User login
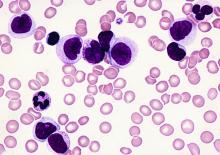
Selinexor trials placed on partial hold
The US Food and Drug Administration (FDA) has placed a partial clinical hold on all trials of selinexor (KPT-330).
Selinexor is an inhibitor being evaluated in multiple trials of patients with relapsed and/or refractory hematologic and solid tumor malignancies.
While the partial clinical hold remains in effect, patients with stable disease or better may remain on selinexor.
However, no new patients may be enrolled in selinexor trials until the hold is lifted.
The FDA has indicated that the partial clinical hold is due to incomplete information in the existing version of the investigator’s brochure, including an incomplete list of serious adverse events associated with selinexor.
Karyopharm Therapeutics Inc., the company developing selinexor, said it has amended the brochure, updated the informed consent documents accordingly, and submitted the documents to the FDA as requested.
As of March 10, Karyopharm had provided all requested materials to the FDA believed to be required to lift the partial clinical hold. By regulation, the FDA has 30 days from the receipt of Karyopharm’s submission to notify the company whether the partial clinical hold is lifted.
Karyopharm said it is working with the FDA to seek the release of the hold and resume enrollment in its selinexor trials as expeditiously as possible. The company believes its previously disclosed enrollment rates and timelines for its ongoing trials will remain materially unchanged.
About selinexor
Selinexor is a selective inhibitor of nuclear export (SINE) XPO1 antagonist. The drug binds with and inhibits XPO1, leading to the accumulation of tumor suppressor proteins in the cell nucleus. This reinitiates and amplifies their tumor suppressor function and is believed to induce apoptosis in cancer cells while largely sparing normal cells.
To date, more than 1900 patients have been treated with selinexor. The drug is currently being evaluated in several trials across multiple cancer indications.
One of these is the phase 2 SOPRA trial, in which selinexor is being compared to investigator’s choice of therapy (1 of 3 potential salvage therapies). The trial is enrolling patients 60 years of age or older with relapsed or refractory acute myeloid leukemia who are ineligible for standard intensive chemotherapy and/or transplant.
The SADAL study is a phase 2b trial comparing high and low doses of selinexor in patients with relapsed and/or refractory de novo diffuse large B-cell lymphoma who have no therapeutic options of demonstrated clinical benefit.
STORM is a phase 2b trial evaluating selinexor and low-dose dexamethasone in patients with heavily pretreated multiple myeloma (MM). And STOMP is a phase 1b/2 study evaluating selinexor in combination with existing therapies across the broader population in MM.
Karyopharm is also planning a randomized, phase 3 study known as BOSTON. In this trial, researchers will compare selinexor plus bortezomib and low-dose dexamethasone to bortezomib and low-dose dexamethasone in MM patients who have had 1 to 3 prior lines of therapy.
Additional phase 1, 2, and 3 studies are ongoing or currently planned.
The US Food and Drug Administration (FDA) has placed a partial clinical hold on all trials of selinexor (KPT-330).
Selinexor is an inhibitor being evaluated in multiple trials of patients with relapsed and/or refractory hematologic and solid tumor malignancies.
While the partial clinical hold remains in effect, patients with stable disease or better may remain on selinexor.
However, no new patients may be enrolled in selinexor trials until the hold is lifted.
The FDA has indicated that the partial clinical hold is due to incomplete information in the existing version of the investigator’s brochure, including an incomplete list of serious adverse events associated with selinexor.
Karyopharm Therapeutics Inc., the company developing selinexor, said it has amended the brochure, updated the informed consent documents accordingly, and submitted the documents to the FDA as requested.
As of March 10, Karyopharm had provided all requested materials to the FDA believed to be required to lift the partial clinical hold. By regulation, the FDA has 30 days from the receipt of Karyopharm’s submission to notify the company whether the partial clinical hold is lifted.
Karyopharm said it is working with the FDA to seek the release of the hold and resume enrollment in its selinexor trials as expeditiously as possible. The company believes its previously disclosed enrollment rates and timelines for its ongoing trials will remain materially unchanged.
About selinexor
Selinexor is a selective inhibitor of nuclear export (SINE) XPO1 antagonist. The drug binds with and inhibits XPO1, leading to the accumulation of tumor suppressor proteins in the cell nucleus. This reinitiates and amplifies their tumor suppressor function and is believed to induce apoptosis in cancer cells while largely sparing normal cells.
To date, more than 1900 patients have been treated with selinexor. The drug is currently being evaluated in several trials across multiple cancer indications.
One of these is the phase 2 SOPRA trial, in which selinexor is being compared to investigator’s choice of therapy (1 of 3 potential salvage therapies). The trial is enrolling patients 60 years of age or older with relapsed or refractory acute myeloid leukemia who are ineligible for standard intensive chemotherapy and/or transplant.
The SADAL study is a phase 2b trial comparing high and low doses of selinexor in patients with relapsed and/or refractory de novo diffuse large B-cell lymphoma who have no therapeutic options of demonstrated clinical benefit.
STORM is a phase 2b trial evaluating selinexor and low-dose dexamethasone in patients with heavily pretreated multiple myeloma (MM). And STOMP is a phase 1b/2 study evaluating selinexor in combination with existing therapies across the broader population in MM.
Karyopharm is also planning a randomized, phase 3 study known as BOSTON. In this trial, researchers will compare selinexor plus bortezomib and low-dose dexamethasone to bortezomib and low-dose dexamethasone in MM patients who have had 1 to 3 prior lines of therapy.
Additional phase 1, 2, and 3 studies are ongoing or currently planned.
The US Food and Drug Administration (FDA) has placed a partial clinical hold on all trials of selinexor (KPT-330).
Selinexor is an inhibitor being evaluated in multiple trials of patients with relapsed and/or refractory hematologic and solid tumor malignancies.
While the partial clinical hold remains in effect, patients with stable disease or better may remain on selinexor.
However, no new patients may be enrolled in selinexor trials until the hold is lifted.
The FDA has indicated that the partial clinical hold is due to incomplete information in the existing version of the investigator’s brochure, including an incomplete list of serious adverse events associated with selinexor.
Karyopharm Therapeutics Inc., the company developing selinexor, said it has amended the brochure, updated the informed consent documents accordingly, and submitted the documents to the FDA as requested.
As of March 10, Karyopharm had provided all requested materials to the FDA believed to be required to lift the partial clinical hold. By regulation, the FDA has 30 days from the receipt of Karyopharm’s submission to notify the company whether the partial clinical hold is lifted.
Karyopharm said it is working with the FDA to seek the release of the hold and resume enrollment in its selinexor trials as expeditiously as possible. The company believes its previously disclosed enrollment rates and timelines for its ongoing trials will remain materially unchanged.
About selinexor
Selinexor is a selective inhibitor of nuclear export (SINE) XPO1 antagonist. The drug binds with and inhibits XPO1, leading to the accumulation of tumor suppressor proteins in the cell nucleus. This reinitiates and amplifies their tumor suppressor function and is believed to induce apoptosis in cancer cells while largely sparing normal cells.
To date, more than 1900 patients have been treated with selinexor. The drug is currently being evaluated in several trials across multiple cancer indications.
One of these is the phase 2 SOPRA trial, in which selinexor is being compared to investigator’s choice of therapy (1 of 3 potential salvage therapies). The trial is enrolling patients 60 years of age or older with relapsed or refractory acute myeloid leukemia who are ineligible for standard intensive chemotherapy and/or transplant.
The SADAL study is a phase 2b trial comparing high and low doses of selinexor in patients with relapsed and/or refractory de novo diffuse large B-cell lymphoma who have no therapeutic options of demonstrated clinical benefit.
STORM is a phase 2b trial evaluating selinexor and low-dose dexamethasone in patients with heavily pretreated multiple myeloma (MM). And STOMP is a phase 1b/2 study evaluating selinexor in combination with existing therapies across the broader population in MM.
Karyopharm is also planning a randomized, phase 3 study known as BOSTON. In this trial, researchers will compare selinexor plus bortezomib and low-dose dexamethasone to bortezomib and low-dose dexamethasone in MM patients who have had 1 to 3 prior lines of therapy.
Additional phase 1, 2, and 3 studies are ongoing or currently planned.
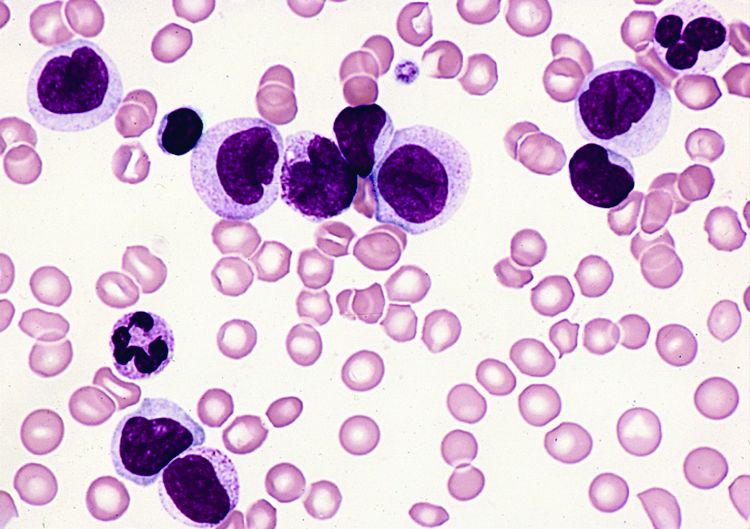
FDA lifts clinical hold on AML trials
The US Food and Drug Administration (FDA) has lifted the clinical hold placed on 3 trials of vadastuximab talirine (SGN-CD33A), an antibody-drug conjugate targeting CD33, in acute myeloid leukemia (AML).
Last December, 1 trial was placed on full clinical hold (enrollment was halted and no further dosing of subjects was allowed), and 2 were placed on partial hold (enrollment was halted, but existing patients could continue treatment with re-consent).
All 3 of the holds were due to the potential risk of hepatotoxicity in patients who underwent allogeneic hematopoietic stem cell transplant (HSCT) before or after treatment with vadastuximab talirine.
In particular, the holds were in response to 6 cases of hepatotoxicity, including several cases of veno-occlusive disease (VOD), with 4 fatal events.
At the time the holds were announced, Seattle Genetics, Inc., the company developing vadastuximab talirine, said it was working with the FDA to determine whether there is an association between hepatotoxicity and treatment with the drug.
The company analyzed data from more than 350 patients treated with vadastuximab talirine and found no such association.
The rate of VOD they observed was “within the background rate of VOD in AML patients receiving allo-transplant,” according to Clay B. Siegall, PhD, president, chief executive officer, and chairman of the board at Seattle Genetics.
Dr Siegall said the company would not disclose the exact rate of VOD in these trials.
Resuming trials
As Seattle Genetics found no evidence to suggest that vadastuximab talirine increased the risk of hepatotoxicity, the FDA lifted the clinical holds on all 3 trials. The 2 trials placed on partial hold will continue, but the trial placed on full hold will not.
One of the trials that will continue is a phase 1 study of vadastuximab talirine alone and in combination with hypomethylating agents in both newly diagnosed and relapsed AML patients.
The other trial is a phase 1 study of vadastuximab talirine in combination with 7+3 chemotherapy in newly diagnosed, younger AML patients. (Results from this trial were presented at the 2016 ASH Annual Meeting.)
Cancelled trial
The trial that will not resume is a phase 1/2 study of vadastuximab talirine monotherapy pre- and post-allogeneic HSCT in patients with relapsed, chemo-resistant AML.
Seattle Genetics said it will not continue with this trial because of the challenges of developing therapies in this specific setting.
“It’s a very small group of patients, and we’re going to focus on the 3 biggest groups of patients [older and younger patients newly diagnosed with AML and patients with myelodysplastic syndromes] so we can really impact AML in the biggest way,” Dr Siegall said.
He noted that this decision does not prevent patients from undergoing HSCT after receiving vadastuximab talirine.
In the phase 1/2 trial, patients received vadastuximab talirine directly before HSCT, a practice that will not continue. However, patients can undergo HSCT as long as the transplant doesn’t occur immediately after treatment with vadastuximab talirine.
Moving forward
Two other trials of vadastuximab talirine were not affected by the clinical holds and have continued to enroll patients.
One is CASCADE, a randomized, phase 3 trial of vadastuximab talirine as front-line therapy in older AML patients. The other is a phase 1/2 trial of vadastuximab talirine as front-line therapy in patients with myelodysplastic syndromes.
Seattle Genetics is also planning to begin a randomized, phase 2 trial comparing 7+3 chemotherapy alone to 7+3 in combination with vadastuximab talirine in younger patients with previously untreated AML. The company plans to start the trial later this year.
Going forward, additional risk mitigation measures will be implemented in all vadastuximab talirine studies, including revised eligibility criteria and stopping rules for VOD.
Specifically, trials will not be stopped if the incidence of VOD is considered within the normal range, and an adjudication committee consisting of 2 experts will be tasked with verifying reports of VOD.
In addition, patients with liver cirrhosis due to alcohol abuse are no longer eligible for trials of vadastuximab talirine. ![]()
The US Food and Drug Administration (FDA) has lifted the clinical hold placed on 3 trials of vadastuximab talirine (SGN-CD33A), an antibody-drug conjugate targeting CD33, in acute myeloid leukemia (AML).
Last December, 1 trial was placed on full clinical hold (enrollment was halted and no further dosing of subjects was allowed), and 2 were placed on partial hold (enrollment was halted, but existing patients could continue treatment with re-consent).
All 3 of the holds were due to the potential risk of hepatotoxicity in patients who underwent allogeneic hematopoietic stem cell transplant (HSCT) before or after treatment with vadastuximab talirine.
In particular, the holds were in response to 6 cases of hepatotoxicity, including several cases of veno-occlusive disease (VOD), with 4 fatal events.
At the time the holds were announced, Seattle Genetics, Inc., the company developing vadastuximab talirine, said it was working with the FDA to determine whether there is an association between hepatotoxicity and treatment with the drug.
The company analyzed data from more than 350 patients treated with vadastuximab talirine and found no such association.
The rate of VOD they observed was “within the background rate of VOD in AML patients receiving allo-transplant,” according to Clay B. Siegall, PhD, president, chief executive officer, and chairman of the board at Seattle Genetics.
Dr Siegall said the company would not disclose the exact rate of VOD in these trials.
Resuming trials
As Seattle Genetics found no evidence to suggest that vadastuximab talirine increased the risk of hepatotoxicity, the FDA lifted the clinical holds on all 3 trials. The 2 trials placed on partial hold will continue, but the trial placed on full hold will not.
One of the trials that will continue is a phase 1 study of vadastuximab talirine alone and in combination with hypomethylating agents in both newly diagnosed and relapsed AML patients.
The other trial is a phase 1 study of vadastuximab talirine in combination with 7+3 chemotherapy in newly diagnosed, younger AML patients. (Results from this trial were presented at the 2016 ASH Annual Meeting.)
Cancelled trial
The trial that will not resume is a phase 1/2 study of vadastuximab talirine monotherapy pre- and post-allogeneic HSCT in patients with relapsed, chemo-resistant AML.
Seattle Genetics said it will not continue with this trial because of the challenges of developing therapies in this specific setting.
“It’s a very small group of patients, and we’re going to focus on the 3 biggest groups of patients [older and younger patients newly diagnosed with AML and patients with myelodysplastic syndromes] so we can really impact AML in the biggest way,” Dr Siegall said.
He noted that this decision does not prevent patients from undergoing HSCT after receiving vadastuximab talirine.
In the phase 1/2 trial, patients received vadastuximab talirine directly before HSCT, a practice that will not continue. However, patients can undergo HSCT as long as the transplant doesn’t occur immediately after treatment with vadastuximab talirine.
Moving forward
Two other trials of vadastuximab talirine were not affected by the clinical holds and have continued to enroll patients.
One is CASCADE, a randomized, phase 3 trial of vadastuximab talirine as front-line therapy in older AML patients. The other is a phase 1/2 trial of vadastuximab talirine as front-line therapy in patients with myelodysplastic syndromes.
Seattle Genetics is also planning to begin a randomized, phase 2 trial comparing 7+3 chemotherapy alone to 7+3 in combination with vadastuximab talirine in younger patients with previously untreated AML. The company plans to start the trial later this year.
Going forward, additional risk mitigation measures will be implemented in all vadastuximab talirine studies, including revised eligibility criteria and stopping rules for VOD.
Specifically, trials will not be stopped if the incidence of VOD is considered within the normal range, and an adjudication committee consisting of 2 experts will be tasked with verifying reports of VOD.
In addition, patients with liver cirrhosis due to alcohol abuse are no longer eligible for trials of vadastuximab talirine. ![]()
The US Food and Drug Administration (FDA) has lifted the clinical hold placed on 3 trials of vadastuximab talirine (SGN-CD33A), an antibody-drug conjugate targeting CD33, in acute myeloid leukemia (AML).
Last December, 1 trial was placed on full clinical hold (enrollment was halted and no further dosing of subjects was allowed), and 2 were placed on partial hold (enrollment was halted, but existing patients could continue treatment with re-consent).
All 3 of the holds were due to the potential risk of hepatotoxicity in patients who underwent allogeneic hematopoietic stem cell transplant (HSCT) before or after treatment with vadastuximab talirine.
In particular, the holds were in response to 6 cases of hepatotoxicity, including several cases of veno-occlusive disease (VOD), with 4 fatal events.
At the time the holds were announced, Seattle Genetics, Inc., the company developing vadastuximab talirine, said it was working with the FDA to determine whether there is an association between hepatotoxicity and treatment with the drug.
The company analyzed data from more than 350 patients treated with vadastuximab talirine and found no such association.
The rate of VOD they observed was “within the background rate of VOD in AML patients receiving allo-transplant,” according to Clay B. Siegall, PhD, president, chief executive officer, and chairman of the board at Seattle Genetics.
Dr Siegall said the company would not disclose the exact rate of VOD in these trials.
Resuming trials
As Seattle Genetics found no evidence to suggest that vadastuximab talirine increased the risk of hepatotoxicity, the FDA lifted the clinical holds on all 3 trials. The 2 trials placed on partial hold will continue, but the trial placed on full hold will not.
One of the trials that will continue is a phase 1 study of vadastuximab talirine alone and in combination with hypomethylating agents in both newly diagnosed and relapsed AML patients.
The other trial is a phase 1 study of vadastuximab talirine in combination with 7+3 chemotherapy in newly diagnosed, younger AML patients. (Results from this trial were presented at the 2016 ASH Annual Meeting.)
Cancelled trial
The trial that will not resume is a phase 1/2 study of vadastuximab talirine monotherapy pre- and post-allogeneic HSCT in patients with relapsed, chemo-resistant AML.
Seattle Genetics said it will not continue with this trial because of the challenges of developing therapies in this specific setting.
“It’s a very small group of patients, and we’re going to focus on the 3 biggest groups of patients [older and younger patients newly diagnosed with AML and patients with myelodysplastic syndromes] so we can really impact AML in the biggest way,” Dr Siegall said.
He noted that this decision does not prevent patients from undergoing HSCT after receiving vadastuximab talirine.
In the phase 1/2 trial, patients received vadastuximab talirine directly before HSCT, a practice that will not continue. However, patients can undergo HSCT as long as the transplant doesn’t occur immediately after treatment with vadastuximab talirine.
Moving forward
Two other trials of vadastuximab talirine were not affected by the clinical holds and have continued to enroll patients.
One is CASCADE, a randomized, phase 3 trial of vadastuximab talirine as front-line therapy in older AML patients. The other is a phase 1/2 trial of vadastuximab talirine as front-line therapy in patients with myelodysplastic syndromes.
Seattle Genetics is also planning to begin a randomized, phase 2 trial comparing 7+3 chemotherapy alone to 7+3 in combination with vadastuximab talirine in younger patients with previously untreated AML. The company plans to start the trial later this year.
Going forward, additional risk mitigation measures will be implemented in all vadastuximab talirine studies, including revised eligibility criteria and stopping rules for VOD.
Specifically, trials will not be stopped if the incidence of VOD is considered within the normal range, and an adjudication committee consisting of 2 experts will be tasked with verifying reports of VOD.
In addition, patients with liver cirrhosis due to alcohol abuse are no longer eligible for trials of vadastuximab talirine. ![]()
Post-transplant drug combo eyed for high-risk AML
ORLANDO – Azacitadine and valproic acid can be safely coadministered as maintenance therapy after allogeneic stem cell transplantation in patients with high-risk acute myelogenous leukemia (AML), according to interim findings from an investigator-initiated phase II study.
One-year relapse-free and overall survival rates were about 80%, and no significant toxicities were reported in 28 such patients who began the treatment at least 40 days after transplant and were treated for 4 months, Patrick A. Hagen, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“As we all know, relapse after allogeneic transplant for AML is a huge and ongoing problem. It’s the primary cause of death following transplant, and unfortunately it really hasn’t decreased over the past couple decades,” Dr. Hagen said, adding that relapse is of increasing concern for biologically high-risk patients and is a “pretty ripe area for improved methodologies or approaches.” The decision to pursue maintenance therapy after transplant is complicated: It’s a good opportunity to improve outcomes, but there are many challenges, including problems with drug interactions and myelosuppression, he said.
The study was undertaken based in part on previously reported findings of synergism of a demethylating agent and a histone dacetylase inhibitor in patients with AML, he said.
The maintenance therapy included up to four 28-day cycles of azacitadine at 40 mg/m2 daily on days 1-5, along with oral valproic acid daily throughout the cycle at a 15-mg/kg dose adjusted to achieve a 100-mcg/mL trough level of bound valproic acid as tolerated. Nineteen patients completed all four cycles of treatment.
“The regimen was pretty well tolerated. The vast majority of the toxicities were grade I/II – 70%. The only grade-4 toxicities were cytopenia related and did not lead to a delay in treatment,” he said.
No patients developed acute graft-versus-host disease after therapy, although 11 (39%) developed chronic GVHD, which was extensive in 8, Dr. Hagen said.
Study participants were adults with high-risk AML with no grade 3-4 acute GVHD. All had adequate organ function after allogeneic stem cell transplantation (allo-SCT) performed 40-60 days prior to the start of maintenance therapy. Those with active or uncontrolled infections, low-risk AML in first relapse, neutrophil counts below 1,500, and platelets below 50,000 were excluded, he said.
The patients’ median age was 44 years, they had a median of two prior chemotherapy regimens, and their median Sorror Comorbidity Index was 2.5. Cytogenetics were mostly intermediate and adverse; only one patient had favorable cytogenetics.
Graft type was mixed, with most patients having matched unrelated donors. Conditioning regimens also were mixed, although they “skewed toward myeloablative,” he said. GVHD prophylaxis was standard for the institution and included tacrolimus and methotrexate.
The promising 1-year overall survival and relapse rate without significant dose-limiting toxicities warrants further evaluation in a phase III trial, Dr. Hagen concluded.
He reported having no disclosures.
ORLANDO – Azacitadine and valproic acid can be safely coadministered as maintenance therapy after allogeneic stem cell transplantation in patients with high-risk acute myelogenous leukemia (AML), according to interim findings from an investigator-initiated phase II study.
One-year relapse-free and overall survival rates were about 80%, and no significant toxicities were reported in 28 such patients who began the treatment at least 40 days after transplant and were treated for 4 months, Patrick A. Hagen, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“As we all know, relapse after allogeneic transplant for AML is a huge and ongoing problem. It’s the primary cause of death following transplant, and unfortunately it really hasn’t decreased over the past couple decades,” Dr. Hagen said, adding that relapse is of increasing concern for biologically high-risk patients and is a “pretty ripe area for improved methodologies or approaches.” The decision to pursue maintenance therapy after transplant is complicated: It’s a good opportunity to improve outcomes, but there are many challenges, including problems with drug interactions and myelosuppression, he said.
The study was undertaken based in part on previously reported findings of synergism of a demethylating agent and a histone dacetylase inhibitor in patients with AML, he said.
The maintenance therapy included up to four 28-day cycles of azacitadine at 40 mg/m2 daily on days 1-5, along with oral valproic acid daily throughout the cycle at a 15-mg/kg dose adjusted to achieve a 100-mcg/mL trough level of bound valproic acid as tolerated. Nineteen patients completed all four cycles of treatment.
“The regimen was pretty well tolerated. The vast majority of the toxicities were grade I/II – 70%. The only grade-4 toxicities were cytopenia related and did not lead to a delay in treatment,” he said.
No patients developed acute graft-versus-host disease after therapy, although 11 (39%) developed chronic GVHD, which was extensive in 8, Dr. Hagen said.
Study participants were adults with high-risk AML with no grade 3-4 acute GVHD. All had adequate organ function after allogeneic stem cell transplantation (allo-SCT) performed 40-60 days prior to the start of maintenance therapy. Those with active or uncontrolled infections, low-risk AML in first relapse, neutrophil counts below 1,500, and platelets below 50,000 were excluded, he said.
The patients’ median age was 44 years, they had a median of two prior chemotherapy regimens, and their median Sorror Comorbidity Index was 2.5. Cytogenetics were mostly intermediate and adverse; only one patient had favorable cytogenetics.
Graft type was mixed, with most patients having matched unrelated donors. Conditioning regimens also were mixed, although they “skewed toward myeloablative,” he said. GVHD prophylaxis was standard for the institution and included tacrolimus and methotrexate.
The promising 1-year overall survival and relapse rate without significant dose-limiting toxicities warrants further evaluation in a phase III trial, Dr. Hagen concluded.
He reported having no disclosures.
ORLANDO – Azacitadine and valproic acid can be safely coadministered as maintenance therapy after allogeneic stem cell transplantation in patients with high-risk acute myelogenous leukemia (AML), according to interim findings from an investigator-initiated phase II study.
One-year relapse-free and overall survival rates were about 80%, and no significant toxicities were reported in 28 such patients who began the treatment at least 40 days after transplant and were treated for 4 months, Patrick A. Hagen, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“As we all know, relapse after allogeneic transplant for AML is a huge and ongoing problem. It’s the primary cause of death following transplant, and unfortunately it really hasn’t decreased over the past couple decades,” Dr. Hagen said, adding that relapse is of increasing concern for biologically high-risk patients and is a “pretty ripe area for improved methodologies or approaches.” The decision to pursue maintenance therapy after transplant is complicated: It’s a good opportunity to improve outcomes, but there are many challenges, including problems with drug interactions and myelosuppression, he said.
The study was undertaken based in part on previously reported findings of synergism of a demethylating agent and a histone dacetylase inhibitor in patients with AML, he said.
The maintenance therapy included up to four 28-day cycles of azacitadine at 40 mg/m2 daily on days 1-5, along with oral valproic acid daily throughout the cycle at a 15-mg/kg dose adjusted to achieve a 100-mcg/mL trough level of bound valproic acid as tolerated. Nineteen patients completed all four cycles of treatment.
“The regimen was pretty well tolerated. The vast majority of the toxicities were grade I/II – 70%. The only grade-4 toxicities were cytopenia related and did not lead to a delay in treatment,” he said.
No patients developed acute graft-versus-host disease after therapy, although 11 (39%) developed chronic GVHD, which was extensive in 8, Dr. Hagen said.
Study participants were adults with high-risk AML with no grade 3-4 acute GVHD. All had adequate organ function after allogeneic stem cell transplantation (allo-SCT) performed 40-60 days prior to the start of maintenance therapy. Those with active or uncontrolled infections, low-risk AML in first relapse, neutrophil counts below 1,500, and platelets below 50,000 were excluded, he said.
The patients’ median age was 44 years, they had a median of two prior chemotherapy regimens, and their median Sorror Comorbidity Index was 2.5. Cytogenetics were mostly intermediate and adverse; only one patient had favorable cytogenetics.
Graft type was mixed, with most patients having matched unrelated donors. Conditioning regimens also were mixed, although they “skewed toward myeloablative,” he said. GVHD prophylaxis was standard for the institution and included tacrolimus and methotrexate.
The promising 1-year overall survival and relapse rate without significant dose-limiting toxicities warrants further evaluation in a phase III trial, Dr. Hagen concluded.
He reported having no disclosures.
AT THE 2017 BMT TANDEM MEETINGS
Key clinical point:
Major finding: 1-year relapse-free survival and 1-year overall survival were about 80%.
Data source: An interim analysis of data from 28 patients in a phase II study.
Disclosures: Dr. Hagen reported having no disclosures.
Study reveals higher rate of early death in kids with cancer
New research suggests that, in the US, early deaths from childhood cancer may be more common than clinical trials suggest.
Researchers found the rate of death within 1 month of diagnosis was higher among patients included in a large national database than among patients enrolled in phase 3 trials.
The data also indicated that early death is more likely in cancer patients under the age of 1 and those belonging to minority racial and ethnic groups.
Adam Green, MD, of the University of Colorado Anschutz Medical Campus in Aurora, and his colleagues conducted this research and reported the results in the Journal of Clinical Oncology.
The researchers analyzed data from the Surveillance, Epidemiology and End Results (SEER) database, which collects about 15% of all cancer outcomes across the US (representing a geographic and socioeconomic cross-section).
The team identified 36,337 patients with pediatric cancer (ages 0 to 19) diagnosed between 1992 and 2011. Of these patients, 555 (1.5%) died within 1 month of diagnosis.
Young age
Overall, the strongest predictor of early death was age below 1 year. The odds ratio (OR) was 4.36 for these patients (P<0.001), 0.75 for patients age 1 to 4, 0.78 for patients age 5 to 9, 1.00 (reference) for those age 10 to 14, and 1.69 for those age 15 to 19.
For hematologic malignancies, the adjusted OR (adjusted by poverty, unemployment, education, and year of diagnosis) was 4.32 for patients younger than 1 (P<0.001), 0.76 for patients age 1 to 4, 0.79 for patients age 5 to 9, 1.00 for those age 10 to 14, and 1.76 for those age 15 to 19 (P=0.002).
“In general, babies are just challenging, clinically, because they can’t tell you what they’re feeling,” Dr Green noted. “Parents and physicians have to pick the ones with cancer from the ones with a cold, without the patient being able to tell you about symptoms that could be diagnostic.”
“Babies tend to get aggressive cancers, it’s hard to tell when they’re getting sick, and some are even born with cancers that have already progressed. These factors combine to make very young age the strongest predictor of early death in our study.”
Race/ethnicity
Black race and Hispanic ethnicity also predicted early death. The OR was 1.48 for black race, 1.00 for white race (reference), and 1.09 for “other” races (P=0.102). The OR was 1.39 for Hispanic patients and 1.00 (reference) for non-Hispanic patients (P=0.007).
For hematologic malignancies, the adjusted OR was 1.68 for black patients (P=0.01) and 1.44 for patients of other, non-white races. The adjusted OR was 1.48 for Hispanic patients (P=0.009).
Dr Green said he hopes future studies will be able to determine the factors responsible for these disparities.
Higher death rates
Dr Green and his colleagues also found the rate of early death due to pediatric cancers is higher than reported in clinical trials, and this was true for all cancer subtypes assessed.
“Most of what we know about outcomes for cancer patients come from clinical trials, which have much more thorough reporting rules than cancer treated outside trials,” Dr Green said. “However, these kids in our study aren’t surviving long enough to join clinical trials.”
The researchers looked at a phase 3 trial (COG AAML0531) of pediatric patients with acute myeloid leukemia (AML) who were studied from August 2006 to June 2010. The early death rate in this trial was 1.6% (16/1022).
In contrast, the SEER database showed an early death rate for pediatric AML patients of 5.9% (15/256) during the same period as the trial and 6.2% (106/1698) for the period from 1992 to 2011. This is almost 4 times as high as the death rate in the trial.
The same effect was observed for acute lymphoblastic leukemia (ALL).
The early death rate for non-infant ALL was 0.7% (13/1790) in a trial (POG 9900) conducted from April 2000 to April 2005, 1.6% (30/1823) in the SEER data covering the same time period, and 1.3% (94/7353) in the SEER data from 1992 to 2011.
For infant ALL, the rates were 2.0% (3/149) in a trial (COG AALL0631) conducted from January 2008 to June 2014, 5.0% (2/40) in the SEER data during the trial period, and 5.4% (12/223) in the SEER data from 1992 to 2011.
“I had a hunch this was a bigger problem than we thought,” Dr Green said. “Now we see that is indeed the case.” ![]()
New research suggests that, in the US, early deaths from childhood cancer may be more common than clinical trials suggest.
Researchers found the rate of death within 1 month of diagnosis was higher among patients included in a large national database than among patients enrolled in phase 3 trials.
The data also indicated that early death is more likely in cancer patients under the age of 1 and those belonging to minority racial and ethnic groups.
Adam Green, MD, of the University of Colorado Anschutz Medical Campus in Aurora, and his colleagues conducted this research and reported the results in the Journal of Clinical Oncology.
The researchers analyzed data from the Surveillance, Epidemiology and End Results (SEER) database, which collects about 15% of all cancer outcomes across the US (representing a geographic and socioeconomic cross-section).
The team identified 36,337 patients with pediatric cancer (ages 0 to 19) diagnosed between 1992 and 2011. Of these patients, 555 (1.5%) died within 1 month of diagnosis.
Young age
Overall, the strongest predictor of early death was age below 1 year. The odds ratio (OR) was 4.36 for these patients (P<0.001), 0.75 for patients age 1 to 4, 0.78 for patients age 5 to 9, 1.00 (reference) for those age 10 to 14, and 1.69 for those age 15 to 19.
For hematologic malignancies, the adjusted OR (adjusted by poverty, unemployment, education, and year of diagnosis) was 4.32 for patients younger than 1 (P<0.001), 0.76 for patients age 1 to 4, 0.79 for patients age 5 to 9, 1.00 for those age 10 to 14, and 1.76 for those age 15 to 19 (P=0.002).
“In general, babies are just challenging, clinically, because they can’t tell you what they’re feeling,” Dr Green noted. “Parents and physicians have to pick the ones with cancer from the ones with a cold, without the patient being able to tell you about symptoms that could be diagnostic.”
“Babies tend to get aggressive cancers, it’s hard to tell when they’re getting sick, and some are even born with cancers that have already progressed. These factors combine to make very young age the strongest predictor of early death in our study.”
Race/ethnicity
Black race and Hispanic ethnicity also predicted early death. The OR was 1.48 for black race, 1.00 for white race (reference), and 1.09 for “other” races (P=0.102). The OR was 1.39 for Hispanic patients and 1.00 (reference) for non-Hispanic patients (P=0.007).
For hematologic malignancies, the adjusted OR was 1.68 for black patients (P=0.01) and 1.44 for patients of other, non-white races. The adjusted OR was 1.48 for Hispanic patients (P=0.009).
Dr Green said he hopes future studies will be able to determine the factors responsible for these disparities.
Higher death rates
Dr Green and his colleagues also found the rate of early death due to pediatric cancers is higher than reported in clinical trials, and this was true for all cancer subtypes assessed.
“Most of what we know about outcomes for cancer patients come from clinical trials, which have much more thorough reporting rules than cancer treated outside trials,” Dr Green said. “However, these kids in our study aren’t surviving long enough to join clinical trials.”
The researchers looked at a phase 3 trial (COG AAML0531) of pediatric patients with acute myeloid leukemia (AML) who were studied from August 2006 to June 2010. The early death rate in this trial was 1.6% (16/1022).
In contrast, the SEER database showed an early death rate for pediatric AML patients of 5.9% (15/256) during the same period as the trial and 6.2% (106/1698) for the period from 1992 to 2011. This is almost 4 times as high as the death rate in the trial.
The same effect was observed for acute lymphoblastic leukemia (ALL).
The early death rate for non-infant ALL was 0.7% (13/1790) in a trial (POG 9900) conducted from April 2000 to April 2005, 1.6% (30/1823) in the SEER data covering the same time period, and 1.3% (94/7353) in the SEER data from 1992 to 2011.
For infant ALL, the rates were 2.0% (3/149) in a trial (COG AALL0631) conducted from January 2008 to June 2014, 5.0% (2/40) in the SEER data during the trial period, and 5.4% (12/223) in the SEER data from 1992 to 2011.
“I had a hunch this was a bigger problem than we thought,” Dr Green said. “Now we see that is indeed the case.” ![]()
New research suggests that, in the US, early deaths from childhood cancer may be more common than clinical trials suggest.
Researchers found the rate of death within 1 month of diagnosis was higher among patients included in a large national database than among patients enrolled in phase 3 trials.
The data also indicated that early death is more likely in cancer patients under the age of 1 and those belonging to minority racial and ethnic groups.
Adam Green, MD, of the University of Colorado Anschutz Medical Campus in Aurora, and his colleagues conducted this research and reported the results in the Journal of Clinical Oncology.
The researchers analyzed data from the Surveillance, Epidemiology and End Results (SEER) database, which collects about 15% of all cancer outcomes across the US (representing a geographic and socioeconomic cross-section).
The team identified 36,337 patients with pediatric cancer (ages 0 to 19) diagnosed between 1992 and 2011. Of these patients, 555 (1.5%) died within 1 month of diagnosis.
Young age
Overall, the strongest predictor of early death was age below 1 year. The odds ratio (OR) was 4.36 for these patients (P<0.001), 0.75 for patients age 1 to 4, 0.78 for patients age 5 to 9, 1.00 (reference) for those age 10 to 14, and 1.69 for those age 15 to 19.
For hematologic malignancies, the adjusted OR (adjusted by poverty, unemployment, education, and year of diagnosis) was 4.32 for patients younger than 1 (P<0.001), 0.76 for patients age 1 to 4, 0.79 for patients age 5 to 9, 1.00 for those age 10 to 14, and 1.76 for those age 15 to 19 (P=0.002).
“In general, babies are just challenging, clinically, because they can’t tell you what they’re feeling,” Dr Green noted. “Parents and physicians have to pick the ones with cancer from the ones with a cold, without the patient being able to tell you about symptoms that could be diagnostic.”
“Babies tend to get aggressive cancers, it’s hard to tell when they’re getting sick, and some are even born with cancers that have already progressed. These factors combine to make very young age the strongest predictor of early death in our study.”
Race/ethnicity
Black race and Hispanic ethnicity also predicted early death. The OR was 1.48 for black race, 1.00 for white race (reference), and 1.09 for “other” races (P=0.102). The OR was 1.39 for Hispanic patients and 1.00 (reference) for non-Hispanic patients (P=0.007).
For hematologic malignancies, the adjusted OR was 1.68 for black patients (P=0.01) and 1.44 for patients of other, non-white races. The adjusted OR was 1.48 for Hispanic patients (P=0.009).
Dr Green said he hopes future studies will be able to determine the factors responsible for these disparities.
Higher death rates
Dr Green and his colleagues also found the rate of early death due to pediatric cancers is higher than reported in clinical trials, and this was true for all cancer subtypes assessed.
“Most of what we know about outcomes for cancer patients come from clinical trials, which have much more thorough reporting rules than cancer treated outside trials,” Dr Green said. “However, these kids in our study aren’t surviving long enough to join clinical trials.”
The researchers looked at a phase 3 trial (COG AAML0531) of pediatric patients with acute myeloid leukemia (AML) who were studied from August 2006 to June 2010. The early death rate in this trial was 1.6% (16/1022).
In contrast, the SEER database showed an early death rate for pediatric AML patients of 5.9% (15/256) during the same period as the trial and 6.2% (106/1698) for the period from 1992 to 2011. This is almost 4 times as high as the death rate in the trial.
The same effect was observed for acute lymphoblastic leukemia (ALL).
The early death rate for non-infant ALL was 0.7% (13/1790) in a trial (POG 9900) conducted from April 2000 to April 2005, 1.6% (30/1823) in the SEER data covering the same time period, and 1.3% (94/7353) in the SEER data from 1992 to 2011.
For infant ALL, the rates were 2.0% (3/149) in a trial (COG AALL0631) conducted from January 2008 to June 2014, 5.0% (2/40) in the SEER data during the trial period, and 5.4% (12/223) in the SEER data from 1992 to 2011.
“I had a hunch this was a bigger problem than we thought,” Dr Green said. “Now we see that is indeed the case.” ![]()
Mixed leukemias can benefit from allo-HST
ORLANDO – Allogeneic hematopoietic stem cell transplantation using a matched donor is a valid treatment option – and potential cure – for leukemias with markers of both myeloid and lymphoid lineages, or mixed phenotype acute leukemias, according to findings from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation (ALWP-EBMT) database.
Treatment outcomes at 3 years in 519 patients from the database who received an allogeneic transplant (allo-HCT) for mixed-phenotype acute leukemia (MPAL) between 2000 and 2014 and were transplanted in complete remission (CR1) included an overall survival of 56.3%, a leukemia-free survival of 46.5%, a relapse incidence of 31.4%, a nonrelapse mortality of 22.1%, and an incidence of chronic graft-versus-host disease (GVHD) of 37.5%, Reinhold Munker, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“The outcome in this large adult study is pretty favorable based upon 519 patients; 45%-65% can expect overall survival at 5 years,” he said.
The median age of the study subjects was 38.1 years (range, 18-75). Transplants were from a matched sibling donor in 54.5% of cases, and from a matched unrelated donor in 45.5% of cases. Myeloablative conditioning was used in 400 patients and included only chemotherapy in 140 patients and chemotherapy with total body irradiation in 260 patients. The remaining patients received nonmyeloablative conditioning, said Dr. Munker of Tulane University, New Orleans.
The source of stem cells was bone marrow in 26% of patients, and peripheral blood in 73%. Grade II-IV acute GVHD developed in 32.5% of patients. Median follow-up was 32 months, he noted.
In univariate analysis, age at transplant was strongly associated with leukemia-free survival, nonrelapse mortality, relapse incidence, and overall survival. The best outcomes were among those aged 18-35 years. The nonrelapse mortality rate and overall survival rate were lower for transplants done in 2005-2014 vs. 2000-2004 (20% vs 33.2% and 58.3 vs 44.7%, respectively). No differences in outcomes were found between related and unrelated donors, but chronic GVHD was more common with female donors and male recipients, with no in vivo T-cell depletion, and with use of peripheral blood stem cells – findings which are not unexpected, Dr. Munker noted.
Use of myeloablative conditioning with total-body irradiation correlated with a lower relapse incidence and with better leukemia-free survival vs. both myeloablative conditioning with only chemotherapy and reduced-intensity conditioning, he said.
In multivariate analysis, younger age and more recent year of transplant were associated with a better leukemia-free survival and overall survival, and use of myeloablative conditioning with total-body irradiation was associated with better leukemia-free survival and with a trend for higher overall survival.
MPALs are rare, accounting for only 2%-5% of all acute leukemias, Dr. Munker said, noting that prognosis is considered to be intermediate in children and unfavorable in adults.
The diagnostic criteria for MPAL were revised by the World Health Organization (WHO) in 2008 and accepted by most centers, but until recently data were lacking with respect to the recommended treatment strategy of induction regimens similar to those used in acute lymphoblastic leukemia, and consolidation by allogeneic transplant, he explained.
However, the Center for International Blood and Marrow Transplant Research last year published a series of 95 cases showing encouraging long-term survival with allo-HCT in MPAL patients with a median age of 20 years.
The current findings confirm and extend those prior findings, Dr. Munker said.
Dr. Munker reported having no disclosures.
ORLANDO – Allogeneic hematopoietic stem cell transplantation using a matched donor is a valid treatment option – and potential cure – for leukemias with markers of both myeloid and lymphoid lineages, or mixed phenotype acute leukemias, according to findings from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation (ALWP-EBMT) database.
Treatment outcomes at 3 years in 519 patients from the database who received an allogeneic transplant (allo-HCT) for mixed-phenotype acute leukemia (MPAL) between 2000 and 2014 and were transplanted in complete remission (CR1) included an overall survival of 56.3%, a leukemia-free survival of 46.5%, a relapse incidence of 31.4%, a nonrelapse mortality of 22.1%, and an incidence of chronic graft-versus-host disease (GVHD) of 37.5%, Reinhold Munker, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“The outcome in this large adult study is pretty favorable based upon 519 patients; 45%-65% can expect overall survival at 5 years,” he said.
The median age of the study subjects was 38.1 years (range, 18-75). Transplants were from a matched sibling donor in 54.5% of cases, and from a matched unrelated donor in 45.5% of cases. Myeloablative conditioning was used in 400 patients and included only chemotherapy in 140 patients and chemotherapy with total body irradiation in 260 patients. The remaining patients received nonmyeloablative conditioning, said Dr. Munker of Tulane University, New Orleans.
The source of stem cells was bone marrow in 26% of patients, and peripheral blood in 73%. Grade II-IV acute GVHD developed in 32.5% of patients. Median follow-up was 32 months, he noted.
In univariate analysis, age at transplant was strongly associated with leukemia-free survival, nonrelapse mortality, relapse incidence, and overall survival. The best outcomes were among those aged 18-35 years. The nonrelapse mortality rate and overall survival rate were lower for transplants done in 2005-2014 vs. 2000-2004 (20% vs 33.2% and 58.3 vs 44.7%, respectively). No differences in outcomes were found between related and unrelated donors, but chronic GVHD was more common with female donors and male recipients, with no in vivo T-cell depletion, and with use of peripheral blood stem cells – findings which are not unexpected, Dr. Munker noted.
Use of myeloablative conditioning with total-body irradiation correlated with a lower relapse incidence and with better leukemia-free survival vs. both myeloablative conditioning with only chemotherapy and reduced-intensity conditioning, he said.
In multivariate analysis, younger age and more recent year of transplant were associated with a better leukemia-free survival and overall survival, and use of myeloablative conditioning with total-body irradiation was associated with better leukemia-free survival and with a trend for higher overall survival.
MPALs are rare, accounting for only 2%-5% of all acute leukemias, Dr. Munker said, noting that prognosis is considered to be intermediate in children and unfavorable in adults.
The diagnostic criteria for MPAL were revised by the World Health Organization (WHO) in 2008 and accepted by most centers, but until recently data were lacking with respect to the recommended treatment strategy of induction regimens similar to those used in acute lymphoblastic leukemia, and consolidation by allogeneic transplant, he explained.
However, the Center for International Blood and Marrow Transplant Research last year published a series of 95 cases showing encouraging long-term survival with allo-HCT in MPAL patients with a median age of 20 years.
The current findings confirm and extend those prior findings, Dr. Munker said.
Dr. Munker reported having no disclosures.
ORLANDO – Allogeneic hematopoietic stem cell transplantation using a matched donor is a valid treatment option – and potential cure – for leukemias with markers of both myeloid and lymphoid lineages, or mixed phenotype acute leukemias, according to findings from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation (ALWP-EBMT) database.
Treatment outcomes at 3 years in 519 patients from the database who received an allogeneic transplant (allo-HCT) for mixed-phenotype acute leukemia (MPAL) between 2000 and 2014 and were transplanted in complete remission (CR1) included an overall survival of 56.3%, a leukemia-free survival of 46.5%, a relapse incidence of 31.4%, a nonrelapse mortality of 22.1%, and an incidence of chronic graft-versus-host disease (GVHD) of 37.5%, Reinhold Munker, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“The outcome in this large adult study is pretty favorable based upon 519 patients; 45%-65% can expect overall survival at 5 years,” he said.
The median age of the study subjects was 38.1 years (range, 18-75). Transplants were from a matched sibling donor in 54.5% of cases, and from a matched unrelated donor in 45.5% of cases. Myeloablative conditioning was used in 400 patients and included only chemotherapy in 140 patients and chemotherapy with total body irradiation in 260 patients. The remaining patients received nonmyeloablative conditioning, said Dr. Munker of Tulane University, New Orleans.
The source of stem cells was bone marrow in 26% of patients, and peripheral blood in 73%. Grade II-IV acute GVHD developed in 32.5% of patients. Median follow-up was 32 months, he noted.
In univariate analysis, age at transplant was strongly associated with leukemia-free survival, nonrelapse mortality, relapse incidence, and overall survival. The best outcomes were among those aged 18-35 years. The nonrelapse mortality rate and overall survival rate were lower for transplants done in 2005-2014 vs. 2000-2004 (20% vs 33.2% and 58.3 vs 44.7%, respectively). No differences in outcomes were found between related and unrelated donors, but chronic GVHD was more common with female donors and male recipients, with no in vivo T-cell depletion, and with use of peripheral blood stem cells – findings which are not unexpected, Dr. Munker noted.
Use of myeloablative conditioning with total-body irradiation correlated with a lower relapse incidence and with better leukemia-free survival vs. both myeloablative conditioning with only chemotherapy and reduced-intensity conditioning, he said.
In multivariate analysis, younger age and more recent year of transplant were associated with a better leukemia-free survival and overall survival, and use of myeloablative conditioning with total-body irradiation was associated with better leukemia-free survival and with a trend for higher overall survival.
MPALs are rare, accounting for only 2%-5% of all acute leukemias, Dr. Munker said, noting that prognosis is considered to be intermediate in children and unfavorable in adults.
The diagnostic criteria for MPAL were revised by the World Health Organization (WHO) in 2008 and accepted by most centers, but until recently data were lacking with respect to the recommended treatment strategy of induction regimens similar to those used in acute lymphoblastic leukemia, and consolidation by allogeneic transplant, he explained.
However, the Center for International Blood and Marrow Transplant Research last year published a series of 95 cases showing encouraging long-term survival with allo-HCT in MPAL patients with a median age of 20 years.
The current findings confirm and extend those prior findings, Dr. Munker said.
Dr. Munker reported having no disclosures.
Key clinical point:
Major finding: Treatment outcomes at 3 years included overall survival of 56.3%, leukemia-free survival of 46.5%, relapse incidence of 31.4%, nonrelapse mortality of 22.1%, and incidence of chronic graft-versus-host disease of 37.5%.
Data source: A review of 519 cases from the ALWP-EBMT database.
Disclosures: Dr. Munker reported having no disclosures.
Drug granted priority review for relapsed/refractory AML
The US Food and Drug Administration (FDA) has granted priority review for the new drug application (NDA) for enasidenib (AG-221), an inhibitor of mutant IDH2.
The drug is under review for the treatment of patients with relapsed or refractory acute myeloid leukemia (AML) with an IDH2 mutation.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10-month period.
The NDA for enasidenib has been given a Prescription Drug User Fee Act action date of August 30, 2017.
Enasidenib is being developed by Celgene Corporation and Agios Pharmaceuticals.
Phase 1/2 trial
The NDA submission for enasidenib is based on results from AG221-C-001, a single-arm, phase 1/2 study of the drug in patients with advanced hematologic malignancies with an IDH2 mutation.
Early data from the relapsed or refractory AML patients in this study were presented at the 2015 ASH Annual Meeting. (The presentation included updated data that differ from the data in the abstract.)
The trial included a dose-escalation phase and 5 expansion cohorts. The first 4 expansion cohorts had completed enrollment as of the presentation.
- Arm 1: 25 patients with IDH2-mutant-positive relapsed or refractory AML age ≥60 years, or any patient with AML regardless of age who relapsed after a bone marrow transplant (BMT)
- Arm 2: 25 patients with IDH2-mutant-positive relapsed or refractory AML age <60 years, excluding patients with AML who relapsed after a BMT
- Arm 3: 25 patients with IDH2-mutant-positive untreated AML age ≥60 years who decline standard of care chemotherapy
- Arm 4: 25 patients with IDH2-mutant-positive advanced hematologic malignancies not eligible for arms 1 to 3
- Arm 5: The phase 2 portion of the trial included 125 patients with IDH2-mutant-positive AML who were in second or later relapse, refractory to second-line induction or reinduction treatment, or relapsed after allogeneic transplant.
The data reported at ASH were from patients receiving enasidenib administered from 50-mg to 650-mg total daily doses in the dose-escalation arm and 100 mg once daily in the first 4 expansion arms, as of September 1, 2015.
The median age of these patients was 69 (range, 19-100). Patients with relapsed or refractory AML received a median of 2 prior lines of therapy (range, 1-6).
Safety data
A safety analysis was conducted for all 231 treated patients. As of the ASH presentation, a maximum tolerated dose of enasidenib had not been reached.
The majority of adverse events were mild to moderate, with the most common being nausea, diarrhea, fatigue, and febrile neutropenia.
Twenty-three percent of patients had treatment-related serious adverse events—notably, differentiation syndrome (4%), leukocytosis (4%), and nausea (2%).
Drug-related grade 5 serious adverse events include atrial flutter (n=1), cardiac tamponade (n=1), pericardial effusion (n=1), and respiratory failure (n=1).
Efficacy Data
Seventy-nine of the 209 response-evaluable patients achieved investigator-assessed objective responses, for an overall response rate of 38%.
There were 37 (18%) complete remissions (CR), 3 CRs with incomplete platelet recovery (CRp), 14 marrow CRs (mCR), 3 CRs with incomplete hematologic recovery (CRi), and 22 partial remissions (PR).
Of the 159 patients with relapsed or refractory AML, 59 (37%) achieved an objective response, including 29 (18%) CRs, 1 CRp, 9 mCRs, 3 CRis, and 17 PRs.
Of the 24 patients with AML who declined standard of care chemotherapy, 10 achieved an objective response, including 4 CRs, 1 CRp, 1 mCR, and 4 PRs.
The median duration of response was 6.9 months in patients with relapsed or refractory AML.
Responding relapsed/refractory AML patients were on study treatment for up to 18 months. The median duration of treatment was 6.8 months (range, 1.8 to 18 months). ![]()
The US Food and Drug Administration (FDA) has granted priority review for the new drug application (NDA) for enasidenib (AG-221), an inhibitor of mutant IDH2.
The drug is under review for the treatment of patients with relapsed or refractory acute myeloid leukemia (AML) with an IDH2 mutation.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10-month period.
The NDA for enasidenib has been given a Prescription Drug User Fee Act action date of August 30, 2017.
Enasidenib is being developed by Celgene Corporation and Agios Pharmaceuticals.
Phase 1/2 trial
The NDA submission for enasidenib is based on results from AG221-C-001, a single-arm, phase 1/2 study of the drug in patients with advanced hematologic malignancies with an IDH2 mutation.
Early data from the relapsed or refractory AML patients in this study were presented at the 2015 ASH Annual Meeting. (The presentation included updated data that differ from the data in the abstract.)
The trial included a dose-escalation phase and 5 expansion cohorts. The first 4 expansion cohorts had completed enrollment as of the presentation.
- Arm 1: 25 patients with IDH2-mutant-positive relapsed or refractory AML age ≥60 years, or any patient with AML regardless of age who relapsed after a bone marrow transplant (BMT)
- Arm 2: 25 patients with IDH2-mutant-positive relapsed or refractory AML age <60 years, excluding patients with AML who relapsed after a BMT
- Arm 3: 25 patients with IDH2-mutant-positive untreated AML age ≥60 years who decline standard of care chemotherapy
- Arm 4: 25 patients with IDH2-mutant-positive advanced hematologic malignancies not eligible for arms 1 to 3
- Arm 5: The phase 2 portion of the trial included 125 patients with IDH2-mutant-positive AML who were in second or later relapse, refractory to second-line induction or reinduction treatment, or relapsed after allogeneic transplant.
The data reported at ASH were from patients receiving enasidenib administered from 50-mg to 650-mg total daily doses in the dose-escalation arm and 100 mg once daily in the first 4 expansion arms, as of September 1, 2015.
The median age of these patients was 69 (range, 19-100). Patients with relapsed or refractory AML received a median of 2 prior lines of therapy (range, 1-6).
Safety data
A safety analysis was conducted for all 231 treated patients. As of the ASH presentation, a maximum tolerated dose of enasidenib had not been reached.
The majority of adverse events were mild to moderate, with the most common being nausea, diarrhea, fatigue, and febrile neutropenia.
Twenty-three percent of patients had treatment-related serious adverse events—notably, differentiation syndrome (4%), leukocytosis (4%), and nausea (2%).
Drug-related grade 5 serious adverse events include atrial flutter (n=1), cardiac tamponade (n=1), pericardial effusion (n=1), and respiratory failure (n=1).
Efficacy Data
Seventy-nine of the 209 response-evaluable patients achieved investigator-assessed objective responses, for an overall response rate of 38%.
There were 37 (18%) complete remissions (CR), 3 CRs with incomplete platelet recovery (CRp), 14 marrow CRs (mCR), 3 CRs with incomplete hematologic recovery (CRi), and 22 partial remissions (PR).
Of the 159 patients with relapsed or refractory AML, 59 (37%) achieved an objective response, including 29 (18%) CRs, 1 CRp, 9 mCRs, 3 CRis, and 17 PRs.
Of the 24 patients with AML who declined standard of care chemotherapy, 10 achieved an objective response, including 4 CRs, 1 CRp, 1 mCR, and 4 PRs.
The median duration of response was 6.9 months in patients with relapsed or refractory AML.
Responding relapsed/refractory AML patients were on study treatment for up to 18 months. The median duration of treatment was 6.8 months (range, 1.8 to 18 months). ![]()
The US Food and Drug Administration (FDA) has granted priority review for the new drug application (NDA) for enasidenib (AG-221), an inhibitor of mutant IDH2.
The drug is under review for the treatment of patients with relapsed or refractory acute myeloid leukemia (AML) with an IDH2 mutation.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10-month period.
The NDA for enasidenib has been given a Prescription Drug User Fee Act action date of August 30, 2017.
Enasidenib is being developed by Celgene Corporation and Agios Pharmaceuticals.
Phase 1/2 trial
The NDA submission for enasidenib is based on results from AG221-C-001, a single-arm, phase 1/2 study of the drug in patients with advanced hematologic malignancies with an IDH2 mutation.
Early data from the relapsed or refractory AML patients in this study were presented at the 2015 ASH Annual Meeting. (The presentation included updated data that differ from the data in the abstract.)
The trial included a dose-escalation phase and 5 expansion cohorts. The first 4 expansion cohorts had completed enrollment as of the presentation.
- Arm 1: 25 patients with IDH2-mutant-positive relapsed or refractory AML age ≥60 years, or any patient with AML regardless of age who relapsed after a bone marrow transplant (BMT)
- Arm 2: 25 patients with IDH2-mutant-positive relapsed or refractory AML age <60 years, excluding patients with AML who relapsed after a BMT
- Arm 3: 25 patients with IDH2-mutant-positive untreated AML age ≥60 years who decline standard of care chemotherapy
- Arm 4: 25 patients with IDH2-mutant-positive advanced hematologic malignancies not eligible for arms 1 to 3
- Arm 5: The phase 2 portion of the trial included 125 patients with IDH2-mutant-positive AML who were in second or later relapse, refractory to second-line induction or reinduction treatment, or relapsed after allogeneic transplant.
The data reported at ASH were from patients receiving enasidenib administered from 50-mg to 650-mg total daily doses in the dose-escalation arm and 100 mg once daily in the first 4 expansion arms, as of September 1, 2015.
The median age of these patients was 69 (range, 19-100). Patients with relapsed or refractory AML received a median of 2 prior lines of therapy (range, 1-6).
Safety data
A safety analysis was conducted for all 231 treated patients. As of the ASH presentation, a maximum tolerated dose of enasidenib had not been reached.
The majority of adverse events were mild to moderate, with the most common being nausea, diarrhea, fatigue, and febrile neutropenia.
Twenty-three percent of patients had treatment-related serious adverse events—notably, differentiation syndrome (4%), leukocytosis (4%), and nausea (2%).
Drug-related grade 5 serious adverse events include atrial flutter (n=1), cardiac tamponade (n=1), pericardial effusion (n=1), and respiratory failure (n=1).
Efficacy Data
Seventy-nine of the 209 response-evaluable patients achieved investigator-assessed objective responses, for an overall response rate of 38%.
There were 37 (18%) complete remissions (CR), 3 CRs with incomplete platelet recovery (CRp), 14 marrow CRs (mCR), 3 CRs with incomplete hematologic recovery (CRi), and 22 partial remissions (PR).
Of the 159 patients with relapsed or refractory AML, 59 (37%) achieved an objective response, including 29 (18%) CRs, 1 CRp, 9 mCRs, 3 CRis, and 17 PRs.
Of the 24 patients with AML who declined standard of care chemotherapy, 10 achieved an objective response, including 4 CRs, 1 CRp, 1 mCR, and 4 PRs.
The median duration of response was 6.9 months in patients with relapsed or refractory AML.
Responding relapsed/refractory AML patients were on study treatment for up to 18 months. The median duration of treatment was 6.8 months (range, 1.8 to 18 months). ![]()
Older AML patients benefit from frailty assessment and interventions
ORLANDO – Performing a comprehensive geriatric assessment of patients with acute myeloid leukemia (AML) does more than provide fine-tuned risk stratification; spotting areas of vulnerability and frailty can also identify targets for prehabilitation, support, and remediation as older patients face transplant.
A program at the University of Chicago termed the Transplant Optimization Program, or TOP, uses the geriatric assessment as the foundation of an interdisciplinary, customized intervention for older adults facing hematopoietic cell transplant (HCT). The team includes the transplant physician and a geriatric oncologist; however, social work, dietetics, psychology, and physical therapy professionals also are brought on board.
“High comorbidities and functional limitations influence overall survival,” Dr. Artz said. “Non-relapse mortality remains a major barrier; of course, the attendant morbidity before it is perhaps a bigger concern, and our patients’ concern.”
Dr. Artz said that he and his colleagues at the University of Chicago, where he is a professor of medicine, had adapted the geriatric assessment developed by the Cancer and Aging Group, and now administer it to all prospective transplant patients aged 50 years and older. Dr. Artz said that, at his center, they have found that 25% of patients aged 50 and older were frail according to the Fried Frailty Index. “That’s what we expect for people in the community who are aged 80 and older, so we’re painting a picture of accelerated aging for patients before they undergo allograft,” he said.
Results of the assessment are then reviewed by a multidisciplinary team, and an individualized prehabilitation and support program is developed based on those results. “Using chronologic and physiologic age in transplant can help us better risk stratify, and think about strategies to mitigate some of those risks,” Dr. Artz said.
The staging tool currently used at the University of Chicago examines seven domains and uses objective tools to deliver information in each domain. Comorbidities are assessed by the Hematopoietic Cell Transplant–Specific Comorbidity Index (HCT-CI) and the Older Americans Resources and Services (OARS) scale. “We are trying to use standardized tools such as the geriatric assessment, because our ‘eyeball test’ is quite poor,” he said.
Physical function is assessed by measuring four-meter walk speed and grip strength; by asking about falls and capacity for instrumental activities of daily living; and by administering the Karnofsky Performance Scale–MD. Patients are given the Mental Health Inventory–17 to assess psychological health.
Neurocognitive testing and the Blessed Orientation-Memory-Concentration test are used to assess cognitive function. The Medical Outcomes Study Social Activity and Social Limitations scales give an idea about social support.
Biomarkers that are tracked include C-reactive protein and ferritin, among others, Dr. Artz said. Nutritional status is assessed by measuring serum albumin as well as any weight loss.
Once the full geriatric assessment data about a patient are gathered, a plan is formulated for impairments in any given domain. For example, if significant comorbid conditions exist, the TOP team seeks subspecialty consultation for management advice in the context of transplant. “This is a consultation – not a clearance!” Dr. Artz said.
Physical and functional impairments are addressed with prehabilitation if time permits, and the home assessment is aligned with patient expectations. Sometimes, the inactivity associated with peritransplant period can worsen conditions such as osteoarthritis, so a patient who’s been functional may find themselves very stiff when going home. Caregivers can encourage early activity to help minimize this effect, Dr. Artz said.
For patients at nutritional risk, it’s vital to have a good nutrition plan for transplant and to make sure medications or social factors aren’t standing in the way of adequate nutrition, Dr. Artz said. “Don’t forget to ask about dentures,” he said, since mucositis can make dentures unbearable in the immediate posttransplant period.
It’s important to be careful when unpacking findings of cognitive impairment, Dr. Artz said, since untreated depression and anxiety can manifest as forgetfulness and perseveration. Eliminating unnecessary medication, having a good delirium protocol in place, and keeping a family member in the room with the patient can help minimize a cognitive downturn during transplant.
As patients age, it can be more common for them to have limited social support. The TOP team calls a family meeting to assess resources and appoint a “team captain” among the patient’s social circle. “We work to enlarge that circle,” by pulling in as many family members and friends as possible, Dr. Artz said. In a discussion after the talk, he said that he feels that having a family member in the hospital with the older transplant patient is important for many reasons. Not only is the patient less likely to fall, he or she may also eat better and feel better. In addition, he said he has a sense that when the caregiver has seen the patient at his or her nadir, taking that patient home isn’t as scary, since it’s easier to see that the trajectory is headed upward by the time of discharge.
Dr. Artz said that other facilities are now beginning to send patients for TOP evaluations; “the aging evaluation informs physiologic age, candidacy for transplant, and may permit optimizing outcomes,” he said. “We’re trying to ... optimize patients both before and after transplant. It’s one thing to say you’re vulnerable, but how do you take that vulnerable patient and improve their outcomes? That’s the question.” Though the proportion of older individuals receiving allogeneic transplants is growing rapidly, research is not keeping up, Dr. Artz said, calling for more prospective studies in older adults.
Dr. Artz reported that he has received research funding from Miltenyi Biotech.
koakes@frontlinemedcom.com
On Twitter @karioakes
ORLANDO – Performing a comprehensive geriatric assessment of patients with acute myeloid leukemia (AML) does more than provide fine-tuned risk stratification; spotting areas of vulnerability and frailty can also identify targets for prehabilitation, support, and remediation as older patients face transplant.
A program at the University of Chicago termed the Transplant Optimization Program, or TOP, uses the geriatric assessment as the foundation of an interdisciplinary, customized intervention for older adults facing hematopoietic cell transplant (HCT). The team includes the transplant physician and a geriatric oncologist; however, social work, dietetics, psychology, and physical therapy professionals also are brought on board.
“High comorbidities and functional limitations influence overall survival,” Dr. Artz said. “Non-relapse mortality remains a major barrier; of course, the attendant morbidity before it is perhaps a bigger concern, and our patients’ concern.”
Dr. Artz said that he and his colleagues at the University of Chicago, where he is a professor of medicine, had adapted the geriatric assessment developed by the Cancer and Aging Group, and now administer it to all prospective transplant patients aged 50 years and older. Dr. Artz said that, at his center, they have found that 25% of patients aged 50 and older were frail according to the Fried Frailty Index. “That’s what we expect for people in the community who are aged 80 and older, so we’re painting a picture of accelerated aging for patients before they undergo allograft,” he said.
Results of the assessment are then reviewed by a multidisciplinary team, and an individualized prehabilitation and support program is developed based on those results. “Using chronologic and physiologic age in transplant can help us better risk stratify, and think about strategies to mitigate some of those risks,” Dr. Artz said.
The staging tool currently used at the University of Chicago examines seven domains and uses objective tools to deliver information in each domain. Comorbidities are assessed by the Hematopoietic Cell Transplant–Specific Comorbidity Index (HCT-CI) and the Older Americans Resources and Services (OARS) scale. “We are trying to use standardized tools such as the geriatric assessment, because our ‘eyeball test’ is quite poor,” he said.
Physical function is assessed by measuring four-meter walk speed and grip strength; by asking about falls and capacity for instrumental activities of daily living; and by administering the Karnofsky Performance Scale–MD. Patients are given the Mental Health Inventory–17 to assess psychological health.
Neurocognitive testing and the Blessed Orientation-Memory-Concentration test are used to assess cognitive function. The Medical Outcomes Study Social Activity and Social Limitations scales give an idea about social support.
Biomarkers that are tracked include C-reactive protein and ferritin, among others, Dr. Artz said. Nutritional status is assessed by measuring serum albumin as well as any weight loss.
Once the full geriatric assessment data about a patient are gathered, a plan is formulated for impairments in any given domain. For example, if significant comorbid conditions exist, the TOP team seeks subspecialty consultation for management advice in the context of transplant. “This is a consultation – not a clearance!” Dr. Artz said.
Physical and functional impairments are addressed with prehabilitation if time permits, and the home assessment is aligned with patient expectations. Sometimes, the inactivity associated with peritransplant period can worsen conditions such as osteoarthritis, so a patient who’s been functional may find themselves very stiff when going home. Caregivers can encourage early activity to help minimize this effect, Dr. Artz said.
For patients at nutritional risk, it’s vital to have a good nutrition plan for transplant and to make sure medications or social factors aren’t standing in the way of adequate nutrition, Dr. Artz said. “Don’t forget to ask about dentures,” he said, since mucositis can make dentures unbearable in the immediate posttransplant period.
It’s important to be careful when unpacking findings of cognitive impairment, Dr. Artz said, since untreated depression and anxiety can manifest as forgetfulness and perseveration. Eliminating unnecessary medication, having a good delirium protocol in place, and keeping a family member in the room with the patient can help minimize a cognitive downturn during transplant.
As patients age, it can be more common for them to have limited social support. The TOP team calls a family meeting to assess resources and appoint a “team captain” among the patient’s social circle. “We work to enlarge that circle,” by pulling in as many family members and friends as possible, Dr. Artz said. In a discussion after the talk, he said that he feels that having a family member in the hospital with the older transplant patient is important for many reasons. Not only is the patient less likely to fall, he or she may also eat better and feel better. In addition, he said he has a sense that when the caregiver has seen the patient at his or her nadir, taking that patient home isn’t as scary, since it’s easier to see that the trajectory is headed upward by the time of discharge.
Dr. Artz said that other facilities are now beginning to send patients for TOP evaluations; “the aging evaluation informs physiologic age, candidacy for transplant, and may permit optimizing outcomes,” he said. “We’re trying to ... optimize patients both before and after transplant. It’s one thing to say you’re vulnerable, but how do you take that vulnerable patient and improve their outcomes? That’s the question.” Though the proportion of older individuals receiving allogeneic transplants is growing rapidly, research is not keeping up, Dr. Artz said, calling for more prospective studies in older adults.
Dr. Artz reported that he has received research funding from Miltenyi Biotech.
koakes@frontlinemedcom.com
On Twitter @karioakes
ORLANDO – Performing a comprehensive geriatric assessment of patients with acute myeloid leukemia (AML) does more than provide fine-tuned risk stratification; spotting areas of vulnerability and frailty can also identify targets for prehabilitation, support, and remediation as older patients face transplant.
A program at the University of Chicago termed the Transplant Optimization Program, or TOP, uses the geriatric assessment as the foundation of an interdisciplinary, customized intervention for older adults facing hematopoietic cell transplant (HCT). The team includes the transplant physician and a geriatric oncologist; however, social work, dietetics, psychology, and physical therapy professionals also are brought on board.
“High comorbidities and functional limitations influence overall survival,” Dr. Artz said. “Non-relapse mortality remains a major barrier; of course, the attendant morbidity before it is perhaps a bigger concern, and our patients’ concern.”
Dr. Artz said that he and his colleagues at the University of Chicago, where he is a professor of medicine, had adapted the geriatric assessment developed by the Cancer and Aging Group, and now administer it to all prospective transplant patients aged 50 years and older. Dr. Artz said that, at his center, they have found that 25% of patients aged 50 and older were frail according to the Fried Frailty Index. “That’s what we expect for people in the community who are aged 80 and older, so we’re painting a picture of accelerated aging for patients before they undergo allograft,” he said.
Results of the assessment are then reviewed by a multidisciplinary team, and an individualized prehabilitation and support program is developed based on those results. “Using chronologic and physiologic age in transplant can help us better risk stratify, and think about strategies to mitigate some of those risks,” Dr. Artz said.
The staging tool currently used at the University of Chicago examines seven domains and uses objective tools to deliver information in each domain. Comorbidities are assessed by the Hematopoietic Cell Transplant–Specific Comorbidity Index (HCT-CI) and the Older Americans Resources and Services (OARS) scale. “We are trying to use standardized tools such as the geriatric assessment, because our ‘eyeball test’ is quite poor,” he said.
Physical function is assessed by measuring four-meter walk speed and grip strength; by asking about falls and capacity for instrumental activities of daily living; and by administering the Karnofsky Performance Scale–MD. Patients are given the Mental Health Inventory–17 to assess psychological health.
Neurocognitive testing and the Blessed Orientation-Memory-Concentration test are used to assess cognitive function. The Medical Outcomes Study Social Activity and Social Limitations scales give an idea about social support.
Biomarkers that are tracked include C-reactive protein and ferritin, among others, Dr. Artz said. Nutritional status is assessed by measuring serum albumin as well as any weight loss.
Once the full geriatric assessment data about a patient are gathered, a plan is formulated for impairments in any given domain. For example, if significant comorbid conditions exist, the TOP team seeks subspecialty consultation for management advice in the context of transplant. “This is a consultation – not a clearance!” Dr. Artz said.
Physical and functional impairments are addressed with prehabilitation if time permits, and the home assessment is aligned with patient expectations. Sometimes, the inactivity associated with peritransplant period can worsen conditions such as osteoarthritis, so a patient who’s been functional may find themselves very stiff when going home. Caregivers can encourage early activity to help minimize this effect, Dr. Artz said.
For patients at nutritional risk, it’s vital to have a good nutrition plan for transplant and to make sure medications or social factors aren’t standing in the way of adequate nutrition, Dr. Artz said. “Don’t forget to ask about dentures,” he said, since mucositis can make dentures unbearable in the immediate posttransplant period.
It’s important to be careful when unpacking findings of cognitive impairment, Dr. Artz said, since untreated depression and anxiety can manifest as forgetfulness and perseveration. Eliminating unnecessary medication, having a good delirium protocol in place, and keeping a family member in the room with the patient can help minimize a cognitive downturn during transplant.
As patients age, it can be more common for them to have limited social support. The TOP team calls a family meeting to assess resources and appoint a “team captain” among the patient’s social circle. “We work to enlarge that circle,” by pulling in as many family members and friends as possible, Dr. Artz said. In a discussion after the talk, he said that he feels that having a family member in the hospital with the older transplant patient is important for many reasons. Not only is the patient less likely to fall, he or she may also eat better and feel better. In addition, he said he has a sense that when the caregiver has seen the patient at his or her nadir, taking that patient home isn’t as scary, since it’s easier to see that the trajectory is headed upward by the time of discharge.
Dr. Artz said that other facilities are now beginning to send patients for TOP evaluations; “the aging evaluation informs physiologic age, candidacy for transplant, and may permit optimizing outcomes,” he said. “We’re trying to ... optimize patients both before and after transplant. It’s one thing to say you’re vulnerable, but how do you take that vulnerable patient and improve their outcomes? That’s the question.” Though the proportion of older individuals receiving allogeneic transplants is growing rapidly, research is not keeping up, Dr. Artz said, calling for more prospective studies in older adults.
Dr. Artz reported that he has received research funding from Miltenyi Biotech.
koakes@frontlinemedcom.com
On Twitter @karioakes
EXPERT ANALYSIS FROM THE 2017 BMT TANDEM MEETINGS
Docs create guideline to aid workup of acute leukemia
Two physician groups have published an evidence-based guideline that addresses the initial workup of acute leukemia.
The guideline includes 27 recommendations intended to aid treating physicians and pathologists involved in the diagnostic and prognostic evaluation of acute leukemia samples, including those from patients with acute lymphoblastic leukemia, acute myeloid leukemia, and acute leukemias of ambiguous lineage.
The guideline, which was developed through a collaboration between the College of American Pathologists (CAP) and the American Society of Hematology (ASH), has been published in the Archives of Pathology and Laboratory Medicine.
The recommendations in the guideline will also be available in a pocket guide and via the ASH Pocket Guides app in March.
“With its multidisciplinary perspective, this guideline reflects contemporary, integrated cancer care, and, therefore, it will also help providers realize efficiencies in test management,” said ASH guideline co-chair James W. Vardiman, MD, of the University of Chicago in Illinois.
To create this guideline, Dr Vardiman and his colleagues sought and reviewed relevant published evidence.
The group set out to answer 6 questions for the initial diagnosis of acute leukemias:
1) What clinical and laboratory information should be available?
2) What samples and specimen types should be evaluated?
3) What tests are required for all patients during the initial evaluation?
4) What tests are required for only a subset of patients?
5) Where should laboratory testing be performed?
6) How should the results be reported?
The authors say the guideline’s 27 recommendations answer these questions, providing a framework for the steps involved in the evaluation of acute leukemia samples.
In particular, the guideline includes steps to coordinate and communicate across clinical teams, specifying information that must be shared—particularly among treating physicians and pathologists—for optimal patient outcomes and to avoid duplicative testing.
“The laboratory testing to diagnose acute leukemia and inform treatment is increasingly complex, making this guideline essential,” said CAP guideline co-chair Daniel A. Arber, MD, of the University of Chicago.
“New gene mutations and protein expressions have been described over the last decade in all types of acute leukemia, and many of them impact diagnosis or inform prognosis.” ![]()
Two physician groups have published an evidence-based guideline that addresses the initial workup of acute leukemia.
The guideline includes 27 recommendations intended to aid treating physicians and pathologists involved in the diagnostic and prognostic evaluation of acute leukemia samples, including those from patients with acute lymphoblastic leukemia, acute myeloid leukemia, and acute leukemias of ambiguous lineage.
The guideline, which was developed through a collaboration between the College of American Pathologists (CAP) and the American Society of Hematology (ASH), has been published in the Archives of Pathology and Laboratory Medicine.
The recommendations in the guideline will also be available in a pocket guide and via the ASH Pocket Guides app in March.
“With its multidisciplinary perspective, this guideline reflects contemporary, integrated cancer care, and, therefore, it will also help providers realize efficiencies in test management,” said ASH guideline co-chair James W. Vardiman, MD, of the University of Chicago in Illinois.
To create this guideline, Dr Vardiman and his colleagues sought and reviewed relevant published evidence.
The group set out to answer 6 questions for the initial diagnosis of acute leukemias:
1) What clinical and laboratory information should be available?
2) What samples and specimen types should be evaluated?
3) What tests are required for all patients during the initial evaluation?
4) What tests are required for only a subset of patients?
5) Where should laboratory testing be performed?
6) How should the results be reported?
The authors say the guideline’s 27 recommendations answer these questions, providing a framework for the steps involved in the evaluation of acute leukemia samples.
In particular, the guideline includes steps to coordinate and communicate across clinical teams, specifying information that must be shared—particularly among treating physicians and pathologists—for optimal patient outcomes and to avoid duplicative testing.
“The laboratory testing to diagnose acute leukemia and inform treatment is increasingly complex, making this guideline essential,” said CAP guideline co-chair Daniel A. Arber, MD, of the University of Chicago.
“New gene mutations and protein expressions have been described over the last decade in all types of acute leukemia, and many of them impact diagnosis or inform prognosis.” ![]()
Two physician groups have published an evidence-based guideline that addresses the initial workup of acute leukemia.
The guideline includes 27 recommendations intended to aid treating physicians and pathologists involved in the diagnostic and prognostic evaluation of acute leukemia samples, including those from patients with acute lymphoblastic leukemia, acute myeloid leukemia, and acute leukemias of ambiguous lineage.
The guideline, which was developed through a collaboration between the College of American Pathologists (CAP) and the American Society of Hematology (ASH), has been published in the Archives of Pathology and Laboratory Medicine.
The recommendations in the guideline will also be available in a pocket guide and via the ASH Pocket Guides app in March.
“With its multidisciplinary perspective, this guideline reflects contemporary, integrated cancer care, and, therefore, it will also help providers realize efficiencies in test management,” said ASH guideline co-chair James W. Vardiman, MD, of the University of Chicago in Illinois.
To create this guideline, Dr Vardiman and his colleagues sought and reviewed relevant published evidence.
The group set out to answer 6 questions for the initial diagnosis of acute leukemias:
1) What clinical and laboratory information should be available?
2) What samples and specimen types should be evaluated?
3) What tests are required for all patients during the initial evaluation?
4) What tests are required for only a subset of patients?
5) Where should laboratory testing be performed?
6) How should the results be reported?
The authors say the guideline’s 27 recommendations answer these questions, providing a framework for the steps involved in the evaluation of acute leukemia samples.
In particular, the guideline includes steps to coordinate and communicate across clinical teams, specifying information that must be shared—particularly among treating physicians and pathologists—for optimal patient outcomes and to avoid duplicative testing.
“The laboratory testing to diagnose acute leukemia and inform treatment is increasingly complex, making this guideline essential,” said CAP guideline co-chair Daniel A. Arber, MD, of the University of Chicago.
“New gene mutations and protein expressions have been described over the last decade in all types of acute leukemia, and many of them impact diagnosis or inform prognosis.” ![]()
Tumor suppressor promotes FLT3-ITD AML
New research indicates that RUNX1, a known tumor suppressor, actually cooperates with internal tandem duplications in the FLT3 receptor tyrosine kinase (FLT3-ITD) to induce acute myeloid leukemia (AML).
Investigators say this discovery suggests that blocking RUNX1 activity will “greatly enhance” current therapeutic approaches using FLT3 inhibitors.
The discovery was published in The Journal of Experimental Medicine.
The research began when investigators noticed that many AML patients with FLT3-ITD also showed increased levels of RUNX1.
“This was unexpected because up to 20% of AML patients carry mutations that inactivate RUNX1, which is generally considered to be a tumor suppressor that prevents the formation of leukemias,” said study author Carol Stocking, PhD, of Heinrich-Pette-Institute-Leibniz Institute for Experimental Virology in Hamburg, Germany.
To investigate this finding, she and her colleagues injected mice with human AML cells expressing FLT3-ITD. The team found that reducing RUNX1 levels attenuated the cells’ ability to form tumors, but elevated RUNX1 levels worked with FLT3-ITD to induce AML.
Mouse hematopoietic stem cells expressing FLT3-ITD were highly proliferative, and co-expression of RUNX1 blocked their differentiation, allowing them to give rise to AML.
Mutant FLT3 appears to stabilize and activate RUNX1 by promoting the transcription factor’s phosphorylation. Active RUNX1 then blocks differentiation, at least in part, by upregulation of another transcription factor, Hhex.
The investigators found that hematopoietic stem cells expressing both Hhex and FLT3-ITD gave rise to AML.
RUNX1 may therefore suppress the initiation of AML but, after being activated by mutant FLT3, block differentiation and promote tumor development.
“Therapies that can reverse this differentiation block may offer significant therapeutic efficacy in AML patients with FLT3 mutations,” Dr Stocking said. “Ablating RUNX1 is toxic to leukemic cells but not to normal hematopoietic stem cells, so inhibiting RUNX1 may be a promising target in combination with FLT3 inhibitors.” ![]()
New research indicates that RUNX1, a known tumor suppressor, actually cooperates with internal tandem duplications in the FLT3 receptor tyrosine kinase (FLT3-ITD) to induce acute myeloid leukemia (AML).
Investigators say this discovery suggests that blocking RUNX1 activity will “greatly enhance” current therapeutic approaches using FLT3 inhibitors.
The discovery was published in The Journal of Experimental Medicine.
The research began when investigators noticed that many AML patients with FLT3-ITD also showed increased levels of RUNX1.
“This was unexpected because up to 20% of AML patients carry mutations that inactivate RUNX1, which is generally considered to be a tumor suppressor that prevents the formation of leukemias,” said study author Carol Stocking, PhD, of Heinrich-Pette-Institute-Leibniz Institute for Experimental Virology in Hamburg, Germany.
To investigate this finding, she and her colleagues injected mice with human AML cells expressing FLT3-ITD. The team found that reducing RUNX1 levels attenuated the cells’ ability to form tumors, but elevated RUNX1 levels worked with FLT3-ITD to induce AML.
Mouse hematopoietic stem cells expressing FLT3-ITD were highly proliferative, and co-expression of RUNX1 blocked their differentiation, allowing them to give rise to AML.
Mutant FLT3 appears to stabilize and activate RUNX1 by promoting the transcription factor’s phosphorylation. Active RUNX1 then blocks differentiation, at least in part, by upregulation of another transcription factor, Hhex.
The investigators found that hematopoietic stem cells expressing both Hhex and FLT3-ITD gave rise to AML.
RUNX1 may therefore suppress the initiation of AML but, after being activated by mutant FLT3, block differentiation and promote tumor development.
“Therapies that can reverse this differentiation block may offer significant therapeutic efficacy in AML patients with FLT3 mutations,” Dr Stocking said. “Ablating RUNX1 is toxic to leukemic cells but not to normal hematopoietic stem cells, so inhibiting RUNX1 may be a promising target in combination with FLT3 inhibitors.” ![]()
New research indicates that RUNX1, a known tumor suppressor, actually cooperates with internal tandem duplications in the FLT3 receptor tyrosine kinase (FLT3-ITD) to induce acute myeloid leukemia (AML).
Investigators say this discovery suggests that blocking RUNX1 activity will “greatly enhance” current therapeutic approaches using FLT3 inhibitors.
The discovery was published in The Journal of Experimental Medicine.
The research began when investigators noticed that many AML patients with FLT3-ITD also showed increased levels of RUNX1.
“This was unexpected because up to 20% of AML patients carry mutations that inactivate RUNX1, which is generally considered to be a tumor suppressor that prevents the formation of leukemias,” said study author Carol Stocking, PhD, of Heinrich-Pette-Institute-Leibniz Institute for Experimental Virology in Hamburg, Germany.
To investigate this finding, she and her colleagues injected mice with human AML cells expressing FLT3-ITD. The team found that reducing RUNX1 levels attenuated the cells’ ability to form tumors, but elevated RUNX1 levels worked with FLT3-ITD to induce AML.
Mouse hematopoietic stem cells expressing FLT3-ITD were highly proliferative, and co-expression of RUNX1 blocked their differentiation, allowing them to give rise to AML.
Mutant FLT3 appears to stabilize and activate RUNX1 by promoting the transcription factor’s phosphorylation. Active RUNX1 then blocks differentiation, at least in part, by upregulation of another transcription factor, Hhex.
The investigators found that hematopoietic stem cells expressing both Hhex and FLT3-ITD gave rise to AML.
RUNX1 may therefore suppress the initiation of AML but, after being activated by mutant FLT3, block differentiation and promote tumor development.
“Therapies that can reverse this differentiation block may offer significant therapeutic efficacy in AML patients with FLT3 mutations,” Dr Stocking said. “Ablating RUNX1 is toxic to leukemic cells but not to normal hematopoietic stem cells, so inhibiting RUNX1 may be a promising target in combination with FLT3 inhibitors.” ![]()
Model illustrates progression to MDS, AML
Researchers say they have created a model that shows the step-by-step progression from normal blood cells to acute myeloid leukemia (AML).
The team generated induced pluripotent stem cell (iPSC) lines capturing disease stages that included preleukemia, low-risk myelodysplastic syndrome (MDS), high-risk MDS, and AML.
The researchers then used CRISPR/Cas9 genome editing to induce disease progression and reversal.
And they used the iPSCs to uncover disease-stage-specific effects of 2 drugs.
Eirini P. Papapetrou, MD, PhD, of the Icahn School of Medicine at Mount Sinai in New York, New York, and her colleagues described this work in Cell Stem Cell.
The researchers first explained how they generated patient-derived iPSCs that represented familial predisposition to myeloid malignancy, low-risk and high-risk MDS, and AML.
By studying these iPSC lines, the team uncovered “a phenotypic road map of disease progression” that led to a “serially transplantable leukemia.”
“We are encouraged by the discovery that it was possible to generate potent, engraftable leukemia derived from AML induced pluripotent stem cells,” said study author Michael G. Kharas, PhD, of the Icahn School of Medicine at Mount Sinai.
The researchers also showed that they could revert a high-risk MDS iPSC line to a premalignant state by correcting a chromosome 7q deletion.
And they could force progression in a preleukemic iPSC line. The team induced progression to low-risk MDS by inactivating the second GATA2 allele and progression to high-risk MDS by deleting chromosome 7q.
“This work shows that integrated patient cell reprogramming and cancer genetics is a powerful way to dissect cancer progression,” Dr Kharas said.
The researchers reported that, ultimately, they were able to model the stepwise progression of normal cells to preleukemia and MDS by sequentially introducing genetic lesions associated with earlier and later disease stages (ASXL1 truncation and chromosome 7q deletion, respectively).
“The new model will empower investigation into the cellular and molecular events underlying the development of leukemia in ways that were not possible before,” Dr Papapetrou said.
She added that the group’s findings provide a framework to aid investigation into disease mechanisms, events driving progression, and drug responses.
In fact, the researchers did use hematopoietic progenitor cells (HPCs) derived from their iPSCs to analyze the disease-stage-specific effects of 2 drugs—5-azacytidine and rigosertib.
The team said they found evidence to suggest that 5-azacytidine may work in low-risk MDS by affecting differentiation, and the drug’s main therapeutic action in high-risk MDS might be mediated through selective inhibition of the MDS clone.
The researchers tested rigosertib in HPCs derived from 2 AML lines (from the same patient) that captured 2 different disease stages. One line was derived from the dominant clone (del 7q), and the other was derived from a KRAS-mutated subclone.
The team found that HPCs derived from the KRAS-mutated line demonstrated “marked sensitivity” to rigosertib, but the other HPCs were “marginally affected.” ![]()
Researchers say they have created a model that shows the step-by-step progression from normal blood cells to acute myeloid leukemia (AML).
The team generated induced pluripotent stem cell (iPSC) lines capturing disease stages that included preleukemia, low-risk myelodysplastic syndrome (MDS), high-risk MDS, and AML.
The researchers then used CRISPR/Cas9 genome editing to induce disease progression and reversal.
And they used the iPSCs to uncover disease-stage-specific effects of 2 drugs.
Eirini P. Papapetrou, MD, PhD, of the Icahn School of Medicine at Mount Sinai in New York, New York, and her colleagues described this work in Cell Stem Cell.
The researchers first explained how they generated patient-derived iPSCs that represented familial predisposition to myeloid malignancy, low-risk and high-risk MDS, and AML.
By studying these iPSC lines, the team uncovered “a phenotypic road map of disease progression” that led to a “serially transplantable leukemia.”
“We are encouraged by the discovery that it was possible to generate potent, engraftable leukemia derived from AML induced pluripotent stem cells,” said study author Michael G. Kharas, PhD, of the Icahn School of Medicine at Mount Sinai.
The researchers also showed that they could revert a high-risk MDS iPSC line to a premalignant state by correcting a chromosome 7q deletion.
And they could force progression in a preleukemic iPSC line. The team induced progression to low-risk MDS by inactivating the second GATA2 allele and progression to high-risk MDS by deleting chromosome 7q.
“This work shows that integrated patient cell reprogramming and cancer genetics is a powerful way to dissect cancer progression,” Dr Kharas said.
The researchers reported that, ultimately, they were able to model the stepwise progression of normal cells to preleukemia and MDS by sequentially introducing genetic lesions associated with earlier and later disease stages (ASXL1 truncation and chromosome 7q deletion, respectively).
“The new model will empower investigation into the cellular and molecular events underlying the development of leukemia in ways that were not possible before,” Dr Papapetrou said.
She added that the group’s findings provide a framework to aid investigation into disease mechanisms, events driving progression, and drug responses.
In fact, the researchers did use hematopoietic progenitor cells (HPCs) derived from their iPSCs to analyze the disease-stage-specific effects of 2 drugs—5-azacytidine and rigosertib.
The team said they found evidence to suggest that 5-azacytidine may work in low-risk MDS by affecting differentiation, and the drug’s main therapeutic action in high-risk MDS might be mediated through selective inhibition of the MDS clone.
The researchers tested rigosertib in HPCs derived from 2 AML lines (from the same patient) that captured 2 different disease stages. One line was derived from the dominant clone (del 7q), and the other was derived from a KRAS-mutated subclone.
The team found that HPCs derived from the KRAS-mutated line demonstrated “marked sensitivity” to rigosertib, but the other HPCs were “marginally affected.” ![]()
Researchers say they have created a model that shows the step-by-step progression from normal blood cells to acute myeloid leukemia (AML).
The team generated induced pluripotent stem cell (iPSC) lines capturing disease stages that included preleukemia, low-risk myelodysplastic syndrome (MDS), high-risk MDS, and AML.
The researchers then used CRISPR/Cas9 genome editing to induce disease progression and reversal.
And they used the iPSCs to uncover disease-stage-specific effects of 2 drugs.
Eirini P. Papapetrou, MD, PhD, of the Icahn School of Medicine at Mount Sinai in New York, New York, and her colleagues described this work in Cell Stem Cell.
The researchers first explained how they generated patient-derived iPSCs that represented familial predisposition to myeloid malignancy, low-risk and high-risk MDS, and AML.
By studying these iPSC lines, the team uncovered “a phenotypic road map of disease progression” that led to a “serially transplantable leukemia.”
“We are encouraged by the discovery that it was possible to generate potent, engraftable leukemia derived from AML induced pluripotent stem cells,” said study author Michael G. Kharas, PhD, of the Icahn School of Medicine at Mount Sinai.
The researchers also showed that they could revert a high-risk MDS iPSC line to a premalignant state by correcting a chromosome 7q deletion.
And they could force progression in a preleukemic iPSC line. The team induced progression to low-risk MDS by inactivating the second GATA2 allele and progression to high-risk MDS by deleting chromosome 7q.
“This work shows that integrated patient cell reprogramming and cancer genetics is a powerful way to dissect cancer progression,” Dr Kharas said.
The researchers reported that, ultimately, they were able to model the stepwise progression of normal cells to preleukemia and MDS by sequentially introducing genetic lesions associated with earlier and later disease stages (ASXL1 truncation and chromosome 7q deletion, respectively).
“The new model will empower investigation into the cellular and molecular events underlying the development of leukemia in ways that were not possible before,” Dr Papapetrou said.
She added that the group’s findings provide a framework to aid investigation into disease mechanisms, events driving progression, and drug responses.
In fact, the researchers did use hematopoietic progenitor cells (HPCs) derived from their iPSCs to analyze the disease-stage-specific effects of 2 drugs—5-azacytidine and rigosertib.
The team said they found evidence to suggest that 5-azacytidine may work in low-risk MDS by affecting differentiation, and the drug’s main therapeutic action in high-risk MDS might be mediated through selective inhibition of the MDS clone.
The researchers tested rigosertib in HPCs derived from 2 AML lines (from the same patient) that captured 2 different disease stages. One line was derived from the dominant clone (del 7q), and the other was derived from a KRAS-mutated subclone.
The team found that HPCs derived from the KRAS-mutated line demonstrated “marked sensitivity” to rigosertib, but the other HPCs were “marginally affected.” ![]()