User login
Collagen filler succeeds against acne scars
SAN DIEGO – An injectable collagen-based filler significantly outperformed saline placebo for treating acne scars, with durable effects at 12 months, according to a randomized, double-blind crossover trial of 147 adults.
The study “successfully demonstrates the effectiveness and safety of polymethylmethacrylate in atrophic acne scars,” said Dr. James Spencer, who conducted the research while at Mount Sinai School of Medicine in New York. Dr. Spencer is now in private practice in St. Petersburg, Fla.
Six months after treatment, 64% of patients who received the polymethylmethacrylate-collagen filler (or PMMA) had at least half their scars improve by at least 2 points on an acne rating scale, Dr. Spencer and his associates said at the annual meeting of the American Society for Dermatologic Surgery. Only 32% of the control group achieved that result (P = .0005). Response rates for the filler were 61% at 9 months and 70% at 12 months, and “crossover subjects subsequently treated with PMMA collagen showed similar response levels,” they said.
The PMMA-collagen filler is easy to administer; “works well on deep, severe scars; and should also work very well on shallow scars,” the researchers noted. The treatment “may enable practitioners to effectively treat acne scarring with no need for capital equipment expenditure or the risks associated with resurfacing procedures,” they said.
About 61% of patients in the study were female, participants averaged 44 years of age, and 20% were Fitzpatrick skin type V or VI. To enter the study, participants had to have at least four facial acne scars that were soft contoured, rolling, distensible, and rated moderate to severe (3-4) on a 4-point acne rating scale.
The patients were treated every 2 weeks for a month, and again at months 3 and 6. At 6 months, patients in the placebo group crossed over and received the filler, and all patients were followed for another 6 months.
Adverse effects were uncommon and included mild transient pain at the injection site, swelling, bruising, and acne, the researchers said. “There was no evidence of granulomas, changes in pigmentation, or hypertrophic scarring,” they added.
No funding source was reported for the study. Dr. Spencer and his coauthors reported financial relationships with Photomedex, Genentech, and Leo Pharma.
SAN DIEGO – An injectable collagen-based filler significantly outperformed saline placebo for treating acne scars, with durable effects at 12 months, according to a randomized, double-blind crossover trial of 147 adults.
The study “successfully demonstrates the effectiveness and safety of polymethylmethacrylate in atrophic acne scars,” said Dr. James Spencer, who conducted the research while at Mount Sinai School of Medicine in New York. Dr. Spencer is now in private practice in St. Petersburg, Fla.
Six months after treatment, 64% of patients who received the polymethylmethacrylate-collagen filler (or PMMA) had at least half their scars improve by at least 2 points on an acne rating scale, Dr. Spencer and his associates said at the annual meeting of the American Society for Dermatologic Surgery. Only 32% of the control group achieved that result (P = .0005). Response rates for the filler were 61% at 9 months and 70% at 12 months, and “crossover subjects subsequently treated with PMMA collagen showed similar response levels,” they said.
The PMMA-collagen filler is easy to administer; “works well on deep, severe scars; and should also work very well on shallow scars,” the researchers noted. The treatment “may enable practitioners to effectively treat acne scarring with no need for capital equipment expenditure or the risks associated with resurfacing procedures,” they said.
About 61% of patients in the study were female, participants averaged 44 years of age, and 20% were Fitzpatrick skin type V or VI. To enter the study, participants had to have at least four facial acne scars that were soft contoured, rolling, distensible, and rated moderate to severe (3-4) on a 4-point acne rating scale.
The patients were treated every 2 weeks for a month, and again at months 3 and 6. At 6 months, patients in the placebo group crossed over and received the filler, and all patients were followed for another 6 months.
Adverse effects were uncommon and included mild transient pain at the injection site, swelling, bruising, and acne, the researchers said. “There was no evidence of granulomas, changes in pigmentation, or hypertrophic scarring,” they added.
No funding source was reported for the study. Dr. Spencer and his coauthors reported financial relationships with Photomedex, Genentech, and Leo Pharma.
SAN DIEGO – An injectable collagen-based filler significantly outperformed saline placebo for treating acne scars, with durable effects at 12 months, according to a randomized, double-blind crossover trial of 147 adults.
The study “successfully demonstrates the effectiveness and safety of polymethylmethacrylate in atrophic acne scars,” said Dr. James Spencer, who conducted the research while at Mount Sinai School of Medicine in New York. Dr. Spencer is now in private practice in St. Petersburg, Fla.
Six months after treatment, 64% of patients who received the polymethylmethacrylate-collagen filler (or PMMA) had at least half their scars improve by at least 2 points on an acne rating scale, Dr. Spencer and his associates said at the annual meeting of the American Society for Dermatologic Surgery. Only 32% of the control group achieved that result (P = .0005). Response rates for the filler were 61% at 9 months and 70% at 12 months, and “crossover subjects subsequently treated with PMMA collagen showed similar response levels,” they said.
The PMMA-collagen filler is easy to administer; “works well on deep, severe scars; and should also work very well on shallow scars,” the researchers noted. The treatment “may enable practitioners to effectively treat acne scarring with no need for capital equipment expenditure or the risks associated with resurfacing procedures,” they said.
About 61% of patients in the study were female, participants averaged 44 years of age, and 20% were Fitzpatrick skin type V or VI. To enter the study, participants had to have at least four facial acne scars that were soft contoured, rolling, distensible, and rated moderate to severe (3-4) on a 4-point acne rating scale.
The patients were treated every 2 weeks for a month, and again at months 3 and 6. At 6 months, patients in the placebo group crossed over and received the filler, and all patients were followed for another 6 months.
Adverse effects were uncommon and included mild transient pain at the injection site, swelling, bruising, and acne, the researchers said. “There was no evidence of granulomas, changes in pigmentation, or hypertrophic scarring,” they added.
No funding source was reported for the study. Dr. Spencer and his coauthors reported financial relationships with Photomedex, Genentech, and Leo Pharma.
Key clinical point: A collagen-based filler significantly improved the appearance of acne scars in adults, with persistent improvement at 12 months.
Major finding: At 6-month evaluation, 64% of the intervention group were considered responders, compared with 32% of the control group (P = .0005).
Data source: A prospective, randomized, double-blind, controlled, multicenter crossover study of 147 adults with acne scars.
Disclosures: The researchers did not report funding sources. Dr. Spencer and his coauthors reported financial relationships with Photomedex, Genentech, and Leo Pharma.
Fractional laser technology reduces facial acne scarring
Fractional laser technology, often used in the removal of unwanted tattoos, can improve the appearance and texture of facial acne scars, based on data published online Nov.19 in JAMA Dermatology.
“The evolution from traditional nanosecond to picosecond lasers has been observed to produce a photomechanical effect that causes fragmentation of tattoo ink or pigment,” wrote Dr. Jeremy A. Brauer, a dermatologist in group practice in New York, and his coinvestigators. “An innovative optical attachment for the picosecond laser, a diffractive lens array, has been developed that gauges distribution of energy to the treatment area. This specialized optic affects more surface area, has a greater pattern density per pulse, and may improve the appearance of acne scars,” they reported.
In a single-center, prospective study, Dr. Brauer and his associates enrolled 20 patients – 15 women and 5 men – based on screenings to ensure no history of skin cancer, keloidal scarring, localized or active infection, immunodeficiency disorders, and light hypersensitivity or use of medications with known phototoxic effects. Of that initial group, 17 completed all six treatments and presented for follow-up visits at 1 and 3 months. Patients were aged 27-66 (mean age, 44 years), and included Fitzpatrick skin types I (one patient), II (seven patients), III (six patients), and IV (three patients).
Subjects mostly had rolling-type scars, boxcar scars, and icepick lesions related to acne. Each subject underwent six treatments with a 755-nanometer alexandrite picosecond laser with a diffractive lens array; each treatment occurred every 4-8 weeks.
Subjects also provided a subjective score for pain experienced during each treatment on a scale of 0 (no pain) to 10 (extreme pain). The patients also used a scale of 0-4 to indicate their satisfaction with improvement of overall skin appearance and texture prior to their final treatment, at the 1-month follow-up, and at the 3-month follow-up (with 0 being total dissatisfaction and 4 total satisfaction).
At the 1-month and 3-month follow-ups, three independent dermatologists gave masked evaluations of each patient’s improvement on a 4-point scale, with 0 = 0%-25%, 1 = 26%-50%, 3 = 51%-75%, and 4 = 76%-100%.
All patients were either “satisfied” or “extremely satisfied” with the appearance and texture of their facial skin after receiving the full treatment regimen, and recorded an average pain score of 2.83 out of 10. The masked assessment scores also were favorable, averaging 1.5 of 3 and 1.4 of 3 at the 1-month and 3-month follow-ups, with a score of 0 indicating 0-25% improvement and a score of 3, greater than 75% improvement, the researchers reported.
Dr. Brauer and his associates also evaluated three-dimensional volumetric data for each subject, which showed an average of 24.0% improvement in scar volume at the 1-month follow-up and 27.2% at the 3-month follow-up. Furthermore, histologic analysis revealed elongation and increased density of elastic fibers, as well as an increase in dermal collagen and mucin.
“This is the first study, to our knowledge, that demonstrates favorable clinical outcomes in acne scar management with the 755[-nm] picosecond laser and diffractive lens array,” the researchers noted. “Observed improvement in pigmentation and texture of the surrounding skin suggests that there may be benefits for indications beyond scarring,” they wrote.
The authors disclosed that funding for this study was provided in part by Cynosure, manufacturer of the Food and Drug Administration–approved 755-nm picosecond alexandrite laser used in the study, and noted that “Cynosure had a role in the design of the study but not the conduct, collection, management, analysis, or interpretation of data. They approved the manuscript but did not prepare or decide to submit.” Dr. Brauer disclosed receiving honoraria from Cynosure/Palomar Medical Technologies and consulting for Miramar. Several other coauthors disclosed financial relationships with multiple companies, including Cynosure/Palomar Medical Technologies.
Fractional laser technology, often used in the removal of unwanted tattoos, can improve the appearance and texture of facial acne scars, based on data published online Nov.19 in JAMA Dermatology.
“The evolution from traditional nanosecond to picosecond lasers has been observed to produce a photomechanical effect that causes fragmentation of tattoo ink or pigment,” wrote Dr. Jeremy A. Brauer, a dermatologist in group practice in New York, and his coinvestigators. “An innovative optical attachment for the picosecond laser, a diffractive lens array, has been developed that gauges distribution of energy to the treatment area. This specialized optic affects more surface area, has a greater pattern density per pulse, and may improve the appearance of acne scars,” they reported.
In a single-center, prospective study, Dr. Brauer and his associates enrolled 20 patients – 15 women and 5 men – based on screenings to ensure no history of skin cancer, keloidal scarring, localized or active infection, immunodeficiency disorders, and light hypersensitivity or use of medications with known phototoxic effects. Of that initial group, 17 completed all six treatments and presented for follow-up visits at 1 and 3 months. Patients were aged 27-66 (mean age, 44 years), and included Fitzpatrick skin types I (one patient), II (seven patients), III (six patients), and IV (three patients).
Subjects mostly had rolling-type scars, boxcar scars, and icepick lesions related to acne. Each subject underwent six treatments with a 755-nanometer alexandrite picosecond laser with a diffractive lens array; each treatment occurred every 4-8 weeks.
Subjects also provided a subjective score for pain experienced during each treatment on a scale of 0 (no pain) to 10 (extreme pain). The patients also used a scale of 0-4 to indicate their satisfaction with improvement of overall skin appearance and texture prior to their final treatment, at the 1-month follow-up, and at the 3-month follow-up (with 0 being total dissatisfaction and 4 total satisfaction).
At the 1-month and 3-month follow-ups, three independent dermatologists gave masked evaluations of each patient’s improvement on a 4-point scale, with 0 = 0%-25%, 1 = 26%-50%, 3 = 51%-75%, and 4 = 76%-100%.
All patients were either “satisfied” or “extremely satisfied” with the appearance and texture of their facial skin after receiving the full treatment regimen, and recorded an average pain score of 2.83 out of 10. The masked assessment scores also were favorable, averaging 1.5 of 3 and 1.4 of 3 at the 1-month and 3-month follow-ups, with a score of 0 indicating 0-25% improvement and a score of 3, greater than 75% improvement, the researchers reported.
Dr. Brauer and his associates also evaluated three-dimensional volumetric data for each subject, which showed an average of 24.0% improvement in scar volume at the 1-month follow-up and 27.2% at the 3-month follow-up. Furthermore, histologic analysis revealed elongation and increased density of elastic fibers, as well as an increase in dermal collagen and mucin.
“This is the first study, to our knowledge, that demonstrates favorable clinical outcomes in acne scar management with the 755[-nm] picosecond laser and diffractive lens array,” the researchers noted. “Observed improvement in pigmentation and texture of the surrounding skin suggests that there may be benefits for indications beyond scarring,” they wrote.
The authors disclosed that funding for this study was provided in part by Cynosure, manufacturer of the Food and Drug Administration–approved 755-nm picosecond alexandrite laser used in the study, and noted that “Cynosure had a role in the design of the study but not the conduct, collection, management, analysis, or interpretation of data. They approved the manuscript but did not prepare or decide to submit.” Dr. Brauer disclosed receiving honoraria from Cynosure/Palomar Medical Technologies and consulting for Miramar. Several other coauthors disclosed financial relationships with multiple companies, including Cynosure/Palomar Medical Technologies.
Fractional laser technology, often used in the removal of unwanted tattoos, can improve the appearance and texture of facial acne scars, based on data published online Nov.19 in JAMA Dermatology.
“The evolution from traditional nanosecond to picosecond lasers has been observed to produce a photomechanical effect that causes fragmentation of tattoo ink or pigment,” wrote Dr. Jeremy A. Brauer, a dermatologist in group practice in New York, and his coinvestigators. “An innovative optical attachment for the picosecond laser, a diffractive lens array, has been developed that gauges distribution of energy to the treatment area. This specialized optic affects more surface area, has a greater pattern density per pulse, and may improve the appearance of acne scars,” they reported.
In a single-center, prospective study, Dr. Brauer and his associates enrolled 20 patients – 15 women and 5 men – based on screenings to ensure no history of skin cancer, keloidal scarring, localized or active infection, immunodeficiency disorders, and light hypersensitivity or use of medications with known phototoxic effects. Of that initial group, 17 completed all six treatments and presented for follow-up visits at 1 and 3 months. Patients were aged 27-66 (mean age, 44 years), and included Fitzpatrick skin types I (one patient), II (seven patients), III (six patients), and IV (three patients).
Subjects mostly had rolling-type scars, boxcar scars, and icepick lesions related to acne. Each subject underwent six treatments with a 755-nanometer alexandrite picosecond laser with a diffractive lens array; each treatment occurred every 4-8 weeks.
Subjects also provided a subjective score for pain experienced during each treatment on a scale of 0 (no pain) to 10 (extreme pain). The patients also used a scale of 0-4 to indicate their satisfaction with improvement of overall skin appearance and texture prior to their final treatment, at the 1-month follow-up, and at the 3-month follow-up (with 0 being total dissatisfaction and 4 total satisfaction).
At the 1-month and 3-month follow-ups, three independent dermatologists gave masked evaluations of each patient’s improvement on a 4-point scale, with 0 = 0%-25%, 1 = 26%-50%, 3 = 51%-75%, and 4 = 76%-100%.
All patients were either “satisfied” or “extremely satisfied” with the appearance and texture of their facial skin after receiving the full treatment regimen, and recorded an average pain score of 2.83 out of 10. The masked assessment scores also were favorable, averaging 1.5 of 3 and 1.4 of 3 at the 1-month and 3-month follow-ups, with a score of 0 indicating 0-25% improvement and a score of 3, greater than 75% improvement, the researchers reported.
Dr. Brauer and his associates also evaluated three-dimensional volumetric data for each subject, which showed an average of 24.0% improvement in scar volume at the 1-month follow-up and 27.2% at the 3-month follow-up. Furthermore, histologic analysis revealed elongation and increased density of elastic fibers, as well as an increase in dermal collagen and mucin.
“This is the first study, to our knowledge, that demonstrates favorable clinical outcomes in acne scar management with the 755[-nm] picosecond laser and diffractive lens array,” the researchers noted. “Observed improvement in pigmentation and texture of the surrounding skin suggests that there may be benefits for indications beyond scarring,” they wrote.
The authors disclosed that funding for this study was provided in part by Cynosure, manufacturer of the Food and Drug Administration–approved 755-nm picosecond alexandrite laser used in the study, and noted that “Cynosure had a role in the design of the study but not the conduct, collection, management, analysis, or interpretation of data. They approved the manuscript but did not prepare or decide to submit.” Dr. Brauer disclosed receiving honoraria from Cynosure/Palomar Medical Technologies and consulting for Miramar. Several other coauthors disclosed financial relationships with multiple companies, including Cynosure/Palomar Medical Technologies.
FROM JAMA DERMATOLOGY
Key clinical point: Treatment of facial acne scars with a diffractive lens array and a picosecond 755-nm alexandrite laser improves the appearance and texture of skin within 3 months.
Major finding: Masked assessments by a dermatologist found a 25%-50% global improvement at the 1-month postoperation follow-up visit, which was maintained at the 3-month follow-up.
Data source: A single-center, prospective study of 20 patients.
Disclosures: This study was supported in part by Cynosure. The authors disclosed several potential conflicts of interest.
Applications of Lasers in Medical Dermatology
The use of lasers in dermatology has had a major impact on the treatment of many dermatologic conditions. In this column practical applications of lasers in medical dermatology will be discussed to give dermatology residents a broad overview of both established indications and the reasoning behind the usage of lasers in treating these skin conditions. The applications for lasers in aesthetic dermatology are numerous and are constantly being refined and developed; they have been discussed extensively in the literature. Given the vast variety of uses of lasers in dermatology today, a comprehensive review of this topic would likely span several volumes. This article will focus on recent evidence regarding the use of lasers in medical dermatology, specifically laser treatment of selected common dermatoses and cutaneous malignancies.
Laser Treatment of Skin Diseases
Many common dermatoses seen in the dermatologist’s office (eg, discoid lupus erythematosus [DLE], morphea, alopecia) already have an established therapeutic ladder, with most patients responding to either first- or second-line therapies; however, a number of patients present with refractory disease that can be difficult to treat due to either treatment resistance or other contraindications to therapy. With the advent and development of modern lasers, we are now able to target many of these conditions and provide a viable safe treatment option for these patients. Although many physicians may be familiar with the use of the excimer laser in the treatment of psoriasis,1 a long-standing and well-accepted treatment modality for this condition, many novel applications for different types of lasers have been developed.
First, it is important to consider what a laser is able to accomplish to modulate the skin. With ablative lasers such as the CO2 laser, it is possible to destroy superficial layers of the skin (ie, the epidermis). It would stand to reason that this approach would be ideal for treating epidermal processes such as viral warts; in fact, this modality has been used for this indication for more than 3 decades, with the earliest references coming from the podiatric and urologic literature.2,3 Despite conflicting reports of the risk for human papillomavirus aerosolization and subsequent contamination of the treatment area,4,5 CO2 laser therapy has been advocated as a nonsurgical approach to difficult-to-treat cases of viral warts.
On the other hand, the pulsed dye laser (PDL) can target blood vessels because the wavelength corresponds to the absorption spectrum of hemoglobin and penetrates to the level of the dermis, while the pulse duration can be set to be shorter than the thermal relaxation time of a small cutaneous blood vessel.6 In clinical practice, the PDL has been used for the treatment of vascular lesions including hemangiomas, nevus flammeus, and other vascular proliferations.7-9 However, the PDL also can be used to target the vessels in cutaneous inflammatory diseases that feature vascular dilation and/or perivascular inflammation as a prominent feature.
Discoid lupus erythematosus is a form of chronic cutaneous lupus erythematosus that may be difficult to treat, with recalcitrant lesions displaying continued inflammation leading to chronic scarring and dyspigmentation. A small study (N=12) presented the efficacy of the PDL in the treatment of DLE lesions, suggesting that it has good efficacy in treating recalcitrant lesions with significant reduction in the cutaneous lupus erythematosus disease area and severity index after 6 weeks of treatment and 6 weeks of follow-up (P<.0001) with decreased erythema and scaling.10 It is important to note, however, that scarring, dyspigmentation, and atrophy were not affected, which suggests that early intervention may be optimal to prevent development of these sequelae. More interestingly, a more recent study expounded on this idea and attempted to examine pathophysiologic mechanisms behind this observed improvement. Evaluation of biopsy specimens before and after treatment and immunohistochemistry revealed that PDL treatment of cutaneous DLE lesions led to a decrease in vascular endothelial proteins—intercellular adhesion molecule 1 and vascular cell adhesion molecule 1—with a coincident reduction in the dermal lymphocytic infiltrate in treated lesions.11 These results offer a somewhat satisfying view on the correlation between the theory and basic science of laser therapy and the subsequent clinical benefits afforded by laser treatment. A case series provided further evidence that PDL or intense pulsed light can ameliorate the cutaneous lesions of DLE in 16 patients in whom all other treatments had failed.12
Several other inflammatory dermatoses can be treated with PDL, though the evidence for most of these conditions is sporadic at best, consisting mostly of case reports and a few case series. Granuloma faciale is one such condition, with evidence of efficacy of the PDL dating back as far as 1999,13 though a more recent case series of 4 patients only showed response in 2 patients.14 Because granuloma faciale features vasculitis as a prominent feature in its pathology, targeting the blood vessels may be helpful, but it is important to remember that there is a complex interplay between multiple factors. For example, treatment with typical fluences used in dermatology can be proinflammatory, leading to tissue damage, necrosis, and posttreatment erythema. However, low-level laser therapy (LLLT) has been shown to downregulate proinflammatory mediators.15 Additionally, the presence of a large burden of inflammatory cells also may alter the effectiveness of the laser. Several case reports also the show effectiveness of both PDL and the CO2 laser in treating lesions of cutaneous sarcoidosis, especially lupus pernio.16-19 Of these 2 modalities, the use of the CO2 laser for effective remodeling of lupus pernio may be more intuitive; however, it is still important to note that the mechanism of action of several of these laser modalities is unclear with regard to the clinical benefit shown. Morphea and scleroderma also have been treated with laser therapy. It is essential to understand that in many cases, laser therapy may be targeted to treat the precise cutaneous manifestations of disease in each individual patient (eg, CO2 laser to treat disabling contractures and calcinosis cutis,20,21 PDL to treat telangiectases related to morphea22). Again, the most critical consideration is that the treatment modality should align with the cutaneous lesion being targeted.
A relatively recent development in the use of lasers has been LLLT, which refers to the use of lasers below levels where they would cause any thermal effects, thereby limiting tissue damage. Although the technology has existed for decades, there has been a recent flurry of reports extolling the many benefits of LLLT; however, the true physiologic effects of LLLT have yet to be determined, with many studies trying to elucidate its numerous effects on various signaling pathways, cell proliferation, and cellular respiration.23-26 Upon reviewing the literature, the list of cutaneous conditions that are being treated with LLLT is vast, spanning acne, vitiligo, wounds, burns, psoriasis, and alopecia, among others.15 It is important to consider that the definition of LLLT in the literature is rather broad with a wide range of wavelengths, fluences, and power densities. As such, the specific laser settings and protocols may vary considerably among different practitioners and therefore the treatment results also may vary. Nevertheless, many studies have hinted at promising results in the use of LLLT in conditions that may have previously been extremely difficult to treat (eg, alopecia). Earlier trials had demonstrated a faster resolution time in patients with alopecia areata when LLLT was added to a topical regimen27; however, the improvement was modest and lesions tended to improve with or without LLLT. Perhaps more compelling is the use of LLLT in treating androgenetic alopecia, a condition for which a satisfying facile treatment would truly carry great impact. Although physicians should be cautious of studies regarding LLLT and hair regrowth that are conducted by groups who may stand to benefit from producing such a device, the results are nonetheless notable, if only for the relative paucity of other therapeutic approaches toward this condition.28,29 A randomized, double-blind, controlled, multicenter trial showed significant improvements in median hair thickness and density with LLLT (P=.01 and P=.003, respectively), though global appearance did not change significantly.30
Laser Treatment of Skin Cancer
Lasers also have been used to treat cutaneous malignancies. Although they may be powerful in the treatment of these conditions, this treatment approach must be used with caution. As with any superficial treatment modality for skin cancer, it is difficult to ascertain if a lesion has been completely treated without any residual cancer cells, and therein lies the main caveat of laser treatment. With the use of a modality that causes a cutaneous response that may mask any underlying process, it is important to ensure that there is a reasonable degree of certainty that this treatment can effectively remove a cancerous lesion in its entirety while avoiding the theoretical risk that disturbing underlying vasculature and/or lymphatics may be modulating the ability of a cancer to metastasize. Thankfully, current evidence does not suggest that there are any downsides to laser treatment for malignancies. Clinically, we know that basal cell carcinomas (BCCs) often feature prominent vasculature, with telangiectases being used as a clinical marker to suggest the diagnosis of a BCC. Capitalizing on this aspect of the clinical lesion, PDL has been used to treat BCCs in 2 small studies with a response rate of approximately 75% for small BCCs in both studies.31,32 A recent randomized controlled trial showed significant superiority of PDL as compared to the control (P<.0001) in treatment of BCC, with nearly 80% (44/56) of cases showing histologically proven complete remission at 6-month follow-up.33 Thus, we have some promising data that suggest PDL may be a viable treatment option in BCC, especially in areas that are difficult to treat surgically.
Additionally, a newer treatment approach for BCC capitalizes on the ability of confocal microscopy to provide a feasible, bedside imaging modality to identify tumor margins. Confocal microscopy has been used as a road map to identify where and how to apply the laser treatment, thus allowing for a higher likelihood of complete destruction of the tumor, at least in theory.34 Although the concept of using confocal microscopy to guide laser treatment of skin cancer has been shown in smaller proof-of-concept case series, it remains to be seen if it is not only an efficacious approach that may be widely adopted but also whether it is pragmatic to do so, as the equipment and expertise involved in using confocal microscopy is not trivial.
Finally, lasers also have been used in the treatment of mycosis fungoides (MF), or cutaneous T-cell lymphoma. It has been suggested that this modality is an excellent treatment option as a skin-directed therapy for stage IA or IB MFs limited to the acral surfaces or MF palmaris et plantaris.35 The reasoning behind this approach was the effectiveness of narrowband UVB for early-stage MF, with an excimer laser operating at a similar wavelength (308 nm) and offering similar therapeutic benefits while limiting adverse effects to surrounding skin.36 More recently, the excimer laser was applied to a small population of 6 patients, with 3 achieving complete response, 1 with partial response, 1 with stable disease, and 1 with progressive disease. The authors were careful to point out that the excimer laser should not be thought of as a replacement for narrowband UVB in early-stage MF but rather as an adjunctive treatment of specific targeted lesional areas.36
Conclusion
Lasers are an important part of the dermatologist’s treatment arsenal. Although much attention has been focused on laser treatment for aesthetic indications, it is important not to overlook the fact that lasers also can be useful in the treatment of refractory skin diseases, as a first-line treatment in some conditions such as vascular lesions, or as an adjunctive treatment modality. There is a great deal of exciting research that may lead to new indications and a better understanding of how to best use these powerful tools, and the outlook is bright for the use of lasers in dermatology.
1. Bonis B, Kemeny L, Dobozy A, et al. 308 nm UVB excimer laser for psoriasis. Lancet. 1997;350:1522.
2. Fuselier HA Jr, McBurney EI, Brannan W, et al. Treatment of condylomata acuminata with carbon dioxide laser. Urology. 1980;15:265-266.
3. Mueller TJ, Carlson BA, Lindy MP. The use of the carbon dioxide surgical laser for the treatment of verrucae. J Am Podiatry Assoc. 1980;70:136-141.
4. Weyandt GH, Tollmann F, Kristen P, et al. Low risk of contamination with human papilloma virus during treatment of condylomata acuminata with multilayer argon plasma coagulation and CO2 laser ablation. Arch Dermatol Res. 2011;303:141-144.
5. Ferenczy A, Bergeron C, Richart RM. Human papillomavirus DNA in CO2 laser-generated plume of smoke and its consequences to the surgeon. Obstet Gynecol. 1990;75:114-118.
6. Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981:263-276.
7. Morelli JG, Tan OT, Garden J, et al. Tunable dye laser (577 nm) treatment of port wine stains. Lasers Surg Med. 1986;6:94-99.
8. Reyes BA, Geronemus R. Treatment of port-wine stains during childhood with the flashlamp-pumped pulsed dye laser. J Am Acad Dermatol. 1990;23:1142-1148.
9. Ashinoff R, Geronemus RG. Capillary hemangiomas and treatment with the flash lamp-pumped pulsed dye laser. Arch Dermatol. 1991;127:202-205.
10. Erceg A, Bovenschen HJ, van de Kerkhof PC, et al. Efficacy and safety of pulsed dye laser treatment for cutaneous discoid lupus erythematosus. J Am Acad Dermatol. 2009;60:626-632.
11. Diez MT, Boixeda P, Moreno C, et al. Histopathology and immunohistochemistry of cutaneous lupus erythematosus after pulsed dye laser treatment. Dermatol Surg. 2011;37:971-981.
12. Ekback MP, Troilius A. Laser therapy for refractory discoid lupus erythematosus when everything else has failed. J Cosmet Laser Ther. 2013;15:260-265.
13. Welsh JH, Schroeder TL, Levy ML. Granuloma faciale in a child successfully treated with the pulsed dye laser. J Am Acad Dermatol. 1999;41:351-353.
14. Cheung ST, Lanigan SW. Granuloma faciale treated with the pulsed-dye laser: a case series. Clin Exp Dermatol. 2005;30:373-375.
15. Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32:41-52.
16. Roos S, Raulin C, Ockenfels HM, et al. Successful treatment of cutaneous sarcoidosis lesions with the flashlamp pumped pulsed dye laser: a case report. Dermatol Surg. 2009;35:1139-1140.
17. Cliff S, Felix RH, Singh L, et al. The successful treatment of lupus pernio with the flashlamp pulsed dye laser. J Cutan Laser Ther. 1999;1:49-52.
18. O’Donoghue NB, Barlow RJ. Laser remodelling of nodular nasal lupus pernio. Clin Exp Dermatol. 2006;31:27-29.
19. Young HS, Chalmers RJ, Griffiths CE, et al. CO2 laser vaporization for disfiguring lupus pernio. J Cosmet Laser Ther. 2002;4:87-90.
20. Kineston D, Kwan JM, Uebelhoer NS, et al. Use of a fractional ablative 10.6-mum carbon dioxide laser in the treatment of a morphea-related contracture. Arch Dermatol. 2011;147:1148-1150.
21. Chamberlain AJ, Walker NP. Successful palliation and significant remission of cutaneous calcinosis in CREST syndrome with carbon dioxide laser. Dermatol Surg. 2003;29:968-970.
22. Ciatti S, Varga J, Greenbaum SS. The 585 nm flashlamp-pumped pulsed dye laser for the treatment of telangiectases in patients with scleroderma. J Am Acad Dermatol. 1996;35:487-488.
23. Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23:355-361.
24. Greco M, Guida G, Perlino E, et al. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem Biophys Res Commun. 1989;163:1428-1434.
25. Karu TI, Pyatibrat LV, Kalendo GS. Photobiological modulation of cell attachment via cytochrome c oxidase. Photochem Photobiol Sci. 2004;3:211-216.
26. Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761-4771.
27. Yamazaki M, Miura Y, Tsuboi R, et al. Linear polarized infrared irradiation using Super Lizer is an effective treatment for multiple-type alopecia areata. Int J Dermatol. 2003;42:738-740.
28. Leavitt M, Charles G, Heyman E, et al. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: a randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29:283-292.
29. Munck A, Gavazzoni MF, Trueb RM. Use of low-level laser therapy as monotherapy or concomitant therapy for male and female androgenetic alopecia. Int J Trichology. 2014;6:45-49.
30. Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
31. Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series [published online ahead of print December 13, 2012]. J Skin Cancer. 2012;2012:286480.
32. Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
33. Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial [published online ahead of print July 12, 2014]. Br J Dermatol. doi:10.1111/bjd.13266.
34. Chen CS, Sierra H, Cordova M, et al. Confocal microscopy-guided laser ablation for superficial and early nodular Basal cell carcinoma: a promising surgical alternative for superficial skin cancers. JAMA Dermatol. 2014;150:994-998.
35. Jin SP, Jeon YK, Cho KH, et al. Excimer laser therapy (308 nm) for mycosis fungoides palmaris et plantaris: a skin-directed and anatomically feasible treatment. Br J Dermatol. 2010;163:651-653.
36. Deaver D, Cauthen A, Cohen G, et al. Excimer laser in the treatment of mycosis fungoides. J Am Acad Dermatol. 2014;70:1058-1060.
The use of lasers in dermatology has had a major impact on the treatment of many dermatologic conditions. In this column practical applications of lasers in medical dermatology will be discussed to give dermatology residents a broad overview of both established indications and the reasoning behind the usage of lasers in treating these skin conditions. The applications for lasers in aesthetic dermatology are numerous and are constantly being refined and developed; they have been discussed extensively in the literature. Given the vast variety of uses of lasers in dermatology today, a comprehensive review of this topic would likely span several volumes. This article will focus on recent evidence regarding the use of lasers in medical dermatology, specifically laser treatment of selected common dermatoses and cutaneous malignancies.
Laser Treatment of Skin Diseases
Many common dermatoses seen in the dermatologist’s office (eg, discoid lupus erythematosus [DLE], morphea, alopecia) already have an established therapeutic ladder, with most patients responding to either first- or second-line therapies; however, a number of patients present with refractory disease that can be difficult to treat due to either treatment resistance or other contraindications to therapy. With the advent and development of modern lasers, we are now able to target many of these conditions and provide a viable safe treatment option for these patients. Although many physicians may be familiar with the use of the excimer laser in the treatment of psoriasis,1 a long-standing and well-accepted treatment modality for this condition, many novel applications for different types of lasers have been developed.
First, it is important to consider what a laser is able to accomplish to modulate the skin. With ablative lasers such as the CO2 laser, it is possible to destroy superficial layers of the skin (ie, the epidermis). It would stand to reason that this approach would be ideal for treating epidermal processes such as viral warts; in fact, this modality has been used for this indication for more than 3 decades, with the earliest references coming from the podiatric and urologic literature.2,3 Despite conflicting reports of the risk for human papillomavirus aerosolization and subsequent contamination of the treatment area,4,5 CO2 laser therapy has been advocated as a nonsurgical approach to difficult-to-treat cases of viral warts.
On the other hand, the pulsed dye laser (PDL) can target blood vessels because the wavelength corresponds to the absorption spectrum of hemoglobin and penetrates to the level of the dermis, while the pulse duration can be set to be shorter than the thermal relaxation time of a small cutaneous blood vessel.6 In clinical practice, the PDL has been used for the treatment of vascular lesions including hemangiomas, nevus flammeus, and other vascular proliferations.7-9 However, the PDL also can be used to target the vessels in cutaneous inflammatory diseases that feature vascular dilation and/or perivascular inflammation as a prominent feature.
Discoid lupus erythematosus is a form of chronic cutaneous lupus erythematosus that may be difficult to treat, with recalcitrant lesions displaying continued inflammation leading to chronic scarring and dyspigmentation. A small study (N=12) presented the efficacy of the PDL in the treatment of DLE lesions, suggesting that it has good efficacy in treating recalcitrant lesions with significant reduction in the cutaneous lupus erythematosus disease area and severity index after 6 weeks of treatment and 6 weeks of follow-up (P<.0001) with decreased erythema and scaling.10 It is important to note, however, that scarring, dyspigmentation, and atrophy were not affected, which suggests that early intervention may be optimal to prevent development of these sequelae. More interestingly, a more recent study expounded on this idea and attempted to examine pathophysiologic mechanisms behind this observed improvement. Evaluation of biopsy specimens before and after treatment and immunohistochemistry revealed that PDL treatment of cutaneous DLE lesions led to a decrease in vascular endothelial proteins—intercellular adhesion molecule 1 and vascular cell adhesion molecule 1—with a coincident reduction in the dermal lymphocytic infiltrate in treated lesions.11 These results offer a somewhat satisfying view on the correlation between the theory and basic science of laser therapy and the subsequent clinical benefits afforded by laser treatment. A case series provided further evidence that PDL or intense pulsed light can ameliorate the cutaneous lesions of DLE in 16 patients in whom all other treatments had failed.12
Several other inflammatory dermatoses can be treated with PDL, though the evidence for most of these conditions is sporadic at best, consisting mostly of case reports and a few case series. Granuloma faciale is one such condition, with evidence of efficacy of the PDL dating back as far as 1999,13 though a more recent case series of 4 patients only showed response in 2 patients.14 Because granuloma faciale features vasculitis as a prominent feature in its pathology, targeting the blood vessels may be helpful, but it is important to remember that there is a complex interplay between multiple factors. For example, treatment with typical fluences used in dermatology can be proinflammatory, leading to tissue damage, necrosis, and posttreatment erythema. However, low-level laser therapy (LLLT) has been shown to downregulate proinflammatory mediators.15 Additionally, the presence of a large burden of inflammatory cells also may alter the effectiveness of the laser. Several case reports also the show effectiveness of both PDL and the CO2 laser in treating lesions of cutaneous sarcoidosis, especially lupus pernio.16-19 Of these 2 modalities, the use of the CO2 laser for effective remodeling of lupus pernio may be more intuitive; however, it is still important to note that the mechanism of action of several of these laser modalities is unclear with regard to the clinical benefit shown. Morphea and scleroderma also have been treated with laser therapy. It is essential to understand that in many cases, laser therapy may be targeted to treat the precise cutaneous manifestations of disease in each individual patient (eg, CO2 laser to treat disabling contractures and calcinosis cutis,20,21 PDL to treat telangiectases related to morphea22). Again, the most critical consideration is that the treatment modality should align with the cutaneous lesion being targeted.
A relatively recent development in the use of lasers has been LLLT, which refers to the use of lasers below levels where they would cause any thermal effects, thereby limiting tissue damage. Although the technology has existed for decades, there has been a recent flurry of reports extolling the many benefits of LLLT; however, the true physiologic effects of LLLT have yet to be determined, with many studies trying to elucidate its numerous effects on various signaling pathways, cell proliferation, and cellular respiration.23-26 Upon reviewing the literature, the list of cutaneous conditions that are being treated with LLLT is vast, spanning acne, vitiligo, wounds, burns, psoriasis, and alopecia, among others.15 It is important to consider that the definition of LLLT in the literature is rather broad with a wide range of wavelengths, fluences, and power densities. As such, the specific laser settings and protocols may vary considerably among different practitioners and therefore the treatment results also may vary. Nevertheless, many studies have hinted at promising results in the use of LLLT in conditions that may have previously been extremely difficult to treat (eg, alopecia). Earlier trials had demonstrated a faster resolution time in patients with alopecia areata when LLLT was added to a topical regimen27; however, the improvement was modest and lesions tended to improve with or without LLLT. Perhaps more compelling is the use of LLLT in treating androgenetic alopecia, a condition for which a satisfying facile treatment would truly carry great impact. Although physicians should be cautious of studies regarding LLLT and hair regrowth that are conducted by groups who may stand to benefit from producing such a device, the results are nonetheless notable, if only for the relative paucity of other therapeutic approaches toward this condition.28,29 A randomized, double-blind, controlled, multicenter trial showed significant improvements in median hair thickness and density with LLLT (P=.01 and P=.003, respectively), though global appearance did not change significantly.30
Laser Treatment of Skin Cancer
Lasers also have been used to treat cutaneous malignancies. Although they may be powerful in the treatment of these conditions, this treatment approach must be used with caution. As with any superficial treatment modality for skin cancer, it is difficult to ascertain if a lesion has been completely treated without any residual cancer cells, and therein lies the main caveat of laser treatment. With the use of a modality that causes a cutaneous response that may mask any underlying process, it is important to ensure that there is a reasonable degree of certainty that this treatment can effectively remove a cancerous lesion in its entirety while avoiding the theoretical risk that disturbing underlying vasculature and/or lymphatics may be modulating the ability of a cancer to metastasize. Thankfully, current evidence does not suggest that there are any downsides to laser treatment for malignancies. Clinically, we know that basal cell carcinomas (BCCs) often feature prominent vasculature, with telangiectases being used as a clinical marker to suggest the diagnosis of a BCC. Capitalizing on this aspect of the clinical lesion, PDL has been used to treat BCCs in 2 small studies with a response rate of approximately 75% for small BCCs in both studies.31,32 A recent randomized controlled trial showed significant superiority of PDL as compared to the control (P<.0001) in treatment of BCC, with nearly 80% (44/56) of cases showing histologically proven complete remission at 6-month follow-up.33 Thus, we have some promising data that suggest PDL may be a viable treatment option in BCC, especially in areas that are difficult to treat surgically.
Additionally, a newer treatment approach for BCC capitalizes on the ability of confocal microscopy to provide a feasible, bedside imaging modality to identify tumor margins. Confocal microscopy has been used as a road map to identify where and how to apply the laser treatment, thus allowing for a higher likelihood of complete destruction of the tumor, at least in theory.34 Although the concept of using confocal microscopy to guide laser treatment of skin cancer has been shown in smaller proof-of-concept case series, it remains to be seen if it is not only an efficacious approach that may be widely adopted but also whether it is pragmatic to do so, as the equipment and expertise involved in using confocal microscopy is not trivial.
Finally, lasers also have been used in the treatment of mycosis fungoides (MF), or cutaneous T-cell lymphoma. It has been suggested that this modality is an excellent treatment option as a skin-directed therapy for stage IA or IB MFs limited to the acral surfaces or MF palmaris et plantaris.35 The reasoning behind this approach was the effectiveness of narrowband UVB for early-stage MF, with an excimer laser operating at a similar wavelength (308 nm) and offering similar therapeutic benefits while limiting adverse effects to surrounding skin.36 More recently, the excimer laser was applied to a small population of 6 patients, with 3 achieving complete response, 1 with partial response, 1 with stable disease, and 1 with progressive disease. The authors were careful to point out that the excimer laser should not be thought of as a replacement for narrowband UVB in early-stage MF but rather as an adjunctive treatment of specific targeted lesional areas.36
Conclusion
Lasers are an important part of the dermatologist’s treatment arsenal. Although much attention has been focused on laser treatment for aesthetic indications, it is important not to overlook the fact that lasers also can be useful in the treatment of refractory skin diseases, as a first-line treatment in some conditions such as vascular lesions, or as an adjunctive treatment modality. There is a great deal of exciting research that may lead to new indications and a better understanding of how to best use these powerful tools, and the outlook is bright for the use of lasers in dermatology.
The use of lasers in dermatology has had a major impact on the treatment of many dermatologic conditions. In this column practical applications of lasers in medical dermatology will be discussed to give dermatology residents a broad overview of both established indications and the reasoning behind the usage of lasers in treating these skin conditions. The applications for lasers in aesthetic dermatology are numerous and are constantly being refined and developed; they have been discussed extensively in the literature. Given the vast variety of uses of lasers in dermatology today, a comprehensive review of this topic would likely span several volumes. This article will focus on recent evidence regarding the use of lasers in medical dermatology, specifically laser treatment of selected common dermatoses and cutaneous malignancies.
Laser Treatment of Skin Diseases
Many common dermatoses seen in the dermatologist’s office (eg, discoid lupus erythematosus [DLE], morphea, alopecia) already have an established therapeutic ladder, with most patients responding to either first- or second-line therapies; however, a number of patients present with refractory disease that can be difficult to treat due to either treatment resistance or other contraindications to therapy. With the advent and development of modern lasers, we are now able to target many of these conditions and provide a viable safe treatment option for these patients. Although many physicians may be familiar with the use of the excimer laser in the treatment of psoriasis,1 a long-standing and well-accepted treatment modality for this condition, many novel applications for different types of lasers have been developed.
First, it is important to consider what a laser is able to accomplish to modulate the skin. With ablative lasers such as the CO2 laser, it is possible to destroy superficial layers of the skin (ie, the epidermis). It would stand to reason that this approach would be ideal for treating epidermal processes such as viral warts; in fact, this modality has been used for this indication for more than 3 decades, with the earliest references coming from the podiatric and urologic literature.2,3 Despite conflicting reports of the risk for human papillomavirus aerosolization and subsequent contamination of the treatment area,4,5 CO2 laser therapy has been advocated as a nonsurgical approach to difficult-to-treat cases of viral warts.
On the other hand, the pulsed dye laser (PDL) can target blood vessels because the wavelength corresponds to the absorption spectrum of hemoglobin and penetrates to the level of the dermis, while the pulse duration can be set to be shorter than the thermal relaxation time of a small cutaneous blood vessel.6 In clinical practice, the PDL has been used for the treatment of vascular lesions including hemangiomas, nevus flammeus, and other vascular proliferations.7-9 However, the PDL also can be used to target the vessels in cutaneous inflammatory diseases that feature vascular dilation and/or perivascular inflammation as a prominent feature.
Discoid lupus erythematosus is a form of chronic cutaneous lupus erythematosus that may be difficult to treat, with recalcitrant lesions displaying continued inflammation leading to chronic scarring and dyspigmentation. A small study (N=12) presented the efficacy of the PDL in the treatment of DLE lesions, suggesting that it has good efficacy in treating recalcitrant lesions with significant reduction in the cutaneous lupus erythematosus disease area and severity index after 6 weeks of treatment and 6 weeks of follow-up (P<.0001) with decreased erythema and scaling.10 It is important to note, however, that scarring, dyspigmentation, and atrophy were not affected, which suggests that early intervention may be optimal to prevent development of these sequelae. More interestingly, a more recent study expounded on this idea and attempted to examine pathophysiologic mechanisms behind this observed improvement. Evaluation of biopsy specimens before and after treatment and immunohistochemistry revealed that PDL treatment of cutaneous DLE lesions led to a decrease in vascular endothelial proteins—intercellular adhesion molecule 1 and vascular cell adhesion molecule 1—with a coincident reduction in the dermal lymphocytic infiltrate in treated lesions.11 These results offer a somewhat satisfying view on the correlation between the theory and basic science of laser therapy and the subsequent clinical benefits afforded by laser treatment. A case series provided further evidence that PDL or intense pulsed light can ameliorate the cutaneous lesions of DLE in 16 patients in whom all other treatments had failed.12
Several other inflammatory dermatoses can be treated with PDL, though the evidence for most of these conditions is sporadic at best, consisting mostly of case reports and a few case series. Granuloma faciale is one such condition, with evidence of efficacy of the PDL dating back as far as 1999,13 though a more recent case series of 4 patients only showed response in 2 patients.14 Because granuloma faciale features vasculitis as a prominent feature in its pathology, targeting the blood vessels may be helpful, but it is important to remember that there is a complex interplay between multiple factors. For example, treatment with typical fluences used in dermatology can be proinflammatory, leading to tissue damage, necrosis, and posttreatment erythema. However, low-level laser therapy (LLLT) has been shown to downregulate proinflammatory mediators.15 Additionally, the presence of a large burden of inflammatory cells also may alter the effectiveness of the laser. Several case reports also the show effectiveness of both PDL and the CO2 laser in treating lesions of cutaneous sarcoidosis, especially lupus pernio.16-19 Of these 2 modalities, the use of the CO2 laser for effective remodeling of lupus pernio may be more intuitive; however, it is still important to note that the mechanism of action of several of these laser modalities is unclear with regard to the clinical benefit shown. Morphea and scleroderma also have been treated with laser therapy. It is essential to understand that in many cases, laser therapy may be targeted to treat the precise cutaneous manifestations of disease in each individual patient (eg, CO2 laser to treat disabling contractures and calcinosis cutis,20,21 PDL to treat telangiectases related to morphea22). Again, the most critical consideration is that the treatment modality should align with the cutaneous lesion being targeted.
A relatively recent development in the use of lasers has been LLLT, which refers to the use of lasers below levels where they would cause any thermal effects, thereby limiting tissue damage. Although the technology has existed for decades, there has been a recent flurry of reports extolling the many benefits of LLLT; however, the true physiologic effects of LLLT have yet to be determined, with many studies trying to elucidate its numerous effects on various signaling pathways, cell proliferation, and cellular respiration.23-26 Upon reviewing the literature, the list of cutaneous conditions that are being treated with LLLT is vast, spanning acne, vitiligo, wounds, burns, psoriasis, and alopecia, among others.15 It is important to consider that the definition of LLLT in the literature is rather broad with a wide range of wavelengths, fluences, and power densities. As such, the specific laser settings and protocols may vary considerably among different practitioners and therefore the treatment results also may vary. Nevertheless, many studies have hinted at promising results in the use of LLLT in conditions that may have previously been extremely difficult to treat (eg, alopecia). Earlier trials had demonstrated a faster resolution time in patients with alopecia areata when LLLT was added to a topical regimen27; however, the improvement was modest and lesions tended to improve with or without LLLT. Perhaps more compelling is the use of LLLT in treating androgenetic alopecia, a condition for which a satisfying facile treatment would truly carry great impact. Although physicians should be cautious of studies regarding LLLT and hair regrowth that are conducted by groups who may stand to benefit from producing such a device, the results are nonetheless notable, if only for the relative paucity of other therapeutic approaches toward this condition.28,29 A randomized, double-blind, controlled, multicenter trial showed significant improvements in median hair thickness and density with LLLT (P=.01 and P=.003, respectively), though global appearance did not change significantly.30
Laser Treatment of Skin Cancer
Lasers also have been used to treat cutaneous malignancies. Although they may be powerful in the treatment of these conditions, this treatment approach must be used with caution. As with any superficial treatment modality for skin cancer, it is difficult to ascertain if a lesion has been completely treated without any residual cancer cells, and therein lies the main caveat of laser treatment. With the use of a modality that causes a cutaneous response that may mask any underlying process, it is important to ensure that there is a reasonable degree of certainty that this treatment can effectively remove a cancerous lesion in its entirety while avoiding the theoretical risk that disturbing underlying vasculature and/or lymphatics may be modulating the ability of a cancer to metastasize. Thankfully, current evidence does not suggest that there are any downsides to laser treatment for malignancies. Clinically, we know that basal cell carcinomas (BCCs) often feature prominent vasculature, with telangiectases being used as a clinical marker to suggest the diagnosis of a BCC. Capitalizing on this aspect of the clinical lesion, PDL has been used to treat BCCs in 2 small studies with a response rate of approximately 75% for small BCCs in both studies.31,32 A recent randomized controlled trial showed significant superiority of PDL as compared to the control (P<.0001) in treatment of BCC, with nearly 80% (44/56) of cases showing histologically proven complete remission at 6-month follow-up.33 Thus, we have some promising data that suggest PDL may be a viable treatment option in BCC, especially in areas that are difficult to treat surgically.
Additionally, a newer treatment approach for BCC capitalizes on the ability of confocal microscopy to provide a feasible, bedside imaging modality to identify tumor margins. Confocal microscopy has been used as a road map to identify where and how to apply the laser treatment, thus allowing for a higher likelihood of complete destruction of the tumor, at least in theory.34 Although the concept of using confocal microscopy to guide laser treatment of skin cancer has been shown in smaller proof-of-concept case series, it remains to be seen if it is not only an efficacious approach that may be widely adopted but also whether it is pragmatic to do so, as the equipment and expertise involved in using confocal microscopy is not trivial.
Finally, lasers also have been used in the treatment of mycosis fungoides (MF), or cutaneous T-cell lymphoma. It has been suggested that this modality is an excellent treatment option as a skin-directed therapy for stage IA or IB MFs limited to the acral surfaces or MF palmaris et plantaris.35 The reasoning behind this approach was the effectiveness of narrowband UVB for early-stage MF, with an excimer laser operating at a similar wavelength (308 nm) and offering similar therapeutic benefits while limiting adverse effects to surrounding skin.36 More recently, the excimer laser was applied to a small population of 6 patients, with 3 achieving complete response, 1 with partial response, 1 with stable disease, and 1 with progressive disease. The authors were careful to point out that the excimer laser should not be thought of as a replacement for narrowband UVB in early-stage MF but rather as an adjunctive treatment of specific targeted lesional areas.36
Conclusion
Lasers are an important part of the dermatologist’s treatment arsenal. Although much attention has been focused on laser treatment for aesthetic indications, it is important not to overlook the fact that lasers also can be useful in the treatment of refractory skin diseases, as a first-line treatment in some conditions such as vascular lesions, or as an adjunctive treatment modality. There is a great deal of exciting research that may lead to new indications and a better understanding of how to best use these powerful tools, and the outlook is bright for the use of lasers in dermatology.
1. Bonis B, Kemeny L, Dobozy A, et al. 308 nm UVB excimer laser for psoriasis. Lancet. 1997;350:1522.
2. Fuselier HA Jr, McBurney EI, Brannan W, et al. Treatment of condylomata acuminata with carbon dioxide laser. Urology. 1980;15:265-266.
3. Mueller TJ, Carlson BA, Lindy MP. The use of the carbon dioxide surgical laser for the treatment of verrucae. J Am Podiatry Assoc. 1980;70:136-141.
4. Weyandt GH, Tollmann F, Kristen P, et al. Low risk of contamination with human papilloma virus during treatment of condylomata acuminata with multilayer argon plasma coagulation and CO2 laser ablation. Arch Dermatol Res. 2011;303:141-144.
5. Ferenczy A, Bergeron C, Richart RM. Human papillomavirus DNA in CO2 laser-generated plume of smoke and its consequences to the surgeon. Obstet Gynecol. 1990;75:114-118.
6. Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981:263-276.
7. Morelli JG, Tan OT, Garden J, et al. Tunable dye laser (577 nm) treatment of port wine stains. Lasers Surg Med. 1986;6:94-99.
8. Reyes BA, Geronemus R. Treatment of port-wine stains during childhood with the flashlamp-pumped pulsed dye laser. J Am Acad Dermatol. 1990;23:1142-1148.
9. Ashinoff R, Geronemus RG. Capillary hemangiomas and treatment with the flash lamp-pumped pulsed dye laser. Arch Dermatol. 1991;127:202-205.
10. Erceg A, Bovenschen HJ, van de Kerkhof PC, et al. Efficacy and safety of pulsed dye laser treatment for cutaneous discoid lupus erythematosus. J Am Acad Dermatol. 2009;60:626-632.
11. Diez MT, Boixeda P, Moreno C, et al. Histopathology and immunohistochemistry of cutaneous lupus erythematosus after pulsed dye laser treatment. Dermatol Surg. 2011;37:971-981.
12. Ekback MP, Troilius A. Laser therapy for refractory discoid lupus erythematosus when everything else has failed. J Cosmet Laser Ther. 2013;15:260-265.
13. Welsh JH, Schroeder TL, Levy ML. Granuloma faciale in a child successfully treated with the pulsed dye laser. J Am Acad Dermatol. 1999;41:351-353.
14. Cheung ST, Lanigan SW. Granuloma faciale treated with the pulsed-dye laser: a case series. Clin Exp Dermatol. 2005;30:373-375.
15. Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32:41-52.
16. Roos S, Raulin C, Ockenfels HM, et al. Successful treatment of cutaneous sarcoidosis lesions with the flashlamp pumped pulsed dye laser: a case report. Dermatol Surg. 2009;35:1139-1140.
17. Cliff S, Felix RH, Singh L, et al. The successful treatment of lupus pernio with the flashlamp pulsed dye laser. J Cutan Laser Ther. 1999;1:49-52.
18. O’Donoghue NB, Barlow RJ. Laser remodelling of nodular nasal lupus pernio. Clin Exp Dermatol. 2006;31:27-29.
19. Young HS, Chalmers RJ, Griffiths CE, et al. CO2 laser vaporization for disfiguring lupus pernio. J Cosmet Laser Ther. 2002;4:87-90.
20. Kineston D, Kwan JM, Uebelhoer NS, et al. Use of a fractional ablative 10.6-mum carbon dioxide laser in the treatment of a morphea-related contracture. Arch Dermatol. 2011;147:1148-1150.
21. Chamberlain AJ, Walker NP. Successful palliation and significant remission of cutaneous calcinosis in CREST syndrome with carbon dioxide laser. Dermatol Surg. 2003;29:968-970.
22. Ciatti S, Varga J, Greenbaum SS. The 585 nm flashlamp-pumped pulsed dye laser for the treatment of telangiectases in patients with scleroderma. J Am Acad Dermatol. 1996;35:487-488.
23. Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23:355-361.
24. Greco M, Guida G, Perlino E, et al. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem Biophys Res Commun. 1989;163:1428-1434.
25. Karu TI, Pyatibrat LV, Kalendo GS. Photobiological modulation of cell attachment via cytochrome c oxidase. Photochem Photobiol Sci. 2004;3:211-216.
26. Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761-4771.
27. Yamazaki M, Miura Y, Tsuboi R, et al. Linear polarized infrared irradiation using Super Lizer is an effective treatment for multiple-type alopecia areata. Int J Dermatol. 2003;42:738-740.
28. Leavitt M, Charles G, Heyman E, et al. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: a randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29:283-292.
29. Munck A, Gavazzoni MF, Trueb RM. Use of low-level laser therapy as monotherapy or concomitant therapy for male and female androgenetic alopecia. Int J Trichology. 2014;6:45-49.
30. Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
31. Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series [published online ahead of print December 13, 2012]. J Skin Cancer. 2012;2012:286480.
32. Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
33. Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial [published online ahead of print July 12, 2014]. Br J Dermatol. doi:10.1111/bjd.13266.
34. Chen CS, Sierra H, Cordova M, et al. Confocal microscopy-guided laser ablation for superficial and early nodular Basal cell carcinoma: a promising surgical alternative for superficial skin cancers. JAMA Dermatol. 2014;150:994-998.
35. Jin SP, Jeon YK, Cho KH, et al. Excimer laser therapy (308 nm) for mycosis fungoides palmaris et plantaris: a skin-directed and anatomically feasible treatment. Br J Dermatol. 2010;163:651-653.
36. Deaver D, Cauthen A, Cohen G, et al. Excimer laser in the treatment of mycosis fungoides. J Am Acad Dermatol. 2014;70:1058-1060.
1. Bonis B, Kemeny L, Dobozy A, et al. 308 nm UVB excimer laser for psoriasis. Lancet. 1997;350:1522.
2. Fuselier HA Jr, McBurney EI, Brannan W, et al. Treatment of condylomata acuminata with carbon dioxide laser. Urology. 1980;15:265-266.
3. Mueller TJ, Carlson BA, Lindy MP. The use of the carbon dioxide surgical laser for the treatment of verrucae. J Am Podiatry Assoc. 1980;70:136-141.
4. Weyandt GH, Tollmann F, Kristen P, et al. Low risk of contamination with human papilloma virus during treatment of condylomata acuminata with multilayer argon plasma coagulation and CO2 laser ablation. Arch Dermatol Res. 2011;303:141-144.
5. Ferenczy A, Bergeron C, Richart RM. Human papillomavirus DNA in CO2 laser-generated plume of smoke and its consequences to the surgeon. Obstet Gynecol. 1990;75:114-118.
6. Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981:263-276.
7. Morelli JG, Tan OT, Garden J, et al. Tunable dye laser (577 nm) treatment of port wine stains. Lasers Surg Med. 1986;6:94-99.
8. Reyes BA, Geronemus R. Treatment of port-wine stains during childhood with the flashlamp-pumped pulsed dye laser. J Am Acad Dermatol. 1990;23:1142-1148.
9. Ashinoff R, Geronemus RG. Capillary hemangiomas and treatment with the flash lamp-pumped pulsed dye laser. Arch Dermatol. 1991;127:202-205.
10. Erceg A, Bovenschen HJ, van de Kerkhof PC, et al. Efficacy and safety of pulsed dye laser treatment for cutaneous discoid lupus erythematosus. J Am Acad Dermatol. 2009;60:626-632.
11. Diez MT, Boixeda P, Moreno C, et al. Histopathology and immunohistochemistry of cutaneous lupus erythematosus after pulsed dye laser treatment. Dermatol Surg. 2011;37:971-981.
12. Ekback MP, Troilius A. Laser therapy for refractory discoid lupus erythematosus when everything else has failed. J Cosmet Laser Ther. 2013;15:260-265.
13. Welsh JH, Schroeder TL, Levy ML. Granuloma faciale in a child successfully treated with the pulsed dye laser. J Am Acad Dermatol. 1999;41:351-353.
14. Cheung ST, Lanigan SW. Granuloma faciale treated with the pulsed-dye laser: a case series. Clin Exp Dermatol. 2005;30:373-375.
15. Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32:41-52.
16. Roos S, Raulin C, Ockenfels HM, et al. Successful treatment of cutaneous sarcoidosis lesions with the flashlamp pumped pulsed dye laser: a case report. Dermatol Surg. 2009;35:1139-1140.
17. Cliff S, Felix RH, Singh L, et al. The successful treatment of lupus pernio with the flashlamp pulsed dye laser. J Cutan Laser Ther. 1999;1:49-52.
18. O’Donoghue NB, Barlow RJ. Laser remodelling of nodular nasal lupus pernio. Clin Exp Dermatol. 2006;31:27-29.
19. Young HS, Chalmers RJ, Griffiths CE, et al. CO2 laser vaporization for disfiguring lupus pernio. J Cosmet Laser Ther. 2002;4:87-90.
20. Kineston D, Kwan JM, Uebelhoer NS, et al. Use of a fractional ablative 10.6-mum carbon dioxide laser in the treatment of a morphea-related contracture. Arch Dermatol. 2011;147:1148-1150.
21. Chamberlain AJ, Walker NP. Successful palliation and significant remission of cutaneous calcinosis in CREST syndrome with carbon dioxide laser. Dermatol Surg. 2003;29:968-970.
22. Ciatti S, Varga J, Greenbaum SS. The 585 nm flashlamp-pumped pulsed dye laser for the treatment of telangiectases in patients with scleroderma. J Am Acad Dermatol. 1996;35:487-488.
23. Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23:355-361.
24. Greco M, Guida G, Perlino E, et al. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem Biophys Res Commun. 1989;163:1428-1434.
25. Karu TI, Pyatibrat LV, Kalendo GS. Photobiological modulation of cell attachment via cytochrome c oxidase. Photochem Photobiol Sci. 2004;3:211-216.
26. Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761-4771.
27. Yamazaki M, Miura Y, Tsuboi R, et al. Linear polarized infrared irradiation using Super Lizer is an effective treatment for multiple-type alopecia areata. Int J Dermatol. 2003;42:738-740.
28. Leavitt M, Charles G, Heyman E, et al. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: a randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29:283-292.
29. Munck A, Gavazzoni MF, Trueb RM. Use of low-level laser therapy as monotherapy or concomitant therapy for male and female androgenetic alopecia. Int J Trichology. 2014;6:45-49.
30. Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
31. Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series [published online ahead of print December 13, 2012]. J Skin Cancer. 2012;2012:286480.
32. Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
33. Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial [published online ahead of print July 12, 2014]. Br J Dermatol. doi:10.1111/bjd.13266.
34. Chen CS, Sierra H, Cordova M, et al. Confocal microscopy-guided laser ablation for superficial and early nodular Basal cell carcinoma: a promising surgical alternative for superficial skin cancers. JAMA Dermatol. 2014;150:994-998.
35. Jin SP, Jeon YK, Cho KH, et al. Excimer laser therapy (308 nm) for mycosis fungoides palmaris et plantaris: a skin-directed and anatomically feasible treatment. Br J Dermatol. 2010;163:651-653.
36. Deaver D, Cauthen A, Cohen G, et al. Excimer laser in the treatment of mycosis fungoides. J Am Acad Dermatol. 2014;70:1058-1060.
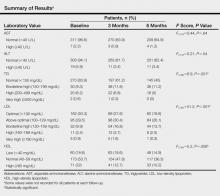
Effects of Oral Isotretinoin on Lipids and Liver Enzymes in Acne Patients
Acne is a chronic inflammatory condition of the pilosebaceous unit affecting approximately 79% to 95% of adolescents in the Western world.1 Treatment of acne depends on its severity. Topical tretinoin, adapalene, benzoyl peroxide, azelaic acid, and topical antibiotics generally are used in cases of noninflammatory or mild inflammatory disease. Isotretinoin is recommended for treatment of severe inflammatory acne (eg, nodulocystic or conglobata acne) and for cases of acne that have proven to be resistant to prior treatment with antibiotics or topical agents. Dosages of isotretinoin range from 0.5 to 2 mg/kg daily for 16 to 24 weeks.1 Isotretinoin reduces the activity and size of the sebaceous glands, normalizes keratinization of the sebaceous follicles, and decreases the number of Propionibacterium acnes. Isotretinoin also may cause clinical side effects and laboratory changes, the most important being teratogenicity. It also may cause mucocutaneous side effects including cracked lips, dryness of the skin and nasal mucosa, skin redness, eye dryness, and eye irritation.1 It also may cause blepharoconjuctivitis, photosensitivity, asteatotic dermatitis, pruritus, telogen effluvium, secondary bacterial colonization, nail fragility, periungual pyogenic granuloma, paronychia, myalgia, intracranial hypertension, nausea, headache, vomiting, depression, psychosis, suicide, constipation, and allergic reactions.2 Isotretinoin treatment may increase serum levels of liver enzymes, triglyc-erides (TGs), and low-density lipoprotein (LDL) cholesterol, and reduce the level of high-density lipoprotein (HDL) cholesterol.1 This retrospective study sought to evaluate the effect of isotretinoin on liver enzymes and lipids over 6 months.
Materials and Methods
Our retrospective study was conducted at the Hospital of Atatürk University in Erzurum, a city located in eastern Turkey. All patients who were treated in the department of dermatology and had received oral isotretinoin between June 2009 and June 2012 were included in the study. The study was based on an evaluation of the patients’ medical records. All patients received oral isotretinoin 0.5 to 1 mg/kg daily; the majority of patients received 30 to 40 mg daily. Patient medical records included age; gender; white blood cell (WBC) count; red blood cell (RBC) count; hemoglobin count; and aspartate aminotransferase (AST), alanine aminotransferase (ALT), TG, LDL, and HDL levels at the beginning of treatment. Aspartate aminotransferase, ALT, TG, LDL, and HDL levels also were measured at 3- and 6-month follow-up. Analysis of AST, ALT, TG, LDL, and HDL levels was based on the National Cholesterol Education Program guidelines.3 Aspartate aminotransferase and ALT levels were classified as normal (<40 U/L) and high (≥40 U/L). Triglyceride levels were classified as normal (<150 mg/dL), borderline high (150–199 mg/dL), high (200–499 mg/dL), and very high (≥500 mg/dL). Low-density lipoprotein levels were classified as optimal (<100 mg/dL), above optimal (100–129 mg/dL), borderline high (130–159 mg/dL), high (160–189 mg/dL), and very high (≥190 mg/dL). High-density lipoprotein levels were classified as low (<40 mg/dL), normal (40–59 mg/dL), and high (≥60 mg/dL). Normal WBC was defined as 3.5 to 12.5×103/mL. Normal hemoglobin count was defined as 11.5 to 15.0×106/mL for women and 13 to 17×106/mL for men. Normal RBC was defined as 4.0 to 5.2×106/mL for women and 4.5 to 5.9×106/mL for men. Statistical analysis was performed using SPSS version 17.0. Repeated measures analysis of variance was used to compare means between 3 groups (baseline, 3-month, and 6-month values). A paired sample t test was used to compare means between any 2 groups. Results with P<.05 were considered statistically significant.
Results
Treatment with oral isotretinoin was initiated in 349 patients at our institution from June 2009 to June 2012. Twenty-seven of these patients were excluded from the study because their medical records were not available. Medical records from 322 patients were obtained. The study population consisted of 226 (70.2%) women and 96 (29.8%) men. Patients ranged in age from 17 to 64 years, with a mean age of 23.9 years. The mean (standard deviation [SD]) age of the women was 23.9 (5.4) years and the mean (SD) age of the men was 23.8 (7.02) years. Most of the patients were on a regimen of 30 or 40 mg of isotretinoin daily. Differences between dosages and laboratory values were not analyzed because we assumed there would not be a significant difference, as most patients received the same dose. The mean (SD) WBC was 8.4 (3.5)×103/mL. The mean (SD) RBC was 4.9 (0.5)×106/mL. The mean (SD) hemoglobin count was 14.3 (1.7)×106/mL (women, 13.6 [1.5]×106/mL; men, 15.9 [1.1]×106/mL).
The study evaluated the effects of isotretinoin on liver enzymes (AST and ALT) and lipids (TGs, LDL, and HDL). Nearly all of the patients (>95%) had normal AST and ALT levels at baseline. The results are outlined in the Table. Some values were not recorded for all patients at each follow-up.
Aspartate Aminotransferase Analysis
Aspartate aminotransferase levels were classified as normal and high. At baseline, mean (SD) AST levels were 20.2 (6.6) U/L, with normal levels in 311 (96.6%) patients and high in 7 (2.2%) patients. At 3-month follow-up, mean (SD) AST levels were 20.7 (5.2) U/L, with normal levels in 270 (83.9%) patients and high levels in 3 (0.9%) patients. At 6-month follow-up, mean (SD) AST levels were 21.3 (5.7) U/L, with normal levels in 209 (64.9%) patients and high levels in 4 (1.2%) patients. Aspartate aminotransferase levels increased at 3- and 6-month follow-up compared to baseline. Differences between AST levels were statistically significant (F2,416=4.2, P=.016). Differences between AST levels at baseline and 3-month follow-up were not statistically significant (P=.3). Differences between AST levels at 3- and 6-month follow-up were not statistically significant (P=.4). Differences between AST levels at baseline and 6-month follow-up were statistically significant (P=.07). Differences between AST classifications at the 3 time points were not statistically significant (F2,416=0.44, P=.64). Overall, the results indicated that AST levels increased over time in patients treated with isotretinoin, but the increase was not above the normal range and was not statistically significant.
Alanine Aminotransferase Analysis
Alanine aminotransferase levels were classified as normal or high. At baseline, mean (SD) ALT levels were 16.8 (11.2) U/L, with normal levels in 303 (94.1%) patients and high in 19 (5.9%) patients. At 3-month follow-up, mean (SD) ALT levels were 16.2 (9.3) U/L, with normal levels in 263 (81.7%) patients and high in 11 (3.4%) patients. At 6-month follow-up, mean (SD) ALT levels were 17.0 (11.3) U/L, with normal levels in 201 (62.4%) patients and high in 11 (3.4%) patients. Alanine aminotransferase levels at 3-month follow-up were lower than baseline but higher at 6-month follow-up compared to baseline and 3-month follow-up. Overall, ALT levels increased with time, but the differences between baseline and 3- and 6-month follow-up were not statistically significant (F2,416=0.32, P=.72). Differences between ALT classifications at each time point were not statistically significant (F2,418=0.21, P=.54). Overall, the results indicated that ALT levels increased over time in patients treated with isotretinoin, but the increase was not statistically significant.
Triglycerides Analysis
Triglyceride levels were classified as normal, borderline high, high, and very high. At baseline, mean (SD) TG levels were 107 (71) mg/dL, with normal levels in 270 (83.9%) patients, borderline high in 30 (9.3%) patients, high in 20 (6.2%) patients, and very high in 2 (0.6%) patients. At 3-month follow-up, mean (SD) TG levels were 117 (60) mg/dL, with normal levels in 197 (61.2%) patients, borderline high in 38 (11.8%) patients, high in 22 (6.8%) patients, and very high in 1 (0.3%) patient. At 6-month follow-up, mean (SD) TG levels were 122 (65) mg/dL, with normal levels in 145 (45%) patients, borderline high in 36 (11.2%) patients, high in 16 (5%) patients, and very high in 0 (0%) patients. Triglyceride levels increased and differences between TG levels at baseline and 3- and 6-month follow-up were statistically significant (F2,384=17, P<.001). Baseline TG levels compared to 3-month follow-up were statistically signif-icant (P<.001). Differences in TG levels at 6-month follow-up versus baseline were statistically significant (P<.001). However, changes in TG levels from 3- to 6-month follow-up were not statistically significant (P=.21). Differences between TG classifications at each time point were statistically significant (F2,386=6.9, P=.001). Overall, TG levels increased from baseline during isotretinoin treatment at 3- and 6-month follow-up, and these increases were above normal range; however, there was no statistically significant increase from 3- to 6-month follow-up.
Low-Density Lipoprotein Analysis
Low-density lipoprotein levels were classified as optimal, above optimal, borderline high, high, and very high. At baseline, mean (SD) LDL levels were 102 (28) mg/dL, with optimal levels in 162 (50.3%) patients, above optimal in 95 (29.5%) patients, borderline high in 32 (9.9%) patients, high in 11 (3.4%) patients, and very high in 3 (0.9%) patients. At 3-month follow-up, mean (SD) LDL levels were 113 (30) mg/dL, with optimal levels in 89 (27.6%) patients, above optimal in 98 (30.4%) patients, borderline high in 54 (16.8%) patients, high in 12 (3.7%) patients, and very high in 5 (1.6%) patients. At 6-month follow-up, mean (SD) LDL levels were 113 (27) mg/dL, with optimal levels in 60 (18.6%) patients, above optimal in 84 (26.1%) patients, borderline high in 44 (13.7%) patients, high in 8 (2.5%) patients, and very high in 1 (0.3%) patient. Overall, there were statistically significant increases in LDL levels at 3- and 6-month follow-up (F2,382<75, P<.001). Differences between baseline LDL levels and 3-month follow-up were statistically significant (P<.001). Differences between baseline LDL levels and 6-month follow-up were statistically significant (P<.001). However, differences in LDL levels at 3- and 6-month follow-up were not statistically significant (P=.74). Differences between LDL classifications at each time point were statistically significant (F2,382=51.2, P<.001). Overall, statistically significant increases in LDL levels from baseline were noted during isotretinoin treatment and this increase was above normal range; however, LDL levels did not significantly increase from 3- to 6-month follow-up.
High-Density Lipoprotein Analysis
High-density lipoprotein levels were classified as low, normal, and high. At baseline, mean (SD) HDL levels were 52.4 (16) mg/dL, with low levels in 60 (18.6%) patients, normal in 173 (53.7%) patients, and high in 71 (22%) patients. At 3-month follow-up, mean (SD) HDL levels were 48 (12) mg/dL, with low levels in 63 (19.6%) patients, normal in 154 (47.8%) patients, and high in 41 (12.7%) patients. At 6-month follow-up, mean (SD) HDL levels were 47.6 (12) mg/dL, with low levels in 48 (14.9%) patients, normal in 117 (36.3%) patients, and high in 33 (10.2%) patients. Overall, statistically significant decreases were noted in HDL levels (F2,384=19, P<.001). Differences between baseline HDL levels compared to 3-month follow-up were statistically significant (P<.001). Differences between baseline HDL levels compared to 6-month follow-up were statistically significant (P<.001). Differences in HDL levels at 3- and 6-month follow-up were statistically significant (P<.001). Differences between HDL classifications at each time point were statistically significant (F2,384=5.2, P=.006). Overall, there were statistically significant decreases in HDL levels during isotretinoin treatment from baseline and this decrease was above normal range; however, HDL levels did not decrease at 3- and 6-month follow-up.
Comment
Studies in the literature evaluating the effects of isotretinoin on liver enzymes and lipids suggested that oral isotretinoin may cause alterations in liver aminotransferases (AST and ALT), TGs, HDL, and LDL in various degrees.1 Zane et al4 studied 13,772 patients with acne undergoing oral isotretinoin therapy between March 1995 and September 2002. The investigators found increased liver transaminase and serum lipid levels. They suggested that these abnormalities were generally transient and reversible.4 Bershad et al5 reported an increase in LDL and TG but a decrease in HDL during isotretinoin therapy. These changes in the lipid profile also appeared to be transient and returned to baseline level 2 months following the end of treatment.5 In another study of 130 patients who were treated with isotretinoin, Vieira et al1 noted an increase in AST, ALT, and TG levels. Most of the studies in the literature that reported effects of isotretinoin on liver enzymes and lipids suggested that the effects were reversible.
Although many studies reported alterations in serum transaminase and lipid levels, other studies reported no effect. In one study of 150 participants, Brito et al2 found no statistically significant changes in liver transaminase, TG, HDL, or LDL levels following treatment with isotretinoin. In another study of 1292 participants by Alcalay et al,6 serum levels of liver enzymes were not elevated to a degree necessitating discontinuation of isotretinoin treatment. In another study of 30 participants, Baxter et al7 reported no significant changes in TG, LDL, or HDL levels measured at baseline or during treatment with isotretinoin.
Some studies suggest that routine laboratory tests are needed when treating patients with isotretinoin due to severe alterations in serum liver transaminase and lipid levels, while other studies conclude that the effects are minimal and laboratory tests are not needed. In the current study, we found that there were statistically significant increases in TG and LDL levels in patients who underwent treatment with isotretinoin. We also found statistically significant decreases in HDL levels. In our study, liver enzymes were less affected than lipids in patients who underwent treatment with isotretinoin. There were statistically significant increases in AST levels, but the clinical classification was not affected. There also were increases in ALT levels, but the changes were not statistically significant.
Overall, we advise dermatologists that isotretinoin can be administered with minimal concern regarding changes in serum transaminase and lipid levels; however, although severe laboratory alterations were not noted in our study, we advise physicians to use caution when administering isotretinoin in patients with a history of abnormal findings.
1. Vieira AS, Beijamini V, Melchiors AC. The effect of isotretinoin on triglyceride and liver aminotransferases. An Bras Dermatol. 2012;87:382-387.
2. Brito MFM, Pessoa IS, Galindo JCS, et al. Evaluation of clinical adverse effects and laboratory alterations in patients with acne vulgaris treated with oral isotretinoin [in English, Portuguese]. An Bras Dermatol. 2010;85:331-337.
3. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
4. Zane LT, Leyden WA, Marqueling AL, et al. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142:1016-1022.
5. Bershad S, Rubinstein A, Paterniti JR, et al. Changes in plasma lipids and lipoproteins during isotretinoin therapy for acne. N Engl J Med. 1985;313:981-985.
6. Alcalay J, Landau M, Zucker A. Analysis of laboratory data in acne patients treated with isotretinoin: is there really a need to perform routine laboratory tests? J Dermatolog Treat. 2001;12:9-12.
7. Baxter KF, Ling TC, Barth JH, et al. Retrospective survey of serum lipids in patients receiving more than three courses of isotretinoin. J Dermatolog Treat. 2003;14:216-218.
Acne is a chronic inflammatory condition of the pilosebaceous unit affecting approximately 79% to 95% of adolescents in the Western world.1 Treatment of acne depends on its severity. Topical tretinoin, adapalene, benzoyl peroxide, azelaic acid, and topical antibiotics generally are used in cases of noninflammatory or mild inflammatory disease. Isotretinoin is recommended for treatment of severe inflammatory acne (eg, nodulocystic or conglobata acne) and for cases of acne that have proven to be resistant to prior treatment with antibiotics or topical agents. Dosages of isotretinoin range from 0.5 to 2 mg/kg daily for 16 to 24 weeks.1 Isotretinoin reduces the activity and size of the sebaceous glands, normalizes keratinization of the sebaceous follicles, and decreases the number of Propionibacterium acnes. Isotretinoin also may cause clinical side effects and laboratory changes, the most important being teratogenicity. It also may cause mucocutaneous side effects including cracked lips, dryness of the skin and nasal mucosa, skin redness, eye dryness, and eye irritation.1 It also may cause blepharoconjuctivitis, photosensitivity, asteatotic dermatitis, pruritus, telogen effluvium, secondary bacterial colonization, nail fragility, periungual pyogenic granuloma, paronychia, myalgia, intracranial hypertension, nausea, headache, vomiting, depression, psychosis, suicide, constipation, and allergic reactions.2 Isotretinoin treatment may increase serum levels of liver enzymes, triglyc-erides (TGs), and low-density lipoprotein (LDL) cholesterol, and reduce the level of high-density lipoprotein (HDL) cholesterol.1 This retrospective study sought to evaluate the effect of isotretinoin on liver enzymes and lipids over 6 months.
Materials and Methods
Our retrospective study was conducted at the Hospital of Atatürk University in Erzurum, a city located in eastern Turkey. All patients who were treated in the department of dermatology and had received oral isotretinoin between June 2009 and June 2012 were included in the study. The study was based on an evaluation of the patients’ medical records. All patients received oral isotretinoin 0.5 to 1 mg/kg daily; the majority of patients received 30 to 40 mg daily. Patient medical records included age; gender; white blood cell (WBC) count; red blood cell (RBC) count; hemoglobin count; and aspartate aminotransferase (AST), alanine aminotransferase (ALT), TG, LDL, and HDL levels at the beginning of treatment. Aspartate aminotransferase, ALT, TG, LDL, and HDL levels also were measured at 3- and 6-month follow-up. Analysis of AST, ALT, TG, LDL, and HDL levels was based on the National Cholesterol Education Program guidelines.3 Aspartate aminotransferase and ALT levels were classified as normal (<40 U/L) and high (≥40 U/L). Triglyceride levels were classified as normal (<150 mg/dL), borderline high (150–199 mg/dL), high (200–499 mg/dL), and very high (≥500 mg/dL). Low-density lipoprotein levels were classified as optimal (<100 mg/dL), above optimal (100–129 mg/dL), borderline high (130–159 mg/dL), high (160–189 mg/dL), and very high (≥190 mg/dL). High-density lipoprotein levels were classified as low (<40 mg/dL), normal (40–59 mg/dL), and high (≥60 mg/dL). Normal WBC was defined as 3.5 to 12.5×103/mL. Normal hemoglobin count was defined as 11.5 to 15.0×106/mL for women and 13 to 17×106/mL for men. Normal RBC was defined as 4.0 to 5.2×106/mL for women and 4.5 to 5.9×106/mL for men. Statistical analysis was performed using SPSS version 17.0. Repeated measures analysis of variance was used to compare means between 3 groups (baseline, 3-month, and 6-month values). A paired sample t test was used to compare means between any 2 groups. Results with P<.05 were considered statistically significant.
Results
Treatment with oral isotretinoin was initiated in 349 patients at our institution from June 2009 to June 2012. Twenty-seven of these patients were excluded from the study because their medical records were not available. Medical records from 322 patients were obtained. The study population consisted of 226 (70.2%) women and 96 (29.8%) men. Patients ranged in age from 17 to 64 years, with a mean age of 23.9 years. The mean (standard deviation [SD]) age of the women was 23.9 (5.4) years and the mean (SD) age of the men was 23.8 (7.02) years. Most of the patients were on a regimen of 30 or 40 mg of isotretinoin daily. Differences between dosages and laboratory values were not analyzed because we assumed there would not be a significant difference, as most patients received the same dose. The mean (SD) WBC was 8.4 (3.5)×103/mL. The mean (SD) RBC was 4.9 (0.5)×106/mL. The mean (SD) hemoglobin count was 14.3 (1.7)×106/mL (women, 13.6 [1.5]×106/mL; men, 15.9 [1.1]×106/mL).
The study evaluated the effects of isotretinoin on liver enzymes (AST and ALT) and lipids (TGs, LDL, and HDL). Nearly all of the patients (>95%) had normal AST and ALT levels at baseline. The results are outlined in the Table. Some values were not recorded for all patients at each follow-up.
Aspartate Aminotransferase Analysis
Aspartate aminotransferase levels were classified as normal and high. At baseline, mean (SD) AST levels were 20.2 (6.6) U/L, with normal levels in 311 (96.6%) patients and high in 7 (2.2%) patients. At 3-month follow-up, mean (SD) AST levels were 20.7 (5.2) U/L, with normal levels in 270 (83.9%) patients and high levels in 3 (0.9%) patients. At 6-month follow-up, mean (SD) AST levels were 21.3 (5.7) U/L, with normal levels in 209 (64.9%) patients and high levels in 4 (1.2%) patients. Aspartate aminotransferase levels increased at 3- and 6-month follow-up compared to baseline. Differences between AST levels were statistically significant (F2,416=4.2, P=.016). Differences between AST levels at baseline and 3-month follow-up were not statistically significant (P=.3). Differences between AST levels at 3- and 6-month follow-up were not statistically significant (P=.4). Differences between AST levels at baseline and 6-month follow-up were statistically significant (P=.07). Differences between AST classifications at the 3 time points were not statistically significant (F2,416=0.44, P=.64). Overall, the results indicated that AST levels increased over time in patients treated with isotretinoin, but the increase was not above the normal range and was not statistically significant.
Alanine Aminotransferase Analysis
Alanine aminotransferase levels were classified as normal or high. At baseline, mean (SD) ALT levels were 16.8 (11.2) U/L, with normal levels in 303 (94.1%) patients and high in 19 (5.9%) patients. At 3-month follow-up, mean (SD) ALT levels were 16.2 (9.3) U/L, with normal levels in 263 (81.7%) patients and high in 11 (3.4%) patients. At 6-month follow-up, mean (SD) ALT levels were 17.0 (11.3) U/L, with normal levels in 201 (62.4%) patients and high in 11 (3.4%) patients. Alanine aminotransferase levels at 3-month follow-up were lower than baseline but higher at 6-month follow-up compared to baseline and 3-month follow-up. Overall, ALT levels increased with time, but the differences between baseline and 3- and 6-month follow-up were not statistically significant (F2,416=0.32, P=.72). Differences between ALT classifications at each time point were not statistically significant (F2,418=0.21, P=.54). Overall, the results indicated that ALT levels increased over time in patients treated with isotretinoin, but the increase was not statistically significant.
Triglycerides Analysis
Triglyceride levels were classified as normal, borderline high, high, and very high. At baseline, mean (SD) TG levels were 107 (71) mg/dL, with normal levels in 270 (83.9%) patients, borderline high in 30 (9.3%) patients, high in 20 (6.2%) patients, and very high in 2 (0.6%) patients. At 3-month follow-up, mean (SD) TG levels were 117 (60) mg/dL, with normal levels in 197 (61.2%) patients, borderline high in 38 (11.8%) patients, high in 22 (6.8%) patients, and very high in 1 (0.3%) patient. At 6-month follow-up, mean (SD) TG levels were 122 (65) mg/dL, with normal levels in 145 (45%) patients, borderline high in 36 (11.2%) patients, high in 16 (5%) patients, and very high in 0 (0%) patients. Triglyceride levels increased and differences between TG levels at baseline and 3- and 6-month follow-up were statistically significant (F2,384=17, P<.001). Baseline TG levels compared to 3-month follow-up were statistically signif-icant (P<.001). Differences in TG levels at 6-month follow-up versus baseline were statistically significant (P<.001). However, changes in TG levels from 3- to 6-month follow-up were not statistically significant (P=.21). Differences between TG classifications at each time point were statistically significant (F2,386=6.9, P=.001). Overall, TG levels increased from baseline during isotretinoin treatment at 3- and 6-month follow-up, and these increases were above normal range; however, there was no statistically significant increase from 3- to 6-month follow-up.
Low-Density Lipoprotein Analysis
Low-density lipoprotein levels were classified as optimal, above optimal, borderline high, high, and very high. At baseline, mean (SD) LDL levels were 102 (28) mg/dL, with optimal levels in 162 (50.3%) patients, above optimal in 95 (29.5%) patients, borderline high in 32 (9.9%) patients, high in 11 (3.4%) patients, and very high in 3 (0.9%) patients. At 3-month follow-up, mean (SD) LDL levels were 113 (30) mg/dL, with optimal levels in 89 (27.6%) patients, above optimal in 98 (30.4%) patients, borderline high in 54 (16.8%) patients, high in 12 (3.7%) patients, and very high in 5 (1.6%) patients. At 6-month follow-up, mean (SD) LDL levels were 113 (27) mg/dL, with optimal levels in 60 (18.6%) patients, above optimal in 84 (26.1%) patients, borderline high in 44 (13.7%) patients, high in 8 (2.5%) patients, and very high in 1 (0.3%) patient. Overall, there were statistically significant increases in LDL levels at 3- and 6-month follow-up (F2,382<75, P<.001). Differences between baseline LDL levels and 3-month follow-up were statistically significant (P<.001). Differences between baseline LDL levels and 6-month follow-up were statistically significant (P<.001). However, differences in LDL levels at 3- and 6-month follow-up were not statistically significant (P=.74). Differences between LDL classifications at each time point were statistically significant (F2,382=51.2, P<.001). Overall, statistically significant increases in LDL levels from baseline were noted during isotretinoin treatment and this increase was above normal range; however, LDL levels did not significantly increase from 3- to 6-month follow-up.
High-Density Lipoprotein Analysis
High-density lipoprotein levels were classified as low, normal, and high. At baseline, mean (SD) HDL levels were 52.4 (16) mg/dL, with low levels in 60 (18.6%) patients, normal in 173 (53.7%) patients, and high in 71 (22%) patients. At 3-month follow-up, mean (SD) HDL levels were 48 (12) mg/dL, with low levels in 63 (19.6%) patients, normal in 154 (47.8%) patients, and high in 41 (12.7%) patients. At 6-month follow-up, mean (SD) HDL levels were 47.6 (12) mg/dL, with low levels in 48 (14.9%) patients, normal in 117 (36.3%) patients, and high in 33 (10.2%) patients. Overall, statistically significant decreases were noted in HDL levels (F2,384=19, P<.001). Differences between baseline HDL levels compared to 3-month follow-up were statistically significant (P<.001). Differences between baseline HDL levels compared to 6-month follow-up were statistically significant (P<.001). Differences in HDL levels at 3- and 6-month follow-up were statistically significant (P<.001). Differences between HDL classifications at each time point were statistically significant (F2,384=5.2, P=.006). Overall, there were statistically significant decreases in HDL levels during isotretinoin treatment from baseline and this decrease was above normal range; however, HDL levels did not decrease at 3- and 6-month follow-up.
Comment
Studies in the literature evaluating the effects of isotretinoin on liver enzymes and lipids suggested that oral isotretinoin may cause alterations in liver aminotransferases (AST and ALT), TGs, HDL, and LDL in various degrees.1 Zane et al4 studied 13,772 patients with acne undergoing oral isotretinoin therapy between March 1995 and September 2002. The investigators found increased liver transaminase and serum lipid levels. They suggested that these abnormalities were generally transient and reversible.4 Bershad et al5 reported an increase in LDL and TG but a decrease in HDL during isotretinoin therapy. These changes in the lipid profile also appeared to be transient and returned to baseline level 2 months following the end of treatment.5 In another study of 130 patients who were treated with isotretinoin, Vieira et al1 noted an increase in AST, ALT, and TG levels. Most of the studies in the literature that reported effects of isotretinoin on liver enzymes and lipids suggested that the effects were reversible.
Although many studies reported alterations in serum transaminase and lipid levels, other studies reported no effect. In one study of 150 participants, Brito et al2 found no statistically significant changes in liver transaminase, TG, HDL, or LDL levels following treatment with isotretinoin. In another study of 1292 participants by Alcalay et al,6 serum levels of liver enzymes were not elevated to a degree necessitating discontinuation of isotretinoin treatment. In another study of 30 participants, Baxter et al7 reported no significant changes in TG, LDL, or HDL levels measured at baseline or during treatment with isotretinoin.
Some studies suggest that routine laboratory tests are needed when treating patients with isotretinoin due to severe alterations in serum liver transaminase and lipid levels, while other studies conclude that the effects are minimal and laboratory tests are not needed. In the current study, we found that there were statistically significant increases in TG and LDL levels in patients who underwent treatment with isotretinoin. We also found statistically significant decreases in HDL levels. In our study, liver enzymes were less affected than lipids in patients who underwent treatment with isotretinoin. There were statistically significant increases in AST levels, but the clinical classification was not affected. There also were increases in ALT levels, but the changes were not statistically significant.
Overall, we advise dermatologists that isotretinoin can be administered with minimal concern regarding changes in serum transaminase and lipid levels; however, although severe laboratory alterations were not noted in our study, we advise physicians to use caution when administering isotretinoin in patients with a history of abnormal findings.
Acne is a chronic inflammatory condition of the pilosebaceous unit affecting approximately 79% to 95% of adolescents in the Western world.1 Treatment of acne depends on its severity. Topical tretinoin, adapalene, benzoyl peroxide, azelaic acid, and topical antibiotics generally are used in cases of noninflammatory or mild inflammatory disease. Isotretinoin is recommended for treatment of severe inflammatory acne (eg, nodulocystic or conglobata acne) and for cases of acne that have proven to be resistant to prior treatment with antibiotics or topical agents. Dosages of isotretinoin range from 0.5 to 2 mg/kg daily for 16 to 24 weeks.1 Isotretinoin reduces the activity and size of the sebaceous glands, normalizes keratinization of the sebaceous follicles, and decreases the number of Propionibacterium acnes. Isotretinoin also may cause clinical side effects and laboratory changes, the most important being teratogenicity. It also may cause mucocutaneous side effects including cracked lips, dryness of the skin and nasal mucosa, skin redness, eye dryness, and eye irritation.1 It also may cause blepharoconjuctivitis, photosensitivity, asteatotic dermatitis, pruritus, telogen effluvium, secondary bacterial colonization, nail fragility, periungual pyogenic granuloma, paronychia, myalgia, intracranial hypertension, nausea, headache, vomiting, depression, psychosis, suicide, constipation, and allergic reactions.2 Isotretinoin treatment may increase serum levels of liver enzymes, triglyc-erides (TGs), and low-density lipoprotein (LDL) cholesterol, and reduce the level of high-density lipoprotein (HDL) cholesterol.1 This retrospective study sought to evaluate the effect of isotretinoin on liver enzymes and lipids over 6 months.
Materials and Methods
Our retrospective study was conducted at the Hospital of Atatürk University in Erzurum, a city located in eastern Turkey. All patients who were treated in the department of dermatology and had received oral isotretinoin between June 2009 and June 2012 were included in the study. The study was based on an evaluation of the patients’ medical records. All patients received oral isotretinoin 0.5 to 1 mg/kg daily; the majority of patients received 30 to 40 mg daily. Patient medical records included age; gender; white blood cell (WBC) count; red blood cell (RBC) count; hemoglobin count; and aspartate aminotransferase (AST), alanine aminotransferase (ALT), TG, LDL, and HDL levels at the beginning of treatment. Aspartate aminotransferase, ALT, TG, LDL, and HDL levels also were measured at 3- and 6-month follow-up. Analysis of AST, ALT, TG, LDL, and HDL levels was based on the National Cholesterol Education Program guidelines.3 Aspartate aminotransferase and ALT levels were classified as normal (<40 U/L) and high (≥40 U/L). Triglyceride levels were classified as normal (<150 mg/dL), borderline high (150–199 mg/dL), high (200–499 mg/dL), and very high (≥500 mg/dL). Low-density lipoprotein levels were classified as optimal (<100 mg/dL), above optimal (100–129 mg/dL), borderline high (130–159 mg/dL), high (160–189 mg/dL), and very high (≥190 mg/dL). High-density lipoprotein levels were classified as low (<40 mg/dL), normal (40–59 mg/dL), and high (≥60 mg/dL). Normal WBC was defined as 3.5 to 12.5×103/mL. Normal hemoglobin count was defined as 11.5 to 15.0×106/mL for women and 13 to 17×106/mL for men. Normal RBC was defined as 4.0 to 5.2×106/mL for women and 4.5 to 5.9×106/mL for men. Statistical analysis was performed using SPSS version 17.0. Repeated measures analysis of variance was used to compare means between 3 groups (baseline, 3-month, and 6-month values). A paired sample t test was used to compare means between any 2 groups. Results with P<.05 were considered statistically significant.
Results
Treatment with oral isotretinoin was initiated in 349 patients at our institution from June 2009 to June 2012. Twenty-seven of these patients were excluded from the study because their medical records were not available. Medical records from 322 patients were obtained. The study population consisted of 226 (70.2%) women and 96 (29.8%) men. Patients ranged in age from 17 to 64 years, with a mean age of 23.9 years. The mean (standard deviation [SD]) age of the women was 23.9 (5.4) years and the mean (SD) age of the men was 23.8 (7.02) years. Most of the patients were on a regimen of 30 or 40 mg of isotretinoin daily. Differences between dosages and laboratory values were not analyzed because we assumed there would not be a significant difference, as most patients received the same dose. The mean (SD) WBC was 8.4 (3.5)×103/mL. The mean (SD) RBC was 4.9 (0.5)×106/mL. The mean (SD) hemoglobin count was 14.3 (1.7)×106/mL (women, 13.6 [1.5]×106/mL; men, 15.9 [1.1]×106/mL).
The study evaluated the effects of isotretinoin on liver enzymes (AST and ALT) and lipids (TGs, LDL, and HDL). Nearly all of the patients (>95%) had normal AST and ALT levels at baseline. The results are outlined in the Table. Some values were not recorded for all patients at each follow-up.
Aspartate Aminotransferase Analysis
Aspartate aminotransferase levels were classified as normal and high. At baseline, mean (SD) AST levels were 20.2 (6.6) U/L, with normal levels in 311 (96.6%) patients and high in 7 (2.2%) patients. At 3-month follow-up, mean (SD) AST levels were 20.7 (5.2) U/L, with normal levels in 270 (83.9%) patients and high levels in 3 (0.9%) patients. At 6-month follow-up, mean (SD) AST levels were 21.3 (5.7) U/L, with normal levels in 209 (64.9%) patients and high levels in 4 (1.2%) patients. Aspartate aminotransferase levels increased at 3- and 6-month follow-up compared to baseline. Differences between AST levels were statistically significant (F2,416=4.2, P=.016). Differences between AST levels at baseline and 3-month follow-up were not statistically significant (P=.3). Differences between AST levels at 3- and 6-month follow-up were not statistically significant (P=.4). Differences between AST levels at baseline and 6-month follow-up were statistically significant (P=.07). Differences between AST classifications at the 3 time points were not statistically significant (F2,416=0.44, P=.64). Overall, the results indicated that AST levels increased over time in patients treated with isotretinoin, but the increase was not above the normal range and was not statistically significant.
Alanine Aminotransferase Analysis
Alanine aminotransferase levels were classified as normal or high. At baseline, mean (SD) ALT levels were 16.8 (11.2) U/L, with normal levels in 303 (94.1%) patients and high in 19 (5.9%) patients. At 3-month follow-up, mean (SD) ALT levels were 16.2 (9.3) U/L, with normal levels in 263 (81.7%) patients and high in 11 (3.4%) patients. At 6-month follow-up, mean (SD) ALT levels were 17.0 (11.3) U/L, with normal levels in 201 (62.4%) patients and high in 11 (3.4%) patients. Alanine aminotransferase levels at 3-month follow-up were lower than baseline but higher at 6-month follow-up compared to baseline and 3-month follow-up. Overall, ALT levels increased with time, but the differences between baseline and 3- and 6-month follow-up were not statistically significant (F2,416=0.32, P=.72). Differences between ALT classifications at each time point were not statistically significant (F2,418=0.21, P=.54). Overall, the results indicated that ALT levels increased over time in patients treated with isotretinoin, but the increase was not statistically significant.
Triglycerides Analysis
Triglyceride levels were classified as normal, borderline high, high, and very high. At baseline, mean (SD) TG levels were 107 (71) mg/dL, with normal levels in 270 (83.9%) patients, borderline high in 30 (9.3%) patients, high in 20 (6.2%) patients, and very high in 2 (0.6%) patients. At 3-month follow-up, mean (SD) TG levels were 117 (60) mg/dL, with normal levels in 197 (61.2%) patients, borderline high in 38 (11.8%) patients, high in 22 (6.8%) patients, and very high in 1 (0.3%) patient. At 6-month follow-up, mean (SD) TG levels were 122 (65) mg/dL, with normal levels in 145 (45%) patients, borderline high in 36 (11.2%) patients, high in 16 (5%) patients, and very high in 0 (0%) patients. Triglyceride levels increased and differences between TG levels at baseline and 3- and 6-month follow-up were statistically significant (F2,384=17, P<.001). Baseline TG levels compared to 3-month follow-up were statistically signif-icant (P<.001). Differences in TG levels at 6-month follow-up versus baseline were statistically significant (P<.001). However, changes in TG levels from 3- to 6-month follow-up were not statistically significant (P=.21). Differences between TG classifications at each time point were statistically significant (F2,386=6.9, P=.001). Overall, TG levels increased from baseline during isotretinoin treatment at 3- and 6-month follow-up, and these increases were above normal range; however, there was no statistically significant increase from 3- to 6-month follow-up.
Low-Density Lipoprotein Analysis
Low-density lipoprotein levels were classified as optimal, above optimal, borderline high, high, and very high. At baseline, mean (SD) LDL levels were 102 (28) mg/dL, with optimal levels in 162 (50.3%) patients, above optimal in 95 (29.5%) patients, borderline high in 32 (9.9%) patients, high in 11 (3.4%) patients, and very high in 3 (0.9%) patients. At 3-month follow-up, mean (SD) LDL levels were 113 (30) mg/dL, with optimal levels in 89 (27.6%) patients, above optimal in 98 (30.4%) patients, borderline high in 54 (16.8%) patients, high in 12 (3.7%) patients, and very high in 5 (1.6%) patients. At 6-month follow-up, mean (SD) LDL levels were 113 (27) mg/dL, with optimal levels in 60 (18.6%) patients, above optimal in 84 (26.1%) patients, borderline high in 44 (13.7%) patients, high in 8 (2.5%) patients, and very high in 1 (0.3%) patient. Overall, there were statistically significant increases in LDL levels at 3- and 6-month follow-up (F2,382<75, P<.001). Differences between baseline LDL levels and 3-month follow-up were statistically significant (P<.001). Differences between baseline LDL levels and 6-month follow-up were statistically significant (P<.001). However, differences in LDL levels at 3- and 6-month follow-up were not statistically significant (P=.74). Differences between LDL classifications at each time point were statistically significant (F2,382=51.2, P<.001). Overall, statistically significant increases in LDL levels from baseline were noted during isotretinoin treatment and this increase was above normal range; however, LDL levels did not significantly increase from 3- to 6-month follow-up.
High-Density Lipoprotein Analysis
High-density lipoprotein levels were classified as low, normal, and high. At baseline, mean (SD) HDL levels were 52.4 (16) mg/dL, with low levels in 60 (18.6%) patients, normal in 173 (53.7%) patients, and high in 71 (22%) patients. At 3-month follow-up, mean (SD) HDL levels were 48 (12) mg/dL, with low levels in 63 (19.6%) patients, normal in 154 (47.8%) patients, and high in 41 (12.7%) patients. At 6-month follow-up, mean (SD) HDL levels were 47.6 (12) mg/dL, with low levels in 48 (14.9%) patients, normal in 117 (36.3%) patients, and high in 33 (10.2%) patients. Overall, statistically significant decreases were noted in HDL levels (F2,384=19, P<.001). Differences between baseline HDL levels compared to 3-month follow-up were statistically significant (P<.001). Differences between baseline HDL levels compared to 6-month follow-up were statistically significant (P<.001). Differences in HDL levels at 3- and 6-month follow-up were statistically significant (P<.001). Differences between HDL classifications at each time point were statistically significant (F2,384=5.2, P=.006). Overall, there were statistically significant decreases in HDL levels during isotretinoin treatment from baseline and this decrease was above normal range; however, HDL levels did not decrease at 3- and 6-month follow-up.
Comment
Studies in the literature evaluating the effects of isotretinoin on liver enzymes and lipids suggested that oral isotretinoin may cause alterations in liver aminotransferases (AST and ALT), TGs, HDL, and LDL in various degrees.1 Zane et al4 studied 13,772 patients with acne undergoing oral isotretinoin therapy between March 1995 and September 2002. The investigators found increased liver transaminase and serum lipid levels. They suggested that these abnormalities were generally transient and reversible.4 Bershad et al5 reported an increase in LDL and TG but a decrease in HDL during isotretinoin therapy. These changes in the lipid profile also appeared to be transient and returned to baseline level 2 months following the end of treatment.5 In another study of 130 patients who were treated with isotretinoin, Vieira et al1 noted an increase in AST, ALT, and TG levels. Most of the studies in the literature that reported effects of isotretinoin on liver enzymes and lipids suggested that the effects were reversible.
Although many studies reported alterations in serum transaminase and lipid levels, other studies reported no effect. In one study of 150 participants, Brito et al2 found no statistically significant changes in liver transaminase, TG, HDL, or LDL levels following treatment with isotretinoin. In another study of 1292 participants by Alcalay et al,6 serum levels of liver enzymes were not elevated to a degree necessitating discontinuation of isotretinoin treatment. In another study of 30 participants, Baxter et al7 reported no significant changes in TG, LDL, or HDL levels measured at baseline or during treatment with isotretinoin.
Some studies suggest that routine laboratory tests are needed when treating patients with isotretinoin due to severe alterations in serum liver transaminase and lipid levels, while other studies conclude that the effects are minimal and laboratory tests are not needed. In the current study, we found that there were statistically significant increases in TG and LDL levels in patients who underwent treatment with isotretinoin. We also found statistically significant decreases in HDL levels. In our study, liver enzymes were less affected than lipids in patients who underwent treatment with isotretinoin. There were statistically significant increases in AST levels, but the clinical classification was not affected. There also were increases in ALT levels, but the changes were not statistically significant.
Overall, we advise dermatologists that isotretinoin can be administered with minimal concern regarding changes in serum transaminase and lipid levels; however, although severe laboratory alterations were not noted in our study, we advise physicians to use caution when administering isotretinoin in patients with a history of abnormal findings.
1. Vieira AS, Beijamini V, Melchiors AC. The effect of isotretinoin on triglyceride and liver aminotransferases. An Bras Dermatol. 2012;87:382-387.
2. Brito MFM, Pessoa IS, Galindo JCS, et al. Evaluation of clinical adverse effects and laboratory alterations in patients with acne vulgaris treated with oral isotretinoin [in English, Portuguese]. An Bras Dermatol. 2010;85:331-337.
3. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
4. Zane LT, Leyden WA, Marqueling AL, et al. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142:1016-1022.
5. Bershad S, Rubinstein A, Paterniti JR, et al. Changes in plasma lipids and lipoproteins during isotretinoin therapy for acne. N Engl J Med. 1985;313:981-985.
6. Alcalay J, Landau M, Zucker A. Analysis of laboratory data in acne patients treated with isotretinoin: is there really a need to perform routine laboratory tests? J Dermatolog Treat. 2001;12:9-12.
7. Baxter KF, Ling TC, Barth JH, et al. Retrospective survey of serum lipids in patients receiving more than three courses of isotretinoin. J Dermatolog Treat. 2003;14:216-218.
1. Vieira AS, Beijamini V, Melchiors AC. The effect of isotretinoin on triglyceride and liver aminotransferases. An Bras Dermatol. 2012;87:382-387.
2. Brito MFM, Pessoa IS, Galindo JCS, et al. Evaluation of clinical adverse effects and laboratory alterations in patients with acne vulgaris treated with oral isotretinoin [in English, Portuguese]. An Bras Dermatol. 2010;85:331-337.
3. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
4. Zane LT, Leyden WA, Marqueling AL, et al. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142:1016-1022.
5. Bershad S, Rubinstein A, Paterniti JR, et al. Changes in plasma lipids and lipoproteins during isotretinoin therapy for acne. N Engl J Med. 1985;313:981-985.
6. Alcalay J, Landau M, Zucker A. Analysis of laboratory data in acne patients treated with isotretinoin: is there really a need to perform routine laboratory tests? J Dermatolog Treat. 2001;12:9-12.
7. Baxter KF, Ling TC, Barth JH, et al. Retrospective survey of serum lipids in patients receiving more than three courses of isotretinoin. J Dermatolog Treat. 2003;14:216-218.
Practice Points
- Isotretinoin is recommended for treatment of severe inflammatory acne and for cases resistant to prior treatment with antibiotics or topical agents; however, it may cause alterations in lipids and liver enzymes.
- In our study, liver enzymes were less affected than lipids in patients who were treated with isotretinoin.
- Use caution when administering isotretinoin in patients with a history of abnormal findings.
VIDEO: Dr. Sheila F. Friedlander discusses when and why to worry about acne in young children
NEWPORT BEACH, CALIF.– “The group we worry about are the 1- to 7-year-olds,” when it comes to new-onset acne, Dr. Sheila Fallon Friedlander said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
In an interview at the meeting, Dr. Friedlander, a professor at the University of California, San Diego, explained the additional clinical signs that can indicate a serious problem, and what questions to ask parents.
Tune in for her tips on how to evaluate children aged 1-7 years with acne.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEWPORT BEACH, CALIF.– “The group we worry about are the 1- to 7-year-olds,” when it comes to new-onset acne, Dr. Sheila Fallon Friedlander said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
In an interview at the meeting, Dr. Friedlander, a professor at the University of California, San Diego, explained the additional clinical signs that can indicate a serious problem, and what questions to ask parents.
Tune in for her tips on how to evaluate children aged 1-7 years with acne.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEWPORT BEACH, CALIF.– “The group we worry about are the 1- to 7-year-olds,” when it comes to new-onset acne, Dr. Sheila Fallon Friedlander said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
In an interview at the meeting, Dr. Friedlander, a professor at the University of California, San Diego, explained the additional clinical signs that can indicate a serious problem, and what questions to ask parents.
Tune in for her tips on how to evaluate children aged 1-7 years with acne.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
VIDEO: What’s unique about treating acne in adult women
NEWPORT BEACH, CALIF. – Do more women today really have acne? Or are they simply more likely to seek help because they learn of new and better medications?
Acne often causes more psychosocial and psychological stress in adult women than in men or adolescents, Dr. Hilary Baldwin of SUNY Downstate Medical Center, Brooklyn, N.Y., said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
In an interview at the meeting, Dr. Baldwin explained what makes the treatment of acne in adult women distinct from acne treatment for men and adolescents, and what underused medications can yield success.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEWPORT BEACH, CALIF. – Do more women today really have acne? Or are they simply more likely to seek help because they learn of new and better medications?
Acne often causes more psychosocial and psychological stress in adult women than in men or adolescents, Dr. Hilary Baldwin of SUNY Downstate Medical Center, Brooklyn, N.Y., said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
In an interview at the meeting, Dr. Baldwin explained what makes the treatment of acne in adult women distinct from acne treatment for men and adolescents, and what underused medications can yield success.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEWPORT BEACH, CALIF. – Do more women today really have acne? Or are they simply more likely to seek help because they learn of new and better medications?
Acne often causes more psychosocial and psychological stress in adult women than in men or adolescents, Dr. Hilary Baldwin of SUNY Downstate Medical Center, Brooklyn, N.Y., said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
In an interview at the meeting, Dr. Baldwin explained what makes the treatment of acne in adult women distinct from acne treatment for men and adolescents, and what underused medications can yield success.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Topical Therapy for Acne in Women: Is There a Role for Clindamycin Phosphate–Benzoyl Peroxide Gel?
The management of acne vulgaris (AV) in women has been the subject of considerable attention over the last few years. It has become increasingly recognized that a greater number of patient encounters in dermatology offices involve women with AV who are beyond their adolescent years. Overall, it is estimated that up to approximately 22% of women in the United States are affected by AV, with approximately half of women in their 20s and one-third of women in their 30s reporting some degree of AV.1-4 Among women, the disease shows no predilection for certain skin types or ethnicities, can start during the preteenaged or adolescent years, can persist or recur in adulthood (persistent acne, 75%), or can start in adulthood (late-onset acne, 25%) in females with minimal or no history of AV occurring earlier in life.3,5-7 In the subpopulation of adult women, AV occurs at a time when many expect to be far beyond this “teenage affliction.” Women who are affected commonly express feeling embarrassed and frustrated.5-8
Most of the emphasis in the literature and in presentations at dermatology meetings regarding the management of AV in adult women has focused on excluding underlying disorders that cause excess androgens (eg, polycystic ovary syndrome, congenital adrenal hyperplasia, tumors, exogenous sources) as well as the use of systemic therapies such as oral contraceptives (OCs) and spironolactone.5-7,9,10 Little attention has been given to the selection of topical therapies in this patient population, especially with regard to evidence from clinical studies. To date, results from published study analyses using topical agents specifically for adult females with facial AV have only included adapalene gel 0.3% applied once daily and dapsone gel 5% applied twice daily.11-13 Both agents have been evaluated in subset analysis comparisons of outcomes in women aged 18 years and older versus adolescents aged 12 to 17 years based on data from 12-week phase 3 pivotal trials.14-16
Are there clinically relevant differences between AV in adult versus adolescent females?
Although much has been written about AV in women, epidemiology, demographics, assessment of clinical presentation, and correlation of clinical presentation with excess androgens have not been emphasized,1-3,5-10,17 likely due to marketing campaigns that emphasize AV as a disorder that predominantly affects teenagers as well as the focus on optimal use of oral spironolactone and/or OCs in the management of AV in the adult female population. Attention to spironolactone use is important because it is not approved by the US Food and Drug Administration for the treatment of AV. Spironolactone carries certain black-box warnings that may not be clinically relevant in all patients but still require attention. It also is associated with risks if taken during pregnancy, and it is a potassium-sparing diuretic with potential for hyperkalemia, especially in patients with reduced renal function or those who are taking potassium supplements or certain other medications.6,9,11,17 Use of OCs to treat AV also is not without potential risks, with specific warnings and relative contraindications reported, especially in relation to increased risks for cardiac complications, stroke, and thromboembolism.6,9,11,12 Because adult females are in a different stage of life than teenagers, there are defined psychosocial and medical considerations in managing AV in these patients compared with adolescents.5-8,17 Importantly for both clinicians and patients, addressing these differences and considerations can have a major impact on whether or not women with AV experience successful treatment outcomes.1-3,5,6,8,10,13 Skin color and ethnicity also can affect the psychosocial and physical factors that influence the overall management of adult female patients with AV, including selection of therapies and handling long-term visible sequelae that occur in some AV patients, such as dyschromia (eg, persistent or postinflammatory erythema or hyperpigmentation) and acne scarring.5-8,13,17-20
Psychosocial Considerations
With regard to psychosocial, emotional, and attitudinal considerations in women with AV, common findings include concern or frustration regarding the presence of AV beyond adolescence; anxiety; symptoms of depression; decreased self-confidence; increased self-consciousness, especially during public interactions or intimate situations; and interference with steady concentration at work or school.5,6,8,13 Long-term complications of AV, such as dyschromia and acne scarring, are more likely to be encountered in adult patients, especially if they had AV as a teenager, with women reporting that they remain conscious of these adverse sequelae.8 It is estimated that approximately three-fourths of women with AV also had AV as teenagers; therefore, most of them have already used many over-the-counter and prescription therapies and are likely to want treatments that are newer, well-tolerated, safe, and known to be effective in adult women.8,16,17 Convenience and simplicity are vital components of treatment selection and regimen design, as many women with AV frequently face time constraints in their daily routines due to family, social, employment, and home-related demands and responsibilities.6-8,17
Medical Considerations
It is apparent from reports in the literature as well as from clinical experience that some women with AV present with a U-shaped pattern of involvement on the face,5-7,10,13,17 which refers to the presence of predominantly inflammatory papules (many of them deep) and some nodules on the lower face, jawline, and anterolateral neck region, with comedones often sparse or absent.5-7 It often is perceived and may be true that women who present with this pattern of distribution are more androgen sensitive despite having normal serum androgen levels or in some cases exhibit detectable excess androgens (eg, in the setting of polycystic ovary syndrome) and may be more likely to respond to hormonal therapies (eg, spironolactone, OCs) than those with mixed facial AV (ie, multiple comedonal and inflammatory acne lesions, not limited to a U-shaped pattern, similar to adolescent AV), but data are limited to support differentiation between the U-shaped pattern group and the conventional mixed facial AV group.5-7,17 Adult and adolescent females in both groups sometimes report perimenstrual flares and frequent persistent papular AV that tends to concentrate on the perioral and chin area.
It is also important to consider that the current literature suggests approximately three-fourths of women with AV report that they also had AV as a teenager, with many indicating the same clinical pattern of AV and approximately one-third reporting AV that is more severe in adulthood than adolescence.5-8,17 The available literature on topical and oral therapies used to treat AV in both adolescent and adult females predominantly focuses on inclusion of both inflammatory and noninflammatory (comedonal) facial AV lesions, does not specifically address or include the U-shaped pattern of AV in adult women for inclusion in studies that evaluate efficacy in this subgroup, and does not include AV involving the neck region and below the jawline margin as part of any study protocols and/or discussions about therapy.5-7,9-12,17,21-26 Involvement of the neck and lower jawline is common in women presenting with the U-shaped pattern of AV, and available studies only evaluate AV involving the face and do not include AV lesions present below the jawline margin. As a result, there is a considerable need for well-designed studies with laboratory assessments to include or exclude underlying detectable excess androgens and to assess the efficacy, tolerability, and safety of specific therapeutic agents both alone and in combination in adult women who present with a U-shaped pattern of AV.17
Other medical considerations that can influence treatment selection and are more likely to be present in adult versus adolescent females include underlying chronic medical disorders; concomitant medications that may interact with other oral agents; potential for pregnancy; age, particularly when prescribing OCs; and the potential desire to stop taking OCs if already used over a prolonged period.6,7
Age-Related Differentiation of Female Subgroups With AV
The age-based dividing line that defines AV in adults versus adolescent females has been described in the literature; however, the basis for published definitions of female subgroups with AV is not well-supported by strong scientific evidence.1-3,5-7,17 The conventional dividing line that was originally selected to define adult females with AV was 25 years of age or older; persistent acne is present both during adolescence and at or after 25 years of age, while late-onset acne is described as AV that first presents at 25 years of age or older.3,5-7
More recently, a range of 18 years or older has been used to classify adult female AV and a range of 12 to 17 years for adolescent female AV in subset analyses that evaluated treatment outcomes in both patient populations from phase 3 pivotal trials completed with adapalene gel 0.3% applied once daily and dapsone gel 5% applied twice daily.14-16 These subanalyses included participants with facial AV that was predominantly moderate in severity, mandated specific lesion count ranges for both comedonal and inflammatory lesions, and included only facial AV that was above the mandibular (jawline) margin.15,16,21,26 Therefore, patients with AV presenting in a U-shaped pattern with involvement below the jawline and on the neck were not included in these study analyses, as these patients were excluded from the phase 3 trials on which the analyses were based. The outcomes of these analyses apply to treatment in women who present with both inflammatory and noninflammatory facial AV lesions, which supports the observation that AV in this patient population is not always predominantly inflammatory and does not always present in a U-shaped distribution.14-16 In fact, a U-shaped pattern of distribution appears to be less common in women with AV than a mixed inflammatory and comedonal distribution that involves the face more diffusely, though more data are needed from well-designed and large-scale epidemiologic and demographic studies.5,14,17
Are there data available on the use of benzoyl peroxide with or without a topical antibiotic in women with AV?
There is a conspicuous absence of prospective clinical trials and retrospective analyses evaluating the specific use of individual AV therapies in adult females, with a particular lack of studies with topical agents (eg, benzoyl peroxide [BP]).14 Subset analyses have been completed for adapalene gel 0.3% and dapsone gel 5%.15,16 Additionally, an age-based subset analysis in females with facial AV also has been completed with clindamycin phosphate (CP) 1.2%–BP 2.5% gel once daily, with data presented but not yet fully published.14
Two identical phase 3, double-blind, randomized, 12-week, 4-arm trials compared treatment outcomes in groups treated with an aqueous-based combination gel formulation containing BP 2.5% and CP 1.2% (n=797), active monad gels (BP [n=809] or CP [n=812]), or vehicle gel (n=395), all applied once daily in patients with facial AV.22 Participants were 12 years or older (mean age range, 19.1–19.6 years; age range, 12.1–70.2 years), were of either gender (approximately 50% split in each study arm), and presented with moderate (approximately 80% of participants) or severe AV (approximately 20% of participants) at baseline. The entry criteria for lesion types and number of lesions were 17 to 40 inflammatory lesions (ie, papules, pustules, <2 nodules)(range of mean number of lesions, 25.8–26.4) and 20 to 100 noninflammatory lesions (ie, closed comedones, open comedones)(range of mean number of lesions, 44.0–47.4). Participant demographics included white (73.9%–77.5%), black/African American (16.1%–20.4%), and Asian (2.1%–3.3%), with the remaining participants distributed among a variety of other ethnic groups such as Native Hawaiian/Native Pacific Islander and Native American Indian/Native Alaskan (collectively <5% in each study arm). Therefore, approximately 1 of every 4 patients had skin of color, which provided good diversity of patients considering the large study size (N=2813). Data analysis included dichotomization of participants by severity rating (moderate or severe based on evaluator global severity score) and skin phototype (Fitzpatrick skin types I–III or IV–VI).22
The pooled results from both studies completed at 68 investigative sites demonstrated that CP 1.2%–BP 2.5% gel was superior in efficacy to each individual monad and to the vehicle in inflammatory, noninflammatory, and total lesion reductions as early as week 4 (P<.001) and at week 12, which was the study end point (P<.001), with superiority also demonstrated in achieving treatment success (defined as a >2 grade improvement according to the evaluator global severity score) compared to the 3 other study arms (P<.001).22 Subject assessments also were consistent with outcomes noted by the investigators. Cutaneous tolerability was favorable and comparable in all 4 study arms with less than 1% of participants discontinuing treatment due to adverse events.22
A subset analysis of the data from the phase 3 pivotal trials with CP 1.2%–BP 2.5% gel was completed to compare reductions in both inflammatory and noninflammatory lesions in female participants who were younger than 25 years and 25 years of age or older in all 4 study arms. This information has been presented14,17 but has not been previously published. Based on the overall results reported in the phase 3 studies, there were no differentiations in skin tolerability or safety based on participant age, gender, or skin type.22 The subanalysis included a total of 1080 females who were younger than 25 years and 395 females who were 25 years of age or older. The lesion reduction outcomes of this subanalysis are presented in the Table. Statistical analyses of the results among these age groups in the 4 study arms were not completed because the objective was to determine if there were any major or obvious differences in reduction of AV lesions based on the conventional dividing line of 25 years of age in adult women as compared to adolescent females treated with CP 1.2%–BP 2.5% gel. In addition, the large difference in numbers of female participants between the 2 age groups (>25 years of age, n=395; <25 years of age, n=1080) at least partially confounds both statistical and observational analysis. Among the women who were 25 years of age or older who were included in the subanalysis, 67.0% and 25.8% were between the ages of 25 to 35 years and 36 to 45 years, respectively. Based on the outcomes reported in the phase 3 trials and in this subgroup analysis, CP 1.2%–BP 2.5% gel applied once daily over a 12-week period appeared overall to be comparably effective in females regardless of age and with no apparent adverse events regarding differences in skin tolerability or safety.14,22 One observation that was noted was the possible trend of greater reduction in both lesion types in women older than 35 years versus younger females with the use of the combination gel or BP alone; however, the number of female participants who were older than 35 years of age was substantially less (n=102) than those who were 35 years of age or younger (n=1345), thus precluding support for any definitive conclusions about this possible trend.22
How can CP 1.2%–BP 2.5% gel be incorporated into a treatment regimen for women with facial AV?
The incorporation of CP 1.2%–BP 2.5% gel into a treatment regimen for women with facial AV is similar to the general use of BP-containing formulations in the overall management of AV.9,14,27,28 Because women with AV commonly present with facial inflammatory lesions and many also with facial comedones, CP 1.2%–BP 2.5% gel is best used once daily in the morning in combination with a topical retinoid in the evening,9,27 which can be achieved with use of CP 1.2%–BP 2.5% gel in the morning and a topical retinoid (ie, tretinoin, adapalene, tazarotene) in the evening or CP 1.2%–tretinoin 0.025% gel in the evening. It is important to note that cutaneous irritation may be more likely if neck lesions are present; the potential for bleaching of colored fabric by BP also is a practical concern.28 In addition, CP 1.2%–BP 2.5% gel may also be used in combination with topical dapsone, but both products should be applied separately at different times of the day to avoid temporary orange discoloration of the skin, which appears to be an uncommon side effect but remains a possibility based on the product information for dapsone gel 5% with regard to its concomitant use with BP.29,30
1. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health. 2012;21:223-230.
2. Zeichner J. Evaluating and treating the adult female patient with acne. J Drugs Dermatol. 2013;12:1416-1427.
3. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41:577-580.
4. Collier CN, Harper J, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
5. Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
6. Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
7. Kim GK, Michaels BB. Post-adolescent acne in women: more common and more clinical considerations. J Drugs Dermatol. 2012;11:708-713.
8. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
9. Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(suppl 1):1-37.
10. Thiboutot DM. Endocrinological evaluation and hormonal therapy for women with difficult acne. J Eur Acad Dermatol Venereol. 2001;15(suppl 3):57-61.
11. Sawaya ME, Samani N. Antiandrogens and androgen receptors. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Saunders-Elsevier; 2012:361-374.
12. Harper JC. Should dermatologists prescribe hormonal contraceptives for acne? Dermatol Ther. 2009;22:452-457.
13. Preneau S, Dreno B. Female acne: a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
14. Del Rosso JQ, Zeichner JA. What’s new in the medicine cabinet?: a panoramic review of clinically relevant information for the busy dermatologist. J Clin Aesthet Dermatol. 2014;7:26-30.
15. Berson D, Alexis A. Adapalene 0.3% for the treatment of acne in women. J Clin Aesthet Dermatol. 2013;6:32-35.
16. Del Rosso JQ, Kircik L, Gallagher C. Facing up to adult women with acne vulgaris: an analysis of pivotal trial data on dapsone 5% gel in the adult female population. Poster presented at: Fall Clinical Dermatology; October 2013; Las Vegas, NV.
17. Del Rosso JQ. Management of acne with oral spironolactone. Presented at: American Academy of Dermatology Summer Meeting; August 2013; Boston, MA.
18. Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
19. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
20. Perkins AC, Cheng CE, Hillebrand GG, et al. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
21. Draelos ZD, Carter E, Maloney JM, et al. Two randomized studies demonstrate the efficacy and safety of dapsone gel, 5% for the treatment of acne vulgaris [published online ahead of print January 17, 2007]. J Am Acad Dermatol. 2007;56:439.e1-439.e10.
22. Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
23. Schlessinger J, Menter A, Gold M, et al. Clinical safety and efficacy studies of a novel formulation combining
1.2% clindamycin phosphate and 0.025% tretinoin for the treatment of acne vulgaris. J Drugs Dermatol. 2007;6:607-615.
24. Fleischer AB Jr, Dinehart S, Stough D, et al. Safety and efficacy of a new extended-release formulation of minocycline. Cutis. 2006;78(suppl 4):21-31.
25. Gollnick HP, Draelos Z, Glenn MJ, et al. Adapalene-benzoyl peroxide, a unique fixed-dose combination topical gel for the treatment of acne vulgaris: a transatlantic, randomized, double-blind, controlled study in 1670 patients. Br J Dermatol. 2009;161:1180-1189.
26. Thiboutot D, Arsonnaud S, Soto P. Efficacy and tolerability of adapalene 0.3% gel compared to tazarotene 0.1% gel in the treatment of acne vulgaris. J Drugs Dermatol. 2008;7(suppl 6):3-10.
27. Zeichner JA. Optimizing topical combination therapy for the treatment of acne vulgaris. J Drugs Dermatol. 2012;11:313-317.
28. Tanghetti EA, Popp KF. A current review of topical benzoyl peroxide: new perspectives on formulation and utilization. Dermatol Clin. 2009;27:17-24.
29. Fleischer AB, Shalita A, Eichenfield LF. Dapsone gel 5% in combination with adapalene gel 0.1%, benzoyl peroxide gel 4% or moisturizer for the treatment of acne vulgaris: a 12-week, randomized, double-blind study. J Drugs Dermatol. 2010;9:33-40.
30. Aczone (dapsone gel 5%) [package insert]. Irvine, CA: Allergan, Inc; 2013.
The management of acne vulgaris (AV) in women has been the subject of considerable attention over the last few years. It has become increasingly recognized that a greater number of patient encounters in dermatology offices involve women with AV who are beyond their adolescent years. Overall, it is estimated that up to approximately 22% of women in the United States are affected by AV, with approximately half of women in their 20s and one-third of women in their 30s reporting some degree of AV.1-4 Among women, the disease shows no predilection for certain skin types or ethnicities, can start during the preteenaged or adolescent years, can persist or recur in adulthood (persistent acne, 75%), or can start in adulthood (late-onset acne, 25%) in females with minimal or no history of AV occurring earlier in life.3,5-7 In the subpopulation of adult women, AV occurs at a time when many expect to be far beyond this “teenage affliction.” Women who are affected commonly express feeling embarrassed and frustrated.5-8
Most of the emphasis in the literature and in presentations at dermatology meetings regarding the management of AV in adult women has focused on excluding underlying disorders that cause excess androgens (eg, polycystic ovary syndrome, congenital adrenal hyperplasia, tumors, exogenous sources) as well as the use of systemic therapies such as oral contraceptives (OCs) and spironolactone.5-7,9,10 Little attention has been given to the selection of topical therapies in this patient population, especially with regard to evidence from clinical studies. To date, results from published study analyses using topical agents specifically for adult females with facial AV have only included adapalene gel 0.3% applied once daily and dapsone gel 5% applied twice daily.11-13 Both agents have been evaluated in subset analysis comparisons of outcomes in women aged 18 years and older versus adolescents aged 12 to 17 years based on data from 12-week phase 3 pivotal trials.14-16
Are there clinically relevant differences between AV in adult versus adolescent females?
Although much has been written about AV in women, epidemiology, demographics, assessment of clinical presentation, and correlation of clinical presentation with excess androgens have not been emphasized,1-3,5-10,17 likely due to marketing campaigns that emphasize AV as a disorder that predominantly affects teenagers as well as the focus on optimal use of oral spironolactone and/or OCs in the management of AV in the adult female population. Attention to spironolactone use is important because it is not approved by the US Food and Drug Administration for the treatment of AV. Spironolactone carries certain black-box warnings that may not be clinically relevant in all patients but still require attention. It also is associated with risks if taken during pregnancy, and it is a potassium-sparing diuretic with potential for hyperkalemia, especially in patients with reduced renal function or those who are taking potassium supplements or certain other medications.6,9,11,17 Use of OCs to treat AV also is not without potential risks, with specific warnings and relative contraindications reported, especially in relation to increased risks for cardiac complications, stroke, and thromboembolism.6,9,11,12 Because adult females are in a different stage of life than teenagers, there are defined psychosocial and medical considerations in managing AV in these patients compared with adolescents.5-8,17 Importantly for both clinicians and patients, addressing these differences and considerations can have a major impact on whether or not women with AV experience successful treatment outcomes.1-3,5,6,8,10,13 Skin color and ethnicity also can affect the psychosocial and physical factors that influence the overall management of adult female patients with AV, including selection of therapies and handling long-term visible sequelae that occur in some AV patients, such as dyschromia (eg, persistent or postinflammatory erythema or hyperpigmentation) and acne scarring.5-8,13,17-20
Psychosocial Considerations
With regard to psychosocial, emotional, and attitudinal considerations in women with AV, common findings include concern or frustration regarding the presence of AV beyond adolescence; anxiety; symptoms of depression; decreased self-confidence; increased self-consciousness, especially during public interactions or intimate situations; and interference with steady concentration at work or school.5,6,8,13 Long-term complications of AV, such as dyschromia and acne scarring, are more likely to be encountered in adult patients, especially if they had AV as a teenager, with women reporting that they remain conscious of these adverse sequelae.8 It is estimated that approximately three-fourths of women with AV also had AV as teenagers; therefore, most of them have already used many over-the-counter and prescription therapies and are likely to want treatments that are newer, well-tolerated, safe, and known to be effective in adult women.8,16,17 Convenience and simplicity are vital components of treatment selection and regimen design, as many women with AV frequently face time constraints in their daily routines due to family, social, employment, and home-related demands and responsibilities.6-8,17
Medical Considerations
It is apparent from reports in the literature as well as from clinical experience that some women with AV present with a U-shaped pattern of involvement on the face,5-7,10,13,17 which refers to the presence of predominantly inflammatory papules (many of them deep) and some nodules on the lower face, jawline, and anterolateral neck region, with comedones often sparse or absent.5-7 It often is perceived and may be true that women who present with this pattern of distribution are more androgen sensitive despite having normal serum androgen levels or in some cases exhibit detectable excess androgens (eg, in the setting of polycystic ovary syndrome) and may be more likely to respond to hormonal therapies (eg, spironolactone, OCs) than those with mixed facial AV (ie, multiple comedonal and inflammatory acne lesions, not limited to a U-shaped pattern, similar to adolescent AV), but data are limited to support differentiation between the U-shaped pattern group and the conventional mixed facial AV group.5-7,17 Adult and adolescent females in both groups sometimes report perimenstrual flares and frequent persistent papular AV that tends to concentrate on the perioral and chin area.
It is also important to consider that the current literature suggests approximately three-fourths of women with AV report that they also had AV as a teenager, with many indicating the same clinical pattern of AV and approximately one-third reporting AV that is more severe in adulthood than adolescence.5-8,17 The available literature on topical and oral therapies used to treat AV in both adolescent and adult females predominantly focuses on inclusion of both inflammatory and noninflammatory (comedonal) facial AV lesions, does not specifically address or include the U-shaped pattern of AV in adult women for inclusion in studies that evaluate efficacy in this subgroup, and does not include AV involving the neck region and below the jawline margin as part of any study protocols and/or discussions about therapy.5-7,9-12,17,21-26 Involvement of the neck and lower jawline is common in women presenting with the U-shaped pattern of AV, and available studies only evaluate AV involving the face and do not include AV lesions present below the jawline margin. As a result, there is a considerable need for well-designed studies with laboratory assessments to include or exclude underlying detectable excess androgens and to assess the efficacy, tolerability, and safety of specific therapeutic agents both alone and in combination in adult women who present with a U-shaped pattern of AV.17
Other medical considerations that can influence treatment selection and are more likely to be present in adult versus adolescent females include underlying chronic medical disorders; concomitant medications that may interact with other oral agents; potential for pregnancy; age, particularly when prescribing OCs; and the potential desire to stop taking OCs if already used over a prolonged period.6,7
Age-Related Differentiation of Female Subgroups With AV
The age-based dividing line that defines AV in adults versus adolescent females has been described in the literature; however, the basis for published definitions of female subgroups with AV is not well-supported by strong scientific evidence.1-3,5-7,17 The conventional dividing line that was originally selected to define adult females with AV was 25 years of age or older; persistent acne is present both during adolescence and at or after 25 years of age, while late-onset acne is described as AV that first presents at 25 years of age or older.3,5-7
More recently, a range of 18 years or older has been used to classify adult female AV and a range of 12 to 17 years for adolescent female AV in subset analyses that evaluated treatment outcomes in both patient populations from phase 3 pivotal trials completed with adapalene gel 0.3% applied once daily and dapsone gel 5% applied twice daily.14-16 These subanalyses included participants with facial AV that was predominantly moderate in severity, mandated specific lesion count ranges for both comedonal and inflammatory lesions, and included only facial AV that was above the mandibular (jawline) margin.15,16,21,26 Therefore, patients with AV presenting in a U-shaped pattern with involvement below the jawline and on the neck were not included in these study analyses, as these patients were excluded from the phase 3 trials on which the analyses were based. The outcomes of these analyses apply to treatment in women who present with both inflammatory and noninflammatory facial AV lesions, which supports the observation that AV in this patient population is not always predominantly inflammatory and does not always present in a U-shaped distribution.14-16 In fact, a U-shaped pattern of distribution appears to be less common in women with AV than a mixed inflammatory and comedonal distribution that involves the face more diffusely, though more data are needed from well-designed and large-scale epidemiologic and demographic studies.5,14,17
Are there data available on the use of benzoyl peroxide with or without a topical antibiotic in women with AV?
There is a conspicuous absence of prospective clinical trials and retrospective analyses evaluating the specific use of individual AV therapies in adult females, with a particular lack of studies with topical agents (eg, benzoyl peroxide [BP]).14 Subset analyses have been completed for adapalene gel 0.3% and dapsone gel 5%.15,16 Additionally, an age-based subset analysis in females with facial AV also has been completed with clindamycin phosphate (CP) 1.2%–BP 2.5% gel once daily, with data presented but not yet fully published.14
Two identical phase 3, double-blind, randomized, 12-week, 4-arm trials compared treatment outcomes in groups treated with an aqueous-based combination gel formulation containing BP 2.5% and CP 1.2% (n=797), active monad gels (BP [n=809] or CP [n=812]), or vehicle gel (n=395), all applied once daily in patients with facial AV.22 Participants were 12 years or older (mean age range, 19.1–19.6 years; age range, 12.1–70.2 years), were of either gender (approximately 50% split in each study arm), and presented with moderate (approximately 80% of participants) or severe AV (approximately 20% of participants) at baseline. The entry criteria for lesion types and number of lesions were 17 to 40 inflammatory lesions (ie, papules, pustules, <2 nodules)(range of mean number of lesions, 25.8–26.4) and 20 to 100 noninflammatory lesions (ie, closed comedones, open comedones)(range of mean number of lesions, 44.0–47.4). Participant demographics included white (73.9%–77.5%), black/African American (16.1%–20.4%), and Asian (2.1%–3.3%), with the remaining participants distributed among a variety of other ethnic groups such as Native Hawaiian/Native Pacific Islander and Native American Indian/Native Alaskan (collectively <5% in each study arm). Therefore, approximately 1 of every 4 patients had skin of color, which provided good diversity of patients considering the large study size (N=2813). Data analysis included dichotomization of participants by severity rating (moderate or severe based on evaluator global severity score) and skin phototype (Fitzpatrick skin types I–III or IV–VI).22
The pooled results from both studies completed at 68 investigative sites demonstrated that CP 1.2%–BP 2.5% gel was superior in efficacy to each individual monad and to the vehicle in inflammatory, noninflammatory, and total lesion reductions as early as week 4 (P<.001) and at week 12, which was the study end point (P<.001), with superiority also demonstrated in achieving treatment success (defined as a >2 grade improvement according to the evaluator global severity score) compared to the 3 other study arms (P<.001).22 Subject assessments also were consistent with outcomes noted by the investigators. Cutaneous tolerability was favorable and comparable in all 4 study arms with less than 1% of participants discontinuing treatment due to adverse events.22
A subset analysis of the data from the phase 3 pivotal trials with CP 1.2%–BP 2.5% gel was completed to compare reductions in both inflammatory and noninflammatory lesions in female participants who were younger than 25 years and 25 years of age or older in all 4 study arms. This information has been presented14,17 but has not been previously published. Based on the overall results reported in the phase 3 studies, there were no differentiations in skin tolerability or safety based on participant age, gender, or skin type.22 The subanalysis included a total of 1080 females who were younger than 25 years and 395 females who were 25 years of age or older. The lesion reduction outcomes of this subanalysis are presented in the Table. Statistical analyses of the results among these age groups in the 4 study arms were not completed because the objective was to determine if there were any major or obvious differences in reduction of AV lesions based on the conventional dividing line of 25 years of age in adult women as compared to adolescent females treated with CP 1.2%–BP 2.5% gel. In addition, the large difference in numbers of female participants between the 2 age groups (>25 years of age, n=395; <25 years of age, n=1080) at least partially confounds both statistical and observational analysis. Among the women who were 25 years of age or older who were included in the subanalysis, 67.0% and 25.8% were between the ages of 25 to 35 years and 36 to 45 years, respectively. Based on the outcomes reported in the phase 3 trials and in this subgroup analysis, CP 1.2%–BP 2.5% gel applied once daily over a 12-week period appeared overall to be comparably effective in females regardless of age and with no apparent adverse events regarding differences in skin tolerability or safety.14,22 One observation that was noted was the possible trend of greater reduction in both lesion types in women older than 35 years versus younger females with the use of the combination gel or BP alone; however, the number of female participants who were older than 35 years of age was substantially less (n=102) than those who were 35 years of age or younger (n=1345), thus precluding support for any definitive conclusions about this possible trend.22
How can CP 1.2%–BP 2.5% gel be incorporated into a treatment regimen for women with facial AV?
The incorporation of CP 1.2%–BP 2.5% gel into a treatment regimen for women with facial AV is similar to the general use of BP-containing formulations in the overall management of AV.9,14,27,28 Because women with AV commonly present with facial inflammatory lesions and many also with facial comedones, CP 1.2%–BP 2.5% gel is best used once daily in the morning in combination with a topical retinoid in the evening,9,27 which can be achieved with use of CP 1.2%–BP 2.5% gel in the morning and a topical retinoid (ie, tretinoin, adapalene, tazarotene) in the evening or CP 1.2%–tretinoin 0.025% gel in the evening. It is important to note that cutaneous irritation may be more likely if neck lesions are present; the potential for bleaching of colored fabric by BP also is a practical concern.28 In addition, CP 1.2%–BP 2.5% gel may also be used in combination with topical dapsone, but both products should be applied separately at different times of the day to avoid temporary orange discoloration of the skin, which appears to be an uncommon side effect but remains a possibility based on the product information for dapsone gel 5% with regard to its concomitant use with BP.29,30
The management of acne vulgaris (AV) in women has been the subject of considerable attention over the last few years. It has become increasingly recognized that a greater number of patient encounters in dermatology offices involve women with AV who are beyond their adolescent years. Overall, it is estimated that up to approximately 22% of women in the United States are affected by AV, with approximately half of women in their 20s and one-third of women in their 30s reporting some degree of AV.1-4 Among women, the disease shows no predilection for certain skin types or ethnicities, can start during the preteenaged or adolescent years, can persist or recur in adulthood (persistent acne, 75%), or can start in adulthood (late-onset acne, 25%) in females with minimal or no history of AV occurring earlier in life.3,5-7 In the subpopulation of adult women, AV occurs at a time when many expect to be far beyond this “teenage affliction.” Women who are affected commonly express feeling embarrassed and frustrated.5-8
Most of the emphasis in the literature and in presentations at dermatology meetings regarding the management of AV in adult women has focused on excluding underlying disorders that cause excess androgens (eg, polycystic ovary syndrome, congenital adrenal hyperplasia, tumors, exogenous sources) as well as the use of systemic therapies such as oral contraceptives (OCs) and spironolactone.5-7,9,10 Little attention has been given to the selection of topical therapies in this patient population, especially with regard to evidence from clinical studies. To date, results from published study analyses using topical agents specifically for adult females with facial AV have only included adapalene gel 0.3% applied once daily and dapsone gel 5% applied twice daily.11-13 Both agents have been evaluated in subset analysis comparisons of outcomes in women aged 18 years and older versus adolescents aged 12 to 17 years based on data from 12-week phase 3 pivotal trials.14-16
Are there clinically relevant differences between AV in adult versus adolescent females?
Although much has been written about AV in women, epidemiology, demographics, assessment of clinical presentation, and correlation of clinical presentation with excess androgens have not been emphasized,1-3,5-10,17 likely due to marketing campaigns that emphasize AV as a disorder that predominantly affects teenagers as well as the focus on optimal use of oral spironolactone and/or OCs in the management of AV in the adult female population. Attention to spironolactone use is important because it is not approved by the US Food and Drug Administration for the treatment of AV. Spironolactone carries certain black-box warnings that may not be clinically relevant in all patients but still require attention. It also is associated with risks if taken during pregnancy, and it is a potassium-sparing diuretic with potential for hyperkalemia, especially in patients with reduced renal function or those who are taking potassium supplements or certain other medications.6,9,11,17 Use of OCs to treat AV also is not without potential risks, with specific warnings and relative contraindications reported, especially in relation to increased risks for cardiac complications, stroke, and thromboembolism.6,9,11,12 Because adult females are in a different stage of life than teenagers, there are defined psychosocial and medical considerations in managing AV in these patients compared with adolescents.5-8,17 Importantly for both clinicians and patients, addressing these differences and considerations can have a major impact on whether or not women with AV experience successful treatment outcomes.1-3,5,6,8,10,13 Skin color and ethnicity also can affect the psychosocial and physical factors that influence the overall management of adult female patients with AV, including selection of therapies and handling long-term visible sequelae that occur in some AV patients, such as dyschromia (eg, persistent or postinflammatory erythema or hyperpigmentation) and acne scarring.5-8,13,17-20
Psychosocial Considerations
With regard to psychosocial, emotional, and attitudinal considerations in women with AV, common findings include concern or frustration regarding the presence of AV beyond adolescence; anxiety; symptoms of depression; decreased self-confidence; increased self-consciousness, especially during public interactions or intimate situations; and interference with steady concentration at work or school.5,6,8,13 Long-term complications of AV, such as dyschromia and acne scarring, are more likely to be encountered in adult patients, especially if they had AV as a teenager, with women reporting that they remain conscious of these adverse sequelae.8 It is estimated that approximately three-fourths of women with AV also had AV as teenagers; therefore, most of them have already used many over-the-counter and prescription therapies and are likely to want treatments that are newer, well-tolerated, safe, and known to be effective in adult women.8,16,17 Convenience and simplicity are vital components of treatment selection and regimen design, as many women with AV frequently face time constraints in their daily routines due to family, social, employment, and home-related demands and responsibilities.6-8,17
Medical Considerations
It is apparent from reports in the literature as well as from clinical experience that some women with AV present with a U-shaped pattern of involvement on the face,5-7,10,13,17 which refers to the presence of predominantly inflammatory papules (many of them deep) and some nodules on the lower face, jawline, and anterolateral neck region, with comedones often sparse or absent.5-7 It often is perceived and may be true that women who present with this pattern of distribution are more androgen sensitive despite having normal serum androgen levels or in some cases exhibit detectable excess androgens (eg, in the setting of polycystic ovary syndrome) and may be more likely to respond to hormonal therapies (eg, spironolactone, OCs) than those with mixed facial AV (ie, multiple comedonal and inflammatory acne lesions, not limited to a U-shaped pattern, similar to adolescent AV), but data are limited to support differentiation between the U-shaped pattern group and the conventional mixed facial AV group.5-7,17 Adult and adolescent females in both groups sometimes report perimenstrual flares and frequent persistent papular AV that tends to concentrate on the perioral and chin area.
It is also important to consider that the current literature suggests approximately three-fourths of women with AV report that they also had AV as a teenager, with many indicating the same clinical pattern of AV and approximately one-third reporting AV that is more severe in adulthood than adolescence.5-8,17 The available literature on topical and oral therapies used to treat AV in both adolescent and adult females predominantly focuses on inclusion of both inflammatory and noninflammatory (comedonal) facial AV lesions, does not specifically address or include the U-shaped pattern of AV in adult women for inclusion in studies that evaluate efficacy in this subgroup, and does not include AV involving the neck region and below the jawline margin as part of any study protocols and/or discussions about therapy.5-7,9-12,17,21-26 Involvement of the neck and lower jawline is common in women presenting with the U-shaped pattern of AV, and available studies only evaluate AV involving the face and do not include AV lesions present below the jawline margin. As a result, there is a considerable need for well-designed studies with laboratory assessments to include or exclude underlying detectable excess androgens and to assess the efficacy, tolerability, and safety of specific therapeutic agents both alone and in combination in adult women who present with a U-shaped pattern of AV.17
Other medical considerations that can influence treatment selection and are more likely to be present in adult versus adolescent females include underlying chronic medical disorders; concomitant medications that may interact with other oral agents; potential for pregnancy; age, particularly when prescribing OCs; and the potential desire to stop taking OCs if already used over a prolonged period.6,7
Age-Related Differentiation of Female Subgroups With AV
The age-based dividing line that defines AV in adults versus adolescent females has been described in the literature; however, the basis for published definitions of female subgroups with AV is not well-supported by strong scientific evidence.1-3,5-7,17 The conventional dividing line that was originally selected to define adult females with AV was 25 years of age or older; persistent acne is present both during adolescence and at or after 25 years of age, while late-onset acne is described as AV that first presents at 25 years of age or older.3,5-7
More recently, a range of 18 years or older has been used to classify adult female AV and a range of 12 to 17 years for adolescent female AV in subset analyses that evaluated treatment outcomes in both patient populations from phase 3 pivotal trials completed with adapalene gel 0.3% applied once daily and dapsone gel 5% applied twice daily.14-16 These subanalyses included participants with facial AV that was predominantly moderate in severity, mandated specific lesion count ranges for both comedonal and inflammatory lesions, and included only facial AV that was above the mandibular (jawline) margin.15,16,21,26 Therefore, patients with AV presenting in a U-shaped pattern with involvement below the jawline and on the neck were not included in these study analyses, as these patients were excluded from the phase 3 trials on which the analyses were based. The outcomes of these analyses apply to treatment in women who present with both inflammatory and noninflammatory facial AV lesions, which supports the observation that AV in this patient population is not always predominantly inflammatory and does not always present in a U-shaped distribution.14-16 In fact, a U-shaped pattern of distribution appears to be less common in women with AV than a mixed inflammatory and comedonal distribution that involves the face more diffusely, though more data are needed from well-designed and large-scale epidemiologic and demographic studies.5,14,17
Are there data available on the use of benzoyl peroxide with or without a topical antibiotic in women with AV?
There is a conspicuous absence of prospective clinical trials and retrospective analyses evaluating the specific use of individual AV therapies in adult females, with a particular lack of studies with topical agents (eg, benzoyl peroxide [BP]).14 Subset analyses have been completed for adapalene gel 0.3% and dapsone gel 5%.15,16 Additionally, an age-based subset analysis in females with facial AV also has been completed with clindamycin phosphate (CP) 1.2%–BP 2.5% gel once daily, with data presented but not yet fully published.14
Two identical phase 3, double-blind, randomized, 12-week, 4-arm trials compared treatment outcomes in groups treated with an aqueous-based combination gel formulation containing BP 2.5% and CP 1.2% (n=797), active monad gels (BP [n=809] or CP [n=812]), or vehicle gel (n=395), all applied once daily in patients with facial AV.22 Participants were 12 years or older (mean age range, 19.1–19.6 years; age range, 12.1–70.2 years), were of either gender (approximately 50% split in each study arm), and presented with moderate (approximately 80% of participants) or severe AV (approximately 20% of participants) at baseline. The entry criteria for lesion types and number of lesions were 17 to 40 inflammatory lesions (ie, papules, pustules, <2 nodules)(range of mean number of lesions, 25.8–26.4) and 20 to 100 noninflammatory lesions (ie, closed comedones, open comedones)(range of mean number of lesions, 44.0–47.4). Participant demographics included white (73.9%–77.5%), black/African American (16.1%–20.4%), and Asian (2.1%–3.3%), with the remaining participants distributed among a variety of other ethnic groups such as Native Hawaiian/Native Pacific Islander and Native American Indian/Native Alaskan (collectively <5% in each study arm). Therefore, approximately 1 of every 4 patients had skin of color, which provided good diversity of patients considering the large study size (N=2813). Data analysis included dichotomization of participants by severity rating (moderate or severe based on evaluator global severity score) and skin phototype (Fitzpatrick skin types I–III or IV–VI).22
The pooled results from both studies completed at 68 investigative sites demonstrated that CP 1.2%–BP 2.5% gel was superior in efficacy to each individual monad and to the vehicle in inflammatory, noninflammatory, and total lesion reductions as early as week 4 (P<.001) and at week 12, which was the study end point (P<.001), with superiority also demonstrated in achieving treatment success (defined as a >2 grade improvement according to the evaluator global severity score) compared to the 3 other study arms (P<.001).22 Subject assessments also were consistent with outcomes noted by the investigators. Cutaneous tolerability was favorable and comparable in all 4 study arms with less than 1% of participants discontinuing treatment due to adverse events.22
A subset analysis of the data from the phase 3 pivotal trials with CP 1.2%–BP 2.5% gel was completed to compare reductions in both inflammatory and noninflammatory lesions in female participants who were younger than 25 years and 25 years of age or older in all 4 study arms. This information has been presented14,17 but has not been previously published. Based on the overall results reported in the phase 3 studies, there were no differentiations in skin tolerability or safety based on participant age, gender, or skin type.22 The subanalysis included a total of 1080 females who were younger than 25 years and 395 females who were 25 years of age or older. The lesion reduction outcomes of this subanalysis are presented in the Table. Statistical analyses of the results among these age groups in the 4 study arms were not completed because the objective was to determine if there were any major or obvious differences in reduction of AV lesions based on the conventional dividing line of 25 years of age in adult women as compared to adolescent females treated with CP 1.2%–BP 2.5% gel. In addition, the large difference in numbers of female participants between the 2 age groups (>25 years of age, n=395; <25 years of age, n=1080) at least partially confounds both statistical and observational analysis. Among the women who were 25 years of age or older who were included in the subanalysis, 67.0% and 25.8% were between the ages of 25 to 35 years and 36 to 45 years, respectively. Based on the outcomes reported in the phase 3 trials and in this subgroup analysis, CP 1.2%–BP 2.5% gel applied once daily over a 12-week period appeared overall to be comparably effective in females regardless of age and with no apparent adverse events regarding differences in skin tolerability or safety.14,22 One observation that was noted was the possible trend of greater reduction in both lesion types in women older than 35 years versus younger females with the use of the combination gel or BP alone; however, the number of female participants who were older than 35 years of age was substantially less (n=102) than those who were 35 years of age or younger (n=1345), thus precluding support for any definitive conclusions about this possible trend.22
How can CP 1.2%–BP 2.5% gel be incorporated into a treatment regimen for women with facial AV?
The incorporation of CP 1.2%–BP 2.5% gel into a treatment regimen for women with facial AV is similar to the general use of BP-containing formulations in the overall management of AV.9,14,27,28 Because women with AV commonly present with facial inflammatory lesions and many also with facial comedones, CP 1.2%–BP 2.5% gel is best used once daily in the morning in combination with a topical retinoid in the evening,9,27 which can be achieved with use of CP 1.2%–BP 2.5% gel in the morning and a topical retinoid (ie, tretinoin, adapalene, tazarotene) in the evening or CP 1.2%–tretinoin 0.025% gel in the evening. It is important to note that cutaneous irritation may be more likely if neck lesions are present; the potential for bleaching of colored fabric by BP also is a practical concern.28 In addition, CP 1.2%–BP 2.5% gel may also be used in combination with topical dapsone, but both products should be applied separately at different times of the day to avoid temporary orange discoloration of the skin, which appears to be an uncommon side effect but remains a possibility based on the product information for dapsone gel 5% with regard to its concomitant use with BP.29,30
1. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health. 2012;21:223-230.
2. Zeichner J. Evaluating and treating the adult female patient with acne. J Drugs Dermatol. 2013;12:1416-1427.
3. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41:577-580.
4. Collier CN, Harper J, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
5. Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
6. Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
7. Kim GK, Michaels BB. Post-adolescent acne in women: more common and more clinical considerations. J Drugs Dermatol. 2012;11:708-713.
8. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
9. Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(suppl 1):1-37.
10. Thiboutot DM. Endocrinological evaluation and hormonal therapy for women with difficult acne. J Eur Acad Dermatol Venereol. 2001;15(suppl 3):57-61.
11. Sawaya ME, Samani N. Antiandrogens and androgen receptors. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Saunders-Elsevier; 2012:361-374.
12. Harper JC. Should dermatologists prescribe hormonal contraceptives for acne? Dermatol Ther. 2009;22:452-457.
13. Preneau S, Dreno B. Female acne: a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
14. Del Rosso JQ, Zeichner JA. What’s new in the medicine cabinet?: a panoramic review of clinically relevant information for the busy dermatologist. J Clin Aesthet Dermatol. 2014;7:26-30.
15. Berson D, Alexis A. Adapalene 0.3% for the treatment of acne in women. J Clin Aesthet Dermatol. 2013;6:32-35.
16. Del Rosso JQ, Kircik L, Gallagher C. Facing up to adult women with acne vulgaris: an analysis of pivotal trial data on dapsone 5% gel in the adult female population. Poster presented at: Fall Clinical Dermatology; October 2013; Las Vegas, NV.
17. Del Rosso JQ. Management of acne with oral spironolactone. Presented at: American Academy of Dermatology Summer Meeting; August 2013; Boston, MA.
18. Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
19. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
20. Perkins AC, Cheng CE, Hillebrand GG, et al. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
21. Draelos ZD, Carter E, Maloney JM, et al. Two randomized studies demonstrate the efficacy and safety of dapsone gel, 5% for the treatment of acne vulgaris [published online ahead of print January 17, 2007]. J Am Acad Dermatol. 2007;56:439.e1-439.e10.
22. Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
23. Schlessinger J, Menter A, Gold M, et al. Clinical safety and efficacy studies of a novel formulation combining
1.2% clindamycin phosphate and 0.025% tretinoin for the treatment of acne vulgaris. J Drugs Dermatol. 2007;6:607-615.
24. Fleischer AB Jr, Dinehart S, Stough D, et al. Safety and efficacy of a new extended-release formulation of minocycline. Cutis. 2006;78(suppl 4):21-31.
25. Gollnick HP, Draelos Z, Glenn MJ, et al. Adapalene-benzoyl peroxide, a unique fixed-dose combination topical gel for the treatment of acne vulgaris: a transatlantic, randomized, double-blind, controlled study in 1670 patients. Br J Dermatol. 2009;161:1180-1189.
26. Thiboutot D, Arsonnaud S, Soto P. Efficacy and tolerability of adapalene 0.3% gel compared to tazarotene 0.1% gel in the treatment of acne vulgaris. J Drugs Dermatol. 2008;7(suppl 6):3-10.
27. Zeichner JA. Optimizing topical combination therapy for the treatment of acne vulgaris. J Drugs Dermatol. 2012;11:313-317.
28. Tanghetti EA, Popp KF. A current review of topical benzoyl peroxide: new perspectives on formulation and utilization. Dermatol Clin. 2009;27:17-24.
29. Fleischer AB, Shalita A, Eichenfield LF. Dapsone gel 5% in combination with adapalene gel 0.1%, benzoyl peroxide gel 4% or moisturizer for the treatment of acne vulgaris: a 12-week, randomized, double-blind study. J Drugs Dermatol. 2010;9:33-40.
30. Aczone (dapsone gel 5%) [package insert]. Irvine, CA: Allergan, Inc; 2013.
1. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health. 2012;21:223-230.
2. Zeichner J. Evaluating and treating the adult female patient with acne. J Drugs Dermatol. 2013;12:1416-1427.
3. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41:577-580.
4. Collier CN, Harper J, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
5. Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
6. Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
7. Kim GK, Michaels BB. Post-adolescent acne in women: more common and more clinical considerations. J Drugs Dermatol. 2012;11:708-713.
8. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
9. Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(suppl 1):1-37.
10. Thiboutot DM. Endocrinological evaluation and hormonal therapy for women with difficult acne. J Eur Acad Dermatol Venereol. 2001;15(suppl 3):57-61.
11. Sawaya ME, Samani N. Antiandrogens and androgen receptors. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Saunders-Elsevier; 2012:361-374.
12. Harper JC. Should dermatologists prescribe hormonal contraceptives for acne? Dermatol Ther. 2009;22:452-457.
13. Preneau S, Dreno B. Female acne: a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
14. Del Rosso JQ, Zeichner JA. What’s new in the medicine cabinet?: a panoramic review of clinically relevant information for the busy dermatologist. J Clin Aesthet Dermatol. 2014;7:26-30.
15. Berson D, Alexis A. Adapalene 0.3% for the treatment of acne in women. J Clin Aesthet Dermatol. 2013;6:32-35.
16. Del Rosso JQ, Kircik L, Gallagher C. Facing up to adult women with acne vulgaris: an analysis of pivotal trial data on dapsone 5% gel in the adult female population. Poster presented at: Fall Clinical Dermatology; October 2013; Las Vegas, NV.
17. Del Rosso JQ. Management of acne with oral spironolactone. Presented at: American Academy of Dermatology Summer Meeting; August 2013; Boston, MA.
18. Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
19. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
20. Perkins AC, Cheng CE, Hillebrand GG, et al. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
21. Draelos ZD, Carter E, Maloney JM, et al. Two randomized studies demonstrate the efficacy and safety of dapsone gel, 5% for the treatment of acne vulgaris [published online ahead of print January 17, 2007]. J Am Acad Dermatol. 2007;56:439.e1-439.e10.
22. Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
23. Schlessinger J, Menter A, Gold M, et al. Clinical safety and efficacy studies of a novel formulation combining
1.2% clindamycin phosphate and 0.025% tretinoin for the treatment of acne vulgaris. J Drugs Dermatol. 2007;6:607-615.
24. Fleischer AB Jr, Dinehart S, Stough D, et al. Safety and efficacy of a new extended-release formulation of minocycline. Cutis. 2006;78(suppl 4):21-31.
25. Gollnick HP, Draelos Z, Glenn MJ, et al. Adapalene-benzoyl peroxide, a unique fixed-dose combination topical gel for the treatment of acne vulgaris: a transatlantic, randomized, double-blind, controlled study in 1670 patients. Br J Dermatol. 2009;161:1180-1189.
26. Thiboutot D, Arsonnaud S, Soto P. Efficacy and tolerability of adapalene 0.3% gel compared to tazarotene 0.1% gel in the treatment of acne vulgaris. J Drugs Dermatol. 2008;7(suppl 6):3-10.
27. Zeichner JA. Optimizing topical combination therapy for the treatment of acne vulgaris. J Drugs Dermatol. 2012;11:313-317.
28. Tanghetti EA, Popp KF. A current review of topical benzoyl peroxide: new perspectives on formulation and utilization. Dermatol Clin. 2009;27:17-24.
29. Fleischer AB, Shalita A, Eichenfield LF. Dapsone gel 5% in combination with adapalene gel 0.1%, benzoyl peroxide gel 4% or moisturizer for the treatment of acne vulgaris: a 12-week, randomized, double-blind study. J Drugs Dermatol. 2010;9:33-40.
30. Aczone (dapsone gel 5%) [package insert]. Irvine, CA: Allergan, Inc; 2013.
Practice Points
- Adult women with acne vulgaris (AV) can present with a U-shaped pattern of predominantly inflammatory papules, pustules, and nodules involving the lower face, jawline region, and anterior and lateral neck; however, many women present with mixed comedonal and inflammatory acne involving the face more diffusely with a presentation similar to what is typically seen in adolescent AV.
- Topical therapy for adult women with acne has been evaluated in subanalyses of data from phase 3 studies with good efficacy and favorable tolerability shown with adapalene gel 0.3% once daily, dapsone gel 5% twice daily, and clindamycin phosphate 1.2%–benzoyl peroxide (BP) 2.5% gel once daily. The latter agent requires cautious use below the jawline margin, as BP can bleach colored fabric of clothing such as shirts, blouses, and sweaters. These topical agents may be used in combination based on the clinical situation, including with systemic therapies for AV.
ICD-10 Codes: More Specificity With More Characters
As I have mentioned in prior columns, the key to success with International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) coding will be additional specificity, which will come in the form of more characters to communicate with payors and statisticians regarding the services that have been provided during the office visit. The addition of characters will permit more information about the visit to be delivered in the code.
Some of these additional characters will be required to submit the code for processing. Specifically look out for codes that communicate accidents or injury. Some common circumstances for dermatologists will be lacerations, abrasions, laceration repairs, and burns. The structure of the ICD-10-CM codes is outlined below to explain circumstances in which the increased number of characters will be most important for dermatologists.
The ICD-10-CM codes will be 3 to 7 characters long.1 The first 3 characters are the general categories. For example, L70 is the 3-character category for acne and L40 is the category for psoriasis. The next 3 characters (ie, characters 4–6) correspond to the related etiology (ie, the cause, set of causes, manner of causation of a disease or condition), anatomic site, severity, and other vital clinical details.1
Take the case of a burn on the hand. With the International Classification of Diseases, Ninth Revision, a first-degree burn on the back of the hand is coded with 5 characters (944.16).2 According to the ICD-10-CM coding system, the code for an initial visit for a first-degree burn on the back of the hand would include 7 characters (T23.169A).1 There will be times when the sixth character may not be necessary; in these instances, an X must be placed in this position as a placeholder, but the absence of a sixth character does not negate the need for a seventh. Thankfully, the seventh character can only be 1 of 3 letters. As illustrated above in the code for a first-degree burn on the back of the hand, the A designates an initial encounter; a D would designate a follow-up visit for this burn, and an S would represent a sequela from the initial burn, such as a postinflammatory change, which would have its own code.1
Conclusion
To be reimbursed, appropriate ICD-10-CM codes must be used. Be sure to master the structure of these codes before the October 1, 2015, compliance deadline; plan now for a training and testing period.
1. Centers for Medicare & Medicaid Services. ICD-10-CM Tabular List of Diseases and Injuries. http://www.cms.gov/Medicare/Coding/ICD10/downloads/6_I10tab2010.pdf. Published 2010. Accessed September 18, 2014.
2. ICD-9 code lookup. Centers for Medicare & Medicaid Services Web site. http://www.cms.gov/medicare-coverage-database/staticpages/icd-9-code-lookup.aspx. Accessed September 18, 2014.
As I have mentioned in prior columns, the key to success with International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) coding will be additional specificity, which will come in the form of more characters to communicate with payors and statisticians regarding the services that have been provided during the office visit. The addition of characters will permit more information about the visit to be delivered in the code.
Some of these additional characters will be required to submit the code for processing. Specifically look out for codes that communicate accidents or injury. Some common circumstances for dermatologists will be lacerations, abrasions, laceration repairs, and burns. The structure of the ICD-10-CM codes is outlined below to explain circumstances in which the increased number of characters will be most important for dermatologists.
The ICD-10-CM codes will be 3 to 7 characters long.1 The first 3 characters are the general categories. For example, L70 is the 3-character category for acne and L40 is the category for psoriasis. The next 3 characters (ie, characters 4–6) correspond to the related etiology (ie, the cause, set of causes, manner of causation of a disease or condition), anatomic site, severity, and other vital clinical details.1
Take the case of a burn on the hand. With the International Classification of Diseases, Ninth Revision, a first-degree burn on the back of the hand is coded with 5 characters (944.16).2 According to the ICD-10-CM coding system, the code for an initial visit for a first-degree burn on the back of the hand would include 7 characters (T23.169A).1 There will be times when the sixth character may not be necessary; in these instances, an X must be placed in this position as a placeholder, but the absence of a sixth character does not negate the need for a seventh. Thankfully, the seventh character can only be 1 of 3 letters. As illustrated above in the code for a first-degree burn on the back of the hand, the A designates an initial encounter; a D would designate a follow-up visit for this burn, and an S would represent a sequela from the initial burn, such as a postinflammatory change, which would have its own code.1
Conclusion
To be reimbursed, appropriate ICD-10-CM codes must be used. Be sure to master the structure of these codes before the October 1, 2015, compliance deadline; plan now for a training and testing period.
As I have mentioned in prior columns, the key to success with International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) coding will be additional specificity, which will come in the form of more characters to communicate with payors and statisticians regarding the services that have been provided during the office visit. The addition of characters will permit more information about the visit to be delivered in the code.
Some of these additional characters will be required to submit the code for processing. Specifically look out for codes that communicate accidents or injury. Some common circumstances for dermatologists will be lacerations, abrasions, laceration repairs, and burns. The structure of the ICD-10-CM codes is outlined below to explain circumstances in which the increased number of characters will be most important for dermatologists.
The ICD-10-CM codes will be 3 to 7 characters long.1 The first 3 characters are the general categories. For example, L70 is the 3-character category for acne and L40 is the category for psoriasis. The next 3 characters (ie, characters 4–6) correspond to the related etiology (ie, the cause, set of causes, manner of causation of a disease or condition), anatomic site, severity, and other vital clinical details.1
Take the case of a burn on the hand. With the International Classification of Diseases, Ninth Revision, a first-degree burn on the back of the hand is coded with 5 characters (944.16).2 According to the ICD-10-CM coding system, the code for an initial visit for a first-degree burn on the back of the hand would include 7 characters (T23.169A).1 There will be times when the sixth character may not be necessary; in these instances, an X must be placed in this position as a placeholder, but the absence of a sixth character does not negate the need for a seventh. Thankfully, the seventh character can only be 1 of 3 letters. As illustrated above in the code for a first-degree burn on the back of the hand, the A designates an initial encounter; a D would designate a follow-up visit for this burn, and an S would represent a sequela from the initial burn, such as a postinflammatory change, which would have its own code.1
Conclusion
To be reimbursed, appropriate ICD-10-CM codes must be used. Be sure to master the structure of these codes before the October 1, 2015, compliance deadline; plan now for a training and testing period.
1. Centers for Medicare & Medicaid Services. ICD-10-CM Tabular List of Diseases and Injuries. http://www.cms.gov/Medicare/Coding/ICD10/downloads/6_I10tab2010.pdf. Published 2010. Accessed September 18, 2014.
2. ICD-9 code lookup. Centers for Medicare & Medicaid Services Web site. http://www.cms.gov/medicare-coverage-database/staticpages/icd-9-code-lookup.aspx. Accessed September 18, 2014.
1. Centers for Medicare & Medicaid Services. ICD-10-CM Tabular List of Diseases and Injuries. http://www.cms.gov/Medicare/Coding/ICD10/downloads/6_I10tab2010.pdf. Published 2010. Accessed September 18, 2014.
2. ICD-9 code lookup. Centers for Medicare & Medicaid Services Web site. http://www.cms.gov/medicare-coverage-database/staticpages/icd-9-code-lookup.aspx. Accessed September 18, 2014.
Practice Points
- The addition of characters in International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes will permit more information about the visit to be communicated to payors and statisticians.
- Codes with ICD-10-CM will be 3 to 7 characters long and indicate the disease category, followed by the related etiology, anatomic site, severity, and other vital clinical details. The last character is 1 of 3 letters to indicate if the visit is an initial encounter, subsequent encounter, or sequela.
Acne and rosacea management for men
In a report released March 2014 by the American Society of Aesthetic Plastic Surgery, the top five surgical procedures for men were liposuction, eyelid surgery, rhinoplasty, male breast reduction, and ear surgery. However, the rate of noninvasive cosmetic procedures and sales of men’s grooming products is one of the leading segments of the beauty industry.
Although most scientific research and media are focused on the female aesthetic, understanding the specific needs of your male patients is key to patient satisfaction. Most men are generally less aware than are women of the treatment options and risks and benefits of procedures. Men also prefer treatments with less downtime and natural-looking results. This column continues our miniseries on aesthetic dermatology for the male patient.
In a general dermatology practice, there are several skin concerns often identified by male patients, and acne and rosacea are among them.
Acne: Men generally have thicker, more sebaceous skin than that of women. Although acne is a very common problem in teens and young men, there is a growing trend of cases of cystic acne in adult men who consume popular protein meal replacement or muscle enhancing shakes that contain whey protein. Whey is a protein derived from cow’s milk. Milk and dairy products act by increasing insulin-like growth factor 1, which has been linked to acne. Although few case reports have shown a link between dietary whey supplementation and acne, in my practice, men with cystic acne who report using whey supplementation products have had almost complete resolution of their acne without medical intervention after discontinuing these products.
Rosacea: Men have a higher density of facial blood vessels than women do, and often seek treatment for telangiectasias and overall facial erythema. For papulopustular rosacea, common treatments include oral antibiotics, topical antibiotics, topical azaleic acid, and topical anti-inflammatory medications. For erythematotelangiectatic rosacea, Mirvaso (brimonidine), a topical vasoconstrictor, can be applied to the skin for 8-12 hours of marked reduction in facial erythema. Although theoretically a great option for patients suffering from erythema, the effects of topical brimonidine are transient, and the gel requires daily application with no long-term benefit. Vascular laser treatments are effective for telangiectasias for both men and women. However, men with more granulomatous or phymatous rosacea often need a combination of treatments including antibiotics, oral isotretinoin and fractional ablative lasers.
Resources:
American Society for Plastic Surgery 2012 statistics.
“Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes,” Cutis 2012;90:70-2.
Dr. Talakoub and Dr. Wesley are cocontributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
In a report released March 2014 by the American Society of Aesthetic Plastic Surgery, the top five surgical procedures for men were liposuction, eyelid surgery, rhinoplasty, male breast reduction, and ear surgery. However, the rate of noninvasive cosmetic procedures and sales of men’s grooming products is one of the leading segments of the beauty industry.
Although most scientific research and media are focused on the female aesthetic, understanding the specific needs of your male patients is key to patient satisfaction. Most men are generally less aware than are women of the treatment options and risks and benefits of procedures. Men also prefer treatments with less downtime and natural-looking results. This column continues our miniseries on aesthetic dermatology for the male patient.
In a general dermatology practice, there are several skin concerns often identified by male patients, and acne and rosacea are among them.
Acne: Men generally have thicker, more sebaceous skin than that of women. Although acne is a very common problem in teens and young men, there is a growing trend of cases of cystic acne in adult men who consume popular protein meal replacement or muscle enhancing shakes that contain whey protein. Whey is a protein derived from cow’s milk. Milk and dairy products act by increasing insulin-like growth factor 1, which has been linked to acne. Although few case reports have shown a link between dietary whey supplementation and acne, in my practice, men with cystic acne who report using whey supplementation products have had almost complete resolution of their acne without medical intervention after discontinuing these products.
Rosacea: Men have a higher density of facial blood vessels than women do, and often seek treatment for telangiectasias and overall facial erythema. For papulopustular rosacea, common treatments include oral antibiotics, topical antibiotics, topical azaleic acid, and topical anti-inflammatory medications. For erythematotelangiectatic rosacea, Mirvaso (brimonidine), a topical vasoconstrictor, can be applied to the skin for 8-12 hours of marked reduction in facial erythema. Although theoretically a great option for patients suffering from erythema, the effects of topical brimonidine are transient, and the gel requires daily application with no long-term benefit. Vascular laser treatments are effective for telangiectasias for both men and women. However, men with more granulomatous or phymatous rosacea often need a combination of treatments including antibiotics, oral isotretinoin and fractional ablative lasers.
Resources:
American Society for Plastic Surgery 2012 statistics.
“Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes,” Cutis 2012;90:70-2.
Dr. Talakoub and Dr. Wesley are cocontributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
In a report released March 2014 by the American Society of Aesthetic Plastic Surgery, the top five surgical procedures for men were liposuction, eyelid surgery, rhinoplasty, male breast reduction, and ear surgery. However, the rate of noninvasive cosmetic procedures and sales of men’s grooming products is one of the leading segments of the beauty industry.
Although most scientific research and media are focused on the female aesthetic, understanding the specific needs of your male patients is key to patient satisfaction. Most men are generally less aware than are women of the treatment options and risks and benefits of procedures. Men also prefer treatments with less downtime and natural-looking results. This column continues our miniseries on aesthetic dermatology for the male patient.
In a general dermatology practice, there are several skin concerns often identified by male patients, and acne and rosacea are among them.
Acne: Men generally have thicker, more sebaceous skin than that of women. Although acne is a very common problem in teens and young men, there is a growing trend of cases of cystic acne in adult men who consume popular protein meal replacement or muscle enhancing shakes that contain whey protein. Whey is a protein derived from cow’s milk. Milk and dairy products act by increasing insulin-like growth factor 1, which has been linked to acne. Although few case reports have shown a link between dietary whey supplementation and acne, in my practice, men with cystic acne who report using whey supplementation products have had almost complete resolution of their acne without medical intervention after discontinuing these products.
Rosacea: Men have a higher density of facial blood vessels than women do, and often seek treatment for telangiectasias and overall facial erythema. For papulopustular rosacea, common treatments include oral antibiotics, topical antibiotics, topical azaleic acid, and topical anti-inflammatory medications. For erythematotelangiectatic rosacea, Mirvaso (brimonidine), a topical vasoconstrictor, can be applied to the skin for 8-12 hours of marked reduction in facial erythema. Although theoretically a great option for patients suffering from erythema, the effects of topical brimonidine are transient, and the gel requires daily application with no long-term benefit. Vascular laser treatments are effective for telangiectasias for both men and women. However, men with more granulomatous or phymatous rosacea often need a combination of treatments including antibiotics, oral isotretinoin and fractional ablative lasers.
Resources:
American Society for Plastic Surgery 2012 statistics.
“Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes,” Cutis 2012;90:70-2.
Dr. Talakoub and Dr. Wesley are cocontributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
Assessing Attributes of Topical Vehicles for the Treatment of Acne, Atopic Dermatitis, and Plaque Psoriasis
The skin care market includes topical product formulations (eg, foams, lotions, ointments, creams) for a number of dermatologic therapeutic targets; however, there is limited information available regarding patient preference for product vehicles by specific dermatologic disease. There are few studies in the literature examining patient adherence to topical medications.1 In the current study, 6 focus groups comprised of patients with 3 dermatologic conditions of interest—acne, atopic dermatitis (AD), or plaque psoriasis (PP)—were surveyed to gain a better understanding of treatment preferences among patients and attributes of various formulations that are most desirable and important to this patient population.
Patient preference for a vehicle is relevant to treatment adherence in patients with conditions such as acne, AD, and PP because noncompliance is a major factor in the high rates of failure that have been associated with topical dermatologic treatments.2 The aesthetic attributes of a given vehicle formulation depend on the disease state being treated, the site of application, and the length of treatment.3 A limited number of studies have linked patient preferences to the attributes of topical medications. In one study, participants preferred an aqueous gel formulation compared with previously used topical treatments for AD.3 In another study, participants ranked the following properties of a hypothetical topical medication as most important: gel formulation, room temperature storage, product life of up to 18 months once opened, application with fingers, and a once-daily regimen.2 A third study found that foams were preferred among other formulations (ie, creams, gels, and ointments) for a variety of disease states, including AD, PP, and seborrheic dermatitis.4 The current study uses a qualitative analysis to further explore patient preferences of vehicle attributes for the topical treatment of acne, AD, and PP.
Methods
Study Participants
Six focus groups were conducted with patients who were using topical prescription medications for the treatment of acne, AD, or PP from March to April 2012. Participants were recruited from Raleigh, North Carolina, and New York, New York, with 3 focus groups (1 for each condition) from each city.
Following institutional review board approval of the study processes and recruitment materials, participants meeting the following criteria were included in the study: 18 years or older; diagnosed with acne, AD, or PP by a physician (participants who currently reported more than 1 of these conditions were not eligible); diagnosed with the respective skin condition at least 6 months prior to screening; current or prior use of topical prescription medications in at least 2 different vehicle formulations (eg, cream and foam, ointment and gel); use of at least 1 topical prescription treatment 5 or more times per month; and English speaking and able to provide written consent.
Study Design
A semistructured discussion guide was developed to ensure consistency in the topics surveyed among all 6 focus groups, and the same 2 moderators conducted the discussion for all 6 groups. At the beginning of the study, after providing written informed consent, participants were given an overview of the study and were asked general questions intended to get the participants talking about their experiences with their respective conditions. To avoid or minimize bias, participants were only asked open-ended questions designed to ascertain what symptoms they experienced in relation to their respective conditions. Finally, the discussions were focused on the topical prescription treatments that participants had tried and the properties of each treatment they liked and disliked.
The focus groups were recorded (audio) and transcribed; transcriptions were then verified through an iterative process of technical and editorial review. Analysis of the results was conducted by evaluation and review of the field notes as well as the transcripts from the focus groups.
Results
A total of 54 participants were surveyed (average age, 40.9 years). Although the average age of participants in the acne and AD groups was generally the same (35.2 and 35.4 years, respectively), the average age of participants in the PP group was higher (52.2 years). The majority of participants were white females who had at least a college degree. On average, participants had been diagnosed with their respective condition approximately 15.5 years prior to screening. Participant demographics and clinical characteristics are presented in more detail in Table 1.
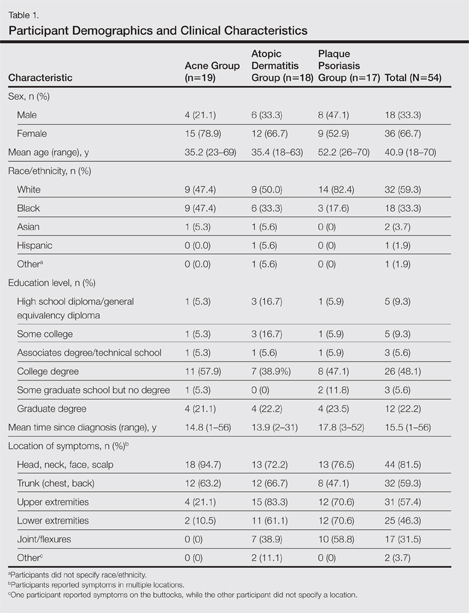
At the time of screening, participants reported prior or present use of topical prescription medications in various formulations for treatment of their respective conditions. The most commonly reported vehicles across all 3 conditions were creams and ointments, followed by lotions, gels, and foams.
Symptoms Across Conditions
At the beginning of the study, participants reported symptoms they experienced in association with their respective skin conditions. Itching and redness were the only symptoms reported across all 3 conditions in all 6 focus groups. Dry skin/dryness, flaking/scaling/peeling, and pain were reported by at least 1 participant in 5 of 6 focus groups. Other common symptoms reported in at least 3 focus groups included pimples/bumps/boils, sensitivity, bleeding, discoloration of skin, cracking/cuts/skin breaking, and buildup of dead or thickened skin/chunks of skin/plaque. As shown in Table 2, there was more variability in the symptoms reported across the 2 acne groups compared with those reported by the AD and PP groups. Table 2 displays all symptoms spontaneously reported across each of the 6 focus groups. Symptoms are listed according to the terms/descriptions provided by focus group participants.
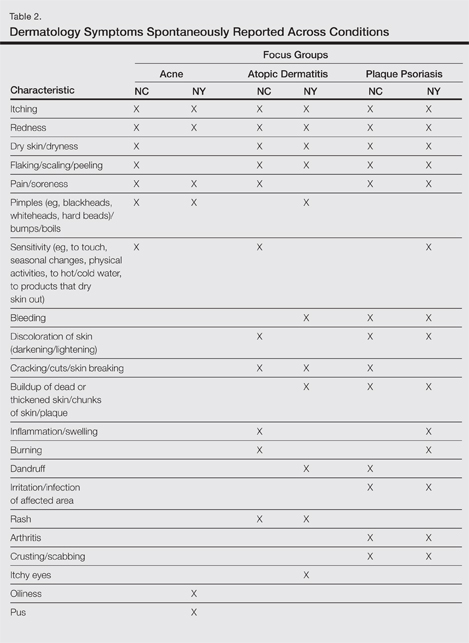
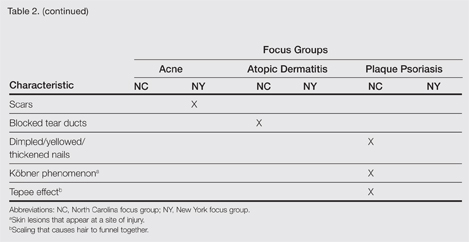
Results by Condition
Unless otherwise noted, participant responses generally were consistent across the North Carolina and New York focus groups. Results from the study, which included a total of 54 participants, suggested a high degree of similarity among preferences for topical treatment attributes across the 3 conditions. Moisturizing was the single attribute that was mentioned across all 3 conditions in all 6 focus groups as an important characteristic in a topical dermatologic treatment. Other attributes mentioned by at least 1 participant in 5 of 6 focus groups included the following: absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy/oily, is not sticky/tacky, is long lasting/long acting/stays on/lasts through sweating or hand washing, is fragrance or odor free, is easy to apply/simple to use, and can use all the time.
A few condition-specific attributes also were noted. An attribute was considered condition specific if it was mentioned by at least 1 participant in both groups for any condition. Preferred properties for topical medications that were specific to acne patients included the following: easy to dispense/dispenses right amount, nondrying, product goes on/spreads smoothly, container (eg, tube, pump) is not easily broken/does not leak, and creamy. Preferred properties that were specific to AD patients included the following: is not noticeable to others/conceals area, good consistency, and cooling. Preferred properties that were specific to 2 conditions included no residue among acne and AD patients and soothing among AD and PP patients. Preferred properties for topical medications across all focus groups are described in further detail in Table 3.
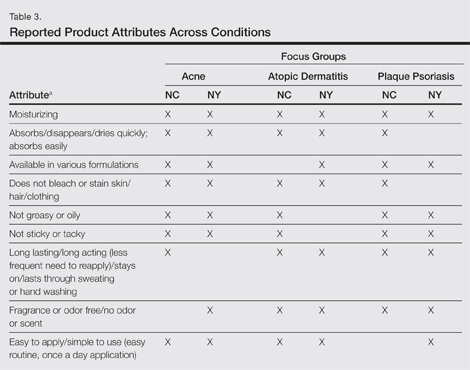
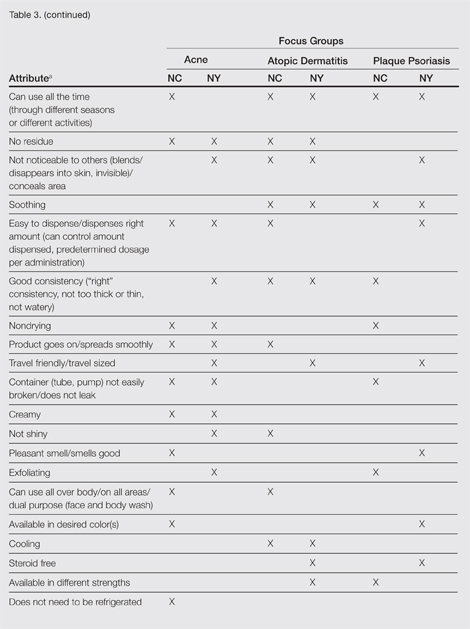
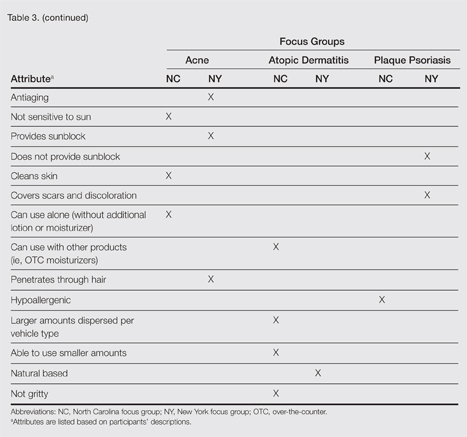
Regarding vehicle-type preferences, acne patients tended to prefer washes, creams, and lotions; AD patients preferred creams; and psoriasis patients preferred creams, ointments, and foams (particularly for the scalp).
Participants across all 3 conditions reported that during daytime hours (versus at night), they would be less likely to use products that are oily, shiny, thick (eg, ointments, oils); bleach or stain the skin/clothing; interfere with makeup, work, or other activities; or are visible to others. Rather, a majority of participants noted that they were more likely to use thinner, less oily products (eg, creams, lotions) during daytime hours, in social situations, or during certain activities (eg, exercise). Participants across all 3 conditions noted that they only used prescription shampoos when they had enough time to repeatedly rinse their hair to eliminate the smell. Participants across all 6 groups noted that they tended to choose creams or lotions over ointments (when available) during the summer because they considered these products to be lighter and less greasy.
Overall, participants indicated that condition-specific symptoms did not influence their preference for topical formulations; rather, they used whatever prescriptions they currently had. However, in some instances, participants noted that the location of the affected area might influence the type of product selected. For example, some participants tended not to use ointments on the scalp or in locations where their clothes might come into contact with the medicated area to avoid clothes sticking to medicated areas. Other participants used lighter, less stringent products on the face versus other locations. Participants noted that foams were preferred for more discrete localized areas.
Comment
Despite the variability in symptoms and conditions across the study population as well as participants’ varying experience with and access to topical treatments, the attributes participants valued most in topical prescription treatments were relatively consistent across the 3 conditions: moisturizing, absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy or oily, is not sticky or tacky, is long lasting/long acting, is fragrance or odor free, is easy to apply/simple to use, and can use all the time. Preferred vehicle attributes were generally consistent across the 3 conditions, but preferences for vehicle types tended to vary by condition. Although some vehicles were more closely associated with specific attributes (eg, the majority considered lotions to be moisturizing), no one vehicle was associated with the complete list of attributes for the ideal topical medication. Regardless of the symptoms experienced or particular situations/activities, participants noted that they tended to use the topical medications that were available to them, even if intended for different locations of the body, and nearly all reported using more than 1 type of topical treatment.
Caution should be used in interpreting these findings, as participant preferences are largely dependent on prior experience with different vehicle types. The small number of participants across the 3 conditions in this analysis also limits conclusions that can be drawn from the data sets, as each condition has different though somewhat overlapping treatment algorithms. A focused inquiry into each of these conditions through separate evaluations may provide for a more robust analysis. Future research might employ questionnaire methodology to develop items assessing the attributes of interest (eg, moisturizing ability, rate of absorption, greasiness/oiliness, stickiness/tackiness, fragrance/odor). The final set of desirable attributes may vary depending on the condition (eg, a moisturizing product may be more important to PP and AD patients than acne patients) as well as comparator products.
Acknowledgment—The authors would like to thank Jennifer Gwazdauskas, MBA, Research Triangle Park, North Carolina, of Stiefel, a GSK company, for her assistance with manuscript review.
- Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Kellett N, West F, Finlay AY. Conjoint analysis: a novel, rigorous tool for determining patient p for topical antibiotic treatment for acne. a randomised controlled trial. Br J Dermatol. 2006;154:524-532.
- Trookman NS, Rizer RL, Ho ET, et al. The importance of vehicle properties to patients with atopic dermatitis. Cutis. 2011;88:13-17.
- Weiss S, Wyres M, Brundage T. A novel foam vehicle is consistently preferred by patients for dermatologic conditions. J Am Acad Dermatol. 2011;64(suppl 1):AB50.
The skin care market includes topical product formulations (eg, foams, lotions, ointments, creams) for a number of dermatologic therapeutic targets; however, there is limited information available regarding patient preference for product vehicles by specific dermatologic disease. There are few studies in the literature examining patient adherence to topical medications.1 In the current study, 6 focus groups comprised of patients with 3 dermatologic conditions of interest—acne, atopic dermatitis (AD), or plaque psoriasis (PP)—were surveyed to gain a better understanding of treatment preferences among patients and attributes of various formulations that are most desirable and important to this patient population.
Patient preference for a vehicle is relevant to treatment adherence in patients with conditions such as acne, AD, and PP because noncompliance is a major factor in the high rates of failure that have been associated with topical dermatologic treatments.2 The aesthetic attributes of a given vehicle formulation depend on the disease state being treated, the site of application, and the length of treatment.3 A limited number of studies have linked patient preferences to the attributes of topical medications. In one study, participants preferred an aqueous gel formulation compared with previously used topical treatments for AD.3 In another study, participants ranked the following properties of a hypothetical topical medication as most important: gel formulation, room temperature storage, product life of up to 18 months once opened, application with fingers, and a once-daily regimen.2 A third study found that foams were preferred among other formulations (ie, creams, gels, and ointments) for a variety of disease states, including AD, PP, and seborrheic dermatitis.4 The current study uses a qualitative analysis to further explore patient preferences of vehicle attributes for the topical treatment of acne, AD, and PP.
Methods
Study Participants
Six focus groups were conducted with patients who were using topical prescription medications for the treatment of acne, AD, or PP from March to April 2012. Participants were recruited from Raleigh, North Carolina, and New York, New York, with 3 focus groups (1 for each condition) from each city.
Following institutional review board approval of the study processes and recruitment materials, participants meeting the following criteria were included in the study: 18 years or older; diagnosed with acne, AD, or PP by a physician (participants who currently reported more than 1 of these conditions were not eligible); diagnosed with the respective skin condition at least 6 months prior to screening; current or prior use of topical prescription medications in at least 2 different vehicle formulations (eg, cream and foam, ointment and gel); use of at least 1 topical prescription treatment 5 or more times per month; and English speaking and able to provide written consent.
Study Design
A semistructured discussion guide was developed to ensure consistency in the topics surveyed among all 6 focus groups, and the same 2 moderators conducted the discussion for all 6 groups. At the beginning of the study, after providing written informed consent, participants were given an overview of the study and were asked general questions intended to get the participants talking about their experiences with their respective conditions. To avoid or minimize bias, participants were only asked open-ended questions designed to ascertain what symptoms they experienced in relation to their respective conditions. Finally, the discussions were focused on the topical prescription treatments that participants had tried and the properties of each treatment they liked and disliked.
The focus groups were recorded (audio) and transcribed; transcriptions were then verified through an iterative process of technical and editorial review. Analysis of the results was conducted by evaluation and review of the field notes as well as the transcripts from the focus groups.
Results
A total of 54 participants were surveyed (average age, 40.9 years). Although the average age of participants in the acne and AD groups was generally the same (35.2 and 35.4 years, respectively), the average age of participants in the PP group was higher (52.2 years). The majority of participants were white females who had at least a college degree. On average, participants had been diagnosed with their respective condition approximately 15.5 years prior to screening. Participant demographics and clinical characteristics are presented in more detail in Table 1.

At the time of screening, participants reported prior or present use of topical prescription medications in various formulations for treatment of their respective conditions. The most commonly reported vehicles across all 3 conditions were creams and ointments, followed by lotions, gels, and foams.
Symptoms Across Conditions
At the beginning of the study, participants reported symptoms they experienced in association with their respective skin conditions. Itching and redness were the only symptoms reported across all 3 conditions in all 6 focus groups. Dry skin/dryness, flaking/scaling/peeling, and pain were reported by at least 1 participant in 5 of 6 focus groups. Other common symptoms reported in at least 3 focus groups included pimples/bumps/boils, sensitivity, bleeding, discoloration of skin, cracking/cuts/skin breaking, and buildup of dead or thickened skin/chunks of skin/plaque. As shown in Table 2, there was more variability in the symptoms reported across the 2 acne groups compared with those reported by the AD and PP groups. Table 2 displays all symptoms spontaneously reported across each of the 6 focus groups. Symptoms are listed according to the terms/descriptions provided by focus group participants.


Results by Condition
Unless otherwise noted, participant responses generally were consistent across the North Carolina and New York focus groups. Results from the study, which included a total of 54 participants, suggested a high degree of similarity among preferences for topical treatment attributes across the 3 conditions. Moisturizing was the single attribute that was mentioned across all 3 conditions in all 6 focus groups as an important characteristic in a topical dermatologic treatment. Other attributes mentioned by at least 1 participant in 5 of 6 focus groups included the following: absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy/oily, is not sticky/tacky, is long lasting/long acting/stays on/lasts through sweating or hand washing, is fragrance or odor free, is easy to apply/simple to use, and can use all the time.
A few condition-specific attributes also were noted. An attribute was considered condition specific if it was mentioned by at least 1 participant in both groups for any condition. Preferred properties for topical medications that were specific to acne patients included the following: easy to dispense/dispenses right amount, nondrying, product goes on/spreads smoothly, container (eg, tube, pump) is not easily broken/does not leak, and creamy. Preferred properties that were specific to AD patients included the following: is not noticeable to others/conceals area, good consistency, and cooling. Preferred properties that were specific to 2 conditions included no residue among acne and AD patients and soothing among AD and PP patients. Preferred properties for topical medications across all focus groups are described in further detail in Table 3.



Regarding vehicle-type preferences, acne patients tended to prefer washes, creams, and lotions; AD patients preferred creams; and psoriasis patients preferred creams, ointments, and foams (particularly for the scalp).
Participants across all 3 conditions reported that during daytime hours (versus at night), they would be less likely to use products that are oily, shiny, thick (eg, ointments, oils); bleach or stain the skin/clothing; interfere with makeup, work, or other activities; or are visible to others. Rather, a majority of participants noted that they were more likely to use thinner, less oily products (eg, creams, lotions) during daytime hours, in social situations, or during certain activities (eg, exercise). Participants across all 3 conditions noted that they only used prescription shampoos when they had enough time to repeatedly rinse their hair to eliminate the smell. Participants across all 6 groups noted that they tended to choose creams or lotions over ointments (when available) during the summer because they considered these products to be lighter and less greasy.
Overall, participants indicated that condition-specific symptoms did not influence their preference for topical formulations; rather, they used whatever prescriptions they currently had. However, in some instances, participants noted that the location of the affected area might influence the type of product selected. For example, some participants tended not to use ointments on the scalp or in locations where their clothes might come into contact with the medicated area to avoid clothes sticking to medicated areas. Other participants used lighter, less stringent products on the face versus other locations. Participants noted that foams were preferred for more discrete localized areas.
Comment
Despite the variability in symptoms and conditions across the study population as well as participants’ varying experience with and access to topical treatments, the attributes participants valued most in topical prescription treatments were relatively consistent across the 3 conditions: moisturizing, absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy or oily, is not sticky or tacky, is long lasting/long acting, is fragrance or odor free, is easy to apply/simple to use, and can use all the time. Preferred vehicle attributes were generally consistent across the 3 conditions, but preferences for vehicle types tended to vary by condition. Although some vehicles were more closely associated with specific attributes (eg, the majority considered lotions to be moisturizing), no one vehicle was associated with the complete list of attributes for the ideal topical medication. Regardless of the symptoms experienced or particular situations/activities, participants noted that they tended to use the topical medications that were available to them, even if intended for different locations of the body, and nearly all reported using more than 1 type of topical treatment.
Caution should be used in interpreting these findings, as participant preferences are largely dependent on prior experience with different vehicle types. The small number of participants across the 3 conditions in this analysis also limits conclusions that can be drawn from the data sets, as each condition has different though somewhat overlapping treatment algorithms. A focused inquiry into each of these conditions through separate evaluations may provide for a more robust analysis. Future research might employ questionnaire methodology to develop items assessing the attributes of interest (eg, moisturizing ability, rate of absorption, greasiness/oiliness, stickiness/tackiness, fragrance/odor). The final set of desirable attributes may vary depending on the condition (eg, a moisturizing product may be more important to PP and AD patients than acne patients) as well as comparator products.
Acknowledgment—The authors would like to thank Jennifer Gwazdauskas, MBA, Research Triangle Park, North Carolina, of Stiefel, a GSK company, for her assistance with manuscript review.
The skin care market includes topical product formulations (eg, foams, lotions, ointments, creams) for a number of dermatologic therapeutic targets; however, there is limited information available regarding patient preference for product vehicles by specific dermatologic disease. There are few studies in the literature examining patient adherence to topical medications.1 In the current study, 6 focus groups comprised of patients with 3 dermatologic conditions of interest—acne, atopic dermatitis (AD), or plaque psoriasis (PP)—were surveyed to gain a better understanding of treatment preferences among patients and attributes of various formulations that are most desirable and important to this patient population.
Patient preference for a vehicle is relevant to treatment adherence in patients with conditions such as acne, AD, and PP because noncompliance is a major factor in the high rates of failure that have been associated with topical dermatologic treatments.2 The aesthetic attributes of a given vehicle formulation depend on the disease state being treated, the site of application, and the length of treatment.3 A limited number of studies have linked patient preferences to the attributes of topical medications. In one study, participants preferred an aqueous gel formulation compared with previously used topical treatments for AD.3 In another study, participants ranked the following properties of a hypothetical topical medication as most important: gel formulation, room temperature storage, product life of up to 18 months once opened, application with fingers, and a once-daily regimen.2 A third study found that foams were preferred among other formulations (ie, creams, gels, and ointments) for a variety of disease states, including AD, PP, and seborrheic dermatitis.4 The current study uses a qualitative analysis to further explore patient preferences of vehicle attributes for the topical treatment of acne, AD, and PP.
Methods
Study Participants
Six focus groups were conducted with patients who were using topical prescription medications for the treatment of acne, AD, or PP from March to April 2012. Participants were recruited from Raleigh, North Carolina, and New York, New York, with 3 focus groups (1 for each condition) from each city.
Following institutional review board approval of the study processes and recruitment materials, participants meeting the following criteria were included in the study: 18 years or older; diagnosed with acne, AD, or PP by a physician (participants who currently reported more than 1 of these conditions were not eligible); diagnosed with the respective skin condition at least 6 months prior to screening; current or prior use of topical prescription medications in at least 2 different vehicle formulations (eg, cream and foam, ointment and gel); use of at least 1 topical prescription treatment 5 or more times per month; and English speaking and able to provide written consent.
Study Design
A semistructured discussion guide was developed to ensure consistency in the topics surveyed among all 6 focus groups, and the same 2 moderators conducted the discussion for all 6 groups. At the beginning of the study, after providing written informed consent, participants were given an overview of the study and were asked general questions intended to get the participants talking about their experiences with their respective conditions. To avoid or minimize bias, participants were only asked open-ended questions designed to ascertain what symptoms they experienced in relation to their respective conditions. Finally, the discussions were focused on the topical prescription treatments that participants had tried and the properties of each treatment they liked and disliked.
The focus groups were recorded (audio) and transcribed; transcriptions were then verified through an iterative process of technical and editorial review. Analysis of the results was conducted by evaluation and review of the field notes as well as the transcripts from the focus groups.
Results
A total of 54 participants were surveyed (average age, 40.9 years). Although the average age of participants in the acne and AD groups was generally the same (35.2 and 35.4 years, respectively), the average age of participants in the PP group was higher (52.2 years). The majority of participants were white females who had at least a college degree. On average, participants had been diagnosed with their respective condition approximately 15.5 years prior to screening. Participant demographics and clinical characteristics are presented in more detail in Table 1.

At the time of screening, participants reported prior or present use of topical prescription medications in various formulations for treatment of their respective conditions. The most commonly reported vehicles across all 3 conditions were creams and ointments, followed by lotions, gels, and foams.
Symptoms Across Conditions
At the beginning of the study, participants reported symptoms they experienced in association with their respective skin conditions. Itching and redness were the only symptoms reported across all 3 conditions in all 6 focus groups. Dry skin/dryness, flaking/scaling/peeling, and pain were reported by at least 1 participant in 5 of 6 focus groups. Other common symptoms reported in at least 3 focus groups included pimples/bumps/boils, sensitivity, bleeding, discoloration of skin, cracking/cuts/skin breaking, and buildup of dead or thickened skin/chunks of skin/plaque. As shown in Table 2, there was more variability in the symptoms reported across the 2 acne groups compared with those reported by the AD and PP groups. Table 2 displays all symptoms spontaneously reported across each of the 6 focus groups. Symptoms are listed according to the terms/descriptions provided by focus group participants.


Results by Condition
Unless otherwise noted, participant responses generally were consistent across the North Carolina and New York focus groups. Results from the study, which included a total of 54 participants, suggested a high degree of similarity among preferences for topical treatment attributes across the 3 conditions. Moisturizing was the single attribute that was mentioned across all 3 conditions in all 6 focus groups as an important characteristic in a topical dermatologic treatment. Other attributes mentioned by at least 1 participant in 5 of 6 focus groups included the following: absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy/oily, is not sticky/tacky, is long lasting/long acting/stays on/lasts through sweating or hand washing, is fragrance or odor free, is easy to apply/simple to use, and can use all the time.
A few condition-specific attributes also were noted. An attribute was considered condition specific if it was mentioned by at least 1 participant in both groups for any condition. Preferred properties for topical medications that were specific to acne patients included the following: easy to dispense/dispenses right amount, nondrying, product goes on/spreads smoothly, container (eg, tube, pump) is not easily broken/does not leak, and creamy. Preferred properties that were specific to AD patients included the following: is not noticeable to others/conceals area, good consistency, and cooling. Preferred properties that were specific to 2 conditions included no residue among acne and AD patients and soothing among AD and PP patients. Preferred properties for topical medications across all focus groups are described in further detail in Table 3.



Regarding vehicle-type preferences, acne patients tended to prefer washes, creams, and lotions; AD patients preferred creams; and psoriasis patients preferred creams, ointments, and foams (particularly for the scalp).
Participants across all 3 conditions reported that during daytime hours (versus at night), they would be less likely to use products that are oily, shiny, thick (eg, ointments, oils); bleach or stain the skin/clothing; interfere with makeup, work, or other activities; or are visible to others. Rather, a majority of participants noted that they were more likely to use thinner, less oily products (eg, creams, lotions) during daytime hours, in social situations, or during certain activities (eg, exercise). Participants across all 3 conditions noted that they only used prescription shampoos when they had enough time to repeatedly rinse their hair to eliminate the smell. Participants across all 6 groups noted that they tended to choose creams or lotions over ointments (when available) during the summer because they considered these products to be lighter and less greasy.
Overall, participants indicated that condition-specific symptoms did not influence their preference for topical formulations; rather, they used whatever prescriptions they currently had. However, in some instances, participants noted that the location of the affected area might influence the type of product selected. For example, some participants tended not to use ointments on the scalp or in locations where their clothes might come into contact with the medicated area to avoid clothes sticking to medicated areas. Other participants used lighter, less stringent products on the face versus other locations. Participants noted that foams were preferred for more discrete localized areas.
Comment
Despite the variability in symptoms and conditions across the study population as well as participants’ varying experience with and access to topical treatments, the attributes participants valued most in topical prescription treatments were relatively consistent across the 3 conditions: moisturizing, absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy or oily, is not sticky or tacky, is long lasting/long acting, is fragrance or odor free, is easy to apply/simple to use, and can use all the time. Preferred vehicle attributes were generally consistent across the 3 conditions, but preferences for vehicle types tended to vary by condition. Although some vehicles were more closely associated with specific attributes (eg, the majority considered lotions to be moisturizing), no one vehicle was associated with the complete list of attributes for the ideal topical medication. Regardless of the symptoms experienced or particular situations/activities, participants noted that they tended to use the topical medications that were available to them, even if intended for different locations of the body, and nearly all reported using more than 1 type of topical treatment.
Caution should be used in interpreting these findings, as participant preferences are largely dependent on prior experience with different vehicle types. The small number of participants across the 3 conditions in this analysis also limits conclusions that can be drawn from the data sets, as each condition has different though somewhat overlapping treatment algorithms. A focused inquiry into each of these conditions through separate evaluations may provide for a more robust analysis. Future research might employ questionnaire methodology to develop items assessing the attributes of interest (eg, moisturizing ability, rate of absorption, greasiness/oiliness, stickiness/tackiness, fragrance/odor). The final set of desirable attributes may vary depending on the condition (eg, a moisturizing product may be more important to PP and AD patients than acne patients) as well as comparator products.
Acknowledgment—The authors would like to thank Jennifer Gwazdauskas, MBA, Research Triangle Park, North Carolina, of Stiefel, a GSK company, for her assistance with manuscript review.
- Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Kellett N, West F, Finlay AY. Conjoint analysis: a novel, rigorous tool for determining patient p for topical antibiotic treatment for acne. a randomised controlled trial. Br J Dermatol. 2006;154:524-532.
- Trookman NS, Rizer RL, Ho ET, et al. The importance of vehicle properties to patients with atopic dermatitis. Cutis. 2011;88:13-17.
- Weiss S, Wyres M, Brundage T. A novel foam vehicle is consistently preferred by patients for dermatologic conditions. J Am Acad Dermatol. 2011;64(suppl 1):AB50.
- Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Kellett N, West F, Finlay AY. Conjoint analysis: a novel, rigorous tool for determining patient p for topical antibiotic treatment for acne. a randomised controlled trial. Br J Dermatol. 2006;154:524-532.
- Trookman NS, Rizer RL, Ho ET, et al. The importance of vehicle properties to patients with atopic dermatitis. Cutis. 2011;88:13-17.
- Weiss S, Wyres M, Brundage T. A novel foam vehicle is consistently preferred by patients for dermatologic conditions. J Am Acad Dermatol. 2011;64(suppl 1):AB50.
- Patient preference for topical product formulation varies by dermatologic condition being treated.
- Most important vehicle attributes cited across study conditions include moisturizing, absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy or oily, is not sticky or tacky, is long lasting/long acting, is fragrance or odor free, is easy to apply/simple to use, and can use all the time.
- Patient preference for product vehicle is relevant to treatment adherence and may vary depending on the condition being treated.