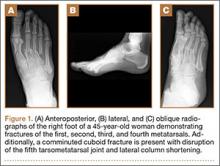
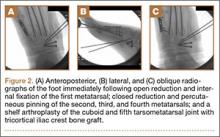
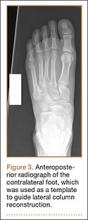
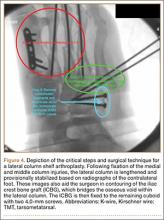
User login
Predicting and Preventing Injury in Major League Baseball
Major league baseball (MLB) is one of the most popular sports in the United States, with an average annual viewership of 11 million for the All-Star game and almost 14 million for the World Series.1 MLB has an average annual revenue of almost $10 billion, while the net worth of all 30 MLB teams combined is estimated at $36 billion; an increase of 48% from 1 year ago.2 As the sport continues to grow in popularity and receives more social media coverage, several issues, specifically injuries to its players, have come to the forefront of the news. Injuries to MLB players, specifically pitchers, have become a significant concern in recent years. The active and extended rosters in MLB include 750 and 1200 athletes, respectively, with approximately 360 active spots taken up by pitchers.3 Hence, MLB employs a large number of elite athletes within its organization. It is important to understand not only what injuries are occurring in these athletes, but also how these injuries may be prevented.
Epidemiology
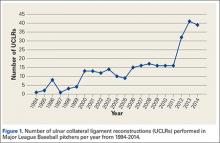
Injuries to MLB players, specifically pitchers, have increased over the past several years.4 Between 2005 and 2008, there was an overall increase of 37% in total number of injuries, with more injuries occurring in pitchers than any other position.5 While position players are more likely to sustain an injury to the lower extremity, pitchers are more likely to sustain an injury to the upper extremity.5 The month with the most injuries to MLB players was April, while the fewest number of injuries occurred in September.5 One injury that has been in the spotlight due to its dramatically increasing incidence is tear of the ulnar collateral ligament (UCL). Several studies have shown that the number of pitchers undergoing ulnar collateral ligament reconstruction (UCLR), commonly known as Tommy John surgery, has significantly increased over the past 20 years (Figure 1).4,6 Between 25% to 33% of all MLB pitchers have undergone UCLR.
While the number of primary UCLR in MLB pitchers has become a significant concern, an even more pressing concern is the number of pitchers undergoing revision UCLR, as this number has increased over the past several years.7 Currently, there is some debate as to how to best address the UCL during primary UCLR (graft type, exposure, treatment of the ulnar nerve, and graft fixation methods) because no study has shown one fixation method or graft type to be superior to others. Similarly, no study has definitively proven how to best manage the ulnar nerve (transpose in all patients, only transpose if preoperative symptoms of numbness/tingling, subluxation, etc. exist). Unfortunately, the results following revision UCLR are inferior to those following primary UCLR.4,7,8 Hence, given this information, it is imperative to both determine and implement strategies aimed at minimizing the need for revision.
Risk Factors for Injury
Although MLB has received more media attention than lower levels of baseball competition, there is relatively sparse evidence surrounding injury risk factors among MLB players. The majority of studies performed have evaluated risk factors for injury in younger baseball athletes (adolescent, high school, and college). The number of athletes at these lower levels sustaining injuries has increased over the past several years as well.9 Several large prospective studies have evaluated risk factors for shoulder and elbow injuries in adolescent baseball players. The risk factors include pitching year-round, pitching more than 100 innings per year, high pitch counts, pitching for multiple teams, geography, pitching on consecutive days, pitching while fatigued, breaking pitches, higher elbow valgus torque, pitching with higher velocity, pitching with supraspinatus weakness, and pitching with a glenohumeral internal rotation deficit (GIRD).10-17 The large majority of these risk factors are essentially part of a pitcher’s cumulative work, which consists of number of games pitched, total pitches thrown, total innings pitched, innings pitched per game, and pitches thrown per game. One prior study has evaluated cumulative work as a predictor for injury in MLB pitchers.18 While there were several issues with the study methodology, the authors found no correlation between a MLB pitcher’s cumulative work and risk for injury.
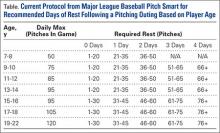
Given our current understanding of repetitive microtrauma as the pathophysiology behind these injuries, it remains unclear why cumulative work would be predictive of injury in youth pitchers but not in MLB pitchers.16 Several potential reasons exist as to why cumulative work may relate to risk of injury in youth pitchers and not MLB pitchers. Achieving MLB status may infer the element of natural selection based on technique and talent that supersedes the effect of “cumulative trauma” in many players. In MLB pitchers, cumulative work is closely monitored. In addition, these players are only playing for a single team and are not pitching competitively year-round, while many youth players play for multiple teams and may pitch year-round. To combat youth injuries, MLB Pitch Smart has developed recommendations on pitch counts and days of rest for pitchers of all age groups (Table).19 While data do not yet exist to clearly demonstrate the effectiveness of these guidelines, given the risk factors previously mentioned, it seems that these recommendations will show some reduction in youth injuries in years to come.
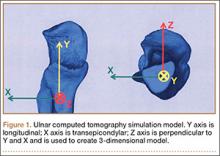
Some studies have evaluated anatomic variation among pitchers as a risk factor for injury. Polster and colleagues20 performed computed tomography (CT) scans with 3-dimensional reconstructions on the humeri of both the throwing and non-throwing arms of 25 MLB pitchers to determine if humeral torsion was related to the incidence and severity of upper extremity injuries in these athletes. The authors defined a severe injury as those which kept the player out for >30 days. Overall, 11 pitchers were injured during the 2-year study period. There was a strong inverse relationship between torsion and injury severity such that lower degrees of dominant humeral torsion correlated with higher injury severity (P = .005). However, neither throwing arm humeral torsion nor the difference in torsion between throwing and non-throwing humeri were predictive of overall injury incidence. While this is a nonmodifiable risk factor, it is important to understand how the pitcher’s anatomy plays a role in risk of injury.20 Understanding nonmodifiable risk factors may be helpful in the future to risk stratify, prognosticate, and modulate modifiable risk factors such as cumulative work.
Elbow
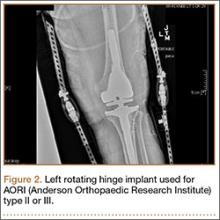
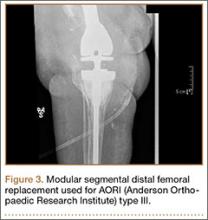
Injuries to the elbow have become more common in recent years amongst MLB players, although the literature regarding risk factors for elbow injuries is sparse.4,6 Wilk and colleagues21 performed a prospective study to determine if deficits in glenohumeral passive range of motion (ROM) increased the risk of elbow injury in MLB pitchers. Between 2005-2012, the authors measured passive shoulder ROM of both the throwing and non-throwing shoulder of 296 major and minor league pitchers and followed them for a median of 53.4 months. In total, 38 players suffered 49 elbow injuries and required 8 surgeries, accounting for a total of 2551 days spent on the disabled list (DL). GIRD and external rotation insufficiency were not correlated with elbow injuries. However, pitchers with deficits of >5° in total rotation between the throwing and non-throwing shoulders had a 2.6 times greater risk for injury (P = .007) and pitchers with deficits of ≥5° in flexion of the throwing shoulder compared to the non-throwing shoulder had a 2.8 times greater risk for injury (P = .008).21 Prior studies have demonstrated trends towards increased elbow injury in professional baseball pitchers with an increase in both elbow valgus torque as well as shoulder external rotation torque; maximum pitch velocity was also shown to be an independent risk factor for elbow injury in professional baseball pitchers.10,11 These injuries typically occur during the late cocking/early acceleration phase of the pitching cycle, when the shoulder and elbow experience the most significant force of any point in time during a pitch (Figure 2).17 At our institution, there are several ongoing studies to determine the relative contributions of pitch velocity, number, and type to elbow injury rates. Prospective studies are also ongoing at other institutions.
Shoulder
Shoulder injuries are one of the most common injuries seen in MLB players, specifically pitchers. Similar to the prior study, Wilk and colleagues22 recently performed a prospective study to determine if passive ROM of the glenohumeral joint in MLB pitchers was predictive of shoulder injury or shoulder surgery. As in the previous study, the authors’ measured passive shoulder ROM of the throwing and non-throwing shoulder of 296 major and minor league pitchers during spring training between 2005-2012 and obtained an average follow-up of 48.4 months. The authors found a total of 75 shoulder injuries and 20 surgeries among 51 pitchers (17%) that resulted in 5570 days on the DL. While total rotation deficit, GIRD, and flexion deficit had no relation to shoulder injury or surgery, pitchers with <5° greater external rotation in the throwing shoulder compared to the non-throwing shoulder were more than 2 times more likely to be placed on the DL for a shoulder injury (P = .014) and were 4 times more likely to require shoulder surgery (P = .009).22 The authors concluded that an insufficient side-to-side difference in external rotation of the throwing shoulder increased a pitcher’s likelihood of shoulder injury as well as surgery.
Other
One area that has not received as much attention as repetitive use injuries of the shoulder and elbow is acute collision injuries. Collision injuries include concussions, hyperextension injuries to the knees, shoulder dislocations, fractures of the foot and ankle, and others.23 Catchers and base runners during scoring plays are at a high risk for collision injury. Recent evidence has shown that catchers average approximately 2.75 collision injuries per 1000 athletic exposures (AE), accounting for an average of 39.1 days on the DL per collision injury.23 However, despite these collision injuries, catchers spend more time on the DL from non-collision injuries (specifically shoulder injuries requiring surgical intervention), as studies have shown 19 different non-collision injuries that accounted for >100 days on the DL for catchers compared to no collision injuries that caused a catcher to be on the DL for >100 days.23 The position of catcher is not an independent risk factor for sustaining an injury in MLB players.5
Preventative Measures
Given that recent evidence has identified certain modifiable risk factors, largely regarding shoulder ROM, for injuries to MLB pitchers, it stands to reason that by modifying these risk factors, the number of injuries to MLB pitchers can be decreased.21,22 However, to the authors’ knowledge, there have been no studies in the current literature that have clearly demonstrated the ability to prevent injuries in MLB players. Based on the prior studies, it seems logical that lowering peak pitch velocity and ensuring proper shoulder ROM would help prevent injuries in MLB players, but this remains speculative. Stretching techniques that have been shown to increase posterior shoulder soft tissue flexibility, including sleeper stretches and modified cross-body stretches, as well as closely monitoring ROM may be helpful in modifying these risk factors.24-26
Although the number of collision injuries is significantly lower than non-collision repetitive use injuries, MLB has implemented rule changes in recent years to prevent injuries to catchers and base runners alike.23,27 The rule change, which went into effect in 2014, prohibits catchers from blocking home plate unless they are actively fielding the ball or are in possession of the ball. Similarly, base runners are not allowed to deviate from their path to collide with the catcher while attempting to score.27 However, no study has analyzed whether this rule change has decreased the number of collision injuries sustained by MLB catchers, so it is unclear if this rule change has accomplished its goal.
Outcomes Following Injuries
One of the driving forces behind injury prevention in MLB players is to allow players to reach and maintain their full potential while minimizing time missed because of injury. Furthermore, as with any sport, the clinical outcomes and return to sport (RTS) rates for MLB players following injuries, especially injuries requiring surgical intervention, can be improved.4,28,29 Several studies have evaluated MLB pitchers following UCLR and have shown that over 80% of pitchers are able to RTS following surgery.4,30 When critically evaluated in multiple statistical parameters upon RTS, these players perform better in some areas and worse in others.4,30 However, the results following revision UCLR are not as encouraging as those following primary UCLR in MLB pitchers.7 Following revision UCLR, only 65% of pitchers were able to RTS, and those who were able to RTS pitched, on average, almost 1 year less than matched controls.7 Unfortunately, results following surgeries about the shoulder in MLB players have been worse than those about the elbow. Cohen and colleagues28 reported on 22 MLB players who underwent labral repair of the shoulder and found that only 32% were able to return to the same or higher level following surgery, while over 45% retired from baseball following surgery. Hence, it is imperative these injuries are prevented, as the RTS rate following treatment is less than ideal.
Future Directions
Although a concerted effort has been made over the past several years to mitigate the number of injuries sustained by MLB players, there is still significant room for improvement. New products are in development/early stages of use that attempt to determine when a pitcher begins to show signs of fatigue to allow the coach to remove him from the game. The mTHROW sleeve (Motus Global), currently used by several MLB teams, is an elastic sleeve that is worn by pitchers on their dominant arm. The sleeve approximates torque, velocity, and workload based upon an accelerometer positioned at the medial elbow and sends this information to a smart phone in real time. This technology theoretically allows players to be intensively monitored and thus may prevent injuries to the UCL by preventing pitchers from throwing while fatigued. However, elbow kinematic parameters may not change significantly as pitchers fatigue, which suggests that this strategy may be suboptimal. Trunk mechanics do change as pitchers become fatigued, opening up the possibility for shoulder and elbow injury.17,31,32 Further products that track hip-to-shoulder separation and trunk fatigue may be necessary to truly lower injury rates. However, no study has proven modifying either parameter leads to a decrease in injury rates.
Conclusion
Injuries to MLB pitchers and position players have become a significant concern over the past several years. Several risk factors for injury have been identified, including loss of shoulder ROM and pitch velocity. Further studies are necessary to determine the effectiveness of modifying these parameters on injury prevention.
1. Statista. Major League Baseball average TV viewership - selected games 2014 season (in million viewers) 2015 [cited 2015 December 12]. Available at: http://www.statista.com/statistics/251536/average-tv-viewership-of-selected-major-league-baseball-games/. Accessed December 12, 2015.
2. Ozanian M. MLB worth $36 billion as team values hit record $1.2 billion average. Forbes website. Available at: http://www.forbes.com/sites/mikeozanian/2015/03/25/mlb-worth-36-billion-as-team-values-hit-record-1-2-billion-average/. Accessed December 12, 2015.
3. Castrovince A. Equitable roster rules needed for September. Major League Baseball website. Available at: http://m.mlb.com/news/article/39009416. Accessed December 12, 2015.
4. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John Surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ Jr, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
7. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
8. Wilson AT, Pidgeon TS, Morrell NT, DaSilva MF. Trends in revision elbow ulnar collateral ligament reconstruction in professional baseball pitchers. J Hand Surg Am. 2015;40(11):2249-2254.
9. Cain EL Jr, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: Results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
10. Anz AW, Bushnell BD, Griffin LP, Noonan TJ, Torry MR, Hawkins RJ. Correlation of torque and elbow injury in professional baseball pitchers. Am J Sports Med. 2010;38(7):1368-1374.
11. Bushnell BD, Anz AW, Noonan TJ, Torry MR, Hawkins RJ. Association of maximum pitch velocity and elbow injury in professional baseball pitchers. Am J Sports Med 2010;38(4):728-732.
12. Byram IR, Bushnell BD, Dugger K, Charron K, Harrell FE Jr, Noonan TJ. Preseason shoulder strength measurements in professional baseball pitchers: identifying players at risk for injury. Am J Sports Med. 2010;38(7):1375-1382.
13. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
14. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2004;32(5):1158-1164.
15. Lyman S, Fleisig GS, Andrews JR, Osinski ED. Effect of pitch type, pitch count, and pitching mechanics on risk of elbow and shoulder pain in youth baseball pitchers. Am J Sports Med. 2002;30(4):463-468.
16. Fleisig GS, Andrews JR, Cutter GR, et al. Risk of serious injury for young baseball pitchers: a 10-year prospective study. Am J Sports Med. 2011;39(2):253-257.
17. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
18. Karakolis T, Bhan S, Crotin RL. An inferential and descriptive statistical examination of the relationship between cumulative work metrics and injury in Major League Baseball pitchers. J Strength Cond Res. 2013;27(8):2113-2118.
19. Smart MP. Guidelines for youth and adolescent pitchers. Major League Baseball website. Available at: http://m.mlb.com/pitchsmart/pitching-guidelines/. Accessed January 3, 2016.
20. Polster JM, Bullen J, Obuchowski NA, Bryan JA, Soloff L, Schickendantz MS. Relationship between humeral torsion and injury in professional baseball pitchers. Am J Sports Med. 2013;41(9):2015-2021.
21. Wilk KE, Macrina LC, Fleisig GS, et al. Deficits in glenohumeral passive range of motion increase risk of elbow injury in professional baseball pitchers: a prospective study. Am J Sports Med. 2014;42(9):2075-2081.
22. Wilk KE, Macrina LC, Fleisig GS, et al. Deficits in glenohumeral passive range of motion increase risk of shoulder injury in professional baseball pitchers: a prospective study. Am J Sports Med. 2015;43(10):2379-2385.
23. Kilcoyne KG, Ebel BG, Bancells RL, Wilckens JH, McFarland EG. Epidemiology of injuries in Major League Baseball catchers. Am J Sports Med. 2015;43(10):2496-2500.
24. Wilk KE, Hooks TR, Macrina LC. The modified sleeper stretch and modified cross-body stretch to increase shoulder internal rotation range of motion in the overhead throwing athlete. J Orthop Sports Phys Ther. 2013;43(12):891-894.
25. Laudner KG, Sipes RC, Wilson JT. The acute effects of sleeper stretches on shoulder range of motion. J Athl Train. 2008;43(4):359-363.
26. McClure P, Balaicuis J, Heiland D, Broersma ME, Thorndike CK, Wood A. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. J Orthop Sports Phys Ther. 2007;37(3):108-114.
27. Major League Baseball. MLB, MLBPA adopt experimental rule 7.13 on home plate collisions. Major League Baseball website. Available from: http://m.mlb.com/news/article/68268622/mlb-mlbpa-adopt-experimental-rule-713-on-home-plate-collisions. Accessed December 2, 2015.
28. Cohen SB, Sheridan S, Ciccotti MG. Return to sports for professional baseball players after surgery of the shoulder or elbow. Sports Health. 2011;3(1):105-111.
29. Wasserman EB, Abar B, Shah MN, Wasserman D, Bazarian JJ. Concussions are associated with decreased batting performance among Major League Baseball Players. Am J Sports Med. 2015;43(5):1127-1133.
30. Jiang JJ, Leland JM. Analysis of pitching velocity in major league baseball players before and after ulnar collateral ligament reconstruction. Am J Sports Med. 2014;42(4):880-885.
31. Crotin RL, Kozlowski K, Horvath P, Ramsey DK. Altered stride length in response to increasing exertion among baseball pitchers. Med Sci Sports Exerc. 2014;46(3):565-571.
32. Escamilla RF, Barrentine SW, Fleisig GS, et al. Pitching biomechanics as a pitcher approaches muscular fatigue during a simulated baseball game. Am J Sports Med. 2007;35(1):23-33.
Major league baseball (MLB) is one of the most popular sports in the United States, with an average annual viewership of 11 million for the All-Star game and almost 14 million for the World Series.1 MLB has an average annual revenue of almost $10 billion, while the net worth of all 30 MLB teams combined is estimated at $36 billion; an increase of 48% from 1 year ago.2 As the sport continues to grow in popularity and receives more social media coverage, several issues, specifically injuries to its players, have come to the forefront of the news. Injuries to MLB players, specifically pitchers, have become a significant concern in recent years. The active and extended rosters in MLB include 750 and 1200 athletes, respectively, with approximately 360 active spots taken up by pitchers.3 Hence, MLB employs a large number of elite athletes within its organization. It is important to understand not only what injuries are occurring in these athletes, but also how these injuries may be prevented.
Epidemiology
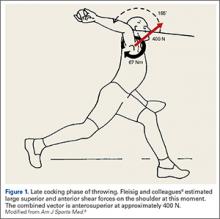
Injuries to MLB players, specifically pitchers, have increased over the past several years.4 Between 2005 and 2008, there was an overall increase of 37% in total number of injuries, with more injuries occurring in pitchers than any other position.5 While position players are more likely to sustain an injury to the lower extremity, pitchers are more likely to sustain an injury to the upper extremity.5 The month with the most injuries to MLB players was April, while the fewest number of injuries occurred in September.5 One injury that has been in the spotlight due to its dramatically increasing incidence is tear of the ulnar collateral ligament (UCL). Several studies have shown that the number of pitchers undergoing ulnar collateral ligament reconstruction (UCLR), commonly known as Tommy John surgery, has significantly increased over the past 20 years (Figure 1).4,6 Between 25% to 33% of all MLB pitchers have undergone UCLR.
While the number of primary UCLR in MLB pitchers has become a significant concern, an even more pressing concern is the number of pitchers undergoing revision UCLR, as this number has increased over the past several years.7 Currently, there is some debate as to how to best address the UCL during primary UCLR (graft type, exposure, treatment of the ulnar nerve, and graft fixation methods) because no study has shown one fixation method or graft type to be superior to others. Similarly, no study has definitively proven how to best manage the ulnar nerve (transpose in all patients, only transpose if preoperative symptoms of numbness/tingling, subluxation, etc. exist). Unfortunately, the results following revision UCLR are inferior to those following primary UCLR.4,7,8 Hence, given this information, it is imperative to both determine and implement strategies aimed at minimizing the need for revision.
Risk Factors for Injury
Although MLB has received more media attention than lower levels of baseball competition, there is relatively sparse evidence surrounding injury risk factors among MLB players. The majority of studies performed have evaluated risk factors for injury in younger baseball athletes (adolescent, high school, and college). The number of athletes at these lower levels sustaining injuries has increased over the past several years as well.9 Several large prospective studies have evaluated risk factors for shoulder and elbow injuries in adolescent baseball players. The risk factors include pitching year-round, pitching more than 100 innings per year, high pitch counts, pitching for multiple teams, geography, pitching on consecutive days, pitching while fatigued, breaking pitches, higher elbow valgus torque, pitching with higher velocity, pitching with supraspinatus weakness, and pitching with a glenohumeral internal rotation deficit (GIRD).10-17 The large majority of these risk factors are essentially part of a pitcher’s cumulative work, which consists of number of games pitched, total pitches thrown, total innings pitched, innings pitched per game, and pitches thrown per game. One prior study has evaluated cumulative work as a predictor for injury in MLB pitchers.18 While there were several issues with the study methodology, the authors found no correlation between a MLB pitcher’s cumulative work and risk for injury.
Given our current understanding of repetitive microtrauma as the pathophysiology behind these injuries, it remains unclear why cumulative work would be predictive of injury in youth pitchers but not in MLB pitchers.16 Several potential reasons exist as to why cumulative work may relate to risk of injury in youth pitchers and not MLB pitchers. Achieving MLB status may infer the element of natural selection based on technique and talent that supersedes the effect of “cumulative trauma” in many players. In MLB pitchers, cumulative work is closely monitored. In addition, these players are only playing for a single team and are not pitching competitively year-round, while many youth players play for multiple teams and may pitch year-round. To combat youth injuries, MLB Pitch Smart has developed recommendations on pitch counts and days of rest for pitchers of all age groups (Table).19 While data do not yet exist to clearly demonstrate the effectiveness of these guidelines, given the risk factors previously mentioned, it seems that these recommendations will show some reduction in youth injuries in years to come.
Some studies have evaluated anatomic variation among pitchers as a risk factor for injury. Polster and colleagues20 performed computed tomography (CT) scans with 3-dimensional reconstructions on the humeri of both the throwing and non-throwing arms of 25 MLB pitchers to determine if humeral torsion was related to the incidence and severity of upper extremity injuries in these athletes. The authors defined a severe injury as those which kept the player out for >30 days. Overall, 11 pitchers were injured during the 2-year study period. There was a strong inverse relationship between torsion and injury severity such that lower degrees of dominant humeral torsion correlated with higher injury severity (P = .005). However, neither throwing arm humeral torsion nor the difference in torsion between throwing and non-throwing humeri were predictive of overall injury incidence. While this is a nonmodifiable risk factor, it is important to understand how the pitcher’s anatomy plays a role in risk of injury.20 Understanding nonmodifiable risk factors may be helpful in the future to risk stratify, prognosticate, and modulate modifiable risk factors such as cumulative work.
Elbow
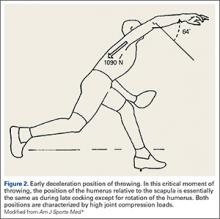
Injuries to the elbow have become more common in recent years amongst MLB players, although the literature regarding risk factors for elbow injuries is sparse.4,6 Wilk and colleagues21 performed a prospective study to determine if deficits in glenohumeral passive range of motion (ROM) increased the risk of elbow injury in MLB pitchers. Between 2005-2012, the authors measured passive shoulder ROM of both the throwing and non-throwing shoulder of 296 major and minor league pitchers and followed them for a median of 53.4 months. In total, 38 players suffered 49 elbow injuries and required 8 surgeries, accounting for a total of 2551 days spent on the disabled list (DL). GIRD and external rotation insufficiency were not correlated with elbow injuries. However, pitchers with deficits of >5° in total rotation between the throwing and non-throwing shoulders had a 2.6 times greater risk for injury (P = .007) and pitchers with deficits of ≥5° in flexion of the throwing shoulder compared to the non-throwing shoulder had a 2.8 times greater risk for injury (P = .008).21 Prior studies have demonstrated trends towards increased elbow injury in professional baseball pitchers with an increase in both elbow valgus torque as well as shoulder external rotation torque; maximum pitch velocity was also shown to be an independent risk factor for elbow injury in professional baseball pitchers.10,11 These injuries typically occur during the late cocking/early acceleration phase of the pitching cycle, when the shoulder and elbow experience the most significant force of any point in time during a pitch (Figure 2).17 At our institution, there are several ongoing studies to determine the relative contributions of pitch velocity, number, and type to elbow injury rates. Prospective studies are also ongoing at other institutions.
Shoulder
Shoulder injuries are one of the most common injuries seen in MLB players, specifically pitchers. Similar to the prior study, Wilk and colleagues22 recently performed a prospective study to determine if passive ROM of the glenohumeral joint in MLB pitchers was predictive of shoulder injury or shoulder surgery. As in the previous study, the authors’ measured passive shoulder ROM of the throwing and non-throwing shoulder of 296 major and minor league pitchers during spring training between 2005-2012 and obtained an average follow-up of 48.4 months. The authors found a total of 75 shoulder injuries and 20 surgeries among 51 pitchers (17%) that resulted in 5570 days on the DL. While total rotation deficit, GIRD, and flexion deficit had no relation to shoulder injury or surgery, pitchers with <5° greater external rotation in the throwing shoulder compared to the non-throwing shoulder were more than 2 times more likely to be placed on the DL for a shoulder injury (P = .014) and were 4 times more likely to require shoulder surgery (P = .009).22 The authors concluded that an insufficient side-to-side difference in external rotation of the throwing shoulder increased a pitcher’s likelihood of shoulder injury as well as surgery.
Other
One area that has not received as much attention as repetitive use injuries of the shoulder and elbow is acute collision injuries. Collision injuries include concussions, hyperextension injuries to the knees, shoulder dislocations, fractures of the foot and ankle, and others.23 Catchers and base runners during scoring plays are at a high risk for collision injury. Recent evidence has shown that catchers average approximately 2.75 collision injuries per 1000 athletic exposures (AE), accounting for an average of 39.1 days on the DL per collision injury.23 However, despite these collision injuries, catchers spend more time on the DL from non-collision injuries (specifically shoulder injuries requiring surgical intervention), as studies have shown 19 different non-collision injuries that accounted for >100 days on the DL for catchers compared to no collision injuries that caused a catcher to be on the DL for >100 days.23 The position of catcher is not an independent risk factor for sustaining an injury in MLB players.5
Preventative Measures
Given that recent evidence has identified certain modifiable risk factors, largely regarding shoulder ROM, for injuries to MLB pitchers, it stands to reason that by modifying these risk factors, the number of injuries to MLB pitchers can be decreased.21,22 However, to the authors’ knowledge, there have been no studies in the current literature that have clearly demonstrated the ability to prevent injuries in MLB players. Based on the prior studies, it seems logical that lowering peak pitch velocity and ensuring proper shoulder ROM would help prevent injuries in MLB players, but this remains speculative. Stretching techniques that have been shown to increase posterior shoulder soft tissue flexibility, including sleeper stretches and modified cross-body stretches, as well as closely monitoring ROM may be helpful in modifying these risk factors.24-26
Although the number of collision injuries is significantly lower than non-collision repetitive use injuries, MLB has implemented rule changes in recent years to prevent injuries to catchers and base runners alike.23,27 The rule change, which went into effect in 2014, prohibits catchers from blocking home plate unless they are actively fielding the ball or are in possession of the ball. Similarly, base runners are not allowed to deviate from their path to collide with the catcher while attempting to score.27 However, no study has analyzed whether this rule change has decreased the number of collision injuries sustained by MLB catchers, so it is unclear if this rule change has accomplished its goal.
Outcomes Following Injuries
One of the driving forces behind injury prevention in MLB players is to allow players to reach and maintain their full potential while minimizing time missed because of injury. Furthermore, as with any sport, the clinical outcomes and return to sport (RTS) rates for MLB players following injuries, especially injuries requiring surgical intervention, can be improved.4,28,29 Several studies have evaluated MLB pitchers following UCLR and have shown that over 80% of pitchers are able to RTS following surgery.4,30 When critically evaluated in multiple statistical parameters upon RTS, these players perform better in some areas and worse in others.4,30 However, the results following revision UCLR are not as encouraging as those following primary UCLR in MLB pitchers.7 Following revision UCLR, only 65% of pitchers were able to RTS, and those who were able to RTS pitched, on average, almost 1 year less than matched controls.7 Unfortunately, results following surgeries about the shoulder in MLB players have been worse than those about the elbow. Cohen and colleagues28 reported on 22 MLB players who underwent labral repair of the shoulder and found that only 32% were able to return to the same or higher level following surgery, while over 45% retired from baseball following surgery. Hence, it is imperative these injuries are prevented, as the RTS rate following treatment is less than ideal.
Future Directions
Although a concerted effort has been made over the past several years to mitigate the number of injuries sustained by MLB players, there is still significant room for improvement. New products are in development/early stages of use that attempt to determine when a pitcher begins to show signs of fatigue to allow the coach to remove him from the game. The mTHROW sleeve (Motus Global), currently used by several MLB teams, is an elastic sleeve that is worn by pitchers on their dominant arm. The sleeve approximates torque, velocity, and workload based upon an accelerometer positioned at the medial elbow and sends this information to a smart phone in real time. This technology theoretically allows players to be intensively monitored and thus may prevent injuries to the UCL by preventing pitchers from throwing while fatigued. However, elbow kinematic parameters may not change significantly as pitchers fatigue, which suggests that this strategy may be suboptimal. Trunk mechanics do change as pitchers become fatigued, opening up the possibility for shoulder and elbow injury.17,31,32 Further products that track hip-to-shoulder separation and trunk fatigue may be necessary to truly lower injury rates. However, no study has proven modifying either parameter leads to a decrease in injury rates.
Conclusion
Injuries to MLB pitchers and position players have become a significant concern over the past several years. Several risk factors for injury have been identified, including loss of shoulder ROM and pitch velocity. Further studies are necessary to determine the effectiveness of modifying these parameters on injury prevention.
Major league baseball (MLB) is one of the most popular sports in the United States, with an average annual viewership of 11 million for the All-Star game and almost 14 million for the World Series.1 MLB has an average annual revenue of almost $10 billion, while the net worth of all 30 MLB teams combined is estimated at $36 billion; an increase of 48% from 1 year ago.2 As the sport continues to grow in popularity and receives more social media coverage, several issues, specifically injuries to its players, have come to the forefront of the news. Injuries to MLB players, specifically pitchers, have become a significant concern in recent years. The active and extended rosters in MLB include 750 and 1200 athletes, respectively, with approximately 360 active spots taken up by pitchers.3 Hence, MLB employs a large number of elite athletes within its organization. It is important to understand not only what injuries are occurring in these athletes, but also how these injuries may be prevented.
Epidemiology
Injuries to MLB players, specifically pitchers, have increased over the past several years.4 Between 2005 and 2008, there was an overall increase of 37% in total number of injuries, with more injuries occurring in pitchers than any other position.5 While position players are more likely to sustain an injury to the lower extremity, pitchers are more likely to sustain an injury to the upper extremity.5 The month with the most injuries to MLB players was April, while the fewest number of injuries occurred in September.5 One injury that has been in the spotlight due to its dramatically increasing incidence is tear of the ulnar collateral ligament (UCL). Several studies have shown that the number of pitchers undergoing ulnar collateral ligament reconstruction (UCLR), commonly known as Tommy John surgery, has significantly increased over the past 20 years (Figure 1).4,6 Between 25% to 33% of all MLB pitchers have undergone UCLR.
While the number of primary UCLR in MLB pitchers has become a significant concern, an even more pressing concern is the number of pitchers undergoing revision UCLR, as this number has increased over the past several years.7 Currently, there is some debate as to how to best address the UCL during primary UCLR (graft type, exposure, treatment of the ulnar nerve, and graft fixation methods) because no study has shown one fixation method or graft type to be superior to others. Similarly, no study has definitively proven how to best manage the ulnar nerve (transpose in all patients, only transpose if preoperative symptoms of numbness/tingling, subluxation, etc. exist). Unfortunately, the results following revision UCLR are inferior to those following primary UCLR.4,7,8 Hence, given this information, it is imperative to both determine and implement strategies aimed at minimizing the need for revision.
Risk Factors for Injury
Although MLB has received more media attention than lower levels of baseball competition, there is relatively sparse evidence surrounding injury risk factors among MLB players. The majority of studies performed have evaluated risk factors for injury in younger baseball athletes (adolescent, high school, and college). The number of athletes at these lower levels sustaining injuries has increased over the past several years as well.9 Several large prospective studies have evaluated risk factors for shoulder and elbow injuries in adolescent baseball players. The risk factors include pitching year-round, pitching more than 100 innings per year, high pitch counts, pitching for multiple teams, geography, pitching on consecutive days, pitching while fatigued, breaking pitches, higher elbow valgus torque, pitching with higher velocity, pitching with supraspinatus weakness, and pitching with a glenohumeral internal rotation deficit (GIRD).10-17 The large majority of these risk factors are essentially part of a pitcher’s cumulative work, which consists of number of games pitched, total pitches thrown, total innings pitched, innings pitched per game, and pitches thrown per game. One prior study has evaluated cumulative work as a predictor for injury in MLB pitchers.18 While there were several issues with the study methodology, the authors found no correlation between a MLB pitcher’s cumulative work and risk for injury.
Given our current understanding of repetitive microtrauma as the pathophysiology behind these injuries, it remains unclear why cumulative work would be predictive of injury in youth pitchers but not in MLB pitchers.16 Several potential reasons exist as to why cumulative work may relate to risk of injury in youth pitchers and not MLB pitchers. Achieving MLB status may infer the element of natural selection based on technique and talent that supersedes the effect of “cumulative trauma” in many players. In MLB pitchers, cumulative work is closely monitored. In addition, these players are only playing for a single team and are not pitching competitively year-round, while many youth players play for multiple teams and may pitch year-round. To combat youth injuries, MLB Pitch Smart has developed recommendations on pitch counts and days of rest for pitchers of all age groups (Table).19 While data do not yet exist to clearly demonstrate the effectiveness of these guidelines, given the risk factors previously mentioned, it seems that these recommendations will show some reduction in youth injuries in years to come.
Some studies have evaluated anatomic variation among pitchers as a risk factor for injury. Polster and colleagues20 performed computed tomography (CT) scans with 3-dimensional reconstructions on the humeri of both the throwing and non-throwing arms of 25 MLB pitchers to determine if humeral torsion was related to the incidence and severity of upper extremity injuries in these athletes. The authors defined a severe injury as those which kept the player out for >30 days. Overall, 11 pitchers were injured during the 2-year study period. There was a strong inverse relationship between torsion and injury severity such that lower degrees of dominant humeral torsion correlated with higher injury severity (P = .005). However, neither throwing arm humeral torsion nor the difference in torsion between throwing and non-throwing humeri were predictive of overall injury incidence. While this is a nonmodifiable risk factor, it is important to understand how the pitcher’s anatomy plays a role in risk of injury.20 Understanding nonmodifiable risk factors may be helpful in the future to risk stratify, prognosticate, and modulate modifiable risk factors such as cumulative work.
Elbow
Injuries to the elbow have become more common in recent years amongst MLB players, although the literature regarding risk factors for elbow injuries is sparse.4,6 Wilk and colleagues21 performed a prospective study to determine if deficits in glenohumeral passive range of motion (ROM) increased the risk of elbow injury in MLB pitchers. Between 2005-2012, the authors measured passive shoulder ROM of both the throwing and non-throwing shoulder of 296 major and minor league pitchers and followed them for a median of 53.4 months. In total, 38 players suffered 49 elbow injuries and required 8 surgeries, accounting for a total of 2551 days spent on the disabled list (DL). GIRD and external rotation insufficiency were not correlated with elbow injuries. However, pitchers with deficits of >5° in total rotation between the throwing and non-throwing shoulders had a 2.6 times greater risk for injury (P = .007) and pitchers with deficits of ≥5° in flexion of the throwing shoulder compared to the non-throwing shoulder had a 2.8 times greater risk for injury (P = .008).21 Prior studies have demonstrated trends towards increased elbow injury in professional baseball pitchers with an increase in both elbow valgus torque as well as shoulder external rotation torque; maximum pitch velocity was also shown to be an independent risk factor for elbow injury in professional baseball pitchers.10,11 These injuries typically occur during the late cocking/early acceleration phase of the pitching cycle, when the shoulder and elbow experience the most significant force of any point in time during a pitch (Figure 2).17 At our institution, there are several ongoing studies to determine the relative contributions of pitch velocity, number, and type to elbow injury rates. Prospective studies are also ongoing at other institutions.
Shoulder
Shoulder injuries are one of the most common injuries seen in MLB players, specifically pitchers. Similar to the prior study, Wilk and colleagues22 recently performed a prospective study to determine if passive ROM of the glenohumeral joint in MLB pitchers was predictive of shoulder injury or shoulder surgery. As in the previous study, the authors’ measured passive shoulder ROM of the throwing and non-throwing shoulder of 296 major and minor league pitchers during spring training between 2005-2012 and obtained an average follow-up of 48.4 months. The authors found a total of 75 shoulder injuries and 20 surgeries among 51 pitchers (17%) that resulted in 5570 days on the DL. While total rotation deficit, GIRD, and flexion deficit had no relation to shoulder injury or surgery, pitchers with <5° greater external rotation in the throwing shoulder compared to the non-throwing shoulder were more than 2 times more likely to be placed on the DL for a shoulder injury (P = .014) and were 4 times more likely to require shoulder surgery (P = .009).22 The authors concluded that an insufficient side-to-side difference in external rotation of the throwing shoulder increased a pitcher’s likelihood of shoulder injury as well as surgery.
Other
One area that has not received as much attention as repetitive use injuries of the shoulder and elbow is acute collision injuries. Collision injuries include concussions, hyperextension injuries to the knees, shoulder dislocations, fractures of the foot and ankle, and others.23 Catchers and base runners during scoring plays are at a high risk for collision injury. Recent evidence has shown that catchers average approximately 2.75 collision injuries per 1000 athletic exposures (AE), accounting for an average of 39.1 days on the DL per collision injury.23 However, despite these collision injuries, catchers spend more time on the DL from non-collision injuries (specifically shoulder injuries requiring surgical intervention), as studies have shown 19 different non-collision injuries that accounted for >100 days on the DL for catchers compared to no collision injuries that caused a catcher to be on the DL for >100 days.23 The position of catcher is not an independent risk factor for sustaining an injury in MLB players.5
Preventative Measures
Given that recent evidence has identified certain modifiable risk factors, largely regarding shoulder ROM, for injuries to MLB pitchers, it stands to reason that by modifying these risk factors, the number of injuries to MLB pitchers can be decreased.21,22 However, to the authors’ knowledge, there have been no studies in the current literature that have clearly demonstrated the ability to prevent injuries in MLB players. Based on the prior studies, it seems logical that lowering peak pitch velocity and ensuring proper shoulder ROM would help prevent injuries in MLB players, but this remains speculative. Stretching techniques that have been shown to increase posterior shoulder soft tissue flexibility, including sleeper stretches and modified cross-body stretches, as well as closely monitoring ROM may be helpful in modifying these risk factors.24-26
Although the number of collision injuries is significantly lower than non-collision repetitive use injuries, MLB has implemented rule changes in recent years to prevent injuries to catchers and base runners alike.23,27 The rule change, which went into effect in 2014, prohibits catchers from blocking home plate unless they are actively fielding the ball or are in possession of the ball. Similarly, base runners are not allowed to deviate from their path to collide with the catcher while attempting to score.27 However, no study has analyzed whether this rule change has decreased the number of collision injuries sustained by MLB catchers, so it is unclear if this rule change has accomplished its goal.
Outcomes Following Injuries
One of the driving forces behind injury prevention in MLB players is to allow players to reach and maintain their full potential while minimizing time missed because of injury. Furthermore, as with any sport, the clinical outcomes and return to sport (RTS) rates for MLB players following injuries, especially injuries requiring surgical intervention, can be improved.4,28,29 Several studies have evaluated MLB pitchers following UCLR and have shown that over 80% of pitchers are able to RTS following surgery.4,30 When critically evaluated in multiple statistical parameters upon RTS, these players perform better in some areas and worse in others.4,30 However, the results following revision UCLR are not as encouraging as those following primary UCLR in MLB pitchers.7 Following revision UCLR, only 65% of pitchers were able to RTS, and those who were able to RTS pitched, on average, almost 1 year less than matched controls.7 Unfortunately, results following surgeries about the shoulder in MLB players have been worse than those about the elbow. Cohen and colleagues28 reported on 22 MLB players who underwent labral repair of the shoulder and found that only 32% were able to return to the same or higher level following surgery, while over 45% retired from baseball following surgery. Hence, it is imperative these injuries are prevented, as the RTS rate following treatment is less than ideal.
Future Directions
Although a concerted effort has been made over the past several years to mitigate the number of injuries sustained by MLB players, there is still significant room for improvement. New products are in development/early stages of use that attempt to determine when a pitcher begins to show signs of fatigue to allow the coach to remove him from the game. The mTHROW sleeve (Motus Global), currently used by several MLB teams, is an elastic sleeve that is worn by pitchers on their dominant arm. The sleeve approximates torque, velocity, and workload based upon an accelerometer positioned at the medial elbow and sends this information to a smart phone in real time. This technology theoretically allows players to be intensively monitored and thus may prevent injuries to the UCL by preventing pitchers from throwing while fatigued. However, elbow kinematic parameters may not change significantly as pitchers fatigue, which suggests that this strategy may be suboptimal. Trunk mechanics do change as pitchers become fatigued, opening up the possibility for shoulder and elbow injury.17,31,32 Further products that track hip-to-shoulder separation and trunk fatigue may be necessary to truly lower injury rates. However, no study has proven modifying either parameter leads to a decrease in injury rates.
Conclusion
Injuries to MLB pitchers and position players have become a significant concern over the past several years. Several risk factors for injury have been identified, including loss of shoulder ROM and pitch velocity. Further studies are necessary to determine the effectiveness of modifying these parameters on injury prevention.
1. Statista. Major League Baseball average TV viewership - selected games 2014 season (in million viewers) 2015 [cited 2015 December 12]. Available at: http://www.statista.com/statistics/251536/average-tv-viewership-of-selected-major-league-baseball-games/. Accessed December 12, 2015.
2. Ozanian M. MLB worth $36 billion as team values hit record $1.2 billion average. Forbes website. Available at: http://www.forbes.com/sites/mikeozanian/2015/03/25/mlb-worth-36-billion-as-team-values-hit-record-1-2-billion-average/. Accessed December 12, 2015.
3. Castrovince A. Equitable roster rules needed for September. Major League Baseball website. Available at: http://m.mlb.com/news/article/39009416. Accessed December 12, 2015.
4. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John Surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ Jr, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
7. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
8. Wilson AT, Pidgeon TS, Morrell NT, DaSilva MF. Trends in revision elbow ulnar collateral ligament reconstruction in professional baseball pitchers. J Hand Surg Am. 2015;40(11):2249-2254.
9. Cain EL Jr, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: Results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
10. Anz AW, Bushnell BD, Griffin LP, Noonan TJ, Torry MR, Hawkins RJ. Correlation of torque and elbow injury in professional baseball pitchers. Am J Sports Med. 2010;38(7):1368-1374.
11. Bushnell BD, Anz AW, Noonan TJ, Torry MR, Hawkins RJ. Association of maximum pitch velocity and elbow injury in professional baseball pitchers. Am J Sports Med 2010;38(4):728-732.
12. Byram IR, Bushnell BD, Dugger K, Charron K, Harrell FE Jr, Noonan TJ. Preseason shoulder strength measurements in professional baseball pitchers: identifying players at risk for injury. Am J Sports Med. 2010;38(7):1375-1382.
13. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
14. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2004;32(5):1158-1164.
15. Lyman S, Fleisig GS, Andrews JR, Osinski ED. Effect of pitch type, pitch count, and pitching mechanics on risk of elbow and shoulder pain in youth baseball pitchers. Am J Sports Med. 2002;30(4):463-468.
16. Fleisig GS, Andrews JR, Cutter GR, et al. Risk of serious injury for young baseball pitchers: a 10-year prospective study. Am J Sports Med. 2011;39(2):253-257.
17. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
18. Karakolis T, Bhan S, Crotin RL. An inferential and descriptive statistical examination of the relationship between cumulative work metrics and injury in Major League Baseball pitchers. J Strength Cond Res. 2013;27(8):2113-2118.
19. Smart MP. Guidelines for youth and adolescent pitchers. Major League Baseball website. Available at: http://m.mlb.com/pitchsmart/pitching-guidelines/. Accessed January 3, 2016.
20. Polster JM, Bullen J, Obuchowski NA, Bryan JA, Soloff L, Schickendantz MS. Relationship between humeral torsion and injury in professional baseball pitchers. Am J Sports Med. 2013;41(9):2015-2021.
21. Wilk KE, Macrina LC, Fleisig GS, et al. Deficits in glenohumeral passive range of motion increase risk of elbow injury in professional baseball pitchers: a prospective study. Am J Sports Med. 2014;42(9):2075-2081.
22. Wilk KE, Macrina LC, Fleisig GS, et al. Deficits in glenohumeral passive range of motion increase risk of shoulder injury in professional baseball pitchers: a prospective study. Am J Sports Med. 2015;43(10):2379-2385.
23. Kilcoyne KG, Ebel BG, Bancells RL, Wilckens JH, McFarland EG. Epidemiology of injuries in Major League Baseball catchers. Am J Sports Med. 2015;43(10):2496-2500.
24. Wilk KE, Hooks TR, Macrina LC. The modified sleeper stretch and modified cross-body stretch to increase shoulder internal rotation range of motion in the overhead throwing athlete. J Orthop Sports Phys Ther. 2013;43(12):891-894.
25. Laudner KG, Sipes RC, Wilson JT. The acute effects of sleeper stretches on shoulder range of motion. J Athl Train. 2008;43(4):359-363.
26. McClure P, Balaicuis J, Heiland D, Broersma ME, Thorndike CK, Wood A. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. J Orthop Sports Phys Ther. 2007;37(3):108-114.
27. Major League Baseball. MLB, MLBPA adopt experimental rule 7.13 on home plate collisions. Major League Baseball website. Available from: http://m.mlb.com/news/article/68268622/mlb-mlbpa-adopt-experimental-rule-713-on-home-plate-collisions. Accessed December 2, 2015.
28. Cohen SB, Sheridan S, Ciccotti MG. Return to sports for professional baseball players after surgery of the shoulder or elbow. Sports Health. 2011;3(1):105-111.
29. Wasserman EB, Abar B, Shah MN, Wasserman D, Bazarian JJ. Concussions are associated with decreased batting performance among Major League Baseball Players. Am J Sports Med. 2015;43(5):1127-1133.
30. Jiang JJ, Leland JM. Analysis of pitching velocity in major league baseball players before and after ulnar collateral ligament reconstruction. Am J Sports Med. 2014;42(4):880-885.
31. Crotin RL, Kozlowski K, Horvath P, Ramsey DK. Altered stride length in response to increasing exertion among baseball pitchers. Med Sci Sports Exerc. 2014;46(3):565-571.
32. Escamilla RF, Barrentine SW, Fleisig GS, et al. Pitching biomechanics as a pitcher approaches muscular fatigue during a simulated baseball game. Am J Sports Med. 2007;35(1):23-33.
1. Statista. Major League Baseball average TV viewership - selected games 2014 season (in million viewers) 2015 [cited 2015 December 12]. Available at: http://www.statista.com/statistics/251536/average-tv-viewership-of-selected-major-league-baseball-games/. Accessed December 12, 2015.
2. Ozanian M. MLB worth $36 billion as team values hit record $1.2 billion average. Forbes website. Available at: http://www.forbes.com/sites/mikeozanian/2015/03/25/mlb-worth-36-billion-as-team-values-hit-record-1-2-billion-average/. Accessed December 12, 2015.
3. Castrovince A. Equitable roster rules needed for September. Major League Baseball website. Available at: http://m.mlb.com/news/article/39009416. Accessed December 12, 2015.
4. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John Surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ Jr, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
7. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
8. Wilson AT, Pidgeon TS, Morrell NT, DaSilva MF. Trends in revision elbow ulnar collateral ligament reconstruction in professional baseball pitchers. J Hand Surg Am. 2015;40(11):2249-2254.
9. Cain EL Jr, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: Results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
10. Anz AW, Bushnell BD, Griffin LP, Noonan TJ, Torry MR, Hawkins RJ. Correlation of torque and elbow injury in professional baseball pitchers. Am J Sports Med. 2010;38(7):1368-1374.
11. Bushnell BD, Anz AW, Noonan TJ, Torry MR, Hawkins RJ. Association of maximum pitch velocity and elbow injury in professional baseball pitchers. Am J Sports Med 2010;38(4):728-732.
12. Byram IR, Bushnell BD, Dugger K, Charron K, Harrell FE Jr, Noonan TJ. Preseason shoulder strength measurements in professional baseball pitchers: identifying players at risk for injury. Am J Sports Med. 2010;38(7):1375-1382.
13. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
14. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2004;32(5):1158-1164.
15. Lyman S, Fleisig GS, Andrews JR, Osinski ED. Effect of pitch type, pitch count, and pitching mechanics on risk of elbow and shoulder pain in youth baseball pitchers. Am J Sports Med. 2002;30(4):463-468.
16. Fleisig GS, Andrews JR, Cutter GR, et al. Risk of serious injury for young baseball pitchers: a 10-year prospective study. Am J Sports Med. 2011;39(2):253-257.
17. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
18. Karakolis T, Bhan S, Crotin RL. An inferential and descriptive statistical examination of the relationship between cumulative work metrics and injury in Major League Baseball pitchers. J Strength Cond Res. 2013;27(8):2113-2118.
19. Smart MP. Guidelines for youth and adolescent pitchers. Major League Baseball website. Available at: http://m.mlb.com/pitchsmart/pitching-guidelines/. Accessed January 3, 2016.
20. Polster JM, Bullen J, Obuchowski NA, Bryan JA, Soloff L, Schickendantz MS. Relationship between humeral torsion and injury in professional baseball pitchers. Am J Sports Med. 2013;41(9):2015-2021.
21. Wilk KE, Macrina LC, Fleisig GS, et al. Deficits in glenohumeral passive range of motion increase risk of elbow injury in professional baseball pitchers: a prospective study. Am J Sports Med. 2014;42(9):2075-2081.
22. Wilk KE, Macrina LC, Fleisig GS, et al. Deficits in glenohumeral passive range of motion increase risk of shoulder injury in professional baseball pitchers: a prospective study. Am J Sports Med. 2015;43(10):2379-2385.
23. Kilcoyne KG, Ebel BG, Bancells RL, Wilckens JH, McFarland EG. Epidemiology of injuries in Major League Baseball catchers. Am J Sports Med. 2015;43(10):2496-2500.
24. Wilk KE, Hooks TR, Macrina LC. The modified sleeper stretch and modified cross-body stretch to increase shoulder internal rotation range of motion in the overhead throwing athlete. J Orthop Sports Phys Ther. 2013;43(12):891-894.
25. Laudner KG, Sipes RC, Wilson JT. The acute effects of sleeper stretches on shoulder range of motion. J Athl Train. 2008;43(4):359-363.
26. McClure P, Balaicuis J, Heiland D, Broersma ME, Thorndike CK, Wood A. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. J Orthop Sports Phys Ther. 2007;37(3):108-114.
27. Major League Baseball. MLB, MLBPA adopt experimental rule 7.13 on home plate collisions. Major League Baseball website. Available from: http://m.mlb.com/news/article/68268622/mlb-mlbpa-adopt-experimental-rule-713-on-home-plate-collisions. Accessed December 2, 2015.
28. Cohen SB, Sheridan S, Ciccotti MG. Return to sports for professional baseball players after surgery of the shoulder or elbow. Sports Health. 2011;3(1):105-111.
29. Wasserman EB, Abar B, Shah MN, Wasserman D, Bazarian JJ. Concussions are associated with decreased batting performance among Major League Baseball Players. Am J Sports Med. 2015;43(5):1127-1133.
30. Jiang JJ, Leland JM. Analysis of pitching velocity in major league baseball players before and after ulnar collateral ligament reconstruction. Am J Sports Med. 2014;42(4):880-885.
31. Crotin RL, Kozlowski K, Horvath P, Ramsey DK. Altered stride length in response to increasing exertion among baseball pitchers. Med Sci Sports Exerc. 2014;46(3):565-571.
32. Escamilla RF, Barrentine SW, Fleisig GS, et al. Pitching biomechanics as a pitcher approaches muscular fatigue during a simulated baseball game. Am J Sports Med. 2007;35(1):23-33.
Valgus Extension Overload in Baseball Players
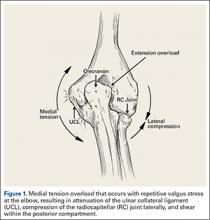
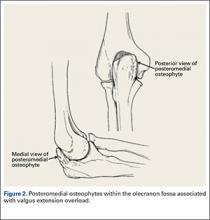
The supraphysiological demands imposed on the elbow of a throwing athlete result in predictable patterns of injury. This is especially true of baseball pitchers. Knowledge of elbow anatomy, as well as the biomechanics of throwing, assist in making diagnostic and therapeutic decisions and also influence surgical technique when surgery is required. During the late cocking and early acceleration phases of throwing, valgus torque can reach 65 Nm with angular velocities of the forearm reaching 5000°/sec, which is considered the fasted recorded human movment.1 The valgus torque and rapid extension synergistically create 3 major forces placed on the elbow. The first is a tensile stress along the medial aspect of elbow affecting the ulnar collateral ligament (UCL), flexor pronator mass, and medial epicondyle. Secondly, compression forces affect the lateral aspect of the elbow at the radiocapitellar joint. Finally, a shearing stress occurs in the posterior compartment at the posterior medial tip of the olecranon and the olecranon fossa.
These forces generated on the elbow result in predictable pathology. The recurring tensile forces applied on the medial aspect on the elbow can compromise the integrity of the UCL. It is well known that injury to the UCL leads to valgus instability. Individuals with valgus instability who continue to throw may trigger and/or aggravate injury in the posterior and lateral components of the elbow. Lateral compression forces can often reach 500 N, resulting in radiocapitellar overload syndrome, which occurs in combination with medial ligament instability and valgus extension overload.2 Radiocapitellar compression may cause chondral or osteochondral fracture with resulting intra-articular loose bodes. This compression also contributes to the etiology of osteochondritis dissecans (OCD) in skeletally immature athletes. In the posterior elbow, throwing forcefully and repeatedly pushes the olecranon into the olecranon fossa. Shear stress on the medial olecranon tip and fossa, due to combined valgus and extension forces, lead to the development of osteophytes. This collection of injuries in the medial, lateral, and posterior aspects of the elbow is known as “valgus extension overload syndrome” or VEO. Symptoms in VEO can be the result of chondral lesions, loose bodies, and marginal exostosis.3
The aim of this review is to provide understanding regarding both the relevant anatomy and pathomechanics of VEO, key aspects to clinical evaluation, and effective treatment options.
Functional Anatomy
A functional comprehension of elbow anatomy and biomechanics is essential to understanding the constellation of injuries in VEO. The osseous anatomy of the elbow permits a variety of movements. These include flexion-extension and pronation-supination, which are mediated by the ulnohumeral and radiocapitellar articulations. While in full extension, the elbow has a normal valgus carrying angle of 11° to 16°. It is important to know that 50% of the elbow’s stability is attributed to the configuration of the bones.4-6 This is especially true in varus stress while the elbow is in full extension. The soft tissues, including muscle and ligaments such as the UCL, lateral UCL, and radial UCL complexes, provide the remaining elbow stability.4-6
The UCL complex is composed of 3 main segments known as the anterior, posterior, and oblique bundles (transverse ligament). Collectively, these bundles are responsible for providing medial elbow stability. However, each of these bundles contributes to medial elbow stability in its own way. The first and arguably the most important bundle is the anterior bundle; its most important function is providing stability against valgus stress.4,5,7 It is composed of parallel fibers inserting on the medial coronoid process.4,5,7 Furthermore, its eccentric location with respect to the axis of elbow allows it to provide stability throughout the full range of elbow motion.6 The anterior bundle can be further divided into individual anterior and posterior bands that have reciprocal functionality.5,8,9 The anterior band acts as the chief restraint to valgus stress up to 90° of flexion.9 Any flexion beyond 90° renders the anterior band’s role secondary in resisting valgus stress.9 The posterior band’s function in resisting valgus stress is most important between 60° and full flexion, while having a secondary role in lesser degrees of flexion.8,9 Notably, the posterior band is isometric and is more important in the overhead-throwing athlete due to the fact its primary role in resisting valgus stress occurs at higher degrees of flexion.10
The remaining posterior and oblique bundles of the UCL complex have lesser roles in maintaining elbow stability. The posterior bundle of the UCL complex is fan-shaped, originates from the medial epicondyle, and inserts onto the medial margin the semi-lunar notch. It is more slender and frailer than the anterior bundle. This is reflected in its functionality, as it plays a secondary role in elbow stability during elbow flexion beyond 90°.4,5,8 In contrast to the anterior and posterior bundles, the oblique bundle, also known as the transverse ligament, does not cross the elbow joint. It is a thickening of the caudal most aspect of the joint capsule, which extends from the medial olecranon to the inferior medial coronoid process and as a result functions in expanding the greater sigmoid notch.6
The musculotendinous components of the elbow are essential to providing dynamic functional resistance to valgus stress.11 These components are flexor-pronator musculature that originate from the medial epicondyle. Listed proximally to distally, the flexor-pronator muscles include pronator teres, flexor carpi radialis (FCR), palmaris longus, flexor digitorum superficialis, and the flexor carpi ulnaris (FCU).
Pathomechanics
Once familiarized with the relevant function anatomy, it is crucial to understand the mechanics of throwing in order to understand the pathomechanics of VEO. The action of overhead throwing has been divided into 6 phases.6,12-16 Phase 4, acceleration, is the most relevant when discussing forces on elbow, since the majority of forces are generated during this state. Phase 4 represents a rapid acceleration of the upper extremity with a large forward-directed force on the arm generated by the shoulder muscles. Additionally, there is internal rotation and adduction of the humerus with rapid elbow extension terminating with ball release. The elbow accelerates up to 600,000°/sec2 in a miniscule time frame of 40 to 50 milliseconds.1,5 Immense valgus forces are exerted on the medial aspect of the elbow. The anterior bundle of the UCL bears the majority of the force, with the flexor pronator mass enabling the transmission.11 The majority of injuries occur during stage 4 as a result of the stress load on the medial elbow structures like the UCL. The proceeding phases 5 (deceleration), and 6 (follow-through) involve eventual dissipation of excess kinetic energy as the elbow completely extends. The deceleration during phase 5 is rapid and powerful, occurring at about 500,000°/sec2 in the short span of 50 milliseconds.1,6,12-16 High-velocity throwing, such as baseball pitching, generates forces in the elbow that are opposed by the articular, ligamentous, and muscular portions of the arm. The ulnohumeral articulation stabilizes motion of the arm from 0° to 20° of flexion and beyond 120° of flexion. Static and dynamic soft tissues maintain stability during the remaining of 100° arc of motion.
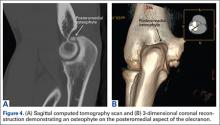
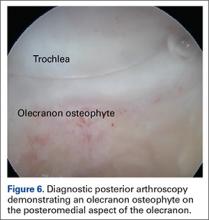
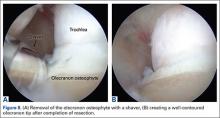
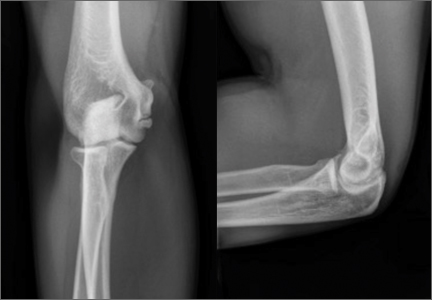
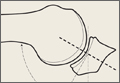
During deceleration, the elbow undergoes terminal extension resulting in the posteromedial olecranon contacting the trochlea and the olecranon fossa with subsequent dissipation of the combined valgus force and angular moment (Figure 1). This dissipation of force creates pathologic shear and compressive forces in the posterior elbow. Poor muscular control and the traumatic abutment that occurs in the posterior compartment may further add to the pathologic forces. Reactive bone formation is induced by the repetitive compression and shear, resulting in osteophytes on the posteromedial tip of the olecranon (Figure 2). Consequent “kissing lesions” of chondromalacia may occur in the olecranon fossa and posteromedial trochlea. The subsequent development of loose bodies may also occur. The presence of osteophytes and/or loose bodies may result in posteromedial impingement (PMI).
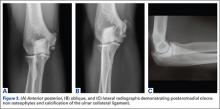
The association between PMI of the olecranon and valgus instability has been elucidated in both clinical and biomechanical investigations.17,18,19,20 Conway18 identified tip exostosis in 24% of lateral radiographs of 135 asymptomatic professional pitchers. Approximately one-fifth (21%) of these pitchers had >1.0 mm increased relative valgus laxity on stress radiographs. Roughly one-third (34%) of players with exostosis had >1.0 mm of increased relative valgus laxity, compared to 16% of players without exostosis formation. These results provide evidence for a probable association between PMI and valgus laxity. In biomechanical research, Ahmad and colleagues17 studied the effect of partial and full thickness UCL injuries on contact forces of the posterior elbow. Posteromedial compartments of cadaver specimens were subjected to physiologic valgus stresses while placed on pressure-senstive film. Contact area and pressure between posteromedial trochlea and olecranon were altered in the setting of UCL insufficiency, helping explain how posteromedial osteophyte formation occurs.
Additional biomechanical studies have also investigated the posteromedial olecranon’s role in functioning as a stabilizing buttress to medial tensile forces. Treating PMI with aggressive bone removal may increase valgus instability as well as strain on the UCL, leading to UCL injury following olecranon resection.19,20 Kamineni and colleagues19 investigated strain on anterior bundle of UCL as a function of increasing applied torque and posteromedial resections of the olecranon. This investigation was done utilizing an electromagnetic tracking placed in cadaver elbows. A nonuniform change in strain was found at 3 mm of resection during flexion and valgus testing. This nonuniform change implied that removal of posteromedial olecranon beyond 3 mm made the UCL more vulnerable to injury. Follow-up investigations looked at kinematic effects of increasing valgus and varus torques and sequential posteromedial olecranon resections.20 Valgus angulation of the elbow increased with all resection levels but no critical amount of olecranon resection was identified. The consensus in the literature indicates that posteromedial articulation of the elbow is a significant stabilizer to valgus stress.17-22 Thus, normal bone should be preserved and only osteophytes should be removed during treatment.
In addition, VEO may lead to injury in the lateral compartment as well. After attenuation and insufficiency of the UCL due to repetitive stress, excessive force transmission to the lateral aspect of the elbow occurs. Compressive and rotatory forces escalate within the radiocapitellar joint, causing synovitis and osteochondral lesions.3,23 These osteochondral lesions include osteochondritis dissecans and osteochondral fractures that may fragment and become loose bodies.
Evaluation of VEO
History
Patients will typically have a history of repetitive throwing or other repetitive overhead activity. VEO is most common in baseball pitchers but may also occur in other sports, such as tennis, football, lacrosse, gymnastics, and javelin throwing. In baseball pitchers, clinical presentation is often preceded by a decrease in pitch velocity, control, and early fatigability. It presents with elbow pain localized to the posteromedial aspect of olecranon after release of the ball, when the elbow reaches terminal extension. Patients also report limited extension, due to impinging posterior osteophytes. Also, locking and catching caused by loose bodies and chondromalacia may be present. VEO may also occur in combination with concomitant valgus instability, as well as in a patient with a prior history of valgus instability. Flexor pronator injury, ulnar neuritis, and subluxation may also be present in a patient with VEO.
Physical Examination
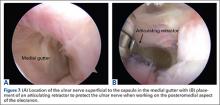
VEO may occur in an isolated fashion or with concomitant pathology. Therefore, a comprehensive physical examination includes evaluating the entire kinetic chain of throwing and a focused examination covering VEO and associated valgus instability. Patients may exhibit crepitus and tenderness over the posteromedial olecranon and a loss of extension with a firm end point. The extension impingement test should be performed where the elbow is snapped into terminal extension. This typically elicits pain in the posterior compartment in a patient with VEO. The arm bar test involves positioning the patient’s shoulder at 90° of forward flexion, full internal rotation, with the patient’s hand placed on the examiner’s shoulder.24 The examiner pulls down on the olecranon, simulating forced extension; pain is indicative of a positive test. It is important to note if there are signs of ulnar neuritis or subluxing ulnar nerve, especially if planning to utilize medial portals during arthroscopic treatment.
Examination maneuvers for valgus instability should also be conducted during evaluation of VEO. The physical examination for valgus instability in the elbow is ideally performed with the patient seated. Secure the patient’s wrist between the examiner’s forearm and trunk, and flex the patient’s elbow between 20° and 30° to unlock the olecranon from its fossa. Proceed to apply valgus stress. This stresses the anterior band of the anterior bundle of the UCL.6,25,26 Palpate the UCL from the medial epicondyle to the proximal ulna as valgus stress is applied. Occasionally, valgus laxity can be appreciated when compared to contralateral side. The milking maneuver is a helpful test to determine UCL injury. Pull on the patient’s thumb while the forearm is supinated, shoulder extended, and the elbow flexed beyond 90°.6 The milking maneuver exerts valgus stress on a flexed elbow. A patient with an injured UCL will experience the subjective feeling of apprehension and instability, with medial elbow pain.
The most sensitive test is the moving valgus stress test. This is performed with the patient in the upright position and the shoulder abducted 90°. Starting with the arm in full flexion, the examiner applies a constant valgus torque to the elbow and then rapidly extends the elbow. Reproduction of pain during range of motion from 120° to 70° represents UCL injury, while pain with extension beyond 70° represents chondral injury to the ulnohumeral joint. Be aware that the absence of increased pain with wrist flexion, along with pain localized slightly posterior to the common flexor origin, differentiates a UCL injury from flexor-pronator muscle injury.6,26,27Examine range of motion in affected and unaffected elbows. Loss of terminal extension may be present, along with secondary to flexion contracture due to repeated attempts at healing and stabilization.25
Imaging
Imaging is essential to the accurate diagnosis of VEO and related conditions. Anterior posterior (AP), lateral, and oblique radiographs of elbow (Figures 3A-3C) may show posteromedial olecranon osteophytes and/or loose bodies. Calcification of ligaments or other soft tissues may also be seen. An AP radiograph with 140° of external rotation may best visualize osteophytes on posteromedial olecranon.18 A computed tomography scan with 2-dimensional sagittal and coronal reconstruction and 3-dimensional surface rendering (Figures 4A, 4B) may best demonstrate morphological abnormalities, loose bodies, and osteophytes. Magnetic resonance imaging (MRI) is essential for assessment of soft tissues and chondral injuries. MRI may detect UCL compromise, synovial plicae, bone edema, olecranon, or stress fractures.
Treatment
Nonoperative Treatment
Treatment consists of both nonoperative and operative modalities. Nonoperative treatment methods are first line in treating VEO. Patients should modify their physical activity and rest from throwing activities. Nonsteroid anti-inflammatory drugs are appropriate to treat pain along with intra-articular corticosteroid injections of the elbow. A wide assessment of pitching mechanics should be performed in an attempt to correct errors in throwing technique and address muscular imbalances. After cessation of the resting period, the patient may initiate a progressive throwing program supervised by an experienced therapist and trainer. A plan for returning to competition should be made upon completion of the throwing program.
Operative Treatment
Surgical treatment is reserved for patients who fail nonoperative treatment. These patients have persistent symptoms of posteromedial impingement and desire to return to pre-injury level of performance. Posteromedial decompression is not recommended when provocative physical examination maneuvers are negative, regardless of presence of olecranon osteophytes on imaging. Osteophytes are an asymptomatic finding typically seen in professional baseball players and do not warrant surgical treatment.18,28 UCL compromise is a relative contraindication to olecranon debridement as UCL injury could become symptomatic following surgery. Surgical options in the appropriate patient to decompress posterior compartment include arthroscopic olecranon debridement or limited incision arthrotomy. Excessive resection of posteromedial osteophytes must be avoided. Arthroscopy has limited morbidity and allows for complete diagnostic assessment. UCL reconstruction should also be considered in combination with posteromedial debridement when the UCL is torn. More challenging indications for UCL reconstruction occur when the UCL is partially torn or torn and asymptomatic. Isolated posteromedial decompression in this setting risks future development of UCL symptoms that would then need to be addressed.
Surgical Technique
As previously mentioned, elbow arthroscopy or limited excision arthrotomy are the preferred operative methods for decompression of the posterior compartment and thus treatment of VEO. Anesthesia and patient positioning should be selected based on the surgeon’s preference. The patient should be positioned supine, prone, or in lateral decubitis. When a UCL reconstruction is expected, supine position is advantageous to avoid repositioning after completing the arthroscopic portion of the procedure. However, arthroscopy can be performed in the lateral position with subsequent repositioning, repeat prepping, and draping for UCL reconstruction (Figures 5A, 5B).
Prepare for elbow arthroscopy by distending the elbow joint with normal saline to aid in protection of neurovascular structures and simplify the insertion of the scope trocar. Perform diagnostic anterior arthroscopy via the proximal anteromedial portal. Assess for presence of loose bodies and osteochondral lesions of the radiocapitellar joint, as well as osteophytes of the coronoid tip and fossa. Utilizing a spinal needle under direct visualization establish a proximal lateral portal with adequate view of the anterior compartment. Proceed to visualize the medial compartment and assess for UCL injury. Apply valgus stress while in 70° of flexion. Visualize the coronoid process and look for medial trochlea gapping of 3 mm or greater, which indicates UCL insufficiency.30
Establish the posterolateral port for visualization of the posterior compartment. A posterior portal is established through the triceps tendon. Proceed to shave and ablate synovitis in order to create an adequate working space. Inspect the posteromedial olecranon, looking for any osteophytes or chondromalacia in the area (Figure 6). Examine the posterior radiocapitellar joint, looking specifically for loose bodies. The presence of loose bodies may require creating an extra mid lateral portal for removal. The ulnar nerve is located superficial to the elbow capsule and can be damaged by instruments utilized in the posteromedial gutter. As a precaution, be sure to remove suction attached to shaver. Place a curved articulating retractor in an accessory posterolateral portal to assist in protecting the ulnar nerve by retracting the capsule away from the surgical field (Figures 7A, 7B).
The osteophyte may be encased in soft tissue. Using a combination of ablation devices and shavers, the osteophyte can be exposed. The olecranon osteophyte can be removed with a small osteotome located at the border of the osteophyte and the normal olecranon. A motorized shaver or burr may also be introduced through the direct posterior portal or the posterolateral portal to complete the contouring of the olecranon (Figures 8A, 8B). Intraoperative lateral radiographs may be obtained for guidance in adequate bone removal and to ensure no bone debris is left in the soft tissues. It is critical that only pathologic osteophyte is removed and that normal olecranon is not compromised. This prevents an increase in UCL strain during valgus loading.19 However, in some non-throwing athletes, more aggressive debridement can be performed due to a smaller risk of UCL injury after posterior decompression.
Often, with the presence of osteophytes on the olecranon, there may be associated chondromalacia of the trochlea. These kissing lesions must be addressed after debridement of osteophytes. Loose flaps or frayed edges are carefully debrided and for any significant lesion the edges are contoured to a stable rim using shavers and curettes. Once altered to a well-shouldered lesion, microfracture is performed. Anterograde drilling of the lesion with perforations separated by 2 to 3 mm allow for the release of marrow elements and induction of a fibrocartilage healing response.
For an isolated posteromedial decompression, early rehabilitation begins with simple elbow flexion and extension exercises. It is important to restore flexor-pronator strength. Six weeks postoperatively, a progressive throwing program that includes plyometric exercises, neuromuscular training, and endurance exercises can be initiated. Patients can typically return to competition 3 to 4 months after surgery, if they have successfully regained preoperative range of motion, preoperative strength in the elbow, and there is no pain or tenderness on stress testing or palpation.
Outcomes
Safety and Advances in Arthroscopy
A clearer understanding of portal placement and proximity to neurovasculature in conjunction with advances in equipment have allowed for continual improvements in elbow arthroscopy techniques. There is plenty of literature indicating that arthroscopic posteromedial decompression is a safe, reliable, effective procedure, with a high rate of patient satisfaction.22,30-34] Andrews and Carson30 published one of the earliest investigations indicating the effectiveness of elbow arthroscopy utilizing objective and subjective outcome scores. They found that preoperative scores indicating patient satisfaction increased from 50% to 83%. Patients who underwent only loose body removal had the best outcomes. Andrews and Timmerman31 later evaluated the results of 72 professional baseball players who underwent either arthroscopic or open elbow surgery. They found that posteromedial olecranon osteophytes and intraarticular loose bodies were the most common diagnoses, present in 65% and 54% of players, respectively. In addition, a 41% reoperation rate was reported after posteromedial olecranon resection, along with 25% a rate of valgus instability necessitating UCL reconstruction. Andrews and Timmerman31 propose that the incidence of UCL injuries is underestimated and that UCL pathology must be treated prior to treating its secondary effects. Recently, Reddy and colleagues32 reviewed the results of 187 arthroscopic procedures. Posterior impingement, loose bodies, and osteoarthritis were the most common problems, occurring in 51%, 31%, and 22% of patients, respectively. Reported results were encouraging, with 87% good to excellent results and 85% of baseball players returning to preinjury levels.
Conclusion
An understanding of the relevant functional anatomy and the biomechanics of throwing is essential to understanding VEO. Potential concomitant valgus instability and UCL injury must be carefully assessed. Only symptomatic patients who have failed conservative treatment should undergo surgery. It is critical to avoid exacerbating and/or causing valgus instability by surgical excessively removing normal bone from the olecranon. Arthroscopy has been shown to be a safe and effective method to treat refractory cases of VEO.
1. Pappas AM, Zawacki RM, Sullivan TJ. Biomechanics of baseball pitching. A preliminary report. Am J Sports Med. 1985;13(4):216-222.
2. Fleisig GS, Barrentine SW, Escamilla RF, Andrews JR. Biomechanics of overhand throwing with implications for injuries. Sports Med. 1996;21(6):421-437.
3. Wilson FD, Andrews JR, Blackburn TA, McCluskey G. Valgus extension overload in the pitching elbow. Am J Sports Med. 1983;11(2):83-88.
4. Morrey BF. Applied anatomy and biomechanics of the elbow joint. Instr Course Lect. 1986,35:59-68.
5. Schwab GH, Bennett JB, Woods GW, Tullos HS. Biomechanics of elbow instability: the role of medial collateral ligament. Clin Orthop Relat Res. 1980;146:42-52.
6. Jobe FW, Kvitne RS. Elbow instability in the athlete. Instr Course Lect. 1991;40:17-23.
7. Søjbjerg JO, Ovesen J, Nielsen S. Experimental elbow instability after transection of the medial collateral ligament. Clin Orthop Relat Res. 1987;218:186-190.
8. Regan WD, Korinek SL, Morrey BF, An KN. Biomechanical study of ligaments around the elbow joint. Clin Orthop Relat Res. 1991;271:170-179.
9. Callaway GH, Field LD, Deng XH, et al. Biomechanical evaluation of the medial collateral ligament of the elbow. J Bone Joint Surg Am. 1997;79(8):1223-1231.
10. Chen FS, Rokito AS, Jobe FW. Medial elbow problems in the overhead-throwing athlete. J Am Acad Orthop Surg. 2001;9(2):99-113.
11. Davidson PA, Pink M, Perry J, Jobe FW. Functional anatomy of the flexor pronator muscle group in relation to the medial collateral ligament of the elbow. Am J Sports Med. 1995;23(2):245-250.
12. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
13. Hamilton CD, Glousman RE, Jobe FW, Brault J, Pink M, Perry J. Dynamic stability of the elbow: electromyographic analysis of the flexor pronator group and the extensor group in pitchers with valgus instability. J Shoulder Elbow Surg. 1996;5(5):347-354.
14. Glousman RE, Barron J, Jobe FW, Perry J, Pink M. An electromyographic analysis of the elbow in normal and injured pitchers with medial collateral ligament insufficiency. Am J Sports Med. 1992;20(3):311-317.
15. DiGiovine NM, Jobe FW, Pink M, Perry J. An electromyographic analysis of the upper extremity in pitching. J Shoulder Elbow Surg. 1992;1(1):15-25.
16. Sisto DJ, Jobe FW, Moynes DR, Antonelli DJ. An electromyographic analysis of the elbow in pitching. Am J Sports Med. 1987;15(3):260-263.
17. Ahmad CS, Park MC, Elattrache NS. Elbow medial ulnar collateral ligament insufficiency alters posteromedial olecranon contact. Am J Sports Med. 2004;32(7):1607–1612.
18. Ahmad CS, Conway J. Elbow arthroscopy: beginners to advanced: valgus extension overload. In: Egol, ed. Instructional Course Lectures; vol 60. Rosemont, IL: American Academy of Orthopaedic Surgeons; submitted 2009.
19. Kamineni S, ElAttrache NS, O’Driscoll S W, et al. Medial collateral ligament strain with partial posteromedial olecranon resection. A biomechanical study. J Bone Joint Surg Am. 2004;86-A(11):2424–2430.
20. Kamineni S, Hirahara H, Pomianowski S, et al. Partial posteromedial olecranon resection: a kinematic study. J Bone Joint Surg Am. 2003;85-A(6):1005–1011.
21. Morrey BF, An KN. Articular and ligamentous contributions to the stability of the elbow joint. Am J Sports Med. 1983;11(5):315–319.
22. O’Driscoll SW, Morrey BF. Arthroscopy of the elbow. Diagnostic and therapeutic benefits and hazards. J Bone Joint Surg Am. 1992;74(1):84–94.
23. Miller CD, Savoie FH 3rd. Valgus extension injuries of the elbow in the throwing athlete. J Am Acad Orthop Surg. 1994;2(5):261-269.
24. O’Driscoll SW. Valgus extension overload and plica. In: Levine WN, ed. The Athlete’s Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:71-83.
25. Boatright JR, D’Alessandro DF. Nerve entrapment syndromes at the elbow. In Jobe FW, Pink MM, Glousman RE, Kvitne RE, Zemel NP, eds. Operative Techniques in Upper Extremity Sports Injuries. St. Louis, MO: Mosby-Year Book; 1996:518-537.
26. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
27. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
28. Kooima CL, Anderson K, Craig JV, Teeter DM, van Holsbeeck M. Evidence of subclinical medial collateral ligament injury and posteromedial impingement in professional baseball players. Am J Sports Med. 2004;32(7):1602-1606.
29. Field LD, Altchek DW. Evaluation of the arthroscopic valgus instability test of the elbow. Am J Sports Med. 1996;24(2):177–181.
30. Andrews JR, Carson WG. Arthroscopy of the elbow. Arthroscopy. 1985;1(2):97-107.
31. Andrews JR, Timmerman LA. Outcome of elbow surgery in professional baseball players. Am J Sports Med. 1995;23(4):407-413.
32. Reddy AS, Kvitne RE, Yocum LA, Elattrache NS, Glousman RE, Jobe FW. Arthroscopy of the elbow: a long-term clinical review. Arthroscopy. 2000;16(6):588-594.
33. Rosenwasser MP, Steinmann S. Elbow arthroscopy in the treatment of posterior olecranon impingement. Paper present at: AANA Annual Meeting; 1991; San Diego, CA.
34. Wilson FD, Andrews JR, Blackburn TA, McCluskey G. Valgus extension overload in the pitching elbow. Am J Sports Med. 1983;11(2):83-88.
The supraphysiological demands imposed on the elbow of a throwing athlete result in predictable patterns of injury. This is especially true of baseball pitchers. Knowledge of elbow anatomy, as well as the biomechanics of throwing, assist in making diagnostic and therapeutic decisions and also influence surgical technique when surgery is required. During the late cocking and early acceleration phases of throwing, valgus torque can reach 65 Nm with angular velocities of the forearm reaching 5000°/sec, which is considered the fasted recorded human movment.1 The valgus torque and rapid extension synergistically create 3 major forces placed on the elbow. The first is a tensile stress along the medial aspect of elbow affecting the ulnar collateral ligament (UCL), flexor pronator mass, and medial epicondyle. Secondly, compression forces affect the lateral aspect of the elbow at the radiocapitellar joint. Finally, a shearing stress occurs in the posterior compartment at the posterior medial tip of the olecranon and the olecranon fossa.
These forces generated on the elbow result in predictable pathology. The recurring tensile forces applied on the medial aspect on the elbow can compromise the integrity of the UCL. It is well known that injury to the UCL leads to valgus instability. Individuals with valgus instability who continue to throw may trigger and/or aggravate injury in the posterior and lateral components of the elbow. Lateral compression forces can often reach 500 N, resulting in radiocapitellar overload syndrome, which occurs in combination with medial ligament instability and valgus extension overload.2 Radiocapitellar compression may cause chondral or osteochondral fracture with resulting intra-articular loose bodes. This compression also contributes to the etiology of osteochondritis dissecans (OCD) in skeletally immature athletes. In the posterior elbow, throwing forcefully and repeatedly pushes the olecranon into the olecranon fossa. Shear stress on the medial olecranon tip and fossa, due to combined valgus and extension forces, lead to the development of osteophytes. This collection of injuries in the medial, lateral, and posterior aspects of the elbow is known as “valgus extension overload syndrome” or VEO. Symptoms in VEO can be the result of chondral lesions, loose bodies, and marginal exostosis.3
The aim of this review is to provide understanding regarding both the relevant anatomy and pathomechanics of VEO, key aspects to clinical evaluation, and effective treatment options.
Functional Anatomy
A functional comprehension of elbow anatomy and biomechanics is essential to understanding the constellation of injuries in VEO. The osseous anatomy of the elbow permits a variety of movements. These include flexion-extension and pronation-supination, which are mediated by the ulnohumeral and radiocapitellar articulations. While in full extension, the elbow has a normal valgus carrying angle of 11° to 16°. It is important to know that 50% of the elbow’s stability is attributed to the configuration of the bones.4-6 This is especially true in varus stress while the elbow is in full extension. The soft tissues, including muscle and ligaments such as the UCL, lateral UCL, and radial UCL complexes, provide the remaining elbow stability.4-6
The UCL complex is composed of 3 main segments known as the anterior, posterior, and oblique bundles (transverse ligament). Collectively, these bundles are responsible for providing medial elbow stability. However, each of these bundles contributes to medial elbow stability in its own way. The first and arguably the most important bundle is the anterior bundle; its most important function is providing stability against valgus stress.4,5,7 It is composed of parallel fibers inserting on the medial coronoid process.4,5,7 Furthermore, its eccentric location with respect to the axis of elbow allows it to provide stability throughout the full range of elbow motion.6 The anterior bundle can be further divided into individual anterior and posterior bands that have reciprocal functionality.5,8,9 The anterior band acts as the chief restraint to valgus stress up to 90° of flexion.9 Any flexion beyond 90° renders the anterior band’s role secondary in resisting valgus stress.9 The posterior band’s function in resisting valgus stress is most important between 60° and full flexion, while having a secondary role in lesser degrees of flexion.8,9 Notably, the posterior band is isometric and is more important in the overhead-throwing athlete due to the fact its primary role in resisting valgus stress occurs at higher degrees of flexion.10
The remaining posterior and oblique bundles of the UCL complex have lesser roles in maintaining elbow stability. The posterior bundle of the UCL complex is fan-shaped, originates from the medial epicondyle, and inserts onto the medial margin the semi-lunar notch. It is more slender and frailer than the anterior bundle. This is reflected in its functionality, as it plays a secondary role in elbow stability during elbow flexion beyond 90°.4,5,8 In contrast to the anterior and posterior bundles, the oblique bundle, also known as the transverse ligament, does not cross the elbow joint. It is a thickening of the caudal most aspect of the joint capsule, which extends from the medial olecranon to the inferior medial coronoid process and as a result functions in expanding the greater sigmoid notch.6
The musculotendinous components of the elbow are essential to providing dynamic functional resistance to valgus stress.11 These components are flexor-pronator musculature that originate from the medial epicondyle. Listed proximally to distally, the flexor-pronator muscles include pronator teres, flexor carpi radialis (FCR), palmaris longus, flexor digitorum superficialis, and the flexor carpi ulnaris (FCU).
Pathomechanics
Once familiarized with the relevant function anatomy, it is crucial to understand the mechanics of throwing in order to understand the pathomechanics of VEO. The action of overhead throwing has been divided into 6 phases.6,12-16 Phase 4, acceleration, is the most relevant when discussing forces on elbow, since the majority of forces are generated during this state. Phase 4 represents a rapid acceleration of the upper extremity with a large forward-directed force on the arm generated by the shoulder muscles. Additionally, there is internal rotation and adduction of the humerus with rapid elbow extension terminating with ball release. The elbow accelerates up to 600,000°/sec2 in a miniscule time frame of 40 to 50 milliseconds.1,5 Immense valgus forces are exerted on the medial aspect of the elbow. The anterior bundle of the UCL bears the majority of the force, with the flexor pronator mass enabling the transmission.11 The majority of injuries occur during stage 4 as a result of the stress load on the medial elbow structures like the UCL. The proceeding phases 5 (deceleration), and 6 (follow-through) involve eventual dissipation of excess kinetic energy as the elbow completely extends. The deceleration during phase 5 is rapid and powerful, occurring at about 500,000°/sec2 in the short span of 50 milliseconds.1,6,12-16 High-velocity throwing, such as baseball pitching, generates forces in the elbow that are opposed by the articular, ligamentous, and muscular portions of the arm. The ulnohumeral articulation stabilizes motion of the arm from 0° to 20° of flexion and beyond 120° of flexion. Static and dynamic soft tissues maintain stability during the remaining of 100° arc of motion.
During deceleration, the elbow undergoes terminal extension resulting in the posteromedial olecranon contacting the trochlea and the olecranon fossa with subsequent dissipation of the combined valgus force and angular moment (Figure 1). This dissipation of force creates pathologic shear and compressive forces in the posterior elbow. Poor muscular control and the traumatic abutment that occurs in the posterior compartment may further add to the pathologic forces. Reactive bone formation is induced by the repetitive compression and shear, resulting in osteophytes on the posteromedial tip of the olecranon (Figure 2). Consequent “kissing lesions” of chondromalacia may occur in the olecranon fossa and posteromedial trochlea. The subsequent development of loose bodies may also occur. The presence of osteophytes and/or loose bodies may result in posteromedial impingement (PMI).
The association between PMI of the olecranon and valgus instability has been elucidated in both clinical and biomechanical investigations.17,18,19,20 Conway18 identified tip exostosis in 24% of lateral radiographs of 135 asymptomatic professional pitchers. Approximately one-fifth (21%) of these pitchers had >1.0 mm increased relative valgus laxity on stress radiographs. Roughly one-third (34%) of players with exostosis had >1.0 mm of increased relative valgus laxity, compared to 16% of players without exostosis formation. These results provide evidence for a probable association between PMI and valgus laxity. In biomechanical research, Ahmad and colleagues17 studied the effect of partial and full thickness UCL injuries on contact forces of the posterior elbow. Posteromedial compartments of cadaver specimens were subjected to physiologic valgus stresses while placed on pressure-senstive film. Contact area and pressure between posteromedial trochlea and olecranon were altered in the setting of UCL insufficiency, helping explain how posteromedial osteophyte formation occurs.
Additional biomechanical studies have also investigated the posteromedial olecranon’s role in functioning as a stabilizing buttress to medial tensile forces. Treating PMI with aggressive bone removal may increase valgus instability as well as strain on the UCL, leading to UCL injury following olecranon resection.19,20 Kamineni and colleagues19 investigated strain on anterior bundle of UCL as a function of increasing applied torque and posteromedial resections of the olecranon. This investigation was done utilizing an electromagnetic tracking placed in cadaver elbows. A nonuniform change in strain was found at 3 mm of resection during flexion and valgus testing. This nonuniform change implied that removal of posteromedial olecranon beyond 3 mm made the UCL more vulnerable to injury. Follow-up investigations looked at kinematic effects of increasing valgus and varus torques and sequential posteromedial olecranon resections.20 Valgus angulation of the elbow increased with all resection levels but no critical amount of olecranon resection was identified. The consensus in the literature indicates that posteromedial articulation of the elbow is a significant stabilizer to valgus stress.17-22 Thus, normal bone should be preserved and only osteophytes should be removed during treatment.
In addition, VEO may lead to injury in the lateral compartment as well. After attenuation and insufficiency of the UCL due to repetitive stress, excessive force transmission to the lateral aspect of the elbow occurs. Compressive and rotatory forces escalate within the radiocapitellar joint, causing synovitis and osteochondral lesions.3,23 These osteochondral lesions include osteochondritis dissecans and osteochondral fractures that may fragment and become loose bodies.
Evaluation of VEO
History
Patients will typically have a history of repetitive throwing or other repetitive overhead activity. VEO is most common in baseball pitchers but may also occur in other sports, such as tennis, football, lacrosse, gymnastics, and javelin throwing. In baseball pitchers, clinical presentation is often preceded by a decrease in pitch velocity, control, and early fatigability. It presents with elbow pain localized to the posteromedial aspect of olecranon after release of the ball, when the elbow reaches terminal extension. Patients also report limited extension, due to impinging posterior osteophytes. Also, locking and catching caused by loose bodies and chondromalacia may be present. VEO may also occur in combination with concomitant valgus instability, as well as in a patient with a prior history of valgus instability. Flexor pronator injury, ulnar neuritis, and subluxation may also be present in a patient with VEO.
Physical Examination
VEO may occur in an isolated fashion or with concomitant pathology. Therefore, a comprehensive physical examination includes evaluating the entire kinetic chain of throwing and a focused examination covering VEO and associated valgus instability. Patients may exhibit crepitus and tenderness over the posteromedial olecranon and a loss of extension with a firm end point. The extension impingement test should be performed where the elbow is snapped into terminal extension. This typically elicits pain in the posterior compartment in a patient with VEO. The arm bar test involves positioning the patient’s shoulder at 90° of forward flexion, full internal rotation, with the patient’s hand placed on the examiner’s shoulder.24 The examiner pulls down on the olecranon, simulating forced extension; pain is indicative of a positive test. It is important to note if there are signs of ulnar neuritis or subluxing ulnar nerve, especially if planning to utilize medial portals during arthroscopic treatment.
Examination maneuvers for valgus instability should also be conducted during evaluation of VEO. The physical examination for valgus instability in the elbow is ideally performed with the patient seated. Secure the patient’s wrist between the examiner’s forearm and trunk, and flex the patient’s elbow between 20° and 30° to unlock the olecranon from its fossa. Proceed to apply valgus stress. This stresses the anterior band of the anterior bundle of the UCL.6,25,26 Palpate the UCL from the medial epicondyle to the proximal ulna as valgus stress is applied. Occasionally, valgus laxity can be appreciated when compared to contralateral side. The milking maneuver is a helpful test to determine UCL injury. Pull on the patient’s thumb while the forearm is supinated, shoulder extended, and the elbow flexed beyond 90°.6 The milking maneuver exerts valgus stress on a flexed elbow. A patient with an injured UCL will experience the subjective feeling of apprehension and instability, with medial elbow pain.
The most sensitive test is the moving valgus stress test. This is performed with the patient in the upright position and the shoulder abducted 90°. Starting with the arm in full flexion, the examiner applies a constant valgus torque to the elbow and then rapidly extends the elbow. Reproduction of pain during range of motion from 120° to 70° represents UCL injury, while pain with extension beyond 70° represents chondral injury to the ulnohumeral joint. Be aware that the absence of increased pain with wrist flexion, along with pain localized slightly posterior to the common flexor origin, differentiates a UCL injury from flexor-pronator muscle injury.6,26,27Examine range of motion in affected and unaffected elbows. Loss of terminal extension may be present, along with secondary to flexion contracture due to repeated attempts at healing and stabilization.25
Imaging
Imaging is essential to the accurate diagnosis of VEO and related conditions. Anterior posterior (AP), lateral, and oblique radiographs of elbow (Figures 3A-3C) may show posteromedial olecranon osteophytes and/or loose bodies. Calcification of ligaments or other soft tissues may also be seen. An AP radiograph with 140° of external rotation may best visualize osteophytes on posteromedial olecranon.18 A computed tomography scan with 2-dimensional sagittal and coronal reconstruction and 3-dimensional surface rendering (Figures 4A, 4B) may best demonstrate morphological abnormalities, loose bodies, and osteophytes. Magnetic resonance imaging (MRI) is essential for assessment of soft tissues and chondral injuries. MRI may detect UCL compromise, synovial plicae, bone edema, olecranon, or stress fractures.
Treatment
Nonoperative Treatment
Treatment consists of both nonoperative and operative modalities. Nonoperative treatment methods are first line in treating VEO. Patients should modify their physical activity and rest from throwing activities. Nonsteroid anti-inflammatory drugs are appropriate to treat pain along with intra-articular corticosteroid injections of the elbow. A wide assessment of pitching mechanics should be performed in an attempt to correct errors in throwing technique and address muscular imbalances. After cessation of the resting period, the patient may initiate a progressive throwing program supervised by an experienced therapist and trainer. A plan for returning to competition should be made upon completion of the throwing program.
Operative Treatment
Surgical treatment is reserved for patients who fail nonoperative treatment. These patients have persistent symptoms of posteromedial impingement and desire to return to pre-injury level of performance. Posteromedial decompression is not recommended when provocative physical examination maneuvers are negative, regardless of presence of olecranon osteophytes on imaging. Osteophytes are an asymptomatic finding typically seen in professional baseball players and do not warrant surgical treatment.18,28 UCL compromise is a relative contraindication to olecranon debridement as UCL injury could become symptomatic following surgery. Surgical options in the appropriate patient to decompress posterior compartment include arthroscopic olecranon debridement or limited incision arthrotomy. Excessive resection of posteromedial osteophytes must be avoided. Arthroscopy has limited morbidity and allows for complete diagnostic assessment. UCL reconstruction should also be considered in combination with posteromedial debridement when the UCL is torn. More challenging indications for UCL reconstruction occur when the UCL is partially torn or torn and asymptomatic. Isolated posteromedial decompression in this setting risks future development of UCL symptoms that would then need to be addressed.
Surgical Technique
As previously mentioned, elbow arthroscopy or limited excision arthrotomy are the preferred operative methods for decompression of the posterior compartment and thus treatment of VEO. Anesthesia and patient positioning should be selected based on the surgeon’s preference. The patient should be positioned supine, prone, or in lateral decubitis. When a UCL reconstruction is expected, supine position is advantageous to avoid repositioning after completing the arthroscopic portion of the procedure. However, arthroscopy can be performed in the lateral position with subsequent repositioning, repeat prepping, and draping for UCL reconstruction (Figures 5A, 5B).
Prepare for elbow arthroscopy by distending the elbow joint with normal saline to aid in protection of neurovascular structures and simplify the insertion of the scope trocar. Perform diagnostic anterior arthroscopy via the proximal anteromedial portal. Assess for presence of loose bodies and osteochondral lesions of the radiocapitellar joint, as well as osteophytes of the coronoid tip and fossa. Utilizing a spinal needle under direct visualization establish a proximal lateral portal with adequate view of the anterior compartment. Proceed to visualize the medial compartment and assess for UCL injury. Apply valgus stress while in 70° of flexion. Visualize the coronoid process and look for medial trochlea gapping of 3 mm or greater, which indicates UCL insufficiency.30
Establish the posterolateral port for visualization of the posterior compartment. A posterior portal is established through the triceps tendon. Proceed to shave and ablate synovitis in order to create an adequate working space. Inspect the posteromedial olecranon, looking for any osteophytes or chondromalacia in the area (Figure 6). Examine the posterior radiocapitellar joint, looking specifically for loose bodies. The presence of loose bodies may require creating an extra mid lateral portal for removal. The ulnar nerve is located superficial to the elbow capsule and can be damaged by instruments utilized in the posteromedial gutter. As a precaution, be sure to remove suction attached to shaver. Place a curved articulating retractor in an accessory posterolateral portal to assist in protecting the ulnar nerve by retracting the capsule away from the surgical field (Figures 7A, 7B).
The osteophyte may be encased in soft tissue. Using a combination of ablation devices and shavers, the osteophyte can be exposed. The olecranon osteophyte can be removed with a small osteotome located at the border of the osteophyte and the normal olecranon. A motorized shaver or burr may also be introduced through the direct posterior portal or the posterolateral portal to complete the contouring of the olecranon (Figures 8A, 8B). Intraoperative lateral radiographs may be obtained for guidance in adequate bone removal and to ensure no bone debris is left in the soft tissues. It is critical that only pathologic osteophyte is removed and that normal olecranon is not compromised. This prevents an increase in UCL strain during valgus loading.19 However, in some non-throwing athletes, more aggressive debridement can be performed due to a smaller risk of UCL injury after posterior decompression.
Often, with the presence of osteophytes on the olecranon, there may be associated chondromalacia of the trochlea. These kissing lesions must be addressed after debridement of osteophytes. Loose flaps or frayed edges are carefully debrided and for any significant lesion the edges are contoured to a stable rim using shavers and curettes. Once altered to a well-shouldered lesion, microfracture is performed. Anterograde drilling of the lesion with perforations separated by 2 to 3 mm allow for the release of marrow elements and induction of a fibrocartilage healing response.
For an isolated posteromedial decompression, early rehabilitation begins with simple elbow flexion and extension exercises. It is important to restore flexor-pronator strength. Six weeks postoperatively, a progressive throwing program that includes plyometric exercises, neuromuscular training, and endurance exercises can be initiated. Patients can typically return to competition 3 to 4 months after surgery, if they have successfully regained preoperative range of motion, preoperative strength in the elbow, and there is no pain or tenderness on stress testing or palpation.
Outcomes
Safety and Advances in Arthroscopy
A clearer understanding of portal placement and proximity to neurovasculature in conjunction with advances in equipment have allowed for continual improvements in elbow arthroscopy techniques. There is plenty of literature indicating that arthroscopic posteromedial decompression is a safe, reliable, effective procedure, with a high rate of patient satisfaction.22,30-34] Andrews and Carson30 published one of the earliest investigations indicating the effectiveness of elbow arthroscopy utilizing objective and subjective outcome scores. They found that preoperative scores indicating patient satisfaction increased from 50% to 83%. Patients who underwent only loose body removal had the best outcomes. Andrews and Timmerman31 later evaluated the results of 72 professional baseball players who underwent either arthroscopic or open elbow surgery. They found that posteromedial olecranon osteophytes and intraarticular loose bodies were the most common diagnoses, present in 65% and 54% of players, respectively. In addition, a 41% reoperation rate was reported after posteromedial olecranon resection, along with 25% a rate of valgus instability necessitating UCL reconstruction. Andrews and Timmerman31 propose that the incidence of UCL injuries is underestimated and that UCL pathology must be treated prior to treating its secondary effects. Recently, Reddy and colleagues32 reviewed the results of 187 arthroscopic procedures. Posterior impingement, loose bodies, and osteoarthritis were the most common problems, occurring in 51%, 31%, and 22% of patients, respectively. Reported results were encouraging, with 87% good to excellent results and 85% of baseball players returning to preinjury levels.
Conclusion
An understanding of the relevant functional anatomy and the biomechanics of throwing is essential to understanding VEO. Potential concomitant valgus instability and UCL injury must be carefully assessed. Only symptomatic patients who have failed conservative treatment should undergo surgery. It is critical to avoid exacerbating and/or causing valgus instability by surgical excessively removing normal bone from the olecranon. Arthroscopy has been shown to be a safe and effective method to treat refractory cases of VEO.
The supraphysiological demands imposed on the elbow of a throwing athlete result in predictable patterns of injury. This is especially true of baseball pitchers. Knowledge of elbow anatomy, as well as the biomechanics of throwing, assist in making diagnostic and therapeutic decisions and also influence surgical technique when surgery is required. During the late cocking and early acceleration phases of throwing, valgus torque can reach 65 Nm with angular velocities of the forearm reaching 5000°/sec, which is considered the fasted recorded human movment.1 The valgus torque and rapid extension synergistically create 3 major forces placed on the elbow. The first is a tensile stress along the medial aspect of elbow affecting the ulnar collateral ligament (UCL), flexor pronator mass, and medial epicondyle. Secondly, compression forces affect the lateral aspect of the elbow at the radiocapitellar joint. Finally, a shearing stress occurs in the posterior compartment at the posterior medial tip of the olecranon and the olecranon fossa.
These forces generated on the elbow result in predictable pathology. The recurring tensile forces applied on the medial aspect on the elbow can compromise the integrity of the UCL. It is well known that injury to the UCL leads to valgus instability. Individuals with valgus instability who continue to throw may trigger and/or aggravate injury in the posterior and lateral components of the elbow. Lateral compression forces can often reach 500 N, resulting in radiocapitellar overload syndrome, which occurs in combination with medial ligament instability and valgus extension overload.2 Radiocapitellar compression may cause chondral or osteochondral fracture with resulting intra-articular loose bodes. This compression also contributes to the etiology of osteochondritis dissecans (OCD) in skeletally immature athletes. In the posterior elbow, throwing forcefully and repeatedly pushes the olecranon into the olecranon fossa. Shear stress on the medial olecranon tip and fossa, due to combined valgus and extension forces, lead to the development of osteophytes. This collection of injuries in the medial, lateral, and posterior aspects of the elbow is known as “valgus extension overload syndrome” or VEO. Symptoms in VEO can be the result of chondral lesions, loose bodies, and marginal exostosis.3
The aim of this review is to provide understanding regarding both the relevant anatomy and pathomechanics of VEO, key aspects to clinical evaluation, and effective treatment options.
Functional Anatomy
A functional comprehension of elbow anatomy and biomechanics is essential to understanding the constellation of injuries in VEO. The osseous anatomy of the elbow permits a variety of movements. These include flexion-extension and pronation-supination, which are mediated by the ulnohumeral and radiocapitellar articulations. While in full extension, the elbow has a normal valgus carrying angle of 11° to 16°. It is important to know that 50% of the elbow’s stability is attributed to the configuration of the bones.4-6 This is especially true in varus stress while the elbow is in full extension. The soft tissues, including muscle and ligaments such as the UCL, lateral UCL, and radial UCL complexes, provide the remaining elbow stability.4-6
The UCL complex is composed of 3 main segments known as the anterior, posterior, and oblique bundles (transverse ligament). Collectively, these bundles are responsible for providing medial elbow stability. However, each of these bundles contributes to medial elbow stability in its own way. The first and arguably the most important bundle is the anterior bundle; its most important function is providing stability against valgus stress.4,5,7 It is composed of parallel fibers inserting on the medial coronoid process.4,5,7 Furthermore, its eccentric location with respect to the axis of elbow allows it to provide stability throughout the full range of elbow motion.6 The anterior bundle can be further divided into individual anterior and posterior bands that have reciprocal functionality.5,8,9 The anterior band acts as the chief restraint to valgus stress up to 90° of flexion.9 Any flexion beyond 90° renders the anterior band’s role secondary in resisting valgus stress.9 The posterior band’s function in resisting valgus stress is most important between 60° and full flexion, while having a secondary role in lesser degrees of flexion.8,9 Notably, the posterior band is isometric and is more important in the overhead-throwing athlete due to the fact its primary role in resisting valgus stress occurs at higher degrees of flexion.10
The remaining posterior and oblique bundles of the UCL complex have lesser roles in maintaining elbow stability. The posterior bundle of the UCL complex is fan-shaped, originates from the medial epicondyle, and inserts onto the medial margin the semi-lunar notch. It is more slender and frailer than the anterior bundle. This is reflected in its functionality, as it plays a secondary role in elbow stability during elbow flexion beyond 90°.4,5,8 In contrast to the anterior and posterior bundles, the oblique bundle, also known as the transverse ligament, does not cross the elbow joint. It is a thickening of the caudal most aspect of the joint capsule, which extends from the medial olecranon to the inferior medial coronoid process and as a result functions in expanding the greater sigmoid notch.6
The musculotendinous components of the elbow are essential to providing dynamic functional resistance to valgus stress.11 These components are flexor-pronator musculature that originate from the medial epicondyle. Listed proximally to distally, the flexor-pronator muscles include pronator teres, flexor carpi radialis (FCR), palmaris longus, flexor digitorum superficialis, and the flexor carpi ulnaris (FCU).
Pathomechanics
Once familiarized with the relevant function anatomy, it is crucial to understand the mechanics of throwing in order to understand the pathomechanics of VEO. The action of overhead throwing has been divided into 6 phases.6,12-16 Phase 4, acceleration, is the most relevant when discussing forces on elbow, since the majority of forces are generated during this state. Phase 4 represents a rapid acceleration of the upper extremity with a large forward-directed force on the arm generated by the shoulder muscles. Additionally, there is internal rotation and adduction of the humerus with rapid elbow extension terminating with ball release. The elbow accelerates up to 600,000°/sec2 in a miniscule time frame of 40 to 50 milliseconds.1,5 Immense valgus forces are exerted on the medial aspect of the elbow. The anterior bundle of the UCL bears the majority of the force, with the flexor pronator mass enabling the transmission.11 The majority of injuries occur during stage 4 as a result of the stress load on the medial elbow structures like the UCL. The proceeding phases 5 (deceleration), and 6 (follow-through) involve eventual dissipation of excess kinetic energy as the elbow completely extends. The deceleration during phase 5 is rapid and powerful, occurring at about 500,000°/sec2 in the short span of 50 milliseconds.1,6,12-16 High-velocity throwing, such as baseball pitching, generates forces in the elbow that are opposed by the articular, ligamentous, and muscular portions of the arm. The ulnohumeral articulation stabilizes motion of the arm from 0° to 20° of flexion and beyond 120° of flexion. Static and dynamic soft tissues maintain stability during the remaining of 100° arc of motion.
During deceleration, the elbow undergoes terminal extension resulting in the posteromedial olecranon contacting the trochlea and the olecranon fossa with subsequent dissipation of the combined valgus force and angular moment (Figure 1). This dissipation of force creates pathologic shear and compressive forces in the posterior elbow. Poor muscular control and the traumatic abutment that occurs in the posterior compartment may further add to the pathologic forces. Reactive bone formation is induced by the repetitive compression and shear, resulting in osteophytes on the posteromedial tip of the olecranon (Figure 2). Consequent “kissing lesions” of chondromalacia may occur in the olecranon fossa and posteromedial trochlea. The subsequent development of loose bodies may also occur. The presence of osteophytes and/or loose bodies may result in posteromedial impingement (PMI).
The association between PMI of the olecranon and valgus instability has been elucidated in both clinical and biomechanical investigations.17,18,19,20 Conway18 identified tip exostosis in 24% of lateral radiographs of 135 asymptomatic professional pitchers. Approximately one-fifth (21%) of these pitchers had >1.0 mm increased relative valgus laxity on stress radiographs. Roughly one-third (34%) of players with exostosis had >1.0 mm of increased relative valgus laxity, compared to 16% of players without exostosis formation. These results provide evidence for a probable association between PMI and valgus laxity. In biomechanical research, Ahmad and colleagues17 studied the effect of partial and full thickness UCL injuries on contact forces of the posterior elbow. Posteromedial compartments of cadaver specimens were subjected to physiologic valgus stresses while placed on pressure-senstive film. Contact area and pressure between posteromedial trochlea and olecranon were altered in the setting of UCL insufficiency, helping explain how posteromedial osteophyte formation occurs.
Additional biomechanical studies have also investigated the posteromedial olecranon’s role in functioning as a stabilizing buttress to medial tensile forces. Treating PMI with aggressive bone removal may increase valgus instability as well as strain on the UCL, leading to UCL injury following olecranon resection.19,20 Kamineni and colleagues19 investigated strain on anterior bundle of UCL as a function of increasing applied torque and posteromedial resections of the olecranon. This investigation was done utilizing an electromagnetic tracking placed in cadaver elbows. A nonuniform change in strain was found at 3 mm of resection during flexion and valgus testing. This nonuniform change implied that removal of posteromedial olecranon beyond 3 mm made the UCL more vulnerable to injury. Follow-up investigations looked at kinematic effects of increasing valgus and varus torques and sequential posteromedial olecranon resections.20 Valgus angulation of the elbow increased with all resection levels but no critical amount of olecranon resection was identified. The consensus in the literature indicates that posteromedial articulation of the elbow is a significant stabilizer to valgus stress.17-22 Thus, normal bone should be preserved and only osteophytes should be removed during treatment.
In addition, VEO may lead to injury in the lateral compartment as well. After attenuation and insufficiency of the UCL due to repetitive stress, excessive force transmission to the lateral aspect of the elbow occurs. Compressive and rotatory forces escalate within the radiocapitellar joint, causing synovitis and osteochondral lesions.3,23 These osteochondral lesions include osteochondritis dissecans and osteochondral fractures that may fragment and become loose bodies.
Evaluation of VEO
History
Patients will typically have a history of repetitive throwing or other repetitive overhead activity. VEO is most common in baseball pitchers but may also occur in other sports, such as tennis, football, lacrosse, gymnastics, and javelin throwing. In baseball pitchers, clinical presentation is often preceded by a decrease in pitch velocity, control, and early fatigability. It presents with elbow pain localized to the posteromedial aspect of olecranon after release of the ball, when the elbow reaches terminal extension. Patients also report limited extension, due to impinging posterior osteophytes. Also, locking and catching caused by loose bodies and chondromalacia may be present. VEO may also occur in combination with concomitant valgus instability, as well as in a patient with a prior history of valgus instability. Flexor pronator injury, ulnar neuritis, and subluxation may also be present in a patient with VEO.
Physical Examination
VEO may occur in an isolated fashion or with concomitant pathology. Therefore, a comprehensive physical examination includes evaluating the entire kinetic chain of throwing and a focused examination covering VEO and associated valgus instability. Patients may exhibit crepitus and tenderness over the posteromedial olecranon and a loss of extension with a firm end point. The extension impingement test should be performed where the elbow is snapped into terminal extension. This typically elicits pain in the posterior compartment in a patient with VEO. The arm bar test involves positioning the patient’s shoulder at 90° of forward flexion, full internal rotation, with the patient’s hand placed on the examiner’s shoulder.24 The examiner pulls down on the olecranon, simulating forced extension; pain is indicative of a positive test. It is important to note if there are signs of ulnar neuritis or subluxing ulnar nerve, especially if planning to utilize medial portals during arthroscopic treatment.
Examination maneuvers for valgus instability should also be conducted during evaluation of VEO. The physical examination for valgus instability in the elbow is ideally performed with the patient seated. Secure the patient’s wrist between the examiner’s forearm and trunk, and flex the patient’s elbow between 20° and 30° to unlock the olecranon from its fossa. Proceed to apply valgus stress. This stresses the anterior band of the anterior bundle of the UCL.6,25,26 Palpate the UCL from the medial epicondyle to the proximal ulna as valgus stress is applied. Occasionally, valgus laxity can be appreciated when compared to contralateral side. The milking maneuver is a helpful test to determine UCL injury. Pull on the patient’s thumb while the forearm is supinated, shoulder extended, and the elbow flexed beyond 90°.6 The milking maneuver exerts valgus stress on a flexed elbow. A patient with an injured UCL will experience the subjective feeling of apprehension and instability, with medial elbow pain.
The most sensitive test is the moving valgus stress test. This is performed with the patient in the upright position and the shoulder abducted 90°. Starting with the arm in full flexion, the examiner applies a constant valgus torque to the elbow and then rapidly extends the elbow. Reproduction of pain during range of motion from 120° to 70° represents UCL injury, while pain with extension beyond 70° represents chondral injury to the ulnohumeral joint. Be aware that the absence of increased pain with wrist flexion, along with pain localized slightly posterior to the common flexor origin, differentiates a UCL injury from flexor-pronator muscle injury.6,26,27Examine range of motion in affected and unaffected elbows. Loss of terminal extension may be present, along with secondary to flexion contracture due to repeated attempts at healing and stabilization.25
Imaging
Imaging is essential to the accurate diagnosis of VEO and related conditions. Anterior posterior (AP), lateral, and oblique radiographs of elbow (Figures 3A-3C) may show posteromedial olecranon osteophytes and/or loose bodies. Calcification of ligaments or other soft tissues may also be seen. An AP radiograph with 140° of external rotation may best visualize osteophytes on posteromedial olecranon.18 A computed tomography scan with 2-dimensional sagittal and coronal reconstruction and 3-dimensional surface rendering (Figures 4A, 4B) may best demonstrate morphological abnormalities, loose bodies, and osteophytes. Magnetic resonance imaging (MRI) is essential for assessment of soft tissues and chondral injuries. MRI may detect UCL compromise, synovial plicae, bone edema, olecranon, or stress fractures.
Treatment
Nonoperative Treatment
Treatment consists of both nonoperative and operative modalities. Nonoperative treatment methods are first line in treating VEO. Patients should modify their physical activity and rest from throwing activities. Nonsteroid anti-inflammatory drugs are appropriate to treat pain along with intra-articular corticosteroid injections of the elbow. A wide assessment of pitching mechanics should be performed in an attempt to correct errors in throwing technique and address muscular imbalances. After cessation of the resting period, the patient may initiate a progressive throwing program supervised by an experienced therapist and trainer. A plan for returning to competition should be made upon completion of the throwing program.
Operative Treatment
Surgical treatment is reserved for patients who fail nonoperative treatment. These patients have persistent symptoms of posteromedial impingement and desire to return to pre-injury level of performance. Posteromedial decompression is not recommended when provocative physical examination maneuvers are negative, regardless of presence of olecranon osteophytes on imaging. Osteophytes are an asymptomatic finding typically seen in professional baseball players and do not warrant surgical treatment.18,28 UCL compromise is a relative contraindication to olecranon debridement as UCL injury could become symptomatic following surgery. Surgical options in the appropriate patient to decompress posterior compartment include arthroscopic olecranon debridement or limited incision arthrotomy. Excessive resection of posteromedial osteophytes must be avoided. Arthroscopy has limited morbidity and allows for complete diagnostic assessment. UCL reconstruction should also be considered in combination with posteromedial debridement when the UCL is torn. More challenging indications for UCL reconstruction occur when the UCL is partially torn or torn and asymptomatic. Isolated posteromedial decompression in this setting risks future development of UCL symptoms that would then need to be addressed.
Surgical Technique
As previously mentioned, elbow arthroscopy or limited excision arthrotomy are the preferred operative methods for decompression of the posterior compartment and thus treatment of VEO. Anesthesia and patient positioning should be selected based on the surgeon’s preference. The patient should be positioned supine, prone, or in lateral decubitis. When a UCL reconstruction is expected, supine position is advantageous to avoid repositioning after completing the arthroscopic portion of the procedure. However, arthroscopy can be performed in the lateral position with subsequent repositioning, repeat prepping, and draping for UCL reconstruction (Figures 5A, 5B).
Prepare for elbow arthroscopy by distending the elbow joint with normal saline to aid in protection of neurovascular structures and simplify the insertion of the scope trocar. Perform diagnostic anterior arthroscopy via the proximal anteromedial portal. Assess for presence of loose bodies and osteochondral lesions of the radiocapitellar joint, as well as osteophytes of the coronoid tip and fossa. Utilizing a spinal needle under direct visualization establish a proximal lateral portal with adequate view of the anterior compartment. Proceed to visualize the medial compartment and assess for UCL injury. Apply valgus stress while in 70° of flexion. Visualize the coronoid process and look for medial trochlea gapping of 3 mm or greater, which indicates UCL insufficiency.30
Establish the posterolateral port for visualization of the posterior compartment. A posterior portal is established through the triceps tendon. Proceed to shave and ablate synovitis in order to create an adequate working space. Inspect the posteromedial olecranon, looking for any osteophytes or chondromalacia in the area (Figure 6). Examine the posterior radiocapitellar joint, looking specifically for loose bodies. The presence of loose bodies may require creating an extra mid lateral portal for removal. The ulnar nerve is located superficial to the elbow capsule and can be damaged by instruments utilized in the posteromedial gutter. As a precaution, be sure to remove suction attached to shaver. Place a curved articulating retractor in an accessory posterolateral portal to assist in protecting the ulnar nerve by retracting the capsule away from the surgical field (Figures 7A, 7B).
The osteophyte may be encased in soft tissue. Using a combination of ablation devices and shavers, the osteophyte can be exposed. The olecranon osteophyte can be removed with a small osteotome located at the border of the osteophyte and the normal olecranon. A motorized shaver or burr may also be introduced through the direct posterior portal or the posterolateral portal to complete the contouring of the olecranon (Figures 8A, 8B). Intraoperative lateral radiographs may be obtained for guidance in adequate bone removal and to ensure no bone debris is left in the soft tissues. It is critical that only pathologic osteophyte is removed and that normal olecranon is not compromised. This prevents an increase in UCL strain during valgus loading.19 However, in some non-throwing athletes, more aggressive debridement can be performed due to a smaller risk of UCL injury after posterior decompression.
Often, with the presence of osteophytes on the olecranon, there may be associated chondromalacia of the trochlea. These kissing lesions must be addressed after debridement of osteophytes. Loose flaps or frayed edges are carefully debrided and for any significant lesion the edges are contoured to a stable rim using shavers and curettes. Once altered to a well-shouldered lesion, microfracture is performed. Anterograde drilling of the lesion with perforations separated by 2 to 3 mm allow for the release of marrow elements and induction of a fibrocartilage healing response.
For an isolated posteromedial decompression, early rehabilitation begins with simple elbow flexion and extension exercises. It is important to restore flexor-pronator strength. Six weeks postoperatively, a progressive throwing program that includes plyometric exercises, neuromuscular training, and endurance exercises can be initiated. Patients can typically return to competition 3 to 4 months after surgery, if they have successfully regained preoperative range of motion, preoperative strength in the elbow, and there is no pain or tenderness on stress testing or palpation.
Outcomes
Safety and Advances in Arthroscopy
A clearer understanding of portal placement and proximity to neurovasculature in conjunction with advances in equipment have allowed for continual improvements in elbow arthroscopy techniques. There is plenty of literature indicating that arthroscopic posteromedial decompression is a safe, reliable, effective procedure, with a high rate of patient satisfaction.22,30-34] Andrews and Carson30 published one of the earliest investigations indicating the effectiveness of elbow arthroscopy utilizing objective and subjective outcome scores. They found that preoperative scores indicating patient satisfaction increased from 50% to 83%. Patients who underwent only loose body removal had the best outcomes. Andrews and Timmerman31 later evaluated the results of 72 professional baseball players who underwent either arthroscopic or open elbow surgery. They found that posteromedial olecranon osteophytes and intraarticular loose bodies were the most common diagnoses, present in 65% and 54% of players, respectively. In addition, a 41% reoperation rate was reported after posteromedial olecranon resection, along with 25% a rate of valgus instability necessitating UCL reconstruction. Andrews and Timmerman31 propose that the incidence of UCL injuries is underestimated and that UCL pathology must be treated prior to treating its secondary effects. Recently, Reddy and colleagues32 reviewed the results of 187 arthroscopic procedures. Posterior impingement, loose bodies, and osteoarthritis were the most common problems, occurring in 51%, 31%, and 22% of patients, respectively. Reported results were encouraging, with 87% good to excellent results and 85% of baseball players returning to preinjury levels.
Conclusion
An understanding of the relevant functional anatomy and the biomechanics of throwing is essential to understanding VEO. Potential concomitant valgus instability and UCL injury must be carefully assessed. Only symptomatic patients who have failed conservative treatment should undergo surgery. It is critical to avoid exacerbating and/or causing valgus instability by surgical excessively removing normal bone from the olecranon. Arthroscopy has been shown to be a safe and effective method to treat refractory cases of VEO.
1. Pappas AM, Zawacki RM, Sullivan TJ. Biomechanics of baseball pitching. A preliminary report. Am J Sports Med. 1985;13(4):216-222.
2. Fleisig GS, Barrentine SW, Escamilla RF, Andrews JR. Biomechanics of overhand throwing with implications for injuries. Sports Med. 1996;21(6):421-437.
3. Wilson FD, Andrews JR, Blackburn TA, McCluskey G. Valgus extension overload in the pitching elbow. Am J Sports Med. 1983;11(2):83-88.
4. Morrey BF. Applied anatomy and biomechanics of the elbow joint. Instr Course Lect. 1986,35:59-68.
5. Schwab GH, Bennett JB, Woods GW, Tullos HS. Biomechanics of elbow instability: the role of medial collateral ligament. Clin Orthop Relat Res. 1980;146:42-52.
6. Jobe FW, Kvitne RS. Elbow instability in the athlete. Instr Course Lect. 1991;40:17-23.
7. Søjbjerg JO, Ovesen J, Nielsen S. Experimental elbow instability after transection of the medial collateral ligament. Clin Orthop Relat Res. 1987;218:186-190.
8. Regan WD, Korinek SL, Morrey BF, An KN. Biomechanical study of ligaments around the elbow joint. Clin Orthop Relat Res. 1991;271:170-179.
9. Callaway GH, Field LD, Deng XH, et al. Biomechanical evaluation of the medial collateral ligament of the elbow. J Bone Joint Surg Am. 1997;79(8):1223-1231.
10. Chen FS, Rokito AS, Jobe FW. Medial elbow problems in the overhead-throwing athlete. J Am Acad Orthop Surg. 2001;9(2):99-113.
11. Davidson PA, Pink M, Perry J, Jobe FW. Functional anatomy of the flexor pronator muscle group in relation to the medial collateral ligament of the elbow. Am J Sports Med. 1995;23(2):245-250.
12. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
13. Hamilton CD, Glousman RE, Jobe FW, Brault J, Pink M, Perry J. Dynamic stability of the elbow: electromyographic analysis of the flexor pronator group and the extensor group in pitchers with valgus instability. J Shoulder Elbow Surg. 1996;5(5):347-354.
14. Glousman RE, Barron J, Jobe FW, Perry J, Pink M. An electromyographic analysis of the elbow in normal and injured pitchers with medial collateral ligament insufficiency. Am J Sports Med. 1992;20(3):311-317.
15. DiGiovine NM, Jobe FW, Pink M, Perry J. An electromyographic analysis of the upper extremity in pitching. J Shoulder Elbow Surg. 1992;1(1):15-25.
16. Sisto DJ, Jobe FW, Moynes DR, Antonelli DJ. An electromyographic analysis of the elbow in pitching. Am J Sports Med. 1987;15(3):260-263.
17. Ahmad CS, Park MC, Elattrache NS. Elbow medial ulnar collateral ligament insufficiency alters posteromedial olecranon contact. Am J Sports Med. 2004;32(7):1607–1612.
18. Ahmad CS, Conway J. Elbow arthroscopy: beginners to advanced: valgus extension overload. In: Egol, ed. Instructional Course Lectures; vol 60. Rosemont, IL: American Academy of Orthopaedic Surgeons; submitted 2009.
19. Kamineni S, ElAttrache NS, O’Driscoll S W, et al. Medial collateral ligament strain with partial posteromedial olecranon resection. A biomechanical study. J Bone Joint Surg Am. 2004;86-A(11):2424–2430.
20. Kamineni S, Hirahara H, Pomianowski S, et al. Partial posteromedial olecranon resection: a kinematic study. J Bone Joint Surg Am. 2003;85-A(6):1005–1011.
21. Morrey BF, An KN. Articular and ligamentous contributions to the stability of the elbow joint. Am J Sports Med. 1983;11(5):315–319.
22. O’Driscoll SW, Morrey BF. Arthroscopy of the elbow. Diagnostic and therapeutic benefits and hazards. J Bone Joint Surg Am. 1992;74(1):84–94.
23. Miller CD, Savoie FH 3rd. Valgus extension injuries of the elbow in the throwing athlete. J Am Acad Orthop Surg. 1994;2(5):261-269.
24. O’Driscoll SW. Valgus extension overload and plica. In: Levine WN, ed. The Athlete’s Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:71-83.
25. Boatright JR, D’Alessandro DF. Nerve entrapment syndromes at the elbow. In Jobe FW, Pink MM, Glousman RE, Kvitne RE, Zemel NP, eds. Operative Techniques in Upper Extremity Sports Injuries. St. Louis, MO: Mosby-Year Book; 1996:518-537.
26. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
27. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
28. Kooima CL, Anderson K, Craig JV, Teeter DM, van Holsbeeck M. Evidence of subclinical medial collateral ligament injury and posteromedial impingement in professional baseball players. Am J Sports Med. 2004;32(7):1602-1606.
29. Field LD, Altchek DW. Evaluation of the arthroscopic valgus instability test of the elbow. Am J Sports Med. 1996;24(2):177–181.
30. Andrews JR, Carson WG. Arthroscopy of the elbow. Arthroscopy. 1985;1(2):97-107.
31. Andrews JR, Timmerman LA. Outcome of elbow surgery in professional baseball players. Am J Sports Med. 1995;23(4):407-413.
32. Reddy AS, Kvitne RE, Yocum LA, Elattrache NS, Glousman RE, Jobe FW. Arthroscopy of the elbow: a long-term clinical review. Arthroscopy. 2000;16(6):588-594.
33. Rosenwasser MP, Steinmann S. Elbow arthroscopy in the treatment of posterior olecranon impingement. Paper present at: AANA Annual Meeting; 1991; San Diego, CA.
34. Wilson FD, Andrews JR, Blackburn TA, McCluskey G. Valgus extension overload in the pitching elbow. Am J Sports Med. 1983;11(2):83-88.
1. Pappas AM, Zawacki RM, Sullivan TJ. Biomechanics of baseball pitching. A preliminary report. Am J Sports Med. 1985;13(4):216-222.
2. Fleisig GS, Barrentine SW, Escamilla RF, Andrews JR. Biomechanics of overhand throwing with implications for injuries. Sports Med. 1996;21(6):421-437.
3. Wilson FD, Andrews JR, Blackburn TA, McCluskey G. Valgus extension overload in the pitching elbow. Am J Sports Med. 1983;11(2):83-88.
4. Morrey BF. Applied anatomy and biomechanics of the elbow joint. Instr Course Lect. 1986,35:59-68.
5. Schwab GH, Bennett JB, Woods GW, Tullos HS. Biomechanics of elbow instability: the role of medial collateral ligament. Clin Orthop Relat Res. 1980;146:42-52.
6. Jobe FW, Kvitne RS. Elbow instability in the athlete. Instr Course Lect. 1991;40:17-23.
7. Søjbjerg JO, Ovesen J, Nielsen S. Experimental elbow instability after transection of the medial collateral ligament. Clin Orthop Relat Res. 1987;218:186-190.
8. Regan WD, Korinek SL, Morrey BF, An KN. Biomechanical study of ligaments around the elbow joint. Clin Orthop Relat Res. 1991;271:170-179.
9. Callaway GH, Field LD, Deng XH, et al. Biomechanical evaluation of the medial collateral ligament of the elbow. J Bone Joint Surg Am. 1997;79(8):1223-1231.
10. Chen FS, Rokito AS, Jobe FW. Medial elbow problems in the overhead-throwing athlete. J Am Acad Orthop Surg. 2001;9(2):99-113.
11. Davidson PA, Pink M, Perry J, Jobe FW. Functional anatomy of the flexor pronator muscle group in relation to the medial collateral ligament of the elbow. Am J Sports Med. 1995;23(2):245-250.
12. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
13. Hamilton CD, Glousman RE, Jobe FW, Brault J, Pink M, Perry J. Dynamic stability of the elbow: electromyographic analysis of the flexor pronator group and the extensor group in pitchers with valgus instability. J Shoulder Elbow Surg. 1996;5(5):347-354.
14. Glousman RE, Barron J, Jobe FW, Perry J, Pink M. An electromyographic analysis of the elbow in normal and injured pitchers with medial collateral ligament insufficiency. Am J Sports Med. 1992;20(3):311-317.
15. DiGiovine NM, Jobe FW, Pink M, Perry J. An electromyographic analysis of the upper extremity in pitching. J Shoulder Elbow Surg. 1992;1(1):15-25.
16. Sisto DJ, Jobe FW, Moynes DR, Antonelli DJ. An electromyographic analysis of the elbow in pitching. Am J Sports Med. 1987;15(3):260-263.
17. Ahmad CS, Park MC, Elattrache NS. Elbow medial ulnar collateral ligament insufficiency alters posteromedial olecranon contact. Am J Sports Med. 2004;32(7):1607–1612.
18. Ahmad CS, Conway J. Elbow arthroscopy: beginners to advanced: valgus extension overload. In: Egol, ed. Instructional Course Lectures; vol 60. Rosemont, IL: American Academy of Orthopaedic Surgeons; submitted 2009.
19. Kamineni S, ElAttrache NS, O’Driscoll S W, et al. Medial collateral ligament strain with partial posteromedial olecranon resection. A biomechanical study. J Bone Joint Surg Am. 2004;86-A(11):2424–2430.
20. Kamineni S, Hirahara H, Pomianowski S, et al. Partial posteromedial olecranon resection: a kinematic study. J Bone Joint Surg Am. 2003;85-A(6):1005–1011.
21. Morrey BF, An KN. Articular and ligamentous contributions to the stability of the elbow joint. Am J Sports Med. 1983;11(5):315–319.
22. O’Driscoll SW, Morrey BF. Arthroscopy of the elbow. Diagnostic and therapeutic benefits and hazards. J Bone Joint Surg Am. 1992;74(1):84–94.
23. Miller CD, Savoie FH 3rd. Valgus extension injuries of the elbow in the throwing athlete. J Am Acad Orthop Surg. 1994;2(5):261-269.
24. O’Driscoll SW. Valgus extension overload and plica. In: Levine WN, ed. The Athlete’s Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:71-83.
25. Boatright JR, D’Alessandro DF. Nerve entrapment syndromes at the elbow. In Jobe FW, Pink MM, Glousman RE, Kvitne RE, Zemel NP, eds. Operative Techniques in Upper Extremity Sports Injuries. St. Louis, MO: Mosby-Year Book; 1996:518-537.
26. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
27. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
28. Kooima CL, Anderson K, Craig JV, Teeter DM, van Holsbeeck M. Evidence of subclinical medial collateral ligament injury and posteromedial impingement in professional baseball players. Am J Sports Med. 2004;32(7):1602-1606.
29. Field LD, Altchek DW. Evaluation of the arthroscopic valgus instability test of the elbow. Am J Sports Med. 1996;24(2):177–181.
30. Andrews JR, Carson WG. Arthroscopy of the elbow. Arthroscopy. 1985;1(2):97-107.
31. Andrews JR, Timmerman LA. Outcome of elbow surgery in professional baseball players. Am J Sports Med. 1995;23(4):407-413.
32. Reddy AS, Kvitne RE, Yocum LA, Elattrache NS, Glousman RE, Jobe FW. Arthroscopy of the elbow: a long-term clinical review. Arthroscopy. 2000;16(6):588-594.
33. Rosenwasser MP, Steinmann S. Elbow arthroscopy in the treatment of posterior olecranon impingement. Paper present at: AANA Annual Meeting; 1991; San Diego, CA.
34. Wilson FD, Andrews JR, Blackburn TA, McCluskey G. Valgus extension overload in the pitching elbow. Am J Sports Med. 1983;11(2):83-88.
Epidemiology, Treatment, and Prevention of Lumbar Spine Injuries in Major League Baseball Players
For the last 20 years, injuries resulting in time out of play have been on the rise in Major League Baseball (MLB), and those affecting the back are no exception.1,2 In the first comprehensive report on injuries in MLB players, back injuries resulted in a mean of 1016 disabled list days per season from 1995 to 1999.1 Similarly, core and back injuries were responsible for 359 disabled list designations from 2002 to 2008. This represented 11.7% of all injuries resulting in time out of play during that time span.2 During that time, back injury prevalence ranked 6th highest of all possible body regions (out of 17), and both position players and pitchers were similarly affected (7.8% and 7.4% of all injuries, respectively).2 These injuries often result in a significant time out of play and can have a tremendous impact on player health. A healthy, stable, and well-functioning lumbar spine is a prerequisite for nearly all baseball-related activities, including pitching, throwing, batting, and running. Accordingly, even minor lumbar spine injuries may profoundly influence baseball performance. Despite this, less is currently known about the true epidemiology and impact of back injuries in professional baseball compared to other professional sporting organizations.3
The most common causes of low back pain and injury in elite baseball players include muscle strains, stress fractures (spondylolysis), annular tears, disc herniation, stenosis, transverse process fractures, facetogenic pain, and sacroiliac (SI) joint arthropathy.4-8 These injuries present in a variety of ways with varying symptomatology. Accordingly, a thorough understanding and comprehensive approach to the diagnosis and treatment of these injuries is necessary. The purpose of this article is to discuss the current state of lumbar spine injuries in professional baseball players. Specifically, we will discuss the critical role of the spine in baseball activities, common causes of injury, tips for making the diagnosis, treatment options, outcomes, and injury prevention and rehabilitation strategies.
Role of the Spine in Baseball
The spine and core musculature are responsible for positioning the head, shoulders, and upper extremities in space over the hips and lower extremities. Proper maintenance of this relationship is required during all phases of throwing, pitching, running, and hitting. During these activities, the spine may dynamically flex, extend, rotate, and laterally bend as needed to keep the body balanced with the head centered over the trunk.
Pitching and Throwing
Whether pitching from the wind-up or the stretch, the head begins centered over the hips and pelvis. As the pitching motion progresses, the hips undergo rotation, flexion, extension, abduction, and circumduction. While this is occurring, the shoulders and upper truck must bend, rotate, and translate toward home plate with the body. Just prior to front foot contact, trunk rotation averages 55 ± 6° with a maximal mean angular acceleration of 11,600 ± 3100°/s2. 9 In order for the body to remain balanced, controlled, and synchronized throughout this delivery, the lumbar spine and core musculature must work diligently to stabilize the entire kinetic chain. Of all the trunk muscles (paraspinal, rectus abdominis, obliques, and glutei), the lumbar paraspinal muscles often work the hardest during the pitching motion, demonstrating activity increases ranging from 100% to 400%.10 Accordingly, it is not uncommon for pitchers to develop SI joint or lumbar facet joint pain due to this high degree of torsional strain exerted on the low back.4 Poor lumbopelvic control has been shown to be a predictor of subsequent injury, and the degree of lumbopelvic dysfunction is proportional to injury severity in MLB pitchers.5
Hitting
Similar to pitching, hitting involves a complex combination of movements from the upper and lower extremities that must be balanced by the core and spine. Although numerous movements occur simultaneously, rotational motion is primarily responsible for generating power. The trunk rotates an average of 46 ± 9° during the swing and reaches a maximal angular acceleration of 7200 ± 2800°/s2 just after contact.9 During this period of rapid torsion, the spine must rotate in conjunction with the hips and shoulders to create a stable cylinder and axis of rotation. The spine and core are responsible for synchronizing rotation to ensure that hip and shoulder parallelism is maintained from swing initiation to ball contact. If the body does not rotate as a unit, the position of the head is affected and the batter’s ability to see the ball may be compromised. Additionally, if delivery of the shoulders lags too far behind that of the hips, the position of the hands (and bat) in space is adversely affected. The entire kinetic chain must remain balanced, coordinated, precisely timed, and standardized throughout the entire swing from initial trigger to final follow-through. The lumbar spine plays a critical role in each of these steps. If lumbar spine mechanics are not sound, this can have significant adverse effects on batting performance and may predispose hitters to injury.4
Common Etiologies for Spinal Injury
The vast majority of baseball players who experience lumbar pain will have injuries that can be classified as mechanical back pain (ie, spondylolysis, annular tears, facetogenic pain, SI joint arthropathy, or muscle injuries) (Table). Although less likely to occur, nerve entrapment or impingement syndromes (ie, disc herniation, stenosis, and peripheral nerve entrapment) have been observed in professional baseball players. Finally, more concerning pathologies such as infection and tumor are extremely rare, but they must not be overlooked in this high-demand patient population.
Stress Fracture or Spondylolysis
In young athletic patients, up to one-third of those with low back pain may have evidence of a lumbar stress fracture on bone scan.11,12 This is particularly true for athletes who undergo repetitive lumbar extension and rotation, such as linemen, gymnasts, wrestlers, weight lifters, and baseball players.4,13 Although the majority of lumbar stress fractures occur at the pars interarticularis, they can occur in the pedicle or articular process (Figure 1). Most spondylolytic lesions do not progress to spondylolisthesis, especially once patients reach skeletal maturity. Because the fifth lumbar vertebra represents the transition from the lumbar to the sacral spine, most stress fractures occur at L5. These typically present as localized low back pain that worsens with flexion, extension, and rotation.
Muscle Injury
One of the most common causes of low back pain in athletes is muscle strains and spasms. Because the lumbar paraspinal muscles are extremely active during throwing and hitting,10 they are particularly susceptible to injury. This is particularly true in deconditioned athletes or those who report to spring training having not adequately maintained strength and flexibility through the off-season.4,5 These injuries typically present in an acute fashion with an obvious inciting incident. Players may have a history of similar muscle injuries in the past. On examination, they tend to have difficulty maintaining normal posture or ranging the spine through a full arc of motion. Localized, superficial tenderness to palpation in the injured muscle is a key component of the diagnosis.
Annular Tears and Disc Herniation
These injuries typically occur as the result of a combination of compressive and rotary forces on the lumbar spine that overcome the ability of the annulus fibrosus to resist hoop stresses. Patients with annular tears typically present with severe lower back pain that may be accompanied by spasm and pain radiation into the buttock or lower extremities. Pain is usually worsened by valsalva, coughing, sneezing, or bearing down.4 Although annular tears can occur in isolation, they can also lead to herniation of the nucleus pulposus into the spinal canal (Figure 2). Depending on the location and severity of the herniation, nerve entrapment or impingement can occur. This may initially present as pain that radiates into the lower extremities in a dermatomal fashion. As the herniation progresses, decreased sensation and weakness may develop.
Facet Joint Pain
Facetogenic pain can occur as the result of degenerative changes, trauma, or joint inflammation. Facet injury typically occurs during rotation while the back is extended.4 This results in localized pain and tenderness that can be reproduced by loading the facet joint (lumbar extension) during the examination, and patients will often demonstrate discomfort and altered motion when extending the flexed back.
Sacroiliac Joint Pain
Although pain in the region of the SI joint is very common, much of this may actually be referred from more centrally located neuromotion segments.4 SI joint pathology can occur as a result of trauma, degeneration, or inflammatory processes as is seen in ankylosing spondylitis (AS). Patients with AS typically present with a gradual onset of progressive stiffness and pain in the low back and hips that is worse in the morning or following periods of inactivity. It is most common in Caucasian males in their second to fourth decades.14 Although 80% to 95% of patients with AS will test positive for human leukocyte antigen B27 (HLA-B27), it is important to note that the vast majority of people with HLA-B27 do not go on to develop AS.14 Regardless of the cause, SI joint pain can be very debilitating and negatively impact all baseball-related activities.
Stenosis
Lumbar stenosis may develop from arthritic changes, disc protrusion, facet hypertrophy, or ligament ossification. In this young, athletic population, congenital stenosis should also be a consideration. Patients with congenital stenosis at baseline are at increased risk for developing neurologic symptoms from disc protrusion or other acquired spinal pathology. Lumbar stenosis generally manifests as a gradual onset of progressive low back pain with radicular symptoms or neurogenic claudication.4
Making the Diagnosis
History
When identifying the cause of any musculoskeletal complaint, the diagnosis begins with a thorough history. In addition to the standard components of the history, such as timing, severity, relation to activity, exacerbating factors, associated symptoms, and prior treatments, Watkins and colleagues4 have outlined a number of key factors that should be determined when specifically evaluating the athlete with low back pain.These include quantification of the morbidity, identification of contributing psychosocial factors, ruling out of urgent diagnoses (ie, neoplasm, infection, rapidly progressive neurologic deficits, cauda equina, and paralysis), determination of injury type and duration, identification of the clinical syndrome/etiology, pinpointing the location of the pathology (what nerve at what level?), and quantification of back versus leg symptoms. Answers to these questions will set the framework for an appropriately directed physical examination, imaging, and diagnostic tests.
Physical Examination
The physical examination begins by observing the patient or player walk across the playing field, training room, or examination room, paying attention to posture, gait, and overall body movement. Many patients with lumbar injuries will demonstrate adaptive patterns of motion in an attempt to accommodate their pain. This may be seen during baseball-related activities such as throwing, batting, or running. The spine should be visualized and palpated for malalignment while standing erect and during forward bending. If possible, motion should be assessed in rotation, lateral bending, and the flexion and extension planes. Special attention should be paid to any positions or maneuvers that reproduce pain or neurologic symptoms. Areas of tenderness and radiating pain should be fully palpated. A full neurologic examination consisting of manual muscle testing, sensory examination, and reflex evaluation of both the upper and lower extremities should be performed. Numerous special tests and neurologic stretch maneuvers that assess specific lumbar nerve roots have been described.15
Imaging and Diagnostic Tests
Depending on the history and physical examination, imaging of the lumbar spine is not always warranted in the acute setting. This is especially the case if muscle injury, herniation, or annular tears are suspected. In cases of persistent pain, trauma, or suspected neoplasia, imaging is generally warranted. When x-rays are negative and spondylolysis is suspected, bone scan with lumbar single photon emission computed tomography (SPECT) is the most sensitive test.16 SPECT scans are positive in active spondylolysis because the radio-nucleotide is taken up by active, bone-forming osteoblasts. Quiescent stress fractures that are not apparent on SPECT scans are generally chronic and painless.4 If the SPECT scan is positive, the injury can be further characterized by computed tomography (CT) (Figure 1), which can distinguish between spondylolysis, osteoid osteoma, osteoblastoma, acute fracture, or arthritic degeneration. When the SPECT is negative, or if neural impingement is suspected, magnetic resonance imaging (MRI) (Figure 2) is likely the best diagnostic imaging tool. MRI allows identification of bone edema, disc herniation, annular disruption, disc desiccation, stenosis, and nerve entrapment. Finally, when attempting to distinguish between central and peripheral nerve entrapment syndromes, an electromyogram (EMG) or nerve conduction study (NCS) is a reliable way to identify the location of injury.
Treatment and Outcomes
The approach to a patient with low back pain begins with identification of the etiology and discontinuation of the activities that reproduce pain.4 Trunk stabilization exercises and anti-inflammatory medications are the mainstays of treatment regardless of the cause of the lumbar spinal injury in the baseball player.4
Stress Fracture or Spondylolysis
Management of symptomatic spondylolysis or spondylolisthesis in the athlete initially consists of conservative treatment, which achieves good to excellent long-term outcomes and return to play in 70% to 90% of athletes, especially for acute injuries.17-19 After stopping the activity that causes the pain, trunk stabilization exercises should be started as soon as tolerated with the use of non-steroidal anti-inflammatory medications (NSAIDs), oral steroids, and spinal injections to control symptoms and permit initiation of the rehabilitation program.4 Although bracing is a commonly used adjunctive treatment, a recent meta-analysis did not demonstrate any difference in clinical outcomes between patients treated with a brace compared to non-braced controls.20
Surgical indications for the treatment of spondylolysis or spondylolisthesis are limited; however, failure of nonoperative treatment after 6 months is a reasonable time to consider surgery.17 The spondylolytic defect can often be repaired directly using hook screws, translaminar screws, wiring, pedicle screws, or image-guided lag screws across the lesion with grafting.4 Lumbar spinal fusion is less successful in professional athletes due to the high demands placed on adjacent levels as well as the time required for the fusion to heal.4 Bony union can be determined by a CT scan at 6 months postoperatively if the patient has met appropriate return to play criteria.4
Muscle Injury
Management of lumbar sprains and strains typically includes restricting painful postures and a rehabilitation program that focuses on core strengthening within a pain-free arc of motion.21 Because acute injuries typically resolve quickly and spontaneously, a short interval of decreased activity, icing, NSAIDs, and stretching followed by focused strength training is appropriate before return to sports activity.22
Annular Tears and Disc Herniation
Initial management of baseball players with acute lumbar disc herniation and/or annular tears consists of rest for up to 5 days followed by physical therapy and NSAIDs, Medrol Dose pak, or epidural injections.4 Professional baseball players return to play at high rates following a herniated lumbar disc.6 Earhart and colleagues6 found that 97.1% of players returned to play at an average time of 6.6 months from the time of injury. When stratified by position, all pitchers (29 of 29) returned to competitive play after operative or nonoperative management, while 38 of 40 hitters returned.6 The average career length after lumbar disc herniation in the professional baseball player is between 4.1 and 5.3 years or between 256 and 471 games.6,23 Other work has suggested that players undergoing operative treatment for lumbar herniation had shorter career lengths; however, patients in the operative group tended to be older at the time of injury.23
Emphasis should be placed on nonoperative management of baseball players with disc pathology except in cases of cauda equina syndrome.4 Hitters and pitchers who require surgery have demonstrated decreased 1-year and 3-year postoperative statistical performance compared to preinjury levels.6 No significant changes in any performance statistic were seen in baseball following nonoperative management.6 Consequently, indications for surgery in the baseball player with lumbar disc pathology includes cauda equina syndrome, progressive neurologic deficit, sufficient morbidity, failure of conservative care, a lesion that can be corrected safely with surgery, and the ability for the patient to comply with a comprehensive postoperative rehabilitation program.4 Operative treatment typically consists of a lumbar microdiscectomy and/or laminotomy. 4,6
Facet Joint Pain
The mainstay of therapy in patients with facet joint pain consists of analgesia and a trunk stabilization program.24 Lumbar zygapophysial joint injections and radiofrequency denervation can be considered if the patient fails 4 weeks of directed conservative treatment.24,25 Injections may be useful in select patients; however, the literature supporting the use of lumbar facet joint injections or radiofrequency denervation for facetogenic pain is limited.24,25
Sacroiliac Joint Pain
Acute injury of the SI joint can be treated with NSAIDs, icing, and relative rest.26 Mobilization of the SI joint in addition to correcting any asymmetries in muscle length or stiffness should be started and progressed as soon as tolerated within a pain-free range of motion.26 Rehabilitation should correct biomechanical deficits and maladaptation with a special focus on agonist and antagonist muscle groups across the sacrum and ilium.26 Treatment of AS in the athlete should emphasize symptom control, as there is no definite treatment. For patients with AS, other long-term therapeutic options include sulfasalazine, methotrexate, thalidomide, and anti-tumor necrosis factor therapies.14
Stenosis
Lumbar spinal stenosis, whether congenital or acquired, should initially be managed conservatively.27 Although they do not alter the progression of the disease, epidural steroids and local injections may temporarily decrease symptoms in approximately 40% of cases.27 Those who fail conservative therapy after 3 months may be candidates for surgical decompression and/or fusion.27,28 However, surgical treatment for lumbar spinal stenosis in elite baseball players has not been thoroughly studied, so the long-term prognosis is not well documented.27
Rehabilitation and Prevention of Injuries
After an appropriate diagnosis has been made, a structured rehabilitation process should commence. During rehabilitation, it is of primary importance that deep core stabilization is established. As an initial step in this process, athletes are trained to initiate deep core stabilization with breathing techniques in a static, supine position.29 Proper diaphragm activation with co-contractions of the transverse abdominis (TA) and pelvic floor has been shown to increase lumbar spine stability.30 This will allow for an increase in intra-abdominal pressure (IAP) and improved stabilization of the lumbar spine, creating a muscular cylinder between the bottom of the rib cage and top of the pelvis. These activities are initiated in the supine position but are soon advanced as upper and lower extremity movement against resistance is added. It is important to make sure IAP and contraction of the TA is maintained throughout this sequence of progression.
Once deep core stabilization has been established, athletes are progressed to global muscle training and kinetic linking in all 3 planes of movement. This is an important phase, as lumbar stability is a result of coordinated muscle activation involving many muscles.31 This program progresses from supine breathing exercises to a modified side bridge position to enhance core activation along with frontal plane stability. Next, athletes are progressed to a half kneeling position and then on to standing. Rotational activities are introduced starting with isometric holds progressing to chops/lifts and rotational medicine ball toss. During these tasks, focus should be on quality of movement and maintenance of core activation. Endurance of these muscles should be trained during this process. Appropriate pain-free and safe cardiovascular exercise, such as walking, biking, swimming, and jogging, should be performed throughout each stage in the rehabilitation process. Activities should be halted with any increase in pain. At the completion of the rehabilitation process, it is important to observe the athlete while performing sport-specific tasks. Spinal stabilization must be translational and monitored by observing maintenance of the “cylinder” from the training room to sports specific movements.
Since poor lumbar control has been associated with increased amount of time on the disabled list,5 it would be ideal to identify those at risk of injury before problems arise. Conte and colleagues32 have shown that core muscle strains could be a result of muscle imbalance or improper pitching or hitting technique. Other work has demonstrated that pitchers with poor lumbopelvic control did not perform as well as those with superior control.33 By assessing spinal stability and biomechanics at baseline, we may be able to identify those at risk. Pitchers with suboptimal spinal stabilization can present with an unstable balance phase, increased amounts of hyperextension of the lumbar spine from the moment of max cocking through ball release, as well as increased lateral trunk tilt at ball release. Correcting these flaws and increasing deep core stabilization can prevent injuries and improve performance.
Summary
A stable, well-functioning lumbar spine is vital to nearly every baseball-related activity, including pitching, throwing, batting, fielding, and running. The spine serves as a critical link in the kinetic chain between the upper and lower extremities. Due to the high demand on the lumbar spine, injuries to this area represent a significant amount of time out of play in MLB. Initial treatment typically consists of a comprehensive nonoperative rehabilitation process involving analgesics, rest, and therapy focusing on core stabilization. Because poor lumbopelvic control and mechanics have been demonstrated to increase injury risk, preemptive spinal and core stabilization is likely an appropriate step towards injury prevention.
1. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
2. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
3. Makhni EC, Buza JA, Byram I, Ahmad CS. Sports reporting: a comprehensive review of the medical literature regarding North American professional sports. Phys Sportsmed. 2014;42(2):154-162.
4. Watkins RG III, Watkins RG IV. Chapter 36: Lumbar injuries. In: Sports Medicine of Baseball. Dines JS, Altchek DW, Andrews JR, ElAttrache NS, Wilk KE, Yocum LA, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2012; 383-398.
5. Chaudhari AMW, McKenzie CS, Pan X, Oñate JA. Lumbopelvic control and days missed because of injury in professional baseball pitchers. Am J Sports Med. 2014;42(11):2734-2740.
6. Earhart JS, Roberts D, Roc G, Gryzlo S, Hsu W. Effects of lumbar disk herniation on the careers of professional baseball players. Orthopedics. 2012;35(1):43-49.
7. Hamid KS, Nwachukwu BU, Hsu E, Edgerton CA, Hobson DR, Lang JE. Orthopedic resident work-shift analysis: Are we making the best use of resident work hours? J Surg Educ. 2014;71(2):205-210.
8. Nair R, Kahlenberg CA, Hsu WK. Outcomes of lumbar discectomy in elite athletes: the need for high-level evidence. Clin Orthop Relat Res. 2015;473(6):1971-1977.
9. Fleisig GS, Hsu WK, Fortenbaugh D, Cordover A, Press JM. Trunk axial rotation in baseball pitching and batting. Sports Biomech. 2013;12(4):324-333.
10. Watkins RG, Dennis S, Dillin WH, et al. Dynamic EMG analysis of torque transfer in professional baseball pitchers. Spine (Phila Pa 1976). 1989;14(4):404-408.
11. Micheli LJ. Back injuries in gymnastics. Clin Sports Med. 1985;4(1):85-93.
12. Papanicolaou N, Wilkinson RH, Emans JB, Treves S, Micheli LJ. Bone scintigraphy and radiography in young athletes with low back pain. AJR Am J Roentgenol. 1985;145(5):1039-1044.
13. Elliott S, Hutson MA, Wastie ML. Bone scintigraphy in the assessment of spondylolysis in patients attending a sports injury clinic. Clin Radiol. 1988;39(3):269-272.
14. Kubiak EN, Moskovich R, Errico TJ, Di Cesare PE. Orthopaedic management of ankylosing spondylitis. J Am Acad Orthop Surg. 2005;13(4):267-278.
15. Miller KJ. Physical assessment of lower extremity radiculopathy and sciatica. J Chiropr Med. 2007;6(2):75-82.
16. Bellah RD, Summerville DA, Treves ST, Micheli LJ. Low-back pain in adolescent athletes: detection of stress injury to the pars interarticularis with SPECT. Radiology. 1991;180(2):509-512.
17. Radcliff KE, Kalantar SB, Reitman CA. Surgical management of spondylolysis and spondylolisthesis in athletes: indications and return to play. Curr Sports Med Rep. 8(1):35-40.
18. Morita T, Ikata T, Katoh S, Miyake R. Lumbar spondylolysis in children and adolescents. J Bone Joint Surg Br. 1995;77(4):620-625.
19. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
20. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29(2):146-156.
21. Bono CM. Low-back pain in athletes. J Bone Joint Surg Am. 2004;86-A(2):382-396.
22. Dreisinger TE, Nelson B. Management of back pain in athletes. Sports Med. 1996;21(4):313-320.
23. Hsu WK, McCarthy KJ, Savage JW, et al. The Professional Athlete Spine Initiative: outcomes after lumbar disc herniation in 342 elite professional athletes. Spine J. 2011;11(3):180-186.
24. Dreyfuss PH, Dreyer SJ; NASS. Lumbar zygapophysial (facet) joint injections. Spine J. 2003;3(3 Suppl):50S-59S.
25. Slipman CW, Bhat AL, Gilchrist R V, Issac Z, Chou L, Lenrow DA. A critical review of the evidence for the use of zygapophysial injections and radiofrequency denervation in the treatment of low back pain. Spine J. 2003;3(4):310-316.
26. Prather H. Sacroiliac joint pain: practical management. Clin J Sport Med. 2003;13(4):252-255.
27. Graw BP, Wiesel SW. Low back pain in the aging athlete. Sports Med Arthrosc. 2008;16(1):39-46.
28. Melancia JL, Francisco AF, Antunes JL. Spinal stenosis. Handb Clin Neurol. 2014;119:541-549.
29. Frank C, Kobesova A, Kolar P. Dynamic neuromuscular stabilization & sports rehabilitation. Int J Sports Phys Ther. 2013;8(1):62-73.
30. Cholewicki J, Juluru K, McGill SM. Intra-abdominal pressure mechanism for stabilizing the lumbar spine. J Biomech. 1999;32(1):13-17.
31. McGill SM, Grenier S, Kavcic N, Cholewicki J. Coordination of muscle activity to assure stability of the lumbar spine. J Electromyogr Kinesiol. 2003;13(4):353-359.
32. Conte SA, Thompson MM, Marks MA, Dines JS. Abdominal muscle strains in professional baseball: 1991-2010. Am J Sports Med. 2012;40(3):650-656.
33. Chaudhari AMW, McKenzie CS, Borchers JR, Best TM. Lumbopelvic control and pitching performance of professional baseball pitchers. J Strength Cond Res. 2011;25(8):2127-2132.
For the last 20 years, injuries resulting in time out of play have been on the rise in Major League Baseball (MLB), and those affecting the back are no exception.1,2 In the first comprehensive report on injuries in MLB players, back injuries resulted in a mean of 1016 disabled list days per season from 1995 to 1999.1 Similarly, core and back injuries were responsible for 359 disabled list designations from 2002 to 2008. This represented 11.7% of all injuries resulting in time out of play during that time span.2 During that time, back injury prevalence ranked 6th highest of all possible body regions (out of 17), and both position players and pitchers were similarly affected (7.8% and 7.4% of all injuries, respectively).2 These injuries often result in a significant time out of play and can have a tremendous impact on player health. A healthy, stable, and well-functioning lumbar spine is a prerequisite for nearly all baseball-related activities, including pitching, throwing, batting, and running. Accordingly, even minor lumbar spine injuries may profoundly influence baseball performance. Despite this, less is currently known about the true epidemiology and impact of back injuries in professional baseball compared to other professional sporting organizations.3
The most common causes of low back pain and injury in elite baseball players include muscle strains, stress fractures (spondylolysis), annular tears, disc herniation, stenosis, transverse process fractures, facetogenic pain, and sacroiliac (SI) joint arthropathy.4-8 These injuries present in a variety of ways with varying symptomatology. Accordingly, a thorough understanding and comprehensive approach to the diagnosis and treatment of these injuries is necessary. The purpose of this article is to discuss the current state of lumbar spine injuries in professional baseball players. Specifically, we will discuss the critical role of the spine in baseball activities, common causes of injury, tips for making the diagnosis, treatment options, outcomes, and injury prevention and rehabilitation strategies.
Role of the Spine in Baseball
The spine and core musculature are responsible for positioning the head, shoulders, and upper extremities in space over the hips and lower extremities. Proper maintenance of this relationship is required during all phases of throwing, pitching, running, and hitting. During these activities, the spine may dynamically flex, extend, rotate, and laterally bend as needed to keep the body balanced with the head centered over the trunk.
Pitching and Throwing
Whether pitching from the wind-up or the stretch, the head begins centered over the hips and pelvis. As the pitching motion progresses, the hips undergo rotation, flexion, extension, abduction, and circumduction. While this is occurring, the shoulders and upper truck must bend, rotate, and translate toward home plate with the body. Just prior to front foot contact, trunk rotation averages 55 ± 6° with a maximal mean angular acceleration of 11,600 ± 3100°/s2. 9 In order for the body to remain balanced, controlled, and synchronized throughout this delivery, the lumbar spine and core musculature must work diligently to stabilize the entire kinetic chain. Of all the trunk muscles (paraspinal, rectus abdominis, obliques, and glutei), the lumbar paraspinal muscles often work the hardest during the pitching motion, demonstrating activity increases ranging from 100% to 400%.10 Accordingly, it is not uncommon for pitchers to develop SI joint or lumbar facet joint pain due to this high degree of torsional strain exerted on the low back.4 Poor lumbopelvic control has been shown to be a predictor of subsequent injury, and the degree of lumbopelvic dysfunction is proportional to injury severity in MLB pitchers.5
Hitting
Similar to pitching, hitting involves a complex combination of movements from the upper and lower extremities that must be balanced by the core and spine. Although numerous movements occur simultaneously, rotational motion is primarily responsible for generating power. The trunk rotates an average of 46 ± 9° during the swing and reaches a maximal angular acceleration of 7200 ± 2800°/s2 just after contact.9 During this period of rapid torsion, the spine must rotate in conjunction with the hips and shoulders to create a stable cylinder and axis of rotation. The spine and core are responsible for synchronizing rotation to ensure that hip and shoulder parallelism is maintained from swing initiation to ball contact. If the body does not rotate as a unit, the position of the head is affected and the batter’s ability to see the ball may be compromised. Additionally, if delivery of the shoulders lags too far behind that of the hips, the position of the hands (and bat) in space is adversely affected. The entire kinetic chain must remain balanced, coordinated, precisely timed, and standardized throughout the entire swing from initial trigger to final follow-through. The lumbar spine plays a critical role in each of these steps. If lumbar spine mechanics are not sound, this can have significant adverse effects on batting performance and may predispose hitters to injury.4
Common Etiologies for Spinal Injury
The vast majority of baseball players who experience lumbar pain will have injuries that can be classified as mechanical back pain (ie, spondylolysis, annular tears, facetogenic pain, SI joint arthropathy, or muscle injuries) (Table). Although less likely to occur, nerve entrapment or impingement syndromes (ie, disc herniation, stenosis, and peripheral nerve entrapment) have been observed in professional baseball players. Finally, more concerning pathologies such as infection and tumor are extremely rare, but they must not be overlooked in this high-demand patient population.
Stress Fracture or Spondylolysis
In young athletic patients, up to one-third of those with low back pain may have evidence of a lumbar stress fracture on bone scan.11,12 This is particularly true for athletes who undergo repetitive lumbar extension and rotation, such as linemen, gymnasts, wrestlers, weight lifters, and baseball players.4,13 Although the majority of lumbar stress fractures occur at the pars interarticularis, they can occur in the pedicle or articular process (Figure 1). Most spondylolytic lesions do not progress to spondylolisthesis, especially once patients reach skeletal maturity. Because the fifth lumbar vertebra represents the transition from the lumbar to the sacral spine, most stress fractures occur at L5. These typically present as localized low back pain that worsens with flexion, extension, and rotation.
Muscle Injury
One of the most common causes of low back pain in athletes is muscle strains and spasms. Because the lumbar paraspinal muscles are extremely active during throwing and hitting,10 they are particularly susceptible to injury. This is particularly true in deconditioned athletes or those who report to spring training having not adequately maintained strength and flexibility through the off-season.4,5 These injuries typically present in an acute fashion with an obvious inciting incident. Players may have a history of similar muscle injuries in the past. On examination, they tend to have difficulty maintaining normal posture or ranging the spine through a full arc of motion. Localized, superficial tenderness to palpation in the injured muscle is a key component of the diagnosis.
Annular Tears and Disc Herniation
These injuries typically occur as the result of a combination of compressive and rotary forces on the lumbar spine that overcome the ability of the annulus fibrosus to resist hoop stresses. Patients with annular tears typically present with severe lower back pain that may be accompanied by spasm and pain radiation into the buttock or lower extremities. Pain is usually worsened by valsalva, coughing, sneezing, or bearing down.4 Although annular tears can occur in isolation, they can also lead to herniation of the nucleus pulposus into the spinal canal (Figure 2). Depending on the location and severity of the herniation, nerve entrapment or impingement can occur. This may initially present as pain that radiates into the lower extremities in a dermatomal fashion. As the herniation progresses, decreased sensation and weakness may develop.
Facet Joint Pain
Facetogenic pain can occur as the result of degenerative changes, trauma, or joint inflammation. Facet injury typically occurs during rotation while the back is extended.4 This results in localized pain and tenderness that can be reproduced by loading the facet joint (lumbar extension) during the examination, and patients will often demonstrate discomfort and altered motion when extending the flexed back.
Sacroiliac Joint Pain
Although pain in the region of the SI joint is very common, much of this may actually be referred from more centrally located neuromotion segments.4 SI joint pathology can occur as a result of trauma, degeneration, or inflammatory processes as is seen in ankylosing spondylitis (AS). Patients with AS typically present with a gradual onset of progressive stiffness and pain in the low back and hips that is worse in the morning or following periods of inactivity. It is most common in Caucasian males in their second to fourth decades.14 Although 80% to 95% of patients with AS will test positive for human leukocyte antigen B27 (HLA-B27), it is important to note that the vast majority of people with HLA-B27 do not go on to develop AS.14 Regardless of the cause, SI joint pain can be very debilitating and negatively impact all baseball-related activities.
Stenosis
Lumbar stenosis may develop from arthritic changes, disc protrusion, facet hypertrophy, or ligament ossification. In this young, athletic population, congenital stenosis should also be a consideration. Patients with congenital stenosis at baseline are at increased risk for developing neurologic symptoms from disc protrusion or other acquired spinal pathology. Lumbar stenosis generally manifests as a gradual onset of progressive low back pain with radicular symptoms or neurogenic claudication.4
Making the Diagnosis
History
When identifying the cause of any musculoskeletal complaint, the diagnosis begins with a thorough history. In addition to the standard components of the history, such as timing, severity, relation to activity, exacerbating factors, associated symptoms, and prior treatments, Watkins and colleagues4 have outlined a number of key factors that should be determined when specifically evaluating the athlete with low back pain.These include quantification of the morbidity, identification of contributing psychosocial factors, ruling out of urgent diagnoses (ie, neoplasm, infection, rapidly progressive neurologic deficits, cauda equina, and paralysis), determination of injury type and duration, identification of the clinical syndrome/etiology, pinpointing the location of the pathology (what nerve at what level?), and quantification of back versus leg symptoms. Answers to these questions will set the framework for an appropriately directed physical examination, imaging, and diagnostic tests.
Physical Examination
The physical examination begins by observing the patient or player walk across the playing field, training room, or examination room, paying attention to posture, gait, and overall body movement. Many patients with lumbar injuries will demonstrate adaptive patterns of motion in an attempt to accommodate their pain. This may be seen during baseball-related activities such as throwing, batting, or running. The spine should be visualized and palpated for malalignment while standing erect and during forward bending. If possible, motion should be assessed in rotation, lateral bending, and the flexion and extension planes. Special attention should be paid to any positions or maneuvers that reproduce pain or neurologic symptoms. Areas of tenderness and radiating pain should be fully palpated. A full neurologic examination consisting of manual muscle testing, sensory examination, and reflex evaluation of both the upper and lower extremities should be performed. Numerous special tests and neurologic stretch maneuvers that assess specific lumbar nerve roots have been described.15
Imaging and Diagnostic Tests
Depending on the history and physical examination, imaging of the lumbar spine is not always warranted in the acute setting. This is especially the case if muscle injury, herniation, or annular tears are suspected. In cases of persistent pain, trauma, or suspected neoplasia, imaging is generally warranted. When x-rays are negative and spondylolysis is suspected, bone scan with lumbar single photon emission computed tomography (SPECT) is the most sensitive test.16 SPECT scans are positive in active spondylolysis because the radio-nucleotide is taken up by active, bone-forming osteoblasts. Quiescent stress fractures that are not apparent on SPECT scans are generally chronic and painless.4 If the SPECT scan is positive, the injury can be further characterized by computed tomography (CT) (Figure 1), which can distinguish between spondylolysis, osteoid osteoma, osteoblastoma, acute fracture, or arthritic degeneration. When the SPECT is negative, or if neural impingement is suspected, magnetic resonance imaging (MRI) (Figure 2) is likely the best diagnostic imaging tool. MRI allows identification of bone edema, disc herniation, annular disruption, disc desiccation, stenosis, and nerve entrapment. Finally, when attempting to distinguish between central and peripheral nerve entrapment syndromes, an electromyogram (EMG) or nerve conduction study (NCS) is a reliable way to identify the location of injury.
Treatment and Outcomes
The approach to a patient with low back pain begins with identification of the etiology and discontinuation of the activities that reproduce pain.4 Trunk stabilization exercises and anti-inflammatory medications are the mainstays of treatment regardless of the cause of the lumbar spinal injury in the baseball player.4
Stress Fracture or Spondylolysis
Management of symptomatic spondylolysis or spondylolisthesis in the athlete initially consists of conservative treatment, which achieves good to excellent long-term outcomes and return to play in 70% to 90% of athletes, especially for acute injuries.17-19 After stopping the activity that causes the pain, trunk stabilization exercises should be started as soon as tolerated with the use of non-steroidal anti-inflammatory medications (NSAIDs), oral steroids, and spinal injections to control symptoms and permit initiation of the rehabilitation program.4 Although bracing is a commonly used adjunctive treatment, a recent meta-analysis did not demonstrate any difference in clinical outcomes between patients treated with a brace compared to non-braced controls.20
Surgical indications for the treatment of spondylolysis or spondylolisthesis are limited; however, failure of nonoperative treatment after 6 months is a reasonable time to consider surgery.17 The spondylolytic defect can often be repaired directly using hook screws, translaminar screws, wiring, pedicle screws, or image-guided lag screws across the lesion with grafting.4 Lumbar spinal fusion is less successful in professional athletes due to the high demands placed on adjacent levels as well as the time required for the fusion to heal.4 Bony union can be determined by a CT scan at 6 months postoperatively if the patient has met appropriate return to play criteria.4
Muscle Injury
Management of lumbar sprains and strains typically includes restricting painful postures and a rehabilitation program that focuses on core strengthening within a pain-free arc of motion.21 Because acute injuries typically resolve quickly and spontaneously, a short interval of decreased activity, icing, NSAIDs, and stretching followed by focused strength training is appropriate before return to sports activity.22
Annular Tears and Disc Herniation
Initial management of baseball players with acute lumbar disc herniation and/or annular tears consists of rest for up to 5 days followed by physical therapy and NSAIDs, Medrol Dose pak, or epidural injections.4 Professional baseball players return to play at high rates following a herniated lumbar disc.6 Earhart and colleagues6 found that 97.1% of players returned to play at an average time of 6.6 months from the time of injury. When stratified by position, all pitchers (29 of 29) returned to competitive play after operative or nonoperative management, while 38 of 40 hitters returned.6 The average career length after lumbar disc herniation in the professional baseball player is between 4.1 and 5.3 years or between 256 and 471 games.6,23 Other work has suggested that players undergoing operative treatment for lumbar herniation had shorter career lengths; however, patients in the operative group tended to be older at the time of injury.23
Emphasis should be placed on nonoperative management of baseball players with disc pathology except in cases of cauda equina syndrome.4 Hitters and pitchers who require surgery have demonstrated decreased 1-year and 3-year postoperative statistical performance compared to preinjury levels.6 No significant changes in any performance statistic were seen in baseball following nonoperative management.6 Consequently, indications for surgery in the baseball player with lumbar disc pathology includes cauda equina syndrome, progressive neurologic deficit, sufficient morbidity, failure of conservative care, a lesion that can be corrected safely with surgery, and the ability for the patient to comply with a comprehensive postoperative rehabilitation program.4 Operative treatment typically consists of a lumbar microdiscectomy and/or laminotomy. 4,6
Facet Joint Pain
The mainstay of therapy in patients with facet joint pain consists of analgesia and a trunk stabilization program.24 Lumbar zygapophysial joint injections and radiofrequency denervation can be considered if the patient fails 4 weeks of directed conservative treatment.24,25 Injections may be useful in select patients; however, the literature supporting the use of lumbar facet joint injections or radiofrequency denervation for facetogenic pain is limited.24,25
Sacroiliac Joint Pain
Acute injury of the SI joint can be treated with NSAIDs, icing, and relative rest.26 Mobilization of the SI joint in addition to correcting any asymmetries in muscle length or stiffness should be started and progressed as soon as tolerated within a pain-free range of motion.26 Rehabilitation should correct biomechanical deficits and maladaptation with a special focus on agonist and antagonist muscle groups across the sacrum and ilium.26 Treatment of AS in the athlete should emphasize symptom control, as there is no definite treatment. For patients with AS, other long-term therapeutic options include sulfasalazine, methotrexate, thalidomide, and anti-tumor necrosis factor therapies.14
Stenosis
Lumbar spinal stenosis, whether congenital or acquired, should initially be managed conservatively.27 Although they do not alter the progression of the disease, epidural steroids and local injections may temporarily decrease symptoms in approximately 40% of cases.27 Those who fail conservative therapy after 3 months may be candidates for surgical decompression and/or fusion.27,28 However, surgical treatment for lumbar spinal stenosis in elite baseball players has not been thoroughly studied, so the long-term prognosis is not well documented.27
Rehabilitation and Prevention of Injuries
After an appropriate diagnosis has been made, a structured rehabilitation process should commence. During rehabilitation, it is of primary importance that deep core stabilization is established. As an initial step in this process, athletes are trained to initiate deep core stabilization with breathing techniques in a static, supine position.29 Proper diaphragm activation with co-contractions of the transverse abdominis (TA) and pelvic floor has been shown to increase lumbar spine stability.30 This will allow for an increase in intra-abdominal pressure (IAP) and improved stabilization of the lumbar spine, creating a muscular cylinder between the bottom of the rib cage and top of the pelvis. These activities are initiated in the supine position but are soon advanced as upper and lower extremity movement against resistance is added. It is important to make sure IAP and contraction of the TA is maintained throughout this sequence of progression.
Once deep core stabilization has been established, athletes are progressed to global muscle training and kinetic linking in all 3 planes of movement. This is an important phase, as lumbar stability is a result of coordinated muscle activation involving many muscles.31 This program progresses from supine breathing exercises to a modified side bridge position to enhance core activation along with frontal plane stability. Next, athletes are progressed to a half kneeling position and then on to standing. Rotational activities are introduced starting with isometric holds progressing to chops/lifts and rotational medicine ball toss. During these tasks, focus should be on quality of movement and maintenance of core activation. Endurance of these muscles should be trained during this process. Appropriate pain-free and safe cardiovascular exercise, such as walking, biking, swimming, and jogging, should be performed throughout each stage in the rehabilitation process. Activities should be halted with any increase in pain. At the completion of the rehabilitation process, it is important to observe the athlete while performing sport-specific tasks. Spinal stabilization must be translational and monitored by observing maintenance of the “cylinder” from the training room to sports specific movements.
Since poor lumbar control has been associated with increased amount of time on the disabled list,5 it would be ideal to identify those at risk of injury before problems arise. Conte and colleagues32 have shown that core muscle strains could be a result of muscle imbalance or improper pitching or hitting technique. Other work has demonstrated that pitchers with poor lumbopelvic control did not perform as well as those with superior control.33 By assessing spinal stability and biomechanics at baseline, we may be able to identify those at risk. Pitchers with suboptimal spinal stabilization can present with an unstable balance phase, increased amounts of hyperextension of the lumbar spine from the moment of max cocking through ball release, as well as increased lateral trunk tilt at ball release. Correcting these flaws and increasing deep core stabilization can prevent injuries and improve performance.
Summary
A stable, well-functioning lumbar spine is vital to nearly every baseball-related activity, including pitching, throwing, batting, fielding, and running. The spine serves as a critical link in the kinetic chain between the upper and lower extremities. Due to the high demand on the lumbar spine, injuries to this area represent a significant amount of time out of play in MLB. Initial treatment typically consists of a comprehensive nonoperative rehabilitation process involving analgesics, rest, and therapy focusing on core stabilization. Because poor lumbopelvic control and mechanics have been demonstrated to increase injury risk, preemptive spinal and core stabilization is likely an appropriate step towards injury prevention.
For the last 20 years, injuries resulting in time out of play have been on the rise in Major League Baseball (MLB), and those affecting the back are no exception.1,2 In the first comprehensive report on injuries in MLB players, back injuries resulted in a mean of 1016 disabled list days per season from 1995 to 1999.1 Similarly, core and back injuries were responsible for 359 disabled list designations from 2002 to 2008. This represented 11.7% of all injuries resulting in time out of play during that time span.2 During that time, back injury prevalence ranked 6th highest of all possible body regions (out of 17), and both position players and pitchers were similarly affected (7.8% and 7.4% of all injuries, respectively).2 These injuries often result in a significant time out of play and can have a tremendous impact on player health. A healthy, stable, and well-functioning lumbar spine is a prerequisite for nearly all baseball-related activities, including pitching, throwing, batting, and running. Accordingly, even minor lumbar spine injuries may profoundly influence baseball performance. Despite this, less is currently known about the true epidemiology and impact of back injuries in professional baseball compared to other professional sporting organizations.3
The most common causes of low back pain and injury in elite baseball players include muscle strains, stress fractures (spondylolysis), annular tears, disc herniation, stenosis, transverse process fractures, facetogenic pain, and sacroiliac (SI) joint arthropathy.4-8 These injuries present in a variety of ways with varying symptomatology. Accordingly, a thorough understanding and comprehensive approach to the diagnosis and treatment of these injuries is necessary. The purpose of this article is to discuss the current state of lumbar spine injuries in professional baseball players. Specifically, we will discuss the critical role of the spine in baseball activities, common causes of injury, tips for making the diagnosis, treatment options, outcomes, and injury prevention and rehabilitation strategies.
Role of the Spine in Baseball
The spine and core musculature are responsible for positioning the head, shoulders, and upper extremities in space over the hips and lower extremities. Proper maintenance of this relationship is required during all phases of throwing, pitching, running, and hitting. During these activities, the spine may dynamically flex, extend, rotate, and laterally bend as needed to keep the body balanced with the head centered over the trunk.
Pitching and Throwing
Whether pitching from the wind-up or the stretch, the head begins centered over the hips and pelvis. As the pitching motion progresses, the hips undergo rotation, flexion, extension, abduction, and circumduction. While this is occurring, the shoulders and upper truck must bend, rotate, and translate toward home plate with the body. Just prior to front foot contact, trunk rotation averages 55 ± 6° with a maximal mean angular acceleration of 11,600 ± 3100°/s2. 9 In order for the body to remain balanced, controlled, and synchronized throughout this delivery, the lumbar spine and core musculature must work diligently to stabilize the entire kinetic chain. Of all the trunk muscles (paraspinal, rectus abdominis, obliques, and glutei), the lumbar paraspinal muscles often work the hardest during the pitching motion, demonstrating activity increases ranging from 100% to 400%.10 Accordingly, it is not uncommon for pitchers to develop SI joint or lumbar facet joint pain due to this high degree of torsional strain exerted on the low back.4 Poor lumbopelvic control has been shown to be a predictor of subsequent injury, and the degree of lumbopelvic dysfunction is proportional to injury severity in MLB pitchers.5
Hitting
Similar to pitching, hitting involves a complex combination of movements from the upper and lower extremities that must be balanced by the core and spine. Although numerous movements occur simultaneously, rotational motion is primarily responsible for generating power. The trunk rotates an average of 46 ± 9° during the swing and reaches a maximal angular acceleration of 7200 ± 2800°/s2 just after contact.9 During this period of rapid torsion, the spine must rotate in conjunction with the hips and shoulders to create a stable cylinder and axis of rotation. The spine and core are responsible for synchronizing rotation to ensure that hip and shoulder parallelism is maintained from swing initiation to ball contact. If the body does not rotate as a unit, the position of the head is affected and the batter’s ability to see the ball may be compromised. Additionally, if delivery of the shoulders lags too far behind that of the hips, the position of the hands (and bat) in space is adversely affected. The entire kinetic chain must remain balanced, coordinated, precisely timed, and standardized throughout the entire swing from initial trigger to final follow-through. The lumbar spine plays a critical role in each of these steps. If lumbar spine mechanics are not sound, this can have significant adverse effects on batting performance and may predispose hitters to injury.4
Common Etiologies for Spinal Injury
The vast majority of baseball players who experience lumbar pain will have injuries that can be classified as mechanical back pain (ie, spondylolysis, annular tears, facetogenic pain, SI joint arthropathy, or muscle injuries) (Table). Although less likely to occur, nerve entrapment or impingement syndromes (ie, disc herniation, stenosis, and peripheral nerve entrapment) have been observed in professional baseball players. Finally, more concerning pathologies such as infection and tumor are extremely rare, but they must not be overlooked in this high-demand patient population.
Stress Fracture or Spondylolysis
In young athletic patients, up to one-third of those with low back pain may have evidence of a lumbar stress fracture on bone scan.11,12 This is particularly true for athletes who undergo repetitive lumbar extension and rotation, such as linemen, gymnasts, wrestlers, weight lifters, and baseball players.4,13 Although the majority of lumbar stress fractures occur at the pars interarticularis, they can occur in the pedicle or articular process (Figure 1). Most spondylolytic lesions do not progress to spondylolisthesis, especially once patients reach skeletal maturity. Because the fifth lumbar vertebra represents the transition from the lumbar to the sacral spine, most stress fractures occur at L5. These typically present as localized low back pain that worsens with flexion, extension, and rotation.
Muscle Injury
One of the most common causes of low back pain in athletes is muscle strains and spasms. Because the lumbar paraspinal muscles are extremely active during throwing and hitting,10 they are particularly susceptible to injury. This is particularly true in deconditioned athletes or those who report to spring training having not adequately maintained strength and flexibility through the off-season.4,5 These injuries typically present in an acute fashion with an obvious inciting incident. Players may have a history of similar muscle injuries in the past. On examination, they tend to have difficulty maintaining normal posture or ranging the spine through a full arc of motion. Localized, superficial tenderness to palpation in the injured muscle is a key component of the diagnosis.
Annular Tears and Disc Herniation
These injuries typically occur as the result of a combination of compressive and rotary forces on the lumbar spine that overcome the ability of the annulus fibrosus to resist hoop stresses. Patients with annular tears typically present with severe lower back pain that may be accompanied by spasm and pain radiation into the buttock or lower extremities. Pain is usually worsened by valsalva, coughing, sneezing, or bearing down.4 Although annular tears can occur in isolation, they can also lead to herniation of the nucleus pulposus into the spinal canal (Figure 2). Depending on the location and severity of the herniation, nerve entrapment or impingement can occur. This may initially present as pain that radiates into the lower extremities in a dermatomal fashion. As the herniation progresses, decreased sensation and weakness may develop.
Facet Joint Pain
Facetogenic pain can occur as the result of degenerative changes, trauma, or joint inflammation. Facet injury typically occurs during rotation while the back is extended.4 This results in localized pain and tenderness that can be reproduced by loading the facet joint (lumbar extension) during the examination, and patients will often demonstrate discomfort and altered motion when extending the flexed back.
Sacroiliac Joint Pain
Although pain in the region of the SI joint is very common, much of this may actually be referred from more centrally located neuromotion segments.4 SI joint pathology can occur as a result of trauma, degeneration, or inflammatory processes as is seen in ankylosing spondylitis (AS). Patients with AS typically present with a gradual onset of progressive stiffness and pain in the low back and hips that is worse in the morning or following periods of inactivity. It is most common in Caucasian males in their second to fourth decades.14 Although 80% to 95% of patients with AS will test positive for human leukocyte antigen B27 (HLA-B27), it is important to note that the vast majority of people with HLA-B27 do not go on to develop AS.14 Regardless of the cause, SI joint pain can be very debilitating and negatively impact all baseball-related activities.
Stenosis
Lumbar stenosis may develop from arthritic changes, disc protrusion, facet hypertrophy, or ligament ossification. In this young, athletic population, congenital stenosis should also be a consideration. Patients with congenital stenosis at baseline are at increased risk for developing neurologic symptoms from disc protrusion or other acquired spinal pathology. Lumbar stenosis generally manifests as a gradual onset of progressive low back pain with radicular symptoms or neurogenic claudication.4
Making the Diagnosis
History
When identifying the cause of any musculoskeletal complaint, the diagnosis begins with a thorough history. In addition to the standard components of the history, such as timing, severity, relation to activity, exacerbating factors, associated symptoms, and prior treatments, Watkins and colleagues4 have outlined a number of key factors that should be determined when specifically evaluating the athlete with low back pain.These include quantification of the morbidity, identification of contributing psychosocial factors, ruling out of urgent diagnoses (ie, neoplasm, infection, rapidly progressive neurologic deficits, cauda equina, and paralysis), determination of injury type and duration, identification of the clinical syndrome/etiology, pinpointing the location of the pathology (what nerve at what level?), and quantification of back versus leg symptoms. Answers to these questions will set the framework for an appropriately directed physical examination, imaging, and diagnostic tests.
Physical Examination
The physical examination begins by observing the patient or player walk across the playing field, training room, or examination room, paying attention to posture, gait, and overall body movement. Many patients with lumbar injuries will demonstrate adaptive patterns of motion in an attempt to accommodate their pain. This may be seen during baseball-related activities such as throwing, batting, or running. The spine should be visualized and palpated for malalignment while standing erect and during forward bending. If possible, motion should be assessed in rotation, lateral bending, and the flexion and extension planes. Special attention should be paid to any positions or maneuvers that reproduce pain or neurologic symptoms. Areas of tenderness and radiating pain should be fully palpated. A full neurologic examination consisting of manual muscle testing, sensory examination, and reflex evaluation of both the upper and lower extremities should be performed. Numerous special tests and neurologic stretch maneuvers that assess specific lumbar nerve roots have been described.15
Imaging and Diagnostic Tests
Depending on the history and physical examination, imaging of the lumbar spine is not always warranted in the acute setting. This is especially the case if muscle injury, herniation, or annular tears are suspected. In cases of persistent pain, trauma, or suspected neoplasia, imaging is generally warranted. When x-rays are negative and spondylolysis is suspected, bone scan with lumbar single photon emission computed tomography (SPECT) is the most sensitive test.16 SPECT scans are positive in active spondylolysis because the radio-nucleotide is taken up by active, bone-forming osteoblasts. Quiescent stress fractures that are not apparent on SPECT scans are generally chronic and painless.4 If the SPECT scan is positive, the injury can be further characterized by computed tomography (CT) (Figure 1), which can distinguish between spondylolysis, osteoid osteoma, osteoblastoma, acute fracture, or arthritic degeneration. When the SPECT is negative, or if neural impingement is suspected, magnetic resonance imaging (MRI) (Figure 2) is likely the best diagnostic imaging tool. MRI allows identification of bone edema, disc herniation, annular disruption, disc desiccation, stenosis, and nerve entrapment. Finally, when attempting to distinguish between central and peripheral nerve entrapment syndromes, an electromyogram (EMG) or nerve conduction study (NCS) is a reliable way to identify the location of injury.
Treatment and Outcomes
The approach to a patient with low back pain begins with identification of the etiology and discontinuation of the activities that reproduce pain.4 Trunk stabilization exercises and anti-inflammatory medications are the mainstays of treatment regardless of the cause of the lumbar spinal injury in the baseball player.4
Stress Fracture or Spondylolysis
Management of symptomatic spondylolysis or spondylolisthesis in the athlete initially consists of conservative treatment, which achieves good to excellent long-term outcomes and return to play in 70% to 90% of athletes, especially for acute injuries.17-19 After stopping the activity that causes the pain, trunk stabilization exercises should be started as soon as tolerated with the use of non-steroidal anti-inflammatory medications (NSAIDs), oral steroids, and spinal injections to control symptoms and permit initiation of the rehabilitation program.4 Although bracing is a commonly used adjunctive treatment, a recent meta-analysis did not demonstrate any difference in clinical outcomes between patients treated with a brace compared to non-braced controls.20
Surgical indications for the treatment of spondylolysis or spondylolisthesis are limited; however, failure of nonoperative treatment after 6 months is a reasonable time to consider surgery.17 The spondylolytic defect can often be repaired directly using hook screws, translaminar screws, wiring, pedicle screws, or image-guided lag screws across the lesion with grafting.4 Lumbar spinal fusion is less successful in professional athletes due to the high demands placed on adjacent levels as well as the time required for the fusion to heal.4 Bony union can be determined by a CT scan at 6 months postoperatively if the patient has met appropriate return to play criteria.4
Muscle Injury
Management of lumbar sprains and strains typically includes restricting painful postures and a rehabilitation program that focuses on core strengthening within a pain-free arc of motion.21 Because acute injuries typically resolve quickly and spontaneously, a short interval of decreased activity, icing, NSAIDs, and stretching followed by focused strength training is appropriate before return to sports activity.22
Annular Tears and Disc Herniation
Initial management of baseball players with acute lumbar disc herniation and/or annular tears consists of rest for up to 5 days followed by physical therapy and NSAIDs, Medrol Dose pak, or epidural injections.4 Professional baseball players return to play at high rates following a herniated lumbar disc.6 Earhart and colleagues6 found that 97.1% of players returned to play at an average time of 6.6 months from the time of injury. When stratified by position, all pitchers (29 of 29) returned to competitive play after operative or nonoperative management, while 38 of 40 hitters returned.6 The average career length after lumbar disc herniation in the professional baseball player is between 4.1 and 5.3 years or between 256 and 471 games.6,23 Other work has suggested that players undergoing operative treatment for lumbar herniation had shorter career lengths; however, patients in the operative group tended to be older at the time of injury.23
Emphasis should be placed on nonoperative management of baseball players with disc pathology except in cases of cauda equina syndrome.4 Hitters and pitchers who require surgery have demonstrated decreased 1-year and 3-year postoperative statistical performance compared to preinjury levels.6 No significant changes in any performance statistic were seen in baseball following nonoperative management.6 Consequently, indications for surgery in the baseball player with lumbar disc pathology includes cauda equina syndrome, progressive neurologic deficit, sufficient morbidity, failure of conservative care, a lesion that can be corrected safely with surgery, and the ability for the patient to comply with a comprehensive postoperative rehabilitation program.4 Operative treatment typically consists of a lumbar microdiscectomy and/or laminotomy. 4,6
Facet Joint Pain
The mainstay of therapy in patients with facet joint pain consists of analgesia and a trunk stabilization program.24 Lumbar zygapophysial joint injections and radiofrequency denervation can be considered if the patient fails 4 weeks of directed conservative treatment.24,25 Injections may be useful in select patients; however, the literature supporting the use of lumbar facet joint injections or radiofrequency denervation for facetogenic pain is limited.24,25
Sacroiliac Joint Pain
Acute injury of the SI joint can be treated with NSAIDs, icing, and relative rest.26 Mobilization of the SI joint in addition to correcting any asymmetries in muscle length or stiffness should be started and progressed as soon as tolerated within a pain-free range of motion.26 Rehabilitation should correct biomechanical deficits and maladaptation with a special focus on agonist and antagonist muscle groups across the sacrum and ilium.26 Treatment of AS in the athlete should emphasize symptom control, as there is no definite treatment. For patients with AS, other long-term therapeutic options include sulfasalazine, methotrexate, thalidomide, and anti-tumor necrosis factor therapies.14
Stenosis
Lumbar spinal stenosis, whether congenital or acquired, should initially be managed conservatively.27 Although they do not alter the progression of the disease, epidural steroids and local injections may temporarily decrease symptoms in approximately 40% of cases.27 Those who fail conservative therapy after 3 months may be candidates for surgical decompression and/or fusion.27,28 However, surgical treatment for lumbar spinal stenosis in elite baseball players has not been thoroughly studied, so the long-term prognosis is not well documented.27
Rehabilitation and Prevention of Injuries
After an appropriate diagnosis has been made, a structured rehabilitation process should commence. During rehabilitation, it is of primary importance that deep core stabilization is established. As an initial step in this process, athletes are trained to initiate deep core stabilization with breathing techniques in a static, supine position.29 Proper diaphragm activation with co-contractions of the transverse abdominis (TA) and pelvic floor has been shown to increase lumbar spine stability.30 This will allow for an increase in intra-abdominal pressure (IAP) and improved stabilization of the lumbar spine, creating a muscular cylinder between the bottom of the rib cage and top of the pelvis. These activities are initiated in the supine position but are soon advanced as upper and lower extremity movement against resistance is added. It is important to make sure IAP and contraction of the TA is maintained throughout this sequence of progression.
Once deep core stabilization has been established, athletes are progressed to global muscle training and kinetic linking in all 3 planes of movement. This is an important phase, as lumbar stability is a result of coordinated muscle activation involving many muscles.31 This program progresses from supine breathing exercises to a modified side bridge position to enhance core activation along with frontal plane stability. Next, athletes are progressed to a half kneeling position and then on to standing. Rotational activities are introduced starting with isometric holds progressing to chops/lifts and rotational medicine ball toss. During these tasks, focus should be on quality of movement and maintenance of core activation. Endurance of these muscles should be trained during this process. Appropriate pain-free and safe cardiovascular exercise, such as walking, biking, swimming, and jogging, should be performed throughout each stage in the rehabilitation process. Activities should be halted with any increase in pain. At the completion of the rehabilitation process, it is important to observe the athlete while performing sport-specific tasks. Spinal stabilization must be translational and monitored by observing maintenance of the “cylinder” from the training room to sports specific movements.
Since poor lumbar control has been associated with increased amount of time on the disabled list,5 it would be ideal to identify those at risk of injury before problems arise. Conte and colleagues32 have shown that core muscle strains could be a result of muscle imbalance or improper pitching or hitting technique. Other work has demonstrated that pitchers with poor lumbopelvic control did not perform as well as those with superior control.33 By assessing spinal stability and biomechanics at baseline, we may be able to identify those at risk. Pitchers with suboptimal spinal stabilization can present with an unstable balance phase, increased amounts of hyperextension of the lumbar spine from the moment of max cocking through ball release, as well as increased lateral trunk tilt at ball release. Correcting these flaws and increasing deep core stabilization can prevent injuries and improve performance.
Summary
A stable, well-functioning lumbar spine is vital to nearly every baseball-related activity, including pitching, throwing, batting, fielding, and running. The spine serves as a critical link in the kinetic chain between the upper and lower extremities. Due to the high demand on the lumbar spine, injuries to this area represent a significant amount of time out of play in MLB. Initial treatment typically consists of a comprehensive nonoperative rehabilitation process involving analgesics, rest, and therapy focusing on core stabilization. Because poor lumbopelvic control and mechanics have been demonstrated to increase injury risk, preemptive spinal and core stabilization is likely an appropriate step towards injury prevention.
1. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
2. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
3. Makhni EC, Buza JA, Byram I, Ahmad CS. Sports reporting: a comprehensive review of the medical literature regarding North American professional sports. Phys Sportsmed. 2014;42(2):154-162.
4. Watkins RG III, Watkins RG IV. Chapter 36: Lumbar injuries. In: Sports Medicine of Baseball. Dines JS, Altchek DW, Andrews JR, ElAttrache NS, Wilk KE, Yocum LA, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2012; 383-398.
5. Chaudhari AMW, McKenzie CS, Pan X, Oñate JA. Lumbopelvic control and days missed because of injury in professional baseball pitchers. Am J Sports Med. 2014;42(11):2734-2740.
6. Earhart JS, Roberts D, Roc G, Gryzlo S, Hsu W. Effects of lumbar disk herniation on the careers of professional baseball players. Orthopedics. 2012;35(1):43-49.
7. Hamid KS, Nwachukwu BU, Hsu E, Edgerton CA, Hobson DR, Lang JE. Orthopedic resident work-shift analysis: Are we making the best use of resident work hours? J Surg Educ. 2014;71(2):205-210.
8. Nair R, Kahlenberg CA, Hsu WK. Outcomes of lumbar discectomy in elite athletes: the need for high-level evidence. Clin Orthop Relat Res. 2015;473(6):1971-1977.
9. Fleisig GS, Hsu WK, Fortenbaugh D, Cordover A, Press JM. Trunk axial rotation in baseball pitching and batting. Sports Biomech. 2013;12(4):324-333.
10. Watkins RG, Dennis S, Dillin WH, et al. Dynamic EMG analysis of torque transfer in professional baseball pitchers. Spine (Phila Pa 1976). 1989;14(4):404-408.
11. Micheli LJ. Back injuries in gymnastics. Clin Sports Med. 1985;4(1):85-93.
12. Papanicolaou N, Wilkinson RH, Emans JB, Treves S, Micheli LJ. Bone scintigraphy and radiography in young athletes with low back pain. AJR Am J Roentgenol. 1985;145(5):1039-1044.
13. Elliott S, Hutson MA, Wastie ML. Bone scintigraphy in the assessment of spondylolysis in patients attending a sports injury clinic. Clin Radiol. 1988;39(3):269-272.
14. Kubiak EN, Moskovich R, Errico TJ, Di Cesare PE. Orthopaedic management of ankylosing spondylitis. J Am Acad Orthop Surg. 2005;13(4):267-278.
15. Miller KJ. Physical assessment of lower extremity radiculopathy and sciatica. J Chiropr Med. 2007;6(2):75-82.
16. Bellah RD, Summerville DA, Treves ST, Micheli LJ. Low-back pain in adolescent athletes: detection of stress injury to the pars interarticularis with SPECT. Radiology. 1991;180(2):509-512.
17. Radcliff KE, Kalantar SB, Reitman CA. Surgical management of spondylolysis and spondylolisthesis in athletes: indications and return to play. Curr Sports Med Rep. 8(1):35-40.
18. Morita T, Ikata T, Katoh S, Miyake R. Lumbar spondylolysis in children and adolescents. J Bone Joint Surg Br. 1995;77(4):620-625.
19. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
20. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29(2):146-156.
21. Bono CM. Low-back pain in athletes. J Bone Joint Surg Am. 2004;86-A(2):382-396.
22. Dreisinger TE, Nelson B. Management of back pain in athletes. Sports Med. 1996;21(4):313-320.
23. Hsu WK, McCarthy KJ, Savage JW, et al. The Professional Athlete Spine Initiative: outcomes after lumbar disc herniation in 342 elite professional athletes. Spine J. 2011;11(3):180-186.
24. Dreyfuss PH, Dreyer SJ; NASS. Lumbar zygapophysial (facet) joint injections. Spine J. 2003;3(3 Suppl):50S-59S.
25. Slipman CW, Bhat AL, Gilchrist R V, Issac Z, Chou L, Lenrow DA. A critical review of the evidence for the use of zygapophysial injections and radiofrequency denervation in the treatment of low back pain. Spine J. 2003;3(4):310-316.
26. Prather H. Sacroiliac joint pain: practical management. Clin J Sport Med. 2003;13(4):252-255.
27. Graw BP, Wiesel SW. Low back pain in the aging athlete. Sports Med Arthrosc. 2008;16(1):39-46.
28. Melancia JL, Francisco AF, Antunes JL. Spinal stenosis. Handb Clin Neurol. 2014;119:541-549.
29. Frank C, Kobesova A, Kolar P. Dynamic neuromuscular stabilization & sports rehabilitation. Int J Sports Phys Ther. 2013;8(1):62-73.
30. Cholewicki J, Juluru K, McGill SM. Intra-abdominal pressure mechanism for stabilizing the lumbar spine. J Biomech. 1999;32(1):13-17.
31. McGill SM, Grenier S, Kavcic N, Cholewicki J. Coordination of muscle activity to assure stability of the lumbar spine. J Electromyogr Kinesiol. 2003;13(4):353-359.
32. Conte SA, Thompson MM, Marks MA, Dines JS. Abdominal muscle strains in professional baseball: 1991-2010. Am J Sports Med. 2012;40(3):650-656.
33. Chaudhari AMW, McKenzie CS, Borchers JR, Best TM. Lumbopelvic control and pitching performance of professional baseball pitchers. J Strength Cond Res. 2011;25(8):2127-2132.
1. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
2. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
3. Makhni EC, Buza JA, Byram I, Ahmad CS. Sports reporting: a comprehensive review of the medical literature regarding North American professional sports. Phys Sportsmed. 2014;42(2):154-162.
4. Watkins RG III, Watkins RG IV. Chapter 36: Lumbar injuries. In: Sports Medicine of Baseball. Dines JS, Altchek DW, Andrews JR, ElAttrache NS, Wilk KE, Yocum LA, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2012; 383-398.
5. Chaudhari AMW, McKenzie CS, Pan X, Oñate JA. Lumbopelvic control and days missed because of injury in professional baseball pitchers. Am J Sports Med. 2014;42(11):2734-2740.
6. Earhart JS, Roberts D, Roc G, Gryzlo S, Hsu W. Effects of lumbar disk herniation on the careers of professional baseball players. Orthopedics. 2012;35(1):43-49.
7. Hamid KS, Nwachukwu BU, Hsu E, Edgerton CA, Hobson DR, Lang JE. Orthopedic resident work-shift analysis: Are we making the best use of resident work hours? J Surg Educ. 2014;71(2):205-210.
8. Nair R, Kahlenberg CA, Hsu WK. Outcomes of lumbar discectomy in elite athletes: the need for high-level evidence. Clin Orthop Relat Res. 2015;473(6):1971-1977.
9. Fleisig GS, Hsu WK, Fortenbaugh D, Cordover A, Press JM. Trunk axial rotation in baseball pitching and batting. Sports Biomech. 2013;12(4):324-333.
10. Watkins RG, Dennis S, Dillin WH, et al. Dynamic EMG analysis of torque transfer in professional baseball pitchers. Spine (Phila Pa 1976). 1989;14(4):404-408.
11. Micheli LJ. Back injuries in gymnastics. Clin Sports Med. 1985;4(1):85-93.
12. Papanicolaou N, Wilkinson RH, Emans JB, Treves S, Micheli LJ. Bone scintigraphy and radiography in young athletes with low back pain. AJR Am J Roentgenol. 1985;145(5):1039-1044.
13. Elliott S, Hutson MA, Wastie ML. Bone scintigraphy in the assessment of spondylolysis in patients attending a sports injury clinic. Clin Radiol. 1988;39(3):269-272.
14. Kubiak EN, Moskovich R, Errico TJ, Di Cesare PE. Orthopaedic management of ankylosing spondylitis. J Am Acad Orthop Surg. 2005;13(4):267-278.
15. Miller KJ. Physical assessment of lower extremity radiculopathy and sciatica. J Chiropr Med. 2007;6(2):75-82.
16. Bellah RD, Summerville DA, Treves ST, Micheli LJ. Low-back pain in adolescent athletes: detection of stress injury to the pars interarticularis with SPECT. Radiology. 1991;180(2):509-512.
17. Radcliff KE, Kalantar SB, Reitman CA. Surgical management of spondylolysis and spondylolisthesis in athletes: indications and return to play. Curr Sports Med Rep. 8(1):35-40.
18. Morita T, Ikata T, Katoh S, Miyake R. Lumbar spondylolysis in children and adolescents. J Bone Joint Surg Br. 1995;77(4):620-625.
19. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
20. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29(2):146-156.
21. Bono CM. Low-back pain in athletes. J Bone Joint Surg Am. 2004;86-A(2):382-396.
22. Dreisinger TE, Nelson B. Management of back pain in athletes. Sports Med. 1996;21(4):313-320.
23. Hsu WK, McCarthy KJ, Savage JW, et al. The Professional Athlete Spine Initiative: outcomes after lumbar disc herniation in 342 elite professional athletes. Spine J. 2011;11(3):180-186.
24. Dreyfuss PH, Dreyer SJ; NASS. Lumbar zygapophysial (facet) joint injections. Spine J. 2003;3(3 Suppl):50S-59S.
25. Slipman CW, Bhat AL, Gilchrist R V, Issac Z, Chou L, Lenrow DA. A critical review of the evidence for the use of zygapophysial injections and radiofrequency denervation in the treatment of low back pain. Spine J. 2003;3(4):310-316.
26. Prather H. Sacroiliac joint pain: practical management. Clin J Sport Med. 2003;13(4):252-255.
27. Graw BP, Wiesel SW. Low back pain in the aging athlete. Sports Med Arthrosc. 2008;16(1):39-46.
28. Melancia JL, Francisco AF, Antunes JL. Spinal stenosis. Handb Clin Neurol. 2014;119:541-549.
29. Frank C, Kobesova A, Kolar P. Dynamic neuromuscular stabilization & sports rehabilitation. Int J Sports Phys Ther. 2013;8(1):62-73.
30. Cholewicki J, Juluru K, McGill SM. Intra-abdominal pressure mechanism for stabilizing the lumbar spine. J Biomech. 1999;32(1):13-17.
31. McGill SM, Grenier S, Kavcic N, Cholewicki J. Coordination of muscle activity to assure stability of the lumbar spine. J Electromyogr Kinesiol. 2003;13(4):353-359.
32. Conte SA, Thompson MM, Marks MA, Dines JS. Abdominal muscle strains in professional baseball: 1991-2010. Am J Sports Med. 2012;40(3):650-656.
33. Chaudhari AMW, McKenzie CS, Borchers JR, Best TM. Lumbopelvic control and pitching performance of professional baseball pitchers. J Strength Cond Res. 2011;25(8):2127-2132.
Throwing, the Shoulder, and Human Evolution
Charles Darwin once said that apes “...are quite unable, as I have myself seen, to throw a stone with precision”.1 Yet humans can throw with precision and speed, a skill that likely had significant advantages: throwing can affect change at a distance—something few species can do. Throwing can provide protection against predators and can allow for predation for food resources. Throwing would be important in contesting other hominids for scarce resources. As such, throwing has been critically important in human evolution and likely is a skill that has been promoted through natural selection.2-5
In the orthopedic literature, most published work on throwing will ask proximate questions: “how, what, who, when, and where?” Evolutionary biologists are concerned with ultimate questions6,7: “why?” Asking ultimate questions provides insight into how a behavior might offer advantages under natural selection, which can then improve our understanding of the proximate questions for that behavior.
With regard to the shoulder, a number of mysteries exist that, to date, proximate studies have not been able to solve. This article argues that the human shoulder has evolved for throwing and by using this frame of reference, many of the mysteries surrounding the anatomy of the shoulder can be understood.
Pitching Kinematics
The mechanics of pitching have been analyzed extensively. Fleisig and colleagues8 performed kinematic and electromyographic analyses of pitchers to identify the critical moments of pitching (defined as where the forces are highest and injury is most likely going to occur). They found 2 moments where the forces about the shoulder are highest during pitching: the late cocking phase (defined by the point where the humerus reaches maximal external rotation); and the early deceleration phase (defined by the point when the ball is released). If throwing is important in natural selection of humans, then the shoulder anatomy should be optimized to withstand the forces generated in these positions.
Late Cocking Phase of Throwing
The early phases of throwing are attempting to maximize external rotation of the abducted arm as the velocity of the pitched ball correlates to the amount of external rotation achieved.9-11 In this position, kinetic energy in external rotation is stored and converted into kinetic energy in internal rotation.12 The position of the shoulder during late cocking is 94 ± 21° of thoracohumeral abduction, 11 ± 11° of horizontal adduction, and a remarkable 165 ± 11° of thoracohumeral external rotation (Figure 1).8
Fleisig and colleagues8 estimated the torque and forces about the shoulder, which are quite high for joint compression (480 ± 130 N). They also analyzed the shear forces and while trying to describe the origin of superior labrum anterior to posterior (SLAP) lesions and anterior labral tears, broke down the major shear vector into an anterior force vector (310 ± 100 N) and a superior force (250 ± 80 N).8 Note that the resulting shear vector is in an anterosuperior direction and is approximately 400 N.
Early Deceleration Phase of Throwing
Interestingly, the position of the humerus during this critical moment of throwing is not much different than the position during the late cocking phase of throwing, with 93 ± 10° of thoracohumeral abduction, 6 ± 8° of horizontal adduction.8 The major difference in the position of the arm is found in the amount of thoracohumeral rotation, which is now 64 ± 35° of external rotation (Figure 2).8
The forces in early deceleration are tremendous, with an estimated 1090 ± 110 N joint compression force, and an anteroinferior shear force of approximately 130 N.8
Clearly, if throwing is an important skill in human evolution, adaptations must exist in the shoulder to withstand the high forces in these 2 critical moments of throwing.
Solving Mysteries of Shoulder Anatomy in the Context of Throwing
There are many anatomic features of the shoulder that remain poorly understood. These include the alignment of the glenohumeral joint, the function of the glenohumeral ligaments, the function of the coracoacromial ligament, the depression of the human greater tuberosity, and the nature and function of the very tendinous subscapularis and long head of the biceps. These mysteries of the human shoulder can be solved if one considers the hypothesis that the shoulder has evolved to throw.
Glenohumeral Joint Alignment
The cartilage of the humeral head is thickest at its center, and thinnest at the periphery (Figure 3A).13,14 Conversely, the cartilage of the glenoid is thinnest at the fovea and thickest in the periphery (Figure 3B).14 It seems obvious that in order to maximally distribute high loads across this joint, the center of the humeral head should rest in the center of the glenoid. Interestingly, this does not occur during most positions of the shoulder. When upright, the center of the humeral head is directed above the glenoid in the coronal plane (Figure 3C). In order to align the glenohumeral joint optimally for the distribution of loads across the joint, the humerus must be abducted approximately 60° relative to the scapula. Assuming a 2:1 glenohumeral to scapulothoracic abduction for arm abduction relative to the thorax,15 this equates to approximately 90° of thoracohumeral abduction—the exact kinematic position of the shoulder during both critical moments of throwing (Figure 3D).
Function of the Glenohumeral Ligaments
The glenohumeral joint capsule has thickenings that help to stabilize the joint. The function of these glenohumeral ligaments has been evaluated biomechanically for their role in preventing translation and instability by a number of authors. The inferior glenohumeral ligament has classically been described as resisting anterior translation of the abducted arm.16 The coracohumeral ligament has been described as important to prevent inferior translation of the adducted arm.17
Interestingly, these ligaments are also the most important ligaments in resisting external rotation of the adducted arm.18 The dominant arm of throwing athletes has been shown to have increased inferior translation19 and increase external rotation.19-22 While the external rotation is partly related to bony adaptation,23,24 the ligamentous restraints to external rotation are likely under tremendous load, which may explain why Dr. Frank Jobe revolutionized the surgical treatment of the throwing athlete by performing an “instability” operation,25,26 as he believed these athletes had “subtle instability” that produced pain, but not symptoms of looseness.27
While these ligaments may exist in part to prevent translation and instability, current thinking suggests that “over-rotation” may lead to internal impingement and may be responsible for symptoms in the thrower’s shoulder,28 as SLAP lesions seem to occur easier with external rotation.29 Again, the importance of maximizing external rotation in throwing and the finding that this position is a critical moment with very high forces suggests that these ligaments may represent an adaptation to restrain external rotation while throwing.
Coracoacromial Ligament
The coracoacromial ligament is unique in that it connects 2 pieces of the same bone, and is only seen in hominids—not other primates.30 Its function has been debated for decades. This ligament is generally thought to limit superior translation of the humeral head,31,32 an effect that is critically important in patients with rotator cuff tears 33,34 Its importance is demonstrated by the fact that it seems to regenerate after it has been resected.35,36 Yet release or resection of this ligament has been a standard treatment for shoulder pain for decades.
Its function becomes clear if one examines the coracoacromial ligament with respect to the kinematics of throwing. As mentioned above, in the late cocking phase of throwing, tremendous shear forces exist in the shoulder. Fleisig and colleagues8 estimated a superior force of 250 ± 80 N, and an anterior shear force of 310 ± 100 N. While Fleisig and colleagues8 analyzed these shear forces with respect to the development of superior and anterior labral tears, it is important to note that these shear forces are vectors that should be combined. When one does this, it becomes apparent that in the late cocking phase of throwing there is shear force in an anterosuperior direction of approximately 400 N (Figure 1). The coracoacromial ligament is positioned to restrain this tremendous force. If throwing is an important adaptation in the evolution of humans, then the function of this ligament and its importance becomes clear.
Depressed Greater Tuberosity and the Pear-Shaped Glenoid
Compared to other primates, the greater tuberosity in humans sits significantly lower (Figure 4). This depression effectively decreases the moment arm of the muscle tendon unit, making the supraspinatus less powerful for raising the arm.37 In addition, by tenting the supraspinatus tendon over the humeral head, a watershed zone is created with decreased vascularity, which is thought to contribute to rotator cuff disease.38 What would be the advantage of the depressed tuberosity?
In primates, a lower tuberosity allows for more motion, particularly for arboreal travel.37 In order to throw with velocity, the humerus must achieve extremes of external rotation. A large tuberosity would limit external rotation of the abducted arm. Similarly, the pear-shaped glenoid cavity allows for the depressed tuberosity to achieve maximal external rotation. It is conceivable that a depressed greater tuberosity that allows for throwing would be an adaptation that could be favorable despite its proclivity toward rotator cuff disease in senescence.
Nature of the Subscapularis and the Role of the Long Head of the Biceps
The subscapularis is unique among rotator cuff muscles in that the upper two-thirds of the muscle is surprisingly tendinous.39 Why should this rotator cuff muscle have so much tendon material? Why is the tendon missing from the inferior one-third of the muscle? This situation is not optimal to prevent anterior glenohumeral instability, where inferior tendon material would be preferred.40
The function of the tendon of the long head of the biceps has long been debated and remains unclear.41-43 Cadaver experiments suggest the long head of the biceps provides glenohumeral joint stability in a variety of directions and positions, yet in vivo studies may not show this effect. Electromyography studies show little activity of the long head of the biceps with shoulder motion when the elbow is immobilized, leading some to suggest it is important as a passive restraint.43 This lack of understanding has led some to believe the biceps is not important and can be sacrificed without much concern.42,43
Again, these questions can be answered if one considers them in the context of throwing. At the point of maximal external rotation, the shoulder quickly moves from external rotation to internal rotation. This occurs by converting kinetic energy of external rotation into stored potential energy in the tissues. This energy is then converted into internal rotation. This elastic energy storage is critical for developing the necessary velocities to launch a projectile. While many structures are responsible for storing this energy,12 the subscapularis and long head of the biceps are particularly important. In fact, these 2 structures are important restraints to external rotation of the abducted arm–and become increasingly important with increased external rotation.45,46
One can think of the long head of the biceps as a spring (muscle), a cable (the long tendon), and a pulley (the bicipital groove). Similarly, one can consider the subscapularis as a similar structure, with the coracoid process serving as the pulley. In the late cocking phase of throwing, an interesting alignment occurs such that the pulleys (coracoid process and bicipital groove) are on opposite sides of the joint, providing glenohumeral joint stability. This system, with the inferior glenohumeral ligament (which is the primary restraint to external rotation of the abducted arm18), produces an incredibly stable envelope, preventing the humeral head from over-rotating and translating during the late cocking phase of throwing when the forces about the shoulder are extremely high. Because the muscles serve as springs, this system is also capable of storing kinetic energy during the late cocking phase of throwing and converting it into kinetic energy for internal rotation.
Summary
While throwing is not as critical to survival in today’s culture, the ability to throw was clearly an important adaptation in human evolution. With this in mind, we can approach human anatomy with this perspective, and in fact, many other lines of thinking suggest that throwing was important in the evolution of the hand,47 the brain,48 bipedalism,49 and even human society.50 The shoulder was highly influenced through natural selection to promote the throwing skill. With this perspective, many of the mysteries about the shoulder can be answered.
1. Darwin C. The Descent of Man, and Selection in Relation to Sex. 2nd ed. London, UK: John Murray; 1874:35.
2. Issac B. Throwing and human evolution. African Archeol Record. 1987;5:3-17.
3. Kirschann E. The human throw and a new model of hominid evolution (German). Homo. 1999;50(1):80-85.
4. Knusel CJ. The throwing hypothesis and hominid origins. Human Evolution. 1992;7(1):1-7.
5. Dunsworth H, Challis J, Walker A. The evolution of throwing: a new look at an old idea. Courier Forschungsinstitut Senckenberg. 2003;243:105-110.
6. Mayr E. Animal Species and Evolution. Cambridge, MA: Harvard University Press; 1963.
7. Tinbergen N. On the aims and methods of ethology. Zeitschrift für Tierpsychologie. 1963;20:410-433.
8. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
9. Atwater AE. Biomechanics of overarm throwing movements and of throwing injuries. Exerc Sport Sci Rev. 1975;7:43-85.
10. Matsuo T, Escamila RF, Fleisig GS, Barrentine SW, Andrews JR. Comparison of kinematic and temporal parameters between different pitch velocity groups. J Appl Biomech. 2001;17:1-13.
11. Wang YT, Ford HT III, Ford HT Jr, Shin DM. Three-dimensional kinematic analysis of baseball pitching in acceleration phase. Percept Mot Skills. 1995;80:43-48.
12. Roach NT, Venkadesan M, Rainbow MJ, Lieberman DE. Elastic energy storage in the shoulder and the evolution of high-speed throwing in Homo. Nature. 2013;498(7455):483-486.
13. Fox JA, Cole BJ, Romeo AA, et al. Articular cartilage thickness of the humeral head: an anatomic study. Orthopedics. 2008;31(3):216.
14. Zumstein V, Kraljevic M, Conzen A, Hoechel S, Müller-Gerbl M. Thickness distribution of the glenohumeral joint cartilage: a quantitative study using computed tomography. Surg Radiol Anat. 2014;36(4):327-331.
15. Inman VT, Saunders M, Abbott LC. Observations on the function of the shoulder joint. J Bone Joint Surg Am. 1944;26:1-30.
16. O’Brien SJ, Schwartz RS, Warren RF, Torzilli PA. Capsular restraints to anterior posterior motion of the abducted shoulder: A biomechanical study. J Shoulder Elbow Surg. 1995;4(4):298-308.
17. Warner JJ, Deng XH, Warren RF, Torzilli PA. Static capsuloligamentous restraints to superior-inferior translation of the glenohumeral joint. Am J Sports Med. 1992;20(6):675-685.
18. Kuhn JE, Bey MJ, Huston LJ, Blasier RB, Soslowsky LJ. Ligamentous restraints to external rotation of the humerus in the late-cocking phase of throwing. A cadaveric biomechanical investigation. Am J Sports Med. 2000;28(2):200-205.
19. Bigliani LU, Codd TP, Connor PM, Levine WN, Littlefield MA, Hershon SJ. Shoulder motion and laxity in the professional baseball player. Am J Sports Med. 1997;25(5):609-613.
20. Borsa PA, Dover GC, Wilk KE, Reinold MM. Glenohumeral range of motion and stiffness in professional baseball pitchers. Med Sci Sports Exerc. 2006;38(1):21-26.
21. Hurd WJ, Kaplan KM, Eiattrache NS, Jobe FW, Morrey BF, Kaufman KR. A profile of glenohumeral internal and external rotation motion in the uninjured high school baseball pitcher, part I: motion. J Athl Train. 2011;46(3):282-288.
22. Wilk KE, Macrina LC, Arrigo C. Passive range of motion characteristics in the overhead baseball pitcher and their implications for rehabilitation. Clin Orthop Relat Res. 2012;470(6):1586-1594.
23. Osbahr DC, Cannon DL, Speer KP. Retroversion of the humerus in the throwing shoulder of college baseball pitchers. Am J Sports Med. 2002;30(3):347-353.
24. Greenberg EM, Fernandez-Fernandez A, Lawrence JT, McClure P. The development of humeral retrotorsion and its relationship to throwing sports. Sports Health. 2015;7(6):489-496.
25. Jobe FW, Pink M. The athlete’s shoulder. J Hand Ther. 1994;7(2):107-110.
26. Montgomery WH 3rd, Jobe FW. Functional outcomes in athletes after modified anterior capsulolabral reconstruction. Am J Sports Med. 1994;22(3):352-358.
27. Jobe FW, Kvitne RS, Giangarra CE. Shoulder pain in the overhand or throwing athlete. The relationship of anterior instability and rotator cuff impingement. Orthop Rev. 1989;18(9):963-975.
28. Reinhold MM, Wilk KE, Dugas JR, Andrews JR. Chapter 11. Internal Impingement. In: Wilk K, Reinold MM, Andrews JR, eds. The Athlete’s Shoulder. 2nd ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2009:126.
29. Kuhn JE, Lindholm SR, Huston LJ, Soslowsky LJ, Blasier RB. Failure of the biceps superior labral complex: a cadaveric biomechanical investigation comparing the late cocking and early deceleration positions of throwing. Arthroscopy. 2003;19(4):373-379.
30. Ciochon RL, Corruccini RS. The coraco-acromial ligament and projection index in man and other anthropoid primates. J Anat. 1977;124(Pt 3):627-632.
31. Moorman CT, Warren RF, Deng XH, Wickiewicz TL, Torzilli PA. Role of coracoacromial ligament and related structures in glenohumeral stability: a cadaveric study. J Surg Orthop Adv. 2012;21(4):210-217.
32. Su WR, Budoff JE, Luo ZP. The effect of coracoacromial ligament excision and acromioplasty on superior and anterosuperior glenohumeral stability. Arthroscopy. 2009;25(1):13-18.
33. Wellmann M, Petersen W, Zantop T, Schanz S, Raschke MJ, Hurschler C. Effect of coracoacromial ligament resection on glenohumeral stability under active muscle loading in an in vitro model. Arthroscopy. 2008;24(11):1258-1264.
34. Fagelman M, Sartori M, Freedman KB, Patwardhan AG, Carandang G, Marra G. Biomechanics of coracoacromial arch modification. J Shoulder Elbow Surg. 2007;16(1):101-116.
35. Bak K, Spring IB, Henderson IP. Re-formation of the coracoacromial ligament after open resection or arthroscopic release. J Shoulder Elbow Surg. 2000;9:289-293.
36. Levy O, Copeland SA. Regeneration of the coracoacromial ligament after acromioplasty and arthroscopic subacromial decompression. J Shoulder Elbow Surg. 2001;10(4):317-320.
37. Larson SG, Stern JT Jr. Role of supraspinatus in the quadrupedal locomotion of vervets (Cercopithecus aethiops): Implications for interpretation of humeral morphology. Am J Phys Anthropol. 1989;79(3):369-377.
38. Chansky HA, Iannotti JP. The vascularity of the rotator cuff. Clin Sports Med. 1991;10(4):807-822.
39. Klapper RC, Jobe FW, Matsuura P. Subscapularis muscle and its glenohumeral ligament-like bands. A histomorphologic study. Am J Sports Med. 1992;20(3):307-310.
40. Halder A, Zobitz ME, Schultz E, An KN. Structural properties of the subscapularis tendon. J Orthop Res. 2000;18(5):
829-834.
41. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
42. Pill SG, Walch G, Hawkins RJ, Kissenberth MJ. The role of the biceps tendon in massive rotator cuff tears. Instr Course Lect. 2012;61:113-120.
43. Krupp RJ, Kevern MA, Gaines MD, Kotara S, Singleton SB. Long head of the biceps tendon pain: differential diagnosis and treatment. J Orthop Sports Phys Ther. 2009;39(2):55-70.
44. Levy AS, Kelly BT, Lintner SA, Osbahr DC, Speer KP. Function of the long head of the biceps at the shoulder: electromyographic analysis. J Shoulder Elbow Surg. 2001;10(3):250-255.
45. Kuhn JE, Huston LJ, Soslowsky LJ, Shyr Y, Blasier RB. External rotation of the glenohumeral joint: ligament restraints and muscle effects in the neutral and abducted positions.
J Shoulder Elbow Surg. 2005;14(1 Suppl S):39S-48S.
46. McGarry MH, Nguyen ML, Quigley RJ, Hanypsiak B, Gupta R, Lee TQ. The effect of long and short head biceps loading on glenohumeral joint rotational range of motion and humeral head postion. Knee Surge Sports Traumatol Arthrosc. 2014 Sep 26. [Epub ahead of print].
47. Young RW. Evolution of the human hand: The role of throwing and clubbing. J Anat. 2003;202:165-174.
48. Calvin WH. Did throwing stones shape hominid brain evolution? Ethology and Sociobiology. 1982;3:115-124.
49. Fifer FC. The adoption of bipedalism by the hominids: A new hypothesis. Human Evolution. 1987;2(2):135-147.
50. Darlington PJ. Group selection, altruism, reinforcement, and throwing in human evolution. Proc Nat Acad Sci. 1973;72(9):3748-3752.
Charles Darwin once said that apes “...are quite unable, as I have myself seen, to throw a stone with precision”.1 Yet humans can throw with precision and speed, a skill that likely had significant advantages: throwing can affect change at a distance—something few species can do. Throwing can provide protection against predators and can allow for predation for food resources. Throwing would be important in contesting other hominids for scarce resources. As such, throwing has been critically important in human evolution and likely is a skill that has been promoted through natural selection.2-5
In the orthopedic literature, most published work on throwing will ask proximate questions: “how, what, who, when, and where?” Evolutionary biologists are concerned with ultimate questions6,7: “why?” Asking ultimate questions provides insight into how a behavior might offer advantages under natural selection, which can then improve our understanding of the proximate questions for that behavior.
With regard to the shoulder, a number of mysteries exist that, to date, proximate studies have not been able to solve. This article argues that the human shoulder has evolved for throwing and by using this frame of reference, many of the mysteries surrounding the anatomy of the shoulder can be understood.
Pitching Kinematics
The mechanics of pitching have been analyzed extensively. Fleisig and colleagues8 performed kinematic and electromyographic analyses of pitchers to identify the critical moments of pitching (defined as where the forces are highest and injury is most likely going to occur). They found 2 moments where the forces about the shoulder are highest during pitching: the late cocking phase (defined by the point where the humerus reaches maximal external rotation); and the early deceleration phase (defined by the point when the ball is released). If throwing is important in natural selection of humans, then the shoulder anatomy should be optimized to withstand the forces generated in these positions.
Late Cocking Phase of Throwing
The early phases of throwing are attempting to maximize external rotation of the abducted arm as the velocity of the pitched ball correlates to the amount of external rotation achieved.9-11 In this position, kinetic energy in external rotation is stored and converted into kinetic energy in internal rotation.12 The position of the shoulder during late cocking is 94 ± 21° of thoracohumeral abduction, 11 ± 11° of horizontal adduction, and a remarkable 165 ± 11° of thoracohumeral external rotation (Figure 1).8
Fleisig and colleagues8 estimated the torque and forces about the shoulder, which are quite high for joint compression (480 ± 130 N). They also analyzed the shear forces and while trying to describe the origin of superior labrum anterior to posterior (SLAP) lesions and anterior labral tears, broke down the major shear vector into an anterior force vector (310 ± 100 N) and a superior force (250 ± 80 N).8 Note that the resulting shear vector is in an anterosuperior direction and is approximately 400 N.
Early Deceleration Phase of Throwing
Interestingly, the position of the humerus during this critical moment of throwing is not much different than the position during the late cocking phase of throwing, with 93 ± 10° of thoracohumeral abduction, 6 ± 8° of horizontal adduction.8 The major difference in the position of the arm is found in the amount of thoracohumeral rotation, which is now 64 ± 35° of external rotation (Figure 2).8
The forces in early deceleration are tremendous, with an estimated 1090 ± 110 N joint compression force, and an anteroinferior shear force of approximately 130 N.8
Clearly, if throwing is an important skill in human evolution, adaptations must exist in the shoulder to withstand the high forces in these 2 critical moments of throwing.
Solving Mysteries of Shoulder Anatomy in the Context of Throwing
There are many anatomic features of the shoulder that remain poorly understood. These include the alignment of the glenohumeral joint, the function of the glenohumeral ligaments, the function of the coracoacromial ligament, the depression of the human greater tuberosity, and the nature and function of the very tendinous subscapularis and long head of the biceps. These mysteries of the human shoulder can be solved if one considers the hypothesis that the shoulder has evolved to throw.
Glenohumeral Joint Alignment
The cartilage of the humeral head is thickest at its center, and thinnest at the periphery (Figure 3A).13,14 Conversely, the cartilage of the glenoid is thinnest at the fovea and thickest in the periphery (Figure 3B).14 It seems obvious that in order to maximally distribute high loads across this joint, the center of the humeral head should rest in the center of the glenoid. Interestingly, this does not occur during most positions of the shoulder. When upright, the center of the humeral head is directed above the glenoid in the coronal plane (Figure 3C). In order to align the glenohumeral joint optimally for the distribution of loads across the joint, the humerus must be abducted approximately 60° relative to the scapula. Assuming a 2:1 glenohumeral to scapulothoracic abduction for arm abduction relative to the thorax,15 this equates to approximately 90° of thoracohumeral abduction—the exact kinematic position of the shoulder during both critical moments of throwing (Figure 3D).
Function of the Glenohumeral Ligaments
The glenohumeral joint capsule has thickenings that help to stabilize the joint. The function of these glenohumeral ligaments has been evaluated biomechanically for their role in preventing translation and instability by a number of authors. The inferior glenohumeral ligament has classically been described as resisting anterior translation of the abducted arm.16 The coracohumeral ligament has been described as important to prevent inferior translation of the adducted arm.17
Interestingly, these ligaments are also the most important ligaments in resisting external rotation of the adducted arm.18 The dominant arm of throwing athletes has been shown to have increased inferior translation19 and increase external rotation.19-22 While the external rotation is partly related to bony adaptation,23,24 the ligamentous restraints to external rotation are likely under tremendous load, which may explain why Dr. Frank Jobe revolutionized the surgical treatment of the throwing athlete by performing an “instability” operation,25,26 as he believed these athletes had “subtle instability” that produced pain, but not symptoms of looseness.27
While these ligaments may exist in part to prevent translation and instability, current thinking suggests that “over-rotation” may lead to internal impingement and may be responsible for symptoms in the thrower’s shoulder,28 as SLAP lesions seem to occur easier with external rotation.29 Again, the importance of maximizing external rotation in throwing and the finding that this position is a critical moment with very high forces suggests that these ligaments may represent an adaptation to restrain external rotation while throwing.
Coracoacromial Ligament
The coracoacromial ligament is unique in that it connects 2 pieces of the same bone, and is only seen in hominids—not other primates.30 Its function has been debated for decades. This ligament is generally thought to limit superior translation of the humeral head,31,32 an effect that is critically important in patients with rotator cuff tears 33,34 Its importance is demonstrated by the fact that it seems to regenerate after it has been resected.35,36 Yet release or resection of this ligament has been a standard treatment for shoulder pain for decades.
Its function becomes clear if one examines the coracoacromial ligament with respect to the kinematics of throwing. As mentioned above, in the late cocking phase of throwing, tremendous shear forces exist in the shoulder. Fleisig and colleagues8 estimated a superior force of 250 ± 80 N, and an anterior shear force of 310 ± 100 N. While Fleisig and colleagues8 analyzed these shear forces with respect to the development of superior and anterior labral tears, it is important to note that these shear forces are vectors that should be combined. When one does this, it becomes apparent that in the late cocking phase of throwing there is shear force in an anterosuperior direction of approximately 400 N (Figure 1). The coracoacromial ligament is positioned to restrain this tremendous force. If throwing is an important adaptation in the evolution of humans, then the function of this ligament and its importance becomes clear.
Depressed Greater Tuberosity and the Pear-Shaped Glenoid
Compared to other primates, the greater tuberosity in humans sits significantly lower (Figure 4). This depression effectively decreases the moment arm of the muscle tendon unit, making the supraspinatus less powerful for raising the arm.37 In addition, by tenting the supraspinatus tendon over the humeral head, a watershed zone is created with decreased vascularity, which is thought to contribute to rotator cuff disease.38 What would be the advantage of the depressed tuberosity?
In primates, a lower tuberosity allows for more motion, particularly for arboreal travel.37 In order to throw with velocity, the humerus must achieve extremes of external rotation. A large tuberosity would limit external rotation of the abducted arm. Similarly, the pear-shaped glenoid cavity allows for the depressed tuberosity to achieve maximal external rotation. It is conceivable that a depressed greater tuberosity that allows for throwing would be an adaptation that could be favorable despite its proclivity toward rotator cuff disease in senescence.
Nature of the Subscapularis and the Role of the Long Head of the Biceps
The subscapularis is unique among rotator cuff muscles in that the upper two-thirds of the muscle is surprisingly tendinous.39 Why should this rotator cuff muscle have so much tendon material? Why is the tendon missing from the inferior one-third of the muscle? This situation is not optimal to prevent anterior glenohumeral instability, where inferior tendon material would be preferred.40
The function of the tendon of the long head of the biceps has long been debated and remains unclear.41-43 Cadaver experiments suggest the long head of the biceps provides glenohumeral joint stability in a variety of directions and positions, yet in vivo studies may not show this effect. Electromyography studies show little activity of the long head of the biceps with shoulder motion when the elbow is immobilized, leading some to suggest it is important as a passive restraint.43 This lack of understanding has led some to believe the biceps is not important and can be sacrificed without much concern.42,43
Again, these questions can be answered if one considers them in the context of throwing. At the point of maximal external rotation, the shoulder quickly moves from external rotation to internal rotation. This occurs by converting kinetic energy of external rotation into stored potential energy in the tissues. This energy is then converted into internal rotation. This elastic energy storage is critical for developing the necessary velocities to launch a projectile. While many structures are responsible for storing this energy,12 the subscapularis and long head of the biceps are particularly important. In fact, these 2 structures are important restraints to external rotation of the abducted arm–and become increasingly important with increased external rotation.45,46
One can think of the long head of the biceps as a spring (muscle), a cable (the long tendon), and a pulley (the bicipital groove). Similarly, one can consider the subscapularis as a similar structure, with the coracoid process serving as the pulley. In the late cocking phase of throwing, an interesting alignment occurs such that the pulleys (coracoid process and bicipital groove) are on opposite sides of the joint, providing glenohumeral joint stability. This system, with the inferior glenohumeral ligament (which is the primary restraint to external rotation of the abducted arm18), produces an incredibly stable envelope, preventing the humeral head from over-rotating and translating during the late cocking phase of throwing when the forces about the shoulder are extremely high. Because the muscles serve as springs, this system is also capable of storing kinetic energy during the late cocking phase of throwing and converting it into kinetic energy for internal rotation.
Summary
While throwing is not as critical to survival in today’s culture, the ability to throw was clearly an important adaptation in human evolution. With this in mind, we can approach human anatomy with this perspective, and in fact, many other lines of thinking suggest that throwing was important in the evolution of the hand,47 the brain,48 bipedalism,49 and even human society.50 The shoulder was highly influenced through natural selection to promote the throwing skill. With this perspective, many of the mysteries about the shoulder can be answered.
Charles Darwin once said that apes “...are quite unable, as I have myself seen, to throw a stone with precision”.1 Yet humans can throw with precision and speed, a skill that likely had significant advantages: throwing can affect change at a distance—something few species can do. Throwing can provide protection against predators and can allow for predation for food resources. Throwing would be important in contesting other hominids for scarce resources. As such, throwing has been critically important in human evolution and likely is a skill that has been promoted through natural selection.2-5
In the orthopedic literature, most published work on throwing will ask proximate questions: “how, what, who, when, and where?” Evolutionary biologists are concerned with ultimate questions6,7: “why?” Asking ultimate questions provides insight into how a behavior might offer advantages under natural selection, which can then improve our understanding of the proximate questions for that behavior.
With regard to the shoulder, a number of mysteries exist that, to date, proximate studies have not been able to solve. This article argues that the human shoulder has evolved for throwing and by using this frame of reference, many of the mysteries surrounding the anatomy of the shoulder can be understood.
Pitching Kinematics
The mechanics of pitching have been analyzed extensively. Fleisig and colleagues8 performed kinematic and electromyographic analyses of pitchers to identify the critical moments of pitching (defined as where the forces are highest and injury is most likely going to occur). They found 2 moments where the forces about the shoulder are highest during pitching: the late cocking phase (defined by the point where the humerus reaches maximal external rotation); and the early deceleration phase (defined by the point when the ball is released). If throwing is important in natural selection of humans, then the shoulder anatomy should be optimized to withstand the forces generated in these positions.
Late Cocking Phase of Throwing
The early phases of throwing are attempting to maximize external rotation of the abducted arm as the velocity of the pitched ball correlates to the amount of external rotation achieved.9-11 In this position, kinetic energy in external rotation is stored and converted into kinetic energy in internal rotation.12 The position of the shoulder during late cocking is 94 ± 21° of thoracohumeral abduction, 11 ± 11° of horizontal adduction, and a remarkable 165 ± 11° of thoracohumeral external rotation (Figure 1).8
Fleisig and colleagues8 estimated the torque and forces about the shoulder, which are quite high for joint compression (480 ± 130 N). They also analyzed the shear forces and while trying to describe the origin of superior labrum anterior to posterior (SLAP) lesions and anterior labral tears, broke down the major shear vector into an anterior force vector (310 ± 100 N) and a superior force (250 ± 80 N).8 Note that the resulting shear vector is in an anterosuperior direction and is approximately 400 N.
Early Deceleration Phase of Throwing
Interestingly, the position of the humerus during this critical moment of throwing is not much different than the position during the late cocking phase of throwing, with 93 ± 10° of thoracohumeral abduction, 6 ± 8° of horizontal adduction.8 The major difference in the position of the arm is found in the amount of thoracohumeral rotation, which is now 64 ± 35° of external rotation (Figure 2).8
The forces in early deceleration are tremendous, with an estimated 1090 ± 110 N joint compression force, and an anteroinferior shear force of approximately 130 N.8
Clearly, if throwing is an important skill in human evolution, adaptations must exist in the shoulder to withstand the high forces in these 2 critical moments of throwing.
Solving Mysteries of Shoulder Anatomy in the Context of Throwing
There are many anatomic features of the shoulder that remain poorly understood. These include the alignment of the glenohumeral joint, the function of the glenohumeral ligaments, the function of the coracoacromial ligament, the depression of the human greater tuberosity, and the nature and function of the very tendinous subscapularis and long head of the biceps. These mysteries of the human shoulder can be solved if one considers the hypothesis that the shoulder has evolved to throw.
Glenohumeral Joint Alignment
The cartilage of the humeral head is thickest at its center, and thinnest at the periphery (Figure 3A).13,14 Conversely, the cartilage of the glenoid is thinnest at the fovea and thickest in the periphery (Figure 3B).14 It seems obvious that in order to maximally distribute high loads across this joint, the center of the humeral head should rest in the center of the glenoid. Interestingly, this does not occur during most positions of the shoulder. When upright, the center of the humeral head is directed above the glenoid in the coronal plane (Figure 3C). In order to align the glenohumeral joint optimally for the distribution of loads across the joint, the humerus must be abducted approximately 60° relative to the scapula. Assuming a 2:1 glenohumeral to scapulothoracic abduction for arm abduction relative to the thorax,15 this equates to approximately 90° of thoracohumeral abduction—the exact kinematic position of the shoulder during both critical moments of throwing (Figure 3D).
Function of the Glenohumeral Ligaments
The glenohumeral joint capsule has thickenings that help to stabilize the joint. The function of these glenohumeral ligaments has been evaluated biomechanically for their role in preventing translation and instability by a number of authors. The inferior glenohumeral ligament has classically been described as resisting anterior translation of the abducted arm.16 The coracohumeral ligament has been described as important to prevent inferior translation of the adducted arm.17
Interestingly, these ligaments are also the most important ligaments in resisting external rotation of the adducted arm.18 The dominant arm of throwing athletes has been shown to have increased inferior translation19 and increase external rotation.19-22 While the external rotation is partly related to bony adaptation,23,24 the ligamentous restraints to external rotation are likely under tremendous load, which may explain why Dr. Frank Jobe revolutionized the surgical treatment of the throwing athlete by performing an “instability” operation,25,26 as he believed these athletes had “subtle instability” that produced pain, but not symptoms of looseness.27
While these ligaments may exist in part to prevent translation and instability, current thinking suggests that “over-rotation” may lead to internal impingement and may be responsible for symptoms in the thrower’s shoulder,28 as SLAP lesions seem to occur easier with external rotation.29 Again, the importance of maximizing external rotation in throwing and the finding that this position is a critical moment with very high forces suggests that these ligaments may represent an adaptation to restrain external rotation while throwing.
Coracoacromial Ligament
The coracoacromial ligament is unique in that it connects 2 pieces of the same bone, and is only seen in hominids—not other primates.30 Its function has been debated for decades. This ligament is generally thought to limit superior translation of the humeral head,31,32 an effect that is critically important in patients with rotator cuff tears 33,34 Its importance is demonstrated by the fact that it seems to regenerate after it has been resected.35,36 Yet release or resection of this ligament has been a standard treatment for shoulder pain for decades.
Its function becomes clear if one examines the coracoacromial ligament with respect to the kinematics of throwing. As mentioned above, in the late cocking phase of throwing, tremendous shear forces exist in the shoulder. Fleisig and colleagues8 estimated a superior force of 250 ± 80 N, and an anterior shear force of 310 ± 100 N. While Fleisig and colleagues8 analyzed these shear forces with respect to the development of superior and anterior labral tears, it is important to note that these shear forces are vectors that should be combined. When one does this, it becomes apparent that in the late cocking phase of throwing there is shear force in an anterosuperior direction of approximately 400 N (Figure 1). The coracoacromial ligament is positioned to restrain this tremendous force. If throwing is an important adaptation in the evolution of humans, then the function of this ligament and its importance becomes clear.
Depressed Greater Tuberosity and the Pear-Shaped Glenoid
Compared to other primates, the greater tuberosity in humans sits significantly lower (Figure 4). This depression effectively decreases the moment arm of the muscle tendon unit, making the supraspinatus less powerful for raising the arm.37 In addition, by tenting the supraspinatus tendon over the humeral head, a watershed zone is created with decreased vascularity, which is thought to contribute to rotator cuff disease.38 What would be the advantage of the depressed tuberosity?
In primates, a lower tuberosity allows for more motion, particularly for arboreal travel.37 In order to throw with velocity, the humerus must achieve extremes of external rotation. A large tuberosity would limit external rotation of the abducted arm. Similarly, the pear-shaped glenoid cavity allows for the depressed tuberosity to achieve maximal external rotation. It is conceivable that a depressed greater tuberosity that allows for throwing would be an adaptation that could be favorable despite its proclivity toward rotator cuff disease in senescence.
Nature of the Subscapularis and the Role of the Long Head of the Biceps
The subscapularis is unique among rotator cuff muscles in that the upper two-thirds of the muscle is surprisingly tendinous.39 Why should this rotator cuff muscle have so much tendon material? Why is the tendon missing from the inferior one-third of the muscle? This situation is not optimal to prevent anterior glenohumeral instability, where inferior tendon material would be preferred.40
The function of the tendon of the long head of the biceps has long been debated and remains unclear.41-43 Cadaver experiments suggest the long head of the biceps provides glenohumeral joint stability in a variety of directions and positions, yet in vivo studies may not show this effect. Electromyography studies show little activity of the long head of the biceps with shoulder motion when the elbow is immobilized, leading some to suggest it is important as a passive restraint.43 This lack of understanding has led some to believe the biceps is not important and can be sacrificed without much concern.42,43
Again, these questions can be answered if one considers them in the context of throwing. At the point of maximal external rotation, the shoulder quickly moves from external rotation to internal rotation. This occurs by converting kinetic energy of external rotation into stored potential energy in the tissues. This energy is then converted into internal rotation. This elastic energy storage is critical for developing the necessary velocities to launch a projectile. While many structures are responsible for storing this energy,12 the subscapularis and long head of the biceps are particularly important. In fact, these 2 structures are important restraints to external rotation of the abducted arm–and become increasingly important with increased external rotation.45,46
One can think of the long head of the biceps as a spring (muscle), a cable (the long tendon), and a pulley (the bicipital groove). Similarly, one can consider the subscapularis as a similar structure, with the coracoid process serving as the pulley. In the late cocking phase of throwing, an interesting alignment occurs such that the pulleys (coracoid process and bicipital groove) are on opposite sides of the joint, providing glenohumeral joint stability. This system, with the inferior glenohumeral ligament (which is the primary restraint to external rotation of the abducted arm18), produces an incredibly stable envelope, preventing the humeral head from over-rotating and translating during the late cocking phase of throwing when the forces about the shoulder are extremely high. Because the muscles serve as springs, this system is also capable of storing kinetic energy during the late cocking phase of throwing and converting it into kinetic energy for internal rotation.
Summary
While throwing is not as critical to survival in today’s culture, the ability to throw was clearly an important adaptation in human evolution. With this in mind, we can approach human anatomy with this perspective, and in fact, many other lines of thinking suggest that throwing was important in the evolution of the hand,47 the brain,48 bipedalism,49 and even human society.50 The shoulder was highly influenced through natural selection to promote the throwing skill. With this perspective, many of the mysteries about the shoulder can be answered.
1. Darwin C. The Descent of Man, and Selection in Relation to Sex. 2nd ed. London, UK: John Murray; 1874:35.
2. Issac B. Throwing and human evolution. African Archeol Record. 1987;5:3-17.
3. Kirschann E. The human throw and a new model of hominid evolution (German). Homo. 1999;50(1):80-85.
4. Knusel CJ. The throwing hypothesis and hominid origins. Human Evolution. 1992;7(1):1-7.
5. Dunsworth H, Challis J, Walker A. The evolution of throwing: a new look at an old idea. Courier Forschungsinstitut Senckenberg. 2003;243:105-110.
6. Mayr E. Animal Species and Evolution. Cambridge, MA: Harvard University Press; 1963.
7. Tinbergen N. On the aims and methods of ethology. Zeitschrift für Tierpsychologie. 1963;20:410-433.
8. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
9. Atwater AE. Biomechanics of overarm throwing movements and of throwing injuries. Exerc Sport Sci Rev. 1975;7:43-85.
10. Matsuo T, Escamila RF, Fleisig GS, Barrentine SW, Andrews JR. Comparison of kinematic and temporal parameters between different pitch velocity groups. J Appl Biomech. 2001;17:1-13.
11. Wang YT, Ford HT III, Ford HT Jr, Shin DM. Three-dimensional kinematic analysis of baseball pitching in acceleration phase. Percept Mot Skills. 1995;80:43-48.
12. Roach NT, Venkadesan M, Rainbow MJ, Lieberman DE. Elastic energy storage in the shoulder and the evolution of high-speed throwing in Homo. Nature. 2013;498(7455):483-486.
13. Fox JA, Cole BJ, Romeo AA, et al. Articular cartilage thickness of the humeral head: an anatomic study. Orthopedics. 2008;31(3):216.
14. Zumstein V, Kraljevic M, Conzen A, Hoechel S, Müller-Gerbl M. Thickness distribution of the glenohumeral joint cartilage: a quantitative study using computed tomography. Surg Radiol Anat. 2014;36(4):327-331.
15. Inman VT, Saunders M, Abbott LC. Observations on the function of the shoulder joint. J Bone Joint Surg Am. 1944;26:1-30.
16. O’Brien SJ, Schwartz RS, Warren RF, Torzilli PA. Capsular restraints to anterior posterior motion of the abducted shoulder: A biomechanical study. J Shoulder Elbow Surg. 1995;4(4):298-308.
17. Warner JJ, Deng XH, Warren RF, Torzilli PA. Static capsuloligamentous restraints to superior-inferior translation of the glenohumeral joint. Am J Sports Med. 1992;20(6):675-685.
18. Kuhn JE, Bey MJ, Huston LJ, Blasier RB, Soslowsky LJ. Ligamentous restraints to external rotation of the humerus in the late-cocking phase of throwing. A cadaveric biomechanical investigation. Am J Sports Med. 2000;28(2):200-205.
19. Bigliani LU, Codd TP, Connor PM, Levine WN, Littlefield MA, Hershon SJ. Shoulder motion and laxity in the professional baseball player. Am J Sports Med. 1997;25(5):609-613.
20. Borsa PA, Dover GC, Wilk KE, Reinold MM. Glenohumeral range of motion and stiffness in professional baseball pitchers. Med Sci Sports Exerc. 2006;38(1):21-26.
21. Hurd WJ, Kaplan KM, Eiattrache NS, Jobe FW, Morrey BF, Kaufman KR. A profile of glenohumeral internal and external rotation motion in the uninjured high school baseball pitcher, part I: motion. J Athl Train. 2011;46(3):282-288.
22. Wilk KE, Macrina LC, Arrigo C. Passive range of motion characteristics in the overhead baseball pitcher and their implications for rehabilitation. Clin Orthop Relat Res. 2012;470(6):1586-1594.
23. Osbahr DC, Cannon DL, Speer KP. Retroversion of the humerus in the throwing shoulder of college baseball pitchers. Am J Sports Med. 2002;30(3):347-353.
24. Greenberg EM, Fernandez-Fernandez A, Lawrence JT, McClure P. The development of humeral retrotorsion and its relationship to throwing sports. Sports Health. 2015;7(6):489-496.
25. Jobe FW, Pink M. The athlete’s shoulder. J Hand Ther. 1994;7(2):107-110.
26. Montgomery WH 3rd, Jobe FW. Functional outcomes in athletes after modified anterior capsulolabral reconstruction. Am J Sports Med. 1994;22(3):352-358.
27. Jobe FW, Kvitne RS, Giangarra CE. Shoulder pain in the overhand or throwing athlete. The relationship of anterior instability and rotator cuff impingement. Orthop Rev. 1989;18(9):963-975.
28. Reinhold MM, Wilk KE, Dugas JR, Andrews JR. Chapter 11. Internal Impingement. In: Wilk K, Reinold MM, Andrews JR, eds. The Athlete’s Shoulder. 2nd ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2009:126.
29. Kuhn JE, Lindholm SR, Huston LJ, Soslowsky LJ, Blasier RB. Failure of the biceps superior labral complex: a cadaveric biomechanical investigation comparing the late cocking and early deceleration positions of throwing. Arthroscopy. 2003;19(4):373-379.
30. Ciochon RL, Corruccini RS. The coraco-acromial ligament and projection index in man and other anthropoid primates. J Anat. 1977;124(Pt 3):627-632.
31. Moorman CT, Warren RF, Deng XH, Wickiewicz TL, Torzilli PA. Role of coracoacromial ligament and related structures in glenohumeral stability: a cadaveric study. J Surg Orthop Adv. 2012;21(4):210-217.
32. Su WR, Budoff JE, Luo ZP. The effect of coracoacromial ligament excision and acromioplasty on superior and anterosuperior glenohumeral stability. Arthroscopy. 2009;25(1):13-18.
33. Wellmann M, Petersen W, Zantop T, Schanz S, Raschke MJ, Hurschler C. Effect of coracoacromial ligament resection on glenohumeral stability under active muscle loading in an in vitro model. Arthroscopy. 2008;24(11):1258-1264.
34. Fagelman M, Sartori M, Freedman KB, Patwardhan AG, Carandang G, Marra G. Biomechanics of coracoacromial arch modification. J Shoulder Elbow Surg. 2007;16(1):101-116.
35. Bak K, Spring IB, Henderson IP. Re-formation of the coracoacromial ligament after open resection or arthroscopic release. J Shoulder Elbow Surg. 2000;9:289-293.
36. Levy O, Copeland SA. Regeneration of the coracoacromial ligament after acromioplasty and arthroscopic subacromial decompression. J Shoulder Elbow Surg. 2001;10(4):317-320.
37. Larson SG, Stern JT Jr. Role of supraspinatus in the quadrupedal locomotion of vervets (Cercopithecus aethiops): Implications for interpretation of humeral morphology. Am J Phys Anthropol. 1989;79(3):369-377.
38. Chansky HA, Iannotti JP. The vascularity of the rotator cuff. Clin Sports Med. 1991;10(4):807-822.
39. Klapper RC, Jobe FW, Matsuura P. Subscapularis muscle and its glenohumeral ligament-like bands. A histomorphologic study. Am J Sports Med. 1992;20(3):307-310.
40. Halder A, Zobitz ME, Schultz E, An KN. Structural properties of the subscapularis tendon. J Orthop Res. 2000;18(5):
829-834.
41. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
42. Pill SG, Walch G, Hawkins RJ, Kissenberth MJ. The role of the biceps tendon in massive rotator cuff tears. Instr Course Lect. 2012;61:113-120.
43. Krupp RJ, Kevern MA, Gaines MD, Kotara S, Singleton SB. Long head of the biceps tendon pain: differential diagnosis and treatment. J Orthop Sports Phys Ther. 2009;39(2):55-70.
44. Levy AS, Kelly BT, Lintner SA, Osbahr DC, Speer KP. Function of the long head of the biceps at the shoulder: electromyographic analysis. J Shoulder Elbow Surg. 2001;10(3):250-255.
45. Kuhn JE, Huston LJ, Soslowsky LJ, Shyr Y, Blasier RB. External rotation of the glenohumeral joint: ligament restraints and muscle effects in the neutral and abducted positions.
J Shoulder Elbow Surg. 2005;14(1 Suppl S):39S-48S.
46. McGarry MH, Nguyen ML, Quigley RJ, Hanypsiak B, Gupta R, Lee TQ. The effect of long and short head biceps loading on glenohumeral joint rotational range of motion and humeral head postion. Knee Surge Sports Traumatol Arthrosc. 2014 Sep 26. [Epub ahead of print].
47. Young RW. Evolution of the human hand: The role of throwing and clubbing. J Anat. 2003;202:165-174.
48. Calvin WH. Did throwing stones shape hominid brain evolution? Ethology and Sociobiology. 1982;3:115-124.
49. Fifer FC. The adoption of bipedalism by the hominids: A new hypothesis. Human Evolution. 1987;2(2):135-147.
50. Darlington PJ. Group selection, altruism, reinforcement, and throwing in human evolution. Proc Nat Acad Sci. 1973;72(9):3748-3752.
1. Darwin C. The Descent of Man, and Selection in Relation to Sex. 2nd ed. London, UK: John Murray; 1874:35.
2. Issac B. Throwing and human evolution. African Archeol Record. 1987;5:3-17.
3. Kirschann E. The human throw and a new model of hominid evolution (German). Homo. 1999;50(1):80-85.
4. Knusel CJ. The throwing hypothesis and hominid origins. Human Evolution. 1992;7(1):1-7.
5. Dunsworth H, Challis J, Walker A. The evolution of throwing: a new look at an old idea. Courier Forschungsinstitut Senckenberg. 2003;243:105-110.
6. Mayr E. Animal Species and Evolution. Cambridge, MA: Harvard University Press; 1963.
7. Tinbergen N. On the aims and methods of ethology. Zeitschrift für Tierpsychologie. 1963;20:410-433.
8. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
9. Atwater AE. Biomechanics of overarm throwing movements and of throwing injuries. Exerc Sport Sci Rev. 1975;7:43-85.
10. Matsuo T, Escamila RF, Fleisig GS, Barrentine SW, Andrews JR. Comparison of kinematic and temporal parameters between different pitch velocity groups. J Appl Biomech. 2001;17:1-13.
11. Wang YT, Ford HT III, Ford HT Jr, Shin DM. Three-dimensional kinematic analysis of baseball pitching in acceleration phase. Percept Mot Skills. 1995;80:43-48.
12. Roach NT, Venkadesan M, Rainbow MJ, Lieberman DE. Elastic energy storage in the shoulder and the evolution of high-speed throwing in Homo. Nature. 2013;498(7455):483-486.
13. Fox JA, Cole BJ, Romeo AA, et al. Articular cartilage thickness of the humeral head: an anatomic study. Orthopedics. 2008;31(3):216.
14. Zumstein V, Kraljevic M, Conzen A, Hoechel S, Müller-Gerbl M. Thickness distribution of the glenohumeral joint cartilage: a quantitative study using computed tomography. Surg Radiol Anat. 2014;36(4):327-331.
15. Inman VT, Saunders M, Abbott LC. Observations on the function of the shoulder joint. J Bone Joint Surg Am. 1944;26:1-30.
16. O’Brien SJ, Schwartz RS, Warren RF, Torzilli PA. Capsular restraints to anterior posterior motion of the abducted shoulder: A biomechanical study. J Shoulder Elbow Surg. 1995;4(4):298-308.
17. Warner JJ, Deng XH, Warren RF, Torzilli PA. Static capsuloligamentous restraints to superior-inferior translation of the glenohumeral joint. Am J Sports Med. 1992;20(6):675-685.
18. Kuhn JE, Bey MJ, Huston LJ, Blasier RB, Soslowsky LJ. Ligamentous restraints to external rotation of the humerus in the late-cocking phase of throwing. A cadaveric biomechanical investigation. Am J Sports Med. 2000;28(2):200-205.
19. Bigliani LU, Codd TP, Connor PM, Levine WN, Littlefield MA, Hershon SJ. Shoulder motion and laxity in the professional baseball player. Am J Sports Med. 1997;25(5):609-613.
20. Borsa PA, Dover GC, Wilk KE, Reinold MM. Glenohumeral range of motion and stiffness in professional baseball pitchers. Med Sci Sports Exerc. 2006;38(1):21-26.
21. Hurd WJ, Kaplan KM, Eiattrache NS, Jobe FW, Morrey BF, Kaufman KR. A profile of glenohumeral internal and external rotation motion in the uninjured high school baseball pitcher, part I: motion. J Athl Train. 2011;46(3):282-288.
22. Wilk KE, Macrina LC, Arrigo C. Passive range of motion characteristics in the overhead baseball pitcher and their implications for rehabilitation. Clin Orthop Relat Res. 2012;470(6):1586-1594.
23. Osbahr DC, Cannon DL, Speer KP. Retroversion of the humerus in the throwing shoulder of college baseball pitchers. Am J Sports Med. 2002;30(3):347-353.
24. Greenberg EM, Fernandez-Fernandez A, Lawrence JT, McClure P. The development of humeral retrotorsion and its relationship to throwing sports. Sports Health. 2015;7(6):489-496.
25. Jobe FW, Pink M. The athlete’s shoulder. J Hand Ther. 1994;7(2):107-110.
26. Montgomery WH 3rd, Jobe FW. Functional outcomes in athletes after modified anterior capsulolabral reconstruction. Am J Sports Med. 1994;22(3):352-358.
27. Jobe FW, Kvitne RS, Giangarra CE. Shoulder pain in the overhand or throwing athlete. The relationship of anterior instability and rotator cuff impingement. Orthop Rev. 1989;18(9):963-975.
28. Reinhold MM, Wilk KE, Dugas JR, Andrews JR. Chapter 11. Internal Impingement. In: Wilk K, Reinold MM, Andrews JR, eds. The Athlete’s Shoulder. 2nd ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2009:126.
29. Kuhn JE, Lindholm SR, Huston LJ, Soslowsky LJ, Blasier RB. Failure of the biceps superior labral complex: a cadaveric biomechanical investigation comparing the late cocking and early deceleration positions of throwing. Arthroscopy. 2003;19(4):373-379.
30. Ciochon RL, Corruccini RS. The coraco-acromial ligament and projection index in man and other anthropoid primates. J Anat. 1977;124(Pt 3):627-632.
31. Moorman CT, Warren RF, Deng XH, Wickiewicz TL, Torzilli PA. Role of coracoacromial ligament and related structures in glenohumeral stability: a cadaveric study. J Surg Orthop Adv. 2012;21(4):210-217.
32. Su WR, Budoff JE, Luo ZP. The effect of coracoacromial ligament excision and acromioplasty on superior and anterosuperior glenohumeral stability. Arthroscopy. 2009;25(1):13-18.
33. Wellmann M, Petersen W, Zantop T, Schanz S, Raschke MJ, Hurschler C. Effect of coracoacromial ligament resection on glenohumeral stability under active muscle loading in an in vitro model. Arthroscopy. 2008;24(11):1258-1264.
34. Fagelman M, Sartori M, Freedman KB, Patwardhan AG, Carandang G, Marra G. Biomechanics of coracoacromial arch modification. J Shoulder Elbow Surg. 2007;16(1):101-116.
35. Bak K, Spring IB, Henderson IP. Re-formation of the coracoacromial ligament after open resection or arthroscopic release. J Shoulder Elbow Surg. 2000;9:289-293.
36. Levy O, Copeland SA. Regeneration of the coracoacromial ligament after acromioplasty and arthroscopic subacromial decompression. J Shoulder Elbow Surg. 2001;10(4):317-320.
37. Larson SG, Stern JT Jr. Role of supraspinatus in the quadrupedal locomotion of vervets (Cercopithecus aethiops): Implications for interpretation of humeral morphology. Am J Phys Anthropol. 1989;79(3):369-377.
38. Chansky HA, Iannotti JP. The vascularity of the rotator cuff. Clin Sports Med. 1991;10(4):807-822.
39. Klapper RC, Jobe FW, Matsuura P. Subscapularis muscle and its glenohumeral ligament-like bands. A histomorphologic study. Am J Sports Med. 1992;20(3):307-310.
40. Halder A, Zobitz ME, Schultz E, An KN. Structural properties of the subscapularis tendon. J Orthop Res. 2000;18(5):
829-834.
41. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
42. Pill SG, Walch G, Hawkins RJ, Kissenberth MJ. The role of the biceps tendon in massive rotator cuff tears. Instr Course Lect. 2012;61:113-120.
43. Krupp RJ, Kevern MA, Gaines MD, Kotara S, Singleton SB. Long head of the biceps tendon pain: differential diagnosis and treatment. J Orthop Sports Phys Ther. 2009;39(2):55-70.
44. Levy AS, Kelly BT, Lintner SA, Osbahr DC, Speer KP. Function of the long head of the biceps at the shoulder: electromyographic analysis. J Shoulder Elbow Surg. 2001;10(3):250-255.
45. Kuhn JE, Huston LJ, Soslowsky LJ, Shyr Y, Blasier RB. External rotation of the glenohumeral joint: ligament restraints and muscle effects in the neutral and abducted positions.
J Shoulder Elbow Surg. 2005;14(1 Suppl S):39S-48S.
46. McGarry MH, Nguyen ML, Quigley RJ, Hanypsiak B, Gupta R, Lee TQ. The effect of long and short head biceps loading on glenohumeral joint rotational range of motion and humeral head postion. Knee Surge Sports Traumatol Arthrosc. 2014 Sep 26. [Epub ahead of print].
47. Young RW. Evolution of the human hand: The role of throwing and clubbing. J Anat. 2003;202:165-174.
48. Calvin WH. Did throwing stones shape hominid brain evolution? Ethology and Sociobiology. 1982;3:115-124.
49. Fifer FC. The adoption of bipedalism by the hominids: A new hypothesis. Human Evolution. 1987;2(2):135-147.
50. Darlington PJ. Group selection, altruism, reinforcement, and throwing in human evolution. Proc Nat Acad Sci. 1973;72(9):3748-3752.
Outcomes and Aseptic Survivorship of Revision Total Knee Arthroplasty
Over the past 3 decades, total knee arthroplasty (TKA) has been considered a safe and effective treatment for end-stage knee arthritis.1 However, as the population, the incidence of obesity, and life expectancy continue to increase, the number of TKAs will rise as well.2,3 It is expected that over the next 16 years, the number of TKAs performed annually will exceed 3 million in the United States alone.4 This projection represents an over 600% increase from 2005 figures.5 Given the demographic shift expected over the next 2 decades, patients are anticipated to undergo these procedures at younger ages compared with previous generations, such that those age 65 years or younger will account for more than 55% of primary TKAs.6 More important, given this exponential growth in primary TKAs, there will be a concordant rise in revision procedures. It is expected that, the annual number has roughly doubled from that recorded for 2005.4
Compared with primary TKAs, however, revision TKAs have had less promising results, with survivorship as low as 60% over shorter periods.7,8 In addition, recent studies have found an even higher degree of dissatisfaction and functional limitations among revision TKA patients than among primary TKA patients, 15% to 30% of whom are unhappy with their procedures.9-11 These shortcomings of revision TKAs are thought to result from several factors, including poor bone quality, insufficient bone stock, ligamentous instability, soft-tissue incompetence, infection, malalignment, problems with extensor mechanisms, and substantial pain of uncertain etiology.
Despite there being several complex factors that can lead to worse outcomes with revision TKAs, surgeons are expected to produce results equivalent to those of primary TKAs. It is therefore imperative to delineate the objective and subjective outcomes of revision techniques to identify areas in need of improvement. In this article, we provide a concise overview of revision TKA outcomes in order to stimulate manufacturers, surgeons, and hospitals to improve on implant designs, surgical techniques, and care guidelines for revision TKA. We review the evidence on 5 points: aseptic survivorship, functional outcomes, patient satisfaction, quality of life (QOL), and economic impact. In addition, we compare available outcome data for revision and primary TKAs.
1. Aseptic survivorship
Fehring and colleagues12 in 2001 and Sharkey and colleagues13 in 2002 evaluated mechanisms of failure for revision TKA and reported many failures resulted from infection or were associated with the implant, and occurred within 2 years after the primary procedure. More recently, Dy and colleagues14 found the most common reason for revision was aseptic loosening, followed by infection. The present review focuses on aseptic femoral and tibial revision.
The failure rate for revision TKA is substantially higher than for primary TKA with the same type of prosthesis because of the complexity of the revision procedure, the increasing constraint of the implant design, and the higher degree of bone loss. (Appendix 1 lists risk factors for revision surgery. Appendix 2 is a complete list of survivorship outcomes of revision TKA.)
Sheng and colleagues15 in 2006 and Koskinen and colleagues16 in 2008 analyzed Finnish Arthroplasty Register data to determine failure rates for revision and primary TKA. Sheng and colleagues15 examined survivorship of 2637 revision TKAs (performed between 1990 and 2002) for all-cause endpoints after first revision procedure. Survivorship rates were 89% (5 years) and 79% (10 years), while Koskinen and colleagues16 noted all-cause survival rates of 80% at 15 years. More recently, in 2013, the New Zealand Orthopaedic Association17 analyzed New Zealand Joint Registry data for revision and re-revision rates (rates of revision per 100 component years) for 64,556 primary TKAs performed between 1999 and 2012. During the period studied, 1684 revisions were performed, reflecting a 2.6% revision rate, a 0.50% rate of revision per 100 component years, and a 13-year Kaplan-Meier survivorship of 94.5%. The most common reasons for revision were pain, deep infection, and tibial component loosening (Table 1).
Posterior stabilized implants
Laskin and Ohnsorge18 retrospectively reviewed the cases of 58 patients who underwent unilateral revision TKA (with a posterior stabilized implant), of which 42% were for coronal instability and 44% for a loose tibial component. At minimum 4-year follow-up, 52 of the 58 patients had anteroposterior instability of less than 5 mm. In addition, 5 years after surgery, aseptic survivorship was 96%. Meijer and colleagues19 conducted a retrospective comparative study of 69 revision TKAs (65 patients) in which 9 knees received a primary implant and 60 received a revision implant with stems and augmentation (60 = 37 posterior stabilized, 20 constrained, 3 rotating hinge). Survival rates for the primary implants were 100% (1 year), 73% (2 years), and 44% (5 years), and survival rates for the revision implants were significantly better: 95% (1 year), 92% (2 years), and 92% (5 years) (hazard ratio, 5.87; P = .008). The authors therefore indicated that it was unclear whether using a primary implant should still be an option in revision TKA and, if it is used, whether it should be limited to less complex situations in which bone loss and ligament damage are minimal (Table 2).
Constrained and semiconstrained implants
In a study of 234 knees (209 patients) with soft-tissue deficiency, Wilke and colleagues20 evaluated the long-term survivorship of revision TKA with use of a semiconstrained modular fixed-bearing implant system. Overall Kaplan-Meier survival rates were 91% (5 years) and 81% (10 years) at a mean follow-up of 9 years. When aseptic revision was evaluated, however, the survival rates increased to 95% (5 years) and 90% (10 years). The authors noted that male sex was the only variable that significantly increased the risk for re-revision (hazard ratio, 2.07; P = .02), which they attributed to potentially higher activity levels. In 2006 and 2011, Lachiewicz and Soileau21,22 evaluated the survival of first- and second-generation constrained condylar prostheses in primary TKA cases with severe valgus deformities, incompetent collateral ligaments, or severe flexion contractures. Of the 54 knees (44 patients) with first-generation prostheses, 42 (34 patients) had a mean follow-up of 9 years (range, 5-16 years). Ten-year survival with failure, defined as component revision for loosening, was 96%. The 27 TKAs using second-generation prostheses had a mean follow-up of about 5 years (range, 2-12 years). At final follow-up, there were no revisions for loosening or patellar problems, but 6 knees (22%) required lateral retinacular release of the patella (Table 3).
Rotating hinge implants
Neumann and colleagues23 evaluated the clinical and radiographic outcomes of 24 rotating hinge prostheses used for aseptic loosening with substantial bone loss and collateral ligament instability. At a mean follow-up of 56 months (range, 3-5 years), there was no evidence of loosening of any implants, and nonprogressive radiolucent lines were found in only 2 tibial components. Kowalczewski and colleagues24 evaluated the clinical and radiologic outcomes of 12 primary TKAs using a rotating hinge knee prosthesis at a minimum follow-up of 10 years. By most recent follow-up, no implants had been revised for loosening, and only 3 had nonprogressive radiolucent lines (Table 4).
Endoprostheses (modular segmental implants)
In a systematic review of 9 studies, Korim and colleagues25 evaluated 241 endoprostheses used for limb salvage under nononcologic conditions. Mean follow-up was about 3 years (range, 1-5 years). The devices were used to treat various conditions, including periprosthetic fracture, bone loss with aseptic loosening, and ligament insufficiency. The overall reoperation rate was 17% (41/241 cases). Mechanical failures were less frequent (6%-19%) (Table 5).
2. Functional outcomes
The goal in both primary and revision TKA is to restore the function and mobility of the knee and to alleviate pain. Whereas primary TKAs are realistically predictable and reproducible in their outcomes, revision TKAs are vastly more complicated, which can result in worse postoperative outcomes and function. In addition, revision TKAs may require extensive surgical exposure, which causes more tissue and muscle damage, prolonging rehabilitation. (Appendix 3 is a complete list of studies of functional outcomes of revision TKA.)
This discrepancy in functional outcomes between primary and revision TKA begins as early as the postoperative inpatient rehabilitation period. Using the functional independence measurement (FIM), which estimates performance of activities of daily living, mobility, and cognition, Vincent and colleagues26 evaluated the functional improvement produced by revision versus primary TKA during inpatient rehabilitation. They compared 424 consecutive primary TKAs with 138 revision TKAs. For both groups, FIM scores increased significantly (P = .015) between admission and discharge. On discharge, however, FIM scores were significantly (P = .01) higher for the primary group than the revision group (29 and 27 points, respectively). Furthermore, in the evaluation of mechanisms of failure, patients who had revision TKA for mechanical or pain-related problems did markedly better than those who had revision TKA for infection.
Compared with primary knee implants, revision implants require increasing constraint. We assume increasing constraint affects knee biomechanics, leading to worsening functional outcomes. In a study of 60 revision TKAs (57 patients) using posterior stabilized, condylar constrained, or rotating hinge prostheses, Vasso and colleagues27 examined functional outcomes at a median follow-up of 9 years (range, 4-12 years). At most recent follow-up, mean International Knee Society (IKS) Knee and Function scores were 81 (range, 48-97) and 79 (range, 56-92), mean Hospital for Special Surgery (HSS) score was 84 (range, 62-98), and mean range of motion (ROM) was 121° (range, 98°-132°) (P < .001). Although there were no significant differences in IKS and HSS scores between prosthesis types, ROM was significantly (P < .01) wider in the posterior stabilized group than in the condylar constrained and rotating hinge groups (127° vs 112° and 108°), suggesting increasing constraint resulted in decreased ROM. Several studies have found increasing constraint might lead to reduced function.28-30
However, Hwang and colleagues31 evaluated functional outcomes in 36 revision TKAs and noted that the cemented posterior stabilized (n = 8), condylar constrained (n = 25), and rotating hinge (n = 13) prostheses used did not differ in their mean Knee Society scores (78, 81, and 83, respectively).
There remains a marked disparity in patient limitations seen after revision versus primary TKA. Given the positive results being obtained with newer implants, studies might suggest recent generations of prostheses have allowed designs to be comparable. As design development continues, we may come closer to achieving outcomes comparable to those of primary TKA.
3. Patient satisfaction
Several recent reports have shown that 10% to 25% of patients who underwent primary TKA were dissatisfied with their surgery30,32; other studies have found patient satisfaction often correlating to function and pain.33-35 Given the worse outcomes for revision TKA (outlined in the preceding section), the substantial pain accompanying a second, more complex procedure, and the extensive rehabilitation expected, we suspect patients who undergo revision TKA are even less satisfied with their surgery than their primary counterparts are. (See Appendix 4 for a complete list of studies of patient satisfaction after revision TKA.)
Barrack and colleagues32 evaluated a consecutive series of 238 patients followed up for at least 1 year after revision TKA. Patients were asked to rate their degree of satisfaction with both their primary procedure and the revision and to indicate their expectations regarding their revision prosthesis. Mean satisfaction score was 7.4 (maximum = 10), with 13% of patients dissatisfied, 18% somewhat satisfied, and 69% satisfied. Seventy-four percent of patients expected their revision prosthesis to last longer than the primary prosthesis.
Greidanus and colleagues36 evaluated patient satisfaction in 60 revision TKA cases and 199 primary TKA cases at 2-year follow-up. The primary TKA group had significantly (P < .01) higher satisfaction scores in a comparison with the revision TKA group: Global (86 vs 73), Pain Relief (88 vs 70), Function (83 vs 67), and Recreation (77 vs 62). These findings support the satisfaction rates reported by Dahm and colleagues33,34: 91% for primary TKA patients and 77% for revision TKA patients.
4. Quality of life
Procedure complexity leads to reduced survivorship, function, and mobility, longer rehabilitation, and decreased QOL for revision TKA patients relative to primary TKA patients.37 (See Appendix 5 for a complete list of studies of QOL outcomes of revision TKA.)
Greidanus and colleagues36 evaluated joint-specific QOL (using the 12-item Oxford Knee Score; OKS) and generic QOL (using the 12-Item Short Form Health Survey; SF-12) in 60 revision TKA cases and 199 primary TKA cases at a mean follow-up of 2 years. (The OKS survey is used to evaluate patient perspectives on TKA outcomes,38 and the multipurpose SF-12 questionnaire is used to assess mental and physical function and general health-related QOL.39) Compared with the revision TKA group, the primary TKA group had significantly higher OKS after surgery (78 vs 68; P = .01) as well as significantly higher SF-12 scores: Global (84 vs 72; P = .01), Mental (54 vs 50; P = .03), and Physical (43 vs 37; P = .01). Similarly, Ghomrawi and colleagues40 evaluated patterns of improvement in 308 patients (318 knees) who had revision TKA. At 24-month follow-up, mean SF-36 Physical and Mental scores were 35 and 52, respectively.
Deehan and colleagues41 used the Nottingham Health Profile (NHP) to compare 94 patients’ health-related QOL scores before revision TKA with their scores 3 months, 1 year, and 5 years after revision. NHP Pain subscale scores were significantly lower 3 and 12 months after surgery than before surgery, but this difference was no longer seen at the 5-year follow-up. There was no significant improvement in scores on the other 5 NHP subscales (Sleep, Energy, Emotion, Mobility, Social Isolation) at any time points.
As shown in the literature, patients’ QOL outcomes improve after revision TKA, but these gains are not at the level of patients who undergo primary TKA.36,41 Given that revision surgery is more extensive, and that perhaps revision patients have poorer muscle function, they usually do not return to the level they attained after their index procedure.
5. Economic impact
Consistent with the outcomes already described, the economic impact of revision TKAs is excess expenditures and costs to patients and health care institutions.42 The sources of this impact are higher implant costs, extra operative trays and times, longer hospital stays, more rehabilitation, and increased medication use.43 Revision TKA costs range from $49,000 to more than $100,000—a tremendous increase over primary TKA costs ($25,000-$30,000).43-45 Furthermore, the annual economic burden associated with revision TKA, now $2.7 billion, is expected to exceed $13 billion by 2030.46 In the United States, about $23.2 billion will be spent on 926,527 primary TKAs in 2015; significantly, the costs associated with revising just 10% of these cases account for almost 50% of the total cost of the primary procedures.46
In a retrospective cost-identification multicenter cohort study, Bozic and colleagues47 found that both-component and single-component revisions, compared with primary procedures, were associated with significantly increased operative time (~265 and 221 minutes vs 200 minutes), use of allograft bone (23% and 14% vs 1%), length of stay (5.4 and 5.7 days vs 5.0 days), and percentage of patients discharged to extended-care facilities (26% and 26% vs 25%) (P < .0001). Hospital costs for both- and single-component revisions were 138% and 114% higher than costs for primary procedures (P < .0001). More recently, Kallala and colleagues44 analyzed UK National Health Service data and compared the costs of revision for infection with revision for other causes (pain, instability, aseptic loosening, fracture). Mean length of stay associated with revision for infection (21.5 days) was more than double that associated with revision for aseptic loosening (9.5 days; P < .0001), and mean cost of revision for septic causes (£30,011) was more than 3 times that of revision for other causes (£9655; P < .0001). The authors concluded that the higher costs of revision knee surgery have a considerable economic impact, especially in infection cases.
With more extensive procedures, long-stem or more constrained prostheses are often needed to obtain adequate fixation and stability. The resulting increased, substantial economic burden is felt by patients and the health care system. Given that health care reimbursements are declining, hospitals that perform revision TKAs can sustain marked financial losses. Some centers are asking whether it is cost-effective to continue to perform these types of procedures. We must find new ways to provide revision procedures using less costly implants and tools so that centers will continue to make these procedures available to patients.
Conclusion
Given the exponential growth in primary TKAs, there will be a concordant increase in revision TKAs in the decades to come. This review provides a concise overview of revision TKA outcomes. Given the low level of evidence regarding revision TKAs, we need further higher quality studies of their prostheses and outcomes. Specifically, we need systematic reviews and meta-analyses to provide higher quality evidence regarding outcomes of using individual prosthetic designs.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Crowninshield RD, Rosenberg AG, Sporer SM. Changing demographics of patients with total joint replacement. Clin Orthop Relat Res. 2006;443:266-272.
3. Ravi B, Croxford R, Reichmann WM, Losina E, Katz JN, Hawker GA. The changing demographics of total joint arthroplasty recipients in the United States and Ontario from 2001 to 2007. Best Pract Res Clin Rheumatol. 2012;26(5):637-647.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
6. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606-2612.
7. Bryan RS, Rand JA. Revision total knee arthroplasty. Clin Orthop Relat Res. 1982;170:116-122.
8. Rand JA, Bryan RS. Revision after total knee arthroplasty. Orthop Clin North Am. 1982;13(1):201-212.
9. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
10. Parvizi J, Nunley RM, Berend KR, et al. High level of residual symptoms in young patients after total knee arthroplasty. Clin Orthop Relat Res. 2014;472(1):133-137.
11. Ali A, Sundberg M, Robertsson O, et al. Dissatisfied patients after total knee arthroplasty: a registry study involving 114 patients with 8-13 years of followup. Acta Orthop. 2014;85(3):229-233.
12. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
13. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
14. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
15. Sheng PY, Konttinen L, Lehto M, et al. Revision total knee arthroplasty: 1990 through 2002. A review of the Finnish Arthroplasty Registry. J Bone Joint Surg Am. 2006;88(7):1425-1430.
16. Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79(4):499-507.
17. New Zealand Orthopaedic Association. The New Zealand Joint Registry Fourteen Year Report (January 1999 to December 2012). http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed December 16, 2015.
18. Laskin RS, Ohnsorge J. The use of standard posterior stabilized implants in revision total knee arthroplasty. Clin Orthop Relat Res. 2005;(440):122-125.
19. Meijer MF, Reininga IH, Boerboom AL, Stevens M, Bulstra SK. Poorer survival after a primary implant during revision total knee arthroplasty. Int Orthop. 2013;37(3):415-419.
20. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
21. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
22. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
23. Neumann DR, Hofstaedter T, Dorn U. Follow-up of a modular rotating hinge knee system in salvage revision total knee arthroplasty. J Arthroplasty. 2012;27(5):814-819.
24. Kowalczewski J, Marczak D, Synder M, Sibinski M. Primary rotating-hinge total knee arthroplasty: good outcomes at mid-term follow-up. J Arthroplasty. 2014;29(6):1202-1206.
25. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. Knee. 2013;20(6):367-375.
26. Vincent KR, Vincent HK, Lee LW, Alfano AP. Inpatient rehabilitation outcomes in primary and revision total knee arthroplasty patients. Clin Orthop Relat Res. 2006;(446):201-207.
27. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
28. Baier C, Luring C, Schaumburger J, et al. Assessing patient-oriented results after revision total knee arthroplasty. J Orthop Sci. 2013;18(6):955-961.
29. Hartford JM, Goodman SB, Schurman DJ, Knoblick G. Complex primary and revision total knee arthroplasty using the condylar constrained prosthesis: an average 5-year follow-up. J Arthroplasty. 1998;13(4):380-387.
30. Haidukewych GJ, Jacofsky DJ, Pagnano MW, Trousdale RT. Functional results after revision of well-fixed components for stiffness after primary total knee arthroplasty. J Arthroplasty. 2005;20(2):133-138.
31. Hwang SC, Kong JY, Nam DC, et al. Revision total knee arthroplasty with a cemented posterior stabilized, condylar constrained or fully constrained prosthesis: a minimum 2-year follow-up analysis. Clin Orthop Surg. 2010;2(2):112-120.
32. Barrack RL, McClure JT, Burak CF, Clohisy JC, Parvizi J, Sharkey P. Revision total knee arthroplasty: the patient’s perspective. Clin Orthop Relat Res. 2007;464:146-150.
33. Dahm DL, Barnes SA, Harrington JR, Berry DJ. Patient reported activity after revision total knee arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):106-110.
34. Dahm DL, Barnes SA, Harrington JR, Sayeed SA, Berry DJ. Patient-reported activity level after total knee arthroplasty. J Arthroplasty. 2008;23(3):401-407.
35. Richards CJ, Garbuz DS, Pugh L, Masri BA. Revision total knee arthroplasty: clinical outcome comparison with and without the use of femoral head structural allograft. J Arthroplasty. 2011;26(8):1299-1304.
36. Greidanus NV, Peterson RC, Masri BA, Garbuz DS. Quality of life outcomes in revision versus primary total knee arthroplasty. J Arthroplasty. 2011;26(4):615-620.
37. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
38. Murray DW, Fitzpatrick R, Rogers K, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br. 2007;89(8):1010-1014.
39. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
40. Ghomrawi HM, Kane RL, Eberly LE, Bershadsky B, Saleh KJ; North American Knee Arthroplasty Revision Study Group. Patterns of functional improvement after revision knee arthroplasty. J Bone Joint Surg Am. 2009;91(12):2838-2845.
41. Deehan DJ, Murray JD, Birdsall PD, Pinder IM. Quality of life after knee revision arthroplasty. Acta Orthop. 2006;77(5):761-766.
42. Kapadia BH, McElroy MJ, Issa K, Johnson AJ, Bozic KJ, Mont MA. The economic impact of periprosthetic infections following total knee arthroplasty at a specialized tertiary-care center. J Arthroplasty. 2014;29(5):929-932.
43. Bhandari M, Smith J, Miller LE, Block JE. Clinical and economic burden of revision knee arthroplasty. Clin Med Insights Arthritis Musculoskelet Disord. 2012;5:89-94.
44. Kallala RF, Vanhegan IS, Ibrahim MS, Sarmah S, Haddad FS. Financial analysis of revision knee surgery based on NHS tariffs and hospital costs: does it pay to provide a revision service? Bone Joint J Br. 2015;97(2):197-201.
45. Ong KL, Mowat FS, Chan N, Lau E, Halpern MT, Kurtz SM. Economic burden of revision hip and knee arthroplasty in Medicare enrollees. Clin Orthop Relat Res. 2006;446:22-28.
46. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
47. Bozic KJ, Durbhakula S, Berry DJ, et al. Differences in patient and procedure characteristics and hospital resource use in primary and revision total joint arthroplasty: a multicenter study. J Arthroplasty. 2005;20(7 suppl 3):17-25.
48. Lee KJ, Moon JY, Song EK, Lim HA, Seon JK. Minimum Two-year Results of Revision Total Knee Arthroplasty Following Infectious or Non-infectious Causes. Knee Surg Relat Res. 2012;24(4):227-234.
49. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
50. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
51. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
52. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
53. Mabry TM, Vessely MB, Schleck CD, Harmsen WS, Berry DJ. Revision total knee arthroplasty with modular cemented stems: long-term follow-up. J Arthroplasty. 2007;22(6 Suppl 2):100-105.
54. Gudnason A, Milbrink J, Hailer NP. Implant survival and outcome after rotating-hinge total knee revision arthroplasty: a minimum 6-year follow-up. Arch Orthop Trauma Surg. 2011;131(11):1601-1607.
55. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125-131.
56. Greene JW, Reynolds SM, Stimac JD, Malkani AL, Massini MA. Midterm results of hybrid cement technique in revision total knee arthroplasty. J Arthroplasty. 2013;28(4):570-574.
57. Dalury DF, Adams MJ. Minimum 6-year follow-up of revision total knee arthroplasty without patella reimplantation. Journal Arthroplasty. 2012;27(8 Suppl):91-94.
58. Whaley AL, Trousdale RT, Rand JA, Hanssen AD. Cemented long-stem revision total knee arthroplasty. J Arthroplasty. 2003;18(5):592-599.
59. Friedman RJ, Hirst P, Poss R, Kelley K, Sledge CB. Results of revision total knee arthroplasty performed for aseptic loosening. Clinical Orthop Relat Res. 1990;255:235-241.
60. Barrack RL, Rorabeck C, Partington P, Sawhney J, Engh G. The results of retaining a well-fixed patellar component in revision total knee arthroplasty. J Arthroplasty. 2000;15(4):413-417.
61. Christensen CP, Crawford JJ, Olin MD, Vail TP. Revision of the stiff total knee arthroplasty. J Arthroplasty. 2002;17(4):409-415.
62. Garcia RM, Hardy BT, Kraay MJ, Goldberg VM. Revision total knee arthroplasty for aseptic and septic causes in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2010;468(1):82-89.
63. Patil N, Lee K, Huddleston JI, Harris AH, Goodman SB. Aseptic versus septic revision total knee arthroplasty: patient satisfaction, outcome and quality of life improvement. Knee. 2010;17(3):200-203.
64. Luque R, Rizo B, Urda A, et al. Predictive factors for failure after total knee replacement revision. Int Orthop. 2014;38(2):429-435.
65. Bistolfi A, Massazza G, Rosso F, Crova M. Rotating-hinge total knee for revision total knee arthroplasty. Orthopedics. 2012;35(3):e325-e330.
66. Bottner F, Laskin R, Windsor RE, Haas SB. Hybrid component fixation in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:127-131.
67. Jensen CL, Winther N, Schroder HM, Petersen MM. Outcome of revision total knee arthroplasty with the use of trabecular metal cone for reconstruction of severe bone loss at the proximal tibia. Knee. 2014;21(6):1233-1237.
68. Howard JL, Kudera J, Lewallen DG, Hanssen AD. Early results of the use of tantalum femoral cones for revision total knee arthroplasty. J Bone Joint Surg Am. 2011;93(5):478-484.
69. Yang JH, Yoon JR, Oh CH, Kim TS. Hybrid component fixation in total knee arthroplasty: minimum of 10-year follow-up study. J Arthroplasty. 2012;27(6):1111-1118.
70. Peters CL, Erickson JA, Gililland JM. Clinical and radiographic results of 184 consecutive revision total knee arthroplasties placed with modular cementless stems. J Arthroplasty. 2009;24(6 Suppl):48-53.
71. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2014. 2014.
72. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2013. 2013.
Over the past 3 decades, total knee arthroplasty (TKA) has been considered a safe and effective treatment for end-stage knee arthritis.1 However, as the population, the incidence of obesity, and life expectancy continue to increase, the number of TKAs will rise as well.2,3 It is expected that over the next 16 years, the number of TKAs performed annually will exceed 3 million in the United States alone.4 This projection represents an over 600% increase from 2005 figures.5 Given the demographic shift expected over the next 2 decades, patients are anticipated to undergo these procedures at younger ages compared with previous generations, such that those age 65 years or younger will account for more than 55% of primary TKAs.6 More important, given this exponential growth in primary TKAs, there will be a concordant rise in revision procedures. It is expected that, the annual number has roughly doubled from that recorded for 2005.4
Compared with primary TKAs, however, revision TKAs have had less promising results, with survivorship as low as 60% over shorter periods.7,8 In addition, recent studies have found an even higher degree of dissatisfaction and functional limitations among revision TKA patients than among primary TKA patients, 15% to 30% of whom are unhappy with their procedures.9-11 These shortcomings of revision TKAs are thought to result from several factors, including poor bone quality, insufficient bone stock, ligamentous instability, soft-tissue incompetence, infection, malalignment, problems with extensor mechanisms, and substantial pain of uncertain etiology.
Despite there being several complex factors that can lead to worse outcomes with revision TKAs, surgeons are expected to produce results equivalent to those of primary TKAs. It is therefore imperative to delineate the objective and subjective outcomes of revision techniques to identify areas in need of improvement. In this article, we provide a concise overview of revision TKA outcomes in order to stimulate manufacturers, surgeons, and hospitals to improve on implant designs, surgical techniques, and care guidelines for revision TKA. We review the evidence on 5 points: aseptic survivorship, functional outcomes, patient satisfaction, quality of life (QOL), and economic impact. In addition, we compare available outcome data for revision and primary TKAs.
1. Aseptic survivorship
Fehring and colleagues12 in 2001 and Sharkey and colleagues13 in 2002 evaluated mechanisms of failure for revision TKA and reported many failures resulted from infection or were associated with the implant, and occurred within 2 years after the primary procedure. More recently, Dy and colleagues14 found the most common reason for revision was aseptic loosening, followed by infection. The present review focuses on aseptic femoral and tibial revision.
The failure rate for revision TKA is substantially higher than for primary TKA with the same type of prosthesis because of the complexity of the revision procedure, the increasing constraint of the implant design, and the higher degree of bone loss. (Appendix 1 lists risk factors for revision surgery. Appendix 2 is a complete list of survivorship outcomes of revision TKA.)
Sheng and colleagues15 in 2006 and Koskinen and colleagues16 in 2008 analyzed Finnish Arthroplasty Register data to determine failure rates for revision and primary TKA. Sheng and colleagues15 examined survivorship of 2637 revision TKAs (performed between 1990 and 2002) for all-cause endpoints after first revision procedure. Survivorship rates were 89% (5 years) and 79% (10 years), while Koskinen and colleagues16 noted all-cause survival rates of 80% at 15 years. More recently, in 2013, the New Zealand Orthopaedic Association17 analyzed New Zealand Joint Registry data for revision and re-revision rates (rates of revision per 100 component years) for 64,556 primary TKAs performed between 1999 and 2012. During the period studied, 1684 revisions were performed, reflecting a 2.6% revision rate, a 0.50% rate of revision per 100 component years, and a 13-year Kaplan-Meier survivorship of 94.5%. The most common reasons for revision were pain, deep infection, and tibial component loosening (Table 1).
Posterior stabilized implants
Laskin and Ohnsorge18 retrospectively reviewed the cases of 58 patients who underwent unilateral revision TKA (with a posterior stabilized implant), of which 42% were for coronal instability and 44% for a loose tibial component. At minimum 4-year follow-up, 52 of the 58 patients had anteroposterior instability of less than 5 mm. In addition, 5 years after surgery, aseptic survivorship was 96%. Meijer and colleagues19 conducted a retrospective comparative study of 69 revision TKAs (65 patients) in which 9 knees received a primary implant and 60 received a revision implant with stems and augmentation (60 = 37 posterior stabilized, 20 constrained, 3 rotating hinge). Survival rates for the primary implants were 100% (1 year), 73% (2 years), and 44% (5 years), and survival rates for the revision implants were significantly better: 95% (1 year), 92% (2 years), and 92% (5 years) (hazard ratio, 5.87; P = .008). The authors therefore indicated that it was unclear whether using a primary implant should still be an option in revision TKA and, if it is used, whether it should be limited to less complex situations in which bone loss and ligament damage are minimal (Table 2).
Constrained and semiconstrained implants
In a study of 234 knees (209 patients) with soft-tissue deficiency, Wilke and colleagues20 evaluated the long-term survivorship of revision TKA with use of a semiconstrained modular fixed-bearing implant system. Overall Kaplan-Meier survival rates were 91% (5 years) and 81% (10 years) at a mean follow-up of 9 years. When aseptic revision was evaluated, however, the survival rates increased to 95% (5 years) and 90% (10 years). The authors noted that male sex was the only variable that significantly increased the risk for re-revision (hazard ratio, 2.07; P = .02), which they attributed to potentially higher activity levels. In 2006 and 2011, Lachiewicz and Soileau21,22 evaluated the survival of first- and second-generation constrained condylar prostheses in primary TKA cases with severe valgus deformities, incompetent collateral ligaments, or severe flexion contractures. Of the 54 knees (44 patients) with first-generation prostheses, 42 (34 patients) had a mean follow-up of 9 years (range, 5-16 years). Ten-year survival with failure, defined as component revision for loosening, was 96%. The 27 TKAs using second-generation prostheses had a mean follow-up of about 5 years (range, 2-12 years). At final follow-up, there were no revisions for loosening or patellar problems, but 6 knees (22%) required lateral retinacular release of the patella (Table 3).
Rotating hinge implants
Neumann and colleagues23 evaluated the clinical and radiographic outcomes of 24 rotating hinge prostheses used for aseptic loosening with substantial bone loss and collateral ligament instability. At a mean follow-up of 56 months (range, 3-5 years), there was no evidence of loosening of any implants, and nonprogressive radiolucent lines were found in only 2 tibial components. Kowalczewski and colleagues24 evaluated the clinical and radiologic outcomes of 12 primary TKAs using a rotating hinge knee prosthesis at a minimum follow-up of 10 years. By most recent follow-up, no implants had been revised for loosening, and only 3 had nonprogressive radiolucent lines (Table 4).
Endoprostheses (modular segmental implants)
In a systematic review of 9 studies, Korim and colleagues25 evaluated 241 endoprostheses used for limb salvage under nononcologic conditions. Mean follow-up was about 3 years (range, 1-5 years). The devices were used to treat various conditions, including periprosthetic fracture, bone loss with aseptic loosening, and ligament insufficiency. The overall reoperation rate was 17% (41/241 cases). Mechanical failures were less frequent (6%-19%) (Table 5).
2. Functional outcomes
The goal in both primary and revision TKA is to restore the function and mobility of the knee and to alleviate pain. Whereas primary TKAs are realistically predictable and reproducible in their outcomes, revision TKAs are vastly more complicated, which can result in worse postoperative outcomes and function. In addition, revision TKAs may require extensive surgical exposure, which causes more tissue and muscle damage, prolonging rehabilitation. (Appendix 3 is a complete list of studies of functional outcomes of revision TKA.)
This discrepancy in functional outcomes between primary and revision TKA begins as early as the postoperative inpatient rehabilitation period. Using the functional independence measurement (FIM), which estimates performance of activities of daily living, mobility, and cognition, Vincent and colleagues26 evaluated the functional improvement produced by revision versus primary TKA during inpatient rehabilitation. They compared 424 consecutive primary TKAs with 138 revision TKAs. For both groups, FIM scores increased significantly (P = .015) between admission and discharge. On discharge, however, FIM scores were significantly (P = .01) higher for the primary group than the revision group (29 and 27 points, respectively). Furthermore, in the evaluation of mechanisms of failure, patients who had revision TKA for mechanical or pain-related problems did markedly better than those who had revision TKA for infection.
Compared with primary knee implants, revision implants require increasing constraint. We assume increasing constraint affects knee biomechanics, leading to worsening functional outcomes. In a study of 60 revision TKAs (57 patients) using posterior stabilized, condylar constrained, or rotating hinge prostheses, Vasso and colleagues27 examined functional outcomes at a median follow-up of 9 years (range, 4-12 years). At most recent follow-up, mean International Knee Society (IKS) Knee and Function scores were 81 (range, 48-97) and 79 (range, 56-92), mean Hospital for Special Surgery (HSS) score was 84 (range, 62-98), and mean range of motion (ROM) was 121° (range, 98°-132°) (P < .001). Although there were no significant differences in IKS and HSS scores between prosthesis types, ROM was significantly (P < .01) wider in the posterior stabilized group than in the condylar constrained and rotating hinge groups (127° vs 112° and 108°), suggesting increasing constraint resulted in decreased ROM. Several studies have found increasing constraint might lead to reduced function.28-30
However, Hwang and colleagues31 evaluated functional outcomes in 36 revision TKAs and noted that the cemented posterior stabilized (n = 8), condylar constrained (n = 25), and rotating hinge (n = 13) prostheses used did not differ in their mean Knee Society scores (78, 81, and 83, respectively).
There remains a marked disparity in patient limitations seen after revision versus primary TKA. Given the positive results being obtained with newer implants, studies might suggest recent generations of prostheses have allowed designs to be comparable. As design development continues, we may come closer to achieving outcomes comparable to those of primary TKA.
3. Patient satisfaction
Several recent reports have shown that 10% to 25% of patients who underwent primary TKA were dissatisfied with their surgery30,32; other studies have found patient satisfaction often correlating to function and pain.33-35 Given the worse outcomes for revision TKA (outlined in the preceding section), the substantial pain accompanying a second, more complex procedure, and the extensive rehabilitation expected, we suspect patients who undergo revision TKA are even less satisfied with their surgery than their primary counterparts are. (See Appendix 4 for a complete list of studies of patient satisfaction after revision TKA.)
Barrack and colleagues32 evaluated a consecutive series of 238 patients followed up for at least 1 year after revision TKA. Patients were asked to rate their degree of satisfaction with both their primary procedure and the revision and to indicate their expectations regarding their revision prosthesis. Mean satisfaction score was 7.4 (maximum = 10), with 13% of patients dissatisfied, 18% somewhat satisfied, and 69% satisfied. Seventy-four percent of patients expected their revision prosthesis to last longer than the primary prosthesis.
Greidanus and colleagues36 evaluated patient satisfaction in 60 revision TKA cases and 199 primary TKA cases at 2-year follow-up. The primary TKA group had significantly (P < .01) higher satisfaction scores in a comparison with the revision TKA group: Global (86 vs 73), Pain Relief (88 vs 70), Function (83 vs 67), and Recreation (77 vs 62). These findings support the satisfaction rates reported by Dahm and colleagues33,34: 91% for primary TKA patients and 77% for revision TKA patients.
4. Quality of life
Procedure complexity leads to reduced survivorship, function, and mobility, longer rehabilitation, and decreased QOL for revision TKA patients relative to primary TKA patients.37 (See Appendix 5 for a complete list of studies of QOL outcomes of revision TKA.)
Greidanus and colleagues36 evaluated joint-specific QOL (using the 12-item Oxford Knee Score; OKS) and generic QOL (using the 12-Item Short Form Health Survey; SF-12) in 60 revision TKA cases and 199 primary TKA cases at a mean follow-up of 2 years. (The OKS survey is used to evaluate patient perspectives on TKA outcomes,38 and the multipurpose SF-12 questionnaire is used to assess mental and physical function and general health-related QOL.39) Compared with the revision TKA group, the primary TKA group had significantly higher OKS after surgery (78 vs 68; P = .01) as well as significantly higher SF-12 scores: Global (84 vs 72; P = .01), Mental (54 vs 50; P = .03), and Physical (43 vs 37; P = .01). Similarly, Ghomrawi and colleagues40 evaluated patterns of improvement in 308 patients (318 knees) who had revision TKA. At 24-month follow-up, mean SF-36 Physical and Mental scores were 35 and 52, respectively.
Deehan and colleagues41 used the Nottingham Health Profile (NHP) to compare 94 patients’ health-related QOL scores before revision TKA with their scores 3 months, 1 year, and 5 years after revision. NHP Pain subscale scores were significantly lower 3 and 12 months after surgery than before surgery, but this difference was no longer seen at the 5-year follow-up. There was no significant improvement in scores on the other 5 NHP subscales (Sleep, Energy, Emotion, Mobility, Social Isolation) at any time points.
As shown in the literature, patients’ QOL outcomes improve after revision TKA, but these gains are not at the level of patients who undergo primary TKA.36,41 Given that revision surgery is more extensive, and that perhaps revision patients have poorer muscle function, they usually do not return to the level they attained after their index procedure.
5. Economic impact
Consistent with the outcomes already described, the economic impact of revision TKAs is excess expenditures and costs to patients and health care institutions.42 The sources of this impact are higher implant costs, extra operative trays and times, longer hospital stays, more rehabilitation, and increased medication use.43 Revision TKA costs range from $49,000 to more than $100,000—a tremendous increase over primary TKA costs ($25,000-$30,000).43-45 Furthermore, the annual economic burden associated with revision TKA, now $2.7 billion, is expected to exceed $13 billion by 2030.46 In the United States, about $23.2 billion will be spent on 926,527 primary TKAs in 2015; significantly, the costs associated with revising just 10% of these cases account for almost 50% of the total cost of the primary procedures.46
In a retrospective cost-identification multicenter cohort study, Bozic and colleagues47 found that both-component and single-component revisions, compared with primary procedures, were associated with significantly increased operative time (~265 and 221 minutes vs 200 minutes), use of allograft bone (23% and 14% vs 1%), length of stay (5.4 and 5.7 days vs 5.0 days), and percentage of patients discharged to extended-care facilities (26% and 26% vs 25%) (P < .0001). Hospital costs for both- and single-component revisions were 138% and 114% higher than costs for primary procedures (P < .0001). More recently, Kallala and colleagues44 analyzed UK National Health Service data and compared the costs of revision for infection with revision for other causes (pain, instability, aseptic loosening, fracture). Mean length of stay associated with revision for infection (21.5 days) was more than double that associated with revision for aseptic loosening (9.5 days; P < .0001), and mean cost of revision for septic causes (£30,011) was more than 3 times that of revision for other causes (£9655; P < .0001). The authors concluded that the higher costs of revision knee surgery have a considerable economic impact, especially in infection cases.
With more extensive procedures, long-stem or more constrained prostheses are often needed to obtain adequate fixation and stability. The resulting increased, substantial economic burden is felt by patients and the health care system. Given that health care reimbursements are declining, hospitals that perform revision TKAs can sustain marked financial losses. Some centers are asking whether it is cost-effective to continue to perform these types of procedures. We must find new ways to provide revision procedures using less costly implants and tools so that centers will continue to make these procedures available to patients.
Conclusion
Given the exponential growth in primary TKAs, there will be a concordant increase in revision TKAs in the decades to come. This review provides a concise overview of revision TKA outcomes. Given the low level of evidence regarding revision TKAs, we need further higher quality studies of their prostheses and outcomes. Specifically, we need systematic reviews and meta-analyses to provide higher quality evidence regarding outcomes of using individual prosthetic designs.
Over the past 3 decades, total knee arthroplasty (TKA) has been considered a safe and effective treatment for end-stage knee arthritis.1 However, as the population, the incidence of obesity, and life expectancy continue to increase, the number of TKAs will rise as well.2,3 It is expected that over the next 16 years, the number of TKAs performed annually will exceed 3 million in the United States alone.4 This projection represents an over 600% increase from 2005 figures.5 Given the demographic shift expected over the next 2 decades, patients are anticipated to undergo these procedures at younger ages compared with previous generations, such that those age 65 years or younger will account for more than 55% of primary TKAs.6 More important, given this exponential growth in primary TKAs, there will be a concordant rise in revision procedures. It is expected that, the annual number has roughly doubled from that recorded for 2005.4
Compared with primary TKAs, however, revision TKAs have had less promising results, with survivorship as low as 60% over shorter periods.7,8 In addition, recent studies have found an even higher degree of dissatisfaction and functional limitations among revision TKA patients than among primary TKA patients, 15% to 30% of whom are unhappy with their procedures.9-11 These shortcomings of revision TKAs are thought to result from several factors, including poor bone quality, insufficient bone stock, ligamentous instability, soft-tissue incompetence, infection, malalignment, problems with extensor mechanisms, and substantial pain of uncertain etiology.
Despite there being several complex factors that can lead to worse outcomes with revision TKAs, surgeons are expected to produce results equivalent to those of primary TKAs. It is therefore imperative to delineate the objective and subjective outcomes of revision techniques to identify areas in need of improvement. In this article, we provide a concise overview of revision TKA outcomes in order to stimulate manufacturers, surgeons, and hospitals to improve on implant designs, surgical techniques, and care guidelines for revision TKA. We review the evidence on 5 points: aseptic survivorship, functional outcomes, patient satisfaction, quality of life (QOL), and economic impact. In addition, we compare available outcome data for revision and primary TKAs.
1. Aseptic survivorship
Fehring and colleagues12 in 2001 and Sharkey and colleagues13 in 2002 evaluated mechanisms of failure for revision TKA and reported many failures resulted from infection or were associated with the implant, and occurred within 2 years after the primary procedure. More recently, Dy and colleagues14 found the most common reason for revision was aseptic loosening, followed by infection. The present review focuses on aseptic femoral and tibial revision.
The failure rate for revision TKA is substantially higher than for primary TKA with the same type of prosthesis because of the complexity of the revision procedure, the increasing constraint of the implant design, and the higher degree of bone loss. (Appendix 1 lists risk factors for revision surgery. Appendix 2 is a complete list of survivorship outcomes of revision TKA.)
Sheng and colleagues15 in 2006 and Koskinen and colleagues16 in 2008 analyzed Finnish Arthroplasty Register data to determine failure rates for revision and primary TKA. Sheng and colleagues15 examined survivorship of 2637 revision TKAs (performed between 1990 and 2002) for all-cause endpoints after first revision procedure. Survivorship rates were 89% (5 years) and 79% (10 years), while Koskinen and colleagues16 noted all-cause survival rates of 80% at 15 years. More recently, in 2013, the New Zealand Orthopaedic Association17 analyzed New Zealand Joint Registry data for revision and re-revision rates (rates of revision per 100 component years) for 64,556 primary TKAs performed between 1999 and 2012. During the period studied, 1684 revisions were performed, reflecting a 2.6% revision rate, a 0.50% rate of revision per 100 component years, and a 13-year Kaplan-Meier survivorship of 94.5%. The most common reasons for revision were pain, deep infection, and tibial component loosening (Table 1).
Posterior stabilized implants
Laskin and Ohnsorge18 retrospectively reviewed the cases of 58 patients who underwent unilateral revision TKA (with a posterior stabilized implant), of which 42% were for coronal instability and 44% for a loose tibial component. At minimum 4-year follow-up, 52 of the 58 patients had anteroposterior instability of less than 5 mm. In addition, 5 years after surgery, aseptic survivorship was 96%. Meijer and colleagues19 conducted a retrospective comparative study of 69 revision TKAs (65 patients) in which 9 knees received a primary implant and 60 received a revision implant with stems and augmentation (60 = 37 posterior stabilized, 20 constrained, 3 rotating hinge). Survival rates for the primary implants were 100% (1 year), 73% (2 years), and 44% (5 years), and survival rates for the revision implants were significantly better: 95% (1 year), 92% (2 years), and 92% (5 years) (hazard ratio, 5.87; P = .008). The authors therefore indicated that it was unclear whether using a primary implant should still be an option in revision TKA and, if it is used, whether it should be limited to less complex situations in which bone loss and ligament damage are minimal (Table 2).
Constrained and semiconstrained implants
In a study of 234 knees (209 patients) with soft-tissue deficiency, Wilke and colleagues20 evaluated the long-term survivorship of revision TKA with use of a semiconstrained modular fixed-bearing implant system. Overall Kaplan-Meier survival rates were 91% (5 years) and 81% (10 years) at a mean follow-up of 9 years. When aseptic revision was evaluated, however, the survival rates increased to 95% (5 years) and 90% (10 years). The authors noted that male sex was the only variable that significantly increased the risk for re-revision (hazard ratio, 2.07; P = .02), which they attributed to potentially higher activity levels. In 2006 and 2011, Lachiewicz and Soileau21,22 evaluated the survival of first- and second-generation constrained condylar prostheses in primary TKA cases with severe valgus deformities, incompetent collateral ligaments, or severe flexion contractures. Of the 54 knees (44 patients) with first-generation prostheses, 42 (34 patients) had a mean follow-up of 9 years (range, 5-16 years). Ten-year survival with failure, defined as component revision for loosening, was 96%. The 27 TKAs using second-generation prostheses had a mean follow-up of about 5 years (range, 2-12 years). At final follow-up, there were no revisions for loosening or patellar problems, but 6 knees (22%) required lateral retinacular release of the patella (Table 3).
Rotating hinge implants
Neumann and colleagues23 evaluated the clinical and radiographic outcomes of 24 rotating hinge prostheses used for aseptic loosening with substantial bone loss and collateral ligament instability. At a mean follow-up of 56 months (range, 3-5 years), there was no evidence of loosening of any implants, and nonprogressive radiolucent lines were found in only 2 tibial components. Kowalczewski and colleagues24 evaluated the clinical and radiologic outcomes of 12 primary TKAs using a rotating hinge knee prosthesis at a minimum follow-up of 10 years. By most recent follow-up, no implants had been revised for loosening, and only 3 had nonprogressive radiolucent lines (Table 4).
Endoprostheses (modular segmental implants)
In a systematic review of 9 studies, Korim and colleagues25 evaluated 241 endoprostheses used for limb salvage under nononcologic conditions. Mean follow-up was about 3 years (range, 1-5 years). The devices were used to treat various conditions, including periprosthetic fracture, bone loss with aseptic loosening, and ligament insufficiency. The overall reoperation rate was 17% (41/241 cases). Mechanical failures were less frequent (6%-19%) (Table 5).
2. Functional outcomes
The goal in both primary and revision TKA is to restore the function and mobility of the knee and to alleviate pain. Whereas primary TKAs are realistically predictable and reproducible in their outcomes, revision TKAs are vastly more complicated, which can result in worse postoperative outcomes and function. In addition, revision TKAs may require extensive surgical exposure, which causes more tissue and muscle damage, prolonging rehabilitation. (Appendix 3 is a complete list of studies of functional outcomes of revision TKA.)
This discrepancy in functional outcomes between primary and revision TKA begins as early as the postoperative inpatient rehabilitation period. Using the functional independence measurement (FIM), which estimates performance of activities of daily living, mobility, and cognition, Vincent and colleagues26 evaluated the functional improvement produced by revision versus primary TKA during inpatient rehabilitation. They compared 424 consecutive primary TKAs with 138 revision TKAs. For both groups, FIM scores increased significantly (P = .015) between admission and discharge. On discharge, however, FIM scores were significantly (P = .01) higher for the primary group than the revision group (29 and 27 points, respectively). Furthermore, in the evaluation of mechanisms of failure, patients who had revision TKA for mechanical or pain-related problems did markedly better than those who had revision TKA for infection.
Compared with primary knee implants, revision implants require increasing constraint. We assume increasing constraint affects knee biomechanics, leading to worsening functional outcomes. In a study of 60 revision TKAs (57 patients) using posterior stabilized, condylar constrained, or rotating hinge prostheses, Vasso and colleagues27 examined functional outcomes at a median follow-up of 9 years (range, 4-12 years). At most recent follow-up, mean International Knee Society (IKS) Knee and Function scores were 81 (range, 48-97) and 79 (range, 56-92), mean Hospital for Special Surgery (HSS) score was 84 (range, 62-98), and mean range of motion (ROM) was 121° (range, 98°-132°) (P < .001). Although there were no significant differences in IKS and HSS scores between prosthesis types, ROM was significantly (P < .01) wider in the posterior stabilized group than in the condylar constrained and rotating hinge groups (127° vs 112° and 108°), suggesting increasing constraint resulted in decreased ROM. Several studies have found increasing constraint might lead to reduced function.28-30
However, Hwang and colleagues31 evaluated functional outcomes in 36 revision TKAs and noted that the cemented posterior stabilized (n = 8), condylar constrained (n = 25), and rotating hinge (n = 13) prostheses used did not differ in their mean Knee Society scores (78, 81, and 83, respectively).
There remains a marked disparity in patient limitations seen after revision versus primary TKA. Given the positive results being obtained with newer implants, studies might suggest recent generations of prostheses have allowed designs to be comparable. As design development continues, we may come closer to achieving outcomes comparable to those of primary TKA.
3. Patient satisfaction
Several recent reports have shown that 10% to 25% of patients who underwent primary TKA were dissatisfied with their surgery30,32; other studies have found patient satisfaction often correlating to function and pain.33-35 Given the worse outcomes for revision TKA (outlined in the preceding section), the substantial pain accompanying a second, more complex procedure, and the extensive rehabilitation expected, we suspect patients who undergo revision TKA are even less satisfied with their surgery than their primary counterparts are. (See Appendix 4 for a complete list of studies of patient satisfaction after revision TKA.)
Barrack and colleagues32 evaluated a consecutive series of 238 patients followed up for at least 1 year after revision TKA. Patients were asked to rate their degree of satisfaction with both their primary procedure and the revision and to indicate their expectations regarding their revision prosthesis. Mean satisfaction score was 7.4 (maximum = 10), with 13% of patients dissatisfied, 18% somewhat satisfied, and 69% satisfied. Seventy-four percent of patients expected their revision prosthesis to last longer than the primary prosthesis.
Greidanus and colleagues36 evaluated patient satisfaction in 60 revision TKA cases and 199 primary TKA cases at 2-year follow-up. The primary TKA group had significantly (P < .01) higher satisfaction scores in a comparison with the revision TKA group: Global (86 vs 73), Pain Relief (88 vs 70), Function (83 vs 67), and Recreation (77 vs 62). These findings support the satisfaction rates reported by Dahm and colleagues33,34: 91% for primary TKA patients and 77% for revision TKA patients.
4. Quality of life
Procedure complexity leads to reduced survivorship, function, and mobility, longer rehabilitation, and decreased QOL for revision TKA patients relative to primary TKA patients.37 (See Appendix 5 for a complete list of studies of QOL outcomes of revision TKA.)
Greidanus and colleagues36 evaluated joint-specific QOL (using the 12-item Oxford Knee Score; OKS) and generic QOL (using the 12-Item Short Form Health Survey; SF-12) in 60 revision TKA cases and 199 primary TKA cases at a mean follow-up of 2 years. (The OKS survey is used to evaluate patient perspectives on TKA outcomes,38 and the multipurpose SF-12 questionnaire is used to assess mental and physical function and general health-related QOL.39) Compared with the revision TKA group, the primary TKA group had significantly higher OKS after surgery (78 vs 68; P = .01) as well as significantly higher SF-12 scores: Global (84 vs 72; P = .01), Mental (54 vs 50; P = .03), and Physical (43 vs 37; P = .01). Similarly, Ghomrawi and colleagues40 evaluated patterns of improvement in 308 patients (318 knees) who had revision TKA. At 24-month follow-up, mean SF-36 Physical and Mental scores were 35 and 52, respectively.
Deehan and colleagues41 used the Nottingham Health Profile (NHP) to compare 94 patients’ health-related QOL scores before revision TKA with their scores 3 months, 1 year, and 5 years after revision. NHP Pain subscale scores were significantly lower 3 and 12 months after surgery than before surgery, but this difference was no longer seen at the 5-year follow-up. There was no significant improvement in scores on the other 5 NHP subscales (Sleep, Energy, Emotion, Mobility, Social Isolation) at any time points.
As shown in the literature, patients’ QOL outcomes improve after revision TKA, but these gains are not at the level of patients who undergo primary TKA.36,41 Given that revision surgery is more extensive, and that perhaps revision patients have poorer muscle function, they usually do not return to the level they attained after their index procedure.
5. Economic impact
Consistent with the outcomes already described, the economic impact of revision TKAs is excess expenditures and costs to patients and health care institutions.42 The sources of this impact are higher implant costs, extra operative trays and times, longer hospital stays, more rehabilitation, and increased medication use.43 Revision TKA costs range from $49,000 to more than $100,000—a tremendous increase over primary TKA costs ($25,000-$30,000).43-45 Furthermore, the annual economic burden associated with revision TKA, now $2.7 billion, is expected to exceed $13 billion by 2030.46 In the United States, about $23.2 billion will be spent on 926,527 primary TKAs in 2015; significantly, the costs associated with revising just 10% of these cases account for almost 50% of the total cost of the primary procedures.46
In a retrospective cost-identification multicenter cohort study, Bozic and colleagues47 found that both-component and single-component revisions, compared with primary procedures, were associated with significantly increased operative time (~265 and 221 minutes vs 200 minutes), use of allograft bone (23% and 14% vs 1%), length of stay (5.4 and 5.7 days vs 5.0 days), and percentage of patients discharged to extended-care facilities (26% and 26% vs 25%) (P < .0001). Hospital costs for both- and single-component revisions were 138% and 114% higher than costs for primary procedures (P < .0001). More recently, Kallala and colleagues44 analyzed UK National Health Service data and compared the costs of revision for infection with revision for other causes (pain, instability, aseptic loosening, fracture). Mean length of stay associated with revision for infection (21.5 days) was more than double that associated with revision for aseptic loosening (9.5 days; P < .0001), and mean cost of revision for septic causes (£30,011) was more than 3 times that of revision for other causes (£9655; P < .0001). The authors concluded that the higher costs of revision knee surgery have a considerable economic impact, especially in infection cases.
With more extensive procedures, long-stem or more constrained prostheses are often needed to obtain adequate fixation and stability. The resulting increased, substantial economic burden is felt by patients and the health care system. Given that health care reimbursements are declining, hospitals that perform revision TKAs can sustain marked financial losses. Some centers are asking whether it is cost-effective to continue to perform these types of procedures. We must find new ways to provide revision procedures using less costly implants and tools so that centers will continue to make these procedures available to patients.
Conclusion
Given the exponential growth in primary TKAs, there will be a concordant increase in revision TKAs in the decades to come. This review provides a concise overview of revision TKA outcomes. Given the low level of evidence regarding revision TKAs, we need further higher quality studies of their prostheses and outcomes. Specifically, we need systematic reviews and meta-analyses to provide higher quality evidence regarding outcomes of using individual prosthetic designs.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Crowninshield RD, Rosenberg AG, Sporer SM. Changing demographics of patients with total joint replacement. Clin Orthop Relat Res. 2006;443:266-272.
3. Ravi B, Croxford R, Reichmann WM, Losina E, Katz JN, Hawker GA. The changing demographics of total joint arthroplasty recipients in the United States and Ontario from 2001 to 2007. Best Pract Res Clin Rheumatol. 2012;26(5):637-647.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
6. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606-2612.
7. Bryan RS, Rand JA. Revision total knee arthroplasty. Clin Orthop Relat Res. 1982;170:116-122.
8. Rand JA, Bryan RS. Revision after total knee arthroplasty. Orthop Clin North Am. 1982;13(1):201-212.
9. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
10. Parvizi J, Nunley RM, Berend KR, et al. High level of residual symptoms in young patients after total knee arthroplasty. Clin Orthop Relat Res. 2014;472(1):133-137.
11. Ali A, Sundberg M, Robertsson O, et al. Dissatisfied patients after total knee arthroplasty: a registry study involving 114 patients with 8-13 years of followup. Acta Orthop. 2014;85(3):229-233.
12. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
13. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
14. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
15. Sheng PY, Konttinen L, Lehto M, et al. Revision total knee arthroplasty: 1990 through 2002. A review of the Finnish Arthroplasty Registry. J Bone Joint Surg Am. 2006;88(7):1425-1430.
16. Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79(4):499-507.
17. New Zealand Orthopaedic Association. The New Zealand Joint Registry Fourteen Year Report (January 1999 to December 2012). http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed December 16, 2015.
18. Laskin RS, Ohnsorge J. The use of standard posterior stabilized implants in revision total knee arthroplasty. Clin Orthop Relat Res. 2005;(440):122-125.
19. Meijer MF, Reininga IH, Boerboom AL, Stevens M, Bulstra SK. Poorer survival after a primary implant during revision total knee arthroplasty. Int Orthop. 2013;37(3):415-419.
20. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
21. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
22. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
23. Neumann DR, Hofstaedter T, Dorn U. Follow-up of a modular rotating hinge knee system in salvage revision total knee arthroplasty. J Arthroplasty. 2012;27(5):814-819.
24. Kowalczewski J, Marczak D, Synder M, Sibinski M. Primary rotating-hinge total knee arthroplasty: good outcomes at mid-term follow-up. J Arthroplasty. 2014;29(6):1202-1206.
25. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. Knee. 2013;20(6):367-375.
26. Vincent KR, Vincent HK, Lee LW, Alfano AP. Inpatient rehabilitation outcomes in primary and revision total knee arthroplasty patients. Clin Orthop Relat Res. 2006;(446):201-207.
27. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
28. Baier C, Luring C, Schaumburger J, et al. Assessing patient-oriented results after revision total knee arthroplasty. J Orthop Sci. 2013;18(6):955-961.
29. Hartford JM, Goodman SB, Schurman DJ, Knoblick G. Complex primary and revision total knee arthroplasty using the condylar constrained prosthesis: an average 5-year follow-up. J Arthroplasty. 1998;13(4):380-387.
30. Haidukewych GJ, Jacofsky DJ, Pagnano MW, Trousdale RT. Functional results after revision of well-fixed components for stiffness after primary total knee arthroplasty. J Arthroplasty. 2005;20(2):133-138.
31. Hwang SC, Kong JY, Nam DC, et al. Revision total knee arthroplasty with a cemented posterior stabilized, condylar constrained or fully constrained prosthesis: a minimum 2-year follow-up analysis. Clin Orthop Surg. 2010;2(2):112-120.
32. Barrack RL, McClure JT, Burak CF, Clohisy JC, Parvizi J, Sharkey P. Revision total knee arthroplasty: the patient’s perspective. Clin Orthop Relat Res. 2007;464:146-150.
33. Dahm DL, Barnes SA, Harrington JR, Berry DJ. Patient reported activity after revision total knee arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):106-110.
34. Dahm DL, Barnes SA, Harrington JR, Sayeed SA, Berry DJ. Patient-reported activity level after total knee arthroplasty. J Arthroplasty. 2008;23(3):401-407.
35. Richards CJ, Garbuz DS, Pugh L, Masri BA. Revision total knee arthroplasty: clinical outcome comparison with and without the use of femoral head structural allograft. J Arthroplasty. 2011;26(8):1299-1304.
36. Greidanus NV, Peterson RC, Masri BA, Garbuz DS. Quality of life outcomes in revision versus primary total knee arthroplasty. J Arthroplasty. 2011;26(4):615-620.
37. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
38. Murray DW, Fitzpatrick R, Rogers K, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br. 2007;89(8):1010-1014.
39. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
40. Ghomrawi HM, Kane RL, Eberly LE, Bershadsky B, Saleh KJ; North American Knee Arthroplasty Revision Study Group. Patterns of functional improvement after revision knee arthroplasty. J Bone Joint Surg Am. 2009;91(12):2838-2845.
41. Deehan DJ, Murray JD, Birdsall PD, Pinder IM. Quality of life after knee revision arthroplasty. Acta Orthop. 2006;77(5):761-766.
42. Kapadia BH, McElroy MJ, Issa K, Johnson AJ, Bozic KJ, Mont MA. The economic impact of periprosthetic infections following total knee arthroplasty at a specialized tertiary-care center. J Arthroplasty. 2014;29(5):929-932.
43. Bhandari M, Smith J, Miller LE, Block JE. Clinical and economic burden of revision knee arthroplasty. Clin Med Insights Arthritis Musculoskelet Disord. 2012;5:89-94.
44. Kallala RF, Vanhegan IS, Ibrahim MS, Sarmah S, Haddad FS. Financial analysis of revision knee surgery based on NHS tariffs and hospital costs: does it pay to provide a revision service? Bone Joint J Br. 2015;97(2):197-201.
45. Ong KL, Mowat FS, Chan N, Lau E, Halpern MT, Kurtz SM. Economic burden of revision hip and knee arthroplasty in Medicare enrollees. Clin Orthop Relat Res. 2006;446:22-28.
46. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
47. Bozic KJ, Durbhakula S, Berry DJ, et al. Differences in patient and procedure characteristics and hospital resource use in primary and revision total joint arthroplasty: a multicenter study. J Arthroplasty. 2005;20(7 suppl 3):17-25.
48. Lee KJ, Moon JY, Song EK, Lim HA, Seon JK. Minimum Two-year Results of Revision Total Knee Arthroplasty Following Infectious or Non-infectious Causes. Knee Surg Relat Res. 2012;24(4):227-234.
49. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
50. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
51. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
52. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
53. Mabry TM, Vessely MB, Schleck CD, Harmsen WS, Berry DJ. Revision total knee arthroplasty with modular cemented stems: long-term follow-up. J Arthroplasty. 2007;22(6 Suppl 2):100-105.
54. Gudnason A, Milbrink J, Hailer NP. Implant survival and outcome after rotating-hinge total knee revision arthroplasty: a minimum 6-year follow-up. Arch Orthop Trauma Surg. 2011;131(11):1601-1607.
55. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125-131.
56. Greene JW, Reynolds SM, Stimac JD, Malkani AL, Massini MA. Midterm results of hybrid cement technique in revision total knee arthroplasty. J Arthroplasty. 2013;28(4):570-574.
57. Dalury DF, Adams MJ. Minimum 6-year follow-up of revision total knee arthroplasty without patella reimplantation. Journal Arthroplasty. 2012;27(8 Suppl):91-94.
58. Whaley AL, Trousdale RT, Rand JA, Hanssen AD. Cemented long-stem revision total knee arthroplasty. J Arthroplasty. 2003;18(5):592-599.
59. Friedman RJ, Hirst P, Poss R, Kelley K, Sledge CB. Results of revision total knee arthroplasty performed for aseptic loosening. Clinical Orthop Relat Res. 1990;255:235-241.
60. Barrack RL, Rorabeck C, Partington P, Sawhney J, Engh G. The results of retaining a well-fixed patellar component in revision total knee arthroplasty. J Arthroplasty. 2000;15(4):413-417.
61. Christensen CP, Crawford JJ, Olin MD, Vail TP. Revision of the stiff total knee arthroplasty. J Arthroplasty. 2002;17(4):409-415.
62. Garcia RM, Hardy BT, Kraay MJ, Goldberg VM. Revision total knee arthroplasty for aseptic and septic causes in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2010;468(1):82-89.
63. Patil N, Lee K, Huddleston JI, Harris AH, Goodman SB. Aseptic versus septic revision total knee arthroplasty: patient satisfaction, outcome and quality of life improvement. Knee. 2010;17(3):200-203.
64. Luque R, Rizo B, Urda A, et al. Predictive factors for failure after total knee replacement revision. Int Orthop. 2014;38(2):429-435.
65. Bistolfi A, Massazza G, Rosso F, Crova M. Rotating-hinge total knee for revision total knee arthroplasty. Orthopedics. 2012;35(3):e325-e330.
66. Bottner F, Laskin R, Windsor RE, Haas SB. Hybrid component fixation in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:127-131.
67. Jensen CL, Winther N, Schroder HM, Petersen MM. Outcome of revision total knee arthroplasty with the use of trabecular metal cone for reconstruction of severe bone loss at the proximal tibia. Knee. 2014;21(6):1233-1237.
68. Howard JL, Kudera J, Lewallen DG, Hanssen AD. Early results of the use of tantalum femoral cones for revision total knee arthroplasty. J Bone Joint Surg Am. 2011;93(5):478-484.
69. Yang JH, Yoon JR, Oh CH, Kim TS. Hybrid component fixation in total knee arthroplasty: minimum of 10-year follow-up study. J Arthroplasty. 2012;27(6):1111-1118.
70. Peters CL, Erickson JA, Gililland JM. Clinical and radiographic results of 184 consecutive revision total knee arthroplasties placed with modular cementless stems. J Arthroplasty. 2009;24(6 Suppl):48-53.
71. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2014. 2014.
72. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2013. 2013.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Crowninshield RD, Rosenberg AG, Sporer SM. Changing demographics of patients with total joint replacement. Clin Orthop Relat Res. 2006;443:266-272.
3. Ravi B, Croxford R, Reichmann WM, Losina E, Katz JN, Hawker GA. The changing demographics of total joint arthroplasty recipients in the United States and Ontario from 2001 to 2007. Best Pract Res Clin Rheumatol. 2012;26(5):637-647.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
6. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606-2612.
7. Bryan RS, Rand JA. Revision total knee arthroplasty. Clin Orthop Relat Res. 1982;170:116-122.
8. Rand JA, Bryan RS. Revision after total knee arthroplasty. Orthop Clin North Am. 1982;13(1):201-212.
9. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
10. Parvizi J, Nunley RM, Berend KR, et al. High level of residual symptoms in young patients after total knee arthroplasty. Clin Orthop Relat Res. 2014;472(1):133-137.
11. Ali A, Sundberg M, Robertsson O, et al. Dissatisfied patients after total knee arthroplasty: a registry study involving 114 patients with 8-13 years of followup. Acta Orthop. 2014;85(3):229-233.
12. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
13. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
14. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
15. Sheng PY, Konttinen L, Lehto M, et al. Revision total knee arthroplasty: 1990 through 2002. A review of the Finnish Arthroplasty Registry. J Bone Joint Surg Am. 2006;88(7):1425-1430.
16. Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79(4):499-507.
17. New Zealand Orthopaedic Association. The New Zealand Joint Registry Fourteen Year Report (January 1999 to December 2012). http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed December 16, 2015.
18. Laskin RS, Ohnsorge J. The use of standard posterior stabilized implants in revision total knee arthroplasty. Clin Orthop Relat Res. 2005;(440):122-125.
19. Meijer MF, Reininga IH, Boerboom AL, Stevens M, Bulstra SK. Poorer survival after a primary implant during revision total knee arthroplasty. Int Orthop. 2013;37(3):415-419.
20. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
21. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
22. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
23. Neumann DR, Hofstaedter T, Dorn U. Follow-up of a modular rotating hinge knee system in salvage revision total knee arthroplasty. J Arthroplasty. 2012;27(5):814-819.
24. Kowalczewski J, Marczak D, Synder M, Sibinski M. Primary rotating-hinge total knee arthroplasty: good outcomes at mid-term follow-up. J Arthroplasty. 2014;29(6):1202-1206.
25. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. Knee. 2013;20(6):367-375.
26. Vincent KR, Vincent HK, Lee LW, Alfano AP. Inpatient rehabilitation outcomes in primary and revision total knee arthroplasty patients. Clin Orthop Relat Res. 2006;(446):201-207.
27. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
28. Baier C, Luring C, Schaumburger J, et al. Assessing patient-oriented results after revision total knee arthroplasty. J Orthop Sci. 2013;18(6):955-961.
29. Hartford JM, Goodman SB, Schurman DJ, Knoblick G. Complex primary and revision total knee arthroplasty using the condylar constrained prosthesis: an average 5-year follow-up. J Arthroplasty. 1998;13(4):380-387.
30. Haidukewych GJ, Jacofsky DJ, Pagnano MW, Trousdale RT. Functional results after revision of well-fixed components for stiffness after primary total knee arthroplasty. J Arthroplasty. 2005;20(2):133-138.
31. Hwang SC, Kong JY, Nam DC, et al. Revision total knee arthroplasty with a cemented posterior stabilized, condylar constrained or fully constrained prosthesis: a minimum 2-year follow-up analysis. Clin Orthop Surg. 2010;2(2):112-120.
32. Barrack RL, McClure JT, Burak CF, Clohisy JC, Parvizi J, Sharkey P. Revision total knee arthroplasty: the patient’s perspective. Clin Orthop Relat Res. 2007;464:146-150.
33. Dahm DL, Barnes SA, Harrington JR, Berry DJ. Patient reported activity after revision total knee arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):106-110.
34. Dahm DL, Barnes SA, Harrington JR, Sayeed SA, Berry DJ. Patient-reported activity level after total knee arthroplasty. J Arthroplasty. 2008;23(3):401-407.
35. Richards CJ, Garbuz DS, Pugh L, Masri BA. Revision total knee arthroplasty: clinical outcome comparison with and without the use of femoral head structural allograft. J Arthroplasty. 2011;26(8):1299-1304.
36. Greidanus NV, Peterson RC, Masri BA, Garbuz DS. Quality of life outcomes in revision versus primary total knee arthroplasty. J Arthroplasty. 2011;26(4):615-620.
37. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
38. Murray DW, Fitzpatrick R, Rogers K, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br. 2007;89(8):1010-1014.
39. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
40. Ghomrawi HM, Kane RL, Eberly LE, Bershadsky B, Saleh KJ; North American Knee Arthroplasty Revision Study Group. Patterns of functional improvement after revision knee arthroplasty. J Bone Joint Surg Am. 2009;91(12):2838-2845.
41. Deehan DJ, Murray JD, Birdsall PD, Pinder IM. Quality of life after knee revision arthroplasty. Acta Orthop. 2006;77(5):761-766.
42. Kapadia BH, McElroy MJ, Issa K, Johnson AJ, Bozic KJ, Mont MA. The economic impact of periprosthetic infections following total knee arthroplasty at a specialized tertiary-care center. J Arthroplasty. 2014;29(5):929-932.
43. Bhandari M, Smith J, Miller LE, Block JE. Clinical and economic burden of revision knee arthroplasty. Clin Med Insights Arthritis Musculoskelet Disord. 2012;5:89-94.
44. Kallala RF, Vanhegan IS, Ibrahim MS, Sarmah S, Haddad FS. Financial analysis of revision knee surgery based on NHS tariffs and hospital costs: does it pay to provide a revision service? Bone Joint J Br. 2015;97(2):197-201.
45. Ong KL, Mowat FS, Chan N, Lau E, Halpern MT, Kurtz SM. Economic burden of revision hip and knee arthroplasty in Medicare enrollees. Clin Orthop Relat Res. 2006;446:22-28.
46. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
47. Bozic KJ, Durbhakula S, Berry DJ, et al. Differences in patient and procedure characteristics and hospital resource use in primary and revision total joint arthroplasty: a multicenter study. J Arthroplasty. 2005;20(7 suppl 3):17-25.
48. Lee KJ, Moon JY, Song EK, Lim HA, Seon JK. Minimum Two-year Results of Revision Total Knee Arthroplasty Following Infectious or Non-infectious Causes. Knee Surg Relat Res. 2012;24(4):227-234.
49. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
50. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
51. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
52. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
53. Mabry TM, Vessely MB, Schleck CD, Harmsen WS, Berry DJ. Revision total knee arthroplasty with modular cemented stems: long-term follow-up. J Arthroplasty. 2007;22(6 Suppl 2):100-105.
54. Gudnason A, Milbrink J, Hailer NP. Implant survival and outcome after rotating-hinge total knee revision arthroplasty: a minimum 6-year follow-up. Arch Orthop Trauma Surg. 2011;131(11):1601-1607.
55. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125-131.
56. Greene JW, Reynolds SM, Stimac JD, Malkani AL, Massini MA. Midterm results of hybrid cement technique in revision total knee arthroplasty. J Arthroplasty. 2013;28(4):570-574.
57. Dalury DF, Adams MJ. Minimum 6-year follow-up of revision total knee arthroplasty without patella reimplantation. Journal Arthroplasty. 2012;27(8 Suppl):91-94.
58. Whaley AL, Trousdale RT, Rand JA, Hanssen AD. Cemented long-stem revision total knee arthroplasty. J Arthroplasty. 2003;18(5):592-599.
59. Friedman RJ, Hirst P, Poss R, Kelley K, Sledge CB. Results of revision total knee arthroplasty performed for aseptic loosening. Clinical Orthop Relat Res. 1990;255:235-241.
60. Barrack RL, Rorabeck C, Partington P, Sawhney J, Engh G. The results of retaining a well-fixed patellar component in revision total knee arthroplasty. J Arthroplasty. 2000;15(4):413-417.
61. Christensen CP, Crawford JJ, Olin MD, Vail TP. Revision of the stiff total knee arthroplasty. J Arthroplasty. 2002;17(4):409-415.
62. Garcia RM, Hardy BT, Kraay MJ, Goldberg VM. Revision total knee arthroplasty for aseptic and septic causes in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2010;468(1):82-89.
63. Patil N, Lee K, Huddleston JI, Harris AH, Goodman SB. Aseptic versus septic revision total knee arthroplasty: patient satisfaction, outcome and quality of life improvement. Knee. 2010;17(3):200-203.
64. Luque R, Rizo B, Urda A, et al. Predictive factors for failure after total knee replacement revision. Int Orthop. 2014;38(2):429-435.
65. Bistolfi A, Massazza G, Rosso F, Crova M. Rotating-hinge total knee for revision total knee arthroplasty. Orthopedics. 2012;35(3):e325-e330.
66. Bottner F, Laskin R, Windsor RE, Haas SB. Hybrid component fixation in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:127-131.
67. Jensen CL, Winther N, Schroder HM, Petersen MM. Outcome of revision total knee arthroplasty with the use of trabecular metal cone for reconstruction of severe bone loss at the proximal tibia. Knee. 2014;21(6):1233-1237.
68. Howard JL, Kudera J, Lewallen DG, Hanssen AD. Early results of the use of tantalum femoral cones for revision total knee arthroplasty. J Bone Joint Surg Am. 2011;93(5):478-484.
69. Yang JH, Yoon JR, Oh CH, Kim TS. Hybrid component fixation in total knee arthroplasty: minimum of 10-year follow-up study. J Arthroplasty. 2012;27(6):1111-1118.
70. Peters CL, Erickson JA, Gililland JM. Clinical and radiographic results of 184 consecutive revision total knee arthroplasties placed with modular cementless stems. J Arthroplasty. 2009;24(6 Suppl):48-53.
71. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2014. 2014.
72. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2013. 2013.
Implant Designs in Revision Total Knee Arthroplasty
Before 1990, a considerable number of revisions were performed, largely for implant-associated failures, in the first few years after index primary knee arthroplasties.1,2 Since then, surgeons, manufacturers, and hospitals have collaborated to improve implant designs, techniques, and care guidelines.3,4 Despite the substantial improvements in designs, which led to implant longevity of more than 15 years in many cases, these devices still have limited life spans. Large studies have estimated that the risk for revision required after primary knee arthroplasty ranges from as low as 5% at 15 years to up to 9% at 10 years.4,5
The surgical goals of revision total knee arthroplasty (TKA) are to obtain stable fixation of the prosthesis to host bone, to obtain a stable range of motion compatible with the patient’s activities of daily living, and to achieve these goals while using the smallest amount of prosthetic augments and constraint so that the soft tissues may share in load transfer.6 As prosthetic constraint increases, the soft tissues participate less in load sharing, and increasing stresses are put on the implant–bone interface, which further increases the risk for early implant loosening.7 Hence, as characteristics of a revision implant become more constrained, there is often a higher rate of aseptic loosening expected.8
Controversy remains regarding the ideal implant type for revision TKA. To ensure the success of revision surgery and to reduce the risks for postoperative dissatisfaction, complications, and re-revision, orthopedists must understand the types of revision implant designs available, particularly as each has its own indications and potential complications.
In this article, we review the classification systems used for revision TKA as well as the types of prosthetic designs that can be used: posterior stabilized, nonlinked constrained, rotating hinge, and modular segmental.
1. Classification of bone loss and soft-tissue integrity
To further understand revision TKA, we must consider the complexity level of these cases, particularly by evaluating degree of bone loss and soft-tissue deficiency. The most accepted way to assess bone loss both before and during surgery is to use the AORI (Anderson Orthopaedic Research Institute) classification system.9 Bone loss can be classified into 3 types: I, in which metaphyseal bone is intact and small bone defects do not compromise component stability; II, in which metaphyseal bone is damaged and cancellous bone loss requires cement fill, augments, or bone graft; and III, in which metaphyseal bone is deficient, and lost bone comprises a major portion of condyle or plateau and occasionally requires bone grafts or custom implants (Table 1). These patterns of bone loss are occasionally associated with detachment of the collateral ligament or patellar tendon.
In addition to understanding bone loss in revision TKA, surgeons must be aware of soft-tissue deficiencies (eg, collateral ligaments, extensor mechanism), which also influence type and amount of prosthesis constraint. Specifically, constraint choice depends on amount of bone loss and on the condition of stabilizing tissues, such as the collateral ligaments. Under conditions of minimal bone loss and intact peripheral ligaments, a less constrained device, such as a primary posterior stabilized system, can be considered. When ligaments are present but insufficient, a semiconstrained device is recommended. In the presence of medial collateral ligament attenuation or complete medial or lateral collateral ligament dysfunction, a fully constrained prosthesis is required.8 Therefore, amount of bone loss or soft-tissue deficiency often dictates which prosthesis to use.
For radiographic classification, the Knee Society roentgenographic evaluation and scoring system10 has been implemented to allow for uniform reporting of radiographic results and to ensure adequate preoperative planning and postoperative assessment of component alignment. This system incorporates the evaluation of alignment in the coronal, sagittal, and patellofemoral planes and assesses radiolucency using zones dividing the implant–bone interface into segments to allow for easier classification of areas of lucency. More recently, a modified version of the Knee Society system was constructed.11 This modification simplifies zone classifications and accommodates more complex revision knee designs and stem extensions.
2. Posterior stabilized designs
Cruciate-retaining prostheses are seldom applicable in the revision TKA setting because of frequent damage to the posterior cruciate ligament, except in the case of simple polyethylene exchanges or, potentially, revisions of failed unicompartmental TKAs. Thus, posterior stabilized designs are the first-line choice for revision TKA (Figure 1). These prostheses are indicated only when the posterior cruciate ligament is incompetent and in the setting of adequate flexion and extension and medial and lateral collateral ligament balancing.
However, studies have shown that posterior stabilized TKAs have a limited role in revision TKAs, as the amount of ligamentous and bony damage is often underestimated in these patients, and use of a primary implant in a revision setting often requires additional augments, all of which may have contributed to the high failure rate. Thus, this design should be used only when the patient has adequate bone stock (AORI type I) and collateral ligament tension. This situation further emphasizes the importance of performing intraoperative testing for ligamentous balance and bone deficit evaluation in order to determine the most appropriate implant (Table 2).
3. Nonlinked constrained designs
Nonlinked constrained (condylar constrained) designs are the devices most commonly used for revision TKAs (>50% of revision knees). These prostheses provide increased articular constraint, which is required in patients with persistent instability, despite appropriate soft-tissue balancing. Increased articular constraint allows for more knee stability by providing progressive varus-valgus, coronal, and rotational stability with the aid of taller and wider tibial posts.12 Specifically, these implants incorporate a tibial post that fits closely between the femoral condyles, allowing for less motion compared with a standard posterior stabilized design.12
In addition, these designs may be used with augments, stems, and allografts when bone loss is more substantial. In particular, stem extensions allow for load distribution to the diaphyseal regions of the tibia and femur and thereby aid in reducing the increased stress at the bone–implant interface, which is a common concern with these implants. However, these extensions cost more, require intramedullary invasion, and are associated with higher rates of leg and thigh pain.12
These prostheses are often implicated in cases involving a high degree of bone loss (eg, AORI type II or III). They are ideally used in cases in which complete revision of both tibial and femoral components is needed and are indicated in cases of incompetent posterior cruciate ligament, partial functional loss of medial or lateral collateral ligaments, or flexion-extension mismatch.13 Furthermore, use of a constrained prosthesis is recommended in the setting of varus or valgus instability, or repeated dislocations of a posterior stabilized design (Table 2).
Ten-year survivorship ranges from 85% to 96%, but this is substantially lower than the 95% to 96% for condylar constrained prostheses used in primary TKAs.14-17 Moreover, the large discrepancy between survivorship of primary TKA and revision TKA with a constrained prosthesis further affirms that the complexity of revision surgery, rather than the prosthesis used, may have more deleterious effects on outcomes. However, surgeons must be aware that increased constraint leads to increased stress on the prosthetic interfaces with associated aseptic loosening and early failure, and this continues to be a legitimate concern.
4. Rotating hinge designs
Many patients who undergo revision TKA can be managed with a posterior stabilizing or nonlinked constrained design. However, in patients who present with severe ligamentous instability and bone loss (AORI type II or III), a rotating hinge prostheses, or highly constrained device, is often recommended (Figure 2).18 By using a rotating mobile-bearing platform, this prosthesis permits axial rotation through a metal-reinforced polyethylene-post articulation in the tibial tray. In addition, it involves use of modular diaphyseal-engaging stems and diaphyseal sleeves, which allow for the bypass of bony defects and areas of bone loss (Table 2).
However, the rigid biomechanics of hinged prostheses is associated with increased risk for aseptic loosening (aseptic 10-year survival, 60%-80%), imparted by the transfer of stresses across the bone. The higher risk for early loosening, osteolysis, and excessive wear—caused by the highly restricted biomechanics of early generations of fixed hinged designs—has led to the development of new devices with mobile mechanics. Prosthetic designs have been improved with an added rotational axis to reduce torsional stress, a patellar resurfacing option, and better stem fixation and patellofemoral kinematics. Overall, these are aimed to improve rates of instability and aseptic loosening, with promising results demonstrated in the literature.
5. Modular segmental arthroplasty designs
Segmental arthroplasty prostheses, which typically are end-of-the-line revision TKA options, are applicable only in cases of extensive bone loss (more than can be treated with allografts or augments; AORI type 3), complete ligamentous disruption/absence, loss of periprosthetic soft tissue, and multiple previous revision procedures (Figure 3). Despite the limited indications for these prostheses, they yield quick return to function without graft nonunion or resorption, and they augment ingrowth/ongrowth. Furthermore, the next surgical option could be fusion or amputation. When failures were specifically evaluated for aseptic loosening across 4 studies, the survival rate ranged from 83% to 99.5%, with the most frequent complication being infection (up to 33% in one series).6,19-21
The major roles for segmental arthroplasty prostheses in primary TKAs are in the setting of oncologic conditions that require bony excision, or unreconstuctable fractures about the knee. Used after ancillary metastatic disease, these prostheses demonstrate positive results, according to several reports.22,23 In the setting of revision TKA, however, these prostheses should be used only when other surgical options are unfeasible, given the high risk for infection and the re-revision rates. Currently, revision TKAs with tumor prostheses have a high failure rate (up to 50%) because of the extensive surgery and the lack of bony and soft-tissue support (Table 2).
Conclusion
Orthopedists performing revision TKAs must consider bone stock and remaining ligament stability. In particular, they should choose implants for least constraint and adequate knee stability, as these are essential in minimizing the stresses on the implant–bone interface. Ultimately, functional outcomes, survivorship, and postoperative satisfaction determine the success of these designs. However, predictors of outcomes of revision surgery are often multifactorial, and surgeons must also consider procedure complexity and patient-specific characteristics.
1. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
2. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28(8 suppl):116-119.
4. Kim TK. CORR Insights(®): risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1208-1209.
5. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
6. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
7. Dennis DA. A stepwise approach to revision total knee arthroplasty. J Arthroplasty. 2007;22(4 suppl 1):32-38.
8. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
9. Engh GA, Ammeen DJ. Bone loss with revision total knee arthroplasty: defect classification and alternatives for reconstruction. Instr Course Lect. 1999;48:167-175.
10. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
11. Meneghini RM, Mont MA, Backstein DB, Bourne RB, Dennis DA, Scuderi GR. Development of a modern Knee Society radiographic evaluation system and methodology for total knee arthroplasty. J Arthroplasty. 2015;30(12):2311-2314.
12. Nam D, Umunna BP, Cross MB, Reinhardt KR, Duggal S, Cornell CN. Clinical results and failure mechanisms of a nonmodular constrained knee without stem extensions. HSS J. 2012;8(2):96-102.
13. Lombardi AV Jr, Berend KR. The role of implant constraint in revision TKA: striking the balance. Orthopedics. 2006;29(9):847-849.
14. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
15. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
16. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
17. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
18. Jones RE. Total knee arthroplasty with modular rotating-platform hinge. Orthopedics. 2006;29(9 suppl):S80-S82.
19. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. The Knee. 2013;20:367-375.
20. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;(430):125-131.
21. Peters CL, Erickson J, Kloepper RG, Mohr RA. Revision total knee arthroplasty with modular components inserted with metaphyseal cement and stems without cement. J Arthroplasty. 2005;20:302-308.
22. Pala E, Trovarelli G, Calabro T, Angelini A, Abati CN, Ruggieri P. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clinical Orthop Relat Res. 2015;473:891-899.
23. Angelini A, Henderson E, Trovarelli G, Ruggieri P. Is there a role for knee arthrodesis with modular endoprostheses for tumor and revision of failed endoprostheses? Clin Orthop Relat Res. 2013;471(10):3326-3335.
Before 1990, a considerable number of revisions were performed, largely for implant-associated failures, in the first few years after index primary knee arthroplasties.1,2 Since then, surgeons, manufacturers, and hospitals have collaborated to improve implant designs, techniques, and care guidelines.3,4 Despite the substantial improvements in designs, which led to implant longevity of more than 15 years in many cases, these devices still have limited life spans. Large studies have estimated that the risk for revision required after primary knee arthroplasty ranges from as low as 5% at 15 years to up to 9% at 10 years.4,5
The surgical goals of revision total knee arthroplasty (TKA) are to obtain stable fixation of the prosthesis to host bone, to obtain a stable range of motion compatible with the patient’s activities of daily living, and to achieve these goals while using the smallest amount of prosthetic augments and constraint so that the soft tissues may share in load transfer.6 As prosthetic constraint increases, the soft tissues participate less in load sharing, and increasing stresses are put on the implant–bone interface, which further increases the risk for early implant loosening.7 Hence, as characteristics of a revision implant become more constrained, there is often a higher rate of aseptic loosening expected.8
Controversy remains regarding the ideal implant type for revision TKA. To ensure the success of revision surgery and to reduce the risks for postoperative dissatisfaction, complications, and re-revision, orthopedists must understand the types of revision implant designs available, particularly as each has its own indications and potential complications.
In this article, we review the classification systems used for revision TKA as well as the types of prosthetic designs that can be used: posterior stabilized, nonlinked constrained, rotating hinge, and modular segmental.
1. Classification of bone loss and soft-tissue integrity
To further understand revision TKA, we must consider the complexity level of these cases, particularly by evaluating degree of bone loss and soft-tissue deficiency. The most accepted way to assess bone loss both before and during surgery is to use the AORI (Anderson Orthopaedic Research Institute) classification system.9 Bone loss can be classified into 3 types: I, in which metaphyseal bone is intact and small bone defects do not compromise component stability; II, in which metaphyseal bone is damaged and cancellous bone loss requires cement fill, augments, or bone graft; and III, in which metaphyseal bone is deficient, and lost bone comprises a major portion of condyle or plateau and occasionally requires bone grafts or custom implants (Table 1). These patterns of bone loss are occasionally associated with detachment of the collateral ligament or patellar tendon.
In addition to understanding bone loss in revision TKA, surgeons must be aware of soft-tissue deficiencies (eg, collateral ligaments, extensor mechanism), which also influence type and amount of prosthesis constraint. Specifically, constraint choice depends on amount of bone loss and on the condition of stabilizing tissues, such as the collateral ligaments. Under conditions of minimal bone loss and intact peripheral ligaments, a less constrained device, such as a primary posterior stabilized system, can be considered. When ligaments are present but insufficient, a semiconstrained device is recommended. In the presence of medial collateral ligament attenuation or complete medial or lateral collateral ligament dysfunction, a fully constrained prosthesis is required.8 Therefore, amount of bone loss or soft-tissue deficiency often dictates which prosthesis to use.
For radiographic classification, the Knee Society roentgenographic evaluation and scoring system10 has been implemented to allow for uniform reporting of radiographic results and to ensure adequate preoperative planning and postoperative assessment of component alignment. This system incorporates the evaluation of alignment in the coronal, sagittal, and patellofemoral planes and assesses radiolucency using zones dividing the implant–bone interface into segments to allow for easier classification of areas of lucency. More recently, a modified version of the Knee Society system was constructed.11 This modification simplifies zone classifications and accommodates more complex revision knee designs and stem extensions.
2. Posterior stabilized designs
Cruciate-retaining prostheses are seldom applicable in the revision TKA setting because of frequent damage to the posterior cruciate ligament, except in the case of simple polyethylene exchanges or, potentially, revisions of failed unicompartmental TKAs. Thus, posterior stabilized designs are the first-line choice for revision TKA (Figure 1). These prostheses are indicated only when the posterior cruciate ligament is incompetent and in the setting of adequate flexion and extension and medial and lateral collateral ligament balancing.
However, studies have shown that posterior stabilized TKAs have a limited role in revision TKAs, as the amount of ligamentous and bony damage is often underestimated in these patients, and use of a primary implant in a revision setting often requires additional augments, all of which may have contributed to the high failure rate. Thus, this design should be used only when the patient has adequate bone stock (AORI type I) and collateral ligament tension. This situation further emphasizes the importance of performing intraoperative testing for ligamentous balance and bone deficit evaluation in order to determine the most appropriate implant (Table 2).
3. Nonlinked constrained designs
Nonlinked constrained (condylar constrained) designs are the devices most commonly used for revision TKAs (>50% of revision knees). These prostheses provide increased articular constraint, which is required in patients with persistent instability, despite appropriate soft-tissue balancing. Increased articular constraint allows for more knee stability by providing progressive varus-valgus, coronal, and rotational stability with the aid of taller and wider tibial posts.12 Specifically, these implants incorporate a tibial post that fits closely between the femoral condyles, allowing for less motion compared with a standard posterior stabilized design.12
In addition, these designs may be used with augments, stems, and allografts when bone loss is more substantial. In particular, stem extensions allow for load distribution to the diaphyseal regions of the tibia and femur and thereby aid in reducing the increased stress at the bone–implant interface, which is a common concern with these implants. However, these extensions cost more, require intramedullary invasion, and are associated with higher rates of leg and thigh pain.12
These prostheses are often implicated in cases involving a high degree of bone loss (eg, AORI type II or III). They are ideally used in cases in which complete revision of both tibial and femoral components is needed and are indicated in cases of incompetent posterior cruciate ligament, partial functional loss of medial or lateral collateral ligaments, or flexion-extension mismatch.13 Furthermore, use of a constrained prosthesis is recommended in the setting of varus or valgus instability, or repeated dislocations of a posterior stabilized design (Table 2).
Ten-year survivorship ranges from 85% to 96%, but this is substantially lower than the 95% to 96% for condylar constrained prostheses used in primary TKAs.14-17 Moreover, the large discrepancy between survivorship of primary TKA and revision TKA with a constrained prosthesis further affirms that the complexity of revision surgery, rather than the prosthesis used, may have more deleterious effects on outcomes. However, surgeons must be aware that increased constraint leads to increased stress on the prosthetic interfaces with associated aseptic loosening and early failure, and this continues to be a legitimate concern.
4. Rotating hinge designs
Many patients who undergo revision TKA can be managed with a posterior stabilizing or nonlinked constrained design. However, in patients who present with severe ligamentous instability and bone loss (AORI type II or III), a rotating hinge prostheses, or highly constrained device, is often recommended (Figure 2).18 By using a rotating mobile-bearing platform, this prosthesis permits axial rotation through a metal-reinforced polyethylene-post articulation in the tibial tray. In addition, it involves use of modular diaphyseal-engaging stems and diaphyseal sleeves, which allow for the bypass of bony defects and areas of bone loss (Table 2).
However, the rigid biomechanics of hinged prostheses is associated with increased risk for aseptic loosening (aseptic 10-year survival, 60%-80%), imparted by the transfer of stresses across the bone. The higher risk for early loosening, osteolysis, and excessive wear—caused by the highly restricted biomechanics of early generations of fixed hinged designs—has led to the development of new devices with mobile mechanics. Prosthetic designs have been improved with an added rotational axis to reduce torsional stress, a patellar resurfacing option, and better stem fixation and patellofemoral kinematics. Overall, these are aimed to improve rates of instability and aseptic loosening, with promising results demonstrated in the literature.
5. Modular segmental arthroplasty designs
Segmental arthroplasty prostheses, which typically are end-of-the-line revision TKA options, are applicable only in cases of extensive bone loss (more than can be treated with allografts or augments; AORI type 3), complete ligamentous disruption/absence, loss of periprosthetic soft tissue, and multiple previous revision procedures (Figure 3). Despite the limited indications for these prostheses, they yield quick return to function without graft nonunion or resorption, and they augment ingrowth/ongrowth. Furthermore, the next surgical option could be fusion or amputation. When failures were specifically evaluated for aseptic loosening across 4 studies, the survival rate ranged from 83% to 99.5%, with the most frequent complication being infection (up to 33% in one series).6,19-21
The major roles for segmental arthroplasty prostheses in primary TKAs are in the setting of oncologic conditions that require bony excision, or unreconstuctable fractures about the knee. Used after ancillary metastatic disease, these prostheses demonstrate positive results, according to several reports.22,23 In the setting of revision TKA, however, these prostheses should be used only when other surgical options are unfeasible, given the high risk for infection and the re-revision rates. Currently, revision TKAs with tumor prostheses have a high failure rate (up to 50%) because of the extensive surgery and the lack of bony and soft-tissue support (Table 2).
Conclusion
Orthopedists performing revision TKAs must consider bone stock and remaining ligament stability. In particular, they should choose implants for least constraint and adequate knee stability, as these are essential in minimizing the stresses on the implant–bone interface. Ultimately, functional outcomes, survivorship, and postoperative satisfaction determine the success of these designs. However, predictors of outcomes of revision surgery are often multifactorial, and surgeons must also consider procedure complexity and patient-specific characteristics.
Before 1990, a considerable number of revisions were performed, largely for implant-associated failures, in the first few years after index primary knee arthroplasties.1,2 Since then, surgeons, manufacturers, and hospitals have collaborated to improve implant designs, techniques, and care guidelines.3,4 Despite the substantial improvements in designs, which led to implant longevity of more than 15 years in many cases, these devices still have limited life spans. Large studies have estimated that the risk for revision required after primary knee arthroplasty ranges from as low as 5% at 15 years to up to 9% at 10 years.4,5
The surgical goals of revision total knee arthroplasty (TKA) are to obtain stable fixation of the prosthesis to host bone, to obtain a stable range of motion compatible with the patient’s activities of daily living, and to achieve these goals while using the smallest amount of prosthetic augments and constraint so that the soft tissues may share in load transfer.6 As prosthetic constraint increases, the soft tissues participate less in load sharing, and increasing stresses are put on the implant–bone interface, which further increases the risk for early implant loosening.7 Hence, as characteristics of a revision implant become more constrained, there is often a higher rate of aseptic loosening expected.8
Controversy remains regarding the ideal implant type for revision TKA. To ensure the success of revision surgery and to reduce the risks for postoperative dissatisfaction, complications, and re-revision, orthopedists must understand the types of revision implant designs available, particularly as each has its own indications and potential complications.
In this article, we review the classification systems used for revision TKA as well as the types of prosthetic designs that can be used: posterior stabilized, nonlinked constrained, rotating hinge, and modular segmental.
1. Classification of bone loss and soft-tissue integrity
To further understand revision TKA, we must consider the complexity level of these cases, particularly by evaluating degree of bone loss and soft-tissue deficiency. The most accepted way to assess bone loss both before and during surgery is to use the AORI (Anderson Orthopaedic Research Institute) classification system.9 Bone loss can be classified into 3 types: I, in which metaphyseal bone is intact and small bone defects do not compromise component stability; II, in which metaphyseal bone is damaged and cancellous bone loss requires cement fill, augments, or bone graft; and III, in which metaphyseal bone is deficient, and lost bone comprises a major portion of condyle or plateau and occasionally requires bone grafts or custom implants (Table 1). These patterns of bone loss are occasionally associated with detachment of the collateral ligament or patellar tendon.
In addition to understanding bone loss in revision TKA, surgeons must be aware of soft-tissue deficiencies (eg, collateral ligaments, extensor mechanism), which also influence type and amount of prosthesis constraint. Specifically, constraint choice depends on amount of bone loss and on the condition of stabilizing tissues, such as the collateral ligaments. Under conditions of minimal bone loss and intact peripheral ligaments, a less constrained device, such as a primary posterior stabilized system, can be considered. When ligaments are present but insufficient, a semiconstrained device is recommended. In the presence of medial collateral ligament attenuation or complete medial or lateral collateral ligament dysfunction, a fully constrained prosthesis is required.8 Therefore, amount of bone loss or soft-tissue deficiency often dictates which prosthesis to use.
For radiographic classification, the Knee Society roentgenographic evaluation and scoring system10 has been implemented to allow for uniform reporting of radiographic results and to ensure adequate preoperative planning and postoperative assessment of component alignment. This system incorporates the evaluation of alignment in the coronal, sagittal, and patellofemoral planes and assesses radiolucency using zones dividing the implant–bone interface into segments to allow for easier classification of areas of lucency. More recently, a modified version of the Knee Society system was constructed.11 This modification simplifies zone classifications and accommodates more complex revision knee designs and stem extensions.
2. Posterior stabilized designs
Cruciate-retaining prostheses are seldom applicable in the revision TKA setting because of frequent damage to the posterior cruciate ligament, except in the case of simple polyethylene exchanges or, potentially, revisions of failed unicompartmental TKAs. Thus, posterior stabilized designs are the first-line choice for revision TKA (Figure 1). These prostheses are indicated only when the posterior cruciate ligament is incompetent and in the setting of adequate flexion and extension and medial and lateral collateral ligament balancing.
However, studies have shown that posterior stabilized TKAs have a limited role in revision TKAs, as the amount of ligamentous and bony damage is often underestimated in these patients, and use of a primary implant in a revision setting often requires additional augments, all of which may have contributed to the high failure rate. Thus, this design should be used only when the patient has adequate bone stock (AORI type I) and collateral ligament tension. This situation further emphasizes the importance of performing intraoperative testing for ligamentous balance and bone deficit evaluation in order to determine the most appropriate implant (Table 2).
3. Nonlinked constrained designs
Nonlinked constrained (condylar constrained) designs are the devices most commonly used for revision TKAs (>50% of revision knees). These prostheses provide increased articular constraint, which is required in patients with persistent instability, despite appropriate soft-tissue balancing. Increased articular constraint allows for more knee stability by providing progressive varus-valgus, coronal, and rotational stability with the aid of taller and wider tibial posts.12 Specifically, these implants incorporate a tibial post that fits closely between the femoral condyles, allowing for less motion compared with a standard posterior stabilized design.12
In addition, these designs may be used with augments, stems, and allografts when bone loss is more substantial. In particular, stem extensions allow for load distribution to the diaphyseal regions of the tibia and femur and thereby aid in reducing the increased stress at the bone–implant interface, which is a common concern with these implants. However, these extensions cost more, require intramedullary invasion, and are associated with higher rates of leg and thigh pain.12
These prostheses are often implicated in cases involving a high degree of bone loss (eg, AORI type II or III). They are ideally used in cases in which complete revision of both tibial and femoral components is needed and are indicated in cases of incompetent posterior cruciate ligament, partial functional loss of medial or lateral collateral ligaments, or flexion-extension mismatch.13 Furthermore, use of a constrained prosthesis is recommended in the setting of varus or valgus instability, or repeated dislocations of a posterior stabilized design (Table 2).
Ten-year survivorship ranges from 85% to 96%, but this is substantially lower than the 95% to 96% for condylar constrained prostheses used in primary TKAs.14-17 Moreover, the large discrepancy between survivorship of primary TKA and revision TKA with a constrained prosthesis further affirms that the complexity of revision surgery, rather than the prosthesis used, may have more deleterious effects on outcomes. However, surgeons must be aware that increased constraint leads to increased stress on the prosthetic interfaces with associated aseptic loosening and early failure, and this continues to be a legitimate concern.
4. Rotating hinge designs
Many patients who undergo revision TKA can be managed with a posterior stabilizing or nonlinked constrained design. However, in patients who present with severe ligamentous instability and bone loss (AORI type II or III), a rotating hinge prostheses, or highly constrained device, is often recommended (Figure 2).18 By using a rotating mobile-bearing platform, this prosthesis permits axial rotation through a metal-reinforced polyethylene-post articulation in the tibial tray. In addition, it involves use of modular diaphyseal-engaging stems and diaphyseal sleeves, which allow for the bypass of bony defects and areas of bone loss (Table 2).
However, the rigid biomechanics of hinged prostheses is associated with increased risk for aseptic loosening (aseptic 10-year survival, 60%-80%), imparted by the transfer of stresses across the bone. The higher risk for early loosening, osteolysis, and excessive wear—caused by the highly restricted biomechanics of early generations of fixed hinged designs—has led to the development of new devices with mobile mechanics. Prosthetic designs have been improved with an added rotational axis to reduce torsional stress, a patellar resurfacing option, and better stem fixation and patellofemoral kinematics. Overall, these are aimed to improve rates of instability and aseptic loosening, with promising results demonstrated in the literature.
5. Modular segmental arthroplasty designs
Segmental arthroplasty prostheses, which typically are end-of-the-line revision TKA options, are applicable only in cases of extensive bone loss (more than can be treated with allografts or augments; AORI type 3), complete ligamentous disruption/absence, loss of periprosthetic soft tissue, and multiple previous revision procedures (Figure 3). Despite the limited indications for these prostheses, they yield quick return to function without graft nonunion or resorption, and they augment ingrowth/ongrowth. Furthermore, the next surgical option could be fusion or amputation. When failures were specifically evaluated for aseptic loosening across 4 studies, the survival rate ranged from 83% to 99.5%, with the most frequent complication being infection (up to 33% in one series).6,19-21
The major roles for segmental arthroplasty prostheses in primary TKAs are in the setting of oncologic conditions that require bony excision, or unreconstuctable fractures about the knee. Used after ancillary metastatic disease, these prostheses demonstrate positive results, according to several reports.22,23 In the setting of revision TKA, however, these prostheses should be used only when other surgical options are unfeasible, given the high risk for infection and the re-revision rates. Currently, revision TKAs with tumor prostheses have a high failure rate (up to 50%) because of the extensive surgery and the lack of bony and soft-tissue support (Table 2).
Conclusion
Orthopedists performing revision TKAs must consider bone stock and remaining ligament stability. In particular, they should choose implants for least constraint and adequate knee stability, as these are essential in minimizing the stresses on the implant–bone interface. Ultimately, functional outcomes, survivorship, and postoperative satisfaction determine the success of these designs. However, predictors of outcomes of revision surgery are often multifactorial, and surgeons must also consider procedure complexity and patient-specific characteristics.
1. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
2. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28(8 suppl):116-119.
4. Kim TK. CORR Insights(®): risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1208-1209.
5. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
6. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
7. Dennis DA. A stepwise approach to revision total knee arthroplasty. J Arthroplasty. 2007;22(4 suppl 1):32-38.
8. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
9. Engh GA, Ammeen DJ. Bone loss with revision total knee arthroplasty: defect classification and alternatives for reconstruction. Instr Course Lect. 1999;48:167-175.
10. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
11. Meneghini RM, Mont MA, Backstein DB, Bourne RB, Dennis DA, Scuderi GR. Development of a modern Knee Society radiographic evaluation system and methodology for total knee arthroplasty. J Arthroplasty. 2015;30(12):2311-2314.
12. Nam D, Umunna BP, Cross MB, Reinhardt KR, Duggal S, Cornell CN. Clinical results and failure mechanisms of a nonmodular constrained knee without stem extensions. HSS J. 2012;8(2):96-102.
13. Lombardi AV Jr, Berend KR. The role of implant constraint in revision TKA: striking the balance. Orthopedics. 2006;29(9):847-849.
14. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
15. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
16. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
17. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
18. Jones RE. Total knee arthroplasty with modular rotating-platform hinge. Orthopedics. 2006;29(9 suppl):S80-S82.
19. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. The Knee. 2013;20:367-375.
20. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;(430):125-131.
21. Peters CL, Erickson J, Kloepper RG, Mohr RA. Revision total knee arthroplasty with modular components inserted with metaphyseal cement and stems without cement. J Arthroplasty. 2005;20:302-308.
22. Pala E, Trovarelli G, Calabro T, Angelini A, Abati CN, Ruggieri P. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clinical Orthop Relat Res. 2015;473:891-899.
23. Angelini A, Henderson E, Trovarelli G, Ruggieri P. Is there a role for knee arthrodesis with modular endoprostheses for tumor and revision of failed endoprostheses? Clin Orthop Relat Res. 2013;471(10):3326-3335.
1. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
2. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28(8 suppl):116-119.
4. Kim TK. CORR Insights(®): risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1208-1209.
5. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
6. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
7. Dennis DA. A stepwise approach to revision total knee arthroplasty. J Arthroplasty. 2007;22(4 suppl 1):32-38.
8. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
9. Engh GA, Ammeen DJ. Bone loss with revision total knee arthroplasty: defect classification and alternatives for reconstruction. Instr Course Lect. 1999;48:167-175.
10. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
11. Meneghini RM, Mont MA, Backstein DB, Bourne RB, Dennis DA, Scuderi GR. Development of a modern Knee Society radiographic evaluation system and methodology for total knee arthroplasty. J Arthroplasty. 2015;30(12):2311-2314.
12. Nam D, Umunna BP, Cross MB, Reinhardt KR, Duggal S, Cornell CN. Clinical results and failure mechanisms of a nonmodular constrained knee without stem extensions. HSS J. 2012;8(2):96-102.
13. Lombardi AV Jr, Berend KR. The role of implant constraint in revision TKA: striking the balance. Orthopedics. 2006;29(9):847-849.
14. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
15. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
16. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
17. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
18. Jones RE. Total knee arthroplasty with modular rotating-platform hinge. Orthopedics. 2006;29(9 suppl):S80-S82.
19. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. The Knee. 2013;20:367-375.
20. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;(430):125-131.
21. Peters CL, Erickson J, Kloepper RG, Mohr RA. Revision total knee arthroplasty with modular components inserted with metaphyseal cement and stems without cement. J Arthroplasty. 2005;20:302-308.
22. Pala E, Trovarelli G, Calabro T, Angelini A, Abati CN, Ruggieri P. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clinical Orthop Relat Res. 2015;473:891-899.
23. Angelini A, Henderson E, Trovarelli G, Ruggieri P. Is there a role for knee arthrodesis with modular endoprostheses for tumor and revision of failed endoprostheses? Clin Orthop Relat Res. 2013;471(10):3326-3335.
Lateral Ulnar Collateral Ligament Reconstruction: An Analysis of Ulnar Tunnel Locations
Posterolateral rotatory instability (PLRI) of the elbow is well recognized1 and is the most common type of chronic elbow instability. PLRI is often an end result of traumatic elbow dislocation.2 The “essential lesion” in patients with PLRI of the elbow is injury to the lateral ulnar collateral ligament (LUCL).1 However, more recent research has emphasized the importance of other ligaments in the lateral ligament complex (radial collateral and annular ligaments) in preventing PLRI.3-5 Nevertheless, when conservative treatment fails, the most commonly used surgical treatment involves LUCL reconstruction.1,6-11
Numerous techniques for LUCL reconstruction have been described.1,7-9,11-13 The chosen technique ideally restores normal anatomy. Therefore, the isometric point of origin at the lateral epicondyle and insertion at the supinator tubercle are important landmarks for creating tunnels that reproduce isometry, function, and normal anatomy. Most often, 2 tunnels are created in the ulna to secure the graft. It has been our experience that ulnar tunnel creation can affect the length of the bony bridge and the orientation of the graft.
We conducted a study to identify the precise proximal ulna tunnel location—anterior to posterior, with the distal tunnel at the supinator tubercle on the crest—that allows for the largest bony bridge and most geometrically favorable construct. We hypothesized that a most posteriorly placed proximal tunnel would increase bony bridge size and allow for a more isosceles graft configuration. An isosceles configuration with the humerus tunnel at the isometric location would allow for anterior and posterior bands of the same length with theoretically equal force distribution.
Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 17 adults with elbow computed tomography (CT) scans for inclusion in this study. The scans were previously performed for diagnostic workup of several pathologies, including valgus instability, olecranon stress fracture, and valgus extension overload. The scan protocol involved 0.5-mm axial cuts with inclusion of the distal humerus through the proximal radius and ulna in the DICOM (Digital Imaging and Communications in Medicine) format. Exclusion criteria included poor CT quality, inadequate visualization of the entire supinator crest, and age under 18 years. Fifteen patients with adequate CT scans met the inclusion criteria. MIMICS (Materialise’s Interactive Medical Image Control System) software was used to convert scans into patient-specific 3-dimensional (3-D) computer models. (Use of this software to produce anatomically accurate models has been verified in shoulder14 and elbow15 models.) These models were uploaded into Magics rapid prototyping software (Materialise) and manipulated for simulated tunnel drilling by precise bone subtraction methods. This software was used to define an ulnar Cartesian coordinate system with anatomical landmarks as reference points in order to standardize the position of each model (Figure 1).16 The y-axis was defined by the longitudinal axis of the ulna, and the x-axis was the transepicondylar axis, defined as the perpendicular line connecting the y-axis with the supinator crest. The z-axis was then established as the line perpendicular to the x- and y-axes—yielding a 3-D coordinate system that allowed us to manipulate the models in standardized fashion, maintaining the exact positions of the ulna while making measurements.
Surgical simulations were performed in the rapid prototyping software by creating a cylinder and placing it at the desired location of each tunnel. Cylinder diameter was 4 mm, matching the diameter of the drill we use to create each tunnel in our practice. The cylinder was inserted into the bone, perpendicular to the surface of the ulna at the point of insertion, so the cylinder’s deepest point entered the medullary canal of the ulna. Using a Boolean operation in the rapid prototyping software, we subtracted cylinder from bone to create a tunnel (Figure 2).15
In a previous study,17 we determined that the radial head junction is reproducibly about 15 mm proximal to the distinct supinator tubercle, which may be absent or not readily appreciated in up to 50% of cases. Therefore, proximal ulnar tunnels were placed 0, 5, and 10 mm posterior to the supinator crest at the radial head junction. Distal tunnels were placed 15 mm anterior to the radial head junction on the supinator crest (Figure 2). The bony bridges created by these tunnels were measured, as was the distance between the distal tunnel and the supinator tubercle.
Ideal graft configuration was described as an isosceles triangle with ulna tunnels perpendicular to the humeral tunnel (Figure 3).11 Location of the humeral origin in the sagittal plane was determined by finding the isometric point of the lateral humerus using only bony landmarks. Similar techniques have been used to find the isometric point on the medial epicondyle for medial ulnar collateral ligament reconstruction.15,18 With a circle fit into the trochlear notch of the ulna, the isometric point can be determined by the center of the circle. This point was then superimposed on the humerus to identify the starting point (Figure 4). In our simulation, we measured the isosceles configuration by drawing a line between the proximal and distal tunnels, and then another line connecting the bisecting point of the first line with the isometric point on the humerus from which the graft would originate. The angle between the 2 lines was measured; if isosceles, the angle was 90° (Figure 5). Length of the more proximal limb of the graft and the more distal limb of the graft was determined by measuring the distance from the isometric point to the proximal and distal tunnels, respectively (Figure 6).
One-way analysis of variance was used to compare all the tunnels’ bony bridge sizes, graft lengths, and angles to the isometric point. For all comparisons, statistical significance was set at P < .05. As no other studies have compared bony bridges by varying tunnel creation parameters, and as the present study is observational and not comparative, no power analysis was performed.
Results
Bony bridges were significantly longer, and angles more perpendicular, with increasing distance from the proximal tunnel to the supinator crest (Table 1, Figure 5, Figure 7). The bony bridge 0 mm posterior to the supinator crest yielded a mean (SE) bony bridge length of 11.0 (0.2) mm. This proximal tunnel also yielded the smallest mean (SE) perpendicular angle to the isometric point, 131.2° (1.9°). The tunnel most posterior to the supinator crest yielded the longest mean (SE) bony bridge, 13.7 (0.2) mm, and the largest mean (SE) degree of perpendicularity, 95.8° (1.4°). The differences between all tunnels’ bony bridges and isometric angles were statistically significant (P < .00001). The difference between the more distal limb and the more proximal limb of the graft was smallest in the more posteriorly placed proximal tunnel (Table 2, Figure 8). In fact, there was no statistical difference between the proximal and distal limbs of the graft when the proximal tunnel was placed 10 mm posterior to the supinator crest: Mean (SE) was 9.4 (0.5) mm at 0 mm (P < .00001) and 1.1 (0.6) mm at 10 mm (P = .24).
Discussion
PLRI of the elbow is best initially managed nonoperatively. However, when nonoperative management fails, the LUCL is often surgically reconstructed. Reconstruction methods vary by fixation method, graft choice, and bone tunnels.1,7-9,11-13 In 1991, O’Driscoll and colleagues1 described a “yoke” technique for LUCL reconstruction. Since then, the docking technique7 and other techniques have been developed. All these techniques emphasize maximizing anatomical precision and isometry with careful placement of tunnels or fixation devices. The humeral fixation site, at the anterior inferior aspect of the lateral epicondyle at the point of isometry, can be accessed relatively reproducibly. By contrast, the ulnar points of fixation are more variable, because of increased bone stock and overlying soft-tissue and bony anatomy.
Among the challenges in determining the points of ulnar fixation is the bony anatomy that is often used for landmarks. In the literature, the supinator crest or the supintor tubercle is the landmark for placing the distal tunnel.1,7-9,11-13 This is a problem for 2 reasons. First, the supintor crest, a longitudinal structure on the lateral aspect of the ulna, originates from the radial head junction and extends tens of millimeters distally; further specification is needed to guide these ulnar tunnels. The second reason is that use of the supinator tubercle, a prominence on the supinator crest, adds specificity to the location of the ulnar tunnels. During surgery, however, the supinator tubercle may not be a reliable, independently prominent structure; instead, it may be indistinguishable from the supinator crest, on which it rests. One study determined that only about 50% of computer models of patient ulnas had a distinct prominence that could be classified as the supinator tubercle.17 The percentage presumably is lower during surgery, with limited exposure and overlying soft tissues.
In a study of patients with a prominent tubercle, mean (SE) distance from radial head junction to tubercle was 15 (2) mm.17 This finding led us to use the radial head junction as the primary bony landmark in determining the location of the proximal tunnel and placing the distal tunnel 15 mm distally—achieving the same fixation described in the literature but using more distinct landmarks. Our study thus provided a reliable, verified approach to locating the ulnar tunnels in the proximal-distal axis.
We also explored the anterior-posterior orientation of the proximal ulnar tunnel. The 2 primary considerations surrounding the varied proximal tunnel placements were the bony bridge formed between the proximal and distal tunnels and the perpendicularity of the triangle formed by the fixation points. Maximizing the bony bridge is obviously ideal in securing and preventing fixation blowout. Achieving an isoceles reconstruction has been reported in the literature on the various fixation techniques for LUCL reconstruction.11 Although the biomechanical advantage of this fixation type is not fully clear, we assume the construct produces graft stands of equal length, tension, and stability. In addition, the larger footprint created by an isoceles reconstructed ligament increases the stability of the radial head.
Results of the present study showed that the more posterior the proximal ulnar tunnel, the longer the bony bridge and the more isoceles the reconstruction. The difference in bony bridge distance from the most anterior to the most posterior tunnel was about 2 mm, or 18%. For every 1 mm of posteriorization, the bony bridge was 0.2 mm longer. The line from the isometric point of humeral fixation bisecting the proximal and distal tunnels was also more perpendicular with the most posterior tunnel, by about 40°. The resulting proximal and distal limbs of the reconstruction were equal in length, as demonstrated by the smaller difference between the limbs. We assume this isoceles reconstruction more likely applies uniform restraint on the radial head. Thus, an effort should be made to posteriorize the proximal ulnar tunnel during reconstruction.
The study was limited by the number of patient-specific elbow models used. However, given the statistical consistency of measurements, sample size was sufficient. Another limitation, inherent to the model, was that only bony anatomy was incorporated. However, the overlying muscles, tendons, and ligaments can significantly alter tunnel placement, and this study provided other means and cues using more reliable landmarks to adequately place the tunnels. As this was a simulation study, we cannot confirm whether these results would make a difference clinically. The strengths of this study include development and verification of reliable landmarks that can be used to guide ulnar tunnel locations during LUCL reconstruction; these landmarks have been used for medial ulnar collateral ligament reconstruction.15 Other strengths include precise and accurate placement of tunnels and measurement of resulting bony bridges—accomplished independently and without compromising specimen quality.
Conclusion
We recommend drilling the proximal ulnar tunnel posterior to the supinator crest at the level of the radial head junction. A reasonable goal is 10 mm posterior to the crest, though the overlying soft tissue must be considered, and care should be taken to aim the drill anteriorly, toward the ulna’s intramedullary canal, to avoid posterior cortical breach. The distal ulnar tunnel should be drilled just posterior to the supinator crest, 15 mm distal to the radial head junction.
1. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
2. O’Driscoll SW. Classification and evaluation of recurrent instability of the elbow. Clin Orthop Relat Res. 2000;370:34-43.
3. Takigawa N, Ryu J, Kish VL, Kinoshita M, Abe M. Functional anatomy of the lateral collateral ligament complex of the elbow: morphology and strain. J Hand Surg Br. 2005;30(2):143-147.
4. McAdams TR, Masters GW, Srivastava S. The effect of arthroscopic sectioning of the lateral ligament complex of the elbow on posterolateral rotatory stability. J Shoulder Elbow Surg. 2005;14(3):298-301.
5. Dunning CE, Zarzour ZD, Patterson SD, Johnson JA, King GJ. Ligamentous stabilizers against posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 2001;83(12):1823-1828.
6. Eygendaal D. Ligamentous reconstruction around the elbow using triceps tendon. Acta Orthop Scand. 2004;75(5):516-523.
7. Jones KJ, Dodson CC, Osbahr DC, et al. The docking technique for lateral ulnar collateral ligament reconstruction: surgical technique and clinical outcomes. J Shoulder Elbow Surg. 2012;21(3):389-395.
8. Lee BP, Teo LH. Surgical reconstruction for posterolateral rotatory instability of the elbow. J Shoulder Elbow Surg. 2003;12(5):476-479.
9. Lin KY, Shen PH, Lee CH, Pan RY, Lin LC, Shen HC. Functional outcomes of surgical reconstruction for posterolateral rotatory instability of the elbow. Injury. 2012;43(10):1657-1661.
10. Olsen BS, Søjbjerg JO. The treatment of recurrent posterolateral instability of the elbow. J Bone Joint Surg Br. 2003;85(3):342-346.
11. Sanchez-Sotelo J, Morrey BF, O’Driscoll SW. Ligamentous repair and reconstruction for posterolateral rotatory instability of the elbow. J Bone Joint Surg Br. 2005;87(1):54-61.
12. Savoie FH 3rd, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Hand Clin. 2009;25(3):323-329.
13. Savoie FH 3rd, O’Brien MJ, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Clin Sports Med. 2010;29(4):611-618.
14. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
15. Byram IR, Khanna K, Gardner TR, Ahmad CS. Characterizing bone tunnel placement in medial ulnar collateral ligament reconstruction using patient-specific 3-dimensional computed tomography modeling. Am J Sports Med. 2013;41(4):894-902.
16. Shiba R, Sorbie C, Siu DW, Bryant JT, Cooke TD, Wevers HW. Geometry of the humeroulnar joint. J Orthop Res. 1988;6(6):897-906.
17. Anakwenze OA, Khanna K, Levine WN, Ahmad CS. Characterization of the supinator tubercle for lateral ulnar collateral ligament reconstruction. Orthop J Sports Med. 2014;2(4):2325967114530969. doi:10.1177/2325967114530969.
18. Sasashige Y, Ochi M, Ikuta Y. Optimal attachment site for reconstruction of the ulnar collateral ligament. A cadaver study. Arch Orthop Trauma Surg. 1994;113(5):265-270.
Posterolateral rotatory instability (PLRI) of the elbow is well recognized1 and is the most common type of chronic elbow instability. PLRI is often an end result of traumatic elbow dislocation.2 The “essential lesion” in patients with PLRI of the elbow is injury to the lateral ulnar collateral ligament (LUCL).1 However, more recent research has emphasized the importance of other ligaments in the lateral ligament complex (radial collateral and annular ligaments) in preventing PLRI.3-5 Nevertheless, when conservative treatment fails, the most commonly used surgical treatment involves LUCL reconstruction.1,6-11
Numerous techniques for LUCL reconstruction have been described.1,7-9,11-13 The chosen technique ideally restores normal anatomy. Therefore, the isometric point of origin at the lateral epicondyle and insertion at the supinator tubercle are important landmarks for creating tunnels that reproduce isometry, function, and normal anatomy. Most often, 2 tunnels are created in the ulna to secure the graft. It has been our experience that ulnar tunnel creation can affect the length of the bony bridge and the orientation of the graft.
We conducted a study to identify the precise proximal ulna tunnel location—anterior to posterior, with the distal tunnel at the supinator tubercle on the crest—that allows for the largest bony bridge and most geometrically favorable construct. We hypothesized that a most posteriorly placed proximal tunnel would increase bony bridge size and allow for a more isosceles graft configuration. An isosceles configuration with the humerus tunnel at the isometric location would allow for anterior and posterior bands of the same length with theoretically equal force distribution.
Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 17 adults with elbow computed tomography (CT) scans for inclusion in this study. The scans were previously performed for diagnostic workup of several pathologies, including valgus instability, olecranon stress fracture, and valgus extension overload. The scan protocol involved 0.5-mm axial cuts with inclusion of the distal humerus through the proximal radius and ulna in the DICOM (Digital Imaging and Communications in Medicine) format. Exclusion criteria included poor CT quality, inadequate visualization of the entire supinator crest, and age under 18 years. Fifteen patients with adequate CT scans met the inclusion criteria. MIMICS (Materialise’s Interactive Medical Image Control System) software was used to convert scans into patient-specific 3-dimensional (3-D) computer models. (Use of this software to produce anatomically accurate models has been verified in shoulder14 and elbow15 models.) These models were uploaded into Magics rapid prototyping software (Materialise) and manipulated for simulated tunnel drilling by precise bone subtraction methods. This software was used to define an ulnar Cartesian coordinate system with anatomical landmarks as reference points in order to standardize the position of each model (Figure 1).16 The y-axis was defined by the longitudinal axis of the ulna, and the x-axis was the transepicondylar axis, defined as the perpendicular line connecting the y-axis with the supinator crest. The z-axis was then established as the line perpendicular to the x- and y-axes—yielding a 3-D coordinate system that allowed us to manipulate the models in standardized fashion, maintaining the exact positions of the ulna while making measurements.
Surgical simulations were performed in the rapid prototyping software by creating a cylinder and placing it at the desired location of each tunnel. Cylinder diameter was 4 mm, matching the diameter of the drill we use to create each tunnel in our practice. The cylinder was inserted into the bone, perpendicular to the surface of the ulna at the point of insertion, so the cylinder’s deepest point entered the medullary canal of the ulna. Using a Boolean operation in the rapid prototyping software, we subtracted cylinder from bone to create a tunnel (Figure 2).15
In a previous study,17 we determined that the radial head junction is reproducibly about 15 mm proximal to the distinct supinator tubercle, which may be absent or not readily appreciated in up to 50% of cases. Therefore, proximal ulnar tunnels were placed 0, 5, and 10 mm posterior to the supinator crest at the radial head junction. Distal tunnels were placed 15 mm anterior to the radial head junction on the supinator crest (Figure 2). The bony bridges created by these tunnels were measured, as was the distance between the distal tunnel and the supinator tubercle.
Ideal graft configuration was described as an isosceles triangle with ulna tunnels perpendicular to the humeral tunnel (Figure 3).11 Location of the humeral origin in the sagittal plane was determined by finding the isometric point of the lateral humerus using only bony landmarks. Similar techniques have been used to find the isometric point on the medial epicondyle for medial ulnar collateral ligament reconstruction.15,18 With a circle fit into the trochlear notch of the ulna, the isometric point can be determined by the center of the circle. This point was then superimposed on the humerus to identify the starting point (Figure 4). In our simulation, we measured the isosceles configuration by drawing a line between the proximal and distal tunnels, and then another line connecting the bisecting point of the first line with the isometric point on the humerus from which the graft would originate. The angle between the 2 lines was measured; if isosceles, the angle was 90° (Figure 5). Length of the more proximal limb of the graft and the more distal limb of the graft was determined by measuring the distance from the isometric point to the proximal and distal tunnels, respectively (Figure 6).
One-way analysis of variance was used to compare all the tunnels’ bony bridge sizes, graft lengths, and angles to the isometric point. For all comparisons, statistical significance was set at P < .05. As no other studies have compared bony bridges by varying tunnel creation parameters, and as the present study is observational and not comparative, no power analysis was performed.
Results
Bony bridges were significantly longer, and angles more perpendicular, with increasing distance from the proximal tunnel to the supinator crest (Table 1, Figure 5, Figure 7). The bony bridge 0 mm posterior to the supinator crest yielded a mean (SE) bony bridge length of 11.0 (0.2) mm. This proximal tunnel also yielded the smallest mean (SE) perpendicular angle to the isometric point, 131.2° (1.9°). The tunnel most posterior to the supinator crest yielded the longest mean (SE) bony bridge, 13.7 (0.2) mm, and the largest mean (SE) degree of perpendicularity, 95.8° (1.4°). The differences between all tunnels’ bony bridges and isometric angles were statistically significant (P < .00001). The difference between the more distal limb and the more proximal limb of the graft was smallest in the more posteriorly placed proximal tunnel (Table 2, Figure 8). In fact, there was no statistical difference between the proximal and distal limbs of the graft when the proximal tunnel was placed 10 mm posterior to the supinator crest: Mean (SE) was 9.4 (0.5) mm at 0 mm (P < .00001) and 1.1 (0.6) mm at 10 mm (P = .24).
Discussion
PLRI of the elbow is best initially managed nonoperatively. However, when nonoperative management fails, the LUCL is often surgically reconstructed. Reconstruction methods vary by fixation method, graft choice, and bone tunnels.1,7-9,11-13 In 1991, O’Driscoll and colleagues1 described a “yoke” technique for LUCL reconstruction. Since then, the docking technique7 and other techniques have been developed. All these techniques emphasize maximizing anatomical precision and isometry with careful placement of tunnels or fixation devices. The humeral fixation site, at the anterior inferior aspect of the lateral epicondyle at the point of isometry, can be accessed relatively reproducibly. By contrast, the ulnar points of fixation are more variable, because of increased bone stock and overlying soft-tissue and bony anatomy.
Among the challenges in determining the points of ulnar fixation is the bony anatomy that is often used for landmarks. In the literature, the supinator crest or the supintor tubercle is the landmark for placing the distal tunnel.1,7-9,11-13 This is a problem for 2 reasons. First, the supintor crest, a longitudinal structure on the lateral aspect of the ulna, originates from the radial head junction and extends tens of millimeters distally; further specification is needed to guide these ulnar tunnels. The second reason is that use of the supinator tubercle, a prominence on the supinator crest, adds specificity to the location of the ulnar tunnels. During surgery, however, the supinator tubercle may not be a reliable, independently prominent structure; instead, it may be indistinguishable from the supinator crest, on which it rests. One study determined that only about 50% of computer models of patient ulnas had a distinct prominence that could be classified as the supinator tubercle.17 The percentage presumably is lower during surgery, with limited exposure and overlying soft tissues.
In a study of patients with a prominent tubercle, mean (SE) distance from radial head junction to tubercle was 15 (2) mm.17 This finding led us to use the radial head junction as the primary bony landmark in determining the location of the proximal tunnel and placing the distal tunnel 15 mm distally—achieving the same fixation described in the literature but using more distinct landmarks. Our study thus provided a reliable, verified approach to locating the ulnar tunnels in the proximal-distal axis.
We also explored the anterior-posterior orientation of the proximal ulnar tunnel. The 2 primary considerations surrounding the varied proximal tunnel placements were the bony bridge formed between the proximal and distal tunnels and the perpendicularity of the triangle formed by the fixation points. Maximizing the bony bridge is obviously ideal in securing and preventing fixation blowout. Achieving an isoceles reconstruction has been reported in the literature on the various fixation techniques for LUCL reconstruction.11 Although the biomechanical advantage of this fixation type is not fully clear, we assume the construct produces graft stands of equal length, tension, and stability. In addition, the larger footprint created by an isoceles reconstructed ligament increases the stability of the radial head.
Results of the present study showed that the more posterior the proximal ulnar tunnel, the longer the bony bridge and the more isoceles the reconstruction. The difference in bony bridge distance from the most anterior to the most posterior tunnel was about 2 mm, or 18%. For every 1 mm of posteriorization, the bony bridge was 0.2 mm longer. The line from the isometric point of humeral fixation bisecting the proximal and distal tunnels was also more perpendicular with the most posterior tunnel, by about 40°. The resulting proximal and distal limbs of the reconstruction were equal in length, as demonstrated by the smaller difference between the limbs. We assume this isoceles reconstruction more likely applies uniform restraint on the radial head. Thus, an effort should be made to posteriorize the proximal ulnar tunnel during reconstruction.
The study was limited by the number of patient-specific elbow models used. However, given the statistical consistency of measurements, sample size was sufficient. Another limitation, inherent to the model, was that only bony anatomy was incorporated. However, the overlying muscles, tendons, and ligaments can significantly alter tunnel placement, and this study provided other means and cues using more reliable landmarks to adequately place the tunnels. As this was a simulation study, we cannot confirm whether these results would make a difference clinically. The strengths of this study include development and verification of reliable landmarks that can be used to guide ulnar tunnel locations during LUCL reconstruction; these landmarks have been used for medial ulnar collateral ligament reconstruction.15 Other strengths include precise and accurate placement of tunnels and measurement of resulting bony bridges—accomplished independently and without compromising specimen quality.
Conclusion
We recommend drilling the proximal ulnar tunnel posterior to the supinator crest at the level of the radial head junction. A reasonable goal is 10 mm posterior to the crest, though the overlying soft tissue must be considered, and care should be taken to aim the drill anteriorly, toward the ulna’s intramedullary canal, to avoid posterior cortical breach. The distal ulnar tunnel should be drilled just posterior to the supinator crest, 15 mm distal to the radial head junction.
Posterolateral rotatory instability (PLRI) of the elbow is well recognized1 and is the most common type of chronic elbow instability. PLRI is often an end result of traumatic elbow dislocation.2 The “essential lesion” in patients with PLRI of the elbow is injury to the lateral ulnar collateral ligament (LUCL).1 However, more recent research has emphasized the importance of other ligaments in the lateral ligament complex (radial collateral and annular ligaments) in preventing PLRI.3-5 Nevertheless, when conservative treatment fails, the most commonly used surgical treatment involves LUCL reconstruction.1,6-11
Numerous techniques for LUCL reconstruction have been described.1,7-9,11-13 The chosen technique ideally restores normal anatomy. Therefore, the isometric point of origin at the lateral epicondyle and insertion at the supinator tubercle are important landmarks for creating tunnels that reproduce isometry, function, and normal anatomy. Most often, 2 tunnels are created in the ulna to secure the graft. It has been our experience that ulnar tunnel creation can affect the length of the bony bridge and the orientation of the graft.
We conducted a study to identify the precise proximal ulna tunnel location—anterior to posterior, with the distal tunnel at the supinator tubercle on the crest—that allows for the largest bony bridge and most geometrically favorable construct. We hypothesized that a most posteriorly placed proximal tunnel would increase bony bridge size and allow for a more isosceles graft configuration. An isosceles configuration with the humerus tunnel at the isometric location would allow for anterior and posterior bands of the same length with theoretically equal force distribution.
Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 17 adults with elbow computed tomography (CT) scans for inclusion in this study. The scans were previously performed for diagnostic workup of several pathologies, including valgus instability, olecranon stress fracture, and valgus extension overload. The scan protocol involved 0.5-mm axial cuts with inclusion of the distal humerus through the proximal radius and ulna in the DICOM (Digital Imaging and Communications in Medicine) format. Exclusion criteria included poor CT quality, inadequate visualization of the entire supinator crest, and age under 18 years. Fifteen patients with adequate CT scans met the inclusion criteria. MIMICS (Materialise’s Interactive Medical Image Control System) software was used to convert scans into patient-specific 3-dimensional (3-D) computer models. (Use of this software to produce anatomically accurate models has been verified in shoulder14 and elbow15 models.) These models were uploaded into Magics rapid prototyping software (Materialise) and manipulated for simulated tunnel drilling by precise bone subtraction methods. This software was used to define an ulnar Cartesian coordinate system with anatomical landmarks as reference points in order to standardize the position of each model (Figure 1).16 The y-axis was defined by the longitudinal axis of the ulna, and the x-axis was the transepicondylar axis, defined as the perpendicular line connecting the y-axis with the supinator crest. The z-axis was then established as the line perpendicular to the x- and y-axes—yielding a 3-D coordinate system that allowed us to manipulate the models in standardized fashion, maintaining the exact positions of the ulna while making measurements.
Surgical simulations were performed in the rapid prototyping software by creating a cylinder and placing it at the desired location of each tunnel. Cylinder diameter was 4 mm, matching the diameter of the drill we use to create each tunnel in our practice. The cylinder was inserted into the bone, perpendicular to the surface of the ulna at the point of insertion, so the cylinder’s deepest point entered the medullary canal of the ulna. Using a Boolean operation in the rapid prototyping software, we subtracted cylinder from bone to create a tunnel (Figure 2).15
In a previous study,17 we determined that the radial head junction is reproducibly about 15 mm proximal to the distinct supinator tubercle, which may be absent or not readily appreciated in up to 50% of cases. Therefore, proximal ulnar tunnels were placed 0, 5, and 10 mm posterior to the supinator crest at the radial head junction. Distal tunnels were placed 15 mm anterior to the radial head junction on the supinator crest (Figure 2). The bony bridges created by these tunnels were measured, as was the distance between the distal tunnel and the supinator tubercle.
Ideal graft configuration was described as an isosceles triangle with ulna tunnels perpendicular to the humeral tunnel (Figure 3).11 Location of the humeral origin in the sagittal plane was determined by finding the isometric point of the lateral humerus using only bony landmarks. Similar techniques have been used to find the isometric point on the medial epicondyle for medial ulnar collateral ligament reconstruction.15,18 With a circle fit into the trochlear notch of the ulna, the isometric point can be determined by the center of the circle. This point was then superimposed on the humerus to identify the starting point (Figure 4). In our simulation, we measured the isosceles configuration by drawing a line between the proximal and distal tunnels, and then another line connecting the bisecting point of the first line with the isometric point on the humerus from which the graft would originate. The angle between the 2 lines was measured; if isosceles, the angle was 90° (Figure 5). Length of the more proximal limb of the graft and the more distal limb of the graft was determined by measuring the distance from the isometric point to the proximal and distal tunnels, respectively (Figure 6).
One-way analysis of variance was used to compare all the tunnels’ bony bridge sizes, graft lengths, and angles to the isometric point. For all comparisons, statistical significance was set at P < .05. As no other studies have compared bony bridges by varying tunnel creation parameters, and as the present study is observational and not comparative, no power analysis was performed.
Results
Bony bridges were significantly longer, and angles more perpendicular, with increasing distance from the proximal tunnel to the supinator crest (Table 1, Figure 5, Figure 7). The bony bridge 0 mm posterior to the supinator crest yielded a mean (SE) bony bridge length of 11.0 (0.2) mm. This proximal tunnel also yielded the smallest mean (SE) perpendicular angle to the isometric point, 131.2° (1.9°). The tunnel most posterior to the supinator crest yielded the longest mean (SE) bony bridge, 13.7 (0.2) mm, and the largest mean (SE) degree of perpendicularity, 95.8° (1.4°). The differences between all tunnels’ bony bridges and isometric angles were statistically significant (P < .00001). The difference between the more distal limb and the more proximal limb of the graft was smallest in the more posteriorly placed proximal tunnel (Table 2, Figure 8). In fact, there was no statistical difference between the proximal and distal limbs of the graft when the proximal tunnel was placed 10 mm posterior to the supinator crest: Mean (SE) was 9.4 (0.5) mm at 0 mm (P < .00001) and 1.1 (0.6) mm at 10 mm (P = .24).
Discussion
PLRI of the elbow is best initially managed nonoperatively. However, when nonoperative management fails, the LUCL is often surgically reconstructed. Reconstruction methods vary by fixation method, graft choice, and bone tunnels.1,7-9,11-13 In 1991, O’Driscoll and colleagues1 described a “yoke” technique for LUCL reconstruction. Since then, the docking technique7 and other techniques have been developed. All these techniques emphasize maximizing anatomical precision and isometry with careful placement of tunnels or fixation devices. The humeral fixation site, at the anterior inferior aspect of the lateral epicondyle at the point of isometry, can be accessed relatively reproducibly. By contrast, the ulnar points of fixation are more variable, because of increased bone stock and overlying soft-tissue and bony anatomy.
Among the challenges in determining the points of ulnar fixation is the bony anatomy that is often used for landmarks. In the literature, the supinator crest or the supintor tubercle is the landmark for placing the distal tunnel.1,7-9,11-13 This is a problem for 2 reasons. First, the supintor crest, a longitudinal structure on the lateral aspect of the ulna, originates from the radial head junction and extends tens of millimeters distally; further specification is needed to guide these ulnar tunnels. The second reason is that use of the supinator tubercle, a prominence on the supinator crest, adds specificity to the location of the ulnar tunnels. During surgery, however, the supinator tubercle may not be a reliable, independently prominent structure; instead, it may be indistinguishable from the supinator crest, on which it rests. One study determined that only about 50% of computer models of patient ulnas had a distinct prominence that could be classified as the supinator tubercle.17 The percentage presumably is lower during surgery, with limited exposure and overlying soft tissues.
In a study of patients with a prominent tubercle, mean (SE) distance from radial head junction to tubercle was 15 (2) mm.17 This finding led us to use the radial head junction as the primary bony landmark in determining the location of the proximal tunnel and placing the distal tunnel 15 mm distally—achieving the same fixation described in the literature but using more distinct landmarks. Our study thus provided a reliable, verified approach to locating the ulnar tunnels in the proximal-distal axis.
We also explored the anterior-posterior orientation of the proximal ulnar tunnel. The 2 primary considerations surrounding the varied proximal tunnel placements were the bony bridge formed between the proximal and distal tunnels and the perpendicularity of the triangle formed by the fixation points. Maximizing the bony bridge is obviously ideal in securing and preventing fixation blowout. Achieving an isoceles reconstruction has been reported in the literature on the various fixation techniques for LUCL reconstruction.11 Although the biomechanical advantage of this fixation type is not fully clear, we assume the construct produces graft stands of equal length, tension, and stability. In addition, the larger footprint created by an isoceles reconstructed ligament increases the stability of the radial head.
Results of the present study showed that the more posterior the proximal ulnar tunnel, the longer the bony bridge and the more isoceles the reconstruction. The difference in bony bridge distance from the most anterior to the most posterior tunnel was about 2 mm, or 18%. For every 1 mm of posteriorization, the bony bridge was 0.2 mm longer. The line from the isometric point of humeral fixation bisecting the proximal and distal tunnels was also more perpendicular with the most posterior tunnel, by about 40°. The resulting proximal and distal limbs of the reconstruction were equal in length, as demonstrated by the smaller difference between the limbs. We assume this isoceles reconstruction more likely applies uniform restraint on the radial head. Thus, an effort should be made to posteriorize the proximal ulnar tunnel during reconstruction.
The study was limited by the number of patient-specific elbow models used. However, given the statistical consistency of measurements, sample size was sufficient. Another limitation, inherent to the model, was that only bony anatomy was incorporated. However, the overlying muscles, tendons, and ligaments can significantly alter tunnel placement, and this study provided other means and cues using more reliable landmarks to adequately place the tunnels. As this was a simulation study, we cannot confirm whether these results would make a difference clinically. The strengths of this study include development and verification of reliable landmarks that can be used to guide ulnar tunnel locations during LUCL reconstruction; these landmarks have been used for medial ulnar collateral ligament reconstruction.15 Other strengths include precise and accurate placement of tunnels and measurement of resulting bony bridges—accomplished independently and without compromising specimen quality.
Conclusion
We recommend drilling the proximal ulnar tunnel posterior to the supinator crest at the level of the radial head junction. A reasonable goal is 10 mm posterior to the crest, though the overlying soft tissue must be considered, and care should be taken to aim the drill anteriorly, toward the ulna’s intramedullary canal, to avoid posterior cortical breach. The distal ulnar tunnel should be drilled just posterior to the supinator crest, 15 mm distal to the radial head junction.
1. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
2. O’Driscoll SW. Classification and evaluation of recurrent instability of the elbow. Clin Orthop Relat Res. 2000;370:34-43.
3. Takigawa N, Ryu J, Kish VL, Kinoshita M, Abe M. Functional anatomy of the lateral collateral ligament complex of the elbow: morphology and strain. J Hand Surg Br. 2005;30(2):143-147.
4. McAdams TR, Masters GW, Srivastava S. The effect of arthroscopic sectioning of the lateral ligament complex of the elbow on posterolateral rotatory stability. J Shoulder Elbow Surg. 2005;14(3):298-301.
5. Dunning CE, Zarzour ZD, Patterson SD, Johnson JA, King GJ. Ligamentous stabilizers against posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 2001;83(12):1823-1828.
6. Eygendaal D. Ligamentous reconstruction around the elbow using triceps tendon. Acta Orthop Scand. 2004;75(5):516-523.
7. Jones KJ, Dodson CC, Osbahr DC, et al. The docking technique for lateral ulnar collateral ligament reconstruction: surgical technique and clinical outcomes. J Shoulder Elbow Surg. 2012;21(3):389-395.
8. Lee BP, Teo LH. Surgical reconstruction for posterolateral rotatory instability of the elbow. J Shoulder Elbow Surg. 2003;12(5):476-479.
9. Lin KY, Shen PH, Lee CH, Pan RY, Lin LC, Shen HC. Functional outcomes of surgical reconstruction for posterolateral rotatory instability of the elbow. Injury. 2012;43(10):1657-1661.
10. Olsen BS, Søjbjerg JO. The treatment of recurrent posterolateral instability of the elbow. J Bone Joint Surg Br. 2003;85(3):342-346.
11. Sanchez-Sotelo J, Morrey BF, O’Driscoll SW. Ligamentous repair and reconstruction for posterolateral rotatory instability of the elbow. J Bone Joint Surg Br. 2005;87(1):54-61.
12. Savoie FH 3rd, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Hand Clin. 2009;25(3):323-329.
13. Savoie FH 3rd, O’Brien MJ, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Clin Sports Med. 2010;29(4):611-618.
14. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
15. Byram IR, Khanna K, Gardner TR, Ahmad CS. Characterizing bone tunnel placement in medial ulnar collateral ligament reconstruction using patient-specific 3-dimensional computed tomography modeling. Am J Sports Med. 2013;41(4):894-902.
16. Shiba R, Sorbie C, Siu DW, Bryant JT, Cooke TD, Wevers HW. Geometry of the humeroulnar joint. J Orthop Res. 1988;6(6):897-906.
17. Anakwenze OA, Khanna K, Levine WN, Ahmad CS. Characterization of the supinator tubercle for lateral ulnar collateral ligament reconstruction. Orthop J Sports Med. 2014;2(4):2325967114530969. doi:10.1177/2325967114530969.
18. Sasashige Y, Ochi M, Ikuta Y. Optimal attachment site for reconstruction of the ulnar collateral ligament. A cadaver study. Arch Orthop Trauma Surg. 1994;113(5):265-270.
1. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
2. O’Driscoll SW. Classification and evaluation of recurrent instability of the elbow. Clin Orthop Relat Res. 2000;370:34-43.
3. Takigawa N, Ryu J, Kish VL, Kinoshita M, Abe M. Functional anatomy of the lateral collateral ligament complex of the elbow: morphology and strain. J Hand Surg Br. 2005;30(2):143-147.
4. McAdams TR, Masters GW, Srivastava S. The effect of arthroscopic sectioning of the lateral ligament complex of the elbow on posterolateral rotatory stability. J Shoulder Elbow Surg. 2005;14(3):298-301.
5. Dunning CE, Zarzour ZD, Patterson SD, Johnson JA, King GJ. Ligamentous stabilizers against posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 2001;83(12):1823-1828.
6. Eygendaal D. Ligamentous reconstruction around the elbow using triceps tendon. Acta Orthop Scand. 2004;75(5):516-523.
7. Jones KJ, Dodson CC, Osbahr DC, et al. The docking technique for lateral ulnar collateral ligament reconstruction: surgical technique and clinical outcomes. J Shoulder Elbow Surg. 2012;21(3):389-395.
8. Lee BP, Teo LH. Surgical reconstruction for posterolateral rotatory instability of the elbow. J Shoulder Elbow Surg. 2003;12(5):476-479.
9. Lin KY, Shen PH, Lee CH, Pan RY, Lin LC, Shen HC. Functional outcomes of surgical reconstruction for posterolateral rotatory instability of the elbow. Injury. 2012;43(10):1657-1661.
10. Olsen BS, Søjbjerg JO. The treatment of recurrent posterolateral instability of the elbow. J Bone Joint Surg Br. 2003;85(3):342-346.
11. Sanchez-Sotelo J, Morrey BF, O’Driscoll SW. Ligamentous repair and reconstruction for posterolateral rotatory instability of the elbow. J Bone Joint Surg Br. 2005;87(1):54-61.
12. Savoie FH 3rd, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Hand Clin. 2009;25(3):323-329.
13. Savoie FH 3rd, O’Brien MJ, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Clin Sports Med. 2010;29(4):611-618.
14. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
15. Byram IR, Khanna K, Gardner TR, Ahmad CS. Characterizing bone tunnel placement in medial ulnar collateral ligament reconstruction using patient-specific 3-dimensional computed tomography modeling. Am J Sports Med. 2013;41(4):894-902.
16. Shiba R, Sorbie C, Siu DW, Bryant JT, Cooke TD, Wevers HW. Geometry of the humeroulnar joint. J Orthop Res. 1988;6(6):897-906.
17. Anakwenze OA, Khanna K, Levine WN, Ahmad CS. Characterization of the supinator tubercle for lateral ulnar collateral ligament reconstruction. Orthop J Sports Med. 2014;2(4):2325967114530969. doi:10.1177/2325967114530969.
18. Sasashige Y, Ochi M, Ikuta Y. Optimal attachment site for reconstruction of the ulnar collateral ligament. A cadaver study. Arch Orthop Trauma Surg. 1994;113(5):265-270.
Reconstructive Shelf Arthroplasty as a Salvage Procedure for Complex Fifth Tarsometatarsal Joint Complex Injuries: A Case Review and Discussion
Fractures of the cuboid bone are uncommon, with an annual incidence of approximately 1.8 per 100,000.1 This is largely attributed to the inherent stability provided by its anatomy and position in the foot’s lateral column, where it functions as a link between the lateral column and transverse plantar arch.2 Regarding its anatomy, the cuboid is a pyramidal-shaped bone with 6 bony surfaces that provide tremendous stability—3 of these are articular, 3 nonarticular.
Although the cuboid bone is susceptible to low-energy avulsion injuries, injuries that occur in the setting of high-energy trauma are most concerning, as they often occur concurrently with other midfoot fractures and dislocations. These less common crush injuries are associated with comminution, articular disruption, and shortening of the lateral column.3-5 Avulsion injuries occur via a twisting mechanism, while the more complex nutcracker fracture evolves via longitudinal compression of the lateral column, with the foot in a position of forced plantarflexion.6 Other comminuted fractures occur from direct impact on the lateral aspect of the foot.
Management of cuboid fractures varies according to etiology, fracture displacement, and articular involvement. Conservative management is reserved solely for stable, nondisplaced fractures.7 Unstable fracture-dislocations and those with associated lateral column shortening necessitate operative treatment, which attempts to restore anatomy, stability, and length of the foot’s lateral column.7-9 However, with the exception of open injuries, fractures tenting the skin, and injuries with concomitant compartment syndrome, the high-energy nature of cuboid fractures often precludes early surgical intervention, as the foot’s soft-tissue envelope is too compromised. For this reason, operative intervention is often performed on a delayed basis only after recovery of the soft tissue.
In this case report and literature review, we describe a reconstructive shelf arthroplasty of the fifth tarsometatarsal (TMT) joint as a primary intervention for crush-type cuboid fractures with associated joint subsidence and lateral column shortening. The shelf arthroplasty, which was first credited to Konig in 1891, has historically been described as a remodeling operation using bone graft wedges for the treatment of nonconcentric acetabular dysplasia.10 Although bone grafting is recognized as an effective means of addressing osseous voids in the setting of comminuted cuboid fractures, its specific application in the form of a shelf arthroplasty has not been described.11 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 45-year-old woman presented to our institution’s emergency department (ED) complaining of right foot pain after a motor vehicle accident. She was the restrained driver in a head-on collision. Primary survey revealed a swollen, ecchymotic, and tender right foot. Radiographs demonstrated fractures of her first, second, third, and fourth metatarsals, and a comminuted cuboid fracture with lateral column shortening and disruption of the fifth TMT joint (Figure 1).
Due to swelling, initial management consisted of soft-tissue management through the use of a well-padded splint. As this was her only injury, she was instructed to remain non-weight-bearing, ambulate with crutches, and return to our outpatient office for close follow-up. The need for delayed surgical intervention of her multiple foot injuries, due to her compromised soft-tissue envelope, was discussed prior to discharge.
Surgical intervention was performed 15 days after the injury, when the soft-tissue swelling had dissipated. The surgical plan included fixation of the multiple metatarsal fractures and lateral column reconstruction and stabilization. With regard to the lateral column, we obtained patient consent for several possible procedures, including fifth TMT joint closed reduction and percutaneous pinning, open reduction and internal fixation (ORIF), and TMT joint reconstruction with iliac crest bone graft (ICBG).
The metatarsals were addressed first via a dorsomedial incision, using a 5-hole 2.7-mm Limited Contact Dynamic Compression Plate (Synthes) to stabilize the first metatarsal and 2.0-mm Kirschner wires (K-wires) to maintain the length and alignment of the second, third, and fourth metatarsals (Figure 2). Closed reduction and percutaneous pinning of the fifth metatarsal was then attempted but abandoned because of persistent instability and subsidence of the cuboid in the proximal and plantar direction. ORIF was then attempted through a dorsolateral incision extending from just distal to the sinus tarsi to the base of the fourth metatarsal. However, the lateral cuboid was too comminuted to accommodate any fixation and prevent fifth TMT joint subluxation and lateral column shortening.
Autograft reconstruction of the lateral column was therefore performed, using radiographs of the patient’s uninjured, contralateral foot as a template for our lateral column shelf arthroplasty (Figure 3). Based on this template, the length and alignment of the lateral column were provisionally maintained with two 2.0-mm K-wires placed between the fifth metatarsal and intact cuboid (Figure 4). Tricortical ICBG was then harvested through an anterior approach to the iliac crest and contoured accordingly to fill the osseous void. To facilitate graft incorporation, comminuted fragments of cuboid bone were removed, with the remaining bone decorticated. The graft was then fixed to the remaining cuboid with two 4.0-mm partially threaded cannulated screws (Synthes; Figures 2, 4). This construct restored the length of the lateral column and effectively buttressed the fifth TMT joint, preventing subsidence and dislocation of the TMT joint.
After a 2-day postoperative course in the hospital, the patient was discharged. She remained non-weight-bearing in a splint with Robert Jones cotton bandage. At her 2-week postoperative visit, all hardware was intact and there was no evidence of infection. Her sutures were removed and she was placed in a new splint. At the patient’s 5-week postoperative visit, all K-wires were removed. At this time she remained non-weight-bearing but was transitioned into a controlled ankle movement (CAM) boot and was allowed to begin active and passive ankle exercises. At her 10-week follow-up, radiographs revealed appropriate interval healing and callus formation. The patient began weight-bearing as tolerated in the CAM boot at that time. At 12 weeks, she was transitioned into a hard-soled shoe for comfort and was allowed to ambulate in the footwear of her choice as tolerated. Her activity levels were slowly advanced, and, at her 12-month follow-up, the patient had returned to playing tennis in her recreational league with no residual sequelae (Figure 5).
Discussion
Although rare, cuboid fractures are critical to identify and can result in significant disability, as they are frequently associated with additional foot trauma, as demonstrated in this case.1-4When isolated cuboid fractures are present, further imaging must be performed, including additional radiographic views and computed tomography, to search for other injuries, such as TMT joint complex disruption.
Only those cuboid fractures that are low-energy, stable, or nondisplaced can be effectively managed conservatively.12In the presence of instability, articular incongruity, or lateral column shortening, operative intervention is warranted. Arthritic degeneration, pain, and deformity result from residual incongruity at the calcaneocuboid or TMT joints, or when lateral column length is not restored.4-6,13 The latter leads to forefoot abduction and lateral subluxation of the lesser metatarsals, with ensuing posttraumatic pes planus or planovalgus deformity, which often necessitates secondary reconstructive procedures or arthrodesis.14,15 Stable reduction and restoration of lateral column length can be challenging, particularly in the setting of comminution and bone loss. Common methods of treatment involve lifting the dorsolateral cortex of the cuboid and buttressing the impacted articular surface with bone graft or bone graft substitutes. Fixation can be achieved with K-wires, small fragment plates and screws, and distraction external fixation.11 The latter is a particularly beneficial technique, as it can be used independent of or in conjunction with ORIF.
In a study by Weber and Locher,11 the short-term to midterm results of cuboid ORIF were assessed in 12 patients. Results were found to be good with respect to restoration of length, joint reconstruction, and overall return to function.11 Admittedly, these authors at times employed a similar but conceptually different approach to our patient. In their 7 patients with severe comminution and lateral column shortening, corticocancellous ICBG was used. However, Weber and Locher11did not describe this as a shelf arthroplasty, but instead as an adjunct to primary ORIF.
In our case, the tricortical ICBG shelf arthroplasty was used as it is in the hip, as a salvage procedure. Although little is known about outcomes following shelf arthroplasty for lateral column reconstruction in the foot, a 50% failure rate has been observed in the hip.16 As such, our preference was to perform an anatomic ORIF of the cuboid and lateral column, with the shelf arthroplasty only indicated if we were unable to achieve this. We believe that the need for tricortical ICBG in the treatment of cuboid fractures is indicative of a more severe injury and that it is a less optimal and more technically demanding intervention compared with primary ORIF. Furthermore, in other studies devoted to the treatment of cuboid fractures, patients requiring reconstruction with structural graft are not included in primary ORIF cohorts.17
As in the hip, suboptimal outcomes may occur when shelf arthroplasty is performed in the foot. There are additional considerations unique to the foot that surgeons must also contemplate when considering shelf arthroplasty. As demonstrated in the literature for adult-acquired flatfoot deformity, lateral column reconstruction is challenging and controversial and is associated with overload, pain, and the need to remove prominent hardware.18 These complications may also occur after shelf arthroplasty for cuboid fractures.
The work by Weber and Locher11 did not elucidate such considerations, and outcomes of ORIF and ICBG reconstruction were not compared. This is a limitation of their study, as differences in functional outcomes between the 2 procedures remain unknown. Given the degree of comminution that precludes ORIF and necessitates a graft reconstruction, we believe that the description of the shelf arthroplasty as a salvage procedure more accurately reflects the severity of injury. This may have implications regarding outcomes and patient expectations that the orthopedic surgeon must address. Future studies must further evaluate the outcomes of this technique, independent of and in comparison with ORIF.
Conclusion
In this case, we describe shelf arthroplasty for cuboid fractures. It is a reconstructive salvage procedure that is indicated when ORIF cannot be achieved. This useful approach to a complex injury must remain in the armamentarium of orthopedic surgeons. As we have demonstrated, it can effectively restore a damaged lateral column, providing length and, in our case, enabling the patient to return to her pre-injury level of activity.
1. Court-Brown C, Zinna S, Ekrol I. Classification and epidemiology of midfoot fractures. Foot. 2006;16(3):138-141.
2. Sarrafian SK. Osteology. In: Kelikian AS, ed. Sarrafian’s Anatomy of the Foot and Ankle. Philadelphia, PA: Lippincott; 1993:65-70.
3. Davis CA, Lubowitz J, Thordarson DB. Midtarsal fracture subluxation. Case report and review of the literature. Clin Orthop Relat Res. 1993;(292):264-268.
4. Dewar FP, Evans DC. Occult fracture-subluxation of the midtarsal joint. J Bone Joint Surg Br. 1968;50(2):386-388.
5. Sangeorzan BJ, Swiontkowski MF. Displaced fractures of the cuboid. J Bone Joint Surg Br. 1990;72(3):376-378.
6. Hermel MB, Gershon-Cohen J. The nutcracker fracture of the cuboid by indirect violence. Radiology. 1953;60(6):850-854.
7. Early J, Reid J. Fractures and dislocations of the midfoot and forefoot. In: Heckman JD, Bucholz RW, Court-Brown CM, Tornetta P, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:2120-2126.
8. Richter M, Wippermann B, Krettek C, Schratt HE, Hufner T, Therman H. Fractures and fracture dislocations of the midfoot: occurrence, causes and long-term results. Foot Ankle Int. 2001;22(5):392-398.
9. Borrelli J Jr, De S, VanPelt M. Fracture of the cuboid. J Am Acad Orthop Surg. 2012;20(7):472-477.
10. Love BRT, Stevens PM, Williams PF. A long-term review of shelf arthroplasty. J Bone Joint Surg Br. 1980;62(3):321-325.
11. Weber M, Locher S. Reconstruction of the cuboid in compression fractures: short to midterm results in 12 patients. Foot Ankle Int. 2002;23(11):1008-1013.
12. Ebizie AO. Crush fractures of the cuboid from indirect violence. Injury. 1991;22(5):414-416.
13. Berlet GC, Hodges Davis W, Anderson RB. Tendon arthroplasty for basal fourth and fifth metatarsal arthritis. Foot Ankle Int. 2002;23(5):440-444.
14. Brunet JA, Wiley JJ. The late results of tarsometatarsal joint injuries. J Bone Joint Surg Br. 1987;69(3):437-440.
15. DeAsla R, Deland J. Anatomy and biomechanics of the foot and ankle. In: Thordarson DB, Tornetta P, Einhorn TA, eds. Orthopaedic Surgery Essentials: Foot & Ankle. Philadelphia, PA: Lippincott William & Wilkins; 2004:18-23.
16. Berton C, Bocquet D, Krantz N, Cotton A, Migaud H, Girard J. Shelf arthroplasties long-term outcome: influence of labral tears. A prospective study at a minimal 16 years’ follows up. Orthop Traumatol Surg Res. 2010;96(7):753-759.
17. van Raaij TM, Duffy PJ, Buckley RE. Displaced isolated cuboid fractures: results of four cases with operative treatment. Foot Ankle Int. 2010;31(3):242-246.
18. Grier KM, Walling AK. The use of tricortical autograft versus allograft in lateral column lengthening for adult acquired flatfoot deformity: an analysis of union rates and complications. Foot Ankle Int. 2010;31(9):760-769.
Fractures of the cuboid bone are uncommon, with an annual incidence of approximately 1.8 per 100,000.1 This is largely attributed to the inherent stability provided by its anatomy and position in the foot’s lateral column, where it functions as a link between the lateral column and transverse plantar arch.2 Regarding its anatomy, the cuboid is a pyramidal-shaped bone with 6 bony surfaces that provide tremendous stability—3 of these are articular, 3 nonarticular.
Although the cuboid bone is susceptible to low-energy avulsion injuries, injuries that occur in the setting of high-energy trauma are most concerning, as they often occur concurrently with other midfoot fractures and dislocations. These less common crush injuries are associated with comminution, articular disruption, and shortening of the lateral column.3-5 Avulsion injuries occur via a twisting mechanism, while the more complex nutcracker fracture evolves via longitudinal compression of the lateral column, with the foot in a position of forced plantarflexion.6 Other comminuted fractures occur from direct impact on the lateral aspect of the foot.
Management of cuboid fractures varies according to etiology, fracture displacement, and articular involvement. Conservative management is reserved solely for stable, nondisplaced fractures.7 Unstable fracture-dislocations and those with associated lateral column shortening necessitate operative treatment, which attempts to restore anatomy, stability, and length of the foot’s lateral column.7-9 However, with the exception of open injuries, fractures tenting the skin, and injuries with concomitant compartment syndrome, the high-energy nature of cuboid fractures often precludes early surgical intervention, as the foot’s soft-tissue envelope is too compromised. For this reason, operative intervention is often performed on a delayed basis only after recovery of the soft tissue.
In this case report and literature review, we describe a reconstructive shelf arthroplasty of the fifth tarsometatarsal (TMT) joint as a primary intervention for crush-type cuboid fractures with associated joint subsidence and lateral column shortening. The shelf arthroplasty, which was first credited to Konig in 1891, has historically been described as a remodeling operation using bone graft wedges for the treatment of nonconcentric acetabular dysplasia.10 Although bone grafting is recognized as an effective means of addressing osseous voids in the setting of comminuted cuboid fractures, its specific application in the form of a shelf arthroplasty has not been described.11 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 45-year-old woman presented to our institution’s emergency department (ED) complaining of right foot pain after a motor vehicle accident. She was the restrained driver in a head-on collision. Primary survey revealed a swollen, ecchymotic, and tender right foot. Radiographs demonstrated fractures of her first, second, third, and fourth metatarsals, and a comminuted cuboid fracture with lateral column shortening and disruption of the fifth TMT joint (Figure 1).
Due to swelling, initial management consisted of soft-tissue management through the use of a well-padded splint. As this was her only injury, she was instructed to remain non-weight-bearing, ambulate with crutches, and return to our outpatient office for close follow-up. The need for delayed surgical intervention of her multiple foot injuries, due to her compromised soft-tissue envelope, was discussed prior to discharge.
Surgical intervention was performed 15 days after the injury, when the soft-tissue swelling had dissipated. The surgical plan included fixation of the multiple metatarsal fractures and lateral column reconstruction and stabilization. With regard to the lateral column, we obtained patient consent for several possible procedures, including fifth TMT joint closed reduction and percutaneous pinning, open reduction and internal fixation (ORIF), and TMT joint reconstruction with iliac crest bone graft (ICBG).
The metatarsals were addressed first via a dorsomedial incision, using a 5-hole 2.7-mm Limited Contact Dynamic Compression Plate (Synthes) to stabilize the first metatarsal and 2.0-mm Kirschner wires (K-wires) to maintain the length and alignment of the second, third, and fourth metatarsals (Figure 2). Closed reduction and percutaneous pinning of the fifth metatarsal was then attempted but abandoned because of persistent instability and subsidence of the cuboid in the proximal and plantar direction. ORIF was then attempted through a dorsolateral incision extending from just distal to the sinus tarsi to the base of the fourth metatarsal. However, the lateral cuboid was too comminuted to accommodate any fixation and prevent fifth TMT joint subluxation and lateral column shortening.
Autograft reconstruction of the lateral column was therefore performed, using radiographs of the patient’s uninjured, contralateral foot as a template for our lateral column shelf arthroplasty (Figure 3). Based on this template, the length and alignment of the lateral column were provisionally maintained with two 2.0-mm K-wires placed between the fifth metatarsal and intact cuboid (Figure 4). Tricortical ICBG was then harvested through an anterior approach to the iliac crest and contoured accordingly to fill the osseous void. To facilitate graft incorporation, comminuted fragments of cuboid bone were removed, with the remaining bone decorticated. The graft was then fixed to the remaining cuboid with two 4.0-mm partially threaded cannulated screws (Synthes; Figures 2, 4). This construct restored the length of the lateral column and effectively buttressed the fifth TMT joint, preventing subsidence and dislocation of the TMT joint.
After a 2-day postoperative course in the hospital, the patient was discharged. She remained non-weight-bearing in a splint with Robert Jones cotton bandage. At her 2-week postoperative visit, all hardware was intact and there was no evidence of infection. Her sutures were removed and she was placed in a new splint. At the patient’s 5-week postoperative visit, all K-wires were removed. At this time she remained non-weight-bearing but was transitioned into a controlled ankle movement (CAM) boot and was allowed to begin active and passive ankle exercises. At her 10-week follow-up, radiographs revealed appropriate interval healing and callus formation. The patient began weight-bearing as tolerated in the CAM boot at that time. At 12 weeks, she was transitioned into a hard-soled shoe for comfort and was allowed to ambulate in the footwear of her choice as tolerated. Her activity levels were slowly advanced, and, at her 12-month follow-up, the patient had returned to playing tennis in her recreational league with no residual sequelae (Figure 5).
Discussion
Although rare, cuboid fractures are critical to identify and can result in significant disability, as they are frequently associated with additional foot trauma, as demonstrated in this case.1-4When isolated cuboid fractures are present, further imaging must be performed, including additional radiographic views and computed tomography, to search for other injuries, such as TMT joint complex disruption.
Only those cuboid fractures that are low-energy, stable, or nondisplaced can be effectively managed conservatively.12In the presence of instability, articular incongruity, or lateral column shortening, operative intervention is warranted. Arthritic degeneration, pain, and deformity result from residual incongruity at the calcaneocuboid or TMT joints, or when lateral column length is not restored.4-6,13 The latter leads to forefoot abduction and lateral subluxation of the lesser metatarsals, with ensuing posttraumatic pes planus or planovalgus deformity, which often necessitates secondary reconstructive procedures or arthrodesis.14,15 Stable reduction and restoration of lateral column length can be challenging, particularly in the setting of comminution and bone loss. Common methods of treatment involve lifting the dorsolateral cortex of the cuboid and buttressing the impacted articular surface with bone graft or bone graft substitutes. Fixation can be achieved with K-wires, small fragment plates and screws, and distraction external fixation.11 The latter is a particularly beneficial technique, as it can be used independent of or in conjunction with ORIF.
In a study by Weber and Locher,11 the short-term to midterm results of cuboid ORIF were assessed in 12 patients. Results were found to be good with respect to restoration of length, joint reconstruction, and overall return to function.11 Admittedly, these authors at times employed a similar but conceptually different approach to our patient. In their 7 patients with severe comminution and lateral column shortening, corticocancellous ICBG was used. However, Weber and Locher11did not describe this as a shelf arthroplasty, but instead as an adjunct to primary ORIF.
In our case, the tricortical ICBG shelf arthroplasty was used as it is in the hip, as a salvage procedure. Although little is known about outcomes following shelf arthroplasty for lateral column reconstruction in the foot, a 50% failure rate has been observed in the hip.16 As such, our preference was to perform an anatomic ORIF of the cuboid and lateral column, with the shelf arthroplasty only indicated if we were unable to achieve this. We believe that the need for tricortical ICBG in the treatment of cuboid fractures is indicative of a more severe injury and that it is a less optimal and more technically demanding intervention compared with primary ORIF. Furthermore, in other studies devoted to the treatment of cuboid fractures, patients requiring reconstruction with structural graft are not included in primary ORIF cohorts.17
As in the hip, suboptimal outcomes may occur when shelf arthroplasty is performed in the foot. There are additional considerations unique to the foot that surgeons must also contemplate when considering shelf arthroplasty. As demonstrated in the literature for adult-acquired flatfoot deformity, lateral column reconstruction is challenging and controversial and is associated with overload, pain, and the need to remove prominent hardware.18 These complications may also occur after shelf arthroplasty for cuboid fractures.
The work by Weber and Locher11 did not elucidate such considerations, and outcomes of ORIF and ICBG reconstruction were not compared. This is a limitation of their study, as differences in functional outcomes between the 2 procedures remain unknown. Given the degree of comminution that precludes ORIF and necessitates a graft reconstruction, we believe that the description of the shelf arthroplasty as a salvage procedure more accurately reflects the severity of injury. This may have implications regarding outcomes and patient expectations that the orthopedic surgeon must address. Future studies must further evaluate the outcomes of this technique, independent of and in comparison with ORIF.
Conclusion
In this case, we describe shelf arthroplasty for cuboid fractures. It is a reconstructive salvage procedure that is indicated when ORIF cannot be achieved. This useful approach to a complex injury must remain in the armamentarium of orthopedic surgeons. As we have demonstrated, it can effectively restore a damaged lateral column, providing length and, in our case, enabling the patient to return to her pre-injury level of activity.
Fractures of the cuboid bone are uncommon, with an annual incidence of approximately 1.8 per 100,000.1 This is largely attributed to the inherent stability provided by its anatomy and position in the foot’s lateral column, where it functions as a link between the lateral column and transverse plantar arch.2 Regarding its anatomy, the cuboid is a pyramidal-shaped bone with 6 bony surfaces that provide tremendous stability—3 of these are articular, 3 nonarticular.
Although the cuboid bone is susceptible to low-energy avulsion injuries, injuries that occur in the setting of high-energy trauma are most concerning, as they often occur concurrently with other midfoot fractures and dislocations. These less common crush injuries are associated with comminution, articular disruption, and shortening of the lateral column.3-5 Avulsion injuries occur via a twisting mechanism, while the more complex nutcracker fracture evolves via longitudinal compression of the lateral column, with the foot in a position of forced plantarflexion.6 Other comminuted fractures occur from direct impact on the lateral aspect of the foot.
Management of cuboid fractures varies according to etiology, fracture displacement, and articular involvement. Conservative management is reserved solely for stable, nondisplaced fractures.7 Unstable fracture-dislocations and those with associated lateral column shortening necessitate operative treatment, which attempts to restore anatomy, stability, and length of the foot’s lateral column.7-9 However, with the exception of open injuries, fractures tenting the skin, and injuries with concomitant compartment syndrome, the high-energy nature of cuboid fractures often precludes early surgical intervention, as the foot’s soft-tissue envelope is too compromised. For this reason, operative intervention is often performed on a delayed basis only after recovery of the soft tissue.
In this case report and literature review, we describe a reconstructive shelf arthroplasty of the fifth tarsometatarsal (TMT) joint as a primary intervention for crush-type cuboid fractures with associated joint subsidence and lateral column shortening. The shelf arthroplasty, which was first credited to Konig in 1891, has historically been described as a remodeling operation using bone graft wedges for the treatment of nonconcentric acetabular dysplasia.10 Although bone grafting is recognized as an effective means of addressing osseous voids in the setting of comminuted cuboid fractures, its specific application in the form of a shelf arthroplasty has not been described.11 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 45-year-old woman presented to our institution’s emergency department (ED) complaining of right foot pain after a motor vehicle accident. She was the restrained driver in a head-on collision. Primary survey revealed a swollen, ecchymotic, and tender right foot. Radiographs demonstrated fractures of her first, second, third, and fourth metatarsals, and a comminuted cuboid fracture with lateral column shortening and disruption of the fifth TMT joint (Figure 1).
Due to swelling, initial management consisted of soft-tissue management through the use of a well-padded splint. As this was her only injury, she was instructed to remain non-weight-bearing, ambulate with crutches, and return to our outpatient office for close follow-up. The need for delayed surgical intervention of her multiple foot injuries, due to her compromised soft-tissue envelope, was discussed prior to discharge.
Surgical intervention was performed 15 days after the injury, when the soft-tissue swelling had dissipated. The surgical plan included fixation of the multiple metatarsal fractures and lateral column reconstruction and stabilization. With regard to the lateral column, we obtained patient consent for several possible procedures, including fifth TMT joint closed reduction and percutaneous pinning, open reduction and internal fixation (ORIF), and TMT joint reconstruction with iliac crest bone graft (ICBG).
The metatarsals were addressed first via a dorsomedial incision, using a 5-hole 2.7-mm Limited Contact Dynamic Compression Plate (Synthes) to stabilize the first metatarsal and 2.0-mm Kirschner wires (K-wires) to maintain the length and alignment of the second, third, and fourth metatarsals (Figure 2). Closed reduction and percutaneous pinning of the fifth metatarsal was then attempted but abandoned because of persistent instability and subsidence of the cuboid in the proximal and plantar direction. ORIF was then attempted through a dorsolateral incision extending from just distal to the sinus tarsi to the base of the fourth metatarsal. However, the lateral cuboid was too comminuted to accommodate any fixation and prevent fifth TMT joint subluxation and lateral column shortening.
Autograft reconstruction of the lateral column was therefore performed, using radiographs of the patient’s uninjured, contralateral foot as a template for our lateral column shelf arthroplasty (Figure 3). Based on this template, the length and alignment of the lateral column were provisionally maintained with two 2.0-mm K-wires placed between the fifth metatarsal and intact cuboid (Figure 4). Tricortical ICBG was then harvested through an anterior approach to the iliac crest and contoured accordingly to fill the osseous void. To facilitate graft incorporation, comminuted fragments of cuboid bone were removed, with the remaining bone decorticated. The graft was then fixed to the remaining cuboid with two 4.0-mm partially threaded cannulated screws (Synthes; Figures 2, 4). This construct restored the length of the lateral column and effectively buttressed the fifth TMT joint, preventing subsidence and dislocation of the TMT joint.
After a 2-day postoperative course in the hospital, the patient was discharged. She remained non-weight-bearing in a splint with Robert Jones cotton bandage. At her 2-week postoperative visit, all hardware was intact and there was no evidence of infection. Her sutures were removed and she was placed in a new splint. At the patient’s 5-week postoperative visit, all K-wires were removed. At this time she remained non-weight-bearing but was transitioned into a controlled ankle movement (CAM) boot and was allowed to begin active and passive ankle exercises. At her 10-week follow-up, radiographs revealed appropriate interval healing and callus formation. The patient began weight-bearing as tolerated in the CAM boot at that time. At 12 weeks, she was transitioned into a hard-soled shoe for comfort and was allowed to ambulate in the footwear of her choice as tolerated. Her activity levels were slowly advanced, and, at her 12-month follow-up, the patient had returned to playing tennis in her recreational league with no residual sequelae (Figure 5).
Discussion
Although rare, cuboid fractures are critical to identify and can result in significant disability, as they are frequently associated with additional foot trauma, as demonstrated in this case.1-4When isolated cuboid fractures are present, further imaging must be performed, including additional radiographic views and computed tomography, to search for other injuries, such as TMT joint complex disruption.
Only those cuboid fractures that are low-energy, stable, or nondisplaced can be effectively managed conservatively.12In the presence of instability, articular incongruity, or lateral column shortening, operative intervention is warranted. Arthritic degeneration, pain, and deformity result from residual incongruity at the calcaneocuboid or TMT joints, or when lateral column length is not restored.4-6,13 The latter leads to forefoot abduction and lateral subluxation of the lesser metatarsals, with ensuing posttraumatic pes planus or planovalgus deformity, which often necessitates secondary reconstructive procedures or arthrodesis.14,15 Stable reduction and restoration of lateral column length can be challenging, particularly in the setting of comminution and bone loss. Common methods of treatment involve lifting the dorsolateral cortex of the cuboid and buttressing the impacted articular surface with bone graft or bone graft substitutes. Fixation can be achieved with K-wires, small fragment plates and screws, and distraction external fixation.11 The latter is a particularly beneficial technique, as it can be used independent of or in conjunction with ORIF.
In a study by Weber and Locher,11 the short-term to midterm results of cuboid ORIF were assessed in 12 patients. Results were found to be good with respect to restoration of length, joint reconstruction, and overall return to function.11 Admittedly, these authors at times employed a similar but conceptually different approach to our patient. In their 7 patients with severe comminution and lateral column shortening, corticocancellous ICBG was used. However, Weber and Locher11did not describe this as a shelf arthroplasty, but instead as an adjunct to primary ORIF.
In our case, the tricortical ICBG shelf arthroplasty was used as it is in the hip, as a salvage procedure. Although little is known about outcomes following shelf arthroplasty for lateral column reconstruction in the foot, a 50% failure rate has been observed in the hip.16 As such, our preference was to perform an anatomic ORIF of the cuboid and lateral column, with the shelf arthroplasty only indicated if we were unable to achieve this. We believe that the need for tricortical ICBG in the treatment of cuboid fractures is indicative of a more severe injury and that it is a less optimal and more technically demanding intervention compared with primary ORIF. Furthermore, in other studies devoted to the treatment of cuboid fractures, patients requiring reconstruction with structural graft are not included in primary ORIF cohorts.17
As in the hip, suboptimal outcomes may occur when shelf arthroplasty is performed in the foot. There are additional considerations unique to the foot that surgeons must also contemplate when considering shelf arthroplasty. As demonstrated in the literature for adult-acquired flatfoot deformity, lateral column reconstruction is challenging and controversial and is associated with overload, pain, and the need to remove prominent hardware.18 These complications may also occur after shelf arthroplasty for cuboid fractures.
The work by Weber and Locher11 did not elucidate such considerations, and outcomes of ORIF and ICBG reconstruction were not compared. This is a limitation of their study, as differences in functional outcomes between the 2 procedures remain unknown. Given the degree of comminution that precludes ORIF and necessitates a graft reconstruction, we believe that the description of the shelf arthroplasty as a salvage procedure more accurately reflects the severity of injury. This may have implications regarding outcomes and patient expectations that the orthopedic surgeon must address. Future studies must further evaluate the outcomes of this technique, independent of and in comparison with ORIF.
Conclusion
In this case, we describe shelf arthroplasty for cuboid fractures. It is a reconstructive salvage procedure that is indicated when ORIF cannot be achieved. This useful approach to a complex injury must remain in the armamentarium of orthopedic surgeons. As we have demonstrated, it can effectively restore a damaged lateral column, providing length and, in our case, enabling the patient to return to her pre-injury level of activity.
1. Court-Brown C, Zinna S, Ekrol I. Classification and epidemiology of midfoot fractures. Foot. 2006;16(3):138-141.
2. Sarrafian SK. Osteology. In: Kelikian AS, ed. Sarrafian’s Anatomy of the Foot and Ankle. Philadelphia, PA: Lippincott; 1993:65-70.
3. Davis CA, Lubowitz J, Thordarson DB. Midtarsal fracture subluxation. Case report and review of the literature. Clin Orthop Relat Res. 1993;(292):264-268.
4. Dewar FP, Evans DC. Occult fracture-subluxation of the midtarsal joint. J Bone Joint Surg Br. 1968;50(2):386-388.
5. Sangeorzan BJ, Swiontkowski MF. Displaced fractures of the cuboid. J Bone Joint Surg Br. 1990;72(3):376-378.
6. Hermel MB, Gershon-Cohen J. The nutcracker fracture of the cuboid by indirect violence. Radiology. 1953;60(6):850-854.
7. Early J, Reid J. Fractures and dislocations of the midfoot and forefoot. In: Heckman JD, Bucholz RW, Court-Brown CM, Tornetta P, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:2120-2126.
8. Richter M, Wippermann B, Krettek C, Schratt HE, Hufner T, Therman H. Fractures and fracture dislocations of the midfoot: occurrence, causes and long-term results. Foot Ankle Int. 2001;22(5):392-398.
9. Borrelli J Jr, De S, VanPelt M. Fracture of the cuboid. J Am Acad Orthop Surg. 2012;20(7):472-477.
10. Love BRT, Stevens PM, Williams PF. A long-term review of shelf arthroplasty. J Bone Joint Surg Br. 1980;62(3):321-325.
11. Weber M, Locher S. Reconstruction of the cuboid in compression fractures: short to midterm results in 12 patients. Foot Ankle Int. 2002;23(11):1008-1013.
12. Ebizie AO. Crush fractures of the cuboid from indirect violence. Injury. 1991;22(5):414-416.
13. Berlet GC, Hodges Davis W, Anderson RB. Tendon arthroplasty for basal fourth and fifth metatarsal arthritis. Foot Ankle Int. 2002;23(5):440-444.
14. Brunet JA, Wiley JJ. The late results of tarsometatarsal joint injuries. J Bone Joint Surg Br. 1987;69(3):437-440.
15. DeAsla R, Deland J. Anatomy and biomechanics of the foot and ankle. In: Thordarson DB, Tornetta P, Einhorn TA, eds. Orthopaedic Surgery Essentials: Foot & Ankle. Philadelphia, PA: Lippincott William & Wilkins; 2004:18-23.
16. Berton C, Bocquet D, Krantz N, Cotton A, Migaud H, Girard J. Shelf arthroplasties long-term outcome: influence of labral tears. A prospective study at a minimal 16 years’ follows up. Orthop Traumatol Surg Res. 2010;96(7):753-759.
17. van Raaij TM, Duffy PJ, Buckley RE. Displaced isolated cuboid fractures: results of four cases with operative treatment. Foot Ankle Int. 2010;31(3):242-246.
18. Grier KM, Walling AK. The use of tricortical autograft versus allograft in lateral column lengthening for adult acquired flatfoot deformity: an analysis of union rates and complications. Foot Ankle Int. 2010;31(9):760-769.
1. Court-Brown C, Zinna S, Ekrol I. Classification and epidemiology of midfoot fractures. Foot. 2006;16(3):138-141.
2. Sarrafian SK. Osteology. In: Kelikian AS, ed. Sarrafian’s Anatomy of the Foot and Ankle. Philadelphia, PA: Lippincott; 1993:65-70.
3. Davis CA, Lubowitz J, Thordarson DB. Midtarsal fracture subluxation. Case report and review of the literature. Clin Orthop Relat Res. 1993;(292):264-268.
4. Dewar FP, Evans DC. Occult fracture-subluxation of the midtarsal joint. J Bone Joint Surg Br. 1968;50(2):386-388.
5. Sangeorzan BJ, Swiontkowski MF. Displaced fractures of the cuboid. J Bone Joint Surg Br. 1990;72(3):376-378.
6. Hermel MB, Gershon-Cohen J. The nutcracker fracture of the cuboid by indirect violence. Radiology. 1953;60(6):850-854.
7. Early J, Reid J. Fractures and dislocations of the midfoot and forefoot. In: Heckman JD, Bucholz RW, Court-Brown CM, Tornetta P, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:2120-2126.
8. Richter M, Wippermann B, Krettek C, Schratt HE, Hufner T, Therman H. Fractures and fracture dislocations of the midfoot: occurrence, causes and long-term results. Foot Ankle Int. 2001;22(5):392-398.
9. Borrelli J Jr, De S, VanPelt M. Fracture of the cuboid. J Am Acad Orthop Surg. 2012;20(7):472-477.
10. Love BRT, Stevens PM, Williams PF. A long-term review of shelf arthroplasty. J Bone Joint Surg Br. 1980;62(3):321-325.
11. Weber M, Locher S. Reconstruction of the cuboid in compression fractures: short to midterm results in 12 patients. Foot Ankle Int. 2002;23(11):1008-1013.
12. Ebizie AO. Crush fractures of the cuboid from indirect violence. Injury. 1991;22(5):414-416.
13. Berlet GC, Hodges Davis W, Anderson RB. Tendon arthroplasty for basal fourth and fifth metatarsal arthritis. Foot Ankle Int. 2002;23(5):440-444.
14. Brunet JA, Wiley JJ. The late results of tarsometatarsal joint injuries. J Bone Joint Surg Br. 1987;69(3):437-440.
15. DeAsla R, Deland J. Anatomy and biomechanics of the foot and ankle. In: Thordarson DB, Tornetta P, Einhorn TA, eds. Orthopaedic Surgery Essentials: Foot & Ankle. Philadelphia, PA: Lippincott William & Wilkins; 2004:18-23.
16. Berton C, Bocquet D, Krantz N, Cotton A, Migaud H, Girard J. Shelf arthroplasties long-term outcome: influence of labral tears. A prospective study at a minimal 16 years’ follows up. Orthop Traumatol Surg Res. 2010;96(7):753-759.
17. van Raaij TM, Duffy PJ, Buckley RE. Displaced isolated cuboid fractures: results of four cases with operative treatment. Foot Ankle Int. 2010;31(3):242-246.
18. Grier KM, Walling AK. The use of tricortical autograft versus allograft in lateral column lengthening for adult acquired flatfoot deformity: an analysis of union rates and complications. Foot Ankle Int. 2010;31(9):760-769.
Phenotype HNPP (Hereditary Neuropathy With Liability to Pressure Palsies) Induced by Medical Procedures
PMP22 is a tetra-span membrane protein primarily expressed in myelinating Schwann cells. Heterozygous deletion of the PMP22 gene (1 copy) causes HNPP (hereditary neuropathy with liability to pressure palsies).1 Interestingly, a reciprocal genetic disorder with 3 copies of human PMP22 causes the most common inherited neuropathy, Charcot-Marie-Tooth disease type 1A (CMT1A).2,3 As the reciprocal mutations occur at initiation of gestation, it is expected that HNPP and CMT1A have a similar prevalence. However, studies have shown HNPP prevalence of 2 to 5 cases per 100,000, far below the CMT1A prevalence of 1:5000.4 This finding prompted speculation that many patients with HNPP may be undiagnosed because of the subtlety of the phenotypes.5
Patients with HNPP typically present with focal sensory loss and muscle weakness related to mechanical stress–induced failure of action potential propagation.6,7 In this article, we report the case of an asymptomatic woman with the HNPP mutation. Her focal neurologic deficits occurred only after total knee arthroplasty (TKA), which in healthy patients is not expected to induce focal sensory and motor symptoms. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient, a healthy 57-year-old woman, had a normal developmental history. For decades, she had practiced ballet without any physical difficulties. She underwent left TKA and woke up with a footdrop on the left side. The left foot was less sensitive to temperature. Ankle strength returned 2 months later. There was no family history of HNPP.
The patient was examined by a local neurologist, who found steppage gait, weak ankle dorsiflexion (4 on Medical Research Council scale), and diminished touch on the lateral aspect of the left leg. Deep tendon reflexes were present in the arms but not the legs.
A nerve conduction study (NCS) performed after the footdrop revealed prolonged distal latency and decreased amplitude in the left peroneal and tibial nerves. The left sural nerve was normal. Needle electromyogram revealed denervation changes in the muscles innervated by the left peroneal nerve (Table). In addition, we also performed an NCS on the arm (Table), which was unaffected by the surgical procedure. This NCS revealed severely prolonged distal latency across the left wrist in the median nerve and focal slowing of conduction velocity of the ulnar nerve across the left elbow. These changes provide evidence of asymptomatic carpal tunnel syndrome and ulnar nerve entrapment, typical electrophysiologic abnormalities of HNPP.8As there was no explanation for the footdrop from the surgery, we had a DNA test performed (Athena Diagnostics). This test identified a heterozygous deletion of chromosome 17p12 containing the PMP22 gene, the HNPP mutation.
Discussion
This case had several important features. First, though the patient developed an electrophysiologic phenotype of HNPP, she was completely asymptomatic clinically and very athletic before her medical procedure. She would not have been diagnosed with HNPP if her clinical deficits had not been induced by TKA. Therefore, the prevalence of HNPP is likely underestimated. Second, for patients with the HNPP mutation, there may be serious neurologic consequences of certain medical procedures. The diagnosis of HNPP should be pursued if there is no explanation from the medical procedure per se. In addition, patients with a family history of HNPP should be carefully evaluated before any procedure that may put them at risk for severe peripheral nerve damage, and they should be counseled regarding the risks. It is important to determine the prevalence of HNPP among patients who develop footdrop after knee arthroplasty, as this information could potentially be used to revise ideas about the etiology of peripheral nerve complications of knee arthroplasty. We now describe possible revisions of these ideas.
Footdrop is a rare complication of TKA. Retrospective studies have found its incidence ranging from 0.3% to 1.3%.9-11 The investigators in those studies postulated 3 main causes for peroneal nerve palsy. First, traction may put pressure on the peroneal nerve during normalization of the mechanical axis of a valgus knee. Our patient did not have a valgus knee. Second, epidural hematoma by anesthetic procedure may compress the spinal roots. Our patient received general anesthesia during the procedure; epidural or spinal anesthesia was not used. Third, postoperative dressing may compress the nerve. Our patient did not develop any signs of constrictive dressing, such as inordinate pain, which can be relieved by removing the dressing, and swelling of the leg distal to the dressing. Therefore, her footdrop likely was not a complication of surgery.
This case demonstrates how a patient with undiagnosed HNPP can manifest the HNPP phenotype only after undergoing a particular surgical procedure. HNPP is unfamiliar to most orthopedic surgeons.
1. Chance PF, Alderson MK, Leppig KA, et al. DNA deletion associated with hereditary neuropathy with liability to pressure palsies. Cell. 1993;72(1):143-151.
2. Lupski JR, de Oca-Luna RM, Slaugenhaupt S, et al. DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell. 1991;66(2):219-232.
3. Raeymaekers P, Timmerman V, Nelis E, et al. Estimation of the size of the chromosome 17p11.2 duplication in Charcot-Marie-Tooth neuropathy type 1a (CMT1a). HMSN Collaborative Research Group. J Med Genet. 1992;29(1):5-11.
4. Meretoja P, Silander K, Kalimo H, Aula P, Meretoja A, Savontaus ML. Epidemiology of hereditary neuropathy with liability to pressure palsies (HNPP) in south western Finland. Neuromuscul Disord. 1997;7(8):529-532.
5. Li J, Parker B, Martyn C, Natarajan C, Guo J. The PMP22 gene and its related diseases. Mol Neurobiol. 2013;47(2):673-698.
6. Bai Y, Zhang X, Katona I, et al. Conduction block in PMP22 deficiency. J Neurosci. 2010;30(2):600-608.
7. Guo J, Wang L, Zhang Y, et al. Abnormal junctions and permeability of myelin in PMP22-deficient nerves. Ann Neurol. 2014;75(2):255-265.
8. Li J, Krajewski K, Shy ME, Lewis RA. Hereditary neuropathy with liability to pressure palsy: the electrophysiology fits the name. Neurology. 2002;58(12):1769-1773.
9. Rose HA, Hood RW, Otis JC, Ranawat CS, Insall JN. Peroneal-nerve palsy following total knee arthroplasty. A review of the Hospital for Special Surgery experience. J Bone Joint Surg Am. 1982;64(3):347-351.
10. Schinsky MF, Macaulay W, Parks ML, Kiernan H, Nercessian OA. Nerve injury after primary total knee arthroplasty. J Arthroplasty. 2001;16(8):1048-1054.
11. Nercessian OA, Ugwonali OF, Park S. Peroneal nerve palsy after total knee arthroplasty. J Arthroplasty. 2005;20(8):1068-1073.
PMP22 is a tetra-span membrane protein primarily expressed in myelinating Schwann cells. Heterozygous deletion of the PMP22 gene (1 copy) causes HNPP (hereditary neuropathy with liability to pressure palsies).1 Interestingly, a reciprocal genetic disorder with 3 copies of human PMP22 causes the most common inherited neuropathy, Charcot-Marie-Tooth disease type 1A (CMT1A).2,3 As the reciprocal mutations occur at initiation of gestation, it is expected that HNPP and CMT1A have a similar prevalence. However, studies have shown HNPP prevalence of 2 to 5 cases per 100,000, far below the CMT1A prevalence of 1:5000.4 This finding prompted speculation that many patients with HNPP may be undiagnosed because of the subtlety of the phenotypes.5
Patients with HNPP typically present with focal sensory loss and muscle weakness related to mechanical stress–induced failure of action potential propagation.6,7 In this article, we report the case of an asymptomatic woman with the HNPP mutation. Her focal neurologic deficits occurred only after total knee arthroplasty (TKA), which in healthy patients is not expected to induce focal sensory and motor symptoms. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient, a healthy 57-year-old woman, had a normal developmental history. For decades, she had practiced ballet without any physical difficulties. She underwent left TKA and woke up with a footdrop on the left side. The left foot was less sensitive to temperature. Ankle strength returned 2 months later. There was no family history of HNPP.
The patient was examined by a local neurologist, who found steppage gait, weak ankle dorsiflexion (4 on Medical Research Council scale), and diminished touch on the lateral aspect of the left leg. Deep tendon reflexes were present in the arms but not the legs.
A nerve conduction study (NCS) performed after the footdrop revealed prolonged distal latency and decreased amplitude in the left peroneal and tibial nerves. The left sural nerve was normal. Needle electromyogram revealed denervation changes in the muscles innervated by the left peroneal nerve (Table). In addition, we also performed an NCS on the arm (Table), which was unaffected by the surgical procedure. This NCS revealed severely prolonged distal latency across the left wrist in the median nerve and focal slowing of conduction velocity of the ulnar nerve across the left elbow. These changes provide evidence of asymptomatic carpal tunnel syndrome and ulnar nerve entrapment, typical electrophysiologic abnormalities of HNPP.8As there was no explanation for the footdrop from the surgery, we had a DNA test performed (Athena Diagnostics). This test identified a heterozygous deletion of chromosome 17p12 containing the PMP22 gene, the HNPP mutation.
Discussion
This case had several important features. First, though the patient developed an electrophysiologic phenotype of HNPP, she was completely asymptomatic clinically and very athletic before her medical procedure. She would not have been diagnosed with HNPP if her clinical deficits had not been induced by TKA. Therefore, the prevalence of HNPP is likely underestimated. Second, for patients with the HNPP mutation, there may be serious neurologic consequences of certain medical procedures. The diagnosis of HNPP should be pursued if there is no explanation from the medical procedure per se. In addition, patients with a family history of HNPP should be carefully evaluated before any procedure that may put them at risk for severe peripheral nerve damage, and they should be counseled regarding the risks. It is important to determine the prevalence of HNPP among patients who develop footdrop after knee arthroplasty, as this information could potentially be used to revise ideas about the etiology of peripheral nerve complications of knee arthroplasty. We now describe possible revisions of these ideas.
Footdrop is a rare complication of TKA. Retrospective studies have found its incidence ranging from 0.3% to 1.3%.9-11 The investigators in those studies postulated 3 main causes for peroneal nerve palsy. First, traction may put pressure on the peroneal nerve during normalization of the mechanical axis of a valgus knee. Our patient did not have a valgus knee. Second, epidural hematoma by anesthetic procedure may compress the spinal roots. Our patient received general anesthesia during the procedure; epidural or spinal anesthesia was not used. Third, postoperative dressing may compress the nerve. Our patient did not develop any signs of constrictive dressing, such as inordinate pain, which can be relieved by removing the dressing, and swelling of the leg distal to the dressing. Therefore, her footdrop likely was not a complication of surgery.
This case demonstrates how a patient with undiagnosed HNPP can manifest the HNPP phenotype only after undergoing a particular surgical procedure. HNPP is unfamiliar to most orthopedic surgeons.
PMP22 is a tetra-span membrane protein primarily expressed in myelinating Schwann cells. Heterozygous deletion of the PMP22 gene (1 copy) causes HNPP (hereditary neuropathy with liability to pressure palsies).1 Interestingly, a reciprocal genetic disorder with 3 copies of human PMP22 causes the most common inherited neuropathy, Charcot-Marie-Tooth disease type 1A (CMT1A).2,3 As the reciprocal mutations occur at initiation of gestation, it is expected that HNPP and CMT1A have a similar prevalence. However, studies have shown HNPP prevalence of 2 to 5 cases per 100,000, far below the CMT1A prevalence of 1:5000.4 This finding prompted speculation that many patients with HNPP may be undiagnosed because of the subtlety of the phenotypes.5
Patients with HNPP typically present with focal sensory loss and muscle weakness related to mechanical stress–induced failure of action potential propagation.6,7 In this article, we report the case of an asymptomatic woman with the HNPP mutation. Her focal neurologic deficits occurred only after total knee arthroplasty (TKA), which in healthy patients is not expected to induce focal sensory and motor symptoms. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient, a healthy 57-year-old woman, had a normal developmental history. For decades, she had practiced ballet without any physical difficulties. She underwent left TKA and woke up with a footdrop on the left side. The left foot was less sensitive to temperature. Ankle strength returned 2 months later. There was no family history of HNPP.
The patient was examined by a local neurologist, who found steppage gait, weak ankle dorsiflexion (4 on Medical Research Council scale), and diminished touch on the lateral aspect of the left leg. Deep tendon reflexes were present in the arms but not the legs.
A nerve conduction study (NCS) performed after the footdrop revealed prolonged distal latency and decreased amplitude in the left peroneal and tibial nerves. The left sural nerve was normal. Needle electromyogram revealed denervation changes in the muscles innervated by the left peroneal nerve (Table). In addition, we also performed an NCS on the arm (Table), which was unaffected by the surgical procedure. This NCS revealed severely prolonged distal latency across the left wrist in the median nerve and focal slowing of conduction velocity of the ulnar nerve across the left elbow. These changes provide evidence of asymptomatic carpal tunnel syndrome and ulnar nerve entrapment, typical electrophysiologic abnormalities of HNPP.8As there was no explanation for the footdrop from the surgery, we had a DNA test performed (Athena Diagnostics). This test identified a heterozygous deletion of chromosome 17p12 containing the PMP22 gene, the HNPP mutation.
Discussion
This case had several important features. First, though the patient developed an electrophysiologic phenotype of HNPP, she was completely asymptomatic clinically and very athletic before her medical procedure. She would not have been diagnosed with HNPP if her clinical deficits had not been induced by TKA. Therefore, the prevalence of HNPP is likely underestimated. Second, for patients with the HNPP mutation, there may be serious neurologic consequences of certain medical procedures. The diagnosis of HNPP should be pursued if there is no explanation from the medical procedure per se. In addition, patients with a family history of HNPP should be carefully evaluated before any procedure that may put them at risk for severe peripheral nerve damage, and they should be counseled regarding the risks. It is important to determine the prevalence of HNPP among patients who develop footdrop after knee arthroplasty, as this information could potentially be used to revise ideas about the etiology of peripheral nerve complications of knee arthroplasty. We now describe possible revisions of these ideas.
Footdrop is a rare complication of TKA. Retrospective studies have found its incidence ranging from 0.3% to 1.3%.9-11 The investigators in those studies postulated 3 main causes for peroneal nerve palsy. First, traction may put pressure on the peroneal nerve during normalization of the mechanical axis of a valgus knee. Our patient did not have a valgus knee. Second, epidural hematoma by anesthetic procedure may compress the spinal roots. Our patient received general anesthesia during the procedure; epidural or spinal anesthesia was not used. Third, postoperative dressing may compress the nerve. Our patient did not develop any signs of constrictive dressing, such as inordinate pain, which can be relieved by removing the dressing, and swelling of the leg distal to the dressing. Therefore, her footdrop likely was not a complication of surgery.
This case demonstrates how a patient with undiagnosed HNPP can manifest the HNPP phenotype only after undergoing a particular surgical procedure. HNPP is unfamiliar to most orthopedic surgeons.
1. Chance PF, Alderson MK, Leppig KA, et al. DNA deletion associated with hereditary neuropathy with liability to pressure palsies. Cell. 1993;72(1):143-151.
2. Lupski JR, de Oca-Luna RM, Slaugenhaupt S, et al. DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell. 1991;66(2):219-232.
3. Raeymaekers P, Timmerman V, Nelis E, et al. Estimation of the size of the chromosome 17p11.2 duplication in Charcot-Marie-Tooth neuropathy type 1a (CMT1a). HMSN Collaborative Research Group. J Med Genet. 1992;29(1):5-11.
4. Meretoja P, Silander K, Kalimo H, Aula P, Meretoja A, Savontaus ML. Epidemiology of hereditary neuropathy with liability to pressure palsies (HNPP) in south western Finland. Neuromuscul Disord. 1997;7(8):529-532.
5. Li J, Parker B, Martyn C, Natarajan C, Guo J. The PMP22 gene and its related diseases. Mol Neurobiol. 2013;47(2):673-698.
6. Bai Y, Zhang X, Katona I, et al. Conduction block in PMP22 deficiency. J Neurosci. 2010;30(2):600-608.
7. Guo J, Wang L, Zhang Y, et al. Abnormal junctions and permeability of myelin in PMP22-deficient nerves. Ann Neurol. 2014;75(2):255-265.
8. Li J, Krajewski K, Shy ME, Lewis RA. Hereditary neuropathy with liability to pressure palsy: the electrophysiology fits the name. Neurology. 2002;58(12):1769-1773.
9. Rose HA, Hood RW, Otis JC, Ranawat CS, Insall JN. Peroneal-nerve palsy following total knee arthroplasty. A review of the Hospital for Special Surgery experience. J Bone Joint Surg Am. 1982;64(3):347-351.
10. Schinsky MF, Macaulay W, Parks ML, Kiernan H, Nercessian OA. Nerve injury after primary total knee arthroplasty. J Arthroplasty. 2001;16(8):1048-1054.
11. Nercessian OA, Ugwonali OF, Park S. Peroneal nerve palsy after total knee arthroplasty. J Arthroplasty. 2005;20(8):1068-1073.
1. Chance PF, Alderson MK, Leppig KA, et al. DNA deletion associated with hereditary neuropathy with liability to pressure palsies. Cell. 1993;72(1):143-151.
2. Lupski JR, de Oca-Luna RM, Slaugenhaupt S, et al. DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell. 1991;66(2):219-232.
3. Raeymaekers P, Timmerman V, Nelis E, et al. Estimation of the size of the chromosome 17p11.2 duplication in Charcot-Marie-Tooth neuropathy type 1a (CMT1a). HMSN Collaborative Research Group. J Med Genet. 1992;29(1):5-11.
4. Meretoja P, Silander K, Kalimo H, Aula P, Meretoja A, Savontaus ML. Epidemiology of hereditary neuropathy with liability to pressure palsies (HNPP) in south western Finland. Neuromuscul Disord. 1997;7(8):529-532.
5. Li J, Parker B, Martyn C, Natarajan C, Guo J. The PMP22 gene and its related diseases. Mol Neurobiol. 2013;47(2):673-698.
6. Bai Y, Zhang X, Katona I, et al. Conduction block in PMP22 deficiency. J Neurosci. 2010;30(2):600-608.
7. Guo J, Wang L, Zhang Y, et al. Abnormal junctions and permeability of myelin in PMP22-deficient nerves. Ann Neurol. 2014;75(2):255-265.
8. Li J, Krajewski K, Shy ME, Lewis RA. Hereditary neuropathy with liability to pressure palsy: the electrophysiology fits the name. Neurology. 2002;58(12):1769-1773.
9. Rose HA, Hood RW, Otis JC, Ranawat CS, Insall JN. Peroneal-nerve palsy following total knee arthroplasty. A review of the Hospital for Special Surgery experience. J Bone Joint Surg Am. 1982;64(3):347-351.
10. Schinsky MF, Macaulay W, Parks ML, Kiernan H, Nercessian OA. Nerve injury after primary total knee arthroplasty. J Arthroplasty. 2001;16(8):1048-1054.
11. Nercessian OA, Ugwonali OF, Park S. Peroneal nerve palsy after total knee arthroplasty. J Arthroplasty. 2005;20(8):1068-1073.
Risk-Stratified VTE Prophylaxis Following Total Joint Replacement Leads to Significant Hospital Cost Reductions and Prevents Deep Vein Thrombosis
DALLAS—Medical Compression Systems, Inc. (Concord, Massachusetts), announced new data that further validates the use of their ActiveCare deep vein thrombosis prophylaxis compression system following total joint replacement procedures. The study results demonstrate that a risk-stratification protocol using a synchronized mobile compression and an aspirin regimen is associated with low rates of venous thromboembolism, lower rates of adverse events, and reduced overall costs compared with a group treated with aggressive anticoagulant agents. Data were presented at the 25th Annual Meeting of the American Association of Hip and Knee Surgeons.
“We’ve established through previous studies that prophylactic treatment with mobile compression and aspirin following total joint replacement can reduce the occurrence of venous thromboembolism and decrease adverse events, infections, and bleeding complications in patients undergoing total joint replacement,” said Richard Iorio, MD, primary study author and Professor of Orthopedic Surgery at NYU School of Medicine in New York.
The study was designed to determine if utilizing a risk-based venous thromboembolism chemoprophylaxis protocol would improve prevention of deep vein thrombosis and pulmonary embolism, quality metrics, and bleeding-related complications in patients undergoing total joint arthroplasty.
The retrospective review evaluated 2,611 patients that were divided into 2 cohorts. Cohort 1 included 1,203 patients who were previously treated with standard aggressive chemoprophylaxis agents (Enoxaparin, Rivaroxaban, Warfarin). Cohort 2 consisted of a risk-stratified group of patients either undergoing treatment with prophylactic synchronized mobile compression and aspirin (n=843) or aggressive prophylaxis (n=565).
Results demonstrated that patients in the risk-stratified protocol had a lower incidence of venous thromboembolism than the group treated with anticoagulation. Patients in this group also experienced fewer adverse events, readmissions, infections, and bleeding-related complications. Hospital costs were significantly lower in the synchronized mobile compression and aspirin subgroup of cohort 2 and overall costs were lower in the risk-stratified cohort, though they did not reach statistical significance.
“These results are significant in that they represent a large study population of more than 2,600 patients and are the first to demonstrate significant reductions in hospital costs, which support the hypothesis that a risk stratification protocol can advance patient-specific therapy and enhance the delivery of value-based care,” Dr. Iorio said.
DALLAS—Medical Compression Systems, Inc. (Concord, Massachusetts), announced new data that further validates the use of their ActiveCare deep vein thrombosis prophylaxis compression system following total joint replacement procedures. The study results demonstrate that a risk-stratification protocol using a synchronized mobile compression and an aspirin regimen is associated with low rates of venous thromboembolism, lower rates of adverse events, and reduced overall costs compared with a group treated with aggressive anticoagulant agents. Data were presented at the 25th Annual Meeting of the American Association of Hip and Knee Surgeons.
“We’ve established through previous studies that prophylactic treatment with mobile compression and aspirin following total joint replacement can reduce the occurrence of venous thromboembolism and decrease adverse events, infections, and bleeding complications in patients undergoing total joint replacement,” said Richard Iorio, MD, primary study author and Professor of Orthopedic Surgery at NYU School of Medicine in New York.
The study was designed to determine if utilizing a risk-based venous thromboembolism chemoprophylaxis protocol would improve prevention of deep vein thrombosis and pulmonary embolism, quality metrics, and bleeding-related complications in patients undergoing total joint arthroplasty.
The retrospective review evaluated 2,611 patients that were divided into 2 cohorts. Cohort 1 included 1,203 patients who were previously treated with standard aggressive chemoprophylaxis agents (Enoxaparin, Rivaroxaban, Warfarin). Cohort 2 consisted of a risk-stratified group of patients either undergoing treatment with prophylactic synchronized mobile compression and aspirin (n=843) or aggressive prophylaxis (n=565).
Results demonstrated that patients in the risk-stratified protocol had a lower incidence of venous thromboembolism than the group treated with anticoagulation. Patients in this group also experienced fewer adverse events, readmissions, infections, and bleeding-related complications. Hospital costs were significantly lower in the synchronized mobile compression and aspirin subgroup of cohort 2 and overall costs were lower in the risk-stratified cohort, though they did not reach statistical significance.
“These results are significant in that they represent a large study population of more than 2,600 patients and are the first to demonstrate significant reductions in hospital costs, which support the hypothesis that a risk stratification protocol can advance patient-specific therapy and enhance the delivery of value-based care,” Dr. Iorio said.
DALLAS—Medical Compression Systems, Inc. (Concord, Massachusetts), announced new data that further validates the use of their ActiveCare deep vein thrombosis prophylaxis compression system following total joint replacement procedures. The study results demonstrate that a risk-stratification protocol using a synchronized mobile compression and an aspirin regimen is associated with low rates of venous thromboembolism, lower rates of adverse events, and reduced overall costs compared with a group treated with aggressive anticoagulant agents. Data were presented at the 25th Annual Meeting of the American Association of Hip and Knee Surgeons.
“We’ve established through previous studies that prophylactic treatment with mobile compression and aspirin following total joint replacement can reduce the occurrence of venous thromboembolism and decrease adverse events, infections, and bleeding complications in patients undergoing total joint replacement,” said Richard Iorio, MD, primary study author and Professor of Orthopedic Surgery at NYU School of Medicine in New York.
The study was designed to determine if utilizing a risk-based venous thromboembolism chemoprophylaxis protocol would improve prevention of deep vein thrombosis and pulmonary embolism, quality metrics, and bleeding-related complications in patients undergoing total joint arthroplasty.
The retrospective review evaluated 2,611 patients that were divided into 2 cohorts. Cohort 1 included 1,203 patients who were previously treated with standard aggressive chemoprophylaxis agents (Enoxaparin, Rivaroxaban, Warfarin). Cohort 2 consisted of a risk-stratified group of patients either undergoing treatment with prophylactic synchronized mobile compression and aspirin (n=843) or aggressive prophylaxis (n=565).
Results demonstrated that patients in the risk-stratified protocol had a lower incidence of venous thromboembolism than the group treated with anticoagulation. Patients in this group also experienced fewer adverse events, readmissions, infections, and bleeding-related complications. Hospital costs were significantly lower in the synchronized mobile compression and aspirin subgroup of cohort 2 and overall costs were lower in the risk-stratified cohort, though they did not reach statistical significance.
“These results are significant in that they represent a large study population of more than 2,600 patients and are the first to demonstrate significant reductions in hospital costs, which support the hypothesis that a risk stratification protocol can advance patient-specific therapy and enhance the delivery of value-based care,” Dr. Iorio said.