User login
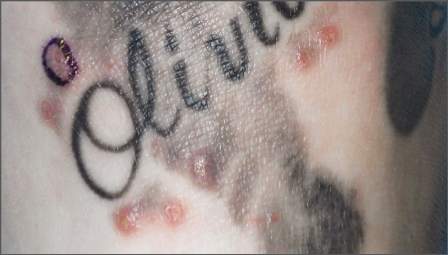
Teenage tattoos are most often regretted
SAN FRANCISCO – The younger people are when they get a tattoo, the more likely they are to regret it later, according to a survey of 501 people in the French Quarter of New Orleans.
All the participants were aged 18 years or older and had at least one tattoo. Overall, 16.2% said they regretted at least one tattoo, but that number rose to more than a third (35%) among the 77 people who got their first tattoo when they were 17 years old or younger. Among the 257 people who waited until they were 18-20 years old, 13.6% regretted one or more of their tattoos.
The rest got their first tattoo when they were at least 21 years old; 11.4% had regrets.
About one in five Americans have a tattoo, with the number increasing to about a third or more in people 18-30 years old, according to Walter Liszewski, a medical student at Tulane University in New Orleans.
Previous studies have found a regret rate of 16%-44%. The association with age suggests that it might be wise, when possible, to counsel teenagers to hold off for a while, Mr. Liszewski said. There’s a chance they will listen to that message, too, if it comes from a dermatologist. In the survey, 93% of people thought that tattoo artists were the best source of information on tattoo complications and how to treat them, but dermatologists weren’t far behind, with about 80% of respondents saying that dermatologists also were a trusted source for reliable information about tattoos. “So don’t hesitate to engage with patients about tattoos, complications, and other questions they might have,” Mr. Liszewski said at the annual meeting of the American Academy of Dermatology.
About 70% of the survey participants said they felt comfortable discussing such issues with primary care providers, and 40% said they felt comfortable discussing such issues with pharmacists.
Age requirements for tattoos vary widely from state to state, but some do allow individuals under 18 years of age to get a tattoo, even without parent permission.
In the first few days of 2015, the investigators asked passersby in the French Quarter’s Jackson Square if they had at least one tattoo, then offered the 18-question survey. They ran the project between 11 a.m. and 3 p.m., when people were less likely to be tipsy, Mr. Liszewski said. Respondents came from 38 states, plus the District of Columbia and Puerto Rico; most were from the Southeast, including 39% from Louisiana. Just over half were women, and the majority were white.
“We were also concerned that 21.2% received a tattoo while intoxicated, and 17.6% had at least one tattoo” done at someplace other than a tattoo parlor, he said. That means that if “patients have a lot of tattoos and you are trying to make small talk, feel free to ask them where they’re getting their tattoos done,” he added.
If tattoos were received at a party, or in prison or someplace else, “you may want to consider counseling them on HIV and hepatitis C testing,” Mr. Liszewski noted.
Just over 3% of the participants reported having had an infected tattoo, and while it’s normal for tattoos to be itchy and a little bit painful for the first week or two, 22.6% of the respondents said they had a pruritic tattoo, and 3.8% said they’d had a painful one a month or more after it was done.
“Tattoo complications are not uncommon,” Mr. Liszewski said. Given how much tattooed people seem to trust dermatologists, “there is an opportunity for dermatologists to manage these complications,” he added.
Mr. Liszewski said he had no disclosures.
SAN FRANCISCO – The younger people are when they get a tattoo, the more likely they are to regret it later, according to a survey of 501 people in the French Quarter of New Orleans.
All the participants were aged 18 years or older and had at least one tattoo. Overall, 16.2% said they regretted at least one tattoo, but that number rose to more than a third (35%) among the 77 people who got their first tattoo when they were 17 years old or younger. Among the 257 people who waited until they were 18-20 years old, 13.6% regretted one or more of their tattoos.
The rest got their first tattoo when they were at least 21 years old; 11.4% had regrets.
About one in five Americans have a tattoo, with the number increasing to about a third or more in people 18-30 years old, according to Walter Liszewski, a medical student at Tulane University in New Orleans.
Previous studies have found a regret rate of 16%-44%. The association with age suggests that it might be wise, when possible, to counsel teenagers to hold off for a while, Mr. Liszewski said. There’s a chance they will listen to that message, too, if it comes from a dermatologist. In the survey, 93% of people thought that tattoo artists were the best source of information on tattoo complications and how to treat them, but dermatologists weren’t far behind, with about 80% of respondents saying that dermatologists also were a trusted source for reliable information about tattoos. “So don’t hesitate to engage with patients about tattoos, complications, and other questions they might have,” Mr. Liszewski said at the annual meeting of the American Academy of Dermatology.
About 70% of the survey participants said they felt comfortable discussing such issues with primary care providers, and 40% said they felt comfortable discussing such issues with pharmacists.
Age requirements for tattoos vary widely from state to state, but some do allow individuals under 18 years of age to get a tattoo, even without parent permission.
In the first few days of 2015, the investigators asked passersby in the French Quarter’s Jackson Square if they had at least one tattoo, then offered the 18-question survey. They ran the project between 11 a.m. and 3 p.m., when people were less likely to be tipsy, Mr. Liszewski said. Respondents came from 38 states, plus the District of Columbia and Puerto Rico; most were from the Southeast, including 39% from Louisiana. Just over half were women, and the majority were white.
“We were also concerned that 21.2% received a tattoo while intoxicated, and 17.6% had at least one tattoo” done at someplace other than a tattoo parlor, he said. That means that if “patients have a lot of tattoos and you are trying to make small talk, feel free to ask them where they’re getting their tattoos done,” he added.
If tattoos were received at a party, or in prison or someplace else, “you may want to consider counseling them on HIV and hepatitis C testing,” Mr. Liszewski noted.
Just over 3% of the participants reported having had an infected tattoo, and while it’s normal for tattoos to be itchy and a little bit painful for the first week or two, 22.6% of the respondents said they had a pruritic tattoo, and 3.8% said they’d had a painful one a month or more after it was done.
“Tattoo complications are not uncommon,” Mr. Liszewski said. Given how much tattooed people seem to trust dermatologists, “there is an opportunity for dermatologists to manage these complications,” he added.
Mr. Liszewski said he had no disclosures.
SAN FRANCISCO – The younger people are when they get a tattoo, the more likely they are to regret it later, according to a survey of 501 people in the French Quarter of New Orleans.
All the participants were aged 18 years or older and had at least one tattoo. Overall, 16.2% said they regretted at least one tattoo, but that number rose to more than a third (35%) among the 77 people who got their first tattoo when they were 17 years old or younger. Among the 257 people who waited until they were 18-20 years old, 13.6% regretted one or more of their tattoos.
The rest got their first tattoo when they were at least 21 years old; 11.4% had regrets.
About one in five Americans have a tattoo, with the number increasing to about a third or more in people 18-30 years old, according to Walter Liszewski, a medical student at Tulane University in New Orleans.
Previous studies have found a regret rate of 16%-44%. The association with age suggests that it might be wise, when possible, to counsel teenagers to hold off for a while, Mr. Liszewski said. There’s a chance they will listen to that message, too, if it comes from a dermatologist. In the survey, 93% of people thought that tattoo artists were the best source of information on tattoo complications and how to treat them, but dermatologists weren’t far behind, with about 80% of respondents saying that dermatologists also were a trusted source for reliable information about tattoos. “So don’t hesitate to engage with patients about tattoos, complications, and other questions they might have,” Mr. Liszewski said at the annual meeting of the American Academy of Dermatology.
About 70% of the survey participants said they felt comfortable discussing such issues with primary care providers, and 40% said they felt comfortable discussing such issues with pharmacists.
Age requirements for tattoos vary widely from state to state, but some do allow individuals under 18 years of age to get a tattoo, even without parent permission.
In the first few days of 2015, the investigators asked passersby in the French Quarter’s Jackson Square if they had at least one tattoo, then offered the 18-question survey. They ran the project between 11 a.m. and 3 p.m., when people were less likely to be tipsy, Mr. Liszewski said. Respondents came from 38 states, plus the District of Columbia and Puerto Rico; most were from the Southeast, including 39% from Louisiana. Just over half were women, and the majority were white.
“We were also concerned that 21.2% received a tattoo while intoxicated, and 17.6% had at least one tattoo” done at someplace other than a tattoo parlor, he said. That means that if “patients have a lot of tattoos and you are trying to make small talk, feel free to ask them where they’re getting their tattoos done,” he added.
If tattoos were received at a party, or in prison or someplace else, “you may want to consider counseling them on HIV and hepatitis C testing,” Mr. Liszewski noted.
Just over 3% of the participants reported having had an infected tattoo, and while it’s normal for tattoos to be itchy and a little bit painful for the first week or two, 22.6% of the respondents said they had a pruritic tattoo, and 3.8% said they’d had a painful one a month or more after it was done.
“Tattoo complications are not uncommon,” Mr. Liszewski said. Given how much tattooed people seem to trust dermatologists, “there is an opportunity for dermatologists to manage these complications,” he added.
Mr. Liszewski said he had no disclosures.
AT THE AAD ANNUAL MEETING
Key clinical point: It might be wise, when possible, to counsel teenagers to postpone tattoos.
Major finding: Overall, 16.2% of tattooed adults said they regretted at least one, but that number rose to 35% among people who got their first tattoo when they were 17 years old or younger.
Data source: An in-person survey of 501 people with at least one tattoo.
Disclosures: The lead investigator had no disclosures.
Cosmetic procedures in pregnancy
Cosmetic procedures in general should be postponed until after pregnancy. Factors to consider in a pregnant patient include the hormonal and physiologic changes of the patient during pregnancy, as well as the risk to the fetus.
Many dermatologic changes occur during a pregnancy. Pregnant women may develop hyperpigmentation, formation of vascular lesions and varicose veins, hirsutism, striae, acne, and increased skin growths. These changes may lead pregnant women to seek cosmetic treatments.
However, physiologic changes such as increased blood volume, decreased hematocrit, increased flushing, increased melanocyte stimulation, and decreased wound healing should prompt a delay of cosmetic procedures until 3-6 months after the postpartum period, when these factors return to normal and the risk of complications is reduced.
The safety of many cosmetic treatments during pregnancy remains unknown. This includes microdermabrasion, chemical peels, and laser treatments. Given the increased risk of postinflammatory hyperpigmentation, as well as poor wound healing and increased risk of hypertrophic and keloidal scarring in pregnancy, these procedures are often avoided.
The safety of injectable treatments during pregnancy, such as liquid sclerosants and fillers, has not been evaluated. However, the manufacturers list pregnancy and breastfeeding as contraindications to treatment. Neurotoxins are also avoided during pregnancy and breastfeeding, based on teratogenicity in animal studies. There have been no controlled trials in humans.
Though there have been incidental exposures of botulinum toxin in women who did not know they were pregnant, no documented reports of fetal anomaly during these incidental exposures has been reported. In addition, no studies have been conducted to evaluate whether the toxin is excreted in breast milk, or when it is safe to use neurotoxins, fillers, or liquid sclerosants prior to conception.
The 10 months of pregnancy and many months of nursing can be a long stretch to wait for women who get regular cosmetic treatments. The skin changes of pregnancy can be bothersome; however, the risks of complications to the mother and the fetus outweigh the transient benefits of cosmetic procedures. The hormonal and physiologic changes of pregnancy are widely different in each woman, and sometimes the long-term side effects and complications can be completely unpredictable. Thus, patience and thorough counseling are the best strategies for treating our pregnant and nursing moms.
References
Nussbaum, R. and Benedetto, A.V. Cosmetic aspects of pregnancy. Clinics in Dermatology 2006;24:133-41.
Morgan, J.C. et al. Botulinum Toxin and Pregnancy Skinmed 2006;5:308.
Monteiro, E. Botulinum toxin A during pregnancy: a survey of treating physicians. J. Neurol. Neurosurg. Psychiatry 2006;77:117-9.
Lee, K.C., et al. Safety of cosmetic dermatologic procedures during pregnancy. Dermatol. Surg. 2013;39:1573-86.
Goldberg, D. and Maloney, M. Dermatologic surgery and cosmetic procedures during pregnancy and the postpartum period. Dermatologic Therapy 2013;26:321-30.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Dermatology News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
Cosmetic procedures in general should be postponed until after pregnancy. Factors to consider in a pregnant patient include the hormonal and physiologic changes of the patient during pregnancy, as well as the risk to the fetus.
Many dermatologic changes occur during a pregnancy. Pregnant women may develop hyperpigmentation, formation of vascular lesions and varicose veins, hirsutism, striae, acne, and increased skin growths. These changes may lead pregnant women to seek cosmetic treatments.
However, physiologic changes such as increased blood volume, decreased hematocrit, increased flushing, increased melanocyte stimulation, and decreased wound healing should prompt a delay of cosmetic procedures until 3-6 months after the postpartum period, when these factors return to normal and the risk of complications is reduced.
The safety of many cosmetic treatments during pregnancy remains unknown. This includes microdermabrasion, chemical peels, and laser treatments. Given the increased risk of postinflammatory hyperpigmentation, as well as poor wound healing and increased risk of hypertrophic and keloidal scarring in pregnancy, these procedures are often avoided.
The safety of injectable treatments during pregnancy, such as liquid sclerosants and fillers, has not been evaluated. However, the manufacturers list pregnancy and breastfeeding as contraindications to treatment. Neurotoxins are also avoided during pregnancy and breastfeeding, based on teratogenicity in animal studies. There have been no controlled trials in humans.
Though there have been incidental exposures of botulinum toxin in women who did not know they were pregnant, no documented reports of fetal anomaly during these incidental exposures has been reported. In addition, no studies have been conducted to evaluate whether the toxin is excreted in breast milk, or when it is safe to use neurotoxins, fillers, or liquid sclerosants prior to conception.
The 10 months of pregnancy and many months of nursing can be a long stretch to wait for women who get regular cosmetic treatments. The skin changes of pregnancy can be bothersome; however, the risks of complications to the mother and the fetus outweigh the transient benefits of cosmetic procedures. The hormonal and physiologic changes of pregnancy are widely different in each woman, and sometimes the long-term side effects and complications can be completely unpredictable. Thus, patience and thorough counseling are the best strategies for treating our pregnant and nursing moms.
References
Nussbaum, R. and Benedetto, A.V. Cosmetic aspects of pregnancy. Clinics in Dermatology 2006;24:133-41.
Morgan, J.C. et al. Botulinum Toxin and Pregnancy Skinmed 2006;5:308.
Monteiro, E. Botulinum toxin A during pregnancy: a survey of treating physicians. J. Neurol. Neurosurg. Psychiatry 2006;77:117-9.
Lee, K.C., et al. Safety of cosmetic dermatologic procedures during pregnancy. Dermatol. Surg. 2013;39:1573-86.
Goldberg, D. and Maloney, M. Dermatologic surgery and cosmetic procedures during pregnancy and the postpartum period. Dermatologic Therapy 2013;26:321-30.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Dermatology News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
Cosmetic procedures in general should be postponed until after pregnancy. Factors to consider in a pregnant patient include the hormonal and physiologic changes of the patient during pregnancy, as well as the risk to the fetus.
Many dermatologic changes occur during a pregnancy. Pregnant women may develop hyperpigmentation, formation of vascular lesions and varicose veins, hirsutism, striae, acne, and increased skin growths. These changes may lead pregnant women to seek cosmetic treatments.
However, physiologic changes such as increased blood volume, decreased hematocrit, increased flushing, increased melanocyte stimulation, and decreased wound healing should prompt a delay of cosmetic procedures until 3-6 months after the postpartum period, when these factors return to normal and the risk of complications is reduced.
The safety of many cosmetic treatments during pregnancy remains unknown. This includes microdermabrasion, chemical peels, and laser treatments. Given the increased risk of postinflammatory hyperpigmentation, as well as poor wound healing and increased risk of hypertrophic and keloidal scarring in pregnancy, these procedures are often avoided.
The safety of injectable treatments during pregnancy, such as liquid sclerosants and fillers, has not been evaluated. However, the manufacturers list pregnancy and breastfeeding as contraindications to treatment. Neurotoxins are also avoided during pregnancy and breastfeeding, based on teratogenicity in animal studies. There have been no controlled trials in humans.
Though there have been incidental exposures of botulinum toxin in women who did not know they were pregnant, no documented reports of fetal anomaly during these incidental exposures has been reported. In addition, no studies have been conducted to evaluate whether the toxin is excreted in breast milk, or when it is safe to use neurotoxins, fillers, or liquid sclerosants prior to conception.
The 10 months of pregnancy and many months of nursing can be a long stretch to wait for women who get regular cosmetic treatments. The skin changes of pregnancy can be bothersome; however, the risks of complications to the mother and the fetus outweigh the transient benefits of cosmetic procedures. The hormonal and physiologic changes of pregnancy are widely different in each woman, and sometimes the long-term side effects and complications can be completely unpredictable. Thus, patience and thorough counseling are the best strategies for treating our pregnant and nursing moms.
References
Nussbaum, R. and Benedetto, A.V. Cosmetic aspects of pregnancy. Clinics in Dermatology 2006;24:133-41.
Morgan, J.C. et al. Botulinum Toxin and Pregnancy Skinmed 2006;5:308.
Monteiro, E. Botulinum toxin A during pregnancy: a survey of treating physicians. J. Neurol. Neurosurg. Psychiatry 2006;77:117-9.
Lee, K.C., et al. Safety of cosmetic dermatologic procedures during pregnancy. Dermatol. Surg. 2013;39:1573-86.
Goldberg, D. and Maloney, M. Dermatologic surgery and cosmetic procedures during pregnancy and the postpartum period. Dermatologic Therapy 2013;26:321-30.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Dermatology News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
Plant-based supplement safely protects against UV radiation
MIAMI BEACH – An oral Polypodium leucotomos extract supplement was safe and effective for sun protection in a randomized, double-blind, placebo-controlled study of 40 participants.
The plant-based supplement, marketed as Heliocare by Ferndale Healthcare, was associated with a significant reduction in the risk of sunburn and the risk of erythema after ultraviolet B exposure, and with a significant increase in sun tolerability, according to study coauthor Dr. Brian Berman, who presented the findings at the South Beach Symposium.
After 2 months of treatment, 8 of 20 participants in the placebo group experienced at least one episode of sunburn, compared with 2 of 20 in the treatment group. At day 28 of treatment, 1 vs. 8 participants in the placebo and treatment groups, respectively, experienced an increased minimal erythema dose (MED), and 3 vs. 10 experienced decreased ultraviolet-induced erythema intensity (J. Clin. Aesthet. Dermatol. 2015;8:19-23).
Study participants were adults aged 18-65 years with Fitzpatrick skin types I-IV. They took 240-mg Heliocare capsules twice daily for 60 days and were assessed by physical examination. Vital signs were measured and clinical laboratory parameters, including hematology, comprehensive metabolic panel, and prothrombin time-partial thromboplastin time, were assessed at baseline and at the end of treatment. Twelve participants in each group also underwent MED testing. The treatment and placebo groups were similar with respect to the prestudy number of sunburns and the number of hours of sun exposure both before and during the study, said Dr. Berman of the University of Miami.
No safety issues associated with treatment were detected. Four participants in the treatment group reported transient mild fatigue, bloating, and headache, and one in the placebo group reported headache.
Polypodium leucotomos is a South American species of fern. Extracts from the fern have been used for at least 4 decades for photoprotection and for treatment of various skin disorders. The current findings suggest that the supplement is a safe and effective means for reducing the damaging effects of ultraviolet radiation, he said.
As with any dietary supplement, Polypodium leucotomos extract is not approved by the Food and Drug Administration to diagnose, treat, cure, or prevent any disease.
Dr. Berman and his colleagues concluded that, based on the excellent safety profile, additional studies assessing higher doses may be warranted.
Dr. Berman is a consultant/speaker for Ferndale Pharmaceuticals and has relationships with several other pharmaceutical companies.
MIAMI BEACH – An oral Polypodium leucotomos extract supplement was safe and effective for sun protection in a randomized, double-blind, placebo-controlled study of 40 participants.
The plant-based supplement, marketed as Heliocare by Ferndale Healthcare, was associated with a significant reduction in the risk of sunburn and the risk of erythema after ultraviolet B exposure, and with a significant increase in sun tolerability, according to study coauthor Dr. Brian Berman, who presented the findings at the South Beach Symposium.
After 2 months of treatment, 8 of 20 participants in the placebo group experienced at least one episode of sunburn, compared with 2 of 20 in the treatment group. At day 28 of treatment, 1 vs. 8 participants in the placebo and treatment groups, respectively, experienced an increased minimal erythema dose (MED), and 3 vs. 10 experienced decreased ultraviolet-induced erythema intensity (J. Clin. Aesthet. Dermatol. 2015;8:19-23).
Study participants were adults aged 18-65 years with Fitzpatrick skin types I-IV. They took 240-mg Heliocare capsules twice daily for 60 days and were assessed by physical examination. Vital signs were measured and clinical laboratory parameters, including hematology, comprehensive metabolic panel, and prothrombin time-partial thromboplastin time, were assessed at baseline and at the end of treatment. Twelve participants in each group also underwent MED testing. The treatment and placebo groups were similar with respect to the prestudy number of sunburns and the number of hours of sun exposure both before and during the study, said Dr. Berman of the University of Miami.
No safety issues associated with treatment were detected. Four participants in the treatment group reported transient mild fatigue, bloating, and headache, and one in the placebo group reported headache.
Polypodium leucotomos is a South American species of fern. Extracts from the fern have been used for at least 4 decades for photoprotection and for treatment of various skin disorders. The current findings suggest that the supplement is a safe and effective means for reducing the damaging effects of ultraviolet radiation, he said.
As with any dietary supplement, Polypodium leucotomos extract is not approved by the Food and Drug Administration to diagnose, treat, cure, or prevent any disease.
Dr. Berman and his colleagues concluded that, based on the excellent safety profile, additional studies assessing higher doses may be warranted.
Dr. Berman is a consultant/speaker for Ferndale Pharmaceuticals and has relationships with several other pharmaceutical companies.
MIAMI BEACH – An oral Polypodium leucotomos extract supplement was safe and effective for sun protection in a randomized, double-blind, placebo-controlled study of 40 participants.
The plant-based supplement, marketed as Heliocare by Ferndale Healthcare, was associated with a significant reduction in the risk of sunburn and the risk of erythema after ultraviolet B exposure, and with a significant increase in sun tolerability, according to study coauthor Dr. Brian Berman, who presented the findings at the South Beach Symposium.
After 2 months of treatment, 8 of 20 participants in the placebo group experienced at least one episode of sunburn, compared with 2 of 20 in the treatment group. At day 28 of treatment, 1 vs. 8 participants in the placebo and treatment groups, respectively, experienced an increased minimal erythema dose (MED), and 3 vs. 10 experienced decreased ultraviolet-induced erythema intensity (J. Clin. Aesthet. Dermatol. 2015;8:19-23).
Study participants were adults aged 18-65 years with Fitzpatrick skin types I-IV. They took 240-mg Heliocare capsules twice daily for 60 days and were assessed by physical examination. Vital signs were measured and clinical laboratory parameters, including hematology, comprehensive metabolic panel, and prothrombin time-partial thromboplastin time, were assessed at baseline and at the end of treatment. Twelve participants in each group also underwent MED testing. The treatment and placebo groups were similar with respect to the prestudy number of sunburns and the number of hours of sun exposure both before and during the study, said Dr. Berman of the University of Miami.
No safety issues associated with treatment were detected. Four participants in the treatment group reported transient mild fatigue, bloating, and headache, and one in the placebo group reported headache.
Polypodium leucotomos is a South American species of fern. Extracts from the fern have been used for at least 4 decades for photoprotection and for treatment of various skin disorders. The current findings suggest that the supplement is a safe and effective means for reducing the damaging effects of ultraviolet radiation, he said.
As with any dietary supplement, Polypodium leucotomos extract is not approved by the Food and Drug Administration to diagnose, treat, cure, or prevent any disease.
Dr. Berman and his colleagues concluded that, based on the excellent safety profile, additional studies assessing higher doses may be warranted.
Dr. Berman is a consultant/speaker for Ferndale Pharmaceuticals and has relationships with several other pharmaceutical companies.
AT THE SOUTH BEACH SYMPOSIUM
Key clinical point: An oral Polypodium leucotomos supplement provides safe and effective photoprotection.
Major finding:. At 2 months, eight placebo-group and two treatment-group participants experienced at least one episode of sunburn.
Data source: A randomized, double-blind, placebo-controlled study of 40 subjects.
Disclosures: Dr. Berman is a consultant/speaker for Ferndale Pharmaceuticals and has relationships with several other pharmaceutical companies.
Skin Rejuvenation With Fat Grafting and Stem Cells
Recently there has been a lot of interest and attention given to the rejuvenation of the face utilizing autologous fat. Much of this interest stems from the understanding of the aging process on facial fat volume, bone density, and muscle thickness. Even in your 30s, volume deficit begins to show as undereye circles and the cheeks take on a submalar hollow, which continues to progress with time.
Charles-de-Sá et al (Plast Reconstr Surg. 2015;135:999-1009) looked at the direct antiaging effects that autologous fat grafting has on skin. The authors observed 6 consecutive patients (5 women and 1 man; mixed ethnic backgrounds; aged 45–65 years; nonsmokers) who presented for face-lifts. A small skin and fat biopsy from the preauricular area on each patient was taken for baseline histologic analysis.
The patients had abdominal fat harvested and processed in 2 manners. One portion was centrifuged at 3000 rpm for 3 minutes. The pellet at the base of the syringe (stromal vascular fraction) was then taken and mixed with 1 mL of adipose tissue and injected into the right preauricular skin in a subdermal fanning technique using a small cannula (1.5 mm) on a 3-mL syringe. The second portion of the fat was sent for stem cell expansion (2×106 mesenchymal cells). Five weeks later, the solution of stem cells (0.4 mL) was diluted with normal saline to a volume of 1 mL and injected in the left preauricular area, also 2 cm in front of the tragus.
Three months after the injections, repeat biopsies in the grafted areas were taken and sent for hematoxylin and eosin stain and electron microscopy. Results showed that at baseline on hematoxylin and eosin, the skin showed evidence of mild solar elastosis. However, after both methods of fat processing, the treated areas showed a reduction in solar elastosis, increased elastin fibers in the papillary dermis, and increased vasculature in the reticular dermis (near the subcutaneous fat). The results were similar in both types of fat processing; there was no statistically significant difference between modalities.
What’s the issue?
Those of us who have performed autologous fat grafting on patients have remarked for years on the improved appearance of the skin and the slowing down of the aging process that is not accounted for by volume replacement alone. This study has shown that fat grafting or stem cell injection in the subcutaneous layer has a beneficial effect on the overlying skin. The fact that both techniques showed similar results is helpful because it demonstrates that improvement can be achieved without having to expand the cells in vitro, thus eliminating all the regulatory issues that accompany cell cultures. A larger study would be extremely beneficial at this point. How does this procedure compare to platelet-rich plasma injections that are also the big fad for skin rejuvenation?
Recently there has been a lot of interest and attention given to the rejuvenation of the face utilizing autologous fat. Much of this interest stems from the understanding of the aging process on facial fat volume, bone density, and muscle thickness. Even in your 30s, volume deficit begins to show as undereye circles and the cheeks take on a submalar hollow, which continues to progress with time.
Charles-de-Sá et al (Plast Reconstr Surg. 2015;135:999-1009) looked at the direct antiaging effects that autologous fat grafting has on skin. The authors observed 6 consecutive patients (5 women and 1 man; mixed ethnic backgrounds; aged 45–65 years; nonsmokers) who presented for face-lifts. A small skin and fat biopsy from the preauricular area on each patient was taken for baseline histologic analysis.
The patients had abdominal fat harvested and processed in 2 manners. One portion was centrifuged at 3000 rpm for 3 minutes. The pellet at the base of the syringe (stromal vascular fraction) was then taken and mixed with 1 mL of adipose tissue and injected into the right preauricular skin in a subdermal fanning technique using a small cannula (1.5 mm) on a 3-mL syringe. The second portion of the fat was sent for stem cell expansion (2×106 mesenchymal cells). Five weeks later, the solution of stem cells (0.4 mL) was diluted with normal saline to a volume of 1 mL and injected in the left preauricular area, also 2 cm in front of the tragus.
Three months after the injections, repeat biopsies in the grafted areas were taken and sent for hematoxylin and eosin stain and electron microscopy. Results showed that at baseline on hematoxylin and eosin, the skin showed evidence of mild solar elastosis. However, after both methods of fat processing, the treated areas showed a reduction in solar elastosis, increased elastin fibers in the papillary dermis, and increased vasculature in the reticular dermis (near the subcutaneous fat). The results were similar in both types of fat processing; there was no statistically significant difference between modalities.
What’s the issue?
Those of us who have performed autologous fat grafting on patients have remarked for years on the improved appearance of the skin and the slowing down of the aging process that is not accounted for by volume replacement alone. This study has shown that fat grafting or stem cell injection in the subcutaneous layer has a beneficial effect on the overlying skin. The fact that both techniques showed similar results is helpful because it demonstrates that improvement can be achieved without having to expand the cells in vitro, thus eliminating all the regulatory issues that accompany cell cultures. A larger study would be extremely beneficial at this point. How does this procedure compare to platelet-rich plasma injections that are also the big fad for skin rejuvenation?
Recently there has been a lot of interest and attention given to the rejuvenation of the face utilizing autologous fat. Much of this interest stems from the understanding of the aging process on facial fat volume, bone density, and muscle thickness. Even in your 30s, volume deficit begins to show as undereye circles and the cheeks take on a submalar hollow, which continues to progress with time.
Charles-de-Sá et al (Plast Reconstr Surg. 2015;135:999-1009) looked at the direct antiaging effects that autologous fat grafting has on skin. The authors observed 6 consecutive patients (5 women and 1 man; mixed ethnic backgrounds; aged 45–65 years; nonsmokers) who presented for face-lifts. A small skin and fat biopsy from the preauricular area on each patient was taken for baseline histologic analysis.
The patients had abdominal fat harvested and processed in 2 manners. One portion was centrifuged at 3000 rpm for 3 minutes. The pellet at the base of the syringe (stromal vascular fraction) was then taken and mixed with 1 mL of adipose tissue and injected into the right preauricular skin in a subdermal fanning technique using a small cannula (1.5 mm) on a 3-mL syringe. The second portion of the fat was sent for stem cell expansion (2×106 mesenchymal cells). Five weeks later, the solution of stem cells (0.4 mL) was diluted with normal saline to a volume of 1 mL and injected in the left preauricular area, also 2 cm in front of the tragus.
Three months after the injections, repeat biopsies in the grafted areas were taken and sent for hematoxylin and eosin stain and electron microscopy. Results showed that at baseline on hematoxylin and eosin, the skin showed evidence of mild solar elastosis. However, after both methods of fat processing, the treated areas showed a reduction in solar elastosis, increased elastin fibers in the papillary dermis, and increased vasculature in the reticular dermis (near the subcutaneous fat). The results were similar in both types of fat processing; there was no statistically significant difference between modalities.
What’s the issue?
Those of us who have performed autologous fat grafting on patients have remarked for years on the improved appearance of the skin and the slowing down of the aging process that is not accounted for by volume replacement alone. This study has shown that fat grafting or stem cell injection in the subcutaneous layer has a beneficial effect on the overlying skin. The fact that both techniques showed similar results is helpful because it demonstrates that improvement can be achieved without having to expand the cells in vitro, thus eliminating all the regulatory issues that accompany cell cultures. A larger study would be extremely beneficial at this point. How does this procedure compare to platelet-rich plasma injections that are also the big fad for skin rejuvenation?
Rapidly Recurring Keratoacanthoma
To the Editor:
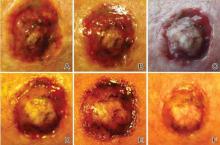
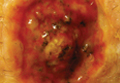
A 61-year-old man with a medical history of type 2 diabetes mellitus presented to us with a 2.5×3.0-cm erythematous, ulcerated, and exophytic tumor on the right dorsal forearm that had rapidly developed over 2 weeks. A tangential biopsy was performed followed by treatment with electrodesiccation and curettage (ED&C). Histology revealed a squamous cell carcinoma (SCC), keratoacanthoma (KA) type. Over the next 11 days the lesion rapidly recurred and the patient returned with his own daily photodocumentation of the KA’s progression (Figure). The lesion was re-excised with 5-mm margins; histology again revealed SCC, KA type, with deep margin involvement. Chest radiograph revealed findings suspicious for metastatic lesions in the right lung. He was referred to oncology for metastatic workup; positron emission tomography was negative and ultimately the lung lesion was found to be benign. The patient underwent adjuvant radia-tion to the KA resection bed and lymph nodes with minimal side effects. The patient has remained cancer free to date.
Keratoacanthomas are rapidly growing, typically painless, cutaneous neoplasms that often develop on sun-exposed areas. They can occur spontaneously or following trauma and have the propensity to regress with time.1-3 They are described as progressing through 3 clinical stages: rapid proliferation, mature/stable, and involution. However, KAs can be aggressive, becoming locally destructive; therefore, KAs are typically treated to avoid further morbidity. Keratoacanthomas may be considered a subtype of SCC, as some have the potential to become locally destructive and metastasize.3-5 There are reports of spontaneous resolution of KAs over weeks to months, though surgical excision is the gold standard of treatment.3,5
Reactive KA is a subtype that is thought to develop at the site of prior trauma, representing a sort of Köbner phenomenon.3,4 We demonstrated a case of a recurrent KA in the setting of recent ED&C. Several reports describe KAs developing after dermatologic surgery, including Mohs micrographic surgery, laser resurfacing, radiation therapy, and after skin grafting.3,4,6 Trauma-induced epidermal injury and dermal inflammation may play a role in postoperative KA formation or recurrence.6
Keratoacanthoma recurrence has been reported in 3% to 8% of cases within a few weeks after treatment, as seen in our current patient.3,5 In our case, the patient photodocumented the regrowth of his lesion (Figure). Treatment of reactive KAs may be therapeutically challenging, as they can form or worsen with repeated surgeries and may require several treatment modalities to eradicate them.4 Treatment options include observation, ED&C, excision, Mohs micrographic surgery, radiation, cryosurgery, laser, isotretinoin, acitretin, imiquimod, 5-fluorouracil, methotrexate, interferon alfa-2b, or bleomycin, to name a few.3,4,7
Combination therapy should be considered in the presence of recurrent and/or aggressive KAs, such as in our case. Our patient has remained disease free after a combination of surgical excision with radiation therapy.
1. Schwartz R. Keratoacanthoma. J Am Acad Dermatol. 1994;30:1-19.
2. Kingman J. Keratoacanthoma. Arch Dermatol. 1984;20:736-740.
3. Goldberg L, Silapunt S, Beyrau K, et al. Keratoacanthoma as a postoperative complication of skin cancer excision. J Am Acad Dermatol. 2004;50:753-758.
4. Hadley J, Tristani-Firouzi P, Florell S, et al. Case series of multiple recurrent reactive keratoacanthomas developing at surgical margins. Dermatol Surg. 2009;35:2019-2024.
5. Karaa A, Khachemoune A. Keratoacanthoma: a tumor in search of a classification. Int J Dermatol. 2007;46:671-678.
6. Chesnut GT, Maggio KL, Turiansky GW. Letter: re: case series of multiple recurrent reactive keratoacanthomas developing at surgical margins. Dermatol Surg. 2011;37:884-885.
7. Lernia V, Ricci C, Albertini G. Spontaneous regression of keratoacanthoma can be promoted by topical treatment with imiquimod cream. J Eur Acad Dermatol Venereol. 2004;18:626-629.
To the Editor:
A 61-year-old man with a medical history of type 2 diabetes mellitus presented to us with a 2.5×3.0-cm erythematous, ulcerated, and exophytic tumor on the right dorsal forearm that had rapidly developed over 2 weeks. A tangential biopsy was performed followed by treatment with electrodesiccation and curettage (ED&C). Histology revealed a squamous cell carcinoma (SCC), keratoacanthoma (KA) type. Over the next 11 days the lesion rapidly recurred and the patient returned with his own daily photodocumentation of the KA’s progression (Figure). The lesion was re-excised with 5-mm margins; histology again revealed SCC, KA type, with deep margin involvement. Chest radiograph revealed findings suspicious for metastatic lesions in the right lung. He was referred to oncology for metastatic workup; positron emission tomography was negative and ultimately the lung lesion was found to be benign. The patient underwent adjuvant radia-tion to the KA resection bed and lymph nodes with minimal side effects. The patient has remained cancer free to date.
Keratoacanthomas are rapidly growing, typically painless, cutaneous neoplasms that often develop on sun-exposed areas. They can occur spontaneously or following trauma and have the propensity to regress with time.1-3 They are described as progressing through 3 clinical stages: rapid proliferation, mature/stable, and involution. However, KAs can be aggressive, becoming locally destructive; therefore, KAs are typically treated to avoid further morbidity. Keratoacanthomas may be considered a subtype of SCC, as some have the potential to become locally destructive and metastasize.3-5 There are reports of spontaneous resolution of KAs over weeks to months, though surgical excision is the gold standard of treatment.3,5
Reactive KA is a subtype that is thought to develop at the site of prior trauma, representing a sort of Köbner phenomenon.3,4 We demonstrated a case of a recurrent KA in the setting of recent ED&C. Several reports describe KAs developing after dermatologic surgery, including Mohs micrographic surgery, laser resurfacing, radiation therapy, and after skin grafting.3,4,6 Trauma-induced epidermal injury and dermal inflammation may play a role in postoperative KA formation or recurrence.6
Keratoacanthoma recurrence has been reported in 3% to 8% of cases within a few weeks after treatment, as seen in our current patient.3,5 In our case, the patient photodocumented the regrowth of his lesion (Figure). Treatment of reactive KAs may be therapeutically challenging, as they can form or worsen with repeated surgeries and may require several treatment modalities to eradicate them.4 Treatment options include observation, ED&C, excision, Mohs micrographic surgery, radiation, cryosurgery, laser, isotretinoin, acitretin, imiquimod, 5-fluorouracil, methotrexate, interferon alfa-2b, or bleomycin, to name a few.3,4,7
Combination therapy should be considered in the presence of recurrent and/or aggressive KAs, such as in our case. Our patient has remained disease free after a combination of surgical excision with radiation therapy.
To the Editor:
A 61-year-old man with a medical history of type 2 diabetes mellitus presented to us with a 2.5×3.0-cm erythematous, ulcerated, and exophytic tumor on the right dorsal forearm that had rapidly developed over 2 weeks. A tangential biopsy was performed followed by treatment with electrodesiccation and curettage (ED&C). Histology revealed a squamous cell carcinoma (SCC), keratoacanthoma (KA) type. Over the next 11 days the lesion rapidly recurred and the patient returned with his own daily photodocumentation of the KA’s progression (Figure). The lesion was re-excised with 5-mm margins; histology again revealed SCC, KA type, with deep margin involvement. Chest radiograph revealed findings suspicious for metastatic lesions in the right lung. He was referred to oncology for metastatic workup; positron emission tomography was negative and ultimately the lung lesion was found to be benign. The patient underwent adjuvant radia-tion to the KA resection bed and lymph nodes with minimal side effects. The patient has remained cancer free to date.
Keratoacanthomas are rapidly growing, typically painless, cutaneous neoplasms that often develop on sun-exposed areas. They can occur spontaneously or following trauma and have the propensity to regress with time.1-3 They are described as progressing through 3 clinical stages: rapid proliferation, mature/stable, and involution. However, KAs can be aggressive, becoming locally destructive; therefore, KAs are typically treated to avoid further morbidity. Keratoacanthomas may be considered a subtype of SCC, as some have the potential to become locally destructive and metastasize.3-5 There are reports of spontaneous resolution of KAs over weeks to months, though surgical excision is the gold standard of treatment.3,5
Reactive KA is a subtype that is thought to develop at the site of prior trauma, representing a sort of Köbner phenomenon.3,4 We demonstrated a case of a recurrent KA in the setting of recent ED&C. Several reports describe KAs developing after dermatologic surgery, including Mohs micrographic surgery, laser resurfacing, radiation therapy, and after skin grafting.3,4,6 Trauma-induced epidermal injury and dermal inflammation may play a role in postoperative KA formation or recurrence.6
Keratoacanthoma recurrence has been reported in 3% to 8% of cases within a few weeks after treatment, as seen in our current patient.3,5 In our case, the patient photodocumented the regrowth of his lesion (Figure). Treatment of reactive KAs may be therapeutically challenging, as they can form or worsen with repeated surgeries and may require several treatment modalities to eradicate them.4 Treatment options include observation, ED&C, excision, Mohs micrographic surgery, radiation, cryosurgery, laser, isotretinoin, acitretin, imiquimod, 5-fluorouracil, methotrexate, interferon alfa-2b, or bleomycin, to name a few.3,4,7
Combination therapy should be considered in the presence of recurrent and/or aggressive KAs, such as in our case. Our patient has remained disease free after a combination of surgical excision with radiation therapy.
1. Schwartz R. Keratoacanthoma. J Am Acad Dermatol. 1994;30:1-19.
2. Kingman J. Keratoacanthoma. Arch Dermatol. 1984;20:736-740.
3. Goldberg L, Silapunt S, Beyrau K, et al. Keratoacanthoma as a postoperative complication of skin cancer excision. J Am Acad Dermatol. 2004;50:753-758.
4. Hadley J, Tristani-Firouzi P, Florell S, et al. Case series of multiple recurrent reactive keratoacanthomas developing at surgical margins. Dermatol Surg. 2009;35:2019-2024.
5. Karaa A, Khachemoune A. Keratoacanthoma: a tumor in search of a classification. Int J Dermatol. 2007;46:671-678.
6. Chesnut GT, Maggio KL, Turiansky GW. Letter: re: case series of multiple recurrent reactive keratoacanthomas developing at surgical margins. Dermatol Surg. 2011;37:884-885.
7. Lernia V, Ricci C, Albertini G. Spontaneous regression of keratoacanthoma can be promoted by topical treatment with imiquimod cream. J Eur Acad Dermatol Venereol. 2004;18:626-629.
1. Schwartz R. Keratoacanthoma. J Am Acad Dermatol. 1994;30:1-19.
2. Kingman J. Keratoacanthoma. Arch Dermatol. 1984;20:736-740.
3. Goldberg L, Silapunt S, Beyrau K, et al. Keratoacanthoma as a postoperative complication of skin cancer excision. J Am Acad Dermatol. 2004;50:753-758.
4. Hadley J, Tristani-Firouzi P, Florell S, et al. Case series of multiple recurrent reactive keratoacanthomas developing at surgical margins. Dermatol Surg. 2009;35:2019-2024.
5. Karaa A, Khachemoune A. Keratoacanthoma: a tumor in search of a classification. Int J Dermatol. 2007;46:671-678.
6. Chesnut GT, Maggio KL, Turiansky GW. Letter: re: case series of multiple recurrent reactive keratoacanthomas developing at surgical margins. Dermatol Surg. 2011;37:884-885.
7. Lernia V, Ricci C, Albertini G. Spontaneous regression of keratoacanthoma can be promoted by topical treatment with imiquimod cream. J Eur Acad Dermatol Venereol. 2004;18:626-629.
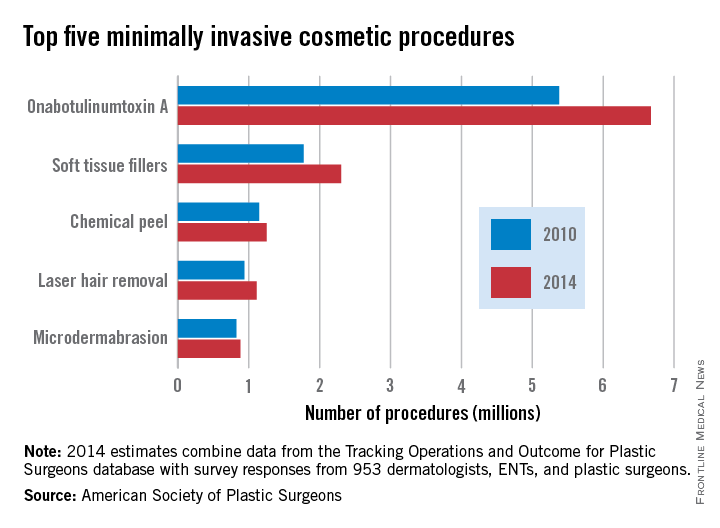
Minimally invasive cosmetic procedures climb by 20% since 2010
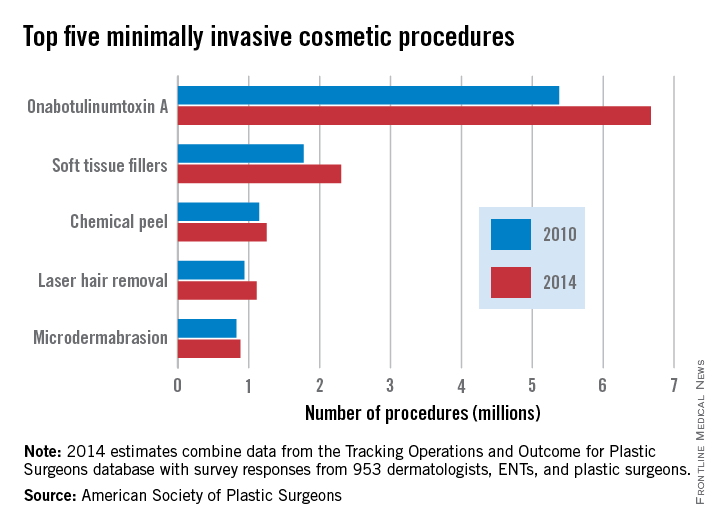
The number of minimally invasive cosmetic procedures increased by well over 2 million from 2010 to 2014, according to a report from the American Society of Plastic Surgeons (ASPS).
Almost 14 million cosmetic procedures were performed in 2014, a 20% increase from 11.6 million in 2010. OnabotulinumtoxinA injection was by far the most common procedure, with 6.7 million cases accounting for nearly half of all the cosmetic minimally invasive procedures in 2014. In addition, with a rise from 5.4 million in 2010, onabotulinumtoxinA injections accounted for approximately half of the overall growth in minimally invasive procedures from 2010 to 2014.
Although all of the five most common minimally invasive cosmetic procedures increased from 2010 to 2014, the number of microdermabrasions decreased from 2013 to 2014, from 970,000 to approximately 880,000. Soft tissue fillers saw the greatest relative change (about 30%), increasing from just under 1.8 million to about 2.3 million.
Overall, the number of cosmetic surgical procedures (including minimally invasive procedures) increased from 1.56 million to 1.68 million from 2010 to 2014, but three of the five most common surgeries in 2014 – breast augmentation, rhinoplasty, and eyelid surgery – decreased, with the number of rhinoplasties falling by nearly 14%. Of the remaining two most common surgeries, the number of liposuctions increased by approximately 4%, and face-lifts increased by nearly 14%.
The ASPS report used data from the society’s Tracking Operations and Outcomes for Plastic Surgeons database and survey results from 953 board-certified physicians.
The number of minimally invasive cosmetic procedures increased by well over 2 million from 2010 to 2014, according to a report from the American Society of Plastic Surgeons (ASPS).
Almost 14 million cosmetic procedures were performed in 2014, a 20% increase from 11.6 million in 2010. OnabotulinumtoxinA injection was by far the most common procedure, with 6.7 million cases accounting for nearly half of all the cosmetic minimally invasive procedures in 2014. In addition, with a rise from 5.4 million in 2010, onabotulinumtoxinA injections accounted for approximately half of the overall growth in minimally invasive procedures from 2010 to 2014.
Although all of the five most common minimally invasive cosmetic procedures increased from 2010 to 2014, the number of microdermabrasions decreased from 2013 to 2014, from 970,000 to approximately 880,000. Soft tissue fillers saw the greatest relative change (about 30%), increasing from just under 1.8 million to about 2.3 million.
Overall, the number of cosmetic surgical procedures (including minimally invasive procedures) increased from 1.56 million to 1.68 million from 2010 to 2014, but three of the five most common surgeries in 2014 – breast augmentation, rhinoplasty, and eyelid surgery – decreased, with the number of rhinoplasties falling by nearly 14%. Of the remaining two most common surgeries, the number of liposuctions increased by approximately 4%, and face-lifts increased by nearly 14%.
The ASPS report used data from the society’s Tracking Operations and Outcomes for Plastic Surgeons database and survey results from 953 board-certified physicians.

The number of minimally invasive cosmetic procedures increased by well over 2 million from 2010 to 2014, according to a report from the American Society of Plastic Surgeons (ASPS).
Almost 14 million cosmetic procedures were performed in 2014, a 20% increase from 11.6 million in 2010. OnabotulinumtoxinA injection was by far the most common procedure, with 6.7 million cases accounting for nearly half of all the cosmetic minimally invasive procedures in 2014. In addition, with a rise from 5.4 million in 2010, onabotulinumtoxinA injections accounted for approximately half of the overall growth in minimally invasive procedures from 2010 to 2014.
Although all of the five most common minimally invasive cosmetic procedures increased from 2010 to 2014, the number of microdermabrasions decreased from 2013 to 2014, from 970,000 to approximately 880,000. Soft tissue fillers saw the greatest relative change (about 30%), increasing from just under 1.8 million to about 2.3 million.
Overall, the number of cosmetic surgical procedures (including minimally invasive procedures) increased from 1.56 million to 1.68 million from 2010 to 2014, but three of the five most common surgeries in 2014 – breast augmentation, rhinoplasty, and eyelid surgery – decreased, with the number of rhinoplasties falling by nearly 14%. Of the remaining two most common surgeries, the number of liposuctions increased by approximately 4%, and face-lifts increased by nearly 14%.
The ASPS report used data from the society’s Tracking Operations and Outcomes for Plastic Surgeons database and survey results from 953 board-certified physicians.

Cosmetic Corner: Dermatologists Weigh in on Antiaging Moisturizers
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top antiaging moisturizers. Consideration must be given to:
- AOX+ Eye Gel
SkinCeuticals
Recommended by Julie Woodward, MD, Durham, North Carolina
- Aveeno Protect + Hydrate Lotion Sunscreen with Broad Spectrum SPF 30
Johnson & Johnson Consumer Companies, Inc
Recommended by Gary Goldenberg, MD, New York, New York
- CeraVe Facial Moisturizing Lotion AM with SPF 30
Valeant Consumer Products, a division of Valeant Pharmaceuticals North America LLC
Recommended by Gary Goldenberg, MD, New York, New York
- Lumière Bio-restorative Eye Cream with PSP
Neocutis S.A.
Recommended by Julie Woodward, MD, Durham, North Carolina
- Recovery Night Moisture Serum
Lifeline Skin Care
“This product contains growth factors from human stem cells, and I recommend it nightly for my patients who are recovering from laser procedures.” —Elizabeth K. Hale, MD, New York, New York
- TNS Essential Serum
SkinMedica
“It is made from a fibroblast growth factor that helps stimulate collagen.”—Anthony M. Rossi, MD, New York, New York
Cutis invites readers to send us their recommendations. Cleansers for rosacea patients, OTC acne preparations, and products for babies will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to cutis@frontlinemedcom.com.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top antiaging moisturizers. Consideration must be given to:
- AOX+ Eye Gel
SkinCeuticals
Recommended by Julie Woodward, MD, Durham, North Carolina
- Aveeno Protect + Hydrate Lotion Sunscreen with Broad Spectrum SPF 30
Johnson & Johnson Consumer Companies, Inc
Recommended by Gary Goldenberg, MD, New York, New York
- CeraVe Facial Moisturizing Lotion AM with SPF 30
Valeant Consumer Products, a division of Valeant Pharmaceuticals North America LLC
Recommended by Gary Goldenberg, MD, New York, New York
- Lumière Bio-restorative Eye Cream with PSP
Neocutis S.A.
Recommended by Julie Woodward, MD, Durham, North Carolina
- Recovery Night Moisture Serum
Lifeline Skin Care
“This product contains growth factors from human stem cells, and I recommend it nightly for my patients who are recovering from laser procedures.” —Elizabeth K. Hale, MD, New York, New York
- TNS Essential Serum
SkinMedica
“It is made from a fibroblast growth factor that helps stimulate collagen.”—Anthony M. Rossi, MD, New York, New York
Cutis invites readers to send us their recommendations. Cleansers for rosacea patients, OTC acne preparations, and products for babies will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to cutis@frontlinemedcom.com.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top antiaging moisturizers. Consideration must be given to:
- AOX+ Eye Gel
SkinCeuticals
Recommended by Julie Woodward, MD, Durham, North Carolina
- Aveeno Protect + Hydrate Lotion Sunscreen with Broad Spectrum SPF 30
Johnson & Johnson Consumer Companies, Inc
Recommended by Gary Goldenberg, MD, New York, New York
- CeraVe Facial Moisturizing Lotion AM with SPF 30
Valeant Consumer Products, a division of Valeant Pharmaceuticals North America LLC
Recommended by Gary Goldenberg, MD, New York, New York
- Lumière Bio-restorative Eye Cream with PSP
Neocutis S.A.
Recommended by Julie Woodward, MD, Durham, North Carolina
- Recovery Night Moisture Serum
Lifeline Skin Care
“This product contains growth factors from human stem cells, and I recommend it nightly for my patients who are recovering from laser procedures.” —Elizabeth K. Hale, MD, New York, New York
- TNS Essential Serum
SkinMedica
“It is made from a fibroblast growth factor that helps stimulate collagen.”—Anthony M. Rossi, MD, New York, New York
Cutis invites readers to send us their recommendations. Cleansers for rosacea patients, OTC acne preparations, and products for babies will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to cutis@frontlinemedcom.com.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
Fellowships After Dermatology Residency: The Traditional and Beyond
Dermatology residents, such as myself, often wonder what we will do after graduation. There are many resources for finding job opportunities, and many of us have received solicitation e-mails from various headhunters and medical groups that are looking to hire. The American Academy of Dermatology (AAD) has a resource called the AAD Career Compass (http://www.healthecareers.com/aad), which is an exhaustive database of job listings for dermatologists. However, I could not locate a definitive resource containing information that might be useful for dermatology residents who are interested in subspecializing or pursuing fellowships.
Subspecialty training is typically pursued after successful completion of a dermatology residency training program. Fellowships are traditionally offered in dermatopathology, pediatric dermatology, micrographic surgery and dermatologic oncology (procedural dermatology), and cosmetic dermatologic surgery. Fellowships also are available in other subspecialties or for those pursuing an academic career. The goal of this article is to help dermatology residents learn more about traditional and nontraditional opportunities for graduate education and certification in various dermatologic subspecialties, with links to sources for more detailed information.
Traditional Fellowship Programs by Subspecialty
Dermatopathology
One- to 2-year dermatopathology fellowship programs are certified by both the American Board of Dermatology (ABD) and the American Board of Pathology and are available to graduates of either dermatology or pathology residency programs. These programs offer combined training in either anatomic pathology (for dermatologists) or clinical dermatology (for pathologists), along with dermatopathology; the majority of time is devoted to the latter. The Accreditation Council for Graduate Medical Education and the ABD have issued specific requirements for graduate medical education and subspecialty certification in dermatopathology.1,2 Fellowship matches are institution dependent, and the application process and match generally takes place during the second year of dermatology residency for those residents who want to start a fellowship program immediately following graduation. The American Society of Dermatopathology offers a dermatopathology fellowship program finder on its Web site.
Pediatric Dermatology
Fellowships in pediatric dermatology are typically 1- to 2-year programs that focus on dermatologic diseases in the pediatric population. Applicants are matched to these programs through the San Francisco Matching Program (SF Match) and the programs are ABD accredited.3,4 (There also are a number of non–ABD-approved training opportunities available.5) On completion of the training program, fellows may qualify for subspecialty board certification in pediatric dermatology. Applications are open starting in January, and the rank order list and match occur in August of the same year. As of 2012, there were 20 participating programs with 28 available positions, while the match included 22 applicants; of these applicants, 15 matched formally into pediatric dermatology fellowships.6
Micrographic Surgery and Dermatologic Oncology (Procedural Dermatology)
There are specific requirements issued by the Accreditation Council for Graduate Medical Education for dermatologic surgery fellowships,7 which are typically 1- to 2-year programs. Many fellowship programs also are accredited by the American College of Mohs Surgery. This subspecialty is not ABD accredited; therefore, there is no certification process upon completion of a fellowship program. The American College of Mohs Surgery sponsors the match process through SF Match. Applicant registration begins in July and the match occurs in December of the same year. As of 2013, there were 47 participating programs offering 55 positions. Of 77 applicants, 49 obtained fellowship positions formally through the match.8 The American Society for Dermatologic Surgery (ASDS) Web site provides the DermSurg Fellowship Finder, which includes information about independent fellowship programs.
Cosmetic Dermatologic Surgery
The ASDS has an accreditation program for fellowships in cosmetic dermatologic surgery,9 which are generally 1-year programs. Certification in this subspecialty is not ABD accredited. Fellowship opportunities can be found using the ASDS DermSurg Fellowship Finder.
Nontraditional Fellowship Programs
The following are fellowship programs that are in nontraditional subspecialties, are only available at certain institutions, and are not accredited. This list is not exhaustive of all available programs but are those that may be of interest to dermatology residents who are drawn to a particular dermatologic subspecialty or have an interest in academic dermatology. There is no formal match process and applications vary by institution.
Clinician Educator Fellowship
The clinician educator fellowship is available at the Department of Dermatology at the University of Pennsylvania (Philadelphia, Pennsylvania) and is intended to foster dermatologic clinician educators. More information can be found on the program’s Web site.
Cutaneous Oncology Fellowship
This 1- to 2-year fellowship program focuses on diagnosis and management of melanoma and nonmelanoma skin cancers as well as cutaneous lymphomas. Fellowships in cutaneous oncology are offered at the University of California, San Francisco (San Francisco, California), Brigham and Women’s Hospital (Boston, Massachusetts), the University of Pennsylvania (Philadelphia, Pennsylvania), Case Western Reserve University (Cleveland, Ohio), the University of Pittsburgh (Pittsburgh, Pennsylvania), and Stanford University Medical Center (Stanford, California).
Dermatology/Rheumatology
The dermatology/rheumatology fellowship offered by Brigham and Women’s Hospital is a 1-year program that focuses on the management of connective-tissue diseases in a multidisciplinary fashion with rheumatology.
Advanced Medical Dermatology/Complex Medical Dermatology
Several programs offer fellowships in medical dermatology under different titles but with a similar curriculum and goal: to foster dermatologists interested in careers as academic medical dermatologists or as future clinician scientists by means of specialized training and mentorship in complex medical and dermatological issues in the outpatient and inpatient settings. The 2-year program at New York University School of Medicine (New York, New York) also gives fellows the opportunity to earn a master of science in clinical investigation degree. The University of California, San Francisco, program offers protected time for career development.
Epidemiology Training Program
Fellows and residents in the University of Pennsylvania’s dermatology training program may elect to work with the Center for Clinical Epidemiology and Biostatistics and have the opportunity to earn a graduate degree (MSCE or PhD).
Contact Dermatitis and Patch Testing Fellowship
The Dermatology Department at the Cleveland Clinic (Cleveland, Ohio) offers a 1-year contact dermatitis and patch testing fellowship that includes clinical research.
Conclusion
Both traditional and nontraditional fellowship opportunities exist after dermatology residency. This guide serves as an overview of the training programs in dermatopathology, pediatric dermatology, micrographic surgery and dermatologic oncology (procedural dermatology), and cosmetic dermatologic surgery, as well as the fellowships offered at certain institutions for those interested in more specific subspecialties or academia.
1. Accreditation Council for Graduate Medical Education. ACGME program requirements for graduate medical education in dermatopathology. http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/100_dermatopathology_2016_1-YR.pdf. Revised April 2014. Effective July 1, 2015. Accessed on February 26, 2015.
2. Subspecialty certification in dermatopathology. The American Board of Dermatology, Inc Web site. http://www.abderm.org/subspecialties/derm.html. Accessed February 26, 2015.
3. American Board of Dermatology (ABD) approved pediatric dermatology fellowship programs. The Society for Pediatric Dermatology Web site. http://pedsderm.net/training/fellowships/abd-approved-pediatric-dermatology-fellowship-programs/. Updated June 9, 2014. Accessed February 26, 2015.
4. Subspecialty certification in pediatric dermatology. The American Board of Dermatology, Inc Web site. http://www.abderm.org/subspecialties/pediatric.html. Accessed February 26, 2015.
5. Non-ABD pediatric dermatology fellowship programs. The Society for Pediatric Dermatology Web site. https://pedsderm.net/training/fellowships/non-abd-pediatric-dermatology-fellowship-programs/. Accessed February 26, 2015.
6. Pediatric dermatology match report. SF Match Web site. https://www.sfmatch.org/SpecialtyInsideAll.aspx?id=16&typ=1&name=Pediatric%20Dermatology#. Accessed March 4, 2015.
7. Accreditation Council for Graduate Medical Education. ACGME program requirements for graduate medical education in procedural dermatology. https://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/081_procedural_derm_1-YR_07012014.pdf. Effective July 2014. Accessed February 26, 2015.
8. Statistics: micrographic surgery & dermatologic oncology fellowship. SF Match Web site. https://www.sfmatch.org/SpecialtyInsideAll.aspx?id=10&typ=1&name=Micrographic%20Surgery%20and%20Dermatologic%20Oncology#. Accessed March 4, 2015.
9. ASDS cosmetic dermatologic surgery fellowship accreditation program. American Society for Dermatologic Surgery Web site. http://www.asds.net/cosmetic-accreditation/. Accessed February 26, 2015.
Dermatology residents, such as myself, often wonder what we will do after graduation. There are many resources for finding job opportunities, and many of us have received solicitation e-mails from various headhunters and medical groups that are looking to hire. The American Academy of Dermatology (AAD) has a resource called the AAD Career Compass (http://www.healthecareers.com/aad), which is an exhaustive database of job listings for dermatologists. However, I could not locate a definitive resource containing information that might be useful for dermatology residents who are interested in subspecializing or pursuing fellowships.
Subspecialty training is typically pursued after successful completion of a dermatology residency training program. Fellowships are traditionally offered in dermatopathology, pediatric dermatology, micrographic surgery and dermatologic oncology (procedural dermatology), and cosmetic dermatologic surgery. Fellowships also are available in other subspecialties or for those pursuing an academic career. The goal of this article is to help dermatology residents learn more about traditional and nontraditional opportunities for graduate education and certification in various dermatologic subspecialties, with links to sources for more detailed information.
Traditional Fellowship Programs by Subspecialty
Dermatopathology
One- to 2-year dermatopathology fellowship programs are certified by both the American Board of Dermatology (ABD) and the American Board of Pathology and are available to graduates of either dermatology or pathology residency programs. These programs offer combined training in either anatomic pathology (for dermatologists) or clinical dermatology (for pathologists), along with dermatopathology; the majority of time is devoted to the latter. The Accreditation Council for Graduate Medical Education and the ABD have issued specific requirements for graduate medical education and subspecialty certification in dermatopathology.1,2 Fellowship matches are institution dependent, and the application process and match generally takes place during the second year of dermatology residency for those residents who want to start a fellowship program immediately following graduation. The American Society of Dermatopathology offers a dermatopathology fellowship program finder on its Web site.
Pediatric Dermatology
Fellowships in pediatric dermatology are typically 1- to 2-year programs that focus on dermatologic diseases in the pediatric population. Applicants are matched to these programs through the San Francisco Matching Program (SF Match) and the programs are ABD accredited.3,4 (There also are a number of non–ABD-approved training opportunities available.5) On completion of the training program, fellows may qualify for subspecialty board certification in pediatric dermatology. Applications are open starting in January, and the rank order list and match occur in August of the same year. As of 2012, there were 20 participating programs with 28 available positions, while the match included 22 applicants; of these applicants, 15 matched formally into pediatric dermatology fellowships.6
Micrographic Surgery and Dermatologic Oncology (Procedural Dermatology)
There are specific requirements issued by the Accreditation Council for Graduate Medical Education for dermatologic surgery fellowships,7 which are typically 1- to 2-year programs. Many fellowship programs also are accredited by the American College of Mohs Surgery. This subspecialty is not ABD accredited; therefore, there is no certification process upon completion of a fellowship program. The American College of Mohs Surgery sponsors the match process through SF Match. Applicant registration begins in July and the match occurs in December of the same year. As of 2013, there were 47 participating programs offering 55 positions. Of 77 applicants, 49 obtained fellowship positions formally through the match.8 The American Society for Dermatologic Surgery (ASDS) Web site provides the DermSurg Fellowship Finder, which includes information about independent fellowship programs.
Cosmetic Dermatologic Surgery
The ASDS has an accreditation program for fellowships in cosmetic dermatologic surgery,9 which are generally 1-year programs. Certification in this subspecialty is not ABD accredited. Fellowship opportunities can be found using the ASDS DermSurg Fellowship Finder.
Nontraditional Fellowship Programs
The following are fellowship programs that are in nontraditional subspecialties, are only available at certain institutions, and are not accredited. This list is not exhaustive of all available programs but are those that may be of interest to dermatology residents who are drawn to a particular dermatologic subspecialty or have an interest in academic dermatology. There is no formal match process and applications vary by institution.
Clinician Educator Fellowship
The clinician educator fellowship is available at the Department of Dermatology at the University of Pennsylvania (Philadelphia, Pennsylvania) and is intended to foster dermatologic clinician educators. More information can be found on the program’s Web site.
Cutaneous Oncology Fellowship
This 1- to 2-year fellowship program focuses on diagnosis and management of melanoma and nonmelanoma skin cancers as well as cutaneous lymphomas. Fellowships in cutaneous oncology are offered at the University of California, San Francisco (San Francisco, California), Brigham and Women’s Hospital (Boston, Massachusetts), the University of Pennsylvania (Philadelphia, Pennsylvania), Case Western Reserve University (Cleveland, Ohio), the University of Pittsburgh (Pittsburgh, Pennsylvania), and Stanford University Medical Center (Stanford, California).
Dermatology/Rheumatology
The dermatology/rheumatology fellowship offered by Brigham and Women’s Hospital is a 1-year program that focuses on the management of connective-tissue diseases in a multidisciplinary fashion with rheumatology.
Advanced Medical Dermatology/Complex Medical Dermatology
Several programs offer fellowships in medical dermatology under different titles but with a similar curriculum and goal: to foster dermatologists interested in careers as academic medical dermatologists or as future clinician scientists by means of specialized training and mentorship in complex medical and dermatological issues in the outpatient and inpatient settings. The 2-year program at New York University School of Medicine (New York, New York) also gives fellows the opportunity to earn a master of science in clinical investigation degree. The University of California, San Francisco, program offers protected time for career development.
Epidemiology Training Program
Fellows and residents in the University of Pennsylvania’s dermatology training program may elect to work with the Center for Clinical Epidemiology and Biostatistics and have the opportunity to earn a graduate degree (MSCE or PhD).
Contact Dermatitis and Patch Testing Fellowship
The Dermatology Department at the Cleveland Clinic (Cleveland, Ohio) offers a 1-year contact dermatitis and patch testing fellowship that includes clinical research.
Conclusion
Both traditional and nontraditional fellowship opportunities exist after dermatology residency. This guide serves as an overview of the training programs in dermatopathology, pediatric dermatology, micrographic surgery and dermatologic oncology (procedural dermatology), and cosmetic dermatologic surgery, as well as the fellowships offered at certain institutions for those interested in more specific subspecialties or academia.
Dermatology residents, such as myself, often wonder what we will do after graduation. There are many resources for finding job opportunities, and many of us have received solicitation e-mails from various headhunters and medical groups that are looking to hire. The American Academy of Dermatology (AAD) has a resource called the AAD Career Compass (http://www.healthecareers.com/aad), which is an exhaustive database of job listings for dermatologists. However, I could not locate a definitive resource containing information that might be useful for dermatology residents who are interested in subspecializing or pursuing fellowships.
Subspecialty training is typically pursued after successful completion of a dermatology residency training program. Fellowships are traditionally offered in dermatopathology, pediatric dermatology, micrographic surgery and dermatologic oncology (procedural dermatology), and cosmetic dermatologic surgery. Fellowships also are available in other subspecialties or for those pursuing an academic career. The goal of this article is to help dermatology residents learn more about traditional and nontraditional opportunities for graduate education and certification in various dermatologic subspecialties, with links to sources for more detailed information.
Traditional Fellowship Programs by Subspecialty
Dermatopathology
One- to 2-year dermatopathology fellowship programs are certified by both the American Board of Dermatology (ABD) and the American Board of Pathology and are available to graduates of either dermatology or pathology residency programs. These programs offer combined training in either anatomic pathology (for dermatologists) or clinical dermatology (for pathologists), along with dermatopathology; the majority of time is devoted to the latter. The Accreditation Council for Graduate Medical Education and the ABD have issued specific requirements for graduate medical education and subspecialty certification in dermatopathology.1,2 Fellowship matches are institution dependent, and the application process and match generally takes place during the second year of dermatology residency for those residents who want to start a fellowship program immediately following graduation. The American Society of Dermatopathology offers a dermatopathology fellowship program finder on its Web site.
Pediatric Dermatology
Fellowships in pediatric dermatology are typically 1- to 2-year programs that focus on dermatologic diseases in the pediatric population. Applicants are matched to these programs through the San Francisco Matching Program (SF Match) and the programs are ABD accredited.3,4 (There also are a number of non–ABD-approved training opportunities available.5) On completion of the training program, fellows may qualify for subspecialty board certification in pediatric dermatology. Applications are open starting in January, and the rank order list and match occur in August of the same year. As of 2012, there were 20 participating programs with 28 available positions, while the match included 22 applicants; of these applicants, 15 matched formally into pediatric dermatology fellowships.6
Micrographic Surgery and Dermatologic Oncology (Procedural Dermatology)
There are specific requirements issued by the Accreditation Council for Graduate Medical Education for dermatologic surgery fellowships,7 which are typically 1- to 2-year programs. Many fellowship programs also are accredited by the American College of Mohs Surgery. This subspecialty is not ABD accredited; therefore, there is no certification process upon completion of a fellowship program. The American College of Mohs Surgery sponsors the match process through SF Match. Applicant registration begins in July and the match occurs in December of the same year. As of 2013, there were 47 participating programs offering 55 positions. Of 77 applicants, 49 obtained fellowship positions formally through the match.8 The American Society for Dermatologic Surgery (ASDS) Web site provides the DermSurg Fellowship Finder, which includes information about independent fellowship programs.
Cosmetic Dermatologic Surgery
The ASDS has an accreditation program for fellowships in cosmetic dermatologic surgery,9 which are generally 1-year programs. Certification in this subspecialty is not ABD accredited. Fellowship opportunities can be found using the ASDS DermSurg Fellowship Finder.
Nontraditional Fellowship Programs
The following are fellowship programs that are in nontraditional subspecialties, are only available at certain institutions, and are not accredited. This list is not exhaustive of all available programs but are those that may be of interest to dermatology residents who are drawn to a particular dermatologic subspecialty or have an interest in academic dermatology. There is no formal match process and applications vary by institution.
Clinician Educator Fellowship
The clinician educator fellowship is available at the Department of Dermatology at the University of Pennsylvania (Philadelphia, Pennsylvania) and is intended to foster dermatologic clinician educators. More information can be found on the program’s Web site.
Cutaneous Oncology Fellowship
This 1- to 2-year fellowship program focuses on diagnosis and management of melanoma and nonmelanoma skin cancers as well as cutaneous lymphomas. Fellowships in cutaneous oncology are offered at the University of California, San Francisco (San Francisco, California), Brigham and Women’s Hospital (Boston, Massachusetts), the University of Pennsylvania (Philadelphia, Pennsylvania), Case Western Reserve University (Cleveland, Ohio), the University of Pittsburgh (Pittsburgh, Pennsylvania), and Stanford University Medical Center (Stanford, California).
Dermatology/Rheumatology
The dermatology/rheumatology fellowship offered by Brigham and Women’s Hospital is a 1-year program that focuses on the management of connective-tissue diseases in a multidisciplinary fashion with rheumatology.
Advanced Medical Dermatology/Complex Medical Dermatology
Several programs offer fellowships in medical dermatology under different titles but with a similar curriculum and goal: to foster dermatologists interested in careers as academic medical dermatologists or as future clinician scientists by means of specialized training and mentorship in complex medical and dermatological issues in the outpatient and inpatient settings. The 2-year program at New York University School of Medicine (New York, New York) also gives fellows the opportunity to earn a master of science in clinical investigation degree. The University of California, San Francisco, program offers protected time for career development.
Epidemiology Training Program
Fellows and residents in the University of Pennsylvania’s dermatology training program may elect to work with the Center for Clinical Epidemiology and Biostatistics and have the opportunity to earn a graduate degree (MSCE or PhD).
Contact Dermatitis and Patch Testing Fellowship
The Dermatology Department at the Cleveland Clinic (Cleveland, Ohio) offers a 1-year contact dermatitis and patch testing fellowship that includes clinical research.
Conclusion
Both traditional and nontraditional fellowship opportunities exist after dermatology residency. This guide serves as an overview of the training programs in dermatopathology, pediatric dermatology, micrographic surgery and dermatologic oncology (procedural dermatology), and cosmetic dermatologic surgery, as well as the fellowships offered at certain institutions for those interested in more specific subspecialties or academia.
1. Accreditation Council for Graduate Medical Education. ACGME program requirements for graduate medical education in dermatopathology. http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/100_dermatopathology_2016_1-YR.pdf. Revised April 2014. Effective July 1, 2015. Accessed on February 26, 2015.
2. Subspecialty certification in dermatopathology. The American Board of Dermatology, Inc Web site. http://www.abderm.org/subspecialties/derm.html. Accessed February 26, 2015.
3. American Board of Dermatology (ABD) approved pediatric dermatology fellowship programs. The Society for Pediatric Dermatology Web site. http://pedsderm.net/training/fellowships/abd-approved-pediatric-dermatology-fellowship-programs/. Updated June 9, 2014. Accessed February 26, 2015.
4. Subspecialty certification in pediatric dermatology. The American Board of Dermatology, Inc Web site. http://www.abderm.org/subspecialties/pediatric.html. Accessed February 26, 2015.
5. Non-ABD pediatric dermatology fellowship programs. The Society for Pediatric Dermatology Web site. https://pedsderm.net/training/fellowships/non-abd-pediatric-dermatology-fellowship-programs/. Accessed February 26, 2015.
6. Pediatric dermatology match report. SF Match Web site. https://www.sfmatch.org/SpecialtyInsideAll.aspx?id=16&typ=1&name=Pediatric%20Dermatology#. Accessed March 4, 2015.
7. Accreditation Council for Graduate Medical Education. ACGME program requirements for graduate medical education in procedural dermatology. https://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/081_procedural_derm_1-YR_07012014.pdf. Effective July 2014. Accessed February 26, 2015.
8. Statistics: micrographic surgery & dermatologic oncology fellowship. SF Match Web site. https://www.sfmatch.org/SpecialtyInsideAll.aspx?id=10&typ=1&name=Micrographic%20Surgery%20and%20Dermatologic%20Oncology#. Accessed March 4, 2015.
9. ASDS cosmetic dermatologic surgery fellowship accreditation program. American Society for Dermatologic Surgery Web site. http://www.asds.net/cosmetic-accreditation/. Accessed February 26, 2015.
1. Accreditation Council for Graduate Medical Education. ACGME program requirements for graduate medical education in dermatopathology. http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/100_dermatopathology_2016_1-YR.pdf. Revised April 2014. Effective July 1, 2015. Accessed on February 26, 2015.
2. Subspecialty certification in dermatopathology. The American Board of Dermatology, Inc Web site. http://www.abderm.org/subspecialties/derm.html. Accessed February 26, 2015.
3. American Board of Dermatology (ABD) approved pediatric dermatology fellowship programs. The Society for Pediatric Dermatology Web site. http://pedsderm.net/training/fellowships/abd-approved-pediatric-dermatology-fellowship-programs/. Updated June 9, 2014. Accessed February 26, 2015.
4. Subspecialty certification in pediatric dermatology. The American Board of Dermatology, Inc Web site. http://www.abderm.org/subspecialties/pediatric.html. Accessed February 26, 2015.
5. Non-ABD pediatric dermatology fellowship programs. The Society for Pediatric Dermatology Web site. https://pedsderm.net/training/fellowships/non-abd-pediatric-dermatology-fellowship-programs/. Accessed February 26, 2015.
6. Pediatric dermatology match report. SF Match Web site. https://www.sfmatch.org/SpecialtyInsideAll.aspx?id=16&typ=1&name=Pediatric%20Dermatology#. Accessed March 4, 2015.
7. Accreditation Council for Graduate Medical Education. ACGME program requirements for graduate medical education in procedural dermatology. https://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/081_procedural_derm_1-YR_07012014.pdf. Effective July 2014. Accessed February 26, 2015.
8. Statistics: micrographic surgery & dermatologic oncology fellowship. SF Match Web site. https://www.sfmatch.org/SpecialtyInsideAll.aspx?id=10&typ=1&name=Micrographic%20Surgery%20and%20Dermatologic%20Oncology#. Accessed March 4, 2015.
9. ASDS cosmetic dermatologic surgery fellowship accreditation program. American Society for Dermatologic Surgery Web site. http://www.asds.net/cosmetic-accreditation/. Accessed February 26, 2015.
VIDEO: Finesse is key to avoiding exfoliation mistakes
SAN FRANCISCO – Sometimes, salicylic acid is the right call for exfoliation, but sometimes not. Other times microdermabrasion or glycolic acid make sense, or maybe mandelic acid, or something else entirely.
In other words, dermatologists have a lot of options for exfoliation, including not to do it at all. The way to go all comes down to understanding your patient’s skin, and that can be tricky.
Dr. Mary Lupo, clinical professor of dermatology at Tulane University School of Medicine, New Orleans, explained the nuances of exfoliation and shared her advice in an interview at the annual meeting of the American Academy of Dermatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN FRANCISCO – Sometimes, salicylic acid is the right call for exfoliation, but sometimes not. Other times microdermabrasion or glycolic acid make sense, or maybe mandelic acid, or something else entirely.
In other words, dermatologists have a lot of options for exfoliation, including not to do it at all. The way to go all comes down to understanding your patient’s skin, and that can be tricky.
Dr. Mary Lupo, clinical professor of dermatology at Tulane University School of Medicine, New Orleans, explained the nuances of exfoliation and shared her advice in an interview at the annual meeting of the American Academy of Dermatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN FRANCISCO – Sometimes, salicylic acid is the right call for exfoliation, but sometimes not. Other times microdermabrasion or glycolic acid make sense, or maybe mandelic acid, or something else entirely.
In other words, dermatologists have a lot of options for exfoliation, including not to do it at all. The way to go all comes down to understanding your patient’s skin, and that can be tricky.
Dr. Mary Lupo, clinical professor of dermatology at Tulane University School of Medicine, New Orleans, explained the nuances of exfoliation and shared her advice in an interview at the annual meeting of the American Academy of Dermatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE AAD ANNUAL MEETING
VIDEO: When to use glycopyrrolate as first-line hyperhidrosis therapy
KAUAI, HAWAII – When should glycopyrrolate be used as first-line therapy in the treatment of hyperhidrosis? And if it’s not the first treatment, what should take its place?
In a video report from the 2015 Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Christopher Zachary and Dr. Joshua Spanogle share their thoughts on the latest in hyperhidrosis treatment.
Dr. Zachary disclosed that he has financial relationships with Cutera and Solta. Dr. Spanogle disclosed he has relationships with Illumina and Quantified Skin. SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
KAUAI, HAWAII – When should glycopyrrolate be used as first-line therapy in the treatment of hyperhidrosis? And if it’s not the first treatment, what should take its place?
In a video report from the 2015 Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Christopher Zachary and Dr. Joshua Spanogle share their thoughts on the latest in hyperhidrosis treatment.
Dr. Zachary disclosed that he has financial relationships with Cutera and Solta. Dr. Spanogle disclosed he has relationships with Illumina and Quantified Skin. SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
KAUAI, HAWAII – When should glycopyrrolate be used as first-line therapy in the treatment of hyperhidrosis? And if it’s not the first treatment, what should take its place?
In a video report from the 2015 Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Christopher Zachary and Dr. Joshua Spanogle share their thoughts on the latest in hyperhidrosis treatment.
Dr. Zachary disclosed that he has financial relationships with Cutera and Solta. Dr. Spanogle disclosed he has relationships with Illumina and Quantified Skin. SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
AT SDEF HAWAII DERMATOLOGY SEMINAR 2015