User login
VIDEO: Sun protection myths debunked
CHAMPIONSGATE, FLA. – Can you separate sun protection myths from facts for your patients? Misperceptions persist, but sunscreen remains a safe and effective tool to help protect the skin from ultraviolet radiation.
In a video interview at the Orlando Dermatology Aesthetic and Clinical Conference, Dr. Adam Friedman of Montefiore/Albert Einstein College of Medicine, New York, debunked several sun protection myths related to oxybenzone, nanoparticles, and vitamin D.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHAMPIONSGATE, FLA. – Can you separate sun protection myths from facts for your patients? Misperceptions persist, but sunscreen remains a safe and effective tool to help protect the skin from ultraviolet radiation.
In a video interview at the Orlando Dermatology Aesthetic and Clinical Conference, Dr. Adam Friedman of Montefiore/Albert Einstein College of Medicine, New York, debunked several sun protection myths related to oxybenzone, nanoparticles, and vitamin D.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHAMPIONSGATE, FLA. – Can you separate sun protection myths from facts for your patients? Misperceptions persist, but sunscreen remains a safe and effective tool to help protect the skin from ultraviolet radiation.
In a video interview at the Orlando Dermatology Aesthetic and Clinical Conference, Dr. Adam Friedman of Montefiore/Albert Einstein College of Medicine, New York, debunked several sun protection myths related to oxybenzone, nanoparticles, and vitamin D.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM THE ODAC CONFERENCE
Reticular Erythematous Mucinosis
Pulmonary Embolism During Temporal Filling: The Middle Temporal Vein
In an article published online on February 27, 2014, in JAMA Facial Plastic Surgery, Jiang et al reported on a potentially fatal complication during the use of autologous fat transfer for facial augmentation. They described 3 patients with a nonthrombotic pulmonary embolism during autologous fat injection to the temple area. Two of 3 patients were under local anesthesia and 1 patient was under general anesthesia. The 2 patients under local anesthesia complained of sudden diaphoresis, dyspnea, and tachypnea. The other patient who was under general anesthesia had sudden cardiac and respiratory arrest and subsequently died. Autopsy confirmed a pulmonary embolism. The authors identified the middle temporal vein (MTV) as the culprit vessel that was cannulized. In this report, 10 cadaveric dissections were done to identify and characterize the MTV.
What’s the issue?
Facial augmentation with autologous fat as well as other filler materials has become increasingly popular. Therefore, knowledge of anatomy and vasculature are of utmost importance to help mitigate potential complications. The anatomic levels of the temporal region from superficial to deep include the epidermis, dermis, subcutaneous adipose, superficial musculoaponeurotic system, superficial temporal fascia, superficial layer of the deep temporal fascia, superficial temporal fat-pad, deep layer of the deep temporal fascia, and temporalis muscle. The MTV arises from 2 to 4 tributaries at the area of the lateral orbital angle. It is lifted by the superficial temporal fat-pad, and because it lies between the superficial and deep layers of the deep temporal fascia, the vein walls are kept patent and do not collapse during injection. The sentinel vein is one of the MTV’s tributaries and has the same characteristics. It lies lateral to the lateral orbital rim and perforates through the superficial layer of the deep temporal fascia. Another reason why the MTV is a risk factor for cannulization is its large caliber. The stem can be as wide as 3.15 +/- 0.13 mm. This study found a mean (standard deviation) of 2.06 (0.17) mm from the point of origin and 3.02 (0.23) mm at the palpebral fissure plane. The authors made the recommendation to use blunt-tipped needles during fat augmentation in this area as well as multiple injection sites with slow injection. This technique also can be applied to filling with other materials. Pulling back on the syringe before injection also can help as well as injecting small amounts in a steady retrograde fashion. Before your next filler patient, is it time for an anatomy review?
In an article published online on February 27, 2014, in JAMA Facial Plastic Surgery, Jiang et al reported on a potentially fatal complication during the use of autologous fat transfer for facial augmentation. They described 3 patients with a nonthrombotic pulmonary embolism during autologous fat injection to the temple area. Two of 3 patients were under local anesthesia and 1 patient was under general anesthesia. The 2 patients under local anesthesia complained of sudden diaphoresis, dyspnea, and tachypnea. The other patient who was under general anesthesia had sudden cardiac and respiratory arrest and subsequently died. Autopsy confirmed a pulmonary embolism. The authors identified the middle temporal vein (MTV) as the culprit vessel that was cannulized. In this report, 10 cadaveric dissections were done to identify and characterize the MTV.
What’s the issue?
Facial augmentation with autologous fat as well as other filler materials has become increasingly popular. Therefore, knowledge of anatomy and vasculature are of utmost importance to help mitigate potential complications. The anatomic levels of the temporal region from superficial to deep include the epidermis, dermis, subcutaneous adipose, superficial musculoaponeurotic system, superficial temporal fascia, superficial layer of the deep temporal fascia, superficial temporal fat-pad, deep layer of the deep temporal fascia, and temporalis muscle. The MTV arises from 2 to 4 tributaries at the area of the lateral orbital angle. It is lifted by the superficial temporal fat-pad, and because it lies between the superficial and deep layers of the deep temporal fascia, the vein walls are kept patent and do not collapse during injection. The sentinel vein is one of the MTV’s tributaries and has the same characteristics. It lies lateral to the lateral orbital rim and perforates through the superficial layer of the deep temporal fascia. Another reason why the MTV is a risk factor for cannulization is its large caliber. The stem can be as wide as 3.15 +/- 0.13 mm. This study found a mean (standard deviation) of 2.06 (0.17) mm from the point of origin and 3.02 (0.23) mm at the palpebral fissure plane. The authors made the recommendation to use blunt-tipped needles during fat augmentation in this area as well as multiple injection sites with slow injection. This technique also can be applied to filling with other materials. Pulling back on the syringe before injection also can help as well as injecting small amounts in a steady retrograde fashion. Before your next filler patient, is it time for an anatomy review?
In an article published online on February 27, 2014, in JAMA Facial Plastic Surgery, Jiang et al reported on a potentially fatal complication during the use of autologous fat transfer for facial augmentation. They described 3 patients with a nonthrombotic pulmonary embolism during autologous fat injection to the temple area. Two of 3 patients were under local anesthesia and 1 patient was under general anesthesia. The 2 patients under local anesthesia complained of sudden diaphoresis, dyspnea, and tachypnea. The other patient who was under general anesthesia had sudden cardiac and respiratory arrest and subsequently died. Autopsy confirmed a pulmonary embolism. The authors identified the middle temporal vein (MTV) as the culprit vessel that was cannulized. In this report, 10 cadaveric dissections were done to identify and characterize the MTV.
What’s the issue?
Facial augmentation with autologous fat as well as other filler materials has become increasingly popular. Therefore, knowledge of anatomy and vasculature are of utmost importance to help mitigate potential complications. The anatomic levels of the temporal region from superficial to deep include the epidermis, dermis, subcutaneous adipose, superficial musculoaponeurotic system, superficial temporal fascia, superficial layer of the deep temporal fascia, superficial temporal fat-pad, deep layer of the deep temporal fascia, and temporalis muscle. The MTV arises from 2 to 4 tributaries at the area of the lateral orbital angle. It is lifted by the superficial temporal fat-pad, and because it lies between the superficial and deep layers of the deep temporal fascia, the vein walls are kept patent and do not collapse during injection. The sentinel vein is one of the MTV’s tributaries and has the same characteristics. It lies lateral to the lateral orbital rim and perforates through the superficial layer of the deep temporal fascia. Another reason why the MTV is a risk factor for cannulization is its large caliber. The stem can be as wide as 3.15 +/- 0.13 mm. This study found a mean (standard deviation) of 2.06 (0.17) mm from the point of origin and 3.02 (0.23) mm at the palpebral fissure plane. The authors made the recommendation to use blunt-tipped needles during fat augmentation in this area as well as multiple injection sites with slow injection. This technique also can be applied to filling with other materials. Pulling back on the syringe before injection also can help as well as injecting small amounts in a steady retrograde fashion. Before your next filler patient, is it time for an anatomy review?
Titanium dioxide
Titanium dioxide (TiO2) and zinc oxide (ZnO) in large-particle form have long been used in various sunscreens to protect the skin by reflecting or physically blocking ultraviolet (UV) radiation. In recent years, TiO2 as well as ZnO nanoparticles have been incorporated into sunscreens and cosmetics to act as a UV shield. They have been shown to be effective barriers against UV-induced damage, and yield stronger protection against UV insult, while leaving less white residue, than previous generations of the physical sunblocks.
However, some data suggest that in nanoparticle form, TiO2 and ZnO absorb UV radiation, leading to photocatalysis and the release of reactive oxygen species (Australas. J. Dermatol. 2011;52:1-6). This column will focus primarily on the safety of TiO2 in nanoparticle form.
While numerous studies examine both TiO2 and ZnO, the primary inorganic sunscreens, the sheer number of separate investigations warrants individual articles, and ZnO was addressed in previous columns. Briefly, though, TiO2 is more photoactive and exhibits a higher refractive index in visible light than ZnO (J. Am. Acad. Dermatol. 1999;40:85-90); therefore, TiO2 appears whiter and is more difficult to incorporate into transparent products.
A 2011 study by Kang et al. showed that TiO2 nanoparticles, but not normal-sized TiO2, and UVA synergistically foster rapid production of reactive oxygen species and breakdown of mitochondrial membrane potential, leading to apoptosis, and that TiO2 nanoparticles are more phototoxic than larger ones (Drug Chem. Toxicol. 2011;34:277-84).
However, also in 2011, Tyner et al. investigated the effects of nanoscale TiO2 use on UV attenuation in simple to complex sunscreen products. They found that barrier function was diminished by none of the formulations, and that optimal UV attenuation resulted when TiO2 particles were stabilized with a coating and evenly dispersed. The researchers concluded that nanoscale TiO2 is nontoxic and may impart greater efficacy (Int. J. Cosmet. Sci. 2011;33:234-44).
In vitro and in vivo studies
In 2010, Tiano et al. evaluated five modified TiO2 particles, developed and marketed for sunscreens. They used different in vitro models, including cultured human skin fibroblasts, to determine potential photocatalytic effects after UVA exposure. The investigators found that the kind of modification to and crystal form of the TiO2 nanoparticle influences its ability to augment or reduce DNA damage, increase or decrease intracellular reactive oxygen species, diminish cell viability, and promote other effects of photocatalysis. In particular, they noted that the anatase crystal form of TiO2 retained photocatalytic activity. The authors suggested that while the debate continues over the penetration of nanosized TiO2 into the viable epidermis, their results help elucidate the potential effects of TiO2 particles at the cellular level (Free Radic. Biol. Med. 2010;49:408-15).
A 2010 study by Senzui et al. using in vitro intact, stripped, and hair-removed skin of Yucatan micropigs to test the skin penetration of four different types of rutile (the most natural form of) TiO2 (two coated, two uncoated) revealed no penetration of TiO2 type in intact and stripped skin. The concentration of titanium in skin was significantly higher when one of the coated forms was applied on hair-removed skin, with titanium penetrating into vacant hair follicles (greater than 1 mm below the skin surface), but not into dermis or viable epidermis (J. Toxicol. Sci. 2010;35:107-13).
Animal studies
In 2009, the Food and Drug Administration Center for Drug Evaluation and Research worked with the National Center for Toxicology Research using minipigs and four sunscreen formulations to determine whether nanoscale TiO2 can penetrate intact skin. Their use of scanning electron microscopy and x-ray diffraction revealed that TiO2 particles were the same size as that observed for the raw materials, implying that the formulation process influenced neither the size nor the shape of TiO2 particles (Drug Dev. Ind. Pharm. 2009;35:1180-9).
In 2010, Sadrieh et al. performed a study of the dermal penetration of three TiO2 particles: uncoated submicrometer-sized, uncoated nano-sized, and dimethicone/methicone copolymer-coated nanosized. The investigators applied 5% by weight of each of the types of particles in a sunscreen on minipigs and found no significant penetration into intact normal epidermis (Toxicol. Sci. 2010;115(1):156-66).
In 2011, Furukawa et al. studied the postinitiation carcinogenic potential of coated and uncoated TiO2 nanoparticles in a two-stage skin carcinogenesis model using 7-week-old CD1 (ICR) female mice. They found that application of coated and uncoated nanoparticles after initiation and promotion with 7,12-dimethylbenz[a]anthracene and 12-O-tetradecanoylphorbol 13-acetate at doses of up to 20 mg/mouse failed to augment nodule development. The investigators concluded that TiO2 nanoparticles do not exhibit postinitiation potential for mouse skin carcinogenesis (Food Chem. Toxicol. 2011;49(4):744-9).
Human data
Given the persistent concerns about possible side effects of coated TiO2 and ZnO nanoparticles used in physical sun blockers, Filipe et al., in 2009, assessed the localization and potential skin penetration of TiO2 and ZnO nanoparticles dispersed in three sunscreen formulations, under realistic in vivo conditions in normal and altered skin. The investigators examined a test hydrophobic formulation containing coated 20-nm TiO2 nanoparticles and two commercially available sunscreen formulations containing TiO2 alone or in combination with ZnO, with respect to how consumers actually used sunscreens compared with the recommended standard condition for the sun protection factor test. They found that traces of the physical blockers could be detected only at the skin surface and uppermost area of the stratum corneum in normal human skin after a 2-hour exposure. After 48 hours of exposure, layers deeper than the stratum corneum contained no detectable TiO2 or ZnO nanoparticles. While preferential deposition of the nanoparticles in the openings of pilosebaceous follicles was noted, no penetration into viable skin tissue was observed. The investigators concluded that significant penetration of TiO2 or ZnO nanoparticles into keratinocytes is improbable (Skin Pharmacol. Physiol. 2009;22:266-75).
The weight of evidence
Current evidence suggests minimal risks to human health from the use of TiO2 or ZnO nanoparticles at concentrations up to 25% in cosmetic preparations or sunscreens, according to Schilling et al., regardless of coatings or crystalline structure. In a safety review of these ingredients, they noted that these nanoparticles formulated in topical products occur as aggregates of primary particles 30-150 nm in size, and bond in such a way that renders them impervious to the force of product application. Thus their structure remains unaffected, and no primary particles are released. The authors also noted that nanoparticles exhibit equivalence with larger particles in terms of distribution and duration and, therefore, recognition and elimination from the body (Photochem. Photobiol. Sci. 2010;9:495-509).
But in 2011, Tran and Salmon, in light of findings that nanoparticles may penetrate the stratum corneum under certain conditions, considered the possible photocarcinogenic results of nanoparticle sunscreens. They noted, though, that most such results were obtained through the use of animal skin models, not investigations with human skin (Australas. J. Dermatol. 2011;52:1-6). To this point, the weight of evidence appears to show that such TiO2 nanoparticles are safe when applied to intact human skin (Semin. Cutan. Med. Surg. 2011;30:210-13).
In response to the increased scrutiny and concern exhibited by the general public and government agencies regarding the safety of TiO2 and ZnO nanoparticles, Newman et al. reviewed the literature and position statements from 1980 to 2008 to ascertain and describe the use, safety, and regulatory state of such ingredients in sunscreens. They found no evidence of significant penetration deeper than the stratum corneum of TiO2 and ZnO nanoparticles, but caution that additional studies simulating real-world conditions (i.e., sunburned skin and under UV exposure) are necessary (J. Am. Acad. Dermatol. 2009;61:685-92).
Conclusion
Titanium dioxide is a well-established, safe, and effective physical sunblock. Nanotechnology has introduced some cause for concern regarding its use in physical sunblocks. In particular, evidence suggesting that photoexcitation of TiO2 nanoparticles leads to the generation of reactive oxygen species that damage DNA, potentially launching a cascade of adverse events, has prompted investigations into the safety of TiO2 in nanoparticle form. However, to date, multiple studies suggest that TiO2 nanoparticles do not penetrate or are highly unlikely to penetrate beyond the stratum corneum.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Titanium dioxide (TiO2) and zinc oxide (ZnO) in large-particle form have long been used in various sunscreens to protect the skin by reflecting or physically blocking ultraviolet (UV) radiation. In recent years, TiO2 as well as ZnO nanoparticles have been incorporated into sunscreens and cosmetics to act as a UV shield. They have been shown to be effective barriers against UV-induced damage, and yield stronger protection against UV insult, while leaving less white residue, than previous generations of the physical sunblocks.
However, some data suggest that in nanoparticle form, TiO2 and ZnO absorb UV radiation, leading to photocatalysis and the release of reactive oxygen species (Australas. J. Dermatol. 2011;52:1-6). This column will focus primarily on the safety of TiO2 in nanoparticle form.
While numerous studies examine both TiO2 and ZnO, the primary inorganic sunscreens, the sheer number of separate investigations warrants individual articles, and ZnO was addressed in previous columns. Briefly, though, TiO2 is more photoactive and exhibits a higher refractive index in visible light than ZnO (J. Am. Acad. Dermatol. 1999;40:85-90); therefore, TiO2 appears whiter and is more difficult to incorporate into transparent products.
A 2011 study by Kang et al. showed that TiO2 nanoparticles, but not normal-sized TiO2, and UVA synergistically foster rapid production of reactive oxygen species and breakdown of mitochondrial membrane potential, leading to apoptosis, and that TiO2 nanoparticles are more phototoxic than larger ones (Drug Chem. Toxicol. 2011;34:277-84).
However, also in 2011, Tyner et al. investigated the effects of nanoscale TiO2 use on UV attenuation in simple to complex sunscreen products. They found that barrier function was diminished by none of the formulations, and that optimal UV attenuation resulted when TiO2 particles were stabilized with a coating and evenly dispersed. The researchers concluded that nanoscale TiO2 is nontoxic and may impart greater efficacy (Int. J. Cosmet. Sci. 2011;33:234-44).
In vitro and in vivo studies
In 2010, Tiano et al. evaluated five modified TiO2 particles, developed and marketed for sunscreens. They used different in vitro models, including cultured human skin fibroblasts, to determine potential photocatalytic effects after UVA exposure. The investigators found that the kind of modification to and crystal form of the TiO2 nanoparticle influences its ability to augment or reduce DNA damage, increase or decrease intracellular reactive oxygen species, diminish cell viability, and promote other effects of photocatalysis. In particular, they noted that the anatase crystal form of TiO2 retained photocatalytic activity. The authors suggested that while the debate continues over the penetration of nanosized TiO2 into the viable epidermis, their results help elucidate the potential effects of TiO2 particles at the cellular level (Free Radic. Biol. Med. 2010;49:408-15).
A 2010 study by Senzui et al. using in vitro intact, stripped, and hair-removed skin of Yucatan micropigs to test the skin penetration of four different types of rutile (the most natural form of) TiO2 (two coated, two uncoated) revealed no penetration of TiO2 type in intact and stripped skin. The concentration of titanium in skin was significantly higher when one of the coated forms was applied on hair-removed skin, with titanium penetrating into vacant hair follicles (greater than 1 mm below the skin surface), but not into dermis or viable epidermis (J. Toxicol. Sci. 2010;35:107-13).
Animal studies
In 2009, the Food and Drug Administration Center for Drug Evaluation and Research worked with the National Center for Toxicology Research using minipigs and four sunscreen formulations to determine whether nanoscale TiO2 can penetrate intact skin. Their use of scanning electron microscopy and x-ray diffraction revealed that TiO2 particles were the same size as that observed for the raw materials, implying that the formulation process influenced neither the size nor the shape of TiO2 particles (Drug Dev. Ind. Pharm. 2009;35:1180-9).
In 2010, Sadrieh et al. performed a study of the dermal penetration of three TiO2 particles: uncoated submicrometer-sized, uncoated nano-sized, and dimethicone/methicone copolymer-coated nanosized. The investigators applied 5% by weight of each of the types of particles in a sunscreen on minipigs and found no significant penetration into intact normal epidermis (Toxicol. Sci. 2010;115(1):156-66).
In 2011, Furukawa et al. studied the postinitiation carcinogenic potential of coated and uncoated TiO2 nanoparticles in a two-stage skin carcinogenesis model using 7-week-old CD1 (ICR) female mice. They found that application of coated and uncoated nanoparticles after initiation and promotion with 7,12-dimethylbenz[a]anthracene and 12-O-tetradecanoylphorbol 13-acetate at doses of up to 20 mg/mouse failed to augment nodule development. The investigators concluded that TiO2 nanoparticles do not exhibit postinitiation potential for mouse skin carcinogenesis (Food Chem. Toxicol. 2011;49(4):744-9).
Human data
Given the persistent concerns about possible side effects of coated TiO2 and ZnO nanoparticles used in physical sun blockers, Filipe et al., in 2009, assessed the localization and potential skin penetration of TiO2 and ZnO nanoparticles dispersed in three sunscreen formulations, under realistic in vivo conditions in normal and altered skin. The investigators examined a test hydrophobic formulation containing coated 20-nm TiO2 nanoparticles and two commercially available sunscreen formulations containing TiO2 alone or in combination with ZnO, with respect to how consumers actually used sunscreens compared with the recommended standard condition for the sun protection factor test. They found that traces of the physical blockers could be detected only at the skin surface and uppermost area of the stratum corneum in normal human skin after a 2-hour exposure. After 48 hours of exposure, layers deeper than the stratum corneum contained no detectable TiO2 or ZnO nanoparticles. While preferential deposition of the nanoparticles in the openings of pilosebaceous follicles was noted, no penetration into viable skin tissue was observed. The investigators concluded that significant penetration of TiO2 or ZnO nanoparticles into keratinocytes is improbable (Skin Pharmacol. Physiol. 2009;22:266-75).
The weight of evidence
Current evidence suggests minimal risks to human health from the use of TiO2 or ZnO nanoparticles at concentrations up to 25% in cosmetic preparations or sunscreens, according to Schilling et al., regardless of coatings or crystalline structure. In a safety review of these ingredients, they noted that these nanoparticles formulated in topical products occur as aggregates of primary particles 30-150 nm in size, and bond in such a way that renders them impervious to the force of product application. Thus their structure remains unaffected, and no primary particles are released. The authors also noted that nanoparticles exhibit equivalence with larger particles in terms of distribution and duration and, therefore, recognition and elimination from the body (Photochem. Photobiol. Sci. 2010;9:495-509).
But in 2011, Tran and Salmon, in light of findings that nanoparticles may penetrate the stratum corneum under certain conditions, considered the possible photocarcinogenic results of nanoparticle sunscreens. They noted, though, that most such results were obtained through the use of animal skin models, not investigations with human skin (Australas. J. Dermatol. 2011;52:1-6). To this point, the weight of evidence appears to show that such TiO2 nanoparticles are safe when applied to intact human skin (Semin. Cutan. Med. Surg. 2011;30:210-13).
In response to the increased scrutiny and concern exhibited by the general public and government agencies regarding the safety of TiO2 and ZnO nanoparticles, Newman et al. reviewed the literature and position statements from 1980 to 2008 to ascertain and describe the use, safety, and regulatory state of such ingredients in sunscreens. They found no evidence of significant penetration deeper than the stratum corneum of TiO2 and ZnO nanoparticles, but caution that additional studies simulating real-world conditions (i.e., sunburned skin and under UV exposure) are necessary (J. Am. Acad. Dermatol. 2009;61:685-92).
Conclusion
Titanium dioxide is a well-established, safe, and effective physical sunblock. Nanotechnology has introduced some cause for concern regarding its use in physical sunblocks. In particular, evidence suggesting that photoexcitation of TiO2 nanoparticles leads to the generation of reactive oxygen species that damage DNA, potentially launching a cascade of adverse events, has prompted investigations into the safety of TiO2 in nanoparticle form. However, to date, multiple studies suggest that TiO2 nanoparticles do not penetrate or are highly unlikely to penetrate beyond the stratum corneum.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Titanium dioxide (TiO2) and zinc oxide (ZnO) in large-particle form have long been used in various sunscreens to protect the skin by reflecting or physically blocking ultraviolet (UV) radiation. In recent years, TiO2 as well as ZnO nanoparticles have been incorporated into sunscreens and cosmetics to act as a UV shield. They have been shown to be effective barriers against UV-induced damage, and yield stronger protection against UV insult, while leaving less white residue, than previous generations of the physical sunblocks.
However, some data suggest that in nanoparticle form, TiO2 and ZnO absorb UV radiation, leading to photocatalysis and the release of reactive oxygen species (Australas. J. Dermatol. 2011;52:1-6). This column will focus primarily on the safety of TiO2 in nanoparticle form.
While numerous studies examine both TiO2 and ZnO, the primary inorganic sunscreens, the sheer number of separate investigations warrants individual articles, and ZnO was addressed in previous columns. Briefly, though, TiO2 is more photoactive and exhibits a higher refractive index in visible light than ZnO (J. Am. Acad. Dermatol. 1999;40:85-90); therefore, TiO2 appears whiter and is more difficult to incorporate into transparent products.
A 2011 study by Kang et al. showed that TiO2 nanoparticles, but not normal-sized TiO2, and UVA synergistically foster rapid production of reactive oxygen species and breakdown of mitochondrial membrane potential, leading to apoptosis, and that TiO2 nanoparticles are more phototoxic than larger ones (Drug Chem. Toxicol. 2011;34:277-84).
However, also in 2011, Tyner et al. investigated the effects of nanoscale TiO2 use on UV attenuation in simple to complex sunscreen products. They found that barrier function was diminished by none of the formulations, and that optimal UV attenuation resulted when TiO2 particles were stabilized with a coating and evenly dispersed. The researchers concluded that nanoscale TiO2 is nontoxic and may impart greater efficacy (Int. J. Cosmet. Sci. 2011;33:234-44).
In vitro and in vivo studies
In 2010, Tiano et al. evaluated five modified TiO2 particles, developed and marketed for sunscreens. They used different in vitro models, including cultured human skin fibroblasts, to determine potential photocatalytic effects after UVA exposure. The investigators found that the kind of modification to and crystal form of the TiO2 nanoparticle influences its ability to augment or reduce DNA damage, increase or decrease intracellular reactive oxygen species, diminish cell viability, and promote other effects of photocatalysis. In particular, they noted that the anatase crystal form of TiO2 retained photocatalytic activity. The authors suggested that while the debate continues over the penetration of nanosized TiO2 into the viable epidermis, their results help elucidate the potential effects of TiO2 particles at the cellular level (Free Radic. Biol. Med. 2010;49:408-15).
A 2010 study by Senzui et al. using in vitro intact, stripped, and hair-removed skin of Yucatan micropigs to test the skin penetration of four different types of rutile (the most natural form of) TiO2 (two coated, two uncoated) revealed no penetration of TiO2 type in intact and stripped skin. The concentration of titanium in skin was significantly higher when one of the coated forms was applied on hair-removed skin, with titanium penetrating into vacant hair follicles (greater than 1 mm below the skin surface), but not into dermis or viable epidermis (J. Toxicol. Sci. 2010;35:107-13).
Animal studies
In 2009, the Food and Drug Administration Center for Drug Evaluation and Research worked with the National Center for Toxicology Research using minipigs and four sunscreen formulations to determine whether nanoscale TiO2 can penetrate intact skin. Their use of scanning electron microscopy and x-ray diffraction revealed that TiO2 particles were the same size as that observed for the raw materials, implying that the formulation process influenced neither the size nor the shape of TiO2 particles (Drug Dev. Ind. Pharm. 2009;35:1180-9).
In 2010, Sadrieh et al. performed a study of the dermal penetration of three TiO2 particles: uncoated submicrometer-sized, uncoated nano-sized, and dimethicone/methicone copolymer-coated nanosized. The investigators applied 5% by weight of each of the types of particles in a sunscreen on minipigs and found no significant penetration into intact normal epidermis (Toxicol. Sci. 2010;115(1):156-66).
In 2011, Furukawa et al. studied the postinitiation carcinogenic potential of coated and uncoated TiO2 nanoparticles in a two-stage skin carcinogenesis model using 7-week-old CD1 (ICR) female mice. They found that application of coated and uncoated nanoparticles after initiation and promotion with 7,12-dimethylbenz[a]anthracene and 12-O-tetradecanoylphorbol 13-acetate at doses of up to 20 mg/mouse failed to augment nodule development. The investigators concluded that TiO2 nanoparticles do not exhibit postinitiation potential for mouse skin carcinogenesis (Food Chem. Toxicol. 2011;49(4):744-9).
Human data
Given the persistent concerns about possible side effects of coated TiO2 and ZnO nanoparticles used in physical sun blockers, Filipe et al., in 2009, assessed the localization and potential skin penetration of TiO2 and ZnO nanoparticles dispersed in three sunscreen formulations, under realistic in vivo conditions in normal and altered skin. The investigators examined a test hydrophobic formulation containing coated 20-nm TiO2 nanoparticles and two commercially available sunscreen formulations containing TiO2 alone or in combination with ZnO, with respect to how consumers actually used sunscreens compared with the recommended standard condition for the sun protection factor test. They found that traces of the physical blockers could be detected only at the skin surface and uppermost area of the stratum corneum in normal human skin after a 2-hour exposure. After 48 hours of exposure, layers deeper than the stratum corneum contained no detectable TiO2 or ZnO nanoparticles. While preferential deposition of the nanoparticles in the openings of pilosebaceous follicles was noted, no penetration into viable skin tissue was observed. The investigators concluded that significant penetration of TiO2 or ZnO nanoparticles into keratinocytes is improbable (Skin Pharmacol. Physiol. 2009;22:266-75).
The weight of evidence
Current evidence suggests minimal risks to human health from the use of TiO2 or ZnO nanoparticles at concentrations up to 25% in cosmetic preparations or sunscreens, according to Schilling et al., regardless of coatings or crystalline structure. In a safety review of these ingredients, they noted that these nanoparticles formulated in topical products occur as aggregates of primary particles 30-150 nm in size, and bond in such a way that renders them impervious to the force of product application. Thus their structure remains unaffected, and no primary particles are released. The authors also noted that nanoparticles exhibit equivalence with larger particles in terms of distribution and duration and, therefore, recognition and elimination from the body (Photochem. Photobiol. Sci. 2010;9:495-509).
But in 2011, Tran and Salmon, in light of findings that nanoparticles may penetrate the stratum corneum under certain conditions, considered the possible photocarcinogenic results of nanoparticle sunscreens. They noted, though, that most such results were obtained through the use of animal skin models, not investigations with human skin (Australas. J. Dermatol. 2011;52:1-6). To this point, the weight of evidence appears to show that such TiO2 nanoparticles are safe when applied to intact human skin (Semin. Cutan. Med. Surg. 2011;30:210-13).
In response to the increased scrutiny and concern exhibited by the general public and government agencies regarding the safety of TiO2 and ZnO nanoparticles, Newman et al. reviewed the literature and position statements from 1980 to 2008 to ascertain and describe the use, safety, and regulatory state of such ingredients in sunscreens. They found no evidence of significant penetration deeper than the stratum corneum of TiO2 and ZnO nanoparticles, but caution that additional studies simulating real-world conditions (i.e., sunburned skin and under UV exposure) are necessary (J. Am. Acad. Dermatol. 2009;61:685-92).
Conclusion
Titanium dioxide is a well-established, safe, and effective physical sunblock. Nanotechnology has introduced some cause for concern regarding its use in physical sunblocks. In particular, evidence suggesting that photoexcitation of TiO2 nanoparticles leads to the generation of reactive oxygen species that damage DNA, potentially launching a cascade of adverse events, has prompted investigations into the safety of TiO2 in nanoparticle form. However, to date, multiple studies suggest that TiO2 nanoparticles do not penetrate or are highly unlikely to penetrate beyond the stratum corneum.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Hair washing – Too much or too little?
Many dermatologists continue to battle an overwashing epidemic. From bar soaps to antibacterial washes, dermatologists continue to educate patients that the extensive lather, the alkaline pH, and the antibacterial components of our washing rituals can strip the natural oils from the skin and leave it dry, cracked, and damaged.
This phenomenon is well reported in the literature, and industry has taken notice by developing more "no-soap" soaps than ever before.
But does the same philosophy apply to hair care practices? Hair washing is more complicated, particularly in skin of color patients.
Overwashing the hair often leads to dry hair, split ends, and the need for compensatory conditioners to replace lost moisture. In African American hair, especially that of patients who use chemical or heat treatments, the lost oil and sebum from overwashing can cause even more damage.
Many skin of color patients wash their hair infrequently to protect it from breakage, and they may use topical oils to smooth and protect the fragile hair shaft.
However, can underwashing the scalp and hair cause problems? Yes, in some cases.
You might see African American patients in your practice who are suffering from scalp folliculitis, itchy scalp, seborrheic dermatitis, or alopecia that can be traced to infrequent hair washing. The infrequency of washing and the application of oils to the hair does help the hair shaft, but the buildup of oils and sebum on the scalp itself can lead to scalp inflammation, follicular plugging, extensive seborrhea, acneiform eruptions, and folliculitis.
Depending on its level and degree, the inflammation can cause pruritus and burning of the scalp and can even lead to temporary or permanent hair loss. Although topical and oral antibiotics, topical steroids, and medicated shampoos do help, proper washing also plays an important preventative role.
For skin of color patients with some of the chronic scalp problems mentioned above, decreasing heat and chemical treatments, along with increasing hair washing to two or three times a week can help prevent scalp dermatitides without compromising the hair integrity. In addition, the use of sulfate-free shampoos, use of shampoo on the scalp only (without lathering the ends of the hair), or use of a dry shampoo between washes can help control the oil and product buildup on the scalp itself.
Ultimately, it may take some trial and error to find the right hair washing regimen for skin of color patients. Determining how often to wash the scalp depends on many patient-specific factors including ethnicity, hair type, frequency of chemical and heat treatments, cost, and level of scalp inflammation. Experimenting with new hair care products and possibly a new hairstyle also may be part of a successful treatment plan.
Dr. Talakoub is in private practice at McLean (Va.) Dermatology Center. A graduate of Boston University School of Medicine, Dr. Talakoub did her residency in dermatology at the University of California, San Francisco. She is the author of multiple scholarly articles and a textbook chapter.
Many dermatologists continue to battle an overwashing epidemic. From bar soaps to antibacterial washes, dermatologists continue to educate patients that the extensive lather, the alkaline pH, and the antibacterial components of our washing rituals can strip the natural oils from the skin and leave it dry, cracked, and damaged.
This phenomenon is well reported in the literature, and industry has taken notice by developing more "no-soap" soaps than ever before.
But does the same philosophy apply to hair care practices? Hair washing is more complicated, particularly in skin of color patients.
Overwashing the hair often leads to dry hair, split ends, and the need for compensatory conditioners to replace lost moisture. In African American hair, especially that of patients who use chemical or heat treatments, the lost oil and sebum from overwashing can cause even more damage.
Many skin of color patients wash their hair infrequently to protect it from breakage, and they may use topical oils to smooth and protect the fragile hair shaft.
However, can underwashing the scalp and hair cause problems? Yes, in some cases.
You might see African American patients in your practice who are suffering from scalp folliculitis, itchy scalp, seborrheic dermatitis, or alopecia that can be traced to infrequent hair washing. The infrequency of washing and the application of oils to the hair does help the hair shaft, but the buildup of oils and sebum on the scalp itself can lead to scalp inflammation, follicular plugging, extensive seborrhea, acneiform eruptions, and folliculitis.
Depending on its level and degree, the inflammation can cause pruritus and burning of the scalp and can even lead to temporary or permanent hair loss. Although topical and oral antibiotics, topical steroids, and medicated shampoos do help, proper washing also plays an important preventative role.
For skin of color patients with some of the chronic scalp problems mentioned above, decreasing heat and chemical treatments, along with increasing hair washing to two or three times a week can help prevent scalp dermatitides without compromising the hair integrity. In addition, the use of sulfate-free shampoos, use of shampoo on the scalp only (without lathering the ends of the hair), or use of a dry shampoo between washes can help control the oil and product buildup on the scalp itself.
Ultimately, it may take some trial and error to find the right hair washing regimen for skin of color patients. Determining how often to wash the scalp depends on many patient-specific factors including ethnicity, hair type, frequency of chemical and heat treatments, cost, and level of scalp inflammation. Experimenting with new hair care products and possibly a new hairstyle also may be part of a successful treatment plan.
Dr. Talakoub is in private practice at McLean (Va.) Dermatology Center. A graduate of Boston University School of Medicine, Dr. Talakoub did her residency in dermatology at the University of California, San Francisco. She is the author of multiple scholarly articles and a textbook chapter.
Many dermatologists continue to battle an overwashing epidemic. From bar soaps to antibacterial washes, dermatologists continue to educate patients that the extensive lather, the alkaline pH, and the antibacterial components of our washing rituals can strip the natural oils from the skin and leave it dry, cracked, and damaged.
This phenomenon is well reported in the literature, and industry has taken notice by developing more "no-soap" soaps than ever before.
But does the same philosophy apply to hair care practices? Hair washing is more complicated, particularly in skin of color patients.
Overwashing the hair often leads to dry hair, split ends, and the need for compensatory conditioners to replace lost moisture. In African American hair, especially that of patients who use chemical or heat treatments, the lost oil and sebum from overwashing can cause even more damage.
Many skin of color patients wash their hair infrequently to protect it from breakage, and they may use topical oils to smooth and protect the fragile hair shaft.
However, can underwashing the scalp and hair cause problems? Yes, in some cases.
You might see African American patients in your practice who are suffering from scalp folliculitis, itchy scalp, seborrheic dermatitis, or alopecia that can be traced to infrequent hair washing. The infrequency of washing and the application of oils to the hair does help the hair shaft, but the buildup of oils and sebum on the scalp itself can lead to scalp inflammation, follicular plugging, extensive seborrhea, acneiform eruptions, and folliculitis.
Depending on its level and degree, the inflammation can cause pruritus and burning of the scalp and can even lead to temporary or permanent hair loss. Although topical and oral antibiotics, topical steroids, and medicated shampoos do help, proper washing also plays an important preventative role.
For skin of color patients with some of the chronic scalp problems mentioned above, decreasing heat and chemical treatments, along with increasing hair washing to two or three times a week can help prevent scalp dermatitides without compromising the hair integrity. In addition, the use of sulfate-free shampoos, use of shampoo on the scalp only (without lathering the ends of the hair), or use of a dry shampoo between washes can help control the oil and product buildup on the scalp itself.
Ultimately, it may take some trial and error to find the right hair washing regimen for skin of color patients. Determining how often to wash the scalp depends on many patient-specific factors including ethnicity, hair type, frequency of chemical and heat treatments, cost, and level of scalp inflammation. Experimenting with new hair care products and possibly a new hairstyle also may be part of a successful treatment plan.
Dr. Talakoub is in private practice at McLean (Va.) Dermatology Center. A graduate of Boston University School of Medicine, Dr. Talakoub did her residency in dermatology at the University of California, San Francisco. She is the author of multiple scholarly articles and a textbook chapter.
Cosmetic Corner: Dermatologists Weigh in on OTC Antifungals
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top OTC antifungal products. Consideration must be given to:
Cutis invites readers to send us their recommendations. Antiperspirants and OTC hair restoration products will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top OTC antifungal products. Consideration must be given to:
Cutis invites readers to send us their recommendations. Antiperspirants and OTC hair restoration products will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top OTC antifungal products. Consideration must be given to:
Cutis invites readers to send us their recommendations. Antiperspirants and OTC hair restoration products will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
Basal Cell Carcinoma: Analysis of Factors Associated With Incomplete Excision at a Referral Hospital in Southern Spain
Cutaneous Squamous Cell Carcinoma With Perineural Invasion: A Case Report and Review of the Literature
Case Report
A 74-year-old man with a history of squamous cell carcinoma (SCC) on the right side of the temple that was treated with Mohs micrographic surgery (MMS) 3 years prior presented with a burning and tingling sensation of 3 months’ duration in the medial border of the repair scar. The patient denied prior anesthesia or muscle weakness of the face as well as any loss or change in vision.
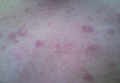
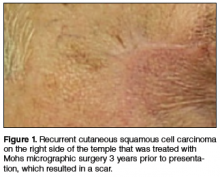
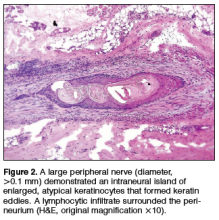
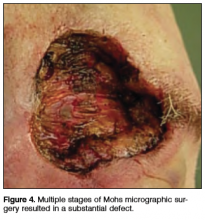
Physical examination revealed a well-healed advancement flap scar with induration at the medial border (Figure 1). Biopsy results were positive for recurrent SCC. Based on anatomic location, clinical symptoms, and tumor recurrence, treatment with MMS was initiated. Mohs sections demonstrated perineural invasion (PNI) (Figures 2 and 3). Multiple treatment stages were required for tumor clearance following the retrograde course of a nerve, which resulted in a substantial defect (Figure 4). The defect was allowed to heal by second intention followed by radiation therapy.
Comment
Incidence and Pathogenesis—Perineural invasion was first described by Cruveilhier1 in a report of invasion of the facial nerve in a patient with mammary carcinoma. Neumann2 reported the first case of a primary cutaneous lesion exhibiting PNI in a patient with a primary carcinoma of the lower lip with invasion and spread along the mental nerve. Perineural invasion is seen in approximately 5% of 200,000 total cases of cutaneous SCC reported annually in the United States.3,4 Other malignancies exhibit PNI more frequently, such as microcystic adnexal carcinoma of the skin, which has been reported to have an 80% rate of perineural growth.5
Perineural invasion can involve nerves of variable thickness, but invasion of larger nerves typically portends a poorer prognosis.6 Characteristics of cutaneous SCC that predispose the lesion to PNI include size greater than 2 cm, male gender, location on the face, and prior treatment of the lesion.6,7 In a study of cutaneous SCC, Leibovitch et al7 found PNI in 4.7% (36/772) of primary lesions and 6.9% (34/491) of recurrent lesions. In another study of 180 SCC tumors of the head and neck with PNI, Carter et al8 found that PNI was most commonly seen in tumors that were greater than 2.5 cm, suggesting that larger lesions have an increased predisposition for PNI.
The mechanism(s) by which PNI develops from these malignancies has not been fully elucidated, but some clues have been found. Vural et al9 showed a statistically significant difference (P<.01) in expression of neural cell adhesion molecules with 93% (38/41) of SCCs with PNI showing evidence of expression versus 36% (9/25) of SCCs without PNI. Chen-Tsai et al10 also suggested that levels of neural cell adhesion molecules may be a factor in determining the metastatic potential of cutaneous SCCs and that levels of neurotrophic tyrosine kinase receptor type 1 (TrkA) may predict PNI, but their study results lacked statistical power to form a firm conclusion.
Diagnosis and Prognosis—Perineural invasion can be diagnosed clinically, radiologically, or microscopically. On clinical examination, PNI is suggested by findings of neuropathy most frequently in cranial nerves V and/or VII, likely due to their extensive subcutaneous distribution.11 Common symptoms include pain, loss of motor skills, anesthesia, dysesthesia, and/or paresthesia (ie, tingling, burning, pricking, numbness).12,13 In a study of 72 cases, Goepfert et al14 found that only 40% (29/72) of patients with pathologically confirmed PNI presented with clinical symptoms and these patients had a poorer prognosis.
Radiologically, PNI can be identified via computed tomography or magnetic resonance imaging through findings of enlargement or abnormal enhancement of the nerve, obliteration of the normal fat plane surrounding the nerve, or erosion or enlargement of its related foramen.15 Magnetic resonance imaging is the preferred method for assessing enhancement of the nerve, while computed tomography is preferred to assess involvement of bone.16,17 Microscopically, there is some debate as to what defines PNI. Suggested findings include the presence of cells inside the epineurium, involvement of nerves outside the main bulk of the tumor, or presence of tumor cells surrounding a nerve.18
These definitions have prognostic significance. Mendenhall et al16 found that patients with radiologic evidence of PNI without clinical symptoms had a higher cure rate using surgery and postoperative irradiation compared to patients with clinical symptoms (80% vs 45%). Although prognosis generally is good in patients with cutaneous SCC without PNI, prognosis is notably poorer when PNI is present due to the association of this finding with increased tumor recurrence and both local and distant metastasis.13 Most frequently, cutaneous SCC with PNI spreads proximally, which can lead to invasion into the base of the brain, but also can extend distally, leading to increased local burden.12,19 In a study of 64 patients with mucosal SCC, Soo et al20 found that patients with lesions that exhibited PNI had a 5-year survival of 16% versus 44% in those without PNI. In their study of SCC of the head and neck, Goepfert et al14 reported that 46% (33/72) of patients with PNI had died or were alive with recurrence at 2 years’ follow-up versus 9.1% (41/448) of patients without PNI. In a systematic review of outcomes, Jambusaria-Pahlajani et al21 reported a disease-specific death rate of 16% for cutaneous SCC with PNI compared to 4% for SCC without PNI.
Perineural invasion can be further classified as clinical or microscopic (incidental) for prognostic purposes. A study by Garcia-Serra et al13 found that patients with clinical PNI had a notably poorer prognosis than those with microscopic (incidental) PNI. The clinical group achieved a local control rate of 55% at 5 years’ follow-up versus 87% in the microscopic group. McCord et al22 found a 5-year local control rate of 78% for microscopic (incidental) PNI versus 50% for clinical PNI; they also found that patients with radiologic evidence of PNI had a worse prognosis, noting that patients with radiologic evidence of PNI were nearly all clinically symptomatic.
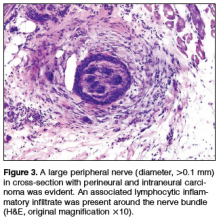
Prognosis also is altered by the diameter of the nerve involved. In a study of 48 patients, Ross et al23 found that patients with cutaneous SCC involving small-caliber nerves (diameter, ≤0.1 mm) had a 0% disease-specific death rate versus 32% in those with large-caliber nerves (>0.1 mm). Perineural involvement of small-caliber nerves (<0.1 mm) was a positive prognostic indicator in that it was associated with smaller tumor diameter, more shallow invasion, and increased likelihood to be primary tumors.23 In a recent study, Jambusaria-Pahlajani et al24 investigated tumor staging for cutaneous SCC and reported that PNI is a statistically independent prognostic risk factor for nodal metastasis (subhazard ratio, 2.2 [95% confidence interval, 0.9-5.1]) and disease-specific death (subhazard ratio, 3.4 [95% confidence interval, 0.9-13.3]). Of interest, this increased risk applied only to PNI in nerves that were greater than 0.1 mm.24
Treatment Options—Management of confirmed cases of cutaneous SCC with PNI is difficult because of the nature of the lesions, including their increased propensity for metastasis, increased frequency of poorly differentiated cell types, highly aggressive nature, and the unique challenge of skip lesions.4,16 Skip lesions are found microscopically and show (or appear to show) neoplastic cells invading a nerve in a discontinuous fashion. This phenomenon has been suggested as one explanation for the relatively higher postsurgical recurrence rate of SCC with PNI compared to lesions without PNI.7 They are of particular interest when removing cutaneous SCC with PNI using MMS and attempting to define clear margins. Despite this limitation, MMS generally is accepted as the primary mode of excision of cutaneous SCCs with PNI, as it has the highest known cure rate.7 Cottel4 did not report any cases of local recurrence over 1 to 42 months in 17 patients who were treated with MMS, in contrast to Rowe et al25 who demonstrated that traditional surgical excision had a 47% (34/72) local recurrence rate; however, it bears noting that the varying follow-up periods in the Cottel4 study may underestimate recurrence rate. Leibovitch et al7 had similar findings in their prospective case series study of 70 patients, which revealed an 8% recurrence rate within 5 years in patients treated with MMS, a rate lower than other non-MMS modalities. In this same study, the authors noted that some researchers believe an additional level should be taken with MMS beyond the appearance of free margins in cases with PNI.7
Jambusaria-Pahlajani et al21 reported that PNI is one of the most common reasons cited for using adjuvant radiation therapy for cases of cutaneous SCC because of the known propensity of local recurrence; however, in 74 reviewed cases, there was no statistically significant difference in outcomes in cases of surgery alone versus surgery and adjuvant irradiation. Radiation therapy is a possible alternative primary treatment of cutaneous SCC with PNI, especially in cases of perineural involvement that is extensive or affects proximal portions of cranial nerves when surgery is a less viable option.17 Mendenhall et al16 suggested that patients with positive margins after excision who display extensive PNI should be treated with adjuvant irradiation locally and along the course of the involved nerve to the skull base.
Conclusion
Physicians should recognize the importance of early detection of PNI in cases of cutaneous SCC. A thorough history with good neurologic examination of the head and neck in patients with cutaneous SCC is imperative so patients can be treated earlier in the course of the lesion, increasing the likelihood of local control, minimizing the risk for future recurrence, and decreasing mortality.
1. Cruveilhier J. Maladies des nerfs. In: Cruveilhier J, ed. Anatomie Pathologique du Corps Humain. 2nd ed. Paris, France: JB Bailliere; 1835:1-3.
2. Neumann E. Secondare cancroid infiltration des nervus mentalis bei einem fall von lippincroid. Arch Pathol Anat. 1862;24:201-205.
3. Salasche S. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1, pt 2):4-7.
4. Cottel WI. Perineural invasion by squamous-cell carcinoma. J Dermatol Surg Oncol. 1982;8:589-600.
5. Cooper PH, Mills SE, Leonard DD, et al. Sclerosing sweat duct (syringomatous) carcinoma. Am J Surg Pathol. 1985;9:422-433.
6. Ross AS, Whalen FM, Elenitsas R, et al. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35:1859-1866.
7. Leibovitch I, Huilgol SC, Selva D, et al. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia II. perineural invasion. J Am Acad Dermatol. 2005;53:261-266.
8. Carter RL, Foster CS, Dinsdale EA, et al. Perineural spread by squamous carcinomas of the head and neck: a morphological study using antiaxonal and antimyelin monoclonal antibodies. J Clin Pathol. 1983;36:269-275.
9. Vural E, Hutcheson J, Korourian S, et al. Correlation of neural cell adhesion molecules with perineural spread of squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg. 2000;122:717-720.
10. Chen-Tsai CP, Colome-Grimmer M, Wagner RF Jr. Correlations among neural cell adhesion molecule, nerve growth factor, and its receptors, TrkA, TrkB, TrkC, and p75, in perineural invasion by basal cell and cutaneous squamous cell carcinomas. Dermatol Surg. 2004;30:1009-1016.
11. McCord M, Mendenhall WM, Parsons JT, et al. Skin cancer of the head and neck with clinical perineural invasion. Int J Radiat Oncol Biol Phys. 2000;47:89-93.
12. Ampil FL, Hardin JC, Peskind SP, et al. Perineural invasion in skin cancer of the head and neck: a review of nine cases. J Oral Maxillofac Surg. 1995;53:34-38.
13. Garcia-Serra A, Hinerman RW, Mendenhall WM, et al. Carcinoma of the skin with perineural invasion. Head Neck. 2003;25:1027-1033.
14. Goepfert H, Dichtel WJ, Medina JE, et al. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148:542-547.
15. Galloway TJ, Morris CG, Mancuso AA, et al. Impact of radiographic findings on prognosis for skin carcinoma with clinical perineural invasion. Cancer. 2005;103:1254-1257.
16. Mendenhall WM, Amdur RJ, Williams LS, et al. Carcinoma of the skin of the head and neck with perineural invasion. Head Neck. 2002;24:78-83.
17. Williams LS, Mancuso AA, Mendenhall WM. Perineural spread of cutaneous squamous and basal cell carcinoma: CT and MR detection and its impact on patient management and prognosis. Int J Radiat Oncol Biol Phys. 2001;49:1061-1069.
18. Veness MJ, Biankin S. Perineural spread leading to orbital invasion from skin cancer. Australasian Radiol. 2000;44:296-302.
19. Feasel AM, Brown TJ, Bogle MA, et al. Perineural invasion of cutaneous malignancies. Dermatol Surg. 2001;27:531-542.
20. Soo K, Carter RL, O’Brien CJ, et al. Prognostic implications of perineural spread in squamous carcinomas of the head and neck. Laryngoscope. 1986;96:1145-1148.
21. Jambusaria-Pahlajani A, Miller CJ, Quon H, et al. Surgical monotherapy versus surgery plus adjuvant radiotherapy in high-risk cutaneous squamous cell carcinoma: a systematic review of outcomes. Dermatol Surg. 2009;35:574-584.
22. McCord MW, Mendenhall WM, Parsons JT, et al. Skin cancer of the head and neck with incidental microscopic perineural invasion. Int J Radiat Oncol Biol Phys. 1999;43:591-595.
23. Ross AS, Whalen FM, Elenitsas R, et al. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35:1859-1866.
24. Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149:402-410.
25. Rowe DE, Carroll RJ, Day CL. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. J Am Acad Dermatol. 1992;26:976-990.
Case Report
A 74-year-old man with a history of squamous cell carcinoma (SCC) on the right side of the temple that was treated with Mohs micrographic surgery (MMS) 3 years prior presented with a burning and tingling sensation of 3 months’ duration in the medial border of the repair scar. The patient denied prior anesthesia or muscle weakness of the face as well as any loss or change in vision.
Physical examination revealed a well-healed advancement flap scar with induration at the medial border (Figure 1). Biopsy results were positive for recurrent SCC. Based on anatomic location, clinical symptoms, and tumor recurrence, treatment with MMS was initiated. Mohs sections demonstrated perineural invasion (PNI) (Figures 2 and 3). Multiple treatment stages were required for tumor clearance following the retrograde course of a nerve, which resulted in a substantial defect (Figure 4). The defect was allowed to heal by second intention followed by radiation therapy.
Comment
Incidence and Pathogenesis—Perineural invasion was first described by Cruveilhier1 in a report of invasion of the facial nerve in a patient with mammary carcinoma. Neumann2 reported the first case of a primary cutaneous lesion exhibiting PNI in a patient with a primary carcinoma of the lower lip with invasion and spread along the mental nerve. Perineural invasion is seen in approximately 5% of 200,000 total cases of cutaneous SCC reported annually in the United States.3,4 Other malignancies exhibit PNI more frequently, such as microcystic adnexal carcinoma of the skin, which has been reported to have an 80% rate of perineural growth.5
Perineural invasion can involve nerves of variable thickness, but invasion of larger nerves typically portends a poorer prognosis.6 Characteristics of cutaneous SCC that predispose the lesion to PNI include size greater than 2 cm, male gender, location on the face, and prior treatment of the lesion.6,7 In a study of cutaneous SCC, Leibovitch et al7 found PNI in 4.7% (36/772) of primary lesions and 6.9% (34/491) of recurrent lesions. In another study of 180 SCC tumors of the head and neck with PNI, Carter et al8 found that PNI was most commonly seen in tumors that were greater than 2.5 cm, suggesting that larger lesions have an increased predisposition for PNI.
The mechanism(s) by which PNI develops from these malignancies has not been fully elucidated, but some clues have been found. Vural et al9 showed a statistically significant difference (P<.01) in expression of neural cell adhesion molecules with 93% (38/41) of SCCs with PNI showing evidence of expression versus 36% (9/25) of SCCs without PNI. Chen-Tsai et al10 also suggested that levels of neural cell adhesion molecules may be a factor in determining the metastatic potential of cutaneous SCCs and that levels of neurotrophic tyrosine kinase receptor type 1 (TrkA) may predict PNI, but their study results lacked statistical power to form a firm conclusion.
Diagnosis and Prognosis—Perineural invasion can be diagnosed clinically, radiologically, or microscopically. On clinical examination, PNI is suggested by findings of neuropathy most frequently in cranial nerves V and/or VII, likely due to their extensive subcutaneous distribution.11 Common symptoms include pain, loss of motor skills, anesthesia, dysesthesia, and/or paresthesia (ie, tingling, burning, pricking, numbness).12,13 In a study of 72 cases, Goepfert et al14 found that only 40% (29/72) of patients with pathologically confirmed PNI presented with clinical symptoms and these patients had a poorer prognosis.
Radiologically, PNI can be identified via computed tomography or magnetic resonance imaging through findings of enlargement or abnormal enhancement of the nerve, obliteration of the normal fat plane surrounding the nerve, or erosion or enlargement of its related foramen.15 Magnetic resonance imaging is the preferred method for assessing enhancement of the nerve, while computed tomography is preferred to assess involvement of bone.16,17 Microscopically, there is some debate as to what defines PNI. Suggested findings include the presence of cells inside the epineurium, involvement of nerves outside the main bulk of the tumor, or presence of tumor cells surrounding a nerve.18
These definitions have prognostic significance. Mendenhall et al16 found that patients with radiologic evidence of PNI without clinical symptoms had a higher cure rate using surgery and postoperative irradiation compared to patients with clinical symptoms (80% vs 45%). Although prognosis generally is good in patients with cutaneous SCC without PNI, prognosis is notably poorer when PNI is present due to the association of this finding with increased tumor recurrence and both local and distant metastasis.13 Most frequently, cutaneous SCC with PNI spreads proximally, which can lead to invasion into the base of the brain, but also can extend distally, leading to increased local burden.12,19 In a study of 64 patients with mucosal SCC, Soo et al20 found that patients with lesions that exhibited PNI had a 5-year survival of 16% versus 44% in those without PNI. In their study of SCC of the head and neck, Goepfert et al14 reported that 46% (33/72) of patients with PNI had died or were alive with recurrence at 2 years’ follow-up versus 9.1% (41/448) of patients without PNI. In a systematic review of outcomes, Jambusaria-Pahlajani et al21 reported a disease-specific death rate of 16% for cutaneous SCC with PNI compared to 4% for SCC without PNI.
Perineural invasion can be further classified as clinical or microscopic (incidental) for prognostic purposes. A study by Garcia-Serra et al13 found that patients with clinical PNI had a notably poorer prognosis than those with microscopic (incidental) PNI. The clinical group achieved a local control rate of 55% at 5 years’ follow-up versus 87% in the microscopic group. McCord et al22 found a 5-year local control rate of 78% for microscopic (incidental) PNI versus 50% for clinical PNI; they also found that patients with radiologic evidence of PNI had a worse prognosis, noting that patients with radiologic evidence of PNI were nearly all clinically symptomatic.
Prognosis also is altered by the diameter of the nerve involved. In a study of 48 patients, Ross et al23 found that patients with cutaneous SCC involving small-caliber nerves (diameter, ≤0.1 mm) had a 0% disease-specific death rate versus 32% in those with large-caliber nerves (>0.1 mm). Perineural involvement of small-caliber nerves (<0.1 mm) was a positive prognostic indicator in that it was associated with smaller tumor diameter, more shallow invasion, and increased likelihood to be primary tumors.23 In a recent study, Jambusaria-Pahlajani et al24 investigated tumor staging for cutaneous SCC and reported that PNI is a statistically independent prognostic risk factor for nodal metastasis (subhazard ratio, 2.2 [95% confidence interval, 0.9-5.1]) and disease-specific death (subhazard ratio, 3.4 [95% confidence interval, 0.9-13.3]). Of interest, this increased risk applied only to PNI in nerves that were greater than 0.1 mm.24
Treatment Options—Management of confirmed cases of cutaneous SCC with PNI is difficult because of the nature of the lesions, including their increased propensity for metastasis, increased frequency of poorly differentiated cell types, highly aggressive nature, and the unique challenge of skip lesions.4,16 Skip lesions are found microscopically and show (or appear to show) neoplastic cells invading a nerve in a discontinuous fashion. This phenomenon has been suggested as one explanation for the relatively higher postsurgical recurrence rate of SCC with PNI compared to lesions without PNI.7 They are of particular interest when removing cutaneous SCC with PNI using MMS and attempting to define clear margins. Despite this limitation, MMS generally is accepted as the primary mode of excision of cutaneous SCCs with PNI, as it has the highest known cure rate.7 Cottel4 did not report any cases of local recurrence over 1 to 42 months in 17 patients who were treated with MMS, in contrast to Rowe et al25 who demonstrated that traditional surgical excision had a 47% (34/72) local recurrence rate; however, it bears noting that the varying follow-up periods in the Cottel4 study may underestimate recurrence rate. Leibovitch et al7 had similar findings in their prospective case series study of 70 patients, which revealed an 8% recurrence rate within 5 years in patients treated with MMS, a rate lower than other non-MMS modalities. In this same study, the authors noted that some researchers believe an additional level should be taken with MMS beyond the appearance of free margins in cases with PNI.7
Jambusaria-Pahlajani et al21 reported that PNI is one of the most common reasons cited for using adjuvant radiation therapy for cases of cutaneous SCC because of the known propensity of local recurrence; however, in 74 reviewed cases, there was no statistically significant difference in outcomes in cases of surgery alone versus surgery and adjuvant irradiation. Radiation therapy is a possible alternative primary treatment of cutaneous SCC with PNI, especially in cases of perineural involvement that is extensive or affects proximal portions of cranial nerves when surgery is a less viable option.17 Mendenhall et al16 suggested that patients with positive margins after excision who display extensive PNI should be treated with adjuvant irradiation locally and along the course of the involved nerve to the skull base.
Conclusion
Physicians should recognize the importance of early detection of PNI in cases of cutaneous SCC. A thorough history with good neurologic examination of the head and neck in patients with cutaneous SCC is imperative so patients can be treated earlier in the course of the lesion, increasing the likelihood of local control, minimizing the risk for future recurrence, and decreasing mortality.
Case Report
A 74-year-old man with a history of squamous cell carcinoma (SCC) on the right side of the temple that was treated with Mohs micrographic surgery (MMS) 3 years prior presented with a burning and tingling sensation of 3 months’ duration in the medial border of the repair scar. The patient denied prior anesthesia or muscle weakness of the face as well as any loss or change in vision.
Physical examination revealed a well-healed advancement flap scar with induration at the medial border (Figure 1). Biopsy results were positive for recurrent SCC. Based on anatomic location, clinical symptoms, and tumor recurrence, treatment with MMS was initiated. Mohs sections demonstrated perineural invasion (PNI) (Figures 2 and 3). Multiple treatment stages were required for tumor clearance following the retrograde course of a nerve, which resulted in a substantial defect (Figure 4). The defect was allowed to heal by second intention followed by radiation therapy.
Comment
Incidence and Pathogenesis—Perineural invasion was first described by Cruveilhier1 in a report of invasion of the facial nerve in a patient with mammary carcinoma. Neumann2 reported the first case of a primary cutaneous lesion exhibiting PNI in a patient with a primary carcinoma of the lower lip with invasion and spread along the mental nerve. Perineural invasion is seen in approximately 5% of 200,000 total cases of cutaneous SCC reported annually in the United States.3,4 Other malignancies exhibit PNI more frequently, such as microcystic adnexal carcinoma of the skin, which has been reported to have an 80% rate of perineural growth.5
Perineural invasion can involve nerves of variable thickness, but invasion of larger nerves typically portends a poorer prognosis.6 Characteristics of cutaneous SCC that predispose the lesion to PNI include size greater than 2 cm, male gender, location on the face, and prior treatment of the lesion.6,7 In a study of cutaneous SCC, Leibovitch et al7 found PNI in 4.7% (36/772) of primary lesions and 6.9% (34/491) of recurrent lesions. In another study of 180 SCC tumors of the head and neck with PNI, Carter et al8 found that PNI was most commonly seen in tumors that were greater than 2.5 cm, suggesting that larger lesions have an increased predisposition for PNI.
The mechanism(s) by which PNI develops from these malignancies has not been fully elucidated, but some clues have been found. Vural et al9 showed a statistically significant difference (P<.01) in expression of neural cell adhesion molecules with 93% (38/41) of SCCs with PNI showing evidence of expression versus 36% (9/25) of SCCs without PNI. Chen-Tsai et al10 also suggested that levels of neural cell adhesion molecules may be a factor in determining the metastatic potential of cutaneous SCCs and that levels of neurotrophic tyrosine kinase receptor type 1 (TrkA) may predict PNI, but their study results lacked statistical power to form a firm conclusion.
Diagnosis and Prognosis—Perineural invasion can be diagnosed clinically, radiologically, or microscopically. On clinical examination, PNI is suggested by findings of neuropathy most frequently in cranial nerves V and/or VII, likely due to their extensive subcutaneous distribution.11 Common symptoms include pain, loss of motor skills, anesthesia, dysesthesia, and/or paresthesia (ie, tingling, burning, pricking, numbness).12,13 In a study of 72 cases, Goepfert et al14 found that only 40% (29/72) of patients with pathologically confirmed PNI presented with clinical symptoms and these patients had a poorer prognosis.
Radiologically, PNI can be identified via computed tomography or magnetic resonance imaging through findings of enlargement or abnormal enhancement of the nerve, obliteration of the normal fat plane surrounding the nerve, or erosion or enlargement of its related foramen.15 Magnetic resonance imaging is the preferred method for assessing enhancement of the nerve, while computed tomography is preferred to assess involvement of bone.16,17 Microscopically, there is some debate as to what defines PNI. Suggested findings include the presence of cells inside the epineurium, involvement of nerves outside the main bulk of the tumor, or presence of tumor cells surrounding a nerve.18
These definitions have prognostic significance. Mendenhall et al16 found that patients with radiologic evidence of PNI without clinical symptoms had a higher cure rate using surgery and postoperative irradiation compared to patients with clinical symptoms (80% vs 45%). Although prognosis generally is good in patients with cutaneous SCC without PNI, prognosis is notably poorer when PNI is present due to the association of this finding with increased tumor recurrence and both local and distant metastasis.13 Most frequently, cutaneous SCC with PNI spreads proximally, which can lead to invasion into the base of the brain, but also can extend distally, leading to increased local burden.12,19 In a study of 64 patients with mucosal SCC, Soo et al20 found that patients with lesions that exhibited PNI had a 5-year survival of 16% versus 44% in those without PNI. In their study of SCC of the head and neck, Goepfert et al14 reported that 46% (33/72) of patients with PNI had died or were alive with recurrence at 2 years’ follow-up versus 9.1% (41/448) of patients without PNI. In a systematic review of outcomes, Jambusaria-Pahlajani et al21 reported a disease-specific death rate of 16% for cutaneous SCC with PNI compared to 4% for SCC without PNI.
Perineural invasion can be further classified as clinical or microscopic (incidental) for prognostic purposes. A study by Garcia-Serra et al13 found that patients with clinical PNI had a notably poorer prognosis than those with microscopic (incidental) PNI. The clinical group achieved a local control rate of 55% at 5 years’ follow-up versus 87% in the microscopic group. McCord et al22 found a 5-year local control rate of 78% for microscopic (incidental) PNI versus 50% for clinical PNI; they also found that patients with radiologic evidence of PNI had a worse prognosis, noting that patients with radiologic evidence of PNI were nearly all clinically symptomatic.
Prognosis also is altered by the diameter of the nerve involved. In a study of 48 patients, Ross et al23 found that patients with cutaneous SCC involving small-caliber nerves (diameter, ≤0.1 mm) had a 0% disease-specific death rate versus 32% in those with large-caliber nerves (>0.1 mm). Perineural involvement of small-caliber nerves (<0.1 mm) was a positive prognostic indicator in that it was associated with smaller tumor diameter, more shallow invasion, and increased likelihood to be primary tumors.23 In a recent study, Jambusaria-Pahlajani et al24 investigated tumor staging for cutaneous SCC and reported that PNI is a statistically independent prognostic risk factor for nodal metastasis (subhazard ratio, 2.2 [95% confidence interval, 0.9-5.1]) and disease-specific death (subhazard ratio, 3.4 [95% confidence interval, 0.9-13.3]). Of interest, this increased risk applied only to PNI in nerves that were greater than 0.1 mm.24
Treatment Options—Management of confirmed cases of cutaneous SCC with PNI is difficult because of the nature of the lesions, including their increased propensity for metastasis, increased frequency of poorly differentiated cell types, highly aggressive nature, and the unique challenge of skip lesions.4,16 Skip lesions are found microscopically and show (or appear to show) neoplastic cells invading a nerve in a discontinuous fashion. This phenomenon has been suggested as one explanation for the relatively higher postsurgical recurrence rate of SCC with PNI compared to lesions without PNI.7 They are of particular interest when removing cutaneous SCC with PNI using MMS and attempting to define clear margins. Despite this limitation, MMS generally is accepted as the primary mode of excision of cutaneous SCCs with PNI, as it has the highest known cure rate.7 Cottel4 did not report any cases of local recurrence over 1 to 42 months in 17 patients who were treated with MMS, in contrast to Rowe et al25 who demonstrated that traditional surgical excision had a 47% (34/72) local recurrence rate; however, it bears noting that the varying follow-up periods in the Cottel4 study may underestimate recurrence rate. Leibovitch et al7 had similar findings in their prospective case series study of 70 patients, which revealed an 8% recurrence rate within 5 years in patients treated with MMS, a rate lower than other non-MMS modalities. In this same study, the authors noted that some researchers believe an additional level should be taken with MMS beyond the appearance of free margins in cases with PNI.7
Jambusaria-Pahlajani et al21 reported that PNI is one of the most common reasons cited for using adjuvant radiation therapy for cases of cutaneous SCC because of the known propensity of local recurrence; however, in 74 reviewed cases, there was no statistically significant difference in outcomes in cases of surgery alone versus surgery and adjuvant irradiation. Radiation therapy is a possible alternative primary treatment of cutaneous SCC with PNI, especially in cases of perineural involvement that is extensive or affects proximal portions of cranial nerves when surgery is a less viable option.17 Mendenhall et al16 suggested that patients with positive margins after excision who display extensive PNI should be treated with adjuvant irradiation locally and along the course of the involved nerve to the skull base.
Conclusion
Physicians should recognize the importance of early detection of PNI in cases of cutaneous SCC. A thorough history with good neurologic examination of the head and neck in patients with cutaneous SCC is imperative so patients can be treated earlier in the course of the lesion, increasing the likelihood of local control, minimizing the risk for future recurrence, and decreasing mortality.
1. Cruveilhier J. Maladies des nerfs. In: Cruveilhier J, ed. Anatomie Pathologique du Corps Humain. 2nd ed. Paris, France: JB Bailliere; 1835:1-3.
2. Neumann E. Secondare cancroid infiltration des nervus mentalis bei einem fall von lippincroid. Arch Pathol Anat. 1862;24:201-205.
3. Salasche S. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1, pt 2):4-7.
4. Cottel WI. Perineural invasion by squamous-cell carcinoma. J Dermatol Surg Oncol. 1982;8:589-600.
5. Cooper PH, Mills SE, Leonard DD, et al. Sclerosing sweat duct (syringomatous) carcinoma. Am J Surg Pathol. 1985;9:422-433.
6. Ross AS, Whalen FM, Elenitsas R, et al. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35:1859-1866.
7. Leibovitch I, Huilgol SC, Selva D, et al. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia II. perineural invasion. J Am Acad Dermatol. 2005;53:261-266.
8. Carter RL, Foster CS, Dinsdale EA, et al. Perineural spread by squamous carcinomas of the head and neck: a morphological study using antiaxonal and antimyelin monoclonal antibodies. J Clin Pathol. 1983;36:269-275.
9. Vural E, Hutcheson J, Korourian S, et al. Correlation of neural cell adhesion molecules with perineural spread of squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg. 2000;122:717-720.
10. Chen-Tsai CP, Colome-Grimmer M, Wagner RF Jr. Correlations among neural cell adhesion molecule, nerve growth factor, and its receptors, TrkA, TrkB, TrkC, and p75, in perineural invasion by basal cell and cutaneous squamous cell carcinomas. Dermatol Surg. 2004;30:1009-1016.
11. McCord M, Mendenhall WM, Parsons JT, et al. Skin cancer of the head and neck with clinical perineural invasion. Int J Radiat Oncol Biol Phys. 2000;47:89-93.
12. Ampil FL, Hardin JC, Peskind SP, et al. Perineural invasion in skin cancer of the head and neck: a review of nine cases. J Oral Maxillofac Surg. 1995;53:34-38.
13. Garcia-Serra A, Hinerman RW, Mendenhall WM, et al. Carcinoma of the skin with perineural invasion. Head Neck. 2003;25:1027-1033.
14. Goepfert H, Dichtel WJ, Medina JE, et al. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148:542-547.
15. Galloway TJ, Morris CG, Mancuso AA, et al. Impact of radiographic findings on prognosis for skin carcinoma with clinical perineural invasion. Cancer. 2005;103:1254-1257.
16. Mendenhall WM, Amdur RJ, Williams LS, et al. Carcinoma of the skin of the head and neck with perineural invasion. Head Neck. 2002;24:78-83.
17. Williams LS, Mancuso AA, Mendenhall WM. Perineural spread of cutaneous squamous and basal cell carcinoma: CT and MR detection and its impact on patient management and prognosis. Int J Radiat Oncol Biol Phys. 2001;49:1061-1069.
18. Veness MJ, Biankin S. Perineural spread leading to orbital invasion from skin cancer. Australasian Radiol. 2000;44:296-302.
19. Feasel AM, Brown TJ, Bogle MA, et al. Perineural invasion of cutaneous malignancies. Dermatol Surg. 2001;27:531-542.
20. Soo K, Carter RL, O’Brien CJ, et al. Prognostic implications of perineural spread in squamous carcinomas of the head and neck. Laryngoscope. 1986;96:1145-1148.
21. Jambusaria-Pahlajani A, Miller CJ, Quon H, et al. Surgical monotherapy versus surgery plus adjuvant radiotherapy in high-risk cutaneous squamous cell carcinoma: a systematic review of outcomes. Dermatol Surg. 2009;35:574-584.
22. McCord MW, Mendenhall WM, Parsons JT, et al. Skin cancer of the head and neck with incidental microscopic perineural invasion. Int J Radiat Oncol Biol Phys. 1999;43:591-595.
23. Ross AS, Whalen FM, Elenitsas R, et al. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35:1859-1866.
24. Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149:402-410.
25. Rowe DE, Carroll RJ, Day CL. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. J Am Acad Dermatol. 1992;26:976-990.
1. Cruveilhier J. Maladies des nerfs. In: Cruveilhier J, ed. Anatomie Pathologique du Corps Humain. 2nd ed. Paris, France: JB Bailliere; 1835:1-3.
2. Neumann E. Secondare cancroid infiltration des nervus mentalis bei einem fall von lippincroid. Arch Pathol Anat. 1862;24:201-205.
3. Salasche S. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1, pt 2):4-7.
4. Cottel WI. Perineural invasion by squamous-cell carcinoma. J Dermatol Surg Oncol. 1982;8:589-600.
5. Cooper PH, Mills SE, Leonard DD, et al. Sclerosing sweat duct (syringomatous) carcinoma. Am J Surg Pathol. 1985;9:422-433.
6. Ross AS, Whalen FM, Elenitsas R, et al. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35:1859-1866.
7. Leibovitch I, Huilgol SC, Selva D, et al. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia II. perineural invasion. J Am Acad Dermatol. 2005;53:261-266.
8. Carter RL, Foster CS, Dinsdale EA, et al. Perineural spread by squamous carcinomas of the head and neck: a morphological study using antiaxonal and antimyelin monoclonal antibodies. J Clin Pathol. 1983;36:269-275.
9. Vural E, Hutcheson J, Korourian S, et al. Correlation of neural cell adhesion molecules with perineural spread of squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg. 2000;122:717-720.
10. Chen-Tsai CP, Colome-Grimmer M, Wagner RF Jr. Correlations among neural cell adhesion molecule, nerve growth factor, and its receptors, TrkA, TrkB, TrkC, and p75, in perineural invasion by basal cell and cutaneous squamous cell carcinomas. Dermatol Surg. 2004;30:1009-1016.
11. McCord M, Mendenhall WM, Parsons JT, et al. Skin cancer of the head and neck with clinical perineural invasion. Int J Radiat Oncol Biol Phys. 2000;47:89-93.
12. Ampil FL, Hardin JC, Peskind SP, et al. Perineural invasion in skin cancer of the head and neck: a review of nine cases. J Oral Maxillofac Surg. 1995;53:34-38.
13. Garcia-Serra A, Hinerman RW, Mendenhall WM, et al. Carcinoma of the skin with perineural invasion. Head Neck. 2003;25:1027-1033.
14. Goepfert H, Dichtel WJ, Medina JE, et al. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148:542-547.
15. Galloway TJ, Morris CG, Mancuso AA, et al. Impact of radiographic findings on prognosis for skin carcinoma with clinical perineural invasion. Cancer. 2005;103:1254-1257.
16. Mendenhall WM, Amdur RJ, Williams LS, et al. Carcinoma of the skin of the head and neck with perineural invasion. Head Neck. 2002;24:78-83.
17. Williams LS, Mancuso AA, Mendenhall WM. Perineural spread of cutaneous squamous and basal cell carcinoma: CT and MR detection and its impact on patient management and prognosis. Int J Radiat Oncol Biol Phys. 2001;49:1061-1069.
18. Veness MJ, Biankin S. Perineural spread leading to orbital invasion from skin cancer. Australasian Radiol. 2000;44:296-302.
19. Feasel AM, Brown TJ, Bogle MA, et al. Perineural invasion of cutaneous malignancies. Dermatol Surg. 2001;27:531-542.
20. Soo K, Carter RL, O’Brien CJ, et al. Prognostic implications of perineural spread in squamous carcinomas of the head and neck. Laryngoscope. 1986;96:1145-1148.
21. Jambusaria-Pahlajani A, Miller CJ, Quon H, et al. Surgical monotherapy versus surgery plus adjuvant radiotherapy in high-risk cutaneous squamous cell carcinoma: a systematic review of outcomes. Dermatol Surg. 2009;35:574-584.
22. McCord MW, Mendenhall WM, Parsons JT, et al. Skin cancer of the head and neck with incidental microscopic perineural invasion. Int J Radiat Oncol Biol Phys. 1999;43:591-595.
23. Ross AS, Whalen FM, Elenitsas R, et al. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35:1859-1866.
24. Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149:402-410.
25. Rowe DE, Carroll RJ, Day CL. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. J Am Acad Dermatol. 1992;26:976-990.
Practice Points
• Patients with suspected cutaneous squamous cell carcinoma should be asked about neurological symptoms including pain, loss of motor skills, anesthesia, dysesthesia, and/or paresthesia, which may indicate perineural invasion.
• Patients with perineural invasion carry a much higher risk for local and distant recurrence and may require more aggressive treatment including Mohs micrographic surgery and adjuvant radiation.
Epithelioid Sarcoma: Report of 3 Cases
VIDEO: Bright future ahead for U.S. sunscreen market
WAIKOLOA, HAWAII – A new wave of better sunscreens may soon wash into the U.S. marketplace, predicted Dr. Susan Swetter of Stanford (Calif.) University. She spoke with us at the Hawaii Dermatology Seminar about what might be in the pipeline for U.S. patients, and she outlined her own choices for sun protection in patients with a history of melanoma or who have very fair skin.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – A new wave of better sunscreens may soon wash into the U.S. marketplace, predicted Dr. Susan Swetter of Stanford (Calif.) University. She spoke with us at the Hawaii Dermatology Seminar about what might be in the pipeline for U.S. patients, and she outlined her own choices for sun protection in patients with a history of melanoma or who have very fair skin.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – A new wave of better sunscreens may soon wash into the U.S. marketplace, predicted Dr. Susan Swetter of Stanford (Calif.) University. She spoke with us at the Hawaii Dermatology Seminar about what might be in the pipeline for U.S. patients, and she outlined her own choices for sun protection in patients with a history of melanoma or who have very fair skin.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR