User login
Adult ADHD: 6 studies of pharmacologic interventions
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder that begins in childhood and continues into adulthood. The clinical presentation is characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional interference.1 ADHD affects patients’ interpersonal and professional lives as well as their daily functioning.2 Adults with ADHD may suffer from excessive self-criticism, low self-esteem, and sensitivity to criticism.3 The overall prevalence of adult ADHD is 4.4%.4 ADHD in adults is frequently associated with comorbid psychiatric disorders.5 The diagnosis of ADHD in adults requires the presence of ≥5 symptoms of inattention and hyperactivity/impulsivity that persist for ≥6 months. Patients must have first had such symptoms before age 12; symptoms need to be present in ≥2 settings and interfere with functioning.1
Treatment of ADHD includes pharmacologic and nonpharmacologic interventions. For most patients, pharmacotherapy—specifically stimulant medications—is advised as first-line treatment,6 with adequate trials of methylphenidate and amphetamines before using second-line agents such as nonstimulants. However, despite these medications’ efficacy in randomized controlled trials (RCTs), adherence is low.7 This could be due to inadequate response or adverse effects.8 Guidelines also recommend the use of nonpharmacologic interventions for adults who cannot adhere to or tolerate medication or have an inadequate response.6 Potential nonpharmacologic interventions include transcranial direct current stimulation, mindfulness, psychoeducation, cognitive-behavioral therapy, and chronotherapy.
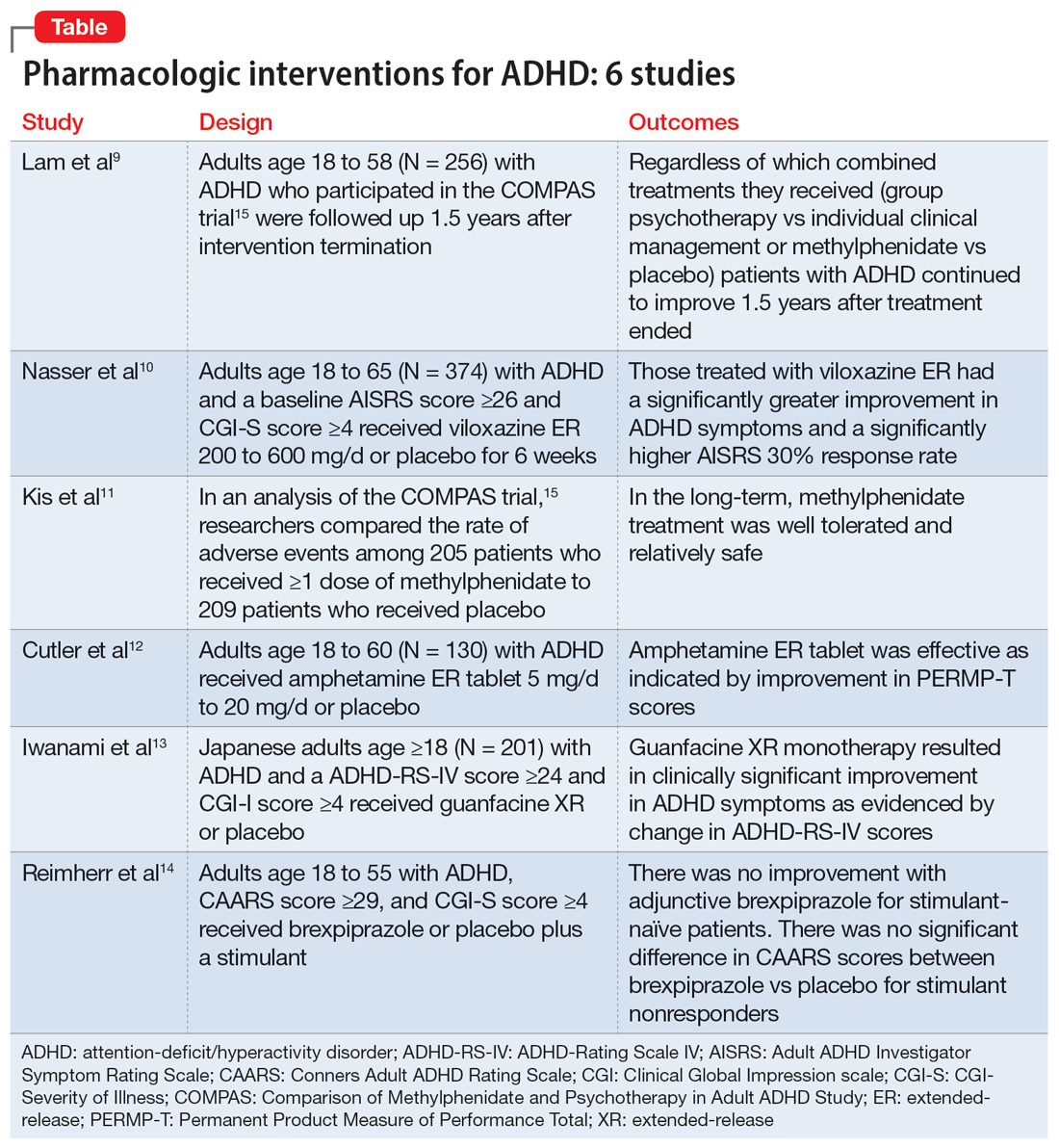
In Part 1 of this 2-part article, we review 6 RCTs of pharmacologic interventions for adult ADHD published within the last 5 years (Table9-14). Part 2 will review nonpharmacologic treatments.
1. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
The Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) was a multicenter prospective, randomized trial of adults age 18 to 58 with ADHD.15 It compared cognitive-behavioral group psychotherapy (GPT) with individual clinical management (CM), and methylphenidate with placebo. When used in conjunction with methylphenidate, psychological treatments produced better results than placebo. However, studies on the long-term effects of multimodal treatment in ADHD are limited. Lam et al9 performed a follow-up analysis of the COMPAS trial.
Study design
- This observer-masked study involved a follow-up of participants in COMPAS 1.5 years after the interventions were terminated. Of the 433 adults with ADHD who participated in COMPAS, 256 participated in this follow-up.
- The inclusion criteria of COMPAS were age 18 to 58; diagnosis of ADHD according to DSM-IV criteria; chronic course of ADHD symptoms from childhood to adulthood; a Wender Utah Rating Scale short version score ≥30; and no pathological abnormality detected on physical examination.
- The exclusion criteria were having an IQ <85; schizophrenia, bipolar disorder (BD), borderline personality disorder, antisocial personality disorder, suicidal or self-injurious behavior, autism, motor tics, or Tourette syndrome; substance abuse/dependence within 6 months prior to screening; positive drug screening; neurologic diseases, seizures, glaucoma, diabetes, hyperlipidemia, uncontrolled arterial hypertension, angina pectoris, tachycardia arrhythmia, or arterial occlusive disease; previous stroke; current bulimia or anorexia; low weight (body mass index [BMI] <20; pregnancy (current or planned) or breastfeeding; treatment with stimulants or ADHD-specific psychotherapy in the past 6 months; methylphenidate intolerance; treatment with antidepressants, norepinephrine reuptake inhibitors, bupropion, antipsychotics, theophylline, amantadine, anticoagulants derived from coumarin, antacids, or alpha-adrenergic agonists in the 2 weeks prior to baseline; and treatment with fluoxetine or monoamine oxidase inhibitors in the 4 weeks prior to baseline.
- The primary outcome was a change from baseline on the ADHD Index of Conners Adult ADHD Rating Scale (CAARS) score. Secondary outcomes were self-ratings on the Beck Depression Inventory (BDI) and observer-masked ratings of the Clinical Global Impression (CGI) scale and other ADHD rating scale scores, such as the Diagnostic Checklist for the diagnosis of ADHD in adults (ADHD-DC) and subscales of the CAARS.
- COMPAS was open regarding patient and therapist assignment to GPT and CM, but double-masked regarding medication. The statistical analysis focused on the 2x2 comparison of GPT vs CM and methylphenidate vs placebo.
Outcomes
- A total of 251 participants had an assessment with the observer-masked CAARS score. The baseline mean (SD) age was 36.3 (10.1), and approximately one-half (49.8%) of participants were male.
- Overall, 9.2% of patients took methylphenidate >31 days from termination of COMPAS before this study but not at the start of this study. Approximately one-third (31.1%) of patients were taking methylphenidate at follow-up. The mean (SD) daily dosage of methylphenidate was 36 (24.77) mg and 0.46 (0.27) mg/kg of body weight.
- The baseline all-group mean ADHD Index of CAARS score was 20.6. At follow-up, it was 14.7 for the CM arm and 14.2 for the GPT arm (difference not significant, P = .48). The mean score decreased to 13.8 for the methylphenidate arm and to 15.2 for the placebo (significant difference, P = .04).
- Overall, methylphenidate was associated with greater improvement in symptoms than placebo. Patients in the GPT arm had fewer severe symptoms as assessed by the self-reported ADHD Symptoms Total Score compared to the CM arm (P = .04).
- There were no significant differences in self-rating CAARS and observer-rated CAARS subscale scores. Compared to CM, GPT significantly decreased pure hyperactive symptoms on the ADHD-DC (P = .08). No significant differences were observed in BDI scores. The difference between GPT and CM remained significant at follow-up in terms of the CGI evaluation of efficacy (P = .04).
Continue to: Conclusions/limitations
Conclusions/limitations
- Regardless of which combined treatments they received, patients with ADHD continued to improve 1.5 years after the 52-week treatment phase ended.
- Patients assigned to methylphenidate performed considerably better on the observer-rated CAARS than patients assigned to placebo.
- Benefits from GPT or CM in addition to methylphenidate therapy lasted 1.5 years. Compared to CM, GPT was not linked to better scores on the CAARS.
- Limitations: Approximately 41% of patients who were recruited did not participate. Daily functioning was measured only by the CGI. There were only marginal differences among the 4 treatments, and the study compared a very regimented approach (GPT) with one that was less focused (CM).
2. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double‐blind, placebo‐controlled trial assessing the efficacy and safety of viloxazine extended‐release capsules in adults with attention‐deficit/hyperactivity disorder. CNS Drugs. 2022;36(8): 897-915. doi:10.1007/s40263-022-00938-w
In 2021, the FDA approved viloxazine extended-release (ER) for treating ADHD in children and adolescents (age 6 to 17). Nasser et al10 reviewed the safety and efficacy of viloxazine ER in adults with ADHD.
Study design
- This phase III, randomized, double-blind, placebo-controlled, multicenter clinical trial included 374 adults with ADHD who received viloxazine ER or placebo.
- Participants were age 18 to 65 and had been given a primary diagnosis of ADHD according to DSM-5 criteria in the last 6 months. Other inclusion criteria were having an Adult ADHD Investigator Symptom Rating Scale (AISRS) total score ≥26 and CGI-Severity of Illness (CGI-S) score ≥4 at baseline, BMI 18 to 35 kg/m2, and being medically healthy.
- Exclusion criteria included having treatment-resistant ADHD, a current diagnosis of any psychiatric disorder other than ADHD, or a history of schizophrenia, schizoaffective disorder, BD, autism, obsessive-compulsive disorder, personality disorder, or posttraumatic stress disorder. Individuals with any significant neurologic disorder, heart condition, arrhythmia, clinically relevant vital sign abnormality, or systemic illness were excluded, as were those with a history (within the past year) or current diagnosis of substance use disorder or a positive drug screen for a drug of abuse. Those with an allergic reaction or intolerance to viloxazine or were breastfeeding, pregnant, or refused to be abstinent or practice birth control were excluded.
- The dosage of viloxazine ER ranged from 200 to 600 mg/d for 6 weeks. This was titrated based on symptom response and adverse effects.
- All individuals received 2 capsules once a day for Week 1 and Week 2. During Week 1 and Week 2, participants in the viloxazine ER group received 200 mg (1 viloxazine ER capsule and 1 placebo capsule) and 400 mg (2 viloxazine ER capsules) of the medication, respectively. Two placebo pills were administered to those in the placebo group. From Week 3 to Week 6, the dose could be titrated or tapered at the investigator’s discretion. Compliance was assessed by comparing the number of pills dispensed vs returned.
- The primary outcome was a change in AISRS score from baselines to Week 6.
- The key secondary outcome was the change in CGI-S score from baseline to Week 6. Scores on the AISRS inattention and hyperactive/impulsivity subscales, Behavioral Regulation Index, Metacognition Index, Behavior Rating Inventory of Executive Function–Adult Version (BRIEF-A), and Generalized Anxiety Disorder-7 item scale (GAD-7) were also evaluated. Also, the rates of 30% and 50% responders on the AISRS (defined as ≥30% or ≥50% reduction from baseline in AISRS total score, respectively), CGI-S scores, and CGI-Improvement (CGI-I) scores were examined.
Outcomes
- Based on change in AISRS total scores, patients who received viloxazine ER had significantly greater improvement in their ADHD symptoms than those taking placebo (P = .0040). Patients in the viloxazine ER group had significantly greater improvement in AISRS hyperactive/impulsive (P = .0380) and inattentive symptoms (P = .0015).
- The decrease in CGI-S score was also significantly greater in the viloxazine ER group than in the placebo group (P = .0023). The viloxazine ER group also had significantly greater improvement in executive function as assessed by the BRIEF-A (P = .0468). The difference in GAD-7 scores between the viloxazine ER group and the placebo group was not significant.
- The viloxazine ER group had a greater AISRS 30% response rate than the placebo group (P = .0395). There were no significant differences between groups in AISRS 50% responder rate or CGI-I responder rate.
- Adverse effects related to viloxazine and occurring in ≥5% of participants included insomnia (14.8%), fatigue (11.6%), nausea, decreased appetite (10.1%), dry mouth (9.0%), and headache (9.0%). The discontinuation rate was 9.0% in the viloxazine ER group vs 4.9% in the placebo group.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, patients treated with viloxazine ER had significantly greater improvements in ADHD symptoms, including both hyperactive/impulsive and inattentive components as well as executive function.
- The viloxazine ER group had a significantly higher AISRS 30% response rate than the placebo group, but there were no significant differences in anxiety symptoms or other measures of response.
- Viloxazine ER was well tolerated and safe.
- Limitations: There was a reduced power to detect differences in treatment due to participants dropping out or discontinuing treatment, a lack of interrater reliability data, and a lack of patient-reported outcome or satisfaction data.
3. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
Kis et al11 analyzed the safety results of COMPAS.15 Details of this trial, including interventions and inclusion/exclusion criteria, are described in the description of Lam et al.9
Study design
- Researchers compared the rate of adverse events (AEs) among 205 patients who received ≥1 dose of methylphenidate with 209 patients who received placebo.
- AEs were documented and analyzed on an “as received” basis during Week 0 to Week 52. Electrocardiogram (ECG) data were recorded at baseline and Week 24. Vital signs were monitored at baseline, every week for the first 12 weeks, then every 4 weeks for the next 52 weeks. Body weight was assessed at Week 6, Week 12, Week 20, Week 28, Week 40, and Week 52. A 12-lead ECG was obtained at baseline and Week 24.
- The sample size was assessed to have 80% power to detect group differences in AEs.
Outcomes
- Overall, 96% of participants in the methylphenidate group and 88% of participants in the placebo group experienced at least 1 AE (difference 8.1%; 95% CI, 2.9% to 13.5%).
- AEs that occurred more frequently with methylphenidate compared to placebo were decreased appetite (22% vs 3.8%); dry mouth (15% vs 4.8%); palpitations (13% vs 3.3%); gastrointestinal (GI) infection (11% vs 4.8%); agitation (11% vs 3.3%); restlessness (10% vs 2.9%); hyperhidrosis, tachycardia, and weight decrease (all 6.3% vs 1.9%); depressive symptoms and influenza (both 4.9% vs 1.0%); and acute tonsillitis (4.4% vs 0.5%). Serious AEs were reported by 7.3% of patients in the methylphenidate group and 4.3% of those in the placebo group, with no difference in frequency (difference 3.0%; 95% CI, 1.6% to 7.9%). The most severe AEs were aggression, depression, somnambulism, and suicidal ideation in the methylphenidate group and car accidents, epicondylitis, and a fall in the placebo group.
- There were no significant differences in AEs between the GPT and CM groups.
- The treatment combinations that included methylphenidate had higher rates of patients experiencing at least 1 AE (CM/methylphenidate 97%, GPT/methylphenidate 96%, CM/placebo 92%, GPT/placebo 84%).
- Overall, 8.8% of patients in the methylphenidate group and 4.8% in the placebo group stopped their medication treatment because of an AE (difference 4.0%; 95% CI, 0.9% to 9.1%). At least 1 dose decrease, increase, or discontinuation was made after an AE in 42% of participants in the placebo group and 69% of those in the methylphenidate group.
- There were no significant differences in clinically pertinent ECG abnormalities between methylphenidate and placebo therapy.
Continue to: Conclusions/limitations
Conclusions/limitations
- AEs were more common in the methylphenidate groups compared to placebo, but there was no significant differences for severe AEs. In the long-term, methylphenidate treatment was well tolerated and relatively safe.
- Limitations: The sample size may have been too small to detect uncommon AEs, all AEs had to be reported and may not have been caused by the treatment, and the original study’s main outcome was efficacy, not safety, which makes this an exploratory analysis of AEs.
4. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
Once-daily dosing of stimulants, which are commonly used to manage adult ADHD,16 can be beneficial because many patients have schedules that limit taking medication multiple times a day. Cutler et al12 looked at the efficacy and safety of amphetamine extended-release tablet (AMPH ER TAB), which is a 3.2:1 mixture of d- and l-amphetamine released by the LiquiXR drug delivery system. This technology allows for a continuous release following an initial quick onset of action.
Study design
- This parallel-study, double-blind study evaluated adults age 18 to 60 who had a diagnosis of ADHD according to DSM-5 criteria and the Adult ADHD Clinical Diagnostic Scale, normal-range IQ, AISRS score ≥26, and baseline CGI-S score ≥4.
- Women were not lactating or pregnant during the study.
- Exclusion criteria included a history of mental illnesses; chronic medical conditions; clinically significant abnormal ECG or cardiac findings on exam; renal or liver disease; family history of sudden death; significant vital sign findings; uncontrolled hypertension or a resting systolic blood pressure (SBP) >140 mmHg or diastolic blood pressure (DBP) >90 mmHg; recent history of or current alcohol or substance use disorder; use of atomoxetine, monoamine oxidase inhibitors, or tricyclic antidepressants within 14 days of study or the use of other stimulant medications within 1 week of screening; use of GI acidifying agents or urinary acidifying agents within 3 days of screening; answering “yes” to questions 4 or 5 of the Suicidal Ideation section of the Columbia Suicide Severity Rating Scale within 2 years prior to the study; taking another investigational medication within 30 days of screening; allergic to amphetamine or components of the study drug, and a lack of prior response to amphetamine.
- Patients were randomized to receive AMPH ER TAB (n = 65) or placebo (n = 65), taken before 10
am . Participants started at 5 mg/d of the drug/placebo and then entered a 5-week titration period in which the medication was increased by 5 mg/d each week until reaching 20 mg/d, and then continued 20 mg/d for 2 weeks. - The primary outcome was the mean Permanent Product Measure of Performance Total (PERMP-T) score averaged across all time points (0.5-, 1-, 2-, 4-, 8-, 10-, 12-, 13-, and 14-hours postdose) at Visit 5.
- Participants underwent AISRS, CGI-S, and safety evaluations at baseline and at the 5 visits at the end of each treatment week.
Outcomes
- Analyses were completed on participants who received ≥1 dose of the medication and who had ≥1 PERMP-T score at Visit 5.
- Predose PERMP-T scores were similar between the AMPH ER TAB group (259.5) and placebo group (260). The mean postdose PERMP-T score in the AMPH ER TAB group (302.8) was significantly higher (P = .0043) than the placebo group (279.6).
- The PERMP-T scores were significantly different at 0.5-, 1-, 2-, 4-, 8-, and 13-hours postdose but not at 10-, 12-, and 14-hours postdose. The first Visit 5 time point at which the difference between groups was statistically different was at 0.5 hours postdose (P = .01), and the last significant time point was 13 hours (P = .006).
- The improvement in CGI-S scores was significantly greater in the AMPH ER TAB group than the placebo group. The improvement in AISRS scores was significantly greater in the AMPH ER TAB group at Visit 3, Visit 4, and Visit 5. More participants in the AMPH ER TAB group had AEs compared to the placebo group (90% vs 60%). The most common AEs (frequency ≥5% and occurring more in the intervention arm) were decreased appetite, insomnia, dry mouth, irritability, headache, anxiety, nausea, dizziness, and tachycardia.
- The AMPH ER TAB group had nonclinically significant increases in SBP (116.8 to 120.7 mmHg), DBP (74.1 to 77.1 mmHg), and heart rate (73.0 to 81.9 bpm) at Visit 5 compared to baseline.
- No serious AEs occurred. Three participants in the AMPH ER TAB group experienced AEs (increased blood pressure, CNS stimulation, and anxiety) that led them to discontinue the study.
Continue to: Conclusions/limitations
Conclusions/limitations
- AMPH ER TAB reduced symptoms in adults with ADHD as assessed by improvement in PERMP-T scores.
- The safety and tolerability profile of AMPH ER TAB were comparable to other stimulants, with expected rises in blood pressure and heart rate.
- Limitations: Patients were required to be titrated to 20 mg/d of AMPH ER TAB, instead of following a flexible titration based on an individual’s response. Some participants may have had greater improvement at a higher or lower dose. This study did not compare AMPH ER TAB to other stimulants. The 5-week duration of this study limited the ability to evaluate long-term efficacy and tolerability. Patients with a wide range of psychiatric or medical comorbidities were excluded.
5. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
Guanfacine extended-release (GXR) is a selective alpha 2A-adrenergic receptor agonist approved for treating ADHD in children and adolescents.17 Iwanami et al13 evaluated the efficacy and safety of GXR for adults.
Study design
- This randomized, double-blinded, placebo-controlled trial enrolled Japanese adults age ≥18 who were diagnosed with ADHD according to DSM-5 criteria and scored ≥24 on the ADHD-Rating Scale IV (ADHD-RS-IV) and ≥4 on CGI- I.
- Exclusion criteria included having anxiety, depression, substance use disorder, tic disorder, BD, personality disorder, schizophrenia, or intellectual disability; a moderate or severe psychiatric disorder requiring treatment other than counseling; seizures; increased risk for suicide; a history of cardiovascular disease, including prolonged QTc/abnormal ECG/abnormal labs, orthostatic hypotension, or continuous bradycardia; or taking medications that affect blood pressure or heart rate.
- Overall, 101 participants were randomized to the GXR group and 100 to the placebo group. Approximately two-thirds of the study population was male. Patients received GXR or placebo once daily at approximately the same time.
- There were 5 phases to the trial. The screening period occurred over 1 to 4 weeks. Part 1 of the treatment period consisted of 5 weeks of medication optimization. Participants were started on GXR 2 mg/d and were required to be receiving a minimum dose of 4 mg/d starting at Week 3. Clinicians were allowed to increase the dose 1 mg/d per week starting at Week 4 based on clinical response to a maximum dosage of 6 mg/d. Part 2 of the treatment period consisted of 5 weeks of maintenance at 4 to 6 mg/d. The tapering period to 2 mg/d occurred over 2 weeks. The follow-up period lasted 1 week.
- Efficacy measurements included the Japanese version of the ADHD-RS-IV and translations of the English-language CAARS, CGI-I, and CGI-S. Participant-reported measures included the Patient Global Impression-Improvement scale (PGI-I), Adult ADHD Quality of Life Questionnaire (AAQoL), and BRIEF-A.
- The primary outcome was the difference in ADHD-RS-IV total score from baseline to the end of the maintenance period (Week 10).
- Safety assessments were completed at Week 5 (end of dose optimization period), Week 10 (end of dose maintenance period), and Week 12 (tapering period).
Outcomes
- The average GXR dose during the maintenance period was 5.07 mg/d.
- Compared to the placebo group, the GXR group had more patients age <30 (47% vs 39%) and fewer patients age ≥40 (17% vs 27%). Baseline ADHD-RS-IV scores in both groups were comparable. At baseline, 51% in the GXR group had a combined inattentive/hyperactive-impulsive presentation and 47% had a predominately inattention presentation, with similar characteristics in the placebo group (49% combined, 49% inattention).
- At Week 10, the least squares mean change from baseline on the ADHD-RS-IV total score was significantly greater in the GXR group than in the placebo group (-11.55 ± 1.10 vs -7.27 ± 1.07; P = .0005), with an effect size of 0.52. There was a greater decrease in the ADHD-RS-IV scores starting at Week 4 and continuing to Week 10 (P < .005).
- There were also significant differences favoring GXR on the ADHD-RS-IV hyperactivity-impulsivity subscale score (P = .0021) and ADHD-RS-IV inattention subscale score (P = .0032).
- There were significant differences in the CAARS total ADHD score (P = .0029) and BRIEF-A scores on the inhibit (P = .0173), initiate (P = .0406), plan/organize (P = .174), and global executive composite index (P = .0404) scales. There was no significant difference in the total AAQoL score (P = .0691), but there was a significant improvement in the AAQoL life productivity subscore (P = .0072).
- At Week 10, there were also significant improvements in the CGI-I scores (P = .0007) and PGI-I scores (P = .0283). The CGI-S scores were similar at all time points.
- Overall, 81.2% of GXR patients reported AEs compared to 62% in the placebo group. There was 1 serious treatment-emergent AE (a suicide attempt) that the authors concluded was unrelated to the study drug. No deaths occurred. The most common AEs (incidence ≥10% in either group) included somnolence, thirst, nasopharyngitis (occurring more in the placebo group), blood pressure decrease, postural dizziness, and constipation. The main AEs leading to discontinuation were somnolence and blood pressure decrease. Overall, 19.8% of patients receiving GXR discontinued treatment due to AEs, compared to 3% in the placebo group.
- Heart rate, blood pressure, and QTc (corrected by the Bazett formula) were decreased in the GXR group at Week 10 while QT and RR intervals increased, and most returned to normal by Week 12.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, GXR monotherapy resulted in clinical improvement in ADHD symptoms, with a moderate effect size.
- The most common AEs were mild to moderate and congruent with known adverse effects of guanfacine. Sedation effects mostly transpired within the first week of medication administration and were transient.
- Limitations: The findings might not be generalizable to non-Japanese patients. The duration of the study was short. Patients with a wide range of psychiatric and medical comorbidities were excluded. Two-thirds of the participants were male, and there was a disparity in participant age in the GXR and placebo groups.
6. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
While stimulants are a mainstay ADHD treatment, some patients have a partial response or do not respond to amphetamines or methylphenidate. Reimherr et el14 assessed the efficacy and safety of adding brexpiprazole (BXP) to a stimulant.
Study design
- This randomized, double-blinded, placebo-controlled trial recruited 559 stimulant-naive patients and 174 patients who had not responded to previous stimulant therapy.
- Participants were adults age 18 to 55 with a primary diagnosis of ADHD according to DSM-IV-TR criteria and the Conners Adult ADHD Diagnostic Interview. Other inclusion criteria were having a CAARS score ≥29 and a CGI-S score ≥4.
- Exclusion criteria included being at risk for suicide; having current substance abuse or positive alcohol/drug screens; a history of good response to prestudy treatment; a clinically significant medical condition; fasting blood glucose >200 mg/dL or hemoglobin A1C >7%; and hospitalization in past 12 months from a diabetic complication, uncontrolled hypertension, ischemic heart disease, or epilepsy. Further exclusion criteria included a history of psychosis, current MDD or BD, current panic disorder, uncontrolled comorbid psychiatric condition, or clinically significant personality disorder. Investigators excluded any patient with severe DSM-IV axis I or II disorders or abnormal/psychopathological behaviors.
- The trial consisted of 3 segments. Part 1 was screening. If the patient was currently receiving a stimulant but not fully responding, the medication was discontinued for at least 5 half-lives.
- Part 2 (5 weeks) involved administering a stimulant plus a single-blind placebo (597 patients completed this phase). The stimulant was chosen by the investigator, who had the option of using 1 of 2 amphetamine derivatives (mixed amphetamine salts capsules or lisdexamfetamine dimesylate capsules) or 1 of 2 methylphenidate derivatives (methylphenidate hydrochloride ER tabs or dexmethylphenidate HCl ER capsules). If a patient did not respond to a particular stimulant prior to the study, they were given a different stimulant from the list. Patients continued the same stimulant throughout the trial. Patients were monitored for a response, defined as a ≥30% decrease in CAARS score or a CAARS score <24, or a CGI-I score of 1 or 2 at Week 5. Patients who did not show this improvement were categorized as open-label nonresponders.
- Part 3 (6 weeks) involved administering a stimulant plus double-blind BXP vs placebo (stimulant-naive n = 167, stimulant nonresponders n = 68). Nonresponders continued the stimulant (at the same dose reached at the end of Part 2) and added either BXP (n = 155) or continued placebo (n = 80). Patients who responded in Part 2 were continued on the stimulant plus placebo and were not randomized. Patients were started on BXP 0.25 mg/d, and the medication could be titrated to 2 mg/d during the following 3 weeks, depending on the benefit vs AE profile. After the third week, the dose could be decreased but not increased.
- The primary outcome was a change in CAARS score. Secondary measurements included the CGI-S, Wender-Reimherr Adult Attention Deficit Disorder Scale (WRAADDS), Montgomery-Åsberg Depression Rating Scale (MADRS), and BDI.
Outcomes
- Stimulant-naive patients were equally divided among the 4 stimulant groups, and previous nonresponders who continued to not respond in Part 2 were more likely to be given methylphenidate HCl or lisdexamfetamine dimesylate.
- Patients with a history of nonresponse had less response to stimulants in Part 2 compared to stimulant-naive patients, as seen by 27% (n = 167) of stimulant-naive patients entering Part 3 compared to 39% of prior nonresponders (n = 68; P = .0249).
- ADHD improvement with BXP appeared to be greater among pretrial nonresponders.
- For stimulant nonresponders before and during the study, at the end of the double-blind endpoint (Part 3; Week 11), WRAADDS total score was significantly improved in the BXP group compared to the placebo group (P = .013; d = 0.74), with most beneficial effects seen in the hyperactivity/restlessness, emotional dysregulation factor, and impulsivity categories.
- For stimulant nonresponders before and during the study, there was no significant difference at the end of Week 11 on the CAARS (P = .64), MADRS (P = .37), or BDI (P = .73). There was a trend toward significance on the CAARS subscale for hyperactive/impulsive (P = .09).
- For prestudy stimulant-naive patients who did not respond to stimulants in Part 2 and were randomized in Part 3, there was not a significant difference between BXP and placebo at Week 11 as assessed on WRAADDS, CAARS, MADRS, or BDI.
- As assessed on WRAADDS, 50% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.334). Under the emotional dysregulation factor category of the WRAADDS, 64% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.064). The attention factor category showed a 40% improvement in the BXP group compared to 32% in the placebo group (Fisher exact = 0.344).
- There were 2 serious AEs in the BXP group (gall bladder inflammation and diarrhea) and 2 in the placebo group (pneumonia and urinary tract infection). There was no statistically significant difference between groups with regards to common AEs (ie, fatigue, heartburn/nausea/stomachache, weight loss), although there was a trend to significant for insomnia in the BXP group (P = .083).
Conclusions/limitations
- Stimulant-naive patients experienced no improvement with adjunctive BXP.
- For prior stimulant nonresponders, there was no significant difference between BXP vs placebo on the primary outcome of the CAARS score, but there was an improvement as observed by assessment with the WRAADDS.
- The largest change in the WRAADDS occurred in the emotional dysregulation factor compared to the attention factor.
- BXP appeared to be well tolerated.
- Limitations: The WRAADDS was administered without the patients’ significant other/collateral. Raters were not trained in the use of the WRAADDS. Patients with a wide range of psychiatric and medical comorbidities were excluded. Fewer patients were recruited in the prior stimulant nonresponder group.
Bottom Line
Recent randomized controlled trials suggest that methylphenidate, amphetamine extended-release, viloxazine extended-release, and guanfacine extended-release improved symptoms of adult attention-deficit/hyperactivity disorder (ADHD). There were no improvements in ADHD symptoms with adjunctive brexpiprazole.
Related Resources
- Parikh AR, Baker SA. Adult ADHD: pharmacologic treatment in the DSM-5 era. Current Psychiatry. 2016;15(10):18-25.
- Akbar HN. Why we should be scrutinizing the rising prevalence of adult ADHD. Current Psychiatry. 2022; 21(7):e1-e2. doi:10.12788/cp.0268
Drug Brand Names
Amantadine • Gocovri
Amphetamine extended-release tablet • Dyanavel XR
Atomoxetine • Strattera
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Dexmethylphenidate • Focalin
Fluoxetine • Prozac
Guanfacine extended- release • Intuniv
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin
Theophylline • Elixophyllin
Viloxazine • Qelbree
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Harpin V, Mazzone L, Raynaud JP, et al. Long-term outcomes of ADHD: a systematic review of self-esteem and social function. J Atten Disord. 2016;20(4):295-305. doi:10.1177/1087054713486516
3. Beaton DM, Sirois F, Milne E. Experiences of criticism in adults with ADHD: a qualitative study. PLoS One. 2022;17(2):e0263366. doi:10.1371/journal.pone.0263366
4. Attention-deficit/hyperactivity disorder (ADHD). National Institute of Mental Health. Accessed February 9, 2023. https://www.nimh.nih.gov/health/statistics/attention-deficit-hyperactivity-disorder-adhd
5. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
6. Attention Deficit Hyperactivity Disorder: Diagnosis and Management. NICE Guideline No. 87. National Institute for Health and Care Excellence (NICE); 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
7. Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgrad Med. 2010;122(1):184-191. doi:10.3810/pgm.2010.01.2112
8. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
9. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
10. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of viloxazine extended-release capsules in adults with attention-deficit/hyperactivity disorder. CNS Drugs. 2022;36(8):897-915. doi:10.1007/s40263-022-00938-w
11. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
12. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
13. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
14. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
15. Philipsen A, Jans T, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Effects of group psychotherapy, individual counseling, methylphenidate, and placebo in the treatment of adult attention-deficit/hyperactivity disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(12):1199-1210.
16. McGough JJ. Treatment controversies in adult ADHD. Am J Psychiatry. 2016;173(10):960-966. doi:10.1176/appi.ajp.2016.15091207
17. Cruz MP. Guanfacine extended-release tablets (Intuniv), a nonstimulant selective alpha2a-adrenergic receptor agonist for attention-deficit/hyperactivity disorder. P T. 2010;35(8):448-451.
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder that begins in childhood and continues into adulthood. The clinical presentation is characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional interference.1 ADHD affects patients’ interpersonal and professional lives as well as their daily functioning.2 Adults with ADHD may suffer from excessive self-criticism, low self-esteem, and sensitivity to criticism.3 The overall prevalence of adult ADHD is 4.4%.4 ADHD in adults is frequently associated with comorbid psychiatric disorders.5 The diagnosis of ADHD in adults requires the presence of ≥5 symptoms of inattention and hyperactivity/impulsivity that persist for ≥6 months. Patients must have first had such symptoms before age 12; symptoms need to be present in ≥2 settings and interfere with functioning.1
Treatment of ADHD includes pharmacologic and nonpharmacologic interventions. For most patients, pharmacotherapy—specifically stimulant medications—is advised as first-line treatment,6 with adequate trials of methylphenidate and amphetamines before using second-line agents such as nonstimulants. However, despite these medications’ efficacy in randomized controlled trials (RCTs), adherence is low.7 This could be due to inadequate response or adverse effects.8 Guidelines also recommend the use of nonpharmacologic interventions for adults who cannot adhere to or tolerate medication or have an inadequate response.6 Potential nonpharmacologic interventions include transcranial direct current stimulation, mindfulness, psychoeducation, cognitive-behavioral therapy, and chronotherapy.
In Part 1 of this 2-part article, we review 6 RCTs of pharmacologic interventions for adult ADHD published within the last 5 years (Table9-14). Part 2 will review nonpharmacologic treatments.

1. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
The Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) was a multicenter prospective, randomized trial of adults age 18 to 58 with ADHD.15 It compared cognitive-behavioral group psychotherapy (GPT) with individual clinical management (CM), and methylphenidate with placebo. When used in conjunction with methylphenidate, psychological treatments produced better results than placebo. However, studies on the long-term effects of multimodal treatment in ADHD are limited. Lam et al9 performed a follow-up analysis of the COMPAS trial.
Study design
- This observer-masked study involved a follow-up of participants in COMPAS 1.5 years after the interventions were terminated. Of the 433 adults with ADHD who participated in COMPAS, 256 participated in this follow-up.
- The inclusion criteria of COMPAS were age 18 to 58; diagnosis of ADHD according to DSM-IV criteria; chronic course of ADHD symptoms from childhood to adulthood; a Wender Utah Rating Scale short version score ≥30; and no pathological abnormality detected on physical examination.
- The exclusion criteria were having an IQ <85; schizophrenia, bipolar disorder (BD), borderline personality disorder, antisocial personality disorder, suicidal or self-injurious behavior, autism, motor tics, or Tourette syndrome; substance abuse/dependence within 6 months prior to screening; positive drug screening; neurologic diseases, seizures, glaucoma, diabetes, hyperlipidemia, uncontrolled arterial hypertension, angina pectoris, tachycardia arrhythmia, or arterial occlusive disease; previous stroke; current bulimia or anorexia; low weight (body mass index [BMI] <20; pregnancy (current or planned) or breastfeeding; treatment with stimulants or ADHD-specific psychotherapy in the past 6 months; methylphenidate intolerance; treatment with antidepressants, norepinephrine reuptake inhibitors, bupropion, antipsychotics, theophylline, amantadine, anticoagulants derived from coumarin, antacids, or alpha-adrenergic agonists in the 2 weeks prior to baseline; and treatment with fluoxetine or monoamine oxidase inhibitors in the 4 weeks prior to baseline.
- The primary outcome was a change from baseline on the ADHD Index of Conners Adult ADHD Rating Scale (CAARS) score. Secondary outcomes were self-ratings on the Beck Depression Inventory (BDI) and observer-masked ratings of the Clinical Global Impression (CGI) scale and other ADHD rating scale scores, such as the Diagnostic Checklist for the diagnosis of ADHD in adults (ADHD-DC) and subscales of the CAARS.
- COMPAS was open regarding patient and therapist assignment to GPT and CM, but double-masked regarding medication. The statistical analysis focused on the 2x2 comparison of GPT vs CM and methylphenidate vs placebo.
Outcomes
- A total of 251 participants had an assessment with the observer-masked CAARS score. The baseline mean (SD) age was 36.3 (10.1), and approximately one-half (49.8%) of participants were male.
- Overall, 9.2% of patients took methylphenidate >31 days from termination of COMPAS before this study but not at the start of this study. Approximately one-third (31.1%) of patients were taking methylphenidate at follow-up. The mean (SD) daily dosage of methylphenidate was 36 (24.77) mg and 0.46 (0.27) mg/kg of body weight.
- The baseline all-group mean ADHD Index of CAARS score was 20.6. At follow-up, it was 14.7 for the CM arm and 14.2 for the GPT arm (difference not significant, P = .48). The mean score decreased to 13.8 for the methylphenidate arm and to 15.2 for the placebo (significant difference, P = .04).
- Overall, methylphenidate was associated with greater improvement in symptoms than placebo. Patients in the GPT arm had fewer severe symptoms as assessed by the self-reported ADHD Symptoms Total Score compared to the CM arm (P = .04).
- There were no significant differences in self-rating CAARS and observer-rated CAARS subscale scores. Compared to CM, GPT significantly decreased pure hyperactive symptoms on the ADHD-DC (P = .08). No significant differences were observed in BDI scores. The difference between GPT and CM remained significant at follow-up in terms of the CGI evaluation of efficacy (P = .04).
Continue to: Conclusions/limitations
Conclusions/limitations
- Regardless of which combined treatments they received, patients with ADHD continued to improve 1.5 years after the 52-week treatment phase ended.
- Patients assigned to methylphenidate performed considerably better on the observer-rated CAARS than patients assigned to placebo.
- Benefits from GPT or CM in addition to methylphenidate therapy lasted 1.5 years. Compared to CM, GPT was not linked to better scores on the CAARS.
- Limitations: Approximately 41% of patients who were recruited did not participate. Daily functioning was measured only by the CGI. There were only marginal differences among the 4 treatments, and the study compared a very regimented approach (GPT) with one that was less focused (CM).
2. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double‐blind, placebo‐controlled trial assessing the efficacy and safety of viloxazine extended‐release capsules in adults with attention‐deficit/hyperactivity disorder. CNS Drugs. 2022;36(8): 897-915. doi:10.1007/s40263-022-00938-w
In 2021, the FDA approved viloxazine extended-release (ER) for treating ADHD in children and adolescents (age 6 to 17). Nasser et al10 reviewed the safety and efficacy of viloxazine ER in adults with ADHD.
Study design
- This phase III, randomized, double-blind, placebo-controlled, multicenter clinical trial included 374 adults with ADHD who received viloxazine ER or placebo.
- Participants were age 18 to 65 and had been given a primary diagnosis of ADHD according to DSM-5 criteria in the last 6 months. Other inclusion criteria were having an Adult ADHD Investigator Symptom Rating Scale (AISRS) total score ≥26 and CGI-Severity of Illness (CGI-S) score ≥4 at baseline, BMI 18 to 35 kg/m2, and being medically healthy.
- Exclusion criteria included having treatment-resistant ADHD, a current diagnosis of any psychiatric disorder other than ADHD, or a history of schizophrenia, schizoaffective disorder, BD, autism, obsessive-compulsive disorder, personality disorder, or posttraumatic stress disorder. Individuals with any significant neurologic disorder, heart condition, arrhythmia, clinically relevant vital sign abnormality, or systemic illness were excluded, as were those with a history (within the past year) or current diagnosis of substance use disorder or a positive drug screen for a drug of abuse. Those with an allergic reaction or intolerance to viloxazine or were breastfeeding, pregnant, or refused to be abstinent or practice birth control were excluded.
- The dosage of viloxazine ER ranged from 200 to 600 mg/d for 6 weeks. This was titrated based on symptom response and adverse effects.
- All individuals received 2 capsules once a day for Week 1 and Week 2. During Week 1 and Week 2, participants in the viloxazine ER group received 200 mg (1 viloxazine ER capsule and 1 placebo capsule) and 400 mg (2 viloxazine ER capsules) of the medication, respectively. Two placebo pills were administered to those in the placebo group. From Week 3 to Week 6, the dose could be titrated or tapered at the investigator’s discretion. Compliance was assessed by comparing the number of pills dispensed vs returned.
- The primary outcome was a change in AISRS score from baselines to Week 6.
- The key secondary outcome was the change in CGI-S score from baseline to Week 6. Scores on the AISRS inattention and hyperactive/impulsivity subscales, Behavioral Regulation Index, Metacognition Index, Behavior Rating Inventory of Executive Function–Adult Version (BRIEF-A), and Generalized Anxiety Disorder-7 item scale (GAD-7) were also evaluated. Also, the rates of 30% and 50% responders on the AISRS (defined as ≥30% or ≥50% reduction from baseline in AISRS total score, respectively), CGI-S scores, and CGI-Improvement (CGI-I) scores were examined.
Outcomes
- Based on change in AISRS total scores, patients who received viloxazine ER had significantly greater improvement in their ADHD symptoms than those taking placebo (P = .0040). Patients in the viloxazine ER group had significantly greater improvement in AISRS hyperactive/impulsive (P = .0380) and inattentive symptoms (P = .0015).
- The decrease in CGI-S score was also significantly greater in the viloxazine ER group than in the placebo group (P = .0023). The viloxazine ER group also had significantly greater improvement in executive function as assessed by the BRIEF-A (P = .0468). The difference in GAD-7 scores between the viloxazine ER group and the placebo group was not significant.
- The viloxazine ER group had a greater AISRS 30% response rate than the placebo group (P = .0395). There were no significant differences between groups in AISRS 50% responder rate or CGI-I responder rate.
- Adverse effects related to viloxazine and occurring in ≥5% of participants included insomnia (14.8%), fatigue (11.6%), nausea, decreased appetite (10.1%), dry mouth (9.0%), and headache (9.0%). The discontinuation rate was 9.0% in the viloxazine ER group vs 4.9% in the placebo group.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, patients treated with viloxazine ER had significantly greater improvements in ADHD symptoms, including both hyperactive/impulsive and inattentive components as well as executive function.
- The viloxazine ER group had a significantly higher AISRS 30% response rate than the placebo group, but there were no significant differences in anxiety symptoms or other measures of response.
- Viloxazine ER was well tolerated and safe.
- Limitations: There was a reduced power to detect differences in treatment due to participants dropping out or discontinuing treatment, a lack of interrater reliability data, and a lack of patient-reported outcome or satisfaction data.
3. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
Kis et al11 analyzed the safety results of COMPAS.15 Details of this trial, including interventions and inclusion/exclusion criteria, are described in the description of Lam et al.9
Study design
- Researchers compared the rate of adverse events (AEs) among 205 patients who received ≥1 dose of methylphenidate with 209 patients who received placebo.
- AEs were documented and analyzed on an “as received” basis during Week 0 to Week 52. Electrocardiogram (ECG) data were recorded at baseline and Week 24. Vital signs were monitored at baseline, every week for the first 12 weeks, then every 4 weeks for the next 52 weeks. Body weight was assessed at Week 6, Week 12, Week 20, Week 28, Week 40, and Week 52. A 12-lead ECG was obtained at baseline and Week 24.
- The sample size was assessed to have 80% power to detect group differences in AEs.
Outcomes
- Overall, 96% of participants in the methylphenidate group and 88% of participants in the placebo group experienced at least 1 AE (difference 8.1%; 95% CI, 2.9% to 13.5%).
- AEs that occurred more frequently with methylphenidate compared to placebo were decreased appetite (22% vs 3.8%); dry mouth (15% vs 4.8%); palpitations (13% vs 3.3%); gastrointestinal (GI) infection (11% vs 4.8%); agitation (11% vs 3.3%); restlessness (10% vs 2.9%); hyperhidrosis, tachycardia, and weight decrease (all 6.3% vs 1.9%); depressive symptoms and influenza (both 4.9% vs 1.0%); and acute tonsillitis (4.4% vs 0.5%). Serious AEs were reported by 7.3% of patients in the methylphenidate group and 4.3% of those in the placebo group, with no difference in frequency (difference 3.0%; 95% CI, 1.6% to 7.9%). The most severe AEs were aggression, depression, somnambulism, and suicidal ideation in the methylphenidate group and car accidents, epicondylitis, and a fall in the placebo group.
- There were no significant differences in AEs between the GPT and CM groups.
- The treatment combinations that included methylphenidate had higher rates of patients experiencing at least 1 AE (CM/methylphenidate 97%, GPT/methylphenidate 96%, CM/placebo 92%, GPT/placebo 84%).
- Overall, 8.8% of patients in the methylphenidate group and 4.8% in the placebo group stopped their medication treatment because of an AE (difference 4.0%; 95% CI, 0.9% to 9.1%). At least 1 dose decrease, increase, or discontinuation was made after an AE in 42% of participants in the placebo group and 69% of those in the methylphenidate group.
- There were no significant differences in clinically pertinent ECG abnormalities between methylphenidate and placebo therapy.
Continue to: Conclusions/limitations
Conclusions/limitations
- AEs were more common in the methylphenidate groups compared to placebo, but there was no significant differences for severe AEs. In the long-term, methylphenidate treatment was well tolerated and relatively safe.
- Limitations: The sample size may have been too small to detect uncommon AEs, all AEs had to be reported and may not have been caused by the treatment, and the original study’s main outcome was efficacy, not safety, which makes this an exploratory analysis of AEs.
4. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
Once-daily dosing of stimulants, which are commonly used to manage adult ADHD,16 can be beneficial because many patients have schedules that limit taking medication multiple times a day. Cutler et al12 looked at the efficacy and safety of amphetamine extended-release tablet (AMPH ER TAB), which is a 3.2:1 mixture of d- and l-amphetamine released by the LiquiXR drug delivery system. This technology allows for a continuous release following an initial quick onset of action.
Study design
- This parallel-study, double-blind study evaluated adults age 18 to 60 who had a diagnosis of ADHD according to DSM-5 criteria and the Adult ADHD Clinical Diagnostic Scale, normal-range IQ, AISRS score ≥26, and baseline CGI-S score ≥4.
- Women were not lactating or pregnant during the study.
- Exclusion criteria included a history of mental illnesses; chronic medical conditions; clinically significant abnormal ECG or cardiac findings on exam; renal or liver disease; family history of sudden death; significant vital sign findings; uncontrolled hypertension or a resting systolic blood pressure (SBP) >140 mmHg or diastolic blood pressure (DBP) >90 mmHg; recent history of or current alcohol or substance use disorder; use of atomoxetine, monoamine oxidase inhibitors, or tricyclic antidepressants within 14 days of study or the use of other stimulant medications within 1 week of screening; use of GI acidifying agents or urinary acidifying agents within 3 days of screening; answering “yes” to questions 4 or 5 of the Suicidal Ideation section of the Columbia Suicide Severity Rating Scale within 2 years prior to the study; taking another investigational medication within 30 days of screening; allergic to amphetamine or components of the study drug, and a lack of prior response to amphetamine.
- Patients were randomized to receive AMPH ER TAB (n = 65) or placebo (n = 65), taken before 10
am . Participants started at 5 mg/d of the drug/placebo and then entered a 5-week titration period in which the medication was increased by 5 mg/d each week until reaching 20 mg/d, and then continued 20 mg/d for 2 weeks. - The primary outcome was the mean Permanent Product Measure of Performance Total (PERMP-T) score averaged across all time points (0.5-, 1-, 2-, 4-, 8-, 10-, 12-, 13-, and 14-hours postdose) at Visit 5.
- Participants underwent AISRS, CGI-S, and safety evaluations at baseline and at the 5 visits at the end of each treatment week.
Outcomes
- Analyses were completed on participants who received ≥1 dose of the medication and who had ≥1 PERMP-T score at Visit 5.
- Predose PERMP-T scores were similar between the AMPH ER TAB group (259.5) and placebo group (260). The mean postdose PERMP-T score in the AMPH ER TAB group (302.8) was significantly higher (P = .0043) than the placebo group (279.6).
- The PERMP-T scores were significantly different at 0.5-, 1-, 2-, 4-, 8-, and 13-hours postdose but not at 10-, 12-, and 14-hours postdose. The first Visit 5 time point at which the difference between groups was statistically different was at 0.5 hours postdose (P = .01), and the last significant time point was 13 hours (P = .006).
- The improvement in CGI-S scores was significantly greater in the AMPH ER TAB group than the placebo group. The improvement in AISRS scores was significantly greater in the AMPH ER TAB group at Visit 3, Visit 4, and Visit 5. More participants in the AMPH ER TAB group had AEs compared to the placebo group (90% vs 60%). The most common AEs (frequency ≥5% and occurring more in the intervention arm) were decreased appetite, insomnia, dry mouth, irritability, headache, anxiety, nausea, dizziness, and tachycardia.
- The AMPH ER TAB group had nonclinically significant increases in SBP (116.8 to 120.7 mmHg), DBP (74.1 to 77.1 mmHg), and heart rate (73.0 to 81.9 bpm) at Visit 5 compared to baseline.
- No serious AEs occurred. Three participants in the AMPH ER TAB group experienced AEs (increased blood pressure, CNS stimulation, and anxiety) that led them to discontinue the study.
Continue to: Conclusions/limitations
Conclusions/limitations
- AMPH ER TAB reduced symptoms in adults with ADHD as assessed by improvement in PERMP-T scores.
- The safety and tolerability profile of AMPH ER TAB were comparable to other stimulants, with expected rises in blood pressure and heart rate.
- Limitations: Patients were required to be titrated to 20 mg/d of AMPH ER TAB, instead of following a flexible titration based on an individual’s response. Some participants may have had greater improvement at a higher or lower dose. This study did not compare AMPH ER TAB to other stimulants. The 5-week duration of this study limited the ability to evaluate long-term efficacy and tolerability. Patients with a wide range of psychiatric or medical comorbidities were excluded.
5. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
Guanfacine extended-release (GXR) is a selective alpha 2A-adrenergic receptor agonist approved for treating ADHD in children and adolescents.17 Iwanami et al13 evaluated the efficacy and safety of GXR for adults.
Study design
- This randomized, double-blinded, placebo-controlled trial enrolled Japanese adults age ≥18 who were diagnosed with ADHD according to DSM-5 criteria and scored ≥24 on the ADHD-Rating Scale IV (ADHD-RS-IV) and ≥4 on CGI- I.
- Exclusion criteria included having anxiety, depression, substance use disorder, tic disorder, BD, personality disorder, schizophrenia, or intellectual disability; a moderate or severe psychiatric disorder requiring treatment other than counseling; seizures; increased risk for suicide; a history of cardiovascular disease, including prolonged QTc/abnormal ECG/abnormal labs, orthostatic hypotension, or continuous bradycardia; or taking medications that affect blood pressure or heart rate.
- Overall, 101 participants were randomized to the GXR group and 100 to the placebo group. Approximately two-thirds of the study population was male. Patients received GXR or placebo once daily at approximately the same time.
- There were 5 phases to the trial. The screening period occurred over 1 to 4 weeks. Part 1 of the treatment period consisted of 5 weeks of medication optimization. Participants were started on GXR 2 mg/d and were required to be receiving a minimum dose of 4 mg/d starting at Week 3. Clinicians were allowed to increase the dose 1 mg/d per week starting at Week 4 based on clinical response to a maximum dosage of 6 mg/d. Part 2 of the treatment period consisted of 5 weeks of maintenance at 4 to 6 mg/d. The tapering period to 2 mg/d occurred over 2 weeks. The follow-up period lasted 1 week.
- Efficacy measurements included the Japanese version of the ADHD-RS-IV and translations of the English-language CAARS, CGI-I, and CGI-S. Participant-reported measures included the Patient Global Impression-Improvement scale (PGI-I), Adult ADHD Quality of Life Questionnaire (AAQoL), and BRIEF-A.
- The primary outcome was the difference in ADHD-RS-IV total score from baseline to the end of the maintenance period (Week 10).
- Safety assessments were completed at Week 5 (end of dose optimization period), Week 10 (end of dose maintenance period), and Week 12 (tapering period).
Outcomes
- The average GXR dose during the maintenance period was 5.07 mg/d.
- Compared to the placebo group, the GXR group had more patients age <30 (47% vs 39%) and fewer patients age ≥40 (17% vs 27%). Baseline ADHD-RS-IV scores in both groups were comparable. At baseline, 51% in the GXR group had a combined inattentive/hyperactive-impulsive presentation and 47% had a predominately inattention presentation, with similar characteristics in the placebo group (49% combined, 49% inattention).
- At Week 10, the least squares mean change from baseline on the ADHD-RS-IV total score was significantly greater in the GXR group than in the placebo group (-11.55 ± 1.10 vs -7.27 ± 1.07; P = .0005), with an effect size of 0.52. There was a greater decrease in the ADHD-RS-IV scores starting at Week 4 and continuing to Week 10 (P < .005).
- There were also significant differences favoring GXR on the ADHD-RS-IV hyperactivity-impulsivity subscale score (P = .0021) and ADHD-RS-IV inattention subscale score (P = .0032).
- There were significant differences in the CAARS total ADHD score (P = .0029) and BRIEF-A scores on the inhibit (P = .0173), initiate (P = .0406), plan/organize (P = .174), and global executive composite index (P = .0404) scales. There was no significant difference in the total AAQoL score (P = .0691), but there was a significant improvement in the AAQoL life productivity subscore (P = .0072).
- At Week 10, there were also significant improvements in the CGI-I scores (P = .0007) and PGI-I scores (P = .0283). The CGI-S scores were similar at all time points.
- Overall, 81.2% of GXR patients reported AEs compared to 62% in the placebo group. There was 1 serious treatment-emergent AE (a suicide attempt) that the authors concluded was unrelated to the study drug. No deaths occurred. The most common AEs (incidence ≥10% in either group) included somnolence, thirst, nasopharyngitis (occurring more in the placebo group), blood pressure decrease, postural dizziness, and constipation. The main AEs leading to discontinuation were somnolence and blood pressure decrease. Overall, 19.8% of patients receiving GXR discontinued treatment due to AEs, compared to 3% in the placebo group.
- Heart rate, blood pressure, and QTc (corrected by the Bazett formula) were decreased in the GXR group at Week 10 while QT and RR intervals increased, and most returned to normal by Week 12.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, GXR monotherapy resulted in clinical improvement in ADHD symptoms, with a moderate effect size.
- The most common AEs were mild to moderate and congruent with known adverse effects of guanfacine. Sedation effects mostly transpired within the first week of medication administration and were transient.
- Limitations: The findings might not be generalizable to non-Japanese patients. The duration of the study was short. Patients with a wide range of psychiatric and medical comorbidities were excluded. Two-thirds of the participants were male, and there was a disparity in participant age in the GXR and placebo groups.
6. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
While stimulants are a mainstay ADHD treatment, some patients have a partial response or do not respond to amphetamines or methylphenidate. Reimherr et el14 assessed the efficacy and safety of adding brexpiprazole (BXP) to a stimulant.
Study design
- This randomized, double-blinded, placebo-controlled trial recruited 559 stimulant-naive patients and 174 patients who had not responded to previous stimulant therapy.
- Participants were adults age 18 to 55 with a primary diagnosis of ADHD according to DSM-IV-TR criteria and the Conners Adult ADHD Diagnostic Interview. Other inclusion criteria were having a CAARS score ≥29 and a CGI-S score ≥4.
- Exclusion criteria included being at risk for suicide; having current substance abuse or positive alcohol/drug screens; a history of good response to prestudy treatment; a clinically significant medical condition; fasting blood glucose >200 mg/dL or hemoglobin A1C >7%; and hospitalization in past 12 months from a diabetic complication, uncontrolled hypertension, ischemic heart disease, or epilepsy. Further exclusion criteria included a history of psychosis, current MDD or BD, current panic disorder, uncontrolled comorbid psychiatric condition, or clinically significant personality disorder. Investigators excluded any patient with severe DSM-IV axis I or II disorders or abnormal/psychopathological behaviors.
- The trial consisted of 3 segments. Part 1 was screening. If the patient was currently receiving a stimulant but not fully responding, the medication was discontinued for at least 5 half-lives.
- Part 2 (5 weeks) involved administering a stimulant plus a single-blind placebo (597 patients completed this phase). The stimulant was chosen by the investigator, who had the option of using 1 of 2 amphetamine derivatives (mixed amphetamine salts capsules or lisdexamfetamine dimesylate capsules) or 1 of 2 methylphenidate derivatives (methylphenidate hydrochloride ER tabs or dexmethylphenidate HCl ER capsules). If a patient did not respond to a particular stimulant prior to the study, they were given a different stimulant from the list. Patients continued the same stimulant throughout the trial. Patients were monitored for a response, defined as a ≥30% decrease in CAARS score or a CAARS score <24, or a CGI-I score of 1 or 2 at Week 5. Patients who did not show this improvement were categorized as open-label nonresponders.
- Part 3 (6 weeks) involved administering a stimulant plus double-blind BXP vs placebo (stimulant-naive n = 167, stimulant nonresponders n = 68). Nonresponders continued the stimulant (at the same dose reached at the end of Part 2) and added either BXP (n = 155) or continued placebo (n = 80). Patients who responded in Part 2 were continued on the stimulant plus placebo and were not randomized. Patients were started on BXP 0.25 mg/d, and the medication could be titrated to 2 mg/d during the following 3 weeks, depending on the benefit vs AE profile. After the third week, the dose could be decreased but not increased.
- The primary outcome was a change in CAARS score. Secondary measurements included the CGI-S, Wender-Reimherr Adult Attention Deficit Disorder Scale (WRAADDS), Montgomery-Åsberg Depression Rating Scale (MADRS), and BDI.
Outcomes
- Stimulant-naive patients were equally divided among the 4 stimulant groups, and previous nonresponders who continued to not respond in Part 2 were more likely to be given methylphenidate HCl or lisdexamfetamine dimesylate.
- Patients with a history of nonresponse had less response to stimulants in Part 2 compared to stimulant-naive patients, as seen by 27% (n = 167) of stimulant-naive patients entering Part 3 compared to 39% of prior nonresponders (n = 68; P = .0249).
- ADHD improvement with BXP appeared to be greater among pretrial nonresponders.
- For stimulant nonresponders before and during the study, at the end of the double-blind endpoint (Part 3; Week 11), WRAADDS total score was significantly improved in the BXP group compared to the placebo group (P = .013; d = 0.74), with most beneficial effects seen in the hyperactivity/restlessness, emotional dysregulation factor, and impulsivity categories.
- For stimulant nonresponders before and during the study, there was no significant difference at the end of Week 11 on the CAARS (P = .64), MADRS (P = .37), or BDI (P = .73). There was a trend toward significance on the CAARS subscale for hyperactive/impulsive (P = .09).
- For prestudy stimulant-naive patients who did not respond to stimulants in Part 2 and were randomized in Part 3, there was not a significant difference between BXP and placebo at Week 11 as assessed on WRAADDS, CAARS, MADRS, or BDI.
- As assessed on WRAADDS, 50% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.334). Under the emotional dysregulation factor category of the WRAADDS, 64% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.064). The attention factor category showed a 40% improvement in the BXP group compared to 32% in the placebo group (Fisher exact = 0.344).
- There were 2 serious AEs in the BXP group (gall bladder inflammation and diarrhea) and 2 in the placebo group (pneumonia and urinary tract infection). There was no statistically significant difference between groups with regards to common AEs (ie, fatigue, heartburn/nausea/stomachache, weight loss), although there was a trend to significant for insomnia in the BXP group (P = .083).
Conclusions/limitations
- Stimulant-naive patients experienced no improvement with adjunctive BXP.
- For prior stimulant nonresponders, there was no significant difference between BXP vs placebo on the primary outcome of the CAARS score, but there was an improvement as observed by assessment with the WRAADDS.
- The largest change in the WRAADDS occurred in the emotional dysregulation factor compared to the attention factor.
- BXP appeared to be well tolerated.
- Limitations: The WRAADDS was administered without the patients’ significant other/collateral. Raters were not trained in the use of the WRAADDS. Patients with a wide range of psychiatric and medical comorbidities were excluded. Fewer patients were recruited in the prior stimulant nonresponder group.
Bottom Line
Recent randomized controlled trials suggest that methylphenidate, amphetamine extended-release, viloxazine extended-release, and guanfacine extended-release improved symptoms of adult attention-deficit/hyperactivity disorder (ADHD). There were no improvements in ADHD symptoms with adjunctive brexpiprazole.
Related Resources
- Parikh AR, Baker SA. Adult ADHD: pharmacologic treatment in the DSM-5 era. Current Psychiatry. 2016;15(10):18-25.
- Akbar HN. Why we should be scrutinizing the rising prevalence of adult ADHD. Current Psychiatry. 2022; 21(7):e1-e2. doi:10.12788/cp.0268
Drug Brand Names
Amantadine • Gocovri
Amphetamine extended-release tablet • Dyanavel XR
Atomoxetine • Strattera
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Dexmethylphenidate • Focalin
Fluoxetine • Prozac
Guanfacine extended- release • Intuniv
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin
Theophylline • Elixophyllin
Viloxazine • Qelbree
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder that begins in childhood and continues into adulthood. The clinical presentation is characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional interference.1 ADHD affects patients’ interpersonal and professional lives as well as their daily functioning.2 Adults with ADHD may suffer from excessive self-criticism, low self-esteem, and sensitivity to criticism.3 The overall prevalence of adult ADHD is 4.4%.4 ADHD in adults is frequently associated with comorbid psychiatric disorders.5 The diagnosis of ADHD in adults requires the presence of ≥5 symptoms of inattention and hyperactivity/impulsivity that persist for ≥6 months. Patients must have first had such symptoms before age 12; symptoms need to be present in ≥2 settings and interfere with functioning.1
Treatment of ADHD includes pharmacologic and nonpharmacologic interventions. For most patients, pharmacotherapy—specifically stimulant medications—is advised as first-line treatment,6 with adequate trials of methylphenidate and amphetamines before using second-line agents such as nonstimulants. However, despite these medications’ efficacy in randomized controlled trials (RCTs), adherence is low.7 This could be due to inadequate response or adverse effects.8 Guidelines also recommend the use of nonpharmacologic interventions for adults who cannot adhere to or tolerate medication or have an inadequate response.6 Potential nonpharmacologic interventions include transcranial direct current stimulation, mindfulness, psychoeducation, cognitive-behavioral therapy, and chronotherapy.
In Part 1 of this 2-part article, we review 6 RCTs of pharmacologic interventions for adult ADHD published within the last 5 years (Table9-14). Part 2 will review nonpharmacologic treatments.

1. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
The Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) was a multicenter prospective, randomized trial of adults age 18 to 58 with ADHD.15 It compared cognitive-behavioral group psychotherapy (GPT) with individual clinical management (CM), and methylphenidate with placebo. When used in conjunction with methylphenidate, psychological treatments produced better results than placebo. However, studies on the long-term effects of multimodal treatment in ADHD are limited. Lam et al9 performed a follow-up analysis of the COMPAS trial.
Study design
- This observer-masked study involved a follow-up of participants in COMPAS 1.5 years after the interventions were terminated. Of the 433 adults with ADHD who participated in COMPAS, 256 participated in this follow-up.
- The inclusion criteria of COMPAS were age 18 to 58; diagnosis of ADHD according to DSM-IV criteria; chronic course of ADHD symptoms from childhood to adulthood; a Wender Utah Rating Scale short version score ≥30; and no pathological abnormality detected on physical examination.
- The exclusion criteria were having an IQ <85; schizophrenia, bipolar disorder (BD), borderline personality disorder, antisocial personality disorder, suicidal or self-injurious behavior, autism, motor tics, or Tourette syndrome; substance abuse/dependence within 6 months prior to screening; positive drug screening; neurologic diseases, seizures, glaucoma, diabetes, hyperlipidemia, uncontrolled arterial hypertension, angina pectoris, tachycardia arrhythmia, or arterial occlusive disease; previous stroke; current bulimia or anorexia; low weight (body mass index [BMI] <20; pregnancy (current or planned) or breastfeeding; treatment with stimulants or ADHD-specific psychotherapy in the past 6 months; methylphenidate intolerance; treatment with antidepressants, norepinephrine reuptake inhibitors, bupropion, antipsychotics, theophylline, amantadine, anticoagulants derived from coumarin, antacids, or alpha-adrenergic agonists in the 2 weeks prior to baseline; and treatment with fluoxetine or monoamine oxidase inhibitors in the 4 weeks prior to baseline.
- The primary outcome was a change from baseline on the ADHD Index of Conners Adult ADHD Rating Scale (CAARS) score. Secondary outcomes were self-ratings on the Beck Depression Inventory (BDI) and observer-masked ratings of the Clinical Global Impression (CGI) scale and other ADHD rating scale scores, such as the Diagnostic Checklist for the diagnosis of ADHD in adults (ADHD-DC) and subscales of the CAARS.
- COMPAS was open regarding patient and therapist assignment to GPT and CM, but double-masked regarding medication. The statistical analysis focused on the 2x2 comparison of GPT vs CM and methylphenidate vs placebo.
Outcomes
- A total of 251 participants had an assessment with the observer-masked CAARS score. The baseline mean (SD) age was 36.3 (10.1), and approximately one-half (49.8%) of participants were male.
- Overall, 9.2% of patients took methylphenidate >31 days from termination of COMPAS before this study but not at the start of this study. Approximately one-third (31.1%) of patients were taking methylphenidate at follow-up. The mean (SD) daily dosage of methylphenidate was 36 (24.77) mg and 0.46 (0.27) mg/kg of body weight.
- The baseline all-group mean ADHD Index of CAARS score was 20.6. At follow-up, it was 14.7 for the CM arm and 14.2 for the GPT arm (difference not significant, P = .48). The mean score decreased to 13.8 for the methylphenidate arm and to 15.2 for the placebo (significant difference, P = .04).
- Overall, methylphenidate was associated with greater improvement in symptoms than placebo. Patients in the GPT arm had fewer severe symptoms as assessed by the self-reported ADHD Symptoms Total Score compared to the CM arm (P = .04).
- There were no significant differences in self-rating CAARS and observer-rated CAARS subscale scores. Compared to CM, GPT significantly decreased pure hyperactive symptoms on the ADHD-DC (P = .08). No significant differences were observed in BDI scores. The difference between GPT and CM remained significant at follow-up in terms of the CGI evaluation of efficacy (P = .04).
Continue to: Conclusions/limitations
Conclusions/limitations
- Regardless of which combined treatments they received, patients with ADHD continued to improve 1.5 years after the 52-week treatment phase ended.
- Patients assigned to methylphenidate performed considerably better on the observer-rated CAARS than patients assigned to placebo.
- Benefits from GPT or CM in addition to methylphenidate therapy lasted 1.5 years. Compared to CM, GPT was not linked to better scores on the CAARS.
- Limitations: Approximately 41% of patients who were recruited did not participate. Daily functioning was measured only by the CGI. There were only marginal differences among the 4 treatments, and the study compared a very regimented approach (GPT) with one that was less focused (CM).
2. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double‐blind, placebo‐controlled trial assessing the efficacy and safety of viloxazine extended‐release capsules in adults with attention‐deficit/hyperactivity disorder. CNS Drugs. 2022;36(8): 897-915. doi:10.1007/s40263-022-00938-w
In 2021, the FDA approved viloxazine extended-release (ER) for treating ADHD in children and adolescents (age 6 to 17). Nasser et al10 reviewed the safety and efficacy of viloxazine ER in adults with ADHD.
Study design
- This phase III, randomized, double-blind, placebo-controlled, multicenter clinical trial included 374 adults with ADHD who received viloxazine ER or placebo.
- Participants were age 18 to 65 and had been given a primary diagnosis of ADHD according to DSM-5 criteria in the last 6 months. Other inclusion criteria were having an Adult ADHD Investigator Symptom Rating Scale (AISRS) total score ≥26 and CGI-Severity of Illness (CGI-S) score ≥4 at baseline, BMI 18 to 35 kg/m2, and being medically healthy.
- Exclusion criteria included having treatment-resistant ADHD, a current diagnosis of any psychiatric disorder other than ADHD, or a history of schizophrenia, schizoaffective disorder, BD, autism, obsessive-compulsive disorder, personality disorder, or posttraumatic stress disorder. Individuals with any significant neurologic disorder, heart condition, arrhythmia, clinically relevant vital sign abnormality, or systemic illness were excluded, as were those with a history (within the past year) or current diagnosis of substance use disorder or a positive drug screen for a drug of abuse. Those with an allergic reaction or intolerance to viloxazine or were breastfeeding, pregnant, or refused to be abstinent or practice birth control were excluded.
- The dosage of viloxazine ER ranged from 200 to 600 mg/d for 6 weeks. This was titrated based on symptom response and adverse effects.
- All individuals received 2 capsules once a day for Week 1 and Week 2. During Week 1 and Week 2, participants in the viloxazine ER group received 200 mg (1 viloxazine ER capsule and 1 placebo capsule) and 400 mg (2 viloxazine ER capsules) of the medication, respectively. Two placebo pills were administered to those in the placebo group. From Week 3 to Week 6, the dose could be titrated or tapered at the investigator’s discretion. Compliance was assessed by comparing the number of pills dispensed vs returned.
- The primary outcome was a change in AISRS score from baselines to Week 6.
- The key secondary outcome was the change in CGI-S score from baseline to Week 6. Scores on the AISRS inattention and hyperactive/impulsivity subscales, Behavioral Regulation Index, Metacognition Index, Behavior Rating Inventory of Executive Function–Adult Version (BRIEF-A), and Generalized Anxiety Disorder-7 item scale (GAD-7) were also evaluated. Also, the rates of 30% and 50% responders on the AISRS (defined as ≥30% or ≥50% reduction from baseline in AISRS total score, respectively), CGI-S scores, and CGI-Improvement (CGI-I) scores were examined.
Outcomes
- Based on change in AISRS total scores, patients who received viloxazine ER had significantly greater improvement in their ADHD symptoms than those taking placebo (P = .0040). Patients in the viloxazine ER group had significantly greater improvement in AISRS hyperactive/impulsive (P = .0380) and inattentive symptoms (P = .0015).
- The decrease in CGI-S score was also significantly greater in the viloxazine ER group than in the placebo group (P = .0023). The viloxazine ER group also had significantly greater improvement in executive function as assessed by the BRIEF-A (P = .0468). The difference in GAD-7 scores between the viloxazine ER group and the placebo group was not significant.
- The viloxazine ER group had a greater AISRS 30% response rate than the placebo group (P = .0395). There were no significant differences between groups in AISRS 50% responder rate or CGI-I responder rate.
- Adverse effects related to viloxazine and occurring in ≥5% of participants included insomnia (14.8%), fatigue (11.6%), nausea, decreased appetite (10.1%), dry mouth (9.0%), and headache (9.0%). The discontinuation rate was 9.0% in the viloxazine ER group vs 4.9% in the placebo group.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, patients treated with viloxazine ER had significantly greater improvements in ADHD symptoms, including both hyperactive/impulsive and inattentive components as well as executive function.
- The viloxazine ER group had a significantly higher AISRS 30% response rate than the placebo group, but there were no significant differences in anxiety symptoms or other measures of response.
- Viloxazine ER was well tolerated and safe.
- Limitations: There was a reduced power to detect differences in treatment due to participants dropping out or discontinuing treatment, a lack of interrater reliability data, and a lack of patient-reported outcome or satisfaction data.
3. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
Kis et al11 analyzed the safety results of COMPAS.15 Details of this trial, including interventions and inclusion/exclusion criteria, are described in the description of Lam et al.9
Study design
- Researchers compared the rate of adverse events (AEs) among 205 patients who received ≥1 dose of methylphenidate with 209 patients who received placebo.
- AEs were documented and analyzed on an “as received” basis during Week 0 to Week 52. Electrocardiogram (ECG) data were recorded at baseline and Week 24. Vital signs were monitored at baseline, every week for the first 12 weeks, then every 4 weeks for the next 52 weeks. Body weight was assessed at Week 6, Week 12, Week 20, Week 28, Week 40, and Week 52. A 12-lead ECG was obtained at baseline and Week 24.
- The sample size was assessed to have 80% power to detect group differences in AEs.
Outcomes
- Overall, 96% of participants in the methylphenidate group and 88% of participants in the placebo group experienced at least 1 AE (difference 8.1%; 95% CI, 2.9% to 13.5%).
- AEs that occurred more frequently with methylphenidate compared to placebo were decreased appetite (22% vs 3.8%); dry mouth (15% vs 4.8%); palpitations (13% vs 3.3%); gastrointestinal (GI) infection (11% vs 4.8%); agitation (11% vs 3.3%); restlessness (10% vs 2.9%); hyperhidrosis, tachycardia, and weight decrease (all 6.3% vs 1.9%); depressive symptoms and influenza (both 4.9% vs 1.0%); and acute tonsillitis (4.4% vs 0.5%). Serious AEs were reported by 7.3% of patients in the methylphenidate group and 4.3% of those in the placebo group, with no difference in frequency (difference 3.0%; 95% CI, 1.6% to 7.9%). The most severe AEs were aggression, depression, somnambulism, and suicidal ideation in the methylphenidate group and car accidents, epicondylitis, and a fall in the placebo group.
- There were no significant differences in AEs between the GPT and CM groups.
- The treatment combinations that included methylphenidate had higher rates of patients experiencing at least 1 AE (CM/methylphenidate 97%, GPT/methylphenidate 96%, CM/placebo 92%, GPT/placebo 84%).
- Overall, 8.8% of patients in the methylphenidate group and 4.8% in the placebo group stopped their medication treatment because of an AE (difference 4.0%; 95% CI, 0.9% to 9.1%). At least 1 dose decrease, increase, or discontinuation was made after an AE in 42% of participants in the placebo group and 69% of those in the methylphenidate group.
- There were no significant differences in clinically pertinent ECG abnormalities between methylphenidate and placebo therapy.
Continue to: Conclusions/limitations
Conclusions/limitations
- AEs were more common in the methylphenidate groups compared to placebo, but there was no significant differences for severe AEs. In the long-term, methylphenidate treatment was well tolerated and relatively safe.
- Limitations: The sample size may have been too small to detect uncommon AEs, all AEs had to be reported and may not have been caused by the treatment, and the original study’s main outcome was efficacy, not safety, which makes this an exploratory analysis of AEs.
4. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
Once-daily dosing of stimulants, which are commonly used to manage adult ADHD,16 can be beneficial because many patients have schedules that limit taking medication multiple times a day. Cutler et al12 looked at the efficacy and safety of amphetamine extended-release tablet (AMPH ER TAB), which is a 3.2:1 mixture of d- and l-amphetamine released by the LiquiXR drug delivery system. This technology allows for a continuous release following an initial quick onset of action.
Study design
- This parallel-study, double-blind study evaluated adults age 18 to 60 who had a diagnosis of ADHD according to DSM-5 criteria and the Adult ADHD Clinical Diagnostic Scale, normal-range IQ, AISRS score ≥26, and baseline CGI-S score ≥4.
- Women were not lactating or pregnant during the study.
- Exclusion criteria included a history of mental illnesses; chronic medical conditions; clinically significant abnormal ECG or cardiac findings on exam; renal or liver disease; family history of sudden death; significant vital sign findings; uncontrolled hypertension or a resting systolic blood pressure (SBP) >140 mmHg or diastolic blood pressure (DBP) >90 mmHg; recent history of or current alcohol or substance use disorder; use of atomoxetine, monoamine oxidase inhibitors, or tricyclic antidepressants within 14 days of study or the use of other stimulant medications within 1 week of screening; use of GI acidifying agents or urinary acidifying agents within 3 days of screening; answering “yes” to questions 4 or 5 of the Suicidal Ideation section of the Columbia Suicide Severity Rating Scale within 2 years prior to the study; taking another investigational medication within 30 days of screening; allergic to amphetamine or components of the study drug, and a lack of prior response to amphetamine.
- Patients were randomized to receive AMPH ER TAB (n = 65) or placebo (n = 65), taken before 10
am . Participants started at 5 mg/d of the drug/placebo and then entered a 5-week titration period in which the medication was increased by 5 mg/d each week until reaching 20 mg/d, and then continued 20 mg/d for 2 weeks. - The primary outcome was the mean Permanent Product Measure of Performance Total (PERMP-T) score averaged across all time points (0.5-, 1-, 2-, 4-, 8-, 10-, 12-, 13-, and 14-hours postdose) at Visit 5.
- Participants underwent AISRS, CGI-S, and safety evaluations at baseline and at the 5 visits at the end of each treatment week.
Outcomes
- Analyses were completed on participants who received ≥1 dose of the medication and who had ≥1 PERMP-T score at Visit 5.
- Predose PERMP-T scores were similar between the AMPH ER TAB group (259.5) and placebo group (260). The mean postdose PERMP-T score in the AMPH ER TAB group (302.8) was significantly higher (P = .0043) than the placebo group (279.6).
- The PERMP-T scores were significantly different at 0.5-, 1-, 2-, 4-, 8-, and 13-hours postdose but not at 10-, 12-, and 14-hours postdose. The first Visit 5 time point at which the difference between groups was statistically different was at 0.5 hours postdose (P = .01), and the last significant time point was 13 hours (P = .006).
- The improvement in CGI-S scores was significantly greater in the AMPH ER TAB group than the placebo group. The improvement in AISRS scores was significantly greater in the AMPH ER TAB group at Visit 3, Visit 4, and Visit 5. More participants in the AMPH ER TAB group had AEs compared to the placebo group (90% vs 60%). The most common AEs (frequency ≥5% and occurring more in the intervention arm) were decreased appetite, insomnia, dry mouth, irritability, headache, anxiety, nausea, dizziness, and tachycardia.
- The AMPH ER TAB group had nonclinically significant increases in SBP (116.8 to 120.7 mmHg), DBP (74.1 to 77.1 mmHg), and heart rate (73.0 to 81.9 bpm) at Visit 5 compared to baseline.
- No serious AEs occurred. Three participants in the AMPH ER TAB group experienced AEs (increased blood pressure, CNS stimulation, and anxiety) that led them to discontinue the study.
Continue to: Conclusions/limitations
Conclusions/limitations
- AMPH ER TAB reduced symptoms in adults with ADHD as assessed by improvement in PERMP-T scores.
- The safety and tolerability profile of AMPH ER TAB were comparable to other stimulants, with expected rises in blood pressure and heart rate.
- Limitations: Patients were required to be titrated to 20 mg/d of AMPH ER TAB, instead of following a flexible titration based on an individual’s response. Some participants may have had greater improvement at a higher or lower dose. This study did not compare AMPH ER TAB to other stimulants. The 5-week duration of this study limited the ability to evaluate long-term efficacy and tolerability. Patients with a wide range of psychiatric or medical comorbidities were excluded.
5. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
Guanfacine extended-release (GXR) is a selective alpha 2A-adrenergic receptor agonist approved for treating ADHD in children and adolescents.17 Iwanami et al13 evaluated the efficacy and safety of GXR for adults.
Study design
- This randomized, double-blinded, placebo-controlled trial enrolled Japanese adults age ≥18 who were diagnosed with ADHD according to DSM-5 criteria and scored ≥24 on the ADHD-Rating Scale IV (ADHD-RS-IV) and ≥4 on CGI- I.
- Exclusion criteria included having anxiety, depression, substance use disorder, tic disorder, BD, personality disorder, schizophrenia, or intellectual disability; a moderate or severe psychiatric disorder requiring treatment other than counseling; seizures; increased risk for suicide; a history of cardiovascular disease, including prolonged QTc/abnormal ECG/abnormal labs, orthostatic hypotension, or continuous bradycardia; or taking medications that affect blood pressure or heart rate.
- Overall, 101 participants were randomized to the GXR group and 100 to the placebo group. Approximately two-thirds of the study population was male. Patients received GXR or placebo once daily at approximately the same time.
- There were 5 phases to the trial. The screening period occurred over 1 to 4 weeks. Part 1 of the treatment period consisted of 5 weeks of medication optimization. Participants were started on GXR 2 mg/d and were required to be receiving a minimum dose of 4 mg/d starting at Week 3. Clinicians were allowed to increase the dose 1 mg/d per week starting at Week 4 based on clinical response to a maximum dosage of 6 mg/d. Part 2 of the treatment period consisted of 5 weeks of maintenance at 4 to 6 mg/d. The tapering period to 2 mg/d occurred over 2 weeks. The follow-up period lasted 1 week.
- Efficacy measurements included the Japanese version of the ADHD-RS-IV and translations of the English-language CAARS, CGI-I, and CGI-S. Participant-reported measures included the Patient Global Impression-Improvement scale (PGI-I), Adult ADHD Quality of Life Questionnaire (AAQoL), and BRIEF-A.
- The primary outcome was the difference in ADHD-RS-IV total score from baseline to the end of the maintenance period (Week 10).
- Safety assessments were completed at Week 5 (end of dose optimization period), Week 10 (end of dose maintenance period), and Week 12 (tapering period).
Outcomes
- The average GXR dose during the maintenance period was 5.07 mg/d.
- Compared to the placebo group, the GXR group had more patients age <30 (47% vs 39%) and fewer patients age ≥40 (17% vs 27%). Baseline ADHD-RS-IV scores in both groups were comparable. At baseline, 51% in the GXR group had a combined inattentive/hyperactive-impulsive presentation and 47% had a predominately inattention presentation, with similar characteristics in the placebo group (49% combined, 49% inattention).
- At Week 10, the least squares mean change from baseline on the ADHD-RS-IV total score was significantly greater in the GXR group than in the placebo group (-11.55 ± 1.10 vs -7.27 ± 1.07; P = .0005), with an effect size of 0.52. There was a greater decrease in the ADHD-RS-IV scores starting at Week 4 and continuing to Week 10 (P < .005).
- There were also significant differences favoring GXR on the ADHD-RS-IV hyperactivity-impulsivity subscale score (P = .0021) and ADHD-RS-IV inattention subscale score (P = .0032).
- There were significant differences in the CAARS total ADHD score (P = .0029) and BRIEF-A scores on the inhibit (P = .0173), initiate (P = .0406), plan/organize (P = .174), and global executive composite index (P = .0404) scales. There was no significant difference in the total AAQoL score (P = .0691), but there was a significant improvement in the AAQoL life productivity subscore (P = .0072).
- At Week 10, there were also significant improvements in the CGI-I scores (P = .0007) and PGI-I scores (P = .0283). The CGI-S scores were similar at all time points.
- Overall, 81.2% of GXR patients reported AEs compared to 62% in the placebo group. There was 1 serious treatment-emergent AE (a suicide attempt) that the authors concluded was unrelated to the study drug. No deaths occurred. The most common AEs (incidence ≥10% in either group) included somnolence, thirst, nasopharyngitis (occurring more in the placebo group), blood pressure decrease, postural dizziness, and constipation. The main AEs leading to discontinuation were somnolence and blood pressure decrease. Overall, 19.8% of patients receiving GXR discontinued treatment due to AEs, compared to 3% in the placebo group.
- Heart rate, blood pressure, and QTc (corrected by the Bazett formula) were decreased in the GXR group at Week 10 while QT and RR intervals increased, and most returned to normal by Week 12.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, GXR monotherapy resulted in clinical improvement in ADHD symptoms, with a moderate effect size.
- The most common AEs were mild to moderate and congruent with known adverse effects of guanfacine. Sedation effects mostly transpired within the first week of medication administration and were transient.
- Limitations: The findings might not be generalizable to non-Japanese patients. The duration of the study was short. Patients with a wide range of psychiatric and medical comorbidities were excluded. Two-thirds of the participants were male, and there was a disparity in participant age in the GXR and placebo groups.
6. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
While stimulants are a mainstay ADHD treatment, some patients have a partial response or do not respond to amphetamines or methylphenidate. Reimherr et el14 assessed the efficacy and safety of adding brexpiprazole (BXP) to a stimulant.
Study design
- This randomized, double-blinded, placebo-controlled trial recruited 559 stimulant-naive patients and 174 patients who had not responded to previous stimulant therapy.
- Participants were adults age 18 to 55 with a primary diagnosis of ADHD according to DSM-IV-TR criteria and the Conners Adult ADHD Diagnostic Interview. Other inclusion criteria were having a CAARS score ≥29 and a CGI-S score ≥4.
- Exclusion criteria included being at risk for suicide; having current substance abuse or positive alcohol/drug screens; a history of good response to prestudy treatment; a clinically significant medical condition; fasting blood glucose >200 mg/dL or hemoglobin A1C >7%; and hospitalization in past 12 months from a diabetic complication, uncontrolled hypertension, ischemic heart disease, or epilepsy. Further exclusion criteria included a history of psychosis, current MDD or BD, current panic disorder, uncontrolled comorbid psychiatric condition, or clinically significant personality disorder. Investigators excluded any patient with severe DSM-IV axis I or II disorders or abnormal/psychopathological behaviors.
- The trial consisted of 3 segments. Part 1 was screening. If the patient was currently receiving a stimulant but not fully responding, the medication was discontinued for at least 5 half-lives.
- Part 2 (5 weeks) involved administering a stimulant plus a single-blind placebo (597 patients completed this phase). The stimulant was chosen by the investigator, who had the option of using 1 of 2 amphetamine derivatives (mixed amphetamine salts capsules or lisdexamfetamine dimesylate capsules) or 1 of 2 methylphenidate derivatives (methylphenidate hydrochloride ER tabs or dexmethylphenidate HCl ER capsules). If a patient did not respond to a particular stimulant prior to the study, they were given a different stimulant from the list. Patients continued the same stimulant throughout the trial. Patients were monitored for a response, defined as a ≥30% decrease in CAARS score or a CAARS score <24, or a CGI-I score of 1 or 2 at Week 5. Patients who did not show this improvement were categorized as open-label nonresponders.
- Part 3 (6 weeks) involved administering a stimulant plus double-blind BXP vs placebo (stimulant-naive n = 167, stimulant nonresponders n = 68). Nonresponders continued the stimulant (at the same dose reached at the end of Part 2) and added either BXP (n = 155) or continued placebo (n = 80). Patients who responded in Part 2 were continued on the stimulant plus placebo and were not randomized. Patients were started on BXP 0.25 mg/d, and the medication could be titrated to 2 mg/d during the following 3 weeks, depending on the benefit vs AE profile. After the third week, the dose could be decreased but not increased.
- The primary outcome was a change in CAARS score. Secondary measurements included the CGI-S, Wender-Reimherr Adult Attention Deficit Disorder Scale (WRAADDS), Montgomery-Åsberg Depression Rating Scale (MADRS), and BDI.
Outcomes
- Stimulant-naive patients were equally divided among the 4 stimulant groups, and previous nonresponders who continued to not respond in Part 2 were more likely to be given methylphenidate HCl or lisdexamfetamine dimesylate.
- Patients with a history of nonresponse had less response to stimulants in Part 2 compared to stimulant-naive patients, as seen by 27% (n = 167) of stimulant-naive patients entering Part 3 compared to 39% of prior nonresponders (n = 68; P = .0249).
- ADHD improvement with BXP appeared to be greater among pretrial nonresponders.
- For stimulant nonresponders before and during the study, at the end of the double-blind endpoint (Part 3; Week 11), WRAADDS total score was significantly improved in the BXP group compared to the placebo group (P = .013; d = 0.74), with most beneficial effects seen in the hyperactivity/restlessness, emotional dysregulation factor, and impulsivity categories.
- For stimulant nonresponders before and during the study, there was no significant difference at the end of Week 11 on the CAARS (P = .64), MADRS (P = .37), or BDI (P = .73). There was a trend toward significance on the CAARS subscale for hyperactive/impulsive (P = .09).
- For prestudy stimulant-naive patients who did not respond to stimulants in Part 2 and were randomized in Part 3, there was not a significant difference between BXP and placebo at Week 11 as assessed on WRAADDS, CAARS, MADRS, or BDI.
- As assessed on WRAADDS, 50% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.334). Under the emotional dysregulation factor category of the WRAADDS, 64% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.064). The attention factor category showed a 40% improvement in the BXP group compared to 32% in the placebo group (Fisher exact = 0.344).
- There were 2 serious AEs in the BXP group (gall bladder inflammation and diarrhea) and 2 in the placebo group (pneumonia and urinary tract infection). There was no statistically significant difference between groups with regards to common AEs (ie, fatigue, heartburn/nausea/stomachache, weight loss), although there was a trend to significant for insomnia in the BXP group (P = .083).
Conclusions/limitations
- Stimulant-naive patients experienced no improvement with adjunctive BXP.
- For prior stimulant nonresponders, there was no significant difference between BXP vs placebo on the primary outcome of the CAARS score, but there was an improvement as observed by assessment with the WRAADDS.
- The largest change in the WRAADDS occurred in the emotional dysregulation factor compared to the attention factor.
- BXP appeared to be well tolerated.
- Limitations: The WRAADDS was administered without the patients’ significant other/collateral. Raters were not trained in the use of the WRAADDS. Patients with a wide range of psychiatric and medical comorbidities were excluded. Fewer patients were recruited in the prior stimulant nonresponder group.
Bottom Line
Recent randomized controlled trials suggest that methylphenidate, amphetamine extended-release, viloxazine extended-release, and guanfacine extended-release improved symptoms of adult attention-deficit/hyperactivity disorder (ADHD). There were no improvements in ADHD symptoms with adjunctive brexpiprazole.
Related Resources
- Parikh AR, Baker SA. Adult ADHD: pharmacologic treatment in the DSM-5 era. Current Psychiatry. 2016;15(10):18-25.
- Akbar HN. Why we should be scrutinizing the rising prevalence of adult ADHD. Current Psychiatry. 2022; 21(7):e1-e2. doi:10.12788/cp.0268
Drug Brand Names
Amantadine • Gocovri
Amphetamine extended-release tablet • Dyanavel XR
Atomoxetine • Strattera
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Dexmethylphenidate • Focalin
Fluoxetine • Prozac
Guanfacine extended- release • Intuniv
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin
Theophylline • Elixophyllin
Viloxazine • Qelbree
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Harpin V, Mazzone L, Raynaud JP, et al. Long-term outcomes of ADHD: a systematic review of self-esteem and social function. J Atten Disord. 2016;20(4):295-305. doi:10.1177/1087054713486516
3. Beaton DM, Sirois F, Milne E. Experiences of criticism in adults with ADHD: a qualitative study. PLoS One. 2022;17(2):e0263366. doi:10.1371/journal.pone.0263366
4. Attention-deficit/hyperactivity disorder (ADHD). National Institute of Mental Health. Accessed February 9, 2023. https://www.nimh.nih.gov/health/statistics/attention-deficit-hyperactivity-disorder-adhd
5. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
6. Attention Deficit Hyperactivity Disorder: Diagnosis and Management. NICE Guideline No. 87. National Institute for Health and Care Excellence (NICE); 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
7. Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgrad Med. 2010;122(1):184-191. doi:10.3810/pgm.2010.01.2112
8. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
9. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
10. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of viloxazine extended-release capsules in adults with attention-deficit/hyperactivity disorder. CNS Drugs. 2022;36(8):897-915. doi:10.1007/s40263-022-00938-w
11. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
12. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
13. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
14. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
15. Philipsen A, Jans T, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Effects of group psychotherapy, individual counseling, methylphenidate, and placebo in the treatment of adult attention-deficit/hyperactivity disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(12):1199-1210.
16. McGough JJ. Treatment controversies in adult ADHD. Am J Psychiatry. 2016;173(10):960-966. doi:10.1176/appi.ajp.2016.15091207
17. Cruz MP. Guanfacine extended-release tablets (Intuniv), a nonstimulant selective alpha2a-adrenergic receptor agonist for attention-deficit/hyperactivity disorder. P T. 2010;35(8):448-451.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Harpin V, Mazzone L, Raynaud JP, et al. Long-term outcomes of ADHD: a systematic review of self-esteem and social function. J Atten Disord. 2016;20(4):295-305. doi:10.1177/1087054713486516
3. Beaton DM, Sirois F, Milne E. Experiences of criticism in adults with ADHD: a qualitative study. PLoS One. 2022;17(2):e0263366. doi:10.1371/journal.pone.0263366
4. Attention-deficit/hyperactivity disorder (ADHD). National Institute of Mental Health. Accessed February 9, 2023. https://www.nimh.nih.gov/health/statistics/attention-deficit-hyperactivity-disorder-adhd
5. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
6. Attention Deficit Hyperactivity Disorder: Diagnosis and Management. NICE Guideline No. 87. National Institute for Health and Care Excellence (NICE); 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
7. Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgrad Med. 2010;122(1):184-191. doi:10.3810/pgm.2010.01.2112
8. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
9. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
10. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of viloxazine extended-release capsules in adults with attention-deficit/hyperactivity disorder. CNS Drugs. 2022;36(8):897-915. doi:10.1007/s40263-022-00938-w
11. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
12. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
13. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
14. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
15. Philipsen A, Jans T, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Effects of group psychotherapy, individual counseling, methylphenidate, and placebo in the treatment of adult attention-deficit/hyperactivity disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(12):1199-1210.
16. McGough JJ. Treatment controversies in adult ADHD. Am J Psychiatry. 2016;173(10):960-966. doi:10.1176/appi.ajp.2016.15091207
17. Cruz MP. Guanfacine extended-release tablets (Intuniv), a nonstimulant selective alpha2a-adrenergic receptor agonist for attention-deficit/hyperactivity disorder. P T. 2010;35(8):448-451.
Clonidine: Off-label uses in pediatric patients
Clonidine is a centrally acting alpha-2 agonist originally developed for treating hypertension. It is believed to work by stimulating alpha-2 receptors in various areas of the brain. It is nonselective, binding alpha-2A, -2B, and -2C receptors, and mediates inattentiveness, hyperactivity, impulsivity, sedation, and hypotension.1 Clonidine is available as immediate-release (IR), extended-release, and patch formulations, with typical doses ranging from 0.1 to 0.4 mg/d. The most common adverse effects are anticholinergic, such as sedation, dry mouth, and constipation. Since clonidine is effective at lowering blood pressure, the main safety concern is the possibility of rebound hypertension if abruptly stopped, which necessitates a short taper period.1
In child and adolescent psychiatry, the only FDA-approved use of clonidine is for treating attention-deficit/hyperactivity disorder (ADHD). Yet this medication has been increasingly used off-label for several common psychiatric ailments in pediatric patients. In this article, we discuss potential uses of clonidine in child and adolescent psychiatry; except for ADHD, all uses we describe are off-label.
ADHD. Clonidine is effective both as a monotherapy and as an adjunctive therapy to stimulants for pediatric ADHD. When used alone, clonidine is better suited for patients who have hyperactivity as their primary concern, whereas stimulants may be better suited for patients with inattentive subtypes. It also can help reduce sleep disturbances associated with the use of stimulants, especially insomnia.1
Tics/Tourette syndrome. Clonidine is a first-line treatment for tics in Tourette syndrome, demonstrating high efficacy with limited or no adverse effects. Furthermore, ADHD is the most common comorbid condition in patients with dystonic tics, which makes clonidine useful for simultaneously treating both conditions.2
Insomnia. Currently, there are no FDA-approved medications for treating sleep disorders in children and adolescents. However, clonidine is among the most used medications for childhood sleep difficulties, second only to antihistamines. The IR formulation is often preferred for this indication due to increased sedation.3
Posttraumatic stress disorder (PTSD). Research has shown clonidine can help reduce hyperarousal symptoms, address sleep difficulties, and reduce PTSD trauma nightmares, anxiety, and irritability.4
Substance detoxification. Clonidine successfully suppresses opiate withdrawal signs and symptoms by reducing sympathetic overactivity. It can help with alcohol withdrawal and smoking cessation.2
Antipsychotic-induced akathisia. Controlled trials have shown that clonidine significantly reduces akathisia associated with the use of antipsychotics.2
Sialorrhea. Due to its anticholinergic effects, clonidine can effectively reduce antipsychotic-induced hypersalivation.2
Behavioral disturbances. Due to its sedative and anti-impulsive properties, clonidine can be used to address broadly defined behavioral issues, including anxiety-related behaviors, aggression, and agitation, although there is a lack of proven efficacy.1,2,4
1. Stahl SM, Grady MM, Muntner N. Stahl’s Essential Psychopharmacology: Prescriber’s Guide: Children and Adolescents. Cambridge University Press; 2019.
2. Naguy A. Clonidine use in psychiatry: panacea or panache. Pharmacology. 2016;98(1-2):87-92. doi:10.1159/000446441
3. Jang YJ, Choi H, Han TS, et al. Effectiveness of clonidine in child and adolescent sleep disorders. Psychiatry Investig. 2022;19(9):738-747. doi:10.30773/pi.2022.0117
4. Bajor LA, Balsara C, Osser DN. An evidence-based approach to psychopharmacology for posttraumatic stress disorder (PTSD) - 2022 update. Psychiatry Res. 2022;317:114840. doi:10.1016/j.psychres.2022.114840
Clonidine is a centrally acting alpha-2 agonist originally developed for treating hypertension. It is believed to work by stimulating alpha-2 receptors in various areas of the brain. It is nonselective, binding alpha-2A, -2B, and -2C receptors, and mediates inattentiveness, hyperactivity, impulsivity, sedation, and hypotension.1 Clonidine is available as immediate-release (IR), extended-release, and patch formulations, with typical doses ranging from 0.1 to 0.4 mg/d. The most common adverse effects are anticholinergic, such as sedation, dry mouth, and constipation. Since clonidine is effective at lowering blood pressure, the main safety concern is the possibility of rebound hypertension if abruptly stopped, which necessitates a short taper period.1
In child and adolescent psychiatry, the only FDA-approved use of clonidine is for treating attention-deficit/hyperactivity disorder (ADHD). Yet this medication has been increasingly used off-label for several common psychiatric ailments in pediatric patients. In this article, we discuss potential uses of clonidine in child and adolescent psychiatry; except for ADHD, all uses we describe are off-label.
ADHD. Clonidine is effective both as a monotherapy and as an adjunctive therapy to stimulants for pediatric ADHD. When used alone, clonidine is better suited for patients who have hyperactivity as their primary concern, whereas stimulants may be better suited for patients with inattentive subtypes. It also can help reduce sleep disturbances associated with the use of stimulants, especially insomnia.1
Tics/Tourette syndrome. Clonidine is a first-line treatment for tics in Tourette syndrome, demonstrating high efficacy with limited or no adverse effects. Furthermore, ADHD is the most common comorbid condition in patients with dystonic tics, which makes clonidine useful for simultaneously treating both conditions.2
Insomnia. Currently, there are no FDA-approved medications for treating sleep disorders in children and adolescents. However, clonidine is among the most used medications for childhood sleep difficulties, second only to antihistamines. The IR formulation is often preferred for this indication due to increased sedation.3
Posttraumatic stress disorder (PTSD). Research has shown clonidine can help reduce hyperarousal symptoms, address sleep difficulties, and reduce PTSD trauma nightmares, anxiety, and irritability.4
Substance detoxification. Clonidine successfully suppresses opiate withdrawal signs and symptoms by reducing sympathetic overactivity. It can help with alcohol withdrawal and smoking cessation.2
Antipsychotic-induced akathisia. Controlled trials have shown that clonidine significantly reduces akathisia associated with the use of antipsychotics.2
Sialorrhea. Due to its anticholinergic effects, clonidine can effectively reduce antipsychotic-induced hypersalivation.2
Behavioral disturbances. Due to its sedative and anti-impulsive properties, clonidine can be used to address broadly defined behavioral issues, including anxiety-related behaviors, aggression, and agitation, although there is a lack of proven efficacy.1,2,4
Clonidine is a centrally acting alpha-2 agonist originally developed for treating hypertension. It is believed to work by stimulating alpha-2 receptors in various areas of the brain. It is nonselective, binding alpha-2A, -2B, and -2C receptors, and mediates inattentiveness, hyperactivity, impulsivity, sedation, and hypotension.1 Clonidine is available as immediate-release (IR), extended-release, and patch formulations, with typical doses ranging from 0.1 to 0.4 mg/d. The most common adverse effects are anticholinergic, such as sedation, dry mouth, and constipation. Since clonidine is effective at lowering blood pressure, the main safety concern is the possibility of rebound hypertension if abruptly stopped, which necessitates a short taper period.1
In child and adolescent psychiatry, the only FDA-approved use of clonidine is for treating attention-deficit/hyperactivity disorder (ADHD). Yet this medication has been increasingly used off-label for several common psychiatric ailments in pediatric patients. In this article, we discuss potential uses of clonidine in child and adolescent psychiatry; except for ADHD, all uses we describe are off-label.
ADHD. Clonidine is effective both as a monotherapy and as an adjunctive therapy to stimulants for pediatric ADHD. When used alone, clonidine is better suited for patients who have hyperactivity as their primary concern, whereas stimulants may be better suited for patients with inattentive subtypes. It also can help reduce sleep disturbances associated with the use of stimulants, especially insomnia.1
Tics/Tourette syndrome. Clonidine is a first-line treatment for tics in Tourette syndrome, demonstrating high efficacy with limited or no adverse effects. Furthermore, ADHD is the most common comorbid condition in patients with dystonic tics, which makes clonidine useful for simultaneously treating both conditions.2
Insomnia. Currently, there are no FDA-approved medications for treating sleep disorders in children and adolescents. However, clonidine is among the most used medications for childhood sleep difficulties, second only to antihistamines. The IR formulation is often preferred for this indication due to increased sedation.3
Posttraumatic stress disorder (PTSD). Research has shown clonidine can help reduce hyperarousal symptoms, address sleep difficulties, and reduce PTSD trauma nightmares, anxiety, and irritability.4
Substance detoxification. Clonidine successfully suppresses opiate withdrawal signs and symptoms by reducing sympathetic overactivity. It can help with alcohol withdrawal and smoking cessation.2
Antipsychotic-induced akathisia. Controlled trials have shown that clonidine significantly reduces akathisia associated with the use of antipsychotics.2
Sialorrhea. Due to its anticholinergic effects, clonidine can effectively reduce antipsychotic-induced hypersalivation.2
Behavioral disturbances. Due to its sedative and anti-impulsive properties, clonidine can be used to address broadly defined behavioral issues, including anxiety-related behaviors, aggression, and agitation, although there is a lack of proven efficacy.1,2,4
1. Stahl SM, Grady MM, Muntner N. Stahl’s Essential Psychopharmacology: Prescriber’s Guide: Children and Adolescents. Cambridge University Press; 2019.
2. Naguy A. Clonidine use in psychiatry: panacea or panache. Pharmacology. 2016;98(1-2):87-92. doi:10.1159/000446441
3. Jang YJ, Choi H, Han TS, et al. Effectiveness of clonidine in child and adolescent sleep disorders. Psychiatry Investig. 2022;19(9):738-747. doi:10.30773/pi.2022.0117
4. Bajor LA, Balsara C, Osser DN. An evidence-based approach to psychopharmacology for posttraumatic stress disorder (PTSD) - 2022 update. Psychiatry Res. 2022;317:114840. doi:10.1016/j.psychres.2022.114840
1. Stahl SM, Grady MM, Muntner N. Stahl’s Essential Psychopharmacology: Prescriber’s Guide: Children and Adolescents. Cambridge University Press; 2019.
2. Naguy A. Clonidine use in psychiatry: panacea or panache. Pharmacology. 2016;98(1-2):87-92. doi:10.1159/000446441
3. Jang YJ, Choi H, Han TS, et al. Effectiveness of clonidine in child and adolescent sleep disorders. Psychiatry Investig. 2022;19(9):738-747. doi:10.30773/pi.2022.0117
4. Bajor LA, Balsara C, Osser DN. An evidence-based approach to psychopharmacology for posttraumatic stress disorder (PTSD) - 2022 update. Psychiatry Res. 2022;317:114840. doi:10.1016/j.psychres.2022.114840
Once-daily stimulant for ADHD safe, effective at 1 year
A once-daily oral stimulant medication for treatment of attention-deficit/hyperactivity disorder in individuals aged 6 years or older is safe and effective after 1 year of treatment, new research shows.
Results from a phase 3, multicenter dose optimization, open-label safety study of Azstarys (KemPharm) found that most treatment-emergent adverse events (TEAEs) were mild to moderate.
“This data show that Azstarys remains safe and effective for the treatment of ADHD when given for up to a year,” lead investigator Ann Childress, MD, president of the Center for Psychiatry and Behavioral Medicine, Las Vegas, said in an interview.
The study was published online in the Journal of Child and Adolescent Psychopharmacology.
Safety at 1 year
The drug is a combination of extended-release serdexmethylphenidate (SDX), KemPharm’s prodrug of dexmethylphenidate (d-MPH), co-formulated with immediate-release d-MPH.
SDX is converted to d-MPH after it is absorbed in the gastrointestinal tract. The d-MPH is released gradually throughout the day, providing quick symptom control with the d-MPH and extended control with SDX.
As reported by this news organization, Azstarys was approved by the U.S. Food and Drug Administration in 2021 on the basis of results from a laboratory classroom phase 3 trial, which showed significant improvement in ADHD symptoms, compared with placebo.
For this study, the second phase 3 trial of Azstarys, investigators analyzed data from 282 children aged 6-12 years in the United States, including 70 who participated in an earlier 1-month efficacy trial as well.
After screening and a 3-week dose-optimization phase for new participants, patients received once-daily treatment with doses of 26.1 mg/5.2 mg, 39.2 mg/7.8 mg, or 52.3 mg/10.4 mg of SDX/d-MPH.
After 1 year of treatment, 60.1% of participants reported at least one TEAE, the majority of which were moderate. Twelve patients reported severe TEAEs. Six children (2.5%) discontinued the study due to a TEAE during the treatment phase.
Investigators also measured growth and changes in sleep with the Children’s Sleep Habits Questionnaire during the 12-month study. Sleep improved on most measures and the impact on growth was mild.
There were no life-threatening TEAEs and no deaths reported during the study.
The most common TEAEs during the treatment phase were decreased appetite, upper respiratory tract infection, nasopharyngitis, decreased weight, irritability, and increased weight.
Efficacy at 1 year
ADHD symptoms improved considerably after 1 month of treatment, with responses continuing at 1 year.
At baseline, participants’ mean ADHD Rating Scale-5 (ADHD-RS-5) score was 41.5. After 1 month of treatment, scores averaged 16.1, a decline of –25.3 (P < .001).
The mean score stabilized in the 12-15 range for the remainder of the study. After 1 year of treatment, ADHD symptoms had decreased approximately 70% from baseline.
Investigators found similar results in clinical severity. After 1 month of treatment, the average Clinical Global Impressions–Severity (CGI-S) scale score was 2.5, a decline of –2.2 (P < .0001).
CGI-S scale scores remained in the 2.2-2.4 range for the remainder of the study.
These results, combined with the results of the original classroom trial, suggest Azstarys may offer advantages over other ADHD drugs, Dr. Childress said.
“In the laboratory classroom trial, subjects taking Azstarys completed significantly more math problems than subjects taking placebo beginning at 30 minutes and up to 13 hours after dosing,” Dr. Childress said. “No other methylphenidate extended-release product currently marketed in the United States has a 13-hour duration of effect.”
‘Reassuring data’
Aditya Pawar, MD, a child and adolescent psychiatrist with the Kennedy Krieger Institute and an assistant professor of psychiatry and behavioral sciences at Johns Hopkins School of Medicine, Baltimore, said that the study suggests the drug may be a valuable addition to ADHD treatment options for pediatric patients.
“The study provides reassuring data on the safety of stimulants in patients without significant history of cardiac events or blood pressure changes, which are usual concerns among patients and clinicians despite the evidence supporting safety, said Dr. Pawar, who was not part of the study.
“Additionally, the 1-year data on efficacy and safety of a new stimulant medication is valuable for clinicians looking for sustained relief for their patients, despite the limitations of an open-label trial,” she added.
Overall, the safety data reported here are fairly consistent with the safety profile of other methylphenidates used for treating ADHD, Dr. Pawar said.
However, she noted, the study does have some limitations, including its open-label design and lack of blinding. The research also excluded children with autism, disruptive mood dysregulation disorders, and other common comorbidities of ADHD, which may limit the generalizability of the results.
“These comorbidities often require stimulants as a part of treatment and yet have a higher risk of side effects,” Dr. Pawar said. “Future studies with a broader population may be needed to better understand treatment effectiveness and potential risks.”
The study was funded by KemPharm. Dr. Childress serves as consultant for Aardvark, Arbor, Attentive, Cingulate, Ironshore, Neos Therapeutics, Neurocentria, Otsuka, Purdue, Rhodes, Sunovion, Tris Pharma, KemPharm, Supernus, Jazz, Corium, Tulex, and Lumos. Full disclosures are reported in the original article.
A version of this article first appeared on Medscape.com.
A once-daily oral stimulant medication for treatment of attention-deficit/hyperactivity disorder in individuals aged 6 years or older is safe and effective after 1 year of treatment, new research shows.
Results from a phase 3, multicenter dose optimization, open-label safety study of Azstarys (KemPharm) found that most treatment-emergent adverse events (TEAEs) were mild to moderate.
“This data show that Azstarys remains safe and effective for the treatment of ADHD when given for up to a year,” lead investigator Ann Childress, MD, president of the Center for Psychiatry and Behavioral Medicine, Las Vegas, said in an interview.
The study was published online in the Journal of Child and Adolescent Psychopharmacology.
Safety at 1 year
The drug is a combination of extended-release serdexmethylphenidate (SDX), KemPharm’s prodrug of dexmethylphenidate (d-MPH), co-formulated with immediate-release d-MPH.
SDX is converted to d-MPH after it is absorbed in the gastrointestinal tract. The d-MPH is released gradually throughout the day, providing quick symptom control with the d-MPH and extended control with SDX.
As reported by this news organization, Azstarys was approved by the U.S. Food and Drug Administration in 2021 on the basis of results from a laboratory classroom phase 3 trial, which showed significant improvement in ADHD symptoms, compared with placebo.
For this study, the second phase 3 trial of Azstarys, investigators analyzed data from 282 children aged 6-12 years in the United States, including 70 who participated in an earlier 1-month efficacy trial as well.
After screening and a 3-week dose-optimization phase for new participants, patients received once-daily treatment with doses of 26.1 mg/5.2 mg, 39.2 mg/7.8 mg, or 52.3 mg/10.4 mg of SDX/d-MPH.
After 1 year of treatment, 60.1% of participants reported at least one TEAE, the majority of which were moderate. Twelve patients reported severe TEAEs. Six children (2.5%) discontinued the study due to a TEAE during the treatment phase.
Investigators also measured growth and changes in sleep with the Children’s Sleep Habits Questionnaire during the 12-month study. Sleep improved on most measures and the impact on growth was mild.
There were no life-threatening TEAEs and no deaths reported during the study.
The most common TEAEs during the treatment phase were decreased appetite, upper respiratory tract infection, nasopharyngitis, decreased weight, irritability, and increased weight.
Efficacy at 1 year
ADHD symptoms improved considerably after 1 month of treatment, with responses continuing at 1 year.
At baseline, participants’ mean ADHD Rating Scale-5 (ADHD-RS-5) score was 41.5. After 1 month of treatment, scores averaged 16.1, a decline of –25.3 (P < .001).
The mean score stabilized in the 12-15 range for the remainder of the study. After 1 year of treatment, ADHD symptoms had decreased approximately 70% from baseline.
Investigators found similar results in clinical severity. After 1 month of treatment, the average Clinical Global Impressions–Severity (CGI-S) scale score was 2.5, a decline of –2.2 (P < .0001).
CGI-S scale scores remained in the 2.2-2.4 range for the remainder of the study.
These results, combined with the results of the original classroom trial, suggest Azstarys may offer advantages over other ADHD drugs, Dr. Childress said.
“In the laboratory classroom trial, subjects taking Azstarys completed significantly more math problems than subjects taking placebo beginning at 30 minutes and up to 13 hours after dosing,” Dr. Childress said. “No other methylphenidate extended-release product currently marketed in the United States has a 13-hour duration of effect.”
‘Reassuring data’
Aditya Pawar, MD, a child and adolescent psychiatrist with the Kennedy Krieger Institute and an assistant professor of psychiatry and behavioral sciences at Johns Hopkins School of Medicine, Baltimore, said that the study suggests the drug may be a valuable addition to ADHD treatment options for pediatric patients.
“The study provides reassuring data on the safety of stimulants in patients without significant history of cardiac events or blood pressure changes, which are usual concerns among patients and clinicians despite the evidence supporting safety, said Dr. Pawar, who was not part of the study.
“Additionally, the 1-year data on efficacy and safety of a new stimulant medication is valuable for clinicians looking for sustained relief for their patients, despite the limitations of an open-label trial,” she added.
Overall, the safety data reported here are fairly consistent with the safety profile of other methylphenidates used for treating ADHD, Dr. Pawar said.
However, she noted, the study does have some limitations, including its open-label design and lack of blinding. The research also excluded children with autism, disruptive mood dysregulation disorders, and other common comorbidities of ADHD, which may limit the generalizability of the results.
“These comorbidities often require stimulants as a part of treatment and yet have a higher risk of side effects,” Dr. Pawar said. “Future studies with a broader population may be needed to better understand treatment effectiveness and potential risks.”
The study was funded by KemPharm. Dr. Childress serves as consultant for Aardvark, Arbor, Attentive, Cingulate, Ironshore, Neos Therapeutics, Neurocentria, Otsuka, Purdue, Rhodes, Sunovion, Tris Pharma, KemPharm, Supernus, Jazz, Corium, Tulex, and Lumos. Full disclosures are reported in the original article.
A version of this article first appeared on Medscape.com.
A once-daily oral stimulant medication for treatment of attention-deficit/hyperactivity disorder in individuals aged 6 years or older is safe and effective after 1 year of treatment, new research shows.
Results from a phase 3, multicenter dose optimization, open-label safety study of Azstarys (KemPharm) found that most treatment-emergent adverse events (TEAEs) were mild to moderate.
“This data show that Azstarys remains safe and effective for the treatment of ADHD when given for up to a year,” lead investigator Ann Childress, MD, president of the Center for Psychiatry and Behavioral Medicine, Las Vegas, said in an interview.
The study was published online in the Journal of Child and Adolescent Psychopharmacology.
Safety at 1 year
The drug is a combination of extended-release serdexmethylphenidate (SDX), KemPharm’s prodrug of dexmethylphenidate (d-MPH), co-formulated with immediate-release d-MPH.
SDX is converted to d-MPH after it is absorbed in the gastrointestinal tract. The d-MPH is released gradually throughout the day, providing quick symptom control with the d-MPH and extended control with SDX.
As reported by this news organization, Azstarys was approved by the U.S. Food and Drug Administration in 2021 on the basis of results from a laboratory classroom phase 3 trial, which showed significant improvement in ADHD symptoms, compared with placebo.
For this study, the second phase 3 trial of Azstarys, investigators analyzed data from 282 children aged 6-12 years in the United States, including 70 who participated in an earlier 1-month efficacy trial as well.
After screening and a 3-week dose-optimization phase for new participants, patients received once-daily treatment with doses of 26.1 mg/5.2 mg, 39.2 mg/7.8 mg, or 52.3 mg/10.4 mg of SDX/d-MPH.
After 1 year of treatment, 60.1% of participants reported at least one TEAE, the majority of which were moderate. Twelve patients reported severe TEAEs. Six children (2.5%) discontinued the study due to a TEAE during the treatment phase.
Investigators also measured growth and changes in sleep with the Children’s Sleep Habits Questionnaire during the 12-month study. Sleep improved on most measures and the impact on growth was mild.
There were no life-threatening TEAEs and no deaths reported during the study.
The most common TEAEs during the treatment phase were decreased appetite, upper respiratory tract infection, nasopharyngitis, decreased weight, irritability, and increased weight.
Efficacy at 1 year
ADHD symptoms improved considerably after 1 month of treatment, with responses continuing at 1 year.
At baseline, participants’ mean ADHD Rating Scale-5 (ADHD-RS-5) score was 41.5. After 1 month of treatment, scores averaged 16.1, a decline of –25.3 (P < .001).
The mean score stabilized in the 12-15 range for the remainder of the study. After 1 year of treatment, ADHD symptoms had decreased approximately 70% from baseline.
Investigators found similar results in clinical severity. After 1 month of treatment, the average Clinical Global Impressions–Severity (CGI-S) scale score was 2.5, a decline of –2.2 (P < .0001).
CGI-S scale scores remained in the 2.2-2.4 range for the remainder of the study.
These results, combined with the results of the original classroom trial, suggest Azstarys may offer advantages over other ADHD drugs, Dr. Childress said.
“In the laboratory classroom trial, subjects taking Azstarys completed significantly more math problems than subjects taking placebo beginning at 30 minutes and up to 13 hours after dosing,” Dr. Childress said. “No other methylphenidate extended-release product currently marketed in the United States has a 13-hour duration of effect.”
‘Reassuring data’
Aditya Pawar, MD, a child and adolescent psychiatrist with the Kennedy Krieger Institute and an assistant professor of psychiatry and behavioral sciences at Johns Hopkins School of Medicine, Baltimore, said that the study suggests the drug may be a valuable addition to ADHD treatment options for pediatric patients.
“The study provides reassuring data on the safety of stimulants in patients without significant history of cardiac events or blood pressure changes, which are usual concerns among patients and clinicians despite the evidence supporting safety, said Dr. Pawar, who was not part of the study.
“Additionally, the 1-year data on efficacy and safety of a new stimulant medication is valuable for clinicians looking for sustained relief for their patients, despite the limitations of an open-label trial,” she added.
Overall, the safety data reported here are fairly consistent with the safety profile of other methylphenidates used for treating ADHD, Dr. Pawar said.
However, she noted, the study does have some limitations, including its open-label design and lack of blinding. The research also excluded children with autism, disruptive mood dysregulation disorders, and other common comorbidities of ADHD, which may limit the generalizability of the results.
“These comorbidities often require stimulants as a part of treatment and yet have a higher risk of side effects,” Dr. Pawar said. “Future studies with a broader population may be needed to better understand treatment effectiveness and potential risks.”
The study was funded by KemPharm. Dr. Childress serves as consultant for Aardvark, Arbor, Attentive, Cingulate, Ironshore, Neos Therapeutics, Neurocentria, Otsuka, Purdue, Rhodes, Sunovion, Tris Pharma, KemPharm, Supernus, Jazz, Corium, Tulex, and Lumos. Full disclosures are reported in the original article.
A version of this article first appeared on Medscape.com.
Once-daily stimulant for ADHD safe, effective at 1 year
new research shows.
Results from a phase 3, multicenter, dose-optimization, open-label safety study of Azstarys (KemPharm) found that most treatment-emergent adverse events (TEAEs) were mild to moderate.
“This data show that Azstarys remains safe and effective for the treatment of ADHD when given for up to a year,” lead investigator Ann Childress, MD, president of the Center for Psychiatry and Behavioral Medicine, Las Vegas, said in an interview.
The study was published online in the Journal of Child and Adolescent Psychopharmacology.
Safety at 1 year
The drug is a combination of extended-release serdexmethylphenidate (SDX), KemPharm’s prodrug of dexmethylphenidate, coformulated with immediate-release d-MPH.
SDX is converted to d-MPH after it is absorbed in the gastrointestinal tract. The d-MPH is released gradually throughout the day, providing quick symptom control with the d-MPH and extended control with SDX.
Azstarys was approved by the Food and Drug Administration in 2021 on the basis of results from a laboratory classroom phase 3 trial, which showed significant improvement in ADHD symptoms, compared with placebo.
For this study, the second phase 3 trial of Azstarys, investigators analyzed data from 282 children aged 6-12 years in the United States, including 70 who participated in an earlier 1-month efficacy trial.
After screening and a 3-week dose-optimization phase for new participants, patients received once-daily treatment with doses of 26.1 mg/5.2 mg, 39.2 mg/7.8 mg, or 52.3 mg/10.4 mg of SDX/d-MPH.
After 1 year of treatment, 60.1% of participants reported at least one TEAE, the majority of which were moderate. Twelve patients reported severe TEAEs. Six children (2.5%) discontinued the study because of a TEAE during the treatment phase.
The investigators also measured growth and changes in sleep with the Children’s Sleep Habits Questionnaire during the 12-month study. Sleep improved on most measures and the impact on growth was mild.
There were no life-threatening TEAEs and no deaths reported during the study.
The most common TEAEs during the treatment phase were decreased appetite, upper respiratory tract infection, nasopharyngitis, decreased weight, irritability, and increased weight.
Efficacy at 1 year
ADHD symptoms improved considerably after 1 month of treatment, with responses continuing at 1 year.
At baseline, participants’ mean ADHD Rating Scale–5 score was 41.5. After 1 month of treatment, scores averaged 16.1, a decline of –25.3 (P < .001).
The mean score stabilized in the 12-15 range for the remainder of the study. After 1 year of treatment, ADHD symptoms had decreased approximately 70% from baseline.
Investigators found similar results in clinical severity. After 1 month of treatment, the average Clinical Global Impressions–Severity (CGI-S) scale score was 2.5, a decline of –2.2 (P < .0001).
CGI-S scale scores remained in the 2.2-2.4 range for the remainder of the study.
These results, combined with the results of the original classroom trial, suggest Azstarys may offer advantages over other ADHD drugs, Dr. Childress said.
“In the laboratory classroom trial, subjects taking Azstarys completed significantly more math problems than subjects taking placebo beginning at 30 minutes and up to 13 hours after dosing,” Dr. Childress said. “No other methylphenidate extended-release product currently marketed in the United States has a 13-hour duration of effect.”
‘Reassuring data’
Commenting on the findings, Aditya Pawar, MD, a child and adolescent psychiatrist with the Kennedy Krieger Institute and an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, said that the study suggests the drug may be a valuable addition to ADHD treatment options for pediatric patients.
“The study provides reassuring data on the safety of stimulants in patients without significant history of cardiac events or blood pressure changes, which are usual concerns among patients and clinicians despite the evidence supporting safety, said Dr. Pawar, who was not part of the study.
“Additionally, the 1-year data on efficacy and safety of a new stimulant medication is valuable for clinicians looking for sustained relief for their patients, despite the limitations of an open-label trial,” she added.
Overall, the safety data reported in the study are fairly consistent with the safety profile of other methylphenidates used for treating ADHD, Dr. Pawar said.
However, she noted, the study does have some limitations, including its open-label design and lack of blinding. The research also excluded children with autism, disruptive mood dysregulation disorders, and other common comorbidities of ADHD, which may limit the generalizability of the results.
“These comorbidities often require stimulants as a part of treatment, and yet have a higher risk of side effects,” Dr. Pawar said. “Future studies with a broader population may be needed to better understand treatment effectiveness and potential risks.”
The study was funded by KemPharm. Dr. Childress serves as consultant for Aardvark, Arbor, Attentive, Cingulate, Ironshore, Neos Therapeutics, Neurocentria, Otsuka, Purdue, Rhodes, Sunovion, Tris Pharma, KemPharm, Supernus, Jazz, Corium, Tulex, and Lumos.
A version of this article first appeared on Medscape.com.
new research shows.
Results from a phase 3, multicenter, dose-optimization, open-label safety study of Azstarys (KemPharm) found that most treatment-emergent adverse events (TEAEs) were mild to moderate.
“This data show that Azstarys remains safe and effective for the treatment of ADHD when given for up to a year,” lead investigator Ann Childress, MD, president of the Center for Psychiatry and Behavioral Medicine, Las Vegas, said in an interview.
The study was published online in the Journal of Child and Adolescent Psychopharmacology.
Safety at 1 year
The drug is a combination of extended-release serdexmethylphenidate (SDX), KemPharm’s prodrug of dexmethylphenidate, coformulated with immediate-release d-MPH.
SDX is converted to d-MPH after it is absorbed in the gastrointestinal tract. The d-MPH is released gradually throughout the day, providing quick symptom control with the d-MPH and extended control with SDX.
Azstarys was approved by the Food and Drug Administration in 2021 on the basis of results from a laboratory classroom phase 3 trial, which showed significant improvement in ADHD symptoms, compared with placebo.
For this study, the second phase 3 trial of Azstarys, investigators analyzed data from 282 children aged 6-12 years in the United States, including 70 who participated in an earlier 1-month efficacy trial.
After screening and a 3-week dose-optimization phase for new participants, patients received once-daily treatment with doses of 26.1 mg/5.2 mg, 39.2 mg/7.8 mg, or 52.3 mg/10.4 mg of SDX/d-MPH.
After 1 year of treatment, 60.1% of participants reported at least one TEAE, the majority of which were moderate. Twelve patients reported severe TEAEs. Six children (2.5%) discontinued the study because of a TEAE during the treatment phase.
The investigators also measured growth and changes in sleep with the Children’s Sleep Habits Questionnaire during the 12-month study. Sleep improved on most measures and the impact on growth was mild.
There were no life-threatening TEAEs and no deaths reported during the study.
The most common TEAEs during the treatment phase were decreased appetite, upper respiratory tract infection, nasopharyngitis, decreased weight, irritability, and increased weight.
Efficacy at 1 year
ADHD symptoms improved considerably after 1 month of treatment, with responses continuing at 1 year.
At baseline, participants’ mean ADHD Rating Scale–5 score was 41.5. After 1 month of treatment, scores averaged 16.1, a decline of –25.3 (P < .001).
The mean score stabilized in the 12-15 range for the remainder of the study. After 1 year of treatment, ADHD symptoms had decreased approximately 70% from baseline.
Investigators found similar results in clinical severity. After 1 month of treatment, the average Clinical Global Impressions–Severity (CGI-S) scale score was 2.5, a decline of –2.2 (P < .0001).
CGI-S scale scores remained in the 2.2-2.4 range for the remainder of the study.
These results, combined with the results of the original classroom trial, suggest Azstarys may offer advantages over other ADHD drugs, Dr. Childress said.
“In the laboratory classroom trial, subjects taking Azstarys completed significantly more math problems than subjects taking placebo beginning at 30 minutes and up to 13 hours after dosing,” Dr. Childress said. “No other methylphenidate extended-release product currently marketed in the United States has a 13-hour duration of effect.”
‘Reassuring data’
Commenting on the findings, Aditya Pawar, MD, a child and adolescent psychiatrist with the Kennedy Krieger Institute and an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, said that the study suggests the drug may be a valuable addition to ADHD treatment options for pediatric patients.
“The study provides reassuring data on the safety of stimulants in patients without significant history of cardiac events or blood pressure changes, which are usual concerns among patients and clinicians despite the evidence supporting safety, said Dr. Pawar, who was not part of the study.
“Additionally, the 1-year data on efficacy and safety of a new stimulant medication is valuable for clinicians looking for sustained relief for their patients, despite the limitations of an open-label trial,” she added.
Overall, the safety data reported in the study are fairly consistent with the safety profile of other methylphenidates used for treating ADHD, Dr. Pawar said.
However, she noted, the study does have some limitations, including its open-label design and lack of blinding. The research also excluded children with autism, disruptive mood dysregulation disorders, and other common comorbidities of ADHD, which may limit the generalizability of the results.
“These comorbidities often require stimulants as a part of treatment, and yet have a higher risk of side effects,” Dr. Pawar said. “Future studies with a broader population may be needed to better understand treatment effectiveness and potential risks.”
The study was funded by KemPharm. Dr. Childress serves as consultant for Aardvark, Arbor, Attentive, Cingulate, Ironshore, Neos Therapeutics, Neurocentria, Otsuka, Purdue, Rhodes, Sunovion, Tris Pharma, KemPharm, Supernus, Jazz, Corium, Tulex, and Lumos.
A version of this article first appeared on Medscape.com.
new research shows.
Results from a phase 3, multicenter, dose-optimization, open-label safety study of Azstarys (KemPharm) found that most treatment-emergent adverse events (TEAEs) were mild to moderate.
“This data show that Azstarys remains safe and effective for the treatment of ADHD when given for up to a year,” lead investigator Ann Childress, MD, president of the Center for Psychiatry and Behavioral Medicine, Las Vegas, said in an interview.
The study was published online in the Journal of Child and Adolescent Psychopharmacology.
Safety at 1 year
The drug is a combination of extended-release serdexmethylphenidate (SDX), KemPharm’s prodrug of dexmethylphenidate, coformulated with immediate-release d-MPH.
SDX is converted to d-MPH after it is absorbed in the gastrointestinal tract. The d-MPH is released gradually throughout the day, providing quick symptom control with the d-MPH and extended control with SDX.
Azstarys was approved by the Food and Drug Administration in 2021 on the basis of results from a laboratory classroom phase 3 trial, which showed significant improvement in ADHD symptoms, compared with placebo.
For this study, the second phase 3 trial of Azstarys, investigators analyzed data from 282 children aged 6-12 years in the United States, including 70 who participated in an earlier 1-month efficacy trial.
After screening and a 3-week dose-optimization phase for new participants, patients received once-daily treatment with doses of 26.1 mg/5.2 mg, 39.2 mg/7.8 mg, or 52.3 mg/10.4 mg of SDX/d-MPH.
After 1 year of treatment, 60.1% of participants reported at least one TEAE, the majority of which were moderate. Twelve patients reported severe TEAEs. Six children (2.5%) discontinued the study because of a TEAE during the treatment phase.
The investigators also measured growth and changes in sleep with the Children’s Sleep Habits Questionnaire during the 12-month study. Sleep improved on most measures and the impact on growth was mild.
There were no life-threatening TEAEs and no deaths reported during the study.
The most common TEAEs during the treatment phase were decreased appetite, upper respiratory tract infection, nasopharyngitis, decreased weight, irritability, and increased weight.
Efficacy at 1 year
ADHD symptoms improved considerably after 1 month of treatment, with responses continuing at 1 year.
At baseline, participants’ mean ADHD Rating Scale–5 score was 41.5. After 1 month of treatment, scores averaged 16.1, a decline of –25.3 (P < .001).
The mean score stabilized in the 12-15 range for the remainder of the study. After 1 year of treatment, ADHD symptoms had decreased approximately 70% from baseline.
Investigators found similar results in clinical severity. After 1 month of treatment, the average Clinical Global Impressions–Severity (CGI-S) scale score was 2.5, a decline of –2.2 (P < .0001).
CGI-S scale scores remained in the 2.2-2.4 range for the remainder of the study.
These results, combined with the results of the original classroom trial, suggest Azstarys may offer advantages over other ADHD drugs, Dr. Childress said.
“In the laboratory classroom trial, subjects taking Azstarys completed significantly more math problems than subjects taking placebo beginning at 30 minutes and up to 13 hours after dosing,” Dr. Childress said. “No other methylphenidate extended-release product currently marketed in the United States has a 13-hour duration of effect.”
‘Reassuring data’
Commenting on the findings, Aditya Pawar, MD, a child and adolescent psychiatrist with the Kennedy Krieger Institute and an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, said that the study suggests the drug may be a valuable addition to ADHD treatment options for pediatric patients.
“The study provides reassuring data on the safety of stimulants in patients without significant history of cardiac events or blood pressure changes, which are usual concerns among patients and clinicians despite the evidence supporting safety, said Dr. Pawar, who was not part of the study.
“Additionally, the 1-year data on efficacy and safety of a new stimulant medication is valuable for clinicians looking for sustained relief for their patients, despite the limitations of an open-label trial,” she added.
Overall, the safety data reported in the study are fairly consistent with the safety profile of other methylphenidates used for treating ADHD, Dr. Pawar said.
However, she noted, the study does have some limitations, including its open-label design and lack of blinding. The research also excluded children with autism, disruptive mood dysregulation disorders, and other common comorbidities of ADHD, which may limit the generalizability of the results.
“These comorbidities often require stimulants as a part of treatment, and yet have a higher risk of side effects,” Dr. Pawar said. “Future studies with a broader population may be needed to better understand treatment effectiveness and potential risks.”
The study was funded by KemPharm. Dr. Childress serves as consultant for Aardvark, Arbor, Attentive, Cingulate, Ironshore, Neos Therapeutics, Neurocentria, Otsuka, Purdue, Rhodes, Sunovion, Tris Pharma, KemPharm, Supernus, Jazz, Corium, Tulex, and Lumos.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CHILD AND ADOLESCENT PSYCHOPHARMACOLOGY
Generic stimulant shortage update: From bad to worse
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
I (MZP) just completed my first semester of medical school. An important lesson imparted in my coursework so far has been to remain a staunch advocate for patients. Yet compared to the rigors of medical school, over the past year it has been far more difficult to help patients locate generic Adderall. Physicians were already overburdened with administrative responsibilities stretching into burnout territory well before the shortage, and now this! Unlike paper prescriptions of old, which patients could take to any pharmacy, e-prescribing apps require selection of a specific pharmacy, and controlled substances such as stimulants require 2-factor authentication. But if the designated pharmacy does not have the medication in stock, the entire process must be repeated with an alternative pharmacy, long after the visit has concluded.
To add insult to injury, the generic stimulant shortage has grown even worse. As of February 2023, generic Adderall remained hard to find and generic Concerta was also in short supply. How did this happen? In 1985, Bulow et al¹ coined the game theory concept of “strategic substitutes,” where (for example) as beef becomes less readily accessible, consumers may switch to eating chicken as their protein. Unable to locate generic Adderall, many patients have turned to generic Concerta as a substitute psychostimulant to continue management of their attention-deficit/hyperactivity disorder.
In addition to the increase in demand, compounding the shortage is that one of the manufacturers of generic Concerta has discontinued production.² Branded methylphenidates and amphetamines, which are much more expensive than their generic counterparts, have remained in ample supply, but many insurers require trials of generics before considering coverage for more expensive brands.
Our approach to this situation
Each morning we call our local and chain pharmacies to take a census of their supply of generic stimulants. Some pharmacies refuse to release this information. Despite these census reports, we have found cases where patients have been turned away from pharmacies when they are not “regular customers,” while patients whom the pharmacies know retain access as “members.” Hence, a patient is unlikely to obtain these medications if their regular pharmacy is out of stock.
We want to share a workaround that has been effective. After unsuccessfully searching for generic stimulants at the patient’s regular pharmacy, I (RLP) write “dispense as written” for the closest branded version and file a prior authorization with the patient’s insurance company, noting “patient unable to trial any generic amphetamines or methylphenidates due to current nationwide shortage.” Even with the most difficult insurers, the response has been “a temporary 3-month authorization has been granted,” which is at least a small victory for our desperate patients and busy prescribers who are both struggling to negotiate a fragmented health care system.
1. Bulow JI, Geanakoplos JD, Klemperer PD. Multimarket oligopoly: strategic substitutes and complements. Journal of Political Economy. 1985;93(3):488-511. https://doi.org/10.1086/261312
2. US Food & Drug Administration. FDA Drug Shortages. Accessed January 7, 2023. https://www.accessdata.fda.gov/scripts/drugshortages/dsp_ActiveIngredientDetails.cfm?AI=Methylphenidate+Hydrochloride+Extended+Release+Tablets&st=d
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
I (MZP) just completed my first semester of medical school. An important lesson imparted in my coursework so far has been to remain a staunch advocate for patients. Yet compared to the rigors of medical school, over the past year it has been far more difficult to help patients locate generic Adderall. Physicians were already overburdened with administrative responsibilities stretching into burnout territory well before the shortage, and now this! Unlike paper prescriptions of old, which patients could take to any pharmacy, e-prescribing apps require selection of a specific pharmacy, and controlled substances such as stimulants require 2-factor authentication. But if the designated pharmacy does not have the medication in stock, the entire process must be repeated with an alternative pharmacy, long after the visit has concluded.
To add insult to injury, the generic stimulant shortage has grown even worse. As of February 2023, generic Adderall remained hard to find and generic Concerta was also in short supply. How did this happen? In 1985, Bulow et al¹ coined the game theory concept of “strategic substitutes,” where (for example) as beef becomes less readily accessible, consumers may switch to eating chicken as their protein. Unable to locate generic Adderall, many patients have turned to generic Concerta as a substitute psychostimulant to continue management of their attention-deficit/hyperactivity disorder.
In addition to the increase in demand, compounding the shortage is that one of the manufacturers of generic Concerta has discontinued production.² Branded methylphenidates and amphetamines, which are much more expensive than their generic counterparts, have remained in ample supply, but many insurers require trials of generics before considering coverage for more expensive brands.
Our approach to this situation
Each morning we call our local and chain pharmacies to take a census of their supply of generic stimulants. Some pharmacies refuse to release this information. Despite these census reports, we have found cases where patients have been turned away from pharmacies when they are not “regular customers,” while patients whom the pharmacies know retain access as “members.” Hence, a patient is unlikely to obtain these medications if their regular pharmacy is out of stock.
We want to share a workaround that has been effective. After unsuccessfully searching for generic stimulants at the patient’s regular pharmacy, I (RLP) write “dispense as written” for the closest branded version and file a prior authorization with the patient’s insurance company, noting “patient unable to trial any generic amphetamines or methylphenidates due to current nationwide shortage.” Even with the most difficult insurers, the response has been “a temporary 3-month authorization has been granted,” which is at least a small victory for our desperate patients and busy prescribers who are both struggling to negotiate a fragmented health care system.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
I (MZP) just completed my first semester of medical school. An important lesson imparted in my coursework so far has been to remain a staunch advocate for patients. Yet compared to the rigors of medical school, over the past year it has been far more difficult to help patients locate generic Adderall. Physicians were already overburdened with administrative responsibilities stretching into burnout territory well before the shortage, and now this! Unlike paper prescriptions of old, which patients could take to any pharmacy, e-prescribing apps require selection of a specific pharmacy, and controlled substances such as stimulants require 2-factor authentication. But if the designated pharmacy does not have the medication in stock, the entire process must be repeated with an alternative pharmacy, long after the visit has concluded.
To add insult to injury, the generic stimulant shortage has grown even worse. As of February 2023, generic Adderall remained hard to find and generic Concerta was also in short supply. How did this happen? In 1985, Bulow et al¹ coined the game theory concept of “strategic substitutes,” where (for example) as beef becomes less readily accessible, consumers may switch to eating chicken as their protein. Unable to locate generic Adderall, many patients have turned to generic Concerta as a substitute psychostimulant to continue management of their attention-deficit/hyperactivity disorder.
In addition to the increase in demand, compounding the shortage is that one of the manufacturers of generic Concerta has discontinued production.² Branded methylphenidates and amphetamines, which are much more expensive than their generic counterparts, have remained in ample supply, but many insurers require trials of generics before considering coverage for more expensive brands.
Our approach to this situation
Each morning we call our local and chain pharmacies to take a census of their supply of generic stimulants. Some pharmacies refuse to release this information. Despite these census reports, we have found cases where patients have been turned away from pharmacies when they are not “regular customers,” while patients whom the pharmacies know retain access as “members.” Hence, a patient is unlikely to obtain these medications if their regular pharmacy is out of stock.
We want to share a workaround that has been effective. After unsuccessfully searching for generic stimulants at the patient’s regular pharmacy, I (RLP) write “dispense as written” for the closest branded version and file a prior authorization with the patient’s insurance company, noting “patient unable to trial any generic amphetamines or methylphenidates due to current nationwide shortage.” Even with the most difficult insurers, the response has been “a temporary 3-month authorization has been granted,” which is at least a small victory for our desperate patients and busy prescribers who are both struggling to negotiate a fragmented health care system.
1. Bulow JI, Geanakoplos JD, Klemperer PD. Multimarket oligopoly: strategic substitutes and complements. Journal of Political Economy. 1985;93(3):488-511. https://doi.org/10.1086/261312
2. US Food & Drug Administration. FDA Drug Shortages. Accessed January 7, 2023. https://www.accessdata.fda.gov/scripts/drugshortages/dsp_ActiveIngredientDetails.cfm?AI=Methylphenidate+Hydrochloride+Extended+Release+Tablets&st=d
1. Bulow JI, Geanakoplos JD, Klemperer PD. Multimarket oligopoly: strategic substitutes and complements. Journal of Political Economy. 1985;93(3):488-511. https://doi.org/10.1086/261312
2. US Food & Drug Administration. FDA Drug Shortages. Accessed January 7, 2023. https://www.accessdata.fda.gov/scripts/drugshortages/dsp_ActiveIngredientDetails.cfm?AI=Methylphenidate+Hydrochloride+Extended+Release+Tablets&st=d
‘Concerning’ uptick in pediatric antipsychotic prescribing
“This study demonstrates a concerning trend in antipsychotic prescribing in children and adolescents,” study investigator Matthias Pierce, PhD, senior research fellow at the University of Manchester (England) Center for Women’s Mental Health, who jointly led the study, said in a news release.
“We do not think the changes in prescribing necessarily relate to changes in clinical need; rather, it may be more likely to reflect changes in prescribing practice by clinicians,” Dr. Pierce said.
The study was published online in The Lancet Psychiatry.
Increase in long-term use
Between 2000 and 2019, prescriptions for antipsychotics nearly doubled from 0.06% to 0.11%.
The investigators note that the U.K.’s National Institute for Health and Care Excellence has approved the use of some antipsychotics in patients younger than age 18 with schizophrenia, bipolar disorder, and severely aggressive behavior attributable to conduct disorder.
However, these data suggest antipsychotics are being prescribed for an increasingly broad range of conditions, most commonly autism, but also for attention-deficit/ hyperactivity disorder, tic disorders like Tourrette syndrome, and learning difficulties.
“Broadening use of antipsychotics in developing young people begs questions about their safety over time and demands more research on this topic,” senior author Kathryn Abel, MBBS, PhD, from the University of Manchester said in the news release.
During the study period, antipsychotic prescribing in primary care increased by an average of 3.3% per year and the rate of first prescriptions increased by 2.2% per year.
The data also suggest that more children and adolescents are taking these powerful drugs for longer periods of time. The proportion receiving antipsychotics for at least 6 months after an initial prescription rose from 41.9% in 2000 to 62.8% in 2018.
Prescribing inequities
From 2009 onwards, more than 90% of prescriptions were for atypical antipsychotics.
Over time, risperidone dominated, with more than 60% of all prescriptions, followed by aripiprazole, quetiapine, olanzapine, and haloperidol as the most prescribed antipsychotics.
Boys and older children aged 15-18 years were most likely to receive an antipsychotic. However, the increasing trends were evident in all groups.
The data also point to inequities in prescribing as a result of deprivation levels, with typical antipsychotics prescribed more frequently in more deprived areas over time.
Dr. Pierce said he hopes this study will “help clinicians to evaluate the prescribing of antipsychotics to children more fully and will encourage them to consider better access to alternatives.”
Dr. Abel noted that antipsychotic medications “continue to have a valuable role in the treatment of serious mental illness. These findings represent a descriptive account of antipsychotic prescribing to children and adolescents in the U.K. today and provide a window onto current practice.”
Findings are no surprise
Emily Simonoff, MD, professor of child and adolescent psychiatry, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, offered perspective on the study in a statement from the U.K. nonprofit Science Media Centre.
“To clinicians, it will not be surprising that the authors demonstrate an increase in rates of prescriptions over that time period, as there has been a steadily emerging evidence base for the benefits of this group of medication for a range of different indications, which has been further supported by new licensing indications and recommendations from NICE,” Dr. Simonoff said.
For example, “there is good evidence for their benefits for other conditions such as irritability in autism spectrum disorder.
“However, it should also be noted that NICE recommendations for their use in many conditions is as part of a multimodal treatment plan, for example including psychological or behavioral interventions. It’s unclear from the study whether such recommendations were being followed or medication was being used on its own,” she added.
Dr. Simonoff also said it’s “reassuring” that prescribing rates remain very low in the youngest children and notes that the authors “rightly highlight the need for high-quality, longer-term studies on efficacy and, most importantly, adverse effects. This should be a research priority.”
The study had no funding. The authors report no relevant financial relationships. Dr. Simonoff is a member of the NICE guideline development group for the management of autism and has published on the efficacy of antipsychotic medication for irritability in autism.
A version of this article first appeared on Medscape.com.
“This study demonstrates a concerning trend in antipsychotic prescribing in children and adolescents,” study investigator Matthias Pierce, PhD, senior research fellow at the University of Manchester (England) Center for Women’s Mental Health, who jointly led the study, said in a news release.
“We do not think the changes in prescribing necessarily relate to changes in clinical need; rather, it may be more likely to reflect changes in prescribing practice by clinicians,” Dr. Pierce said.
The study was published online in The Lancet Psychiatry.
Increase in long-term use
Between 2000 and 2019, prescriptions for antipsychotics nearly doubled from 0.06% to 0.11%.
The investigators note that the U.K.’s National Institute for Health and Care Excellence has approved the use of some antipsychotics in patients younger than age 18 with schizophrenia, bipolar disorder, and severely aggressive behavior attributable to conduct disorder.
However, these data suggest antipsychotics are being prescribed for an increasingly broad range of conditions, most commonly autism, but also for attention-deficit/ hyperactivity disorder, tic disorders like Tourrette syndrome, and learning difficulties.
“Broadening use of antipsychotics in developing young people begs questions about their safety over time and demands more research on this topic,” senior author Kathryn Abel, MBBS, PhD, from the University of Manchester said in the news release.
During the study period, antipsychotic prescribing in primary care increased by an average of 3.3% per year and the rate of first prescriptions increased by 2.2% per year.
The data also suggest that more children and adolescents are taking these powerful drugs for longer periods of time. The proportion receiving antipsychotics for at least 6 months after an initial prescription rose from 41.9% in 2000 to 62.8% in 2018.
Prescribing inequities
From 2009 onwards, more than 90% of prescriptions were for atypical antipsychotics.
Over time, risperidone dominated, with more than 60% of all prescriptions, followed by aripiprazole, quetiapine, olanzapine, and haloperidol as the most prescribed antipsychotics.
Boys and older children aged 15-18 years were most likely to receive an antipsychotic. However, the increasing trends were evident in all groups.
The data also point to inequities in prescribing as a result of deprivation levels, with typical antipsychotics prescribed more frequently in more deprived areas over time.
Dr. Pierce said he hopes this study will “help clinicians to evaluate the prescribing of antipsychotics to children more fully and will encourage them to consider better access to alternatives.”
Dr. Abel noted that antipsychotic medications “continue to have a valuable role in the treatment of serious mental illness. These findings represent a descriptive account of antipsychotic prescribing to children and adolescents in the U.K. today and provide a window onto current practice.”
Findings are no surprise
Emily Simonoff, MD, professor of child and adolescent psychiatry, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, offered perspective on the study in a statement from the U.K. nonprofit Science Media Centre.
“To clinicians, it will not be surprising that the authors demonstrate an increase in rates of prescriptions over that time period, as there has been a steadily emerging evidence base for the benefits of this group of medication for a range of different indications, which has been further supported by new licensing indications and recommendations from NICE,” Dr. Simonoff said.
For example, “there is good evidence for their benefits for other conditions such as irritability in autism spectrum disorder.
“However, it should also be noted that NICE recommendations for their use in many conditions is as part of a multimodal treatment plan, for example including psychological or behavioral interventions. It’s unclear from the study whether such recommendations were being followed or medication was being used on its own,” she added.
Dr. Simonoff also said it’s “reassuring” that prescribing rates remain very low in the youngest children and notes that the authors “rightly highlight the need for high-quality, longer-term studies on efficacy and, most importantly, adverse effects. This should be a research priority.”
The study had no funding. The authors report no relevant financial relationships. Dr. Simonoff is a member of the NICE guideline development group for the management of autism and has published on the efficacy of antipsychotic medication for irritability in autism.
A version of this article first appeared on Medscape.com.
“This study demonstrates a concerning trend in antipsychotic prescribing in children and adolescents,” study investigator Matthias Pierce, PhD, senior research fellow at the University of Manchester (England) Center for Women’s Mental Health, who jointly led the study, said in a news release.
“We do not think the changes in prescribing necessarily relate to changes in clinical need; rather, it may be more likely to reflect changes in prescribing practice by clinicians,” Dr. Pierce said.
The study was published online in The Lancet Psychiatry.
Increase in long-term use
Between 2000 and 2019, prescriptions for antipsychotics nearly doubled from 0.06% to 0.11%.
The investigators note that the U.K.’s National Institute for Health and Care Excellence has approved the use of some antipsychotics in patients younger than age 18 with schizophrenia, bipolar disorder, and severely aggressive behavior attributable to conduct disorder.
However, these data suggest antipsychotics are being prescribed for an increasingly broad range of conditions, most commonly autism, but also for attention-deficit/ hyperactivity disorder, tic disorders like Tourrette syndrome, and learning difficulties.
“Broadening use of antipsychotics in developing young people begs questions about their safety over time and demands more research on this topic,” senior author Kathryn Abel, MBBS, PhD, from the University of Manchester said in the news release.
During the study period, antipsychotic prescribing in primary care increased by an average of 3.3% per year and the rate of first prescriptions increased by 2.2% per year.
The data also suggest that more children and adolescents are taking these powerful drugs for longer periods of time. The proportion receiving antipsychotics for at least 6 months after an initial prescription rose from 41.9% in 2000 to 62.8% in 2018.
Prescribing inequities
From 2009 onwards, more than 90% of prescriptions were for atypical antipsychotics.
Over time, risperidone dominated, with more than 60% of all prescriptions, followed by aripiprazole, quetiapine, olanzapine, and haloperidol as the most prescribed antipsychotics.
Boys and older children aged 15-18 years were most likely to receive an antipsychotic. However, the increasing trends were evident in all groups.
The data also point to inequities in prescribing as a result of deprivation levels, with typical antipsychotics prescribed more frequently in more deprived areas over time.
Dr. Pierce said he hopes this study will “help clinicians to evaluate the prescribing of antipsychotics to children more fully and will encourage them to consider better access to alternatives.”
Dr. Abel noted that antipsychotic medications “continue to have a valuable role in the treatment of serious mental illness. These findings represent a descriptive account of antipsychotic prescribing to children and adolescents in the U.K. today and provide a window onto current practice.”
Findings are no surprise
Emily Simonoff, MD, professor of child and adolescent psychiatry, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, offered perspective on the study in a statement from the U.K. nonprofit Science Media Centre.
“To clinicians, it will not be surprising that the authors demonstrate an increase in rates of prescriptions over that time period, as there has been a steadily emerging evidence base for the benefits of this group of medication for a range of different indications, which has been further supported by new licensing indications and recommendations from NICE,” Dr. Simonoff said.
For example, “there is good evidence for their benefits for other conditions such as irritability in autism spectrum disorder.
“However, it should also be noted that NICE recommendations for their use in many conditions is as part of a multimodal treatment plan, for example including psychological or behavioral interventions. It’s unclear from the study whether such recommendations were being followed or medication was being used on its own,” she added.
Dr. Simonoff also said it’s “reassuring” that prescribing rates remain very low in the youngest children and notes that the authors “rightly highlight the need for high-quality, longer-term studies on efficacy and, most importantly, adverse effects. This should be a research priority.”
The study had no funding. The authors report no relevant financial relationships. Dr. Simonoff is a member of the NICE guideline development group for the management of autism and has published on the efficacy of antipsychotic medication for irritability in autism.
A version of this article first appeared on Medscape.com.
FROM THE LANCET PSYCHIATRY
ADHD beyond medications
Attention-deficit/hyperactivity disorder (ADHD) is often a very challenging condition for parents to manage, both because of the “gleeful mayhem” children with ADHD manifest and because of the nature of effective treatments. Multiple randomized controlled studies and meta-analyses have demonstrated that stimulant medication with behavioral interventions is the optimal first-line treatment for children with both subtypes of ADHD, and that medications alone are superior to behavioral interventions alone. By improving attention and impulse control, the medications effectively decrease the many negative interactions with teachers, peers, and parents, aiding development and healthy self-esteem.
But many parents feel anxious about treating their young children with stimulants. Importantly, how children with ADHD will fare as adults is not predicted by their symptom level, but instead by the quality of their relationships with their parents, their ability to perform at school, and their social skills. Bring this framework to parents as you listen to their questions and help them decide on the best approach for their family. To assist you in these conversations, we will review the evidence for (or against) several of the most common alternatives to medication that parents are likely to ask about.
Diets and supplements
Dietary modifications are among the most popular “natural” approaches to managing ADHD in children. Diets that eliminate processed sugars or food additives (particularly artificial food coloring) are among the most common approaches discussed in the lay press. These diets are usually very time-consuming and disruptive for families to follow, and there is no evidence to support their general use in ADHD management. Those studies that rigorously examined them suggest that, for children with severe impairment who have failed to respond to medications for ADHD, a workup for food intolerance or nutritional deficits may reveal a different problem underlying their behavioral difficulties.1
Similarly, supplementation with high-dose omega-3 fatty acids is modestly helpful only in a subset of children with ADHD symptoms, and not nearly as effective as medications or behavioral interventions. Spending time on an exacting diet or buying expensive supplements is very unlikely to relieve the children’s symptoms and may only add to their stress at home. The “sugar high” parents note may be the rare joy of eating a candy bar and not sugar causing ADHD. Offer parents the guidance to focus on a healthy diet, high in fruits and vegetables, whole grains, and healthy protein, and on meals that emphasize family time instead of struggles around food.
Neurofeedback
Neurofeedback is an approach that grew out of the observation that many adults with ADHD had resting patterns of brain wave activity different from those of neurotypical adults. In neurofeedback, patients learn strategies that amplify the brain waves associated with focused mental activity, rather than listless or hyperactive states. Businesses market this service for all sorts of illnesses and challenges, ADHD chief among them. Despite the marketing, there are very few randomized controlled studies of this intervention for ADHD in youth, and those have shown only the possibility of a benefit.
While there is no evidence of serious side effects, these treatments are time-consuming and expensive and unlikely to be covered by any insurance. You might suggest to parents that they could achieve some of the same theoretical benefits by looking for hobbies that invite sustained focus in their children. That is, they should think about activities that interest the children, such as music lessons or karate, that they could practice in classes and at home. If the children find these activities even somewhat interesting (or just enjoy the reward of their parents’ or teachers’ attention), regular practice will be supporting their developing attention while building social skills and authentic self-confidence, rather than the activities feeling like a treatment for an illness or condition.
Sleep and exercise
There are not many businesses or books selling worried and exhausted parents a quick nonmedication solution for their children’s ADHD in the form of healthy sleep and exercise habits. But these are safe and healthy ways to reduce symptoms and support development. Children with ADHD often enjoy and benefit from participating in a sport, and daily exercise can help with sleep and regulating their energy. They also often have difficulty with sleep initiation, and commonly do not get adequate or restful sleep. Inadequate sleep exacerbates inattention, distractibility, and irritability. Children with untreated ADHD also often spend a lot of time on screens, as it is difficult for them to shift away from rewarding activities, and parents can find screen time to be a welcome break from hyperactivity and negative interactions. But excessive screen time, especially close to bedtime, can worsen irritability and make sleep more difficult. Talk with parents about the value of establishing a routine around screen time, modest daily physical activity, and sleep that everyone can follow. If their family life is currently marked by late bedtimes and long hours in front of video games, this change will take effort. But within a few weeks, it could lead to significant improvements in energy, attention, and interactions at home.
Behavioral treatments
Effective behavioral treatments for ADHD do not change ADHD symptoms, but they do help children learn how to manage them. In “parent management training,” younger children and parents learn together how to avoid negative cycles of behavior (i.e., temper outbursts) by focusing on consistent routines and consequences that support children calmly learning to manage their impulses. The only other evidence-based treatment focuses on helping school age and older children develop executive functions – their planning, organization, and time management skills – with a range of age-appropriate tools. Both of these therapies may be more effective if the children are also receiving medication, but medication is not necessary for them to be helpful. It is important to note that play therapy and other evidence-based psychotherapies are not effective for management of ADHD, although they may treat comorbid problems.
Parent treatment
You may have diagnosed children with ADHD only to hear their parents respond by saying that they suspect (or know) that they (or their spouses) also have ADHD. This would not be surprising, as ADHD has one of the highest rates of heritability of psychiatric disorders, at 80%. Somewhere between 25% and 50% of parents of children with ADHD have ADHD themselves.2 Screening for adults with ADHD, such as the Adult ADHD Self-Report Scale, is widely available and free. Speak with parents about the fact that behavioral treatments for their children’s ADHD are demanding. Such treatments require patience, calm, organization, and consistency.
If parents have ADHD, it may be very helpful for them to prioritize their own effective treatments, so that their attention and impulse control will support their parenting. They may be interested in learning about how treatment might also improve their performance at work and even the quality of their relationships. While there is some evidence that their children’s treatment outcome will hinge on the parents’ treatment,3 they deserve good care independent of the expectations of parenting.
Families benefit from a comprehensive “ADHD plan” for their children. This would start with an assessment of the severity of their children’s symptoms, specifying their impairment at home, school, and in social relationships. It would include their nonacademic performance, exploration of interests, and developing self-confidence. All of these considerations lead to setting reasonable expectations so the children can feel successful. Parents should think about how best to structure their children’s schedules to promote healthy sleep, exercise, and nutrition, and to expand opportunities for building their frustration tolerance, social skills, and executive function.
Parents will need to consider what kind of supports they themselves need to offer this structure. There are good resources available online for information and support, including Children and Adults with ADHD (chadd.org) and the ADHD Resource Center from the American Academy of Child and Adolescent Psychiatry (aacap.org). This approach may help parents to evaluate the potential risks and benefits of medications as a component of treatment. Most of the quick fixes for childhood ADHD on the market will take a family’s time and money without providing meaningful improvement. Parents should focus instead on the tried-and-true routines and supports that will help them to create the setting at home that will enable their children to flourish.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at pdnews@mdedge.com.
References
1. Millichap JG and Yee MM. Pediatrics. 2012 Feb;129(2):330-7.
2. Grimm O et al. Curr Psychiatry Rep. 2020 Feb 27;22(4):18.
3. Chronis-Tuscano A et al. J Abnorm Child Psychol. 2017 Apr;45(3):501-7.
Attention-deficit/hyperactivity disorder (ADHD) is often a very challenging condition for parents to manage, both because of the “gleeful mayhem” children with ADHD manifest and because of the nature of effective treatments. Multiple randomized controlled studies and meta-analyses have demonstrated that stimulant medication with behavioral interventions is the optimal first-line treatment for children with both subtypes of ADHD, and that medications alone are superior to behavioral interventions alone. By improving attention and impulse control, the medications effectively decrease the many negative interactions with teachers, peers, and parents, aiding development and healthy self-esteem.
But many parents feel anxious about treating their young children with stimulants. Importantly, how children with ADHD will fare as adults is not predicted by their symptom level, but instead by the quality of their relationships with their parents, their ability to perform at school, and their social skills. Bring this framework to parents as you listen to their questions and help them decide on the best approach for their family. To assist you in these conversations, we will review the evidence for (or against) several of the most common alternatives to medication that parents are likely to ask about.
Diets and supplements
Dietary modifications are among the most popular “natural” approaches to managing ADHD in children. Diets that eliminate processed sugars or food additives (particularly artificial food coloring) are among the most common approaches discussed in the lay press. These diets are usually very time-consuming and disruptive for families to follow, and there is no evidence to support their general use in ADHD management. Those studies that rigorously examined them suggest that, for children with severe impairment who have failed to respond to medications for ADHD, a workup for food intolerance or nutritional deficits may reveal a different problem underlying their behavioral difficulties.1
Similarly, supplementation with high-dose omega-3 fatty acids is modestly helpful only in a subset of children with ADHD symptoms, and not nearly as effective as medications or behavioral interventions. Spending time on an exacting diet or buying expensive supplements is very unlikely to relieve the children’s symptoms and may only add to their stress at home. The “sugar high” parents note may be the rare joy of eating a candy bar and not sugar causing ADHD. Offer parents the guidance to focus on a healthy diet, high in fruits and vegetables, whole grains, and healthy protein, and on meals that emphasize family time instead of struggles around food.
Neurofeedback
Neurofeedback is an approach that grew out of the observation that many adults with ADHD had resting patterns of brain wave activity different from those of neurotypical adults. In neurofeedback, patients learn strategies that amplify the brain waves associated with focused mental activity, rather than listless or hyperactive states. Businesses market this service for all sorts of illnesses and challenges, ADHD chief among them. Despite the marketing, there are very few randomized controlled studies of this intervention for ADHD in youth, and those have shown only the possibility of a benefit.
While there is no evidence of serious side effects, these treatments are time-consuming and expensive and unlikely to be covered by any insurance. You might suggest to parents that they could achieve some of the same theoretical benefits by looking for hobbies that invite sustained focus in their children. That is, they should think about activities that interest the children, such as music lessons or karate, that they could practice in classes and at home. If the children find these activities even somewhat interesting (or just enjoy the reward of their parents’ or teachers’ attention), regular practice will be supporting their developing attention while building social skills and authentic self-confidence, rather than the activities feeling like a treatment for an illness or condition.
Sleep and exercise
There are not many businesses or books selling worried and exhausted parents a quick nonmedication solution for their children’s ADHD in the form of healthy sleep and exercise habits. But these are safe and healthy ways to reduce symptoms and support development. Children with ADHD often enjoy and benefit from participating in a sport, and daily exercise can help with sleep and regulating their energy. They also often have difficulty with sleep initiation, and commonly do not get adequate or restful sleep. Inadequate sleep exacerbates inattention, distractibility, and irritability. Children with untreated ADHD also often spend a lot of time on screens, as it is difficult for them to shift away from rewarding activities, and parents can find screen time to be a welcome break from hyperactivity and negative interactions. But excessive screen time, especially close to bedtime, can worsen irritability and make sleep more difficult. Talk with parents about the value of establishing a routine around screen time, modest daily physical activity, and sleep that everyone can follow. If their family life is currently marked by late bedtimes and long hours in front of video games, this change will take effort. But within a few weeks, it could lead to significant improvements in energy, attention, and interactions at home.
Behavioral treatments
Effective behavioral treatments for ADHD do not change ADHD symptoms, but they do help children learn how to manage them. In “parent management training,” younger children and parents learn together how to avoid negative cycles of behavior (i.e., temper outbursts) by focusing on consistent routines and consequences that support children calmly learning to manage their impulses. The only other evidence-based treatment focuses on helping school age and older children develop executive functions – their planning, organization, and time management skills – with a range of age-appropriate tools. Both of these therapies may be more effective if the children are also receiving medication, but medication is not necessary for them to be helpful. It is important to note that play therapy and other evidence-based psychotherapies are not effective for management of ADHD, although they may treat comorbid problems.
Parent treatment
You may have diagnosed children with ADHD only to hear their parents respond by saying that they suspect (or know) that they (or their spouses) also have ADHD. This would not be surprising, as ADHD has one of the highest rates of heritability of psychiatric disorders, at 80%. Somewhere between 25% and 50% of parents of children with ADHD have ADHD themselves.2 Screening for adults with ADHD, such as the Adult ADHD Self-Report Scale, is widely available and free. Speak with parents about the fact that behavioral treatments for their children’s ADHD are demanding. Such treatments require patience, calm, organization, and consistency.
If parents have ADHD, it may be very helpful for them to prioritize their own effective treatments, so that their attention and impulse control will support their parenting. They may be interested in learning about how treatment might also improve their performance at work and even the quality of their relationships. While there is some evidence that their children’s treatment outcome will hinge on the parents’ treatment,3 they deserve good care independent of the expectations of parenting.
Families benefit from a comprehensive “ADHD plan” for their children. This would start with an assessment of the severity of their children’s symptoms, specifying their impairment at home, school, and in social relationships. It would include their nonacademic performance, exploration of interests, and developing self-confidence. All of these considerations lead to setting reasonable expectations so the children can feel successful. Parents should think about how best to structure their children’s schedules to promote healthy sleep, exercise, and nutrition, and to expand opportunities for building their frustration tolerance, social skills, and executive function.
Parents will need to consider what kind of supports they themselves need to offer this structure. There are good resources available online for information and support, including Children and Adults with ADHD (chadd.org) and the ADHD Resource Center from the American Academy of Child and Adolescent Psychiatry (aacap.org). This approach may help parents to evaluate the potential risks and benefits of medications as a component of treatment. Most of the quick fixes for childhood ADHD on the market will take a family’s time and money without providing meaningful improvement. Parents should focus instead on the tried-and-true routines and supports that will help them to create the setting at home that will enable their children to flourish.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at pdnews@mdedge.com.
References
1. Millichap JG and Yee MM. Pediatrics. 2012 Feb;129(2):330-7.
2. Grimm O et al. Curr Psychiatry Rep. 2020 Feb 27;22(4):18.
3. Chronis-Tuscano A et al. J Abnorm Child Psychol. 2017 Apr;45(3):501-7.
Attention-deficit/hyperactivity disorder (ADHD) is often a very challenging condition for parents to manage, both because of the “gleeful mayhem” children with ADHD manifest and because of the nature of effective treatments. Multiple randomized controlled studies and meta-analyses have demonstrated that stimulant medication with behavioral interventions is the optimal first-line treatment for children with both subtypes of ADHD, and that medications alone are superior to behavioral interventions alone. By improving attention and impulse control, the medications effectively decrease the many negative interactions with teachers, peers, and parents, aiding development and healthy self-esteem.
But many parents feel anxious about treating their young children with stimulants. Importantly, how children with ADHD will fare as adults is not predicted by their symptom level, but instead by the quality of their relationships with their parents, their ability to perform at school, and their social skills. Bring this framework to parents as you listen to their questions and help them decide on the best approach for their family. To assist you in these conversations, we will review the evidence for (or against) several of the most common alternatives to medication that parents are likely to ask about.
Diets and supplements
Dietary modifications are among the most popular “natural” approaches to managing ADHD in children. Diets that eliminate processed sugars or food additives (particularly artificial food coloring) are among the most common approaches discussed in the lay press. These diets are usually very time-consuming and disruptive for families to follow, and there is no evidence to support their general use in ADHD management. Those studies that rigorously examined them suggest that, for children with severe impairment who have failed to respond to medications for ADHD, a workup for food intolerance or nutritional deficits may reveal a different problem underlying their behavioral difficulties.1
Similarly, supplementation with high-dose omega-3 fatty acids is modestly helpful only in a subset of children with ADHD symptoms, and not nearly as effective as medications or behavioral interventions. Spending time on an exacting diet or buying expensive supplements is very unlikely to relieve the children’s symptoms and may only add to their stress at home. The “sugar high” parents note may be the rare joy of eating a candy bar and not sugar causing ADHD. Offer parents the guidance to focus on a healthy diet, high in fruits and vegetables, whole grains, and healthy protein, and on meals that emphasize family time instead of struggles around food.
Neurofeedback
Neurofeedback is an approach that grew out of the observation that many adults with ADHD had resting patterns of brain wave activity different from those of neurotypical adults. In neurofeedback, patients learn strategies that amplify the brain waves associated with focused mental activity, rather than listless or hyperactive states. Businesses market this service for all sorts of illnesses and challenges, ADHD chief among them. Despite the marketing, there are very few randomized controlled studies of this intervention for ADHD in youth, and those have shown only the possibility of a benefit.
While there is no evidence of serious side effects, these treatments are time-consuming and expensive and unlikely to be covered by any insurance. You might suggest to parents that they could achieve some of the same theoretical benefits by looking for hobbies that invite sustained focus in their children. That is, they should think about activities that interest the children, such as music lessons or karate, that they could practice in classes and at home. If the children find these activities even somewhat interesting (or just enjoy the reward of their parents’ or teachers’ attention), regular practice will be supporting their developing attention while building social skills and authentic self-confidence, rather than the activities feeling like a treatment for an illness or condition.
Sleep and exercise
There are not many businesses or books selling worried and exhausted parents a quick nonmedication solution for their children’s ADHD in the form of healthy sleep and exercise habits. But these are safe and healthy ways to reduce symptoms and support development. Children with ADHD often enjoy and benefit from participating in a sport, and daily exercise can help with sleep and regulating their energy. They also often have difficulty with sleep initiation, and commonly do not get adequate or restful sleep. Inadequate sleep exacerbates inattention, distractibility, and irritability. Children with untreated ADHD also often spend a lot of time on screens, as it is difficult for them to shift away from rewarding activities, and parents can find screen time to be a welcome break from hyperactivity and negative interactions. But excessive screen time, especially close to bedtime, can worsen irritability and make sleep more difficult. Talk with parents about the value of establishing a routine around screen time, modest daily physical activity, and sleep that everyone can follow. If their family life is currently marked by late bedtimes and long hours in front of video games, this change will take effort. But within a few weeks, it could lead to significant improvements in energy, attention, and interactions at home.
Behavioral treatments
Effective behavioral treatments for ADHD do not change ADHD symptoms, but they do help children learn how to manage them. In “parent management training,” younger children and parents learn together how to avoid negative cycles of behavior (i.e., temper outbursts) by focusing on consistent routines and consequences that support children calmly learning to manage their impulses. The only other evidence-based treatment focuses on helping school age and older children develop executive functions – their planning, organization, and time management skills – with a range of age-appropriate tools. Both of these therapies may be more effective if the children are also receiving medication, but medication is not necessary for them to be helpful. It is important to note that play therapy and other evidence-based psychotherapies are not effective for management of ADHD, although they may treat comorbid problems.
Parent treatment
You may have diagnosed children with ADHD only to hear their parents respond by saying that they suspect (or know) that they (or their spouses) also have ADHD. This would not be surprising, as ADHD has one of the highest rates of heritability of psychiatric disorders, at 80%. Somewhere between 25% and 50% of parents of children with ADHD have ADHD themselves.2 Screening for adults with ADHD, such as the Adult ADHD Self-Report Scale, is widely available and free. Speak with parents about the fact that behavioral treatments for their children’s ADHD are demanding. Such treatments require patience, calm, organization, and consistency.
If parents have ADHD, it may be very helpful for them to prioritize their own effective treatments, so that their attention and impulse control will support their parenting. They may be interested in learning about how treatment might also improve their performance at work and even the quality of their relationships. While there is some evidence that their children’s treatment outcome will hinge on the parents’ treatment,3 they deserve good care independent of the expectations of parenting.
Families benefit from a comprehensive “ADHD plan” for their children. This would start with an assessment of the severity of their children’s symptoms, specifying their impairment at home, school, and in social relationships. It would include their nonacademic performance, exploration of interests, and developing self-confidence. All of these considerations lead to setting reasonable expectations so the children can feel successful. Parents should think about how best to structure their children’s schedules to promote healthy sleep, exercise, and nutrition, and to expand opportunities for building their frustration tolerance, social skills, and executive function.
Parents will need to consider what kind of supports they themselves need to offer this structure. There are good resources available online for information and support, including Children and Adults with ADHD (chadd.org) and the ADHD Resource Center from the American Academy of Child and Adolescent Psychiatry (aacap.org). This approach may help parents to evaluate the potential risks and benefits of medications as a component of treatment. Most of the quick fixes for childhood ADHD on the market will take a family’s time and money without providing meaningful improvement. Parents should focus instead on the tried-and-true routines and supports that will help them to create the setting at home that will enable their children to flourish.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at pdnews@mdedge.com.
References
1. Millichap JG and Yee MM. Pediatrics. 2012 Feb;129(2):330-7.
2. Grimm O et al. Curr Psychiatry Rep. 2020 Feb 27;22(4):18.
3. Chronis-Tuscano A et al. J Abnorm Child Psychol. 2017 Apr;45(3):501-7.
Warning: Watch out for ‘medication substitution reaction’
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
I (MZP) recently started medical school, and one of the first things we learned in our Human Dimension class was to listen to our patients. While this may seem prosaic to seasoned practitioners, I quickly realized the important, real-world consequences of doing so.
Clinicians rightfully presume that when they send a prescription to a pharmacy, the patient will receive what they have ordered or the generic equivalent unless it is ordered “Dispense as written.” Unfortunately, a confluence of increased demand and supply chain disruptions has produced nationwide shortages of generic Adderall extended-release (XR) and Adderall, which are commonly prescribed to patients with attention-deficit/hyperactivity disorder (ADHD).1 While pharmacies should notify patients when they do not have these medications in stock, we have encountered numerous cases where due to shortages, prescriptions for generic dextroamphetamine/amphetamine salts XR or immediate-release (IR) have been filled with the same milligrams of only dextroamphetamine XR or IR, respectively, without notifying the patient or the prescribing clinician. Pharmacies have included several national chains and local independent stores in the New York/New Jersey region.
Over the past several months, we have encountered patients who had been well stabilized on their ADHD medication regimen who began to report anxiety, jitteriness, agitation, fatigue, poor concentration, and/or hyperactivity, and who also reported that their pills “look different.” First, we considered their symptoms could be attributed to a switch between generic manufacturers. However, upon further inspection, we discovered that the medication name printed on the label was different from what had been prescribed. We confirmed this by checking the Prescription Monitoring Program database.
Pharmacists have recently won prescribing privileges for nirmatrelvir/ritonavir (Paxlovid) to treat COVID-19, but they certainly are not permitted to fill prescriptions for psychoactive controlled substances that have different pharmacologic profiles than the medication the clinician ordered. Adderall contains D-amphetamine and L-amphetamine in a ratio of 3:1, which makes it different in potency from dextroamphetamine alone and requires adjustment to the dosage and potentially to the frequency to achieve near equivalency.
Once we realized the issue and helped our patients locate a pharmacy that had generic Adderall XR and Adderall in stock so they could resume their previous regimen, their symptoms resolved.
It is important for all clinicians to add “medication substitution reaction” to their differential diagnosis of new-onset ADHD-related symptoms in previously stable patients.
1. Pharmaceutical Commerce. Innovative solutions for pandemic-driven pharmacy drug shortages. Published February 28, 2022. Accessed September 8, 2022. https://www.pharmaceuticalcommerce.com/view/innovative-solutions-for-pandemic-driven-pharmacy-drug-shortages
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
I (MZP) recently started medical school, and one of the first things we learned in our Human Dimension class was to listen to our patients. While this may seem prosaic to seasoned practitioners, I quickly realized the important, real-world consequences of doing so.
Clinicians rightfully presume that when they send a prescription to a pharmacy, the patient will receive what they have ordered or the generic equivalent unless it is ordered “Dispense as written.” Unfortunately, a confluence of increased demand and supply chain disruptions has produced nationwide shortages of generic Adderall extended-release (XR) and Adderall, which are commonly prescribed to patients with attention-deficit/hyperactivity disorder (ADHD).1 While pharmacies should notify patients when they do not have these medications in stock, we have encountered numerous cases where due to shortages, prescriptions for generic dextroamphetamine/amphetamine salts XR or immediate-release (IR) have been filled with the same milligrams of only dextroamphetamine XR or IR, respectively, without notifying the patient or the prescribing clinician. Pharmacies have included several national chains and local independent stores in the New York/New Jersey region.
Over the past several months, we have encountered patients who had been well stabilized on their ADHD medication regimen who began to report anxiety, jitteriness, agitation, fatigue, poor concentration, and/or hyperactivity, and who also reported that their pills “look different.” First, we considered their symptoms could be attributed to a switch between generic manufacturers. However, upon further inspection, we discovered that the medication name printed on the label was different from what had been prescribed. We confirmed this by checking the Prescription Monitoring Program database.
Pharmacists have recently won prescribing privileges for nirmatrelvir/ritonavir (Paxlovid) to treat COVID-19, but they certainly are not permitted to fill prescriptions for psychoactive controlled substances that have different pharmacologic profiles than the medication the clinician ordered. Adderall contains D-amphetamine and L-amphetamine in a ratio of 3:1, which makes it different in potency from dextroamphetamine alone and requires adjustment to the dosage and potentially to the frequency to achieve near equivalency.
Once we realized the issue and helped our patients locate a pharmacy that had generic Adderall XR and Adderall in stock so they could resume their previous regimen, their symptoms resolved.
It is important for all clinicians to add “medication substitution reaction” to their differential diagnosis of new-onset ADHD-related symptoms in previously stable patients.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
I (MZP) recently started medical school, and one of the first things we learned in our Human Dimension class was to listen to our patients. While this may seem prosaic to seasoned practitioners, I quickly realized the important, real-world consequences of doing so.
Clinicians rightfully presume that when they send a prescription to a pharmacy, the patient will receive what they have ordered or the generic equivalent unless it is ordered “Dispense as written.” Unfortunately, a confluence of increased demand and supply chain disruptions has produced nationwide shortages of generic Adderall extended-release (XR) and Adderall, which are commonly prescribed to patients with attention-deficit/hyperactivity disorder (ADHD).1 While pharmacies should notify patients when they do not have these medications in stock, we have encountered numerous cases where due to shortages, prescriptions for generic dextroamphetamine/amphetamine salts XR or immediate-release (IR) have been filled with the same milligrams of only dextroamphetamine XR or IR, respectively, without notifying the patient or the prescribing clinician. Pharmacies have included several national chains and local independent stores in the New York/New Jersey region.
Over the past several months, we have encountered patients who had been well stabilized on their ADHD medication regimen who began to report anxiety, jitteriness, agitation, fatigue, poor concentration, and/or hyperactivity, and who also reported that their pills “look different.” First, we considered their symptoms could be attributed to a switch between generic manufacturers. However, upon further inspection, we discovered that the medication name printed on the label was different from what had been prescribed. We confirmed this by checking the Prescription Monitoring Program database.
Pharmacists have recently won prescribing privileges for nirmatrelvir/ritonavir (Paxlovid) to treat COVID-19, but they certainly are not permitted to fill prescriptions for psychoactive controlled substances that have different pharmacologic profiles than the medication the clinician ordered. Adderall contains D-amphetamine and L-amphetamine in a ratio of 3:1, which makes it different in potency from dextroamphetamine alone and requires adjustment to the dosage and potentially to the frequency to achieve near equivalency.
Once we realized the issue and helped our patients locate a pharmacy that had generic Adderall XR and Adderall in stock so they could resume their previous regimen, their symptoms resolved.
It is important for all clinicians to add “medication substitution reaction” to their differential diagnosis of new-onset ADHD-related symptoms in previously stable patients.
1. Pharmaceutical Commerce. Innovative solutions for pandemic-driven pharmacy drug shortages. Published February 28, 2022. Accessed September 8, 2022. https://www.pharmaceuticalcommerce.com/view/innovative-solutions-for-pandemic-driven-pharmacy-drug-shortages
1. Pharmaceutical Commerce. Innovative solutions for pandemic-driven pharmacy drug shortages. Published February 28, 2022. Accessed September 8, 2022. https://www.pharmaceuticalcommerce.com/view/innovative-solutions-for-pandemic-driven-pharmacy-drug-shortages
Cognition-boosting ‘smart drugs’ not so smart for healthy people
VIENNA – , new research suggests.
In a randomized controlled trial, 40 healthy adults were given the attention-deficit/hyperactivity disorder (ADHD) treatments methylphenidate or dexamphetamine or the wakefulness-promoting drug modafinil vs. placebo.
While receiving the so-called “smart drugs,” participants spent more time and made more moves more quickly while solving each problem on a complex cognitive task than when given the placebo. But with no significant improvement in overall performance, all drugs were associated with a significant reduction in efficiency.
The findings “reinforce the idea that, while the drugs administered were motivational, the resulting increase in effort came at a cost in the loss of productivity,” said study presenter David Coghill, MD, PhD, chair of developmental mental health, the University of Melbourne.
This was especially true for individuals who scored high when receiving placebo, “who ended up producing below average productivity when on the drugs,” he noted.
“Overall, these drugs don’t increase the performance. Instead, they cause a regression to the mean, and appear to have a more negative effect on those who performed best at baseline,” Dr. Coghill added.
He presented the findings at the 35th European College of Neuropsychopharmacology (ECNP) Congress.
Past evidence ambiguous
Dr. Coghill noted that prescription-only stimulant drugs are increasingly used by employees and students as “smart drugs” to enhance workplace or academic productivity.
He conducted the study with colleagues from the department of economics at his institution, because of “their interest in people using cognitive enhancers within the financial industry, in the hope that that would increase their productivity in what is a very competitive industry on the floor of the trading rooms.”
However, while “there’s a subjective belief” that these drugs are effective as cognitive enhancers, the evidence to actually demonstrate that in healthy individuals “is, at best, ambiguous,” he told meeting attendees.
Improvements in cognitive capacities, such as working memory and improved planning, are most evident in clinical populations such as those with ADHD, which could be due to a “ceiling effect” of the cognitive tasks in healthy individuals, Dr. Coghill noted.
To investigate further, the researchers conducted a randomized, double-blinded trial of standard adult doses of methylphenidate (30 mg), dexamphetamine (15 mg), and modafinil (200 mg) vs. placebo. The healthy participants (n = 40), all of whom were aged 18-35 years, crossed to each of the other treatment groups over the course of four intervention sessions.
All were asked to solve eight instances of the knapsack task, the aim of which is to place theoretical objects in a knapsack to achieve the maximum value within a certain weight limit.
“This looks very simple but as the number of items increases, it becomes incredibly complex to compute, and actually is not computable using standard approaches. You have to deal with trial and error,” Dr. Coghill said.
The participants also completed several CANTAB cognitive tasks.
‘Surprising’ findings
Results showed that, overall, the drugs did not have a significant effect on task performance (slope = –0.16; P = .011).
Moreover, the drugs, both individually and collectively, had a significant negative effect on the value attained during any one attempt at the knapsack task (slope = –0.003; P = .02), an effect that extended “across the whole range” of task complexity, Dr. Coghill reported.
He went on to show that “participants actually looked as if they were working harder” when they took the three active drugs than when they were given a placebo. They also “spent more time solving each problem,” he added.
When taking the active drugs, participants made more moves during each task than when taking placebo, and made their moves more quickly.
“So these medications increased motivation,” Dr. Coghill said. “If you were sitting [and] watching this person, you would think that they were working harder.”
Yet their productivity, defined as the average gain in value per move on the knapsack task, was lower. Regression analysis identified a “significant and sizable drop in productivity” vs. placebo, Dr. Coghill noted.
This was the case for methylphenidate (P < .001), dexamphetamine (P < .001), and modafinil (P < .05), “whether you looked at the mean or median performance,” he said.
“Breaking it down a little bit more, when you looked at the individual participant level, you find substantial heterogeneity across participants,” noted Dr. Coghill.
“More than that, we found a significant negative correlation between productivity under methylphenidate compared to productivity under placebo, and this suggests a regression to the mean,” with participants who performed better under placebo performing worse with methylphenidate, he explained.
While the relationship was “exactly the same with modafinil,” it was not found with dexamphetamine, with a strong negative correlation between the productivity effects between dexamphetamine and methylphenidate (slope = –0.29; P < .0001).
“This is surprising because we assume that methylphenidate and dexamphetamine are working in very similar ways,” Dr. Coghill said.
Time to rethink, rewind?
Commenting for this article, session chair John F. Cryan, PhD, department of anatomy and neuroscience, University College Cork, Ireland, said that, based on the current data, “we might need to rethink [how] ‘smart’ psychopharmacological agents are.”
Dr. Cryan, chair of the ECNP Scientific Program Committee, added that there may be a need to revisit the difficulty of different types of cognitive tasks used in studies assessing the abilities of cognitive enhancing drugs and to “rewind conventional wisdom” around them.
Also commenting, Andrew Westbrook, PhD, of the department of cognitive linguistics and psychological sciences, Brown University, Providence, R.I., said the results seem “reasonable” and are “consistent with my own perspective.”
However, he told this news organization, “some caveats are warranted,” not least that the context of the task can have an impact on the results it obtains.
“We have hypothesized that pharmacologically-enhanced striatal dopamine signaling can boost a kind of cognitive impulsivity, leading to errors and diminished performance, especially for people who already have high striatal dopamine functioning.”
He added that this impulsivity can also lead to errors “in situations where there are highly likely actions, thoughts, or behaviors” in a task, “which they would have to override to be successful” in performing it.
Dr. Westbrook gave the example of the “Stroop task where you are presented with words presented in some color ink and your job is to name the color of the ink but not read the word.”
If the word “green,” for example, was presented in green ink, “you may have no trouble naming the ink color,” but if it was presented in red ink “then you may impulsively read the word, because that is what we normally do with words.
“Overriding this kind of habitual action can be particularly slippery business when striatal dopamine signaling is pharmacologically enhanced,” Dr. Westbrook said.
No funding for the study was reported. Dr. Coghill reported relationships with Medice, Novartis, Servier, Takeda/Shire Cambridge University Press, and Oxford University Press.
A version of this article first appeared on Medscape.com.
VIENNA – , new research suggests.
In a randomized controlled trial, 40 healthy adults were given the attention-deficit/hyperactivity disorder (ADHD) treatments methylphenidate or dexamphetamine or the wakefulness-promoting drug modafinil vs. placebo.
While receiving the so-called “smart drugs,” participants spent more time and made more moves more quickly while solving each problem on a complex cognitive task than when given the placebo. But with no significant improvement in overall performance, all drugs were associated with a significant reduction in efficiency.
The findings “reinforce the idea that, while the drugs administered were motivational, the resulting increase in effort came at a cost in the loss of productivity,” said study presenter David Coghill, MD, PhD, chair of developmental mental health, the University of Melbourne.
This was especially true for individuals who scored high when receiving placebo, “who ended up producing below average productivity when on the drugs,” he noted.
“Overall, these drugs don’t increase the performance. Instead, they cause a regression to the mean, and appear to have a more negative effect on those who performed best at baseline,” Dr. Coghill added.
He presented the findings at the 35th European College of Neuropsychopharmacology (ECNP) Congress.
Past evidence ambiguous
Dr. Coghill noted that prescription-only stimulant drugs are increasingly used by employees and students as “smart drugs” to enhance workplace or academic productivity.
He conducted the study with colleagues from the department of economics at his institution, because of “their interest in people using cognitive enhancers within the financial industry, in the hope that that would increase their productivity in what is a very competitive industry on the floor of the trading rooms.”
However, while “there’s a subjective belief” that these drugs are effective as cognitive enhancers, the evidence to actually demonstrate that in healthy individuals “is, at best, ambiguous,” he told meeting attendees.
Improvements in cognitive capacities, such as working memory and improved planning, are most evident in clinical populations such as those with ADHD, which could be due to a “ceiling effect” of the cognitive tasks in healthy individuals, Dr. Coghill noted.
To investigate further, the researchers conducted a randomized, double-blinded trial of standard adult doses of methylphenidate (30 mg), dexamphetamine (15 mg), and modafinil (200 mg) vs. placebo. The healthy participants (n = 40), all of whom were aged 18-35 years, crossed to each of the other treatment groups over the course of four intervention sessions.
All were asked to solve eight instances of the knapsack task, the aim of which is to place theoretical objects in a knapsack to achieve the maximum value within a certain weight limit.
“This looks very simple but as the number of items increases, it becomes incredibly complex to compute, and actually is not computable using standard approaches. You have to deal with trial and error,” Dr. Coghill said.
The participants also completed several CANTAB cognitive tasks.
‘Surprising’ findings
Results showed that, overall, the drugs did not have a significant effect on task performance (slope = –0.16; P = .011).
Moreover, the drugs, both individually and collectively, had a significant negative effect on the value attained during any one attempt at the knapsack task (slope = –0.003; P = .02), an effect that extended “across the whole range” of task complexity, Dr. Coghill reported.
He went on to show that “participants actually looked as if they were working harder” when they took the three active drugs than when they were given a placebo. They also “spent more time solving each problem,” he added.
When taking the active drugs, participants made more moves during each task than when taking placebo, and made their moves more quickly.
“So these medications increased motivation,” Dr. Coghill said. “If you were sitting [and] watching this person, you would think that they were working harder.”
Yet their productivity, defined as the average gain in value per move on the knapsack task, was lower. Regression analysis identified a “significant and sizable drop in productivity” vs. placebo, Dr. Coghill noted.
This was the case for methylphenidate (P < .001), dexamphetamine (P < .001), and modafinil (P < .05), “whether you looked at the mean or median performance,” he said.
“Breaking it down a little bit more, when you looked at the individual participant level, you find substantial heterogeneity across participants,” noted Dr. Coghill.
“More than that, we found a significant negative correlation between productivity under methylphenidate compared to productivity under placebo, and this suggests a regression to the mean,” with participants who performed better under placebo performing worse with methylphenidate, he explained.
While the relationship was “exactly the same with modafinil,” it was not found with dexamphetamine, with a strong negative correlation between the productivity effects between dexamphetamine and methylphenidate (slope = –0.29; P < .0001).
“This is surprising because we assume that methylphenidate and dexamphetamine are working in very similar ways,” Dr. Coghill said.
Time to rethink, rewind?
Commenting for this article, session chair John F. Cryan, PhD, department of anatomy and neuroscience, University College Cork, Ireland, said that, based on the current data, “we might need to rethink [how] ‘smart’ psychopharmacological agents are.”
Dr. Cryan, chair of the ECNP Scientific Program Committee, added that there may be a need to revisit the difficulty of different types of cognitive tasks used in studies assessing the abilities of cognitive enhancing drugs and to “rewind conventional wisdom” around them.
Also commenting, Andrew Westbrook, PhD, of the department of cognitive linguistics and psychological sciences, Brown University, Providence, R.I., said the results seem “reasonable” and are “consistent with my own perspective.”
However, he told this news organization, “some caveats are warranted,” not least that the context of the task can have an impact on the results it obtains.
“We have hypothesized that pharmacologically-enhanced striatal dopamine signaling can boost a kind of cognitive impulsivity, leading to errors and diminished performance, especially for people who already have high striatal dopamine functioning.”
He added that this impulsivity can also lead to errors “in situations where there are highly likely actions, thoughts, or behaviors” in a task, “which they would have to override to be successful” in performing it.
Dr. Westbrook gave the example of the “Stroop task where you are presented with words presented in some color ink and your job is to name the color of the ink but not read the word.”
If the word “green,” for example, was presented in green ink, “you may have no trouble naming the ink color,” but if it was presented in red ink “then you may impulsively read the word, because that is what we normally do with words.
“Overriding this kind of habitual action can be particularly slippery business when striatal dopamine signaling is pharmacologically enhanced,” Dr. Westbrook said.
No funding for the study was reported. Dr. Coghill reported relationships with Medice, Novartis, Servier, Takeda/Shire Cambridge University Press, and Oxford University Press.
A version of this article first appeared on Medscape.com.
VIENNA – , new research suggests.
In a randomized controlled trial, 40 healthy adults were given the attention-deficit/hyperactivity disorder (ADHD) treatments methylphenidate or dexamphetamine or the wakefulness-promoting drug modafinil vs. placebo.
While receiving the so-called “smart drugs,” participants spent more time and made more moves more quickly while solving each problem on a complex cognitive task than when given the placebo. But with no significant improvement in overall performance, all drugs were associated with a significant reduction in efficiency.
The findings “reinforce the idea that, while the drugs administered were motivational, the resulting increase in effort came at a cost in the loss of productivity,” said study presenter David Coghill, MD, PhD, chair of developmental mental health, the University of Melbourne.
This was especially true for individuals who scored high when receiving placebo, “who ended up producing below average productivity when on the drugs,” he noted.
“Overall, these drugs don’t increase the performance. Instead, they cause a regression to the mean, and appear to have a more negative effect on those who performed best at baseline,” Dr. Coghill added.
He presented the findings at the 35th European College of Neuropsychopharmacology (ECNP) Congress.
Past evidence ambiguous
Dr. Coghill noted that prescription-only stimulant drugs are increasingly used by employees and students as “smart drugs” to enhance workplace or academic productivity.
He conducted the study with colleagues from the department of economics at his institution, because of “their interest in people using cognitive enhancers within the financial industry, in the hope that that would increase their productivity in what is a very competitive industry on the floor of the trading rooms.”
However, while “there’s a subjective belief” that these drugs are effective as cognitive enhancers, the evidence to actually demonstrate that in healthy individuals “is, at best, ambiguous,” he told meeting attendees.
Improvements in cognitive capacities, such as working memory and improved planning, are most evident in clinical populations such as those with ADHD, which could be due to a “ceiling effect” of the cognitive tasks in healthy individuals, Dr. Coghill noted.
To investigate further, the researchers conducted a randomized, double-blinded trial of standard adult doses of methylphenidate (30 mg), dexamphetamine (15 mg), and modafinil (200 mg) vs. placebo. The healthy participants (n = 40), all of whom were aged 18-35 years, crossed to each of the other treatment groups over the course of four intervention sessions.
All were asked to solve eight instances of the knapsack task, the aim of which is to place theoretical objects in a knapsack to achieve the maximum value within a certain weight limit.
“This looks very simple but as the number of items increases, it becomes incredibly complex to compute, and actually is not computable using standard approaches. You have to deal with trial and error,” Dr. Coghill said.
The participants also completed several CANTAB cognitive tasks.
‘Surprising’ findings
Results showed that, overall, the drugs did not have a significant effect on task performance (slope = –0.16; P = .011).
Moreover, the drugs, both individually and collectively, had a significant negative effect on the value attained during any one attempt at the knapsack task (slope = –0.003; P = .02), an effect that extended “across the whole range” of task complexity, Dr. Coghill reported.
He went on to show that “participants actually looked as if they were working harder” when they took the three active drugs than when they were given a placebo. They also “spent more time solving each problem,” he added.
When taking the active drugs, participants made more moves during each task than when taking placebo, and made their moves more quickly.
“So these medications increased motivation,” Dr. Coghill said. “If you were sitting [and] watching this person, you would think that they were working harder.”
Yet their productivity, defined as the average gain in value per move on the knapsack task, was lower. Regression analysis identified a “significant and sizable drop in productivity” vs. placebo, Dr. Coghill noted.
This was the case for methylphenidate (P < .001), dexamphetamine (P < .001), and modafinil (P < .05), “whether you looked at the mean or median performance,” he said.
“Breaking it down a little bit more, when you looked at the individual participant level, you find substantial heterogeneity across participants,” noted Dr. Coghill.
“More than that, we found a significant negative correlation between productivity under methylphenidate compared to productivity under placebo, and this suggests a regression to the mean,” with participants who performed better under placebo performing worse with methylphenidate, he explained.
While the relationship was “exactly the same with modafinil,” it was not found with dexamphetamine, with a strong negative correlation between the productivity effects between dexamphetamine and methylphenidate (slope = –0.29; P < .0001).
“This is surprising because we assume that methylphenidate and dexamphetamine are working in very similar ways,” Dr. Coghill said.
Time to rethink, rewind?
Commenting for this article, session chair John F. Cryan, PhD, department of anatomy and neuroscience, University College Cork, Ireland, said that, based on the current data, “we might need to rethink [how] ‘smart’ psychopharmacological agents are.”
Dr. Cryan, chair of the ECNP Scientific Program Committee, added that there may be a need to revisit the difficulty of different types of cognitive tasks used in studies assessing the abilities of cognitive enhancing drugs and to “rewind conventional wisdom” around them.
Also commenting, Andrew Westbrook, PhD, of the department of cognitive linguistics and psychological sciences, Brown University, Providence, R.I., said the results seem “reasonable” and are “consistent with my own perspective.”
However, he told this news organization, “some caveats are warranted,” not least that the context of the task can have an impact on the results it obtains.
“We have hypothesized that pharmacologically-enhanced striatal dopamine signaling can boost a kind of cognitive impulsivity, leading to errors and diminished performance, especially for people who already have high striatal dopamine functioning.”
He added that this impulsivity can also lead to errors “in situations where there are highly likely actions, thoughts, or behaviors” in a task, “which they would have to override to be successful” in performing it.
Dr. Westbrook gave the example of the “Stroop task where you are presented with words presented in some color ink and your job is to name the color of the ink but not read the word.”
If the word “green,” for example, was presented in green ink, “you may have no trouble naming the ink color,” but if it was presented in red ink “then you may impulsively read the word, because that is what we normally do with words.
“Overriding this kind of habitual action can be particularly slippery business when striatal dopamine signaling is pharmacologically enhanced,” Dr. Westbrook said.
No funding for the study was reported. Dr. Coghill reported relationships with Medice, Novartis, Servier, Takeda/Shire Cambridge University Press, and Oxford University Press.
A version of this article first appeared on Medscape.com.
AT ECNP 2022
Teens with diagnosed and undiagnosed ADHD report similar quality of life
The results align with findings from other studies suggesting lower quality of life (QOL) in teens with ADHD, but the current study is the first known to focus on the association between ADHD diagnosis itself vs. ADHD symptoms, and QOL, the researchers wrote. The findings show that at least some of the reduced QOL is associated with the diagnosis itself, they explained.
The researchers directly compared 393 teens with a childhood ADHD diagnosis to 393 matched teens with no ADHD diagnosis but who had hyperactive/inattentive behaviors.
The researchers reviewed self-reports from individuals who were enrolled in a population-based prospective study in Australia. The primary outcome was quality of life at age 14-15, which was measured with Child Health Utility 9D (CHU9D), a validated quality of life measure.
Study results
Overall, teens with and without an ADHD diagnosis reported similar levels of overall quality of life; the mean difference in the primary outcome CHU9D score was –0.03 (P = .10). Teens with and without an ADHD diagnosis also showed similar scores on measures of general health, happiness, and peer trust, the researchers noted.
The researchers also reviewed eight other prespecified, self-reported measures: academic self-concept, global health, negative social behaviors, overall happiness, peer trust, psychological sense of school membership, self-efficacy, and self-harm.
Teens diagnosed with ADHD in childhood were more than twice as likely to report self-harm (odds ratio 2.53, P less than .001) and displayed significantly more negative social behaviors (mean difference 1.56, P = .002), compared with teens without an ADHD diagnosis.
Teens diagnosed with ADHD in childhood also scored significantly worse on measures of sense of school membership (mean difference −2.58, P less than .001), academic self-concept (mean difference, −0.14; P = .02), and self-efficacy (mean difference −0.20; P = .007), compared to teens without an ADHD diagnosis.
The average age at ADHD diagnosis was 10 years, and 72% of the ADHD-diagnosed group were boys. No significant differences were noted for levels of hyperactive/inattentive behaviors and between girls and boys, but girls overall and children with the highest levels of hyperactive and inattentive behaviors reported generally worse outcomes, regardless of ADHD diagnosis, the researchers noted.
Don’t rush to diagnosis
Although rates of ADHD diagnosis in children continue to rise, the prevalence of hyperactivity and inattentive behaviors appears stable, which suggests a problem with diagnosis, senior author Alexandra Barratt, MBBS, MPH, PhD, professor of public health at the University of Sydney, Australia, said in an interview.
“Our hypothesis was that children who had been diagnosed, and we assume treated for, ADHD would have better outcomes, compared to children matched for hyperactivity/inattention behaviors who were left undiagnosed and untreated, but we were surprised to find that, at best, outcomes were unchanged, and for some outcomes, worse,” Dr. Barratt said.
“Our study provides evidence that diagnosing ADHD may lead, inadvertently, to long-term harms, particularly for children with mild or borderline hyperactivity and inattention behaviors,” she emphasized.
“We can’t say from this study what to do instead, but previously one of our team has looked at stepped diagnosis as an alternative option for children with mild or borderline hyperactivity and inattention behaviors,” she said.
The stepped diagnosis includes such actions as gathering behavior data from multiple sources, and conducting a period of watchful waiting without presumption of a diagnosis or active treatment.
Given the findings of the new study, “I would ask that health professionals considering a child who may have ADHD be aware that there is an evidence gap around the long-term impact of an ADHD diagnosis on children, and to proceed cautiously,” Dr. Barratt said. As for additional research, independent, high-quality, randomized controlled trials of ADHD diagnosis in children with mild or borderline hyperactivity/inattention behaviors are urgently needed, with long-term, patient-centered outcomes including quality of life she noted.
ADHD screening needs improvement
The incidence and prevalence of ADHD is on the rise, but much of the perceived increase in ADHD may be due to overdiagnosis, “and a lack of robust thorough psychological testing as standard of care for diagnosis,” Peter Loper, MD, a pediatrician and psychiatrist at the University of South Carolina, Columbia, said in an interview.
The current study “reinforces the necessity of consistent screening for comorbid mental health problems, and specifically for thoughts of self-harm, in those children who are diagnosed with ADHD,” he said.
Expressing his lack of astonishment about the study findings, Dr. Loper said: “Previous data indicates that while following initial diagnosis of a medical or mental health problem, patients may experience a sense of relief; however, this is followed shortly thereafter by feelings of insufficiency or anxiety related to their specific diagnosis.”
“As it stands now, ADHD is often diagnosed in children and adolescents using basic screening questionnaires,” said Dr. Loper. “The findings of this study may bolster calls for more robust and thorough psychological testing for supporting the diagnosis of ADHD,” he said.
Individuals diagnosed with ADHD can sometimes have difficulty with social skills and relating to others, said Dr. Loper. “They may be more prone to internalize their poor school performance as due to being ‘stupid’ or ‘dumb,’ ” he said. Children and teens with ADHD should, whenever possible, be involved in extracurricular activities that support the development of social skills, he said. Parents’ praise of the process/effort, rather than focusing only on outcomes such as grades, is very important for the esteem of children and teens with ADHD, he added.
The study limitations included the use of observational data vs. data from randomized trials, and the potential for confounding factors in propensity scoring, the researchers wrote. Additional limitations include the size of the sample, which may have been too small to detect additional differences between diagnosed teens and matched controls, they noted.
“As the study authors appropriately cite, a large, randomized trial would be very helpful in supporting additional understanding of this issue,” Dr. Loper added.
The study was supported by the National Health and Medical Research Council The researchers and Dr. Loper had no financial conflicts to disclose.
The results align with findings from other studies suggesting lower quality of life (QOL) in teens with ADHD, but the current study is the first known to focus on the association between ADHD diagnosis itself vs. ADHD symptoms, and QOL, the researchers wrote. The findings show that at least some of the reduced QOL is associated with the diagnosis itself, they explained.
The researchers directly compared 393 teens with a childhood ADHD diagnosis to 393 matched teens with no ADHD diagnosis but who had hyperactive/inattentive behaviors.
The researchers reviewed self-reports from individuals who were enrolled in a population-based prospective study in Australia. The primary outcome was quality of life at age 14-15, which was measured with Child Health Utility 9D (CHU9D), a validated quality of life measure.
Study results
Overall, teens with and without an ADHD diagnosis reported similar levels of overall quality of life; the mean difference in the primary outcome CHU9D score was –0.03 (P = .10). Teens with and without an ADHD diagnosis also showed similar scores on measures of general health, happiness, and peer trust, the researchers noted.
The researchers also reviewed eight other prespecified, self-reported measures: academic self-concept, global health, negative social behaviors, overall happiness, peer trust, psychological sense of school membership, self-efficacy, and self-harm.
Teens diagnosed with ADHD in childhood were more than twice as likely to report self-harm (odds ratio 2.53, P less than .001) and displayed significantly more negative social behaviors (mean difference 1.56, P = .002), compared with teens without an ADHD diagnosis.
Teens diagnosed with ADHD in childhood also scored significantly worse on measures of sense of school membership (mean difference −2.58, P less than .001), academic self-concept (mean difference, −0.14; P = .02), and self-efficacy (mean difference −0.20; P = .007), compared to teens without an ADHD diagnosis.
The average age at ADHD diagnosis was 10 years, and 72% of the ADHD-diagnosed group were boys. No significant differences were noted for levels of hyperactive/inattentive behaviors and between girls and boys, but girls overall and children with the highest levels of hyperactive and inattentive behaviors reported generally worse outcomes, regardless of ADHD diagnosis, the researchers noted.
Don’t rush to diagnosis
Although rates of ADHD diagnosis in children continue to rise, the prevalence of hyperactivity and inattentive behaviors appears stable, which suggests a problem with diagnosis, senior author Alexandra Barratt, MBBS, MPH, PhD, professor of public health at the University of Sydney, Australia, said in an interview.
“Our hypothesis was that children who had been diagnosed, and we assume treated for, ADHD would have better outcomes, compared to children matched for hyperactivity/inattention behaviors who were left undiagnosed and untreated, but we were surprised to find that, at best, outcomes were unchanged, and for some outcomes, worse,” Dr. Barratt said.
“Our study provides evidence that diagnosing ADHD may lead, inadvertently, to long-term harms, particularly for children with mild or borderline hyperactivity and inattention behaviors,” she emphasized.
“We can’t say from this study what to do instead, but previously one of our team has looked at stepped diagnosis as an alternative option for children with mild or borderline hyperactivity and inattention behaviors,” she said.
The stepped diagnosis includes such actions as gathering behavior data from multiple sources, and conducting a period of watchful waiting without presumption of a diagnosis or active treatment.
Given the findings of the new study, “I would ask that health professionals considering a child who may have ADHD be aware that there is an evidence gap around the long-term impact of an ADHD diagnosis on children, and to proceed cautiously,” Dr. Barratt said. As for additional research, independent, high-quality, randomized controlled trials of ADHD diagnosis in children with mild or borderline hyperactivity/inattention behaviors are urgently needed, with long-term, patient-centered outcomes including quality of life she noted.
ADHD screening needs improvement
The incidence and prevalence of ADHD is on the rise, but much of the perceived increase in ADHD may be due to overdiagnosis, “and a lack of robust thorough psychological testing as standard of care for diagnosis,” Peter Loper, MD, a pediatrician and psychiatrist at the University of South Carolina, Columbia, said in an interview.
The current study “reinforces the necessity of consistent screening for comorbid mental health problems, and specifically for thoughts of self-harm, in those children who are diagnosed with ADHD,” he said.
Expressing his lack of astonishment about the study findings, Dr. Loper said: “Previous data indicates that while following initial diagnosis of a medical or mental health problem, patients may experience a sense of relief; however, this is followed shortly thereafter by feelings of insufficiency or anxiety related to their specific diagnosis.”
“As it stands now, ADHD is often diagnosed in children and adolescents using basic screening questionnaires,” said Dr. Loper. “The findings of this study may bolster calls for more robust and thorough psychological testing for supporting the diagnosis of ADHD,” he said.
Individuals diagnosed with ADHD can sometimes have difficulty with social skills and relating to others, said Dr. Loper. “They may be more prone to internalize their poor school performance as due to being ‘stupid’ or ‘dumb,’ ” he said. Children and teens with ADHD should, whenever possible, be involved in extracurricular activities that support the development of social skills, he said. Parents’ praise of the process/effort, rather than focusing only on outcomes such as grades, is very important for the esteem of children and teens with ADHD, he added.
The study limitations included the use of observational data vs. data from randomized trials, and the potential for confounding factors in propensity scoring, the researchers wrote. Additional limitations include the size of the sample, which may have been too small to detect additional differences between diagnosed teens and matched controls, they noted.
“As the study authors appropriately cite, a large, randomized trial would be very helpful in supporting additional understanding of this issue,” Dr. Loper added.
The study was supported by the National Health and Medical Research Council The researchers and Dr. Loper had no financial conflicts to disclose.
The results align with findings from other studies suggesting lower quality of life (QOL) in teens with ADHD, but the current study is the first known to focus on the association between ADHD diagnosis itself vs. ADHD symptoms, and QOL, the researchers wrote. The findings show that at least some of the reduced QOL is associated with the diagnosis itself, they explained.
The researchers directly compared 393 teens with a childhood ADHD diagnosis to 393 matched teens with no ADHD diagnosis but who had hyperactive/inattentive behaviors.
The researchers reviewed self-reports from individuals who were enrolled in a population-based prospective study in Australia. The primary outcome was quality of life at age 14-15, which was measured with Child Health Utility 9D (CHU9D), a validated quality of life measure.
Study results
Overall, teens with and without an ADHD diagnosis reported similar levels of overall quality of life; the mean difference in the primary outcome CHU9D score was –0.03 (P = .10). Teens with and without an ADHD diagnosis also showed similar scores on measures of general health, happiness, and peer trust, the researchers noted.
The researchers also reviewed eight other prespecified, self-reported measures: academic self-concept, global health, negative social behaviors, overall happiness, peer trust, psychological sense of school membership, self-efficacy, and self-harm.
Teens diagnosed with ADHD in childhood were more than twice as likely to report self-harm (odds ratio 2.53, P less than .001) and displayed significantly more negative social behaviors (mean difference 1.56, P = .002), compared with teens without an ADHD diagnosis.
Teens diagnosed with ADHD in childhood also scored significantly worse on measures of sense of school membership (mean difference −2.58, P less than .001), academic self-concept (mean difference, −0.14; P = .02), and self-efficacy (mean difference −0.20; P = .007), compared to teens without an ADHD diagnosis.
The average age at ADHD diagnosis was 10 years, and 72% of the ADHD-diagnosed group were boys. No significant differences were noted for levels of hyperactive/inattentive behaviors and between girls and boys, but girls overall and children with the highest levels of hyperactive and inattentive behaviors reported generally worse outcomes, regardless of ADHD diagnosis, the researchers noted.
Don’t rush to diagnosis
Although rates of ADHD diagnosis in children continue to rise, the prevalence of hyperactivity and inattentive behaviors appears stable, which suggests a problem with diagnosis, senior author Alexandra Barratt, MBBS, MPH, PhD, professor of public health at the University of Sydney, Australia, said in an interview.
“Our hypothesis was that children who had been diagnosed, and we assume treated for, ADHD would have better outcomes, compared to children matched for hyperactivity/inattention behaviors who were left undiagnosed and untreated, but we were surprised to find that, at best, outcomes were unchanged, and for some outcomes, worse,” Dr. Barratt said.
“Our study provides evidence that diagnosing ADHD may lead, inadvertently, to long-term harms, particularly for children with mild or borderline hyperactivity and inattention behaviors,” she emphasized.
“We can’t say from this study what to do instead, but previously one of our team has looked at stepped diagnosis as an alternative option for children with mild or borderline hyperactivity and inattention behaviors,” she said.
The stepped diagnosis includes such actions as gathering behavior data from multiple sources, and conducting a period of watchful waiting without presumption of a diagnosis or active treatment.
Given the findings of the new study, “I would ask that health professionals considering a child who may have ADHD be aware that there is an evidence gap around the long-term impact of an ADHD diagnosis on children, and to proceed cautiously,” Dr. Barratt said. As for additional research, independent, high-quality, randomized controlled trials of ADHD diagnosis in children with mild or borderline hyperactivity/inattention behaviors are urgently needed, with long-term, patient-centered outcomes including quality of life she noted.
ADHD screening needs improvement
The incidence and prevalence of ADHD is on the rise, but much of the perceived increase in ADHD may be due to overdiagnosis, “and a lack of robust thorough psychological testing as standard of care for diagnosis,” Peter Loper, MD, a pediatrician and psychiatrist at the University of South Carolina, Columbia, said in an interview.
The current study “reinforces the necessity of consistent screening for comorbid mental health problems, and specifically for thoughts of self-harm, in those children who are diagnosed with ADHD,” he said.
Expressing his lack of astonishment about the study findings, Dr. Loper said: “Previous data indicates that while following initial diagnosis of a medical or mental health problem, patients may experience a sense of relief; however, this is followed shortly thereafter by feelings of insufficiency or anxiety related to their specific diagnosis.”
“As it stands now, ADHD is often diagnosed in children and adolescents using basic screening questionnaires,” said Dr. Loper. “The findings of this study may bolster calls for more robust and thorough psychological testing for supporting the diagnosis of ADHD,” he said.
Individuals diagnosed with ADHD can sometimes have difficulty with social skills and relating to others, said Dr. Loper. “They may be more prone to internalize their poor school performance as due to being ‘stupid’ or ‘dumb,’ ” he said. Children and teens with ADHD should, whenever possible, be involved in extracurricular activities that support the development of social skills, he said. Parents’ praise of the process/effort, rather than focusing only on outcomes such as grades, is very important for the esteem of children and teens with ADHD, he added.
The study limitations included the use of observational data vs. data from randomized trials, and the potential for confounding factors in propensity scoring, the researchers wrote. Additional limitations include the size of the sample, which may have been too small to detect additional differences between diagnosed teens and matched controls, they noted.
“As the study authors appropriately cite, a large, randomized trial would be very helpful in supporting additional understanding of this issue,” Dr. Loper added.
The study was supported by the National Health and Medical Research Council The researchers and Dr. Loper had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN









