User login
Drug receives orphan designation for AML
The US Food and Drug Administration (FDA) has granted orphan designation to MAX-40279 for the treatment of acute myeloid leukemia (AML).
MAX-40279 is a multi-target kinase inhibitor being developed by MaxiNovel Pharmaceuticals, Inc.
The drug mainly targets FMS-related tyrosine kinase 3 (FLT3) and fibroblast growth factor receptor (FGFR).
MAX-40279 demonstrated “potent” inhibition of FLT3 and FGFR in preclinical testing, according to MaxiNovel Pharmaceuticals, Inc.
The company is currently testing MAX-40279 in a phase 1 trial of patients with AML (NCT03412292).
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted orphan designation to MAX-40279 for the treatment of acute myeloid leukemia (AML).
MAX-40279 is a multi-target kinase inhibitor being developed by MaxiNovel Pharmaceuticals, Inc.
The drug mainly targets FMS-related tyrosine kinase 3 (FLT3) and fibroblast growth factor receptor (FGFR).
MAX-40279 demonstrated “potent” inhibition of FLT3 and FGFR in preclinical testing, according to MaxiNovel Pharmaceuticals, Inc.
The company is currently testing MAX-40279 in a phase 1 trial of patients with AML (NCT03412292).
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted orphan designation to MAX-40279 for the treatment of acute myeloid leukemia (AML).
MAX-40279 is a multi-target kinase inhibitor being developed by MaxiNovel Pharmaceuticals, Inc.
The drug mainly targets FMS-related tyrosine kinase 3 (FLT3) and fibroblast growth factor receptor (FGFR).
MAX-40279 demonstrated “potent” inhibition of FLT3 and FGFR in preclinical testing, according to MaxiNovel Pharmaceuticals, Inc.
The company is currently testing MAX-40279 in a phase 1 trial of patients with AML (NCT03412292).
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
MRD may indicate relapse risk in AML
Detecting molecular minimal residual disease among patients in complete remission was a significant independent predictor of relapse and survival in acute myeloid leukemia (AML), findings from a new study suggest.
Mojca Jongen-Lavrencic, MD, PhD, of the Erasmus University in the Netherlands, and colleagues conducted targeted next-generation sequencing on bone marrow or peripheral blood samples to detect minimal residual disease in 482 newly diagnosed AML patients aged 18-65 years. The sampling was conducted at diagnosis and again after induction therapy. The study endpoints included 4-year rates of relapse, relapse-free survival, and overall survival. The findings are reported in the New England Journal of Medicine.
Overall, at least one mutation was found in 430 patients (89.2%). Persistent DTA mutations, which are associated with age-related hematopoiesis, were not significantly associated with higher 4-year relapse rates, compared with no detection of DTA mutations (P = .29). However, having a persistent DTA mutation and a coexisting persistent non-DTA mutation was a significant predictor of relapse, with a 4-year relapse rate of 66.7% versus 39.4% for no detection (P = .002).
Similarly, having a persistent non-DTA mutation at any allele frequency was linked to an increase risk of relapse at 4 years (55.7% with detection versus 34.6% without detection, P = .001). Non-DTA mutation was also significantly associated with reduced relapsed-free survival and reduced overall survival.
The study was funded by the Queen Wilhelmina Fund Foundation of the Dutch Cancer, among others. There was no commercial funding for the study.
SOURCE: Jongen-Lavrencic M et al. N Engl J Med. 2018 Mar 29;378:1189-99.
Detecting molecular minimal residual disease among patients in complete remission was a significant independent predictor of relapse and survival in acute myeloid leukemia (AML), findings from a new study suggest.
Mojca Jongen-Lavrencic, MD, PhD, of the Erasmus University in the Netherlands, and colleagues conducted targeted next-generation sequencing on bone marrow or peripheral blood samples to detect minimal residual disease in 482 newly diagnosed AML patients aged 18-65 years. The sampling was conducted at diagnosis and again after induction therapy. The study endpoints included 4-year rates of relapse, relapse-free survival, and overall survival. The findings are reported in the New England Journal of Medicine.
Overall, at least one mutation was found in 430 patients (89.2%). Persistent DTA mutations, which are associated with age-related hematopoiesis, were not significantly associated with higher 4-year relapse rates, compared with no detection of DTA mutations (P = .29). However, having a persistent DTA mutation and a coexisting persistent non-DTA mutation was a significant predictor of relapse, with a 4-year relapse rate of 66.7% versus 39.4% for no detection (P = .002).
Similarly, having a persistent non-DTA mutation at any allele frequency was linked to an increase risk of relapse at 4 years (55.7% with detection versus 34.6% without detection, P = .001). Non-DTA mutation was also significantly associated with reduced relapsed-free survival and reduced overall survival.
The study was funded by the Queen Wilhelmina Fund Foundation of the Dutch Cancer, among others. There was no commercial funding for the study.
SOURCE: Jongen-Lavrencic M et al. N Engl J Med. 2018 Mar 29;378:1189-99.
Detecting molecular minimal residual disease among patients in complete remission was a significant independent predictor of relapse and survival in acute myeloid leukemia (AML), findings from a new study suggest.
Mojca Jongen-Lavrencic, MD, PhD, of the Erasmus University in the Netherlands, and colleagues conducted targeted next-generation sequencing on bone marrow or peripheral blood samples to detect minimal residual disease in 482 newly diagnosed AML patients aged 18-65 years. The sampling was conducted at diagnosis and again after induction therapy. The study endpoints included 4-year rates of relapse, relapse-free survival, and overall survival. The findings are reported in the New England Journal of Medicine.
Overall, at least one mutation was found in 430 patients (89.2%). Persistent DTA mutations, which are associated with age-related hematopoiesis, were not significantly associated with higher 4-year relapse rates, compared with no detection of DTA mutations (P = .29). However, having a persistent DTA mutation and a coexisting persistent non-DTA mutation was a significant predictor of relapse, with a 4-year relapse rate of 66.7% versus 39.4% for no detection (P = .002).
Similarly, having a persistent non-DTA mutation at any allele frequency was linked to an increase risk of relapse at 4 years (55.7% with detection versus 34.6% without detection, P = .001). Non-DTA mutation was also significantly associated with reduced relapsed-free survival and reduced overall survival.
The study was funded by the Queen Wilhelmina Fund Foundation of the Dutch Cancer, among others. There was no commercial funding for the study.
SOURCE: Jongen-Lavrencic M et al. N Engl J Med. 2018 Mar 29;378:1189-99.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Leukemia research pioneer dies at 92
James F. Holland, MD, passed away last week, at the age of 92, due to complications of cardiovascular disease.
Dr Holland has been called a pioneer in the field of leukemia research.
He and his colleagues are credited with using combination chemotherapy to transform pediatric acute lymphoblastic leukemia from an incurable illness to one with a high survival rate.
Dr Holland and his colleagues also developed the 7+3 regimen—3 daily injections of daunorubicin and 7 days of intravenous cytarabine—for patients with acute myeloid leukemia.
Dr Holland was born on May 16, 1925, in Morristown, New Jersey. He graduated from Princeton University in 1944 and earned his medical degree from Columbia University College of Physicians and Surgeons in 1947.
Dr Holland was a captain in the US Army Medical Corps from 1949 to 1951. After that, he worked at Francis Delafield Hospital (which closed in 1975) in New York, New York. He joined the National Cancer Institute (NCI) in 1953. Two years later, he began working at Roswell Park Cancer Institute in Buffalo, New York.
Dr Holland became Roswell Park’s chief of medicine and director of the Cancer Clinical Research Center. But he continued to work with the NCI, conducting research as part of Acute Leukemia Group B, which later became Cancer and Leukemia Group B.
After spending a year on an oncology exchange program in the Soviet Union, Dr Holland started at Mount Sinai in New York, New York, in 1973. While there, he established the Department of Neoplastic Diseases at The Tisch Cancer Institute, Icahn School of Medicine.
Most recently, Dr Holland was a distinguished professor of neoplastic diseases at Mount Sinai. He saw patients but also conducted research on the human mammary tumor virus.
Dr Holland collaborated with Emil Frei III to publish the textbook Cancer Medicine, which is now in its ninth edition. Dr Holland served as president of the American Association for Cancer Research and the American Society of Clinical Oncology. He was a co-founder of the African Organization for Research and Training in Cancer as well.
Dr Holland was married to Jimmie C. Holland, MD, who is credited with founding the field of psycho-oncology. Jimmie passed away in December 2017. The couple is survived by 6 children and 9 grandchildren.
James F. Holland, MD, passed away last week, at the age of 92, due to complications of cardiovascular disease.
Dr Holland has been called a pioneer in the field of leukemia research.
He and his colleagues are credited with using combination chemotherapy to transform pediatric acute lymphoblastic leukemia from an incurable illness to one with a high survival rate.
Dr Holland and his colleagues also developed the 7+3 regimen—3 daily injections of daunorubicin and 7 days of intravenous cytarabine—for patients with acute myeloid leukemia.
Dr Holland was born on May 16, 1925, in Morristown, New Jersey. He graduated from Princeton University in 1944 and earned his medical degree from Columbia University College of Physicians and Surgeons in 1947.
Dr Holland was a captain in the US Army Medical Corps from 1949 to 1951. After that, he worked at Francis Delafield Hospital (which closed in 1975) in New York, New York. He joined the National Cancer Institute (NCI) in 1953. Two years later, he began working at Roswell Park Cancer Institute in Buffalo, New York.
Dr Holland became Roswell Park’s chief of medicine and director of the Cancer Clinical Research Center. But he continued to work with the NCI, conducting research as part of Acute Leukemia Group B, which later became Cancer and Leukemia Group B.
After spending a year on an oncology exchange program in the Soviet Union, Dr Holland started at Mount Sinai in New York, New York, in 1973. While there, he established the Department of Neoplastic Diseases at The Tisch Cancer Institute, Icahn School of Medicine.
Most recently, Dr Holland was a distinguished professor of neoplastic diseases at Mount Sinai. He saw patients but also conducted research on the human mammary tumor virus.
Dr Holland collaborated with Emil Frei III to publish the textbook Cancer Medicine, which is now in its ninth edition. Dr Holland served as president of the American Association for Cancer Research and the American Society of Clinical Oncology. He was a co-founder of the African Organization for Research and Training in Cancer as well.
Dr Holland was married to Jimmie C. Holland, MD, who is credited with founding the field of psycho-oncology. Jimmie passed away in December 2017. The couple is survived by 6 children and 9 grandchildren.
James F. Holland, MD, passed away last week, at the age of 92, due to complications of cardiovascular disease.
Dr Holland has been called a pioneer in the field of leukemia research.
He and his colleagues are credited with using combination chemotherapy to transform pediatric acute lymphoblastic leukemia from an incurable illness to one with a high survival rate.
Dr Holland and his colleagues also developed the 7+3 regimen—3 daily injections of daunorubicin and 7 days of intravenous cytarabine—for patients with acute myeloid leukemia.
Dr Holland was born on May 16, 1925, in Morristown, New Jersey. He graduated from Princeton University in 1944 and earned his medical degree from Columbia University College of Physicians and Surgeons in 1947.
Dr Holland was a captain in the US Army Medical Corps from 1949 to 1951. After that, he worked at Francis Delafield Hospital (which closed in 1975) in New York, New York. He joined the National Cancer Institute (NCI) in 1953. Two years later, he began working at Roswell Park Cancer Institute in Buffalo, New York.
Dr Holland became Roswell Park’s chief of medicine and director of the Cancer Clinical Research Center. But he continued to work with the NCI, conducting research as part of Acute Leukemia Group B, which later became Cancer and Leukemia Group B.
After spending a year on an oncology exchange program in the Soviet Union, Dr Holland started at Mount Sinai in New York, New York, in 1973. While there, he established the Department of Neoplastic Diseases at The Tisch Cancer Institute, Icahn School of Medicine.
Most recently, Dr Holland was a distinguished professor of neoplastic diseases at Mount Sinai. He saw patients but also conducted research on the human mammary tumor virus.
Dr Holland collaborated with Emil Frei III to publish the textbook Cancer Medicine, which is now in its ninth edition. Dr Holland served as president of the American Association for Cancer Research and the American Society of Clinical Oncology. He was a co-founder of the African Organization for Research and Training in Cancer as well.
Dr Holland was married to Jimmie C. Holland, MD, who is credited with founding the field of psycho-oncology. Jimmie passed away in December 2017. The couple is survived by 6 children and 9 grandchildren.
Manufactured graft deemed safe in blood cancer patients
LISBON—Phase 1 results suggest a programmed cellular therapy is safe for use in patients with hematologic malignancies.
The therapy, ProTmune, is being developed as a next-generation allogeneic graft intended to reduce the incidence and severity of acute graft-versus-host disease (GVHD) after hematopoietic stem cell transplant (HSCT).
Three of 7 patients who received ProTmune in this trial did develop acute GVHD, and 2 patients died.
However, the remaining 5 patients were still alive and disease-free at last follow-up.
There were no serious adverse events (AEs) attributed to ProTmune. The most common AEs were nausea, vomiting, and chest pain.
These results were presented at the 44th Annual Meeting of the EBMT (abstract A401*).
The trial, known as PROTECT, is sponsored by Fate Therapeutics, the company developing ProTmune.
The phase 1 portion of PROTECT enrolled 7 adults with hematologic malignancies—1 with myelodysplastic syndrome, 3 with acute lymphoblastic leukemia, and 3 with acute myeloid leukemia.
Patients were set to undergo matched, unrelated donor HSCT and received ProTmune as the graft. ProTmune is manufactured by modulating a mobilized peripheral blood graft with 2 small molecules, FT1050 and FT4145.
The patients ranged in age from 34 to 69, and most (n=5) were female. For conditioning, patients received fludarabine/busulfan (n=1), busulfan/cyclophosphamide (n=1), fludarabine/melphalan (n=3), or cyclophosphamide/total body irradiation (n=2).
Results
The data cut-off was February 26, 2018. The median time on study was 228 days (range, 151 to 353).
None of the patients had graft failure. The median time to neutrophil engraftment was 18 days (range, 14 to 22).
Three patients had acute GVHD at day 100 after HSCT. Two patients had grade 2 skin GVHD, and 1 had grade 3 GVHD in the skin and gut.
All 3 patients responded to steroid treatment. GVHD resolved in 5 days for the patient with grade 3 GVHD. For the grade 2 patients, GVHD resolved in 7 days and 8 days, respectively.
None of the patients relapsed, but 2 died—1 of pulmonary edema and 1 of atrial fibrillation.
AEs related to ProTmune included grade 1 vomiting (n=2), grade 2 nausea (n=2), and grade 2 chest pain (n=1).
Phase 2
The phase 2 portion of PROTECT is ongoing. This is a randomized, controlled, double-blinded trial designed to assess the safety and efficacy of ProTmune in up to 60 adults with hematologic malignancies undergoing matched, unrelated donor HSCT following myeloablative conditioning.
Patients are being randomized, in a 1:1 ratio, to receive either ProTmune or a conventional, mobilized peripheral blood cell graft from a matched, unrelated donor.
The primary efficacy endpoint is the cumulative incidence of grade 2-4 acute GVHD by day 100 post-HSCT. Rates of chronic GVHD, cancer relapse, disease-free survival, and overall survival are also being assessed.
*Some data in the abstract differ from the presentation.
LISBON—Phase 1 results suggest a programmed cellular therapy is safe for use in patients with hematologic malignancies.
The therapy, ProTmune, is being developed as a next-generation allogeneic graft intended to reduce the incidence and severity of acute graft-versus-host disease (GVHD) after hematopoietic stem cell transplant (HSCT).
Three of 7 patients who received ProTmune in this trial did develop acute GVHD, and 2 patients died.
However, the remaining 5 patients were still alive and disease-free at last follow-up.
There were no serious adverse events (AEs) attributed to ProTmune. The most common AEs were nausea, vomiting, and chest pain.
These results were presented at the 44th Annual Meeting of the EBMT (abstract A401*).
The trial, known as PROTECT, is sponsored by Fate Therapeutics, the company developing ProTmune.
The phase 1 portion of PROTECT enrolled 7 adults with hematologic malignancies—1 with myelodysplastic syndrome, 3 with acute lymphoblastic leukemia, and 3 with acute myeloid leukemia.
Patients were set to undergo matched, unrelated donor HSCT and received ProTmune as the graft. ProTmune is manufactured by modulating a mobilized peripheral blood graft with 2 small molecules, FT1050 and FT4145.
The patients ranged in age from 34 to 69, and most (n=5) were female. For conditioning, patients received fludarabine/busulfan (n=1), busulfan/cyclophosphamide (n=1), fludarabine/melphalan (n=3), or cyclophosphamide/total body irradiation (n=2).
Results
The data cut-off was February 26, 2018. The median time on study was 228 days (range, 151 to 353).
None of the patients had graft failure. The median time to neutrophil engraftment was 18 days (range, 14 to 22).
Three patients had acute GVHD at day 100 after HSCT. Two patients had grade 2 skin GVHD, and 1 had grade 3 GVHD in the skin and gut.
All 3 patients responded to steroid treatment. GVHD resolved in 5 days for the patient with grade 3 GVHD. For the grade 2 patients, GVHD resolved in 7 days and 8 days, respectively.
None of the patients relapsed, but 2 died—1 of pulmonary edema and 1 of atrial fibrillation.
AEs related to ProTmune included grade 1 vomiting (n=2), grade 2 nausea (n=2), and grade 2 chest pain (n=1).
Phase 2
The phase 2 portion of PROTECT is ongoing. This is a randomized, controlled, double-blinded trial designed to assess the safety and efficacy of ProTmune in up to 60 adults with hematologic malignancies undergoing matched, unrelated donor HSCT following myeloablative conditioning.
Patients are being randomized, in a 1:1 ratio, to receive either ProTmune or a conventional, mobilized peripheral blood cell graft from a matched, unrelated donor.
The primary efficacy endpoint is the cumulative incidence of grade 2-4 acute GVHD by day 100 post-HSCT. Rates of chronic GVHD, cancer relapse, disease-free survival, and overall survival are also being assessed.
*Some data in the abstract differ from the presentation.
LISBON—Phase 1 results suggest a programmed cellular therapy is safe for use in patients with hematologic malignancies.
The therapy, ProTmune, is being developed as a next-generation allogeneic graft intended to reduce the incidence and severity of acute graft-versus-host disease (GVHD) after hematopoietic stem cell transplant (HSCT).
Three of 7 patients who received ProTmune in this trial did develop acute GVHD, and 2 patients died.
However, the remaining 5 patients were still alive and disease-free at last follow-up.
There were no serious adverse events (AEs) attributed to ProTmune. The most common AEs were nausea, vomiting, and chest pain.
These results were presented at the 44th Annual Meeting of the EBMT (abstract A401*).
The trial, known as PROTECT, is sponsored by Fate Therapeutics, the company developing ProTmune.
The phase 1 portion of PROTECT enrolled 7 adults with hematologic malignancies—1 with myelodysplastic syndrome, 3 with acute lymphoblastic leukemia, and 3 with acute myeloid leukemia.
Patients were set to undergo matched, unrelated donor HSCT and received ProTmune as the graft. ProTmune is manufactured by modulating a mobilized peripheral blood graft with 2 small molecules, FT1050 and FT4145.
The patients ranged in age from 34 to 69, and most (n=5) were female. For conditioning, patients received fludarabine/busulfan (n=1), busulfan/cyclophosphamide (n=1), fludarabine/melphalan (n=3), or cyclophosphamide/total body irradiation (n=2).
Results
The data cut-off was February 26, 2018. The median time on study was 228 days (range, 151 to 353).
None of the patients had graft failure. The median time to neutrophil engraftment was 18 days (range, 14 to 22).
Three patients had acute GVHD at day 100 after HSCT. Two patients had grade 2 skin GVHD, and 1 had grade 3 GVHD in the skin and gut.
All 3 patients responded to steroid treatment. GVHD resolved in 5 days for the patient with grade 3 GVHD. For the grade 2 patients, GVHD resolved in 7 days and 8 days, respectively.
None of the patients relapsed, but 2 died—1 of pulmonary edema and 1 of atrial fibrillation.
AEs related to ProTmune included grade 1 vomiting (n=2), grade 2 nausea (n=2), and grade 2 chest pain (n=1).
Phase 2
The phase 2 portion of PROTECT is ongoing. This is a randomized, controlled, double-blinded trial designed to assess the safety and efficacy of ProTmune in up to 60 adults with hematologic malignancies undergoing matched, unrelated donor HSCT following myeloablative conditioning.
Patients are being randomized, in a 1:1 ratio, to receive either ProTmune or a conventional, mobilized peripheral blood cell graft from a matched, unrelated donor.
The primary efficacy endpoint is the cumulative incidence of grade 2-4 acute GVHD by day 100 post-HSCT. Rates of chronic GVHD, cancer relapse, disease-free survival, and overall survival are also being assessed.
*Some data in the abstract differ from the presentation.
AML patients may fare better at NCI centers
New research suggests patients with acute myeloid leukemia (AML) may have a lower risk of early mortality if they receive treatment at a National Cancer Institute (NCI) cancer center.
In a study of AML patients in California, the risk of 60-day mortality was 53% lower among patients treated at NCI cancer centers than among those treated at other centers.
These findings were reported in Cancer.
“We found the early mortality, deaths less than 60 days after diagnosis, was significantly lower at the NCI-designated cancer centers compared to non-NCI-designated cancer centers in California,” said study author Brian Jonas, MD, PhD, of the University of California at Davis School of Medicine in Sacramento, California.
To conduct this study, Dr Jonas and his colleagues analyzed data from the California Cancer Registry and the California Office of Statewide Health Planning and Development Patient Discharge Database.
The California Cancer Registry provides sociodemographic and clinical data for all California cancer patients. The California Office of Statewide Health Planning and Development Patient Discharge Database has data on diagnoses and procedures for all hospital patients in California, excluding 14 Veterans Affairs and military hospitals.
Patients
The study included data on AML patients 18 and older who received inpatient chemotherapy between 1999 and 2014. There were 7007 patients, 1762 (25%) of whom were treated at NCI-designated cancer centers.
The median number of new AML patients per year was 13.5 (range, 0-43) at the NCI centers and 2 (range, 1-17) at non-NCI centers that admitted at least 1 patient with AML. More than half of the non-NCI centers had a median of 0 new AML patients per year.
NCI patients were more likely to be younger (≤65) than non-NCI patients (P<0.0001), to live in neighborhoods with higher socioeconomic status (P<0.0001), have fewer comorbidities (P<0.0001), and have public health insurance (P<0.0001).
Results
There were several types of complications that differed significantly between center types.
Patients treated at NCI centers were significantly more likely to have leukapheresis (5.5% vs 2.7%; P<0.001) and renal failure (22.8% vs 19.9%; P=0.010).
But they were significantly less likely to have respiratory failure (11.6% vs 14.3%; P=0.003) and cardiac arrest (1.1% vs 2.0%; P=0.014).
Sixty-day survival was significantly higher among NCI patients (88.0% vs 76.3%; P<0.001).
In an inverse-probability-weighted analysis adjusted for sociodemographic factors and comorbidities, treatment at an NCI center was associated with significantly lower early mortality, with an odds ratio (OR) of 0.46 (P<0.001).
This analysis also revealed a significant association between increased early mortality and major bleeding (OR=1.79, P<0.001), renal failure (OR=2.33, P<0.001), respiratory failure (OR=6.46, P<0.001), and cardiac arrest (OR=13.33, P<0.001).
For the most part, the impact of complications on early mortality did not differ significantly by treatment center.
The exception was respiratory failure. Patients with respiratory failure had a significantly greater risk of early mortality if they were treated at a non-NCI center (OR=9.48) than at an NCI center (OR=4.20).
Potential explanations
The researchers believe the variations in early mortality they observed point to inconsistent supportive care. However, more work must be done to fully understand the differences in care driving these issues.
“This is clearly provocative data that makes you want to understand exactly why,” Dr Jonas said. “We’re going to have to dive into that question in a more significant way.”
In the absence of data that could identify the exact causes, the researchers noted that other studies have shown higher patient volumes may contribute to better care.
“I see 60 or more AML cases per year,” Dr Jonas said. “High volume/low volume must play a role.”
The researchers believe other potential contributing factors could be access to clinical trials, better nursing ratios, and more sophisticated intensive care units.
The team hopes this research will spawn more intensive efforts to identify the causes that underlie variations in early mortality between hospital sites.
“This is a provocative and hopeful paper in terms of improving outcomes,” Dr Jonas said. “It sends a positive message that there are things we could probably do that could help everyone.”
New research suggests patients with acute myeloid leukemia (AML) may have a lower risk of early mortality if they receive treatment at a National Cancer Institute (NCI) cancer center.
In a study of AML patients in California, the risk of 60-day mortality was 53% lower among patients treated at NCI cancer centers than among those treated at other centers.
These findings were reported in Cancer.
“We found the early mortality, deaths less than 60 days after diagnosis, was significantly lower at the NCI-designated cancer centers compared to non-NCI-designated cancer centers in California,” said study author Brian Jonas, MD, PhD, of the University of California at Davis School of Medicine in Sacramento, California.
To conduct this study, Dr Jonas and his colleagues analyzed data from the California Cancer Registry and the California Office of Statewide Health Planning and Development Patient Discharge Database.
The California Cancer Registry provides sociodemographic and clinical data for all California cancer patients. The California Office of Statewide Health Planning and Development Patient Discharge Database has data on diagnoses and procedures for all hospital patients in California, excluding 14 Veterans Affairs and military hospitals.
Patients
The study included data on AML patients 18 and older who received inpatient chemotherapy between 1999 and 2014. There were 7007 patients, 1762 (25%) of whom were treated at NCI-designated cancer centers.
The median number of new AML patients per year was 13.5 (range, 0-43) at the NCI centers and 2 (range, 1-17) at non-NCI centers that admitted at least 1 patient with AML. More than half of the non-NCI centers had a median of 0 new AML patients per year.
NCI patients were more likely to be younger (≤65) than non-NCI patients (P<0.0001), to live in neighborhoods with higher socioeconomic status (P<0.0001), have fewer comorbidities (P<0.0001), and have public health insurance (P<0.0001).
Results
There were several types of complications that differed significantly between center types.
Patients treated at NCI centers were significantly more likely to have leukapheresis (5.5% vs 2.7%; P<0.001) and renal failure (22.8% vs 19.9%; P=0.010).
But they were significantly less likely to have respiratory failure (11.6% vs 14.3%; P=0.003) and cardiac arrest (1.1% vs 2.0%; P=0.014).
Sixty-day survival was significantly higher among NCI patients (88.0% vs 76.3%; P<0.001).
In an inverse-probability-weighted analysis adjusted for sociodemographic factors and comorbidities, treatment at an NCI center was associated with significantly lower early mortality, with an odds ratio (OR) of 0.46 (P<0.001).
This analysis also revealed a significant association between increased early mortality and major bleeding (OR=1.79, P<0.001), renal failure (OR=2.33, P<0.001), respiratory failure (OR=6.46, P<0.001), and cardiac arrest (OR=13.33, P<0.001).
For the most part, the impact of complications on early mortality did not differ significantly by treatment center.
The exception was respiratory failure. Patients with respiratory failure had a significantly greater risk of early mortality if they were treated at a non-NCI center (OR=9.48) than at an NCI center (OR=4.20).
Potential explanations
The researchers believe the variations in early mortality they observed point to inconsistent supportive care. However, more work must be done to fully understand the differences in care driving these issues.
“This is clearly provocative data that makes you want to understand exactly why,” Dr Jonas said. “We’re going to have to dive into that question in a more significant way.”
In the absence of data that could identify the exact causes, the researchers noted that other studies have shown higher patient volumes may contribute to better care.
“I see 60 or more AML cases per year,” Dr Jonas said. “High volume/low volume must play a role.”
The researchers believe other potential contributing factors could be access to clinical trials, better nursing ratios, and more sophisticated intensive care units.
The team hopes this research will spawn more intensive efforts to identify the causes that underlie variations in early mortality between hospital sites.
“This is a provocative and hopeful paper in terms of improving outcomes,” Dr Jonas said. “It sends a positive message that there are things we could probably do that could help everyone.”
New research suggests patients with acute myeloid leukemia (AML) may have a lower risk of early mortality if they receive treatment at a National Cancer Institute (NCI) cancer center.
In a study of AML patients in California, the risk of 60-day mortality was 53% lower among patients treated at NCI cancer centers than among those treated at other centers.
These findings were reported in Cancer.
“We found the early mortality, deaths less than 60 days after diagnosis, was significantly lower at the NCI-designated cancer centers compared to non-NCI-designated cancer centers in California,” said study author Brian Jonas, MD, PhD, of the University of California at Davis School of Medicine in Sacramento, California.
To conduct this study, Dr Jonas and his colleagues analyzed data from the California Cancer Registry and the California Office of Statewide Health Planning and Development Patient Discharge Database.
The California Cancer Registry provides sociodemographic and clinical data for all California cancer patients. The California Office of Statewide Health Planning and Development Patient Discharge Database has data on diagnoses and procedures for all hospital patients in California, excluding 14 Veterans Affairs and military hospitals.
Patients
The study included data on AML patients 18 and older who received inpatient chemotherapy between 1999 and 2014. There were 7007 patients, 1762 (25%) of whom were treated at NCI-designated cancer centers.
The median number of new AML patients per year was 13.5 (range, 0-43) at the NCI centers and 2 (range, 1-17) at non-NCI centers that admitted at least 1 patient with AML. More than half of the non-NCI centers had a median of 0 new AML patients per year.
NCI patients were more likely to be younger (≤65) than non-NCI patients (P<0.0001), to live in neighborhoods with higher socioeconomic status (P<0.0001), have fewer comorbidities (P<0.0001), and have public health insurance (P<0.0001).
Results
There were several types of complications that differed significantly between center types.
Patients treated at NCI centers were significantly more likely to have leukapheresis (5.5% vs 2.7%; P<0.001) and renal failure (22.8% vs 19.9%; P=0.010).
But they were significantly less likely to have respiratory failure (11.6% vs 14.3%; P=0.003) and cardiac arrest (1.1% vs 2.0%; P=0.014).
Sixty-day survival was significantly higher among NCI patients (88.0% vs 76.3%; P<0.001).
In an inverse-probability-weighted analysis adjusted for sociodemographic factors and comorbidities, treatment at an NCI center was associated with significantly lower early mortality, with an odds ratio (OR) of 0.46 (P<0.001).
This analysis also revealed a significant association between increased early mortality and major bleeding (OR=1.79, P<0.001), renal failure (OR=2.33, P<0.001), respiratory failure (OR=6.46, P<0.001), and cardiac arrest (OR=13.33, P<0.001).
For the most part, the impact of complications on early mortality did not differ significantly by treatment center.
The exception was respiratory failure. Patients with respiratory failure had a significantly greater risk of early mortality if they were treated at a non-NCI center (OR=9.48) than at an NCI center (OR=4.20).
Potential explanations
The researchers believe the variations in early mortality they observed point to inconsistent supportive care. However, more work must be done to fully understand the differences in care driving these issues.
“This is clearly provocative data that makes you want to understand exactly why,” Dr Jonas said. “We’re going to have to dive into that question in a more significant way.”
In the absence of data that could identify the exact causes, the researchers noted that other studies have shown higher patient volumes may contribute to better care.
“I see 60 or more AML cases per year,” Dr Jonas said. “High volume/low volume must play a role.”
The researchers believe other potential contributing factors could be access to clinical trials, better nursing ratios, and more sophisticated intensive care units.
The team hopes this research will spawn more intensive efforts to identify the causes that underlie variations in early mortality between hospital sites.
“This is a provocative and hopeful paper in terms of improving outcomes,” Dr Jonas said. “It sends a positive message that there are things we could probably do that could help everyone.”
A global snapshot of leukemia incidence
, according to an analysis of World Health Organization cancer databases.
Incidence also is generally higher in males, with a global male to female ratio of 1.4. For men, the highest regional leukemia rate – estimated at 11.3 per 100,000 population for 2012 – was found in Australia and New Zealand, with northern America (the United States and Canada) next at 10.5 per 100,000. Australia/New Zealand and northern America had the highest rate for women at 7.2 per 100,000, followed by western Europe and northern Europe at 6.0 per 100,000, reported Adalberto Miranda-Filho, PhD, of the WHO’s International Agency for Research on Cancer in Lyon, France, and his associates.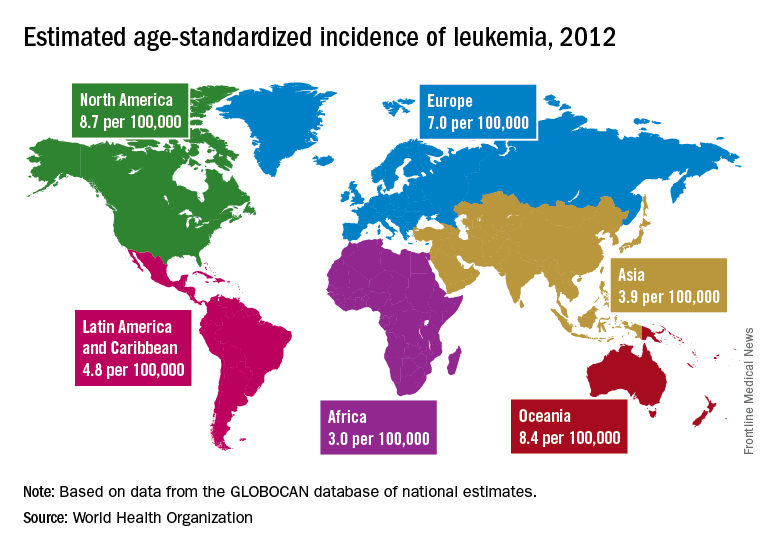
The lowest regional rates for women were found in western Africa (1.2 per 100,000), middle Africa (1.8), and Micronesia/Polynesia (2.1). For men, leukemia incidence was lowest in western Africa (1.4 per 100,000), middle Africa (2.6), and south-central Asia (3.4), according to data from the WHO’s GLOBOCAN database. The report was published in The Lancet Haematology.
Estimates for leukemia subtypes in 2003-2007 – calculated for 54 countries, not regions – also showed a great deal of variation. For acute lymphoblastic leukemia, Ecuador had the highest rates for both males (2.8 per 100,000) and females (3.3), with high rates seen in Costa Rica, Columbia, and Cyprus. Rates in the United States were near the top: 2.1 for males and 1.6 for females. Rates were lowest for men in Jamaica (0.4) and Serbia (0.6) and for women in India (0.5) and Serbia and Cuba (0.6), Dr. Miranda-Filho and his associates said.
Incidence rates for acute myeloid leukemia were highest in Australia for men (2.8 per 100,000) and Austria for women (2.2), with the United States near the top for both men (2.6) and women (1.9). The lowest rates occurred in Cuba and Egypt for men (0.9 per 100,000) and Cuba for women (0.4), data from the WHO’s Cancer Incidence in Five Continents Volume X show.
Chronic lymphocytic leukemia incidence was highest for men in Canada (4.5 per 100,000), Ireland and Lithuania (4.4), and Slovakia (4.3). The incidence was highest for women in Lithuania (2.5), Canada (2.3), and Slovakia and Denmark (2.1). Incidence in the United States was 3.5 for men and 1.8 for women. At the other end of the scale, the lowest rates for both men and women were in Japan and Malaysia (0.1), the investigators’ analysis showed.
Chronic myeloid leukemia rates were the lowest of the subtypes, and Tunisia was the lowest for men at 0.4 per 100,000 and tied for lowest with Serbia, Slovenia, and Puerto Rico for women at 0.3. Incidence was highest for men in Australia at 1.8 per 100,000 and highest for women in Uruguay at 1.1. Rates in the United States were 1.3 for men and 0.8 for women, Dr. Miranda-Filho and his associates said.
“The higher incidence of acute lymphoblastic leukaemia in parts of South America, as well as of chronic lymphocytic leukaemia in populations across North America and Oceania, alongside a lower incidence in Asia, might be important markers for further epidemiological study, and a means to better understand the underlying factors to support future cancer prevention strategies,” the investigators wrote.
SOURCE: Miranda-Filho A et al. Lancet Haematol. 2018;5:e14-24.
, according to an analysis of World Health Organization cancer databases.
Incidence also is generally higher in males, with a global male to female ratio of 1.4. For men, the highest regional leukemia rate – estimated at 11.3 per 100,000 population for 2012 – was found in Australia and New Zealand, with northern America (the United States and Canada) next at 10.5 per 100,000. Australia/New Zealand and northern America had the highest rate for women at 7.2 per 100,000, followed by western Europe and northern Europe at 6.0 per 100,000, reported Adalberto Miranda-Filho, PhD, of the WHO’s International Agency for Research on Cancer in Lyon, France, and his associates.
The lowest regional rates for women were found in western Africa (1.2 per 100,000), middle Africa (1.8), and Micronesia/Polynesia (2.1). For men, leukemia incidence was lowest in western Africa (1.4 per 100,000), middle Africa (2.6), and south-central Asia (3.4), according to data from the WHO’s GLOBOCAN database. The report was published in The Lancet Haematology.
Estimates for leukemia subtypes in 2003-2007 – calculated for 54 countries, not regions – also showed a great deal of variation. For acute lymphoblastic leukemia, Ecuador had the highest rates for both males (2.8 per 100,000) and females (3.3), with high rates seen in Costa Rica, Columbia, and Cyprus. Rates in the United States were near the top: 2.1 for males and 1.6 for females. Rates were lowest for men in Jamaica (0.4) and Serbia (0.6) and for women in India (0.5) and Serbia and Cuba (0.6), Dr. Miranda-Filho and his associates said.
Incidence rates for acute myeloid leukemia were highest in Australia for men (2.8 per 100,000) and Austria for women (2.2), with the United States near the top for both men (2.6) and women (1.9). The lowest rates occurred in Cuba and Egypt for men (0.9 per 100,000) and Cuba for women (0.4), data from the WHO’s Cancer Incidence in Five Continents Volume X show.
Chronic lymphocytic leukemia incidence was highest for men in Canada (4.5 per 100,000), Ireland and Lithuania (4.4), and Slovakia (4.3). The incidence was highest for women in Lithuania (2.5), Canada (2.3), and Slovakia and Denmark (2.1). Incidence in the United States was 3.5 for men and 1.8 for women. At the other end of the scale, the lowest rates for both men and women were in Japan and Malaysia (0.1), the investigators’ analysis showed.
Chronic myeloid leukemia rates were the lowest of the subtypes, and Tunisia was the lowest for men at 0.4 per 100,000 and tied for lowest with Serbia, Slovenia, and Puerto Rico for women at 0.3. Incidence was highest for men in Australia at 1.8 per 100,000 and highest for women in Uruguay at 1.1. Rates in the United States were 1.3 for men and 0.8 for women, Dr. Miranda-Filho and his associates said.
“The higher incidence of acute lymphoblastic leukaemia in parts of South America, as well as of chronic lymphocytic leukaemia in populations across North America and Oceania, alongside a lower incidence in Asia, might be important markers for further epidemiological study, and a means to better understand the underlying factors to support future cancer prevention strategies,” the investigators wrote.
SOURCE: Miranda-Filho A et al. Lancet Haematol. 2018;5:e14-24.
, according to an analysis of World Health Organization cancer databases.
Incidence also is generally higher in males, with a global male to female ratio of 1.4. For men, the highest regional leukemia rate – estimated at 11.3 per 100,000 population for 2012 – was found in Australia and New Zealand, with northern America (the United States and Canada) next at 10.5 per 100,000. Australia/New Zealand and northern America had the highest rate for women at 7.2 per 100,000, followed by western Europe and northern Europe at 6.0 per 100,000, reported Adalberto Miranda-Filho, PhD, of the WHO’s International Agency for Research on Cancer in Lyon, France, and his associates.
The lowest regional rates for women were found in western Africa (1.2 per 100,000), middle Africa (1.8), and Micronesia/Polynesia (2.1). For men, leukemia incidence was lowest in western Africa (1.4 per 100,000), middle Africa (2.6), and south-central Asia (3.4), according to data from the WHO’s GLOBOCAN database. The report was published in The Lancet Haematology.
Estimates for leukemia subtypes in 2003-2007 – calculated for 54 countries, not regions – also showed a great deal of variation. For acute lymphoblastic leukemia, Ecuador had the highest rates for both males (2.8 per 100,000) and females (3.3), with high rates seen in Costa Rica, Columbia, and Cyprus. Rates in the United States were near the top: 2.1 for males and 1.6 for females. Rates were lowest for men in Jamaica (0.4) and Serbia (0.6) and for women in India (0.5) and Serbia and Cuba (0.6), Dr. Miranda-Filho and his associates said.
Incidence rates for acute myeloid leukemia were highest in Australia for men (2.8 per 100,000) and Austria for women (2.2), with the United States near the top for both men (2.6) and women (1.9). The lowest rates occurred in Cuba and Egypt for men (0.9 per 100,000) and Cuba for women (0.4), data from the WHO’s Cancer Incidence in Five Continents Volume X show.
Chronic lymphocytic leukemia incidence was highest for men in Canada (4.5 per 100,000), Ireland and Lithuania (4.4), and Slovakia (4.3). The incidence was highest for women in Lithuania (2.5), Canada (2.3), and Slovakia and Denmark (2.1). Incidence in the United States was 3.5 for men and 1.8 for women. At the other end of the scale, the lowest rates for both men and women were in Japan and Malaysia (0.1), the investigators’ analysis showed.
Chronic myeloid leukemia rates were the lowest of the subtypes, and Tunisia was the lowest for men at 0.4 per 100,000 and tied for lowest with Serbia, Slovenia, and Puerto Rico for women at 0.3. Incidence was highest for men in Australia at 1.8 per 100,000 and highest for women in Uruguay at 1.1. Rates in the United States were 1.3 for men and 0.8 for women, Dr. Miranda-Filho and his associates said.
“The higher incidence of acute lymphoblastic leukaemia in parts of South America, as well as of chronic lymphocytic leukaemia in populations across North America and Oceania, alongside a lower incidence in Asia, might be important markers for further epidemiological study, and a means to better understand the underlying factors to support future cancer prevention strategies,” the investigators wrote.
SOURCE: Miranda-Filho A et al. Lancet Haematol. 2018;5:e14-24.
FROM THE LANCET HAEMATOLOGY
Team targets transcription factor in AML
Researchers say they have discovered a way to target the transcription factor MEF2C in acute myeloid leukemia (AML).
The team found they could stop the growth of MEF2C-driven AML cells by blocking either LKB1 or the salt-inducible kinases SIK3 and SIK2.
Christopher Vakoc, MD, PhD, of Cold Spring Harbor Laboratory in Cold Spring Harbor, New York, and his colleagues described this research in Molecular Cell.
The current discoveries are the result of a broad search for potential therapeutic strategies against AML that began several years ago in Dr Vakoc’s lab.
In 2013, his team devised a system based on CRISPR gene editing tools. They used this system to screen large numbers of genes, seeking to discover their impact on cancer cell survival.
Now, the system has revealed that LKB1 and SIK are critical for the survival of certain AML cells. These enzymes had not previously been linked to AML, but the researchers learned that LKB1 and SIK help control MEF2C.
The team observed overlapping LKB1, SIK, and MEF2C dependencies in AML cell lines, particularly MLL fusion lines (MOLM-13, MV4-11, NOMO-1, and THP-1). And the researchers found the transcriptional output of MEF2C could be suppressed by inhibition of LKB1 or SIK.
“At the end of project, we realized we’d actually discovered a way to control a transcription factor,” Dr Vakoc said.
He and his colleagues found that SIK3 inactivation had the strongest effect on MEF2C. Two hours of exposure to the SIK inhibitor HG-9-91-01 (100 nM) was enough to suppress the MEF2C signature.
The researchers also noted that the effect of SIK3 targeting on transcription was attenuated if it was performed in cells deficient in HDAC4. This and related findings suggested that LKB1-SIK3 signaling supports the transcriptional output of MEF2C through inhibition of HDAC4.
Dr Vakoc and his colleagues said the “potency and selectivity of AML growth arrest” they observed after targeting LKB1 or SIK2 and SIK3 resembles the effects of targeting other validated kinase oncogenes in AML, such as FLT3.
The team also said the sensitivity of AML cell lines to HG-9-91-01 “compares favorably” to the sensitivity of cancer cell lines to kinase inhibitors already approved for oncology indications. However, “additional optimization” of HG-9-91-01 is needed.
Researchers say they have discovered a way to target the transcription factor MEF2C in acute myeloid leukemia (AML).
The team found they could stop the growth of MEF2C-driven AML cells by blocking either LKB1 or the salt-inducible kinases SIK3 and SIK2.
Christopher Vakoc, MD, PhD, of Cold Spring Harbor Laboratory in Cold Spring Harbor, New York, and his colleagues described this research in Molecular Cell.
The current discoveries are the result of a broad search for potential therapeutic strategies against AML that began several years ago in Dr Vakoc’s lab.
In 2013, his team devised a system based on CRISPR gene editing tools. They used this system to screen large numbers of genes, seeking to discover their impact on cancer cell survival.
Now, the system has revealed that LKB1 and SIK are critical for the survival of certain AML cells. These enzymes had not previously been linked to AML, but the researchers learned that LKB1 and SIK help control MEF2C.
The team observed overlapping LKB1, SIK, and MEF2C dependencies in AML cell lines, particularly MLL fusion lines (MOLM-13, MV4-11, NOMO-1, and THP-1). And the researchers found the transcriptional output of MEF2C could be suppressed by inhibition of LKB1 or SIK.
“At the end of project, we realized we’d actually discovered a way to control a transcription factor,” Dr Vakoc said.
He and his colleagues found that SIK3 inactivation had the strongest effect on MEF2C. Two hours of exposure to the SIK inhibitor HG-9-91-01 (100 nM) was enough to suppress the MEF2C signature.
The researchers also noted that the effect of SIK3 targeting on transcription was attenuated if it was performed in cells deficient in HDAC4. This and related findings suggested that LKB1-SIK3 signaling supports the transcriptional output of MEF2C through inhibition of HDAC4.
Dr Vakoc and his colleagues said the “potency and selectivity of AML growth arrest” they observed after targeting LKB1 or SIK2 and SIK3 resembles the effects of targeting other validated kinase oncogenes in AML, such as FLT3.
The team also said the sensitivity of AML cell lines to HG-9-91-01 “compares favorably” to the sensitivity of cancer cell lines to kinase inhibitors already approved for oncology indications. However, “additional optimization” of HG-9-91-01 is needed.
Researchers say they have discovered a way to target the transcription factor MEF2C in acute myeloid leukemia (AML).
The team found they could stop the growth of MEF2C-driven AML cells by blocking either LKB1 or the salt-inducible kinases SIK3 and SIK2.
Christopher Vakoc, MD, PhD, of Cold Spring Harbor Laboratory in Cold Spring Harbor, New York, and his colleagues described this research in Molecular Cell.
The current discoveries are the result of a broad search for potential therapeutic strategies against AML that began several years ago in Dr Vakoc’s lab.
In 2013, his team devised a system based on CRISPR gene editing tools. They used this system to screen large numbers of genes, seeking to discover their impact on cancer cell survival.
Now, the system has revealed that LKB1 and SIK are critical for the survival of certain AML cells. These enzymes had not previously been linked to AML, but the researchers learned that LKB1 and SIK help control MEF2C.
The team observed overlapping LKB1, SIK, and MEF2C dependencies in AML cell lines, particularly MLL fusion lines (MOLM-13, MV4-11, NOMO-1, and THP-1). And the researchers found the transcriptional output of MEF2C could be suppressed by inhibition of LKB1 or SIK.
“At the end of project, we realized we’d actually discovered a way to control a transcription factor,” Dr Vakoc said.
He and his colleagues found that SIK3 inactivation had the strongest effect on MEF2C. Two hours of exposure to the SIK inhibitor HG-9-91-01 (100 nM) was enough to suppress the MEF2C signature.
The researchers also noted that the effect of SIK3 targeting on transcription was attenuated if it was performed in cells deficient in HDAC4. This and related findings suggested that LKB1-SIK3 signaling supports the transcriptional output of MEF2C through inhibition of HDAC4.
Dr Vakoc and his colleagues said the “potency and selectivity of AML growth arrest” they observed after targeting LKB1 or SIK2 and SIK3 resembles the effects of targeting other validated kinase oncogenes in AML, such as FLT3.
The team also said the sensitivity of AML cell lines to HG-9-91-01 “compares favorably” to the sensitivity of cancer cell lines to kinase inhibitors already approved for oncology indications. However, “additional optimization” of HG-9-91-01 is needed.
MEC plus ixazomib looks promising in relapsed/refractory AML
ATLANTA – Mitoxantrone, etoposide, and cytarabine (MEC) in combination with the second-generation proteasome inhibitor ixazomib was well tolerated and effective in a phase 1 expansion study of patients with relapsed or refractory acute myeloid leukemia.
The overall response rate in 30 patients enrolled in the study and treated with the novel combination was 53%; 11 patients had a complete response (CR), and 5 had a complete response with incomplete blood count recovery (CRi). The median overall survival was 4.9 months, Anjali S. Advani, MD, reported at the annual meeting of the American Society of Hematology.
Thirteen patients proceeded to allogeneic hematopoietic cell transplant (AHCT), and one received a donor lymphocyte infusion. Seven of these 14 patients are alive with a median follow-up of 14.5 months, said Dr. Advani of Taussig Cancer Institute, Cleveland Clinic.
The patients, who had a median age of 58 years (range of 18-70 years), were eligible for the study if they had relapsed/refractory acute myeloid leukemia (AML), adequate organ function, and cardiac ejection fraction of at least 45%. The median time from initial diagnosis to enrollment was 7.6 months.
Eight patients had a history of an antecedent hematologic disorder; 14 were in their first relapse; and 13 had disease that was refractory to their last treatment. Two had received a prior AHCT; seven had FLT3 internal tandem duplication (ITD) mutations indicative of particularly poor prognosis; and seven had adverse cytogenetics, she said.
They received one cycle of the therapy, which included 8mg/m2 of mitoxantrone, 80 mg/m2 of etoposide, and 1,000 mg/m2 of cytarabine given intravenously on days 1-6, plus ixazomib at doses of 1 mg (27 patients) or 2 mg (3 patients) given orally on days 1, 4, 8, and 11. An additional 18 patients were treated at the maximum tolerated dose (1 mg, as determined in phase 1 of the trial), Dr. Advani said.
The treatment was well tolerated in most patients. Grade 3-5 nonhematologic toxicities occurred in at least 15% of patients and included infection in 74%, febrile neutropenia in 85%, hypotension in 18%, hypoxia in 19%, mucositis in 15%, hypokalemia in 33%, and hypoalbuminemia in 30%, she said. The early mortality rate was 10%.
Of note, prior studies have demonstrated that the number of mutations in DNMT3A, TP53, ASXL1, and NRAS is associated with a worse response to salvage therapy. Of 21 patients in the current study who had available data, 10 patients had at least one of these mutations, and 8 of those 10 patients achieved CR or CRi, Dr. Advani said.
“To identify a signature predictive of response to treatment, we performed RNA sequencing on pretreatment samples from 17 patients, and on posttreatment samples from 11 patients,” she said. “We found that genes were differentially expressed between resistant and responding patients in 314 genes in the pretreatment samples, in 217 genes in the posttreatment samples, and in 72 genes at both time points.”
Gene set enrichment analysis identified significantly differentially expressed genes clustering in heme-metabolism and erythroblast differentiation, inflammatory response, cytokine/STAT signaling, nuclear factor-kappa beta (NF-kappaB), and hypoxia. Two genes – gamma-interferon–inducible lysosomal thiol reductase (IFI30) and retinoic acid–related orphan receptor A (ROR-alpha) – were found to be significantly different between responding and resistant patients, and could potentially classify response, she noted.
“IFI30, which may increase the levels of antioxidants and lead to a decreased ER [endoplasmic reticulum] stress response to therapy, was more highly expressed in resistant patients, and ROR-alpha, a tumor-suppressor gene, was down regulated in resistant patients,” she said.
Ixazomib was combined with the AML salvage regimen MEC in this study because proteasome inhibitors like ixazomib induce cell death in AML cells through inhibition of NF-kappaB, and also increase chemosensitivity to anthracyclines and cytarabines, Dr. Advani explained.
The findings are encouraging and suggest that results from gene expression profiling may help identify resistant patients and provide further therapeutic targets, she said, noting that in vitro studies are planned to clarify whether the use of ROR-alpha agonists may help sensitize resistant cells to treatment.
Dr. Advani reported receiving research funding from Takeda/Millenium, and serving as a consultant for Pfizer.
ATLANTA – Mitoxantrone, etoposide, and cytarabine (MEC) in combination with the second-generation proteasome inhibitor ixazomib was well tolerated and effective in a phase 1 expansion study of patients with relapsed or refractory acute myeloid leukemia.
The overall response rate in 30 patients enrolled in the study and treated with the novel combination was 53%; 11 patients had a complete response (CR), and 5 had a complete response with incomplete blood count recovery (CRi). The median overall survival was 4.9 months, Anjali S. Advani, MD, reported at the annual meeting of the American Society of Hematology.
Thirteen patients proceeded to allogeneic hematopoietic cell transplant (AHCT), and one received a donor lymphocyte infusion. Seven of these 14 patients are alive with a median follow-up of 14.5 months, said Dr. Advani of Taussig Cancer Institute, Cleveland Clinic.
The patients, who had a median age of 58 years (range of 18-70 years), were eligible for the study if they had relapsed/refractory acute myeloid leukemia (AML), adequate organ function, and cardiac ejection fraction of at least 45%. The median time from initial diagnosis to enrollment was 7.6 months.
Eight patients had a history of an antecedent hematologic disorder; 14 were in their first relapse; and 13 had disease that was refractory to their last treatment. Two had received a prior AHCT; seven had FLT3 internal tandem duplication (ITD) mutations indicative of particularly poor prognosis; and seven had adverse cytogenetics, she said.
They received one cycle of the therapy, which included 8mg/m2 of mitoxantrone, 80 mg/m2 of etoposide, and 1,000 mg/m2 of cytarabine given intravenously on days 1-6, plus ixazomib at doses of 1 mg (27 patients) or 2 mg (3 patients) given orally on days 1, 4, 8, and 11. An additional 18 patients were treated at the maximum tolerated dose (1 mg, as determined in phase 1 of the trial), Dr. Advani said.
The treatment was well tolerated in most patients. Grade 3-5 nonhematologic toxicities occurred in at least 15% of patients and included infection in 74%, febrile neutropenia in 85%, hypotension in 18%, hypoxia in 19%, mucositis in 15%, hypokalemia in 33%, and hypoalbuminemia in 30%, she said. The early mortality rate was 10%.
Of note, prior studies have demonstrated that the number of mutations in DNMT3A, TP53, ASXL1, and NRAS is associated with a worse response to salvage therapy. Of 21 patients in the current study who had available data, 10 patients had at least one of these mutations, and 8 of those 10 patients achieved CR or CRi, Dr. Advani said.
“To identify a signature predictive of response to treatment, we performed RNA sequencing on pretreatment samples from 17 patients, and on posttreatment samples from 11 patients,” she said. “We found that genes were differentially expressed between resistant and responding patients in 314 genes in the pretreatment samples, in 217 genes in the posttreatment samples, and in 72 genes at both time points.”
Gene set enrichment analysis identified significantly differentially expressed genes clustering in heme-metabolism and erythroblast differentiation, inflammatory response, cytokine/STAT signaling, nuclear factor-kappa beta (NF-kappaB), and hypoxia. Two genes – gamma-interferon–inducible lysosomal thiol reductase (IFI30) and retinoic acid–related orphan receptor A (ROR-alpha) – were found to be significantly different between responding and resistant patients, and could potentially classify response, she noted.
“IFI30, which may increase the levels of antioxidants and lead to a decreased ER [endoplasmic reticulum] stress response to therapy, was more highly expressed in resistant patients, and ROR-alpha, a tumor-suppressor gene, was down regulated in resistant patients,” she said.
Ixazomib was combined with the AML salvage regimen MEC in this study because proteasome inhibitors like ixazomib induce cell death in AML cells through inhibition of NF-kappaB, and also increase chemosensitivity to anthracyclines and cytarabines, Dr. Advani explained.
The findings are encouraging and suggest that results from gene expression profiling may help identify resistant patients and provide further therapeutic targets, she said, noting that in vitro studies are planned to clarify whether the use of ROR-alpha agonists may help sensitize resistant cells to treatment.
Dr. Advani reported receiving research funding from Takeda/Millenium, and serving as a consultant for Pfizer.
ATLANTA – Mitoxantrone, etoposide, and cytarabine (MEC) in combination with the second-generation proteasome inhibitor ixazomib was well tolerated and effective in a phase 1 expansion study of patients with relapsed or refractory acute myeloid leukemia.
The overall response rate in 30 patients enrolled in the study and treated with the novel combination was 53%; 11 patients had a complete response (CR), and 5 had a complete response with incomplete blood count recovery (CRi). The median overall survival was 4.9 months, Anjali S. Advani, MD, reported at the annual meeting of the American Society of Hematology.
Thirteen patients proceeded to allogeneic hematopoietic cell transplant (AHCT), and one received a donor lymphocyte infusion. Seven of these 14 patients are alive with a median follow-up of 14.5 months, said Dr. Advani of Taussig Cancer Institute, Cleveland Clinic.
The patients, who had a median age of 58 years (range of 18-70 years), were eligible for the study if they had relapsed/refractory acute myeloid leukemia (AML), adequate organ function, and cardiac ejection fraction of at least 45%. The median time from initial diagnosis to enrollment was 7.6 months.
Eight patients had a history of an antecedent hematologic disorder; 14 were in their first relapse; and 13 had disease that was refractory to their last treatment. Two had received a prior AHCT; seven had FLT3 internal tandem duplication (ITD) mutations indicative of particularly poor prognosis; and seven had adverse cytogenetics, she said.
They received one cycle of the therapy, which included 8mg/m2 of mitoxantrone, 80 mg/m2 of etoposide, and 1,000 mg/m2 of cytarabine given intravenously on days 1-6, plus ixazomib at doses of 1 mg (27 patients) or 2 mg (3 patients) given orally on days 1, 4, 8, and 11. An additional 18 patients were treated at the maximum tolerated dose (1 mg, as determined in phase 1 of the trial), Dr. Advani said.
The treatment was well tolerated in most patients. Grade 3-5 nonhematologic toxicities occurred in at least 15% of patients and included infection in 74%, febrile neutropenia in 85%, hypotension in 18%, hypoxia in 19%, mucositis in 15%, hypokalemia in 33%, and hypoalbuminemia in 30%, she said. The early mortality rate was 10%.
Of note, prior studies have demonstrated that the number of mutations in DNMT3A, TP53, ASXL1, and NRAS is associated with a worse response to salvage therapy. Of 21 patients in the current study who had available data, 10 patients had at least one of these mutations, and 8 of those 10 patients achieved CR or CRi, Dr. Advani said.
“To identify a signature predictive of response to treatment, we performed RNA sequencing on pretreatment samples from 17 patients, and on posttreatment samples from 11 patients,” she said. “We found that genes were differentially expressed between resistant and responding patients in 314 genes in the pretreatment samples, in 217 genes in the posttreatment samples, and in 72 genes at both time points.”
Gene set enrichment analysis identified significantly differentially expressed genes clustering in heme-metabolism and erythroblast differentiation, inflammatory response, cytokine/STAT signaling, nuclear factor-kappa beta (NF-kappaB), and hypoxia. Two genes – gamma-interferon–inducible lysosomal thiol reductase (IFI30) and retinoic acid–related orphan receptor A (ROR-alpha) – were found to be significantly different between responding and resistant patients, and could potentially classify response, she noted.
“IFI30, which may increase the levels of antioxidants and lead to a decreased ER [endoplasmic reticulum] stress response to therapy, was more highly expressed in resistant patients, and ROR-alpha, a tumor-suppressor gene, was down regulated in resistant patients,” she said.
Ixazomib was combined with the AML salvage regimen MEC in this study because proteasome inhibitors like ixazomib induce cell death in AML cells through inhibition of NF-kappaB, and also increase chemosensitivity to anthracyclines and cytarabines, Dr. Advani explained.
The findings are encouraging and suggest that results from gene expression profiling may help identify resistant patients and provide further therapeutic targets, she said, noting that in vitro studies are planned to clarify whether the use of ROR-alpha agonists may help sensitize resistant cells to treatment.
Dr. Advani reported receiving research funding from Takeda/Millenium, and serving as a consultant for Pfizer.
REPORTING FROM ASH 2017
Key clinical point: Major finding: The overall response rate was 53%.
Study details: A phase 1 trial involving 30 patients.
Disclosures: Dr. Advani reported receiving research funding from Takeda/Millenium, and serving as a consultant for Pfizer.
Source: Advani A et al. ASH 2017, Abstract 150.
GCLAM therapy shows promise for relapsed/refractory AML
ATLANTA – GCLAM – the combined use of granulocyte colony-stimulating factor (G-CSF), cladribine, high-dose cytarabine, and mitoxantrone – was well tolerated and had potent antileukemia activity in a phase 1/2 trial of adults with relapsed/refractory acute myeloid leukemia or high-grade myeloid neoplasms.
Of 40 patients who were treated with GCLAM (with mitoxantrone at the maximum tolerated dose of 16 mg/m2 per day as established in phase 1 of the trial), 11 achieved a complete response (CR), and 13 achieved a complete response with incomplete blood count recovery (CRi), for an overall response rate of 60%, Anna B. Halpern, MD, reported at the annual meeting of the American Society of Hematology.
“Nine of the 11 CR patients and 11 of 13 with CRis were negative for minimal residual disease, for an overall MRD-negative CR rate of 23%,” said Dr. Halpern of the University of Washington and the Fred Hutchinson Cancer Research Center, both in Seattle.
Resistant disease occurred in 11 patients, she noted.
Median overall survival was 11.5 months, and the treatment-related mortality (TRM) rate was 5%.
Overall, 21 of 40 patients went to transplant, with a 49% 1-year survival rate, she said.
The patients had a median age of 63 years. Thirty-four had acute myeloid leukemia (AML), and 6 had high-grade myelodysplastic syndrome; 28 had secondary disease. Nineteen had primary refractory disease, 21 had relapsed disease after a median initial CR duration of 12 months, and 7 had prior allogeneic transplant. The median TRM score for all patients was 2.0, indicating a low risk for treatment-related mortality.
“Cytogenetics distribution, based on Medical Research Council criteria, was as expected,” she added.
At the mitoxantrone maximum tolerated dose of 16 mg/m2 per day – which was established during phase 1 in 26 patients in whom higher dose levels (18 mg/m2 per day) led to dose-limiting encephalopathy and cardiogenic shock – the most common grade 3 or greater adverse events included neutropenic fever, infectious complications, and hypoxia during therapy. This was largely attributed to volume overload and infection, Dr. Halpern said.
“Although three patients did have decreased ejection fraction in cycle 2, this was largely in the setting of sepsis, making the etiology difficult to ascribe to the anthracycline versus sepsis physiology,” she noted.
The median times to an absolute neutrophil count of 500/mcL or greater and platelet count of 50,000/mcL or greater were 29 days each, she noted.
A multivariable analysis controlling for baseline prognostic features showed that the mitoxantrone dose of 16 mg/m2 per day was associated with significantly better overall survival, compared with a dose of 10 mg/m2 per day used in a historical cohort according to standard GCLAM dosing (hazard ratio for death, 0.45). Additionally, more of those receiving a dose of 16 mg/m2 per day went on to transplant (52% vs. 37%), she said. The overall response rate was also higher with the 16-mg/m2 dose, but the difference was not statistically significant (odds ratio, 1.87).
“Further, the outcomes appear to be as good or better with GCLAM with mitoxantrone at 16 mg/m2 compared to other salvage regimens used at our institution, including decitabine priming plus mitoxantrone, etoposide, and cytarabine [d/MEC] and G-CSF with clofarabine and high-dose cytarabine [GCLAC],” she said, noting that the examination is currently ongoing in a larger sample.
The initial analysis, however, showed that, after controlling for age, cytogenetic risk, first CR duration, and prior hematopoietic cell transplant, overall response and overall survival rates were better with GCLAM than with d/MEC (OR, 3.23; HR for death, 0.64) and that the overall response rate was similar between GCLAM and GCLAC (OR, 1.75), she said.
The findings are encouraging because outcomes with standard chemotherapies for relapsed/refractory myeloid neoplasms are poor, with complete remission rates rarely exceeding 15%-20%, Dr. Halpern said.
The current study was undertaken based on promising results from a previous phase 2 study in poor-risk relapsed/refractory AML, which also showed encouraging activity with GCLAM and based on data suggesting benefit with escalated doses of anthracyclines in AML, she explained.
Patients 18 years and older were eligible if they had adequate organ function and a TRM score of 6.9 or lower, which corresponds to a predicted 28-day mortality of no more than 6.9% with standard induction chemotherapy. Those with uncontrolled infection or concomitant illness with expected survival of less than 1 year were excluded.
The phase 1 dose escalation involved cohorts of 6-12 patients who were assigned to receive mitoxantrone dose levels of 12, 14, 16, or 18 mg/m2 per day on days 1-3. The doses of the remaining drugs in the combination were fixed at 300 mcg or 480 mcg of G-CSF on days 0-5, 5 mg/m2 of cladribine on days 1-5, and 2 mg/m2 of cytarabine on days 1-5.
“All patients received GCLAM induction at their assigned mitoxantrone dose level. If CR wasn’t achieved with cycle 1, a second identical course of GCLAM was given,” Dr. Halpern explained, noting that patients with resistant disease after 2 cycles were taken off the study.
If CR or CRi was achieved within 1-2 cycles of induction, up to 4 cycles of consolidation with G-CLA (mitoxantrone omitted) were allowed, and responders could proceed with transplant at any time.
In phase 2, patients received the maximum tolerated dose of mitoxantrone (16mg/m2 per day), as defined in phase 1.
“Relapsed and refractory AML and high grade myeloid neoplasms are a challenging disease to treat. With an overall response rate of 60%, this regimen showed efficacy in a heavily pretreated patient population,” Dr. Halpern said. “And many of the responders were able to go on to receive a stem cell transplant, the only known curative option in this situation.”
A follow-up study is currently exploring the relative value of decitabine priming followed by GCLAM in this setting, she said.
Dr. Halpern reported having no relevant financial disclosures.
sworcester@frontlinemedcom.com
SOURCE: Halpern AB et al. ASH 2017, Abstract 149
ATLANTA – GCLAM – the combined use of granulocyte colony-stimulating factor (G-CSF), cladribine, high-dose cytarabine, and mitoxantrone – was well tolerated and had potent antileukemia activity in a phase 1/2 trial of adults with relapsed/refractory acute myeloid leukemia or high-grade myeloid neoplasms.
Of 40 patients who were treated with GCLAM (with mitoxantrone at the maximum tolerated dose of 16 mg/m2 per day as established in phase 1 of the trial), 11 achieved a complete response (CR), and 13 achieved a complete response with incomplete blood count recovery (CRi), for an overall response rate of 60%, Anna B. Halpern, MD, reported at the annual meeting of the American Society of Hematology.
“Nine of the 11 CR patients and 11 of 13 with CRis were negative for minimal residual disease, for an overall MRD-negative CR rate of 23%,” said Dr. Halpern of the University of Washington and the Fred Hutchinson Cancer Research Center, both in Seattle.
Resistant disease occurred in 11 patients, she noted.
Median overall survival was 11.5 months, and the treatment-related mortality (TRM) rate was 5%.
Overall, 21 of 40 patients went to transplant, with a 49% 1-year survival rate, she said.
The patients had a median age of 63 years. Thirty-four had acute myeloid leukemia (AML), and 6 had high-grade myelodysplastic syndrome; 28 had secondary disease. Nineteen had primary refractory disease, 21 had relapsed disease after a median initial CR duration of 12 months, and 7 had prior allogeneic transplant. The median TRM score for all patients was 2.0, indicating a low risk for treatment-related mortality.
“Cytogenetics distribution, based on Medical Research Council criteria, was as expected,” she added.
At the mitoxantrone maximum tolerated dose of 16 mg/m2 per day – which was established during phase 1 in 26 patients in whom higher dose levels (18 mg/m2 per day) led to dose-limiting encephalopathy and cardiogenic shock – the most common grade 3 or greater adverse events included neutropenic fever, infectious complications, and hypoxia during therapy. This was largely attributed to volume overload and infection, Dr. Halpern said.
“Although three patients did have decreased ejection fraction in cycle 2, this was largely in the setting of sepsis, making the etiology difficult to ascribe to the anthracycline versus sepsis physiology,” she noted.
The median times to an absolute neutrophil count of 500/mcL or greater and platelet count of 50,000/mcL or greater were 29 days each, she noted.
A multivariable analysis controlling for baseline prognostic features showed that the mitoxantrone dose of 16 mg/m2 per day was associated with significantly better overall survival, compared with a dose of 10 mg/m2 per day used in a historical cohort according to standard GCLAM dosing (hazard ratio for death, 0.45). Additionally, more of those receiving a dose of 16 mg/m2 per day went on to transplant (52% vs. 37%), she said. The overall response rate was also higher with the 16-mg/m2 dose, but the difference was not statistically significant (odds ratio, 1.87).
“Further, the outcomes appear to be as good or better with GCLAM with mitoxantrone at 16 mg/m2 compared to other salvage regimens used at our institution, including decitabine priming plus mitoxantrone, etoposide, and cytarabine [d/MEC] and G-CSF with clofarabine and high-dose cytarabine [GCLAC],” she said, noting that the examination is currently ongoing in a larger sample.
The initial analysis, however, showed that, after controlling for age, cytogenetic risk, first CR duration, and prior hematopoietic cell transplant, overall response and overall survival rates were better with GCLAM than with d/MEC (OR, 3.23; HR for death, 0.64) and that the overall response rate was similar between GCLAM and GCLAC (OR, 1.75), she said.
The findings are encouraging because outcomes with standard chemotherapies for relapsed/refractory myeloid neoplasms are poor, with complete remission rates rarely exceeding 15%-20%, Dr. Halpern said.
The current study was undertaken based on promising results from a previous phase 2 study in poor-risk relapsed/refractory AML, which also showed encouraging activity with GCLAM and based on data suggesting benefit with escalated doses of anthracyclines in AML, she explained.
Patients 18 years and older were eligible if they had adequate organ function and a TRM score of 6.9 or lower, which corresponds to a predicted 28-day mortality of no more than 6.9% with standard induction chemotherapy. Those with uncontrolled infection or concomitant illness with expected survival of less than 1 year were excluded.
The phase 1 dose escalation involved cohorts of 6-12 patients who were assigned to receive mitoxantrone dose levels of 12, 14, 16, or 18 mg/m2 per day on days 1-3. The doses of the remaining drugs in the combination were fixed at 300 mcg or 480 mcg of G-CSF on days 0-5, 5 mg/m2 of cladribine on days 1-5, and 2 mg/m2 of cytarabine on days 1-5.
“All patients received GCLAM induction at their assigned mitoxantrone dose level. If CR wasn’t achieved with cycle 1, a second identical course of GCLAM was given,” Dr. Halpern explained, noting that patients with resistant disease after 2 cycles were taken off the study.
If CR or CRi was achieved within 1-2 cycles of induction, up to 4 cycles of consolidation with G-CLA (mitoxantrone omitted) were allowed, and responders could proceed with transplant at any time.
In phase 2, patients received the maximum tolerated dose of mitoxantrone (16mg/m2 per day), as defined in phase 1.
“Relapsed and refractory AML and high grade myeloid neoplasms are a challenging disease to treat. With an overall response rate of 60%, this regimen showed efficacy in a heavily pretreated patient population,” Dr. Halpern said. “And many of the responders were able to go on to receive a stem cell transplant, the only known curative option in this situation.”
A follow-up study is currently exploring the relative value of decitabine priming followed by GCLAM in this setting, she said.
Dr. Halpern reported having no relevant financial disclosures.
sworcester@frontlinemedcom.com
SOURCE: Halpern AB et al. ASH 2017, Abstract 149
ATLANTA – GCLAM – the combined use of granulocyte colony-stimulating factor (G-CSF), cladribine, high-dose cytarabine, and mitoxantrone – was well tolerated and had potent antileukemia activity in a phase 1/2 trial of adults with relapsed/refractory acute myeloid leukemia or high-grade myeloid neoplasms.
Of 40 patients who were treated with GCLAM (with mitoxantrone at the maximum tolerated dose of 16 mg/m2 per day as established in phase 1 of the trial), 11 achieved a complete response (CR), and 13 achieved a complete response with incomplete blood count recovery (CRi), for an overall response rate of 60%, Anna B. Halpern, MD, reported at the annual meeting of the American Society of Hematology.
“Nine of the 11 CR patients and 11 of 13 with CRis were negative for minimal residual disease, for an overall MRD-negative CR rate of 23%,” said Dr. Halpern of the University of Washington and the Fred Hutchinson Cancer Research Center, both in Seattle.
Resistant disease occurred in 11 patients, she noted.
Median overall survival was 11.5 months, and the treatment-related mortality (TRM) rate was 5%.
Overall, 21 of 40 patients went to transplant, with a 49% 1-year survival rate, she said.
The patients had a median age of 63 years. Thirty-four had acute myeloid leukemia (AML), and 6 had high-grade myelodysplastic syndrome; 28 had secondary disease. Nineteen had primary refractory disease, 21 had relapsed disease after a median initial CR duration of 12 months, and 7 had prior allogeneic transplant. The median TRM score for all patients was 2.0, indicating a low risk for treatment-related mortality.
“Cytogenetics distribution, based on Medical Research Council criteria, was as expected,” she added.
At the mitoxantrone maximum tolerated dose of 16 mg/m2 per day – which was established during phase 1 in 26 patients in whom higher dose levels (18 mg/m2 per day) led to dose-limiting encephalopathy and cardiogenic shock – the most common grade 3 or greater adverse events included neutropenic fever, infectious complications, and hypoxia during therapy. This was largely attributed to volume overload and infection, Dr. Halpern said.
“Although three patients did have decreased ejection fraction in cycle 2, this was largely in the setting of sepsis, making the etiology difficult to ascribe to the anthracycline versus sepsis physiology,” she noted.
The median times to an absolute neutrophil count of 500/mcL or greater and platelet count of 50,000/mcL or greater were 29 days each, she noted.
A multivariable analysis controlling for baseline prognostic features showed that the mitoxantrone dose of 16 mg/m2 per day was associated with significantly better overall survival, compared with a dose of 10 mg/m2 per day used in a historical cohort according to standard GCLAM dosing (hazard ratio for death, 0.45). Additionally, more of those receiving a dose of 16 mg/m2 per day went on to transplant (52% vs. 37%), she said. The overall response rate was also higher with the 16-mg/m2 dose, but the difference was not statistically significant (odds ratio, 1.87).
“Further, the outcomes appear to be as good or better with GCLAM with mitoxantrone at 16 mg/m2 compared to other salvage regimens used at our institution, including decitabine priming plus mitoxantrone, etoposide, and cytarabine [d/MEC] and G-CSF with clofarabine and high-dose cytarabine [GCLAC],” she said, noting that the examination is currently ongoing in a larger sample.
The initial analysis, however, showed that, after controlling for age, cytogenetic risk, first CR duration, and prior hematopoietic cell transplant, overall response and overall survival rates were better with GCLAM than with d/MEC (OR, 3.23; HR for death, 0.64) and that the overall response rate was similar between GCLAM and GCLAC (OR, 1.75), she said.
The findings are encouraging because outcomes with standard chemotherapies for relapsed/refractory myeloid neoplasms are poor, with complete remission rates rarely exceeding 15%-20%, Dr. Halpern said.
The current study was undertaken based on promising results from a previous phase 2 study in poor-risk relapsed/refractory AML, which also showed encouraging activity with GCLAM and based on data suggesting benefit with escalated doses of anthracyclines in AML, she explained.
Patients 18 years and older were eligible if they had adequate organ function and a TRM score of 6.9 or lower, which corresponds to a predicted 28-day mortality of no more than 6.9% with standard induction chemotherapy. Those with uncontrolled infection or concomitant illness with expected survival of less than 1 year were excluded.
The phase 1 dose escalation involved cohorts of 6-12 patients who were assigned to receive mitoxantrone dose levels of 12, 14, 16, or 18 mg/m2 per day on days 1-3. The doses of the remaining drugs in the combination were fixed at 300 mcg or 480 mcg of G-CSF on days 0-5, 5 mg/m2 of cladribine on days 1-5, and 2 mg/m2 of cytarabine on days 1-5.
“All patients received GCLAM induction at their assigned mitoxantrone dose level. If CR wasn’t achieved with cycle 1, a second identical course of GCLAM was given,” Dr. Halpern explained, noting that patients with resistant disease after 2 cycles were taken off the study.
If CR or CRi was achieved within 1-2 cycles of induction, up to 4 cycles of consolidation with G-CLA (mitoxantrone omitted) were allowed, and responders could proceed with transplant at any time.
In phase 2, patients received the maximum tolerated dose of mitoxantrone (16mg/m2 per day), as defined in phase 1.
“Relapsed and refractory AML and high grade myeloid neoplasms are a challenging disease to treat. With an overall response rate of 60%, this regimen showed efficacy in a heavily pretreated patient population,” Dr. Halpern said. “And many of the responders were able to go on to receive a stem cell transplant, the only known curative option in this situation.”
A follow-up study is currently exploring the relative value of decitabine priming followed by GCLAM in this setting, she said.
Dr. Halpern reported having no relevant financial disclosures.
sworcester@frontlinemedcom.com
SOURCE: Halpern AB et al. ASH 2017, Abstract 149
REPORTING FROM ASH 2017
Key clinical point: GCLAM was well tolerated and had potent antileukemia activity in a phase 1/2 trial.
Major finding: The overall response rate was 60%.
Study details: A phase 1/2 study of 40 patients.
Disclosures: Dr. Halpern reported having no financial disclosures.
Source: Halpern AB et al. ASH 2017, Abstract 149.
HSCT approach provides ‘excellent’ survival in FA
SALT LAKE CITY—A “risk-adjusted” approach leads to “excellent” survival in patients with Fanconi anemia (FA) undergoing alternative donor hematopoietic stem cell transplant (HSCT), according to a speaker at the 2018 BMT Tandem Meetings.
All FA patients who received personalized doses of busulfan in place of total body irradiation (TBI) were alive and disease-free after undergoing HSCT for bone marrow failure or myelodysplastic syndrome (MDS).
None of the patients developed graft-vs-host disease (GVHD), and the most common toxicity was viral infection.
Parinda A. Mehta, MD, of Cincinnati Children’s Hospital Medical Center in Ohio, presented these results at this year’s BMT Tandem Meetings as abstract 109.*
“We all know that inherent chemotherapy and radiation sensitivity makes transplant for patients with Fanconi anemia quite challenging,” Dr Mehta began. “In our recently published, prospective, multi-institutional study, we showed excellent outcomes of alternative donor transplant in patients with Fanconi anemia without using radiation.”
“In that study,** TBI was replaced by pharmacokinetically adjusted busulfan. It proved that, yes, we can do alternative donor transplant successfully without radiation by showing an overall survival of 80% for a total of 45 patients. We were quite ecstatic to see these numbers.”
The study also showed that younger patients fared better with this regimen, and younger patients did best with the lowest dose of busulfan tested (0.6 mg/kg vs 0.8 to 1.0 mg/kg). In addition, patients who underwent HSCT for bone marrow failure had better outcomes than patients who had MDS.
This led Dr Mehta and her colleagues to hypothesize that adjusting busulfan dosing based on a patient’s age and disease status at HSCT could minimize toxicity and improve outcomes.
Patients
The researchers tested their theory in 22 FA patients. They had a median age of 7 (range, 4-27), and most (n=13) were female.
Twelve patients had pancytopenia, 6 had severe single-lineage cytopenia, 3 had low-grade MDS, and 1 patient had acute myeloid leukemia (AML).
Eighteen patients had a history of transfusions, and 3 had a history of androgen use.
Treatment
The preparative regimen consisted of 4 doses of busulfan (every 12 hours on day -7 to -6), followed by cyclophosphamide at 10 mg/kg/day (on day -5 to -2), fludarabine at 35 mg/m2/day (on day -5 to -2), and rabbit antithymocyte globulin at 2.5 mg/kg/day (on day -5 to -2).
Busulfan doses were adjusted according to age and disease status.
Children (age 18 and younger) with bone marrow failure received busulfan at 0.6 to 0.8 mg/kg. Children with MDS/AML received busulfan at 0.8 to 1.0 mg/kg. Adults (19 and older) received the lowest dose of busulfan—0.4 mg/kg—regardless of disease status.
“At the first sight, this will look counterintuitive . . . ,” Dr Mehta said. “However, based on our previous experience, in general and also from results of our previous study, this was specifically designed to avoid upfront TRM [transplant-related mortality] for these adult patients.”
All 22 patients received CD34-selected, T-cell-depleted peripheral blood stem cells from unrelated donors. Eleven patients received a fully matched graft (10/10), 8 patients had a 9/10 match, and 3 had an 8/10 match.
The median number of CD34+ cells/kg was 23.9 x 106 (range, 4.9-76.6), and the median number of CD3 cells/kg was 1 x 104 (range, 0.003-3.1).
T-cell depletion was the only GVHD prophylaxis used.
Patients with MDS/AML could receive azacitidine at day 42 after HSCT, an option intended to prevent relapse in these patients.
Toxicity
There were no cases of acute or chronic GVHD.
Toxicities included infections (n=24), oral mucositis (n=14), hyperbilirubinemia (n=2), pulmonary hemorrhage (n=1), and sinusoidal obstruction syndrome (n=1).
There were 20 viral infections, 4 bacterial infections, and no fungal infections. Viral infections included BK virus (n=7), cytomegalovirus (n=6), Epstein-Barr virus (n=6), and adenovirus (n=1).
Dr Mehta noted that viral infections are “not unexpected in a T-cell-depleted graft setting.”
“Because we know this complication [can occur], and we worry about our patients, one of the things that, in recent years, we have done is, we manufacture viral-specific CTLs [cytotoxic T lymphocytes] for all of these patients ahead of time whenever possible,” she said.
“To give you an example, 19 out of these 20 patients’ viral infections—or rather, viremias—are completely under control with the use of either antivirals or donor-specific CTLs, including a third-party CTL in one of the patients.”
Response and survival
All 22 patients engrafted. The median time to neutrophil engraftment was 9 days (range, 8-10), and the median time to platelet engraftment was 16 days (range, 11-40).
Twenty-one of the 22 patients (95%) were alive and disease-free at last follow-up. The median follow-up was 21 months (range, 6-44).
The single AML patient achieved remission but died of post-transplant lymphoproliferative disorder (PTLD) on day 202 after HSCT. Dr Mehta said this was due to partial loss of follow-up and noncompliance with medical recommendations during PTLD treatment.
The AML patient also had “significant upfront toxicity” but “recovered very nicely,” according to Dr Mehta. He had severe mucositis, herpetic stomatitis, and sinusoidal obstruction syndrome that responded to defibrotide.
“Overall, we are quite excited to see 95% overall survival for this cohort and conclude that the current risk-adjusted approach leads to excellent overall survival and disease-free survival in patients undergoing alternative donor transplant either for marrow failure or MDS/AML,” Dr Mehta said.
“Enrollment is ongoing, and we hope to see continued success in patients with MDS/AML as well as in adult patients.”
*Data in the abstract differ from the presentation.
**Mehta PA et al. Radiation-free, alternative donor HCT for Fanconi anemia patients: results from a prospective multi-institutional study. Blood 2017; doi: https://doi.org/10.1182/blood-2016-09-743112.
SALT LAKE CITY—A “risk-adjusted” approach leads to “excellent” survival in patients with Fanconi anemia (FA) undergoing alternative donor hematopoietic stem cell transplant (HSCT), according to a speaker at the 2018 BMT Tandem Meetings.
All FA patients who received personalized doses of busulfan in place of total body irradiation (TBI) were alive and disease-free after undergoing HSCT for bone marrow failure or myelodysplastic syndrome (MDS).
None of the patients developed graft-vs-host disease (GVHD), and the most common toxicity was viral infection.
Parinda A. Mehta, MD, of Cincinnati Children’s Hospital Medical Center in Ohio, presented these results at this year’s BMT Tandem Meetings as abstract 109.*
“We all know that inherent chemotherapy and radiation sensitivity makes transplant for patients with Fanconi anemia quite challenging,” Dr Mehta began. “In our recently published, prospective, multi-institutional study, we showed excellent outcomes of alternative donor transplant in patients with Fanconi anemia without using radiation.”
“In that study,** TBI was replaced by pharmacokinetically adjusted busulfan. It proved that, yes, we can do alternative donor transplant successfully without radiation by showing an overall survival of 80% for a total of 45 patients. We were quite ecstatic to see these numbers.”
The study also showed that younger patients fared better with this regimen, and younger patients did best with the lowest dose of busulfan tested (0.6 mg/kg vs 0.8 to 1.0 mg/kg). In addition, patients who underwent HSCT for bone marrow failure had better outcomes than patients who had MDS.
This led Dr Mehta and her colleagues to hypothesize that adjusting busulfan dosing based on a patient’s age and disease status at HSCT could minimize toxicity and improve outcomes.
Patients
The researchers tested their theory in 22 FA patients. They had a median age of 7 (range, 4-27), and most (n=13) were female.
Twelve patients had pancytopenia, 6 had severe single-lineage cytopenia, 3 had low-grade MDS, and 1 patient had acute myeloid leukemia (AML).
Eighteen patients had a history of transfusions, and 3 had a history of androgen use.
Treatment
The preparative regimen consisted of 4 doses of busulfan (every 12 hours on day -7 to -6), followed by cyclophosphamide at 10 mg/kg/day (on day -5 to -2), fludarabine at 35 mg/m2/day (on day -5 to -2), and rabbit antithymocyte globulin at 2.5 mg/kg/day (on day -5 to -2).
Busulfan doses were adjusted according to age and disease status.
Children (age 18 and younger) with bone marrow failure received busulfan at 0.6 to 0.8 mg/kg. Children with MDS/AML received busulfan at 0.8 to 1.0 mg/kg. Adults (19 and older) received the lowest dose of busulfan—0.4 mg/kg—regardless of disease status.
“At the first sight, this will look counterintuitive . . . ,” Dr Mehta said. “However, based on our previous experience, in general and also from results of our previous study, this was specifically designed to avoid upfront TRM [transplant-related mortality] for these adult patients.”
All 22 patients received CD34-selected, T-cell-depleted peripheral blood stem cells from unrelated donors. Eleven patients received a fully matched graft (10/10), 8 patients had a 9/10 match, and 3 had an 8/10 match.
The median number of CD34+ cells/kg was 23.9 x 106 (range, 4.9-76.6), and the median number of CD3 cells/kg was 1 x 104 (range, 0.003-3.1).
T-cell depletion was the only GVHD prophylaxis used.
Patients with MDS/AML could receive azacitidine at day 42 after HSCT, an option intended to prevent relapse in these patients.
Toxicity
There were no cases of acute or chronic GVHD.
Toxicities included infections (n=24), oral mucositis (n=14), hyperbilirubinemia (n=2), pulmonary hemorrhage (n=1), and sinusoidal obstruction syndrome (n=1).
There were 20 viral infections, 4 bacterial infections, and no fungal infections. Viral infections included BK virus (n=7), cytomegalovirus (n=6), Epstein-Barr virus (n=6), and adenovirus (n=1).
Dr Mehta noted that viral infections are “not unexpected in a T-cell-depleted graft setting.”
“Because we know this complication [can occur], and we worry about our patients, one of the things that, in recent years, we have done is, we manufacture viral-specific CTLs [cytotoxic T lymphocytes] for all of these patients ahead of time whenever possible,” she said.
“To give you an example, 19 out of these 20 patients’ viral infections—or rather, viremias—are completely under control with the use of either antivirals or donor-specific CTLs, including a third-party CTL in one of the patients.”
Response and survival
All 22 patients engrafted. The median time to neutrophil engraftment was 9 days (range, 8-10), and the median time to platelet engraftment was 16 days (range, 11-40).
Twenty-one of the 22 patients (95%) were alive and disease-free at last follow-up. The median follow-up was 21 months (range, 6-44).
The single AML patient achieved remission but died of post-transplant lymphoproliferative disorder (PTLD) on day 202 after HSCT. Dr Mehta said this was due to partial loss of follow-up and noncompliance with medical recommendations during PTLD treatment.
The AML patient also had “significant upfront toxicity” but “recovered very nicely,” according to Dr Mehta. He had severe mucositis, herpetic stomatitis, and sinusoidal obstruction syndrome that responded to defibrotide.
“Overall, we are quite excited to see 95% overall survival for this cohort and conclude that the current risk-adjusted approach leads to excellent overall survival and disease-free survival in patients undergoing alternative donor transplant either for marrow failure or MDS/AML,” Dr Mehta said.
“Enrollment is ongoing, and we hope to see continued success in patients with MDS/AML as well as in adult patients.”
*Data in the abstract differ from the presentation.
**Mehta PA et al. Radiation-free, alternative donor HCT for Fanconi anemia patients: results from a prospective multi-institutional study. Blood 2017; doi: https://doi.org/10.1182/blood-2016-09-743112.
SALT LAKE CITY—A “risk-adjusted” approach leads to “excellent” survival in patients with Fanconi anemia (FA) undergoing alternative donor hematopoietic stem cell transplant (HSCT), according to a speaker at the 2018 BMT Tandem Meetings.
All FA patients who received personalized doses of busulfan in place of total body irradiation (TBI) were alive and disease-free after undergoing HSCT for bone marrow failure or myelodysplastic syndrome (MDS).
None of the patients developed graft-vs-host disease (GVHD), and the most common toxicity was viral infection.
Parinda A. Mehta, MD, of Cincinnati Children’s Hospital Medical Center in Ohio, presented these results at this year’s BMT Tandem Meetings as abstract 109.*
“We all know that inherent chemotherapy and radiation sensitivity makes transplant for patients with Fanconi anemia quite challenging,” Dr Mehta began. “In our recently published, prospective, multi-institutional study, we showed excellent outcomes of alternative donor transplant in patients with Fanconi anemia without using radiation.”
“In that study,** TBI was replaced by pharmacokinetically adjusted busulfan. It proved that, yes, we can do alternative donor transplant successfully without radiation by showing an overall survival of 80% for a total of 45 patients. We were quite ecstatic to see these numbers.”
The study also showed that younger patients fared better with this regimen, and younger patients did best with the lowest dose of busulfan tested (0.6 mg/kg vs 0.8 to 1.0 mg/kg). In addition, patients who underwent HSCT for bone marrow failure had better outcomes than patients who had MDS.
This led Dr Mehta and her colleagues to hypothesize that adjusting busulfan dosing based on a patient’s age and disease status at HSCT could minimize toxicity and improve outcomes.
Patients
The researchers tested their theory in 22 FA patients. They had a median age of 7 (range, 4-27), and most (n=13) were female.
Twelve patients had pancytopenia, 6 had severe single-lineage cytopenia, 3 had low-grade MDS, and 1 patient had acute myeloid leukemia (AML).
Eighteen patients had a history of transfusions, and 3 had a history of androgen use.
Treatment
The preparative regimen consisted of 4 doses of busulfan (every 12 hours on day -7 to -6), followed by cyclophosphamide at 10 mg/kg/day (on day -5 to -2), fludarabine at 35 mg/m2/day (on day -5 to -2), and rabbit antithymocyte globulin at 2.5 mg/kg/day (on day -5 to -2).
Busulfan doses were adjusted according to age and disease status.
Children (age 18 and younger) with bone marrow failure received busulfan at 0.6 to 0.8 mg/kg. Children with MDS/AML received busulfan at 0.8 to 1.0 mg/kg. Adults (19 and older) received the lowest dose of busulfan—0.4 mg/kg—regardless of disease status.
“At the first sight, this will look counterintuitive . . . ,” Dr Mehta said. “However, based on our previous experience, in general and also from results of our previous study, this was specifically designed to avoid upfront TRM [transplant-related mortality] for these adult patients.”
All 22 patients received CD34-selected, T-cell-depleted peripheral blood stem cells from unrelated donors. Eleven patients received a fully matched graft (10/10), 8 patients had a 9/10 match, and 3 had an 8/10 match.
The median number of CD34+ cells/kg was 23.9 x 106 (range, 4.9-76.6), and the median number of CD3 cells/kg was 1 x 104 (range, 0.003-3.1).
T-cell depletion was the only GVHD prophylaxis used.
Patients with MDS/AML could receive azacitidine at day 42 after HSCT, an option intended to prevent relapse in these patients.
Toxicity
There were no cases of acute or chronic GVHD.
Toxicities included infections (n=24), oral mucositis (n=14), hyperbilirubinemia (n=2), pulmonary hemorrhage (n=1), and sinusoidal obstruction syndrome (n=1).
There were 20 viral infections, 4 bacterial infections, and no fungal infections. Viral infections included BK virus (n=7), cytomegalovirus (n=6), Epstein-Barr virus (n=6), and adenovirus (n=1).
Dr Mehta noted that viral infections are “not unexpected in a T-cell-depleted graft setting.”
“Because we know this complication [can occur], and we worry about our patients, one of the things that, in recent years, we have done is, we manufacture viral-specific CTLs [cytotoxic T lymphocytes] for all of these patients ahead of time whenever possible,” she said.
“To give you an example, 19 out of these 20 patients’ viral infections—or rather, viremias—are completely under control with the use of either antivirals or donor-specific CTLs, including a third-party CTL in one of the patients.”
Response and survival
All 22 patients engrafted. The median time to neutrophil engraftment was 9 days (range, 8-10), and the median time to platelet engraftment was 16 days (range, 11-40).
Twenty-one of the 22 patients (95%) were alive and disease-free at last follow-up. The median follow-up was 21 months (range, 6-44).
The single AML patient achieved remission but died of post-transplant lymphoproliferative disorder (PTLD) on day 202 after HSCT. Dr Mehta said this was due to partial loss of follow-up and noncompliance with medical recommendations during PTLD treatment.
The AML patient also had “significant upfront toxicity” but “recovered very nicely,” according to Dr Mehta. He had severe mucositis, herpetic stomatitis, and sinusoidal obstruction syndrome that responded to defibrotide.
“Overall, we are quite excited to see 95% overall survival for this cohort and conclude that the current risk-adjusted approach leads to excellent overall survival and disease-free survival in patients undergoing alternative donor transplant either for marrow failure or MDS/AML,” Dr Mehta said.
“Enrollment is ongoing, and we hope to see continued success in patients with MDS/AML as well as in adult patients.”
*Data in the abstract differ from the presentation.
**Mehta PA et al. Radiation-free, alternative donor HCT for Fanconi anemia patients: results from a prospective multi-institutional study. Blood 2017; doi: https://doi.org/10.1182/blood-2016-09-743112.