User login
Ingredient in aspirin may help fight leukemia

Photo credit: Jill Watson
Researchers say they may have found a new use for an ancient anti-inflammatory drug, salicylate, which was first described by the Greek physician Hippocrates.
“Salicylic acid is one of the oldest drugs on the planet,” said senior author Eric Verdin, MD, of the Gladstone Institutes/UCSF, “dating back to the Egyptians and the Greeks, but we're still discovering new things about it.”
Its derivatives, acetylsalicylic acid, or aspirin, and diflunisal suppress 2 key proteins, CREB-binding protein (CBP) and p300, which control levels of proteins that cause inflammation or are involved in cell growth.
By inhibiting p300 and CBP, salicylic acid and diflunisal block the activation of these proteins and prevent cellular damage caused by inflammation. The research team believes that both p300 and CBP can be targeted by drugs, which would have important clinical implications.
Earlier research conducted by coauthor Stephen D. Nimer, MD, of the University of Miami Miller School of Medicine in Florida, and colleagues found a link between p300 and the leukemia-promoting protein AML1-ETO.
In the current study, published in eLife, researchers tested whether suppressing p300 with diflunisal would suppress leukemia growth in mice.
The research team inoculated SCID mice with Kasumi-1 cells, an AML cell line with the t(8;21) translocation, which represents one of the subtypes of CBF leukemia.
Three weeks later, they started treating the mice with diflunisal. Diflunisal reduced tumor size in a dose-dependent manner, and after 3 weeks of treatment, the tumors were significantly smaller than in the vehicle-treated control mice.
In fact, the researchers noted most tumors disappeared in mice treated with higher doses of diflunisal. The researchers also pointed out that diflunisal, an FDA-approved drug containing a salicylic acid substructure, inhibited CBP/p300 more potently than salicylate.
The researchers concluded that diflunisal and salicylate have promise as an oral therapy for AML patients with a t(8;21) translocation, and called it “an exciting potential application” for this new characterization of older drugs.
“Uncovering this pathway of inflammation that salicylic acid acts upon opens up a host of new clinical possibilities for these drugs,” Dr Verdin added.
The scientists are now pursuing a clinical trial to test whether salicylic acid can treat patients with leukemia as part of novel combination therapies. ![]()

Photo credit: Jill Watson
Researchers say they may have found a new use for an ancient anti-inflammatory drug, salicylate, which was first described by the Greek physician Hippocrates.
“Salicylic acid is one of the oldest drugs on the planet,” said senior author Eric Verdin, MD, of the Gladstone Institutes/UCSF, “dating back to the Egyptians and the Greeks, but we're still discovering new things about it.”
Its derivatives, acetylsalicylic acid, or aspirin, and diflunisal suppress 2 key proteins, CREB-binding protein (CBP) and p300, which control levels of proteins that cause inflammation or are involved in cell growth.
By inhibiting p300 and CBP, salicylic acid and diflunisal block the activation of these proteins and prevent cellular damage caused by inflammation. The research team believes that both p300 and CBP can be targeted by drugs, which would have important clinical implications.
Earlier research conducted by coauthor Stephen D. Nimer, MD, of the University of Miami Miller School of Medicine in Florida, and colleagues found a link between p300 and the leukemia-promoting protein AML1-ETO.
In the current study, published in eLife, researchers tested whether suppressing p300 with diflunisal would suppress leukemia growth in mice.
The research team inoculated SCID mice with Kasumi-1 cells, an AML cell line with the t(8;21) translocation, which represents one of the subtypes of CBF leukemia.
Three weeks later, they started treating the mice with diflunisal. Diflunisal reduced tumor size in a dose-dependent manner, and after 3 weeks of treatment, the tumors were significantly smaller than in the vehicle-treated control mice.
In fact, the researchers noted most tumors disappeared in mice treated with higher doses of diflunisal. The researchers also pointed out that diflunisal, an FDA-approved drug containing a salicylic acid substructure, inhibited CBP/p300 more potently than salicylate.
The researchers concluded that diflunisal and salicylate have promise as an oral therapy for AML patients with a t(8;21) translocation, and called it “an exciting potential application” for this new characterization of older drugs.
“Uncovering this pathway of inflammation that salicylic acid acts upon opens up a host of new clinical possibilities for these drugs,” Dr Verdin added.
The scientists are now pursuing a clinical trial to test whether salicylic acid can treat patients with leukemia as part of novel combination therapies. ![]()

Photo credit: Jill Watson
Researchers say they may have found a new use for an ancient anti-inflammatory drug, salicylate, which was first described by the Greek physician Hippocrates.
“Salicylic acid is one of the oldest drugs on the planet,” said senior author Eric Verdin, MD, of the Gladstone Institutes/UCSF, “dating back to the Egyptians and the Greeks, but we're still discovering new things about it.”
Its derivatives, acetylsalicylic acid, or aspirin, and diflunisal suppress 2 key proteins, CREB-binding protein (CBP) and p300, which control levels of proteins that cause inflammation or are involved in cell growth.
By inhibiting p300 and CBP, salicylic acid and diflunisal block the activation of these proteins and prevent cellular damage caused by inflammation. The research team believes that both p300 and CBP can be targeted by drugs, which would have important clinical implications.
Earlier research conducted by coauthor Stephen D. Nimer, MD, of the University of Miami Miller School of Medicine in Florida, and colleagues found a link between p300 and the leukemia-promoting protein AML1-ETO.
In the current study, published in eLife, researchers tested whether suppressing p300 with diflunisal would suppress leukemia growth in mice.
The research team inoculated SCID mice with Kasumi-1 cells, an AML cell line with the t(8;21) translocation, which represents one of the subtypes of CBF leukemia.
Three weeks later, they started treating the mice with diflunisal. Diflunisal reduced tumor size in a dose-dependent manner, and after 3 weeks of treatment, the tumors were significantly smaller than in the vehicle-treated control mice.
In fact, the researchers noted most tumors disappeared in mice treated with higher doses of diflunisal. The researchers also pointed out that diflunisal, an FDA-approved drug containing a salicylic acid substructure, inhibited CBP/p300 more potently than salicylate.
The researchers concluded that diflunisal and salicylate have promise as an oral therapy for AML patients with a t(8;21) translocation, and called it “an exciting potential application” for this new characterization of older drugs.
“Uncovering this pathway of inflammation that salicylic acid acts upon opens up a host of new clinical possibilities for these drugs,” Dr Verdin added.
The scientists are now pursuing a clinical trial to test whether salicylic acid can treat patients with leukemia as part of novel combination therapies. ![]()
AYAs still fare worse than kids with leukemia, lymphoma

patient and her father
Photo by Rhoda Baer
Adolescents and young adults (AYAs) are less likely than children to survive 8 relatively common types of cancer, according to a long-running study of cancer survival across Europe.
The study showed that AYAs had significantly worse survival rates than children if they were diagnosed with acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin or non-Hodgkin lymphoma (NHL), and 4 types of solid tumor malignancies.
The study’s authors say that variations in survival between age groups are due to a number of factors, including delays in diagnosis and treatment, a lack of treatment guidelines and clinical trials specifically for AYAs, and differences in the biology of some cancers.
“The good news is that the number of children, adolescents, and young adults surviving for at least 5 years after diagnosis has risen steadily over time in Europe,” said author Annalisa Trama, PhD, of The National Institute of Cancer (Istituto Nazionale dei Tumori: Fondazione IRCCS) in Milan, Italy.
“Across all cancers, the level of improvement is similar in these age groups. This contrasts with earlier results that adolescents and young adults diagnosed up to the 1990s were lagging behind children in terms of survival.”
“However, we found that adolescents and young adults still tend to die earlier than children for several cancers common to these age groups, particularly blood cancers like leukemias and non-Hodgkin’s lymphoma.”
Dr Trama and her colleagues reported these findings in The Lancet Oncology.
The researchers compared survival between AYAs (ages 15 to 39), children (ages 0 to 14), and adults (ages 40 to 69) who were diagnosed from 2000 to 2007 and followed up to at least 2008.
The team analyzed data from population-based cancer registries covering all or part of 27 European countries* and estimated 5-year survival for 56,505 cancer cases in children; 312,483 in AYAs; and 3,567,383 in adults. The researchers also analyzed changes in survival over time from 1999 to 2007.
For AYAs, survival at 5 years from diagnosis for all cancers combined was 82% for 2005-2007, which is up from 79% for 1999-2001 (P<0.0001). In children, survival improved from 76% to 79% over the same time period (P<0.0001).
Survival improved significantly in children and AYAs for ALL (P<0.0001) and NHL (P<0.0001 in AYAs and P=0.023 in children). On the other hand, between 1999 and 2007, survival rates remained unchanged for AYAs with AML (around 50%).
Overall, AYAs had slightly better 5-year survival than children because they were diagnosed more often with cancers with fairly good prognoses—Hodgkin lymphoma, NHL, germ cell tumors, melanoma, thyroid cancer, and breast cancer.
However, the overall survival rates conceal differences between specific cancers. Survival was significantly worse for AYAs than for children when it came to 8 relatively common cancers affecting both age groups:
- ALL—55.6% for AYAs and 85.8% for children (P<0.0001)
- AML—49.8% and 60.5%, respectively (P<0.0001)
- Hodgkin lymphoma—92.9% and 95.1%, respectively (P<0.0001)
- NHL—77.4% and 83.0%, respectively (P<0.0001)
- Astrocytomas—46.4% and 61.9%, respectively (P<0.0001)
- Ewing’s sarcoma of bone—49.3% and 66.6%, respectively (P<0.0001)
- Rhabdomyosarcoma—37.8% and 66.6%, respectively (P<0.0001)
- Osteosarcoma—61.5% and 66.8%, respectively (P=0.011).
AYAs had a survival advantage over adults for almost all major cancers affecting both age groups, supporting the idea that younger patients with few other illnesses are likely to fare better than older patients.
There are only 2 types of cancer for which AYAs were at a survival disadvantage—breast (83.5% vs 87.0%) and prostate (79.9% vs 89.8%).
Dr Trama and her colleagues pointed out that this analysis pre-dates recent initiatives to improve outcomes for AYAs that have been implemented in several European countries.
“The European Network for Teenagers and Young Adults with Cancer is advocating collaboration between pediatric and adult oncologists, greater access to clinical trials and research to improve treatments for this specific age group, as well as developing adolescent and young adult-specific practice guidelines, encouraging healthier lifestyles and the greater involvement of patients and patients support groups,” Dr Trama said.
“This study will provide an important starting point from which to evaluate whether these initiatives will reduce the gulf in survival between European adolescents and young adults and children with cancer.” ![]()
*Finland, Iceland, Norway, Sweden, England, Ireland, Northern Ireland, Scotland, Wales, Austria, Belgium, France, Germany, Netherlands, Switzerland, Croatia, Italy, Malta, Portugal, Slovenia, Spain, Bulgaria, Estonia, Latvia, Lithuania, Poland, and Slovakia

patient and her father
Photo by Rhoda Baer
Adolescents and young adults (AYAs) are less likely than children to survive 8 relatively common types of cancer, according to a long-running study of cancer survival across Europe.
The study showed that AYAs had significantly worse survival rates than children if they were diagnosed with acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin or non-Hodgkin lymphoma (NHL), and 4 types of solid tumor malignancies.
The study’s authors say that variations in survival between age groups are due to a number of factors, including delays in diagnosis and treatment, a lack of treatment guidelines and clinical trials specifically for AYAs, and differences in the biology of some cancers.
“The good news is that the number of children, adolescents, and young adults surviving for at least 5 years after diagnosis has risen steadily over time in Europe,” said author Annalisa Trama, PhD, of The National Institute of Cancer (Istituto Nazionale dei Tumori: Fondazione IRCCS) in Milan, Italy.
“Across all cancers, the level of improvement is similar in these age groups. This contrasts with earlier results that adolescents and young adults diagnosed up to the 1990s were lagging behind children in terms of survival.”
“However, we found that adolescents and young adults still tend to die earlier than children for several cancers common to these age groups, particularly blood cancers like leukemias and non-Hodgkin’s lymphoma.”
Dr Trama and her colleagues reported these findings in The Lancet Oncology.
The researchers compared survival between AYAs (ages 15 to 39), children (ages 0 to 14), and adults (ages 40 to 69) who were diagnosed from 2000 to 2007 and followed up to at least 2008.
The team analyzed data from population-based cancer registries covering all or part of 27 European countries* and estimated 5-year survival for 56,505 cancer cases in children; 312,483 in AYAs; and 3,567,383 in adults. The researchers also analyzed changes in survival over time from 1999 to 2007.
For AYAs, survival at 5 years from diagnosis for all cancers combined was 82% for 2005-2007, which is up from 79% for 1999-2001 (P<0.0001). In children, survival improved from 76% to 79% over the same time period (P<0.0001).
Survival improved significantly in children and AYAs for ALL (P<0.0001) and NHL (P<0.0001 in AYAs and P=0.023 in children). On the other hand, between 1999 and 2007, survival rates remained unchanged for AYAs with AML (around 50%).
Overall, AYAs had slightly better 5-year survival than children because they were diagnosed more often with cancers with fairly good prognoses—Hodgkin lymphoma, NHL, germ cell tumors, melanoma, thyroid cancer, and breast cancer.
However, the overall survival rates conceal differences between specific cancers. Survival was significantly worse for AYAs than for children when it came to 8 relatively common cancers affecting both age groups:
- ALL—55.6% for AYAs and 85.8% for children (P<0.0001)
- AML—49.8% and 60.5%, respectively (P<0.0001)
- Hodgkin lymphoma—92.9% and 95.1%, respectively (P<0.0001)
- NHL—77.4% and 83.0%, respectively (P<0.0001)
- Astrocytomas—46.4% and 61.9%, respectively (P<0.0001)
- Ewing’s sarcoma of bone—49.3% and 66.6%, respectively (P<0.0001)
- Rhabdomyosarcoma—37.8% and 66.6%, respectively (P<0.0001)
- Osteosarcoma—61.5% and 66.8%, respectively (P=0.011).
AYAs had a survival advantage over adults for almost all major cancers affecting both age groups, supporting the idea that younger patients with few other illnesses are likely to fare better than older patients.
There are only 2 types of cancer for which AYAs were at a survival disadvantage—breast (83.5% vs 87.0%) and prostate (79.9% vs 89.8%).
Dr Trama and her colleagues pointed out that this analysis pre-dates recent initiatives to improve outcomes for AYAs that have been implemented in several European countries.
“The European Network for Teenagers and Young Adults with Cancer is advocating collaboration between pediatric and adult oncologists, greater access to clinical trials and research to improve treatments for this specific age group, as well as developing adolescent and young adult-specific practice guidelines, encouraging healthier lifestyles and the greater involvement of patients and patients support groups,” Dr Trama said.
“This study will provide an important starting point from which to evaluate whether these initiatives will reduce the gulf in survival between European adolescents and young adults and children with cancer.” ![]()
*Finland, Iceland, Norway, Sweden, England, Ireland, Northern Ireland, Scotland, Wales, Austria, Belgium, France, Germany, Netherlands, Switzerland, Croatia, Italy, Malta, Portugal, Slovenia, Spain, Bulgaria, Estonia, Latvia, Lithuania, Poland, and Slovakia

patient and her father
Photo by Rhoda Baer
Adolescents and young adults (AYAs) are less likely than children to survive 8 relatively common types of cancer, according to a long-running study of cancer survival across Europe.
The study showed that AYAs had significantly worse survival rates than children if they were diagnosed with acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin or non-Hodgkin lymphoma (NHL), and 4 types of solid tumor malignancies.
The study’s authors say that variations in survival between age groups are due to a number of factors, including delays in diagnosis and treatment, a lack of treatment guidelines and clinical trials specifically for AYAs, and differences in the biology of some cancers.
“The good news is that the number of children, adolescents, and young adults surviving for at least 5 years after diagnosis has risen steadily over time in Europe,” said author Annalisa Trama, PhD, of The National Institute of Cancer (Istituto Nazionale dei Tumori: Fondazione IRCCS) in Milan, Italy.
“Across all cancers, the level of improvement is similar in these age groups. This contrasts with earlier results that adolescents and young adults diagnosed up to the 1990s were lagging behind children in terms of survival.”
“However, we found that adolescents and young adults still tend to die earlier than children for several cancers common to these age groups, particularly blood cancers like leukemias and non-Hodgkin’s lymphoma.”
Dr Trama and her colleagues reported these findings in The Lancet Oncology.
The researchers compared survival between AYAs (ages 15 to 39), children (ages 0 to 14), and adults (ages 40 to 69) who were diagnosed from 2000 to 2007 and followed up to at least 2008.
The team analyzed data from population-based cancer registries covering all or part of 27 European countries* and estimated 5-year survival for 56,505 cancer cases in children; 312,483 in AYAs; and 3,567,383 in adults. The researchers also analyzed changes in survival over time from 1999 to 2007.
For AYAs, survival at 5 years from diagnosis for all cancers combined was 82% for 2005-2007, which is up from 79% for 1999-2001 (P<0.0001). In children, survival improved from 76% to 79% over the same time period (P<0.0001).
Survival improved significantly in children and AYAs for ALL (P<0.0001) and NHL (P<0.0001 in AYAs and P=0.023 in children). On the other hand, between 1999 and 2007, survival rates remained unchanged for AYAs with AML (around 50%).
Overall, AYAs had slightly better 5-year survival than children because they were diagnosed more often with cancers with fairly good prognoses—Hodgkin lymphoma, NHL, germ cell tumors, melanoma, thyroid cancer, and breast cancer.
However, the overall survival rates conceal differences between specific cancers. Survival was significantly worse for AYAs than for children when it came to 8 relatively common cancers affecting both age groups:
- ALL—55.6% for AYAs and 85.8% for children (P<0.0001)
- AML—49.8% and 60.5%, respectively (P<0.0001)
- Hodgkin lymphoma—92.9% and 95.1%, respectively (P<0.0001)
- NHL—77.4% and 83.0%, respectively (P<0.0001)
- Astrocytomas—46.4% and 61.9%, respectively (P<0.0001)
- Ewing’s sarcoma of bone—49.3% and 66.6%, respectively (P<0.0001)
- Rhabdomyosarcoma—37.8% and 66.6%, respectively (P<0.0001)
- Osteosarcoma—61.5% and 66.8%, respectively (P=0.011).
AYAs had a survival advantage over adults for almost all major cancers affecting both age groups, supporting the idea that younger patients with few other illnesses are likely to fare better than older patients.
There are only 2 types of cancer for which AYAs were at a survival disadvantage—breast (83.5% vs 87.0%) and prostate (79.9% vs 89.8%).
Dr Trama and her colleagues pointed out that this analysis pre-dates recent initiatives to improve outcomes for AYAs that have been implemented in several European countries.
“The European Network for Teenagers and Young Adults with Cancer is advocating collaboration between pediatric and adult oncologists, greater access to clinical trials and research to improve treatments for this specific age group, as well as developing adolescent and young adult-specific practice guidelines, encouraging healthier lifestyles and the greater involvement of patients and patients support groups,” Dr Trama said.
“This study will provide an important starting point from which to evaluate whether these initiatives will reduce the gulf in survival between European adolescents and young adults and children with cancer.” ![]()
*Finland, Iceland, Norway, Sweden, England, Ireland, Northern Ireland, Scotland, Wales, Austria, Belgium, France, Germany, Netherlands, Switzerland, Croatia, Italy, Malta, Portugal, Slovenia, Spain, Bulgaria, Estonia, Latvia, Lithuania, Poland, and Slovakia
Dr. Matt Kalaycio’s top 10 hematologic oncology abstracts for ASCO 2016
Hematology News’ Editor-in-Chief Matt Kalaycio selected the following as his “top 10” picks for hematologic oncology abstracts at ASCO 2016:
Abstract 7000: Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML
Comment: When any treatment appears to improve survival, compared with 7+3 for AML, all must take notice.
Abstract 7001: Treatment-free remission (TFR) in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) treated with frontline nilotinib: Results from the ENESTFreedom study
Comment: About 50% of the CML patients treated with frontline nilotinib are eventually able to stop the drug and successfully stay off of it. That means more patients in treatment-free remission, compared with those initially treated with imatinib.
Link to abstract 7001
Abstract 7007: Phase Ib/2 study of venetoclax with low-dose cytarabine in treatment-naive patients age ≥ 65 with acute myelogenous leukemia
Abstract 7009: Results of a phase 1b study of venetoclax plus decitabine or azacitidine in untreated acute myeloid leukemia patients ≥ 65 years ineligible for standard induction therapy
Comment: The response rates in these older AML patients are remarkable and challenge results typically seen with 7+3 in a younger population.
Link to abstract 7007 and 7009
Abstract 7501: A prospective, multicenter, randomized study of anti-CCR4 monoclonal antibody mogamulizumab (moga) vs investigator’s choice (IC) in the treatment of patients (pts) with relapsed/refractory (R/R) adult T-cell leukemia-lymphoma (ATL)
Comment: The response rate to mogamulizumab was outstanding in the largest randomized clinical trial thus far conducted for this cancer. Although rare in the USA, ATL is more common in Asia.
Link to abstract 7501
Abstract 7507: Effect of bortezomib on complete remission (CR) rate when added to bendamustine-rituximab (BR) in previously untreated high-risk (HR) follicular lymphoma (FL): A randomized phase II trial of the ECOG-ACRIN Cancer Research Group (E2408)
Comment: This interesting observation of improved complete remission needs longer follow-up.
Link to abstract 7507
Abstract 7519: Venetoclax activity in CLL patients who have relapsed after or are refractory to ibrutinib or idelalisib
Comment: This study has implications for practice. Venetoclax elicits a 50%-60% response rate after patients with CLL progress during treatment with B-cell receptor pathway inhibitors.
Link to abstract 7519
Abstract 7521: Acalabrutinib, a second-generation bruton tyrosine kinase (Btk) inhibitor, in previously untreated chronic lymphocytic leukemia (CLL)
Comment: This next-generation variation on ibrutinib was associated with a 96% overall response rate with fewer adverse effects such as atrial fibrillation.
Link to abstract 7521
Abstract 8000: Upfront autologous stem cell transplantation (ASCT) versus novel agent-based therapy for multiple myeloma (MM): A randomized phase 3 study of the European Myeloma Network (EMN02/HO95 MM trial)
Comment: Other trials are underway to address the role of upfront ASCT for newly diagnosed multiple myeloma. While the last word on this issue has yet to be written, ASCT remains the standard of care for MM patients after induction.
Link to abstract 8000
LBA4: Phase III randomized controlled study of daratumumab, bortezomib, and dexamethasone (DVd) versus bortezomib and dexamethasone (Vd) in patients (pts) with relapsed or refractory multiple myeloma (RRMM): CASTOR study
Comment: As predicted by most, the addition of daratumumab to bortezomib-based therapy increases response rates, compared with bortezomib-based alone. Efficacy is becoming less of a concern with myeloma treatment than is economics..
Look for the full, final text of this abstract to be posted online at 7:30 AM (EDT) on Sunday, June 5.
Hematology News’ Editor-in-Chief Matt Kalaycio selected the following as his “top 10” picks for hematologic oncology abstracts at ASCO 2016:
Abstract 7000: Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML
Comment: When any treatment appears to improve survival, compared with 7+3 for AML, all must take notice.
Abstract 7001: Treatment-free remission (TFR) in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) treated with frontline nilotinib: Results from the ENESTFreedom study
Comment: About 50% of the CML patients treated with frontline nilotinib are eventually able to stop the drug and successfully stay off of it. That means more patients in treatment-free remission, compared with those initially treated with imatinib.
Link to abstract 7001
Abstract 7007: Phase Ib/2 study of venetoclax with low-dose cytarabine in treatment-naive patients age ≥ 65 with acute myelogenous leukemia
Abstract 7009: Results of a phase 1b study of venetoclax plus decitabine or azacitidine in untreated acute myeloid leukemia patients ≥ 65 years ineligible for standard induction therapy
Comment: The response rates in these older AML patients are remarkable and challenge results typically seen with 7+3 in a younger population.
Link to abstract 7007 and 7009
Abstract 7501: A prospective, multicenter, randomized study of anti-CCR4 monoclonal antibody mogamulizumab (moga) vs investigator’s choice (IC) in the treatment of patients (pts) with relapsed/refractory (R/R) adult T-cell leukemia-lymphoma (ATL)
Comment: The response rate to mogamulizumab was outstanding in the largest randomized clinical trial thus far conducted for this cancer. Although rare in the USA, ATL is more common in Asia.
Link to abstract 7501
Abstract 7507: Effect of bortezomib on complete remission (CR) rate when added to bendamustine-rituximab (BR) in previously untreated high-risk (HR) follicular lymphoma (FL): A randomized phase II trial of the ECOG-ACRIN Cancer Research Group (E2408)
Comment: This interesting observation of improved complete remission needs longer follow-up.
Link to abstract 7507
Abstract 7519: Venetoclax activity in CLL patients who have relapsed after or are refractory to ibrutinib or idelalisib
Comment: This study has implications for practice. Venetoclax elicits a 50%-60% response rate after patients with CLL progress during treatment with B-cell receptor pathway inhibitors.
Link to abstract 7519
Abstract 7521: Acalabrutinib, a second-generation bruton tyrosine kinase (Btk) inhibitor, in previously untreated chronic lymphocytic leukemia (CLL)
Comment: This next-generation variation on ibrutinib was associated with a 96% overall response rate with fewer adverse effects such as atrial fibrillation.
Link to abstract 7521
Abstract 8000: Upfront autologous stem cell transplantation (ASCT) versus novel agent-based therapy for multiple myeloma (MM): A randomized phase 3 study of the European Myeloma Network (EMN02/HO95 MM trial)
Comment: Other trials are underway to address the role of upfront ASCT for newly diagnosed multiple myeloma. While the last word on this issue has yet to be written, ASCT remains the standard of care for MM patients after induction.
Link to abstract 8000
LBA4: Phase III randomized controlled study of daratumumab, bortezomib, and dexamethasone (DVd) versus bortezomib and dexamethasone (Vd) in patients (pts) with relapsed or refractory multiple myeloma (RRMM): CASTOR study
Comment: As predicted by most, the addition of daratumumab to bortezomib-based therapy increases response rates, compared with bortezomib-based alone. Efficacy is becoming less of a concern with myeloma treatment than is economics..
Look for the full, final text of this abstract to be posted online at 7:30 AM (EDT) on Sunday, June 5.
Hematology News’ Editor-in-Chief Matt Kalaycio selected the following as his “top 10” picks for hematologic oncology abstracts at ASCO 2016:
Abstract 7000: Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML
Comment: When any treatment appears to improve survival, compared with 7+3 for AML, all must take notice.
Abstract 7001: Treatment-free remission (TFR) in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) treated with frontline nilotinib: Results from the ENESTFreedom study
Comment: About 50% of the CML patients treated with frontline nilotinib are eventually able to stop the drug and successfully stay off of it. That means more patients in treatment-free remission, compared with those initially treated with imatinib.
Link to abstract 7001
Abstract 7007: Phase Ib/2 study of venetoclax with low-dose cytarabine in treatment-naive patients age ≥ 65 with acute myelogenous leukemia
Abstract 7009: Results of a phase 1b study of venetoclax plus decitabine or azacitidine in untreated acute myeloid leukemia patients ≥ 65 years ineligible for standard induction therapy
Comment: The response rates in these older AML patients are remarkable and challenge results typically seen with 7+3 in a younger population.
Link to abstract 7007 and 7009
Abstract 7501: A prospective, multicenter, randomized study of anti-CCR4 monoclonal antibody mogamulizumab (moga) vs investigator’s choice (IC) in the treatment of patients (pts) with relapsed/refractory (R/R) adult T-cell leukemia-lymphoma (ATL)
Comment: The response rate to mogamulizumab was outstanding in the largest randomized clinical trial thus far conducted for this cancer. Although rare in the USA, ATL is more common in Asia.
Link to abstract 7501
Abstract 7507: Effect of bortezomib on complete remission (CR) rate when added to bendamustine-rituximab (BR) in previously untreated high-risk (HR) follicular lymphoma (FL): A randomized phase II trial of the ECOG-ACRIN Cancer Research Group (E2408)
Comment: This interesting observation of improved complete remission needs longer follow-up.
Link to abstract 7507
Abstract 7519: Venetoclax activity in CLL patients who have relapsed after or are refractory to ibrutinib or idelalisib
Comment: This study has implications for practice. Venetoclax elicits a 50%-60% response rate after patients with CLL progress during treatment with B-cell receptor pathway inhibitors.
Link to abstract 7519
Abstract 7521: Acalabrutinib, a second-generation bruton tyrosine kinase (Btk) inhibitor, in previously untreated chronic lymphocytic leukemia (CLL)
Comment: This next-generation variation on ibrutinib was associated with a 96% overall response rate with fewer adverse effects such as atrial fibrillation.
Link to abstract 7521
Abstract 8000: Upfront autologous stem cell transplantation (ASCT) versus novel agent-based therapy for multiple myeloma (MM): A randomized phase 3 study of the European Myeloma Network (EMN02/HO95 MM trial)
Comment: Other trials are underway to address the role of upfront ASCT for newly diagnosed multiple myeloma. While the last word on this issue has yet to be written, ASCT remains the standard of care for MM patients after induction.
Link to abstract 8000
LBA4: Phase III randomized controlled study of daratumumab, bortezomib, and dexamethasone (DVd) versus bortezomib and dexamethasone (Vd) in patients (pts) with relapsed or refractory multiple myeloma (RRMM): CASTOR study
Comment: As predicted by most, the addition of daratumumab to bortezomib-based therapy increases response rates, compared with bortezomib-based alone. Efficacy is becoming less of a concern with myeloma treatment than is economics..
Look for the full, final text of this abstract to be posted online at 7:30 AM (EDT) on Sunday, June 5.
Team simplifies synthesis of anticancer agent

Photo courtesy of Jeff Fitlow
and Rice University
Researchers say they have streamlined synthesis of delta12-prostaglandin J3, a molecule that has been shown to kill leukemia cells.
Total synthesis of the molecule now requires only 6 steps from commercially available starting materials.
The researchers say this work sets the stage for large-scale synthesis of the molecule—a lipid found in nearly all animal tissues—and related compounds that can be produced as potential anticancer agents.
K.C. Nicolaou, PhD, of Rice University in Houston, Texas, and his colleagues described the work in Chemistry - A European Journal.
The prostaglandin the researchers synthesized had been isolated in 2011 as a secondary metabolite formed from eicosapentaenoic acid, which is found primarily in fish oil.
The team reported the first total synthesis of the molecule in 2014. That allowed them to confirm its structure.
Now, the researchers have established techniques to develop related disease-fighting compounds and ramp up bulk production if necessary.
Several such prostaglandin derivatives under consideration as preclinical drug candidates were detailed in a second paper published in the Journal of the American Chemical Society.
That publication described the synthesis of dozens of prostaglandin derivatives that were tested against a range of cancer cells by the National Cancer Institute.
One such derivative, macrolactone 11, is currently under evaluation as a preclinical drug candidate. Related compounds macrolactone 33 and 44 showed evidence of even higher potency against leukemia, lung cancer, colon cancer, melanoma, renal, and prostate cancer.
“The macrolactones are very good—better than the natural product—and now we’re following this lead to optimize the potency while minimizing toxicity,” Dr Nicolaou said. “It’s a balancing act.”
In addition, he and his colleagues are developing other drug candidates based on prostaglandin.
“In the process, we’ve developed a lot of nice chemistry, and we know a lot more about the biology of this compound,” Dr Nicolaou said. “We’ve advanced organic synthesis in general and also enriched the knowledge about how these kinds of molecules behave. We hope the papers provide some ideas and leads and inspiration for others to follow.” ![]()

Photo courtesy of Jeff Fitlow
and Rice University
Researchers say they have streamlined synthesis of delta12-prostaglandin J3, a molecule that has been shown to kill leukemia cells.
Total synthesis of the molecule now requires only 6 steps from commercially available starting materials.
The researchers say this work sets the stage for large-scale synthesis of the molecule—a lipid found in nearly all animal tissues—and related compounds that can be produced as potential anticancer agents.
K.C. Nicolaou, PhD, of Rice University in Houston, Texas, and his colleagues described the work in Chemistry - A European Journal.
The prostaglandin the researchers synthesized had been isolated in 2011 as a secondary metabolite formed from eicosapentaenoic acid, which is found primarily in fish oil.
The team reported the first total synthesis of the molecule in 2014. That allowed them to confirm its structure.
Now, the researchers have established techniques to develop related disease-fighting compounds and ramp up bulk production if necessary.
Several such prostaglandin derivatives under consideration as preclinical drug candidates were detailed in a second paper published in the Journal of the American Chemical Society.
That publication described the synthesis of dozens of prostaglandin derivatives that were tested against a range of cancer cells by the National Cancer Institute.
One such derivative, macrolactone 11, is currently under evaluation as a preclinical drug candidate. Related compounds macrolactone 33 and 44 showed evidence of even higher potency against leukemia, lung cancer, colon cancer, melanoma, renal, and prostate cancer.
“The macrolactones are very good—better than the natural product—and now we’re following this lead to optimize the potency while minimizing toxicity,” Dr Nicolaou said. “It’s a balancing act.”
In addition, he and his colleagues are developing other drug candidates based on prostaglandin.
“In the process, we’ve developed a lot of nice chemistry, and we know a lot more about the biology of this compound,” Dr Nicolaou said. “We’ve advanced organic synthesis in general and also enriched the knowledge about how these kinds of molecules behave. We hope the papers provide some ideas and leads and inspiration for others to follow.” ![]()

Photo courtesy of Jeff Fitlow
and Rice University
Researchers say they have streamlined synthesis of delta12-prostaglandin J3, a molecule that has been shown to kill leukemia cells.
Total synthesis of the molecule now requires only 6 steps from commercially available starting materials.
The researchers say this work sets the stage for large-scale synthesis of the molecule—a lipid found in nearly all animal tissues—and related compounds that can be produced as potential anticancer agents.
K.C. Nicolaou, PhD, of Rice University in Houston, Texas, and his colleagues described the work in Chemistry - A European Journal.
The prostaglandin the researchers synthesized had been isolated in 2011 as a secondary metabolite formed from eicosapentaenoic acid, which is found primarily in fish oil.
The team reported the first total synthesis of the molecule in 2014. That allowed them to confirm its structure.
Now, the researchers have established techniques to develop related disease-fighting compounds and ramp up bulk production if necessary.
Several such prostaglandin derivatives under consideration as preclinical drug candidates were detailed in a second paper published in the Journal of the American Chemical Society.
That publication described the synthesis of dozens of prostaglandin derivatives that were tested against a range of cancer cells by the National Cancer Institute.
One such derivative, macrolactone 11, is currently under evaluation as a preclinical drug candidate. Related compounds macrolactone 33 and 44 showed evidence of even higher potency against leukemia, lung cancer, colon cancer, melanoma, renal, and prostate cancer.
“The macrolactones are very good—better than the natural product—and now we’re following this lead to optimize the potency while minimizing toxicity,” Dr Nicolaou said. “It’s a balancing act.”
In addition, he and his colleagues are developing other drug candidates based on prostaglandin.
“In the process, we’ve developed a lot of nice chemistry, and we know a lot more about the biology of this compound,” Dr Nicolaou said. “We’ve advanced organic synthesis in general and also enriched the knowledge about how these kinds of molecules behave. We hope the papers provide some ideas and leads and inspiration for others to follow.” ![]()
Drug granted breakthrough designation for AML

Image by Lance Liotta
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for CPX-351 (Vyxeos), a fixed-ratio combination of cytarabine and daunorubicin inside a lipid vesicle, to treat adults with therapy-related acute myeloid leukemia (AML) or AML with myelodysplasia-related changes.
The FDA’s breakthrough therapy designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
To earn the designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
Phase 3 trial
The breakthrough designation for CPX-351 is primarily based on positive results from a phase 3 trial in older patients with previously untreated, high-risk, secondary AML.
The trial was sponsored by Celator Pharmaceuticals, Inc., the company developing CPX-351.
The company has released some results from the trial, and additional data are scheduled to be presented at the 2016 ASCO Annual Meeting (abstract 7000).
The trial enrolled 309 patients, ages 60 to 75, with one of the following:
- Untreated AML and a history of prior cytotoxic treatment
- Antecedent myelodysplastic syndrome (MDS) or chronic myelomonocytic leukemia, with or without prior hypomethylating therapy
- AML with WHO-defined, MDS-related cytogenetic abnormalities.
One hundred and fifty-three patients were randomized to receive CPX-351, and 156 were randomized to 7+3 (cytarabine continuously for 7 days and a single dose of daunorubicin for the first 3 days).
The treatment arms were well-balanced for sex, race, age, performance status, AML subtype, MDS-related cytogenetics, and prior hypomethylating therapy.
The trial met its primary endpoint, demonstrating a significant improvement in overall survival with CPX-351. The median overall survival was 9.56 months in the CPX-351 arm and 5.95 months in the 7+3 arm. The hazard ratio was 0.69 (P=0.005).
According to researchers, there was no substantial difference between the treatment arms with regard to grade 3-5 adverse events.
About CPX-351
CPX-351 is a liposomal formulation of a 5:1 molar ratio of cytarabine and daunorubicin.
In one phase 2 trial, CPX-351 conferred a significant improvement in survival—over investigator’s choice of therapy—when used as salvage therapy in poor-risk patients with AML.
In another phase 2 trial, CPX-351 conferred a significant survival benefit—over 7+3—in patients with secondary AML.
The FDA previously granted CPX-351 fast track designation to treat elderly patients with secondary AML. The agency established the fast track designation process to expedite the review of drugs that are intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs.
The designation allows a drug’s developer to submit sections of a new drug application (NDA) on a rolling basis, so the FDA can review portions of the NDA as they are received instead of waiting for the entire NDA submission. A fast-track-designated product could be eligible for priority review if supported by clinical data at the time of NDA submission. ![]()

Image by Lance Liotta
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for CPX-351 (Vyxeos), a fixed-ratio combination of cytarabine and daunorubicin inside a lipid vesicle, to treat adults with therapy-related acute myeloid leukemia (AML) or AML with myelodysplasia-related changes.
The FDA’s breakthrough therapy designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
To earn the designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
Phase 3 trial
The breakthrough designation for CPX-351 is primarily based on positive results from a phase 3 trial in older patients with previously untreated, high-risk, secondary AML.
The trial was sponsored by Celator Pharmaceuticals, Inc., the company developing CPX-351.
The company has released some results from the trial, and additional data are scheduled to be presented at the 2016 ASCO Annual Meeting (abstract 7000).
The trial enrolled 309 patients, ages 60 to 75, with one of the following:
- Untreated AML and a history of prior cytotoxic treatment
- Antecedent myelodysplastic syndrome (MDS) or chronic myelomonocytic leukemia, with or without prior hypomethylating therapy
- AML with WHO-defined, MDS-related cytogenetic abnormalities.
One hundred and fifty-three patients were randomized to receive CPX-351, and 156 were randomized to 7+3 (cytarabine continuously for 7 days and a single dose of daunorubicin for the first 3 days).
The treatment arms were well-balanced for sex, race, age, performance status, AML subtype, MDS-related cytogenetics, and prior hypomethylating therapy.
The trial met its primary endpoint, demonstrating a significant improvement in overall survival with CPX-351. The median overall survival was 9.56 months in the CPX-351 arm and 5.95 months in the 7+3 arm. The hazard ratio was 0.69 (P=0.005).
According to researchers, there was no substantial difference between the treatment arms with regard to grade 3-5 adverse events.
About CPX-351
CPX-351 is a liposomal formulation of a 5:1 molar ratio of cytarabine and daunorubicin.
In one phase 2 trial, CPX-351 conferred a significant improvement in survival—over investigator’s choice of therapy—when used as salvage therapy in poor-risk patients with AML.
In another phase 2 trial, CPX-351 conferred a significant survival benefit—over 7+3—in patients with secondary AML.
The FDA previously granted CPX-351 fast track designation to treat elderly patients with secondary AML. The agency established the fast track designation process to expedite the review of drugs that are intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs.
The designation allows a drug’s developer to submit sections of a new drug application (NDA) on a rolling basis, so the FDA can review portions of the NDA as they are received instead of waiting for the entire NDA submission. A fast-track-designated product could be eligible for priority review if supported by clinical data at the time of NDA submission. ![]()

Image by Lance Liotta
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for CPX-351 (Vyxeos), a fixed-ratio combination of cytarabine and daunorubicin inside a lipid vesicle, to treat adults with therapy-related acute myeloid leukemia (AML) or AML with myelodysplasia-related changes.
The FDA’s breakthrough therapy designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
To earn the designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
Phase 3 trial
The breakthrough designation for CPX-351 is primarily based on positive results from a phase 3 trial in older patients with previously untreated, high-risk, secondary AML.
The trial was sponsored by Celator Pharmaceuticals, Inc., the company developing CPX-351.
The company has released some results from the trial, and additional data are scheduled to be presented at the 2016 ASCO Annual Meeting (abstract 7000).
The trial enrolled 309 patients, ages 60 to 75, with one of the following:
- Untreated AML and a history of prior cytotoxic treatment
- Antecedent myelodysplastic syndrome (MDS) or chronic myelomonocytic leukemia, with or without prior hypomethylating therapy
- AML with WHO-defined, MDS-related cytogenetic abnormalities.
One hundred and fifty-three patients were randomized to receive CPX-351, and 156 were randomized to 7+3 (cytarabine continuously for 7 days and a single dose of daunorubicin for the first 3 days).
The treatment arms were well-balanced for sex, race, age, performance status, AML subtype, MDS-related cytogenetics, and prior hypomethylating therapy.
The trial met its primary endpoint, demonstrating a significant improvement in overall survival with CPX-351. The median overall survival was 9.56 months in the CPX-351 arm and 5.95 months in the 7+3 arm. The hazard ratio was 0.69 (P=0.005).
According to researchers, there was no substantial difference between the treatment arms with regard to grade 3-5 adverse events.
About CPX-351
CPX-351 is a liposomal formulation of a 5:1 molar ratio of cytarabine and daunorubicin.
In one phase 2 trial, CPX-351 conferred a significant improvement in survival—over investigator’s choice of therapy—when used as salvage therapy in poor-risk patients with AML.
In another phase 2 trial, CPX-351 conferred a significant survival benefit—over 7+3—in patients with secondary AML.
The FDA previously granted CPX-351 fast track designation to treat elderly patients with secondary AML. The agency established the fast track designation process to expedite the review of drugs that are intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs.
The designation allows a drug’s developer to submit sections of a new drug application (NDA) on a rolling basis, so the FDA can review portions of the NDA as they are received instead of waiting for the entire NDA submission. A fast-track-designated product could be eligible for priority review if supported by clinical data at the time of NDA submission. ![]()
Breast cancer drug could treat AML
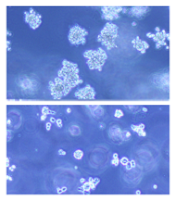
(below) palbociclib treatment
Images courtesy of Iris Uras
and Vetmeduni Vienna
Palbociclib, a CDK4/6 kinase inhibitor approved to treat breast cancer, could also be used to treat acute myeloid leukemia (AML), according to research published in Blood.
The drug induced apoptosis in FLT3-mutant AML cells and inhibited tumor growth in mouse models of FLT3-ITD+ AML.
Palbociclib also demonstrated synergy with a range of FLT3 inhibitors.
The researchers said these results can be explained by the fact that CDK6 is “absolutely required” for the viability of FLT3-dependent leukemic cells and FLT3-ITD-induced leukemogenesis.
“We found a novel therapeutic window that attacks the dependency of a cancer cell on its growth regulator,” said study author Iris Uras, PhD, of Vetmeduni Vienna in Austria.
Dr Uras and her colleagues first found that palbociclib acts specifically on FLT3-ITD+ AML cells, inhibiting their viability in a dose-dependent manner. Palbociclib induced cell-cycle arrest and apoptosis in these cells.
Palbociclib also arrested tumor growth in mouse models of FLT3-ITD+ AML, significantly decreasing tumor size when compared to untreated controls (P<0.05).
Further investigation revealed that CDK6—but not CDK4—directly regulates FLT3 expression.
CDK6 acts as a transcriptional regulator of FLT3 and the serine threonine kinase PIM1, which also plays a role in leukemogenesis. As palbociclib inhibits CDK6, it downregulates FLT3 and reduces PIM1 transcription.
To build upon these findings, the researchers tested palbociclib in combination with FLT3 inhibitors.
They observed “pronounced in vitro synergy” between palbociclib and TCS-359, tandutinib, and quizartinib in FLT3-ITD+ AML cell lines and samples from patients with FLT3-ITD+ AML.
“We are attacking FLT3 from two sides there—blocking its expression and inhibiting its activity,” Dr Uras said. “A combination therapy could be a breakthrough for many patients suffering from leukemia.” ![]()

(below) palbociclib treatment
Images courtesy of Iris Uras
and Vetmeduni Vienna
Palbociclib, a CDK4/6 kinase inhibitor approved to treat breast cancer, could also be used to treat acute myeloid leukemia (AML), according to research published in Blood.
The drug induced apoptosis in FLT3-mutant AML cells and inhibited tumor growth in mouse models of FLT3-ITD+ AML.
Palbociclib also demonstrated synergy with a range of FLT3 inhibitors.
The researchers said these results can be explained by the fact that CDK6 is “absolutely required” for the viability of FLT3-dependent leukemic cells and FLT3-ITD-induced leukemogenesis.
“We found a novel therapeutic window that attacks the dependency of a cancer cell on its growth regulator,” said study author Iris Uras, PhD, of Vetmeduni Vienna in Austria.
Dr Uras and her colleagues first found that palbociclib acts specifically on FLT3-ITD+ AML cells, inhibiting their viability in a dose-dependent manner. Palbociclib induced cell-cycle arrest and apoptosis in these cells.
Palbociclib also arrested tumor growth in mouse models of FLT3-ITD+ AML, significantly decreasing tumor size when compared to untreated controls (P<0.05).
Further investigation revealed that CDK6—but not CDK4—directly regulates FLT3 expression.
CDK6 acts as a transcriptional regulator of FLT3 and the serine threonine kinase PIM1, which also plays a role in leukemogenesis. As palbociclib inhibits CDK6, it downregulates FLT3 and reduces PIM1 transcription.
To build upon these findings, the researchers tested palbociclib in combination with FLT3 inhibitors.
They observed “pronounced in vitro synergy” between palbociclib and TCS-359, tandutinib, and quizartinib in FLT3-ITD+ AML cell lines and samples from patients with FLT3-ITD+ AML.
“We are attacking FLT3 from two sides there—blocking its expression and inhibiting its activity,” Dr Uras said. “A combination therapy could be a breakthrough for many patients suffering from leukemia.” ![]()

(below) palbociclib treatment
Images courtesy of Iris Uras
and Vetmeduni Vienna
Palbociclib, a CDK4/6 kinase inhibitor approved to treat breast cancer, could also be used to treat acute myeloid leukemia (AML), according to research published in Blood.
The drug induced apoptosis in FLT3-mutant AML cells and inhibited tumor growth in mouse models of FLT3-ITD+ AML.
Palbociclib also demonstrated synergy with a range of FLT3 inhibitors.
The researchers said these results can be explained by the fact that CDK6 is “absolutely required” for the viability of FLT3-dependent leukemic cells and FLT3-ITD-induced leukemogenesis.
“We found a novel therapeutic window that attacks the dependency of a cancer cell on its growth regulator,” said study author Iris Uras, PhD, of Vetmeduni Vienna in Austria.
Dr Uras and her colleagues first found that palbociclib acts specifically on FLT3-ITD+ AML cells, inhibiting their viability in a dose-dependent manner. Palbociclib induced cell-cycle arrest and apoptosis in these cells.
Palbociclib also arrested tumor growth in mouse models of FLT3-ITD+ AML, significantly decreasing tumor size when compared to untreated controls (P<0.05).
Further investigation revealed that CDK6—but not CDK4—directly regulates FLT3 expression.
CDK6 acts as a transcriptional regulator of FLT3 and the serine threonine kinase PIM1, which also plays a role in leukemogenesis. As palbociclib inhibits CDK6, it downregulates FLT3 and reduces PIM1 transcription.
To build upon these findings, the researchers tested palbociclib in combination with FLT3 inhibitors.
They observed “pronounced in vitro synergy” between palbociclib and TCS-359, tandutinib, and quizartinib in FLT3-ITD+ AML cell lines and samples from patients with FLT3-ITD+ AML.
“We are attacking FLT3 from two sides there—blocking its expression and inhibiting its activity,” Dr Uras said. “A combination therapy could be a breakthrough for many patients suffering from leukemia.” ![]()
On the road to harnessing CRISPR gene editing to treat cancer
CRISPR technology, a simple, yet incredibly powerful tool for genetic engineering, is not only allowing cancer researchers to screen for drug targets more efficiently, but is also opening the door for direct cancer treatment through gene interference or activation.
“The pace at which this technology is developing is astounding and almost every cancer research lab is now using some version of it in their studies,” Dr. Scott A. Armstrong, director of Memorial Sloan Kettering Leukemia Center, New York, said in an interview.
Ancient defense mechanism
While the term CRISPR is now synonymous with the editing of human genes, it actually refers to a sort of primitive immune system used by bacteria for billions of years. CRISPR is an acronym for Clustered Regularly Interspaced Short Palindromic Repeats, but the complex terminology belies an elegantly simple mechanism by which bacterial cells destroy invading pathogens.
The bacterial genome contains regions with short repetitive stretches of DNA that are separated by spacers. Researchers made the startling discovery that the spacers are often composed of bits of foreign DNA and it transpired that bacteria use it as a molecular memory of prior infection.
When the same pathogen is encountered again, the stretches of repeats and spacers are transcribed to form CRISPR RNAs (crRNA). Together with a transactivating RNA (tracrRNA), it forms a kind of GPS system for a series of CRISPR-associated (Cas) proteins that function like molecular scissors, destroying the target DNA sequence in the invader’s genome.
There are three CRISPR systems – type I, II, and III – that are associated with different sets of Cas proteins and each employ unique methods for achieving the same ultimate function. The type II system that pairs with Cas9 has received the most attention.
Cut and paste gene editing
The discovery that CRISPR could be exploited as a tool for genetic manipulation in mammalian cells sparked a revolution in the genome editing field. “The CRISPR-Cas9 system has been adapted to specifically edit the genomes of mammalian cells, allowing one to make targeted changes to almost any gene,” Dr. Armstrong said.
The use of CRISPR-Cas9 as a genome editing tool is simplified by joining the crRNA and tracrRNA together so they are transcribed in a single guide RNA (gRNA). The GPS coordinates of the gRNA can be preprogrammed to target a gene of interest, specifically directing the co-transcribed Cas9 protein to cut at that location, and introducing a double-strand break (DSB) in the DNA. Cells employ a number of different mechanisms to repair DSBs and these can then be exploited for genome editing purposes, allowing researchers to introduce changes to the DNA as it is repaired.
The CRISPR-Cas9 system excels in its simplicity – allowing alterations to be made to the genome much more easily, quickly, and cheaply than ever before, plagued by far fewer off-target effects. It also allows researchers to examine the function of multiple genes at once, where before they were mostly limited to a single gene.
“Cancer genomics has identified a large number of genes that are mutated in human cancer,” Tyler Jacks, Ph.D., director of the Koch Institute for Integrative Cancer Research at MIT, Boston, said in an interview. “CRISPR allows us to study these genes in cancer cells and in whole animals much more efficiently than the methods that were in use just a few years ago.”
But the potential of the CRISPR-Cas9 system doesn’t stop there. “At the moment, a particularly exciting application is the use of this approach to inactive genes in very specific fashion to assess the function of a given protein in a cancer cell, which should speed the identification of proteins that are important for cancer cells and thus potentially aid drug discovery efforts,” Dr. Armstrong said.
CRISPR at AACR
The latest developments in the use of the CRISPR-Cas9 system were highlighted at the annual meeting of the American Association of Cancer Research. Dr. David Sabatini, professor of biology at MIT, Boston, described his own lab’s method for using CRISPR-Cas9 to seek out the essential genes involved in different types of cancers. In a study recently published in Science, he and his colleagues employed this method in chronic myelogenous leukemia and Burkitt’s lymphoma cell lines. The gRNA library targeted just over 18,000 genes and roughly 10% of these proved to be essential. Mostly, these genes were linked to key cellular processes (Science 2015;350[6264]:1096-1101).
Dr. Christopher Vakoc of Cold Spring Harbor (N.Y.) Laboratory, presented a slightly different kind of CRISPR screen for drug targets. Most commonly, CRISPR introduces edits at the start of the gene, which may or may not change the DNA enough to produce a nonfunctional protein. Dr. Vakoc’s lab has developed a system that instead edits functional protein domains, which present ideal drug targets. Mutated domains can be identified that are essential for cancer cell survival and small molecule inhibitors designed that bind to them to kill cancer cells.
The technique has already been used to identify such a domain on the BRD4 protein and inhibitors that bind to this domain had significant antitumor activity in leukemia, Dr. Vakoc reported. A screen targeting 192 chromatin regulatory domains expressed in mouse acute myeloid leukemia cells was subsequently performed and identified 25 domains that impacted survival, 6 that are already being therapeutically targeted, and 19 novel potential targets.
Another development in CRISPR-Cas9 technology creates an inactive version of the Cas9 enzyme, one that has lost the ability to cut DNA. Though it seems counterintuitive, this has opened up a wealth of new possible uses. Jonathan S. Weissman, Ph.D., professor of cellular and molecular pharmacology, University of California, San Francisco, part of the group to develop this ‘dead’ Cas9 (dCas9), published a description of the use of two new tools dubbed CRISPR interference and CRISPR activation (Cell 2013;152[5]:1173-83).
Essentially, by fusing dCas9 with different proteins, such as epigenetic modifiers or transcriptional activators or repressors, it can be used as a delivery system to fine-tune gene expression, instead of editing the gene sequence.
Treating cancer?
Ultimately, CRISPR-Cas9 could be used to treat cancers by cutting out defective genes and replacing them with a wild-type version, or by repairing mutations, though for the time being this is theoretical. Studies have suggested it is possible with other types of diseases, however.
“It is not clear exactly how the CRISPR system would be used to directly treat cancer, but the discoveries that come from its use will likely lead to new ways to treat cancer,” said Dr Armstrong.
Dr Jacks highlighted the technical challenges that will need to be overcome first. “In principle, CRISPR-based genome editing could be used to correct cancer-causing mutations in tumors in vivo or to inactivate activated cancer genes,” he said. “At this point, however, we lack the technology necessary to deliver the CRISPR system to all cancer cells in the body. Improvements in this so-called ‘delivery problem’ may allow CRISPR to become a powerful anticancer therapy strategy.”
CRISPR technology, a simple, yet incredibly powerful tool for genetic engineering, is not only allowing cancer researchers to screen for drug targets more efficiently, but is also opening the door for direct cancer treatment through gene interference or activation.
“The pace at which this technology is developing is astounding and almost every cancer research lab is now using some version of it in their studies,” Dr. Scott A. Armstrong, director of Memorial Sloan Kettering Leukemia Center, New York, said in an interview.
Ancient defense mechanism
While the term CRISPR is now synonymous with the editing of human genes, it actually refers to a sort of primitive immune system used by bacteria for billions of years. CRISPR is an acronym for Clustered Regularly Interspaced Short Palindromic Repeats, but the complex terminology belies an elegantly simple mechanism by which bacterial cells destroy invading pathogens.
The bacterial genome contains regions with short repetitive stretches of DNA that are separated by spacers. Researchers made the startling discovery that the spacers are often composed of bits of foreign DNA and it transpired that bacteria use it as a molecular memory of prior infection.
When the same pathogen is encountered again, the stretches of repeats and spacers are transcribed to form CRISPR RNAs (crRNA). Together with a transactivating RNA (tracrRNA), it forms a kind of GPS system for a series of CRISPR-associated (Cas) proteins that function like molecular scissors, destroying the target DNA sequence in the invader’s genome.
There are three CRISPR systems – type I, II, and III – that are associated with different sets of Cas proteins and each employ unique methods for achieving the same ultimate function. The type II system that pairs with Cas9 has received the most attention.
Cut and paste gene editing
The discovery that CRISPR could be exploited as a tool for genetic manipulation in mammalian cells sparked a revolution in the genome editing field. “The CRISPR-Cas9 system has been adapted to specifically edit the genomes of mammalian cells, allowing one to make targeted changes to almost any gene,” Dr. Armstrong said.
The use of CRISPR-Cas9 as a genome editing tool is simplified by joining the crRNA and tracrRNA together so they are transcribed in a single guide RNA (gRNA). The GPS coordinates of the gRNA can be preprogrammed to target a gene of interest, specifically directing the co-transcribed Cas9 protein to cut at that location, and introducing a double-strand break (DSB) in the DNA. Cells employ a number of different mechanisms to repair DSBs and these can then be exploited for genome editing purposes, allowing researchers to introduce changes to the DNA as it is repaired.
The CRISPR-Cas9 system excels in its simplicity – allowing alterations to be made to the genome much more easily, quickly, and cheaply than ever before, plagued by far fewer off-target effects. It also allows researchers to examine the function of multiple genes at once, where before they were mostly limited to a single gene.
“Cancer genomics has identified a large number of genes that are mutated in human cancer,” Tyler Jacks, Ph.D., director of the Koch Institute for Integrative Cancer Research at MIT, Boston, said in an interview. “CRISPR allows us to study these genes in cancer cells and in whole animals much more efficiently than the methods that were in use just a few years ago.”
But the potential of the CRISPR-Cas9 system doesn’t stop there. “At the moment, a particularly exciting application is the use of this approach to inactive genes in very specific fashion to assess the function of a given protein in a cancer cell, which should speed the identification of proteins that are important for cancer cells and thus potentially aid drug discovery efforts,” Dr. Armstrong said.
CRISPR at AACR
The latest developments in the use of the CRISPR-Cas9 system were highlighted at the annual meeting of the American Association of Cancer Research. Dr. David Sabatini, professor of biology at MIT, Boston, described his own lab’s method for using CRISPR-Cas9 to seek out the essential genes involved in different types of cancers. In a study recently published in Science, he and his colleagues employed this method in chronic myelogenous leukemia and Burkitt’s lymphoma cell lines. The gRNA library targeted just over 18,000 genes and roughly 10% of these proved to be essential. Mostly, these genes were linked to key cellular processes (Science 2015;350[6264]:1096-1101).
Dr. Christopher Vakoc of Cold Spring Harbor (N.Y.) Laboratory, presented a slightly different kind of CRISPR screen for drug targets. Most commonly, CRISPR introduces edits at the start of the gene, which may or may not change the DNA enough to produce a nonfunctional protein. Dr. Vakoc’s lab has developed a system that instead edits functional protein domains, which present ideal drug targets. Mutated domains can be identified that are essential for cancer cell survival and small molecule inhibitors designed that bind to them to kill cancer cells.
The technique has already been used to identify such a domain on the BRD4 protein and inhibitors that bind to this domain had significant antitumor activity in leukemia, Dr. Vakoc reported. A screen targeting 192 chromatin regulatory domains expressed in mouse acute myeloid leukemia cells was subsequently performed and identified 25 domains that impacted survival, 6 that are already being therapeutically targeted, and 19 novel potential targets.
Another development in CRISPR-Cas9 technology creates an inactive version of the Cas9 enzyme, one that has lost the ability to cut DNA. Though it seems counterintuitive, this has opened up a wealth of new possible uses. Jonathan S. Weissman, Ph.D., professor of cellular and molecular pharmacology, University of California, San Francisco, part of the group to develop this ‘dead’ Cas9 (dCas9), published a description of the use of two new tools dubbed CRISPR interference and CRISPR activation (Cell 2013;152[5]:1173-83).
Essentially, by fusing dCas9 with different proteins, such as epigenetic modifiers or transcriptional activators or repressors, it can be used as a delivery system to fine-tune gene expression, instead of editing the gene sequence.
Treating cancer?
Ultimately, CRISPR-Cas9 could be used to treat cancers by cutting out defective genes and replacing them with a wild-type version, or by repairing mutations, though for the time being this is theoretical. Studies have suggested it is possible with other types of diseases, however.
“It is not clear exactly how the CRISPR system would be used to directly treat cancer, but the discoveries that come from its use will likely lead to new ways to treat cancer,” said Dr Armstrong.
Dr Jacks highlighted the technical challenges that will need to be overcome first. “In principle, CRISPR-based genome editing could be used to correct cancer-causing mutations in tumors in vivo or to inactivate activated cancer genes,” he said. “At this point, however, we lack the technology necessary to deliver the CRISPR system to all cancer cells in the body. Improvements in this so-called ‘delivery problem’ may allow CRISPR to become a powerful anticancer therapy strategy.”
CRISPR technology, a simple, yet incredibly powerful tool for genetic engineering, is not only allowing cancer researchers to screen for drug targets more efficiently, but is also opening the door for direct cancer treatment through gene interference or activation.
“The pace at which this technology is developing is astounding and almost every cancer research lab is now using some version of it in their studies,” Dr. Scott A. Armstrong, director of Memorial Sloan Kettering Leukemia Center, New York, said in an interview.
Ancient defense mechanism
While the term CRISPR is now synonymous with the editing of human genes, it actually refers to a sort of primitive immune system used by bacteria for billions of years. CRISPR is an acronym for Clustered Regularly Interspaced Short Palindromic Repeats, but the complex terminology belies an elegantly simple mechanism by which bacterial cells destroy invading pathogens.
The bacterial genome contains regions with short repetitive stretches of DNA that are separated by spacers. Researchers made the startling discovery that the spacers are often composed of bits of foreign DNA and it transpired that bacteria use it as a molecular memory of prior infection.
When the same pathogen is encountered again, the stretches of repeats and spacers are transcribed to form CRISPR RNAs (crRNA). Together with a transactivating RNA (tracrRNA), it forms a kind of GPS system for a series of CRISPR-associated (Cas) proteins that function like molecular scissors, destroying the target DNA sequence in the invader’s genome.
There are three CRISPR systems – type I, II, and III – that are associated with different sets of Cas proteins and each employ unique methods for achieving the same ultimate function. The type II system that pairs with Cas9 has received the most attention.
Cut and paste gene editing
The discovery that CRISPR could be exploited as a tool for genetic manipulation in mammalian cells sparked a revolution in the genome editing field. “The CRISPR-Cas9 system has been adapted to specifically edit the genomes of mammalian cells, allowing one to make targeted changes to almost any gene,” Dr. Armstrong said.
The use of CRISPR-Cas9 as a genome editing tool is simplified by joining the crRNA and tracrRNA together so they are transcribed in a single guide RNA (gRNA). The GPS coordinates of the gRNA can be preprogrammed to target a gene of interest, specifically directing the co-transcribed Cas9 protein to cut at that location, and introducing a double-strand break (DSB) in the DNA. Cells employ a number of different mechanisms to repair DSBs and these can then be exploited for genome editing purposes, allowing researchers to introduce changes to the DNA as it is repaired.
The CRISPR-Cas9 system excels in its simplicity – allowing alterations to be made to the genome much more easily, quickly, and cheaply than ever before, plagued by far fewer off-target effects. It also allows researchers to examine the function of multiple genes at once, where before they were mostly limited to a single gene.
“Cancer genomics has identified a large number of genes that are mutated in human cancer,” Tyler Jacks, Ph.D., director of the Koch Institute for Integrative Cancer Research at MIT, Boston, said in an interview. “CRISPR allows us to study these genes in cancer cells and in whole animals much more efficiently than the methods that were in use just a few years ago.”
But the potential of the CRISPR-Cas9 system doesn’t stop there. “At the moment, a particularly exciting application is the use of this approach to inactive genes in very specific fashion to assess the function of a given protein in a cancer cell, which should speed the identification of proteins that are important for cancer cells and thus potentially aid drug discovery efforts,” Dr. Armstrong said.
CRISPR at AACR
The latest developments in the use of the CRISPR-Cas9 system were highlighted at the annual meeting of the American Association of Cancer Research. Dr. David Sabatini, professor of biology at MIT, Boston, described his own lab’s method for using CRISPR-Cas9 to seek out the essential genes involved in different types of cancers. In a study recently published in Science, he and his colleagues employed this method in chronic myelogenous leukemia and Burkitt’s lymphoma cell lines. The gRNA library targeted just over 18,000 genes and roughly 10% of these proved to be essential. Mostly, these genes were linked to key cellular processes (Science 2015;350[6264]:1096-1101).
Dr. Christopher Vakoc of Cold Spring Harbor (N.Y.) Laboratory, presented a slightly different kind of CRISPR screen for drug targets. Most commonly, CRISPR introduces edits at the start of the gene, which may or may not change the DNA enough to produce a nonfunctional protein. Dr. Vakoc’s lab has developed a system that instead edits functional protein domains, which present ideal drug targets. Mutated domains can be identified that are essential for cancer cell survival and small molecule inhibitors designed that bind to them to kill cancer cells.
The technique has already been used to identify such a domain on the BRD4 protein and inhibitors that bind to this domain had significant antitumor activity in leukemia, Dr. Vakoc reported. A screen targeting 192 chromatin regulatory domains expressed in mouse acute myeloid leukemia cells was subsequently performed and identified 25 domains that impacted survival, 6 that are already being therapeutically targeted, and 19 novel potential targets.
Another development in CRISPR-Cas9 technology creates an inactive version of the Cas9 enzyme, one that has lost the ability to cut DNA. Though it seems counterintuitive, this has opened up a wealth of new possible uses. Jonathan S. Weissman, Ph.D., professor of cellular and molecular pharmacology, University of California, San Francisco, part of the group to develop this ‘dead’ Cas9 (dCas9), published a description of the use of two new tools dubbed CRISPR interference and CRISPR activation (Cell 2013;152[5]:1173-83).
Essentially, by fusing dCas9 with different proteins, such as epigenetic modifiers or transcriptional activators or repressors, it can be used as a delivery system to fine-tune gene expression, instead of editing the gene sequence.
Treating cancer?
Ultimately, CRISPR-Cas9 could be used to treat cancers by cutting out defective genes and replacing them with a wild-type version, or by repairing mutations, though for the time being this is theoretical. Studies have suggested it is possible with other types of diseases, however.
“It is not clear exactly how the CRISPR system would be used to directly treat cancer, but the discoveries that come from its use will likely lead to new ways to treat cancer,” said Dr Armstrong.
Dr Jacks highlighted the technical challenges that will need to be overcome first. “In principle, CRISPR-based genome editing could be used to correct cancer-causing mutations in tumors in vivo or to inactivate activated cancer genes,” he said. “At this point, however, we lack the technology necessary to deliver the CRISPR system to all cancer cells in the body. Improvements in this so-called ‘delivery problem’ may allow CRISPR to become a powerful anticancer therapy strategy.”
Manipulating a microRNA to treat AML

Image by Su Jung Song
The microRNA miR-22 is “an essential antitumor gatekeeper” in acute myeloid leukemia (AML), researchers have reported in Nature Communications.
The team found that miR-22 was significantly downregulated in AML, and forced expression of miR-22 produced antileukemic effects in AML cells
and mouse models of the disease.
Futhermore, nanoparticles carrying miR-22 oligonucleotides appeared to cure AML in some mice.
“Previous research has shown that microRNA miR-22 is linked to breast cancer and other blood disorders [myelodysplastic syndromes], which sometimes turn into AML,” said study author Jianjun Chen, PhD, of the University of Cincinnati in Ohio.
“But we found in this study that it could be an essential antitumor gatekeeper in AML when it is downregulated. When we forced miR-22 expression, we saw difficulty in leukemia cells developing, growing, and thriving.”
Dr Chen and his colleagues first found that miR-22 was significantly downregulated (P<0.05) in samples from AML patients, when compared with normal CD34+ hematopoietic stem/progenitor cells, CD33+ myeloid progenitor cells, and mononuclear cells. The set of AML samples included MLL, t(15;17), t(8;21), and inv(16) AML.
When the researchers forced expression of miR-22 in human AML cells, they found the microRNA significantly inhibited cell viability, growth, and proliferation, while promoting apoptosis.
The team also investigated the role of miR-22 in colony formation induced by MLL-AF10/t(10;11), PML-RARA/t(15;17), and AML1-ETO9a/t(8;21). They found that forced expression of miR-22 significantly inhibited colony formation induced by all of these oncogenic fusion genes.
In mice, forced expression of miR-22 blocked MLL-AF9-mediated leukemogenesis and MLL-AF10-mediated leukemogenesis.
Forced expression of miR-22 also inhibited progression of AML induced by MLL-AF9, AE9a, or FLT3-ITD/NPM1c+ in secondary recipient mice. The researchers said this resulted in “largely normal” morphologies in the peripheral blood, bone marrow, spleen, and liver tissues of these mice.
In addition, the team found that nanoparticles carrying miR-22 oligonucleotides significantly delayed AML progression in secondary recipient mice with MLL-AF9 and AE9a-induced AML. At least 40% of the mice appeared to be completely cured.
In a xenotransplantation model, miR-22 nanoparticles significantly delayed AML progression induced by human MV4;11/t(4;11) cells.
Further investigation into the role miR-22 plays in AML revealed that 3 oncogenes—CRTC1, FLT3, and MYCBP—are “functionally important” targets of miR-22 in AML. And miR-22 represses the CREB and MYC signaling pathways.
The researchers also found DNA copy-number loss in the miR-22 gene locus in AML cases, and they discovered the expression of miR-22 is epigenetically repressed in AML.
“The downregulation, or decreased output, of miR-22 in AML is caused by the loss of the number of DNA being copied and/or stopping their expression through a pathway called TET1/GFI1/EZH2/SIN3A,” Dr Chen explained.
“Our study uncovers a previously unappreciated signaling pathway—TET1/GFI1/EZH2/SIN3A/miR-22/CREB-MYC—and provides new insights into genetic mechanisms causing and progressing AML and also highlights the clinical potential of miR-22-based AML therapy. More research on this pathway and ways to target it are necessary.” ![]()

Image by Su Jung Song
The microRNA miR-22 is “an essential antitumor gatekeeper” in acute myeloid leukemia (AML), researchers have reported in Nature Communications.
The team found that miR-22 was significantly downregulated in AML, and forced expression of miR-22 produced antileukemic effects in AML cells
and mouse models of the disease.
Futhermore, nanoparticles carrying miR-22 oligonucleotides appeared to cure AML in some mice.
“Previous research has shown that microRNA miR-22 is linked to breast cancer and other blood disorders [myelodysplastic syndromes], which sometimes turn into AML,” said study author Jianjun Chen, PhD, of the University of Cincinnati in Ohio.
“But we found in this study that it could be an essential antitumor gatekeeper in AML when it is downregulated. When we forced miR-22 expression, we saw difficulty in leukemia cells developing, growing, and thriving.”
Dr Chen and his colleagues first found that miR-22 was significantly downregulated (P<0.05) in samples from AML patients, when compared with normal CD34+ hematopoietic stem/progenitor cells, CD33+ myeloid progenitor cells, and mononuclear cells. The set of AML samples included MLL, t(15;17), t(8;21), and inv(16) AML.
When the researchers forced expression of miR-22 in human AML cells, they found the microRNA significantly inhibited cell viability, growth, and proliferation, while promoting apoptosis.
The team also investigated the role of miR-22 in colony formation induced by MLL-AF10/t(10;11), PML-RARA/t(15;17), and AML1-ETO9a/t(8;21). They found that forced expression of miR-22 significantly inhibited colony formation induced by all of these oncogenic fusion genes.
In mice, forced expression of miR-22 blocked MLL-AF9-mediated leukemogenesis and MLL-AF10-mediated leukemogenesis.
Forced expression of miR-22 also inhibited progression of AML induced by MLL-AF9, AE9a, or FLT3-ITD/NPM1c+ in secondary recipient mice. The researchers said this resulted in “largely normal” morphologies in the peripheral blood, bone marrow, spleen, and liver tissues of these mice.
In addition, the team found that nanoparticles carrying miR-22 oligonucleotides significantly delayed AML progression in secondary recipient mice with MLL-AF9 and AE9a-induced AML. At least 40% of the mice appeared to be completely cured.
In a xenotransplantation model, miR-22 nanoparticles significantly delayed AML progression induced by human MV4;11/t(4;11) cells.
Further investigation into the role miR-22 plays in AML revealed that 3 oncogenes—CRTC1, FLT3, and MYCBP—are “functionally important” targets of miR-22 in AML. And miR-22 represses the CREB and MYC signaling pathways.
The researchers also found DNA copy-number loss in the miR-22 gene locus in AML cases, and they discovered the expression of miR-22 is epigenetically repressed in AML.
“The downregulation, or decreased output, of miR-22 in AML is caused by the loss of the number of DNA being copied and/or stopping their expression through a pathway called TET1/GFI1/EZH2/SIN3A,” Dr Chen explained.
“Our study uncovers a previously unappreciated signaling pathway—TET1/GFI1/EZH2/SIN3A/miR-22/CREB-MYC—and provides new insights into genetic mechanisms causing and progressing AML and also highlights the clinical potential of miR-22-based AML therapy. More research on this pathway and ways to target it are necessary.” ![]()

Image by Su Jung Song
The microRNA miR-22 is “an essential antitumor gatekeeper” in acute myeloid leukemia (AML), researchers have reported in Nature Communications.
The team found that miR-22 was significantly downregulated in AML, and forced expression of miR-22 produced antileukemic effects in AML cells
and mouse models of the disease.
Futhermore, nanoparticles carrying miR-22 oligonucleotides appeared to cure AML in some mice.
“Previous research has shown that microRNA miR-22 is linked to breast cancer and other blood disorders [myelodysplastic syndromes], which sometimes turn into AML,” said study author Jianjun Chen, PhD, of the University of Cincinnati in Ohio.
“But we found in this study that it could be an essential antitumor gatekeeper in AML when it is downregulated. When we forced miR-22 expression, we saw difficulty in leukemia cells developing, growing, and thriving.”
Dr Chen and his colleagues first found that miR-22 was significantly downregulated (P<0.05) in samples from AML patients, when compared with normal CD34+ hematopoietic stem/progenitor cells, CD33+ myeloid progenitor cells, and mononuclear cells. The set of AML samples included MLL, t(15;17), t(8;21), and inv(16) AML.
When the researchers forced expression of miR-22 in human AML cells, they found the microRNA significantly inhibited cell viability, growth, and proliferation, while promoting apoptosis.
The team also investigated the role of miR-22 in colony formation induced by MLL-AF10/t(10;11), PML-RARA/t(15;17), and AML1-ETO9a/t(8;21). They found that forced expression of miR-22 significantly inhibited colony formation induced by all of these oncogenic fusion genes.
In mice, forced expression of miR-22 blocked MLL-AF9-mediated leukemogenesis and MLL-AF10-mediated leukemogenesis.
Forced expression of miR-22 also inhibited progression of AML induced by MLL-AF9, AE9a, or FLT3-ITD/NPM1c+ in secondary recipient mice. The researchers said this resulted in “largely normal” morphologies in the peripheral blood, bone marrow, spleen, and liver tissues of these mice.
In addition, the team found that nanoparticles carrying miR-22 oligonucleotides significantly delayed AML progression in secondary recipient mice with MLL-AF9 and AE9a-induced AML. At least 40% of the mice appeared to be completely cured.
In a xenotransplantation model, miR-22 nanoparticles significantly delayed AML progression induced by human MV4;11/t(4;11) cells.
Further investigation into the role miR-22 plays in AML revealed that 3 oncogenes—CRTC1, FLT3, and MYCBP—are “functionally important” targets of miR-22 in AML. And miR-22 represses the CREB and MYC signaling pathways.
The researchers also found DNA copy-number loss in the miR-22 gene locus in AML cases, and they discovered the expression of miR-22 is epigenetically repressed in AML.
“The downregulation, or decreased output, of miR-22 in AML is caused by the loss of the number of DNA being copied and/or stopping their expression through a pathway called TET1/GFI1/EZH2/SIN3A,” Dr Chen explained.
“Our study uncovers a previously unappreciated signaling pathway—TET1/GFI1/EZH2/SIN3A/miR-22/CREB-MYC—and provides new insights into genetic mechanisms causing and progressing AML and also highlights the clinical potential of miR-22-based AML therapy. More research on this pathway and ways to target it are necessary.” ![]()
Childhood cancer risk linked to mother’s birthplace

Photo by Nina Matthews
New research suggests a mother’s birthplace may affect the risk of certain cancers for Hispanic children.
The study showed that children of Hispanic mothers who were not born in the US had lower risks of brain cancers, neuroblastoma, and Wilms tumor, when compared to children of US-born Hispanic mothers and non-Hispanic white mothers born in the US.
However, all Hispanic children, regardless of where their mothers were born, had higher risks of acute leukemias and Hodgkin lymphoma but a lower risk of non-Hodgkin lymphoma (NHL).
Julia E. Heck, PhD, of the University of California, Los Angeles, and her colleagues reported these findings in JAMA Pediatrics.
The researchers used California birth records to identify children born from 1983 through 2011. Information on cancer cases came from California Cancer Registry records from 1988 to 2012.
The team restricted their analysis to children of US-born white, US-born Hispanic, and non-US-born Hispanic mothers. The study included 13,666 cases of children diagnosed with cancer before the age of 6 and 15,513,718 children who served as control subjects.
To assess the hazard ratios (HRs) for various cancers, the researchers used children of non-Hispanic white mothers as a reference (HR=1.00) and compared them to the children of non-US-born Hispanic mothers and US-born Hispanic mothers.
For children of non-US-born Hispanic mothers, the HR was 0.50 for glioma, 0.43 for astrocytoma, 0.47 for neuroblastoma, and 0.70 for Wilms tumor. For children of US-born Hispanic mothers, the HR was 0.71 for glioma, 0.62 for astrocytoma, 0.66 for neuroblastoma, and 0.88 for Wilms tumor.
When compared to non-Hispanic white children, Hispanic children had an increased risk of acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), and Hodgkin lymphoma but lower risks of NHL and Burkitt lymphoma.
For children of US-born Hispanic mothers, the HR was 1.20 for ALL, 1.28 for AML, 2.49 for Hodgkin lymphoma, 0.79 for NHL, and 0.69 for Burkitt lymphoma.
For children of non-US-born Hispanic mothers, the HR was 1.06 for ALL, 1.05 for AML, 2.35 for Hodgkin lymphoma, 0.76 for NHL, and 0.73 for Burkitt lymphoma.
The researchers said the differences observed between children of US-born and non-US-born Hispanic mothers may be explained by lifestyle differences and varying environmental exposures.
These factors may explain the differences in cancer incidence between Hispanic children and white children as well, but the differences may also be a result of genetic variation and infection exposures early in life. ![]()

Photo by Nina Matthews
New research suggests a mother’s birthplace may affect the risk of certain cancers for Hispanic children.
The study showed that children of Hispanic mothers who were not born in the US had lower risks of brain cancers, neuroblastoma, and Wilms tumor, when compared to children of US-born Hispanic mothers and non-Hispanic white mothers born in the US.
However, all Hispanic children, regardless of where their mothers were born, had higher risks of acute leukemias and Hodgkin lymphoma but a lower risk of non-Hodgkin lymphoma (NHL).
Julia E. Heck, PhD, of the University of California, Los Angeles, and her colleagues reported these findings in JAMA Pediatrics.
The researchers used California birth records to identify children born from 1983 through 2011. Information on cancer cases came from California Cancer Registry records from 1988 to 2012.
The team restricted their analysis to children of US-born white, US-born Hispanic, and non-US-born Hispanic mothers. The study included 13,666 cases of children diagnosed with cancer before the age of 6 and 15,513,718 children who served as control subjects.
To assess the hazard ratios (HRs) for various cancers, the researchers used children of non-Hispanic white mothers as a reference (HR=1.00) and compared them to the children of non-US-born Hispanic mothers and US-born Hispanic mothers.
For children of non-US-born Hispanic mothers, the HR was 0.50 for glioma, 0.43 for astrocytoma, 0.47 for neuroblastoma, and 0.70 for Wilms tumor. For children of US-born Hispanic mothers, the HR was 0.71 for glioma, 0.62 for astrocytoma, 0.66 for neuroblastoma, and 0.88 for Wilms tumor.
When compared to non-Hispanic white children, Hispanic children had an increased risk of acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), and Hodgkin lymphoma but lower risks of NHL and Burkitt lymphoma.
For children of US-born Hispanic mothers, the HR was 1.20 for ALL, 1.28 for AML, 2.49 for Hodgkin lymphoma, 0.79 for NHL, and 0.69 for Burkitt lymphoma.
For children of non-US-born Hispanic mothers, the HR was 1.06 for ALL, 1.05 for AML, 2.35 for Hodgkin lymphoma, 0.76 for NHL, and 0.73 for Burkitt lymphoma.
The researchers said the differences observed between children of US-born and non-US-born Hispanic mothers may be explained by lifestyle differences and varying environmental exposures.
These factors may explain the differences in cancer incidence between Hispanic children and white children as well, but the differences may also be a result of genetic variation and infection exposures early in life. ![]()

Photo by Nina Matthews
New research suggests a mother’s birthplace may affect the risk of certain cancers for Hispanic children.
The study showed that children of Hispanic mothers who were not born in the US had lower risks of brain cancers, neuroblastoma, and Wilms tumor, when compared to children of US-born Hispanic mothers and non-Hispanic white mothers born in the US.
However, all Hispanic children, regardless of where their mothers were born, had higher risks of acute leukemias and Hodgkin lymphoma but a lower risk of non-Hodgkin lymphoma (NHL).
Julia E. Heck, PhD, of the University of California, Los Angeles, and her colleagues reported these findings in JAMA Pediatrics.
The researchers used California birth records to identify children born from 1983 through 2011. Information on cancer cases came from California Cancer Registry records from 1988 to 2012.
The team restricted their analysis to children of US-born white, US-born Hispanic, and non-US-born Hispanic mothers. The study included 13,666 cases of children diagnosed with cancer before the age of 6 and 15,513,718 children who served as control subjects.
To assess the hazard ratios (HRs) for various cancers, the researchers used children of non-Hispanic white mothers as a reference (HR=1.00) and compared them to the children of non-US-born Hispanic mothers and US-born Hispanic mothers.
For children of non-US-born Hispanic mothers, the HR was 0.50 for glioma, 0.43 for astrocytoma, 0.47 for neuroblastoma, and 0.70 for Wilms tumor. For children of US-born Hispanic mothers, the HR was 0.71 for glioma, 0.62 for astrocytoma, 0.66 for neuroblastoma, and 0.88 for Wilms tumor.
When compared to non-Hispanic white children, Hispanic children had an increased risk of acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), and Hodgkin lymphoma but lower risks of NHL and Burkitt lymphoma.
For children of US-born Hispanic mothers, the HR was 1.20 for ALL, 1.28 for AML, 2.49 for Hodgkin lymphoma, 0.79 for NHL, and 0.69 for Burkitt lymphoma.
For children of non-US-born Hispanic mothers, the HR was 1.06 for ALL, 1.05 for AML, 2.35 for Hodgkin lymphoma, 0.76 for NHL, and 0.73 for Burkitt lymphoma.
The researchers said the differences observed between children of US-born and non-US-born Hispanic mothers may be explained by lifestyle differences and varying environmental exposures.
These factors may explain the differences in cancer incidence between Hispanic children and white children as well, but the differences may also be a result of genetic variation and infection exposures early in life.
Change in T-cell distribution predicts LFS, OS in AML

NEW ORLEANS—A phase 4 study has revealed biomarkers that appear to predict the efficacy of treatment with histamine dihydrochloride (HDC) and interleukin-2 (IL-2) in patients with acute myeloid leukemia (AML).
Researchers found that patients who remained in complete remission after 1 cycle of HDC/IL-2 experienced a significant reduction in effector memory T cells (TEM) and a concomitant increase in effector T cells (Teff) during therapy.
This “TEM to Teff transition” was associated with favorable leukemia-free survival (LFS) and overall survival (OS), especially among patients older than 60.
Frida Ewald Sander, PhD, of University of Gothenburg in Sweden, and her colleagues presented this research at the 2016 AACR Annual Meeting (abstract CT116*).
The study was supported by The Swedish Research Council, The Swedish Cancer Society, The Swedish Society for Medical Research, Meda Pharma, and Immune Pharmaceuticals, which markets HDC as Ceplene®.
The combination of HDC and IL-2 is currently approved in more than 30 countries to prevent relapse in AML patients. In this phase 4 study (Re:MISSION), the researchers set out to assess the immunomodulatory properties of this treatment and correlate potential biomarkers with clinical outcomes.
The study included 84 non-transplanted AML patients (ages 18 to 79) in first complete remission. The patients received HDC (0.5 mg SC BID) and human recombinant IL-2 (1MIU SC BID) in 3-week cycles for 18 months. The patients were followed for at least 2 years from the start of immunotherapy to evaluate survival.
The researchers collected blood from the patients before they began HDC/IL-2 therapy and at the end of the first treatment cycle. From these samples, the team assessed the frequency of CD8+ T cells, including naïve T cells (CD45RA+CCR7+), central memory T cells (CD45RO+CCR7+), TEM cells (CD45RO+CCR7-), and Teff cells (CD45RA+CCR7-).
The researchers found that non-relapsing patients experienced a significant reduction in TEM cells (P=0.001) and a significant increase in Teff cells (P=0.007) during cycle 1. However, this effect was not observed in patients who did relapse.
Further analysis revealed that the reduction in TEM cells was significantly associated with favorable LFS and OS in the entire cohort (P=0.0007 and P=0.005, respectively) and among patients over 60 (P<0.0001 and P=0.002, respectively).
Likewise, the increase in Teff cells was associated with favorable LFS and OS in the entire cohort (P=0.07 and P=0.04, respectively) and among patients over 60 (P=0.004 and P=0.0001, respectively).
The concomitant reduction of TEM cells and induction of Teff cells—the TEM to Teff transition—was associated with superior LFS and OS in the overall cohort (P=0.0002 and P=0.002, respectively) and in the over-60 population (P<0.0001 for LFS and OS).
The researchers said these predictors of outcome remained significant when they adjusted for potential confounders (age, risk group classification, number of induction courses required to achieve complete response, and number of consolidation courses).
Therefore, the team concluded that the altered distribution of cytotoxic T cells during treatment with HDC/IL-2 can prognosticate LFS and OS in AML patients, particularly those over 60.
“We believe that the new data may allow a personalized approach to selection of patients who are most likely to benefit from Ceplene/IL-2 treatment in AML—in particular, the older patient population, who have demonstrated almost 100% survival when positive for the T-cell transition biomarkers,” said Miri Ben-Ami, MD, executive vice president of oncology at Immune Pharmaceuticals.
“In addition, current research is revealing the potential synergy between immune checkpoint inhibitors and Ceplene, which could open the possibility of additional therapeutic indications for this combination.”
*Information in the abstract differs from that presented at the meeting.

NEW ORLEANS—A phase 4 study has revealed biomarkers that appear to predict the efficacy of treatment with histamine dihydrochloride (HDC) and interleukin-2 (IL-2) in patients with acute myeloid leukemia (AML).
Researchers found that patients who remained in complete remission after 1 cycle of HDC/IL-2 experienced a significant reduction in effector memory T cells (TEM) and a concomitant increase in effector T cells (Teff) during therapy.
This “TEM to Teff transition” was associated with favorable leukemia-free survival (LFS) and overall survival (OS), especially among patients older than 60.
Frida Ewald Sander, PhD, of University of Gothenburg in Sweden, and her colleagues presented this research at the 2016 AACR Annual Meeting (abstract CT116*).
The study was supported by The Swedish Research Council, The Swedish Cancer Society, The Swedish Society for Medical Research, Meda Pharma, and Immune Pharmaceuticals, which markets HDC as Ceplene®.
The combination of HDC and IL-2 is currently approved in more than 30 countries to prevent relapse in AML patients. In this phase 4 study (Re:MISSION), the researchers set out to assess the immunomodulatory properties of this treatment and correlate potential biomarkers with clinical outcomes.
The study included 84 non-transplanted AML patients (ages 18 to 79) in first complete remission. The patients received HDC (0.5 mg SC BID) and human recombinant IL-2 (1MIU SC BID) in 3-week cycles for 18 months. The patients were followed for at least 2 years from the start of immunotherapy to evaluate survival.
The researchers collected blood from the patients before they began HDC/IL-2 therapy and at the end of the first treatment cycle. From these samples, the team assessed the frequency of CD8+ T cells, including naïve T cells (CD45RA+CCR7+), central memory T cells (CD45RO+CCR7+), TEM cells (CD45RO+CCR7-), and Teff cells (CD45RA+CCR7-).
The researchers found that non-relapsing patients experienced a significant reduction in TEM cells (P=0.001) and a significant increase in Teff cells (P=0.007) during cycle 1. However, this effect was not observed in patients who did relapse.
Further analysis revealed that the reduction in TEM cells was significantly associated with favorable LFS and OS in the entire cohort (P=0.0007 and P=0.005, respectively) and among patients over 60 (P<0.0001 and P=0.002, respectively).
Likewise, the increase in Teff cells was associated with favorable LFS and OS in the entire cohort (P=0.07 and P=0.04, respectively) and among patients over 60 (P=0.004 and P=0.0001, respectively).
The concomitant reduction of TEM cells and induction of Teff cells—the TEM to Teff transition—was associated with superior LFS and OS in the overall cohort (P=0.0002 and P=0.002, respectively) and in the over-60 population (P<0.0001 for LFS and OS).
The researchers said these predictors of outcome remained significant when they adjusted for potential confounders (age, risk group classification, number of induction courses required to achieve complete response, and number of consolidation courses).
Therefore, the team concluded that the altered distribution of cytotoxic T cells during treatment with HDC/IL-2 can prognosticate LFS and OS in AML patients, particularly those over 60.
“We believe that the new data may allow a personalized approach to selection of patients who are most likely to benefit from Ceplene/IL-2 treatment in AML—in particular, the older patient population, who have demonstrated almost 100% survival when positive for the T-cell transition biomarkers,” said Miri Ben-Ami, MD, executive vice president of oncology at Immune Pharmaceuticals.
“In addition, current research is revealing the potential synergy between immune checkpoint inhibitors and Ceplene, which could open the possibility of additional therapeutic indications for this combination.”
*Information in the abstract differs from that presented at the meeting.

NEW ORLEANS—A phase 4 study has revealed biomarkers that appear to predict the efficacy of treatment with histamine dihydrochloride (HDC) and interleukin-2 (IL-2) in patients with acute myeloid leukemia (AML).
Researchers found that patients who remained in complete remission after 1 cycle of HDC/IL-2 experienced a significant reduction in effector memory T cells (TEM) and a concomitant increase in effector T cells (Teff) during therapy.
This “TEM to Teff transition” was associated with favorable leukemia-free survival (LFS) and overall survival (OS), especially among patients older than 60.
Frida Ewald Sander, PhD, of University of Gothenburg in Sweden, and her colleagues presented this research at the 2016 AACR Annual Meeting (abstract CT116*).
The study was supported by The Swedish Research Council, The Swedish Cancer Society, The Swedish Society for Medical Research, Meda Pharma, and Immune Pharmaceuticals, which markets HDC as Ceplene®.
The combination of HDC and IL-2 is currently approved in more than 30 countries to prevent relapse in AML patients. In this phase 4 study (Re:MISSION), the researchers set out to assess the immunomodulatory properties of this treatment and correlate potential biomarkers with clinical outcomes.
The study included 84 non-transplanted AML patients (ages 18 to 79) in first complete remission. The patients received HDC (0.5 mg SC BID) and human recombinant IL-2 (1MIU SC BID) in 3-week cycles for 18 months. The patients were followed for at least 2 years from the start of immunotherapy to evaluate survival.
The researchers collected blood from the patients before they began HDC/IL-2 therapy and at the end of the first treatment cycle. From these samples, the team assessed the frequency of CD8+ T cells, including naïve T cells (CD45RA+CCR7+), central memory T cells (CD45RO+CCR7+), TEM cells (CD45RO+CCR7-), and Teff cells (CD45RA+CCR7-).
The researchers found that non-relapsing patients experienced a significant reduction in TEM cells (P=0.001) and a significant increase in Teff cells (P=0.007) during cycle 1. However, this effect was not observed in patients who did relapse.
Further analysis revealed that the reduction in TEM cells was significantly associated with favorable LFS and OS in the entire cohort (P=0.0007 and P=0.005, respectively) and among patients over 60 (P<0.0001 and P=0.002, respectively).
Likewise, the increase in Teff cells was associated with favorable LFS and OS in the entire cohort (P=0.07 and P=0.04, respectively) and among patients over 60 (P=0.004 and P=0.0001, respectively).
The concomitant reduction of TEM cells and induction of Teff cells—the TEM to Teff transition—was associated with superior LFS and OS in the overall cohort (P=0.0002 and P=0.002, respectively) and in the over-60 population (P<0.0001 for LFS and OS).
The researchers said these predictors of outcome remained significant when they adjusted for potential confounders (age, risk group classification, number of induction courses required to achieve complete response, and number of consolidation courses).
Therefore, the team concluded that the altered distribution of cytotoxic T cells during treatment with HDC/IL-2 can prognosticate LFS and OS in AML patients, particularly those over 60.
“We believe that the new data may allow a personalized approach to selection of patients who are most likely to benefit from Ceplene/IL-2 treatment in AML—in particular, the older patient population, who have demonstrated almost 100% survival when positive for the T-cell transition biomarkers,” said Miri Ben-Ami, MD, executive vice president of oncology at Immune Pharmaceuticals.
“In addition, current research is revealing the potential synergy between immune checkpoint inhibitors and Ceplene, which could open the possibility of additional therapeutic indications for this combination.”
*Information in the abstract differs from that presented at the meeting.


