User login
Comorbidities, Racial Disparities, and Geographic Differences in Asthma
- Wenzel M. Gasping for a diagnosis: pediatric vocal cord dysfunction. J Pediatr Health Care. 2019;33(1):5-13. doi:10.1016/j.pedhc.2018.03.002
- Mogensen I, James A, Malinovschi A. Systemic and breath biomarkers for asthma: an update. Curr Opin Allergy Clin Immunol. 2020;20(1):71-79. doi:10.1097/ACI.0000000000000599
- Gibson PG, McDonald VM, Granchelli A, Olin JT. Asthma and comorbid conditions—pulmonary comorbidity. J Allergy Clin Immunol Pract. 2021;9(11):3868-3875. doi:10.1016/j. jaip.2021.08.028
- Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141(4):1169-1179. doi:10.1016/j.jaci.2018.02.004
- Adult obesity facts. Centers for Disease Control and Prevention. Published May 17, 2022. Accessed June 7, 2022. https://www.cdc.gov/obesity/data/adult.html
- Sharma V, Cowan DC. Obesity, inflammation, and severe asthma: an update. Curr Allergy Asthma Rep. 2021;21(12):46. doi:10.1007/s11882-021-01024-9
- Assari S, Chalian H, Bazargan M. Race, ethnicity, socioeconomic status, and chronic lung disease in the U.S. Res Health Sci. 2020;5(1):48-63. doi:10.22158/rhs.v5n1p48
- Bleecker ER, Gandhi H, Gilbert I, Murphy KR, Chupp GL. Mapping geographic variability of severe uncontrolled asthma in the United States: management implications. Ann Allergy Asthma Immunol. 2022;128(1):78-88. doi:10.1016/j.anai.2021.09.025
- Wenzel M. Gasping for a diagnosis: pediatric vocal cord dysfunction. J Pediatr Health Care. 2019;33(1):5-13. doi:10.1016/j.pedhc.2018.03.002
- Mogensen I, James A, Malinovschi A. Systemic and breath biomarkers for asthma: an update. Curr Opin Allergy Clin Immunol. 2020;20(1):71-79. doi:10.1097/ACI.0000000000000599
- Gibson PG, McDonald VM, Granchelli A, Olin JT. Asthma and comorbid conditions—pulmonary comorbidity. J Allergy Clin Immunol Pract. 2021;9(11):3868-3875. doi:10.1016/j. jaip.2021.08.028
- Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141(4):1169-1179. doi:10.1016/j.jaci.2018.02.004
- Adult obesity facts. Centers for Disease Control and Prevention. Published May 17, 2022. Accessed June 7, 2022. https://www.cdc.gov/obesity/data/adult.html
- Sharma V, Cowan DC. Obesity, inflammation, and severe asthma: an update. Curr Allergy Asthma Rep. 2021;21(12):46. doi:10.1007/s11882-021-01024-9
- Assari S, Chalian H, Bazargan M. Race, ethnicity, socioeconomic status, and chronic lung disease in the U.S. Res Health Sci. 2020;5(1):48-63. doi:10.22158/rhs.v5n1p48
- Bleecker ER, Gandhi H, Gilbert I, Murphy KR, Chupp GL. Mapping geographic variability of severe uncontrolled asthma in the United States: management implications. Ann Allergy Asthma Immunol. 2022;128(1):78-88. doi:10.1016/j.anai.2021.09.025
- Wenzel M. Gasping for a diagnosis: pediatric vocal cord dysfunction. J Pediatr Health Care. 2019;33(1):5-13. doi:10.1016/j.pedhc.2018.03.002
- Mogensen I, James A, Malinovschi A. Systemic and breath biomarkers for asthma: an update. Curr Opin Allergy Clin Immunol. 2020;20(1):71-79. doi:10.1097/ACI.0000000000000599
- Gibson PG, McDonald VM, Granchelli A, Olin JT. Asthma and comorbid conditions—pulmonary comorbidity. J Allergy Clin Immunol Pract. 2021;9(11):3868-3875. doi:10.1016/j. jaip.2021.08.028
- Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141(4):1169-1179. doi:10.1016/j.jaci.2018.02.004
- Adult obesity facts. Centers for Disease Control and Prevention. Published May 17, 2022. Accessed June 7, 2022. https://www.cdc.gov/obesity/data/adult.html
- Sharma V, Cowan DC. Obesity, inflammation, and severe asthma: an update. Curr Allergy Asthma Rep. 2021;21(12):46. doi:10.1007/s11882-021-01024-9
- Assari S, Chalian H, Bazargan M. Race, ethnicity, socioeconomic status, and chronic lung disease in the U.S. Res Health Sci. 2020;5(1):48-63. doi:10.22158/rhs.v5n1p48
- Bleecker ER, Gandhi H, Gilbert I, Murphy KR, Chupp GL. Mapping geographic variability of severe uncontrolled asthma in the United States: management implications. Ann Allergy Asthma Immunol. 2022;128(1):78-88. doi:10.1016/j.anai.2021.09.025
Two-year dupilumab data: Continued response for moderate to severe pediatric asthma
NASHVILLE, TENN. – For children with uncontrolled asthma on standard therapies and meeting criteria of a type 2 (T2) inflammatory phenotype, a prospective 1-year extension from a phase 3 trial supports the biologic dupilumab as a potential treatment standard, according to the investigator who presented the findings at the annual meeting of the American College of Chest Physicians (CHEST).
said Leonard B. Bacharier, MD, section chief, division of pediatric allergy, immunology, and pulmonary medicine, Vanderbilt University Medical Center, Nashville, Tenn.
By T2 inflammatory phenotype, Dr. Bacharier specified that key features include an eosinophil count of at least 150 cells/mL and a FENO level of at least 20 ppb. If children meet these and inadequate standard-therapy response criteria, Dr. Bacharier thinks the extension data support dupilumab as a routine therapy despite the cost.
“As a pediatrician, I think it is really important that children with asthma finish their childhood with the best bone health and the lowest risk of other steroid-associated adverse events,” Dr. Bacharier said.
Over the course of the 1-year extension, called EXCURSION, there was no evidence of diminished efficacy nor of any new safety signal. In other words, patients have remained well controlled for 2 years with a well-tolerated therapy. Dr. Bacharier pointed out, however, that one of the most compelling reasons to consider this as a potential standard was the very low rates at which patients required a course of steroids.
At the end of 1 year in the extension trial, called VOYAGE, the unadjusted annualized total number of steroid courses per patient was 0.414 in the dupilumab group vs. 0.816 in the placebo group. At the end of EXCURSION, following an additional year of therapy, the rate was 0.152.
“This means that fewer than 2 patients out of 10 required prednisone in the previous year,” Dr. Bacharier said.
The EXCURSION extension study did not capture data on steroid-related adverse events, but Dr. Bacharier said that these data are reassuring for both acute and long-term risks of steroid exposure.
“We know that the adverse effects associated with oral steroids are related to cumulative exposure. The more you receive, the greater the risk of adverse effects,” he said.
In patients who were randomly assigned to placebo in the VOYAGE trial and then switched to dupilumab in the EXCURSION extension, steroid exposure was also very low, but whether evaluated as annualized total courses (0.152 vs. 0.181) or by proportion of patients with any steroid intake (10.5% vs. 13.2%), there was a numerical advantage for starting and remaining on dupilumab over the 2-year follow-up.
In VOYAGE, which was published last year in the New England Journal of Medicine, 408 children from ages 6 to 11 years were randomly assigned in a 2:1 ratio to dupilumab or matching placebo. For children weighing less than 30 kg, the dose was 200 mg. For those who weighed less, the dose was 100 mg. Both doses were administered every 2 weeks.
As previously reported, the study met the primary endpoint of annualized rate of severe asthma exacerbations, which was 0.31 in the dupilumab group vs. 0.75 in the placebo group, a relative reduction of 59.3% (P < .001). Dupilumab was also superior on several secondary endpoints, including measures of lung function and asthma control.
The EXCURSION extension study enrolled 365 of the patients who participated in VOYAGE. This included 125 of the 135 randomly assigned to placebo and 240 of the 273 randomly assigned to dupilumab. Those initially randomly assigned to placebo were transitioned to dupilumab. The same weight-based dosing was employed.
At baseline, the children enrolled in VOYAGE had an annualized rate of 2.560 severe exacerbations. At the end of VOYAGE, this rate was 0.330. At the end of EXCURSION after 2 years on dupilumab, the rate was 0.118. In the group switched from placebo to dupilumab, the rate was 0.124.
During EXCURSION, treatment-emergent adverse events occurred in 2.5% of those who remained on dupilumab and 0.8% of those switched from placebo to dupilumab. Three patients (1.3%) permanently discontinued therapy because of a treatment-related event. The most common adverse events involved upper respiratory complaints, such as nasopharyngitis, pharyngitis, upper respiratory tract infections, and rhinitis influenza, but all were reported in fewer than 10% of patients. Other reported side effects, such as injection-site reactions and diarrhea, occurred in 5% or fewer of patients.
“Over the 2 years, dupilumab was well tolerated, and there was evidence of an increased risk of adverse events for longer exposure,” Dr. Bacharier reported.
It is for this reason that Dr. Bacharier concluded that children with repeated exacerbations requiring steroids despite standard therapies should be considered for dupilumab if they also meet criteria for the T2 inflammatory phenotype. This last point is important.
“In children with low levels of eosinophil and low phenol, we are not seeing these kinds of response,” Dr. Bacharier said. Rather, in the absence of eosinophilia, “there is probably no difference between dupilumab and placebo.”
An important steroid-sparing effect is “suggested” by the data, but Sally E. Wenzel, MD, director of the University of Pittsburgh Asthma and Environmental Lung Health Institute in Pittsburgh, characterized the idea that dupilumab is emerging to be a standard in uncontrolled asthma in children with the T2 phenotype as “a bit premature.”
She challenged the conclusion that the EXCURSION data associated dupilumab with a reduction in annualized steroid courses over time. While the number was lower after 2 years of treatment than after 1, Dr. Wenzel pointed out that all patients were on dupilumab in the second year, “so we don’t know what really happens without treatment.” She said there are other potential explanations, including the possibility that aging children have less active disease.
More importantly, Dr. Wenzel said in an interview that she would also hesitate to urge biologics in every child who meets the criteria that Dr. Bacharier outlined.
“The most important concern is that we do not know how long one should continue the dupilumab and if the long-term treatment adversely or positively affects a growing immune system,” she said.
There is reason to be concerned that blockage of an entire immune pathway with a biologic could adversely affect autoimmunity as well as susceptibility to cancer, according to Dr. Wenzel. She hopes this does not prove to be the case, but she encouraged prudence until there are more data to judge.
While extension data for dupilumab “sound good,” she thinks moving toward any type of standard of care with biologics in children “has to be done with caution and constant evaluation and reevaluation.”
Dr. Bacharier disclosed relationships with AstraZeneca, GlaxoSmithKline, Regeneron and Sanofi. The two latter companies collaborated on the development and marketing of dupilumab. Dr. Wenzel disclosed relationships with AstraZeneca, GlaxoSmithKline, Knopp Pharmaceuticals, Pieris, and Sanofi-Regeneron.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – For children with uncontrolled asthma on standard therapies and meeting criteria of a type 2 (T2) inflammatory phenotype, a prospective 1-year extension from a phase 3 trial supports the biologic dupilumab as a potential treatment standard, according to the investigator who presented the findings at the annual meeting of the American College of Chest Physicians (CHEST).
said Leonard B. Bacharier, MD, section chief, division of pediatric allergy, immunology, and pulmonary medicine, Vanderbilt University Medical Center, Nashville, Tenn.
By T2 inflammatory phenotype, Dr. Bacharier specified that key features include an eosinophil count of at least 150 cells/mL and a FENO level of at least 20 ppb. If children meet these and inadequate standard-therapy response criteria, Dr. Bacharier thinks the extension data support dupilumab as a routine therapy despite the cost.
“As a pediatrician, I think it is really important that children with asthma finish their childhood with the best bone health and the lowest risk of other steroid-associated adverse events,” Dr. Bacharier said.
Over the course of the 1-year extension, called EXCURSION, there was no evidence of diminished efficacy nor of any new safety signal. In other words, patients have remained well controlled for 2 years with a well-tolerated therapy. Dr. Bacharier pointed out, however, that one of the most compelling reasons to consider this as a potential standard was the very low rates at which patients required a course of steroids.
At the end of 1 year in the extension trial, called VOYAGE, the unadjusted annualized total number of steroid courses per patient was 0.414 in the dupilumab group vs. 0.816 in the placebo group. At the end of EXCURSION, following an additional year of therapy, the rate was 0.152.
“This means that fewer than 2 patients out of 10 required prednisone in the previous year,” Dr. Bacharier said.
The EXCURSION extension study did not capture data on steroid-related adverse events, but Dr. Bacharier said that these data are reassuring for both acute and long-term risks of steroid exposure.
“We know that the adverse effects associated with oral steroids are related to cumulative exposure. The more you receive, the greater the risk of adverse effects,” he said.
In patients who were randomly assigned to placebo in the VOYAGE trial and then switched to dupilumab in the EXCURSION extension, steroid exposure was also very low, but whether evaluated as annualized total courses (0.152 vs. 0.181) or by proportion of patients with any steroid intake (10.5% vs. 13.2%), there was a numerical advantage for starting and remaining on dupilumab over the 2-year follow-up.
In VOYAGE, which was published last year in the New England Journal of Medicine, 408 children from ages 6 to 11 years were randomly assigned in a 2:1 ratio to dupilumab or matching placebo. For children weighing less than 30 kg, the dose was 200 mg. For those who weighed less, the dose was 100 mg. Both doses were administered every 2 weeks.
As previously reported, the study met the primary endpoint of annualized rate of severe asthma exacerbations, which was 0.31 in the dupilumab group vs. 0.75 in the placebo group, a relative reduction of 59.3% (P < .001). Dupilumab was also superior on several secondary endpoints, including measures of lung function and asthma control.
The EXCURSION extension study enrolled 365 of the patients who participated in VOYAGE. This included 125 of the 135 randomly assigned to placebo and 240 of the 273 randomly assigned to dupilumab. Those initially randomly assigned to placebo were transitioned to dupilumab. The same weight-based dosing was employed.
At baseline, the children enrolled in VOYAGE had an annualized rate of 2.560 severe exacerbations. At the end of VOYAGE, this rate was 0.330. At the end of EXCURSION after 2 years on dupilumab, the rate was 0.118. In the group switched from placebo to dupilumab, the rate was 0.124.
During EXCURSION, treatment-emergent adverse events occurred in 2.5% of those who remained on dupilumab and 0.8% of those switched from placebo to dupilumab. Three patients (1.3%) permanently discontinued therapy because of a treatment-related event. The most common adverse events involved upper respiratory complaints, such as nasopharyngitis, pharyngitis, upper respiratory tract infections, and rhinitis influenza, but all were reported in fewer than 10% of patients. Other reported side effects, such as injection-site reactions and diarrhea, occurred in 5% or fewer of patients.
“Over the 2 years, dupilumab was well tolerated, and there was evidence of an increased risk of adverse events for longer exposure,” Dr. Bacharier reported.
It is for this reason that Dr. Bacharier concluded that children with repeated exacerbations requiring steroids despite standard therapies should be considered for dupilumab if they also meet criteria for the T2 inflammatory phenotype. This last point is important.
“In children with low levels of eosinophil and low phenol, we are not seeing these kinds of response,” Dr. Bacharier said. Rather, in the absence of eosinophilia, “there is probably no difference between dupilumab and placebo.”
An important steroid-sparing effect is “suggested” by the data, but Sally E. Wenzel, MD, director of the University of Pittsburgh Asthma and Environmental Lung Health Institute in Pittsburgh, characterized the idea that dupilumab is emerging to be a standard in uncontrolled asthma in children with the T2 phenotype as “a bit premature.”
She challenged the conclusion that the EXCURSION data associated dupilumab with a reduction in annualized steroid courses over time. While the number was lower after 2 years of treatment than after 1, Dr. Wenzel pointed out that all patients were on dupilumab in the second year, “so we don’t know what really happens without treatment.” She said there are other potential explanations, including the possibility that aging children have less active disease.
More importantly, Dr. Wenzel said in an interview that she would also hesitate to urge biologics in every child who meets the criteria that Dr. Bacharier outlined.
“The most important concern is that we do not know how long one should continue the dupilumab and if the long-term treatment adversely or positively affects a growing immune system,” she said.
There is reason to be concerned that blockage of an entire immune pathway with a biologic could adversely affect autoimmunity as well as susceptibility to cancer, according to Dr. Wenzel. She hopes this does not prove to be the case, but she encouraged prudence until there are more data to judge.
While extension data for dupilumab “sound good,” she thinks moving toward any type of standard of care with biologics in children “has to be done with caution and constant evaluation and reevaluation.”
Dr. Bacharier disclosed relationships with AstraZeneca, GlaxoSmithKline, Regeneron and Sanofi. The two latter companies collaborated on the development and marketing of dupilumab. Dr. Wenzel disclosed relationships with AstraZeneca, GlaxoSmithKline, Knopp Pharmaceuticals, Pieris, and Sanofi-Regeneron.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – For children with uncontrolled asthma on standard therapies and meeting criteria of a type 2 (T2) inflammatory phenotype, a prospective 1-year extension from a phase 3 trial supports the biologic dupilumab as a potential treatment standard, according to the investigator who presented the findings at the annual meeting of the American College of Chest Physicians (CHEST).
said Leonard B. Bacharier, MD, section chief, division of pediatric allergy, immunology, and pulmonary medicine, Vanderbilt University Medical Center, Nashville, Tenn.
By T2 inflammatory phenotype, Dr. Bacharier specified that key features include an eosinophil count of at least 150 cells/mL and a FENO level of at least 20 ppb. If children meet these and inadequate standard-therapy response criteria, Dr. Bacharier thinks the extension data support dupilumab as a routine therapy despite the cost.
“As a pediatrician, I think it is really important that children with asthma finish their childhood with the best bone health and the lowest risk of other steroid-associated adverse events,” Dr. Bacharier said.
Over the course of the 1-year extension, called EXCURSION, there was no evidence of diminished efficacy nor of any new safety signal. In other words, patients have remained well controlled for 2 years with a well-tolerated therapy. Dr. Bacharier pointed out, however, that one of the most compelling reasons to consider this as a potential standard was the very low rates at which patients required a course of steroids.
At the end of 1 year in the extension trial, called VOYAGE, the unadjusted annualized total number of steroid courses per patient was 0.414 in the dupilumab group vs. 0.816 in the placebo group. At the end of EXCURSION, following an additional year of therapy, the rate was 0.152.
“This means that fewer than 2 patients out of 10 required prednisone in the previous year,” Dr. Bacharier said.
The EXCURSION extension study did not capture data on steroid-related adverse events, but Dr. Bacharier said that these data are reassuring for both acute and long-term risks of steroid exposure.
“We know that the adverse effects associated with oral steroids are related to cumulative exposure. The more you receive, the greater the risk of adverse effects,” he said.
In patients who were randomly assigned to placebo in the VOYAGE trial and then switched to dupilumab in the EXCURSION extension, steroid exposure was also very low, but whether evaluated as annualized total courses (0.152 vs. 0.181) or by proportion of patients with any steroid intake (10.5% vs. 13.2%), there was a numerical advantage for starting and remaining on dupilumab over the 2-year follow-up.
In VOYAGE, which was published last year in the New England Journal of Medicine, 408 children from ages 6 to 11 years were randomly assigned in a 2:1 ratio to dupilumab or matching placebo. For children weighing less than 30 kg, the dose was 200 mg. For those who weighed less, the dose was 100 mg. Both doses were administered every 2 weeks.
As previously reported, the study met the primary endpoint of annualized rate of severe asthma exacerbations, which was 0.31 in the dupilumab group vs. 0.75 in the placebo group, a relative reduction of 59.3% (P < .001). Dupilumab was also superior on several secondary endpoints, including measures of lung function and asthma control.
The EXCURSION extension study enrolled 365 of the patients who participated in VOYAGE. This included 125 of the 135 randomly assigned to placebo and 240 of the 273 randomly assigned to dupilumab. Those initially randomly assigned to placebo were transitioned to dupilumab. The same weight-based dosing was employed.
At baseline, the children enrolled in VOYAGE had an annualized rate of 2.560 severe exacerbations. At the end of VOYAGE, this rate was 0.330. At the end of EXCURSION after 2 years on dupilumab, the rate was 0.118. In the group switched from placebo to dupilumab, the rate was 0.124.
During EXCURSION, treatment-emergent adverse events occurred in 2.5% of those who remained on dupilumab and 0.8% of those switched from placebo to dupilumab. Three patients (1.3%) permanently discontinued therapy because of a treatment-related event. The most common adverse events involved upper respiratory complaints, such as nasopharyngitis, pharyngitis, upper respiratory tract infections, and rhinitis influenza, but all were reported in fewer than 10% of patients. Other reported side effects, such as injection-site reactions and diarrhea, occurred in 5% or fewer of patients.
“Over the 2 years, dupilumab was well tolerated, and there was evidence of an increased risk of adverse events for longer exposure,” Dr. Bacharier reported.
It is for this reason that Dr. Bacharier concluded that children with repeated exacerbations requiring steroids despite standard therapies should be considered for dupilumab if they also meet criteria for the T2 inflammatory phenotype. This last point is important.
“In children with low levels of eosinophil and low phenol, we are not seeing these kinds of response,” Dr. Bacharier said. Rather, in the absence of eosinophilia, “there is probably no difference between dupilumab and placebo.”
An important steroid-sparing effect is “suggested” by the data, but Sally E. Wenzel, MD, director of the University of Pittsburgh Asthma and Environmental Lung Health Institute in Pittsburgh, characterized the idea that dupilumab is emerging to be a standard in uncontrolled asthma in children with the T2 phenotype as “a bit premature.”
She challenged the conclusion that the EXCURSION data associated dupilumab with a reduction in annualized steroid courses over time. While the number was lower after 2 years of treatment than after 1, Dr. Wenzel pointed out that all patients were on dupilumab in the second year, “so we don’t know what really happens without treatment.” She said there are other potential explanations, including the possibility that aging children have less active disease.
More importantly, Dr. Wenzel said in an interview that she would also hesitate to urge biologics in every child who meets the criteria that Dr. Bacharier outlined.
“The most important concern is that we do not know how long one should continue the dupilumab and if the long-term treatment adversely or positively affects a growing immune system,” she said.
There is reason to be concerned that blockage of an entire immune pathway with a biologic could adversely affect autoimmunity as well as susceptibility to cancer, according to Dr. Wenzel. She hopes this does not prove to be the case, but she encouraged prudence until there are more data to judge.
While extension data for dupilumab “sound good,” she thinks moving toward any type of standard of care with biologics in children “has to be done with caution and constant evaluation and reevaluation.”
Dr. Bacharier disclosed relationships with AstraZeneca, GlaxoSmithKline, Regeneron and Sanofi. The two latter companies collaborated on the development and marketing of dupilumab. Dr. Wenzel disclosed relationships with AstraZeneca, GlaxoSmithKline, Knopp Pharmaceuticals, Pieris, and Sanofi-Regeneron.
A version of this article first appeared on Medscape.com.
AT CHEST 2022
Asthma ED visits predict failed housing inspections
, according to a new study presented at the annual meeting of the American College of Emergency Physicians.
While links between asthma and low-quality housing prone to harboring allergens have been well-documented, the current study takes the extra step of looking at housing down to the level of individual land parcels and suggests that asthma hospital visits can be used to identify hazardous housing earlier.
“Emergency department visits for asthma provide a leading indicator that can be used by health departments or housing authorities to direct housing inspections and remediation of poor housing conditions, track improvements in housing quality, measure housing department performance, support resident grievances, and inform funding allocation decisions,” said the study’s lead researcher, Elizabeth Samuels, MD, who is assistant professor of epidemiology and emergency medicine at Brown University, Providence, R.I.
Researchers retrospectively looked at cases of children and adults in the Greater New Haven area of Connecticut seen at the Yale New Haven Hospital ED for asthma-related problems between March 2013 and August 2017. The region has the fifth-highest prevalence of asthma in the United States, the researchers point out, due to its air quality, pollens, and quality of its housing. More than half of residences were built before 1,940, compared with about 13% nationally. Patient addresses were matched with HUD inspection records.
The review encompassed 11,429 ED visits by 6,366 individuals; 54% were insured by Medicaid, and 42% were Black. Controlling for patient and neighborhood data, researchers found that increased asthma ED visits at the parcel level were associated with decreased HUD inspection scores to a highly significant degree (P < .001).
They also found that there was a relationship in terms of timing between asthma ED visits and inspection scores: asthma ED visits increased more than 1 year before a failed HUD inspection. They also found that asthma ED visits were not elevated at housing units that passed inspection. Using asthma ED visits to predict failed housing inspections produced a specificity rate of 92.3% in an adjusted model, Dr. Samuels noted.
“This approach represents a novel method of early identification of dangerous housing conditions, which could aid in the prevention of asthma-related morbidity and mortality,” Dr. Samuels said.
The investigators noted that, of the parcels with the top three incidence rates of asthma ED visits, “all of them have been closed or demolished.”
In addition to limiting exposure of patients with asthma to the allergens of mold, mice and rats, and cockroaches, improving poor-quality housing earlier could help asthma by reducing stress, she said.
“There is also an increasing evidence base that psychosocial stress increases the risk of asthma attacks, and it’s therefore possible that living in poor housing conditions – often highly stressful situations – drives exacerbation risk via this pathway,” she said. “Synergistic effects between these pathways are also possible or even likely.”
Neeta Thakur, MD, associate professor of medicine at the University of California, San Francisco, who researches asthma, said the findings could lead to a strategy for improving poor-quality housing more quickly. As it is, inspections are too infrequent, often prompted by resident complaints.
“Once the complaints get to a certain threshold, then there might be an inspection that happens, and if there is a periodic review of the buildings, they often happen few and far between,” she said. “We could actually use some of the information that we’re already getting from something like ED visits and see if there is a pattern.”
An important follow-up would be to see whether asthma outcomes improve after housing deficiencies are addressed and whether the predictive capacity of ED visits occurs in other places.
“Would you then see a decline in the ED visit rates from individuals living in those buildings?” Dr. Thakur said. “It’s important to find a leading indicator, but you want to be sure that that leading indicator is useful as something that can be intervened upon.”
Dr. Samuels and Dr. Thakur have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new study presented at the annual meeting of the American College of Emergency Physicians.
While links between asthma and low-quality housing prone to harboring allergens have been well-documented, the current study takes the extra step of looking at housing down to the level of individual land parcels and suggests that asthma hospital visits can be used to identify hazardous housing earlier.
“Emergency department visits for asthma provide a leading indicator that can be used by health departments or housing authorities to direct housing inspections and remediation of poor housing conditions, track improvements in housing quality, measure housing department performance, support resident grievances, and inform funding allocation decisions,” said the study’s lead researcher, Elizabeth Samuels, MD, who is assistant professor of epidemiology and emergency medicine at Brown University, Providence, R.I.
Researchers retrospectively looked at cases of children and adults in the Greater New Haven area of Connecticut seen at the Yale New Haven Hospital ED for asthma-related problems between March 2013 and August 2017. The region has the fifth-highest prevalence of asthma in the United States, the researchers point out, due to its air quality, pollens, and quality of its housing. More than half of residences were built before 1,940, compared with about 13% nationally. Patient addresses were matched with HUD inspection records.
The review encompassed 11,429 ED visits by 6,366 individuals; 54% were insured by Medicaid, and 42% were Black. Controlling for patient and neighborhood data, researchers found that increased asthma ED visits at the parcel level were associated with decreased HUD inspection scores to a highly significant degree (P < .001).
They also found that there was a relationship in terms of timing between asthma ED visits and inspection scores: asthma ED visits increased more than 1 year before a failed HUD inspection. They also found that asthma ED visits were not elevated at housing units that passed inspection. Using asthma ED visits to predict failed housing inspections produced a specificity rate of 92.3% in an adjusted model, Dr. Samuels noted.
“This approach represents a novel method of early identification of dangerous housing conditions, which could aid in the prevention of asthma-related morbidity and mortality,” Dr. Samuels said.
The investigators noted that, of the parcels with the top three incidence rates of asthma ED visits, “all of them have been closed or demolished.”
In addition to limiting exposure of patients with asthma to the allergens of mold, mice and rats, and cockroaches, improving poor-quality housing earlier could help asthma by reducing stress, she said.
“There is also an increasing evidence base that psychosocial stress increases the risk of asthma attacks, and it’s therefore possible that living in poor housing conditions – often highly stressful situations – drives exacerbation risk via this pathway,” she said. “Synergistic effects between these pathways are also possible or even likely.”
Neeta Thakur, MD, associate professor of medicine at the University of California, San Francisco, who researches asthma, said the findings could lead to a strategy for improving poor-quality housing more quickly. As it is, inspections are too infrequent, often prompted by resident complaints.
“Once the complaints get to a certain threshold, then there might be an inspection that happens, and if there is a periodic review of the buildings, they often happen few and far between,” she said. “We could actually use some of the information that we’re already getting from something like ED visits and see if there is a pattern.”
An important follow-up would be to see whether asthma outcomes improve after housing deficiencies are addressed and whether the predictive capacity of ED visits occurs in other places.
“Would you then see a decline in the ED visit rates from individuals living in those buildings?” Dr. Thakur said. “It’s important to find a leading indicator, but you want to be sure that that leading indicator is useful as something that can be intervened upon.”
Dr. Samuels and Dr. Thakur have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new study presented at the annual meeting of the American College of Emergency Physicians.
While links between asthma and low-quality housing prone to harboring allergens have been well-documented, the current study takes the extra step of looking at housing down to the level of individual land parcels and suggests that asthma hospital visits can be used to identify hazardous housing earlier.
“Emergency department visits for asthma provide a leading indicator that can be used by health departments or housing authorities to direct housing inspections and remediation of poor housing conditions, track improvements in housing quality, measure housing department performance, support resident grievances, and inform funding allocation decisions,” said the study’s lead researcher, Elizabeth Samuels, MD, who is assistant professor of epidemiology and emergency medicine at Brown University, Providence, R.I.
Researchers retrospectively looked at cases of children and adults in the Greater New Haven area of Connecticut seen at the Yale New Haven Hospital ED for asthma-related problems between March 2013 and August 2017. The region has the fifth-highest prevalence of asthma in the United States, the researchers point out, due to its air quality, pollens, and quality of its housing. More than half of residences were built before 1,940, compared with about 13% nationally. Patient addresses were matched with HUD inspection records.
The review encompassed 11,429 ED visits by 6,366 individuals; 54% were insured by Medicaid, and 42% were Black. Controlling for patient and neighborhood data, researchers found that increased asthma ED visits at the parcel level were associated with decreased HUD inspection scores to a highly significant degree (P < .001).
They also found that there was a relationship in terms of timing between asthma ED visits and inspection scores: asthma ED visits increased more than 1 year before a failed HUD inspection. They also found that asthma ED visits were not elevated at housing units that passed inspection. Using asthma ED visits to predict failed housing inspections produced a specificity rate of 92.3% in an adjusted model, Dr. Samuels noted.
“This approach represents a novel method of early identification of dangerous housing conditions, which could aid in the prevention of asthma-related morbidity and mortality,” Dr. Samuels said.
The investigators noted that, of the parcels with the top three incidence rates of asthma ED visits, “all of them have been closed or demolished.”
In addition to limiting exposure of patients with asthma to the allergens of mold, mice and rats, and cockroaches, improving poor-quality housing earlier could help asthma by reducing stress, she said.
“There is also an increasing evidence base that psychosocial stress increases the risk of asthma attacks, and it’s therefore possible that living in poor housing conditions – often highly stressful situations – drives exacerbation risk via this pathway,” she said. “Synergistic effects between these pathways are also possible or even likely.”
Neeta Thakur, MD, associate professor of medicine at the University of California, San Francisco, who researches asthma, said the findings could lead to a strategy for improving poor-quality housing more quickly. As it is, inspections are too infrequent, often prompted by resident complaints.
“Once the complaints get to a certain threshold, then there might be an inspection that happens, and if there is a periodic review of the buildings, they often happen few and far between,” she said. “We could actually use some of the information that we’re already getting from something like ED visits and see if there is a pattern.”
An important follow-up would be to see whether asthma outcomes improve after housing deficiencies are addressed and whether the predictive capacity of ED visits occurs in other places.
“Would you then see a decline in the ED visit rates from individuals living in those buildings?” Dr. Thakur said. “It’s important to find a leading indicator, but you want to be sure that that leading indicator is useful as something that can be intervened upon.”
Dr. Samuels and Dr. Thakur have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACEP 2022
Climate change magnifies health effects of wildfire smoke in care deserts
DRESSLERVILLE, NEV. – Smoke began billowing into the skies of northwestern Nevada in September, clouding the mountains, dimming the sun – and quashing residents’ hopes that they would be spared from wildfires and the awful air quality the blazes produce.
The lung-irritating particles were blowing in from burning forests in California and settling in Douglas County, Nevada, home to nearly 50,000 people, prompting warnings that air quality had reached hazardous levels.
Those levels meant the air was very unhealthy, bad enough to raise alarms about people’s immediate health care needs and questions about whether worsening pollution could result in long-term health issues. People could increasingly face such risks as climate change makes wildfires, drought, dust storms, and floods more frequent across the United States and the world.
Some people simply feel powerless.
“There’s not much we could do about it,” said Serrell Smokey, chairman of the Washoe Tribe of Nevada and California. The tribe’s land straddles the border between California and Nevada near Lake Tahoe and extends into Douglas County, about 60 miles south of Reno.
Tribe members and other area residents are among millions of people nationwide who this year will experience poor air quality because of wildfires. In September, as smoke settled over Nevada, fire-related air quality alerts were dispatched in six other states: California, Idaho, Montana, Oregon, Washington, and Wyoming.
Yet, by one measure, people who live in Douglas County are better off than those in some other hard-hit areas. Douglas County residents must drive 30 minutes, on average, for medical care from lung specialists called pulmonologists. In other parts of the West and Upper Midwest, however, patients must drive an hour or more, according to data analyzed by GoodRx, a website that tracks prescription drug prices and conducts research.
Specifically, the research found that about 5.5 million Americans live in the 488 counties where drive times to pulmonologists are an hour or more. Much of Nevada and large parts of Montana fall into those gaps between specialists – places that have recently grappled with wildfires that fill the air with smoke and ash, which can cause lung problems or exacerbate existing ones.
Data from the Association of American Medical Colleges shows the number of pulmonary disease specialists in the United States dropped nearly 11% from 2014 to 2019. The group, which is based in Washington, D.C., and represents the academic medicine community, noted that the decline might not be as high as it appears because some physicians are opting to practice pulmonary critical care rather than just pulmonology. Many of those types of pulmonologists work in hospital intensive care units.
About 15,000 pulmonologists are practicing in the United States, according to the GoodRx report. Yet vast swaths of the country have few or none.
“New Mexico has one pulmonologist for the entire southeastern part of state, not counting Las Cruces, which is closer to El Paso,” said Victor Test, MD, a pulmonologist at Texas Tech Physicians.
Dr. Test, one of 13 pulmonologists in the Lubbock, Tex., region, said that his patients from within Texas sometimes drive 4 hours for an appointment and that other people travel from “New Mexico, Oklahoma, even far western Kansas.”
Increases in wildfires and their intensity will likely expand the need for pulmonologists.
“Climate change is going to affect lung disease,” said Nicholas Kenyon, MD, a professor of pulmonary, critical care, and sleep medicine at the University of California, Davis, where he and several other researchers are tracking the effects of wildfires. At his Sacramento practice, Dr. Kenyon said, he sees patients from far northern parts of California, including Eureka, a 5-hour drive from the state capital.
The short-term effects of breathing smoke are pretty well known. People show up in emergency rooms with asthma attacks, exacerbation of COPD, bronchitis, and even pneumonia, Dr. Kenyon said. Some have chest pain or other cardiac concerns.
“But we have very little understanding of what happens over the longer term,” he said. “If people get 2 or 3 weeks of wildfire exposure for 2 or 3 years, does that lead to worsening of asthma or COPD? We just don’t know.”
Fires release multiple pollutants, including carbon dioxide, carbon monoxide, and chemicals like benzene. All fires send particles into the air. Health researchers and air quality experts are most concerned about tiny pieces referred to as particulate matter 2.5. Far smaller than a human hair, the particles can lodge deep in the lungs and have been linked to heart and lung conditions.
Increases in those tiny particles are associated with a greater risk of death from all causes, excluding accidents, homicides, and other nonaccidental causes, for up to 4 days after a population is exposed, according to a 2020 New England Journal of Medicine overview.
The concentration of fine particulate matter is one of five gauges used to calculate the Air Quality Index, a numerical and color-coded index used to let the public know about local air pollution levels. Green denotes good air quality and is given if the total index is 50 or less. When the measurement exceeds 100, the air quality gets an orange label and may be bad for certain groups. Levels over 200 get a red label and are considered unhealthy for everyone.
Government agencies track those levels, as do people who use apps or websites to determine whether it’s safe to go outside.
When the AQI rises above 150, Farah Madhani-Lovely, MD, a pulmonologist, said, Renown Regional Medical Center in Reno shuts its outpatient pulmonary rehabilitation clinic because it doesn’t want to encourage patients to drive in. Some patients from Douglas County opt for care near home, about an hour away. “We don’t want these patients exposed outside because just 1 minute of exposure to the smoke can trigger an exacerbation of their chronic disease,” Dr. Madhani-Lovely said.
Mr. Smokey said connecting with pulmonologists can be difficult for Washoe Tribe members, particularly those who live on the California side of the reservation. “We cannot find providers for them,” he said. “We end up referring them out and sending them hundreds of miles out of their way just to get care that we should be able to provide here.”
Recruiting specialists to rural areas or smaller cities has long been difficult. For one thing, a specialist might be the only one for miles around, “so there’s a tremendous burden in terms of coverage and days off,” Dr. Test said.
Another concern is that physicians tend to train in larger cities and often want to practice in similar places. Even recruiting pulmonary physicians to Lubbock, a city of 260,000 in West Texas, is a challenge, Dr. Test said.
“I love Lubbock,” he said. “But I tell people who have never been here, I say, ‘It’s really flat.’ They don’t understand flat until they get here.”
In Nevada, on days when the air quality is bad, Washoe tribal members try to protect themselves with makeshift air purifiers created from fans, duct tape, and air filters, Mr. Smokey said.
Longer term, Mr. Smokey and other tribal leaders are pushing the Indian Health Service to establish a specialty care hospital in northern Nevada. The closest specialty care hospital for Washoe tribal members is more than 700 miles away, in Phoenix.
It’s difficult because “there’s a need we should be taking care of,” Mr. Smokey said. “But we have to fight for it. And sometimes that fight takes years, years, and years to accomplish.”
A version of this article first appeared on Medscape.com.
DRESSLERVILLE, NEV. – Smoke began billowing into the skies of northwestern Nevada in September, clouding the mountains, dimming the sun – and quashing residents’ hopes that they would be spared from wildfires and the awful air quality the blazes produce.
The lung-irritating particles were blowing in from burning forests in California and settling in Douglas County, Nevada, home to nearly 50,000 people, prompting warnings that air quality had reached hazardous levels.
Those levels meant the air was very unhealthy, bad enough to raise alarms about people’s immediate health care needs and questions about whether worsening pollution could result in long-term health issues. People could increasingly face such risks as climate change makes wildfires, drought, dust storms, and floods more frequent across the United States and the world.
Some people simply feel powerless.
“There’s not much we could do about it,” said Serrell Smokey, chairman of the Washoe Tribe of Nevada and California. The tribe’s land straddles the border between California and Nevada near Lake Tahoe and extends into Douglas County, about 60 miles south of Reno.
Tribe members and other area residents are among millions of people nationwide who this year will experience poor air quality because of wildfires. In September, as smoke settled over Nevada, fire-related air quality alerts were dispatched in six other states: California, Idaho, Montana, Oregon, Washington, and Wyoming.
Yet, by one measure, people who live in Douglas County are better off than those in some other hard-hit areas. Douglas County residents must drive 30 minutes, on average, for medical care from lung specialists called pulmonologists. In other parts of the West and Upper Midwest, however, patients must drive an hour or more, according to data analyzed by GoodRx, a website that tracks prescription drug prices and conducts research.
Specifically, the research found that about 5.5 million Americans live in the 488 counties where drive times to pulmonologists are an hour or more. Much of Nevada and large parts of Montana fall into those gaps between specialists – places that have recently grappled with wildfires that fill the air with smoke and ash, which can cause lung problems or exacerbate existing ones.
Data from the Association of American Medical Colleges shows the number of pulmonary disease specialists in the United States dropped nearly 11% from 2014 to 2019. The group, which is based in Washington, D.C., and represents the academic medicine community, noted that the decline might not be as high as it appears because some physicians are opting to practice pulmonary critical care rather than just pulmonology. Many of those types of pulmonologists work in hospital intensive care units.
About 15,000 pulmonologists are practicing in the United States, according to the GoodRx report. Yet vast swaths of the country have few or none.
“New Mexico has one pulmonologist for the entire southeastern part of state, not counting Las Cruces, which is closer to El Paso,” said Victor Test, MD, a pulmonologist at Texas Tech Physicians.
Dr. Test, one of 13 pulmonologists in the Lubbock, Tex., region, said that his patients from within Texas sometimes drive 4 hours for an appointment and that other people travel from “New Mexico, Oklahoma, even far western Kansas.”
Increases in wildfires and their intensity will likely expand the need for pulmonologists.
“Climate change is going to affect lung disease,” said Nicholas Kenyon, MD, a professor of pulmonary, critical care, and sleep medicine at the University of California, Davis, where he and several other researchers are tracking the effects of wildfires. At his Sacramento practice, Dr. Kenyon said, he sees patients from far northern parts of California, including Eureka, a 5-hour drive from the state capital.
The short-term effects of breathing smoke are pretty well known. People show up in emergency rooms with asthma attacks, exacerbation of COPD, bronchitis, and even pneumonia, Dr. Kenyon said. Some have chest pain or other cardiac concerns.
“But we have very little understanding of what happens over the longer term,” he said. “If people get 2 or 3 weeks of wildfire exposure for 2 or 3 years, does that lead to worsening of asthma or COPD? We just don’t know.”
Fires release multiple pollutants, including carbon dioxide, carbon monoxide, and chemicals like benzene. All fires send particles into the air. Health researchers and air quality experts are most concerned about tiny pieces referred to as particulate matter 2.5. Far smaller than a human hair, the particles can lodge deep in the lungs and have been linked to heart and lung conditions.
Increases in those tiny particles are associated with a greater risk of death from all causes, excluding accidents, homicides, and other nonaccidental causes, for up to 4 days after a population is exposed, according to a 2020 New England Journal of Medicine overview.
The concentration of fine particulate matter is one of five gauges used to calculate the Air Quality Index, a numerical and color-coded index used to let the public know about local air pollution levels. Green denotes good air quality and is given if the total index is 50 or less. When the measurement exceeds 100, the air quality gets an orange label and may be bad for certain groups. Levels over 200 get a red label and are considered unhealthy for everyone.
Government agencies track those levels, as do people who use apps or websites to determine whether it’s safe to go outside.
When the AQI rises above 150, Farah Madhani-Lovely, MD, a pulmonologist, said, Renown Regional Medical Center in Reno shuts its outpatient pulmonary rehabilitation clinic because it doesn’t want to encourage patients to drive in. Some patients from Douglas County opt for care near home, about an hour away. “We don’t want these patients exposed outside because just 1 minute of exposure to the smoke can trigger an exacerbation of their chronic disease,” Dr. Madhani-Lovely said.
Mr. Smokey said connecting with pulmonologists can be difficult for Washoe Tribe members, particularly those who live on the California side of the reservation. “We cannot find providers for them,” he said. “We end up referring them out and sending them hundreds of miles out of their way just to get care that we should be able to provide here.”
Recruiting specialists to rural areas or smaller cities has long been difficult. For one thing, a specialist might be the only one for miles around, “so there’s a tremendous burden in terms of coverage and days off,” Dr. Test said.
Another concern is that physicians tend to train in larger cities and often want to practice in similar places. Even recruiting pulmonary physicians to Lubbock, a city of 260,000 in West Texas, is a challenge, Dr. Test said.
“I love Lubbock,” he said. “But I tell people who have never been here, I say, ‘It’s really flat.’ They don’t understand flat until they get here.”
In Nevada, on days when the air quality is bad, Washoe tribal members try to protect themselves with makeshift air purifiers created from fans, duct tape, and air filters, Mr. Smokey said.
Longer term, Mr. Smokey and other tribal leaders are pushing the Indian Health Service to establish a specialty care hospital in northern Nevada. The closest specialty care hospital for Washoe tribal members is more than 700 miles away, in Phoenix.
It’s difficult because “there’s a need we should be taking care of,” Mr. Smokey said. “But we have to fight for it. And sometimes that fight takes years, years, and years to accomplish.”
A version of this article first appeared on Medscape.com.
DRESSLERVILLE, NEV. – Smoke began billowing into the skies of northwestern Nevada in September, clouding the mountains, dimming the sun – and quashing residents’ hopes that they would be spared from wildfires and the awful air quality the blazes produce.
The lung-irritating particles were blowing in from burning forests in California and settling in Douglas County, Nevada, home to nearly 50,000 people, prompting warnings that air quality had reached hazardous levels.
Those levels meant the air was very unhealthy, bad enough to raise alarms about people’s immediate health care needs and questions about whether worsening pollution could result in long-term health issues. People could increasingly face such risks as climate change makes wildfires, drought, dust storms, and floods more frequent across the United States and the world.
Some people simply feel powerless.
“There’s not much we could do about it,” said Serrell Smokey, chairman of the Washoe Tribe of Nevada and California. The tribe’s land straddles the border between California and Nevada near Lake Tahoe and extends into Douglas County, about 60 miles south of Reno.
Tribe members and other area residents are among millions of people nationwide who this year will experience poor air quality because of wildfires. In September, as smoke settled over Nevada, fire-related air quality alerts were dispatched in six other states: California, Idaho, Montana, Oregon, Washington, and Wyoming.
Yet, by one measure, people who live in Douglas County are better off than those in some other hard-hit areas. Douglas County residents must drive 30 minutes, on average, for medical care from lung specialists called pulmonologists. In other parts of the West and Upper Midwest, however, patients must drive an hour or more, according to data analyzed by GoodRx, a website that tracks prescription drug prices and conducts research.
Specifically, the research found that about 5.5 million Americans live in the 488 counties where drive times to pulmonologists are an hour or more. Much of Nevada and large parts of Montana fall into those gaps between specialists – places that have recently grappled with wildfires that fill the air with smoke and ash, which can cause lung problems or exacerbate existing ones.
Data from the Association of American Medical Colleges shows the number of pulmonary disease specialists in the United States dropped nearly 11% from 2014 to 2019. The group, which is based in Washington, D.C., and represents the academic medicine community, noted that the decline might not be as high as it appears because some physicians are opting to practice pulmonary critical care rather than just pulmonology. Many of those types of pulmonologists work in hospital intensive care units.
About 15,000 pulmonologists are practicing in the United States, according to the GoodRx report. Yet vast swaths of the country have few or none.
“New Mexico has one pulmonologist for the entire southeastern part of state, not counting Las Cruces, which is closer to El Paso,” said Victor Test, MD, a pulmonologist at Texas Tech Physicians.
Dr. Test, one of 13 pulmonologists in the Lubbock, Tex., region, said that his patients from within Texas sometimes drive 4 hours for an appointment and that other people travel from “New Mexico, Oklahoma, even far western Kansas.”
Increases in wildfires and their intensity will likely expand the need for pulmonologists.
“Climate change is going to affect lung disease,” said Nicholas Kenyon, MD, a professor of pulmonary, critical care, and sleep medicine at the University of California, Davis, where he and several other researchers are tracking the effects of wildfires. At his Sacramento practice, Dr. Kenyon said, he sees patients from far northern parts of California, including Eureka, a 5-hour drive from the state capital.
The short-term effects of breathing smoke are pretty well known. People show up in emergency rooms with asthma attacks, exacerbation of COPD, bronchitis, and even pneumonia, Dr. Kenyon said. Some have chest pain or other cardiac concerns.
“But we have very little understanding of what happens over the longer term,” he said. “If people get 2 or 3 weeks of wildfire exposure for 2 or 3 years, does that lead to worsening of asthma or COPD? We just don’t know.”
Fires release multiple pollutants, including carbon dioxide, carbon monoxide, and chemicals like benzene. All fires send particles into the air. Health researchers and air quality experts are most concerned about tiny pieces referred to as particulate matter 2.5. Far smaller than a human hair, the particles can lodge deep in the lungs and have been linked to heart and lung conditions.
Increases in those tiny particles are associated with a greater risk of death from all causes, excluding accidents, homicides, and other nonaccidental causes, for up to 4 days after a population is exposed, according to a 2020 New England Journal of Medicine overview.
The concentration of fine particulate matter is one of five gauges used to calculate the Air Quality Index, a numerical and color-coded index used to let the public know about local air pollution levels. Green denotes good air quality and is given if the total index is 50 or less. When the measurement exceeds 100, the air quality gets an orange label and may be bad for certain groups. Levels over 200 get a red label and are considered unhealthy for everyone.
Government agencies track those levels, as do people who use apps or websites to determine whether it’s safe to go outside.
When the AQI rises above 150, Farah Madhani-Lovely, MD, a pulmonologist, said, Renown Regional Medical Center in Reno shuts its outpatient pulmonary rehabilitation clinic because it doesn’t want to encourage patients to drive in. Some patients from Douglas County opt for care near home, about an hour away. “We don’t want these patients exposed outside because just 1 minute of exposure to the smoke can trigger an exacerbation of their chronic disease,” Dr. Madhani-Lovely said.
Mr. Smokey said connecting with pulmonologists can be difficult for Washoe Tribe members, particularly those who live on the California side of the reservation. “We cannot find providers for them,” he said. “We end up referring them out and sending them hundreds of miles out of their way just to get care that we should be able to provide here.”
Recruiting specialists to rural areas or smaller cities has long been difficult. For one thing, a specialist might be the only one for miles around, “so there’s a tremendous burden in terms of coverage and days off,” Dr. Test said.
Another concern is that physicians tend to train in larger cities and often want to practice in similar places. Even recruiting pulmonary physicians to Lubbock, a city of 260,000 in West Texas, is a challenge, Dr. Test said.
“I love Lubbock,” he said. “But I tell people who have never been here, I say, ‘It’s really flat.’ They don’t understand flat until they get here.”
In Nevada, on days when the air quality is bad, Washoe tribal members try to protect themselves with makeshift air purifiers created from fans, duct tape, and air filters, Mr. Smokey said.
Longer term, Mr. Smokey and other tribal leaders are pushing the Indian Health Service to establish a specialty care hospital in northern Nevada. The closest specialty care hospital for Washoe tribal members is more than 700 miles away, in Phoenix.
It’s difficult because “there’s a need we should be taking care of,” Mr. Smokey said. “But we have to fight for it. And sometimes that fight takes years, years, and years to accomplish.”
A version of this article first appeared on Medscape.com.
New screening tool identifies asthma risk in toddlers
A symptom-based screening tool can identify 2-year-olds at increased risk of asthma, persistent symptoms of wheeze, and health care burden by the age of 5, according to researchers.
The validated CHILDhood Asthma Risk Tool (CHART) determines high, moderate, or low risk of asthma based on symptoms reported before the age of 3 years. It also recommends follow-up.
Potentially, CHART could be used “to identify children who need monitoring, timely symptom control, and introduction of preventive therapies,” said Padmaja Subbarao, MD, MSc, associate chief of clinical research at the Hospital for Sick Children, Toronto, and colleagues in JAMA Network Open.
“The implementation of CHART as a first-step screening tool in general practice could promote timely treatment control and, in turn, improve quality of life for patients and reduce the clinical and economic burden of asthma,” they wrote.
Dr. Subbarao and colleagues developed CHART using data from parent questionnaires and 3- and 5-year clinic visits in the CHILD study. Children were categorized as “high risk” when they experienced two or more episodes of wheeze annually at both 3 and 5 years of age, concurrent with ED visits, hospitalizations, asthma medication, or frequent dry cough. Children with only cough episodes or with cough episodes plus one episode of wheeze in the past 12 months were categorized as “low risk.”
“Our unique approach to classification of wheeze symptoms is important because it helps busy practitioners identify the smaller subset of children with more frequent or severe wheezing episodes who have a higher probability of continued symptoms and impaired lung function in adult life among most children with infrequent wheeze,” Dr. Sabbarao and coauthors said.
Their diagnostic study to evaluate CHART’s predictive capacity showed that the tool had the highest proportion of true-positive asthma at 5 years (sensitivity, 50.0%), compared with physicians’ diagnosis at 3 years (sensitivity, 43.5%), and positive standardized modified Asthma Predictive Index (mAPI) at 3 years (sensitivity, 24.4%).
CHART also outperformed physician assessments and mAPI for predicting persistent wheeze at 5 years and provided the highest predictive capacity for subsequent health care use at 5 years of age. The study showed that it identified 20% more children with emergency department visits or hospitalizations than the standardized mAPI (sensitivity 45.5% vs. 25.0%), and approximately 10% more at-risk children than physician diagnosis.
“These findings are especially important given that many hospitalizations are avoidable if appropriate treatment and management of asthma are implemented at primary care,” Dr. Subbarao and colleagues wrote.
CHART has been validated in two external cohorts: a general-population cohort of 2,185 children from the Raine Study in Australia at 5 years of age; and the other a high-risk cohort of 349 children from the Canadian Asthma Primary Prevention Study at 7 years of age.
“We want to highlight the importance of periodic monitoring of wheeze symptoms and simplify the identification of high-risk children for primary care providers and parents or caregivers,” said Dr. Subbarao, who is director of the CHILD study and professor of pediatrics at the University of Toronto.
The tool “does not identify the underlying biology, which could impact the efficacy of our current standard asthma treatment,” Dr. Subbarao emphasized. CHART has not been tested in low-prevalence settings or in countries in which the term “wheeze” is not commonly recognized, she added.
“CHART helps you focus your crystal ball a little bit, look into the future, and see what’s going to happen,” said Harold Farber, MD, a pediatric pulmonologist who was not involved in the study. “It’s useful even if it just confirms what I’m already doing clinically.”
Dr. Farber, who is professor of pediatrics at Baylor College of Medicine and the Texas Children’s Hospital, Houston, cautioned that the predictive value of CHART is based on the diagnosis of asthma, and that this can differ across health care communities. “Between the extremes and what’s considered borderline, there’s a lot of diagnostic variation in what we call asthma,” he explained in an interview. “The diagnosis is, to some extent, subjective.”
However, Dr. Farber agreed that two or more wheezing episodes in the past 12 months – enough to require treatment – puts a child at very high risk for future wheezing. “Kids with a bunch of wheezing problems at 3 years are likely to have wheezing problems at 5. We have to think about what we can do for a toddler today to keep him from wheezing later.”
CHART is simple to use, the investigators said. The information needed can be easily gathered through interviews and parent-reported questionnaires, then put into the electronic medical record to flag children at high risk for further investigation, and well as those at low or moderate risk for monitoring.
Parents and caregivers can also use CHART to document symptoms every 6 months in children older than 1 year of age, said Dr. Subbarao. This information can be brought to the attention of the doctor “to facilitate a deeper discussion,” she suggested.
This study was funded by the Canadian Institutes of Health Research, Allergy, Genes and Environment Network of Centers of Excellence; Don and Debbie Morrison; Women’s and Children Health Research Institute; and Canada Research Chairs. Dr Subbarao reported having no potential conflicts of interest. Coauthor Vanessa Breton, PhD, disclosed being employed by F. Hoffmann-La Roche Ltd., and coauthor Elinor Simons, MD, PhD, reported membership on the Sanofi-Genzyme Data Monitoring Board. No other conflicts of interest were reported by the study authors. Dr Farber disclosed having no potential conflicts of interest.
A symptom-based screening tool can identify 2-year-olds at increased risk of asthma, persistent symptoms of wheeze, and health care burden by the age of 5, according to researchers.
The validated CHILDhood Asthma Risk Tool (CHART) determines high, moderate, or low risk of asthma based on symptoms reported before the age of 3 years. It also recommends follow-up.
Potentially, CHART could be used “to identify children who need monitoring, timely symptom control, and introduction of preventive therapies,” said Padmaja Subbarao, MD, MSc, associate chief of clinical research at the Hospital for Sick Children, Toronto, and colleagues in JAMA Network Open.
“The implementation of CHART as a first-step screening tool in general practice could promote timely treatment control and, in turn, improve quality of life for patients and reduce the clinical and economic burden of asthma,” they wrote.
Dr. Subbarao and colleagues developed CHART using data from parent questionnaires and 3- and 5-year clinic visits in the CHILD study. Children were categorized as “high risk” when they experienced two or more episodes of wheeze annually at both 3 and 5 years of age, concurrent with ED visits, hospitalizations, asthma medication, or frequent dry cough. Children with only cough episodes or with cough episodes plus one episode of wheeze in the past 12 months were categorized as “low risk.”
“Our unique approach to classification of wheeze symptoms is important because it helps busy practitioners identify the smaller subset of children with more frequent or severe wheezing episodes who have a higher probability of continued symptoms and impaired lung function in adult life among most children with infrequent wheeze,” Dr. Sabbarao and coauthors said.
Their diagnostic study to evaluate CHART’s predictive capacity showed that the tool had the highest proportion of true-positive asthma at 5 years (sensitivity, 50.0%), compared with physicians’ diagnosis at 3 years (sensitivity, 43.5%), and positive standardized modified Asthma Predictive Index (mAPI) at 3 years (sensitivity, 24.4%).
CHART also outperformed physician assessments and mAPI for predicting persistent wheeze at 5 years and provided the highest predictive capacity for subsequent health care use at 5 years of age. The study showed that it identified 20% more children with emergency department visits or hospitalizations than the standardized mAPI (sensitivity 45.5% vs. 25.0%), and approximately 10% more at-risk children than physician diagnosis.
“These findings are especially important given that many hospitalizations are avoidable if appropriate treatment and management of asthma are implemented at primary care,” Dr. Subbarao and colleagues wrote.
CHART has been validated in two external cohorts: a general-population cohort of 2,185 children from the Raine Study in Australia at 5 years of age; and the other a high-risk cohort of 349 children from the Canadian Asthma Primary Prevention Study at 7 years of age.
“We want to highlight the importance of periodic monitoring of wheeze symptoms and simplify the identification of high-risk children for primary care providers and parents or caregivers,” said Dr. Subbarao, who is director of the CHILD study and professor of pediatrics at the University of Toronto.
The tool “does not identify the underlying biology, which could impact the efficacy of our current standard asthma treatment,” Dr. Subbarao emphasized. CHART has not been tested in low-prevalence settings or in countries in which the term “wheeze” is not commonly recognized, she added.
“CHART helps you focus your crystal ball a little bit, look into the future, and see what’s going to happen,” said Harold Farber, MD, a pediatric pulmonologist who was not involved in the study. “It’s useful even if it just confirms what I’m already doing clinically.”
Dr. Farber, who is professor of pediatrics at Baylor College of Medicine and the Texas Children’s Hospital, Houston, cautioned that the predictive value of CHART is based on the diagnosis of asthma, and that this can differ across health care communities. “Between the extremes and what’s considered borderline, there’s a lot of diagnostic variation in what we call asthma,” he explained in an interview. “The diagnosis is, to some extent, subjective.”
However, Dr. Farber agreed that two or more wheezing episodes in the past 12 months – enough to require treatment – puts a child at very high risk for future wheezing. “Kids with a bunch of wheezing problems at 3 years are likely to have wheezing problems at 5. We have to think about what we can do for a toddler today to keep him from wheezing later.”
CHART is simple to use, the investigators said. The information needed can be easily gathered through interviews and parent-reported questionnaires, then put into the electronic medical record to flag children at high risk for further investigation, and well as those at low or moderate risk for monitoring.
Parents and caregivers can also use CHART to document symptoms every 6 months in children older than 1 year of age, said Dr. Subbarao. This information can be brought to the attention of the doctor “to facilitate a deeper discussion,” she suggested.
This study was funded by the Canadian Institutes of Health Research, Allergy, Genes and Environment Network of Centers of Excellence; Don and Debbie Morrison; Women’s and Children Health Research Institute; and Canada Research Chairs. Dr Subbarao reported having no potential conflicts of interest. Coauthor Vanessa Breton, PhD, disclosed being employed by F. Hoffmann-La Roche Ltd., and coauthor Elinor Simons, MD, PhD, reported membership on the Sanofi-Genzyme Data Monitoring Board. No other conflicts of interest were reported by the study authors. Dr Farber disclosed having no potential conflicts of interest.
A symptom-based screening tool can identify 2-year-olds at increased risk of asthma, persistent symptoms of wheeze, and health care burden by the age of 5, according to researchers.
The validated CHILDhood Asthma Risk Tool (CHART) determines high, moderate, or low risk of asthma based on symptoms reported before the age of 3 years. It also recommends follow-up.
Potentially, CHART could be used “to identify children who need monitoring, timely symptom control, and introduction of preventive therapies,” said Padmaja Subbarao, MD, MSc, associate chief of clinical research at the Hospital for Sick Children, Toronto, and colleagues in JAMA Network Open.
“The implementation of CHART as a first-step screening tool in general practice could promote timely treatment control and, in turn, improve quality of life for patients and reduce the clinical and economic burden of asthma,” they wrote.
Dr. Subbarao and colleagues developed CHART using data from parent questionnaires and 3- and 5-year clinic visits in the CHILD study. Children were categorized as “high risk” when they experienced two or more episodes of wheeze annually at both 3 and 5 years of age, concurrent with ED visits, hospitalizations, asthma medication, or frequent dry cough. Children with only cough episodes or with cough episodes plus one episode of wheeze in the past 12 months were categorized as “low risk.”
“Our unique approach to classification of wheeze symptoms is important because it helps busy practitioners identify the smaller subset of children with more frequent or severe wheezing episodes who have a higher probability of continued symptoms and impaired lung function in adult life among most children with infrequent wheeze,” Dr. Sabbarao and coauthors said.
Their diagnostic study to evaluate CHART’s predictive capacity showed that the tool had the highest proportion of true-positive asthma at 5 years (sensitivity, 50.0%), compared with physicians’ diagnosis at 3 years (sensitivity, 43.5%), and positive standardized modified Asthma Predictive Index (mAPI) at 3 years (sensitivity, 24.4%).
CHART also outperformed physician assessments and mAPI for predicting persistent wheeze at 5 years and provided the highest predictive capacity for subsequent health care use at 5 years of age. The study showed that it identified 20% more children with emergency department visits or hospitalizations than the standardized mAPI (sensitivity 45.5% vs. 25.0%), and approximately 10% more at-risk children than physician diagnosis.
“These findings are especially important given that many hospitalizations are avoidable if appropriate treatment and management of asthma are implemented at primary care,” Dr. Subbarao and colleagues wrote.
CHART has been validated in two external cohorts: a general-population cohort of 2,185 children from the Raine Study in Australia at 5 years of age; and the other a high-risk cohort of 349 children from the Canadian Asthma Primary Prevention Study at 7 years of age.
“We want to highlight the importance of periodic monitoring of wheeze symptoms and simplify the identification of high-risk children for primary care providers and parents or caregivers,” said Dr. Subbarao, who is director of the CHILD study and professor of pediatrics at the University of Toronto.
The tool “does not identify the underlying biology, which could impact the efficacy of our current standard asthma treatment,” Dr. Subbarao emphasized. CHART has not been tested in low-prevalence settings or in countries in which the term “wheeze” is not commonly recognized, she added.
“CHART helps you focus your crystal ball a little bit, look into the future, and see what’s going to happen,” said Harold Farber, MD, a pediatric pulmonologist who was not involved in the study. “It’s useful even if it just confirms what I’m already doing clinically.”
Dr. Farber, who is professor of pediatrics at Baylor College of Medicine and the Texas Children’s Hospital, Houston, cautioned that the predictive value of CHART is based on the diagnosis of asthma, and that this can differ across health care communities. “Between the extremes and what’s considered borderline, there’s a lot of diagnostic variation in what we call asthma,” he explained in an interview. “The diagnosis is, to some extent, subjective.”
However, Dr. Farber agreed that two or more wheezing episodes in the past 12 months – enough to require treatment – puts a child at very high risk for future wheezing. “Kids with a bunch of wheezing problems at 3 years are likely to have wheezing problems at 5. We have to think about what we can do for a toddler today to keep him from wheezing later.”
CHART is simple to use, the investigators said. The information needed can be easily gathered through interviews and parent-reported questionnaires, then put into the electronic medical record to flag children at high risk for further investigation, and well as those at low or moderate risk for monitoring.
Parents and caregivers can also use CHART to document symptoms every 6 months in children older than 1 year of age, said Dr. Subbarao. This information can be brought to the attention of the doctor “to facilitate a deeper discussion,” she suggested.
This study was funded by the Canadian Institutes of Health Research, Allergy, Genes and Environment Network of Centers of Excellence; Don and Debbie Morrison; Women’s and Children Health Research Institute; and Canada Research Chairs. Dr Subbarao reported having no potential conflicts of interest. Coauthor Vanessa Breton, PhD, disclosed being employed by F. Hoffmann-La Roche Ltd., and coauthor Elinor Simons, MD, PhD, reported membership on the Sanofi-Genzyme Data Monitoring Board. No other conflicts of interest were reported by the study authors. Dr Farber disclosed having no potential conflicts of interest.
FROM JAMA NETWORK OPEN
Would your patient benefit from a monoclonal antibody?
Small-molecule drugs such as aspirin, albuterol, atorvastatin, and lisinopril are the backbone of disease management in family medicine.1 However, large-molecule biological drugs such as monoclonal antibodies (MAbs) are increasingly prescribed to treat common conditions. In the past decade, MAbs comprised 20% of all drug approvals by the US Food and Drug Administration (FDA), and today they represent more than half of drugs currently in development.2 Fifteen MAbs have been approved by the FDA over the past decade for asthma, atopic dermatitis (AD), hyperlipidemia, osteoporosis, and migraine prevention.3 This review details what makes MAbs unique and what you should know about them.
The uniqueness of monoclonal antibodies
MAbs are biologics, but not all biologics are MAbs—eg, adalimumab (Humira) is a MAb, but etanercept (Enbrel) is not. MAbs are therapeutic proteins made possible by hybridoma technology used to create an antibody with single specificity.4-6 Monoclonal antibodies differ from small-molecule drugs in structure, dosing, route of administration, manufacturing, metabolism, drug interactions, and elimination (TABLE 17-9).
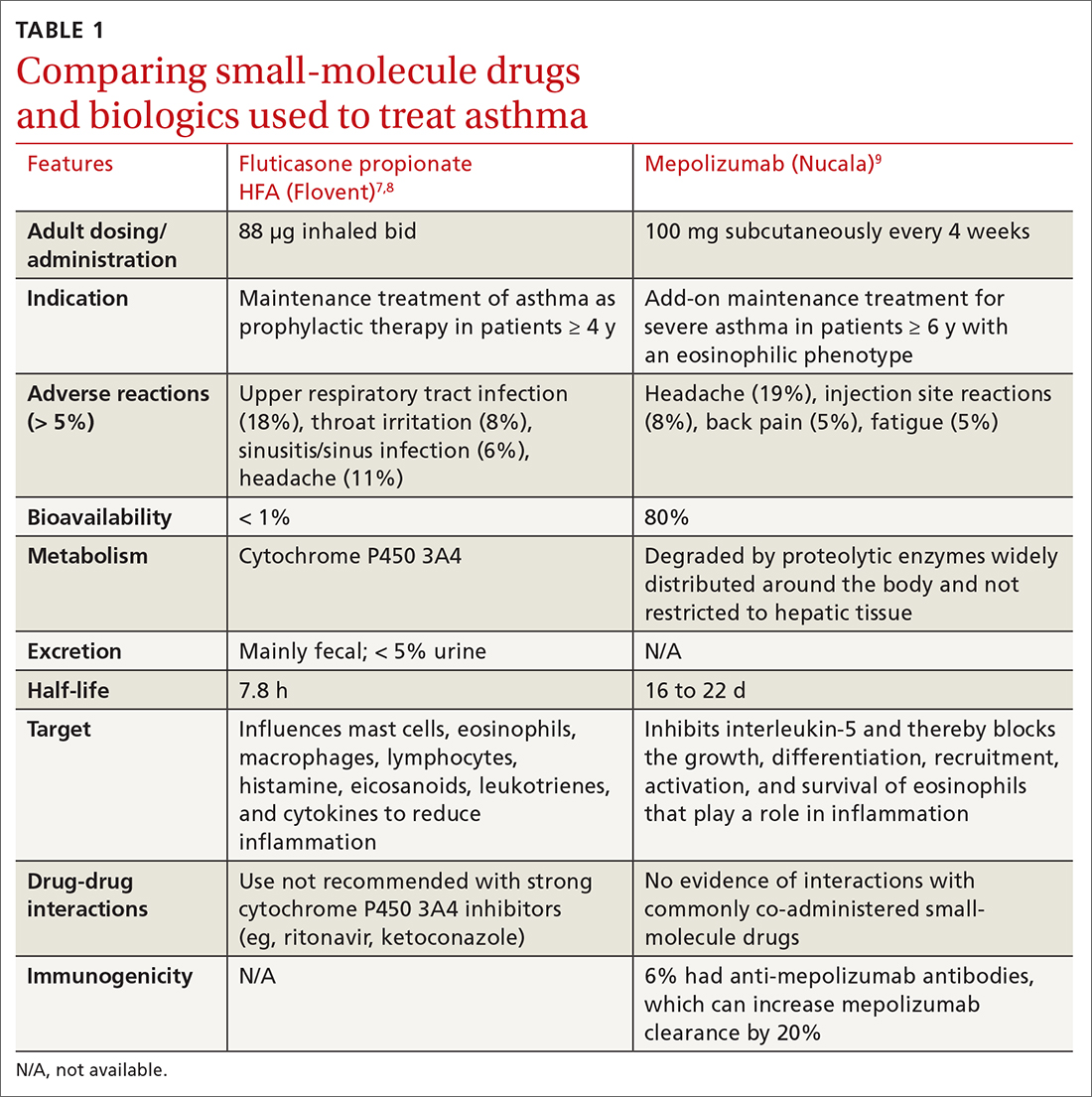
MAbs can be classified as naked, “without any drug or radioactive material attached to them,” or conjugated, “joined to a chemotherapy drug, radioactive isotope, or toxin.”10 MAbs work in several ways, including competitively inhibiting ligand-receptor binding, receptor blockade, or cell elimination from indirect immune system activities such as antibody-dependent cell-mediated cytotoxicity.11,12
Monoclonal antibody uses in family medicine
Asthma
Several MAbs have been approved for use in severe asthma, including but not limited to: omalizumab (Xolair),13 mepolizumab (Nucala),9,14 and dupilumab (Dupixent).15
Omalizumab is a humanized MAb that prevents IgE antibodies from binding to mast cells and basophils, thereby reducing inflammatory mediators.13 A systematic review found that, compared with placebo, omalizumab used in patients with inadequately controlled moderate-to-severe asthma led to significantly fewer asthma exacerbations (absolute risk reduction [ARR], 16% vs 26%; odds ratio [OR] = 0.55; 95% CI, 0.42-0.60; number needed to treat [NNT] = 10) and fewer hospitalizations (ARR, 0.5% vs 3%; OR = 0.16; 95% CI, 0.06-0.42; NNT = 40).13
Significantly more patients in the omalizumab group were able to withdraw from, or reduce, the dose of ICS. GINA recommends omalizumab for patients with positive skin sensitization, total serum IgE ≥ 30 IU/mL, weight within 30 kg to 150 kg, history of childhood asthma and recent exacerbations, and blood eosinophils ≥ 260/mcL.16 Omalizumab is also approved for use in chronic spontaneous urticaria and nasal polyps.
Mepolizumab
Continue to: Another trial found that...
Another trial found that mepolizumab reduced total OCS doses in patients with severe asthma by 50% without increasing exacerbations or worsening asthma control.18 All 3 anti-IL-5 drugs—including not only mepolizumab, but also benralizumab (Fasenra) and reslizumab (Cinqair)—appear to yield similar improvements. A 2017 systematic review found all anti-IL-5 treatments reduced rates of clinically significant asthma exacerbations (treatment with OCS for ≥ 3 days) by roughly 50% in patients with severe eosinophilic asthma and a history of ≥ 2 exacerbations in the past year.
Dupilumab is a humanized MAb that inhibits IL-4 and IL-13, which influence multiple cell types involved in inflammation (eg, mast cells, eosinophils) and inflammatory mediators (histamine, leukotrienes, cytokines).15 In a recent study of patients with uncontrolled asthma, dupilumab 200 mg every 2 weeks compared with placebo showed a modest reduction in the annualized rate of severe asthma exacerbations (0.46 exacerbations vs 0.87, respectively). Dupilumab was effective in patients with blood eosinophil counts ≥ 150/μL but was ineffective in patients with eosinophil counts < 150/μL.15
For patients ≥ 12 years old with severe eosinophilic asthma, GINA recommends using dupilumab as add-on therapy for an initial trial of 4 months at doses of 200 or 300 mg SC every 2 weeks, with preference for 300 mg SC every 2 weeks for OCS-dependent asthma. Dupilumab is approved for use in AD and chronic rhinosinusitis with nasal polyposis. If a biologic agent is not successful after a 4-month trial, consider a 6- to 12-month trial. If efficacy is still minimal, consider switching to an alternative biologic therapy approved for asthma.16
❯ Asthma: Test your skills
Subjective findings: A 19-year-old man presents to your clinic. He has a history of nasal polyps and allergic asthma. At age 18, he was given a diagnosis of severe persistent asthma. He has shortness of breath during waking hours 4 times per week, and treats each of these episodes with albuterol. He also wakes up about twice a week with shortness of breath and has some limitations in normal activities. He reports missing his prescribed fluticasone/salmeterol 500/50 μg, 1 inhalation bid, only once each month. In the last year, he has had 2 exacerbations requiring oral steroids.
Medications: Albuterol 90 μg, 1-2 inhalations, q6h prn; fluticasone/salmeterol 500/50 μg, 1 inhalation bid; tiotropium 1.25 μg, 2 puffs/d; montelukast 10 mg every morning; prednisone 10 mg/d.
Continue to: Objective data
Objective data: Patient is in no apparent distress and afebrile, and oxygen saturation on room air is 97%. Ht, 70 inches; wt, 75 kg. Labs: IgE, 15 IU/mL; serum eosinophils, 315/μL.
Which MAb would be appropriate for this patient? Given that the patient has a blood eosinophil level ≥ 300/μL and severe exacerbations, adult-onset asthma, nasal polyposis, and maintenance OCS at baseline, it would be reasonable to initiate mepolizumab 100 mg SC every 4 weeks, or dupilumab 600 mg once, then 300 mg SC every 2 weeks. Both agents can be self-administered.
Atopic dermatitis
Two MAbs—dupilumab and tralokinumab (Adbry; inhibits IL-13)—are approved for treatment of AD in adults that is uncontrolled with conventional therapy.15,19 Dupilumab is also approved for children ≥ 6 months old.20 Both MAbs are dosed at 600 mg SC, followed by 300 mg every 2 weeks. Dupilumab was compared with placebo in adult patients who had moderate-to-severe AD inadequately controlled on topical corticosteroids (TCSs), to determine the proportion of patients in each group achieving improvement of either 0 or 1 points or ≥ 2 points in the 5-point Investigator Global Assessment (IGA) score from baseline to 16 weeks.21 Thirty-seven percent of patients receiving dupilumab 300 mg SC weekly and 38% of patients receiving dupilumab 300 mg SC every 2 weeks achieved the primary outcome, compared with 10% of those receiving placebo (P < .001).21 Similar IGA scores were reported when dupilumab was combined with TCS, compared with placebo.22
It would be reasonable to consider dupilumab or tralokinumab in patients with: cutaneous atrophy or hypothalamic-pituitary-adrenal axis suppression with TCS, concerns of malignancy with topical calcineurin inhibitors, or problems with the alternative systemic therapies (cyclosporine-induced hypertension, nephrotoxicity, or immunosuppression; azathioprine-induced malignancy; or methotrexate-induced bone marrow suppression, renal impairment, hepatotoxicity, pneumonitis, or gastrointestinal toxicity).23
A distinct advantage of MAbs over other systemic agents in the management of AD is that MAbs do not require frequent monitoring of blood pressure, renal or liver function, complete blood count with differential, electrolytes, or uric acid. Additionally, MAbs have fewer black box warnings and adverse reactions when compared with other systemic agents.
Continue to: Hyperlipidemia
Hyperlipidemia
Three MAbs are approved for use in hyperlipidemia: the angiopoietin-like protein 3 (ANGPTL3) inhibitor evinacumab (Evkeeza)24 and 2 proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, evolocumab (Repatha)25 and alirocumab (Praluent).26
ANGPTL3 inhibitors block ANGPTL3 and reduce endothelial lipase and lipoprotein lipase activity, which in turn decreases low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride formation. PCSK9 inhibitors prevent PCSK9 from binding to LDL receptors, thereby maintaining the number of active LDL receptors and increasing LDL-C removal.
Evinacumab is indicated for homozygous familial hypercholesterolemia and is administered intravenously every 4 weeks. Evinacumab has not been evaluated for effects on cardiovascular morbidity and mortality.
Evolocumab 140 mg SC every 2 weeks or 420 mg SC monthly has been studied in patients on statin therapy with LDL-C ≥ 70 mg/dL. Patients on evolocumab experienced significantly less of the composite endpoint of cardiovascular death, myocardial infarction (MI), stroke, hospitalization for unstable angina, or coronary revascularization compared with placebo (9.8% vs 11.3%; hazard ratio [HR] = 0.85; 95% CI, 0.79-0.92; NNT = 67.27
Alirocumab 75 mg SC every 2 weeks has also been studied in patients receiving statin therapy with LDL-C ≥ 70 mg/dL. Patients taking alirocumab experienced significantly less of the composite endpoint of death from coronary heart disease, nonfatal MI, ischemic stroke, or hospitalization for unstable angina compared with placebo (9.5% vs 11.1%; HR = 0.85; 95% CI, 0.78-0.93; NNT = 63).
Continue to: According to the 2018...
According to the 2018 AHA Cholesterol Guidelines, PCSK9 inhibitors are indicated for patients receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe) with LDL-C ≥ 70 mg/dL, if they have had multiple atherosclerotic cardiovascular disease (ASCVD) events or 1 major ASCVD event with multiple high-risk conditions (eg, heterozygous familial hypercholesterolemia, history of coronary artery bypass grafting or percutaneous coronary intervention, hypertension, estimated glomerular filtration rate of 15 to 59 mL/min/1.73m2).29 For patients without prior ASCVD events or high-risk conditions who are receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe), PCSK9 inhibitors are indicated if the LDL-C remains ≥ 100 mg/dL.
Osteoporosis
The 2 MAbs approved for use in osteoporosis are the receptor activator of nuclear factor kB ligand (RANKL) inhibitor denosumab (Prolia)30 and the sclerostin inhibitor romosozumab (Evenity).31
Denosumab prevents RANKL from binding to the RANK receptor, thereby inhibiting osteoclast formation and decreasing bone resorption. Denosumab is approved for use in women and men who are at high risk of osteoporotic fracture, including those taking OCSs, men receiving androgen deprivation therapy for prostate cancer, and women receiving adjuvant aromatase inhibitor therapy for breast cancer.
In a 3-year randomized trial, denosumab 60 mg SC every 6 months was compared with placebo in postmenopausal women with T-scores < –2.5, but not < –4.0 at the lumbar spine or total hip. Denosumab significantly reduced new radiographic vertebral fractures (2.3% vs 7.2%; risk ratio [RR] = 0.32; 95% CI, 0.26-0.41; NNT = 21), hip fracture (0.7% vs 1.2%), and nonvertebral fracture (6.5% vs 8.0%).32 Denosumab carries an increased risk of multiple vertebral fractures following discontinuation, skin infections, dermatologic reactions, and severe bone, joint, and muscle pain.
Romosozumab inhibits sclerostin, thereby increasing bone formation and, to a lesser degree, decreasing bone resorption. Romosozumab is approved for use in postmenopausal women at high risk for fracture (ie, those with a history of osteoporotic fracture or multiple risk factors for fracture) or in patients who have not benefited from or are intolerant of other therapies. In one study, postmenopausal women with a T-score of –2.5 to –3.5 at the total hip or femoral neck were randomly assigned to receive either romosozumab 210 mg SC or placebo for 12 months, then each group was switched to denosumab 60 mg SC for 12 months. After the first year, prior to initiating denosumab, patients taking romosozumab experienced significantly fewer new vertebral fractures than patients taking placebo (0.5% vs 1.8%; RR = 0.27; 95% CI, 0.16-0.47; NNT = 77); however, there was no significant difference between the 2 groups with nonvertebral fractures (HR = 0.75; 95% CI, 0.53-1.05).33
Continue to: In another study...
In another study, romosozumab 210 mg SC was compared with alendronate 70 mg weekly, followed by alendronate 70 mg weekly in both groups. Over the first 12 months, patients treated with romosozumab saw a significant reduction in the incidence of new vertebral fractures (4% vs 6.3%; RR = 0.63, P < .003; NNT = 44). Patients treated with romosozumab with alendronate added for another 12 months also saw a significant reduction in new incidence of vertebral fractures (6.2% vs 11.9%; RR = 0.52; P < .001; NNT = 18).34 There was a higher risk of cardiovascular events among patients receiving romosozumab compared with those treated with alendronate, so romosozumab should not be used in individuals who have had an MI or stroke within the previous year.34 Denosumab and romosozumab offer an advantage over some bisphosphonates in that they require less frequent dosing and can be used in patients with renal impairment (creatinine clearance < 35 mL/min, in which zoledronic acid is contraindicated and alendronate is not recommended; < 30 mL/min, in which risedronate and ibandronate are not recommended).
Migraine prevention
Four
Erenumab, fremanezumab, and galcanezumab are all available in subcutaneous autoinjectors (or syringe with fremanezumab). Eptinezumab is an intravenous (IV) infusion given every 3 months.
Erenumab is available in both 70-mg and 140-mg dosing options. Fremanezumab can be given as 225 mg monthly or 675 mg quarterly. Galcanezumab has an initial loading dose of 240 mg followed by 120 mg given monthly. Erenumab targets the CGRP receptor; the others target the CGRP ligand. Eptinezumab has 100% bioavailability and reaches maximum serum concentration sooner than the other antagonists (due to its route of administration), but it must be given in an infusion center. Few insurers approve the use of eptinezumab unless a trial of least 1 of the monthly injectables has failed.
There are no head-to-head studies of the medications in this class. Additionally, differing study designs, definitions, statistical analyses, endpoints, and responder-rate calculations make it challenging to compare them directly against one another. At the very least, all of the CGRP MAbs have efficacy comparable to conventional preventive migraine medications such as propranolol, amitriptyline, and topiramate.40
Continue to: The most commonly reported adverse...
The most commonly reported adverse effect for all 4 CGRPs is injection site reaction, which was highest with the quarterly fremanezumab dose (45%).37 Constipation was most notable with the 140-mg dose of erenumab (3%)35; with the other CGRP MAbs it is comparable to that seen with placebo (< 1%).
Erenumab-induced hypertension has been identified in 61 cases reported through the FDA Adverse Event Reporting System (FAERS) as of 2021.41 This was not reported during MAb development programs, nor was it noted during clinical trials. Blood pressure elevation was seen within 1 week of injection in nearly 50% of the cases, and nearly one-third had pre-existing hypertension.41 Due to these findings, the erenumab prescribing information was updated to include hypertension in its warnings and precautions. It is possible that hypertension could be a class effect, although trial data and posthoc studies have yet to bear that out. Since erenumab was the first CGRP antagonist brought to market (May 2018 vs September 2018 for fremanezumab and galcanezumab), it may have accumulated more FAERS reports. Nearly all studies exclude patients with older age, uncontrolled hypertension, and unstable cardiovascular disease, which could impact data.41
Overall, this class of medications is very well tolerated, easy to use (again, excluding eptinezumab), and maintains a low adverse effect profile, giving added value compared with conventional preventive migraine medications.
The American Headache Society recommends a preventive oral therapy for at least 3 months before trying an alternative medication. After treatment failure with at least 2 oral agents, CGRP MAbs are recommended.42 CGRP antagonists offer convenient dosing, bypass gastrointestinal metabolism (which is useful in patients with nausea/vomiting), and have fewer adverse effects than traditional oral medications.
Worth noting. Several newer oral agents have been recently approved for migraine prevention, including atogepant (Qulipta) and rimegepant (Nurtec), which are also CGRP antagonists. Rimegepant is approved for both acute migraine treatment and prevention.
Continue to: Migraine
❯ Migraine: Test your skills
Subjective findings: A 25-year-old woman presents to your clinic for management of episodic migraines with aura. Her baseline average migraine frequency is 9 headache days/month. Her migraines are becoming more frequent despite treatment. She fears IV medication use and avoids hospitals.
History: Hypertension, irritable bowel syndrome with constipation (IBS-C), and depression. The patient is not pregnant or trying to get pregnant.
Medications: Current medications (for previous 4 months) include propranolol 40 mg at bedtime, linaclotide 145 μg/d, citalopram 20 mg/d, and sumatriptan 50 mg prn. Past medications include venlafaxine 150 mg po bid for 5 months.
What would be appropriate for this patient? This patient meets the criteria for using a CGRP antagonist because she has tried 2 preventive treatments for more than 60 to 90 days. Erenumab is not the best option, given the patient’s history of hypertension and IBS-C. The patient fears hospitals and IV medications, making eptinezumab a less-than-ideal choice. Depending on her insurance, fremanezumab or galcanezumab would be good options at this time.
CGRP antagonists have not been studied or evaluated in pregnancy, but if this patient becomes pregnant, a first-line agent for prevention would be propranolol, and a second-line agent would be a tricyclic antidepressant, memantine, or verapamil. Avoid ergotamines and antiepileptics (topiramate or valproate) in pregnancy.43,44
Continue to: The challenges associated with MAbs
The challenges associated with MAbs
MAbs can be expensive (TABLE 2),45 some prohibitively so. On a population scale, biologics account for around 40% of prescription drug spending and may cost 22 times more than small-molecule drugs.46 Estimates in 2016 showed that MAbs comprise $90.2 billion (43%) of the biologic market.46
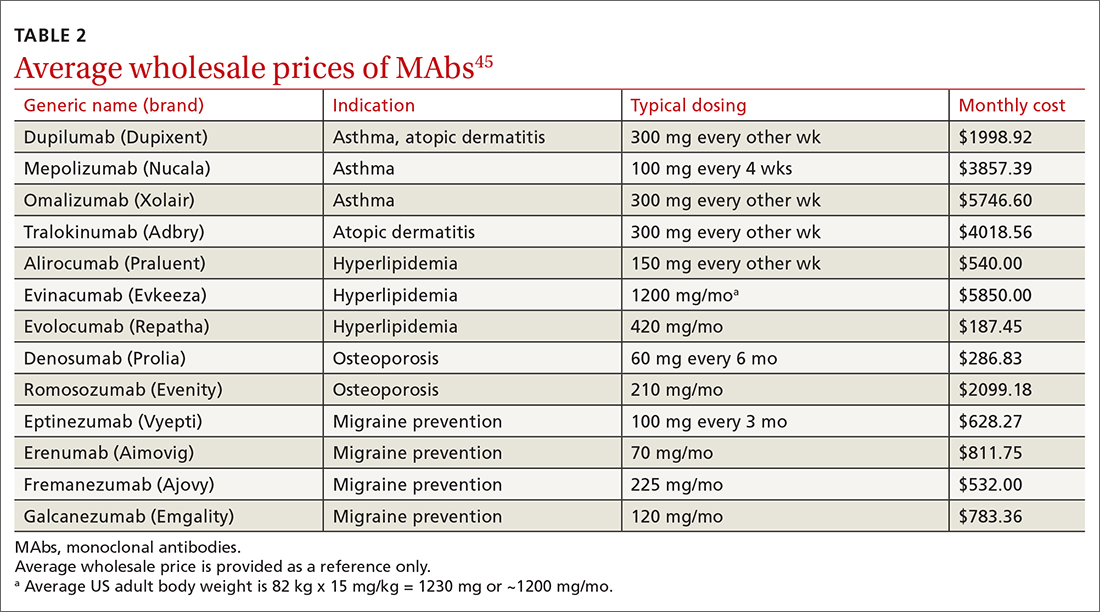
MAbs also require prior authorization forms to be submitted. Prior authorization criteria vary by state and by insurance plan. In my (ES) experience, submitting letters of medical necessity justifying the need for therapy or expertise in the disease states for which the MAb is being prescribed help your patient get the medication they need.
Expect to see additional MAbs approved in the future. If the costs come down, adoption of these agents into practice will likely increase.
CORRESPONDENCE
Evelyn Sbar, MD, Texas Tech University Health Sciences Center, 1400 South Coulter Street, Suite 5100, Amarillo, TX 79106; evelyn.sbar@ttuhsc.edu
1. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. National Center for Health Statistics. Accessed June 15, 2022. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
2. IDBS. The future of biologics drug development is today. June 27, 2018. Accessed June 15, 2022. www.idbs.com/blog/2018/06/the-future-of-biologics-drug-development-is-today/
3. Antibody therapeutics approved or in regulatory review in the EU or US. Antibody Society. Accessed June 15, 2022. www.antibodysociety.org/resources/approved-antibodies/
4. FDA. Code of Federal Regulations, Title 21, Chapter I, Subchapter F biologics. March 29, 2022. Accessed June 15, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=600.3
5. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495-497. doi: 10.1038/256495a0
6. Raejewsky K. The advent and rise of monoclonal antibodies. Nature. November 4, 2019. Accessed June 15, 2022. www.nature.com/articles/d41586-019-02840-w
7. Flovent. Prescribing information. GlaxoSmithKline; 2010. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2010/021433s015lbl.pdf
8. NLM. National Center for Biotechnology Information. PubChem. Method for the preparation of fluticasone and related 17beta-carbothioic esters using a novel carbothioic acid synthesis and novel purification methods. Accessed June 15, 2022. pubchem.ncbi.nlm.nih.gov/patent/WO-0162722-A2
9. Nucala. Prescribing information. GlaxoSmithKline; 2019. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761122s000lbl.pdf
10. Argyriou AA, Kalofonos HP. Recent advances relating to the clinical application of naked monoclonal antibodies in solid tumors. Mol Med. 2009;15:183-191. doi: 10.2119/molmed.2009.00007
11. Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548-558. doi: 10.1038/clpt.2008.170
12. Zahavi D, AlDeghaither D, O’Connell A, et al. Enhancing antibody-dependent cell-mediated cytotoxicity: a strategy for improving antibody-based immunotherapy. Antib Ther. 2018;1:7-12. doi: 10.1093/abt/tby002
13. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014:CD003559. doi: 10.1002/14651858.CD003559.pub4
14. Farne HA, Wilson A, Powell C, et al. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:CD010834. doi: 10.1002/14651858.CD010834.pub3
15. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486-2496. doi: 10.1056/NEJMoa1804092
16. GINA. Global strategy for asthma management and prevention. 2022 Difficult-to-treat and severe asthma guide—slide set. Accessed June 23, 2022. https://ginasthma.org/severeasthma/
17. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207. doi: 10.1056/NEJMoa1403290
18. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197. doi: 10.1056/NEJMoa1403291
19. Adbry. Prescribing information. Leo Pharma Inc; 2021. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761180Orig1s000lbl.pdf
20. Dupixent. Prescribing information. Regeneron Pharmaceuticals; 2022. Accessed October 5, 2022. https://www.regeneron.com/downloads/dupixent_fpi.pdf
21. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348. doi: 10.1056/NEJMoa1610020
22. Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303. doi: 10.1016/s0140-6736(17)31191-1
23. Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi: 10.1016/j.jaad.2014.03.030
24. Evkeeza. Prescribing information. Regeneron Pharmaceuticals; 2021. Accessed June 24, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761181s000lbl.pdf
25. Repatha. Prescribing information. Amgen; 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125522s014lbl.pdf
26. Praluent. Prescribing information. Sanofi Aventis and Regeneron Pharmaceuticals. 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125559s002lbl.pdf
27. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722. doi: 10.1056/NEJMoa1615664
28. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097-2107. doi:10.1056/NEJMoa1801174
29. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
30. Prolia. Prescribing information. Amgen; 2010. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2013/125320s094lbl.pdf
31. Evenity. Prescribing information. Amgen; 2019. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761062s000lbl.pdf
32. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
33. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543. doi: 10.1056/NEJMoa1607948
34. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427. doi: 10.1056/NEJMoa1708322
35. Aimovig. Prescribing information. Amgen; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761077s000lbl.pdf
36. Vyepti. Prescribing information. Lundbeck Seattle BioPharmaceuticals; 2020. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2020/761119s000lbl.pdf
37. Ajovy. Prescribing information. Teva Pharmaceuticals; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.pdf
38. Emgality. Prescribing information. Eli Lilly and Co.; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761063s000lbl.pdf
39. Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338-350. doi: 10.1038/s41582-018-0003-1
40. Vandervorst F. Van Deun L, Van Dycke A, et al. CGRP monoclonal antibodies in migraine: an efficacy and tolerability comparison with standard prophylactic drugs. J Headache Pain. 2021;22:128. doi: 10.1186/s10194-021-01335-2
41. Saely S, Croteau D, Jawidzik L, et al. Hypertension: a new safety risk for patients treated with erenumab. Headache. 2021;61:202-208. doi: 10.1111/head.14051
42. American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1-18. doi: 10.1111/head.13456
43. Burch R. Headache in pregnancy and the puerperium. Neurol Clin. 2019;37:31-51. doi: 10.1016/j.ncl.2018.09.004
44. Burch R. Epidemiology and treatment of menstrual migraine and migraine during pregnancy and lactation: a narrative review. Headache. 2020;60:200-216. doi: 10.1111/head.13665
45. Lexi-Comp. Lexi-drug database. Accessed April 4, 2022. https://online.lexi.com/lco/action/login
46. Walker N. Biologics: driving force in pharma. Pharma’s Almanac. June 5, 2017. Accessed June 15, 2020. www.pharmasalmanac.com/articles/biologics-driving-force-in-pharma
Small-molecule drugs such as aspirin, albuterol, atorvastatin, and lisinopril are the backbone of disease management in family medicine.1 However, large-molecule biological drugs such as monoclonal antibodies (MAbs) are increasingly prescribed to treat common conditions. In the past decade, MAbs comprised 20% of all drug approvals by the US Food and Drug Administration (FDA), and today they represent more than half of drugs currently in development.2 Fifteen MAbs have been approved by the FDA over the past decade for asthma, atopic dermatitis (AD), hyperlipidemia, osteoporosis, and migraine prevention.3 This review details what makes MAbs unique and what you should know about them.
The uniqueness of monoclonal antibodies
MAbs are biologics, but not all biologics are MAbs—eg, adalimumab (Humira) is a MAb, but etanercept (Enbrel) is not. MAbs are therapeutic proteins made possible by hybridoma technology used to create an antibody with single specificity.4-6 Monoclonal antibodies differ from small-molecule drugs in structure, dosing, route of administration, manufacturing, metabolism, drug interactions, and elimination (TABLE 17-9).

MAbs can be classified as naked, “without any drug or radioactive material attached to them,” or conjugated, “joined to a chemotherapy drug, radioactive isotope, or toxin.”10 MAbs work in several ways, including competitively inhibiting ligand-receptor binding, receptor blockade, or cell elimination from indirect immune system activities such as antibody-dependent cell-mediated cytotoxicity.11,12
Monoclonal antibody uses in family medicine
Asthma
Several MAbs have been approved for use in severe asthma, including but not limited to: omalizumab (Xolair),13 mepolizumab (Nucala),9,14 and dupilumab (Dupixent).15
Omalizumab is a humanized MAb that prevents IgE antibodies from binding to mast cells and basophils, thereby reducing inflammatory mediators.13 A systematic review found that, compared with placebo, omalizumab used in patients with inadequately controlled moderate-to-severe asthma led to significantly fewer asthma exacerbations (absolute risk reduction [ARR], 16% vs 26%; odds ratio [OR] = 0.55; 95% CI, 0.42-0.60; number needed to treat [NNT] = 10) and fewer hospitalizations (ARR, 0.5% vs 3%; OR = 0.16; 95% CI, 0.06-0.42; NNT = 40).13
Significantly more patients in the omalizumab group were able to withdraw from, or reduce, the dose of ICS. GINA recommends omalizumab for patients with positive skin sensitization, total serum IgE ≥ 30 IU/mL, weight within 30 kg to 150 kg, history of childhood asthma and recent exacerbations, and blood eosinophils ≥ 260/mcL.16 Omalizumab is also approved for use in chronic spontaneous urticaria and nasal polyps.
Mepolizumab
Continue to: Another trial found that...
Another trial found that mepolizumab reduced total OCS doses in patients with severe asthma by 50% without increasing exacerbations or worsening asthma control.18 All 3 anti-IL-5 drugs—including not only mepolizumab, but also benralizumab (Fasenra) and reslizumab (Cinqair)—appear to yield similar improvements. A 2017 systematic review found all anti-IL-5 treatments reduced rates of clinically significant asthma exacerbations (treatment with OCS for ≥ 3 days) by roughly 50% in patients with severe eosinophilic asthma and a history of ≥ 2 exacerbations in the past year.
Dupilumab is a humanized MAb that inhibits IL-4 and IL-13, which influence multiple cell types involved in inflammation (eg, mast cells, eosinophils) and inflammatory mediators (histamine, leukotrienes, cytokines).15 In a recent study of patients with uncontrolled asthma, dupilumab 200 mg every 2 weeks compared with placebo showed a modest reduction in the annualized rate of severe asthma exacerbations (0.46 exacerbations vs 0.87, respectively). Dupilumab was effective in patients with blood eosinophil counts ≥ 150/μL but was ineffective in patients with eosinophil counts < 150/μL.15
For patients ≥ 12 years old with severe eosinophilic asthma, GINA recommends using dupilumab as add-on therapy for an initial trial of 4 months at doses of 200 or 300 mg SC every 2 weeks, with preference for 300 mg SC every 2 weeks for OCS-dependent asthma. Dupilumab is approved for use in AD and chronic rhinosinusitis with nasal polyposis. If a biologic agent is not successful after a 4-month trial, consider a 6- to 12-month trial. If efficacy is still minimal, consider switching to an alternative biologic therapy approved for asthma.16
❯ Asthma: Test your skills
Subjective findings: A 19-year-old man presents to your clinic. He has a history of nasal polyps and allergic asthma. At age 18, he was given a diagnosis of severe persistent asthma. He has shortness of breath during waking hours 4 times per week, and treats each of these episodes with albuterol. He also wakes up about twice a week with shortness of breath and has some limitations in normal activities. He reports missing his prescribed fluticasone/salmeterol 500/50 μg, 1 inhalation bid, only once each month. In the last year, he has had 2 exacerbations requiring oral steroids.
Medications: Albuterol 90 μg, 1-2 inhalations, q6h prn; fluticasone/salmeterol 500/50 μg, 1 inhalation bid; tiotropium 1.25 μg, 2 puffs/d; montelukast 10 mg every morning; prednisone 10 mg/d.
Continue to: Objective data
Objective data: Patient is in no apparent distress and afebrile, and oxygen saturation on room air is 97%. Ht, 70 inches; wt, 75 kg. Labs: IgE, 15 IU/mL; serum eosinophils, 315/μL.
Which MAb would be appropriate for this patient? Given that the patient has a blood eosinophil level ≥ 300/μL and severe exacerbations, adult-onset asthma, nasal polyposis, and maintenance OCS at baseline, it would be reasonable to initiate mepolizumab 100 mg SC every 4 weeks, or dupilumab 600 mg once, then 300 mg SC every 2 weeks. Both agents can be self-administered.
Atopic dermatitis
Two MAbs—dupilumab and tralokinumab (Adbry; inhibits IL-13)—are approved for treatment of AD in adults that is uncontrolled with conventional therapy.15,19 Dupilumab is also approved for children ≥ 6 months old.20 Both MAbs are dosed at 600 mg SC, followed by 300 mg every 2 weeks. Dupilumab was compared with placebo in adult patients who had moderate-to-severe AD inadequately controlled on topical corticosteroids (TCSs), to determine the proportion of patients in each group achieving improvement of either 0 or 1 points or ≥ 2 points in the 5-point Investigator Global Assessment (IGA) score from baseline to 16 weeks.21 Thirty-seven percent of patients receiving dupilumab 300 mg SC weekly and 38% of patients receiving dupilumab 300 mg SC every 2 weeks achieved the primary outcome, compared with 10% of those receiving placebo (P < .001).21 Similar IGA scores were reported when dupilumab was combined with TCS, compared with placebo.22
It would be reasonable to consider dupilumab or tralokinumab in patients with: cutaneous atrophy or hypothalamic-pituitary-adrenal axis suppression with TCS, concerns of malignancy with topical calcineurin inhibitors, or problems with the alternative systemic therapies (cyclosporine-induced hypertension, nephrotoxicity, or immunosuppression; azathioprine-induced malignancy; or methotrexate-induced bone marrow suppression, renal impairment, hepatotoxicity, pneumonitis, or gastrointestinal toxicity).23
A distinct advantage of MAbs over other systemic agents in the management of AD is that MAbs do not require frequent monitoring of blood pressure, renal or liver function, complete blood count with differential, electrolytes, or uric acid. Additionally, MAbs have fewer black box warnings and adverse reactions when compared with other systemic agents.
Continue to: Hyperlipidemia
Hyperlipidemia
Three MAbs are approved for use in hyperlipidemia: the angiopoietin-like protein 3 (ANGPTL3) inhibitor evinacumab (Evkeeza)24 and 2 proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, evolocumab (Repatha)25 and alirocumab (Praluent).26
ANGPTL3 inhibitors block ANGPTL3 and reduce endothelial lipase and lipoprotein lipase activity, which in turn decreases low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride formation. PCSK9 inhibitors prevent PCSK9 from binding to LDL receptors, thereby maintaining the number of active LDL receptors and increasing LDL-C removal.
Evinacumab is indicated for homozygous familial hypercholesterolemia and is administered intravenously every 4 weeks. Evinacumab has not been evaluated for effects on cardiovascular morbidity and mortality.
Evolocumab 140 mg SC every 2 weeks or 420 mg SC monthly has been studied in patients on statin therapy with LDL-C ≥ 70 mg/dL. Patients on evolocumab experienced significantly less of the composite endpoint of cardiovascular death, myocardial infarction (MI), stroke, hospitalization for unstable angina, or coronary revascularization compared with placebo (9.8% vs 11.3%; hazard ratio [HR] = 0.85; 95% CI, 0.79-0.92; NNT = 67.27
Alirocumab 75 mg SC every 2 weeks has also been studied in patients receiving statin therapy with LDL-C ≥ 70 mg/dL. Patients taking alirocumab experienced significantly less of the composite endpoint of death from coronary heart disease, nonfatal MI, ischemic stroke, or hospitalization for unstable angina compared with placebo (9.5% vs 11.1%; HR = 0.85; 95% CI, 0.78-0.93; NNT = 63).
Continue to: According to the 2018...
According to the 2018 AHA Cholesterol Guidelines, PCSK9 inhibitors are indicated for patients receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe) with LDL-C ≥ 70 mg/dL, if they have had multiple atherosclerotic cardiovascular disease (ASCVD) events or 1 major ASCVD event with multiple high-risk conditions (eg, heterozygous familial hypercholesterolemia, history of coronary artery bypass grafting or percutaneous coronary intervention, hypertension, estimated glomerular filtration rate of 15 to 59 mL/min/1.73m2).29 For patients without prior ASCVD events or high-risk conditions who are receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe), PCSK9 inhibitors are indicated if the LDL-C remains ≥ 100 mg/dL.
Osteoporosis
The 2 MAbs approved for use in osteoporosis are the receptor activator of nuclear factor kB ligand (RANKL) inhibitor denosumab (Prolia)30 and the sclerostin inhibitor romosozumab (Evenity).31
Denosumab prevents RANKL from binding to the RANK receptor, thereby inhibiting osteoclast formation and decreasing bone resorption. Denosumab is approved for use in women and men who are at high risk of osteoporotic fracture, including those taking OCSs, men receiving androgen deprivation therapy for prostate cancer, and women receiving adjuvant aromatase inhibitor therapy for breast cancer.
In a 3-year randomized trial, denosumab 60 mg SC every 6 months was compared with placebo in postmenopausal women with T-scores < –2.5, but not < –4.0 at the lumbar spine or total hip. Denosumab significantly reduced new radiographic vertebral fractures (2.3% vs 7.2%; risk ratio [RR] = 0.32; 95% CI, 0.26-0.41; NNT = 21), hip fracture (0.7% vs 1.2%), and nonvertebral fracture (6.5% vs 8.0%).32 Denosumab carries an increased risk of multiple vertebral fractures following discontinuation, skin infections, dermatologic reactions, and severe bone, joint, and muscle pain.
Romosozumab inhibits sclerostin, thereby increasing bone formation and, to a lesser degree, decreasing bone resorption. Romosozumab is approved for use in postmenopausal women at high risk for fracture (ie, those with a history of osteoporotic fracture or multiple risk factors for fracture) or in patients who have not benefited from or are intolerant of other therapies. In one study, postmenopausal women with a T-score of –2.5 to –3.5 at the total hip or femoral neck were randomly assigned to receive either romosozumab 210 mg SC or placebo for 12 months, then each group was switched to denosumab 60 mg SC for 12 months. After the first year, prior to initiating denosumab, patients taking romosozumab experienced significantly fewer new vertebral fractures than patients taking placebo (0.5% vs 1.8%; RR = 0.27; 95% CI, 0.16-0.47; NNT = 77); however, there was no significant difference between the 2 groups with nonvertebral fractures (HR = 0.75; 95% CI, 0.53-1.05).33
Continue to: In another study...
In another study, romosozumab 210 mg SC was compared with alendronate 70 mg weekly, followed by alendronate 70 mg weekly in both groups. Over the first 12 months, patients treated with romosozumab saw a significant reduction in the incidence of new vertebral fractures (4% vs 6.3%; RR = 0.63, P < .003; NNT = 44). Patients treated with romosozumab with alendronate added for another 12 months also saw a significant reduction in new incidence of vertebral fractures (6.2% vs 11.9%; RR = 0.52; P < .001; NNT = 18).34 There was a higher risk of cardiovascular events among patients receiving romosozumab compared with those treated with alendronate, so romosozumab should not be used in individuals who have had an MI or stroke within the previous year.34 Denosumab and romosozumab offer an advantage over some bisphosphonates in that they require less frequent dosing and can be used in patients with renal impairment (creatinine clearance < 35 mL/min, in which zoledronic acid is contraindicated and alendronate is not recommended; < 30 mL/min, in which risedronate and ibandronate are not recommended).
Migraine prevention
Four
Erenumab, fremanezumab, and galcanezumab are all available in subcutaneous autoinjectors (or syringe with fremanezumab). Eptinezumab is an intravenous (IV) infusion given every 3 months.
Erenumab is available in both 70-mg and 140-mg dosing options. Fremanezumab can be given as 225 mg monthly or 675 mg quarterly. Galcanezumab has an initial loading dose of 240 mg followed by 120 mg given monthly. Erenumab targets the CGRP receptor; the others target the CGRP ligand. Eptinezumab has 100% bioavailability and reaches maximum serum concentration sooner than the other antagonists (due to its route of administration), but it must be given in an infusion center. Few insurers approve the use of eptinezumab unless a trial of least 1 of the monthly injectables has failed.
There are no head-to-head studies of the medications in this class. Additionally, differing study designs, definitions, statistical analyses, endpoints, and responder-rate calculations make it challenging to compare them directly against one another. At the very least, all of the CGRP MAbs have efficacy comparable to conventional preventive migraine medications such as propranolol, amitriptyline, and topiramate.40
Continue to: The most commonly reported adverse...
The most commonly reported adverse effect for all 4 CGRPs is injection site reaction, which was highest with the quarterly fremanezumab dose (45%).37 Constipation was most notable with the 140-mg dose of erenumab (3%)35; with the other CGRP MAbs it is comparable to that seen with placebo (< 1%).
Erenumab-induced hypertension has been identified in 61 cases reported through the FDA Adverse Event Reporting System (FAERS) as of 2021.41 This was not reported during MAb development programs, nor was it noted during clinical trials. Blood pressure elevation was seen within 1 week of injection in nearly 50% of the cases, and nearly one-third had pre-existing hypertension.41 Due to these findings, the erenumab prescribing information was updated to include hypertension in its warnings and precautions. It is possible that hypertension could be a class effect, although trial data and posthoc studies have yet to bear that out. Since erenumab was the first CGRP antagonist brought to market (May 2018 vs September 2018 for fremanezumab and galcanezumab), it may have accumulated more FAERS reports. Nearly all studies exclude patients with older age, uncontrolled hypertension, and unstable cardiovascular disease, which could impact data.41
Overall, this class of medications is very well tolerated, easy to use (again, excluding eptinezumab), and maintains a low adverse effect profile, giving added value compared with conventional preventive migraine medications.
The American Headache Society recommends a preventive oral therapy for at least 3 months before trying an alternative medication. After treatment failure with at least 2 oral agents, CGRP MAbs are recommended.42 CGRP antagonists offer convenient dosing, bypass gastrointestinal metabolism (which is useful in patients with nausea/vomiting), and have fewer adverse effects than traditional oral medications.
Worth noting. Several newer oral agents have been recently approved for migraine prevention, including atogepant (Qulipta) and rimegepant (Nurtec), which are also CGRP antagonists. Rimegepant is approved for both acute migraine treatment and prevention.
Continue to: Migraine
❯ Migraine: Test your skills
Subjective findings: A 25-year-old woman presents to your clinic for management of episodic migraines with aura. Her baseline average migraine frequency is 9 headache days/month. Her migraines are becoming more frequent despite treatment. She fears IV medication use and avoids hospitals.
History: Hypertension, irritable bowel syndrome with constipation (IBS-C), and depression. The patient is not pregnant or trying to get pregnant.
Medications: Current medications (for previous 4 months) include propranolol 40 mg at bedtime, linaclotide 145 μg/d, citalopram 20 mg/d, and sumatriptan 50 mg prn. Past medications include venlafaxine 150 mg po bid for 5 months.
What would be appropriate for this patient? This patient meets the criteria for using a CGRP antagonist because she has tried 2 preventive treatments for more than 60 to 90 days. Erenumab is not the best option, given the patient’s history of hypertension and IBS-C. The patient fears hospitals and IV medications, making eptinezumab a less-than-ideal choice. Depending on her insurance, fremanezumab or galcanezumab would be good options at this time.
CGRP antagonists have not been studied or evaluated in pregnancy, but if this patient becomes pregnant, a first-line agent for prevention would be propranolol, and a second-line agent would be a tricyclic antidepressant, memantine, or verapamil. Avoid ergotamines and antiepileptics (topiramate or valproate) in pregnancy.43,44
Continue to: The challenges associated with MAbs
The challenges associated with MAbs
MAbs can be expensive (TABLE 2),45 some prohibitively so. On a population scale, biologics account for around 40% of prescription drug spending and may cost 22 times more than small-molecule drugs.46 Estimates in 2016 showed that MAbs comprise $90.2 billion (43%) of the biologic market.46

MAbs also require prior authorization forms to be submitted. Prior authorization criteria vary by state and by insurance plan. In my (ES) experience, submitting letters of medical necessity justifying the need for therapy or expertise in the disease states for which the MAb is being prescribed help your patient get the medication they need.
Expect to see additional MAbs approved in the future. If the costs come down, adoption of these agents into practice will likely increase.
CORRESPONDENCE
Evelyn Sbar, MD, Texas Tech University Health Sciences Center, 1400 South Coulter Street, Suite 5100, Amarillo, TX 79106; evelyn.sbar@ttuhsc.edu
Small-molecule drugs such as aspirin, albuterol, atorvastatin, and lisinopril are the backbone of disease management in family medicine.1 However, large-molecule biological drugs such as monoclonal antibodies (MAbs) are increasingly prescribed to treat common conditions. In the past decade, MAbs comprised 20% of all drug approvals by the US Food and Drug Administration (FDA), and today they represent more than half of drugs currently in development.2 Fifteen MAbs have been approved by the FDA over the past decade for asthma, atopic dermatitis (AD), hyperlipidemia, osteoporosis, and migraine prevention.3 This review details what makes MAbs unique and what you should know about them.
The uniqueness of monoclonal antibodies
MAbs are biologics, but not all biologics are MAbs—eg, adalimumab (Humira) is a MAb, but etanercept (Enbrel) is not. MAbs are therapeutic proteins made possible by hybridoma technology used to create an antibody with single specificity.4-6 Monoclonal antibodies differ from small-molecule drugs in structure, dosing, route of administration, manufacturing, metabolism, drug interactions, and elimination (TABLE 17-9).

MAbs can be classified as naked, “without any drug or radioactive material attached to them,” or conjugated, “joined to a chemotherapy drug, radioactive isotope, or toxin.”10 MAbs work in several ways, including competitively inhibiting ligand-receptor binding, receptor blockade, or cell elimination from indirect immune system activities such as antibody-dependent cell-mediated cytotoxicity.11,12
Monoclonal antibody uses in family medicine
Asthma
Several MAbs have been approved for use in severe asthma, including but not limited to: omalizumab (Xolair),13 mepolizumab (Nucala),9,14 and dupilumab (Dupixent).15
Omalizumab is a humanized MAb that prevents IgE antibodies from binding to mast cells and basophils, thereby reducing inflammatory mediators.13 A systematic review found that, compared with placebo, omalizumab used in patients with inadequately controlled moderate-to-severe asthma led to significantly fewer asthma exacerbations (absolute risk reduction [ARR], 16% vs 26%; odds ratio [OR] = 0.55; 95% CI, 0.42-0.60; number needed to treat [NNT] = 10) and fewer hospitalizations (ARR, 0.5% vs 3%; OR = 0.16; 95% CI, 0.06-0.42; NNT = 40).13
Significantly more patients in the omalizumab group were able to withdraw from, or reduce, the dose of ICS. GINA recommends omalizumab for patients with positive skin sensitization, total serum IgE ≥ 30 IU/mL, weight within 30 kg to 150 kg, history of childhood asthma and recent exacerbations, and blood eosinophils ≥ 260/mcL.16 Omalizumab is also approved for use in chronic spontaneous urticaria and nasal polyps.
Mepolizumab
Continue to: Another trial found that...
Another trial found that mepolizumab reduced total OCS doses in patients with severe asthma by 50% without increasing exacerbations or worsening asthma control.18 All 3 anti-IL-5 drugs—including not only mepolizumab, but also benralizumab (Fasenra) and reslizumab (Cinqair)—appear to yield similar improvements. A 2017 systematic review found all anti-IL-5 treatments reduced rates of clinically significant asthma exacerbations (treatment with OCS for ≥ 3 days) by roughly 50% in patients with severe eosinophilic asthma and a history of ≥ 2 exacerbations in the past year.
Dupilumab is a humanized MAb that inhibits IL-4 and IL-13, which influence multiple cell types involved in inflammation (eg, mast cells, eosinophils) and inflammatory mediators (histamine, leukotrienes, cytokines).15 In a recent study of patients with uncontrolled asthma, dupilumab 200 mg every 2 weeks compared with placebo showed a modest reduction in the annualized rate of severe asthma exacerbations (0.46 exacerbations vs 0.87, respectively). Dupilumab was effective in patients with blood eosinophil counts ≥ 150/μL but was ineffective in patients with eosinophil counts < 150/μL.15
For patients ≥ 12 years old with severe eosinophilic asthma, GINA recommends using dupilumab as add-on therapy for an initial trial of 4 months at doses of 200 or 300 mg SC every 2 weeks, with preference for 300 mg SC every 2 weeks for OCS-dependent asthma. Dupilumab is approved for use in AD and chronic rhinosinusitis with nasal polyposis. If a biologic agent is not successful after a 4-month trial, consider a 6- to 12-month trial. If efficacy is still minimal, consider switching to an alternative biologic therapy approved for asthma.16
❯ Asthma: Test your skills
Subjective findings: A 19-year-old man presents to your clinic. He has a history of nasal polyps and allergic asthma. At age 18, he was given a diagnosis of severe persistent asthma. He has shortness of breath during waking hours 4 times per week, and treats each of these episodes with albuterol. He also wakes up about twice a week with shortness of breath and has some limitations in normal activities. He reports missing his prescribed fluticasone/salmeterol 500/50 μg, 1 inhalation bid, only once each month. In the last year, he has had 2 exacerbations requiring oral steroids.
Medications: Albuterol 90 μg, 1-2 inhalations, q6h prn; fluticasone/salmeterol 500/50 μg, 1 inhalation bid; tiotropium 1.25 μg, 2 puffs/d; montelukast 10 mg every morning; prednisone 10 mg/d.
Continue to: Objective data
Objective data: Patient is in no apparent distress and afebrile, and oxygen saturation on room air is 97%. Ht, 70 inches; wt, 75 kg. Labs: IgE, 15 IU/mL; serum eosinophils, 315/μL.
Which MAb would be appropriate for this patient? Given that the patient has a blood eosinophil level ≥ 300/μL and severe exacerbations, adult-onset asthma, nasal polyposis, and maintenance OCS at baseline, it would be reasonable to initiate mepolizumab 100 mg SC every 4 weeks, or dupilumab 600 mg once, then 300 mg SC every 2 weeks. Both agents can be self-administered.
Atopic dermatitis
Two MAbs—dupilumab and tralokinumab (Adbry; inhibits IL-13)—are approved for treatment of AD in adults that is uncontrolled with conventional therapy.15,19 Dupilumab is also approved for children ≥ 6 months old.20 Both MAbs are dosed at 600 mg SC, followed by 300 mg every 2 weeks. Dupilumab was compared with placebo in adult patients who had moderate-to-severe AD inadequately controlled on topical corticosteroids (TCSs), to determine the proportion of patients in each group achieving improvement of either 0 or 1 points or ≥ 2 points in the 5-point Investigator Global Assessment (IGA) score from baseline to 16 weeks.21 Thirty-seven percent of patients receiving dupilumab 300 mg SC weekly and 38% of patients receiving dupilumab 300 mg SC every 2 weeks achieved the primary outcome, compared with 10% of those receiving placebo (P < .001).21 Similar IGA scores were reported when dupilumab was combined with TCS, compared with placebo.22
It would be reasonable to consider dupilumab or tralokinumab in patients with: cutaneous atrophy or hypothalamic-pituitary-adrenal axis suppression with TCS, concerns of malignancy with topical calcineurin inhibitors, or problems with the alternative systemic therapies (cyclosporine-induced hypertension, nephrotoxicity, or immunosuppression; azathioprine-induced malignancy; or methotrexate-induced bone marrow suppression, renal impairment, hepatotoxicity, pneumonitis, or gastrointestinal toxicity).23
A distinct advantage of MAbs over other systemic agents in the management of AD is that MAbs do not require frequent monitoring of blood pressure, renal or liver function, complete blood count with differential, electrolytes, or uric acid. Additionally, MAbs have fewer black box warnings and adverse reactions when compared with other systemic agents.
Continue to: Hyperlipidemia
Hyperlipidemia
Three MAbs are approved for use in hyperlipidemia: the angiopoietin-like protein 3 (ANGPTL3) inhibitor evinacumab (Evkeeza)24 and 2 proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, evolocumab (Repatha)25 and alirocumab (Praluent).26
ANGPTL3 inhibitors block ANGPTL3 and reduce endothelial lipase and lipoprotein lipase activity, which in turn decreases low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride formation. PCSK9 inhibitors prevent PCSK9 from binding to LDL receptors, thereby maintaining the number of active LDL receptors and increasing LDL-C removal.
Evinacumab is indicated for homozygous familial hypercholesterolemia and is administered intravenously every 4 weeks. Evinacumab has not been evaluated for effects on cardiovascular morbidity and mortality.
Evolocumab 140 mg SC every 2 weeks or 420 mg SC monthly has been studied in patients on statin therapy with LDL-C ≥ 70 mg/dL. Patients on evolocumab experienced significantly less of the composite endpoint of cardiovascular death, myocardial infarction (MI), stroke, hospitalization for unstable angina, or coronary revascularization compared with placebo (9.8% vs 11.3%; hazard ratio [HR] = 0.85; 95% CI, 0.79-0.92; NNT = 67.27
Alirocumab 75 mg SC every 2 weeks has also been studied in patients receiving statin therapy with LDL-C ≥ 70 mg/dL. Patients taking alirocumab experienced significantly less of the composite endpoint of death from coronary heart disease, nonfatal MI, ischemic stroke, or hospitalization for unstable angina compared with placebo (9.5% vs 11.1%; HR = 0.85; 95% CI, 0.78-0.93; NNT = 63).
Continue to: According to the 2018...
According to the 2018 AHA Cholesterol Guidelines, PCSK9 inhibitors are indicated for patients receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe) with LDL-C ≥ 70 mg/dL, if they have had multiple atherosclerotic cardiovascular disease (ASCVD) events or 1 major ASCVD event with multiple high-risk conditions (eg, heterozygous familial hypercholesterolemia, history of coronary artery bypass grafting or percutaneous coronary intervention, hypertension, estimated glomerular filtration rate of 15 to 59 mL/min/1.73m2).29 For patients without prior ASCVD events or high-risk conditions who are receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe), PCSK9 inhibitors are indicated if the LDL-C remains ≥ 100 mg/dL.
Osteoporosis
The 2 MAbs approved for use in osteoporosis are the receptor activator of nuclear factor kB ligand (RANKL) inhibitor denosumab (Prolia)30 and the sclerostin inhibitor romosozumab (Evenity).31
Denosumab prevents RANKL from binding to the RANK receptor, thereby inhibiting osteoclast formation and decreasing bone resorption. Denosumab is approved for use in women and men who are at high risk of osteoporotic fracture, including those taking OCSs, men receiving androgen deprivation therapy for prostate cancer, and women receiving adjuvant aromatase inhibitor therapy for breast cancer.
In a 3-year randomized trial, denosumab 60 mg SC every 6 months was compared with placebo in postmenopausal women with T-scores < –2.5, but not < –4.0 at the lumbar spine or total hip. Denosumab significantly reduced new radiographic vertebral fractures (2.3% vs 7.2%; risk ratio [RR] = 0.32; 95% CI, 0.26-0.41; NNT = 21), hip fracture (0.7% vs 1.2%), and nonvertebral fracture (6.5% vs 8.0%).32 Denosumab carries an increased risk of multiple vertebral fractures following discontinuation, skin infections, dermatologic reactions, and severe bone, joint, and muscle pain.
Romosozumab inhibits sclerostin, thereby increasing bone formation and, to a lesser degree, decreasing bone resorption. Romosozumab is approved for use in postmenopausal women at high risk for fracture (ie, those with a history of osteoporotic fracture or multiple risk factors for fracture) or in patients who have not benefited from or are intolerant of other therapies. In one study, postmenopausal women with a T-score of –2.5 to –3.5 at the total hip or femoral neck were randomly assigned to receive either romosozumab 210 mg SC or placebo for 12 months, then each group was switched to denosumab 60 mg SC for 12 months. After the first year, prior to initiating denosumab, patients taking romosozumab experienced significantly fewer new vertebral fractures than patients taking placebo (0.5% vs 1.8%; RR = 0.27; 95% CI, 0.16-0.47; NNT = 77); however, there was no significant difference between the 2 groups with nonvertebral fractures (HR = 0.75; 95% CI, 0.53-1.05).33
Continue to: In another study...
In another study, romosozumab 210 mg SC was compared with alendronate 70 mg weekly, followed by alendronate 70 mg weekly in both groups. Over the first 12 months, patients treated with romosozumab saw a significant reduction in the incidence of new vertebral fractures (4% vs 6.3%; RR = 0.63, P < .003; NNT = 44). Patients treated with romosozumab with alendronate added for another 12 months also saw a significant reduction in new incidence of vertebral fractures (6.2% vs 11.9%; RR = 0.52; P < .001; NNT = 18).34 There was a higher risk of cardiovascular events among patients receiving romosozumab compared with those treated with alendronate, so romosozumab should not be used in individuals who have had an MI or stroke within the previous year.34 Denosumab and romosozumab offer an advantage over some bisphosphonates in that they require less frequent dosing and can be used in patients with renal impairment (creatinine clearance < 35 mL/min, in which zoledronic acid is contraindicated and alendronate is not recommended; < 30 mL/min, in which risedronate and ibandronate are not recommended).
Migraine prevention
Four
Erenumab, fremanezumab, and galcanezumab are all available in subcutaneous autoinjectors (or syringe with fremanezumab). Eptinezumab is an intravenous (IV) infusion given every 3 months.
Erenumab is available in both 70-mg and 140-mg dosing options. Fremanezumab can be given as 225 mg monthly or 675 mg quarterly. Galcanezumab has an initial loading dose of 240 mg followed by 120 mg given monthly. Erenumab targets the CGRP receptor; the others target the CGRP ligand. Eptinezumab has 100% bioavailability and reaches maximum serum concentration sooner than the other antagonists (due to its route of administration), but it must be given in an infusion center. Few insurers approve the use of eptinezumab unless a trial of least 1 of the monthly injectables has failed.
There are no head-to-head studies of the medications in this class. Additionally, differing study designs, definitions, statistical analyses, endpoints, and responder-rate calculations make it challenging to compare them directly against one another. At the very least, all of the CGRP MAbs have efficacy comparable to conventional preventive migraine medications such as propranolol, amitriptyline, and topiramate.40
Continue to: The most commonly reported adverse...
The most commonly reported adverse effect for all 4 CGRPs is injection site reaction, which was highest with the quarterly fremanezumab dose (45%).37 Constipation was most notable with the 140-mg dose of erenumab (3%)35; with the other CGRP MAbs it is comparable to that seen with placebo (< 1%).
Erenumab-induced hypertension has been identified in 61 cases reported through the FDA Adverse Event Reporting System (FAERS) as of 2021.41 This was not reported during MAb development programs, nor was it noted during clinical trials. Blood pressure elevation was seen within 1 week of injection in nearly 50% of the cases, and nearly one-third had pre-existing hypertension.41 Due to these findings, the erenumab prescribing information was updated to include hypertension in its warnings and precautions. It is possible that hypertension could be a class effect, although trial data and posthoc studies have yet to bear that out. Since erenumab was the first CGRP antagonist brought to market (May 2018 vs September 2018 for fremanezumab and galcanezumab), it may have accumulated more FAERS reports. Nearly all studies exclude patients with older age, uncontrolled hypertension, and unstable cardiovascular disease, which could impact data.41
Overall, this class of medications is very well tolerated, easy to use (again, excluding eptinezumab), and maintains a low adverse effect profile, giving added value compared with conventional preventive migraine medications.
The American Headache Society recommends a preventive oral therapy for at least 3 months before trying an alternative medication. After treatment failure with at least 2 oral agents, CGRP MAbs are recommended.42 CGRP antagonists offer convenient dosing, bypass gastrointestinal metabolism (which is useful in patients with nausea/vomiting), and have fewer adverse effects than traditional oral medications.
Worth noting. Several newer oral agents have been recently approved for migraine prevention, including atogepant (Qulipta) and rimegepant (Nurtec), which are also CGRP antagonists. Rimegepant is approved for both acute migraine treatment and prevention.
Continue to: Migraine
❯ Migraine: Test your skills
Subjective findings: A 25-year-old woman presents to your clinic for management of episodic migraines with aura. Her baseline average migraine frequency is 9 headache days/month. Her migraines are becoming more frequent despite treatment. She fears IV medication use and avoids hospitals.
History: Hypertension, irritable bowel syndrome with constipation (IBS-C), and depression. The patient is not pregnant or trying to get pregnant.
Medications: Current medications (for previous 4 months) include propranolol 40 mg at bedtime, linaclotide 145 μg/d, citalopram 20 mg/d, and sumatriptan 50 mg prn. Past medications include venlafaxine 150 mg po bid for 5 months.
What would be appropriate for this patient? This patient meets the criteria for using a CGRP antagonist because she has tried 2 preventive treatments for more than 60 to 90 days. Erenumab is not the best option, given the patient’s history of hypertension and IBS-C. The patient fears hospitals and IV medications, making eptinezumab a less-than-ideal choice. Depending on her insurance, fremanezumab or galcanezumab would be good options at this time.
CGRP antagonists have not been studied or evaluated in pregnancy, but if this patient becomes pregnant, a first-line agent for prevention would be propranolol, and a second-line agent would be a tricyclic antidepressant, memantine, or verapamil. Avoid ergotamines and antiepileptics (topiramate or valproate) in pregnancy.43,44
Continue to: The challenges associated with MAbs
The challenges associated with MAbs
MAbs can be expensive (TABLE 2),45 some prohibitively so. On a population scale, biologics account for around 40% of prescription drug spending and may cost 22 times more than small-molecule drugs.46 Estimates in 2016 showed that MAbs comprise $90.2 billion (43%) of the biologic market.46

MAbs also require prior authorization forms to be submitted. Prior authorization criteria vary by state and by insurance plan. In my (ES) experience, submitting letters of medical necessity justifying the need for therapy or expertise in the disease states for which the MAb is being prescribed help your patient get the medication they need.
Expect to see additional MAbs approved in the future. If the costs come down, adoption of these agents into practice will likely increase.
CORRESPONDENCE
Evelyn Sbar, MD, Texas Tech University Health Sciences Center, 1400 South Coulter Street, Suite 5100, Amarillo, TX 79106; evelyn.sbar@ttuhsc.edu
1. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. National Center for Health Statistics. Accessed June 15, 2022. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
2. IDBS. The future of biologics drug development is today. June 27, 2018. Accessed June 15, 2022. www.idbs.com/blog/2018/06/the-future-of-biologics-drug-development-is-today/
3. Antibody therapeutics approved or in regulatory review in the EU or US. Antibody Society. Accessed June 15, 2022. www.antibodysociety.org/resources/approved-antibodies/
4. FDA. Code of Federal Regulations, Title 21, Chapter I, Subchapter F biologics. March 29, 2022. Accessed June 15, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=600.3
5. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495-497. doi: 10.1038/256495a0
6. Raejewsky K. The advent and rise of monoclonal antibodies. Nature. November 4, 2019. Accessed June 15, 2022. www.nature.com/articles/d41586-019-02840-w
7. Flovent. Prescribing information. GlaxoSmithKline; 2010. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2010/021433s015lbl.pdf
8. NLM. National Center for Biotechnology Information. PubChem. Method for the preparation of fluticasone and related 17beta-carbothioic esters using a novel carbothioic acid synthesis and novel purification methods. Accessed June 15, 2022. pubchem.ncbi.nlm.nih.gov/patent/WO-0162722-A2
9. Nucala. Prescribing information. GlaxoSmithKline; 2019. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761122s000lbl.pdf
10. Argyriou AA, Kalofonos HP. Recent advances relating to the clinical application of naked monoclonal antibodies in solid tumors. Mol Med. 2009;15:183-191. doi: 10.2119/molmed.2009.00007
11. Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548-558. doi: 10.1038/clpt.2008.170
12. Zahavi D, AlDeghaither D, O’Connell A, et al. Enhancing antibody-dependent cell-mediated cytotoxicity: a strategy for improving antibody-based immunotherapy. Antib Ther. 2018;1:7-12. doi: 10.1093/abt/tby002
13. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014:CD003559. doi: 10.1002/14651858.CD003559.pub4
14. Farne HA, Wilson A, Powell C, et al. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:CD010834. doi: 10.1002/14651858.CD010834.pub3
15. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486-2496. doi: 10.1056/NEJMoa1804092
16. GINA. Global strategy for asthma management and prevention. 2022 Difficult-to-treat and severe asthma guide—slide set. Accessed June 23, 2022. https://ginasthma.org/severeasthma/
17. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207. doi: 10.1056/NEJMoa1403290
18. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197. doi: 10.1056/NEJMoa1403291
19. Adbry. Prescribing information. Leo Pharma Inc; 2021. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761180Orig1s000lbl.pdf
20. Dupixent. Prescribing information. Regeneron Pharmaceuticals; 2022. Accessed October 5, 2022. https://www.regeneron.com/downloads/dupixent_fpi.pdf
21. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348. doi: 10.1056/NEJMoa1610020
22. Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303. doi: 10.1016/s0140-6736(17)31191-1
23. Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi: 10.1016/j.jaad.2014.03.030
24. Evkeeza. Prescribing information. Regeneron Pharmaceuticals; 2021. Accessed June 24, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761181s000lbl.pdf
25. Repatha. Prescribing information. Amgen; 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125522s014lbl.pdf
26. Praluent. Prescribing information. Sanofi Aventis and Regeneron Pharmaceuticals. 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125559s002lbl.pdf
27. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722. doi: 10.1056/NEJMoa1615664
28. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097-2107. doi:10.1056/NEJMoa1801174
29. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
30. Prolia. Prescribing information. Amgen; 2010. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2013/125320s094lbl.pdf
31. Evenity. Prescribing information. Amgen; 2019. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761062s000lbl.pdf
32. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
33. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543. doi: 10.1056/NEJMoa1607948
34. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427. doi: 10.1056/NEJMoa1708322
35. Aimovig. Prescribing information. Amgen; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761077s000lbl.pdf
36. Vyepti. Prescribing information. Lundbeck Seattle BioPharmaceuticals; 2020. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2020/761119s000lbl.pdf
37. Ajovy. Prescribing information. Teva Pharmaceuticals; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.pdf
38. Emgality. Prescribing information. Eli Lilly and Co.; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761063s000lbl.pdf
39. Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338-350. doi: 10.1038/s41582-018-0003-1
40. Vandervorst F. Van Deun L, Van Dycke A, et al. CGRP monoclonal antibodies in migraine: an efficacy and tolerability comparison with standard prophylactic drugs. J Headache Pain. 2021;22:128. doi: 10.1186/s10194-021-01335-2
41. Saely S, Croteau D, Jawidzik L, et al. Hypertension: a new safety risk for patients treated with erenumab. Headache. 2021;61:202-208. doi: 10.1111/head.14051
42. American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1-18. doi: 10.1111/head.13456
43. Burch R. Headache in pregnancy and the puerperium. Neurol Clin. 2019;37:31-51. doi: 10.1016/j.ncl.2018.09.004
44. Burch R. Epidemiology and treatment of menstrual migraine and migraine during pregnancy and lactation: a narrative review. Headache. 2020;60:200-216. doi: 10.1111/head.13665
45. Lexi-Comp. Lexi-drug database. Accessed April 4, 2022. https://online.lexi.com/lco/action/login
46. Walker N. Biologics: driving force in pharma. Pharma’s Almanac. June 5, 2017. Accessed June 15, 2020. www.pharmasalmanac.com/articles/biologics-driving-force-in-pharma
1. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. National Center for Health Statistics. Accessed June 15, 2022. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
2. IDBS. The future of biologics drug development is today. June 27, 2018. Accessed June 15, 2022. www.idbs.com/blog/2018/06/the-future-of-biologics-drug-development-is-today/
3. Antibody therapeutics approved or in regulatory review in the EU or US. Antibody Society. Accessed June 15, 2022. www.antibodysociety.org/resources/approved-antibodies/
4. FDA. Code of Federal Regulations, Title 21, Chapter I, Subchapter F biologics. March 29, 2022. Accessed June 15, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=600.3
5. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495-497. doi: 10.1038/256495a0
6. Raejewsky K. The advent and rise of monoclonal antibodies. Nature. November 4, 2019. Accessed June 15, 2022. www.nature.com/articles/d41586-019-02840-w
7. Flovent. Prescribing information. GlaxoSmithKline; 2010. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2010/021433s015lbl.pdf
8. NLM. National Center for Biotechnology Information. PubChem. Method for the preparation of fluticasone and related 17beta-carbothioic esters using a novel carbothioic acid synthesis and novel purification methods. Accessed June 15, 2022. pubchem.ncbi.nlm.nih.gov/patent/WO-0162722-A2
9. Nucala. Prescribing information. GlaxoSmithKline; 2019. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761122s000lbl.pdf
10. Argyriou AA, Kalofonos HP. Recent advances relating to the clinical application of naked monoclonal antibodies in solid tumors. Mol Med. 2009;15:183-191. doi: 10.2119/molmed.2009.00007
11. Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548-558. doi: 10.1038/clpt.2008.170
12. Zahavi D, AlDeghaither D, O’Connell A, et al. Enhancing antibody-dependent cell-mediated cytotoxicity: a strategy for improving antibody-based immunotherapy. Antib Ther. 2018;1:7-12. doi: 10.1093/abt/tby002
13. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014:CD003559. doi: 10.1002/14651858.CD003559.pub4
14. Farne HA, Wilson A, Powell C, et al. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:CD010834. doi: 10.1002/14651858.CD010834.pub3
15. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486-2496. doi: 10.1056/NEJMoa1804092
16. GINA. Global strategy for asthma management and prevention. 2022 Difficult-to-treat and severe asthma guide—slide set. Accessed June 23, 2022. https://ginasthma.org/severeasthma/
17. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207. doi: 10.1056/NEJMoa1403290
18. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197. doi: 10.1056/NEJMoa1403291
19. Adbry. Prescribing information. Leo Pharma Inc; 2021. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761180Orig1s000lbl.pdf
20. Dupixent. Prescribing information. Regeneron Pharmaceuticals; 2022. Accessed October 5, 2022. https://www.regeneron.com/downloads/dupixent_fpi.pdf
21. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348. doi: 10.1056/NEJMoa1610020
22. Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303. doi: 10.1016/s0140-6736(17)31191-1
23. Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi: 10.1016/j.jaad.2014.03.030
24. Evkeeza. Prescribing information. Regeneron Pharmaceuticals; 2021. Accessed June 24, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761181s000lbl.pdf
25. Repatha. Prescribing information. Amgen; 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125522s014lbl.pdf
26. Praluent. Prescribing information. Sanofi Aventis and Regeneron Pharmaceuticals. 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125559s002lbl.pdf
27. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722. doi: 10.1056/NEJMoa1615664
28. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097-2107. doi:10.1056/NEJMoa1801174
29. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
30. Prolia. Prescribing information. Amgen; 2010. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2013/125320s094lbl.pdf
31. Evenity. Prescribing information. Amgen; 2019. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761062s000lbl.pdf
32. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
33. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543. doi: 10.1056/NEJMoa1607948
34. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427. doi: 10.1056/NEJMoa1708322
35. Aimovig. Prescribing information. Amgen; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761077s000lbl.pdf
36. Vyepti. Prescribing information. Lundbeck Seattle BioPharmaceuticals; 2020. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2020/761119s000lbl.pdf
37. Ajovy. Prescribing information. Teva Pharmaceuticals; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.pdf
38. Emgality. Prescribing information. Eli Lilly and Co.; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761063s000lbl.pdf
39. Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338-350. doi: 10.1038/s41582-018-0003-1
40. Vandervorst F. Van Deun L, Van Dycke A, et al. CGRP monoclonal antibodies in migraine: an efficacy and tolerability comparison with standard prophylactic drugs. J Headache Pain. 2021;22:128. doi: 10.1186/s10194-021-01335-2
41. Saely S, Croteau D, Jawidzik L, et al. Hypertension: a new safety risk for patients treated with erenumab. Headache. 2021;61:202-208. doi: 10.1111/head.14051
42. American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1-18. doi: 10.1111/head.13456
43. Burch R. Headache in pregnancy and the puerperium. Neurol Clin. 2019;37:31-51. doi: 10.1016/j.ncl.2018.09.004
44. Burch R. Epidemiology and treatment of menstrual migraine and migraine during pregnancy and lactation: a narrative review. Headache. 2020;60:200-216. doi: 10.1111/head.13665
45. Lexi-Comp. Lexi-drug database. Accessed April 4, 2022. https://online.lexi.com/lco/action/login
46. Walker N. Biologics: driving force in pharma. Pharma’s Almanac. June 5, 2017. Accessed June 15, 2020. www.pharmasalmanac.com/articles/biologics-driving-force-in-pharma
PRACTICE RECOMMENDATIONS
› Consider anti-immunoglobulin E, anti-interleukin 5, or anti-interleukin 4/interleukin 13 for patients with moderate-to-severe asthma and type 2 airway inflammation. B
› Consider dupilumab for patients with moderate-to-severe atopic dermatitis (with or without topical corticosteroids), or when traditional oral therapies are inadequate or contraindicated. B
› Consider proprotein convertase subtilisin/kexin type 9 inhibitors for patients with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease when maximally tolerated statins or ezetimibe have not lowered low-density lipoprotein cholesterol levels far enough. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Childhood peanut allergy linked with other legume allergies
French children with peanut allergy tend to have reactions to other legumes, including soy, lentil, pea, bean, lupin, and fenugreek, and those other allergies often lead to anaphylactic reactions, a retrospective study from France reports.
“Among children allergic to peanut, at least two-thirds were sensitized to one other legume, and legume allergy was diagnosed in one-quarter of the sensitized patients,” wrote senior study author Amandine Divaret-Chauveau, MD, of Centre Hospitalier Universitaire de Nancy, Vandoeuvre-les-Nancy, and colleagues. The report is in Pediatric Allergy and Immunology.
People worldwide are eating more legumes these days, the authors noted. High in protein, low in unsaturated fats, with low production costs, legumes are important components of increasingly vegetarian, healthy, sustainable diets.
Food allergens are the most common childhood triggers of allergic reactions. Among children in France, legumes cause 14.6% of food-related anaphylactic reactions, with peanut as the main allergen, they added.
Dr. Divaret-Chauveau and colleagues assessed the prevalence and relevance of sensitization to legumes among all children and adolescents aged 1-17 years who had peanut allergy and had been admitted to one academic pediatric allergy department over roughly 3 years, beginning in early 2017. For the 195 study participants, peanut allergy had been confirmed, and they had been documented to have consumed or to have sensitization to at least one non-peanut legume; 69.7% were boys.
The researchers analyzed data on consumption history, skin prick tests, specific immunoglobulin E status, prior allergic reactions, and oral food challenges for each legume. They found the following:
- Among the 195 children with peanut allergy, 98.4% had at least one other atopic disease.
- Of the 195 children with peanut allergy, 122 (63.9%) were sensitized to at least one other legume. Of these 122 children, 66.3% were sensitized to fenugreek, 42.2% to lentil, 39.9% to soy, and 34.2% to lupin.
- Allergy to one or more legumes was confirmed for 27.9% of the 122 sensitized children, including 4.9% who had multiple legume allergies. Lentil, lupin, and pea were the main allergens.
- Of the 118 children also having a non-legume food allergy, the main food allergens were egg (57.6%), cow’s milk (33.0%), cashew (39.0%), pistachio (23.7%), and hazelnut (30.5%).
- Fifty percent of allergic reactions to non-peanut legumes were severe, often showing as asthma. Atopic comorbidities, including asthma, in most participants may have contributed to the severity of allergic reactions, the authors noted.
Allergy awareness needs to grow with plant-based diets
“The high prevalence of legume sensitization reported in our study highlights the need to explore legume consumption in children with PA [peanut allergy], and the need to investigate sensitization in the absence of consumption,” they added.
Jodi A. Shroba, MSN, APRN, CPNP, coordinator for the Food Allergy Program at Children’s Mercy Kansas City, in Missouri, told this news organization that few data are available in the literature regarding allergies to legumes other than peanut.
“It was interesting that these authors found such a high legume sensitization in their peanut-allergic patients,” Ms. Shroba, who was not involved in the study, said by email. “As more people are starting to eat plant-based diets, it is important that we better understand their allergenicity and cross-reactivity so we can better help guide patient management and education.”
Deborah Albright, MD, assistant professor of pediatrics at the University of Pittsburgh, agreed.
“As plant-based protein consumption broadens worldwide, awareness of the potential for cross-reactivity and co-allergy amongst legumes will become increasingly important,” she said by email.
“However, positive allergy tests do not reliably correlate with true food allergy; therefore, the diagnosis of legume co-allergy should be confirmed by the individual patient’s history, a formal food challenge, or both,” advised Dr. Albright. She was not involved in the study.
“Cross-sensitization to other legumes in patients with a single legume allergy is common; however, true clinical reactivity is often not present,” she added. “Also, legume allergy test sensitization rates and objective reactivity on food challenge can vary by region, depending on diet and pollen aeroallergen exposure.
“Systematic exploration of tolerance versus co-allergy to other legumes should be considered in patients allergic to peanut or other legumes,” Dr. Albright said.
The authors recommend further research and registry data collection of legume anaphylaxis.
Details regarding funding for the study were not provided. The authors, Ms. Shroba, and Dr. Albright report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
French children with peanut allergy tend to have reactions to other legumes, including soy, lentil, pea, bean, lupin, and fenugreek, and those other allergies often lead to anaphylactic reactions, a retrospective study from France reports.
“Among children allergic to peanut, at least two-thirds were sensitized to one other legume, and legume allergy was diagnosed in one-quarter of the sensitized patients,” wrote senior study author Amandine Divaret-Chauveau, MD, of Centre Hospitalier Universitaire de Nancy, Vandoeuvre-les-Nancy, and colleagues. The report is in Pediatric Allergy and Immunology.
People worldwide are eating more legumes these days, the authors noted. High in protein, low in unsaturated fats, with low production costs, legumes are important components of increasingly vegetarian, healthy, sustainable diets.
Food allergens are the most common childhood triggers of allergic reactions. Among children in France, legumes cause 14.6% of food-related anaphylactic reactions, with peanut as the main allergen, they added.
Dr. Divaret-Chauveau and colleagues assessed the prevalence and relevance of sensitization to legumes among all children and adolescents aged 1-17 years who had peanut allergy and had been admitted to one academic pediatric allergy department over roughly 3 years, beginning in early 2017. For the 195 study participants, peanut allergy had been confirmed, and they had been documented to have consumed or to have sensitization to at least one non-peanut legume; 69.7% were boys.
The researchers analyzed data on consumption history, skin prick tests, specific immunoglobulin E status, prior allergic reactions, and oral food challenges for each legume. They found the following:
- Among the 195 children with peanut allergy, 98.4% had at least one other atopic disease.
- Of the 195 children with peanut allergy, 122 (63.9%) were sensitized to at least one other legume. Of these 122 children, 66.3% were sensitized to fenugreek, 42.2% to lentil, 39.9% to soy, and 34.2% to lupin.
- Allergy to one or more legumes was confirmed for 27.9% of the 122 sensitized children, including 4.9% who had multiple legume allergies. Lentil, lupin, and pea were the main allergens.
- Of the 118 children also having a non-legume food allergy, the main food allergens were egg (57.6%), cow’s milk (33.0%), cashew (39.0%), pistachio (23.7%), and hazelnut (30.5%).
- Fifty percent of allergic reactions to non-peanut legumes were severe, often showing as asthma. Atopic comorbidities, including asthma, in most participants may have contributed to the severity of allergic reactions, the authors noted.
Allergy awareness needs to grow with plant-based diets
“The high prevalence of legume sensitization reported in our study highlights the need to explore legume consumption in children with PA [peanut allergy], and the need to investigate sensitization in the absence of consumption,” they added.
Jodi A. Shroba, MSN, APRN, CPNP, coordinator for the Food Allergy Program at Children’s Mercy Kansas City, in Missouri, told this news organization that few data are available in the literature regarding allergies to legumes other than peanut.
“It was interesting that these authors found such a high legume sensitization in their peanut-allergic patients,” Ms. Shroba, who was not involved in the study, said by email. “As more people are starting to eat plant-based diets, it is important that we better understand their allergenicity and cross-reactivity so we can better help guide patient management and education.”
Deborah Albright, MD, assistant professor of pediatrics at the University of Pittsburgh, agreed.
“As plant-based protein consumption broadens worldwide, awareness of the potential for cross-reactivity and co-allergy amongst legumes will become increasingly important,” she said by email.
“However, positive allergy tests do not reliably correlate with true food allergy; therefore, the diagnosis of legume co-allergy should be confirmed by the individual patient’s history, a formal food challenge, or both,” advised Dr. Albright. She was not involved in the study.
“Cross-sensitization to other legumes in patients with a single legume allergy is common; however, true clinical reactivity is often not present,” she added. “Also, legume allergy test sensitization rates and objective reactivity on food challenge can vary by region, depending on diet and pollen aeroallergen exposure.
“Systematic exploration of tolerance versus co-allergy to other legumes should be considered in patients allergic to peanut or other legumes,” Dr. Albright said.
The authors recommend further research and registry data collection of legume anaphylaxis.
Details regarding funding for the study were not provided. The authors, Ms. Shroba, and Dr. Albright report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
French children with peanut allergy tend to have reactions to other legumes, including soy, lentil, pea, bean, lupin, and fenugreek, and those other allergies often lead to anaphylactic reactions, a retrospective study from France reports.
“Among children allergic to peanut, at least two-thirds were sensitized to one other legume, and legume allergy was diagnosed in one-quarter of the sensitized patients,” wrote senior study author Amandine Divaret-Chauveau, MD, of Centre Hospitalier Universitaire de Nancy, Vandoeuvre-les-Nancy, and colleagues. The report is in Pediatric Allergy and Immunology.
People worldwide are eating more legumes these days, the authors noted. High in protein, low in unsaturated fats, with low production costs, legumes are important components of increasingly vegetarian, healthy, sustainable diets.
Food allergens are the most common childhood triggers of allergic reactions. Among children in France, legumes cause 14.6% of food-related anaphylactic reactions, with peanut as the main allergen, they added.
Dr. Divaret-Chauveau and colleagues assessed the prevalence and relevance of sensitization to legumes among all children and adolescents aged 1-17 years who had peanut allergy and had been admitted to one academic pediatric allergy department over roughly 3 years, beginning in early 2017. For the 195 study participants, peanut allergy had been confirmed, and they had been documented to have consumed or to have sensitization to at least one non-peanut legume; 69.7% were boys.
The researchers analyzed data on consumption history, skin prick tests, specific immunoglobulin E status, prior allergic reactions, and oral food challenges for each legume. They found the following:
- Among the 195 children with peanut allergy, 98.4% had at least one other atopic disease.
- Of the 195 children with peanut allergy, 122 (63.9%) were sensitized to at least one other legume. Of these 122 children, 66.3% were sensitized to fenugreek, 42.2% to lentil, 39.9% to soy, and 34.2% to lupin.
- Allergy to one or more legumes was confirmed for 27.9% of the 122 sensitized children, including 4.9% who had multiple legume allergies. Lentil, lupin, and pea were the main allergens.
- Of the 118 children also having a non-legume food allergy, the main food allergens were egg (57.6%), cow’s milk (33.0%), cashew (39.0%), pistachio (23.7%), and hazelnut (30.5%).
- Fifty percent of allergic reactions to non-peanut legumes were severe, often showing as asthma. Atopic comorbidities, including asthma, in most participants may have contributed to the severity of allergic reactions, the authors noted.
Allergy awareness needs to grow with plant-based diets
“The high prevalence of legume sensitization reported in our study highlights the need to explore legume consumption in children with PA [peanut allergy], and the need to investigate sensitization in the absence of consumption,” they added.
Jodi A. Shroba, MSN, APRN, CPNP, coordinator for the Food Allergy Program at Children’s Mercy Kansas City, in Missouri, told this news organization that few data are available in the literature regarding allergies to legumes other than peanut.
“It was interesting that these authors found such a high legume sensitization in their peanut-allergic patients,” Ms. Shroba, who was not involved in the study, said by email. “As more people are starting to eat plant-based diets, it is important that we better understand their allergenicity and cross-reactivity so we can better help guide patient management and education.”
Deborah Albright, MD, assistant professor of pediatrics at the University of Pittsburgh, agreed.
“As plant-based protein consumption broadens worldwide, awareness of the potential for cross-reactivity and co-allergy amongst legumes will become increasingly important,” she said by email.
“However, positive allergy tests do not reliably correlate with true food allergy; therefore, the diagnosis of legume co-allergy should be confirmed by the individual patient’s history, a formal food challenge, or both,” advised Dr. Albright. She was not involved in the study.
“Cross-sensitization to other legumes in patients with a single legume allergy is common; however, true clinical reactivity is often not present,” she added. “Also, legume allergy test sensitization rates and objective reactivity on food challenge can vary by region, depending on diet and pollen aeroallergen exposure.
“Systematic exploration of tolerance versus co-allergy to other legumes should be considered in patients allergic to peanut or other legumes,” Dr. Albright said.
The authors recommend further research and registry data collection of legume anaphylaxis.
Details regarding funding for the study were not provided. The authors, Ms. Shroba, and Dr. Albright report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Mindfulness eases asthma burden
Adults with asthma who received mindfulness training showed significant improvement in symptoms compared to those who did not receive such training, based on data from 73 individuals.
Although previous research shows the contribution of psychological factors to poor asthma control and exacerbations, the ability of mindfulness-based stress reduction (MBSR) to improve asthma symptoms in particular has not been well studied, wrote Estelle T. Higgins, BA, of the University of Wisconsin, Madison, and colleagues.
they wrote. The researchers hypothesized that MBSR training would reduce the effect of psychological distress on asthma control and inflammation compared to asthma patients in a waitlist control group.
In a study published in Brain, Behavior, & Immunity – Health, the researchers randomized 38 adults with asthma to a program of MBSR and 24 to a waitlist. The participants ranged in age from 18 to 65 years, with a mean of 38.1 years, and 43 were female. All patients had an asthma diagnosis for at least 6 months; airway inflammation was based on measures of fraction of exhaled nitric oxide (FeNO) ≥ 30 ppb, 138 blood eosinophil count ≥ 150 cells/mcL, or percent sputum eosinophils ≥ 2% of total leukocytes. Individuals with ongoing medical conditions other than asthma were excluded.
The MBSR group had seven clinical data collection visits at approximately 1-month intervals. MBSR training sessions occurred within classes offered to the community over a period of 8 weekly sessions and one 6-hour retreat, and included breath-focused attention, body scan, and mindful awareness in seated positions, walking, and yoga. Participants completed questionnaires about mindfulness, distress, depression, and anxiety symptoms. These were assessed at baseline, post intervention, and at study completion. Chronic stress level was determined at baseline only.
The primary outcome was asthma control based on the Asthma Control Questionnaire 6-item version (ACQ6) Minimally Important Difference.
Overall, asthma control improved significantly among those randomized to MBSR compared to waitlisted controls (P = .01) and this difference persisted at 4 months after the intervention.
Nearly one-third (32%) of the MBSR participants met the criteria for clinically significant improvement in asthma symptoms, compared to 12% of those on the wait list.
In addition, MBSR-related improvement in asthma control was significantly associated with a reduced distress (P = .043), and was especially effective for individuals with the highest levels of depressive symptoms at baseline, the researchers noted. Individuals who received MBSR also showed significantly reduced levels of exhaled nitric oxide compared to waitlist controls (P < .05).
The study findings were limited by the lack of an active control group, the researchers noted. “Though a wait-list control group was employed to control for variation in outcome measures over time, it is possible that effects reported here were driven by factors that are not specific to training in mindfulness, such as social support or expectancy effects,” they wrote. However, the results support the value of mindfulness in reducing psychological stress, FeNO, and impairments related to asthma that were sustained over time, they said. The results also support the potential of MBSR to both augment and reduce the need for pharmacological treatment in asthma, and mindfulness may be an effective way to improve overall disease control by reducing the contribution of psychological factors to asthma morbidity, they concluded.
The study was supported by the National Center for Complementary and Integrative Health. Coauthor Richard J. Davidson, MD, is the founder and president, and serves on the board of directors for the nonprofit organization, Healthy Minds Innovations Inc. The researchers had no financial conflicts to disclose.
Adults with asthma who received mindfulness training showed significant improvement in symptoms compared to those who did not receive such training, based on data from 73 individuals.
Although previous research shows the contribution of psychological factors to poor asthma control and exacerbations, the ability of mindfulness-based stress reduction (MBSR) to improve asthma symptoms in particular has not been well studied, wrote Estelle T. Higgins, BA, of the University of Wisconsin, Madison, and colleagues.
they wrote. The researchers hypothesized that MBSR training would reduce the effect of psychological distress on asthma control and inflammation compared to asthma patients in a waitlist control group.
In a study published in Brain, Behavior, & Immunity – Health, the researchers randomized 38 adults with asthma to a program of MBSR and 24 to a waitlist. The participants ranged in age from 18 to 65 years, with a mean of 38.1 years, and 43 were female. All patients had an asthma diagnosis for at least 6 months; airway inflammation was based on measures of fraction of exhaled nitric oxide (FeNO) ≥ 30 ppb, 138 blood eosinophil count ≥ 150 cells/mcL, or percent sputum eosinophils ≥ 2% of total leukocytes. Individuals with ongoing medical conditions other than asthma were excluded.
The MBSR group had seven clinical data collection visits at approximately 1-month intervals. MBSR training sessions occurred within classes offered to the community over a period of 8 weekly sessions and one 6-hour retreat, and included breath-focused attention, body scan, and mindful awareness in seated positions, walking, and yoga. Participants completed questionnaires about mindfulness, distress, depression, and anxiety symptoms. These were assessed at baseline, post intervention, and at study completion. Chronic stress level was determined at baseline only.
The primary outcome was asthma control based on the Asthma Control Questionnaire 6-item version (ACQ6) Minimally Important Difference.
Overall, asthma control improved significantly among those randomized to MBSR compared to waitlisted controls (P = .01) and this difference persisted at 4 months after the intervention.
Nearly one-third (32%) of the MBSR participants met the criteria for clinically significant improvement in asthma symptoms, compared to 12% of those on the wait list.
In addition, MBSR-related improvement in asthma control was significantly associated with a reduced distress (P = .043), and was especially effective for individuals with the highest levels of depressive symptoms at baseline, the researchers noted. Individuals who received MBSR also showed significantly reduced levels of exhaled nitric oxide compared to waitlist controls (P < .05).
The study findings were limited by the lack of an active control group, the researchers noted. “Though a wait-list control group was employed to control for variation in outcome measures over time, it is possible that effects reported here were driven by factors that are not specific to training in mindfulness, such as social support or expectancy effects,” they wrote. However, the results support the value of mindfulness in reducing psychological stress, FeNO, and impairments related to asthma that were sustained over time, they said. The results also support the potential of MBSR to both augment and reduce the need for pharmacological treatment in asthma, and mindfulness may be an effective way to improve overall disease control by reducing the contribution of psychological factors to asthma morbidity, they concluded.
The study was supported by the National Center for Complementary and Integrative Health. Coauthor Richard J. Davidson, MD, is the founder and president, and serves on the board of directors for the nonprofit organization, Healthy Minds Innovations Inc. The researchers had no financial conflicts to disclose.
Adults with asthma who received mindfulness training showed significant improvement in symptoms compared to those who did not receive such training, based on data from 73 individuals.
Although previous research shows the contribution of psychological factors to poor asthma control and exacerbations, the ability of mindfulness-based stress reduction (MBSR) to improve asthma symptoms in particular has not been well studied, wrote Estelle T. Higgins, BA, of the University of Wisconsin, Madison, and colleagues.
they wrote. The researchers hypothesized that MBSR training would reduce the effect of psychological distress on asthma control and inflammation compared to asthma patients in a waitlist control group.
In a study published in Brain, Behavior, & Immunity – Health, the researchers randomized 38 adults with asthma to a program of MBSR and 24 to a waitlist. The participants ranged in age from 18 to 65 years, with a mean of 38.1 years, and 43 were female. All patients had an asthma diagnosis for at least 6 months; airway inflammation was based on measures of fraction of exhaled nitric oxide (FeNO) ≥ 30 ppb, 138 blood eosinophil count ≥ 150 cells/mcL, or percent sputum eosinophils ≥ 2% of total leukocytes. Individuals with ongoing medical conditions other than asthma were excluded.
The MBSR group had seven clinical data collection visits at approximately 1-month intervals. MBSR training sessions occurred within classes offered to the community over a period of 8 weekly sessions and one 6-hour retreat, and included breath-focused attention, body scan, and mindful awareness in seated positions, walking, and yoga. Participants completed questionnaires about mindfulness, distress, depression, and anxiety symptoms. These were assessed at baseline, post intervention, and at study completion. Chronic stress level was determined at baseline only.
The primary outcome was asthma control based on the Asthma Control Questionnaire 6-item version (ACQ6) Minimally Important Difference.
Overall, asthma control improved significantly among those randomized to MBSR compared to waitlisted controls (P = .01) and this difference persisted at 4 months after the intervention.
Nearly one-third (32%) of the MBSR participants met the criteria for clinically significant improvement in asthma symptoms, compared to 12% of those on the wait list.
In addition, MBSR-related improvement in asthma control was significantly associated with a reduced distress (P = .043), and was especially effective for individuals with the highest levels of depressive symptoms at baseline, the researchers noted. Individuals who received MBSR also showed significantly reduced levels of exhaled nitric oxide compared to waitlist controls (P < .05).
The study findings were limited by the lack of an active control group, the researchers noted. “Though a wait-list control group was employed to control for variation in outcome measures over time, it is possible that effects reported here were driven by factors that are not specific to training in mindfulness, such as social support or expectancy effects,” they wrote. However, the results support the value of mindfulness in reducing psychological stress, FeNO, and impairments related to asthma that were sustained over time, they said. The results also support the potential of MBSR to both augment and reduce the need for pharmacological treatment in asthma, and mindfulness may be an effective way to improve overall disease control by reducing the contribution of psychological factors to asthma morbidity, they concluded.
The study was supported by the National Center for Complementary and Integrative Health. Coauthor Richard J. Davidson, MD, is the founder and president, and serves on the board of directors for the nonprofit organization, Healthy Minds Innovations Inc. The researchers had no financial conflicts to disclose.
FROM BRAIN, BEHAVIOR, & IMMUNITY – HEALTH
Inhaled, systemic steroids linked to changes in brain structure
New research links the use of glucocorticoids with changes in white matter microstructure – which may explain the development of anxiety, depression, and other neuropsychiatric side effects related to these drugs, investigators say.
Results from a cross-sectional study showed that use of both systemic and inhaled glucocorticoids was associated with widespread reductions in fractional anisotropy (FA) and increases in mean diffusivity.
Glucocorticoids have “a whole catalogue” of adverse events, and effects on brain structure “adds to the list,” co-investigator Onno C. Meijer, PhD, professor of molecular neuroendocrinology of corticosteroids, department of medicine, Leiden University Medical Center, the Netherlands, told this news organization.
The findings should encourage clinicians to consider whether doses they are prescribing are too high, said Dr. Meijer. He added that the negative effect of glucocorticoids on the brain was also found in those using inhalers, such as patients with asthma.
The findings were published online in the BMJ Open.
Serious side effects
Glucocorticoids, a class of synthetic steroids with immunosuppressive properties, are prescribed for a wide range of conditions, including rheumatoid arthritis and asthma.
However, they are also associated with potentially serious metabolic, cardiovascular, and musculoskeletal side effects as well as neuropsychiatric side effects such as depression, mania, and cognitive impairment.
About 1 in 3 patients exposed to “quite a lot of these drugs” will experience neuropsychiatric symptoms, Dr. Meijer said.
Most previous studies that investigated effects from high levels of glucocorticoids on brain structure have been small and involved selected populations, such as those with Cushing disease.
The new study included participants from the UK Biobank, a large population-based cohort. Participants had undergone imaging and did not have a history of psychiatric disease – although they could have conditions associated with glucocorticoid use, including anxiety, depression, mania, or delirium.
The analysis included 222 patients using oral or parenteral glucocorticoids at the time of imaging (systemic group), 557 using inhaled glucocorticoids, and 24,106 not using glucocorticoids (the control group).
Inhaled steroids target the lungs, whereas a steroid in pill form “travels in the blood and reaches each and every organ and cell in the body and typically requires higher doses,” Dr. Meijer noted.
The groups were similar with respect to sex, education, and smoking status. However, the systemic glucocorticoid group was slightly older (mean age, 66.1 years vs. 63.3 years for inhaled glucocorticoid users and 63.5 years for the control group).
In addition to age, researchers adjusted for sex, education level, head position in the scanner, head size, assessment center, and year of imaging.
Imaging analyses
Imaging analyses showed systemic glucocorticoid use was associated with reduced global FA (adjusted mean difference, -3.7e-3; 95% confidence interval, -6.4e-3 to 1.0e-3), and reductions in regional FA in the body and genu of the corpus callosum versus the control group.
Inhaled glucocorticoid use was associated with reduced global FA (AMD, -2.3e-3; 95% CI, -4.0e-3 to -5.7e-4), and lower FA in the splenium of the corpus callosum and the cingulum of the hippocampus.
Global mean diffusivity was higher in systemic glucocorticoid users (AMD, 7.2e-6; 95% CI, 3.2e-6 to 1.1e-5) and inhaled glucocorticoid users (AMD, 2.7e-6; 95% CI, 1.7e-7 to 5.2e-6), compared with the control group.
The effects of glucocorticoids on white matter were “pervasive,” and the “most important finding” of the study, Dr. Meijer said. “We were impressed by the fact white matter is so sensitive to these drugs.”
He noted that it is likely that functional connectivity between brain regions is affected by use of glucocorticoids. “You could say communication between brain regions is probably somewhat impaired or challenged,” he said.
Subgroup analyses among participants using glucocorticoids chronically, defined as reported at two consecutive visits, suggested a potential dose-dependent or duration-dependent effect of glucocorticoids on white matter microstructure.
Systemic glucocorticoid use was also associated with an increase in total and grey matter volume of the caudate nucleus.
In addition, there was a significant association between inhaled glucocorticoid use and decreased grey matter volume of the amygdala, which Dr. Meijer said was surprising because studies have shown that glucocorticoids “can drive amygdala big time.”
Move away from ‘one dose for all’?
Another surprise was that the results showed no hippocampal volume differences with steroid use, Dr. Meijer noted.
The modest association between glucocorticoid use and brain volumes could indicate that white matter integrity is more sensitive to glucocorticoids than is grey matter volume, “at least at the structural level,” he said.
He added that longer use or higher doses may be necessary to also induce volumetric changes.
Participants also completed a questionnaire to assess mood over the previous 2 weeks. Systemic glucocorticoid users had more depressive symptoms, disinterest, tenseness/restlessness, and tiredness/lethargy, compared with the control group. Inhaled glucocorticoid users only reported more tiredness/lethargy.
The investigators note that mood-related effects could be linked to the condition for which glucocorticoids were prescribed: for example, rheumatoid arthritis or chronic obstructive pulmonary disease.
In terms of cognition, systemic glucocorticoid users performed significantly worse on the symbol digit substitution task, compared with participants in the control group.
In light of these findings, pharmaceutical companies that make inhaled corticosteroids “should perhaps find out if glucocorticoids can be dosed by kilogram body weight rather than simply one dose fits all,” which is currently the case, Dr. Meijer said.
Impressive, but several limitations
Commenting on the findings, E. Sherwood Brown, MD, PhD, Distinguished Chair in Psychiatric Research and professor and vice chair for clinical research, department of psychiatry, The University of Texas Southwestern Medical Center, Dallas, called the study sample size “impressive.”
In addition, the study is the first to look at systemic as well as inhaled corticosteroids, said Dr. Brown, who was not involved with the research. He noted that previously, there had been only case reports of psychiatric symptoms with inhaled corticosteroids.
That results are in the same direction but greater with systemic, compared with inhaled corticosteroids, is “particularly interesting” because this might suggest dose-dependent effects, Dr. Brown said.
He noted that cognitive differences were also only observed with systemic corticosteroids.
Some study observations, such as smaller amygdala volume with inhaled but not systemic corticosteroids, “are harder to understand,” said Dr. Brown.
However, he pointed out some study limitations. For example, data were apparently unavailable for verbal and declarative memory test data, despite corticosteroids probably affecting the hippocampus and causing memory changes.
Other drawbacks were that the dose and duration of corticosteroid use, as well as the medical histories of study participants, were not available, Dr. Brown said.
No study funding was reported. Dr. Meijer has received research grants and honorariums from Corcept Therapeutics and a speakers’ fee from Ipsen. Dr. Brown is on an advisory board for Sage Pharmaceuticals, which is developing neurosteroids (not corticosteroids) for mood disorders. He is also on a Medscape advisory board related to bipolar disorder.
A version of this article first appeared on Medscape.com.
New research links the use of glucocorticoids with changes in white matter microstructure – which may explain the development of anxiety, depression, and other neuropsychiatric side effects related to these drugs, investigators say.
Results from a cross-sectional study showed that use of both systemic and inhaled glucocorticoids was associated with widespread reductions in fractional anisotropy (FA) and increases in mean diffusivity.
Glucocorticoids have “a whole catalogue” of adverse events, and effects on brain structure “adds to the list,” co-investigator Onno C. Meijer, PhD, professor of molecular neuroendocrinology of corticosteroids, department of medicine, Leiden University Medical Center, the Netherlands, told this news organization.
The findings should encourage clinicians to consider whether doses they are prescribing are too high, said Dr. Meijer. He added that the negative effect of glucocorticoids on the brain was also found in those using inhalers, such as patients with asthma.
The findings were published online in the BMJ Open.
Serious side effects
Glucocorticoids, a class of synthetic steroids with immunosuppressive properties, are prescribed for a wide range of conditions, including rheumatoid arthritis and asthma.
However, they are also associated with potentially serious metabolic, cardiovascular, and musculoskeletal side effects as well as neuropsychiatric side effects such as depression, mania, and cognitive impairment.
About 1 in 3 patients exposed to “quite a lot of these drugs” will experience neuropsychiatric symptoms, Dr. Meijer said.
Most previous studies that investigated effects from high levels of glucocorticoids on brain structure have been small and involved selected populations, such as those with Cushing disease.
The new study included participants from the UK Biobank, a large population-based cohort. Participants had undergone imaging and did not have a history of psychiatric disease – although they could have conditions associated with glucocorticoid use, including anxiety, depression, mania, or delirium.
The analysis included 222 patients using oral or parenteral glucocorticoids at the time of imaging (systemic group), 557 using inhaled glucocorticoids, and 24,106 not using glucocorticoids (the control group).
Inhaled steroids target the lungs, whereas a steroid in pill form “travels in the blood and reaches each and every organ and cell in the body and typically requires higher doses,” Dr. Meijer noted.
The groups were similar with respect to sex, education, and smoking status. However, the systemic glucocorticoid group was slightly older (mean age, 66.1 years vs. 63.3 years for inhaled glucocorticoid users and 63.5 years for the control group).
In addition to age, researchers adjusted for sex, education level, head position in the scanner, head size, assessment center, and year of imaging.
Imaging analyses
Imaging analyses showed systemic glucocorticoid use was associated with reduced global FA (adjusted mean difference, -3.7e-3; 95% confidence interval, -6.4e-3 to 1.0e-3), and reductions in regional FA in the body and genu of the corpus callosum versus the control group.
Inhaled glucocorticoid use was associated with reduced global FA (AMD, -2.3e-3; 95% CI, -4.0e-3 to -5.7e-4), and lower FA in the splenium of the corpus callosum and the cingulum of the hippocampus.
Global mean diffusivity was higher in systemic glucocorticoid users (AMD, 7.2e-6; 95% CI, 3.2e-6 to 1.1e-5) and inhaled glucocorticoid users (AMD, 2.7e-6; 95% CI, 1.7e-7 to 5.2e-6), compared with the control group.
The effects of glucocorticoids on white matter were “pervasive,” and the “most important finding” of the study, Dr. Meijer said. “We were impressed by the fact white matter is so sensitive to these drugs.”
He noted that it is likely that functional connectivity between brain regions is affected by use of glucocorticoids. “You could say communication between brain regions is probably somewhat impaired or challenged,” he said.
Subgroup analyses among participants using glucocorticoids chronically, defined as reported at two consecutive visits, suggested a potential dose-dependent or duration-dependent effect of glucocorticoids on white matter microstructure.
Systemic glucocorticoid use was also associated with an increase in total and grey matter volume of the caudate nucleus.
In addition, there was a significant association between inhaled glucocorticoid use and decreased grey matter volume of the amygdala, which Dr. Meijer said was surprising because studies have shown that glucocorticoids “can drive amygdala big time.”
Move away from ‘one dose for all’?
Another surprise was that the results showed no hippocampal volume differences with steroid use, Dr. Meijer noted.
The modest association between glucocorticoid use and brain volumes could indicate that white matter integrity is more sensitive to glucocorticoids than is grey matter volume, “at least at the structural level,” he said.
He added that longer use or higher doses may be necessary to also induce volumetric changes.
Participants also completed a questionnaire to assess mood over the previous 2 weeks. Systemic glucocorticoid users had more depressive symptoms, disinterest, tenseness/restlessness, and tiredness/lethargy, compared with the control group. Inhaled glucocorticoid users only reported more tiredness/lethargy.
The investigators note that mood-related effects could be linked to the condition for which glucocorticoids were prescribed: for example, rheumatoid arthritis or chronic obstructive pulmonary disease.
In terms of cognition, systemic glucocorticoid users performed significantly worse on the symbol digit substitution task, compared with participants in the control group.
In light of these findings, pharmaceutical companies that make inhaled corticosteroids “should perhaps find out if glucocorticoids can be dosed by kilogram body weight rather than simply one dose fits all,” which is currently the case, Dr. Meijer said.
Impressive, but several limitations
Commenting on the findings, E. Sherwood Brown, MD, PhD, Distinguished Chair in Psychiatric Research and professor and vice chair for clinical research, department of psychiatry, The University of Texas Southwestern Medical Center, Dallas, called the study sample size “impressive.”
In addition, the study is the first to look at systemic as well as inhaled corticosteroids, said Dr. Brown, who was not involved with the research. He noted that previously, there had been only case reports of psychiatric symptoms with inhaled corticosteroids.
That results are in the same direction but greater with systemic, compared with inhaled corticosteroids, is “particularly interesting” because this might suggest dose-dependent effects, Dr. Brown said.
He noted that cognitive differences were also only observed with systemic corticosteroids.
Some study observations, such as smaller amygdala volume with inhaled but not systemic corticosteroids, “are harder to understand,” said Dr. Brown.
However, he pointed out some study limitations. For example, data were apparently unavailable for verbal and declarative memory test data, despite corticosteroids probably affecting the hippocampus and causing memory changes.
Other drawbacks were that the dose and duration of corticosteroid use, as well as the medical histories of study participants, were not available, Dr. Brown said.
No study funding was reported. Dr. Meijer has received research grants and honorariums from Corcept Therapeutics and a speakers’ fee from Ipsen. Dr. Brown is on an advisory board for Sage Pharmaceuticals, which is developing neurosteroids (not corticosteroids) for mood disorders. He is also on a Medscape advisory board related to bipolar disorder.
A version of this article first appeared on Medscape.com.
New research links the use of glucocorticoids with changes in white matter microstructure – which may explain the development of anxiety, depression, and other neuropsychiatric side effects related to these drugs, investigators say.
Results from a cross-sectional study showed that use of both systemic and inhaled glucocorticoids was associated with widespread reductions in fractional anisotropy (FA) and increases in mean diffusivity.
Glucocorticoids have “a whole catalogue” of adverse events, and effects on brain structure “adds to the list,” co-investigator Onno C. Meijer, PhD, professor of molecular neuroendocrinology of corticosteroids, department of medicine, Leiden University Medical Center, the Netherlands, told this news organization.
The findings should encourage clinicians to consider whether doses they are prescribing are too high, said Dr. Meijer. He added that the negative effect of glucocorticoids on the brain was also found in those using inhalers, such as patients with asthma.
The findings were published online in the BMJ Open.
Serious side effects
Glucocorticoids, a class of synthetic steroids with immunosuppressive properties, are prescribed for a wide range of conditions, including rheumatoid arthritis and asthma.
However, they are also associated with potentially serious metabolic, cardiovascular, and musculoskeletal side effects as well as neuropsychiatric side effects such as depression, mania, and cognitive impairment.
About 1 in 3 patients exposed to “quite a lot of these drugs” will experience neuropsychiatric symptoms, Dr. Meijer said.
Most previous studies that investigated effects from high levels of glucocorticoids on brain structure have been small and involved selected populations, such as those with Cushing disease.
The new study included participants from the UK Biobank, a large population-based cohort. Participants had undergone imaging and did not have a history of psychiatric disease – although they could have conditions associated with glucocorticoid use, including anxiety, depression, mania, or delirium.
The analysis included 222 patients using oral or parenteral glucocorticoids at the time of imaging (systemic group), 557 using inhaled glucocorticoids, and 24,106 not using glucocorticoids (the control group).
Inhaled steroids target the lungs, whereas a steroid in pill form “travels in the blood and reaches each and every organ and cell in the body and typically requires higher doses,” Dr. Meijer noted.
The groups were similar with respect to sex, education, and smoking status. However, the systemic glucocorticoid group was slightly older (mean age, 66.1 years vs. 63.3 years for inhaled glucocorticoid users and 63.5 years for the control group).
In addition to age, researchers adjusted for sex, education level, head position in the scanner, head size, assessment center, and year of imaging.
Imaging analyses
Imaging analyses showed systemic glucocorticoid use was associated with reduced global FA (adjusted mean difference, -3.7e-3; 95% confidence interval, -6.4e-3 to 1.0e-3), and reductions in regional FA in the body and genu of the corpus callosum versus the control group.
Inhaled glucocorticoid use was associated with reduced global FA (AMD, -2.3e-3; 95% CI, -4.0e-3 to -5.7e-4), and lower FA in the splenium of the corpus callosum and the cingulum of the hippocampus.
Global mean diffusivity was higher in systemic glucocorticoid users (AMD, 7.2e-6; 95% CI, 3.2e-6 to 1.1e-5) and inhaled glucocorticoid users (AMD, 2.7e-6; 95% CI, 1.7e-7 to 5.2e-6), compared with the control group.
The effects of glucocorticoids on white matter were “pervasive,” and the “most important finding” of the study, Dr. Meijer said. “We were impressed by the fact white matter is so sensitive to these drugs.”
He noted that it is likely that functional connectivity between brain regions is affected by use of glucocorticoids. “You could say communication between brain regions is probably somewhat impaired or challenged,” he said.
Subgroup analyses among participants using glucocorticoids chronically, defined as reported at two consecutive visits, suggested a potential dose-dependent or duration-dependent effect of glucocorticoids on white matter microstructure.
Systemic glucocorticoid use was also associated with an increase in total and grey matter volume of the caudate nucleus.
In addition, there was a significant association between inhaled glucocorticoid use and decreased grey matter volume of the amygdala, which Dr. Meijer said was surprising because studies have shown that glucocorticoids “can drive amygdala big time.”
Move away from ‘one dose for all’?
Another surprise was that the results showed no hippocampal volume differences with steroid use, Dr. Meijer noted.
The modest association between glucocorticoid use and brain volumes could indicate that white matter integrity is more sensitive to glucocorticoids than is grey matter volume, “at least at the structural level,” he said.
He added that longer use or higher doses may be necessary to also induce volumetric changes.
Participants also completed a questionnaire to assess mood over the previous 2 weeks. Systemic glucocorticoid users had more depressive symptoms, disinterest, tenseness/restlessness, and tiredness/lethargy, compared with the control group. Inhaled glucocorticoid users only reported more tiredness/lethargy.
The investigators note that mood-related effects could be linked to the condition for which glucocorticoids were prescribed: for example, rheumatoid arthritis or chronic obstructive pulmonary disease.
In terms of cognition, systemic glucocorticoid users performed significantly worse on the symbol digit substitution task, compared with participants in the control group.
In light of these findings, pharmaceutical companies that make inhaled corticosteroids “should perhaps find out if glucocorticoids can be dosed by kilogram body weight rather than simply one dose fits all,” which is currently the case, Dr. Meijer said.
Impressive, but several limitations
Commenting on the findings, E. Sherwood Brown, MD, PhD, Distinguished Chair in Psychiatric Research and professor and vice chair for clinical research, department of psychiatry, The University of Texas Southwestern Medical Center, Dallas, called the study sample size “impressive.”
In addition, the study is the first to look at systemic as well as inhaled corticosteroids, said Dr. Brown, who was not involved with the research. He noted that previously, there had been only case reports of psychiatric symptoms with inhaled corticosteroids.
That results are in the same direction but greater with systemic, compared with inhaled corticosteroids, is “particularly interesting” because this might suggest dose-dependent effects, Dr. Brown said.
He noted that cognitive differences were also only observed with systemic corticosteroids.
Some study observations, such as smaller amygdala volume with inhaled but not systemic corticosteroids, “are harder to understand,” said Dr. Brown.
However, he pointed out some study limitations. For example, data were apparently unavailable for verbal and declarative memory test data, despite corticosteroids probably affecting the hippocampus and causing memory changes.
Other drawbacks were that the dose and duration of corticosteroid use, as well as the medical histories of study participants, were not available, Dr. Brown said.
No study funding was reported. Dr. Meijer has received research grants and honorariums from Corcept Therapeutics and a speakers’ fee from Ipsen. Dr. Brown is on an advisory board for Sage Pharmaceuticals, which is developing neurosteroids (not corticosteroids) for mood disorders. He is also on a Medscape advisory board related to bipolar disorder.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN
Minimal differences between biologics approved for severe asthma
Differences in the safety and efficacy between the biologics approved for the treatment of severe eosinophilic asthma are so minimal as to not meet clinically important thresholds, a network meta-analysis shows.
“We know relatively little of the comparative effectiveness or safety of biologics approved for the treatment of asthma [but since] the opportunities to use these biologics will only continue to increase, and we need to know more about their comparative effectiveness to optimize their use,” Ayobami Akenroye, MD, MPH, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, said in an interview.
“But the decision to use one biologic or not is complex and goes beyond comparative effectiveness, and factors such as insurance coverage, convenience of self-administration, and comorbidities all play a role in the choice of biologics,” she said, adding that all the outcomes assessed in the study contribute to or reflect a patient’s underlying asthma control.
The study was published online in the Journal of Allergy and Clinical Immunology.
Interleukin pathways
Drugs that target various interleukin signaling pathways involved in the pathogenesis of asthma include mepolizumab (Nucala), benralizumab (Fasenra), and dupilumab (Dupixent), all of which have been shown to decrease exacerbation rates, improve lung function, and enhance quality of life for patients with severe eosinophilic asthma. In a Bayesian network meta-analysis that allows for simultaneous comparisons of these three treatments, investigators analyzed eight randomized, placebo-controlled trials that compared each of the drugs with placebo. In total, the trials involved 6,461 patients; the duration of follow-up was between 24 and 56 weeks.
“In the subgroup of patients with eosinophil counts of ≥ 300 cells/mcL, all three biologics were significantly better than placebo in reducing exacerbations,” Dr. Akenroye and colleagues reported. For example, dupilumab reduced the exacerbation risk by 68% at a risk ratio of 0.32 (95% confidence interval, 0.23-0.45), while mepolizumab reduced it by almost as much at 63% (RR, 0.37; 95% CI, 0.30-0.45).
Benralizumab was slightly less effective than the other two biologics, reducing exacerbation risk by 51% (RR, 0.49; 95% CI, 0.43-0.55). “In patients with eosinophil counts of ≥ 300 cells/mcL, all three biologics had a probability of 1 in improving the exacerbation rate by 20% or more ... in comparison to placebo,” the authors wrote.
Regarding each drug’s effect in improving forced expiratory volume in 1 second (FEV1), the mean difference in milliliters with dupilumab before and after treatment was 230 (95% CI, 160-300), while for benralizumab, the MD was 150 (95% CI, 100-220) before and after treatment. With mepolizumab, the MD in FEV1 before and after treatment was also 150. In the same subgroup of patients with eosinophil counts of at least300 cells/mcL, all three biologics again had a probability of 1 in improving FEV1 by 50 mL or more above the placebo effect. A third endpoint that was analyzed was the potential reduction in asthma control questionnaire (ACQ) scores. With mepolizumab, the MD before and after treatment was –0.65 (95% CI, –0.81 to –0.45); with dupilumab, it was –0.48 (95% CI, –0.83 to –0.14); and with dupilumab, it was –0.32 (95% CI, –0.43 to –0.21).
“Dupilumab was significantly better than benralizumab in improving exacerbations,” the authors noted (RR, 0.66; 95% CI, 0.47-0.94), while mepolizumab was also better than benralizumab (RR, 0.75; 95% CI, 0.60-0.95). On the other hand, both dupilumab and benralizumab led to greater improvements in FEV1 than mepolizumab, although the effects of dupilumab and benralizumab on ACQ scores were not significantly different for patients whose lower eosinophil counts were between 150 and 299 cells/mcL.
As for safety outcomes, both mepolizumab and benralizumab were associated with a lower risk of serious adverse events, but dupilumab was not different from placebo in terms of overall safety, according to the authors. “The ultimate choice of biologic for each patient would ... depend on multiple factors including cost considerations and timing of administration.
“[However], these results may be helpful to clinicians as they optimize patient care,” they concluded. Limitations to the analysis include the fact that indirect comparisons cannot replace randomized trials that compare the three drugs directly.
It’s estimated that 5%-10% of the 26 million individuals with asthma in the United States have severe disease.
Dr. Akenroye disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Differences in the safety and efficacy between the biologics approved for the treatment of severe eosinophilic asthma are so minimal as to not meet clinically important thresholds, a network meta-analysis shows.
“We know relatively little of the comparative effectiveness or safety of biologics approved for the treatment of asthma [but since] the opportunities to use these biologics will only continue to increase, and we need to know more about their comparative effectiveness to optimize their use,” Ayobami Akenroye, MD, MPH, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, said in an interview.
“But the decision to use one biologic or not is complex and goes beyond comparative effectiveness, and factors such as insurance coverage, convenience of self-administration, and comorbidities all play a role in the choice of biologics,” she said, adding that all the outcomes assessed in the study contribute to or reflect a patient’s underlying asthma control.
The study was published online in the Journal of Allergy and Clinical Immunology.
Interleukin pathways
Drugs that target various interleukin signaling pathways involved in the pathogenesis of asthma include mepolizumab (Nucala), benralizumab (Fasenra), and dupilumab (Dupixent), all of which have been shown to decrease exacerbation rates, improve lung function, and enhance quality of life for patients with severe eosinophilic asthma. In a Bayesian network meta-analysis that allows for simultaneous comparisons of these three treatments, investigators analyzed eight randomized, placebo-controlled trials that compared each of the drugs with placebo. In total, the trials involved 6,461 patients; the duration of follow-up was between 24 and 56 weeks.
“In the subgroup of patients with eosinophil counts of ≥ 300 cells/mcL, all three biologics were significantly better than placebo in reducing exacerbations,” Dr. Akenroye and colleagues reported. For example, dupilumab reduced the exacerbation risk by 68% at a risk ratio of 0.32 (95% confidence interval, 0.23-0.45), while mepolizumab reduced it by almost as much at 63% (RR, 0.37; 95% CI, 0.30-0.45).
Benralizumab was slightly less effective than the other two biologics, reducing exacerbation risk by 51% (RR, 0.49; 95% CI, 0.43-0.55). “In patients with eosinophil counts of ≥ 300 cells/mcL, all three biologics had a probability of 1 in improving the exacerbation rate by 20% or more ... in comparison to placebo,” the authors wrote.
Regarding each drug’s effect in improving forced expiratory volume in 1 second (FEV1), the mean difference in milliliters with dupilumab before and after treatment was 230 (95% CI, 160-300), while for benralizumab, the MD was 150 (95% CI, 100-220) before and after treatment. With mepolizumab, the MD in FEV1 before and after treatment was also 150. In the same subgroup of patients with eosinophil counts of at least300 cells/mcL, all three biologics again had a probability of 1 in improving FEV1 by 50 mL or more above the placebo effect. A third endpoint that was analyzed was the potential reduction in asthma control questionnaire (ACQ) scores. With mepolizumab, the MD before and after treatment was –0.65 (95% CI, –0.81 to –0.45); with dupilumab, it was –0.48 (95% CI, –0.83 to –0.14); and with dupilumab, it was –0.32 (95% CI, –0.43 to –0.21).
“Dupilumab was significantly better than benralizumab in improving exacerbations,” the authors noted (RR, 0.66; 95% CI, 0.47-0.94), while mepolizumab was also better than benralizumab (RR, 0.75; 95% CI, 0.60-0.95). On the other hand, both dupilumab and benralizumab led to greater improvements in FEV1 than mepolizumab, although the effects of dupilumab and benralizumab on ACQ scores were not significantly different for patients whose lower eosinophil counts were between 150 and 299 cells/mcL.
As for safety outcomes, both mepolizumab and benralizumab were associated with a lower risk of serious adverse events, but dupilumab was not different from placebo in terms of overall safety, according to the authors. “The ultimate choice of biologic for each patient would ... depend on multiple factors including cost considerations and timing of administration.
“[However], these results may be helpful to clinicians as they optimize patient care,” they concluded. Limitations to the analysis include the fact that indirect comparisons cannot replace randomized trials that compare the three drugs directly.
It’s estimated that 5%-10% of the 26 million individuals with asthma in the United States have severe disease.
Dr. Akenroye disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Differences in the safety and efficacy between the biologics approved for the treatment of severe eosinophilic asthma are so minimal as to not meet clinically important thresholds, a network meta-analysis shows.
“We know relatively little of the comparative effectiveness or safety of biologics approved for the treatment of asthma [but since] the opportunities to use these biologics will only continue to increase, and we need to know more about their comparative effectiveness to optimize their use,” Ayobami Akenroye, MD, MPH, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, said in an interview.
“But the decision to use one biologic or not is complex and goes beyond comparative effectiveness, and factors such as insurance coverage, convenience of self-administration, and comorbidities all play a role in the choice of biologics,” she said, adding that all the outcomes assessed in the study contribute to or reflect a patient’s underlying asthma control.
The study was published online in the Journal of Allergy and Clinical Immunology.
Interleukin pathways
Drugs that target various interleukin signaling pathways involved in the pathogenesis of asthma include mepolizumab (Nucala), benralizumab (Fasenra), and dupilumab (Dupixent), all of which have been shown to decrease exacerbation rates, improve lung function, and enhance quality of life for patients with severe eosinophilic asthma. In a Bayesian network meta-analysis that allows for simultaneous comparisons of these three treatments, investigators analyzed eight randomized, placebo-controlled trials that compared each of the drugs with placebo. In total, the trials involved 6,461 patients; the duration of follow-up was between 24 and 56 weeks.
“In the subgroup of patients with eosinophil counts of ≥ 300 cells/mcL, all three biologics were significantly better than placebo in reducing exacerbations,” Dr. Akenroye and colleagues reported. For example, dupilumab reduced the exacerbation risk by 68% at a risk ratio of 0.32 (95% confidence interval, 0.23-0.45), while mepolizumab reduced it by almost as much at 63% (RR, 0.37; 95% CI, 0.30-0.45).
Benralizumab was slightly less effective than the other two biologics, reducing exacerbation risk by 51% (RR, 0.49; 95% CI, 0.43-0.55). “In patients with eosinophil counts of ≥ 300 cells/mcL, all three biologics had a probability of 1 in improving the exacerbation rate by 20% or more ... in comparison to placebo,” the authors wrote.
Regarding each drug’s effect in improving forced expiratory volume in 1 second (FEV1), the mean difference in milliliters with dupilumab before and after treatment was 230 (95% CI, 160-300), while for benralizumab, the MD was 150 (95% CI, 100-220) before and after treatment. With mepolizumab, the MD in FEV1 before and after treatment was also 150. In the same subgroup of patients with eosinophil counts of at least300 cells/mcL, all three biologics again had a probability of 1 in improving FEV1 by 50 mL or more above the placebo effect. A third endpoint that was analyzed was the potential reduction in asthma control questionnaire (ACQ) scores. With mepolizumab, the MD before and after treatment was –0.65 (95% CI, –0.81 to –0.45); with dupilumab, it was –0.48 (95% CI, –0.83 to –0.14); and with dupilumab, it was –0.32 (95% CI, –0.43 to –0.21).
“Dupilumab was significantly better than benralizumab in improving exacerbations,” the authors noted (RR, 0.66; 95% CI, 0.47-0.94), while mepolizumab was also better than benralizumab (RR, 0.75; 95% CI, 0.60-0.95). On the other hand, both dupilumab and benralizumab led to greater improvements in FEV1 than mepolizumab, although the effects of dupilumab and benralizumab on ACQ scores were not significantly different for patients whose lower eosinophil counts were between 150 and 299 cells/mcL.
As for safety outcomes, both mepolizumab and benralizumab were associated with a lower risk of serious adverse events, but dupilumab was not different from placebo in terms of overall safety, according to the authors. “The ultimate choice of biologic for each patient would ... depend on multiple factors including cost considerations and timing of administration.
“[However], these results may be helpful to clinicians as they optimize patient care,” they concluded. Limitations to the analysis include the fact that indirect comparisons cannot replace randomized trials that compare the three drugs directly.
It’s estimated that 5%-10% of the 26 million individuals with asthma in the United States have severe disease.
Dr. Akenroye disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY