User login
Basics of Lasers in Dermatology
Lasers have become a critical part of the dermatologist’s armamentarium for modulating cutaneous biology, both in treating skin disorders and providing tangible cosmetic alterations to the skin. Although advances in technology and convenient user interfaces have made modern lasers relatively straightforward to use, they are in fact quite complex and powerful instruments that are capable of considerable damage if not used correctly. Thus it is necessary to establish a framework for the safe and responsible use of lasers in dermatology; fundamental to this tenet is an understanding of the development and physics of lasers. In this article, the fundamental concepts of lasers as well as their interactions with the skin will be discussed to impart a working knowledge of lasers to allow for better, safer use of these important tools.
Development of Lasers
The term laser is an acronym for “light amplification by the stimulated emission of radiation.” Albert Einstein established the framework for the functioning of lasers in his seminal work, “On the Quantum Theory of Radiation,”1 in which he described how an electron in an atom in an excited state can return to a lower state by emitting energy in the form of a photon of light. Light comprises a portion of the electromagnetic spectrum, rangingfrom UV (200–400 nm) to visible (400 to about 700 nm) to infrared light (about 700 to >3000 nm). The unique properties of light that affect the function of lasers include reflection (eg, seeing a mirror image of a mountain on the surface of a still lake) and refraction (eg, your hand looking larger under the surface of a pool of water).
Despite early theories on lasers, it was not until the late 1950s that the technology finally started to catch up to the science. Researchers experimenting with microwave fields were able to generate a beam of excited ammonia molecules through a resonant cavity, resulting in a uniform (albeit low power) emission of radiation.2 Maiman3 expounded on this development by building the first working prototype of a device that radiated light without the use of a microwave. So how exactly do lasers work?
Basic Physics of Lasers
To understand how lasers work, one must have a rudimentary understanding of quantum mechanics. Bohr4 revealed that an atom is comprised of a nucleus that is orbited by electrons at discrete distances (ie, only at specific radii), which have corresponding energy levels that increase as the distance from the nucleus increases. With the application of energy, an electron may be excited to a higher energy level, thus increasing its distance from the nucleus, but will then spontaneously return to the lower energy level. By the law of conservation of energy, the excess energy is released as a photon. Although this small amount of energy would not be of much interest at the single particle level, Einstein and Bose discovered that photons were uniquely “gregarious” with the tendency to join together in a common state, leading to the ability to generate a coherent beam of light by simultaneously exciting multiple atoms and their electrons, whereby the return of one electron to a lower energy state generated a chain reaction among the other excited electrons, subsequently prompting the release of photons with the same characteristics as the initial incident photon and returning to a lower energy state.5 This process requires several steps to occur in order. First, absorption of energy has to occur among a population of atoms, thus exciting the electrons to higher energy states. When one of the electrons returns to a lower energy level, spontaneous emission will occur with the release of a photon of light. The photon has a certain probability of colliding with other atoms, thereby causing their electrons to return to a lower energy state and release additional photons of light with the same wavelength and in the same direction as the incident photon in a process that is referred to as stimulated emission.6 When this process occurs in a cavity with a large number of atoms, the result may lead to a high-energy beam of photons, which becomes the laser beam.
There are some caveats to consider regarding electron population dynamics as outlined by the Boltzmann principle whereby only a small proportion of molecules are in the first excited state and the vast majority are in the ground state (lowest energy) at any given time, but the details of higher-energy transitions in quantum mechanics are beyond the scope of this article.7 Primarily, it is important to understand that the ultimate power of a laser’s output depends largely on the population of electrons that are residing at a higher energy state at any given point in time, and the goal of many types of lasers is to achieve a large number of electrons in a high-energy state as opposed to their usual ground state, a process known as population inversion.8
This process leads to the fundamental construction of a laser: a population of atoms in a resonant cavity flanked by reflectors that are exposed to some sort of excitation mechanism (known as the pump) with an output mechanism for the laser beam to exit. In practice, the material used to supply the atoms (known as the gain medium) varies and also determines the wavelength and properties of the laser beam due to differences in the discrete energy states of orbiting electrons. Whatever the gain medium being used, the important properties of a laser resulting from these principles is that the beam is monochromatic (consisting of a single wavelength or a very narrow band), coherent (the light is emitted in the same phase and direction), collimated (a narrow beam diameter with limited divergence), and intense (high power per unit area). Consider the differences between a laser pointer and a flashlight; from across the room, the laser pointer output is a small spot of light on the wall whereas the flashlight has long dispersed to a weak, broad swath of light.
Types of Lasers
Different gain media have been used to create a variety of lasers with different properties. In general, lasers fall into 1 of 4 categories: gas discharge, diode, dye, and solid-state lasers.9
Although a gas discharge laser theoretically is the simplest laser, whereby a gas is excited by an electric discharge and the excited particles of gas create the laser beam, there are practical considerations such as excessive heat production, which may necessitate the use of cooling coils or some other method for heat dispersion. The excimer laser is a specific type of gas discharge laser in which a noble gas is mixed with halogen and high-current pulses are used to generate excited dimers, hence the term excimer. The excited dimers consisting of 1 halogen molecule and 1 noble gas molecule are only linked in the excited state, thus allowing for more stability in the excited state and enabling a higher proportion of molecules to be in that state at any given time, which increases population inversion and thus helps to maximize the output energy.
Diode lasers employ the use of diodes, or semiconductors that allow current to flow in one direction but not the other (theoretically with infinite resistance in one direction and no resistance in the other direction), thus creating a downstream method to achieve a high-power laser output; however, despite its theoretical efficiency, the use of diode lasers has been limited due to practical considerations of the divergence and quality of the output.
Dye lasers consist of a liquid solution of organic dye in a solvent that is pumped by an optical source. While gas discharge lasers involve excitation of a gas, there is a clear corollary with dye lasers with liquid taking the place of the gas; however, this modality has certain limitations, including the use of toxic materials that degrade naturally; the need to switch cuvettes when changing gain media, which serve as the lasing medium; and a relatively low-power output. One benefit of the dye laser, as alluded to above, is the operator’s capability to switch out cuvettes containing different dyes, thus using one machine to generate widely varying laser beams.
Solid-state lasers are most often used in dermatology. These devices utilize a conducting medium (eg, garnet, sapphire, ruby) doped with trivalent rare-earth ions or transition metal ions (eg, neodymium, ytterbium, erbium, titanium, chromium). This process is a relatively reliable and flexible methodology for generating stable lasers, thus explaining its widespread use. Additionally, these solid-state lasers are particularly amenable to modifications (eg, Q-switching).
Considerations for Lasers
Quality switching (known as Q-switching) is a method used to generate a shorter burst of a higher-power laser output.10 The longer the electrons have to become excited within a resonant cavity, the higher the number that may end up in an excited state, thus allowing for a higher ultimate energy output to a certain point. The quality of a medium, in general, refers to the ability of light exiting a medium to return. Within a cavity, the ability of light to go back and forth through the lasing medium is critical in achieving stimulated emission and thus laser beam output; however, in a low-quality medium, population inversion can still occur to allow a greater proportion of electrons to reside in a higher energy state. There are multiple mechanisms to switch the quality of a medium, but the ultimate result always is for the quality to be switched to high so that the light beams can immediately start achieving stimulated emission of a “primed” population of high-energy, population-inverted electrons, resulting in a much higher output power.
Selective thermolysis is critical for understanding modern laser use. To fully comprehend its meaning, one must first understand that the interaction of a laser with the skin depends on a number of factors, including the power density of the laser itself (the beam characteristics), the length of time of exposure, and the physical properties of the targeted molecules. Although there is some modulation of laser function via the wavelength of the laser (eg, higher wavelengths penetrate deeper), the properties of the target molecule can allow for precise control of the laser’s action. The framework for understanding this principle was outlined by Anderson and Parrish11 in 1983. Fundamentally, a laser causes damage to a target molecule via application of large amounts of energy; however, the laser beam does not discriminate between different molecules in its path. Rather the size and other properties of the molecule play a critical role in determining the amount of energy it is able to absorb before dissipating the excess energy as heat. This excess heat energy is what causes damage to surrounding tissues, or collateral damage. Conceptually, being able to target a molecule without damaging surrounding tissues is our goal as practitioners when using lasers in dermatology. It is accomplished by heating a molecule to just under its thermal relaxation time (ie, the time needed for a molecule to dissipate half of the energy applied), thus allowing for acceptable results with regard to efficacy balanced with side effects.12
Conclusion
Lasers are an important treatment modality, and their use in dermatology is becoming widespread for many possible indications; however, lasers are complex mechanical devices that have the potential to cause great harm when used incorrectly. By gaining a thorough understanding of the basic physics of lasers, the different types of lasers that are available, and critical concepts regarding the cutaneous application of lasers, physicians can better understand these devices and approach their use confidently and safely.
1. Einstein A. Zur quantentheorie der Strahlung. Physik Zeitschr. 1917;18:121-128.
2. Schawlow AL, Townes CH. Infrared and optical masers. Phys Rev. 1958;112:1940.
3. Maiman TH. Stimulated optical radiation in ruby. Nature. 1960;187:493-494.
4. Bohr N. On the constitution of atoms and molecules. Philos Mag. 1913;26:1-25.
5. Lewenstein M. Atomic physics: the social life of atoms. Nature. 2007;445:372-375.
6. Chang WSC. Principles of Lasers and Optics. Cambridge, United Kingdom: Cambridge University Press; 2005.
7. Svelto O. Principles of Lasers. 5th ed. New York, NY: Springer; 2010.
8. Rentzepis PM. Lasers in chemistry. Photochem Photobiol. 1968;8:579-588.
9. Tanzi EL, Lupton JR, Alster TS. Lasers in dermatology: four decades of progress. J Am Acad Dermatol. 2003;49:1-31.
10. Saedi N, Green JB, Dover JS, et al. The evolution of quality-switched lasers. J Drugs Dermatol. 2012;11:1296-1299.
11. Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-527.
12. Babilas P, Shafirstein G, Baumler W, et al. Selective photothermolysis of blood vessels following flashlamp-pumped pulsed dye laser irradiation: in vivo results and mathematical modelling are in agreement. J Invest Dermatol. 2005;125:343-352.
Lasers have become a critical part of the dermatologist’s armamentarium for modulating cutaneous biology, both in treating skin disorders and providing tangible cosmetic alterations to the skin. Although advances in technology and convenient user interfaces have made modern lasers relatively straightforward to use, they are in fact quite complex and powerful instruments that are capable of considerable damage if not used correctly. Thus it is necessary to establish a framework for the safe and responsible use of lasers in dermatology; fundamental to this tenet is an understanding of the development and physics of lasers. In this article, the fundamental concepts of lasers as well as their interactions with the skin will be discussed to impart a working knowledge of lasers to allow for better, safer use of these important tools.
Development of Lasers
The term laser is an acronym for “light amplification by the stimulated emission of radiation.” Albert Einstein established the framework for the functioning of lasers in his seminal work, “On the Quantum Theory of Radiation,”1 in which he described how an electron in an atom in an excited state can return to a lower state by emitting energy in the form of a photon of light. Light comprises a portion of the electromagnetic spectrum, rangingfrom UV (200–400 nm) to visible (400 to about 700 nm) to infrared light (about 700 to >3000 nm). The unique properties of light that affect the function of lasers include reflection (eg, seeing a mirror image of a mountain on the surface of a still lake) and refraction (eg, your hand looking larger under the surface of a pool of water).
Despite early theories on lasers, it was not until the late 1950s that the technology finally started to catch up to the science. Researchers experimenting with microwave fields were able to generate a beam of excited ammonia molecules through a resonant cavity, resulting in a uniform (albeit low power) emission of radiation.2 Maiman3 expounded on this development by building the first working prototype of a device that radiated light without the use of a microwave. So how exactly do lasers work?
Basic Physics of Lasers
To understand how lasers work, one must have a rudimentary understanding of quantum mechanics. Bohr4 revealed that an atom is comprised of a nucleus that is orbited by electrons at discrete distances (ie, only at specific radii), which have corresponding energy levels that increase as the distance from the nucleus increases. With the application of energy, an electron may be excited to a higher energy level, thus increasing its distance from the nucleus, but will then spontaneously return to the lower energy level. By the law of conservation of energy, the excess energy is released as a photon. Although this small amount of energy would not be of much interest at the single particle level, Einstein and Bose discovered that photons were uniquely “gregarious” with the tendency to join together in a common state, leading to the ability to generate a coherent beam of light by simultaneously exciting multiple atoms and their electrons, whereby the return of one electron to a lower energy state generated a chain reaction among the other excited electrons, subsequently prompting the release of photons with the same characteristics as the initial incident photon and returning to a lower energy state.5 This process requires several steps to occur in order. First, absorption of energy has to occur among a population of atoms, thus exciting the electrons to higher energy states. When one of the electrons returns to a lower energy level, spontaneous emission will occur with the release of a photon of light. The photon has a certain probability of colliding with other atoms, thereby causing their electrons to return to a lower energy state and release additional photons of light with the same wavelength and in the same direction as the incident photon in a process that is referred to as stimulated emission.6 When this process occurs in a cavity with a large number of atoms, the result may lead to a high-energy beam of photons, which becomes the laser beam.
There are some caveats to consider regarding electron population dynamics as outlined by the Boltzmann principle whereby only a small proportion of molecules are in the first excited state and the vast majority are in the ground state (lowest energy) at any given time, but the details of higher-energy transitions in quantum mechanics are beyond the scope of this article.7 Primarily, it is important to understand that the ultimate power of a laser’s output depends largely on the population of electrons that are residing at a higher energy state at any given point in time, and the goal of many types of lasers is to achieve a large number of electrons in a high-energy state as opposed to their usual ground state, a process known as population inversion.8
This process leads to the fundamental construction of a laser: a population of atoms in a resonant cavity flanked by reflectors that are exposed to some sort of excitation mechanism (known as the pump) with an output mechanism for the laser beam to exit. In practice, the material used to supply the atoms (known as the gain medium) varies and also determines the wavelength and properties of the laser beam due to differences in the discrete energy states of orbiting electrons. Whatever the gain medium being used, the important properties of a laser resulting from these principles is that the beam is monochromatic (consisting of a single wavelength or a very narrow band), coherent (the light is emitted in the same phase and direction), collimated (a narrow beam diameter with limited divergence), and intense (high power per unit area). Consider the differences between a laser pointer and a flashlight; from across the room, the laser pointer output is a small spot of light on the wall whereas the flashlight has long dispersed to a weak, broad swath of light.
Types of Lasers
Different gain media have been used to create a variety of lasers with different properties. In general, lasers fall into 1 of 4 categories: gas discharge, diode, dye, and solid-state lasers.9
Although a gas discharge laser theoretically is the simplest laser, whereby a gas is excited by an electric discharge and the excited particles of gas create the laser beam, there are practical considerations such as excessive heat production, which may necessitate the use of cooling coils or some other method for heat dispersion. The excimer laser is a specific type of gas discharge laser in which a noble gas is mixed with halogen and high-current pulses are used to generate excited dimers, hence the term excimer. The excited dimers consisting of 1 halogen molecule and 1 noble gas molecule are only linked in the excited state, thus allowing for more stability in the excited state and enabling a higher proportion of molecules to be in that state at any given time, which increases population inversion and thus helps to maximize the output energy.
Diode lasers employ the use of diodes, or semiconductors that allow current to flow in one direction but not the other (theoretically with infinite resistance in one direction and no resistance in the other direction), thus creating a downstream method to achieve a high-power laser output; however, despite its theoretical efficiency, the use of diode lasers has been limited due to practical considerations of the divergence and quality of the output.
Dye lasers consist of a liquid solution of organic dye in a solvent that is pumped by an optical source. While gas discharge lasers involve excitation of a gas, there is a clear corollary with dye lasers with liquid taking the place of the gas; however, this modality has certain limitations, including the use of toxic materials that degrade naturally; the need to switch cuvettes when changing gain media, which serve as the lasing medium; and a relatively low-power output. One benefit of the dye laser, as alluded to above, is the operator’s capability to switch out cuvettes containing different dyes, thus using one machine to generate widely varying laser beams.
Solid-state lasers are most often used in dermatology. These devices utilize a conducting medium (eg, garnet, sapphire, ruby) doped with trivalent rare-earth ions or transition metal ions (eg, neodymium, ytterbium, erbium, titanium, chromium). This process is a relatively reliable and flexible methodology for generating stable lasers, thus explaining its widespread use. Additionally, these solid-state lasers are particularly amenable to modifications (eg, Q-switching).
Considerations for Lasers
Quality switching (known as Q-switching) is a method used to generate a shorter burst of a higher-power laser output.10 The longer the electrons have to become excited within a resonant cavity, the higher the number that may end up in an excited state, thus allowing for a higher ultimate energy output to a certain point. The quality of a medium, in general, refers to the ability of light exiting a medium to return. Within a cavity, the ability of light to go back and forth through the lasing medium is critical in achieving stimulated emission and thus laser beam output; however, in a low-quality medium, population inversion can still occur to allow a greater proportion of electrons to reside in a higher energy state. There are multiple mechanisms to switch the quality of a medium, but the ultimate result always is for the quality to be switched to high so that the light beams can immediately start achieving stimulated emission of a “primed” population of high-energy, population-inverted electrons, resulting in a much higher output power.
Selective thermolysis is critical for understanding modern laser use. To fully comprehend its meaning, one must first understand that the interaction of a laser with the skin depends on a number of factors, including the power density of the laser itself (the beam characteristics), the length of time of exposure, and the physical properties of the targeted molecules. Although there is some modulation of laser function via the wavelength of the laser (eg, higher wavelengths penetrate deeper), the properties of the target molecule can allow for precise control of the laser’s action. The framework for understanding this principle was outlined by Anderson and Parrish11 in 1983. Fundamentally, a laser causes damage to a target molecule via application of large amounts of energy; however, the laser beam does not discriminate between different molecules in its path. Rather the size and other properties of the molecule play a critical role in determining the amount of energy it is able to absorb before dissipating the excess energy as heat. This excess heat energy is what causes damage to surrounding tissues, or collateral damage. Conceptually, being able to target a molecule without damaging surrounding tissues is our goal as practitioners when using lasers in dermatology. It is accomplished by heating a molecule to just under its thermal relaxation time (ie, the time needed for a molecule to dissipate half of the energy applied), thus allowing for acceptable results with regard to efficacy balanced with side effects.12
Conclusion
Lasers are an important treatment modality, and their use in dermatology is becoming widespread for many possible indications; however, lasers are complex mechanical devices that have the potential to cause great harm when used incorrectly. By gaining a thorough understanding of the basic physics of lasers, the different types of lasers that are available, and critical concepts regarding the cutaneous application of lasers, physicians can better understand these devices and approach their use confidently and safely.
Lasers have become a critical part of the dermatologist’s armamentarium for modulating cutaneous biology, both in treating skin disorders and providing tangible cosmetic alterations to the skin. Although advances in technology and convenient user interfaces have made modern lasers relatively straightforward to use, they are in fact quite complex and powerful instruments that are capable of considerable damage if not used correctly. Thus it is necessary to establish a framework for the safe and responsible use of lasers in dermatology; fundamental to this tenet is an understanding of the development and physics of lasers. In this article, the fundamental concepts of lasers as well as their interactions with the skin will be discussed to impart a working knowledge of lasers to allow for better, safer use of these important tools.
Development of Lasers
The term laser is an acronym for “light amplification by the stimulated emission of radiation.” Albert Einstein established the framework for the functioning of lasers in his seminal work, “On the Quantum Theory of Radiation,”1 in which he described how an electron in an atom in an excited state can return to a lower state by emitting energy in the form of a photon of light. Light comprises a portion of the electromagnetic spectrum, rangingfrom UV (200–400 nm) to visible (400 to about 700 nm) to infrared light (about 700 to >3000 nm). The unique properties of light that affect the function of lasers include reflection (eg, seeing a mirror image of a mountain on the surface of a still lake) and refraction (eg, your hand looking larger under the surface of a pool of water).
Despite early theories on lasers, it was not until the late 1950s that the technology finally started to catch up to the science. Researchers experimenting with microwave fields were able to generate a beam of excited ammonia molecules through a resonant cavity, resulting in a uniform (albeit low power) emission of radiation.2 Maiman3 expounded on this development by building the first working prototype of a device that radiated light without the use of a microwave. So how exactly do lasers work?
Basic Physics of Lasers
To understand how lasers work, one must have a rudimentary understanding of quantum mechanics. Bohr4 revealed that an atom is comprised of a nucleus that is orbited by electrons at discrete distances (ie, only at specific radii), which have corresponding energy levels that increase as the distance from the nucleus increases. With the application of energy, an electron may be excited to a higher energy level, thus increasing its distance from the nucleus, but will then spontaneously return to the lower energy level. By the law of conservation of energy, the excess energy is released as a photon. Although this small amount of energy would not be of much interest at the single particle level, Einstein and Bose discovered that photons were uniquely “gregarious” with the tendency to join together in a common state, leading to the ability to generate a coherent beam of light by simultaneously exciting multiple atoms and their electrons, whereby the return of one electron to a lower energy state generated a chain reaction among the other excited electrons, subsequently prompting the release of photons with the same characteristics as the initial incident photon and returning to a lower energy state.5 This process requires several steps to occur in order. First, absorption of energy has to occur among a population of atoms, thus exciting the electrons to higher energy states. When one of the electrons returns to a lower energy level, spontaneous emission will occur with the release of a photon of light. The photon has a certain probability of colliding with other atoms, thereby causing their electrons to return to a lower energy state and release additional photons of light with the same wavelength and in the same direction as the incident photon in a process that is referred to as stimulated emission.6 When this process occurs in a cavity with a large number of atoms, the result may lead to a high-energy beam of photons, which becomes the laser beam.
There are some caveats to consider regarding electron population dynamics as outlined by the Boltzmann principle whereby only a small proportion of molecules are in the first excited state and the vast majority are in the ground state (lowest energy) at any given time, but the details of higher-energy transitions in quantum mechanics are beyond the scope of this article.7 Primarily, it is important to understand that the ultimate power of a laser’s output depends largely on the population of electrons that are residing at a higher energy state at any given point in time, and the goal of many types of lasers is to achieve a large number of electrons in a high-energy state as opposed to their usual ground state, a process known as population inversion.8
This process leads to the fundamental construction of a laser: a population of atoms in a resonant cavity flanked by reflectors that are exposed to some sort of excitation mechanism (known as the pump) with an output mechanism for the laser beam to exit. In practice, the material used to supply the atoms (known as the gain medium) varies and also determines the wavelength and properties of the laser beam due to differences in the discrete energy states of orbiting electrons. Whatever the gain medium being used, the important properties of a laser resulting from these principles is that the beam is monochromatic (consisting of a single wavelength or a very narrow band), coherent (the light is emitted in the same phase and direction), collimated (a narrow beam diameter with limited divergence), and intense (high power per unit area). Consider the differences between a laser pointer and a flashlight; from across the room, the laser pointer output is a small spot of light on the wall whereas the flashlight has long dispersed to a weak, broad swath of light.
Types of Lasers
Different gain media have been used to create a variety of lasers with different properties. In general, lasers fall into 1 of 4 categories: gas discharge, diode, dye, and solid-state lasers.9
Although a gas discharge laser theoretically is the simplest laser, whereby a gas is excited by an electric discharge and the excited particles of gas create the laser beam, there are practical considerations such as excessive heat production, which may necessitate the use of cooling coils or some other method for heat dispersion. The excimer laser is a specific type of gas discharge laser in which a noble gas is mixed with halogen and high-current pulses are used to generate excited dimers, hence the term excimer. The excited dimers consisting of 1 halogen molecule and 1 noble gas molecule are only linked in the excited state, thus allowing for more stability in the excited state and enabling a higher proportion of molecules to be in that state at any given time, which increases population inversion and thus helps to maximize the output energy.
Diode lasers employ the use of diodes, or semiconductors that allow current to flow in one direction but not the other (theoretically with infinite resistance in one direction and no resistance in the other direction), thus creating a downstream method to achieve a high-power laser output; however, despite its theoretical efficiency, the use of diode lasers has been limited due to practical considerations of the divergence and quality of the output.
Dye lasers consist of a liquid solution of organic dye in a solvent that is pumped by an optical source. While gas discharge lasers involve excitation of a gas, there is a clear corollary with dye lasers with liquid taking the place of the gas; however, this modality has certain limitations, including the use of toxic materials that degrade naturally; the need to switch cuvettes when changing gain media, which serve as the lasing medium; and a relatively low-power output. One benefit of the dye laser, as alluded to above, is the operator’s capability to switch out cuvettes containing different dyes, thus using one machine to generate widely varying laser beams.
Solid-state lasers are most often used in dermatology. These devices utilize a conducting medium (eg, garnet, sapphire, ruby) doped with trivalent rare-earth ions or transition metal ions (eg, neodymium, ytterbium, erbium, titanium, chromium). This process is a relatively reliable and flexible methodology for generating stable lasers, thus explaining its widespread use. Additionally, these solid-state lasers are particularly amenable to modifications (eg, Q-switching).
Considerations for Lasers
Quality switching (known as Q-switching) is a method used to generate a shorter burst of a higher-power laser output.10 The longer the electrons have to become excited within a resonant cavity, the higher the number that may end up in an excited state, thus allowing for a higher ultimate energy output to a certain point. The quality of a medium, in general, refers to the ability of light exiting a medium to return. Within a cavity, the ability of light to go back and forth through the lasing medium is critical in achieving stimulated emission and thus laser beam output; however, in a low-quality medium, population inversion can still occur to allow a greater proportion of electrons to reside in a higher energy state. There are multiple mechanisms to switch the quality of a medium, but the ultimate result always is for the quality to be switched to high so that the light beams can immediately start achieving stimulated emission of a “primed” population of high-energy, population-inverted electrons, resulting in a much higher output power.
Selective thermolysis is critical for understanding modern laser use. To fully comprehend its meaning, one must first understand that the interaction of a laser with the skin depends on a number of factors, including the power density of the laser itself (the beam characteristics), the length of time of exposure, and the physical properties of the targeted molecules. Although there is some modulation of laser function via the wavelength of the laser (eg, higher wavelengths penetrate deeper), the properties of the target molecule can allow for precise control of the laser’s action. The framework for understanding this principle was outlined by Anderson and Parrish11 in 1983. Fundamentally, a laser causes damage to a target molecule via application of large amounts of energy; however, the laser beam does not discriminate between different molecules in its path. Rather the size and other properties of the molecule play a critical role in determining the amount of energy it is able to absorb before dissipating the excess energy as heat. This excess heat energy is what causes damage to surrounding tissues, or collateral damage. Conceptually, being able to target a molecule without damaging surrounding tissues is our goal as practitioners when using lasers in dermatology. It is accomplished by heating a molecule to just under its thermal relaxation time (ie, the time needed for a molecule to dissipate half of the energy applied), thus allowing for acceptable results with regard to efficacy balanced with side effects.12
Conclusion
Lasers are an important treatment modality, and their use in dermatology is becoming widespread for many possible indications; however, lasers are complex mechanical devices that have the potential to cause great harm when used incorrectly. By gaining a thorough understanding of the basic physics of lasers, the different types of lasers that are available, and critical concepts regarding the cutaneous application of lasers, physicians can better understand these devices and approach their use confidently and safely.
1. Einstein A. Zur quantentheorie der Strahlung. Physik Zeitschr. 1917;18:121-128.
2. Schawlow AL, Townes CH. Infrared and optical masers. Phys Rev. 1958;112:1940.
3. Maiman TH. Stimulated optical radiation in ruby. Nature. 1960;187:493-494.
4. Bohr N. On the constitution of atoms and molecules. Philos Mag. 1913;26:1-25.
5. Lewenstein M. Atomic physics: the social life of atoms. Nature. 2007;445:372-375.
6. Chang WSC. Principles of Lasers and Optics. Cambridge, United Kingdom: Cambridge University Press; 2005.
7. Svelto O. Principles of Lasers. 5th ed. New York, NY: Springer; 2010.
8. Rentzepis PM. Lasers in chemistry. Photochem Photobiol. 1968;8:579-588.
9. Tanzi EL, Lupton JR, Alster TS. Lasers in dermatology: four decades of progress. J Am Acad Dermatol. 2003;49:1-31.
10. Saedi N, Green JB, Dover JS, et al. The evolution of quality-switched lasers. J Drugs Dermatol. 2012;11:1296-1299.
11. Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-527.
12. Babilas P, Shafirstein G, Baumler W, et al. Selective photothermolysis of blood vessels following flashlamp-pumped pulsed dye laser irradiation: in vivo results and mathematical modelling are in agreement. J Invest Dermatol. 2005;125:343-352.
1. Einstein A. Zur quantentheorie der Strahlung. Physik Zeitschr. 1917;18:121-128.
2. Schawlow AL, Townes CH. Infrared and optical masers. Phys Rev. 1958;112:1940.
3. Maiman TH. Stimulated optical radiation in ruby. Nature. 1960;187:493-494.
4. Bohr N. On the constitution of atoms and molecules. Philos Mag. 1913;26:1-25.
5. Lewenstein M. Atomic physics: the social life of atoms. Nature. 2007;445:372-375.
6. Chang WSC. Principles of Lasers and Optics. Cambridge, United Kingdom: Cambridge University Press; 2005.
7. Svelto O. Principles of Lasers. 5th ed. New York, NY: Springer; 2010.
8. Rentzepis PM. Lasers in chemistry. Photochem Photobiol. 1968;8:579-588.
9. Tanzi EL, Lupton JR, Alster TS. Lasers in dermatology: four decades of progress. J Am Acad Dermatol. 2003;49:1-31.
10. Saedi N, Green JB, Dover JS, et al. The evolution of quality-switched lasers. J Drugs Dermatol. 2012;11:1296-1299.
11. Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-527.
12. Babilas P, Shafirstein G, Baumler W, et al. Selective photothermolysis of blood vessels following flashlamp-pumped pulsed dye laser irradiation: in vivo results and mathematical modelling are in agreement. J Invest Dermatol. 2005;125:343-352.
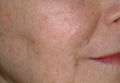
Closing large dermal defects much like a Victorian corset
EDINBURGH – Barbed absorbable sutures are a useful new tool to facilitate dermal closure of facial and nonfacial defects following tumor resection.
“These are not the bad old sutures that you might of heard about before, that were nonabsorbable sutures and attempted for use in cosmetic procedures,” Dr. John Strasswimmer said at the 15th World Congress on Cancers of the Skin.
Last year, Dr. Strasswimmer, medical director of melanoma and cutaneous oncology at the Lynn Cancer Institute in Boca Raton, Fla., reported his initial experience using a procedure he calls “Corseta” to close a large Mohs defect on the trunk of an 83-year-old man (JAMA Dermatol. 2013;149:853-4).
The procedure employs a barbed, bioabsorbable suture (Ethicon’s Stratafix and Covidien’s V-Loc) that is run in a continuous vertical looping manner in the subcutaneous layer, with minimal to no undermining of the wound. Undermining is typically used in cutaneous surgery to relieve tension or provide structure around anatomical landmarks, but it can increase the risk of bleeding, swelling, and patient discomfort, he said.
Instead, the first suture pass is placed in the deepest portion of the subcutaneous tissue and brought out within the more superficial subcutaneous layer. Each bite of the barbed suture extends peripherally at least 2.0 cm from the edge of the wound, so the point of tension is lateral to the wound margins. At every two passes, tension is placed evenly across the sutures to close the deepest layer of tissue and to engage the barbs, much like closing of a Victorian corset, Dr. Strasswimmer said.
The second arm of the suture is passed in a similar manner in the subcutaneous plane, superficial to the first pass.
“This is a lacing, not a suturing technique,” he said. “You get tissue approximation, but more importantly, because we’re bringing in all that deep tissue, you automatically get beautiful wound-edge eversion and very nice cosmetic results.”
Because the sutures have barbs cut into them, however, a 0-0 weight polydioxane or other absorbable material suture can have a breaking strength of a #2-0 suture. “You have to look very carefully at the manufacturer’s sizing and strength requirements,” Dr. Strasswimmer cautioned.
Since their initial case report, Dr. Strasswimmer and his colleagues have expanded use of the Corseta technique to more than 600 facial and nonfacial reconstructions. The Corseta procedure is not as helpful for curved topography such as the central face or scalp, he said in an interview. Still, of the 600 or so cases, none required conversion to another closure technique.
“The traditional closure technique would not have worked in those challenging cases,” Dr. Strasswimmer said. “In the most difficult situations, such as older patients with severely atrophic skin, even the best suturing won’t work. In that case, the Corseta at least produces a partial closure, thereby reducing the wound and accelerating healing.” The Corseta procedure is often coupled with tumescent anesthesia to decrease the risk of bleeding, particularly in patients on anticoagulation, he noted.
The conference was sponsored by the Skin Cancer Foundation.
EDINBURGH – Barbed absorbable sutures are a useful new tool to facilitate dermal closure of facial and nonfacial defects following tumor resection.
“These are not the bad old sutures that you might of heard about before, that were nonabsorbable sutures and attempted for use in cosmetic procedures,” Dr. John Strasswimmer said at the 15th World Congress on Cancers of the Skin.
Last year, Dr. Strasswimmer, medical director of melanoma and cutaneous oncology at the Lynn Cancer Institute in Boca Raton, Fla., reported his initial experience using a procedure he calls “Corseta” to close a large Mohs defect on the trunk of an 83-year-old man (JAMA Dermatol. 2013;149:853-4).
The procedure employs a barbed, bioabsorbable suture (Ethicon’s Stratafix and Covidien’s V-Loc) that is run in a continuous vertical looping manner in the subcutaneous layer, with minimal to no undermining of the wound. Undermining is typically used in cutaneous surgery to relieve tension or provide structure around anatomical landmarks, but it can increase the risk of bleeding, swelling, and patient discomfort, he said.
Instead, the first suture pass is placed in the deepest portion of the subcutaneous tissue and brought out within the more superficial subcutaneous layer. Each bite of the barbed suture extends peripherally at least 2.0 cm from the edge of the wound, so the point of tension is lateral to the wound margins. At every two passes, tension is placed evenly across the sutures to close the deepest layer of tissue and to engage the barbs, much like closing of a Victorian corset, Dr. Strasswimmer said.
The second arm of the suture is passed in a similar manner in the subcutaneous plane, superficial to the first pass.
“This is a lacing, not a suturing technique,” he said. “You get tissue approximation, but more importantly, because we’re bringing in all that deep tissue, you automatically get beautiful wound-edge eversion and very nice cosmetic results.”
Because the sutures have barbs cut into them, however, a 0-0 weight polydioxane or other absorbable material suture can have a breaking strength of a #2-0 suture. “You have to look very carefully at the manufacturer’s sizing and strength requirements,” Dr. Strasswimmer cautioned.
Since their initial case report, Dr. Strasswimmer and his colleagues have expanded use of the Corseta technique to more than 600 facial and nonfacial reconstructions. The Corseta procedure is not as helpful for curved topography such as the central face or scalp, he said in an interview. Still, of the 600 or so cases, none required conversion to another closure technique.
“The traditional closure technique would not have worked in those challenging cases,” Dr. Strasswimmer said. “In the most difficult situations, such as older patients with severely atrophic skin, even the best suturing won’t work. In that case, the Corseta at least produces a partial closure, thereby reducing the wound and accelerating healing.” The Corseta procedure is often coupled with tumescent anesthesia to decrease the risk of bleeding, particularly in patients on anticoagulation, he noted.
The conference was sponsored by the Skin Cancer Foundation.
EDINBURGH – Barbed absorbable sutures are a useful new tool to facilitate dermal closure of facial and nonfacial defects following tumor resection.
“These are not the bad old sutures that you might of heard about before, that were nonabsorbable sutures and attempted for use in cosmetic procedures,” Dr. John Strasswimmer said at the 15th World Congress on Cancers of the Skin.
Last year, Dr. Strasswimmer, medical director of melanoma and cutaneous oncology at the Lynn Cancer Institute in Boca Raton, Fla., reported his initial experience using a procedure he calls “Corseta” to close a large Mohs defect on the trunk of an 83-year-old man (JAMA Dermatol. 2013;149:853-4).
The procedure employs a barbed, bioabsorbable suture (Ethicon’s Stratafix and Covidien’s V-Loc) that is run in a continuous vertical looping manner in the subcutaneous layer, with minimal to no undermining of the wound. Undermining is typically used in cutaneous surgery to relieve tension or provide structure around anatomical landmarks, but it can increase the risk of bleeding, swelling, and patient discomfort, he said.
Instead, the first suture pass is placed in the deepest portion of the subcutaneous tissue and brought out within the more superficial subcutaneous layer. Each bite of the barbed suture extends peripherally at least 2.0 cm from the edge of the wound, so the point of tension is lateral to the wound margins. At every two passes, tension is placed evenly across the sutures to close the deepest layer of tissue and to engage the barbs, much like closing of a Victorian corset, Dr. Strasswimmer said.
The second arm of the suture is passed in a similar manner in the subcutaneous plane, superficial to the first pass.
“This is a lacing, not a suturing technique,” he said. “You get tissue approximation, but more importantly, because we’re bringing in all that deep tissue, you automatically get beautiful wound-edge eversion and very nice cosmetic results.”
Because the sutures have barbs cut into them, however, a 0-0 weight polydioxane or other absorbable material suture can have a breaking strength of a #2-0 suture. “You have to look very carefully at the manufacturer’s sizing and strength requirements,” Dr. Strasswimmer cautioned.
Since their initial case report, Dr. Strasswimmer and his colleagues have expanded use of the Corseta technique to more than 600 facial and nonfacial reconstructions. The Corseta procedure is not as helpful for curved topography such as the central face or scalp, he said in an interview. Still, of the 600 or so cases, none required conversion to another closure technique.
“The traditional closure technique would not have worked in those challenging cases,” Dr. Strasswimmer said. “In the most difficult situations, such as older patients with severely atrophic skin, even the best suturing won’t work. In that case, the Corseta at least produces a partial closure, thereby reducing the wound and accelerating healing.” The Corseta procedure is often coupled with tumescent anesthesia to decrease the risk of bleeding, particularly in patients on anticoagulation, he noted.
The conference was sponsored by the Skin Cancer Foundation.
EXPERT ANALYSIS FROM WCCS 2014
Practice Question Answers: Electrosurgery
1. A 71-year-old woman with a pacemaker/defibrillator is undergoing Mohs micrographic surgery. Which method is the safest for hemostasis during surgery?
a. biterminal electrocoagulation
b. electrocautery
c. electrodesiccation
d. monopolar electrocoagulation
e. monoterminal electrocoagulation
2. Which method usually causes the deepest level of tissue damage during hemostasis?
a. electrocautery
b. electrocoagulation
c. electrodesiccation
d. electrofulguration
e. electrosection
3. Which electrosurgical mode has the highest maximum output power?
a. bipolar mode
b. coagulation mode
c. cutting mode
d. fulguration mode
e. monoterminal mode
4. A 59-year-old woman is scheduled for curettage and electrodesiccation of a superficial basal cell carcinoma on the upper back. Which method is preferred for deep electrodesiccation?
a. large-tip electrode and coagulation current
b. large-tip electrode in fulguration mode
c. small-tip electrode and coagulation current
d. small-tip electrode and cutting current
e. small-tip electrode in fulguration mode
5. A 32-year-old Asian woman is scheduled for superficial electrosurgical destruction of a small flat seborrheic keratosis on the face. Which treatment method is preferred?
a. large-tip electrode and coagulation current
b. large-tip electrode and cutting current
c. large-tip electrode and fulguration current in noncontact mode at the maximum power
d. small-tip electrode and cutting current
e. small-tip electrode and fulguration current at the maximum power
1. A 71-year-old woman with a pacemaker/defibrillator is undergoing Mohs micrographic surgery. Which method is the safest for hemostasis during surgery?
a. biterminal electrocoagulation
b. electrocautery
c. electrodesiccation
d. monopolar electrocoagulation
e. monoterminal electrocoagulation
2. Which method usually causes the deepest level of tissue damage during hemostasis?
a. electrocautery
b. electrocoagulation
c. electrodesiccation
d. electrofulguration
e. electrosection
3. Which electrosurgical mode has the highest maximum output power?
a. bipolar mode
b. coagulation mode
c. cutting mode
d. fulguration mode
e. monoterminal mode
4. A 59-year-old woman is scheduled for curettage and electrodesiccation of a superficial basal cell carcinoma on the upper back. Which method is preferred for deep electrodesiccation?
a. large-tip electrode and coagulation current
b. large-tip electrode in fulguration mode
c. small-tip electrode and coagulation current
d. small-tip electrode and cutting current
e. small-tip electrode in fulguration mode
5. A 32-year-old Asian woman is scheduled for superficial electrosurgical destruction of a small flat seborrheic keratosis on the face. Which treatment method is preferred?
a. large-tip electrode and coagulation current
b. large-tip electrode and cutting current
c. large-tip electrode and fulguration current in noncontact mode at the maximum power
d. small-tip electrode and cutting current
e. small-tip electrode and fulguration current at the maximum power
1. A 71-year-old woman with a pacemaker/defibrillator is undergoing Mohs micrographic surgery. Which method is the safest for hemostasis during surgery?
a. biterminal electrocoagulation
b. electrocautery
c. electrodesiccation
d. monopolar electrocoagulation
e. monoterminal electrocoagulation
2. Which method usually causes the deepest level of tissue damage during hemostasis?
a. electrocautery
b. electrocoagulation
c. electrodesiccation
d. electrofulguration
e. electrosection
3. Which electrosurgical mode has the highest maximum output power?
a. bipolar mode
b. coagulation mode
c. cutting mode
d. fulguration mode
e. monoterminal mode
4. A 59-year-old woman is scheduled for curettage and electrodesiccation of a superficial basal cell carcinoma on the upper back. Which method is preferred for deep electrodesiccation?
a. large-tip electrode and coagulation current
b. large-tip electrode in fulguration mode
c. small-tip electrode and coagulation current
d. small-tip electrode and cutting current
e. small-tip electrode in fulguration mode
5. A 32-year-old Asian woman is scheduled for superficial electrosurgical destruction of a small flat seborrheic keratosis on the face. Which treatment method is preferred?
a. large-tip electrode and coagulation current
b. large-tip electrode and cutting current
c. large-tip electrode and fulguration current in noncontact mode at the maximum power
d. small-tip electrode and cutting current
e. small-tip electrode and fulguration current at the maximum power
Electrosurgery
Acne and rosacea management for men
In a report released March 2014 by the American Society of Aesthetic Plastic Surgery, the top five surgical procedures for men were liposuction, eyelid surgery, rhinoplasty, male breast reduction, and ear surgery. However, the rate of noninvasive cosmetic procedures and sales of men’s grooming products is one of the leading segments of the beauty industry.
Although most scientific research and media are focused on the female aesthetic, understanding the specific needs of your male patients is key to patient satisfaction. Most men are generally less aware than are women of the treatment options and risks and benefits of procedures. Men also prefer treatments with less downtime and natural-looking results. This column continues our miniseries on aesthetic dermatology for the male patient.
In a general dermatology practice, there are several skin concerns often identified by male patients, and acne and rosacea are among them.
Acne: Men generally have thicker, more sebaceous skin than that of women. Although acne is a very common problem in teens and young men, there is a growing trend of cases of cystic acne in adult men who consume popular protein meal replacement or muscle enhancing shakes that contain whey protein. Whey is a protein derived from cow’s milk. Milk and dairy products act by increasing insulin-like growth factor 1, which has been linked to acne. Although few case reports have shown a link between dietary whey supplementation and acne, in my practice, men with cystic acne who report using whey supplementation products have had almost complete resolution of their acne without medical intervention after discontinuing these products.
Rosacea: Men have a higher density of facial blood vessels than women do, and often seek treatment for telangiectasias and overall facial erythema. For papulopustular rosacea, common treatments include oral antibiotics, topical antibiotics, topical azaleic acid, and topical anti-inflammatory medications. For erythematotelangiectatic rosacea, Mirvaso (brimonidine), a topical vasoconstrictor, can be applied to the skin for 8-12 hours of marked reduction in facial erythema. Although theoretically a great option for patients suffering from erythema, the effects of topical brimonidine are transient, and the gel requires daily application with no long-term benefit. Vascular laser treatments are effective for telangiectasias for both men and women. However, men with more granulomatous or phymatous rosacea often need a combination of treatments including antibiotics, oral isotretinoin and fractional ablative lasers.
Resources:
American Society for Plastic Surgery 2012 statistics.
“Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes,” Cutis 2012;90:70-2.
Dr. Talakoub and Dr. Wesley are cocontributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
In a report released March 2014 by the American Society of Aesthetic Plastic Surgery, the top five surgical procedures for men were liposuction, eyelid surgery, rhinoplasty, male breast reduction, and ear surgery. However, the rate of noninvasive cosmetic procedures and sales of men’s grooming products is one of the leading segments of the beauty industry.
Although most scientific research and media are focused on the female aesthetic, understanding the specific needs of your male patients is key to patient satisfaction. Most men are generally less aware than are women of the treatment options and risks and benefits of procedures. Men also prefer treatments with less downtime and natural-looking results. This column continues our miniseries on aesthetic dermatology for the male patient.
In a general dermatology practice, there are several skin concerns often identified by male patients, and acne and rosacea are among them.
Acne: Men generally have thicker, more sebaceous skin than that of women. Although acne is a very common problem in teens and young men, there is a growing trend of cases of cystic acne in adult men who consume popular protein meal replacement or muscle enhancing shakes that contain whey protein. Whey is a protein derived from cow’s milk. Milk and dairy products act by increasing insulin-like growth factor 1, which has been linked to acne. Although few case reports have shown a link between dietary whey supplementation and acne, in my practice, men with cystic acne who report using whey supplementation products have had almost complete resolution of their acne without medical intervention after discontinuing these products.
Rosacea: Men have a higher density of facial blood vessels than women do, and often seek treatment for telangiectasias and overall facial erythema. For papulopustular rosacea, common treatments include oral antibiotics, topical antibiotics, topical azaleic acid, and topical anti-inflammatory medications. For erythematotelangiectatic rosacea, Mirvaso (brimonidine), a topical vasoconstrictor, can be applied to the skin for 8-12 hours of marked reduction in facial erythema. Although theoretically a great option for patients suffering from erythema, the effects of topical brimonidine are transient, and the gel requires daily application with no long-term benefit. Vascular laser treatments are effective for telangiectasias for both men and women. However, men with more granulomatous or phymatous rosacea often need a combination of treatments including antibiotics, oral isotretinoin and fractional ablative lasers.
Resources:
American Society for Plastic Surgery 2012 statistics.
“Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes,” Cutis 2012;90:70-2.
Dr. Talakoub and Dr. Wesley are cocontributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
In a report released March 2014 by the American Society of Aesthetic Plastic Surgery, the top five surgical procedures for men were liposuction, eyelid surgery, rhinoplasty, male breast reduction, and ear surgery. However, the rate of noninvasive cosmetic procedures and sales of men’s grooming products is one of the leading segments of the beauty industry.
Although most scientific research and media are focused on the female aesthetic, understanding the specific needs of your male patients is key to patient satisfaction. Most men are generally less aware than are women of the treatment options and risks and benefits of procedures. Men also prefer treatments with less downtime and natural-looking results. This column continues our miniseries on aesthetic dermatology for the male patient.
In a general dermatology practice, there are several skin concerns often identified by male patients, and acne and rosacea are among them.
Acne: Men generally have thicker, more sebaceous skin than that of women. Although acne is a very common problem in teens and young men, there is a growing trend of cases of cystic acne in adult men who consume popular protein meal replacement or muscle enhancing shakes that contain whey protein. Whey is a protein derived from cow’s milk. Milk and dairy products act by increasing insulin-like growth factor 1, which has been linked to acne. Although few case reports have shown a link between dietary whey supplementation and acne, in my practice, men with cystic acne who report using whey supplementation products have had almost complete resolution of their acne without medical intervention after discontinuing these products.
Rosacea: Men have a higher density of facial blood vessels than women do, and often seek treatment for telangiectasias and overall facial erythema. For papulopustular rosacea, common treatments include oral antibiotics, topical antibiotics, topical azaleic acid, and topical anti-inflammatory medications. For erythematotelangiectatic rosacea, Mirvaso (brimonidine), a topical vasoconstrictor, can be applied to the skin for 8-12 hours of marked reduction in facial erythema. Although theoretically a great option for patients suffering from erythema, the effects of topical brimonidine are transient, and the gel requires daily application with no long-term benefit. Vascular laser treatments are effective for telangiectasias for both men and women. However, men with more granulomatous or phymatous rosacea often need a combination of treatments including antibiotics, oral isotretinoin and fractional ablative lasers.
Resources:
American Society for Plastic Surgery 2012 statistics.
“Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes,” Cutis 2012;90:70-2.
Dr. Talakoub and Dr. Wesley are cocontributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
Cosmetic Corner: Dermatologists Weigh in on OTC Antioxidants
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top OTC antioxidants. Consideration must be given to:
- Active C
La Roche-Posay Laboratoire Dermatologique
“It has 5% vitamin C, which is a known potent antioxidant for skin. It is available in most pharmacies and has a reasonable price point for patients.”—Anthony M. Rossi, MD, New York, New York
- Super Serum Advance
iS Clinical
“Super Serum Advance not only provides antioxidants to the skin, but it also has specifically formulated peptides to increase collagen production. This allows Super Serum Advance to reduce the appearance of fine lines, wrinkles, scar tissue, stretch marks, and uneven pigmentation while providing superior antioxidant protection to the skin.”—Wm. Philip Werschler, Seattle, Washington
- Urbane Renewal
Biopelle, Inc
It has an array of antioxidants but also DNA repair. Plus it goes on very nicely as a serum.”—Joel L. Cohen, Englewood, Colorado
Cutis invites readers to send us their recommendations. Over-the-counter pigment control, facial scrubs, and mineral makeup will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to cutis@frontlinemedcom.com.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top OTC antioxidants. Consideration must be given to:
- Active C
La Roche-Posay Laboratoire Dermatologique
“It has 5% vitamin C, which is a known potent antioxidant for skin. It is available in most pharmacies and has a reasonable price point for patients.”—Anthony M. Rossi, MD, New York, New York
- Super Serum Advance
iS Clinical
“Super Serum Advance not only provides antioxidants to the skin, but it also has specifically formulated peptides to increase collagen production. This allows Super Serum Advance to reduce the appearance of fine lines, wrinkles, scar tissue, stretch marks, and uneven pigmentation while providing superior antioxidant protection to the skin.”—Wm. Philip Werschler, Seattle, Washington
- Urbane Renewal
Biopelle, Inc
It has an array of antioxidants but also DNA repair. Plus it goes on very nicely as a serum.”—Joel L. Cohen, Englewood, Colorado
Cutis invites readers to send us their recommendations. Over-the-counter pigment control, facial scrubs, and mineral makeup will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to cutis@frontlinemedcom.com.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top OTC antioxidants. Consideration must be given to:
- Active C
La Roche-Posay Laboratoire Dermatologique
“It has 5% vitamin C, which is a known potent antioxidant for skin. It is available in most pharmacies and has a reasonable price point for patients.”—Anthony M. Rossi, MD, New York, New York
- Super Serum Advance
iS Clinical
“Super Serum Advance not only provides antioxidants to the skin, but it also has specifically formulated peptides to increase collagen production. This allows Super Serum Advance to reduce the appearance of fine lines, wrinkles, scar tissue, stretch marks, and uneven pigmentation while providing superior antioxidant protection to the skin.”—Wm. Philip Werschler, Seattle, Washington
- Urbane Renewal
Biopelle, Inc
It has an array of antioxidants but also DNA repair. Plus it goes on very nicely as a serum.”—Joel L. Cohen, Englewood, Colorado
Cutis invites readers to send us their recommendations. Over-the-counter pigment control, facial scrubs, and mineral makeup will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to cutis@frontlinemedcom.com.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
Adding second pain med to HA filler disappoints
CHICAGO – Adding epinephrine to a specific formulation of hyaluronic acid already mixed with lidocaine failed to reduce the severity of adverse events following correction of perioral lines in a blinded study.
"Our results using split-face comparison did not reveal a large difference in bruising and pain scores," Dr. Azadeh Shirazi of Scripps Clinic in La Jolla, Calif., reported at the American Academy of Dermatology summer meeting.
Three groups of 10 women with mild to severe lip wrinkles were treated with 1.0 mL of cohesive polydensified matrix hyaluronic acid (CPMHA) alone; CPMHA plus 0.3 mL of lidocaine HCl 1%; or CPMHA plus lidocaine and epinephrine 1:100,000. The volumes in each syringe were adjusted to 1 mL in total.
All injections were performed in the dermis, using serial punctures and linear threading techniques. An entire syringe of one mixture was used on one side, followed by injection of a different mixture on the contralateral side.
Outside evaluators, physician investigators, and patients were blinded to treatment. Bruising was assessed on a 4-point, nonvalidated scale, with 0 being "no visible bruising" and 3 "severe" bruising.
On day 1 post procedure, outside evaluators gave the lowest bruising score to CPMHA alone at about 1.375, followed by CPMHA plus lidocaine and epinephrine at about 1.4, and CPMHA plus lidocaine at about 1.45.
Physicians favored the hyaluronic acid with both pain medications, while patients rated the lidocaine formulation as causing the least bruising.
By day 7, bruising was lower in all groups. The outside evaluators again rated CPMHA alone as causing the least bruising on day 7, while patients and physicians perceived less bruising with the CPMHA-lidocaine formulation. Pain scores followed a similar pattern.
Although not a variable in the study, short-lived edema also was noted in most patients.
It’s possible that there was not enough time for the epinephrine to take full effect as a vasoconstrictor to decrease bruising, Dr. Shirazi said in an interview.
Larger studies are warranted to determine differences between the different formulations, the Dr. Shirazi and her colleagues concluded in the poster.
The study was supported by Merz North America, makers of the CPMHA formulation. Dr. Shirazi has served as an investigator for Allergan, Medicis, and Merz, and as an advisory board member for several pharmaceutical and device companies.
CHICAGO – Adding epinephrine to a specific formulation of hyaluronic acid already mixed with lidocaine failed to reduce the severity of adverse events following correction of perioral lines in a blinded study.
"Our results using split-face comparison did not reveal a large difference in bruising and pain scores," Dr. Azadeh Shirazi of Scripps Clinic in La Jolla, Calif., reported at the American Academy of Dermatology summer meeting.
Three groups of 10 women with mild to severe lip wrinkles were treated with 1.0 mL of cohesive polydensified matrix hyaluronic acid (CPMHA) alone; CPMHA plus 0.3 mL of lidocaine HCl 1%; or CPMHA plus lidocaine and epinephrine 1:100,000. The volumes in each syringe were adjusted to 1 mL in total.
All injections were performed in the dermis, using serial punctures and linear threading techniques. An entire syringe of one mixture was used on one side, followed by injection of a different mixture on the contralateral side.
Outside evaluators, physician investigators, and patients were blinded to treatment. Bruising was assessed on a 4-point, nonvalidated scale, with 0 being "no visible bruising" and 3 "severe" bruising.
On day 1 post procedure, outside evaluators gave the lowest bruising score to CPMHA alone at about 1.375, followed by CPMHA plus lidocaine and epinephrine at about 1.4, and CPMHA plus lidocaine at about 1.45.
Physicians favored the hyaluronic acid with both pain medications, while patients rated the lidocaine formulation as causing the least bruising.
By day 7, bruising was lower in all groups. The outside evaluators again rated CPMHA alone as causing the least bruising on day 7, while patients and physicians perceived less bruising with the CPMHA-lidocaine formulation. Pain scores followed a similar pattern.
Although not a variable in the study, short-lived edema also was noted in most patients.
It’s possible that there was not enough time for the epinephrine to take full effect as a vasoconstrictor to decrease bruising, Dr. Shirazi said in an interview.
Larger studies are warranted to determine differences between the different formulations, the Dr. Shirazi and her colleagues concluded in the poster.
The study was supported by Merz North America, makers of the CPMHA formulation. Dr. Shirazi has served as an investigator for Allergan, Medicis, and Merz, and as an advisory board member for several pharmaceutical and device companies.
CHICAGO – Adding epinephrine to a specific formulation of hyaluronic acid already mixed with lidocaine failed to reduce the severity of adverse events following correction of perioral lines in a blinded study.
"Our results using split-face comparison did not reveal a large difference in bruising and pain scores," Dr. Azadeh Shirazi of Scripps Clinic in La Jolla, Calif., reported at the American Academy of Dermatology summer meeting.
Three groups of 10 women with mild to severe lip wrinkles were treated with 1.0 mL of cohesive polydensified matrix hyaluronic acid (CPMHA) alone; CPMHA plus 0.3 mL of lidocaine HCl 1%; or CPMHA plus lidocaine and epinephrine 1:100,000. The volumes in each syringe were adjusted to 1 mL in total.
All injections were performed in the dermis, using serial punctures and linear threading techniques. An entire syringe of one mixture was used on one side, followed by injection of a different mixture on the contralateral side.
Outside evaluators, physician investigators, and patients were blinded to treatment. Bruising was assessed on a 4-point, nonvalidated scale, with 0 being "no visible bruising" and 3 "severe" bruising.
On day 1 post procedure, outside evaluators gave the lowest bruising score to CPMHA alone at about 1.375, followed by CPMHA plus lidocaine and epinephrine at about 1.4, and CPMHA plus lidocaine at about 1.45.
Physicians favored the hyaluronic acid with both pain medications, while patients rated the lidocaine formulation as causing the least bruising.
By day 7, bruising was lower in all groups. The outside evaluators again rated CPMHA alone as causing the least bruising on day 7, while patients and physicians perceived less bruising with the CPMHA-lidocaine formulation. Pain scores followed a similar pattern.
Although not a variable in the study, short-lived edema also was noted in most patients.
It’s possible that there was not enough time for the epinephrine to take full effect as a vasoconstrictor to decrease bruising, Dr. Shirazi said in an interview.
Larger studies are warranted to determine differences between the different formulations, the Dr. Shirazi and her colleagues concluded in the poster.
The study was supported by Merz North America, makers of the CPMHA formulation. Dr. Shirazi has served as an investigator for Allergan, Medicis, and Merz, and as an advisory board member for several pharmaceutical and device companies.
AT THE AAD SUMMER ACADEMY 2014
Key clinical point: Adding epinephrine to hyaluronic acid with lidocaine did not affect the severity of bruising or pain scores.
Major finding: On a scale of 0-3, bruising scores on day 1 post procedure were 1.375 with CPMHA, 1.4 with CPMHA plus lidocaine and epinephrine, and 1.45 with CPMHA plus lidocaine.
Data source: Blinded, split-face study in 30 women treated for correction of perioral lines.
Disclosures: The study was supported by Merz North America, makers of the CPMHA formulation. Dr. Shirazi has served as an investigator for Allergan, Medicis, and Merz, and as an advisory board member for several pharmaceutical and device companies.
Ergonomic solutions limit Mohs surgeons’ aches and pains
VANCOUVER, B.C. – Attention to ergonomics may reduce risks for a wide range of musculoskeletal problems and headaches that afflict Mohs surgeons, Dr. Mariusz Sapijaszko said at the annual meeting of the Pacific Dermatologic Association.
The demands of performing Mohs surgery were first detailed in a study of 17 Mohs surgeons at the Mayo Clinic. Nearly two-thirds (59%) had chronic neck pain, half had shoulder pain (53%), and nearly half had lower back pain (41%). About a third experienced eye fatigue and one-fourth had headaches (Dermatol. Surg. 2007;33:1304-13).
Since then, a survey of American College of Mohs Surgery members found that 90% reported some type of musculoskeletal symptoms or injuries (Dermatol. Surg. 2012;38:240-8).
Dr. Sapijaszko of the Western Canada Dermatology Institute, Edmonton, Alberta, offered the following ergonomic tips based on his own clinical practice as well as recommendations offered by researchers who conducted the 2007 Mayo Clinic study and those focused on optimizing the operating theater environment (ANZ J. Surg. 2010;80:917-24).
To reduce neck-related symptoms:
• Keep your gaze angle between 15 and 30 degrees below horizontal.
• Position the patient close to you.
• Take short surgery breaks to stretch and adjust your posture.
• Use a stool with sternal support or a sit/stand stool.
To avoid lower back pain:
• Change positions frequently.
• Use a foot rest or foot rail.
• Use a stool with sternal support.
To prevent eye fatigue:
• Decrease the intensity of surgical lighting with a dimmer switch.
• Use goggles or glasses that contain antiglare film.
• Use brushed steel instead of polished steel instruments.
To minimize peripheral edema:
• Wear compression stockings.
• Use a foot rest or foot rail.
• Use gel insoles, or antifatigue floor mats.
To reduce headaches:
• Keep ambient noise below 56 dB.
• Select music based on the preference of the surgeon, patient, and other OR staff.
To optimize your comfort, optimize patient comfort:
• Select a procedure table that has adjustable positions for knee, hip, and neck angles as well as good lower back and lumbar support and a comfortable pillow type and position.
"You need to lie down on your own table and find out how good or bad it feels," Dr. Sapijaszko advised. He favors fully adjustable tables such as those used in the massage industry, and recommends a 12-degree tilt for the patient’s head.
On Twitter @dougbrunk
VANCOUVER, B.C. – Attention to ergonomics may reduce risks for a wide range of musculoskeletal problems and headaches that afflict Mohs surgeons, Dr. Mariusz Sapijaszko said at the annual meeting of the Pacific Dermatologic Association.
The demands of performing Mohs surgery were first detailed in a study of 17 Mohs surgeons at the Mayo Clinic. Nearly two-thirds (59%) had chronic neck pain, half had shoulder pain (53%), and nearly half had lower back pain (41%). About a third experienced eye fatigue and one-fourth had headaches (Dermatol. Surg. 2007;33:1304-13).
Since then, a survey of American College of Mohs Surgery members found that 90% reported some type of musculoskeletal symptoms or injuries (Dermatol. Surg. 2012;38:240-8).
Dr. Sapijaszko of the Western Canada Dermatology Institute, Edmonton, Alberta, offered the following ergonomic tips based on his own clinical practice as well as recommendations offered by researchers who conducted the 2007 Mayo Clinic study and those focused on optimizing the operating theater environment (ANZ J. Surg. 2010;80:917-24).
To reduce neck-related symptoms:
• Keep your gaze angle between 15 and 30 degrees below horizontal.
• Position the patient close to you.
• Take short surgery breaks to stretch and adjust your posture.
• Use a stool with sternal support or a sit/stand stool.
To avoid lower back pain:
• Change positions frequently.
• Use a foot rest or foot rail.
• Use a stool with sternal support.
To prevent eye fatigue:
• Decrease the intensity of surgical lighting with a dimmer switch.
• Use goggles or glasses that contain antiglare film.
• Use brushed steel instead of polished steel instruments.
To minimize peripheral edema:
• Wear compression stockings.
• Use a foot rest or foot rail.
• Use gel insoles, or antifatigue floor mats.
To reduce headaches:
• Keep ambient noise below 56 dB.
• Select music based on the preference of the surgeon, patient, and other OR staff.
To optimize your comfort, optimize patient comfort:
• Select a procedure table that has adjustable positions for knee, hip, and neck angles as well as good lower back and lumbar support and a comfortable pillow type and position.
"You need to lie down on your own table and find out how good or bad it feels," Dr. Sapijaszko advised. He favors fully adjustable tables such as those used in the massage industry, and recommends a 12-degree tilt for the patient’s head.
On Twitter @dougbrunk
VANCOUVER, B.C. – Attention to ergonomics may reduce risks for a wide range of musculoskeletal problems and headaches that afflict Mohs surgeons, Dr. Mariusz Sapijaszko said at the annual meeting of the Pacific Dermatologic Association.
The demands of performing Mohs surgery were first detailed in a study of 17 Mohs surgeons at the Mayo Clinic. Nearly two-thirds (59%) had chronic neck pain, half had shoulder pain (53%), and nearly half had lower back pain (41%). About a third experienced eye fatigue and one-fourth had headaches (Dermatol. Surg. 2007;33:1304-13).
Since then, a survey of American College of Mohs Surgery members found that 90% reported some type of musculoskeletal symptoms or injuries (Dermatol. Surg. 2012;38:240-8).
Dr. Sapijaszko of the Western Canada Dermatology Institute, Edmonton, Alberta, offered the following ergonomic tips based on his own clinical practice as well as recommendations offered by researchers who conducted the 2007 Mayo Clinic study and those focused on optimizing the operating theater environment (ANZ J. Surg. 2010;80:917-24).
To reduce neck-related symptoms:
• Keep your gaze angle between 15 and 30 degrees below horizontal.
• Position the patient close to you.
• Take short surgery breaks to stretch and adjust your posture.
• Use a stool with sternal support or a sit/stand stool.
To avoid lower back pain:
• Change positions frequently.
• Use a foot rest or foot rail.
• Use a stool with sternal support.
To prevent eye fatigue:
• Decrease the intensity of surgical lighting with a dimmer switch.
• Use goggles or glasses that contain antiglare film.
• Use brushed steel instead of polished steel instruments.
To minimize peripheral edema:
• Wear compression stockings.
• Use a foot rest or foot rail.
• Use gel insoles, or antifatigue floor mats.
To reduce headaches:
• Keep ambient noise below 56 dB.
• Select music based on the preference of the surgeon, patient, and other OR staff.
To optimize your comfort, optimize patient comfort:
• Select a procedure table that has adjustable positions for knee, hip, and neck angles as well as good lower back and lumbar support and a comfortable pillow type and position.
"You need to lie down on your own table and find out how good or bad it feels," Dr. Sapijaszko advised. He favors fully adjustable tables such as those used in the massage industry, and recommends a 12-degree tilt for the patient’s head.
On Twitter @dougbrunk
EXPERT ANALYSIS AT THE PDA ANNUAL MEETING
Facial Rejuvenation: Combining Cosmeceuticals With Cosmetic Procedures
Today’s cosmetic patient wants to look more youthful every day without spending a lot of money, feeling any pain, or having any postprocedure downtime. With continued technological improvements, dermatologists have been able to provide our patients with the more youthful appearance they desire; however, many of these procedures still are costly, painful, and may require some downtime. New cosmeceutical therapies can be used as adjuncts to these procedures, making antiaging regimens less painful for patients and requiring less postprocedure healing time. In this article, the use of cosmeceuticals in conjunction with chemical peels, lasers, and injectables will be discussed.
Chemical Peels
Chemical peels are used to create an injury of specific skin depth with a goal of stimulating new skin growth and improving surface texture and appearance. They generally are classified as superficial, medium, or deep according to the depth of action. Currently available agents for superficial chemical peels include α-hydroxy acids (AHAs)(eg, glycolic acid [GA]) and β-hydroxy acids (BHAs)(eg, salicylic acid). β-Lipohydroxy acid (up to 10%), a derivative of salicylic acid, is widely used in Europe. Trichloroacetic acid (TCA) can be used for superficial peels (10%–20%) and for medium-depth peels (35%). Combination peels such as Monheit combination (Jessner solution plus TCA), Brody combination (solid CO2 plus TCA), Coleman combination (GA 70% plus TCA), and Jessner solution with GA can be used as medium-depth peels. Deep peels typically are performed with phenol-based solutions, including the Baker-Gordon phenol peel and the Hetter peel (phenol or croton oil peel).
Specific agents for chemical peels should be selected based on the disorder being treated and should be administered using an appropriate peel depth determined by the histologic level or severity of skin pathology to maximize treatment success.1 However, other considerations, such as skin characteristics, area of skin to be treated, safety concerns, healing time, and patient adherence also should be taken into account to achieve the best overall results. Although many of the deeper peels recently have been replaced by laser-based ablative treatments, superficial to medium-depth peels still are commonly used in the treatment of fine lines, uneven texture, and dyspigmentation.2
Superficial peels are reasonably safe and well tolerated, usually with only mild discomfort (eg, transient burning, irritation, erythema). Scarring, postinflammatory hyperpigmentation (PIH), and infection are rare with superficial peels.1 Postinflammatory hyperpigmentation can be exacerbated by sun exposure, making it important for patients to be educated about sun protection and closely monitored during the recovery phase. In medium and deep peels, lines of demarcation related to the administration technique can occur. Feathering the chemical peel solution at junctions with nonpeeled skin can help to avoid this effect.1 Side effects associated with deeper chemical peels can include pigmentary changes, infections, allergic reactions, improper healing, hypersensitivity, and underlying disease exacerbation. The best way to prevent complications is to identify patients who are at risk and maintain an appropriate peel depth that balances efficacy with known adverse events.1
Many adjunctive agents (eg, AHAs, BHAs, retinoids, skin-bleaching preparations) can be used to enhance chemical peels and decrease the incidence of PIH. α-Hydroxy acids and BHAs can be beneficial when applied prior to chemical peels. Moisturizers containing AHAs and BHAs can be used for 2 to 3 weeks before superficial or medium-depth chemical peels.2 These agents cause thinning of the stratum corneum, thereby creating a more uniform cutaneous surface and allowing for deeper penetration of the chemical peeling agent. Retinoids also are superior prepeeling agents; however, retinoids also can increase the likelihood of irritation, which can be minimized by discontinuing retinoids for 1 week following chemical peels.2 A combination of chemical peels and topical bleaching agents has been shown to be effective in treating hyperpigmentation. The chemical peel causes superficial exfoliation, which allows the lightening agent to penetrate more deeply.2
Hydroquinone (HQ) is the gold standard for improvement of existing pigmentation.3 It is one of the most effective inhibitors of melanogenesis both in vitro and in vivo and is widely used for the treatment of melanosis and other hyperpigmentary disorders. It is widely accepted that the depigmentation activity of HQ may partly be related to its ability to act as an alternate substrate of tyrosinase, thereby competing for tyrosine oxidation in active melanocytes.3 Using HQ at a 4% concentration and combining it with retinoids is quite efficacious.2 Other commonly used depigmenting agents include kojic acid, ascorbic acid (vitamin C), and niacinamide, which often can be used as adjuncts with or maintenance therapy after HQ treatment.2,3
The risk for PIH is imminent for chemical peels and cosmetic laser treatments; therefore, it is crucial to educate patients about the importance of daily and aggressive sun protection. There are several methods of reducing or eliminating postprocedure melanin formation, such as inhibiting tyrosinase synthesis, using complex copper to inhibit tyrosinase function, eliminating oxidation reactions that lead to polymer formation, slowing down the transfer of melanosomes to keratinocytes, or acting upstream on the hormone that stimulates melanogenesis.3 Most of the depigmenting agents presently on the market act by inhibiting tyrosinase via one of these mechanisms.
Skin-lightening agents are primarily formulated as emulsions that have a higher aesthetic appeal. Many of the ingredients get better dispersions with emulsions, which is an added feature of these products. Recently, gel-based formulations also are being considered for their suitability in certain skin types. Efficacy studies for skin-lightening formulations are being carried out through clinical trials that utilize devices that measure skin color in addition to the dermatologist’s assessment.4 Other skin parameters (eg, moisturization, texture, barrier integrity, pH) also are being evaluated to give physicians a picture of skin health after the use of skin-lightening agents. With advances in technology and measurement techniques, it is becoming easier to identify the efficacy of these formulations in different skin types.4
Lasers
The ultimate goal of laser therapy often is to improve the canvas and color of the skin. Ablative laser resurfacing is reliably the most effective procedure for sun-damaged skin.2 This technique causes thermally induced full-thickness epidermal and dermal denudation, which in turn facilitates cytokine-led dermal collagen formation and reepithelialization. Various nonablative modalities also are used for treating photodamaged skin. The epidermis remains unaffected by these nonablative methods, thus decreasing the need for extensive wound care and downtime that is required with ablative treatments. Combining nonablative laser treatments with topical cosmeceuticals has been proven more effective than using either method alone.2 The use of topical retinoids prior to ablative laser resurfacing often results in remarkably faster postprocedure healing and reepithelialization (Figure). Retinoids are best applied nightly for at least 2 weeks and optimally for 3 months before ablative laser treatment. Application should be discontinued for 1 week immediately prior to the procedure.
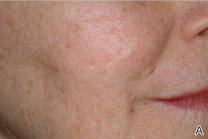 |
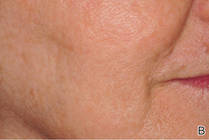 |
| Before (A) and after (B) treatment with a fractional laser in combination with a pre- and postprocedure skin care regimen consisting of retinoids and sunscreen. |
Topical retinoids also are effective in reducing erythema and increasing dermal thickness after nonablative treatments. When used prior to laser treatments, retinoids have been shown to decrease the risk for postoperative milia and hyperpigmentation as well as to allow for better penetration of the laser beam secondary to a thinner stratum corneum.2 Following ablative resurfacing, retinoid use should be discontinued for several weeks to allow for reepithelialization and adequate healing.
Postprocedure Wound Healing
Most of the recommended products that help decrease postprocedural inflammation are cosmeceuticals containing both antioxidants and anti-inflammatories to help decrease redness and inflammation, including various barrier repair moisturizers. Restoring barrier integrity improves the overall appearance of the skin. The ingredients normally recommended in barrier repair moisturizers are epidermal lipids such as ceramides; hyaluronic acid (HA), which is a humectant; and occlusives for patients with very dry skin. Some of the ingredients in over-the-counter cosmeceuticals that can help decrease redness and inflammation include vitamin C, vitamin E, and vitamin B or niacinamide, which will help plump the barrier and also have anti-inflammatory properties. Additionally, polyphenolic flavonoids such as soy and green tea can help decrease inflammation, along with a number of other organic ingredients, such as caffeine, feverfew, and licorice.5 If topical vitamin C is being considered for postprocedure use, the non–ascorbic acid variant should be administered. The magnesium ascorbyl phosphate and ascorbyl palmitate forms of vitamin C have a neutral pH and tend to be better tolerated by patients.
In addition to current prescription and over-the-counter cosmeceuticals used for postprocedure irritation and inflammation, copper peptides and other well-tolerated and effective naturally occurring compounds are being investigated and tried. Copper is a biocide that regulates keratinocyte integrins for epithelization and extracellular matrix remodeling. The extracellular matrix consists of the structural fibrillar collagens and is remodeled or degraded by matrix metalloproteinases (MMPs) that facilitate epithelization. The predominant classes of MMPs include collagenases (ie, MMP-1) and gelatinases (ie, MMP-2, MMP-9) that degrade interstitial collagen and basement membrane proteins.6 The MMPs are endogenously inhibited by tissue inhibitors of metalloproteinases (TIMPs). Copper is a cofactor to lysyl oxidase, which cross-links collagen and stimulates expression of MMP-2 and collagen in a complex with a matrix-derived tripeptide (glycyl-histidyl-lysine or Gly-His-Lys [GHK]) in fibroblasts.6 Much attention has been focused on the tripeptides, such as GHK and Gly-Gly-His, and their copper complexes, which have high activity and good skin tolerance. These complexes have been shown to play a physiological role in the process of wound healing, tissue repair, and skin inflammation. Gly-Gly-His, GHK, copper chloride, and their copper complexes decrease tumor necrosis factor α–dependent IL-6 secretion in fibroblasts.7 IL-6 is crucial for normal wound healing, skin inflammation, and UVB-induced erythema. Because of their anti-inflammatory properties, these copper peptides could potentially be used in place of corticosteroids or nonsteroidal anti-inflammatory drugs, which have more side effects.
Botulinum Neurotoxin and Other Injectable Fillers
Acetyl Hexapeptide-3: A Topical Complement to Botulinum Neurotoxin
Acetyl hexapeptide-3 (Ac-Glu-Glu-Met-Gln-Arg-Arg-NH2) was discovered when looking for a less toxic variation of botulinum neurotoxin (BoNT) to treat aging skin.8 It is patterned from the N-terminal end of the synaptosome-associated protein of molecular weight 25 kDa (SNAP-25), which is essential for docking and fusion of synaptic vesicles to the presynaptic membrane for acetylcholine release.9 It prevents formation and stability of the soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) complex, inhibiting vesicle docking and calcium-dependent catecholamine exocytosis.8 It also has been found to substantially inhibit the repetitive muscular contraction of facial expression similar to BoNT type A but with somewhat lower efficacy. Acetyl hexapeptide-3 was shown to inhibit 30% of total catecholamine exocytosis and had a remarkable capacity to permeate the skin.10 Thus this topical form of BoNT is a useful complement to intramuscular BoNT.
Studies showing the efficacy and safety of acetyl hexapeptide-3 have demonstrated reductions in wrinkle intensity, mainly in the lateral periorbital areas. In one early study, 10 women applied an emulsion containing 10% of the hexapeptide to one lateral periorbital region and the same emulsion without the hexapeptide to the contralateral side, both twice daily for 30 days.10 A 30% decrease in the depth of skin wrinkles was seen on the hexapeptide side compared with a 10% decrease in the depth of wrinkles on the side treated without hexapeptide. No irritation or toxicity was noted.10 In another trial, 10 women applied an acetyl hexapeptide-3 cream 5% twice daily to lateral periorbital rhytides, with a 27% improvement in wrinkle depth after a 30-day treatment period.9 A double-blind, placebo-controlled study of 60 women assessing the safety and efficacy of topical hexapeptide showed a total antiwrinkle efficacy of 48.9% on the side treated with an emulsion containing 10% of the hexapeptide compared with 0% efficacy on the placebo side.8 Similar to Blanes-Mira et al,10 no adverse events such as skin irritation or toxicity were seen.8 In all of these studies, wrinkle depth was measured by silicone replica analysis.
Topical acetyl hexapeptide-3 is effective in decreasing wrinkles, and its best use will likely be as an adjunct to intramuscular BoNT, as the intramuscular form likely has higher efficacy with the toxin injected directly into the target muscle; however, patients who want the effects of BoNT without the pain of injections may choose to use topical acetyl hexapeptide-3 alone. Patients who do use acetyl hexapeptide-3 as a complement to their intramuscular BoNT regimen may not need as many units of BoNT with each treatment or may not need certain areas injected as often, leading to fewer injections and less pain with each visit. Skin irritation was not seen as a side effect in these trials. Additionally, the topical form has insignificant acute toxicity (≥2000 mg/kg) compared to BoNT type A (20 ng/kg), and genotoxicity was not seen with testing, making it a safe complementary option to an injectable regimen.8
Topical Hyaluronic Acid: A Complement to Injectable Fillers
Hyaluronic acid (HA) is a glycosaminoglycan found in the extracellular matrix of the skin that greatly contributes to tissue hydration. Additionally, it plays a crucial role in the synthesis of extracellular matrix molecules and epidermal cell interaction with the environment.11 The water-binding capacity of HA approximates 1000 times its volume or 6 L of water per gram of HA; however, once an individual reaches adulthood, the amount of HA decreases to 5% of baseline levels, thus contributing to xerosis, loss of skin elasticity, and atrophy.11,12 Although photoaged skin can have increased glycosaminoglycans due to an increase in chondroitin sulfate proteoglycans, they are abnormally deposited on elastotic material in the superficial dermis rather than diffusely scattered, as seen in youthful skin.12
Many topical antiaging products contain HA, though evidence for efficacy in reducing wrinkles has been lacking, along with concerns that HA cannot penetrate the skin. This concern stems from the fact that the original molecule is 3000 nm in diameter and the intercellular space is only 15 to 50 nm. This space is only 6 to 10 nm at the hyaline membrane. Recently, scientists in Japan found a way to reduce the size of HA molecules to 5 nm (nano-HA) without changing its structure. A study of 33 women who applied the topical nano-HA twice daily for 8 weeks to one periorbital area while the contralateral side was left untreated showed improved hydration of the treated side that continued to increase when measured at 2, 4, and 8 weeks using corneometry.11 Roughness decreased and elasticity increased after week 2, which were maintained throughout the study. Additionally, erythema was measured using a chroma meter, which was found to have decreased at day 57 versus day 1.11 An earlier study by Pavicic et al12 evaluated the efficacy of topical hyalu-ronan 0.1% formulations of different molecular weights—50, 130, 300, 800, or 2000 kDa—in the periocular area. A randomized group of 76 women were treated twice daily for 2 months with HA cream on one side of the periocular area and placebo cream on the other. With regard to antiwrinkle properties, only the 50- and 130-kDa HA formulations showed marked effects compared with placebo after 2 months.12
Topical HA would be an effective addition to an antiwrinkle regimen, especially in patients who are averse to needles or are just starting to get wrinkles and are looking for a noninvasive therapy. Additionally, it would be beneficial for patients who have an injectable filler and BoNT regimen, as these patients will be able to target wrinkles simultaneously with both topical cosmeceuticals and injectables and likely will need fewer units of BoNT and/or filler and possibly fewer injections over time, which translates to decreased pain and adverse outcomes for patients.
Conclusion
The myriad of options dermatologists have to offer patients for cosmetic enhancement provides alternatives for patients who have contraindications to certain treatments, are needle averse, or have lifestyles that do not afford them a great deal of postprocedural healing time. Being knowledgeable about these options and how to combine them for improved outcomes is essential to any cosmetic practice.
1. Rendon MI, Berson DS, Cohen JL, et al. Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. J Clin Aesthet Dermatol. 2010;3:32-43.
2. Lupo MP, Jacob LG. Cosmeceuticals for enhancing cosmetic procedures. In: Farris PK, ed. Cosmeceuticals and Cosmetic Practice. Oxford, United Kingdom: Wiley-Blackwell; 2014:268-276.
3. Gruber JV, Holtz R. Examining the impact of skin lighteners in vitro [published online ahead of print April 28, 2013]. Oxid Med Cell Longev. 2013;2013:702120.
4. Antonio JR, Antonio CR, Cardeal ILS, et al. Nanotechnology in dermatology. An Bras Dermatol. 2014;89:126-136.
5. Ganceviciene R, Liakou AI, Theodoridis A, et al. Skin anti-aging strategies. Dermatoendocrinol. 2012;4:308-319.
6. Gruchlik A, Jurzak M, Chodurek, E, et al. Effect of GLY-GLY-HIS, GLY-HIS-LYS and their copper complexes on TNF-α-dependant IL-6 secretion in normal human dermal fibroblasts. Acta Pol Pharm. 2012;69:1303-1306.
7. Philips N, Hwang H, Chauhan S, et al. Stimulation of cell proliferation and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect Tissue Res. 2010;51:224-229.
8. Wang Y, Wang M, Xiao S, et al. The anti-wrinkle efficacy of Argireline, a synthetic hexapeptide, in Chinese subjects. Am J Clin Dermatol. 2013;14:147-153.
9. Lupo MP, Cole A. Cosmeceutical peptides. Dermatol Ther. 2007;20:343-349.
10. Blanes-Mira C, Clemente J, Jodas G, et al. A synthetic hexapeptide (Argireline) with antiwrinkle activity. Int J Cosmet Sci. 2002;24:303-310.
11. Jegasothy SM, Zabolotniaia V, Bielfeldt S. Efficacy of a new topical nano-hyaluronic acid in humans. J Clin Aesthet Dermatol. 2014;7:27-29.
12. Pavicic T, Gauglitz G, Lersch P, et al. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. J Drugs Dermatol. 2011;10:990-1000.
Today’s cosmetic patient wants to look more youthful every day without spending a lot of money, feeling any pain, or having any postprocedure downtime. With continued technological improvements, dermatologists have been able to provide our patients with the more youthful appearance they desire; however, many of these procedures still are costly, painful, and may require some downtime. New cosmeceutical therapies can be used as adjuncts to these procedures, making antiaging regimens less painful for patients and requiring less postprocedure healing time. In this article, the use of cosmeceuticals in conjunction with chemical peels, lasers, and injectables will be discussed.
Chemical Peels
Chemical peels are used to create an injury of specific skin depth with a goal of stimulating new skin growth and improving surface texture and appearance. They generally are classified as superficial, medium, or deep according to the depth of action. Currently available agents for superficial chemical peels include α-hydroxy acids (AHAs)(eg, glycolic acid [GA]) and β-hydroxy acids (BHAs)(eg, salicylic acid). β-Lipohydroxy acid (up to 10%), a derivative of salicylic acid, is widely used in Europe. Trichloroacetic acid (TCA) can be used for superficial peels (10%–20%) and for medium-depth peels (35%). Combination peels such as Monheit combination (Jessner solution plus TCA), Brody combination (solid CO2 plus TCA), Coleman combination (GA 70% plus TCA), and Jessner solution with GA can be used as medium-depth peels. Deep peels typically are performed with phenol-based solutions, including the Baker-Gordon phenol peel and the Hetter peel (phenol or croton oil peel).
Specific agents for chemical peels should be selected based on the disorder being treated and should be administered using an appropriate peel depth determined by the histologic level or severity of skin pathology to maximize treatment success.1 However, other considerations, such as skin characteristics, area of skin to be treated, safety concerns, healing time, and patient adherence also should be taken into account to achieve the best overall results. Although many of the deeper peels recently have been replaced by laser-based ablative treatments, superficial to medium-depth peels still are commonly used in the treatment of fine lines, uneven texture, and dyspigmentation.2
Superficial peels are reasonably safe and well tolerated, usually with only mild discomfort (eg, transient burning, irritation, erythema). Scarring, postinflammatory hyperpigmentation (PIH), and infection are rare with superficial peels.1 Postinflammatory hyperpigmentation can be exacerbated by sun exposure, making it important for patients to be educated about sun protection and closely monitored during the recovery phase. In medium and deep peels, lines of demarcation related to the administration technique can occur. Feathering the chemical peel solution at junctions with nonpeeled skin can help to avoid this effect.1 Side effects associated with deeper chemical peels can include pigmentary changes, infections, allergic reactions, improper healing, hypersensitivity, and underlying disease exacerbation. The best way to prevent complications is to identify patients who are at risk and maintain an appropriate peel depth that balances efficacy with known adverse events.1
Many adjunctive agents (eg, AHAs, BHAs, retinoids, skin-bleaching preparations) can be used to enhance chemical peels and decrease the incidence of PIH. α-Hydroxy acids and BHAs can be beneficial when applied prior to chemical peels. Moisturizers containing AHAs and BHAs can be used for 2 to 3 weeks before superficial or medium-depth chemical peels.2 These agents cause thinning of the stratum corneum, thereby creating a more uniform cutaneous surface and allowing for deeper penetration of the chemical peeling agent. Retinoids also are superior prepeeling agents; however, retinoids also can increase the likelihood of irritation, which can be minimized by discontinuing retinoids for 1 week following chemical peels.2 A combination of chemical peels and topical bleaching agents has been shown to be effective in treating hyperpigmentation. The chemical peel causes superficial exfoliation, which allows the lightening agent to penetrate more deeply.2
Hydroquinone (HQ) is the gold standard for improvement of existing pigmentation.3 It is one of the most effective inhibitors of melanogenesis both in vitro and in vivo and is widely used for the treatment of melanosis and other hyperpigmentary disorders. It is widely accepted that the depigmentation activity of HQ may partly be related to its ability to act as an alternate substrate of tyrosinase, thereby competing for tyrosine oxidation in active melanocytes.3 Using HQ at a 4% concentration and combining it with retinoids is quite efficacious.2 Other commonly used depigmenting agents include kojic acid, ascorbic acid (vitamin C), and niacinamide, which often can be used as adjuncts with or maintenance therapy after HQ treatment.2,3
The risk for PIH is imminent for chemical peels and cosmetic laser treatments; therefore, it is crucial to educate patients about the importance of daily and aggressive sun protection. There are several methods of reducing or eliminating postprocedure melanin formation, such as inhibiting tyrosinase synthesis, using complex copper to inhibit tyrosinase function, eliminating oxidation reactions that lead to polymer formation, slowing down the transfer of melanosomes to keratinocytes, or acting upstream on the hormone that stimulates melanogenesis.3 Most of the depigmenting agents presently on the market act by inhibiting tyrosinase via one of these mechanisms.
Skin-lightening agents are primarily formulated as emulsions that have a higher aesthetic appeal. Many of the ingredients get better dispersions with emulsions, which is an added feature of these products. Recently, gel-based formulations also are being considered for their suitability in certain skin types. Efficacy studies for skin-lightening formulations are being carried out through clinical trials that utilize devices that measure skin color in addition to the dermatologist’s assessment.4 Other skin parameters (eg, moisturization, texture, barrier integrity, pH) also are being evaluated to give physicians a picture of skin health after the use of skin-lightening agents. With advances in technology and measurement techniques, it is becoming easier to identify the efficacy of these formulations in different skin types.4
Lasers
The ultimate goal of laser therapy often is to improve the canvas and color of the skin. Ablative laser resurfacing is reliably the most effective procedure for sun-damaged skin.2 This technique causes thermally induced full-thickness epidermal and dermal denudation, which in turn facilitates cytokine-led dermal collagen formation and reepithelialization. Various nonablative modalities also are used for treating photodamaged skin. The epidermis remains unaffected by these nonablative methods, thus decreasing the need for extensive wound care and downtime that is required with ablative treatments. Combining nonablative laser treatments with topical cosmeceuticals has been proven more effective than using either method alone.2 The use of topical retinoids prior to ablative laser resurfacing often results in remarkably faster postprocedure healing and reepithelialization (Figure). Retinoids are best applied nightly for at least 2 weeks and optimally for 3 months before ablative laser treatment. Application should be discontinued for 1 week immediately prior to the procedure.
 |
 |
| Before (A) and after (B) treatment with a fractional laser in combination with a pre- and postprocedure skin care regimen consisting of retinoids and sunscreen. |
Topical retinoids also are effective in reducing erythema and increasing dermal thickness after nonablative treatments. When used prior to laser treatments, retinoids have been shown to decrease the risk for postoperative milia and hyperpigmentation as well as to allow for better penetration of the laser beam secondary to a thinner stratum corneum.2 Following ablative resurfacing, retinoid use should be discontinued for several weeks to allow for reepithelialization and adequate healing.
Postprocedure Wound Healing
Most of the recommended products that help decrease postprocedural inflammation are cosmeceuticals containing both antioxidants and anti-inflammatories to help decrease redness and inflammation, including various barrier repair moisturizers. Restoring barrier integrity improves the overall appearance of the skin. The ingredients normally recommended in barrier repair moisturizers are epidermal lipids such as ceramides; hyaluronic acid (HA), which is a humectant; and occlusives for patients with very dry skin. Some of the ingredients in over-the-counter cosmeceuticals that can help decrease redness and inflammation include vitamin C, vitamin E, and vitamin B or niacinamide, which will help plump the barrier and also have anti-inflammatory properties. Additionally, polyphenolic flavonoids such as soy and green tea can help decrease inflammation, along with a number of other organic ingredients, such as caffeine, feverfew, and licorice.5 If topical vitamin C is being considered for postprocedure use, the non–ascorbic acid variant should be administered. The magnesium ascorbyl phosphate and ascorbyl palmitate forms of vitamin C have a neutral pH and tend to be better tolerated by patients.
In addition to current prescription and over-the-counter cosmeceuticals used for postprocedure irritation and inflammation, copper peptides and other well-tolerated and effective naturally occurring compounds are being investigated and tried. Copper is a biocide that regulates keratinocyte integrins for epithelization and extracellular matrix remodeling. The extracellular matrix consists of the structural fibrillar collagens and is remodeled or degraded by matrix metalloproteinases (MMPs) that facilitate epithelization. The predominant classes of MMPs include collagenases (ie, MMP-1) and gelatinases (ie, MMP-2, MMP-9) that degrade interstitial collagen and basement membrane proteins.6 The MMPs are endogenously inhibited by tissue inhibitors of metalloproteinases (TIMPs). Copper is a cofactor to lysyl oxidase, which cross-links collagen and stimulates expression of MMP-2 and collagen in a complex with a matrix-derived tripeptide (glycyl-histidyl-lysine or Gly-His-Lys [GHK]) in fibroblasts.6 Much attention has been focused on the tripeptides, such as GHK and Gly-Gly-His, and their copper complexes, which have high activity and good skin tolerance. These complexes have been shown to play a physiological role in the process of wound healing, tissue repair, and skin inflammation. Gly-Gly-His, GHK, copper chloride, and their copper complexes decrease tumor necrosis factor α–dependent IL-6 secretion in fibroblasts.7 IL-6 is crucial for normal wound healing, skin inflammation, and UVB-induced erythema. Because of their anti-inflammatory properties, these copper peptides could potentially be used in place of corticosteroids or nonsteroidal anti-inflammatory drugs, which have more side effects.
Botulinum Neurotoxin and Other Injectable Fillers
Acetyl Hexapeptide-3: A Topical Complement to Botulinum Neurotoxin
Acetyl hexapeptide-3 (Ac-Glu-Glu-Met-Gln-Arg-Arg-NH2) was discovered when looking for a less toxic variation of botulinum neurotoxin (BoNT) to treat aging skin.8 It is patterned from the N-terminal end of the synaptosome-associated protein of molecular weight 25 kDa (SNAP-25), which is essential for docking and fusion of synaptic vesicles to the presynaptic membrane for acetylcholine release.9 It prevents formation and stability of the soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) complex, inhibiting vesicle docking and calcium-dependent catecholamine exocytosis.8 It also has been found to substantially inhibit the repetitive muscular contraction of facial expression similar to BoNT type A but with somewhat lower efficacy. Acetyl hexapeptide-3 was shown to inhibit 30% of total catecholamine exocytosis and had a remarkable capacity to permeate the skin.10 Thus this topical form of BoNT is a useful complement to intramuscular BoNT.
Studies showing the efficacy and safety of acetyl hexapeptide-3 have demonstrated reductions in wrinkle intensity, mainly in the lateral periorbital areas. In one early study, 10 women applied an emulsion containing 10% of the hexapeptide to one lateral periorbital region and the same emulsion without the hexapeptide to the contralateral side, both twice daily for 30 days.10 A 30% decrease in the depth of skin wrinkles was seen on the hexapeptide side compared with a 10% decrease in the depth of wrinkles on the side treated without hexapeptide. No irritation or toxicity was noted.10 In another trial, 10 women applied an acetyl hexapeptide-3 cream 5% twice daily to lateral periorbital rhytides, with a 27% improvement in wrinkle depth after a 30-day treatment period.9 A double-blind, placebo-controlled study of 60 women assessing the safety and efficacy of topical hexapeptide showed a total antiwrinkle efficacy of 48.9% on the side treated with an emulsion containing 10% of the hexapeptide compared with 0% efficacy on the placebo side.8 Similar to Blanes-Mira et al,10 no adverse events such as skin irritation or toxicity were seen.8 In all of these studies, wrinkle depth was measured by silicone replica analysis.
Topical acetyl hexapeptide-3 is effective in decreasing wrinkles, and its best use will likely be as an adjunct to intramuscular BoNT, as the intramuscular form likely has higher efficacy with the toxin injected directly into the target muscle; however, patients who want the effects of BoNT without the pain of injections may choose to use topical acetyl hexapeptide-3 alone. Patients who do use acetyl hexapeptide-3 as a complement to their intramuscular BoNT regimen may not need as many units of BoNT with each treatment or may not need certain areas injected as often, leading to fewer injections and less pain with each visit. Skin irritation was not seen as a side effect in these trials. Additionally, the topical form has insignificant acute toxicity (≥2000 mg/kg) compared to BoNT type A (20 ng/kg), and genotoxicity was not seen with testing, making it a safe complementary option to an injectable regimen.8
Topical Hyaluronic Acid: A Complement to Injectable Fillers
Hyaluronic acid (HA) is a glycosaminoglycan found in the extracellular matrix of the skin that greatly contributes to tissue hydration. Additionally, it plays a crucial role in the synthesis of extracellular matrix molecules and epidermal cell interaction with the environment.11 The water-binding capacity of HA approximates 1000 times its volume or 6 L of water per gram of HA; however, once an individual reaches adulthood, the amount of HA decreases to 5% of baseline levels, thus contributing to xerosis, loss of skin elasticity, and atrophy.11,12 Although photoaged skin can have increased glycosaminoglycans due to an increase in chondroitin sulfate proteoglycans, they are abnormally deposited on elastotic material in the superficial dermis rather than diffusely scattered, as seen in youthful skin.12
Many topical antiaging products contain HA, though evidence for efficacy in reducing wrinkles has been lacking, along with concerns that HA cannot penetrate the skin. This concern stems from the fact that the original molecule is 3000 nm in diameter and the intercellular space is only 15 to 50 nm. This space is only 6 to 10 nm at the hyaline membrane. Recently, scientists in Japan found a way to reduce the size of HA molecules to 5 nm (nano-HA) without changing its structure. A study of 33 women who applied the topical nano-HA twice daily for 8 weeks to one periorbital area while the contralateral side was left untreated showed improved hydration of the treated side that continued to increase when measured at 2, 4, and 8 weeks using corneometry.11 Roughness decreased and elasticity increased after week 2, which were maintained throughout the study. Additionally, erythema was measured using a chroma meter, which was found to have decreased at day 57 versus day 1.11 An earlier study by Pavicic et al12 evaluated the efficacy of topical hyalu-ronan 0.1% formulations of different molecular weights—50, 130, 300, 800, or 2000 kDa—in the periocular area. A randomized group of 76 women were treated twice daily for 2 months with HA cream on one side of the periocular area and placebo cream on the other. With regard to antiwrinkle properties, only the 50- and 130-kDa HA formulations showed marked effects compared with placebo after 2 months.12
Topical HA would be an effective addition to an antiwrinkle regimen, especially in patients who are averse to needles or are just starting to get wrinkles and are looking for a noninvasive therapy. Additionally, it would be beneficial for patients who have an injectable filler and BoNT regimen, as these patients will be able to target wrinkles simultaneously with both topical cosmeceuticals and injectables and likely will need fewer units of BoNT and/or filler and possibly fewer injections over time, which translates to decreased pain and adverse outcomes for patients.
Conclusion
The myriad of options dermatologists have to offer patients for cosmetic enhancement provides alternatives for patients who have contraindications to certain treatments, are needle averse, or have lifestyles that do not afford them a great deal of postprocedural healing time. Being knowledgeable about these options and how to combine them for improved outcomes is essential to any cosmetic practice.
Today’s cosmetic patient wants to look more youthful every day without spending a lot of money, feeling any pain, or having any postprocedure downtime. With continued technological improvements, dermatologists have been able to provide our patients with the more youthful appearance they desire; however, many of these procedures still are costly, painful, and may require some downtime. New cosmeceutical therapies can be used as adjuncts to these procedures, making antiaging regimens less painful for patients and requiring less postprocedure healing time. In this article, the use of cosmeceuticals in conjunction with chemical peels, lasers, and injectables will be discussed.
Chemical Peels
Chemical peels are used to create an injury of specific skin depth with a goal of stimulating new skin growth and improving surface texture and appearance. They generally are classified as superficial, medium, or deep according to the depth of action. Currently available agents for superficial chemical peels include α-hydroxy acids (AHAs)(eg, glycolic acid [GA]) and β-hydroxy acids (BHAs)(eg, salicylic acid). β-Lipohydroxy acid (up to 10%), a derivative of salicylic acid, is widely used in Europe. Trichloroacetic acid (TCA) can be used for superficial peels (10%–20%) and for medium-depth peels (35%). Combination peels such as Monheit combination (Jessner solution plus TCA), Brody combination (solid CO2 plus TCA), Coleman combination (GA 70% plus TCA), and Jessner solution with GA can be used as medium-depth peels. Deep peels typically are performed with phenol-based solutions, including the Baker-Gordon phenol peel and the Hetter peel (phenol or croton oil peel).
Specific agents for chemical peels should be selected based on the disorder being treated and should be administered using an appropriate peel depth determined by the histologic level or severity of skin pathology to maximize treatment success.1 However, other considerations, such as skin characteristics, area of skin to be treated, safety concerns, healing time, and patient adherence also should be taken into account to achieve the best overall results. Although many of the deeper peels recently have been replaced by laser-based ablative treatments, superficial to medium-depth peels still are commonly used in the treatment of fine lines, uneven texture, and dyspigmentation.2
Superficial peels are reasonably safe and well tolerated, usually with only mild discomfort (eg, transient burning, irritation, erythema). Scarring, postinflammatory hyperpigmentation (PIH), and infection are rare with superficial peels.1 Postinflammatory hyperpigmentation can be exacerbated by sun exposure, making it important for patients to be educated about sun protection and closely monitored during the recovery phase. In medium and deep peels, lines of demarcation related to the administration technique can occur. Feathering the chemical peel solution at junctions with nonpeeled skin can help to avoid this effect.1 Side effects associated with deeper chemical peels can include pigmentary changes, infections, allergic reactions, improper healing, hypersensitivity, and underlying disease exacerbation. The best way to prevent complications is to identify patients who are at risk and maintain an appropriate peel depth that balances efficacy with known adverse events.1
Many adjunctive agents (eg, AHAs, BHAs, retinoids, skin-bleaching preparations) can be used to enhance chemical peels and decrease the incidence of PIH. α-Hydroxy acids and BHAs can be beneficial when applied prior to chemical peels. Moisturizers containing AHAs and BHAs can be used for 2 to 3 weeks before superficial or medium-depth chemical peels.2 These agents cause thinning of the stratum corneum, thereby creating a more uniform cutaneous surface and allowing for deeper penetration of the chemical peeling agent. Retinoids also are superior prepeeling agents; however, retinoids also can increase the likelihood of irritation, which can be minimized by discontinuing retinoids for 1 week following chemical peels.2 A combination of chemical peels and topical bleaching agents has been shown to be effective in treating hyperpigmentation. The chemical peel causes superficial exfoliation, which allows the lightening agent to penetrate more deeply.2
Hydroquinone (HQ) is the gold standard for improvement of existing pigmentation.3 It is one of the most effective inhibitors of melanogenesis both in vitro and in vivo and is widely used for the treatment of melanosis and other hyperpigmentary disorders. It is widely accepted that the depigmentation activity of HQ may partly be related to its ability to act as an alternate substrate of tyrosinase, thereby competing for tyrosine oxidation in active melanocytes.3 Using HQ at a 4% concentration and combining it with retinoids is quite efficacious.2 Other commonly used depigmenting agents include kojic acid, ascorbic acid (vitamin C), and niacinamide, which often can be used as adjuncts with or maintenance therapy after HQ treatment.2,3
The risk for PIH is imminent for chemical peels and cosmetic laser treatments; therefore, it is crucial to educate patients about the importance of daily and aggressive sun protection. There are several methods of reducing or eliminating postprocedure melanin formation, such as inhibiting tyrosinase synthesis, using complex copper to inhibit tyrosinase function, eliminating oxidation reactions that lead to polymer formation, slowing down the transfer of melanosomes to keratinocytes, or acting upstream on the hormone that stimulates melanogenesis.3 Most of the depigmenting agents presently on the market act by inhibiting tyrosinase via one of these mechanisms.
Skin-lightening agents are primarily formulated as emulsions that have a higher aesthetic appeal. Many of the ingredients get better dispersions with emulsions, which is an added feature of these products. Recently, gel-based formulations also are being considered for their suitability in certain skin types. Efficacy studies for skin-lightening formulations are being carried out through clinical trials that utilize devices that measure skin color in addition to the dermatologist’s assessment.4 Other skin parameters (eg, moisturization, texture, barrier integrity, pH) also are being evaluated to give physicians a picture of skin health after the use of skin-lightening agents. With advances in technology and measurement techniques, it is becoming easier to identify the efficacy of these formulations in different skin types.4
Lasers
The ultimate goal of laser therapy often is to improve the canvas and color of the skin. Ablative laser resurfacing is reliably the most effective procedure for sun-damaged skin.2 This technique causes thermally induced full-thickness epidermal and dermal denudation, which in turn facilitates cytokine-led dermal collagen formation and reepithelialization. Various nonablative modalities also are used for treating photodamaged skin. The epidermis remains unaffected by these nonablative methods, thus decreasing the need for extensive wound care and downtime that is required with ablative treatments. Combining nonablative laser treatments with topical cosmeceuticals has been proven more effective than using either method alone.2 The use of topical retinoids prior to ablative laser resurfacing often results in remarkably faster postprocedure healing and reepithelialization (Figure). Retinoids are best applied nightly for at least 2 weeks and optimally for 3 months before ablative laser treatment. Application should be discontinued for 1 week immediately prior to the procedure.
 |
 |
| Before (A) and after (B) treatment with a fractional laser in combination with a pre- and postprocedure skin care regimen consisting of retinoids and sunscreen. |
Topical retinoids also are effective in reducing erythema and increasing dermal thickness after nonablative treatments. When used prior to laser treatments, retinoids have been shown to decrease the risk for postoperative milia and hyperpigmentation as well as to allow for better penetration of the laser beam secondary to a thinner stratum corneum.2 Following ablative resurfacing, retinoid use should be discontinued for several weeks to allow for reepithelialization and adequate healing.
Postprocedure Wound Healing
Most of the recommended products that help decrease postprocedural inflammation are cosmeceuticals containing both antioxidants and anti-inflammatories to help decrease redness and inflammation, including various barrier repair moisturizers. Restoring barrier integrity improves the overall appearance of the skin. The ingredients normally recommended in barrier repair moisturizers are epidermal lipids such as ceramides; hyaluronic acid (HA), which is a humectant; and occlusives for patients with very dry skin. Some of the ingredients in over-the-counter cosmeceuticals that can help decrease redness and inflammation include vitamin C, vitamin E, and vitamin B or niacinamide, which will help plump the barrier and also have anti-inflammatory properties. Additionally, polyphenolic flavonoids such as soy and green tea can help decrease inflammation, along with a number of other organic ingredients, such as caffeine, feverfew, and licorice.5 If topical vitamin C is being considered for postprocedure use, the non–ascorbic acid variant should be administered. The magnesium ascorbyl phosphate and ascorbyl palmitate forms of vitamin C have a neutral pH and tend to be better tolerated by patients.
In addition to current prescription and over-the-counter cosmeceuticals used for postprocedure irritation and inflammation, copper peptides and other well-tolerated and effective naturally occurring compounds are being investigated and tried. Copper is a biocide that regulates keratinocyte integrins for epithelization and extracellular matrix remodeling. The extracellular matrix consists of the structural fibrillar collagens and is remodeled or degraded by matrix metalloproteinases (MMPs) that facilitate epithelization. The predominant classes of MMPs include collagenases (ie, MMP-1) and gelatinases (ie, MMP-2, MMP-9) that degrade interstitial collagen and basement membrane proteins.6 The MMPs are endogenously inhibited by tissue inhibitors of metalloproteinases (TIMPs). Copper is a cofactor to lysyl oxidase, which cross-links collagen and stimulates expression of MMP-2 and collagen in a complex with a matrix-derived tripeptide (glycyl-histidyl-lysine or Gly-His-Lys [GHK]) in fibroblasts.6 Much attention has been focused on the tripeptides, such as GHK and Gly-Gly-His, and their copper complexes, which have high activity and good skin tolerance. These complexes have been shown to play a physiological role in the process of wound healing, tissue repair, and skin inflammation. Gly-Gly-His, GHK, copper chloride, and their copper complexes decrease tumor necrosis factor α–dependent IL-6 secretion in fibroblasts.7 IL-6 is crucial for normal wound healing, skin inflammation, and UVB-induced erythema. Because of their anti-inflammatory properties, these copper peptides could potentially be used in place of corticosteroids or nonsteroidal anti-inflammatory drugs, which have more side effects.
Botulinum Neurotoxin and Other Injectable Fillers
Acetyl Hexapeptide-3: A Topical Complement to Botulinum Neurotoxin
Acetyl hexapeptide-3 (Ac-Glu-Glu-Met-Gln-Arg-Arg-NH2) was discovered when looking for a less toxic variation of botulinum neurotoxin (BoNT) to treat aging skin.8 It is patterned from the N-terminal end of the synaptosome-associated protein of molecular weight 25 kDa (SNAP-25), which is essential for docking and fusion of synaptic vesicles to the presynaptic membrane for acetylcholine release.9 It prevents formation and stability of the soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) complex, inhibiting vesicle docking and calcium-dependent catecholamine exocytosis.8 It also has been found to substantially inhibit the repetitive muscular contraction of facial expression similar to BoNT type A but with somewhat lower efficacy. Acetyl hexapeptide-3 was shown to inhibit 30% of total catecholamine exocytosis and had a remarkable capacity to permeate the skin.10 Thus this topical form of BoNT is a useful complement to intramuscular BoNT.
Studies showing the efficacy and safety of acetyl hexapeptide-3 have demonstrated reductions in wrinkle intensity, mainly in the lateral periorbital areas. In one early study, 10 women applied an emulsion containing 10% of the hexapeptide to one lateral periorbital region and the same emulsion without the hexapeptide to the contralateral side, both twice daily for 30 days.10 A 30% decrease in the depth of skin wrinkles was seen on the hexapeptide side compared with a 10% decrease in the depth of wrinkles on the side treated without hexapeptide. No irritation or toxicity was noted.10 In another trial, 10 women applied an acetyl hexapeptide-3 cream 5% twice daily to lateral periorbital rhytides, with a 27% improvement in wrinkle depth after a 30-day treatment period.9 A double-blind, placebo-controlled study of 60 women assessing the safety and efficacy of topical hexapeptide showed a total antiwrinkle efficacy of 48.9% on the side treated with an emulsion containing 10% of the hexapeptide compared with 0% efficacy on the placebo side.8 Similar to Blanes-Mira et al,10 no adverse events such as skin irritation or toxicity were seen.8 In all of these studies, wrinkle depth was measured by silicone replica analysis.
Topical acetyl hexapeptide-3 is effective in decreasing wrinkles, and its best use will likely be as an adjunct to intramuscular BoNT, as the intramuscular form likely has higher efficacy with the toxin injected directly into the target muscle; however, patients who want the effects of BoNT without the pain of injections may choose to use topical acetyl hexapeptide-3 alone. Patients who do use acetyl hexapeptide-3 as a complement to their intramuscular BoNT regimen may not need as many units of BoNT with each treatment or may not need certain areas injected as often, leading to fewer injections and less pain with each visit. Skin irritation was not seen as a side effect in these trials. Additionally, the topical form has insignificant acute toxicity (≥2000 mg/kg) compared to BoNT type A (20 ng/kg), and genotoxicity was not seen with testing, making it a safe complementary option to an injectable regimen.8
Topical Hyaluronic Acid: A Complement to Injectable Fillers
Hyaluronic acid (HA) is a glycosaminoglycan found in the extracellular matrix of the skin that greatly contributes to tissue hydration. Additionally, it plays a crucial role in the synthesis of extracellular matrix molecules and epidermal cell interaction with the environment.11 The water-binding capacity of HA approximates 1000 times its volume or 6 L of water per gram of HA; however, once an individual reaches adulthood, the amount of HA decreases to 5% of baseline levels, thus contributing to xerosis, loss of skin elasticity, and atrophy.11,12 Although photoaged skin can have increased glycosaminoglycans due to an increase in chondroitin sulfate proteoglycans, they are abnormally deposited on elastotic material in the superficial dermis rather than diffusely scattered, as seen in youthful skin.12
Many topical antiaging products contain HA, though evidence for efficacy in reducing wrinkles has been lacking, along with concerns that HA cannot penetrate the skin. This concern stems from the fact that the original molecule is 3000 nm in diameter and the intercellular space is only 15 to 50 nm. This space is only 6 to 10 nm at the hyaline membrane. Recently, scientists in Japan found a way to reduce the size of HA molecules to 5 nm (nano-HA) without changing its structure. A study of 33 women who applied the topical nano-HA twice daily for 8 weeks to one periorbital area while the contralateral side was left untreated showed improved hydration of the treated side that continued to increase when measured at 2, 4, and 8 weeks using corneometry.11 Roughness decreased and elasticity increased after week 2, which were maintained throughout the study. Additionally, erythema was measured using a chroma meter, which was found to have decreased at day 57 versus day 1.11 An earlier study by Pavicic et al12 evaluated the efficacy of topical hyalu-ronan 0.1% formulations of different molecular weights—50, 130, 300, 800, or 2000 kDa—in the periocular area. A randomized group of 76 women were treated twice daily for 2 months with HA cream on one side of the periocular area and placebo cream on the other. With regard to antiwrinkle properties, only the 50- and 130-kDa HA formulations showed marked effects compared with placebo after 2 months.12
Topical HA would be an effective addition to an antiwrinkle regimen, especially in patients who are averse to needles or are just starting to get wrinkles and are looking for a noninvasive therapy. Additionally, it would be beneficial for patients who have an injectable filler and BoNT regimen, as these patients will be able to target wrinkles simultaneously with both topical cosmeceuticals and injectables and likely will need fewer units of BoNT and/or filler and possibly fewer injections over time, which translates to decreased pain and adverse outcomes for patients.
Conclusion
The myriad of options dermatologists have to offer patients for cosmetic enhancement provides alternatives for patients who have contraindications to certain treatments, are needle averse, or have lifestyles that do not afford them a great deal of postprocedural healing time. Being knowledgeable about these options and how to combine them for improved outcomes is essential to any cosmetic practice.
1. Rendon MI, Berson DS, Cohen JL, et al. Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. J Clin Aesthet Dermatol. 2010;3:32-43.
2. Lupo MP, Jacob LG. Cosmeceuticals for enhancing cosmetic procedures. In: Farris PK, ed. Cosmeceuticals and Cosmetic Practice. Oxford, United Kingdom: Wiley-Blackwell; 2014:268-276.
3. Gruber JV, Holtz R. Examining the impact of skin lighteners in vitro [published online ahead of print April 28, 2013]. Oxid Med Cell Longev. 2013;2013:702120.
4. Antonio JR, Antonio CR, Cardeal ILS, et al. Nanotechnology in dermatology. An Bras Dermatol. 2014;89:126-136.
5. Ganceviciene R, Liakou AI, Theodoridis A, et al. Skin anti-aging strategies. Dermatoendocrinol. 2012;4:308-319.
6. Gruchlik A, Jurzak M, Chodurek, E, et al. Effect of GLY-GLY-HIS, GLY-HIS-LYS and their copper complexes on TNF-α-dependant IL-6 secretion in normal human dermal fibroblasts. Acta Pol Pharm. 2012;69:1303-1306.
7. Philips N, Hwang H, Chauhan S, et al. Stimulation of cell proliferation and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect Tissue Res. 2010;51:224-229.
8. Wang Y, Wang M, Xiao S, et al. The anti-wrinkle efficacy of Argireline, a synthetic hexapeptide, in Chinese subjects. Am J Clin Dermatol. 2013;14:147-153.
9. Lupo MP, Cole A. Cosmeceutical peptides. Dermatol Ther. 2007;20:343-349.
10. Blanes-Mira C, Clemente J, Jodas G, et al. A synthetic hexapeptide (Argireline) with antiwrinkle activity. Int J Cosmet Sci. 2002;24:303-310.
11. Jegasothy SM, Zabolotniaia V, Bielfeldt S. Efficacy of a new topical nano-hyaluronic acid in humans. J Clin Aesthet Dermatol. 2014;7:27-29.
12. Pavicic T, Gauglitz G, Lersch P, et al. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. J Drugs Dermatol. 2011;10:990-1000.
1. Rendon MI, Berson DS, Cohen JL, et al. Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. J Clin Aesthet Dermatol. 2010;3:32-43.
2. Lupo MP, Jacob LG. Cosmeceuticals for enhancing cosmetic procedures. In: Farris PK, ed. Cosmeceuticals and Cosmetic Practice. Oxford, United Kingdom: Wiley-Blackwell; 2014:268-276.
3. Gruber JV, Holtz R. Examining the impact of skin lighteners in vitro [published online ahead of print April 28, 2013]. Oxid Med Cell Longev. 2013;2013:702120.
4. Antonio JR, Antonio CR, Cardeal ILS, et al. Nanotechnology in dermatology. An Bras Dermatol. 2014;89:126-136.
5. Ganceviciene R, Liakou AI, Theodoridis A, et al. Skin anti-aging strategies. Dermatoendocrinol. 2012;4:308-319.
6. Gruchlik A, Jurzak M, Chodurek, E, et al. Effect of GLY-GLY-HIS, GLY-HIS-LYS and their copper complexes on TNF-α-dependant IL-6 secretion in normal human dermal fibroblasts. Acta Pol Pharm. 2012;69:1303-1306.
7. Philips N, Hwang H, Chauhan S, et al. Stimulation of cell proliferation and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect Tissue Res. 2010;51:224-229.
8. Wang Y, Wang M, Xiao S, et al. The anti-wrinkle efficacy of Argireline, a synthetic hexapeptide, in Chinese subjects. Am J Clin Dermatol. 2013;14:147-153.
9. Lupo MP, Cole A. Cosmeceutical peptides. Dermatol Ther. 2007;20:343-349.
10. Blanes-Mira C, Clemente J, Jodas G, et al. A synthetic hexapeptide (Argireline) with antiwrinkle activity. Int J Cosmet Sci. 2002;24:303-310.
11. Jegasothy SM, Zabolotniaia V, Bielfeldt S. Efficacy of a new topical nano-hyaluronic acid in humans. J Clin Aesthet Dermatol. 2014;7:27-29.
12. Pavicic T, Gauglitz G, Lersch P, et al. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. J Drugs Dermatol. 2011;10:990-1000.
Practice Points
- Copper peptides could potentially be used in place of corticosteroids or nonsteroidal anti-inflammatory drugs for postprocedure irritation and inflammation.
- Acetyl hexapeptide-3 is a topical variation of botulinum toxin to be used on its own or adjunctively with the injectable form.
- Topical hyaluronic acid can be used on its own or adjunctively with injectable fillers.
UV Radiation Transmittance: Regular Clothing Versus Sun-Protective Clothing
Dermatologists frequently encounter patients who inquire about the need to buy special clothing and hats that claim to block UV light rather than using their regular clothing and hats. A patient may argue that he/she has never gotten sunburned through his/her favorite T-shirt while fishing, so why does he/she need to buy special clothing? The answer to this question is not straightforward. The dermatologist could easily say yes and advise patients to buy special sun-protective clothing, which could be especially tempting if a practitioner actually sells these items in the office. However, when considering evidence-based medicine, one needs to look at the data to appropriately answer the question.
Although it is still evolving, a standard has been set for UV protection factor (UPF) in the United States as well as other countries.1 Clothing with the maximum UPF rating of 50 blocks 98% of UVA/UVB radiation. Although there are data published in the literature regarding sun-protective clothing, there are scant data in the clinical dermatologic literature.2-5 To give patients an educated answer to this question, we measured and compared UVA/UVB radiation transmittance through regular (ie, non–UPF rated) clothing versus sun-protective clothing with a UPF rating.
Materials and Methods
A digital handheld UVA/UVB meter with an absorption spectrum of 280 to 400 nm was used to measure UV energy transmitted through sample clothing articles. The meter measured UVA/UVB light with a maximum reading of 40 mW/cm2. Clothing articles were selected of varied material/color and intended use.
Regular clothing articles included a straw golf hat (Figure 1A), an off-white and blue baseball hat (70% wool)(Figure 1B), a black baseball hat (100% wool)(Figure 1C), a white athletic tank shirt (100% cotton), a white T-shirt/undershirt (100% cotton), a thin-weave blue T-shirt (100% cotton), and a conventional-weave blue T-shirt (100% cotton)(Figure 1D). The regular clothing items, with the exception of the hats, had been laundered in conventional (ie, non–UV blocking) laundry detergent and no chemicals were applied to enhance UVA/UVB blocking properties. The exact number of times the items were laundered was unknown.
 |
 |
 |
 |
| Figure 1. Regular clothing articles included a straw golf hat (A), an off-white and blue baseball hat (B), a black baseball hat (C), and white and blue T-shirts (D). |
Sun-protective clothing articles included a polyester floral splash bucket hat and a polyester ruffled swim romper (Figure 2), both with a UPF rating of 50+. These items were purchased from a manufacturer who regularly promotes sun-protective clothing to both dermatologists and the general public. The company “guarantees” a UPF rating of 50 and advertises that these clothing articles block 98% of harmful UV rays. These items were not laundered prior to the study, and no chemicals were applied to enhance UVA/UVB blocking properties.
The UVA/UVB meter was calibrated on a clear cloudless July day in Frankfort, Illinois. An initial reading was taken without any obstruction to the sunlight. The regular and sun-protective clothing articles then were placed over the meter to measure the amount of UVA/UVB transmitted through each item. Measurements were taken for each article of clothing after the meter was covered by the respective material for 10 seconds. Care was taken to cover the meter with only 1 layer of material for each article, which was intended to mimic the degree of UVA/UVB blocking and transmittance during normal wear.
Results
The full results from the study are outlined in the Table. The unobstructed sunlight exposure exceeded the maximum measure of 40 mW/cm2, indicating there was a sufficient amount of sunlight to conduct testing.
The data show that both regular and sun-protective clothing blocked UVA/UVB rays in the 280- to 400-nm range. The Table outlines the level of UVA/UVB transmittance for each article of clothing; a lower number indicates less UVA/UVB transmittance occurred and more radiation was blocked.
Several of the regular clothing blocked more UV radiation than the sun-protective clothing; specifically, the data indicate that the baseball hats or the straw golf hat provided better protection than the sun-protective bucket hat. The black baseball hat provided the best UV protection. However, the straw golf hat provided adequate protection and better coverage, making it the best recommendation for patients.
Comment
Several of the regular items included in the study allowed less UVA/UVB transmission than the sun-protective clothing. Although our small study tested a limited number and type of articles, we assert that similar regular clothing would have similar transmittance.
There are various factors that affect UVA/UVB transmittance. Fabric construction, weight, thickness, composition, and color will affect the degree of UVA/UVB transmittance.1 In our study, the thickness, weave, and color of the fabric of the regular hats may have contributed to the superior results compared with the sun-protective hat. It could be postulated that cotton is inherently a superior fabric to the polyester sun-protective clothing fabric. With regard to the regular T-shirts, thickness, weave, and color also may have played a role in blocking UVA/UVB transmittance.
Patients may be assured of a sufficient amount of UVA/UVB blocking with sun-protective clothing. However, our study indicated that the regular clothing articles we tested provided similar, if not better, protection against UV radiation compared with the sun-protective clothing articles.
Conclusion
Based on the data, we would advise patients that they do not need to buy special sun-protective clothing that claims to block UV radiation, as regular clothing will provide equivalent protection against UVA/UVB radiation. However, these findings do not suggest that the claims for sun-protective clothing are inaccurate. Nevertheless, similar UVA/UVB blocking may be achieved with clothing already owned by patients.
1. Gies P. Photoprotection by clothing. Photodermatol Photoimmunol Photomed. 2007;23:264-274.
2. Wilson CA, Bevin NK, Laing RM, et al. Solar protection—effect of selected fabric and use characteristics on ultraviolet transmission. Textile Research Journal. 2008;78:95-104.
3. Ghazi S, Couteau C, Coiffard LJ. How to guarantee sun protection for a young sportsperson. J Dtsch Dermatol Ges. 2011;9:470-474.
4. Ghazi S, Couteau C, Coiffard LJ. What level of protection can be obtained using sun protective clothing? determining effectiveness using an in vitro method. Int J Pharm. 2010;397:144-146.
5. Morison WL. Photoprotection by clothing. Dermatol Ther. 2003;16:16-22.
Dermatologists frequently encounter patients who inquire about the need to buy special clothing and hats that claim to block UV light rather than using their regular clothing and hats. A patient may argue that he/she has never gotten sunburned through his/her favorite T-shirt while fishing, so why does he/she need to buy special clothing? The answer to this question is not straightforward. The dermatologist could easily say yes and advise patients to buy special sun-protective clothing, which could be especially tempting if a practitioner actually sells these items in the office. However, when considering evidence-based medicine, one needs to look at the data to appropriately answer the question.
Although it is still evolving, a standard has been set for UV protection factor (UPF) in the United States as well as other countries.1 Clothing with the maximum UPF rating of 50 blocks 98% of UVA/UVB radiation. Although there are data published in the literature regarding sun-protective clothing, there are scant data in the clinical dermatologic literature.2-5 To give patients an educated answer to this question, we measured and compared UVA/UVB radiation transmittance through regular (ie, non–UPF rated) clothing versus sun-protective clothing with a UPF rating.
Materials and Methods
A digital handheld UVA/UVB meter with an absorption spectrum of 280 to 400 nm was used to measure UV energy transmitted through sample clothing articles. The meter measured UVA/UVB light with a maximum reading of 40 mW/cm2. Clothing articles were selected of varied material/color and intended use.
Regular clothing articles included a straw golf hat (Figure 1A), an off-white and blue baseball hat (70% wool)(Figure 1B), a black baseball hat (100% wool)(Figure 1C), a white athletic tank shirt (100% cotton), a white T-shirt/undershirt (100% cotton), a thin-weave blue T-shirt (100% cotton), and a conventional-weave blue T-shirt (100% cotton)(Figure 1D). The regular clothing items, with the exception of the hats, had been laundered in conventional (ie, non–UV blocking) laundry detergent and no chemicals were applied to enhance UVA/UVB blocking properties. The exact number of times the items were laundered was unknown.
 |
 |
 |
 |
| Figure 1. Regular clothing articles included a straw golf hat (A), an off-white and blue baseball hat (B), a black baseball hat (C), and white and blue T-shirts (D). |
Sun-protective clothing articles included a polyester floral splash bucket hat and a polyester ruffled swim romper (Figure 2), both with a UPF rating of 50+. These items were purchased from a manufacturer who regularly promotes sun-protective clothing to both dermatologists and the general public. The company “guarantees” a UPF rating of 50 and advertises that these clothing articles block 98% of harmful UV rays. These items were not laundered prior to the study, and no chemicals were applied to enhance UVA/UVB blocking properties.
The UVA/UVB meter was calibrated on a clear cloudless July day in Frankfort, Illinois. An initial reading was taken without any obstruction to the sunlight. The regular and sun-protective clothing articles then were placed over the meter to measure the amount of UVA/UVB transmitted through each item. Measurements were taken for each article of clothing after the meter was covered by the respective material for 10 seconds. Care was taken to cover the meter with only 1 layer of material for each article, which was intended to mimic the degree of UVA/UVB blocking and transmittance during normal wear.
Results
The full results from the study are outlined in the Table. The unobstructed sunlight exposure exceeded the maximum measure of 40 mW/cm2, indicating there was a sufficient amount of sunlight to conduct testing.
The data show that both regular and sun-protective clothing blocked UVA/UVB rays in the 280- to 400-nm range. The Table outlines the level of UVA/UVB transmittance for each article of clothing; a lower number indicates less UVA/UVB transmittance occurred and more radiation was blocked.
Several of the regular clothing blocked more UV radiation than the sun-protective clothing; specifically, the data indicate that the baseball hats or the straw golf hat provided better protection than the sun-protective bucket hat. The black baseball hat provided the best UV protection. However, the straw golf hat provided adequate protection and better coverage, making it the best recommendation for patients.
Comment
Several of the regular items included in the study allowed less UVA/UVB transmission than the sun-protective clothing. Although our small study tested a limited number and type of articles, we assert that similar regular clothing would have similar transmittance.
There are various factors that affect UVA/UVB transmittance. Fabric construction, weight, thickness, composition, and color will affect the degree of UVA/UVB transmittance.1 In our study, the thickness, weave, and color of the fabric of the regular hats may have contributed to the superior results compared with the sun-protective hat. It could be postulated that cotton is inherently a superior fabric to the polyester sun-protective clothing fabric. With regard to the regular T-shirts, thickness, weave, and color also may have played a role in blocking UVA/UVB transmittance.
Patients may be assured of a sufficient amount of UVA/UVB blocking with sun-protective clothing. However, our study indicated that the regular clothing articles we tested provided similar, if not better, protection against UV radiation compared with the sun-protective clothing articles.
Conclusion
Based on the data, we would advise patients that they do not need to buy special sun-protective clothing that claims to block UV radiation, as regular clothing will provide equivalent protection against UVA/UVB radiation. However, these findings do not suggest that the claims for sun-protective clothing are inaccurate. Nevertheless, similar UVA/UVB blocking may be achieved with clothing already owned by patients.
Dermatologists frequently encounter patients who inquire about the need to buy special clothing and hats that claim to block UV light rather than using their regular clothing and hats. A patient may argue that he/she has never gotten sunburned through his/her favorite T-shirt while fishing, so why does he/she need to buy special clothing? The answer to this question is not straightforward. The dermatologist could easily say yes and advise patients to buy special sun-protective clothing, which could be especially tempting if a practitioner actually sells these items in the office. However, when considering evidence-based medicine, one needs to look at the data to appropriately answer the question.
Although it is still evolving, a standard has been set for UV protection factor (UPF) in the United States as well as other countries.1 Clothing with the maximum UPF rating of 50 blocks 98% of UVA/UVB radiation. Although there are data published in the literature regarding sun-protective clothing, there are scant data in the clinical dermatologic literature.2-5 To give patients an educated answer to this question, we measured and compared UVA/UVB radiation transmittance through regular (ie, non–UPF rated) clothing versus sun-protective clothing with a UPF rating.
Materials and Methods
A digital handheld UVA/UVB meter with an absorption spectrum of 280 to 400 nm was used to measure UV energy transmitted through sample clothing articles. The meter measured UVA/UVB light with a maximum reading of 40 mW/cm2. Clothing articles were selected of varied material/color and intended use.
Regular clothing articles included a straw golf hat (Figure 1A), an off-white and blue baseball hat (70% wool)(Figure 1B), a black baseball hat (100% wool)(Figure 1C), a white athletic tank shirt (100% cotton), a white T-shirt/undershirt (100% cotton), a thin-weave blue T-shirt (100% cotton), and a conventional-weave blue T-shirt (100% cotton)(Figure 1D). The regular clothing items, with the exception of the hats, had been laundered in conventional (ie, non–UV blocking) laundry detergent and no chemicals were applied to enhance UVA/UVB blocking properties. The exact number of times the items were laundered was unknown.
 |
 |
 |
 |
| Figure 1. Regular clothing articles included a straw golf hat (A), an off-white and blue baseball hat (B), a black baseball hat (C), and white and blue T-shirts (D). |
Sun-protective clothing articles included a polyester floral splash bucket hat and a polyester ruffled swim romper (Figure 2), both with a UPF rating of 50+. These items were purchased from a manufacturer who regularly promotes sun-protective clothing to both dermatologists and the general public. The company “guarantees” a UPF rating of 50 and advertises that these clothing articles block 98% of harmful UV rays. These items were not laundered prior to the study, and no chemicals were applied to enhance UVA/UVB blocking properties.
The UVA/UVB meter was calibrated on a clear cloudless July day in Frankfort, Illinois. An initial reading was taken without any obstruction to the sunlight. The regular and sun-protective clothing articles then were placed over the meter to measure the amount of UVA/UVB transmitted through each item. Measurements were taken for each article of clothing after the meter was covered by the respective material for 10 seconds. Care was taken to cover the meter with only 1 layer of material for each article, which was intended to mimic the degree of UVA/UVB blocking and transmittance during normal wear.
Results
The full results from the study are outlined in the Table. The unobstructed sunlight exposure exceeded the maximum measure of 40 mW/cm2, indicating there was a sufficient amount of sunlight to conduct testing.
The data show that both regular and sun-protective clothing blocked UVA/UVB rays in the 280- to 400-nm range. The Table outlines the level of UVA/UVB transmittance for each article of clothing; a lower number indicates less UVA/UVB transmittance occurred and more radiation was blocked.
Several of the regular clothing blocked more UV radiation than the sun-protective clothing; specifically, the data indicate that the baseball hats or the straw golf hat provided better protection than the sun-protective bucket hat. The black baseball hat provided the best UV protection. However, the straw golf hat provided adequate protection and better coverage, making it the best recommendation for patients.
Comment
Several of the regular items included in the study allowed less UVA/UVB transmission than the sun-protective clothing. Although our small study tested a limited number and type of articles, we assert that similar regular clothing would have similar transmittance.
There are various factors that affect UVA/UVB transmittance. Fabric construction, weight, thickness, composition, and color will affect the degree of UVA/UVB transmittance.1 In our study, the thickness, weave, and color of the fabric of the regular hats may have contributed to the superior results compared with the sun-protective hat. It could be postulated that cotton is inherently a superior fabric to the polyester sun-protective clothing fabric. With regard to the regular T-shirts, thickness, weave, and color also may have played a role in blocking UVA/UVB transmittance.
Patients may be assured of a sufficient amount of UVA/UVB blocking with sun-protective clothing. However, our study indicated that the regular clothing articles we tested provided similar, if not better, protection against UV radiation compared with the sun-protective clothing articles.
Conclusion
Based on the data, we would advise patients that they do not need to buy special sun-protective clothing that claims to block UV radiation, as regular clothing will provide equivalent protection against UVA/UVB radiation. However, these findings do not suggest that the claims for sun-protective clothing are inaccurate. Nevertheless, similar UVA/UVB blocking may be achieved with clothing already owned by patients.
1. Gies P. Photoprotection by clothing. Photodermatol Photoimmunol Photomed. 2007;23:264-274.
2. Wilson CA, Bevin NK, Laing RM, et al. Solar protection—effect of selected fabric and use characteristics on ultraviolet transmission. Textile Research Journal. 2008;78:95-104.
3. Ghazi S, Couteau C, Coiffard LJ. How to guarantee sun protection for a young sportsperson. J Dtsch Dermatol Ges. 2011;9:470-474.
4. Ghazi S, Couteau C, Coiffard LJ. What level of protection can be obtained using sun protective clothing? determining effectiveness using an in vitro method. Int J Pharm. 2010;397:144-146.
5. Morison WL. Photoprotection by clothing. Dermatol Ther. 2003;16:16-22.
1. Gies P. Photoprotection by clothing. Photodermatol Photoimmunol Photomed. 2007;23:264-274.
2. Wilson CA, Bevin NK, Laing RM, et al. Solar protection—effect of selected fabric and use characteristics on ultraviolet transmission. Textile Research Journal. 2008;78:95-104.
3. Ghazi S, Couteau C, Coiffard LJ. How to guarantee sun protection for a young sportsperson. J Dtsch Dermatol Ges. 2011;9:470-474.
4. Ghazi S, Couteau C, Coiffard LJ. What level of protection can be obtained using sun protective clothing? determining effectiveness using an in vitro method. Int J Pharm. 2010;397:144-146.
5. Morison WL. Photoprotection by clothing. Dermatol Ther. 2003;16:16-22.
- Dermatologists routinely advise patients that clothing is a method of UVA/UVB protection.
- Regular clothing items provide similar, if not superior, UVA/UVB protection compared to sun-protective clothing.
- Physicians may confidently inform patients that regular clothing items will provide adequate UVA/UVB protection.