User login
Glomus Tumor of Uncertain Malignant Potential on the Forehead
Glomus tumors (GTs) are uncommon benign tumors originating in the neuromyoarterial elements of the glomus body, an arteriovenous shunt specialized in thermoregulation.1 Glomus tumors usually occur in the distal extremities of young adults2 and rarely are seen in the deep soft tissue or viscera. Malignant GTs are rare and highly aggressive tumors that have been associated with both local recurrence and distant metastasis.1-11 Glomus tumors have been subdivided into 3 groups with different prognoses2: (1) malignant GT with metastatic potential (subfascial or visceral location, >2 cm in size, atypical mitotic figures, >5 mitoses per 50 high-power fields [HPFs], marked nuclear atypia); (2) symplastic GT (benign tumor with nuclear pleomorphism without mitotic activity); and (3) GT of uncertain malignant potential (GTUMP)(absence of metastatic disease, favorable prognosis, at least 1 feature of malignant GTs other than marked nuclear atypia [eg, high mitotic activity, >2 cm in size, deep location]).1,2
We report a case of GTUMP with unusual clinicopathologic features in a 74-year-old man that was treated via wide surgical excision. No local recurrence or distant metastasis was noted at 3-year follow-up.
Case Report
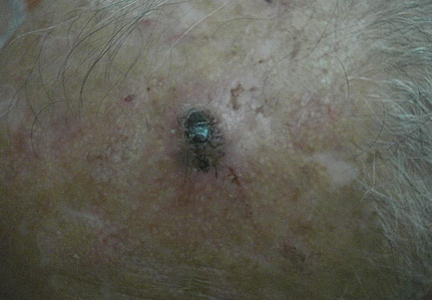
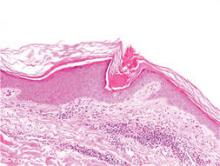
A 74-year-old man presented to the Unit of Surgery with a slowly progressing, painful, ulcerated, 2.5-cm, red-blue nodule on the forehead (Figure 1). An excisional biopsy of the nodule was performed. Histologically, the dermis and superficial subcutis were filled with a proliferation of atypical epithelioid cells to slightly spindled cells. Both cells displayed a weakly eosinophilic cytoplasm with indistinct membranes and larger ovoid nuclei, some with prominent nucleoli. Neoplastic cells showed a disordered arrangement or were organized in short fascicles separated by slitlike spaces, vascular lumens of various sizes, or hemorrhagic stroma resembling angiosarcoma or Kaposi sarcoma (Figure 2). No areas of necrosis were noted. Pleomorphic nuclei and some mitotic figures also were identified, but they were not atypical and showed fewer than 5 mitoses per 50 HPFs. Immunohistochemically, the neoplastic cells stained positive for vimentin, caldesmon (Figure 3), and a–smooth muscle actin, and they stained negative for cytokeratins, desmin, CD34, factor VIII–related antigen, S-100 protein, and the latent nuclear antigen of Kaposi sarcoma–associated herpesvirus. The Ki-67 labeling index revealed less than 20% positive cells.
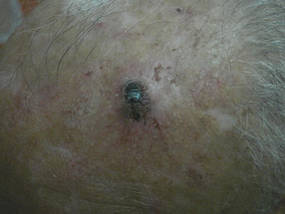 |
| Figure 1. A painful, red-blue, ulcerated nodule on the forehead of a 74-year-old man. |
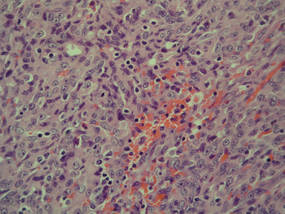 |
| Figure 2. The tumor was composed of atypical epithelioid cells to slightly spindled cells with indistinct membranes and larger ovoid nuclei, some with prominent nucleoli in a disordered arrangement or rather in short fascicles separated in a hemorrhagic background (H&E, original magnification ×40). |
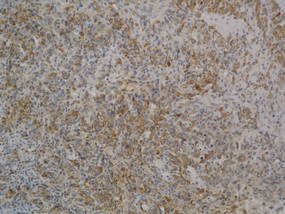 |
| Figure 3. The neoplastic cells stained positive for caldesmon (original magnification ×20). |
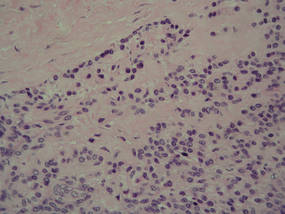 |
| Figure 4. A focus of round to polygonal tumor cells reminiscent of a preexisting benign-appearing glomus tumor was found on biopsy following reexcision (H&E, original magnification ×20). |
Because the tumor involved margins of excision, the patient successfully underwent wide reexcision with adequate margins. Histological examination of the reexcised specimen showed a focus of bland, round to polygonal tumor cells with the features of glomus cells (Figure 4). On the basis of the histologic features of both specimens, the lesion was classified as a GT. The biggest problem in our case was the classification of the lesion according to established pathologic criteria. We considered this case to be borderline because the lesion was greater than 2 cm but the location was superficial; marked atypia with sarcomatoid features also were present, but there was an absence of necrosis and fewer than 5 mitoses per 50 HPFs. For these reasons, we diagnosed this problematic lesion as a GTUMP. Following reexcision, the patient underwent strict follow-up. Wound healing was uncomplicated and the patient showed no local recurrence or distant metastasis at 3-year follow-up.
Comment
Clinically metastatic and histologically malignant GTs are exceptional.3-11 The classification system for GTs based on histologic criteria subdivided these tumors into 3 groups with different prognoses.2 Malignant GTs are highly aggressive tumors with metastatic potential, symplastic GTs are considered to be a degenerative phenomenon, and GTUMPs have a favorable clinical outcome and absence of metastatic disease.12
Diagnosis of malignant GTs and symplastic GTs is relatively easy in the presence of typical uniform, small, round epithelioid cells (glomus cells) located around blood vessels. Immunohistochemistry may be useful, as GTs express smooth muscle actin and caldesmon. Over the years the existence and diagnosis of malignant GT has been questioned because a residual component of benign GT in the surgical biopsy is useful in diagnosis but is not always present1-3 and because an unusual pattern may be present in malignant tumors with prevalent spindle cells resembling fibrosarcoma, leiomyosarcoma, spindle cell angiosarcoma, and spindle cell melanoma.1 In the absence of a preexisting GT, the differential diagnosis may be difficult; in such cases, a panel of immunohistochemical markers including smooth muscle actin, caldesmon, desmin, S-100, human melanoma black-45 (HMB-45), CD34, and CD31 is always necessary. The GTUMP category was introduced for GTs that demonstrate marked nuclear atypia but do not fulfill histologic criteria for malignancy. Along with other tumors of uncertain malignant potential, borderline cases should be considered GTUMPs to guarantee wide excision of the tumor with negative margins and an adequate follow-up due to the possibility of local recurrence or distant metastasis. In our patient, a diagnosis of GTUMP was made. Additionally, our case demonstrates some previously unreported features of GTUMPs, such as spindled cells in short fascicles separated by slitlike spaces, small vessels, and hemorrhagic stroma resembling Kaposi sarcoma. Along with these unusual sarcomatous features, the superficial location of the lesion, absence of necrosis, and a mitotic count of less than 5 per 50 HPFs were suggestive of an uncertain malignant potential for this tumor.
Distinction between malignant GTs and GTUMPs in the presence of unusual histologic features may be difficult.12 Glomus tumors that do not fulfill criteria for malignancy but have at least 1 atypical feature other than nuclear pleomorphism should be named GTUMPs. According to classification criteria, a true malignant GT is a highly aggressive tumor with metastatic potential. In a case series reported by Folpe et al,2 38% (20/52) of malignant GTs showed metastases, while metastatic disease was not observed in the tumors classified as GTUMPs. Wide surgical excision or Mohs micrographic surgery13 are the treatments of choice for malignant GTs and GTUMPs. Complete excision of the lesion with negative margins is always necessary in cases of GTUMPs. After the diagnosis of GTUMP, adequate follow-up should berecommended due to the possibility of local recurrence or distant metastasis.
Conclusion
Malignant GTs and GTUMPs are rare, and the nomenclature and classification of these tumors is controversial. These findings and the difficulty of differential diagnosis in a continuum between benignity and malignancy prompted our report.
1. Weiss SW, Goldblum JR. Perivascular tumors. In: Enzinger FM, Weiss SW, eds. Enzinger and Weiss’s Soft Tissue Tumors. 5th ed. St Louis, MO: Mosby; 2008:751-768.
2. Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12.
3. Aiba M, Hirayama A, Kuramochi S. Glomangiosarcoma in a glomus tumor. an immunohistochemical and ultrastructural study. Cancer. 1988;61:1467-1471.
4. Gould EW, Manivel JC, Albores-Saavedra J, et al. Locally infiltrative glomus tumors and glomangiosarcomas. a clinical, ultrastructural, and immunohistochemical study. Cancer. 1990;65:310-318.
5. Noer H, Krogdahl A. Glomangiosarcoma of the lower extremity. Histopathology. 1991;18:365-366.
6. Hiruta N, Kameda N, Tokudome T, et al. Malignant glomus tumor: a case report and review of the literature. Am J Surg Pathol. 1997;21:1096-1103.
7. Watanabe K, Sugino T, Saito A, et al. Glomangiosarcoma of the hip: report of a highly aggressive tumour with widespread distant metastases. Br J Dermatol. 1998;139:1097-1101.
8. Park JH, Oh SH, Yang MH, et al. Glomangiosarcoma of the hand: a case report and review of the literature. J Dermatol. 2003;30:827-833.
9. Kayal JD, Hampton RW, Sheehan DJ, et al. Malignant glomus tumor: a case report and review of the literature. Dermatol Surg. 2001;27:837-840.
10. Pérez de la Fuente T, Vega C, Gutierrez Palacios A, et al. Glomangiosarcoma of the hypothenar eminence: a case report. Chir Main. 2005;24:199-202.
11. Terada T, Fujimoto J, Shirakashi Y, et al. Malignant glomus tumor of the palm: a case report. J Cutan Pathol. 2011;38:381-384.
12. Gill J, Van Vliet C. Infiltrating glomus tumor of uncertain malignant potential arising in the kidney. Hum Pathol. 2010;41:145-149.
13. Cecchi R, Pavesi M, Apicella P. Malignant glomus tumor of the trunk treated with Mohs micrographic surgery [in English, German]. J Dtsch Dermatol Ges. 2011;9:391-392.
Glomus tumors (GTs) are uncommon benign tumors originating in the neuromyoarterial elements of the glomus body, an arteriovenous shunt specialized in thermoregulation.1 Glomus tumors usually occur in the distal extremities of young adults2 and rarely are seen in the deep soft tissue or viscera. Malignant GTs are rare and highly aggressive tumors that have been associated with both local recurrence and distant metastasis.1-11 Glomus tumors have been subdivided into 3 groups with different prognoses2: (1) malignant GT with metastatic potential (subfascial or visceral location, >2 cm in size, atypical mitotic figures, >5 mitoses per 50 high-power fields [HPFs], marked nuclear atypia); (2) symplastic GT (benign tumor with nuclear pleomorphism without mitotic activity); and (3) GT of uncertain malignant potential (GTUMP)(absence of metastatic disease, favorable prognosis, at least 1 feature of malignant GTs other than marked nuclear atypia [eg, high mitotic activity, >2 cm in size, deep location]).1,2
We report a case of GTUMP with unusual clinicopathologic features in a 74-year-old man that was treated via wide surgical excision. No local recurrence or distant metastasis was noted at 3-year follow-up.
Case Report
A 74-year-old man presented to the Unit of Surgery with a slowly progressing, painful, ulcerated, 2.5-cm, red-blue nodule on the forehead (Figure 1). An excisional biopsy of the nodule was performed. Histologically, the dermis and superficial subcutis were filled with a proliferation of atypical epithelioid cells to slightly spindled cells. Both cells displayed a weakly eosinophilic cytoplasm with indistinct membranes and larger ovoid nuclei, some with prominent nucleoli. Neoplastic cells showed a disordered arrangement or were organized in short fascicles separated by slitlike spaces, vascular lumens of various sizes, or hemorrhagic stroma resembling angiosarcoma or Kaposi sarcoma (Figure 2). No areas of necrosis were noted. Pleomorphic nuclei and some mitotic figures also were identified, but they were not atypical and showed fewer than 5 mitoses per 50 HPFs. Immunohistochemically, the neoplastic cells stained positive for vimentin, caldesmon (Figure 3), and a–smooth muscle actin, and they stained negative for cytokeratins, desmin, CD34, factor VIII–related antigen, S-100 protein, and the latent nuclear antigen of Kaposi sarcoma–associated herpesvirus. The Ki-67 labeling index revealed less than 20% positive cells.
 |
| Figure 1. A painful, red-blue, ulcerated nodule on the forehead of a 74-year-old man. |
 |
| Figure 2. The tumor was composed of atypical epithelioid cells to slightly spindled cells with indistinct membranes and larger ovoid nuclei, some with prominent nucleoli in a disordered arrangement or rather in short fascicles separated in a hemorrhagic background (H&E, original magnification ×40). |
 |
| Figure 3. The neoplastic cells stained positive for caldesmon (original magnification ×20). |
 |
| Figure 4. A focus of round to polygonal tumor cells reminiscent of a preexisting benign-appearing glomus tumor was found on biopsy following reexcision (H&E, original magnification ×20). |
Because the tumor involved margins of excision, the patient successfully underwent wide reexcision with adequate margins. Histological examination of the reexcised specimen showed a focus of bland, round to polygonal tumor cells with the features of glomus cells (Figure 4). On the basis of the histologic features of both specimens, the lesion was classified as a GT. The biggest problem in our case was the classification of the lesion according to established pathologic criteria. We considered this case to be borderline because the lesion was greater than 2 cm but the location was superficial; marked atypia with sarcomatoid features also were present, but there was an absence of necrosis and fewer than 5 mitoses per 50 HPFs. For these reasons, we diagnosed this problematic lesion as a GTUMP. Following reexcision, the patient underwent strict follow-up. Wound healing was uncomplicated and the patient showed no local recurrence or distant metastasis at 3-year follow-up.
Comment
Clinically metastatic and histologically malignant GTs are exceptional.3-11 The classification system for GTs based on histologic criteria subdivided these tumors into 3 groups with different prognoses.2 Malignant GTs are highly aggressive tumors with metastatic potential, symplastic GTs are considered to be a degenerative phenomenon, and GTUMPs have a favorable clinical outcome and absence of metastatic disease.12
Diagnosis of malignant GTs and symplastic GTs is relatively easy in the presence of typical uniform, small, round epithelioid cells (glomus cells) located around blood vessels. Immunohistochemistry may be useful, as GTs express smooth muscle actin and caldesmon. Over the years the existence and diagnosis of malignant GT has been questioned because a residual component of benign GT in the surgical biopsy is useful in diagnosis but is not always present1-3 and because an unusual pattern may be present in malignant tumors with prevalent spindle cells resembling fibrosarcoma, leiomyosarcoma, spindle cell angiosarcoma, and spindle cell melanoma.1 In the absence of a preexisting GT, the differential diagnosis may be difficult; in such cases, a panel of immunohistochemical markers including smooth muscle actin, caldesmon, desmin, S-100, human melanoma black-45 (HMB-45), CD34, and CD31 is always necessary. The GTUMP category was introduced for GTs that demonstrate marked nuclear atypia but do not fulfill histologic criteria for malignancy. Along with other tumors of uncertain malignant potential, borderline cases should be considered GTUMPs to guarantee wide excision of the tumor with negative margins and an adequate follow-up due to the possibility of local recurrence or distant metastasis. In our patient, a diagnosis of GTUMP was made. Additionally, our case demonstrates some previously unreported features of GTUMPs, such as spindled cells in short fascicles separated by slitlike spaces, small vessels, and hemorrhagic stroma resembling Kaposi sarcoma. Along with these unusual sarcomatous features, the superficial location of the lesion, absence of necrosis, and a mitotic count of less than 5 per 50 HPFs were suggestive of an uncertain malignant potential for this tumor.
Distinction between malignant GTs and GTUMPs in the presence of unusual histologic features may be difficult.12 Glomus tumors that do not fulfill criteria for malignancy but have at least 1 atypical feature other than nuclear pleomorphism should be named GTUMPs. According to classification criteria, a true malignant GT is a highly aggressive tumor with metastatic potential. In a case series reported by Folpe et al,2 38% (20/52) of malignant GTs showed metastases, while metastatic disease was not observed in the tumors classified as GTUMPs. Wide surgical excision or Mohs micrographic surgery13 are the treatments of choice for malignant GTs and GTUMPs. Complete excision of the lesion with negative margins is always necessary in cases of GTUMPs. After the diagnosis of GTUMP, adequate follow-up should berecommended due to the possibility of local recurrence or distant metastasis.
Conclusion
Malignant GTs and GTUMPs are rare, and the nomenclature and classification of these tumors is controversial. These findings and the difficulty of differential diagnosis in a continuum between benignity and malignancy prompted our report.
Glomus tumors (GTs) are uncommon benign tumors originating in the neuromyoarterial elements of the glomus body, an arteriovenous shunt specialized in thermoregulation.1 Glomus tumors usually occur in the distal extremities of young adults2 and rarely are seen in the deep soft tissue or viscera. Malignant GTs are rare and highly aggressive tumors that have been associated with both local recurrence and distant metastasis.1-11 Glomus tumors have been subdivided into 3 groups with different prognoses2: (1) malignant GT with metastatic potential (subfascial or visceral location, >2 cm in size, atypical mitotic figures, >5 mitoses per 50 high-power fields [HPFs], marked nuclear atypia); (2) symplastic GT (benign tumor with nuclear pleomorphism without mitotic activity); and (3) GT of uncertain malignant potential (GTUMP)(absence of metastatic disease, favorable prognosis, at least 1 feature of malignant GTs other than marked nuclear atypia [eg, high mitotic activity, >2 cm in size, deep location]).1,2
We report a case of GTUMP with unusual clinicopathologic features in a 74-year-old man that was treated via wide surgical excision. No local recurrence or distant metastasis was noted at 3-year follow-up.
Case Report
A 74-year-old man presented to the Unit of Surgery with a slowly progressing, painful, ulcerated, 2.5-cm, red-blue nodule on the forehead (Figure 1). An excisional biopsy of the nodule was performed. Histologically, the dermis and superficial subcutis were filled with a proliferation of atypical epithelioid cells to slightly spindled cells. Both cells displayed a weakly eosinophilic cytoplasm with indistinct membranes and larger ovoid nuclei, some with prominent nucleoli. Neoplastic cells showed a disordered arrangement or were organized in short fascicles separated by slitlike spaces, vascular lumens of various sizes, or hemorrhagic stroma resembling angiosarcoma or Kaposi sarcoma (Figure 2). No areas of necrosis were noted. Pleomorphic nuclei and some mitotic figures also were identified, but they were not atypical and showed fewer than 5 mitoses per 50 HPFs. Immunohistochemically, the neoplastic cells stained positive for vimentin, caldesmon (Figure 3), and a–smooth muscle actin, and they stained negative for cytokeratins, desmin, CD34, factor VIII–related antigen, S-100 protein, and the latent nuclear antigen of Kaposi sarcoma–associated herpesvirus. The Ki-67 labeling index revealed less than 20% positive cells.
 |
| Figure 1. A painful, red-blue, ulcerated nodule on the forehead of a 74-year-old man. |
 |
| Figure 2. The tumor was composed of atypical epithelioid cells to slightly spindled cells with indistinct membranes and larger ovoid nuclei, some with prominent nucleoli in a disordered arrangement or rather in short fascicles separated in a hemorrhagic background (H&E, original magnification ×40). |
 |
| Figure 3. The neoplastic cells stained positive for caldesmon (original magnification ×20). |
 |
| Figure 4. A focus of round to polygonal tumor cells reminiscent of a preexisting benign-appearing glomus tumor was found on biopsy following reexcision (H&E, original magnification ×20). |
Because the tumor involved margins of excision, the patient successfully underwent wide reexcision with adequate margins. Histological examination of the reexcised specimen showed a focus of bland, round to polygonal tumor cells with the features of glomus cells (Figure 4). On the basis of the histologic features of both specimens, the lesion was classified as a GT. The biggest problem in our case was the classification of the lesion according to established pathologic criteria. We considered this case to be borderline because the lesion was greater than 2 cm but the location was superficial; marked atypia with sarcomatoid features also were present, but there was an absence of necrosis and fewer than 5 mitoses per 50 HPFs. For these reasons, we diagnosed this problematic lesion as a GTUMP. Following reexcision, the patient underwent strict follow-up. Wound healing was uncomplicated and the patient showed no local recurrence or distant metastasis at 3-year follow-up.
Comment
Clinically metastatic and histologically malignant GTs are exceptional.3-11 The classification system for GTs based on histologic criteria subdivided these tumors into 3 groups with different prognoses.2 Malignant GTs are highly aggressive tumors with metastatic potential, symplastic GTs are considered to be a degenerative phenomenon, and GTUMPs have a favorable clinical outcome and absence of metastatic disease.12
Diagnosis of malignant GTs and symplastic GTs is relatively easy in the presence of typical uniform, small, round epithelioid cells (glomus cells) located around blood vessels. Immunohistochemistry may be useful, as GTs express smooth muscle actin and caldesmon. Over the years the existence and diagnosis of malignant GT has been questioned because a residual component of benign GT in the surgical biopsy is useful in diagnosis but is not always present1-3 and because an unusual pattern may be present in malignant tumors with prevalent spindle cells resembling fibrosarcoma, leiomyosarcoma, spindle cell angiosarcoma, and spindle cell melanoma.1 In the absence of a preexisting GT, the differential diagnosis may be difficult; in such cases, a panel of immunohistochemical markers including smooth muscle actin, caldesmon, desmin, S-100, human melanoma black-45 (HMB-45), CD34, and CD31 is always necessary. The GTUMP category was introduced for GTs that demonstrate marked nuclear atypia but do not fulfill histologic criteria for malignancy. Along with other tumors of uncertain malignant potential, borderline cases should be considered GTUMPs to guarantee wide excision of the tumor with negative margins and an adequate follow-up due to the possibility of local recurrence or distant metastasis. In our patient, a diagnosis of GTUMP was made. Additionally, our case demonstrates some previously unreported features of GTUMPs, such as spindled cells in short fascicles separated by slitlike spaces, small vessels, and hemorrhagic stroma resembling Kaposi sarcoma. Along with these unusual sarcomatous features, the superficial location of the lesion, absence of necrosis, and a mitotic count of less than 5 per 50 HPFs were suggestive of an uncertain malignant potential for this tumor.
Distinction between malignant GTs and GTUMPs in the presence of unusual histologic features may be difficult.12 Glomus tumors that do not fulfill criteria for malignancy but have at least 1 atypical feature other than nuclear pleomorphism should be named GTUMPs. According to classification criteria, a true malignant GT is a highly aggressive tumor with metastatic potential. In a case series reported by Folpe et al,2 38% (20/52) of malignant GTs showed metastases, while metastatic disease was not observed in the tumors classified as GTUMPs. Wide surgical excision or Mohs micrographic surgery13 are the treatments of choice for malignant GTs and GTUMPs. Complete excision of the lesion with negative margins is always necessary in cases of GTUMPs. After the diagnosis of GTUMP, adequate follow-up should berecommended due to the possibility of local recurrence or distant metastasis.
Conclusion
Malignant GTs and GTUMPs are rare, and the nomenclature and classification of these tumors is controversial. These findings and the difficulty of differential diagnosis in a continuum between benignity and malignancy prompted our report.
1. Weiss SW, Goldblum JR. Perivascular tumors. In: Enzinger FM, Weiss SW, eds. Enzinger and Weiss’s Soft Tissue Tumors. 5th ed. St Louis, MO: Mosby; 2008:751-768.
2. Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12.
3. Aiba M, Hirayama A, Kuramochi S. Glomangiosarcoma in a glomus tumor. an immunohistochemical and ultrastructural study. Cancer. 1988;61:1467-1471.
4. Gould EW, Manivel JC, Albores-Saavedra J, et al. Locally infiltrative glomus tumors and glomangiosarcomas. a clinical, ultrastructural, and immunohistochemical study. Cancer. 1990;65:310-318.
5. Noer H, Krogdahl A. Glomangiosarcoma of the lower extremity. Histopathology. 1991;18:365-366.
6. Hiruta N, Kameda N, Tokudome T, et al. Malignant glomus tumor: a case report and review of the literature. Am J Surg Pathol. 1997;21:1096-1103.
7. Watanabe K, Sugino T, Saito A, et al. Glomangiosarcoma of the hip: report of a highly aggressive tumour with widespread distant metastases. Br J Dermatol. 1998;139:1097-1101.
8. Park JH, Oh SH, Yang MH, et al. Glomangiosarcoma of the hand: a case report and review of the literature. J Dermatol. 2003;30:827-833.
9. Kayal JD, Hampton RW, Sheehan DJ, et al. Malignant glomus tumor: a case report and review of the literature. Dermatol Surg. 2001;27:837-840.
10. Pérez de la Fuente T, Vega C, Gutierrez Palacios A, et al. Glomangiosarcoma of the hypothenar eminence: a case report. Chir Main. 2005;24:199-202.
11. Terada T, Fujimoto J, Shirakashi Y, et al. Malignant glomus tumor of the palm: a case report. J Cutan Pathol. 2011;38:381-384.
12. Gill J, Van Vliet C. Infiltrating glomus tumor of uncertain malignant potential arising in the kidney. Hum Pathol. 2010;41:145-149.
13. Cecchi R, Pavesi M, Apicella P. Malignant glomus tumor of the trunk treated with Mohs micrographic surgery [in English, German]. J Dtsch Dermatol Ges. 2011;9:391-392.
1. Weiss SW, Goldblum JR. Perivascular tumors. In: Enzinger FM, Weiss SW, eds. Enzinger and Weiss’s Soft Tissue Tumors. 5th ed. St Louis, MO: Mosby; 2008:751-768.
2. Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12.
3. Aiba M, Hirayama A, Kuramochi S. Glomangiosarcoma in a glomus tumor. an immunohistochemical and ultrastructural study. Cancer. 1988;61:1467-1471.
4. Gould EW, Manivel JC, Albores-Saavedra J, et al. Locally infiltrative glomus tumors and glomangiosarcomas. a clinical, ultrastructural, and immunohistochemical study. Cancer. 1990;65:310-318.
5. Noer H, Krogdahl A. Glomangiosarcoma of the lower extremity. Histopathology. 1991;18:365-366.
6. Hiruta N, Kameda N, Tokudome T, et al. Malignant glomus tumor: a case report and review of the literature. Am J Surg Pathol. 1997;21:1096-1103.
7. Watanabe K, Sugino T, Saito A, et al. Glomangiosarcoma of the hip: report of a highly aggressive tumour with widespread distant metastases. Br J Dermatol. 1998;139:1097-1101.
8. Park JH, Oh SH, Yang MH, et al. Glomangiosarcoma of the hand: a case report and review of the literature. J Dermatol. 2003;30:827-833.
9. Kayal JD, Hampton RW, Sheehan DJ, et al. Malignant glomus tumor: a case report and review of the literature. Dermatol Surg. 2001;27:837-840.
10. Pérez de la Fuente T, Vega C, Gutierrez Palacios A, et al. Glomangiosarcoma of the hypothenar eminence: a case report. Chir Main. 2005;24:199-202.
11. Terada T, Fujimoto J, Shirakashi Y, et al. Malignant glomus tumor of the palm: a case report. J Cutan Pathol. 2011;38:381-384.
12. Gill J, Van Vliet C. Infiltrating glomus tumor of uncertain malignant potential arising in the kidney. Hum Pathol. 2010;41:145-149.
13. Cecchi R, Pavesi M, Apicella P. Malignant glomus tumor of the trunk treated with Mohs micrographic surgery [in English, German]. J Dtsch Dermatol Ges. 2011;9:391-392.
Practice Points
- Glomus tumors have been subdivided into 3 groups with different prognoses.
- The term glomus tumor of uncertain malignant potential (GTUMP) was introduced to describe glomus tumors that demonstrate marked nuclear atypia but do not fulfill histologic criteria for malignancy.
- Complete excision with negative margins is always necessary in cases of GTUMPs.
Papular Eruption Following Excessive Tanning Bed Use
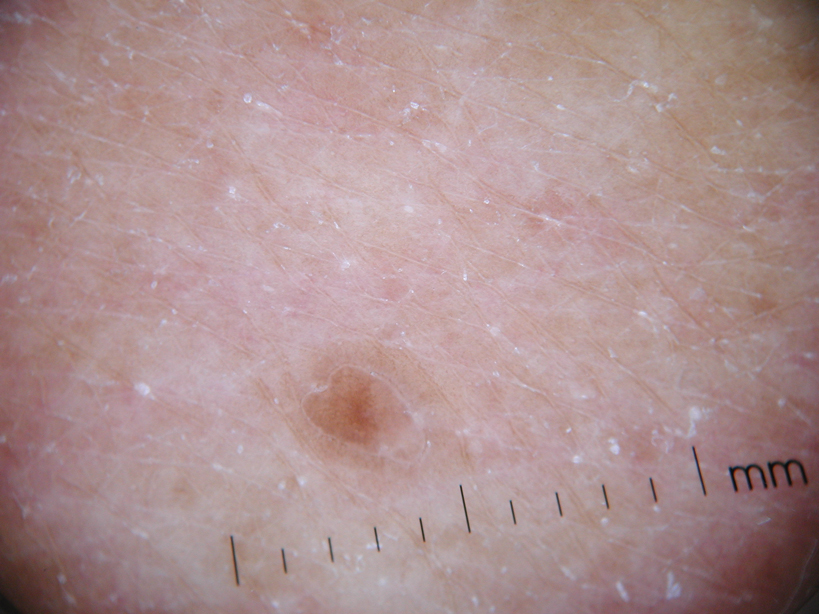
The Diagnosis: Disseminated Superficial Actinic Porokeratosis
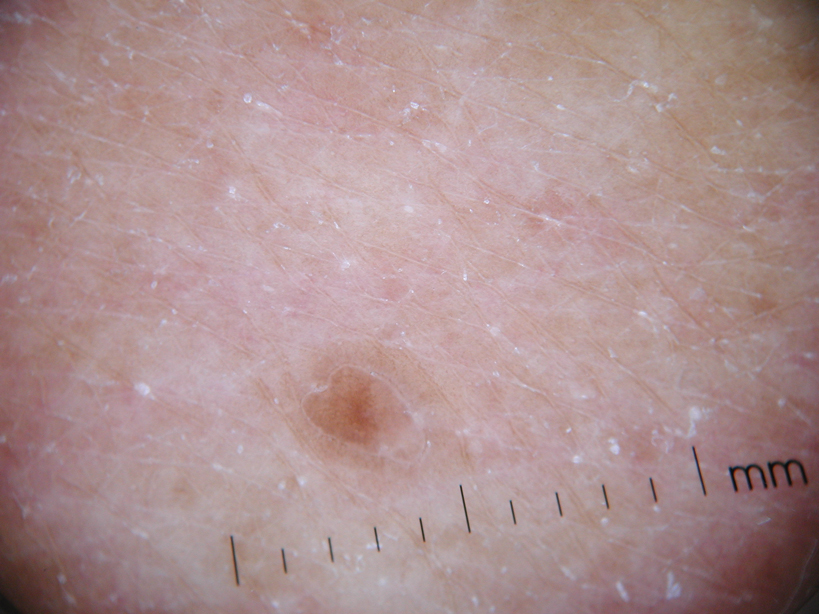
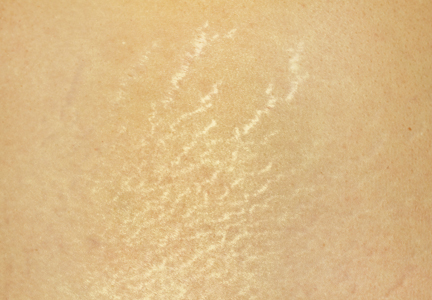
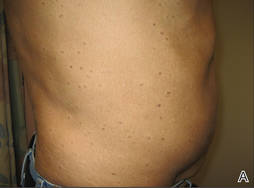
Physical examination after 7 years of tanning salon use showed a tanned white man with multiple 4- to 5-mm, discrete, round to oval, reddish brown papules on the chest, back, abdomen, arms, and legs that were rough to palpate (Figure 1), with a peripheral rim of scale seen more prominently on dermoscopy. There were no lesions on the palms or soles. A subsequent 4-mm punch biopsy was done on the right abdomen and right thigh, which showed focal thinning of the epidermis, loss of the granular layer, a discrete column of parakeratosis and a characteristic feature of a coronoid lamella in the epidermis (Figure 2). The patient received 14 narrowband UVB (NB-UVB) treatments; however, he could not continue due to transportation issues. He visited the clinic sporadically for 6 months thereafter and reportedly went to tanning salons daily. The patient was subsequently lost to follow-up.
 |
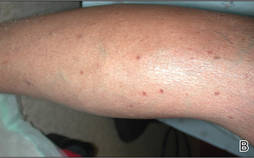 |
| Figure 1. Multiple 4- to 5-mm, discrete, round to oval, reddish brown papules on the abdomen (A) and leg (B) that were rough to palpate. |
Disseminated porokeratosis, or disseminated superficial actinic porokeratosis (DSAP), was first described in 1967 by Chernosky and Freeman.1 It is the most common variant of porokeratosis. Other variants include Mibelli type, porokeratosis palmaris et plantaris disseminata, punctuate porokeratosis, and linear porokeratosis.2-4 Porokeratosis is inherited in an autosomal-dominant fashion, presenting in the third or fourth decades of life; however, most cases are sporadic.5 Pruritus is a common symptom and can be debilitating.5
Disseminated superficial actinic porokeratosis can be precipitated by excessive sun exposure, with a reported increase in lesions during summer months and resolution during the winter months.6 The lesions of DSAP can be experimentally induced by exposure to daily use of artificial UV sunlamps.7 Patients with psoriasis undergoing psoralen plus UVA and NB-UVB treatments also have been reported to trigger DSAP.8 A study by Neumann et al6 suggested that a combination of both UVB and UVA wavelengths may be most effective in inducing DSAP. Exposure to UVA and UVB light may explain an increased number of DSAP lesions in patients who excessively visit tanning salons, as the bulbs emit a combination of wavelengths with UVA in much greater amounts than UVB.
Our patient developed DSAP secondary to artificial UV light exposure from excessive tanning salon use. Medications (allopurinol and lisinopril) were initially thought to be etiologic agents for the eruption and also corroborated with histologic findings of a drug eruption on the initial biopsy. However, new lesions continued to develop even after cessation of medications and NB-UVB treatments. A subsequent biopsy and further history of daily tanning salon use confirmed the diagnosis of DSAP.
Therapies for this condition are limited with variable degrees of success. Cryotherapy, 5-fluorouracil cream, imiquimod cream 5%, Q-switched ruby laser, diclofenac gel 3%, and acitretin for more widespread or refractory lesions have been used with partial to complete resolution of DSAP.9
We present this case to highlight the occurrence of DSAP secondary to UV light exposure from excessive tanning salon use.
1. Chernosky ME, Freeman RG. Disseminated superficial actinic porokeratosis (DSAP). Arch Dermatol. 1967;96:611-624.
2. Guss SB, Osbourn RA, Lutzner MA. Porokeratosis plantaris, palmaris et disseminata: a third type of porokeratosis. Arch Dermatol. 1971;104:366-373.
3. Brown FC. Punctate keratoderma. Arch Dermatol. 1971;104:682-683.
4. Eyre WG, Carson WE. Linear porokeratosis of Mibelli. Arch Dermatol. 1972;105:426-429.
5. Anderson DE, Chernosky ME. Disseminated superficial actinic porokeratosis. Arch Dermatol. 1969;99:408-412.
6. Neumann RA, Knobler RM, Jurecka W, et al. Disseminated superficial actinic porokeratosis: experimental induction and exacerbation of skin lesions. J Am Acad Dermatol. 1989;21:1182-1188.
7. Chernosky ME, Anderson DE. Disseminated superficial actinic porokeratosis: clinical studies and experimental production of lesions. Arch Dermatol. 1969;99:401-407.
8. Allen LA, Glaser DA. Disseminated superficial actinic porokeratosis associated with topical PUVA. J Am Acad Dermatol. 2000;43:720-722.
9. Arun B, Pearson J, Chalmers R. Disseminated superficial actinic porokeratosis treated effectively with topical imiquimod 5% cream. Clin Exp Dermatol. 2011;36:509-511.
The Diagnosis: Disseminated Superficial Actinic Porokeratosis
Physical examination after 7 years of tanning salon use showed a tanned white man with multiple 4- to 5-mm, discrete, round to oval, reddish brown papules on the chest, back, abdomen, arms, and legs that were rough to palpate (Figure 1), with a peripheral rim of scale seen more prominently on dermoscopy. There were no lesions on the palms or soles. A subsequent 4-mm punch biopsy was done on the right abdomen and right thigh, which showed focal thinning of the epidermis, loss of the granular layer, a discrete column of parakeratosis and a characteristic feature of a coronoid lamella in the epidermis (Figure 2). The patient received 14 narrowband UVB (NB-UVB) treatments; however, he could not continue due to transportation issues. He visited the clinic sporadically for 6 months thereafter and reportedly went to tanning salons daily. The patient was subsequently lost to follow-up.
 |
 |
| Figure 1. Multiple 4- to 5-mm, discrete, round to oval, reddish brown papules on the abdomen (A) and leg (B) that were rough to palpate. |
Disseminated porokeratosis, or disseminated superficial actinic porokeratosis (DSAP), was first described in 1967 by Chernosky and Freeman.1 It is the most common variant of porokeratosis. Other variants include Mibelli type, porokeratosis palmaris et plantaris disseminata, punctuate porokeratosis, and linear porokeratosis.2-4 Porokeratosis is inherited in an autosomal-dominant fashion, presenting in the third or fourth decades of life; however, most cases are sporadic.5 Pruritus is a common symptom and can be debilitating.5
Disseminated superficial actinic porokeratosis can be precipitated by excessive sun exposure, with a reported increase in lesions during summer months and resolution during the winter months.6 The lesions of DSAP can be experimentally induced by exposure to daily use of artificial UV sunlamps.7 Patients with psoriasis undergoing psoralen plus UVA and NB-UVB treatments also have been reported to trigger DSAP.8 A study by Neumann et al6 suggested that a combination of both UVB and UVA wavelengths may be most effective in inducing DSAP. Exposure to UVA and UVB light may explain an increased number of DSAP lesions in patients who excessively visit tanning salons, as the bulbs emit a combination of wavelengths with UVA in much greater amounts than UVB.
Our patient developed DSAP secondary to artificial UV light exposure from excessive tanning salon use. Medications (allopurinol and lisinopril) were initially thought to be etiologic agents for the eruption and also corroborated with histologic findings of a drug eruption on the initial biopsy. However, new lesions continued to develop even after cessation of medications and NB-UVB treatments. A subsequent biopsy and further history of daily tanning salon use confirmed the diagnosis of DSAP.
Therapies for this condition are limited with variable degrees of success. Cryotherapy, 5-fluorouracil cream, imiquimod cream 5%, Q-switched ruby laser, diclofenac gel 3%, and acitretin for more widespread or refractory lesions have been used with partial to complete resolution of DSAP.9
We present this case to highlight the occurrence of DSAP secondary to UV light exposure from excessive tanning salon use.
The Diagnosis: Disseminated Superficial Actinic Porokeratosis
Physical examination after 7 years of tanning salon use showed a tanned white man with multiple 4- to 5-mm, discrete, round to oval, reddish brown papules on the chest, back, abdomen, arms, and legs that were rough to palpate (Figure 1), with a peripheral rim of scale seen more prominently on dermoscopy. There were no lesions on the palms or soles. A subsequent 4-mm punch biopsy was done on the right abdomen and right thigh, which showed focal thinning of the epidermis, loss of the granular layer, a discrete column of parakeratosis and a characteristic feature of a coronoid lamella in the epidermis (Figure 2). The patient received 14 narrowband UVB (NB-UVB) treatments; however, he could not continue due to transportation issues. He visited the clinic sporadically for 6 months thereafter and reportedly went to tanning salons daily. The patient was subsequently lost to follow-up.
 |
 |
| Figure 1. Multiple 4- to 5-mm, discrete, round to oval, reddish brown papules on the abdomen (A) and leg (B) that were rough to palpate. |
Disseminated porokeratosis, or disseminated superficial actinic porokeratosis (DSAP), was first described in 1967 by Chernosky and Freeman.1 It is the most common variant of porokeratosis. Other variants include Mibelli type, porokeratosis palmaris et plantaris disseminata, punctuate porokeratosis, and linear porokeratosis.2-4 Porokeratosis is inherited in an autosomal-dominant fashion, presenting in the third or fourth decades of life; however, most cases are sporadic.5 Pruritus is a common symptom and can be debilitating.5
Disseminated superficial actinic porokeratosis can be precipitated by excessive sun exposure, with a reported increase in lesions during summer months and resolution during the winter months.6 The lesions of DSAP can be experimentally induced by exposure to daily use of artificial UV sunlamps.7 Patients with psoriasis undergoing psoralen plus UVA and NB-UVB treatments also have been reported to trigger DSAP.8 A study by Neumann et al6 suggested that a combination of both UVB and UVA wavelengths may be most effective in inducing DSAP. Exposure to UVA and UVB light may explain an increased number of DSAP lesions in patients who excessively visit tanning salons, as the bulbs emit a combination of wavelengths with UVA in much greater amounts than UVB.
Our patient developed DSAP secondary to artificial UV light exposure from excessive tanning salon use. Medications (allopurinol and lisinopril) were initially thought to be etiologic agents for the eruption and also corroborated with histologic findings of a drug eruption on the initial biopsy. However, new lesions continued to develop even after cessation of medications and NB-UVB treatments. A subsequent biopsy and further history of daily tanning salon use confirmed the diagnosis of DSAP.
Therapies for this condition are limited with variable degrees of success. Cryotherapy, 5-fluorouracil cream, imiquimod cream 5%, Q-switched ruby laser, diclofenac gel 3%, and acitretin for more widespread or refractory lesions have been used with partial to complete resolution of DSAP.9
We present this case to highlight the occurrence of DSAP secondary to UV light exposure from excessive tanning salon use.
1. Chernosky ME, Freeman RG. Disseminated superficial actinic porokeratosis (DSAP). Arch Dermatol. 1967;96:611-624.
2. Guss SB, Osbourn RA, Lutzner MA. Porokeratosis plantaris, palmaris et disseminata: a third type of porokeratosis. Arch Dermatol. 1971;104:366-373.
3. Brown FC. Punctate keratoderma. Arch Dermatol. 1971;104:682-683.
4. Eyre WG, Carson WE. Linear porokeratosis of Mibelli. Arch Dermatol. 1972;105:426-429.
5. Anderson DE, Chernosky ME. Disseminated superficial actinic porokeratosis. Arch Dermatol. 1969;99:408-412.
6. Neumann RA, Knobler RM, Jurecka W, et al. Disseminated superficial actinic porokeratosis: experimental induction and exacerbation of skin lesions. J Am Acad Dermatol. 1989;21:1182-1188.
7. Chernosky ME, Anderson DE. Disseminated superficial actinic porokeratosis: clinical studies and experimental production of lesions. Arch Dermatol. 1969;99:401-407.
8. Allen LA, Glaser DA. Disseminated superficial actinic porokeratosis associated with topical PUVA. J Am Acad Dermatol. 2000;43:720-722.
9. Arun B, Pearson J, Chalmers R. Disseminated superficial actinic porokeratosis treated effectively with topical imiquimod 5% cream. Clin Exp Dermatol. 2011;36:509-511.
1. Chernosky ME, Freeman RG. Disseminated superficial actinic porokeratosis (DSAP). Arch Dermatol. 1967;96:611-624.
2. Guss SB, Osbourn RA, Lutzner MA. Porokeratosis plantaris, palmaris et disseminata: a third type of porokeratosis. Arch Dermatol. 1971;104:366-373.
3. Brown FC. Punctate keratoderma. Arch Dermatol. 1971;104:682-683.
4. Eyre WG, Carson WE. Linear porokeratosis of Mibelli. Arch Dermatol. 1972;105:426-429.
5. Anderson DE, Chernosky ME. Disseminated superficial actinic porokeratosis. Arch Dermatol. 1969;99:408-412.
6. Neumann RA, Knobler RM, Jurecka W, et al. Disseminated superficial actinic porokeratosis: experimental induction and exacerbation of skin lesions. J Am Acad Dermatol. 1989;21:1182-1188.
7. Chernosky ME, Anderson DE. Disseminated superficial actinic porokeratosis: clinical studies and experimental production of lesions. Arch Dermatol. 1969;99:401-407.
8. Allen LA, Glaser DA. Disseminated superficial actinic porokeratosis associated with topical PUVA. J Am Acad Dermatol. 2000;43:720-722.
9. Arun B, Pearson J, Chalmers R. Disseminated superficial actinic porokeratosis treated effectively with topical imiquimod 5% cream. Clin Exp Dermatol. 2011;36:509-511.
A 78-year-old man with Fitzpatrick skin type III presented to the dermatology department for evaluation of a pruritic, erythematous, papular eruption on the chest, back, abdomen, arms, and legs of 5 years’ duration. His medications include clonazepam, lisinopril, allopurinol, omeprazole, tramadol, and mirtazapine. The lesions did not respond to topical corticosteroids; however, the pruritus improved with narrowband UVB (NB-UVB) treatments. Review of systems did not reveal any abnormalities. The patient’s medical history included gout, hypertension, anxiety, esophageal stricture, and emphysema. He reported a history of tanning salon use at least 3 times weekly for 7 years. After initial consultation, the patient was treated with clobetasol propionate cream 0.05% twice daily and hydroxyzine 10 mg 3 times daily. Following 1 month of treatment, the eruption did not improve. A 4-mm punch biopsy of the left upper arm revealed a dense infiltrate in the upper dermis with prominent parakeratosis, lymphocytes, and numerous eosinophils, suggestive of a drug eruption. As a result, allopurinol was discontinued as a causative agent; however, the eruption presented prior to taking allopurinol. Because the patient experienced intense pruritus, he was started on NB-UVB treatments. After 14 treatments of NB-UVB 3 times weekly, the patient noticed some improvement with respect to pruritus, but the lesions did not resolve. A complete blood cell count indicated 7.6% eosinophils (reference range, 0%–5%). Liver function tests, complete metabolic profile, and renal function were within reference range. Lisinopril was then discontinued as a likely culprit for persistent drug eruption; however, new lesions continued to develop.
Best Practices in Pulsed Dye Laser Treatment
For more information, access Dr. Ezra's article from the August 2014 issue, "Linear Scarring Following Treatment With a 595-nm Pulsed Dye Laser."
For more information, access Dr. Ezra's article from the August 2014 issue, "Linear Scarring Following Treatment With a 595-nm Pulsed Dye Laser."
For more information, access Dr. Ezra's article from the August 2014 issue, "Linear Scarring Following Treatment With a 595-nm Pulsed Dye Laser."
Mastering the Masseter Muscle: Tailored Treatment of Masseter Hypertrophy

In the article “Classification of Masseter Hypertrophy for Tailored Botulinum Toxin Type A Treatment” (Plast Reconstr Surg. 2014;134:209e-218e), Xie et al systemically classified and compared degrees of masseter hypertrophy and then prospectively used this system to tailor treatment with botulinum toxin type A. The authors identified 5 different bulging types of a contracted masseter: minimal, mono, double, triple, and excessive. Ultrasound studies and cadaver dissections were used to identify the bulging type and showed that the masseter consists of 3 different muscle layers that exhibit different directions of muscle contraction. These muscle layers are innervated by separate nerve branches that originate from the nervus massetericus, the most prominent part of the masseter bulge corresponding to the distribution region of the nervus massetericus in the central lower one-third region. The authors concluded that an injection close to the nerve endings at this site of insertion would allow for a reduced injection dosage and limited dispersion. Therefore, they chose the most prominent point of the bulging masseter while clenching as the ideal initial injection point.
What’s the issue?
The off-label use of botulinum toxin type A for the treatment of masseter hypertrophy is becoming more popular. It has been used for aesthetic volume reduction of the masseter and lower-face recontouring. Masseter hypertrophy can cause a prominent mandibular angle resulting in a wide counter of the lower face, which can be a cause of aesthetic concern in women and men as well as individuals of many ethnicities, such as those of Asian descent. The reported doses of neurotoxin as well as injection points and techniques vary, making it difficult to translate into clinical practice. Xie et al have suggested a tailored approach to the treatment of masseter hypertrophy rather than injecting in a prototypical approach. The authors had the patient clench and palpated the masseter to classify it into 1 of 5 types. Then the main injection point was into the belly of the most prominent bulge. Further injection points (range, 1–3) were used depending on the type of masseter hypertrophy. The minimal dose of botulinum toxin type A used was 20 U with the highest amount being 40 U. The greatest effect in reduction of masseter hypertrophy occurred at 3 months. Adverse effects encountered were on par with prior reports and included unnatural smile, concavity below the zygomatic arch, and even paradoxical bulging. The overall complication rate was 9.1% (20/220), but it was 60% among patients who received higher doses. With this classification system and injection pattern described, the authors noted a reduction in injection dose and therefore complication rates as well as significantly reduced masseter volume and improved lower face contour (P<.01). When it comes to masseter injections and lower-face shaping, what is your method?

In the article “Classification of Masseter Hypertrophy for Tailored Botulinum Toxin Type A Treatment” (Plast Reconstr Surg. 2014;134:209e-218e), Xie et al systemically classified and compared degrees of masseter hypertrophy and then prospectively used this system to tailor treatment with botulinum toxin type A. The authors identified 5 different bulging types of a contracted masseter: minimal, mono, double, triple, and excessive. Ultrasound studies and cadaver dissections were used to identify the bulging type and showed that the masseter consists of 3 different muscle layers that exhibit different directions of muscle contraction. These muscle layers are innervated by separate nerve branches that originate from the nervus massetericus, the most prominent part of the masseter bulge corresponding to the distribution region of the nervus massetericus in the central lower one-third region. The authors concluded that an injection close to the nerve endings at this site of insertion would allow for a reduced injection dosage and limited dispersion. Therefore, they chose the most prominent point of the bulging masseter while clenching as the ideal initial injection point.
What’s the issue?
The off-label use of botulinum toxin type A for the treatment of masseter hypertrophy is becoming more popular. It has been used for aesthetic volume reduction of the masseter and lower-face recontouring. Masseter hypertrophy can cause a prominent mandibular angle resulting in a wide counter of the lower face, which can be a cause of aesthetic concern in women and men as well as individuals of many ethnicities, such as those of Asian descent. The reported doses of neurotoxin as well as injection points and techniques vary, making it difficult to translate into clinical practice. Xie et al have suggested a tailored approach to the treatment of masseter hypertrophy rather than injecting in a prototypical approach. The authors had the patient clench and palpated the masseter to classify it into 1 of 5 types. Then the main injection point was into the belly of the most prominent bulge. Further injection points (range, 1–3) were used depending on the type of masseter hypertrophy. The minimal dose of botulinum toxin type A used was 20 U with the highest amount being 40 U. The greatest effect in reduction of masseter hypertrophy occurred at 3 months. Adverse effects encountered were on par with prior reports and included unnatural smile, concavity below the zygomatic arch, and even paradoxical bulging. The overall complication rate was 9.1% (20/220), but it was 60% among patients who received higher doses. With this classification system and injection pattern described, the authors noted a reduction in injection dose and therefore complication rates as well as significantly reduced masseter volume and improved lower face contour (P<.01). When it comes to masseter injections and lower-face shaping, what is your method?

In the article “Classification of Masseter Hypertrophy for Tailored Botulinum Toxin Type A Treatment” (Plast Reconstr Surg. 2014;134:209e-218e), Xie et al systemically classified and compared degrees of masseter hypertrophy and then prospectively used this system to tailor treatment with botulinum toxin type A. The authors identified 5 different bulging types of a contracted masseter: minimal, mono, double, triple, and excessive. Ultrasound studies and cadaver dissections were used to identify the bulging type and showed that the masseter consists of 3 different muscle layers that exhibit different directions of muscle contraction. These muscle layers are innervated by separate nerve branches that originate from the nervus massetericus, the most prominent part of the masseter bulge corresponding to the distribution region of the nervus massetericus in the central lower one-third region. The authors concluded that an injection close to the nerve endings at this site of insertion would allow for a reduced injection dosage and limited dispersion. Therefore, they chose the most prominent point of the bulging masseter while clenching as the ideal initial injection point.
What’s the issue?
The off-label use of botulinum toxin type A for the treatment of masseter hypertrophy is becoming more popular. It has been used for aesthetic volume reduction of the masseter and lower-face recontouring. Masseter hypertrophy can cause a prominent mandibular angle resulting in a wide counter of the lower face, which can be a cause of aesthetic concern in women and men as well as individuals of many ethnicities, such as those of Asian descent. The reported doses of neurotoxin as well as injection points and techniques vary, making it difficult to translate into clinical practice. Xie et al have suggested a tailored approach to the treatment of masseter hypertrophy rather than injecting in a prototypical approach. The authors had the patient clench and palpated the masseter to classify it into 1 of 5 types. Then the main injection point was into the belly of the most prominent bulge. Further injection points (range, 1–3) were used depending on the type of masseter hypertrophy. The minimal dose of botulinum toxin type A used was 20 U with the highest amount being 40 U. The greatest effect in reduction of masseter hypertrophy occurred at 3 months. Adverse effects encountered were on par with prior reports and included unnatural smile, concavity below the zygomatic arch, and even paradoxical bulging. The overall complication rate was 9.1% (20/220), but it was 60% among patients who received higher doses. With this classification system and injection pattern described, the authors noted a reduction in injection dose and therefore complication rates as well as significantly reduced masseter volume and improved lower face contour (P<.01). When it comes to masseter injections and lower-face shaping, what is your method?
Cosmetic Corner: Dermatologists Weigh in on Toners
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top toners. Consideration must be given to:
- Calendula Herbal Extract Alcohol-Free Toner
Kiehl’s
“There is no alcohol base in this toner, which makes it far less drying than most, but it still is suitable for patients with more sebaceous/oily skin.”—Anthony M. Rossi, MD, New York, New York
- Copper Firming Mist
Innovative Skincare
“Copper Firming Mist is a unique toner that contains copper to promote collagen production in the skin. Also, Copper Firming Mist assists to hydrate tissue while regulating sebaceous gland activity. This product is especially good to use after laser treatments, as it assists in healing and hydrating after a procedure.”—Wm. Philip Werschler, MD, Seattle, Washington
- Effaclar Toner
La Roche-Posay Laboratoire Dermatologique
“This toner has lipohydroxy acid, which helps with mild acne and enlarged pores.”—Gary Goldenberg, MD, New York, New York
- Teoxane RHA Prime Solution
Alphaeon Corporation
“[The product] doesn’t just cleanse and normalize pH but delivers resilient hyaluronic acid, vitamin B6, zinc, and copper, as well as peptides and antioxidants.”—Mary P. Lupo, MD, New Orleans, Louisiana
Cutis invites readers to send us their recommendations. Over-the-counter antioxidants and facial scrubs will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to cutis@frontlinemedcom.com.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top toners. Consideration must be given to:
- Calendula Herbal Extract Alcohol-Free Toner
Kiehl’s
“There is no alcohol base in this toner, which makes it far less drying than most, but it still is suitable for patients with more sebaceous/oily skin.”—Anthony M. Rossi, MD, New York, New York
- Copper Firming Mist
Innovative Skincare
“Copper Firming Mist is a unique toner that contains copper to promote collagen production in the skin. Also, Copper Firming Mist assists to hydrate tissue while regulating sebaceous gland activity. This product is especially good to use after laser treatments, as it assists in healing and hydrating after a procedure.”—Wm. Philip Werschler, MD, Seattle, Washington
- Effaclar Toner
La Roche-Posay Laboratoire Dermatologique
“This toner has lipohydroxy acid, which helps with mild acne and enlarged pores.”—Gary Goldenberg, MD, New York, New York
- Teoxane RHA Prime Solution
Alphaeon Corporation
“[The product] doesn’t just cleanse and normalize pH but delivers resilient hyaluronic acid, vitamin B6, zinc, and copper, as well as peptides and antioxidants.”—Mary P. Lupo, MD, New Orleans, Louisiana
Cutis invites readers to send us their recommendations. Over-the-counter antioxidants and facial scrubs will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to cutis@frontlinemedcom.com.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top toners. Consideration must be given to:
- Calendula Herbal Extract Alcohol-Free Toner
Kiehl’s
“There is no alcohol base in this toner, which makes it far less drying than most, but it still is suitable for patients with more sebaceous/oily skin.”—Anthony M. Rossi, MD, New York, New York
- Copper Firming Mist
Innovative Skincare
“Copper Firming Mist is a unique toner that contains copper to promote collagen production in the skin. Also, Copper Firming Mist assists to hydrate tissue while regulating sebaceous gland activity. This product is especially good to use after laser treatments, as it assists in healing and hydrating after a procedure.”—Wm. Philip Werschler, MD, Seattle, Washington
- Effaclar Toner
La Roche-Posay Laboratoire Dermatologique
“This toner has lipohydroxy acid, which helps with mild acne and enlarged pores.”—Gary Goldenberg, MD, New York, New York
- Teoxane RHA Prime Solution
Alphaeon Corporation
“[The product] doesn’t just cleanse and normalize pH but delivers resilient hyaluronic acid, vitamin B6, zinc, and copper, as well as peptides and antioxidants.”—Mary P. Lupo, MD, New Orleans, Louisiana
Cutis invites readers to send us their recommendations. Over-the-counter antioxidants and facial scrubs will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to cutis@frontlinemedcom.com.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
Effective treatments abound for children with heavy sweating in the hands and feet
COEUR D’ALENE, IDAHO – By the time patients seek a physician’s help for palmar/plantar focal hyperhidrosis, they may be well beyond the point at which even potent topical antiperspirants will help.
"Most people think there is nothing that can be done at all [for these patients]. But there are effective options. Even if you’re not going to offer them, get your patients to someone who will. We can make a profound difference for children and adolescents," Dr. Jane S. Bellet said at the annual meeting of the Society for Pediatric Dermatology.
In fact, more potent therapies are available, ranging from iontophoresis to oral medications, botulinum toxin A injections, and surgical thoracic sympathetectomy.
Oral medications: "I’ve really changed my practice in the last 5 years. I didn’t use systemic medications very much. I use them much more frequently now. I think you can get a very nice response," said Dr. Bellet, a pediatric dermatologist at Duke University in Durham, N.C.
She typically turns to the anticholinergic agents glycopyrrolate and oxybutynin. Glycopyrrolate, marketed as Cuvposa in a cherry-flavored solution at 1 mg/5 mL, does not have an indication from the Food and Drug Administration for treatment of pediatric hyperhidrosis, she noted. However, it is FDA-approved in 3- to 16-year-olds for severe chronic drooling caused by neurologic disorders.
"Although we don’t have an indication for hyperhidrosis, we do have a pediatric indication, and I think many times that puts our parents at ease," she observed.
Glycopyrrolate is cost effective and painless, although approximately 30% of children treated for hyperhidrosis will develop dry mouth and/or dry eyes.
"I would definitely add glycopyrrolate to your armamentarium. I think the biggest concern most of us have is the side effects. Speak about them with the family ahead of time, guide them as to what to expect, and stop if it becomes intolerable," Dr. Bellet said.
A recent randomized, prospective, controlled clinical trial in 45 children aged 7-14 years with palmar hyperhidrosis showed excellent outcomes with oxybutynin (Ditropan), with more than 85% experiencing at least moderate improvement in sweating and 80% gaining improved quality of life (Pediatr. Dermatol. 2014;31:48-53).
Iontophoresis: "This is a wonderful treatment for palms and soles," said Dr. Bellet.
The treatment entails placing the hands or feet in a water bath tray filled with tap water through which direct electric current is running. The proposed mechanism of benefit is that hydrolysis of the bath water results in accumulation of hydrogen ions, which then induce sweat gland destruction.
The chief disadvantage of iontophoresis is that it is labor intensive. Unless the family rents or buys a home unit, the patient typically must visit the physician’s office on a daily basis initially, placing the hands or feet in the bath for 10 minutes, followed by a second 10-minute round after the polarity is switched. Eventually, many patients can step down to two or three 20- to 30-minute sessions per week, then perhaps once-weekly maintenance therapy, she said.
Adding glycopyrrolate to the water bath has been shown to result in longer improvement in a study in both children and adults (Australas. J. Dermatol. 2004;45:208-12); however, this poses a greater risk of systemic absorption in children.
"Interestingly enough, some insurance companies will cover iontophoresis if you add glycopyrrolate to the water, but they won’t cover regular iontophoresis," Dr. Bellet said.
In response to audience inquiries, she indicated she uses the Fischer MD-1a galvanic unit. It’s simple to operate, costs about $700 including accessories, and can be rented with the payments applied to a later purchase.
Botulinum toxin A: First using this product for hyperhidrosis is off-label therapy, Dr. Bellet emphasized. Second, the doses required to treat palmar/plantar hyperhidrosis – 75-100 units per palm or sole – are much higher than in treating the axillae. Also, injecting botulinum toxin A into the palms and soles is extraordinarily painful. Children typically require general anesthesia, because the alternative methods employed with mixed results in adults, including EMLA cream, ice packs, and ethyl chloride spray, don’t cut it in younger patients, Dr. Bellet noted.
That being said, botulinum toxin A therapy works quite well in children and adolescents. However, it’s important to explain to patients and parents up front that the injections can cause transient weakness of the hand muscles because of the diffusion of the toxin from dermis to muscles; for a budding pianist or baseball pitcher, that can be a deal-breaker, she said. Also, Dr. Bellet added, repeated injections can result in atrophy of the thenar and hypothenar eminences, with resultant irreversible weakness.
Thoracic sympathectomy: Not many American thoracic surgeons do sympathectomy to treat hyperhidrosis on a frequent basis, but those with extensive experience obtain outstanding results, Dr. Bellet said. To treat palmar hyperhidrosis, the nerve is clipped at the top of the third or fourth rib; for the soles, it’s at the fourth and fifth rib. The youngest reported treated patients have been 8 years old.
Dr. Bellet recommended the International Hyperhidrosis Society (www.sweathelp.org) as an excellent source of further information.
Dr. Bellet reported having no financial conflicts of interest regarding her presentation.
How do you treat palmar/plantar hyperhidrosis in children? Take our Quick Poll on the Skin & Allergy News homepage.
COEUR D’ALENE, IDAHO – By the time patients seek a physician’s help for palmar/plantar focal hyperhidrosis, they may be well beyond the point at which even potent topical antiperspirants will help.
"Most people think there is nothing that can be done at all [for these patients]. But there are effective options. Even if you’re not going to offer them, get your patients to someone who will. We can make a profound difference for children and adolescents," Dr. Jane S. Bellet said at the annual meeting of the Society for Pediatric Dermatology.
In fact, more potent therapies are available, ranging from iontophoresis to oral medications, botulinum toxin A injections, and surgical thoracic sympathetectomy.
Oral medications: "I’ve really changed my practice in the last 5 years. I didn’t use systemic medications very much. I use them much more frequently now. I think you can get a very nice response," said Dr. Bellet, a pediatric dermatologist at Duke University in Durham, N.C.
She typically turns to the anticholinergic agents glycopyrrolate and oxybutynin. Glycopyrrolate, marketed as Cuvposa in a cherry-flavored solution at 1 mg/5 mL, does not have an indication from the Food and Drug Administration for treatment of pediatric hyperhidrosis, she noted. However, it is FDA-approved in 3- to 16-year-olds for severe chronic drooling caused by neurologic disorders.
"Although we don’t have an indication for hyperhidrosis, we do have a pediatric indication, and I think many times that puts our parents at ease," she observed.
Glycopyrrolate is cost effective and painless, although approximately 30% of children treated for hyperhidrosis will develop dry mouth and/or dry eyes.
"I would definitely add glycopyrrolate to your armamentarium. I think the biggest concern most of us have is the side effects. Speak about them with the family ahead of time, guide them as to what to expect, and stop if it becomes intolerable," Dr. Bellet said.
A recent randomized, prospective, controlled clinical trial in 45 children aged 7-14 years with palmar hyperhidrosis showed excellent outcomes with oxybutynin (Ditropan), with more than 85% experiencing at least moderate improvement in sweating and 80% gaining improved quality of life (Pediatr. Dermatol. 2014;31:48-53).
Iontophoresis: "This is a wonderful treatment for palms and soles," said Dr. Bellet.
The treatment entails placing the hands or feet in a water bath tray filled with tap water through which direct electric current is running. The proposed mechanism of benefit is that hydrolysis of the bath water results in accumulation of hydrogen ions, which then induce sweat gland destruction.
The chief disadvantage of iontophoresis is that it is labor intensive. Unless the family rents or buys a home unit, the patient typically must visit the physician’s office on a daily basis initially, placing the hands or feet in the bath for 10 minutes, followed by a second 10-minute round after the polarity is switched. Eventually, many patients can step down to two or three 20- to 30-minute sessions per week, then perhaps once-weekly maintenance therapy, she said.
Adding glycopyrrolate to the water bath has been shown to result in longer improvement in a study in both children and adults (Australas. J. Dermatol. 2004;45:208-12); however, this poses a greater risk of systemic absorption in children.
"Interestingly enough, some insurance companies will cover iontophoresis if you add glycopyrrolate to the water, but they won’t cover regular iontophoresis," Dr. Bellet said.
In response to audience inquiries, she indicated she uses the Fischer MD-1a galvanic unit. It’s simple to operate, costs about $700 including accessories, and can be rented with the payments applied to a later purchase.
Botulinum toxin A: First using this product for hyperhidrosis is off-label therapy, Dr. Bellet emphasized. Second, the doses required to treat palmar/plantar hyperhidrosis – 75-100 units per palm or sole – are much higher than in treating the axillae. Also, injecting botulinum toxin A into the palms and soles is extraordinarily painful. Children typically require general anesthesia, because the alternative methods employed with mixed results in adults, including EMLA cream, ice packs, and ethyl chloride spray, don’t cut it in younger patients, Dr. Bellet noted.
That being said, botulinum toxin A therapy works quite well in children and adolescents. However, it’s important to explain to patients and parents up front that the injections can cause transient weakness of the hand muscles because of the diffusion of the toxin from dermis to muscles; for a budding pianist or baseball pitcher, that can be a deal-breaker, she said. Also, Dr. Bellet added, repeated injections can result in atrophy of the thenar and hypothenar eminences, with resultant irreversible weakness.
Thoracic sympathectomy: Not many American thoracic surgeons do sympathectomy to treat hyperhidrosis on a frequent basis, but those with extensive experience obtain outstanding results, Dr. Bellet said. To treat palmar hyperhidrosis, the nerve is clipped at the top of the third or fourth rib; for the soles, it’s at the fourth and fifth rib. The youngest reported treated patients have been 8 years old.
Dr. Bellet recommended the International Hyperhidrosis Society (www.sweathelp.org) as an excellent source of further information.
Dr. Bellet reported having no financial conflicts of interest regarding her presentation.
How do you treat palmar/plantar hyperhidrosis in children? Take our Quick Poll on the Skin & Allergy News homepage.
COEUR D’ALENE, IDAHO – By the time patients seek a physician’s help for palmar/plantar focal hyperhidrosis, they may be well beyond the point at which even potent topical antiperspirants will help.
"Most people think there is nothing that can be done at all [for these patients]. But there are effective options. Even if you’re not going to offer them, get your patients to someone who will. We can make a profound difference for children and adolescents," Dr. Jane S. Bellet said at the annual meeting of the Society for Pediatric Dermatology.
In fact, more potent therapies are available, ranging from iontophoresis to oral medications, botulinum toxin A injections, and surgical thoracic sympathetectomy.
Oral medications: "I’ve really changed my practice in the last 5 years. I didn’t use systemic medications very much. I use them much more frequently now. I think you can get a very nice response," said Dr. Bellet, a pediatric dermatologist at Duke University in Durham, N.C.
She typically turns to the anticholinergic agents glycopyrrolate and oxybutynin. Glycopyrrolate, marketed as Cuvposa in a cherry-flavored solution at 1 mg/5 mL, does not have an indication from the Food and Drug Administration for treatment of pediatric hyperhidrosis, she noted. However, it is FDA-approved in 3- to 16-year-olds for severe chronic drooling caused by neurologic disorders.
"Although we don’t have an indication for hyperhidrosis, we do have a pediatric indication, and I think many times that puts our parents at ease," she observed.
Glycopyrrolate is cost effective and painless, although approximately 30% of children treated for hyperhidrosis will develop dry mouth and/or dry eyes.
"I would definitely add glycopyrrolate to your armamentarium. I think the biggest concern most of us have is the side effects. Speak about them with the family ahead of time, guide them as to what to expect, and stop if it becomes intolerable," Dr. Bellet said.
A recent randomized, prospective, controlled clinical trial in 45 children aged 7-14 years with palmar hyperhidrosis showed excellent outcomes with oxybutynin (Ditropan), with more than 85% experiencing at least moderate improvement in sweating and 80% gaining improved quality of life (Pediatr. Dermatol. 2014;31:48-53).
Iontophoresis: "This is a wonderful treatment for palms and soles," said Dr. Bellet.
The treatment entails placing the hands or feet in a water bath tray filled with tap water through which direct electric current is running. The proposed mechanism of benefit is that hydrolysis of the bath water results in accumulation of hydrogen ions, which then induce sweat gland destruction.
The chief disadvantage of iontophoresis is that it is labor intensive. Unless the family rents or buys a home unit, the patient typically must visit the physician’s office on a daily basis initially, placing the hands or feet in the bath for 10 minutes, followed by a second 10-minute round after the polarity is switched. Eventually, many patients can step down to two or three 20- to 30-minute sessions per week, then perhaps once-weekly maintenance therapy, she said.
Adding glycopyrrolate to the water bath has been shown to result in longer improvement in a study in both children and adults (Australas. J. Dermatol. 2004;45:208-12); however, this poses a greater risk of systemic absorption in children.
"Interestingly enough, some insurance companies will cover iontophoresis if you add glycopyrrolate to the water, but they won’t cover regular iontophoresis," Dr. Bellet said.
In response to audience inquiries, she indicated she uses the Fischer MD-1a galvanic unit. It’s simple to operate, costs about $700 including accessories, and can be rented with the payments applied to a later purchase.
Botulinum toxin A: First using this product for hyperhidrosis is off-label therapy, Dr. Bellet emphasized. Second, the doses required to treat palmar/plantar hyperhidrosis – 75-100 units per palm or sole – are much higher than in treating the axillae. Also, injecting botulinum toxin A into the palms and soles is extraordinarily painful. Children typically require general anesthesia, because the alternative methods employed with mixed results in adults, including EMLA cream, ice packs, and ethyl chloride spray, don’t cut it in younger patients, Dr. Bellet noted.
That being said, botulinum toxin A therapy works quite well in children and adolescents. However, it’s important to explain to patients and parents up front that the injections can cause transient weakness of the hand muscles because of the diffusion of the toxin from dermis to muscles; for a budding pianist or baseball pitcher, that can be a deal-breaker, she said. Also, Dr. Bellet added, repeated injections can result in atrophy of the thenar and hypothenar eminences, with resultant irreversible weakness.
Thoracic sympathectomy: Not many American thoracic surgeons do sympathectomy to treat hyperhidrosis on a frequent basis, but those with extensive experience obtain outstanding results, Dr. Bellet said. To treat palmar hyperhidrosis, the nerve is clipped at the top of the third or fourth rib; for the soles, it’s at the fourth and fifth rib. The youngest reported treated patients have been 8 years old.
Dr. Bellet recommended the International Hyperhidrosis Society (www.sweathelp.org) as an excellent source of further information.
Dr. Bellet reported having no financial conflicts of interest regarding her presentation.
How do you treat palmar/plantar hyperhidrosis in children? Take our Quick Poll on the Skin & Allergy News homepage.
EXPERT ANALYSIS FROM THE SPD ANNUAL MEETING
Botulinum toxin for men
Men make up an increasing number of dermatology patients seeking cosmetic procedures. According to data from the American Society for Dermatologic Surgery and the American Society of Plastic Surgery, 9%-10% of all cosmetic procedures performed in the United States in 2013 were on men, a 104% increase since 2000. Botulinum toxin is currently the most common minimally invasive cosmetic procedure performed in men. While the overall percentage of men undergoing treatment, compared with women, is relatively small, more than 385,000 botulinum toxin treatments were performed on men last year in the United States, an increase of 310% since 2000.
Studies have shown that men often require more units of onabotulinumtoxinA than women when treating the glabella. In a 2005 study by Alstair and Jean Carruthers, 80 men were randomized to receive 20, 40, 60, or 80 units of onabotulinumtoxinA (Botox or Vistabel). The 40-, 60-, and 80-U doses were consistently more effective than the 20-U dose was in reducing glabellar lines (duration, peak response rate, and improvement from baseline). I find this to be true in my practice. Men may often require 20-60 U in the superficial corrugator and procerus muscles, compared with 20-30 units of onabotulinumtoxinA in the same muscles in women. For the frontalis muscles, I may use 5-20 U in men, compared with 5-10 U of onabotulinumtoxinA in women, but I take care not to inject too inferiorly to avoid a heavy brow or brow ptosis. The orbicularis oculi muscles often require the similar doses of between 6-15 U (most often 12 U/side) depending on degree of muscle contraction and severity of rhytids.
In addition to differences in botulinum toxin dosing between men and women, placement of toxin also may vary. Placement in the superficial corrugator, procerus, frontalis, and orbicularis oculi muscles are often the similar and patient dependent, based on a visual assessment of where their muscles move/contract. The superficial corrugator may insert more laterally in some men, and the brow is often straighter or less arched. The difference in injection site is often at the lateral brow. In women, botulinum toxin is often injected at the lateral brow at the junction where the lateral superior portion of the orbicularis oculi muscle and frontalis meet, in order to help give the brow a "lift." Men, however, often do not want raised or arched brow. Therefore, an injection is often placed about 1 cm above the lateral brow to maintain brow position and avoid overarching, or what some may regard as feminization of the male brow.
The depressor anguli oris and orbicularis oris muscles are injected less frequently in men than in women in my practice because of seemingly decreased rhytid formation in men in these locations. The platysmal muscles may be injected in men, but their average age is older compared with women who receive injections in this location.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
Men make up an increasing number of dermatology patients seeking cosmetic procedures. According to data from the American Society for Dermatologic Surgery and the American Society of Plastic Surgery, 9%-10% of all cosmetic procedures performed in the United States in 2013 were on men, a 104% increase since 2000. Botulinum toxin is currently the most common minimally invasive cosmetic procedure performed in men. While the overall percentage of men undergoing treatment, compared with women, is relatively small, more than 385,000 botulinum toxin treatments were performed on men last year in the United States, an increase of 310% since 2000.
Studies have shown that men often require more units of onabotulinumtoxinA than women when treating the glabella. In a 2005 study by Alstair and Jean Carruthers, 80 men were randomized to receive 20, 40, 60, or 80 units of onabotulinumtoxinA (Botox or Vistabel). The 40-, 60-, and 80-U doses were consistently more effective than the 20-U dose was in reducing glabellar lines (duration, peak response rate, and improvement from baseline). I find this to be true in my practice. Men may often require 20-60 U in the superficial corrugator and procerus muscles, compared with 20-30 units of onabotulinumtoxinA in the same muscles in women. For the frontalis muscles, I may use 5-20 U in men, compared with 5-10 U of onabotulinumtoxinA in women, but I take care not to inject too inferiorly to avoid a heavy brow or brow ptosis. The orbicularis oculi muscles often require the similar doses of between 6-15 U (most often 12 U/side) depending on degree of muscle contraction and severity of rhytids.
In addition to differences in botulinum toxin dosing between men and women, placement of toxin also may vary. Placement in the superficial corrugator, procerus, frontalis, and orbicularis oculi muscles are often the similar and patient dependent, based on a visual assessment of where their muscles move/contract. The superficial corrugator may insert more laterally in some men, and the brow is often straighter or less arched. The difference in injection site is often at the lateral brow. In women, botulinum toxin is often injected at the lateral brow at the junction where the lateral superior portion of the orbicularis oculi muscle and frontalis meet, in order to help give the brow a "lift." Men, however, often do not want raised or arched brow. Therefore, an injection is often placed about 1 cm above the lateral brow to maintain brow position and avoid overarching, or what some may regard as feminization of the male brow.
The depressor anguli oris and orbicularis oris muscles are injected less frequently in men than in women in my practice because of seemingly decreased rhytid formation in men in these locations. The platysmal muscles may be injected in men, but their average age is older compared with women who receive injections in this location.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
Men make up an increasing number of dermatology patients seeking cosmetic procedures. According to data from the American Society for Dermatologic Surgery and the American Society of Plastic Surgery, 9%-10% of all cosmetic procedures performed in the United States in 2013 were on men, a 104% increase since 2000. Botulinum toxin is currently the most common minimally invasive cosmetic procedure performed in men. While the overall percentage of men undergoing treatment, compared with women, is relatively small, more than 385,000 botulinum toxin treatments were performed on men last year in the United States, an increase of 310% since 2000.
Studies have shown that men often require more units of onabotulinumtoxinA than women when treating the glabella. In a 2005 study by Alstair and Jean Carruthers, 80 men were randomized to receive 20, 40, 60, or 80 units of onabotulinumtoxinA (Botox or Vistabel). The 40-, 60-, and 80-U doses were consistently more effective than the 20-U dose was in reducing glabellar lines (duration, peak response rate, and improvement from baseline). I find this to be true in my practice. Men may often require 20-60 U in the superficial corrugator and procerus muscles, compared with 20-30 units of onabotulinumtoxinA in the same muscles in women. For the frontalis muscles, I may use 5-20 U in men, compared with 5-10 U of onabotulinumtoxinA in women, but I take care not to inject too inferiorly to avoid a heavy brow or brow ptosis. The orbicularis oculi muscles often require the similar doses of between 6-15 U (most often 12 U/side) depending on degree of muscle contraction and severity of rhytids.
In addition to differences in botulinum toxin dosing between men and women, placement of toxin also may vary. Placement in the superficial corrugator, procerus, frontalis, and orbicularis oculi muscles are often the similar and patient dependent, based on a visual assessment of where their muscles move/contract. The superficial corrugator may insert more laterally in some men, and the brow is often straighter or less arched. The difference in injection site is often at the lateral brow. In women, botulinum toxin is often injected at the lateral brow at the junction where the lateral superior portion of the orbicularis oculi muscle and frontalis meet, in order to help give the brow a "lift." Men, however, often do not want raised or arched brow. Therefore, an injection is often placed about 1 cm above the lateral brow to maintain brow position and avoid overarching, or what some may regard as feminization of the male brow.
The depressor anguli oris and orbicularis oris muscles are injected less frequently in men than in women in my practice because of seemingly decreased rhytid formation in men in these locations. The platysmal muscles may be injected in men, but their average age is older compared with women who receive injections in this location.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
AUDIO: Unusual approaches to unusual tumors
CHICAGO – Doctors who don’t abide by accepted norms can sometimes achieve better outcomes when treating patients who have unusual and tenacious tumors. This was the theme of a session covering cases of confounding tumors at the American Academy of Dermatology summer meeting.
In an interview after the session, panel moderator and case presenter Dr. John A. Carucci, chief of Mohs and dermatologic surgery at New York (N.Y.) University, shared his thoughts on when certain kinds of imaging are more appropriate than others, even if it’s not the "usual way."
Dr. Carucci also addressed the use of postsurgical negative pressure wound therapy, radiation therapy in conjunction with Mohs surgery, and potentially controversial topics such as the use of a protein kinase inhibitor as a neoadjuvant therapy.
And what new information on staging squamous cell carcinomas has Dr. Carucci and his colleagues excited? Listen and find out. Dr. Carucci said that he had no financial conflicts to disclose.
On Twitter @whitneymcknight
CHICAGO – Doctors who don’t abide by accepted norms can sometimes achieve better outcomes when treating patients who have unusual and tenacious tumors. This was the theme of a session covering cases of confounding tumors at the American Academy of Dermatology summer meeting.
In an interview after the session, panel moderator and case presenter Dr. John A. Carucci, chief of Mohs and dermatologic surgery at New York (N.Y.) University, shared his thoughts on when certain kinds of imaging are more appropriate than others, even if it’s not the "usual way."
Dr. Carucci also addressed the use of postsurgical negative pressure wound therapy, radiation therapy in conjunction with Mohs surgery, and potentially controversial topics such as the use of a protein kinase inhibitor as a neoadjuvant therapy.
And what new information on staging squamous cell carcinomas has Dr. Carucci and his colleagues excited? Listen and find out. Dr. Carucci said that he had no financial conflicts to disclose.
On Twitter @whitneymcknight
CHICAGO – Doctors who don’t abide by accepted norms can sometimes achieve better outcomes when treating patients who have unusual and tenacious tumors. This was the theme of a session covering cases of confounding tumors at the American Academy of Dermatology summer meeting.
In an interview after the session, panel moderator and case presenter Dr. John A. Carucci, chief of Mohs and dermatologic surgery at New York (N.Y.) University, shared his thoughts on when certain kinds of imaging are more appropriate than others, even if it’s not the "usual way."
Dr. Carucci also addressed the use of postsurgical negative pressure wound therapy, radiation therapy in conjunction with Mohs surgery, and potentially controversial topics such as the use of a protein kinase inhibitor as a neoadjuvant therapy.
And what new information on staging squamous cell carcinomas has Dr. Carucci and his colleagues excited? Listen and find out. Dr. Carucci said that he had no financial conflicts to disclose.
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2014
Linear Scarring Following Treatment With a 595-nm Pulsed Dye Laser
The pulsed dye laser (PDL) has been widely used in the treatment of port-wine stains, telangiectases, and other cutaneous vascular lesions since the late 1980s.1 This treatment modality generally is considered to have few serious adverse effects. There have been few reports of PDL treatment with subsequent complications,1-3 which may include ulceration developing immediately after treatment as well as scarring with a spotlike pattern caused by laser therapy. Numerous studies within the last 2 decades have documented improvement in the appearance of scars and telangiectases following treatment with PDL.4-6 We report the case of a 42-year-old woman who developed atrophic linear scarring of the nasal ala following cosmetic treatment with a 595-nm PDL.
Case Report
A healthy 42-year-old woman presented with atrophic linear scarring of the bilateral nasal alae following treatment with a 595-nm PDL. The patient had initially presented to an outside clinic 13 months prior for treatment of multiple telangiectases in this area. She received a single, 1-pass treatment with the 595-nm PDL (spot size, 3×10 mm; fluence, 11 J/cm2; pulse duration, 1.5 milliseconds) and returned to the clinic approximately 2 months later for a second treatment with the same settings. Seven months later she returned for a third treatment of the recalcitrant alar telangiectases with the same settings to maximize clinical outcome. Dynamic cooling was used during all treatment sessions with 30/20 setting. After the third treatment, immediate blanching followed by purpura was noted in the treated area. The patient initially was lost to follow-up but returned to the outside clinic 6 months later. On physical examination white atrophic skin with linear scarred depressions were noted on the nasofacial angle of the nasal alae (Figure). The patient denied any postoperative complications such as scabbing, blistering, or pain. At that time she was referred to our office for evaluation, and treatment with a hyaluronic acid filler was initiated. Examination and medical history were otherwise unremarkable at the time of presentation to our office. Resolution of skin atrophy and excellent correction of the depressions was maintained at a follow-up 2 months later. She declined photographs at that time.
 |
 |
 |
| Right (A), left (B), and frontal (C) views of atrophic linear scars (arrows) caused by high-energy purpuric doses of a 595-nm pulsed dye laser. |
Comment
The PDL often is employed in the treatment of vascular lesions such as telangiectases.7 The most common adverse effect is postinflammatory hyperpigmentation; atrophic and hypertrophic scarring rarely are seen.1,8,9 In a study of adverse reactions following pulsed tunable dye laser treatment of port-wine stains in 701 patients, atrophic scarring occurred in 5% of patients and 0.83% of treatments; clinical resolution was noted over the following 6 to 12 months in 30% of patients.8
Following treatment with the PDL, thermal damage occurs primarily to vessel walls with little or no damage to surrounding nonvascular structures. The depth of vascular injury after PDL treatment has been shown to be approximately 1.2 mm.10
Although scarring in our patient was a result of PDL treatment, PDL therapy is commonly used as a treatment option for scars. In conjunction with intralesional steroids directed at flattening hypertrophic scars and keloids, the PDL is used to reduce scar redness and enhance pliability.11 Although redness and telangiectases that develop in surgical scars usually spontaneously remit, they often can show prolonged and incomplete healing. Surgical scars have been shown to benefit from PDL treatment as it advances the end point closer to the complete absence of redness.11-14
The off-label use of hyaluronic acid filler in our patient is notable, as the injection of the nasal ala is not an ordinary injection site for this filler material. It can be associated with risk for necrosis and thus must be performed by an experienced injector combined with informed consent from the patient. The nasal ala is particularly sebaceous and consists of fibrofatty tissue, which is not easily amenable to infiltration. Despite this usual characteristic of the nasal area, the scarring in our patient was fortuitously lateral to the nasal ala and easily filled with hyaluronic acid, as a linear tract was created by the high energies and linear spot size used to treat the patient.
We report a 595-nm PDL treatment that resulted in atrophic linear scarring in a distribution mimicking the linear spot size used by the laser operator. No adverse effects were noted following the first 2 treatments, thereby suggesting either a cumulative insult or more likely cutaneous necrosis from excessive fluence and short pulse durations due to operator inexperience. Other possibilities include rapid and overlapping passes with the laser leading to bulk heating and thermal injury to the skin.
Alternative laser treatment protocols have been proposed in the literature. Rohrer et al15 recommended multiple passes at subpurpuric doses for treatment of facial telangiectases with the PDL. It has been suggested that multiple stacked pulses at lower fluences may have similar effects on targets as a single pulse at a higher fluence, thereby minimizing thermal injury and leading to decreased risk for adverse events such as scarring. When treating vascular lesions such as telangiectases, increasing the fluence will increase the risk for purpura due to the constant pulse duration. Stacking pulses of lower fluence may have the advantage of heating vessels to a critical temperature without creating purpura, leading to similar clearance rates with decreased adverse risk profiles.15
It may be better to err on the side of safety by performing a greater number of treatment sessions with increased pulse width and decreased fluence (subpurpuric treatment settings) to minimize the risk for atrophic scarring from treatment with the PDL. Treating superficial facial telangiectases with a pulse-stacking technique may improve clinical results without a remarkable increase in adverse effects. It may be wrongfully intuitive to try to maximize results by using high fluences and purpuric narrow pulse durations; this case report reiterates the danger of using these settings in an attempt to rapidly achieve clearance of telangiectases. Lastly, this case underscores the value of verbal and written postoperative instructions that should be given to every patient prior to undergoing laser therapy. Specifically, with regard to our case, the laser operator must be aware at all times of potential adverse events, which may be foreseen during treatment if persistent or prolonged blanching and/or blistering occurs. The physician operator and patient must be prepared to rapidly respond to adverse reactions such as skin necrosis or blistering. Meticulous wound care is necessary if skin breakdown occurs. We recommend using a hydrating petrolatum ointment or a topical emulsion to minimize the risks for scarring, if possible.
1. Levine VJ, Geronemus RG. Adverse effects associated with the 577- and 585-nanometer pulsed dye laser in the treatment of cutaneous vascular lesions: a study of 500 patients. J Am Acad Dermatol. 1995;32:613-617.
2. Witman PM, Wagner AM, Scherer K, et al. Complications following pulsed dye laser treatment of superficial hemangiomas. Lasers Surg Med. 2006;38:116-123.
3. Sommer S, Sheehan-Dare RA. Atrophie blanche-like scarring after pulsed dye laser treatment. J Am Acad Dermatol. 1999;41:100-102.
4. Dover JS, Geronemus R, Stern RS, et al. Dye laser treatment of port-wine stains: comparison of the continuous-wave dye laser with a robotized scanning device and the pulsed dye laser. J Am Acad Dermatol. 1995;32(2, pt 1):237-240.
5. Dierickx C, Goldman MP, Fitzpatrick RE. Laser treatment of erythematous/hypertrophic and pigmented scars in 26 patients. Plast Reconstr Surg. 1995;95:84-90.
6. Wittenberg GP, Fabian BG, Bogomilsky JL, et al. Prospective, single-blind, randomized, controlled study to assess the efficacy of the 585-nm flashlamp-pumped pulsed-dye laser and silicone gel sheeting in hypertrophic scar treatment. Arch Dermatol. 1999;135:1049-1055.
7. Ross EV, Uebelhoer NS, Domankevitz Y. Use of a novel pulse dye laser for rapid single-pass purpura-free treatment of telangiectases. Dermatol Surg. 2007;33:1466-1469.
8. Seukeran DC, Collins P, Sheehan-Dare RA. Adverse reactions following pulsed tunable dye laser treatment of port wine stains in 701 patients. Br J Dermatol. 1997;136:725-729.
9. Wlotzke U, Hohenleutner U, Abd-El-Raheem TA, et al. Side-effects and complications of flashlamp-pumped pulsed dye laser therapy of port-wine stains. a prospective study. Br J Dermatol. 1996;134:475-480.
10. Tan OT, Morrison P, Kurban AK. 585 nm for the treatment of port-wine stains. Plast Reconstr Surg. 1990;86:1112-1117.
11. Alam M, Pon K, Van Laborde S, et al. Clinical effect of a single pulsed dye laser treatment of fresh surgical scars: randomized controlled trial. Dermatol Surg. 2006;32:21-25.
12. Bowes LE, Nouri K, Berman B, et al. Treatment of pigmented hypertrophic scars with the 585 nm pulsed dye laser and the 532 nm frequency-doubled Nd:YAG laser in the Q-switched and variable pulse modes: a comparative study. Dermatol Surg. 2002;28:714-719.
13. Alster TS, Williams CM. Treatment of keloid sternotomy scars with 585 nm flashlamp-pumped pulsed-dye laser. Lancet. 1995;345:1198-1200.
14. Manuskiatti W, Fitzpatrick RE. Treatment response of keloidal and hypertrophic sternotomy scars: comparison among intralesional corticosteroid, 5-fluorouracil, and 585-nm flashlamp-pumped pulsed-dye laser treatments. Arch Dermatol. 2002;138:1149-1155.
15. Rohrer TE, Chatrath V, Iyengar V. Does pulse stacking improve the results of treatment with variable-pulse pulsed-dye lasers? Dermatol Surg. 2004;30(2, pt 1):163-167.
The pulsed dye laser (PDL) has been widely used in the treatment of port-wine stains, telangiectases, and other cutaneous vascular lesions since the late 1980s.1 This treatment modality generally is considered to have few serious adverse effects. There have been few reports of PDL treatment with subsequent complications,1-3 which may include ulceration developing immediately after treatment as well as scarring with a spotlike pattern caused by laser therapy. Numerous studies within the last 2 decades have documented improvement in the appearance of scars and telangiectases following treatment with PDL.4-6 We report the case of a 42-year-old woman who developed atrophic linear scarring of the nasal ala following cosmetic treatment with a 595-nm PDL.
Case Report
A healthy 42-year-old woman presented with atrophic linear scarring of the bilateral nasal alae following treatment with a 595-nm PDL. The patient had initially presented to an outside clinic 13 months prior for treatment of multiple telangiectases in this area. She received a single, 1-pass treatment with the 595-nm PDL (spot size, 3×10 mm; fluence, 11 J/cm2; pulse duration, 1.5 milliseconds) and returned to the clinic approximately 2 months later for a second treatment with the same settings. Seven months later she returned for a third treatment of the recalcitrant alar telangiectases with the same settings to maximize clinical outcome. Dynamic cooling was used during all treatment sessions with 30/20 setting. After the third treatment, immediate blanching followed by purpura was noted in the treated area. The patient initially was lost to follow-up but returned to the outside clinic 6 months later. On physical examination white atrophic skin with linear scarred depressions were noted on the nasofacial angle of the nasal alae (Figure). The patient denied any postoperative complications such as scabbing, blistering, or pain. At that time she was referred to our office for evaluation, and treatment with a hyaluronic acid filler was initiated. Examination and medical history were otherwise unremarkable at the time of presentation to our office. Resolution of skin atrophy and excellent correction of the depressions was maintained at a follow-up 2 months later. She declined photographs at that time.
 |
 |
 |
| Right (A), left (B), and frontal (C) views of atrophic linear scars (arrows) caused by high-energy purpuric doses of a 595-nm pulsed dye laser. |
Comment
The PDL often is employed in the treatment of vascular lesions such as telangiectases.7 The most common adverse effect is postinflammatory hyperpigmentation; atrophic and hypertrophic scarring rarely are seen.1,8,9 In a study of adverse reactions following pulsed tunable dye laser treatment of port-wine stains in 701 patients, atrophic scarring occurred in 5% of patients and 0.83% of treatments; clinical resolution was noted over the following 6 to 12 months in 30% of patients.8
Following treatment with the PDL, thermal damage occurs primarily to vessel walls with little or no damage to surrounding nonvascular structures. The depth of vascular injury after PDL treatment has been shown to be approximately 1.2 mm.10
Although scarring in our patient was a result of PDL treatment, PDL therapy is commonly used as a treatment option for scars. In conjunction with intralesional steroids directed at flattening hypertrophic scars and keloids, the PDL is used to reduce scar redness and enhance pliability.11 Although redness and telangiectases that develop in surgical scars usually spontaneously remit, they often can show prolonged and incomplete healing. Surgical scars have been shown to benefit from PDL treatment as it advances the end point closer to the complete absence of redness.11-14
The off-label use of hyaluronic acid filler in our patient is notable, as the injection of the nasal ala is not an ordinary injection site for this filler material. It can be associated with risk for necrosis and thus must be performed by an experienced injector combined with informed consent from the patient. The nasal ala is particularly sebaceous and consists of fibrofatty tissue, which is not easily amenable to infiltration. Despite this usual characteristic of the nasal area, the scarring in our patient was fortuitously lateral to the nasal ala and easily filled with hyaluronic acid, as a linear tract was created by the high energies and linear spot size used to treat the patient.
We report a 595-nm PDL treatment that resulted in atrophic linear scarring in a distribution mimicking the linear spot size used by the laser operator. No adverse effects were noted following the first 2 treatments, thereby suggesting either a cumulative insult or more likely cutaneous necrosis from excessive fluence and short pulse durations due to operator inexperience. Other possibilities include rapid and overlapping passes with the laser leading to bulk heating and thermal injury to the skin.
Alternative laser treatment protocols have been proposed in the literature. Rohrer et al15 recommended multiple passes at subpurpuric doses for treatment of facial telangiectases with the PDL. It has been suggested that multiple stacked pulses at lower fluences may have similar effects on targets as a single pulse at a higher fluence, thereby minimizing thermal injury and leading to decreased risk for adverse events such as scarring. When treating vascular lesions such as telangiectases, increasing the fluence will increase the risk for purpura due to the constant pulse duration. Stacking pulses of lower fluence may have the advantage of heating vessels to a critical temperature without creating purpura, leading to similar clearance rates with decreased adverse risk profiles.15
It may be better to err on the side of safety by performing a greater number of treatment sessions with increased pulse width and decreased fluence (subpurpuric treatment settings) to minimize the risk for atrophic scarring from treatment with the PDL. Treating superficial facial telangiectases with a pulse-stacking technique may improve clinical results without a remarkable increase in adverse effects. It may be wrongfully intuitive to try to maximize results by using high fluences and purpuric narrow pulse durations; this case report reiterates the danger of using these settings in an attempt to rapidly achieve clearance of telangiectases. Lastly, this case underscores the value of verbal and written postoperative instructions that should be given to every patient prior to undergoing laser therapy. Specifically, with regard to our case, the laser operator must be aware at all times of potential adverse events, which may be foreseen during treatment if persistent or prolonged blanching and/or blistering occurs. The physician operator and patient must be prepared to rapidly respond to adverse reactions such as skin necrosis or blistering. Meticulous wound care is necessary if skin breakdown occurs. We recommend using a hydrating petrolatum ointment or a topical emulsion to minimize the risks for scarring, if possible.
The pulsed dye laser (PDL) has been widely used in the treatment of port-wine stains, telangiectases, and other cutaneous vascular lesions since the late 1980s.1 This treatment modality generally is considered to have few serious adverse effects. There have been few reports of PDL treatment with subsequent complications,1-3 which may include ulceration developing immediately after treatment as well as scarring with a spotlike pattern caused by laser therapy. Numerous studies within the last 2 decades have documented improvement in the appearance of scars and telangiectases following treatment with PDL.4-6 We report the case of a 42-year-old woman who developed atrophic linear scarring of the nasal ala following cosmetic treatment with a 595-nm PDL.
Case Report
A healthy 42-year-old woman presented with atrophic linear scarring of the bilateral nasal alae following treatment with a 595-nm PDL. The patient had initially presented to an outside clinic 13 months prior for treatment of multiple telangiectases in this area. She received a single, 1-pass treatment with the 595-nm PDL (spot size, 3×10 mm; fluence, 11 J/cm2; pulse duration, 1.5 milliseconds) and returned to the clinic approximately 2 months later for a second treatment with the same settings. Seven months later she returned for a third treatment of the recalcitrant alar telangiectases with the same settings to maximize clinical outcome. Dynamic cooling was used during all treatment sessions with 30/20 setting. After the third treatment, immediate blanching followed by purpura was noted in the treated area. The patient initially was lost to follow-up but returned to the outside clinic 6 months later. On physical examination white atrophic skin with linear scarred depressions were noted on the nasofacial angle of the nasal alae (Figure). The patient denied any postoperative complications such as scabbing, blistering, or pain. At that time she was referred to our office for evaluation, and treatment with a hyaluronic acid filler was initiated. Examination and medical history were otherwise unremarkable at the time of presentation to our office. Resolution of skin atrophy and excellent correction of the depressions was maintained at a follow-up 2 months later. She declined photographs at that time.
 |
 |
 |
| Right (A), left (B), and frontal (C) views of atrophic linear scars (arrows) caused by high-energy purpuric doses of a 595-nm pulsed dye laser. |
Comment
The PDL often is employed in the treatment of vascular lesions such as telangiectases.7 The most common adverse effect is postinflammatory hyperpigmentation; atrophic and hypertrophic scarring rarely are seen.1,8,9 In a study of adverse reactions following pulsed tunable dye laser treatment of port-wine stains in 701 patients, atrophic scarring occurred in 5% of patients and 0.83% of treatments; clinical resolution was noted over the following 6 to 12 months in 30% of patients.8
Following treatment with the PDL, thermal damage occurs primarily to vessel walls with little or no damage to surrounding nonvascular structures. The depth of vascular injury after PDL treatment has been shown to be approximately 1.2 mm.10
Although scarring in our patient was a result of PDL treatment, PDL therapy is commonly used as a treatment option for scars. In conjunction with intralesional steroids directed at flattening hypertrophic scars and keloids, the PDL is used to reduce scar redness and enhance pliability.11 Although redness and telangiectases that develop in surgical scars usually spontaneously remit, they often can show prolonged and incomplete healing. Surgical scars have been shown to benefit from PDL treatment as it advances the end point closer to the complete absence of redness.11-14
The off-label use of hyaluronic acid filler in our patient is notable, as the injection of the nasal ala is not an ordinary injection site for this filler material. It can be associated with risk for necrosis and thus must be performed by an experienced injector combined with informed consent from the patient. The nasal ala is particularly sebaceous and consists of fibrofatty tissue, which is not easily amenable to infiltration. Despite this usual characteristic of the nasal area, the scarring in our patient was fortuitously lateral to the nasal ala and easily filled with hyaluronic acid, as a linear tract was created by the high energies and linear spot size used to treat the patient.
We report a 595-nm PDL treatment that resulted in atrophic linear scarring in a distribution mimicking the linear spot size used by the laser operator. No adverse effects were noted following the first 2 treatments, thereby suggesting either a cumulative insult or more likely cutaneous necrosis from excessive fluence and short pulse durations due to operator inexperience. Other possibilities include rapid and overlapping passes with the laser leading to bulk heating and thermal injury to the skin.
Alternative laser treatment protocols have been proposed in the literature. Rohrer et al15 recommended multiple passes at subpurpuric doses for treatment of facial telangiectases with the PDL. It has been suggested that multiple stacked pulses at lower fluences may have similar effects on targets as a single pulse at a higher fluence, thereby minimizing thermal injury and leading to decreased risk for adverse events such as scarring. When treating vascular lesions such as telangiectases, increasing the fluence will increase the risk for purpura due to the constant pulse duration. Stacking pulses of lower fluence may have the advantage of heating vessels to a critical temperature without creating purpura, leading to similar clearance rates with decreased adverse risk profiles.15
It may be better to err on the side of safety by performing a greater number of treatment sessions with increased pulse width and decreased fluence (subpurpuric treatment settings) to minimize the risk for atrophic scarring from treatment with the PDL. Treating superficial facial telangiectases with a pulse-stacking technique may improve clinical results without a remarkable increase in adverse effects. It may be wrongfully intuitive to try to maximize results by using high fluences and purpuric narrow pulse durations; this case report reiterates the danger of using these settings in an attempt to rapidly achieve clearance of telangiectases. Lastly, this case underscores the value of verbal and written postoperative instructions that should be given to every patient prior to undergoing laser therapy. Specifically, with regard to our case, the laser operator must be aware at all times of potential adverse events, which may be foreseen during treatment if persistent or prolonged blanching and/or blistering occurs. The physician operator and patient must be prepared to rapidly respond to adverse reactions such as skin necrosis or blistering. Meticulous wound care is necessary if skin breakdown occurs. We recommend using a hydrating petrolatum ointment or a topical emulsion to minimize the risks for scarring, if possible.
1. Levine VJ, Geronemus RG. Adverse effects associated with the 577- and 585-nanometer pulsed dye laser in the treatment of cutaneous vascular lesions: a study of 500 patients. J Am Acad Dermatol. 1995;32:613-617.
2. Witman PM, Wagner AM, Scherer K, et al. Complications following pulsed dye laser treatment of superficial hemangiomas. Lasers Surg Med. 2006;38:116-123.
3. Sommer S, Sheehan-Dare RA. Atrophie blanche-like scarring after pulsed dye laser treatment. J Am Acad Dermatol. 1999;41:100-102.
4. Dover JS, Geronemus R, Stern RS, et al. Dye laser treatment of port-wine stains: comparison of the continuous-wave dye laser with a robotized scanning device and the pulsed dye laser. J Am Acad Dermatol. 1995;32(2, pt 1):237-240.
5. Dierickx C, Goldman MP, Fitzpatrick RE. Laser treatment of erythematous/hypertrophic and pigmented scars in 26 patients. Plast Reconstr Surg. 1995;95:84-90.
6. Wittenberg GP, Fabian BG, Bogomilsky JL, et al. Prospective, single-blind, randomized, controlled study to assess the efficacy of the 585-nm flashlamp-pumped pulsed-dye laser and silicone gel sheeting in hypertrophic scar treatment. Arch Dermatol. 1999;135:1049-1055.
7. Ross EV, Uebelhoer NS, Domankevitz Y. Use of a novel pulse dye laser for rapid single-pass purpura-free treatment of telangiectases. Dermatol Surg. 2007;33:1466-1469.
8. Seukeran DC, Collins P, Sheehan-Dare RA. Adverse reactions following pulsed tunable dye laser treatment of port wine stains in 701 patients. Br J Dermatol. 1997;136:725-729.
9. Wlotzke U, Hohenleutner U, Abd-El-Raheem TA, et al. Side-effects and complications of flashlamp-pumped pulsed dye laser therapy of port-wine stains. a prospective study. Br J Dermatol. 1996;134:475-480.
10. Tan OT, Morrison P, Kurban AK. 585 nm for the treatment of port-wine stains. Plast Reconstr Surg. 1990;86:1112-1117.
11. Alam M, Pon K, Van Laborde S, et al. Clinical effect of a single pulsed dye laser treatment of fresh surgical scars: randomized controlled trial. Dermatol Surg. 2006;32:21-25.
12. Bowes LE, Nouri K, Berman B, et al. Treatment of pigmented hypertrophic scars with the 585 nm pulsed dye laser and the 532 nm frequency-doubled Nd:YAG laser in the Q-switched and variable pulse modes: a comparative study. Dermatol Surg. 2002;28:714-719.
13. Alster TS, Williams CM. Treatment of keloid sternotomy scars with 585 nm flashlamp-pumped pulsed-dye laser. Lancet. 1995;345:1198-1200.
14. Manuskiatti W, Fitzpatrick RE. Treatment response of keloidal and hypertrophic sternotomy scars: comparison among intralesional corticosteroid, 5-fluorouracil, and 585-nm flashlamp-pumped pulsed-dye laser treatments. Arch Dermatol. 2002;138:1149-1155.
15. Rohrer TE, Chatrath V, Iyengar V. Does pulse stacking improve the results of treatment with variable-pulse pulsed-dye lasers? Dermatol Surg. 2004;30(2, pt 1):163-167.
1. Levine VJ, Geronemus RG. Adverse effects associated with the 577- and 585-nanometer pulsed dye laser in the treatment of cutaneous vascular lesions: a study of 500 patients. J Am Acad Dermatol. 1995;32:613-617.
2. Witman PM, Wagner AM, Scherer K, et al. Complications following pulsed dye laser treatment of superficial hemangiomas. Lasers Surg Med. 2006;38:116-123.
3. Sommer S, Sheehan-Dare RA. Atrophie blanche-like scarring after pulsed dye laser treatment. J Am Acad Dermatol. 1999;41:100-102.
4. Dover JS, Geronemus R, Stern RS, et al. Dye laser treatment of port-wine stains: comparison of the continuous-wave dye laser with a robotized scanning device and the pulsed dye laser. J Am Acad Dermatol. 1995;32(2, pt 1):237-240.
5. Dierickx C, Goldman MP, Fitzpatrick RE. Laser treatment of erythematous/hypertrophic and pigmented scars in 26 patients. Plast Reconstr Surg. 1995;95:84-90.
6. Wittenberg GP, Fabian BG, Bogomilsky JL, et al. Prospective, single-blind, randomized, controlled study to assess the efficacy of the 585-nm flashlamp-pumped pulsed-dye laser and silicone gel sheeting in hypertrophic scar treatment. Arch Dermatol. 1999;135:1049-1055.
7. Ross EV, Uebelhoer NS, Domankevitz Y. Use of a novel pulse dye laser for rapid single-pass purpura-free treatment of telangiectases. Dermatol Surg. 2007;33:1466-1469.
8. Seukeran DC, Collins P, Sheehan-Dare RA. Adverse reactions following pulsed tunable dye laser treatment of port wine stains in 701 patients. Br J Dermatol. 1997;136:725-729.
9. Wlotzke U, Hohenleutner U, Abd-El-Raheem TA, et al. Side-effects and complications of flashlamp-pumped pulsed dye laser therapy of port-wine stains. a prospective study. Br J Dermatol. 1996;134:475-480.
10. Tan OT, Morrison P, Kurban AK. 585 nm for the treatment of port-wine stains. Plast Reconstr Surg. 1990;86:1112-1117.
11. Alam M, Pon K, Van Laborde S, et al. Clinical effect of a single pulsed dye laser treatment of fresh surgical scars: randomized controlled trial. Dermatol Surg. 2006;32:21-25.
12. Bowes LE, Nouri K, Berman B, et al. Treatment of pigmented hypertrophic scars with the 585 nm pulsed dye laser and the 532 nm frequency-doubled Nd:YAG laser in the Q-switched and variable pulse modes: a comparative study. Dermatol Surg. 2002;28:714-719.
13. Alster TS, Williams CM. Treatment of keloid sternotomy scars with 585 nm flashlamp-pumped pulsed-dye laser. Lancet. 1995;345:1198-1200.
14. Manuskiatti W, Fitzpatrick RE. Treatment response of keloidal and hypertrophic sternotomy scars: comparison among intralesional corticosteroid, 5-fluorouracil, and 585-nm flashlamp-pumped pulsed-dye laser treatments. Arch Dermatol. 2002;138:1149-1155.
15. Rohrer TE, Chatrath V, Iyengar V. Does pulse stacking improve the results of treatment with variable-pulse pulsed-dye lasers? Dermatol Surg. 2004;30(2, pt 1):163-167.
Practice Points
- Lasers should be used by experienced operators and treatments should be tailored to individual patient needs.
- Multiple passes at subpurpuric settings with the pulsed dye laser may lead to safer results with fewer adverse events and at the same time more tolerable treatments for the patient by minimizing downtime associated with purpura.
- Although scarring is rare, it can occur and should be part of the patient’s informed consent prior to treatment.
Interventions for the Treatment of Stretch Marks: A Systematic Review
Stretch marks (striae cutis distensae) are a common disfiguring skin condition characterized by linear bands of atrophic-appearing skin.1 The prevalence of stretch marks associated with pregnancy ranges from 50% to 90%.2 Although stretch marks do not pose a health risk, they often cause burning, itching, and emotional distress, and they can have a deep psychological impact on patients, particularly in young healthy women who are commonly affected by this condition.3
The cause of stretch marks currently is unknown, but they are known to develop in a variety of physiological and pathological states (eg, pregnancy, adolescent growth spurts, obesity, large weight gain, Cushing syndrome, Marfan syndrome, diabetes mellitus, long-term systemic or topical steroid use).2-5 Clinically, newly formed stretch marks present as pink or purple linear lesions without substantial depression of the skin (striae rubra). Over time, the lesions lose their pigmentation, becoming depressed, atrophic, and white (striae alba).2,3,6 The most commonly affected sites are the breasts, upper arms, abdomen, buttocks, and thighs.3,4
Regardless of the etiology, the same histologic changes can be noted in the epidermis of all stretch marks, such as atrophy and loss of rete ridges, with features that are similar to scarring.3 Additionally, reorganization and diminution of the elastic fiber network of skin can be observed.7
A variety of treatment strategies are available for stretch marks, including topical preparations (eg, tretinoin, glycolic acid) and lasers.4 With current methods, no consistently effective therapies have been established yet. In this article, we present the results of a systematic review of the literature to address the effectiveness and safety of the available treatment options for stretch marks.
Methods
A literature search for randomized controlled trials (RCTs) related to the treatment of stretch marks was conducted on March 13, 2013, using the Cochrane Central Register of Controlled Trials, PubMed (from 1966), Embase (from 1974), Chinese Biomedical Literature Database (from 1978), China National Knowledge Infrastructure (from 1994), Chinese Science Journals Database (from 1989), and Wanfang Data (from 1995). Search terms included stretch marks, stretch mark, striae atrophicae, striae distensae, striae gravidarum, striae rubra, striae alba, lineae albicantes, striae, kikkisa, and random*.
We attempted to contact the original investigators of the 25 articles assessed for eligibility by e-mail to identify the randomization and answer other methodology questions to ensure that the studies included in the analysis were RCTs. Each of the 8 RCTs selected for inclusion was assessed independently by 2 investigators (L.L. and H.M.), and data extraction also was performed independently. Any differences in opinion were resolved by discussion. The risk of biases were assessed and 5 domains—random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data—were judged for each study included in the analysis using the Cochrane Collaboration’s domain-based evaluation tool as described in the Cochrane Handbook for Systematic Reviews of Interventions.8 Publication bias was not assessed due to insufficient data.
Studies ultimately were classified into 3 categories based on the risk of bias: (1) low risk of bias or low risk of bias for all key domains; (2) unclear risk of bias or unclear risk of bias for 1 or more key domains; and (3) high risk of bias or high risk of 1 or more key domains.
Results
Search Results
Figure 1 presents the literature search results. Of 300 total search results, 8 RCTs were selected for assessment,9-16 which included a total of 240 patients (Table). The investigators of all 8 reports were contacted, but only 2 responses were received.11,14 The full text of one article could not be obtained; therefore, we could not confirm that it was a true RCT and excluded it.17
Risk of Bias
The risk of bias in methodology was evaluated for all 8 RCTs and the judgments were given for each domain (Figure 2). All the included studies claimed to be RCTs, but only 37.5% (3/8) of them used adequate randomizations, which were from a including computer-generated code,10 a table of randomized numbers,13 or the Microsoft Excel RND function (from the author by e-mail).14 The randomization methods in the other 5 studies were unclear. Allocation concealment was adequate in 1 trial13 but was unclear in the others. Three trials were double-blinded with the participants and outcome assessors blinded10,13,16; in 2 of these studies investigators also were blinded.10,13 There were 5 single-blinded trials; in 3 of these trials the outcome assessors were blinded12,14,15 and 1 was investigator-blinded.9 The other study was stated to be single-blinded but with no further detail.11 Due to the nature of the experimental design in 2 of the trials12,15 (ie, effects of laser therapy compared to topical treatment or no therapy), participants could not be blinded to treatment types; however, participants were blinded in 1 trial that compared different types of lasers.16 Investigators from all studies reported participants who did not complete the trial or were lost to follow-up, ranging from 0% to 65.6%. Two trials reported no loss of follow-up.11,12 Most trials had losses less than 20% except Pribanich et al13 who reported a loss of 65.6% of participants. One trial included a full analysis set,9 and none of the studies included an intention-to-treat analysis.
The overall risk of bias was assessed for each study and none could be categorized as low risk. Six studies had 1 or more domains assessed as high risk of bias and were classified as high risk of bias.9,11-15 The remaining 2 studies without high-risk domains had one or more domains assessed as unclear10,16 and were therefore considered to be at unclear risk of bias overall.
Effects of Treatments
Among the 8 studies we assessed, there were different treatments, methods of comparison, product concentrations, and times of application. The methods for assessing outcomes (eg, the size and severity of stretch marks) also were varied. Therefore, it is difficult to perform a meta-analysis of the data, and all the evidence was from individual studies. A summary of the results is presented in the Table.
All of the studies we evaluated assessed clinical improvement. Three studies reported the effects of topical tretinoin on stretch marks.10,12,13 A small parallel study with unclear risk of bias indicated that white participants with erythematous stretch marks seemed to have a better response to treatment with tretinoin cream 0.1% for 24 weeks versus placebo.10 However, there was no significant difference between tretinoin cream 0.025% and placebo for patients with abdominal striae in another trial.13 The latter trial was performed with low risk of bias in methodology, but the dropout rate was high (65.6%), with only 11 of 32 participants completing the trial. It is likely that the small number of patients makes the power too low to detect significant differences between tretinoin cream 0.025% and placebo if such a difference indeed existed.13 Because the outcomes in these 2 trials were assessed in different ways, it is difficult to perform a meta-analysis on the data. More adverse effects, mainly erythema and scaling associated with itching or burning sensations, were reported with the higher concentration (0.05%) of tretinoin.10 Another study at a high risk of bias found that the combined use of tretinoin cream 0.05% and glycolic acid 10% was not as effective as fractional CO2 laser therapy in improving the appearance of striae alba.12 There also were 3 studies comparing the effects of laser therapy with another treatment or no treatment.12,15,16 Two within-participant comparison studies with unclear or high risk of bias compared CO2 fractional laser therapy with other active treatment methods in female participants with striae alba.12,16 No difference between the fractional CO2 laser and the 1550-nm nonablative fractional erbium:glass laser was reported,16 but the fractional CO2 laser may be more effective than the topical therapy.12 A small study (11 participants) at high risk of bias reported negative results for the 1450-nm mid-infrared diode laser compared to no treatment.15 Data on the adverse effects of laser therapy were available from these studies. Postinflammatory hyperpigmentation was found in all the 3 studies12,15,16 and posttreatment erythema was mentioned in 2 studies.15,16 Based on the individual studies, treatment with a cosmetic oil formulation was more effective than a moisturizer in improving clinical presentation of stretch marks in white patients.14 Women with striae rubra showed better response to treatment with onion extract cream versus no treatment.9 Limited data from 1 study showed that combined use of Active B (sodium ascorbyl phosphate, 3-aminopropyl-L-ascorbyl phosphate, carboxybetaglucan, hyaluronic acid) and Active A (hyaluronic acid, sodium salt 2 mg, sodium carboxymethyl betaglucan 0.1 mg, ascorbic acid 0.5 mg, arginine 1 mg, sodium chloride 9 mg, sterile water) might be more effective than the use of Active B or placebo.11 These 3 studies are at high risk of bias and no obvious adverse effects were reported.9,11,14
Comment
In the 8 trials included in our assessment, 5 used a within-participant design in which 2 different treatments were randomly administered to the left and right sides of the body, respectively.9,12,14-16 Because the comparison of treatments was made based on results in the same patient versus 2 different treatment groups, the results may be more accurate. In the studies we reviewed, only 3 were placebo-controlled, which may only provide limited evidence on the comparative efficacy of the treatments used in these studies.10,11,13 Most treatments were evaluated in single studies, and most studies had a small number of participants (range, 6–66 participants). A considerable number of the total participants withdrew from their respective studies or were lost during follow-up. In some cases, no reason was given,13 but in the others, it was because of an obvious side effect16 or noncompliance.14 Overall, the methodology quality was low, especially the methods of randomization and allocation concealment. Unsuccessful attempts to contact the original investigators made it difficult to make accurate assessments of the risk of bias in most of the studies included in our assessment. No study met all the risk of bias criteria, and none were classified as having a low risk of bias.
The impact of industry sponsorship on the direction and completeness of the results of the studies we reviewed is unclear. One study was funded by a grant from the manufacturer of the study product,14 and the medication used in another study was supplied by the manufacturer.13 Another study was supported in part by a company that had no part in the conduct, analysis, or reporting of the study.10 In one instance, the authors were employees of the manufacturer of the study product.9 The remaining studies made no declaration.11,12,15,16
Thus the evidence from this review was insufficient to provide clear guidelines for practice. Because the results were based on a small number of patients and were of high or unclear risk of bias, caution must be taken when comparing the efficacy of the treatments administered in these studies; however, given the negligible reported side effects, tretinoin cream 0.1%, a cosmetic oil formulation, onion extract cream, or the combined use of Active A and Active B could reasonably be considered for the treatment of stretch marks. Laser therapies such as the fractional CO2 laser or the 1550-nm fractional erbium:glass laser may be another effective choice.
Conclusion
In future investigations of stretch mark treatments, more high-quality, placebo-controlled trials are needed. One important issue is the varied outcome assessment among different studies, which makes the evaluation and pooling of different studies difficult. Therefore, future RCTs should measure clinical features with a uniform score system such as the visual analog scale or the patient and observer scar assessment scale and try to avoid individual made-up system to assess the outcome. Furthermore, quality-of-life assessment was not included in any of the reports we evaluated; rather all 8 studies focused on changes in the appearance of stretch marks only. Given the deep psychological impact that stretch marks can have on patients, measures for quality-of-life assessment, such as the dermatology life quality index, should be incorporated into future study designs to improve the relevance of the trial and allow comparisons among studies using different interventions.
1. Viennet C, Bride J, Cohen-Letessier A, et al. Mechanical behavior of fibroblasts included in collagen lattices. J Soc Biol. 2001;195:427-430.
2. Chang AL, Agredano YZ, Kimball AB. Risk factors associated with striae gravidarum. J Am Acad Dermatol. 2004;51:881-885.
3. Salter SA, Kimball AB. Striae gravidarum. Clin Dermatol. 2006;24:97-100.
4. Elsaie ML, Baumann LS, Elsaaiee LT. Striae distensae (stretch marks) and different modalities of therapy: an update. Dermatol Surg. 2009;35:563-573.
5. Singh G, Kumar LP. Striae distensae. Indian J Dermatol Venereol Leprol. 2005;71:370-372.
6. Jiménez GP, Flores F, Berman B, et al. Treatment of striae rubra and striae alba with the 585-nm pulsed-dye laser. Dermatol Surg. 2003;29:362-365.
7. Watson RE, Parry EJ, Humphries JD, et al. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. 1998;138:931-937.
8. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Oxford, United Kingdom: The Cochrane Collaboration; 2011. http://handbook.cochrane.org/. Accessed July 8, 2014.
9. Draelos ZD, Gold MH, Kaur M, et al. Evaluation of an onion extract, Centella asiatica, and hyaluronic acid cream in the appearance of striae rubra. Skinmed. 2010;8:80-86.
10. Kang S, Kim KJ, Griffiths CE, et al. Topical tretinoin (retinoic acid) improves early stretch marks. Arch Dermatol. 1996;132:519-526.
11. Morganti P, Palombo P, Fabrizi G, et al. Biweekly in-office injectable treatment of striae distensae vs a long-term daily use of topical vitamin C. J Appl Cosmetol. 2001;19:107-112.
12. Naein FF, Soghrati M. Fractional CO2 laser as an effective modality in treatment of striae alba in skin types III and IV. J Res Med Sci. 2012;17:928-933.
13. Pribanich S, Simpson FG, Held B, et al. Low-dose tretinoin does not improve striae distensae: a double-blind, placebo-controlled study. Cutis. 1994;54:121-124.
14. Summers B, Lategan M. The effect of a topically-applied cosmetic oil formulation on striae distensae. SA Fam Pract. 2009;51:332-336.
15. Tay YK, Kwok C, Tan E. Non-ablative 1,450-nm diode laser treatment of striae distensae. Lasers Surg Med. 2006;38:196-199.
16. Yang YJ, Lee GY. Treatment of striae distensae with nonablative fractional laser versus ablative CO2 fractional laser: a randomized controlled trial. Ann Dermatol. 2011;23:481-489.
17. Joshi J, Donga SB, Pandya MA. A comparative study of Savarnakara Ghrita and Savarnakara Cream in the management of Kikkisa w.s.r. to Striae Gravidarum. Ayu. 2008;29:260-265.
Stretch marks (striae cutis distensae) are a common disfiguring skin condition characterized by linear bands of atrophic-appearing skin.1 The prevalence of stretch marks associated with pregnancy ranges from 50% to 90%.2 Although stretch marks do not pose a health risk, they often cause burning, itching, and emotional distress, and they can have a deep psychological impact on patients, particularly in young healthy women who are commonly affected by this condition.3
The cause of stretch marks currently is unknown, but they are known to develop in a variety of physiological and pathological states (eg, pregnancy, adolescent growth spurts, obesity, large weight gain, Cushing syndrome, Marfan syndrome, diabetes mellitus, long-term systemic or topical steroid use).2-5 Clinically, newly formed stretch marks present as pink or purple linear lesions without substantial depression of the skin (striae rubra). Over time, the lesions lose their pigmentation, becoming depressed, atrophic, and white (striae alba).2,3,6 The most commonly affected sites are the breasts, upper arms, abdomen, buttocks, and thighs.3,4
Regardless of the etiology, the same histologic changes can be noted in the epidermis of all stretch marks, such as atrophy and loss of rete ridges, with features that are similar to scarring.3 Additionally, reorganization and diminution of the elastic fiber network of skin can be observed.7
A variety of treatment strategies are available for stretch marks, including topical preparations (eg, tretinoin, glycolic acid) and lasers.4 With current methods, no consistently effective therapies have been established yet. In this article, we present the results of a systematic review of the literature to address the effectiveness and safety of the available treatment options for stretch marks.
Methods
A literature search for randomized controlled trials (RCTs) related to the treatment of stretch marks was conducted on March 13, 2013, using the Cochrane Central Register of Controlled Trials, PubMed (from 1966), Embase (from 1974), Chinese Biomedical Literature Database (from 1978), China National Knowledge Infrastructure (from 1994), Chinese Science Journals Database (from 1989), and Wanfang Data (from 1995). Search terms included stretch marks, stretch mark, striae atrophicae, striae distensae, striae gravidarum, striae rubra, striae alba, lineae albicantes, striae, kikkisa, and random*.
We attempted to contact the original investigators of the 25 articles assessed for eligibility by e-mail to identify the randomization and answer other methodology questions to ensure that the studies included in the analysis were RCTs. Each of the 8 RCTs selected for inclusion was assessed independently by 2 investigators (L.L. and H.M.), and data extraction also was performed independently. Any differences in opinion were resolved by discussion. The risk of biases were assessed and 5 domains—random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data—were judged for each study included in the analysis using the Cochrane Collaboration’s domain-based evaluation tool as described in the Cochrane Handbook for Systematic Reviews of Interventions.8 Publication bias was not assessed due to insufficient data.
Studies ultimately were classified into 3 categories based on the risk of bias: (1) low risk of bias or low risk of bias for all key domains; (2) unclear risk of bias or unclear risk of bias for 1 or more key domains; and (3) high risk of bias or high risk of 1 or more key domains.
Results
Search Results
Figure 1 presents the literature search results. Of 300 total search results, 8 RCTs were selected for assessment,9-16 which included a total of 240 patients (Table). The investigators of all 8 reports were contacted, but only 2 responses were received.11,14 The full text of one article could not be obtained; therefore, we could not confirm that it was a true RCT and excluded it.17
Risk of Bias
The risk of bias in methodology was evaluated for all 8 RCTs and the judgments were given for each domain (Figure 2). All the included studies claimed to be RCTs, but only 37.5% (3/8) of them used adequate randomizations, which were from a including computer-generated code,10 a table of randomized numbers,13 or the Microsoft Excel RND function (from the author by e-mail).14 The randomization methods in the other 5 studies were unclear. Allocation concealment was adequate in 1 trial13 but was unclear in the others. Three trials were double-blinded with the participants and outcome assessors blinded10,13,16; in 2 of these studies investigators also were blinded.10,13 There were 5 single-blinded trials; in 3 of these trials the outcome assessors were blinded12,14,15 and 1 was investigator-blinded.9 The other study was stated to be single-blinded but with no further detail.11 Due to the nature of the experimental design in 2 of the trials12,15 (ie, effects of laser therapy compared to topical treatment or no therapy), participants could not be blinded to treatment types; however, participants were blinded in 1 trial that compared different types of lasers.16 Investigators from all studies reported participants who did not complete the trial or were lost to follow-up, ranging from 0% to 65.6%. Two trials reported no loss of follow-up.11,12 Most trials had losses less than 20% except Pribanich et al13 who reported a loss of 65.6% of participants. One trial included a full analysis set,9 and none of the studies included an intention-to-treat analysis.
The overall risk of bias was assessed for each study and none could be categorized as low risk. Six studies had 1 or more domains assessed as high risk of bias and were classified as high risk of bias.9,11-15 The remaining 2 studies without high-risk domains had one or more domains assessed as unclear10,16 and were therefore considered to be at unclear risk of bias overall.
Effects of Treatments
Among the 8 studies we assessed, there were different treatments, methods of comparison, product concentrations, and times of application. The methods for assessing outcomes (eg, the size and severity of stretch marks) also were varied. Therefore, it is difficult to perform a meta-analysis of the data, and all the evidence was from individual studies. A summary of the results is presented in the Table.
All of the studies we evaluated assessed clinical improvement. Three studies reported the effects of topical tretinoin on stretch marks.10,12,13 A small parallel study with unclear risk of bias indicated that white participants with erythematous stretch marks seemed to have a better response to treatment with tretinoin cream 0.1% for 24 weeks versus placebo.10 However, there was no significant difference between tretinoin cream 0.025% and placebo for patients with abdominal striae in another trial.13 The latter trial was performed with low risk of bias in methodology, but the dropout rate was high (65.6%), with only 11 of 32 participants completing the trial. It is likely that the small number of patients makes the power too low to detect significant differences between tretinoin cream 0.025% and placebo if such a difference indeed existed.13 Because the outcomes in these 2 trials were assessed in different ways, it is difficult to perform a meta-analysis on the data. More adverse effects, mainly erythema and scaling associated with itching or burning sensations, were reported with the higher concentration (0.05%) of tretinoin.10 Another study at a high risk of bias found that the combined use of tretinoin cream 0.05% and glycolic acid 10% was not as effective as fractional CO2 laser therapy in improving the appearance of striae alba.12 There also were 3 studies comparing the effects of laser therapy with another treatment or no treatment.12,15,16 Two within-participant comparison studies with unclear or high risk of bias compared CO2 fractional laser therapy with other active treatment methods in female participants with striae alba.12,16 No difference between the fractional CO2 laser and the 1550-nm nonablative fractional erbium:glass laser was reported,16 but the fractional CO2 laser may be more effective than the topical therapy.12 A small study (11 participants) at high risk of bias reported negative results for the 1450-nm mid-infrared diode laser compared to no treatment.15 Data on the adverse effects of laser therapy were available from these studies. Postinflammatory hyperpigmentation was found in all the 3 studies12,15,16 and posttreatment erythema was mentioned in 2 studies.15,16 Based on the individual studies, treatment with a cosmetic oil formulation was more effective than a moisturizer in improving clinical presentation of stretch marks in white patients.14 Women with striae rubra showed better response to treatment with onion extract cream versus no treatment.9 Limited data from 1 study showed that combined use of Active B (sodium ascorbyl phosphate, 3-aminopropyl-L-ascorbyl phosphate, carboxybetaglucan, hyaluronic acid) and Active A (hyaluronic acid, sodium salt 2 mg, sodium carboxymethyl betaglucan 0.1 mg, ascorbic acid 0.5 mg, arginine 1 mg, sodium chloride 9 mg, sterile water) might be more effective than the use of Active B or placebo.11 These 3 studies are at high risk of bias and no obvious adverse effects were reported.9,11,14
Comment
In the 8 trials included in our assessment, 5 used a within-participant design in which 2 different treatments were randomly administered to the left and right sides of the body, respectively.9,12,14-16 Because the comparison of treatments was made based on results in the same patient versus 2 different treatment groups, the results may be more accurate. In the studies we reviewed, only 3 were placebo-controlled, which may only provide limited evidence on the comparative efficacy of the treatments used in these studies.10,11,13 Most treatments were evaluated in single studies, and most studies had a small number of participants (range, 6–66 participants). A considerable number of the total participants withdrew from their respective studies or were lost during follow-up. In some cases, no reason was given,13 but in the others, it was because of an obvious side effect16 or noncompliance.14 Overall, the methodology quality was low, especially the methods of randomization and allocation concealment. Unsuccessful attempts to contact the original investigators made it difficult to make accurate assessments of the risk of bias in most of the studies included in our assessment. No study met all the risk of bias criteria, and none were classified as having a low risk of bias.
The impact of industry sponsorship on the direction and completeness of the results of the studies we reviewed is unclear. One study was funded by a grant from the manufacturer of the study product,14 and the medication used in another study was supplied by the manufacturer.13 Another study was supported in part by a company that had no part in the conduct, analysis, or reporting of the study.10 In one instance, the authors were employees of the manufacturer of the study product.9 The remaining studies made no declaration.11,12,15,16
Thus the evidence from this review was insufficient to provide clear guidelines for practice. Because the results were based on a small number of patients and were of high or unclear risk of bias, caution must be taken when comparing the efficacy of the treatments administered in these studies; however, given the negligible reported side effects, tretinoin cream 0.1%, a cosmetic oil formulation, onion extract cream, or the combined use of Active A and Active B could reasonably be considered for the treatment of stretch marks. Laser therapies such as the fractional CO2 laser or the 1550-nm fractional erbium:glass laser may be another effective choice.
Conclusion
In future investigations of stretch mark treatments, more high-quality, placebo-controlled trials are needed. One important issue is the varied outcome assessment among different studies, which makes the evaluation and pooling of different studies difficult. Therefore, future RCTs should measure clinical features with a uniform score system such as the visual analog scale or the patient and observer scar assessment scale and try to avoid individual made-up system to assess the outcome. Furthermore, quality-of-life assessment was not included in any of the reports we evaluated; rather all 8 studies focused on changes in the appearance of stretch marks only. Given the deep psychological impact that stretch marks can have on patients, measures for quality-of-life assessment, such as the dermatology life quality index, should be incorporated into future study designs to improve the relevance of the trial and allow comparisons among studies using different interventions.
Stretch marks (striae cutis distensae) are a common disfiguring skin condition characterized by linear bands of atrophic-appearing skin.1 The prevalence of stretch marks associated with pregnancy ranges from 50% to 90%.2 Although stretch marks do not pose a health risk, they often cause burning, itching, and emotional distress, and they can have a deep psychological impact on patients, particularly in young healthy women who are commonly affected by this condition.3
The cause of stretch marks currently is unknown, but they are known to develop in a variety of physiological and pathological states (eg, pregnancy, adolescent growth spurts, obesity, large weight gain, Cushing syndrome, Marfan syndrome, diabetes mellitus, long-term systemic or topical steroid use).2-5 Clinically, newly formed stretch marks present as pink or purple linear lesions without substantial depression of the skin (striae rubra). Over time, the lesions lose their pigmentation, becoming depressed, atrophic, and white (striae alba).2,3,6 The most commonly affected sites are the breasts, upper arms, abdomen, buttocks, and thighs.3,4
Regardless of the etiology, the same histologic changes can be noted in the epidermis of all stretch marks, such as atrophy and loss of rete ridges, with features that are similar to scarring.3 Additionally, reorganization and diminution of the elastic fiber network of skin can be observed.7
A variety of treatment strategies are available for stretch marks, including topical preparations (eg, tretinoin, glycolic acid) and lasers.4 With current methods, no consistently effective therapies have been established yet. In this article, we present the results of a systematic review of the literature to address the effectiveness and safety of the available treatment options for stretch marks.
Methods
A literature search for randomized controlled trials (RCTs) related to the treatment of stretch marks was conducted on March 13, 2013, using the Cochrane Central Register of Controlled Trials, PubMed (from 1966), Embase (from 1974), Chinese Biomedical Literature Database (from 1978), China National Knowledge Infrastructure (from 1994), Chinese Science Journals Database (from 1989), and Wanfang Data (from 1995). Search terms included stretch marks, stretch mark, striae atrophicae, striae distensae, striae gravidarum, striae rubra, striae alba, lineae albicantes, striae, kikkisa, and random*.
We attempted to contact the original investigators of the 25 articles assessed for eligibility by e-mail to identify the randomization and answer other methodology questions to ensure that the studies included in the analysis were RCTs. Each of the 8 RCTs selected for inclusion was assessed independently by 2 investigators (L.L. and H.M.), and data extraction also was performed independently. Any differences in opinion were resolved by discussion. The risk of biases were assessed and 5 domains—random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data—were judged for each study included in the analysis using the Cochrane Collaboration’s domain-based evaluation tool as described in the Cochrane Handbook for Systematic Reviews of Interventions.8 Publication bias was not assessed due to insufficient data.
Studies ultimately were classified into 3 categories based on the risk of bias: (1) low risk of bias or low risk of bias for all key domains; (2) unclear risk of bias or unclear risk of bias for 1 or more key domains; and (3) high risk of bias or high risk of 1 or more key domains.
Results
Search Results
Figure 1 presents the literature search results. Of 300 total search results, 8 RCTs were selected for assessment,9-16 which included a total of 240 patients (Table). The investigators of all 8 reports were contacted, but only 2 responses were received.11,14 The full text of one article could not be obtained; therefore, we could not confirm that it was a true RCT and excluded it.17
Risk of Bias
The risk of bias in methodology was evaluated for all 8 RCTs and the judgments were given for each domain (Figure 2). All the included studies claimed to be RCTs, but only 37.5% (3/8) of them used adequate randomizations, which were from a including computer-generated code,10 a table of randomized numbers,13 or the Microsoft Excel RND function (from the author by e-mail).14 The randomization methods in the other 5 studies were unclear. Allocation concealment was adequate in 1 trial13 but was unclear in the others. Three trials were double-blinded with the participants and outcome assessors blinded10,13,16; in 2 of these studies investigators also were blinded.10,13 There were 5 single-blinded trials; in 3 of these trials the outcome assessors were blinded12,14,15 and 1 was investigator-blinded.9 The other study was stated to be single-blinded but with no further detail.11 Due to the nature of the experimental design in 2 of the trials12,15 (ie, effects of laser therapy compared to topical treatment or no therapy), participants could not be blinded to treatment types; however, participants were blinded in 1 trial that compared different types of lasers.16 Investigators from all studies reported participants who did not complete the trial or were lost to follow-up, ranging from 0% to 65.6%. Two trials reported no loss of follow-up.11,12 Most trials had losses less than 20% except Pribanich et al13 who reported a loss of 65.6% of participants. One trial included a full analysis set,9 and none of the studies included an intention-to-treat analysis.
The overall risk of bias was assessed for each study and none could be categorized as low risk. Six studies had 1 or more domains assessed as high risk of bias and were classified as high risk of bias.9,11-15 The remaining 2 studies without high-risk domains had one or more domains assessed as unclear10,16 and were therefore considered to be at unclear risk of bias overall.
Effects of Treatments
Among the 8 studies we assessed, there were different treatments, methods of comparison, product concentrations, and times of application. The methods for assessing outcomes (eg, the size and severity of stretch marks) also were varied. Therefore, it is difficult to perform a meta-analysis of the data, and all the evidence was from individual studies. A summary of the results is presented in the Table.
All of the studies we evaluated assessed clinical improvement. Three studies reported the effects of topical tretinoin on stretch marks.10,12,13 A small parallel study with unclear risk of bias indicated that white participants with erythematous stretch marks seemed to have a better response to treatment with tretinoin cream 0.1% for 24 weeks versus placebo.10 However, there was no significant difference between tretinoin cream 0.025% and placebo for patients with abdominal striae in another trial.13 The latter trial was performed with low risk of bias in methodology, but the dropout rate was high (65.6%), with only 11 of 32 participants completing the trial. It is likely that the small number of patients makes the power too low to detect significant differences between tretinoin cream 0.025% and placebo if such a difference indeed existed.13 Because the outcomes in these 2 trials were assessed in different ways, it is difficult to perform a meta-analysis on the data. More adverse effects, mainly erythema and scaling associated with itching or burning sensations, were reported with the higher concentration (0.05%) of tretinoin.10 Another study at a high risk of bias found that the combined use of tretinoin cream 0.05% and glycolic acid 10% was not as effective as fractional CO2 laser therapy in improving the appearance of striae alba.12 There also were 3 studies comparing the effects of laser therapy with another treatment or no treatment.12,15,16 Two within-participant comparison studies with unclear or high risk of bias compared CO2 fractional laser therapy with other active treatment methods in female participants with striae alba.12,16 No difference between the fractional CO2 laser and the 1550-nm nonablative fractional erbium:glass laser was reported,16 but the fractional CO2 laser may be more effective than the topical therapy.12 A small study (11 participants) at high risk of bias reported negative results for the 1450-nm mid-infrared diode laser compared to no treatment.15 Data on the adverse effects of laser therapy were available from these studies. Postinflammatory hyperpigmentation was found in all the 3 studies12,15,16 and posttreatment erythema was mentioned in 2 studies.15,16 Based on the individual studies, treatment with a cosmetic oil formulation was more effective than a moisturizer in improving clinical presentation of stretch marks in white patients.14 Women with striae rubra showed better response to treatment with onion extract cream versus no treatment.9 Limited data from 1 study showed that combined use of Active B (sodium ascorbyl phosphate, 3-aminopropyl-L-ascorbyl phosphate, carboxybetaglucan, hyaluronic acid) and Active A (hyaluronic acid, sodium salt 2 mg, sodium carboxymethyl betaglucan 0.1 mg, ascorbic acid 0.5 mg, arginine 1 mg, sodium chloride 9 mg, sterile water) might be more effective than the use of Active B or placebo.11 These 3 studies are at high risk of bias and no obvious adverse effects were reported.9,11,14
Comment
In the 8 trials included in our assessment, 5 used a within-participant design in which 2 different treatments were randomly administered to the left and right sides of the body, respectively.9,12,14-16 Because the comparison of treatments was made based on results in the same patient versus 2 different treatment groups, the results may be more accurate. In the studies we reviewed, only 3 were placebo-controlled, which may only provide limited evidence on the comparative efficacy of the treatments used in these studies.10,11,13 Most treatments were evaluated in single studies, and most studies had a small number of participants (range, 6–66 participants). A considerable number of the total participants withdrew from their respective studies or were lost during follow-up. In some cases, no reason was given,13 but in the others, it was because of an obvious side effect16 or noncompliance.14 Overall, the methodology quality was low, especially the methods of randomization and allocation concealment. Unsuccessful attempts to contact the original investigators made it difficult to make accurate assessments of the risk of bias in most of the studies included in our assessment. No study met all the risk of bias criteria, and none were classified as having a low risk of bias.
The impact of industry sponsorship on the direction and completeness of the results of the studies we reviewed is unclear. One study was funded by a grant from the manufacturer of the study product,14 and the medication used in another study was supplied by the manufacturer.13 Another study was supported in part by a company that had no part in the conduct, analysis, or reporting of the study.10 In one instance, the authors were employees of the manufacturer of the study product.9 The remaining studies made no declaration.11,12,15,16
Thus the evidence from this review was insufficient to provide clear guidelines for practice. Because the results were based on a small number of patients and were of high or unclear risk of bias, caution must be taken when comparing the efficacy of the treatments administered in these studies; however, given the negligible reported side effects, tretinoin cream 0.1%, a cosmetic oil formulation, onion extract cream, or the combined use of Active A and Active B could reasonably be considered for the treatment of stretch marks. Laser therapies such as the fractional CO2 laser or the 1550-nm fractional erbium:glass laser may be another effective choice.
Conclusion
In future investigations of stretch mark treatments, more high-quality, placebo-controlled trials are needed. One important issue is the varied outcome assessment among different studies, which makes the evaluation and pooling of different studies difficult. Therefore, future RCTs should measure clinical features with a uniform score system such as the visual analog scale or the patient and observer scar assessment scale and try to avoid individual made-up system to assess the outcome. Furthermore, quality-of-life assessment was not included in any of the reports we evaluated; rather all 8 studies focused on changes in the appearance of stretch marks only. Given the deep psychological impact that stretch marks can have on patients, measures for quality-of-life assessment, such as the dermatology life quality index, should be incorporated into future study designs to improve the relevance of the trial and allow comparisons among studies using different interventions.
1. Viennet C, Bride J, Cohen-Letessier A, et al. Mechanical behavior of fibroblasts included in collagen lattices. J Soc Biol. 2001;195:427-430.
2. Chang AL, Agredano YZ, Kimball AB. Risk factors associated with striae gravidarum. J Am Acad Dermatol. 2004;51:881-885.
3. Salter SA, Kimball AB. Striae gravidarum. Clin Dermatol. 2006;24:97-100.
4. Elsaie ML, Baumann LS, Elsaaiee LT. Striae distensae (stretch marks) and different modalities of therapy: an update. Dermatol Surg. 2009;35:563-573.
5. Singh G, Kumar LP. Striae distensae. Indian J Dermatol Venereol Leprol. 2005;71:370-372.
6. Jiménez GP, Flores F, Berman B, et al. Treatment of striae rubra and striae alba with the 585-nm pulsed-dye laser. Dermatol Surg. 2003;29:362-365.
7. Watson RE, Parry EJ, Humphries JD, et al. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. 1998;138:931-937.
8. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Oxford, United Kingdom: The Cochrane Collaboration; 2011. http://handbook.cochrane.org/. Accessed July 8, 2014.
9. Draelos ZD, Gold MH, Kaur M, et al. Evaluation of an onion extract, Centella asiatica, and hyaluronic acid cream in the appearance of striae rubra. Skinmed. 2010;8:80-86.
10. Kang S, Kim KJ, Griffiths CE, et al. Topical tretinoin (retinoic acid) improves early stretch marks. Arch Dermatol. 1996;132:519-526.
11. Morganti P, Palombo P, Fabrizi G, et al. Biweekly in-office injectable treatment of striae distensae vs a long-term daily use of topical vitamin C. J Appl Cosmetol. 2001;19:107-112.
12. Naein FF, Soghrati M. Fractional CO2 laser as an effective modality in treatment of striae alba in skin types III and IV. J Res Med Sci. 2012;17:928-933.
13. Pribanich S, Simpson FG, Held B, et al. Low-dose tretinoin does not improve striae distensae: a double-blind, placebo-controlled study. Cutis. 1994;54:121-124.
14. Summers B, Lategan M. The effect of a topically-applied cosmetic oil formulation on striae distensae. SA Fam Pract. 2009;51:332-336.
15. Tay YK, Kwok C, Tan E. Non-ablative 1,450-nm diode laser treatment of striae distensae. Lasers Surg Med. 2006;38:196-199.
16. Yang YJ, Lee GY. Treatment of striae distensae with nonablative fractional laser versus ablative CO2 fractional laser: a randomized controlled trial. Ann Dermatol. 2011;23:481-489.
17. Joshi J, Donga SB, Pandya MA. A comparative study of Savarnakara Ghrita and Savarnakara Cream in the management of Kikkisa w.s.r. to Striae Gravidarum. Ayu. 2008;29:260-265.
1. Viennet C, Bride J, Cohen-Letessier A, et al. Mechanical behavior of fibroblasts included in collagen lattices. J Soc Biol. 2001;195:427-430.
2. Chang AL, Agredano YZ, Kimball AB. Risk factors associated with striae gravidarum. J Am Acad Dermatol. 2004;51:881-885.
3. Salter SA, Kimball AB. Striae gravidarum. Clin Dermatol. 2006;24:97-100.
4. Elsaie ML, Baumann LS, Elsaaiee LT. Striae distensae (stretch marks) and different modalities of therapy: an update. Dermatol Surg. 2009;35:563-573.
5. Singh G, Kumar LP. Striae distensae. Indian J Dermatol Venereol Leprol. 2005;71:370-372.
6. Jiménez GP, Flores F, Berman B, et al. Treatment of striae rubra and striae alba with the 585-nm pulsed-dye laser. Dermatol Surg. 2003;29:362-365.
7. Watson RE, Parry EJ, Humphries JD, et al. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. 1998;138:931-937.
8. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Oxford, United Kingdom: The Cochrane Collaboration; 2011. http://handbook.cochrane.org/. Accessed July 8, 2014.
9. Draelos ZD, Gold MH, Kaur M, et al. Evaluation of an onion extract, Centella asiatica, and hyaluronic acid cream in the appearance of striae rubra. Skinmed. 2010;8:80-86.
10. Kang S, Kim KJ, Griffiths CE, et al. Topical tretinoin (retinoic acid) improves early stretch marks. Arch Dermatol. 1996;132:519-526.
11. Morganti P, Palombo P, Fabrizi G, et al. Biweekly in-office injectable treatment of striae distensae vs a long-term daily use of topical vitamin C. J Appl Cosmetol. 2001;19:107-112.
12. Naein FF, Soghrati M. Fractional CO2 laser as an effective modality in treatment of striae alba in skin types III and IV. J Res Med Sci. 2012;17:928-933.
13. Pribanich S, Simpson FG, Held B, et al. Low-dose tretinoin does not improve striae distensae: a double-blind, placebo-controlled study. Cutis. 1994;54:121-124.
14. Summers B, Lategan M. The effect of a topically-applied cosmetic oil formulation on striae distensae. SA Fam Pract. 2009;51:332-336.
15. Tay YK, Kwok C, Tan E. Non-ablative 1,450-nm diode laser treatment of striae distensae. Lasers Surg Med. 2006;38:196-199.
16. Yang YJ, Lee GY. Treatment of striae distensae with nonablative fractional laser versus ablative CO2 fractional laser: a randomized controlled trial. Ann Dermatol. 2011;23:481-489.
17. Joshi J, Donga SB, Pandya MA. A comparative study of Savarnakara Ghrita and Savarnakara Cream in the management of Kikkisa w.s.r. to Striae Gravidarum. Ayu. 2008;29:260-265.
Practice Points
- Given the negligible reported side effects, tretinoin cream 0.1%, a cosmetic oil formulation, onion extract cream, or the combined use of Active A and Active B could be considered for the treatment of stretch marks, though the evidence is insufficient.
- High-quality, randomized, placebo-controlled trials are needed in the future.