User login
The Evolution of Image-Free Robotic Assistance in Unicompartmental Knee Arthroplasty
The concept of robotics is relatively new in medical practice. The term “robot” itself is less than 100 years old, having been first introduced to popular culture in 1917 by Joseph Capek in the science fiction story Opilec.1,2 Robots eventually transitioned from this initial fictional literary setting to reality in 1958, when General Motors began adding automated machines to its assembly lines.1 However, it was not until the 1980s that robotics and their exacting efficiencies would be introduced in the medical field, and it would take another decade before they would enter the specialty of orthopedics.1-4
The first robotic-assisted orthopedic surgery was reportedly performed in 1992, when the Robodoc autonomous system was utilized for total hip arthroplasty.2-4 A robotic system for total knee arthroplasty (TKA) was first described in 1993, but it would take several more years until a system for unicompartmental knee arthroplasty (UKA) would be commercialized and used clinically.5,6 The rationale for advancement of robotic technology for isolated medial or lateral knee arthritis stems from the recognition that while UKA is effective and durable when components and limb are well aligned and soft tissues appropriately balanced, they are less forgiving of even slight component malalignment of as little as 2° to 3° and prone to premature loosening or wear in those circumstances.7-13,14 In the mid 2000s, Cobb and colleagues6 reported using a semiautonomous robot for UKA. Since then, emergence of other semiautonomous robotic systems has led to greater market penetration and technology utilization.15
Currently, an estimated 15% to 20% of UKA surgeries are being performed with robotic assistance.16 Further, patent activity and peer-reviewed publications related to robotic technology in UKA (which can be considered surrogate measures of interest and evolving development and experience with robotic technologies) have increased dramatically over the past few years.2,6,14,17,18-34 To date, while the most dramatic growth of robotic utilization and case volumes has occurred in the subspecialty of UKA, semiautonomous robotic systems have been used with increasing frequency for patellofemoral and bicompartmental knee arthroplasty.35,36 Robotics have been used sparingly for TKA, and limited to autonomous systems;37,38 however, it is anticipated that emergence of semiautonomous platforms for TKA will further expand the role of robotics over the next decade, particularly as our focus shifts beyond component and limb alignment in TKA and more towards the role of robotics in soft tissue balancing, reduction in instrumentation and inventory and its attendant cost savings, and surgical efficiencies. One semiautonomous robotic technology first used in 2006 (Mako, Stryker) reported a 130% increase in robotic volume from 2011 to 2012; another, first used in 2013, reported growth of 480% between 2013 and 2014, due to its improved cost structure, ease of use, smaller footprint, image-free platform and applicability in ambulatory surgery centers (Navio, Smith & Nephew; data supplied by manufacturer), demonstrating the growing popularity of robotic technology.17,39 Further, a recent analysis of potential market penetration over the next decade published by Medical Device and Diagnostic Industry (http://www.mddionline.com) projected that nearly 37% of UKAs and 23% of TKAs will be performed with robotics in 10 years.
Distinction Between Robotic-Assisted Technologies
Autonomous systems involve pre-programming the system with parameters that define the amount and orientation of bone to be removed, after which the system prepares the surfaces independent of surgeon control, other than having access to a “shutdown” switch. There are currently no autonomous robotic tools approved by the US Food and Drug Administration (FDA) for knee arthroplasty.
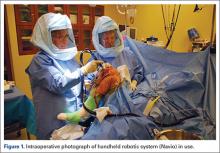
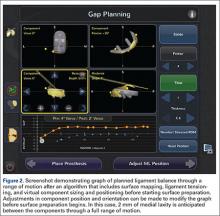
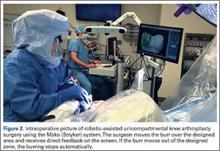
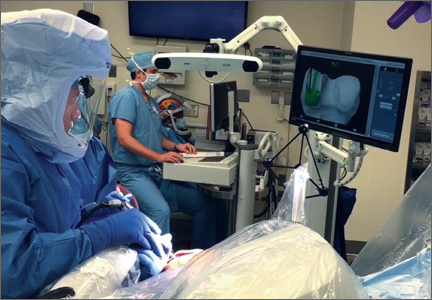
Semiautonomous systems involve the mapping of condylar landmarks and determination of alignment indices, which also defines the volume and orientation of bone to be removed. While the systems remove bone and cartilage within the pre-established parameters, the robotic tools are controlled and manipulated by the surgeon (Figure 1). The predetermined safe zones modulate and safeguard the surgical actions. These systems also provide real-time quantification of soft tissue balancing, which may contribute to the reported successful clinical and functional outcomes with semiautonomous systems (Figure 2).2,4,19,22 There are several semiautonomous robotic systems that are approved for use by the FDA.
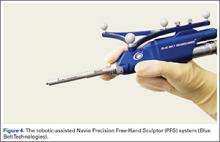
Each robotic-assisted surgery (RAS) system utilizes some sort of 3-dimensional digital map of the surgical surfaces after a process of surface mapping and landmark registration.2 In the case of Mako, this planning process also requires a preoperative computed tomography (CT) scan. Over the past few years, the requirement of a CT scan has proven problematic and costly, as increasingly third-party payers and insurers are denying coverage for additional studies used for preoperative planning, leaving the burden of cost on the patients and/or hospitals. Additionally, in an era in which bundled payment arrangements are commonplace or in which providers are held accountable for costly care, the use of costly preoperative imaging is untenable. Furthermore, there is a growing concern regarding the risk of radiation exposure from CT scans that makes image-free technologies, such as Navio, an alternative for stakeholders.40
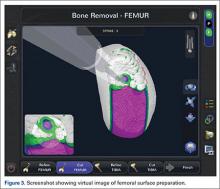
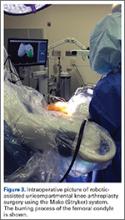
At this time, the 2 semiautonomous systems in use for UKA employ different methods to safeguard against inadvertent bone preparation: one by providing haptic constraint beyond which movement of the bur is limited (Mako); the other by modulating the exposure or speed of the handheld robotic bur (Navio) (Figure 3).
Outcomes of RAS in UKA
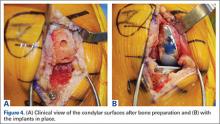
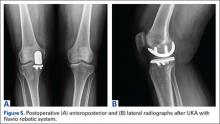
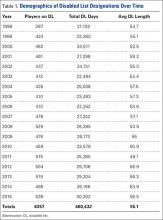
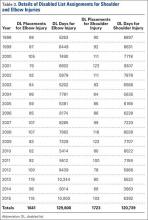
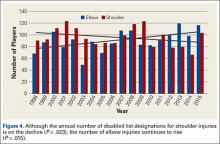
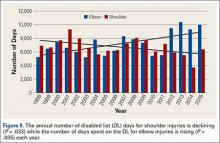
Compared to conventional UKA, robotic assistance has consistently demonstrated improved surgical accuracy, even through minimally invasive incisions (Figures 4, 5).6,20-28 Several studies have found substantial reduction in variability and error of component positioning with use of semiautonomous robotic tools.6,21,25 In fact, precision appears to be comparable regardless of whether an image-free system or one requiring a preoperative CT scan is used (Table). Further, in addition to improving component and limb alignment, Plate and colleagues22 demonstrated that RAS-based UKA systems can help the surgeon precisely reproduce plans for soft-tissue balancing. The authors reported ligament balancing to be accurate up to .53 mm compared to the preoperative plan, with approximately 83% of cases balanced within 1 mm of the plan through a full range of flexion.22
When evaluating advanced and novel technologies, there is undoubtedly concern that there will be increased operative time and a substantial learning curve with those technologies. Karia and colleagues30 found that when inexperienced surgeons performed UKA on synthetic bone models using robotics, the mean compound rotational and translational errors were lower than when conventional techniques were used. Among those using conventional techniques, although surgical times improved during the learning period, positional inaccuracies persisted. On the other hand, robotic assistance enabled surgeons to achieve precision and accuracy when positioning UKA components irrespective of their learning experience.30 Another study, by Coon,31 similarly suggested a shorter learning curve and greater accuracy with RAS using the Mako system compared to conventional techniques. A prospective, multicenter, observational study evaluated the operative times of 11 surgeons during their initial clinical cases using Navio robotic technology for medial UKA after a period of training using cadaver knees and sawbones.41 The learning curve for total surgical time (tracker placement to implant trial phase) indicates that it takes 8 cases to achieve 95% of total learning and maintain a steady state surgical time.
Potential Disadvantages of RAS in UKA
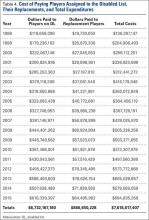
RAS for UKA has several potential disadvantages that must be weighed against their potential benefits. One major barrier to broader use of RAS is the increased cost associated with the technologies.17,19,27,32 Capital and maintenance costs for these systems can be high, and those that require additional advanced imaging, such as CT scans, further challenge the return on investment.17,19,32 In a Markov analysis of one robotic system (Mako), Moschetti and colleagues17 found that if one assumes a system cost of $1.362 million, value can be attained due to slightly better outcomes despite being more expensive than traditional methods. Nonetheless, their analysis of the Mako system estimated that each robot-assisted UKA case cost $19,219, compared to $16,476 with traditional UKA, and was associated with an incremental cost of $47,180 per quality-adjusted life-year. Their analysis further demonstrated that the cost-effectiveness was very sensitive to case volume, with lower costs realized once volumes surpassed 94 cases per year. On the other hand, costs (and thus value) will also obviously vary depending on the capital costs, annual service charges, and avoidance of unnecessary preoperative scans.19 For instance, assuming a cost of $500,000 for the image-free Navio robotic system, return on investment is achievable within 25 cases annually, roughly one-quarter of the cases necessary with the image-based system.
Another disadvantage of RAS systems in UKA is the unique complications associated with their use. Both RAS and conventional UKA can be complicated by similar problems such as component loosening, polyethylene wear, progressive arthritis, infection, stiffness, instability, and thromboembolism. RAS systems, however, carry the additional risk of specific robot-related issues.19,27 Perhaps most notably, the pin tracts for the required optical tracking arrays can create a stress riser in the cortical bone,19,27,33,42 highlighting the importance of inserting these pins in metaphyseal, and not diaphyseal, bone. Soft tissue complications have been reported during bone preparation with autonomous systems in total knee and hip arthroplasty;37,38 however, the senior author (JHL) has not observed that in 1000 consecutive cases with either semiautonomous surgeon-driven robotic tool.19
Finally, systems that require a preoperative CT scan pose an increased radiation risk.40 Ponzio and Lonner40 recently reported that each preoperative CT scan for robotic-assisted knee arthroplasty (using a Mako protocol) is associated with a mean effective dose of radiation of 4.8 mSv, which is approximately equivalent to 48 chest radiographs.34 Further, in that study, at least 25% of patients had been subjected to multiple scans, with some being exposed to cumulative effective doses of up to 103 mSv. This risk should not be considered completely negligible given that 10 mSv may be associated with an increase in the possibility of fatal cancer, and an estimated 29,000 excess cancer cases in the United States annually are reportedly caused by CT scans.40,43,44 However, this increased radiation risk is not inherent to all RAS systems. Image-free systems, such as Navio, do not require CT scans and are thus not associated with this potential disadvantage.
Conclusion
Robotics has come a long way from its humble conceptual beginnings nearly a century ago. Rapid advances in medical technology over the past 10 years have led to the development and growing popularity of RAS in orthopedic surgery, particularly during UKA. Component placement, quantified soft tissue balance, and radiographic alignment appear to be improved and the incidence of outliers reduced with the use of RAS during UKA. Further assessment is needed to determine whether the improved alignment and balance will impact clinical function and/or durability. Early results are very promising, though, creating optimism that the full benefits of RAS in UKA will be further confirmed with additional time and research.
1. Hockstein NG, Gourin CG, Faust RA, Terris DJ. A history of robots: from science fiction to surgical robotics. J Robot Surg. 2007;1(2):113-118.
2. Tamam C, Poehling GG. Robotic-assisted unicompartmental knee arthroplasty. Sports Med Arthrosc. 2014;22(4):219-222.
3. Beasley RA. Medical robots: current systems and research directions. Journal of Robotics. 2012. doi:10.1155/2012/401613.
4. Bargar WL. Robots in orthopaedic surgery: past, present, and future. Clin Orthop Relat Res. 2007;463:31-36.
5. Matsen FA 3rd, Garbini JL, Sidles JA, Pratt B, Baumgarten D, Kaiura R. Robotic assistance in orthopaedic surgery. A proof of principle using distal femoral arthroplasty. Clin Orthop Relat Res. 1993;(296):178-186.
6. Cobb J, Henckel J, Gomes P, et al. Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br. 2006;88(2):188-197.
7. Borus T, Thornhill T. Unicompartmental knee arthroplasty.
J Am Acad Orthop Surg. 2008;16(1):9-18.
8. Berger RA, Meneghini RM, Jacobs JJ, et al. Results of unicompartmental knee arthroplasty at a minimum of ten years of follow-up. J Bone Joint Surg Am. 2005;87(5):999-1006.
9. Price AJ, Waite JC, Svard U. Long-term clinical results of the medial Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2005;(435):171-180.
10. Collier MB, Eickmann TH, Sukezaki F, McAuley JP, Engh GA. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):108-115.
11. Hamilton WG, Collier MB, Tarabee E, McAuley JP, Engh CA Jr, Engh GA. Incidence and reasons for reoperation after minimally invasive unicompartmental knee arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):98-107.
12. Hernigou P, Deschamps G. Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res. 2004;(423):161-165.
13. Hernigou P, Deschamps G. Posterior slope of the tibial implant and the outcome of unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(3):506-511.
14. Lonner JH. Indications for unicompartmental knee arthroplasty and rationale for robotic arm-assisted technology. Am J Orthop. 2009;38(2 Suppl):3-6.
15. Lonner JH. Robotically-assisted unicompartmental knee arthroplasty with a hand-held image-free sculpting tool. Orthop Clin North Am. 2016;47(1):29-40.
16. Orthopedic Network News. 2013 Hip and Knee Implant Review. http://www.OrthopedicNetworkNews.com. Published July 2013. Accessed March 7, 2016.
17. Moschetti WE, Konopka JF, Rubash HE, Genuario JW. Can robot-assisted unicompartmental knee arthroplasty be cost-effective? A Markov decision analysis. J Arthroplasty. 2016;31(4):759-765.
18. Roche M. Robotic-assisted unicompartmental knee arthroplasty: the MAKO experience. Orthop Clin North Am. 2015;46(1):125-131.
19. Lonner JH. Robotically assisted unicompartmental knee arthroplasty with a handheld image-free sculpting tool. Oper Tech Orthop. 2015;25:104-113.
20. Mofidi A, Plate JF, Lu B, et al. Assessment of accuracy of robotically assisted unicompartmental arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22(8):1918-1925.
21. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
22. Plate JF, Mofidi A, Mannava S, et al. Achieving accurate ligament balancing using robotic-assisted unicompartmental knee arthroplasty. Adv Orthop. 2013;2013:837167.
23. Smith JR, Riches PE, Rowe PJ. Accuracy of a freehand sculpting tool for unicondylar knee replacement. Int J Med Robot. 2014;10(2):162-169.
24. Smith JR, Picard F, Lonner J, et al. The accuracy of a robotically-controlled freehand sculpting tool for unicondylar knee arthroplasty. J Bone Joint Surg Br. 2014;96-B(Suppl 16):12.
25. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
26. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
27. Sinha RK. Outcomes of robotic arm-assisted unicompartmental knee arthroplasty. Am J Orthop. 2009;38(2 Suppl):20-22.
28. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
29. Mozes A, Chang TC, Arata L, Zhao W. Three-dimensional A-mode ultrasound calibration and registration for robotic orthopaedic knee surgery. Int J Med Robot. 2010;6(1):91-101.
30. Karia M, Masjedi M, Andrews B, Jaffry Z, Cobb J. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop. 2013;2013:481039.
31. Coon TM. Integrating robotic technology into the operating room. Am J Orthop. 2009;38(2 Suppl):7-9.
32. Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop. 2009;38(2 Suppl):32-36.
33. Roche M, Augustin D, Conditt M. One year outcomes of robotically guided UKA. In: Proceedings of the 21st Annual Congress of the International Society of Technology in Arthroplasty. Sacramento, CA: International Society for Technology in Arthroplasty; 2008:175.
34. Dalton DM, Burke TP, Kelly EG, Curtin PD. Quantitative analysis of technological innovation in knee arthroplasty: using patent and publication metrics to identify developments and trends. J Arthroplasty. 2015. [Epub ahead of print]
35. Lonner JH. Modular bicompartmental knee arthroplasty with robotic arm assistance. Am J Orthop. 2009;38(2 Suppl):28-31.
36. Kamath AF, Levack A, John T, Thomas BS, Lonner JH. Minimum two-year outcomes of modular bicompartmental knee arthroplasty. J Arthroplasty. 2014;29(1):75-79.
37. Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471(1):118-126.
38. Chun YS, Kim KI, Cho YJ, Kim YH, Yoo MC, Rhyu KH. Causes and patterns of aborting a robot-assisted arthroplasty. J Arthroplasty. 2011;26(4):621-625.
39. MAKO Surgical Corp. Fact Sheet. http://www.makosurgical.com/assets/files/Company/newsroom/Corporate_Fact_Sheet_208578r00.pdf. Published 2013. Accessed March 7, 2016.
40. Ponzio DY, Lonner JH. Preoperative mapping in unicompartmental knee arthroplasty using computed tomography scans is associated with radiation exposure and carries high cost. J Arthroplasty. 2015;30(6):964-967.
41. Wallace D, Gregori A, Picard F, et al. The learning curve of a novel handheld robotic system for unicondylar knee arthroplasty. Paper presented at: 14th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery. June 18-21, 2014; Milan, Italy.
42. Wysocki RW, Sheinkop MB, Virkus WW, Della Valle CJ. Femoral fracture through a previous pin site after computer-assisted total knee arthroplasty. J Arthroplasty. 2008;23(3):462-465.
43. Costello JE, Cecava ND, Tucker JE, Bau JL. CT radiation dose: current controversies and dose reduction strategies. AJR Am J Roentgenol. 2013;201(6):1283-1290.
44. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
The concept of robotics is relatively new in medical practice. The term “robot” itself is less than 100 years old, having been first introduced to popular culture in 1917 by Joseph Capek in the science fiction story Opilec.1,2 Robots eventually transitioned from this initial fictional literary setting to reality in 1958, when General Motors began adding automated machines to its assembly lines.1 However, it was not until the 1980s that robotics and their exacting efficiencies would be introduced in the medical field, and it would take another decade before they would enter the specialty of orthopedics.1-4
The first robotic-assisted orthopedic surgery was reportedly performed in 1992, when the Robodoc autonomous system was utilized for total hip arthroplasty.2-4 A robotic system for total knee arthroplasty (TKA) was first described in 1993, but it would take several more years until a system for unicompartmental knee arthroplasty (UKA) would be commercialized and used clinically.5,6 The rationale for advancement of robotic technology for isolated medial or lateral knee arthritis stems from the recognition that while UKA is effective and durable when components and limb are well aligned and soft tissues appropriately balanced, they are less forgiving of even slight component malalignment of as little as 2° to 3° and prone to premature loosening or wear in those circumstances.7-13,14 In the mid 2000s, Cobb and colleagues6 reported using a semiautonomous robot for UKA. Since then, emergence of other semiautonomous robotic systems has led to greater market penetration and technology utilization.15
Currently, an estimated 15% to 20% of UKA surgeries are being performed with robotic assistance.16 Further, patent activity and peer-reviewed publications related to robotic technology in UKA (which can be considered surrogate measures of interest and evolving development and experience with robotic technologies) have increased dramatically over the past few years.2,6,14,17,18-34 To date, while the most dramatic growth of robotic utilization and case volumes has occurred in the subspecialty of UKA, semiautonomous robotic systems have been used with increasing frequency for patellofemoral and bicompartmental knee arthroplasty.35,36 Robotics have been used sparingly for TKA, and limited to autonomous systems;37,38 however, it is anticipated that emergence of semiautonomous platforms for TKA will further expand the role of robotics over the next decade, particularly as our focus shifts beyond component and limb alignment in TKA and more towards the role of robotics in soft tissue balancing, reduction in instrumentation and inventory and its attendant cost savings, and surgical efficiencies. One semiautonomous robotic technology first used in 2006 (Mako, Stryker) reported a 130% increase in robotic volume from 2011 to 2012; another, first used in 2013, reported growth of 480% between 2013 and 2014, due to its improved cost structure, ease of use, smaller footprint, image-free platform and applicability in ambulatory surgery centers (Navio, Smith & Nephew; data supplied by manufacturer), demonstrating the growing popularity of robotic technology.17,39 Further, a recent analysis of potential market penetration over the next decade published by Medical Device and Diagnostic Industry (http://www.mddionline.com) projected that nearly 37% of UKAs and 23% of TKAs will be performed with robotics in 10 years.
Distinction Between Robotic-Assisted Technologies
Autonomous systems involve pre-programming the system with parameters that define the amount and orientation of bone to be removed, after which the system prepares the surfaces independent of surgeon control, other than having access to a “shutdown” switch. There are currently no autonomous robotic tools approved by the US Food and Drug Administration (FDA) for knee arthroplasty.
Semiautonomous systems involve the mapping of condylar landmarks and determination of alignment indices, which also defines the volume and orientation of bone to be removed. While the systems remove bone and cartilage within the pre-established parameters, the robotic tools are controlled and manipulated by the surgeon (Figure 1). The predetermined safe zones modulate and safeguard the surgical actions. These systems also provide real-time quantification of soft tissue balancing, which may contribute to the reported successful clinical and functional outcomes with semiautonomous systems (Figure 2).2,4,19,22 There are several semiautonomous robotic systems that are approved for use by the FDA.
Each robotic-assisted surgery (RAS) system utilizes some sort of 3-dimensional digital map of the surgical surfaces after a process of surface mapping and landmark registration.2 In the case of Mako, this planning process also requires a preoperative computed tomography (CT) scan. Over the past few years, the requirement of a CT scan has proven problematic and costly, as increasingly third-party payers and insurers are denying coverage for additional studies used for preoperative planning, leaving the burden of cost on the patients and/or hospitals. Additionally, in an era in which bundled payment arrangements are commonplace or in which providers are held accountable for costly care, the use of costly preoperative imaging is untenable. Furthermore, there is a growing concern regarding the risk of radiation exposure from CT scans that makes image-free technologies, such as Navio, an alternative for stakeholders.40
At this time, the 2 semiautonomous systems in use for UKA employ different methods to safeguard against inadvertent bone preparation: one by providing haptic constraint beyond which movement of the bur is limited (Mako); the other by modulating the exposure or speed of the handheld robotic bur (Navio) (Figure 3).
Outcomes of RAS in UKA
Compared to conventional UKA, robotic assistance has consistently demonstrated improved surgical accuracy, even through minimally invasive incisions (Figures 4, 5).6,20-28 Several studies have found substantial reduction in variability and error of component positioning with use of semiautonomous robotic tools.6,21,25 In fact, precision appears to be comparable regardless of whether an image-free system or one requiring a preoperative CT scan is used (Table). Further, in addition to improving component and limb alignment, Plate and colleagues22 demonstrated that RAS-based UKA systems can help the surgeon precisely reproduce plans for soft-tissue balancing. The authors reported ligament balancing to be accurate up to .53 mm compared to the preoperative plan, with approximately 83% of cases balanced within 1 mm of the plan through a full range of flexion.22
When evaluating advanced and novel technologies, there is undoubtedly concern that there will be increased operative time and a substantial learning curve with those technologies. Karia and colleagues30 found that when inexperienced surgeons performed UKA on synthetic bone models using robotics, the mean compound rotational and translational errors were lower than when conventional techniques were used. Among those using conventional techniques, although surgical times improved during the learning period, positional inaccuracies persisted. On the other hand, robotic assistance enabled surgeons to achieve precision and accuracy when positioning UKA components irrespective of their learning experience.30 Another study, by Coon,31 similarly suggested a shorter learning curve and greater accuracy with RAS using the Mako system compared to conventional techniques. A prospective, multicenter, observational study evaluated the operative times of 11 surgeons during their initial clinical cases using Navio robotic technology for medial UKA after a period of training using cadaver knees and sawbones.41 The learning curve for total surgical time (tracker placement to implant trial phase) indicates that it takes 8 cases to achieve 95% of total learning and maintain a steady state surgical time.
Potential Disadvantages of RAS in UKA
RAS for UKA has several potential disadvantages that must be weighed against their potential benefits. One major barrier to broader use of RAS is the increased cost associated with the technologies.17,19,27,32 Capital and maintenance costs for these systems can be high, and those that require additional advanced imaging, such as CT scans, further challenge the return on investment.17,19,32 In a Markov analysis of one robotic system (Mako), Moschetti and colleagues17 found that if one assumes a system cost of $1.362 million, value can be attained due to slightly better outcomes despite being more expensive than traditional methods. Nonetheless, their analysis of the Mako system estimated that each robot-assisted UKA case cost $19,219, compared to $16,476 with traditional UKA, and was associated with an incremental cost of $47,180 per quality-adjusted life-year. Their analysis further demonstrated that the cost-effectiveness was very sensitive to case volume, with lower costs realized once volumes surpassed 94 cases per year. On the other hand, costs (and thus value) will also obviously vary depending on the capital costs, annual service charges, and avoidance of unnecessary preoperative scans.19 For instance, assuming a cost of $500,000 for the image-free Navio robotic system, return on investment is achievable within 25 cases annually, roughly one-quarter of the cases necessary with the image-based system.
Another disadvantage of RAS systems in UKA is the unique complications associated with their use. Both RAS and conventional UKA can be complicated by similar problems such as component loosening, polyethylene wear, progressive arthritis, infection, stiffness, instability, and thromboembolism. RAS systems, however, carry the additional risk of specific robot-related issues.19,27 Perhaps most notably, the pin tracts for the required optical tracking arrays can create a stress riser in the cortical bone,19,27,33,42 highlighting the importance of inserting these pins in metaphyseal, and not diaphyseal, bone. Soft tissue complications have been reported during bone preparation with autonomous systems in total knee and hip arthroplasty;37,38 however, the senior author (JHL) has not observed that in 1000 consecutive cases with either semiautonomous surgeon-driven robotic tool.19
Finally, systems that require a preoperative CT scan pose an increased radiation risk.40 Ponzio and Lonner40 recently reported that each preoperative CT scan for robotic-assisted knee arthroplasty (using a Mako protocol) is associated with a mean effective dose of radiation of 4.8 mSv, which is approximately equivalent to 48 chest radiographs.34 Further, in that study, at least 25% of patients had been subjected to multiple scans, with some being exposed to cumulative effective doses of up to 103 mSv. This risk should not be considered completely negligible given that 10 mSv may be associated with an increase in the possibility of fatal cancer, and an estimated 29,000 excess cancer cases in the United States annually are reportedly caused by CT scans.40,43,44 However, this increased radiation risk is not inherent to all RAS systems. Image-free systems, such as Navio, do not require CT scans and are thus not associated with this potential disadvantage.
Conclusion
Robotics has come a long way from its humble conceptual beginnings nearly a century ago. Rapid advances in medical technology over the past 10 years have led to the development and growing popularity of RAS in orthopedic surgery, particularly during UKA. Component placement, quantified soft tissue balance, and radiographic alignment appear to be improved and the incidence of outliers reduced with the use of RAS during UKA. Further assessment is needed to determine whether the improved alignment and balance will impact clinical function and/or durability. Early results are very promising, though, creating optimism that the full benefits of RAS in UKA will be further confirmed with additional time and research.
The concept of robotics is relatively new in medical practice. The term “robot” itself is less than 100 years old, having been first introduced to popular culture in 1917 by Joseph Capek in the science fiction story Opilec.1,2 Robots eventually transitioned from this initial fictional literary setting to reality in 1958, when General Motors began adding automated machines to its assembly lines.1 However, it was not until the 1980s that robotics and their exacting efficiencies would be introduced in the medical field, and it would take another decade before they would enter the specialty of orthopedics.1-4
The first robotic-assisted orthopedic surgery was reportedly performed in 1992, when the Robodoc autonomous system was utilized for total hip arthroplasty.2-4 A robotic system for total knee arthroplasty (TKA) was first described in 1993, but it would take several more years until a system for unicompartmental knee arthroplasty (UKA) would be commercialized and used clinically.5,6 The rationale for advancement of robotic technology for isolated medial or lateral knee arthritis stems from the recognition that while UKA is effective and durable when components and limb are well aligned and soft tissues appropriately balanced, they are less forgiving of even slight component malalignment of as little as 2° to 3° and prone to premature loosening or wear in those circumstances.7-13,14 In the mid 2000s, Cobb and colleagues6 reported using a semiautonomous robot for UKA. Since then, emergence of other semiautonomous robotic systems has led to greater market penetration and technology utilization.15
Currently, an estimated 15% to 20% of UKA surgeries are being performed with robotic assistance.16 Further, patent activity and peer-reviewed publications related to robotic technology in UKA (which can be considered surrogate measures of interest and evolving development and experience with robotic technologies) have increased dramatically over the past few years.2,6,14,17,18-34 To date, while the most dramatic growth of robotic utilization and case volumes has occurred in the subspecialty of UKA, semiautonomous robotic systems have been used with increasing frequency for patellofemoral and bicompartmental knee arthroplasty.35,36 Robotics have been used sparingly for TKA, and limited to autonomous systems;37,38 however, it is anticipated that emergence of semiautonomous platforms for TKA will further expand the role of robotics over the next decade, particularly as our focus shifts beyond component and limb alignment in TKA and more towards the role of robotics in soft tissue balancing, reduction in instrumentation and inventory and its attendant cost savings, and surgical efficiencies. One semiautonomous robotic technology first used in 2006 (Mako, Stryker) reported a 130% increase in robotic volume from 2011 to 2012; another, first used in 2013, reported growth of 480% between 2013 and 2014, due to its improved cost structure, ease of use, smaller footprint, image-free platform and applicability in ambulatory surgery centers (Navio, Smith & Nephew; data supplied by manufacturer), demonstrating the growing popularity of robotic technology.17,39 Further, a recent analysis of potential market penetration over the next decade published by Medical Device and Diagnostic Industry (http://www.mddionline.com) projected that nearly 37% of UKAs and 23% of TKAs will be performed with robotics in 10 years.
Distinction Between Robotic-Assisted Technologies
Autonomous systems involve pre-programming the system with parameters that define the amount and orientation of bone to be removed, after which the system prepares the surfaces independent of surgeon control, other than having access to a “shutdown” switch. There are currently no autonomous robotic tools approved by the US Food and Drug Administration (FDA) for knee arthroplasty.
Semiautonomous systems involve the mapping of condylar landmarks and determination of alignment indices, which also defines the volume and orientation of bone to be removed. While the systems remove bone and cartilage within the pre-established parameters, the robotic tools are controlled and manipulated by the surgeon (Figure 1). The predetermined safe zones modulate and safeguard the surgical actions. These systems also provide real-time quantification of soft tissue balancing, which may contribute to the reported successful clinical and functional outcomes with semiautonomous systems (Figure 2).2,4,19,22 There are several semiautonomous robotic systems that are approved for use by the FDA.
Each robotic-assisted surgery (RAS) system utilizes some sort of 3-dimensional digital map of the surgical surfaces after a process of surface mapping and landmark registration.2 In the case of Mako, this planning process also requires a preoperative computed tomography (CT) scan. Over the past few years, the requirement of a CT scan has proven problematic and costly, as increasingly third-party payers and insurers are denying coverage for additional studies used for preoperative planning, leaving the burden of cost on the patients and/or hospitals. Additionally, in an era in which bundled payment arrangements are commonplace or in which providers are held accountable for costly care, the use of costly preoperative imaging is untenable. Furthermore, there is a growing concern regarding the risk of radiation exposure from CT scans that makes image-free technologies, such as Navio, an alternative for stakeholders.40
At this time, the 2 semiautonomous systems in use for UKA employ different methods to safeguard against inadvertent bone preparation: one by providing haptic constraint beyond which movement of the bur is limited (Mako); the other by modulating the exposure or speed of the handheld robotic bur (Navio) (Figure 3).
Outcomes of RAS in UKA
Compared to conventional UKA, robotic assistance has consistently demonstrated improved surgical accuracy, even through minimally invasive incisions (Figures 4, 5).6,20-28 Several studies have found substantial reduction in variability and error of component positioning with use of semiautonomous robotic tools.6,21,25 In fact, precision appears to be comparable regardless of whether an image-free system or one requiring a preoperative CT scan is used (Table). Further, in addition to improving component and limb alignment, Plate and colleagues22 demonstrated that RAS-based UKA systems can help the surgeon precisely reproduce plans for soft-tissue balancing. The authors reported ligament balancing to be accurate up to .53 mm compared to the preoperative plan, with approximately 83% of cases balanced within 1 mm of the plan through a full range of flexion.22
When evaluating advanced and novel technologies, there is undoubtedly concern that there will be increased operative time and a substantial learning curve with those technologies. Karia and colleagues30 found that when inexperienced surgeons performed UKA on synthetic bone models using robotics, the mean compound rotational and translational errors were lower than when conventional techniques were used. Among those using conventional techniques, although surgical times improved during the learning period, positional inaccuracies persisted. On the other hand, robotic assistance enabled surgeons to achieve precision and accuracy when positioning UKA components irrespective of their learning experience.30 Another study, by Coon,31 similarly suggested a shorter learning curve and greater accuracy with RAS using the Mako system compared to conventional techniques. A prospective, multicenter, observational study evaluated the operative times of 11 surgeons during their initial clinical cases using Navio robotic technology for medial UKA after a period of training using cadaver knees and sawbones.41 The learning curve for total surgical time (tracker placement to implant trial phase) indicates that it takes 8 cases to achieve 95% of total learning and maintain a steady state surgical time.
Potential Disadvantages of RAS in UKA
RAS for UKA has several potential disadvantages that must be weighed against their potential benefits. One major barrier to broader use of RAS is the increased cost associated with the technologies.17,19,27,32 Capital and maintenance costs for these systems can be high, and those that require additional advanced imaging, such as CT scans, further challenge the return on investment.17,19,32 In a Markov analysis of one robotic system (Mako), Moschetti and colleagues17 found that if one assumes a system cost of $1.362 million, value can be attained due to slightly better outcomes despite being more expensive than traditional methods. Nonetheless, their analysis of the Mako system estimated that each robot-assisted UKA case cost $19,219, compared to $16,476 with traditional UKA, and was associated with an incremental cost of $47,180 per quality-adjusted life-year. Their analysis further demonstrated that the cost-effectiveness was very sensitive to case volume, with lower costs realized once volumes surpassed 94 cases per year. On the other hand, costs (and thus value) will also obviously vary depending on the capital costs, annual service charges, and avoidance of unnecessary preoperative scans.19 For instance, assuming a cost of $500,000 for the image-free Navio robotic system, return on investment is achievable within 25 cases annually, roughly one-quarter of the cases necessary with the image-based system.
Another disadvantage of RAS systems in UKA is the unique complications associated with their use. Both RAS and conventional UKA can be complicated by similar problems such as component loosening, polyethylene wear, progressive arthritis, infection, stiffness, instability, and thromboembolism. RAS systems, however, carry the additional risk of specific robot-related issues.19,27 Perhaps most notably, the pin tracts for the required optical tracking arrays can create a stress riser in the cortical bone,19,27,33,42 highlighting the importance of inserting these pins in metaphyseal, and not diaphyseal, bone. Soft tissue complications have been reported during bone preparation with autonomous systems in total knee and hip arthroplasty;37,38 however, the senior author (JHL) has not observed that in 1000 consecutive cases with either semiautonomous surgeon-driven robotic tool.19
Finally, systems that require a preoperative CT scan pose an increased radiation risk.40 Ponzio and Lonner40 recently reported that each preoperative CT scan for robotic-assisted knee arthroplasty (using a Mako protocol) is associated with a mean effective dose of radiation of 4.8 mSv, which is approximately equivalent to 48 chest radiographs.34 Further, in that study, at least 25% of patients had been subjected to multiple scans, with some being exposed to cumulative effective doses of up to 103 mSv. This risk should not be considered completely negligible given that 10 mSv may be associated with an increase in the possibility of fatal cancer, and an estimated 29,000 excess cancer cases in the United States annually are reportedly caused by CT scans.40,43,44 However, this increased radiation risk is not inherent to all RAS systems. Image-free systems, such as Navio, do not require CT scans and are thus not associated with this potential disadvantage.
Conclusion
Robotics has come a long way from its humble conceptual beginnings nearly a century ago. Rapid advances in medical technology over the past 10 years have led to the development and growing popularity of RAS in orthopedic surgery, particularly during UKA. Component placement, quantified soft tissue balance, and radiographic alignment appear to be improved and the incidence of outliers reduced with the use of RAS during UKA. Further assessment is needed to determine whether the improved alignment and balance will impact clinical function and/or durability. Early results are very promising, though, creating optimism that the full benefits of RAS in UKA will be further confirmed with additional time and research.
1. Hockstein NG, Gourin CG, Faust RA, Terris DJ. A history of robots: from science fiction to surgical robotics. J Robot Surg. 2007;1(2):113-118.
2. Tamam C, Poehling GG. Robotic-assisted unicompartmental knee arthroplasty. Sports Med Arthrosc. 2014;22(4):219-222.
3. Beasley RA. Medical robots: current systems and research directions. Journal of Robotics. 2012. doi:10.1155/2012/401613.
4. Bargar WL. Robots in orthopaedic surgery: past, present, and future. Clin Orthop Relat Res. 2007;463:31-36.
5. Matsen FA 3rd, Garbini JL, Sidles JA, Pratt B, Baumgarten D, Kaiura R. Robotic assistance in orthopaedic surgery. A proof of principle using distal femoral arthroplasty. Clin Orthop Relat Res. 1993;(296):178-186.
6. Cobb J, Henckel J, Gomes P, et al. Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br. 2006;88(2):188-197.
7. Borus T, Thornhill T. Unicompartmental knee arthroplasty.
J Am Acad Orthop Surg. 2008;16(1):9-18.
8. Berger RA, Meneghini RM, Jacobs JJ, et al. Results of unicompartmental knee arthroplasty at a minimum of ten years of follow-up. J Bone Joint Surg Am. 2005;87(5):999-1006.
9. Price AJ, Waite JC, Svard U. Long-term clinical results of the medial Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2005;(435):171-180.
10. Collier MB, Eickmann TH, Sukezaki F, McAuley JP, Engh GA. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):108-115.
11. Hamilton WG, Collier MB, Tarabee E, McAuley JP, Engh CA Jr, Engh GA. Incidence and reasons for reoperation after minimally invasive unicompartmental knee arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):98-107.
12. Hernigou P, Deschamps G. Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res. 2004;(423):161-165.
13. Hernigou P, Deschamps G. Posterior slope of the tibial implant and the outcome of unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(3):506-511.
14. Lonner JH. Indications for unicompartmental knee arthroplasty and rationale for robotic arm-assisted technology. Am J Orthop. 2009;38(2 Suppl):3-6.
15. Lonner JH. Robotically-assisted unicompartmental knee arthroplasty with a hand-held image-free sculpting tool. Orthop Clin North Am. 2016;47(1):29-40.
16. Orthopedic Network News. 2013 Hip and Knee Implant Review. http://www.OrthopedicNetworkNews.com. Published July 2013. Accessed March 7, 2016.
17. Moschetti WE, Konopka JF, Rubash HE, Genuario JW. Can robot-assisted unicompartmental knee arthroplasty be cost-effective? A Markov decision analysis. J Arthroplasty. 2016;31(4):759-765.
18. Roche M. Robotic-assisted unicompartmental knee arthroplasty: the MAKO experience. Orthop Clin North Am. 2015;46(1):125-131.
19. Lonner JH. Robotically assisted unicompartmental knee arthroplasty with a handheld image-free sculpting tool. Oper Tech Orthop. 2015;25:104-113.
20. Mofidi A, Plate JF, Lu B, et al. Assessment of accuracy of robotically assisted unicompartmental arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22(8):1918-1925.
21. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
22. Plate JF, Mofidi A, Mannava S, et al. Achieving accurate ligament balancing using robotic-assisted unicompartmental knee arthroplasty. Adv Orthop. 2013;2013:837167.
23. Smith JR, Riches PE, Rowe PJ. Accuracy of a freehand sculpting tool for unicondylar knee replacement. Int J Med Robot. 2014;10(2):162-169.
24. Smith JR, Picard F, Lonner J, et al. The accuracy of a robotically-controlled freehand sculpting tool for unicondylar knee arthroplasty. J Bone Joint Surg Br. 2014;96-B(Suppl 16):12.
25. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
26. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
27. Sinha RK. Outcomes of robotic arm-assisted unicompartmental knee arthroplasty. Am J Orthop. 2009;38(2 Suppl):20-22.
28. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
29. Mozes A, Chang TC, Arata L, Zhao W. Three-dimensional A-mode ultrasound calibration and registration for robotic orthopaedic knee surgery. Int J Med Robot. 2010;6(1):91-101.
30. Karia M, Masjedi M, Andrews B, Jaffry Z, Cobb J. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop. 2013;2013:481039.
31. Coon TM. Integrating robotic technology into the operating room. Am J Orthop. 2009;38(2 Suppl):7-9.
32. Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop. 2009;38(2 Suppl):32-36.
33. Roche M, Augustin D, Conditt M. One year outcomes of robotically guided UKA. In: Proceedings of the 21st Annual Congress of the International Society of Technology in Arthroplasty. Sacramento, CA: International Society for Technology in Arthroplasty; 2008:175.
34. Dalton DM, Burke TP, Kelly EG, Curtin PD. Quantitative analysis of technological innovation in knee arthroplasty: using patent and publication metrics to identify developments and trends. J Arthroplasty. 2015. [Epub ahead of print]
35. Lonner JH. Modular bicompartmental knee arthroplasty with robotic arm assistance. Am J Orthop. 2009;38(2 Suppl):28-31.
36. Kamath AF, Levack A, John T, Thomas BS, Lonner JH. Minimum two-year outcomes of modular bicompartmental knee arthroplasty. J Arthroplasty. 2014;29(1):75-79.
37. Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471(1):118-126.
38. Chun YS, Kim KI, Cho YJ, Kim YH, Yoo MC, Rhyu KH. Causes and patterns of aborting a robot-assisted arthroplasty. J Arthroplasty. 2011;26(4):621-625.
39. MAKO Surgical Corp. Fact Sheet. http://www.makosurgical.com/assets/files/Company/newsroom/Corporate_Fact_Sheet_208578r00.pdf. Published 2013. Accessed March 7, 2016.
40. Ponzio DY, Lonner JH. Preoperative mapping in unicompartmental knee arthroplasty using computed tomography scans is associated with radiation exposure and carries high cost. J Arthroplasty. 2015;30(6):964-967.
41. Wallace D, Gregori A, Picard F, et al. The learning curve of a novel handheld robotic system for unicondylar knee arthroplasty. Paper presented at: 14th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery. June 18-21, 2014; Milan, Italy.
42. Wysocki RW, Sheinkop MB, Virkus WW, Della Valle CJ. Femoral fracture through a previous pin site after computer-assisted total knee arthroplasty. J Arthroplasty. 2008;23(3):462-465.
43. Costello JE, Cecava ND, Tucker JE, Bau JL. CT radiation dose: current controversies and dose reduction strategies. AJR Am J Roentgenol. 2013;201(6):1283-1290.
44. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
1. Hockstein NG, Gourin CG, Faust RA, Terris DJ. A history of robots: from science fiction to surgical robotics. J Robot Surg. 2007;1(2):113-118.
2. Tamam C, Poehling GG. Robotic-assisted unicompartmental knee arthroplasty. Sports Med Arthrosc. 2014;22(4):219-222.
3. Beasley RA. Medical robots: current systems and research directions. Journal of Robotics. 2012. doi:10.1155/2012/401613.
4. Bargar WL. Robots in orthopaedic surgery: past, present, and future. Clin Orthop Relat Res. 2007;463:31-36.
5. Matsen FA 3rd, Garbini JL, Sidles JA, Pratt B, Baumgarten D, Kaiura R. Robotic assistance in orthopaedic surgery. A proof of principle using distal femoral arthroplasty. Clin Orthop Relat Res. 1993;(296):178-186.
6. Cobb J, Henckel J, Gomes P, et al. Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br. 2006;88(2):188-197.
7. Borus T, Thornhill T. Unicompartmental knee arthroplasty.
J Am Acad Orthop Surg. 2008;16(1):9-18.
8. Berger RA, Meneghini RM, Jacobs JJ, et al. Results of unicompartmental knee arthroplasty at a minimum of ten years of follow-up. J Bone Joint Surg Am. 2005;87(5):999-1006.
9. Price AJ, Waite JC, Svard U. Long-term clinical results of the medial Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2005;(435):171-180.
10. Collier MB, Eickmann TH, Sukezaki F, McAuley JP, Engh GA. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):108-115.
11. Hamilton WG, Collier MB, Tarabee E, McAuley JP, Engh CA Jr, Engh GA. Incidence and reasons for reoperation after minimally invasive unicompartmental knee arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):98-107.
12. Hernigou P, Deschamps G. Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res. 2004;(423):161-165.
13. Hernigou P, Deschamps G. Posterior slope of the tibial implant and the outcome of unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(3):506-511.
14. Lonner JH. Indications for unicompartmental knee arthroplasty and rationale for robotic arm-assisted technology. Am J Orthop. 2009;38(2 Suppl):3-6.
15. Lonner JH. Robotically-assisted unicompartmental knee arthroplasty with a hand-held image-free sculpting tool. Orthop Clin North Am. 2016;47(1):29-40.
16. Orthopedic Network News. 2013 Hip and Knee Implant Review. http://www.OrthopedicNetworkNews.com. Published July 2013. Accessed March 7, 2016.
17. Moschetti WE, Konopka JF, Rubash HE, Genuario JW. Can robot-assisted unicompartmental knee arthroplasty be cost-effective? A Markov decision analysis. J Arthroplasty. 2016;31(4):759-765.
18. Roche M. Robotic-assisted unicompartmental knee arthroplasty: the MAKO experience. Orthop Clin North Am. 2015;46(1):125-131.
19. Lonner JH. Robotically assisted unicompartmental knee arthroplasty with a handheld image-free sculpting tool. Oper Tech Orthop. 2015;25:104-113.
20. Mofidi A, Plate JF, Lu B, et al. Assessment of accuracy of robotically assisted unicompartmental arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22(8):1918-1925.
21. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
22. Plate JF, Mofidi A, Mannava S, et al. Achieving accurate ligament balancing using robotic-assisted unicompartmental knee arthroplasty. Adv Orthop. 2013;2013:837167.
23. Smith JR, Riches PE, Rowe PJ. Accuracy of a freehand sculpting tool for unicondylar knee replacement. Int J Med Robot. 2014;10(2):162-169.
24. Smith JR, Picard F, Lonner J, et al. The accuracy of a robotically-controlled freehand sculpting tool for unicondylar knee arthroplasty. J Bone Joint Surg Br. 2014;96-B(Suppl 16):12.
25. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
26. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
27. Sinha RK. Outcomes of robotic arm-assisted unicompartmental knee arthroplasty. Am J Orthop. 2009;38(2 Suppl):20-22.
28. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
29. Mozes A, Chang TC, Arata L, Zhao W. Three-dimensional A-mode ultrasound calibration and registration for robotic orthopaedic knee surgery. Int J Med Robot. 2010;6(1):91-101.
30. Karia M, Masjedi M, Andrews B, Jaffry Z, Cobb J. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop. 2013;2013:481039.
31. Coon TM. Integrating robotic technology into the operating room. Am J Orthop. 2009;38(2 Suppl):7-9.
32. Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop. 2009;38(2 Suppl):32-36.
33. Roche M, Augustin D, Conditt M. One year outcomes of robotically guided UKA. In: Proceedings of the 21st Annual Congress of the International Society of Technology in Arthroplasty. Sacramento, CA: International Society for Technology in Arthroplasty; 2008:175.
34. Dalton DM, Burke TP, Kelly EG, Curtin PD. Quantitative analysis of technological innovation in knee arthroplasty: using patent and publication metrics to identify developments and trends. J Arthroplasty. 2015. [Epub ahead of print]
35. Lonner JH. Modular bicompartmental knee arthroplasty with robotic arm assistance. Am J Orthop. 2009;38(2 Suppl):28-31.
36. Kamath AF, Levack A, John T, Thomas BS, Lonner JH. Minimum two-year outcomes of modular bicompartmental knee arthroplasty. J Arthroplasty. 2014;29(1):75-79.
37. Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471(1):118-126.
38. Chun YS, Kim KI, Cho YJ, Kim YH, Yoo MC, Rhyu KH. Causes and patterns of aborting a robot-assisted arthroplasty. J Arthroplasty. 2011;26(4):621-625.
39. MAKO Surgical Corp. Fact Sheet. http://www.makosurgical.com/assets/files/Company/newsroom/Corporate_Fact_Sheet_208578r00.pdf. Published 2013. Accessed March 7, 2016.
40. Ponzio DY, Lonner JH. Preoperative mapping in unicompartmental knee arthroplasty using computed tomography scans is associated with radiation exposure and carries high cost. J Arthroplasty. 2015;30(6):964-967.
41. Wallace D, Gregori A, Picard F, et al. The learning curve of a novel handheld robotic system for unicondylar knee arthroplasty. Paper presented at: 14th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery. June 18-21, 2014; Milan, Italy.
42. Wysocki RW, Sheinkop MB, Virkus WW, Della Valle CJ. Femoral fracture through a previous pin site after computer-assisted total knee arthroplasty. J Arthroplasty. 2008;23(3):462-465.
43. Costello JE, Cecava ND, Tucker JE, Bau JL. CT radiation dose: current controversies and dose reduction strategies. AJR Am J Roentgenol. 2013;201(6):1283-1290.
44. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
Disposable Navigation for Total Knee Arthroplasty
Total knee arthroplasty (TKA) continues to be a widely utilized treatment option for end-stage knee osteoarthritis, and the number of patients undergoing TKA is projected to continually increase over the next decade.1 Although TKA is highly successful for many patients, studies continue to report that approximately 20% of patients are dissatisfied after undergoing TKA, and nearly 25% of knee revisions are performed for instability or malalignment.2,3 Technological advances have been developed to help improve clinical outcomes and implant survivorship. Over the past decade, computer navigation and intraoperative guides have been introduced to help control surgical variables and overcome the limitations and inaccuracies of traditional mechanical instrumentation. Currently, there are a variety of technologies available to assist surgeons with component alignment, including extramedullary devices, computer-assisted navigation systems (CAS), and patient-specific instrumentation (PSI) that help achieve desired alignment goals.4,5
Computer-assisted navigation tools were introduced in an effort to improve implant alignment and clinical outcomes compared to traditional mechanical guides. Some argue that the use of computer-assisted surgery has a steep learning curve and successful use is dependent on the user’s experience; however, studies have suggested computer-assisted surgery may allow less experienced surgeons to reliably achieve anticipated intraoperative alignment goals with a low complication rate.6,7 Various studies have looked at computer-assisted TKA at short to mid-term follow-up, but few studies have reported long-term outcomes.6-9 de Steiger and colleagues10 recently found that computer-assisted TKA reduced the overall revision rate for aseptic loosening following TKA in patients younger than age 65 years, which suggests benefit of CAS for younger patients. Short-term follow-up has also shown the benefit of CAS TKA in patients with severe extra-articular deformity, where traditional instrumentation cannot be utilized.11 Currently, there is no consensus that computer-assisted TKA leads to improved postoperative patient reported outcomes, because many studies are limited by study design or small cohorts; however, current literature does show an improvement in component alignment as compared to mechanical instrumentation.9,12,13 As future implant and position targets are defined to improve implant survivorship and clinical outcomes in total joint arthroplasty, computer-assisted devices will be useful to help achieve more precise and accurate component positioning.
In addition to CAS devices, some companies have sought to improve TKA surgery by introducing PSI. PSI was introduced to improve component alignment in TKA, with the purported advantages of a shorter surgical time, decrease in the number of instruments needed, and improved clinical outcomes. PSI accuracy remains variable, which may be attributed to the various systems and implant designs in each study.14-17 In addition, advanced preoperative imaging is necessary, which further adds to the overall cost.17 While the recent advancement in technology may provide decreased costs at the time of surgery, the increased cost and time incurred by the patient preoperatively has not resulted in significantly better clinical outcomes.18,19 Additionally, recent work has not shown PSI to have superior accuracy as compared to currently available CAS devices.14 These findings suggest that the additional cost and time incurred by patients may limit the widespread use of PSI.
Although computer navigation has been shown to be more accurate than conventional instrumentation and PSI, the lack of improvement in long-term clinical outcome data has limited its use. In a meta-analysis, Bauwens and colleagues20 suggested that while navigated TKAs have improved component alignment outcomes as compared to conventional surgery, the clinical benefit remains unclear. Less than 5% of surgeons are currently using navigation systems due to the perceived learning curve, cost, additional surgical time, and imaging required to utilize these systems. Certain navigation systems can be seemingly cumbersome, with large consoles, increased number of instruments required, and optical instruments with line-of-sight issues. Recent technological advances have worked to decrease this challenge by using accelerometer- and gyroscope-based electronic components, which combine the accuracy of computer-assisted technology with the ease of use of mechanical guides.
Accelerometer and gyroscope technology systems, such as the iAssist system, are portable devices that do not require a large computer console or navigation arrays. This technology relies on accelerator-based navigation without additional preoperative imaging. A recent study demonstrated the iAssist had reproducible accuracy in component alignment that could be easily incorporated into the operating room without optical trackers.21 The use of portable computer-assisted devices provides a more compact and easily accessible technology that can be used to achieve accurate component alignment without additional large equipment in the operating room.22 These new handheld intraoperative tools have been introduced to place implants according to a preoperative plan in order to minimize failure due to preoperative extra-articular deformity or intraoperative technical miscues.23 Nam and colleagues24 used an accelerometer-based surgical navigation system to perform tibial resections in cadaveric models, and found that the accelerometer-based guide was accurate for tibial resection in both the coronal and sagittal planes. In a prospective randomized controlled trial evaluating 100 patients undergoing a TKA using either an accelerometer-based guide or conventional alignment methods, the authors showed that the accelerometer-based guide decreased outliers in tibial component alignment compared to conventional guides.25 In the accelerometer-based guide cohort, 95.7% of tibial components were within 2° of perpendicular to the tibial mechanical axis, compared to 68.1% in the conventional group (P < .001). These results suggested that portable accelerometer-based navigation allows surgeons to achieve satisfactory tibial component alignment with a decrease in the number of potential outliers.24,25 Similarly, Bugbee and colleagues26 found that accelerometer-based handheld navigation was accurate for tibial coronal and sagittal alignment and no additional surgical time was required compared to conventional techniques.
The relationship between knee alignment and clinical outcomes for TKA remains controversial. Regardless of the surgeon’s alignment preference, it is important to reliably and accurately execute the preoperative plan in a reproducible fashion. Advances in technology that assist with intraoperative component alignment can be useful, and may help decrease the incidence of implant malalignment in clinical practice.
Preoperative Planning and Intraoperative Technique
Preoperative planning is carried out in a manner identical to the use of conventional mechanical guides. Long leg films are taken for evaluation of overall limb alignment, and calibrated lateral images are taken for templating implant sizes. Lines are drawn on the images to determine the difference between the mechanical and anatomic axis of the femur, and a line drawn perpendicular to the mechanical axis is placed to show the expected bone cut. In similar fashion a perpendicular line to the tibial mechanical axis is also drawn to show the expected tibial resection. This preoperative plan allows the surgeon to have an additional intraoperative guide to ensure accuracy of the computer-assisted device.
After standard exposure, the distal femoral or proximal tibial cut can be made based on surgeon preference. The system being demonstrated in the accompanying photos is the KneeAlign 2 system (OrthAlign).
Distal Femoral Cut
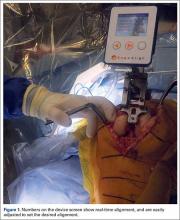
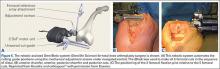
The KneeAlign 2 femoral cutting guide is attached to the distal femur with a central pin that is placed in the middle of the distal femur measured from medial to lateral, and 1 cm anterior to the intercondylar notch. It is important to note that this spot is often more medial than traditionally used for insertion of an intramedullary rod. This central point sets the distal point of the femoral mechanical axis. The device is then held in place with 2 oblique pins, and is solidly fixed to the bone. Using a rotating motion, the femur is rotated around the hip joint. The accelerometer and gyroscope in the unit are able to determine the center of the hip joint from this motion, creating the proximal point of the mechanical axis of the femur. Once the mechanical axis of the femur is determined, varus/valgus and flexion/extension can be adjusted on the guide. One adjustment screw is available for varus/valgus, and a second is available for flexion/extension. Numbers on the device screen show real-time alignment, and are easily adjusted to set the desired alignment (Figure 1). Once alignment is obtained, a mechanical stylus is used to determine depth of resection, and the distal femoral cutting block is pinned. After pinning the block, the 3 pins in the device are removed, and the device is removed from the bone. This leaves only the distal femoral cutting block in place. In experienced hands, this part of the procedure takes less than 3 minutes.
Proximal Tibial Cut
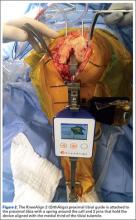
The KneeAlign 2 proximal tibial guide is similar in appearance to a standard mechanical tibial cutting guide. It is attached to the proximal tibia with a spring around the calf and 2 pins that hold the device aligned with the medial third of the tibial tubercle. A stylus is then centered on the anterior cruciate ligament (ACL) footprint, which sets the proximal mechanical axis point of the tibia (Figure 2). An offset number is read off the stylus on the ACL footprint, and this number is matched on the ankle offset portion of the guide. The device has 2 sensors. One sensor is on the chassis of the device, and the other is on a mobile arm. Movements between the 2 are monitored by the accelerometers, allowing for accurate maintenance of alignment position even with motion in the leg. A point is taken from the lateral malleolus and then a second point is taken from the medial malleolus. These points are used to determine the center of the ankle joint, which sets the distal mechanical axis point. Once mechanical axis of the tibia is determined, the tibial cutting guide is pinned in place, and can be adjusted with real-time guidance of the varus/valgus and posterior slope values seen on the device (Figure 3). Cut depth is once again determined with a mechanical stylus.
Limitations
Although these devices have proven to be very accurate, surgeons must continue to recognize that all tools can have errors. With computerized guides of any sort, these errors are usually user errors that cannot be detected by the device. Surgeons need to be able to recognize this and always double-check bone cuts for accuracy. Templating the bone cuts prior to surgery is an effective double-check. In addition, many handheld accelerometer devices do not currently assist with the rotational alignment of the femoral component. This is still performed using the surgeon’s preferred technique, and is a limitation of these systems.
Discussion
Currently, TKA provides satisfactory 10-year results with modern implant designs and survival rates as high as 90% to 95%.27,28 Even with good survival rates, a percentage of patients fail within the first 5 years.3 At a single institution, 50% of revision TKAs were related to instability, malalignment, or failure of fixation that occurred less than 2 years after the index procedure.29 In general, TKA with mechanical instrumentation provides satisfactory pain relief and postoperative knee function; however, studies have consistently shown that the use of advanced technology decreases the risk of implant malalignment, which may decrease early implant failure rates as compared to mechanical and some PSI.13,14,22 While there is a paucity of literature that has shown better clinical outcomes with the use of advanced technology, there are studies supporting the notion that proper limb alignment and component positioning improves implant survivorship.23,30
CAS may have additional advantages if the surgeon chooses to place the TKA in an alignment other than a neutral mechanical axis. Kinematic alignment for TKA has gained increasing popularity, where the target of a neutral mechanical axis alignment is not always the goal.31,32 The reported benefit is a more natural ligament tension with the hope of improving patient satisfaction. One concern with this technique is that it is a departure from the long-held teaching that a TKA aligned to a neutral mechanical axis is necessary for long-term implant survivorship.33,34 In addition, if the goal of surgery is to cut the tibia and femur at a specific varus/valgus cut, standard instrumentation may not allow for this level of accuracy. This in turn increases the risk of having a tibial or femoral cut that is outside the commonly accepted standards of alignment, which may lead to early implant failure. If further research suggests alignment is a variable that differs from patient to patient, the use of precise tools to ensure accuracy of executing the preoperatively templated alignment becomes even more important.
As the number of TKAs continues to rise each year, even a small percentage of malaligned knees that go on to revision surgery will create a large burden on the healthcare system.1,3 Although the short-term clinical benefits of CAS have not shown substantial differences as compared to conventional TKA, the number of knees aligned outside of a desired neutral mechanical axis alignment has been shown in multiple studies to be decreased with the use of advanced technology.7,12,34 Although CAS is an additional cost to a primary TKA, if the orthopedic community can decrease the number of TKA revisions due to malalignment from 6.6% to nearly zero, this may decrease the revision burden and overall cost to the healthcare system.1,3
TKA technology continues to evolve, and we must continue to assess each new advance not only to understand how it works, but also to ensure it addresses a specific clinical problem, and to be aware of the costs associated before incorporating it into routine practice. Some argue that the use of advanced technology requires increased surgical time, which in turn ultimately increases costs; however, one study has documented no increase in surgical time with handheld navigation while maintaining the accuracy of the device.34 In addition, no significant radiographic or clinical differences have been found between handheld navigation and larger console CAS systems, but large console systems have been associated with increased surgical times.22 The use of handheld accelerometer- and gyroscope-based guides has proven to provide reliable coronal and sagittal implant alignment that can easily be incorporated into the operating room. More widespread use of such technology will help decrease alignment outliers for TKA, and future long-term clinical outcome studies will be necessary to assess functional outcomes.
Conclusion
Advanced computer based technology offers an additional tool to the surgeon for reliably improving component positioning during TKA. The use of handheld accelerometer- and gyroscope-based guides increases the accuracy of component placement while decreasing the incidence of outliers compared to standard mechanical guides, without the need for a large computer console. Long-term radiographic and patient-reported outcomes are necessary to further validate these devices.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28( 8 Suppl):116-119.
4. Sassoon A, Nam D, Nunley R, Barrack R. Systematic review of patient-specific instrumentation in total knee arthroplasty: new but not improved. Clin Orthop Relat Res. 2015;473(1):151-158.
5. Anderson KC, Buehler KC, Markel DC. Computer assisted navigation in total knee arthroplasty: comparison with conventional methods. J Arthroplasty. 2005;20(7 Suppl 3):132-138.
6. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
7. Khakha RS, Chowdhry M, Sivaprakasam M, Kheiran A, Chauhan SK. Radiological and functional outcomes in computer assisted total knee arthroplasty between consultants and trainees - a prospective randomized controlled trial. J Arthroplasty. 2015;30(8):1344-1347.
8. Zhu M, Ang CL, Yeo SJ, Lo NN, Chia SL, Chong HC. Minimally invasive computer-assisted total knee arthroplasty compared with conventional total knee arthroplasty: a prospective 9-year follow-up. J Arthroplasty. 2015. [Epub ahead of print]
9. Roberts TD, Clatworthy MG, Frampton CM, Young SW. Does computer assisted navigation improve functional outcomes and implant survivability after total knee arthroplasty? J Arthroplasty. 2015;30(9 Suppl):59-63.
10. de Steiger RN, Liu YL, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635-642.
11. Fehring TK, Mason JB, Moskal J, Pollock DC, Mann J, Williams VJ. When computer-assisted knee replacement is the best alternative. Clin Orthop Relat Res. 2006;452:132-136.
12. Iorio R, Mazza D, Drogo P, et al. Clinical and radiographic outcomes of an accelerometer-based system for the tibial resection in total knee arthroplasty. Int Orthop. 2015;39(3):461-466.
13. Haaker RG, Stockheim M, Kamp M, Proff G, Breitenfelder J, Ottersbach A. Computer-assisted navigation increases precision of component placement in total knee arthroplasty. Clin Orthop Relat Res. 2005;433:152-159.
14. Ollivier M, Tribot-Laspiere Q, Amzallag J, Boisrenoult P, Pujol N, Beaufils P. Abnormal rate of intraoperative and postoperative implant positioning outliers using “MRI-based patient-specific” compared to “computer assisted” instrumentation in total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
15. Nunley RM, Ellison BS, Zhu J, Ruh EL, Howell SM, Barrack RL. Do patient-specific guides improve coronal alignment in total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):895-902.
16. Nunley RM, Ellison BS, Ruh EL, et al. Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):889-894.
17. Barrack RL, Ruh EL, Williams BM, Ford AD, Foreman K, Nunley RM. Patient specific cutting blocks are currently of no proven value. J Bone Joint Surg Br. 2012;94(11 Suppl A):95-99.
18. Chen JY, Chin PL, Tay DK, Chia SL, Lo NN, Yeo SJ. Functional outcome and quality of life after patient-specific instrumentation in total knee arthroplasty. J Arthroplasty. 2015;30(10):1724-1728.
19. Goyal N, Patel AR, Yaffe MA, Luo MY, Stulberg SD. Does implant design influence the accuracy of patient specific instrumentation in total knee arthroplasty? J Arthroplasty. 2015;30(9):1526-1530.
20. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
21. Scuderi GR, Fallaha M, Masse V, Lavigne P, Amiot LP, Berthiaume MJ. Total knee arthroplasty with a novel navigation system within the surgical field. Orthop Clin North Am. 2014;45(2):167-173.
22. Goh GS, Liow MH, Lim WS, Tay DK, Yeo SJ, Tan MH. Accelerometer-based navigation is as accurate as optical computer navigation in restoring the joint line and mechanical axis after total knee arthroplasty: a prospective matched study. J Arthroplasty. 2016;31(1):92-97.
23. Berend KR, Lombardi AV Jr. Liberal indications for minimally invasive oxford unicondylar arthroplasty provide rapid functional recovery and pain relief. Surg Technol Int. 2007;16:193-197.
24. Nam D, Jerabek SA, Cross MB, Mayman DJ. Cadaveric analysis of an accelerometer-based portable navigation device for distal femoral cutting block alignment in total knee arthroplasty. Comput Aided Surg. 2012;17(4):205-210.
25. Nam D, Cody EA, Nguyen JT, Figgie MP, Mayman DJ. Extramedullary guides versus portable, accelerometer-based navigation for tibial alignment in total knee arthroplasty: a randomized, controlled trial: winner of the 2013 HAP PAUL award. J Arthroplasty. 2014;29(2):288-294.
26. Bugbee WD, Kermanshahi AY, Munro MM, McCauley JC, Copp SN. Accuracy of a hand-held surgical navigation system for tibial resection in total knee arthroplasty. Knee. 2014;21(6):1225-1228.
27. Schai PA, Thornhill TS, Scott RD. Total knee arthroplasty with the PFC system. Results at a minimum of ten years and survivorship analysis. J Bone Joint Surg Br. 1998;80(5):850-858.
28. Pradhan NR, Gambhir A, Porter ML. Survivorship analysis of 3234 primary knee arthroplasties implanted over a 26-year period: a study of eight different implant designs. Knee. 2006;13(1):7-11.
29. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
30. Fang DM, Ritter MA, Davis KE. Coronal alignment in total knee arthroplasty: just how important is it? J Arthroplasty. 2009;24(6 Suppl):39-43.
31. Cherian JJ, Kapadia BH, Banerjee S, Jauregui JJ, Issa K, Mont MA. Mechanical, anatomical, and kinematic axis in TKA: concepts and practical applications. Curr Rev Musculoskelet Med. 2014;7(2):89-95.
32. Howell SM, Papadopoulos S, Kuznik K, Ghaly LR, Hull ML. Does varus alignment adversely affect implant survival and function six years after kinematically aligned total knee arthroplasty? Int Orthop. 2015;39(11):2117-2124.
33. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
34. Huang EH, Copp SN, Bugbee WD. Accuracy of a handheld accelerometer-based navigation system for femoral and tibial resection in total knee arthroplasty. J Arthroplasty. 2015;30(11):1906-1910.
Total knee arthroplasty (TKA) continues to be a widely utilized treatment option for end-stage knee osteoarthritis, and the number of patients undergoing TKA is projected to continually increase over the next decade.1 Although TKA is highly successful for many patients, studies continue to report that approximately 20% of patients are dissatisfied after undergoing TKA, and nearly 25% of knee revisions are performed for instability or malalignment.2,3 Technological advances have been developed to help improve clinical outcomes and implant survivorship. Over the past decade, computer navigation and intraoperative guides have been introduced to help control surgical variables and overcome the limitations and inaccuracies of traditional mechanical instrumentation. Currently, there are a variety of technologies available to assist surgeons with component alignment, including extramedullary devices, computer-assisted navigation systems (CAS), and patient-specific instrumentation (PSI) that help achieve desired alignment goals.4,5
Computer-assisted navigation tools were introduced in an effort to improve implant alignment and clinical outcomes compared to traditional mechanical guides. Some argue that the use of computer-assisted surgery has a steep learning curve and successful use is dependent on the user’s experience; however, studies have suggested computer-assisted surgery may allow less experienced surgeons to reliably achieve anticipated intraoperative alignment goals with a low complication rate.6,7 Various studies have looked at computer-assisted TKA at short to mid-term follow-up, but few studies have reported long-term outcomes.6-9 de Steiger and colleagues10 recently found that computer-assisted TKA reduced the overall revision rate for aseptic loosening following TKA in patients younger than age 65 years, which suggests benefit of CAS for younger patients. Short-term follow-up has also shown the benefit of CAS TKA in patients with severe extra-articular deformity, where traditional instrumentation cannot be utilized.11 Currently, there is no consensus that computer-assisted TKA leads to improved postoperative patient reported outcomes, because many studies are limited by study design or small cohorts; however, current literature does show an improvement in component alignment as compared to mechanical instrumentation.9,12,13 As future implant and position targets are defined to improve implant survivorship and clinical outcomes in total joint arthroplasty, computer-assisted devices will be useful to help achieve more precise and accurate component positioning.
In addition to CAS devices, some companies have sought to improve TKA surgery by introducing PSI. PSI was introduced to improve component alignment in TKA, with the purported advantages of a shorter surgical time, decrease in the number of instruments needed, and improved clinical outcomes. PSI accuracy remains variable, which may be attributed to the various systems and implant designs in each study.14-17 In addition, advanced preoperative imaging is necessary, which further adds to the overall cost.17 While the recent advancement in technology may provide decreased costs at the time of surgery, the increased cost and time incurred by the patient preoperatively has not resulted in significantly better clinical outcomes.18,19 Additionally, recent work has not shown PSI to have superior accuracy as compared to currently available CAS devices.14 These findings suggest that the additional cost and time incurred by patients may limit the widespread use of PSI.
Although computer navigation has been shown to be more accurate than conventional instrumentation and PSI, the lack of improvement in long-term clinical outcome data has limited its use. In a meta-analysis, Bauwens and colleagues20 suggested that while navigated TKAs have improved component alignment outcomes as compared to conventional surgery, the clinical benefit remains unclear. Less than 5% of surgeons are currently using navigation systems due to the perceived learning curve, cost, additional surgical time, and imaging required to utilize these systems. Certain navigation systems can be seemingly cumbersome, with large consoles, increased number of instruments required, and optical instruments with line-of-sight issues. Recent technological advances have worked to decrease this challenge by using accelerometer- and gyroscope-based electronic components, which combine the accuracy of computer-assisted technology with the ease of use of mechanical guides.
Accelerometer and gyroscope technology systems, such as the iAssist system, are portable devices that do not require a large computer console or navigation arrays. This technology relies on accelerator-based navigation without additional preoperative imaging. A recent study demonstrated the iAssist had reproducible accuracy in component alignment that could be easily incorporated into the operating room without optical trackers.21 The use of portable computer-assisted devices provides a more compact and easily accessible technology that can be used to achieve accurate component alignment without additional large equipment in the operating room.22 These new handheld intraoperative tools have been introduced to place implants according to a preoperative plan in order to minimize failure due to preoperative extra-articular deformity or intraoperative technical miscues.23 Nam and colleagues24 used an accelerometer-based surgical navigation system to perform tibial resections in cadaveric models, and found that the accelerometer-based guide was accurate for tibial resection in both the coronal and sagittal planes. In a prospective randomized controlled trial evaluating 100 patients undergoing a TKA using either an accelerometer-based guide or conventional alignment methods, the authors showed that the accelerometer-based guide decreased outliers in tibial component alignment compared to conventional guides.25 In the accelerometer-based guide cohort, 95.7% of tibial components were within 2° of perpendicular to the tibial mechanical axis, compared to 68.1% in the conventional group (P < .001). These results suggested that portable accelerometer-based navigation allows surgeons to achieve satisfactory tibial component alignment with a decrease in the number of potential outliers.24,25 Similarly, Bugbee and colleagues26 found that accelerometer-based handheld navigation was accurate for tibial coronal and sagittal alignment and no additional surgical time was required compared to conventional techniques.
The relationship between knee alignment and clinical outcomes for TKA remains controversial. Regardless of the surgeon’s alignment preference, it is important to reliably and accurately execute the preoperative plan in a reproducible fashion. Advances in technology that assist with intraoperative component alignment can be useful, and may help decrease the incidence of implant malalignment in clinical practice.
Preoperative Planning and Intraoperative Technique
Preoperative planning is carried out in a manner identical to the use of conventional mechanical guides. Long leg films are taken for evaluation of overall limb alignment, and calibrated lateral images are taken for templating implant sizes. Lines are drawn on the images to determine the difference between the mechanical and anatomic axis of the femur, and a line drawn perpendicular to the mechanical axis is placed to show the expected bone cut. In similar fashion a perpendicular line to the tibial mechanical axis is also drawn to show the expected tibial resection. This preoperative plan allows the surgeon to have an additional intraoperative guide to ensure accuracy of the computer-assisted device.
After standard exposure, the distal femoral or proximal tibial cut can be made based on surgeon preference. The system being demonstrated in the accompanying photos is the KneeAlign 2 system (OrthAlign).
Distal Femoral Cut
The KneeAlign 2 femoral cutting guide is attached to the distal femur with a central pin that is placed in the middle of the distal femur measured from medial to lateral, and 1 cm anterior to the intercondylar notch. It is important to note that this spot is often more medial than traditionally used for insertion of an intramedullary rod. This central point sets the distal point of the femoral mechanical axis. The device is then held in place with 2 oblique pins, and is solidly fixed to the bone. Using a rotating motion, the femur is rotated around the hip joint. The accelerometer and gyroscope in the unit are able to determine the center of the hip joint from this motion, creating the proximal point of the mechanical axis of the femur. Once the mechanical axis of the femur is determined, varus/valgus and flexion/extension can be adjusted on the guide. One adjustment screw is available for varus/valgus, and a second is available for flexion/extension. Numbers on the device screen show real-time alignment, and are easily adjusted to set the desired alignment (Figure 1). Once alignment is obtained, a mechanical stylus is used to determine depth of resection, and the distal femoral cutting block is pinned. After pinning the block, the 3 pins in the device are removed, and the device is removed from the bone. This leaves only the distal femoral cutting block in place. In experienced hands, this part of the procedure takes less than 3 minutes.
Proximal Tibial Cut
The KneeAlign 2 proximal tibial guide is similar in appearance to a standard mechanical tibial cutting guide. It is attached to the proximal tibia with a spring around the calf and 2 pins that hold the device aligned with the medial third of the tibial tubercle. A stylus is then centered on the anterior cruciate ligament (ACL) footprint, which sets the proximal mechanical axis point of the tibia (Figure 2). An offset number is read off the stylus on the ACL footprint, and this number is matched on the ankle offset portion of the guide. The device has 2 sensors. One sensor is on the chassis of the device, and the other is on a mobile arm. Movements between the 2 are monitored by the accelerometers, allowing for accurate maintenance of alignment position even with motion in the leg. A point is taken from the lateral malleolus and then a second point is taken from the medial malleolus. These points are used to determine the center of the ankle joint, which sets the distal mechanical axis point. Once mechanical axis of the tibia is determined, the tibial cutting guide is pinned in place, and can be adjusted with real-time guidance of the varus/valgus and posterior slope values seen on the device (Figure 3). Cut depth is once again determined with a mechanical stylus.
Limitations
Although these devices have proven to be very accurate, surgeons must continue to recognize that all tools can have errors. With computerized guides of any sort, these errors are usually user errors that cannot be detected by the device. Surgeons need to be able to recognize this and always double-check bone cuts for accuracy. Templating the bone cuts prior to surgery is an effective double-check. In addition, many handheld accelerometer devices do not currently assist with the rotational alignment of the femoral component. This is still performed using the surgeon’s preferred technique, and is a limitation of these systems.
Discussion
Currently, TKA provides satisfactory 10-year results with modern implant designs and survival rates as high as 90% to 95%.27,28 Even with good survival rates, a percentage of patients fail within the first 5 years.3 At a single institution, 50% of revision TKAs were related to instability, malalignment, or failure of fixation that occurred less than 2 years after the index procedure.29 In general, TKA with mechanical instrumentation provides satisfactory pain relief and postoperative knee function; however, studies have consistently shown that the use of advanced technology decreases the risk of implant malalignment, which may decrease early implant failure rates as compared to mechanical and some PSI.13,14,22 While there is a paucity of literature that has shown better clinical outcomes with the use of advanced technology, there are studies supporting the notion that proper limb alignment and component positioning improves implant survivorship.23,30
CAS may have additional advantages if the surgeon chooses to place the TKA in an alignment other than a neutral mechanical axis. Kinematic alignment for TKA has gained increasing popularity, where the target of a neutral mechanical axis alignment is not always the goal.31,32 The reported benefit is a more natural ligament tension with the hope of improving patient satisfaction. One concern with this technique is that it is a departure from the long-held teaching that a TKA aligned to a neutral mechanical axis is necessary for long-term implant survivorship.33,34 In addition, if the goal of surgery is to cut the tibia and femur at a specific varus/valgus cut, standard instrumentation may not allow for this level of accuracy. This in turn increases the risk of having a tibial or femoral cut that is outside the commonly accepted standards of alignment, which may lead to early implant failure. If further research suggests alignment is a variable that differs from patient to patient, the use of precise tools to ensure accuracy of executing the preoperatively templated alignment becomes even more important.
As the number of TKAs continues to rise each year, even a small percentage of malaligned knees that go on to revision surgery will create a large burden on the healthcare system.1,3 Although the short-term clinical benefits of CAS have not shown substantial differences as compared to conventional TKA, the number of knees aligned outside of a desired neutral mechanical axis alignment has been shown in multiple studies to be decreased with the use of advanced technology.7,12,34 Although CAS is an additional cost to a primary TKA, if the orthopedic community can decrease the number of TKA revisions due to malalignment from 6.6% to nearly zero, this may decrease the revision burden and overall cost to the healthcare system.1,3
TKA technology continues to evolve, and we must continue to assess each new advance not only to understand how it works, but also to ensure it addresses a specific clinical problem, and to be aware of the costs associated before incorporating it into routine practice. Some argue that the use of advanced technology requires increased surgical time, which in turn ultimately increases costs; however, one study has documented no increase in surgical time with handheld navigation while maintaining the accuracy of the device.34 In addition, no significant radiographic or clinical differences have been found between handheld navigation and larger console CAS systems, but large console systems have been associated with increased surgical times.22 The use of handheld accelerometer- and gyroscope-based guides has proven to provide reliable coronal and sagittal implant alignment that can easily be incorporated into the operating room. More widespread use of such technology will help decrease alignment outliers for TKA, and future long-term clinical outcome studies will be necessary to assess functional outcomes.
Conclusion
Advanced computer based technology offers an additional tool to the surgeon for reliably improving component positioning during TKA. The use of handheld accelerometer- and gyroscope-based guides increases the accuracy of component placement while decreasing the incidence of outliers compared to standard mechanical guides, without the need for a large computer console. Long-term radiographic and patient-reported outcomes are necessary to further validate these devices.
Total knee arthroplasty (TKA) continues to be a widely utilized treatment option for end-stage knee osteoarthritis, and the number of patients undergoing TKA is projected to continually increase over the next decade.1 Although TKA is highly successful for many patients, studies continue to report that approximately 20% of patients are dissatisfied after undergoing TKA, and nearly 25% of knee revisions are performed for instability or malalignment.2,3 Technological advances have been developed to help improve clinical outcomes and implant survivorship. Over the past decade, computer navigation and intraoperative guides have been introduced to help control surgical variables and overcome the limitations and inaccuracies of traditional mechanical instrumentation. Currently, there are a variety of technologies available to assist surgeons with component alignment, including extramedullary devices, computer-assisted navigation systems (CAS), and patient-specific instrumentation (PSI) that help achieve desired alignment goals.4,5
Computer-assisted navigation tools were introduced in an effort to improve implant alignment and clinical outcomes compared to traditional mechanical guides. Some argue that the use of computer-assisted surgery has a steep learning curve and successful use is dependent on the user’s experience; however, studies have suggested computer-assisted surgery may allow less experienced surgeons to reliably achieve anticipated intraoperative alignment goals with a low complication rate.6,7 Various studies have looked at computer-assisted TKA at short to mid-term follow-up, but few studies have reported long-term outcomes.6-9 de Steiger and colleagues10 recently found that computer-assisted TKA reduced the overall revision rate for aseptic loosening following TKA in patients younger than age 65 years, which suggests benefit of CAS for younger patients. Short-term follow-up has also shown the benefit of CAS TKA in patients with severe extra-articular deformity, where traditional instrumentation cannot be utilized.11 Currently, there is no consensus that computer-assisted TKA leads to improved postoperative patient reported outcomes, because many studies are limited by study design or small cohorts; however, current literature does show an improvement in component alignment as compared to mechanical instrumentation.9,12,13 As future implant and position targets are defined to improve implant survivorship and clinical outcomes in total joint arthroplasty, computer-assisted devices will be useful to help achieve more precise and accurate component positioning.
In addition to CAS devices, some companies have sought to improve TKA surgery by introducing PSI. PSI was introduced to improve component alignment in TKA, with the purported advantages of a shorter surgical time, decrease in the number of instruments needed, and improved clinical outcomes. PSI accuracy remains variable, which may be attributed to the various systems and implant designs in each study.14-17 In addition, advanced preoperative imaging is necessary, which further adds to the overall cost.17 While the recent advancement in technology may provide decreased costs at the time of surgery, the increased cost and time incurred by the patient preoperatively has not resulted in significantly better clinical outcomes.18,19 Additionally, recent work has not shown PSI to have superior accuracy as compared to currently available CAS devices.14 These findings suggest that the additional cost and time incurred by patients may limit the widespread use of PSI.
Although computer navigation has been shown to be more accurate than conventional instrumentation and PSI, the lack of improvement in long-term clinical outcome data has limited its use. In a meta-analysis, Bauwens and colleagues20 suggested that while navigated TKAs have improved component alignment outcomes as compared to conventional surgery, the clinical benefit remains unclear. Less than 5% of surgeons are currently using navigation systems due to the perceived learning curve, cost, additional surgical time, and imaging required to utilize these systems. Certain navigation systems can be seemingly cumbersome, with large consoles, increased number of instruments required, and optical instruments with line-of-sight issues. Recent technological advances have worked to decrease this challenge by using accelerometer- and gyroscope-based electronic components, which combine the accuracy of computer-assisted technology with the ease of use of mechanical guides.
Accelerometer and gyroscope technology systems, such as the iAssist system, are portable devices that do not require a large computer console or navigation arrays. This technology relies on accelerator-based navigation without additional preoperative imaging. A recent study demonstrated the iAssist had reproducible accuracy in component alignment that could be easily incorporated into the operating room without optical trackers.21 The use of portable computer-assisted devices provides a more compact and easily accessible technology that can be used to achieve accurate component alignment without additional large equipment in the operating room.22 These new handheld intraoperative tools have been introduced to place implants according to a preoperative plan in order to minimize failure due to preoperative extra-articular deformity or intraoperative technical miscues.23 Nam and colleagues24 used an accelerometer-based surgical navigation system to perform tibial resections in cadaveric models, and found that the accelerometer-based guide was accurate for tibial resection in both the coronal and sagittal planes. In a prospective randomized controlled trial evaluating 100 patients undergoing a TKA using either an accelerometer-based guide or conventional alignment methods, the authors showed that the accelerometer-based guide decreased outliers in tibial component alignment compared to conventional guides.25 In the accelerometer-based guide cohort, 95.7% of tibial components were within 2° of perpendicular to the tibial mechanical axis, compared to 68.1% in the conventional group (P < .001). These results suggested that portable accelerometer-based navigation allows surgeons to achieve satisfactory tibial component alignment with a decrease in the number of potential outliers.24,25 Similarly, Bugbee and colleagues26 found that accelerometer-based handheld navigation was accurate for tibial coronal and sagittal alignment and no additional surgical time was required compared to conventional techniques.
The relationship between knee alignment and clinical outcomes for TKA remains controversial. Regardless of the surgeon’s alignment preference, it is important to reliably and accurately execute the preoperative plan in a reproducible fashion. Advances in technology that assist with intraoperative component alignment can be useful, and may help decrease the incidence of implant malalignment in clinical practice.
Preoperative Planning and Intraoperative Technique
Preoperative planning is carried out in a manner identical to the use of conventional mechanical guides. Long leg films are taken for evaluation of overall limb alignment, and calibrated lateral images are taken for templating implant sizes. Lines are drawn on the images to determine the difference between the mechanical and anatomic axis of the femur, and a line drawn perpendicular to the mechanical axis is placed to show the expected bone cut. In similar fashion a perpendicular line to the tibial mechanical axis is also drawn to show the expected tibial resection. This preoperative plan allows the surgeon to have an additional intraoperative guide to ensure accuracy of the computer-assisted device.
After standard exposure, the distal femoral or proximal tibial cut can be made based on surgeon preference. The system being demonstrated in the accompanying photos is the KneeAlign 2 system (OrthAlign).
Distal Femoral Cut
The KneeAlign 2 femoral cutting guide is attached to the distal femur with a central pin that is placed in the middle of the distal femur measured from medial to lateral, and 1 cm anterior to the intercondylar notch. It is important to note that this spot is often more medial than traditionally used for insertion of an intramedullary rod. This central point sets the distal point of the femoral mechanical axis. The device is then held in place with 2 oblique pins, and is solidly fixed to the bone. Using a rotating motion, the femur is rotated around the hip joint. The accelerometer and gyroscope in the unit are able to determine the center of the hip joint from this motion, creating the proximal point of the mechanical axis of the femur. Once the mechanical axis of the femur is determined, varus/valgus and flexion/extension can be adjusted on the guide. One adjustment screw is available for varus/valgus, and a second is available for flexion/extension. Numbers on the device screen show real-time alignment, and are easily adjusted to set the desired alignment (Figure 1). Once alignment is obtained, a mechanical stylus is used to determine depth of resection, and the distal femoral cutting block is pinned. After pinning the block, the 3 pins in the device are removed, and the device is removed from the bone. This leaves only the distal femoral cutting block in place. In experienced hands, this part of the procedure takes less than 3 minutes.
Proximal Tibial Cut
The KneeAlign 2 proximal tibial guide is similar in appearance to a standard mechanical tibial cutting guide. It is attached to the proximal tibia with a spring around the calf and 2 pins that hold the device aligned with the medial third of the tibial tubercle. A stylus is then centered on the anterior cruciate ligament (ACL) footprint, which sets the proximal mechanical axis point of the tibia (Figure 2). An offset number is read off the stylus on the ACL footprint, and this number is matched on the ankle offset portion of the guide. The device has 2 sensors. One sensor is on the chassis of the device, and the other is on a mobile arm. Movements between the 2 are monitored by the accelerometers, allowing for accurate maintenance of alignment position even with motion in the leg. A point is taken from the lateral malleolus and then a second point is taken from the medial malleolus. These points are used to determine the center of the ankle joint, which sets the distal mechanical axis point. Once mechanical axis of the tibia is determined, the tibial cutting guide is pinned in place, and can be adjusted with real-time guidance of the varus/valgus and posterior slope values seen on the device (Figure 3). Cut depth is once again determined with a mechanical stylus.
Limitations
Although these devices have proven to be very accurate, surgeons must continue to recognize that all tools can have errors. With computerized guides of any sort, these errors are usually user errors that cannot be detected by the device. Surgeons need to be able to recognize this and always double-check bone cuts for accuracy. Templating the bone cuts prior to surgery is an effective double-check. In addition, many handheld accelerometer devices do not currently assist with the rotational alignment of the femoral component. This is still performed using the surgeon’s preferred technique, and is a limitation of these systems.
Discussion
Currently, TKA provides satisfactory 10-year results with modern implant designs and survival rates as high as 90% to 95%.27,28 Even with good survival rates, a percentage of patients fail within the first 5 years.3 At a single institution, 50% of revision TKAs were related to instability, malalignment, or failure of fixation that occurred less than 2 years after the index procedure.29 In general, TKA with mechanical instrumentation provides satisfactory pain relief and postoperative knee function; however, studies have consistently shown that the use of advanced technology decreases the risk of implant malalignment, which may decrease early implant failure rates as compared to mechanical and some PSI.13,14,22 While there is a paucity of literature that has shown better clinical outcomes with the use of advanced technology, there are studies supporting the notion that proper limb alignment and component positioning improves implant survivorship.23,30
CAS may have additional advantages if the surgeon chooses to place the TKA in an alignment other than a neutral mechanical axis. Kinematic alignment for TKA has gained increasing popularity, where the target of a neutral mechanical axis alignment is not always the goal.31,32 The reported benefit is a more natural ligament tension with the hope of improving patient satisfaction. One concern with this technique is that it is a departure from the long-held teaching that a TKA aligned to a neutral mechanical axis is necessary for long-term implant survivorship.33,34 In addition, if the goal of surgery is to cut the tibia and femur at a specific varus/valgus cut, standard instrumentation may not allow for this level of accuracy. This in turn increases the risk of having a tibial or femoral cut that is outside the commonly accepted standards of alignment, which may lead to early implant failure. If further research suggests alignment is a variable that differs from patient to patient, the use of precise tools to ensure accuracy of executing the preoperatively templated alignment becomes even more important.
As the number of TKAs continues to rise each year, even a small percentage of malaligned knees that go on to revision surgery will create a large burden on the healthcare system.1,3 Although the short-term clinical benefits of CAS have not shown substantial differences as compared to conventional TKA, the number of knees aligned outside of a desired neutral mechanical axis alignment has been shown in multiple studies to be decreased with the use of advanced technology.7,12,34 Although CAS is an additional cost to a primary TKA, if the orthopedic community can decrease the number of TKA revisions due to malalignment from 6.6% to nearly zero, this may decrease the revision burden and overall cost to the healthcare system.1,3
TKA technology continues to evolve, and we must continue to assess each new advance not only to understand how it works, but also to ensure it addresses a specific clinical problem, and to be aware of the costs associated before incorporating it into routine practice. Some argue that the use of advanced technology requires increased surgical time, which in turn ultimately increases costs; however, one study has documented no increase in surgical time with handheld navigation while maintaining the accuracy of the device.34 In addition, no significant radiographic or clinical differences have been found between handheld navigation and larger console CAS systems, but large console systems have been associated with increased surgical times.22 The use of handheld accelerometer- and gyroscope-based guides has proven to provide reliable coronal and sagittal implant alignment that can easily be incorporated into the operating room. More widespread use of such technology will help decrease alignment outliers for TKA, and future long-term clinical outcome studies will be necessary to assess functional outcomes.
Conclusion
Advanced computer based technology offers an additional tool to the surgeon for reliably improving component positioning during TKA. The use of handheld accelerometer- and gyroscope-based guides increases the accuracy of component placement while decreasing the incidence of outliers compared to standard mechanical guides, without the need for a large computer console. Long-term radiographic and patient-reported outcomes are necessary to further validate these devices.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28( 8 Suppl):116-119.
4. Sassoon A, Nam D, Nunley R, Barrack R. Systematic review of patient-specific instrumentation in total knee arthroplasty: new but not improved. Clin Orthop Relat Res. 2015;473(1):151-158.
5. Anderson KC, Buehler KC, Markel DC. Computer assisted navigation in total knee arthroplasty: comparison with conventional methods. J Arthroplasty. 2005;20(7 Suppl 3):132-138.
6. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
7. Khakha RS, Chowdhry M, Sivaprakasam M, Kheiran A, Chauhan SK. Radiological and functional outcomes in computer assisted total knee arthroplasty between consultants and trainees - a prospective randomized controlled trial. J Arthroplasty. 2015;30(8):1344-1347.
8. Zhu M, Ang CL, Yeo SJ, Lo NN, Chia SL, Chong HC. Minimally invasive computer-assisted total knee arthroplasty compared with conventional total knee arthroplasty: a prospective 9-year follow-up. J Arthroplasty. 2015. [Epub ahead of print]
9. Roberts TD, Clatworthy MG, Frampton CM, Young SW. Does computer assisted navigation improve functional outcomes and implant survivability after total knee arthroplasty? J Arthroplasty. 2015;30(9 Suppl):59-63.
10. de Steiger RN, Liu YL, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635-642.
11. Fehring TK, Mason JB, Moskal J, Pollock DC, Mann J, Williams VJ. When computer-assisted knee replacement is the best alternative. Clin Orthop Relat Res. 2006;452:132-136.
12. Iorio R, Mazza D, Drogo P, et al. Clinical and radiographic outcomes of an accelerometer-based system for the tibial resection in total knee arthroplasty. Int Orthop. 2015;39(3):461-466.
13. Haaker RG, Stockheim M, Kamp M, Proff G, Breitenfelder J, Ottersbach A. Computer-assisted navigation increases precision of component placement in total knee arthroplasty. Clin Orthop Relat Res. 2005;433:152-159.
14. Ollivier M, Tribot-Laspiere Q, Amzallag J, Boisrenoult P, Pujol N, Beaufils P. Abnormal rate of intraoperative and postoperative implant positioning outliers using “MRI-based patient-specific” compared to “computer assisted” instrumentation in total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
15. Nunley RM, Ellison BS, Zhu J, Ruh EL, Howell SM, Barrack RL. Do patient-specific guides improve coronal alignment in total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):895-902.
16. Nunley RM, Ellison BS, Ruh EL, et al. Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):889-894.
17. Barrack RL, Ruh EL, Williams BM, Ford AD, Foreman K, Nunley RM. Patient specific cutting blocks are currently of no proven value. J Bone Joint Surg Br. 2012;94(11 Suppl A):95-99.
18. Chen JY, Chin PL, Tay DK, Chia SL, Lo NN, Yeo SJ. Functional outcome and quality of life after patient-specific instrumentation in total knee arthroplasty. J Arthroplasty. 2015;30(10):1724-1728.
19. Goyal N, Patel AR, Yaffe MA, Luo MY, Stulberg SD. Does implant design influence the accuracy of patient specific instrumentation in total knee arthroplasty? J Arthroplasty. 2015;30(9):1526-1530.
20. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
21. Scuderi GR, Fallaha M, Masse V, Lavigne P, Amiot LP, Berthiaume MJ. Total knee arthroplasty with a novel navigation system within the surgical field. Orthop Clin North Am. 2014;45(2):167-173.
22. Goh GS, Liow MH, Lim WS, Tay DK, Yeo SJ, Tan MH. Accelerometer-based navigation is as accurate as optical computer navigation in restoring the joint line and mechanical axis after total knee arthroplasty: a prospective matched study. J Arthroplasty. 2016;31(1):92-97.
23. Berend KR, Lombardi AV Jr. Liberal indications for minimally invasive oxford unicondylar arthroplasty provide rapid functional recovery and pain relief. Surg Technol Int. 2007;16:193-197.
24. Nam D, Jerabek SA, Cross MB, Mayman DJ. Cadaveric analysis of an accelerometer-based portable navigation device for distal femoral cutting block alignment in total knee arthroplasty. Comput Aided Surg. 2012;17(4):205-210.
25. Nam D, Cody EA, Nguyen JT, Figgie MP, Mayman DJ. Extramedullary guides versus portable, accelerometer-based navigation for tibial alignment in total knee arthroplasty: a randomized, controlled trial: winner of the 2013 HAP PAUL award. J Arthroplasty. 2014;29(2):288-294.
26. Bugbee WD, Kermanshahi AY, Munro MM, McCauley JC, Copp SN. Accuracy of a hand-held surgical navigation system for tibial resection in total knee arthroplasty. Knee. 2014;21(6):1225-1228.
27. Schai PA, Thornhill TS, Scott RD. Total knee arthroplasty with the PFC system. Results at a minimum of ten years and survivorship analysis. J Bone Joint Surg Br. 1998;80(5):850-858.
28. Pradhan NR, Gambhir A, Porter ML. Survivorship analysis of 3234 primary knee arthroplasties implanted over a 26-year period: a study of eight different implant designs. Knee. 2006;13(1):7-11.
29. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
30. Fang DM, Ritter MA, Davis KE. Coronal alignment in total knee arthroplasty: just how important is it? J Arthroplasty. 2009;24(6 Suppl):39-43.
31. Cherian JJ, Kapadia BH, Banerjee S, Jauregui JJ, Issa K, Mont MA. Mechanical, anatomical, and kinematic axis in TKA: concepts and practical applications. Curr Rev Musculoskelet Med. 2014;7(2):89-95.
32. Howell SM, Papadopoulos S, Kuznik K, Ghaly LR, Hull ML. Does varus alignment adversely affect implant survival and function six years after kinematically aligned total knee arthroplasty? Int Orthop. 2015;39(11):2117-2124.
33. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
34. Huang EH, Copp SN, Bugbee WD. Accuracy of a handheld accelerometer-based navigation system for femoral and tibial resection in total knee arthroplasty. J Arthroplasty. 2015;30(11):1906-1910.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28( 8 Suppl):116-119.
4. Sassoon A, Nam D, Nunley R, Barrack R. Systematic review of patient-specific instrumentation in total knee arthroplasty: new but not improved. Clin Orthop Relat Res. 2015;473(1):151-158.
5. Anderson KC, Buehler KC, Markel DC. Computer assisted navigation in total knee arthroplasty: comparison with conventional methods. J Arthroplasty. 2005;20(7 Suppl 3):132-138.
6. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
7. Khakha RS, Chowdhry M, Sivaprakasam M, Kheiran A, Chauhan SK. Radiological and functional outcomes in computer assisted total knee arthroplasty between consultants and trainees - a prospective randomized controlled trial. J Arthroplasty. 2015;30(8):1344-1347.
8. Zhu M, Ang CL, Yeo SJ, Lo NN, Chia SL, Chong HC. Minimally invasive computer-assisted total knee arthroplasty compared with conventional total knee arthroplasty: a prospective 9-year follow-up. J Arthroplasty. 2015. [Epub ahead of print]
9. Roberts TD, Clatworthy MG, Frampton CM, Young SW. Does computer assisted navigation improve functional outcomes and implant survivability after total knee arthroplasty? J Arthroplasty. 2015;30(9 Suppl):59-63.
10. de Steiger RN, Liu YL, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635-642.
11. Fehring TK, Mason JB, Moskal J, Pollock DC, Mann J, Williams VJ. When computer-assisted knee replacement is the best alternative. Clin Orthop Relat Res. 2006;452:132-136.
12. Iorio R, Mazza D, Drogo P, et al. Clinical and radiographic outcomes of an accelerometer-based system for the tibial resection in total knee arthroplasty. Int Orthop. 2015;39(3):461-466.
13. Haaker RG, Stockheim M, Kamp M, Proff G, Breitenfelder J, Ottersbach A. Computer-assisted navigation increases precision of component placement in total knee arthroplasty. Clin Orthop Relat Res. 2005;433:152-159.
14. Ollivier M, Tribot-Laspiere Q, Amzallag J, Boisrenoult P, Pujol N, Beaufils P. Abnormal rate of intraoperative and postoperative implant positioning outliers using “MRI-based patient-specific” compared to “computer assisted” instrumentation in total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
15. Nunley RM, Ellison BS, Zhu J, Ruh EL, Howell SM, Barrack RL. Do patient-specific guides improve coronal alignment in total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):895-902.
16. Nunley RM, Ellison BS, Ruh EL, et al. Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):889-894.
17. Barrack RL, Ruh EL, Williams BM, Ford AD, Foreman K, Nunley RM. Patient specific cutting blocks are currently of no proven value. J Bone Joint Surg Br. 2012;94(11 Suppl A):95-99.
18. Chen JY, Chin PL, Tay DK, Chia SL, Lo NN, Yeo SJ. Functional outcome and quality of life after patient-specific instrumentation in total knee arthroplasty. J Arthroplasty. 2015;30(10):1724-1728.
19. Goyal N, Patel AR, Yaffe MA, Luo MY, Stulberg SD. Does implant design influence the accuracy of patient specific instrumentation in total knee arthroplasty? J Arthroplasty. 2015;30(9):1526-1530.
20. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
21. Scuderi GR, Fallaha M, Masse V, Lavigne P, Amiot LP, Berthiaume MJ. Total knee arthroplasty with a novel navigation system within the surgical field. Orthop Clin North Am. 2014;45(2):167-173.
22. Goh GS, Liow MH, Lim WS, Tay DK, Yeo SJ, Tan MH. Accelerometer-based navigation is as accurate as optical computer navigation in restoring the joint line and mechanical axis after total knee arthroplasty: a prospective matched study. J Arthroplasty. 2016;31(1):92-97.
23. Berend KR, Lombardi AV Jr. Liberal indications for minimally invasive oxford unicondylar arthroplasty provide rapid functional recovery and pain relief. Surg Technol Int. 2007;16:193-197.
24. Nam D, Jerabek SA, Cross MB, Mayman DJ. Cadaveric analysis of an accelerometer-based portable navigation device for distal femoral cutting block alignment in total knee arthroplasty. Comput Aided Surg. 2012;17(4):205-210.
25. Nam D, Cody EA, Nguyen JT, Figgie MP, Mayman DJ. Extramedullary guides versus portable, accelerometer-based navigation for tibial alignment in total knee arthroplasty: a randomized, controlled trial: winner of the 2013 HAP PAUL award. J Arthroplasty. 2014;29(2):288-294.
26. Bugbee WD, Kermanshahi AY, Munro MM, McCauley JC, Copp SN. Accuracy of a hand-held surgical navigation system for tibial resection in total knee arthroplasty. Knee. 2014;21(6):1225-1228.
27. Schai PA, Thornhill TS, Scott RD. Total knee arthroplasty with the PFC system. Results at a minimum of ten years and survivorship analysis. J Bone Joint Surg Br. 1998;80(5):850-858.
28. Pradhan NR, Gambhir A, Porter ML. Survivorship analysis of 3234 primary knee arthroplasties implanted over a 26-year period: a study of eight different implant designs. Knee. 2006;13(1):7-11.
29. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
30. Fang DM, Ritter MA, Davis KE. Coronal alignment in total knee arthroplasty: just how important is it? J Arthroplasty. 2009;24(6 Suppl):39-43.
31. Cherian JJ, Kapadia BH, Banerjee S, Jauregui JJ, Issa K, Mont MA. Mechanical, anatomical, and kinematic axis in TKA: concepts and practical applications. Curr Rev Musculoskelet Med. 2014;7(2):89-95.
32. Howell SM, Papadopoulos S, Kuznik K, Ghaly LR, Hull ML. Does varus alignment adversely affect implant survival and function six years after kinematically aligned total knee arthroplasty? Int Orthop. 2015;39(11):2117-2124.
33. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
34. Huang EH, Copp SN, Bugbee WD. Accuracy of a handheld accelerometer-based navigation system for femoral and tibial resection in total knee arthroplasty. J Arthroplasty. 2015;30(11):1906-1910.
Transitions (The Future of Orthopedics)
A transition is underway at AJO. As we discuss the future of the “new journal,” I often think about the future of orthopedics. I’ve decided my vision of the future is centered on 3 components. First, there will be a change in our training paradigm from an apprenticeship model to standardized training, where core competencies must be demonstrated for certification. Second, robots and computers will improve our diagnostic accuracy and will allow us to perform surgery with improved component positioning, while biologics and genetic analysis will accelerate nature’s ability to heal, and perhaps regenerate, injured tissue. Finally, computerized algorithms and technologically improved surgical outcomes will allow us to deliver high-quality healthcare at a lower cost, producing the value our current health systems are striving for, and leveling the playing field between high-volume centers and rural institutions forced to offer complete service lines.
In this issue, we examine robotic-assisted arthroplasty and its role in modern healthcare. I think the best argument for robots in the operating room might come from the airline industry. I’m sitting on a plane as I write this, without once thinking about how much experience my pilot has in the cockpit. I know our pilot has demonstrated the core competencies required to safely operate the plane, trained on emergency simulations, and logged the necessary hours before being handed the controls. I also know that the instrumentation is so good that the plane can essentially fly itself, making pilot skill and experience less relevant. In short, technology has, in all but rare circumstances, made our pilots virtually interchangeable.
Unfortunately, none of the above is true in orthopedics. Our residents are not required to demonstrate their skills before any licensing authority, simulator training is not available in all programs, and we’ve limited resident work hours. Yet it’s that same interchangeability that most healthcare models assume. No one argues that high-volume centers have better results when it comes to arthroplasty, but only a small percentage of total joints are currently performed at these centers. Surgeon training remains a virtual apprenticeship, lacking standardization, and resulting in a wide variation in skill and experience. Surgical residencies are not awarded based on dexterity, and work hour restrictions, Relative Value Unit-based academic contracts, patient expectations, and staffing pressures can lead to reduced hands-on experience for trainees. The results: an entire generation of surgeons with decreased repetitions in the operating room when compared to their predecessors.
That’s why I believe we are on the cusp of a transition in the operating room, and that computer-assisted surgery is here to stay. While studies exist showing robots have tighter control over virtually every identifiable metric, little data currently exists supporting enhanced long-term outcomes. But as long as component malposition remains a leading cause of early failure, there will be a place for technologies that enhance accuracy of component placement. At odds with the drive for increased technology is the necessity of cost containment, leading us to question the value of robotic-assisted surgery, and whether the improved metrics are clinically important and the additional potential complications are worth the risk.
In the articles in this issue, we will take a critical look at the benefits and drawbacks of robotic surgery. As you read, think about the future of orthopedics and how you will implement new technology into your practice. A transition is coming, and I invite each of you to consider leading it.
A transition is underway at AJO. As we discuss the future of the “new journal,” I often think about the future of orthopedics. I’ve decided my vision of the future is centered on 3 components. First, there will be a change in our training paradigm from an apprenticeship model to standardized training, where core competencies must be demonstrated for certification. Second, robots and computers will improve our diagnostic accuracy and will allow us to perform surgery with improved component positioning, while biologics and genetic analysis will accelerate nature’s ability to heal, and perhaps regenerate, injured tissue. Finally, computerized algorithms and technologically improved surgical outcomes will allow us to deliver high-quality healthcare at a lower cost, producing the value our current health systems are striving for, and leveling the playing field between high-volume centers and rural institutions forced to offer complete service lines.
In this issue, we examine robotic-assisted arthroplasty and its role in modern healthcare. I think the best argument for robots in the operating room might come from the airline industry. I’m sitting on a plane as I write this, without once thinking about how much experience my pilot has in the cockpit. I know our pilot has demonstrated the core competencies required to safely operate the plane, trained on emergency simulations, and logged the necessary hours before being handed the controls. I also know that the instrumentation is so good that the plane can essentially fly itself, making pilot skill and experience less relevant. In short, technology has, in all but rare circumstances, made our pilots virtually interchangeable.
Unfortunately, none of the above is true in orthopedics. Our residents are not required to demonstrate their skills before any licensing authority, simulator training is not available in all programs, and we’ve limited resident work hours. Yet it’s that same interchangeability that most healthcare models assume. No one argues that high-volume centers have better results when it comes to arthroplasty, but only a small percentage of total joints are currently performed at these centers. Surgeon training remains a virtual apprenticeship, lacking standardization, and resulting in a wide variation in skill and experience. Surgical residencies are not awarded based on dexterity, and work hour restrictions, Relative Value Unit-based academic contracts, patient expectations, and staffing pressures can lead to reduced hands-on experience for trainees. The results: an entire generation of surgeons with decreased repetitions in the operating room when compared to their predecessors.
That’s why I believe we are on the cusp of a transition in the operating room, and that computer-assisted surgery is here to stay. While studies exist showing robots have tighter control over virtually every identifiable metric, little data currently exists supporting enhanced long-term outcomes. But as long as component malposition remains a leading cause of early failure, there will be a place for technologies that enhance accuracy of component placement. At odds with the drive for increased technology is the necessity of cost containment, leading us to question the value of robotic-assisted surgery, and whether the improved metrics are clinically important and the additional potential complications are worth the risk.
In the articles in this issue, we will take a critical look at the benefits and drawbacks of robotic surgery. As you read, think about the future of orthopedics and how you will implement new technology into your practice. A transition is coming, and I invite each of you to consider leading it.
A transition is underway at AJO. As we discuss the future of the “new journal,” I often think about the future of orthopedics. I’ve decided my vision of the future is centered on 3 components. First, there will be a change in our training paradigm from an apprenticeship model to standardized training, where core competencies must be demonstrated for certification. Second, robots and computers will improve our diagnostic accuracy and will allow us to perform surgery with improved component positioning, while biologics and genetic analysis will accelerate nature’s ability to heal, and perhaps regenerate, injured tissue. Finally, computerized algorithms and technologically improved surgical outcomes will allow us to deliver high-quality healthcare at a lower cost, producing the value our current health systems are striving for, and leveling the playing field between high-volume centers and rural institutions forced to offer complete service lines.
In this issue, we examine robotic-assisted arthroplasty and its role in modern healthcare. I think the best argument for robots in the operating room might come from the airline industry. I’m sitting on a plane as I write this, without once thinking about how much experience my pilot has in the cockpit. I know our pilot has demonstrated the core competencies required to safely operate the plane, trained on emergency simulations, and logged the necessary hours before being handed the controls. I also know that the instrumentation is so good that the plane can essentially fly itself, making pilot skill and experience less relevant. In short, technology has, in all but rare circumstances, made our pilots virtually interchangeable.
Unfortunately, none of the above is true in orthopedics. Our residents are not required to demonstrate their skills before any licensing authority, simulator training is not available in all programs, and we’ve limited resident work hours. Yet it’s that same interchangeability that most healthcare models assume. No one argues that high-volume centers have better results when it comes to arthroplasty, but only a small percentage of total joints are currently performed at these centers. Surgeon training remains a virtual apprenticeship, lacking standardization, and resulting in a wide variation in skill and experience. Surgical residencies are not awarded based on dexterity, and work hour restrictions, Relative Value Unit-based academic contracts, patient expectations, and staffing pressures can lead to reduced hands-on experience for trainees. The results: an entire generation of surgeons with decreased repetitions in the operating room when compared to their predecessors.
That’s why I believe we are on the cusp of a transition in the operating room, and that computer-assisted surgery is here to stay. While studies exist showing robots have tighter control over virtually every identifiable metric, little data currently exists supporting enhanced long-term outcomes. But as long as component malposition remains a leading cause of early failure, there will be a place for technologies that enhance accuracy of component placement. At odds with the drive for increased technology is the necessity of cost containment, leading us to question the value of robotic-assisted surgery, and whether the improved metrics are clinically important and the additional potential complications are worth the risk.
In the articles in this issue, we will take a critical look at the benefits and drawbacks of robotic surgery. As you read, think about the future of orthopedics and how you will implement new technology into your practice. A transition is coming, and I invite each of you to consider leading it.
Robotic-Assisted Knee Arthroplasty: An Overview
Unicompartmental knee arthroplasty (UKA) and total knee arthroplasty (TKA) are 2 reliable treatment options for patients with primary osteoarthritis. Recently published systematic reviews of cohort studies have shown that 10-year survivorship of medial and lateral UKA is 92% and 91%, respectively,1 while 10-year survivorship of TKA in cohort studies is 95%.2 National and annual registries show a similar trend, although the reported survivorship is lower.1,3-7
In order to improve these survivorship rates, the surgical variables that can intraoperatively be controlled by the orthopedic surgeon have been evaluated. These variables include lower leg alignment, soft tissue balance, joint line maintenance, and alignment, size, and fixation of the tibial and femoral component. Several studies have shown that tight control of lower leg alignment,8-14 balancing of the soft tissues,15-19 joint line maintenance,20-23 component alignment,24-28 component size,29-34 and component fixation35-40 can improve the outcomes of UKA and TKA. As a result, over the past 2 decades, several computer-assisted surgery systems have been developed with the goal of more accurate and reliable control of these factors, and thus improved outcomes of knee arthroplasty.
These systems differ with regard to the number and type of variables they control. Computer navigation systems aim to control one or more of these surgical variables, and several meta-analyses have shown that these systems, when compared to conventional surgery, improve mechanical axis accuracy, decrease the risk for mechanical axis outliers, and improve component positioning in TKA41-49 and UKA surgery.50,51 Interestingly, however, meta-analyses have failed to show the expected superiority in clinical outcomes following computer navigation compared to conventional knee arthroplasty.48,52-55 Furthermore, authors have shown that, despite the fact that computer-navigated surgery increases the accuracy of mechanical alignment and surgical cutting, there is still room for improvement.56 As a consequence, robotic-assisted systems have been developed.
Similar to computer navigation, these robotic-assisted systems aim to control the surgical variables; in addition, they aim to improve the surgical precision of the procedure. Interestingly, 2 recent studies have shown that robotic-assisted systems are superior to computer navigation systems with regard to less cutting time and less resection deviations in coronal and sagittal plane in a cadaveric study,57 and shorter total surgery time, more accurate mechanical axis, and shorter hospital stay in a clinical study.58 Although these results are promising, the exact role of robotic surgery in knee arthroplasty remains unclear. In this review, we aim to report the current state of robotic-assisted knee arthroplasty by discussing (1) the different robotic-assisted knee arthroplasty systems that are available for UKA and TKA surgery, (2) studies that assessed the role of robotic-assisted knee arthroplasty in controlling the aforementioned surgical variables, (3) cadaveric and clinical comparative studies that compared how accurate robotic-assisted and conventional knee surgery control these surgical variables, and (4) studies that assessed the cost-effectiveness of robotic-assisted knee arthroplasty surgery.
Robotic-Assisted Knee Arthroplasty Systems
Several systems have been developed over the years for knee arthroplasty, and these are usually defined as active, semi-active, or passive.59 Active systems are capable of performing tasks or processes autonomously under the watchful eye of the surgeon, while passive systems do not perform actions independently but provide the surgeon with information. In semi-active systems, the surgical action is physically constrained in order to follow a predefined strategy.
In the United States, 3 robotic systems are FDA-approved for knee arthroplasty. The Stryker/Mako haptic guided robot (Mako Surgical Corp.) was introduced in 2005 and has been used for over 50,000 UKA procedures (Figure 1). There are nearly 300 robotic systems used nationally, as it has 20% of the market share for UKA in the United States. The Mako system is a semi-active tactile robotic system that requires preoperative imaging, after which a preoperative planning is performed. Intraoperatively, the robotic arm is under direct surgeon control and gives real-time tactile feedback during the procedure (Figure 2).
Furthermore, the surgeon can intraoperatively virtually adjust component positioning and alignment and move the knee through the range of motion, after which the system can provide information on alignment, component position, and balance of the soft tissue (eg, if the knee is overtight or lax through the flexion-arch).60 This system has a burr that resects the bone and when the surgeon directs the burr outside the preplanned area, the burr stops and prevents unnecessary and unwanted resections (Figure 3).
The Navio Precision Free-Hand Sculptor (PFS) system (Blue Belt Technologies) has been used for 1500 UKA procedures, with 50 robots in use in the United States (Figure 4). This system is an image-free semi-active robotic system and has the same characteristics as the aforementioned Mako system.61 Finally, the OmniBotic robotic system (Omnilife Science) has been released for TKA and has been used for over 7300 procedures (Figure 5). This system has an automated cutting-guide technique in which the surgeon designs a virtual plan on the computer system. After this, the cutting-guides are placed by the robotic system at the planned location for all 5 femoral cuts (ie, distal, anterior chamfer, anterior, posterior chamfer, and posterior) and the surgeon then makes the final cuts.57,62
Three robotic systems for knee arthroplasty surgery have been used in Europe. The Caspar system (URS Ortho) is an active robotic system in which a computed tomography (CT) scan is performed preoperatively, after which a virtual implantation is performed on the screen. The surgeon can then obtain information on lower leg alignment, gap balancing, and component positioning, and after an operative plan is made, the surgical resections are performed by the robot. Reflective markers are attached to the leg and all robotic movements are monitored using an infrared camera system. Any undesired motion will be detected by this camera system and will stop all movements.63 A second and more frequently reported system in the literature is the active Robodoc surgical system (Curexo Technology Corporation). This system is designed for TKA and total hip arthroplasty (THA) surgery. Although initial studies reported a high incidence of system-related complications in THA,64 the use of this system for TKA has frequently been reported in the literature.56,63,65-69 A third robotic system that has been used in Europe is the Acrobot surgical system (Acrobot Company Ltd), which is an image-based semi-active robotic system70 used for both UKA and TKA surgery.70,71
Accuracy of Controlling Surgical Variables in Robotic-Assisted Knee Arthroplasty
Several studies have assessed the accuracy of robotic-assisted surgery in UKA surgery with regard to control of the aforementioned surgical variables. Pearle and colleagues72 assessed the mechanical axis accuracy of the Mako system in 10 patients undergoing medial UKA robotic-assisted surgery. They reported that the intraoperative registration lasted 7.5 minutes and the duration of time needed for robotic-assisted burring was 34.8 minutes. They compared the actual postoperative alignment at 6 weeks follow-up with the planned lower leg alignment and found that all measurements were within 1.6° of the planned lower leg alignment. Dunbar and colleagues73 assessed the accuracy of component positioning of the Mako system in 20 patients undergoing medial UKA surgery by comparing preoperative and postoperative 3-dimensional CT scans. They found that the femoral component was within 0.8 mm and 0.9° in all directions and that the tibial component was within 0.9 mm and 1.7° in all directions. They concluded that the accuracy of component positioning with the Mako system was excellent. Finally, Plate and colleagues17 assessed the accuracy of soft tissue balancing in the Mako system in 52 patients undergoing medial UKA surgery. They compared the balance plan with the soft tissue balance after implantation and the Mako system quantified soft tissue balance as the amount of mm of the knee being tight or loose at 0°, 30°, 60°, 90°, and 110° of flexion. They found that at all flexion angles the ligament balancing was accurate up to 0.53 mm of the original plan. Furthermore, they noted that in 83% of cases the accuracy was within 1 mm at all flexion angles.
For the Navio system, Smith and colleagues74 assessed the accuracy of component positioning using 20 synthetic femurs and tibia. They reported a maximum rotational error of 3.2°, an angular error of 1.46° in all orientations, and a maximum translational error of 1.18 mm for both the tibial and femoral implants. Lonner and colleagues75 assessed the accuracy of component positioning in 25 cadaveric specimens. They found similar results as were found in the study of Smith and colleagues74 and concluded that these results were similar to other semi-active robotic systems designed for UKA.
For TKA surgery, Ponder and colleagues76 assessed the accuracy of the OmniBotic system and found that the average error in the anterior-posterior dimension between the targeted and measured cuts was -0.14 mm, and that the standard deviation in guide positioning for the distal, anterior chamfer, and posterior chamfer resections was 0.03° and 0.17 mm. Koenig and colleagues62 assessed the accuracy of the OmniBotic system in the first 100 cases and found that 98% of the cases were within 3° of the neutral mechanical axis. Furthermore, they found a learning curve with regard to tourniquet time between the first and second 10 patients in which they performed robotic-assisted TKA surgery. Siebert and colleagues63 assessed the accuracy of mechanical alignment in the Caspar system in 70 patients treated with the robotic system. They found that the difference between preoperatively planned and postoperatively achieved mechanical alignment was 0.8°. Similarly, Bellemans and colleagues77 assessed mechanical alignment and the positioning and rotation of the tibial and femoral components in a clinical study of 25 cases using the Caspar system. They noted that none of the patients had mechanical alignment, tibial or femoral component positioning, or rotation beyond 1° of the neutral axis. Liow and colleagues56 assessed the accuracy of mechanical axis alignment and component sizing accuracy using the Robodoc system in 25 patients. They reported that the mean postoperative alignment was 0.4° valgus and that all cases were within 3° of the neutral mechanical axis. Furthermore, they reported a mean surgical time of 96 minutes and reported that preoperative planning yielded femoral and tibial component size accuracy of 100%.
These studies have shown that robotic systems for UKA and TKA are accurate in the surgical variables they aim to control. These studies validated tight control of mechanical axis alignment, decrease for outliers, and component positioning and rotation, and also found that the balancing of soft tissues was improved using robotic-assisted surgery.
Robotic-Assisted vs Conventional Knee Arthroplasty
Despite the fact that these systems are accurate in the variables they aim to control, these systems have to be compared to the gold standard of conventional knee arthroplasty. For UKA, Cobb and colleagues70 performed a randomized clinical trial for patients treated undergoing UKA with robotic-assistance of the Acrobot systems compared to conventional UKA and assessed differences in mechanical accuracy. A total of 27 patients were randomly assigned to one of both treatments. They found that in the group of robotic-assisted surgery, 100% of the patients had a mechanical axis within 2° of neutral, while this was only 40% in the conventional UKA groups (difference P < .001). They also assessed the increase in functional outcomes and noted a trend towards improvement in performance with increasing accuracy at 6 weeks and 3 months postoperatively. Lonner and colleagues78 also compared the tibial component positioning between robotic-assisted UKA surgery using the Mako system and conventional UKA surgery. The authors found that the variance in tibial slope, in coronal plane of the tibial component, and varus/valgus alignment were all larger with conventional UKA when compared to robotic-assisted UKA. Citak and colleagues79 compared the accuracy of tibial and femoral implant positioning between robotic-assisted surgery using the Mako system and conventional UKA in a cadaveric study. They reported that the root mean square (RMS) error of femoral component was 1.9 mm and 3.7° in robotic-assisted surgery and 5.4 mm and 10.2° for conventional UKA, while the RMS error for tibial component were 1.4 mm and 5.0° for robotic-assisted surgery and 5.7 mm and 19.2° for conventional UKA surgery. MacCallum and colleagues80 compared the tibial base plate position in a prospective clinical study of 177 patients treated with conventional UKA and 87 patients treated with robotic-assisted surgery using the Mako system. They found that surgery with robotic-assistance was more precise in the coronal and sagittal plane and was more accurate in coronal alignment when compared to conventional UKA. Finally, the first results of robotic-assisted UKA surgery have been presented. Coon and colleagues81 reported the preliminary results of a multicenter study of 854 patients and found a survivorship of 98.9% and satisfaction rate of 92% at minimum 2-year follow-up. Comparing these results to other large conventional UKA cohorts82,83 suggests that robotic-assisted surgery may improve survivorship at short-term follow-up. However, comparative studies and studies with longer follow-up are necessary to assess the additional value of robotic-assisted UKA surgery. Due to the relatively new concept of robotic-assisted surgery, these studies have not been performed or published yet.
For TKA, several studies also have compared how these robotic-systems control the surgical variables compared to conventional TKA surgery. Siebert and colleagues63 assessed mechanical axis accuracy and mechanical outliers following robotic-assisted TKA surgery using the Caspar system and conventional TKA surgery. They reported the difference between preoperative planned and postoperative achieved alignment was 0.8° for robotic-assisted surgery and 2.6° for conventional TKA surgery. Furthermore, they showed that 1 patient in the robotic-assisted group (1.4%) and 18 patients in the conventional TKA group (35%) had mechanical alignment greater than 3° from the neutral mechanical axis. Liow and colleagues56 found similar differences in their prospective randomized study in which they reported that 0% outliers greater than 3° from the neutral mechanical axis were found in the robotic-assisted group while 19.4% of the patients in the conventional TKA group had mechanical axis outliers. They also assessed the joint-line outliers in both procedures and found that 3.2% had joint-line outliers greater than 5 mm in the robotic-assisted group compared to 20.6% in the conventional TKA group. Kim and colleagues65 assessed implant accuracy in robotic-assisted surgery using the ROBODOC system and in conventional surgery and reported higher implant accuracy and fewer outliers using robotic-assisted surgery. Moon and colleagues66 compared robotic-assisted TKA surgery using the Robodoc system with conventional TKA surgery in 10 cadavers. They found that robotic-assisted surgery had excellent precision in all planes and had better accuracy in femoral rotation alignment compared to conventional TKA surgery. Park and Lee67 compared Robodoc robotic-assisted TKA surgery with conventional TKA surgery in a randomized clinical trial of 72 patients. They found that robotic-assisted surgery had definitive advantages in preoperative planning, accuracy of the procedure, and postoperative follow-up regarding femoral and tibial component flexion angles. Finally, Song and colleagues68,69 performed 2 randomized clinical trials in which they compared mechanical axis alignment, component positioning, soft tissue balancing, and patient preference between conventional TKA surgery and robotic-assisted surgery using the Robodoc system. In the first study,68 they simultaneously performed robotic-assisted surgery in one leg and conventional TKA surgery in the other leg. They found that robotic-assisted surgery resulted in less outlier in mechanical axis and component positioning. Furthermore, they found at latest follow-up of 2 years that 12 patients preferred the leg treated with robotic-assisted surgery while 6 preferred the conventional leg. Despite this finding, no significant differences in functional outcome scores were detected between both treatment options. Furthermore, they found that flexion-extension balance was achieved in 92% of patients treated with robotic-assisted TKA surgery and in 77% of patients treated with conventional TKA surgery. In the other study,69 the authors found that more patients treated with robotic-assisted surgery had <2 mm flexion-extension gap and more satisfactory posterior cruciate ligament tension when compared to conventional surgery.
These studies have shown that robotic-assisted surgery is accurate in controlling surgical variables, such as mechanical lower leg alignment, maintaining joint-line, implant positioning, and soft tissue balancing. Furthermore, these studies have shown that controlling these variables is better than the current gold standard of manual knee arthroplasty. Until now, not many studies have assessed survivorship of robotic-assisted surgery. Furthermore, no studies have, to our knowledge, compared survivorship of robotic-assisted with conventional knee replacement surgery. Finally, studies comparing functional outcomes following robotic-assisted surgery and conventional knee arthroplasty surgery are frequently underpowered due to their small sample sizes.68,70 Since many studies have shown that the surgical variables are more tightly controlled using robotic-assisted surgery when compared to conventional surgery, large comparative studies are necessary to assess the role of robotic-assisted surgery in functional outcomes and survivorship of UKA and TKA.
Cost-Effectiveness of Robotic-Assisted Surgery
High initial capital costs of robotic-assisted surgery is one of the factors that constitute a barrier to the widespread implementation of this technique. Multiple authors have suggested that improved implant survivorship afforded by robotic-assisted surgery may justify the expenditure from both societal and provider perspective.84-86 Two studies have performed a cost-effectiveness analysis for UKA surgery. Swank and colleagues84 reviewed the hospital expenditures and profits associated with robot-assisted knee arthroplasty, citing upfront costs of approximately $800,000. The authors estimated a mean per-case contribution profit of $5790 for robotic-assisted UKA, assuming an inpatient-to-outpatient ratio of 1 to 3. Based on this data, Swank and colleagues84 proposed that the capital costs of robotic-assisted UKA may be recovered in as little as 2 years when in the first 3 consecutive years 50, 70, and 90 cases were performed using robotic-assisted UKA. Moschetti and colleagues85 recently published the first formal cost-effectiveness analysis of robotic-assisted compared to manual UKA. The authors used an annual revision risk of 0.55% for the first 2 years following robot-assisted UKA, based on the aforementioned presented data by Coon and colleagues.81 They based their data on the Mako system and assumed an initial capital expenditure of $934,728 with annual servicing costs of 10% (discounted annually) for 4 years thereafter, resulting in a total cost of the robotic system of $1.362 million. These costs were divided by the number of patients estimated to undergo robotic-assisted UKA per year, which was varied to estimate the effect of case volume on cost-effectiveness. The authors reported that robotic-assisted UKA was associated with higher lifetime costs and net utilities compared to manual UKA, at an incremental cost-effectiveness ratio of $47,180 per quality-adjusted life year (QALY) in a high-volume center. This falls well within the societal willingness-to-pay threshold of $100,000/QALY. Sensitivity analysis showed that robotic-assisted UKA is cost-effective under the following conditions: (1) centers performing at least 94 cases annually, (2) in patients younger than age 67 years, and (3) 2-year revision rate does not exceed 1.2%. While the results of this initial analysis are promising, follow-up cost-effectiveness analysis studies will be required as long-term survivorship data become available.
Conclusion
Tighter control of intraoperative surgical variables, such as lower leg alignment, soft tissue balance, joint-line maintenance, and component alignment and positioning, have been associated with improved survivorship and functional outcomes. Upon reviewing the available literature on robotic-assisted surgery, it becomes clear that this technique can improve the accuracy of these surgical variables and is superior to conventional manual UKA and TKA. Although larger and comparative survivorship studies are necessary to compare robotic-assisted knee arthroplasty to conventional techniques, the early results and cost-effectiveness analysis seem promising.
1. van der List JP, McDonald LS, Pearle AD. Systematic review of medial versus lateral survivorship in unicompartmental knee arthroplasty. Knee. 2015;22(6):454-460.
2. Mont MA, Pivec R, Issa K, Kapadia BH, Maheshwari A, Harwin SF. Long-term implant survivorship of cementless total knee arthroplasty: a systematic review of the literature and meta-analysis. J Knee Surg. 2014;27(5):369-376.
3. Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2014 Australian Hip and Knee Arthroplasty Register. https://aoanjrr.sahmri.com/documents/10180/172286/Annual%20Report%202014. Accessed April 6, 2016.
4. The Swedish Knee Arthroplasty Register. Annual Report 2015 Swedish Knee Arthroplasty Register. http://www.myknee.se/pdf/SVK_2015_Eng_1.0.pdf. Published December 1, 2015. Accessed April 6, 2016.
5. Centre of excellence of joint replacements. The Norwegian Arthroplasty Register. http://nrlweb.ihelse.net/eng/Report_2010.pdf. Published June 2010. Accessed June 3, 2015.
6. National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 12th Annual Report 2015. http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/12th%20annual%20report/NJR%20Online%20Annual%20Report%202015.pdf. Accessed April 6, 2016.
7. The New Zealand Joint Registry. Fourteen Year Report January 1999 to December 2012. http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed April 6, 2016.
8. Jeffery RS, Morris RW, Denham RA. Coronal alignment after total knee replacement. J Bone Joint Surg Br. 1991;73(5):709-714.
9. Rand JA, Coventry MB. Ten-year evaluation of geometric total knee arthroplasty. Clin Orthop Relat Res. 1988;232:168-173.
10. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
11. Ryd L, Lindstrand A, Stenström A, Selvik G. Porous coated anatomic tricompartmental tibial components. The relationship between prosthetic position and micromotion. Clin Orthop Relat Res. 1990;251:189-197.
12. van der List JP, Chawla H, Villa JC, Zuiderbaan HA, Pearle AD. Early functional outcome after lateral UKA is sensitive to postoperative lower limb alignment. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
13. van der List JP, Zuiderbaan HA, Pearle AD. Why do medial unicompartmental knee arthroplasties fail today? J Arthroplasty. 2015. [Epub ahead of print]
14. Vasso M, Del Regno C, D’Amelio A, Viggiano D, Corona K, Schiavone Panni A. Minor varus alignment provides better results than neutral alignment in medial UKA. Knee. 2015;22(2):117-121.
15. Attfield SF, Wilton TJ, Pratt DJ, Sambatakakis A. Soft-tissue balance and recovery of proprioception after total knee replacement. J Bone Joint Surg Br. 1996;78(4):540-545.
16. Pagnano MW, Hanssen AD, Lewallen DG, Stuart MJ. Flexion instability after primary posterior cruciate retaining total knee arthroplasty. Clin Orthop Relat Res. 1998;356:39-46.
17. Plate JF, Mofidi A, Mannava S, et al. Achieving accurate ligament balancing using robotic-assisted unicompartmental knee arthroplasty. Adv Orthop. 2013;2013:837167.
18. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
19. Wasielewski RC, Galante JO, Leighty RM, Natarajan RN, Rosenberg AG. Wear patterns on retrieved polyethylene tibial inserts and their relationship to technical considerations during total knee arthroplasty. Clin Orthop Relat Res. 1994;299:31-43.
20. Ji HM, Han J, Jin DS, Seo H, Won YY. Kinematically aligned TKA can align knee joint line to horizontal. Knee Surg Sports Traumatol Arthrosc. 2016. [Epub ahead of print]
21. Khamaisy S, Zuiderbaan HA, van der List JP, Nam D, Pearle AD. Medial unicompartmental knee arthroplasty improves congruence and restores joint space width of the lateral compartment. Knee. 2016. [Epub ahead of print]
22. Niinimaki TT, Murray DW, Partanen J, Pajala A, Leppilahti JI. Unicompartmental knee arthroplasties implanted for osteoarthritis with partial loss of joint space have high re-operation rates. Knee. 2011;18(6):432-435.
23. Zuiderbaan HA, Khamaisy S, Thein R, Nawabi DH, Pearle AD. Congruence and joint space width alterations of the medial compartment following lateral unicompartmental knee arthroplasty. Bone Joint J. 2015;97-B(1):50-55.
24. Barbadoro P, Ensini A, Leardini A, et al. Tibial component alignment and risk of loosening in unicompartmental knee arthroplasty: a radiographic and radiostereometric study. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3157-3162.
25. Collier MB, Eickmann TH, Sukezaki F, McAuley JP, Engh GA. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):108-115.
26. Nedopil AJ, Howell SM, Hull ML. Does malrotation of the tibial and femoral components compromise function in kinematically aligned total knee arthroplasty? Orthop Clin North Am. 2016;47(1):41-50.
27. Rosskopf J, Singh PK, Wolf P, Strauch M, Graichen H. Influence of intentional femoral component flexion in navigated TKA on gap balance and sagittal anatomy. Knee Surg Sports Traumatol Arthrosc. 2014;22(3):687-693.
28. Zihlmann MS, Stacoff A, Romero J, Quervain IK, Stüssi E. Biomechanical background and clinical observations of rotational malalignment in TKA: literature review and consequences. Clin Biomech (Bristol, Avon). 2005;20(7):661-668.
29. Bonnin MP, Saffarini M, Shepherd D, Bossard N, Dantony E. Oversizing the tibial component in TKAs: incidence, consequences and risk factors. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
30. Bonnin MP, Schmidt A, Basiglini L, Bossard N, Dantony E. Mediolateral oversizing influences pain, function, and flexion after TKA. Knee Surg Sports Traumatol Arthrosc. 2013;21(10):2314-2324.
31. Chau R, Gulati A, Pandit H, et al. Tibial component overhang following unicompartmental knee replacement--does it matter? Knee. 2009;16(5):310-313.
32. Mueller JK, Wentorf FA, Moore RE. Femoral and tibial insert downsizing increases the laxity envelope in TKA. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3003-3011.
33. Sriphirom P, Raungthong N, Chutchawan P, Thiranon C, Sukandhavesa N. Influence of a secondary downsizing of the femoral component on the extension gap: a cadaveric study. Orthopedics. 2012;35(10 Suppl):56-59.
34. Young SW, Clarke HD, Graves SE, Liu YL, de Steiger RN. Higher rate of revision in PFC sigma primary total knee arthroplasty with mismatch of femoro-tibial component sizes. J Arthroplasty. 2015;30(5):813-817.
35. Barink M, Verdonschot N, de Waal Malefijt M. A different fixation of the femoral component in total knee arthroplasty may lead to preservation of femoral bone stock. Proc Inst Mech Eng H. 2003;217(5):325-332.
36. Eagar P, Hull ML, Howell SM. How the fixation method stiffness and initial tension affect anterior load-displacement of the knee and tension in anterior cruciate ligament grafts: a study in cadaveric knees using a double-loop hamstrings graft. J Orthop Res. 2004;22(3):613-624.
37. Fricka KB, Sritulanondha S, McAsey CJ. To cement or not? Two-year results of a prospective, randomized study comparing cemented vs. cementless total knee arthroplasty (TKA). J Arthroplasty. 2015;30(9 Suppl):55-58.
38. Kendrick BJ, Kaptein BL, Valstar ER, et al. Cemented versus cementless Oxford unicompartmental knee arthroplasty using radiostereometric analysis: a randomised controlled trial. Bone Joint J. 2015;97-B(2):185-191.
39. Kim TK, Chang CB, Kang YG, Chung BJ, Cho HJ, Seong SC. Execution accuracy of bone resection and implant fixation in computer assisted minimally invasive total knee arthroplasty. Knee. 2010;17(1):23-28.
40. Whiteside LA. Making your next unicompartmental knee arthroplasty last: three keys to success. J Arthroplasty. 2005;20(4 Suppl 2):2-3.
41. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
42. Brin YS, Nikolaou VS, Joseph L, Zukor DJ, Antoniou J. Imageless computer assisted versus conventional total knee replacement. A Bayesian meta-analysis of 23 comparative studies. Int Orthop. 2011;35(3):331-339.
43. Cheng T, Zhang G, Zhang X. Imageless navigation system does not improve component rotational alignment in total knee arthroplasty. J Surg Res. 2011;171(2):590-600.
44. Conteduca F, Iorio R, Mazza D, Ferretti A. Patient-specific instruments in total knee arthroplasty. Int Orthop. 2014;38(2):259-265.
45. Fu Y, Wang M, Liu Y, Fu Q. Alignment outcomes in navigated total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(6):1075-1082.
46. Hetaimish BM, Khan MM, Simunovic N, Al-Harbi HH, Bhandari M, Zalzal PK. Meta-analysis of navigation vs conventional total knee arthroplasty. J Arthroplasty. 2012;27(6):1177-1182.
47. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
48. Moskal JT, Capps SG, Mann JW, Scanelli JA. Navigated versus conventional total knee arthroplasty. J Knee Surg. 2014;27(3):235-248.
49. Shi J, Wei Y, Wang S, et al. Computer navigation and total knee arthroplasty. Orthopedics. 2014;37(1):e39-e43.
50. Nair R, Tripathy G, Deysine GR. Computer navigation systems in unicompartmental knee arthroplasty: a systematic review. Am J Orthop. 2014;43(6):256-261.
51. Weber P, Crispin A, Schmidutz F, et al. Improved accuracy in computer-assisted unicondylar knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2453-2461.
52. Alcelik IA, Blomfield MI, Diana G, Gibbon AJ, Carrington N, Burr S. A comparison of short-term outcomes of minimally invasive computer-assisted vs minimally invasive conventional instrumentation for primary total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2016;31(2):410-418.
53. Cheng T, Pan XY, Mao X, Zhang GY, Zhang XL. Little clinical advantage of computer-assisted navigation over conventional instrumentation in primary total knee arthroplasty at early follow-up. Knee. 2012;19(4):237-245.
54. Rebal BA, Babatunde OM, Lee JH, Geller JA, Patrick DA Jr, Macaulay W. Imageless computer navigation in total knee arthroplasty provides superior short term functional outcomes: a meta-analysis. J Arthroplasty. 2014;29(5):938-944.
55. Zamora LA, Humphreys KJ, Watt AM, Forel D, Cameron AL. Systematic review of computer-navigated total knee arthroplasty. ANZ J Surg. 2013;83(1-2):22-30.
56. Liow MH, Xia Z, Wong MK, Tay KJ, Yeo SJ, Chin PL. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis. A prospective randomised study. J Arthroplasty. 2014;29(12):2373-2377.
57. Koulalis D, O’Loughlin PF, Plaskos C, Kendoff D, Cross MB, Pearle AD. Sequential versus automated cutting guides in computer-assisted total knee arthroplasty. Knee. 2011;18(6):436-442.
58. Clark TC, Schmidt FH. Robot-assisted navigation versus computer-assisted navigation in primary total knee arthroplasty: efficiency and accuracy. ISRN Orthop. 2013;2013:794827.
59. DiGioia AM 3rd, Jaramaz B, Colgan BD. Computer assisted orthopaedic surgery. Image guided and robotic assistive technologies. Clin Orthop Relat Res. 1998(354):8-16.
60. Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 1:63-68.
61. Lonner JH. Robotically assisted unicompartmental knee arthroplasty with a handheld image-free sculpting tool. Orthop Clin North Am. 2016;47(1):29-40.
62. Koenig JA, Suero EM, Plaskos C. Surgical accuracy and efficiency of computer-navigated TKA with a robotic cutting guide–report on the first 100 cases. J Bone Joint Surg Br. 2012;94-B(SUPP XLIV):103. Available at: http://www.bjjprocs.boneandjoint.org.uk/content/94-B/SUPP_XLIV/103. Accessed April 6, 2016.
63. Siebert W, Mai S, Kober R, Heeckt PF. Technique and first clinical results of robot-assisted total knee replacement. Knee. 2002;9(3):173-180.
64. Schulz AP, Seide K, Queitsch C, et al. Results of total hip replacement using the Robodoc surgical assistant system: clinical outcome and evaluation of complications for 97 procedures. Int J Med Robot. 2007;3(4):301-306.
65. Kim SM, Park YS, Ha CW, Lim SJ, Moon YW. Robot-assisted implantation improves the precision of component position in minimally invasive TKA. Orthopedics. 2012;35(9):e1334-e1339.
66. Moon YW, Ha CW, Do KH, et al. Comparison of robot-assisted and conventional total knee arthroplasty: a controlled cadaver study using multiparameter quantitative three-dimensional CT assessment of alignment. Comput Aided Surg. 2012;17(2):86-95.
67. Park SE, Lee CT. Comparison of robotic-assisted and conventional manual implantation of a primary total knee arthroplasty. J Arthroplasty. 2007;22(7):1054-1059.
68. Song EK, Seon JK, Park SJ, Jung WB, Park HW, Lee GW. Simultaneous bilateral total knee arthroplasty with robotic and conventional techniques: a prospective, randomized study. Knee Surg Sports Traumatol Arthrosc. 2011;19(7):1069-1076.
69. Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471(1):118-126.
70. Cobb J, Henckel J, Gomes P, et al. Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br. 2006;88(2):188-197.
71. Jakopec M, Harris SJ, Rodriguez y Baena F, Gomes P, Cobb J, Davies BL. The first clinical application of a “hands-on” robotic knee surgery system. Comput Aided Surg. 2001;6(6):329-339.
72. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
73. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
74. Smith JR, Riches PE, Rowe PJ. Accuracy of a freehand sculpting tool for unicondylar knee replacement. Int J Med Robot. 2014;10(2):162-169.
75. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
76. Ponder C, Plaskos C, Cheal E. Press-fit total knee arthroplasty with a robotic-cutting guide: proof of concept and initial clinical experience. Bone & Joint Journal Orthopaedic Proceedings Supplement. 2013;95(SUPP 28):61. Available at: http://www.bjjprocs.boneandjoint.org.uk/content/95-B/SUPP_28/61.abstract. Accessed April 6, 2016.
77. Bellemans J, Vandenneucker H, Vanlauwe J. Robot-assisted total knee arthroplasty. Clin Orthop Relat Res. 2007;464:111-116.
78. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
79. Citak M, Suero EM, Citak M, et al. Unicompartmental knee arthroplasty: is robotic technology more accurate than conventional technique? Knee. 2013;20(4):268-271.
80. MacCallum KP, Danoff JR, Geller JA. Tibial baseplate positioning in robotic-assisted and conventional unicompartmental knee arthroplasty. Eur J Orthop Surg Traumatol. 2016;26(1):93-98.
81. Coon T, Roche M, Pearle AD, Dounchis J, Borus T, Buechel F Jr. Two year survivorship of robotically guided unicompartmental knee arthroplasty. Paper presented at: International Society for Technology in Arthroplasty 26th Annual Congress; October 16-19, 2013; Palm Beach, FL.
82. Pandit H, Jenkins C, Gill HS, Barker K, Dodd CA, Murray DW. Minimally invasive Oxford phase 3 unicompartmental knee replacement: results of 1000 cases. J Bone Joint Surg Br. 2011;93(2):198-204.
83. Yoshida K, Tada M, Yoshida H, Takei S, Fukuoka S, Nakamura H. Oxford phase 3 unicompartmental knee arthroplasty in Japan--clinical results in greater than one thousand cases over ten years. J Arthroplasty. 2013;28(9 Suppl):168-171.
84. Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop. 2009;38(2 Suppl):32-36.
85. Moschetti WE, Konopka JF, Rubash HE, Genuario JW. Can robot-assisted unicompartmental knee arthroplasty be cost-effective? A markovdecision analysis. J Arthroplasty. 2015. [Epub ahead of print]
86. Thienpont E. Improving Accuracy in Knee Arthroplasty. 1st ed. New Delhi, India: Jaypee Brothers Medical Publishers; 2012.
Unicompartmental knee arthroplasty (UKA) and total knee arthroplasty (TKA) are 2 reliable treatment options for patients with primary osteoarthritis. Recently published systematic reviews of cohort studies have shown that 10-year survivorship of medial and lateral UKA is 92% and 91%, respectively,1 while 10-year survivorship of TKA in cohort studies is 95%.2 National and annual registries show a similar trend, although the reported survivorship is lower.1,3-7
In order to improve these survivorship rates, the surgical variables that can intraoperatively be controlled by the orthopedic surgeon have been evaluated. These variables include lower leg alignment, soft tissue balance, joint line maintenance, and alignment, size, and fixation of the tibial and femoral component. Several studies have shown that tight control of lower leg alignment,8-14 balancing of the soft tissues,15-19 joint line maintenance,20-23 component alignment,24-28 component size,29-34 and component fixation35-40 can improve the outcomes of UKA and TKA. As a result, over the past 2 decades, several computer-assisted surgery systems have been developed with the goal of more accurate and reliable control of these factors, and thus improved outcomes of knee arthroplasty.
These systems differ with regard to the number and type of variables they control. Computer navigation systems aim to control one or more of these surgical variables, and several meta-analyses have shown that these systems, when compared to conventional surgery, improve mechanical axis accuracy, decrease the risk for mechanical axis outliers, and improve component positioning in TKA41-49 and UKA surgery.50,51 Interestingly, however, meta-analyses have failed to show the expected superiority in clinical outcomes following computer navigation compared to conventional knee arthroplasty.48,52-55 Furthermore, authors have shown that, despite the fact that computer-navigated surgery increases the accuracy of mechanical alignment and surgical cutting, there is still room for improvement.56 As a consequence, robotic-assisted systems have been developed.
Similar to computer navigation, these robotic-assisted systems aim to control the surgical variables; in addition, they aim to improve the surgical precision of the procedure. Interestingly, 2 recent studies have shown that robotic-assisted systems are superior to computer navigation systems with regard to less cutting time and less resection deviations in coronal and sagittal plane in a cadaveric study,57 and shorter total surgery time, more accurate mechanical axis, and shorter hospital stay in a clinical study.58 Although these results are promising, the exact role of robotic surgery in knee arthroplasty remains unclear. In this review, we aim to report the current state of robotic-assisted knee arthroplasty by discussing (1) the different robotic-assisted knee arthroplasty systems that are available for UKA and TKA surgery, (2) studies that assessed the role of robotic-assisted knee arthroplasty in controlling the aforementioned surgical variables, (3) cadaveric and clinical comparative studies that compared how accurate robotic-assisted and conventional knee surgery control these surgical variables, and (4) studies that assessed the cost-effectiveness of robotic-assisted knee arthroplasty surgery.
Robotic-Assisted Knee Arthroplasty Systems
Several systems have been developed over the years for knee arthroplasty, and these are usually defined as active, semi-active, or passive.59 Active systems are capable of performing tasks or processes autonomously under the watchful eye of the surgeon, while passive systems do not perform actions independently but provide the surgeon with information. In semi-active systems, the surgical action is physically constrained in order to follow a predefined strategy.
In the United States, 3 robotic systems are FDA-approved for knee arthroplasty. The Stryker/Mako haptic guided robot (Mako Surgical Corp.) was introduced in 2005 and has been used for over 50,000 UKA procedures (Figure 1). There are nearly 300 robotic systems used nationally, as it has 20% of the market share for UKA in the United States. The Mako system is a semi-active tactile robotic system that requires preoperative imaging, after which a preoperative planning is performed. Intraoperatively, the robotic arm is under direct surgeon control and gives real-time tactile feedback during the procedure (Figure 2).
Furthermore, the surgeon can intraoperatively virtually adjust component positioning and alignment and move the knee through the range of motion, after which the system can provide information on alignment, component position, and balance of the soft tissue (eg, if the knee is overtight or lax through the flexion-arch).60 This system has a burr that resects the bone and when the surgeon directs the burr outside the preplanned area, the burr stops and prevents unnecessary and unwanted resections (Figure 3).
The Navio Precision Free-Hand Sculptor (PFS) system (Blue Belt Technologies) has been used for 1500 UKA procedures, with 50 robots in use in the United States (Figure 4). This system is an image-free semi-active robotic system and has the same characteristics as the aforementioned Mako system.61 Finally, the OmniBotic robotic system (Omnilife Science) has been released for TKA and has been used for over 7300 procedures (Figure 5). This system has an automated cutting-guide technique in which the surgeon designs a virtual plan on the computer system. After this, the cutting-guides are placed by the robotic system at the planned location for all 5 femoral cuts (ie, distal, anterior chamfer, anterior, posterior chamfer, and posterior) and the surgeon then makes the final cuts.57,62
Three robotic systems for knee arthroplasty surgery have been used in Europe. The Caspar system (URS Ortho) is an active robotic system in which a computed tomography (CT) scan is performed preoperatively, after which a virtual implantation is performed on the screen. The surgeon can then obtain information on lower leg alignment, gap balancing, and component positioning, and after an operative plan is made, the surgical resections are performed by the robot. Reflective markers are attached to the leg and all robotic movements are monitored using an infrared camera system. Any undesired motion will be detected by this camera system and will stop all movements.63 A second and more frequently reported system in the literature is the active Robodoc surgical system (Curexo Technology Corporation). This system is designed for TKA and total hip arthroplasty (THA) surgery. Although initial studies reported a high incidence of system-related complications in THA,64 the use of this system for TKA has frequently been reported in the literature.56,63,65-69 A third robotic system that has been used in Europe is the Acrobot surgical system (Acrobot Company Ltd), which is an image-based semi-active robotic system70 used for both UKA and TKA surgery.70,71
Accuracy of Controlling Surgical Variables in Robotic-Assisted Knee Arthroplasty
Several studies have assessed the accuracy of robotic-assisted surgery in UKA surgery with regard to control of the aforementioned surgical variables. Pearle and colleagues72 assessed the mechanical axis accuracy of the Mako system in 10 patients undergoing medial UKA robotic-assisted surgery. They reported that the intraoperative registration lasted 7.5 minutes and the duration of time needed for robotic-assisted burring was 34.8 minutes. They compared the actual postoperative alignment at 6 weeks follow-up with the planned lower leg alignment and found that all measurements were within 1.6° of the planned lower leg alignment. Dunbar and colleagues73 assessed the accuracy of component positioning of the Mako system in 20 patients undergoing medial UKA surgery by comparing preoperative and postoperative 3-dimensional CT scans. They found that the femoral component was within 0.8 mm and 0.9° in all directions and that the tibial component was within 0.9 mm and 1.7° in all directions. They concluded that the accuracy of component positioning with the Mako system was excellent. Finally, Plate and colleagues17 assessed the accuracy of soft tissue balancing in the Mako system in 52 patients undergoing medial UKA surgery. They compared the balance plan with the soft tissue balance after implantation and the Mako system quantified soft tissue balance as the amount of mm of the knee being tight or loose at 0°, 30°, 60°, 90°, and 110° of flexion. They found that at all flexion angles the ligament balancing was accurate up to 0.53 mm of the original plan. Furthermore, they noted that in 83% of cases the accuracy was within 1 mm at all flexion angles.
For the Navio system, Smith and colleagues74 assessed the accuracy of component positioning using 20 synthetic femurs and tibia. They reported a maximum rotational error of 3.2°, an angular error of 1.46° in all orientations, and a maximum translational error of 1.18 mm for both the tibial and femoral implants. Lonner and colleagues75 assessed the accuracy of component positioning in 25 cadaveric specimens. They found similar results as were found in the study of Smith and colleagues74 and concluded that these results were similar to other semi-active robotic systems designed for UKA.
For TKA surgery, Ponder and colleagues76 assessed the accuracy of the OmniBotic system and found that the average error in the anterior-posterior dimension between the targeted and measured cuts was -0.14 mm, and that the standard deviation in guide positioning for the distal, anterior chamfer, and posterior chamfer resections was 0.03° and 0.17 mm. Koenig and colleagues62 assessed the accuracy of the OmniBotic system in the first 100 cases and found that 98% of the cases were within 3° of the neutral mechanical axis. Furthermore, they found a learning curve with regard to tourniquet time between the first and second 10 patients in which they performed robotic-assisted TKA surgery. Siebert and colleagues63 assessed the accuracy of mechanical alignment in the Caspar system in 70 patients treated with the robotic system. They found that the difference between preoperatively planned and postoperatively achieved mechanical alignment was 0.8°. Similarly, Bellemans and colleagues77 assessed mechanical alignment and the positioning and rotation of the tibial and femoral components in a clinical study of 25 cases using the Caspar system. They noted that none of the patients had mechanical alignment, tibial or femoral component positioning, or rotation beyond 1° of the neutral axis. Liow and colleagues56 assessed the accuracy of mechanical axis alignment and component sizing accuracy using the Robodoc system in 25 patients. They reported that the mean postoperative alignment was 0.4° valgus and that all cases were within 3° of the neutral mechanical axis. Furthermore, they reported a mean surgical time of 96 minutes and reported that preoperative planning yielded femoral and tibial component size accuracy of 100%.
These studies have shown that robotic systems for UKA and TKA are accurate in the surgical variables they aim to control. These studies validated tight control of mechanical axis alignment, decrease for outliers, and component positioning and rotation, and also found that the balancing of soft tissues was improved using robotic-assisted surgery.
Robotic-Assisted vs Conventional Knee Arthroplasty
Despite the fact that these systems are accurate in the variables they aim to control, these systems have to be compared to the gold standard of conventional knee arthroplasty. For UKA, Cobb and colleagues70 performed a randomized clinical trial for patients treated undergoing UKA with robotic-assistance of the Acrobot systems compared to conventional UKA and assessed differences in mechanical accuracy. A total of 27 patients were randomly assigned to one of both treatments. They found that in the group of robotic-assisted surgery, 100% of the patients had a mechanical axis within 2° of neutral, while this was only 40% in the conventional UKA groups (difference P < .001). They also assessed the increase in functional outcomes and noted a trend towards improvement in performance with increasing accuracy at 6 weeks and 3 months postoperatively. Lonner and colleagues78 also compared the tibial component positioning between robotic-assisted UKA surgery using the Mako system and conventional UKA surgery. The authors found that the variance in tibial slope, in coronal plane of the tibial component, and varus/valgus alignment were all larger with conventional UKA when compared to robotic-assisted UKA. Citak and colleagues79 compared the accuracy of tibial and femoral implant positioning between robotic-assisted surgery using the Mako system and conventional UKA in a cadaveric study. They reported that the root mean square (RMS) error of femoral component was 1.9 mm and 3.7° in robotic-assisted surgery and 5.4 mm and 10.2° for conventional UKA, while the RMS error for tibial component were 1.4 mm and 5.0° for robotic-assisted surgery and 5.7 mm and 19.2° for conventional UKA surgery. MacCallum and colleagues80 compared the tibial base plate position in a prospective clinical study of 177 patients treated with conventional UKA and 87 patients treated with robotic-assisted surgery using the Mako system. They found that surgery with robotic-assistance was more precise in the coronal and sagittal plane and was more accurate in coronal alignment when compared to conventional UKA. Finally, the first results of robotic-assisted UKA surgery have been presented. Coon and colleagues81 reported the preliminary results of a multicenter study of 854 patients and found a survivorship of 98.9% and satisfaction rate of 92% at minimum 2-year follow-up. Comparing these results to other large conventional UKA cohorts82,83 suggests that robotic-assisted surgery may improve survivorship at short-term follow-up. However, comparative studies and studies with longer follow-up are necessary to assess the additional value of robotic-assisted UKA surgery. Due to the relatively new concept of robotic-assisted surgery, these studies have not been performed or published yet.
For TKA, several studies also have compared how these robotic-systems control the surgical variables compared to conventional TKA surgery. Siebert and colleagues63 assessed mechanical axis accuracy and mechanical outliers following robotic-assisted TKA surgery using the Caspar system and conventional TKA surgery. They reported the difference between preoperative planned and postoperative achieved alignment was 0.8° for robotic-assisted surgery and 2.6° for conventional TKA surgery. Furthermore, they showed that 1 patient in the robotic-assisted group (1.4%) and 18 patients in the conventional TKA group (35%) had mechanical alignment greater than 3° from the neutral mechanical axis. Liow and colleagues56 found similar differences in their prospective randomized study in which they reported that 0% outliers greater than 3° from the neutral mechanical axis were found in the robotic-assisted group while 19.4% of the patients in the conventional TKA group had mechanical axis outliers. They also assessed the joint-line outliers in both procedures and found that 3.2% had joint-line outliers greater than 5 mm in the robotic-assisted group compared to 20.6% in the conventional TKA group. Kim and colleagues65 assessed implant accuracy in robotic-assisted surgery using the ROBODOC system and in conventional surgery and reported higher implant accuracy and fewer outliers using robotic-assisted surgery. Moon and colleagues66 compared robotic-assisted TKA surgery using the Robodoc system with conventional TKA surgery in 10 cadavers. They found that robotic-assisted surgery had excellent precision in all planes and had better accuracy in femoral rotation alignment compared to conventional TKA surgery. Park and Lee67 compared Robodoc robotic-assisted TKA surgery with conventional TKA surgery in a randomized clinical trial of 72 patients. They found that robotic-assisted surgery had definitive advantages in preoperative planning, accuracy of the procedure, and postoperative follow-up regarding femoral and tibial component flexion angles. Finally, Song and colleagues68,69 performed 2 randomized clinical trials in which they compared mechanical axis alignment, component positioning, soft tissue balancing, and patient preference between conventional TKA surgery and robotic-assisted surgery using the Robodoc system. In the first study,68 they simultaneously performed robotic-assisted surgery in one leg and conventional TKA surgery in the other leg. They found that robotic-assisted surgery resulted in less outlier in mechanical axis and component positioning. Furthermore, they found at latest follow-up of 2 years that 12 patients preferred the leg treated with robotic-assisted surgery while 6 preferred the conventional leg. Despite this finding, no significant differences in functional outcome scores were detected between both treatment options. Furthermore, they found that flexion-extension balance was achieved in 92% of patients treated with robotic-assisted TKA surgery and in 77% of patients treated with conventional TKA surgery. In the other study,69 the authors found that more patients treated with robotic-assisted surgery had <2 mm flexion-extension gap and more satisfactory posterior cruciate ligament tension when compared to conventional surgery.
These studies have shown that robotic-assisted surgery is accurate in controlling surgical variables, such as mechanical lower leg alignment, maintaining joint-line, implant positioning, and soft tissue balancing. Furthermore, these studies have shown that controlling these variables is better than the current gold standard of manual knee arthroplasty. Until now, not many studies have assessed survivorship of robotic-assisted surgery. Furthermore, no studies have, to our knowledge, compared survivorship of robotic-assisted with conventional knee replacement surgery. Finally, studies comparing functional outcomes following robotic-assisted surgery and conventional knee arthroplasty surgery are frequently underpowered due to their small sample sizes.68,70 Since many studies have shown that the surgical variables are more tightly controlled using robotic-assisted surgery when compared to conventional surgery, large comparative studies are necessary to assess the role of robotic-assisted surgery in functional outcomes and survivorship of UKA and TKA.
Cost-Effectiveness of Robotic-Assisted Surgery
High initial capital costs of robotic-assisted surgery is one of the factors that constitute a barrier to the widespread implementation of this technique. Multiple authors have suggested that improved implant survivorship afforded by robotic-assisted surgery may justify the expenditure from both societal and provider perspective.84-86 Two studies have performed a cost-effectiveness analysis for UKA surgery. Swank and colleagues84 reviewed the hospital expenditures and profits associated with robot-assisted knee arthroplasty, citing upfront costs of approximately $800,000. The authors estimated a mean per-case contribution profit of $5790 for robotic-assisted UKA, assuming an inpatient-to-outpatient ratio of 1 to 3. Based on this data, Swank and colleagues84 proposed that the capital costs of robotic-assisted UKA may be recovered in as little as 2 years when in the first 3 consecutive years 50, 70, and 90 cases were performed using robotic-assisted UKA. Moschetti and colleagues85 recently published the first formal cost-effectiveness analysis of robotic-assisted compared to manual UKA. The authors used an annual revision risk of 0.55% for the first 2 years following robot-assisted UKA, based on the aforementioned presented data by Coon and colleagues.81 They based their data on the Mako system and assumed an initial capital expenditure of $934,728 with annual servicing costs of 10% (discounted annually) for 4 years thereafter, resulting in a total cost of the robotic system of $1.362 million. These costs were divided by the number of patients estimated to undergo robotic-assisted UKA per year, which was varied to estimate the effect of case volume on cost-effectiveness. The authors reported that robotic-assisted UKA was associated with higher lifetime costs and net utilities compared to manual UKA, at an incremental cost-effectiveness ratio of $47,180 per quality-adjusted life year (QALY) in a high-volume center. This falls well within the societal willingness-to-pay threshold of $100,000/QALY. Sensitivity analysis showed that robotic-assisted UKA is cost-effective under the following conditions: (1) centers performing at least 94 cases annually, (2) in patients younger than age 67 years, and (3) 2-year revision rate does not exceed 1.2%. While the results of this initial analysis are promising, follow-up cost-effectiveness analysis studies will be required as long-term survivorship data become available.
Conclusion
Tighter control of intraoperative surgical variables, such as lower leg alignment, soft tissue balance, joint-line maintenance, and component alignment and positioning, have been associated with improved survivorship and functional outcomes. Upon reviewing the available literature on robotic-assisted surgery, it becomes clear that this technique can improve the accuracy of these surgical variables and is superior to conventional manual UKA and TKA. Although larger and comparative survivorship studies are necessary to compare robotic-assisted knee arthroplasty to conventional techniques, the early results and cost-effectiveness analysis seem promising.
Unicompartmental knee arthroplasty (UKA) and total knee arthroplasty (TKA) are 2 reliable treatment options for patients with primary osteoarthritis. Recently published systematic reviews of cohort studies have shown that 10-year survivorship of medial and lateral UKA is 92% and 91%, respectively,1 while 10-year survivorship of TKA in cohort studies is 95%.2 National and annual registries show a similar trend, although the reported survivorship is lower.1,3-7
In order to improve these survivorship rates, the surgical variables that can intraoperatively be controlled by the orthopedic surgeon have been evaluated. These variables include lower leg alignment, soft tissue balance, joint line maintenance, and alignment, size, and fixation of the tibial and femoral component. Several studies have shown that tight control of lower leg alignment,8-14 balancing of the soft tissues,15-19 joint line maintenance,20-23 component alignment,24-28 component size,29-34 and component fixation35-40 can improve the outcomes of UKA and TKA. As a result, over the past 2 decades, several computer-assisted surgery systems have been developed with the goal of more accurate and reliable control of these factors, and thus improved outcomes of knee arthroplasty.
These systems differ with regard to the number and type of variables they control. Computer navigation systems aim to control one or more of these surgical variables, and several meta-analyses have shown that these systems, when compared to conventional surgery, improve mechanical axis accuracy, decrease the risk for mechanical axis outliers, and improve component positioning in TKA41-49 and UKA surgery.50,51 Interestingly, however, meta-analyses have failed to show the expected superiority in clinical outcomes following computer navigation compared to conventional knee arthroplasty.48,52-55 Furthermore, authors have shown that, despite the fact that computer-navigated surgery increases the accuracy of mechanical alignment and surgical cutting, there is still room for improvement.56 As a consequence, robotic-assisted systems have been developed.
Similar to computer navigation, these robotic-assisted systems aim to control the surgical variables; in addition, they aim to improve the surgical precision of the procedure. Interestingly, 2 recent studies have shown that robotic-assisted systems are superior to computer navigation systems with regard to less cutting time and less resection deviations in coronal and sagittal plane in a cadaveric study,57 and shorter total surgery time, more accurate mechanical axis, and shorter hospital stay in a clinical study.58 Although these results are promising, the exact role of robotic surgery in knee arthroplasty remains unclear. In this review, we aim to report the current state of robotic-assisted knee arthroplasty by discussing (1) the different robotic-assisted knee arthroplasty systems that are available for UKA and TKA surgery, (2) studies that assessed the role of robotic-assisted knee arthroplasty in controlling the aforementioned surgical variables, (3) cadaveric and clinical comparative studies that compared how accurate robotic-assisted and conventional knee surgery control these surgical variables, and (4) studies that assessed the cost-effectiveness of robotic-assisted knee arthroplasty surgery.
Robotic-Assisted Knee Arthroplasty Systems
Several systems have been developed over the years for knee arthroplasty, and these are usually defined as active, semi-active, or passive.59 Active systems are capable of performing tasks or processes autonomously under the watchful eye of the surgeon, while passive systems do not perform actions independently but provide the surgeon with information. In semi-active systems, the surgical action is physically constrained in order to follow a predefined strategy.
In the United States, 3 robotic systems are FDA-approved for knee arthroplasty. The Stryker/Mako haptic guided robot (Mako Surgical Corp.) was introduced in 2005 and has been used for over 50,000 UKA procedures (Figure 1). There are nearly 300 robotic systems used nationally, as it has 20% of the market share for UKA in the United States. The Mako system is a semi-active tactile robotic system that requires preoperative imaging, after which a preoperative planning is performed. Intraoperatively, the robotic arm is under direct surgeon control and gives real-time tactile feedback during the procedure (Figure 2).
Furthermore, the surgeon can intraoperatively virtually adjust component positioning and alignment and move the knee through the range of motion, after which the system can provide information on alignment, component position, and balance of the soft tissue (eg, if the knee is overtight or lax through the flexion-arch).60 This system has a burr that resects the bone and when the surgeon directs the burr outside the preplanned area, the burr stops and prevents unnecessary and unwanted resections (Figure 3).
The Navio Precision Free-Hand Sculptor (PFS) system (Blue Belt Technologies) has been used for 1500 UKA procedures, with 50 robots in use in the United States (Figure 4). This system is an image-free semi-active robotic system and has the same characteristics as the aforementioned Mako system.61 Finally, the OmniBotic robotic system (Omnilife Science) has been released for TKA and has been used for over 7300 procedures (Figure 5). This system has an automated cutting-guide technique in which the surgeon designs a virtual plan on the computer system. After this, the cutting-guides are placed by the robotic system at the planned location for all 5 femoral cuts (ie, distal, anterior chamfer, anterior, posterior chamfer, and posterior) and the surgeon then makes the final cuts.57,62
Three robotic systems for knee arthroplasty surgery have been used in Europe. The Caspar system (URS Ortho) is an active robotic system in which a computed tomography (CT) scan is performed preoperatively, after which a virtual implantation is performed on the screen. The surgeon can then obtain information on lower leg alignment, gap balancing, and component positioning, and after an operative plan is made, the surgical resections are performed by the robot. Reflective markers are attached to the leg and all robotic movements are monitored using an infrared camera system. Any undesired motion will be detected by this camera system and will stop all movements.63 A second and more frequently reported system in the literature is the active Robodoc surgical system (Curexo Technology Corporation). This system is designed for TKA and total hip arthroplasty (THA) surgery. Although initial studies reported a high incidence of system-related complications in THA,64 the use of this system for TKA has frequently been reported in the literature.56,63,65-69 A third robotic system that has been used in Europe is the Acrobot surgical system (Acrobot Company Ltd), which is an image-based semi-active robotic system70 used for both UKA and TKA surgery.70,71
Accuracy of Controlling Surgical Variables in Robotic-Assisted Knee Arthroplasty
Several studies have assessed the accuracy of robotic-assisted surgery in UKA surgery with regard to control of the aforementioned surgical variables. Pearle and colleagues72 assessed the mechanical axis accuracy of the Mako system in 10 patients undergoing medial UKA robotic-assisted surgery. They reported that the intraoperative registration lasted 7.5 minutes and the duration of time needed for robotic-assisted burring was 34.8 minutes. They compared the actual postoperative alignment at 6 weeks follow-up with the planned lower leg alignment and found that all measurements were within 1.6° of the planned lower leg alignment. Dunbar and colleagues73 assessed the accuracy of component positioning of the Mako system in 20 patients undergoing medial UKA surgery by comparing preoperative and postoperative 3-dimensional CT scans. They found that the femoral component was within 0.8 mm and 0.9° in all directions and that the tibial component was within 0.9 mm and 1.7° in all directions. They concluded that the accuracy of component positioning with the Mako system was excellent. Finally, Plate and colleagues17 assessed the accuracy of soft tissue balancing in the Mako system in 52 patients undergoing medial UKA surgery. They compared the balance plan with the soft tissue balance after implantation and the Mako system quantified soft tissue balance as the amount of mm of the knee being tight or loose at 0°, 30°, 60°, 90°, and 110° of flexion. They found that at all flexion angles the ligament balancing was accurate up to 0.53 mm of the original plan. Furthermore, they noted that in 83% of cases the accuracy was within 1 mm at all flexion angles.
For the Navio system, Smith and colleagues74 assessed the accuracy of component positioning using 20 synthetic femurs and tibia. They reported a maximum rotational error of 3.2°, an angular error of 1.46° in all orientations, and a maximum translational error of 1.18 mm for both the tibial and femoral implants. Lonner and colleagues75 assessed the accuracy of component positioning in 25 cadaveric specimens. They found similar results as were found in the study of Smith and colleagues74 and concluded that these results were similar to other semi-active robotic systems designed for UKA.
For TKA surgery, Ponder and colleagues76 assessed the accuracy of the OmniBotic system and found that the average error in the anterior-posterior dimension between the targeted and measured cuts was -0.14 mm, and that the standard deviation in guide positioning for the distal, anterior chamfer, and posterior chamfer resections was 0.03° and 0.17 mm. Koenig and colleagues62 assessed the accuracy of the OmniBotic system in the first 100 cases and found that 98% of the cases were within 3° of the neutral mechanical axis. Furthermore, they found a learning curve with regard to tourniquet time between the first and second 10 patients in which they performed robotic-assisted TKA surgery. Siebert and colleagues63 assessed the accuracy of mechanical alignment in the Caspar system in 70 patients treated with the robotic system. They found that the difference between preoperatively planned and postoperatively achieved mechanical alignment was 0.8°. Similarly, Bellemans and colleagues77 assessed mechanical alignment and the positioning and rotation of the tibial and femoral components in a clinical study of 25 cases using the Caspar system. They noted that none of the patients had mechanical alignment, tibial or femoral component positioning, or rotation beyond 1° of the neutral axis. Liow and colleagues56 assessed the accuracy of mechanical axis alignment and component sizing accuracy using the Robodoc system in 25 patients. They reported that the mean postoperative alignment was 0.4° valgus and that all cases were within 3° of the neutral mechanical axis. Furthermore, they reported a mean surgical time of 96 minutes and reported that preoperative planning yielded femoral and tibial component size accuracy of 100%.
These studies have shown that robotic systems for UKA and TKA are accurate in the surgical variables they aim to control. These studies validated tight control of mechanical axis alignment, decrease for outliers, and component positioning and rotation, and also found that the balancing of soft tissues was improved using robotic-assisted surgery.
Robotic-Assisted vs Conventional Knee Arthroplasty
Despite the fact that these systems are accurate in the variables they aim to control, these systems have to be compared to the gold standard of conventional knee arthroplasty. For UKA, Cobb and colleagues70 performed a randomized clinical trial for patients treated undergoing UKA with robotic-assistance of the Acrobot systems compared to conventional UKA and assessed differences in mechanical accuracy. A total of 27 patients were randomly assigned to one of both treatments. They found that in the group of robotic-assisted surgery, 100% of the patients had a mechanical axis within 2° of neutral, while this was only 40% in the conventional UKA groups (difference P < .001). They also assessed the increase in functional outcomes and noted a trend towards improvement in performance with increasing accuracy at 6 weeks and 3 months postoperatively. Lonner and colleagues78 also compared the tibial component positioning between robotic-assisted UKA surgery using the Mako system and conventional UKA surgery. The authors found that the variance in tibial slope, in coronal plane of the tibial component, and varus/valgus alignment were all larger with conventional UKA when compared to robotic-assisted UKA. Citak and colleagues79 compared the accuracy of tibial and femoral implant positioning between robotic-assisted surgery using the Mako system and conventional UKA in a cadaveric study. They reported that the root mean square (RMS) error of femoral component was 1.9 mm and 3.7° in robotic-assisted surgery and 5.4 mm and 10.2° for conventional UKA, while the RMS error for tibial component were 1.4 mm and 5.0° for robotic-assisted surgery and 5.7 mm and 19.2° for conventional UKA surgery. MacCallum and colleagues80 compared the tibial base plate position in a prospective clinical study of 177 patients treated with conventional UKA and 87 patients treated with robotic-assisted surgery using the Mako system. They found that surgery with robotic-assistance was more precise in the coronal and sagittal plane and was more accurate in coronal alignment when compared to conventional UKA. Finally, the first results of robotic-assisted UKA surgery have been presented. Coon and colleagues81 reported the preliminary results of a multicenter study of 854 patients and found a survivorship of 98.9% and satisfaction rate of 92% at minimum 2-year follow-up. Comparing these results to other large conventional UKA cohorts82,83 suggests that robotic-assisted surgery may improve survivorship at short-term follow-up. However, comparative studies and studies with longer follow-up are necessary to assess the additional value of robotic-assisted UKA surgery. Due to the relatively new concept of robotic-assisted surgery, these studies have not been performed or published yet.
For TKA, several studies also have compared how these robotic-systems control the surgical variables compared to conventional TKA surgery. Siebert and colleagues63 assessed mechanical axis accuracy and mechanical outliers following robotic-assisted TKA surgery using the Caspar system and conventional TKA surgery. They reported the difference between preoperative planned and postoperative achieved alignment was 0.8° for robotic-assisted surgery and 2.6° for conventional TKA surgery. Furthermore, they showed that 1 patient in the robotic-assisted group (1.4%) and 18 patients in the conventional TKA group (35%) had mechanical alignment greater than 3° from the neutral mechanical axis. Liow and colleagues56 found similar differences in their prospective randomized study in which they reported that 0% outliers greater than 3° from the neutral mechanical axis were found in the robotic-assisted group while 19.4% of the patients in the conventional TKA group had mechanical axis outliers. They also assessed the joint-line outliers in both procedures and found that 3.2% had joint-line outliers greater than 5 mm in the robotic-assisted group compared to 20.6% in the conventional TKA group. Kim and colleagues65 assessed implant accuracy in robotic-assisted surgery using the ROBODOC system and in conventional surgery and reported higher implant accuracy and fewer outliers using robotic-assisted surgery. Moon and colleagues66 compared robotic-assisted TKA surgery using the Robodoc system with conventional TKA surgery in 10 cadavers. They found that robotic-assisted surgery had excellent precision in all planes and had better accuracy in femoral rotation alignment compared to conventional TKA surgery. Park and Lee67 compared Robodoc robotic-assisted TKA surgery with conventional TKA surgery in a randomized clinical trial of 72 patients. They found that robotic-assisted surgery had definitive advantages in preoperative planning, accuracy of the procedure, and postoperative follow-up regarding femoral and tibial component flexion angles. Finally, Song and colleagues68,69 performed 2 randomized clinical trials in which they compared mechanical axis alignment, component positioning, soft tissue balancing, and patient preference between conventional TKA surgery and robotic-assisted surgery using the Robodoc system. In the first study,68 they simultaneously performed robotic-assisted surgery in one leg and conventional TKA surgery in the other leg. They found that robotic-assisted surgery resulted in less outlier in mechanical axis and component positioning. Furthermore, they found at latest follow-up of 2 years that 12 patients preferred the leg treated with robotic-assisted surgery while 6 preferred the conventional leg. Despite this finding, no significant differences in functional outcome scores were detected between both treatment options. Furthermore, they found that flexion-extension balance was achieved in 92% of patients treated with robotic-assisted TKA surgery and in 77% of patients treated with conventional TKA surgery. In the other study,69 the authors found that more patients treated with robotic-assisted surgery had <2 mm flexion-extension gap and more satisfactory posterior cruciate ligament tension when compared to conventional surgery.
These studies have shown that robotic-assisted surgery is accurate in controlling surgical variables, such as mechanical lower leg alignment, maintaining joint-line, implant positioning, and soft tissue balancing. Furthermore, these studies have shown that controlling these variables is better than the current gold standard of manual knee arthroplasty. Until now, not many studies have assessed survivorship of robotic-assisted surgery. Furthermore, no studies have, to our knowledge, compared survivorship of robotic-assisted with conventional knee replacement surgery. Finally, studies comparing functional outcomes following robotic-assisted surgery and conventional knee arthroplasty surgery are frequently underpowered due to their small sample sizes.68,70 Since many studies have shown that the surgical variables are more tightly controlled using robotic-assisted surgery when compared to conventional surgery, large comparative studies are necessary to assess the role of robotic-assisted surgery in functional outcomes and survivorship of UKA and TKA.
Cost-Effectiveness of Robotic-Assisted Surgery
High initial capital costs of robotic-assisted surgery is one of the factors that constitute a barrier to the widespread implementation of this technique. Multiple authors have suggested that improved implant survivorship afforded by robotic-assisted surgery may justify the expenditure from both societal and provider perspective.84-86 Two studies have performed a cost-effectiveness analysis for UKA surgery. Swank and colleagues84 reviewed the hospital expenditures and profits associated with robot-assisted knee arthroplasty, citing upfront costs of approximately $800,000. The authors estimated a mean per-case contribution profit of $5790 for robotic-assisted UKA, assuming an inpatient-to-outpatient ratio of 1 to 3. Based on this data, Swank and colleagues84 proposed that the capital costs of robotic-assisted UKA may be recovered in as little as 2 years when in the first 3 consecutive years 50, 70, and 90 cases were performed using robotic-assisted UKA. Moschetti and colleagues85 recently published the first formal cost-effectiveness analysis of robotic-assisted compared to manual UKA. The authors used an annual revision risk of 0.55% for the first 2 years following robot-assisted UKA, based on the aforementioned presented data by Coon and colleagues.81 They based their data on the Mako system and assumed an initial capital expenditure of $934,728 with annual servicing costs of 10% (discounted annually) for 4 years thereafter, resulting in a total cost of the robotic system of $1.362 million. These costs were divided by the number of patients estimated to undergo robotic-assisted UKA per year, which was varied to estimate the effect of case volume on cost-effectiveness. The authors reported that robotic-assisted UKA was associated with higher lifetime costs and net utilities compared to manual UKA, at an incremental cost-effectiveness ratio of $47,180 per quality-adjusted life year (QALY) in a high-volume center. This falls well within the societal willingness-to-pay threshold of $100,000/QALY. Sensitivity analysis showed that robotic-assisted UKA is cost-effective under the following conditions: (1) centers performing at least 94 cases annually, (2) in patients younger than age 67 years, and (3) 2-year revision rate does not exceed 1.2%. While the results of this initial analysis are promising, follow-up cost-effectiveness analysis studies will be required as long-term survivorship data become available.
Conclusion
Tighter control of intraoperative surgical variables, such as lower leg alignment, soft tissue balance, joint-line maintenance, and component alignment and positioning, have been associated with improved survivorship and functional outcomes. Upon reviewing the available literature on robotic-assisted surgery, it becomes clear that this technique can improve the accuracy of these surgical variables and is superior to conventional manual UKA and TKA. Although larger and comparative survivorship studies are necessary to compare robotic-assisted knee arthroplasty to conventional techniques, the early results and cost-effectiveness analysis seem promising.
1. van der List JP, McDonald LS, Pearle AD. Systematic review of medial versus lateral survivorship in unicompartmental knee arthroplasty. Knee. 2015;22(6):454-460.
2. Mont MA, Pivec R, Issa K, Kapadia BH, Maheshwari A, Harwin SF. Long-term implant survivorship of cementless total knee arthroplasty: a systematic review of the literature and meta-analysis. J Knee Surg. 2014;27(5):369-376.
3. Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2014 Australian Hip and Knee Arthroplasty Register. https://aoanjrr.sahmri.com/documents/10180/172286/Annual%20Report%202014. Accessed April 6, 2016.
4. The Swedish Knee Arthroplasty Register. Annual Report 2015 Swedish Knee Arthroplasty Register. http://www.myknee.se/pdf/SVK_2015_Eng_1.0.pdf. Published December 1, 2015. Accessed April 6, 2016.
5. Centre of excellence of joint replacements. The Norwegian Arthroplasty Register. http://nrlweb.ihelse.net/eng/Report_2010.pdf. Published June 2010. Accessed June 3, 2015.
6. National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 12th Annual Report 2015. http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/12th%20annual%20report/NJR%20Online%20Annual%20Report%202015.pdf. Accessed April 6, 2016.
7. The New Zealand Joint Registry. Fourteen Year Report January 1999 to December 2012. http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed April 6, 2016.
8. Jeffery RS, Morris RW, Denham RA. Coronal alignment after total knee replacement. J Bone Joint Surg Br. 1991;73(5):709-714.
9. Rand JA, Coventry MB. Ten-year evaluation of geometric total knee arthroplasty. Clin Orthop Relat Res. 1988;232:168-173.
10. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
11. Ryd L, Lindstrand A, Stenström A, Selvik G. Porous coated anatomic tricompartmental tibial components. The relationship between prosthetic position and micromotion. Clin Orthop Relat Res. 1990;251:189-197.
12. van der List JP, Chawla H, Villa JC, Zuiderbaan HA, Pearle AD. Early functional outcome after lateral UKA is sensitive to postoperative lower limb alignment. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
13. van der List JP, Zuiderbaan HA, Pearle AD. Why do medial unicompartmental knee arthroplasties fail today? J Arthroplasty. 2015. [Epub ahead of print]
14. Vasso M, Del Regno C, D’Amelio A, Viggiano D, Corona K, Schiavone Panni A. Minor varus alignment provides better results than neutral alignment in medial UKA. Knee. 2015;22(2):117-121.
15. Attfield SF, Wilton TJ, Pratt DJ, Sambatakakis A. Soft-tissue balance and recovery of proprioception after total knee replacement. J Bone Joint Surg Br. 1996;78(4):540-545.
16. Pagnano MW, Hanssen AD, Lewallen DG, Stuart MJ. Flexion instability after primary posterior cruciate retaining total knee arthroplasty. Clin Orthop Relat Res. 1998;356:39-46.
17. Plate JF, Mofidi A, Mannava S, et al. Achieving accurate ligament balancing using robotic-assisted unicompartmental knee arthroplasty. Adv Orthop. 2013;2013:837167.
18. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
19. Wasielewski RC, Galante JO, Leighty RM, Natarajan RN, Rosenberg AG. Wear patterns on retrieved polyethylene tibial inserts and their relationship to technical considerations during total knee arthroplasty. Clin Orthop Relat Res. 1994;299:31-43.
20. Ji HM, Han J, Jin DS, Seo H, Won YY. Kinematically aligned TKA can align knee joint line to horizontal. Knee Surg Sports Traumatol Arthrosc. 2016. [Epub ahead of print]
21. Khamaisy S, Zuiderbaan HA, van der List JP, Nam D, Pearle AD. Medial unicompartmental knee arthroplasty improves congruence and restores joint space width of the lateral compartment. Knee. 2016. [Epub ahead of print]
22. Niinimaki TT, Murray DW, Partanen J, Pajala A, Leppilahti JI. Unicompartmental knee arthroplasties implanted for osteoarthritis with partial loss of joint space have high re-operation rates. Knee. 2011;18(6):432-435.
23. Zuiderbaan HA, Khamaisy S, Thein R, Nawabi DH, Pearle AD. Congruence and joint space width alterations of the medial compartment following lateral unicompartmental knee arthroplasty. Bone Joint J. 2015;97-B(1):50-55.
24. Barbadoro P, Ensini A, Leardini A, et al. Tibial component alignment and risk of loosening in unicompartmental knee arthroplasty: a radiographic and radiostereometric study. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3157-3162.
25. Collier MB, Eickmann TH, Sukezaki F, McAuley JP, Engh GA. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):108-115.
26. Nedopil AJ, Howell SM, Hull ML. Does malrotation of the tibial and femoral components compromise function in kinematically aligned total knee arthroplasty? Orthop Clin North Am. 2016;47(1):41-50.
27. Rosskopf J, Singh PK, Wolf P, Strauch M, Graichen H. Influence of intentional femoral component flexion in navigated TKA on gap balance and sagittal anatomy. Knee Surg Sports Traumatol Arthrosc. 2014;22(3):687-693.
28. Zihlmann MS, Stacoff A, Romero J, Quervain IK, Stüssi E. Biomechanical background and clinical observations of rotational malalignment in TKA: literature review and consequences. Clin Biomech (Bristol, Avon). 2005;20(7):661-668.
29. Bonnin MP, Saffarini M, Shepherd D, Bossard N, Dantony E. Oversizing the tibial component in TKAs: incidence, consequences and risk factors. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
30. Bonnin MP, Schmidt A, Basiglini L, Bossard N, Dantony E. Mediolateral oversizing influences pain, function, and flexion after TKA. Knee Surg Sports Traumatol Arthrosc. 2013;21(10):2314-2324.
31. Chau R, Gulati A, Pandit H, et al. Tibial component overhang following unicompartmental knee replacement--does it matter? Knee. 2009;16(5):310-313.
32. Mueller JK, Wentorf FA, Moore RE. Femoral and tibial insert downsizing increases the laxity envelope in TKA. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3003-3011.
33. Sriphirom P, Raungthong N, Chutchawan P, Thiranon C, Sukandhavesa N. Influence of a secondary downsizing of the femoral component on the extension gap: a cadaveric study. Orthopedics. 2012;35(10 Suppl):56-59.
34. Young SW, Clarke HD, Graves SE, Liu YL, de Steiger RN. Higher rate of revision in PFC sigma primary total knee arthroplasty with mismatch of femoro-tibial component sizes. J Arthroplasty. 2015;30(5):813-817.
35. Barink M, Verdonschot N, de Waal Malefijt M. A different fixation of the femoral component in total knee arthroplasty may lead to preservation of femoral bone stock. Proc Inst Mech Eng H. 2003;217(5):325-332.
36. Eagar P, Hull ML, Howell SM. How the fixation method stiffness and initial tension affect anterior load-displacement of the knee and tension in anterior cruciate ligament grafts: a study in cadaveric knees using a double-loop hamstrings graft. J Orthop Res. 2004;22(3):613-624.
37. Fricka KB, Sritulanondha S, McAsey CJ. To cement or not? Two-year results of a prospective, randomized study comparing cemented vs. cementless total knee arthroplasty (TKA). J Arthroplasty. 2015;30(9 Suppl):55-58.
38. Kendrick BJ, Kaptein BL, Valstar ER, et al. Cemented versus cementless Oxford unicompartmental knee arthroplasty using radiostereometric analysis: a randomised controlled trial. Bone Joint J. 2015;97-B(2):185-191.
39. Kim TK, Chang CB, Kang YG, Chung BJ, Cho HJ, Seong SC. Execution accuracy of bone resection and implant fixation in computer assisted minimally invasive total knee arthroplasty. Knee. 2010;17(1):23-28.
40. Whiteside LA. Making your next unicompartmental knee arthroplasty last: three keys to success. J Arthroplasty. 2005;20(4 Suppl 2):2-3.
41. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
42. Brin YS, Nikolaou VS, Joseph L, Zukor DJ, Antoniou J. Imageless computer assisted versus conventional total knee replacement. A Bayesian meta-analysis of 23 comparative studies. Int Orthop. 2011;35(3):331-339.
43. Cheng T, Zhang G, Zhang X. Imageless navigation system does not improve component rotational alignment in total knee arthroplasty. J Surg Res. 2011;171(2):590-600.
44. Conteduca F, Iorio R, Mazza D, Ferretti A. Patient-specific instruments in total knee arthroplasty. Int Orthop. 2014;38(2):259-265.
45. Fu Y, Wang M, Liu Y, Fu Q. Alignment outcomes in navigated total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(6):1075-1082.
46. Hetaimish BM, Khan MM, Simunovic N, Al-Harbi HH, Bhandari M, Zalzal PK. Meta-analysis of navigation vs conventional total knee arthroplasty. J Arthroplasty. 2012;27(6):1177-1182.
47. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
48. Moskal JT, Capps SG, Mann JW, Scanelli JA. Navigated versus conventional total knee arthroplasty. J Knee Surg. 2014;27(3):235-248.
49. Shi J, Wei Y, Wang S, et al. Computer navigation and total knee arthroplasty. Orthopedics. 2014;37(1):e39-e43.
50. Nair R, Tripathy G, Deysine GR. Computer navigation systems in unicompartmental knee arthroplasty: a systematic review. Am J Orthop. 2014;43(6):256-261.
51. Weber P, Crispin A, Schmidutz F, et al. Improved accuracy in computer-assisted unicondylar knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2453-2461.
52. Alcelik IA, Blomfield MI, Diana G, Gibbon AJ, Carrington N, Burr S. A comparison of short-term outcomes of minimally invasive computer-assisted vs minimally invasive conventional instrumentation for primary total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2016;31(2):410-418.
53. Cheng T, Pan XY, Mao X, Zhang GY, Zhang XL. Little clinical advantage of computer-assisted navigation over conventional instrumentation in primary total knee arthroplasty at early follow-up. Knee. 2012;19(4):237-245.
54. Rebal BA, Babatunde OM, Lee JH, Geller JA, Patrick DA Jr, Macaulay W. Imageless computer navigation in total knee arthroplasty provides superior short term functional outcomes: a meta-analysis. J Arthroplasty. 2014;29(5):938-944.
55. Zamora LA, Humphreys KJ, Watt AM, Forel D, Cameron AL. Systematic review of computer-navigated total knee arthroplasty. ANZ J Surg. 2013;83(1-2):22-30.
56. Liow MH, Xia Z, Wong MK, Tay KJ, Yeo SJ, Chin PL. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis. A prospective randomised study. J Arthroplasty. 2014;29(12):2373-2377.
57. Koulalis D, O’Loughlin PF, Plaskos C, Kendoff D, Cross MB, Pearle AD. Sequential versus automated cutting guides in computer-assisted total knee arthroplasty. Knee. 2011;18(6):436-442.
58. Clark TC, Schmidt FH. Robot-assisted navigation versus computer-assisted navigation in primary total knee arthroplasty: efficiency and accuracy. ISRN Orthop. 2013;2013:794827.
59. DiGioia AM 3rd, Jaramaz B, Colgan BD. Computer assisted orthopaedic surgery. Image guided and robotic assistive technologies. Clin Orthop Relat Res. 1998(354):8-16.
60. Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 1:63-68.
61. Lonner JH. Robotically assisted unicompartmental knee arthroplasty with a handheld image-free sculpting tool. Orthop Clin North Am. 2016;47(1):29-40.
62. Koenig JA, Suero EM, Plaskos C. Surgical accuracy and efficiency of computer-navigated TKA with a robotic cutting guide–report on the first 100 cases. J Bone Joint Surg Br. 2012;94-B(SUPP XLIV):103. Available at: http://www.bjjprocs.boneandjoint.org.uk/content/94-B/SUPP_XLIV/103. Accessed April 6, 2016.
63. Siebert W, Mai S, Kober R, Heeckt PF. Technique and first clinical results of robot-assisted total knee replacement. Knee. 2002;9(3):173-180.
64. Schulz AP, Seide K, Queitsch C, et al. Results of total hip replacement using the Robodoc surgical assistant system: clinical outcome and evaluation of complications for 97 procedures. Int J Med Robot. 2007;3(4):301-306.
65. Kim SM, Park YS, Ha CW, Lim SJ, Moon YW. Robot-assisted implantation improves the precision of component position in minimally invasive TKA. Orthopedics. 2012;35(9):e1334-e1339.
66. Moon YW, Ha CW, Do KH, et al. Comparison of robot-assisted and conventional total knee arthroplasty: a controlled cadaver study using multiparameter quantitative three-dimensional CT assessment of alignment. Comput Aided Surg. 2012;17(2):86-95.
67. Park SE, Lee CT. Comparison of robotic-assisted and conventional manual implantation of a primary total knee arthroplasty. J Arthroplasty. 2007;22(7):1054-1059.
68. Song EK, Seon JK, Park SJ, Jung WB, Park HW, Lee GW. Simultaneous bilateral total knee arthroplasty with robotic and conventional techniques: a prospective, randomized study. Knee Surg Sports Traumatol Arthrosc. 2011;19(7):1069-1076.
69. Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471(1):118-126.
70. Cobb J, Henckel J, Gomes P, et al. Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br. 2006;88(2):188-197.
71. Jakopec M, Harris SJ, Rodriguez y Baena F, Gomes P, Cobb J, Davies BL. The first clinical application of a “hands-on” robotic knee surgery system. Comput Aided Surg. 2001;6(6):329-339.
72. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
73. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
74. Smith JR, Riches PE, Rowe PJ. Accuracy of a freehand sculpting tool for unicondylar knee replacement. Int J Med Robot. 2014;10(2):162-169.
75. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
76. Ponder C, Plaskos C, Cheal E. Press-fit total knee arthroplasty with a robotic-cutting guide: proof of concept and initial clinical experience. Bone & Joint Journal Orthopaedic Proceedings Supplement. 2013;95(SUPP 28):61. Available at: http://www.bjjprocs.boneandjoint.org.uk/content/95-B/SUPP_28/61.abstract. Accessed April 6, 2016.
77. Bellemans J, Vandenneucker H, Vanlauwe J. Robot-assisted total knee arthroplasty. Clin Orthop Relat Res. 2007;464:111-116.
78. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
79. Citak M, Suero EM, Citak M, et al. Unicompartmental knee arthroplasty: is robotic technology more accurate than conventional technique? Knee. 2013;20(4):268-271.
80. MacCallum KP, Danoff JR, Geller JA. Tibial baseplate positioning in robotic-assisted and conventional unicompartmental knee arthroplasty. Eur J Orthop Surg Traumatol. 2016;26(1):93-98.
81. Coon T, Roche M, Pearle AD, Dounchis J, Borus T, Buechel F Jr. Two year survivorship of robotically guided unicompartmental knee arthroplasty. Paper presented at: International Society for Technology in Arthroplasty 26th Annual Congress; October 16-19, 2013; Palm Beach, FL.
82. Pandit H, Jenkins C, Gill HS, Barker K, Dodd CA, Murray DW. Minimally invasive Oxford phase 3 unicompartmental knee replacement: results of 1000 cases. J Bone Joint Surg Br. 2011;93(2):198-204.
83. Yoshida K, Tada M, Yoshida H, Takei S, Fukuoka S, Nakamura H. Oxford phase 3 unicompartmental knee arthroplasty in Japan--clinical results in greater than one thousand cases over ten years. J Arthroplasty. 2013;28(9 Suppl):168-171.
84. Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop. 2009;38(2 Suppl):32-36.
85. Moschetti WE, Konopka JF, Rubash HE, Genuario JW. Can robot-assisted unicompartmental knee arthroplasty be cost-effective? A markovdecision analysis. J Arthroplasty. 2015. [Epub ahead of print]
86. Thienpont E. Improving Accuracy in Knee Arthroplasty. 1st ed. New Delhi, India: Jaypee Brothers Medical Publishers; 2012.
1. van der List JP, McDonald LS, Pearle AD. Systematic review of medial versus lateral survivorship in unicompartmental knee arthroplasty. Knee. 2015;22(6):454-460.
2. Mont MA, Pivec R, Issa K, Kapadia BH, Maheshwari A, Harwin SF. Long-term implant survivorship of cementless total knee arthroplasty: a systematic review of the literature and meta-analysis. J Knee Surg. 2014;27(5):369-376.
3. Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2014 Australian Hip and Knee Arthroplasty Register. https://aoanjrr.sahmri.com/documents/10180/172286/Annual%20Report%202014. Accessed April 6, 2016.
4. The Swedish Knee Arthroplasty Register. Annual Report 2015 Swedish Knee Arthroplasty Register. http://www.myknee.se/pdf/SVK_2015_Eng_1.0.pdf. Published December 1, 2015. Accessed April 6, 2016.
5. Centre of excellence of joint replacements. The Norwegian Arthroplasty Register. http://nrlweb.ihelse.net/eng/Report_2010.pdf. Published June 2010. Accessed June 3, 2015.
6. National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 12th Annual Report 2015. http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/12th%20annual%20report/NJR%20Online%20Annual%20Report%202015.pdf. Accessed April 6, 2016.
7. The New Zealand Joint Registry. Fourteen Year Report January 1999 to December 2012. http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed April 6, 2016.
8. Jeffery RS, Morris RW, Denham RA. Coronal alignment after total knee replacement. J Bone Joint Surg Br. 1991;73(5):709-714.
9. Rand JA, Coventry MB. Ten-year evaluation of geometric total knee arthroplasty. Clin Orthop Relat Res. 1988;232:168-173.
10. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
11. Ryd L, Lindstrand A, Stenström A, Selvik G. Porous coated anatomic tricompartmental tibial components. The relationship between prosthetic position and micromotion. Clin Orthop Relat Res. 1990;251:189-197.
12. van der List JP, Chawla H, Villa JC, Zuiderbaan HA, Pearle AD. Early functional outcome after lateral UKA is sensitive to postoperative lower limb alignment. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
13. van der List JP, Zuiderbaan HA, Pearle AD. Why do medial unicompartmental knee arthroplasties fail today? J Arthroplasty. 2015. [Epub ahead of print]
14. Vasso M, Del Regno C, D’Amelio A, Viggiano D, Corona K, Schiavone Panni A. Minor varus alignment provides better results than neutral alignment in medial UKA. Knee. 2015;22(2):117-121.
15. Attfield SF, Wilton TJ, Pratt DJ, Sambatakakis A. Soft-tissue balance and recovery of proprioception after total knee replacement. J Bone Joint Surg Br. 1996;78(4):540-545.
16. Pagnano MW, Hanssen AD, Lewallen DG, Stuart MJ. Flexion instability after primary posterior cruciate retaining total knee arthroplasty. Clin Orthop Relat Res. 1998;356:39-46.
17. Plate JF, Mofidi A, Mannava S, et al. Achieving accurate ligament balancing using robotic-assisted unicompartmental knee arthroplasty. Adv Orthop. 2013;2013:837167.
18. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
19. Wasielewski RC, Galante JO, Leighty RM, Natarajan RN, Rosenberg AG. Wear patterns on retrieved polyethylene tibial inserts and their relationship to technical considerations during total knee arthroplasty. Clin Orthop Relat Res. 1994;299:31-43.
20. Ji HM, Han J, Jin DS, Seo H, Won YY. Kinematically aligned TKA can align knee joint line to horizontal. Knee Surg Sports Traumatol Arthrosc. 2016. [Epub ahead of print]
21. Khamaisy S, Zuiderbaan HA, van der List JP, Nam D, Pearle AD. Medial unicompartmental knee arthroplasty improves congruence and restores joint space width of the lateral compartment. Knee. 2016. [Epub ahead of print]
22. Niinimaki TT, Murray DW, Partanen J, Pajala A, Leppilahti JI. Unicompartmental knee arthroplasties implanted for osteoarthritis with partial loss of joint space have high re-operation rates. Knee. 2011;18(6):432-435.
23. Zuiderbaan HA, Khamaisy S, Thein R, Nawabi DH, Pearle AD. Congruence and joint space width alterations of the medial compartment following lateral unicompartmental knee arthroplasty. Bone Joint J. 2015;97-B(1):50-55.
24. Barbadoro P, Ensini A, Leardini A, et al. Tibial component alignment and risk of loosening in unicompartmental knee arthroplasty: a radiographic and radiostereometric study. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3157-3162.
25. Collier MB, Eickmann TH, Sukezaki F, McAuley JP, Engh GA. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):108-115.
26. Nedopil AJ, Howell SM, Hull ML. Does malrotation of the tibial and femoral components compromise function in kinematically aligned total knee arthroplasty? Orthop Clin North Am. 2016;47(1):41-50.
27. Rosskopf J, Singh PK, Wolf P, Strauch M, Graichen H. Influence of intentional femoral component flexion in navigated TKA on gap balance and sagittal anatomy. Knee Surg Sports Traumatol Arthrosc. 2014;22(3):687-693.
28. Zihlmann MS, Stacoff A, Romero J, Quervain IK, Stüssi E. Biomechanical background and clinical observations of rotational malalignment in TKA: literature review and consequences. Clin Biomech (Bristol, Avon). 2005;20(7):661-668.
29. Bonnin MP, Saffarini M, Shepherd D, Bossard N, Dantony E. Oversizing the tibial component in TKAs: incidence, consequences and risk factors. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
30. Bonnin MP, Schmidt A, Basiglini L, Bossard N, Dantony E. Mediolateral oversizing influences pain, function, and flexion after TKA. Knee Surg Sports Traumatol Arthrosc. 2013;21(10):2314-2324.
31. Chau R, Gulati A, Pandit H, et al. Tibial component overhang following unicompartmental knee replacement--does it matter? Knee. 2009;16(5):310-313.
32. Mueller JK, Wentorf FA, Moore RE. Femoral and tibial insert downsizing increases the laxity envelope in TKA. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3003-3011.
33. Sriphirom P, Raungthong N, Chutchawan P, Thiranon C, Sukandhavesa N. Influence of a secondary downsizing of the femoral component on the extension gap: a cadaveric study. Orthopedics. 2012;35(10 Suppl):56-59.
34. Young SW, Clarke HD, Graves SE, Liu YL, de Steiger RN. Higher rate of revision in PFC sigma primary total knee arthroplasty with mismatch of femoro-tibial component sizes. J Arthroplasty. 2015;30(5):813-817.
35. Barink M, Verdonschot N, de Waal Malefijt M. A different fixation of the femoral component in total knee arthroplasty may lead to preservation of femoral bone stock. Proc Inst Mech Eng H. 2003;217(5):325-332.
36. Eagar P, Hull ML, Howell SM. How the fixation method stiffness and initial tension affect anterior load-displacement of the knee and tension in anterior cruciate ligament grafts: a study in cadaveric knees using a double-loop hamstrings graft. J Orthop Res. 2004;22(3):613-624.
37. Fricka KB, Sritulanondha S, McAsey CJ. To cement or not? Two-year results of a prospective, randomized study comparing cemented vs. cementless total knee arthroplasty (TKA). J Arthroplasty. 2015;30(9 Suppl):55-58.
38. Kendrick BJ, Kaptein BL, Valstar ER, et al. Cemented versus cementless Oxford unicompartmental knee arthroplasty using radiostereometric analysis: a randomised controlled trial. Bone Joint J. 2015;97-B(2):185-191.
39. Kim TK, Chang CB, Kang YG, Chung BJ, Cho HJ, Seong SC. Execution accuracy of bone resection and implant fixation in computer assisted minimally invasive total knee arthroplasty. Knee. 2010;17(1):23-28.
40. Whiteside LA. Making your next unicompartmental knee arthroplasty last: three keys to success. J Arthroplasty. 2005;20(4 Suppl 2):2-3.
41. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
42. Brin YS, Nikolaou VS, Joseph L, Zukor DJ, Antoniou J. Imageless computer assisted versus conventional total knee replacement. A Bayesian meta-analysis of 23 comparative studies. Int Orthop. 2011;35(3):331-339.
43. Cheng T, Zhang G, Zhang X. Imageless navigation system does not improve component rotational alignment in total knee arthroplasty. J Surg Res. 2011;171(2):590-600.
44. Conteduca F, Iorio R, Mazza D, Ferretti A. Patient-specific instruments in total knee arthroplasty. Int Orthop. 2014;38(2):259-265.
45. Fu Y, Wang M, Liu Y, Fu Q. Alignment outcomes in navigated total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(6):1075-1082.
46. Hetaimish BM, Khan MM, Simunovic N, Al-Harbi HH, Bhandari M, Zalzal PK. Meta-analysis of navigation vs conventional total knee arthroplasty. J Arthroplasty. 2012;27(6):1177-1182.
47. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
48. Moskal JT, Capps SG, Mann JW, Scanelli JA. Navigated versus conventional total knee arthroplasty. J Knee Surg. 2014;27(3):235-248.
49. Shi J, Wei Y, Wang S, et al. Computer navigation and total knee arthroplasty. Orthopedics. 2014;37(1):e39-e43.
50. Nair R, Tripathy G, Deysine GR. Computer navigation systems in unicompartmental knee arthroplasty: a systematic review. Am J Orthop. 2014;43(6):256-261.
51. Weber P, Crispin A, Schmidutz F, et al. Improved accuracy in computer-assisted unicondylar knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2453-2461.
52. Alcelik IA, Blomfield MI, Diana G, Gibbon AJ, Carrington N, Burr S. A comparison of short-term outcomes of minimally invasive computer-assisted vs minimally invasive conventional instrumentation for primary total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2016;31(2):410-418.
53. Cheng T, Pan XY, Mao X, Zhang GY, Zhang XL. Little clinical advantage of computer-assisted navigation over conventional instrumentation in primary total knee arthroplasty at early follow-up. Knee. 2012;19(4):237-245.
54. Rebal BA, Babatunde OM, Lee JH, Geller JA, Patrick DA Jr, Macaulay W. Imageless computer navigation in total knee arthroplasty provides superior short term functional outcomes: a meta-analysis. J Arthroplasty. 2014;29(5):938-944.
55. Zamora LA, Humphreys KJ, Watt AM, Forel D, Cameron AL. Systematic review of computer-navigated total knee arthroplasty. ANZ J Surg. 2013;83(1-2):22-30.
56. Liow MH, Xia Z, Wong MK, Tay KJ, Yeo SJ, Chin PL. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis. A prospective randomised study. J Arthroplasty. 2014;29(12):2373-2377.
57. Koulalis D, O’Loughlin PF, Plaskos C, Kendoff D, Cross MB, Pearle AD. Sequential versus automated cutting guides in computer-assisted total knee arthroplasty. Knee. 2011;18(6):436-442.
58. Clark TC, Schmidt FH. Robot-assisted navigation versus computer-assisted navigation in primary total knee arthroplasty: efficiency and accuracy. ISRN Orthop. 2013;2013:794827.
59. DiGioia AM 3rd, Jaramaz B, Colgan BD. Computer assisted orthopaedic surgery. Image guided and robotic assistive technologies. Clin Orthop Relat Res. 1998(354):8-16.
60. Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 1:63-68.
61. Lonner JH. Robotically assisted unicompartmental knee arthroplasty with a handheld image-free sculpting tool. Orthop Clin North Am. 2016;47(1):29-40.
62. Koenig JA, Suero EM, Plaskos C. Surgical accuracy and efficiency of computer-navigated TKA with a robotic cutting guide–report on the first 100 cases. J Bone Joint Surg Br. 2012;94-B(SUPP XLIV):103. Available at: http://www.bjjprocs.boneandjoint.org.uk/content/94-B/SUPP_XLIV/103. Accessed April 6, 2016.
63. Siebert W, Mai S, Kober R, Heeckt PF. Technique and first clinical results of robot-assisted total knee replacement. Knee. 2002;9(3):173-180.
64. Schulz AP, Seide K, Queitsch C, et al. Results of total hip replacement using the Robodoc surgical assistant system: clinical outcome and evaluation of complications for 97 procedures. Int J Med Robot. 2007;3(4):301-306.
65. Kim SM, Park YS, Ha CW, Lim SJ, Moon YW. Robot-assisted implantation improves the precision of component position in minimally invasive TKA. Orthopedics. 2012;35(9):e1334-e1339.
66. Moon YW, Ha CW, Do KH, et al. Comparison of robot-assisted and conventional total knee arthroplasty: a controlled cadaver study using multiparameter quantitative three-dimensional CT assessment of alignment. Comput Aided Surg. 2012;17(2):86-95.
67. Park SE, Lee CT. Comparison of robotic-assisted and conventional manual implantation of a primary total knee arthroplasty. J Arthroplasty. 2007;22(7):1054-1059.
68. Song EK, Seon JK, Park SJ, Jung WB, Park HW, Lee GW. Simultaneous bilateral total knee arthroplasty with robotic and conventional techniques: a prospective, randomized study. Knee Surg Sports Traumatol Arthrosc. 2011;19(7):1069-1076.
69. Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471(1):118-126.
70. Cobb J, Henckel J, Gomes P, et al. Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br. 2006;88(2):188-197.
71. Jakopec M, Harris SJ, Rodriguez y Baena F, Gomes P, Cobb J, Davies BL. The first clinical application of a “hands-on” robotic knee surgery system. Comput Aided Surg. 2001;6(6):329-339.
72. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
73. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
74. Smith JR, Riches PE, Rowe PJ. Accuracy of a freehand sculpting tool for unicondylar knee replacement. Int J Med Robot. 2014;10(2):162-169.
75. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
76. Ponder C, Plaskos C, Cheal E. Press-fit total knee arthroplasty with a robotic-cutting guide: proof of concept and initial clinical experience. Bone & Joint Journal Orthopaedic Proceedings Supplement. 2013;95(SUPP 28):61. Available at: http://www.bjjprocs.boneandjoint.org.uk/content/95-B/SUPP_28/61.abstract. Accessed April 6, 2016.
77. Bellemans J, Vandenneucker H, Vanlauwe J. Robot-assisted total knee arthroplasty. Clin Orthop Relat Res. 2007;464:111-116.
78. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
79. Citak M, Suero EM, Citak M, et al. Unicompartmental knee arthroplasty: is robotic technology more accurate than conventional technique? Knee. 2013;20(4):268-271.
80. MacCallum KP, Danoff JR, Geller JA. Tibial baseplate positioning in robotic-assisted and conventional unicompartmental knee arthroplasty. Eur J Orthop Surg Traumatol. 2016;26(1):93-98.
81. Coon T, Roche M, Pearle AD, Dounchis J, Borus T, Buechel F Jr. Two year survivorship of robotically guided unicompartmental knee arthroplasty. Paper presented at: International Society for Technology in Arthroplasty 26th Annual Congress; October 16-19, 2013; Palm Beach, FL.
82. Pandit H, Jenkins C, Gill HS, Barker K, Dodd CA, Murray DW. Minimally invasive Oxford phase 3 unicompartmental knee replacement: results of 1000 cases. J Bone Joint Surg Br. 2011;93(2):198-204.
83. Yoshida K, Tada M, Yoshida H, Takei S, Fukuoka S, Nakamura H. Oxford phase 3 unicompartmental knee arthroplasty in Japan--clinical results in greater than one thousand cases over ten years. J Arthroplasty. 2013;28(9 Suppl):168-171.
84. Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop. 2009;38(2 Suppl):32-36.
85. Moschetti WE, Konopka JF, Rubash HE, Genuario JW. Can robot-assisted unicompartmental knee arthroplasty be cost-effective? A markovdecision analysis. J Arthroplasty. 2015. [Epub ahead of print]
86. Thienpont E. Improving Accuracy in Knee Arthroplasty. 1st ed. New Delhi, India: Jaypee Brothers Medical Publishers; 2012.
Rate of Tommy John Surgeries in Young Athletes Is on the Rise
An increasing number of adolescents are undergoing ulnar lateral ligament (UCL) surgery to repair a pitching-related elbow injury, according to a study published online ahead of print in the March issue of the American Journal of Sports Medicine.
Analyzing a database of all ambulatory discharges in New York state, researchers found that 444 patients underwent surgery to repair their UCL between 2002 and 2011. The median age of study participants was 21; most were male.
The total volume of UCL surgeries increased nearly 200% during that time, while the number of UCL reconstructions per 100,000 people tripled from 0.15 to 0.45. Almost all of the growth occurred in two age groups, 17- to 18-year-olds and 19- to 20-year-olds. Patients who had private insurance were 25 times more likely to undergo UCL construction than those with Medicaid.
Suggested Reading
Hodgins JL, Vitale M, Arons RR, et al. Epidemiology of Medial Ulnar Collateral Ligament Reconstruction. Am J Sports Med. 2016 Mar;44(3):729-34. [Epub ahead of print].
An increasing number of adolescents are undergoing ulnar lateral ligament (UCL) surgery to repair a pitching-related elbow injury, according to a study published online ahead of print in the March issue of the American Journal of Sports Medicine.
Analyzing a database of all ambulatory discharges in New York state, researchers found that 444 patients underwent surgery to repair their UCL between 2002 and 2011. The median age of study participants was 21; most were male.
The total volume of UCL surgeries increased nearly 200% during that time, while the number of UCL reconstructions per 100,000 people tripled from 0.15 to 0.45. Almost all of the growth occurred in two age groups, 17- to 18-year-olds and 19- to 20-year-olds. Patients who had private insurance were 25 times more likely to undergo UCL construction than those with Medicaid.
An increasing number of adolescents are undergoing ulnar lateral ligament (UCL) surgery to repair a pitching-related elbow injury, according to a study published online ahead of print in the March issue of the American Journal of Sports Medicine.
Analyzing a database of all ambulatory discharges in New York state, researchers found that 444 patients underwent surgery to repair their UCL between 2002 and 2011. The median age of study participants was 21; most were male.
The total volume of UCL surgeries increased nearly 200% during that time, while the number of UCL reconstructions per 100,000 people tripled from 0.15 to 0.45. Almost all of the growth occurred in two age groups, 17- to 18-year-olds and 19- to 20-year-olds. Patients who had private insurance were 25 times more likely to undergo UCL construction than those with Medicaid.
Suggested Reading
Hodgins JL, Vitale M, Arons RR, et al. Epidemiology of Medial Ulnar Collateral Ligament Reconstruction. Am J Sports Med. 2016 Mar;44(3):729-34. [Epub ahead of print].
Suggested Reading
Hodgins JL, Vitale M, Arons RR, et al. Epidemiology of Medial Ulnar Collateral Ligament Reconstruction. Am J Sports Med. 2016 Mar;44(3):729-34. [Epub ahead of print].
ADHD Medications Are Linked to Diminished Bone Density in Young Patients
Children and adolescents who take medication for attention-deficit hyperactivity disorder (ADHD) show decreased bone density, according to a large cross-sectional study presented at the 2016 Annual Meeting of the American Academy of Orthopaedic Surgeons in Orlando.
Researchers identified 5,315 pediatric patients in the Center for Disease Control and Prevention’s National Health and Nutrition Examination Survey (NHANES) and compared children who were taking an ADHD medication (methylphenidate, desmethylphenidate, dextroamphetamine, atomoxetine, or lisdexamfetamine) with those who were not.
Children taking ADHD medication had lower bone mineral density in the femur, femoral neck, and lumbar spine. Approximately 25% of survey participants who were taking ADHD medication met criteria for osteopenia.
Researchers were able to rule out other potential causes of low bone density in these children, including age, sex, race/ethnicity, and poverty levels. However, the study did not include information on dose, duration of use, or changes in therapy because of the limitations of the NHANES survey data.
Children and adolescents who take medication for attention-deficit hyperactivity disorder (ADHD) show decreased bone density, according to a large cross-sectional study presented at the 2016 Annual Meeting of the American Academy of Orthopaedic Surgeons in Orlando.
Researchers identified 5,315 pediatric patients in the Center for Disease Control and Prevention’s National Health and Nutrition Examination Survey (NHANES) and compared children who were taking an ADHD medication (methylphenidate, desmethylphenidate, dextroamphetamine, atomoxetine, or lisdexamfetamine) with those who were not.
Children taking ADHD medication had lower bone mineral density in the femur, femoral neck, and lumbar spine. Approximately 25% of survey participants who were taking ADHD medication met criteria for osteopenia.
Researchers were able to rule out other potential causes of low bone density in these children, including age, sex, race/ethnicity, and poverty levels. However, the study did not include information on dose, duration of use, or changes in therapy because of the limitations of the NHANES survey data.
Children and adolescents who take medication for attention-deficit hyperactivity disorder (ADHD) show decreased bone density, according to a large cross-sectional study presented at the 2016 Annual Meeting of the American Academy of Orthopaedic Surgeons in Orlando.
Researchers identified 5,315 pediatric patients in the Center for Disease Control and Prevention’s National Health and Nutrition Examination Survey (NHANES) and compared children who were taking an ADHD medication (methylphenidate, desmethylphenidate, dextroamphetamine, atomoxetine, or lisdexamfetamine) with those who were not.
Children taking ADHD medication had lower bone mineral density in the femur, femoral neck, and lumbar spine. Approximately 25% of survey participants who were taking ADHD medication met criteria for osteopenia.
Researchers were able to rule out other potential causes of low bone density in these children, including age, sex, race/ethnicity, and poverty levels. However, the study did not include information on dose, duration of use, or changes in therapy because of the limitations of the NHANES survey data.
Progressive Cardiomyopathy in a Patient With Elevated Cobalt Ion Levels and Bilateral Metal-on-Metal Hip Arthroplasties
Systemic cobalt toxicity has been reported in the literature after hip arthroplasty revisions for failed ceramic components secondary to third-body abrasive wear of cobalt-chrome (CoCr) components, as well as with metal-on-metal (MOM) hip arthroplasty designs. There have been several cases of systemic cobalt toxicity after revision for fractured ceramic components.1,2 Of these 7 reported cases, all patients had neurologic complaints and 4 patients developed cardiomyopathy secondary to toxic cobalt levels, with 1 case being fatal.1 MOM hip prostheses have also been associated with local and systemic problems secondary to metal debris. Adverse local tissue reactions have been reported to occur in up to 59% of patients, and, in some registries, the failure rate of MOM arthroplasty caused by these soft-tissue reactions is 2 to 3 times that of conventional metal-on-polyethylene design failures.3,4 The occurrence of systemic complications from MOM total hip arthroplasty (THA) wear debris is much less common. There have been 6 cases of systemic cobalt toxicity reported in the literature resulting from MOM total hip prosthesis design.1,2
We present a case of biopsy-confirmed cardiomyopathy secondary to cobalt toxicity from a MOM THA design with subsequent requirement for left ventricular assist device (LVAD) implantation despite prosthesis removal. To our knowledge, this is the first report in the literature of this specific implant design causing systemic cobalt toxicity. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a healthy nondiabetic man age 54 years who presented to our clinic 6 years after undergoing left THA and 5 years after undergoing right THA with the Biomet M2a-Magnum MOM prosthesis at an outside facility. The left-side components placed at the index procedure were a size 50 cup, 44 magnum head, 10 Taperloc stem (Biomet), and +9 neck. The right-side components were a size 52 cup, 46 magnum head, 10 Taperloc stem, and +3 neck. The patient emphasized that he was very happy with his hip prostheses and denied groin or thigh pain. His medical history was significant for exogenous obesity, and he denied any history of alcohol, tobacco, steroid, or recreational drug use.
The patient’s review of systems suggested that, approximately 11 months prior to presentation at our facility, he began having difficulty with his activities of daily living secondary to chest pressure with exertion, fatigue, and associated diaphoresis. He complained of decreased sensation in his feet bilaterally but denied any hearing loss, tinnitus, or vision changes. He underwent evaluation of the new-onset chest discomfort with a cardiac stress test that suggested no active cardiac ischemia. An echocardiogram revealed mitral regurgitation, stage II diastolic dysfunction with a left ventricular ejection fraction of 55%. Additionally, during this time period, the patient was being followed by his local orthopedic surgeon for an elevated cobalt level of 120 ppb and a chromium level of 109 ppb. The patient was referred to our clinic for recommendations regarding the elevated metal-ion levels. Upon initial evaluation, the patient denied any hip or groin pain. His physical examination revealed a nonantalgic gait with full range of motion and no signs of instability, tenderness, or masses. The patient was also noted to have no vibratory sensation in his feet bilaterally. The plain radiographs indicated bilateral MOM THA with acetabular inclination levels of 55º on the right and left sides. No cystic changes or other worrisome signs that would suggest implant loosening or failure were present (Figure 1). The serum metal levels were repeated and showed a cobalt level of 189 ppb and a chromium level of 71 ppb. Whole venous blood samples were drawn at our request using trace element tubes and were sent to Medtox Laboratories Inc. for analysis. Other pertinent laboratory values, including hematocrit and thyroid levels, were within normal limits. Because of concerns of systemic toxicity from significantly elevated cobalt and chromium levels, the patient elected to proceed with revision of the MOM components.
During the preoperative medical evaluation, the patient’s cardiac status was a concern, and the etiology of the cardiac dysfunction was unclear. Cardiac magnetic resonance imaging (MRI), which was performed to evaluate the extent and etiology of cardiac dysfunction, showed biventricular dysfunction. To evaluate the underlying myocardial tissue characteristics, delayed contrast imaging was performed and showed diffuse myocardial hyperenhancement of the anterior, lateral, and apical walls, with sparing of the base and midseptum. This type of extensive hyperenhancement is commonly seen with cardiac amyloidosis; however, the blood-pool kinetics during contrast administration is unusual for amyloidosis, as well as the diffuse edema noted on T2-weighted MRI. Importantly, cardiac MRI is very specific in excluding alternative diagnoses, such as postinfarct, infiltrative, acquired, viral, or alcoholic/drugs of abuse etiologies. In the absence of amyloidosis, the only other pattern that would be consistent with symptoms was diffuse, fulminant myocarditis of toxic origin lacking clinical evidence for an infectious origin. The patient’s prior exposure to cobalt was noted. Thus, the hyperenhancement and edema could be strong supportive evidence of cobalt infiltration, despite no reported cases in the literature of cobalt cardiomyopathy found on cardiac MRI.
Additional workup was initiated, and cardiac catheterization showed that the patient continued to decompensate, with worsening global left ventricular dysfunction with an ejection fraction of 30% without evidence of coronary artery disease. Also, he was noted to have mild renal impairment with a blood urea nitrogen level of 31 mg/dL and a creatinine level of 1.7 mg/dL. The etiology of the renal impairment was unknown and had not been established, according to the patient and his wife. The renal impairment was not thought to be caused by the elevated metal ions levels but likely resulted from prerenal azotemia secondary to decreased cardiac output. During catheterization, an endomyocardial biopsy was performed and the tissue sent to the Mayo Clinic pathology department for analysis. The sample showed myocyte hypertrophy and interstitial fibrosis with scattered myofibers containing large cytoplasmic vacuoles. Also present was karyomegaly consistent with myocyte hypertrophy (Figures 2A, 2B). Trichrome stain confirmed replacement of myofibers by collagen (Figure 2C). Electron microscopy performed on a paraffin block showed reduced contractile elements, vacuolar spaces, and increased lipofuscin. The findings were very consistent with, but not specific for, cardiomyopathy from cobalt toxicity. No evidence of an inflammatory infiltrate was identified. The diagnosis was cobalt cardiomyopathy based on biopsy, presentation, cobalt levels, and intraoperative findings.
The patient was admitted to the cardiac intensive care unit preoperatively and optimized with inotropic agents. A multidisciplinary consultation with the cardiology and anesthesia departments was obtained. Both recommended cardiac anesthesia with intraoperative Swan-Ganz catheter and transesophageal echo monitoring. Assuming that the patient remained hemodynamically stable with limited blood loss and the first hip was timely performed, the cardiology department recommended a single surgery, because fewer risks and complications could be expected than from a staged procedure. Subsequently, surgery was performed on the left hip via a conservative anterior approach on the fracture table. The patient remained stable with limited blood loss. During the same operating room time, revision of the right hip was performed using an anterior approach. The intraoperative findings showed evidence of pseudotumors in the adjacent soft tissues and abundant brown, creamy fluid upon entering the joint capsule, consistent with a metallic appearance. Both hips showed similar prosthetic findings. There was no significant visible wear of the large diameter metal heads or gross abnormality of the acetabular components. The trunnion area on both femoral implants was abnormal, revealing a black coating suggestive of marked corrosion. The components were all well fixed, without visible damage, and, because of his fragile cardiac status, the patient’s acetabular components were not revised. The trunnions were cleaned and the femoral heads were revised to active articulation dual-mobility metal-on-polyethylene constructs using 28-mm Biolox Option ceramic (CeramTec). The tissue specimens from the operation showed chronic inflammation with areas of fibroconnective tissue and bland fibrinoid necrosis with extensive brown pigment-laden macrophage reaction. The intraoperative cultures were negative.
The patient tolerated the surgery without complication, and his postoperative period was without incident. Nine months after surgery, the patient’s cobalt and chromium levels had declined to 16 ppb and 32 ppb, respectively (normal, <1 ppb). However, his cardiac status continued to worsen with significant shortness of breath and bilateral lower extremity edema despite diuresis. Follow-up cardiac MRI indicated progressive left and right dysfunction with ejection fractions of 23% and 25%, respectively. After progressive heart-failure symptoms, the patient was admitted to the hospital for severe congestive heart failure and underwent implantation of a HeartWare LVAD with tricuspid valve repair using an Edwards annuloplasty ring. He has since had a cardiac transplant and is doing well.
Discussion
To our knowledge, this is the first reported case of cardiomyopathy in a patient with elevated cobalt ion levels and a Biomet M2a-Magnum hip prosthesis. This is also the first reported case of cardiac MRI–defined cobalt cardiomyopathy. The cobalt levels seen in this patient were similar to those of other cases with systemic cobalt toxicity from a MOM hip construct. Mao and colleagues5 reported 2 cases of systemic cobalt toxicity in 2 patients with articular surface replacement hip prostheses.One patient presented with mild groin pain, neurologic symptoms, and a cobalt level of 410 ppb 5 years after her index procedure. The other patient presented with cardiac and neurologic symptoms but no hip complaints. The patient’s cobalt levels ranged from 185 ppb to 210 ppb. Both patients improved after their revision surgery, and their cobalt levels decreased. The 2 patients in Tower’s report6 were 49-year-old men who had articular surface replacement implants (DePuy). One patient who presented with progressive hip pain 11 months postoperatively developed neurologic symptoms and cardiomyopathy, with cobalt levels of 83 ppb before revision surgery 43 months after his index procedure. The other patient presented with hip pain and vertigo, headaches, fatigue, and dyspnea. He underwent hip revision 40 months postoperatively and required closed reduction under sedation for dislocation. Finally, and most recently, Allen and colleagues2 reported a 59-year-old woman with a cobalt level of 287 ppb whose symptoms did not resolve after implantation of an LVAD or cardiac transplantation but only after removal of her bilateral hip prosthesis. Our case is most similar to this report but significantly adds to the literature in 2 distinct manners: (1) Biomet M2a-Magnum has not been implicated in cobalt toxicity; and (2) this is the first reported use of dedicated cardiac MRI to noninvasively define underlying cardiac pathology.
The cardiac manifestations secondary to systemic cobalt toxicity in this patient represent a frightening consequence of MOM prosthetic wear. The effects of cobalt toxicity on cardiac tissues were first described in a series of alcoholic patients from Manchester in 1900;7 however, it was not until 1967, in a series of patients in Quebec, that cobalt was found to be the inciting factor. In the modern era, hip arthroplasty techniques resulting in excessive cobalt and chromium wear have demonstrated the same findings of myocyte hypertrophy, interstitial fibrosis, and scattered myofibers containing large cytoplasmic inclusions.8,9 The patient presented here has pathologic findings consistent with previous cases of cobalt cardiomyopathy; however, in the other cases of cardiomyopathy due to MOM total hip components, the patients’ cardiac conditions improved after the prostheses were revised and the cobalt levels began to diminish.5,6In our case, the patient has sustained permanent damage to his myocardium and a progressive decline in his cardiac status, which is a deviation from reported cases as of 2014.
While there is no guideline to unequivocally diagnose cobalt cardiomyopathy, the constellation of findings, including pathologic, biologic, blood levels, imaging, and surgical, all uniformly indicate a unifying diagnosis. The lack of improvement after prosthetic device removal supports a diagnosis of permanent myocardial damage, which is consistent with cardiomyopathy of advanced toxic etiology.
Conclusion
This case presents a patient with bilateral MOM THAs, acetabular cup inclinations of greater than 55º, renal impairment, and cobalt levels greater than 60 ppb, with occult cardiac failure leading to LVAD implantation as a prelude to cardiac transplantation in order to avoid certain death. These factors have been shown, in prior case reports, to be associated with cardiac damage that may be reversible.6 However; it is important for orthopedic surgeons to recognize that certain hip prostheses can be associated or lead to irreversible cardiac damage.
1. Zywiel MG, Brandt JM, Overgaard CB, Cheung AC, Turgeon TR, Syed KA. Fatal cardiomyopathy after revision total hip replacement for fracture of a ceramic liner. Bone Joint J. 2013;95(1):31-37.
2. Allen LA, Ambardekar AV, Devaraj KM, Maleszewski JJ, Wolfel EE. Clinical problem-solving. Missing elements of the history. N Engl J Med. 2014;370(6):559-566.
3. Hart AJ, Satchihananda K, Liddle AD, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94(4);317-325.
4. Kwon MK, Jacobs JJ, MacDonald SJ, Potter HG, Fehring TK, Lombardi AV. Evidence-based understanding of management perils for metal-on-metal hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):20-25.
5. Mao X, Wong AA, Crawford RW. Cobalt toxicity- -an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
6. Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847-2851.
7. Morin Y, Daniel P. Quebec beer-drinkers’ cardiomyopathy: etiological considerations. Can Med Assoc J. 1967;97(15):926-928.
8. Gilbert C, Cheung A, Butany J, et al. Hip pain and heart failure: the missing link. Can J Cardiol. 2013;29(5):639.e1-e2.
9. Seghizzi P, D’Adda F, Borleri D, Barbic F, Mosconi G. Cobalt myocardiopathy. A critical review of literature. Sci Total Environ. 1994;150(1-3):105-109.
Systemic cobalt toxicity has been reported in the literature after hip arthroplasty revisions for failed ceramic components secondary to third-body abrasive wear of cobalt-chrome (CoCr) components, as well as with metal-on-metal (MOM) hip arthroplasty designs. There have been several cases of systemic cobalt toxicity after revision for fractured ceramic components.1,2 Of these 7 reported cases, all patients had neurologic complaints and 4 patients developed cardiomyopathy secondary to toxic cobalt levels, with 1 case being fatal.1 MOM hip prostheses have also been associated with local and systemic problems secondary to metal debris. Adverse local tissue reactions have been reported to occur in up to 59% of patients, and, in some registries, the failure rate of MOM arthroplasty caused by these soft-tissue reactions is 2 to 3 times that of conventional metal-on-polyethylene design failures.3,4 The occurrence of systemic complications from MOM total hip arthroplasty (THA) wear debris is much less common. There have been 6 cases of systemic cobalt toxicity reported in the literature resulting from MOM total hip prosthesis design.1,2
We present a case of biopsy-confirmed cardiomyopathy secondary to cobalt toxicity from a MOM THA design with subsequent requirement for left ventricular assist device (LVAD) implantation despite prosthesis removal. To our knowledge, this is the first report in the literature of this specific implant design causing systemic cobalt toxicity. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a healthy nondiabetic man age 54 years who presented to our clinic 6 years after undergoing left THA and 5 years after undergoing right THA with the Biomet M2a-Magnum MOM prosthesis at an outside facility. The left-side components placed at the index procedure were a size 50 cup, 44 magnum head, 10 Taperloc stem (Biomet), and +9 neck. The right-side components were a size 52 cup, 46 magnum head, 10 Taperloc stem, and +3 neck. The patient emphasized that he was very happy with his hip prostheses and denied groin or thigh pain. His medical history was significant for exogenous obesity, and he denied any history of alcohol, tobacco, steroid, or recreational drug use.
The patient’s review of systems suggested that, approximately 11 months prior to presentation at our facility, he began having difficulty with his activities of daily living secondary to chest pressure with exertion, fatigue, and associated diaphoresis. He complained of decreased sensation in his feet bilaterally but denied any hearing loss, tinnitus, or vision changes. He underwent evaluation of the new-onset chest discomfort with a cardiac stress test that suggested no active cardiac ischemia. An echocardiogram revealed mitral regurgitation, stage II diastolic dysfunction with a left ventricular ejection fraction of 55%. Additionally, during this time period, the patient was being followed by his local orthopedic surgeon for an elevated cobalt level of 120 ppb and a chromium level of 109 ppb. The patient was referred to our clinic for recommendations regarding the elevated metal-ion levels. Upon initial evaluation, the patient denied any hip or groin pain. His physical examination revealed a nonantalgic gait with full range of motion and no signs of instability, tenderness, or masses. The patient was also noted to have no vibratory sensation in his feet bilaterally. The plain radiographs indicated bilateral MOM THA with acetabular inclination levels of 55º on the right and left sides. No cystic changes or other worrisome signs that would suggest implant loosening or failure were present (Figure 1). The serum metal levels were repeated and showed a cobalt level of 189 ppb and a chromium level of 71 ppb. Whole venous blood samples were drawn at our request using trace element tubes and were sent to Medtox Laboratories Inc. for analysis. Other pertinent laboratory values, including hematocrit and thyroid levels, were within normal limits. Because of concerns of systemic toxicity from significantly elevated cobalt and chromium levels, the patient elected to proceed with revision of the MOM components.
During the preoperative medical evaluation, the patient’s cardiac status was a concern, and the etiology of the cardiac dysfunction was unclear. Cardiac magnetic resonance imaging (MRI), which was performed to evaluate the extent and etiology of cardiac dysfunction, showed biventricular dysfunction. To evaluate the underlying myocardial tissue characteristics, delayed contrast imaging was performed and showed diffuse myocardial hyperenhancement of the anterior, lateral, and apical walls, with sparing of the base and midseptum. This type of extensive hyperenhancement is commonly seen with cardiac amyloidosis; however, the blood-pool kinetics during contrast administration is unusual for amyloidosis, as well as the diffuse edema noted on T2-weighted MRI. Importantly, cardiac MRI is very specific in excluding alternative diagnoses, such as postinfarct, infiltrative, acquired, viral, or alcoholic/drugs of abuse etiologies. In the absence of amyloidosis, the only other pattern that would be consistent with symptoms was diffuse, fulminant myocarditis of toxic origin lacking clinical evidence for an infectious origin. The patient’s prior exposure to cobalt was noted. Thus, the hyperenhancement and edema could be strong supportive evidence of cobalt infiltration, despite no reported cases in the literature of cobalt cardiomyopathy found on cardiac MRI.
Additional workup was initiated, and cardiac catheterization showed that the patient continued to decompensate, with worsening global left ventricular dysfunction with an ejection fraction of 30% without evidence of coronary artery disease. Also, he was noted to have mild renal impairment with a blood urea nitrogen level of 31 mg/dL and a creatinine level of 1.7 mg/dL. The etiology of the renal impairment was unknown and had not been established, according to the patient and his wife. The renal impairment was not thought to be caused by the elevated metal ions levels but likely resulted from prerenal azotemia secondary to decreased cardiac output. During catheterization, an endomyocardial biopsy was performed and the tissue sent to the Mayo Clinic pathology department for analysis. The sample showed myocyte hypertrophy and interstitial fibrosis with scattered myofibers containing large cytoplasmic vacuoles. Also present was karyomegaly consistent with myocyte hypertrophy (Figures 2A, 2B). Trichrome stain confirmed replacement of myofibers by collagen (Figure 2C). Electron microscopy performed on a paraffin block showed reduced contractile elements, vacuolar spaces, and increased lipofuscin. The findings were very consistent with, but not specific for, cardiomyopathy from cobalt toxicity. No evidence of an inflammatory infiltrate was identified. The diagnosis was cobalt cardiomyopathy based on biopsy, presentation, cobalt levels, and intraoperative findings.
The patient was admitted to the cardiac intensive care unit preoperatively and optimized with inotropic agents. A multidisciplinary consultation with the cardiology and anesthesia departments was obtained. Both recommended cardiac anesthesia with intraoperative Swan-Ganz catheter and transesophageal echo monitoring. Assuming that the patient remained hemodynamically stable with limited blood loss and the first hip was timely performed, the cardiology department recommended a single surgery, because fewer risks and complications could be expected than from a staged procedure. Subsequently, surgery was performed on the left hip via a conservative anterior approach on the fracture table. The patient remained stable with limited blood loss. During the same operating room time, revision of the right hip was performed using an anterior approach. The intraoperative findings showed evidence of pseudotumors in the adjacent soft tissues and abundant brown, creamy fluid upon entering the joint capsule, consistent with a metallic appearance. Both hips showed similar prosthetic findings. There was no significant visible wear of the large diameter metal heads or gross abnormality of the acetabular components. The trunnion area on both femoral implants was abnormal, revealing a black coating suggestive of marked corrosion. The components were all well fixed, without visible damage, and, because of his fragile cardiac status, the patient’s acetabular components were not revised. The trunnions were cleaned and the femoral heads were revised to active articulation dual-mobility metal-on-polyethylene constructs using 28-mm Biolox Option ceramic (CeramTec). The tissue specimens from the operation showed chronic inflammation with areas of fibroconnective tissue and bland fibrinoid necrosis with extensive brown pigment-laden macrophage reaction. The intraoperative cultures were negative.
The patient tolerated the surgery without complication, and his postoperative period was without incident. Nine months after surgery, the patient’s cobalt and chromium levels had declined to 16 ppb and 32 ppb, respectively (normal, <1 ppb). However, his cardiac status continued to worsen with significant shortness of breath and bilateral lower extremity edema despite diuresis. Follow-up cardiac MRI indicated progressive left and right dysfunction with ejection fractions of 23% and 25%, respectively. After progressive heart-failure symptoms, the patient was admitted to the hospital for severe congestive heart failure and underwent implantation of a HeartWare LVAD with tricuspid valve repair using an Edwards annuloplasty ring. He has since had a cardiac transplant and is doing well.
Discussion
To our knowledge, this is the first reported case of cardiomyopathy in a patient with elevated cobalt ion levels and a Biomet M2a-Magnum hip prosthesis. This is also the first reported case of cardiac MRI–defined cobalt cardiomyopathy. The cobalt levels seen in this patient were similar to those of other cases with systemic cobalt toxicity from a MOM hip construct. Mao and colleagues5 reported 2 cases of systemic cobalt toxicity in 2 patients with articular surface replacement hip prostheses.One patient presented with mild groin pain, neurologic symptoms, and a cobalt level of 410 ppb 5 years after her index procedure. The other patient presented with cardiac and neurologic symptoms but no hip complaints. The patient’s cobalt levels ranged from 185 ppb to 210 ppb. Both patients improved after their revision surgery, and their cobalt levels decreased. The 2 patients in Tower’s report6 were 49-year-old men who had articular surface replacement implants (DePuy). One patient who presented with progressive hip pain 11 months postoperatively developed neurologic symptoms and cardiomyopathy, with cobalt levels of 83 ppb before revision surgery 43 months after his index procedure. The other patient presented with hip pain and vertigo, headaches, fatigue, and dyspnea. He underwent hip revision 40 months postoperatively and required closed reduction under sedation for dislocation. Finally, and most recently, Allen and colleagues2 reported a 59-year-old woman with a cobalt level of 287 ppb whose symptoms did not resolve after implantation of an LVAD or cardiac transplantation but only after removal of her bilateral hip prosthesis. Our case is most similar to this report but significantly adds to the literature in 2 distinct manners: (1) Biomet M2a-Magnum has not been implicated in cobalt toxicity; and (2) this is the first reported use of dedicated cardiac MRI to noninvasively define underlying cardiac pathology.
The cardiac manifestations secondary to systemic cobalt toxicity in this patient represent a frightening consequence of MOM prosthetic wear. The effects of cobalt toxicity on cardiac tissues were first described in a series of alcoholic patients from Manchester in 1900;7 however, it was not until 1967, in a series of patients in Quebec, that cobalt was found to be the inciting factor. In the modern era, hip arthroplasty techniques resulting in excessive cobalt and chromium wear have demonstrated the same findings of myocyte hypertrophy, interstitial fibrosis, and scattered myofibers containing large cytoplasmic inclusions.8,9 The patient presented here has pathologic findings consistent with previous cases of cobalt cardiomyopathy; however, in the other cases of cardiomyopathy due to MOM total hip components, the patients’ cardiac conditions improved after the prostheses were revised and the cobalt levels began to diminish.5,6In our case, the patient has sustained permanent damage to his myocardium and a progressive decline in his cardiac status, which is a deviation from reported cases as of 2014.
While there is no guideline to unequivocally diagnose cobalt cardiomyopathy, the constellation of findings, including pathologic, biologic, blood levels, imaging, and surgical, all uniformly indicate a unifying diagnosis. The lack of improvement after prosthetic device removal supports a diagnosis of permanent myocardial damage, which is consistent with cardiomyopathy of advanced toxic etiology.
Conclusion
This case presents a patient with bilateral MOM THAs, acetabular cup inclinations of greater than 55º, renal impairment, and cobalt levels greater than 60 ppb, with occult cardiac failure leading to LVAD implantation as a prelude to cardiac transplantation in order to avoid certain death. These factors have been shown, in prior case reports, to be associated with cardiac damage that may be reversible.6 However; it is important for orthopedic surgeons to recognize that certain hip prostheses can be associated or lead to irreversible cardiac damage.
Systemic cobalt toxicity has been reported in the literature after hip arthroplasty revisions for failed ceramic components secondary to third-body abrasive wear of cobalt-chrome (CoCr) components, as well as with metal-on-metal (MOM) hip arthroplasty designs. There have been several cases of systemic cobalt toxicity after revision for fractured ceramic components.1,2 Of these 7 reported cases, all patients had neurologic complaints and 4 patients developed cardiomyopathy secondary to toxic cobalt levels, with 1 case being fatal.1 MOM hip prostheses have also been associated with local and systemic problems secondary to metal debris. Adverse local tissue reactions have been reported to occur in up to 59% of patients, and, in some registries, the failure rate of MOM arthroplasty caused by these soft-tissue reactions is 2 to 3 times that of conventional metal-on-polyethylene design failures.3,4 The occurrence of systemic complications from MOM total hip arthroplasty (THA) wear debris is much less common. There have been 6 cases of systemic cobalt toxicity reported in the literature resulting from MOM total hip prosthesis design.1,2
We present a case of biopsy-confirmed cardiomyopathy secondary to cobalt toxicity from a MOM THA design with subsequent requirement for left ventricular assist device (LVAD) implantation despite prosthesis removal. To our knowledge, this is the first report in the literature of this specific implant design causing systemic cobalt toxicity. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a healthy nondiabetic man age 54 years who presented to our clinic 6 years after undergoing left THA and 5 years after undergoing right THA with the Biomet M2a-Magnum MOM prosthesis at an outside facility. The left-side components placed at the index procedure were a size 50 cup, 44 magnum head, 10 Taperloc stem (Biomet), and +9 neck. The right-side components were a size 52 cup, 46 magnum head, 10 Taperloc stem, and +3 neck. The patient emphasized that he was very happy with his hip prostheses and denied groin or thigh pain. His medical history was significant for exogenous obesity, and he denied any history of alcohol, tobacco, steroid, or recreational drug use.
The patient’s review of systems suggested that, approximately 11 months prior to presentation at our facility, he began having difficulty with his activities of daily living secondary to chest pressure with exertion, fatigue, and associated diaphoresis. He complained of decreased sensation in his feet bilaterally but denied any hearing loss, tinnitus, or vision changes. He underwent evaluation of the new-onset chest discomfort with a cardiac stress test that suggested no active cardiac ischemia. An echocardiogram revealed mitral regurgitation, stage II diastolic dysfunction with a left ventricular ejection fraction of 55%. Additionally, during this time period, the patient was being followed by his local orthopedic surgeon for an elevated cobalt level of 120 ppb and a chromium level of 109 ppb. The patient was referred to our clinic for recommendations regarding the elevated metal-ion levels. Upon initial evaluation, the patient denied any hip or groin pain. His physical examination revealed a nonantalgic gait with full range of motion and no signs of instability, tenderness, or masses. The patient was also noted to have no vibratory sensation in his feet bilaterally. The plain radiographs indicated bilateral MOM THA with acetabular inclination levels of 55º on the right and left sides. No cystic changes or other worrisome signs that would suggest implant loosening or failure were present (Figure 1). The serum metal levels were repeated and showed a cobalt level of 189 ppb and a chromium level of 71 ppb. Whole venous blood samples were drawn at our request using trace element tubes and were sent to Medtox Laboratories Inc. for analysis. Other pertinent laboratory values, including hematocrit and thyroid levels, were within normal limits. Because of concerns of systemic toxicity from significantly elevated cobalt and chromium levels, the patient elected to proceed with revision of the MOM components.
During the preoperative medical evaluation, the patient’s cardiac status was a concern, and the etiology of the cardiac dysfunction was unclear. Cardiac magnetic resonance imaging (MRI), which was performed to evaluate the extent and etiology of cardiac dysfunction, showed biventricular dysfunction. To evaluate the underlying myocardial tissue characteristics, delayed contrast imaging was performed and showed diffuse myocardial hyperenhancement of the anterior, lateral, and apical walls, with sparing of the base and midseptum. This type of extensive hyperenhancement is commonly seen with cardiac amyloidosis; however, the blood-pool kinetics during contrast administration is unusual for amyloidosis, as well as the diffuse edema noted on T2-weighted MRI. Importantly, cardiac MRI is very specific in excluding alternative diagnoses, such as postinfarct, infiltrative, acquired, viral, or alcoholic/drugs of abuse etiologies. In the absence of amyloidosis, the only other pattern that would be consistent with symptoms was diffuse, fulminant myocarditis of toxic origin lacking clinical evidence for an infectious origin. The patient’s prior exposure to cobalt was noted. Thus, the hyperenhancement and edema could be strong supportive evidence of cobalt infiltration, despite no reported cases in the literature of cobalt cardiomyopathy found on cardiac MRI.
Additional workup was initiated, and cardiac catheterization showed that the patient continued to decompensate, with worsening global left ventricular dysfunction with an ejection fraction of 30% without evidence of coronary artery disease. Also, he was noted to have mild renal impairment with a blood urea nitrogen level of 31 mg/dL and a creatinine level of 1.7 mg/dL. The etiology of the renal impairment was unknown and had not been established, according to the patient and his wife. The renal impairment was not thought to be caused by the elevated metal ions levels but likely resulted from prerenal azotemia secondary to decreased cardiac output. During catheterization, an endomyocardial biopsy was performed and the tissue sent to the Mayo Clinic pathology department for analysis. The sample showed myocyte hypertrophy and interstitial fibrosis with scattered myofibers containing large cytoplasmic vacuoles. Also present was karyomegaly consistent with myocyte hypertrophy (Figures 2A, 2B). Trichrome stain confirmed replacement of myofibers by collagen (Figure 2C). Electron microscopy performed on a paraffin block showed reduced contractile elements, vacuolar spaces, and increased lipofuscin. The findings were very consistent with, but not specific for, cardiomyopathy from cobalt toxicity. No evidence of an inflammatory infiltrate was identified. The diagnosis was cobalt cardiomyopathy based on biopsy, presentation, cobalt levels, and intraoperative findings.
The patient was admitted to the cardiac intensive care unit preoperatively and optimized with inotropic agents. A multidisciplinary consultation with the cardiology and anesthesia departments was obtained. Both recommended cardiac anesthesia with intraoperative Swan-Ganz catheter and transesophageal echo monitoring. Assuming that the patient remained hemodynamically stable with limited blood loss and the first hip was timely performed, the cardiology department recommended a single surgery, because fewer risks and complications could be expected than from a staged procedure. Subsequently, surgery was performed on the left hip via a conservative anterior approach on the fracture table. The patient remained stable with limited blood loss. During the same operating room time, revision of the right hip was performed using an anterior approach. The intraoperative findings showed evidence of pseudotumors in the adjacent soft tissues and abundant brown, creamy fluid upon entering the joint capsule, consistent with a metallic appearance. Both hips showed similar prosthetic findings. There was no significant visible wear of the large diameter metal heads or gross abnormality of the acetabular components. The trunnion area on both femoral implants was abnormal, revealing a black coating suggestive of marked corrosion. The components were all well fixed, without visible damage, and, because of his fragile cardiac status, the patient’s acetabular components were not revised. The trunnions were cleaned and the femoral heads were revised to active articulation dual-mobility metal-on-polyethylene constructs using 28-mm Biolox Option ceramic (CeramTec). The tissue specimens from the operation showed chronic inflammation with areas of fibroconnective tissue and bland fibrinoid necrosis with extensive brown pigment-laden macrophage reaction. The intraoperative cultures were negative.
The patient tolerated the surgery without complication, and his postoperative period was without incident. Nine months after surgery, the patient’s cobalt and chromium levels had declined to 16 ppb and 32 ppb, respectively (normal, <1 ppb). However, his cardiac status continued to worsen with significant shortness of breath and bilateral lower extremity edema despite diuresis. Follow-up cardiac MRI indicated progressive left and right dysfunction with ejection fractions of 23% and 25%, respectively. After progressive heart-failure symptoms, the patient was admitted to the hospital for severe congestive heart failure and underwent implantation of a HeartWare LVAD with tricuspid valve repair using an Edwards annuloplasty ring. He has since had a cardiac transplant and is doing well.
Discussion
To our knowledge, this is the first reported case of cardiomyopathy in a patient with elevated cobalt ion levels and a Biomet M2a-Magnum hip prosthesis. This is also the first reported case of cardiac MRI–defined cobalt cardiomyopathy. The cobalt levels seen in this patient were similar to those of other cases with systemic cobalt toxicity from a MOM hip construct. Mao and colleagues5 reported 2 cases of systemic cobalt toxicity in 2 patients with articular surface replacement hip prostheses.One patient presented with mild groin pain, neurologic symptoms, and a cobalt level of 410 ppb 5 years after her index procedure. The other patient presented with cardiac and neurologic symptoms but no hip complaints. The patient’s cobalt levels ranged from 185 ppb to 210 ppb. Both patients improved after their revision surgery, and their cobalt levels decreased. The 2 patients in Tower’s report6 were 49-year-old men who had articular surface replacement implants (DePuy). One patient who presented with progressive hip pain 11 months postoperatively developed neurologic symptoms and cardiomyopathy, with cobalt levels of 83 ppb before revision surgery 43 months after his index procedure. The other patient presented with hip pain and vertigo, headaches, fatigue, and dyspnea. He underwent hip revision 40 months postoperatively and required closed reduction under sedation for dislocation. Finally, and most recently, Allen and colleagues2 reported a 59-year-old woman with a cobalt level of 287 ppb whose symptoms did not resolve after implantation of an LVAD or cardiac transplantation but only after removal of her bilateral hip prosthesis. Our case is most similar to this report but significantly adds to the literature in 2 distinct manners: (1) Biomet M2a-Magnum has not been implicated in cobalt toxicity; and (2) this is the first reported use of dedicated cardiac MRI to noninvasively define underlying cardiac pathology.
The cardiac manifestations secondary to systemic cobalt toxicity in this patient represent a frightening consequence of MOM prosthetic wear. The effects of cobalt toxicity on cardiac tissues were first described in a series of alcoholic patients from Manchester in 1900;7 however, it was not until 1967, in a series of patients in Quebec, that cobalt was found to be the inciting factor. In the modern era, hip arthroplasty techniques resulting in excessive cobalt and chromium wear have demonstrated the same findings of myocyte hypertrophy, interstitial fibrosis, and scattered myofibers containing large cytoplasmic inclusions.8,9 The patient presented here has pathologic findings consistent with previous cases of cobalt cardiomyopathy; however, in the other cases of cardiomyopathy due to MOM total hip components, the patients’ cardiac conditions improved after the prostheses were revised and the cobalt levels began to diminish.5,6In our case, the patient has sustained permanent damage to his myocardium and a progressive decline in his cardiac status, which is a deviation from reported cases as of 2014.
While there is no guideline to unequivocally diagnose cobalt cardiomyopathy, the constellation of findings, including pathologic, biologic, blood levels, imaging, and surgical, all uniformly indicate a unifying diagnosis. The lack of improvement after prosthetic device removal supports a diagnosis of permanent myocardial damage, which is consistent with cardiomyopathy of advanced toxic etiology.
Conclusion
This case presents a patient with bilateral MOM THAs, acetabular cup inclinations of greater than 55º, renal impairment, and cobalt levels greater than 60 ppb, with occult cardiac failure leading to LVAD implantation as a prelude to cardiac transplantation in order to avoid certain death. These factors have been shown, in prior case reports, to be associated with cardiac damage that may be reversible.6 However; it is important for orthopedic surgeons to recognize that certain hip prostheses can be associated or lead to irreversible cardiac damage.
1. Zywiel MG, Brandt JM, Overgaard CB, Cheung AC, Turgeon TR, Syed KA. Fatal cardiomyopathy after revision total hip replacement for fracture of a ceramic liner. Bone Joint J. 2013;95(1):31-37.
2. Allen LA, Ambardekar AV, Devaraj KM, Maleszewski JJ, Wolfel EE. Clinical problem-solving. Missing elements of the history. N Engl J Med. 2014;370(6):559-566.
3. Hart AJ, Satchihananda K, Liddle AD, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94(4);317-325.
4. Kwon MK, Jacobs JJ, MacDonald SJ, Potter HG, Fehring TK, Lombardi AV. Evidence-based understanding of management perils for metal-on-metal hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):20-25.
5. Mao X, Wong AA, Crawford RW. Cobalt toxicity- -an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
6. Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847-2851.
7. Morin Y, Daniel P. Quebec beer-drinkers’ cardiomyopathy: etiological considerations. Can Med Assoc J. 1967;97(15):926-928.
8. Gilbert C, Cheung A, Butany J, et al. Hip pain and heart failure: the missing link. Can J Cardiol. 2013;29(5):639.e1-e2.
9. Seghizzi P, D’Adda F, Borleri D, Barbic F, Mosconi G. Cobalt myocardiopathy. A critical review of literature. Sci Total Environ. 1994;150(1-3):105-109.
1. Zywiel MG, Brandt JM, Overgaard CB, Cheung AC, Turgeon TR, Syed KA. Fatal cardiomyopathy after revision total hip replacement for fracture of a ceramic liner. Bone Joint J. 2013;95(1):31-37.
2. Allen LA, Ambardekar AV, Devaraj KM, Maleszewski JJ, Wolfel EE. Clinical problem-solving. Missing elements of the history. N Engl J Med. 2014;370(6):559-566.
3. Hart AJ, Satchihananda K, Liddle AD, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94(4);317-325.
4. Kwon MK, Jacobs JJ, MacDonald SJ, Potter HG, Fehring TK, Lombardi AV. Evidence-based understanding of management perils for metal-on-metal hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):20-25.
5. Mao X, Wong AA, Crawford RW. Cobalt toxicity- -an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
6. Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847-2851.
7. Morin Y, Daniel P. Quebec beer-drinkers’ cardiomyopathy: etiological considerations. Can Med Assoc J. 1967;97(15):926-928.
8. Gilbert C, Cheung A, Butany J, et al. Hip pain and heart failure: the missing link. Can J Cardiol. 2013;29(5):639.e1-e2.
9. Seghizzi P, D’Adda F, Borleri D, Barbic F, Mosconi G. Cobalt myocardiopathy. A critical review of literature. Sci Total Environ. 1994;150(1-3):105-109.
For Men, Exercise-related Bone Loading During Adolescence Reaps Benefits Later in Life
Men who continuously participate in high-impact activities, such as jogging and tennis, during adolescence and young adulthood have greater hip and lumbar spine bone mineral density than those who do not take part in such activities, according to a study published in American Journal of Men’s Health.
In a cross-sectional study, researchers analyzed the physical histories of 203 healthy, physically active males ages 30 to 65. Participants’ sports and exercise histories varied in the type and level of activity and the length of time spent doing various physical activities.
Exercise-associated bone loading scores were calculated based on the biomechanical ground-reaction forces of the patients’ past and current physical activities. Current bone mineral density (BMD) was measured using dual-energy x-ray absorptiometry. In addition, participants were grouped based on current participation in a high-impact activity, resistance training, both, or neither.
Bone loading during adolescence and young adulthood were significant, positive predictors of BMD of the whole body, total hip, and lumbar spine, adjusting for lean body mass and/or age Individuals who currently participate in a high-impact activity had greater lumbar spine BMD than nonparticipants. Men who continuously participated in a high-impact activity had greater hip and lumbar spine BMD than those who did not.
Suggested Reading
Matthew A. Strope, Peggy Nigh, Melissa I. Carter, et al. Physical activity–associated bone loading during adolescence and young adulthood is positively associated with adult bone mineral density in men. Am J Mens Health. 2015 November. [Epub ahead of print].
Men who continuously participate in high-impact activities, such as jogging and tennis, during adolescence and young adulthood have greater hip and lumbar spine bone mineral density than those who do not take part in such activities, according to a study published in American Journal of Men’s Health.
In a cross-sectional study, researchers analyzed the physical histories of 203 healthy, physically active males ages 30 to 65. Participants’ sports and exercise histories varied in the type and level of activity and the length of time spent doing various physical activities.
Exercise-associated bone loading scores were calculated based on the biomechanical ground-reaction forces of the patients’ past and current physical activities. Current bone mineral density (BMD) was measured using dual-energy x-ray absorptiometry. In addition, participants were grouped based on current participation in a high-impact activity, resistance training, both, or neither.
Bone loading during adolescence and young adulthood were significant, positive predictors of BMD of the whole body, total hip, and lumbar spine, adjusting for lean body mass and/or age Individuals who currently participate in a high-impact activity had greater lumbar spine BMD than nonparticipants. Men who continuously participated in a high-impact activity had greater hip and lumbar spine BMD than those who did not.
Men who continuously participate in high-impact activities, such as jogging and tennis, during adolescence and young adulthood have greater hip and lumbar spine bone mineral density than those who do not take part in such activities, according to a study published in American Journal of Men’s Health.
In a cross-sectional study, researchers analyzed the physical histories of 203 healthy, physically active males ages 30 to 65. Participants’ sports and exercise histories varied in the type and level of activity and the length of time spent doing various physical activities.
Exercise-associated bone loading scores were calculated based on the biomechanical ground-reaction forces of the patients’ past and current physical activities. Current bone mineral density (BMD) was measured using dual-energy x-ray absorptiometry. In addition, participants were grouped based on current participation in a high-impact activity, resistance training, both, or neither.
Bone loading during adolescence and young adulthood were significant, positive predictors of BMD of the whole body, total hip, and lumbar spine, adjusting for lean body mass and/or age Individuals who currently participate in a high-impact activity had greater lumbar spine BMD than nonparticipants. Men who continuously participated in a high-impact activity had greater hip and lumbar spine BMD than those who did not.
Suggested Reading
Matthew A. Strope, Peggy Nigh, Melissa I. Carter, et al. Physical activity–associated bone loading during adolescence and young adulthood is positively associated with adult bone mineral density in men. Am J Mens Health. 2015 November. [Epub ahead of print].
Suggested Reading
Matthew A. Strope, Peggy Nigh, Melissa I. Carter, et al. Physical activity–associated bone loading during adolescence and young adulthood is positively associated with adult bone mineral density in men. Am J Mens Health. 2015 November. [Epub ahead of print].
Optimizing Outcomes of Total Joint Arthroplasty Under the Comprehensive Care for Joint Replacement
On July 9, 2015, the Centers for Medicare and Medicaid Services announced the Comprehensive Care for Joint Replacement model, which aims to improve coordination of the whole episode of care for total hip and knee replacement.1 At stake is the fact that hip and knee replacements are the most common inpatient procedures among Medicare beneficiaries, costing over $7 billion in 20141 and projected to grow to $50 billion by 2030.2 Under Medicare’s new initiative, hospitals and physicians are held accountable for the quality and cost of care delivered from the time of surgery through 90 days after discharge. For the first time in the history of our profession, large-scale reimbursement is based on outcomes and value rather than fee-for-service. As a result, a hospital can either earn a reward or be held liable for added expenses related to events such as prolonged hospitalization, readmissions, and complications.
How can we optimize outcomes for total joint arthroplasty (TJA) patients in this era of Medicare (r)evolution? A good outcome starts with good patient selection. Numerous studies have been published on patient-related risk factors for postoperative TJA complications including obesity, congestive heart failure, lung disease, and depression.3,4 The risks and benefits of TJA should be carefully weighed in high-risk patients and surgery delayed until appropriate medical optimization has been achieved. Following the famous saying, “Good surgeons know how to operate, better surgeons know when to operate, and the best surgeons know when not to operate,” one cannot overemphasize the need for an objective assessment of the likelihood of patient outcome weighed against patient risk factors.
Moderating patient expectation is another crucial component given the changing demographics of our country. Patients seeking TJA today are younger, more obese, and better educated; live longer; and have higher expectations.5 Unrealistic expectations can have a profound impact on surgical outcomes, leading to frustration, dissatisfaction, and unnecessary resource utilization. For example, despite alleviating pain and restoring function in a severely degenerative joint, TJA does not necessarily translate to weight loss. There is currently conflicting evidence on this topic,6-8 and the expectation of weight loss after TJA cannot be supported. There is also a paucity of data regarding return to athletic activity after TJA and the effect of athletic activity on TJA survivorship.9 Communication and transparency are needed to moderate unrealistic expectations before surgery, outlining clear and achievable goals.
Clinical pathways for TJA have seen tremendous improvements in the past decade with the advent of multimodal analgesia, rapid recovery programs, use of spinal and regional anesthesia, and evidence-based guidelines for prevention of venous thromboembolic disease. Adequate pain control is critical to recovery. In a prospective, randomized controlled trial, Lamplot and colleagues10 showed that the use of multimodal analgesia correlated with improved pain scores, decreased narcotic usage, faster functional recovery, and higher patient satisfaction after total knee arthroplasty (TKA). In another study, Quack and colleagues11 performed a systematic review of the literature on fast-track rehabilitation and found that it reduced both inpatient length of stay and costs after TKA. With respect to anesthetic choice, Pugely and colleagues12 reviewed a national database of 14,052 cases of primary TKA and found that patients with multiple comorbidities were at higher risk of complications after general anesthesia when compared with spinal anesthesia. We should continue to invest in safer and more effective modalities for pain control and functional recovery.
Last but not least, in today’s era of Medicare’s Comprehensive Care for Joint Replacement, the role of low-volume orthopedic surgeons performing TJA deserves special mention. Over the next few years, we could likely see a decline in the role of low-volume surgeons in favor of high-volume surgeons. While most orthopedic surgeons are comfortable doing primary TJA, failed cases and complications are frequently referred to larger centers, which may create frustration among patients owing to fragmentation of care. The economic pressures related to bundled payments could further influence this transition. Given the lack of a widespread, long-standing national joint registry, the incidence of failed TJA performed by low-volume orthopedic surgeons compared with high-volume orthopedic surgeons is unknown. However, multiple studies have shown surgeon volume to be associated with lower rates of complication, mortality, readmission, reoperation, and discharge to postacute facilities.13-16 As hospitals assume further financial risk, considerable data on physician performance will undoubtedly be gathered and leveraged. Time and data will determine the value of this transition of care.
Today, more than ever, we are challenged to provide efficient, high-quality, patient-centered care. As our nation grapples with reforming a broken health care system, initiatives like the Comprehensive Care for Joint Replacement will continue to emerge in the future. Orthopedic surgeons are the gatekeepers of the system and therefore hold significant responsibility to patients and society. Ensuring good outcomes should be a top priority not just from a financial standpoint, but as a moral obligation. We shall continue to be leaders in the face of challenges, using innovation and integrity to produce the best results and advance our profession.
1. Comprehensive Care for Joint Replacement model. Centers for Medicare and Medicaid Services website. https://innovation.cms.gov/initiatives/cjr. Updated December 21, 2015. Accessed December 30, 2015.
2. Wilson NA, Schneller ES, Montgomery K, Bozic KJ. Hip and knee implants: current trends and policy considerations. Health Aff. 2008;27(6):1587-1598.
3. Bozic KJ, Lau E, Ong K, et al. Risk factors for early revision after primary total hip arthroplasty in Medicare patients. Clin Orthop Relat Res. 2014;472(2):449-454.
4. Bozic KJ, Lau E, Ong K, et al. Risk factors for early revision after primary TKA in Medicare patients. Clin Orthop Relat Res. 2014;472(1):232-237.
5. Mason JB. The new demands by patients in the modern era of total joint arthroplasty: a point of view. Clin Orthop Relat Res. 2008;466(1):146-152.
6. Riddle DL, Singh JA, Harmsen WS, Schleck CD, Lewallen DG. Clinically important body weight gain following knee arthroplasty: a five-year comparative cohort study. Arthritis Care Res. 2013;65(5):669-677.
7. Zeni JA Jr, Snyder-Mackler L. Most patients gain weight in the 2 years after total knee arthroplasty: comparison to a healthy control group. Osteoarthritis Cartilage. 2010;18(4):510-514.
8. Ast MP, Abdel MP, Lee YY, Lyman S, Ruel AV, Westrich GH. Weight changes after total hip or knee arthroplasty: prevalence, predictors, and effects on outcomes. J Bone Joint Surg Am. 2015;97(11):911-919.
9. Healy WL, Sharma S, Schwartz B, Iorio R. Athletic activity after total joint arthroplasty. J Bone Joint Surg Am. 2008;90(10):2245-2252.
10. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334.
11. Quack V, Ippendorf AV, Betsch M, et al. Multidisciplinary rehabilitation and fast-track rehabilitation after knee replacement: faster, better, cheaper? A survey and systematic review of literature [in German]. Rehabilitation (Stuttg). 2015;54(4):245-251.
12. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
13. Katz JN, Losina E, Barrett J, et al. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2001;83(11):1622-1629.
14. Manley M, Ong K, Lau E, Kurtz SM. Effect of volume on total hip arthroplasty revision rates in the United States Medicare population. J Bone Joint Surg Am. 2008;90(11):2446-2451.
15. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
16. Lau RL, Perruccio AV, Gandhi R, Mahomed NN. The role of surgeon volume on patient outcome in total knee arthroplasty: a systematic review of the literature. BMC Musculoskelet Disord. 2012;13:250.
On July 9, 2015, the Centers for Medicare and Medicaid Services announced the Comprehensive Care for Joint Replacement model, which aims to improve coordination of the whole episode of care for total hip and knee replacement.1 At stake is the fact that hip and knee replacements are the most common inpatient procedures among Medicare beneficiaries, costing over $7 billion in 20141 and projected to grow to $50 billion by 2030.2 Under Medicare’s new initiative, hospitals and physicians are held accountable for the quality and cost of care delivered from the time of surgery through 90 days after discharge. For the first time in the history of our profession, large-scale reimbursement is based on outcomes and value rather than fee-for-service. As a result, a hospital can either earn a reward or be held liable for added expenses related to events such as prolonged hospitalization, readmissions, and complications.
How can we optimize outcomes for total joint arthroplasty (TJA) patients in this era of Medicare (r)evolution? A good outcome starts with good patient selection. Numerous studies have been published on patient-related risk factors for postoperative TJA complications including obesity, congestive heart failure, lung disease, and depression.3,4 The risks and benefits of TJA should be carefully weighed in high-risk patients and surgery delayed until appropriate medical optimization has been achieved. Following the famous saying, “Good surgeons know how to operate, better surgeons know when to operate, and the best surgeons know when not to operate,” one cannot overemphasize the need for an objective assessment of the likelihood of patient outcome weighed against patient risk factors.
Moderating patient expectation is another crucial component given the changing demographics of our country. Patients seeking TJA today are younger, more obese, and better educated; live longer; and have higher expectations.5 Unrealistic expectations can have a profound impact on surgical outcomes, leading to frustration, dissatisfaction, and unnecessary resource utilization. For example, despite alleviating pain and restoring function in a severely degenerative joint, TJA does not necessarily translate to weight loss. There is currently conflicting evidence on this topic,6-8 and the expectation of weight loss after TJA cannot be supported. There is also a paucity of data regarding return to athletic activity after TJA and the effect of athletic activity on TJA survivorship.9 Communication and transparency are needed to moderate unrealistic expectations before surgery, outlining clear and achievable goals.
Clinical pathways for TJA have seen tremendous improvements in the past decade with the advent of multimodal analgesia, rapid recovery programs, use of spinal and regional anesthesia, and evidence-based guidelines for prevention of venous thromboembolic disease. Adequate pain control is critical to recovery. In a prospective, randomized controlled trial, Lamplot and colleagues10 showed that the use of multimodal analgesia correlated with improved pain scores, decreased narcotic usage, faster functional recovery, and higher patient satisfaction after total knee arthroplasty (TKA). In another study, Quack and colleagues11 performed a systematic review of the literature on fast-track rehabilitation and found that it reduced both inpatient length of stay and costs after TKA. With respect to anesthetic choice, Pugely and colleagues12 reviewed a national database of 14,052 cases of primary TKA and found that patients with multiple comorbidities were at higher risk of complications after general anesthesia when compared with spinal anesthesia. We should continue to invest in safer and more effective modalities for pain control and functional recovery.
Last but not least, in today’s era of Medicare’s Comprehensive Care for Joint Replacement, the role of low-volume orthopedic surgeons performing TJA deserves special mention. Over the next few years, we could likely see a decline in the role of low-volume surgeons in favor of high-volume surgeons. While most orthopedic surgeons are comfortable doing primary TJA, failed cases and complications are frequently referred to larger centers, which may create frustration among patients owing to fragmentation of care. The economic pressures related to bundled payments could further influence this transition. Given the lack of a widespread, long-standing national joint registry, the incidence of failed TJA performed by low-volume orthopedic surgeons compared with high-volume orthopedic surgeons is unknown. However, multiple studies have shown surgeon volume to be associated with lower rates of complication, mortality, readmission, reoperation, and discharge to postacute facilities.13-16 As hospitals assume further financial risk, considerable data on physician performance will undoubtedly be gathered and leveraged. Time and data will determine the value of this transition of care.
Today, more than ever, we are challenged to provide efficient, high-quality, patient-centered care. As our nation grapples with reforming a broken health care system, initiatives like the Comprehensive Care for Joint Replacement will continue to emerge in the future. Orthopedic surgeons are the gatekeepers of the system and therefore hold significant responsibility to patients and society. Ensuring good outcomes should be a top priority not just from a financial standpoint, but as a moral obligation. We shall continue to be leaders in the face of challenges, using innovation and integrity to produce the best results and advance our profession.
On July 9, 2015, the Centers for Medicare and Medicaid Services announced the Comprehensive Care for Joint Replacement model, which aims to improve coordination of the whole episode of care for total hip and knee replacement.1 At stake is the fact that hip and knee replacements are the most common inpatient procedures among Medicare beneficiaries, costing over $7 billion in 20141 and projected to grow to $50 billion by 2030.2 Under Medicare’s new initiative, hospitals and physicians are held accountable for the quality and cost of care delivered from the time of surgery through 90 days after discharge. For the first time in the history of our profession, large-scale reimbursement is based on outcomes and value rather than fee-for-service. As a result, a hospital can either earn a reward or be held liable for added expenses related to events such as prolonged hospitalization, readmissions, and complications.
How can we optimize outcomes for total joint arthroplasty (TJA) patients in this era of Medicare (r)evolution? A good outcome starts with good patient selection. Numerous studies have been published on patient-related risk factors for postoperative TJA complications including obesity, congestive heart failure, lung disease, and depression.3,4 The risks and benefits of TJA should be carefully weighed in high-risk patients and surgery delayed until appropriate medical optimization has been achieved. Following the famous saying, “Good surgeons know how to operate, better surgeons know when to operate, and the best surgeons know when not to operate,” one cannot overemphasize the need for an objective assessment of the likelihood of patient outcome weighed against patient risk factors.
Moderating patient expectation is another crucial component given the changing demographics of our country. Patients seeking TJA today are younger, more obese, and better educated; live longer; and have higher expectations.5 Unrealistic expectations can have a profound impact on surgical outcomes, leading to frustration, dissatisfaction, and unnecessary resource utilization. For example, despite alleviating pain and restoring function in a severely degenerative joint, TJA does not necessarily translate to weight loss. There is currently conflicting evidence on this topic,6-8 and the expectation of weight loss after TJA cannot be supported. There is also a paucity of data regarding return to athletic activity after TJA and the effect of athletic activity on TJA survivorship.9 Communication and transparency are needed to moderate unrealistic expectations before surgery, outlining clear and achievable goals.
Clinical pathways for TJA have seen tremendous improvements in the past decade with the advent of multimodal analgesia, rapid recovery programs, use of spinal and regional anesthesia, and evidence-based guidelines for prevention of venous thromboembolic disease. Adequate pain control is critical to recovery. In a prospective, randomized controlled trial, Lamplot and colleagues10 showed that the use of multimodal analgesia correlated with improved pain scores, decreased narcotic usage, faster functional recovery, and higher patient satisfaction after total knee arthroplasty (TKA). In another study, Quack and colleagues11 performed a systematic review of the literature on fast-track rehabilitation and found that it reduced both inpatient length of stay and costs after TKA. With respect to anesthetic choice, Pugely and colleagues12 reviewed a national database of 14,052 cases of primary TKA and found that patients with multiple comorbidities were at higher risk of complications after general anesthesia when compared with spinal anesthesia. We should continue to invest in safer and more effective modalities for pain control and functional recovery.
Last but not least, in today’s era of Medicare’s Comprehensive Care for Joint Replacement, the role of low-volume orthopedic surgeons performing TJA deserves special mention. Over the next few years, we could likely see a decline in the role of low-volume surgeons in favor of high-volume surgeons. While most orthopedic surgeons are comfortable doing primary TJA, failed cases and complications are frequently referred to larger centers, which may create frustration among patients owing to fragmentation of care. The economic pressures related to bundled payments could further influence this transition. Given the lack of a widespread, long-standing national joint registry, the incidence of failed TJA performed by low-volume orthopedic surgeons compared with high-volume orthopedic surgeons is unknown. However, multiple studies have shown surgeon volume to be associated with lower rates of complication, mortality, readmission, reoperation, and discharge to postacute facilities.13-16 As hospitals assume further financial risk, considerable data on physician performance will undoubtedly be gathered and leveraged. Time and data will determine the value of this transition of care.
Today, more than ever, we are challenged to provide efficient, high-quality, patient-centered care. As our nation grapples with reforming a broken health care system, initiatives like the Comprehensive Care for Joint Replacement will continue to emerge in the future. Orthopedic surgeons are the gatekeepers of the system and therefore hold significant responsibility to patients and society. Ensuring good outcomes should be a top priority not just from a financial standpoint, but as a moral obligation. We shall continue to be leaders in the face of challenges, using innovation and integrity to produce the best results and advance our profession.
1. Comprehensive Care for Joint Replacement model. Centers for Medicare and Medicaid Services website. https://innovation.cms.gov/initiatives/cjr. Updated December 21, 2015. Accessed December 30, 2015.
2. Wilson NA, Schneller ES, Montgomery K, Bozic KJ. Hip and knee implants: current trends and policy considerations. Health Aff. 2008;27(6):1587-1598.
3. Bozic KJ, Lau E, Ong K, et al. Risk factors for early revision after primary total hip arthroplasty in Medicare patients. Clin Orthop Relat Res. 2014;472(2):449-454.
4. Bozic KJ, Lau E, Ong K, et al. Risk factors for early revision after primary TKA in Medicare patients. Clin Orthop Relat Res. 2014;472(1):232-237.
5. Mason JB. The new demands by patients in the modern era of total joint arthroplasty: a point of view. Clin Orthop Relat Res. 2008;466(1):146-152.
6. Riddle DL, Singh JA, Harmsen WS, Schleck CD, Lewallen DG. Clinically important body weight gain following knee arthroplasty: a five-year comparative cohort study. Arthritis Care Res. 2013;65(5):669-677.
7. Zeni JA Jr, Snyder-Mackler L. Most patients gain weight in the 2 years after total knee arthroplasty: comparison to a healthy control group. Osteoarthritis Cartilage. 2010;18(4):510-514.
8. Ast MP, Abdel MP, Lee YY, Lyman S, Ruel AV, Westrich GH. Weight changes after total hip or knee arthroplasty: prevalence, predictors, and effects on outcomes. J Bone Joint Surg Am. 2015;97(11):911-919.
9. Healy WL, Sharma S, Schwartz B, Iorio R. Athletic activity after total joint arthroplasty. J Bone Joint Surg Am. 2008;90(10):2245-2252.
10. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334.
11. Quack V, Ippendorf AV, Betsch M, et al. Multidisciplinary rehabilitation and fast-track rehabilitation after knee replacement: faster, better, cheaper? A survey and systematic review of literature [in German]. Rehabilitation (Stuttg). 2015;54(4):245-251.
12. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
13. Katz JN, Losina E, Barrett J, et al. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2001;83(11):1622-1629.
14. Manley M, Ong K, Lau E, Kurtz SM. Effect of volume on total hip arthroplasty revision rates in the United States Medicare population. J Bone Joint Surg Am. 2008;90(11):2446-2451.
15. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
16. Lau RL, Perruccio AV, Gandhi R, Mahomed NN. The role of surgeon volume on patient outcome in total knee arthroplasty: a systematic review of the literature. BMC Musculoskelet Disord. 2012;13:250.
1. Comprehensive Care for Joint Replacement model. Centers for Medicare and Medicaid Services website. https://innovation.cms.gov/initiatives/cjr. Updated December 21, 2015. Accessed December 30, 2015.
2. Wilson NA, Schneller ES, Montgomery K, Bozic KJ. Hip and knee implants: current trends and policy considerations. Health Aff. 2008;27(6):1587-1598.
3. Bozic KJ, Lau E, Ong K, et al. Risk factors for early revision after primary total hip arthroplasty in Medicare patients. Clin Orthop Relat Res. 2014;472(2):449-454.
4. Bozic KJ, Lau E, Ong K, et al. Risk factors for early revision after primary TKA in Medicare patients. Clin Orthop Relat Res. 2014;472(1):232-237.
5. Mason JB. The new demands by patients in the modern era of total joint arthroplasty: a point of view. Clin Orthop Relat Res. 2008;466(1):146-152.
6. Riddle DL, Singh JA, Harmsen WS, Schleck CD, Lewallen DG. Clinically important body weight gain following knee arthroplasty: a five-year comparative cohort study. Arthritis Care Res. 2013;65(5):669-677.
7. Zeni JA Jr, Snyder-Mackler L. Most patients gain weight in the 2 years after total knee arthroplasty: comparison to a healthy control group. Osteoarthritis Cartilage. 2010;18(4):510-514.
8. Ast MP, Abdel MP, Lee YY, Lyman S, Ruel AV, Westrich GH. Weight changes after total hip or knee arthroplasty: prevalence, predictors, and effects on outcomes. J Bone Joint Surg Am. 2015;97(11):911-919.
9. Healy WL, Sharma S, Schwartz B, Iorio R. Athletic activity after total joint arthroplasty. J Bone Joint Surg Am. 2008;90(10):2245-2252.
10. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334.
11. Quack V, Ippendorf AV, Betsch M, et al. Multidisciplinary rehabilitation and fast-track rehabilitation after knee replacement: faster, better, cheaper? A survey and systematic review of literature [in German]. Rehabilitation (Stuttg). 2015;54(4):245-251.
12. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
13. Katz JN, Losina E, Barrett J, et al. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2001;83(11):1622-1629.
14. Manley M, Ong K, Lau E, Kurtz SM. Effect of volume on total hip arthroplasty revision rates in the United States Medicare population. J Bone Joint Surg Am. 2008;90(11):2446-2451.
15. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
16. Lau RL, Perruccio AV, Gandhi R, Mahomed NN. The role of surgeon volume on patient outcome in total knee arthroplasty: a systematic review of the literature. BMC Musculoskelet Disord. 2012;13:250.
Injury Trends in Major League Baseball Over 18 Seasons: 1998-2015
While the exact origins of the game of baseball are commonly debated, one thing is certain: statistics have been an integral part of the game since its existence.1-3 This is true at nearly every level of baseball, especially in Major League Baseball (MLB). As our knowledge and technical capabilities advance, new statistical measures of baseball performance are added at a rapid pace.1,3 One example is the Pitch f/x video tracking system (Sportvision, Inc.), which now analyzes over 60 variables on each of the estimated 660,000 pitches thrown in the MLB annually. In addition to measuring performance and production, these advancements are being leveraged to better understand the epidemiology and impact of injuries in MLB players.4,5 As with any sport, performance at the most elite level is highly dependent upon player health and functional capacity. Accordingly, player injuries can have a profound impact not only on individual performance but also on the success of the team as a whole.
The first epidemiologic study of injuries in professional baseball was published by Conte and colleagues4 in 2001. This work utilized publically available disabled list (DL) data to perform a comprehensive review of injury patterns in MLB from 1989 to 1999. They demonstrated that injuries were on the rise and that pitchers were more commonly injured (48.4% of all DL reports) and had greater time out of play compared to players of other positions.4 Shoulder and elbow injuries were responsible for 49.8% of all DL assignments, distantly followed by knee (7.3%), wrist/hand (6.1%), and back (5.0%).4 In a later study, Posner and colleagues5 analyzed DL data spanning the 2002 to 2008 seasons. Similarly, they found that injuries continued to increase, and over half (51.2%) of DL assignments occurred secondary to upper extremity injuries.5 Although the DL is primarily designed as a roster management tool rather than an injury database, it has provided valuable epidemiologic injury information through the years. Out of concern for player health and well-being, MLB and the MLB Players Association (MLBPA) worked together to create and implement an electronic medical record and Health and Injury Tracking System (HITS) for all MLB and Minor League Baseball (MiLB) players. Now active for over 5 seasons, this database has provided valuable, detailed reports regarding specific injuries occurring in professional baseball, such as hamstring strains and concussions.6,7
With shoulder and elbow injuries in pitchers representing the greatest proportion of DL assignments in recent years, a large body of literature on these injuries, particularly medial ulnar collateral ligament (MUCL) injuries, has been published.8-13 Since the initial description of MUCL reconstruction, or “Tommy John surgery,” by Dr. Frank Jobe in 1986, much has been done to improve the technique and rehabilitation to maximize player performance following surgery.10,14-16 Despite this increased attention, large-scale epidemiologic reporting of MUCL injuries in MLB is lacking, but such a report is desirable. The purpose of this work is to: 1) provide a large-scale analysis of injuries occurring in MLB baseball over the course of 18 seasons (1998-2015); 2) highlight the financial implications of these injuries; and 3) detail the evolution of MUCL injuries and reconstructive surgery since it was first performed on a MLB pitcher in 1974. Our study represents the largest longitudinal analysis of MLB injuries since the league expanded to its current level of 30 teams in 1998. It is our hope that this work will serve as a framework for future study of the most common and highest impact injuries occurring in baseball.
Materials And Methods
We performed a retrospective review of the MLB DL from 1998 to 2015. Data analyzed included player demographics such as club, year of placement, age, and position. Injury-specific variables included date of placement on DL, length of time on DL, date of reinstatement, body part injured, diagnosis, and cost of replacement. If a player was put on the DL multiple times during a season, each placement was viewed as a different injury, even if it was to the same body part. If a player was put on the DL for injuries to multiple body parts, the primary injury was analyzed.
Disabled List Data
Although the DL has existed since 1916, this current study covers 18 seasons from 1998 to 2015. The 1998 season was chosen as a starting point because this is the year when MLB expanded to 30 teams. Since then, the number of teams and the active roster limits (25 players) have remained constant, allowing for reliable comparisons across seasons. Initially designed as a roster management tool to allow injured players to temporarily be replaced with healthy players, the DL was not created as an injury database. However, the rules and regulations of the DL have remained fairly constant over the last 18 years, allowing reasonable comparisons of injury data and trends across this timespan. In order for a player to be assigned to the DL, the nature and extent of injury must be certified by a physician. Once designated for the DL, a player cannot return to the major league team for a minimum of 15 days. If the injury is severe, the player can remain on the DL for the remainder of the season or until he is deemed healthy enough to return to play by a physician. One notable exception is the treatment of concussions. Since 2011, a player diagnosed with a concussion may be placed on the DL for a minimum of 7 days rather than 15. The introduction of the HITS database in 2010 should allow for more detailed and reliable study of injuries in baseball moving forward. Although it contains robust data for every injury that has occurred in MLB and MiLB over the last 5 seasons, it does not allow for epidemiologic and longitudinal study of injury patterns and trends in baseball prior to 2010.
Cost of Placing Players on the DL
The dollars lost were calculated by prorating the injured player’s daily salary and multiplying by the number of days missed on the DL. For example, if a player’s annual salary is $1,820,000, his daily salary for the 182 day season is $10,000. If assigned to the DL for 15 days, $150,000 is paid to that player while he is inactive and unable to play. An additional cost is the salary of the replacement player who fills the roster spot. For this work, the replacement player’s prorated, daily salary was assumed to be the league minimum for that specific year. For example, if the league minimum for a given season is $182,000, and the season is 182 days long, a replacement player earns a minimum of $1,000 per day while he is on the 25-man active roster. Thus, the dollars paid to the replacement would be $15,000. In this scenario, that brings the team’s total cost to $165,000 ($150,000 plus $15,000). Because the league minimum salary changes year to year, salaries specific to the year of injury were utilized in this analysis.
MUCL Injury Analysis
In order to better understand the evaluation of MUCL injuries over time, all MLB players undergoing MUCL reconstruction (“Tommy John surgery”) were analyzed separately. Similar to prior studies of UCL injuries, these players were identified using DL data, team websites, and publically available internet databases (primarily www.heatmaps.com).9,12,17-19 Variables studied include the number of procedures, year of surgery, player position, and mean time until return to play at the MLB level. All MLB players undergoing MUCL reconstruction since 1974 (the year the first procedure was performed) were included.
Statistical Methods
Epidemiologic data are reported using descriptive statistics (mean, range, and percentage) where indicated. To determine the significance of trends over time, a best-fit line was generated to illustrate the change over the years. These lines are reported with corresponding R2 values. To assess the trend for significance, the slope was compared to a line with a slope of zero (no change over time) using t tests. For all statistical comparisons, the threshold for alpha was set to P < .05.
Results
Between 1998 and 2015, there were 8357 placements of players on the DL, at an average rate of 464 designations per year (Table 1, Figure 1). This resulted in 460,432 days lost to injury, with a mean of 25,186 days out of play per season (Table 1, Figure 2). The mean length of DL assignment per year was 55.1 days per injury, with a low of 49.1 days in 2011 and a high of 59.2 days in 2001 (Table 1, Figure 3). During the study period, the number of players placed on the DL and the total number of DL days steadily increased (P < .001 and P = .003, respectively), while the average length of DL assignments remained steady (P = .647). When analyzing the data by body region injured, the shoulder (20.6%) and elbow (19.6%) were the 2 leading causes of time out of play (Table 2). This was followed distantly by the chest/back/spine (13.7%), wrist/hand/fingers (10.1%), lower leg/knee (9.8%), and the upper leg/thigh (9.5%). Although the percentage of injuries occurring to the upper extremity remained stable, the rate of shoulder injuries steadily decreased (P = .023) as elbow injuries increased (P = .015) (Table 3, Figure 4). This inverse relationship was also demonstrated for the annual number of DL days for shoulder (P = .033) and elbow (P = 0.005) injuries (Figure 5).
Regarding the financial impact of these injuries, the mean annual cost of replacing players on the DL was $423,267,633.78 (Table 4). This ranged from a low of $136,397,147 in 1998 to a high of $694,835,359 in 2015. There was a steady increase in the cost of replacement during the study period (P < .001) that coincides with the increasing salaries during that time span (Figure 6). In total, $6,732,167,180 was paid to players assigned to the DL and $886,650,228 was spent to fill their positions. This brings the total cost of DL assignments to $7,618,817,407 for the study period.
Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed on MLB players since the procedure was first developed in 1974. The vast majority of these were performed in pitchers (n = 361, 90.3%) followed by outfielders (n = 16, 4.0%), infielders (n = 14, 3.5%) and catchers (n = 9, 2.3%) (Table 5). The mean time to return to competition at the MLB level was 17.8 months for pitchers, 11.1 months for outfielders, 9.6 months for infielders, and 10.5 months for catchers. The overall mean time to return was 17.1 months. The annual number of MUCL reconstructions continues to rise dramatically (P < .001) (Figure 7). During the first 12 years (1974-1985), a total of 8 (2.0%) MUCL reconstructions were performed on MLB players. In subsequent decades, this number increased to 44 (11.0%) from 1986-1995, 123 (30.8%) from 1996-2005, and 225 (56.3%) from 2006-2015. Of all Tommy John surgeries performed over 42 years, nearly one-third (n = 131, 32.75%) were performed in the last 5 years alone (2011-2015).
Discussion
To date, a number of studies have been published on injuries in professional baseball. These can primarily be categorized as either studies with a detailed focus on a single injury type or body region6-13,17,19 or broader reviews that are limited by the relatively short time span covered.4,5 The purpose of this work was to provide a comprehensive review of injury trends in MLB since the league expanded to 30 teams in 1998 while paying special attention to the financial impact of those injuries. Additionally, we sought to provide an up-to-date review of MUCL injuries and surgeries since the procedure was first developed in 1974. Ultimately, this data demonstrates that injuries continue to rise in MLB and this increase is accompanied by increased expense for teams. Thankfully, the rates of DL assignments for shoulder injuries are on the decline; however, this decrease is countered by a reciprocal increase in elbow injuries. Similarly, the rates of MUCL reconstruction have also risen dramatically in recent years.
The fact that injury rates are on the rise is confirmed by other published reports. This trend was demonstrated in prior analyses of DL data from the 1989 to 19984 and 2002 to 2008 seasons.5 These 2 studies represent the only comprehensive reviews of MLB injury trends to date, and each provides valuable information. Both are consistent with the current study findings that pitchers are the most commonly injured players and that shoulder and elbow injuries represent about half of all injuries.4,5 Similar injury rates and characteristics have been reported at the collegiate20 and minor league levels.21 Despite this consistency, this analysis of injuries from 1998 to 2015 is the first to report that DL designations for shoulder injuries are on the decline while designations for elbow injuries continue to rise. Although the exact etiology of this decline in shoulder injuries remains unknown, there are a number of possible explanations. In recent years, increased emphasis has been placed on shoulder rehabilitation, reduction of glenohumeral internal rotation deficits, scapular stabilization, and overall kinetic chain balance and coordination. However, this does not explain why elbow injuries continue to rise annually.
With this increase in injuries, the cost of maintaining an active 25-man roster is also climbing. As expected, this growing expense is primarily due to the increased number of DL days each year as well as the increase in league salaries. Fortunately, this increased financial strain has been met with steadily increased annual revenues in professional baseball. In 2014, the prorated salary cost to players designated to the DL and their replacements was $579,568,059. This figure represents an estimated 6.4% of the $9 billion in total revenue for MLB that same year.22 Although this may represent a small percentage of the whole, it still embodies an exceptionally large financial responsibility. This does not include the medical expenses incurred to treat and rehabilitate the players’ injuries.
Every injury that occurs in MLB players has the potential to adversely affect players, teams, and MLB as a whole. With its increasing prevalence, need for surgical treatment, and prolonged return to play, injuries to the MUCL of the elbow may represent the most costly of all injuries. Although a multitude of reports on MUCL injuries, treatments, techniques, rehabilitation, and outcomes have been reported,8,9,12,14-19,23-25 to our knowledge, a comprehensive and longitudinal incidence study in MLB players has not yet been published. By including every MUCL reconstruction that has been performed on a MLB player, our study demonstrates the dramatic increase in the annual incidence of MUCL surgeries. Studies performed over shorter time intervals corroborate these findings. A recent review of a privately insured patient database revealed an annual increase in MUCL reconstructions of 4.2% in that cohort.26 When looking specifically at the MLB, a recent survey of all 30 clubs found that 25% (96 of 382) of MLB pitchers and 15% (341 of 2324) of minor league pitchers have undergone MUCL reconstruction.8 Because it occurs so frequently and requires a mean of 17 months to return to sport, MUCL injuries represent a very significant cause of time out of play.
While this study represents a unique epidemiologic report on injuries in baseball, it is certainly not without its limitations. As stated previously, it relies on DL data that was initially intended to serve as a roster management tool rather than an injury database. Accordingly, detailed and specific information about every injury is not always available. The limitations of DL data will largely be overcome in future studies thanks to the implementation of the HITS database in 2010. Moving forward, this system will allow for more detailed analysis of injury patterns, characteristics, time out of play, treatments rendered, etc. Its main limitation is that the earliest data dates back to 2010, making it less applicable for longitudinal studies like the present one. Another limitation of this study is the estimations used for the cost of replacing players designated to the DL. For each injury, it was assumed that the replacement player was paid a prorated portion of the league minimum salary while on the major league roster, but in some instances, that may not have been the case. It is possible that some players filling roster spots were already under contract for amounts higher than the league minimum. Since that player would be making that amount regardless of the level of play, the team may not have paid them any additional salary while filling the position of the injured player. The strengths of this study are its comprehensive nature and inclusion of 18 years of data, making it the longest such study of injuries in MLB. It also represents the first report of cost of replacement for players designated to the DL. To our knowledge, this study also represents the first comprehensive report of every MUCL surgery that has been performed on MLB players.
Conclusion
Injury rates continue to rise in MLB, and upper extremity injuries continue to represent approximately half of all injuries resulting in time out of play. Although shoulder injuries have been on the decline in recent years, this decline is offset by a steady increase in elbow injuries. Each year, MLB players are designated to the DL an average of 464 times for a total of 25,579.6 days. This results in a mean annual cost of over $400 million dollars to replace players lost to injury. Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed in the MLB since 1974, and nearly one-third of these were performed in the last 5 years. Pitchers represent 90.3% of players requiring MUCL surgery, and the average time to return to sport for all players is 17 months. These data may serve as a foundation for identifying appropriate targets for continued study into the etiologies, strategies for prevention, and optimal treatments of injuries commonly affecting professional baseball players.
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. Vol 1. New York, NY: W. W. Norton & Company; 2004.
2. Block D. Baseball Before We Knew It: A Search for the Roots of the Game. Vol 1. Lincoln, NE: Bison Books; 2006.
3. James B. The New Bill James Historical Baseball Abstract. Vol 2. Detroit, MI: Free Press; 2003.
4. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
7. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
8. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
9. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elb Surg. 2013;22(5):642-646.
10. Jones KJ, Osbahr DC, Schrumpf MA, Dines JS, Altchek DW. Ulnar collateral ligament reconstruction in throwing athletes: a review of current concepts. AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(8):e49.
11. Dodson CC, Thomas A, Dines JS, Nho SJ, Williams RJ 3rd, Altchek DW. Medial ulnar collateral ligament reconstruction of the elbow in throwing athletes. Am J Sports Med. 2006;34(12):1926-1932.
12. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
13. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: A review of 147 cases. Am J Sports Med. 2014;42(6):
1323-1332.
14. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Rohrbough JT, Altchek DW, Hyman J, Williams RJ 3rd, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30(4):541-548.
16. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
17. Keller RA, Steffes MJ, Zhuo D, Bey MJ, Moutzouros V. The effects of medial ulnar collateral ligament reconstruction on Major League pitching performance. J Shoulder Elbow Surg. 2014;23(11):1591-1598.
18. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
19. Liu JN, Garcia GH, Conte S, ElAttrache N, Altchek DW, Dines JS. Outcomes in revision Tommy John surgery in Major League Baseball pitchers. J Shoulder Elbow Surg. 2016;25(1):90-97.
20. McFarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
21. Chambless KM, Knudtson J, Eck JC, Covington LA. Rate of injury in minor league baseball by level of play. Am J Orthop. 2000;29(11):869-872.
22. Brown M. Major League Baseball Sees Record $9 Billion In Revenues For 2014. Forbes. http://www.forbes.com/sites/maurybrown/2014/12/10/major-league-baseball-sees-record-9-billion-in-revenues-for-2014/. Published December 10, 2014. Accessed February 3, 2016.
23. Jones KJ, Dines JS, Rebolledo BJ, et al. Operative management of ulnar collateral ligament insufficiency in adolescent athletes. Am J Sports Med. 2014;42(1):117-121.
24. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
25. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
26. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
While the exact origins of the game of baseball are commonly debated, one thing is certain: statistics have been an integral part of the game since its existence.1-3 This is true at nearly every level of baseball, especially in Major League Baseball (MLB). As our knowledge and technical capabilities advance, new statistical measures of baseball performance are added at a rapid pace.1,3 One example is the Pitch f/x video tracking system (Sportvision, Inc.), which now analyzes over 60 variables on each of the estimated 660,000 pitches thrown in the MLB annually. In addition to measuring performance and production, these advancements are being leveraged to better understand the epidemiology and impact of injuries in MLB players.4,5 As with any sport, performance at the most elite level is highly dependent upon player health and functional capacity. Accordingly, player injuries can have a profound impact not only on individual performance but also on the success of the team as a whole.
The first epidemiologic study of injuries in professional baseball was published by Conte and colleagues4 in 2001. This work utilized publically available disabled list (DL) data to perform a comprehensive review of injury patterns in MLB from 1989 to 1999. They demonstrated that injuries were on the rise and that pitchers were more commonly injured (48.4% of all DL reports) and had greater time out of play compared to players of other positions.4 Shoulder and elbow injuries were responsible for 49.8% of all DL assignments, distantly followed by knee (7.3%), wrist/hand (6.1%), and back (5.0%).4 In a later study, Posner and colleagues5 analyzed DL data spanning the 2002 to 2008 seasons. Similarly, they found that injuries continued to increase, and over half (51.2%) of DL assignments occurred secondary to upper extremity injuries.5 Although the DL is primarily designed as a roster management tool rather than an injury database, it has provided valuable epidemiologic injury information through the years. Out of concern for player health and well-being, MLB and the MLB Players Association (MLBPA) worked together to create and implement an electronic medical record and Health and Injury Tracking System (HITS) for all MLB and Minor League Baseball (MiLB) players. Now active for over 5 seasons, this database has provided valuable, detailed reports regarding specific injuries occurring in professional baseball, such as hamstring strains and concussions.6,7
With shoulder and elbow injuries in pitchers representing the greatest proportion of DL assignments in recent years, a large body of literature on these injuries, particularly medial ulnar collateral ligament (MUCL) injuries, has been published.8-13 Since the initial description of MUCL reconstruction, or “Tommy John surgery,” by Dr. Frank Jobe in 1986, much has been done to improve the technique and rehabilitation to maximize player performance following surgery.10,14-16 Despite this increased attention, large-scale epidemiologic reporting of MUCL injuries in MLB is lacking, but such a report is desirable. The purpose of this work is to: 1) provide a large-scale analysis of injuries occurring in MLB baseball over the course of 18 seasons (1998-2015); 2) highlight the financial implications of these injuries; and 3) detail the evolution of MUCL injuries and reconstructive surgery since it was first performed on a MLB pitcher in 1974. Our study represents the largest longitudinal analysis of MLB injuries since the league expanded to its current level of 30 teams in 1998. It is our hope that this work will serve as a framework for future study of the most common and highest impact injuries occurring in baseball.
Materials And Methods
We performed a retrospective review of the MLB DL from 1998 to 2015. Data analyzed included player demographics such as club, year of placement, age, and position. Injury-specific variables included date of placement on DL, length of time on DL, date of reinstatement, body part injured, diagnosis, and cost of replacement. If a player was put on the DL multiple times during a season, each placement was viewed as a different injury, even if it was to the same body part. If a player was put on the DL for injuries to multiple body parts, the primary injury was analyzed.
Disabled List Data
Although the DL has existed since 1916, this current study covers 18 seasons from 1998 to 2015. The 1998 season was chosen as a starting point because this is the year when MLB expanded to 30 teams. Since then, the number of teams and the active roster limits (25 players) have remained constant, allowing for reliable comparisons across seasons. Initially designed as a roster management tool to allow injured players to temporarily be replaced with healthy players, the DL was not created as an injury database. However, the rules and regulations of the DL have remained fairly constant over the last 18 years, allowing reasonable comparisons of injury data and trends across this timespan. In order for a player to be assigned to the DL, the nature and extent of injury must be certified by a physician. Once designated for the DL, a player cannot return to the major league team for a minimum of 15 days. If the injury is severe, the player can remain on the DL for the remainder of the season or until he is deemed healthy enough to return to play by a physician. One notable exception is the treatment of concussions. Since 2011, a player diagnosed with a concussion may be placed on the DL for a minimum of 7 days rather than 15. The introduction of the HITS database in 2010 should allow for more detailed and reliable study of injuries in baseball moving forward. Although it contains robust data for every injury that has occurred in MLB and MiLB over the last 5 seasons, it does not allow for epidemiologic and longitudinal study of injury patterns and trends in baseball prior to 2010.
Cost of Placing Players on the DL
The dollars lost were calculated by prorating the injured player’s daily salary and multiplying by the number of days missed on the DL. For example, if a player’s annual salary is $1,820,000, his daily salary for the 182 day season is $10,000. If assigned to the DL for 15 days, $150,000 is paid to that player while he is inactive and unable to play. An additional cost is the salary of the replacement player who fills the roster spot. For this work, the replacement player’s prorated, daily salary was assumed to be the league minimum for that specific year. For example, if the league minimum for a given season is $182,000, and the season is 182 days long, a replacement player earns a minimum of $1,000 per day while he is on the 25-man active roster. Thus, the dollars paid to the replacement would be $15,000. In this scenario, that brings the team’s total cost to $165,000 ($150,000 plus $15,000). Because the league minimum salary changes year to year, salaries specific to the year of injury were utilized in this analysis.
MUCL Injury Analysis
In order to better understand the evaluation of MUCL injuries over time, all MLB players undergoing MUCL reconstruction (“Tommy John surgery”) were analyzed separately. Similar to prior studies of UCL injuries, these players were identified using DL data, team websites, and publically available internet databases (primarily www.heatmaps.com).9,12,17-19 Variables studied include the number of procedures, year of surgery, player position, and mean time until return to play at the MLB level. All MLB players undergoing MUCL reconstruction since 1974 (the year the first procedure was performed) were included.
Statistical Methods
Epidemiologic data are reported using descriptive statistics (mean, range, and percentage) where indicated. To determine the significance of trends over time, a best-fit line was generated to illustrate the change over the years. These lines are reported with corresponding R2 values. To assess the trend for significance, the slope was compared to a line with a slope of zero (no change over time) using t tests. For all statistical comparisons, the threshold for alpha was set to P < .05.
Results
Between 1998 and 2015, there were 8357 placements of players on the DL, at an average rate of 464 designations per year (Table 1, Figure 1). This resulted in 460,432 days lost to injury, with a mean of 25,186 days out of play per season (Table 1, Figure 2). The mean length of DL assignment per year was 55.1 days per injury, with a low of 49.1 days in 2011 and a high of 59.2 days in 2001 (Table 1, Figure 3). During the study period, the number of players placed on the DL and the total number of DL days steadily increased (P < .001 and P = .003, respectively), while the average length of DL assignments remained steady (P = .647). When analyzing the data by body region injured, the shoulder (20.6%) and elbow (19.6%) were the 2 leading causes of time out of play (Table 2). This was followed distantly by the chest/back/spine (13.7%), wrist/hand/fingers (10.1%), lower leg/knee (9.8%), and the upper leg/thigh (9.5%). Although the percentage of injuries occurring to the upper extremity remained stable, the rate of shoulder injuries steadily decreased (P = .023) as elbow injuries increased (P = .015) (Table 3, Figure 4). This inverse relationship was also demonstrated for the annual number of DL days for shoulder (P = .033) and elbow (P = 0.005) injuries (Figure 5).
Regarding the financial impact of these injuries, the mean annual cost of replacing players on the DL was $423,267,633.78 (Table 4). This ranged from a low of $136,397,147 in 1998 to a high of $694,835,359 in 2015. There was a steady increase in the cost of replacement during the study period (P < .001) that coincides with the increasing salaries during that time span (Figure 6). In total, $6,732,167,180 was paid to players assigned to the DL and $886,650,228 was spent to fill their positions. This brings the total cost of DL assignments to $7,618,817,407 for the study period.
Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed on MLB players since the procedure was first developed in 1974. The vast majority of these were performed in pitchers (n = 361, 90.3%) followed by outfielders (n = 16, 4.0%), infielders (n = 14, 3.5%) and catchers (n = 9, 2.3%) (Table 5). The mean time to return to competition at the MLB level was 17.8 months for pitchers, 11.1 months for outfielders, 9.6 months for infielders, and 10.5 months for catchers. The overall mean time to return was 17.1 months. The annual number of MUCL reconstructions continues to rise dramatically (P < .001) (Figure 7). During the first 12 years (1974-1985), a total of 8 (2.0%) MUCL reconstructions were performed on MLB players. In subsequent decades, this number increased to 44 (11.0%) from 1986-1995, 123 (30.8%) from 1996-2005, and 225 (56.3%) from 2006-2015. Of all Tommy John surgeries performed over 42 years, nearly one-third (n = 131, 32.75%) were performed in the last 5 years alone (2011-2015).
Discussion
To date, a number of studies have been published on injuries in professional baseball. These can primarily be categorized as either studies with a detailed focus on a single injury type or body region6-13,17,19 or broader reviews that are limited by the relatively short time span covered.4,5 The purpose of this work was to provide a comprehensive review of injury trends in MLB since the league expanded to 30 teams in 1998 while paying special attention to the financial impact of those injuries. Additionally, we sought to provide an up-to-date review of MUCL injuries and surgeries since the procedure was first developed in 1974. Ultimately, this data demonstrates that injuries continue to rise in MLB and this increase is accompanied by increased expense for teams. Thankfully, the rates of DL assignments for shoulder injuries are on the decline; however, this decrease is countered by a reciprocal increase in elbow injuries. Similarly, the rates of MUCL reconstruction have also risen dramatically in recent years.
The fact that injury rates are on the rise is confirmed by other published reports. This trend was demonstrated in prior analyses of DL data from the 1989 to 19984 and 2002 to 2008 seasons.5 These 2 studies represent the only comprehensive reviews of MLB injury trends to date, and each provides valuable information. Both are consistent with the current study findings that pitchers are the most commonly injured players and that shoulder and elbow injuries represent about half of all injuries.4,5 Similar injury rates and characteristics have been reported at the collegiate20 and minor league levels.21 Despite this consistency, this analysis of injuries from 1998 to 2015 is the first to report that DL designations for shoulder injuries are on the decline while designations for elbow injuries continue to rise. Although the exact etiology of this decline in shoulder injuries remains unknown, there are a number of possible explanations. In recent years, increased emphasis has been placed on shoulder rehabilitation, reduction of glenohumeral internal rotation deficits, scapular stabilization, and overall kinetic chain balance and coordination. However, this does not explain why elbow injuries continue to rise annually.
With this increase in injuries, the cost of maintaining an active 25-man roster is also climbing. As expected, this growing expense is primarily due to the increased number of DL days each year as well as the increase in league salaries. Fortunately, this increased financial strain has been met with steadily increased annual revenues in professional baseball. In 2014, the prorated salary cost to players designated to the DL and their replacements was $579,568,059. This figure represents an estimated 6.4% of the $9 billion in total revenue for MLB that same year.22 Although this may represent a small percentage of the whole, it still embodies an exceptionally large financial responsibility. This does not include the medical expenses incurred to treat and rehabilitate the players’ injuries.
Every injury that occurs in MLB players has the potential to adversely affect players, teams, and MLB as a whole. With its increasing prevalence, need for surgical treatment, and prolonged return to play, injuries to the MUCL of the elbow may represent the most costly of all injuries. Although a multitude of reports on MUCL injuries, treatments, techniques, rehabilitation, and outcomes have been reported,8,9,12,14-19,23-25 to our knowledge, a comprehensive and longitudinal incidence study in MLB players has not yet been published. By including every MUCL reconstruction that has been performed on a MLB player, our study demonstrates the dramatic increase in the annual incidence of MUCL surgeries. Studies performed over shorter time intervals corroborate these findings. A recent review of a privately insured patient database revealed an annual increase in MUCL reconstructions of 4.2% in that cohort.26 When looking specifically at the MLB, a recent survey of all 30 clubs found that 25% (96 of 382) of MLB pitchers and 15% (341 of 2324) of minor league pitchers have undergone MUCL reconstruction.8 Because it occurs so frequently and requires a mean of 17 months to return to sport, MUCL injuries represent a very significant cause of time out of play.
While this study represents a unique epidemiologic report on injuries in baseball, it is certainly not without its limitations. As stated previously, it relies on DL data that was initially intended to serve as a roster management tool rather than an injury database. Accordingly, detailed and specific information about every injury is not always available. The limitations of DL data will largely be overcome in future studies thanks to the implementation of the HITS database in 2010. Moving forward, this system will allow for more detailed analysis of injury patterns, characteristics, time out of play, treatments rendered, etc. Its main limitation is that the earliest data dates back to 2010, making it less applicable for longitudinal studies like the present one. Another limitation of this study is the estimations used for the cost of replacing players designated to the DL. For each injury, it was assumed that the replacement player was paid a prorated portion of the league minimum salary while on the major league roster, but in some instances, that may not have been the case. It is possible that some players filling roster spots were already under contract for amounts higher than the league minimum. Since that player would be making that amount regardless of the level of play, the team may not have paid them any additional salary while filling the position of the injured player. The strengths of this study are its comprehensive nature and inclusion of 18 years of data, making it the longest such study of injuries in MLB. It also represents the first report of cost of replacement for players designated to the DL. To our knowledge, this study also represents the first comprehensive report of every MUCL surgery that has been performed on MLB players.
Conclusion
Injury rates continue to rise in MLB, and upper extremity injuries continue to represent approximately half of all injuries resulting in time out of play. Although shoulder injuries have been on the decline in recent years, this decline is offset by a steady increase in elbow injuries. Each year, MLB players are designated to the DL an average of 464 times for a total of 25,579.6 days. This results in a mean annual cost of over $400 million dollars to replace players lost to injury. Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed in the MLB since 1974, and nearly one-third of these were performed in the last 5 years. Pitchers represent 90.3% of players requiring MUCL surgery, and the average time to return to sport for all players is 17 months. These data may serve as a foundation for identifying appropriate targets for continued study into the etiologies, strategies for prevention, and optimal treatments of injuries commonly affecting professional baseball players.
While the exact origins of the game of baseball are commonly debated, one thing is certain: statistics have been an integral part of the game since its existence.1-3 This is true at nearly every level of baseball, especially in Major League Baseball (MLB). As our knowledge and technical capabilities advance, new statistical measures of baseball performance are added at a rapid pace.1,3 One example is the Pitch f/x video tracking system (Sportvision, Inc.), which now analyzes over 60 variables on each of the estimated 660,000 pitches thrown in the MLB annually. In addition to measuring performance and production, these advancements are being leveraged to better understand the epidemiology and impact of injuries in MLB players.4,5 As with any sport, performance at the most elite level is highly dependent upon player health and functional capacity. Accordingly, player injuries can have a profound impact not only on individual performance but also on the success of the team as a whole.
The first epidemiologic study of injuries in professional baseball was published by Conte and colleagues4 in 2001. This work utilized publically available disabled list (DL) data to perform a comprehensive review of injury patterns in MLB from 1989 to 1999. They demonstrated that injuries were on the rise and that pitchers were more commonly injured (48.4% of all DL reports) and had greater time out of play compared to players of other positions.4 Shoulder and elbow injuries were responsible for 49.8% of all DL assignments, distantly followed by knee (7.3%), wrist/hand (6.1%), and back (5.0%).4 In a later study, Posner and colleagues5 analyzed DL data spanning the 2002 to 2008 seasons. Similarly, they found that injuries continued to increase, and over half (51.2%) of DL assignments occurred secondary to upper extremity injuries.5 Although the DL is primarily designed as a roster management tool rather than an injury database, it has provided valuable epidemiologic injury information through the years. Out of concern for player health and well-being, MLB and the MLB Players Association (MLBPA) worked together to create and implement an electronic medical record and Health and Injury Tracking System (HITS) for all MLB and Minor League Baseball (MiLB) players. Now active for over 5 seasons, this database has provided valuable, detailed reports regarding specific injuries occurring in professional baseball, such as hamstring strains and concussions.6,7
With shoulder and elbow injuries in pitchers representing the greatest proportion of DL assignments in recent years, a large body of literature on these injuries, particularly medial ulnar collateral ligament (MUCL) injuries, has been published.8-13 Since the initial description of MUCL reconstruction, or “Tommy John surgery,” by Dr. Frank Jobe in 1986, much has been done to improve the technique and rehabilitation to maximize player performance following surgery.10,14-16 Despite this increased attention, large-scale epidemiologic reporting of MUCL injuries in MLB is lacking, but such a report is desirable. The purpose of this work is to: 1) provide a large-scale analysis of injuries occurring in MLB baseball over the course of 18 seasons (1998-2015); 2) highlight the financial implications of these injuries; and 3) detail the evolution of MUCL injuries and reconstructive surgery since it was first performed on a MLB pitcher in 1974. Our study represents the largest longitudinal analysis of MLB injuries since the league expanded to its current level of 30 teams in 1998. It is our hope that this work will serve as a framework for future study of the most common and highest impact injuries occurring in baseball.
Materials And Methods
We performed a retrospective review of the MLB DL from 1998 to 2015. Data analyzed included player demographics such as club, year of placement, age, and position. Injury-specific variables included date of placement on DL, length of time on DL, date of reinstatement, body part injured, diagnosis, and cost of replacement. If a player was put on the DL multiple times during a season, each placement was viewed as a different injury, even if it was to the same body part. If a player was put on the DL for injuries to multiple body parts, the primary injury was analyzed.
Disabled List Data
Although the DL has existed since 1916, this current study covers 18 seasons from 1998 to 2015. The 1998 season was chosen as a starting point because this is the year when MLB expanded to 30 teams. Since then, the number of teams and the active roster limits (25 players) have remained constant, allowing for reliable comparisons across seasons. Initially designed as a roster management tool to allow injured players to temporarily be replaced with healthy players, the DL was not created as an injury database. However, the rules and regulations of the DL have remained fairly constant over the last 18 years, allowing reasonable comparisons of injury data and trends across this timespan. In order for a player to be assigned to the DL, the nature and extent of injury must be certified by a physician. Once designated for the DL, a player cannot return to the major league team for a minimum of 15 days. If the injury is severe, the player can remain on the DL for the remainder of the season or until he is deemed healthy enough to return to play by a physician. One notable exception is the treatment of concussions. Since 2011, a player diagnosed with a concussion may be placed on the DL for a minimum of 7 days rather than 15. The introduction of the HITS database in 2010 should allow for more detailed and reliable study of injuries in baseball moving forward. Although it contains robust data for every injury that has occurred in MLB and MiLB over the last 5 seasons, it does not allow for epidemiologic and longitudinal study of injury patterns and trends in baseball prior to 2010.
Cost of Placing Players on the DL
The dollars lost were calculated by prorating the injured player’s daily salary and multiplying by the number of days missed on the DL. For example, if a player’s annual salary is $1,820,000, his daily salary for the 182 day season is $10,000. If assigned to the DL for 15 days, $150,000 is paid to that player while he is inactive and unable to play. An additional cost is the salary of the replacement player who fills the roster spot. For this work, the replacement player’s prorated, daily salary was assumed to be the league minimum for that specific year. For example, if the league minimum for a given season is $182,000, and the season is 182 days long, a replacement player earns a minimum of $1,000 per day while he is on the 25-man active roster. Thus, the dollars paid to the replacement would be $15,000. In this scenario, that brings the team’s total cost to $165,000 ($150,000 plus $15,000). Because the league minimum salary changes year to year, salaries specific to the year of injury were utilized in this analysis.
MUCL Injury Analysis
In order to better understand the evaluation of MUCL injuries over time, all MLB players undergoing MUCL reconstruction (“Tommy John surgery”) were analyzed separately. Similar to prior studies of UCL injuries, these players were identified using DL data, team websites, and publically available internet databases (primarily www.heatmaps.com).9,12,17-19 Variables studied include the number of procedures, year of surgery, player position, and mean time until return to play at the MLB level. All MLB players undergoing MUCL reconstruction since 1974 (the year the first procedure was performed) were included.
Statistical Methods
Epidemiologic data are reported using descriptive statistics (mean, range, and percentage) where indicated. To determine the significance of trends over time, a best-fit line was generated to illustrate the change over the years. These lines are reported with corresponding R2 values. To assess the trend for significance, the slope was compared to a line with a slope of zero (no change over time) using t tests. For all statistical comparisons, the threshold for alpha was set to P < .05.
Results
Between 1998 and 2015, there were 8357 placements of players on the DL, at an average rate of 464 designations per year (Table 1, Figure 1). This resulted in 460,432 days lost to injury, with a mean of 25,186 days out of play per season (Table 1, Figure 2). The mean length of DL assignment per year was 55.1 days per injury, with a low of 49.1 days in 2011 and a high of 59.2 days in 2001 (Table 1, Figure 3). During the study period, the number of players placed on the DL and the total number of DL days steadily increased (P < .001 and P = .003, respectively), while the average length of DL assignments remained steady (P = .647). When analyzing the data by body region injured, the shoulder (20.6%) and elbow (19.6%) were the 2 leading causes of time out of play (Table 2). This was followed distantly by the chest/back/spine (13.7%), wrist/hand/fingers (10.1%), lower leg/knee (9.8%), and the upper leg/thigh (9.5%). Although the percentage of injuries occurring to the upper extremity remained stable, the rate of shoulder injuries steadily decreased (P = .023) as elbow injuries increased (P = .015) (Table 3, Figure 4). This inverse relationship was also demonstrated for the annual number of DL days for shoulder (P = .033) and elbow (P = 0.005) injuries (Figure 5).
Regarding the financial impact of these injuries, the mean annual cost of replacing players on the DL was $423,267,633.78 (Table 4). This ranged from a low of $136,397,147 in 1998 to a high of $694,835,359 in 2015. There was a steady increase in the cost of replacement during the study period (P < .001) that coincides with the increasing salaries during that time span (Figure 6). In total, $6,732,167,180 was paid to players assigned to the DL and $886,650,228 was spent to fill their positions. This brings the total cost of DL assignments to $7,618,817,407 for the study period.
Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed on MLB players since the procedure was first developed in 1974. The vast majority of these were performed in pitchers (n = 361, 90.3%) followed by outfielders (n = 16, 4.0%), infielders (n = 14, 3.5%) and catchers (n = 9, 2.3%) (Table 5). The mean time to return to competition at the MLB level was 17.8 months for pitchers, 11.1 months for outfielders, 9.6 months for infielders, and 10.5 months for catchers. The overall mean time to return was 17.1 months. The annual number of MUCL reconstructions continues to rise dramatically (P < .001) (Figure 7). During the first 12 years (1974-1985), a total of 8 (2.0%) MUCL reconstructions were performed on MLB players. In subsequent decades, this number increased to 44 (11.0%) from 1986-1995, 123 (30.8%) from 1996-2005, and 225 (56.3%) from 2006-2015. Of all Tommy John surgeries performed over 42 years, nearly one-third (n = 131, 32.75%) were performed in the last 5 years alone (2011-2015).
Discussion
To date, a number of studies have been published on injuries in professional baseball. These can primarily be categorized as either studies with a detailed focus on a single injury type or body region6-13,17,19 or broader reviews that are limited by the relatively short time span covered.4,5 The purpose of this work was to provide a comprehensive review of injury trends in MLB since the league expanded to 30 teams in 1998 while paying special attention to the financial impact of those injuries. Additionally, we sought to provide an up-to-date review of MUCL injuries and surgeries since the procedure was first developed in 1974. Ultimately, this data demonstrates that injuries continue to rise in MLB and this increase is accompanied by increased expense for teams. Thankfully, the rates of DL assignments for shoulder injuries are on the decline; however, this decrease is countered by a reciprocal increase in elbow injuries. Similarly, the rates of MUCL reconstruction have also risen dramatically in recent years.
The fact that injury rates are on the rise is confirmed by other published reports. This trend was demonstrated in prior analyses of DL data from the 1989 to 19984 and 2002 to 2008 seasons.5 These 2 studies represent the only comprehensive reviews of MLB injury trends to date, and each provides valuable information. Both are consistent with the current study findings that pitchers are the most commonly injured players and that shoulder and elbow injuries represent about half of all injuries.4,5 Similar injury rates and characteristics have been reported at the collegiate20 and minor league levels.21 Despite this consistency, this analysis of injuries from 1998 to 2015 is the first to report that DL designations for shoulder injuries are on the decline while designations for elbow injuries continue to rise. Although the exact etiology of this decline in shoulder injuries remains unknown, there are a number of possible explanations. In recent years, increased emphasis has been placed on shoulder rehabilitation, reduction of glenohumeral internal rotation deficits, scapular stabilization, and overall kinetic chain balance and coordination. However, this does not explain why elbow injuries continue to rise annually.
With this increase in injuries, the cost of maintaining an active 25-man roster is also climbing. As expected, this growing expense is primarily due to the increased number of DL days each year as well as the increase in league salaries. Fortunately, this increased financial strain has been met with steadily increased annual revenues in professional baseball. In 2014, the prorated salary cost to players designated to the DL and their replacements was $579,568,059. This figure represents an estimated 6.4% of the $9 billion in total revenue for MLB that same year.22 Although this may represent a small percentage of the whole, it still embodies an exceptionally large financial responsibility. This does not include the medical expenses incurred to treat and rehabilitate the players’ injuries.
Every injury that occurs in MLB players has the potential to adversely affect players, teams, and MLB as a whole. With its increasing prevalence, need for surgical treatment, and prolonged return to play, injuries to the MUCL of the elbow may represent the most costly of all injuries. Although a multitude of reports on MUCL injuries, treatments, techniques, rehabilitation, and outcomes have been reported,8,9,12,14-19,23-25 to our knowledge, a comprehensive and longitudinal incidence study in MLB players has not yet been published. By including every MUCL reconstruction that has been performed on a MLB player, our study demonstrates the dramatic increase in the annual incidence of MUCL surgeries. Studies performed over shorter time intervals corroborate these findings. A recent review of a privately insured patient database revealed an annual increase in MUCL reconstructions of 4.2% in that cohort.26 When looking specifically at the MLB, a recent survey of all 30 clubs found that 25% (96 of 382) of MLB pitchers and 15% (341 of 2324) of minor league pitchers have undergone MUCL reconstruction.8 Because it occurs so frequently and requires a mean of 17 months to return to sport, MUCL injuries represent a very significant cause of time out of play.
While this study represents a unique epidemiologic report on injuries in baseball, it is certainly not without its limitations. As stated previously, it relies on DL data that was initially intended to serve as a roster management tool rather than an injury database. Accordingly, detailed and specific information about every injury is not always available. The limitations of DL data will largely be overcome in future studies thanks to the implementation of the HITS database in 2010. Moving forward, this system will allow for more detailed analysis of injury patterns, characteristics, time out of play, treatments rendered, etc. Its main limitation is that the earliest data dates back to 2010, making it less applicable for longitudinal studies like the present one. Another limitation of this study is the estimations used for the cost of replacing players designated to the DL. For each injury, it was assumed that the replacement player was paid a prorated portion of the league minimum salary while on the major league roster, but in some instances, that may not have been the case. It is possible that some players filling roster spots were already under contract for amounts higher than the league minimum. Since that player would be making that amount regardless of the level of play, the team may not have paid them any additional salary while filling the position of the injured player. The strengths of this study are its comprehensive nature and inclusion of 18 years of data, making it the longest such study of injuries in MLB. It also represents the first report of cost of replacement for players designated to the DL. To our knowledge, this study also represents the first comprehensive report of every MUCL surgery that has been performed on MLB players.
Conclusion
Injury rates continue to rise in MLB, and upper extremity injuries continue to represent approximately half of all injuries resulting in time out of play. Although shoulder injuries have been on the decline in recent years, this decline is offset by a steady increase in elbow injuries. Each year, MLB players are designated to the DL an average of 464 times for a total of 25,579.6 days. This results in a mean annual cost of over $400 million dollars to replace players lost to injury. Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed in the MLB since 1974, and nearly one-third of these were performed in the last 5 years. Pitchers represent 90.3% of players requiring MUCL surgery, and the average time to return to sport for all players is 17 months. These data may serve as a foundation for identifying appropriate targets for continued study into the etiologies, strategies for prevention, and optimal treatments of injuries commonly affecting professional baseball players.
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. Vol 1. New York, NY: W. W. Norton & Company; 2004.
2. Block D. Baseball Before We Knew It: A Search for the Roots of the Game. Vol 1. Lincoln, NE: Bison Books; 2006.
3. James B. The New Bill James Historical Baseball Abstract. Vol 2. Detroit, MI: Free Press; 2003.
4. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
7. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
8. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
9. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elb Surg. 2013;22(5):642-646.
10. Jones KJ, Osbahr DC, Schrumpf MA, Dines JS, Altchek DW. Ulnar collateral ligament reconstruction in throwing athletes: a review of current concepts. AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(8):e49.
11. Dodson CC, Thomas A, Dines JS, Nho SJ, Williams RJ 3rd, Altchek DW. Medial ulnar collateral ligament reconstruction of the elbow in throwing athletes. Am J Sports Med. 2006;34(12):1926-1932.
12. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
13. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: A review of 147 cases. Am J Sports Med. 2014;42(6):
1323-1332.
14. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Rohrbough JT, Altchek DW, Hyman J, Williams RJ 3rd, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30(4):541-548.
16. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
17. Keller RA, Steffes MJ, Zhuo D, Bey MJ, Moutzouros V. The effects of medial ulnar collateral ligament reconstruction on Major League pitching performance. J Shoulder Elbow Surg. 2014;23(11):1591-1598.
18. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
19. Liu JN, Garcia GH, Conte S, ElAttrache N, Altchek DW, Dines JS. Outcomes in revision Tommy John surgery in Major League Baseball pitchers. J Shoulder Elbow Surg. 2016;25(1):90-97.
20. McFarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
21. Chambless KM, Knudtson J, Eck JC, Covington LA. Rate of injury in minor league baseball by level of play. Am J Orthop. 2000;29(11):869-872.
22. Brown M. Major League Baseball Sees Record $9 Billion In Revenues For 2014. Forbes. http://www.forbes.com/sites/maurybrown/2014/12/10/major-league-baseball-sees-record-9-billion-in-revenues-for-2014/. Published December 10, 2014. Accessed February 3, 2016.
23. Jones KJ, Dines JS, Rebolledo BJ, et al. Operative management of ulnar collateral ligament insufficiency in adolescent athletes. Am J Sports Med. 2014;42(1):117-121.
24. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
25. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
26. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. Vol 1. New York, NY: W. W. Norton & Company; 2004.
2. Block D. Baseball Before We Knew It: A Search for the Roots of the Game. Vol 1. Lincoln, NE: Bison Books; 2006.
3. James B. The New Bill James Historical Baseball Abstract. Vol 2. Detroit, MI: Free Press; 2003.
4. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
7. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
8. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
9. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elb Surg. 2013;22(5):642-646.
10. Jones KJ, Osbahr DC, Schrumpf MA, Dines JS, Altchek DW. Ulnar collateral ligament reconstruction in throwing athletes: a review of current concepts. AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(8):e49.
11. Dodson CC, Thomas A, Dines JS, Nho SJ, Williams RJ 3rd, Altchek DW. Medial ulnar collateral ligament reconstruction of the elbow in throwing athletes. Am J Sports Med. 2006;34(12):1926-1932.
12. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
13. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: A review of 147 cases. Am J Sports Med. 2014;42(6):
1323-1332.
14. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Rohrbough JT, Altchek DW, Hyman J, Williams RJ 3rd, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30(4):541-548.
16. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
17. Keller RA, Steffes MJ, Zhuo D, Bey MJ, Moutzouros V. The effects of medial ulnar collateral ligament reconstruction on Major League pitching performance. J Shoulder Elbow Surg. 2014;23(11):1591-1598.
18. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
19. Liu JN, Garcia GH, Conte S, ElAttrache N, Altchek DW, Dines JS. Outcomes in revision Tommy John surgery in Major League Baseball pitchers. J Shoulder Elbow Surg. 2016;25(1):90-97.
20. McFarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
21. Chambless KM, Knudtson J, Eck JC, Covington LA. Rate of injury in minor league baseball by level of play. Am J Orthop. 2000;29(11):869-872.
22. Brown M. Major League Baseball Sees Record $9 Billion In Revenues For 2014. Forbes. http://www.forbes.com/sites/maurybrown/2014/12/10/major-league-baseball-sees-record-9-billion-in-revenues-for-2014/. Published December 10, 2014. Accessed February 3, 2016.
23. Jones KJ, Dines JS, Rebolledo BJ, et al. Operative management of ulnar collateral ligament insufficiency in adolescent athletes. Am J Sports Med. 2014;42(1):117-121.
24. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
25. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
26. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.