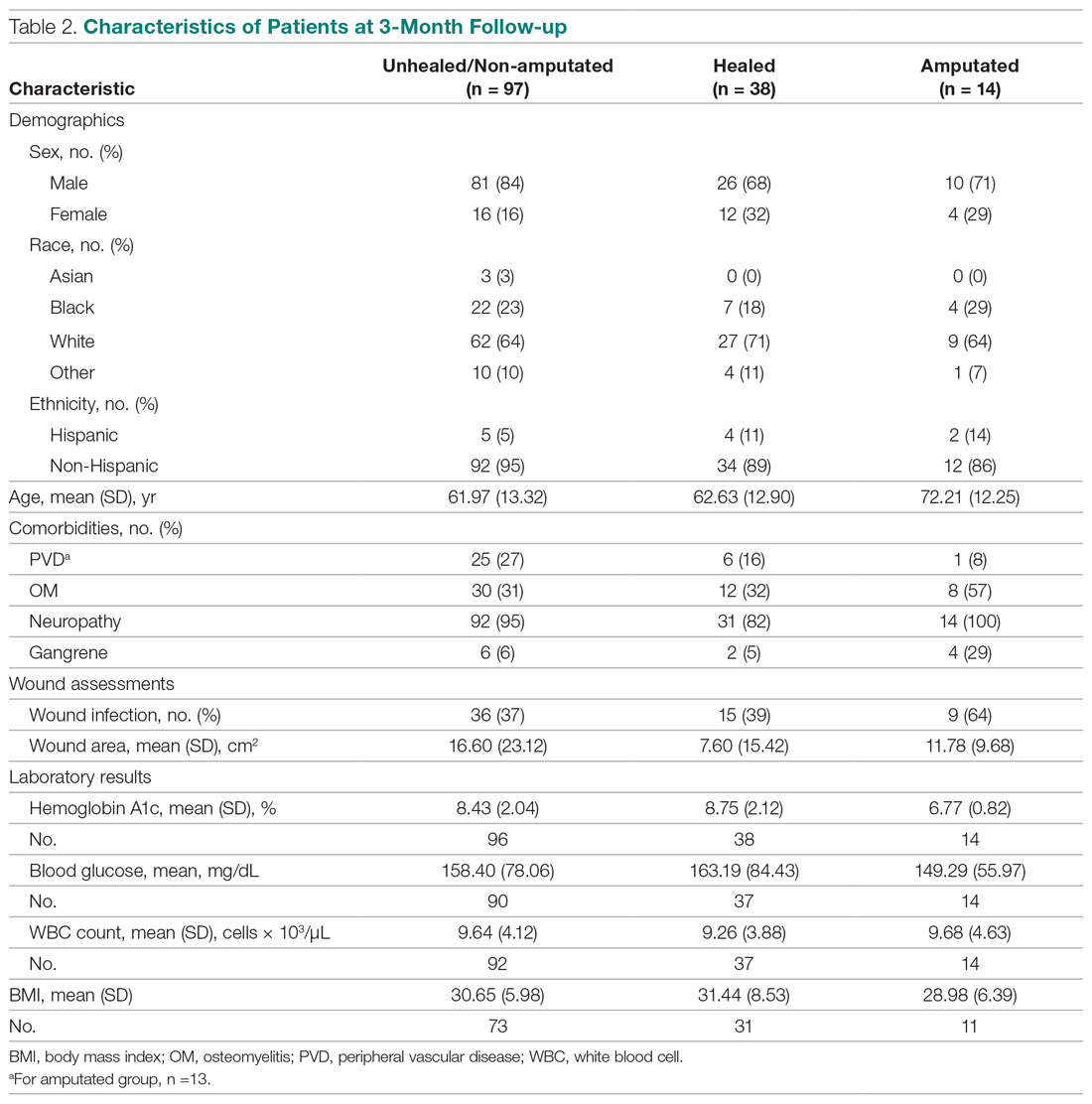
Treatment continued until the patient healed, required an LEA, or phased out of the study, neither healing nor undergoing an amputation. Of the 149 patients who completed the study, 38 had healed ulcers, 14 underwent amputation, and 97 neither healed nor underwent an amputation. All patients were treated under the care of vascular and/or podiatric surgeons. - Measurements: Variables included wound status (healed, amputated, and unhealed/non-amputated); size of wound area; age, gender, race, and ethnicity; white blood cell (WBC) count, hemoglobin A1c (HbA1c), blood glucose, and body mass index (BMI); and presence of osteomyelitis, gangrene, and peripheral vascular disease.
- Results: As compared to the healed and unhealed/non-amputated group, the group of patients who underwent LEA was older and had higher percentages of males, Hispanics, and African Americans; had a higher WBC count, larger wound area, and higher rates of wound infection, osteomyelitis, and neuropathy; and had lower average values of HbA1c, blood glucose, and BMI and a lower rate of peripheral vascular disease.
- Conclusion: The association between HbA1c and LEA highlights a window of relative safety among an at-risk population. By identifying and focusing on factors associated with LEA, health care professionals may be able to decrease the prevalence of LEA in patients with diabetes.
Keywords: diabetic foot ulcer; lower-extremity amputation; risk factors; HbA1c.
An estimated 30.3 million people, or 9.4% of the US population, has diabetes. In 2014, approximately 108,000 amputations were performed on adults with diagnosed diabetes.1 Furthermore, patients with diabetes have a 10-fold increased risk for lower-extremity amputation (LEA), as compared with patients without diabetes.2 The frequency of amputations in the diabetic population is a public health crisis.
Amputation has significant, life-altering consequences. Patients who undergo LEA often face debilitation in their daily activities and must undergo intense rehabilitation to learn basic tasks. Amputations can also impact individuals’ psychological well-being as they come to terms with their altered body and may face challenges in self-perception, confidence, self-esteem, work life, and relationships. In addition, the mortality rate for patients with diabetes 5 years after undergoing LEA is 30%.2 However, public health studies estimate that more than half of LEAs in patients with diabetes are preventable.3
Although studies have explored the relationship between diabetes and LEA, few have sought to identify factors directly correlated with wound care. In the United States, patients with diabetic ulcerations are typically treated in wound care facilities; however, previous studies have concentrated on the conditions that lead to the formation of an ulcer or amputation, viewing amputation and ulcer as 2 separate entities. Our study took into account systemic variables, patient demographics, and specific wound characteristics to explore factors associated with LEA in a high-risk group of patients with diabetes. This study was designed to assess ailments that are prevalent in patients who require a LEA.
Methods
Patients and Setting
A total of 169 patients who were treated at the Comprehensive Wound Healing and Hyperbaric Center (Lake Success, NY), a tertiary facility of the Northwell Health system, participated in this retrospective study. The data for this study were obtained in conjunction with the development of the New York University School of Medicine’s Online Wound Electronic Medical Record to Decrease Limb Amputations in Persons with Diabetes (OWEMR) database. The OWEMR collects individual patient data from satellite locations across the country. Using this database, researchers can analyze similarities and differences between patients who undergo LEA.
This study utilized patient data specific to the Northwell Health facility. All of the patients in our study were enrolled under the criteria of the OWEMR database. In order to be included in the OWEMR database, patients had to be diagnosed with type 1 or type 2 diabetes; have a break in the skin ≥ 0.5 cm2; be 18 years of age or older; and have a measured hemoglobin A1c (HbA1c) value within the past 120 days. Study patients signed an informed consent and committed to being available for follow-up visits to the wound care facility for 6 months after entering the study. Patients were enrolled between 2012 and 2014, and each patient was monitored for a period of 6 months within this time period. Participants were treated with current standards of care using diet, lifestyle, and pharmacologic interventions. This study was approved by the Northwell Health System Institutional Review Board Human Research Protection Program (Manhasset, NY).
Data Collection
On their first visit to the facility, patients were given a physical examination and initial interview regarding their medical history. Clinicians were required to select 1 ulcer that would be examined for the duration of the study. The selection of the ulcer was based on a point system that awarded points for pedal pulses, the ability to be probed to the bone, the location of the ulcer (ie, located on the foot rather than a toe), and the presence of multiple ulcerations. The ulcer with the highest score was selected for the study. If numerous ulcers were evaluated with the same score, the largest and deepest was selected. Wagner classification of the wound was recorded at baseline and taken at each subsequent patient visit. In addition, peripheral sensation was assessed for signs of neuropathy using Semmes-Weinstein monofilament testing.
Once selected, the wound was clinically evaluated, samples for culture were obtained, and blood tests were performed to detect the presence of wound infection. The patient’s blood was drawn for a full laboratory analysis, including white blood cell (WBC) count and measurement of blood glucose and HbA1c levels. Bone biopsy, magnetic resonance imaging, and bone scans were used to detect the presence of osteomyelitis at the discretion of the health care provider. Wounds suspected of infection, underlying osteomyelitis, or gangrene at baseline were excluded. Patients would then return for follow-up visits at least once every 6 weeks, plus or minus 2 weeks, for a maximum of 6 months.
Statistical Analysis
Utilizing SAS version 9.3 (Cary, NC), descriptive statistics (minimum, maximum, mean, median, and SD) were calculated for the following variables: age, WBC count, wound area, HbA1c, blood glucose, and body mass index (BMI). These variables were collected for each patient as per the OWEMR protocol and provided a basis for which to compare patients who underwent amputation and those who did not. Twenty patients were lost to follow-up, and therefore we altered the window of our statistics from 6 months to 3 months to provide the most accurate data, as 6-month follow-up data were limited. The patients were classified into the following categories: healed, amputated, and unhealed/non-amputated. Descriptive statistics were calculated for these 3 groups, analyzing the same variables (age, WBC count, wound area, HbA1c, blood glucose, and BMI). Additional statistical computations were utilized in order to show the prevalence and frequency of our categorical variables: gender, race, ethnicity, osteomyelitis, gangrene, and peripheral vascular disease. The baseline values of WBC count, HbA1c, wound area, and BMI of the 3 groups were analyzed with descriptive statistics for comparison. A multinomial logistic regression was then performed using a 3-level outcome variable: healed, amputated, or unhealed/non-amputated. Each predictor variable was analyzed independently due to the small sample size.
Results
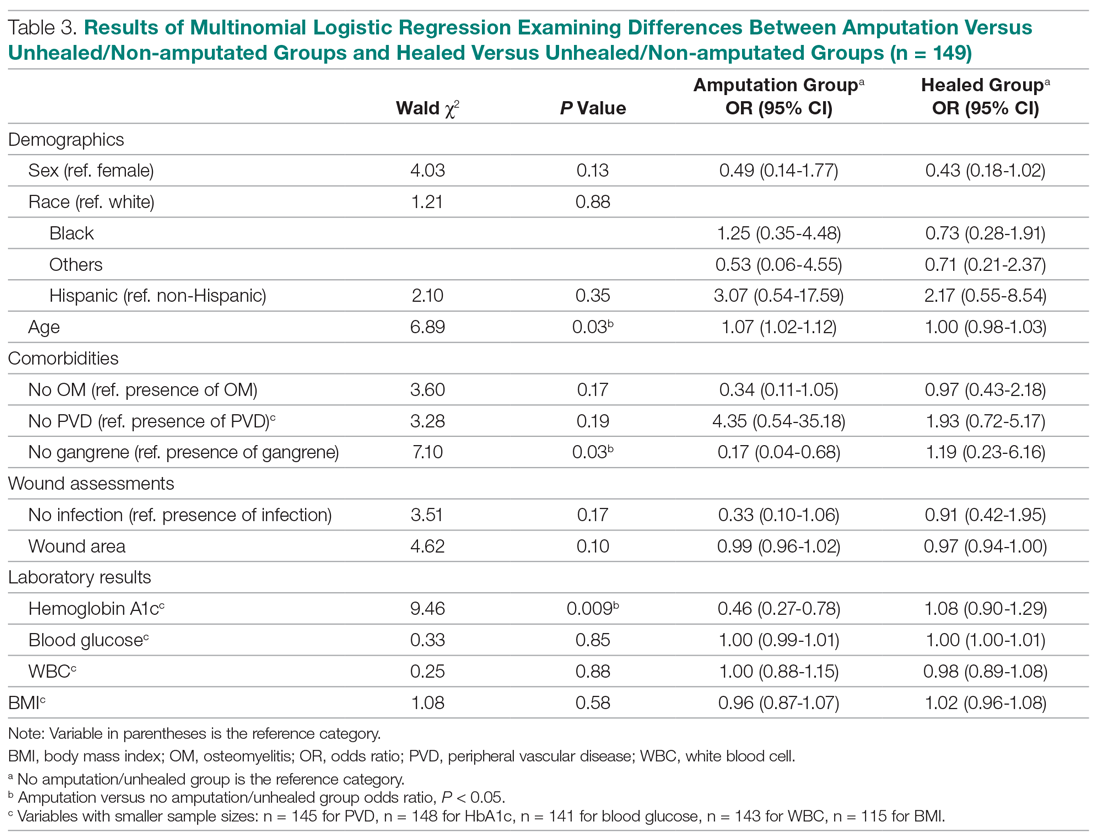
Of the 169 registered patients treated at the Northwell Health facility, all qualified for the OWEMR study and met the study criteria. In the original 169 patients, there were 19 amputations: 6 toe, 6 trans-metatarsal, 6 below knee, and 1 above knee (Table 1).
The descriptive statistics of 149 patients grouped into 3 categories (healed, amputated, unhealed/non-amputated) are shown in Table 2.
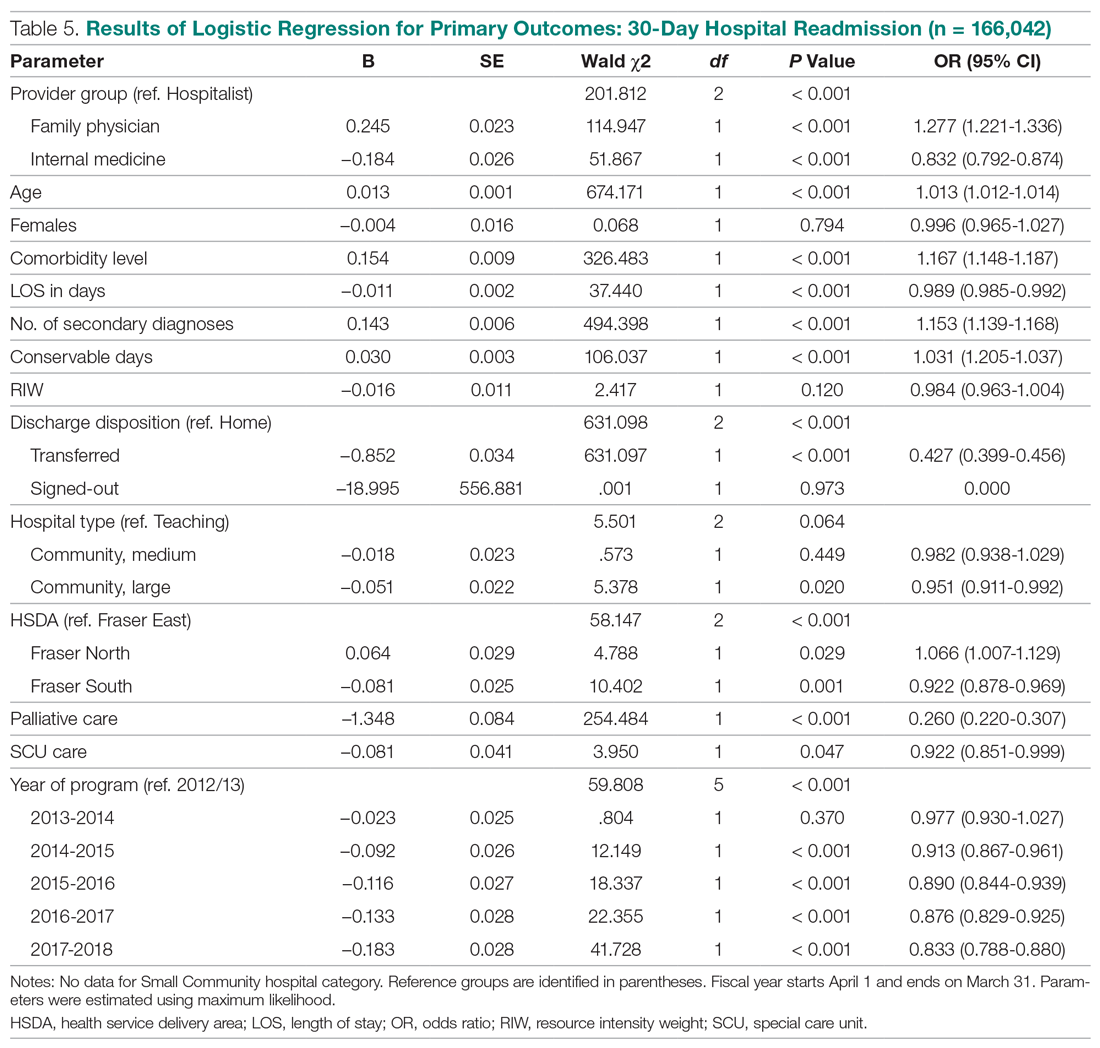
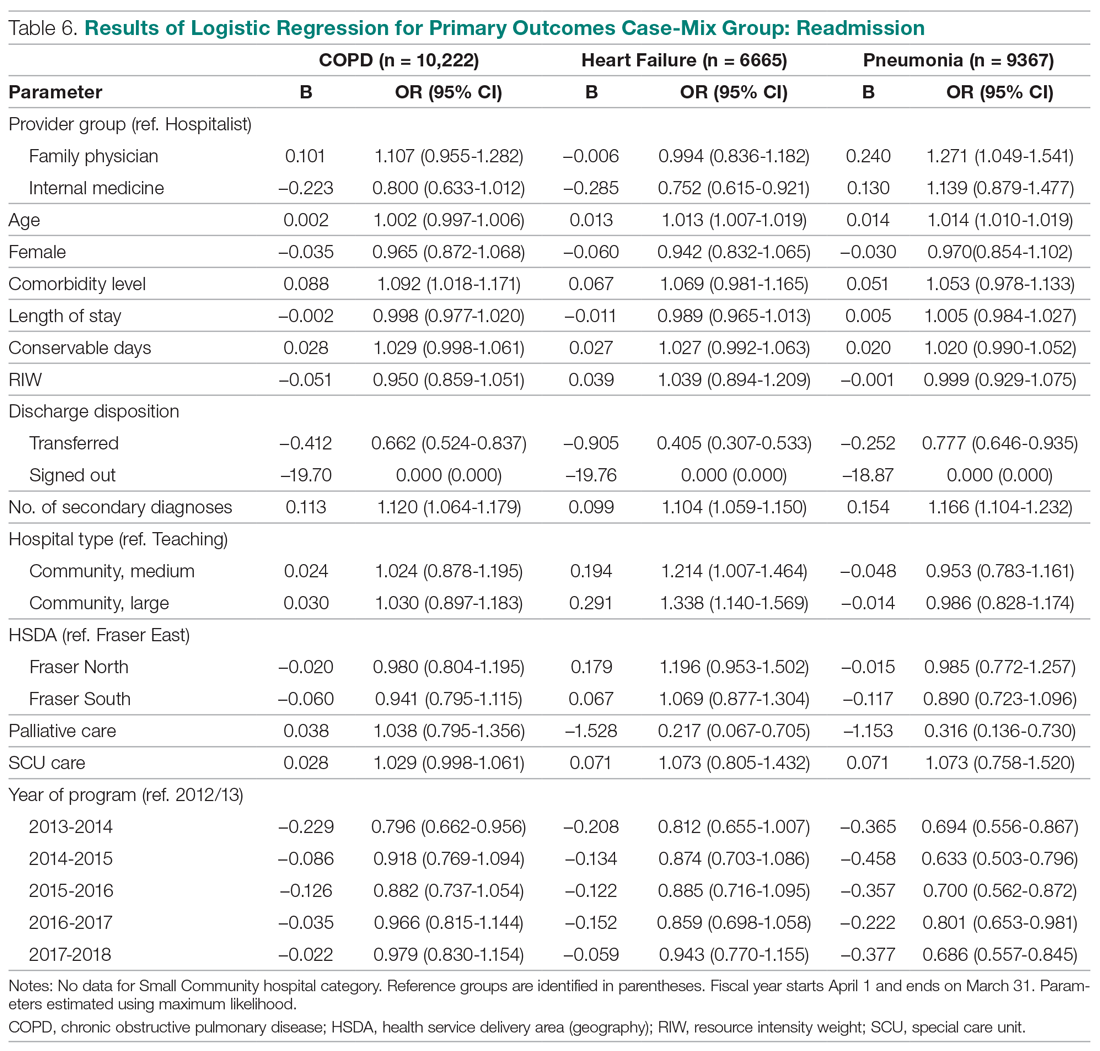
The results of the logistic regression exploring the differences between the amputation and healed groups and the unhealed/non-amputated group are shown in Table 3. The amputation group had a higher mean age and WBC count and greater wound area. Increased age was determined to be a significant predictor of the odds of amputation (P = 0.0089). For each year increase in age, the odds of amputation increased by 6.5% (odds ratio, 1.07 [95% confidence interval {CI}, 1.02-1.12]). Patients in the amputation group were more likely to be male, Hispanic, and African American and to have wound infections and comorbidities (osteomyelitis, neuropathy, and gangrene).
The presence of gangrene was significantly associated with LEA (P = 0.03). Specifically, the odds of patients without gangrene undergoing a LEA were substantially lower compared with their counterparts with gangrene (odds ratio, 0.17; 95% CI, 0.04-0.68; P = 0.0131). However, the presence of gangrene was not associated with the odds of healing compared with the odds of neither healing nor undergoing amputation (P = 0.84; not shown in Table 3).
The amputation group had lower mean values for HbA1c, BMI, and blood glucose levels and a lower rate of peripheral vascular disease. Only the relationship between lower HbA1c and increased odds of amputation versus not healing/non-amputation was found to be statistically significant (95% CI, 0.27-0.78; P = 0.009).
Discussion
This retrospective study was undertaken to evaluate factors associated with LEA in patients with diabetic foot ulcers. Patients with diabetes being treated at a wound care facility often require continuous surgical and metabolic intervention to promote optimal healing: drainage, surgical debridement, irrigation, culturing for infection, and monitoring of blood glucose levels. This treatment requires strict compliance with medical directions and, oftentimes, additional care, such as home-care nursing visits, to maintain a curative environment for the wound. Frequently, wounds on the lower extremity further complicate the healing process by reducing the patient’s mobility and daily life. Due to these factors, many patients progress to LEA. The link between diabetic ulcers and amputation has already been well described in previous studies, with studies showing that history of diabetic foot ulcer significantly predisposes an individual to LEA.4 However, few studies have further investigated demographic factors associated with risk for an amputation. Our study analyzed several categories of patient data taken from a baseline visit. We found that those with highly elevated HbA1c values were less likely to have an amputation than persons with relatively lower levels, a finding that is contrary to previous studies.
Our study’s findings suggest a higher risk for LEA with increased age. The amputation group was, on average, 7 years older than the other 2 groups. A recent study showed that risk for amputation is directly correlated to patient age, as is the mortality rate after undergoing LEA (2.3%; P < 0.05).5 Our study found that with each increase in age of 1 year, the odds of amputation increased by 6.5%. However, recent evidence on LEA risk and aging suggests that age is of less consequence than the duration of diabetes. One study found that the propensity to develop diabetic foot ulcers increases with the duration of diabetes.6 The same study found that prevalence of ulceration was correlated with age, but the relationship between age and LEA was less significant. A follow-up study for LEA could be done to examine the role of disease duration versus age in LEA.
A consensus among previous studies is that men have a higher risk for LEA.5,7 Men comprised the majority in all 3 groups in our study. In addition, the amputation group in our study had the lowest BMI. Higher BMI generally is associated with an increased risk for health complications. However, a past study conducted in Taiwan reported that obese patients with diabetes were less likely to undergo LEA than those within the normal range for BMI.8 Neither study suggests that obesity is a deterrent for LEA, but both studies may suggest that risk of amputation may approach a maximum frequency at a specific BMI range, and then decrease. This unconfirmed “cyclic” relationship should be evaluated further in a larger sample size.
Most patients in our analysis were Caucasian, followed by African American and South Asian. African Americans were the only racial group with an increased frequency in the amputation group. This finding is supported by a previous study that found that the rate of LEA among patients with diabetes in low-income, predominantly African-American neighborhoods was nearly double that in wealthier, predominantly Caucasian areas.9 A potential problem in the comparison between our data with previous studies is that the studies did not analyze patients with our inclusion criteria. All patients with diabetes in previous investigations were grouped by race, but were not necessarily required to have 1 or more ulcers. Multiple ulcers may predispose an individual to a greater risk for amputation.
Multinomial logistic regression did not suggest an association between initial size of a patient’s wound and the risk of amputation. However, the descriptive data suggests a trend. Patients who did not heal or require an amputation had the largest average wound area. This finding is not surprising in that our study followed individuals for only 3 months. Many wounds require a long course of treatment, especially in patients with diabetes, who may have poor vascularization. However, in comparison to the healed patients, the patients who required an amputation had a larger average wound area. A larger wound requires a plentiful vascular supply for the delivery of clotting factors and nutrients to the damaged area. As wound size increases, an individual’s body must transmit an increased quantity of these factors and nutrients for the regeneration of tissue. In addition, wounds that possess a larger surface area require more debridement and present a greater opportunity for infection. This may also foreshadow a longer, more costly course of treatment. Additionally, individuals coping with large ulcerations are burdened by more elaborate and complex wound dressings.
Elevated levels of HbA1c are associated with increased adverse effects of diabetes, including end-stage renal disease, neuropathy, and infection.10 In a previous study, the risk for amputation was 1.2 times higher in patients with elevated HbA1c.11 In contrast, our study suggested the odds of LEA versus not healing/not undergoing amputation decreased as HbA1c increased. As a patient’s HbA1c level increased by a value of 1, their odds for LEA decreased by 54.3%. This finding contradicts prior studies that have found a positive association between HbA1c and LEA risk, including a study where each percentage increase in HbA1c correlated with a 13% to 15% increased risk of LEA.12 The finding that patients who underwent amputation in our study had lower levels of HbA1c and blood glucose cannot be fully explained. The maximum HbA1c value in the amputated group was 7.9%. The average values for healed patients and those who underwent LEA were 8.75% and 6.77%, respectively.
Blood glucose levels were also found to be the lowest in the amputated group in our study (mean, 149.29 mg/dL vs 163.19 mg/dL in the healed group). Similar results were found in a Brazilian study, in which patients who did not require amputation had higher HbA1c levels. This study also found an association between blood glucose levels above 200 mg/dL and amputations.3 These findings provide interesting opportunities for repeat studies, preferably with a larger number of participants.
Our study is limited by the small sample size. The sample population had to be reduced, as many patients were lost to follow-up. Although this paring down of the sample size can introduce bias, we are confident that our study is representative of the demographic of patients treated in our facility. The loss of patients to follow-up in turn caused the window of analysis to be narrowed, as long-term outcome data were not available. A multisite study observing various population samples can better explore the relationship between HbA1c and risk of amputation.
Conclusion
This retrospective study exploring factors associated with LEA was unique in that all our participants had 1 or more diabetic foot ulcerations, and thus already had an extremely high risk for amputation, in contrast to previous studies that followed persons at risk for developing diabetic foot ulcerations. In contrast to several previous studies, we found that the risk for amputation actually decreased as baseline measurements of HbA1c increased. The results of this study offer many opportunities for future investigations, preferably with a larger sample size. By further isolating and scrutinizing specific factors associated with LEA, researchers can help clinicians focus on providing wound care that promotes limb salvage.
Corresponding author: Alisha Oropallo, MD, MS, Northwell Health Comprehensive Wound Care Healing Center and Hyperbarics, 1999 Marcus Avenue, Suite M6, Lake Success, NY 11042; aoropallo@northwell.edu.
Financial disclosures: Funding for this research was provided by a multi-institutional AHRQ governmental grant.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA: US Department of Health and Human Services; 2017.
2. Uccioli L, Giurato L, Meloni M, et al. Comment on Hoffstad et al. Diabetes, lower-extremity amputation, and death. Diabetes Care. 2015;38:1852-1857.
3. Gamba MA, Gotlieb SLD, Bergamaschi DP, Vianna LAC. Lower extremity amputations in diabetic patients: a case-control study. Rev Saúde Pública. 2004;38:399-404.
4. Martins-Mendes D, Monteiro-Soares M, Boyko EJ, et al. The independent contribution of diabetic foot ulcer on lower extremity amputation and mortality risk. J Diabetes Complications. 2014;28:632-638.
5. Lipsky BA, Weigelt JA, Sun X, et al. Developing and validating a risk score for lower-extremity amputation in patients hospitalized for a diabetic foot infection. Diabetes Care. 2011;34:1695-1700.
6. Al-Rubeaan K, Al Derwish M, Ouizi S, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015;10:e0124446.
7. Pickwell K, Siersma V, Kars M, et al. Predictors of lower-extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care. 2015;38:852-857.
8. Lin C, Hsu BR, Tsai J, et al. Effect of limb preservation status and body mass index on the survival of patients with limb-threatening diabetic foot ulcers. J Diabetes Complications. 2017;31:180-185.
9. Stevens CD, Schriger DL, Raffetto B, et al. Geographic clustering of diabetic lower-extremity amputations in low-income regions of California. Health Aff. 2014;33:1383-1390.
10. Liao L, Li C, Liu C, et al. Extreme levels of HbA1c increase incident ESRD risk in Chinese patients with type 2 diabetes: competing risk analysis in national cohort of Taiwan diabetes study. PLoS One. 2015; 10:e0130828.
11. Miyajima S, Shirai A, Yamamoto S, et al. Risk factors for major limb amputations in diabetic foot gangrene patients. Diabetes Res Clin Pract. 2006;71:272-279.
12. Zhao W, Katzmarzyk PT, Horswell R, et al. HbA1c and lower-extremity amputation risk in low-income patients with diabetes. Diabetes Care. 2013;36:3591-3598.
From Northwell Health System, Lake Success, NY.
Abstract
- Objective: To explore factors associated with lower-extremity amputation (LEA) in patients with diabetic foot ulcers using data from the Online Wound Electronic Medical Record Database.
- Design: Retrospective analysis of medical records.
- Setting and participants: Data from 169 individuals with previously diagnosed diabetes mellitus who received wound care for a 6-month period within a span of 2 years was analyzed. A baseline evaluation was obtained and wound(s) were treated, managed, and monitored.
Treatment continued until the patient healed, required an LEA, or phased out of the study, neither healing nor undergoing an amputation. Of the 149 patients who completed the study, 38 had healed ulcers, 14 underwent amputation, and 97 neither healed nor underwent an amputation. All patients were treated under the care of vascular and/or podiatric surgeons. - Measurements: Variables included wound status (healed, amputated, and unhealed/non-amputated); size of wound area; age, gender, race, and ethnicity; white blood cell (WBC) count, hemoglobin A1c (HbA1c), blood glucose, and body mass index (BMI); and presence of osteomyelitis, gangrene, and peripheral vascular disease.
- Results: As compared to the healed and unhealed/non-amputated group, the group of patients who underwent LEA was older and had higher percentages of males, Hispanics, and African Americans; had a higher WBC count, larger wound area, and higher rates of wound infection, osteomyelitis, and neuropathy; and had lower average values of HbA1c, blood glucose, and BMI and a lower rate of peripheral vascular disease.
- Conclusion: The association between HbA1c and LEA highlights a window of relative safety among an at-risk population. By identifying and focusing on factors associated with LEA, health care professionals may be able to decrease the prevalence of LEA in patients with diabetes.
Keywords: diabetic foot ulcer; lower-extremity amputation; risk factors; HbA1c.
An estimated 30.3 million people, or 9.4% of the US population, has diabetes. In 2014, approximately 108,000 amputations were performed on adults with diagnosed diabetes.1 Furthermore, patients with diabetes have a 10-fold increased risk for lower-extremity amputation (LEA), as compared with patients without diabetes.2 The frequency of amputations in the diabetic population is a public health crisis.
Amputation has significant, life-altering consequences. Patients who undergo LEA often face debilitation in their daily activities and must undergo intense rehabilitation to learn basic tasks. Amputations can also impact individuals’ psychological well-being as they come to terms with their altered body and may face challenges in self-perception, confidence, self-esteem, work life, and relationships. In addition, the mortality rate for patients with diabetes 5 years after undergoing LEA is 30%.2 However, public health studies estimate that more than half of LEAs in patients with diabetes are preventable.3
Although studies have explored the relationship between diabetes and LEA, few have sought to identify factors directly correlated with wound care. In the United States, patients with diabetic ulcerations are typically treated in wound care facilities; however, previous studies have concentrated on the conditions that lead to the formation of an ulcer or amputation, viewing amputation and ulcer as 2 separate entities. Our study took into account systemic variables, patient demographics, and specific wound characteristics to explore factors associated with LEA in a high-risk group of patients with diabetes. This study was designed to assess ailments that are prevalent in patients who require a LEA.
Methods
Patients and Setting
A total of 169 patients who were treated at the Comprehensive Wound Healing and Hyperbaric Center (Lake Success, NY), a tertiary facility of the Northwell Health system, participated in this retrospective study. The data for this study were obtained in conjunction with the development of the New York University School of Medicine’s Online Wound Electronic Medical Record to Decrease Limb Amputations in Persons with Diabetes (OWEMR) database. The OWEMR collects individual patient data from satellite locations across the country. Using this database, researchers can analyze similarities and differences between patients who undergo LEA.
This study utilized patient data specific to the Northwell Health facility. All of the patients in our study were enrolled under the criteria of the OWEMR database. In order to be included in the OWEMR database, patients had to be diagnosed with type 1 or type 2 diabetes; have a break in the skin ≥ 0.5 cm2; be 18 years of age or older; and have a measured hemoglobin A1c (HbA1c) value within the past 120 days. Study patients signed an informed consent and committed to being available for follow-up visits to the wound care facility for 6 months after entering the study. Patients were enrolled between 2012 and 2014, and each patient was monitored for a period of 6 months within this time period. Participants were treated with current standards of care using diet, lifestyle, and pharmacologic interventions. This study was approved by the Northwell Health System Institutional Review Board Human Research Protection Program (Manhasset, NY).
Data Collection
On their first visit to the facility, patients were given a physical examination and initial interview regarding their medical history. Clinicians were required to select 1 ulcer that would be examined for the duration of the study. The selection of the ulcer was based on a point system that awarded points for pedal pulses, the ability to be probed to the bone, the location of the ulcer (ie, located on the foot rather than a toe), and the presence of multiple ulcerations. The ulcer with the highest score was selected for the study. If numerous ulcers were evaluated with the same score, the largest and deepest was selected. Wagner classification of the wound was recorded at baseline and taken at each subsequent patient visit. In addition, peripheral sensation was assessed for signs of neuropathy using Semmes-Weinstein monofilament testing.
Once selected, the wound was clinically evaluated, samples for culture were obtained, and blood tests were performed to detect the presence of wound infection. The patient’s blood was drawn for a full laboratory analysis, including white blood cell (WBC) count and measurement of blood glucose and HbA1c levels. Bone biopsy, magnetic resonance imaging, and bone scans were used to detect the presence of osteomyelitis at the discretion of the health care provider. Wounds suspected of infection, underlying osteomyelitis, or gangrene at baseline were excluded. Patients would then return for follow-up visits at least once every 6 weeks, plus or minus 2 weeks, for a maximum of 6 months.
Statistical Analysis
Utilizing SAS version 9.3 (Cary, NC), descriptive statistics (minimum, maximum, mean, median, and SD) were calculated for the following variables: age, WBC count, wound area, HbA1c, blood glucose, and body mass index (BMI). These variables were collected for each patient as per the OWEMR protocol and provided a basis for which to compare patients who underwent amputation and those who did not. Twenty patients were lost to follow-up, and therefore we altered the window of our statistics from 6 months to 3 months to provide the most accurate data, as 6-month follow-up data were limited. The patients were classified into the following categories: healed, amputated, and unhealed/non-amputated. Descriptive statistics were calculated for these 3 groups, analyzing the same variables (age, WBC count, wound area, HbA1c, blood glucose, and BMI). Additional statistical computations were utilized in order to show the prevalence and frequency of our categorical variables: gender, race, ethnicity, osteomyelitis, gangrene, and peripheral vascular disease. The baseline values of WBC count, HbA1c, wound area, and BMI of the 3 groups were analyzed with descriptive statistics for comparison. A multinomial logistic regression was then performed using a 3-level outcome variable: healed, amputated, or unhealed/non-amputated. Each predictor variable was analyzed independently due to the small sample size.
Results
Of the 169 registered patients treated at the Northwell Health facility, all qualified for the OWEMR study and met the study criteria. In the original 169 patients, there were 19 amputations: 6 toe, 6 trans-metatarsal, 6 below knee, and 1 above knee (Table 1).
The descriptive statistics of 149 patients grouped into 3 categories (healed, amputated, unhealed/non-amputated) are shown in Table 2.
The results of the logistic regression exploring the differences between the amputation and healed groups and the unhealed/non-amputated group are shown in Table 3. The amputation group had a higher mean age and WBC count and greater wound area. Increased age was determined to be a significant predictor of the odds of amputation (P = 0.0089). For each year increase in age, the odds of amputation increased by 6.5% (odds ratio, 1.07 [95% confidence interval {CI}, 1.02-1.12]). Patients in the amputation group were more likely to be male, Hispanic, and African American and to have wound infections and comorbidities (osteomyelitis, neuropathy, and gangrene).
The presence of gangrene was significantly associated with LEA (P = 0.03). Specifically, the odds of patients without gangrene undergoing a LEA were substantially lower compared with their counterparts with gangrene (odds ratio, 0.17; 95% CI, 0.04-0.68; P = 0.0131). However, the presence of gangrene was not associated with the odds of healing compared with the odds of neither healing nor undergoing amputation (P = 0.84; not shown in Table 3).
The amputation group had lower mean values for HbA1c, BMI, and blood glucose levels and a lower rate of peripheral vascular disease. Only the relationship between lower HbA1c and increased odds of amputation versus not healing/non-amputation was found to be statistically significant (95% CI, 0.27-0.78; P = 0.009).
Discussion
This retrospective study was undertaken to evaluate factors associated with LEA in patients with diabetic foot ulcers. Patients with diabetes being treated at a wound care facility often require continuous surgical and metabolic intervention to promote optimal healing: drainage, surgical debridement, irrigation, culturing for infection, and monitoring of blood glucose levels. This treatment requires strict compliance with medical directions and, oftentimes, additional care, such as home-care nursing visits, to maintain a curative environment for the wound. Frequently, wounds on the lower extremity further complicate the healing process by reducing the patient’s mobility and daily life. Due to these factors, many patients progress to LEA. The link between diabetic ulcers and amputation has already been well described in previous studies, with studies showing that history of diabetic foot ulcer significantly predisposes an individual to LEA.4 However, few studies have further investigated demographic factors associated with risk for an amputation. Our study analyzed several categories of patient data taken from a baseline visit. We found that those with highly elevated HbA1c values were less likely to have an amputation than persons with relatively lower levels, a finding that is contrary to previous studies.
Our study’s findings suggest a higher risk for LEA with increased age. The amputation group was, on average, 7 years older than the other 2 groups. A recent study showed that risk for amputation is directly correlated to patient age, as is the mortality rate after undergoing LEA (2.3%; P < 0.05).5 Our study found that with each increase in age of 1 year, the odds of amputation increased by 6.5%. However, recent evidence on LEA risk and aging suggests that age is of less consequence than the duration of diabetes. One study found that the propensity to develop diabetic foot ulcers increases with the duration of diabetes.6 The same study found that prevalence of ulceration was correlated with age, but the relationship between age and LEA was less significant. A follow-up study for LEA could be done to examine the role of disease duration versus age in LEA.
A consensus among previous studies is that men have a higher risk for LEA.5,7 Men comprised the majority in all 3 groups in our study. In addition, the amputation group in our study had the lowest BMI. Higher BMI generally is associated with an increased risk for health complications. However, a past study conducted in Taiwan reported that obese patients with diabetes were less likely to undergo LEA than those within the normal range for BMI.8 Neither study suggests that obesity is a deterrent for LEA, but both studies may suggest that risk of amputation may approach a maximum frequency at a specific BMI range, and then decrease. This unconfirmed “cyclic” relationship should be evaluated further in a larger sample size.
Most patients in our analysis were Caucasian, followed by African American and South Asian. African Americans were the only racial group with an increased frequency in the amputation group. This finding is supported by a previous study that found that the rate of LEA among patients with diabetes in low-income, predominantly African-American neighborhoods was nearly double that in wealthier, predominantly Caucasian areas.9 A potential problem in the comparison between our data with previous studies is that the studies did not analyze patients with our inclusion criteria. All patients with diabetes in previous investigations were grouped by race, but were not necessarily required to have 1 or more ulcers. Multiple ulcers may predispose an individual to a greater risk for amputation.
Multinomial logistic regression did not suggest an association between initial size of a patient’s wound and the risk of amputation. However, the descriptive data suggests a trend. Patients who did not heal or require an amputation had the largest average wound area. This finding is not surprising in that our study followed individuals for only 3 months. Many wounds require a long course of treatment, especially in patients with diabetes, who may have poor vascularization. However, in comparison to the healed patients, the patients who required an amputation had a larger average wound area. A larger wound requires a plentiful vascular supply for the delivery of clotting factors and nutrients to the damaged area. As wound size increases, an individual’s body must transmit an increased quantity of these factors and nutrients for the regeneration of tissue. In addition, wounds that possess a larger surface area require more debridement and present a greater opportunity for infection. This may also foreshadow a longer, more costly course of treatment. Additionally, individuals coping with large ulcerations are burdened by more elaborate and complex wound dressings.
Elevated levels of HbA1c are associated with increased adverse effects of diabetes, including end-stage renal disease, neuropathy, and infection.10 In a previous study, the risk for amputation was 1.2 times higher in patients with elevated HbA1c.11 In contrast, our study suggested the odds of LEA versus not healing/not undergoing amputation decreased as HbA1c increased. As a patient’s HbA1c level increased by a value of 1, their odds for LEA decreased by 54.3%. This finding contradicts prior studies that have found a positive association between HbA1c and LEA risk, including a study where each percentage increase in HbA1c correlated with a 13% to 15% increased risk of LEA.12 The finding that patients who underwent amputation in our study had lower levels of HbA1c and blood glucose cannot be fully explained. The maximum HbA1c value in the amputated group was 7.9%. The average values for healed patients and those who underwent LEA were 8.75% and 6.77%, respectively.
Blood glucose levels were also found to be the lowest in the amputated group in our study (mean, 149.29 mg/dL vs 163.19 mg/dL in the healed group). Similar results were found in a Brazilian study, in which patients who did not require amputation had higher HbA1c levels. This study also found an association between blood glucose levels above 200 mg/dL and amputations.3 These findings provide interesting opportunities for repeat studies, preferably with a larger number of participants.
Our study is limited by the small sample size. The sample population had to be reduced, as many patients were lost to follow-up. Although this paring down of the sample size can introduce bias, we are confident that our study is representative of the demographic of patients treated in our facility. The loss of patients to follow-up in turn caused the window of analysis to be narrowed, as long-term outcome data were not available. A multisite study observing various population samples can better explore the relationship between HbA1c and risk of amputation.
Conclusion
This retrospective study exploring factors associated with LEA was unique in that all our participants had 1 or more diabetic foot ulcerations, and thus already had an extremely high risk for amputation, in contrast to previous studies that followed persons at risk for developing diabetic foot ulcerations. In contrast to several previous studies, we found that the risk for amputation actually decreased as baseline measurements of HbA1c increased. The results of this study offer many opportunities for future investigations, preferably with a larger sample size. By further isolating and scrutinizing specific factors associated with LEA, researchers can help clinicians focus on providing wound care that promotes limb salvage.
Corresponding author: Alisha Oropallo, MD, MS, Northwell Health Comprehensive Wound Care Healing Center and Hyperbarics, 1999 Marcus Avenue, Suite M6, Lake Success, NY 11042; aoropallo@northwell.edu.
Financial disclosures: Funding for this research was provided by a multi-institutional AHRQ governmental grant.
From Northwell Health System, Lake Success, NY.
Abstract
- Objective: To explore factors associated with lower-extremity amputation (LEA) in patients with diabetic foot ulcers using data from the Online Wound Electronic Medical Record Database.
- Design: Retrospective analysis of medical records.
- Setting and participants: Data from 169 individuals with previously diagnosed diabetes mellitus who received wound care for a 6-month period within a span of 2 years was analyzed. A baseline evaluation was obtained and wound(s) were treated, managed, and monitored.
Treatment continued until the patient healed, required an LEA, or phased out of the study, neither healing nor undergoing an amputation. Of the 149 patients who completed the study, 38 had healed ulcers, 14 underwent amputation, and 97 neither healed nor underwent an amputation. All patients were treated under the care of vascular and/or podiatric surgeons. - Measurements: Variables included wound status (healed, amputated, and unhealed/non-amputated); size of wound area; age, gender, race, and ethnicity; white blood cell (WBC) count, hemoglobin A1c (HbA1c), blood glucose, and body mass index (BMI); and presence of osteomyelitis, gangrene, and peripheral vascular disease.
- Results: As compared to the healed and unhealed/non-amputated group, the group of patients who underwent LEA was older and had higher percentages of males, Hispanics, and African Americans; had a higher WBC count, larger wound area, and higher rates of wound infection, osteomyelitis, and neuropathy; and had lower average values of HbA1c, blood glucose, and BMI and a lower rate of peripheral vascular disease.
- Conclusion: The association between HbA1c and LEA highlights a window of relative safety among an at-risk population. By identifying and focusing on factors associated with LEA, health care professionals may be able to decrease the prevalence of LEA in patients with diabetes.
Keywords: diabetic foot ulcer; lower-extremity amputation; risk factors; HbA1c.
An estimated 30.3 million people, or 9.4% of the US population, has diabetes. In 2014, approximately 108,000 amputations were performed on adults with diagnosed diabetes.1 Furthermore, patients with diabetes have a 10-fold increased risk for lower-extremity amputation (LEA), as compared with patients without diabetes.2 The frequency of amputations in the diabetic population is a public health crisis.
Amputation has significant, life-altering consequences. Patients who undergo LEA often face debilitation in their daily activities and must undergo intense rehabilitation to learn basic tasks. Amputations can also impact individuals’ psychological well-being as they come to terms with their altered body and may face challenges in self-perception, confidence, self-esteem, work life, and relationships. In addition, the mortality rate for patients with diabetes 5 years after undergoing LEA is 30%.2 However, public health studies estimate that more than half of LEAs in patients with diabetes are preventable.3
Although studies have explored the relationship between diabetes and LEA, few have sought to identify factors directly correlated with wound care. In the United States, patients with diabetic ulcerations are typically treated in wound care facilities; however, previous studies have concentrated on the conditions that lead to the formation of an ulcer or amputation, viewing amputation and ulcer as 2 separate entities. Our study took into account systemic variables, patient demographics, and specific wound characteristics to explore factors associated with LEA in a high-risk group of patients with diabetes. This study was designed to assess ailments that are prevalent in patients who require a LEA.
Methods
Patients and Setting
A total of 169 patients who were treated at the Comprehensive Wound Healing and Hyperbaric Center (Lake Success, NY), a tertiary facility of the Northwell Health system, participated in this retrospective study. The data for this study were obtained in conjunction with the development of the New York University School of Medicine’s Online Wound Electronic Medical Record to Decrease Limb Amputations in Persons with Diabetes (OWEMR) database. The OWEMR collects individual patient data from satellite locations across the country. Using this database, researchers can analyze similarities and differences between patients who undergo LEA.
This study utilized patient data specific to the Northwell Health facility. All of the patients in our study were enrolled under the criteria of the OWEMR database. In order to be included in the OWEMR database, patients had to be diagnosed with type 1 or type 2 diabetes; have a break in the skin ≥ 0.5 cm2; be 18 years of age or older; and have a measured hemoglobin A1c (HbA1c) value within the past 120 days. Study patients signed an informed consent and committed to being available for follow-up visits to the wound care facility for 6 months after entering the study. Patients were enrolled between 2012 and 2014, and each patient was monitored for a period of 6 months within this time period. Participants were treated with current standards of care using diet, lifestyle, and pharmacologic interventions. This study was approved by the Northwell Health System Institutional Review Board Human Research Protection Program (Manhasset, NY).
Data Collection
On their first visit to the facility, patients were given a physical examination and initial interview regarding their medical history. Clinicians were required to select 1 ulcer that would be examined for the duration of the study. The selection of the ulcer was based on a point system that awarded points for pedal pulses, the ability to be probed to the bone, the location of the ulcer (ie, located on the foot rather than a toe), and the presence of multiple ulcerations. The ulcer with the highest score was selected for the study. If numerous ulcers were evaluated with the same score, the largest and deepest was selected. Wagner classification of the wound was recorded at baseline and taken at each subsequent patient visit. In addition, peripheral sensation was assessed for signs of neuropathy using Semmes-Weinstein monofilament testing.
Once selected, the wound was clinically evaluated, samples for culture were obtained, and blood tests were performed to detect the presence of wound infection. The patient’s blood was drawn for a full laboratory analysis, including white blood cell (WBC) count and measurement of blood glucose and HbA1c levels. Bone biopsy, magnetic resonance imaging, and bone scans were used to detect the presence of osteomyelitis at the discretion of the health care provider. Wounds suspected of infection, underlying osteomyelitis, or gangrene at baseline were excluded. Patients would then return for follow-up visits at least once every 6 weeks, plus or minus 2 weeks, for a maximum of 6 months.
Statistical Analysis
Utilizing SAS version 9.3 (Cary, NC), descriptive statistics (minimum, maximum, mean, median, and SD) were calculated for the following variables: age, WBC count, wound area, HbA1c, blood glucose, and body mass index (BMI). These variables were collected for each patient as per the OWEMR protocol and provided a basis for which to compare patients who underwent amputation and those who did not. Twenty patients were lost to follow-up, and therefore we altered the window of our statistics from 6 months to 3 months to provide the most accurate data, as 6-month follow-up data were limited. The patients were classified into the following categories: healed, amputated, and unhealed/non-amputated. Descriptive statistics were calculated for these 3 groups, analyzing the same variables (age, WBC count, wound area, HbA1c, blood glucose, and BMI). Additional statistical computations were utilized in order to show the prevalence and frequency of our categorical variables: gender, race, ethnicity, osteomyelitis, gangrene, and peripheral vascular disease. The baseline values of WBC count, HbA1c, wound area, and BMI of the 3 groups were analyzed with descriptive statistics for comparison. A multinomial logistic regression was then performed using a 3-level outcome variable: healed, amputated, or unhealed/non-amputated. Each predictor variable was analyzed independently due to the small sample size.
Results
Of the 169 registered patients treated at the Northwell Health facility, all qualified for the OWEMR study and met the study criteria. In the original 169 patients, there were 19 amputations: 6 toe, 6 trans-metatarsal, 6 below knee, and 1 above knee (Table 1).
The descriptive statistics of 149 patients grouped into 3 categories (healed, amputated, unhealed/non-amputated) are shown in Table 2.
The results of the logistic regression exploring the differences between the amputation and healed groups and the unhealed/non-amputated group are shown in Table 3. The amputation group had a higher mean age and WBC count and greater wound area. Increased age was determined to be a significant predictor of the odds of amputation (P = 0.0089). For each year increase in age, the odds of amputation increased by 6.5% (odds ratio, 1.07 [95% confidence interval {CI}, 1.02-1.12]). Patients in the amputation group were more likely to be male, Hispanic, and African American and to have wound infections and comorbidities (osteomyelitis, neuropathy, and gangrene).
The presence of gangrene was significantly associated with LEA (P = 0.03). Specifically, the odds of patients without gangrene undergoing a LEA were substantially lower compared with their counterparts with gangrene (odds ratio, 0.17; 95% CI, 0.04-0.68; P = 0.0131). However, the presence of gangrene was not associated with the odds of healing compared with the odds of neither healing nor undergoing amputation (P = 0.84; not shown in Table 3).
The amputation group had lower mean values for HbA1c, BMI, and blood glucose levels and a lower rate of peripheral vascular disease. Only the relationship between lower HbA1c and increased odds of amputation versus not healing/non-amputation was found to be statistically significant (95% CI, 0.27-0.78; P = 0.009).
Discussion
This retrospective study was undertaken to evaluate factors associated with LEA in patients with diabetic foot ulcers. Patients with diabetes being treated at a wound care facility often require continuous surgical and metabolic intervention to promote optimal healing: drainage, surgical debridement, irrigation, culturing for infection, and monitoring of blood glucose levels. This treatment requires strict compliance with medical directions and, oftentimes, additional care, such as home-care nursing visits, to maintain a curative environment for the wound. Frequently, wounds on the lower extremity further complicate the healing process by reducing the patient’s mobility and daily life. Due to these factors, many patients progress to LEA. The link between diabetic ulcers and amputation has already been well described in previous studies, with studies showing that history of diabetic foot ulcer significantly predisposes an individual to LEA.4 However, few studies have further investigated demographic factors associated with risk for an amputation. Our study analyzed several categories of patient data taken from a baseline visit. We found that those with highly elevated HbA1c values were less likely to have an amputation than persons with relatively lower levels, a finding that is contrary to previous studies.
Our study’s findings suggest a higher risk for LEA with increased age. The amputation group was, on average, 7 years older than the other 2 groups. A recent study showed that risk for amputation is directly correlated to patient age, as is the mortality rate after undergoing LEA (2.3%; P < 0.05).5 Our study found that with each increase in age of 1 year, the odds of amputation increased by 6.5%. However, recent evidence on LEA risk and aging suggests that age is of less consequence than the duration of diabetes. One study found that the propensity to develop diabetic foot ulcers increases with the duration of diabetes.6 The same study found that prevalence of ulceration was correlated with age, but the relationship between age and LEA was less significant. A follow-up study for LEA could be done to examine the role of disease duration versus age in LEA.
A consensus among previous studies is that men have a higher risk for LEA.5,7 Men comprised the majority in all 3 groups in our study. In addition, the amputation group in our study had the lowest BMI. Higher BMI generally is associated with an increased risk for health complications. However, a past study conducted in Taiwan reported that obese patients with diabetes were less likely to undergo LEA than those within the normal range for BMI.8 Neither study suggests that obesity is a deterrent for LEA, but both studies may suggest that risk of amputation may approach a maximum frequency at a specific BMI range, and then decrease. This unconfirmed “cyclic” relationship should be evaluated further in a larger sample size.
Most patients in our analysis were Caucasian, followed by African American and South Asian. African Americans were the only racial group with an increased frequency in the amputation group. This finding is supported by a previous study that found that the rate of LEA among patients with diabetes in low-income, predominantly African-American neighborhoods was nearly double that in wealthier, predominantly Caucasian areas.9 A potential problem in the comparison between our data with previous studies is that the studies did not analyze patients with our inclusion criteria. All patients with diabetes in previous investigations were grouped by race, but were not necessarily required to have 1 or more ulcers. Multiple ulcers may predispose an individual to a greater risk for amputation.
Multinomial logistic regression did not suggest an association between initial size of a patient’s wound and the risk of amputation. However, the descriptive data suggests a trend. Patients who did not heal or require an amputation had the largest average wound area. This finding is not surprising in that our study followed individuals for only 3 months. Many wounds require a long course of treatment, especially in patients with diabetes, who may have poor vascularization. However, in comparison to the healed patients, the patients who required an amputation had a larger average wound area. A larger wound requires a plentiful vascular supply for the delivery of clotting factors and nutrients to the damaged area. As wound size increases, an individual’s body must transmit an increased quantity of these factors and nutrients for the regeneration of tissue. In addition, wounds that possess a larger surface area require more debridement and present a greater opportunity for infection. This may also foreshadow a longer, more costly course of treatment. Additionally, individuals coping with large ulcerations are burdened by more elaborate and complex wound dressings.
Elevated levels of HbA1c are associated with increased adverse effects of diabetes, including end-stage renal disease, neuropathy, and infection.10 In a previous study, the risk for amputation was 1.2 times higher in patients with elevated HbA1c.11 In contrast, our study suggested the odds of LEA versus not healing/not undergoing amputation decreased as HbA1c increased. As a patient’s HbA1c level increased by a value of 1, their odds for LEA decreased by 54.3%. This finding contradicts prior studies that have found a positive association between HbA1c and LEA risk, including a study where each percentage increase in HbA1c correlated with a 13% to 15% increased risk of LEA.12 The finding that patients who underwent amputation in our study had lower levels of HbA1c and blood glucose cannot be fully explained. The maximum HbA1c value in the amputated group was 7.9%. The average values for healed patients and those who underwent LEA were 8.75% and 6.77%, respectively.
Blood glucose levels were also found to be the lowest in the amputated group in our study (mean, 149.29 mg/dL vs 163.19 mg/dL in the healed group). Similar results were found in a Brazilian study, in which patients who did not require amputation had higher HbA1c levels. This study also found an association between blood glucose levels above 200 mg/dL and amputations.3 These findings provide interesting opportunities for repeat studies, preferably with a larger number of participants.
Our study is limited by the small sample size. The sample population had to be reduced, as many patients were lost to follow-up. Although this paring down of the sample size can introduce bias, we are confident that our study is representative of the demographic of patients treated in our facility. The loss of patients to follow-up in turn caused the window of analysis to be narrowed, as long-term outcome data were not available. A multisite study observing various population samples can better explore the relationship between HbA1c and risk of amputation.
Conclusion
This retrospective study exploring factors associated with LEA was unique in that all our participants had 1 or more diabetic foot ulcerations, and thus already had an extremely high risk for amputation, in contrast to previous studies that followed persons at risk for developing diabetic foot ulcerations. In contrast to several previous studies, we found that the risk for amputation actually decreased as baseline measurements of HbA1c increased. The results of this study offer many opportunities for future investigations, preferably with a larger sample size. By further isolating and scrutinizing specific factors associated with LEA, researchers can help clinicians focus on providing wound care that promotes limb salvage.
Corresponding author: Alisha Oropallo, MD, MS, Northwell Health Comprehensive Wound Care Healing Center and Hyperbarics, 1999 Marcus Avenue, Suite M6, Lake Success, NY 11042; aoropallo@northwell.edu.
Financial disclosures: Funding for this research was provided by a multi-institutional AHRQ governmental grant.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA: US Department of Health and Human Services; 2017.
2. Uccioli L, Giurato L, Meloni M, et al. Comment on Hoffstad et al. Diabetes, lower-extremity amputation, and death. Diabetes Care. 2015;38:1852-1857.
3. Gamba MA, Gotlieb SLD, Bergamaschi DP, Vianna LAC. Lower extremity amputations in diabetic patients: a case-control study. Rev Saúde Pública. 2004;38:399-404.
4. Martins-Mendes D, Monteiro-Soares M, Boyko EJ, et al. The independent contribution of diabetic foot ulcer on lower extremity amputation and mortality risk. J Diabetes Complications. 2014;28:632-638.
5. Lipsky BA, Weigelt JA, Sun X, et al. Developing and validating a risk score for lower-extremity amputation in patients hospitalized for a diabetic foot infection. Diabetes Care. 2011;34:1695-1700.
6. Al-Rubeaan K, Al Derwish M, Ouizi S, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015;10:e0124446.
7. Pickwell K, Siersma V, Kars M, et al. Predictors of lower-extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care. 2015;38:852-857.
8. Lin C, Hsu BR, Tsai J, et al. Effect of limb preservation status and body mass index on the survival of patients with limb-threatening diabetic foot ulcers. J Diabetes Complications. 2017;31:180-185.
9. Stevens CD, Schriger DL, Raffetto B, et al. Geographic clustering of diabetic lower-extremity amputations in low-income regions of California. Health Aff. 2014;33:1383-1390.
10. Liao L, Li C, Liu C, et al. Extreme levels of HbA1c increase incident ESRD risk in Chinese patients with type 2 diabetes: competing risk analysis in national cohort of Taiwan diabetes study. PLoS One. 2015; 10:e0130828.
11. Miyajima S, Shirai A, Yamamoto S, et al. Risk factors for major limb amputations in diabetic foot gangrene patients. Diabetes Res Clin Pract. 2006;71:272-279.
12. Zhao W, Katzmarzyk PT, Horswell R, et al. HbA1c and lower-extremity amputation risk in low-income patients with diabetes. Diabetes Care. 2013;36:3591-3598.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA: US Department of Health and Human Services; 2017.
2. Uccioli L, Giurato L, Meloni M, et al. Comment on Hoffstad et al. Diabetes, lower-extremity amputation, and death. Diabetes Care. 2015;38:1852-1857.
3. Gamba MA, Gotlieb SLD, Bergamaschi DP, Vianna LAC. Lower extremity amputations in diabetic patients: a case-control study. Rev Saúde Pública. 2004;38:399-404.
4. Martins-Mendes D, Monteiro-Soares M, Boyko EJ, et al. The independent contribution of diabetic foot ulcer on lower extremity amputation and mortality risk. J Diabetes Complications. 2014;28:632-638.
5. Lipsky BA, Weigelt JA, Sun X, et al. Developing and validating a risk score for lower-extremity amputation in patients hospitalized for a diabetic foot infection. Diabetes Care. 2011;34:1695-1700.
6. Al-Rubeaan K, Al Derwish M, Ouizi S, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015;10:e0124446.
7. Pickwell K, Siersma V, Kars M, et al. Predictors of lower-extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care. 2015;38:852-857.
8. Lin C, Hsu BR, Tsai J, et al. Effect of limb preservation status and body mass index on the survival of patients with limb-threatening diabetic foot ulcers. J Diabetes Complications. 2017;31:180-185.
9. Stevens CD, Schriger DL, Raffetto B, et al. Geographic clustering of diabetic lower-extremity amputations in low-income regions of California. Health Aff. 2014;33:1383-1390.
10. Liao L, Li C, Liu C, et al. Extreme levels of HbA1c increase incident ESRD risk in Chinese patients with type 2 diabetes: competing risk analysis in national cohort of Taiwan diabetes study. PLoS One. 2015; 10:e0130828.
11. Miyajima S, Shirai A, Yamamoto S, et al. Risk factors for major limb amputations in diabetic foot gangrene patients. Diabetes Res Clin Pract. 2006;71:272-279.
12. Zhao W, Katzmarzyk PT, Horswell R, et al. HbA1c and lower-extremity amputation risk in low-income patients with diabetes. Diabetes Care. 2013;36:3591-3598.
Impact of Hospitalists on Care Outcomes in a Large Integrated Health System in British Columbia
From the Fraser Health Authority, Surrey, British Columbia, Canada.
Abstract
- Objective: To study care outcomes associated with a network of hospitalist services compared to traditional providers.
- Design: Retrospective review of administrative data.
- Setting and participants: Patients from a large integrated health care system in British Columbia in western Canada admitted and cared for by 3 provider groups between April 1, 2012, and March 31, 2018: hospitalists, family physicians (FP), and internal medicine (IM) physicians:
- Measurements: Average total length of stay (LOS), 30-day readmission, in-hospital mortality, and hospital standardized mortality ratio (HSMR) were the study outcome measures. Multiple logistic regression or generalized regression were completed to determine the relationship between provider groups and outcomes.
- Results: A total of 248,412 hospitalizations were included. Compared to patients admitted to hospitalists, patients admitted to other providers had higher odds of mortality (odds ratio [OR] for FP, 1.29; 95% confidence interval [CI], 1.21-1.37; OR for IM, 1.24; 95% CI, 1.15-1.33). Compared to hospitalist care, FP care was associated with higher readmission (OR, 1.27; 95% CI, 1.22-1.33), while IM care showed lower odds of readmission (OR, 0.83; 95% CI, 0.79-0.87). Patients admitted to the IM group had significantly lower total LOS (mean, 5.13 days; 95% CI, 5.04-5.21) compared to patients admitted to hospitalists (mean, 7.37 days; CI, 7.26-7.49) and FPs (mean, 7.30 days; 95% CI, 7.19-7.41). In a subgroup analysis of patients presenting with congestive heart failure, chronic obstructive pulmonary disease, and pneumonia, these general tendencies broadly persisted for mortality and LOS comparisons between FPs and hospitalists, but results were mixed for hospital readmissions.
- Conclusion: Care provided by hospitalists was associated with lower mortality and readmission rates compared with care provided by FPs, despite similar LOS. These findings may reflect differences in volume of services delivered by individual physicians, on-site availability to address urgent medical issues, and evolving specialization of clinical and nonclinical care processes in the acute care setting.
Keywords: hospital medicine; length of stay; readmission; mortality.
The hospitalist model of care has undergone rapid growth globally in recent years.1 The first hospitalist programs in Canada began around the same time as those in the United States and share many similarities in design and operations with their counterparts.2-4 However, unlike in the United States, where the hospitalist model has successfully established itself as an emerging specialty, debates about the merits of the model and its value proposition continue among Canadian observers.5-9
Historically, the type of physicians who acted as the most responsible provider (MRP) in Canadian hospitals depended on setting and geography.10 In large urban areas, groups of general internists or specialists have historically looked after general medicine patients as part of university-affiliated teaching services.11,12 Patients admitted to community hospitals have traditionally been cared for by their own primary care providers, typically general practitioners or family physicians (FPs). In the mid-1990s, many primary care providers in urban centers began to withdraw from inpatient care and primarily focused their practices in the outpatient setting.13-15 Hospitalist programs emerged as health care administrators sought to fill the resulting gap in MRP coverage.2,10
To date, attempts to understand the impact of hospitalist programs in Canada have been limited. A number of early studies aimed to describe16 the role of hospitalists in Canada and suggested improvements in length of stay (LOS) and staff satisfaction.17 However, these studies relied on unadjusted before-after comparisons and lacked methodological rigor to draw robust conclusions. More recently, a few studies have evaluated care outcomes associated with hospitalists using administrative databases, which attempted to control for potential confounding factors.18-21
While these studies are beginning to shed some light on the impact of hospital medicine programs in Canada, there are a number of issues that limit their generalizability. For example, the majority of studies to date focus on hospital medicine programs in Canada’s largest province (Ontario), and most describe experiences from single institutions. Since each of the 13 provincial and territorial governments organizes its health care system differently,22 results from 1 province may not be generalizable to other parts of the country. Moreover, hospitalists in Ontario are more diverse in their training backgrounds, with a larger percentage having trained in general internal medicine (IM), as compared to other parts of Canada, where the majority of hospitalists are overwhelmingly trained as FPs.3
We aimed to study care outcomes associated with a network of hospitalist services compared to “traditional” providers (community-based FPs and IM specialists) in a large integrated health care system in the province of British Columbia in western Canada. The hospital medicine services in this network span a range of community and academic hospitals, and collectively constitute 1 of the largest regional programs in the country. This provides a unique opportunity to understand the impact of hospitalists on outcome measures across a range of acute care institutions.
Methods
Setting and Population
Fraser Health Authority is 1 of 5 regional health authorities in British Columbia that emerged in 2001.23,24 It operates a network of hospitalist programs in 10 of its 12 acute care hospitals. In addition to hospitalists, there are a variable number of “traditional” physician providers who continue to act as MRPs. These include community-based FPs who continue to see their own patients in the hospital, either as part of a solo-practice model or a clinic-based call group. There are also a number of general internists and other subspecialists who accept MRP roles for general medicine patients who may present with higher-acuity conditions. As a result, patients requiring hospitalization due to nonsurgical or noncritical care conditions at each Fraser Health hospital may be cared for by a physician belonging to 1 of 3 groups, depending on local circumstances: an FP, a hospitalist, or an internist.
Inclusion and Exclusion Criteria
In order to evaluate comparative outcomes associated with hospitalist care, we included all patients admitted to a physician in each of the 3 provider groups between April 1, 2012, and March 31, 2018. We chose this time period for 2 reasons: first, we wanted to ensure comparability over an extended period of time, given the methodological changes implemented in 2009 by the Canadian Institute for Health Information (CIHI), the federal organization in the country responsible for setting standards for health care measures.25 Second, previous internal reviews had suggested that data quality prior to this year was inconsistent. We only considered hospitalizations where patients were admitted to and discharged by the same service, and excluded 2 acute care facilities and 1 free-standing rehabilitation facility without a hospitalist service during this period. We also excluded patients who resided in a location beyond the geographic catchment area of Fraser Health. Further details about data collection are outlined in the Appendix.
Measures
We used the framework developed by White and Glazier26 to inform the selection of our outcome measures, as well as relevant variables that may impact them. This framework proposes that the design of the inpatient care model (structures and processes of care) directly affects care outcomes. The model also proposes that patient and provider attributes can modulate this relationship, and suggests that a comprehensive evaluation of hospitalist performance needs to take these factors into account. We identified average total LOS, 30-day readmission rate, in-hospital mortality, and hospital standardized mortality ratio (HSMR)27 as primary outcome measures. HSMR is defined as actual over expected mortality and is measured by CIHI through a formula that takes into account patient illness attributes (eg, the most responsible diagnosis, comorbidity levels) and baseline population mortality rates.27 We chose these measures because they are clinically relevant and easy to obtain and have been utilized in previous similar studies in Canada and the United States.18-21,26
Statistical Analysis
Baseline demographic and clinical differences in patient outcomes were examined using independent t-tests or chi-square tests. Furthermore, baseline differences based on provider groups were explored using analysis of variance or chi-square tests. Multiple logistic regression analyses were completed to determine the relationship between provider groups and readmission and mortality, while the relationship between provider groups and hospital LOS was determined with generalized linear regression (using gamma distribution and a log link). Gamma distribution with a log link analysis is appropriate with outcome measures that are positively skewed (eg, hospital LOS). It assumes that data are sampled from an exponential family of distributions, thus mimicking a log-normal distribution, and minimizes estimation bias and standard errors. These analyses were completed while controlling for the effects of age, gender, and other potential confounding factors.
We initially attempted to control for case mix by incorporating case-mix groups (CMGs) in our multivariate analysis. However, we identified 475 CMGs with at least 1 patient in our study population. We then explored the inclusion of major clinical categories (MCCs) that broadly group CMGs into various higher order/organ-system level categories (eg, diseases of the respiratory system); however, we could not aggregate them into sufficiently homogenous groups to be entered into regression models. Instead, we conducted subgroup analyses on patients in our study population who were hospitalized with 1 of the following 3 CMGs: chronic obstructive pulmonary disease (COPD, n = 11,404 patients), congestive heart failure without coronary angiography (CHF, n = 7680), and pneumonia (itself an aggregate of 3 separate CMGs: aspiration pneumonia, bacterial pneumonia, viral/unspecified pneumonia, n = 11,155). We chose these CMGs as they are among the top 8 presentations for all 3 provider groups.
For all outcome measures, we excluded atypical patients (defined by CIHI as those with atypically long stays) and patients who had been transferred between facilities. For the readmission analysis, we also excluded patients who died in the hospital (Appendix A). Data analyses were completed in IBM SPSS, version 21. For all analyses, significance was determined using 2-tailed test and alpha < 0.05.
Ethics
The Fraser Health Department of Research and Evaluation reviewed this project to determine need for formal Ethics Review Board review, and granted an exemption based on institutional guidelines for program evaluations.
Results
A total of 132,178 patients were admitted to and discharged by 1 of the 3 study provider groups during the study period, accounting for a total of 248,412 hospitalizations. After excluding patients cared for in Fraser Health facilities without a hospitalist service and those who resided in a geographic area beyond Fraser Health, a total of 224,214 admissions were included in the final analysis.
Patient Characteristics
The demographic and clinical characteristics of patients by provider group are summarized in Table 1. Patients admitted to IM providers were substantially younger than those admitted to either FPs or hospitalists (61.00 vs 70.86 and 71.22 years, respectively; P < 0.005). However, patients admitted to hospitalists had higher degrees of complexity (as measured by higher comorbidity levels, number of secondary diagnoses, and higher resource intensity weights [RIWs]; P < 000.1 for all comparisons). Overall, the most common CMGs seen by FPs and hospitalists were similar, while IM providers primarily saw patients with cardiac conditions (Table 2).
Trends Over Time
During the study period, the number of patients admitted to the hospitalist services increased by 24%, while admissions to FPs and IM providers declined steadily (Figure). During this time, LOS for hospitalists progressively declined, while LOS for FPs and IM providers increased. Similar trends were observed for measures of mortality, while readmission rates remained constant for FPs, despite a decline observed for other providers.
Mortality
Table 3 summarizes the relationship between provider groups and in-hospital mortality (n = 183,779). Controlling for other variables, patients admitted to FP and IM providers had higher odds of mortality when compared to hospitalists (odds ratio [OR] for FPs, 1.29; 95% confidence interval [CI], 1.21-1.37; OR for IM, 1.24; 95% CI, 1.15-1.33). Older age, higher comorbidity level, higher number of secondary diagnoses, higher use of hospital resources (as measured by RIWs), longer than expected hospital stay (as measured by conservable days), and male gender were also associated with higher mortality. Similarly, patients receiving palliative care and those who spent at least 1 day in a special care unit (critical care, observation, and monitored care units) also had higher odds of mortality. On the other hand, admission to nonteaching medium facilities and longer hospital stay were associated with lower mortality. Compared to the first year of this analysis, lower mortality rates were observed in subsequent fiscal years. Finally, there appear to be geographic variations in mortality within Fraser Health.
Our analysis of patients with COPD, CHF, and pneumonia showed mixed results (Table 4). Patients admitted to the FP provider group with CHF and pneumonia had higher mortality compared to hospitalists (OR for CHF, 1.77; 95% CI, 1.38-2.27; OR for pneumonia, 1.53; 95% CI, 1.25-1.88), with a similar but nonstatistically significant trend observed for patients with COPD (OR, 1.29; 95% CI, 0.99-1.70). On the other hand, the higher observed mortality associated with the IM provider group in the overall study population only persisted for patients with COPD (OR, 2.71; 95% CI, 1.94-3.80), with no statistically significant differences for patients with CHF (OR, 1.18; 95% CI, 0.84-1.65) and pneumonia (OR, 0.93; 95% CI, 0.69-1.25).
We also studied adjusted mortality as measured by HSMRs. Currently, our Health Information Management system calculates an HSMR value for each patient admitted to our acute care facilities using the methodology developed by CIHI. Prior internal audits demonstrated that our internal calculations closely approximate those reported nationally. Our analysis suggests that over time, HSMR rates for the 3 provider groups have diverged, with patients admitted to IM providers having a higher mortality rate than what would be expected based on the presenting clinical conditions and comorbidity levels (Figure, part D).
Readmission
The results of our multiple logistic regression for readmission are summarized in Table 5 (n = 166,042). The impact of provider group on 30-day readmission is mixed, with higher odds associated with FPs compared to hospitalists (OR, 1.27; 95% CI, 1.22-1.34) and lower odds associated with IM physicians (OR, 0.83; 95% CI, 0.79-0.87). Gender and RIW did not show any significant associations, but increasing age, higher number of secondary diagnoses, higher comorbidity levels, and longer than expected LOS (as measure by conservable days) were associated with higher odds of readmission. Conversely, longer hospitalization, admission to a large community hospital, palliative status, admission to a special care unit, geography, and fiscal year were associated with lower odds of readmission.
The above differences between provider groups were no longer consistently present when we analyzed patients presenting with COPD, CHF, and pneumonias (Table 6). Only patients admitted to the FP provider group with pneumonia had higher odds of readmission compared to hospitalists (OR, 1.27; 95% CI, 1.05-1.54). Conversely, only patients admitted to the IM provider group with CHF showed lower readmission (OR, 0.75; 95% CI, 0.62-0.92).
Total LOS
Results using generalized linear regressions for total LOS are presented in Table 7 (n = 183,779). Patients admitted to the IM provider group had significantly lower total LOS (mean, 5.13 days; 95% CI, 5.04-5.21) compared to the hospitalist (mean, 7.37 days; 95% CI, 7.26-7.49) and FP (mean, 7.30 days; 95% CI, 7.19-7.41) groups, with no significant differences between the latter 2 groups. Older patients, females, patients with higher comorbidity levels or number of secondary diagnoses, higher RIW, palliative patients, and discharge to a facility other than the patient’s home were associated with a significantly longer LOS. On the other hand, admission to nonteaching hospitals and admission to a special care unit was associated with lower LOS.
When we compared total LOS for patients admitted with COPD, CHF, and pneumonias, the same differences observed for the broader comparisons persisted: IM patients consistently showed shorter LOS compared to hospitalist patients, while LOS associated with FP patients was similar (Table 8).
Discussion
To our knowledge, our evaluation is the largest study to date designed to understand outcomes associated with hospitalist care in Canada. Our analyses suggest that patients admitted to our large network of hospitalist services present with clinical conditions that are very similar to those of general medicine patients in other Canadian provinces.28,29 They also show that patients cared for by hospitalists experience lower mortality rates compared to those cared for by FPs. Our findings are similar to previous studies, which have suggested a 12% to 75% reduction in odds of mortality associated with hospitalist care.18,19 These differences persisted even when we focused on patients presenting with specific clinical conditions (CHF, COPD, and pneumonias).
White and colleagues have previously demonstrated that generalist physicians who had higher volumes of inpatient care activity also had lower mortality rates compared to those who cared for hospitalized patients less frequently.19 An association between higher physician caseloads and better outcomes has been established for many surgical and medical conditions.30-32 Given that 85% of hospitalists in our program have post-graduate medical training in family medicine (internal department surveys, data not shown), it is less likely that training background can explain differences in outcomes. Instead, differences in patient volumes and the dedicated focus of hospitalists on acute care are likely more important contributors to lower mortality. In our program, a full-time hospitalist spends an average of 2000 hours annually providing services in the hospital setting. The continuous on-site presence of hospitalists enhances their clinical experience with regards to the management of common medical conditions, and increases their exposure to less common presentations of illnesses. The ability to respond to deteriorating patients in a timely manner may be another factor in explaining the differences in mortality rates between dedicated hospital-based generalist providers and similarly trained physicians with a primarily community-based focus.
In our study, hospitalist care was also broadly associated with lower mortality compared to the IM providers, although these differences were not consistently present when patients with specific diagnoses were compared. This may be partly explained by the relationship between caseload and outcomes, but other factors may also be important. For example, patients admitted by IM providers spend significantly more time in specialized units. They also predominantly present with cardiac conditions, and as such may have higher acuity levels and require more invasive interventions. While this may explain the higher observed mortality, a within-group comparison still suggests higher than expected mortality for IM patients. The HSMR methodology measures actual mortality rates compared to what would be expected based on clinical presentation and baseline population characteristics. Calculating HSMR is highly dependent on proper documentation and chart abstraction,33,34 and it is possible that some of the differences observed are due to incomplete physician documentation. However, a more in-depth analysis of care processes will be required to clarify the observed trends.
Compared to hospitalists, patients cared for by FPs also had higher odds of readmission within 30 days, which is consistent with prior studies.18,19 One of the criticisms of the hospitalist model has been the inherent discontinuity of care that is built into the model, which can contribute to suboptimal transitions of care between the acute and community settings.35 The expectation is that FPs who admit their own patients do not face this challenge, and as a result their patients should be readmitted less frequently after discharge. Our data and those from previous studies do not support this hypothesis. At the same time, when we studied patients with specific clinical diagnoses, only those hospitalized for pneumonias continued to demonstrate higher readmission odds. This suggests that hospital readmission rate is a complex measure that may be influenced by a multitude of hospital and community factors, and may be different for patients who present with different clinical diagnoses. Further research is required to better understand the relationship between provider type and experience with hospital readmission for patients with various clinical presentations.
Unlike the United States, where hospitalist care has been associated with reductions in LOS,26,36 studies in the Canadian health care setting have shown mixed results.17-21 In our evaluation, hospitalist care is not associated with reductions in total LOS compared to care provided by FPs or IM physicians. This could be due to a number of factors. First, unlike FPs, who know their patients, hospitalists may have a more conservative risk tolerance in discharging patients with whom they are not familiar. Similarly, physicians who have trained in IM may have a lower threshold for discharging patients than hospitalists, whose training background is mainly rooted in family medicine.3 Second, discontinuity of care has been associated with longer LOS for hospitalized patients.37,38 Hospitalists generally work for 7- to 10-day rotations. As a result, a patient may see a number of different hospitalists during the same hospital stay, which could nullify any gains in LOS that may be expected from better familiarity with hospital processes. Third, whereas a FP or an internist may only have a few inpatients under their care at any given time, each hospitalist typically cares for 17 to 22 patients every day. Increasing hospitalist workload has been shown to negatively impact LOS and may result in lower efficiency.39 Finally, many patients in our health system who require more time to recuperate or need complex discharge planning are usually transferred to the care of the hospitalist service from other services, or are preferentially admitted to hospitalists from the emergency department. As a result, hospitalists may look after a disproportionately higher number of long-stay patients. Despite all this, hospitalists in our population perform similarly to FPs, regardless of the clinical diagnoses of hospitalized patients.
Our study has a number of notable limitations. First, we used administrative data to conduct our evaluation and could only control for factors that are available in our data systems. As a result, some potential confounders may not have been taken into consideration. For example, our databases do not contain provider characteristics (eg, age, years of clinical experience) that have been deemed to be relevant by White and Glazier.26 Similarly, we did not have all the necessary information about the characteristics of the various MRP programs (eg, number of physicians involved in group practices, the schedule model of community FP call groups) and were not able to account for the potential impact of these on observed outcomes. Second, although our findings mirror prior studies from other parts of Canada, they may not be applicable to hospitalist programs in other jurisdictions or in health systems that are not regionalized or integrated. Third, our IM provider group is heterogeneous, with a number of different IM subspecialties (cardiologists, gastroenterologists, general internists) grouped under the IM category in our database. As a result, comparisons between the IM provider group and the other 2 provider groups, which are more homogenous, should be interpreted with caution.
Finally, we included only patients admitted to facilities in which a hospitalist service existed during the study period. As a result, a medium-size community hospital without a hospitalist service where patients are cared for exclusively by FPs and IM physicians was not included in the comparisons, and in 4 of the 10 facilities included, the number of FP patients was less than 10% of total hospitalized patients at the site (Appendix A). This may have resulted in an under-representation of FP patients.
Conclusion
Debates about the merits of the hospitalist model in Canada continue, and are in part fueled by a paucity of robust evidence about its impact on care outcomes compared to more traditional ways of providing inpatient care. In our evaluation, care provided by hospitalists is associated with lower mortality and readmission rates, despite similar LOS compared with FPs. Hospitalist care is also associated with lower mortality compared to IM providers. Hospitalists also demonstrated progressive improvement over time, with decreasing LOS and mortality rates and a stable readmission rate. Our results suggest that physicians with a focus on inpatient care can have positive contributions to quality and efficiency of care in Canada.
Corresponding author: Vandad Yousefi MD, CCFP, FHM, Fraser Health Authority, 400, 13450–102 Avenue, Surrey BC V3T 0H1, Canada.
Financial disclosures: None.
1. Kisuule F, Howell E. Hospital medicine beyond the United States. Int J Gen Med. 2018;11:65-71.
2. Yousefi V, Wilton D. Dedesigning hospital care: learning from the experience of hospital medicine in Canada. J Global Health Care Syst. 2011;1(3).
3. Soong C, Fan E, Howell E, et al. Characteristics of hospitalists and hospitalist programs in the United States and Canada. J Clin Outcomes Manag. 2009;16:69-76.
4. Yousefi V. How Canadian hospitalists spend their time - A work-sampling study within a hospital medicine program in Ontario. J Clin Outcomes Manag. 2011;18:159-166.
5. Wilson G. Are inpatients’ needs better served by hospitalists than by their family doctors? No. Can Fam Physician. 2008;54:1101-1103.
6. Samoil D. Are inpatients’ needs better served by hospitalists than by their family doctors: Yes? Can Fam Physician. 2008;54:1100-1101.
7. Nicolson B. Where’s Marcus Welby when you need him? BC Medical J. 2016;58:63-64.
8. Lemire F. Enhanced skills in family medicine: Update. Can Fam Physician. 2018;64:160.
9. Lerner J. Wanting family medicine without primary care. Can Fam Physician. 2018; 64:155.
10. Canadian Society of Hospital Medicine. Core Competencies in Hospital Medicine - Care of the Medical Inpatient. 2015.
11. Redelmeier DA. A Canadian perspective on the American hospitalist movement. Arch Intern Med. 1999;159:1665-1668.
12. Ghali WA, Greenberg PB, Mejia R, et al. International perspectives on general internal medicine and the case for “globalization” of a discipline. J Gen Intern Med. 2006;21:197-200.
13. Day A, MacMillan L. Neglect of the inpatient: The hospitalist movement in Canada responds. Hosp Q. 2001;4:36.
14. Sullivan P. Enter the hospitalist: New type of patient creating a new type of specialist. CMAJ. 2000;162:1345-1346.
15. Chan BTB. The declining comprehensiveness of primary care. CMAJ. 2002;166:429-434.
16. Abenhaim HA, Kahn SR, Raffoul J, Becker MR. Program description: A hospitalist-run, medical short-stay unit in a teaching hospital. CMAJ. 2000;163:1477-1480.
17. McGowan B, Nightingale M. The hospitalist program a new specialty on the horizon in acute care medicine a hospital case study. BC Med J. 2003;45:391-394.
18. Yousefi V, Chong C. Does implementation of a hospitalist program in a Canadian community hospital improve measures of quality of care and utilization? An observational comparative analysis of hospitalists vs. traditional care providers. BMC Health Serv Res. 2013;13:204.
19. White HL. Assessing the prevalence, penetration and performance of hospital physicians in Ontario: Implications for the quality and efficiency of inpatient care. ProQuest Dissertations Publishing; 2016.
20. Gutierrez CA, Norris M, Chail M. Impact of a newly established hospitalist training program on patient LOS and RIW. Poster presented at the 9th Annual Canadian Society of Hospital Medicine Conference, September 23-25, 2011; Banff, Alberta.
21. Seth P, Nicholson K, Habbous S, Menard J. Implementation of a hospitalist medicine model in a full-service community hospital: Examining impact two years post-implementation on health resource use andpatient satisfaction. Poster presented at the 13th Annual Canadian Society of Hospital Medicine Conference. 2015; Niagara Falls, Ontario.
22. Lewis S. A system in name only--access, variation, and reform in Canada’s provinces. N Engl J Med. 2015;372:497-500.
23. Lewis S, Kouri D. Regionalization: Making sense of the Canadian experience. Healthcare Papers. 2004;5:12-31.
24. Fraser Health Authority. About Fraser health. www.fraserhealth.ca/about-us/about-fraser-health#.XFJrl9JKiUk. Updated 2018. Accessed January 30, 2019.
25. Canadian Institute for Health Information. CMG+. https://www.cihi.ca/en/cmg. Accessed January 30, 2019.
26. White HL, Glazier RH. Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9:58.
27. Canadian Institute for Health Information. Hospital standardized mortality ratio technical notes. 2008. www.cihi.ca/sites/default/files/document/hsmr-tech-notes_en_0.pdf.
28. McAlister FA, Youngson E, Bakal JA, et al. Physician experience and outcomes among patients admitted to general internal medicine teaching wards. CMAJ. 2015;187:1041-1048.
29. Verma AA, Guo Y, Kwan JL, et al. Patient characteristics, resource use and outcomes associated with general internal medicine hospital care: The general medicine inpatient initiative (GEMINI) retrospective cohort study. CMAJ Open. 2017;5:E849.
30. Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: A systematic review of systematic reviews. Syst Rev. 2016;5:204.
31. Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511-520.
32. Chen CH, Chen YH, Lin HC, Lin HC. Association between physician caseload and patient outcome for sepsis treatment. Infect Control Hosp Epidemiol. 2009;30:556-562.
33. van Gestel YR, Lemmens VE, Lingsma HF, et al. The hospital standardized mortality ratio fallacy: A narrative review. Med Care. 2012;50:662-667.
34. Scott IA, Brand CA, Phelps GE, et al. Using hospital standardised mortality ratios to assess quality of care—proceed with extreme caution. Med J Aust. 2011; 194:645-648.
35. Wachter RM. Hospitalists in the United States -- mission accomplished or work in progress? N Engl J Med. 2004;350:1935-1936.
36. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84:248-254.
37. Chandra S, Wright SM, Howell EE. The creating incentives and continuity leading to efficiency staffing model: A quality improvement initiative in hospital medicine. Mayo Clin Proc. 2012;87:364-371.
38. Epstein K, Juarez E, Epstein A, et al. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5:335-338.
39. Elliott DJ, Young RS, Brice J, et al. Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174:786-793.
From the Fraser Health Authority, Surrey, British Columbia, Canada.
Abstract
- Objective: To study care outcomes associated with a network of hospitalist services compared to traditional providers.
- Design: Retrospective review of administrative data.
- Setting and participants: Patients from a large integrated health care system in British Columbia in western Canada admitted and cared for by 3 provider groups between April 1, 2012, and March 31, 2018: hospitalists, family physicians (FP), and internal medicine (IM) physicians:
- Measurements: Average total length of stay (LOS), 30-day readmission, in-hospital mortality, and hospital standardized mortality ratio (HSMR) were the study outcome measures. Multiple logistic regression or generalized regression were completed to determine the relationship between provider groups and outcomes.
- Results: A total of 248,412 hospitalizations were included. Compared to patients admitted to hospitalists, patients admitted to other providers had higher odds of mortality (odds ratio [OR] for FP, 1.29; 95% confidence interval [CI], 1.21-1.37; OR for IM, 1.24; 95% CI, 1.15-1.33). Compared to hospitalist care, FP care was associated with higher readmission (OR, 1.27; 95% CI, 1.22-1.33), while IM care showed lower odds of readmission (OR, 0.83; 95% CI, 0.79-0.87). Patients admitted to the IM group had significantly lower total LOS (mean, 5.13 days; 95% CI, 5.04-5.21) compared to patients admitted to hospitalists (mean, 7.37 days; CI, 7.26-7.49) and FPs (mean, 7.30 days; 95% CI, 7.19-7.41). In a subgroup analysis of patients presenting with congestive heart failure, chronic obstructive pulmonary disease, and pneumonia, these general tendencies broadly persisted for mortality and LOS comparisons between FPs and hospitalists, but results were mixed for hospital readmissions.
- Conclusion: Care provided by hospitalists was associated with lower mortality and readmission rates compared with care provided by FPs, despite similar LOS. These findings may reflect differences in volume of services delivered by individual physicians, on-site availability to address urgent medical issues, and evolving specialization of clinical and nonclinical care processes in the acute care setting.
Keywords: hospital medicine; length of stay; readmission; mortality.
The hospitalist model of care has undergone rapid growth globally in recent years.1 The first hospitalist programs in Canada began around the same time as those in the United States and share many similarities in design and operations with their counterparts.2-4 However, unlike in the United States, where the hospitalist model has successfully established itself as an emerging specialty, debates about the merits of the model and its value proposition continue among Canadian observers.5-9
Historically, the type of physicians who acted as the most responsible provider (MRP) in Canadian hospitals depended on setting and geography.10 In large urban areas, groups of general internists or specialists have historically looked after general medicine patients as part of university-affiliated teaching services.11,12 Patients admitted to community hospitals have traditionally been cared for by their own primary care providers, typically general practitioners or family physicians (FPs). In the mid-1990s, many primary care providers in urban centers began to withdraw from inpatient care and primarily focused their practices in the outpatient setting.13-15 Hospitalist programs emerged as health care administrators sought to fill the resulting gap in MRP coverage.2,10
To date, attempts to understand the impact of hospitalist programs in Canada have been limited. A number of early studies aimed to describe16 the role of hospitalists in Canada and suggested improvements in length of stay (LOS) and staff satisfaction.17 However, these studies relied on unadjusted before-after comparisons and lacked methodological rigor to draw robust conclusions. More recently, a few studies have evaluated care outcomes associated with hospitalists using administrative databases, which attempted to control for potential confounding factors.18-21
While these studies are beginning to shed some light on the impact of hospital medicine programs in Canada, there are a number of issues that limit their generalizability. For example, the majority of studies to date focus on hospital medicine programs in Canada’s largest province (Ontario), and most describe experiences from single institutions. Since each of the 13 provincial and territorial governments organizes its health care system differently,22 results from 1 province may not be generalizable to other parts of the country. Moreover, hospitalists in Ontario are more diverse in their training backgrounds, with a larger percentage having trained in general internal medicine (IM), as compared to other parts of Canada, where the majority of hospitalists are overwhelmingly trained as FPs.3
We aimed to study care outcomes associated with a network of hospitalist services compared to “traditional” providers (community-based FPs and IM specialists) in a large integrated health care system in the province of British Columbia in western Canada. The hospital medicine services in this network span a range of community and academic hospitals, and collectively constitute 1 of the largest regional programs in the country. This provides a unique opportunity to understand the impact of hospitalists on outcome measures across a range of acute care institutions.
Methods
Setting and Population
Fraser Health Authority is 1 of 5 regional health authorities in British Columbia that emerged in 2001.23,24 It operates a network of hospitalist programs in 10 of its 12 acute care hospitals. In addition to hospitalists, there are a variable number of “traditional” physician providers who continue to act as MRPs. These include community-based FPs who continue to see their own patients in the hospital, either as part of a solo-practice model or a clinic-based call group. There are also a number of general internists and other subspecialists who accept MRP roles for general medicine patients who may present with higher-acuity conditions. As a result, patients requiring hospitalization due to nonsurgical or noncritical care conditions at each Fraser Health hospital may be cared for by a physician belonging to 1 of 3 groups, depending on local circumstances: an FP, a hospitalist, or an internist.
Inclusion and Exclusion Criteria
In order to evaluate comparative outcomes associated with hospitalist care, we included all patients admitted to a physician in each of the 3 provider groups between April 1, 2012, and March 31, 2018. We chose this time period for 2 reasons: first, we wanted to ensure comparability over an extended period of time, given the methodological changes implemented in 2009 by the Canadian Institute for Health Information (CIHI), the federal organization in the country responsible for setting standards for health care measures.25 Second, previous internal reviews had suggested that data quality prior to this year was inconsistent. We only considered hospitalizations where patients were admitted to and discharged by the same service, and excluded 2 acute care facilities and 1 free-standing rehabilitation facility without a hospitalist service during this period. We also excluded patients who resided in a location beyond the geographic catchment area of Fraser Health. Further details about data collection are outlined in the Appendix.
Measures
We used the framework developed by White and Glazier26 to inform the selection of our outcome measures, as well as relevant variables that may impact them. This framework proposes that the design of the inpatient care model (structures and processes of care) directly affects care outcomes. The model also proposes that patient and provider attributes can modulate this relationship, and suggests that a comprehensive evaluation of hospitalist performance needs to take these factors into account. We identified average total LOS, 30-day readmission rate, in-hospital mortality, and hospital standardized mortality ratio (HSMR)27 as primary outcome measures. HSMR is defined as actual over expected mortality and is measured by CIHI through a formula that takes into account patient illness attributes (eg, the most responsible diagnosis, comorbidity levels) and baseline population mortality rates.27 We chose these measures because they are clinically relevant and easy to obtain and have been utilized in previous similar studies in Canada and the United States.18-21,26
Statistical Analysis
Baseline demographic and clinical differences in patient outcomes were examined using independent t-tests or chi-square tests. Furthermore, baseline differences based on provider groups were explored using analysis of variance or chi-square tests. Multiple logistic regression analyses were completed to determine the relationship between provider groups and readmission and mortality, while the relationship between provider groups and hospital LOS was determined with generalized linear regression (using gamma distribution and a log link). Gamma distribution with a log link analysis is appropriate with outcome measures that are positively skewed (eg, hospital LOS). It assumes that data are sampled from an exponential family of distributions, thus mimicking a log-normal distribution, and minimizes estimation bias and standard errors. These analyses were completed while controlling for the effects of age, gender, and other potential confounding factors.
We initially attempted to control for case mix by incorporating case-mix groups (CMGs) in our multivariate analysis. However, we identified 475 CMGs with at least 1 patient in our study population. We then explored the inclusion of major clinical categories (MCCs) that broadly group CMGs into various higher order/organ-system level categories (eg, diseases of the respiratory system); however, we could not aggregate them into sufficiently homogenous groups to be entered into regression models. Instead, we conducted subgroup analyses on patients in our study population who were hospitalized with 1 of the following 3 CMGs: chronic obstructive pulmonary disease (COPD, n = 11,404 patients), congestive heart failure without coronary angiography (CHF, n = 7680), and pneumonia (itself an aggregate of 3 separate CMGs: aspiration pneumonia, bacterial pneumonia, viral/unspecified pneumonia, n = 11,155). We chose these CMGs as they are among the top 8 presentations for all 3 provider groups.
For all outcome measures, we excluded atypical patients (defined by CIHI as those with atypically long stays) and patients who had been transferred between facilities. For the readmission analysis, we also excluded patients who died in the hospital (Appendix A). Data analyses were completed in IBM SPSS, version 21. For all analyses, significance was determined using 2-tailed test and alpha < 0.05.
Ethics
The Fraser Health Department of Research and Evaluation reviewed this project to determine need for formal Ethics Review Board review, and granted an exemption based on institutional guidelines for program evaluations.
Results
A total of 132,178 patients were admitted to and discharged by 1 of the 3 study provider groups during the study period, accounting for a total of 248,412 hospitalizations. After excluding patients cared for in Fraser Health facilities without a hospitalist service and those who resided in a geographic area beyond Fraser Health, a total of 224,214 admissions were included in the final analysis.
Patient Characteristics
The demographic and clinical characteristics of patients by provider group are summarized in Table 1. Patients admitted to IM providers were substantially younger than those admitted to either FPs or hospitalists (61.00 vs 70.86 and 71.22 years, respectively; P < 0.005). However, patients admitted to hospitalists had higher degrees of complexity (as measured by higher comorbidity levels, number of secondary diagnoses, and higher resource intensity weights [RIWs]; P < 000.1 for all comparisons). Overall, the most common CMGs seen by FPs and hospitalists were similar, while IM providers primarily saw patients with cardiac conditions (Table 2).
Trends Over Time
During the study period, the number of patients admitted to the hospitalist services increased by 24%, while admissions to FPs and IM providers declined steadily (Figure). During this time, LOS for hospitalists progressively declined, while LOS for FPs and IM providers increased. Similar trends were observed for measures of mortality, while readmission rates remained constant for FPs, despite a decline observed for other providers.
Mortality
Table 3 summarizes the relationship between provider groups and in-hospital mortality (n = 183,779). Controlling for other variables, patients admitted to FP and IM providers had higher odds of mortality when compared to hospitalists (odds ratio [OR] for FPs, 1.29; 95% confidence interval [CI], 1.21-1.37; OR for IM, 1.24; 95% CI, 1.15-1.33). Older age, higher comorbidity level, higher number of secondary diagnoses, higher use of hospital resources (as measured by RIWs), longer than expected hospital stay (as measured by conservable days), and male gender were also associated with higher mortality. Similarly, patients receiving palliative care and those who spent at least 1 day in a special care unit (critical care, observation, and monitored care units) also had higher odds of mortality. On the other hand, admission to nonteaching medium facilities and longer hospital stay were associated with lower mortality. Compared to the first year of this analysis, lower mortality rates were observed in subsequent fiscal years. Finally, there appear to be geographic variations in mortality within Fraser Health.
Our analysis of patients with COPD, CHF, and pneumonia showed mixed results (Table 4). Patients admitted to the FP provider group with CHF and pneumonia had higher mortality compared to hospitalists (OR for CHF, 1.77; 95% CI, 1.38-2.27; OR for pneumonia, 1.53; 95% CI, 1.25-1.88), with a similar but nonstatistically significant trend observed for patients with COPD (OR, 1.29; 95% CI, 0.99-1.70). On the other hand, the higher observed mortality associated with the IM provider group in the overall study population only persisted for patients with COPD (OR, 2.71; 95% CI, 1.94-3.80), with no statistically significant differences for patients with CHF (OR, 1.18; 95% CI, 0.84-1.65) and pneumonia (OR, 0.93; 95% CI, 0.69-1.25).
We also studied adjusted mortality as measured by HSMRs. Currently, our Health Information Management system calculates an HSMR value for each patient admitted to our acute care facilities using the methodology developed by CIHI. Prior internal audits demonstrated that our internal calculations closely approximate those reported nationally. Our analysis suggests that over time, HSMR rates for the 3 provider groups have diverged, with patients admitted to IM providers having a higher mortality rate than what would be expected based on the presenting clinical conditions and comorbidity levels (Figure, part D).
Readmission
The results of our multiple logistic regression for readmission are summarized in Table 5 (n = 166,042). The impact of provider group on 30-day readmission is mixed, with higher odds associated with FPs compared to hospitalists (OR, 1.27; 95% CI, 1.22-1.34) and lower odds associated with IM physicians (OR, 0.83; 95% CI, 0.79-0.87). Gender and RIW did not show any significant associations, but increasing age, higher number of secondary diagnoses, higher comorbidity levels, and longer than expected LOS (as measure by conservable days) were associated with higher odds of readmission. Conversely, longer hospitalization, admission to a large community hospital, palliative status, admission to a special care unit, geography, and fiscal year were associated with lower odds of readmission.
The above differences between provider groups were no longer consistently present when we analyzed patients presenting with COPD, CHF, and pneumonias (Table 6). Only patients admitted to the FP provider group with pneumonia had higher odds of readmission compared to hospitalists (OR, 1.27; 95% CI, 1.05-1.54). Conversely, only patients admitted to the IM provider group with CHF showed lower readmission (OR, 0.75; 95% CI, 0.62-0.92).
Total LOS
Results using generalized linear regressions for total LOS are presented in Table 7 (n = 183,779). Patients admitted to the IM provider group had significantly lower total LOS (mean, 5.13 days; 95% CI, 5.04-5.21) compared to the hospitalist (mean, 7.37 days; 95% CI, 7.26-7.49) and FP (mean, 7.30 days; 95% CI, 7.19-7.41) groups, with no significant differences between the latter 2 groups. Older patients, females, patients with higher comorbidity levels or number of secondary diagnoses, higher RIW, palliative patients, and discharge to a facility other than the patient’s home were associated with a significantly longer LOS. On the other hand, admission to nonteaching hospitals and admission to a special care unit was associated with lower LOS.
When we compared total LOS for patients admitted with COPD, CHF, and pneumonias, the same differences observed for the broader comparisons persisted: IM patients consistently showed shorter LOS compared to hospitalist patients, while LOS associated with FP patients was similar (Table 8).
Discussion
To our knowledge, our evaluation is the largest study to date designed to understand outcomes associated with hospitalist care in Canada. Our analyses suggest that patients admitted to our large network of hospitalist services present with clinical conditions that are very similar to those of general medicine patients in other Canadian provinces.28,29 They also show that patients cared for by hospitalists experience lower mortality rates compared to those cared for by FPs. Our findings are similar to previous studies, which have suggested a 12% to 75% reduction in odds of mortality associated with hospitalist care.18,19 These differences persisted even when we focused on patients presenting with specific clinical conditions (CHF, COPD, and pneumonias).
White and colleagues have previously demonstrated that generalist physicians who had higher volumes of inpatient care activity also had lower mortality rates compared to those who cared for hospitalized patients less frequently.19 An association between higher physician caseloads and better outcomes has been established for many surgical and medical conditions.30-32 Given that 85% of hospitalists in our program have post-graduate medical training in family medicine (internal department surveys, data not shown), it is less likely that training background can explain differences in outcomes. Instead, differences in patient volumes and the dedicated focus of hospitalists on acute care are likely more important contributors to lower mortality. In our program, a full-time hospitalist spends an average of 2000 hours annually providing services in the hospital setting. The continuous on-site presence of hospitalists enhances their clinical experience with regards to the management of common medical conditions, and increases their exposure to less common presentations of illnesses. The ability to respond to deteriorating patients in a timely manner may be another factor in explaining the differences in mortality rates between dedicated hospital-based generalist providers and similarly trained physicians with a primarily community-based focus.
In our study, hospitalist care was also broadly associated with lower mortality compared to the IM providers, although these differences were not consistently present when patients with specific diagnoses were compared. This may be partly explained by the relationship between caseload and outcomes, but other factors may also be important. For example, patients admitted by IM providers spend significantly more time in specialized units. They also predominantly present with cardiac conditions, and as such may have higher acuity levels and require more invasive interventions. While this may explain the higher observed mortality, a within-group comparison still suggests higher than expected mortality for IM patients. The HSMR methodology measures actual mortality rates compared to what would be expected based on clinical presentation and baseline population characteristics. Calculating HSMR is highly dependent on proper documentation and chart abstraction,33,34 and it is possible that some of the differences observed are due to incomplete physician documentation. However, a more in-depth analysis of care processes will be required to clarify the observed trends.
Compared to hospitalists, patients cared for by FPs also had higher odds of readmission within 30 days, which is consistent with prior studies.18,19 One of the criticisms of the hospitalist model has been the inherent discontinuity of care that is built into the model, which can contribute to suboptimal transitions of care between the acute and community settings.35 The expectation is that FPs who admit their own patients do not face this challenge, and as a result their patients should be readmitted less frequently after discharge. Our data and those from previous studies do not support this hypothesis. At the same time, when we studied patients with specific clinical diagnoses, only those hospitalized for pneumonias continued to demonstrate higher readmission odds. This suggests that hospital readmission rate is a complex measure that may be influenced by a multitude of hospital and community factors, and may be different for patients who present with different clinical diagnoses. Further research is required to better understand the relationship between provider type and experience with hospital readmission for patients with various clinical presentations.
Unlike the United States, where hospitalist care has been associated with reductions in LOS,26,36 studies in the Canadian health care setting have shown mixed results.17-21 In our evaluation, hospitalist care is not associated with reductions in total LOS compared to care provided by FPs or IM physicians. This could be due to a number of factors. First, unlike FPs, who know their patients, hospitalists may have a more conservative risk tolerance in discharging patients with whom they are not familiar. Similarly, physicians who have trained in IM may have a lower threshold for discharging patients than hospitalists, whose training background is mainly rooted in family medicine.3 Second, discontinuity of care has been associated with longer LOS for hospitalized patients.37,38 Hospitalists generally work for 7- to 10-day rotations. As a result, a patient may see a number of different hospitalists during the same hospital stay, which could nullify any gains in LOS that may be expected from better familiarity with hospital processes. Third, whereas a FP or an internist may only have a few inpatients under their care at any given time, each hospitalist typically cares for 17 to 22 patients every day. Increasing hospitalist workload has been shown to negatively impact LOS and may result in lower efficiency.39 Finally, many patients in our health system who require more time to recuperate or need complex discharge planning are usually transferred to the care of the hospitalist service from other services, or are preferentially admitted to hospitalists from the emergency department. As a result, hospitalists may look after a disproportionately higher number of long-stay patients. Despite all this, hospitalists in our population perform similarly to FPs, regardless of the clinical diagnoses of hospitalized patients.
Our study has a number of notable limitations. First, we used administrative data to conduct our evaluation and could only control for factors that are available in our data systems. As a result, some potential confounders may not have been taken into consideration. For example, our databases do not contain provider characteristics (eg, age, years of clinical experience) that have been deemed to be relevant by White and Glazier.26 Similarly, we did not have all the necessary information about the characteristics of the various MRP programs (eg, number of physicians involved in group practices, the schedule model of community FP call groups) and were not able to account for the potential impact of these on observed outcomes. Second, although our findings mirror prior studies from other parts of Canada, they may not be applicable to hospitalist programs in other jurisdictions or in health systems that are not regionalized or integrated. Third, our IM provider group is heterogeneous, with a number of different IM subspecialties (cardiologists, gastroenterologists, general internists) grouped under the IM category in our database. As a result, comparisons between the IM provider group and the other 2 provider groups, which are more homogenous, should be interpreted with caution.
Finally, we included only patients admitted to facilities in which a hospitalist service existed during the study period. As a result, a medium-size community hospital without a hospitalist service where patients are cared for exclusively by FPs and IM physicians was not included in the comparisons, and in 4 of the 10 facilities included, the number of FP patients was less than 10% of total hospitalized patients at the site (Appendix A). This may have resulted in an under-representation of FP patients.
Conclusion
Debates about the merits of the hospitalist model in Canada continue, and are in part fueled by a paucity of robust evidence about its impact on care outcomes compared to more traditional ways of providing inpatient care. In our evaluation, care provided by hospitalists is associated with lower mortality and readmission rates, despite similar LOS compared with FPs. Hospitalist care is also associated with lower mortality compared to IM providers. Hospitalists also demonstrated progressive improvement over time, with decreasing LOS and mortality rates and a stable readmission rate. Our results suggest that physicians with a focus on inpatient care can have positive contributions to quality and efficiency of care in Canada.
Corresponding author: Vandad Yousefi MD, CCFP, FHM, Fraser Health Authority, 400, 13450–102 Avenue, Surrey BC V3T 0H1, Canada.
Financial disclosures: None.
From the Fraser Health Authority, Surrey, British Columbia, Canada.
Abstract
- Objective: To study care outcomes associated with a network of hospitalist services compared to traditional providers.
- Design: Retrospective review of administrative data.
- Setting and participants: Patients from a large integrated health care system in British Columbia in western Canada admitted and cared for by 3 provider groups between April 1, 2012, and March 31, 2018: hospitalists, family physicians (FP), and internal medicine (IM) physicians:
- Measurements: Average total length of stay (LOS), 30-day readmission, in-hospital mortality, and hospital standardized mortality ratio (HSMR) were the study outcome measures. Multiple logistic regression or generalized regression were completed to determine the relationship between provider groups and outcomes.
- Results: A total of 248,412 hospitalizations were included. Compared to patients admitted to hospitalists, patients admitted to other providers had higher odds of mortality (odds ratio [OR] for FP, 1.29; 95% confidence interval [CI], 1.21-1.37; OR for IM, 1.24; 95% CI, 1.15-1.33). Compared to hospitalist care, FP care was associated with higher readmission (OR, 1.27; 95% CI, 1.22-1.33), while IM care showed lower odds of readmission (OR, 0.83; 95% CI, 0.79-0.87). Patients admitted to the IM group had significantly lower total LOS (mean, 5.13 days; 95% CI, 5.04-5.21) compared to patients admitted to hospitalists (mean, 7.37 days; CI, 7.26-7.49) and FPs (mean, 7.30 days; 95% CI, 7.19-7.41). In a subgroup analysis of patients presenting with congestive heart failure, chronic obstructive pulmonary disease, and pneumonia, these general tendencies broadly persisted for mortality and LOS comparisons between FPs and hospitalists, but results were mixed for hospital readmissions.
- Conclusion: Care provided by hospitalists was associated with lower mortality and readmission rates compared with care provided by FPs, despite similar LOS. These findings may reflect differences in volume of services delivered by individual physicians, on-site availability to address urgent medical issues, and evolving specialization of clinical and nonclinical care processes in the acute care setting.
Keywords: hospital medicine; length of stay; readmission; mortality.
The hospitalist model of care has undergone rapid growth globally in recent years.1 The first hospitalist programs in Canada began around the same time as those in the United States and share many similarities in design and operations with their counterparts.2-4 However, unlike in the United States, where the hospitalist model has successfully established itself as an emerging specialty, debates about the merits of the model and its value proposition continue among Canadian observers.5-9
Historically, the type of physicians who acted as the most responsible provider (MRP) in Canadian hospitals depended on setting and geography.10 In large urban areas, groups of general internists or specialists have historically looked after general medicine patients as part of university-affiliated teaching services.11,12 Patients admitted to community hospitals have traditionally been cared for by their own primary care providers, typically general practitioners or family physicians (FPs). In the mid-1990s, many primary care providers in urban centers began to withdraw from inpatient care and primarily focused their practices in the outpatient setting.13-15 Hospitalist programs emerged as health care administrators sought to fill the resulting gap in MRP coverage.2,10
To date, attempts to understand the impact of hospitalist programs in Canada have been limited. A number of early studies aimed to describe16 the role of hospitalists in Canada and suggested improvements in length of stay (LOS) and staff satisfaction.17 However, these studies relied on unadjusted before-after comparisons and lacked methodological rigor to draw robust conclusions. More recently, a few studies have evaluated care outcomes associated with hospitalists using administrative databases, which attempted to control for potential confounding factors.18-21
While these studies are beginning to shed some light on the impact of hospital medicine programs in Canada, there are a number of issues that limit their generalizability. For example, the majority of studies to date focus on hospital medicine programs in Canada’s largest province (Ontario), and most describe experiences from single institutions. Since each of the 13 provincial and territorial governments organizes its health care system differently,22 results from 1 province may not be generalizable to other parts of the country. Moreover, hospitalists in Ontario are more diverse in their training backgrounds, with a larger percentage having trained in general internal medicine (IM), as compared to other parts of Canada, where the majority of hospitalists are overwhelmingly trained as FPs.3
We aimed to study care outcomes associated with a network of hospitalist services compared to “traditional” providers (community-based FPs and IM specialists) in a large integrated health care system in the province of British Columbia in western Canada. The hospital medicine services in this network span a range of community and academic hospitals, and collectively constitute 1 of the largest regional programs in the country. This provides a unique opportunity to understand the impact of hospitalists on outcome measures across a range of acute care institutions.
Methods
Setting and Population
Fraser Health Authority is 1 of 5 regional health authorities in British Columbia that emerged in 2001.23,24 It operates a network of hospitalist programs in 10 of its 12 acute care hospitals. In addition to hospitalists, there are a variable number of “traditional” physician providers who continue to act as MRPs. These include community-based FPs who continue to see their own patients in the hospital, either as part of a solo-practice model or a clinic-based call group. There are also a number of general internists and other subspecialists who accept MRP roles for general medicine patients who may present with higher-acuity conditions. As a result, patients requiring hospitalization due to nonsurgical or noncritical care conditions at each Fraser Health hospital may be cared for by a physician belonging to 1 of 3 groups, depending on local circumstances: an FP, a hospitalist, or an internist.
Inclusion and Exclusion Criteria
In order to evaluate comparative outcomes associated with hospitalist care, we included all patients admitted to a physician in each of the 3 provider groups between April 1, 2012, and March 31, 2018. We chose this time period for 2 reasons: first, we wanted to ensure comparability over an extended period of time, given the methodological changes implemented in 2009 by the Canadian Institute for Health Information (CIHI), the federal organization in the country responsible for setting standards for health care measures.25 Second, previous internal reviews had suggested that data quality prior to this year was inconsistent. We only considered hospitalizations where patients were admitted to and discharged by the same service, and excluded 2 acute care facilities and 1 free-standing rehabilitation facility without a hospitalist service during this period. We also excluded patients who resided in a location beyond the geographic catchment area of Fraser Health. Further details about data collection are outlined in the Appendix.
Measures
We used the framework developed by White and Glazier26 to inform the selection of our outcome measures, as well as relevant variables that may impact them. This framework proposes that the design of the inpatient care model (structures and processes of care) directly affects care outcomes. The model also proposes that patient and provider attributes can modulate this relationship, and suggests that a comprehensive evaluation of hospitalist performance needs to take these factors into account. We identified average total LOS, 30-day readmission rate, in-hospital mortality, and hospital standardized mortality ratio (HSMR)27 as primary outcome measures. HSMR is defined as actual over expected mortality and is measured by CIHI through a formula that takes into account patient illness attributes (eg, the most responsible diagnosis, comorbidity levels) and baseline population mortality rates.27 We chose these measures because they are clinically relevant and easy to obtain and have been utilized in previous similar studies in Canada and the United States.18-21,26
Statistical Analysis
Baseline demographic and clinical differences in patient outcomes were examined using independent t-tests or chi-square tests. Furthermore, baseline differences based on provider groups were explored using analysis of variance or chi-square tests. Multiple logistic regression analyses were completed to determine the relationship between provider groups and readmission and mortality, while the relationship between provider groups and hospital LOS was determined with generalized linear regression (using gamma distribution and a log link). Gamma distribution with a log link analysis is appropriate with outcome measures that are positively skewed (eg, hospital LOS). It assumes that data are sampled from an exponential family of distributions, thus mimicking a log-normal distribution, and minimizes estimation bias and standard errors. These analyses were completed while controlling for the effects of age, gender, and other potential confounding factors.
We initially attempted to control for case mix by incorporating case-mix groups (CMGs) in our multivariate analysis. However, we identified 475 CMGs with at least 1 patient in our study population. We then explored the inclusion of major clinical categories (MCCs) that broadly group CMGs into various higher order/organ-system level categories (eg, diseases of the respiratory system); however, we could not aggregate them into sufficiently homogenous groups to be entered into regression models. Instead, we conducted subgroup analyses on patients in our study population who were hospitalized with 1 of the following 3 CMGs: chronic obstructive pulmonary disease (COPD, n = 11,404 patients), congestive heart failure without coronary angiography (CHF, n = 7680), and pneumonia (itself an aggregate of 3 separate CMGs: aspiration pneumonia, bacterial pneumonia, viral/unspecified pneumonia, n = 11,155). We chose these CMGs as they are among the top 8 presentations for all 3 provider groups.
For all outcome measures, we excluded atypical patients (defined by CIHI as those with atypically long stays) and patients who had been transferred between facilities. For the readmission analysis, we also excluded patients who died in the hospital (Appendix A). Data analyses were completed in IBM SPSS, version 21. For all analyses, significance was determined using 2-tailed test and alpha < 0.05.
Ethics
The Fraser Health Department of Research and Evaluation reviewed this project to determine need for formal Ethics Review Board review, and granted an exemption based on institutional guidelines for program evaluations.
Results
A total of 132,178 patients were admitted to and discharged by 1 of the 3 study provider groups during the study period, accounting for a total of 248,412 hospitalizations. After excluding patients cared for in Fraser Health facilities without a hospitalist service and those who resided in a geographic area beyond Fraser Health, a total of 224,214 admissions were included in the final analysis.
Patient Characteristics
The demographic and clinical characteristics of patients by provider group are summarized in Table 1. Patients admitted to IM providers were substantially younger than those admitted to either FPs or hospitalists (61.00 vs 70.86 and 71.22 years, respectively; P < 0.005). However, patients admitted to hospitalists had higher degrees of complexity (as measured by higher comorbidity levels, number of secondary diagnoses, and higher resource intensity weights [RIWs]; P < 000.1 for all comparisons). Overall, the most common CMGs seen by FPs and hospitalists were similar, while IM providers primarily saw patients with cardiac conditions (Table 2).
Trends Over Time
During the study period, the number of patients admitted to the hospitalist services increased by 24%, while admissions to FPs and IM providers declined steadily (Figure). During this time, LOS for hospitalists progressively declined, while LOS for FPs and IM providers increased. Similar trends were observed for measures of mortality, while readmission rates remained constant for FPs, despite a decline observed for other providers.
Mortality
Table 3 summarizes the relationship between provider groups and in-hospital mortality (n = 183,779). Controlling for other variables, patients admitted to FP and IM providers had higher odds of mortality when compared to hospitalists (odds ratio [OR] for FPs, 1.29; 95% confidence interval [CI], 1.21-1.37; OR for IM, 1.24; 95% CI, 1.15-1.33). Older age, higher comorbidity level, higher number of secondary diagnoses, higher use of hospital resources (as measured by RIWs), longer than expected hospital stay (as measured by conservable days), and male gender were also associated with higher mortality. Similarly, patients receiving palliative care and those who spent at least 1 day in a special care unit (critical care, observation, and monitored care units) also had higher odds of mortality. On the other hand, admission to nonteaching medium facilities and longer hospital stay were associated with lower mortality. Compared to the first year of this analysis, lower mortality rates were observed in subsequent fiscal years. Finally, there appear to be geographic variations in mortality within Fraser Health.
Our analysis of patients with COPD, CHF, and pneumonia showed mixed results (Table 4). Patients admitted to the FP provider group with CHF and pneumonia had higher mortality compared to hospitalists (OR for CHF, 1.77; 95% CI, 1.38-2.27; OR for pneumonia, 1.53; 95% CI, 1.25-1.88), with a similar but nonstatistically significant trend observed for patients with COPD (OR, 1.29; 95% CI, 0.99-1.70). On the other hand, the higher observed mortality associated with the IM provider group in the overall study population only persisted for patients with COPD (OR, 2.71; 95% CI, 1.94-3.80), with no statistically significant differences for patients with CHF (OR, 1.18; 95% CI, 0.84-1.65) and pneumonia (OR, 0.93; 95% CI, 0.69-1.25).
We also studied adjusted mortality as measured by HSMRs. Currently, our Health Information Management system calculates an HSMR value for each patient admitted to our acute care facilities using the methodology developed by CIHI. Prior internal audits demonstrated that our internal calculations closely approximate those reported nationally. Our analysis suggests that over time, HSMR rates for the 3 provider groups have diverged, with patients admitted to IM providers having a higher mortality rate than what would be expected based on the presenting clinical conditions and comorbidity levels (Figure, part D).
Readmission
The results of our multiple logistic regression for readmission are summarized in Table 5 (n = 166,042). The impact of provider group on 30-day readmission is mixed, with higher odds associated with FPs compared to hospitalists (OR, 1.27; 95% CI, 1.22-1.34) and lower odds associated with IM physicians (OR, 0.83; 95% CI, 0.79-0.87). Gender and RIW did not show any significant associations, but increasing age, higher number of secondary diagnoses, higher comorbidity levels, and longer than expected LOS (as measure by conservable days) were associated with higher odds of readmission. Conversely, longer hospitalization, admission to a large community hospital, palliative status, admission to a special care unit, geography, and fiscal year were associated with lower odds of readmission.
The above differences between provider groups were no longer consistently present when we analyzed patients presenting with COPD, CHF, and pneumonias (Table 6). Only patients admitted to the FP provider group with pneumonia had higher odds of readmission compared to hospitalists (OR, 1.27; 95% CI, 1.05-1.54). Conversely, only patients admitted to the IM provider group with CHF showed lower readmission (OR, 0.75; 95% CI, 0.62-0.92).
Total LOS
Results using generalized linear regressions for total LOS are presented in Table 7 (n = 183,779). Patients admitted to the IM provider group had significantly lower total LOS (mean, 5.13 days; 95% CI, 5.04-5.21) compared to the hospitalist (mean, 7.37 days; 95% CI, 7.26-7.49) and FP (mean, 7.30 days; 95% CI, 7.19-7.41) groups, with no significant differences between the latter 2 groups. Older patients, females, patients with higher comorbidity levels or number of secondary diagnoses, higher RIW, palliative patients, and discharge to a facility other than the patient’s home were associated with a significantly longer LOS. On the other hand, admission to nonteaching hospitals and admission to a special care unit was associated with lower LOS.
When we compared total LOS for patients admitted with COPD, CHF, and pneumonias, the same differences observed for the broader comparisons persisted: IM patients consistently showed shorter LOS compared to hospitalist patients, while LOS associated with FP patients was similar (Table 8).
Discussion
To our knowledge, our evaluation is the largest study to date designed to understand outcomes associated with hospitalist care in Canada. Our analyses suggest that patients admitted to our large network of hospitalist services present with clinical conditions that are very similar to those of general medicine patients in other Canadian provinces.28,29 They also show that patients cared for by hospitalists experience lower mortality rates compared to those cared for by FPs. Our findings are similar to previous studies, which have suggested a 12% to 75% reduction in odds of mortality associated with hospitalist care.18,19 These differences persisted even when we focused on patients presenting with specific clinical conditions (CHF, COPD, and pneumonias).
White and colleagues have previously demonstrated that generalist physicians who had higher volumes of inpatient care activity also had lower mortality rates compared to those who cared for hospitalized patients less frequently.19 An association between higher physician caseloads and better outcomes has been established for many surgical and medical conditions.30-32 Given that 85% of hospitalists in our program have post-graduate medical training in family medicine (internal department surveys, data not shown), it is less likely that training background can explain differences in outcomes. Instead, differences in patient volumes and the dedicated focus of hospitalists on acute care are likely more important contributors to lower mortality. In our program, a full-time hospitalist spends an average of 2000 hours annually providing services in the hospital setting. The continuous on-site presence of hospitalists enhances their clinical experience with regards to the management of common medical conditions, and increases their exposure to less common presentations of illnesses. The ability to respond to deteriorating patients in a timely manner may be another factor in explaining the differences in mortality rates between dedicated hospital-based generalist providers and similarly trained physicians with a primarily community-based focus.
In our study, hospitalist care was also broadly associated with lower mortality compared to the IM providers, although these differences were not consistently present when patients with specific diagnoses were compared. This may be partly explained by the relationship between caseload and outcomes, but other factors may also be important. For example, patients admitted by IM providers spend significantly more time in specialized units. They also predominantly present with cardiac conditions, and as such may have higher acuity levels and require more invasive interventions. While this may explain the higher observed mortality, a within-group comparison still suggests higher than expected mortality for IM patients. The HSMR methodology measures actual mortality rates compared to what would be expected based on clinical presentation and baseline population characteristics. Calculating HSMR is highly dependent on proper documentation and chart abstraction,33,34 and it is possible that some of the differences observed are due to incomplete physician documentation. However, a more in-depth analysis of care processes will be required to clarify the observed trends.
Compared to hospitalists, patients cared for by FPs also had higher odds of readmission within 30 days, which is consistent with prior studies.18,19 One of the criticisms of the hospitalist model has been the inherent discontinuity of care that is built into the model, which can contribute to suboptimal transitions of care between the acute and community settings.35 The expectation is that FPs who admit their own patients do not face this challenge, and as a result their patients should be readmitted less frequently after discharge. Our data and those from previous studies do not support this hypothesis. At the same time, when we studied patients with specific clinical diagnoses, only those hospitalized for pneumonias continued to demonstrate higher readmission odds. This suggests that hospital readmission rate is a complex measure that may be influenced by a multitude of hospital and community factors, and may be different for patients who present with different clinical diagnoses. Further research is required to better understand the relationship between provider type and experience with hospital readmission for patients with various clinical presentations.
Unlike the United States, where hospitalist care has been associated with reductions in LOS,26,36 studies in the Canadian health care setting have shown mixed results.17-21 In our evaluation, hospitalist care is not associated with reductions in total LOS compared to care provided by FPs or IM physicians. This could be due to a number of factors. First, unlike FPs, who know their patients, hospitalists may have a more conservative risk tolerance in discharging patients with whom they are not familiar. Similarly, physicians who have trained in IM may have a lower threshold for discharging patients than hospitalists, whose training background is mainly rooted in family medicine.3 Second, discontinuity of care has been associated with longer LOS for hospitalized patients.37,38 Hospitalists generally work for 7- to 10-day rotations. As a result, a patient may see a number of different hospitalists during the same hospital stay, which could nullify any gains in LOS that may be expected from better familiarity with hospital processes. Third, whereas a FP or an internist may only have a few inpatients under their care at any given time, each hospitalist typically cares for 17 to 22 patients every day. Increasing hospitalist workload has been shown to negatively impact LOS and may result in lower efficiency.39 Finally, many patients in our health system who require more time to recuperate or need complex discharge planning are usually transferred to the care of the hospitalist service from other services, or are preferentially admitted to hospitalists from the emergency department. As a result, hospitalists may look after a disproportionately higher number of long-stay patients. Despite all this, hospitalists in our population perform similarly to FPs, regardless of the clinical diagnoses of hospitalized patients.
Our study has a number of notable limitations. First, we used administrative data to conduct our evaluation and could only control for factors that are available in our data systems. As a result, some potential confounders may not have been taken into consideration. For example, our databases do not contain provider characteristics (eg, age, years of clinical experience) that have been deemed to be relevant by White and Glazier.26 Similarly, we did not have all the necessary information about the characteristics of the various MRP programs (eg, number of physicians involved in group practices, the schedule model of community FP call groups) and were not able to account for the potential impact of these on observed outcomes. Second, although our findings mirror prior studies from other parts of Canada, they may not be applicable to hospitalist programs in other jurisdictions or in health systems that are not regionalized or integrated. Third, our IM provider group is heterogeneous, with a number of different IM subspecialties (cardiologists, gastroenterologists, general internists) grouped under the IM category in our database. As a result, comparisons between the IM provider group and the other 2 provider groups, which are more homogenous, should be interpreted with caution.
Finally, we included only patients admitted to facilities in which a hospitalist service existed during the study period. As a result, a medium-size community hospital without a hospitalist service where patients are cared for exclusively by FPs and IM physicians was not included in the comparisons, and in 4 of the 10 facilities included, the number of FP patients was less than 10% of total hospitalized patients at the site (Appendix A). This may have resulted in an under-representation of FP patients.
Conclusion
Debates about the merits of the hospitalist model in Canada continue, and are in part fueled by a paucity of robust evidence about its impact on care outcomes compared to more traditional ways of providing inpatient care. In our evaluation, care provided by hospitalists is associated with lower mortality and readmission rates, despite similar LOS compared with FPs. Hospitalist care is also associated with lower mortality compared to IM providers. Hospitalists also demonstrated progressive improvement over time, with decreasing LOS and mortality rates and a stable readmission rate. Our results suggest that physicians with a focus on inpatient care can have positive contributions to quality and efficiency of care in Canada.
Corresponding author: Vandad Yousefi MD, CCFP, FHM, Fraser Health Authority, 400, 13450–102 Avenue, Surrey BC V3T 0H1, Canada.
Financial disclosures: None.
1. Kisuule F, Howell E. Hospital medicine beyond the United States. Int J Gen Med. 2018;11:65-71.
2. Yousefi V, Wilton D. Dedesigning hospital care: learning from the experience of hospital medicine in Canada. J Global Health Care Syst. 2011;1(3).
3. Soong C, Fan E, Howell E, et al. Characteristics of hospitalists and hospitalist programs in the United States and Canada. J Clin Outcomes Manag. 2009;16:69-76.
4. Yousefi V. How Canadian hospitalists spend their time - A work-sampling study within a hospital medicine program in Ontario. J Clin Outcomes Manag. 2011;18:159-166.
5. Wilson G. Are inpatients’ needs better served by hospitalists than by their family doctors? No. Can Fam Physician. 2008;54:1101-1103.
6. Samoil D. Are inpatients’ needs better served by hospitalists than by their family doctors: Yes? Can Fam Physician. 2008;54:1100-1101.
7. Nicolson B. Where’s Marcus Welby when you need him? BC Medical J. 2016;58:63-64.
8. Lemire F. Enhanced skills in family medicine: Update. Can Fam Physician. 2018;64:160.
9. Lerner J. Wanting family medicine without primary care. Can Fam Physician. 2018; 64:155.
10. Canadian Society of Hospital Medicine. Core Competencies in Hospital Medicine - Care of the Medical Inpatient. 2015.
11. Redelmeier DA. A Canadian perspective on the American hospitalist movement. Arch Intern Med. 1999;159:1665-1668.
12. Ghali WA, Greenberg PB, Mejia R, et al. International perspectives on general internal medicine and the case for “globalization” of a discipline. J Gen Intern Med. 2006;21:197-200.
13. Day A, MacMillan L. Neglect of the inpatient: The hospitalist movement in Canada responds. Hosp Q. 2001;4:36.
14. Sullivan P. Enter the hospitalist: New type of patient creating a new type of specialist. CMAJ. 2000;162:1345-1346.
15. Chan BTB. The declining comprehensiveness of primary care. CMAJ. 2002;166:429-434.
16. Abenhaim HA, Kahn SR, Raffoul J, Becker MR. Program description: A hospitalist-run, medical short-stay unit in a teaching hospital. CMAJ. 2000;163:1477-1480.
17. McGowan B, Nightingale M. The hospitalist program a new specialty on the horizon in acute care medicine a hospital case study. BC Med J. 2003;45:391-394.
18. Yousefi V, Chong C. Does implementation of a hospitalist program in a Canadian community hospital improve measures of quality of care and utilization? An observational comparative analysis of hospitalists vs. traditional care providers. BMC Health Serv Res. 2013;13:204.
19. White HL. Assessing the prevalence, penetration and performance of hospital physicians in Ontario: Implications for the quality and efficiency of inpatient care. ProQuest Dissertations Publishing; 2016.
20. Gutierrez CA, Norris M, Chail M. Impact of a newly established hospitalist training program on patient LOS and RIW. Poster presented at the 9th Annual Canadian Society of Hospital Medicine Conference, September 23-25, 2011; Banff, Alberta.
21. Seth P, Nicholson K, Habbous S, Menard J. Implementation of a hospitalist medicine model in a full-service community hospital: Examining impact two years post-implementation on health resource use andpatient satisfaction. Poster presented at the 13th Annual Canadian Society of Hospital Medicine Conference. 2015; Niagara Falls, Ontario.
22. Lewis S. A system in name only--access, variation, and reform in Canada’s provinces. N Engl J Med. 2015;372:497-500.
23. Lewis S, Kouri D. Regionalization: Making sense of the Canadian experience. Healthcare Papers. 2004;5:12-31.
24. Fraser Health Authority. About Fraser health. www.fraserhealth.ca/about-us/about-fraser-health#.XFJrl9JKiUk. Updated 2018. Accessed January 30, 2019.
25. Canadian Institute for Health Information. CMG+. https://www.cihi.ca/en/cmg. Accessed January 30, 2019.
26. White HL, Glazier RH. Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9:58.
27. Canadian Institute for Health Information. Hospital standardized mortality ratio technical notes. 2008. www.cihi.ca/sites/default/files/document/hsmr-tech-notes_en_0.pdf.
28. McAlister FA, Youngson E, Bakal JA, et al. Physician experience and outcomes among patients admitted to general internal medicine teaching wards. CMAJ. 2015;187:1041-1048.
29. Verma AA, Guo Y, Kwan JL, et al. Patient characteristics, resource use and outcomes associated with general internal medicine hospital care: The general medicine inpatient initiative (GEMINI) retrospective cohort study. CMAJ Open. 2017;5:E849.
30. Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: A systematic review of systematic reviews. Syst Rev. 2016;5:204.
31. Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511-520.
32. Chen CH, Chen YH, Lin HC, Lin HC. Association between physician caseload and patient outcome for sepsis treatment. Infect Control Hosp Epidemiol. 2009;30:556-562.
33. van Gestel YR, Lemmens VE, Lingsma HF, et al. The hospital standardized mortality ratio fallacy: A narrative review. Med Care. 2012;50:662-667.
34. Scott IA, Brand CA, Phelps GE, et al. Using hospital standardised mortality ratios to assess quality of care—proceed with extreme caution. Med J Aust. 2011; 194:645-648.
35. Wachter RM. Hospitalists in the United States -- mission accomplished or work in progress? N Engl J Med. 2004;350:1935-1936.
36. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84:248-254.
37. Chandra S, Wright SM, Howell EE. The creating incentives and continuity leading to efficiency staffing model: A quality improvement initiative in hospital medicine. Mayo Clin Proc. 2012;87:364-371.
38. Epstein K, Juarez E, Epstein A, et al. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5:335-338.
39. Elliott DJ, Young RS, Brice J, et al. Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174:786-793.
1. Kisuule F, Howell E. Hospital medicine beyond the United States. Int J Gen Med. 2018;11:65-71.
2. Yousefi V, Wilton D. Dedesigning hospital care: learning from the experience of hospital medicine in Canada. J Global Health Care Syst. 2011;1(3).
3. Soong C, Fan E, Howell E, et al. Characteristics of hospitalists and hospitalist programs in the United States and Canada. J Clin Outcomes Manag. 2009;16:69-76.
4. Yousefi V. How Canadian hospitalists spend their time - A work-sampling study within a hospital medicine program in Ontario. J Clin Outcomes Manag. 2011;18:159-166.
5. Wilson G. Are inpatients’ needs better served by hospitalists than by their family doctors? No. Can Fam Physician. 2008;54:1101-1103.
6. Samoil D. Are inpatients’ needs better served by hospitalists than by their family doctors: Yes? Can Fam Physician. 2008;54:1100-1101.
7. Nicolson B. Where’s Marcus Welby when you need him? BC Medical J. 2016;58:63-64.
8. Lemire F. Enhanced skills in family medicine: Update. Can Fam Physician. 2018;64:160.
9. Lerner J. Wanting family medicine without primary care. Can Fam Physician. 2018; 64:155.
10. Canadian Society of Hospital Medicine. Core Competencies in Hospital Medicine - Care of the Medical Inpatient. 2015.
11. Redelmeier DA. A Canadian perspective on the American hospitalist movement. Arch Intern Med. 1999;159:1665-1668.
12. Ghali WA, Greenberg PB, Mejia R, et al. International perspectives on general internal medicine and the case for “globalization” of a discipline. J Gen Intern Med. 2006;21:197-200.
13. Day A, MacMillan L. Neglect of the inpatient: The hospitalist movement in Canada responds. Hosp Q. 2001;4:36.
14. Sullivan P. Enter the hospitalist: New type of patient creating a new type of specialist. CMAJ. 2000;162:1345-1346.
15. Chan BTB. The declining comprehensiveness of primary care. CMAJ. 2002;166:429-434.
16. Abenhaim HA, Kahn SR, Raffoul J, Becker MR. Program description: A hospitalist-run, medical short-stay unit in a teaching hospital. CMAJ. 2000;163:1477-1480.
17. McGowan B, Nightingale M. The hospitalist program a new specialty on the horizon in acute care medicine a hospital case study. BC Med J. 2003;45:391-394.
18. Yousefi V, Chong C. Does implementation of a hospitalist program in a Canadian community hospital improve measures of quality of care and utilization? An observational comparative analysis of hospitalists vs. traditional care providers. BMC Health Serv Res. 2013;13:204.
19. White HL. Assessing the prevalence, penetration and performance of hospital physicians in Ontario: Implications for the quality and efficiency of inpatient care. ProQuest Dissertations Publishing; 2016.
20. Gutierrez CA, Norris M, Chail M. Impact of a newly established hospitalist training program on patient LOS and RIW. Poster presented at the 9th Annual Canadian Society of Hospital Medicine Conference, September 23-25, 2011; Banff, Alberta.
21. Seth P, Nicholson K, Habbous S, Menard J. Implementation of a hospitalist medicine model in a full-service community hospital: Examining impact two years post-implementation on health resource use andpatient satisfaction. Poster presented at the 13th Annual Canadian Society of Hospital Medicine Conference. 2015; Niagara Falls, Ontario.
22. Lewis S. A system in name only--access, variation, and reform in Canada’s provinces. N Engl J Med. 2015;372:497-500.
23. Lewis S, Kouri D. Regionalization: Making sense of the Canadian experience. Healthcare Papers. 2004;5:12-31.
24. Fraser Health Authority. About Fraser health. www.fraserhealth.ca/about-us/about-fraser-health#.XFJrl9JKiUk. Updated 2018. Accessed January 30, 2019.
25. Canadian Institute for Health Information. CMG+. https://www.cihi.ca/en/cmg. Accessed January 30, 2019.
26. White HL, Glazier RH. Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9:58.
27. Canadian Institute for Health Information. Hospital standardized mortality ratio technical notes. 2008. www.cihi.ca/sites/default/files/document/hsmr-tech-notes_en_0.pdf.
28. McAlister FA, Youngson E, Bakal JA, et al. Physician experience and outcomes among patients admitted to general internal medicine teaching wards. CMAJ. 2015;187:1041-1048.
29. Verma AA, Guo Y, Kwan JL, et al. Patient characteristics, resource use and outcomes associated with general internal medicine hospital care: The general medicine inpatient initiative (GEMINI) retrospective cohort study. CMAJ Open. 2017;5:E849.
30. Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: A systematic review of systematic reviews. Syst Rev. 2016;5:204.
31. Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511-520.
32. Chen CH, Chen YH, Lin HC, Lin HC. Association between physician caseload and patient outcome for sepsis treatment. Infect Control Hosp Epidemiol. 2009;30:556-562.
33. van Gestel YR, Lemmens VE, Lingsma HF, et al. The hospital standardized mortality ratio fallacy: A narrative review. Med Care. 2012;50:662-667.
34. Scott IA, Brand CA, Phelps GE, et al. Using hospital standardised mortality ratios to assess quality of care—proceed with extreme caution. Med J Aust. 2011; 194:645-648.
35. Wachter RM. Hospitalists in the United States -- mission accomplished or work in progress? N Engl J Med. 2004;350:1935-1936.
36. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84:248-254.
37. Chandra S, Wright SM, Howell EE. The creating incentives and continuity leading to efficiency staffing model: A quality improvement initiative in hospital medicine. Mayo Clin Proc. 2012;87:364-371.
38. Epstein K, Juarez E, Epstein A, et al. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5:335-338.
39. Elliott DJ, Young RS, Brice J, et al. Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174:786-793.
SPEAKers at the National Society of Hospital Medicine Meeting: A Follow-UP Study of Gender Equity for Conference Speakers from 2015 to 2019. The SPEAK UP Study
Persistent gender disparities exist in pay,1,2 leadership opportunities,3,4 promotion,5 and speaking opportunities.6 While the gender distribution of the hospitalist workforce may be approaching parity,3,7,8 gender differences in leadership, speakership, and authorship have already been noted in hospital medicine.3 Between 2006 and 2012, women constituted less than a third (26%) of the presenters at the national conferences of the Society of Hospital Medicine (SHM) and the Society of General Internal Medicine (SGIM).3
The SHM Annual Meeting has historically had an “open call” peer review process for workshop presenters with the goal of increasing the diversity of presenters. In 2019, this process was expanded to include didactic speakers. Our aim in this study was to assess whether these open call procedures resulted in improved representation of women speakers and how the proportion of women speakers affects the overall evaluation scores of the conference. Our hypothesis was that the introduction of an open call process for the SHM conference didactic speakers would be associated with an increased proportion of women speakers, compared with the closed call processes, without a negative impact on conference scores.
METHODS
The study is a retrospective evaluation of data collected regarding speakers at the annual SHM conference from 2015 to 2019. The SHM national conference typically has two main types of offerings: workshops and didactics. Workshop presenters from 2015 to 2019 were selected via an open call process as defined below. Didactic speakers (except for plenary speakers) were selected using the open call process for 2019 only.
We aimed to compare (1) the number and proportion of women speakers, compared with men speakers, over time and (2) the proportion of women speakers when open call processes were utilized versus that seen with closed call processes. Open call included workshops for all years and didactics for 2019; closed call included didactics for 2015 to 2018 and plenary sessions 2015 to 2019 (Table). The speaker list for the conferences was obtained from conference pamphlets or agendas available via Internet searches or obtained through attendance at the conference.
Speaker Categories and Identification Process
We determined whether each individual was a featured speaker (one whose talk was unopposed by other sessions), plenary speaker (defined as such in the conference pamphlets), whether they spoke in a group format, and whether the speaking opportunity type was a workshop or a didactic session. Numbers of featured and plenary speakers were combined because of low numbers. SHM provided deidentified conference evaluation data for each year studied. For the purposes of this study, we analyzed all speakers which included physicians, advanced practice providers, and professionals such as nurses and other interdisciplinary team members. The same speaker could be included multiple times if they had multiple speaking opportunities.
Open Call Process
We defined the “open call process” (referred to as “open call” here forward) as the process utilized by SHM that includes the following two components: (1) advertisements to members of SHM and to the medical community at large through a variety of mechanisms including emails, websites, and social media outlets and (2) an online submission process that includes names of proposed speakers and their topic and, in the case of workshops, session objectives as well as an outline of the proposed workshop. SHM committees may also submit suggestions for topics and speakers. Annual Conference Committee members then review and rate submissions on the categories of topic, organization and clarity, objectives, and speaker qualifications (with a focus on institutional, geographic, and gender diversity). Scores are assigned from 1 to 5 (with 5 being the best score) for each category and a section for comments is available. All submissions are also evaluated by the course director.
After initial committee reviews, scores with marked reviewer discrepancies are rereviewed and discussed by the committee and course director. A cutoff score is then calculated with proposals falling below the cutoff threshold omitted from further consideration. Weekly calls are then focused on subcategories (ie tracks) with emphasis on clinical and educational content. Each of the tracks have a subcommittee with track leads to curate the best content first and then focus on final speaker selection. More recently, templates are shared with the track leads that include a location to call out gender and institutional diversity. Weekly calls are held to hone the content and determine the speakers.
For the purposes of this study, when the above process was not used, the authors refer to it as “closed call.” Closed call processes do not typically involve open invitations or a peer review process. (Table)
Gender
Gender was assigned based on the speaker’s self-identification by the pronouns used in their biography submitted to the conference or on their institutional website or other websites where the speaker was referenced. Persons using she/her/hers pronouns were noted as women and persons using he/him/his were noted as men. For the purposes of this study, we conceptualized gender as binary (ie woman/man) given the limited information we had from online sources.
ANALYSIS
REDCap, a secure, Web-based application for building and managing online survey and databases, was used to collect and manage all study data.9
All analyses were performed using SAS Enterprise Guide 8.1 (SAS Institute, Inc., Cary, North Carolina) using retrospectively collected data. A Cochran-Armitage test for trend was used to evaluate the proportion of women speakers from 2015 to 2019. A chi-square test was used to assess the proportion of women speakers for open call processes versus that seen with closed call. One-way analysis of variance (ANOVA) was used to evaluate annual conference evaluation scores from 2015 to 2019. Either numbers with proportions or means with standard deviations have been reported. Bonferroni’s correction for multiple comparisons was applied, with a P < .008 considered statistically significant.
RESULTS
Between 2015 and 2019, a total of 709 workshop and didactic presentations were given by 1,261 speakers at the annual Society of Hospital Medicine Conference. Of these, 505 (40%) were women; 756 (60%) were men. There were no missing data.
From 2015 to 2019, representation of women speakers increased from 35% of all speakers to 47% of all speakers (P = .0068). Women plenary speakers increased from 23% in 2015 to 45% in 2019 (P = .0396).
The proportion of women presenters for workshops (which have utilized an open call process throughout the study period), ranged from 43% to 53% from 2015 to 2019 with no statistically significant difference in gender distribution across years (Figure).
A greater proportion of speakers selected by an open call process were women compared to when speakers were selected by a closed call process (261 (47%) vs 244 (34%); P < .0001).
Of didactics or workshops given in a group format (N = 299), 82 (27%) were given by all-men groups and 38 (13%) were given by all-women groups. Women speakers participating in all-women group talks accounted for 21% of all women speakers; whereas men speakers participating in all-men group talks account for 26% of all men speakers (P = .02). We found that all-men group speaking opportunities did decrease from 41% of group talks in 2015 to 21% of group talks in 2019 (P = .0065).
We saw an average 3% annual increase in women speakers from 2015 to 2019, an 8% increase from 2018 to 2019 for all speakers, and an 11% increase in women speakers specific to didactic sessions. Overall conference ratings increased from a mean of 4.3 ± 0.24 in 2015 to a mean of 4.6 ± 0.14 in 2019 (n = 1,202; P < .0001; Figure).
DISCUSSION
The important findings of this study are that there has been an increase in women speakers over the last 5 years at the annual Society of Hospital Medicine Conference, that women had higher representation as speakers when open call processes were followed, and that conference scores continued to improve during the time frame studied. These findings suggest that a systematic open call process helps to support equitable speaking opportunities for men and women at a national hospital medicine conference without a negative impact on conference quality.
To recruit more diverse speakers, open call and peer review processes were used in addition to deliberate efforts at ensuring diversity in speakers. We found that over time, the proportion of women with speaking opportunities increased from 2015 to 2019. Interestingly, workshops, which had open call processes in place for the duration of the study period, had almost equal numbers of men and women presenting in all years. We also found that the number of all-men speaking groups decreased between 2015 and 2019.
A single process change can impact gender equity, but the target of true equity is expected to require additional measures such as assessment of committee structures and diversity, checklists, and reporting structures (data analysis and plans when goals not achieved).10-13 For instance, the American Society for Microbiology General Meeting was able to achieve gender equity in speakers by a multifold approach including ensuring the program committee was aware of gender statistics, increasing female representation among session convener teams, and direct instruction to try to avoid all-male sessions.11
It is important to acknowledge that these processes do require valuable resources including time. SHM has historically used committee volunteers to conduct the peer review process with each committee member reviewing 20 to 30 workshop submissions and 30 to 50 didactic sessions. While open processes with peer review seem to generate improved gender equity, ensuring processes are in place during the selection process is also key.
Several recent notable efforts to enhance gender equity and to increase diversity have been proposed. One such example of a process that may further improve gender equity was proposed by editors at the Journal of Hospital Medicine to assess current representation via demographics including gender, race, and ethnicity of authors with plans to assess patterns in the coming years.14 The American College of Physicians also published a position paper on achieving gender equity with a recommendation that organizational policies and procedures should be implemented that address implicit bias.15
Our study showed that, from 2015 to 2019, conference evaluations saw a significant increase in the score concurrently with the rise in proportion of women speakers. This finding suggests that quality does not seem to be affected by this new methodology for speaker selection and in fact this methodology may actually help improve the overall quality of the conference. To our knowledge, this is one of the first studies to concurrently evaluate speaker gender equity with conference quality.
Our study offers several strengths. This study took a pragmatic approach to understanding how processes can impact gender equity, and we were able to take advantage of the evolution of the open call system (ie workshops which have been an open call process for the duration of the study versus speaking opportunities that were not).
Our study also has several limitations. First, this study is retrospective in nature and thus other processes could have contributed to the improved gender equity, such as an organization’s priorities over time. During this study period, the SHM conference saw an average 3% increase annually in women speakers and an increase of 8% from 2018 to 2019 for all speakers compared to national trends of approximately 1%,6 which suggests that the open call processes in place could be contributing to the overall increases seen. Similarly, because of the retrospective nature of the study, we cannot be certain that the improvements in conference scores were directly the result of improved gender equity, although it does suggest that the improvements in gender equity did not have an adverse impact on the scores. We also did not assess how the composition of selection committee members for the meeting could have impacted the overall composition of the speakers. Our study looked at diversity only from the perspective of gender in a binary fashion, and thus additional studies are needed to assess how to improve diversity overall. It is unclear how this new open call for speakers affects race and ethnic diversity specifically. Identifying gender for the purposes of this study was facilitated by speakers providing their own biographies and the respective pronouns used in those biographies, and thus gender was easier to ascertain than race and ethnicity, which are not as readily available. For organizations to understand their diversity, equity, and inclusion efforts, enhancing the ability to fairly track and measure diversity will be key. Lastly, understanding of the exact composition of hospitalists from both a gender and race/ethnicity perspective is lacking. Studies have suggested that, based upon those surveyed or studied, there is a fairly equal balance of men and women albeit in academic groups.3
CONCLUSIONS
An open call approach to speakers at a national hospitalist conference seems to have contributed to improvements regarding gender equity in speaking opportunities with a concurrent improvement in overall rating of the conference. The open call system is a potential mechanism that other institutions and organizations could employ to enhance their diversity efforts.
Acknowledgments
Society of Hospital Medicine Diversity, Equity, Inclusion Special Interest Group
Work Group for SPEAK UP: Marisha Burden, MD, Daniel Cabrera, MD, Amira del Pino-Jones, MD, Areeba Kara, MD, Angela Keniston, MSPH, Keshav Khanijow, MD, Flora Kisuule, MD, Chiara Mandel, Benji Mathews, MD, David Paje, MD, Stephan Papp, MD, Snehal Patel, MD, Suchita Shah Sata, MD, Dustin Smith, MD, Kevin Vuernick
1. Weaver AC, Wetterneck TB, Whelan CT, Hinami K. A matter of priorities? Exploring the persistent gender pay gap in hospital medicine. J Hosp Med. 2015;10(8):486-490. https://doi.org/10.1002/jhm.2400.
2. Jena AB, Olenski AR, Blumenthal DM. Sex differences in physician salary in US public medical schools. JAMA Intern Med. 2016;176(9):1294-1304. https://doi.org/10.1001/jamainternmed.2016.3284.
3. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340.
4. Silver JK, Ghalib R, Poorman JA, et al. Analysis of gender equity in leadership of physician-focused medical specialty societies, 2008-2017. JAMA Intern Med. 2019;179(3):433-435. https://doi.org/10.1001/jamainternmed.2018.5303.
5. Jena AB, Khullar D, Ho O, Olenski AR, Blumenthal DM. Sex differences in academic rank in US medical schools in 2014. JAMA. 2015;314(11):1149-1158. https://doi.org/10.1001/jama.2015.10680.
6. Ruzycki SM, Fletcher S, Earp M, Bharwani A, Lithgow KC. Trends in the Proportion of Female Speakers at Medical Conferences in the United States and in Canada, 2007 to 2017. JAMA Netw Open. 2019;2(4):e192103. https://doi.org/10.1001/jamanetworkopen.2019.2103
7. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5.
8. Today’s Hospitalist 2018 Compensation and Career Survey Results. https://www.todayshospitalist.com/salary-survey-results/. Accessed September 28, 2019.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
10. Burden M, del Pino-Jones A, Shafer M, Sheth S, Rexrode K. Association of American Medical Colleagues (AAMC) Group on Women in Medicine and Science. Recruitment Toolkit: https://www.aamc.org/download/492864/data/equityinrecruitmenttoolkit.pdf. Accessed July 27, 2019.
11. Casadevall A. Achieving speaker gender equity at the american society for microbiology general meeting. MBio. 2015;6:e01146. https://doi.org/10.1128/mBio.01146-15.
12. Westring A, McDonald JM, Carr P, Grisso JA. An integrated framework for gender equity in academic medicine. Acad Med. 2016;91(8):1041-1044. https://doi.org/10.1097/ACM.0000000000001275.
13. Martin JL. Ten simple rules to achieve conference speaker gender balance. PLoS Comput Biol. 2014;10(11):e1003903. https://doi.org/10.1371/journal.pcbi.1003903.
14. Shah SS, Shaughnessy EE, Spector ND. Leading by example: how medical journals can improve representation in academic medicine. J Hosp Med. 2019;14(7):393. https://doi.org/10.12788/jhm.3247.
15. Butkus R, Serchen J, Moyer DV, et al. Achieving gender equity in physician compensation and career advancement: a position paper of the American College of Physicians. Ann Intern Med. 2018;168:721-723. https://doi.org/10.7326/M17-3438.
Persistent gender disparities exist in pay,1,2 leadership opportunities,3,4 promotion,5 and speaking opportunities.6 While the gender distribution of the hospitalist workforce may be approaching parity,3,7,8 gender differences in leadership, speakership, and authorship have already been noted in hospital medicine.3 Between 2006 and 2012, women constituted less than a third (26%) of the presenters at the national conferences of the Society of Hospital Medicine (SHM) and the Society of General Internal Medicine (SGIM).3
The SHM Annual Meeting has historically had an “open call” peer review process for workshop presenters with the goal of increasing the diversity of presenters. In 2019, this process was expanded to include didactic speakers. Our aim in this study was to assess whether these open call procedures resulted in improved representation of women speakers and how the proportion of women speakers affects the overall evaluation scores of the conference. Our hypothesis was that the introduction of an open call process for the SHM conference didactic speakers would be associated with an increased proportion of women speakers, compared with the closed call processes, without a negative impact on conference scores.
METHODS
The study is a retrospective evaluation of data collected regarding speakers at the annual SHM conference from 2015 to 2019. The SHM national conference typically has two main types of offerings: workshops and didactics. Workshop presenters from 2015 to 2019 were selected via an open call process as defined below. Didactic speakers (except for plenary speakers) were selected using the open call process for 2019 only.
We aimed to compare (1) the number and proportion of women speakers, compared with men speakers, over time and (2) the proportion of women speakers when open call processes were utilized versus that seen with closed call processes. Open call included workshops for all years and didactics for 2019; closed call included didactics for 2015 to 2018 and plenary sessions 2015 to 2019 (Table). The speaker list for the conferences was obtained from conference pamphlets or agendas available via Internet searches or obtained through attendance at the conference.
Speaker Categories and Identification Process
We determined whether each individual was a featured speaker (one whose talk was unopposed by other sessions), plenary speaker (defined as such in the conference pamphlets), whether they spoke in a group format, and whether the speaking opportunity type was a workshop or a didactic session. Numbers of featured and plenary speakers were combined because of low numbers. SHM provided deidentified conference evaluation data for each year studied. For the purposes of this study, we analyzed all speakers which included physicians, advanced practice providers, and professionals such as nurses and other interdisciplinary team members. The same speaker could be included multiple times if they had multiple speaking opportunities.
Open Call Process
We defined the “open call process” (referred to as “open call” here forward) as the process utilized by SHM that includes the following two components: (1) advertisements to members of SHM and to the medical community at large through a variety of mechanisms including emails, websites, and social media outlets and (2) an online submission process that includes names of proposed speakers and their topic and, in the case of workshops, session objectives as well as an outline of the proposed workshop. SHM committees may also submit suggestions for topics and speakers. Annual Conference Committee members then review and rate submissions on the categories of topic, organization and clarity, objectives, and speaker qualifications (with a focus on institutional, geographic, and gender diversity). Scores are assigned from 1 to 5 (with 5 being the best score) for each category and a section for comments is available. All submissions are also evaluated by the course director.
After initial committee reviews, scores with marked reviewer discrepancies are rereviewed and discussed by the committee and course director. A cutoff score is then calculated with proposals falling below the cutoff threshold omitted from further consideration. Weekly calls are then focused on subcategories (ie tracks) with emphasis on clinical and educational content. Each of the tracks have a subcommittee with track leads to curate the best content first and then focus on final speaker selection. More recently, templates are shared with the track leads that include a location to call out gender and institutional diversity. Weekly calls are held to hone the content and determine the speakers.
For the purposes of this study, when the above process was not used, the authors refer to it as “closed call.” Closed call processes do not typically involve open invitations or a peer review process. (Table)
Gender
Gender was assigned based on the speaker’s self-identification by the pronouns used in their biography submitted to the conference or on their institutional website or other websites where the speaker was referenced. Persons using she/her/hers pronouns were noted as women and persons using he/him/his were noted as men. For the purposes of this study, we conceptualized gender as binary (ie woman/man) given the limited information we had from online sources.
ANALYSIS
REDCap, a secure, Web-based application for building and managing online survey and databases, was used to collect and manage all study data.9
All analyses were performed using SAS Enterprise Guide 8.1 (SAS Institute, Inc., Cary, North Carolina) using retrospectively collected data. A Cochran-Armitage test for trend was used to evaluate the proportion of women speakers from 2015 to 2019. A chi-square test was used to assess the proportion of women speakers for open call processes versus that seen with closed call. One-way analysis of variance (ANOVA) was used to evaluate annual conference evaluation scores from 2015 to 2019. Either numbers with proportions or means with standard deviations have been reported. Bonferroni’s correction for multiple comparisons was applied, with a P < .008 considered statistically significant.
RESULTS
Between 2015 and 2019, a total of 709 workshop and didactic presentations were given by 1,261 speakers at the annual Society of Hospital Medicine Conference. Of these, 505 (40%) were women; 756 (60%) were men. There were no missing data.
From 2015 to 2019, representation of women speakers increased from 35% of all speakers to 47% of all speakers (P = .0068). Women plenary speakers increased from 23% in 2015 to 45% in 2019 (P = .0396).
The proportion of women presenters for workshops (which have utilized an open call process throughout the study period), ranged from 43% to 53% from 2015 to 2019 with no statistically significant difference in gender distribution across years (Figure).
A greater proportion of speakers selected by an open call process were women compared to when speakers were selected by a closed call process (261 (47%) vs 244 (34%); P < .0001).
Of didactics or workshops given in a group format (N = 299), 82 (27%) were given by all-men groups and 38 (13%) were given by all-women groups. Women speakers participating in all-women group talks accounted for 21% of all women speakers; whereas men speakers participating in all-men group talks account for 26% of all men speakers (P = .02). We found that all-men group speaking opportunities did decrease from 41% of group talks in 2015 to 21% of group talks in 2019 (P = .0065).
We saw an average 3% annual increase in women speakers from 2015 to 2019, an 8% increase from 2018 to 2019 for all speakers, and an 11% increase in women speakers specific to didactic sessions. Overall conference ratings increased from a mean of 4.3 ± 0.24 in 2015 to a mean of 4.6 ± 0.14 in 2019 (n = 1,202; P < .0001; Figure).
DISCUSSION
The important findings of this study are that there has been an increase in women speakers over the last 5 years at the annual Society of Hospital Medicine Conference, that women had higher representation as speakers when open call processes were followed, and that conference scores continued to improve during the time frame studied. These findings suggest that a systematic open call process helps to support equitable speaking opportunities for men and women at a national hospital medicine conference without a negative impact on conference quality.
To recruit more diverse speakers, open call and peer review processes were used in addition to deliberate efforts at ensuring diversity in speakers. We found that over time, the proportion of women with speaking opportunities increased from 2015 to 2019. Interestingly, workshops, which had open call processes in place for the duration of the study period, had almost equal numbers of men and women presenting in all years. We also found that the number of all-men speaking groups decreased between 2015 and 2019.
A single process change can impact gender equity, but the target of true equity is expected to require additional measures such as assessment of committee structures and diversity, checklists, and reporting structures (data analysis and plans when goals not achieved).10-13 For instance, the American Society for Microbiology General Meeting was able to achieve gender equity in speakers by a multifold approach including ensuring the program committee was aware of gender statistics, increasing female representation among session convener teams, and direct instruction to try to avoid all-male sessions.11
It is important to acknowledge that these processes do require valuable resources including time. SHM has historically used committee volunteers to conduct the peer review process with each committee member reviewing 20 to 30 workshop submissions and 30 to 50 didactic sessions. While open processes with peer review seem to generate improved gender equity, ensuring processes are in place during the selection process is also key.
Several recent notable efforts to enhance gender equity and to increase diversity have been proposed. One such example of a process that may further improve gender equity was proposed by editors at the Journal of Hospital Medicine to assess current representation via demographics including gender, race, and ethnicity of authors with plans to assess patterns in the coming years.14 The American College of Physicians also published a position paper on achieving gender equity with a recommendation that organizational policies and procedures should be implemented that address implicit bias.15
Our study showed that, from 2015 to 2019, conference evaluations saw a significant increase in the score concurrently with the rise in proportion of women speakers. This finding suggests that quality does not seem to be affected by this new methodology for speaker selection and in fact this methodology may actually help improve the overall quality of the conference. To our knowledge, this is one of the first studies to concurrently evaluate speaker gender equity with conference quality.
Our study offers several strengths. This study took a pragmatic approach to understanding how processes can impact gender equity, and we were able to take advantage of the evolution of the open call system (ie workshops which have been an open call process for the duration of the study versus speaking opportunities that were not).
Our study also has several limitations. First, this study is retrospective in nature and thus other processes could have contributed to the improved gender equity, such as an organization’s priorities over time. During this study period, the SHM conference saw an average 3% increase annually in women speakers and an increase of 8% from 2018 to 2019 for all speakers compared to national trends of approximately 1%,6 which suggests that the open call processes in place could be contributing to the overall increases seen. Similarly, because of the retrospective nature of the study, we cannot be certain that the improvements in conference scores were directly the result of improved gender equity, although it does suggest that the improvements in gender equity did not have an adverse impact on the scores. We also did not assess how the composition of selection committee members for the meeting could have impacted the overall composition of the speakers. Our study looked at diversity only from the perspective of gender in a binary fashion, and thus additional studies are needed to assess how to improve diversity overall. It is unclear how this new open call for speakers affects race and ethnic diversity specifically. Identifying gender for the purposes of this study was facilitated by speakers providing their own biographies and the respective pronouns used in those biographies, and thus gender was easier to ascertain than race and ethnicity, which are not as readily available. For organizations to understand their diversity, equity, and inclusion efforts, enhancing the ability to fairly track and measure diversity will be key. Lastly, understanding of the exact composition of hospitalists from both a gender and race/ethnicity perspective is lacking. Studies have suggested that, based upon those surveyed or studied, there is a fairly equal balance of men and women albeit in academic groups.3
CONCLUSIONS
An open call approach to speakers at a national hospitalist conference seems to have contributed to improvements regarding gender equity in speaking opportunities with a concurrent improvement in overall rating of the conference. The open call system is a potential mechanism that other institutions and organizations could employ to enhance their diversity efforts.
Acknowledgments
Society of Hospital Medicine Diversity, Equity, Inclusion Special Interest Group
Work Group for SPEAK UP: Marisha Burden, MD, Daniel Cabrera, MD, Amira del Pino-Jones, MD, Areeba Kara, MD, Angela Keniston, MSPH, Keshav Khanijow, MD, Flora Kisuule, MD, Chiara Mandel, Benji Mathews, MD, David Paje, MD, Stephan Papp, MD, Snehal Patel, MD, Suchita Shah Sata, MD, Dustin Smith, MD, Kevin Vuernick
Persistent gender disparities exist in pay,1,2 leadership opportunities,3,4 promotion,5 and speaking opportunities.6 While the gender distribution of the hospitalist workforce may be approaching parity,3,7,8 gender differences in leadership, speakership, and authorship have already been noted in hospital medicine.3 Between 2006 and 2012, women constituted less than a third (26%) of the presenters at the national conferences of the Society of Hospital Medicine (SHM) and the Society of General Internal Medicine (SGIM).3
The SHM Annual Meeting has historically had an “open call” peer review process for workshop presenters with the goal of increasing the diversity of presenters. In 2019, this process was expanded to include didactic speakers. Our aim in this study was to assess whether these open call procedures resulted in improved representation of women speakers and how the proportion of women speakers affects the overall evaluation scores of the conference. Our hypothesis was that the introduction of an open call process for the SHM conference didactic speakers would be associated with an increased proportion of women speakers, compared with the closed call processes, without a negative impact on conference scores.
METHODS
The study is a retrospective evaluation of data collected regarding speakers at the annual SHM conference from 2015 to 2019. The SHM national conference typically has two main types of offerings: workshops and didactics. Workshop presenters from 2015 to 2019 were selected via an open call process as defined below. Didactic speakers (except for plenary speakers) were selected using the open call process for 2019 only.
We aimed to compare (1) the number and proportion of women speakers, compared with men speakers, over time and (2) the proportion of women speakers when open call processes were utilized versus that seen with closed call processes. Open call included workshops for all years and didactics for 2019; closed call included didactics for 2015 to 2018 and plenary sessions 2015 to 2019 (Table). The speaker list for the conferences was obtained from conference pamphlets or agendas available via Internet searches or obtained through attendance at the conference.
Speaker Categories and Identification Process
We determined whether each individual was a featured speaker (one whose talk was unopposed by other sessions), plenary speaker (defined as such in the conference pamphlets), whether they spoke in a group format, and whether the speaking opportunity type was a workshop or a didactic session. Numbers of featured and plenary speakers were combined because of low numbers. SHM provided deidentified conference evaluation data for each year studied. For the purposes of this study, we analyzed all speakers which included physicians, advanced practice providers, and professionals such as nurses and other interdisciplinary team members. The same speaker could be included multiple times if they had multiple speaking opportunities.
Open Call Process
We defined the “open call process” (referred to as “open call” here forward) as the process utilized by SHM that includes the following two components: (1) advertisements to members of SHM and to the medical community at large through a variety of mechanisms including emails, websites, and social media outlets and (2) an online submission process that includes names of proposed speakers and their topic and, in the case of workshops, session objectives as well as an outline of the proposed workshop. SHM committees may also submit suggestions for topics and speakers. Annual Conference Committee members then review and rate submissions on the categories of topic, organization and clarity, objectives, and speaker qualifications (with a focus on institutional, geographic, and gender diversity). Scores are assigned from 1 to 5 (with 5 being the best score) for each category and a section for comments is available. All submissions are also evaluated by the course director.
After initial committee reviews, scores with marked reviewer discrepancies are rereviewed and discussed by the committee and course director. A cutoff score is then calculated with proposals falling below the cutoff threshold omitted from further consideration. Weekly calls are then focused on subcategories (ie tracks) with emphasis on clinical and educational content. Each of the tracks have a subcommittee with track leads to curate the best content first and then focus on final speaker selection. More recently, templates are shared with the track leads that include a location to call out gender and institutional diversity. Weekly calls are held to hone the content and determine the speakers.
For the purposes of this study, when the above process was not used, the authors refer to it as “closed call.” Closed call processes do not typically involve open invitations or a peer review process. (Table)
Gender
Gender was assigned based on the speaker’s self-identification by the pronouns used in their biography submitted to the conference or on their institutional website or other websites where the speaker was referenced. Persons using she/her/hers pronouns were noted as women and persons using he/him/his were noted as men. For the purposes of this study, we conceptualized gender as binary (ie woman/man) given the limited information we had from online sources.
ANALYSIS
REDCap, a secure, Web-based application for building and managing online survey and databases, was used to collect and manage all study data.9
All analyses were performed using SAS Enterprise Guide 8.1 (SAS Institute, Inc., Cary, North Carolina) using retrospectively collected data. A Cochran-Armitage test for trend was used to evaluate the proportion of women speakers from 2015 to 2019. A chi-square test was used to assess the proportion of women speakers for open call processes versus that seen with closed call. One-way analysis of variance (ANOVA) was used to evaluate annual conference evaluation scores from 2015 to 2019. Either numbers with proportions or means with standard deviations have been reported. Bonferroni’s correction for multiple comparisons was applied, with a P < .008 considered statistically significant.
RESULTS
Between 2015 and 2019, a total of 709 workshop and didactic presentations were given by 1,261 speakers at the annual Society of Hospital Medicine Conference. Of these, 505 (40%) were women; 756 (60%) were men. There were no missing data.
From 2015 to 2019, representation of women speakers increased from 35% of all speakers to 47% of all speakers (P = .0068). Women plenary speakers increased from 23% in 2015 to 45% in 2019 (P = .0396).
The proportion of women presenters for workshops (which have utilized an open call process throughout the study period), ranged from 43% to 53% from 2015 to 2019 with no statistically significant difference in gender distribution across years (Figure).
A greater proportion of speakers selected by an open call process were women compared to when speakers were selected by a closed call process (261 (47%) vs 244 (34%); P < .0001).
Of didactics or workshops given in a group format (N = 299), 82 (27%) were given by all-men groups and 38 (13%) were given by all-women groups. Women speakers participating in all-women group talks accounted for 21% of all women speakers; whereas men speakers participating in all-men group talks account for 26% of all men speakers (P = .02). We found that all-men group speaking opportunities did decrease from 41% of group talks in 2015 to 21% of group talks in 2019 (P = .0065).
We saw an average 3% annual increase in women speakers from 2015 to 2019, an 8% increase from 2018 to 2019 for all speakers, and an 11% increase in women speakers specific to didactic sessions. Overall conference ratings increased from a mean of 4.3 ± 0.24 in 2015 to a mean of 4.6 ± 0.14 in 2019 (n = 1,202; P < .0001; Figure).
DISCUSSION
The important findings of this study are that there has been an increase in women speakers over the last 5 years at the annual Society of Hospital Medicine Conference, that women had higher representation as speakers when open call processes were followed, and that conference scores continued to improve during the time frame studied. These findings suggest that a systematic open call process helps to support equitable speaking opportunities for men and women at a national hospital medicine conference without a negative impact on conference quality.
To recruit more diverse speakers, open call and peer review processes were used in addition to deliberate efforts at ensuring diversity in speakers. We found that over time, the proportion of women with speaking opportunities increased from 2015 to 2019. Interestingly, workshops, which had open call processes in place for the duration of the study period, had almost equal numbers of men and women presenting in all years. We also found that the number of all-men speaking groups decreased between 2015 and 2019.
A single process change can impact gender equity, but the target of true equity is expected to require additional measures such as assessment of committee structures and diversity, checklists, and reporting structures (data analysis and plans when goals not achieved).10-13 For instance, the American Society for Microbiology General Meeting was able to achieve gender equity in speakers by a multifold approach including ensuring the program committee was aware of gender statistics, increasing female representation among session convener teams, and direct instruction to try to avoid all-male sessions.11
It is important to acknowledge that these processes do require valuable resources including time. SHM has historically used committee volunteers to conduct the peer review process with each committee member reviewing 20 to 30 workshop submissions and 30 to 50 didactic sessions. While open processes with peer review seem to generate improved gender equity, ensuring processes are in place during the selection process is also key.
Several recent notable efforts to enhance gender equity and to increase diversity have been proposed. One such example of a process that may further improve gender equity was proposed by editors at the Journal of Hospital Medicine to assess current representation via demographics including gender, race, and ethnicity of authors with plans to assess patterns in the coming years.14 The American College of Physicians also published a position paper on achieving gender equity with a recommendation that organizational policies and procedures should be implemented that address implicit bias.15
Our study showed that, from 2015 to 2019, conference evaluations saw a significant increase in the score concurrently with the rise in proportion of women speakers. This finding suggests that quality does not seem to be affected by this new methodology for speaker selection and in fact this methodology may actually help improve the overall quality of the conference. To our knowledge, this is one of the first studies to concurrently evaluate speaker gender equity with conference quality.
Our study offers several strengths. This study took a pragmatic approach to understanding how processes can impact gender equity, and we were able to take advantage of the evolution of the open call system (ie workshops which have been an open call process for the duration of the study versus speaking opportunities that were not).
Our study also has several limitations. First, this study is retrospective in nature and thus other processes could have contributed to the improved gender equity, such as an organization’s priorities over time. During this study period, the SHM conference saw an average 3% increase annually in women speakers and an increase of 8% from 2018 to 2019 for all speakers compared to national trends of approximately 1%,6 which suggests that the open call processes in place could be contributing to the overall increases seen. Similarly, because of the retrospective nature of the study, we cannot be certain that the improvements in conference scores were directly the result of improved gender equity, although it does suggest that the improvements in gender equity did not have an adverse impact on the scores. We also did not assess how the composition of selection committee members for the meeting could have impacted the overall composition of the speakers. Our study looked at diversity only from the perspective of gender in a binary fashion, and thus additional studies are needed to assess how to improve diversity overall. It is unclear how this new open call for speakers affects race and ethnic diversity specifically. Identifying gender for the purposes of this study was facilitated by speakers providing their own biographies and the respective pronouns used in those biographies, and thus gender was easier to ascertain than race and ethnicity, which are not as readily available. For organizations to understand their diversity, equity, and inclusion efforts, enhancing the ability to fairly track and measure diversity will be key. Lastly, understanding of the exact composition of hospitalists from both a gender and race/ethnicity perspective is lacking. Studies have suggested that, based upon those surveyed or studied, there is a fairly equal balance of men and women albeit in academic groups.3
CONCLUSIONS
An open call approach to speakers at a national hospitalist conference seems to have contributed to improvements regarding gender equity in speaking opportunities with a concurrent improvement in overall rating of the conference. The open call system is a potential mechanism that other institutions and organizations could employ to enhance their diversity efforts.
Acknowledgments
Society of Hospital Medicine Diversity, Equity, Inclusion Special Interest Group
Work Group for SPEAK UP: Marisha Burden, MD, Daniel Cabrera, MD, Amira del Pino-Jones, MD, Areeba Kara, MD, Angela Keniston, MSPH, Keshav Khanijow, MD, Flora Kisuule, MD, Chiara Mandel, Benji Mathews, MD, David Paje, MD, Stephan Papp, MD, Snehal Patel, MD, Suchita Shah Sata, MD, Dustin Smith, MD, Kevin Vuernick
1. Weaver AC, Wetterneck TB, Whelan CT, Hinami K. A matter of priorities? Exploring the persistent gender pay gap in hospital medicine. J Hosp Med. 2015;10(8):486-490. https://doi.org/10.1002/jhm.2400.
2. Jena AB, Olenski AR, Blumenthal DM. Sex differences in physician salary in US public medical schools. JAMA Intern Med. 2016;176(9):1294-1304. https://doi.org/10.1001/jamainternmed.2016.3284.
3. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340.
4. Silver JK, Ghalib R, Poorman JA, et al. Analysis of gender equity in leadership of physician-focused medical specialty societies, 2008-2017. JAMA Intern Med. 2019;179(3):433-435. https://doi.org/10.1001/jamainternmed.2018.5303.
5. Jena AB, Khullar D, Ho O, Olenski AR, Blumenthal DM. Sex differences in academic rank in US medical schools in 2014. JAMA. 2015;314(11):1149-1158. https://doi.org/10.1001/jama.2015.10680.
6. Ruzycki SM, Fletcher S, Earp M, Bharwani A, Lithgow KC. Trends in the Proportion of Female Speakers at Medical Conferences in the United States and in Canada, 2007 to 2017. JAMA Netw Open. 2019;2(4):e192103. https://doi.org/10.1001/jamanetworkopen.2019.2103
7. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5.
8. Today’s Hospitalist 2018 Compensation and Career Survey Results. https://www.todayshospitalist.com/salary-survey-results/. Accessed September 28, 2019.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
10. Burden M, del Pino-Jones A, Shafer M, Sheth S, Rexrode K. Association of American Medical Colleagues (AAMC) Group on Women in Medicine and Science. Recruitment Toolkit: https://www.aamc.org/download/492864/data/equityinrecruitmenttoolkit.pdf. Accessed July 27, 2019.
11. Casadevall A. Achieving speaker gender equity at the american society for microbiology general meeting. MBio. 2015;6:e01146. https://doi.org/10.1128/mBio.01146-15.
12. Westring A, McDonald JM, Carr P, Grisso JA. An integrated framework for gender equity in academic medicine. Acad Med. 2016;91(8):1041-1044. https://doi.org/10.1097/ACM.0000000000001275.
13. Martin JL. Ten simple rules to achieve conference speaker gender balance. PLoS Comput Biol. 2014;10(11):e1003903. https://doi.org/10.1371/journal.pcbi.1003903.
14. Shah SS, Shaughnessy EE, Spector ND. Leading by example: how medical journals can improve representation in academic medicine. J Hosp Med. 2019;14(7):393. https://doi.org/10.12788/jhm.3247.
15. Butkus R, Serchen J, Moyer DV, et al. Achieving gender equity in physician compensation and career advancement: a position paper of the American College of Physicians. Ann Intern Med. 2018;168:721-723. https://doi.org/10.7326/M17-3438.
1. Weaver AC, Wetterneck TB, Whelan CT, Hinami K. A matter of priorities? Exploring the persistent gender pay gap in hospital medicine. J Hosp Med. 2015;10(8):486-490. https://doi.org/10.1002/jhm.2400.
2. Jena AB, Olenski AR, Blumenthal DM. Sex differences in physician salary in US public medical schools. JAMA Intern Med. 2016;176(9):1294-1304. https://doi.org/10.1001/jamainternmed.2016.3284.
3. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340.
4. Silver JK, Ghalib R, Poorman JA, et al. Analysis of gender equity in leadership of physician-focused medical specialty societies, 2008-2017. JAMA Intern Med. 2019;179(3):433-435. https://doi.org/10.1001/jamainternmed.2018.5303.
5. Jena AB, Khullar D, Ho O, Olenski AR, Blumenthal DM. Sex differences in academic rank in US medical schools in 2014. JAMA. 2015;314(11):1149-1158. https://doi.org/10.1001/jama.2015.10680.
6. Ruzycki SM, Fletcher S, Earp M, Bharwani A, Lithgow KC. Trends in the Proportion of Female Speakers at Medical Conferences in the United States and in Canada, 2007 to 2017. JAMA Netw Open. 2019;2(4):e192103. https://doi.org/10.1001/jamanetworkopen.2019.2103
7. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5.
8. Today’s Hospitalist 2018 Compensation and Career Survey Results. https://www.todayshospitalist.com/salary-survey-results/. Accessed September 28, 2019.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
10. Burden M, del Pino-Jones A, Shafer M, Sheth S, Rexrode K. Association of American Medical Colleagues (AAMC) Group on Women in Medicine and Science. Recruitment Toolkit: https://www.aamc.org/download/492864/data/equityinrecruitmenttoolkit.pdf. Accessed July 27, 2019.
11. Casadevall A. Achieving speaker gender equity at the american society for microbiology general meeting. MBio. 2015;6:e01146. https://doi.org/10.1128/mBio.01146-15.
12. Westring A, McDonald JM, Carr P, Grisso JA. An integrated framework for gender equity in academic medicine. Acad Med. 2016;91(8):1041-1044. https://doi.org/10.1097/ACM.0000000000001275.
13. Martin JL. Ten simple rules to achieve conference speaker gender balance. PLoS Comput Biol. 2014;10(11):e1003903. https://doi.org/10.1371/journal.pcbi.1003903.
14. Shah SS, Shaughnessy EE, Spector ND. Leading by example: how medical journals can improve representation in academic medicine. J Hosp Med. 2019;14(7):393. https://doi.org/10.12788/jhm.3247.
15. Butkus R, Serchen J, Moyer DV, et al. Achieving gender equity in physician compensation and career advancement: a position paper of the American College of Physicians. Ann Intern Med. 2018;168:721-723. https://doi.org/10.7326/M17-3438.
© 2020 Society of Hospital Medicine
Nationwide Hospital Performance on Publicly Reported Episode Spending Measures
Amid the continued shift from fee-for-service toward value-based payment, policymakers such as the Centers for Medicare & Medicaid Services have initiated strategies to contain spending on episodes of care. This episode focus has led to nationwide implementation of payment models such as bundled payments, which hold hospitals accountable for quality and costs across procedure-based (eg, coronary artery bypass surgery) and condition-based (eg, congestive heart failure) episodes, which begin with hospitalization and encompass subsequent hospital and postdischarge care.
Simultaneously, Medicare has increased its emphasis on similarly designed episodes of care (eg, those spanning hospitalization and postdischarge care) using other strategies, such as public reporting and use of episode-based measures to evaluate hospital cost performance. In 2017, Medicare trialed the implementation of six Clinical Episode-Based Payment (CEBP) measures in the national Hospital Inpatient Quality Reporting Program in order to assess hospital and clinician spending on procedure and condition episodes.1,2
CEBP measures reflect episode-specific spending, conveying “how expensive a hospital is” by capturing facility and professional payments for a given episode spanning between 3 days prior to hospitalization and 30 days following discharge. Given standard payment rates used in Medicare, the variation in episode spending reflects differences in quantity and type of services utilized within an episode. Medicare has specified episode-related services and designed CEBP measures via logic and definition rules informed by a combination of claims and procedures-based grouping, as well as by physician input. For example, the CEBP measure for cellulitis encompasses services related to diagnosing and treating the infection within the episode window, but not unrelated services such as eye exams for coexisting glaucoma. To increase clinical salience, CEBP measures are subdivided to reflect differing complexity when possible. For instance, cellulitis measures are divided into episodes with or without major complications or comorbidities and further subdivided into subtypes for episodes reflecting cellulitis in patients with diabetes, patients with decubitus ulcers, or neither.
CEBPs are similar to other spending measures used in payment programs, such as the Medicare Spending Per Beneficiary, but are more clinically relevant because their focus on episodes more closely reflects clinical practice. CEBPs and Medicare Spending Per Beneficiary have similar designs (eg, same episode windows) and purpose (eg, to capture the cost efficiency of hospital care).3 However, unlike CEBPs, Medicare Spending Per Beneficiary is a “global” measure that summarizes a hospital’s cost efficiency aggregated across all inpatient episodes rather than represent it based on specific conditions or procedures.4 The limitations of publicly reported global hospital measures—for instance, the poor correlation between hospital performance on distinct publicly reported quality measures5—highlight the potential utility of episode-specific spending measures such as CEBP.
Compared with episode-based payment models, initiatives such as CEBP measures have gone largely unstudied. However, they represent signals of Medicare’s growing commitment to addressing care episodes, tested without potentially tedious rulemaking required to change payment. In fact, publicly reported episode spending measures offer policymakers several interrelated benefits: the ability to rapidly evaluate performance at a large number of hospitals (eg, Medicare scaling up CEBP measures among all eligible hospitals nationwide), the option of leveraging publicly reported feedback to prompt clinical improvements (eg, by including CEBP measures in the Hospital Inpatient Quality Reporting Program), and the platform for developing and testing promising spending measures for subsequent use in formal payment models (eg, by using CEBP measures that possess large variation or cost-reduction opportunities in future bundled payment programs).
Despite these benefits, little is known about hospital performance on publicly reported episode-specific spending measures. We addressed this knowledge gap by providing what is, to our knowledge, the first nationwide description of hospital performance on such measures. We also evaluated which episode components accounted for spending variation in procedural vs condition episodes, examined whether CEBP measures can be used to effectively identify high- vs low-cost hospitals, and compared spending performance on CEBPs vs Medicare Spending Per Beneficiary.
METHODS
Data and Study Sample
We utilized publicly available data from Hospital Compare, which include information about hospital-level CEBP and Medicare Spending Per Beneficiary performance for Medicare-certified acute care hospitals nationwide.5 Our analysis evaluated the six CEBP measures tested by Medicare in 2017: three conditions (cellulitis, kidney/urinary tract infection [UTI], gastrointestinal hemorrhage) and three procedures (spinal fusion, cholecystectomy and common duct exploration, and aortic aneurysm repair). Per Medicare rules, CEBP measures are calculated only for hospitals with requisite volume for targeted conditions (minimum of 40 episodes) and procedures (minimum of 25 episodes) and are reported on Hospital Compare in risk-adjusted (eg, for age, hierarchical condition categories in alignment with existing Medicare methodology) and payment-standardized form (ie, accounts for wage index, medical education, disproportionate share hospital payments) . Each CEBP encompasses episodes with or without major complications/comorbidities.
For each hospital, CEBP spending is reported as average total episode spending, as well as average spending on specific components. We grouped components into three groups: hospitalization, skilled nursing facility (SNF) use, and other (encompassing postdischarge readmissions, emergency department visits, and home health agency use), with a focus on SNF given existing evidence from episode-based payment models about the opportunity for savings from reduced SNF care. Hospital Compare also provides information about the national CEBP measure performance (ie, average spending for a given episode type among all eligible hospitals nationwide).
Hospital Groups
To evaluate hospitals’ CEBP performance for specific episode types, we categorized hospitals as either “below average spending” if their average episode spending was below the national average or “above average spending” if spending was above the national average. According to this approach, a hospital could have below average spending for some episodes but above average spending for others.
To compare hospitals across episode types simultaneously, we categorized hospitals as “low cost” if episode spending was below the national average for all applicable measures, “high cost” if episode spending was above the national average for all applicable measures, or “mixed cost” if episode spending was above the national average for some measures and below for others.
We also conducted sensitivity analyses using alternative hospital group definitions. For comparisons of specific episode types, we categorized hospitals as “high spending” (top quartile of average episode spending among eligible hospitals) or “other spending” (all others). For comparisons across all episode types, we focused on SNF care and categorized hospitals as “high SNF cost” (top quartile of episode spending attributed to SNF care) and “other SNF cost” (all others). We applied a similar approach to Medicare Spending Per Beneficiary, categorizing hospitals as either “low MSPB cost” if their episode spending was below the national average for Medicare Spending Per Beneficiary or “high MSPB cost” if not.
Statistical Analysis
We assessed variation by describing the distribution of total episode spending across eligible hospitals for each individual episode type, as well as the proportion of spending attributed to SNF care across all episode types. We reported the difference between the 10th and 90th percentile for each distribution to quantify variation. To evaluate how individual episode components contributed to overall spending variation, we used linear regression and applied analysis of variance to each episode component. Specifically, we regressed episode spending on each episode component (hospital, SNF, other) separately and used these results to generate predicted episode spending values for each hospital based on its value for each spending component. We then calculated the differen-ces (ie, residuals) between predicted and actual total episode spending values. We plotted residuals for each component, with lower residual plot variation (ie, a flatter curve) representing larger contribution of a spending component to overall spending variation.
Pearson correlation coefficients were used to assess within-hospital CEBP correlation (ie, the extent to which performance was hospital specific). We evaluated if and how components of spending varied across hospitals by comparing spending groups (for individual episode types) and cost groups (for all episode types). To test the robustness of these categories, we conducted sensitivity analyses using high spending vs other spending groups (for individual episode types) and high SNF cost vs low SNF cost groups (for all episode types).
To assess concordance between CEBP and Medicare Spending Per Beneficiary, we cross tabulated hospital CEBP performance (high vs low vs mixed cost) and Medicare Spending Per Beneficiary performance (high vs low MSPB cost). This approached allowed us to quantify the number of hospitals that have concordant performance for both types of spending measures (ie, high cost or low cost on both) and the number with discordant performance (eg, high cost on one spending measure but low cost on the other). We used Pearson correlation coefficients to assess correlation between CEBP and Medicare Spending Per Beneficiary, with evaluation of CEBP performance in aggregate form (ie, hospitals’ average CEBP performance across all eligible episode types) and by individual episode types.
Chi-square and Kruskal-Wallis tests were used to compare categorical and continuous variables, respectively. To compare spending amounts, we evaluated the distribution of total episode spending (Appendix Figure 1) and used ordinary least squares regression with spending as the dependent variable and hospital group, episode components, and their interaction as independent variables. Because CEBP dollar amounts are reported through Hospital Compare on a risk-adjusted and payment-standardized basis, no additional adjustments were applied. Analyses were performed using SAS version 9.4 (SAS Institute; Cary, NC) and all tests of significance were two-tailed at alpha=0.05.
RESULTS
Of 3,129 hospitals, 1,778 achieved minimum thresholds and had CEBPs calculated for at least one of the six CEBP episode types.
Variation in CEBP Performance
For each episode type, spending varied across eligible hospitals (Appendix Figure 2). In particular, the difference between the 10th and 90th percentile values for cellulitis, kidney/UTI, and gastrointestinal hemorrhage were $2,873, $3,514, and $2,982, respectively. Differences were greater for procedural episodes of aortic aneurysm ($17,860), spinal fusion ($11,893), and cholecystectomy ($3,689). Evaluated across all episode types, the proportion of episode spending attributed to SNF care also varied across hospitals (Appendix Figure 3), with a difference of 24.7% between the 10th (4.5%) and 90th (29.2%) percentile values.
Residual plots demonstrated differences in which episode components accounted for variation in overall spending. For aortic aneurysm episodes, variation in the SNF episode component best explained variation in episode spending and thus had the lowest residual plot variation, followed by other and hospital components (Figure). Similar patterns were observed for spinal fusion and cholecystectomy episodes. In contrast, for cellulitis episodes, all three components had comparable residual-plot variation, which indicates that the variation in the components explained episode spending variation similarly (Figure)—a pattern reflected in kidney/UTI and gastrointestinal hemorrhage episodes.
Correlation in Performance on CEBP Measures
Across hospitals in our sample, within-hospital correlations were generally low (Appendix Table 1). In particular, correlations ranged from
CEBP Performance by Hospital Groups
Overall spending on specific episode types varied across hospital groups (Table). Spending for aortic aneurysm episodes was $42,633 at hospitals with above average spending and $37,730 at those with below average spending, while spending for spinal fusion episodes was $39,231 at those with above average spending and $34,832 at those with below average spending. In comparison, spending at hospitals deemed above and below average spending for cellulitis episodes was $10,763 and $9,064, respectively, and $11,223 and $9,161 at hospitals deemed above and below average spending for kidney/UTI episodes, respectively.
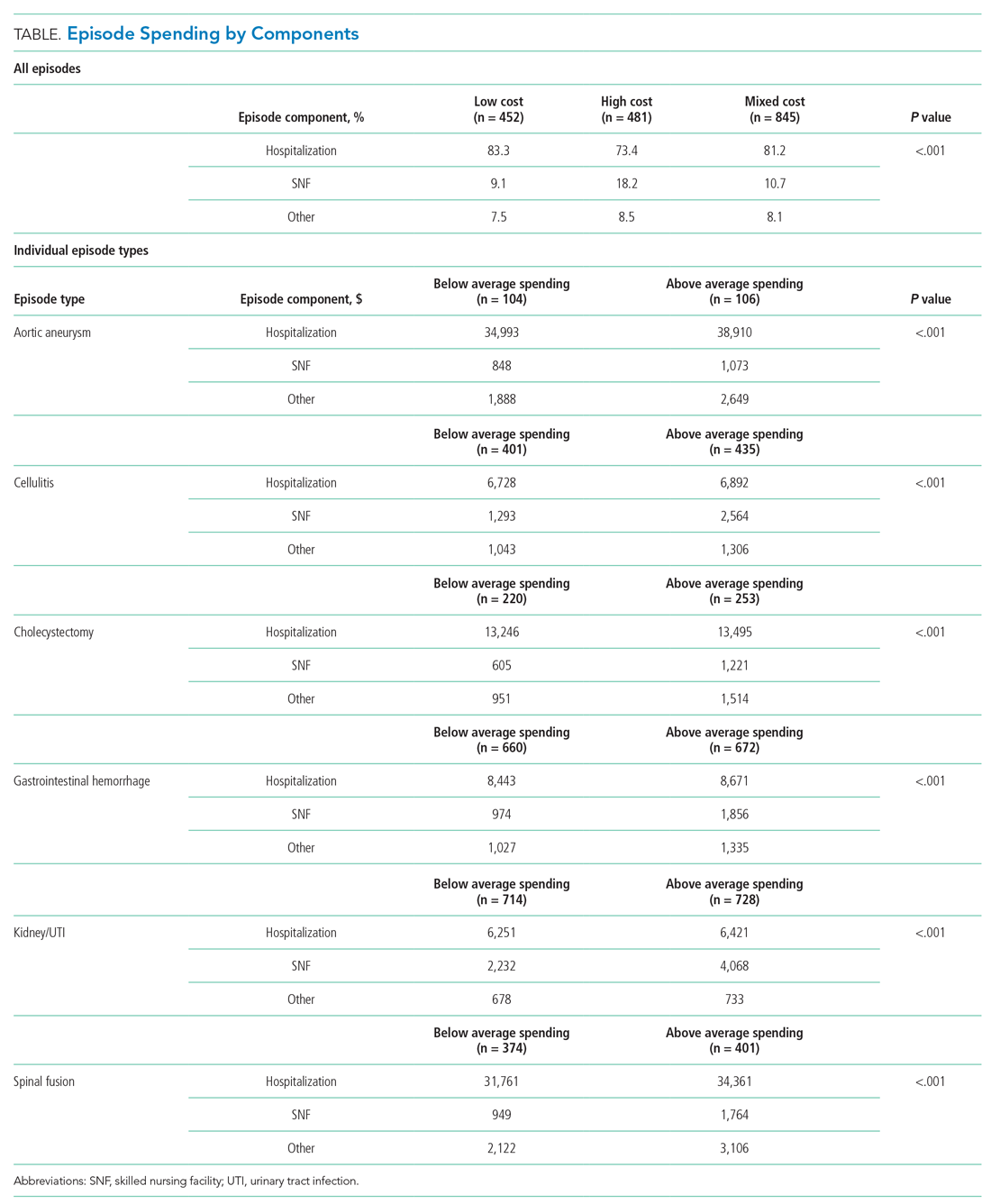
Spending on specific episode components also differed by hospital group (Table). Though the magnitude of absolute spending amounts and differences varied by specific episode, hospitals with above average spending tended to spend more on SNF than did those with below average spending. For example, hospitals with above average spending for cellulitis episodes spent an average of $2,564 on SNF (24% of overall episode spending) vs $1,293 (14% of episode spending) among those with below average spending. Similarly, hospitals with above and below average spending for kidney/UTI episodes spent $4,068 (36% of episode spending) and $2,232 (24% of episode spending) on SNF, respectively (P < .001 for both episode types). Findings were qualitatively similar in sensitivity analyses (Appendix Table 2).
Among hospitals in our sample, we categorized 481 as high cost (27%), 452 as low cost (25%), and 845 as mixed cost (48%), with hospital groups distributed broadly nationwide (Appendix Figure 4). Evaluated on performance across all six episode types, hospital groups also demonstrated differences in spending by cost components (Table). In particular, spending in SNF ranged from 18.1% of overall episode spending among high-cost hospitals to 10.7% among mixed-cost hospitals and 9.2% among low-cost hospitals. Additionally, spending on hospitalization accounted for 83.3% of overall episode spending among low-cost hospitals, compared with 81.2% and 73.4% among mixed-cost and high-cost hospitals, respectively (P < .001). Comparisons were qualitatively similar in sensitivity analyses (Appendix Table 3).
Comparison of CEBP and Medicare Spending Per Beneficiary Performance
Correlation between Medicare Spending Per Beneficiary and aggregated CEBPs was 0.42 and, for individual episode types, ranged between 0.14 and 0.36 (Appendix Table 2). There was low concordance between hospital performance on CEBP and Medicare Spending Per Beneficiary. Across all eligible hospitals, only 16.3% (290/1778) had positive concordance between performance on the two measure types (ie, low cost for both), while 16.5% (293/1778) had negative concordance (ie, high cost for both). There was discordant performance in most instances (67.2%; 1195/1778), which reflecting favorable performance on one measure type but not the other.
DISCUSSION
To our knowledge, this study is the first to describe hospitals’ episode-specific spending performance nationwide. It demonstrated significant variation across hospitals driven by different episode components for different episode types. It also showed low correlation between individual episode spending measures and poor concordance between episode-specific and global hospital spending measures. Two practice and policy implications are noteworthy.
First, our findings corroborate and build upon evidence from bundled payment programs about the opportunity for hospitals to improve their cost efficiency. Findings from bundled payment evaluations of surgical episodes suggest that the major area for cost savings is in the reduction of institutional post-acute care use such as that of SNFs.7-9 We demonstrated similar opportunity in a national sample of hospitals, finding that, for the three evaluated procedural CEBPs, SNF care accounted for more variation in overall episode spending than did other components. While variation may imply opportunity for greater efficiency and standardization, it is important to note that variation itself is not inherently problematic. Additional studies are needed to distinguish between warranted and unwarranted variation in procedural episodes, as well as identify strategies for reducing the latter.
Though bundled payment evaluations have predominantly emphasized procedural episodes, existing evidence suggests that participation in medical condition bundles has not been associated with cost savings or utilization changes.7-15 Findings from our analysis of variance—that there appear to be smaller variation-reduction opportunities for condition episodes than for procedural episodes—offer insight into this issue. Existing episodes are initiated by hospitalization and extend into the postacute period, a design that may not afford substantial post-acute care savings opportunities for condition episodes. This is an important insight as policymakers consider how to best design condition-based episodes in the future (eg, whether to use non–hospital based episode triggers). Future work should evaluate whether our findings reflect inherent differences between condition and procedural episodes16 or whether interventions can still optimize SNF care for these episodes despite smaller variation.
Second, our results highlight the potential limitations of global performance measures such as Medicare Spending Per Beneficiary. As a general measure of hospital spending, Medicare Spending Per Beneficiary is based on the premise that hospitals can be categorized as high or low cost with consideration of all inpatient episodic care. However, our analyses suggest that hospitals may be high cost for certain episodes and low cost for others—a fact highlighted by the low correlation and high discordance observed between hospital CEBP and Medicare Spending Per Beneficiary performance. Because overarching measures may miss spending differen-ces related to underlying clinical scenarios, episode-specific spending measures would provide important perspective and complements to global measures for assessing hospital cost performance, particularly in an era of value-based payments. Policymakers should consider prioritizing the development and implementation of such measures.
Our study has limitations. First, it is descriptive in nature, and future work should evaluate the association between episode-specific spending measure performance and clinical and quality outcomes. Second, we evaluated all CEBP-eligible hospitals nationwide to provide a broad view of episode-specific spending. However, future studies should assess performance among hospital subtypes, such as vertically integrated or safety-net organizations, because they may be more or less able to perform on these spending measures. Third, though findings may not be generalizable to other clinical episodes, our results were qualitatively consistent across episode types and broadly consistent with evidence from episode-based payment models. Fourth, we analyzed cost from the perspective of utilization and did not incorporate price considerations, which may be more relevant for commercial insurers than it is for Medicare.
Nonetheless, the emergence of CEBPs reflects the ongoing shift in policymaker attention toward episode-specific spending. In particular, though further scale or use of CEBP measures has been put on hold amid other payment reform changes, their nationwide implementation in 2017 signals Medicare’s broad interest in evaluating all hospitals on episode-specific spending efficiency, in addition to other facets of spending, quality, safety, and patient experience. Importantly, such efforts complement other ongoing nationwide initiatives for emphasizing episode spending, such as use of episode-based cost measures within the Merit-Based Incentive Payment System17 to score clinicians and groups in part based on their episode-specific spending efficiency. Insight about episode spending performance could help hospitals prepare for environments with increasing focus on episode spending and as policymakers incorporate this perspective into quality and value-based payment policies.
1. Centers for Medicare & Medicaid Services. Fiscal Year 2019 Clinical Episode-Based Payment Measures Overview. https://www.qualityreportingcenter.com/globalassets/migrated-pdf/cepb_slides_npc-6.17.2018_5.22.18_vfinal508.pdf. Accessed November 26, 2019.
2. Centers for Medicare & Medicaid Services. Hospital Inpatient Quality Reporting Program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/HospitalRHQDAPU.html. Accessed November 23, 2019.
3. Centers for Medicare & Medicaid Services. Medicare Spending Per Beneficiary (MSPB) Spending Breakdown by Claim Type. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/hospital-value-based-purchasing/Downloads/Fact-Sheet-MSPB-Spending-Breakdowns-by-Claim-Type-Dec-2014.pdf. Accessed November 25, 2019.
4. Hu J, Jordan J, Rubinfeld I, Schreiber M, Waterman B, Nerenz D. Correlations among hospital quality measure: What “Hospital Compare” data tell us. Am J Med Qual. 2017;32(6):605-610. https://doi.org/10.1177/1062860616684012.
5. Centers for Medicare & Medicaid Services. Hospital Compare datasets. https://data.medicare.gov/data/hospital-compare. Accessed November 26, 2019.
6. American Hospital Association. AHA Data Products. https://www.aha.org/data-insights/aha-data-products. Accessed November 25, 2019.
7. Dummit LA, Kahvecioglu D, Marrufo G, et al. Bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016; 316(12):1267-1278. https://doi.org/10.1001/jama.2016.12717.
8. Finkelstein A, Ji Y, Mahoney N, Skinner J. Mandatory medicare bundled payment program for lower extremity joint replacement and discharge to institutional postacute care: Interim analysis of the first year of a 5-year randomized trial. JAMA. 2018;320(9):892-900. https://doi.org/10.1001/jama.2018.12346.
9. Navathe AS, Troxel AB, Liao JM, et al. Cost of joint replacement using bundled payment models. JAMA Intern Med. 2017;177(2):214-222. https://doi.org/10.1001/jamainternmed.2016.8263.
10. Liao JM, Emanuel EJ, Polsky DE, et al. National representativeness of hospitals and markets in Medicare’s mandatory bundled payment program. Health Aff. 2019;38(1):44-53.
11. Barnett ML, Wilcock A, McWilliams JM, et al. Two-year evaluation of mandatory bundled payments for joint replacement. N Engl J Med. 2019;380(3):252-262. https://doi.org/10.1056/NEJMsa1809010.
12. Navathe AS, Liao JM, Polsky D, et al. Comparison of hospitals participating in Medicare’s voluntary and mandatory orthopedic bundle programs. Health Aff. 2018;37(6):854-863. https://www.doi.org/10.1377/hlthaff.2017.1358.
13. Joynt Maddox KE, Orav EJ, Zheng J, Epstein AM. Participation and Dropout in the Bundled Payments for Care Improvement Initiative. JAMA. 2018;319(2):191-193. https://doi.org/10.1001/jama.2017.14771.
14. Navathe AS, Liao JM, Dykstra SE, et al. Association of hospital participation in a Medicare bundled payment program with volume and case mix of lower extremity joint replacement episodes. JAMA. 2018;320(9):901-910. https://doi.org/10.1001/jama.2018.12345.
15. Joynt Maddox KE, Orav EJ, Epstein AM. Medicare’s bundled payments initiative for medical conditions. N Engl J Med. 2018;379(18):e33. https://doi.org/10.1056/NEJMc1811049.
16. Navathe AS, Shan E, Liao JM. What have we learned about bundling medical conditions? Health Affairs Blog. https://www.healthaffairs.org/do/10.1377/hblog20180828.844613/full/. Accessed November 25, 2019.
17. Centers for Medicare & Medicaid Services. MACRA. https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/value-based-programs/macra-mips-and-apms/macra-mips-and-apms.html. Accessed November 26, 2019.
Amid the continued shift from fee-for-service toward value-based payment, policymakers such as the Centers for Medicare & Medicaid Services have initiated strategies to contain spending on episodes of care. This episode focus has led to nationwide implementation of payment models such as bundled payments, which hold hospitals accountable for quality and costs across procedure-based (eg, coronary artery bypass surgery) and condition-based (eg, congestive heart failure) episodes, which begin with hospitalization and encompass subsequent hospital and postdischarge care.
Simultaneously, Medicare has increased its emphasis on similarly designed episodes of care (eg, those spanning hospitalization and postdischarge care) using other strategies, such as public reporting and use of episode-based measures to evaluate hospital cost performance. In 2017, Medicare trialed the implementation of six Clinical Episode-Based Payment (CEBP) measures in the national Hospital Inpatient Quality Reporting Program in order to assess hospital and clinician spending on procedure and condition episodes.1,2
CEBP measures reflect episode-specific spending, conveying “how expensive a hospital is” by capturing facility and professional payments for a given episode spanning between 3 days prior to hospitalization and 30 days following discharge. Given standard payment rates used in Medicare, the variation in episode spending reflects differences in quantity and type of services utilized within an episode. Medicare has specified episode-related services and designed CEBP measures via logic and definition rules informed by a combination of claims and procedures-based grouping, as well as by physician input. For example, the CEBP measure for cellulitis encompasses services related to diagnosing and treating the infection within the episode window, but not unrelated services such as eye exams for coexisting glaucoma. To increase clinical salience, CEBP measures are subdivided to reflect differing complexity when possible. For instance, cellulitis measures are divided into episodes with or without major complications or comorbidities and further subdivided into subtypes for episodes reflecting cellulitis in patients with diabetes, patients with decubitus ulcers, or neither.
CEBPs are similar to other spending measures used in payment programs, such as the Medicare Spending Per Beneficiary, but are more clinically relevant because their focus on episodes more closely reflects clinical practice. CEBPs and Medicare Spending Per Beneficiary have similar designs (eg, same episode windows) and purpose (eg, to capture the cost efficiency of hospital care).3 However, unlike CEBPs, Medicare Spending Per Beneficiary is a “global” measure that summarizes a hospital’s cost efficiency aggregated across all inpatient episodes rather than represent it based on specific conditions or procedures.4 The limitations of publicly reported global hospital measures—for instance, the poor correlation between hospital performance on distinct publicly reported quality measures5—highlight the potential utility of episode-specific spending measures such as CEBP.
Compared with episode-based payment models, initiatives such as CEBP measures have gone largely unstudied. However, they represent signals of Medicare’s growing commitment to addressing care episodes, tested without potentially tedious rulemaking required to change payment. In fact, publicly reported episode spending measures offer policymakers several interrelated benefits: the ability to rapidly evaluate performance at a large number of hospitals (eg, Medicare scaling up CEBP measures among all eligible hospitals nationwide), the option of leveraging publicly reported feedback to prompt clinical improvements (eg, by including CEBP measures in the Hospital Inpatient Quality Reporting Program), and the platform for developing and testing promising spending measures for subsequent use in formal payment models (eg, by using CEBP measures that possess large variation or cost-reduction opportunities in future bundled payment programs).
Despite these benefits, little is known about hospital performance on publicly reported episode-specific spending measures. We addressed this knowledge gap by providing what is, to our knowledge, the first nationwide description of hospital performance on such measures. We also evaluated which episode components accounted for spending variation in procedural vs condition episodes, examined whether CEBP measures can be used to effectively identify high- vs low-cost hospitals, and compared spending performance on CEBPs vs Medicare Spending Per Beneficiary.
METHODS
Data and Study Sample
We utilized publicly available data from Hospital Compare, which include information about hospital-level CEBP and Medicare Spending Per Beneficiary performance for Medicare-certified acute care hospitals nationwide.5 Our analysis evaluated the six CEBP measures tested by Medicare in 2017: three conditions (cellulitis, kidney/urinary tract infection [UTI], gastrointestinal hemorrhage) and three procedures (spinal fusion, cholecystectomy and common duct exploration, and aortic aneurysm repair). Per Medicare rules, CEBP measures are calculated only for hospitals with requisite volume for targeted conditions (minimum of 40 episodes) and procedures (minimum of 25 episodes) and are reported on Hospital Compare in risk-adjusted (eg, for age, hierarchical condition categories in alignment with existing Medicare methodology) and payment-standardized form (ie, accounts for wage index, medical education, disproportionate share hospital payments) . Each CEBP encompasses episodes with or without major complications/comorbidities.
For each hospital, CEBP spending is reported as average total episode spending, as well as average spending on specific components. We grouped components into three groups: hospitalization, skilled nursing facility (SNF) use, and other (encompassing postdischarge readmissions, emergency department visits, and home health agency use), with a focus on SNF given existing evidence from episode-based payment models about the opportunity for savings from reduced SNF care. Hospital Compare also provides information about the national CEBP measure performance (ie, average spending for a given episode type among all eligible hospitals nationwide).
Hospital Groups
To evaluate hospitals’ CEBP performance for specific episode types, we categorized hospitals as either “below average spending” if their average episode spending was below the national average or “above average spending” if spending was above the national average. According to this approach, a hospital could have below average spending for some episodes but above average spending for others.
To compare hospitals across episode types simultaneously, we categorized hospitals as “low cost” if episode spending was below the national average for all applicable measures, “high cost” if episode spending was above the national average for all applicable measures, or “mixed cost” if episode spending was above the national average for some measures and below for others.
We also conducted sensitivity analyses using alternative hospital group definitions. For comparisons of specific episode types, we categorized hospitals as “high spending” (top quartile of average episode spending among eligible hospitals) or “other spending” (all others). For comparisons across all episode types, we focused on SNF care and categorized hospitals as “high SNF cost” (top quartile of episode spending attributed to SNF care) and “other SNF cost” (all others). We applied a similar approach to Medicare Spending Per Beneficiary, categorizing hospitals as either “low MSPB cost” if their episode spending was below the national average for Medicare Spending Per Beneficiary or “high MSPB cost” if not.
Statistical Analysis
We assessed variation by describing the distribution of total episode spending across eligible hospitals for each individual episode type, as well as the proportion of spending attributed to SNF care across all episode types. We reported the difference between the 10th and 90th percentile for each distribution to quantify variation. To evaluate how individual episode components contributed to overall spending variation, we used linear regression and applied analysis of variance to each episode component. Specifically, we regressed episode spending on each episode component (hospital, SNF, other) separately and used these results to generate predicted episode spending values for each hospital based on its value for each spending component. We then calculated the differen-ces (ie, residuals) between predicted and actual total episode spending values. We plotted residuals for each component, with lower residual plot variation (ie, a flatter curve) representing larger contribution of a spending component to overall spending variation.
Pearson correlation coefficients were used to assess within-hospital CEBP correlation (ie, the extent to which performance was hospital specific). We evaluated if and how components of spending varied across hospitals by comparing spending groups (for individual episode types) and cost groups (for all episode types). To test the robustness of these categories, we conducted sensitivity analyses using high spending vs other spending groups (for individual episode types) and high SNF cost vs low SNF cost groups (for all episode types).
To assess concordance between CEBP and Medicare Spending Per Beneficiary, we cross tabulated hospital CEBP performance (high vs low vs mixed cost) and Medicare Spending Per Beneficiary performance (high vs low MSPB cost). This approached allowed us to quantify the number of hospitals that have concordant performance for both types of spending measures (ie, high cost or low cost on both) and the number with discordant performance (eg, high cost on one spending measure but low cost on the other). We used Pearson correlation coefficients to assess correlation between CEBP and Medicare Spending Per Beneficiary, with evaluation of CEBP performance in aggregate form (ie, hospitals’ average CEBP performance across all eligible episode types) and by individual episode types.
Chi-square and Kruskal-Wallis tests were used to compare categorical and continuous variables, respectively. To compare spending amounts, we evaluated the distribution of total episode spending (Appendix Figure 1) and used ordinary least squares regression with spending as the dependent variable and hospital group, episode components, and their interaction as independent variables. Because CEBP dollar amounts are reported through Hospital Compare on a risk-adjusted and payment-standardized basis, no additional adjustments were applied. Analyses were performed using SAS version 9.4 (SAS Institute; Cary, NC) and all tests of significance were two-tailed at alpha=0.05.
RESULTS
Of 3,129 hospitals, 1,778 achieved minimum thresholds and had CEBPs calculated for at least one of the six CEBP episode types.
Variation in CEBP Performance
For each episode type, spending varied across eligible hospitals (Appendix Figure 2). In particular, the difference between the 10th and 90th percentile values for cellulitis, kidney/UTI, and gastrointestinal hemorrhage were $2,873, $3,514, and $2,982, respectively. Differences were greater for procedural episodes of aortic aneurysm ($17,860), spinal fusion ($11,893), and cholecystectomy ($3,689). Evaluated across all episode types, the proportion of episode spending attributed to SNF care also varied across hospitals (Appendix Figure 3), with a difference of 24.7% between the 10th (4.5%) and 90th (29.2%) percentile values.
Residual plots demonstrated differences in which episode components accounted for variation in overall spending. For aortic aneurysm episodes, variation in the SNF episode component best explained variation in episode spending and thus had the lowest residual plot variation, followed by other and hospital components (Figure). Similar patterns were observed for spinal fusion and cholecystectomy episodes. In contrast, for cellulitis episodes, all three components had comparable residual-plot variation, which indicates that the variation in the components explained episode spending variation similarly (Figure)—a pattern reflected in kidney/UTI and gastrointestinal hemorrhage episodes.
Correlation in Performance on CEBP Measures
Across hospitals in our sample, within-hospital correlations were generally low (Appendix Table 1). In particular, correlations ranged from
CEBP Performance by Hospital Groups
Overall spending on specific episode types varied across hospital groups (Table). Spending for aortic aneurysm episodes was $42,633 at hospitals with above average spending and $37,730 at those with below average spending, while spending for spinal fusion episodes was $39,231 at those with above average spending and $34,832 at those with below average spending. In comparison, spending at hospitals deemed above and below average spending for cellulitis episodes was $10,763 and $9,064, respectively, and $11,223 and $9,161 at hospitals deemed above and below average spending for kidney/UTI episodes, respectively.

Spending on specific episode components also differed by hospital group (Table). Though the magnitude of absolute spending amounts and differences varied by specific episode, hospitals with above average spending tended to spend more on SNF than did those with below average spending. For example, hospitals with above average spending for cellulitis episodes spent an average of $2,564 on SNF (24% of overall episode spending) vs $1,293 (14% of episode spending) among those with below average spending. Similarly, hospitals with above and below average spending for kidney/UTI episodes spent $4,068 (36% of episode spending) and $2,232 (24% of episode spending) on SNF, respectively (P < .001 for both episode types). Findings were qualitatively similar in sensitivity analyses (Appendix Table 2).
Among hospitals in our sample, we categorized 481 as high cost (27%), 452 as low cost (25%), and 845 as mixed cost (48%), with hospital groups distributed broadly nationwide (Appendix Figure 4). Evaluated on performance across all six episode types, hospital groups also demonstrated differences in spending by cost components (Table). In particular, spending in SNF ranged from 18.1% of overall episode spending among high-cost hospitals to 10.7% among mixed-cost hospitals and 9.2% among low-cost hospitals. Additionally, spending on hospitalization accounted for 83.3% of overall episode spending among low-cost hospitals, compared with 81.2% and 73.4% among mixed-cost and high-cost hospitals, respectively (P < .001). Comparisons were qualitatively similar in sensitivity analyses (Appendix Table 3).
Comparison of CEBP and Medicare Spending Per Beneficiary Performance
Correlation between Medicare Spending Per Beneficiary and aggregated CEBPs was 0.42 and, for individual episode types, ranged between 0.14 and 0.36 (Appendix Table 2). There was low concordance between hospital performance on CEBP and Medicare Spending Per Beneficiary. Across all eligible hospitals, only 16.3% (290/1778) had positive concordance between performance on the two measure types (ie, low cost for both), while 16.5% (293/1778) had negative concordance (ie, high cost for both). There was discordant performance in most instances (67.2%; 1195/1778), which reflecting favorable performance on one measure type but not the other.
DISCUSSION
To our knowledge, this study is the first to describe hospitals’ episode-specific spending performance nationwide. It demonstrated significant variation across hospitals driven by different episode components for different episode types. It also showed low correlation between individual episode spending measures and poor concordance between episode-specific and global hospital spending measures. Two practice and policy implications are noteworthy.
First, our findings corroborate and build upon evidence from bundled payment programs about the opportunity for hospitals to improve their cost efficiency. Findings from bundled payment evaluations of surgical episodes suggest that the major area for cost savings is in the reduction of institutional post-acute care use such as that of SNFs.7-9 We demonstrated similar opportunity in a national sample of hospitals, finding that, for the three evaluated procedural CEBPs, SNF care accounted for more variation in overall episode spending than did other components. While variation may imply opportunity for greater efficiency and standardization, it is important to note that variation itself is not inherently problematic. Additional studies are needed to distinguish between warranted and unwarranted variation in procedural episodes, as well as identify strategies for reducing the latter.
Though bundled payment evaluations have predominantly emphasized procedural episodes, existing evidence suggests that participation in medical condition bundles has not been associated with cost savings or utilization changes.7-15 Findings from our analysis of variance—that there appear to be smaller variation-reduction opportunities for condition episodes than for procedural episodes—offer insight into this issue. Existing episodes are initiated by hospitalization and extend into the postacute period, a design that may not afford substantial post-acute care savings opportunities for condition episodes. This is an important insight as policymakers consider how to best design condition-based episodes in the future (eg, whether to use non–hospital based episode triggers). Future work should evaluate whether our findings reflect inherent differences between condition and procedural episodes16 or whether interventions can still optimize SNF care for these episodes despite smaller variation.
Second, our results highlight the potential limitations of global performance measures such as Medicare Spending Per Beneficiary. As a general measure of hospital spending, Medicare Spending Per Beneficiary is based on the premise that hospitals can be categorized as high or low cost with consideration of all inpatient episodic care. However, our analyses suggest that hospitals may be high cost for certain episodes and low cost for others—a fact highlighted by the low correlation and high discordance observed between hospital CEBP and Medicare Spending Per Beneficiary performance. Because overarching measures may miss spending differen-ces related to underlying clinical scenarios, episode-specific spending measures would provide important perspective and complements to global measures for assessing hospital cost performance, particularly in an era of value-based payments. Policymakers should consider prioritizing the development and implementation of such measures.
Our study has limitations. First, it is descriptive in nature, and future work should evaluate the association between episode-specific spending measure performance and clinical and quality outcomes. Second, we evaluated all CEBP-eligible hospitals nationwide to provide a broad view of episode-specific spending. However, future studies should assess performance among hospital subtypes, such as vertically integrated or safety-net organizations, because they may be more or less able to perform on these spending measures. Third, though findings may not be generalizable to other clinical episodes, our results were qualitatively consistent across episode types and broadly consistent with evidence from episode-based payment models. Fourth, we analyzed cost from the perspective of utilization and did not incorporate price considerations, which may be more relevant for commercial insurers than it is for Medicare.
Nonetheless, the emergence of CEBPs reflects the ongoing shift in policymaker attention toward episode-specific spending. In particular, though further scale or use of CEBP measures has been put on hold amid other payment reform changes, their nationwide implementation in 2017 signals Medicare’s broad interest in evaluating all hospitals on episode-specific spending efficiency, in addition to other facets of spending, quality, safety, and patient experience. Importantly, such efforts complement other ongoing nationwide initiatives for emphasizing episode spending, such as use of episode-based cost measures within the Merit-Based Incentive Payment System17 to score clinicians and groups in part based on their episode-specific spending efficiency. Insight about episode spending performance could help hospitals prepare for environments with increasing focus on episode spending and as policymakers incorporate this perspective into quality and value-based payment policies.
Amid the continued shift from fee-for-service toward value-based payment, policymakers such as the Centers for Medicare & Medicaid Services have initiated strategies to contain spending on episodes of care. This episode focus has led to nationwide implementation of payment models such as bundled payments, which hold hospitals accountable for quality and costs across procedure-based (eg, coronary artery bypass surgery) and condition-based (eg, congestive heart failure) episodes, which begin with hospitalization and encompass subsequent hospital and postdischarge care.
Simultaneously, Medicare has increased its emphasis on similarly designed episodes of care (eg, those spanning hospitalization and postdischarge care) using other strategies, such as public reporting and use of episode-based measures to evaluate hospital cost performance. In 2017, Medicare trialed the implementation of six Clinical Episode-Based Payment (CEBP) measures in the national Hospital Inpatient Quality Reporting Program in order to assess hospital and clinician spending on procedure and condition episodes.1,2
CEBP measures reflect episode-specific spending, conveying “how expensive a hospital is” by capturing facility and professional payments for a given episode spanning between 3 days prior to hospitalization and 30 days following discharge. Given standard payment rates used in Medicare, the variation in episode spending reflects differences in quantity and type of services utilized within an episode. Medicare has specified episode-related services and designed CEBP measures via logic and definition rules informed by a combination of claims and procedures-based grouping, as well as by physician input. For example, the CEBP measure for cellulitis encompasses services related to diagnosing and treating the infection within the episode window, but not unrelated services such as eye exams for coexisting glaucoma. To increase clinical salience, CEBP measures are subdivided to reflect differing complexity when possible. For instance, cellulitis measures are divided into episodes with or without major complications or comorbidities and further subdivided into subtypes for episodes reflecting cellulitis in patients with diabetes, patients with decubitus ulcers, or neither.
CEBPs are similar to other spending measures used in payment programs, such as the Medicare Spending Per Beneficiary, but are more clinically relevant because their focus on episodes more closely reflects clinical practice. CEBPs and Medicare Spending Per Beneficiary have similar designs (eg, same episode windows) and purpose (eg, to capture the cost efficiency of hospital care).3 However, unlike CEBPs, Medicare Spending Per Beneficiary is a “global” measure that summarizes a hospital’s cost efficiency aggregated across all inpatient episodes rather than represent it based on specific conditions or procedures.4 The limitations of publicly reported global hospital measures—for instance, the poor correlation between hospital performance on distinct publicly reported quality measures5—highlight the potential utility of episode-specific spending measures such as CEBP.
Compared with episode-based payment models, initiatives such as CEBP measures have gone largely unstudied. However, they represent signals of Medicare’s growing commitment to addressing care episodes, tested without potentially tedious rulemaking required to change payment. In fact, publicly reported episode spending measures offer policymakers several interrelated benefits: the ability to rapidly evaluate performance at a large number of hospitals (eg, Medicare scaling up CEBP measures among all eligible hospitals nationwide), the option of leveraging publicly reported feedback to prompt clinical improvements (eg, by including CEBP measures in the Hospital Inpatient Quality Reporting Program), and the platform for developing and testing promising spending measures for subsequent use in formal payment models (eg, by using CEBP measures that possess large variation or cost-reduction opportunities in future bundled payment programs).
Despite these benefits, little is known about hospital performance on publicly reported episode-specific spending measures. We addressed this knowledge gap by providing what is, to our knowledge, the first nationwide description of hospital performance on such measures. We also evaluated which episode components accounted for spending variation in procedural vs condition episodes, examined whether CEBP measures can be used to effectively identify high- vs low-cost hospitals, and compared spending performance on CEBPs vs Medicare Spending Per Beneficiary.
METHODS
Data and Study Sample
We utilized publicly available data from Hospital Compare, which include information about hospital-level CEBP and Medicare Spending Per Beneficiary performance for Medicare-certified acute care hospitals nationwide.5 Our analysis evaluated the six CEBP measures tested by Medicare in 2017: three conditions (cellulitis, kidney/urinary tract infection [UTI], gastrointestinal hemorrhage) and three procedures (spinal fusion, cholecystectomy and common duct exploration, and aortic aneurysm repair). Per Medicare rules, CEBP measures are calculated only for hospitals with requisite volume for targeted conditions (minimum of 40 episodes) and procedures (minimum of 25 episodes) and are reported on Hospital Compare in risk-adjusted (eg, for age, hierarchical condition categories in alignment with existing Medicare methodology) and payment-standardized form (ie, accounts for wage index, medical education, disproportionate share hospital payments) . Each CEBP encompasses episodes with or without major complications/comorbidities.
For each hospital, CEBP spending is reported as average total episode spending, as well as average spending on specific components. We grouped components into three groups: hospitalization, skilled nursing facility (SNF) use, and other (encompassing postdischarge readmissions, emergency department visits, and home health agency use), with a focus on SNF given existing evidence from episode-based payment models about the opportunity for savings from reduced SNF care. Hospital Compare also provides information about the national CEBP measure performance (ie, average spending for a given episode type among all eligible hospitals nationwide).
Hospital Groups
To evaluate hospitals’ CEBP performance for specific episode types, we categorized hospitals as either “below average spending” if their average episode spending was below the national average or “above average spending” if spending was above the national average. According to this approach, a hospital could have below average spending for some episodes but above average spending for others.
To compare hospitals across episode types simultaneously, we categorized hospitals as “low cost” if episode spending was below the national average for all applicable measures, “high cost” if episode spending was above the national average for all applicable measures, or “mixed cost” if episode spending was above the national average for some measures and below for others.
We also conducted sensitivity analyses using alternative hospital group definitions. For comparisons of specific episode types, we categorized hospitals as “high spending” (top quartile of average episode spending among eligible hospitals) or “other spending” (all others). For comparisons across all episode types, we focused on SNF care and categorized hospitals as “high SNF cost” (top quartile of episode spending attributed to SNF care) and “other SNF cost” (all others). We applied a similar approach to Medicare Spending Per Beneficiary, categorizing hospitals as either “low MSPB cost” if their episode spending was below the national average for Medicare Spending Per Beneficiary or “high MSPB cost” if not.
Statistical Analysis
We assessed variation by describing the distribution of total episode spending across eligible hospitals for each individual episode type, as well as the proportion of spending attributed to SNF care across all episode types. We reported the difference between the 10th and 90th percentile for each distribution to quantify variation. To evaluate how individual episode components contributed to overall spending variation, we used linear regression and applied analysis of variance to each episode component. Specifically, we regressed episode spending on each episode component (hospital, SNF, other) separately and used these results to generate predicted episode spending values for each hospital based on its value for each spending component. We then calculated the differen-ces (ie, residuals) between predicted and actual total episode spending values. We plotted residuals for each component, with lower residual plot variation (ie, a flatter curve) representing larger contribution of a spending component to overall spending variation.
Pearson correlation coefficients were used to assess within-hospital CEBP correlation (ie, the extent to which performance was hospital specific). We evaluated if and how components of spending varied across hospitals by comparing spending groups (for individual episode types) and cost groups (for all episode types). To test the robustness of these categories, we conducted sensitivity analyses using high spending vs other spending groups (for individual episode types) and high SNF cost vs low SNF cost groups (for all episode types).
To assess concordance between CEBP and Medicare Spending Per Beneficiary, we cross tabulated hospital CEBP performance (high vs low vs mixed cost) and Medicare Spending Per Beneficiary performance (high vs low MSPB cost). This approached allowed us to quantify the number of hospitals that have concordant performance for both types of spending measures (ie, high cost or low cost on both) and the number with discordant performance (eg, high cost on one spending measure but low cost on the other). We used Pearson correlation coefficients to assess correlation between CEBP and Medicare Spending Per Beneficiary, with evaluation of CEBP performance in aggregate form (ie, hospitals’ average CEBP performance across all eligible episode types) and by individual episode types.
Chi-square and Kruskal-Wallis tests were used to compare categorical and continuous variables, respectively. To compare spending amounts, we evaluated the distribution of total episode spending (Appendix Figure 1) and used ordinary least squares regression with spending as the dependent variable and hospital group, episode components, and their interaction as independent variables. Because CEBP dollar amounts are reported through Hospital Compare on a risk-adjusted and payment-standardized basis, no additional adjustments were applied. Analyses were performed using SAS version 9.4 (SAS Institute; Cary, NC) and all tests of significance were two-tailed at alpha=0.05.
RESULTS
Of 3,129 hospitals, 1,778 achieved minimum thresholds and had CEBPs calculated for at least one of the six CEBP episode types.
Variation in CEBP Performance
For each episode type, spending varied across eligible hospitals (Appendix Figure 2). In particular, the difference between the 10th and 90th percentile values for cellulitis, kidney/UTI, and gastrointestinal hemorrhage were $2,873, $3,514, and $2,982, respectively. Differences were greater for procedural episodes of aortic aneurysm ($17,860), spinal fusion ($11,893), and cholecystectomy ($3,689). Evaluated across all episode types, the proportion of episode spending attributed to SNF care also varied across hospitals (Appendix Figure 3), with a difference of 24.7% between the 10th (4.5%) and 90th (29.2%) percentile values.
Residual plots demonstrated differences in which episode components accounted for variation in overall spending. For aortic aneurysm episodes, variation in the SNF episode component best explained variation in episode spending and thus had the lowest residual plot variation, followed by other and hospital components (Figure). Similar patterns were observed for spinal fusion and cholecystectomy episodes. In contrast, for cellulitis episodes, all three components had comparable residual-plot variation, which indicates that the variation in the components explained episode spending variation similarly (Figure)—a pattern reflected in kidney/UTI and gastrointestinal hemorrhage episodes.
Correlation in Performance on CEBP Measures
Across hospitals in our sample, within-hospital correlations were generally low (Appendix Table 1). In particular, correlations ranged from
CEBP Performance by Hospital Groups
Overall spending on specific episode types varied across hospital groups (Table). Spending for aortic aneurysm episodes was $42,633 at hospitals with above average spending and $37,730 at those with below average spending, while spending for spinal fusion episodes was $39,231 at those with above average spending and $34,832 at those with below average spending. In comparison, spending at hospitals deemed above and below average spending for cellulitis episodes was $10,763 and $9,064, respectively, and $11,223 and $9,161 at hospitals deemed above and below average spending for kidney/UTI episodes, respectively.

Spending on specific episode components also differed by hospital group (Table). Though the magnitude of absolute spending amounts and differences varied by specific episode, hospitals with above average spending tended to spend more on SNF than did those with below average spending. For example, hospitals with above average spending for cellulitis episodes spent an average of $2,564 on SNF (24% of overall episode spending) vs $1,293 (14% of episode spending) among those with below average spending. Similarly, hospitals with above and below average spending for kidney/UTI episodes spent $4,068 (36% of episode spending) and $2,232 (24% of episode spending) on SNF, respectively (P < .001 for both episode types). Findings were qualitatively similar in sensitivity analyses (Appendix Table 2).
Among hospitals in our sample, we categorized 481 as high cost (27%), 452 as low cost (25%), and 845 as mixed cost (48%), with hospital groups distributed broadly nationwide (Appendix Figure 4). Evaluated on performance across all six episode types, hospital groups also demonstrated differences in spending by cost components (Table). In particular, spending in SNF ranged from 18.1% of overall episode spending among high-cost hospitals to 10.7% among mixed-cost hospitals and 9.2% among low-cost hospitals. Additionally, spending on hospitalization accounted for 83.3% of overall episode spending among low-cost hospitals, compared with 81.2% and 73.4% among mixed-cost and high-cost hospitals, respectively (P < .001). Comparisons were qualitatively similar in sensitivity analyses (Appendix Table 3).
Comparison of CEBP and Medicare Spending Per Beneficiary Performance
Correlation between Medicare Spending Per Beneficiary and aggregated CEBPs was 0.42 and, for individual episode types, ranged between 0.14 and 0.36 (Appendix Table 2). There was low concordance between hospital performance on CEBP and Medicare Spending Per Beneficiary. Across all eligible hospitals, only 16.3% (290/1778) had positive concordance between performance on the two measure types (ie, low cost for both), while 16.5% (293/1778) had negative concordance (ie, high cost for both). There was discordant performance in most instances (67.2%; 1195/1778), which reflecting favorable performance on one measure type but not the other.
DISCUSSION
To our knowledge, this study is the first to describe hospitals’ episode-specific spending performance nationwide. It demonstrated significant variation across hospitals driven by different episode components for different episode types. It also showed low correlation between individual episode spending measures and poor concordance between episode-specific and global hospital spending measures. Two practice and policy implications are noteworthy.
First, our findings corroborate and build upon evidence from bundled payment programs about the opportunity for hospitals to improve their cost efficiency. Findings from bundled payment evaluations of surgical episodes suggest that the major area for cost savings is in the reduction of institutional post-acute care use such as that of SNFs.7-9 We demonstrated similar opportunity in a national sample of hospitals, finding that, for the three evaluated procedural CEBPs, SNF care accounted for more variation in overall episode spending than did other components. While variation may imply opportunity for greater efficiency and standardization, it is important to note that variation itself is not inherently problematic. Additional studies are needed to distinguish between warranted and unwarranted variation in procedural episodes, as well as identify strategies for reducing the latter.
Though bundled payment evaluations have predominantly emphasized procedural episodes, existing evidence suggests that participation in medical condition bundles has not been associated with cost savings or utilization changes.7-15 Findings from our analysis of variance—that there appear to be smaller variation-reduction opportunities for condition episodes than for procedural episodes—offer insight into this issue. Existing episodes are initiated by hospitalization and extend into the postacute period, a design that may not afford substantial post-acute care savings opportunities for condition episodes. This is an important insight as policymakers consider how to best design condition-based episodes in the future (eg, whether to use non–hospital based episode triggers). Future work should evaluate whether our findings reflect inherent differences between condition and procedural episodes16 or whether interventions can still optimize SNF care for these episodes despite smaller variation.
Second, our results highlight the potential limitations of global performance measures such as Medicare Spending Per Beneficiary. As a general measure of hospital spending, Medicare Spending Per Beneficiary is based on the premise that hospitals can be categorized as high or low cost with consideration of all inpatient episodic care. However, our analyses suggest that hospitals may be high cost for certain episodes and low cost for others—a fact highlighted by the low correlation and high discordance observed between hospital CEBP and Medicare Spending Per Beneficiary performance. Because overarching measures may miss spending differen-ces related to underlying clinical scenarios, episode-specific spending measures would provide important perspective and complements to global measures for assessing hospital cost performance, particularly in an era of value-based payments. Policymakers should consider prioritizing the development and implementation of such measures.
Our study has limitations. First, it is descriptive in nature, and future work should evaluate the association between episode-specific spending measure performance and clinical and quality outcomes. Second, we evaluated all CEBP-eligible hospitals nationwide to provide a broad view of episode-specific spending. However, future studies should assess performance among hospital subtypes, such as vertically integrated or safety-net organizations, because they may be more or less able to perform on these spending measures. Third, though findings may not be generalizable to other clinical episodes, our results were qualitatively consistent across episode types and broadly consistent with evidence from episode-based payment models. Fourth, we analyzed cost from the perspective of utilization and did not incorporate price considerations, which may be more relevant for commercial insurers than it is for Medicare.
Nonetheless, the emergence of CEBPs reflects the ongoing shift in policymaker attention toward episode-specific spending. In particular, though further scale or use of CEBP measures has been put on hold amid other payment reform changes, their nationwide implementation in 2017 signals Medicare’s broad interest in evaluating all hospitals on episode-specific spending efficiency, in addition to other facets of spending, quality, safety, and patient experience. Importantly, such efforts complement other ongoing nationwide initiatives for emphasizing episode spending, such as use of episode-based cost measures within the Merit-Based Incentive Payment System17 to score clinicians and groups in part based on their episode-specific spending efficiency. Insight about episode spending performance could help hospitals prepare for environments with increasing focus on episode spending and as policymakers incorporate this perspective into quality and value-based payment policies.
1. Centers for Medicare & Medicaid Services. Fiscal Year 2019 Clinical Episode-Based Payment Measures Overview. https://www.qualityreportingcenter.com/globalassets/migrated-pdf/cepb_slides_npc-6.17.2018_5.22.18_vfinal508.pdf. Accessed November 26, 2019.
2. Centers for Medicare & Medicaid Services. Hospital Inpatient Quality Reporting Program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/HospitalRHQDAPU.html. Accessed November 23, 2019.
3. Centers for Medicare & Medicaid Services. Medicare Spending Per Beneficiary (MSPB) Spending Breakdown by Claim Type. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/hospital-value-based-purchasing/Downloads/Fact-Sheet-MSPB-Spending-Breakdowns-by-Claim-Type-Dec-2014.pdf. Accessed November 25, 2019.
4. Hu J, Jordan J, Rubinfeld I, Schreiber M, Waterman B, Nerenz D. Correlations among hospital quality measure: What “Hospital Compare” data tell us. Am J Med Qual. 2017;32(6):605-610. https://doi.org/10.1177/1062860616684012.
5. Centers for Medicare & Medicaid Services. Hospital Compare datasets. https://data.medicare.gov/data/hospital-compare. Accessed November 26, 2019.
6. American Hospital Association. AHA Data Products. https://www.aha.org/data-insights/aha-data-products. Accessed November 25, 2019.
7. Dummit LA, Kahvecioglu D, Marrufo G, et al. Bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016; 316(12):1267-1278. https://doi.org/10.1001/jama.2016.12717.
8. Finkelstein A, Ji Y, Mahoney N, Skinner J. Mandatory medicare bundled payment program for lower extremity joint replacement and discharge to institutional postacute care: Interim analysis of the first year of a 5-year randomized trial. JAMA. 2018;320(9):892-900. https://doi.org/10.1001/jama.2018.12346.
9. Navathe AS, Troxel AB, Liao JM, et al. Cost of joint replacement using bundled payment models. JAMA Intern Med. 2017;177(2):214-222. https://doi.org/10.1001/jamainternmed.2016.8263.
10. Liao JM, Emanuel EJ, Polsky DE, et al. National representativeness of hospitals and markets in Medicare’s mandatory bundled payment program. Health Aff. 2019;38(1):44-53.
11. Barnett ML, Wilcock A, McWilliams JM, et al. Two-year evaluation of mandatory bundled payments for joint replacement. N Engl J Med. 2019;380(3):252-262. https://doi.org/10.1056/NEJMsa1809010.
12. Navathe AS, Liao JM, Polsky D, et al. Comparison of hospitals participating in Medicare’s voluntary and mandatory orthopedic bundle programs. Health Aff. 2018;37(6):854-863. https://www.doi.org/10.1377/hlthaff.2017.1358.
13. Joynt Maddox KE, Orav EJ, Zheng J, Epstein AM. Participation and Dropout in the Bundled Payments for Care Improvement Initiative. JAMA. 2018;319(2):191-193. https://doi.org/10.1001/jama.2017.14771.
14. Navathe AS, Liao JM, Dykstra SE, et al. Association of hospital participation in a Medicare bundled payment program with volume and case mix of lower extremity joint replacement episodes. JAMA. 2018;320(9):901-910. https://doi.org/10.1001/jama.2018.12345.
15. Joynt Maddox KE, Orav EJ, Epstein AM. Medicare’s bundled payments initiative for medical conditions. N Engl J Med. 2018;379(18):e33. https://doi.org/10.1056/NEJMc1811049.
16. Navathe AS, Shan E, Liao JM. What have we learned about bundling medical conditions? Health Affairs Blog. https://www.healthaffairs.org/do/10.1377/hblog20180828.844613/full/. Accessed November 25, 2019.
17. Centers for Medicare & Medicaid Services. MACRA. https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/value-based-programs/macra-mips-and-apms/macra-mips-and-apms.html. Accessed November 26, 2019.
1. Centers for Medicare & Medicaid Services. Fiscal Year 2019 Clinical Episode-Based Payment Measures Overview. https://www.qualityreportingcenter.com/globalassets/migrated-pdf/cepb_slides_npc-6.17.2018_5.22.18_vfinal508.pdf. Accessed November 26, 2019.
2. Centers for Medicare & Medicaid Services. Hospital Inpatient Quality Reporting Program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/HospitalRHQDAPU.html. Accessed November 23, 2019.
3. Centers for Medicare & Medicaid Services. Medicare Spending Per Beneficiary (MSPB) Spending Breakdown by Claim Type. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/hospital-value-based-purchasing/Downloads/Fact-Sheet-MSPB-Spending-Breakdowns-by-Claim-Type-Dec-2014.pdf. Accessed November 25, 2019.
4. Hu J, Jordan J, Rubinfeld I, Schreiber M, Waterman B, Nerenz D. Correlations among hospital quality measure: What “Hospital Compare” data tell us. Am J Med Qual. 2017;32(6):605-610. https://doi.org/10.1177/1062860616684012.
5. Centers for Medicare & Medicaid Services. Hospital Compare datasets. https://data.medicare.gov/data/hospital-compare. Accessed November 26, 2019.
6. American Hospital Association. AHA Data Products. https://www.aha.org/data-insights/aha-data-products. Accessed November 25, 2019.
7. Dummit LA, Kahvecioglu D, Marrufo G, et al. Bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016; 316(12):1267-1278. https://doi.org/10.1001/jama.2016.12717.
8. Finkelstein A, Ji Y, Mahoney N, Skinner J. Mandatory medicare bundled payment program for lower extremity joint replacement and discharge to institutional postacute care: Interim analysis of the first year of a 5-year randomized trial. JAMA. 2018;320(9):892-900. https://doi.org/10.1001/jama.2018.12346.
9. Navathe AS, Troxel AB, Liao JM, et al. Cost of joint replacement using bundled payment models. JAMA Intern Med. 2017;177(2):214-222. https://doi.org/10.1001/jamainternmed.2016.8263.
10. Liao JM, Emanuel EJ, Polsky DE, et al. National representativeness of hospitals and markets in Medicare’s mandatory bundled payment program. Health Aff. 2019;38(1):44-53.
11. Barnett ML, Wilcock A, McWilliams JM, et al. Two-year evaluation of mandatory bundled payments for joint replacement. N Engl J Med. 2019;380(3):252-262. https://doi.org/10.1056/NEJMsa1809010.
12. Navathe AS, Liao JM, Polsky D, et al. Comparison of hospitals participating in Medicare’s voluntary and mandatory orthopedic bundle programs. Health Aff. 2018;37(6):854-863. https://www.doi.org/10.1377/hlthaff.2017.1358.
13. Joynt Maddox KE, Orav EJ, Zheng J, Epstein AM. Participation and Dropout in the Bundled Payments for Care Improvement Initiative. JAMA. 2018;319(2):191-193. https://doi.org/10.1001/jama.2017.14771.
14. Navathe AS, Liao JM, Dykstra SE, et al. Association of hospital participation in a Medicare bundled payment program with volume and case mix of lower extremity joint replacement episodes. JAMA. 2018;320(9):901-910. https://doi.org/10.1001/jama.2018.12345.
15. Joynt Maddox KE, Orav EJ, Epstein AM. Medicare’s bundled payments initiative for medical conditions. N Engl J Med. 2018;379(18):e33. https://doi.org/10.1056/NEJMc1811049.
16. Navathe AS, Shan E, Liao JM. What have we learned about bundling medical conditions? Health Affairs Blog. https://www.healthaffairs.org/do/10.1377/hblog20180828.844613/full/. Accessed November 25, 2019.
17. Centers for Medicare & Medicaid Services. MACRA. https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/value-based-programs/macra-mips-and-apms/macra-mips-and-apms.html. Accessed November 26, 2019.
© 2020 Society of Hospital Medicine
Two-Year Experience of 14 French Pigtail Catheters Placed by Procedure-Focused Hospitalists
Over the last 15 years, studies have demonstrated the efficacy of small-bore chest tubes (SBCTs), or pigtail catheters (PCs, most commonly ≤14 French), in treating pneumothorax (PTX),1-5 traumatic hemothorax (THTX), hemopneumothorax (HPTX),6,7 parapneumonic effusions (PPEs),8,9 pleural infections,10 and symptomatic malignant pleural effusions.11 A randomized, controlled trial also showed that PC placement resulted in better pain scores, compared with large-bore chest tubes (LBCTs), for traumatic PTX.5 The British Thoracic Society does state that LBCTs may be needed for PTXs with very large air leaks, especially postoperatively. Further, LBCTs may be indicated if small-bore drainage fails, but otherwise they recommend PCs as first-line therapy for PTX, free flowing pleural effusions, and pleural infections.12
BEDSIDE PROCEDURE SERVICE DEVELOPMENT
The Medical College of Wisconsin (MCW) provides hospitalist services to
BPS Pigtail Catheter Training
CT surgery initially trained the BPS director in PC placement using the Seldinger technique in 2015. The director’s training period with CT surgery included direct observation by CT surgery providers for 5 PC placements. Prior to placing PCs, the director had performed approximately 400 ultrasound-guided thoracenteses. The BPS director then independently trained the remaining BPS and has placed or supervised over half of the service’s 124 PCs. Initial credentialing for each BPS physician requires 5 PC placements and 20 thoracenteses under direct supervision of credentialed BPS members. Credentialing is maintained by BPS physicians completing 3 PCs and 15 thoracenteses per year.
Newly credentialed providers are capable of independently placing most PCs. However, the requirements for credentialing are minimal and newly credentialed physicians still encounter PC placements with challenging factors not addressed in their training, such as anterior approach, small effusions, atypical effusion location, mild to moderate coagulopathy, recent therapeutic anticoagulation, and large body habitus. To address these challenges, the BPS has instituted an “on call” system. This system is typically staffed by the BPS director or associate director, already attending on a separate medical service. When needed, the “on call” physician will supervise the newer BPS members to ensure safety while the less experienced physician places the PC. Although rare, if an “on call” member is not available, then it is the practice of the BPS to recommend IR for PC placement.
BPS Operation
Daily BPS operation consists of one attending hospitalist, two internal medicine residents, and a third-year medical student. PCs are placed primarily (95%) by the attending on service under ultrasound guidance using the Seldinger technique with lidocaine for anesthetic. For all PC consults, the attending BPS physician reviews the indication prior to placement. If not a direct consult from surgical services, most PC consults are appropriate referrals to the service after the primary medicine service has consulted CT-surgery or p ulmonary consult teams. After review, the primary role of the BPS is assessing safety of PC placement, including whether the patient can tolerate PC placement without procedural sedation. The BPS’s additional standards for safe PC placement are listed in Table 1.
Additionally, it is not routine practice of the BPS to recommend PC placement when consulted for a thoracentesis. The exception to this rule is patients whose PPE sonographic imaging demonstrates loculation or septations. This is consistent with the latest review on pleural disease.13 In addition, the institution’s CT surgery services prefer to initially treat septated PPEs with PCs and fibrinolytic therapy rather than immediate video-assisted thoracoscopic surgery (VATS).
The BPS operates a partnership with CT surgery in which, after successful PC placement, CT surgery manages the PC immediately and until removal including the negative pressure applied and need for fibrinolytic therapy. CT surgery also determines if secondary therapy, commonly second PC or VATS, is required. After PC placement, a portable chest x-ray (CXR) is taken and then BPS follows the patient in person the following day to note any insertion-related complications (IRCs).
In this paper, data on the consults to the BPS for PC placement over a 2-year period are presented. Primary outcomes included numbers of and indications for PCs consulted—attempted or not attempted—consulting services, IRCs, unsuccessful attempts (UAs), and adverse outcomes (AOs). PC duration, fluid drainage, need for fibrinolytic therapy, or need for secondary therapy were not measured because these decisions were managed by the CT surgery service.
PATIENTS AND METHODS
Institutional review board approval of this retrospective study was granted by MCW/Froedtert Hospital Institutional Review Board #5 on January 14, 2019 (MCW IRB #PRO00033496). Adult patients hospitalized at Froedtert Hospital whose primary team determined they would clinically benefit from a PC and consulted the BPS service for placement were included. There were no exclusion criteria.
The authors conducted a retrospective review of two secure BPS databases. The first database is a record of all procedure consults, while the second database contains information about all attempted PCs. Initial review of the BPS’s consult database found 142 PC consults. Consults were classified as “declined” or “attempted.”
RESULTS
Over a 2-year period, the BPS was consulted to place 142 PCs. After resolution of the 3 discrepancies, total consults remained 142, PC attempts totaled 124 (87.3%), and declined consults totaled 18 (12.7%).
The 18 declined consults were not performed for reasons relating to procedural safety. These included 15 (83.3%) for insufficient fluid depth, 1 (5.6%) poor window for PTX, and 1 (5.6%) patient unstable per BPS attending judgement. One (5.6%) final consult had a previous drain in same hemithorax that resumed functioning.
The manual chart review of procedures performed 48 hours after declined PC consults found only 3 of 17 (17.6%) patients received a PC within the subsequent 48 hours. The 18th patient was unable to be followed in our electronic medical record because his medical record number was recorded incorrectly.
The remaining 124 consults were deemed safe for PC placement. Indications for PC placement varied; the most common indications were complicated effusion (36.3%), large or recurrent effusions (21.8%), PTX (17%), and hemothorax (HTX; 17%). The most common teams who consulted the BPS for PC were medicine/hospitalists (42.7%) and CT surgery (40.3%).
Three UAs were charted in the database, but on review it was determined that only 2 (1.6%) qualified as UAs (Table 3). A PC was attempted with the UA patient No. 3 for a loculated apical PTX. It is clear in the procedure note that the pleural space was accessed, air was appropriately drained, and a PC was advanced safely into the pleural space; however, the PC then stopped draining air. CXR interpretation also noted “pneumothorax described on prior exam is less evident.” Because the pleural space was accessed safely and had a partially therapeutic response, we do not count this PC placement as a UA. The PC may count as “failed,” but determination of a “failure rate” is not the intent of this paper. This point is further discussed in the Discussion section.
In addition, chart review demonstrated that UA patient No. 3 required intubation within the 24-hour period after our PC attempt, which is an AO. Approximately 10 hours after our PC was placed and removed, CT surgery placed a second PC, and 3 hours after their PC placement, the patient was intubated with subsequent bronchoscopy. The patient was extubated after only 17 hours. This sequence of events suggests mucus plugging as a more likely cause for respiratory failure than our PC attempt, but we have included it as an AO given the time frame.
Overall, the AO rate was low. Out of 124 attempted PC placements only 3 (2.4%) had an AO, and as noted above, it is believed that 2 of these patients had an AO caused by other medical problems rather than by PC placement.
DISCUSSION
To our knowledge, this is the first report of the experience of procedure-focused hospitalists with PC placement in a partnership with CT surgery. We believe that, at high volume, tertiary care centers similar to Froedtert Hospital, internal medicine–trained, procedure-focused hospitalists can serve as adjuncts to surgery, pulmonary, and IR services in the placement of PCs in hospitalized patients that do not require procedural sedation.
Given the development of this service and the nature of its shared operations with CT surgery, we do not believe that the BPS has an appropriate comparison in the literature; however, the IRCs are similar to previous papers describing PC placement.5-7,14 Notably, the IRC and AO rates were low, both 2.4%, which indicates safe placement of PCs. Kulvatunyou et al and Bauman et al reported on PC placement from a surgical perspective and reported IRC rates of 4%-10%.5-7,14 These higher IRC rates likely have a few reasons. First, Kulvatunyou et al and Bauman et al did not use ultrasound guidance. Use of ultrasound guidance may have significantly lowered their IRC rate. Second, the definition of IRC used by Kulvatunyou et al and Bauman et al included dislodgements, but we do not believe this to be an IRC. Dislodgements can happen for several reasons, frequently a result of patient movement or forgetfulness, not because of improper placement. Third, the PCs with this BPS are placed primarily by attending physicians. Resident roles on our BPS in PC placement are primarily as assistants, whereas Kulvatunyou et al and Bauman et al note that both attendings and residents, under attending supervision, placed PCs; however, it is not clear what percentage of PCs were placed by attendings or residents in their studies. Finally, this BPS’s IRCs are self-reported, so they could be perceived as falsely low, but given the small number of physicians involved in the group and its standardized follow-up, we do not suspect this is truly contributing to the low rates.
Other complication rates regarding the use of wire-guided SBCTs and PCs range from 0% to 42%15-20; however, several differences including tube size, physician training, and PC indication make these studies imperfect comparisons. The most notable difference in our opinion is the variable definition, or lack of definition, of a complication. One study did not define their complications,19 while other studies list subjective measures like pain,16,20 cough,16 bleeding, 16,20 and hematomas4,15 as complications. We believe that the lack of consensus definition for PC complication or IRC contributes to the large range of complication rates in the literature. This problem is likely not unique to PC placement, but is instead true across all bedside procedures. In a shared-practice model between hospitalists and CT surgeons, we believe the definition of IRC in this paper is adequate in capturing most complications. The only complication we are currently unable to track well is infection. We consider other items discussed previously, such as pain, cough (often from lung re-expansion), minor bleeding, and even small hematomas, to be a part of the procedure and not a complication.
Finally, regarding the IRCs and associated death, this was a tragic event. Complications for all of the BPS’s procedures are infrequent (0.35% over the same time period) and reviewed between the BPS director and the attending who performed the procedure; in addition, given this mortality, the case was reviewed immediately in detail with our CT surgery colleagues. On review, it was easy to determine that the operator had found a clear lung tip and sonographic signs of PTX; however, CXR review did demonstrate a medial placement of the PC. This was judged to be a poor placement location (even with imaging demonstrating PTX in that area) given the well-known “triangle of safety” defined by the British Thoracic Society.12
After review, the primary emphasis for PC placement was safe location. The BPS now strives to place PCs for PTX only in the “triangle of safety.” The BPS believe that most PTXs can be addressed with this placement. In the rare case of a PTX requiring an anterior approach, only the BPS director currently places apical PCs for PTX while on service or “on call.” He discusses the placement with pulmonary and CT surgery directly to determine that the PC is of absolute necessity.
Given the focus on appropriate location, no formal changes were made to the procedural imaging practice described in Table 1. We realize that vascular imaging would seem necessary after this patient’s mammary artery laceration; however, safe location, in addition to the BPS’s current image requirements, is believed to minimize this risk. We feel the imaging criteria align with recommendation No. 5 of the Society of Hospital Medicine’s Position Statement for Ultrasound Guidance for Adult Thoracentesis.21 Some BPS members use vascular ultrasound imaging to confirm absence of vascularity, but it is not required and occasionally not possible, such as in the occasional case of PTX with subcutaneous emphysema.
The UA rate is low without a natural comparator in the literature. It is important to clarify the difference between the UAs and the frequently mentioned “failure rate” (FR) in Kulvatunyou et al and Bauman et al6,7,14 We classify UAs as the inability, for any reason, to access the pleural space and insert a PC. At this stage, these UAs appear to reflect the service’s new experience with PC placement and inability to provide procedural sedation. Kulvatunyou et al and Bauman et al’s FR is defined as an initial PC successfully placed into the pleural space that then required a second PC or intervention (frequently VATS) to resolve the PTX or retained HTX.
We believe calculating the failure rate will be helpful in demonstrating the value of our BPS and our shared-practice model. We look forward to publishing this and other future research, including determination of the cost and time saved by the BPS for PCs and other procedures.
Limitations of this study include its retrospective nature, results from a single center’s experience, and lack of a comparison group.
Our institution feels that there is great benefit in having a BPS operated by procedure-focused hospitalists. It would also be important to determine if our model can be replicated by another institution.
Acknowledgments
The authors thank CT surgery for helping to develop this shared-practice model and to both CT surgery and IR physicians here at the Medical College of Wisconsin and Froedtert Hospital who assist us with both IRCs and UAs of pigtail catheters.
The authors also thank Dr. Ricardo Franco-Sadud for his oversight and thoughtful improvements to the paper.
1. Chang SH, Kang YN, Chiu HY, Chiu YH. A Systematic Review and Meta-Analysis Comparing Pigtail Catheter and Chest Tube as the Initial Treatment for Pneumothorax. Chest. 2018;153(5):1201-1212. https://doi.org/10.1016/j.chest.2018.01.048.
2. Voisin F, Sohier L, Rochas Y, et al. Ambulatory management of large spontaneous pneumothorax with pigtail catheters. Ann Emerg Med. 2014;64(3):222-228. https://doi.org/10.1016/j.annemergmed.2013.12.017.
3. Lin YC, Tu CY, Liang SJ, et al. Pigtail catheter for the management of pneumothorax in mechanically ventilated patients. Am J Emerg Med. 2010;28(4):466-471. https://doi.org/10.1016/j.ajem.2009.01.033.
4. Tsai WK, Chen W, Lee JC, et al. Pigtail catheters vs large-bore chest tubes for management of secondary spontaneous pneumothoraces in adults. Am J Emerg Med. 2006;24(7):795-800. https://doi.org/10.1016/j.ajem.2006.04.006.
5. Kulvatunyou N, Erickson L, Vijayasekaran A, et al. Randomized clinical trial of pigtail catheter versus chest tube in injured patients with uncomplicated traumatic pneumothorax. Br J Surg. 2014;101(2):17-22. https://doi.org/10.1002/bjs.9377.
6. Kulvatunyou N, Joseph B, Friese RS, et al. 14 French pigtail catheters placed by surgeons to drain blood on trauma patients: is 14-Fr too small? J Trauma Acute Care Surg. 2012;73(6):1423-1427. https://doi.org/10.1097/TA.0b013e318271c1c7.
7. Bauman ZM, Kulvatunyou N, Joseph B, et al. A Prospective Study of 7-Year Experience Using Percutaneous 14-French Pigtail Catheters for Traumatic Hemothorax/Hemopneumothorax at a Level-1 Trauma Center: Size Still Does Not Matter. World J Surg. 2018;42(1):107-113. https://doi.org/10.1007/s00268-017-4168-3.
8. Fysh ET, Smith NA, Lee YC. Optimal chest drain size: the rise of the small-bore pleural catheter. Semin Respir Crit Care Med. 2010;31(6):760-768. https://doi.org/10.1055/s-0030-1269836.
9. Ozkan OS, Ozmen MN, Akhan O. Percutaneous management of parapneumonic effusions. Eur J Radiol. 2005;55(3):311-320. https://doi.org/10.1016/j.ejrad.2005.03.004.
10. Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest. 2010;137(3):536-543. https://doi.org/10.1378/chest.09-1044.
11. Saffran L, Ost DE, Fein AM, Schiff MJ. Outpatient pleurodesis of malignant pleural effusions using a small-bore pigtail catheter. Chest. 2000;118(2):417-421. https://doi.org/10.1378/chest.118.2.417.
12. Havelock T, Teoh R, Laws D, Gleeson F, Group BPDG. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii61-76. https://doi.org/10.1136/thx.2010.137026.
13. Feller-Kopman D, Light R. Pleural disease. N Engl J Med. 2018;378(8):740-751. https://doi.org/10.1056/NEJMra1403503.
14. Kulvatunyou N, Vijayasekaran A, Hansen A, et al. Two-year experience of using pigtail catheters to treat traumatic pneumothorax: A changing trend. J Trauma. 2011;71(5):1104-1107; discussion 1107. https://doi.org/10.1097/TA.0b013e31822dd130.
15. Cantin L, Chartrand-Lefebvre C, Lepanto L, et al. Chest tube drainage under radiological guidance for pleural effusion and pneumothorax in a tertiary care university teaching hospital: Review of 51 cases. Can Respir J. 2005;12(1):29-33. https://doi.org/10.1155/2005/498709.
16. Horsley A, Jones L, White J, Henry M. Efficacy and complications of small-bore, wire-guided chest drains. Chest. 2006;130(6):1857-1863. https://doi.org/10.1378/chest.130.6.1857.
17. Merriam MA, Cronan JJ, Dorfman GS, Lambiase RE, Haas RA. Radiographically guided percutaneous catheter drainage of pleural fluid collections. Am J Roentgenol. 1988;151(6):1113-1116. https://doi.org/10.2214/ajr.151.6.1113.
18. Petel D, Li P, Emil S. Percutaneous pigtail catheter versus tube thoracostomy for pediatric empyema: A comparison of outcomes. Surgery. 2013;154(4):655-660; discussion 660-651. https://doi.org/10.1016/j.surg.2013.04.032.
19. Gammie JS, Banks MC, Fuhrman CR, et al. The pigtail catheter for pleural drainage: a less invasive alternative to tube thoracostomy. JSLS. 1999;3(1):57-61.
20. Davies HE, Merchant S, McGown A. A study of the complications of small bore ‘Seldinger’ intercostal chest drains. Respirology. 2008;13(4):603-607. https://doi.org/10.1111/j.1440-1843.2008.01296.x.
21. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the Use of Ultrasound Guidance for Adult Thoracentesis: A Position Statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940.
Over the last 15 years, studies have demonstrated the efficacy of small-bore chest tubes (SBCTs), or pigtail catheters (PCs, most commonly ≤14 French), in treating pneumothorax (PTX),1-5 traumatic hemothorax (THTX), hemopneumothorax (HPTX),6,7 parapneumonic effusions (PPEs),8,9 pleural infections,10 and symptomatic malignant pleural effusions.11 A randomized, controlled trial also showed that PC placement resulted in better pain scores, compared with large-bore chest tubes (LBCTs), for traumatic PTX.5 The British Thoracic Society does state that LBCTs may be needed for PTXs with very large air leaks, especially postoperatively. Further, LBCTs may be indicated if small-bore drainage fails, but otherwise they recommend PCs as first-line therapy for PTX, free flowing pleural effusions, and pleural infections.12
BEDSIDE PROCEDURE SERVICE DEVELOPMENT
The Medical College of Wisconsin (MCW) provides hospitalist services to
BPS Pigtail Catheter Training
CT surgery initially trained the BPS director in PC placement using the Seldinger technique in 2015. The director’s training period with CT surgery included direct observation by CT surgery providers for 5 PC placements. Prior to placing PCs, the director had performed approximately 400 ultrasound-guided thoracenteses. The BPS director then independently trained the remaining BPS and has placed or supervised over half of the service’s 124 PCs. Initial credentialing for each BPS physician requires 5 PC placements and 20 thoracenteses under direct supervision of credentialed BPS members. Credentialing is maintained by BPS physicians completing 3 PCs and 15 thoracenteses per year.
Newly credentialed providers are capable of independently placing most PCs. However, the requirements for credentialing are minimal and newly credentialed physicians still encounter PC placements with challenging factors not addressed in their training, such as anterior approach, small effusions, atypical effusion location, mild to moderate coagulopathy, recent therapeutic anticoagulation, and large body habitus. To address these challenges, the BPS has instituted an “on call” system. This system is typically staffed by the BPS director or associate director, already attending on a separate medical service. When needed, the “on call” physician will supervise the newer BPS members to ensure safety while the less experienced physician places the PC. Although rare, if an “on call” member is not available, then it is the practice of the BPS to recommend IR for PC placement.
BPS Operation
Daily BPS operation consists of one attending hospitalist, two internal medicine residents, and a third-year medical student. PCs are placed primarily (95%) by the attending on service under ultrasound guidance using the Seldinger technique with lidocaine for anesthetic. For all PC consults, the attending BPS physician reviews the indication prior to placement. If not a direct consult from surgical services, most PC consults are appropriate referrals to the service after the primary medicine service has consulted CT-surgery or p ulmonary consult teams. After review, the primary role of the BPS is assessing safety of PC placement, including whether the patient can tolerate PC placement without procedural sedation. The BPS’s additional standards for safe PC placement are listed in Table 1.
Additionally, it is not routine practice of the BPS to recommend PC placement when consulted for a thoracentesis. The exception to this rule is patients whose PPE sonographic imaging demonstrates loculation or septations. This is consistent with the latest review on pleural disease.13 In addition, the institution’s CT surgery services prefer to initially treat septated PPEs with PCs and fibrinolytic therapy rather than immediate video-assisted thoracoscopic surgery (VATS).
The BPS operates a partnership with CT surgery in which, after successful PC placement, CT surgery manages the PC immediately and until removal including the negative pressure applied and need for fibrinolytic therapy. CT surgery also determines if secondary therapy, commonly second PC or VATS, is required. After PC placement, a portable chest x-ray (CXR) is taken and then BPS follows the patient in person the following day to note any insertion-related complications (IRCs).
In this paper, data on the consults to the BPS for PC placement over a 2-year period are presented. Primary outcomes included numbers of and indications for PCs consulted—attempted or not attempted—consulting services, IRCs, unsuccessful attempts (UAs), and adverse outcomes (AOs). PC duration, fluid drainage, need for fibrinolytic therapy, or need for secondary therapy were not measured because these decisions were managed by the CT surgery service.
PATIENTS AND METHODS
Institutional review board approval of this retrospective study was granted by MCW/Froedtert Hospital Institutional Review Board #5 on January 14, 2019 (MCW IRB #PRO00033496). Adult patients hospitalized at Froedtert Hospital whose primary team determined they would clinically benefit from a PC and consulted the BPS service for placement were included. There were no exclusion criteria.
The authors conducted a retrospective review of two secure BPS databases. The first database is a record of all procedure consults, while the second database contains information about all attempted PCs. Initial review of the BPS’s consult database found 142 PC consults. Consults were classified as “declined” or “attempted.”
RESULTS
Over a 2-year period, the BPS was consulted to place 142 PCs. After resolution of the 3 discrepancies, total consults remained 142, PC attempts totaled 124 (87.3%), and declined consults totaled 18 (12.7%).
The 18 declined consults were not performed for reasons relating to procedural safety. These included 15 (83.3%) for insufficient fluid depth, 1 (5.6%) poor window for PTX, and 1 (5.6%) patient unstable per BPS attending judgement. One (5.6%) final consult had a previous drain in same hemithorax that resumed functioning.
The manual chart review of procedures performed 48 hours after declined PC consults found only 3 of 17 (17.6%) patients received a PC within the subsequent 48 hours. The 18th patient was unable to be followed in our electronic medical record because his medical record number was recorded incorrectly.
The remaining 124 consults were deemed safe for PC placement. Indications for PC placement varied; the most common indications were complicated effusion (36.3%), large or recurrent effusions (21.8%), PTX (17%), and hemothorax (HTX; 17%). The most common teams who consulted the BPS for PC were medicine/hospitalists (42.7%) and CT surgery (40.3%).
Three UAs were charted in the database, but on review it was determined that only 2 (1.6%) qualified as UAs (Table 3). A PC was attempted with the UA patient No. 3 for a loculated apical PTX. It is clear in the procedure note that the pleural space was accessed, air was appropriately drained, and a PC was advanced safely into the pleural space; however, the PC then stopped draining air. CXR interpretation also noted “pneumothorax described on prior exam is less evident.” Because the pleural space was accessed safely and had a partially therapeutic response, we do not count this PC placement as a UA. The PC may count as “failed,” but determination of a “failure rate” is not the intent of this paper. This point is further discussed in the Discussion section.
In addition, chart review demonstrated that UA patient No. 3 required intubation within the 24-hour period after our PC attempt, which is an AO. Approximately 10 hours after our PC was placed and removed, CT surgery placed a second PC, and 3 hours after their PC placement, the patient was intubated with subsequent bronchoscopy. The patient was extubated after only 17 hours. This sequence of events suggests mucus plugging as a more likely cause for respiratory failure than our PC attempt, but we have included it as an AO given the time frame.
Overall, the AO rate was low. Out of 124 attempted PC placements only 3 (2.4%) had an AO, and as noted above, it is believed that 2 of these patients had an AO caused by other medical problems rather than by PC placement.
DISCUSSION
To our knowledge, this is the first report of the experience of procedure-focused hospitalists with PC placement in a partnership with CT surgery. We believe that, at high volume, tertiary care centers similar to Froedtert Hospital, internal medicine–trained, procedure-focused hospitalists can serve as adjuncts to surgery, pulmonary, and IR services in the placement of PCs in hospitalized patients that do not require procedural sedation.
Given the development of this service and the nature of its shared operations with CT surgery, we do not believe that the BPS has an appropriate comparison in the literature; however, the IRCs are similar to previous papers describing PC placement.5-7,14 Notably, the IRC and AO rates were low, both 2.4%, which indicates safe placement of PCs. Kulvatunyou et al and Bauman et al reported on PC placement from a surgical perspective and reported IRC rates of 4%-10%.5-7,14 These higher IRC rates likely have a few reasons. First, Kulvatunyou et al and Bauman et al did not use ultrasound guidance. Use of ultrasound guidance may have significantly lowered their IRC rate. Second, the definition of IRC used by Kulvatunyou et al and Bauman et al included dislodgements, but we do not believe this to be an IRC. Dislodgements can happen for several reasons, frequently a result of patient movement or forgetfulness, not because of improper placement. Third, the PCs with this BPS are placed primarily by attending physicians. Resident roles on our BPS in PC placement are primarily as assistants, whereas Kulvatunyou et al and Bauman et al note that both attendings and residents, under attending supervision, placed PCs; however, it is not clear what percentage of PCs were placed by attendings or residents in their studies. Finally, this BPS’s IRCs are self-reported, so they could be perceived as falsely low, but given the small number of physicians involved in the group and its standardized follow-up, we do not suspect this is truly contributing to the low rates.
Other complication rates regarding the use of wire-guided SBCTs and PCs range from 0% to 42%15-20; however, several differences including tube size, physician training, and PC indication make these studies imperfect comparisons. The most notable difference in our opinion is the variable definition, or lack of definition, of a complication. One study did not define their complications,19 while other studies list subjective measures like pain,16,20 cough,16 bleeding, 16,20 and hematomas4,15 as complications. We believe that the lack of consensus definition for PC complication or IRC contributes to the large range of complication rates in the literature. This problem is likely not unique to PC placement, but is instead true across all bedside procedures. In a shared-practice model between hospitalists and CT surgeons, we believe the definition of IRC in this paper is adequate in capturing most complications. The only complication we are currently unable to track well is infection. We consider other items discussed previously, such as pain, cough (often from lung re-expansion), minor bleeding, and even small hematomas, to be a part of the procedure and not a complication.
Finally, regarding the IRCs and associated death, this was a tragic event. Complications for all of the BPS’s procedures are infrequent (0.35% over the same time period) and reviewed between the BPS director and the attending who performed the procedure; in addition, given this mortality, the case was reviewed immediately in detail with our CT surgery colleagues. On review, it was easy to determine that the operator had found a clear lung tip and sonographic signs of PTX; however, CXR review did demonstrate a medial placement of the PC. This was judged to be a poor placement location (even with imaging demonstrating PTX in that area) given the well-known “triangle of safety” defined by the British Thoracic Society.12
After review, the primary emphasis for PC placement was safe location. The BPS now strives to place PCs for PTX only in the “triangle of safety.” The BPS believe that most PTXs can be addressed with this placement. In the rare case of a PTX requiring an anterior approach, only the BPS director currently places apical PCs for PTX while on service or “on call.” He discusses the placement with pulmonary and CT surgery directly to determine that the PC is of absolute necessity.
Given the focus on appropriate location, no formal changes were made to the procedural imaging practice described in Table 1. We realize that vascular imaging would seem necessary after this patient’s mammary artery laceration; however, safe location, in addition to the BPS’s current image requirements, is believed to minimize this risk. We feel the imaging criteria align with recommendation No. 5 of the Society of Hospital Medicine’s Position Statement for Ultrasound Guidance for Adult Thoracentesis.21 Some BPS members use vascular ultrasound imaging to confirm absence of vascularity, but it is not required and occasionally not possible, such as in the occasional case of PTX with subcutaneous emphysema.
The UA rate is low without a natural comparator in the literature. It is important to clarify the difference between the UAs and the frequently mentioned “failure rate” (FR) in Kulvatunyou et al and Bauman et al6,7,14 We classify UAs as the inability, for any reason, to access the pleural space and insert a PC. At this stage, these UAs appear to reflect the service’s new experience with PC placement and inability to provide procedural sedation. Kulvatunyou et al and Bauman et al’s FR is defined as an initial PC successfully placed into the pleural space that then required a second PC or intervention (frequently VATS) to resolve the PTX or retained HTX.
We believe calculating the failure rate will be helpful in demonstrating the value of our BPS and our shared-practice model. We look forward to publishing this and other future research, including determination of the cost and time saved by the BPS for PCs and other procedures.
Limitations of this study include its retrospective nature, results from a single center’s experience, and lack of a comparison group.
Our institution feels that there is great benefit in having a BPS operated by procedure-focused hospitalists. It would also be important to determine if our model can be replicated by another institution.
Acknowledgments
The authors thank CT surgery for helping to develop this shared-practice model and to both CT surgery and IR physicians here at the Medical College of Wisconsin and Froedtert Hospital who assist us with both IRCs and UAs of pigtail catheters.
The authors also thank Dr. Ricardo Franco-Sadud for his oversight and thoughtful improvements to the paper.
Over the last 15 years, studies have demonstrated the efficacy of small-bore chest tubes (SBCTs), or pigtail catheters (PCs, most commonly ≤14 French), in treating pneumothorax (PTX),1-5 traumatic hemothorax (THTX), hemopneumothorax (HPTX),6,7 parapneumonic effusions (PPEs),8,9 pleural infections,10 and symptomatic malignant pleural effusions.11 A randomized, controlled trial also showed that PC placement resulted in better pain scores, compared with large-bore chest tubes (LBCTs), for traumatic PTX.5 The British Thoracic Society does state that LBCTs may be needed for PTXs with very large air leaks, especially postoperatively. Further, LBCTs may be indicated if small-bore drainage fails, but otherwise they recommend PCs as first-line therapy for PTX, free flowing pleural effusions, and pleural infections.12
BEDSIDE PROCEDURE SERVICE DEVELOPMENT
The Medical College of Wisconsin (MCW) provides hospitalist services to
BPS Pigtail Catheter Training
CT surgery initially trained the BPS director in PC placement using the Seldinger technique in 2015. The director’s training period with CT surgery included direct observation by CT surgery providers for 5 PC placements. Prior to placing PCs, the director had performed approximately 400 ultrasound-guided thoracenteses. The BPS director then independently trained the remaining BPS and has placed or supervised over half of the service’s 124 PCs. Initial credentialing for each BPS physician requires 5 PC placements and 20 thoracenteses under direct supervision of credentialed BPS members. Credentialing is maintained by BPS physicians completing 3 PCs and 15 thoracenteses per year.
Newly credentialed providers are capable of independently placing most PCs. However, the requirements for credentialing are minimal and newly credentialed physicians still encounter PC placements with challenging factors not addressed in their training, such as anterior approach, small effusions, atypical effusion location, mild to moderate coagulopathy, recent therapeutic anticoagulation, and large body habitus. To address these challenges, the BPS has instituted an “on call” system. This system is typically staffed by the BPS director or associate director, already attending on a separate medical service. When needed, the “on call” physician will supervise the newer BPS members to ensure safety while the less experienced physician places the PC. Although rare, if an “on call” member is not available, then it is the practice of the BPS to recommend IR for PC placement.
BPS Operation
Daily BPS operation consists of one attending hospitalist, two internal medicine residents, and a third-year medical student. PCs are placed primarily (95%) by the attending on service under ultrasound guidance using the Seldinger technique with lidocaine for anesthetic. For all PC consults, the attending BPS physician reviews the indication prior to placement. If not a direct consult from surgical services, most PC consults are appropriate referrals to the service after the primary medicine service has consulted CT-surgery or p ulmonary consult teams. After review, the primary role of the BPS is assessing safety of PC placement, including whether the patient can tolerate PC placement without procedural sedation. The BPS’s additional standards for safe PC placement are listed in Table 1.
Additionally, it is not routine practice of the BPS to recommend PC placement when consulted for a thoracentesis. The exception to this rule is patients whose PPE sonographic imaging demonstrates loculation or septations. This is consistent with the latest review on pleural disease.13 In addition, the institution’s CT surgery services prefer to initially treat septated PPEs with PCs and fibrinolytic therapy rather than immediate video-assisted thoracoscopic surgery (VATS).
The BPS operates a partnership with CT surgery in which, after successful PC placement, CT surgery manages the PC immediately and until removal including the negative pressure applied and need for fibrinolytic therapy. CT surgery also determines if secondary therapy, commonly second PC or VATS, is required. After PC placement, a portable chest x-ray (CXR) is taken and then BPS follows the patient in person the following day to note any insertion-related complications (IRCs).
In this paper, data on the consults to the BPS for PC placement over a 2-year period are presented. Primary outcomes included numbers of and indications for PCs consulted—attempted or not attempted—consulting services, IRCs, unsuccessful attempts (UAs), and adverse outcomes (AOs). PC duration, fluid drainage, need for fibrinolytic therapy, or need for secondary therapy were not measured because these decisions were managed by the CT surgery service.
PATIENTS AND METHODS
Institutional review board approval of this retrospective study was granted by MCW/Froedtert Hospital Institutional Review Board #5 on January 14, 2019 (MCW IRB #PRO00033496). Adult patients hospitalized at Froedtert Hospital whose primary team determined they would clinically benefit from a PC and consulted the BPS service for placement were included. There were no exclusion criteria.
The authors conducted a retrospective review of two secure BPS databases. The first database is a record of all procedure consults, while the second database contains information about all attempted PCs. Initial review of the BPS’s consult database found 142 PC consults. Consults were classified as “declined” or “attempted.”
RESULTS
Over a 2-year period, the BPS was consulted to place 142 PCs. After resolution of the 3 discrepancies, total consults remained 142, PC attempts totaled 124 (87.3%), and declined consults totaled 18 (12.7%).
The 18 declined consults were not performed for reasons relating to procedural safety. These included 15 (83.3%) for insufficient fluid depth, 1 (5.6%) poor window for PTX, and 1 (5.6%) patient unstable per BPS attending judgement. One (5.6%) final consult had a previous drain in same hemithorax that resumed functioning.
The manual chart review of procedures performed 48 hours after declined PC consults found only 3 of 17 (17.6%) patients received a PC within the subsequent 48 hours. The 18th patient was unable to be followed in our electronic medical record because his medical record number was recorded incorrectly.
The remaining 124 consults were deemed safe for PC placement. Indications for PC placement varied; the most common indications were complicated effusion (36.3%), large or recurrent effusions (21.8%), PTX (17%), and hemothorax (HTX; 17%). The most common teams who consulted the BPS for PC were medicine/hospitalists (42.7%) and CT surgery (40.3%).
Three UAs were charted in the database, but on review it was determined that only 2 (1.6%) qualified as UAs (Table 3). A PC was attempted with the UA patient No. 3 for a loculated apical PTX. It is clear in the procedure note that the pleural space was accessed, air was appropriately drained, and a PC was advanced safely into the pleural space; however, the PC then stopped draining air. CXR interpretation also noted “pneumothorax described on prior exam is less evident.” Because the pleural space was accessed safely and had a partially therapeutic response, we do not count this PC placement as a UA. The PC may count as “failed,” but determination of a “failure rate” is not the intent of this paper. This point is further discussed in the Discussion section.
In addition, chart review demonstrated that UA patient No. 3 required intubation within the 24-hour period after our PC attempt, which is an AO. Approximately 10 hours after our PC was placed and removed, CT surgery placed a second PC, and 3 hours after their PC placement, the patient was intubated with subsequent bronchoscopy. The patient was extubated after only 17 hours. This sequence of events suggests mucus plugging as a more likely cause for respiratory failure than our PC attempt, but we have included it as an AO given the time frame.
Overall, the AO rate was low. Out of 124 attempted PC placements only 3 (2.4%) had an AO, and as noted above, it is believed that 2 of these patients had an AO caused by other medical problems rather than by PC placement.
DISCUSSION
To our knowledge, this is the first report of the experience of procedure-focused hospitalists with PC placement in a partnership with CT surgery. We believe that, at high volume, tertiary care centers similar to Froedtert Hospital, internal medicine–trained, procedure-focused hospitalists can serve as adjuncts to surgery, pulmonary, and IR services in the placement of PCs in hospitalized patients that do not require procedural sedation.
Given the development of this service and the nature of its shared operations with CT surgery, we do not believe that the BPS has an appropriate comparison in the literature; however, the IRCs are similar to previous papers describing PC placement.5-7,14 Notably, the IRC and AO rates were low, both 2.4%, which indicates safe placement of PCs. Kulvatunyou et al and Bauman et al reported on PC placement from a surgical perspective and reported IRC rates of 4%-10%.5-7,14 These higher IRC rates likely have a few reasons. First, Kulvatunyou et al and Bauman et al did not use ultrasound guidance. Use of ultrasound guidance may have significantly lowered their IRC rate. Second, the definition of IRC used by Kulvatunyou et al and Bauman et al included dislodgements, but we do not believe this to be an IRC. Dislodgements can happen for several reasons, frequently a result of patient movement or forgetfulness, not because of improper placement. Third, the PCs with this BPS are placed primarily by attending physicians. Resident roles on our BPS in PC placement are primarily as assistants, whereas Kulvatunyou et al and Bauman et al note that both attendings and residents, under attending supervision, placed PCs; however, it is not clear what percentage of PCs were placed by attendings or residents in their studies. Finally, this BPS’s IRCs are self-reported, so they could be perceived as falsely low, but given the small number of physicians involved in the group and its standardized follow-up, we do not suspect this is truly contributing to the low rates.
Other complication rates regarding the use of wire-guided SBCTs and PCs range from 0% to 42%15-20; however, several differences including tube size, physician training, and PC indication make these studies imperfect comparisons. The most notable difference in our opinion is the variable definition, or lack of definition, of a complication. One study did not define their complications,19 while other studies list subjective measures like pain,16,20 cough,16 bleeding, 16,20 and hematomas4,15 as complications. We believe that the lack of consensus definition for PC complication or IRC contributes to the large range of complication rates in the literature. This problem is likely not unique to PC placement, but is instead true across all bedside procedures. In a shared-practice model between hospitalists and CT surgeons, we believe the definition of IRC in this paper is adequate in capturing most complications. The only complication we are currently unable to track well is infection. We consider other items discussed previously, such as pain, cough (often from lung re-expansion), minor bleeding, and even small hematomas, to be a part of the procedure and not a complication.
Finally, regarding the IRCs and associated death, this was a tragic event. Complications for all of the BPS’s procedures are infrequent (0.35% over the same time period) and reviewed between the BPS director and the attending who performed the procedure; in addition, given this mortality, the case was reviewed immediately in detail with our CT surgery colleagues. On review, it was easy to determine that the operator had found a clear lung tip and sonographic signs of PTX; however, CXR review did demonstrate a medial placement of the PC. This was judged to be a poor placement location (even with imaging demonstrating PTX in that area) given the well-known “triangle of safety” defined by the British Thoracic Society.12
After review, the primary emphasis for PC placement was safe location. The BPS now strives to place PCs for PTX only in the “triangle of safety.” The BPS believe that most PTXs can be addressed with this placement. In the rare case of a PTX requiring an anterior approach, only the BPS director currently places apical PCs for PTX while on service or “on call.” He discusses the placement with pulmonary and CT surgery directly to determine that the PC is of absolute necessity.
Given the focus on appropriate location, no formal changes were made to the procedural imaging practice described in Table 1. We realize that vascular imaging would seem necessary after this patient’s mammary artery laceration; however, safe location, in addition to the BPS’s current image requirements, is believed to minimize this risk. We feel the imaging criteria align with recommendation No. 5 of the Society of Hospital Medicine’s Position Statement for Ultrasound Guidance for Adult Thoracentesis.21 Some BPS members use vascular ultrasound imaging to confirm absence of vascularity, but it is not required and occasionally not possible, such as in the occasional case of PTX with subcutaneous emphysema.
The UA rate is low without a natural comparator in the literature. It is important to clarify the difference between the UAs and the frequently mentioned “failure rate” (FR) in Kulvatunyou et al and Bauman et al6,7,14 We classify UAs as the inability, for any reason, to access the pleural space and insert a PC. At this stage, these UAs appear to reflect the service’s new experience with PC placement and inability to provide procedural sedation. Kulvatunyou et al and Bauman et al’s FR is defined as an initial PC successfully placed into the pleural space that then required a second PC or intervention (frequently VATS) to resolve the PTX or retained HTX.
We believe calculating the failure rate will be helpful in demonstrating the value of our BPS and our shared-practice model. We look forward to publishing this and other future research, including determination of the cost and time saved by the BPS for PCs and other procedures.
Limitations of this study include its retrospective nature, results from a single center’s experience, and lack of a comparison group.
Our institution feels that there is great benefit in having a BPS operated by procedure-focused hospitalists. It would also be important to determine if our model can be replicated by another institution.
Acknowledgments
The authors thank CT surgery for helping to develop this shared-practice model and to both CT surgery and IR physicians here at the Medical College of Wisconsin and Froedtert Hospital who assist us with both IRCs and UAs of pigtail catheters.
The authors also thank Dr. Ricardo Franco-Sadud for his oversight and thoughtful improvements to the paper.
1. Chang SH, Kang YN, Chiu HY, Chiu YH. A Systematic Review and Meta-Analysis Comparing Pigtail Catheter and Chest Tube as the Initial Treatment for Pneumothorax. Chest. 2018;153(5):1201-1212. https://doi.org/10.1016/j.chest.2018.01.048.
2. Voisin F, Sohier L, Rochas Y, et al. Ambulatory management of large spontaneous pneumothorax with pigtail catheters. Ann Emerg Med. 2014;64(3):222-228. https://doi.org/10.1016/j.annemergmed.2013.12.017.
3. Lin YC, Tu CY, Liang SJ, et al. Pigtail catheter for the management of pneumothorax in mechanically ventilated patients. Am J Emerg Med. 2010;28(4):466-471. https://doi.org/10.1016/j.ajem.2009.01.033.
4. Tsai WK, Chen W, Lee JC, et al. Pigtail catheters vs large-bore chest tubes for management of secondary spontaneous pneumothoraces in adults. Am J Emerg Med. 2006;24(7):795-800. https://doi.org/10.1016/j.ajem.2006.04.006.
5. Kulvatunyou N, Erickson L, Vijayasekaran A, et al. Randomized clinical trial of pigtail catheter versus chest tube in injured patients with uncomplicated traumatic pneumothorax. Br J Surg. 2014;101(2):17-22. https://doi.org/10.1002/bjs.9377.
6. Kulvatunyou N, Joseph B, Friese RS, et al. 14 French pigtail catheters placed by surgeons to drain blood on trauma patients: is 14-Fr too small? J Trauma Acute Care Surg. 2012;73(6):1423-1427. https://doi.org/10.1097/TA.0b013e318271c1c7.
7. Bauman ZM, Kulvatunyou N, Joseph B, et al. A Prospective Study of 7-Year Experience Using Percutaneous 14-French Pigtail Catheters for Traumatic Hemothorax/Hemopneumothorax at a Level-1 Trauma Center: Size Still Does Not Matter. World J Surg. 2018;42(1):107-113. https://doi.org/10.1007/s00268-017-4168-3.
8. Fysh ET, Smith NA, Lee YC. Optimal chest drain size: the rise of the small-bore pleural catheter. Semin Respir Crit Care Med. 2010;31(6):760-768. https://doi.org/10.1055/s-0030-1269836.
9. Ozkan OS, Ozmen MN, Akhan O. Percutaneous management of parapneumonic effusions. Eur J Radiol. 2005;55(3):311-320. https://doi.org/10.1016/j.ejrad.2005.03.004.
10. Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest. 2010;137(3):536-543. https://doi.org/10.1378/chest.09-1044.
11. Saffran L, Ost DE, Fein AM, Schiff MJ. Outpatient pleurodesis of malignant pleural effusions using a small-bore pigtail catheter. Chest. 2000;118(2):417-421. https://doi.org/10.1378/chest.118.2.417.
12. Havelock T, Teoh R, Laws D, Gleeson F, Group BPDG. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii61-76. https://doi.org/10.1136/thx.2010.137026.
13. Feller-Kopman D, Light R. Pleural disease. N Engl J Med. 2018;378(8):740-751. https://doi.org/10.1056/NEJMra1403503.
14. Kulvatunyou N, Vijayasekaran A, Hansen A, et al. Two-year experience of using pigtail catheters to treat traumatic pneumothorax: A changing trend. J Trauma. 2011;71(5):1104-1107; discussion 1107. https://doi.org/10.1097/TA.0b013e31822dd130.
15. Cantin L, Chartrand-Lefebvre C, Lepanto L, et al. Chest tube drainage under radiological guidance for pleural effusion and pneumothorax in a tertiary care university teaching hospital: Review of 51 cases. Can Respir J. 2005;12(1):29-33. https://doi.org/10.1155/2005/498709.
16. Horsley A, Jones L, White J, Henry M. Efficacy and complications of small-bore, wire-guided chest drains. Chest. 2006;130(6):1857-1863. https://doi.org/10.1378/chest.130.6.1857.
17. Merriam MA, Cronan JJ, Dorfman GS, Lambiase RE, Haas RA. Radiographically guided percutaneous catheter drainage of pleural fluid collections. Am J Roentgenol. 1988;151(6):1113-1116. https://doi.org/10.2214/ajr.151.6.1113.
18. Petel D, Li P, Emil S. Percutaneous pigtail catheter versus tube thoracostomy for pediatric empyema: A comparison of outcomes. Surgery. 2013;154(4):655-660; discussion 660-651. https://doi.org/10.1016/j.surg.2013.04.032.
19. Gammie JS, Banks MC, Fuhrman CR, et al. The pigtail catheter for pleural drainage: a less invasive alternative to tube thoracostomy. JSLS. 1999;3(1):57-61.
20. Davies HE, Merchant S, McGown A. A study of the complications of small bore ‘Seldinger’ intercostal chest drains. Respirology. 2008;13(4):603-607. https://doi.org/10.1111/j.1440-1843.2008.01296.x.
21. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the Use of Ultrasound Guidance for Adult Thoracentesis: A Position Statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940.
1. Chang SH, Kang YN, Chiu HY, Chiu YH. A Systematic Review and Meta-Analysis Comparing Pigtail Catheter and Chest Tube as the Initial Treatment for Pneumothorax. Chest. 2018;153(5):1201-1212. https://doi.org/10.1016/j.chest.2018.01.048.
2. Voisin F, Sohier L, Rochas Y, et al. Ambulatory management of large spontaneous pneumothorax with pigtail catheters. Ann Emerg Med. 2014;64(3):222-228. https://doi.org/10.1016/j.annemergmed.2013.12.017.
3. Lin YC, Tu CY, Liang SJ, et al. Pigtail catheter for the management of pneumothorax in mechanically ventilated patients. Am J Emerg Med. 2010;28(4):466-471. https://doi.org/10.1016/j.ajem.2009.01.033.
4. Tsai WK, Chen W, Lee JC, et al. Pigtail catheters vs large-bore chest tubes for management of secondary spontaneous pneumothoraces in adults. Am J Emerg Med. 2006;24(7):795-800. https://doi.org/10.1016/j.ajem.2006.04.006.
5. Kulvatunyou N, Erickson L, Vijayasekaran A, et al. Randomized clinical trial of pigtail catheter versus chest tube in injured patients with uncomplicated traumatic pneumothorax. Br J Surg. 2014;101(2):17-22. https://doi.org/10.1002/bjs.9377.
6. Kulvatunyou N, Joseph B, Friese RS, et al. 14 French pigtail catheters placed by surgeons to drain blood on trauma patients: is 14-Fr too small? J Trauma Acute Care Surg. 2012;73(6):1423-1427. https://doi.org/10.1097/TA.0b013e318271c1c7.
7. Bauman ZM, Kulvatunyou N, Joseph B, et al. A Prospective Study of 7-Year Experience Using Percutaneous 14-French Pigtail Catheters for Traumatic Hemothorax/Hemopneumothorax at a Level-1 Trauma Center: Size Still Does Not Matter. World J Surg. 2018;42(1):107-113. https://doi.org/10.1007/s00268-017-4168-3.
8. Fysh ET, Smith NA, Lee YC. Optimal chest drain size: the rise of the small-bore pleural catheter. Semin Respir Crit Care Med. 2010;31(6):760-768. https://doi.org/10.1055/s-0030-1269836.
9. Ozkan OS, Ozmen MN, Akhan O. Percutaneous management of parapneumonic effusions. Eur J Radiol. 2005;55(3):311-320. https://doi.org/10.1016/j.ejrad.2005.03.004.
10. Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest. 2010;137(3):536-543. https://doi.org/10.1378/chest.09-1044.
11. Saffran L, Ost DE, Fein AM, Schiff MJ. Outpatient pleurodesis of malignant pleural effusions using a small-bore pigtail catheter. Chest. 2000;118(2):417-421. https://doi.org/10.1378/chest.118.2.417.
12. Havelock T, Teoh R, Laws D, Gleeson F, Group BPDG. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii61-76. https://doi.org/10.1136/thx.2010.137026.
13. Feller-Kopman D, Light R. Pleural disease. N Engl J Med. 2018;378(8):740-751. https://doi.org/10.1056/NEJMra1403503.
14. Kulvatunyou N, Vijayasekaran A, Hansen A, et al. Two-year experience of using pigtail catheters to treat traumatic pneumothorax: A changing trend. J Trauma. 2011;71(5):1104-1107; discussion 1107. https://doi.org/10.1097/TA.0b013e31822dd130.
15. Cantin L, Chartrand-Lefebvre C, Lepanto L, et al. Chest tube drainage under radiological guidance for pleural effusion and pneumothorax in a tertiary care university teaching hospital: Review of 51 cases. Can Respir J. 2005;12(1):29-33. https://doi.org/10.1155/2005/498709.
16. Horsley A, Jones L, White J, Henry M. Efficacy and complications of small-bore, wire-guided chest drains. Chest. 2006;130(6):1857-1863. https://doi.org/10.1378/chest.130.6.1857.
17. Merriam MA, Cronan JJ, Dorfman GS, Lambiase RE, Haas RA. Radiographically guided percutaneous catheter drainage of pleural fluid collections. Am J Roentgenol. 1988;151(6):1113-1116. https://doi.org/10.2214/ajr.151.6.1113.
18. Petel D, Li P, Emil S. Percutaneous pigtail catheter versus tube thoracostomy for pediatric empyema: A comparison of outcomes. Surgery. 2013;154(4):655-660; discussion 660-651. https://doi.org/10.1016/j.surg.2013.04.032.
19. Gammie JS, Banks MC, Fuhrman CR, et al. The pigtail catheter for pleural drainage: a less invasive alternative to tube thoracostomy. JSLS. 1999;3(1):57-61.
20. Davies HE, Merchant S, McGown A. A study of the complications of small bore ‘Seldinger’ intercostal chest drains. Respirology. 2008;13(4):603-607. https://doi.org/10.1111/j.1440-1843.2008.01296.x.
21. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the Use of Ultrasound Guidance for Adult Thoracentesis: A Position Statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940.
© 2020 Society of Hospital Medicine
A Qualitative Study of Increased Pediatric Reutilization After a Postdischarge Home Nurse Visit
Readmission rates are used as metrics for care quality and reimbursement, with penalties applied to hospitals with higher than expected rates1 and up to 30% of pediatric readmissions deemed potentially preventable.2 There is a paucity of information on how to prevent pediatric readmissions,3 yet pediatric hospitals are tasked with implementing interventions for readmission reduction.
The Hospital to Home Outcomes (H2O) trial was a 2-arm, randomized controlled trial in which patients discharged from hospital medicine and neuroscience services at a single institution were randomized to receive a single home visit from a registered nurse (RN) within 96 hours of discharge.4 RNs completed a structured nurse visit designed specifically for the trial. Lists of “red flags” or warning signs associated with common diagnoses were provided to assist RNs in standardizing education about when to seek additional care. The hypothesis was that the postdischarge visits would result in lower reutilization rates (unplanned readmissions, emergency department [ED] visits, and urgent care visits).5
Unexpectedly, children randomized to receive the postdischarge nurse visit had higher rates of 30-day unplanned healthcare reutilization, with children randomly assigned to the intervention demonstrating higher odds of 30-day healthcare use (OR 1.33; 95% CI 1.003-1.76).4 We sought to understand perspectives on these unanticipated findings by obtaining input from relevant stakeholders. There were 2 goals for the qualitative analysis: first, to understand possible explanations of the increased reutilization finding; second, to elicit suggestions for improving the nurse visit intervention.
METHODS
We selected an in-depth qualitative approach, using interviews and focus groups to explore underlying explanations for the increase in 30-day unplanned healthcare reutilization among those randomized to receive the postdischarge nurse visit during the H2O trial.4 Input was sought from 4 stakeholder groups—parents, primary care physicians (PCPs), hospital medicine physicians, and home care RNs—in an effort to triangulate data sources and elicit rich and diverse opinions. Approval was obtained from the Institutional Review Board prior to conducting the study.
Recruitment
Parents
Because we conducted interviews approximately 1 year after the trial’s conclusion, we purposefully selected families who were enrolled in the latter portion of the H2O trial in order to enhance recall. Beginning with the last families in the study, we sequentially contacted families in reverse order. We contacted 10 families in each of 4 categories (intervention/reutilization, intervention/no reutilization, control/reutilization, control/no reutilization). A total of 3 attempts were made by telephone to contact each family. Participants received a grocery store gift card for participating in the study.
Primary Care Physicians
We conducted focus groups with a purposive sample of physicians recruited from 2 community practices and 1 hospital-owned practice.
Hospital Medicine Physicians
We conducted focus groups with a purposive sample of physicians from our Division of Hospital Medicine. There was a varying level of knowledge of the original trial; however, none of the participants were collaborators in the trial.
Home Care RNs
We conducted focus groups with a subset of RNs who were involved with trial visits. All RNs were members of the pediatric home care division associated with the hospital with specific training in caring for patients at home.
Data Collection
The study team designed question guides for each stakeholder group (Appendix 1). While questions were tailored for specific stakeholders, all guides included the following topics: benefits and challenges of nurse visits, suggestions for improving the intervention in future trials, and reactions to the trial results (once presented to participants). Only the results of the intention-to-treat (ITT) analysis were shared with stakeholders because ITT is considered the gold standard for trial analysis and allows easy understanding of the results.
A single investigator (A.L.) conducted parental interviews by telephone. Focus groups for PCPs, hospital medicine physicians, and RN groups were held at practice locations in private conference rooms and were conducted by trained moderators (S.N.S., A.L., and H.T.C.). Moderators probed responses to the open-ended questions to delve deeply into issues. The question guides were modified in an iterative fashion to include new concepts raised during interviews or focus groups. All interviews and focus groups were recorded and transcribed verbatim with all identifiable information redacted.
Data Analysis
During multiple cycles of inductive thematic analysis,6 we examined, discussed, interpreted, and organized responses to the open-ended questions,6,7 analyzing each stakeholder group separately. First, transcripts were shared with and reviewed by the entire multidisciplinary team (12 members) which included hospital medicine physicians, PCPs, home care nursing leaders, a nurse scientist, a parent representative, research coordinators, and a qualitative research methodologist. Second, team members convened to discuss overall concepts and ideas and created the preliminary coding frameworks. Third, a smaller subgroup (research coordinator [A.L]., hospital medicine physician [S.R.], parent representative [M.M.], and qualitative research methodologist [S.N.S.]), refined the unique coding framework for each stakeholder group and then independently applied codes to participant comments. This subgroup met regularly to reach consensus about the assigned codes and to further refine the codebooks. The codes were organized into major and minor themes based on recurring patterns in the data and the salience or emphasis given by participants. The subgroup’s work was reviewed and discussed on an ongoing basis by the entire multidisciplinary team. Triangulation of the data was achieved in multiple ways. The preliminary results were shared in several forums, and feedback was solicited and incorporated. Two of 4 members of the subgroup analytic team were not part of the trial planning or data collection, providing a potentially broader perspective. All coding decisions were maintained in an electronic database, and an audit trail was created to document codebook revisions.
RESULTS
A total of 33 parents participated in the interviews (intervention/readmit [8], intervention/no readmit [8], control/readmit [8], and control/no readmit [9]). Although we selected families from all 4 categories, we were not able to explore qualitative differences between these groups because of the relatively low numbers of participants. Parent data was very limited as interviews were brief and “control” parents had not received the intervention. Three focus groups were held with PCPs (7 participants in total), 2 focus groups were held with hospital medicine physicians (12 participants), and 2 focus groups were held with RNs (10 participants).
Goal 1: Explanation of Reutilization Rates
During interviews and focus groups, the results of the H2O trial were discussed, and stakeholders were asked to comment on potential explanations of the findings. 4 major themes and 5 minor themes emerged from analysis of the transcripts (summarized in Table 1).
Theme 1: Appropriateness of Patient Reutilization
Hospital medicine physicians and home care RNs questioned whether the reutilization events were clinically indicated. RNs wondered whether children who reutilized the ED were also readmitted to the hospital; many perceived that if the child was ill enough to be readmitted, then the ED revisit was warranted (Table 2). Parents commented on parental decision-making and changes in clinical status of the child leading to reutilization (Table 2).
Theme 2: Impact of Red Flags/Warning Sign Instructions on Family’s Reutilization Decisions
Theme 3: Hospital-Affiliated RNs “Directing Traffic” Back to Hospital
Both physician groups were concerned that, because the study was conducted by hospital-employed nurses, families might have been more likely to reaccess care at the hospital. Thus, the connection with the hospital was strengthened in the H2O model, potentially at the expense of the connection with PCPs. Physicians hypothesized that families might “still feel part of the medical system,” so families would return to the hospital if there was a problem. PCPs emphasized that there may have been straightforward situations that could have been handled appropriately in the outpatient office (Table 2).
Theme 4: Home Visit RNs Had a Low Threshold for Escalating Care
Parents and PCPs hypothesized that RNs are more conservative and, therefore, would have had a low threshold to refer back to the hospital if there were concerns in the home. One parent commented: “I guess, nurses are just by trade accustomed to erring on the side of caution and medical intervention instead of letting time take its course. … They’re more apt to say it’s better off to go to the hospital and have everything be fine” (Table 2).
Minor Themes
Participants also explained reutilization in ways that coalesced into 5 minor themes: (1) families receiving a visit might perceive that their child was sicker; (2) patients in the control group did not reutilize enough; (3) receiving more education on a child’s illness drives reutilization; (4) provider access issues; and (5) variability of RN experience may determine whether escalated care. Supportive quotations found in Appendix 2.
We directly asked parents if they would want a nurse home visit in the future after discussing the results of the study. Almost all of the parents in the intervention group and most of the parents in the control group were in favor of receiving a visit, even knowing that patients who had received a visit were more likely to reutilize care.
Goal 2: Suggestions for Improving Intervention Design
Three major themes and 3 minor themes were related to improving the design of the intervention (Table 1).
Theme 1: Need for Improved Postdischarge Communication
All stakeholder groups highlighted postdischarge communication as an area that could be improved. Parents were frustrated with regard to attempts to connect with inpatient physicians after discharge. PCPs suggested developing pathways for the RN to connect with the primary care office as opposed to the hospital. Hospital medicine physicians discussed a lack of consensus regarding patient ownership following discharge and were uncertain about what types of postdischarge symptoms PCPs would be comfortable managing. RNs described specific situations when they had difficulty contacting a physician to escalate care (Table 3).
Theme 2: Individualizing Home Visits—One Size Does Not Fit All
All stakeholder groups also encouraged “individualization” of home visits according to patient and family characteristics, diagnosis, and both timing and severity of illness. PCPs recommended visits only for certain diagnoses. Hospital medicine physicians voiced similar sentiments as the PCPs and added that worrisome family dynamics during a hospitalization, such as a lack of engagement with the medical team, might also warrant a visit. RNs suggested visits for those families with more concerns, for example, those with young children or children recovering from an acute respiratory illness (Table 3).
Theme 3: Providing Context for and Framing of Red Flags
Physicians and nurses suggested providing more context to “red flag” instructions and education. RNs emphasized that some families seemed to benefit from the opportunity to discuss their postdischarge concerns with a medical professional. Others appreciated concrete written instructions that spelled out how to respond in certain situations (Table 3).
Minor Themes
Three minor themes were revealed regarding intervention design improvement (Table 1): (1) streamlining the discharge process; (2) improving the definition of the scope and goal of intervention; and (3) extending inpatient team expertise post discharge. Supportive quotations can be found in Appendix 3.
DISCUSSION
When stakeholders were asked about why postdischarge RN visits led to increased postdischarge urgent healthcare visits, they questioned the appropriateness of the reutilization events, wondered about the lack of context for the warning signs that nurses provided families as part of the intervention, worried that families were encouraged to return to the hospital because of the ties of the trial to the hospital, and suggested that RNs had a low threshold to refer patients back to the hospital. When asked about how to design an improved nurse visit to better support families, stakeholders emphasized improving communication, individualizing the visit, and providing context around the red-flag discussion, enabling more nuanced instructions about how to respond to specific events.
A synthesis of themes suggests that potential drivers for increased utilization rates may lie in the design and goals of the initial project. The intervention was designed to support families and enhance education after discharge, with components derived from pretrial focus groups with families after a hospital discharge.8 The intervention was not designed to divert patients from the ED nor did it enhance access to the PCP. A second trial of the intervention adapted to a phone call also failed to decrease reutilization rates.9 Both physician stakeholder groups perceived that the intervention directed traffic back to the hospital because of the intervention design. Coupled with the perception that the red flags may have changed a family’s threshold for seeking care and/or that an RN may be more apt to refer back to care, this failure to push utilization to the primary care office may explain the unexpected trial results. Despite the stakeholders’ perception of enhanced connection back to the hospital as a result of the nurse visit, in analysis of visit referral patterns, a referral was made directly back to the ED in only 4 of the 651 trial visits (Tubbs-Cooley H, Riddle SR, Gold JM, et al.; under review. Pediatric clinical and social concerns identified by home visit nurses in the immediate postdischarge period 2020).
Both H2O trials demonstrated improved recall of red flags by parents who received the intervention, which may be important given the stakeholders’ perspectives that the red flags may not have been contextualized well enough. Yet neither trial demonstrated any differences in postdischarge coping or time to return to normal routine. In interviews with parents, despite the clearly stated results of increased reutilization, intervention parents endorsed a desire for a home visit in the future, raising the possibility that our outcome measures did not capture parents’ priorities adequately.
When asked to recommend design improvements of the intervention, 2 major themes (improvement in communication and individualization of visits) were discussed by all stakeholder groups, providing actionable information to modify or create new interventions. Focus groups with clinicians suggested that communication challenges may have influenced reutilization likelihood during the postdischarge period. RNs expressed uncertainty about who to call with problems or questions at the time of a home visit. This was compounded by difficulty reaching physicians. Both hospital medicine physicians and PCPs identified system challenges including questions of patient ownership, variable PCP practice communication preferences, and difficulty in identifying a partnered staff member (on either end of the inpatient-outpatient continuum) who was familiar with a specific patient. While the communication issues raised may reflect difficulties in our local healthcare system, there is broad evidence of postdischarge communication challenges. In adults, postdischarge communication failures between home health staff and physicians are associated with an increased risk of readmission.10 The real or perceived lack of communication between inpatient and outpatient providers can add to parental confusion post discharge.11 Although there have been efforts to improve the reliability of communication across this gulf,12,13 it is not clear whether changes to discharge communication could help to avoid pediatric reutilization events.14
The theme of individualization of the home nurse visit is consistent with evidence regarding the impact of focusing the intervention on patients with specific diagnoses or demographics. In adults, reduced reutilization associated with postdischarge home nurse visits has been described in specific populations such as patients with heart failure and chronic obstructive pulmonary disease.15 Impact of home nurse visits on patients within diagnosis-specific populations with certain demographics (such as advanced age) has also been described.16 In the pediatric population, readmission rates vary widely by diagnosis.17 A systematic review of interventions to reduce pediatric readmissions found increased impact of discharge interventions in specific populations (asthma, oncology, and NICU).3
Next steps may lie in interventions in targeted populations that function as part of a care continuum bridging the patient from the inpatient to the outpatient setting. A home nurse visit as part of this discharge structure may prove to have more impact on reducing reutilization. One population which accounts for a large proportion of readmissions and where there has been recent focus on discharge transition of care has been children with medical complexity.18 This group was largely excluded from the H2O trial. Postdischarge home nurse visits in this population have been found to be feasible and address many questions and problems, but the effect on readmission is less clear.19 Family priorities and preferences related to preparation for discharge, including family engagement, respect for discharge readiness, and goal of returning to normal routines, may be areas on which to focus with future interventions in this population.20 In summary, although widespread postdischarge interventions (home nurse visit4 and nurse telephone call9) have not been found to be effective, targeting interventions to specific populations by diagnosis or demographic factors may prove to be more effective in reducing pediatric reutilization.
There were several strengths to this study. This qualitative approach allowed us to elucidate potential explanations for the H2O trial results from multiple perspectives. The multidisciplinary composition of our analytic team and the use of an iterative process sparked diverse contributions in a dynamic, ongoing discussion and interpretation of our data.
This study should be considered in the context of several limitations. For families and RNs, there was a time lag between participation in the trial and participation in the qualitative study call or focus group which could lead to difficulty recalling details. Only families who received the intervention could give opinions on their experience of the nurse visit, while families in the control group were asked to hypothesize. Focus groups with hospital medicine physicians and PCPs were purposive samples, and complete demographic information of participants was not collected.
CONCLUSION
Key stakeholders reflecting on a postdischarge RN visit trial suggested multiple potential explanations for the unexpected increase in reutilization in children randomized to the intervention. Certain participants questioned whether all reutilization events were appropriate or necessary. Others expressed concerns that the H2O intervention lacked context and directed children back to the hospital instead of the PCP. Parents, PCPs, hospital medicine physicians, and RNs all suggested that future transition-focused interventions should enhance postdischarge communication, strengthen connection to the PCP, and be more effectively tailored to the needs of the individual patient and family.
Acknowledgments
Collaborators: H2O Trial Study Group: Joanne Bachus, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Monica L Borell, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Lenisa V Chang, MA, PhD; Patricia Crawford, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Sarah A Ferris, BA, Division of Hospital Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Judy A Heilman BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Jane C Khoury, PhD, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Karen Lawley, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Lynne O’Donnell, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Hadley S Sauers-Ford, MPH, Department of Pediatrics, UC Davis Health, Sacramento, California; Anita N Shah, DO, MPH, Division of Hospital Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Lauren G Solan, MD, Med, University of Rochester, Rochester, New York; Heidi J Sucharew, PhD, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Karen P Sullivan, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Christine M White, MD, MAT, Division of Hospital Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio.
1. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (re)admissions network. Pediatrics. 2015;135(1):164-175. https://doi.org/10.1542/peds.2014-1887.
2. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a Children’s Hospital. Pediatrics. 2016;138(2). https://doi.org/10.1542/peds.2015-4182.
3. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9(4):251-260. https://doi.org/10.1002/jhm.2134.
4. Auger KA, Simmons JM, Tubbs-Cooley HL, et al. Postdischarge nurse home visits and reuse: the Hospital to Home Outcomes (H2O) trial. Pediatrics. 2018;142(1). https://doi.org/10.1542/peds.2017-3919.
5. Tubbs-Cooley HL, Pickler RH, Simmons JM, et al. Testing a post-discharge nurse-led transitional home visit in acute care pediatrics: the Hospital-To-Home Outcomes (H2O) study protocol. J Adv Nurs. 2016;72(4):915-925. https://doi.org/10.1111/jan.12882.
6. Guest G. Collecting Qualitative Data: A Field Manual for Applied Research. Thousand Oaks, CA: SAGE Publications, Inc.; 2013.
7. Patton M. Qualitative Research and Evaluation Methods. 4th ed. Thousand Oaks, CA: SAGE Publications, Inc.; 2014.
8. Solan LG, Beck AF, Brunswick SA, et al. The family perspective on Hospital to Home Transitions: a qualitative study. Pediatrics. 2015;136(6):e1539-e1549. https://doi.org/10.1542/peds.2015-2098.
9. Auger KA, Shah SS, Tubbs-Cooley HL, et al. Effects of a 1-time nurse-led telephone call after pediatric discharge: the H2O II randomized clinical trial. JAMA Pediatr. 2018;172(9):e181482. https://doi.org/10.1001/jamapediatrics.2018.1482.
10. Pesko MF, Gerber LM, Peng TR, Press MJ. Home health care: nurse-physician communication, patient severity, and hospital readmission. Health Serv Res. 2018;53(2):1008-1024. https://doi.org/10.1111/1475-6773.12667.
11. Solan LG, Beck AF, Shardo SA, et al. Caregiver perspectives on communication during hospitalization at an academic pediatric institution: a qualitative study. J Hosp Med. 2018;13(5):304-311. https://doi.org/10.12788/jhm.2919.
12. Zackoff MW, Graham C, Warrick D, et al. Increasing PCP and hospital medicine physician verbal communication during hospital admissions. Hosp Pediatr. 2018;8(4):220-226. https://doi.org/10.1542/hpeds.2017-0119.
13. Mussman GM, Vossmeyer MT, Brady PW, et al. Improving the reliability of verbal communication between primary care physicians and pediatric hospitalists at hospital discharge. J Hosp Med. 2015;10(9):574-580. https://doi.org/10.1002/jhm.2392.
14. Coller RJ, Klitzner TS, Saenz AA, et al. Discharge handoff communication and pediatric readmissions. J Hosp Med. 2017;12(1):29-35. https://doi.org/10.1002/jhm.2670.
15. Yang F, Xiong ZF, Yang C, et al. Continuity of care to prevent readmissions for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. COPD. 2017;14(2):251-261. https://doi.org/10.1080/15412555.2016.1256384.
16. Finlayson K, Chang AM, Courtney MD, et al. Transitional care interventions reduce unplanned hospital readmissions in high-risk older adults. BMC Health Serv Res. 2018;18(1):956. https://doi.org/10.1186/s12913-018-3771-9.
17. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351.
18. Coller RJ, Nelson BB, Sklansky DJ, et al. Preventing hospitalizations in children with medical complexity: a systematic review. Pediatrics. 2014;134(6):e1628-e1647. https://doi.org/10.1542/peds.2014-1956.
19. Wells S, O’Neill M, Rogers J, et al. Nursing-led home visits post-hospitalization for children with medical complexity. J Pediatr Nurs. 2017;34:10-16. https://doi.org/10.1016/j.pedn.2017.03.003.
20. Leyenaar JK, O’Brien ER, Leslie LK, Lindenauer PK, Mangione-Smith RM. Families’ priorities regarding hospital-to-home transitions for children with medical complexity. Pediatrics. 2017;139(1). https://doi.org/10.1542/peds.2016-1581.
Readmission rates are used as metrics for care quality and reimbursement, with penalties applied to hospitals with higher than expected rates1 and up to 30% of pediatric readmissions deemed potentially preventable.2 There is a paucity of information on how to prevent pediatric readmissions,3 yet pediatric hospitals are tasked with implementing interventions for readmission reduction.
The Hospital to Home Outcomes (H2O) trial was a 2-arm, randomized controlled trial in which patients discharged from hospital medicine and neuroscience services at a single institution were randomized to receive a single home visit from a registered nurse (RN) within 96 hours of discharge.4 RNs completed a structured nurse visit designed specifically for the trial. Lists of “red flags” or warning signs associated with common diagnoses were provided to assist RNs in standardizing education about when to seek additional care. The hypothesis was that the postdischarge visits would result in lower reutilization rates (unplanned readmissions, emergency department [ED] visits, and urgent care visits).5
Unexpectedly, children randomized to receive the postdischarge nurse visit had higher rates of 30-day unplanned healthcare reutilization, with children randomly assigned to the intervention demonstrating higher odds of 30-day healthcare use (OR 1.33; 95% CI 1.003-1.76).4 We sought to understand perspectives on these unanticipated findings by obtaining input from relevant stakeholders. There were 2 goals for the qualitative analysis: first, to understand possible explanations of the increased reutilization finding; second, to elicit suggestions for improving the nurse visit intervention.
METHODS
We selected an in-depth qualitative approach, using interviews and focus groups to explore underlying explanations for the increase in 30-day unplanned healthcare reutilization among those randomized to receive the postdischarge nurse visit during the H2O trial.4 Input was sought from 4 stakeholder groups—parents, primary care physicians (PCPs), hospital medicine physicians, and home care RNs—in an effort to triangulate data sources and elicit rich and diverse opinions. Approval was obtained from the Institutional Review Board prior to conducting the study.
Recruitment
Parents
Because we conducted interviews approximately 1 year after the trial’s conclusion, we purposefully selected families who were enrolled in the latter portion of the H2O trial in order to enhance recall. Beginning with the last families in the study, we sequentially contacted families in reverse order. We contacted 10 families in each of 4 categories (intervention/reutilization, intervention/no reutilization, control/reutilization, control/no reutilization). A total of 3 attempts were made by telephone to contact each family. Participants received a grocery store gift card for participating in the study.
Primary Care Physicians
We conducted focus groups with a purposive sample of physicians recruited from 2 community practices and 1 hospital-owned practice.
Hospital Medicine Physicians
We conducted focus groups with a purposive sample of physicians from our Division of Hospital Medicine. There was a varying level of knowledge of the original trial; however, none of the participants were collaborators in the trial.
Home Care RNs
We conducted focus groups with a subset of RNs who were involved with trial visits. All RNs were members of the pediatric home care division associated with the hospital with specific training in caring for patients at home.
Data Collection
The study team designed question guides for each stakeholder group (Appendix 1). While questions were tailored for specific stakeholders, all guides included the following topics: benefits and challenges of nurse visits, suggestions for improving the intervention in future trials, and reactions to the trial results (once presented to participants). Only the results of the intention-to-treat (ITT) analysis were shared with stakeholders because ITT is considered the gold standard for trial analysis and allows easy understanding of the results.
A single investigator (A.L.) conducted parental interviews by telephone. Focus groups for PCPs, hospital medicine physicians, and RN groups were held at practice locations in private conference rooms and were conducted by trained moderators (S.N.S., A.L., and H.T.C.). Moderators probed responses to the open-ended questions to delve deeply into issues. The question guides were modified in an iterative fashion to include new concepts raised during interviews or focus groups. All interviews and focus groups were recorded and transcribed verbatim with all identifiable information redacted.
Data Analysis
During multiple cycles of inductive thematic analysis,6 we examined, discussed, interpreted, and organized responses to the open-ended questions,6,7 analyzing each stakeholder group separately. First, transcripts were shared with and reviewed by the entire multidisciplinary team (12 members) which included hospital medicine physicians, PCPs, home care nursing leaders, a nurse scientist, a parent representative, research coordinators, and a qualitative research methodologist. Second, team members convened to discuss overall concepts and ideas and created the preliminary coding frameworks. Third, a smaller subgroup (research coordinator [A.L]., hospital medicine physician [S.R.], parent representative [M.M.], and qualitative research methodologist [S.N.S.]), refined the unique coding framework for each stakeholder group and then independently applied codes to participant comments. This subgroup met regularly to reach consensus about the assigned codes and to further refine the codebooks. The codes were organized into major and minor themes based on recurring patterns in the data and the salience or emphasis given by participants. The subgroup’s work was reviewed and discussed on an ongoing basis by the entire multidisciplinary team. Triangulation of the data was achieved in multiple ways. The preliminary results were shared in several forums, and feedback was solicited and incorporated. Two of 4 members of the subgroup analytic team were not part of the trial planning or data collection, providing a potentially broader perspective. All coding decisions were maintained in an electronic database, and an audit trail was created to document codebook revisions.
RESULTS
A total of 33 parents participated in the interviews (intervention/readmit [8], intervention/no readmit [8], control/readmit [8], and control/no readmit [9]). Although we selected families from all 4 categories, we were not able to explore qualitative differences between these groups because of the relatively low numbers of participants. Parent data was very limited as interviews were brief and “control” parents had not received the intervention. Three focus groups were held with PCPs (7 participants in total), 2 focus groups were held with hospital medicine physicians (12 participants), and 2 focus groups were held with RNs (10 participants).
Goal 1: Explanation of Reutilization Rates
During interviews and focus groups, the results of the H2O trial were discussed, and stakeholders were asked to comment on potential explanations of the findings. 4 major themes and 5 minor themes emerged from analysis of the transcripts (summarized in Table 1).
Theme 1: Appropriateness of Patient Reutilization
Hospital medicine physicians and home care RNs questioned whether the reutilization events were clinically indicated. RNs wondered whether children who reutilized the ED were also readmitted to the hospital; many perceived that if the child was ill enough to be readmitted, then the ED revisit was warranted (Table 2). Parents commented on parental decision-making and changes in clinical status of the child leading to reutilization (Table 2).
Theme 2: Impact of Red Flags/Warning Sign Instructions on Family’s Reutilization Decisions
Theme 3: Hospital-Affiliated RNs “Directing Traffic” Back to Hospital
Both physician groups were concerned that, because the study was conducted by hospital-employed nurses, families might have been more likely to reaccess care at the hospital. Thus, the connection with the hospital was strengthened in the H2O model, potentially at the expense of the connection with PCPs. Physicians hypothesized that families might “still feel part of the medical system,” so families would return to the hospital if there was a problem. PCPs emphasized that there may have been straightforward situations that could have been handled appropriately in the outpatient office (Table 2).
Theme 4: Home Visit RNs Had a Low Threshold for Escalating Care
Parents and PCPs hypothesized that RNs are more conservative and, therefore, would have had a low threshold to refer back to the hospital if there were concerns in the home. One parent commented: “I guess, nurses are just by trade accustomed to erring on the side of caution and medical intervention instead of letting time take its course. … They’re more apt to say it’s better off to go to the hospital and have everything be fine” (Table 2).
Minor Themes
Participants also explained reutilization in ways that coalesced into 5 minor themes: (1) families receiving a visit might perceive that their child was sicker; (2) patients in the control group did not reutilize enough; (3) receiving more education on a child’s illness drives reutilization; (4) provider access issues; and (5) variability of RN experience may determine whether escalated care. Supportive quotations found in Appendix 2.
We directly asked parents if they would want a nurse home visit in the future after discussing the results of the study. Almost all of the parents in the intervention group and most of the parents in the control group were in favor of receiving a visit, even knowing that patients who had received a visit were more likely to reutilize care.
Goal 2: Suggestions for Improving Intervention Design
Three major themes and 3 minor themes were related to improving the design of the intervention (Table 1).
Theme 1: Need for Improved Postdischarge Communication
All stakeholder groups highlighted postdischarge communication as an area that could be improved. Parents were frustrated with regard to attempts to connect with inpatient physicians after discharge. PCPs suggested developing pathways for the RN to connect with the primary care office as opposed to the hospital. Hospital medicine physicians discussed a lack of consensus regarding patient ownership following discharge and were uncertain about what types of postdischarge symptoms PCPs would be comfortable managing. RNs described specific situations when they had difficulty contacting a physician to escalate care (Table 3).
Theme 2: Individualizing Home Visits—One Size Does Not Fit All
All stakeholder groups also encouraged “individualization” of home visits according to patient and family characteristics, diagnosis, and both timing and severity of illness. PCPs recommended visits only for certain diagnoses. Hospital medicine physicians voiced similar sentiments as the PCPs and added that worrisome family dynamics during a hospitalization, such as a lack of engagement with the medical team, might also warrant a visit. RNs suggested visits for those families with more concerns, for example, those with young children or children recovering from an acute respiratory illness (Table 3).
Theme 3: Providing Context for and Framing of Red Flags
Physicians and nurses suggested providing more context to “red flag” instructions and education. RNs emphasized that some families seemed to benefit from the opportunity to discuss their postdischarge concerns with a medical professional. Others appreciated concrete written instructions that spelled out how to respond in certain situations (Table 3).
Minor Themes
Three minor themes were revealed regarding intervention design improvement (Table 1): (1) streamlining the discharge process; (2) improving the definition of the scope and goal of intervention; and (3) extending inpatient team expertise post discharge. Supportive quotations can be found in Appendix 3.
DISCUSSION
When stakeholders were asked about why postdischarge RN visits led to increased postdischarge urgent healthcare visits, they questioned the appropriateness of the reutilization events, wondered about the lack of context for the warning signs that nurses provided families as part of the intervention, worried that families were encouraged to return to the hospital because of the ties of the trial to the hospital, and suggested that RNs had a low threshold to refer patients back to the hospital. When asked about how to design an improved nurse visit to better support families, stakeholders emphasized improving communication, individualizing the visit, and providing context around the red-flag discussion, enabling more nuanced instructions about how to respond to specific events.
A synthesis of themes suggests that potential drivers for increased utilization rates may lie in the design and goals of the initial project. The intervention was designed to support families and enhance education after discharge, with components derived from pretrial focus groups with families after a hospital discharge.8 The intervention was not designed to divert patients from the ED nor did it enhance access to the PCP. A second trial of the intervention adapted to a phone call also failed to decrease reutilization rates.9 Both physician stakeholder groups perceived that the intervention directed traffic back to the hospital because of the intervention design. Coupled with the perception that the red flags may have changed a family’s threshold for seeking care and/or that an RN may be more apt to refer back to care, this failure to push utilization to the primary care office may explain the unexpected trial results. Despite the stakeholders’ perception of enhanced connection back to the hospital as a result of the nurse visit, in analysis of visit referral patterns, a referral was made directly back to the ED in only 4 of the 651 trial visits (Tubbs-Cooley H, Riddle SR, Gold JM, et al.; under review. Pediatric clinical and social concerns identified by home visit nurses in the immediate postdischarge period 2020).
Both H2O trials demonstrated improved recall of red flags by parents who received the intervention, which may be important given the stakeholders’ perspectives that the red flags may not have been contextualized well enough. Yet neither trial demonstrated any differences in postdischarge coping or time to return to normal routine. In interviews with parents, despite the clearly stated results of increased reutilization, intervention parents endorsed a desire for a home visit in the future, raising the possibility that our outcome measures did not capture parents’ priorities adequately.
When asked to recommend design improvements of the intervention, 2 major themes (improvement in communication and individualization of visits) were discussed by all stakeholder groups, providing actionable information to modify or create new interventions. Focus groups with clinicians suggested that communication challenges may have influenced reutilization likelihood during the postdischarge period. RNs expressed uncertainty about who to call with problems or questions at the time of a home visit. This was compounded by difficulty reaching physicians. Both hospital medicine physicians and PCPs identified system challenges including questions of patient ownership, variable PCP practice communication preferences, and difficulty in identifying a partnered staff member (on either end of the inpatient-outpatient continuum) who was familiar with a specific patient. While the communication issues raised may reflect difficulties in our local healthcare system, there is broad evidence of postdischarge communication challenges. In adults, postdischarge communication failures between home health staff and physicians are associated with an increased risk of readmission.10 The real or perceived lack of communication between inpatient and outpatient providers can add to parental confusion post discharge.11 Although there have been efforts to improve the reliability of communication across this gulf,12,13 it is not clear whether changes to discharge communication could help to avoid pediatric reutilization events.14
The theme of individualization of the home nurse visit is consistent with evidence regarding the impact of focusing the intervention on patients with specific diagnoses or demographics. In adults, reduced reutilization associated with postdischarge home nurse visits has been described in specific populations such as patients with heart failure and chronic obstructive pulmonary disease.15 Impact of home nurse visits on patients within diagnosis-specific populations with certain demographics (such as advanced age) has also been described.16 In the pediatric population, readmission rates vary widely by diagnosis.17 A systematic review of interventions to reduce pediatric readmissions found increased impact of discharge interventions in specific populations (asthma, oncology, and NICU).3
Next steps may lie in interventions in targeted populations that function as part of a care continuum bridging the patient from the inpatient to the outpatient setting. A home nurse visit as part of this discharge structure may prove to have more impact on reducing reutilization. One population which accounts for a large proportion of readmissions and where there has been recent focus on discharge transition of care has been children with medical complexity.18 This group was largely excluded from the H2O trial. Postdischarge home nurse visits in this population have been found to be feasible and address many questions and problems, but the effect on readmission is less clear.19 Family priorities and preferences related to preparation for discharge, including family engagement, respect for discharge readiness, and goal of returning to normal routines, may be areas on which to focus with future interventions in this population.20 In summary, although widespread postdischarge interventions (home nurse visit4 and nurse telephone call9) have not been found to be effective, targeting interventions to specific populations by diagnosis or demographic factors may prove to be more effective in reducing pediatric reutilization.
There were several strengths to this study. This qualitative approach allowed us to elucidate potential explanations for the H2O trial results from multiple perspectives. The multidisciplinary composition of our analytic team and the use of an iterative process sparked diverse contributions in a dynamic, ongoing discussion and interpretation of our data.
This study should be considered in the context of several limitations. For families and RNs, there was a time lag between participation in the trial and participation in the qualitative study call or focus group which could lead to difficulty recalling details. Only families who received the intervention could give opinions on their experience of the nurse visit, while families in the control group were asked to hypothesize. Focus groups with hospital medicine physicians and PCPs were purposive samples, and complete demographic information of participants was not collected.
CONCLUSION
Key stakeholders reflecting on a postdischarge RN visit trial suggested multiple potential explanations for the unexpected increase in reutilization in children randomized to the intervention. Certain participants questioned whether all reutilization events were appropriate or necessary. Others expressed concerns that the H2O intervention lacked context and directed children back to the hospital instead of the PCP. Parents, PCPs, hospital medicine physicians, and RNs all suggested that future transition-focused interventions should enhance postdischarge communication, strengthen connection to the PCP, and be more effectively tailored to the needs of the individual patient and family.
Acknowledgments
Collaborators: H2O Trial Study Group: Joanne Bachus, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Monica L Borell, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Lenisa V Chang, MA, PhD; Patricia Crawford, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Sarah A Ferris, BA, Division of Hospital Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Judy A Heilman BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Jane C Khoury, PhD, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Karen Lawley, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Lynne O’Donnell, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Hadley S Sauers-Ford, MPH, Department of Pediatrics, UC Davis Health, Sacramento, California; Anita N Shah, DO, MPH, Division of Hospital Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Lauren G Solan, MD, Med, University of Rochester, Rochester, New York; Heidi J Sucharew, PhD, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Karen P Sullivan, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Christine M White, MD, MAT, Division of Hospital Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio.
Readmission rates are used as metrics for care quality and reimbursement, with penalties applied to hospitals with higher than expected rates1 and up to 30% of pediatric readmissions deemed potentially preventable.2 There is a paucity of information on how to prevent pediatric readmissions,3 yet pediatric hospitals are tasked with implementing interventions for readmission reduction.
The Hospital to Home Outcomes (H2O) trial was a 2-arm, randomized controlled trial in which patients discharged from hospital medicine and neuroscience services at a single institution were randomized to receive a single home visit from a registered nurse (RN) within 96 hours of discharge.4 RNs completed a structured nurse visit designed specifically for the trial. Lists of “red flags” or warning signs associated with common diagnoses were provided to assist RNs in standardizing education about when to seek additional care. The hypothesis was that the postdischarge visits would result in lower reutilization rates (unplanned readmissions, emergency department [ED] visits, and urgent care visits).5
Unexpectedly, children randomized to receive the postdischarge nurse visit had higher rates of 30-day unplanned healthcare reutilization, with children randomly assigned to the intervention demonstrating higher odds of 30-day healthcare use (OR 1.33; 95% CI 1.003-1.76).4 We sought to understand perspectives on these unanticipated findings by obtaining input from relevant stakeholders. There were 2 goals for the qualitative analysis: first, to understand possible explanations of the increased reutilization finding; second, to elicit suggestions for improving the nurse visit intervention.
METHODS
We selected an in-depth qualitative approach, using interviews and focus groups to explore underlying explanations for the increase in 30-day unplanned healthcare reutilization among those randomized to receive the postdischarge nurse visit during the H2O trial.4 Input was sought from 4 stakeholder groups—parents, primary care physicians (PCPs), hospital medicine physicians, and home care RNs—in an effort to triangulate data sources and elicit rich and diverse opinions. Approval was obtained from the Institutional Review Board prior to conducting the study.
Recruitment
Parents
Because we conducted interviews approximately 1 year after the trial’s conclusion, we purposefully selected families who were enrolled in the latter portion of the H2O trial in order to enhance recall. Beginning with the last families in the study, we sequentially contacted families in reverse order. We contacted 10 families in each of 4 categories (intervention/reutilization, intervention/no reutilization, control/reutilization, control/no reutilization). A total of 3 attempts were made by telephone to contact each family. Participants received a grocery store gift card for participating in the study.
Primary Care Physicians
We conducted focus groups with a purposive sample of physicians recruited from 2 community practices and 1 hospital-owned practice.
Hospital Medicine Physicians
We conducted focus groups with a purposive sample of physicians from our Division of Hospital Medicine. There was a varying level of knowledge of the original trial; however, none of the participants were collaborators in the trial.
Home Care RNs
We conducted focus groups with a subset of RNs who were involved with trial visits. All RNs were members of the pediatric home care division associated with the hospital with specific training in caring for patients at home.
Data Collection
The study team designed question guides for each stakeholder group (Appendix 1). While questions were tailored for specific stakeholders, all guides included the following topics: benefits and challenges of nurse visits, suggestions for improving the intervention in future trials, and reactions to the trial results (once presented to participants). Only the results of the intention-to-treat (ITT) analysis were shared with stakeholders because ITT is considered the gold standard for trial analysis and allows easy understanding of the results.
A single investigator (A.L.) conducted parental interviews by telephone. Focus groups for PCPs, hospital medicine physicians, and RN groups were held at practice locations in private conference rooms and were conducted by trained moderators (S.N.S., A.L., and H.T.C.). Moderators probed responses to the open-ended questions to delve deeply into issues. The question guides were modified in an iterative fashion to include new concepts raised during interviews or focus groups. All interviews and focus groups were recorded and transcribed verbatim with all identifiable information redacted.
Data Analysis
During multiple cycles of inductive thematic analysis,6 we examined, discussed, interpreted, and organized responses to the open-ended questions,6,7 analyzing each stakeholder group separately. First, transcripts were shared with and reviewed by the entire multidisciplinary team (12 members) which included hospital medicine physicians, PCPs, home care nursing leaders, a nurse scientist, a parent representative, research coordinators, and a qualitative research methodologist. Second, team members convened to discuss overall concepts and ideas and created the preliminary coding frameworks. Third, a smaller subgroup (research coordinator [A.L]., hospital medicine physician [S.R.], parent representative [M.M.], and qualitative research methodologist [S.N.S.]), refined the unique coding framework for each stakeholder group and then independently applied codes to participant comments. This subgroup met regularly to reach consensus about the assigned codes and to further refine the codebooks. The codes were organized into major and minor themes based on recurring patterns in the data and the salience or emphasis given by participants. The subgroup’s work was reviewed and discussed on an ongoing basis by the entire multidisciplinary team. Triangulation of the data was achieved in multiple ways. The preliminary results were shared in several forums, and feedback was solicited and incorporated. Two of 4 members of the subgroup analytic team were not part of the trial planning or data collection, providing a potentially broader perspective. All coding decisions were maintained in an electronic database, and an audit trail was created to document codebook revisions.
RESULTS
A total of 33 parents participated in the interviews (intervention/readmit [8], intervention/no readmit [8], control/readmit [8], and control/no readmit [9]). Although we selected families from all 4 categories, we were not able to explore qualitative differences between these groups because of the relatively low numbers of participants. Parent data was very limited as interviews were brief and “control” parents had not received the intervention. Three focus groups were held with PCPs (7 participants in total), 2 focus groups were held with hospital medicine physicians (12 participants), and 2 focus groups were held with RNs (10 participants).
Goal 1: Explanation of Reutilization Rates
During interviews and focus groups, the results of the H2O trial were discussed, and stakeholders were asked to comment on potential explanations of the findings. 4 major themes and 5 minor themes emerged from analysis of the transcripts (summarized in Table 1).
Theme 1: Appropriateness of Patient Reutilization
Hospital medicine physicians and home care RNs questioned whether the reutilization events were clinically indicated. RNs wondered whether children who reutilized the ED were also readmitted to the hospital; many perceived that if the child was ill enough to be readmitted, then the ED revisit was warranted (Table 2). Parents commented on parental decision-making and changes in clinical status of the child leading to reutilization (Table 2).
Theme 2: Impact of Red Flags/Warning Sign Instructions on Family’s Reutilization Decisions
Theme 3: Hospital-Affiliated RNs “Directing Traffic” Back to Hospital
Both physician groups were concerned that, because the study was conducted by hospital-employed nurses, families might have been more likely to reaccess care at the hospital. Thus, the connection with the hospital was strengthened in the H2O model, potentially at the expense of the connection with PCPs. Physicians hypothesized that families might “still feel part of the medical system,” so families would return to the hospital if there was a problem. PCPs emphasized that there may have been straightforward situations that could have been handled appropriately in the outpatient office (Table 2).
Theme 4: Home Visit RNs Had a Low Threshold for Escalating Care
Parents and PCPs hypothesized that RNs are more conservative and, therefore, would have had a low threshold to refer back to the hospital if there were concerns in the home. One parent commented: “I guess, nurses are just by trade accustomed to erring on the side of caution and medical intervention instead of letting time take its course. … They’re more apt to say it’s better off to go to the hospital and have everything be fine” (Table 2).
Minor Themes
Participants also explained reutilization in ways that coalesced into 5 minor themes: (1) families receiving a visit might perceive that their child was sicker; (2) patients in the control group did not reutilize enough; (3) receiving more education on a child’s illness drives reutilization; (4) provider access issues; and (5) variability of RN experience may determine whether escalated care. Supportive quotations found in Appendix 2.
We directly asked parents if they would want a nurse home visit in the future after discussing the results of the study. Almost all of the parents in the intervention group and most of the parents in the control group were in favor of receiving a visit, even knowing that patients who had received a visit were more likely to reutilize care.
Goal 2: Suggestions for Improving Intervention Design
Three major themes and 3 minor themes were related to improving the design of the intervention (Table 1).
Theme 1: Need for Improved Postdischarge Communication
All stakeholder groups highlighted postdischarge communication as an area that could be improved. Parents were frustrated with regard to attempts to connect with inpatient physicians after discharge. PCPs suggested developing pathways for the RN to connect with the primary care office as opposed to the hospital. Hospital medicine physicians discussed a lack of consensus regarding patient ownership following discharge and were uncertain about what types of postdischarge symptoms PCPs would be comfortable managing. RNs described specific situations when they had difficulty contacting a physician to escalate care (Table 3).
Theme 2: Individualizing Home Visits—One Size Does Not Fit All
All stakeholder groups also encouraged “individualization” of home visits according to patient and family characteristics, diagnosis, and both timing and severity of illness. PCPs recommended visits only for certain diagnoses. Hospital medicine physicians voiced similar sentiments as the PCPs and added that worrisome family dynamics during a hospitalization, such as a lack of engagement with the medical team, might also warrant a visit. RNs suggested visits for those families with more concerns, for example, those with young children or children recovering from an acute respiratory illness (Table 3).
Theme 3: Providing Context for and Framing of Red Flags
Physicians and nurses suggested providing more context to “red flag” instructions and education. RNs emphasized that some families seemed to benefit from the opportunity to discuss their postdischarge concerns with a medical professional. Others appreciated concrete written instructions that spelled out how to respond in certain situations (Table 3).
Minor Themes
Three minor themes were revealed regarding intervention design improvement (Table 1): (1) streamlining the discharge process; (2) improving the definition of the scope and goal of intervention; and (3) extending inpatient team expertise post discharge. Supportive quotations can be found in Appendix 3.
DISCUSSION
When stakeholders were asked about why postdischarge RN visits led to increased postdischarge urgent healthcare visits, they questioned the appropriateness of the reutilization events, wondered about the lack of context for the warning signs that nurses provided families as part of the intervention, worried that families were encouraged to return to the hospital because of the ties of the trial to the hospital, and suggested that RNs had a low threshold to refer patients back to the hospital. When asked about how to design an improved nurse visit to better support families, stakeholders emphasized improving communication, individualizing the visit, and providing context around the red-flag discussion, enabling more nuanced instructions about how to respond to specific events.
A synthesis of themes suggests that potential drivers for increased utilization rates may lie in the design and goals of the initial project. The intervention was designed to support families and enhance education after discharge, with components derived from pretrial focus groups with families after a hospital discharge.8 The intervention was not designed to divert patients from the ED nor did it enhance access to the PCP. A second trial of the intervention adapted to a phone call also failed to decrease reutilization rates.9 Both physician stakeholder groups perceived that the intervention directed traffic back to the hospital because of the intervention design. Coupled with the perception that the red flags may have changed a family’s threshold for seeking care and/or that an RN may be more apt to refer back to care, this failure to push utilization to the primary care office may explain the unexpected trial results. Despite the stakeholders’ perception of enhanced connection back to the hospital as a result of the nurse visit, in analysis of visit referral patterns, a referral was made directly back to the ED in only 4 of the 651 trial visits (Tubbs-Cooley H, Riddle SR, Gold JM, et al.; under review. Pediatric clinical and social concerns identified by home visit nurses in the immediate postdischarge period 2020).
Both H2O trials demonstrated improved recall of red flags by parents who received the intervention, which may be important given the stakeholders’ perspectives that the red flags may not have been contextualized well enough. Yet neither trial demonstrated any differences in postdischarge coping or time to return to normal routine. In interviews with parents, despite the clearly stated results of increased reutilization, intervention parents endorsed a desire for a home visit in the future, raising the possibility that our outcome measures did not capture parents’ priorities adequately.
When asked to recommend design improvements of the intervention, 2 major themes (improvement in communication and individualization of visits) were discussed by all stakeholder groups, providing actionable information to modify or create new interventions. Focus groups with clinicians suggested that communication challenges may have influenced reutilization likelihood during the postdischarge period. RNs expressed uncertainty about who to call with problems or questions at the time of a home visit. This was compounded by difficulty reaching physicians. Both hospital medicine physicians and PCPs identified system challenges including questions of patient ownership, variable PCP practice communication preferences, and difficulty in identifying a partnered staff member (on either end of the inpatient-outpatient continuum) who was familiar with a specific patient. While the communication issues raised may reflect difficulties in our local healthcare system, there is broad evidence of postdischarge communication challenges. In adults, postdischarge communication failures between home health staff and physicians are associated with an increased risk of readmission.10 The real or perceived lack of communication between inpatient and outpatient providers can add to parental confusion post discharge.11 Although there have been efforts to improve the reliability of communication across this gulf,12,13 it is not clear whether changes to discharge communication could help to avoid pediatric reutilization events.14
The theme of individualization of the home nurse visit is consistent with evidence regarding the impact of focusing the intervention on patients with specific diagnoses or demographics. In adults, reduced reutilization associated with postdischarge home nurse visits has been described in specific populations such as patients with heart failure and chronic obstructive pulmonary disease.15 Impact of home nurse visits on patients within diagnosis-specific populations with certain demographics (such as advanced age) has also been described.16 In the pediatric population, readmission rates vary widely by diagnosis.17 A systematic review of interventions to reduce pediatric readmissions found increased impact of discharge interventions in specific populations (asthma, oncology, and NICU).3
Next steps may lie in interventions in targeted populations that function as part of a care continuum bridging the patient from the inpatient to the outpatient setting. A home nurse visit as part of this discharge structure may prove to have more impact on reducing reutilization. One population which accounts for a large proportion of readmissions and where there has been recent focus on discharge transition of care has been children with medical complexity.18 This group was largely excluded from the H2O trial. Postdischarge home nurse visits in this population have been found to be feasible and address many questions and problems, but the effect on readmission is less clear.19 Family priorities and preferences related to preparation for discharge, including family engagement, respect for discharge readiness, and goal of returning to normal routines, may be areas on which to focus with future interventions in this population.20 In summary, although widespread postdischarge interventions (home nurse visit4 and nurse telephone call9) have not been found to be effective, targeting interventions to specific populations by diagnosis or demographic factors may prove to be more effective in reducing pediatric reutilization.
There were several strengths to this study. This qualitative approach allowed us to elucidate potential explanations for the H2O trial results from multiple perspectives. The multidisciplinary composition of our analytic team and the use of an iterative process sparked diverse contributions in a dynamic, ongoing discussion and interpretation of our data.
This study should be considered in the context of several limitations. For families and RNs, there was a time lag between participation in the trial and participation in the qualitative study call or focus group which could lead to difficulty recalling details. Only families who received the intervention could give opinions on their experience of the nurse visit, while families in the control group were asked to hypothesize. Focus groups with hospital medicine physicians and PCPs were purposive samples, and complete demographic information of participants was not collected.
CONCLUSION
Key stakeholders reflecting on a postdischarge RN visit trial suggested multiple potential explanations for the unexpected increase in reutilization in children randomized to the intervention. Certain participants questioned whether all reutilization events were appropriate or necessary. Others expressed concerns that the H2O intervention lacked context and directed children back to the hospital instead of the PCP. Parents, PCPs, hospital medicine physicians, and RNs all suggested that future transition-focused interventions should enhance postdischarge communication, strengthen connection to the PCP, and be more effectively tailored to the needs of the individual patient and family.
Acknowledgments
Collaborators: H2O Trial Study Group: Joanne Bachus, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Monica L Borell, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Lenisa V Chang, MA, PhD; Patricia Crawford, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Sarah A Ferris, BA, Division of Hospital Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Judy A Heilman BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Jane C Khoury, PhD, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Karen Lawley, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Lynne O’Donnell, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Hadley S Sauers-Ford, MPH, Department of Pediatrics, UC Davis Health, Sacramento, California; Anita N Shah, DO, MPH, Division of Hospital Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Lauren G Solan, MD, Med, University of Rochester, Rochester, New York; Heidi J Sucharew, PhD, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Karen P Sullivan, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Christine M White, MD, MAT, Division of Hospital Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio.
1. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (re)admissions network. Pediatrics. 2015;135(1):164-175. https://doi.org/10.1542/peds.2014-1887.
2. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a Children’s Hospital. Pediatrics. 2016;138(2). https://doi.org/10.1542/peds.2015-4182.
3. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9(4):251-260. https://doi.org/10.1002/jhm.2134.
4. Auger KA, Simmons JM, Tubbs-Cooley HL, et al. Postdischarge nurse home visits and reuse: the Hospital to Home Outcomes (H2O) trial. Pediatrics. 2018;142(1). https://doi.org/10.1542/peds.2017-3919.
5. Tubbs-Cooley HL, Pickler RH, Simmons JM, et al. Testing a post-discharge nurse-led transitional home visit in acute care pediatrics: the Hospital-To-Home Outcomes (H2O) study protocol. J Adv Nurs. 2016;72(4):915-925. https://doi.org/10.1111/jan.12882.
6. Guest G. Collecting Qualitative Data: A Field Manual for Applied Research. Thousand Oaks, CA: SAGE Publications, Inc.; 2013.
7. Patton M. Qualitative Research and Evaluation Methods. 4th ed. Thousand Oaks, CA: SAGE Publications, Inc.; 2014.
8. Solan LG, Beck AF, Brunswick SA, et al. The family perspective on Hospital to Home Transitions: a qualitative study. Pediatrics. 2015;136(6):e1539-e1549. https://doi.org/10.1542/peds.2015-2098.
9. Auger KA, Shah SS, Tubbs-Cooley HL, et al. Effects of a 1-time nurse-led telephone call after pediatric discharge: the H2O II randomized clinical trial. JAMA Pediatr. 2018;172(9):e181482. https://doi.org/10.1001/jamapediatrics.2018.1482.
10. Pesko MF, Gerber LM, Peng TR, Press MJ. Home health care: nurse-physician communication, patient severity, and hospital readmission. Health Serv Res. 2018;53(2):1008-1024. https://doi.org/10.1111/1475-6773.12667.
11. Solan LG, Beck AF, Shardo SA, et al. Caregiver perspectives on communication during hospitalization at an academic pediatric institution: a qualitative study. J Hosp Med. 2018;13(5):304-311. https://doi.org/10.12788/jhm.2919.
12. Zackoff MW, Graham C, Warrick D, et al. Increasing PCP and hospital medicine physician verbal communication during hospital admissions. Hosp Pediatr. 2018;8(4):220-226. https://doi.org/10.1542/hpeds.2017-0119.
13. Mussman GM, Vossmeyer MT, Brady PW, et al. Improving the reliability of verbal communication between primary care physicians and pediatric hospitalists at hospital discharge. J Hosp Med. 2015;10(9):574-580. https://doi.org/10.1002/jhm.2392.
14. Coller RJ, Klitzner TS, Saenz AA, et al. Discharge handoff communication and pediatric readmissions. J Hosp Med. 2017;12(1):29-35. https://doi.org/10.1002/jhm.2670.
15. Yang F, Xiong ZF, Yang C, et al. Continuity of care to prevent readmissions for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. COPD. 2017;14(2):251-261. https://doi.org/10.1080/15412555.2016.1256384.
16. Finlayson K, Chang AM, Courtney MD, et al. Transitional care interventions reduce unplanned hospital readmissions in high-risk older adults. BMC Health Serv Res. 2018;18(1):956. https://doi.org/10.1186/s12913-018-3771-9.
17. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351.
18. Coller RJ, Nelson BB, Sklansky DJ, et al. Preventing hospitalizations in children with medical complexity: a systematic review. Pediatrics. 2014;134(6):e1628-e1647. https://doi.org/10.1542/peds.2014-1956.
19. Wells S, O’Neill M, Rogers J, et al. Nursing-led home visits post-hospitalization for children with medical complexity. J Pediatr Nurs. 2017;34:10-16. https://doi.org/10.1016/j.pedn.2017.03.003.
20. Leyenaar JK, O’Brien ER, Leslie LK, Lindenauer PK, Mangione-Smith RM. Families’ priorities regarding hospital-to-home transitions for children with medical complexity. Pediatrics. 2017;139(1). https://doi.org/10.1542/peds.2016-1581.
1. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (re)admissions network. Pediatrics. 2015;135(1):164-175. https://doi.org/10.1542/peds.2014-1887.
2. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a Children’s Hospital. Pediatrics. 2016;138(2). https://doi.org/10.1542/peds.2015-4182.
3. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9(4):251-260. https://doi.org/10.1002/jhm.2134.
4. Auger KA, Simmons JM, Tubbs-Cooley HL, et al. Postdischarge nurse home visits and reuse: the Hospital to Home Outcomes (H2O) trial. Pediatrics. 2018;142(1). https://doi.org/10.1542/peds.2017-3919.
5. Tubbs-Cooley HL, Pickler RH, Simmons JM, et al. Testing a post-discharge nurse-led transitional home visit in acute care pediatrics: the Hospital-To-Home Outcomes (H2O) study protocol. J Adv Nurs. 2016;72(4):915-925. https://doi.org/10.1111/jan.12882.
6. Guest G. Collecting Qualitative Data: A Field Manual for Applied Research. Thousand Oaks, CA: SAGE Publications, Inc.; 2013.
7. Patton M. Qualitative Research and Evaluation Methods. 4th ed. Thousand Oaks, CA: SAGE Publications, Inc.; 2014.
8. Solan LG, Beck AF, Brunswick SA, et al. The family perspective on Hospital to Home Transitions: a qualitative study. Pediatrics. 2015;136(6):e1539-e1549. https://doi.org/10.1542/peds.2015-2098.
9. Auger KA, Shah SS, Tubbs-Cooley HL, et al. Effects of a 1-time nurse-led telephone call after pediatric discharge: the H2O II randomized clinical trial. JAMA Pediatr. 2018;172(9):e181482. https://doi.org/10.1001/jamapediatrics.2018.1482.
10. Pesko MF, Gerber LM, Peng TR, Press MJ. Home health care: nurse-physician communication, patient severity, and hospital readmission. Health Serv Res. 2018;53(2):1008-1024. https://doi.org/10.1111/1475-6773.12667.
11. Solan LG, Beck AF, Shardo SA, et al. Caregiver perspectives on communication during hospitalization at an academic pediatric institution: a qualitative study. J Hosp Med. 2018;13(5):304-311. https://doi.org/10.12788/jhm.2919.
12. Zackoff MW, Graham C, Warrick D, et al. Increasing PCP and hospital medicine physician verbal communication during hospital admissions. Hosp Pediatr. 2018;8(4):220-226. https://doi.org/10.1542/hpeds.2017-0119.
13. Mussman GM, Vossmeyer MT, Brady PW, et al. Improving the reliability of verbal communication between primary care physicians and pediatric hospitalists at hospital discharge. J Hosp Med. 2015;10(9):574-580. https://doi.org/10.1002/jhm.2392.
14. Coller RJ, Klitzner TS, Saenz AA, et al. Discharge handoff communication and pediatric readmissions. J Hosp Med. 2017;12(1):29-35. https://doi.org/10.1002/jhm.2670.
15. Yang F, Xiong ZF, Yang C, et al. Continuity of care to prevent readmissions for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. COPD. 2017;14(2):251-261. https://doi.org/10.1080/15412555.2016.1256384.
16. Finlayson K, Chang AM, Courtney MD, et al. Transitional care interventions reduce unplanned hospital readmissions in high-risk older adults. BMC Health Serv Res. 2018;18(1):956. https://doi.org/10.1186/s12913-018-3771-9.
17. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351.
18. Coller RJ, Nelson BB, Sklansky DJ, et al. Preventing hospitalizations in children with medical complexity: a systematic review. Pediatrics. 2014;134(6):e1628-e1647. https://doi.org/10.1542/peds.2014-1956.
19. Wells S, O’Neill M, Rogers J, et al. Nursing-led home visits post-hospitalization for children with medical complexity. J Pediatr Nurs. 2017;34:10-16. https://doi.org/10.1016/j.pedn.2017.03.003.
20. Leyenaar JK, O’Brien ER, Leslie LK, Lindenauer PK, Mangione-Smith RM. Families’ priorities regarding hospital-to-home transitions for children with medical complexity. Pediatrics. 2017;139(1). https://doi.org/10.1542/peds.2016-1581.
© 2020 Society of Hospital Medicine
Refractive Outcomes for Cataract Surgery With Toric Intraocular Lenses at a Veterans Affairs Medical Center
Cataract surgery is one of the most common ambulatory procedures performed in the US.1-3 With the aging of the US population, the number of Americans with cataracts is projected to increase from 24.4 million in 2010 to 38.7 million in 2030.4
Approximately 20% of all cataract patients have preoperative astigmatism of > 1.5 diopters (D), underscoring the importance of training residents in the placement of toric intraocular lenses (IOLs).5 However, the implantation of toric IOLs is more challenging than monofocal IOLs, requiring precise surgical alignment of the IOL.6 Successful toric IOL implantation also requires accurate calculation of the IOL cylinder power and target axis of alignment. It is unclear which toric IOL calculation formula offers the most accurate refractive predictions, and practitioners have designed strategies to apply different formulae depending on the biometric dimensions of the target eye.7-9
Previous studies of resident-performed cataract surgery using toric IOLs6,10-13 and studies that compare the performance of the Barrett and Holladay toric formulae have been limited by their small sample sizes (< 107 eyes).7,14-16 Moreover, none of the studies that evaluate the comparative effectiveness of these biometric formulae were conducted at a teaching hospital.7,14-16
Given the added complexity of toric IOL placement and variable surgical experience of residents as ophthalmologists-in-training, it is important to assess outcomes in teaching hospitals.13 The primary aims of this study were to assess the visual and refractive outcomes of cataract surgery using toric IOLs in a US Department of Veterans Affairs (VA) teaching hospital and to compare the relative accuracy of the Holladay 2 or Barrett toric biometric formulae in predicting postoperative refraction outcomes.
Methods
The Providence VA Medical Center (PVAMC) Institutional Review Board approved this study. This retrospective chart review included patients with cataract and corneal astigmatism who underwent cataract surgery using Acrysof toric IOLs, model SN6AT (Alcon) at the PVAMC teaching hospital between November 2013 and May 2018.
Only 1 eye was included from each study subject to avoid compounding of data with the use of bilateral eyes.17 In addition, bilateral cataract surgery was only performed on some patients at the PVAMC, so including both eyes from eligible patients would disproportionately weigh those patients’ outcomes. If both eyes had cataract surgery and their postoperative visual acuities were unequal, we chose the eye with the better postoperative visual acuity since refraction accuracy decreases with worsening best-corrected visual acuity (BCVA). If both eyes had cataract surgery and the postoperative visual acuity was the same, the first operated eye was chosen.17,18
Exclusion criteria included worse than 20/40 BCVA, posterior capsular rupture, sulcus IOL, history of corneal disease, history of refractive surgery (laser-assisted in situ keratomileusis [LASIK]/photorefractive keratectomy [PRK]), axial length not measurable by the Lenstar optical biometer (Haag-Streit USA), or no postoperative refraction within 3 weeks to 4 months.19,20
Patient age, race/ethnicity, gender, preoperative refraction, preoperative BCVA, postoperative refraction, postoperative BCVA, and IOL power were recorded from patient charts (Table 1). Preoperative and postoperative refractive values were converted to spherical equivalents. The preoperative biometry and most of the postoperative refractions were performed by experienced technicians certified by the Joint Commission on Allied Health Personnel in Ophthalmology. The main outcomes for the assessment of surgeries included the postoperative BCVA, postoperative spherical equivalent refraction, and postoperative residual refractive astigmatism.
Axial length (AL), preoperative anterior chamber depth (ACD), preoperative flat corneal front power (K1), preoperative steep corneal front power (K2), lens thickness, horizontal white-to-white (WTW) corneal diameter, and central corneal thickness (CCT) were recorded from the Lenstar biometric device. Predicted postoperative refractions for the Holladay 2 formula were calculated using Holladay IOL Consultant software (Holladay Consulting). Predicted postoperative refractions for the Barrett toric IOL formula were calculated using the online Barrett toric formula calculator.21 Since previous studies have shown that both the Holladay and Barrett formulae account for posterior corneal astigmatism, a comparison of refractive outcomes in eyes with against-the-rule astigmatism vs with-the-rule astigmatism was not performed.14 An estimated standardized value for surgically-induced astigmatism was entered into both formulae; 0.3 diopter (D) was chosen based on previously published averages.22-24
A formula’s prediction error is defined as the predicted postoperative refraction minus the actual postoperative refraction. The mean absolute prediction error (MAE), defined as the mean of the absolute values of the prediction errors, and the median absolute prediction error (MedAE), defined as the median of the absolute values of the prediction errors, were used to assess the overall accuracy of each formula. Also, the percentages of eyes with postoperative refraction within ≥ 0.25 D, ≥ 0.50 D, and ≥ 1.0 D were calculated for both formulae. Two-tailed t tests were performed to compare the MAE between the formulae. Subgroup analyses were performed for short eyes (AL < 22 mm), medium length eyes (AL = 22-25 mm), and long eyes (AL > 25 mm). Statistical analysis was performed using STATA 11 (STATA Corp). The preoperative corneal astigmatism and postoperative refractive astigmatism of all the cases were compared in double-angle plots to assess how well the toric IOL neutralized the corneal astigmatism.
Results
Of 325 charts reviewed during the study period, 34 patients were excluded due to lack of postoperative refraction within the designated follow-up period, 5 for worse than 20/40 postoperative BCVA (4 had preexisting ocular disease), 2 for complications, and 1 for missing data. We included 283 eyes from 283 patients in the final study. Resident ophthalmologists were the primary surgeons in 87.6% (248/283) of the cases.
The median postoperative BCVA was 20/20, and 92% of patients had a postoperative BCVA of 20/25 or better. The prediction outcomes of the toric SN6AT IOLs are shown in Table 2. The Barrett toric formula had a lower MAE than the Holladay 2 formula, but this difference was not statistically significant. The Barrett toric formula also predicted a higher percentage of eyes with postoperative refraction within ≥ 0.25 D (53.2%), ≥ 0.5 D (77.3%), and ≥ 1.0 D (96.1%). For both formulae, > 95% of eyes had prediction errors that fell within 1.0 D.
While the Barrett formula demonstrated a lower MAE in all 3 AL groups, no statistically significant differences were found between the Barrett and Holladay formulae (P = .94, P = .49, and P = .08 for short, medium, and long eyes, respectively). Both formulae produced the lowest MAE in the long AL group: Barrett had a MAE of 0.221 D and Holladay 2 had one of 0.329 D. The Barrett formula produced its highest percentage of eyes with prediction errors falling within 0.25 D and 0.5 D in the long AL group. In comparison, both formulae had the highest MAEs in the short AL group (Barrett toric, 0.598 D; Holladay 2, 0.613 D) and produced the lowest percentage of eyes with prediction errors falling within ≥ 0.25 D and ≥ 0.5 D in the short AL group.
A cumulative histogram of the preoperative corneal and postoperative refractive astigmatism magnitude is shown in Figure 1. The same data are presented as double-angle plots in the Appendix, which shows that the centroid values for preoperative corneal astigmatism were greatlyreduced when compared with the postoperative refractive astigmatism (mean absolute value of 1.77 D ≥ 0.73 D to 0.5 D ≥ 0.50 D).
Preoperative corneal astigmatism and postoperative refractive astigmatism were compared since preoperative refractive astigmatism has noncorneal contributions, including lenticular astigmatism, and there is minimal expected change between preoperative and postoperative corneal astigmatism.14 For comparison, double-angle plots of postoperative refractive astigmatism prediction errors for the Holladay and Barrett formulae are shown in Figure 2.
Discussion
To our knowledge, this is the largest study of resident-performed cataract surgery using toric IOLs, the largest study that compared the performance of the Barrett toric and Holladay 2 formulae, and the first that compared these formulae in a teaching hospital setting. This study found no significant difference in the predictive accuracy of the Barrett and Holladay 2 biometric formulae for cataract surgery using toric IOLs. In addition, our refractive outcomes were consistent with the results of previous toric IOL outcome studies conducted in teaching and nonteaching hospital settings.6,10-13
In 4 previous studies that compared the MAE of the Barrett and Holladay formulae for toric IOLs, the Barrett formula produced a lower MAE than the Holladay 2 formula.7,14-16 However, this difference was significant in only 2 of the studies, which had sample sizes of only 68 and 107 eyes.14,16 Furthermore, the Barrett toric formula produced the lower MAE for the entire AL range, though this was not statistically significant at our sample size. In addition, both formulae produced the lowest MAE in the long AL group and the highest MAE in the short AL group. The unique anatomy and high IOL power needed in short eyes may explain the challenges in attaining accurate IOL power predictions in this AL group.19,25
Limitations
The sample size of this study may have prevented us from detecting statistically significant differences in the performance of the Barrett and Holladay formulae. However, our findings are consistent with studies that compare the accuracy of these formulae in teaching and nonteaching hospital settings. Second, the study was conducted at a VA hospital, and a high proportion of patients were male; thus, our findings may not be generalizable to patients who receive cataract surgery with toric IOLs in other settings.
Conclusions
In a single VA teaching hospital, the Barrett and Holladay 2 biometric formulae provide similar refractive predictions for cataract surgery using toric IOLs. Larger studies would be necessary to detect smaller differences in the relative performance of the biometric formulae.
1. Schein OD, Cassard SD, Tielsch JM, Gower EW. Cataract surgery among Medicare beneficiaries. Ophthalmic Epidemiol. 2012;19(5):257-264.
2. Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477-485.
3. Congdon N, Vingerling JR, Klein BE, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122(4):487-494.
4. National Eye Institute. Cataract tables: cataract defined. https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/cataract-data-and-statistics/cataract-tables. Updated February 7, 2020. Accessed February 10, 2020.
5. Ostri C, Falck L, Boberg-Ans G, Kessel L. The need for toric intra-ocular lens implantation in public ophthalmology departments. Acta Ophthalmol. 2015;93(5):e396-e397.
6. Sundy M, McKnight D, Eck C, Rieger F 3rd. Visual acuity outcomes of toric lens implantation in patients undergoing cataract surgery at a residency training program. Mo Med. 2016;113(1):40-43.
7. Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of methodologies using estimated or measured values of total corneal astigmatism for toric intraocular lens power calculation. J Refract Surg. 2017;33(12):794-800.
8. Reitblat O, Levy A, Kleinmann G, Abulafia A, Assia EI. Effect of posterior corneal astigmatism on power calculation and alignment of toric intraocular lenses: comparison of methodologies. J Cataract Refract Surg. 2016;42(2):217-225.
9. Aristodemou P, Knox Cartwright NE, Sparrow JM, Johnston RL. Formula choice: Hoffer Q, Holladay 1, or SRK/T and refractive outcomes in 8108 eyes after cataract surgery with biometry by partial coherence interferometry. J Cataract Refract Surg. 2011;37(1):63-71.
10. Moreira HR, Hatch KM, Greenberg PB. Benchmarking outcomes in resident-performed cataract surgery with toric intraocular lenses [published correction appears in: Clin Experiment Ophthalmol. 2013;41(8):819]. Clin Exp Ophthalmol. 2013;41(6):624-626.
11. Retzlaff JA, Sanders DR, Kraff MC. Development of the SRK/T intraocular lens implant power calculation formula [published correction appears in: J Cataract Refract Surg. 1990;16(4):528]. J Cataract Refract Surg. 1990;16(3):333-340.
12. Roensch MA, Charton JW, Blomquist PH, Aggarwal NK, McCulley JP. Resident experience with toric and multifocal intraocular lenses in a public county hospital system. J Cataract Refract Surg. 2012;38(5):793-798.
13. Pouyeh B, Galor A, Junk AK, et al. Surgical and refractive outcomes of cataract surgery with toric intraocular lens implantation at a resident-teaching institution. J Cataract Refract Surg. 2011;37(9):1623-1628.
14. Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of astigmatic prediction errors associated with new calculation methods for toric intraocular lenses. J Cataract Refract Surg. 2017;43(3):340-347.
15. Abulafia A, Hill WE, Franchina M, Barrett GD. Comparison of methods to predict residual astigmatism after intraocular lens implantation. J Refract Surg. 2015;31(10):699-707.
16. Abulafia A, Barrett GD, Kleinmann G, et al. Prediction of refractive outcomes with toric intraocular lens implantation. J Cataract Refract Surg. 2015;41(5):936-944.
17. Wang Q, Jiang W, Lin T, Wu X, Lin H, Chen W. Meta-analysis of accuracy of intraocular lens power calculation formulas in short eyes. Clin Exp Ophthalmol. 2018;46(4):356-363.
18. Melles RB, Holladay JT, Chang WJ. Accuracy of intraocular lens calculation formulas. Ophthalmology. 2018;125(2):169-178.
19. Hoffer KJ. The Hoffer Q formula: a comparison of theoretic and regression formulas. J Cataract Refract Surg. 1993;19(6):700-712.
20. Cooke DL, Cooke TL. Comparison of 9 intraocular lens power calculation formulas. J Cataract Refract Surg. 2016;42(8):1157-1164.
21. American Society of Cataract and Refractive Surgery. Barrett toric calculator. www.ascrs.org/barrett-toric-calculator. Accessed February 5, 2020.
22. Holladay JT, Pettit G. Improving toric intraocular lens calculations using total surgically induced astigmatism for a 2.5 mm temporal incision. J Cataract Refract Surg. 2019;45(3):272-283.
23. Canovas C, Alarcon A, Rosén R, et al. New algorithm for toric intraocular lens power calculation considering the posterior corneal astigmatism. J Cataract Refract Surg. 2018;44(2):168-174.
24. Visser N, Berendschot TT, Bauer NJ, Nuijts RM. Vector analysis of corneal and refractive astigmatism changes following toric pseudophakic and toric phakic IOL implantation. Invest Ophthalmol Vis Sci. 2012;53(4):1865-1873.
25. Narváez J, Zimmerman G, Stulting RD, Chang DH. Accuracy of intraocular lens power prediction using the Hoffer Q, Holladay 1, Holladay 2, and SRK/T formulas. J Cataract Refract Surg. 2006;32(12):2050-2053.
Cataract surgery is one of the most common ambulatory procedures performed in the US.1-3 With the aging of the US population, the number of Americans with cataracts is projected to increase from 24.4 million in 2010 to 38.7 million in 2030.4
Approximately 20% of all cataract patients have preoperative astigmatism of > 1.5 diopters (D), underscoring the importance of training residents in the placement of toric intraocular lenses (IOLs).5 However, the implantation of toric IOLs is more challenging than monofocal IOLs, requiring precise surgical alignment of the IOL.6 Successful toric IOL implantation also requires accurate calculation of the IOL cylinder power and target axis of alignment. It is unclear which toric IOL calculation formula offers the most accurate refractive predictions, and practitioners have designed strategies to apply different formulae depending on the biometric dimensions of the target eye.7-9
Previous studies of resident-performed cataract surgery using toric IOLs6,10-13 and studies that compare the performance of the Barrett and Holladay toric formulae have been limited by their small sample sizes (< 107 eyes).7,14-16 Moreover, none of the studies that evaluate the comparative effectiveness of these biometric formulae were conducted at a teaching hospital.7,14-16
Given the added complexity of toric IOL placement and variable surgical experience of residents as ophthalmologists-in-training, it is important to assess outcomes in teaching hospitals.13 The primary aims of this study were to assess the visual and refractive outcomes of cataract surgery using toric IOLs in a US Department of Veterans Affairs (VA) teaching hospital and to compare the relative accuracy of the Holladay 2 or Barrett toric biometric formulae in predicting postoperative refraction outcomes.
Methods
The Providence VA Medical Center (PVAMC) Institutional Review Board approved this study. This retrospective chart review included patients with cataract and corneal astigmatism who underwent cataract surgery using Acrysof toric IOLs, model SN6AT (Alcon) at the PVAMC teaching hospital between November 2013 and May 2018.
Only 1 eye was included from each study subject to avoid compounding of data with the use of bilateral eyes.17 In addition, bilateral cataract surgery was only performed on some patients at the PVAMC, so including both eyes from eligible patients would disproportionately weigh those patients’ outcomes. If both eyes had cataract surgery and their postoperative visual acuities were unequal, we chose the eye with the better postoperative visual acuity since refraction accuracy decreases with worsening best-corrected visual acuity (BCVA). If both eyes had cataract surgery and the postoperative visual acuity was the same, the first operated eye was chosen.17,18
Exclusion criteria included worse than 20/40 BCVA, posterior capsular rupture, sulcus IOL, history of corneal disease, history of refractive surgery (laser-assisted in situ keratomileusis [LASIK]/photorefractive keratectomy [PRK]), axial length not measurable by the Lenstar optical biometer (Haag-Streit USA), or no postoperative refraction within 3 weeks to 4 months.19,20
Patient age, race/ethnicity, gender, preoperative refraction, preoperative BCVA, postoperative refraction, postoperative BCVA, and IOL power were recorded from patient charts (Table 1). Preoperative and postoperative refractive values were converted to spherical equivalents. The preoperative biometry and most of the postoperative refractions were performed by experienced technicians certified by the Joint Commission on Allied Health Personnel in Ophthalmology. The main outcomes for the assessment of surgeries included the postoperative BCVA, postoperative spherical equivalent refraction, and postoperative residual refractive astigmatism.
Axial length (AL), preoperative anterior chamber depth (ACD), preoperative flat corneal front power (K1), preoperative steep corneal front power (K2), lens thickness, horizontal white-to-white (WTW) corneal diameter, and central corneal thickness (CCT) were recorded from the Lenstar biometric device. Predicted postoperative refractions for the Holladay 2 formula were calculated using Holladay IOL Consultant software (Holladay Consulting). Predicted postoperative refractions for the Barrett toric IOL formula were calculated using the online Barrett toric formula calculator.21 Since previous studies have shown that both the Holladay and Barrett formulae account for posterior corneal astigmatism, a comparison of refractive outcomes in eyes with against-the-rule astigmatism vs with-the-rule astigmatism was not performed.14 An estimated standardized value for surgically-induced astigmatism was entered into both formulae; 0.3 diopter (D) was chosen based on previously published averages.22-24
A formula’s prediction error is defined as the predicted postoperative refraction minus the actual postoperative refraction. The mean absolute prediction error (MAE), defined as the mean of the absolute values of the prediction errors, and the median absolute prediction error (MedAE), defined as the median of the absolute values of the prediction errors, were used to assess the overall accuracy of each formula. Also, the percentages of eyes with postoperative refraction within ≥ 0.25 D, ≥ 0.50 D, and ≥ 1.0 D were calculated for both formulae. Two-tailed t tests were performed to compare the MAE between the formulae. Subgroup analyses were performed for short eyes (AL < 22 mm), medium length eyes (AL = 22-25 mm), and long eyes (AL > 25 mm). Statistical analysis was performed using STATA 11 (STATA Corp). The preoperative corneal astigmatism and postoperative refractive astigmatism of all the cases were compared in double-angle plots to assess how well the toric IOL neutralized the corneal astigmatism.
Results
Of 325 charts reviewed during the study period, 34 patients were excluded due to lack of postoperative refraction within the designated follow-up period, 5 for worse than 20/40 postoperative BCVA (4 had preexisting ocular disease), 2 for complications, and 1 for missing data. We included 283 eyes from 283 patients in the final study. Resident ophthalmologists were the primary surgeons in 87.6% (248/283) of the cases.
The median postoperative BCVA was 20/20, and 92% of patients had a postoperative BCVA of 20/25 or better. The prediction outcomes of the toric SN6AT IOLs are shown in Table 2. The Barrett toric formula had a lower MAE than the Holladay 2 formula, but this difference was not statistically significant. The Barrett toric formula also predicted a higher percentage of eyes with postoperative refraction within ≥ 0.25 D (53.2%), ≥ 0.5 D (77.3%), and ≥ 1.0 D (96.1%). For both formulae, > 95% of eyes had prediction errors that fell within 1.0 D.
While the Barrett formula demonstrated a lower MAE in all 3 AL groups, no statistically significant differences were found between the Barrett and Holladay formulae (P = .94, P = .49, and P = .08 for short, medium, and long eyes, respectively). Both formulae produced the lowest MAE in the long AL group: Barrett had a MAE of 0.221 D and Holladay 2 had one of 0.329 D. The Barrett formula produced its highest percentage of eyes with prediction errors falling within 0.25 D and 0.5 D in the long AL group. In comparison, both formulae had the highest MAEs in the short AL group (Barrett toric, 0.598 D; Holladay 2, 0.613 D) and produced the lowest percentage of eyes with prediction errors falling within ≥ 0.25 D and ≥ 0.5 D in the short AL group.
A cumulative histogram of the preoperative corneal and postoperative refractive astigmatism magnitude is shown in Figure 1. The same data are presented as double-angle plots in the Appendix, which shows that the centroid values for preoperative corneal astigmatism were greatlyreduced when compared with the postoperative refractive astigmatism (mean absolute value of 1.77 D ≥ 0.73 D to 0.5 D ≥ 0.50 D).
Preoperative corneal astigmatism and postoperative refractive astigmatism were compared since preoperative refractive astigmatism has noncorneal contributions, including lenticular astigmatism, and there is minimal expected change between preoperative and postoperative corneal astigmatism.14 For comparison, double-angle plots of postoperative refractive astigmatism prediction errors for the Holladay and Barrett formulae are shown in Figure 2.
Discussion
To our knowledge, this is the largest study of resident-performed cataract surgery using toric IOLs, the largest study that compared the performance of the Barrett toric and Holladay 2 formulae, and the first that compared these formulae in a teaching hospital setting. This study found no significant difference in the predictive accuracy of the Barrett and Holladay 2 biometric formulae for cataract surgery using toric IOLs. In addition, our refractive outcomes were consistent with the results of previous toric IOL outcome studies conducted in teaching and nonteaching hospital settings.6,10-13
In 4 previous studies that compared the MAE of the Barrett and Holladay formulae for toric IOLs, the Barrett formula produced a lower MAE than the Holladay 2 formula.7,14-16 However, this difference was significant in only 2 of the studies, which had sample sizes of only 68 and 107 eyes.14,16 Furthermore, the Barrett toric formula produced the lower MAE for the entire AL range, though this was not statistically significant at our sample size. In addition, both formulae produced the lowest MAE in the long AL group and the highest MAE in the short AL group. The unique anatomy and high IOL power needed in short eyes may explain the challenges in attaining accurate IOL power predictions in this AL group.19,25
Limitations
The sample size of this study may have prevented us from detecting statistically significant differences in the performance of the Barrett and Holladay formulae. However, our findings are consistent with studies that compare the accuracy of these formulae in teaching and nonteaching hospital settings. Second, the study was conducted at a VA hospital, and a high proportion of patients were male; thus, our findings may not be generalizable to patients who receive cataract surgery with toric IOLs in other settings.
Conclusions
In a single VA teaching hospital, the Barrett and Holladay 2 biometric formulae provide similar refractive predictions for cataract surgery using toric IOLs. Larger studies would be necessary to detect smaller differences in the relative performance of the biometric formulae.
Cataract surgery is one of the most common ambulatory procedures performed in the US.1-3 With the aging of the US population, the number of Americans with cataracts is projected to increase from 24.4 million in 2010 to 38.7 million in 2030.4
Approximately 20% of all cataract patients have preoperative astigmatism of > 1.5 diopters (D), underscoring the importance of training residents in the placement of toric intraocular lenses (IOLs).5 However, the implantation of toric IOLs is more challenging than monofocal IOLs, requiring precise surgical alignment of the IOL.6 Successful toric IOL implantation also requires accurate calculation of the IOL cylinder power and target axis of alignment. It is unclear which toric IOL calculation formula offers the most accurate refractive predictions, and practitioners have designed strategies to apply different formulae depending on the biometric dimensions of the target eye.7-9
Previous studies of resident-performed cataract surgery using toric IOLs6,10-13 and studies that compare the performance of the Barrett and Holladay toric formulae have been limited by their small sample sizes (< 107 eyes).7,14-16 Moreover, none of the studies that evaluate the comparative effectiveness of these biometric formulae were conducted at a teaching hospital.7,14-16
Given the added complexity of toric IOL placement and variable surgical experience of residents as ophthalmologists-in-training, it is important to assess outcomes in teaching hospitals.13 The primary aims of this study were to assess the visual and refractive outcomes of cataract surgery using toric IOLs in a US Department of Veterans Affairs (VA) teaching hospital and to compare the relative accuracy of the Holladay 2 or Barrett toric biometric formulae in predicting postoperative refraction outcomes.
Methods
The Providence VA Medical Center (PVAMC) Institutional Review Board approved this study. This retrospective chart review included patients with cataract and corneal astigmatism who underwent cataract surgery using Acrysof toric IOLs, model SN6AT (Alcon) at the PVAMC teaching hospital between November 2013 and May 2018.
Only 1 eye was included from each study subject to avoid compounding of data with the use of bilateral eyes.17 In addition, bilateral cataract surgery was only performed on some patients at the PVAMC, so including both eyes from eligible patients would disproportionately weigh those patients’ outcomes. If both eyes had cataract surgery and their postoperative visual acuities were unequal, we chose the eye with the better postoperative visual acuity since refraction accuracy decreases with worsening best-corrected visual acuity (BCVA). If both eyes had cataract surgery and the postoperative visual acuity was the same, the first operated eye was chosen.17,18
Exclusion criteria included worse than 20/40 BCVA, posterior capsular rupture, sulcus IOL, history of corneal disease, history of refractive surgery (laser-assisted in situ keratomileusis [LASIK]/photorefractive keratectomy [PRK]), axial length not measurable by the Lenstar optical biometer (Haag-Streit USA), or no postoperative refraction within 3 weeks to 4 months.19,20
Patient age, race/ethnicity, gender, preoperative refraction, preoperative BCVA, postoperative refraction, postoperative BCVA, and IOL power were recorded from patient charts (Table 1). Preoperative and postoperative refractive values were converted to spherical equivalents. The preoperative biometry and most of the postoperative refractions were performed by experienced technicians certified by the Joint Commission on Allied Health Personnel in Ophthalmology. The main outcomes for the assessment of surgeries included the postoperative BCVA, postoperative spherical equivalent refraction, and postoperative residual refractive astigmatism.
Axial length (AL), preoperative anterior chamber depth (ACD), preoperative flat corneal front power (K1), preoperative steep corneal front power (K2), lens thickness, horizontal white-to-white (WTW) corneal diameter, and central corneal thickness (CCT) were recorded from the Lenstar biometric device. Predicted postoperative refractions for the Holladay 2 formula were calculated using Holladay IOL Consultant software (Holladay Consulting). Predicted postoperative refractions for the Barrett toric IOL formula were calculated using the online Barrett toric formula calculator.21 Since previous studies have shown that both the Holladay and Barrett formulae account for posterior corneal astigmatism, a comparison of refractive outcomes in eyes with against-the-rule astigmatism vs with-the-rule astigmatism was not performed.14 An estimated standardized value for surgically-induced astigmatism was entered into both formulae; 0.3 diopter (D) was chosen based on previously published averages.22-24
A formula’s prediction error is defined as the predicted postoperative refraction minus the actual postoperative refraction. The mean absolute prediction error (MAE), defined as the mean of the absolute values of the prediction errors, and the median absolute prediction error (MedAE), defined as the median of the absolute values of the prediction errors, were used to assess the overall accuracy of each formula. Also, the percentages of eyes with postoperative refraction within ≥ 0.25 D, ≥ 0.50 D, and ≥ 1.0 D were calculated for both formulae. Two-tailed t tests were performed to compare the MAE between the formulae. Subgroup analyses were performed for short eyes (AL < 22 mm), medium length eyes (AL = 22-25 mm), and long eyes (AL > 25 mm). Statistical analysis was performed using STATA 11 (STATA Corp). The preoperative corneal astigmatism and postoperative refractive astigmatism of all the cases were compared in double-angle plots to assess how well the toric IOL neutralized the corneal astigmatism.
Results
Of 325 charts reviewed during the study period, 34 patients were excluded due to lack of postoperative refraction within the designated follow-up period, 5 for worse than 20/40 postoperative BCVA (4 had preexisting ocular disease), 2 for complications, and 1 for missing data. We included 283 eyes from 283 patients in the final study. Resident ophthalmologists were the primary surgeons in 87.6% (248/283) of the cases.
The median postoperative BCVA was 20/20, and 92% of patients had a postoperative BCVA of 20/25 or better. The prediction outcomes of the toric SN6AT IOLs are shown in Table 2. The Barrett toric formula had a lower MAE than the Holladay 2 formula, but this difference was not statistically significant. The Barrett toric formula also predicted a higher percentage of eyes with postoperative refraction within ≥ 0.25 D (53.2%), ≥ 0.5 D (77.3%), and ≥ 1.0 D (96.1%). For both formulae, > 95% of eyes had prediction errors that fell within 1.0 D.
While the Barrett formula demonstrated a lower MAE in all 3 AL groups, no statistically significant differences were found between the Barrett and Holladay formulae (P = .94, P = .49, and P = .08 for short, medium, and long eyes, respectively). Both formulae produced the lowest MAE in the long AL group: Barrett had a MAE of 0.221 D and Holladay 2 had one of 0.329 D. The Barrett formula produced its highest percentage of eyes with prediction errors falling within 0.25 D and 0.5 D in the long AL group. In comparison, both formulae had the highest MAEs in the short AL group (Barrett toric, 0.598 D; Holladay 2, 0.613 D) and produced the lowest percentage of eyes with prediction errors falling within ≥ 0.25 D and ≥ 0.5 D in the short AL group.
A cumulative histogram of the preoperative corneal and postoperative refractive astigmatism magnitude is shown in Figure 1. The same data are presented as double-angle plots in the Appendix, which shows that the centroid values for preoperative corneal astigmatism were greatlyreduced when compared with the postoperative refractive astigmatism (mean absolute value of 1.77 D ≥ 0.73 D to 0.5 D ≥ 0.50 D).
Preoperative corneal astigmatism and postoperative refractive astigmatism were compared since preoperative refractive astigmatism has noncorneal contributions, including lenticular astigmatism, and there is minimal expected change between preoperative and postoperative corneal astigmatism.14 For comparison, double-angle plots of postoperative refractive astigmatism prediction errors for the Holladay and Barrett formulae are shown in Figure 2.
Discussion
To our knowledge, this is the largest study of resident-performed cataract surgery using toric IOLs, the largest study that compared the performance of the Barrett toric and Holladay 2 formulae, and the first that compared these formulae in a teaching hospital setting. This study found no significant difference in the predictive accuracy of the Barrett and Holladay 2 biometric formulae for cataract surgery using toric IOLs. In addition, our refractive outcomes were consistent with the results of previous toric IOL outcome studies conducted in teaching and nonteaching hospital settings.6,10-13
In 4 previous studies that compared the MAE of the Barrett and Holladay formulae for toric IOLs, the Barrett formula produced a lower MAE than the Holladay 2 formula.7,14-16 However, this difference was significant in only 2 of the studies, which had sample sizes of only 68 and 107 eyes.14,16 Furthermore, the Barrett toric formula produced the lower MAE for the entire AL range, though this was not statistically significant at our sample size. In addition, both formulae produced the lowest MAE in the long AL group and the highest MAE in the short AL group. The unique anatomy and high IOL power needed in short eyes may explain the challenges in attaining accurate IOL power predictions in this AL group.19,25
Limitations
The sample size of this study may have prevented us from detecting statistically significant differences in the performance of the Barrett and Holladay formulae. However, our findings are consistent with studies that compare the accuracy of these formulae in teaching and nonteaching hospital settings. Second, the study was conducted at a VA hospital, and a high proportion of patients were male; thus, our findings may not be generalizable to patients who receive cataract surgery with toric IOLs in other settings.
Conclusions
In a single VA teaching hospital, the Barrett and Holladay 2 biometric formulae provide similar refractive predictions for cataract surgery using toric IOLs. Larger studies would be necessary to detect smaller differences in the relative performance of the biometric formulae.
1. Schein OD, Cassard SD, Tielsch JM, Gower EW. Cataract surgery among Medicare beneficiaries. Ophthalmic Epidemiol. 2012;19(5):257-264.
2. Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477-485.
3. Congdon N, Vingerling JR, Klein BE, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122(4):487-494.
4. National Eye Institute. Cataract tables: cataract defined. https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/cataract-data-and-statistics/cataract-tables. Updated February 7, 2020. Accessed February 10, 2020.
5. Ostri C, Falck L, Boberg-Ans G, Kessel L. The need for toric intra-ocular lens implantation in public ophthalmology departments. Acta Ophthalmol. 2015;93(5):e396-e397.
6. Sundy M, McKnight D, Eck C, Rieger F 3rd. Visual acuity outcomes of toric lens implantation in patients undergoing cataract surgery at a residency training program. Mo Med. 2016;113(1):40-43.
7. Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of methodologies using estimated or measured values of total corneal astigmatism for toric intraocular lens power calculation. J Refract Surg. 2017;33(12):794-800.
8. Reitblat O, Levy A, Kleinmann G, Abulafia A, Assia EI. Effect of posterior corneal astigmatism on power calculation and alignment of toric intraocular lenses: comparison of methodologies. J Cataract Refract Surg. 2016;42(2):217-225.
9. Aristodemou P, Knox Cartwright NE, Sparrow JM, Johnston RL. Formula choice: Hoffer Q, Holladay 1, or SRK/T and refractive outcomes in 8108 eyes after cataract surgery with biometry by partial coherence interferometry. J Cataract Refract Surg. 2011;37(1):63-71.
10. Moreira HR, Hatch KM, Greenberg PB. Benchmarking outcomes in resident-performed cataract surgery with toric intraocular lenses [published correction appears in: Clin Experiment Ophthalmol. 2013;41(8):819]. Clin Exp Ophthalmol. 2013;41(6):624-626.
11. Retzlaff JA, Sanders DR, Kraff MC. Development of the SRK/T intraocular lens implant power calculation formula [published correction appears in: J Cataract Refract Surg. 1990;16(4):528]. J Cataract Refract Surg. 1990;16(3):333-340.
12. Roensch MA, Charton JW, Blomquist PH, Aggarwal NK, McCulley JP. Resident experience with toric and multifocal intraocular lenses in a public county hospital system. J Cataract Refract Surg. 2012;38(5):793-798.
13. Pouyeh B, Galor A, Junk AK, et al. Surgical and refractive outcomes of cataract surgery with toric intraocular lens implantation at a resident-teaching institution. J Cataract Refract Surg. 2011;37(9):1623-1628.
14. Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of astigmatic prediction errors associated with new calculation methods for toric intraocular lenses. J Cataract Refract Surg. 2017;43(3):340-347.
15. Abulafia A, Hill WE, Franchina M, Barrett GD. Comparison of methods to predict residual astigmatism after intraocular lens implantation. J Refract Surg. 2015;31(10):699-707.
16. Abulafia A, Barrett GD, Kleinmann G, et al. Prediction of refractive outcomes with toric intraocular lens implantation. J Cataract Refract Surg. 2015;41(5):936-944.
17. Wang Q, Jiang W, Lin T, Wu X, Lin H, Chen W. Meta-analysis of accuracy of intraocular lens power calculation formulas in short eyes. Clin Exp Ophthalmol. 2018;46(4):356-363.
18. Melles RB, Holladay JT, Chang WJ. Accuracy of intraocular lens calculation formulas. Ophthalmology. 2018;125(2):169-178.
19. Hoffer KJ. The Hoffer Q formula: a comparison of theoretic and regression formulas. J Cataract Refract Surg. 1993;19(6):700-712.
20. Cooke DL, Cooke TL. Comparison of 9 intraocular lens power calculation formulas. J Cataract Refract Surg. 2016;42(8):1157-1164.
21. American Society of Cataract and Refractive Surgery. Barrett toric calculator. www.ascrs.org/barrett-toric-calculator. Accessed February 5, 2020.
22. Holladay JT, Pettit G. Improving toric intraocular lens calculations using total surgically induced astigmatism for a 2.5 mm temporal incision. J Cataract Refract Surg. 2019;45(3):272-283.
23. Canovas C, Alarcon A, Rosén R, et al. New algorithm for toric intraocular lens power calculation considering the posterior corneal astigmatism. J Cataract Refract Surg. 2018;44(2):168-174.
24. Visser N, Berendschot TT, Bauer NJ, Nuijts RM. Vector analysis of corneal and refractive astigmatism changes following toric pseudophakic and toric phakic IOL implantation. Invest Ophthalmol Vis Sci. 2012;53(4):1865-1873.
25. Narváez J, Zimmerman G, Stulting RD, Chang DH. Accuracy of intraocular lens power prediction using the Hoffer Q, Holladay 1, Holladay 2, and SRK/T formulas. J Cataract Refract Surg. 2006;32(12):2050-2053.
1. Schein OD, Cassard SD, Tielsch JM, Gower EW. Cataract surgery among Medicare beneficiaries. Ophthalmic Epidemiol. 2012;19(5):257-264.
2. Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477-485.
3. Congdon N, Vingerling JR, Klein BE, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122(4):487-494.
4. National Eye Institute. Cataract tables: cataract defined. https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/cataract-data-and-statistics/cataract-tables. Updated February 7, 2020. Accessed February 10, 2020.
5. Ostri C, Falck L, Boberg-Ans G, Kessel L. The need for toric intra-ocular lens implantation in public ophthalmology departments. Acta Ophthalmol. 2015;93(5):e396-e397.
6. Sundy M, McKnight D, Eck C, Rieger F 3rd. Visual acuity outcomes of toric lens implantation in patients undergoing cataract surgery at a residency training program. Mo Med. 2016;113(1):40-43.
7. Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of methodologies using estimated or measured values of total corneal astigmatism for toric intraocular lens power calculation. J Refract Surg. 2017;33(12):794-800.
8. Reitblat O, Levy A, Kleinmann G, Abulafia A, Assia EI. Effect of posterior corneal astigmatism on power calculation and alignment of toric intraocular lenses: comparison of methodologies. J Cataract Refract Surg. 2016;42(2):217-225.
9. Aristodemou P, Knox Cartwright NE, Sparrow JM, Johnston RL. Formula choice: Hoffer Q, Holladay 1, or SRK/T and refractive outcomes in 8108 eyes after cataract surgery with biometry by partial coherence interferometry. J Cataract Refract Surg. 2011;37(1):63-71.
10. Moreira HR, Hatch KM, Greenberg PB. Benchmarking outcomes in resident-performed cataract surgery with toric intraocular lenses [published correction appears in: Clin Experiment Ophthalmol. 2013;41(8):819]. Clin Exp Ophthalmol. 2013;41(6):624-626.
11. Retzlaff JA, Sanders DR, Kraff MC. Development of the SRK/T intraocular lens implant power calculation formula [published correction appears in: J Cataract Refract Surg. 1990;16(4):528]. J Cataract Refract Surg. 1990;16(3):333-340.
12. Roensch MA, Charton JW, Blomquist PH, Aggarwal NK, McCulley JP. Resident experience with toric and multifocal intraocular lenses in a public county hospital system. J Cataract Refract Surg. 2012;38(5):793-798.
13. Pouyeh B, Galor A, Junk AK, et al. Surgical and refractive outcomes of cataract surgery with toric intraocular lens implantation at a resident-teaching institution. J Cataract Refract Surg. 2011;37(9):1623-1628.
14. Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of astigmatic prediction errors associated with new calculation methods for toric intraocular lenses. J Cataract Refract Surg. 2017;43(3):340-347.
15. Abulafia A, Hill WE, Franchina M, Barrett GD. Comparison of methods to predict residual astigmatism after intraocular lens implantation. J Refract Surg. 2015;31(10):699-707.
16. Abulafia A, Barrett GD, Kleinmann G, et al. Prediction of refractive outcomes with toric intraocular lens implantation. J Cataract Refract Surg. 2015;41(5):936-944.
17. Wang Q, Jiang W, Lin T, Wu X, Lin H, Chen W. Meta-analysis of accuracy of intraocular lens power calculation formulas in short eyes. Clin Exp Ophthalmol. 2018;46(4):356-363.
18. Melles RB, Holladay JT, Chang WJ. Accuracy of intraocular lens calculation formulas. Ophthalmology. 2018;125(2):169-178.
19. Hoffer KJ. The Hoffer Q formula: a comparison of theoretic and regression formulas. J Cataract Refract Surg. 1993;19(6):700-712.
20. Cooke DL, Cooke TL. Comparison of 9 intraocular lens power calculation formulas. J Cataract Refract Surg. 2016;42(8):1157-1164.
21. American Society of Cataract and Refractive Surgery. Barrett toric calculator. www.ascrs.org/barrett-toric-calculator. Accessed February 5, 2020.
22. Holladay JT, Pettit G. Improving toric intraocular lens calculations using total surgically induced astigmatism for a 2.5 mm temporal incision. J Cataract Refract Surg. 2019;45(3):272-283.
23. Canovas C, Alarcon A, Rosén R, et al. New algorithm for toric intraocular lens power calculation considering the posterior corneal astigmatism. J Cataract Refract Surg. 2018;44(2):168-174.
24. Visser N, Berendschot TT, Bauer NJ, Nuijts RM. Vector analysis of corneal and refractive astigmatism changes following toric pseudophakic and toric phakic IOL implantation. Invest Ophthalmol Vis Sci. 2012;53(4):1865-1873.
25. Narváez J, Zimmerman G, Stulting RD, Chang DH. Accuracy of intraocular lens power prediction using the Hoffer Q, Holladay 1, Holladay 2, and SRK/T formulas. J Cataract Refract Surg. 2006;32(12):2050-2053.
Demographic Profile and Service-Connection Trends of Posttraumatic Stress Disorder and Traumatic Brain Injury in US Veterans Pre- and Post-9/11
The nature of combat and associated injuries in Operation Iraqi Freedom (OIF), Operation Enduring Freedom (OEF), Operation New Dawn (OND), and Afghanistan War is different from previous conflicts. Multiple protracted deployments with infrequent breaks after September 11, 2001 (9/11) have further compounded the problem.
Posttraumatic stress disorder (PTSD) and traumatic brain injury (TBI) are the signature wounds of recent wars, with a higher incidence among the veterans of OEF and OIF compared with those from previous conflicts.1,2 More than 2.7 million who served in Iraq and Afghanistan suffer from PTSD.3,4 Symptoms of PTSD may appear within the first 3 months after exposure to a traumatic event or after many months and, in some cases, after a delay of many years and continue for life.5 Although delayed onset of PTSD in the absence of prior symptoms is rare,6,7 its incidence rises with increasing frequency of exposure to traumatic events8,9 and over time.10
According to the Brain Injury Association of America, TBI is “an alteration in brain function, or other evidence of brain pathology, caused by an external force.”8 TBI is often associated with increased risk of PTSD, depression, and posttraumatic headache,11-13 which may lead to broader cognitive, somatic, neurobiological, and psychosocial dysfunctions.14-17 According to Veterans Health Administration (VHA) data, 201,435 veterans from all eras enrolled with the US Department of Veterans Affairs (VA) have a diagnosis associated with TBI and 56,695 OEF/OIF veterans have been evaluated for a TBI-related condition.2 According to the Defense and Veterans Brain Injury Center (DVBIC), > 361,000 veterans have been diagnosed with TBI, with a peak of 32,000 cases in 2011.1,18 Moreover, the reported incidence and prevalence of PTSD and TBI among US veterans are not consistent. The incidence of PTSD has been estimated at 15% to 20% in recent wars3,19 compared with 10% to 30% in previous wars.3,19,20
When PTSD or TBI is deemed “related” to military service, the veteran may receive a service-connected disability rating ranging from 0% (no life-interfering symptoms due to injury) to 100% (totally disabling injury). The percentage of service connection associated with an injury is a quantifiable measure of the debilitating effect of injury on the individual. A significant majority (94%) of those who seek mental health services and treatment at VHA clinics apply for PTSD-related disability benefits.21 The estimated cost related to PTSD/TBI service-connected pensions is $20.28 billion per year and approximately $514 billion over 50 years.22 The cost of VA and Social Security disability payments combined with health care costs and treatment of PTSD is estimated to exceed $1 trillion over the next 30 years.22
The National Vietnam Veterans Readjustment Study (NVVRS) provided valuable information on prevalence rates of PTSD and other postwar psychological problems.23 Meanwhile, there have been no recent large-scale studies to compare the demographics of veterans diagnosed with PTSD and TBI who served prior to and after 9/11. A better understanding of demographic changes is considered essential for designing and tailoring therapeutic interventions to manage the rising cost.22
The present study focused on identifying changing trends in the demographics of veterans who served prior to and after 9/11 and who received a VA inpatient or outpatient diagnosis of PTSD and/or TBI. Specifically, this study addressed the changes in demographics of veterans with PTSD, TBI, or PTSD+TBI seen at the VHA clinics between December 1,1998 and May 31, 2014 (before and after September 11, 2001) for diagnosis, treatment and health care policy issues.
Methods
This study was approved by the Kansas City VA Medical Center Institutional Review Board. VHA data from the Corporate Data Warehouse (CDW) and the National Patient Care Database were extracted using the VA Informatics and Computing Infrastructure (VINCI) workspace. CDW uses a unique identifier to identify veterans across treatment episodes at more than 1,400 VHA centers organized under 21 Veterans Integrated Service Networks (VISNs). These sources of VA data are widely used for retrospective longitudinal studies.
Study Population
The study population consisted of 1,339,937 veterans with a VA inpatient/outpatient diagnosis of PTSD or TBI using International Statistical Classification of Diseases and Related Health Problems, Ninth Revision (ICD-9) codes between December 1, 1998 and May 31, 2014. Demographic (gender classification, race, ethnicity, marital status, age at date of data extraction, and date of death if indicated), service-connection disability rating, and geographic distribution within VISN data on each veteran were then extracted.
Veterans in the cohort were assigned to 1 of 4 US military services period groups. The pre-9/11 group included veterans who entered and left the military prior to September 11, 2001. This group mostly included veterans from World War II, Korean War, Vietnam War, and the first Gulf War (1990-1991). The post-9/11 group included veterans who first entered military services after September 11, 2001. The overlap group included veterans who entered military services prior to 9/11, remained in service and left after September 11, 2001. The reentered group included veterans who entered and left service prior to September 11, 2001, and then reentered military service after September 11, 2001 (Figure 1). Using ICD-9 codes, veterans also were placed into the following categories: PTSD alone (ICD-9 309.81 only), TBI alone (ICD-9 850.0-859.9, V15.52), and PTSD+TBI (any combination of ICD-9 codes from the other categories).
Statistical Analysis
Descriptive statistics were applied using proportions and means. Relationships between variables were examined using χ2 tests, t tests, analysis of variance, and nonparametric tests. All hypotheses were 2-sided at 95% CI. Results are presented as absolute numbers.
Results
PTSD only (n = 1,132,356, 85%) was the predominant diagnosis category followed by PTSD+TBI (n = 106,792, 8%) and TBI only (n = 100,789, 7%) (Figure 2). Most of the veterans in the study served pre-9/11 (77%), followed by post-9/11 (15%); 7% were in the overlap group, and 1% in the reentered group (Table 1). It is notable that the proportion of veterans diagnosed with PTSD decreased from pre-9/11 (88%) to post-9/11 (71%), overlap (77%), and reentered (74%) service periods. Increases were noted in those with PTSD+TBI diagnosis category from pre-9/11 (4%) to post-9/11 (23%), overlap (17%), and reentered (22%) service periods (Figure 3). In general, the relative distribution of diagnostic categories in all the service periods showed a similar trend, with the majority of veterans diagnosed with PTSD only. Across all service periods, significantly smaller proportions of veterans were diagnosed with TBI only (P < .001).
Distribution by Gender and Age
The cohort was 92% male (n = 1,239,295), but there was a marked increase in the percentage of nonmale veterans in post-9/11 groups. Study population ages ranged from 18 to 99 years based on date of birth to the date data were obtained; or date of birth to date of death, for those who were reported deceased at the time the data were obtained. The average (SD) ages for veterans in the pre-9/11 group were significantly older (66.3 [11.2] years) compared with the ages of veterans in the post-9/11 group (36.1 [8.7] years), the overlap group (41.4 [8.2] years), and the reentered group (46.9 [9.2] years), respectively.
Distribution by Race and Marital Status
The cohort identified as 65.7% white and 18.2% African American with much smaller percentages of Asians, American Indian/Alaska Natives (AI/AN) and Native Hawaiian/Pacific Islanders (Table 2). The relative proportion of AI/AN and Native Hawaiian/Pacific Islanders remained constant across all groups, whereas the number of Asians diagnosed with PTSD, TBI, or PTSD+TBI increased in the post-9/11 group. The number of African Americans diagnosed with PTSD, TBI, or both markedly increased in the overlap and reentered groups when compared with the pre-9/11 group, yet it went down in the post-9/11/group.
Half the cohort identified themselves as married (n = 675,145) (Table 3). A slightly larger proportion of those diagnosed with PTSD alone were married (51.7%), compared with those diagnosed with TBI only (40.3%), or PTSD+TBI (45.8%). Veterans in the post-9/11 group were less likely to identify as married (45.2%) compared with the pre-9/11 (51.2%), overlap (52.6%), or reentered (53.2%) groups. Divorce rates among pre-9/11 group, overlap group, and reentered group were higher compared with that of the post-9/11 group in all diagnosis categories.
Geographic Distribution
Veterans diagnosed with PTSD, TBI, or both were not evenly distributed across the VISNs VISNs 7, 8, 10, and 22 treated the most veterans, whereas VISN 9 and 15 treated the fewest. Taken together, the top 3 VISNs accounted for 27% to 28% of the total while lowest 3 accounted for 8% to 9% of the total cohort.
Service-Connected Disability
Of 1,339,937 veterans in the cohort, 1,067,691 had a service-connected disability rating for PTSD and/or TBI. Most were diagnosed with PTSD (n = 923,523, 86.5%) followed by both PTSD+TBI (n = 94,051, 8.8%). Three-quarters of the veterans with a service-connected disability were in the pre-9/11 group. Nearly 80% of veterans with a service-connected disability rating had a rating of > 50%. The average (SD) age of veterans with PTSD+TBI and a > 50% service-connected disability was 66.3 (11.2) years in the pre-9/11 group compared with 36.1 (8.7) years in the post-9/11 group.
Discussion
The demographic profile of veterans diagnosed with PTSD+TBI has changed across the service periods covered in this study. Compared with pre-9/11 veterans, the post-9/11 cohort: (1) higher percentage were diagnosed with PTSD+TBI; (2) higher proportion were nonmale veterans; (3) included more young veterans with > 50% service-connected disability; (4) were more racially diverse; and (5) were less likely to be married and divorced and more likely to be self-identified as single. Additionally, data revealed that veterans tended to locate more to some geographic regions than to others.
The nature of the warfare has changed remarkably over the past few decades. Gunshot wounds accounted for 65% of all injuries in World War I, 35% during Vietnam War, and 16% to 23% in the First Gulf War.24 In post-9/11 military conflicts, 81% of injuries were explosion related.24,25 Although improvements in personal protective gear and battlefield trauma care led to increased survival, several factors may have contributed to increased reporting of TBI, which peaked in 2011 at 32,000 cases.24-26
Increases in PTSD Diagnosis
Increasing media awareness, mandatory battlefield concussion screening programs instituted by the US Department of Defense (DoD), and stressful conditions that exacerbate mild TBI (mTBI) may have all contributed to the increase in numbers of veterans seeking evaluations and being diagnosed with PTSD and/or TBI in the post-9/11 groups. Additionally, the 2007 National Defense Authorization Act requested the Secretary of Defense to develop a comprehensive, systematic approach for the identification, treatment, disposition, and documentation of TBI in combat and peacetime. By a conservative estimate, significant numbers of veterans will continue to be seen for mTBI at about 20,000 new cases per year.25-27
More frequent diagnosis of mTBI may have contributed to the increase in veterans diagnosed with PTSD+TBI in the post-9/11 groups. A recent study found that almost 44% of US Army infantry soldiers in Iraq did not lose consciousness but reported symptoms consistent with TBI.14 Compared with veterans of previous wars, veterans of the post-9/11 conflicts (OIF, OED, and OND) have experienced multiple, protracted deployments with infrequent breaks that can have a cumulative effect on the development of PTSD.8-10
The findings from the NVVRS study led to creation of specialized PTSD programs in the late 1980s. Since then, there has been an explosion of knowledge and awareness about PTSD, TBI, and the associated service-connected disability ratings and benefits, leading to an increased number of veterans seeking care for PTSD. For example, media coverage of the 50th anniversary of the D-day celebrations resulted in a surge of World War II veterans seeking treatment for PTSD and a surge of Vietnam veterans sought treatment for PTSD during the wars in Iraq and Afghanistan.28 An increased number of veterans reporting PTSD symptoms prompted the DoD to increase screening for PTSD, and to encourage service members to seek treatment when appropriate.
The VA has instituted training programs for clinicians and psychologists to screen and provide care for PTSD. Beginning in 2007, the VA implemented mandatory TBI screening for all veterans who served in combat operations and separated from active-duty service after September 11, 2001. The 4-question screen identifies veterans who are at increased risk of TBI and who experience symptoms that may be related to specific event(s).29 A positive screen does not diagnose TBI but rather indicates a need for further evaluation, which may or may not be responsible for inflated reporting of TBI. Renewed research also has led providers to recognize and study PTSD resulting from noncombat trauma and moral injury. The possibility of delayed onset also drives up the number of veterans diagnosed with PTSD.5-7
Prevalence
A wide variability exists in the reported prevalence of PTSD among US war veterans with estimates ranging from 15% to 20% of veterans from recent conflicts3,20 and 10% to 30% of veterans from previous wars.3,19 These rates are higher than estimates from allied forces from other countries.19 Meta-analyses suggest that the prevalence of PTSD is 2% to 15% among Vietnam War veterans, 1% to 13% among first (pre-9/11) Gulf War veterans, 4% to 17% among OEF/OIF/OND veterans; these veterans have a lifetime prevalence of 6% to 31%.3,11,19,30-38 The prevalence of PTSD is 2 to 4 times higher among the US veterans19,39 when compared with that of civilians.40,41 According to one study, concomitant PTSD and TBI appears to be much higher in US war veterans (4%-17%) compared with United Kingdom Iraq War veterans (3%-6%).19
This study’s finding of an increase in nonmale soldiers with PTSD and/or TBI was not surprising. There is a paucity of data on the effect of war zone exposure on women veterans. Recently, women have been more actively involved in combat roles with 41,000 women deployed to a combat zone. Results of this study indicate a 2- to 3-fold increase in veterans identifying themselves as nonmale in post-9/11 groups with a higher proportion diagnosed with either PTSD alone or PTSD and TBI. Women are at a higher risk for PTSD than are men due in part to exposure to abuse/trauma prior to deployment, experience of higher rates of discrimination, and/or sexual assault.31-33 One study involving First Gulf War female veterans reported higher precombat psychiatric histories as well as higher rates of physical and sexual abuse when compared with that of men.31
In this study, the average age of veterans adjudicated and compensated for PTSD and/or TBI pre-9/11, was 66 years compared with 36 years for post-9/11 veterans. Sixty-six percent of veterans from the post-9/11 group had ≥ 50% service-connected disability at age 36 years; 75% of veterans from the overlap group had ≥ 50% service-connected disability at age 41 years; and 76% veterans from the reentered group had ≥ 50% service-connected disability at age 46 years. Younger age at diagnosis and higher rates of disability not only pose unique challenges for veterans and family members, but also suggest implications for career prospects, family earnings, loss of productivity, and disease-adjusted life years. Also noted in the results, this younger cohort has a higher percentage of single/unmarried veterans, suggesting familial support systems may be more parental than spousal. Treatment for this younger cohort will likely need to focus on early and sustained rehabilitation that can be integrated with career plans.
For treatment to be effective, there must be evidence for veterans enrolling, remaining, and reporting benefits from the treatment. Limited research has shown currently advocated evidence-based therapies to have low enrollment rates, high drop-out rates, and mixed outcomes.42
Results showing a gradual increase in the proportion of nonwhite, non-African American veterans diagnosed with PTSD alone, TBI alone, or both, likely reflect the changing demographic profile of the US as well as the Army. However, the reason that more African Americans were diagnosed with PTSD and/or TBI in the overlap and reentered groups when compared with the pre-9/11 group could not be ascertained. It is possible that more veterans identified themselves as African Americans as evident from a decrease in the number of veterans in the unknown category post-9/11 when compared with the pre-9/11 group. In 2016, the American Community Survey showed that Hispanic and African American veterans were more likely to use VA health care and other benefits than were any other racial group.40 Improved screening for PTSD and TBI diagnoses, increased awareness, and education about the availability of VA services and benefits may have contributed to the increased use of VA benefits in these groups.
Data from this study are concordant with data from the National Center for Veterans Analysis and Statistics reporting on the younger age of diagnosis and higher rates of initial service-connected disability in veterans with PTSD and PTSD+TBI.43 One study analyzing records from 1999 to 2004 showed that the number of PTSD cases grew by 79.5%, resulting in 148.7% increase in benefits payment from $1.7 billion to $4.3 billion per year.44 In contrast, the compensation cost for all other disability categories increased by only 41.7% over this period. This study also revealed that while veterans with PTSD represented only 8.7% of compensation recipients, they received 20.5% of all compensation payments, driven in large part by an increase in > 50% service-connected disability ratings.44
Thus, from financial as well as treatment points of view, the change in the demographic profile of the veteran must be considered when developing PTSD treatment strategies. While treatment in the past focused solely on addressing trauma-associated psychiatric issues, TBI and PTSD association will likely shift the focus to concurrent psychiatric and physical symptomology. Similarly, PTSD/TBI treatment modalities must consider that the profile of post-9/11 service members includes more women, younger age, and a greater racial diversity. For instance, younger age for a disabled veteran brings additional challenges, including reliance on parental or buddy support systems vs a spousal support system, integrating career with treatment, selecting geographic locations that can support both career and treatment, sustaining rehabilitation over time. The treatment needs of a 35-year-old soldier with PTSD and/or TBI, whether male or female, Asian or African American are likely to be very different from the treatment needs of a 65-year-old white male. Newer treatment approaches will have to address the needs of all soldiers.
Limitations
Our study may underestimate the actual PTSD and/or TBI disease burden because of the social stigma associated with diagnosis, military culture, limitations in data collection.45-50 In addition, in this retrospective database cohort study, we considered and tried to minimize the impact of any of the usual potential limitations, including (1) accuracy of data quality and linkage; (2) identifying cohort appropriately (study groups); (3) defining endpoints clearly to avoid misclassifications; and (4) incorporating all important confounders. We identified veterans utilizing medical services at VA hospitals during a defined period and diagnosed with PTSD and TBI using ICD-9 codes and divided in 4 well-defined groups. In addition, another limitation of our study is to not accurately capture the veterans who have alternative health coverage and may choose not to enroll and/or participate in VA health care. In addition, some service members leaving war zones may not disclose or downplay the mental health symptoms to avoid any delay in their return home.
Conclusions
This study highlights the changing profile of the soldier diagnosed with PTSD and/or TBI who served pre-9/11 compared with that of those who served post-9/11. Treatment modalities must address the changes in warfare and demographics of US service members. Future treatment will need to focus more on concurrent PTSD/TBI therapies, the needs of younger soldiers, the needs of women injured in combat, and the needs of a more racially and ethnically diverse population. Severe injuries at a younger age will require early detection and rehabilitation for return to optimum functioning over a lifetime. The current study underscores a need for identifying the gaps in ongoing programs and services, developing alternatives, and implementing improved systems of care. More studies are needed to identify the cost implications and the effectiveness of current therapies for PTSD and/or TBI.
Acknowledgments
This study was supported by VA Medical Center and Midwest BioMedical Research Foundation (MBRF), Kansas City, Missouri. The manuscript received support, in part, from NIH-RO1 DK107490. These agencies did not participate in the design/conduct of the study or, in the interpretation of the data.
1. Bagalman E. Traumatic brain injury among veterans. http://www.ncsl.org/documents/statefed/health/TBI_Vets2013.pdf. Published January 4, 2013. Accessed February 3, 2020.
2. Veterans Health Administration, Support Service Center. Workload files fiscal year 2008-fiscal year 2012. [Source not verified.]
3. Tanielian T, Jaycox LH, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008.
4. Bagalman E. Health care for veterans: traumatic brain injury. https://fas.org/sgp/crs/misc/R40941.pdf. Published March 9, 2015. Accessed February 4, 2020.
5. Ikin JF, Sim MR, McKenzie DP, et al. Anxiety, post-traumatic stress disorder and depression in Korean War veterans 50 years after the war. Br J Psychiatry. 2007;190(6):475-483.
6. Andrews B, Brewin CR, Philpott R, Stewart L. Delayed-onset posttraumatic stress disorder: a systematic review of the evidence. Am J Psychiatry. 2007;164(9):1319-1326.
7. Frueh BC, Grubaugh AL, Yeager DE, Magruder KM. Delayed-onset post-traumatic stress disorder among war veterans in primary care clinics. Br J Psychiatry. 2009;194(6):515-520.
8. McAllister TW. Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci. 2011;13(3):287-300.
9. Schlenger WE, Kulka RA, Fairbank JA, et al. The prevalence of posttraumatic stress disorder in the Vietnam generation: a multimethod, multisource assessment of psychiatric disorder. J Trauma Stress. 1992;5(3):333-363.
10. Friedman MJ, Resick PA, Bryant RA, Strain J, Horowitz M, Spiegel D. Classification of trauma and stressor-related disorders in DSM-5. Depress Anxiety. 2011;28(9):737-749.
11. Lew HL, Otis JD, Tun C, Kerns RD, Clark ME, Cifu DX. Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in OIF/OEF veterans: polytrauma clinical triad. J Rehabil Res Dev. 2009;46(6):697-702.
12. Carlson K, Kehle S, Meis L, et al. The Assessment and Treatment of Individuals with History of Traumatic Brain Injury and Post-Traumatic Stress Disorder: A Systematic Review of the Evidence. Washington, DC: US Department of Veterans Affairs; 2009.
13. Gironda RJ, Clark ME, Ruff RL, et al. Traumatic brain injury, polytrauma, and pain: challenges and treatment strategies for the polytrauma rehabilitation. Rehabil Psychol. 2009;54(3):247-258.
14. Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358(5):453-463.
15. Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil. 2009;24(6):439-451.
16. Peskind ER, Brody D, Cernak I, McKee A, Ruff RL. Military- and sports-related mild traumatic brain injury: clinical presentation, management, and long-term consequences. J Clin Psychiatry. 2013;74(2):180-188.
17. Riggio S. Traumatic brain injury and its neurobehavioral sequelae. Neurol Clin. 2011;29(1):35-47, vii.
18. Helmick KM, Spells CA, Malik SZ, Davies CA, Marion DW, Hinds SR. Traumatic brain injury in the US military: epidemiology and key clinical and research programs. Brain Imaging Behav. 2015;9(3):358-366.
19. Richardson LK, Frueh BC, Acierno R. Prevalence estimates of combat-related post-traumatic stress disorder: critical review. Aust N Z J Psychiatry. 2010;44(1):4-19.
20. Thompson WW, Gottesman II, Zalewski C. Reconciling disparate prevalence rates of PTSD in large samples of US male Vietnam veterans and their controls. BMC Psychiatry. 2006;6:19.
21. Frueh BC, Elhai JD, Gold PB, et al Disability compensation seeking among veterans evaluated for posttraumatic stress disorder. Psychiatr Serv. 2003;54(1):84-91.
22. Thakur H, Oni O, Singh V, et al. Increases in the service connection disability and treatment costs associated with posttraumatic stress disorder and/or traumatic brain injury in United States veterans pre- and post-9/11: the strong need for a novel therapeutic approach. Epidemiology (Sunnyvale). 2018;8(4):353.
23. Schlenger WE, Kulka RA, Fairbank JA, et al. The prevalence of post-traumatic stress disorder in the Vietnam generation: a multimethod, multisource assessment of psychiatric disorder. J Trauma Stress. 1992;5(3):333-363.
24. Belmont PJ, Schoenfeld AJ, Goodman G. Epidemiology of combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom: orthopaedic burden of disease. J Surg Orthop Adv. 2010;19(1):2-7.
25. Owens BD, Kragh JG Jr, Wenke JC, Macaitis J, Wade CE, Holcomb JB. Combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma. 2008;64(2):295-299.
26. Defense Health Agency, Defense and Veterans Brain Injury Center. DOD worldwide numbers for TBI since 2000. https://dvbic.dcoe.mil/dod-worldwide-numbers-tbi. Updated February 14, 2020. Accessed February 14, 2020.
27. Armed Forces Health Surveillance Center. Deployment-related conditions of special surveillance interest, U.S. armed forces, by month and service, January 2003-December 2012 (data as of 22 January 2013). MSMR. 2013;20(1):16-19.
28. Harvey JH, Stein SK, Scott PK. Fifty years of grief: accounts and reported psychological reactions of Normandy invasion veterans. J Narrative Life History. 1995;5(4):321-332.
29. US Department of Veterans Affairs. Polytrauma/TBI system of care. https://www.polytrauma.va.gov/system-of-care/index.asp. Updated June 3, 2015. Accessed February 4, 2020.
30. Wolfe J, Erickson DJ, Sharkansky EJ, King DW, King LA. Course and predictors of posttraumatic stress disorder among Gulf War veterans: a prospective analysis. J Consult Clin Psychol. 1999;67(4):520-528.
31. Breslau N, Davis GC, Peterson EL, Schultz L. Psychiatric sequelae of posttraumatic stress disorder in women. Arch Gen Psychiatry. 1997;54(1):81-87.
32. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060.
33. Wolfe J, Kimerling R. Gender issues in the assessment of posttraumatic stress disorder. In: Wilson J, Keane TM, eds. Assessing Psychological Trauma and PTSD. New York: Guilford; 2004:192-238.
34. Engel CC Jr, Engel AL, Campbell SJ, McFall ME, Russo J, Katon W. Posttraumatic stress disorder symptoms and precombat sexual and physical abuse in Desert Storm veterans. J Nerv Ment Dis. 1993;181(11):683-688.
35. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of veterans: 2016 data from the American Community Survey. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2016.pdf. Published February 2018. Accessed February 4, 2020.
36. US Department of Commerce Economics and Statistics Administration, US Census Bureau, Geography Division. 2010 population distribution in the United States and Puerto Rico. https://www2.census.gov/geo/maps/dc10_thematic/2010_Nighttime_PopDist/2010_Nighttime_PopDist_Page_Map.pdf. Accessed February 4, 2020.
37. Cifu DX, Taylor BC, Carne WF, et al. Traumatic brain injury, posttraumatic stress disorder, and pain diagnoses in OIF/OEF/OND veterans. J Rehabil Res Dev. 2013;50(9):1169-1176.
38. Dohrenwend BP, Turner JB, Turse NA, Adams BG, Koenen KC, Marshall R. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science. 2006;313(5789):979-982.
39. Magruder KM, Frueh BC, Knapp RG, et al. Prevalence of posttraumatic stress disorder in Veterans Affairs primary care clinics. Gen Hosp Psychiatry. 2005;27(3):169-179.
40. Norris FH. Epidemiology of trauma: frequency and impact of different potentially traumatic events on different demographic groups. J Consult Clin Psychol. 1992;60(3):409-418.
41. Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psychol. 1993;61(6):984-991.
42. Najavits LM. The problem of dropout from “gold standard” PTSD therapies. F1000Prime Rep. 2015;7:43.
43. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Trends in veterans with a service-connected disability: 1985 to 2014. https://www.va.gov/vetdata/docs/QuickFacts/SCD_trends_FINAL_2014.PDF. Published June 2015. Accessed February 4, 2020.
44. US Department of Veterans Affairs, Office of Inspector General. Review of state variances in VA disability compensation payments. Report 05-00765-137. https://www.va.gov/oig/52/reports/2005/VAOIG-05-00765-137.pdf. Published May 19, 2015. Accessed February 4, 2020.
45. McNally RJ. Progress and controversy in the study of posttraumatic stress disorder. Annu Rev Psychol. 2003;54:229-252.
46. Freeman T, Powell M, Kimbrell T. Measuring symptom exaggeration in veterans with chronic posttraumatic stress disorder. Psychiatry Res. 2008;158(3):374-380.
47. Frueh BC, Elhai JD, Grubaugh AL, et al. Documented combat exposure of US veterans seeking treatment for combat-related post-traumatic stress disorder. Br J Psychiatry. 2005;186(6):467-475.
48. Frueh BC, Hamner MB, Cahill SP, Gold PB, Hamlin KL. Apparent symptom overreporting in combat veterans evaluated for PTSD. Clin Psychol Rev. 2000;20(7):853-885.
49. Sparr L, Pankratz LD. Factitious posttraumatic stress disorder. Am J Psychiatry. 1983;140(8):1016-1019.
50. Baggaley M. ‘Military Munchausen’s’: assessment of factitious claims of military service in psychiatric patients. Psychiatr Bull. 1998;22(3):153-154.
The nature of combat and associated injuries in Operation Iraqi Freedom (OIF), Operation Enduring Freedom (OEF), Operation New Dawn (OND), and Afghanistan War is different from previous conflicts. Multiple protracted deployments with infrequent breaks after September 11, 2001 (9/11) have further compounded the problem.
Posttraumatic stress disorder (PTSD) and traumatic brain injury (TBI) are the signature wounds of recent wars, with a higher incidence among the veterans of OEF and OIF compared with those from previous conflicts.1,2 More than 2.7 million who served in Iraq and Afghanistan suffer from PTSD.3,4 Symptoms of PTSD may appear within the first 3 months after exposure to a traumatic event or after many months and, in some cases, after a delay of many years and continue for life.5 Although delayed onset of PTSD in the absence of prior symptoms is rare,6,7 its incidence rises with increasing frequency of exposure to traumatic events8,9 and over time.10
According to the Brain Injury Association of America, TBI is “an alteration in brain function, or other evidence of brain pathology, caused by an external force.”8 TBI is often associated with increased risk of PTSD, depression, and posttraumatic headache,11-13 which may lead to broader cognitive, somatic, neurobiological, and psychosocial dysfunctions.14-17 According to Veterans Health Administration (VHA) data, 201,435 veterans from all eras enrolled with the US Department of Veterans Affairs (VA) have a diagnosis associated with TBI and 56,695 OEF/OIF veterans have been evaluated for a TBI-related condition.2 According to the Defense and Veterans Brain Injury Center (DVBIC), > 361,000 veterans have been diagnosed with TBI, with a peak of 32,000 cases in 2011.1,18 Moreover, the reported incidence and prevalence of PTSD and TBI among US veterans are not consistent. The incidence of PTSD has been estimated at 15% to 20% in recent wars3,19 compared with 10% to 30% in previous wars.3,19,20
When PTSD or TBI is deemed “related” to military service, the veteran may receive a service-connected disability rating ranging from 0% (no life-interfering symptoms due to injury) to 100% (totally disabling injury). The percentage of service connection associated with an injury is a quantifiable measure of the debilitating effect of injury on the individual. A significant majority (94%) of those who seek mental health services and treatment at VHA clinics apply for PTSD-related disability benefits.21 The estimated cost related to PTSD/TBI service-connected pensions is $20.28 billion per year and approximately $514 billion over 50 years.22 The cost of VA and Social Security disability payments combined with health care costs and treatment of PTSD is estimated to exceed $1 trillion over the next 30 years.22
The National Vietnam Veterans Readjustment Study (NVVRS) provided valuable information on prevalence rates of PTSD and other postwar psychological problems.23 Meanwhile, there have been no recent large-scale studies to compare the demographics of veterans diagnosed with PTSD and TBI who served prior to and after 9/11. A better understanding of demographic changes is considered essential for designing and tailoring therapeutic interventions to manage the rising cost.22
The present study focused on identifying changing trends in the demographics of veterans who served prior to and after 9/11 and who received a VA inpatient or outpatient diagnosis of PTSD and/or TBI. Specifically, this study addressed the changes in demographics of veterans with PTSD, TBI, or PTSD+TBI seen at the VHA clinics between December 1,1998 and May 31, 2014 (before and after September 11, 2001) for diagnosis, treatment and health care policy issues.
Methods
This study was approved by the Kansas City VA Medical Center Institutional Review Board. VHA data from the Corporate Data Warehouse (CDW) and the National Patient Care Database were extracted using the VA Informatics and Computing Infrastructure (VINCI) workspace. CDW uses a unique identifier to identify veterans across treatment episodes at more than 1,400 VHA centers organized under 21 Veterans Integrated Service Networks (VISNs). These sources of VA data are widely used for retrospective longitudinal studies.
Study Population
The study population consisted of 1,339,937 veterans with a VA inpatient/outpatient diagnosis of PTSD or TBI using International Statistical Classification of Diseases and Related Health Problems, Ninth Revision (ICD-9) codes between December 1, 1998 and May 31, 2014. Demographic (gender classification, race, ethnicity, marital status, age at date of data extraction, and date of death if indicated), service-connection disability rating, and geographic distribution within VISN data on each veteran were then extracted.
Veterans in the cohort were assigned to 1 of 4 US military services period groups. The pre-9/11 group included veterans who entered and left the military prior to September 11, 2001. This group mostly included veterans from World War II, Korean War, Vietnam War, and the first Gulf War (1990-1991). The post-9/11 group included veterans who first entered military services after September 11, 2001. The overlap group included veterans who entered military services prior to 9/11, remained in service and left after September 11, 2001. The reentered group included veterans who entered and left service prior to September 11, 2001, and then reentered military service after September 11, 2001 (Figure 1). Using ICD-9 codes, veterans also were placed into the following categories: PTSD alone (ICD-9 309.81 only), TBI alone (ICD-9 850.0-859.9, V15.52), and PTSD+TBI (any combination of ICD-9 codes from the other categories).
Statistical Analysis
Descriptive statistics were applied using proportions and means. Relationships between variables were examined using χ2 tests, t tests, analysis of variance, and nonparametric tests. All hypotheses were 2-sided at 95% CI. Results are presented as absolute numbers.
Results
PTSD only (n = 1,132,356, 85%) was the predominant diagnosis category followed by PTSD+TBI (n = 106,792, 8%) and TBI only (n = 100,789, 7%) (Figure 2). Most of the veterans in the study served pre-9/11 (77%), followed by post-9/11 (15%); 7% were in the overlap group, and 1% in the reentered group (Table 1). It is notable that the proportion of veterans diagnosed with PTSD decreased from pre-9/11 (88%) to post-9/11 (71%), overlap (77%), and reentered (74%) service periods. Increases were noted in those with PTSD+TBI diagnosis category from pre-9/11 (4%) to post-9/11 (23%), overlap (17%), and reentered (22%) service periods (Figure 3). In general, the relative distribution of diagnostic categories in all the service periods showed a similar trend, with the majority of veterans diagnosed with PTSD only. Across all service periods, significantly smaller proportions of veterans were diagnosed with TBI only (P < .001).
Distribution by Gender and Age
The cohort was 92% male (n = 1,239,295), but there was a marked increase in the percentage of nonmale veterans in post-9/11 groups. Study population ages ranged from 18 to 99 years based on date of birth to the date data were obtained; or date of birth to date of death, for those who were reported deceased at the time the data were obtained. The average (SD) ages for veterans in the pre-9/11 group were significantly older (66.3 [11.2] years) compared with the ages of veterans in the post-9/11 group (36.1 [8.7] years), the overlap group (41.4 [8.2] years), and the reentered group (46.9 [9.2] years), respectively.
Distribution by Race and Marital Status
The cohort identified as 65.7% white and 18.2% African American with much smaller percentages of Asians, American Indian/Alaska Natives (AI/AN) and Native Hawaiian/Pacific Islanders (Table 2). The relative proportion of AI/AN and Native Hawaiian/Pacific Islanders remained constant across all groups, whereas the number of Asians diagnosed with PTSD, TBI, or PTSD+TBI increased in the post-9/11 group. The number of African Americans diagnosed with PTSD, TBI, or both markedly increased in the overlap and reentered groups when compared with the pre-9/11 group, yet it went down in the post-9/11/group.
Half the cohort identified themselves as married (n = 675,145) (Table 3). A slightly larger proportion of those diagnosed with PTSD alone were married (51.7%), compared with those diagnosed with TBI only (40.3%), or PTSD+TBI (45.8%). Veterans in the post-9/11 group were less likely to identify as married (45.2%) compared with the pre-9/11 (51.2%), overlap (52.6%), or reentered (53.2%) groups. Divorce rates among pre-9/11 group, overlap group, and reentered group were higher compared with that of the post-9/11 group in all diagnosis categories.
Geographic Distribution
Veterans diagnosed with PTSD, TBI, or both were not evenly distributed across the VISNs VISNs 7, 8, 10, and 22 treated the most veterans, whereas VISN 9 and 15 treated the fewest. Taken together, the top 3 VISNs accounted for 27% to 28% of the total while lowest 3 accounted for 8% to 9% of the total cohort.
Service-Connected Disability
Of 1,339,937 veterans in the cohort, 1,067,691 had a service-connected disability rating for PTSD and/or TBI. Most were diagnosed with PTSD (n = 923,523, 86.5%) followed by both PTSD+TBI (n = 94,051, 8.8%). Three-quarters of the veterans with a service-connected disability were in the pre-9/11 group. Nearly 80% of veterans with a service-connected disability rating had a rating of > 50%. The average (SD) age of veterans with PTSD+TBI and a > 50% service-connected disability was 66.3 (11.2) years in the pre-9/11 group compared with 36.1 (8.7) years in the post-9/11 group.
Discussion
The demographic profile of veterans diagnosed with PTSD+TBI has changed across the service periods covered in this study. Compared with pre-9/11 veterans, the post-9/11 cohort: (1) higher percentage were diagnosed with PTSD+TBI; (2) higher proportion were nonmale veterans; (3) included more young veterans with > 50% service-connected disability; (4) were more racially diverse; and (5) were less likely to be married and divorced and more likely to be self-identified as single. Additionally, data revealed that veterans tended to locate more to some geographic regions than to others.
The nature of the warfare has changed remarkably over the past few decades. Gunshot wounds accounted for 65% of all injuries in World War I, 35% during Vietnam War, and 16% to 23% in the First Gulf War.24 In post-9/11 military conflicts, 81% of injuries were explosion related.24,25 Although improvements in personal protective gear and battlefield trauma care led to increased survival, several factors may have contributed to increased reporting of TBI, which peaked in 2011 at 32,000 cases.24-26
Increases in PTSD Diagnosis
Increasing media awareness, mandatory battlefield concussion screening programs instituted by the US Department of Defense (DoD), and stressful conditions that exacerbate mild TBI (mTBI) may have all contributed to the increase in numbers of veterans seeking evaluations and being diagnosed with PTSD and/or TBI in the post-9/11 groups. Additionally, the 2007 National Defense Authorization Act requested the Secretary of Defense to develop a comprehensive, systematic approach for the identification, treatment, disposition, and documentation of TBI in combat and peacetime. By a conservative estimate, significant numbers of veterans will continue to be seen for mTBI at about 20,000 new cases per year.25-27
More frequent diagnosis of mTBI may have contributed to the increase in veterans diagnosed with PTSD+TBI in the post-9/11 groups. A recent study found that almost 44% of US Army infantry soldiers in Iraq did not lose consciousness but reported symptoms consistent with TBI.14 Compared with veterans of previous wars, veterans of the post-9/11 conflicts (OIF, OED, and OND) have experienced multiple, protracted deployments with infrequent breaks that can have a cumulative effect on the development of PTSD.8-10
The findings from the NVVRS study led to creation of specialized PTSD programs in the late 1980s. Since then, there has been an explosion of knowledge and awareness about PTSD, TBI, and the associated service-connected disability ratings and benefits, leading to an increased number of veterans seeking care for PTSD. For example, media coverage of the 50th anniversary of the D-day celebrations resulted in a surge of World War II veterans seeking treatment for PTSD and a surge of Vietnam veterans sought treatment for PTSD during the wars in Iraq and Afghanistan.28 An increased number of veterans reporting PTSD symptoms prompted the DoD to increase screening for PTSD, and to encourage service members to seek treatment when appropriate.
The VA has instituted training programs for clinicians and psychologists to screen and provide care for PTSD. Beginning in 2007, the VA implemented mandatory TBI screening for all veterans who served in combat operations and separated from active-duty service after September 11, 2001. The 4-question screen identifies veterans who are at increased risk of TBI and who experience symptoms that may be related to specific event(s).29 A positive screen does not diagnose TBI but rather indicates a need for further evaluation, which may or may not be responsible for inflated reporting of TBI. Renewed research also has led providers to recognize and study PTSD resulting from noncombat trauma and moral injury. The possibility of delayed onset also drives up the number of veterans diagnosed with PTSD.5-7
Prevalence
A wide variability exists in the reported prevalence of PTSD among US war veterans with estimates ranging from 15% to 20% of veterans from recent conflicts3,20 and 10% to 30% of veterans from previous wars.3,19 These rates are higher than estimates from allied forces from other countries.19 Meta-analyses suggest that the prevalence of PTSD is 2% to 15% among Vietnam War veterans, 1% to 13% among first (pre-9/11) Gulf War veterans, 4% to 17% among OEF/OIF/OND veterans; these veterans have a lifetime prevalence of 6% to 31%.3,11,19,30-38 The prevalence of PTSD is 2 to 4 times higher among the US veterans19,39 when compared with that of civilians.40,41 According to one study, concomitant PTSD and TBI appears to be much higher in US war veterans (4%-17%) compared with United Kingdom Iraq War veterans (3%-6%).19
This study’s finding of an increase in nonmale soldiers with PTSD and/or TBI was not surprising. There is a paucity of data on the effect of war zone exposure on women veterans. Recently, women have been more actively involved in combat roles with 41,000 women deployed to a combat zone. Results of this study indicate a 2- to 3-fold increase in veterans identifying themselves as nonmale in post-9/11 groups with a higher proportion diagnosed with either PTSD alone or PTSD and TBI. Women are at a higher risk for PTSD than are men due in part to exposure to abuse/trauma prior to deployment, experience of higher rates of discrimination, and/or sexual assault.31-33 One study involving First Gulf War female veterans reported higher precombat psychiatric histories as well as higher rates of physical and sexual abuse when compared with that of men.31
In this study, the average age of veterans adjudicated and compensated for PTSD and/or TBI pre-9/11, was 66 years compared with 36 years for post-9/11 veterans. Sixty-six percent of veterans from the post-9/11 group had ≥ 50% service-connected disability at age 36 years; 75% of veterans from the overlap group had ≥ 50% service-connected disability at age 41 years; and 76% veterans from the reentered group had ≥ 50% service-connected disability at age 46 years. Younger age at diagnosis and higher rates of disability not only pose unique challenges for veterans and family members, but also suggest implications for career prospects, family earnings, loss of productivity, and disease-adjusted life years. Also noted in the results, this younger cohort has a higher percentage of single/unmarried veterans, suggesting familial support systems may be more parental than spousal. Treatment for this younger cohort will likely need to focus on early and sustained rehabilitation that can be integrated with career plans.
For treatment to be effective, there must be evidence for veterans enrolling, remaining, and reporting benefits from the treatment. Limited research has shown currently advocated evidence-based therapies to have low enrollment rates, high drop-out rates, and mixed outcomes.42
Results showing a gradual increase in the proportion of nonwhite, non-African American veterans diagnosed with PTSD alone, TBI alone, or both, likely reflect the changing demographic profile of the US as well as the Army. However, the reason that more African Americans were diagnosed with PTSD and/or TBI in the overlap and reentered groups when compared with the pre-9/11 group could not be ascertained. It is possible that more veterans identified themselves as African Americans as evident from a decrease in the number of veterans in the unknown category post-9/11 when compared with the pre-9/11 group. In 2016, the American Community Survey showed that Hispanic and African American veterans were more likely to use VA health care and other benefits than were any other racial group.40 Improved screening for PTSD and TBI diagnoses, increased awareness, and education about the availability of VA services and benefits may have contributed to the increased use of VA benefits in these groups.
Data from this study are concordant with data from the National Center for Veterans Analysis and Statistics reporting on the younger age of diagnosis and higher rates of initial service-connected disability in veterans with PTSD and PTSD+TBI.43 One study analyzing records from 1999 to 2004 showed that the number of PTSD cases grew by 79.5%, resulting in 148.7% increase in benefits payment from $1.7 billion to $4.3 billion per year.44 In contrast, the compensation cost for all other disability categories increased by only 41.7% over this period. This study also revealed that while veterans with PTSD represented only 8.7% of compensation recipients, they received 20.5% of all compensation payments, driven in large part by an increase in > 50% service-connected disability ratings.44
Thus, from financial as well as treatment points of view, the change in the demographic profile of the veteran must be considered when developing PTSD treatment strategies. While treatment in the past focused solely on addressing trauma-associated psychiatric issues, TBI and PTSD association will likely shift the focus to concurrent psychiatric and physical symptomology. Similarly, PTSD/TBI treatment modalities must consider that the profile of post-9/11 service members includes more women, younger age, and a greater racial diversity. For instance, younger age for a disabled veteran brings additional challenges, including reliance on parental or buddy support systems vs a spousal support system, integrating career with treatment, selecting geographic locations that can support both career and treatment, sustaining rehabilitation over time. The treatment needs of a 35-year-old soldier with PTSD and/or TBI, whether male or female, Asian or African American are likely to be very different from the treatment needs of a 65-year-old white male. Newer treatment approaches will have to address the needs of all soldiers.
Limitations
Our study may underestimate the actual PTSD and/or TBI disease burden because of the social stigma associated with diagnosis, military culture, limitations in data collection.45-50 In addition, in this retrospective database cohort study, we considered and tried to minimize the impact of any of the usual potential limitations, including (1) accuracy of data quality and linkage; (2) identifying cohort appropriately (study groups); (3) defining endpoints clearly to avoid misclassifications; and (4) incorporating all important confounders. We identified veterans utilizing medical services at VA hospitals during a defined period and diagnosed with PTSD and TBI using ICD-9 codes and divided in 4 well-defined groups. In addition, another limitation of our study is to not accurately capture the veterans who have alternative health coverage and may choose not to enroll and/or participate in VA health care. In addition, some service members leaving war zones may not disclose or downplay the mental health symptoms to avoid any delay in their return home.
Conclusions
This study highlights the changing profile of the soldier diagnosed with PTSD and/or TBI who served pre-9/11 compared with that of those who served post-9/11. Treatment modalities must address the changes in warfare and demographics of US service members. Future treatment will need to focus more on concurrent PTSD/TBI therapies, the needs of younger soldiers, the needs of women injured in combat, and the needs of a more racially and ethnically diverse population. Severe injuries at a younger age will require early detection and rehabilitation for return to optimum functioning over a lifetime. The current study underscores a need for identifying the gaps in ongoing programs and services, developing alternatives, and implementing improved systems of care. More studies are needed to identify the cost implications and the effectiveness of current therapies for PTSD and/or TBI.
Acknowledgments
This study was supported by VA Medical Center and Midwest BioMedical Research Foundation (MBRF), Kansas City, Missouri. The manuscript received support, in part, from NIH-RO1 DK107490. These agencies did not participate in the design/conduct of the study or, in the interpretation of the data.
The nature of combat and associated injuries in Operation Iraqi Freedom (OIF), Operation Enduring Freedom (OEF), Operation New Dawn (OND), and Afghanistan War is different from previous conflicts. Multiple protracted deployments with infrequent breaks after September 11, 2001 (9/11) have further compounded the problem.
Posttraumatic stress disorder (PTSD) and traumatic brain injury (TBI) are the signature wounds of recent wars, with a higher incidence among the veterans of OEF and OIF compared with those from previous conflicts.1,2 More than 2.7 million who served in Iraq and Afghanistan suffer from PTSD.3,4 Symptoms of PTSD may appear within the first 3 months after exposure to a traumatic event or after many months and, in some cases, after a delay of many years and continue for life.5 Although delayed onset of PTSD in the absence of prior symptoms is rare,6,7 its incidence rises with increasing frequency of exposure to traumatic events8,9 and over time.10
According to the Brain Injury Association of America, TBI is “an alteration in brain function, or other evidence of brain pathology, caused by an external force.”8 TBI is often associated with increased risk of PTSD, depression, and posttraumatic headache,11-13 which may lead to broader cognitive, somatic, neurobiological, and psychosocial dysfunctions.14-17 According to Veterans Health Administration (VHA) data, 201,435 veterans from all eras enrolled with the US Department of Veterans Affairs (VA) have a diagnosis associated with TBI and 56,695 OEF/OIF veterans have been evaluated for a TBI-related condition.2 According to the Defense and Veterans Brain Injury Center (DVBIC), > 361,000 veterans have been diagnosed with TBI, with a peak of 32,000 cases in 2011.1,18 Moreover, the reported incidence and prevalence of PTSD and TBI among US veterans are not consistent. The incidence of PTSD has been estimated at 15% to 20% in recent wars3,19 compared with 10% to 30% in previous wars.3,19,20
When PTSD or TBI is deemed “related” to military service, the veteran may receive a service-connected disability rating ranging from 0% (no life-interfering symptoms due to injury) to 100% (totally disabling injury). The percentage of service connection associated with an injury is a quantifiable measure of the debilitating effect of injury on the individual. A significant majority (94%) of those who seek mental health services and treatment at VHA clinics apply for PTSD-related disability benefits.21 The estimated cost related to PTSD/TBI service-connected pensions is $20.28 billion per year and approximately $514 billion over 50 years.22 The cost of VA and Social Security disability payments combined with health care costs and treatment of PTSD is estimated to exceed $1 trillion over the next 30 years.22
The National Vietnam Veterans Readjustment Study (NVVRS) provided valuable information on prevalence rates of PTSD and other postwar psychological problems.23 Meanwhile, there have been no recent large-scale studies to compare the demographics of veterans diagnosed with PTSD and TBI who served prior to and after 9/11. A better understanding of demographic changes is considered essential for designing and tailoring therapeutic interventions to manage the rising cost.22
The present study focused on identifying changing trends in the demographics of veterans who served prior to and after 9/11 and who received a VA inpatient or outpatient diagnosis of PTSD and/or TBI. Specifically, this study addressed the changes in demographics of veterans with PTSD, TBI, or PTSD+TBI seen at the VHA clinics between December 1,1998 and May 31, 2014 (before and after September 11, 2001) for diagnosis, treatment and health care policy issues.
Methods
This study was approved by the Kansas City VA Medical Center Institutional Review Board. VHA data from the Corporate Data Warehouse (CDW) and the National Patient Care Database were extracted using the VA Informatics and Computing Infrastructure (VINCI) workspace. CDW uses a unique identifier to identify veterans across treatment episodes at more than 1,400 VHA centers organized under 21 Veterans Integrated Service Networks (VISNs). These sources of VA data are widely used for retrospective longitudinal studies.
Study Population
The study population consisted of 1,339,937 veterans with a VA inpatient/outpatient diagnosis of PTSD or TBI using International Statistical Classification of Diseases and Related Health Problems, Ninth Revision (ICD-9) codes between December 1, 1998 and May 31, 2014. Demographic (gender classification, race, ethnicity, marital status, age at date of data extraction, and date of death if indicated), service-connection disability rating, and geographic distribution within VISN data on each veteran were then extracted.
Veterans in the cohort were assigned to 1 of 4 US military services period groups. The pre-9/11 group included veterans who entered and left the military prior to September 11, 2001. This group mostly included veterans from World War II, Korean War, Vietnam War, and the first Gulf War (1990-1991). The post-9/11 group included veterans who first entered military services after September 11, 2001. The overlap group included veterans who entered military services prior to 9/11, remained in service and left after September 11, 2001. The reentered group included veterans who entered and left service prior to September 11, 2001, and then reentered military service after September 11, 2001 (Figure 1). Using ICD-9 codes, veterans also were placed into the following categories: PTSD alone (ICD-9 309.81 only), TBI alone (ICD-9 850.0-859.9, V15.52), and PTSD+TBI (any combination of ICD-9 codes from the other categories).
Statistical Analysis
Descriptive statistics were applied using proportions and means. Relationships between variables were examined using χ2 tests, t tests, analysis of variance, and nonparametric tests. All hypotheses were 2-sided at 95% CI. Results are presented as absolute numbers.
Results
PTSD only (n = 1,132,356, 85%) was the predominant diagnosis category followed by PTSD+TBI (n = 106,792, 8%) and TBI only (n = 100,789, 7%) (Figure 2). Most of the veterans in the study served pre-9/11 (77%), followed by post-9/11 (15%); 7% were in the overlap group, and 1% in the reentered group (Table 1). It is notable that the proportion of veterans diagnosed with PTSD decreased from pre-9/11 (88%) to post-9/11 (71%), overlap (77%), and reentered (74%) service periods. Increases were noted in those with PTSD+TBI diagnosis category from pre-9/11 (4%) to post-9/11 (23%), overlap (17%), and reentered (22%) service periods (Figure 3). In general, the relative distribution of diagnostic categories in all the service periods showed a similar trend, with the majority of veterans diagnosed with PTSD only. Across all service periods, significantly smaller proportions of veterans were diagnosed with TBI only (P < .001).
Distribution by Gender and Age
The cohort was 92% male (n = 1,239,295), but there was a marked increase in the percentage of nonmale veterans in post-9/11 groups. Study population ages ranged from 18 to 99 years based on date of birth to the date data were obtained; or date of birth to date of death, for those who were reported deceased at the time the data were obtained. The average (SD) ages for veterans in the pre-9/11 group were significantly older (66.3 [11.2] years) compared with the ages of veterans in the post-9/11 group (36.1 [8.7] years), the overlap group (41.4 [8.2] years), and the reentered group (46.9 [9.2] years), respectively.
Distribution by Race and Marital Status
The cohort identified as 65.7% white and 18.2% African American with much smaller percentages of Asians, American Indian/Alaska Natives (AI/AN) and Native Hawaiian/Pacific Islanders (Table 2). The relative proportion of AI/AN and Native Hawaiian/Pacific Islanders remained constant across all groups, whereas the number of Asians diagnosed with PTSD, TBI, or PTSD+TBI increased in the post-9/11 group. The number of African Americans diagnosed with PTSD, TBI, or both markedly increased in the overlap and reentered groups when compared with the pre-9/11 group, yet it went down in the post-9/11/group.
Half the cohort identified themselves as married (n = 675,145) (Table 3). A slightly larger proportion of those diagnosed with PTSD alone were married (51.7%), compared with those diagnosed with TBI only (40.3%), or PTSD+TBI (45.8%). Veterans in the post-9/11 group were less likely to identify as married (45.2%) compared with the pre-9/11 (51.2%), overlap (52.6%), or reentered (53.2%) groups. Divorce rates among pre-9/11 group, overlap group, and reentered group were higher compared with that of the post-9/11 group in all diagnosis categories.
Geographic Distribution
Veterans diagnosed with PTSD, TBI, or both were not evenly distributed across the VISNs VISNs 7, 8, 10, and 22 treated the most veterans, whereas VISN 9 and 15 treated the fewest. Taken together, the top 3 VISNs accounted for 27% to 28% of the total while lowest 3 accounted for 8% to 9% of the total cohort.
Service-Connected Disability
Of 1,339,937 veterans in the cohort, 1,067,691 had a service-connected disability rating for PTSD and/or TBI. Most were diagnosed with PTSD (n = 923,523, 86.5%) followed by both PTSD+TBI (n = 94,051, 8.8%). Three-quarters of the veterans with a service-connected disability were in the pre-9/11 group. Nearly 80% of veterans with a service-connected disability rating had a rating of > 50%. The average (SD) age of veterans with PTSD+TBI and a > 50% service-connected disability was 66.3 (11.2) years in the pre-9/11 group compared with 36.1 (8.7) years in the post-9/11 group.
Discussion
The demographic profile of veterans diagnosed with PTSD+TBI has changed across the service periods covered in this study. Compared with pre-9/11 veterans, the post-9/11 cohort: (1) higher percentage were diagnosed with PTSD+TBI; (2) higher proportion were nonmale veterans; (3) included more young veterans with > 50% service-connected disability; (4) were more racially diverse; and (5) were less likely to be married and divorced and more likely to be self-identified as single. Additionally, data revealed that veterans tended to locate more to some geographic regions than to others.
The nature of the warfare has changed remarkably over the past few decades. Gunshot wounds accounted for 65% of all injuries in World War I, 35% during Vietnam War, and 16% to 23% in the First Gulf War.24 In post-9/11 military conflicts, 81% of injuries were explosion related.24,25 Although improvements in personal protective gear and battlefield trauma care led to increased survival, several factors may have contributed to increased reporting of TBI, which peaked in 2011 at 32,000 cases.24-26
Increases in PTSD Diagnosis
Increasing media awareness, mandatory battlefield concussion screening programs instituted by the US Department of Defense (DoD), and stressful conditions that exacerbate mild TBI (mTBI) may have all contributed to the increase in numbers of veterans seeking evaluations and being diagnosed with PTSD and/or TBI in the post-9/11 groups. Additionally, the 2007 National Defense Authorization Act requested the Secretary of Defense to develop a comprehensive, systematic approach for the identification, treatment, disposition, and documentation of TBI in combat and peacetime. By a conservative estimate, significant numbers of veterans will continue to be seen for mTBI at about 20,000 new cases per year.25-27
More frequent diagnosis of mTBI may have contributed to the increase in veterans diagnosed with PTSD+TBI in the post-9/11 groups. A recent study found that almost 44% of US Army infantry soldiers in Iraq did not lose consciousness but reported symptoms consistent with TBI.14 Compared with veterans of previous wars, veterans of the post-9/11 conflicts (OIF, OED, and OND) have experienced multiple, protracted deployments with infrequent breaks that can have a cumulative effect on the development of PTSD.8-10
The findings from the NVVRS study led to creation of specialized PTSD programs in the late 1980s. Since then, there has been an explosion of knowledge and awareness about PTSD, TBI, and the associated service-connected disability ratings and benefits, leading to an increased number of veterans seeking care for PTSD. For example, media coverage of the 50th anniversary of the D-day celebrations resulted in a surge of World War II veterans seeking treatment for PTSD and a surge of Vietnam veterans sought treatment for PTSD during the wars in Iraq and Afghanistan.28 An increased number of veterans reporting PTSD symptoms prompted the DoD to increase screening for PTSD, and to encourage service members to seek treatment when appropriate.
The VA has instituted training programs for clinicians and psychologists to screen and provide care for PTSD. Beginning in 2007, the VA implemented mandatory TBI screening for all veterans who served in combat operations and separated from active-duty service after September 11, 2001. The 4-question screen identifies veterans who are at increased risk of TBI and who experience symptoms that may be related to specific event(s).29 A positive screen does not diagnose TBI but rather indicates a need for further evaluation, which may or may not be responsible for inflated reporting of TBI. Renewed research also has led providers to recognize and study PTSD resulting from noncombat trauma and moral injury. The possibility of delayed onset also drives up the number of veterans diagnosed with PTSD.5-7
Prevalence
A wide variability exists in the reported prevalence of PTSD among US war veterans with estimates ranging from 15% to 20% of veterans from recent conflicts3,20 and 10% to 30% of veterans from previous wars.3,19 These rates are higher than estimates from allied forces from other countries.19 Meta-analyses suggest that the prevalence of PTSD is 2% to 15% among Vietnam War veterans, 1% to 13% among first (pre-9/11) Gulf War veterans, 4% to 17% among OEF/OIF/OND veterans; these veterans have a lifetime prevalence of 6% to 31%.3,11,19,30-38 The prevalence of PTSD is 2 to 4 times higher among the US veterans19,39 when compared with that of civilians.40,41 According to one study, concomitant PTSD and TBI appears to be much higher in US war veterans (4%-17%) compared with United Kingdom Iraq War veterans (3%-6%).19
This study’s finding of an increase in nonmale soldiers with PTSD and/or TBI was not surprising. There is a paucity of data on the effect of war zone exposure on women veterans. Recently, women have been more actively involved in combat roles with 41,000 women deployed to a combat zone. Results of this study indicate a 2- to 3-fold increase in veterans identifying themselves as nonmale in post-9/11 groups with a higher proportion diagnosed with either PTSD alone or PTSD and TBI. Women are at a higher risk for PTSD than are men due in part to exposure to abuse/trauma prior to deployment, experience of higher rates of discrimination, and/or sexual assault.31-33 One study involving First Gulf War female veterans reported higher precombat psychiatric histories as well as higher rates of physical and sexual abuse when compared with that of men.31
In this study, the average age of veterans adjudicated and compensated for PTSD and/or TBI pre-9/11, was 66 years compared with 36 years for post-9/11 veterans. Sixty-six percent of veterans from the post-9/11 group had ≥ 50% service-connected disability at age 36 years; 75% of veterans from the overlap group had ≥ 50% service-connected disability at age 41 years; and 76% veterans from the reentered group had ≥ 50% service-connected disability at age 46 years. Younger age at diagnosis and higher rates of disability not only pose unique challenges for veterans and family members, but also suggest implications for career prospects, family earnings, loss of productivity, and disease-adjusted life years. Also noted in the results, this younger cohort has a higher percentage of single/unmarried veterans, suggesting familial support systems may be more parental than spousal. Treatment for this younger cohort will likely need to focus on early and sustained rehabilitation that can be integrated with career plans.
For treatment to be effective, there must be evidence for veterans enrolling, remaining, and reporting benefits from the treatment. Limited research has shown currently advocated evidence-based therapies to have low enrollment rates, high drop-out rates, and mixed outcomes.42
Results showing a gradual increase in the proportion of nonwhite, non-African American veterans diagnosed with PTSD alone, TBI alone, or both, likely reflect the changing demographic profile of the US as well as the Army. However, the reason that more African Americans were diagnosed with PTSD and/or TBI in the overlap and reentered groups when compared with the pre-9/11 group could not be ascertained. It is possible that more veterans identified themselves as African Americans as evident from a decrease in the number of veterans in the unknown category post-9/11 when compared with the pre-9/11 group. In 2016, the American Community Survey showed that Hispanic and African American veterans were more likely to use VA health care and other benefits than were any other racial group.40 Improved screening for PTSD and TBI diagnoses, increased awareness, and education about the availability of VA services and benefits may have contributed to the increased use of VA benefits in these groups.
Data from this study are concordant with data from the National Center for Veterans Analysis and Statistics reporting on the younger age of diagnosis and higher rates of initial service-connected disability in veterans with PTSD and PTSD+TBI.43 One study analyzing records from 1999 to 2004 showed that the number of PTSD cases grew by 79.5%, resulting in 148.7% increase in benefits payment from $1.7 billion to $4.3 billion per year.44 In contrast, the compensation cost for all other disability categories increased by only 41.7% over this period. This study also revealed that while veterans with PTSD represented only 8.7% of compensation recipients, they received 20.5% of all compensation payments, driven in large part by an increase in > 50% service-connected disability ratings.44
Thus, from financial as well as treatment points of view, the change in the demographic profile of the veteran must be considered when developing PTSD treatment strategies. While treatment in the past focused solely on addressing trauma-associated psychiatric issues, TBI and PTSD association will likely shift the focus to concurrent psychiatric and physical symptomology. Similarly, PTSD/TBI treatment modalities must consider that the profile of post-9/11 service members includes more women, younger age, and a greater racial diversity. For instance, younger age for a disabled veteran brings additional challenges, including reliance on parental or buddy support systems vs a spousal support system, integrating career with treatment, selecting geographic locations that can support both career and treatment, sustaining rehabilitation over time. The treatment needs of a 35-year-old soldier with PTSD and/or TBI, whether male or female, Asian or African American are likely to be very different from the treatment needs of a 65-year-old white male. Newer treatment approaches will have to address the needs of all soldiers.
Limitations
Our study may underestimate the actual PTSD and/or TBI disease burden because of the social stigma associated with diagnosis, military culture, limitations in data collection.45-50 In addition, in this retrospective database cohort study, we considered and tried to minimize the impact of any of the usual potential limitations, including (1) accuracy of data quality and linkage; (2) identifying cohort appropriately (study groups); (3) defining endpoints clearly to avoid misclassifications; and (4) incorporating all important confounders. We identified veterans utilizing medical services at VA hospitals during a defined period and diagnosed with PTSD and TBI using ICD-9 codes and divided in 4 well-defined groups. In addition, another limitation of our study is to not accurately capture the veterans who have alternative health coverage and may choose not to enroll and/or participate in VA health care. In addition, some service members leaving war zones may not disclose or downplay the mental health symptoms to avoid any delay in their return home.
Conclusions
This study highlights the changing profile of the soldier diagnosed with PTSD and/or TBI who served pre-9/11 compared with that of those who served post-9/11. Treatment modalities must address the changes in warfare and demographics of US service members. Future treatment will need to focus more on concurrent PTSD/TBI therapies, the needs of younger soldiers, the needs of women injured in combat, and the needs of a more racially and ethnically diverse population. Severe injuries at a younger age will require early detection and rehabilitation for return to optimum functioning over a lifetime. The current study underscores a need for identifying the gaps in ongoing programs and services, developing alternatives, and implementing improved systems of care. More studies are needed to identify the cost implications and the effectiveness of current therapies for PTSD and/or TBI.
Acknowledgments
This study was supported by VA Medical Center and Midwest BioMedical Research Foundation (MBRF), Kansas City, Missouri. The manuscript received support, in part, from NIH-RO1 DK107490. These agencies did not participate in the design/conduct of the study or, in the interpretation of the data.
1. Bagalman E. Traumatic brain injury among veterans. http://www.ncsl.org/documents/statefed/health/TBI_Vets2013.pdf. Published January 4, 2013. Accessed February 3, 2020.
2. Veterans Health Administration, Support Service Center. Workload files fiscal year 2008-fiscal year 2012. [Source not verified.]
3. Tanielian T, Jaycox LH, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008.
4. Bagalman E. Health care for veterans: traumatic brain injury. https://fas.org/sgp/crs/misc/R40941.pdf. Published March 9, 2015. Accessed February 4, 2020.
5. Ikin JF, Sim MR, McKenzie DP, et al. Anxiety, post-traumatic stress disorder and depression in Korean War veterans 50 years after the war. Br J Psychiatry. 2007;190(6):475-483.
6. Andrews B, Brewin CR, Philpott R, Stewart L. Delayed-onset posttraumatic stress disorder: a systematic review of the evidence. Am J Psychiatry. 2007;164(9):1319-1326.
7. Frueh BC, Grubaugh AL, Yeager DE, Magruder KM. Delayed-onset post-traumatic stress disorder among war veterans in primary care clinics. Br J Psychiatry. 2009;194(6):515-520.
8. McAllister TW. Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci. 2011;13(3):287-300.
9. Schlenger WE, Kulka RA, Fairbank JA, et al. The prevalence of posttraumatic stress disorder in the Vietnam generation: a multimethod, multisource assessment of psychiatric disorder. J Trauma Stress. 1992;5(3):333-363.
10. Friedman MJ, Resick PA, Bryant RA, Strain J, Horowitz M, Spiegel D. Classification of trauma and stressor-related disorders in DSM-5. Depress Anxiety. 2011;28(9):737-749.
11. Lew HL, Otis JD, Tun C, Kerns RD, Clark ME, Cifu DX. Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in OIF/OEF veterans: polytrauma clinical triad. J Rehabil Res Dev. 2009;46(6):697-702.
12. Carlson K, Kehle S, Meis L, et al. The Assessment and Treatment of Individuals with History of Traumatic Brain Injury and Post-Traumatic Stress Disorder: A Systematic Review of the Evidence. Washington, DC: US Department of Veterans Affairs; 2009.
13. Gironda RJ, Clark ME, Ruff RL, et al. Traumatic brain injury, polytrauma, and pain: challenges and treatment strategies for the polytrauma rehabilitation. Rehabil Psychol. 2009;54(3):247-258.
14. Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358(5):453-463.
15. Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil. 2009;24(6):439-451.
16. Peskind ER, Brody D, Cernak I, McKee A, Ruff RL. Military- and sports-related mild traumatic brain injury: clinical presentation, management, and long-term consequences. J Clin Psychiatry. 2013;74(2):180-188.
17. Riggio S. Traumatic brain injury and its neurobehavioral sequelae. Neurol Clin. 2011;29(1):35-47, vii.
18. Helmick KM, Spells CA, Malik SZ, Davies CA, Marion DW, Hinds SR. Traumatic brain injury in the US military: epidemiology and key clinical and research programs. Brain Imaging Behav. 2015;9(3):358-366.
19. Richardson LK, Frueh BC, Acierno R. Prevalence estimates of combat-related post-traumatic stress disorder: critical review. Aust N Z J Psychiatry. 2010;44(1):4-19.
20. Thompson WW, Gottesman II, Zalewski C. Reconciling disparate prevalence rates of PTSD in large samples of US male Vietnam veterans and their controls. BMC Psychiatry. 2006;6:19.
21. Frueh BC, Elhai JD, Gold PB, et al Disability compensation seeking among veterans evaluated for posttraumatic stress disorder. Psychiatr Serv. 2003;54(1):84-91.
22. Thakur H, Oni O, Singh V, et al. Increases in the service connection disability and treatment costs associated with posttraumatic stress disorder and/or traumatic brain injury in United States veterans pre- and post-9/11: the strong need for a novel therapeutic approach. Epidemiology (Sunnyvale). 2018;8(4):353.
23. Schlenger WE, Kulka RA, Fairbank JA, et al. The prevalence of post-traumatic stress disorder in the Vietnam generation: a multimethod, multisource assessment of psychiatric disorder. J Trauma Stress. 1992;5(3):333-363.
24. Belmont PJ, Schoenfeld AJ, Goodman G. Epidemiology of combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom: orthopaedic burden of disease. J Surg Orthop Adv. 2010;19(1):2-7.
25. Owens BD, Kragh JG Jr, Wenke JC, Macaitis J, Wade CE, Holcomb JB. Combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma. 2008;64(2):295-299.
26. Defense Health Agency, Defense and Veterans Brain Injury Center. DOD worldwide numbers for TBI since 2000. https://dvbic.dcoe.mil/dod-worldwide-numbers-tbi. Updated February 14, 2020. Accessed February 14, 2020.
27. Armed Forces Health Surveillance Center. Deployment-related conditions of special surveillance interest, U.S. armed forces, by month and service, January 2003-December 2012 (data as of 22 January 2013). MSMR. 2013;20(1):16-19.
28. Harvey JH, Stein SK, Scott PK. Fifty years of grief: accounts and reported psychological reactions of Normandy invasion veterans. J Narrative Life History. 1995;5(4):321-332.
29. US Department of Veterans Affairs. Polytrauma/TBI system of care. https://www.polytrauma.va.gov/system-of-care/index.asp. Updated June 3, 2015. Accessed February 4, 2020.
30. Wolfe J, Erickson DJ, Sharkansky EJ, King DW, King LA. Course and predictors of posttraumatic stress disorder among Gulf War veterans: a prospective analysis. J Consult Clin Psychol. 1999;67(4):520-528.
31. Breslau N, Davis GC, Peterson EL, Schultz L. Psychiatric sequelae of posttraumatic stress disorder in women. Arch Gen Psychiatry. 1997;54(1):81-87.
32. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060.
33. Wolfe J, Kimerling R. Gender issues in the assessment of posttraumatic stress disorder. In: Wilson J, Keane TM, eds. Assessing Psychological Trauma and PTSD. New York: Guilford; 2004:192-238.
34. Engel CC Jr, Engel AL, Campbell SJ, McFall ME, Russo J, Katon W. Posttraumatic stress disorder symptoms and precombat sexual and physical abuse in Desert Storm veterans. J Nerv Ment Dis. 1993;181(11):683-688.
35. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of veterans: 2016 data from the American Community Survey. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2016.pdf. Published February 2018. Accessed February 4, 2020.
36. US Department of Commerce Economics and Statistics Administration, US Census Bureau, Geography Division. 2010 population distribution in the United States and Puerto Rico. https://www2.census.gov/geo/maps/dc10_thematic/2010_Nighttime_PopDist/2010_Nighttime_PopDist_Page_Map.pdf. Accessed February 4, 2020.
37. Cifu DX, Taylor BC, Carne WF, et al. Traumatic brain injury, posttraumatic stress disorder, and pain diagnoses in OIF/OEF/OND veterans. J Rehabil Res Dev. 2013;50(9):1169-1176.
38. Dohrenwend BP, Turner JB, Turse NA, Adams BG, Koenen KC, Marshall R. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science. 2006;313(5789):979-982.
39. Magruder KM, Frueh BC, Knapp RG, et al. Prevalence of posttraumatic stress disorder in Veterans Affairs primary care clinics. Gen Hosp Psychiatry. 2005;27(3):169-179.
40. Norris FH. Epidemiology of trauma: frequency and impact of different potentially traumatic events on different demographic groups. J Consult Clin Psychol. 1992;60(3):409-418.
41. Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psychol. 1993;61(6):984-991.
42. Najavits LM. The problem of dropout from “gold standard” PTSD therapies. F1000Prime Rep. 2015;7:43.
43. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Trends in veterans with a service-connected disability: 1985 to 2014. https://www.va.gov/vetdata/docs/QuickFacts/SCD_trends_FINAL_2014.PDF. Published June 2015. Accessed February 4, 2020.
44. US Department of Veterans Affairs, Office of Inspector General. Review of state variances in VA disability compensation payments. Report 05-00765-137. https://www.va.gov/oig/52/reports/2005/VAOIG-05-00765-137.pdf. Published May 19, 2015. Accessed February 4, 2020.
45. McNally RJ. Progress and controversy in the study of posttraumatic stress disorder. Annu Rev Psychol. 2003;54:229-252.
46. Freeman T, Powell M, Kimbrell T. Measuring symptom exaggeration in veterans with chronic posttraumatic stress disorder. Psychiatry Res. 2008;158(3):374-380.
47. Frueh BC, Elhai JD, Grubaugh AL, et al. Documented combat exposure of US veterans seeking treatment for combat-related post-traumatic stress disorder. Br J Psychiatry. 2005;186(6):467-475.
48. Frueh BC, Hamner MB, Cahill SP, Gold PB, Hamlin KL. Apparent symptom overreporting in combat veterans evaluated for PTSD. Clin Psychol Rev. 2000;20(7):853-885.
49. Sparr L, Pankratz LD. Factitious posttraumatic stress disorder. Am J Psychiatry. 1983;140(8):1016-1019.
50. Baggaley M. ‘Military Munchausen’s’: assessment of factitious claims of military service in psychiatric patients. Psychiatr Bull. 1998;22(3):153-154.
1. Bagalman E. Traumatic brain injury among veterans. http://www.ncsl.org/documents/statefed/health/TBI_Vets2013.pdf. Published January 4, 2013. Accessed February 3, 2020.
2. Veterans Health Administration, Support Service Center. Workload files fiscal year 2008-fiscal year 2012. [Source not verified.]
3. Tanielian T, Jaycox LH, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008.
4. Bagalman E. Health care for veterans: traumatic brain injury. https://fas.org/sgp/crs/misc/R40941.pdf. Published March 9, 2015. Accessed February 4, 2020.
5. Ikin JF, Sim MR, McKenzie DP, et al. Anxiety, post-traumatic stress disorder and depression in Korean War veterans 50 years after the war. Br J Psychiatry. 2007;190(6):475-483.
6. Andrews B, Brewin CR, Philpott R, Stewart L. Delayed-onset posttraumatic stress disorder: a systematic review of the evidence. Am J Psychiatry. 2007;164(9):1319-1326.
7. Frueh BC, Grubaugh AL, Yeager DE, Magruder KM. Delayed-onset post-traumatic stress disorder among war veterans in primary care clinics. Br J Psychiatry. 2009;194(6):515-520.
8. McAllister TW. Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci. 2011;13(3):287-300.
9. Schlenger WE, Kulka RA, Fairbank JA, et al. The prevalence of posttraumatic stress disorder in the Vietnam generation: a multimethod, multisource assessment of psychiatric disorder. J Trauma Stress. 1992;5(3):333-363.
10. Friedman MJ, Resick PA, Bryant RA, Strain J, Horowitz M, Spiegel D. Classification of trauma and stressor-related disorders in DSM-5. Depress Anxiety. 2011;28(9):737-749.
11. Lew HL, Otis JD, Tun C, Kerns RD, Clark ME, Cifu DX. Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in OIF/OEF veterans: polytrauma clinical triad. J Rehabil Res Dev. 2009;46(6):697-702.
12. Carlson K, Kehle S, Meis L, et al. The Assessment and Treatment of Individuals with History of Traumatic Brain Injury and Post-Traumatic Stress Disorder: A Systematic Review of the Evidence. Washington, DC: US Department of Veterans Affairs; 2009.
13. Gironda RJ, Clark ME, Ruff RL, et al. Traumatic brain injury, polytrauma, and pain: challenges and treatment strategies for the polytrauma rehabilitation. Rehabil Psychol. 2009;54(3):247-258.
14. Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358(5):453-463.
15. Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil. 2009;24(6):439-451.
16. Peskind ER, Brody D, Cernak I, McKee A, Ruff RL. Military- and sports-related mild traumatic brain injury: clinical presentation, management, and long-term consequences. J Clin Psychiatry. 2013;74(2):180-188.
17. Riggio S. Traumatic brain injury and its neurobehavioral sequelae. Neurol Clin. 2011;29(1):35-47, vii.
18. Helmick KM, Spells CA, Malik SZ, Davies CA, Marion DW, Hinds SR. Traumatic brain injury in the US military: epidemiology and key clinical and research programs. Brain Imaging Behav. 2015;9(3):358-366.
19. Richardson LK, Frueh BC, Acierno R. Prevalence estimates of combat-related post-traumatic stress disorder: critical review. Aust N Z J Psychiatry. 2010;44(1):4-19.
20. Thompson WW, Gottesman II, Zalewski C. Reconciling disparate prevalence rates of PTSD in large samples of US male Vietnam veterans and their controls. BMC Psychiatry. 2006;6:19.
21. Frueh BC, Elhai JD, Gold PB, et al Disability compensation seeking among veterans evaluated for posttraumatic stress disorder. Psychiatr Serv. 2003;54(1):84-91.
22. Thakur H, Oni O, Singh V, et al. Increases in the service connection disability and treatment costs associated with posttraumatic stress disorder and/or traumatic brain injury in United States veterans pre- and post-9/11: the strong need for a novel therapeutic approach. Epidemiology (Sunnyvale). 2018;8(4):353.
23. Schlenger WE, Kulka RA, Fairbank JA, et al. The prevalence of post-traumatic stress disorder in the Vietnam generation: a multimethod, multisource assessment of psychiatric disorder. J Trauma Stress. 1992;5(3):333-363.
24. Belmont PJ, Schoenfeld AJ, Goodman G. Epidemiology of combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom: orthopaedic burden of disease. J Surg Orthop Adv. 2010;19(1):2-7.
25. Owens BD, Kragh JG Jr, Wenke JC, Macaitis J, Wade CE, Holcomb JB. Combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma. 2008;64(2):295-299.
26. Defense Health Agency, Defense and Veterans Brain Injury Center. DOD worldwide numbers for TBI since 2000. https://dvbic.dcoe.mil/dod-worldwide-numbers-tbi. Updated February 14, 2020. Accessed February 14, 2020.
27. Armed Forces Health Surveillance Center. Deployment-related conditions of special surveillance interest, U.S. armed forces, by month and service, January 2003-December 2012 (data as of 22 January 2013). MSMR. 2013;20(1):16-19.
28. Harvey JH, Stein SK, Scott PK. Fifty years of grief: accounts and reported psychological reactions of Normandy invasion veterans. J Narrative Life History. 1995;5(4):321-332.
29. US Department of Veterans Affairs. Polytrauma/TBI system of care. https://www.polytrauma.va.gov/system-of-care/index.asp. Updated June 3, 2015. Accessed February 4, 2020.
30. Wolfe J, Erickson DJ, Sharkansky EJ, King DW, King LA. Course and predictors of posttraumatic stress disorder among Gulf War veterans: a prospective analysis. J Consult Clin Psychol. 1999;67(4):520-528.
31. Breslau N, Davis GC, Peterson EL, Schultz L. Psychiatric sequelae of posttraumatic stress disorder in women. Arch Gen Psychiatry. 1997;54(1):81-87.
32. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060.
33. Wolfe J, Kimerling R. Gender issues in the assessment of posttraumatic stress disorder. In: Wilson J, Keane TM, eds. Assessing Psychological Trauma and PTSD. New York: Guilford; 2004:192-238.
34. Engel CC Jr, Engel AL, Campbell SJ, McFall ME, Russo J, Katon W. Posttraumatic stress disorder symptoms and precombat sexual and physical abuse in Desert Storm veterans. J Nerv Ment Dis. 1993;181(11):683-688.
35. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of veterans: 2016 data from the American Community Survey. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2016.pdf. Published February 2018. Accessed February 4, 2020.
36. US Department of Commerce Economics and Statistics Administration, US Census Bureau, Geography Division. 2010 population distribution in the United States and Puerto Rico. https://www2.census.gov/geo/maps/dc10_thematic/2010_Nighttime_PopDist/2010_Nighttime_PopDist_Page_Map.pdf. Accessed February 4, 2020.
37. Cifu DX, Taylor BC, Carne WF, et al. Traumatic brain injury, posttraumatic stress disorder, and pain diagnoses in OIF/OEF/OND veterans. J Rehabil Res Dev. 2013;50(9):1169-1176.
38. Dohrenwend BP, Turner JB, Turse NA, Adams BG, Koenen KC, Marshall R. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science. 2006;313(5789):979-982.
39. Magruder KM, Frueh BC, Knapp RG, et al. Prevalence of posttraumatic stress disorder in Veterans Affairs primary care clinics. Gen Hosp Psychiatry. 2005;27(3):169-179.
40. Norris FH. Epidemiology of trauma: frequency and impact of different potentially traumatic events on different demographic groups. J Consult Clin Psychol. 1992;60(3):409-418.
41. Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psychol. 1993;61(6):984-991.
42. Najavits LM. The problem of dropout from “gold standard” PTSD therapies. F1000Prime Rep. 2015;7:43.
43. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Trends in veterans with a service-connected disability: 1985 to 2014. https://www.va.gov/vetdata/docs/QuickFacts/SCD_trends_FINAL_2014.PDF. Published June 2015. Accessed February 4, 2020.
44. US Department of Veterans Affairs, Office of Inspector General. Review of state variances in VA disability compensation payments. Report 05-00765-137. https://www.va.gov/oig/52/reports/2005/VAOIG-05-00765-137.pdf. Published May 19, 2015. Accessed February 4, 2020.
45. McNally RJ. Progress and controversy in the study of posttraumatic stress disorder. Annu Rev Psychol. 2003;54:229-252.
46. Freeman T, Powell M, Kimbrell T. Measuring symptom exaggeration in veterans with chronic posttraumatic stress disorder. Psychiatry Res. 2008;158(3):374-380.
47. Frueh BC, Elhai JD, Grubaugh AL, et al. Documented combat exposure of US veterans seeking treatment for combat-related post-traumatic stress disorder. Br J Psychiatry. 2005;186(6):467-475.
48. Frueh BC, Hamner MB, Cahill SP, Gold PB, Hamlin KL. Apparent symptom overreporting in combat veterans evaluated for PTSD. Clin Psychol Rev. 2000;20(7):853-885.
49. Sparr L, Pankratz LD. Factitious posttraumatic stress disorder. Am J Psychiatry. 1983;140(8):1016-1019.
50. Baggaley M. ‘Military Munchausen’s’: assessment of factitious claims of military service in psychiatric patients. Psychiatr Bull. 1998;22(3):153-154.