User login
Impact of psoriasis on sexual activity
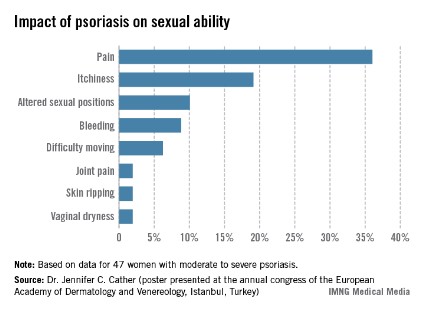
One third of a group of women with psoriasis reported that the pain associated with their condition interfered with their sexual activity, according to findings from a survey presented by Dr. Jennifer C. Cather.
Based on responses from a survey of 60 women with moderate to severe psoriasis, the specific complaints that were the most common ways in which psoriasis interfered with sexual activity were itchiness (19%), the need to adjust sexual position (10%), and bleeding (9%), Dr. Cather reported at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar. The survey was part of an effort to determine the impact of psoriasis on women’s sexual activity, desires, and relationships.
The data were previously presented in a poster at the annual congress of the European Academy of Dermatology and Venereology (Istanbul.
SDEF and this news organization are owned by Frontline Medical Communications. Dr. Cather disclosed that she is a consultant, speaker, or researcher for AbbVie, Novartis, Leo, Janssen, Amgen, Celgene, Merck, and Pfizer.
One third of a group of women with psoriasis reported that the pain associated with their condition interfered with their sexual activity, according to findings from a survey presented by Dr. Jennifer C. Cather.
Based on responses from a survey of 60 women with moderate to severe psoriasis, the specific complaints that were the most common ways in which psoriasis interfered with sexual activity were itchiness (19%), the need to adjust sexual position (10%), and bleeding (9%), Dr. Cather reported at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar. The survey was part of an effort to determine the impact of psoriasis on women’s sexual activity, desires, and relationships.
The data were previously presented in a poster at the annual congress of the European Academy of Dermatology and Venereology (Istanbul.
SDEF and this news organization are owned by Frontline Medical Communications. Dr. Cather disclosed that she is a consultant, speaker, or researcher for AbbVie, Novartis, Leo, Janssen, Amgen, Celgene, Merck, and Pfizer.

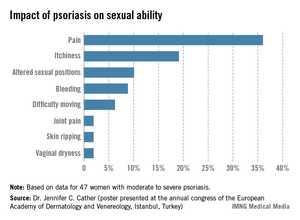
One third of a group of women with psoriasis reported that the pain associated with their condition interfered with their sexual activity, according to findings from a survey presented by Dr. Jennifer C. Cather.
Based on responses from a survey of 60 women with moderate to severe psoriasis, the specific complaints that were the most common ways in which psoriasis interfered with sexual activity were itchiness (19%), the need to adjust sexual position (10%), and bleeding (9%), Dr. Cather reported at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar. The survey was part of an effort to determine the impact of psoriasis on women’s sexual activity, desires, and relationships.
The data were previously presented in a poster at the annual congress of the European Academy of Dermatology and Venereology (Istanbul.
SDEF and this news organization are owned by Frontline Medical Communications. Dr. Cather disclosed that she is a consultant, speaker, or researcher for AbbVie, Novartis, Leo, Janssen, Amgen, Celgene, Merck, and Pfizer.

EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Product News: 11 2013
Cimzia
UCB, Inc, obtains US Food and Drug Administration approval of Cimzia (certolizumab pegol), a tumor necrosis factor blocker for the treatment of active psoriatic arthritis in adults. Cimzia also is indicated in adults for the treatment of moderate to severe rheumatoid arthritis and to reduce signs and symptoms of moderately to severely active Crohn disease. For more information, visit www.cimzia.com.
La Roche-Posay Antiaging Products
La Roche-Posay Laboratoire Dermatologique releases 3 antiaging products: Mela-D Deep Cleansing Brightening Foaming Cream, Redermic [R] Eyes, and Substiane [+] Serum. The Mela-D product is a deep-cleansing and brightening formula with lipohy-droxy acid that combats dark spots and gives the skin a clean and smooth appearance. Redermic [R] Eyes combines 0.01% pure retinol with a retinol booster complex and caffeine to reduce crow’s-feet and dark circles to rejuvenate the eye area. Substiane [+] Serum increases the volume and elasticity of the skin. All products are physician dispensed or available in select drugstores and online. For more information, visit www.laroche-posay.us.
Otrexup
Antares Pharma Inc obtains US Food and Drug Administration approval of Otrexup (methotrexate), a folate analog metabolic inhibitor for the treatment of severe, recalcitrant, disabling psoriasis in adults who have not responded to other therapies. Otrexup is self-administered subcutaneously once weekly via an easy-to-use, single-dose, disposable autoinjector, which utilizes Vibex Medi-Jet technology. Otrexup also is indicated for adults with severe active rheumatoid arthritis and for children with active polyarticular juvenile idiopathic arthritis. For more information, visit www.otrexup.com.
Valeant Partners With National Coalition Against Domestic
Violence Valeant Pharmaceuticals International, Inc, announces a partnership with the National Coalition Against Domestic Violence (NCADV) to help improve the lives of women. Valeant pledges to donate a portion of sales from Medicis and Obagi products to support the initiatives of the NCADV. For more information, visit enddomesticviolence.valeant.com.
If you would like your product included in Product News, please e-mail a press release to Melissa Steiger at msteiger@frontlinemedcom.com.
Cimzia
UCB, Inc, obtains US Food and Drug Administration approval of Cimzia (certolizumab pegol), a tumor necrosis factor blocker for the treatment of active psoriatic arthritis in adults. Cimzia also is indicated in adults for the treatment of moderate to severe rheumatoid arthritis and to reduce signs and symptoms of moderately to severely active Crohn disease. For more information, visit www.cimzia.com.
La Roche-Posay Antiaging Products
La Roche-Posay Laboratoire Dermatologique releases 3 antiaging products: Mela-D Deep Cleansing Brightening Foaming Cream, Redermic [R] Eyes, and Substiane [+] Serum. The Mela-D product is a deep-cleansing and brightening formula with lipohy-droxy acid that combats dark spots and gives the skin a clean and smooth appearance. Redermic [R] Eyes combines 0.01% pure retinol with a retinol booster complex and caffeine to reduce crow’s-feet and dark circles to rejuvenate the eye area. Substiane [+] Serum increases the volume and elasticity of the skin. All products are physician dispensed or available in select drugstores and online. For more information, visit www.laroche-posay.us.
Otrexup
Antares Pharma Inc obtains US Food and Drug Administration approval of Otrexup (methotrexate), a folate analog metabolic inhibitor for the treatment of severe, recalcitrant, disabling psoriasis in adults who have not responded to other therapies. Otrexup is self-administered subcutaneously once weekly via an easy-to-use, single-dose, disposable autoinjector, which utilizes Vibex Medi-Jet technology. Otrexup also is indicated for adults with severe active rheumatoid arthritis and for children with active polyarticular juvenile idiopathic arthritis. For more information, visit www.otrexup.com.
Valeant Partners With National Coalition Against Domestic
Violence Valeant Pharmaceuticals International, Inc, announces a partnership with the National Coalition Against Domestic Violence (NCADV) to help improve the lives of women. Valeant pledges to donate a portion of sales from Medicis and Obagi products to support the initiatives of the NCADV. For more information, visit enddomesticviolence.valeant.com.
If you would like your product included in Product News, please e-mail a press release to Melissa Steiger at msteiger@frontlinemedcom.com.
Cimzia
UCB, Inc, obtains US Food and Drug Administration approval of Cimzia (certolizumab pegol), a tumor necrosis factor blocker for the treatment of active psoriatic arthritis in adults. Cimzia also is indicated in adults for the treatment of moderate to severe rheumatoid arthritis and to reduce signs and symptoms of moderately to severely active Crohn disease. For more information, visit www.cimzia.com.
La Roche-Posay Antiaging Products
La Roche-Posay Laboratoire Dermatologique releases 3 antiaging products: Mela-D Deep Cleansing Brightening Foaming Cream, Redermic [R] Eyes, and Substiane [+] Serum. The Mela-D product is a deep-cleansing and brightening formula with lipohy-droxy acid that combats dark spots and gives the skin a clean and smooth appearance. Redermic [R] Eyes combines 0.01% pure retinol with a retinol booster complex and caffeine to reduce crow’s-feet and dark circles to rejuvenate the eye area. Substiane [+] Serum increases the volume and elasticity of the skin. All products are physician dispensed or available in select drugstores and online. For more information, visit www.laroche-posay.us.
Otrexup
Antares Pharma Inc obtains US Food and Drug Administration approval of Otrexup (methotrexate), a folate analog metabolic inhibitor for the treatment of severe, recalcitrant, disabling psoriasis in adults who have not responded to other therapies. Otrexup is self-administered subcutaneously once weekly via an easy-to-use, single-dose, disposable autoinjector, which utilizes Vibex Medi-Jet technology. Otrexup also is indicated for adults with severe active rheumatoid arthritis and for children with active polyarticular juvenile idiopathic arthritis. For more information, visit www.otrexup.com.
Valeant Partners With National Coalition Against Domestic
Violence Valeant Pharmaceuticals International, Inc, announces a partnership with the National Coalition Against Domestic Violence (NCADV) to help improve the lives of women. Valeant pledges to donate a portion of sales from Medicis and Obagi products to support the initiatives of the NCADV. For more information, visit enddomesticviolence.valeant.com.
If you would like your product included in Product News, please e-mail a press release to Melissa Steiger at msteiger@frontlinemedcom.com.
Superficial Plantar Fibromatosis
Management of keloids draws on clinical wisdom
WOODINVILLE, WASH. – Make decisions about whether to surgically or medically manage keloids according to features of the lesion and patient-related factors, especially likely treatment compliance, Dr. Hilary E. Baldwin advised.
"Step one when we are dealing with our patients with keloids is trying to decide what the patients actually hope for," Dr. Baldwin said at the annual Coastal Dermatology Symposium. That might be eradication of the keloid; palliation of symptoms such as itching or pain; flattening, lightening, or softening of the keloid; ability to wear clip-on earrings; or restoration of mobility.
"Most patients, in my opinion, are actually not surgical candidates, and most need to be convinced to pursue other options," said Dr. Baldwin of the dermatology department at the State University of New York, Brooklyn.
Surgical management
Size and shape should factor into decisions about surgical removal of keloids, said Dr. Baldwin. Larger keloids are not much more difficult to remove than smaller ones, and pedunculated keloids are generally the most amenable to surgery, she noted.
Keloid age also comes into play, as the older, mature, brown lesions are less likely to recur after surgery than the younger, pink, symptomatic ones. Location affects outcome as well, Dr. Baldwin said. Keloids on the jaw, the deltoid, mid-back, mid-chest, and upper back are most likely to recur if treated surgically, and those in low-tension areas are less likely to do so.
Surgery also may be considered for keloids that are truly unresponsive to intralesional corticosteroids, but those are uncommon as previous lack of response is usually because of an inadequate dose, Dr. Baldwin explained.
"Most important is patient commitment. They often believe that cutting is a quick fix and they are not going to have to do anything afterward, and they are poor flight risks because of that," she said. Statistics suggest that the nearly 100% of keloids recur with surgery alone, but the value falls to 50% with adjunctive corticosteroids, and 20% with adjunctive radiation therapy, she added.
Adjunctive therapies
When adjunctive corticosteroids are used after surgery, the steroid is injected into the base and walls of the excision site, according to Dr. Baldwin; her preference is to use 40 mg/cc triamcinolone, with injections given for at least 6 months.
"Radiation therapy ... works really well postop; in fact, it’s our best adjunctive therapy, if you’re not afraid of it and the patient’s not afraid of it," she said. At her institution, this therapy is completed within a week after surgery, "so even if patients are a flight risk, they always return for that first visit, and then they are done with at least one adjunctive therapy."
The main barrier to use has been a fear of secondary malignancy. But reports of cancer after radiation therapy are rare and questionably associated with the treatment, Dr. Baldwin said.
Pressure dressings also are effective, and these are most practical in the form of compression earrings worn after excision of earlobe keloids, usually for at least 6 months. The earrings also come in a form called "sleepers" that may be more acceptable to patients.
Interferon injections also can help prevent recurrence. "I treat the very first day ... into the wall and into the base, just as I would with steroids, and then repeat 1 week later," Dr. Baldwin explained. "So if you use this and radiation therapy, 1 week later, you have finished two adjunctive therapies."
Especially challenging are keloids nearly obliterating normal tissue, such as extensive earlobe keloids. "I call them last-chance keloids," Dr. Baldwin commented. "If I don’t do a good job and don’t have good adjunctive therapy, it’s going to regrow and that’s the last time I am going to be able to cut. The earlobe will be eaten away with keloid, and we are never going to be able to give [the patient] an earlobe on which to hang an earring."
After excision of last-chance keloids, "I recommend that you do everything ... Use everything that is available to you," Dr. Baldwin advised. In addition to excision, that includes "intralesional steroids, intralesional interferon, radiation therapy, and earrings. Why pick one? Use them all if appropriate, and you are much less likely to have a recurrence," she said.
Medical management
Medical therapy is indicated for large, sessile keloids and for those located in areas where surgery could lead to complications, according to Dr. Baldwin.
In these cases, intralesional corticosteroids are the mainstay, and her preference is to use a high dose of triamcinolone. "I only use 40 [mg/cc]—that’s it. I never use anything less," she said. "I don’t care where the keloid is: A keloid on the face is the same as a keloid on the back. Most of the failures that I see are due to inadequate concentration. It’s almost always on the face where people are a little bit shy about using the extraordinarily high doses," she added.
Dr. Baldwin dismissed the dogma of not exceeding 40 mg per month, given that this translates to a dose of just 50 mg of prednisone. "I will sometimes use 80 [mg] in one sitting. I don’t really have that kind of a restriction because it doesn’t make a ton of sense," she said.
For pre-injection anesthesia, Dr. Baldwin recommended applying topical anesthetic followed by a lidocaine ring block. When treating hard keloids, to prevent backwash of the corticosteroid along the needle track, she said she preapplies Krazy Glue and, immediately after removing the needle, applies a strip of precut tape.
Patients should be advised of the possible complications of hypopigmentation, telangiectasia, and atrophy. "You also have to warn your patients about what I call the Play-Doh effect," whereby the keloid spreads as it softens, Dr. Baldwin said. "So the footprint is ultimately much bigger than it was originally, although it’s now flatter."
Intralesional injection of 5-fluorouracil can be used alone or with admixed steroids. However, Dr. Baldwin said that in her hands, the combination has been disappointing, perhaps because the triamcinolone is diluted in the process. "So I’ve reconsidered recently the possibility of doing the steroids the way I like it and following [with 5-fluoruracil] in addition to it, rather than diluting my triamcinolone," she said.
The newest nonsurgical therapy is intralesional cryotherapy, whereby the keloid is frozen from the inside out with a probe attached to a cryotherapy device, so it eventually undergoes necrosis while the skin is preserved. The reduction in volume achieved has ranged from 51% to 93% across studies, with minimal to no hypopigmentation.
"The probe itself unfortunately costs $450 and is not reusable, which is a problem. At the present time this is not covered by insurance," said Dr. Baldwin, who disclosed no relevant conflicts of interest. "But for keloids that really can’t be treated any other way, it has been quite helpful."
The meeting was presented by the Caribbean Dermatology Symposium.
WOODINVILLE, WASH. – Make decisions about whether to surgically or medically manage keloids according to features of the lesion and patient-related factors, especially likely treatment compliance, Dr. Hilary E. Baldwin advised.
"Step one when we are dealing with our patients with keloids is trying to decide what the patients actually hope for," Dr. Baldwin said at the annual Coastal Dermatology Symposium. That might be eradication of the keloid; palliation of symptoms such as itching or pain; flattening, lightening, or softening of the keloid; ability to wear clip-on earrings; or restoration of mobility.
"Most patients, in my opinion, are actually not surgical candidates, and most need to be convinced to pursue other options," said Dr. Baldwin of the dermatology department at the State University of New York, Brooklyn.
Surgical management
Size and shape should factor into decisions about surgical removal of keloids, said Dr. Baldwin. Larger keloids are not much more difficult to remove than smaller ones, and pedunculated keloids are generally the most amenable to surgery, she noted.
Keloid age also comes into play, as the older, mature, brown lesions are less likely to recur after surgery than the younger, pink, symptomatic ones. Location affects outcome as well, Dr. Baldwin said. Keloids on the jaw, the deltoid, mid-back, mid-chest, and upper back are most likely to recur if treated surgically, and those in low-tension areas are less likely to do so.
Surgery also may be considered for keloids that are truly unresponsive to intralesional corticosteroids, but those are uncommon as previous lack of response is usually because of an inadequate dose, Dr. Baldwin explained.
"Most important is patient commitment. They often believe that cutting is a quick fix and they are not going to have to do anything afterward, and they are poor flight risks because of that," she said. Statistics suggest that the nearly 100% of keloids recur with surgery alone, but the value falls to 50% with adjunctive corticosteroids, and 20% with adjunctive radiation therapy, she added.
Adjunctive therapies
When adjunctive corticosteroids are used after surgery, the steroid is injected into the base and walls of the excision site, according to Dr. Baldwin; her preference is to use 40 mg/cc triamcinolone, with injections given for at least 6 months.
"Radiation therapy ... works really well postop; in fact, it’s our best adjunctive therapy, if you’re not afraid of it and the patient’s not afraid of it," she said. At her institution, this therapy is completed within a week after surgery, "so even if patients are a flight risk, they always return for that first visit, and then they are done with at least one adjunctive therapy."
The main barrier to use has been a fear of secondary malignancy. But reports of cancer after radiation therapy are rare and questionably associated with the treatment, Dr. Baldwin said.
Pressure dressings also are effective, and these are most practical in the form of compression earrings worn after excision of earlobe keloids, usually for at least 6 months. The earrings also come in a form called "sleepers" that may be more acceptable to patients.
Interferon injections also can help prevent recurrence. "I treat the very first day ... into the wall and into the base, just as I would with steroids, and then repeat 1 week later," Dr. Baldwin explained. "So if you use this and radiation therapy, 1 week later, you have finished two adjunctive therapies."
Especially challenging are keloids nearly obliterating normal tissue, such as extensive earlobe keloids. "I call them last-chance keloids," Dr. Baldwin commented. "If I don’t do a good job and don’t have good adjunctive therapy, it’s going to regrow and that’s the last time I am going to be able to cut. The earlobe will be eaten away with keloid, and we are never going to be able to give [the patient] an earlobe on which to hang an earring."
After excision of last-chance keloids, "I recommend that you do everything ... Use everything that is available to you," Dr. Baldwin advised. In addition to excision, that includes "intralesional steroids, intralesional interferon, radiation therapy, and earrings. Why pick one? Use them all if appropriate, and you are much less likely to have a recurrence," she said.
Medical management
Medical therapy is indicated for large, sessile keloids and for those located in areas where surgery could lead to complications, according to Dr. Baldwin.
In these cases, intralesional corticosteroids are the mainstay, and her preference is to use a high dose of triamcinolone. "I only use 40 [mg/cc]—that’s it. I never use anything less," she said. "I don’t care where the keloid is: A keloid on the face is the same as a keloid on the back. Most of the failures that I see are due to inadequate concentration. It’s almost always on the face where people are a little bit shy about using the extraordinarily high doses," she added.
Dr. Baldwin dismissed the dogma of not exceeding 40 mg per month, given that this translates to a dose of just 50 mg of prednisone. "I will sometimes use 80 [mg] in one sitting. I don’t really have that kind of a restriction because it doesn’t make a ton of sense," she said.
For pre-injection anesthesia, Dr. Baldwin recommended applying topical anesthetic followed by a lidocaine ring block. When treating hard keloids, to prevent backwash of the corticosteroid along the needle track, she said she preapplies Krazy Glue and, immediately after removing the needle, applies a strip of precut tape.
Patients should be advised of the possible complications of hypopigmentation, telangiectasia, and atrophy. "You also have to warn your patients about what I call the Play-Doh effect," whereby the keloid spreads as it softens, Dr. Baldwin said. "So the footprint is ultimately much bigger than it was originally, although it’s now flatter."
Intralesional injection of 5-fluorouracil can be used alone or with admixed steroids. However, Dr. Baldwin said that in her hands, the combination has been disappointing, perhaps because the triamcinolone is diluted in the process. "So I’ve reconsidered recently the possibility of doing the steroids the way I like it and following [with 5-fluoruracil] in addition to it, rather than diluting my triamcinolone," she said.
The newest nonsurgical therapy is intralesional cryotherapy, whereby the keloid is frozen from the inside out with a probe attached to a cryotherapy device, so it eventually undergoes necrosis while the skin is preserved. The reduction in volume achieved has ranged from 51% to 93% across studies, with minimal to no hypopigmentation.
"The probe itself unfortunately costs $450 and is not reusable, which is a problem. At the present time this is not covered by insurance," said Dr. Baldwin, who disclosed no relevant conflicts of interest. "But for keloids that really can’t be treated any other way, it has been quite helpful."
The meeting was presented by the Caribbean Dermatology Symposium.
WOODINVILLE, WASH. – Make decisions about whether to surgically or medically manage keloids according to features of the lesion and patient-related factors, especially likely treatment compliance, Dr. Hilary E. Baldwin advised.
"Step one when we are dealing with our patients with keloids is trying to decide what the patients actually hope for," Dr. Baldwin said at the annual Coastal Dermatology Symposium. That might be eradication of the keloid; palliation of symptoms such as itching or pain; flattening, lightening, or softening of the keloid; ability to wear clip-on earrings; or restoration of mobility.
"Most patients, in my opinion, are actually not surgical candidates, and most need to be convinced to pursue other options," said Dr. Baldwin of the dermatology department at the State University of New York, Brooklyn.
Surgical management
Size and shape should factor into decisions about surgical removal of keloids, said Dr. Baldwin. Larger keloids are not much more difficult to remove than smaller ones, and pedunculated keloids are generally the most amenable to surgery, she noted.
Keloid age also comes into play, as the older, mature, brown lesions are less likely to recur after surgery than the younger, pink, symptomatic ones. Location affects outcome as well, Dr. Baldwin said. Keloids on the jaw, the deltoid, mid-back, mid-chest, and upper back are most likely to recur if treated surgically, and those in low-tension areas are less likely to do so.
Surgery also may be considered for keloids that are truly unresponsive to intralesional corticosteroids, but those are uncommon as previous lack of response is usually because of an inadequate dose, Dr. Baldwin explained.
"Most important is patient commitment. They often believe that cutting is a quick fix and they are not going to have to do anything afterward, and they are poor flight risks because of that," she said. Statistics suggest that the nearly 100% of keloids recur with surgery alone, but the value falls to 50% with adjunctive corticosteroids, and 20% with adjunctive radiation therapy, she added.
Adjunctive therapies
When adjunctive corticosteroids are used after surgery, the steroid is injected into the base and walls of the excision site, according to Dr. Baldwin; her preference is to use 40 mg/cc triamcinolone, with injections given for at least 6 months.
"Radiation therapy ... works really well postop; in fact, it’s our best adjunctive therapy, if you’re not afraid of it and the patient’s not afraid of it," she said. At her institution, this therapy is completed within a week after surgery, "so even if patients are a flight risk, they always return for that first visit, and then they are done with at least one adjunctive therapy."
The main barrier to use has been a fear of secondary malignancy. But reports of cancer after radiation therapy are rare and questionably associated with the treatment, Dr. Baldwin said.
Pressure dressings also are effective, and these are most practical in the form of compression earrings worn after excision of earlobe keloids, usually for at least 6 months. The earrings also come in a form called "sleepers" that may be more acceptable to patients.
Interferon injections also can help prevent recurrence. "I treat the very first day ... into the wall and into the base, just as I would with steroids, and then repeat 1 week later," Dr. Baldwin explained. "So if you use this and radiation therapy, 1 week later, you have finished two adjunctive therapies."
Especially challenging are keloids nearly obliterating normal tissue, such as extensive earlobe keloids. "I call them last-chance keloids," Dr. Baldwin commented. "If I don’t do a good job and don’t have good adjunctive therapy, it’s going to regrow and that’s the last time I am going to be able to cut. The earlobe will be eaten away with keloid, and we are never going to be able to give [the patient] an earlobe on which to hang an earring."
After excision of last-chance keloids, "I recommend that you do everything ... Use everything that is available to you," Dr. Baldwin advised. In addition to excision, that includes "intralesional steroids, intralesional interferon, radiation therapy, and earrings. Why pick one? Use them all if appropriate, and you are much less likely to have a recurrence," she said.
Medical management
Medical therapy is indicated for large, sessile keloids and for those located in areas where surgery could lead to complications, according to Dr. Baldwin.
In these cases, intralesional corticosteroids are the mainstay, and her preference is to use a high dose of triamcinolone. "I only use 40 [mg/cc]—that’s it. I never use anything less," she said. "I don’t care where the keloid is: A keloid on the face is the same as a keloid on the back. Most of the failures that I see are due to inadequate concentration. It’s almost always on the face where people are a little bit shy about using the extraordinarily high doses," she added.
Dr. Baldwin dismissed the dogma of not exceeding 40 mg per month, given that this translates to a dose of just 50 mg of prednisone. "I will sometimes use 80 [mg] in one sitting. I don’t really have that kind of a restriction because it doesn’t make a ton of sense," she said.
For pre-injection anesthesia, Dr. Baldwin recommended applying topical anesthetic followed by a lidocaine ring block. When treating hard keloids, to prevent backwash of the corticosteroid along the needle track, she said she preapplies Krazy Glue and, immediately after removing the needle, applies a strip of precut tape.
Patients should be advised of the possible complications of hypopigmentation, telangiectasia, and atrophy. "You also have to warn your patients about what I call the Play-Doh effect," whereby the keloid spreads as it softens, Dr. Baldwin said. "So the footprint is ultimately much bigger than it was originally, although it’s now flatter."
Intralesional injection of 5-fluorouracil can be used alone or with admixed steroids. However, Dr. Baldwin said that in her hands, the combination has been disappointing, perhaps because the triamcinolone is diluted in the process. "So I’ve reconsidered recently the possibility of doing the steroids the way I like it and following [with 5-fluoruracil] in addition to it, rather than diluting my triamcinolone," she said.
The newest nonsurgical therapy is intralesional cryotherapy, whereby the keloid is frozen from the inside out with a probe attached to a cryotherapy device, so it eventually undergoes necrosis while the skin is preserved. The reduction in volume achieved has ranged from 51% to 93% across studies, with minimal to no hypopigmentation.
"The probe itself unfortunately costs $450 and is not reusable, which is a problem. At the present time this is not covered by insurance," said Dr. Baldwin, who disclosed no relevant conflicts of interest. "But for keloids that really can’t be treated any other way, it has been quite helpful."
The meeting was presented by the Caribbean Dermatology Symposium.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
Novel PDT method proves superior for difficult AKs
ISTANBUL – A novel form of photodynamic therapy achieved a much higher cure rate than did conventional PDT, based on a randomized controlled trial of organ transplant recipients with numerous actinic keratoses.
The novel approach combines pretreatment with ablative fractional laser resurfacing followed immediately by application of a photosensitizing agent and natural light exposure. In addition to its efficacy, the novel PDT approach was painless. Conventional PDT can be particularly painful for organ transplant recipients as they tend to have particularly thick and widespread lesions located at pain-sensitive sites including the underarms, dorsal hands, and face, Dr. Merete Haedersdal reported at the annual congress of the European Academy of Dermatology and Venereology.
"From a clinical point of view, we are struggling with these patients. ... Now we can individualize PDT. We already have a wonderful conventional PDT, but now we’re better off with these combined techniques, especially when we are going to treat larger areas as in this high-risk group of patients," said Dr. Haedersdal, professor of dermatology at the University of Copenhagen.
As a result of their long-term immunosuppressive therapy, organ transplant recipients have a 100-fold higher risk than the general population for squamous cell carcinoma. Further, their skin tumors grow aggressively; 6%-8% of organ transplant recipients die due to nonmelanoma skin cancers.
The novel PDT approach begins with ablative fractional laser resurfacing to create tiny holes in the skin, allowing increased uptake of the photosensitizer. Dr. Haedersdal and her coworkers used methyl aminolevulinate 16% cream, applied immediately after laser treatment. It takes 30 minutes to start the biochemical process of protoporphyrin-9 production, and the patient then goes outside for a full 2 hours of natural light exposure. This photoactivation period averaged 62,583 lux in the study. The patient then covers the treated area or stays inside for the rest of the day.
Dr. Haedersdal reported on 16 patients with a total of 542 actinic keratoses (AKs) on the scalp, chest, and extremities. The patients had received a donor organ a median of 14 years earlier. In each patient, four symmetrical skin areas within the same anatomic region were mapped out and randomized to receive a single treatment with either the novel laser-assisted daylight PDT, conventional PDT with a red light–emitting diode light source, PDT with daylight rather than the LED light source, or ablative fractional laser therapy alone.
The primary endpoint was the cure rate, or complete lesion response of all AKs at 3 months post treatment. The cure rate was 74% in the laser-assisted daylight PDT group compared with 50% with daylight PDT, 48% with conventional PDT, and 0 with laser-only treatment. When the analysis was restricted to the thicker, grade II and III AKs, the cure rate was 67% with the novel therapy, 50% with conventional PDT, 40% with daylight PDT, and 0 with laser-only treatment.
Median pain scores on a 0-10 visual analog scale were 0 for the areas treated with laser-assisted daylight PDT, laser only, and daylight PDT compared with 4.5 for the portion of skin targeted with conventional PDT. The key to pain-free PDT, according to Dr. Haedersdal, is to do the laser resurfacing at a very low fluence, allowing for continuous daylight photobleaching. The fractional laser therapy is delivered at a total of 5.6 mJ using 4.6 mJ per pulse, 1.15 W, a 50-microsecond pulse duration, 500 Hz, and 2.4% density using a 2,940-nm erbium laser. The laser holes are drilled into the epidermal layer, but not below the basement membrane.
Crusting and erythema developed in all PDT-treated patients but were slightly more intense in the areas treated with laser-assisted PDT. One patient in the laser-assisted PDT group had transient hypopigmentation.
Based on blinded assessments at 3 months, the laser-assisted daylight PDT areas displayed significantly greater skin smoothening and rejuvenation with less dyschromia than the other treatment zones. Cosmesis was rated "excellent" in the laser-treated zones of 10 out of 16 patients, a much higher rate than seen in the other treatment areas.
Dr. Haedersdal reported having received research grants from roughly half a dozen medical device and pharmaceutical companies.
ISTANBUL – A novel form of photodynamic therapy achieved a much higher cure rate than did conventional PDT, based on a randomized controlled trial of organ transplant recipients with numerous actinic keratoses.
The novel approach combines pretreatment with ablative fractional laser resurfacing followed immediately by application of a photosensitizing agent and natural light exposure. In addition to its efficacy, the novel PDT approach was painless. Conventional PDT can be particularly painful for organ transplant recipients as they tend to have particularly thick and widespread lesions located at pain-sensitive sites including the underarms, dorsal hands, and face, Dr. Merete Haedersdal reported at the annual congress of the European Academy of Dermatology and Venereology.
"From a clinical point of view, we are struggling with these patients. ... Now we can individualize PDT. We already have a wonderful conventional PDT, but now we’re better off with these combined techniques, especially when we are going to treat larger areas as in this high-risk group of patients," said Dr. Haedersdal, professor of dermatology at the University of Copenhagen.
As a result of their long-term immunosuppressive therapy, organ transplant recipients have a 100-fold higher risk than the general population for squamous cell carcinoma. Further, their skin tumors grow aggressively; 6%-8% of organ transplant recipients die due to nonmelanoma skin cancers.
The novel PDT approach begins with ablative fractional laser resurfacing to create tiny holes in the skin, allowing increased uptake of the photosensitizer. Dr. Haedersdal and her coworkers used methyl aminolevulinate 16% cream, applied immediately after laser treatment. It takes 30 minutes to start the biochemical process of protoporphyrin-9 production, and the patient then goes outside for a full 2 hours of natural light exposure. This photoactivation period averaged 62,583 lux in the study. The patient then covers the treated area or stays inside for the rest of the day.
Dr. Haedersdal reported on 16 patients with a total of 542 actinic keratoses (AKs) on the scalp, chest, and extremities. The patients had received a donor organ a median of 14 years earlier. In each patient, four symmetrical skin areas within the same anatomic region were mapped out and randomized to receive a single treatment with either the novel laser-assisted daylight PDT, conventional PDT with a red light–emitting diode light source, PDT with daylight rather than the LED light source, or ablative fractional laser therapy alone.
The primary endpoint was the cure rate, or complete lesion response of all AKs at 3 months post treatment. The cure rate was 74% in the laser-assisted daylight PDT group compared with 50% with daylight PDT, 48% with conventional PDT, and 0 with laser-only treatment. When the analysis was restricted to the thicker, grade II and III AKs, the cure rate was 67% with the novel therapy, 50% with conventional PDT, 40% with daylight PDT, and 0 with laser-only treatment.
Median pain scores on a 0-10 visual analog scale were 0 for the areas treated with laser-assisted daylight PDT, laser only, and daylight PDT compared with 4.5 for the portion of skin targeted with conventional PDT. The key to pain-free PDT, according to Dr. Haedersdal, is to do the laser resurfacing at a very low fluence, allowing for continuous daylight photobleaching. The fractional laser therapy is delivered at a total of 5.6 mJ using 4.6 mJ per pulse, 1.15 W, a 50-microsecond pulse duration, 500 Hz, and 2.4% density using a 2,940-nm erbium laser. The laser holes are drilled into the epidermal layer, but not below the basement membrane.
Crusting and erythema developed in all PDT-treated patients but were slightly more intense in the areas treated with laser-assisted PDT. One patient in the laser-assisted PDT group had transient hypopigmentation.
Based on blinded assessments at 3 months, the laser-assisted daylight PDT areas displayed significantly greater skin smoothening and rejuvenation with less dyschromia than the other treatment zones. Cosmesis was rated "excellent" in the laser-treated zones of 10 out of 16 patients, a much higher rate than seen in the other treatment areas.
Dr. Haedersdal reported having received research grants from roughly half a dozen medical device and pharmaceutical companies.
ISTANBUL – A novel form of photodynamic therapy achieved a much higher cure rate than did conventional PDT, based on a randomized controlled trial of organ transplant recipients with numerous actinic keratoses.
The novel approach combines pretreatment with ablative fractional laser resurfacing followed immediately by application of a photosensitizing agent and natural light exposure. In addition to its efficacy, the novel PDT approach was painless. Conventional PDT can be particularly painful for organ transplant recipients as they tend to have particularly thick and widespread lesions located at pain-sensitive sites including the underarms, dorsal hands, and face, Dr. Merete Haedersdal reported at the annual congress of the European Academy of Dermatology and Venereology.
"From a clinical point of view, we are struggling with these patients. ... Now we can individualize PDT. We already have a wonderful conventional PDT, but now we’re better off with these combined techniques, especially when we are going to treat larger areas as in this high-risk group of patients," said Dr. Haedersdal, professor of dermatology at the University of Copenhagen.
As a result of their long-term immunosuppressive therapy, organ transplant recipients have a 100-fold higher risk than the general population for squamous cell carcinoma. Further, their skin tumors grow aggressively; 6%-8% of organ transplant recipients die due to nonmelanoma skin cancers.
The novel PDT approach begins with ablative fractional laser resurfacing to create tiny holes in the skin, allowing increased uptake of the photosensitizer. Dr. Haedersdal and her coworkers used methyl aminolevulinate 16% cream, applied immediately after laser treatment. It takes 30 minutes to start the biochemical process of protoporphyrin-9 production, and the patient then goes outside for a full 2 hours of natural light exposure. This photoactivation period averaged 62,583 lux in the study. The patient then covers the treated area or stays inside for the rest of the day.
Dr. Haedersdal reported on 16 patients with a total of 542 actinic keratoses (AKs) on the scalp, chest, and extremities. The patients had received a donor organ a median of 14 years earlier. In each patient, four symmetrical skin areas within the same anatomic region were mapped out and randomized to receive a single treatment with either the novel laser-assisted daylight PDT, conventional PDT with a red light–emitting diode light source, PDT with daylight rather than the LED light source, or ablative fractional laser therapy alone.
The primary endpoint was the cure rate, or complete lesion response of all AKs at 3 months post treatment. The cure rate was 74% in the laser-assisted daylight PDT group compared with 50% with daylight PDT, 48% with conventional PDT, and 0 with laser-only treatment. When the analysis was restricted to the thicker, grade II and III AKs, the cure rate was 67% with the novel therapy, 50% with conventional PDT, 40% with daylight PDT, and 0 with laser-only treatment.
Median pain scores on a 0-10 visual analog scale were 0 for the areas treated with laser-assisted daylight PDT, laser only, and daylight PDT compared with 4.5 for the portion of skin targeted with conventional PDT. The key to pain-free PDT, according to Dr. Haedersdal, is to do the laser resurfacing at a very low fluence, allowing for continuous daylight photobleaching. The fractional laser therapy is delivered at a total of 5.6 mJ using 4.6 mJ per pulse, 1.15 W, a 50-microsecond pulse duration, 500 Hz, and 2.4% density using a 2,940-nm erbium laser. The laser holes are drilled into the epidermal layer, but not below the basement membrane.
Crusting and erythema developed in all PDT-treated patients but were slightly more intense in the areas treated with laser-assisted PDT. One patient in the laser-assisted PDT group had transient hypopigmentation.
Based on blinded assessments at 3 months, the laser-assisted daylight PDT areas displayed significantly greater skin smoothening and rejuvenation with less dyschromia than the other treatment zones. Cosmesis was rated "excellent" in the laser-treated zones of 10 out of 16 patients, a much higher rate than seen in the other treatment areas.
Dr. Haedersdal reported having received research grants from roughly half a dozen medical device and pharmaceutical companies.
AT THE EADV CONGRESS
Major finding: Three months post treatment, the actinic keratosis cure rate was 74% in organ transplant recipients who underwent a novel form of ablative fractional resurfacing–assisted photodynamic therapy with daylight as a photoactivator, compared with 48% with conventional PDT.
Data source: A randomized trial including 16 organ transplant recipients with a total of 542 actinic keratoses.
Disclosures: This study was funded by a Danish research foundation. The presenter has received research grants from half a dozen medical device and pharmaceutical companies.
Cosmetic Corner: Dermatologists Weigh in on Shampoos and Conditioners
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top shampoos and conditioners. Consideration must be given to:
Johnson & Johnson Consumer Companies, Inc
Recommended by Marian Northington, MD, Birmingham, Alabama
Procter & Gamble
“Head & Shoulders shampoo and conditioner is by far one of the best formulations on the market in terms of a therapeutic shampoo. It is not only gentle and cleanses but clears dandruff quickly. There are a lot of new products in this line to help with different hair and scalp needs.”—Marta I. Rendon, MD, Miami, Florida
• Série Expert Intense Repair Conditioner
L'Oréal Paris Professional
“This is an excellent shampoo for dry and color-treated hair because it is extremely hydrating and moisturizing.”—Marian Northington, MD, Birmingham, Alabama
Aveda Corporation
“This shampoo is free of sodium lauryl sulfate, which can be a contact allergen for some people, and adds moisture to the hair with ingredients such as aloe.”—Anthony M. Rossi, MD, New York, New York
Guthy-Renker
“This product is a 5-in-1 formula that takes the place of your shampoo, conditioner, deep conditioner, detangler, and leave-in conditioner. It cleanses thoroughly without stripping hair and scalp of their natural oils.”—Basil M. Hantash, MD, PhD, Turlock, California
Cutis invites readers to send us their recommendations. Facial moisturizers, scar treatments, and over-the-counter antifungals will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to msteiger@frontlinemedcom.com.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top shampoos and conditioners. Consideration must be given to:
Johnson & Johnson Consumer Companies, Inc
Recommended by Marian Northington, MD, Birmingham, Alabama
Procter & Gamble
“Head & Shoulders shampoo and conditioner is by far one of the best formulations on the market in terms of a therapeutic shampoo. It is not only gentle and cleanses but clears dandruff quickly. There are a lot of new products in this line to help with different hair and scalp needs.”—Marta I. Rendon, MD, Miami, Florida
• Série Expert Intense Repair Conditioner
L'Oréal Paris Professional
“This is an excellent shampoo for dry and color-treated hair because it is extremely hydrating and moisturizing.”—Marian Northington, MD, Birmingham, Alabama
Aveda Corporation
“This shampoo is free of sodium lauryl sulfate, which can be a contact allergen for some people, and adds moisture to the hair with ingredients such as aloe.”—Anthony M. Rossi, MD, New York, New York
Guthy-Renker
“This product is a 5-in-1 formula that takes the place of your shampoo, conditioner, deep conditioner, detangler, and leave-in conditioner. It cleanses thoroughly without stripping hair and scalp of their natural oils.”—Basil M. Hantash, MD, PhD, Turlock, California
Cutis invites readers to send us their recommendations. Facial moisturizers, scar treatments, and over-the-counter antifungals will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to msteiger@frontlinemedcom.com.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top shampoos and conditioners. Consideration must be given to:
Johnson & Johnson Consumer Companies, Inc
Recommended by Marian Northington, MD, Birmingham, Alabama
Procter & Gamble
“Head & Shoulders shampoo and conditioner is by far one of the best formulations on the market in terms of a therapeutic shampoo. It is not only gentle and cleanses but clears dandruff quickly. There are a lot of new products in this line to help with different hair and scalp needs.”—Marta I. Rendon, MD, Miami, Florida
• Série Expert Intense Repair Conditioner
L'Oréal Paris Professional
“This is an excellent shampoo for dry and color-treated hair because it is extremely hydrating and moisturizing.”—Marian Northington, MD, Birmingham, Alabama
Aveda Corporation
“This shampoo is free of sodium lauryl sulfate, which can be a contact allergen for some people, and adds moisture to the hair with ingredients such as aloe.”—Anthony M. Rossi, MD, New York, New York
Guthy-Renker
“This product is a 5-in-1 formula that takes the place of your shampoo, conditioner, deep conditioner, detangler, and leave-in conditioner. It cleanses thoroughly without stripping hair and scalp of their natural oils.”—Basil M. Hantash, MD, PhD, Turlock, California
Cutis invites readers to send us their recommendations. Facial moisturizers, scar treatments, and over-the-counter antifungals will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to msteiger@frontlinemedcom.com.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
Pseudofolliculitis barbae – tips for patients
Pseudofolliculitis barbae (PFB) is a common complaint among darker-skinned patients with coarse curly hair. Patients present with follicular papules in the beard from ingrown hairs that can eventually result in postinflammatory pigmentary alternation and scarring. While these symptoms are most common in men, women may be affected as well, as PFB is not limited to the beard area; it may occur in any other area with thick, coarse curly hair, including the bikini area and axillae.
Some tips for treating PFB:
If the patient doesn’t mind growing a beard, advise him to grow one! The chances of having ingrown hairs that stimulate this condition are less if the hairs are not plucked or shaved, or are kept at least a few millimeters long.
If hair removal/grooming is a must, options include clipping the hairs with a protector; using a self-cleaning electric razor (replacing the blades at least every 2 years); and using thick shaving gel with either a single or twin blade razor, or a chemical depilatory.
Laser hair removal is also an option in the right candidate, particularly with longer pulsed (1,064 nm or 810 nm) lasers in darker-skinned individuals. Eflornithine 12% twice daily for 16 weeks has been shown to work synergistically with laser hair removal. Electrolysis may be helpful for hairs that do not respond to laser hair removal with longer pulsed lasers, such as grey hairs.
If shaving is a must, advise patients to:
• Apply warm compresses to the beard area for a few minutes prior to shaving. In addition, using a mild exfoliant or loofah or toothbrush in a circular motion will help allow any ingrown hairs to be more easily plucked or released at the skin surface.
• Use shaving gel and a sharp razor each time.
• Do not pull the skin taut.
• Do not shave against the direction of hair growth.
• Take short strokes and do not shave back and forth over the same areas.
• After shaving, use a soothing aftershave or hydrocortisone 1% lotion.
Products such as PFB Vanish, which contain salicylic, glycolic, and/or lactic acid, are helpful in some patients after hair removal to prevent ingrown hairs. One version of PFB Vanish contains antipigment ingredients to also address hyperpigmentation.
If inflammatory papules or pustules are present, a combination benzoyl peroxide/clindamycin topical gels (such as Benzaclin, Duac, or Acanya) can be used. Patients with severe inflammation may require oral antibiotics.
Using a topical retinoid at night or a combination retinoid product with hydroquinone can be helpful especially in cases of postinflammatory hyperpigmentation. However, use caution when prescribing retinoids for patients with darker skin, as irritation from these products may lead to postinflammatory pigmentary alteration. Remind patients to avoid drying products, such as toners, if topical retinoids are used.
For severe or refractory postinflammatory hyperpigmentation or inflammatory papules, chemical peels with 20%-30% salicylic acid can be helpful.
What are your PFB solutions? The more we share our clinical insights, the better we will be able to achieve improved treatment results for our patients.
Dr. Wesley practices dermatology in Beverly Hills, Calif. Do you have questions about treating patients with dark skin? If so, send them to sknews@frontlinemedcom.com.
Pseudofolliculitis barbae (PFB) is a common complaint among darker-skinned patients with coarse curly hair. Patients present with follicular papules in the beard from ingrown hairs that can eventually result in postinflammatory pigmentary alternation and scarring. While these symptoms are most common in men, women may be affected as well, as PFB is not limited to the beard area; it may occur in any other area with thick, coarse curly hair, including the bikini area and axillae.
Some tips for treating PFB:
If the patient doesn’t mind growing a beard, advise him to grow one! The chances of having ingrown hairs that stimulate this condition are less if the hairs are not plucked or shaved, or are kept at least a few millimeters long.
If hair removal/grooming is a must, options include clipping the hairs with a protector; using a self-cleaning electric razor (replacing the blades at least every 2 years); and using thick shaving gel with either a single or twin blade razor, or a chemical depilatory.
Laser hair removal is also an option in the right candidate, particularly with longer pulsed (1,064 nm or 810 nm) lasers in darker-skinned individuals. Eflornithine 12% twice daily for 16 weeks has been shown to work synergistically with laser hair removal. Electrolysis may be helpful for hairs that do not respond to laser hair removal with longer pulsed lasers, such as grey hairs.
If shaving is a must, advise patients to:
• Apply warm compresses to the beard area for a few minutes prior to shaving. In addition, using a mild exfoliant or loofah or toothbrush in a circular motion will help allow any ingrown hairs to be more easily plucked or released at the skin surface.
• Use shaving gel and a sharp razor each time.
• Do not pull the skin taut.
• Do not shave against the direction of hair growth.
• Take short strokes and do not shave back and forth over the same areas.
• After shaving, use a soothing aftershave or hydrocortisone 1% lotion.
Products such as PFB Vanish, which contain salicylic, glycolic, and/or lactic acid, are helpful in some patients after hair removal to prevent ingrown hairs. One version of PFB Vanish contains antipigment ingredients to also address hyperpigmentation.
If inflammatory papules or pustules are present, a combination benzoyl peroxide/clindamycin topical gels (such as Benzaclin, Duac, or Acanya) can be used. Patients with severe inflammation may require oral antibiotics.
Using a topical retinoid at night or a combination retinoid product with hydroquinone can be helpful especially in cases of postinflammatory hyperpigmentation. However, use caution when prescribing retinoids for patients with darker skin, as irritation from these products may lead to postinflammatory pigmentary alteration. Remind patients to avoid drying products, such as toners, if topical retinoids are used.
For severe or refractory postinflammatory hyperpigmentation or inflammatory papules, chemical peels with 20%-30% salicylic acid can be helpful.
What are your PFB solutions? The more we share our clinical insights, the better we will be able to achieve improved treatment results for our patients.
Dr. Wesley practices dermatology in Beverly Hills, Calif. Do you have questions about treating patients with dark skin? If so, send them to sknews@frontlinemedcom.com.
Pseudofolliculitis barbae (PFB) is a common complaint among darker-skinned patients with coarse curly hair. Patients present with follicular papules in the beard from ingrown hairs that can eventually result in postinflammatory pigmentary alternation and scarring. While these symptoms are most common in men, women may be affected as well, as PFB is not limited to the beard area; it may occur in any other area with thick, coarse curly hair, including the bikini area and axillae.
Some tips for treating PFB:
If the patient doesn’t mind growing a beard, advise him to grow one! The chances of having ingrown hairs that stimulate this condition are less if the hairs are not plucked or shaved, or are kept at least a few millimeters long.
If hair removal/grooming is a must, options include clipping the hairs with a protector; using a self-cleaning electric razor (replacing the blades at least every 2 years); and using thick shaving gel with either a single or twin blade razor, or a chemical depilatory.
Laser hair removal is also an option in the right candidate, particularly with longer pulsed (1,064 nm or 810 nm) lasers in darker-skinned individuals. Eflornithine 12% twice daily for 16 weeks has been shown to work synergistically with laser hair removal. Electrolysis may be helpful for hairs that do not respond to laser hair removal with longer pulsed lasers, such as grey hairs.
If shaving is a must, advise patients to:
• Apply warm compresses to the beard area for a few minutes prior to shaving. In addition, using a mild exfoliant or loofah or toothbrush in a circular motion will help allow any ingrown hairs to be more easily plucked or released at the skin surface.
• Use shaving gel and a sharp razor each time.
• Do not pull the skin taut.
• Do not shave against the direction of hair growth.
• Take short strokes and do not shave back and forth over the same areas.
• After shaving, use a soothing aftershave or hydrocortisone 1% lotion.
Products such as PFB Vanish, which contain salicylic, glycolic, and/or lactic acid, are helpful in some patients after hair removal to prevent ingrown hairs. One version of PFB Vanish contains antipigment ingredients to also address hyperpigmentation.
If inflammatory papules or pustules are present, a combination benzoyl peroxide/clindamycin topical gels (such as Benzaclin, Duac, or Acanya) can be used. Patients with severe inflammation may require oral antibiotics.
Using a topical retinoid at night or a combination retinoid product with hydroquinone can be helpful especially in cases of postinflammatory hyperpigmentation. However, use caution when prescribing retinoids for patients with darker skin, as irritation from these products may lead to postinflammatory pigmentary alteration. Remind patients to avoid drying products, such as toners, if topical retinoids are used.
For severe or refractory postinflammatory hyperpigmentation or inflammatory papules, chemical peels with 20%-30% salicylic acid can be helpful.
What are your PFB solutions? The more we share our clinical insights, the better we will be able to achieve improved treatment results for our patients.
Dr. Wesley practices dermatology in Beverly Hills, Calif. Do you have questions about treating patients with dark skin? If so, send them to sknews@frontlinemedcom.com.
Use lasers? Lawsuits soar when staff does the procedure
Litigation concerning cutaneous laser surgery has surged in recent years, and the proportion involving nonphysician operators soared from 36% in 1999 to 78% in 2012, according to a report published online Oct. 16 in JAMA Dermatology.
For the most commonly performed laser procedure – hair removal – the proportion of lawsuits involving nonphysician operators is even higher. A "remarkable" 91% of the cases filed in the most recent year for which complete data are available were brought against nonphysician operators, said Dr. H. Ray Jalian of the division of dermatology, University of California, Los Angeles, and his associates.
"The overall trend in increased litigation for laser surgery is in part explained by greater numbers of nonphysician operators performing these procedures, in particular those practicing without direct supervision in ‘medical spas,’ " the investigators said.
Dr. Jalian and his colleagues searched a single legal database and identified 175 cases concerning injury arising from cutaneous laser surgery since 1999. Nonphysician operators included registered nurses, nurse practitioners, medical assistants, electrologists, technicians, aestheticians, and others.
Overall, nonphysician operators were sued in 43% of all cases. But this total is somewhat misleading, because that percentage has risen markedly over time. It represented 78% of all cases in 2011 and both of the two cases filed in early 2012.
Hair removal was the most frequent laser procedure involved in litigation. A total of 76% of the cases brought for hair removal–associated injury in 2004-2012 involved nonphysician operators. Again, this number is markedly higher when the data are categorized by time period: 86% of all lawsuits in 2008-2012 and 91% of all lawsuits in 2010-2012 involved nonphysicians performing laser hair removal.
When the data were analyzed by the location where laser procedures took place, the majority (64%) of cases involving nonphysician operators occurred at facilities such as spas and salons. Again, in more recent years (2008-2012), "nonphysician-operator procedures performed in ‘medical spas’ represented 77% of lawsuits," Dr. Jalian and his associates said (JAMA Dermatology 2013 Oct. 16 [doi:10.1001/jamadermatol.2013. 7117]).
Clinicians also should be aware that almost all the malpractice cases arising from nonphysician operators’ negligence extend beyond the operator to his or her employer, be that a physician, a medical facility, or a spa.
By far the most common allegation against physicians was failure to supervise a nonphysician operator (27 cases), followed by failure to train or hire appropriate staff (23 cases).
The investigators noted that "the actual data likely understate the true incidence of nonphysician operator laser complications" since they were culled from the large legal database that excluded complaints that were handled outside the judicial system.
Laws regulating the use of cutaneous lasers vary widely by state, from edicts specifying that only physicians may operate lasers for hair removal (Maine) to no regulations at all on laser use (Nevada). Even when clinicians supervise so-called physician extenders, rules concerning the degree of supervision, protocol requirements, and employee training are ambiguous.
"Because laws and regulations are constantly evolving, it is imperative for [those] who use physician extenders to be up to date," the researchers said.
Dr. Jalian reported no relevant financial conflicts of interest. One of his associates reported ties to Zeltiq Aesthetics, Cytrellis Biosystems, Unilever, and Allergan.
Litigation concerning cutaneous laser surgery has surged in recent years, and the proportion involving nonphysician operators soared from 36% in 1999 to 78% in 2012, according to a report published online Oct. 16 in JAMA Dermatology.
For the most commonly performed laser procedure – hair removal – the proportion of lawsuits involving nonphysician operators is even higher. A "remarkable" 91% of the cases filed in the most recent year for which complete data are available were brought against nonphysician operators, said Dr. H. Ray Jalian of the division of dermatology, University of California, Los Angeles, and his associates.
"The overall trend in increased litigation for laser surgery is in part explained by greater numbers of nonphysician operators performing these procedures, in particular those practicing without direct supervision in ‘medical spas,’ " the investigators said.
Dr. Jalian and his colleagues searched a single legal database and identified 175 cases concerning injury arising from cutaneous laser surgery since 1999. Nonphysician operators included registered nurses, nurse practitioners, medical assistants, electrologists, technicians, aestheticians, and others.
Overall, nonphysician operators were sued in 43% of all cases. But this total is somewhat misleading, because that percentage has risen markedly over time. It represented 78% of all cases in 2011 and both of the two cases filed in early 2012.
Hair removal was the most frequent laser procedure involved in litigation. A total of 76% of the cases brought for hair removal–associated injury in 2004-2012 involved nonphysician operators. Again, this number is markedly higher when the data are categorized by time period: 86% of all lawsuits in 2008-2012 and 91% of all lawsuits in 2010-2012 involved nonphysicians performing laser hair removal.
When the data were analyzed by the location where laser procedures took place, the majority (64%) of cases involving nonphysician operators occurred at facilities such as spas and salons. Again, in more recent years (2008-2012), "nonphysician-operator procedures performed in ‘medical spas’ represented 77% of lawsuits," Dr. Jalian and his associates said (JAMA Dermatology 2013 Oct. 16 [doi:10.1001/jamadermatol.2013. 7117]).
Clinicians also should be aware that almost all the malpractice cases arising from nonphysician operators’ negligence extend beyond the operator to his or her employer, be that a physician, a medical facility, or a spa.
By far the most common allegation against physicians was failure to supervise a nonphysician operator (27 cases), followed by failure to train or hire appropriate staff (23 cases).
The investigators noted that "the actual data likely understate the true incidence of nonphysician operator laser complications" since they were culled from the large legal database that excluded complaints that were handled outside the judicial system.
Laws regulating the use of cutaneous lasers vary widely by state, from edicts specifying that only physicians may operate lasers for hair removal (Maine) to no regulations at all on laser use (Nevada). Even when clinicians supervise so-called physician extenders, rules concerning the degree of supervision, protocol requirements, and employee training are ambiguous.
"Because laws and regulations are constantly evolving, it is imperative for [those] who use physician extenders to be up to date," the researchers said.
Dr. Jalian reported no relevant financial conflicts of interest. One of his associates reported ties to Zeltiq Aesthetics, Cytrellis Biosystems, Unilever, and Allergan.
Litigation concerning cutaneous laser surgery has surged in recent years, and the proportion involving nonphysician operators soared from 36% in 1999 to 78% in 2012, according to a report published online Oct. 16 in JAMA Dermatology.
For the most commonly performed laser procedure – hair removal – the proportion of lawsuits involving nonphysician operators is even higher. A "remarkable" 91% of the cases filed in the most recent year for which complete data are available were brought against nonphysician operators, said Dr. H. Ray Jalian of the division of dermatology, University of California, Los Angeles, and his associates.
"The overall trend in increased litigation for laser surgery is in part explained by greater numbers of nonphysician operators performing these procedures, in particular those practicing without direct supervision in ‘medical spas,’ " the investigators said.
Dr. Jalian and his colleagues searched a single legal database and identified 175 cases concerning injury arising from cutaneous laser surgery since 1999. Nonphysician operators included registered nurses, nurse practitioners, medical assistants, electrologists, technicians, aestheticians, and others.
Overall, nonphysician operators were sued in 43% of all cases. But this total is somewhat misleading, because that percentage has risen markedly over time. It represented 78% of all cases in 2011 and both of the two cases filed in early 2012.
Hair removal was the most frequent laser procedure involved in litigation. A total of 76% of the cases brought for hair removal–associated injury in 2004-2012 involved nonphysician operators. Again, this number is markedly higher when the data are categorized by time period: 86% of all lawsuits in 2008-2012 and 91% of all lawsuits in 2010-2012 involved nonphysicians performing laser hair removal.
When the data were analyzed by the location where laser procedures took place, the majority (64%) of cases involving nonphysician operators occurred at facilities such as spas and salons. Again, in more recent years (2008-2012), "nonphysician-operator procedures performed in ‘medical spas’ represented 77% of lawsuits," Dr. Jalian and his associates said (JAMA Dermatology 2013 Oct. 16 [doi:10.1001/jamadermatol.2013. 7117]).
Clinicians also should be aware that almost all the malpractice cases arising from nonphysician operators’ negligence extend beyond the operator to his or her employer, be that a physician, a medical facility, or a spa.
By far the most common allegation against physicians was failure to supervise a nonphysician operator (27 cases), followed by failure to train or hire appropriate staff (23 cases).
The investigators noted that "the actual data likely understate the true incidence of nonphysician operator laser complications" since they were culled from the large legal database that excluded complaints that were handled outside the judicial system.
Laws regulating the use of cutaneous lasers vary widely by state, from edicts specifying that only physicians may operate lasers for hair removal (Maine) to no regulations at all on laser use (Nevada). Even when clinicians supervise so-called physician extenders, rules concerning the degree of supervision, protocol requirements, and employee training are ambiguous.
"Because laws and regulations are constantly evolving, it is imperative for [those] who use physician extenders to be up to date," the researchers said.
Dr. Jalian reported no relevant financial conflicts of interest. One of his associates reported ties to Zeltiq Aesthetics, Cytrellis Biosystems, Unilever, and Allergan.
FROM JAMA DERMATOLOGY
Major finding: The proportion of lawsuits concerning cutaneous laser procedures that have been brought against nonphysician operators rose from 36% in 1999 to 78% in 2012.
Data source: A retrospective review of 175 legal claims concerning cutaneous laser surgery that were brought throughout the United States in 1999-2012.
Disclosures: Dr. Jalian reported no relevant financial conflicts of interest. One of his associates reported ties to Zeltiq Aesthetics, Cytrellis Biosystems, Unilever, and Allergan.
Stearic acid
Stearic acid, a waxlike fatty acid also known as octadecanoic acid, is an important component of stratum corneum lipids. Stearic acid is also found in cocoa butter, shea butter, and other vegetable fats, as well as animal tallow. As an FDA-approved ingredient in several cosmetic products, it is used as a surfactant and emulsifying agent for fragrance and as the base for other fatty acid ingredients that are synthesized into emollients and lubricants. Stearic acid is used most often to thicken and retain the shape of soaps (indirectly, through saponification of triglycerides composed of stearic acid esters), and it is also used in shampoos, shaving creams, and detergents.
There is limited evidence for the potential of exogenously produced stearic acid to play a significant role as a topical dermatologic therapeutic agent. Stearic acid is thought to be associated with behenyltrimethylammonium chloride through salt bridges, and the combination is believed to have the capacity to build bilayer vesicles with the aid of hinokitiol (beta-thujaplicin), a natural monoterpenoid found in the wood of trees in the Cupressaceae family that has been shown to exert topical inhibitory activity against Chlamydia trachomatis (Antimicrob. Agents Chemother. 2005;49:2519-21). These vesicles, used to enhance the skin permeation of hinokitiol, were tested in hairless mice and appear to have the potential to promote hair growth (Drug Dev. Ind. Pharm. 2010;36:556-62).
In 2000, Khalil et al. studied the effects of cream formulations on chemically induced burns in mice based on reports that the ingredients, docosanol or stearic acid, were associated with antiviral and anti-inflammatory activity. Burns were engendered by painting murine abdomens with a chloroform solution of phenol. Investigators then topically applied the test formulations 0.5, 3, and 6 hours after injury. They found that the docosanol- and stearic acid–containing creams significantly mitigated the severity and progression of skin lesions compared with untreated sites, yielding, respectively, 76% and 57% declines in mean lesion scores (Contact Dermatitis 2000;43:79-81).
In 2001, Fluhr et al. studied the effects of the free fatty acid pool on stratum corneum (SC) acidification and function by topically applying two phospholipase inhibitors – bromphenacylbromide and 1-hexadecyl-3-trifluoroethylglycero-sn-2-phosphomethanol – for 3 days to murine skin. This raised skin pH and yielded permeability barrier abnormality, altered SC integrity, and reduced SC cohesion. All malfunctions were normalized, including SC pH, with the coapplication of either palmitic, stearic, or linoleic acids along with the inhibiting agents (J. Invest. Dermatol. 2001;117:44-51).
In 2010, Mukherjee et al. evaluated a recently marketed mild, moisturizing body wash containing stearic acid and emollient soybean oil to ascertain the location and amount of stearic acid deposited in the SC after in vivo usage of the product. They conducted clinical cleansing studies for 1 and 5 consecutive days using the soybean product or petroleum jelly. The deuterated variant of stearic acid replaced the free stearic acid in the soybean formulation. The researchers detected deuterated stearic acid in all 10 consecutive layers of SC, with a total stearic acid level measured at 0.33 mcg/cm2 after five washes with the soybean oil product. They concluded that the estimated total fatty acid delivered to the skin from cleansing, probably incorporated into the SC lipid phase, is comparable to the fatty acid amount in an SC layer (J. Cosmet. Dermatol. 2010;9:202-10).
Stearic acid is incorporated into several over-the-counter products, including formulations by Aveda (Green Science Firming Face Cream), Yves Rocher (Les Plaisirs Nature), Kiss My Face (with alpha hydroxy acid), Valeant Pharmaceuticals’ Kinerase line (including Clear Skin Regulating Mask), Buster’s Skin Care for Men (peptide complex organic face moisturizer), and Dermalogica (Soothing Shaving Cream with Daily Defense Block), among others.
Conclusion
While stearic acid is an important component in stratum corneum lipids and a widely used ingredient in skin care products, there is a dearth of data on its significance, if any, in the topical dermatologic armamentarium beyond its primary activity as a surfactant and emulsifying agent. Specifically, it remains to be seen whether stearic acid can be replenished in the stratum corneum through topical treatment. Much more research is needed in this area to assess the potential of stearic acid as a therapeutic agent.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2009), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. She has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever. E-mail sknews@frontlinemedcom.com to contact Dr. Baumann or to suggest topics for a future column.
Stearic acid, a waxlike fatty acid also known as octadecanoic acid, is an important component of stratum corneum lipids. Stearic acid is also found in cocoa butter, shea butter, and other vegetable fats, as well as animal tallow. As an FDA-approved ingredient in several cosmetic products, it is used as a surfactant and emulsifying agent for fragrance and as the base for other fatty acid ingredients that are synthesized into emollients and lubricants. Stearic acid is used most often to thicken and retain the shape of soaps (indirectly, through saponification of triglycerides composed of stearic acid esters), and it is also used in shampoos, shaving creams, and detergents.
There is limited evidence for the potential of exogenously produced stearic acid to play a significant role as a topical dermatologic therapeutic agent. Stearic acid is thought to be associated with behenyltrimethylammonium chloride through salt bridges, and the combination is believed to have the capacity to build bilayer vesicles with the aid of hinokitiol (beta-thujaplicin), a natural monoterpenoid found in the wood of trees in the Cupressaceae family that has been shown to exert topical inhibitory activity against Chlamydia trachomatis (Antimicrob. Agents Chemother. 2005;49:2519-21). These vesicles, used to enhance the skin permeation of hinokitiol, were tested in hairless mice and appear to have the potential to promote hair growth (Drug Dev. Ind. Pharm. 2010;36:556-62).
In 2000, Khalil et al. studied the effects of cream formulations on chemically induced burns in mice based on reports that the ingredients, docosanol or stearic acid, were associated with antiviral and anti-inflammatory activity. Burns were engendered by painting murine abdomens with a chloroform solution of phenol. Investigators then topically applied the test formulations 0.5, 3, and 6 hours after injury. They found that the docosanol- and stearic acid–containing creams significantly mitigated the severity and progression of skin lesions compared with untreated sites, yielding, respectively, 76% and 57% declines in mean lesion scores (Contact Dermatitis 2000;43:79-81).
In 2001, Fluhr et al. studied the effects of the free fatty acid pool on stratum corneum (SC) acidification and function by topically applying two phospholipase inhibitors – bromphenacylbromide and 1-hexadecyl-3-trifluoroethylglycero-sn-2-phosphomethanol – for 3 days to murine skin. This raised skin pH and yielded permeability barrier abnormality, altered SC integrity, and reduced SC cohesion. All malfunctions were normalized, including SC pH, with the coapplication of either palmitic, stearic, or linoleic acids along with the inhibiting agents (J. Invest. Dermatol. 2001;117:44-51).
In 2010, Mukherjee et al. evaluated a recently marketed mild, moisturizing body wash containing stearic acid and emollient soybean oil to ascertain the location and amount of stearic acid deposited in the SC after in vivo usage of the product. They conducted clinical cleansing studies for 1 and 5 consecutive days using the soybean product or petroleum jelly. The deuterated variant of stearic acid replaced the free stearic acid in the soybean formulation. The researchers detected deuterated stearic acid in all 10 consecutive layers of SC, with a total stearic acid level measured at 0.33 mcg/cm2 after five washes with the soybean oil product. They concluded that the estimated total fatty acid delivered to the skin from cleansing, probably incorporated into the SC lipid phase, is comparable to the fatty acid amount in an SC layer (J. Cosmet. Dermatol. 2010;9:202-10).
Stearic acid is incorporated into several over-the-counter products, including formulations by Aveda (Green Science Firming Face Cream), Yves Rocher (Les Plaisirs Nature), Kiss My Face (with alpha hydroxy acid), Valeant Pharmaceuticals’ Kinerase line (including Clear Skin Regulating Mask), Buster’s Skin Care for Men (peptide complex organic face moisturizer), and Dermalogica (Soothing Shaving Cream with Daily Defense Block), among others.
Conclusion
While stearic acid is an important component in stratum corneum lipids and a widely used ingredient in skin care products, there is a dearth of data on its significance, if any, in the topical dermatologic armamentarium beyond its primary activity as a surfactant and emulsifying agent. Specifically, it remains to be seen whether stearic acid can be replenished in the stratum corneum through topical treatment. Much more research is needed in this area to assess the potential of stearic acid as a therapeutic agent.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2009), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. She has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever. E-mail sknews@frontlinemedcom.com to contact Dr. Baumann or to suggest topics for a future column.
Stearic acid, a waxlike fatty acid also known as octadecanoic acid, is an important component of stratum corneum lipids. Stearic acid is also found in cocoa butter, shea butter, and other vegetable fats, as well as animal tallow. As an FDA-approved ingredient in several cosmetic products, it is used as a surfactant and emulsifying agent for fragrance and as the base for other fatty acid ingredients that are synthesized into emollients and lubricants. Stearic acid is used most often to thicken and retain the shape of soaps (indirectly, through saponification of triglycerides composed of stearic acid esters), and it is also used in shampoos, shaving creams, and detergents.
There is limited evidence for the potential of exogenously produced stearic acid to play a significant role as a topical dermatologic therapeutic agent. Stearic acid is thought to be associated with behenyltrimethylammonium chloride through salt bridges, and the combination is believed to have the capacity to build bilayer vesicles with the aid of hinokitiol (beta-thujaplicin), a natural monoterpenoid found in the wood of trees in the Cupressaceae family that has been shown to exert topical inhibitory activity against Chlamydia trachomatis (Antimicrob. Agents Chemother. 2005;49:2519-21). These vesicles, used to enhance the skin permeation of hinokitiol, were tested in hairless mice and appear to have the potential to promote hair growth (Drug Dev. Ind. Pharm. 2010;36:556-62).
In 2000, Khalil et al. studied the effects of cream formulations on chemically induced burns in mice based on reports that the ingredients, docosanol or stearic acid, were associated with antiviral and anti-inflammatory activity. Burns were engendered by painting murine abdomens with a chloroform solution of phenol. Investigators then topically applied the test formulations 0.5, 3, and 6 hours after injury. They found that the docosanol- and stearic acid–containing creams significantly mitigated the severity and progression of skin lesions compared with untreated sites, yielding, respectively, 76% and 57% declines in mean lesion scores (Contact Dermatitis 2000;43:79-81).
In 2001, Fluhr et al. studied the effects of the free fatty acid pool on stratum corneum (SC) acidification and function by topically applying two phospholipase inhibitors – bromphenacylbromide and 1-hexadecyl-3-trifluoroethylglycero-sn-2-phosphomethanol – for 3 days to murine skin. This raised skin pH and yielded permeability barrier abnormality, altered SC integrity, and reduced SC cohesion. All malfunctions were normalized, including SC pH, with the coapplication of either palmitic, stearic, or linoleic acids along with the inhibiting agents (J. Invest. Dermatol. 2001;117:44-51).
In 2010, Mukherjee et al. evaluated a recently marketed mild, moisturizing body wash containing stearic acid and emollient soybean oil to ascertain the location and amount of stearic acid deposited in the SC after in vivo usage of the product. They conducted clinical cleansing studies for 1 and 5 consecutive days using the soybean product or petroleum jelly. The deuterated variant of stearic acid replaced the free stearic acid in the soybean formulation. The researchers detected deuterated stearic acid in all 10 consecutive layers of SC, with a total stearic acid level measured at 0.33 mcg/cm2 after five washes with the soybean oil product. They concluded that the estimated total fatty acid delivered to the skin from cleansing, probably incorporated into the SC lipid phase, is comparable to the fatty acid amount in an SC layer (J. Cosmet. Dermatol. 2010;9:202-10).
Stearic acid is incorporated into several over-the-counter products, including formulations by Aveda (Green Science Firming Face Cream), Yves Rocher (Les Plaisirs Nature), Kiss My Face (with alpha hydroxy acid), Valeant Pharmaceuticals’ Kinerase line (including Clear Skin Regulating Mask), Buster’s Skin Care for Men (peptide complex organic face moisturizer), and Dermalogica (Soothing Shaving Cream with Daily Defense Block), among others.
Conclusion
While stearic acid is an important component in stratum corneum lipids and a widely used ingredient in skin care products, there is a dearth of data on its significance, if any, in the topical dermatologic armamentarium beyond its primary activity as a surfactant and emulsifying agent. Specifically, it remains to be seen whether stearic acid can be replenished in the stratum corneum through topical treatment. Much more research is needed in this area to assess the potential of stearic acid as a therapeutic agent.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2009), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. She has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever. E-mail sknews@frontlinemedcom.com to contact Dr. Baumann or to suggest topics for a future column.
ASDS 2013 Roundup with Dr. Kavita Mariwalla
Dr. Kavita Mariwalla, the 2013 ASDS annual meeting chair, provides a 3-minute summary of the hot topics discussed at the American Society of Dermatologic Surgery, held in Chicago. For more, visit http://www.skindandallergynews.com.
Dr. Kavita Mariwalla, the 2013 ASDS annual meeting chair, provides a 3-minute summary of the hot topics discussed at the American Society of Dermatologic Surgery, held in Chicago. For more, visit http://www.skindandallergynews.com.
Dr. Kavita Mariwalla, the 2013 ASDS annual meeting chair, provides a 3-minute summary of the hot topics discussed at the American Society of Dermatologic Surgery, held in Chicago. For more, visit http://www.skindandallergynews.com.