User login
Adverse events remain rare for noninvasive and minimally invasive cosmetic procedures
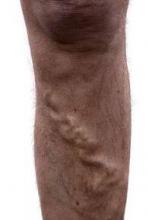
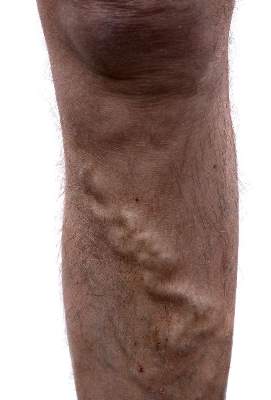
Noninvasive and minimally invasive cosmetic dermatology procedures – such as laser treatments, neurotoxin injections, and soft tissue augmentation – are associated with an aggregate adverse event rate of less than 1% when performed by experienced, board-certified dermatologists, based on data from a multicenter prospective cohort study of more than 20,000 procedures.
Researchers used a web-based data collection system to record relevant procedures and adverse events at eight private and institutional dermatology outpatient clinical practices. They identified a total of 48 adverse events from 20,399 procedures, representing an overall adverse event rate of 0.24%.
The most common adverse events were hyperpigmentation after laser or energy treatments and lumps, nodules, or beading after treatment with neurotoxins or fillers, with adverse events most commonly occurring after procedures on the cheeks, nasolabial folds, and eyelids. No serious adverse events were reported.
“Patients seeking such [noninvasive and minimally invasive] procedures can be reassured that, at least in the hands of trained board-certified dermatologists, they pose minimal risk,” wrote Dr. Murad Alam of Northwestern University, Chicago, and associates. The findings were published online November 5 in JAMA Dermatology (doi:10.1001/jamadermatol.2014.2494).
The study was supported by departmental research funds from the Feinberg School of Medicine, Northwestern University. Dr. Alam declared consultancies with Amway and Leo Pharma, and has been principal investigator on studies funded in part by Allergan, Bioform, Medicis, and Ulthera. There were no other conflicts of interest declared.
Noninvasive and minimally invasive cosmetic dermatology procedures – such as laser treatments, neurotoxin injections, and soft tissue augmentation – are associated with an aggregate adverse event rate of less than 1% when performed by experienced, board-certified dermatologists, based on data from a multicenter prospective cohort study of more than 20,000 procedures.
Researchers used a web-based data collection system to record relevant procedures and adverse events at eight private and institutional dermatology outpatient clinical practices. They identified a total of 48 adverse events from 20,399 procedures, representing an overall adverse event rate of 0.24%.
The most common adverse events were hyperpigmentation after laser or energy treatments and lumps, nodules, or beading after treatment with neurotoxins or fillers, with adverse events most commonly occurring after procedures on the cheeks, nasolabial folds, and eyelids. No serious adverse events were reported.
“Patients seeking such [noninvasive and minimally invasive] procedures can be reassured that, at least in the hands of trained board-certified dermatologists, they pose minimal risk,” wrote Dr. Murad Alam of Northwestern University, Chicago, and associates. The findings were published online November 5 in JAMA Dermatology (doi:10.1001/jamadermatol.2014.2494).
The study was supported by departmental research funds from the Feinberg School of Medicine, Northwestern University. Dr. Alam declared consultancies with Amway and Leo Pharma, and has been principal investigator on studies funded in part by Allergan, Bioform, Medicis, and Ulthera. There were no other conflicts of interest declared.
Noninvasive and minimally invasive cosmetic dermatology procedures – such as laser treatments, neurotoxin injections, and soft tissue augmentation – are associated with an aggregate adverse event rate of less than 1% when performed by experienced, board-certified dermatologists, based on data from a multicenter prospective cohort study of more than 20,000 procedures.
Researchers used a web-based data collection system to record relevant procedures and adverse events at eight private and institutional dermatology outpatient clinical practices. They identified a total of 48 adverse events from 20,399 procedures, representing an overall adverse event rate of 0.24%.
The most common adverse events were hyperpigmentation after laser or energy treatments and lumps, nodules, or beading after treatment with neurotoxins or fillers, with adverse events most commonly occurring after procedures on the cheeks, nasolabial folds, and eyelids. No serious adverse events were reported.
“Patients seeking such [noninvasive and minimally invasive] procedures can be reassured that, at least in the hands of trained board-certified dermatologists, they pose minimal risk,” wrote Dr. Murad Alam of Northwestern University, Chicago, and associates. The findings were published online November 5 in JAMA Dermatology (doi:10.1001/jamadermatol.2014.2494).
The study was supported by departmental research funds from the Feinberg School of Medicine, Northwestern University. Dr. Alam declared consultancies with Amway and Leo Pharma, and has been principal investigator on studies funded in part by Allergan, Bioform, Medicis, and Ulthera. There were no other conflicts of interest declared.
FROM JAMA DERMATOLOGY
Key clinical point: Noninvasive and minimally invasive cosmetic dermatology procedures are safe when performed by experienced dermatologists.
Major finding: Noninvasive and minimally invasive cosmetic dermatology procedures were associated with a 0.24% adverse event rate.
Data source: Multicenter prospective cohort study of 20,399 procedures at eight institutions.
Disclosures: The study was supported by departmental research funds from the Feinberg School of Medicine, Northwestern University. Dr. Alam declared consultancies with Amway and Leo Pharma, and has been principal investigator on studies funded in part by Allergan, Bioform, Medicis, and Ulthera. There were no other conflicts of interest declared.
Hypertrophic Scar Treatment With Intralesional Triamcinolone Acetonide and Pulsed Dye Laser Results in Necrosis
To the Editor:
Intralesional corticosteroids and pulsed dye laser (PDL)(585–595 nm), either as monotherapy or combination therapy, are commonly used to treat hypertrophic scars.1 We describe an unusual adverse effect of protracted necrosis following combination therapy with intralesional triamcinolone acetonide and PDL for hypertrophic scar revision.
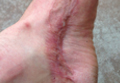
A 29-year-old healthy man presented after sustaining a contaminated crush trauma to the medial aspect of the left foot (Figure, A), requiring staged reconstruction with split-thickness skin grafting. The site healed with a hypertrophic scar (Figure, B), and treatment was pursued 18 months later with a community dermatologist with laser experience. Intralesional triamcinolone acetonide from a new vial diluted with 2% lidocaine was injected into the hypertrophic portions of the scar (20 mg/mL; 1.5 cc injected). The scar was then treated with a PDL at settings of 595 nm, 7-mm spot size, 10 J/cm2, pulse duration of 1.5 milliseconds, 30-millisecond spray with a 20-millisecond delay of cryogen spray, and double-stacked pulses with a 2- to 3-second delay between pulses. The patient reported that purpura was present in treated areas immediately after treatment.
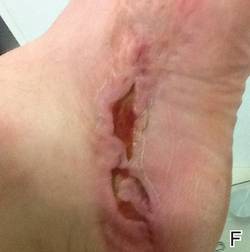
At 6 days posttreatment, the first evidence of possible necrosis with tissue depression in mid posterior portions of the scar appeared (Figure, C). At day 14, deep ulcerations became evident (Figure, D), and by day 21, an exudative, yellow, fibrinopurulent membrane appeared (Figure, E). By 7 weeks posttreatment, ulceration size was only slightly reduced (Figure, F).
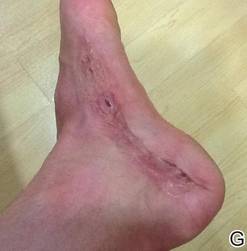
The necrosis was treated with debridement and local wound care using collagen matrix dressings (protease modulating matrix). Seven months after attempted scar revision, a deep 4-mm ulcer with a narrow tunnel-like connection to the skin surface remained anteriorly, disconnected from earlier mid posterior ulcerations, which was indicative of delayed onset of necrosis (Figure, G).
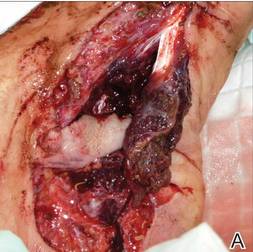 |  | 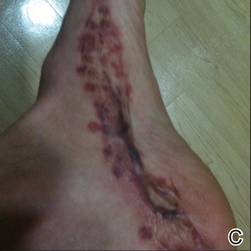 | ||
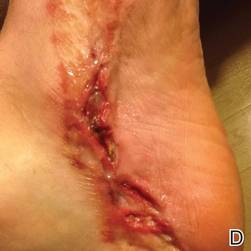 | 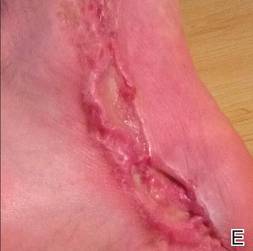 | The medial aspect of the left foot sustained a contaminated crush trauma (A). Following reconstruction, the site healed with a hypertrophic scar (B). Six days after treatment with intralesional triamcinolone acetonide and pulsed dye laser, purpura and tissue depression were observed in mid posterior portions of the scar (C). At 14 days posttreatment, deep ulcerations were evident (D). At 21 days posttreatment, exudative fibrinopurulent membrane appeared (E). At 7 weeks posttreatment, ulceration size was slightly reduced (F). At 7 months posttreatment, a delayed-onset, deep ulceration remained anteriorly (G). | ||
 |  |
Intralesional corticosteroids have been associated with several side effects including pain, dermal atrophy, infection, pigmentation changes, and telangiectases.2 Pulsed dye laser therapy is generally described as safe with mild and transient side effects including purpura (resolves in 7–10 days), edema (diminishes in 48 hours), hyperpigmentation, and pain.3 More serious complications such as ulceration and scarring were initially described with PDL for purely vascular cutaneous lesions,4 and reports of necrosis and scarring have followed with PDL treatment utilizing higher fluences, shorter pulse durations, and stacking of pulses.5
Combination therapy for scar revision with intralesional triamcinolone acetonide and PDL reduces the corticosteroid dose and has been shown to be more efficacious than either modality alone with minimal to no complications.6 In our case, we believe the therapeutic combination resulted in several effects including bulk heating, vascular compromise, and inhibition of wound healing that together contributed to protracted necrosis. Triamcinolone acetonide was injected first; the corticosteroid fluid bolus likely conducted heat energy from the PDL spot leading to tissue damage, which corresponded clinically to the deep, delayed-onset necrosis. The PDL’s destructive effect on the vasculature after stacked pulses likely led to the initial necrosis, with wound healing time potentially extended by the anti-inflammatory effect of the corticosteroids. The location on the lower extremity, an area with limited vasculature and subcutaneous tissue, may have further contributed to impaired wound healing. Finally, the underlying scar tissue was by nature devoid of adnexal structures to expedite wound healing. Although the combination of intralesional corticosteroids and PDL is widely used and considered safe, we suggest that dermatologists carefully choose PDL parameters and pulse delivery methods when utilizing these modalities for scar revision.
1. Tziotzios C, Profyris C, Sterling J. Cutaneous scarring: pathophysiology, molecular mechanisms, and scar reduction therapeutics part II. strategies to reduce scar formation after dermatologic procedures. J Am Acad Dermatol.2012;66:13-24.
2. Chowdri NA, Masarat M, Mattoo A, et al. Keloids and hypertrophic scars: results with intraoperative and serial postoperative corticosteroid injection therapy. Aust N Z J Surg. 1999;69:655-659.
3. Liu A, Moy RL, Ross EV, et al. Pulsed dye laser and pulsed dye laser-mediated photodynamic therapy in the treatment of dermatologic disorders. Dermatol Surg. 2012;38:351-366.
4. Lamb SR, Sheehan-Dare RA. Leg ulceration after pulsed dye laser treatment of a vascular malformation. Lasers Surg Med. 2003;32:396-398.
5. Witman PM, Wagner AM, Scherer K, et al. Complications following pulsed dye laser treatment of superficial hemangiomas. Lasers Surg Med. 2006;38:116-123.
6. Asilian A, Darougheh A, Shariati F. New combination of triamcinolone, 5-fluorouracil, and pulsed-dye laser for treatment of keloid and hypertrophic scars. Dermatol Surg. 2006;32:907-915.
To the Editor:
Intralesional corticosteroids and pulsed dye laser (PDL)(585–595 nm), either as monotherapy or combination therapy, are commonly used to treat hypertrophic scars.1 We describe an unusual adverse effect of protracted necrosis following combination therapy with intralesional triamcinolone acetonide and PDL for hypertrophic scar revision.
A 29-year-old healthy man presented after sustaining a contaminated crush trauma to the medial aspect of the left foot (Figure, A), requiring staged reconstruction with split-thickness skin grafting. The site healed with a hypertrophic scar (Figure, B), and treatment was pursued 18 months later with a community dermatologist with laser experience. Intralesional triamcinolone acetonide from a new vial diluted with 2% lidocaine was injected into the hypertrophic portions of the scar (20 mg/mL; 1.5 cc injected). The scar was then treated with a PDL at settings of 595 nm, 7-mm spot size, 10 J/cm2, pulse duration of 1.5 milliseconds, 30-millisecond spray with a 20-millisecond delay of cryogen spray, and double-stacked pulses with a 2- to 3-second delay between pulses. The patient reported that purpura was present in treated areas immediately after treatment.
At 6 days posttreatment, the first evidence of possible necrosis with tissue depression in mid posterior portions of the scar appeared (Figure, C). At day 14, deep ulcerations became evident (Figure, D), and by day 21, an exudative, yellow, fibrinopurulent membrane appeared (Figure, E). By 7 weeks posttreatment, ulceration size was only slightly reduced (Figure, F).
The necrosis was treated with debridement and local wound care using collagen matrix dressings (protease modulating matrix). Seven months after attempted scar revision, a deep 4-mm ulcer with a narrow tunnel-like connection to the skin surface remained anteriorly, disconnected from earlier mid posterior ulcerations, which was indicative of delayed onset of necrosis (Figure, G).
 |  |  | ||
 |  | The medial aspect of the left foot sustained a contaminated crush trauma (A). Following reconstruction, the site healed with a hypertrophic scar (B). Six days after treatment with intralesional triamcinolone acetonide and pulsed dye laser, purpura and tissue depression were observed in mid posterior portions of the scar (C). At 14 days posttreatment, deep ulcerations were evident (D). At 21 days posttreatment, exudative fibrinopurulent membrane appeared (E). At 7 weeks posttreatment, ulceration size was slightly reduced (F). At 7 months posttreatment, a delayed-onset, deep ulceration remained anteriorly (G). | ||
 |  |
Intralesional corticosteroids have been associated with several side effects including pain, dermal atrophy, infection, pigmentation changes, and telangiectases.2 Pulsed dye laser therapy is generally described as safe with mild and transient side effects including purpura (resolves in 7–10 days), edema (diminishes in 48 hours), hyperpigmentation, and pain.3 More serious complications such as ulceration and scarring were initially described with PDL for purely vascular cutaneous lesions,4 and reports of necrosis and scarring have followed with PDL treatment utilizing higher fluences, shorter pulse durations, and stacking of pulses.5
Combination therapy for scar revision with intralesional triamcinolone acetonide and PDL reduces the corticosteroid dose and has been shown to be more efficacious than either modality alone with minimal to no complications.6 In our case, we believe the therapeutic combination resulted in several effects including bulk heating, vascular compromise, and inhibition of wound healing that together contributed to protracted necrosis. Triamcinolone acetonide was injected first; the corticosteroid fluid bolus likely conducted heat energy from the PDL spot leading to tissue damage, which corresponded clinically to the deep, delayed-onset necrosis. The PDL’s destructive effect on the vasculature after stacked pulses likely led to the initial necrosis, with wound healing time potentially extended by the anti-inflammatory effect of the corticosteroids. The location on the lower extremity, an area with limited vasculature and subcutaneous tissue, may have further contributed to impaired wound healing. Finally, the underlying scar tissue was by nature devoid of adnexal structures to expedite wound healing. Although the combination of intralesional corticosteroids and PDL is widely used and considered safe, we suggest that dermatologists carefully choose PDL parameters and pulse delivery methods when utilizing these modalities for scar revision.
To the Editor:
Intralesional corticosteroids and pulsed dye laser (PDL)(585–595 nm), either as monotherapy or combination therapy, are commonly used to treat hypertrophic scars.1 We describe an unusual adverse effect of protracted necrosis following combination therapy with intralesional triamcinolone acetonide and PDL for hypertrophic scar revision.
A 29-year-old healthy man presented after sustaining a contaminated crush trauma to the medial aspect of the left foot (Figure, A), requiring staged reconstruction with split-thickness skin grafting. The site healed with a hypertrophic scar (Figure, B), and treatment was pursued 18 months later with a community dermatologist with laser experience. Intralesional triamcinolone acetonide from a new vial diluted with 2% lidocaine was injected into the hypertrophic portions of the scar (20 mg/mL; 1.5 cc injected). The scar was then treated with a PDL at settings of 595 nm, 7-mm spot size, 10 J/cm2, pulse duration of 1.5 milliseconds, 30-millisecond spray with a 20-millisecond delay of cryogen spray, and double-stacked pulses with a 2- to 3-second delay between pulses. The patient reported that purpura was present in treated areas immediately after treatment.
At 6 days posttreatment, the first evidence of possible necrosis with tissue depression in mid posterior portions of the scar appeared (Figure, C). At day 14, deep ulcerations became evident (Figure, D), and by day 21, an exudative, yellow, fibrinopurulent membrane appeared (Figure, E). By 7 weeks posttreatment, ulceration size was only slightly reduced (Figure, F).
The necrosis was treated with debridement and local wound care using collagen matrix dressings (protease modulating matrix). Seven months after attempted scar revision, a deep 4-mm ulcer with a narrow tunnel-like connection to the skin surface remained anteriorly, disconnected from earlier mid posterior ulcerations, which was indicative of delayed onset of necrosis (Figure, G).
 |  |  | ||
 |  | The medial aspect of the left foot sustained a contaminated crush trauma (A). Following reconstruction, the site healed with a hypertrophic scar (B). Six days after treatment with intralesional triamcinolone acetonide and pulsed dye laser, purpura and tissue depression were observed in mid posterior portions of the scar (C). At 14 days posttreatment, deep ulcerations were evident (D). At 21 days posttreatment, exudative fibrinopurulent membrane appeared (E). At 7 weeks posttreatment, ulceration size was slightly reduced (F). At 7 months posttreatment, a delayed-onset, deep ulceration remained anteriorly (G). | ||
 |  |
Intralesional corticosteroids have been associated with several side effects including pain, dermal atrophy, infection, pigmentation changes, and telangiectases.2 Pulsed dye laser therapy is generally described as safe with mild and transient side effects including purpura (resolves in 7–10 days), edema (diminishes in 48 hours), hyperpigmentation, and pain.3 More serious complications such as ulceration and scarring were initially described with PDL for purely vascular cutaneous lesions,4 and reports of necrosis and scarring have followed with PDL treatment utilizing higher fluences, shorter pulse durations, and stacking of pulses.5
Combination therapy for scar revision with intralesional triamcinolone acetonide and PDL reduces the corticosteroid dose and has been shown to be more efficacious than either modality alone with minimal to no complications.6 In our case, we believe the therapeutic combination resulted in several effects including bulk heating, vascular compromise, and inhibition of wound healing that together contributed to protracted necrosis. Triamcinolone acetonide was injected first; the corticosteroid fluid bolus likely conducted heat energy from the PDL spot leading to tissue damage, which corresponded clinically to the deep, delayed-onset necrosis. The PDL’s destructive effect on the vasculature after stacked pulses likely led to the initial necrosis, with wound healing time potentially extended by the anti-inflammatory effect of the corticosteroids. The location on the lower extremity, an area with limited vasculature and subcutaneous tissue, may have further contributed to impaired wound healing. Finally, the underlying scar tissue was by nature devoid of adnexal structures to expedite wound healing. Although the combination of intralesional corticosteroids and PDL is widely used and considered safe, we suggest that dermatologists carefully choose PDL parameters and pulse delivery methods when utilizing these modalities for scar revision.
1. Tziotzios C, Profyris C, Sterling J. Cutaneous scarring: pathophysiology, molecular mechanisms, and scar reduction therapeutics part II. strategies to reduce scar formation after dermatologic procedures. J Am Acad Dermatol.2012;66:13-24.
2. Chowdri NA, Masarat M, Mattoo A, et al. Keloids and hypertrophic scars: results with intraoperative and serial postoperative corticosteroid injection therapy. Aust N Z J Surg. 1999;69:655-659.
3. Liu A, Moy RL, Ross EV, et al. Pulsed dye laser and pulsed dye laser-mediated photodynamic therapy in the treatment of dermatologic disorders. Dermatol Surg. 2012;38:351-366.
4. Lamb SR, Sheehan-Dare RA. Leg ulceration after pulsed dye laser treatment of a vascular malformation. Lasers Surg Med. 2003;32:396-398.
5. Witman PM, Wagner AM, Scherer K, et al. Complications following pulsed dye laser treatment of superficial hemangiomas. Lasers Surg Med. 2006;38:116-123.
6. Asilian A, Darougheh A, Shariati F. New combination of triamcinolone, 5-fluorouracil, and pulsed-dye laser for treatment of keloid and hypertrophic scars. Dermatol Surg. 2006;32:907-915.
1. Tziotzios C, Profyris C, Sterling J. Cutaneous scarring: pathophysiology, molecular mechanisms, and scar reduction therapeutics part II. strategies to reduce scar formation after dermatologic procedures. J Am Acad Dermatol.2012;66:13-24.
2. Chowdri NA, Masarat M, Mattoo A, et al. Keloids and hypertrophic scars: results with intraoperative and serial postoperative corticosteroid injection therapy. Aust N Z J Surg. 1999;69:655-659.
3. Liu A, Moy RL, Ross EV, et al. Pulsed dye laser and pulsed dye laser-mediated photodynamic therapy in the treatment of dermatologic disorders. Dermatol Surg. 2012;38:351-366.
4. Lamb SR, Sheehan-Dare RA. Leg ulceration after pulsed dye laser treatment of a vascular malformation. Lasers Surg Med. 2003;32:396-398.
5. Witman PM, Wagner AM, Scherer K, et al. Complications following pulsed dye laser treatment of superficial hemangiomas. Lasers Surg Med. 2006;38:116-123.
6. Asilian A, Darougheh A, Shariati F. New combination of triamcinolone, 5-fluorouracil, and pulsed-dye laser for treatment of keloid and hypertrophic scars. Dermatol Surg. 2006;32:907-915.
VIDEO: What works now and what’s up next for laser treatments of vascular malformations
LAS VEGAS – Pulsed dye lasers have stood the test of time for treating vascular malformations, rosacea, and telangectases, Dr. Christopher Zachary said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
However, Dr. Zachary noted in an interview at the meeting that the field of laser therapy is on the cusp of new innovation, not only in devices, but also in how the devices are used. Different wavelengths and combination treatments will provide more options for patients, he explained. “These are great times for those who are interested in treating vascular problems,” he said.
Dr. Zachary disclosed serving as a consultant for Cutera and Zeltiq, on the speakers bureau for Cynosure and Solta, and on the advisory board for Zeltiq.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Pulsed dye lasers have stood the test of time for treating vascular malformations, rosacea, and telangectases, Dr. Christopher Zachary said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
However, Dr. Zachary noted in an interview at the meeting that the field of laser therapy is on the cusp of new innovation, not only in devices, but also in how the devices are used. Different wavelengths and combination treatments will provide more options for patients, he explained. “These are great times for those who are interested in treating vascular problems,” he said.
Dr. Zachary disclosed serving as a consultant for Cutera and Zeltiq, on the speakers bureau for Cynosure and Solta, and on the advisory board for Zeltiq.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Pulsed dye lasers have stood the test of time for treating vascular malformations, rosacea, and telangectases, Dr. Christopher Zachary said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
However, Dr. Zachary noted in an interview at the meeting that the field of laser therapy is on the cusp of new innovation, not only in devices, but also in how the devices are used. Different wavelengths and combination treatments will provide more options for patients, he explained. “These are great times for those who are interested in treating vascular problems,” he said.
Dr. Zachary disclosed serving as a consultant for Cutera and Zeltiq, on the speakers bureau for Cynosure and Solta, and on the advisory board for Zeltiq.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Fillers for men
Idealized masculine facial features tend to include an overhanging, horizontal brow with minimal arch, deeper-set eyes that look closer together, a somewhat larger nose, a wider mouth, a squared lower face, and a beard or coarser texture to the lower facial skin. A major component of aesthetic disharmony in the aging face in both men and women, however, is the loss or redistribution of subcutaneous fat. Detailed studies by Rohrich and Pessa have demonstrated that facial fat (unlike fat elsewhere in the body) is partitioned into discrete compartments that may age independently of one another. Redistribution and loss of these fat pads contribute to formation of the nasojugal fold, malar crease, nasolabial fold, prejowl sulcus and marionette lines, as well as wasting of the temples, superior brow, and buccal fat. These changes can be visualized most strikingly in cases of cachexia and severe HIV-associated lipodystrophy.
When using fillers to restore volume loss and wrinkles in men, care must be taken to not overfeminize the male face. Filler placement in the anteromedial cheek, submalar cheek, temples, tear trough area, nasolabial folds, and marionette areas are often similar to filler placement in women if the patient has an issue with volume loss in those areas. The main difference is placement in the zygomaticomalar region of the cheek (or the point where the maximal light reflection is off of the highest point of the zygoma, or cheek bone) and the lips. Care must be taken not to overvolumize this region in men.
Also, even within ethnic groups, the male lip is typically not as tall and curvy as the female lip; it often appears less full, with more of a shadow cast by the lower lip.
Men tend to prefer treatments with less downtime and more natural results and are less risk tolerant than women are. Keeping these points in mind can increase patient satisfaction when offering fillers to male patients.
Source: Rohrich R., Pessa J. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. J. Plast. Reconstr. Surg. 2007;119:2219-27.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
Idealized masculine facial features tend to include an overhanging, horizontal brow with minimal arch, deeper-set eyes that look closer together, a somewhat larger nose, a wider mouth, a squared lower face, and a beard or coarser texture to the lower facial skin. A major component of aesthetic disharmony in the aging face in both men and women, however, is the loss or redistribution of subcutaneous fat. Detailed studies by Rohrich and Pessa have demonstrated that facial fat (unlike fat elsewhere in the body) is partitioned into discrete compartments that may age independently of one another. Redistribution and loss of these fat pads contribute to formation of the nasojugal fold, malar crease, nasolabial fold, prejowl sulcus and marionette lines, as well as wasting of the temples, superior brow, and buccal fat. These changes can be visualized most strikingly in cases of cachexia and severe HIV-associated lipodystrophy.
When using fillers to restore volume loss and wrinkles in men, care must be taken to not overfeminize the male face. Filler placement in the anteromedial cheek, submalar cheek, temples, tear trough area, nasolabial folds, and marionette areas are often similar to filler placement in women if the patient has an issue with volume loss in those areas. The main difference is placement in the zygomaticomalar region of the cheek (or the point where the maximal light reflection is off of the highest point of the zygoma, or cheek bone) and the lips. Care must be taken not to overvolumize this region in men.
Also, even within ethnic groups, the male lip is typically not as tall and curvy as the female lip; it often appears less full, with more of a shadow cast by the lower lip.
Men tend to prefer treatments with less downtime and more natural results and are less risk tolerant than women are. Keeping these points in mind can increase patient satisfaction when offering fillers to male patients.
Source: Rohrich R., Pessa J. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. J. Plast. Reconstr. Surg. 2007;119:2219-27.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
Idealized masculine facial features tend to include an overhanging, horizontal brow with minimal arch, deeper-set eyes that look closer together, a somewhat larger nose, a wider mouth, a squared lower face, and a beard or coarser texture to the lower facial skin. A major component of aesthetic disharmony in the aging face in both men and women, however, is the loss or redistribution of subcutaneous fat. Detailed studies by Rohrich and Pessa have demonstrated that facial fat (unlike fat elsewhere in the body) is partitioned into discrete compartments that may age independently of one another. Redistribution and loss of these fat pads contribute to formation of the nasojugal fold, malar crease, nasolabial fold, prejowl sulcus and marionette lines, as well as wasting of the temples, superior brow, and buccal fat. These changes can be visualized most strikingly in cases of cachexia and severe HIV-associated lipodystrophy.
When using fillers to restore volume loss and wrinkles in men, care must be taken to not overfeminize the male face. Filler placement in the anteromedial cheek, submalar cheek, temples, tear trough area, nasolabial folds, and marionette areas are often similar to filler placement in women if the patient has an issue with volume loss in those areas. The main difference is placement in the zygomaticomalar region of the cheek (or the point where the maximal light reflection is off of the highest point of the zygoma, or cheek bone) and the lips. Care must be taken not to overvolumize this region in men.
Also, even within ethnic groups, the male lip is typically not as tall and curvy as the female lip; it often appears less full, with more of a shadow cast by the lower lip.
Men tend to prefer treatments with less downtime and more natural results and are less risk tolerant than women are. Keeping these points in mind can increase patient satisfaction when offering fillers to male patients.
Source: Rohrich R., Pessa J. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. J. Plast. Reconstr. Surg. 2007;119:2219-27.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
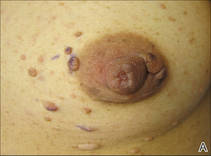
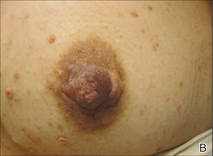
Don’t bypass breasts and nipples in routine skin exams
ORLANDO – As dermatologists do their routine skin checks, they also should pay attention to the breast and the nipple, Dr. David T. Harvey advised in a presentation at the annual meeting of the Florida Society of Dermatologic Surgeons.
Although nipple and areolar tumors are relatively rare, “we have an important role in their evaluation and a wonderful opportunity to impact breast cancer awareness and detection,” said Dr. Harvey, a dermatologist and cosmetic surgeon in Newnan, Ga.
Literature on nipple tumors is limited, but in general, benign breast lesions are much more frequent than the malignant ones. The differential diagnosis can be confusing, “so you don’t want to over or underreact,” while still giving patients the best advice, Dr. Harvey said.
Begin with history
The first step in assessing a lesion on the nipple or areola is to take a thorough history and physical.
The questions to ask include: Is there a discharge? Is there atrophy? Is there a cutaneous erosion? Has the lesion been changing in size? Is the patient pregnant? Is the lesion painful? Perform a visual inspection of the breast and areolar skin, looking for unusual lumps and masses. If the patient will allow, cursory palpation of the breast tissue is helpful.
Ask patients if they have a record of their latest mammograms or MRI. “Get on the phone if you’re not sure. Call a trusted breast specialist, general plastic surgeon, or a surgical oncologist,” he said.
If there’s a lesion that’s erosive and has been there for 6 or 7 months, get a full-thickness incisional biopsy. It also is important to document the presence or absence of axillary lymphadenopathy.
The nipple discharge can be cultured, although most times cultures don’t yield specific data.
And finally, educate patients about the benefits and risks of undergoing regular breast cancer screenings.
Benign tumors
Common benign tumors of the nipple and areola include leiomyoma, erosive adenomatosis, angiolipoma, glomus tumor, neurofibromas, cherry angioma, tags, and epidermal nevus.
Hyperkeratosis around the nipple is another benign condition and can be caused by lack of hygiene. “In these cases, education about how to clean and care for this area is helpful,” Dr. Harvey said.
Erosive adenoma of the nipple is another rare condition. It is usually 0.5-1.5 cm in diameter and arises from apocrine sweat ducts of the nipple’s epithelium. Its clinical symptoms include serosanguinous discharge, bleeding, crusting, erythema, swelling, ulceration, and, in some cases, recurrence because of incomplete removal. This tumor usually occurs in middle-aged women and sometimes is associated with fibrocystic breast disease, breast cancer, and supernumerary nipple. It also can be bilateral. The best treatment is Mohs micrographic surgery.
Malignant tumors
Malignant tumors of the breast and nipple usually present as a lump, hard knot, or thickening inside the breast or underarm area. There’s usually swelling, warmth, redness, or skin hyperpigmentation, in addition to change in the size or shape of the breast.
Other clinical features include dimpling and puckering of the skin and an itchy, scaling sore or rash on the nipple, said Dr. Harvey.
Look for the “pulling in” of the nipple or other parts of the breast, nipple discharge, and discomfort in the breast that doesn’t resolve, he said.
Examples of malignant tumors of the nipple include intraductal breast carcinoma, well-differentiated adenocarcinoma, melanoma, leiomyosarcoma, and squamous cell carcinoma.
Paget’s disease (PD), another breast malignancy, is rare and can mimic eczema or dermatitis. In PD, the nipple may be flattened or eroded. This condition often presents with a discharge and affects older women.
Between 50% and 70% of patients with biopsy-proven mammary PD show positive findings on mammography. The majority of patients who have biopsy-proven Paget’s disease of the nipple as the only physical finding have an underlying deeper breast carcinoma, said Dr. Harvey.
Negative preoperative mammography findings do not reliably exclude an underlying carcinoma. “MRI of the involved breast is a more sensitive way to detect occult PD and is an important tool to help with treatment planning for patients with PD,” he said.
The treatment is radical or modified mastectomy, and lymph node clearance for mammary PD with a palpable mass and underlying invasive breast carcinoma. Estrogen receptor antagonists also can be used in selected cases.
In summary, Dr. Harvey said that it is vital to biopsy a nipple or areolar lesion that is not responding to traditional therapies.
And don’t limit your examination to women
Dr. Christopher Moeller of Wichita, Kan., said that he had diagnosed three men with breast cancer, none of whom where aware of it. One patient had a retracted nipple, another had one breast larger than the other, and he found another lump through palpation.
“Always ask patients,” about their breast health and examine the area, he advised.
Dr. Harvey and Dr. Moeller had no relevant financial disclosures.
ORLANDO – As dermatologists do their routine skin checks, they also should pay attention to the breast and the nipple, Dr. David T. Harvey advised in a presentation at the annual meeting of the Florida Society of Dermatologic Surgeons.
Although nipple and areolar tumors are relatively rare, “we have an important role in their evaluation and a wonderful opportunity to impact breast cancer awareness and detection,” said Dr. Harvey, a dermatologist and cosmetic surgeon in Newnan, Ga.
Literature on nipple tumors is limited, but in general, benign breast lesions are much more frequent than the malignant ones. The differential diagnosis can be confusing, “so you don’t want to over or underreact,” while still giving patients the best advice, Dr. Harvey said.
Begin with history
The first step in assessing a lesion on the nipple or areola is to take a thorough history and physical.
The questions to ask include: Is there a discharge? Is there atrophy? Is there a cutaneous erosion? Has the lesion been changing in size? Is the patient pregnant? Is the lesion painful? Perform a visual inspection of the breast and areolar skin, looking for unusual lumps and masses. If the patient will allow, cursory palpation of the breast tissue is helpful.
Ask patients if they have a record of their latest mammograms or MRI. “Get on the phone if you’re not sure. Call a trusted breast specialist, general plastic surgeon, or a surgical oncologist,” he said.
If there’s a lesion that’s erosive and has been there for 6 or 7 months, get a full-thickness incisional biopsy. It also is important to document the presence or absence of axillary lymphadenopathy.
The nipple discharge can be cultured, although most times cultures don’t yield specific data.
And finally, educate patients about the benefits and risks of undergoing regular breast cancer screenings.
Benign tumors
Common benign tumors of the nipple and areola include leiomyoma, erosive adenomatosis, angiolipoma, glomus tumor, neurofibromas, cherry angioma, tags, and epidermal nevus.
Hyperkeratosis around the nipple is another benign condition and can be caused by lack of hygiene. “In these cases, education about how to clean and care for this area is helpful,” Dr. Harvey said.
Erosive adenoma of the nipple is another rare condition. It is usually 0.5-1.5 cm in diameter and arises from apocrine sweat ducts of the nipple’s epithelium. Its clinical symptoms include serosanguinous discharge, bleeding, crusting, erythema, swelling, ulceration, and, in some cases, recurrence because of incomplete removal. This tumor usually occurs in middle-aged women and sometimes is associated with fibrocystic breast disease, breast cancer, and supernumerary nipple. It also can be bilateral. The best treatment is Mohs micrographic surgery.
Malignant tumors
Malignant tumors of the breast and nipple usually present as a lump, hard knot, or thickening inside the breast or underarm area. There’s usually swelling, warmth, redness, or skin hyperpigmentation, in addition to change in the size or shape of the breast.
Other clinical features include dimpling and puckering of the skin and an itchy, scaling sore or rash on the nipple, said Dr. Harvey.
Look for the “pulling in” of the nipple or other parts of the breast, nipple discharge, and discomfort in the breast that doesn’t resolve, he said.
Examples of malignant tumors of the nipple include intraductal breast carcinoma, well-differentiated adenocarcinoma, melanoma, leiomyosarcoma, and squamous cell carcinoma.
Paget’s disease (PD), another breast malignancy, is rare and can mimic eczema or dermatitis. In PD, the nipple may be flattened or eroded. This condition often presents with a discharge and affects older women.
Between 50% and 70% of patients with biopsy-proven mammary PD show positive findings on mammography. The majority of patients who have biopsy-proven Paget’s disease of the nipple as the only physical finding have an underlying deeper breast carcinoma, said Dr. Harvey.
Negative preoperative mammography findings do not reliably exclude an underlying carcinoma. “MRI of the involved breast is a more sensitive way to detect occult PD and is an important tool to help with treatment planning for patients with PD,” he said.
The treatment is radical or modified mastectomy, and lymph node clearance for mammary PD with a palpable mass and underlying invasive breast carcinoma. Estrogen receptor antagonists also can be used in selected cases.
In summary, Dr. Harvey said that it is vital to biopsy a nipple or areolar lesion that is not responding to traditional therapies.
And don’t limit your examination to women
Dr. Christopher Moeller of Wichita, Kan., said that he had diagnosed three men with breast cancer, none of whom where aware of it. One patient had a retracted nipple, another had one breast larger than the other, and he found another lump through palpation.
“Always ask patients,” about their breast health and examine the area, he advised.
Dr. Harvey and Dr. Moeller had no relevant financial disclosures.
ORLANDO – As dermatologists do their routine skin checks, they also should pay attention to the breast and the nipple, Dr. David T. Harvey advised in a presentation at the annual meeting of the Florida Society of Dermatologic Surgeons.
Although nipple and areolar tumors are relatively rare, “we have an important role in their evaluation and a wonderful opportunity to impact breast cancer awareness and detection,” said Dr. Harvey, a dermatologist and cosmetic surgeon in Newnan, Ga.
Literature on nipple tumors is limited, but in general, benign breast lesions are much more frequent than the malignant ones. The differential diagnosis can be confusing, “so you don’t want to over or underreact,” while still giving patients the best advice, Dr. Harvey said.
Begin with history
The first step in assessing a lesion on the nipple or areola is to take a thorough history and physical.
The questions to ask include: Is there a discharge? Is there atrophy? Is there a cutaneous erosion? Has the lesion been changing in size? Is the patient pregnant? Is the lesion painful? Perform a visual inspection of the breast and areolar skin, looking for unusual lumps and masses. If the patient will allow, cursory palpation of the breast tissue is helpful.
Ask patients if they have a record of their latest mammograms or MRI. “Get on the phone if you’re not sure. Call a trusted breast specialist, general plastic surgeon, or a surgical oncologist,” he said.
If there’s a lesion that’s erosive and has been there for 6 or 7 months, get a full-thickness incisional biopsy. It also is important to document the presence or absence of axillary lymphadenopathy.
The nipple discharge can be cultured, although most times cultures don’t yield specific data.
And finally, educate patients about the benefits and risks of undergoing regular breast cancer screenings.
Benign tumors
Common benign tumors of the nipple and areola include leiomyoma, erosive adenomatosis, angiolipoma, glomus tumor, neurofibromas, cherry angioma, tags, and epidermal nevus.
Hyperkeratosis around the nipple is another benign condition and can be caused by lack of hygiene. “In these cases, education about how to clean and care for this area is helpful,” Dr. Harvey said.
Erosive adenoma of the nipple is another rare condition. It is usually 0.5-1.5 cm in diameter and arises from apocrine sweat ducts of the nipple’s epithelium. Its clinical symptoms include serosanguinous discharge, bleeding, crusting, erythema, swelling, ulceration, and, in some cases, recurrence because of incomplete removal. This tumor usually occurs in middle-aged women and sometimes is associated with fibrocystic breast disease, breast cancer, and supernumerary nipple. It also can be bilateral. The best treatment is Mohs micrographic surgery.
Malignant tumors
Malignant tumors of the breast and nipple usually present as a lump, hard knot, or thickening inside the breast or underarm area. There’s usually swelling, warmth, redness, or skin hyperpigmentation, in addition to change in the size or shape of the breast.
Other clinical features include dimpling and puckering of the skin and an itchy, scaling sore or rash on the nipple, said Dr. Harvey.
Look for the “pulling in” of the nipple or other parts of the breast, nipple discharge, and discomfort in the breast that doesn’t resolve, he said.
Examples of malignant tumors of the nipple include intraductal breast carcinoma, well-differentiated adenocarcinoma, melanoma, leiomyosarcoma, and squamous cell carcinoma.
Paget’s disease (PD), another breast malignancy, is rare and can mimic eczema or dermatitis. In PD, the nipple may be flattened or eroded. This condition often presents with a discharge and affects older women.
Between 50% and 70% of patients with biopsy-proven mammary PD show positive findings on mammography. The majority of patients who have biopsy-proven Paget’s disease of the nipple as the only physical finding have an underlying deeper breast carcinoma, said Dr. Harvey.
Negative preoperative mammography findings do not reliably exclude an underlying carcinoma. “MRI of the involved breast is a more sensitive way to detect occult PD and is an important tool to help with treatment planning for patients with PD,” he said.
The treatment is radical or modified mastectomy, and lymph node clearance for mammary PD with a palpable mass and underlying invasive breast carcinoma. Estrogen receptor antagonists also can be used in selected cases.
In summary, Dr. Harvey said that it is vital to biopsy a nipple or areolar lesion that is not responding to traditional therapies.
And don’t limit your examination to women
Dr. Christopher Moeller of Wichita, Kan., said that he had diagnosed three men with breast cancer, none of whom where aware of it. One patient had a retracted nipple, another had one breast larger than the other, and he found another lump through palpation.
“Always ask patients,” about their breast health and examine the area, he advised.
Dr. Harvey and Dr. Moeller had no relevant financial disclosures.
EXPERT ANALYSIS FROM THE FSDS ANNUAL MEETING
Cosmetic Corner: Dermatologists Weigh in on OTC Pigment Control Products
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top OTC pigment control products. Consideration must be given to:
- Even Better
Clinique Laboratories, LLC
“It also is useful as prevention and offers many different options.”—Antonella Tosti, MD, Miami, Florida
“These OTC products have good clinical data to support use for hyperpigmentation. Patients tell me that they feel good on their skin and aren’t irritating.”—Gary Goldenberg, MD, New York, New York
- Lumixyl Brightening System
Envy Medical, Inc
“A great option for patients who may be experiencing modest issues with pigmentation. Use of a retinoid with this product also may enhance its efficacy.”—Joel Schlessinger, MD, Omaha, Nebraska
- Lytera Skin Brightening Complex
SkinMedica
“With key ingredients such as hexylresorcinol, retinol, and niacinamide, it has been clinically shown to lighten dark patches in its trials as well as adding luminosity to the skin.”—Anthony Rossi, MD, New York, New York
Recommended by Elizabeth K. Hale, MD, New York, New York
- Pigmentclar Serum
La-Roche Posay Laboratoire Dermatologique
“It attacks pigment production at every stage.”—Whitney Bowe, MD, Brooklyn, New York
Cutis invites readers to send us their recommendations. Mineral makeup, eyelash enhancers, and facial scrubs will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to cutis@frontlinemedcom.com.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top OTC pigment control products. Consideration must be given to:
- Even Better
Clinique Laboratories, LLC
“It also is useful as prevention and offers many different options.”—Antonella Tosti, MD, Miami, Florida
“These OTC products have good clinical data to support use for hyperpigmentation. Patients tell me that they feel good on their skin and aren’t irritating.”—Gary Goldenberg, MD, New York, New York
- Lumixyl Brightening System
Envy Medical, Inc
“A great option for patients who may be experiencing modest issues with pigmentation. Use of a retinoid with this product also may enhance its efficacy.”—Joel Schlessinger, MD, Omaha, Nebraska
- Lytera Skin Brightening Complex
SkinMedica
“With key ingredients such as hexylresorcinol, retinol, and niacinamide, it has been clinically shown to lighten dark patches in its trials as well as adding luminosity to the skin.”—Anthony Rossi, MD, New York, New York
Recommended by Elizabeth K. Hale, MD, New York, New York
- Pigmentclar Serum
La-Roche Posay Laboratoire Dermatologique
“It attacks pigment production at every stage.”—Whitney Bowe, MD, Brooklyn, New York
Cutis invites readers to send us their recommendations. Mineral makeup, eyelash enhancers, and facial scrubs will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to cutis@frontlinemedcom.com.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top OTC pigment control products. Consideration must be given to:
- Even Better
Clinique Laboratories, LLC
“It also is useful as prevention and offers many different options.”—Antonella Tosti, MD, Miami, Florida
“These OTC products have good clinical data to support use for hyperpigmentation. Patients tell me that they feel good on their skin and aren’t irritating.”—Gary Goldenberg, MD, New York, New York
- Lumixyl Brightening System
Envy Medical, Inc
“A great option for patients who may be experiencing modest issues with pigmentation. Use of a retinoid with this product also may enhance its efficacy.”—Joel Schlessinger, MD, Omaha, Nebraska
- Lytera Skin Brightening Complex
SkinMedica
“With key ingredients such as hexylresorcinol, retinol, and niacinamide, it has been clinically shown to lighten dark patches in its trials as well as adding luminosity to the skin.”—Anthony Rossi, MD, New York, New York
Recommended by Elizabeth K. Hale, MD, New York, New York
- Pigmentclar Serum
La-Roche Posay Laboratoire Dermatologique
“It attacks pigment production at every stage.”—Whitney Bowe, MD, Brooklyn, New York
Cutis invites readers to send us their recommendations. Mineral makeup, eyelash enhancers, and facial scrubs will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to cutis@frontlinemedcom.com.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
Dermabrasion Versus Tretinoin: The Stretch Mark Showdown

Striae cutis distensae, colloquially known as stretch marks, are extremely common and are difficult to treat. Hexsel et al (Dermatol Surg. 2014;40:537-544) published a randomized pilot study to evaluate the efficacy of superficial dermabrasion versus topical tretinoin in the treatment of striae cutis distensae. Thirty-two women were enrolled and all had early (<6 months) striae rubra measuring up to a maximum of 5 mm in length. Exclusion criteria included any striae alba in the area. Two treatment groups were randomized: one received 16 weekly sessions of localized superficial dermabrasion, and the other group applied tretinoin cream 0.05% daily for 16 weeks. All participants were assessed at 4, 8, 12, and 16 weeks. A 3-mm diamond tip rotating at 10,000 revolutions per minute was used for the dermabrasion. The clinical end point of the superficial dermabrasion was no pain or bleeding. Tretinoin was applied once daily at night. The 5-point global aesthetic improvement scale was used to assess patient improvement and biopsies were done in willing participants.
Both groups experienced significant reduction in the width and length of the striae cutis distensae from baseline (P<.05). No differences were seen between the 2 groups, and the global aesthetic improvement scale score did not differ between the 2 groups. Fourteen participants had adverse events including pruritus, erythema, burning, scaling, crusts, swelling, and papules. There was no statistically significant difference in adverse events between treatments; however, the tretinoin group did experience more scaling, pruritus, and erythema. Eight biopsies were done in the dermabrasion group and 1 was done in the tretinoin group. The dermabrasion group showed histologic evidence of epidermal and dermal improvement with a decrease in elastolysis, collagen fragmentation, and epidermal atrophy, as well as an increase in neocollagenesis.
What’s the issue?
Striae cutis distensae is a common and often distressing skin condition. It is well known that treating early striae rubra may be more efficacious than treating later striae alba. Many methods have been employed to treat striae rubra including lasers and creams. This study places superficial dermabrasion against tretinoin in a prospective manner. Although both treatment groups saw improvement, the tretinoin group was noted to experience more scaling, pruritus, and erythema, which can affect treatment adherence and compliance. Dermabrasion was shown to have beneficial effects via histologic examination, not just on the appearance of the striae but also on the skin structures. Dermabrasion can be a suitable option for patients who cannot pursue more costly laser treatments or expensive tretinoin prescriptions. When a patient presents with striae, what is your go-to treatment?
We want to know your views! Tell us what you think.
Reader Comment
How do you rationalize that dermabrasion is less expensive than tretinoin? Patients can purchase it online if their insurance won't cover it.
--Deborah Ohlhausen, MD
Author Comment
This article points out that dermabrasion can be a suitable treatment option. Tretinoin can be expensive if not covered by insurance. Online purchase is not recommended due to the question of safety and efficacy. Therefore, dermabrasion may be a suitable alternative as pointed out by this study.
--Anthony M. Rossi, MD

Striae cutis distensae, colloquially known as stretch marks, are extremely common and are difficult to treat. Hexsel et al (Dermatol Surg. 2014;40:537-544) published a randomized pilot study to evaluate the efficacy of superficial dermabrasion versus topical tretinoin in the treatment of striae cutis distensae. Thirty-two women were enrolled and all had early (<6 months) striae rubra measuring up to a maximum of 5 mm in length. Exclusion criteria included any striae alba in the area. Two treatment groups were randomized: one received 16 weekly sessions of localized superficial dermabrasion, and the other group applied tretinoin cream 0.05% daily for 16 weeks. All participants were assessed at 4, 8, 12, and 16 weeks. A 3-mm diamond tip rotating at 10,000 revolutions per minute was used for the dermabrasion. The clinical end point of the superficial dermabrasion was no pain or bleeding. Tretinoin was applied once daily at night. The 5-point global aesthetic improvement scale was used to assess patient improvement and biopsies were done in willing participants.
Both groups experienced significant reduction in the width and length of the striae cutis distensae from baseline (P<.05). No differences were seen between the 2 groups, and the global aesthetic improvement scale score did not differ between the 2 groups. Fourteen participants had adverse events including pruritus, erythema, burning, scaling, crusts, swelling, and papules. There was no statistically significant difference in adverse events between treatments; however, the tretinoin group did experience more scaling, pruritus, and erythema. Eight biopsies were done in the dermabrasion group and 1 was done in the tretinoin group. The dermabrasion group showed histologic evidence of epidermal and dermal improvement with a decrease in elastolysis, collagen fragmentation, and epidermal atrophy, as well as an increase in neocollagenesis.
What’s the issue?
Striae cutis distensae is a common and often distressing skin condition. It is well known that treating early striae rubra may be more efficacious than treating later striae alba. Many methods have been employed to treat striae rubra including lasers and creams. This study places superficial dermabrasion against tretinoin in a prospective manner. Although both treatment groups saw improvement, the tretinoin group was noted to experience more scaling, pruritus, and erythema, which can affect treatment adherence and compliance. Dermabrasion was shown to have beneficial effects via histologic examination, not just on the appearance of the striae but also on the skin structures. Dermabrasion can be a suitable option for patients who cannot pursue more costly laser treatments or expensive tretinoin prescriptions. When a patient presents with striae, what is your go-to treatment?
We want to know your views! Tell us what you think.
Reader Comment
How do you rationalize that dermabrasion is less expensive than tretinoin? Patients can purchase it online if their insurance won't cover it.
--Deborah Ohlhausen, MD
Author Comment
This article points out that dermabrasion can be a suitable treatment option. Tretinoin can be expensive if not covered by insurance. Online purchase is not recommended due to the question of safety and efficacy. Therefore, dermabrasion may be a suitable alternative as pointed out by this study.
--Anthony M. Rossi, MD

Striae cutis distensae, colloquially known as stretch marks, are extremely common and are difficult to treat. Hexsel et al (Dermatol Surg. 2014;40:537-544) published a randomized pilot study to evaluate the efficacy of superficial dermabrasion versus topical tretinoin in the treatment of striae cutis distensae. Thirty-two women were enrolled and all had early (<6 months) striae rubra measuring up to a maximum of 5 mm in length. Exclusion criteria included any striae alba in the area. Two treatment groups were randomized: one received 16 weekly sessions of localized superficial dermabrasion, and the other group applied tretinoin cream 0.05% daily for 16 weeks. All participants were assessed at 4, 8, 12, and 16 weeks. A 3-mm diamond tip rotating at 10,000 revolutions per minute was used for the dermabrasion. The clinical end point of the superficial dermabrasion was no pain or bleeding. Tretinoin was applied once daily at night. The 5-point global aesthetic improvement scale was used to assess patient improvement and biopsies were done in willing participants.
Both groups experienced significant reduction in the width and length of the striae cutis distensae from baseline (P<.05). No differences were seen between the 2 groups, and the global aesthetic improvement scale score did not differ between the 2 groups. Fourteen participants had adverse events including pruritus, erythema, burning, scaling, crusts, swelling, and papules. There was no statistically significant difference in adverse events between treatments; however, the tretinoin group did experience more scaling, pruritus, and erythema. Eight biopsies were done in the dermabrasion group and 1 was done in the tretinoin group. The dermabrasion group showed histologic evidence of epidermal and dermal improvement with a decrease in elastolysis, collagen fragmentation, and epidermal atrophy, as well as an increase in neocollagenesis.
What’s the issue?
Striae cutis distensae is a common and often distressing skin condition. It is well known that treating early striae rubra may be more efficacious than treating later striae alba. Many methods have been employed to treat striae rubra including lasers and creams. This study places superficial dermabrasion against tretinoin in a prospective manner. Although both treatment groups saw improvement, the tretinoin group was noted to experience more scaling, pruritus, and erythema, which can affect treatment adherence and compliance. Dermabrasion was shown to have beneficial effects via histologic examination, not just on the appearance of the striae but also on the skin structures. Dermabrasion can be a suitable option for patients who cannot pursue more costly laser treatments or expensive tretinoin prescriptions. When a patient presents with striae, what is your go-to treatment?
We want to know your views! Tell us what you think.
Reader Comment
How do you rationalize that dermabrasion is less expensive than tretinoin? Patients can purchase it online if their insurance won't cover it.
--Deborah Ohlhausen, MD
Author Comment
This article points out that dermabrasion can be a suitable treatment option. Tretinoin can be expensive if not covered by insurance. Online purchase is not recommended due to the question of safety and efficacy. Therefore, dermabrasion may be a suitable alternative as pointed out by this study.
--Anthony M. Rossi, MD
Shave Removal Plus Electrodesiccation for the Treatment of Cutaneous Neurofibromas
To the Editor:
Cutaneous neurofibromas are a clinical feature of both neurofibromatosis type I (NF-1) and neurofibromatosis type II (NF-2). Neurofibromatosis type I occurs in 1 in 3000 live births and NF-2 occurs in 1 in 40,000 live births.1 Neurofibromas are discrete masses that arise from peripheral nerves and are composed of Schwann cells, mast cells, fibroblasts, and perineural cells. They commonly appear during or after puberty in the majority of patients and increase in number as patients age.1,2 Cutaneous neurofibromas can be painful and pruritic as well as cosmetically unappealing. Current treatment of neurofibromas consists primarily of standard surgical excision or laser therapy. Reports of loop electrocoagulation in the operating room, combination erbium:YAG and CO2 laser, and Nd:YAG laser treatments of neurofibromas have been published in the literature.3-5 These treatments can be effective, but the bothersome lesions in patients with neurofibromatosis often are multiple, making these procedures time consuming and costly. A removal technique that is time efficient, cost effective, and poses little discomfort or functional impairment for the patient is desirable. We describe the technique of shave removal with electrodesiccation for multiple neurofibromas.
A 24-year-old woman with NF-1 presented to the dermatology clinic for treatment of several pruritic neurofibromas of the trunk, including the bilateral breasts (Figure, A). We performed shave removal of each lesion with electrodesiccation of the lesion base with successful treatment of symptoms and acceptable cosmetic outcome (Figure, B). The patient identified symptomatic and cosmetically bothersome neurofibromas. The lesions were disinfected with an alcohol swab and anesthetized with 1% lidocaine and a 1:200,000 dilution of epinephrine. A shave biopsy blade was used to remove each neurofibroma at the level of the surrounding skin. Toothed forceps were utilized to lift the lesion and provide traction. Electrodesiccation was then used for destruction of the base or any residual gelatinous component of the lesion to the level of the surrounding skin, thus achieving hemostasis. Lastly, petrolatum was applied to the wound and covered with an adhesive dressing. Daily dressing change was performed for 2 weeks or until healed.
 |
 |
| A patient with neurofibromatosis type I with neurofibromas on the breast before (A) and approximately 6 months after treatment with shave removal plus electrodesiccation of the lesions (B). |
Patients with neurofibromatoses often present to dermatologists for management of symptomatic or cosmetically bothersome neurofibromas, which are often numerous and diffusely distributed. There are few published practical descriptions of removal techniques to manage these multiple and recurrent manifestations of the disease. The shave removal plus electrodesiccation technique we described is straightforward for practitioners and provides a well-tolerated mechanism for treating multiple lesions at once, while also offering patients acceptable functional and cosmetic results.
1. Listernick R, Charrow J. The neurofibromatoses. In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill Companies; 2007:1331-1339.
2. Pinson S, Wolkenstein P. Neurofibromatosis type 1 or Von Recklinghausen’s disease [in French]. Rev Med Interne. 2005;26:196-215.
3. Elwakil TF, Samy NA, Elbasiouny MS. Non-excision treatment of multiple cutaneous neurofibromas by laser photocoagulation [published online ahead of print August 15, 2007]. Lasers Med Sci. 2008;23:301-306.
4. Meissner M, Ochsendorf F, Kaufmann R. Quick and effective treatment of small neurofibromas [published online ahead of print November 23, 2010]. J Dtsch Dermatol Ges. 2011;9:167-168.
5. Roberts AH, Crockett DJ. An operation for the treatment of cutaneous neurofibromatosis. Br J Plast Surg. 1985;38:292-293.
To the Editor:
Cutaneous neurofibromas are a clinical feature of both neurofibromatosis type I (NF-1) and neurofibromatosis type II (NF-2). Neurofibromatosis type I occurs in 1 in 3000 live births and NF-2 occurs in 1 in 40,000 live births.1 Neurofibromas are discrete masses that arise from peripheral nerves and are composed of Schwann cells, mast cells, fibroblasts, and perineural cells. They commonly appear during or after puberty in the majority of patients and increase in number as patients age.1,2 Cutaneous neurofibromas can be painful and pruritic as well as cosmetically unappealing. Current treatment of neurofibromas consists primarily of standard surgical excision or laser therapy. Reports of loop electrocoagulation in the operating room, combination erbium:YAG and CO2 laser, and Nd:YAG laser treatments of neurofibromas have been published in the literature.3-5 These treatments can be effective, but the bothersome lesions in patients with neurofibromatosis often are multiple, making these procedures time consuming and costly. A removal technique that is time efficient, cost effective, and poses little discomfort or functional impairment for the patient is desirable. We describe the technique of shave removal with electrodesiccation for multiple neurofibromas.
A 24-year-old woman with NF-1 presented to the dermatology clinic for treatment of several pruritic neurofibromas of the trunk, including the bilateral breasts (Figure, A). We performed shave removal of each lesion with electrodesiccation of the lesion base with successful treatment of symptoms and acceptable cosmetic outcome (Figure, B). The patient identified symptomatic and cosmetically bothersome neurofibromas. The lesions were disinfected with an alcohol swab and anesthetized with 1% lidocaine and a 1:200,000 dilution of epinephrine. A shave biopsy blade was used to remove each neurofibroma at the level of the surrounding skin. Toothed forceps were utilized to lift the lesion and provide traction. Electrodesiccation was then used for destruction of the base or any residual gelatinous component of the lesion to the level of the surrounding skin, thus achieving hemostasis. Lastly, petrolatum was applied to the wound and covered with an adhesive dressing. Daily dressing change was performed for 2 weeks or until healed.
 |
 |
| A patient with neurofibromatosis type I with neurofibromas on the breast before (A) and approximately 6 months after treatment with shave removal plus electrodesiccation of the lesions (B). |
Patients with neurofibromatoses often present to dermatologists for management of symptomatic or cosmetically bothersome neurofibromas, which are often numerous and diffusely distributed. There are few published practical descriptions of removal techniques to manage these multiple and recurrent manifestations of the disease. The shave removal plus electrodesiccation technique we described is straightforward for practitioners and provides a well-tolerated mechanism for treating multiple lesions at once, while also offering patients acceptable functional and cosmetic results.
To the Editor:
Cutaneous neurofibromas are a clinical feature of both neurofibromatosis type I (NF-1) and neurofibromatosis type II (NF-2). Neurofibromatosis type I occurs in 1 in 3000 live births and NF-2 occurs in 1 in 40,000 live births.1 Neurofibromas are discrete masses that arise from peripheral nerves and are composed of Schwann cells, mast cells, fibroblasts, and perineural cells. They commonly appear during or after puberty in the majority of patients and increase in number as patients age.1,2 Cutaneous neurofibromas can be painful and pruritic as well as cosmetically unappealing. Current treatment of neurofibromas consists primarily of standard surgical excision or laser therapy. Reports of loop electrocoagulation in the operating room, combination erbium:YAG and CO2 laser, and Nd:YAG laser treatments of neurofibromas have been published in the literature.3-5 These treatments can be effective, but the bothersome lesions in patients with neurofibromatosis often are multiple, making these procedures time consuming and costly. A removal technique that is time efficient, cost effective, and poses little discomfort or functional impairment for the patient is desirable. We describe the technique of shave removal with electrodesiccation for multiple neurofibromas.
A 24-year-old woman with NF-1 presented to the dermatology clinic for treatment of several pruritic neurofibromas of the trunk, including the bilateral breasts (Figure, A). We performed shave removal of each lesion with electrodesiccation of the lesion base with successful treatment of symptoms and acceptable cosmetic outcome (Figure, B). The patient identified symptomatic and cosmetically bothersome neurofibromas. The lesions were disinfected with an alcohol swab and anesthetized with 1% lidocaine and a 1:200,000 dilution of epinephrine. A shave biopsy blade was used to remove each neurofibroma at the level of the surrounding skin. Toothed forceps were utilized to lift the lesion and provide traction. Electrodesiccation was then used for destruction of the base or any residual gelatinous component of the lesion to the level of the surrounding skin, thus achieving hemostasis. Lastly, petrolatum was applied to the wound and covered with an adhesive dressing. Daily dressing change was performed for 2 weeks or until healed.
 |
 |
| A patient with neurofibromatosis type I with neurofibromas on the breast before (A) and approximately 6 months after treatment with shave removal plus electrodesiccation of the lesions (B). |
Patients with neurofibromatoses often present to dermatologists for management of symptomatic or cosmetically bothersome neurofibromas, which are often numerous and diffusely distributed. There are few published practical descriptions of removal techniques to manage these multiple and recurrent manifestations of the disease. The shave removal plus electrodesiccation technique we described is straightforward for practitioners and provides a well-tolerated mechanism for treating multiple lesions at once, while also offering patients acceptable functional and cosmetic results.
1. Listernick R, Charrow J. The neurofibromatoses. In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill Companies; 2007:1331-1339.
2. Pinson S, Wolkenstein P. Neurofibromatosis type 1 or Von Recklinghausen’s disease [in French]. Rev Med Interne. 2005;26:196-215.
3. Elwakil TF, Samy NA, Elbasiouny MS. Non-excision treatment of multiple cutaneous neurofibromas by laser photocoagulation [published online ahead of print August 15, 2007]. Lasers Med Sci. 2008;23:301-306.
4. Meissner M, Ochsendorf F, Kaufmann R. Quick and effective treatment of small neurofibromas [published online ahead of print November 23, 2010]. J Dtsch Dermatol Ges. 2011;9:167-168.
5. Roberts AH, Crockett DJ. An operation for the treatment of cutaneous neurofibromatosis. Br J Plast Surg. 1985;38:292-293.
1. Listernick R, Charrow J. The neurofibromatoses. In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill Companies; 2007:1331-1339.
2. Pinson S, Wolkenstein P. Neurofibromatosis type 1 or Von Recklinghausen’s disease [in French]. Rev Med Interne. 2005;26:196-215.
3. Elwakil TF, Samy NA, Elbasiouny MS. Non-excision treatment of multiple cutaneous neurofibromas by laser photocoagulation [published online ahead of print August 15, 2007]. Lasers Med Sci. 2008;23:301-306.
4. Meissner M, Ochsendorf F, Kaufmann R. Quick and effective treatment of small neurofibromas [published online ahead of print November 23, 2010]. J Dtsch Dermatol Ges. 2011;9:167-168.
5. Roberts AH, Crockett DJ. An operation for the treatment of cutaneous neurofibromatosis. Br J Plast Surg. 1985;38:292-293.
3-D images aid total body mole mapping
EDINBURGH – The advent of three-dimensional total body photography overcomes many, but not all, of the drawbacks of two-dimensional imaging as an aid to melanoma detection.
“There are challenges to using two-dimensional images, both in terms of acquiring them and reviewing them, but the simplest of which is a 5-mm lesion that’s perfectly perpendicular to the camera will not look like 5 mm if it’s at an angle to the camera, an inherent problem of consistency in looking at size over time on curved surfaces,” Dr. Allan Halpern said at the 15th World Congress on Cancers of the Skin.
Over the last several months, the dermatology service Dr. Halpern heads at Memorial Sloan-Kettering Cancer Center in New York has shifted to 3-D total body photography in patients at high risk of melanoma.
“The new system (Vectra WB360, Canfield Imaging Systems) uses 40 some odd cameras behind a series of panels, and in 3 milliseconds allows the clinician to image the entire patient in 3-D,” he said.
A touch-screen interface allows for additional close-up pictures of areas of particular interest and tags their location to the corresponding body map photographs. The onscreen 3-D images also can be used for consultation and patient education.
In a recent open-ended survey of 199 of his patients who had already undergone 2-D imaging, participants said 3-D mole mapping was faster, easier, less invasive, and required fewer poses, Dr. Halpern said.
Among their concerns, the admittedly motivated patient cohort wanted even better resolution and more images to catch moles in body folds or on the soles of their feet.
Dr. Halpern was quick to acknowledge that, like population-based melanoma screening, total body photography (TBP) has not been shown in a definitive trial to reduce mortality. In fact, clinical practice guidelines issued this year in Germany and Australia strongly recommend training and use of dermoscopy for examination of pigmented lesions, but note that the value of TBP in melanoma-risk patients remains ‘unproven.’
Research also has shown that uptake of TBP in clinical practice has remained relatively flat in the United States since 2000 (J. Am. Acad. Dermatol. 2010:62:794-803). Obstacles include expense, privacy, and security of stored images, and a lack of imaging standards.
The International Society for Digital Imaging of the Skin, however, is working with dermatology specialists, informatics experts, and imaging technology developers through its Melanoma Project to develop international imaging standards and a public-access archive of clinical and dermoscopic images of skin lesions, Dr. Halpern said.
“One of the encouraging things to me is that the CEOs of six of those imaging developers have already signed on. They recognize the importance of having international standards and interchangeability of images,” he said at the meeting sponsored by the Skin Cancer Foundation.
Dr. Halpern has consulted for and shared research grants with Canfield Scientific and consulted for Caliber I.D., Scibase, and DermTech.
EDINBURGH – The advent of three-dimensional total body photography overcomes many, but not all, of the drawbacks of two-dimensional imaging as an aid to melanoma detection.
“There are challenges to using two-dimensional images, both in terms of acquiring them and reviewing them, but the simplest of which is a 5-mm lesion that’s perfectly perpendicular to the camera will not look like 5 mm if it’s at an angle to the camera, an inherent problem of consistency in looking at size over time on curved surfaces,” Dr. Allan Halpern said at the 15th World Congress on Cancers of the Skin.
Over the last several months, the dermatology service Dr. Halpern heads at Memorial Sloan-Kettering Cancer Center in New York has shifted to 3-D total body photography in patients at high risk of melanoma.
“The new system (Vectra WB360, Canfield Imaging Systems) uses 40 some odd cameras behind a series of panels, and in 3 milliseconds allows the clinician to image the entire patient in 3-D,” he said.
A touch-screen interface allows for additional close-up pictures of areas of particular interest and tags their location to the corresponding body map photographs. The onscreen 3-D images also can be used for consultation and patient education.
In a recent open-ended survey of 199 of his patients who had already undergone 2-D imaging, participants said 3-D mole mapping was faster, easier, less invasive, and required fewer poses, Dr. Halpern said.
Among their concerns, the admittedly motivated patient cohort wanted even better resolution and more images to catch moles in body folds or on the soles of their feet.
Dr. Halpern was quick to acknowledge that, like population-based melanoma screening, total body photography (TBP) has not been shown in a definitive trial to reduce mortality. In fact, clinical practice guidelines issued this year in Germany and Australia strongly recommend training and use of dermoscopy for examination of pigmented lesions, but note that the value of TBP in melanoma-risk patients remains ‘unproven.’
Research also has shown that uptake of TBP in clinical practice has remained relatively flat in the United States since 2000 (J. Am. Acad. Dermatol. 2010:62:794-803). Obstacles include expense, privacy, and security of stored images, and a lack of imaging standards.
The International Society for Digital Imaging of the Skin, however, is working with dermatology specialists, informatics experts, and imaging technology developers through its Melanoma Project to develop international imaging standards and a public-access archive of clinical and dermoscopic images of skin lesions, Dr. Halpern said.
“One of the encouraging things to me is that the CEOs of six of those imaging developers have already signed on. They recognize the importance of having international standards and interchangeability of images,” he said at the meeting sponsored by the Skin Cancer Foundation.
Dr. Halpern has consulted for and shared research grants with Canfield Scientific and consulted for Caliber I.D., Scibase, and DermTech.
EDINBURGH – The advent of three-dimensional total body photography overcomes many, but not all, of the drawbacks of two-dimensional imaging as an aid to melanoma detection.
“There are challenges to using two-dimensional images, both in terms of acquiring them and reviewing them, but the simplest of which is a 5-mm lesion that’s perfectly perpendicular to the camera will not look like 5 mm if it’s at an angle to the camera, an inherent problem of consistency in looking at size over time on curved surfaces,” Dr. Allan Halpern said at the 15th World Congress on Cancers of the Skin.
Over the last several months, the dermatology service Dr. Halpern heads at Memorial Sloan-Kettering Cancer Center in New York has shifted to 3-D total body photography in patients at high risk of melanoma.
“The new system (Vectra WB360, Canfield Imaging Systems) uses 40 some odd cameras behind a series of panels, and in 3 milliseconds allows the clinician to image the entire patient in 3-D,” he said.
A touch-screen interface allows for additional close-up pictures of areas of particular interest and tags their location to the corresponding body map photographs. The onscreen 3-D images also can be used for consultation and patient education.
In a recent open-ended survey of 199 of his patients who had already undergone 2-D imaging, participants said 3-D mole mapping was faster, easier, less invasive, and required fewer poses, Dr. Halpern said.
Among their concerns, the admittedly motivated patient cohort wanted even better resolution and more images to catch moles in body folds or on the soles of their feet.
Dr. Halpern was quick to acknowledge that, like population-based melanoma screening, total body photography (TBP) has not been shown in a definitive trial to reduce mortality. In fact, clinical practice guidelines issued this year in Germany and Australia strongly recommend training and use of dermoscopy for examination of pigmented lesions, but note that the value of TBP in melanoma-risk patients remains ‘unproven.’
Research also has shown that uptake of TBP in clinical practice has remained relatively flat in the United States since 2000 (J. Am. Acad. Dermatol. 2010:62:794-803). Obstacles include expense, privacy, and security of stored images, and a lack of imaging standards.
The International Society for Digital Imaging of the Skin, however, is working with dermatology specialists, informatics experts, and imaging technology developers through its Melanoma Project to develop international imaging standards and a public-access archive of clinical and dermoscopic images of skin lesions, Dr. Halpern said.
“One of the encouraging things to me is that the CEOs of six of those imaging developers have already signed on. They recognize the importance of having international standards and interchangeability of images,” he said at the meeting sponsored by the Skin Cancer Foundation.
Dr. Halpern has consulted for and shared research grants with Canfield Scientific and consulted for Caliber I.D., Scibase, and DermTech.
AT WCCS 2014
Various varicose vein treatments show similar efficacy
Ultrasound-guided foam sclerotherapy, endovenous laser ablation, and surgery for the treatment of varicose veins were generally similar with respect to quality of life measures and clinical efficacy and safety in a randomized trial involving 785 patients.
At 6 months, disease-specific quality of life was slightly worse after treatment with foam than after surgery (effect size, –1.74; P = .006). A post hoc analysis showed slightly better generic quality of life after laser ablation than after foam sclerotherapy (effect size, 1.54; P = .048).
In addition, a lower rate of procedural complications occurred with laser ablation (1%, compared with 6% and 7% for foam sclerotherapy and surgery, respectively), and a lower rate of completely successful ablation of great saphenous veins at 6 weeks occurred after foam treatment (55%, compared with 84% and 83% for the surgery and laser groups, respectively).
Dr. Julie Brittenden of the University of Aberdeen, Scotland, and her colleagues reported the findings online Sept. 25 in the New England Journal of Medicine (N. Engl. J. Med. 2014 Sept. 25 [doi: 10/1056/NEJMoa1400781]).
Subjects in the Comparison of Laser, Surgery, and Foam Sclerotherapy (CLASS) trial were adults with primary varicose veins who were treated at 11 centers in the United Kingdom between November 2008 and October 2012. They were evaluated at 6 weeks and 6 months after treatment.
The baseline scores and overall improvements in quality of life seen in the CLASS trial were comparable to those in smaller European randomized trials, which supports the generalizability of the findings, the investigators said.
This study was supported by a grant from the Health Technology Assessment Programme of the U.K. National Institute for Health Research. Dr. Brittenden reported having no other disclosures.
Ultrasound-guided foam sclerotherapy, endovenous laser ablation, and surgery for the treatment of varicose veins were generally similar with respect to quality of life measures and clinical efficacy and safety in a randomized trial involving 785 patients.
At 6 months, disease-specific quality of life was slightly worse after treatment with foam than after surgery (effect size, –1.74; P = .006). A post hoc analysis showed slightly better generic quality of life after laser ablation than after foam sclerotherapy (effect size, 1.54; P = .048).
In addition, a lower rate of procedural complications occurred with laser ablation (1%, compared with 6% and 7% for foam sclerotherapy and surgery, respectively), and a lower rate of completely successful ablation of great saphenous veins at 6 weeks occurred after foam treatment (55%, compared with 84% and 83% for the surgery and laser groups, respectively).
Dr. Julie Brittenden of the University of Aberdeen, Scotland, and her colleagues reported the findings online Sept. 25 in the New England Journal of Medicine (N. Engl. J. Med. 2014 Sept. 25 [doi: 10/1056/NEJMoa1400781]).
Subjects in the Comparison of Laser, Surgery, and Foam Sclerotherapy (CLASS) trial were adults with primary varicose veins who were treated at 11 centers in the United Kingdom between November 2008 and October 2012. They were evaluated at 6 weeks and 6 months after treatment.
The baseline scores and overall improvements in quality of life seen in the CLASS trial were comparable to those in smaller European randomized trials, which supports the generalizability of the findings, the investigators said.
This study was supported by a grant from the Health Technology Assessment Programme of the U.K. National Institute for Health Research. Dr. Brittenden reported having no other disclosures.
Ultrasound-guided foam sclerotherapy, endovenous laser ablation, and surgery for the treatment of varicose veins were generally similar with respect to quality of life measures and clinical efficacy and safety in a randomized trial involving 785 patients.
At 6 months, disease-specific quality of life was slightly worse after treatment with foam than after surgery (effect size, –1.74; P = .006). A post hoc analysis showed slightly better generic quality of life after laser ablation than after foam sclerotherapy (effect size, 1.54; P = .048).
In addition, a lower rate of procedural complications occurred with laser ablation (1%, compared with 6% and 7% for foam sclerotherapy and surgery, respectively), and a lower rate of completely successful ablation of great saphenous veins at 6 weeks occurred after foam treatment (55%, compared with 84% and 83% for the surgery and laser groups, respectively).
Dr. Julie Brittenden of the University of Aberdeen, Scotland, and her colleagues reported the findings online Sept. 25 in the New England Journal of Medicine (N. Engl. J. Med. 2014 Sept. 25 [doi: 10/1056/NEJMoa1400781]).
Subjects in the Comparison of Laser, Surgery, and Foam Sclerotherapy (CLASS) trial were adults with primary varicose veins who were treated at 11 centers in the United Kingdom between November 2008 and October 2012. They were evaluated at 6 weeks and 6 months after treatment.
The baseline scores and overall improvements in quality of life seen in the CLASS trial were comparable to those in smaller European randomized trials, which supports the generalizability of the findings, the investigators said.
This study was supported by a grant from the Health Technology Assessment Programme of the U.K. National Institute for Health Research. Dr. Brittenden reported having no other disclosures.
Key clinical point: Quality of life outcomes don’t differ substantially between varicose vein treatments.
Major finding: The rate of complications was lower with laser ablation (1%) than with foam sclerotherapy and surgery (6% and 7%, respectively).
Data source: The randomized CLASS trial involving 785 subjects.
Disclosures: This study was supported by a grant from the Health Technology Assessment Programme of the National Institute for Health Research. Dr. Brittenden reported having no other disclosures.