User login
Ionizing radiation linked to BCC
SAN DIEGO – Patients treated with ionizing radiation were 2.65 to 3.78 times more likely to develop basal cell carcinoma than were controls, and exposure at younger ages or relatively high doses further increased that risk, according to a pooled analysis presented at the annual meeting of the American Society for Dermatologic Surgery.
“Concomitant exposure to ultraviolet radiation may potentiate this effect,” said Dr. Min Deng, a dermatology resident at the University of Chicago. “With newer treatment protocols and improved shielding, it would be interesting to see if the effect of ionizing radiation has changed, or whether we as dermatologists should more actively screen this at-risk population.”
Basal cell carcinoma (BCC) is the most common skin cancer worldwide, but relatively few dermatology papers have assessed the effects of ionizing radiation on the incidence of BCC, said Dr. Deng and coauthor Dr. Diana Bolotin, also of the University of Chicago.
To better understand the link, the researchers searched PubMed for controlled studies on the topic by using the terms “radiation,” “risk,” and “basal cell carcinoma.” They excluded case reports, animal studies, studies published in languages other than English, and trials of radiation as a treatment of BCC, they said. They also excluded studies of atomic bomb survivors, because exposure was uncontrolled and methods to estimate exposure in this group have changed over time, they noted.
In all, six studies published between 1991 and 2012 met the inclusion criteria, and all six showed a statistically significant relative risk or odds of BCC with ionizing radiation exposure, the investigators reported. Three analyses calculated the relative risk of BCC in patients who received radiation for tinea capitis or who underwent total-body irradiation before hematopoietic cell transplantation, they said. Two studies evaluated the odds of BCC in patients with a past history of radiation exposure, and one study assessed time to subsequent BCCs in patients with a history of BCC.
For the three studies that calculated relative risk, the researchers calculated a pooled RR of BCC after ionizing radiation treatment of 2.65 (95% confidence interval, 1.22-5.72) compared with controls. The study of total-body irradiation (TBI) did not report or control for the primary disease for which patients were treated, “which may have confounded the results,” they said. When they excluded that study from their calculation, the overall RR rose to 3.78 (95% CI, 2.62 to 5.44).
Notably, in the two studies of patients with tinea capitis, the relative risk of BCC fell by 12% to 16% for every additional year of age at which patients received radiation treatment, the researchers said. Similarly, risk of BCC dropped by 10.9% for every 1-year increase in age at total-body irradiation prior to cell transplantation.
The combined odds ratio for the next two studies was 4.28 (1.45-12.63). “Likewise, both studies found an elevated odds ratio with younger age at radiation exposure,” the researchers added. One study that stratified patients by type of medical condition detected a “markedly elevated” 8.7 odds of BCC after radiation treatment for acne (2.0 to 38.0), they noted.
The sixth study was a nested case-control analysis that found a statistically significant increase in the odds of BCC starting at a 1-Gy dose of ionizing radiation, and rising linearly up to doses of 35-63.3 Gy. “The risk for developing multiple BCCs also appears to be elevated in patients with a history of radiation therapy,” the researchers said. Patients who had been exposed to ionizing ratio were 2.3 times more likely to develop new BCCs compared with unexposed patients (1.7 to 3.1), they said.
The investigators reported no funding sources or conflicts of interest.
SAN DIEGO – Patients treated with ionizing radiation were 2.65 to 3.78 times more likely to develop basal cell carcinoma than were controls, and exposure at younger ages or relatively high doses further increased that risk, according to a pooled analysis presented at the annual meeting of the American Society for Dermatologic Surgery.
“Concomitant exposure to ultraviolet radiation may potentiate this effect,” said Dr. Min Deng, a dermatology resident at the University of Chicago. “With newer treatment protocols and improved shielding, it would be interesting to see if the effect of ionizing radiation has changed, or whether we as dermatologists should more actively screen this at-risk population.”
Basal cell carcinoma (BCC) is the most common skin cancer worldwide, but relatively few dermatology papers have assessed the effects of ionizing radiation on the incidence of BCC, said Dr. Deng and coauthor Dr. Diana Bolotin, also of the University of Chicago.
To better understand the link, the researchers searched PubMed for controlled studies on the topic by using the terms “radiation,” “risk,” and “basal cell carcinoma.” They excluded case reports, animal studies, studies published in languages other than English, and trials of radiation as a treatment of BCC, they said. They also excluded studies of atomic bomb survivors, because exposure was uncontrolled and methods to estimate exposure in this group have changed over time, they noted.
In all, six studies published between 1991 and 2012 met the inclusion criteria, and all six showed a statistically significant relative risk or odds of BCC with ionizing radiation exposure, the investigators reported. Three analyses calculated the relative risk of BCC in patients who received radiation for tinea capitis or who underwent total-body irradiation before hematopoietic cell transplantation, they said. Two studies evaluated the odds of BCC in patients with a past history of radiation exposure, and one study assessed time to subsequent BCCs in patients with a history of BCC.
For the three studies that calculated relative risk, the researchers calculated a pooled RR of BCC after ionizing radiation treatment of 2.65 (95% confidence interval, 1.22-5.72) compared with controls. The study of total-body irradiation (TBI) did not report or control for the primary disease for which patients were treated, “which may have confounded the results,” they said. When they excluded that study from their calculation, the overall RR rose to 3.78 (95% CI, 2.62 to 5.44).
Notably, in the two studies of patients with tinea capitis, the relative risk of BCC fell by 12% to 16% for every additional year of age at which patients received radiation treatment, the researchers said. Similarly, risk of BCC dropped by 10.9% for every 1-year increase in age at total-body irradiation prior to cell transplantation.
The combined odds ratio for the next two studies was 4.28 (1.45-12.63). “Likewise, both studies found an elevated odds ratio with younger age at radiation exposure,” the researchers added. One study that stratified patients by type of medical condition detected a “markedly elevated” 8.7 odds of BCC after radiation treatment for acne (2.0 to 38.0), they noted.
The sixth study was a nested case-control analysis that found a statistically significant increase in the odds of BCC starting at a 1-Gy dose of ionizing radiation, and rising linearly up to doses of 35-63.3 Gy. “The risk for developing multiple BCCs also appears to be elevated in patients with a history of radiation therapy,” the researchers said. Patients who had been exposed to ionizing ratio were 2.3 times more likely to develop new BCCs compared with unexposed patients (1.7 to 3.1), they said.
The investigators reported no funding sources or conflicts of interest.
SAN DIEGO – Patients treated with ionizing radiation were 2.65 to 3.78 times more likely to develop basal cell carcinoma than were controls, and exposure at younger ages or relatively high doses further increased that risk, according to a pooled analysis presented at the annual meeting of the American Society for Dermatologic Surgery.
“Concomitant exposure to ultraviolet radiation may potentiate this effect,” said Dr. Min Deng, a dermatology resident at the University of Chicago. “With newer treatment protocols and improved shielding, it would be interesting to see if the effect of ionizing radiation has changed, or whether we as dermatologists should more actively screen this at-risk population.”
Basal cell carcinoma (BCC) is the most common skin cancer worldwide, but relatively few dermatology papers have assessed the effects of ionizing radiation on the incidence of BCC, said Dr. Deng and coauthor Dr. Diana Bolotin, also of the University of Chicago.
To better understand the link, the researchers searched PubMed for controlled studies on the topic by using the terms “radiation,” “risk,” and “basal cell carcinoma.” They excluded case reports, animal studies, studies published in languages other than English, and trials of radiation as a treatment of BCC, they said. They also excluded studies of atomic bomb survivors, because exposure was uncontrolled and methods to estimate exposure in this group have changed over time, they noted.
In all, six studies published between 1991 and 2012 met the inclusion criteria, and all six showed a statistically significant relative risk or odds of BCC with ionizing radiation exposure, the investigators reported. Three analyses calculated the relative risk of BCC in patients who received radiation for tinea capitis or who underwent total-body irradiation before hematopoietic cell transplantation, they said. Two studies evaluated the odds of BCC in patients with a past history of radiation exposure, and one study assessed time to subsequent BCCs in patients with a history of BCC.
For the three studies that calculated relative risk, the researchers calculated a pooled RR of BCC after ionizing radiation treatment of 2.65 (95% confidence interval, 1.22-5.72) compared with controls. The study of total-body irradiation (TBI) did not report or control for the primary disease for which patients were treated, “which may have confounded the results,” they said. When they excluded that study from their calculation, the overall RR rose to 3.78 (95% CI, 2.62 to 5.44).
Notably, in the two studies of patients with tinea capitis, the relative risk of BCC fell by 12% to 16% for every additional year of age at which patients received radiation treatment, the researchers said. Similarly, risk of BCC dropped by 10.9% for every 1-year increase in age at total-body irradiation prior to cell transplantation.
The combined odds ratio for the next two studies was 4.28 (1.45-12.63). “Likewise, both studies found an elevated odds ratio with younger age at radiation exposure,” the researchers added. One study that stratified patients by type of medical condition detected a “markedly elevated” 8.7 odds of BCC after radiation treatment for acne (2.0 to 38.0), they noted.
The sixth study was a nested case-control analysis that found a statistically significant increase in the odds of BCC starting at a 1-Gy dose of ionizing radiation, and rising linearly up to doses of 35-63.3 Gy. “The risk for developing multiple BCCs also appears to be elevated in patients with a history of radiation therapy,” the researchers said. Patients who had been exposed to ionizing ratio were 2.3 times more likely to develop new BCCs compared with unexposed patients (1.7 to 3.1), they said.
The investigators reported no funding sources or conflicts of interest.
Key clinical point: Ionizing radiation therapy significantly increased risk of basal cell carcinoma, especially when patients were younger or were treated at relatively high doses.
Major finding: The pooled relative risk of BCC after ionizing radiation treatment was 2.65 (95% confidence interval, 1.22-5.72) compared with controls.
Data source: Pooled analysis of six studies of ionizing radiation exposure and risk or odds of basal cell carcinoma.
Disclosures: The researchers reported no funding sources or conflicts of interest.
Burns, fainting, eyes most common indoor tanning injuries
Skin burns, fainting, and eye injuries are the most common indoor tanning injuries requiring a trip to the local emergency department, according to a research letter published online Dec. 15 in JAMA Internal Medicine.
Indoor tanning exposes users to intense ultraviolet radiation, a known carcinogen, but little is known about the more immediate adverse events related to tanning, wrote Gery P. Guy Jr., Ph.D., of the Centers for Disease Control and Prevention, and coauthors.
After analyzing data from a nationally representative sample of hospital emergency departments from 2003 to 2012, the investigators estimated an average 3,234 indoor tanning–related injuries were treated each year in U.S. hospitals.
The most common injury was skin burns (79.5%), followed by syncope (9.5%) and eye injuries (5.8%).
Injuries occurred most commonly among younger adults and non-Hispanic white females, the populations with the highest rates of indoor tanning.
However, indoor tanning injuries were on the decline, decreasing from 6,487 in 2003 to 1,957 in 2012 (P < .001), a finding that was most likely due to a decline in the use of indoor sun beds, the researchers said.
Most patients did not require hospitalization, but burns severe enough to warrant a trip to the emergency department indicate an overexposure to ultraviolet radiation and an increased risk of skin cancer, the investigators added.
Although the Food and Drug Administration required tanning device manufacturers to install timers to limit exposure, several cases described patients falling asleep while tanning, raising concerns about timers malfunctioning or being intentionally overridden, the researchers noted.
The researchers said they had no relevant financial conflicts to disclose.
Skin burns, fainting, and eye injuries are the most common indoor tanning injuries requiring a trip to the local emergency department, according to a research letter published online Dec. 15 in JAMA Internal Medicine.
Indoor tanning exposes users to intense ultraviolet radiation, a known carcinogen, but little is known about the more immediate adverse events related to tanning, wrote Gery P. Guy Jr., Ph.D., of the Centers for Disease Control and Prevention, and coauthors.
After analyzing data from a nationally representative sample of hospital emergency departments from 2003 to 2012, the investigators estimated an average 3,234 indoor tanning–related injuries were treated each year in U.S. hospitals.
The most common injury was skin burns (79.5%), followed by syncope (9.5%) and eye injuries (5.8%).
Injuries occurred most commonly among younger adults and non-Hispanic white females, the populations with the highest rates of indoor tanning.
However, indoor tanning injuries were on the decline, decreasing from 6,487 in 2003 to 1,957 in 2012 (P < .001), a finding that was most likely due to a decline in the use of indoor sun beds, the researchers said.
Most patients did not require hospitalization, but burns severe enough to warrant a trip to the emergency department indicate an overexposure to ultraviolet radiation and an increased risk of skin cancer, the investigators added.
Although the Food and Drug Administration required tanning device manufacturers to install timers to limit exposure, several cases described patients falling asleep while tanning, raising concerns about timers malfunctioning or being intentionally overridden, the researchers noted.
The researchers said they had no relevant financial conflicts to disclose.
Skin burns, fainting, and eye injuries are the most common indoor tanning injuries requiring a trip to the local emergency department, according to a research letter published online Dec. 15 in JAMA Internal Medicine.
Indoor tanning exposes users to intense ultraviolet radiation, a known carcinogen, but little is known about the more immediate adverse events related to tanning, wrote Gery P. Guy Jr., Ph.D., of the Centers for Disease Control and Prevention, and coauthors.
After analyzing data from a nationally representative sample of hospital emergency departments from 2003 to 2012, the investigators estimated an average 3,234 indoor tanning–related injuries were treated each year in U.S. hospitals.
The most common injury was skin burns (79.5%), followed by syncope (9.5%) and eye injuries (5.8%).
Injuries occurred most commonly among younger adults and non-Hispanic white females, the populations with the highest rates of indoor tanning.
However, indoor tanning injuries were on the decline, decreasing from 6,487 in 2003 to 1,957 in 2012 (P < .001), a finding that was most likely due to a decline in the use of indoor sun beds, the researchers said.
Most patients did not require hospitalization, but burns severe enough to warrant a trip to the emergency department indicate an overexposure to ultraviolet radiation and an increased risk of skin cancer, the investigators added.
Although the Food and Drug Administration required tanning device manufacturers to install timers to limit exposure, several cases described patients falling asleep while tanning, raising concerns about timers malfunctioning or being intentionally overridden, the researchers noted.
The researchers said they had no relevant financial conflicts to disclose.
FROM JAMA INTERNAL MEDICINE
Alpinia officinarum
Alpinia officinarum (and its close relative Alpinia galanga), a member of the Zingiberaceae family (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5), has long been used in Chinese medicinals (Bioorg. Med. Chem. 2009;17:6048-53). Specifically, the plant is used in traditional Chinese medicine as an aphrodisiac, abortifacient, carminative, antipyretic, anti-inflammatory, and emmenagogue as well as to treat disorders of the heart and kidneys, bronchitis, chronic enteritis, renal calculus, diabetes, and rheumatism (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5; Bioorg. Med. Chem. 2009;17:6048-53). Stomach ailments are the most typical application of the herb in traditional Chinese and Thai medicine; it is also used in Ayurveda and Sidda medicine. A. officinarum is widely cultivated throughout Asia, including China, Thailand, India, Sri Lanka, Malaysia, and Indonesia, as well as the Middle East and Northern Africa (Saudi Arabia and Egypt, respectively) (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5).
The flavonoid galangin (3,5,7-trihydroxyflavone) is the primary active constituent of A. officinarum (Phytother. Res. 2014;28:1533-8; J. Cell Biochem. 2013;114:152-61). In vitro, it has demonstrated a cytotoxic effect on multiple cancer cell lines (J. Cell Biochem. 2013;114[1]:152-61). Traditional Uighur medicine in China has incorporated galangin for the treatment of vitiligo (Phytother. Res. 2014;28:1533-8). Overall, A. officinarum rhizomes have been associated with antiemetic, antigenotoxic, antimutagenic, and antioxidant activity, as well as inhibitory effects on prostaglandin and leukotriene biosynthesis, and modulatory effects on cytochrome P450 enzymes (Bioorg. Med. Chem. 2009;17:6048-53; J. Cell Biochem. 2013;114:152-61). The rhizomes of A. officinarum have been used externally to treat skin infections, gum diseases, and skin cancer (J. Nat. Med. 2008;62:374-8).
Constituents
The rhizomes of the plant, commonly referred to as galangal, contain several key active constituents, including essential oils, tannins, neolignans, phenol, glycosides, monoterpenes, diarylheptanoids, phenylpropanoids, carbohydrates, gallic acid glycoside, galangoisoflavonoid, beta-sitosterol, galangin, alpinin, zerumbone, and kampferide (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5; Bioorg. Med. Chem. 2009;17:6048-53; J. Nat. Med. 2008;62:374-8).
In 2009, Matsuda et al. reported that the 80% aqueous acetone extract of the rhizomes of A. officinarum suppressed melanogenesis in theophylline-stimulated murine B16 melanoma 4A5 cells. They found that several isolated constituents had significant IC50 values (10-48 mcm) for inhibiting melanogenesis, including four diarylheptanoids (5-hydroxy-1,7-diphenyl-3-heptanone, 7-(4(‘’)-hydroxy-3(‘’)-methoxyphenyl)-1-phenylhept-4-en-3-one, 5-hydroxy-7-(4(‘’)-hydroxy-3(‘’)-methoxyphenyl)-1-phenyl-3-heptanone, and 3,5-dihydroxy-1,7-diphenylheptane) and two flavonol constituents (kaempferide and galangin). The mRNA expression of tyrosinase and tyrosinase-related proteins-1 and -2 was also hindered by 7-(4(‘’)-hydroxy-3(‘’)-methoxyphenyl)-1-phenylhept-4-en-3-one, kaempferide, and galangin, as was the protein level of a microphthalmia-associated transcription factor, the authors noted (Bioorg. Med. Chem. 2009;17:6048-53).
Biologic activity
Penetration enhancement: In 2000, Shen et al. found that volatile oils from galangal, among other herbs, were effective in enhancing the skin permeation of 5-fluorouracil and notably more effective than azone (Zhong Yao Cai 2000;23:697-9).
Anti-inflammatory effects: In 2008, Yasukawa et al. examined the inhibitory effect of galangal in a two-stage in vivo carcinogenesis model in mice. They observed that the A. officinarum rhizomes displayed significant antitumor-promoting activity against 7,12-dimethylbenz[a]anthracene (DMBA)-initiated and 12-O-tetradecanoylphorbol-13-acetate (TPA)–promoted lesions. Seven diarylheptanoids isolated from the active fraction of the methanol extracts demonstrated significant anti-inflammatory effects against TPA-induced inflammation (J. Nat. Med. 2008;62:374-8).
Cancer prevention and pigmentary effects: Heo et al. reported in 2001 that in vitro and in vivo studies have demonstrated that the flavonoid galangin, found in high concentrations in A. officinarum, as well as the bee product propolis, exhibits significant antioxidant activity and can influence enzyme activities and inhibit genotoxicity without introducing a pro-oxidant effect. They concluded that galangin warrants consideration for its potential as a chemical cancer-preventing agent (Mutat. Res. 2001;488:135-50).
In 2007, Lu et al. investigated the whitening effects of the flavonoid components of A. officinarum on melanin biosynthesis in B16 mouse melanoma cells, tyrosinase inhibition, and UV absorption. They found that galangin and the flavonoid mixture both decreased melanin content more than controls and also lowered melanin production, with galangin more effective than the flavonoid mixture. In addition, galangin and the flavonoid mixture exerted greater tyrosinase inhibition at lower concentrations. The A. officinarum constituents also displayed a broad absorption band in the UVB area (270 to 290 nm). The researchers concluded that galangin may be a viable whitening agent with the potential to prevent skin cancer (J. Enzyme Inhib. Med. Chem. 2007;22:433-8).
Six years later, Zhang et al. noted that various doses of galangin resulted in the inhibition of B16F10 melanoma cell proliferation. The investigators also showed that galangin achieved an antimetastatic effect in vivo in C57BL/6J mice, reducing focal adhesion kinase. They concluded that focal adhesion kinase is a viable target in melanoma therapy, with B16F10 melanoma metastasis apparently checked by galangin in mice and in cell cultures (J. Cell Biochem. 2013;114:152-61).
In 2014, Huo et al. tested galangin in a mouse model of vitiligo induced in C57BL/6 mice through the daily topical application of hydroquinone (2.5%) on shaved dorsal skin for 60 days. Thirty days after the final hydroquinone application, investigators began oral administration of galangin for 30 days. Hair grew back after treatment darker than the original color, with histologic analysis revealing that mice treated with galangin and the positive control 8-methoxypsoralen had an increased number of melanin-containing hair follicles, compared with untreated animals. In addition, galangin treatment was associated with significant increases in the number of cutaneous basal layer melanocytes and melanin-containing epidermal cells. Compared with controls, treatment with galangin and 8-methoxypsoralen led to increased serum levels of tyrosinase and decreased levels of malondialdehyde and lower cholinesterase activity. Galangin and 8-methoxypsoralen use also increased the expression of tyrosinase protein in treated skin. The investigators concluded that galangin improved hydroquinone-induced vitiligo in mice and warrants further study as a potential vitiligo treatment in humans (Phytother. Res. 2014 28:1533-8).
Conclusion
Alpinia officinarum is one of many botanical agents with a long history of applications in traditional folk medicine, particularly in Asia. There is a relative paucity of evidence regarding the dermatologic applications of this plant, but recent findings support continued research into its various potential cutaneous benefits, with particular focus on the main active ingredient galangin.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy,Topix Pharmaceuticals, and Unilever.
Alpinia officinarum (and its close relative Alpinia galanga), a member of the Zingiberaceae family (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5), has long been used in Chinese medicinals (Bioorg. Med. Chem. 2009;17:6048-53). Specifically, the plant is used in traditional Chinese medicine as an aphrodisiac, abortifacient, carminative, antipyretic, anti-inflammatory, and emmenagogue as well as to treat disorders of the heart and kidneys, bronchitis, chronic enteritis, renal calculus, diabetes, and rheumatism (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5; Bioorg. Med. Chem. 2009;17:6048-53). Stomach ailments are the most typical application of the herb in traditional Chinese and Thai medicine; it is also used in Ayurveda and Sidda medicine. A. officinarum is widely cultivated throughout Asia, including China, Thailand, India, Sri Lanka, Malaysia, and Indonesia, as well as the Middle East and Northern Africa (Saudi Arabia and Egypt, respectively) (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5).
The flavonoid galangin (3,5,7-trihydroxyflavone) is the primary active constituent of A. officinarum (Phytother. Res. 2014;28:1533-8; J. Cell Biochem. 2013;114:152-61). In vitro, it has demonstrated a cytotoxic effect on multiple cancer cell lines (J. Cell Biochem. 2013;114[1]:152-61). Traditional Uighur medicine in China has incorporated galangin for the treatment of vitiligo (Phytother. Res. 2014;28:1533-8). Overall, A. officinarum rhizomes have been associated with antiemetic, antigenotoxic, antimutagenic, and antioxidant activity, as well as inhibitory effects on prostaglandin and leukotriene biosynthesis, and modulatory effects on cytochrome P450 enzymes (Bioorg. Med. Chem. 2009;17:6048-53; J. Cell Biochem. 2013;114:152-61). The rhizomes of A. officinarum have been used externally to treat skin infections, gum diseases, and skin cancer (J. Nat. Med. 2008;62:374-8).
Constituents
The rhizomes of the plant, commonly referred to as galangal, contain several key active constituents, including essential oils, tannins, neolignans, phenol, glycosides, monoterpenes, diarylheptanoids, phenylpropanoids, carbohydrates, gallic acid glycoside, galangoisoflavonoid, beta-sitosterol, galangin, alpinin, zerumbone, and kampferide (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5; Bioorg. Med. Chem. 2009;17:6048-53; J. Nat. Med. 2008;62:374-8).
In 2009, Matsuda et al. reported that the 80% aqueous acetone extract of the rhizomes of A. officinarum suppressed melanogenesis in theophylline-stimulated murine B16 melanoma 4A5 cells. They found that several isolated constituents had significant IC50 values (10-48 mcm) for inhibiting melanogenesis, including four diarylheptanoids (5-hydroxy-1,7-diphenyl-3-heptanone, 7-(4(‘’)-hydroxy-3(‘’)-methoxyphenyl)-1-phenylhept-4-en-3-one, 5-hydroxy-7-(4(‘’)-hydroxy-3(‘’)-methoxyphenyl)-1-phenyl-3-heptanone, and 3,5-dihydroxy-1,7-diphenylheptane) and two flavonol constituents (kaempferide and galangin). The mRNA expression of tyrosinase and tyrosinase-related proteins-1 and -2 was also hindered by 7-(4(‘’)-hydroxy-3(‘’)-methoxyphenyl)-1-phenylhept-4-en-3-one, kaempferide, and galangin, as was the protein level of a microphthalmia-associated transcription factor, the authors noted (Bioorg. Med. Chem. 2009;17:6048-53).
Biologic activity
Penetration enhancement: In 2000, Shen et al. found that volatile oils from galangal, among other herbs, were effective in enhancing the skin permeation of 5-fluorouracil and notably more effective than azone (Zhong Yao Cai 2000;23:697-9).
Anti-inflammatory effects: In 2008, Yasukawa et al. examined the inhibitory effect of galangal in a two-stage in vivo carcinogenesis model in mice. They observed that the A. officinarum rhizomes displayed significant antitumor-promoting activity against 7,12-dimethylbenz[a]anthracene (DMBA)-initiated and 12-O-tetradecanoylphorbol-13-acetate (TPA)–promoted lesions. Seven diarylheptanoids isolated from the active fraction of the methanol extracts demonstrated significant anti-inflammatory effects against TPA-induced inflammation (J. Nat. Med. 2008;62:374-8).
Cancer prevention and pigmentary effects: Heo et al. reported in 2001 that in vitro and in vivo studies have demonstrated that the flavonoid galangin, found in high concentrations in A. officinarum, as well as the bee product propolis, exhibits significant antioxidant activity and can influence enzyme activities and inhibit genotoxicity without introducing a pro-oxidant effect. They concluded that galangin warrants consideration for its potential as a chemical cancer-preventing agent (Mutat. Res. 2001;488:135-50).
In 2007, Lu et al. investigated the whitening effects of the flavonoid components of A. officinarum on melanin biosynthesis in B16 mouse melanoma cells, tyrosinase inhibition, and UV absorption. They found that galangin and the flavonoid mixture both decreased melanin content more than controls and also lowered melanin production, with galangin more effective than the flavonoid mixture. In addition, galangin and the flavonoid mixture exerted greater tyrosinase inhibition at lower concentrations. The A. officinarum constituents also displayed a broad absorption band in the UVB area (270 to 290 nm). The researchers concluded that galangin may be a viable whitening agent with the potential to prevent skin cancer (J. Enzyme Inhib. Med. Chem. 2007;22:433-8).
Six years later, Zhang et al. noted that various doses of galangin resulted in the inhibition of B16F10 melanoma cell proliferation. The investigators also showed that galangin achieved an antimetastatic effect in vivo in C57BL/6J mice, reducing focal adhesion kinase. They concluded that focal adhesion kinase is a viable target in melanoma therapy, with B16F10 melanoma metastasis apparently checked by galangin in mice and in cell cultures (J. Cell Biochem. 2013;114:152-61).
In 2014, Huo et al. tested galangin in a mouse model of vitiligo induced in C57BL/6 mice through the daily topical application of hydroquinone (2.5%) on shaved dorsal skin for 60 days. Thirty days after the final hydroquinone application, investigators began oral administration of galangin for 30 days. Hair grew back after treatment darker than the original color, with histologic analysis revealing that mice treated with galangin and the positive control 8-methoxypsoralen had an increased number of melanin-containing hair follicles, compared with untreated animals. In addition, galangin treatment was associated with significant increases in the number of cutaneous basal layer melanocytes and melanin-containing epidermal cells. Compared with controls, treatment with galangin and 8-methoxypsoralen led to increased serum levels of tyrosinase and decreased levels of malondialdehyde and lower cholinesterase activity. Galangin and 8-methoxypsoralen use also increased the expression of tyrosinase protein in treated skin. The investigators concluded that galangin improved hydroquinone-induced vitiligo in mice and warrants further study as a potential vitiligo treatment in humans (Phytother. Res. 2014 28:1533-8).
Conclusion
Alpinia officinarum is one of many botanical agents with a long history of applications in traditional folk medicine, particularly in Asia. There is a relative paucity of evidence regarding the dermatologic applications of this plant, but recent findings support continued research into its various potential cutaneous benefits, with particular focus on the main active ingredient galangin.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy,Topix Pharmaceuticals, and Unilever.
Alpinia officinarum (and its close relative Alpinia galanga), a member of the Zingiberaceae family (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5), has long been used in Chinese medicinals (Bioorg. Med. Chem. 2009;17:6048-53). Specifically, the plant is used in traditional Chinese medicine as an aphrodisiac, abortifacient, carminative, antipyretic, anti-inflammatory, and emmenagogue as well as to treat disorders of the heart and kidneys, bronchitis, chronic enteritis, renal calculus, diabetes, and rheumatism (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5; Bioorg. Med. Chem. 2009;17:6048-53). Stomach ailments are the most typical application of the herb in traditional Chinese and Thai medicine; it is also used in Ayurveda and Sidda medicine. A. officinarum is widely cultivated throughout Asia, including China, Thailand, India, Sri Lanka, Malaysia, and Indonesia, as well as the Middle East and Northern Africa (Saudi Arabia and Egypt, respectively) (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5).
The flavonoid galangin (3,5,7-trihydroxyflavone) is the primary active constituent of A. officinarum (Phytother. Res. 2014;28:1533-8; J. Cell Biochem. 2013;114:152-61). In vitro, it has demonstrated a cytotoxic effect on multiple cancer cell lines (J. Cell Biochem. 2013;114[1]:152-61). Traditional Uighur medicine in China has incorporated galangin for the treatment of vitiligo (Phytother. Res. 2014;28:1533-8). Overall, A. officinarum rhizomes have been associated with antiemetic, antigenotoxic, antimutagenic, and antioxidant activity, as well as inhibitory effects on prostaglandin and leukotriene biosynthesis, and modulatory effects on cytochrome P450 enzymes (Bioorg. Med. Chem. 2009;17:6048-53; J. Cell Biochem. 2013;114:152-61). The rhizomes of A. officinarum have been used externally to treat skin infections, gum diseases, and skin cancer (J. Nat. Med. 2008;62:374-8).
Constituents
The rhizomes of the plant, commonly referred to as galangal, contain several key active constituents, including essential oils, tannins, neolignans, phenol, glycosides, monoterpenes, diarylheptanoids, phenylpropanoids, carbohydrates, gallic acid glycoside, galangoisoflavonoid, beta-sitosterol, galangin, alpinin, zerumbone, and kampferide (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5; Bioorg. Med. Chem. 2009;17:6048-53; J. Nat. Med. 2008;62:374-8).
In 2009, Matsuda et al. reported that the 80% aqueous acetone extract of the rhizomes of A. officinarum suppressed melanogenesis in theophylline-stimulated murine B16 melanoma 4A5 cells. They found that several isolated constituents had significant IC50 values (10-48 mcm) for inhibiting melanogenesis, including four diarylheptanoids (5-hydroxy-1,7-diphenyl-3-heptanone, 7-(4(‘’)-hydroxy-3(‘’)-methoxyphenyl)-1-phenylhept-4-en-3-one, 5-hydroxy-7-(4(‘’)-hydroxy-3(‘’)-methoxyphenyl)-1-phenyl-3-heptanone, and 3,5-dihydroxy-1,7-diphenylheptane) and two flavonol constituents (kaempferide and galangin). The mRNA expression of tyrosinase and tyrosinase-related proteins-1 and -2 was also hindered by 7-(4(‘’)-hydroxy-3(‘’)-methoxyphenyl)-1-phenylhept-4-en-3-one, kaempferide, and galangin, as was the protein level of a microphthalmia-associated transcription factor, the authors noted (Bioorg. Med. Chem. 2009;17:6048-53).
Biologic activity
Penetration enhancement: In 2000, Shen et al. found that volatile oils from galangal, among other herbs, were effective in enhancing the skin permeation of 5-fluorouracil and notably more effective than azone (Zhong Yao Cai 2000;23:697-9).
Anti-inflammatory effects: In 2008, Yasukawa et al. examined the inhibitory effect of galangal in a two-stage in vivo carcinogenesis model in mice. They observed that the A. officinarum rhizomes displayed significant antitumor-promoting activity against 7,12-dimethylbenz[a]anthracene (DMBA)-initiated and 12-O-tetradecanoylphorbol-13-acetate (TPA)–promoted lesions. Seven diarylheptanoids isolated from the active fraction of the methanol extracts demonstrated significant anti-inflammatory effects against TPA-induced inflammation (J. Nat. Med. 2008;62:374-8).
Cancer prevention and pigmentary effects: Heo et al. reported in 2001 that in vitro and in vivo studies have demonstrated that the flavonoid galangin, found in high concentrations in A. officinarum, as well as the bee product propolis, exhibits significant antioxidant activity and can influence enzyme activities and inhibit genotoxicity without introducing a pro-oxidant effect. They concluded that galangin warrants consideration for its potential as a chemical cancer-preventing agent (Mutat. Res. 2001;488:135-50).
In 2007, Lu et al. investigated the whitening effects of the flavonoid components of A. officinarum on melanin biosynthesis in B16 mouse melanoma cells, tyrosinase inhibition, and UV absorption. They found that galangin and the flavonoid mixture both decreased melanin content more than controls and also lowered melanin production, with galangin more effective than the flavonoid mixture. In addition, galangin and the flavonoid mixture exerted greater tyrosinase inhibition at lower concentrations. The A. officinarum constituents also displayed a broad absorption band in the UVB area (270 to 290 nm). The researchers concluded that galangin may be a viable whitening agent with the potential to prevent skin cancer (J. Enzyme Inhib. Med. Chem. 2007;22:433-8).
Six years later, Zhang et al. noted that various doses of galangin resulted in the inhibition of B16F10 melanoma cell proliferation. The investigators also showed that galangin achieved an antimetastatic effect in vivo in C57BL/6J mice, reducing focal adhesion kinase. They concluded that focal adhesion kinase is a viable target in melanoma therapy, with B16F10 melanoma metastasis apparently checked by galangin in mice and in cell cultures (J. Cell Biochem. 2013;114:152-61).
In 2014, Huo et al. tested galangin in a mouse model of vitiligo induced in C57BL/6 mice through the daily topical application of hydroquinone (2.5%) on shaved dorsal skin for 60 days. Thirty days after the final hydroquinone application, investigators began oral administration of galangin for 30 days. Hair grew back after treatment darker than the original color, with histologic analysis revealing that mice treated with galangin and the positive control 8-methoxypsoralen had an increased number of melanin-containing hair follicles, compared with untreated animals. In addition, galangin treatment was associated with significant increases in the number of cutaneous basal layer melanocytes and melanin-containing epidermal cells. Compared with controls, treatment with galangin and 8-methoxypsoralen led to increased serum levels of tyrosinase and decreased levels of malondialdehyde and lower cholinesterase activity. Galangin and 8-methoxypsoralen use also increased the expression of tyrosinase protein in treated skin. The investigators concluded that galangin improved hydroquinone-induced vitiligo in mice and warrants further study as a potential vitiligo treatment in humans (Phytother. Res. 2014 28:1533-8).
Conclusion
Alpinia officinarum is one of many botanical agents with a long history of applications in traditional folk medicine, particularly in Asia. There is a relative paucity of evidence regarding the dermatologic applications of this plant, but recent findings support continued research into its various potential cutaneous benefits, with particular focus on the main active ingredient galangin.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy,Topix Pharmaceuticals, and Unilever.
What Is Your Diagnosis? Clear Cell Hidradenoma
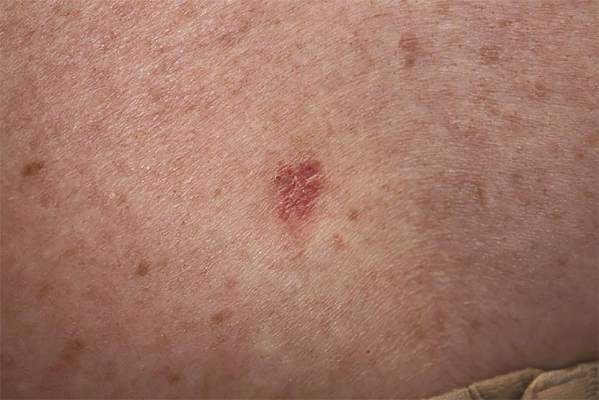
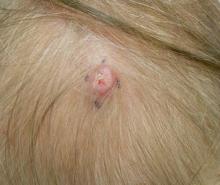
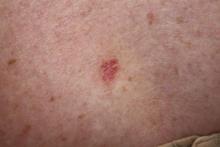
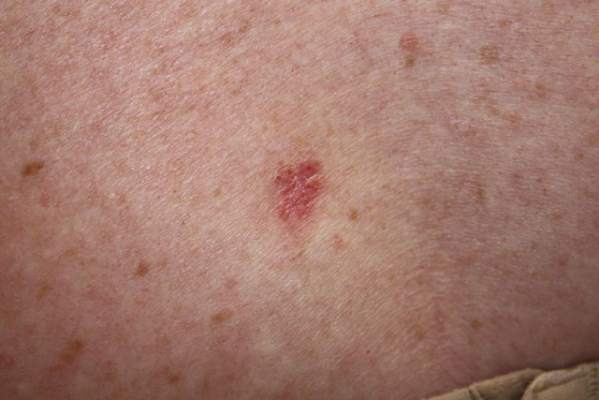
A 70-year-old woman presented to our dermatology clinic with an enlarging lesion on the left anterior aspect of the scalp of 4 years’ duration. She had a history of breast carcinoma in the left breast with positive lymph nodes 2 years prior. Physical examination revealed a 2.5-cm pink, pearly, exophytic plaque on the left anterior aspect of the scalp. The lesion was removed with clear margins by excisional surgery.
The Diagnosis: Clear Cell Hidradenoma
Clear cell hidradenoma (CCH) is a variant of nodular hidradenoma that may contain varying quantities of solid and cystic components and comprises approximately one-third of hidradenomas.1 Clear cell hidradenomas are slow-growing and fairly uncommon adnexal neoplasms derived from either eccrine sweat glands or apocrine glands. Some researchers have regarded hidradenomas as apocrine tumors due to evidence of apocrine decapitation secretion, whereas others note the lack of apocrine and ultrastructural features of immature eccrine glands.2 Clear cell hidradenomas typically develop between the fourth and eighth decades of life, usually peaking during the sixth decade.3 Clear cell hidradenomas usually range in size from 5 to 30 mm and frequently present on the scalp, head, chest, and abdomen; rarely, CCHs present on the joint spaces of the shoulders and knees.3-5 This neoplasm is more common in women than men3 and generally has a flesh-colored, erythematous, red-brown or blue appearance with a tendency to ulcerate and exude a serous discharge (Figure 1).5 The clinical differential diagnosis includes metastatic cancer (eg, renal cell carcinoma, keratoacanthoma, trichoblastoma, trichilemmoma) or other benign adnexal neoplasms.
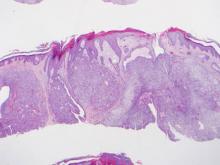
Histopathologic examination of a CCH generally reveals an unencapsulated and circumscribed neoplasm in the mid or upper dermis with occasional extensions into the subcutaneous fat (Figure 2). The tumor typically presents with 2 types of cells: (1) round, fusiform, or polygonal cells with vesicular nuclei and eosinophilic cytoplasm, and (2) cells with clear cytoplasm and basophilic, often eccentrically located nuclei.6 Ducts are scattered within the neoplasm and are lined by a layer of cuboidal cells that can be highlighted on carcinoembryonic antigen and epithelial membrane antigen immunostaining.6 The tumor cells themselves are highlighted on cytokeratin AE1/AE3 staining.
Malignant transformation rarely is associated with CCH, with de novo clear cell hidradenocarcinoma being more common. Only approximately 6.7% of CCHs have been shown to be malignant, and the malignant tumors feature nuclear atypia, abnormal mitotic figures, necrosis, and infiltration.1,7 Although CCH is a benign adnexal neoplasm, it has a high recurrence rate (approximately 10%) following excision.7 The treatment of choice is complete surgical excision, though Mohs micrographic surgery is advocated, as it promotes thorough examination of the tumor margin to ensure complete tumor removal.8 Our case illustrates the importance of a broad differential diagnosis when treating patients with CCH as well as keeping in mind nonmalignant lesions are far more common than malignant lesions.
1. Volmar K, Cummings TJ, Wang WH, et al. Clear cell hidradenoma: a mimic of metastatic clear cell tumors. Arch Pathol Lab Med. 2005;129:E113-E116.
2. Goh SG, Carr R, Dayrit JF, et al. Mucinous hidradenoma: a report of three cases. J Cutan Pathol. 2007;34:497-502.
3. Gonul M, Cakmak SK, Gul U, et al. A skin tumor in a young girl. diagnosis: clear cell hidradenoma. Indian J Dermatol Venereol Leprol. 2010;76:445-446.
4. Singhal V, Sharma SC, Anil J, et al. Giant benign nodular hidradenoma of the shoulder: a rare tumor of orthopedic practice. Int J Shoulder Surg. 2010;4:93-96.
5. Yu G, Goodloe S Jr, D’Angelis CA, et al. Giant clear cell hidradenoma of the knee. J Cutan Pathol. 2010;37:E37-E41.
6. McKee PH, Calonje E, Granter SR. Tumors of the sweat glands. In: McKee PH, Calonje E, Granter SR. Pathology of the Skin With Clinical Correlations. 3rd ed. Philadelphia, PA: Elsevier Mosby; 2005:1632-1635.
7. Ozawa T, Fujiwara M, Nose K, et al. Clear-cell hidradenoma of the forearm in a young boy. Pediatr Dermatol. 2005;22:450-452.
8. Yavel R, Hinshaw M, Rao V, et al. Hidradenoma and a hidradenocarcinoma of the scalp managed by Mohs micrographic surgery and a multidisciplinary approach: case reports and review of the literature. Dermatol Surg. 2009;35:273-281.
A 70-year-old woman presented to our dermatology clinic with an enlarging lesion on the left anterior aspect of the scalp of 4 years’ duration. She had a history of breast carcinoma in the left breast with positive lymph nodes 2 years prior. Physical examination revealed a 2.5-cm pink, pearly, exophytic plaque on the left anterior aspect of the scalp. The lesion was removed with clear margins by excisional surgery.
The Diagnosis: Clear Cell Hidradenoma
Clear cell hidradenoma (CCH) is a variant of nodular hidradenoma that may contain varying quantities of solid and cystic components and comprises approximately one-third of hidradenomas.1 Clear cell hidradenomas are slow-growing and fairly uncommon adnexal neoplasms derived from either eccrine sweat glands or apocrine glands. Some researchers have regarded hidradenomas as apocrine tumors due to evidence of apocrine decapitation secretion, whereas others note the lack of apocrine and ultrastructural features of immature eccrine glands.2 Clear cell hidradenomas typically develop between the fourth and eighth decades of life, usually peaking during the sixth decade.3 Clear cell hidradenomas usually range in size from 5 to 30 mm and frequently present on the scalp, head, chest, and abdomen; rarely, CCHs present on the joint spaces of the shoulders and knees.3-5 This neoplasm is more common in women than men3 and generally has a flesh-colored, erythematous, red-brown or blue appearance with a tendency to ulcerate and exude a serous discharge (Figure 1).5 The clinical differential diagnosis includes metastatic cancer (eg, renal cell carcinoma, keratoacanthoma, trichoblastoma, trichilemmoma) or other benign adnexal neoplasms.
Histopathologic examination of a CCH generally reveals an unencapsulated and circumscribed neoplasm in the mid or upper dermis with occasional extensions into the subcutaneous fat (Figure 2). The tumor typically presents with 2 types of cells: (1) round, fusiform, or polygonal cells with vesicular nuclei and eosinophilic cytoplasm, and (2) cells with clear cytoplasm and basophilic, often eccentrically located nuclei.6 Ducts are scattered within the neoplasm and are lined by a layer of cuboidal cells that can be highlighted on carcinoembryonic antigen and epithelial membrane antigen immunostaining.6 The tumor cells themselves are highlighted on cytokeratin AE1/AE3 staining.
Malignant transformation rarely is associated with CCH, with de novo clear cell hidradenocarcinoma being more common. Only approximately 6.7% of CCHs have been shown to be malignant, and the malignant tumors feature nuclear atypia, abnormal mitotic figures, necrosis, and infiltration.1,7 Although CCH is a benign adnexal neoplasm, it has a high recurrence rate (approximately 10%) following excision.7 The treatment of choice is complete surgical excision, though Mohs micrographic surgery is advocated, as it promotes thorough examination of the tumor margin to ensure complete tumor removal.8 Our case illustrates the importance of a broad differential diagnosis when treating patients with CCH as well as keeping in mind nonmalignant lesions are far more common than malignant lesions.
A 70-year-old woman presented to our dermatology clinic with an enlarging lesion on the left anterior aspect of the scalp of 4 years’ duration. She had a history of breast carcinoma in the left breast with positive lymph nodes 2 years prior. Physical examination revealed a 2.5-cm pink, pearly, exophytic plaque on the left anterior aspect of the scalp. The lesion was removed with clear margins by excisional surgery.
The Diagnosis: Clear Cell Hidradenoma
Clear cell hidradenoma (CCH) is a variant of nodular hidradenoma that may contain varying quantities of solid and cystic components and comprises approximately one-third of hidradenomas.1 Clear cell hidradenomas are slow-growing and fairly uncommon adnexal neoplasms derived from either eccrine sweat glands or apocrine glands. Some researchers have regarded hidradenomas as apocrine tumors due to evidence of apocrine decapitation secretion, whereas others note the lack of apocrine and ultrastructural features of immature eccrine glands.2 Clear cell hidradenomas typically develop between the fourth and eighth decades of life, usually peaking during the sixth decade.3 Clear cell hidradenomas usually range in size from 5 to 30 mm and frequently present on the scalp, head, chest, and abdomen; rarely, CCHs present on the joint spaces of the shoulders and knees.3-5 This neoplasm is more common in women than men3 and generally has a flesh-colored, erythematous, red-brown or blue appearance with a tendency to ulcerate and exude a serous discharge (Figure 1).5 The clinical differential diagnosis includes metastatic cancer (eg, renal cell carcinoma, keratoacanthoma, trichoblastoma, trichilemmoma) or other benign adnexal neoplasms.
Histopathologic examination of a CCH generally reveals an unencapsulated and circumscribed neoplasm in the mid or upper dermis with occasional extensions into the subcutaneous fat (Figure 2). The tumor typically presents with 2 types of cells: (1) round, fusiform, or polygonal cells with vesicular nuclei and eosinophilic cytoplasm, and (2) cells with clear cytoplasm and basophilic, often eccentrically located nuclei.6 Ducts are scattered within the neoplasm and are lined by a layer of cuboidal cells that can be highlighted on carcinoembryonic antigen and epithelial membrane antigen immunostaining.6 The tumor cells themselves are highlighted on cytokeratin AE1/AE3 staining.
Malignant transformation rarely is associated with CCH, with de novo clear cell hidradenocarcinoma being more common. Only approximately 6.7% of CCHs have been shown to be malignant, and the malignant tumors feature nuclear atypia, abnormal mitotic figures, necrosis, and infiltration.1,7 Although CCH is a benign adnexal neoplasm, it has a high recurrence rate (approximately 10%) following excision.7 The treatment of choice is complete surgical excision, though Mohs micrographic surgery is advocated, as it promotes thorough examination of the tumor margin to ensure complete tumor removal.8 Our case illustrates the importance of a broad differential diagnosis when treating patients with CCH as well as keeping in mind nonmalignant lesions are far more common than malignant lesions.
1. Volmar K, Cummings TJ, Wang WH, et al. Clear cell hidradenoma: a mimic of metastatic clear cell tumors. Arch Pathol Lab Med. 2005;129:E113-E116.
2. Goh SG, Carr R, Dayrit JF, et al. Mucinous hidradenoma: a report of three cases. J Cutan Pathol. 2007;34:497-502.
3. Gonul M, Cakmak SK, Gul U, et al. A skin tumor in a young girl. diagnosis: clear cell hidradenoma. Indian J Dermatol Venereol Leprol. 2010;76:445-446.
4. Singhal V, Sharma SC, Anil J, et al. Giant benign nodular hidradenoma of the shoulder: a rare tumor of orthopedic practice. Int J Shoulder Surg. 2010;4:93-96.
5. Yu G, Goodloe S Jr, D’Angelis CA, et al. Giant clear cell hidradenoma of the knee. J Cutan Pathol. 2010;37:E37-E41.
6. McKee PH, Calonje E, Granter SR. Tumors of the sweat glands. In: McKee PH, Calonje E, Granter SR. Pathology of the Skin With Clinical Correlations. 3rd ed. Philadelphia, PA: Elsevier Mosby; 2005:1632-1635.
7. Ozawa T, Fujiwara M, Nose K, et al. Clear-cell hidradenoma of the forearm in a young boy. Pediatr Dermatol. 2005;22:450-452.
8. Yavel R, Hinshaw M, Rao V, et al. Hidradenoma and a hidradenocarcinoma of the scalp managed by Mohs micrographic surgery and a multidisciplinary approach: case reports and review of the literature. Dermatol Surg. 2009;35:273-281.
1. Volmar K, Cummings TJ, Wang WH, et al. Clear cell hidradenoma: a mimic of metastatic clear cell tumors. Arch Pathol Lab Med. 2005;129:E113-E116.
2. Goh SG, Carr R, Dayrit JF, et al. Mucinous hidradenoma: a report of three cases. J Cutan Pathol. 2007;34:497-502.
3. Gonul M, Cakmak SK, Gul U, et al. A skin tumor in a young girl. diagnosis: clear cell hidradenoma. Indian J Dermatol Venereol Leprol. 2010;76:445-446.
4. Singhal V, Sharma SC, Anil J, et al. Giant benign nodular hidradenoma of the shoulder: a rare tumor of orthopedic practice. Int J Shoulder Surg. 2010;4:93-96.
5. Yu G, Goodloe S Jr, D’Angelis CA, et al. Giant clear cell hidradenoma of the knee. J Cutan Pathol. 2010;37:E37-E41.
6. McKee PH, Calonje E, Granter SR. Tumors of the sweat glands. In: McKee PH, Calonje E, Granter SR. Pathology of the Skin With Clinical Correlations. 3rd ed. Philadelphia, PA: Elsevier Mosby; 2005:1632-1635.
7. Ozawa T, Fujiwara M, Nose K, et al. Clear-cell hidradenoma of the forearm in a young boy. Pediatr Dermatol. 2005;22:450-452.
8. Yavel R, Hinshaw M, Rao V, et al. Hidradenoma and a hidradenocarcinoma of the scalp managed by Mohs micrographic surgery and a multidisciplinary approach: case reports and review of the literature. Dermatol Surg. 2009;35:273-281.
At-home radiofrequency devices
The field of body contouring and tissue tightening has expanded over the years, with many new devices appearing on the market that utilize radiofrequency (RF) energy to tighten and rejuvenate the skin. What originally began with a single monopolar RF device has progressed to a world in which there are skin-tightening devices that use bipolar energy and tripolar energy, as well as monopolar, and newer machines that boast five and eight poles of RF energy.
In addition to in-office radiofrequency devices, at-home devices are now available.
Radiofrequency energy uses the tissue’s resistance within the various layers of the skin to transform the RF energy given to the skin into thermal energy. This process induces collagen remodeling and neocollagenesis, resulting in skin tightening. Since RF energy produces an electrical current instead of a light source like lasers, tissue damage can be minimized, and epidermal melanin is not targeted or typically damaged. Therefore, RF energies can be used for patients of all skin types and colors. Adverse events to RF therapy in general may include pain, erythema, swelling, and rare reports of burns or fat atrophy with first-generation devices.
Many at-home devices delivering RF energy have been developed and are now on the market for skin tightening and rejuvenation. These devices range in cost from about $30 to more than $1,000, and are marketed for skin tightening as well as body contouring. Most machines require multiple uses, daily or weekly, to achieve desired results, compared with in-office devices that are typically used once, or not more than once every 6 months. A recent study published in the Journal of Drugs in Dermatology of a newer at-home device that uses phase-controlled multisource radiofrequency technology found statistically significant improvement using a Fitzpatrick wrinkle and elastosis scale of 62 patients when pre- and post-photographs of 62 patients were evaluated by three independent board-certified dermatologists.
At-home devices do not deliver energies as high as in-office devices, and no head-to-head studies comparing in-office versus at-home RF devices are currently available. As even in-office radiofrequency device results can be subtle, or occur over 6 months, patient expectations should be managed, and clinicians should be realistic when counseling patients about the use of these devices. Patient selection is key for successful therapy. If skin laxity is severe enough that the patient warrants a face lift or surgical correction to achieve the desired results, then they may not be the best candidate for RF therapy alone.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
The field of body contouring and tissue tightening has expanded over the years, with many new devices appearing on the market that utilize radiofrequency (RF) energy to tighten and rejuvenate the skin. What originally began with a single monopolar RF device has progressed to a world in which there are skin-tightening devices that use bipolar energy and tripolar energy, as well as monopolar, and newer machines that boast five and eight poles of RF energy.
In addition to in-office radiofrequency devices, at-home devices are now available.
Radiofrequency energy uses the tissue’s resistance within the various layers of the skin to transform the RF energy given to the skin into thermal energy. This process induces collagen remodeling and neocollagenesis, resulting in skin tightening. Since RF energy produces an electrical current instead of a light source like lasers, tissue damage can be minimized, and epidermal melanin is not targeted or typically damaged. Therefore, RF energies can be used for patients of all skin types and colors. Adverse events to RF therapy in general may include pain, erythema, swelling, and rare reports of burns or fat atrophy with first-generation devices.
Many at-home devices delivering RF energy have been developed and are now on the market for skin tightening and rejuvenation. These devices range in cost from about $30 to more than $1,000, and are marketed for skin tightening as well as body contouring. Most machines require multiple uses, daily or weekly, to achieve desired results, compared with in-office devices that are typically used once, or not more than once every 6 months. A recent study published in the Journal of Drugs in Dermatology of a newer at-home device that uses phase-controlled multisource radiofrequency technology found statistically significant improvement using a Fitzpatrick wrinkle and elastosis scale of 62 patients when pre- and post-photographs of 62 patients were evaluated by three independent board-certified dermatologists.
At-home devices do not deliver energies as high as in-office devices, and no head-to-head studies comparing in-office versus at-home RF devices are currently available. As even in-office radiofrequency device results can be subtle, or occur over 6 months, patient expectations should be managed, and clinicians should be realistic when counseling patients about the use of these devices. Patient selection is key for successful therapy. If skin laxity is severe enough that the patient warrants a face lift or surgical correction to achieve the desired results, then they may not be the best candidate for RF therapy alone.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
The field of body contouring and tissue tightening has expanded over the years, with many new devices appearing on the market that utilize radiofrequency (RF) energy to tighten and rejuvenate the skin. What originally began with a single monopolar RF device has progressed to a world in which there are skin-tightening devices that use bipolar energy and tripolar energy, as well as monopolar, and newer machines that boast five and eight poles of RF energy.
In addition to in-office radiofrequency devices, at-home devices are now available.
Radiofrequency energy uses the tissue’s resistance within the various layers of the skin to transform the RF energy given to the skin into thermal energy. This process induces collagen remodeling and neocollagenesis, resulting in skin tightening. Since RF energy produces an electrical current instead of a light source like lasers, tissue damage can be minimized, and epidermal melanin is not targeted or typically damaged. Therefore, RF energies can be used for patients of all skin types and colors. Adverse events to RF therapy in general may include pain, erythema, swelling, and rare reports of burns or fat atrophy with first-generation devices.
Many at-home devices delivering RF energy have been developed and are now on the market for skin tightening and rejuvenation. These devices range in cost from about $30 to more than $1,000, and are marketed for skin tightening as well as body contouring. Most machines require multiple uses, daily or weekly, to achieve desired results, compared with in-office devices that are typically used once, or not more than once every 6 months. A recent study published in the Journal of Drugs in Dermatology of a newer at-home device that uses phase-controlled multisource radiofrequency technology found statistically significant improvement using a Fitzpatrick wrinkle and elastosis scale of 62 patients when pre- and post-photographs of 62 patients were evaluated by three independent board-certified dermatologists.
At-home devices do not deliver energies as high as in-office devices, and no head-to-head studies comparing in-office versus at-home RF devices are currently available. As even in-office radiofrequency device results can be subtle, or occur over 6 months, patient expectations should be managed, and clinicians should be realistic when counseling patients about the use of these devices. Patient selection is key for successful therapy. If skin laxity is severe enough that the patient warrants a face lift or surgical correction to achieve the desired results, then they may not be the best candidate for RF therapy alone.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
Large Subcutaneous Masses
The Diagnosis: Madelung Disease (Benign Symmetric Lipomatosis)
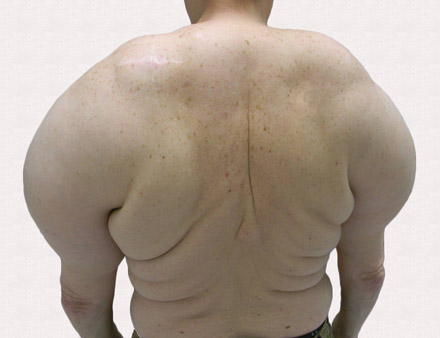
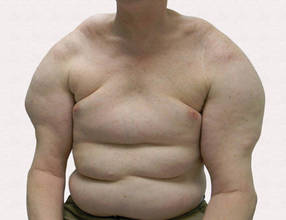
A 56-year-old man presented for evaluation of massive subcutaneous nodules the bilateral upper arms, shoulders, chest, abdomen, and lateral aspect of the proximal thighs (Figures 1 and 2) that developed over the last 12 to 18 months and continued to enlarge. In addition, he was beginning to develop symptoms of neuropathy of the bilateral hands. The patient had a long-standing history of alcohol abuse. Biopsies performed by the patient’s primary care physician revealed benign adipose tissue. He was referred to the dermatology clinic and subsequently diagnosed with Madelung disease.
 |
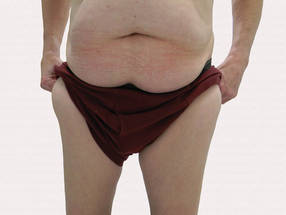 |
Madelung disease, also known as benign symmetric lipomatosis and Launois-Bensaude syndrome, is characterized by multiple large masses of nonencapsulated adipose tissue. These masses are symmetric and most prominent on the head, neck, trunk, and proximal extremities. Classically, a pseudoathletic appearance is described. Madelung disease most frequently affects men aged 30 to 60 years. In more than 90% of cases, it is associated with alcoholism.
In general, the masses of adipose tissue are asymptomatic. However, airway compression and dysphagia requiring surgical intervention has been reported in the otolaryngology literature.1 In addition, neuropathy develops in 84% of cases.2 Nerve biopsies from patients with Madelung disease have revealed a pattern of axonopathy that is distinct from alcohol-induced neuropathy.3 This neuropathy can involve sensory and motor nerves, with the most prominent findings being muscle weakness, tendon areflexia, interosseous muscle atrophy, vibratory sensation loss, and hypoesthesia. Furthermore, dysfunction of the autonomic nervous system can lead to segmental hyperhidrosis, gustatory sweating, and abnormal autonomic cardiovascular reflexes.2 Many patients who develop neuropathy will eventually become incapacitated.
The etiology of Madelung disease is not fully understood. There are several theories on the pathogenesis of this disease, most describing metabolic disturbances induced by alcohol. Specifically, studies have revealed chronic alcohol use causes numerous deletions in mitochondrial DNA.4,5 The mitochondrial DNA damage may explain both the resistance of the lipomatous masses to lipolysis and the nerve-related changes. Comparisons between human immunodeficiency virus/highly active antiretroviral therapy–associated lipodystrophy and Madelung disease lend credence to the metabolic disturbance theory and may help clarify the specific mechanisms involved.6
There have been no cases of spontaneous resolution, even in patients who stop consuming alcohol. For this reason, most patients are referred to a surgeon. Many surgeons prefer open excision for debulking large lipomatous masses, but this technique typically requires general anesthesia. For those in whom this treatment is not an appropriate option, liposuction can be considered with tumescent anesthesia.7 A combination of these surgical modalities also is an option.
Madelung disease is a distinctive disorder typically affecting chronic alcoholics. Recognition of this clinical entity is important, as severe neuropathy and airway compromise may ensue. Although surgical excision is an attractive option for cosmesis and airway compromise, the associated neuropathy can be extremely difficult to treat and can be quite debilitating.
1. Palacios E, Neitzschman HR, Nguyen J. Madelung disease: multiple symmetric lipomatosis. Ear Nose Throat J. 2014;93:94-96.
2. Enzi G, Angelini C, Negrin P, et al. Sensory, motor, and autonomic neuropathy in patients with multiple symmetric lipomatosis. Medicine (Baltimore). 1985;64:388-393.
3. Pollock M, Nicholson GI, Nukada H, et al. Neuropathy in multiple symmetric lipomatosis. Madelung’s disease. Brain. 1988;111:1157-1171.
4. Klopstock T, Naumann M, Schalke B, et al. Multiple symmetric lipomatosis: abnormalities in complex IV and multiple deletions in mitochondrial DNA. Neurology. 1994;44:862-866.
5. Mansouri A, Fromenty B, Berson A, et al. Multiple hepatic mitochondrial DNA deletions suggest premature oxidative aging in alcoholic patients. J Hepatol. 1997;27:96-102.
6. Urso R, Gentile M. Are ‘buffalo hump’ syndrome, Madelung's disease and multiple symmetrical lipomatosis variants of the same dysmetabolism? AIDS. 2001;15:290-291.
7. Grassegger A, Häussler R, Schmalzl F. Tumescent liposuction in a patient with Launois-Bensaude syndrome and severe hepatopathy. Dermatol Surg. 2007;33:982-985.
The Diagnosis: Madelung Disease (Benign Symmetric Lipomatosis)
A 56-year-old man presented for evaluation of massive subcutaneous nodules the bilateral upper arms, shoulders, chest, abdomen, and lateral aspect of the proximal thighs (Figures 1 and 2) that developed over the last 12 to 18 months and continued to enlarge. In addition, he was beginning to develop symptoms of neuropathy of the bilateral hands. The patient had a long-standing history of alcohol abuse. Biopsies performed by the patient’s primary care physician revealed benign adipose tissue. He was referred to the dermatology clinic and subsequently diagnosed with Madelung disease.
 |
 |
Madelung disease, also known as benign symmetric lipomatosis and Launois-Bensaude syndrome, is characterized by multiple large masses of nonencapsulated adipose tissue. These masses are symmetric and most prominent on the head, neck, trunk, and proximal extremities. Classically, a pseudoathletic appearance is described. Madelung disease most frequently affects men aged 30 to 60 years. In more than 90% of cases, it is associated with alcoholism.
In general, the masses of adipose tissue are asymptomatic. However, airway compression and dysphagia requiring surgical intervention has been reported in the otolaryngology literature.1 In addition, neuropathy develops in 84% of cases.2 Nerve biopsies from patients with Madelung disease have revealed a pattern of axonopathy that is distinct from alcohol-induced neuropathy.3 This neuropathy can involve sensory and motor nerves, with the most prominent findings being muscle weakness, tendon areflexia, interosseous muscle atrophy, vibratory sensation loss, and hypoesthesia. Furthermore, dysfunction of the autonomic nervous system can lead to segmental hyperhidrosis, gustatory sweating, and abnormal autonomic cardiovascular reflexes.2 Many patients who develop neuropathy will eventually become incapacitated.
The etiology of Madelung disease is not fully understood. There are several theories on the pathogenesis of this disease, most describing metabolic disturbances induced by alcohol. Specifically, studies have revealed chronic alcohol use causes numerous deletions in mitochondrial DNA.4,5 The mitochondrial DNA damage may explain both the resistance of the lipomatous masses to lipolysis and the nerve-related changes. Comparisons between human immunodeficiency virus/highly active antiretroviral therapy–associated lipodystrophy and Madelung disease lend credence to the metabolic disturbance theory and may help clarify the specific mechanisms involved.6
There have been no cases of spontaneous resolution, even in patients who stop consuming alcohol. For this reason, most patients are referred to a surgeon. Many surgeons prefer open excision for debulking large lipomatous masses, but this technique typically requires general anesthesia. For those in whom this treatment is not an appropriate option, liposuction can be considered with tumescent anesthesia.7 A combination of these surgical modalities also is an option.
Madelung disease is a distinctive disorder typically affecting chronic alcoholics. Recognition of this clinical entity is important, as severe neuropathy and airway compromise may ensue. Although surgical excision is an attractive option for cosmesis and airway compromise, the associated neuropathy can be extremely difficult to treat and can be quite debilitating.
The Diagnosis: Madelung Disease (Benign Symmetric Lipomatosis)
A 56-year-old man presented for evaluation of massive subcutaneous nodules the bilateral upper arms, shoulders, chest, abdomen, and lateral aspect of the proximal thighs (Figures 1 and 2) that developed over the last 12 to 18 months and continued to enlarge. In addition, he was beginning to develop symptoms of neuropathy of the bilateral hands. The patient had a long-standing history of alcohol abuse. Biopsies performed by the patient’s primary care physician revealed benign adipose tissue. He was referred to the dermatology clinic and subsequently diagnosed with Madelung disease.
 |
 |
Madelung disease, also known as benign symmetric lipomatosis and Launois-Bensaude syndrome, is characterized by multiple large masses of nonencapsulated adipose tissue. These masses are symmetric and most prominent on the head, neck, trunk, and proximal extremities. Classically, a pseudoathletic appearance is described. Madelung disease most frequently affects men aged 30 to 60 years. In more than 90% of cases, it is associated with alcoholism.
In general, the masses of adipose tissue are asymptomatic. However, airway compression and dysphagia requiring surgical intervention has been reported in the otolaryngology literature.1 In addition, neuropathy develops in 84% of cases.2 Nerve biopsies from patients with Madelung disease have revealed a pattern of axonopathy that is distinct from alcohol-induced neuropathy.3 This neuropathy can involve sensory and motor nerves, with the most prominent findings being muscle weakness, tendon areflexia, interosseous muscle atrophy, vibratory sensation loss, and hypoesthesia. Furthermore, dysfunction of the autonomic nervous system can lead to segmental hyperhidrosis, gustatory sweating, and abnormal autonomic cardiovascular reflexes.2 Many patients who develop neuropathy will eventually become incapacitated.
The etiology of Madelung disease is not fully understood. There are several theories on the pathogenesis of this disease, most describing metabolic disturbances induced by alcohol. Specifically, studies have revealed chronic alcohol use causes numerous deletions in mitochondrial DNA.4,5 The mitochondrial DNA damage may explain both the resistance of the lipomatous masses to lipolysis and the nerve-related changes. Comparisons between human immunodeficiency virus/highly active antiretroviral therapy–associated lipodystrophy and Madelung disease lend credence to the metabolic disturbance theory and may help clarify the specific mechanisms involved.6
There have been no cases of spontaneous resolution, even in patients who stop consuming alcohol. For this reason, most patients are referred to a surgeon. Many surgeons prefer open excision for debulking large lipomatous masses, but this technique typically requires general anesthesia. For those in whom this treatment is not an appropriate option, liposuction can be considered with tumescent anesthesia.7 A combination of these surgical modalities also is an option.
Madelung disease is a distinctive disorder typically affecting chronic alcoholics. Recognition of this clinical entity is important, as severe neuropathy and airway compromise may ensue. Although surgical excision is an attractive option for cosmesis and airway compromise, the associated neuropathy can be extremely difficult to treat and can be quite debilitating.
1. Palacios E, Neitzschman HR, Nguyen J. Madelung disease: multiple symmetric lipomatosis. Ear Nose Throat J. 2014;93:94-96.
2. Enzi G, Angelini C, Negrin P, et al. Sensory, motor, and autonomic neuropathy in patients with multiple symmetric lipomatosis. Medicine (Baltimore). 1985;64:388-393.
3. Pollock M, Nicholson GI, Nukada H, et al. Neuropathy in multiple symmetric lipomatosis. Madelung’s disease. Brain. 1988;111:1157-1171.
4. Klopstock T, Naumann M, Schalke B, et al. Multiple symmetric lipomatosis: abnormalities in complex IV and multiple deletions in mitochondrial DNA. Neurology. 1994;44:862-866.
5. Mansouri A, Fromenty B, Berson A, et al. Multiple hepatic mitochondrial DNA deletions suggest premature oxidative aging in alcoholic patients. J Hepatol. 1997;27:96-102.
6. Urso R, Gentile M. Are ‘buffalo hump’ syndrome, Madelung's disease and multiple symmetrical lipomatosis variants of the same dysmetabolism? AIDS. 2001;15:290-291.
7. Grassegger A, Häussler R, Schmalzl F. Tumescent liposuction in a patient with Launois-Bensaude syndrome and severe hepatopathy. Dermatol Surg. 2007;33:982-985.
1. Palacios E, Neitzschman HR, Nguyen J. Madelung disease: multiple symmetric lipomatosis. Ear Nose Throat J. 2014;93:94-96.
2. Enzi G, Angelini C, Negrin P, et al. Sensory, motor, and autonomic neuropathy in patients with multiple symmetric lipomatosis. Medicine (Baltimore). 1985;64:388-393.
3. Pollock M, Nicholson GI, Nukada H, et al. Neuropathy in multiple symmetric lipomatosis. Madelung’s disease. Brain. 1988;111:1157-1171.
4. Klopstock T, Naumann M, Schalke B, et al. Multiple symmetric lipomatosis: abnormalities in complex IV and multiple deletions in mitochondrial DNA. Neurology. 1994;44:862-866.
5. Mansouri A, Fromenty B, Berson A, et al. Multiple hepatic mitochondrial DNA deletions suggest premature oxidative aging in alcoholic patients. J Hepatol. 1997;27:96-102.
6. Urso R, Gentile M. Are ‘buffalo hump’ syndrome, Madelung's disease and multiple symmetrical lipomatosis variants of the same dysmetabolism? AIDS. 2001;15:290-291.
7. Grassegger A, Häussler R, Schmalzl F. Tumescent liposuction in a patient with Launois-Bensaude syndrome and severe hepatopathy. Dermatol Surg. 2007;33:982-985.
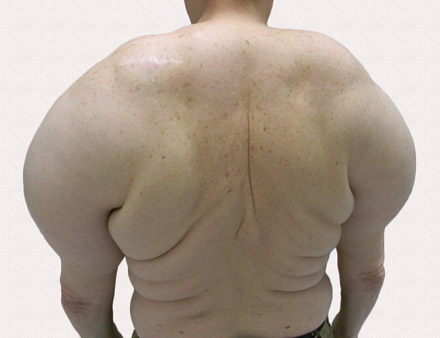
A 56-year-old man presented for evaluation of massive subcutaneous nodules on the bilateral upper arms, shoulders, chest, abdomen, and lateral aspect of the proximal thighs that developed over the last 12 to 18 months and continued to enlarge. His medical history was remarkable for alcoholism, hyperlipidemia, and hypertension.
Ultrasound offers noninvasive option for body contouring, skin tightening
SAN DIEGO – Ultrasound treatments are an alternative for patients looking to avoid the pain and prolonged recovery associated with surgical body contouring or skin tightening procedures, according to Dr. Elizabeth L. Tanzi. Currently, several types of ultrasound devices are available for such procedures, she said at the annual meeting of the American Society for Dermatologic Surgery.
High-intensity, focused ultrasound and pulsed focused ultrasound devices are used for body contouring, and the micro-focused high-intensity ultrasound device is used for skin tightening, Dr. Tanzi explained. Body contouring has rapidly gained popularity in the United States and elsewhere, but surgical contouring procedures can be painful and can require prolonged recovery times, she noted.
Ultrasound for body contouring works via two noninvasive mechanisms, both of which cause selective damage of adipose tissue, said Dr. Tanzi, codirector of the Washington (D.C.) Institute of Dermatologic Laser Surgery.
First, adipose tissue directly absorbs ultrasonic energy during treatment, leading to immediate thermal coagulation and necrosis of fat cells, she said. Mechanical processes also break down tissue, such as when shear forces are created at high levels of pressure during treatment. Circulating macrophages then absorb the damaged adipocytes and the triglycerides they release, leading to a gradual reduction of the fat layer at the treatment site, she added.
When using ultrasound for body contouring, “patient selection is paramount,” Dr. Tanzi emphasized. Although the procedure carries no risk of infection or scarring, ultrasound yields subtler cosmetic results than would liposuction, and patients should understand that difference up front, she said. “This is body contouring, not weight loss,” she added. Patients can typically expect to see a slow reduction in adipose tissue at the treatment area about 1-4 months after treatment, and they also should understand that they might need multiple treatments to achieve their cosmetic goals, Dr. Tanzi said.
“Discuss side effects with patients,” Dr. Tanzi added. When used for body contouring, ultrasound can cause temporary erythema, bruising at the treatment site, soreness, and contour defects, although the latter are rare, she noted. Dr. Tanzi said she now treats patients with lower levels of ultrasonic energy to improve their comfort. “We are much more aggressive now with the overall number of lines we place on the body,” she added. “Go over the areas again and again.”
For example, in one clinical study, high-intensity focused ultrasound yielded statistically significant decreases in waist circumference at 12 weeks with treatment of 150-180 Joules per square centimeter, she noted. However, six passes at just 30 J/cm2 was more comfortable and gave similar results, she said (Dermatol. Surg. 2014;40:641-51).
Micro-focused high-intensity ultrasound can be used for skin tightening, particularly on the face. The procedure targets precise areas of adipose tissue within the skin to create small foci of thermal necrosis, and also stimulates localized collagen production for about 3-6 months afterward, Dr. Tanzi said. As with body contouring, patients should expect subtler results than would be expected from a surgical procedure, but the absence of scarring and the opportunity to immediately return to regular activities can bolster their satisfaction, she said.
Dr. Tanzi is a consultant for Ulthera, Solta, Cynosure/Palomar, Syneron & Candela, and Cutera, and has received financial support from Zeltiq, miraDry, and Clarisonic.
SAN DIEGO – Ultrasound treatments are an alternative for patients looking to avoid the pain and prolonged recovery associated with surgical body contouring or skin tightening procedures, according to Dr. Elizabeth L. Tanzi. Currently, several types of ultrasound devices are available for such procedures, she said at the annual meeting of the American Society for Dermatologic Surgery.
High-intensity, focused ultrasound and pulsed focused ultrasound devices are used for body contouring, and the micro-focused high-intensity ultrasound device is used for skin tightening, Dr. Tanzi explained. Body contouring has rapidly gained popularity in the United States and elsewhere, but surgical contouring procedures can be painful and can require prolonged recovery times, she noted.
Ultrasound for body contouring works via two noninvasive mechanisms, both of which cause selective damage of adipose tissue, said Dr. Tanzi, codirector of the Washington (D.C.) Institute of Dermatologic Laser Surgery.
First, adipose tissue directly absorbs ultrasonic energy during treatment, leading to immediate thermal coagulation and necrosis of fat cells, she said. Mechanical processes also break down tissue, such as when shear forces are created at high levels of pressure during treatment. Circulating macrophages then absorb the damaged adipocytes and the triglycerides they release, leading to a gradual reduction of the fat layer at the treatment site, she added.
When using ultrasound for body contouring, “patient selection is paramount,” Dr. Tanzi emphasized. Although the procedure carries no risk of infection or scarring, ultrasound yields subtler cosmetic results than would liposuction, and patients should understand that difference up front, she said. “This is body contouring, not weight loss,” she added. Patients can typically expect to see a slow reduction in adipose tissue at the treatment area about 1-4 months after treatment, and they also should understand that they might need multiple treatments to achieve their cosmetic goals, Dr. Tanzi said.
“Discuss side effects with patients,” Dr. Tanzi added. When used for body contouring, ultrasound can cause temporary erythema, bruising at the treatment site, soreness, and contour defects, although the latter are rare, she noted. Dr. Tanzi said she now treats patients with lower levels of ultrasonic energy to improve their comfort. “We are much more aggressive now with the overall number of lines we place on the body,” she added. “Go over the areas again and again.”
For example, in one clinical study, high-intensity focused ultrasound yielded statistically significant decreases in waist circumference at 12 weeks with treatment of 150-180 Joules per square centimeter, she noted. However, six passes at just 30 J/cm2 was more comfortable and gave similar results, she said (Dermatol. Surg. 2014;40:641-51).
Micro-focused high-intensity ultrasound can be used for skin tightening, particularly on the face. The procedure targets precise areas of adipose tissue within the skin to create small foci of thermal necrosis, and also stimulates localized collagen production for about 3-6 months afterward, Dr. Tanzi said. As with body contouring, patients should expect subtler results than would be expected from a surgical procedure, but the absence of scarring and the opportunity to immediately return to regular activities can bolster their satisfaction, she said.
Dr. Tanzi is a consultant for Ulthera, Solta, Cynosure/Palomar, Syneron & Candela, and Cutera, and has received financial support from Zeltiq, miraDry, and Clarisonic.
SAN DIEGO – Ultrasound treatments are an alternative for patients looking to avoid the pain and prolonged recovery associated with surgical body contouring or skin tightening procedures, according to Dr. Elizabeth L. Tanzi. Currently, several types of ultrasound devices are available for such procedures, she said at the annual meeting of the American Society for Dermatologic Surgery.
High-intensity, focused ultrasound and pulsed focused ultrasound devices are used for body contouring, and the micro-focused high-intensity ultrasound device is used for skin tightening, Dr. Tanzi explained. Body contouring has rapidly gained popularity in the United States and elsewhere, but surgical contouring procedures can be painful and can require prolonged recovery times, she noted.
Ultrasound for body contouring works via two noninvasive mechanisms, both of which cause selective damage of adipose tissue, said Dr. Tanzi, codirector of the Washington (D.C.) Institute of Dermatologic Laser Surgery.
First, adipose tissue directly absorbs ultrasonic energy during treatment, leading to immediate thermal coagulation and necrosis of fat cells, she said. Mechanical processes also break down tissue, such as when shear forces are created at high levels of pressure during treatment. Circulating macrophages then absorb the damaged adipocytes and the triglycerides they release, leading to a gradual reduction of the fat layer at the treatment site, she added.
When using ultrasound for body contouring, “patient selection is paramount,” Dr. Tanzi emphasized. Although the procedure carries no risk of infection or scarring, ultrasound yields subtler cosmetic results than would liposuction, and patients should understand that difference up front, she said. “This is body contouring, not weight loss,” she added. Patients can typically expect to see a slow reduction in adipose tissue at the treatment area about 1-4 months after treatment, and they also should understand that they might need multiple treatments to achieve their cosmetic goals, Dr. Tanzi said.
“Discuss side effects with patients,” Dr. Tanzi added. When used for body contouring, ultrasound can cause temporary erythema, bruising at the treatment site, soreness, and contour defects, although the latter are rare, she noted. Dr. Tanzi said she now treats patients with lower levels of ultrasonic energy to improve their comfort. “We are much more aggressive now with the overall number of lines we place on the body,” she added. “Go over the areas again and again.”
For example, in one clinical study, high-intensity focused ultrasound yielded statistically significant decreases in waist circumference at 12 weeks with treatment of 150-180 Joules per square centimeter, she noted. However, six passes at just 30 J/cm2 was more comfortable and gave similar results, she said (Dermatol. Surg. 2014;40:641-51).
Micro-focused high-intensity ultrasound can be used for skin tightening, particularly on the face. The procedure targets precise areas of adipose tissue within the skin to create small foci of thermal necrosis, and also stimulates localized collagen production for about 3-6 months afterward, Dr. Tanzi said. As with body contouring, patients should expect subtler results than would be expected from a surgical procedure, but the absence of scarring and the opportunity to immediately return to regular activities can bolster their satisfaction, she said.
Dr. Tanzi is a consultant for Ulthera, Solta, Cynosure/Palomar, Syneron & Candela, and Cutera, and has received financial support from Zeltiq, miraDry, and Clarisonic.
Adapalene/benzoyl peroxide + doxycycline outscores oral isotretinoin
AMSTERDAM – The combination of fixed-dose adapalene/benzoyl peroxide plus oral doxycyline is a reasonable alternative to oral isotretinoin in patients with severe nodular acne, according to the findings of a phase IIIb randomized head-to-head comparative study.
The topical/oral combination is a particularly attractive option for those patients who aren’t candidates for the potent oral retinoid, are unwilling to take it, or can’t tolerate its side effects, Dr. Jerry Tan said at the annual congress of the European Academy of Dermatology and Venereology.
Dr. Tan presented the results of the phase IIIb POWER trial, a 20-week, multicenter, randomized investigator-blinded study involving 266 patients with an average age of 19 years. Participants were randomized to adapalene 0.1%/benzoyl peroxide 2.5% gel (Epiduo)plus oral doxycyline hyclate at 200 mg/day or to placebo gel plus oral isotretinoin at 0.5 mg/kg per day for the first 4 weeks and 1.0 mg/kg per day for the remaining 16 weeks.
At 2 weeks, patients in the combination treatment group averaged a 40% reduction in facial nodules, compared with baseline, versus a 24% decrease with isotretinoin. The combination treatment group also averaged a 27% reduction in facial papules and pustules, an effect size twice that seen in the isotretinoin group at that point. The combination therapy group had a 13% decrease in comedones, compared with 6% with isotretinoin, reported Dr. Tan, a dermatologist at the University of Western Ontario, London.
By the study’s end at week 20, however, isotretinoin showed superior efficacy. But this came at the cost of a significantly worse safety profile. Indeed, the only truly serious treatment-related adverse event seen in the study – a case of Stevens-Johnson syndrome requiring hospitalization – occurred in a patient on isotretinoin.
In an effort to capture both the strengths and drawbacks of each treatment strategy in a single metric, Dr. Tan and his coinvestigators came up with a prespecified novel endpoint they termed “the composite success rate.” They defined it as at least a 75% reduction in a patient’s nodule count, plus the absence of treatment-related, medically relevant adverse events. Based on this measure, the fixed-dose topical plus oral doxycycline regimen was more effective, with a 74% composite success rate, compared with 58% for isotretinoin.
The trial was funded by Galderma. Dr. Tan disclosed serving as a paid researcher for and consultant to Galderma and other pharmaceutical companies.
AMSTERDAM – The combination of fixed-dose adapalene/benzoyl peroxide plus oral doxycyline is a reasonable alternative to oral isotretinoin in patients with severe nodular acne, according to the findings of a phase IIIb randomized head-to-head comparative study.
The topical/oral combination is a particularly attractive option for those patients who aren’t candidates for the potent oral retinoid, are unwilling to take it, or can’t tolerate its side effects, Dr. Jerry Tan said at the annual congress of the European Academy of Dermatology and Venereology.
Dr. Tan presented the results of the phase IIIb POWER trial, a 20-week, multicenter, randomized investigator-blinded study involving 266 patients with an average age of 19 years. Participants were randomized to adapalene 0.1%/benzoyl peroxide 2.5% gel (Epiduo)plus oral doxycyline hyclate at 200 mg/day or to placebo gel plus oral isotretinoin at 0.5 mg/kg per day for the first 4 weeks and 1.0 mg/kg per day for the remaining 16 weeks.
At 2 weeks, patients in the combination treatment group averaged a 40% reduction in facial nodules, compared with baseline, versus a 24% decrease with isotretinoin. The combination treatment group also averaged a 27% reduction in facial papules and pustules, an effect size twice that seen in the isotretinoin group at that point. The combination therapy group had a 13% decrease in comedones, compared with 6% with isotretinoin, reported Dr. Tan, a dermatologist at the University of Western Ontario, London.
By the study’s end at week 20, however, isotretinoin showed superior efficacy. But this came at the cost of a significantly worse safety profile. Indeed, the only truly serious treatment-related adverse event seen in the study – a case of Stevens-Johnson syndrome requiring hospitalization – occurred in a patient on isotretinoin.
In an effort to capture both the strengths and drawbacks of each treatment strategy in a single metric, Dr. Tan and his coinvestigators came up with a prespecified novel endpoint they termed “the composite success rate.” They defined it as at least a 75% reduction in a patient’s nodule count, plus the absence of treatment-related, medically relevant adverse events. Based on this measure, the fixed-dose topical plus oral doxycycline regimen was more effective, with a 74% composite success rate, compared with 58% for isotretinoin.
The trial was funded by Galderma. Dr. Tan disclosed serving as a paid researcher for and consultant to Galderma and other pharmaceutical companies.
AMSTERDAM – The combination of fixed-dose adapalene/benzoyl peroxide plus oral doxycyline is a reasonable alternative to oral isotretinoin in patients with severe nodular acne, according to the findings of a phase IIIb randomized head-to-head comparative study.
The topical/oral combination is a particularly attractive option for those patients who aren’t candidates for the potent oral retinoid, are unwilling to take it, or can’t tolerate its side effects, Dr. Jerry Tan said at the annual congress of the European Academy of Dermatology and Venereology.
Dr. Tan presented the results of the phase IIIb POWER trial, a 20-week, multicenter, randomized investigator-blinded study involving 266 patients with an average age of 19 years. Participants were randomized to adapalene 0.1%/benzoyl peroxide 2.5% gel (Epiduo)plus oral doxycyline hyclate at 200 mg/day or to placebo gel plus oral isotretinoin at 0.5 mg/kg per day for the first 4 weeks and 1.0 mg/kg per day for the remaining 16 weeks.
At 2 weeks, patients in the combination treatment group averaged a 40% reduction in facial nodules, compared with baseline, versus a 24% decrease with isotretinoin. The combination treatment group also averaged a 27% reduction in facial papules and pustules, an effect size twice that seen in the isotretinoin group at that point. The combination therapy group had a 13% decrease in comedones, compared with 6% with isotretinoin, reported Dr. Tan, a dermatologist at the University of Western Ontario, London.
By the study’s end at week 20, however, isotretinoin showed superior efficacy. But this came at the cost of a significantly worse safety profile. Indeed, the only truly serious treatment-related adverse event seen in the study – a case of Stevens-Johnson syndrome requiring hospitalization – occurred in a patient on isotretinoin.
In an effort to capture both the strengths and drawbacks of each treatment strategy in a single metric, Dr. Tan and his coinvestigators came up with a prespecified novel endpoint they termed “the composite success rate.” They defined it as at least a 75% reduction in a patient’s nodule count, plus the absence of treatment-related, medically relevant adverse events. Based on this measure, the fixed-dose topical plus oral doxycycline regimen was more effective, with a 74% composite success rate, compared with 58% for isotretinoin.
The trial was funded by Galderma. Dr. Tan disclosed serving as a paid researcher for and consultant to Galderma and other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point: The combination of fixed-dose adapalene/benzoyl peroxide plus oral doxycyline is an alternative to oral isotretinoin in patients with severe nodular acne.
Major finding: The composite success rate – a novel metric balancing efficacy and safety – was 74% in acne patients treated for 20 weeks with fixed-dose adapalene/benzoyl peroxide gel plus oral doxycycline, compared with 58% in those treated with oral isotretinoin.
Data source: A 20-week, multicenter, randomized, placebo-controlled, investigator-blinded, phase IIIb clinical trial involving 266 patients with severe nodular acne.
Disclosures: The POWER study was funded by Galderma. The presenter has received research grants from and serves as a consultant to Galderma and other pharmaceutical companies.
Biopsy can underestimate diversity, aggressiveness of basal cell carcinomas
SAN DIEGO – Histology of basal cell carcinomas removed by Mohs micrographic surgery showed that presurgical biopsies had not revealed all tumor subtypes in 64% of cases, and had underestimated the aggressiveness of the tumors 24% of the time, according to data from a large, multicenter, retrospective study.
“Unfortunately, while cheap and cost-effective, biopsies are a subsample of the full malignancy,” said Dr. Murad Alam, professor of dermatology, otolaryngology, and surgery at Northwestern University in Chicago. “Skin biopsy of basal cell carcinoma [BCC] may fail to detect all BCC subtypes, and as such may underestimate the aggressiveness of an individual BCC tumor.”
Basal cell carcinoma is the most common skin cancer worldwide, and can broadly be grouped into aggressive and indolent types, Dr. Alam said at the annual meeting of the American Society for Dermatologic Surgery. But tumors often show mixed histology, and cancer treatment needs to target the most aggressive subtype present in the tumor, he added. Results of past studies suggested that biopsies of BCCs could miss tumor subtypes, but the current research is the first large, multicenter study to confirm these findings, he and his associates said.
For the study, the investigators compared biopsy reports and microscopic slides of Mohs micrographic surgery (MMS) specimens from 871 consecutive cases of BCC treated at three hospitals in Illinois from 2013 to 2014. Patients first underwent biopsies, followed by complete excision of their tumors during MMS. Almost 59% of patients were male, and tumors were most commonly removed from the nose or cheek. In all, 78% of biopsies were obtained by the shave technique, but punch and excisional biopsies also were performed, the researchers noted.
Using standard definitions of BCC subtypes, the investigators compared levels of concordance between biopsy and MMS histology findings, Dr. Alam said. They also grouped tumor specimens as high risk (that is, infiltrative, morpheic, micronodular, basosquamous) or low risk (superficial or nodular), and determined whether tumor biopsy and MMS histology yielded the same or discordant risk assessments, he added.
Biopsies identified only 18% of tumors as being of mixed histology, compared with 57% of MMS specimens, said Dr. Alam. Biopsy results matched MMS histologies in only 31% of cases, while in 64% of cases, the MMS specimen yielded more tumor subtypes than the biopsy specimen. The researchers noted that biopsy yielded more subtypes than did MMS in 4% of cases, and that MMS and biopsy subtypes were fully discordant in only four cases.
Dr. Alam and his associates declared no external funding sources or conflicts of interest.
SAN DIEGO – Histology of basal cell carcinomas removed by Mohs micrographic surgery showed that presurgical biopsies had not revealed all tumor subtypes in 64% of cases, and had underestimated the aggressiveness of the tumors 24% of the time, according to data from a large, multicenter, retrospective study.
“Unfortunately, while cheap and cost-effective, biopsies are a subsample of the full malignancy,” said Dr. Murad Alam, professor of dermatology, otolaryngology, and surgery at Northwestern University in Chicago. “Skin biopsy of basal cell carcinoma [BCC] may fail to detect all BCC subtypes, and as such may underestimate the aggressiveness of an individual BCC tumor.”
Basal cell carcinoma is the most common skin cancer worldwide, and can broadly be grouped into aggressive and indolent types, Dr. Alam said at the annual meeting of the American Society for Dermatologic Surgery. But tumors often show mixed histology, and cancer treatment needs to target the most aggressive subtype present in the tumor, he added. Results of past studies suggested that biopsies of BCCs could miss tumor subtypes, but the current research is the first large, multicenter study to confirm these findings, he and his associates said.
For the study, the investigators compared biopsy reports and microscopic slides of Mohs micrographic surgery (MMS) specimens from 871 consecutive cases of BCC treated at three hospitals in Illinois from 2013 to 2014. Patients first underwent biopsies, followed by complete excision of their tumors during MMS. Almost 59% of patients were male, and tumors were most commonly removed from the nose or cheek. In all, 78% of biopsies were obtained by the shave technique, but punch and excisional biopsies also were performed, the researchers noted.
Using standard definitions of BCC subtypes, the investigators compared levels of concordance between biopsy and MMS histology findings, Dr. Alam said. They also grouped tumor specimens as high risk (that is, infiltrative, morpheic, micronodular, basosquamous) or low risk (superficial or nodular), and determined whether tumor biopsy and MMS histology yielded the same or discordant risk assessments, he added.
Biopsies identified only 18% of tumors as being of mixed histology, compared with 57% of MMS specimens, said Dr. Alam. Biopsy results matched MMS histologies in only 31% of cases, while in 64% of cases, the MMS specimen yielded more tumor subtypes than the biopsy specimen. The researchers noted that biopsy yielded more subtypes than did MMS in 4% of cases, and that MMS and biopsy subtypes were fully discordant in only four cases.
Dr. Alam and his associates declared no external funding sources or conflicts of interest.
SAN DIEGO – Histology of basal cell carcinomas removed by Mohs micrographic surgery showed that presurgical biopsies had not revealed all tumor subtypes in 64% of cases, and had underestimated the aggressiveness of the tumors 24% of the time, according to data from a large, multicenter, retrospective study.
“Unfortunately, while cheap and cost-effective, biopsies are a subsample of the full malignancy,” said Dr. Murad Alam, professor of dermatology, otolaryngology, and surgery at Northwestern University in Chicago. “Skin biopsy of basal cell carcinoma [BCC] may fail to detect all BCC subtypes, and as such may underestimate the aggressiveness of an individual BCC tumor.”
Basal cell carcinoma is the most common skin cancer worldwide, and can broadly be grouped into aggressive and indolent types, Dr. Alam said at the annual meeting of the American Society for Dermatologic Surgery. But tumors often show mixed histology, and cancer treatment needs to target the most aggressive subtype present in the tumor, he added. Results of past studies suggested that biopsies of BCCs could miss tumor subtypes, but the current research is the first large, multicenter study to confirm these findings, he and his associates said.
For the study, the investigators compared biopsy reports and microscopic slides of Mohs micrographic surgery (MMS) specimens from 871 consecutive cases of BCC treated at three hospitals in Illinois from 2013 to 2014. Patients first underwent biopsies, followed by complete excision of their tumors during MMS. Almost 59% of patients were male, and tumors were most commonly removed from the nose or cheek. In all, 78% of biopsies were obtained by the shave technique, but punch and excisional biopsies also were performed, the researchers noted.
Using standard definitions of BCC subtypes, the investigators compared levels of concordance between biopsy and MMS histology findings, Dr. Alam said. They also grouped tumor specimens as high risk (that is, infiltrative, morpheic, micronodular, basosquamous) or low risk (superficial or nodular), and determined whether tumor biopsy and MMS histology yielded the same or discordant risk assessments, he added.
Biopsies identified only 18% of tumors as being of mixed histology, compared with 57% of MMS specimens, said Dr. Alam. Biopsy results matched MMS histologies in only 31% of cases, while in 64% of cases, the MMS specimen yielded more tumor subtypes than the biopsy specimen. The researchers noted that biopsy yielded more subtypes than did MMS in 4% of cases, and that MMS and biopsy subtypes were fully discordant in only four cases.
Dr. Alam and his associates declared no external funding sources or conflicts of interest.
Key clinical point: Definitive excision by Mohs micrographic surgery reveals more information about basal cell carcinoma subtypes and tumor behavior than does biopsy.
Major finding: Compared with Mohs specimens, biopsy underestimated the diversity of tumor subtypes in 64% of cases, and underestimated tumor aggressiveness in 24% of cases.
Data source: Multicenter retrospective study of 871 basal cell carcinomas that were biopsied and then removed by Mohs micrographic surgery.
Disclosures: The investigators declared no external funding sources or conflicts of interest.
Cosmetic Corner: Dermatologists Weigh in on Facial Scrubs
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top facial scrubs. Consideration must be given to:
- Facial Fuel Energizing Scrub
Kiehl’s
“This product isn’t terribly expensive and is great for men with all skin types. Patients love the mild stinging the product produces—that’s how they know it’s working!”—Gary Goldenberg, MD, New York, New York
“It is great for women and men. It has menthol and caffeine that gives a refreshing feel to the skin and great exfoliating without excessive irritation.”—Anthony M. Rossi, MD, New York, New York
- NIA24 Physical Cleansing Scrub
Niadyne, Inc.
“It offers a beaded but gentle approach to a facial scrub and is highly rated by our patients and customers.”—Joel Schlessinger, MD, Omaha, Nebraska
- Olay Skin Smoothing Cream Scrub
Proctor & Gamble
“Effective, not expensive, and does not dry the skin.”—Antonella Tosti, MD, Miami, Florida
“This product is inexpensive and great before applying moisturizer or after wearing makeup.”—Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Mineral makeup and eyelash enhancers will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to cutis@frontlinemedcom.com.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top facial scrubs. Consideration must be given to:
- Facial Fuel Energizing Scrub
Kiehl’s
“This product isn’t terribly expensive and is great for men with all skin types. Patients love the mild stinging the product produces—that’s how they know it’s working!”—Gary Goldenberg, MD, New York, New York
“It is great for women and men. It has menthol and caffeine that gives a refreshing feel to the skin and great exfoliating without excessive irritation.”—Anthony M. Rossi, MD, New York, New York
- NIA24 Physical Cleansing Scrub
Niadyne, Inc.
“It offers a beaded but gentle approach to a facial scrub and is highly rated by our patients and customers.”—Joel Schlessinger, MD, Omaha, Nebraska
- Olay Skin Smoothing Cream Scrub
Proctor & Gamble
“Effective, not expensive, and does not dry the skin.”—Antonella Tosti, MD, Miami, Florida
“This product is inexpensive and great before applying moisturizer or after wearing makeup.”—Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Mineral makeup and eyelash enhancers will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to cutis@frontlinemedcom.com.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top facial scrubs. Consideration must be given to:
- Facial Fuel Energizing Scrub
Kiehl’s
“This product isn’t terribly expensive and is great for men with all skin types. Patients love the mild stinging the product produces—that’s how they know it’s working!”—Gary Goldenberg, MD, New York, New York
“It is great for women and men. It has menthol and caffeine that gives a refreshing feel to the skin and great exfoliating without excessive irritation.”—Anthony M. Rossi, MD, New York, New York
- NIA24 Physical Cleansing Scrub
Niadyne, Inc.
“It offers a beaded but gentle approach to a facial scrub and is highly rated by our patients and customers.”—Joel Schlessinger, MD, Omaha, Nebraska
- Olay Skin Smoothing Cream Scrub
Proctor & Gamble
“Effective, not expensive, and does not dry the skin.”—Antonella Tosti, MD, Miami, Florida
“This product is inexpensive and great before applying moisturizer or after wearing makeup.”—Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Mineral makeup and eyelash enhancers will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to cutis@frontlinemedcom.com.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.