User login
Dermal filler approved for treating acne scars
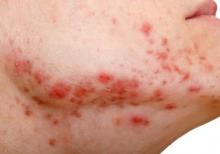
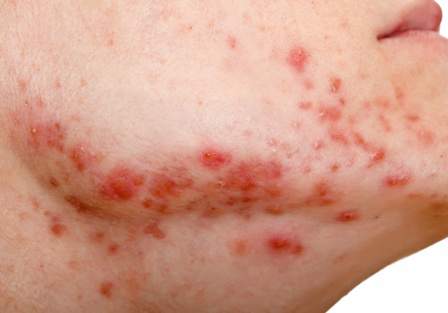
A collagen dermal filler already on the market has been approved for improving the appearance of acne scars in people aged 21 years and older.
The filler, made of bovine collagen and nonabsorbable polymethylmethacrylate beads (PMMA microspheres), with a small amount of lidocaine, was approved for the correction of “moderate to severe, atrophic, distensible facial acne scars on the cheek in patients over the age of 21 years,” according to the Food and Drug Administration’s approval letter, dated Dec. 23, 2014.
The filler is marketed as Bellafill by Suneva Medical; it was formerly marketed as ArteFill. In December, the brand name was changed to Bellafill, according to the company. The filler was approved in 2006 for the correction of nasolabial folds and is the first permanent filler approved by the FDA for treating acne scars, an FDA spokesperson said.
In a clinical trial, one or two injections were needed to improve the appearance of acne scars, and the effect lasted more than 1 year, according to the FDA’s summary of approval-related information. Side effects included lumps at the injection site, redness, swelling, pain, tenderness, and itching. Contraindications included severe allergies, a positive response to the Bellafill skin test (which is required), allergies to lidocaine or cow tissue products, and bleeding disorders.
Approval was based on the multicenter U.S. study of patients aged 21 years and older with moderate to severe atrophic distensible facial acne scars on the cheek. The patients were randomized to Bellafill or sterile saline injections. Their mean age was 45 years, and about 60% were women.
At 6 months, 56 of 87 patients (64%) treated with Bellafill had at least a 2-point improvement on the 4-point Acne Scar Rating Scale for at least half of the treated scars (as assessed by a blinded evaluator), the primary endpoint. Of the 46 controls, 15 (33%) met this endpoint, and this difference was statistically significant.
Patient responses were among the endpoints evaluated in the study. At 6 months, 77% of those treated with Bellafill thought the appearance of scars had “improved” or had “much improved,” based on the Subject Global Aesthetic Improvement Scale ( a secondary endpoint), compared with 41% of controls.
Information on the approval is available on the FDA website at www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfTopic/pma/pma.cfm?num=P020012S009.
Adverse events for this product or other drugs and devices should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/Safety/MedWatch/.
A collagen dermal filler already on the market has been approved for improving the appearance of acne scars in people aged 21 years and older.
The filler, made of bovine collagen and nonabsorbable polymethylmethacrylate beads (PMMA microspheres), with a small amount of lidocaine, was approved for the correction of “moderate to severe, atrophic, distensible facial acne scars on the cheek in patients over the age of 21 years,” according to the Food and Drug Administration’s approval letter, dated Dec. 23, 2014.
The filler is marketed as Bellafill by Suneva Medical; it was formerly marketed as ArteFill. In December, the brand name was changed to Bellafill, according to the company. The filler was approved in 2006 for the correction of nasolabial folds and is the first permanent filler approved by the FDA for treating acne scars, an FDA spokesperson said.
In a clinical trial, one or two injections were needed to improve the appearance of acne scars, and the effect lasted more than 1 year, according to the FDA’s summary of approval-related information. Side effects included lumps at the injection site, redness, swelling, pain, tenderness, and itching. Contraindications included severe allergies, a positive response to the Bellafill skin test (which is required), allergies to lidocaine or cow tissue products, and bleeding disorders.
Approval was based on the multicenter U.S. study of patients aged 21 years and older with moderate to severe atrophic distensible facial acne scars on the cheek. The patients were randomized to Bellafill or sterile saline injections. Their mean age was 45 years, and about 60% were women.
At 6 months, 56 of 87 patients (64%) treated with Bellafill had at least a 2-point improvement on the 4-point Acne Scar Rating Scale for at least half of the treated scars (as assessed by a blinded evaluator), the primary endpoint. Of the 46 controls, 15 (33%) met this endpoint, and this difference was statistically significant.
Patient responses were among the endpoints evaluated in the study. At 6 months, 77% of those treated with Bellafill thought the appearance of scars had “improved” or had “much improved,” based on the Subject Global Aesthetic Improvement Scale ( a secondary endpoint), compared with 41% of controls.
Information on the approval is available on the FDA website at www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfTopic/pma/pma.cfm?num=P020012S009.
Adverse events for this product or other drugs and devices should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/Safety/MedWatch/.
A collagen dermal filler already on the market has been approved for improving the appearance of acne scars in people aged 21 years and older.
The filler, made of bovine collagen and nonabsorbable polymethylmethacrylate beads (PMMA microspheres), with a small amount of lidocaine, was approved for the correction of “moderate to severe, atrophic, distensible facial acne scars on the cheek in patients over the age of 21 years,” according to the Food and Drug Administration’s approval letter, dated Dec. 23, 2014.
The filler is marketed as Bellafill by Suneva Medical; it was formerly marketed as ArteFill. In December, the brand name was changed to Bellafill, according to the company. The filler was approved in 2006 for the correction of nasolabial folds and is the first permanent filler approved by the FDA for treating acne scars, an FDA spokesperson said.
In a clinical trial, one or two injections were needed to improve the appearance of acne scars, and the effect lasted more than 1 year, according to the FDA’s summary of approval-related information. Side effects included lumps at the injection site, redness, swelling, pain, tenderness, and itching. Contraindications included severe allergies, a positive response to the Bellafill skin test (which is required), allergies to lidocaine or cow tissue products, and bleeding disorders.
Approval was based on the multicenter U.S. study of patients aged 21 years and older with moderate to severe atrophic distensible facial acne scars on the cheek. The patients were randomized to Bellafill or sterile saline injections. Their mean age was 45 years, and about 60% were women.
At 6 months, 56 of 87 patients (64%) treated with Bellafill had at least a 2-point improvement on the 4-point Acne Scar Rating Scale for at least half of the treated scars (as assessed by a blinded evaluator), the primary endpoint. Of the 46 controls, 15 (33%) met this endpoint, and this difference was statistically significant.
Patient responses were among the endpoints evaluated in the study. At 6 months, 77% of those treated with Bellafill thought the appearance of scars had “improved” or had “much improved,” based on the Subject Global Aesthetic Improvement Scale ( a secondary endpoint), compared with 41% of controls.
Information on the approval is available on the FDA website at www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfTopic/pma/pma.cfm?num=P020012S009.
Adverse events for this product or other drugs and devices should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/Safety/MedWatch/.
The Role of Diet in Acne: We Get It, But What Should We Do About It?
The role of diet in acne, both as a causative agent and therapeutic intervention, has been the topic of discussion in both the dermatology community as well as the laypress for decades. There is ample evidence highlighting the association of acne and high glycemic loads, certain dairy products, and refined sugar product ingestion. In the most recent edition to the repository, Grossi et al (J Eur Acad Dermatol Venereol. doi:10.1111/jdv.12878) reanalyzed data from their case-control study among young patients (age range, 10–24 years; N=563) with a diagnosis of moderate to severe acne versus control (participants with no or mild acne) between March 2009 and February 2010 that was originally published in 2012 (J Am Acad Dermatol. 2012;67:1129-1135). The unique element was how they evaluated the data. The investigators utilized a semantic connectivity map approach derived from artificial neural network computational models, which allowed for a better understanding of the complex connections between all of the studied variables. (The assumption of a given relation between any variables would not influence the results.) The data were presented on an Auto Semantic Connectivity Map that resembled a 4-leaf clover, representing “explanatory” information pertaining to the cases and controls and “residual” information of less importance. It is worth seeing in the manuscript to better appreciate the data.
What did they find? There is a close association between moderate to severe acne and a high intake of milk, other dairy products, sweets, and chocolate. Obesity and the low consumption of fish were linked to the presence of moderate to severe acne, while high consumption of fish (1 d/wk or more), high intake of fruits and vegetables, and body mass index lower than 18.5 were all associated with limited or no acne.
What’s the issue?
By adopting a different analytic approach, it was shown once again that diet plays a substantial role in acne, indicating that some food items may stimulate selected acne-promoting pathways. But what now? Here is the evidence yet again, but where is the medicine of “evidence-based medicine”? It is time to recommend guidelines for screening and counseling. In a recent article, Bronsnick et al (J Am Acad Dermatol. 2014;71:1039.e1-1039.e12) found that the level of evidence supporting the benefit of a low-glycemic, low-carbohydrate diet was sufficient to recommend to acne patients. How many dermatologists feel comfortable providing dietary guidance to their acne patients? A consensus statement from relevant organizations such as the American Academy of Dermatology, the Society for Investigative Dermatology, and the American Acne & Rosacea Society that provides tangible and realistic screening tools to identify those who would benefit from dietary intervention and implementation and practice guidelines for Dr. Derm seeing 40 to 50 patients a day in Springfield, USA (homage to The Simpsons) based on the level of evidence available would be useful. What are your thoughts?
We want to know your views! Tell us what you think.
Suggested Readings
- Bowe WP, Joshi SS, Shalita AR. Diet and acne. J Am Acad Dermatol. 2010;63:124-141.
- Ferdowsian HR, Levin S. Does diet really affect acne? Skin Therapy Lett. 2010;15:1-2, 5.
- Melnik B. Dietary intervention in acne: attenuation of increased mTORC1 signaling promoted by Western diet. Dermatoendocrinol. 2012;4:20-32.
- Veith WB, Silverberg NB. The association of acne vulgaris with diet. Cutis. 2011;88:84-91.
The role of diet in acne, both as a causative agent and therapeutic intervention, has been the topic of discussion in both the dermatology community as well as the laypress for decades. There is ample evidence highlighting the association of acne and high glycemic loads, certain dairy products, and refined sugar product ingestion. In the most recent edition to the repository, Grossi et al (J Eur Acad Dermatol Venereol. doi:10.1111/jdv.12878) reanalyzed data from their case-control study among young patients (age range, 10–24 years; N=563) with a diagnosis of moderate to severe acne versus control (participants with no or mild acne) between March 2009 and February 2010 that was originally published in 2012 (J Am Acad Dermatol. 2012;67:1129-1135). The unique element was how they evaluated the data. The investigators utilized a semantic connectivity map approach derived from artificial neural network computational models, which allowed for a better understanding of the complex connections between all of the studied variables. (The assumption of a given relation between any variables would not influence the results.) The data were presented on an Auto Semantic Connectivity Map that resembled a 4-leaf clover, representing “explanatory” information pertaining to the cases and controls and “residual” information of less importance. It is worth seeing in the manuscript to better appreciate the data.
What did they find? There is a close association between moderate to severe acne and a high intake of milk, other dairy products, sweets, and chocolate. Obesity and the low consumption of fish were linked to the presence of moderate to severe acne, while high consumption of fish (1 d/wk or more), high intake of fruits and vegetables, and body mass index lower than 18.5 were all associated with limited or no acne.
What’s the issue?
By adopting a different analytic approach, it was shown once again that diet plays a substantial role in acne, indicating that some food items may stimulate selected acne-promoting pathways. But what now? Here is the evidence yet again, but where is the medicine of “evidence-based medicine”? It is time to recommend guidelines for screening and counseling. In a recent article, Bronsnick et al (J Am Acad Dermatol. 2014;71:1039.e1-1039.e12) found that the level of evidence supporting the benefit of a low-glycemic, low-carbohydrate diet was sufficient to recommend to acne patients. How many dermatologists feel comfortable providing dietary guidance to their acne patients? A consensus statement from relevant organizations such as the American Academy of Dermatology, the Society for Investigative Dermatology, and the American Acne & Rosacea Society that provides tangible and realistic screening tools to identify those who would benefit from dietary intervention and implementation and practice guidelines for Dr. Derm seeing 40 to 50 patients a day in Springfield, USA (homage to The Simpsons) based on the level of evidence available would be useful. What are your thoughts?
We want to know your views! Tell us what you think.
Suggested Readings
- Bowe WP, Joshi SS, Shalita AR. Diet and acne. J Am Acad Dermatol. 2010;63:124-141.
- Ferdowsian HR, Levin S. Does diet really affect acne? Skin Therapy Lett. 2010;15:1-2, 5.
- Melnik B. Dietary intervention in acne: attenuation of increased mTORC1 signaling promoted by Western diet. Dermatoendocrinol. 2012;4:20-32.
- Veith WB, Silverberg NB. The association of acne vulgaris with diet. Cutis. 2011;88:84-91.
The role of diet in acne, both as a causative agent and therapeutic intervention, has been the topic of discussion in both the dermatology community as well as the laypress for decades. There is ample evidence highlighting the association of acne and high glycemic loads, certain dairy products, and refined sugar product ingestion. In the most recent edition to the repository, Grossi et al (J Eur Acad Dermatol Venereol. doi:10.1111/jdv.12878) reanalyzed data from their case-control study among young patients (age range, 10–24 years; N=563) with a diagnosis of moderate to severe acne versus control (participants with no or mild acne) between March 2009 and February 2010 that was originally published in 2012 (J Am Acad Dermatol. 2012;67:1129-1135). The unique element was how they evaluated the data. The investigators utilized a semantic connectivity map approach derived from artificial neural network computational models, which allowed for a better understanding of the complex connections between all of the studied variables. (The assumption of a given relation between any variables would not influence the results.) The data were presented on an Auto Semantic Connectivity Map that resembled a 4-leaf clover, representing “explanatory” information pertaining to the cases and controls and “residual” information of less importance. It is worth seeing in the manuscript to better appreciate the data.
What did they find? There is a close association between moderate to severe acne and a high intake of milk, other dairy products, sweets, and chocolate. Obesity and the low consumption of fish were linked to the presence of moderate to severe acne, while high consumption of fish (1 d/wk or more), high intake of fruits and vegetables, and body mass index lower than 18.5 were all associated with limited or no acne.
What’s the issue?
By adopting a different analytic approach, it was shown once again that diet plays a substantial role in acne, indicating that some food items may stimulate selected acne-promoting pathways. But what now? Here is the evidence yet again, but where is the medicine of “evidence-based medicine”? It is time to recommend guidelines for screening and counseling. In a recent article, Bronsnick et al (J Am Acad Dermatol. 2014;71:1039.e1-1039.e12) found that the level of evidence supporting the benefit of a low-glycemic, low-carbohydrate diet was sufficient to recommend to acne patients. How many dermatologists feel comfortable providing dietary guidance to their acne patients? A consensus statement from relevant organizations such as the American Academy of Dermatology, the Society for Investigative Dermatology, and the American Acne & Rosacea Society that provides tangible and realistic screening tools to identify those who would benefit from dietary intervention and implementation and practice guidelines for Dr. Derm seeing 40 to 50 patients a day in Springfield, USA (homage to The Simpsons) based on the level of evidence available would be useful. What are your thoughts?
We want to know your views! Tell us what you think.
Suggested Readings
- Bowe WP, Joshi SS, Shalita AR. Diet and acne. J Am Acad Dermatol. 2010;63:124-141.
- Ferdowsian HR, Levin S. Does diet really affect acne? Skin Therapy Lett. 2010;15:1-2, 5.
- Melnik B. Dietary intervention in acne: attenuation of increased mTORC1 signaling promoted by Western diet. Dermatoendocrinol. 2012;4:20-32.
- Veith WB, Silverberg NB. The association of acne vulgaris with diet. Cutis. 2011;88:84-91.
Manage Your Dermatology Practice: Managing Difficult Patient Encounters
Difficult patient encounters in the dermatology office can be navigated through honest physician-patient communication regarding problems within the office and insurance coverage. Dr. Gary Goldenberg provides tips on communicating with patients about cosmetic procedures that may be noncovered services as well as diagnoses such as melanoma and psoriasis. He also advises how to work through a long list of questions patients may bring to their visit.
Difficult patient encounters in the dermatology office can be navigated through honest physician-patient communication regarding problems within the office and insurance coverage. Dr. Gary Goldenberg provides tips on communicating with patients about cosmetic procedures that may be noncovered services as well as diagnoses such as melanoma and psoriasis. He also advises how to work through a long list of questions patients may bring to their visit.
Difficult patient encounters in the dermatology office can be navigated through honest physician-patient communication regarding problems within the office and insurance coverage. Dr. Gary Goldenberg provides tips on communicating with patients about cosmetic procedures that may be noncovered services as well as diagnoses such as melanoma and psoriasis. He also advises how to work through a long list of questions patients may bring to their visit.
Adolescents’ acne knowledge improves with online counseling
Internet-based patient education sessions – specifically, “virtual counseling” – appear to be an effective method of improving general knowledge about acne among adolescents, according to Mr. William Tuong and his associates.
To compare the effectiveness of approaches in improving patient knowledge of acne vulgaris, the researchers instructed 97 high school students to visit either a standard website or an automated counseling website to learn about acne, and submit a multiple-choice questionnaire designed to assess any changes in acne knowledge.
Both groups demonstrated significantly improved knowledge after a 12-week follow-up, although the automated counseling website group rated the educational material more useful and more enjoyable to view than did the standard website group.
Read the full article at American Journal of Clinical Dermatology (2015;55-60 [doi:10.1007/s40257-014-0104-6]).
Internet-based patient education sessions – specifically, “virtual counseling” – appear to be an effective method of improving general knowledge about acne among adolescents, according to Mr. William Tuong and his associates.
To compare the effectiveness of approaches in improving patient knowledge of acne vulgaris, the researchers instructed 97 high school students to visit either a standard website or an automated counseling website to learn about acne, and submit a multiple-choice questionnaire designed to assess any changes in acne knowledge.
Both groups demonstrated significantly improved knowledge after a 12-week follow-up, although the automated counseling website group rated the educational material more useful and more enjoyable to view than did the standard website group.
Read the full article at American Journal of Clinical Dermatology (2015;55-60 [doi:10.1007/s40257-014-0104-6]).
Internet-based patient education sessions – specifically, “virtual counseling” – appear to be an effective method of improving general knowledge about acne among adolescents, according to Mr. William Tuong and his associates.
To compare the effectiveness of approaches in improving patient knowledge of acne vulgaris, the researchers instructed 97 high school students to visit either a standard website or an automated counseling website to learn about acne, and submit a multiple-choice questionnaire designed to assess any changes in acne knowledge.
Both groups demonstrated significantly improved knowledge after a 12-week follow-up, although the automated counseling website group rated the educational material more useful and more enjoyable to view than did the standard website group.
Read the full article at American Journal of Clinical Dermatology (2015;55-60 [doi:10.1007/s40257-014-0104-6]).
Wrinkle filler also works for acne scars
ORLANDO – A non-resorbable wrinkle filler proved highly effective and durable for the treatment of atrophic acne scars in a randomized, controlled, multicenter study.
At 1 month after treatment with polymethylmethacrylate-collagen, or PMMA-collagen (Artefill, Suneva Medical, Inc.), nearly 70% of 97 subjects showed at least a 2-point improvement on the validated 4-point Acne Scar Rating Scale, compared with about 40% of 50 control subjects injected with saline. At 6 months, the response rate remained above 60% in the PMMA-collagen group, but dropped closer to 30% among those in the control group, Dr. James M. Spencer of Mount Sinai School of Medicine, New York reported in a poster at the Orlando Dermatology Aesthetic and Clinical Conference.
The control group subjects were then crossed over to the treatment group, and, at 12 months, the response rates were about 70% and nearly 60% in the treatment and control groups, respectively, he said.
Similarly, both Physician and Subject Global Aesthetic Improvement Scale (PGAIS/SGAIS) scores diverged during a 6-month evaluator-blinded phase of the study, then converged after crossover by the control group subjects. For example, the percentage of treatment and control group subjects with improvement at 1 month and 6 months according to the 5-point PGAIS was about 90% vs. less than 65%, and about 80% vs. about 30%, respectively. More than 90% in both groups showed improvement at 12 months, after control group crossover.
Additionally, subject satisfaction at 1 and 6 months in the treatment and control groups based on assessment of scar correction using Patient Satisfaction Scale scores was above 80% vs. about 60%, and about 80% vs. about 50%, respectively. Satisfaction in both groups was between 80% and 90% at 12 months, after control group crossover.
Study subjects, who had a mean age of 44 years, were enrolled from 10 U.S. centers and were treated during one injection session. An additional touch-up injection was allowed as needed. A total of 1,292 scars were treated in the 97 treatment group subjects, and 424 were treated in the 50 control group subjects. Participants were evaluated by blinded assessors at 2 weeks and 1, 3, and 6 months, after which control group subjects were treated with PMMA-collagen. Assessments were made in open-label fashion at 9 and 12 months. Most subjects (61%) were women, and 20% had Fitzpatrick skin types 5 or 6.
During the blinded portion of the study, six treatment-related adverse events were reported among treatment group subjects, and two were reported among control group subjects. None of the subjects experienced granulomas, changes in pigmentation, or hypertrophic scarring.
This study is the first randomized, blinded study of PMMA-collagen for treating acne scars, Dr. Spencer noted, adding that the findings demonstrate the efficacy and safety of PMMA-collagen for this purpose.
“The improvement is durable, lasting for 12 months,” he wrote, noting that the filler is easily administered and requires minimal training in those who are familiar with dermal fillers.
“PMMA-collagen works very well on deep, severe acne scars, and should also work very well on shallow scars,” he said.
The product may enable practitioners to effectively treat acne scarring without a large capital equipment expenditure and without the risks associated with resurfacing procedures,” he said.
This study was sponsored by Suneva Medical, Inc.
ORLANDO – A non-resorbable wrinkle filler proved highly effective and durable for the treatment of atrophic acne scars in a randomized, controlled, multicenter study.
At 1 month after treatment with polymethylmethacrylate-collagen, or PMMA-collagen (Artefill, Suneva Medical, Inc.), nearly 70% of 97 subjects showed at least a 2-point improvement on the validated 4-point Acne Scar Rating Scale, compared with about 40% of 50 control subjects injected with saline. At 6 months, the response rate remained above 60% in the PMMA-collagen group, but dropped closer to 30% among those in the control group, Dr. James M. Spencer of Mount Sinai School of Medicine, New York reported in a poster at the Orlando Dermatology Aesthetic and Clinical Conference.
The control group subjects were then crossed over to the treatment group, and, at 12 months, the response rates were about 70% and nearly 60% in the treatment and control groups, respectively, he said.
Similarly, both Physician and Subject Global Aesthetic Improvement Scale (PGAIS/SGAIS) scores diverged during a 6-month evaluator-blinded phase of the study, then converged after crossover by the control group subjects. For example, the percentage of treatment and control group subjects with improvement at 1 month and 6 months according to the 5-point PGAIS was about 90% vs. less than 65%, and about 80% vs. about 30%, respectively. More than 90% in both groups showed improvement at 12 months, after control group crossover.
Additionally, subject satisfaction at 1 and 6 months in the treatment and control groups based on assessment of scar correction using Patient Satisfaction Scale scores was above 80% vs. about 60%, and about 80% vs. about 50%, respectively. Satisfaction in both groups was between 80% and 90% at 12 months, after control group crossover.
Study subjects, who had a mean age of 44 years, were enrolled from 10 U.S. centers and were treated during one injection session. An additional touch-up injection was allowed as needed. A total of 1,292 scars were treated in the 97 treatment group subjects, and 424 were treated in the 50 control group subjects. Participants were evaluated by blinded assessors at 2 weeks and 1, 3, and 6 months, after which control group subjects were treated with PMMA-collagen. Assessments were made in open-label fashion at 9 and 12 months. Most subjects (61%) were women, and 20% had Fitzpatrick skin types 5 or 6.
During the blinded portion of the study, six treatment-related adverse events were reported among treatment group subjects, and two were reported among control group subjects. None of the subjects experienced granulomas, changes in pigmentation, or hypertrophic scarring.
This study is the first randomized, blinded study of PMMA-collagen for treating acne scars, Dr. Spencer noted, adding that the findings demonstrate the efficacy and safety of PMMA-collagen for this purpose.
“The improvement is durable, lasting for 12 months,” he wrote, noting that the filler is easily administered and requires minimal training in those who are familiar with dermal fillers.
“PMMA-collagen works very well on deep, severe acne scars, and should also work very well on shallow scars,” he said.
The product may enable practitioners to effectively treat acne scarring without a large capital equipment expenditure and without the risks associated with resurfacing procedures,” he said.
This study was sponsored by Suneva Medical, Inc.
ORLANDO – A non-resorbable wrinkle filler proved highly effective and durable for the treatment of atrophic acne scars in a randomized, controlled, multicenter study.
At 1 month after treatment with polymethylmethacrylate-collagen, or PMMA-collagen (Artefill, Suneva Medical, Inc.), nearly 70% of 97 subjects showed at least a 2-point improvement on the validated 4-point Acne Scar Rating Scale, compared with about 40% of 50 control subjects injected with saline. At 6 months, the response rate remained above 60% in the PMMA-collagen group, but dropped closer to 30% among those in the control group, Dr. James M. Spencer of Mount Sinai School of Medicine, New York reported in a poster at the Orlando Dermatology Aesthetic and Clinical Conference.
The control group subjects were then crossed over to the treatment group, and, at 12 months, the response rates were about 70% and nearly 60% in the treatment and control groups, respectively, he said.
Similarly, both Physician and Subject Global Aesthetic Improvement Scale (PGAIS/SGAIS) scores diverged during a 6-month evaluator-blinded phase of the study, then converged after crossover by the control group subjects. For example, the percentage of treatment and control group subjects with improvement at 1 month and 6 months according to the 5-point PGAIS was about 90% vs. less than 65%, and about 80% vs. about 30%, respectively. More than 90% in both groups showed improvement at 12 months, after control group crossover.
Additionally, subject satisfaction at 1 and 6 months in the treatment and control groups based on assessment of scar correction using Patient Satisfaction Scale scores was above 80% vs. about 60%, and about 80% vs. about 50%, respectively. Satisfaction in both groups was between 80% and 90% at 12 months, after control group crossover.
Study subjects, who had a mean age of 44 years, were enrolled from 10 U.S. centers and were treated during one injection session. An additional touch-up injection was allowed as needed. A total of 1,292 scars were treated in the 97 treatment group subjects, and 424 were treated in the 50 control group subjects. Participants were evaluated by blinded assessors at 2 weeks and 1, 3, and 6 months, after which control group subjects were treated with PMMA-collagen. Assessments were made in open-label fashion at 9 and 12 months. Most subjects (61%) were women, and 20% had Fitzpatrick skin types 5 or 6.
During the blinded portion of the study, six treatment-related adverse events were reported among treatment group subjects, and two were reported among control group subjects. None of the subjects experienced granulomas, changes in pigmentation, or hypertrophic scarring.
This study is the first randomized, blinded study of PMMA-collagen for treating acne scars, Dr. Spencer noted, adding that the findings demonstrate the efficacy and safety of PMMA-collagen for this purpose.
“The improvement is durable, lasting for 12 months,” he wrote, noting that the filler is easily administered and requires minimal training in those who are familiar with dermal fillers.
“PMMA-collagen works very well on deep, severe acne scars, and should also work very well on shallow scars,” he said.
The product may enable practitioners to effectively treat acne scarring without a large capital equipment expenditure and without the risks associated with resurfacing procedures,” he said.
This study was sponsored by Suneva Medical, Inc.
AT THE ODAC CONFERENCE
Key clinical point: PMMA-collagen is safe, effective, and practical for treating atrophic acne scars.
Major finding: Nearly 70% of treated subjects vs. 40% of controls showed at least a 2-point improvement on the Acne Scar Rating Scale
Data source: A randomized, controlled, multicenter study of 147 subjects.
Disclosures: This study was sponsored by Suneva Medical, Inc.
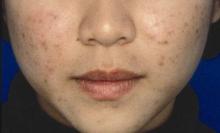
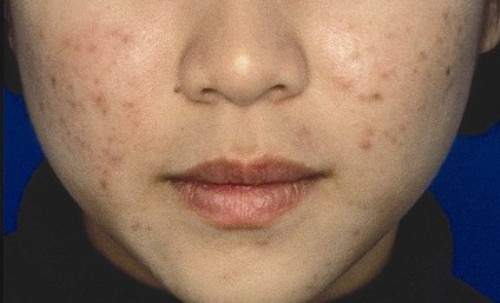
Combined OTC, prescription acne regimen satisfied young patients
ORLANDO – A three-component combined over-the-counter and prescription skin care regimen was safe, effective, well tolerated, and well liked by acne vulgaris patients aged 12 years and older in an open-label multicenter study.
Of 81 participants with a mean age of 19 years, mild or moderate facial acne vulgaris, and mean acne vulgaris duration of 4.4 years, 89.2% agreed or strongly agreed that they liked the regimen, 87.9% reported overall satisfaction, and 87.9% said they would recommend the regimen to others.
Nearly all participants (95.9%) said the regimen was easy to use, 73% reported improved skin texture, and 70.3% said the regimen met their needs, Dr. Michael H. Gold of Nashville, Tenn., reported in a poster at the Orlando Dermatology Aesthetic and Clinical Conference.
Treatment involved once-daily application of a prescription topical gel containing adapalene 1% and benzoyl peroxide 2.5%, as well as the use of two over-the-counter products designed for acne-prone skin: a foaming cleanser used twice daily and a moisturizer with broad spectrum SPF 30 sunscreen used once daily. Treatment continued for 8 weeks.
In addition to the subjective patient satisfaction questionnaire, subjects were assessed based on total inflammatory and noninflammatory lesion counts; photographic evaluation of skin shininess, texture, and presence of Propionibacterium acnes; cutaneous tolerability scores for stinging, burning, erythema, scaling, and dryness; and adverse events.
The therapeutic effect was evident in most patients at 2 weeks, with a reduction in the number of lesions. After 8 weeks, total inflammatory and noninflammatory lesion counts were significantly reduced, compared with baseline. Skin shininess and P. acnes also were significantly reduced by 8 weeks, Dr. Gold noted.
Most patients had no cutaneous irritation; the proportion of patients experiencing irritation was smaller than in prior phase II and III studies that evaluated benzoyl peroxide once-daily gel with or without moisturizer. Of 553 patients from those prior studies, 4% and 1% reported moderate and severe erythema, respectively, compared with 1% and 0% of the patients in the current study. In addition 3% and 1% of patients in earlier phase II and III studies, respectively, reported moderate or severe stinging/burning, compared with none of the patients in the current study.
A total of 18 adverse events were reported by 13 patients in the current study, and 17 events were considered to be related to the skin care regimen. None of the affected patients discontinued the regimen, and none of the patients reported serious adverse events.
Of note, 70% of patients agreed or strongly agreed that a sunscreen made for acne-prone skin was important to them, Dr. Gold said.
He and his colleagues concluded that a complete three-component, acne-specific regimen designed to clean, moisturize, medicate, and photoprotect is important for optimizing patient outcomes, and that the regimen used in this study was associated with good outcomes and high levels of patient satisfaction.
This study was funded by Galderma Laboratories.
ORLANDO – A three-component combined over-the-counter and prescription skin care regimen was safe, effective, well tolerated, and well liked by acne vulgaris patients aged 12 years and older in an open-label multicenter study.
Of 81 participants with a mean age of 19 years, mild or moderate facial acne vulgaris, and mean acne vulgaris duration of 4.4 years, 89.2% agreed or strongly agreed that they liked the regimen, 87.9% reported overall satisfaction, and 87.9% said they would recommend the regimen to others.
Nearly all participants (95.9%) said the regimen was easy to use, 73% reported improved skin texture, and 70.3% said the regimen met their needs, Dr. Michael H. Gold of Nashville, Tenn., reported in a poster at the Orlando Dermatology Aesthetic and Clinical Conference.
Treatment involved once-daily application of a prescription topical gel containing adapalene 1% and benzoyl peroxide 2.5%, as well as the use of two over-the-counter products designed for acne-prone skin: a foaming cleanser used twice daily and a moisturizer with broad spectrum SPF 30 sunscreen used once daily. Treatment continued for 8 weeks.
In addition to the subjective patient satisfaction questionnaire, subjects were assessed based on total inflammatory and noninflammatory lesion counts; photographic evaluation of skin shininess, texture, and presence of Propionibacterium acnes; cutaneous tolerability scores for stinging, burning, erythema, scaling, and dryness; and adverse events.
The therapeutic effect was evident in most patients at 2 weeks, with a reduction in the number of lesions. After 8 weeks, total inflammatory and noninflammatory lesion counts were significantly reduced, compared with baseline. Skin shininess and P. acnes also were significantly reduced by 8 weeks, Dr. Gold noted.
Most patients had no cutaneous irritation; the proportion of patients experiencing irritation was smaller than in prior phase II and III studies that evaluated benzoyl peroxide once-daily gel with or without moisturizer. Of 553 patients from those prior studies, 4% and 1% reported moderate and severe erythema, respectively, compared with 1% and 0% of the patients in the current study. In addition 3% and 1% of patients in earlier phase II and III studies, respectively, reported moderate or severe stinging/burning, compared with none of the patients in the current study.
A total of 18 adverse events were reported by 13 patients in the current study, and 17 events were considered to be related to the skin care regimen. None of the affected patients discontinued the regimen, and none of the patients reported serious adverse events.
Of note, 70% of patients agreed or strongly agreed that a sunscreen made for acne-prone skin was important to them, Dr. Gold said.
He and his colleagues concluded that a complete three-component, acne-specific regimen designed to clean, moisturize, medicate, and photoprotect is important for optimizing patient outcomes, and that the regimen used in this study was associated with good outcomes and high levels of patient satisfaction.
This study was funded by Galderma Laboratories.
ORLANDO – A three-component combined over-the-counter and prescription skin care regimen was safe, effective, well tolerated, and well liked by acne vulgaris patients aged 12 years and older in an open-label multicenter study.
Of 81 participants with a mean age of 19 years, mild or moderate facial acne vulgaris, and mean acne vulgaris duration of 4.4 years, 89.2% agreed or strongly agreed that they liked the regimen, 87.9% reported overall satisfaction, and 87.9% said they would recommend the regimen to others.
Nearly all participants (95.9%) said the regimen was easy to use, 73% reported improved skin texture, and 70.3% said the regimen met their needs, Dr. Michael H. Gold of Nashville, Tenn., reported in a poster at the Orlando Dermatology Aesthetic and Clinical Conference.
Treatment involved once-daily application of a prescription topical gel containing adapalene 1% and benzoyl peroxide 2.5%, as well as the use of two over-the-counter products designed for acne-prone skin: a foaming cleanser used twice daily and a moisturizer with broad spectrum SPF 30 sunscreen used once daily. Treatment continued for 8 weeks.
In addition to the subjective patient satisfaction questionnaire, subjects were assessed based on total inflammatory and noninflammatory lesion counts; photographic evaluation of skin shininess, texture, and presence of Propionibacterium acnes; cutaneous tolerability scores for stinging, burning, erythema, scaling, and dryness; and adverse events.
The therapeutic effect was evident in most patients at 2 weeks, with a reduction in the number of lesions. After 8 weeks, total inflammatory and noninflammatory lesion counts were significantly reduced, compared with baseline. Skin shininess and P. acnes also were significantly reduced by 8 weeks, Dr. Gold noted.
Most patients had no cutaneous irritation; the proportion of patients experiencing irritation was smaller than in prior phase II and III studies that evaluated benzoyl peroxide once-daily gel with or without moisturizer. Of 553 patients from those prior studies, 4% and 1% reported moderate and severe erythema, respectively, compared with 1% and 0% of the patients in the current study. In addition 3% and 1% of patients in earlier phase II and III studies, respectively, reported moderate or severe stinging/burning, compared with none of the patients in the current study.
A total of 18 adverse events were reported by 13 patients in the current study, and 17 events were considered to be related to the skin care regimen. None of the affected patients discontinued the regimen, and none of the patients reported serious adverse events.
Of note, 70% of patients agreed or strongly agreed that a sunscreen made for acne-prone skin was important to them, Dr. Gold said.
He and his colleagues concluded that a complete three-component, acne-specific regimen designed to clean, moisturize, medicate, and photoprotect is important for optimizing patient outcomes, and that the regimen used in this study was associated with good outcomes and high levels of patient satisfaction.
This study was funded by Galderma Laboratories.
AT THE ODAC CONFERENCE
Key clinical point: Children and young adults with acne vulgaris adhered to a three-part skin care regimen involving prescription and OTC products.
Major finding: 87.9% of patients reported overall satisfaction.
Data source: An open-label, multicenter study.
Disclosures: The study was funded by Galderma Laboratories.
OTC acne product equals benzoyl peroxide with clindamycin
ORLANDO – An over-the-counter 5.5% benzoyl peroxide preparation with lipohydroxy acid was a safe and effective alternative to prescription 5% benzoyl peroxide with 1% clindamycin in a 12-week randomized, double-blind, multicenter study of patients with mild to moderate acne vulgaris who also were treated with generic 0.025% tretinoin.
Statistically significant improvement on a variety of efficacy endpoints was seen at weeks 8 and 12 in 60 men and women aged 18-50 years who were randomized to either the OTC-based or prescription-based regimen, Susana Raab of L’Oreal Research & Innovation, Clark, N.J., reported in a poster at the Orlando Dermatology Aesthetic and Clinical Conference.
The mean reduction in total acne lesion count at week 8 was approximately 60% with the OTC regimen and 64% in the prescription regimen. At week 12, the mean reduction was about 72% in both groups. The mean improvement in global acne assessment was approximately 31% and 33% in the groups, respectively, at 8 weeks, and about 33% in both groups at 12 weeks.
The mean improvement in overall appearance was 37%-38% in both groups at 8 weeks, and was close to 50% in both groups at 12 weeks, Ms. Raab said.
All skin types and a range of ethnicities were represented in the study population. Subjects applied the OTC benzoyl peroxide with lipohydroxy acid or the prescription benzoyl peroxide and clindamycin twice daily for 12 weeks. The tretinoin was applied daily at night after the other products were dried and absorbed. Three blinded board-certified dermatologists assessed the subjects for tolerability, and lesion counts were performed on the entire face to assess for open and closed comedones, papules, and pustules.
The dermatologists also assessed for tone, smoothness, brightness, appearance of pores, global acne, overall skin appearance, and tolerability.
The degree of acne relapse was assessed during a 4-week regression phase at which time the treatment was discontinued.
Both treatments improved inflammatory and noninflammatory lesions to a similar degree within 2 weeks of use. Dryness and peeling were seen with both treatments, but resolved by week 12. Stinging, tingling, itching, and burning also were common with both treatments, and largely resolved in both groups by week 8, except for significant stinging in the OTC treatment group. No significant overall differences were seen between the groups with respect to tolerance parameters, Ms. Raab noted.
“When compared, both treatments were at parity in improvement of all efficacy attributes at all time points,” she and her colleagues concluded.
ORLANDO – An over-the-counter 5.5% benzoyl peroxide preparation with lipohydroxy acid was a safe and effective alternative to prescription 5% benzoyl peroxide with 1% clindamycin in a 12-week randomized, double-blind, multicenter study of patients with mild to moderate acne vulgaris who also were treated with generic 0.025% tretinoin.
Statistically significant improvement on a variety of efficacy endpoints was seen at weeks 8 and 12 in 60 men and women aged 18-50 years who were randomized to either the OTC-based or prescription-based regimen, Susana Raab of L’Oreal Research & Innovation, Clark, N.J., reported in a poster at the Orlando Dermatology Aesthetic and Clinical Conference.
The mean reduction in total acne lesion count at week 8 was approximately 60% with the OTC regimen and 64% in the prescription regimen. At week 12, the mean reduction was about 72% in both groups. The mean improvement in global acne assessment was approximately 31% and 33% in the groups, respectively, at 8 weeks, and about 33% in both groups at 12 weeks.
The mean improvement in overall appearance was 37%-38% in both groups at 8 weeks, and was close to 50% in both groups at 12 weeks, Ms. Raab said.
All skin types and a range of ethnicities were represented in the study population. Subjects applied the OTC benzoyl peroxide with lipohydroxy acid or the prescription benzoyl peroxide and clindamycin twice daily for 12 weeks. The tretinoin was applied daily at night after the other products were dried and absorbed. Three blinded board-certified dermatologists assessed the subjects for tolerability, and lesion counts were performed on the entire face to assess for open and closed comedones, papules, and pustules.
The dermatologists also assessed for tone, smoothness, brightness, appearance of pores, global acne, overall skin appearance, and tolerability.
The degree of acne relapse was assessed during a 4-week regression phase at which time the treatment was discontinued.
Both treatments improved inflammatory and noninflammatory lesions to a similar degree within 2 weeks of use. Dryness and peeling were seen with both treatments, but resolved by week 12. Stinging, tingling, itching, and burning also were common with both treatments, and largely resolved in both groups by week 8, except for significant stinging in the OTC treatment group. No significant overall differences were seen between the groups with respect to tolerance parameters, Ms. Raab noted.
“When compared, both treatments were at parity in improvement of all efficacy attributes at all time points,” she and her colleagues concluded.
ORLANDO – An over-the-counter 5.5% benzoyl peroxide preparation with lipohydroxy acid was a safe and effective alternative to prescription 5% benzoyl peroxide with 1% clindamycin in a 12-week randomized, double-blind, multicenter study of patients with mild to moderate acne vulgaris who also were treated with generic 0.025% tretinoin.
Statistically significant improvement on a variety of efficacy endpoints was seen at weeks 8 and 12 in 60 men and women aged 18-50 years who were randomized to either the OTC-based or prescription-based regimen, Susana Raab of L’Oreal Research & Innovation, Clark, N.J., reported in a poster at the Orlando Dermatology Aesthetic and Clinical Conference.
The mean reduction in total acne lesion count at week 8 was approximately 60% with the OTC regimen and 64% in the prescription regimen. At week 12, the mean reduction was about 72% in both groups. The mean improvement in global acne assessment was approximately 31% and 33% in the groups, respectively, at 8 weeks, and about 33% in both groups at 12 weeks.
The mean improvement in overall appearance was 37%-38% in both groups at 8 weeks, and was close to 50% in both groups at 12 weeks, Ms. Raab said.
All skin types and a range of ethnicities were represented in the study population. Subjects applied the OTC benzoyl peroxide with lipohydroxy acid or the prescription benzoyl peroxide and clindamycin twice daily for 12 weeks. The tretinoin was applied daily at night after the other products were dried and absorbed. Three blinded board-certified dermatologists assessed the subjects for tolerability, and lesion counts were performed on the entire face to assess for open and closed comedones, papules, and pustules.
The dermatologists also assessed for tone, smoothness, brightness, appearance of pores, global acne, overall skin appearance, and tolerability.
The degree of acne relapse was assessed during a 4-week regression phase at which time the treatment was discontinued.
Both treatments improved inflammatory and noninflammatory lesions to a similar degree within 2 weeks of use. Dryness and peeling were seen with both treatments, but resolved by week 12. Stinging, tingling, itching, and burning also were common with both treatments, and largely resolved in both groups by week 8, except for significant stinging in the OTC treatment group. No significant overall differences were seen between the groups with respect to tolerance parameters, Ms. Raab noted.
“When compared, both treatments were at parity in improvement of all efficacy attributes at all time points,” she and her colleagues concluded.
Key clinical point: An OTC benzoyl peroxide–based product compared well with a prescription benzoyl peroxide/clindamycin product for acne vulgaris.
Major finding: The mean improvement in overall appearance was 37%-38% in both treatment groups at 8 weeks and was close to 50% in both groups at 12 weeks.
Data source: A randomized, double-blind, multicenter study of 60 adults.
Disclosures: The lead author is employed by L’Oreal Research & Innovation, manufacturer of the OTC product.
Cosmetic Corner: Dermatologists Weigh in on Mineral Makeup
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top mineral makeup products. Consideration must be given to:
- bareMinerals
Bare Escentuals Beauty, Inc
“I recommend bareMinerals everyday to patients with various facial blemishes, acne, and dermatoses. This brand has products for patients of all skin types and colors.”—Gary Goldenberg, MD, New York, New York
Recommended by Elizabeth K. Hale, MD, New York, New York
“Very light and noncomedogenic and does not cake up.”—Anthony M. Rossi, MD, New York, New York
- Jane Iredale
Iredale Mineral Cosmetics, Ltd
“My patients and staff love this brand as a whole. They find the options are terrific for various skin types and the company support is very strong, which is a huge part of the equation. The brush-on sunscreen is terrific.”—Joel Schlessinger, MD, Omaha, Nebraska
- Météorites Powder
Guerlain
“Adds brightness but does not dry the skin.”—Antonella Tosti, MD, Miami, Florida
- Sheer Cover Studio
Guthy-Renker
“It provides very effective coverage without appearing heavy.”—Whitney Bowe, MD, Brooklyn, New York
Cutis invites readers to send us their recommendations. Skin care products for babies, eyelash enhancers, and cleansing pads will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top mineral makeup products. Consideration must be given to:
- bareMinerals
Bare Escentuals Beauty, Inc
“I recommend bareMinerals everyday to patients with various facial blemishes, acne, and dermatoses. This brand has products for patients of all skin types and colors.”—Gary Goldenberg, MD, New York, New York
Recommended by Elizabeth K. Hale, MD, New York, New York
“Very light and noncomedogenic and does not cake up.”—Anthony M. Rossi, MD, New York, New York
- Jane Iredale
Iredale Mineral Cosmetics, Ltd
“My patients and staff love this brand as a whole. They find the options are terrific for various skin types and the company support is very strong, which is a huge part of the equation. The brush-on sunscreen is terrific.”—Joel Schlessinger, MD, Omaha, Nebraska
- Météorites Powder
Guerlain
“Adds brightness but does not dry the skin.”—Antonella Tosti, MD, Miami, Florida
- Sheer Cover Studio
Guthy-Renker
“It provides very effective coverage without appearing heavy.”—Whitney Bowe, MD, Brooklyn, New York
Cutis invites readers to send us their recommendations. Skin care products for babies, eyelash enhancers, and cleansing pads will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top mineral makeup products. Consideration must be given to:
- bareMinerals
Bare Escentuals Beauty, Inc
“I recommend bareMinerals everyday to patients with various facial blemishes, acne, and dermatoses. This brand has products for patients of all skin types and colors.”—Gary Goldenberg, MD, New York, New York
Recommended by Elizabeth K. Hale, MD, New York, New York
“Very light and noncomedogenic and does not cake up.”—Anthony M. Rossi, MD, New York, New York
- Jane Iredale
Iredale Mineral Cosmetics, Ltd
“My patients and staff love this brand as a whole. They find the options are terrific for various skin types and the company support is very strong, which is a huge part of the equation. The brush-on sunscreen is terrific.”—Joel Schlessinger, MD, Omaha, Nebraska
- Météorites Powder
Guerlain
“Adds brightness but does not dry the skin.”—Antonella Tosti, MD, Miami, Florida
- Sheer Cover Studio
Guthy-Renker
“It provides very effective coverage without appearing heavy.”—Whitney Bowe, MD, Brooklyn, New York
Cutis invites readers to send us their recommendations. Skin care products for babies, eyelash enhancers, and cleansing pads will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
Inflammatory Acne: New Developments in Pathogenesis and Treatment
Acne vulgaris is a chronic inflammatory disease that affects the majority of the population at some point in their lifetime. It is characterized by comedones, pustules, and papules. Acne pathogenesis is multifactorial with 4 primary factors that play a pivotal role in the formation of acne lesions: excess sebum production, abnormal keratinization, inflammation, and bacterial colonization of Propionibacterium acnes in the pilosebaceous unit.1 Although there is a general consensus on the pathogenic factors, the sequence of events in acne development is controversial. Traditionally it was believed that abnormal keratinization resulted in the creation of the microcomedone, the earliest subclinical acne lesion.2 Activation of sebaceous glands by androgens, excess sebum production, and keratin plug formation then were followed by P acnes colonization, with induction of the innate immune system culminating in inflammation.2 Androgen-induced sebum production and follicular hyperkeratinization and plugging have been cited as initial events that alter the pilosebaceous milieu, favoring the proliferation of P acnes1,3; however, evidence suggests inflammation as the inciting factor, with proof of significant inflammatory factors surrounding the pilosebaceous unit even in clinically uninvolved skin units in acne patients.4 Herein we will briefly review the most recent data and translational applications pertaining to the P acnes–triggered innate immune response via activation of toll-like receptor 2 (TLR2)5 and importantly the inflammasome.6,7
A new understanding of how P acnes induces the inflammatory cascade may represent a paradigm shift in the management of acne. Recognition of microbes, namely P acnes, by the innate immune system is the body’s first line of defense against pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs).6 Although these pathways combat infection and prevent foreign invasion, they also result in inflammation and tissue injury. The inflammatory response to PAMPs and DAMPs is mediated by the inflammasome, a caspase 1–activating cytoplasmic complex that induces the secretion of crucial proinflammatory cytokines.7 The exact mechanism by which P acnes exerts its proinflammatory activity has been somewhat unclear, though P acnes–induced inflammation has been shown to be mediated by proinflammatory cytokines tumor necrosis factor α, IL-1, IL-6, IL-8, and IL-12.5,8 However, remarkable evidence recently was presented regarding triggers of inflammation and the precise mechanism involved. Qin et al9 showed that P acnes is a potent trigger of IL-1β generation via activation of the NLRP3 (NOD-like receptor family, pyrin domain containing 3) inflammasome. Specifically, the study showed that human monocytes respond to P acnes by upregulating caspase 1, an inflammatory caspase required for proteolytic cleavage of IL-1β. The authors correlated their in vitro findings with clinical evidence of caspase 1 and NLRP3 expression in the dermis surrounding the pilosebaceous units of biopsied lesions.9 Kistowska et al6 confirmed and expanded on these data by showing the inability of NLRP3-deficient myeloid cells to secrete IL-1β and induce an inflammatory response in vivo. A recent investigation demonstrated that human sebocytes can function as constituents of the innate immune response, with P acnes triggering sebocyte NLRP3-inflammasome activation and subsequent IL-1β secretion. These observations were further confirmed in vivo with NLRP3-deficient mice displaying an impaired inflammatory response to P acnes.10
Our understanding of TLR2 signaling in the pathogenesis of acne also has expanded. It is well established that recognition of extracellular PAMPs and DAMPs is mediated by the expression of toll-like receptors on the surface of a variety of cells within the skin.11 Prior research demonstrated how P acnes increases TLR2 expression in keratinocytes, even in vivo.12 Stimulation by P acnes was shown to induce secretion of IL-8 (promoting a TH1 response) and IL-12 (promoting neutrophil chemotaxis) via TLR2 activation.5 Selway et al11 validated this finding by demonstrating that infundibular keratinocytes secrete IL-1α in response to the peptidoglycan cell wall of P acnes. Interestingly, Qin et al9 determined TLR2 inhibition resulted in partial suppression of IL-1β, possibly providing new evidence of TLR2-mediated activation of the NLRP3 inflammasome. Therefore, P acnes activates both extracellular and intracellular triggers of the innate immune response: TLR2 activation (requiring extracellular recognition of pathogens) and inflammasome-mediated activation (requiring internalization and access of the bacterium to the interior compartments of the cells).
Overall, these findings suggest that P acnes–induced inflammation can be selectively targeted by agents directed at inflammasome components, IL-1β, or toll-like receptors. A phase 2 double-blind, placebo-controlled trial assessing the efficacy of the anti–IL-1β monoclonal antibody gevokizumab found that 0.6 mg/kg administered subcutaneously resulted in a significant reduction in mean inflammatory lesion count compared to placebo (P=.077).13 The success of the IL-1 receptor antagonist anakinra against rare genetic autoinflammatory syndromes such as PAPA (pyogenic sterile arthritis, pyoderma gangrenosum, and acne) syndrome, an NLRP3 inflammasomopathy, sheds light onto new therapeutics that may be used to target acne vulgaris.14 Current topical therapies such as retinoids, which have already proven efficacious in the treatment of inflammatory acne, target these pathways. In vivo data revealed that treatment with isotretinoin significantly decreased TLR2 expression in monocytes (P<.001) and suppressed inflammatory cytokine responses to P acnes (P<.001).15 Adapalene, with or without benzoyl peroxide, also was shown to exert anti-inflammatory effects via TLR2 downregulation.16
These data and observations highlight a paradigm shift in our perception of acne. All acne is truly inflammatory, and by identifying aberrations in the immune response, we can develop targeted treatments for this chronic debilitating disease.
1. Kurokawa I, Danby FW, Ju Q, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821-832.
2. Cunliffe WJ, Holland DB, Clark SM, et al. Comedogenesis: some aetiological, clinical and therapeutic strategies. Dermatology. 2003;206:11-16.
3. Bowe W, Kober M. Therapeutic update: acne. J Drugs Dermatol. 2014;13:235-238.
4. Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20-27.
5. Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
6. Kistowska M, Gehrke S, Jankovic D, et al. IL-1beta drives inflammatory responses to Propionibacterium acnes in vitro and in vivo. J Invest Dermatol. 2014;134:677-685.
7. Contassot E, French LE. New insights into acne pathogenesis: Propionibacterium acnes activates the inflammasome. J Invest Dermatol. 2014;134:310-313.
8. Vowels BR, Yang S, Leyden JJ. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: implications for chronic inflammatory acne. Infect Immun. 1995;63:3158-3165.
9. Qin M, Pirouz A, Kim MH, et al. Propionibacterium acnes induces IL-1beta secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol. 2014;134:381-388.
10. Li ZJ, Choi DK, Sohn KC, et al. Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. J Invest Dermatol. 2014;134:2747-2756.
11. Selway JL, Kurczab T, Kealey T, et al. Toll-like receptor 2 activation and comedogenesis: implications for the pathogenesis of acne. BMC Dermatol. 2013;13:10.
12. Jugeau S, Tenaud I, Knol AC, et al. Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol. 2005;153:1105-1113.
13. XOMA announces encouraging interim results from gevokizumab phase 2 study for moderate to severe acne vulgaris [press release]. Berkley, CA: XOMA Corporation; January 7, 2013. http://www.servier.com/content/xoma-announces-encouraging-interim-results-gevokizumab-phase-2-study-moderate-severe-acne. Accessed November 5, 2014.
14. Leemans JC, Cassel SL, Sutterwala FS. Sensing damage by the NLRP3 inflammasome. Immunol Rev. 2011;243:152-162.
15. Dispenza MC, Wolpert EB, Gilliland KL, et al. Systemic isotretinoin therapy normalizes exaggerated TLR-2-mediated innate immune responses in acne patients. J Invest Dermatol. 2012;132:2198-2205.
16. Zuliani T, Khammari A, Chaussy H, et al. Ex vivo demonstration of a synergistic effect of adapalene and benzoyl peroxide on inflammatory acne lesions. Exp Dermatol. 2011;20:850-853.
Acne vulgaris is a chronic inflammatory disease that affects the majority of the population at some point in their lifetime. It is characterized by comedones, pustules, and papules. Acne pathogenesis is multifactorial with 4 primary factors that play a pivotal role in the formation of acne lesions: excess sebum production, abnormal keratinization, inflammation, and bacterial colonization of Propionibacterium acnes in the pilosebaceous unit.1 Although there is a general consensus on the pathogenic factors, the sequence of events in acne development is controversial. Traditionally it was believed that abnormal keratinization resulted in the creation of the microcomedone, the earliest subclinical acne lesion.2 Activation of sebaceous glands by androgens, excess sebum production, and keratin plug formation then were followed by P acnes colonization, with induction of the innate immune system culminating in inflammation.2 Androgen-induced sebum production and follicular hyperkeratinization and plugging have been cited as initial events that alter the pilosebaceous milieu, favoring the proliferation of P acnes1,3; however, evidence suggests inflammation as the inciting factor, with proof of significant inflammatory factors surrounding the pilosebaceous unit even in clinically uninvolved skin units in acne patients.4 Herein we will briefly review the most recent data and translational applications pertaining to the P acnes–triggered innate immune response via activation of toll-like receptor 2 (TLR2)5 and importantly the inflammasome.6,7
A new understanding of how P acnes induces the inflammatory cascade may represent a paradigm shift in the management of acne. Recognition of microbes, namely P acnes, by the innate immune system is the body’s first line of defense against pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs).6 Although these pathways combat infection and prevent foreign invasion, they also result in inflammation and tissue injury. The inflammatory response to PAMPs and DAMPs is mediated by the inflammasome, a caspase 1–activating cytoplasmic complex that induces the secretion of crucial proinflammatory cytokines.7 The exact mechanism by which P acnes exerts its proinflammatory activity has been somewhat unclear, though P acnes–induced inflammation has been shown to be mediated by proinflammatory cytokines tumor necrosis factor α, IL-1, IL-6, IL-8, and IL-12.5,8 However, remarkable evidence recently was presented regarding triggers of inflammation and the precise mechanism involved. Qin et al9 showed that P acnes is a potent trigger of IL-1β generation via activation of the NLRP3 (NOD-like receptor family, pyrin domain containing 3) inflammasome. Specifically, the study showed that human monocytes respond to P acnes by upregulating caspase 1, an inflammatory caspase required for proteolytic cleavage of IL-1β. The authors correlated their in vitro findings with clinical evidence of caspase 1 and NLRP3 expression in the dermis surrounding the pilosebaceous units of biopsied lesions.9 Kistowska et al6 confirmed and expanded on these data by showing the inability of NLRP3-deficient myeloid cells to secrete IL-1β and induce an inflammatory response in vivo. A recent investigation demonstrated that human sebocytes can function as constituents of the innate immune response, with P acnes triggering sebocyte NLRP3-inflammasome activation and subsequent IL-1β secretion. These observations were further confirmed in vivo with NLRP3-deficient mice displaying an impaired inflammatory response to P acnes.10
Our understanding of TLR2 signaling in the pathogenesis of acne also has expanded. It is well established that recognition of extracellular PAMPs and DAMPs is mediated by the expression of toll-like receptors on the surface of a variety of cells within the skin.11 Prior research demonstrated how P acnes increases TLR2 expression in keratinocytes, even in vivo.12 Stimulation by P acnes was shown to induce secretion of IL-8 (promoting a TH1 response) and IL-12 (promoting neutrophil chemotaxis) via TLR2 activation.5 Selway et al11 validated this finding by demonstrating that infundibular keratinocytes secrete IL-1α in response to the peptidoglycan cell wall of P acnes. Interestingly, Qin et al9 determined TLR2 inhibition resulted in partial suppression of IL-1β, possibly providing new evidence of TLR2-mediated activation of the NLRP3 inflammasome. Therefore, P acnes activates both extracellular and intracellular triggers of the innate immune response: TLR2 activation (requiring extracellular recognition of pathogens) and inflammasome-mediated activation (requiring internalization and access of the bacterium to the interior compartments of the cells).
Overall, these findings suggest that P acnes–induced inflammation can be selectively targeted by agents directed at inflammasome components, IL-1β, or toll-like receptors. A phase 2 double-blind, placebo-controlled trial assessing the efficacy of the anti–IL-1β monoclonal antibody gevokizumab found that 0.6 mg/kg administered subcutaneously resulted in a significant reduction in mean inflammatory lesion count compared to placebo (P=.077).13 The success of the IL-1 receptor antagonist anakinra against rare genetic autoinflammatory syndromes such as PAPA (pyogenic sterile arthritis, pyoderma gangrenosum, and acne) syndrome, an NLRP3 inflammasomopathy, sheds light onto new therapeutics that may be used to target acne vulgaris.14 Current topical therapies such as retinoids, which have already proven efficacious in the treatment of inflammatory acne, target these pathways. In vivo data revealed that treatment with isotretinoin significantly decreased TLR2 expression in monocytes (P<.001) and suppressed inflammatory cytokine responses to P acnes (P<.001).15 Adapalene, with or without benzoyl peroxide, also was shown to exert anti-inflammatory effects via TLR2 downregulation.16
These data and observations highlight a paradigm shift in our perception of acne. All acne is truly inflammatory, and by identifying aberrations in the immune response, we can develop targeted treatments for this chronic debilitating disease.
Acne vulgaris is a chronic inflammatory disease that affects the majority of the population at some point in their lifetime. It is characterized by comedones, pustules, and papules. Acne pathogenesis is multifactorial with 4 primary factors that play a pivotal role in the formation of acne lesions: excess sebum production, abnormal keratinization, inflammation, and bacterial colonization of Propionibacterium acnes in the pilosebaceous unit.1 Although there is a general consensus on the pathogenic factors, the sequence of events in acne development is controversial. Traditionally it was believed that abnormal keratinization resulted in the creation of the microcomedone, the earliest subclinical acne lesion.2 Activation of sebaceous glands by androgens, excess sebum production, and keratin plug formation then were followed by P acnes colonization, with induction of the innate immune system culminating in inflammation.2 Androgen-induced sebum production and follicular hyperkeratinization and plugging have been cited as initial events that alter the pilosebaceous milieu, favoring the proliferation of P acnes1,3; however, evidence suggests inflammation as the inciting factor, with proof of significant inflammatory factors surrounding the pilosebaceous unit even in clinically uninvolved skin units in acne patients.4 Herein we will briefly review the most recent data and translational applications pertaining to the P acnes–triggered innate immune response via activation of toll-like receptor 2 (TLR2)5 and importantly the inflammasome.6,7
A new understanding of how P acnes induces the inflammatory cascade may represent a paradigm shift in the management of acne. Recognition of microbes, namely P acnes, by the innate immune system is the body’s first line of defense against pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs).6 Although these pathways combat infection and prevent foreign invasion, they also result in inflammation and tissue injury. The inflammatory response to PAMPs and DAMPs is mediated by the inflammasome, a caspase 1–activating cytoplasmic complex that induces the secretion of crucial proinflammatory cytokines.7 The exact mechanism by which P acnes exerts its proinflammatory activity has been somewhat unclear, though P acnes–induced inflammation has been shown to be mediated by proinflammatory cytokines tumor necrosis factor α, IL-1, IL-6, IL-8, and IL-12.5,8 However, remarkable evidence recently was presented regarding triggers of inflammation and the precise mechanism involved. Qin et al9 showed that P acnes is a potent trigger of IL-1β generation via activation of the NLRP3 (NOD-like receptor family, pyrin domain containing 3) inflammasome. Specifically, the study showed that human monocytes respond to P acnes by upregulating caspase 1, an inflammatory caspase required for proteolytic cleavage of IL-1β. The authors correlated their in vitro findings with clinical evidence of caspase 1 and NLRP3 expression in the dermis surrounding the pilosebaceous units of biopsied lesions.9 Kistowska et al6 confirmed and expanded on these data by showing the inability of NLRP3-deficient myeloid cells to secrete IL-1β and induce an inflammatory response in vivo. A recent investigation demonstrated that human sebocytes can function as constituents of the innate immune response, with P acnes triggering sebocyte NLRP3-inflammasome activation and subsequent IL-1β secretion. These observations were further confirmed in vivo with NLRP3-deficient mice displaying an impaired inflammatory response to P acnes.10
Our understanding of TLR2 signaling in the pathogenesis of acne also has expanded. It is well established that recognition of extracellular PAMPs and DAMPs is mediated by the expression of toll-like receptors on the surface of a variety of cells within the skin.11 Prior research demonstrated how P acnes increases TLR2 expression in keratinocytes, even in vivo.12 Stimulation by P acnes was shown to induce secretion of IL-8 (promoting a TH1 response) and IL-12 (promoting neutrophil chemotaxis) via TLR2 activation.5 Selway et al11 validated this finding by demonstrating that infundibular keratinocytes secrete IL-1α in response to the peptidoglycan cell wall of P acnes. Interestingly, Qin et al9 determined TLR2 inhibition resulted in partial suppression of IL-1β, possibly providing new evidence of TLR2-mediated activation of the NLRP3 inflammasome. Therefore, P acnes activates both extracellular and intracellular triggers of the innate immune response: TLR2 activation (requiring extracellular recognition of pathogens) and inflammasome-mediated activation (requiring internalization and access of the bacterium to the interior compartments of the cells).
Overall, these findings suggest that P acnes–induced inflammation can be selectively targeted by agents directed at inflammasome components, IL-1β, or toll-like receptors. A phase 2 double-blind, placebo-controlled trial assessing the efficacy of the anti–IL-1β monoclonal antibody gevokizumab found that 0.6 mg/kg administered subcutaneously resulted in a significant reduction in mean inflammatory lesion count compared to placebo (P=.077).13 The success of the IL-1 receptor antagonist anakinra against rare genetic autoinflammatory syndromes such as PAPA (pyogenic sterile arthritis, pyoderma gangrenosum, and acne) syndrome, an NLRP3 inflammasomopathy, sheds light onto new therapeutics that may be used to target acne vulgaris.14 Current topical therapies such as retinoids, which have already proven efficacious in the treatment of inflammatory acne, target these pathways. In vivo data revealed that treatment with isotretinoin significantly decreased TLR2 expression in monocytes (P<.001) and suppressed inflammatory cytokine responses to P acnes (P<.001).15 Adapalene, with or without benzoyl peroxide, also was shown to exert anti-inflammatory effects via TLR2 downregulation.16
These data and observations highlight a paradigm shift in our perception of acne. All acne is truly inflammatory, and by identifying aberrations in the immune response, we can develop targeted treatments for this chronic debilitating disease.
1. Kurokawa I, Danby FW, Ju Q, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821-832.
2. Cunliffe WJ, Holland DB, Clark SM, et al. Comedogenesis: some aetiological, clinical and therapeutic strategies. Dermatology. 2003;206:11-16.
3. Bowe W, Kober M. Therapeutic update: acne. J Drugs Dermatol. 2014;13:235-238.
4. Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20-27.
5. Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
6. Kistowska M, Gehrke S, Jankovic D, et al. IL-1beta drives inflammatory responses to Propionibacterium acnes in vitro and in vivo. J Invest Dermatol. 2014;134:677-685.
7. Contassot E, French LE. New insights into acne pathogenesis: Propionibacterium acnes activates the inflammasome. J Invest Dermatol. 2014;134:310-313.
8. Vowels BR, Yang S, Leyden JJ. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: implications for chronic inflammatory acne. Infect Immun. 1995;63:3158-3165.
9. Qin M, Pirouz A, Kim MH, et al. Propionibacterium acnes induces IL-1beta secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol. 2014;134:381-388.
10. Li ZJ, Choi DK, Sohn KC, et al. Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. J Invest Dermatol. 2014;134:2747-2756.
11. Selway JL, Kurczab T, Kealey T, et al. Toll-like receptor 2 activation and comedogenesis: implications for the pathogenesis of acne. BMC Dermatol. 2013;13:10.
12. Jugeau S, Tenaud I, Knol AC, et al. Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol. 2005;153:1105-1113.
13. XOMA announces encouraging interim results from gevokizumab phase 2 study for moderate to severe acne vulgaris [press release]. Berkley, CA: XOMA Corporation; January 7, 2013. http://www.servier.com/content/xoma-announces-encouraging-interim-results-gevokizumab-phase-2-study-moderate-severe-acne. Accessed November 5, 2014.
14. Leemans JC, Cassel SL, Sutterwala FS. Sensing damage by the NLRP3 inflammasome. Immunol Rev. 2011;243:152-162.
15. Dispenza MC, Wolpert EB, Gilliland KL, et al. Systemic isotretinoin therapy normalizes exaggerated TLR-2-mediated innate immune responses in acne patients. J Invest Dermatol. 2012;132:2198-2205.
16. Zuliani T, Khammari A, Chaussy H, et al. Ex vivo demonstration of a synergistic effect of adapalene and benzoyl peroxide on inflammatory acne lesions. Exp Dermatol. 2011;20:850-853.
1. Kurokawa I, Danby FW, Ju Q, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821-832.
2. Cunliffe WJ, Holland DB, Clark SM, et al. Comedogenesis: some aetiological, clinical and therapeutic strategies. Dermatology. 2003;206:11-16.
3. Bowe W, Kober M. Therapeutic update: acne. J Drugs Dermatol. 2014;13:235-238.
4. Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20-27.
5. Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
6. Kistowska M, Gehrke S, Jankovic D, et al. IL-1beta drives inflammatory responses to Propionibacterium acnes in vitro and in vivo. J Invest Dermatol. 2014;134:677-685.
7. Contassot E, French LE. New insights into acne pathogenesis: Propionibacterium acnes activates the inflammasome. J Invest Dermatol. 2014;134:310-313.
8. Vowels BR, Yang S, Leyden JJ. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: implications for chronic inflammatory acne. Infect Immun. 1995;63:3158-3165.
9. Qin M, Pirouz A, Kim MH, et al. Propionibacterium acnes induces IL-1beta secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol. 2014;134:381-388.
10. Li ZJ, Choi DK, Sohn KC, et al. Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. J Invest Dermatol. 2014;134:2747-2756.
11. Selway JL, Kurczab T, Kealey T, et al. Toll-like receptor 2 activation and comedogenesis: implications for the pathogenesis of acne. BMC Dermatol. 2013;13:10.
12. Jugeau S, Tenaud I, Knol AC, et al. Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol. 2005;153:1105-1113.
13. XOMA announces encouraging interim results from gevokizumab phase 2 study for moderate to severe acne vulgaris [press release]. Berkley, CA: XOMA Corporation; January 7, 2013. http://www.servier.com/content/xoma-announces-encouraging-interim-results-gevokizumab-phase-2-study-moderate-severe-acne. Accessed November 5, 2014.
14. Leemans JC, Cassel SL, Sutterwala FS. Sensing damage by the NLRP3 inflammasome. Immunol Rev. 2011;243:152-162.
15. Dispenza MC, Wolpert EB, Gilliland KL, et al. Systemic isotretinoin therapy normalizes exaggerated TLR-2-mediated innate immune responses in acne patients. J Invest Dermatol. 2012;132:2198-2205.
16. Zuliani T, Khammari A, Chaussy H, et al. Ex vivo demonstration of a synergistic effect of adapalene and benzoyl peroxide on inflammatory acne lesions. Exp Dermatol. 2011;20:850-853.
Most Common Dermatologic Conditions Encountered by Dermatologists and Nondermatologists
Skin diseases are highly prevalent in the United States, affecting an estimated 1 in 3 Americans at any given time.1,2 In 2009 the direct medical costs associated with skin-related diseases, including health services and prescriptions, was approximately $22 billion; the annual total economic burden was estimated to be closer to $96 billion when factoring in the cost of lost productivity and pay for symptom relief.3,4 Effective and efficient management of skin disease is essential to minimizing cost and morbidity. Nondermatologists traditionally have diagnosed the majority of skin diseases.5,6 In particular, primary care physicians commonly manage dermatologic conditions and often are the first health care providers to encounter patients presenting with skin problems. A predicted shortage of dermatologists will likely contribute to an increase in this trend.7,8 Therefore, it is important to adequately prepare nondermatologists to evaluate and treat the skin conditions that they are most likely to encounter in their scope of practice.
Residents, particularly in primary care specialties, often have opportunities to spend 2 to 4 weeks with a dermatologist to learn about skin diseases; however, the skin conditions most often encountered by dermatologists may differ from those most often encountered by physicians in other specialties. For instance, one study demonstrated a disparity between the most common skin problems seen by dermatologists and internists.9 These dissimilarities should be recognized and addressed in curriculum content. The purpose of this study was to identify and compare the 20 most common dermatologic conditions reported by dermatologists versus those reported by nondermatologists (ie, internists, pediatricians, family physicians, emergency medicine physicians, general surgeons, otolaryngologists) from 2001 to 2010. Data also were analyzed to determine the top 20 conditions referred to dermatologists by nondermatologists as a potential indicator for areas of further improvement within medical education. With this knowledge, we hope educational curricula and self-study can be modified to reflect the current epidemiology of cutaneous diseases, thereby improving patient care.
Methods
Data from 2001 to 2010 were extracted from the National Ambulatory Medical Care Survey (NAMCS), which is an ongoing survey conducted by the National Center for Health Statistics. The NAMCS collects descriptive data regarding ambulatory visits to nonfederal office-based physicians in the United States. Participating physicians are instructed to record information about patient visits for a 1-week period, including patient demographics, insurance status, reason for visit, diagnoses, procedures, therapeutics, and referrals made at that time. Data collected for the NAMCS are entered into a multistage probability sample to produce national estimates. Within dermatology, an average of 118 dermatologists are sampled each year, and over the last 10 years, participation rates have ranged from 47% to 77%.
International Classification of Diseases, Ninth Revision, Clinical Modification codes were identified to determine the diagnoses that could be classified as dermatologic conditions. Select infectious and neoplastic disorders of the skin and mucous membrane conditions were included as well as the codes for skin diseases. Nondermatologic diagnoses and V codes were not included in the study. Data for all providers were studied to identify outpatient visits associated with the primary diagnosis of a dermatologic condition. Minor diagnoses that were considered to be subsets of major diagnoses were combined to allow better analysis of the data. For example, all tinea infections (ie, dermatophytosis of various sites, dermatomycosis unspecified) were combined into 1 diagnosis referred to as tinea because the recognition and treatment of this disease does not vary tremendously by anatomic location. Visits to dermatologists that listed nonspecific diagnoses and codes (eg, other postsurgical status [V45.89], neoplasm of uncertain behavior site unspecified [238.9]) were assumed to be for dermatologic problems.
Sampling weights were applied to obtain estimates for the number of each diagnosis made nationally. All data analyses were performed using SAS software and linear regression models were generated using SAS PROC SURVEYREG.
Data were analyzed to determine the dermatologic conditions most commonly encountered by dermatologists and nondermatologists in emergency medicine, family medicine, general surgery, internal medicine, otolaryngology, and pediatrics; these specialties include physicians who are known to commonly diagnose and treat skin diseases.10 Data also were analyzed to determine the most common conditions referred to dermatologists for treatment by nondermatologists from the selected specialties. Permission to conduct this study was obtained from the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Results
From 2001 to 2010, more than 700 million outpatient visits for skin-related problems were identified, with 676.3 million visits to dermatologists, emergency medicine physicians, family practitioners, general surgeons, internists, otolaryngologists, and pediatricians. More than half (52.9%) of all skin-related visits were addressed by nondermatologists during this time. Among nondermatologists, family practitioners encountered the greatest number of skin diseases (20.5%), followed by pediatricians (11.3%), internists (9.2%), general surgeons (3.4%), otolaryngologists (1.0%), and emergency medicine physicians (0.2%)(Table 1).
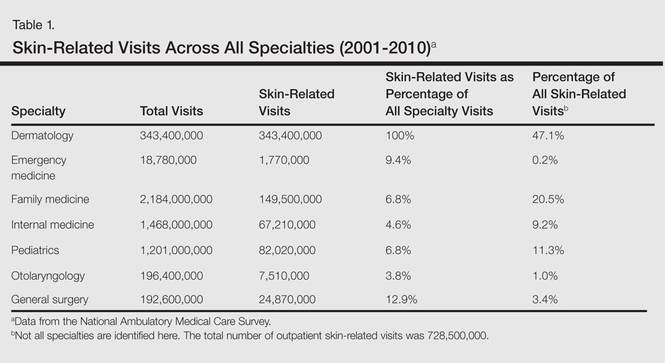
Benign tumors and acne were the most common cutaneous conditions referred to dermatologists by nondermatologists (10.6% and 10.1% of all dermatology referrals, respectively), followed by nonmelanoma skin cancers (9.7%), contact dermatitis (8.8%), and actinic keratosis (7.8%)(Table 2). The top 20 conditions referred to dermatologists accounted for 83.7% of all outpatient referrals to dermatologists.
Among the diseases most frequently reported by nondermatologists, contact dermatitis was the most common (12.0%), with twice the number of visits to nondermatologists for contact dermatitis than to dermatologists (51.6 million vs 25.3 million). In terms of disease categories, infectious skin diseases (ie, bacterial [cellulitis/abscess], viral [warts, herpesvirus], fungal [tinea] and yeast [candida] etiologies) were the most common dermatologic conditions reported by nondermatologists (Table 2).
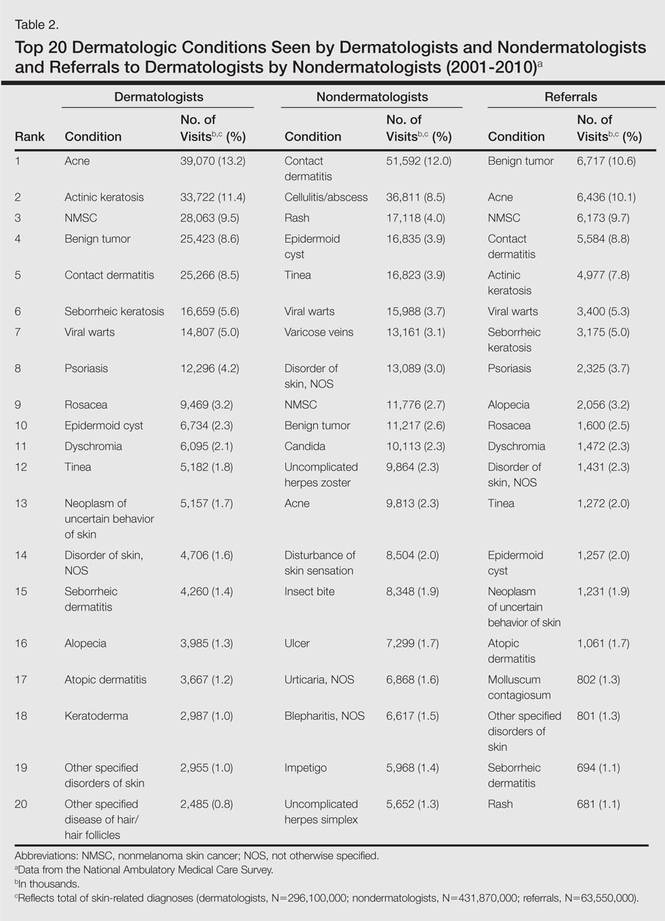
The top 20 dermatologic conditions reported by dermatologists accounted for 85.4% of all diagnoses made by dermatologists. Diseases that were among the top 20 conditions encountered by dermatologists but were not among the top 20 for nondermatologists included actinic keratosis, seborrheic keratosis, atopic dermatitis, psoriasis, alopecia, rosacea, dyschromia, seborrheic dermatitis, follicular disease, and neoplasm of uncertain behavior of skin. Additionally, 5 of the top 20 conditions encountered by dermatologists also were among the top 20 for only 1 individual nondermatologic specialty; these included atopic dermatitis (pediatrics), seborrheic dermatitis (pediatrics), psoriasis (internal medicine), rosacea (otolaryngology), and keratoderma (general surgery). Seborrheic dermatitis, psoriasis, and rosacea also were among the top 20 conditions most commonly referred to dermatologists for treatment by nondermatologists. Table 3 shows the top 20 dermatologic conditions encountered by nondermatologists by comparison.
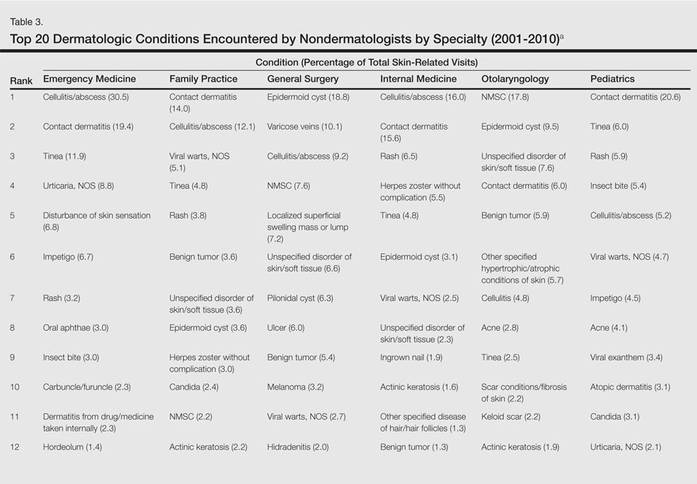
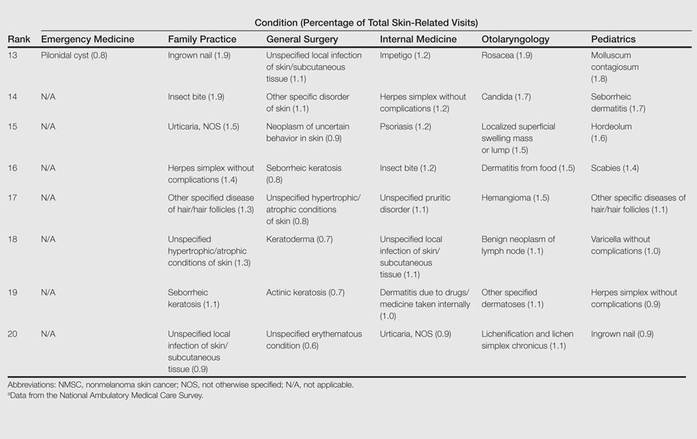
Comment
According to NAMCS data from 2001 to 2010, visits to nondermatologists accounted for more than half of total outpatient visits for cutaneous diseases in the United States, whereas visits to dermatologists accounted for 47.1%. These findings are consistent with historical data indicating that 30% to 40% of skin-related visits are to dermatologists, and the majority of patients with skin disease are diagnosed by nondermatologists.5,6
Past data indicate that most visits to dermatologists were for evaluation of acne, infections, psoriasis, and neoplasms, whereas most visits to nondermatologists were for evaluation of epidermoid cysts, impetigo, plant dermatitis, cellulitis, and diaper rash.9 Over the last 10 years, acne has been more commonly encountered by nondermatologists, especially pediatricians. Additionally, infectious etiologies have been seen in larger volume by nondermatologists.9 Together, infectious cutaneous conditions make up nearly one-fourth of dermatologic encounters by emergency medicine physicians, internists, and family practitioners but are not within the top 20 diagnoses referred to dermatologists, which suggests that uncomplicated cases of cellulitis, herpes zoster, and other skin-related infections are largely managed by nondermatologists.5,6 Contact dermatitis, often caused by specific allergens such as detergents, solvents, and topical products, was one of the most common reported dermatologic encounters among dermatologists and nondermatologists and also was the fourth most common condition referred to dermatologists by nondermatologists for treatment; however, there may be an element of overuse of the International Classification of Diseases, Ninth Revision code, as any presumed contact dermatitis of unspecified cause can be reported under 692.9 defined as contact dermatitis and other eczema, unspecified cause. The high rate of referrals to dermatologists by nondermatologists may be for patch testing and further management. Additionally, there are no specific codes for allergic or irritant dermatitis, thus these diseases may be lumped together.
Although nearly half of all dermatologic encounters were seen by nondermatologists, dermatologists see a much larger proportion of patients with skin disease than nondermatologists and nondermatologists often have limited exposure to the field of dermatology during residency training. Studies have demonstrated differences in the abilities of dermatologists and nondermatologists to correctly diagnose common cutaneous diseases, which unsurprisingly revealed greater diagnostic accuracy demonstrated by dermatologists.11-16 The increase in acne and skin-related infections reported by nondermatologists is consistent with possible efforts to increase formal training in frequently encountered skin diseases. In one study evaluating the impact of a formal 3-week dermatology curriculum on an internal medicine department, internists demonstrated 100% accuracy in the diagnosis of acne and herpes zoster in contrast to 29% for tinea and 12% for lichen planus.5,6
The current Accreditation Council for Graduate Medical Education guidelines place little emphasis on exposure to dermatology training during residency for internists and pediatricians, as this training is not a required component of these programs.17 Two core problems with current training regarding the evaluation and management of cutaneous disease are minimal exposure to dermatologic conditions in medical school and residency and lack of consensus on the core topics that should be taught to nondermatologists.18 Exposure to dermatologic conditions through rotations in medical school has been shown to increase residents’ self-reported confidence in diagnosing and treating alopecia, cutaneous drug eruptions, warts, acne, rosacea, nonmelanoma skin cancers, sun damage, psoriasis, seborrhea, atopic dermatitis, and contact dermatitis; however, the majority of primary care residents surveyed still felt that this exposure in medical school was inadequate.19
In creating a core curriculum for dermatology training for nondermatologists, it is important to consider the dermatologic conditions that are most frequently encountered by these specialties. Our study revealed that the most commonly encountered dermatologic conditions differ among dermatologists and nondermatologists, with a fair degree of variation even among individual specialties. Failure to recognize these discrepancies has likely contributed to the challenges faced by nondermatologists in the diagnosis and management of dermatologic disease. In this study, contact dermatitis, epidermoid cysts, and skin infections were the most common dermatologic conditions encountered by nondermatologists and also were among the top skin diseases referred to dermatologists by nondermatologists. This finding suggests that nondermatologists are able to identify these conditions but have a tendency to refer approximately 10% of these patients to dermatology for further management. Clinical evaluation and medical management of these cutaneous diseases may be an important area of focus for medical school curricula, as the treatment of these diseases is within the capabilities of the nondermatologist. For example, initial management of dermatitis requires determination of the type of dermatitis (ie, essential, contact, atopic, seborrheic, stasis) and selection of an appropriate topical steroid, with referral to a dermatologist needed for questionable or refractory cases. Although a curriculum cannot be built solely on a list of the top 20 diagnoses provided here, these data may serve as a preliminary platform for medical school dermatology curriculum design. The curriculum also should include serious skin diseases, such as melanoma and severe drug eruptions. Although these conditions are less commonly encountered by nondermatologists, missed diagnosis and/or improper management can be life threatening.
The use of NAMCS data presents a few limitations. For instance, these data only represent outpatient management of skin disease. There is the potential for misdiagnosis and coding errors by the reporting physicians. The volume of data (ie, billions of office visits) prevents verification of diagnostic accuracy. The coding system requires physicians to give a diagnosis but does not provide any means by which to determine the physician’s confidence in that diagnosis. There is no code for “uncertain” or “diagnosis not determined.” Additionally, an “unspecified” diagnosis may reflect uncertainty or may simply imply that no other code accurately described the condition. Despite these limitations, the NAMCS database is a large, nationally representative survey of actual patient visits and represents some of the best data available for a study such as ours.
Conclusion
This study provides an important analysis of the most common outpatient dermatologic conditions encountered by dermatologists and nondermatologists of various specialties and offers a foundation from which to construct curricula for dermatology training tailored to individual specialties based on their needs. In the future, identification of the most common inpatient dermatologic conditions managed by each specialty also may benefit curriculum design.
- Thorpe KE, Florence CS, Joski P. Which medical conditions account for the rise in health care spending? Health Aff (Millwood). 2004;(suppl web exclusives):W4-437-445.
- Johnson ML. Defining the burden of skin disease in the United States—a historical perspective. J Investig Dermatol Symp Proc. 2004;9:108-110.
- Agency for Healthcare Research and Quality. Medical expenditure panel survey. US Department of Health & Human Services Web site. http://meps.ahrq.gov. Accessed November 17, 2014.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Johnson ML. On teaching dermatology to nondermatologists. Arch Dermatol. 1994;130:850-852.
- Ramsay DL, Weary PE. Primary care in dermatology: whose role should it be? J Am Acad Dermatol. 1996;35:1005-1008.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Kimball AB. Who else is providing care in dermatology practices? trends in the use of nonphysician clinicians. J Am Acad Dermatol. 2008;58:211-216.
- Feldman SR, Fleischer AB Jr, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730.
- Ahn CS, Davis SA, Debade TS, et al. Noncosmetic skin-related procedures performed in the United States: an analysis of national ambulatory medical care survey data from 1995 to 2010. Dermatol Surg. 2013;39:1912-1921.
- Antic M, Conen D, Itin PH. Teaching effects of dermatological consultations on nondermatologists in the field of internal medicine. a study of 1290 inpatients. Dermatology. 2004;208:32-37.
- Federman DG, Concato J, Kirsner RS. Comparison of dermatologic diagnoses by primary care practitioners and dermatologists. a review of the literature. Arch Fam Med. 1999;8:170-172.
- Fleischer AB Jr, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by nondermatologists. Am J Manag Care. 2000;6:1149-1156.
- Kirsner RS, Federman DG. Lack of correlation between internists’ ability in dermatology and their patterns of treating patients with skin disease. Arch Dermatol. 1996;132:1043-1046.
- McCarthy GM, Lamb GC, Russell TJ, et al. Primary care-based dermatology practice: internists need more training. J Gen Intern Med. 1991;6:52-56.
- Sellheyer K, Bergfeld WF. A retrospective biopsy study of the clinical diagnostic accuracy of common skin diseases by different specialties compared with dermatology. J Am Acad Dermatol. 2005;52:823-830.
- Medical specialties. Accreditation Council for Graduate Medical Education Web site. http://www.acgme.org/acgmeweb/tabid/368ProgramandInstitutionalGuidelines/MedicalAccreditation.aspx. Accessed November 17, 2014.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
Skin diseases are highly prevalent in the United States, affecting an estimated 1 in 3 Americans at any given time.1,2 In 2009 the direct medical costs associated with skin-related diseases, including health services and prescriptions, was approximately $22 billion; the annual total economic burden was estimated to be closer to $96 billion when factoring in the cost of lost productivity and pay for symptom relief.3,4 Effective and efficient management of skin disease is essential to minimizing cost and morbidity. Nondermatologists traditionally have diagnosed the majority of skin diseases.5,6 In particular, primary care physicians commonly manage dermatologic conditions and often are the first health care providers to encounter patients presenting with skin problems. A predicted shortage of dermatologists will likely contribute to an increase in this trend.7,8 Therefore, it is important to adequately prepare nondermatologists to evaluate and treat the skin conditions that they are most likely to encounter in their scope of practice.
Residents, particularly in primary care specialties, often have opportunities to spend 2 to 4 weeks with a dermatologist to learn about skin diseases; however, the skin conditions most often encountered by dermatologists may differ from those most often encountered by physicians in other specialties. For instance, one study demonstrated a disparity between the most common skin problems seen by dermatologists and internists.9 These dissimilarities should be recognized and addressed in curriculum content. The purpose of this study was to identify and compare the 20 most common dermatologic conditions reported by dermatologists versus those reported by nondermatologists (ie, internists, pediatricians, family physicians, emergency medicine physicians, general surgeons, otolaryngologists) from 2001 to 2010. Data also were analyzed to determine the top 20 conditions referred to dermatologists by nondermatologists as a potential indicator for areas of further improvement within medical education. With this knowledge, we hope educational curricula and self-study can be modified to reflect the current epidemiology of cutaneous diseases, thereby improving patient care.
Methods
Data from 2001 to 2010 were extracted from the National Ambulatory Medical Care Survey (NAMCS), which is an ongoing survey conducted by the National Center for Health Statistics. The NAMCS collects descriptive data regarding ambulatory visits to nonfederal office-based physicians in the United States. Participating physicians are instructed to record information about patient visits for a 1-week period, including patient demographics, insurance status, reason for visit, diagnoses, procedures, therapeutics, and referrals made at that time. Data collected for the NAMCS are entered into a multistage probability sample to produce national estimates. Within dermatology, an average of 118 dermatologists are sampled each year, and over the last 10 years, participation rates have ranged from 47% to 77%.
International Classification of Diseases, Ninth Revision, Clinical Modification codes were identified to determine the diagnoses that could be classified as dermatologic conditions. Select infectious and neoplastic disorders of the skin and mucous membrane conditions were included as well as the codes for skin diseases. Nondermatologic diagnoses and V codes were not included in the study. Data for all providers were studied to identify outpatient visits associated with the primary diagnosis of a dermatologic condition. Minor diagnoses that were considered to be subsets of major diagnoses were combined to allow better analysis of the data. For example, all tinea infections (ie, dermatophytosis of various sites, dermatomycosis unspecified) were combined into 1 diagnosis referred to as tinea because the recognition and treatment of this disease does not vary tremendously by anatomic location. Visits to dermatologists that listed nonspecific diagnoses and codes (eg, other postsurgical status [V45.89], neoplasm of uncertain behavior site unspecified [238.9]) were assumed to be for dermatologic problems.
Sampling weights were applied to obtain estimates for the number of each diagnosis made nationally. All data analyses were performed using SAS software and linear regression models were generated using SAS PROC SURVEYREG.
Data were analyzed to determine the dermatologic conditions most commonly encountered by dermatologists and nondermatologists in emergency medicine, family medicine, general surgery, internal medicine, otolaryngology, and pediatrics; these specialties include physicians who are known to commonly diagnose and treat skin diseases.10 Data also were analyzed to determine the most common conditions referred to dermatologists for treatment by nondermatologists from the selected specialties. Permission to conduct this study was obtained from the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Results
From 2001 to 2010, more than 700 million outpatient visits for skin-related problems were identified, with 676.3 million visits to dermatologists, emergency medicine physicians, family practitioners, general surgeons, internists, otolaryngologists, and pediatricians. More than half (52.9%) of all skin-related visits were addressed by nondermatologists during this time. Among nondermatologists, family practitioners encountered the greatest number of skin diseases (20.5%), followed by pediatricians (11.3%), internists (9.2%), general surgeons (3.4%), otolaryngologists (1.0%), and emergency medicine physicians (0.2%)(Table 1).

Benign tumors and acne were the most common cutaneous conditions referred to dermatologists by nondermatologists (10.6% and 10.1% of all dermatology referrals, respectively), followed by nonmelanoma skin cancers (9.7%), contact dermatitis (8.8%), and actinic keratosis (7.8%)(Table 2). The top 20 conditions referred to dermatologists accounted for 83.7% of all outpatient referrals to dermatologists.
Among the diseases most frequently reported by nondermatologists, contact dermatitis was the most common (12.0%), with twice the number of visits to nondermatologists for contact dermatitis than to dermatologists (51.6 million vs 25.3 million). In terms of disease categories, infectious skin diseases (ie, bacterial [cellulitis/abscess], viral [warts, herpesvirus], fungal [tinea] and yeast [candida] etiologies) were the most common dermatologic conditions reported by nondermatologists (Table 2).

The top 20 dermatologic conditions reported by dermatologists accounted for 85.4% of all diagnoses made by dermatologists. Diseases that were among the top 20 conditions encountered by dermatologists but were not among the top 20 for nondermatologists included actinic keratosis, seborrheic keratosis, atopic dermatitis, psoriasis, alopecia, rosacea, dyschromia, seborrheic dermatitis, follicular disease, and neoplasm of uncertain behavior of skin. Additionally, 5 of the top 20 conditions encountered by dermatologists also were among the top 20 for only 1 individual nondermatologic specialty; these included atopic dermatitis (pediatrics), seborrheic dermatitis (pediatrics), psoriasis (internal medicine), rosacea (otolaryngology), and keratoderma (general surgery). Seborrheic dermatitis, psoriasis, and rosacea also were among the top 20 conditions most commonly referred to dermatologists for treatment by nondermatologists. Table 3 shows the top 20 dermatologic conditions encountered by nondermatologists by comparison.


Comment
According to NAMCS data from 2001 to 2010, visits to nondermatologists accounted for more than half of total outpatient visits for cutaneous diseases in the United States, whereas visits to dermatologists accounted for 47.1%. These findings are consistent with historical data indicating that 30% to 40% of skin-related visits are to dermatologists, and the majority of patients with skin disease are diagnosed by nondermatologists.5,6
Past data indicate that most visits to dermatologists were for evaluation of acne, infections, psoriasis, and neoplasms, whereas most visits to nondermatologists were for evaluation of epidermoid cysts, impetigo, plant dermatitis, cellulitis, and diaper rash.9 Over the last 10 years, acne has been more commonly encountered by nondermatologists, especially pediatricians. Additionally, infectious etiologies have been seen in larger volume by nondermatologists.9 Together, infectious cutaneous conditions make up nearly one-fourth of dermatologic encounters by emergency medicine physicians, internists, and family practitioners but are not within the top 20 diagnoses referred to dermatologists, which suggests that uncomplicated cases of cellulitis, herpes zoster, and other skin-related infections are largely managed by nondermatologists.5,6 Contact dermatitis, often caused by specific allergens such as detergents, solvents, and topical products, was one of the most common reported dermatologic encounters among dermatologists and nondermatologists and also was the fourth most common condition referred to dermatologists by nondermatologists for treatment; however, there may be an element of overuse of the International Classification of Diseases, Ninth Revision code, as any presumed contact dermatitis of unspecified cause can be reported under 692.9 defined as contact dermatitis and other eczema, unspecified cause. The high rate of referrals to dermatologists by nondermatologists may be for patch testing and further management. Additionally, there are no specific codes for allergic or irritant dermatitis, thus these diseases may be lumped together.
Although nearly half of all dermatologic encounters were seen by nondermatologists, dermatologists see a much larger proportion of patients with skin disease than nondermatologists and nondermatologists often have limited exposure to the field of dermatology during residency training. Studies have demonstrated differences in the abilities of dermatologists and nondermatologists to correctly diagnose common cutaneous diseases, which unsurprisingly revealed greater diagnostic accuracy demonstrated by dermatologists.11-16 The increase in acne and skin-related infections reported by nondermatologists is consistent with possible efforts to increase formal training in frequently encountered skin diseases. In one study evaluating the impact of a formal 3-week dermatology curriculum on an internal medicine department, internists demonstrated 100% accuracy in the diagnosis of acne and herpes zoster in contrast to 29% for tinea and 12% for lichen planus.5,6
The current Accreditation Council for Graduate Medical Education guidelines place little emphasis on exposure to dermatology training during residency for internists and pediatricians, as this training is not a required component of these programs.17 Two core problems with current training regarding the evaluation and management of cutaneous disease are minimal exposure to dermatologic conditions in medical school and residency and lack of consensus on the core topics that should be taught to nondermatologists.18 Exposure to dermatologic conditions through rotations in medical school has been shown to increase residents’ self-reported confidence in diagnosing and treating alopecia, cutaneous drug eruptions, warts, acne, rosacea, nonmelanoma skin cancers, sun damage, psoriasis, seborrhea, atopic dermatitis, and contact dermatitis; however, the majority of primary care residents surveyed still felt that this exposure in medical school was inadequate.19
In creating a core curriculum for dermatology training for nondermatologists, it is important to consider the dermatologic conditions that are most frequently encountered by these specialties. Our study revealed that the most commonly encountered dermatologic conditions differ among dermatologists and nondermatologists, with a fair degree of variation even among individual specialties. Failure to recognize these discrepancies has likely contributed to the challenges faced by nondermatologists in the diagnosis and management of dermatologic disease. In this study, contact dermatitis, epidermoid cysts, and skin infections were the most common dermatologic conditions encountered by nondermatologists and also were among the top skin diseases referred to dermatologists by nondermatologists. This finding suggests that nondermatologists are able to identify these conditions but have a tendency to refer approximately 10% of these patients to dermatology for further management. Clinical evaluation and medical management of these cutaneous diseases may be an important area of focus for medical school curricula, as the treatment of these diseases is within the capabilities of the nondermatologist. For example, initial management of dermatitis requires determination of the type of dermatitis (ie, essential, contact, atopic, seborrheic, stasis) and selection of an appropriate topical steroid, with referral to a dermatologist needed for questionable or refractory cases. Although a curriculum cannot be built solely on a list of the top 20 diagnoses provided here, these data may serve as a preliminary platform for medical school dermatology curriculum design. The curriculum also should include serious skin diseases, such as melanoma and severe drug eruptions. Although these conditions are less commonly encountered by nondermatologists, missed diagnosis and/or improper management can be life threatening.
The use of NAMCS data presents a few limitations. For instance, these data only represent outpatient management of skin disease. There is the potential for misdiagnosis and coding errors by the reporting physicians. The volume of data (ie, billions of office visits) prevents verification of diagnostic accuracy. The coding system requires physicians to give a diagnosis but does not provide any means by which to determine the physician’s confidence in that diagnosis. There is no code for “uncertain” or “diagnosis not determined.” Additionally, an “unspecified” diagnosis may reflect uncertainty or may simply imply that no other code accurately described the condition. Despite these limitations, the NAMCS database is a large, nationally representative survey of actual patient visits and represents some of the best data available for a study such as ours.
Conclusion
This study provides an important analysis of the most common outpatient dermatologic conditions encountered by dermatologists and nondermatologists of various specialties and offers a foundation from which to construct curricula for dermatology training tailored to individual specialties based on their needs. In the future, identification of the most common inpatient dermatologic conditions managed by each specialty also may benefit curriculum design.
Skin diseases are highly prevalent in the United States, affecting an estimated 1 in 3 Americans at any given time.1,2 In 2009 the direct medical costs associated with skin-related diseases, including health services and prescriptions, was approximately $22 billion; the annual total economic burden was estimated to be closer to $96 billion when factoring in the cost of lost productivity and pay for symptom relief.3,4 Effective and efficient management of skin disease is essential to minimizing cost and morbidity. Nondermatologists traditionally have diagnosed the majority of skin diseases.5,6 In particular, primary care physicians commonly manage dermatologic conditions and often are the first health care providers to encounter patients presenting with skin problems. A predicted shortage of dermatologists will likely contribute to an increase in this trend.7,8 Therefore, it is important to adequately prepare nondermatologists to evaluate and treat the skin conditions that they are most likely to encounter in their scope of practice.
Residents, particularly in primary care specialties, often have opportunities to spend 2 to 4 weeks with a dermatologist to learn about skin diseases; however, the skin conditions most often encountered by dermatologists may differ from those most often encountered by physicians in other specialties. For instance, one study demonstrated a disparity between the most common skin problems seen by dermatologists and internists.9 These dissimilarities should be recognized and addressed in curriculum content. The purpose of this study was to identify and compare the 20 most common dermatologic conditions reported by dermatologists versus those reported by nondermatologists (ie, internists, pediatricians, family physicians, emergency medicine physicians, general surgeons, otolaryngologists) from 2001 to 2010. Data also were analyzed to determine the top 20 conditions referred to dermatologists by nondermatologists as a potential indicator for areas of further improvement within medical education. With this knowledge, we hope educational curricula and self-study can be modified to reflect the current epidemiology of cutaneous diseases, thereby improving patient care.
Methods
Data from 2001 to 2010 were extracted from the National Ambulatory Medical Care Survey (NAMCS), which is an ongoing survey conducted by the National Center for Health Statistics. The NAMCS collects descriptive data regarding ambulatory visits to nonfederal office-based physicians in the United States. Participating physicians are instructed to record information about patient visits for a 1-week period, including patient demographics, insurance status, reason for visit, diagnoses, procedures, therapeutics, and referrals made at that time. Data collected for the NAMCS are entered into a multistage probability sample to produce national estimates. Within dermatology, an average of 118 dermatologists are sampled each year, and over the last 10 years, participation rates have ranged from 47% to 77%.
International Classification of Diseases, Ninth Revision, Clinical Modification codes were identified to determine the diagnoses that could be classified as dermatologic conditions. Select infectious and neoplastic disorders of the skin and mucous membrane conditions were included as well as the codes for skin diseases. Nondermatologic diagnoses and V codes were not included in the study. Data for all providers were studied to identify outpatient visits associated with the primary diagnosis of a dermatologic condition. Minor diagnoses that were considered to be subsets of major diagnoses were combined to allow better analysis of the data. For example, all tinea infections (ie, dermatophytosis of various sites, dermatomycosis unspecified) were combined into 1 diagnosis referred to as tinea because the recognition and treatment of this disease does not vary tremendously by anatomic location. Visits to dermatologists that listed nonspecific diagnoses and codes (eg, other postsurgical status [V45.89], neoplasm of uncertain behavior site unspecified [238.9]) were assumed to be for dermatologic problems.
Sampling weights were applied to obtain estimates for the number of each diagnosis made nationally. All data analyses were performed using SAS software and linear regression models were generated using SAS PROC SURVEYREG.
Data were analyzed to determine the dermatologic conditions most commonly encountered by dermatologists and nondermatologists in emergency medicine, family medicine, general surgery, internal medicine, otolaryngology, and pediatrics; these specialties include physicians who are known to commonly diagnose and treat skin diseases.10 Data also were analyzed to determine the most common conditions referred to dermatologists for treatment by nondermatologists from the selected specialties. Permission to conduct this study was obtained from the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Results
From 2001 to 2010, more than 700 million outpatient visits for skin-related problems were identified, with 676.3 million visits to dermatologists, emergency medicine physicians, family practitioners, general surgeons, internists, otolaryngologists, and pediatricians. More than half (52.9%) of all skin-related visits were addressed by nondermatologists during this time. Among nondermatologists, family practitioners encountered the greatest number of skin diseases (20.5%), followed by pediatricians (11.3%), internists (9.2%), general surgeons (3.4%), otolaryngologists (1.0%), and emergency medicine physicians (0.2%)(Table 1).

Benign tumors and acne were the most common cutaneous conditions referred to dermatologists by nondermatologists (10.6% and 10.1% of all dermatology referrals, respectively), followed by nonmelanoma skin cancers (9.7%), contact dermatitis (8.8%), and actinic keratosis (7.8%)(Table 2). The top 20 conditions referred to dermatologists accounted for 83.7% of all outpatient referrals to dermatologists.
Among the diseases most frequently reported by nondermatologists, contact dermatitis was the most common (12.0%), with twice the number of visits to nondermatologists for contact dermatitis than to dermatologists (51.6 million vs 25.3 million). In terms of disease categories, infectious skin diseases (ie, bacterial [cellulitis/abscess], viral [warts, herpesvirus], fungal [tinea] and yeast [candida] etiologies) were the most common dermatologic conditions reported by nondermatologists (Table 2).

The top 20 dermatologic conditions reported by dermatologists accounted for 85.4% of all diagnoses made by dermatologists. Diseases that were among the top 20 conditions encountered by dermatologists but were not among the top 20 for nondermatologists included actinic keratosis, seborrheic keratosis, atopic dermatitis, psoriasis, alopecia, rosacea, dyschromia, seborrheic dermatitis, follicular disease, and neoplasm of uncertain behavior of skin. Additionally, 5 of the top 20 conditions encountered by dermatologists also were among the top 20 for only 1 individual nondermatologic specialty; these included atopic dermatitis (pediatrics), seborrheic dermatitis (pediatrics), psoriasis (internal medicine), rosacea (otolaryngology), and keratoderma (general surgery). Seborrheic dermatitis, psoriasis, and rosacea also were among the top 20 conditions most commonly referred to dermatologists for treatment by nondermatologists. Table 3 shows the top 20 dermatologic conditions encountered by nondermatologists by comparison.


Comment
According to NAMCS data from 2001 to 2010, visits to nondermatologists accounted for more than half of total outpatient visits for cutaneous diseases in the United States, whereas visits to dermatologists accounted for 47.1%. These findings are consistent with historical data indicating that 30% to 40% of skin-related visits are to dermatologists, and the majority of patients with skin disease are diagnosed by nondermatologists.5,6
Past data indicate that most visits to dermatologists were for evaluation of acne, infections, psoriasis, and neoplasms, whereas most visits to nondermatologists were for evaluation of epidermoid cysts, impetigo, plant dermatitis, cellulitis, and diaper rash.9 Over the last 10 years, acne has been more commonly encountered by nondermatologists, especially pediatricians. Additionally, infectious etiologies have been seen in larger volume by nondermatologists.9 Together, infectious cutaneous conditions make up nearly one-fourth of dermatologic encounters by emergency medicine physicians, internists, and family practitioners but are not within the top 20 diagnoses referred to dermatologists, which suggests that uncomplicated cases of cellulitis, herpes zoster, and other skin-related infections are largely managed by nondermatologists.5,6 Contact dermatitis, often caused by specific allergens such as detergents, solvents, and topical products, was one of the most common reported dermatologic encounters among dermatologists and nondermatologists and also was the fourth most common condition referred to dermatologists by nondermatologists for treatment; however, there may be an element of overuse of the International Classification of Diseases, Ninth Revision code, as any presumed contact dermatitis of unspecified cause can be reported under 692.9 defined as contact dermatitis and other eczema, unspecified cause. The high rate of referrals to dermatologists by nondermatologists may be for patch testing and further management. Additionally, there are no specific codes for allergic or irritant dermatitis, thus these diseases may be lumped together.
Although nearly half of all dermatologic encounters were seen by nondermatologists, dermatologists see a much larger proportion of patients with skin disease than nondermatologists and nondermatologists often have limited exposure to the field of dermatology during residency training. Studies have demonstrated differences in the abilities of dermatologists and nondermatologists to correctly diagnose common cutaneous diseases, which unsurprisingly revealed greater diagnostic accuracy demonstrated by dermatologists.11-16 The increase in acne and skin-related infections reported by nondermatologists is consistent with possible efforts to increase formal training in frequently encountered skin diseases. In one study evaluating the impact of a formal 3-week dermatology curriculum on an internal medicine department, internists demonstrated 100% accuracy in the diagnosis of acne and herpes zoster in contrast to 29% for tinea and 12% for lichen planus.5,6
The current Accreditation Council for Graduate Medical Education guidelines place little emphasis on exposure to dermatology training during residency for internists and pediatricians, as this training is not a required component of these programs.17 Two core problems with current training regarding the evaluation and management of cutaneous disease are minimal exposure to dermatologic conditions in medical school and residency and lack of consensus on the core topics that should be taught to nondermatologists.18 Exposure to dermatologic conditions through rotations in medical school has been shown to increase residents’ self-reported confidence in diagnosing and treating alopecia, cutaneous drug eruptions, warts, acne, rosacea, nonmelanoma skin cancers, sun damage, psoriasis, seborrhea, atopic dermatitis, and contact dermatitis; however, the majority of primary care residents surveyed still felt that this exposure in medical school was inadequate.19
In creating a core curriculum for dermatology training for nondermatologists, it is important to consider the dermatologic conditions that are most frequently encountered by these specialties. Our study revealed that the most commonly encountered dermatologic conditions differ among dermatologists and nondermatologists, with a fair degree of variation even among individual specialties. Failure to recognize these discrepancies has likely contributed to the challenges faced by nondermatologists in the diagnosis and management of dermatologic disease. In this study, contact dermatitis, epidermoid cysts, and skin infections were the most common dermatologic conditions encountered by nondermatologists and also were among the top skin diseases referred to dermatologists by nondermatologists. This finding suggests that nondermatologists are able to identify these conditions but have a tendency to refer approximately 10% of these patients to dermatology for further management. Clinical evaluation and medical management of these cutaneous diseases may be an important area of focus for medical school curricula, as the treatment of these diseases is within the capabilities of the nondermatologist. For example, initial management of dermatitis requires determination of the type of dermatitis (ie, essential, contact, atopic, seborrheic, stasis) and selection of an appropriate topical steroid, with referral to a dermatologist needed for questionable or refractory cases. Although a curriculum cannot be built solely on a list of the top 20 diagnoses provided here, these data may serve as a preliminary platform for medical school dermatology curriculum design. The curriculum also should include serious skin diseases, such as melanoma and severe drug eruptions. Although these conditions are less commonly encountered by nondermatologists, missed diagnosis and/or improper management can be life threatening.
The use of NAMCS data presents a few limitations. For instance, these data only represent outpatient management of skin disease. There is the potential for misdiagnosis and coding errors by the reporting physicians. The volume of data (ie, billions of office visits) prevents verification of diagnostic accuracy. The coding system requires physicians to give a diagnosis but does not provide any means by which to determine the physician’s confidence in that diagnosis. There is no code for “uncertain” or “diagnosis not determined.” Additionally, an “unspecified” diagnosis may reflect uncertainty or may simply imply that no other code accurately described the condition. Despite these limitations, the NAMCS database is a large, nationally representative survey of actual patient visits and represents some of the best data available for a study such as ours.
Conclusion
This study provides an important analysis of the most common outpatient dermatologic conditions encountered by dermatologists and nondermatologists of various specialties and offers a foundation from which to construct curricula for dermatology training tailored to individual specialties based on their needs. In the future, identification of the most common inpatient dermatologic conditions managed by each specialty also may benefit curriculum design.
- Thorpe KE, Florence CS, Joski P. Which medical conditions account for the rise in health care spending? Health Aff (Millwood). 2004;(suppl web exclusives):W4-437-445.
- Johnson ML. Defining the burden of skin disease in the United States—a historical perspective. J Investig Dermatol Symp Proc. 2004;9:108-110.
- Agency for Healthcare Research and Quality. Medical expenditure panel survey. US Department of Health & Human Services Web site. http://meps.ahrq.gov. Accessed November 17, 2014.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Johnson ML. On teaching dermatology to nondermatologists. Arch Dermatol. 1994;130:850-852.
- Ramsay DL, Weary PE. Primary care in dermatology: whose role should it be? J Am Acad Dermatol. 1996;35:1005-1008.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Kimball AB. Who else is providing care in dermatology practices? trends in the use of nonphysician clinicians. J Am Acad Dermatol. 2008;58:211-216.
- Feldman SR, Fleischer AB Jr, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730.
- Ahn CS, Davis SA, Debade TS, et al. Noncosmetic skin-related procedures performed in the United States: an analysis of national ambulatory medical care survey data from 1995 to 2010. Dermatol Surg. 2013;39:1912-1921.
- Antic M, Conen D, Itin PH. Teaching effects of dermatological consultations on nondermatologists in the field of internal medicine. a study of 1290 inpatients. Dermatology. 2004;208:32-37.
- Federman DG, Concato J, Kirsner RS. Comparison of dermatologic diagnoses by primary care practitioners and dermatologists. a review of the literature. Arch Fam Med. 1999;8:170-172.
- Fleischer AB Jr, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by nondermatologists. Am J Manag Care. 2000;6:1149-1156.
- Kirsner RS, Federman DG. Lack of correlation between internists’ ability in dermatology and their patterns of treating patients with skin disease. Arch Dermatol. 1996;132:1043-1046.
- McCarthy GM, Lamb GC, Russell TJ, et al. Primary care-based dermatology practice: internists need more training. J Gen Intern Med. 1991;6:52-56.
- Sellheyer K, Bergfeld WF. A retrospective biopsy study of the clinical diagnostic accuracy of common skin diseases by different specialties compared with dermatology. J Am Acad Dermatol. 2005;52:823-830.
- Medical specialties. Accreditation Council for Graduate Medical Education Web site. http://www.acgme.org/acgmeweb/tabid/368ProgramandInstitutionalGuidelines/MedicalAccreditation.aspx. Accessed November 17, 2014.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Thorpe KE, Florence CS, Joski P. Which medical conditions account for the rise in health care spending? Health Aff (Millwood). 2004;(suppl web exclusives):W4-437-445.
- Johnson ML. Defining the burden of skin disease in the United States—a historical perspective. J Investig Dermatol Symp Proc. 2004;9:108-110.
- Agency for Healthcare Research and Quality. Medical expenditure panel survey. US Department of Health & Human Services Web site. http://meps.ahrq.gov. Accessed November 17, 2014.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Johnson ML. On teaching dermatology to nondermatologists. Arch Dermatol. 1994;130:850-852.
- Ramsay DL, Weary PE. Primary care in dermatology: whose role should it be? J Am Acad Dermatol. 1996;35:1005-1008.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Kimball AB. Who else is providing care in dermatology practices? trends in the use of nonphysician clinicians. J Am Acad Dermatol. 2008;58:211-216.
- Feldman SR, Fleischer AB Jr, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730.
- Ahn CS, Davis SA, Debade TS, et al. Noncosmetic skin-related procedures performed in the United States: an analysis of national ambulatory medical care survey data from 1995 to 2010. Dermatol Surg. 2013;39:1912-1921.
- Antic M, Conen D, Itin PH. Teaching effects of dermatological consultations on nondermatologists in the field of internal medicine. a study of 1290 inpatients. Dermatology. 2004;208:32-37.
- Federman DG, Concato J, Kirsner RS. Comparison of dermatologic diagnoses by primary care practitioners and dermatologists. a review of the literature. Arch Fam Med. 1999;8:170-172.
- Fleischer AB Jr, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by nondermatologists. Am J Manag Care. 2000;6:1149-1156.
- Kirsner RS, Federman DG. Lack of correlation between internists’ ability in dermatology and their patterns of treating patients with skin disease. Arch Dermatol. 1996;132:1043-1046.
- McCarthy GM, Lamb GC, Russell TJ, et al. Primary care-based dermatology practice: internists need more training. J Gen Intern Med. 1991;6:52-56.
- Sellheyer K, Bergfeld WF. A retrospective biopsy study of the clinical diagnostic accuracy of common skin diseases by different specialties compared with dermatology. J Am Acad Dermatol. 2005;52:823-830.
- Medical specialties. Accreditation Council for Graduate Medical Education Web site. http://www.acgme.org/acgmeweb/tabid/368ProgramandInstitutionalGuidelines/MedicalAccreditation.aspx. Accessed November 17, 2014.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
Practice Points
- Approximately half of skin-related visits are to nondermatologists, such as family medicine physicians, pediatricians, and internists.
- Skin conditions that most frequently present to nondermatologists are different from those seen by dermatologists.
- Education efforts in nondermatology specialties should be targeted toward the common skin diseases that present to these specialties to maximize the yield of medical education and improve diagnostic accuracy and patient outcomes.