User login
Dark circles under the eyes
How many times a week are we asked by our patients about “dark circles” under the eyes? The term “dark circles” is a catch-all term that refers to problems that have a vast range of genetic, environmental, and skin causes. However, it is a common frustrating problem with little structure in its definition and few foolproof treatments.
We propose a classification system for the definition of dark circles, and offer some clinical pearls in their treatment. Most patients, however, have dark circles with multifactorial causes that need to be addressed.
I. Infraorbital fat pad protrusion (“bags under my eyes”)
Blepharoplasty is the best solution and for now, the only solution for fat pad prominence. The fat may be removed in lower lid blepharoplasty or repositioned. Referral to a board certified plastic surgeon, oculoplastic surgeon, or dermatologic surgeon is recommended. If there is also significant tear trough deformity, fillers may be placed in the tear trough to help “camouflage” the appearance of the fat pad protrusion but it does not rid the patient of the fat pads.
II. Infraorbital edema (“puffiness”)
The infraorbital skin is very thin and highly sensitive to fluid compartmentalization. Seasonal allergies, sinus infections, crying, or water retention from high blood pressure or consumption of high sodium foods are some of the reasons the loose, thin epidermis becomes edematous. Recommendations for patients:
• Treat seasonal allergies with over-the-counter allergy medications, or see your doctor for prescription medications for resistant allergies or possible sinus infections.
• Switch your sleep position. Sleep position can be contributing to under-eye bags through gravity. Sleeping on your side or stomach can encourage fluids to collect under your eyes. If you’re a side sleeper, you may notice a heavier bag on the side you sleep on. Patients who wake up with puffy eyes can sleep on their backs and add an extra pillow under the head.
• Avoid rubbing eyes frequently, going to bed with makeup on, and harsh cleansers. Anything that irritates the eyes can cause fluids to pool. Sleeping in eye makeup can irritate eyes, causing undereye edema.
• Eye bags might be a sign of an underlying medical condition, if they appear suddenly and none of the above conditions apply. Thyroid, cardiovascular, or kidney problems can cause under-eye fluid retention and the patients need to see their primary care doctors for further evaluation.
• Place an ice pack, slices of cucumbers, chilled tea bags, or even a package of frozen peas on eyes. This can constrict leaky blood vessels and lessen the periorbital edema.
• A few topical eye creams have been developed, such as Neotensil, that temporarily reduce the appearance of lower eyelid puffiness. The product is a blend of polymers that provide compression, smoothing, and hydrating benefits to the skin. In addition, a makeup is often applied over it to reduce the appearance further.
III: Periorbital hyperpigmentation (“dark circles”)
Pigmentation of the periorbital skin is very common in skin of color because of the increased melanin content. Genetics, rubbing, and inflammatory skin diseases such as eczema may play a role in exacerbating the pigmentation of the thin under-eye skin. Recommendations for patients:
• Remind them to avoid rubbing the area – chronic rubbing and the development of lichen simplex chronicus can lead to dark, thickened under-eye skin.
• Retinoic acid creams can help slough the dark pigmented skin. However, it should be used in very small amounts with increasing use over several weeks to avoid severe irritation.
• Skin lightening creams with azaleic acid, kojic acid, and glycolic acid, can be found in varying strengths in dermatologist office preparations, over-the-counter creams, or prescriptions. Hydroquinone creams have demonstrated success in lightening under-eye hyperpigmentation. Strengths in over-the-counter preparations start at 1%-2% and in prescription strength can be compounded to higher than 4%.
• Chemical peels: Light chemical peels such as glycolic acid and Jessner’s peels will assist in lightening dark under-eye pigmentation. Dermatologists also can use peels with hydroquinone or retinoic acid for an added lightening benefit.
• Intense pulsed light (IPL) can help minimize under eye pigmentation, particularly UV-induced pigmentation.
IV: Infraorbital tear trough depression
Most often, dark circles aren’t about changes in the color of the skin at all. Instead, they’re created by a loss of volume in the area around the eye. This exposes the underlying blue veins and orbital bone, creating a hollow trough that shows up as a dark circle. These changes are often caused by genetics; however, significant weight loss and aging with resorption or displacement of the infraorbital fat pads can also expose under-eye tear trough depressions.
The best way to treat this problem is with a small amount of a hyaluronic acid filler placed by a dermatologist in the trough. Very small aliquots are needed in even the deepest trough but can give outstanding results. Caution however, must be taken as this is a highly specialized technique and injector dependent procedure. There are crucial vascular structures around the eye that need to be avoided, and overfilled troughs will give patients a puffy appearance that may pose a worse and more difficult problem to fix. Hyaluronic acid fillers are not approved by the Food and Drug Administration for treatment of under-eye depressions, so patients should be educated about the risks and benefits prior to undergoing these procedures.
V: Periorbital vascular prominence
With age, the skin around the eye becomes thinner, exposing the small capillaries and venules just below the thin epidermal layer. Vascular prominence can leave a bluish undertone to the infraorbital skin which can cast dark shadows and make the area appear dark or sallow.
• Eye creams that contain caffeine can constrict the underlying blood vessels and temporarily diminish small vessel prominence.
• For large blue veins, vascular lasers such as a long pulse Nd:Yag lasers can be recommended. But in darker skin types these lasers can cause hyperpigmented scars if not used with adequate skin cooling techniques. Proper eye protection should also be used.
VI: Periorbital static and dynamic rhytids
• Botulinum toxin placed in small aliquots around the orbital rim will reduce the dynamic rhytids in this area. Treatments spaced 3 months apart will ensure long-lasting benefits as botulinum toxin often wears off.
• Laser resurfacing with CO2, fractionated CO2, or erbium lasers may also be used to treat periorbital rhytides.
Additional tips for your patients:
• For most of the types of infraorbital issues, makeup can help conceal some skin imperfections. Patients should choose a concealer that matches or is slightly lighter than their skin tone. If the patient has mild discoloration, choose a liquid formula. For more prominent imperfections, a cream full-coverage concealer works best.
• Recommend that patients avoid smoking, which dehydrates the skin and causes premature aging and collagen degradation.
• Remind patients to apply a sunscreen around the eye area. Hyperpigmentation and tear troughs can accentuate with UV-induced skin pigmentation. Physical blocking sunscreens may be less irritating than chemical blockers for those with sensitive eyelid skin.
• Remind patients to apply a moisturizer to the eye area nightly to keep the skin from becoming dry, irritated, and dehydrated.
• Advise patients not to break the bank with over-the-counter creams that promise cures for under-eye circles. Most over-the-counter preparations provide temporary, mild benefits at most, and often do not provide any lasting benefit.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Dermatology News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is an update by Dr. Wesley of a previous column by Dr. Talakoub.
How many times a week are we asked by our patients about “dark circles” under the eyes? The term “dark circles” is a catch-all term that refers to problems that have a vast range of genetic, environmental, and skin causes. However, it is a common frustrating problem with little structure in its definition and few foolproof treatments.
We propose a classification system for the definition of dark circles, and offer some clinical pearls in their treatment. Most patients, however, have dark circles with multifactorial causes that need to be addressed.
I. Infraorbital fat pad protrusion (“bags under my eyes”)
Blepharoplasty is the best solution and for now, the only solution for fat pad prominence. The fat may be removed in lower lid blepharoplasty or repositioned. Referral to a board certified plastic surgeon, oculoplastic surgeon, or dermatologic surgeon is recommended. If there is also significant tear trough deformity, fillers may be placed in the tear trough to help “camouflage” the appearance of the fat pad protrusion but it does not rid the patient of the fat pads.
II. Infraorbital edema (“puffiness”)
The infraorbital skin is very thin and highly sensitive to fluid compartmentalization. Seasonal allergies, sinus infections, crying, or water retention from high blood pressure or consumption of high sodium foods are some of the reasons the loose, thin epidermis becomes edematous. Recommendations for patients:
• Treat seasonal allergies with over-the-counter allergy medications, or see your doctor for prescription medications for resistant allergies or possible sinus infections.
• Switch your sleep position. Sleep position can be contributing to under-eye bags through gravity. Sleeping on your side or stomach can encourage fluids to collect under your eyes. If you’re a side sleeper, you may notice a heavier bag on the side you sleep on. Patients who wake up with puffy eyes can sleep on their backs and add an extra pillow under the head.
• Avoid rubbing eyes frequently, going to bed with makeup on, and harsh cleansers. Anything that irritates the eyes can cause fluids to pool. Sleeping in eye makeup can irritate eyes, causing undereye edema.
• Eye bags might be a sign of an underlying medical condition, if they appear suddenly and none of the above conditions apply. Thyroid, cardiovascular, or kidney problems can cause under-eye fluid retention and the patients need to see their primary care doctors for further evaluation.
• Place an ice pack, slices of cucumbers, chilled tea bags, or even a package of frozen peas on eyes. This can constrict leaky blood vessels and lessen the periorbital edema.
• A few topical eye creams have been developed, such as Neotensil, that temporarily reduce the appearance of lower eyelid puffiness. The product is a blend of polymers that provide compression, smoothing, and hydrating benefits to the skin. In addition, a makeup is often applied over it to reduce the appearance further.
III: Periorbital hyperpigmentation (“dark circles”)
Pigmentation of the periorbital skin is very common in skin of color because of the increased melanin content. Genetics, rubbing, and inflammatory skin diseases such as eczema may play a role in exacerbating the pigmentation of the thin under-eye skin. Recommendations for patients:
• Remind them to avoid rubbing the area – chronic rubbing and the development of lichen simplex chronicus can lead to dark, thickened under-eye skin.
• Retinoic acid creams can help slough the dark pigmented skin. However, it should be used in very small amounts with increasing use over several weeks to avoid severe irritation.
• Skin lightening creams with azaleic acid, kojic acid, and glycolic acid, can be found in varying strengths in dermatologist office preparations, over-the-counter creams, or prescriptions. Hydroquinone creams have demonstrated success in lightening under-eye hyperpigmentation. Strengths in over-the-counter preparations start at 1%-2% and in prescription strength can be compounded to higher than 4%.
• Chemical peels: Light chemical peels such as glycolic acid and Jessner’s peels will assist in lightening dark under-eye pigmentation. Dermatologists also can use peels with hydroquinone or retinoic acid for an added lightening benefit.
• Intense pulsed light (IPL) can help minimize under eye pigmentation, particularly UV-induced pigmentation.
IV: Infraorbital tear trough depression
Most often, dark circles aren’t about changes in the color of the skin at all. Instead, they’re created by a loss of volume in the area around the eye. This exposes the underlying blue veins and orbital bone, creating a hollow trough that shows up as a dark circle. These changes are often caused by genetics; however, significant weight loss and aging with resorption or displacement of the infraorbital fat pads can also expose under-eye tear trough depressions.
The best way to treat this problem is with a small amount of a hyaluronic acid filler placed by a dermatologist in the trough. Very small aliquots are needed in even the deepest trough but can give outstanding results. Caution however, must be taken as this is a highly specialized technique and injector dependent procedure. There are crucial vascular structures around the eye that need to be avoided, and overfilled troughs will give patients a puffy appearance that may pose a worse and more difficult problem to fix. Hyaluronic acid fillers are not approved by the Food and Drug Administration for treatment of under-eye depressions, so patients should be educated about the risks and benefits prior to undergoing these procedures.
V: Periorbital vascular prominence
With age, the skin around the eye becomes thinner, exposing the small capillaries and venules just below the thin epidermal layer. Vascular prominence can leave a bluish undertone to the infraorbital skin which can cast dark shadows and make the area appear dark or sallow.
• Eye creams that contain caffeine can constrict the underlying blood vessels and temporarily diminish small vessel prominence.
• For large blue veins, vascular lasers such as a long pulse Nd:Yag lasers can be recommended. But in darker skin types these lasers can cause hyperpigmented scars if not used with adequate skin cooling techniques. Proper eye protection should also be used.
VI: Periorbital static and dynamic rhytids
• Botulinum toxin placed in small aliquots around the orbital rim will reduce the dynamic rhytids in this area. Treatments spaced 3 months apart will ensure long-lasting benefits as botulinum toxin often wears off.
• Laser resurfacing with CO2, fractionated CO2, or erbium lasers may also be used to treat periorbital rhytides.
Additional tips for your patients:
• For most of the types of infraorbital issues, makeup can help conceal some skin imperfections. Patients should choose a concealer that matches or is slightly lighter than their skin tone. If the patient has mild discoloration, choose a liquid formula. For more prominent imperfections, a cream full-coverage concealer works best.
• Recommend that patients avoid smoking, which dehydrates the skin and causes premature aging and collagen degradation.
• Remind patients to apply a sunscreen around the eye area. Hyperpigmentation and tear troughs can accentuate with UV-induced skin pigmentation. Physical blocking sunscreens may be less irritating than chemical blockers for those with sensitive eyelid skin.
• Remind patients to apply a moisturizer to the eye area nightly to keep the skin from becoming dry, irritated, and dehydrated.
• Advise patients not to break the bank with over-the-counter creams that promise cures for under-eye circles. Most over-the-counter preparations provide temporary, mild benefits at most, and often do not provide any lasting benefit.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Dermatology News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is an update by Dr. Wesley of a previous column by Dr. Talakoub.
How many times a week are we asked by our patients about “dark circles” under the eyes? The term “dark circles” is a catch-all term that refers to problems that have a vast range of genetic, environmental, and skin causes. However, it is a common frustrating problem with little structure in its definition and few foolproof treatments.
We propose a classification system for the definition of dark circles, and offer some clinical pearls in their treatment. Most patients, however, have dark circles with multifactorial causes that need to be addressed.
I. Infraorbital fat pad protrusion (“bags under my eyes”)
Blepharoplasty is the best solution and for now, the only solution for fat pad prominence. The fat may be removed in lower lid blepharoplasty or repositioned. Referral to a board certified plastic surgeon, oculoplastic surgeon, or dermatologic surgeon is recommended. If there is also significant tear trough deformity, fillers may be placed in the tear trough to help “camouflage” the appearance of the fat pad protrusion but it does not rid the patient of the fat pads.
II. Infraorbital edema (“puffiness”)
The infraorbital skin is very thin and highly sensitive to fluid compartmentalization. Seasonal allergies, sinus infections, crying, or water retention from high blood pressure or consumption of high sodium foods are some of the reasons the loose, thin epidermis becomes edematous. Recommendations for patients:
• Treat seasonal allergies with over-the-counter allergy medications, or see your doctor for prescription medications for resistant allergies or possible sinus infections.
• Switch your sleep position. Sleep position can be contributing to under-eye bags through gravity. Sleeping on your side or stomach can encourage fluids to collect under your eyes. If you’re a side sleeper, you may notice a heavier bag on the side you sleep on. Patients who wake up with puffy eyes can sleep on their backs and add an extra pillow under the head.
• Avoid rubbing eyes frequently, going to bed with makeup on, and harsh cleansers. Anything that irritates the eyes can cause fluids to pool. Sleeping in eye makeup can irritate eyes, causing undereye edema.
• Eye bags might be a sign of an underlying medical condition, if they appear suddenly and none of the above conditions apply. Thyroid, cardiovascular, or kidney problems can cause under-eye fluid retention and the patients need to see their primary care doctors for further evaluation.
• Place an ice pack, slices of cucumbers, chilled tea bags, or even a package of frozen peas on eyes. This can constrict leaky blood vessels and lessen the periorbital edema.
• A few topical eye creams have been developed, such as Neotensil, that temporarily reduce the appearance of lower eyelid puffiness. The product is a blend of polymers that provide compression, smoothing, and hydrating benefits to the skin. In addition, a makeup is often applied over it to reduce the appearance further.
III: Periorbital hyperpigmentation (“dark circles”)
Pigmentation of the periorbital skin is very common in skin of color because of the increased melanin content. Genetics, rubbing, and inflammatory skin diseases such as eczema may play a role in exacerbating the pigmentation of the thin under-eye skin. Recommendations for patients:
• Remind them to avoid rubbing the area – chronic rubbing and the development of lichen simplex chronicus can lead to dark, thickened under-eye skin.
• Retinoic acid creams can help slough the dark pigmented skin. However, it should be used in very small amounts with increasing use over several weeks to avoid severe irritation.
• Skin lightening creams with azaleic acid, kojic acid, and glycolic acid, can be found in varying strengths in dermatologist office preparations, over-the-counter creams, or prescriptions. Hydroquinone creams have demonstrated success in lightening under-eye hyperpigmentation. Strengths in over-the-counter preparations start at 1%-2% and in prescription strength can be compounded to higher than 4%.
• Chemical peels: Light chemical peels such as glycolic acid and Jessner’s peels will assist in lightening dark under-eye pigmentation. Dermatologists also can use peels with hydroquinone or retinoic acid for an added lightening benefit.
• Intense pulsed light (IPL) can help minimize under eye pigmentation, particularly UV-induced pigmentation.
IV: Infraorbital tear trough depression
Most often, dark circles aren’t about changes in the color of the skin at all. Instead, they’re created by a loss of volume in the area around the eye. This exposes the underlying blue veins and orbital bone, creating a hollow trough that shows up as a dark circle. These changes are often caused by genetics; however, significant weight loss and aging with resorption or displacement of the infraorbital fat pads can also expose under-eye tear trough depressions.
The best way to treat this problem is with a small amount of a hyaluronic acid filler placed by a dermatologist in the trough. Very small aliquots are needed in even the deepest trough but can give outstanding results. Caution however, must be taken as this is a highly specialized technique and injector dependent procedure. There are crucial vascular structures around the eye that need to be avoided, and overfilled troughs will give patients a puffy appearance that may pose a worse and more difficult problem to fix. Hyaluronic acid fillers are not approved by the Food and Drug Administration for treatment of under-eye depressions, so patients should be educated about the risks and benefits prior to undergoing these procedures.
V: Periorbital vascular prominence
With age, the skin around the eye becomes thinner, exposing the small capillaries and venules just below the thin epidermal layer. Vascular prominence can leave a bluish undertone to the infraorbital skin which can cast dark shadows and make the area appear dark or sallow.
• Eye creams that contain caffeine can constrict the underlying blood vessels and temporarily diminish small vessel prominence.
• For large blue veins, vascular lasers such as a long pulse Nd:Yag lasers can be recommended. But in darker skin types these lasers can cause hyperpigmented scars if not used with adequate skin cooling techniques. Proper eye protection should also be used.
VI: Periorbital static and dynamic rhytids
• Botulinum toxin placed in small aliquots around the orbital rim will reduce the dynamic rhytids in this area. Treatments spaced 3 months apart will ensure long-lasting benefits as botulinum toxin often wears off.
• Laser resurfacing with CO2, fractionated CO2, or erbium lasers may also be used to treat periorbital rhytides.
Additional tips for your patients:
• For most of the types of infraorbital issues, makeup can help conceal some skin imperfections. Patients should choose a concealer that matches or is slightly lighter than their skin tone. If the patient has mild discoloration, choose a liquid formula. For more prominent imperfections, a cream full-coverage concealer works best.
• Recommend that patients avoid smoking, which dehydrates the skin and causes premature aging and collagen degradation.
• Remind patients to apply a sunscreen around the eye area. Hyperpigmentation and tear troughs can accentuate with UV-induced skin pigmentation. Physical blocking sunscreens may be less irritating than chemical blockers for those with sensitive eyelid skin.
• Remind patients to apply a moisturizer to the eye area nightly to keep the skin from becoming dry, irritated, and dehydrated.
• Advise patients not to break the bank with over-the-counter creams that promise cures for under-eye circles. Most over-the-counter preparations provide temporary, mild benefits at most, and often do not provide any lasting benefit.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Dermatology News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is an update by Dr. Wesley of a previous column by Dr. Talakoub.
Nail Biopsy: 6 Techniques to Biopsy the Nail Matrix
Nail matrix biopsies are performed to confirm a diagnosis or surgically remove a skin lesion that is affecting the growth of the nail plate. The procedure may be used to identify:
- Inflammatory conditions such as nail psoriasis and lichen planus
- Benign tumors
- Solitary melanonychia
- Squamous cell carcinoma (SCC)
- Other nail disorders
Nail biopsy can lead to complications such as bleeding, infection, or scarring. Postoperative scarring can cause permanent nail splitting, dystrophy, or both.
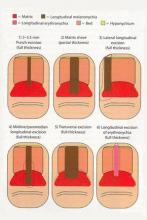
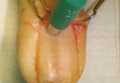
In a Cosmetic Dermatology article, “Matrix Biopsy of Longitudinal Melanonychia and Longitudinal Erythronychia: A Step-by-Step Approach,” Drs. Siobhan C. Collins and Nathaniel J. Jellinek review 6 techniques used to biopsy the nail matrix.
- Punch excision
- Matrix shave
- Lateral longitudinal excision
- Midline/paramedian longitudinal excision
- Transverse excision
- Longitudinal excision of erythronychia
In the setting of longitudinal melanonychia (to diagnose nail melanoma or SCC) and longitudinal erythronychia (to diagnose SCC and rarely amelanotic melanoma or basal cell carcinoma), the techniques they describe accomplish 3 fundamental goals of nail surgery:
- Obtain adequate tissue via an excisional biopsy to make an accurate diagnosis and avoid sampling error
- Avoid unnecessary trauma to surrounding nail tissues by the judicious use of partial plate avulsions whenever feasible
- Avoid unnecessary postoperative nail scarring whenever possible
Dermatologists must be confident when performing nail biopsies and the techniques discussed by the authors will help approach nail surgery with more certainty.
At the 73rd Annual Meeting of the American Academy of Dermatology, Dr. Jellinek provides a hands-on approach to nail surgery. On Saturday, March 21, he will provide tips for nail surgeries at the “Medical and Surgical Management of Nail Disorders” lecture.
For more information, read the Collins and Jellinek article from Cosmetic Dermatology.
Nail matrix biopsies are performed to confirm a diagnosis or surgically remove a skin lesion that is affecting the growth of the nail plate. The procedure may be used to identify:
- Inflammatory conditions such as nail psoriasis and lichen planus
- Benign tumors
- Solitary melanonychia
- Squamous cell carcinoma (SCC)
- Other nail disorders
Nail biopsy can lead to complications such as bleeding, infection, or scarring. Postoperative scarring can cause permanent nail splitting, dystrophy, or both.
In a Cosmetic Dermatology article, “Matrix Biopsy of Longitudinal Melanonychia and Longitudinal Erythronychia: A Step-by-Step Approach,” Drs. Siobhan C. Collins and Nathaniel J. Jellinek review 6 techniques used to biopsy the nail matrix.
- Punch excision
- Matrix shave
- Lateral longitudinal excision
- Midline/paramedian longitudinal excision
- Transverse excision
- Longitudinal excision of erythronychia
In the setting of longitudinal melanonychia (to diagnose nail melanoma or SCC) and longitudinal erythronychia (to diagnose SCC and rarely amelanotic melanoma or basal cell carcinoma), the techniques they describe accomplish 3 fundamental goals of nail surgery:
- Obtain adequate tissue via an excisional biopsy to make an accurate diagnosis and avoid sampling error
- Avoid unnecessary trauma to surrounding nail tissues by the judicious use of partial plate avulsions whenever feasible
- Avoid unnecessary postoperative nail scarring whenever possible
Dermatologists must be confident when performing nail biopsies and the techniques discussed by the authors will help approach nail surgery with more certainty.
At the 73rd Annual Meeting of the American Academy of Dermatology, Dr. Jellinek provides a hands-on approach to nail surgery. On Saturday, March 21, he will provide tips for nail surgeries at the “Medical and Surgical Management of Nail Disorders” lecture.
For more information, read the Collins and Jellinek article from Cosmetic Dermatology.
Nail matrix biopsies are performed to confirm a diagnosis or surgically remove a skin lesion that is affecting the growth of the nail plate. The procedure may be used to identify:
- Inflammatory conditions such as nail psoriasis and lichen planus
- Benign tumors
- Solitary melanonychia
- Squamous cell carcinoma (SCC)
- Other nail disorders
Nail biopsy can lead to complications such as bleeding, infection, or scarring. Postoperative scarring can cause permanent nail splitting, dystrophy, or both.
In a Cosmetic Dermatology article, “Matrix Biopsy of Longitudinal Melanonychia and Longitudinal Erythronychia: A Step-by-Step Approach,” Drs. Siobhan C. Collins and Nathaniel J. Jellinek review 6 techniques used to biopsy the nail matrix.
- Punch excision
- Matrix shave
- Lateral longitudinal excision
- Midline/paramedian longitudinal excision
- Transverse excision
- Longitudinal excision of erythronychia
In the setting of longitudinal melanonychia (to diagnose nail melanoma or SCC) and longitudinal erythronychia (to diagnose SCC and rarely amelanotic melanoma or basal cell carcinoma), the techniques they describe accomplish 3 fundamental goals of nail surgery:
- Obtain adequate tissue via an excisional biopsy to make an accurate diagnosis and avoid sampling error
- Avoid unnecessary trauma to surrounding nail tissues by the judicious use of partial plate avulsions whenever feasible
- Avoid unnecessary postoperative nail scarring whenever possible
Dermatologists must be confident when performing nail biopsies and the techniques discussed by the authors will help approach nail surgery with more certainty.
At the 73rd Annual Meeting of the American Academy of Dermatology, Dr. Jellinek provides a hands-on approach to nail surgery. On Saturday, March 21, he will provide tips for nail surgeries at the “Medical and Surgical Management of Nail Disorders” lecture.
For more information, read the Collins and Jellinek article from Cosmetic Dermatology.
Kinetin and the skin
Kinetin (N6-furfuryladenine or 6-furfurylaminopurine) is a plant cytokinin or phytohormone that promotes cell division, delays senescence in plants, and is reputed to aid in the restoration of skin barrier function and, possibly, in reducing the signs and symptoms of rosacea (Clin. Exp. Dermatol. 2007;32:693-5; Plant Sci. 1999;148:37-45).
Kinetin is believed to develop in cellular DNA as a product of the oxidative, secondary modification of DNA (Plant Sci. 1999;148:37-45). In 1955, it became the first cytokinin isolated from DNA (from herring sperm) as an artifactual rearrangement product of the autoclaving process (J. Cosmet. Dermatol. 2007;6:243-9; Int. J. Biol. Macromol. 2007;40:182-92).
It has since been found to be present in human urine as well as DNA freshly extracted from human cells (Int. J. Biol. Macromol. 2007;40:182-92). The preponderance of amassed experimental evidence suggests that endogenous kinetin acts in vitro and in vivo as a potent antioxidant (Plant Sci. 1999;148:37-45). Currently, it is used as an anti-aging agent in various cosmetic products (J. Cosmet. Dermatol. 2007;6:243-9; J. Cosmet. Dermatol. 2010;9:218-25). Synthetic kinetin is thought to have the capacity to neutralize free radicals as well as limit the damage to DNA and fibroblasts (Photochem. Photobiol. 2012;88:748-52).
In vitro results
Olsen et al. demonstrated in vitro in 1999 that kinetin dose-dependently protected DNA against oxidative damage mediated by the Fenton reaction, and noted that kinetin had previously been linked to anti-aging activity in plants, fruit flies, and human cells in culture (Biochem. Biophys. Res. Commun. 1999;265:499-502). The following year, Verbeke et al. showed in vitro that kinetin potently inhibited damage caused by oxidation and glycoxidation (Biochem. Biophys. Res. Commun. 2000;276:1265-70).
In 2006, Vicanova et al. analyzed the effects of active ingredients from topical and systemic skin care formulations in vitro, finding that kinetin affected the upper dermis by enhancing deposits of fibrillin-1 and elastin fibers as well as their organization perpendicular to the dermal-epidermal junction. In the epidermis, kinetin stimulated keratinocyte production. Further, the investigators noted that the combination of topically applied kinetin with Imedeen Time Perfection ingredients (i.e., BioMarine Complex, grape seed extract, tomato extract, and vitamin C) supplemented systemically into culture medium yielded complementary benefits to dermal and epidermal development (Ann. N.Y. Acad. Sci. 2006;1067:337-42).
It is worth noting that in a study by Tournas et al. published the same month, investigators found that the topical application of a combination of vitamins C and E and ferulic acid yielded photoprotection to pig skin at 5 times the minimal erythema dose (MED) while individual antioxidants to which it was compared (i.e., coenzyme Q10, idebenone, and kinetin) delivered no photoprotective effects (J. Invest. Dermatol. 2006;126:1185-7). Nevertheless, Barciszewski et al. have observed that kinetin is the first stable secondary DNA damage product characterized by well defined cytokinin and anti-aging activity, with data showing that it has delayed human cellular aging in culture (Int. J. Biol. Macromol. 2007;40:182-92).
Rosacea
In 2007, Wu et al. performed a 12-week open-label study in 15 women and 3 men (aged 30-67 years) to ascertain the tolerability and efficacy of kinetin 0.1% lotion in the treatment of mild to moderate facial rosacea. Patients (17 of whom completed the study) applied the lotion twice daily, also daily applying an SPF 30 sunscreen. By week 4, significant improvements were observed in the reduction of skin roughness and mottled hyperpigmentation. Subject assessments at each 4-week interval after baseline and after 12 weeks revealed that kinetin 0.1% was well tolerated and effective for mild to moderate inflammatory rosacea (Clin. Exp. Dermatol. 2007;32:693-5).
Anti-aging
A 2002 study by J.L. McCullough and G.D. Weinstein represented the first evidence of the efficacy of topical kinetin in human beings, with twice-daily application for 24 weeks found to ameliorate skin texture, color, and blotchiness while diminishing rhytides and transepidermal water loss (J. Cosmet. Dermatol. 2002;15:29-32).
Two years later, T. Kimura and K. Doi showed that topical administration of kinetin improved the texture, wrinkling, and pigmentation of aged skin of hairless descendants of Mexican hairless dogs, resulting in notable depigmentation and rejuvenation after 100 days of treatment (Rejuvenation Res. 2004;7:32-9).In 2007, Chiu et al. conducted a randomized, double-blind, placebo-controlled, split-face comparative study in 52 Taiwanese subjects aged 30-60 years (90% of whom were female, all of whom had Fitzpatrick skin types II, III, or IV) to evaluate the clinical anti-aging effects and efficacy differences between kinetin plus niacinamide (kinetin 0.03%, niacinamide 4%) and niacinamide 4% alone versus vehicle placebo.
In the combination group, significant and sustained decreases were observed in counts of spots, pores, wrinkles, and evenness as well as persistent reductions in erythema index at weeks 8 and 12. At week 12, stratum corneum hydration status also was significantly enhanced in this group. In the niacinamide-only group, pore and evenness counts were significantly decreased at week 8, with declines in wrinkle counts emerging at week 12. The investigators concluded that kinetin and niacinamide display synergistic and dynamic anti-aging effects, showing substantial potential as topical anti-aging cosmeceutical agents (J. Cosmet. Dermatol. 2007;6:243-9).
However, Levin et al. noted in 2010 that while the effects of kinetin have been established in plants and its antioxidant properties have been displayed in vitro, the anti-aging effects and clinical efficacy ascribed to kinetin have been based on limited evidence, with no studies extant on the percutaneous absorption of kinetin. They added that research elucidating the mechanisms through which kinetin appears to improve skin barrier function, texture, and pigmentation also are lacking (J. Clin. Aesthet. Dermatol. 2010;3:22-41).
In 2012, Campos et al. assessed the effects on hydration, viscoelastic characteristics, and photoprotection of cosmetic preparations containing a dispersion of liposome with magnesium ascorbyl phosphate, alpha-lipoic acid, and kinetin. They observed that the formulation protected hairless mouse skin barrier function against UV harm. After 4 weeks of application on human skin, the combination product was found to have improved moisturization of the stratum corneum, also delivering hydration effects to deeper skin layers. The researchers concluded that the cosmetic formulation containing kinetin shows promise as a cutaneous anti-aging product (Photochem. Photobiol. 2012;88:748-52).
Conclusion
While some experimental and clinical results appear to suggest an anti-aging effect exerted by topically applied kinetin, much more research – particularly randomized controlled and comparison studies – are needed to provide a clearer picture as to the mechanisms and appropriate role of kinetin in the dermatologic armamentarium.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy,Topix Pharmaceuticals, and Unilever.
Kinetin (N6-furfuryladenine or 6-furfurylaminopurine) is a plant cytokinin or phytohormone that promotes cell division, delays senescence in plants, and is reputed to aid in the restoration of skin barrier function and, possibly, in reducing the signs and symptoms of rosacea (Clin. Exp. Dermatol. 2007;32:693-5; Plant Sci. 1999;148:37-45).
Kinetin is believed to develop in cellular DNA as a product of the oxidative, secondary modification of DNA (Plant Sci. 1999;148:37-45). In 1955, it became the first cytokinin isolated from DNA (from herring sperm) as an artifactual rearrangement product of the autoclaving process (J. Cosmet. Dermatol. 2007;6:243-9; Int. J. Biol. Macromol. 2007;40:182-92).
It has since been found to be present in human urine as well as DNA freshly extracted from human cells (Int. J. Biol. Macromol. 2007;40:182-92). The preponderance of amassed experimental evidence suggests that endogenous kinetin acts in vitro and in vivo as a potent antioxidant (Plant Sci. 1999;148:37-45). Currently, it is used as an anti-aging agent in various cosmetic products (J. Cosmet. Dermatol. 2007;6:243-9; J. Cosmet. Dermatol. 2010;9:218-25). Synthetic kinetin is thought to have the capacity to neutralize free radicals as well as limit the damage to DNA and fibroblasts (Photochem. Photobiol. 2012;88:748-52).
In vitro results
Olsen et al. demonstrated in vitro in 1999 that kinetin dose-dependently protected DNA against oxidative damage mediated by the Fenton reaction, and noted that kinetin had previously been linked to anti-aging activity in plants, fruit flies, and human cells in culture (Biochem. Biophys. Res. Commun. 1999;265:499-502). The following year, Verbeke et al. showed in vitro that kinetin potently inhibited damage caused by oxidation and glycoxidation (Biochem. Biophys. Res. Commun. 2000;276:1265-70).
In 2006, Vicanova et al. analyzed the effects of active ingredients from topical and systemic skin care formulations in vitro, finding that kinetin affected the upper dermis by enhancing deposits of fibrillin-1 and elastin fibers as well as their organization perpendicular to the dermal-epidermal junction. In the epidermis, kinetin stimulated keratinocyte production. Further, the investigators noted that the combination of topically applied kinetin with Imedeen Time Perfection ingredients (i.e., BioMarine Complex, grape seed extract, tomato extract, and vitamin C) supplemented systemically into culture medium yielded complementary benefits to dermal and epidermal development (Ann. N.Y. Acad. Sci. 2006;1067:337-42).
It is worth noting that in a study by Tournas et al. published the same month, investigators found that the topical application of a combination of vitamins C and E and ferulic acid yielded photoprotection to pig skin at 5 times the minimal erythema dose (MED) while individual antioxidants to which it was compared (i.e., coenzyme Q10, idebenone, and kinetin) delivered no photoprotective effects (J. Invest. Dermatol. 2006;126:1185-7). Nevertheless, Barciszewski et al. have observed that kinetin is the first stable secondary DNA damage product characterized by well defined cytokinin and anti-aging activity, with data showing that it has delayed human cellular aging in culture (Int. J. Biol. Macromol. 2007;40:182-92).
Rosacea
In 2007, Wu et al. performed a 12-week open-label study in 15 women and 3 men (aged 30-67 years) to ascertain the tolerability and efficacy of kinetin 0.1% lotion in the treatment of mild to moderate facial rosacea. Patients (17 of whom completed the study) applied the lotion twice daily, also daily applying an SPF 30 sunscreen. By week 4, significant improvements were observed in the reduction of skin roughness and mottled hyperpigmentation. Subject assessments at each 4-week interval after baseline and after 12 weeks revealed that kinetin 0.1% was well tolerated and effective for mild to moderate inflammatory rosacea (Clin. Exp. Dermatol. 2007;32:693-5).
Anti-aging
A 2002 study by J.L. McCullough and G.D. Weinstein represented the first evidence of the efficacy of topical kinetin in human beings, with twice-daily application for 24 weeks found to ameliorate skin texture, color, and blotchiness while diminishing rhytides and transepidermal water loss (J. Cosmet. Dermatol. 2002;15:29-32).
Two years later, T. Kimura and K. Doi showed that topical administration of kinetin improved the texture, wrinkling, and pigmentation of aged skin of hairless descendants of Mexican hairless dogs, resulting in notable depigmentation and rejuvenation after 100 days of treatment (Rejuvenation Res. 2004;7:32-9).In 2007, Chiu et al. conducted a randomized, double-blind, placebo-controlled, split-face comparative study in 52 Taiwanese subjects aged 30-60 years (90% of whom were female, all of whom had Fitzpatrick skin types II, III, or IV) to evaluate the clinical anti-aging effects and efficacy differences between kinetin plus niacinamide (kinetin 0.03%, niacinamide 4%) and niacinamide 4% alone versus vehicle placebo.
In the combination group, significant and sustained decreases were observed in counts of spots, pores, wrinkles, and evenness as well as persistent reductions in erythema index at weeks 8 and 12. At week 12, stratum corneum hydration status also was significantly enhanced in this group. In the niacinamide-only group, pore and evenness counts were significantly decreased at week 8, with declines in wrinkle counts emerging at week 12. The investigators concluded that kinetin and niacinamide display synergistic and dynamic anti-aging effects, showing substantial potential as topical anti-aging cosmeceutical agents (J. Cosmet. Dermatol. 2007;6:243-9).
However, Levin et al. noted in 2010 that while the effects of kinetin have been established in plants and its antioxidant properties have been displayed in vitro, the anti-aging effects and clinical efficacy ascribed to kinetin have been based on limited evidence, with no studies extant on the percutaneous absorption of kinetin. They added that research elucidating the mechanisms through which kinetin appears to improve skin barrier function, texture, and pigmentation also are lacking (J. Clin. Aesthet. Dermatol. 2010;3:22-41).
In 2012, Campos et al. assessed the effects on hydration, viscoelastic characteristics, and photoprotection of cosmetic preparations containing a dispersion of liposome with magnesium ascorbyl phosphate, alpha-lipoic acid, and kinetin. They observed that the formulation protected hairless mouse skin barrier function against UV harm. After 4 weeks of application on human skin, the combination product was found to have improved moisturization of the stratum corneum, also delivering hydration effects to deeper skin layers. The researchers concluded that the cosmetic formulation containing kinetin shows promise as a cutaneous anti-aging product (Photochem. Photobiol. 2012;88:748-52).
Conclusion
While some experimental and clinical results appear to suggest an anti-aging effect exerted by topically applied kinetin, much more research – particularly randomized controlled and comparison studies – are needed to provide a clearer picture as to the mechanisms and appropriate role of kinetin in the dermatologic armamentarium.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy,Topix Pharmaceuticals, and Unilever.
Kinetin (N6-furfuryladenine or 6-furfurylaminopurine) is a plant cytokinin or phytohormone that promotes cell division, delays senescence in plants, and is reputed to aid in the restoration of skin barrier function and, possibly, in reducing the signs and symptoms of rosacea (Clin. Exp. Dermatol. 2007;32:693-5; Plant Sci. 1999;148:37-45).
Kinetin is believed to develop in cellular DNA as a product of the oxidative, secondary modification of DNA (Plant Sci. 1999;148:37-45). In 1955, it became the first cytokinin isolated from DNA (from herring sperm) as an artifactual rearrangement product of the autoclaving process (J. Cosmet. Dermatol. 2007;6:243-9; Int. J. Biol. Macromol. 2007;40:182-92).
It has since been found to be present in human urine as well as DNA freshly extracted from human cells (Int. J. Biol. Macromol. 2007;40:182-92). The preponderance of amassed experimental evidence suggests that endogenous kinetin acts in vitro and in vivo as a potent antioxidant (Plant Sci. 1999;148:37-45). Currently, it is used as an anti-aging agent in various cosmetic products (J. Cosmet. Dermatol. 2007;6:243-9; J. Cosmet. Dermatol. 2010;9:218-25). Synthetic kinetin is thought to have the capacity to neutralize free radicals as well as limit the damage to DNA and fibroblasts (Photochem. Photobiol. 2012;88:748-52).
In vitro results
Olsen et al. demonstrated in vitro in 1999 that kinetin dose-dependently protected DNA against oxidative damage mediated by the Fenton reaction, and noted that kinetin had previously been linked to anti-aging activity in plants, fruit flies, and human cells in culture (Biochem. Biophys. Res. Commun. 1999;265:499-502). The following year, Verbeke et al. showed in vitro that kinetin potently inhibited damage caused by oxidation and glycoxidation (Biochem. Biophys. Res. Commun. 2000;276:1265-70).
In 2006, Vicanova et al. analyzed the effects of active ingredients from topical and systemic skin care formulations in vitro, finding that kinetin affected the upper dermis by enhancing deposits of fibrillin-1 and elastin fibers as well as their organization perpendicular to the dermal-epidermal junction. In the epidermis, kinetin stimulated keratinocyte production. Further, the investigators noted that the combination of topically applied kinetin with Imedeen Time Perfection ingredients (i.e., BioMarine Complex, grape seed extract, tomato extract, and vitamin C) supplemented systemically into culture medium yielded complementary benefits to dermal and epidermal development (Ann. N.Y. Acad. Sci. 2006;1067:337-42).
It is worth noting that in a study by Tournas et al. published the same month, investigators found that the topical application of a combination of vitamins C and E and ferulic acid yielded photoprotection to pig skin at 5 times the minimal erythema dose (MED) while individual antioxidants to which it was compared (i.e., coenzyme Q10, idebenone, and kinetin) delivered no photoprotective effects (J. Invest. Dermatol. 2006;126:1185-7). Nevertheless, Barciszewski et al. have observed that kinetin is the first stable secondary DNA damage product characterized by well defined cytokinin and anti-aging activity, with data showing that it has delayed human cellular aging in culture (Int. J. Biol. Macromol. 2007;40:182-92).
Rosacea
In 2007, Wu et al. performed a 12-week open-label study in 15 women and 3 men (aged 30-67 years) to ascertain the tolerability and efficacy of kinetin 0.1% lotion in the treatment of mild to moderate facial rosacea. Patients (17 of whom completed the study) applied the lotion twice daily, also daily applying an SPF 30 sunscreen. By week 4, significant improvements were observed in the reduction of skin roughness and mottled hyperpigmentation. Subject assessments at each 4-week interval after baseline and after 12 weeks revealed that kinetin 0.1% was well tolerated and effective for mild to moderate inflammatory rosacea (Clin. Exp. Dermatol. 2007;32:693-5).
Anti-aging
A 2002 study by J.L. McCullough and G.D. Weinstein represented the first evidence of the efficacy of topical kinetin in human beings, with twice-daily application for 24 weeks found to ameliorate skin texture, color, and blotchiness while diminishing rhytides and transepidermal water loss (J. Cosmet. Dermatol. 2002;15:29-32).
Two years later, T. Kimura and K. Doi showed that topical administration of kinetin improved the texture, wrinkling, and pigmentation of aged skin of hairless descendants of Mexican hairless dogs, resulting in notable depigmentation and rejuvenation after 100 days of treatment (Rejuvenation Res. 2004;7:32-9).In 2007, Chiu et al. conducted a randomized, double-blind, placebo-controlled, split-face comparative study in 52 Taiwanese subjects aged 30-60 years (90% of whom were female, all of whom had Fitzpatrick skin types II, III, or IV) to evaluate the clinical anti-aging effects and efficacy differences between kinetin plus niacinamide (kinetin 0.03%, niacinamide 4%) and niacinamide 4% alone versus vehicle placebo.
In the combination group, significant and sustained decreases were observed in counts of spots, pores, wrinkles, and evenness as well as persistent reductions in erythema index at weeks 8 and 12. At week 12, stratum corneum hydration status also was significantly enhanced in this group. In the niacinamide-only group, pore and evenness counts were significantly decreased at week 8, with declines in wrinkle counts emerging at week 12. The investigators concluded that kinetin and niacinamide display synergistic and dynamic anti-aging effects, showing substantial potential as topical anti-aging cosmeceutical agents (J. Cosmet. Dermatol. 2007;6:243-9).
However, Levin et al. noted in 2010 that while the effects of kinetin have been established in plants and its antioxidant properties have been displayed in vitro, the anti-aging effects and clinical efficacy ascribed to kinetin have been based on limited evidence, with no studies extant on the percutaneous absorption of kinetin. They added that research elucidating the mechanisms through which kinetin appears to improve skin barrier function, texture, and pigmentation also are lacking (J. Clin. Aesthet. Dermatol. 2010;3:22-41).
In 2012, Campos et al. assessed the effects on hydration, viscoelastic characteristics, and photoprotection of cosmetic preparations containing a dispersion of liposome with magnesium ascorbyl phosphate, alpha-lipoic acid, and kinetin. They observed that the formulation protected hairless mouse skin barrier function against UV harm. After 4 weeks of application on human skin, the combination product was found to have improved moisturization of the stratum corneum, also delivering hydration effects to deeper skin layers. The researchers concluded that the cosmetic formulation containing kinetin shows promise as a cutaneous anti-aging product (Photochem. Photobiol. 2012;88:748-52).
Conclusion
While some experimental and clinical results appear to suggest an anti-aging effect exerted by topically applied kinetin, much more research – particularly randomized controlled and comparison studies – are needed to provide a clearer picture as to the mechanisms and appropriate role of kinetin in the dermatologic armamentarium.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy,Topix Pharmaceuticals, and Unilever.
FDA panel backs injectable treatment for chin fat
SILVER SPRING, MD – A less-invasive alternative to liposuction and other cosmetic treatments for submental fat will likely become available in the United States in the wake of a Food and Drug Administration advisory panel’s unanimous support for approval of deoxycholic acid as an injection into subcutaneous fat under the chin.
At a meeting on March 9, the FDA’s dermatologic and ophthalmic drugs advisory committee voted 17-0 that the efficacy and safety data on 1% deoxycholic acid (DCA) injection supported approval for the proposed indication, “the improvement in the appearance of moderate to severe convexity or fullness associated with submental fat” in adults.
The product is a synthetic version of naturally occurring DCA, an endogenous secondary bile acid that “serves to emulsify and solubilize dietary fat,” and for cosmetic use, “disrupts cell membranes of adipocytes, causing destruction of fat cells,” according to the manufacturer, Kythera Biopharmaceuticals.
The regimen proposed by Kythera is up to six treatment sessions with 4-week intervals between sessions. Each session would involve up to 50 injections, with 0.2 mL of DCA solution per injection, administered subcutaneously using a grid to control spacing of injections. The product would be the first drug approved for treatment of submental fat.
A pair of phase III studies including about 1,000 adults (median age, 48-49 years) randomized patients to treatment with DCA or placebo saline injections of 6 or fewer treatments, one month apart. The effects were evaluated with validated Clinician-Reported and a Patient-Reported Submental Fat Rating Scales (CR-SMFRS and PR-SMFRS), which Kythera developed in consultation with the FDA. On the CR-SMFRS, a 5-point rating scale ranged from 0 (no localized submental fat evident) to 4 (extreme submental convexity).
Most patients enrolled were rated as 2 (moderate, with prominent localized submental fat) or 3 (severe, with marked localized submental fat); the company is not seeking an indication for extreme submental convexity. On the PR-SMFRS, patients had five options to check off, based on how much fat they thought they had under their chin when looking in a mirror, ranging from “no chin fat at all” to a “very large amount of fat.”
The coprimary efficacy endpoints were those who had at least a 2-grade improvement on both scales, or at least a 1-grade improvement in both scales, 12 weeks after the first treatment.
In the two studies, 70% and 66% of those treated with DCA achieved at least a 1-grade improvement in both scales, vs.19% and 22%, respectively, in the placebo group. The difference was statistically significant. In addition, 13% and 19% of those treated with DCA in the two studies had at least a 2-grade improvement in both scales at that time, vs. 0-3% of those in the placebo group, also statistically significant differences.
One issue raised at the meeting was that the scales were somewhat subjective. A more objective secondary endpoint used MRI volumetric measurements to evaluate results in 449 patients from both studies; this measure determined that 40%-46% of those treated with DCA achieved at least a 10% reduction in MRI volume of submental fat, vs. 5% of those on placebo.
Most patients in the placebo and DCA groups experienced adverse events that were mostly mild and were related to the injections. The rate of moderate adverse events was 17% in the DCA-treated patients and 10% in the placebo group. Injection site reactions more common in the DCA group vs. placebo included pain (70% vs. 31%), numbness (66% vs. 6%), edema (60% vs. 29%), and swelling (33% vs. 16%). There were 11 cases of dysphagia, all of which resolved, except for 1 patient who withdrew from the study and was not available for follow-up. There have been no cases of glandular injuries, including salivary or thyroid injuries, according to the company.
In a safety database of almost 2,000 patients, including 1,050 treated with DCA, the rate of marginal mandibular nerve injuries has been 4% in those treated with DCA. The nerve injuries lasted a median of 45 days (1-298 days) and all resolved. The rate of dysphagia was 2% among those on DCA , lasting a median of 3 days, vs. fewer than 1% among those on placebo, according to the FDA reviewers. One patient did not resolve, but the company noted that this patient dropped out of the study and was not followed. There were no cases of anaphylactic reactions; injection site urticaria was rare and resolved.
In addition, the potential risk of marginal mandibular nerve injuries, issues raised during the meeting included the concerns that the treatment would be used by clinicians who were not adequately trained to provide the treatment or not adequately familiar with the anatomy of the treated area. Other concerns include potential use of the product in areas that include the periorbitum, as well as off-label use of large amounts in different parts of the body, such as the abdomen or thighs. The company said that the product will not be provided to clinicians who have not gone through the planned training program and that it was better suited to small areas of the body.
While there are some questions that still need to be answered and concerns about off-label use and the need for proper training, the panel agreed this was safe and effective if used properly with appropriate training and appropriate precautions, said the panel chair, Dr. Lynn A. Drake of the department of dermatology, Massachusetts General Hospital, Boston.
DCA should not be used off label, a concern that could be at least partially addressed in the label, and “without appropriate training, this product could be misused and mishandled and cause damage,” she added. In addition, because of some “very novel” aspects to this treatment, postmarketing data are needed, addressing questions that include whether it causes scarring that could affect surgery and other interventions in the future, she said.
The FDA usually follows the recommendations of its advisory panels. None of the panel members had relevant disclosures.
SILVER SPRING, MD – A less-invasive alternative to liposuction and other cosmetic treatments for submental fat will likely become available in the United States in the wake of a Food and Drug Administration advisory panel’s unanimous support for approval of deoxycholic acid as an injection into subcutaneous fat under the chin.
At a meeting on March 9, the FDA’s dermatologic and ophthalmic drugs advisory committee voted 17-0 that the efficacy and safety data on 1% deoxycholic acid (DCA) injection supported approval for the proposed indication, “the improvement in the appearance of moderate to severe convexity or fullness associated with submental fat” in adults.
The product is a synthetic version of naturally occurring DCA, an endogenous secondary bile acid that “serves to emulsify and solubilize dietary fat,” and for cosmetic use, “disrupts cell membranes of adipocytes, causing destruction of fat cells,” according to the manufacturer, Kythera Biopharmaceuticals.
The regimen proposed by Kythera is up to six treatment sessions with 4-week intervals between sessions. Each session would involve up to 50 injections, with 0.2 mL of DCA solution per injection, administered subcutaneously using a grid to control spacing of injections. The product would be the first drug approved for treatment of submental fat.
A pair of phase III studies including about 1,000 adults (median age, 48-49 years) randomized patients to treatment with DCA or placebo saline injections of 6 or fewer treatments, one month apart. The effects were evaluated with validated Clinician-Reported and a Patient-Reported Submental Fat Rating Scales (CR-SMFRS and PR-SMFRS), which Kythera developed in consultation with the FDA. On the CR-SMFRS, a 5-point rating scale ranged from 0 (no localized submental fat evident) to 4 (extreme submental convexity).
Most patients enrolled were rated as 2 (moderate, with prominent localized submental fat) or 3 (severe, with marked localized submental fat); the company is not seeking an indication for extreme submental convexity. On the PR-SMFRS, patients had five options to check off, based on how much fat they thought they had under their chin when looking in a mirror, ranging from “no chin fat at all” to a “very large amount of fat.”
The coprimary efficacy endpoints were those who had at least a 2-grade improvement on both scales, or at least a 1-grade improvement in both scales, 12 weeks after the first treatment.
In the two studies, 70% and 66% of those treated with DCA achieved at least a 1-grade improvement in both scales, vs.19% and 22%, respectively, in the placebo group. The difference was statistically significant. In addition, 13% and 19% of those treated with DCA in the two studies had at least a 2-grade improvement in both scales at that time, vs. 0-3% of those in the placebo group, also statistically significant differences.
One issue raised at the meeting was that the scales were somewhat subjective. A more objective secondary endpoint used MRI volumetric measurements to evaluate results in 449 patients from both studies; this measure determined that 40%-46% of those treated with DCA achieved at least a 10% reduction in MRI volume of submental fat, vs. 5% of those on placebo.
Most patients in the placebo and DCA groups experienced adverse events that were mostly mild and were related to the injections. The rate of moderate adverse events was 17% in the DCA-treated patients and 10% in the placebo group. Injection site reactions more common in the DCA group vs. placebo included pain (70% vs. 31%), numbness (66% vs. 6%), edema (60% vs. 29%), and swelling (33% vs. 16%). There were 11 cases of dysphagia, all of which resolved, except for 1 patient who withdrew from the study and was not available for follow-up. There have been no cases of glandular injuries, including salivary or thyroid injuries, according to the company.
In a safety database of almost 2,000 patients, including 1,050 treated with DCA, the rate of marginal mandibular nerve injuries has been 4% in those treated with DCA. The nerve injuries lasted a median of 45 days (1-298 days) and all resolved. The rate of dysphagia was 2% among those on DCA , lasting a median of 3 days, vs. fewer than 1% among those on placebo, according to the FDA reviewers. One patient did not resolve, but the company noted that this patient dropped out of the study and was not followed. There were no cases of anaphylactic reactions; injection site urticaria was rare and resolved.
In addition, the potential risk of marginal mandibular nerve injuries, issues raised during the meeting included the concerns that the treatment would be used by clinicians who were not adequately trained to provide the treatment or not adequately familiar with the anatomy of the treated area. Other concerns include potential use of the product in areas that include the periorbitum, as well as off-label use of large amounts in different parts of the body, such as the abdomen or thighs. The company said that the product will not be provided to clinicians who have not gone through the planned training program and that it was better suited to small areas of the body.
While there are some questions that still need to be answered and concerns about off-label use and the need for proper training, the panel agreed this was safe and effective if used properly with appropriate training and appropriate precautions, said the panel chair, Dr. Lynn A. Drake of the department of dermatology, Massachusetts General Hospital, Boston.
DCA should not be used off label, a concern that could be at least partially addressed in the label, and “without appropriate training, this product could be misused and mishandled and cause damage,” she added. In addition, because of some “very novel” aspects to this treatment, postmarketing data are needed, addressing questions that include whether it causes scarring that could affect surgery and other interventions in the future, she said.
The FDA usually follows the recommendations of its advisory panels. None of the panel members had relevant disclosures.
SILVER SPRING, MD – A less-invasive alternative to liposuction and other cosmetic treatments for submental fat will likely become available in the United States in the wake of a Food and Drug Administration advisory panel’s unanimous support for approval of deoxycholic acid as an injection into subcutaneous fat under the chin.
At a meeting on March 9, the FDA’s dermatologic and ophthalmic drugs advisory committee voted 17-0 that the efficacy and safety data on 1% deoxycholic acid (DCA) injection supported approval for the proposed indication, “the improvement in the appearance of moderate to severe convexity or fullness associated with submental fat” in adults.
The product is a synthetic version of naturally occurring DCA, an endogenous secondary bile acid that “serves to emulsify and solubilize dietary fat,” and for cosmetic use, “disrupts cell membranes of adipocytes, causing destruction of fat cells,” according to the manufacturer, Kythera Biopharmaceuticals.
The regimen proposed by Kythera is up to six treatment sessions with 4-week intervals between sessions. Each session would involve up to 50 injections, with 0.2 mL of DCA solution per injection, administered subcutaneously using a grid to control spacing of injections. The product would be the first drug approved for treatment of submental fat.
A pair of phase III studies including about 1,000 adults (median age, 48-49 years) randomized patients to treatment with DCA or placebo saline injections of 6 or fewer treatments, one month apart. The effects were evaluated with validated Clinician-Reported and a Patient-Reported Submental Fat Rating Scales (CR-SMFRS and PR-SMFRS), which Kythera developed in consultation with the FDA. On the CR-SMFRS, a 5-point rating scale ranged from 0 (no localized submental fat evident) to 4 (extreme submental convexity).
Most patients enrolled were rated as 2 (moderate, with prominent localized submental fat) or 3 (severe, with marked localized submental fat); the company is not seeking an indication for extreme submental convexity. On the PR-SMFRS, patients had five options to check off, based on how much fat they thought they had under their chin when looking in a mirror, ranging from “no chin fat at all” to a “very large amount of fat.”
The coprimary efficacy endpoints were those who had at least a 2-grade improvement on both scales, or at least a 1-grade improvement in both scales, 12 weeks after the first treatment.
In the two studies, 70% and 66% of those treated with DCA achieved at least a 1-grade improvement in both scales, vs.19% and 22%, respectively, in the placebo group. The difference was statistically significant. In addition, 13% and 19% of those treated with DCA in the two studies had at least a 2-grade improvement in both scales at that time, vs. 0-3% of those in the placebo group, also statistically significant differences.
One issue raised at the meeting was that the scales were somewhat subjective. A more objective secondary endpoint used MRI volumetric measurements to evaluate results in 449 patients from both studies; this measure determined that 40%-46% of those treated with DCA achieved at least a 10% reduction in MRI volume of submental fat, vs. 5% of those on placebo.
Most patients in the placebo and DCA groups experienced adverse events that were mostly mild and were related to the injections. The rate of moderate adverse events was 17% in the DCA-treated patients and 10% in the placebo group. Injection site reactions more common in the DCA group vs. placebo included pain (70% vs. 31%), numbness (66% vs. 6%), edema (60% vs. 29%), and swelling (33% vs. 16%). There were 11 cases of dysphagia, all of which resolved, except for 1 patient who withdrew from the study and was not available for follow-up. There have been no cases of glandular injuries, including salivary or thyroid injuries, according to the company.
In a safety database of almost 2,000 patients, including 1,050 treated with DCA, the rate of marginal mandibular nerve injuries has been 4% in those treated with DCA. The nerve injuries lasted a median of 45 days (1-298 days) and all resolved. The rate of dysphagia was 2% among those on DCA , lasting a median of 3 days, vs. fewer than 1% among those on placebo, according to the FDA reviewers. One patient did not resolve, but the company noted that this patient dropped out of the study and was not followed. There were no cases of anaphylactic reactions; injection site urticaria was rare and resolved.
In addition, the potential risk of marginal mandibular nerve injuries, issues raised during the meeting included the concerns that the treatment would be used by clinicians who were not adequately trained to provide the treatment or not adequately familiar with the anatomy of the treated area. Other concerns include potential use of the product in areas that include the periorbitum, as well as off-label use of large amounts in different parts of the body, such as the abdomen or thighs. The company said that the product will not be provided to clinicians who have not gone through the planned training program and that it was better suited to small areas of the body.
While there are some questions that still need to be answered and concerns about off-label use and the need for proper training, the panel agreed this was safe and effective if used properly with appropriate training and appropriate precautions, said the panel chair, Dr. Lynn A. Drake of the department of dermatology, Massachusetts General Hospital, Boston.
DCA should not be used off label, a concern that could be at least partially addressed in the label, and “without appropriate training, this product could be misused and mishandled and cause damage,” she added. In addition, because of some “very novel” aspects to this treatment, postmarketing data are needed, addressing questions that include whether it causes scarring that could affect surgery and other interventions in the future, she said.
The FDA usually follows the recommendations of its advisory panels. None of the panel members had relevant disclosures.
AT AN FDA ADVISORY COMMITTEE MEETING
VIDEO: Expert tips to avoid eye ptosis
KAUAI, HAWAII– Ptosis can occur if toxins injected around the eyes are imprecisely placed, according to Dr. Brooke Sikora.
In this video report from the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Sikora, a private practice dermatologist in Boston discusses how to avoid adverse events when injecting toxins around the lids and brows, and what to do should the procedure not go according to plan.
Dr. Sikora had no relevant financial conflicts to disclose.
SDEF and this news organ
On Twitter @whitneymcknight
KAUAI, HAWAII– Ptosis can occur if toxins injected around the eyes are imprecisely placed, according to Dr. Brooke Sikora.
In this video report from the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Sikora, a private practice dermatologist in Boston discusses how to avoid adverse events when injecting toxins around the lids and brows, and what to do should the procedure not go according to plan.
Dr. Sikora had no relevant financial conflicts to disclose.
SDEF and this news organ
On Twitter @whitneymcknight
KAUAI, HAWAII– Ptosis can occur if toxins injected around the eyes are imprecisely placed, according to Dr. Brooke Sikora.
In this video report from the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Sikora, a private practice dermatologist in Boston discusses how to avoid adverse events when injecting toxins around the lids and brows, and what to do should the procedure not go according to plan.
Dr. Sikora had no relevant financial conflicts to disclose.
SDEF and this news organ
On Twitter @whitneymcknight
AT SDEF HAWAII DERMATOLOGY SEMINAR
Identification of Cutaneous Warts: Cryotherapy-Induced Acetowhitelike Epithelium
To the Editor:
Cutaneous warts are benign proliferations of the epidermis that occur secondary to human papillomavirus (HPV) infection. The diagnosis of cutaneous warts is generally based on clinical appearance. Occasionally subtle lesions, particularly those of verruca plana, escape clinical identification leading to incomplete treatment and spreading. The acetic acid test (sometimes called the acetic acid visual inspection) causes epithelial whitening of HPV-infected areas after application of a 3% to 5% aqueous solution of acetic acid and has been used to detect subclinical HPV infection.1 Although the acetic acid test can support the diagnosis of cutaneous warts, it is more effective at detecting hyperplastic rather than flat warts and may be cumbersome to use routinely.2 We describe a simple clinical maneuver to help confirm the presence of subtle warts using gentle liquid nitrogen cryotherapy to induce epithelial whitening in areas of HPV infection.
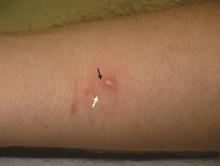
A 22-year-old man presented for evaluation of a 5-mm verrucous papule on the right wrist. He was diagnosed with verruca vulgaris. During treatment, small satellite verrucous papules were visualized by differential whitening from the surrounding uninfected skin (Figure). A brief light spray of liquid nitrogen cryotherapy (-196°C) was applied over areas containing suspicious lesions for confirmation. This acetowhitelike change from indirect collateral cryotherapy allowed for identification and treatment of these subtle warts.
Cutaneous warts represent foci of epithelial proliferation, and acetowhite changes are thought to occur from extravasation of intracellular water with subsequent tissue whitening in areas of high nuclear density.3 Acetowhite epithelium also has been reported after other ablative wart therapies.4 Similarly, acetowhitelike changes after cryotherapy may be secondary to cellular dehydration from ice crystal formation,5 with HPV-infected areas demonstrating increased susceptibility to freezing because of increased cellular water content in areas of hyperkeratosis. In addition, it has been demonstrated that cryotherapy alters the composition of the epithelium by destroying neutral and acidic mucopolysaccharides, which may subsequently induce the characteristic acetowhitelike changes in the epithelium of cutaneous warts.6
We propose that gentle painless sprays of liquid nitrogen to areas with suspicious lesions can help confirm the presence of subtle warts through cryotherapy-induced epithelial whitening. Although this test is a valuable diagnostic pearl, it should be noted that cryotherapy may accentuate an area of hyperkeratosis from causes other than an HPV infection. As such, clinical judgment is required.
1. Allan BM. Acetowhite epithelium. Gynecol Oncol. 2004;95:691-694.
2. Kumar B, Gupta S. The acetowhite test in genital human papillomavirus infection in men: what does it add? J Eur Acad Dermatol Venereol. 2001;15:27-29.
3. O’Connor DM. A tissue basis for colposcopic findings. Obstet Gynecol Clin North Am. 2008;35:565-582.
4. MacLean AB. Healing of the cervical epithelium after laser treatment of cervical intraepithelial neoplasia. Br J Obstet Gynaecol. 1984;91:697-706.
5. Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37:171-186.
6. Ciecierski L. Histochemical studies on acid and neutral mucopolysaccharides in the acanthotic epidermis of warts before and after cryotherapy with liquid nitrogen. Przegl Dermatol. 1970;57:11-15.
To the Editor:
Cutaneous warts are benign proliferations of the epidermis that occur secondary to human papillomavirus (HPV) infection. The diagnosis of cutaneous warts is generally based on clinical appearance. Occasionally subtle lesions, particularly those of verruca plana, escape clinical identification leading to incomplete treatment and spreading. The acetic acid test (sometimes called the acetic acid visual inspection) causes epithelial whitening of HPV-infected areas after application of a 3% to 5% aqueous solution of acetic acid and has been used to detect subclinical HPV infection.1 Although the acetic acid test can support the diagnosis of cutaneous warts, it is more effective at detecting hyperplastic rather than flat warts and may be cumbersome to use routinely.2 We describe a simple clinical maneuver to help confirm the presence of subtle warts using gentle liquid nitrogen cryotherapy to induce epithelial whitening in areas of HPV infection.
A 22-year-old man presented for evaluation of a 5-mm verrucous papule on the right wrist. He was diagnosed with verruca vulgaris. During treatment, small satellite verrucous papules were visualized by differential whitening from the surrounding uninfected skin (Figure). A brief light spray of liquid nitrogen cryotherapy (-196°C) was applied over areas containing suspicious lesions for confirmation. This acetowhitelike change from indirect collateral cryotherapy allowed for identification and treatment of these subtle warts.
Cutaneous warts represent foci of epithelial proliferation, and acetowhite changes are thought to occur from extravasation of intracellular water with subsequent tissue whitening in areas of high nuclear density.3 Acetowhite epithelium also has been reported after other ablative wart therapies.4 Similarly, acetowhitelike changes after cryotherapy may be secondary to cellular dehydration from ice crystal formation,5 with HPV-infected areas demonstrating increased susceptibility to freezing because of increased cellular water content in areas of hyperkeratosis. In addition, it has been demonstrated that cryotherapy alters the composition of the epithelium by destroying neutral and acidic mucopolysaccharides, which may subsequently induce the characteristic acetowhitelike changes in the epithelium of cutaneous warts.6
We propose that gentle painless sprays of liquid nitrogen to areas with suspicious lesions can help confirm the presence of subtle warts through cryotherapy-induced epithelial whitening. Although this test is a valuable diagnostic pearl, it should be noted that cryotherapy may accentuate an area of hyperkeratosis from causes other than an HPV infection. As such, clinical judgment is required.
To the Editor:
Cutaneous warts are benign proliferations of the epidermis that occur secondary to human papillomavirus (HPV) infection. The diagnosis of cutaneous warts is generally based on clinical appearance. Occasionally subtle lesions, particularly those of verruca plana, escape clinical identification leading to incomplete treatment and spreading. The acetic acid test (sometimes called the acetic acid visual inspection) causes epithelial whitening of HPV-infected areas after application of a 3% to 5% aqueous solution of acetic acid and has been used to detect subclinical HPV infection.1 Although the acetic acid test can support the diagnosis of cutaneous warts, it is more effective at detecting hyperplastic rather than flat warts and may be cumbersome to use routinely.2 We describe a simple clinical maneuver to help confirm the presence of subtle warts using gentle liquid nitrogen cryotherapy to induce epithelial whitening in areas of HPV infection.
A 22-year-old man presented for evaluation of a 5-mm verrucous papule on the right wrist. He was diagnosed with verruca vulgaris. During treatment, small satellite verrucous papules were visualized by differential whitening from the surrounding uninfected skin (Figure). A brief light spray of liquid nitrogen cryotherapy (-196°C) was applied over areas containing suspicious lesions for confirmation. This acetowhitelike change from indirect collateral cryotherapy allowed for identification and treatment of these subtle warts.
Cutaneous warts represent foci of epithelial proliferation, and acetowhite changes are thought to occur from extravasation of intracellular water with subsequent tissue whitening in areas of high nuclear density.3 Acetowhite epithelium also has been reported after other ablative wart therapies.4 Similarly, acetowhitelike changes after cryotherapy may be secondary to cellular dehydration from ice crystal formation,5 with HPV-infected areas demonstrating increased susceptibility to freezing because of increased cellular water content in areas of hyperkeratosis. In addition, it has been demonstrated that cryotherapy alters the composition of the epithelium by destroying neutral and acidic mucopolysaccharides, which may subsequently induce the characteristic acetowhitelike changes in the epithelium of cutaneous warts.6
We propose that gentle painless sprays of liquid nitrogen to areas with suspicious lesions can help confirm the presence of subtle warts through cryotherapy-induced epithelial whitening. Although this test is a valuable diagnostic pearl, it should be noted that cryotherapy may accentuate an area of hyperkeratosis from causes other than an HPV infection. As such, clinical judgment is required.
1. Allan BM. Acetowhite epithelium. Gynecol Oncol. 2004;95:691-694.
2. Kumar B, Gupta S. The acetowhite test in genital human papillomavirus infection in men: what does it add? J Eur Acad Dermatol Venereol. 2001;15:27-29.
3. O’Connor DM. A tissue basis for colposcopic findings. Obstet Gynecol Clin North Am. 2008;35:565-582.
4. MacLean AB. Healing of the cervical epithelium after laser treatment of cervical intraepithelial neoplasia. Br J Obstet Gynaecol. 1984;91:697-706.
5. Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37:171-186.
6. Ciecierski L. Histochemical studies on acid and neutral mucopolysaccharides in the acanthotic epidermis of warts before and after cryotherapy with liquid nitrogen. Przegl Dermatol. 1970;57:11-15.
1. Allan BM. Acetowhite epithelium. Gynecol Oncol. 2004;95:691-694.
2. Kumar B, Gupta S. The acetowhite test in genital human papillomavirus infection in men: what does it add? J Eur Acad Dermatol Venereol. 2001;15:27-29.
3. O’Connor DM. A tissue basis for colposcopic findings. Obstet Gynecol Clin North Am. 2008;35:565-582.
4. MacLean AB. Healing of the cervical epithelium after laser treatment of cervical intraepithelial neoplasia. Br J Obstet Gynaecol. 1984;91:697-706.
5. Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37:171-186.
6. Ciecierski L. Histochemical studies on acid and neutral mucopolysaccharides in the acanthotic epidermis of warts before and after cryotherapy with liquid nitrogen. Przegl Dermatol. 1970;57:11-15.
Customizable features enhance the latest fillers
Be prepared for more dermal fillers featuring a mix of low- and high-molecular-weight hyaluronic acid, as well as options for customization, said Dr. Nowell Solish.
Such a combination results in a highly crosslinked product that promotes greater duration with less swelling of the product and improved spreadability, he said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
“There is a role for dermal fillers with these characteristics,” said Dr. Solish of the University of Toronto. He compared the uses of several combination products, including Voluma, Volbella, and Volift.
Dr. Solish’s take on Voluma: “Great for volume,” particularly for the chest and midface, with a low and manageable rate of adverse events.
By contrast, Volbella, which is not yet approved for use in the United States, is a fast-flowing filler suited for lip hydration and perioral lines, rather than volume, he said. In a 2012 study, 48% of 58 adult patients showed an improvement of 1 point or higher on the Lip Fullness Scale at 1 year, based on physician assessment, he said (Clin. Cosmet. Investig. Dermatol. 2012;5;167-72).
Volift, another combination high/low-molecular-weight hyaluronic acid product, is “likely last to get approved” in the United States, Dr. Solish said. The product, however, is “great for marionette lines and smile lines,” as well as for improving the appearance of mild to moderate lines around the face.
Emervel is an example of a product in which the varying particle size allows for customization. The hyaluronic acid concentration of Emervel is consistent, but the product is available in three particle sizes for three depths of injection and varying lifting capacity, he explained.
Finally, the use of a filler containing polymethylmethacrylate (PMMA) microspheres was associated with significant improvements in atrophic facial acne scars, Dr. Solish said, citing a recent randomized controlled trial of 147 subjects (J. Am. Acad. Dermatol. 2014;71:77-83). The microspheres are designed to be consistent is size (30-50 mcm). Treatment-related adverse events were generally mild and transient.
The calcium microsphere filler Radiesse with lidocaine was recently approved by the Food and Drug Administration and should be available soon, he added.
Dr. Solish disclosed serving as an investigator and consultant for Allergan, Medicis, Revance Therapeutics, Marz, and Indeed. SDEF and this news organization are owned by the same parent company.
Be prepared for more dermal fillers featuring a mix of low- and high-molecular-weight hyaluronic acid, as well as options for customization, said Dr. Nowell Solish.
Such a combination results in a highly crosslinked product that promotes greater duration with less swelling of the product and improved spreadability, he said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
“There is a role for dermal fillers with these characteristics,” said Dr. Solish of the University of Toronto. He compared the uses of several combination products, including Voluma, Volbella, and Volift.
Dr. Solish’s take on Voluma: “Great for volume,” particularly for the chest and midface, with a low and manageable rate of adverse events.
By contrast, Volbella, which is not yet approved for use in the United States, is a fast-flowing filler suited for lip hydration and perioral lines, rather than volume, he said. In a 2012 study, 48% of 58 adult patients showed an improvement of 1 point or higher on the Lip Fullness Scale at 1 year, based on physician assessment, he said (Clin. Cosmet. Investig. Dermatol. 2012;5;167-72).
Volift, another combination high/low-molecular-weight hyaluronic acid product, is “likely last to get approved” in the United States, Dr. Solish said. The product, however, is “great for marionette lines and smile lines,” as well as for improving the appearance of mild to moderate lines around the face.
Emervel is an example of a product in which the varying particle size allows for customization. The hyaluronic acid concentration of Emervel is consistent, but the product is available in three particle sizes for three depths of injection and varying lifting capacity, he explained.
Finally, the use of a filler containing polymethylmethacrylate (PMMA) microspheres was associated with significant improvements in atrophic facial acne scars, Dr. Solish said, citing a recent randomized controlled trial of 147 subjects (J. Am. Acad. Dermatol. 2014;71:77-83). The microspheres are designed to be consistent is size (30-50 mcm). Treatment-related adverse events were generally mild and transient.
The calcium microsphere filler Radiesse with lidocaine was recently approved by the Food and Drug Administration and should be available soon, he added.
Dr. Solish disclosed serving as an investigator and consultant for Allergan, Medicis, Revance Therapeutics, Marz, and Indeed. SDEF and this news organization are owned by the same parent company.
Be prepared for more dermal fillers featuring a mix of low- and high-molecular-weight hyaluronic acid, as well as options for customization, said Dr. Nowell Solish.
Such a combination results in a highly crosslinked product that promotes greater duration with less swelling of the product and improved spreadability, he said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
“There is a role for dermal fillers with these characteristics,” said Dr. Solish of the University of Toronto. He compared the uses of several combination products, including Voluma, Volbella, and Volift.
Dr. Solish’s take on Voluma: “Great for volume,” particularly for the chest and midface, with a low and manageable rate of adverse events.
By contrast, Volbella, which is not yet approved for use in the United States, is a fast-flowing filler suited for lip hydration and perioral lines, rather than volume, he said. In a 2012 study, 48% of 58 adult patients showed an improvement of 1 point or higher on the Lip Fullness Scale at 1 year, based on physician assessment, he said (Clin. Cosmet. Investig. Dermatol. 2012;5;167-72).
Volift, another combination high/low-molecular-weight hyaluronic acid product, is “likely last to get approved” in the United States, Dr. Solish said. The product, however, is “great for marionette lines and smile lines,” as well as for improving the appearance of mild to moderate lines around the face.
Emervel is an example of a product in which the varying particle size allows for customization. The hyaluronic acid concentration of Emervel is consistent, but the product is available in three particle sizes for three depths of injection and varying lifting capacity, he explained.
Finally, the use of a filler containing polymethylmethacrylate (PMMA) microspheres was associated with significant improvements in atrophic facial acne scars, Dr. Solish said, citing a recent randomized controlled trial of 147 subjects (J. Am. Acad. Dermatol. 2014;71:77-83). The microspheres are designed to be consistent is size (30-50 mcm). Treatment-related adverse events were generally mild and transient.
The calcium microsphere filler Radiesse with lidocaine was recently approved by the Food and Drug Administration and should be available soon, he added.
Dr. Solish disclosed serving as an investigator and consultant for Allergan, Medicis, Revance Therapeutics, Marz, and Indeed. SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Novel ‘soft anticholinergic’ shows promise for axillary hyperhidrosis
MIAMI BEACH – A promising new treatment for axillary hyperhidrosis will soon be evaluated in a clinical trial.
The novel molecular compound – a “soft anticholinergic” – was safe and effective as a topical treatment in a recent pilot study, Dr. Brian Berman reported in a session on innovations in clinical therapeutics at the South Beach Symposium.
Currently known as BBI-4000, the product is locally active but quickly metabolized if absorbed, which reduces the risk of treatment-related adverse events, said Dr. Berman of the University of Miami.
No comparable first-line topical prescription treatment exists for axillary hyperhidrosis, he noted.
In the pilot study – the results of which were reported in September by the manufacturer, Brickell Biotech – 18 subjects were randomized to receive once-daily treatment with either a high topical concentration, a low topical concentration, or topical vehicle for 14 days. Of the 12 patients receiving active treatment, 75% achieved at least a 50% sweat reduction, compared with 33% of the 6 patients in the vehicle group.
Further, 67% of those receiving active treatment, compared with 33% in the vehicle group, achieved at least a two-point improvement in their Hyperhidrosis Disease Severity Scale score, indicating substantial improvement, Dr. Berman said.
No treatment-related adverse events were reported, and none of the patients discontinued treatment because of adverse events.
Based on these findings, Brickell Biotech has initiated a randomized, double-blind, vehicle-controlled phase IIb clinical trial with plans to enroll 180 patients with primary axillary hyperhidrosis. The trial will assess the safety and efficacy of three different concentrations of BBI-4000, compared with vehicle.
Dr. Berman is a consultant, researcher, and/or speaker for Beiersdorf, DUSA Pharmaceuticals, Ferndale Pharmaceuticals, Galderma Laboratories, Genentech, Halscion, LEO Pharmaceuticals, Onset Therapeutics, Sensus Healthcare, Tigercat Pharma, and TopMD Skin Care. He also owns stock in TopMD Skin Care.
MIAMI BEACH – A promising new treatment for axillary hyperhidrosis will soon be evaluated in a clinical trial.
The novel molecular compound – a “soft anticholinergic” – was safe and effective as a topical treatment in a recent pilot study, Dr. Brian Berman reported in a session on innovations in clinical therapeutics at the South Beach Symposium.
Currently known as BBI-4000, the product is locally active but quickly metabolized if absorbed, which reduces the risk of treatment-related adverse events, said Dr. Berman of the University of Miami.
No comparable first-line topical prescription treatment exists for axillary hyperhidrosis, he noted.
In the pilot study – the results of which were reported in September by the manufacturer, Brickell Biotech – 18 subjects were randomized to receive once-daily treatment with either a high topical concentration, a low topical concentration, or topical vehicle for 14 days. Of the 12 patients receiving active treatment, 75% achieved at least a 50% sweat reduction, compared with 33% of the 6 patients in the vehicle group.
Further, 67% of those receiving active treatment, compared with 33% in the vehicle group, achieved at least a two-point improvement in their Hyperhidrosis Disease Severity Scale score, indicating substantial improvement, Dr. Berman said.
No treatment-related adverse events were reported, and none of the patients discontinued treatment because of adverse events.
Based on these findings, Brickell Biotech has initiated a randomized, double-blind, vehicle-controlled phase IIb clinical trial with plans to enroll 180 patients with primary axillary hyperhidrosis. The trial will assess the safety and efficacy of three different concentrations of BBI-4000, compared with vehicle.
Dr. Berman is a consultant, researcher, and/or speaker for Beiersdorf, DUSA Pharmaceuticals, Ferndale Pharmaceuticals, Galderma Laboratories, Genentech, Halscion, LEO Pharmaceuticals, Onset Therapeutics, Sensus Healthcare, Tigercat Pharma, and TopMD Skin Care. He also owns stock in TopMD Skin Care.
MIAMI BEACH – A promising new treatment for axillary hyperhidrosis will soon be evaluated in a clinical trial.
The novel molecular compound – a “soft anticholinergic” – was safe and effective as a topical treatment in a recent pilot study, Dr. Brian Berman reported in a session on innovations in clinical therapeutics at the South Beach Symposium.
Currently known as BBI-4000, the product is locally active but quickly metabolized if absorbed, which reduces the risk of treatment-related adverse events, said Dr. Berman of the University of Miami.
No comparable first-line topical prescription treatment exists for axillary hyperhidrosis, he noted.
In the pilot study – the results of which were reported in September by the manufacturer, Brickell Biotech – 18 subjects were randomized to receive once-daily treatment with either a high topical concentration, a low topical concentration, or topical vehicle for 14 days. Of the 12 patients receiving active treatment, 75% achieved at least a 50% sweat reduction, compared with 33% of the 6 patients in the vehicle group.
Further, 67% of those receiving active treatment, compared with 33% in the vehicle group, achieved at least a two-point improvement in their Hyperhidrosis Disease Severity Scale score, indicating substantial improvement, Dr. Berman said.
No treatment-related adverse events were reported, and none of the patients discontinued treatment because of adverse events.
Based on these findings, Brickell Biotech has initiated a randomized, double-blind, vehicle-controlled phase IIb clinical trial with plans to enroll 180 patients with primary axillary hyperhidrosis. The trial will assess the safety and efficacy of three different concentrations of BBI-4000, compared with vehicle.
Dr. Berman is a consultant, researcher, and/or speaker for Beiersdorf, DUSA Pharmaceuticals, Ferndale Pharmaceuticals, Galderma Laboratories, Genentech, Halscion, LEO Pharmaceuticals, Onset Therapeutics, Sensus Healthcare, Tigercat Pharma, and TopMD Skin Care. He also owns stock in TopMD Skin Care.
AT THE SOUTH BEACH SYMPOSIUM
Careful planning, technique, and counseling can minimize pediatric scarring
It’s a myth that children don’t scar like adults, according to Dr. Jon A. Dyer.
In fact, children often scar worse than adults, he said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
Children have more aggressive healing and inflammatory responses, a highly elastic dermis and connective tissues, and a high activity level that increases the risk of stressing the wound, and they may pay less attention to it, he explained.
Additionally, children can be more difficult to treat for scars because they are often more fearful and anxious, they have a short attention span, and they move around – a lot, he said. This leads to greater anxiety on the part of both the dermatologist and the family, which can lead to rapidly completing a procedure, said Dr. Dyer of the University of Missouri, Columbia.
Successful treatment of a wound or lesion – that is, getting the best initial scar possible – requires good planning, proactive application of surgical principles, and careful patient and family counseling and follow-up, he said.
Among Dr. Dyer’s tips for minimizing scarring when treating lesions in children:
• Do the simplest possible procedure.
• Make the scar as small as possible, keeping in mind that pediatric skin is more elastic.
• Remember that fusiform closures in children do not always require a 3:1 ratio. Tips settle, and divots fill in in young children, he said.
• Operate before puberty when the lesion is located in a cosmetically important area.
Also, consider performing a staged excision if possible; this allows a reduction in final scar length, provides tissue expansion, and allows assessment of individual wound healing. Take as much of the lesion as possible the first time, and remember that central excision is preferable to lateral excision, Dr. Dyer said.
The ideal time to wait between stage 1 and 2 is 4-6 weeks. Longer intervals will lead to more spread and more hypertrophy, and will negate the benefits of staging, he said.
Keep in mind that purse-string sutures can be particularly effective for suturing small spaces and for closing dead spaces, he said, adding that use of a purse-string closure for a round defect can reduce the final scar length by at least 50%.
Scar prevention or minimization requires strict adherence to good surgical principles, Dr. Dyer said, explaining that attention must be paid to perfecting the excision through proper use of skin tension lines, clean wound edges, sharp corners with no or minimal boating, undermining, and removing bulk if necessary.
Track marks are more likely on nonfacial skin and can be avoided by using running subcuticular sutures; in some cases of excellent approximation with no tension, the wound surface can be secured with Dermabond, he said.
Dr. Dyer also outlined the best closure choices for specific areas. For the scalp, use running cuticular sutures; for the extremities, trunk, axilla, or groin, use running subcuticular sutures; for the face, feet, or hands, use interrupted sutures; and for a punch biopsy, consider not using any closure, he advised.
If cuticular stitches are used, minimize scarring by pulling them within 5 days on the face, and within 7-10 days on the body. If dehiscence is a concern, pull the cuticulars and follow with either Dermabond or SteriStrips, he said, but strongly consider using running subcuticular sutures.
Once a wound is closed, secure it with Dermabond or with Steri-Strips and Mastisol and provide protective dressing.
“The bulkier and more colorful the dressing, the happier and more compliant the child,” he said.
Minimize postoperative wound tension in high-risk scars or sites and restrict movement and trauma for as long as possible; 6 weeks is ideal, he added, noting that movement restriction is critical for ensuring a good outcome.
To reduce the trauma of suture removal, “cut ’em long and leave ’em long,” and be sure to use proper scissors, he said.
Running subcuticular suture removal doesn’t hurt; enlist parents’ help in convincing the child of this, he suggested.
Finally, be specific when counseling patients about restrictions, discuss possible complications, provide a written summary including contact numbers, address the scar, and give the follow-up appointment time in writing, he said.
If dehiscence occurs – and it’s not uncommon in children – let it heal and restart the process, he said, noting that prevention is best and can be achieved in many cases with aggressive pre- and postop education, restriction of movement, and proper dressing.
Dr. Dyer stressed that good surgical principles for use in children can also be utilized to improve results in adult patients.
Dr. Dyer reported having no disclosures. SDEF and this new organization are owned by the same parent company.
It’s a myth that children don’t scar like adults, according to Dr. Jon A. Dyer.
In fact, children often scar worse than adults, he said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
Children have more aggressive healing and inflammatory responses, a highly elastic dermis and connective tissues, and a high activity level that increases the risk of stressing the wound, and they may pay less attention to it, he explained.
Additionally, children can be more difficult to treat for scars because they are often more fearful and anxious, they have a short attention span, and they move around – a lot, he said. This leads to greater anxiety on the part of both the dermatologist and the family, which can lead to rapidly completing a procedure, said Dr. Dyer of the University of Missouri, Columbia.
Successful treatment of a wound or lesion – that is, getting the best initial scar possible – requires good planning, proactive application of surgical principles, and careful patient and family counseling and follow-up, he said.
Among Dr. Dyer’s tips for minimizing scarring when treating lesions in children:
• Do the simplest possible procedure.
• Make the scar as small as possible, keeping in mind that pediatric skin is more elastic.
• Remember that fusiform closures in children do not always require a 3:1 ratio. Tips settle, and divots fill in in young children, he said.
• Operate before puberty when the lesion is located in a cosmetically important area.
Also, consider performing a staged excision if possible; this allows a reduction in final scar length, provides tissue expansion, and allows assessment of individual wound healing. Take as much of the lesion as possible the first time, and remember that central excision is preferable to lateral excision, Dr. Dyer said.
The ideal time to wait between stage 1 and 2 is 4-6 weeks. Longer intervals will lead to more spread and more hypertrophy, and will negate the benefits of staging, he said.
Keep in mind that purse-string sutures can be particularly effective for suturing small spaces and for closing dead spaces, he said, adding that use of a purse-string closure for a round defect can reduce the final scar length by at least 50%.
Scar prevention or minimization requires strict adherence to good surgical principles, Dr. Dyer said, explaining that attention must be paid to perfecting the excision through proper use of skin tension lines, clean wound edges, sharp corners with no or minimal boating, undermining, and removing bulk if necessary.
Track marks are more likely on nonfacial skin and can be avoided by using running subcuticular sutures; in some cases of excellent approximation with no tension, the wound surface can be secured with Dermabond, he said.
Dr. Dyer also outlined the best closure choices for specific areas. For the scalp, use running cuticular sutures; for the extremities, trunk, axilla, or groin, use running subcuticular sutures; for the face, feet, or hands, use interrupted sutures; and for a punch biopsy, consider not using any closure, he advised.
If cuticular stitches are used, minimize scarring by pulling them within 5 days on the face, and within 7-10 days on the body. If dehiscence is a concern, pull the cuticulars and follow with either Dermabond or SteriStrips, he said, but strongly consider using running subcuticular sutures.
Once a wound is closed, secure it with Dermabond or with Steri-Strips and Mastisol and provide protective dressing.
“The bulkier and more colorful the dressing, the happier and more compliant the child,” he said.
Minimize postoperative wound tension in high-risk scars or sites and restrict movement and trauma for as long as possible; 6 weeks is ideal, he added, noting that movement restriction is critical for ensuring a good outcome.
To reduce the trauma of suture removal, “cut ’em long and leave ’em long,” and be sure to use proper scissors, he said.
Running subcuticular suture removal doesn’t hurt; enlist parents’ help in convincing the child of this, he suggested.
Finally, be specific when counseling patients about restrictions, discuss possible complications, provide a written summary including contact numbers, address the scar, and give the follow-up appointment time in writing, he said.
If dehiscence occurs – and it’s not uncommon in children – let it heal and restart the process, he said, noting that prevention is best and can be achieved in many cases with aggressive pre- and postop education, restriction of movement, and proper dressing.
Dr. Dyer stressed that good surgical principles for use in children can also be utilized to improve results in adult patients.
Dr. Dyer reported having no disclosures. SDEF and this new organization are owned by the same parent company.
It’s a myth that children don’t scar like adults, according to Dr. Jon A. Dyer.
In fact, children often scar worse than adults, he said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
Children have more aggressive healing and inflammatory responses, a highly elastic dermis and connective tissues, and a high activity level that increases the risk of stressing the wound, and they may pay less attention to it, he explained.
Additionally, children can be more difficult to treat for scars because they are often more fearful and anxious, they have a short attention span, and they move around – a lot, he said. This leads to greater anxiety on the part of both the dermatologist and the family, which can lead to rapidly completing a procedure, said Dr. Dyer of the University of Missouri, Columbia.
Successful treatment of a wound or lesion – that is, getting the best initial scar possible – requires good planning, proactive application of surgical principles, and careful patient and family counseling and follow-up, he said.
Among Dr. Dyer’s tips for minimizing scarring when treating lesions in children:
• Do the simplest possible procedure.
• Make the scar as small as possible, keeping in mind that pediatric skin is more elastic.
• Remember that fusiform closures in children do not always require a 3:1 ratio. Tips settle, and divots fill in in young children, he said.
• Operate before puberty when the lesion is located in a cosmetically important area.
Also, consider performing a staged excision if possible; this allows a reduction in final scar length, provides tissue expansion, and allows assessment of individual wound healing. Take as much of the lesion as possible the first time, and remember that central excision is preferable to lateral excision, Dr. Dyer said.
The ideal time to wait between stage 1 and 2 is 4-6 weeks. Longer intervals will lead to more spread and more hypertrophy, and will negate the benefits of staging, he said.
Keep in mind that purse-string sutures can be particularly effective for suturing small spaces and for closing dead spaces, he said, adding that use of a purse-string closure for a round defect can reduce the final scar length by at least 50%.
Scar prevention or minimization requires strict adherence to good surgical principles, Dr. Dyer said, explaining that attention must be paid to perfecting the excision through proper use of skin tension lines, clean wound edges, sharp corners with no or minimal boating, undermining, and removing bulk if necessary.
Track marks are more likely on nonfacial skin and can be avoided by using running subcuticular sutures; in some cases of excellent approximation with no tension, the wound surface can be secured with Dermabond, he said.
Dr. Dyer also outlined the best closure choices for specific areas. For the scalp, use running cuticular sutures; for the extremities, trunk, axilla, or groin, use running subcuticular sutures; for the face, feet, or hands, use interrupted sutures; and for a punch biopsy, consider not using any closure, he advised.
If cuticular stitches are used, minimize scarring by pulling them within 5 days on the face, and within 7-10 days on the body. If dehiscence is a concern, pull the cuticulars and follow with either Dermabond or SteriStrips, he said, but strongly consider using running subcuticular sutures.
Once a wound is closed, secure it with Dermabond or with Steri-Strips and Mastisol and provide protective dressing.
“The bulkier and more colorful the dressing, the happier and more compliant the child,” he said.
Minimize postoperative wound tension in high-risk scars or sites and restrict movement and trauma for as long as possible; 6 weeks is ideal, he added, noting that movement restriction is critical for ensuring a good outcome.
To reduce the trauma of suture removal, “cut ’em long and leave ’em long,” and be sure to use proper scissors, he said.
Running subcuticular suture removal doesn’t hurt; enlist parents’ help in convincing the child of this, he suggested.
Finally, be specific when counseling patients about restrictions, discuss possible complications, provide a written summary including contact numbers, address the scar, and give the follow-up appointment time in writing, he said.
If dehiscence occurs – and it’s not uncommon in children – let it heal and restart the process, he said, noting that prevention is best and can be achieved in many cases with aggressive pre- and postop education, restriction of movement, and proper dressing.
Dr. Dyer stressed that good surgical principles for use in children can also be utilized to improve results in adult patients.
Dr. Dyer reported having no disclosures. SDEF and this new organization are owned by the same parent company.
Hyaluronic acid wrinkle filler also appears useful for midface augmentation
MIAMI BEACH – A large gel particle hyaluronic acid filler with lidocaine (LGP-HAL) was safe and effective for midface augmentation and correction of midface contour deficiencies in a randomized, multicenter, pivotal study.
The product (Perlane, Galderma Laboratories), which is currently approved for moderate to severe facial folds and wrinkles, provided visible, clinically meaningful, aesthetic results for at least 12 months based on assessment by a blinded evaluator, Dr. David E. Bank reported in a poster at the South Beach Symposium.
At 8 weeks after initial treatment, 89% of 150 treated subjects experienced at least a one-point improvement in their combined right and left side Medicis Midface Volume Scale (MMVS) score, compared with 16% of 50 patients who were not treated.
Further, the treated subjects were assessed as having greater midface fullness than the nontreated subjects at every measurement during the first 12 months after treatment, according to Dr. Bank of New York–Presbyterian Hospital/Columbia University Medical Center, New York.
Study participants were men and women aged 18-65 years (mean of 53 years) with MMVS scores of two, three, or four on the validated one- to four-point scale, with one indicating a fairly full midface and four indicating substantial loss of fullness in the midface area. At the initial treatment, the 150 treatment group subjects were injected with a mean volume of 4.21 mL. All but one received subcutaneous injections, and 115 received supraperiosteal injections.
They were evaluated at weeks 2 (when an optional touch-up was allowed) and 4, and months 2, 4, 6, 8, 10, and 12 following the initial treatment.
The treatment group subjects were treated again at 12 months, along with those not treated initially. Another touch-up was allowed at a 2-week assessment, and additional assessments were conducted at weeks 3 and 4.
Treatment was well tolerated. More than 80% of events reported after treatment were mild in severity, and the adverse events that occurred in more than 5% of subjects included implant site hematoma, hemorrhage, pain, swelling, and headache.
One subject had two cases of implant site inflammation that were deemed related by the investigators, and one experienced a serious adverse event (implant site hematoma) deemed related to the procedure as opposed to the device.
No additional risk was seen in those who were treated twice, Dr. Bank noted.
“Facial shape plays an important role in facial attractiveness,” he wrote, adding that full cheeks, in particular, play an important role.
Aging, however, is associated with increased tissue laxity, soft-tissue descent and deflation, and loss of bone, which all contribute to deficiencies in the midface.
Hyaluronic fillers have been used successfully to correct such deficiencies, and the current findings suggest that LGP-HAL also can be used safely to enhance midface aesthetics, he concluded.
This study was funded by Galderma Laboratories.
MIAMI BEACH – A large gel particle hyaluronic acid filler with lidocaine (LGP-HAL) was safe and effective for midface augmentation and correction of midface contour deficiencies in a randomized, multicenter, pivotal study.
The product (Perlane, Galderma Laboratories), which is currently approved for moderate to severe facial folds and wrinkles, provided visible, clinically meaningful, aesthetic results for at least 12 months based on assessment by a blinded evaluator, Dr. David E. Bank reported in a poster at the South Beach Symposium.
At 8 weeks after initial treatment, 89% of 150 treated subjects experienced at least a one-point improvement in their combined right and left side Medicis Midface Volume Scale (MMVS) score, compared with 16% of 50 patients who were not treated.
Further, the treated subjects were assessed as having greater midface fullness than the nontreated subjects at every measurement during the first 12 months after treatment, according to Dr. Bank of New York–Presbyterian Hospital/Columbia University Medical Center, New York.
Study participants were men and women aged 18-65 years (mean of 53 years) with MMVS scores of two, three, or four on the validated one- to four-point scale, with one indicating a fairly full midface and four indicating substantial loss of fullness in the midface area. At the initial treatment, the 150 treatment group subjects were injected with a mean volume of 4.21 mL. All but one received subcutaneous injections, and 115 received supraperiosteal injections.
They were evaluated at weeks 2 (when an optional touch-up was allowed) and 4, and months 2, 4, 6, 8, 10, and 12 following the initial treatment.
The treatment group subjects were treated again at 12 months, along with those not treated initially. Another touch-up was allowed at a 2-week assessment, and additional assessments were conducted at weeks 3 and 4.
Treatment was well tolerated. More than 80% of events reported after treatment were mild in severity, and the adverse events that occurred in more than 5% of subjects included implant site hematoma, hemorrhage, pain, swelling, and headache.
One subject had two cases of implant site inflammation that were deemed related by the investigators, and one experienced a serious adverse event (implant site hematoma) deemed related to the procedure as opposed to the device.
No additional risk was seen in those who were treated twice, Dr. Bank noted.
“Facial shape plays an important role in facial attractiveness,” he wrote, adding that full cheeks, in particular, play an important role.
Aging, however, is associated with increased tissue laxity, soft-tissue descent and deflation, and loss of bone, which all contribute to deficiencies in the midface.
Hyaluronic fillers have been used successfully to correct such deficiencies, and the current findings suggest that LGP-HAL also can be used safely to enhance midface aesthetics, he concluded.
This study was funded by Galderma Laboratories.
MIAMI BEACH – A large gel particle hyaluronic acid filler with lidocaine (LGP-HAL) was safe and effective for midface augmentation and correction of midface contour deficiencies in a randomized, multicenter, pivotal study.
The product (Perlane, Galderma Laboratories), which is currently approved for moderate to severe facial folds and wrinkles, provided visible, clinically meaningful, aesthetic results for at least 12 months based on assessment by a blinded evaluator, Dr. David E. Bank reported in a poster at the South Beach Symposium.
At 8 weeks after initial treatment, 89% of 150 treated subjects experienced at least a one-point improvement in their combined right and left side Medicis Midface Volume Scale (MMVS) score, compared with 16% of 50 patients who were not treated.
Further, the treated subjects were assessed as having greater midface fullness than the nontreated subjects at every measurement during the first 12 months after treatment, according to Dr. Bank of New York–Presbyterian Hospital/Columbia University Medical Center, New York.
Study participants were men and women aged 18-65 years (mean of 53 years) with MMVS scores of two, three, or four on the validated one- to four-point scale, with one indicating a fairly full midface and four indicating substantial loss of fullness in the midface area. At the initial treatment, the 150 treatment group subjects were injected with a mean volume of 4.21 mL. All but one received subcutaneous injections, and 115 received supraperiosteal injections.
They were evaluated at weeks 2 (when an optional touch-up was allowed) and 4, and months 2, 4, 6, 8, 10, and 12 following the initial treatment.
The treatment group subjects were treated again at 12 months, along with those not treated initially. Another touch-up was allowed at a 2-week assessment, and additional assessments were conducted at weeks 3 and 4.
Treatment was well tolerated. More than 80% of events reported after treatment were mild in severity, and the adverse events that occurred in more than 5% of subjects included implant site hematoma, hemorrhage, pain, swelling, and headache.
One subject had two cases of implant site inflammation that were deemed related by the investigators, and one experienced a serious adverse event (implant site hematoma) deemed related to the procedure as opposed to the device.
No additional risk was seen in those who were treated twice, Dr. Bank noted.
“Facial shape plays an important role in facial attractiveness,” he wrote, adding that full cheeks, in particular, play an important role.
Aging, however, is associated with increased tissue laxity, soft-tissue descent and deflation, and loss of bone, which all contribute to deficiencies in the midface.
Hyaluronic fillers have been used successfully to correct such deficiencies, and the current findings suggest that LGP-HAL also can be used safely to enhance midface aesthetics, he concluded.
This study was funded by Galderma Laboratories.
Key clinical point: LGP-HAL (Perlane) is safe and effective for midface augmentation.
Major finding: At 8 weeks, 89% of treated subjects vs. 16% of controls experienced at least a one-point improvement in MMVS score.
Data source: A randomized, multicenter, pivotal study of 200 subjects.
Disclosures This study was funded by Galderma Laboratories.