User login
Collagen filler succeeds against acne scars
SAN DIEGO – An injectable collagen-based filler significantly outperformed saline placebo for treating acne scars, with durable effects at 12 months, according to a randomized, double-blind crossover trial of 147 adults.
The study “successfully demonstrates the effectiveness and safety of polymethylmethacrylate in atrophic acne scars,” said Dr. James Spencer, who conducted the research while at Mount Sinai School of Medicine in New York. Dr. Spencer is now in private practice in St. Petersburg, Fla.
Six months after treatment, 64% of patients who received the polymethylmethacrylate-collagen filler (or PMMA) had at least half their scars improve by at least 2 points on an acne rating scale, Dr. Spencer and his associates said at the annual meeting of the American Society for Dermatologic Surgery. Only 32% of the control group achieved that result (P = .0005). Response rates for the filler were 61% at 9 months and 70% at 12 months, and “crossover subjects subsequently treated with PMMA collagen showed similar response levels,” they said.
The PMMA-collagen filler is easy to administer; “works well on deep, severe scars; and should also work very well on shallow scars,” the researchers noted. The treatment “may enable practitioners to effectively treat acne scarring with no need for capital equipment expenditure or the risks associated with resurfacing procedures,” they said.
About 61% of patients in the study were female, participants averaged 44 years of age, and 20% were Fitzpatrick skin type V or VI. To enter the study, participants had to have at least four facial acne scars that were soft contoured, rolling, distensible, and rated moderate to severe (3-4) on a 4-point acne rating scale.
The patients were treated every 2 weeks for a month, and again at months 3 and 6. At 6 months, patients in the placebo group crossed over and received the filler, and all patients were followed for another 6 months.
Adverse effects were uncommon and included mild transient pain at the injection site, swelling, bruising, and acne, the researchers said. “There was no evidence of granulomas, changes in pigmentation, or hypertrophic scarring,” they added.
No funding source was reported for the study. Dr. Spencer and his coauthors reported financial relationships with Photomedex, Genentech, and Leo Pharma.
SAN DIEGO – An injectable collagen-based filler significantly outperformed saline placebo for treating acne scars, with durable effects at 12 months, according to a randomized, double-blind crossover trial of 147 adults.
The study “successfully demonstrates the effectiveness and safety of polymethylmethacrylate in atrophic acne scars,” said Dr. James Spencer, who conducted the research while at Mount Sinai School of Medicine in New York. Dr. Spencer is now in private practice in St. Petersburg, Fla.
Six months after treatment, 64% of patients who received the polymethylmethacrylate-collagen filler (or PMMA) had at least half their scars improve by at least 2 points on an acne rating scale, Dr. Spencer and his associates said at the annual meeting of the American Society for Dermatologic Surgery. Only 32% of the control group achieved that result (P = .0005). Response rates for the filler were 61% at 9 months and 70% at 12 months, and “crossover subjects subsequently treated with PMMA collagen showed similar response levels,” they said.
The PMMA-collagen filler is easy to administer; “works well on deep, severe scars; and should also work very well on shallow scars,” the researchers noted. The treatment “may enable practitioners to effectively treat acne scarring with no need for capital equipment expenditure or the risks associated with resurfacing procedures,” they said.
About 61% of patients in the study were female, participants averaged 44 years of age, and 20% were Fitzpatrick skin type V or VI. To enter the study, participants had to have at least four facial acne scars that were soft contoured, rolling, distensible, and rated moderate to severe (3-4) on a 4-point acne rating scale.
The patients were treated every 2 weeks for a month, and again at months 3 and 6. At 6 months, patients in the placebo group crossed over and received the filler, and all patients were followed for another 6 months.
Adverse effects were uncommon and included mild transient pain at the injection site, swelling, bruising, and acne, the researchers said. “There was no evidence of granulomas, changes in pigmentation, or hypertrophic scarring,” they added.
No funding source was reported for the study. Dr. Spencer and his coauthors reported financial relationships with Photomedex, Genentech, and Leo Pharma.
SAN DIEGO – An injectable collagen-based filler significantly outperformed saline placebo for treating acne scars, with durable effects at 12 months, according to a randomized, double-blind crossover trial of 147 adults.
The study “successfully demonstrates the effectiveness and safety of polymethylmethacrylate in atrophic acne scars,” said Dr. James Spencer, who conducted the research while at Mount Sinai School of Medicine in New York. Dr. Spencer is now in private practice in St. Petersburg, Fla.
Six months after treatment, 64% of patients who received the polymethylmethacrylate-collagen filler (or PMMA) had at least half their scars improve by at least 2 points on an acne rating scale, Dr. Spencer and his associates said at the annual meeting of the American Society for Dermatologic Surgery. Only 32% of the control group achieved that result (P = .0005). Response rates for the filler were 61% at 9 months and 70% at 12 months, and “crossover subjects subsequently treated with PMMA collagen showed similar response levels,” they said.
The PMMA-collagen filler is easy to administer; “works well on deep, severe scars; and should also work very well on shallow scars,” the researchers noted. The treatment “may enable practitioners to effectively treat acne scarring with no need for capital equipment expenditure or the risks associated with resurfacing procedures,” they said.
About 61% of patients in the study were female, participants averaged 44 years of age, and 20% were Fitzpatrick skin type V or VI. To enter the study, participants had to have at least four facial acne scars that were soft contoured, rolling, distensible, and rated moderate to severe (3-4) on a 4-point acne rating scale.
The patients were treated every 2 weeks for a month, and again at months 3 and 6. At 6 months, patients in the placebo group crossed over and received the filler, and all patients were followed for another 6 months.
Adverse effects were uncommon and included mild transient pain at the injection site, swelling, bruising, and acne, the researchers said. “There was no evidence of granulomas, changes in pigmentation, or hypertrophic scarring,” they added.
No funding source was reported for the study. Dr. Spencer and his coauthors reported financial relationships with Photomedex, Genentech, and Leo Pharma.
Key clinical point: A collagen-based filler significantly improved the appearance of acne scars in adults, with persistent improvement at 12 months.
Major finding: At 6-month evaluation, 64% of the intervention group were considered responders, compared with 32% of the control group (P = .0005).
Data source: A prospective, randomized, double-blind, controlled, multicenter crossover study of 147 adults with acne scars.
Disclosures: The researchers did not report funding sources. Dr. Spencer and his coauthors reported financial relationships with Photomedex, Genentech, and Leo Pharma.
Checklists to Improve Laser Practices

Hamilton and Dover (Dermatol Surg. 2014;40:1173-1174) discussed the use of checklists for improving laser and light-based procedures in their November 2014 editorial. The authors discussed how the use of checklists has become pervasive in many fields of medicine, including surgery. However, they also highlighted that the utility of checklists and their success do not come from just checking off boxes but rather from actively performing the tasks. The implementation of a laser checklist can seem daunting, but Hamilton and Dover proposed that for a dermatology practice or dermatology department it becomes easier because the dermatologist and staff work closely already, making communication less of a challenge. Also, the procedure lends itself to a systematic approach. In doing so, one can routinely ensure that the necessary practices are being done to ensure both patient and staff safety.
What’s the issue?
The implementation of checklists for medical procedures may seem straightforward; however, to be effective there has to be pertinent information gathered or actions taken. Our department utilizes checklists for our laser procedures. The different steps that we have included help to ensure that the nurses who prepare the patients will communicate the pertinent information to the physician. Our checklist starts with ensuring proper setup, such as connecting the necessary pieces, before starting the laser. Next is making sure all the necessary equipment is in place, such as the correct laser goggles for the patient and staff. We label all of our goggles with the name of the laser on them so it is readily apparent what to use. We also have a check box that ensures the proper signage is outside on the door and the laser shades are drawn. There also is a section relating to the patient. Depending on the type of laser being utilized the checklist may include the following: skin type, recent history of tanning, history of oral herpes, isotretinoin use, and time since last treatment. The next check box ensures consent is signed and pretreatment photographs are taken. Any prior complications also are noted. We have a section for treatment parameters used during the current visit and then another section for posttreatment instructions.
Although a checklist may seem like unnecessary work, we found that it actually helps to enhance productivity and ensures proper laser safety for the physician and patient. We have a different one for each laser, as it also serves as a documentation tool for the procedure. Do you utilize checklists for your laser procedures? If so, what else do you include?

Hamilton and Dover (Dermatol Surg. 2014;40:1173-1174) discussed the use of checklists for improving laser and light-based procedures in their November 2014 editorial. The authors discussed how the use of checklists has become pervasive in many fields of medicine, including surgery. However, they also highlighted that the utility of checklists and their success do not come from just checking off boxes but rather from actively performing the tasks. The implementation of a laser checklist can seem daunting, but Hamilton and Dover proposed that for a dermatology practice or dermatology department it becomes easier because the dermatologist and staff work closely already, making communication less of a challenge. Also, the procedure lends itself to a systematic approach. In doing so, one can routinely ensure that the necessary practices are being done to ensure both patient and staff safety.
What’s the issue?
The implementation of checklists for medical procedures may seem straightforward; however, to be effective there has to be pertinent information gathered or actions taken. Our department utilizes checklists for our laser procedures. The different steps that we have included help to ensure that the nurses who prepare the patients will communicate the pertinent information to the physician. Our checklist starts with ensuring proper setup, such as connecting the necessary pieces, before starting the laser. Next is making sure all the necessary equipment is in place, such as the correct laser goggles for the patient and staff. We label all of our goggles with the name of the laser on them so it is readily apparent what to use. We also have a check box that ensures the proper signage is outside on the door and the laser shades are drawn. There also is a section relating to the patient. Depending on the type of laser being utilized the checklist may include the following: skin type, recent history of tanning, history of oral herpes, isotretinoin use, and time since last treatment. The next check box ensures consent is signed and pretreatment photographs are taken. Any prior complications also are noted. We have a section for treatment parameters used during the current visit and then another section for posttreatment instructions.
Although a checklist may seem like unnecessary work, we found that it actually helps to enhance productivity and ensures proper laser safety for the physician and patient. We have a different one for each laser, as it also serves as a documentation tool for the procedure. Do you utilize checklists for your laser procedures? If so, what else do you include?

Hamilton and Dover (Dermatol Surg. 2014;40:1173-1174) discussed the use of checklists for improving laser and light-based procedures in their November 2014 editorial. The authors discussed how the use of checklists has become pervasive in many fields of medicine, including surgery. However, they also highlighted that the utility of checklists and their success do not come from just checking off boxes but rather from actively performing the tasks. The implementation of a laser checklist can seem daunting, but Hamilton and Dover proposed that for a dermatology practice or dermatology department it becomes easier because the dermatologist and staff work closely already, making communication less of a challenge. Also, the procedure lends itself to a systematic approach. In doing so, one can routinely ensure that the necessary practices are being done to ensure both patient and staff safety.
What’s the issue?
The implementation of checklists for medical procedures may seem straightforward; however, to be effective there has to be pertinent information gathered or actions taken. Our department utilizes checklists for our laser procedures. The different steps that we have included help to ensure that the nurses who prepare the patients will communicate the pertinent information to the physician. Our checklist starts with ensuring proper setup, such as connecting the necessary pieces, before starting the laser. Next is making sure all the necessary equipment is in place, such as the correct laser goggles for the patient and staff. We label all of our goggles with the name of the laser on them so it is readily apparent what to use. We also have a check box that ensures the proper signage is outside on the door and the laser shades are drawn. There also is a section relating to the patient. Depending on the type of laser being utilized the checklist may include the following: skin type, recent history of tanning, history of oral herpes, isotretinoin use, and time since last treatment. The next check box ensures consent is signed and pretreatment photographs are taken. Any prior complications also are noted. We have a section for treatment parameters used during the current visit and then another section for posttreatment instructions.
Although a checklist may seem like unnecessary work, we found that it actually helps to enhance productivity and ensures proper laser safety for the physician and patient. We have a different one for each laser, as it also serves as a documentation tool for the procedure. Do you utilize checklists for your laser procedures? If so, what else do you include?
Fractional laser technology reduces facial acne scarring
Fractional laser technology, often used in the removal of unwanted tattoos, can improve the appearance and texture of facial acne scars, based on data published online Nov.19 in JAMA Dermatology.
“The evolution from traditional nanosecond to picosecond lasers has been observed to produce a photomechanical effect that causes fragmentation of tattoo ink or pigment,” wrote Dr. Jeremy A. Brauer, a dermatologist in group practice in New York, and his coinvestigators. “An innovative optical attachment for the picosecond laser, a diffractive lens array, has been developed that gauges distribution of energy to the treatment area. This specialized optic affects more surface area, has a greater pattern density per pulse, and may improve the appearance of acne scars,” they reported.
In a single-center, prospective study, Dr. Brauer and his associates enrolled 20 patients – 15 women and 5 men – based on screenings to ensure no history of skin cancer, keloidal scarring, localized or active infection, immunodeficiency disorders, and light hypersensitivity or use of medications with known phototoxic effects. Of that initial group, 17 completed all six treatments and presented for follow-up visits at 1 and 3 months. Patients were aged 27-66 (mean age, 44 years), and included Fitzpatrick skin types I (one patient), II (seven patients), III (six patients), and IV (three patients).
Subjects mostly had rolling-type scars, boxcar scars, and icepick lesions related to acne. Each subject underwent six treatments with a 755-nanometer alexandrite picosecond laser with a diffractive lens array; each treatment occurred every 4-8 weeks.
Subjects also provided a subjective score for pain experienced during each treatment on a scale of 0 (no pain) to 10 (extreme pain). The patients also used a scale of 0-4 to indicate their satisfaction with improvement of overall skin appearance and texture prior to their final treatment, at the 1-month follow-up, and at the 3-month follow-up (with 0 being total dissatisfaction and 4 total satisfaction).
At the 1-month and 3-month follow-ups, three independent dermatologists gave masked evaluations of each patient’s improvement on a 4-point scale, with 0 = 0%-25%, 1 = 26%-50%, 3 = 51%-75%, and 4 = 76%-100%.
All patients were either “satisfied” or “extremely satisfied” with the appearance and texture of their facial skin after receiving the full treatment regimen, and recorded an average pain score of 2.83 out of 10. The masked assessment scores also were favorable, averaging 1.5 of 3 and 1.4 of 3 at the 1-month and 3-month follow-ups, with a score of 0 indicating 0-25% improvement and a score of 3, greater than 75% improvement, the researchers reported.
Dr. Brauer and his associates also evaluated three-dimensional volumetric data for each subject, which showed an average of 24.0% improvement in scar volume at the 1-month follow-up and 27.2% at the 3-month follow-up. Furthermore, histologic analysis revealed elongation and increased density of elastic fibers, as well as an increase in dermal collagen and mucin.
“This is the first study, to our knowledge, that demonstrates favorable clinical outcomes in acne scar management with the 755[-nm] picosecond laser and diffractive lens array,” the researchers noted. “Observed improvement in pigmentation and texture of the surrounding skin suggests that there may be benefits for indications beyond scarring,” they wrote.
The authors disclosed that funding for this study was provided in part by Cynosure, manufacturer of the Food and Drug Administration–approved 755-nm picosecond alexandrite laser used in the study, and noted that “Cynosure had a role in the design of the study but not the conduct, collection, management, analysis, or interpretation of data. They approved the manuscript but did not prepare or decide to submit.” Dr. Brauer disclosed receiving honoraria from Cynosure/Palomar Medical Technologies and consulting for Miramar. Several other coauthors disclosed financial relationships with multiple companies, including Cynosure/Palomar Medical Technologies.
Fractional laser technology, often used in the removal of unwanted tattoos, can improve the appearance and texture of facial acne scars, based on data published online Nov.19 in JAMA Dermatology.
“The evolution from traditional nanosecond to picosecond lasers has been observed to produce a photomechanical effect that causes fragmentation of tattoo ink or pigment,” wrote Dr. Jeremy A. Brauer, a dermatologist in group practice in New York, and his coinvestigators. “An innovative optical attachment for the picosecond laser, a diffractive lens array, has been developed that gauges distribution of energy to the treatment area. This specialized optic affects more surface area, has a greater pattern density per pulse, and may improve the appearance of acne scars,” they reported.
In a single-center, prospective study, Dr. Brauer and his associates enrolled 20 patients – 15 women and 5 men – based on screenings to ensure no history of skin cancer, keloidal scarring, localized or active infection, immunodeficiency disorders, and light hypersensitivity or use of medications with known phototoxic effects. Of that initial group, 17 completed all six treatments and presented for follow-up visits at 1 and 3 months. Patients were aged 27-66 (mean age, 44 years), and included Fitzpatrick skin types I (one patient), II (seven patients), III (six patients), and IV (three patients).
Subjects mostly had rolling-type scars, boxcar scars, and icepick lesions related to acne. Each subject underwent six treatments with a 755-nanometer alexandrite picosecond laser with a diffractive lens array; each treatment occurred every 4-8 weeks.
Subjects also provided a subjective score for pain experienced during each treatment on a scale of 0 (no pain) to 10 (extreme pain). The patients also used a scale of 0-4 to indicate their satisfaction with improvement of overall skin appearance and texture prior to their final treatment, at the 1-month follow-up, and at the 3-month follow-up (with 0 being total dissatisfaction and 4 total satisfaction).
At the 1-month and 3-month follow-ups, three independent dermatologists gave masked evaluations of each patient’s improvement on a 4-point scale, with 0 = 0%-25%, 1 = 26%-50%, 3 = 51%-75%, and 4 = 76%-100%.
All patients were either “satisfied” or “extremely satisfied” with the appearance and texture of their facial skin after receiving the full treatment regimen, and recorded an average pain score of 2.83 out of 10. The masked assessment scores also were favorable, averaging 1.5 of 3 and 1.4 of 3 at the 1-month and 3-month follow-ups, with a score of 0 indicating 0-25% improvement and a score of 3, greater than 75% improvement, the researchers reported.
Dr. Brauer and his associates also evaluated three-dimensional volumetric data for each subject, which showed an average of 24.0% improvement in scar volume at the 1-month follow-up and 27.2% at the 3-month follow-up. Furthermore, histologic analysis revealed elongation and increased density of elastic fibers, as well as an increase in dermal collagen and mucin.
“This is the first study, to our knowledge, that demonstrates favorable clinical outcomes in acne scar management with the 755[-nm] picosecond laser and diffractive lens array,” the researchers noted. “Observed improvement in pigmentation and texture of the surrounding skin suggests that there may be benefits for indications beyond scarring,” they wrote.
The authors disclosed that funding for this study was provided in part by Cynosure, manufacturer of the Food and Drug Administration–approved 755-nm picosecond alexandrite laser used in the study, and noted that “Cynosure had a role in the design of the study but not the conduct, collection, management, analysis, or interpretation of data. They approved the manuscript but did not prepare or decide to submit.” Dr. Brauer disclosed receiving honoraria from Cynosure/Palomar Medical Technologies and consulting for Miramar. Several other coauthors disclosed financial relationships with multiple companies, including Cynosure/Palomar Medical Technologies.
Fractional laser technology, often used in the removal of unwanted tattoos, can improve the appearance and texture of facial acne scars, based on data published online Nov.19 in JAMA Dermatology.
“The evolution from traditional nanosecond to picosecond lasers has been observed to produce a photomechanical effect that causes fragmentation of tattoo ink or pigment,” wrote Dr. Jeremy A. Brauer, a dermatologist in group practice in New York, and his coinvestigators. “An innovative optical attachment for the picosecond laser, a diffractive lens array, has been developed that gauges distribution of energy to the treatment area. This specialized optic affects more surface area, has a greater pattern density per pulse, and may improve the appearance of acne scars,” they reported.
In a single-center, prospective study, Dr. Brauer and his associates enrolled 20 patients – 15 women and 5 men – based on screenings to ensure no history of skin cancer, keloidal scarring, localized or active infection, immunodeficiency disorders, and light hypersensitivity or use of medications with known phototoxic effects. Of that initial group, 17 completed all six treatments and presented for follow-up visits at 1 and 3 months. Patients were aged 27-66 (mean age, 44 years), and included Fitzpatrick skin types I (one patient), II (seven patients), III (six patients), and IV (three patients).
Subjects mostly had rolling-type scars, boxcar scars, and icepick lesions related to acne. Each subject underwent six treatments with a 755-nanometer alexandrite picosecond laser with a diffractive lens array; each treatment occurred every 4-8 weeks.
Subjects also provided a subjective score for pain experienced during each treatment on a scale of 0 (no pain) to 10 (extreme pain). The patients also used a scale of 0-4 to indicate their satisfaction with improvement of overall skin appearance and texture prior to their final treatment, at the 1-month follow-up, and at the 3-month follow-up (with 0 being total dissatisfaction and 4 total satisfaction).
At the 1-month and 3-month follow-ups, three independent dermatologists gave masked evaluations of each patient’s improvement on a 4-point scale, with 0 = 0%-25%, 1 = 26%-50%, 3 = 51%-75%, and 4 = 76%-100%.
All patients were either “satisfied” or “extremely satisfied” with the appearance and texture of their facial skin after receiving the full treatment regimen, and recorded an average pain score of 2.83 out of 10. The masked assessment scores also were favorable, averaging 1.5 of 3 and 1.4 of 3 at the 1-month and 3-month follow-ups, with a score of 0 indicating 0-25% improvement and a score of 3, greater than 75% improvement, the researchers reported.
Dr. Brauer and his associates also evaluated three-dimensional volumetric data for each subject, which showed an average of 24.0% improvement in scar volume at the 1-month follow-up and 27.2% at the 3-month follow-up. Furthermore, histologic analysis revealed elongation and increased density of elastic fibers, as well as an increase in dermal collagen and mucin.
“This is the first study, to our knowledge, that demonstrates favorable clinical outcomes in acne scar management with the 755[-nm] picosecond laser and diffractive lens array,” the researchers noted. “Observed improvement in pigmentation and texture of the surrounding skin suggests that there may be benefits for indications beyond scarring,” they wrote.
The authors disclosed that funding for this study was provided in part by Cynosure, manufacturer of the Food and Drug Administration–approved 755-nm picosecond alexandrite laser used in the study, and noted that “Cynosure had a role in the design of the study but not the conduct, collection, management, analysis, or interpretation of data. They approved the manuscript but did not prepare or decide to submit.” Dr. Brauer disclosed receiving honoraria from Cynosure/Palomar Medical Technologies and consulting for Miramar. Several other coauthors disclosed financial relationships with multiple companies, including Cynosure/Palomar Medical Technologies.
FROM JAMA DERMATOLOGY
Key clinical point: Treatment of facial acne scars with a diffractive lens array and a picosecond 755-nm alexandrite laser improves the appearance and texture of skin within 3 months.
Major finding: Masked assessments by a dermatologist found a 25%-50% global improvement at the 1-month postoperation follow-up visit, which was maintained at the 3-month follow-up.
Data source: A single-center, prospective study of 20 patients.
Disclosures: This study was supported in part by Cynosure. The authors disclosed several potential conflicts of interest.
Beta-blockers expand treatment options for infantile hemangiomas
VANCOUVER, B.C. – Propranolol may have revolutionized the treatment of infantile hemangiomas over the past decade, but other beta-blockers are making inroads in the treatment of these and other pediatric dermatologic disorders.
One such beta-blocker is atenolol, a hydrophilic cardioselective agent that acts principally on beta1-adrenergic receptors, “and theoretically has less chance of hypoglycemia and bronchoconstriction,” Dr. Joseph M. Lam said at the annual meeting of the Pacific Dermatologic Association. Atenolol does not cross the blood-brain barrier and features once-daily dosing, he noted.
A recent noninferiority trial in 23 patients with infantile hemangiomas compared atenolol 1 mg/kg/day with propranolol 2 mg/kg/day, and followed the patients for 6 months (J. Am. Acad. Dermatol. 2014;70:1045-9). Patients in both groups achieved a similar rate of complete response (54% in the atenolol group, compared with 60% in the propranolol group) and there were no significant adverse effects in either group. “There was rebound growth in 26% of patients once the medication was withdrawn,” said Dr. Lam of the department of pediatrics at British Columbia Children’s Hospital, Vancouver, who was not affiliated with the study. “Initially there was more rebound growth in the propranolol group versus the atenolol group (four vs. two cases, respectively), but that was not significant.”
Another study compared oral nadolol with propranolol in patients aged 1-12 months: 10 on nadolol and 9 matched controls on propranolol (Br. J. Dermatol; 2013:168:222-4). After 24 weeks of treatment patients in the nadolol group had statistically superior lesion shrinkage, compared with those in the propranolol group (P less than .0001). Dr. Lam described nadolol as a nonselective beta-blocker that “has no intrinsic sympathomimetic activity, little myocardial depressant activity, and does not cross the blood-brain barrier. So theoretically, you have less sleep disturbance than with propranolol. Practically, it’s easier dosing than propranolol. It’s 2 mg/kg per day and you divide that b.i.d. The solution is 10 mg/mL.”
Researchers also are studying topical application of beta-blockers for hemangiomas and other skin conditions, Dr. Lam noted. A pilot study of six patients with infantile hemangiomas on the face and neck demonstrated that twice-daily topical administration of timolol maleate 0.5% was safe and effective (Arch. Dermatol. 2010;146:564-5). Dr. Lam characterized timolol as “a hydrophilic molecule that permeates poorly across intact skin, but doesn’t work great for deep hemangiomas. It’s a good choice for superficial hemangiomas, and anyone can use this.”
A larger, retrospective, multicenter cohort study of timolol maleate 0.5% or 0.1% gel-forming solution for infantile hemangiomas also demonstrated positive results (Pediatr. Dermatol. 2012; 29:28-31). In that study, 72 of 73 treated patients improved, with better response seen in those who received the 0.5% solution.
In another pilot study, Chinese researchers assessed the efficacy of fractional carbon dioxide laser–assisted drug delivery of topical timolol solution for the treatment of deep infantile hemangioma (Pediatr. Dermatol. 2014;31:286-91). The regimen consisted of one pass without overlap delivered from a 10,600-nm fractional CO2 laser to a .12-mm spot size with a single pulse of 25-30 mJ. The researchers rated the results as “excellent” in 44% of patients. “good” in 44%, and “moderate” in 11%. Plasma timolol levels were detectable in all patients.
Additional data from an unrelated case series found that use of topical timolol successfully treated pediatric pyogenic granulomas (Pediatric Dermatol. 2014; 31:203-7). The agent “doesn’t work as fast as with hemangiomas, but it works as well, which is great because most pyogenic granulomas in young children are on the face or neck area,” Dr. Lam noted. “Some took up to 6 months to respond, but all patients in this series had no further bleeding. That’s usually the main concern for families.”
Current evidence suggests that timolol does not work well for port wine stains and other vascular malformations. To date, it has shown mixed results for other vascular tumors such as kaposiform hemangioendothelioma, tufted angioma, and Kasabach-Merritt phenomenon.
Dr. Lam disclosed that he is a member of the scientific advisory board for Johnson & Johnson, and is a speaker for the company. He also is a member of the Eczema Society of Canada’s Board of Directors.
VANCOUVER, B.C. – Propranolol may have revolutionized the treatment of infantile hemangiomas over the past decade, but other beta-blockers are making inroads in the treatment of these and other pediatric dermatologic disorders.
One such beta-blocker is atenolol, a hydrophilic cardioselective agent that acts principally on beta1-adrenergic receptors, “and theoretically has less chance of hypoglycemia and bronchoconstriction,” Dr. Joseph M. Lam said at the annual meeting of the Pacific Dermatologic Association. Atenolol does not cross the blood-brain barrier and features once-daily dosing, he noted.
A recent noninferiority trial in 23 patients with infantile hemangiomas compared atenolol 1 mg/kg/day with propranolol 2 mg/kg/day, and followed the patients for 6 months (J. Am. Acad. Dermatol. 2014;70:1045-9). Patients in both groups achieved a similar rate of complete response (54% in the atenolol group, compared with 60% in the propranolol group) and there were no significant adverse effects in either group. “There was rebound growth in 26% of patients once the medication was withdrawn,” said Dr. Lam of the department of pediatrics at British Columbia Children’s Hospital, Vancouver, who was not affiliated with the study. “Initially there was more rebound growth in the propranolol group versus the atenolol group (four vs. two cases, respectively), but that was not significant.”
Another study compared oral nadolol with propranolol in patients aged 1-12 months: 10 on nadolol and 9 matched controls on propranolol (Br. J. Dermatol; 2013:168:222-4). After 24 weeks of treatment patients in the nadolol group had statistically superior lesion shrinkage, compared with those in the propranolol group (P less than .0001). Dr. Lam described nadolol as a nonselective beta-blocker that “has no intrinsic sympathomimetic activity, little myocardial depressant activity, and does not cross the blood-brain barrier. So theoretically, you have less sleep disturbance than with propranolol. Practically, it’s easier dosing than propranolol. It’s 2 mg/kg per day and you divide that b.i.d. The solution is 10 mg/mL.”
Researchers also are studying topical application of beta-blockers for hemangiomas and other skin conditions, Dr. Lam noted. A pilot study of six patients with infantile hemangiomas on the face and neck demonstrated that twice-daily topical administration of timolol maleate 0.5% was safe and effective (Arch. Dermatol. 2010;146:564-5). Dr. Lam characterized timolol as “a hydrophilic molecule that permeates poorly across intact skin, but doesn’t work great for deep hemangiomas. It’s a good choice for superficial hemangiomas, and anyone can use this.”
A larger, retrospective, multicenter cohort study of timolol maleate 0.5% or 0.1% gel-forming solution for infantile hemangiomas also demonstrated positive results (Pediatr. Dermatol. 2012; 29:28-31). In that study, 72 of 73 treated patients improved, with better response seen in those who received the 0.5% solution.
In another pilot study, Chinese researchers assessed the efficacy of fractional carbon dioxide laser–assisted drug delivery of topical timolol solution for the treatment of deep infantile hemangioma (Pediatr. Dermatol. 2014;31:286-91). The regimen consisted of one pass without overlap delivered from a 10,600-nm fractional CO2 laser to a .12-mm spot size with a single pulse of 25-30 mJ. The researchers rated the results as “excellent” in 44% of patients. “good” in 44%, and “moderate” in 11%. Plasma timolol levels were detectable in all patients.
Additional data from an unrelated case series found that use of topical timolol successfully treated pediatric pyogenic granulomas (Pediatric Dermatol. 2014; 31:203-7). The agent “doesn’t work as fast as with hemangiomas, but it works as well, which is great because most pyogenic granulomas in young children are on the face or neck area,” Dr. Lam noted. “Some took up to 6 months to respond, but all patients in this series had no further bleeding. That’s usually the main concern for families.”
Current evidence suggests that timolol does not work well for port wine stains and other vascular malformations. To date, it has shown mixed results for other vascular tumors such as kaposiform hemangioendothelioma, tufted angioma, and Kasabach-Merritt phenomenon.
Dr. Lam disclosed that he is a member of the scientific advisory board for Johnson & Johnson, and is a speaker for the company. He also is a member of the Eczema Society of Canada’s Board of Directors.
VANCOUVER, B.C. – Propranolol may have revolutionized the treatment of infantile hemangiomas over the past decade, but other beta-blockers are making inroads in the treatment of these and other pediatric dermatologic disorders.
One such beta-blocker is atenolol, a hydrophilic cardioselective agent that acts principally on beta1-adrenergic receptors, “and theoretically has less chance of hypoglycemia and bronchoconstriction,” Dr. Joseph M. Lam said at the annual meeting of the Pacific Dermatologic Association. Atenolol does not cross the blood-brain barrier and features once-daily dosing, he noted.
A recent noninferiority trial in 23 patients with infantile hemangiomas compared atenolol 1 mg/kg/day with propranolol 2 mg/kg/day, and followed the patients for 6 months (J. Am. Acad. Dermatol. 2014;70:1045-9). Patients in both groups achieved a similar rate of complete response (54% in the atenolol group, compared with 60% in the propranolol group) and there were no significant adverse effects in either group. “There was rebound growth in 26% of patients once the medication was withdrawn,” said Dr. Lam of the department of pediatrics at British Columbia Children’s Hospital, Vancouver, who was not affiliated with the study. “Initially there was more rebound growth in the propranolol group versus the atenolol group (four vs. two cases, respectively), but that was not significant.”
Another study compared oral nadolol with propranolol in patients aged 1-12 months: 10 on nadolol and 9 matched controls on propranolol (Br. J. Dermatol; 2013:168:222-4). After 24 weeks of treatment patients in the nadolol group had statistically superior lesion shrinkage, compared with those in the propranolol group (P less than .0001). Dr. Lam described nadolol as a nonselective beta-blocker that “has no intrinsic sympathomimetic activity, little myocardial depressant activity, and does not cross the blood-brain barrier. So theoretically, you have less sleep disturbance than with propranolol. Practically, it’s easier dosing than propranolol. It’s 2 mg/kg per day and you divide that b.i.d. The solution is 10 mg/mL.”
Researchers also are studying topical application of beta-blockers for hemangiomas and other skin conditions, Dr. Lam noted. A pilot study of six patients with infantile hemangiomas on the face and neck demonstrated that twice-daily topical administration of timolol maleate 0.5% was safe and effective (Arch. Dermatol. 2010;146:564-5). Dr. Lam characterized timolol as “a hydrophilic molecule that permeates poorly across intact skin, but doesn’t work great for deep hemangiomas. It’s a good choice for superficial hemangiomas, and anyone can use this.”
A larger, retrospective, multicenter cohort study of timolol maleate 0.5% or 0.1% gel-forming solution for infantile hemangiomas also demonstrated positive results (Pediatr. Dermatol. 2012; 29:28-31). In that study, 72 of 73 treated patients improved, with better response seen in those who received the 0.5% solution.
In another pilot study, Chinese researchers assessed the efficacy of fractional carbon dioxide laser–assisted drug delivery of topical timolol solution for the treatment of deep infantile hemangioma (Pediatr. Dermatol. 2014;31:286-91). The regimen consisted of one pass without overlap delivered from a 10,600-nm fractional CO2 laser to a .12-mm spot size with a single pulse of 25-30 mJ. The researchers rated the results as “excellent” in 44% of patients. “good” in 44%, and “moderate” in 11%. Plasma timolol levels were detectable in all patients.
Additional data from an unrelated case series found that use of topical timolol successfully treated pediatric pyogenic granulomas (Pediatric Dermatol. 2014; 31:203-7). The agent “doesn’t work as fast as with hemangiomas, but it works as well, which is great because most pyogenic granulomas in young children are on the face or neck area,” Dr. Lam noted. “Some took up to 6 months to respond, but all patients in this series had no further bleeding. That’s usually the main concern for families.”
Current evidence suggests that timolol does not work well for port wine stains and other vascular malformations. To date, it has shown mixed results for other vascular tumors such as kaposiform hemangioendothelioma, tufted angioma, and Kasabach-Merritt phenomenon.
Dr. Lam disclosed that he is a member of the scientific advisory board for Johnson & Johnson, and is a speaker for the company. He also is a member of the Eczema Society of Canada’s Board of Directors.
Fatigue checks assist in preventing wrong-site surgery
EDINBURGH – It’s 4 o’clock in the afternoon on a long Mohs surgery day, and you’ve got another patient with innumerable stages plus reconstructions to do.
Performing a fatigue check that gives nursing and biomedical staff the opportunity to admit they’re too tired may be the best next step to prevent wrong-site surgery, Dr. Colin Fleming, president of the British Society for Dermatological Surgery (BSDS), posited at the 15th World Congress on Cancers of the Skin.
“When we introduced this, it gave great power to the staff, whom we as doctors were asking to work hard on our behalf,” he said. “It’s been a really valuable tool in increasing safety at the end of a long operating day.”
The fatigue check is separate from the oft-recommended preprocedural surgical pause. Though helpful, research has shown that the surgical pause failed to prevent wrong-site surgery in 10% to a third of cases, said Dr. Fleming, a consultant dermatologist and Mohs surgeon at Ninewells Hospital, Dundee, Scotland.
A study on the usefulness of proposed methods for correct biopsy site identification in cutaneous surgery with the Delphi consensus method was recently published (JAMA Dermatol. 2014;150:550-8), but “I’m not convinced it really tells us anything,” he remarked.
Dr. Fleming suggested coupling the surgical pause with a site check, and he emphasized the value of good quality preoperative photographs of the index lesion. Today’s ubiquitous “selfie” may even have a role, as patients themselves often misidentify the biopsy site.
In addition, dermatology departments should have a protocol incorporating a variety of safety features, he said.
However, only 54% of surgeons said they have a written protocol in place to identify the correct surgical site, based on data from a recent survey of BSDS members conducted by Dr. Fleming and his colleagues.
More than a half of respondents (60/114) acknowledged it was “sometimes” difficult to locate the surgical site, with 47 respondents saying it was difficult to locate the surgical site in 1%-10% of cases and 13 admitting it was difficult to do so in 10%-25% of cases.
The face, scalp, and back were reported as the most challenging areas in which to locate the exact surgical site, Dr. Fleming said at the meeting, sponsored by the Skin Cancer Foundation.
When asked what steps they take in their local written protocols to avoid wrong site surgery, 82 respondents said they check with the patient, 66 use drawings/templates with the site marked, 50 use a mirror or photographs, and 39 respondents double-check with a colleague.
Overall, slightly less than 40% of respondents recalled having no patient in the past 5 years who underwent wrong-site surgery. Approximately 28% had 1 wrong-site surgery patient, 28% had 2-3 patients, 3% had 4-5 patients, and 1% had more than 10 such patients.
“Wrong-site surgery in U.K. dermatology departments is infrequent,” Dr. Fleming concluded, adding that only a small proportion led to formal complaints or a medicolegal case.
The response rate to the web-based survey was 37.5% (115/306 members); 32 respondents were Mohs surgeons, 75 were non-Mohs surgeons, 68 worked at a teaching hospital, and 110 had a regular surgical list of between 500 and 2,000 procedures per year that they were responsible for or did themselves.
EDINBURGH – It’s 4 o’clock in the afternoon on a long Mohs surgery day, and you’ve got another patient with innumerable stages plus reconstructions to do.
Performing a fatigue check that gives nursing and biomedical staff the opportunity to admit they’re too tired may be the best next step to prevent wrong-site surgery, Dr. Colin Fleming, president of the British Society for Dermatological Surgery (BSDS), posited at the 15th World Congress on Cancers of the Skin.
“When we introduced this, it gave great power to the staff, whom we as doctors were asking to work hard on our behalf,” he said. “It’s been a really valuable tool in increasing safety at the end of a long operating day.”
The fatigue check is separate from the oft-recommended preprocedural surgical pause. Though helpful, research has shown that the surgical pause failed to prevent wrong-site surgery in 10% to a third of cases, said Dr. Fleming, a consultant dermatologist and Mohs surgeon at Ninewells Hospital, Dundee, Scotland.
A study on the usefulness of proposed methods for correct biopsy site identification in cutaneous surgery with the Delphi consensus method was recently published (JAMA Dermatol. 2014;150:550-8), but “I’m not convinced it really tells us anything,” he remarked.
Dr. Fleming suggested coupling the surgical pause with a site check, and he emphasized the value of good quality preoperative photographs of the index lesion. Today’s ubiquitous “selfie” may even have a role, as patients themselves often misidentify the biopsy site.
In addition, dermatology departments should have a protocol incorporating a variety of safety features, he said.
However, only 54% of surgeons said they have a written protocol in place to identify the correct surgical site, based on data from a recent survey of BSDS members conducted by Dr. Fleming and his colleagues.
More than a half of respondents (60/114) acknowledged it was “sometimes” difficult to locate the surgical site, with 47 respondents saying it was difficult to locate the surgical site in 1%-10% of cases and 13 admitting it was difficult to do so in 10%-25% of cases.
The face, scalp, and back were reported as the most challenging areas in which to locate the exact surgical site, Dr. Fleming said at the meeting, sponsored by the Skin Cancer Foundation.
When asked what steps they take in their local written protocols to avoid wrong site surgery, 82 respondents said they check with the patient, 66 use drawings/templates with the site marked, 50 use a mirror or photographs, and 39 respondents double-check with a colleague.
Overall, slightly less than 40% of respondents recalled having no patient in the past 5 years who underwent wrong-site surgery. Approximately 28% had 1 wrong-site surgery patient, 28% had 2-3 patients, 3% had 4-5 patients, and 1% had more than 10 such patients.
“Wrong-site surgery in U.K. dermatology departments is infrequent,” Dr. Fleming concluded, adding that only a small proportion led to formal complaints or a medicolegal case.
The response rate to the web-based survey was 37.5% (115/306 members); 32 respondents were Mohs surgeons, 75 were non-Mohs surgeons, 68 worked at a teaching hospital, and 110 had a regular surgical list of between 500 and 2,000 procedures per year that they were responsible for or did themselves.
EDINBURGH – It’s 4 o’clock in the afternoon on a long Mohs surgery day, and you’ve got another patient with innumerable stages plus reconstructions to do.
Performing a fatigue check that gives nursing and biomedical staff the opportunity to admit they’re too tired may be the best next step to prevent wrong-site surgery, Dr. Colin Fleming, president of the British Society for Dermatological Surgery (BSDS), posited at the 15th World Congress on Cancers of the Skin.
“When we introduced this, it gave great power to the staff, whom we as doctors were asking to work hard on our behalf,” he said. “It’s been a really valuable tool in increasing safety at the end of a long operating day.”
The fatigue check is separate from the oft-recommended preprocedural surgical pause. Though helpful, research has shown that the surgical pause failed to prevent wrong-site surgery in 10% to a third of cases, said Dr. Fleming, a consultant dermatologist and Mohs surgeon at Ninewells Hospital, Dundee, Scotland.
A study on the usefulness of proposed methods for correct biopsy site identification in cutaneous surgery with the Delphi consensus method was recently published (JAMA Dermatol. 2014;150:550-8), but “I’m not convinced it really tells us anything,” he remarked.
Dr. Fleming suggested coupling the surgical pause with a site check, and he emphasized the value of good quality preoperative photographs of the index lesion. Today’s ubiquitous “selfie” may even have a role, as patients themselves often misidentify the biopsy site.
In addition, dermatology departments should have a protocol incorporating a variety of safety features, he said.
However, only 54% of surgeons said they have a written protocol in place to identify the correct surgical site, based on data from a recent survey of BSDS members conducted by Dr. Fleming and his colleagues.
More than a half of respondents (60/114) acknowledged it was “sometimes” difficult to locate the surgical site, with 47 respondents saying it was difficult to locate the surgical site in 1%-10% of cases and 13 admitting it was difficult to do so in 10%-25% of cases.
The face, scalp, and back were reported as the most challenging areas in which to locate the exact surgical site, Dr. Fleming said at the meeting, sponsored by the Skin Cancer Foundation.
When asked what steps they take in their local written protocols to avoid wrong site surgery, 82 respondents said they check with the patient, 66 use drawings/templates with the site marked, 50 use a mirror or photographs, and 39 respondents double-check with a colleague.
Overall, slightly less than 40% of respondents recalled having no patient in the past 5 years who underwent wrong-site surgery. Approximately 28% had 1 wrong-site surgery patient, 28% had 2-3 patients, 3% had 4-5 patients, and 1% had more than 10 such patients.
“Wrong-site surgery in U.K. dermatology departments is infrequent,” Dr. Fleming concluded, adding that only a small proportion led to formal complaints or a medicolegal case.
The response rate to the web-based survey was 37.5% (115/306 members); 32 respondents were Mohs surgeons, 75 were non-Mohs surgeons, 68 worked at a teaching hospital, and 110 had a regular surgical list of between 500 and 2,000 procedures per year that they were responsible for or did themselves.
AT THE WCCS 2014
Applications of Lasers in Medical Dermatology
The use of lasers in dermatology has had a major impact on the treatment of many dermatologic conditions. In this column practical applications of lasers in medical dermatology will be discussed to give dermatology residents a broad overview of both established indications and the reasoning behind the usage of lasers in treating these skin conditions. The applications for lasers in aesthetic dermatology are numerous and are constantly being refined and developed; they have been discussed extensively in the literature. Given the vast variety of uses of lasers in dermatology today, a comprehensive review of this topic would likely span several volumes. This article will focus on recent evidence regarding the use of lasers in medical dermatology, specifically laser treatment of selected common dermatoses and cutaneous malignancies.
Laser Treatment of Skin Diseases
Many common dermatoses seen in the dermatologist’s office (eg, discoid lupus erythematosus [DLE], morphea, alopecia) already have an established therapeutic ladder, with most patients responding to either first- or second-line therapies; however, a number of patients present with refractory disease that can be difficult to treat due to either treatment resistance or other contraindications to therapy. With the advent and development of modern lasers, we are now able to target many of these conditions and provide a viable safe treatment option for these patients. Although many physicians may be familiar with the use of the excimer laser in the treatment of psoriasis,1 a long-standing and well-accepted treatment modality for this condition, many novel applications for different types of lasers have been developed.
First, it is important to consider what a laser is able to accomplish to modulate the skin. With ablative lasers such as the CO2 laser, it is possible to destroy superficial layers of the skin (ie, the epidermis). It would stand to reason that this approach would be ideal for treating epidermal processes such as viral warts; in fact, this modality has been used for this indication for more than 3 decades, with the earliest references coming from the podiatric and urologic literature.2,3 Despite conflicting reports of the risk for human papillomavirus aerosolization and subsequent contamination of the treatment area,4,5 CO2 laser therapy has been advocated as a nonsurgical approach to difficult-to-treat cases of viral warts.
On the other hand, the pulsed dye laser (PDL) can target blood vessels because the wavelength corresponds to the absorption spectrum of hemoglobin and penetrates to the level of the dermis, while the pulse duration can be set to be shorter than the thermal relaxation time of a small cutaneous blood vessel.6 In clinical practice, the PDL has been used for the treatment of vascular lesions including hemangiomas, nevus flammeus, and other vascular proliferations.7-9 However, the PDL also can be used to target the vessels in cutaneous inflammatory diseases that feature vascular dilation and/or perivascular inflammation as a prominent feature.
Discoid lupus erythematosus is a form of chronic cutaneous lupus erythematosus that may be difficult to treat, with recalcitrant lesions displaying continued inflammation leading to chronic scarring and dyspigmentation. A small study (N=12) presented the efficacy of the PDL in the treatment of DLE lesions, suggesting that it has good efficacy in treating recalcitrant lesions with significant reduction in the cutaneous lupus erythematosus disease area and severity index after 6 weeks of treatment and 6 weeks of follow-up (P<.0001) with decreased erythema and scaling.10 It is important to note, however, that scarring, dyspigmentation, and atrophy were not affected, which suggests that early intervention may be optimal to prevent development of these sequelae. More interestingly, a more recent study expounded on this idea and attempted to examine pathophysiologic mechanisms behind this observed improvement. Evaluation of biopsy specimens before and after treatment and immunohistochemistry revealed that PDL treatment of cutaneous DLE lesions led to a decrease in vascular endothelial proteins—intercellular adhesion molecule 1 and vascular cell adhesion molecule 1—with a coincident reduction in the dermal lymphocytic infiltrate in treated lesions.11 These results offer a somewhat satisfying view on the correlation between the theory and basic science of laser therapy and the subsequent clinical benefits afforded by laser treatment. A case series provided further evidence that PDL or intense pulsed light can ameliorate the cutaneous lesions of DLE in 16 patients in whom all other treatments had failed.12
Several other inflammatory dermatoses can be treated with PDL, though the evidence for most of these conditions is sporadic at best, consisting mostly of case reports and a few case series. Granuloma faciale is one such condition, with evidence of efficacy of the PDL dating back as far as 1999,13 though a more recent case series of 4 patients only showed response in 2 patients.14 Because granuloma faciale features vasculitis as a prominent feature in its pathology, targeting the blood vessels may be helpful, but it is important to remember that there is a complex interplay between multiple factors. For example, treatment with typical fluences used in dermatology can be proinflammatory, leading to tissue damage, necrosis, and posttreatment erythema. However, low-level laser therapy (LLLT) has been shown to downregulate proinflammatory mediators.15 Additionally, the presence of a large burden of inflammatory cells also may alter the effectiveness of the laser. Several case reports also the show effectiveness of both PDL and the CO2 laser in treating lesions of cutaneous sarcoidosis, especially lupus pernio.16-19 Of these 2 modalities, the use of the CO2 laser for effective remodeling of lupus pernio may be more intuitive; however, it is still important to note that the mechanism of action of several of these laser modalities is unclear with regard to the clinical benefit shown. Morphea and scleroderma also have been treated with laser therapy. It is essential to understand that in many cases, laser therapy may be targeted to treat the precise cutaneous manifestations of disease in each individual patient (eg, CO2 laser to treat disabling contractures and calcinosis cutis,20,21 PDL to treat telangiectases related to morphea22). Again, the most critical consideration is that the treatment modality should align with the cutaneous lesion being targeted.
A relatively recent development in the use of lasers has been LLLT, which refers to the use of lasers below levels where they would cause any thermal effects, thereby limiting tissue damage. Although the technology has existed for decades, there has been a recent flurry of reports extolling the many benefits of LLLT; however, the true physiologic effects of LLLT have yet to be determined, with many studies trying to elucidate its numerous effects on various signaling pathways, cell proliferation, and cellular respiration.23-26 Upon reviewing the literature, the list of cutaneous conditions that are being treated with LLLT is vast, spanning acne, vitiligo, wounds, burns, psoriasis, and alopecia, among others.15 It is important to consider that the definition of LLLT in the literature is rather broad with a wide range of wavelengths, fluences, and power densities. As such, the specific laser settings and protocols may vary considerably among different practitioners and therefore the treatment results also may vary. Nevertheless, many studies have hinted at promising results in the use of LLLT in conditions that may have previously been extremely difficult to treat (eg, alopecia). Earlier trials had demonstrated a faster resolution time in patients with alopecia areata when LLLT was added to a topical regimen27; however, the improvement was modest and lesions tended to improve with or without LLLT. Perhaps more compelling is the use of LLLT in treating androgenetic alopecia, a condition for which a satisfying facile treatment would truly carry great impact. Although physicians should be cautious of studies regarding LLLT and hair regrowth that are conducted by groups who may stand to benefit from producing such a device, the results are nonetheless notable, if only for the relative paucity of other therapeutic approaches toward this condition.28,29 A randomized, double-blind, controlled, multicenter trial showed significant improvements in median hair thickness and density with LLLT (P=.01 and P=.003, respectively), though global appearance did not change significantly.30
Laser Treatment of Skin Cancer
Lasers also have been used to treat cutaneous malignancies. Although they may be powerful in the treatment of these conditions, this treatment approach must be used with caution. As with any superficial treatment modality for skin cancer, it is difficult to ascertain if a lesion has been completely treated without any residual cancer cells, and therein lies the main caveat of laser treatment. With the use of a modality that causes a cutaneous response that may mask any underlying process, it is important to ensure that there is a reasonable degree of certainty that this treatment can effectively remove a cancerous lesion in its entirety while avoiding the theoretical risk that disturbing underlying vasculature and/or lymphatics may be modulating the ability of a cancer to metastasize. Thankfully, current evidence does not suggest that there are any downsides to laser treatment for malignancies. Clinically, we know that basal cell carcinomas (BCCs) often feature prominent vasculature, with telangiectases being used as a clinical marker to suggest the diagnosis of a BCC. Capitalizing on this aspect of the clinical lesion, PDL has been used to treat BCCs in 2 small studies with a response rate of approximately 75% for small BCCs in both studies.31,32 A recent randomized controlled trial showed significant superiority of PDL as compared to the control (P<.0001) in treatment of BCC, with nearly 80% (44/56) of cases showing histologically proven complete remission at 6-month follow-up.33 Thus, we have some promising data that suggest PDL may be a viable treatment option in BCC, especially in areas that are difficult to treat surgically.
Additionally, a newer treatment approach for BCC capitalizes on the ability of confocal microscopy to provide a feasible, bedside imaging modality to identify tumor margins. Confocal microscopy has been used as a road map to identify where and how to apply the laser treatment, thus allowing for a higher likelihood of complete destruction of the tumor, at least in theory.34 Although the concept of using confocal microscopy to guide laser treatment of skin cancer has been shown in smaller proof-of-concept case series, it remains to be seen if it is not only an efficacious approach that may be widely adopted but also whether it is pragmatic to do so, as the equipment and expertise involved in using confocal microscopy is not trivial.
Finally, lasers also have been used in the treatment of mycosis fungoides (MF), or cutaneous T-cell lymphoma. It has been suggested that this modality is an excellent treatment option as a skin-directed therapy for stage IA or IB MFs limited to the acral surfaces or MF palmaris et plantaris.35 The reasoning behind this approach was the effectiveness of narrowband UVB for early-stage MF, with an excimer laser operating at a similar wavelength (308 nm) and offering similar therapeutic benefits while limiting adverse effects to surrounding skin.36 More recently, the excimer laser was applied to a small population of 6 patients, with 3 achieving complete response, 1 with partial response, 1 with stable disease, and 1 with progressive disease. The authors were careful to point out that the excimer laser should not be thought of as a replacement for narrowband UVB in early-stage MF but rather as an adjunctive treatment of specific targeted lesional areas.36
Conclusion
Lasers are an important part of the dermatologist’s treatment arsenal. Although much attention has been focused on laser treatment for aesthetic indications, it is important not to overlook the fact that lasers also can be useful in the treatment of refractory skin diseases, as a first-line treatment in some conditions such as vascular lesions, or as an adjunctive treatment modality. There is a great deal of exciting research that may lead to new indications and a better understanding of how to best use these powerful tools, and the outlook is bright for the use of lasers in dermatology.
1. Bonis B, Kemeny L, Dobozy A, et al. 308 nm UVB excimer laser for psoriasis. Lancet. 1997;350:1522.
2. Fuselier HA Jr, McBurney EI, Brannan W, et al. Treatment of condylomata acuminata with carbon dioxide laser. Urology. 1980;15:265-266.
3. Mueller TJ, Carlson BA, Lindy MP. The use of the carbon dioxide surgical laser for the treatment of verrucae. J Am Podiatry Assoc. 1980;70:136-141.
4. Weyandt GH, Tollmann F, Kristen P, et al. Low risk of contamination with human papilloma virus during treatment of condylomata acuminata with multilayer argon plasma coagulation and CO2 laser ablation. Arch Dermatol Res. 2011;303:141-144.
5. Ferenczy A, Bergeron C, Richart RM. Human papillomavirus DNA in CO2 laser-generated plume of smoke and its consequences to the surgeon. Obstet Gynecol. 1990;75:114-118.
6. Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981:263-276.
7. Morelli JG, Tan OT, Garden J, et al. Tunable dye laser (577 nm) treatment of port wine stains. Lasers Surg Med. 1986;6:94-99.
8. Reyes BA, Geronemus R. Treatment of port-wine stains during childhood with the flashlamp-pumped pulsed dye laser. J Am Acad Dermatol. 1990;23:1142-1148.
9. Ashinoff R, Geronemus RG. Capillary hemangiomas and treatment with the flash lamp-pumped pulsed dye laser. Arch Dermatol. 1991;127:202-205.
10. Erceg A, Bovenschen HJ, van de Kerkhof PC, et al. Efficacy and safety of pulsed dye laser treatment for cutaneous discoid lupus erythematosus. J Am Acad Dermatol. 2009;60:626-632.
11. Diez MT, Boixeda P, Moreno C, et al. Histopathology and immunohistochemistry of cutaneous lupus erythematosus after pulsed dye laser treatment. Dermatol Surg. 2011;37:971-981.
12. Ekback MP, Troilius A. Laser therapy for refractory discoid lupus erythematosus when everything else has failed. J Cosmet Laser Ther. 2013;15:260-265.
13. Welsh JH, Schroeder TL, Levy ML. Granuloma faciale in a child successfully treated with the pulsed dye laser. J Am Acad Dermatol. 1999;41:351-353.
14. Cheung ST, Lanigan SW. Granuloma faciale treated with the pulsed-dye laser: a case series. Clin Exp Dermatol. 2005;30:373-375.
15. Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32:41-52.
16. Roos S, Raulin C, Ockenfels HM, et al. Successful treatment of cutaneous sarcoidosis lesions with the flashlamp pumped pulsed dye laser: a case report. Dermatol Surg. 2009;35:1139-1140.
17. Cliff S, Felix RH, Singh L, et al. The successful treatment of lupus pernio with the flashlamp pulsed dye laser. J Cutan Laser Ther. 1999;1:49-52.
18. O’Donoghue NB, Barlow RJ. Laser remodelling of nodular nasal lupus pernio. Clin Exp Dermatol. 2006;31:27-29.
19. Young HS, Chalmers RJ, Griffiths CE, et al. CO2 laser vaporization for disfiguring lupus pernio. J Cosmet Laser Ther. 2002;4:87-90.
20. Kineston D, Kwan JM, Uebelhoer NS, et al. Use of a fractional ablative 10.6-mum carbon dioxide laser in the treatment of a morphea-related contracture. Arch Dermatol. 2011;147:1148-1150.
21. Chamberlain AJ, Walker NP. Successful palliation and significant remission of cutaneous calcinosis in CREST syndrome with carbon dioxide laser. Dermatol Surg. 2003;29:968-970.
22. Ciatti S, Varga J, Greenbaum SS. The 585 nm flashlamp-pumped pulsed dye laser for the treatment of telangiectases in patients with scleroderma. J Am Acad Dermatol. 1996;35:487-488.
23. Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23:355-361.
24. Greco M, Guida G, Perlino E, et al. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem Biophys Res Commun. 1989;163:1428-1434.
25. Karu TI, Pyatibrat LV, Kalendo GS. Photobiological modulation of cell attachment via cytochrome c oxidase. Photochem Photobiol Sci. 2004;3:211-216.
26. Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761-4771.
27. Yamazaki M, Miura Y, Tsuboi R, et al. Linear polarized infrared irradiation using Super Lizer is an effective treatment for multiple-type alopecia areata. Int J Dermatol. 2003;42:738-740.
28. Leavitt M, Charles G, Heyman E, et al. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: a randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29:283-292.
29. Munck A, Gavazzoni MF, Trueb RM. Use of low-level laser therapy as monotherapy or concomitant therapy for male and female androgenetic alopecia. Int J Trichology. 2014;6:45-49.
30. Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
31. Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series [published online ahead of print December 13, 2012]. J Skin Cancer. 2012;2012:286480.
32. Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
33. Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial [published online ahead of print July 12, 2014]. Br J Dermatol. doi:10.1111/bjd.13266.
34. Chen CS, Sierra H, Cordova M, et al. Confocal microscopy-guided laser ablation for superficial and early nodular Basal cell carcinoma: a promising surgical alternative for superficial skin cancers. JAMA Dermatol. 2014;150:994-998.
35. Jin SP, Jeon YK, Cho KH, et al. Excimer laser therapy (308 nm) for mycosis fungoides palmaris et plantaris: a skin-directed and anatomically feasible treatment. Br J Dermatol. 2010;163:651-653.
36. Deaver D, Cauthen A, Cohen G, et al. Excimer laser in the treatment of mycosis fungoides. J Am Acad Dermatol. 2014;70:1058-1060.
The use of lasers in dermatology has had a major impact on the treatment of many dermatologic conditions. In this column practical applications of lasers in medical dermatology will be discussed to give dermatology residents a broad overview of both established indications and the reasoning behind the usage of lasers in treating these skin conditions. The applications for lasers in aesthetic dermatology are numerous and are constantly being refined and developed; they have been discussed extensively in the literature. Given the vast variety of uses of lasers in dermatology today, a comprehensive review of this topic would likely span several volumes. This article will focus on recent evidence regarding the use of lasers in medical dermatology, specifically laser treatment of selected common dermatoses and cutaneous malignancies.
Laser Treatment of Skin Diseases
Many common dermatoses seen in the dermatologist’s office (eg, discoid lupus erythematosus [DLE], morphea, alopecia) already have an established therapeutic ladder, with most patients responding to either first- or second-line therapies; however, a number of patients present with refractory disease that can be difficult to treat due to either treatment resistance or other contraindications to therapy. With the advent and development of modern lasers, we are now able to target many of these conditions and provide a viable safe treatment option for these patients. Although many physicians may be familiar with the use of the excimer laser in the treatment of psoriasis,1 a long-standing and well-accepted treatment modality for this condition, many novel applications for different types of lasers have been developed.
First, it is important to consider what a laser is able to accomplish to modulate the skin. With ablative lasers such as the CO2 laser, it is possible to destroy superficial layers of the skin (ie, the epidermis). It would stand to reason that this approach would be ideal for treating epidermal processes such as viral warts; in fact, this modality has been used for this indication for more than 3 decades, with the earliest references coming from the podiatric and urologic literature.2,3 Despite conflicting reports of the risk for human papillomavirus aerosolization and subsequent contamination of the treatment area,4,5 CO2 laser therapy has been advocated as a nonsurgical approach to difficult-to-treat cases of viral warts.
On the other hand, the pulsed dye laser (PDL) can target blood vessels because the wavelength corresponds to the absorption spectrum of hemoglobin and penetrates to the level of the dermis, while the pulse duration can be set to be shorter than the thermal relaxation time of a small cutaneous blood vessel.6 In clinical practice, the PDL has been used for the treatment of vascular lesions including hemangiomas, nevus flammeus, and other vascular proliferations.7-9 However, the PDL also can be used to target the vessels in cutaneous inflammatory diseases that feature vascular dilation and/or perivascular inflammation as a prominent feature.
Discoid lupus erythematosus is a form of chronic cutaneous lupus erythematosus that may be difficult to treat, with recalcitrant lesions displaying continued inflammation leading to chronic scarring and dyspigmentation. A small study (N=12) presented the efficacy of the PDL in the treatment of DLE lesions, suggesting that it has good efficacy in treating recalcitrant lesions with significant reduction in the cutaneous lupus erythematosus disease area and severity index after 6 weeks of treatment and 6 weeks of follow-up (P<.0001) with decreased erythema and scaling.10 It is important to note, however, that scarring, dyspigmentation, and atrophy were not affected, which suggests that early intervention may be optimal to prevent development of these sequelae. More interestingly, a more recent study expounded on this idea and attempted to examine pathophysiologic mechanisms behind this observed improvement. Evaluation of biopsy specimens before and after treatment and immunohistochemistry revealed that PDL treatment of cutaneous DLE lesions led to a decrease in vascular endothelial proteins—intercellular adhesion molecule 1 and vascular cell adhesion molecule 1—with a coincident reduction in the dermal lymphocytic infiltrate in treated lesions.11 These results offer a somewhat satisfying view on the correlation between the theory and basic science of laser therapy and the subsequent clinical benefits afforded by laser treatment. A case series provided further evidence that PDL or intense pulsed light can ameliorate the cutaneous lesions of DLE in 16 patients in whom all other treatments had failed.12
Several other inflammatory dermatoses can be treated with PDL, though the evidence for most of these conditions is sporadic at best, consisting mostly of case reports and a few case series. Granuloma faciale is one such condition, with evidence of efficacy of the PDL dating back as far as 1999,13 though a more recent case series of 4 patients only showed response in 2 patients.14 Because granuloma faciale features vasculitis as a prominent feature in its pathology, targeting the blood vessels may be helpful, but it is important to remember that there is a complex interplay between multiple factors. For example, treatment with typical fluences used in dermatology can be proinflammatory, leading to tissue damage, necrosis, and posttreatment erythema. However, low-level laser therapy (LLLT) has been shown to downregulate proinflammatory mediators.15 Additionally, the presence of a large burden of inflammatory cells also may alter the effectiveness of the laser. Several case reports also the show effectiveness of both PDL and the CO2 laser in treating lesions of cutaneous sarcoidosis, especially lupus pernio.16-19 Of these 2 modalities, the use of the CO2 laser for effective remodeling of lupus pernio may be more intuitive; however, it is still important to note that the mechanism of action of several of these laser modalities is unclear with regard to the clinical benefit shown. Morphea and scleroderma also have been treated with laser therapy. It is essential to understand that in many cases, laser therapy may be targeted to treat the precise cutaneous manifestations of disease in each individual patient (eg, CO2 laser to treat disabling contractures and calcinosis cutis,20,21 PDL to treat telangiectases related to morphea22). Again, the most critical consideration is that the treatment modality should align with the cutaneous lesion being targeted.
A relatively recent development in the use of lasers has been LLLT, which refers to the use of lasers below levels where they would cause any thermal effects, thereby limiting tissue damage. Although the technology has existed for decades, there has been a recent flurry of reports extolling the many benefits of LLLT; however, the true physiologic effects of LLLT have yet to be determined, with many studies trying to elucidate its numerous effects on various signaling pathways, cell proliferation, and cellular respiration.23-26 Upon reviewing the literature, the list of cutaneous conditions that are being treated with LLLT is vast, spanning acne, vitiligo, wounds, burns, psoriasis, and alopecia, among others.15 It is important to consider that the definition of LLLT in the literature is rather broad with a wide range of wavelengths, fluences, and power densities. As such, the specific laser settings and protocols may vary considerably among different practitioners and therefore the treatment results also may vary. Nevertheless, many studies have hinted at promising results in the use of LLLT in conditions that may have previously been extremely difficult to treat (eg, alopecia). Earlier trials had demonstrated a faster resolution time in patients with alopecia areata when LLLT was added to a topical regimen27; however, the improvement was modest and lesions tended to improve with or without LLLT. Perhaps more compelling is the use of LLLT in treating androgenetic alopecia, a condition for which a satisfying facile treatment would truly carry great impact. Although physicians should be cautious of studies regarding LLLT and hair regrowth that are conducted by groups who may stand to benefit from producing such a device, the results are nonetheless notable, if only for the relative paucity of other therapeutic approaches toward this condition.28,29 A randomized, double-blind, controlled, multicenter trial showed significant improvements in median hair thickness and density with LLLT (P=.01 and P=.003, respectively), though global appearance did not change significantly.30
Laser Treatment of Skin Cancer
Lasers also have been used to treat cutaneous malignancies. Although they may be powerful in the treatment of these conditions, this treatment approach must be used with caution. As with any superficial treatment modality for skin cancer, it is difficult to ascertain if a lesion has been completely treated without any residual cancer cells, and therein lies the main caveat of laser treatment. With the use of a modality that causes a cutaneous response that may mask any underlying process, it is important to ensure that there is a reasonable degree of certainty that this treatment can effectively remove a cancerous lesion in its entirety while avoiding the theoretical risk that disturbing underlying vasculature and/or lymphatics may be modulating the ability of a cancer to metastasize. Thankfully, current evidence does not suggest that there are any downsides to laser treatment for malignancies. Clinically, we know that basal cell carcinomas (BCCs) often feature prominent vasculature, with telangiectases being used as a clinical marker to suggest the diagnosis of a BCC. Capitalizing on this aspect of the clinical lesion, PDL has been used to treat BCCs in 2 small studies with a response rate of approximately 75% for small BCCs in both studies.31,32 A recent randomized controlled trial showed significant superiority of PDL as compared to the control (P<.0001) in treatment of BCC, with nearly 80% (44/56) of cases showing histologically proven complete remission at 6-month follow-up.33 Thus, we have some promising data that suggest PDL may be a viable treatment option in BCC, especially in areas that are difficult to treat surgically.
Additionally, a newer treatment approach for BCC capitalizes on the ability of confocal microscopy to provide a feasible, bedside imaging modality to identify tumor margins. Confocal microscopy has been used as a road map to identify where and how to apply the laser treatment, thus allowing for a higher likelihood of complete destruction of the tumor, at least in theory.34 Although the concept of using confocal microscopy to guide laser treatment of skin cancer has been shown in smaller proof-of-concept case series, it remains to be seen if it is not only an efficacious approach that may be widely adopted but also whether it is pragmatic to do so, as the equipment and expertise involved in using confocal microscopy is not trivial.
Finally, lasers also have been used in the treatment of mycosis fungoides (MF), or cutaneous T-cell lymphoma. It has been suggested that this modality is an excellent treatment option as a skin-directed therapy for stage IA or IB MFs limited to the acral surfaces or MF palmaris et plantaris.35 The reasoning behind this approach was the effectiveness of narrowband UVB for early-stage MF, with an excimer laser operating at a similar wavelength (308 nm) and offering similar therapeutic benefits while limiting adverse effects to surrounding skin.36 More recently, the excimer laser was applied to a small population of 6 patients, with 3 achieving complete response, 1 with partial response, 1 with stable disease, and 1 with progressive disease. The authors were careful to point out that the excimer laser should not be thought of as a replacement for narrowband UVB in early-stage MF but rather as an adjunctive treatment of specific targeted lesional areas.36
Conclusion
Lasers are an important part of the dermatologist’s treatment arsenal. Although much attention has been focused on laser treatment for aesthetic indications, it is important not to overlook the fact that lasers also can be useful in the treatment of refractory skin diseases, as a first-line treatment in some conditions such as vascular lesions, or as an adjunctive treatment modality. There is a great deal of exciting research that may lead to new indications and a better understanding of how to best use these powerful tools, and the outlook is bright for the use of lasers in dermatology.
The use of lasers in dermatology has had a major impact on the treatment of many dermatologic conditions. In this column practical applications of lasers in medical dermatology will be discussed to give dermatology residents a broad overview of both established indications and the reasoning behind the usage of lasers in treating these skin conditions. The applications for lasers in aesthetic dermatology are numerous and are constantly being refined and developed; they have been discussed extensively in the literature. Given the vast variety of uses of lasers in dermatology today, a comprehensive review of this topic would likely span several volumes. This article will focus on recent evidence regarding the use of lasers in medical dermatology, specifically laser treatment of selected common dermatoses and cutaneous malignancies.
Laser Treatment of Skin Diseases
Many common dermatoses seen in the dermatologist’s office (eg, discoid lupus erythematosus [DLE], morphea, alopecia) already have an established therapeutic ladder, with most patients responding to either first- or second-line therapies; however, a number of patients present with refractory disease that can be difficult to treat due to either treatment resistance or other contraindications to therapy. With the advent and development of modern lasers, we are now able to target many of these conditions and provide a viable safe treatment option for these patients. Although many physicians may be familiar with the use of the excimer laser in the treatment of psoriasis,1 a long-standing and well-accepted treatment modality for this condition, many novel applications for different types of lasers have been developed.
First, it is important to consider what a laser is able to accomplish to modulate the skin. With ablative lasers such as the CO2 laser, it is possible to destroy superficial layers of the skin (ie, the epidermis). It would stand to reason that this approach would be ideal for treating epidermal processes such as viral warts; in fact, this modality has been used for this indication for more than 3 decades, with the earliest references coming from the podiatric and urologic literature.2,3 Despite conflicting reports of the risk for human papillomavirus aerosolization and subsequent contamination of the treatment area,4,5 CO2 laser therapy has been advocated as a nonsurgical approach to difficult-to-treat cases of viral warts.
On the other hand, the pulsed dye laser (PDL) can target blood vessels because the wavelength corresponds to the absorption spectrum of hemoglobin and penetrates to the level of the dermis, while the pulse duration can be set to be shorter than the thermal relaxation time of a small cutaneous blood vessel.6 In clinical practice, the PDL has been used for the treatment of vascular lesions including hemangiomas, nevus flammeus, and other vascular proliferations.7-9 However, the PDL also can be used to target the vessels in cutaneous inflammatory diseases that feature vascular dilation and/or perivascular inflammation as a prominent feature.
Discoid lupus erythematosus is a form of chronic cutaneous lupus erythematosus that may be difficult to treat, with recalcitrant lesions displaying continued inflammation leading to chronic scarring and dyspigmentation. A small study (N=12) presented the efficacy of the PDL in the treatment of DLE lesions, suggesting that it has good efficacy in treating recalcitrant lesions with significant reduction in the cutaneous lupus erythematosus disease area and severity index after 6 weeks of treatment and 6 weeks of follow-up (P<.0001) with decreased erythema and scaling.10 It is important to note, however, that scarring, dyspigmentation, and atrophy were not affected, which suggests that early intervention may be optimal to prevent development of these sequelae. More interestingly, a more recent study expounded on this idea and attempted to examine pathophysiologic mechanisms behind this observed improvement. Evaluation of biopsy specimens before and after treatment and immunohistochemistry revealed that PDL treatment of cutaneous DLE lesions led to a decrease in vascular endothelial proteins—intercellular adhesion molecule 1 and vascular cell adhesion molecule 1—with a coincident reduction in the dermal lymphocytic infiltrate in treated lesions.11 These results offer a somewhat satisfying view on the correlation between the theory and basic science of laser therapy and the subsequent clinical benefits afforded by laser treatment. A case series provided further evidence that PDL or intense pulsed light can ameliorate the cutaneous lesions of DLE in 16 patients in whom all other treatments had failed.12
Several other inflammatory dermatoses can be treated with PDL, though the evidence for most of these conditions is sporadic at best, consisting mostly of case reports and a few case series. Granuloma faciale is one such condition, with evidence of efficacy of the PDL dating back as far as 1999,13 though a more recent case series of 4 patients only showed response in 2 patients.14 Because granuloma faciale features vasculitis as a prominent feature in its pathology, targeting the blood vessels may be helpful, but it is important to remember that there is a complex interplay between multiple factors. For example, treatment with typical fluences used in dermatology can be proinflammatory, leading to tissue damage, necrosis, and posttreatment erythema. However, low-level laser therapy (LLLT) has been shown to downregulate proinflammatory mediators.15 Additionally, the presence of a large burden of inflammatory cells also may alter the effectiveness of the laser. Several case reports also the show effectiveness of both PDL and the CO2 laser in treating lesions of cutaneous sarcoidosis, especially lupus pernio.16-19 Of these 2 modalities, the use of the CO2 laser for effective remodeling of lupus pernio may be more intuitive; however, it is still important to note that the mechanism of action of several of these laser modalities is unclear with regard to the clinical benefit shown. Morphea and scleroderma also have been treated with laser therapy. It is essential to understand that in many cases, laser therapy may be targeted to treat the precise cutaneous manifestations of disease in each individual patient (eg, CO2 laser to treat disabling contractures and calcinosis cutis,20,21 PDL to treat telangiectases related to morphea22). Again, the most critical consideration is that the treatment modality should align with the cutaneous lesion being targeted.
A relatively recent development in the use of lasers has been LLLT, which refers to the use of lasers below levels where they would cause any thermal effects, thereby limiting tissue damage. Although the technology has existed for decades, there has been a recent flurry of reports extolling the many benefits of LLLT; however, the true physiologic effects of LLLT have yet to be determined, with many studies trying to elucidate its numerous effects on various signaling pathways, cell proliferation, and cellular respiration.23-26 Upon reviewing the literature, the list of cutaneous conditions that are being treated with LLLT is vast, spanning acne, vitiligo, wounds, burns, psoriasis, and alopecia, among others.15 It is important to consider that the definition of LLLT in the literature is rather broad with a wide range of wavelengths, fluences, and power densities. As such, the specific laser settings and protocols may vary considerably among different practitioners and therefore the treatment results also may vary. Nevertheless, many studies have hinted at promising results in the use of LLLT in conditions that may have previously been extremely difficult to treat (eg, alopecia). Earlier trials had demonstrated a faster resolution time in patients with alopecia areata when LLLT was added to a topical regimen27; however, the improvement was modest and lesions tended to improve with or without LLLT. Perhaps more compelling is the use of LLLT in treating androgenetic alopecia, a condition for which a satisfying facile treatment would truly carry great impact. Although physicians should be cautious of studies regarding LLLT and hair regrowth that are conducted by groups who may stand to benefit from producing such a device, the results are nonetheless notable, if only for the relative paucity of other therapeutic approaches toward this condition.28,29 A randomized, double-blind, controlled, multicenter trial showed significant improvements in median hair thickness and density with LLLT (P=.01 and P=.003, respectively), though global appearance did not change significantly.30
Laser Treatment of Skin Cancer
Lasers also have been used to treat cutaneous malignancies. Although they may be powerful in the treatment of these conditions, this treatment approach must be used with caution. As with any superficial treatment modality for skin cancer, it is difficult to ascertain if a lesion has been completely treated without any residual cancer cells, and therein lies the main caveat of laser treatment. With the use of a modality that causes a cutaneous response that may mask any underlying process, it is important to ensure that there is a reasonable degree of certainty that this treatment can effectively remove a cancerous lesion in its entirety while avoiding the theoretical risk that disturbing underlying vasculature and/or lymphatics may be modulating the ability of a cancer to metastasize. Thankfully, current evidence does not suggest that there are any downsides to laser treatment for malignancies. Clinically, we know that basal cell carcinomas (BCCs) often feature prominent vasculature, with telangiectases being used as a clinical marker to suggest the diagnosis of a BCC. Capitalizing on this aspect of the clinical lesion, PDL has been used to treat BCCs in 2 small studies with a response rate of approximately 75% for small BCCs in both studies.31,32 A recent randomized controlled trial showed significant superiority of PDL as compared to the control (P<.0001) in treatment of BCC, with nearly 80% (44/56) of cases showing histologically proven complete remission at 6-month follow-up.33 Thus, we have some promising data that suggest PDL may be a viable treatment option in BCC, especially in areas that are difficult to treat surgically.
Additionally, a newer treatment approach for BCC capitalizes on the ability of confocal microscopy to provide a feasible, bedside imaging modality to identify tumor margins. Confocal microscopy has been used as a road map to identify where and how to apply the laser treatment, thus allowing for a higher likelihood of complete destruction of the tumor, at least in theory.34 Although the concept of using confocal microscopy to guide laser treatment of skin cancer has been shown in smaller proof-of-concept case series, it remains to be seen if it is not only an efficacious approach that may be widely adopted but also whether it is pragmatic to do so, as the equipment and expertise involved in using confocal microscopy is not trivial.
Finally, lasers also have been used in the treatment of mycosis fungoides (MF), or cutaneous T-cell lymphoma. It has been suggested that this modality is an excellent treatment option as a skin-directed therapy for stage IA or IB MFs limited to the acral surfaces or MF palmaris et plantaris.35 The reasoning behind this approach was the effectiveness of narrowband UVB for early-stage MF, with an excimer laser operating at a similar wavelength (308 nm) and offering similar therapeutic benefits while limiting adverse effects to surrounding skin.36 More recently, the excimer laser was applied to a small population of 6 patients, with 3 achieving complete response, 1 with partial response, 1 with stable disease, and 1 with progressive disease. The authors were careful to point out that the excimer laser should not be thought of as a replacement for narrowband UVB in early-stage MF but rather as an adjunctive treatment of specific targeted lesional areas.36
Conclusion
Lasers are an important part of the dermatologist’s treatment arsenal. Although much attention has been focused on laser treatment for aesthetic indications, it is important not to overlook the fact that lasers also can be useful in the treatment of refractory skin diseases, as a first-line treatment in some conditions such as vascular lesions, or as an adjunctive treatment modality. There is a great deal of exciting research that may lead to new indications and a better understanding of how to best use these powerful tools, and the outlook is bright for the use of lasers in dermatology.
1. Bonis B, Kemeny L, Dobozy A, et al. 308 nm UVB excimer laser for psoriasis. Lancet. 1997;350:1522.
2. Fuselier HA Jr, McBurney EI, Brannan W, et al. Treatment of condylomata acuminata with carbon dioxide laser. Urology. 1980;15:265-266.
3. Mueller TJ, Carlson BA, Lindy MP. The use of the carbon dioxide surgical laser for the treatment of verrucae. J Am Podiatry Assoc. 1980;70:136-141.
4. Weyandt GH, Tollmann F, Kristen P, et al. Low risk of contamination with human papilloma virus during treatment of condylomata acuminata with multilayer argon plasma coagulation and CO2 laser ablation. Arch Dermatol Res. 2011;303:141-144.
5. Ferenczy A, Bergeron C, Richart RM. Human papillomavirus DNA in CO2 laser-generated plume of smoke and its consequences to the surgeon. Obstet Gynecol. 1990;75:114-118.
6. Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981:263-276.
7. Morelli JG, Tan OT, Garden J, et al. Tunable dye laser (577 nm) treatment of port wine stains. Lasers Surg Med. 1986;6:94-99.
8. Reyes BA, Geronemus R. Treatment of port-wine stains during childhood with the flashlamp-pumped pulsed dye laser. J Am Acad Dermatol. 1990;23:1142-1148.
9. Ashinoff R, Geronemus RG. Capillary hemangiomas and treatment with the flash lamp-pumped pulsed dye laser. Arch Dermatol. 1991;127:202-205.
10. Erceg A, Bovenschen HJ, van de Kerkhof PC, et al. Efficacy and safety of pulsed dye laser treatment for cutaneous discoid lupus erythematosus. J Am Acad Dermatol. 2009;60:626-632.
11. Diez MT, Boixeda P, Moreno C, et al. Histopathology and immunohistochemistry of cutaneous lupus erythematosus after pulsed dye laser treatment. Dermatol Surg. 2011;37:971-981.
12. Ekback MP, Troilius A. Laser therapy for refractory discoid lupus erythematosus when everything else has failed. J Cosmet Laser Ther. 2013;15:260-265.
13. Welsh JH, Schroeder TL, Levy ML. Granuloma faciale in a child successfully treated with the pulsed dye laser. J Am Acad Dermatol. 1999;41:351-353.
14. Cheung ST, Lanigan SW. Granuloma faciale treated with the pulsed-dye laser: a case series. Clin Exp Dermatol. 2005;30:373-375.
15. Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32:41-52.
16. Roos S, Raulin C, Ockenfels HM, et al. Successful treatment of cutaneous sarcoidosis lesions with the flashlamp pumped pulsed dye laser: a case report. Dermatol Surg. 2009;35:1139-1140.
17. Cliff S, Felix RH, Singh L, et al. The successful treatment of lupus pernio with the flashlamp pulsed dye laser. J Cutan Laser Ther. 1999;1:49-52.
18. O’Donoghue NB, Barlow RJ. Laser remodelling of nodular nasal lupus pernio. Clin Exp Dermatol. 2006;31:27-29.
19. Young HS, Chalmers RJ, Griffiths CE, et al. CO2 laser vaporization for disfiguring lupus pernio. J Cosmet Laser Ther. 2002;4:87-90.
20. Kineston D, Kwan JM, Uebelhoer NS, et al. Use of a fractional ablative 10.6-mum carbon dioxide laser in the treatment of a morphea-related contracture. Arch Dermatol. 2011;147:1148-1150.
21. Chamberlain AJ, Walker NP. Successful palliation and significant remission of cutaneous calcinosis in CREST syndrome with carbon dioxide laser. Dermatol Surg. 2003;29:968-970.
22. Ciatti S, Varga J, Greenbaum SS. The 585 nm flashlamp-pumped pulsed dye laser for the treatment of telangiectases in patients with scleroderma. J Am Acad Dermatol. 1996;35:487-488.
23. Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23:355-361.
24. Greco M, Guida G, Perlino E, et al. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem Biophys Res Commun. 1989;163:1428-1434.
25. Karu TI, Pyatibrat LV, Kalendo GS. Photobiological modulation of cell attachment via cytochrome c oxidase. Photochem Photobiol Sci. 2004;3:211-216.
26. Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761-4771.
27. Yamazaki M, Miura Y, Tsuboi R, et al. Linear polarized infrared irradiation using Super Lizer is an effective treatment for multiple-type alopecia areata. Int J Dermatol. 2003;42:738-740.
28. Leavitt M, Charles G, Heyman E, et al. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: a randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29:283-292.
29. Munck A, Gavazzoni MF, Trueb RM. Use of low-level laser therapy as monotherapy or concomitant therapy for male and female androgenetic alopecia. Int J Trichology. 2014;6:45-49.
30. Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
31. Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series [published online ahead of print December 13, 2012]. J Skin Cancer. 2012;2012:286480.
32. Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
33. Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial [published online ahead of print July 12, 2014]. Br J Dermatol. doi:10.1111/bjd.13266.
34. Chen CS, Sierra H, Cordova M, et al. Confocal microscopy-guided laser ablation for superficial and early nodular Basal cell carcinoma: a promising surgical alternative for superficial skin cancers. JAMA Dermatol. 2014;150:994-998.
35. Jin SP, Jeon YK, Cho KH, et al. Excimer laser therapy (308 nm) for mycosis fungoides palmaris et plantaris: a skin-directed and anatomically feasible treatment. Br J Dermatol. 2010;163:651-653.
36. Deaver D, Cauthen A, Cohen G, et al. Excimer laser in the treatment of mycosis fungoides. J Am Acad Dermatol. 2014;70:1058-1060.
1. Bonis B, Kemeny L, Dobozy A, et al. 308 nm UVB excimer laser for psoriasis. Lancet. 1997;350:1522.
2. Fuselier HA Jr, McBurney EI, Brannan W, et al. Treatment of condylomata acuminata with carbon dioxide laser. Urology. 1980;15:265-266.
3. Mueller TJ, Carlson BA, Lindy MP. The use of the carbon dioxide surgical laser for the treatment of verrucae. J Am Podiatry Assoc. 1980;70:136-141.
4. Weyandt GH, Tollmann F, Kristen P, et al. Low risk of contamination with human papilloma virus during treatment of condylomata acuminata with multilayer argon plasma coagulation and CO2 laser ablation. Arch Dermatol Res. 2011;303:141-144.
5. Ferenczy A, Bergeron C, Richart RM. Human papillomavirus DNA in CO2 laser-generated plume of smoke and its consequences to the surgeon. Obstet Gynecol. 1990;75:114-118.
6. Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981:263-276.
7. Morelli JG, Tan OT, Garden J, et al. Tunable dye laser (577 nm) treatment of port wine stains. Lasers Surg Med. 1986;6:94-99.
8. Reyes BA, Geronemus R. Treatment of port-wine stains during childhood with the flashlamp-pumped pulsed dye laser. J Am Acad Dermatol. 1990;23:1142-1148.
9. Ashinoff R, Geronemus RG. Capillary hemangiomas and treatment with the flash lamp-pumped pulsed dye laser. Arch Dermatol. 1991;127:202-205.
10. Erceg A, Bovenschen HJ, van de Kerkhof PC, et al. Efficacy and safety of pulsed dye laser treatment for cutaneous discoid lupus erythematosus. J Am Acad Dermatol. 2009;60:626-632.
11. Diez MT, Boixeda P, Moreno C, et al. Histopathology and immunohistochemistry of cutaneous lupus erythematosus after pulsed dye laser treatment. Dermatol Surg. 2011;37:971-981.
12. Ekback MP, Troilius A. Laser therapy for refractory discoid lupus erythematosus when everything else has failed. J Cosmet Laser Ther. 2013;15:260-265.
13. Welsh JH, Schroeder TL, Levy ML. Granuloma faciale in a child successfully treated with the pulsed dye laser. J Am Acad Dermatol. 1999;41:351-353.
14. Cheung ST, Lanigan SW. Granuloma faciale treated with the pulsed-dye laser: a case series. Clin Exp Dermatol. 2005;30:373-375.
15. Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32:41-52.
16. Roos S, Raulin C, Ockenfels HM, et al. Successful treatment of cutaneous sarcoidosis lesions with the flashlamp pumped pulsed dye laser: a case report. Dermatol Surg. 2009;35:1139-1140.
17. Cliff S, Felix RH, Singh L, et al. The successful treatment of lupus pernio with the flashlamp pulsed dye laser. J Cutan Laser Ther. 1999;1:49-52.
18. O’Donoghue NB, Barlow RJ. Laser remodelling of nodular nasal lupus pernio. Clin Exp Dermatol. 2006;31:27-29.
19. Young HS, Chalmers RJ, Griffiths CE, et al. CO2 laser vaporization for disfiguring lupus pernio. J Cosmet Laser Ther. 2002;4:87-90.
20. Kineston D, Kwan JM, Uebelhoer NS, et al. Use of a fractional ablative 10.6-mum carbon dioxide laser in the treatment of a morphea-related contracture. Arch Dermatol. 2011;147:1148-1150.
21. Chamberlain AJ, Walker NP. Successful palliation and significant remission of cutaneous calcinosis in CREST syndrome with carbon dioxide laser. Dermatol Surg. 2003;29:968-970.
22. Ciatti S, Varga J, Greenbaum SS. The 585 nm flashlamp-pumped pulsed dye laser for the treatment of telangiectases in patients with scleroderma. J Am Acad Dermatol. 1996;35:487-488.
23. Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23:355-361.
24. Greco M, Guida G, Perlino E, et al. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem Biophys Res Commun. 1989;163:1428-1434.
25. Karu TI, Pyatibrat LV, Kalendo GS. Photobiological modulation of cell attachment via cytochrome c oxidase. Photochem Photobiol Sci. 2004;3:211-216.
26. Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761-4771.
27. Yamazaki M, Miura Y, Tsuboi R, et al. Linear polarized infrared irradiation using Super Lizer is an effective treatment for multiple-type alopecia areata. Int J Dermatol. 2003;42:738-740.
28. Leavitt M, Charles G, Heyman E, et al. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: a randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29:283-292.
29. Munck A, Gavazzoni MF, Trueb RM. Use of low-level laser therapy as monotherapy or concomitant therapy for male and female androgenetic alopecia. Int J Trichology. 2014;6:45-49.
30. Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
31. Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series [published online ahead of print December 13, 2012]. J Skin Cancer. 2012;2012:286480.
32. Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
33. Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial [published online ahead of print July 12, 2014]. Br J Dermatol. doi:10.1111/bjd.13266.
34. Chen CS, Sierra H, Cordova M, et al. Confocal microscopy-guided laser ablation for superficial and early nodular Basal cell carcinoma: a promising surgical alternative for superficial skin cancers. JAMA Dermatol. 2014;150:994-998.
35. Jin SP, Jeon YK, Cho KH, et al. Excimer laser therapy (308 nm) for mycosis fungoides palmaris et plantaris: a skin-directed and anatomically feasible treatment. Br J Dermatol. 2010;163:651-653.
36. Deaver D, Cauthen A, Cohen G, et al. Excimer laser in the treatment of mycosis fungoides. J Am Acad Dermatol. 2014;70:1058-1060.
Laser treatments for men
In our final segment on male dermatology, we will be focusing on laser treatments in men. There has been a steady increase in cosmetic procedures in men over the last decade, and laser procedures tend to be some of the most popular. In general, laser treatments provide faster results than topical or oral treatments and offer subtle aesthetic improvements with little to no downtime depending on the procedure. These factors appeal to male patients, who generally are generally less risk tolerant than women, and want masculinizing treatments with little downtime and natural results.
• Hair growth. Men tend to have highly pigmented, thicker hair in contrast to women, and often seek laser hair removal for excess body hair. Common sites include the back, upper arms, posterior hairline, lower beardline, and chest. Similar precautions apply to both men and women, such as proper cooling of the skin and avoidance of tanned skin. However, laser settings for male patients may need to be adjusted given the thicker, darkly pigmented hairs and often lower pain threshold. In addition, proper counseling of men is necessary with laser hair removal, because men often need more treatments than women and may need a topical anesthetic for highly sensitive areas.
• Body contouring. Men tend to deposit fat in hard-to-lose areas, such as the central abdomen and flanks. The expanding array of noninvasive devices using cold temperatures to freeze the fat, or ultrasound and radiofrequency devices to heat and thereby tighten the subcutaneous tissue have made body contouring one of the fastest growing cosmetic markets for men. Men are great candidates for these procedures given the fast results, minor discomfort, and noninvasive nature. Although many men have visceral abdominal fat that does not respond to these treatments, areas often treated with great long-term results include the upper and lower abdomen, flanks, arms, chest, and back.
• Rosacea. Men have a higher density of facial blood vessels than women, and they often seek treatment for telangiectasias and overall facial erythema. For noninflammatory erythematotelangiectatic rosacea, vascular laser treatments are the most effective treatments. Pulsed dye laser is often the best laser to target both large and small facial blood vessels and flushing erythema. Intense pulsed light (IPL) lasers are often a more popular choice for men because they involve less downtime and can treat brown spots as well. However, IPL must be used with caution in skin of color and tanned skin because of the risks of scarring and hyperpigmentation. Men may need more treatments and higher energy settings than women. Men also prefer minimal downtime and thus more frequent nonpurpuric settings are often preferred with any vascular laser. In addition, with IPL, men should be warned of the possibility of the laser temporarily stunting hair growth or causing hair to grow in patchy temporarily when using the device in the beard or mustache area.
• Laser resurfacing. Laser skin resurfacing can be performed for acne scars, rhytids, age sports, sun spots, melasma, and overall skin laxity. Options include ablative and nonablative skin resurfacing. The choice of procedure depends on the type of problem being treated, skin type, and downtime. Ablative CO2, erbium:YAG, and fractional ablative lasers provide the best results for deep rhytids, acne scars, surgical scars, and skin laxity. However, men often shy away from these procedures given the pain, postprocedure care necessary, and downtime. Nonablative lasers may be a better choice for men, particularly for fine rhytids, melasma, and sun spots. With multiple treatments, they also may be used for scars and skin laxity. Postprocedure skincare and downtime are the critical factors for men when choosing resurfacing procedures, and detailed review of the care, complexity, and side effects are essential in the care of male patients.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
In our final segment on male dermatology, we will be focusing on laser treatments in men. There has been a steady increase in cosmetic procedures in men over the last decade, and laser procedures tend to be some of the most popular. In general, laser treatments provide faster results than topical or oral treatments and offer subtle aesthetic improvements with little to no downtime depending on the procedure. These factors appeal to male patients, who generally are generally less risk tolerant than women, and want masculinizing treatments with little downtime and natural results.
• Hair growth. Men tend to have highly pigmented, thicker hair in contrast to women, and often seek laser hair removal for excess body hair. Common sites include the back, upper arms, posterior hairline, lower beardline, and chest. Similar precautions apply to both men and women, such as proper cooling of the skin and avoidance of tanned skin. However, laser settings for male patients may need to be adjusted given the thicker, darkly pigmented hairs and often lower pain threshold. In addition, proper counseling of men is necessary with laser hair removal, because men often need more treatments than women and may need a topical anesthetic for highly sensitive areas.
• Body contouring. Men tend to deposit fat in hard-to-lose areas, such as the central abdomen and flanks. The expanding array of noninvasive devices using cold temperatures to freeze the fat, or ultrasound and radiofrequency devices to heat and thereby tighten the subcutaneous tissue have made body contouring one of the fastest growing cosmetic markets for men. Men are great candidates for these procedures given the fast results, minor discomfort, and noninvasive nature. Although many men have visceral abdominal fat that does not respond to these treatments, areas often treated with great long-term results include the upper and lower abdomen, flanks, arms, chest, and back.
• Rosacea. Men have a higher density of facial blood vessels than women, and they often seek treatment for telangiectasias and overall facial erythema. For noninflammatory erythematotelangiectatic rosacea, vascular laser treatments are the most effective treatments. Pulsed dye laser is often the best laser to target both large and small facial blood vessels and flushing erythema. Intense pulsed light (IPL) lasers are often a more popular choice for men because they involve less downtime and can treat brown spots as well. However, IPL must be used with caution in skin of color and tanned skin because of the risks of scarring and hyperpigmentation. Men may need more treatments and higher energy settings than women. Men also prefer minimal downtime and thus more frequent nonpurpuric settings are often preferred with any vascular laser. In addition, with IPL, men should be warned of the possibility of the laser temporarily stunting hair growth or causing hair to grow in patchy temporarily when using the device in the beard or mustache area.
• Laser resurfacing. Laser skin resurfacing can be performed for acne scars, rhytids, age sports, sun spots, melasma, and overall skin laxity. Options include ablative and nonablative skin resurfacing. The choice of procedure depends on the type of problem being treated, skin type, and downtime. Ablative CO2, erbium:YAG, and fractional ablative lasers provide the best results for deep rhytids, acne scars, surgical scars, and skin laxity. However, men often shy away from these procedures given the pain, postprocedure care necessary, and downtime. Nonablative lasers may be a better choice for men, particularly for fine rhytids, melasma, and sun spots. With multiple treatments, they also may be used for scars and skin laxity. Postprocedure skincare and downtime are the critical factors for men when choosing resurfacing procedures, and detailed review of the care, complexity, and side effects are essential in the care of male patients.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
In our final segment on male dermatology, we will be focusing on laser treatments in men. There has been a steady increase in cosmetic procedures in men over the last decade, and laser procedures tend to be some of the most popular. In general, laser treatments provide faster results than topical or oral treatments and offer subtle aesthetic improvements with little to no downtime depending on the procedure. These factors appeal to male patients, who generally are generally less risk tolerant than women, and want masculinizing treatments with little downtime and natural results.
• Hair growth. Men tend to have highly pigmented, thicker hair in contrast to women, and often seek laser hair removal for excess body hair. Common sites include the back, upper arms, posterior hairline, lower beardline, and chest. Similar precautions apply to both men and women, such as proper cooling of the skin and avoidance of tanned skin. However, laser settings for male patients may need to be adjusted given the thicker, darkly pigmented hairs and often lower pain threshold. In addition, proper counseling of men is necessary with laser hair removal, because men often need more treatments than women and may need a topical anesthetic for highly sensitive areas.
• Body contouring. Men tend to deposit fat in hard-to-lose areas, such as the central abdomen and flanks. The expanding array of noninvasive devices using cold temperatures to freeze the fat, or ultrasound and radiofrequency devices to heat and thereby tighten the subcutaneous tissue have made body contouring one of the fastest growing cosmetic markets for men. Men are great candidates for these procedures given the fast results, minor discomfort, and noninvasive nature. Although many men have visceral abdominal fat that does not respond to these treatments, areas often treated with great long-term results include the upper and lower abdomen, flanks, arms, chest, and back.
• Rosacea. Men have a higher density of facial blood vessels than women, and they often seek treatment for telangiectasias and overall facial erythema. For noninflammatory erythematotelangiectatic rosacea, vascular laser treatments are the most effective treatments. Pulsed dye laser is often the best laser to target both large and small facial blood vessels and flushing erythema. Intense pulsed light (IPL) lasers are often a more popular choice for men because they involve less downtime and can treat brown spots as well. However, IPL must be used with caution in skin of color and tanned skin because of the risks of scarring and hyperpigmentation. Men may need more treatments and higher energy settings than women. Men also prefer minimal downtime and thus more frequent nonpurpuric settings are often preferred with any vascular laser. In addition, with IPL, men should be warned of the possibility of the laser temporarily stunting hair growth or causing hair to grow in patchy temporarily when using the device in the beard or mustache area.
• Laser resurfacing. Laser skin resurfacing can be performed for acne scars, rhytids, age sports, sun spots, melasma, and overall skin laxity. Options include ablative and nonablative skin resurfacing. The choice of procedure depends on the type of problem being treated, skin type, and downtime. Ablative CO2, erbium:YAG, and fractional ablative lasers provide the best results for deep rhytids, acne scars, surgical scars, and skin laxity. However, men often shy away from these procedures given the pain, postprocedure care necessary, and downtime. Nonablative lasers may be a better choice for men, particularly for fine rhytids, melasma, and sun spots. With multiple treatments, they also may be used for scars and skin laxity. Postprocedure skincare and downtime are the critical factors for men when choosing resurfacing procedures, and detailed review of the care, complexity, and side effects are essential in the care of male patients.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
Counsel cryolipolysis patients about paradoxical adipose hyperplasia
SAN DIEGO – Clinicians who perform cryolipolysis should inform patients about the risk of paradoxical adipose hyperplasia, “a newly described, rare side effect” consisting of a “usually permanent” increase in fatty tissue at the treatment site, said Dr. Andrew Nelson, a dermatologist in private practice in St. Petersburg, Fla.
Based on case reports to date, the estimated incidence of paradoxical adipose hyperplasia (PAH) is only about 0.0051%, or 1 case per 20,000 treatment cycles, Dr. Nelson added. But use of cryolipolysis is growing rapidly, including use among physician extenders, he noted. “We should be counseling all our patients on the possibility of PAH, and we need to generate more data to determine its cause and the best treatment options.”
Even a single cycle of cryolipolysis can trigger PAH, which seems to occur at least 2-3 months later and requires invasive treatment to resolve, Dr. Nelson said at the annual meeting of the American Society for Dermatologic Surgery (ASDS). “Most patients report an initial reduction of fat before PAH develops,” he added.
Histology at the site of PAH typically shows disorganized adipocytes that vary more in size and shape than does normal panniculus, but the overlying dermis and epidermis appear normal. About half of cases reported to date have involved men, “but women are treated at a higher rate than men, so there may be a preponderance of men,” he said.
No one knows why PAH occurs, Dr. Nelson noted. Possibilities include recruitment of local or circulating preadipocytes or stem cells; changes in the expression of receptors or soluble factors that play a role in adipocyte metabolism; reduced sympathetic innervation; and hypoxic injury, he said. Hypoxic injury is known to spur capillary growth, which can cause fat hypertrophy, he added.
In a case reported at the ASDS meeting by Dr. Brian Raphael, a 75-year-old woman developed PAH approximately 4 months after undergoing two sessions of cryolipolysis that had been spaced 1 month apart. The adipose tissue had accumulated in the center of the treatment site “in a distribution identical to the device applicator,” Dr. Raphael and his associates reported. They performed a wedge biopsy to confirm that the fat accumulation was benign, and then carried out a pannectomy to remove the unwanted fat, they said.
Cryolipolysis works by slowly cooling adipocytes, which are more sensitive to temperature decreases than epidermal and dermal cells, Dr. Nelson said. The adipocytes crystallize, which is thought to trigger their apoptosis and gradual elimination from the body in the months after treatment. Adverse effects besides PAH are usually mild and include edema, pain, and temporary redness at the treatment site, he noted. About two-thirds of patients also have a temporary, localized loss of sensation that can last up to 8 weeks, he added.
Dr. Nelson and Dr. Raphael reported no conflicts of interest.
SAN DIEGO – Clinicians who perform cryolipolysis should inform patients about the risk of paradoxical adipose hyperplasia, “a newly described, rare side effect” consisting of a “usually permanent” increase in fatty tissue at the treatment site, said Dr. Andrew Nelson, a dermatologist in private practice in St. Petersburg, Fla.
Based on case reports to date, the estimated incidence of paradoxical adipose hyperplasia (PAH) is only about 0.0051%, or 1 case per 20,000 treatment cycles, Dr. Nelson added. But use of cryolipolysis is growing rapidly, including use among physician extenders, he noted. “We should be counseling all our patients on the possibility of PAH, and we need to generate more data to determine its cause and the best treatment options.”
Even a single cycle of cryolipolysis can trigger PAH, which seems to occur at least 2-3 months later and requires invasive treatment to resolve, Dr. Nelson said at the annual meeting of the American Society for Dermatologic Surgery (ASDS). “Most patients report an initial reduction of fat before PAH develops,” he added.
Histology at the site of PAH typically shows disorganized adipocytes that vary more in size and shape than does normal panniculus, but the overlying dermis and epidermis appear normal. About half of cases reported to date have involved men, “but women are treated at a higher rate than men, so there may be a preponderance of men,” he said.
No one knows why PAH occurs, Dr. Nelson noted. Possibilities include recruitment of local or circulating preadipocytes or stem cells; changes in the expression of receptors or soluble factors that play a role in adipocyte metabolism; reduced sympathetic innervation; and hypoxic injury, he said. Hypoxic injury is known to spur capillary growth, which can cause fat hypertrophy, he added.
In a case reported at the ASDS meeting by Dr. Brian Raphael, a 75-year-old woman developed PAH approximately 4 months after undergoing two sessions of cryolipolysis that had been spaced 1 month apart. The adipose tissue had accumulated in the center of the treatment site “in a distribution identical to the device applicator,” Dr. Raphael and his associates reported. They performed a wedge biopsy to confirm that the fat accumulation was benign, and then carried out a pannectomy to remove the unwanted fat, they said.
Cryolipolysis works by slowly cooling adipocytes, which are more sensitive to temperature decreases than epidermal and dermal cells, Dr. Nelson said. The adipocytes crystallize, which is thought to trigger their apoptosis and gradual elimination from the body in the months after treatment. Adverse effects besides PAH are usually mild and include edema, pain, and temporary redness at the treatment site, he noted. About two-thirds of patients also have a temporary, localized loss of sensation that can last up to 8 weeks, he added.
Dr. Nelson and Dr. Raphael reported no conflicts of interest.
SAN DIEGO – Clinicians who perform cryolipolysis should inform patients about the risk of paradoxical adipose hyperplasia, “a newly described, rare side effect” consisting of a “usually permanent” increase in fatty tissue at the treatment site, said Dr. Andrew Nelson, a dermatologist in private practice in St. Petersburg, Fla.
Based on case reports to date, the estimated incidence of paradoxical adipose hyperplasia (PAH) is only about 0.0051%, or 1 case per 20,000 treatment cycles, Dr. Nelson added. But use of cryolipolysis is growing rapidly, including use among physician extenders, he noted. “We should be counseling all our patients on the possibility of PAH, and we need to generate more data to determine its cause and the best treatment options.”
Even a single cycle of cryolipolysis can trigger PAH, which seems to occur at least 2-3 months later and requires invasive treatment to resolve, Dr. Nelson said at the annual meeting of the American Society for Dermatologic Surgery (ASDS). “Most patients report an initial reduction of fat before PAH develops,” he added.
Histology at the site of PAH typically shows disorganized adipocytes that vary more in size and shape than does normal panniculus, but the overlying dermis and epidermis appear normal. About half of cases reported to date have involved men, “but women are treated at a higher rate than men, so there may be a preponderance of men,” he said.
No one knows why PAH occurs, Dr. Nelson noted. Possibilities include recruitment of local or circulating preadipocytes or stem cells; changes in the expression of receptors or soluble factors that play a role in adipocyte metabolism; reduced sympathetic innervation; and hypoxic injury, he said. Hypoxic injury is known to spur capillary growth, which can cause fat hypertrophy, he added.
In a case reported at the ASDS meeting by Dr. Brian Raphael, a 75-year-old woman developed PAH approximately 4 months after undergoing two sessions of cryolipolysis that had been spaced 1 month apart. The adipose tissue had accumulated in the center of the treatment site “in a distribution identical to the device applicator,” Dr. Raphael and his associates reported. They performed a wedge biopsy to confirm that the fat accumulation was benign, and then carried out a pannectomy to remove the unwanted fat, they said.
Cryolipolysis works by slowly cooling adipocytes, which are more sensitive to temperature decreases than epidermal and dermal cells, Dr. Nelson said. The adipocytes crystallize, which is thought to trigger their apoptosis and gradual elimination from the body in the months after treatment. Adverse effects besides PAH are usually mild and include edema, pain, and temporary redness at the treatment site, he noted. About two-thirds of patients also have a temporary, localized loss of sensation that can last up to 8 weeks, he added.
Dr. Nelson and Dr. Raphael reported no conflicts of interest.
AT THE ASDS ANNUAL MEETING
Risk Factors for Malignant Melanoma and Preventive Methods
Cutaneous melanoma is a malignant tumor of the skin that develops from melanin-producing pigment cells known as melanocytes. The development of melanoma is a multifactorial process. External factors, genetic predisposition, or both may cause damage to DNA in melanoma cells. Genetic mutations may occur de novo or can be transferred from generation to generation. The most important environmental risk factor is UV radiation, both natural and artificial. Other risk factors include skin type, ethnicity, number of melanocytic nevi, number and severity of sunburns, frequency and duration of UV exposure, geographic location, and level of awareness about malignant melanoma (MM) and its risk factors.1
Melanoma accounts for only 1% to 2% of all tumors but is known for its rapidly increasing incidence.2 White individuals who reside in sunny areas of North America, northern Europe, Australia, and New Zealand seem to be at the highest risk for developing melanoma.3 The global incidence of MM from 2004 to 2008 was 20.8 individuals per 100,000 people.4 In Central Europe, 10 to 12 individuals per 100,000 people were diagnosed with melanoma, and 50 to 60 individuals per 100,000 people were diagnosed in Australia. In 2011, the lifetime risk of being diagnosed with melanoma was 1% in Central Europe and 4% in Australia.2 The incidence of melanoma is lower in populations with darker skin types (ie, Africans, Asians). In some parts of the world, the overall incidence and/or severity of melanoma has been declining over the last few decades, possibly reflecting improved public awareness.5
Cutaneous MM is an aggressive skin cancer that has fatal consequences if diagnosed late. Chances of survival, however, increase dramatically when melanoma is detected early. Collecting and analyzing data about a certain disease leads to a better understanding of the condition and encourages the development of prevention strategies. Epidemiologic research helps to improve patient care by measuring the occurrence of an event and by investigating the relationship between the occurrence of an event and associated factors; in doing so, epidemiologic research directly enables a better understanding of the disease and promotes effective preventive and therapeutic approaches.6
Although risk factors for melanoma are well established, current epidemiologic research shows that information on UV exposure and its association with this disease in many parts of the world, including Central Europe, is lacking. The aim of this study was to investigate behavioral and sociodemographic factors associated with the development of MM in the Czech Republic and Germany.
Materials and Methods
This hospital-based, case-control study was conducted in the largest dermatology departments in the Czech Republic (Clinic of Dermatology and Venereology, Third Faculty of Medicine, Charles University, Prague) and Germany (Department of Dermatology and Allergology, Ludwig Maximilian University, Munich). Data from the Czech Republic and Germany were not evaluated separately. These 2 countries were chosen as a representative sample population from Central Europe.
Study Population
The study population included 207 patients (103 men; 104 women) aged 31 to 94 years who were consecutively diagnosed with MM (cases). Patients with acral lentiginous melanoma were excluded from the study due to the generally accepted theory that the condition is not linked to UV exposure. Melanoma diagnosis was based on histopathologic examination. The study population also included 235 randomly selected controls (110 men; 125 women) from the same 2 study centers who had been hospitalized due to other dermatologic diagnoses with no history of any skin cancer. Among patients asked to take part in the study, the participation rates were 83% among cases and 62% among controls.
Assessment
Various sociodemographic factors and factors related to UV exposure were assessed via administration of a structured questionnaire that was completed by all 442 patients.
Four statistical models concerning variables were constructed. The basic model, which was part of all subsequent models, included age, sex, education, and history of skin tumors. Variables included in the biological model were eye color (light vs dark) and Fitzpatrick skin type (I–V). Variables included in the lifestyle model were the use of sunscreen (never and rarely; often; always; always and repetitively), sun exposure during work (yes/no), and seaside vacation (never, rarely, regularly, more than once per year). The variable in the exposure model was the number of sunburns during childhood and adolescence (none, 1–5 times, 6–10 times, ≥11 times).
Sociodemographic characteristics (sex, age, education) and prior incidence of skin tumor were included in each model. Although there were no statistically significant differences in the incidence of melanoma associated with sex and age, those variables were kept in the models to control the impact of other variables by sex and age.
Other variables were added into the model one by one, and the likelihood ratio was tested step-by-step. Only the variables that improved the model fit were kept in the final model. Impact of variables on dependent variables also was tested; variables with no significant impact on dependent variables were left out of the model.
Statistical Analysis
The association between risk factors and MM was assessed using multivariate logistic regression. In total, 4 models were included in the results, which were presented as odds ratios (ORs) and 95% confidence intervals (CIs). A significance level of α=.05 was chosen. The statistical program Stata 11 was used for all analyses.
Results
Descriptive data on the 442 patients surveyed are shown in Table 1. The results of the logistic regression in all studied models are shown in Table 2.
Basic Model
There was no difference in the proportion of men and women in the melanoma and control groups. We observed that more patients in the melanoma group had a university degree than patients in the control group. Patients in the melanoma group with a history of MM showed 4.2 times higher risk for developing another melanoma.
Biological Model
Eye color and Fitzpatrick skin type were the focus of the biological model. The odds of being diagnosed with melanoma were 2.5 times greater in respondents with a light eye color (ie, blue, green, gray) than in respondents with a dark eye color (ie, brown, black). Respondents with Fitzpatrick skin types I and II had a significantly higher association with melanoma (OR, 4.25 and 6.98; 95% CI, 2.13-8.51 and 3.78-12.88) than Fitzpatrick skin type III (OR, 1.0)(P<.001 for both). Respondents with darker skin types (IV and V) also were present in our study population. The numbers were low, and the CI was too wide; nevertheless, the results were statistically significant (P<.001).
Lifestyle Model
The lifestyle model included patients’ use of sunscreen and level of sun exposure at work and on vacation. Respondents who did not use sunscreen were 12 times more likely to develop melanoma than those who always used it (95% CI, 5.56-27.14); however, individuals who used sunscreen always and repetitively (ie, more than once during 1 period of sun exposure) had a higher likelihood of melanoma compared to those who always used it. The incidence of melanoma was lower in respondents who regularly spent their vacations by the sea than those who did not vacation in seaside regions. Respondents who worked in direct sunlight were approximately 2 times more likely to present with melanoma than individuals who did not work outside.
Exposure Model
The number of sunburns sustained during childhood and adolescence was assessed in the exposure model. Respondents with a history of 1 to 5 sunburns during childhood and adolescence did not show a statistically significant increase in the incidence of melanoma diagnosis; however, those with a history of 6 or more sunburns during these periods showed a significant increase in the odds of developing melanoma (OR, 4.95 and 25.52; 95% CI, 2.29-10.71 and 12.16-53.54)(P<.001 for both).
Comment
In this study, we concentrated on UV exposure and various sociodemographic factors that were possibly connected to a higher risk for developing melanoma. We observed that the majority of patients in the melanoma group had achieved a higher level of education than the control group. Most of the melanoma group patients had light-colored eyes and spent more time in direct sunlight at work. Although seaside vacations did not correlate with a higher occurrence of melanoma, it was noted that the melanoma patients used sunscreen much less often than the control group. Major differences among respondents in the melanoma group versus the control group were seen in the reported number of sunburns sustained in childhood and adolescence. More sunburns during these periods seemed to play the most important role in the risk for melanoma. Some of the patient responses to the questionnaire may be biased, as respondents answered the questions by themselves.
Because risk factors for and preventive methods against melanoma are well established, one would assume that general knowledge regarding melanoma is adequate. On the contrary, it has been shown that knowledge about melanoma is insufficient, even among professionals and individuals with higher levels of education. In a study based on a questionnaire administered to plastic surgeons, only 37.5% (27/72) of respondents correctly identified the duration of action of sunscreen to be 3 to 4 hours.7 Approximately half of the respondents (37/72) did not know that geographical conditions such as altitude and latitude as well as shade can alter sunscreen efficacy and also were not aware of the protective action of clothing. These results are alarming and indicate that even medical professionals, who should play a main role in improving the health knowledge of the general population, have an unsatisfactory level of education in prevention of melanoma. Another important part of better education of specialists treating skin disorders is good knowledge of dermatoscopy. In fact, the Annual Skin Cancer Conference 2011 in Australia emphasized the importance of dermatoscopy in primary and secondary prevention of skin cancer.8 Teaching dermatoscopy should be part of melanoma campaigns for professionals.
Our basic model demonstrated that a higher level of education was connected to a higher occurrence of MM, which may seem surprising, considering that most diseases, along with their incidence, prevalence, and mortality, usually are associated with lower levels of education or lower socioeconomic status. A similar trend also was reported in prior studies, with higher socioeconomic groups showing higher incidences of cutaneous melanoma; colon cancer; brain cancer in men; and breast and ovarian cancer in women. Additionally, patients with higher socioeconomic status have been shown to have a survival advantage.9 Individuals with higher socioeconomic status can afford to travel more often for vacation and are more frequently exposed to direct sun. Individuals with higher levels of education also are generally more aware of the importance of disease prevention and therefore go for preventive checkups more often. The detection of melanoma in this socioeconomic group should be higher.
Our biological model demonstrated that respondents with lighter eyes had melanoma almost 3 times more often than individuals with darker eyes. Fitzpatrick skin types I and II also were significantly associated with the development of melanoma (P<.001). These findings are generally confirmed in the literature. In a study of the incidence of melanoma in Spain, statistically significant risk factors included blonde or red hair (P=.002), multiple melanocytic nevi (P=.002), Fitzpatrick skin types I and II (P=.002), and a history of actinic keratosis (P=.021) or nonmelanoma skin cancer (P=.002).10 A group in Italy also has investigated the main risk factors for melanoma. This study suggested dividing patients into high-risk subgroups to help minimize exposure to UV radiation and diagnose melanoma in its early stage.11
The results from our study confirmed the importance of concentrating melanoma prevention campaign efforts on high-risk patients. Dividing these patients into subgroups (eg, individuals who play outdoor sports, individuals with occupations associated with UV exposure, individuals who use indoor tanning beds, individuals with a family history of melanoma) may be helpful. A case-control study on sun-seeking behavior in the Czech Republic showed that the most alarming risk factors were all-day sun exposure during adolescence, frequent holidays spent in the mountains, and inadequate use of sunscreen in adulthood.12 We investigated the effects of sunscreen use on the incidence of melanoma in our lifestyle model and discovered that it decreased the risk for melanoma. Respondents who used it always had a much lower risk for developing melanoma than those who never or rarely applied it. Individuals who used sunscreen always and repetitively (ie, more than once per period of sun exposure) did not show a lower risk than those who used it once per period of sun exposure. This finding could mean that patients who are known to get sunburns or who feel a certain discomfort on direct exposure to the sun tend to use sunscreen always and repetitively.
It is important to note that some investigators disagree with the importance of some generally accepted means of prevention, such as the effect of sunscreen products. Due to insufficient evidence, the role of sunscreen use in reducing the risk for skin cancer, especially cutaneous MM, is controversial.13 Although we could prove there is a considerable difference in the incidence of melanoma in patients who claimed to use sunscreen always versus those who never use it, we agree that more evidence on this topic is needed. Furthermore, it has been reported that risk for melanoma has increased with rising intermittent sun exposure and indoor tanning bed use.14,15
Respondents who regularly traveled to seaside regions showed a surprisingly lower incidence of melanoma than respondents who did not spend their vacations in seaside locations. It is possible that individuals who choose not to spend their vacations at the seaside are more prone to sunburns and therefore do not prefer to spend their free time in direct sunlight. Another possible explanation is that individuals who regularly travel to seaside regions actively try to protect themselves from sunlight and sunburns. A higher incidence of melanoma also was observed in respondents who reported sun exposure during work.
In our exposure model, we demonstrated that a history of sunburns is the strongest risk factor for melanoma. Frequent sunburns during childhood and adolescence were strongly associated with the development of MM. This association has been supported in a systematic review on sun exposure during childhood and associated risks.16
Conclusion
To improve patient knowledge about melanoma prevention, we suggest directing targeted campaigns that address high-risk population groups, such as individuals with red hair and/or light eyes, people with an occupation associated with frequent UV exposure, and individuals with higher levels of education. With regard to younger populations, parents as well as physicians and teachers should be aware that frequent sunburns during childhood and adolescence and use of tanning beds are 2 main risk factors for MM.
1. IARC monographs on the evaluation of carcinogenic risks to humans. solar and ultraviolet radiation. IARC Monogr Eval Carcinog Risks Hum. 1992;55:1-316.
2. Kunte C, Geimer T, Baumert J, et al. Analysis of predictive factors for the outcome of complete lymph node dissection in melanoma patients with metastatic sentinel lymph nodes. J Am Acad Dermatol. 2011;64:655-662.
3. Parkin D, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108.
4. SEER Stat Fact Sheets: melanoma of the skin. Bethesda, MD: National Cancer Institute; 2013. http://seer.cancer.gov/statfacts/html/melan.html#incidence-mortality. Accessed October 25, 2014.
5. Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917.
6. Nijsten T, Stern RS. How epidemiology has contributed to a better understanding of skin disease [published online ahead of print December 8, 2011]. J Invest Dermatol. 2012;132(3, pt 2):994-1002.
7. Magdum A, Leonforte F, McNaughton E, et al. Sun protection–do we know enough [published online ahead of print February 8, 2012]? J Plast Reconstr Aesthet Surg. 2012;65:1384-1389.
8. Zalaudek I, Whiteman D, Rosendahl C, et al. Update on melanoma and non-melanoma skin cancer. Annual Skin Cancer Conference 2011, Hamilton Island, Australia, 2011. Expert Rev Anticancer Ther. 2011;11:1829-1832.
9. Mackenbach JP. Health inequalities: Europe in profile. http://ec.europa.eu/health/ph_determinants/socio_economics/documents/ev_060302_rd06_en.pdf. Published February 2006. Accessed October 25, 2014.
10. Ballester I, Oliver V, Bañuls J, et al. Multicenter case-control study of risk factors for cutaneous melanoma in Valencia, Spain [published online ahead of print May 22, 2012]. Actas Dermosifiliogr. 2012;103:790-797.
11. Jaimes N, Marghoob AA. An update on risk factors, prognosis and management of melanoma patients. G Ital Dermatol Venereol. 2012;147:1-19.
12. Vranova J, Arenbergerova M, Arenberger P, et al. Incidence of cutaneous malignant melanoma in the Czech Republic: the risks of sun exposure for adolescents. Neoplasma. 2012;59:316-325.
13. Planta MB. Sunscreen and melanoma: is our prevention message correct? J Am Board Fam Med. 2011;24:735-739.
14. Veierød MB, Adami HO, Lund E, et al. Sun and solarium exposure and melanoma risk: effects of age, pigmentary characteristics, and nevi. Cancer Epidemiol Biomarkers Prev. 2010;19:111-120.
15. Doré JF, Chignol MC. Tanning salons and skin cancer [published online ahead of print August 15, 2011]. Photochem Photobiol Sci. 2012;11:30-37.
16. Whiteman DC, Whiteman CA, Green AC. Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiologic studies. Cancer Causes Control. 2001;12:69-82.
Cutaneous melanoma is a malignant tumor of the skin that develops from melanin-producing pigment cells known as melanocytes. The development of melanoma is a multifactorial process. External factors, genetic predisposition, or both may cause damage to DNA in melanoma cells. Genetic mutations may occur de novo or can be transferred from generation to generation. The most important environmental risk factor is UV radiation, both natural and artificial. Other risk factors include skin type, ethnicity, number of melanocytic nevi, number and severity of sunburns, frequency and duration of UV exposure, geographic location, and level of awareness about malignant melanoma (MM) and its risk factors.1
Melanoma accounts for only 1% to 2% of all tumors but is known for its rapidly increasing incidence.2 White individuals who reside in sunny areas of North America, northern Europe, Australia, and New Zealand seem to be at the highest risk for developing melanoma.3 The global incidence of MM from 2004 to 2008 was 20.8 individuals per 100,000 people.4 In Central Europe, 10 to 12 individuals per 100,000 people were diagnosed with melanoma, and 50 to 60 individuals per 100,000 people were diagnosed in Australia. In 2011, the lifetime risk of being diagnosed with melanoma was 1% in Central Europe and 4% in Australia.2 The incidence of melanoma is lower in populations with darker skin types (ie, Africans, Asians). In some parts of the world, the overall incidence and/or severity of melanoma has been declining over the last few decades, possibly reflecting improved public awareness.5
Cutaneous MM is an aggressive skin cancer that has fatal consequences if diagnosed late. Chances of survival, however, increase dramatically when melanoma is detected early. Collecting and analyzing data about a certain disease leads to a better understanding of the condition and encourages the development of prevention strategies. Epidemiologic research helps to improve patient care by measuring the occurrence of an event and by investigating the relationship between the occurrence of an event and associated factors; in doing so, epidemiologic research directly enables a better understanding of the disease and promotes effective preventive and therapeutic approaches.6
Although risk factors for melanoma are well established, current epidemiologic research shows that information on UV exposure and its association with this disease in many parts of the world, including Central Europe, is lacking. The aim of this study was to investigate behavioral and sociodemographic factors associated with the development of MM in the Czech Republic and Germany.
Materials and Methods
This hospital-based, case-control study was conducted in the largest dermatology departments in the Czech Republic (Clinic of Dermatology and Venereology, Third Faculty of Medicine, Charles University, Prague) and Germany (Department of Dermatology and Allergology, Ludwig Maximilian University, Munich). Data from the Czech Republic and Germany were not evaluated separately. These 2 countries were chosen as a representative sample population from Central Europe.
Study Population
The study population included 207 patients (103 men; 104 women) aged 31 to 94 years who were consecutively diagnosed with MM (cases). Patients with acral lentiginous melanoma were excluded from the study due to the generally accepted theory that the condition is not linked to UV exposure. Melanoma diagnosis was based on histopathologic examination. The study population also included 235 randomly selected controls (110 men; 125 women) from the same 2 study centers who had been hospitalized due to other dermatologic diagnoses with no history of any skin cancer. Among patients asked to take part in the study, the participation rates were 83% among cases and 62% among controls.
Assessment
Various sociodemographic factors and factors related to UV exposure were assessed via administration of a structured questionnaire that was completed by all 442 patients.
Four statistical models concerning variables were constructed. The basic model, which was part of all subsequent models, included age, sex, education, and history of skin tumors. Variables included in the biological model were eye color (light vs dark) and Fitzpatrick skin type (I–V). Variables included in the lifestyle model were the use of sunscreen (never and rarely; often; always; always and repetitively), sun exposure during work (yes/no), and seaside vacation (never, rarely, regularly, more than once per year). The variable in the exposure model was the number of sunburns during childhood and adolescence (none, 1–5 times, 6–10 times, ≥11 times).
Sociodemographic characteristics (sex, age, education) and prior incidence of skin tumor were included in each model. Although there were no statistically significant differences in the incidence of melanoma associated with sex and age, those variables were kept in the models to control the impact of other variables by sex and age.
Other variables were added into the model one by one, and the likelihood ratio was tested step-by-step. Only the variables that improved the model fit were kept in the final model. Impact of variables on dependent variables also was tested; variables with no significant impact on dependent variables were left out of the model.
Statistical Analysis
The association between risk factors and MM was assessed using multivariate logistic regression. In total, 4 models were included in the results, which were presented as odds ratios (ORs) and 95% confidence intervals (CIs). A significance level of α=.05 was chosen. The statistical program Stata 11 was used for all analyses.
Results
Descriptive data on the 442 patients surveyed are shown in Table 1. The results of the logistic regression in all studied models are shown in Table 2.
Basic Model
There was no difference in the proportion of men and women in the melanoma and control groups. We observed that more patients in the melanoma group had a university degree than patients in the control group. Patients in the melanoma group with a history of MM showed 4.2 times higher risk for developing another melanoma.
Biological Model
Eye color and Fitzpatrick skin type were the focus of the biological model. The odds of being diagnosed with melanoma were 2.5 times greater in respondents with a light eye color (ie, blue, green, gray) than in respondents with a dark eye color (ie, brown, black). Respondents with Fitzpatrick skin types I and II had a significantly higher association with melanoma (OR, 4.25 and 6.98; 95% CI, 2.13-8.51 and 3.78-12.88) than Fitzpatrick skin type III (OR, 1.0)(P<.001 for both). Respondents with darker skin types (IV and V) also were present in our study population. The numbers were low, and the CI was too wide; nevertheless, the results were statistically significant (P<.001).
Lifestyle Model
The lifestyle model included patients’ use of sunscreen and level of sun exposure at work and on vacation. Respondents who did not use sunscreen were 12 times more likely to develop melanoma than those who always used it (95% CI, 5.56-27.14); however, individuals who used sunscreen always and repetitively (ie, more than once during 1 period of sun exposure) had a higher likelihood of melanoma compared to those who always used it. The incidence of melanoma was lower in respondents who regularly spent their vacations by the sea than those who did not vacation in seaside regions. Respondents who worked in direct sunlight were approximately 2 times more likely to present with melanoma than individuals who did not work outside.
Exposure Model
The number of sunburns sustained during childhood and adolescence was assessed in the exposure model. Respondents with a history of 1 to 5 sunburns during childhood and adolescence did not show a statistically significant increase in the incidence of melanoma diagnosis; however, those with a history of 6 or more sunburns during these periods showed a significant increase in the odds of developing melanoma (OR, 4.95 and 25.52; 95% CI, 2.29-10.71 and 12.16-53.54)(P<.001 for both).
Comment
In this study, we concentrated on UV exposure and various sociodemographic factors that were possibly connected to a higher risk for developing melanoma. We observed that the majority of patients in the melanoma group had achieved a higher level of education than the control group. Most of the melanoma group patients had light-colored eyes and spent more time in direct sunlight at work. Although seaside vacations did not correlate with a higher occurrence of melanoma, it was noted that the melanoma patients used sunscreen much less often than the control group. Major differences among respondents in the melanoma group versus the control group were seen in the reported number of sunburns sustained in childhood and adolescence. More sunburns during these periods seemed to play the most important role in the risk for melanoma. Some of the patient responses to the questionnaire may be biased, as respondents answered the questions by themselves.
Because risk factors for and preventive methods against melanoma are well established, one would assume that general knowledge regarding melanoma is adequate. On the contrary, it has been shown that knowledge about melanoma is insufficient, even among professionals and individuals with higher levels of education. In a study based on a questionnaire administered to plastic surgeons, only 37.5% (27/72) of respondents correctly identified the duration of action of sunscreen to be 3 to 4 hours.7 Approximately half of the respondents (37/72) did not know that geographical conditions such as altitude and latitude as well as shade can alter sunscreen efficacy and also were not aware of the protective action of clothing. These results are alarming and indicate that even medical professionals, who should play a main role in improving the health knowledge of the general population, have an unsatisfactory level of education in prevention of melanoma. Another important part of better education of specialists treating skin disorders is good knowledge of dermatoscopy. In fact, the Annual Skin Cancer Conference 2011 in Australia emphasized the importance of dermatoscopy in primary and secondary prevention of skin cancer.8 Teaching dermatoscopy should be part of melanoma campaigns for professionals.
Our basic model demonstrated that a higher level of education was connected to a higher occurrence of MM, which may seem surprising, considering that most diseases, along with their incidence, prevalence, and mortality, usually are associated with lower levels of education or lower socioeconomic status. A similar trend also was reported in prior studies, with higher socioeconomic groups showing higher incidences of cutaneous melanoma; colon cancer; brain cancer in men; and breast and ovarian cancer in women. Additionally, patients with higher socioeconomic status have been shown to have a survival advantage.9 Individuals with higher socioeconomic status can afford to travel more often for vacation and are more frequently exposed to direct sun. Individuals with higher levels of education also are generally more aware of the importance of disease prevention and therefore go for preventive checkups more often. The detection of melanoma in this socioeconomic group should be higher.
Our biological model demonstrated that respondents with lighter eyes had melanoma almost 3 times more often than individuals with darker eyes. Fitzpatrick skin types I and II also were significantly associated with the development of melanoma (P<.001). These findings are generally confirmed in the literature. In a study of the incidence of melanoma in Spain, statistically significant risk factors included blonde or red hair (P=.002), multiple melanocytic nevi (P=.002), Fitzpatrick skin types I and II (P=.002), and a history of actinic keratosis (P=.021) or nonmelanoma skin cancer (P=.002).10 A group in Italy also has investigated the main risk factors for melanoma. This study suggested dividing patients into high-risk subgroups to help minimize exposure to UV radiation and diagnose melanoma in its early stage.11
The results from our study confirmed the importance of concentrating melanoma prevention campaign efforts on high-risk patients. Dividing these patients into subgroups (eg, individuals who play outdoor sports, individuals with occupations associated with UV exposure, individuals who use indoor tanning beds, individuals with a family history of melanoma) may be helpful. A case-control study on sun-seeking behavior in the Czech Republic showed that the most alarming risk factors were all-day sun exposure during adolescence, frequent holidays spent in the mountains, and inadequate use of sunscreen in adulthood.12 We investigated the effects of sunscreen use on the incidence of melanoma in our lifestyle model and discovered that it decreased the risk for melanoma. Respondents who used it always had a much lower risk for developing melanoma than those who never or rarely applied it. Individuals who used sunscreen always and repetitively (ie, more than once per period of sun exposure) did not show a lower risk than those who used it once per period of sun exposure. This finding could mean that patients who are known to get sunburns or who feel a certain discomfort on direct exposure to the sun tend to use sunscreen always and repetitively.
It is important to note that some investigators disagree with the importance of some generally accepted means of prevention, such as the effect of sunscreen products. Due to insufficient evidence, the role of sunscreen use in reducing the risk for skin cancer, especially cutaneous MM, is controversial.13 Although we could prove there is a considerable difference in the incidence of melanoma in patients who claimed to use sunscreen always versus those who never use it, we agree that more evidence on this topic is needed. Furthermore, it has been reported that risk for melanoma has increased with rising intermittent sun exposure and indoor tanning bed use.14,15
Respondents who regularly traveled to seaside regions showed a surprisingly lower incidence of melanoma than respondents who did not spend their vacations in seaside locations. It is possible that individuals who choose not to spend their vacations at the seaside are more prone to sunburns and therefore do not prefer to spend their free time in direct sunlight. Another possible explanation is that individuals who regularly travel to seaside regions actively try to protect themselves from sunlight and sunburns. A higher incidence of melanoma also was observed in respondents who reported sun exposure during work.
In our exposure model, we demonstrated that a history of sunburns is the strongest risk factor for melanoma. Frequent sunburns during childhood and adolescence were strongly associated with the development of MM. This association has been supported in a systematic review on sun exposure during childhood and associated risks.16
Conclusion
To improve patient knowledge about melanoma prevention, we suggest directing targeted campaigns that address high-risk population groups, such as individuals with red hair and/or light eyes, people with an occupation associated with frequent UV exposure, and individuals with higher levels of education. With regard to younger populations, parents as well as physicians and teachers should be aware that frequent sunburns during childhood and adolescence and use of tanning beds are 2 main risk factors for MM.
Cutaneous melanoma is a malignant tumor of the skin that develops from melanin-producing pigment cells known as melanocytes. The development of melanoma is a multifactorial process. External factors, genetic predisposition, or both may cause damage to DNA in melanoma cells. Genetic mutations may occur de novo or can be transferred from generation to generation. The most important environmental risk factor is UV radiation, both natural and artificial. Other risk factors include skin type, ethnicity, number of melanocytic nevi, number and severity of sunburns, frequency and duration of UV exposure, geographic location, and level of awareness about malignant melanoma (MM) and its risk factors.1
Melanoma accounts for only 1% to 2% of all tumors but is known for its rapidly increasing incidence.2 White individuals who reside in sunny areas of North America, northern Europe, Australia, and New Zealand seem to be at the highest risk for developing melanoma.3 The global incidence of MM from 2004 to 2008 was 20.8 individuals per 100,000 people.4 In Central Europe, 10 to 12 individuals per 100,000 people were diagnosed with melanoma, and 50 to 60 individuals per 100,000 people were diagnosed in Australia. In 2011, the lifetime risk of being diagnosed with melanoma was 1% in Central Europe and 4% in Australia.2 The incidence of melanoma is lower in populations with darker skin types (ie, Africans, Asians). In some parts of the world, the overall incidence and/or severity of melanoma has been declining over the last few decades, possibly reflecting improved public awareness.5
Cutaneous MM is an aggressive skin cancer that has fatal consequences if diagnosed late. Chances of survival, however, increase dramatically when melanoma is detected early. Collecting and analyzing data about a certain disease leads to a better understanding of the condition and encourages the development of prevention strategies. Epidemiologic research helps to improve patient care by measuring the occurrence of an event and by investigating the relationship between the occurrence of an event and associated factors; in doing so, epidemiologic research directly enables a better understanding of the disease and promotes effective preventive and therapeutic approaches.6
Although risk factors for melanoma are well established, current epidemiologic research shows that information on UV exposure and its association with this disease in many parts of the world, including Central Europe, is lacking. The aim of this study was to investigate behavioral and sociodemographic factors associated with the development of MM in the Czech Republic and Germany.
Materials and Methods
This hospital-based, case-control study was conducted in the largest dermatology departments in the Czech Republic (Clinic of Dermatology and Venereology, Third Faculty of Medicine, Charles University, Prague) and Germany (Department of Dermatology and Allergology, Ludwig Maximilian University, Munich). Data from the Czech Republic and Germany were not evaluated separately. These 2 countries were chosen as a representative sample population from Central Europe.
Study Population
The study population included 207 patients (103 men; 104 women) aged 31 to 94 years who were consecutively diagnosed with MM (cases). Patients with acral lentiginous melanoma were excluded from the study due to the generally accepted theory that the condition is not linked to UV exposure. Melanoma diagnosis was based on histopathologic examination. The study population also included 235 randomly selected controls (110 men; 125 women) from the same 2 study centers who had been hospitalized due to other dermatologic diagnoses with no history of any skin cancer. Among patients asked to take part in the study, the participation rates were 83% among cases and 62% among controls.
Assessment
Various sociodemographic factors and factors related to UV exposure were assessed via administration of a structured questionnaire that was completed by all 442 patients.
Four statistical models concerning variables were constructed. The basic model, which was part of all subsequent models, included age, sex, education, and history of skin tumors. Variables included in the biological model were eye color (light vs dark) and Fitzpatrick skin type (I–V). Variables included in the lifestyle model were the use of sunscreen (never and rarely; often; always; always and repetitively), sun exposure during work (yes/no), and seaside vacation (never, rarely, regularly, more than once per year). The variable in the exposure model was the number of sunburns during childhood and adolescence (none, 1–5 times, 6–10 times, ≥11 times).
Sociodemographic characteristics (sex, age, education) and prior incidence of skin tumor were included in each model. Although there were no statistically significant differences in the incidence of melanoma associated with sex and age, those variables were kept in the models to control the impact of other variables by sex and age.
Other variables were added into the model one by one, and the likelihood ratio was tested step-by-step. Only the variables that improved the model fit were kept in the final model. Impact of variables on dependent variables also was tested; variables with no significant impact on dependent variables were left out of the model.
Statistical Analysis
The association between risk factors and MM was assessed using multivariate logistic regression. In total, 4 models were included in the results, which were presented as odds ratios (ORs) and 95% confidence intervals (CIs). A significance level of α=.05 was chosen. The statistical program Stata 11 was used for all analyses.
Results
Descriptive data on the 442 patients surveyed are shown in Table 1. The results of the logistic regression in all studied models are shown in Table 2.
Basic Model
There was no difference in the proportion of men and women in the melanoma and control groups. We observed that more patients in the melanoma group had a university degree than patients in the control group. Patients in the melanoma group with a history of MM showed 4.2 times higher risk for developing another melanoma.
Biological Model
Eye color and Fitzpatrick skin type were the focus of the biological model. The odds of being diagnosed with melanoma were 2.5 times greater in respondents with a light eye color (ie, blue, green, gray) than in respondents with a dark eye color (ie, brown, black). Respondents with Fitzpatrick skin types I and II had a significantly higher association with melanoma (OR, 4.25 and 6.98; 95% CI, 2.13-8.51 and 3.78-12.88) than Fitzpatrick skin type III (OR, 1.0)(P<.001 for both). Respondents with darker skin types (IV and V) also were present in our study population. The numbers were low, and the CI was too wide; nevertheless, the results were statistically significant (P<.001).
Lifestyle Model
The lifestyle model included patients’ use of sunscreen and level of sun exposure at work and on vacation. Respondents who did not use sunscreen were 12 times more likely to develop melanoma than those who always used it (95% CI, 5.56-27.14); however, individuals who used sunscreen always and repetitively (ie, more than once during 1 period of sun exposure) had a higher likelihood of melanoma compared to those who always used it. The incidence of melanoma was lower in respondents who regularly spent their vacations by the sea than those who did not vacation in seaside regions. Respondents who worked in direct sunlight were approximately 2 times more likely to present with melanoma than individuals who did not work outside.
Exposure Model
The number of sunburns sustained during childhood and adolescence was assessed in the exposure model. Respondents with a history of 1 to 5 sunburns during childhood and adolescence did not show a statistically significant increase in the incidence of melanoma diagnosis; however, those with a history of 6 or more sunburns during these periods showed a significant increase in the odds of developing melanoma (OR, 4.95 and 25.52; 95% CI, 2.29-10.71 and 12.16-53.54)(P<.001 for both).
Comment
In this study, we concentrated on UV exposure and various sociodemographic factors that were possibly connected to a higher risk for developing melanoma. We observed that the majority of patients in the melanoma group had achieved a higher level of education than the control group. Most of the melanoma group patients had light-colored eyes and spent more time in direct sunlight at work. Although seaside vacations did not correlate with a higher occurrence of melanoma, it was noted that the melanoma patients used sunscreen much less often than the control group. Major differences among respondents in the melanoma group versus the control group were seen in the reported number of sunburns sustained in childhood and adolescence. More sunburns during these periods seemed to play the most important role in the risk for melanoma. Some of the patient responses to the questionnaire may be biased, as respondents answered the questions by themselves.
Because risk factors for and preventive methods against melanoma are well established, one would assume that general knowledge regarding melanoma is adequate. On the contrary, it has been shown that knowledge about melanoma is insufficient, even among professionals and individuals with higher levels of education. In a study based on a questionnaire administered to plastic surgeons, only 37.5% (27/72) of respondents correctly identified the duration of action of sunscreen to be 3 to 4 hours.7 Approximately half of the respondents (37/72) did not know that geographical conditions such as altitude and latitude as well as shade can alter sunscreen efficacy and also were not aware of the protective action of clothing. These results are alarming and indicate that even medical professionals, who should play a main role in improving the health knowledge of the general population, have an unsatisfactory level of education in prevention of melanoma. Another important part of better education of specialists treating skin disorders is good knowledge of dermatoscopy. In fact, the Annual Skin Cancer Conference 2011 in Australia emphasized the importance of dermatoscopy in primary and secondary prevention of skin cancer.8 Teaching dermatoscopy should be part of melanoma campaigns for professionals.
Our basic model demonstrated that a higher level of education was connected to a higher occurrence of MM, which may seem surprising, considering that most diseases, along with their incidence, prevalence, and mortality, usually are associated with lower levels of education or lower socioeconomic status. A similar trend also was reported in prior studies, with higher socioeconomic groups showing higher incidences of cutaneous melanoma; colon cancer; brain cancer in men; and breast and ovarian cancer in women. Additionally, patients with higher socioeconomic status have been shown to have a survival advantage.9 Individuals with higher socioeconomic status can afford to travel more often for vacation and are more frequently exposed to direct sun. Individuals with higher levels of education also are generally more aware of the importance of disease prevention and therefore go for preventive checkups more often. The detection of melanoma in this socioeconomic group should be higher.
Our biological model demonstrated that respondents with lighter eyes had melanoma almost 3 times more often than individuals with darker eyes. Fitzpatrick skin types I and II also were significantly associated with the development of melanoma (P<.001). These findings are generally confirmed in the literature. In a study of the incidence of melanoma in Spain, statistically significant risk factors included blonde or red hair (P=.002), multiple melanocytic nevi (P=.002), Fitzpatrick skin types I and II (P=.002), and a history of actinic keratosis (P=.021) or nonmelanoma skin cancer (P=.002).10 A group in Italy also has investigated the main risk factors for melanoma. This study suggested dividing patients into high-risk subgroups to help minimize exposure to UV radiation and diagnose melanoma in its early stage.11
The results from our study confirmed the importance of concentrating melanoma prevention campaign efforts on high-risk patients. Dividing these patients into subgroups (eg, individuals who play outdoor sports, individuals with occupations associated with UV exposure, individuals who use indoor tanning beds, individuals with a family history of melanoma) may be helpful. A case-control study on sun-seeking behavior in the Czech Republic showed that the most alarming risk factors were all-day sun exposure during adolescence, frequent holidays spent in the mountains, and inadequate use of sunscreen in adulthood.12 We investigated the effects of sunscreen use on the incidence of melanoma in our lifestyle model and discovered that it decreased the risk for melanoma. Respondents who used it always had a much lower risk for developing melanoma than those who never or rarely applied it. Individuals who used sunscreen always and repetitively (ie, more than once per period of sun exposure) did not show a lower risk than those who used it once per period of sun exposure. This finding could mean that patients who are known to get sunburns or who feel a certain discomfort on direct exposure to the sun tend to use sunscreen always and repetitively.
It is important to note that some investigators disagree with the importance of some generally accepted means of prevention, such as the effect of sunscreen products. Due to insufficient evidence, the role of sunscreen use in reducing the risk for skin cancer, especially cutaneous MM, is controversial.13 Although we could prove there is a considerable difference in the incidence of melanoma in patients who claimed to use sunscreen always versus those who never use it, we agree that more evidence on this topic is needed. Furthermore, it has been reported that risk for melanoma has increased with rising intermittent sun exposure and indoor tanning bed use.14,15
Respondents who regularly traveled to seaside regions showed a surprisingly lower incidence of melanoma than respondents who did not spend their vacations in seaside locations. It is possible that individuals who choose not to spend their vacations at the seaside are more prone to sunburns and therefore do not prefer to spend their free time in direct sunlight. Another possible explanation is that individuals who regularly travel to seaside regions actively try to protect themselves from sunlight and sunburns. A higher incidence of melanoma also was observed in respondents who reported sun exposure during work.
In our exposure model, we demonstrated that a history of sunburns is the strongest risk factor for melanoma. Frequent sunburns during childhood and adolescence were strongly associated with the development of MM. This association has been supported in a systematic review on sun exposure during childhood and associated risks.16
Conclusion
To improve patient knowledge about melanoma prevention, we suggest directing targeted campaigns that address high-risk population groups, such as individuals with red hair and/or light eyes, people with an occupation associated with frequent UV exposure, and individuals with higher levels of education. With regard to younger populations, parents as well as physicians and teachers should be aware that frequent sunburns during childhood and adolescence and use of tanning beds are 2 main risk factors for MM.
1. IARC monographs on the evaluation of carcinogenic risks to humans. solar and ultraviolet radiation. IARC Monogr Eval Carcinog Risks Hum. 1992;55:1-316.
2. Kunte C, Geimer T, Baumert J, et al. Analysis of predictive factors for the outcome of complete lymph node dissection in melanoma patients with metastatic sentinel lymph nodes. J Am Acad Dermatol. 2011;64:655-662.
3. Parkin D, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108.
4. SEER Stat Fact Sheets: melanoma of the skin. Bethesda, MD: National Cancer Institute; 2013. http://seer.cancer.gov/statfacts/html/melan.html#incidence-mortality. Accessed October 25, 2014.
5. Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917.
6. Nijsten T, Stern RS. How epidemiology has contributed to a better understanding of skin disease [published online ahead of print December 8, 2011]. J Invest Dermatol. 2012;132(3, pt 2):994-1002.
7. Magdum A, Leonforte F, McNaughton E, et al. Sun protection–do we know enough [published online ahead of print February 8, 2012]? J Plast Reconstr Aesthet Surg. 2012;65:1384-1389.
8. Zalaudek I, Whiteman D, Rosendahl C, et al. Update on melanoma and non-melanoma skin cancer. Annual Skin Cancer Conference 2011, Hamilton Island, Australia, 2011. Expert Rev Anticancer Ther. 2011;11:1829-1832.
9. Mackenbach JP. Health inequalities: Europe in profile. http://ec.europa.eu/health/ph_determinants/socio_economics/documents/ev_060302_rd06_en.pdf. Published February 2006. Accessed October 25, 2014.
10. Ballester I, Oliver V, Bañuls J, et al. Multicenter case-control study of risk factors for cutaneous melanoma in Valencia, Spain [published online ahead of print May 22, 2012]. Actas Dermosifiliogr. 2012;103:790-797.
11. Jaimes N, Marghoob AA. An update on risk factors, prognosis and management of melanoma patients. G Ital Dermatol Venereol. 2012;147:1-19.
12. Vranova J, Arenbergerova M, Arenberger P, et al. Incidence of cutaneous malignant melanoma in the Czech Republic: the risks of sun exposure for adolescents. Neoplasma. 2012;59:316-325.
13. Planta MB. Sunscreen and melanoma: is our prevention message correct? J Am Board Fam Med. 2011;24:735-739.
14. Veierød MB, Adami HO, Lund E, et al. Sun and solarium exposure and melanoma risk: effects of age, pigmentary characteristics, and nevi. Cancer Epidemiol Biomarkers Prev. 2010;19:111-120.
15. Doré JF, Chignol MC. Tanning salons and skin cancer [published online ahead of print August 15, 2011]. Photochem Photobiol Sci. 2012;11:30-37.
16. Whiteman DC, Whiteman CA, Green AC. Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiologic studies. Cancer Causes Control. 2001;12:69-82.
1. IARC monographs on the evaluation of carcinogenic risks to humans. solar and ultraviolet radiation. IARC Monogr Eval Carcinog Risks Hum. 1992;55:1-316.
2. Kunte C, Geimer T, Baumert J, et al. Analysis of predictive factors for the outcome of complete lymph node dissection in melanoma patients with metastatic sentinel lymph nodes. J Am Acad Dermatol. 2011;64:655-662.
3. Parkin D, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108.
4. SEER Stat Fact Sheets: melanoma of the skin. Bethesda, MD: National Cancer Institute; 2013. http://seer.cancer.gov/statfacts/html/melan.html#incidence-mortality. Accessed October 25, 2014.
5. Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917.
6. Nijsten T, Stern RS. How epidemiology has contributed to a better understanding of skin disease [published online ahead of print December 8, 2011]. J Invest Dermatol. 2012;132(3, pt 2):994-1002.
7. Magdum A, Leonforte F, McNaughton E, et al. Sun protection–do we know enough [published online ahead of print February 8, 2012]? J Plast Reconstr Aesthet Surg. 2012;65:1384-1389.
8. Zalaudek I, Whiteman D, Rosendahl C, et al. Update on melanoma and non-melanoma skin cancer. Annual Skin Cancer Conference 2011, Hamilton Island, Australia, 2011. Expert Rev Anticancer Ther. 2011;11:1829-1832.
9. Mackenbach JP. Health inequalities: Europe in profile. http://ec.europa.eu/health/ph_determinants/socio_economics/documents/ev_060302_rd06_en.pdf. Published February 2006. Accessed October 25, 2014.
10. Ballester I, Oliver V, Bañuls J, et al. Multicenter case-control study of risk factors for cutaneous melanoma in Valencia, Spain [published online ahead of print May 22, 2012]. Actas Dermosifiliogr. 2012;103:790-797.
11. Jaimes N, Marghoob AA. An update on risk factors, prognosis and management of melanoma patients. G Ital Dermatol Venereol. 2012;147:1-19.
12. Vranova J, Arenbergerova M, Arenberger P, et al. Incidence of cutaneous malignant melanoma in the Czech Republic: the risks of sun exposure for adolescents. Neoplasma. 2012;59:316-325.
13. Planta MB. Sunscreen and melanoma: is our prevention message correct? J Am Board Fam Med. 2011;24:735-739.
14. Veierød MB, Adami HO, Lund E, et al. Sun and solarium exposure and melanoma risk: effects of age, pigmentary characteristics, and nevi. Cancer Epidemiol Biomarkers Prev. 2010;19:111-120.
15. Doré JF, Chignol MC. Tanning salons and skin cancer [published online ahead of print August 15, 2011]. Photochem Photobiol Sci. 2012;11:30-37.
16. Whiteman DC, Whiteman CA, Green AC. Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiologic studies. Cancer Causes Control. 2001;12:69-82.
Practice Points
- Our study revealed the following common risk factors associated with higher melanoma incidence: light eye color (ie, blue, green, gray), Fitzpatrick skin types I and II, frequent sunburns during childhood and adolescence, and higher level of education.
- Prevention campaigns should be implemented to improve awareness of melanoma to reduce exposure to UV radiation among high-risk patient populations.
Cutaneous Adenosquamous Carcinoma: A Rare Neoplasm With Biphasic Differentiation
Case Report
An 85-year-old woman presented with a painless red plaque on the right bicep of 5 years’ duration. The patient had not seen a physician in the last 63 years and had unsuccessfully attempted to treat the plaque by occlusion with an adhesive bandage. A review of systems was negative for pain, pruritus, bleeding, fever, unexplained weight loss, and night sweats. Physical examination revealed a raised, 2×4×1-cm, red, nontender, ulcerated plaque with slight exudate and gelatinous texture on the right bicep (Figure 1). Full-body skin examination revealed erythema and swelling of the right wrist and forearm consistent with cellulitis as well as tinea pedis and onychomycosis of the toenails of both feet.
 Figure 1. A raised, red, nontender, ulcerated plaque with slight exudate and gelatinous texture on the right bicep. 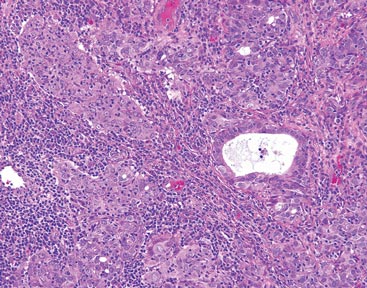 Figure 2. The tumor was comprised of gland-forming cells and exhibited crowding, pleomorphism, enlarged hyperchromatic nuclei, and mitotic division figures (H&E, original magnification ×100). 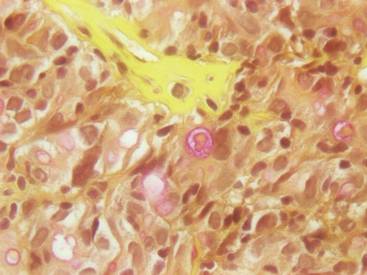 Figure 3. Mucicarmine staining highlighted sialomucin within the glandular component (original magnification ×400). |
Hematoxylin and eosin as well as mucicarmine staining of a shave biopsy from the lesion demonstrated an invasive epithelial neoplasm comprised of squamoid and gland-forming cells broadly attached to the epidermis, which suggested a primary cutaneous origin (Figures 2 and 3). The tumor cells were arranged in infiltrating cords and nests; they exhibited crowding, pleomorphism, enlarged hyperchromatic nuclei, and mitotic division figures. Epithelial mucin (sialomucin) within the glandular component was highlighted on mucicarmine staining. The gland-forming segment of the tumor was strongly positive for cytokeratin (CK) 7. Gastrointestinal tumors were excluded on negative CDX2 and CK20 staining, pulmonary and thyroid tumors were excluded on negative thyroid transcription factor 1 staining, and endometrial and ovarian tumors were excluded with negative estrogen receptor staining. On physical examination the breasts were soft, nontender, and without deformity. A chest radiograph demonstrated normal heart size and pulmonary vasculature with mild bibasilar atelectasis and no areas of consolidation. Given these clinical findings along with a negative history of cancer and negative estrogen receptor staining, breast cancer was excluded from the differential diagnosis, and the diagnosis of cASC was made. The tumor was excised using Mohs micrographic surgery and was free of recurrence at 6- and 12-month follow-up.
Comment
Primary cutaneous adenosquamous carcinoma (cASC) is an aggressive subtype of squamous cell carcinoma that was first described in 1985.1 It typically presents as an erythematous, indurated, keratotic papule or plaque with a predilection for the face, scalp, and upper extremities of immunocompromised individuals and elderly men.2,3 Biopsies generally demonstrate a malignant epithelial neoplasm arising from the epidermis and exhibiting squamous and glandular differentiation. The glandular segment usually is indistinguishable from adenocarcinoma and can be highlighted on CK7, carcinoembryonic antigen, mucicarmine, and periodic acid–Schiff staining. The squamous segment typically is indistinguishable from squamous cell carcinoma and shows aberrant keratinization and intercellular bridges. Tumors often are deeply invasive, poorly differentiated, and associated with a desmoplastic stromal reaction. Local recurrence rates are between 22% and 26%,4 but metastasis is rare. Surgical excision is the mainstay of therapy. When clear margins cannot be obtained using Mohs micrographic surgery, adjuvant external beam radiation therapy and epidermal growth factor receptor inhibitors can be used to treat locally recurrent cASCs.2
The differential diagnosis for cASC includes cutaneous mucoepidermoid carcinoma, cutaneous acantholytic squamous cell carcinoma, and cutaneous manifestations of metastatic visceral adenosquamous carcinoma. Mucoepidermoid carcinoma sometimes is used interchangeably with cASC in the literature, but it is a different cutaneous neoplasm that forms goblet cells, intermediate cells, and squamous cells. It is considered the cutaneous analogue of salivary gland mucoepidermoid carcinoma and does not exhibit the anaplasia, stromal desmoplasia, and aggressive course of cASC.5 The acantholytic subtype of squamous cell carcinoma forms glandlike spaces due to poor adhesion between keratinocytes, but the glandlike spaces do not form mucin or stain positive for CK7 or carcinoembryonic antigen. Adenosquamous carcinomas are well recognized in the lungs, breasts, genitourinary tract, pancreas, and gastroenteric system. Visceral tumor metastasis to the skin should be excluded by appropriate screening.
Conclusion
Although cASCs are not commonly encountered in clinical practice, accurate diagnosis of these lesions is important due to their potentially aggressive behavior. Misdiagnosis and improper treatment could be attributed to lack of awareness of this type of lesion.
1. Weidner N, Foucar E. Adenosquamous carcinoma of the skin. an aggressive mucin-and gland-forming squamous carcinoma. Arch Dermatol. 1985;121:775-779.
2. Fu JM, McCalmont T, Siegrid YS. Adenosquamous carcinoma of the skin: a case series. Arch Dermatol. 2009;145:1152-1158.
3. Ko JK, Leffel DJ, McNiff JM. Adenosquamous carcinoma: a report of 9 cases with p63 and cytokeratin 5/6 staining. J Cutan Pathol. 2009;36:448-452.
4. Banks ER, Cooper PH. Adenosquamous carcinoma of the skin: a report of 10 cases. J Cutan Pathol. 1991;18:227-234.
5. Riedlinger WF, Hurley MY, Dehner LP, et al. Muco-epidermoid carcinoma of the skin: a distinct entity from adenosquamous carcinoma: a case study with a review of the literature. Am J Surg Pathol. 2005;29:131-135.
Case Report
An 85-year-old woman presented with a painless red plaque on the right bicep of 5 years’ duration. The patient had not seen a physician in the last 63 years and had unsuccessfully attempted to treat the plaque by occlusion with an adhesive bandage. A review of systems was negative for pain, pruritus, bleeding, fever, unexplained weight loss, and night sweats. Physical examination revealed a raised, 2×4×1-cm, red, nontender, ulcerated plaque with slight exudate and gelatinous texture on the right bicep (Figure 1). Full-body skin examination revealed erythema and swelling of the right wrist and forearm consistent with cellulitis as well as tinea pedis and onychomycosis of the toenails of both feet.
 Figure 1. A raised, red, nontender, ulcerated plaque with slight exudate and gelatinous texture on the right bicep.  Figure 2. The tumor was comprised of gland-forming cells and exhibited crowding, pleomorphism, enlarged hyperchromatic nuclei, and mitotic division figures (H&E, original magnification ×100).  Figure 3. Mucicarmine staining highlighted sialomucin within the glandular component (original magnification ×400). |
Hematoxylin and eosin as well as mucicarmine staining of a shave biopsy from the lesion demonstrated an invasive epithelial neoplasm comprised of squamoid and gland-forming cells broadly attached to the epidermis, which suggested a primary cutaneous origin (Figures 2 and 3). The tumor cells were arranged in infiltrating cords and nests; they exhibited crowding, pleomorphism, enlarged hyperchromatic nuclei, and mitotic division figures. Epithelial mucin (sialomucin) within the glandular component was highlighted on mucicarmine staining. The gland-forming segment of the tumor was strongly positive for cytokeratin (CK) 7. Gastrointestinal tumors were excluded on negative CDX2 and CK20 staining, pulmonary and thyroid tumors were excluded on negative thyroid transcription factor 1 staining, and endometrial and ovarian tumors were excluded with negative estrogen receptor staining. On physical examination the breasts were soft, nontender, and without deformity. A chest radiograph demonstrated normal heart size and pulmonary vasculature with mild bibasilar atelectasis and no areas of consolidation. Given these clinical findings along with a negative history of cancer and negative estrogen receptor staining, breast cancer was excluded from the differential diagnosis, and the diagnosis of cASC was made. The tumor was excised using Mohs micrographic surgery and was free of recurrence at 6- and 12-month follow-up.
Comment
Primary cutaneous adenosquamous carcinoma (cASC) is an aggressive subtype of squamous cell carcinoma that was first described in 1985.1 It typically presents as an erythematous, indurated, keratotic papule or plaque with a predilection for the face, scalp, and upper extremities of immunocompromised individuals and elderly men.2,3 Biopsies generally demonstrate a malignant epithelial neoplasm arising from the epidermis and exhibiting squamous and glandular differentiation. The glandular segment usually is indistinguishable from adenocarcinoma and can be highlighted on CK7, carcinoembryonic antigen, mucicarmine, and periodic acid–Schiff staining. The squamous segment typically is indistinguishable from squamous cell carcinoma and shows aberrant keratinization and intercellular bridges. Tumors often are deeply invasive, poorly differentiated, and associated with a desmoplastic stromal reaction. Local recurrence rates are between 22% and 26%,4 but metastasis is rare. Surgical excision is the mainstay of therapy. When clear margins cannot be obtained using Mohs micrographic surgery, adjuvant external beam radiation therapy and epidermal growth factor receptor inhibitors can be used to treat locally recurrent cASCs.2
The differential diagnosis for cASC includes cutaneous mucoepidermoid carcinoma, cutaneous acantholytic squamous cell carcinoma, and cutaneous manifestations of metastatic visceral adenosquamous carcinoma. Mucoepidermoid carcinoma sometimes is used interchangeably with cASC in the literature, but it is a different cutaneous neoplasm that forms goblet cells, intermediate cells, and squamous cells. It is considered the cutaneous analogue of salivary gland mucoepidermoid carcinoma and does not exhibit the anaplasia, stromal desmoplasia, and aggressive course of cASC.5 The acantholytic subtype of squamous cell carcinoma forms glandlike spaces due to poor adhesion between keratinocytes, but the glandlike spaces do not form mucin or stain positive for CK7 or carcinoembryonic antigen. Adenosquamous carcinomas are well recognized in the lungs, breasts, genitourinary tract, pancreas, and gastroenteric system. Visceral tumor metastasis to the skin should be excluded by appropriate screening.
Conclusion
Although cASCs are not commonly encountered in clinical practice, accurate diagnosis of these lesions is important due to their potentially aggressive behavior. Misdiagnosis and improper treatment could be attributed to lack of awareness of this type of lesion.
Case Report
An 85-year-old woman presented with a painless red plaque on the right bicep of 5 years’ duration. The patient had not seen a physician in the last 63 years and had unsuccessfully attempted to treat the plaque by occlusion with an adhesive bandage. A review of systems was negative for pain, pruritus, bleeding, fever, unexplained weight loss, and night sweats. Physical examination revealed a raised, 2×4×1-cm, red, nontender, ulcerated plaque with slight exudate and gelatinous texture on the right bicep (Figure 1). Full-body skin examination revealed erythema and swelling of the right wrist and forearm consistent with cellulitis as well as tinea pedis and onychomycosis of the toenails of both feet.
 Figure 1. A raised, red, nontender, ulcerated plaque with slight exudate and gelatinous texture on the right bicep.  Figure 2. The tumor was comprised of gland-forming cells and exhibited crowding, pleomorphism, enlarged hyperchromatic nuclei, and mitotic division figures (H&E, original magnification ×100).  Figure 3. Mucicarmine staining highlighted sialomucin within the glandular component (original magnification ×400). |
Hematoxylin and eosin as well as mucicarmine staining of a shave biopsy from the lesion demonstrated an invasive epithelial neoplasm comprised of squamoid and gland-forming cells broadly attached to the epidermis, which suggested a primary cutaneous origin (Figures 2 and 3). The tumor cells were arranged in infiltrating cords and nests; they exhibited crowding, pleomorphism, enlarged hyperchromatic nuclei, and mitotic division figures. Epithelial mucin (sialomucin) within the glandular component was highlighted on mucicarmine staining. The gland-forming segment of the tumor was strongly positive for cytokeratin (CK) 7. Gastrointestinal tumors were excluded on negative CDX2 and CK20 staining, pulmonary and thyroid tumors were excluded on negative thyroid transcription factor 1 staining, and endometrial and ovarian tumors were excluded with negative estrogen receptor staining. On physical examination the breasts were soft, nontender, and without deformity. A chest radiograph demonstrated normal heart size and pulmonary vasculature with mild bibasilar atelectasis and no areas of consolidation. Given these clinical findings along with a negative history of cancer and negative estrogen receptor staining, breast cancer was excluded from the differential diagnosis, and the diagnosis of cASC was made. The tumor was excised using Mohs micrographic surgery and was free of recurrence at 6- and 12-month follow-up.
Comment
Primary cutaneous adenosquamous carcinoma (cASC) is an aggressive subtype of squamous cell carcinoma that was first described in 1985.1 It typically presents as an erythematous, indurated, keratotic papule or plaque with a predilection for the face, scalp, and upper extremities of immunocompromised individuals and elderly men.2,3 Biopsies generally demonstrate a malignant epithelial neoplasm arising from the epidermis and exhibiting squamous and glandular differentiation. The glandular segment usually is indistinguishable from adenocarcinoma and can be highlighted on CK7, carcinoembryonic antigen, mucicarmine, and periodic acid–Schiff staining. The squamous segment typically is indistinguishable from squamous cell carcinoma and shows aberrant keratinization and intercellular bridges. Tumors often are deeply invasive, poorly differentiated, and associated with a desmoplastic stromal reaction. Local recurrence rates are between 22% and 26%,4 but metastasis is rare. Surgical excision is the mainstay of therapy. When clear margins cannot be obtained using Mohs micrographic surgery, adjuvant external beam radiation therapy and epidermal growth factor receptor inhibitors can be used to treat locally recurrent cASCs.2
The differential diagnosis for cASC includes cutaneous mucoepidermoid carcinoma, cutaneous acantholytic squamous cell carcinoma, and cutaneous manifestations of metastatic visceral adenosquamous carcinoma. Mucoepidermoid carcinoma sometimes is used interchangeably with cASC in the literature, but it is a different cutaneous neoplasm that forms goblet cells, intermediate cells, and squamous cells. It is considered the cutaneous analogue of salivary gland mucoepidermoid carcinoma and does not exhibit the anaplasia, stromal desmoplasia, and aggressive course of cASC.5 The acantholytic subtype of squamous cell carcinoma forms glandlike spaces due to poor adhesion between keratinocytes, but the glandlike spaces do not form mucin or stain positive for CK7 or carcinoembryonic antigen. Adenosquamous carcinomas are well recognized in the lungs, breasts, genitourinary tract, pancreas, and gastroenteric system. Visceral tumor metastasis to the skin should be excluded by appropriate screening.
Conclusion
Although cASCs are not commonly encountered in clinical practice, accurate diagnosis of these lesions is important due to their potentially aggressive behavior. Misdiagnosis and improper treatment could be attributed to lack of awareness of this type of lesion.
1. Weidner N, Foucar E. Adenosquamous carcinoma of the skin. an aggressive mucin-and gland-forming squamous carcinoma. Arch Dermatol. 1985;121:775-779.
2. Fu JM, McCalmont T, Siegrid YS. Adenosquamous carcinoma of the skin: a case series. Arch Dermatol. 2009;145:1152-1158.
3. Ko JK, Leffel DJ, McNiff JM. Adenosquamous carcinoma: a report of 9 cases with p63 and cytokeratin 5/6 staining. J Cutan Pathol. 2009;36:448-452.
4. Banks ER, Cooper PH. Adenosquamous carcinoma of the skin: a report of 10 cases. J Cutan Pathol. 1991;18:227-234.
5. Riedlinger WF, Hurley MY, Dehner LP, et al. Muco-epidermoid carcinoma of the skin: a distinct entity from adenosquamous carcinoma: a case study with a review of the literature. Am J Surg Pathol. 2005;29:131-135.
1. Weidner N, Foucar E. Adenosquamous carcinoma of the skin. an aggressive mucin-and gland-forming squamous carcinoma. Arch Dermatol. 1985;121:775-779.
2. Fu JM, McCalmont T, Siegrid YS. Adenosquamous carcinoma of the skin: a case series. Arch Dermatol. 2009;145:1152-1158.
3. Ko JK, Leffel DJ, McNiff JM. Adenosquamous carcinoma: a report of 9 cases with p63 and cytokeratin 5/6 staining. J Cutan Pathol. 2009;36:448-452.
4. Banks ER, Cooper PH. Adenosquamous carcinoma of the skin: a report of 10 cases. J Cutan Pathol. 1991;18:227-234.
5. Riedlinger WF, Hurley MY, Dehner LP, et al. Muco-epidermoid carcinoma of the skin: a distinct entity from adenosquamous carcinoma: a case study with a review of the literature. Am J Surg Pathol. 2005;29:131-135.
Practice Points
- Cutaneous adenosquamous carcinoma (cASC) is an extremely rare malignant neoplasm with histologic similarities to both squamous cell carcinoma and adenocarcinoma.
- Mohs micrographic surgery for excision is recommended; however, adjuvant external beam radiation therapy and epidermal growth factor receptor inhibitors also have been used to treat locally recurrent cASCs.