User login
Sleeping on animal skins might protect against childhood asthma, hay fever
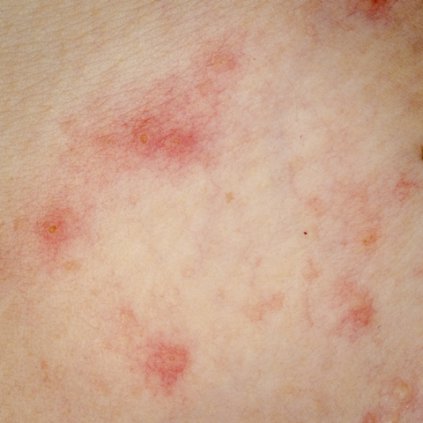
Babies who slept on animal skins during their first 3 months of life were almost 40% less likely to have asthma by the time they were 10 years old, according to a population-based cohort study.
Sleeping on animal skins during infancy also was linked to lower odds of wheezing and hay fever, but did not seem to affect eczema or sensitivity to airborne antigens, Dr. Christina Tischer reported at the annual meeting of the European Respiratory Society.
"Early exposure to animal fur could be a simple, cheap, and effective way to resemble an environment with higher microbial exposure," said Dr. Tischer, a researcher at the German Institute for Environmental Health in Neuherberg, Germany. "It might follow similar protective mechanisms in relation to asthma and allergy as it has been observed in farm and rural environments."
The investigators studied 2,441 children in Germany who were up to 10 years old; parents answered a series of questionnaires about asthma and respiratory risk factors and health outcomes. In all, 55% of the children slept on animal skins or animal furs during their first 3 months of life, Dr. Tischer and her associates reported.
By age 10 years, children who slept on animal skins or animal fur as infants had a 25% lower odds of ever having wheezed (adjusted odds ratio, 0.75), a 38% lower odds of having been diagnosed with asthma (aOR, 0.62), and a 35% lower odds of having been diagnosed with hay fever (aOR, 0.65) compared with children who did not sleep on animals skins or furs as infants, the investigators reported.
Funding information for the study was not available. Dr. Tischer reported no conflicts of interest.
Sleeping on animal skins or fur in the first 3 months of life may decrease the risk of childhood atopy BUT increases the risk of death related to Sudden Infant Death Syndrome (SIDS). This study is from Germany, which may explain cultural differences regarding bedding for infants, but there are reports of cultural diversity regarding bedding for infants even in the United States.
The journal Pediatrics published a study looking at data in 2011 discussing African American parental decisions about infant bedding and sleep surfaces (Pediatrics 2011;128;494).The American Academy of Pediatrics (AAP) supports the policy that infants should sleep on firm bedding. Specifically, infants should not be placed on soft bedding, including blankets, pillows, or sheepskin, for example. In addition, crib bumpers should not be used. Finally, once the AAP promoted the “Back to Sleep” program (meaning babies should NOT be placed on their tummies to sleep), SIDS deaths in the United States decreased.
Sleeping on animal skins or fur in the first 3 months of life may decrease the risk of childhood atopy BUT increases the risk of death related to Sudden Infant Death Syndrome (SIDS). This study is from Germany, which may explain cultural differences regarding bedding for infants, but there are reports of cultural diversity regarding bedding for infants even in the United States.
The journal Pediatrics published a study looking at data in 2011 discussing African American parental decisions about infant bedding and sleep surfaces (Pediatrics 2011;128;494).The American Academy of Pediatrics (AAP) supports the policy that infants should sleep on firm bedding. Specifically, infants should not be placed on soft bedding, including blankets, pillows, or sheepskin, for example. In addition, crib bumpers should not be used. Finally, once the AAP promoted the “Back to Sleep” program (meaning babies should NOT be placed on their tummies to sleep), SIDS deaths in the United States decreased.
Sleeping on animal skins or fur in the first 3 months of life may decrease the risk of childhood atopy BUT increases the risk of death related to Sudden Infant Death Syndrome (SIDS). This study is from Germany, which may explain cultural differences regarding bedding for infants, but there are reports of cultural diversity regarding bedding for infants even in the United States.
The journal Pediatrics published a study looking at data in 2011 discussing African American parental decisions about infant bedding and sleep surfaces (Pediatrics 2011;128;494).The American Academy of Pediatrics (AAP) supports the policy that infants should sleep on firm bedding. Specifically, infants should not be placed on soft bedding, including blankets, pillows, or sheepskin, for example. In addition, crib bumpers should not be used. Finally, once the AAP promoted the “Back to Sleep” program (meaning babies should NOT be placed on their tummies to sleep), SIDS deaths in the United States decreased.
Babies who slept on animal skins during their first 3 months of life were almost 40% less likely to have asthma by the time they were 10 years old, according to a population-based cohort study.
Sleeping on animal skins during infancy also was linked to lower odds of wheezing and hay fever, but did not seem to affect eczema or sensitivity to airborne antigens, Dr. Christina Tischer reported at the annual meeting of the European Respiratory Society.
"Early exposure to animal fur could be a simple, cheap, and effective way to resemble an environment with higher microbial exposure," said Dr. Tischer, a researcher at the German Institute for Environmental Health in Neuherberg, Germany. "It might follow similar protective mechanisms in relation to asthma and allergy as it has been observed in farm and rural environments."
The investigators studied 2,441 children in Germany who were up to 10 years old; parents answered a series of questionnaires about asthma and respiratory risk factors and health outcomes. In all, 55% of the children slept on animal skins or animal furs during their first 3 months of life, Dr. Tischer and her associates reported.
By age 10 years, children who slept on animal skins or animal fur as infants had a 25% lower odds of ever having wheezed (adjusted odds ratio, 0.75), a 38% lower odds of having been diagnosed with asthma (aOR, 0.62), and a 35% lower odds of having been diagnosed with hay fever (aOR, 0.65) compared with children who did not sleep on animals skins or furs as infants, the investigators reported.
Funding information for the study was not available. Dr. Tischer reported no conflicts of interest.
Babies who slept on animal skins during their first 3 months of life were almost 40% less likely to have asthma by the time they were 10 years old, according to a population-based cohort study.
Sleeping on animal skins during infancy also was linked to lower odds of wheezing and hay fever, but did not seem to affect eczema or sensitivity to airborne antigens, Dr. Christina Tischer reported at the annual meeting of the European Respiratory Society.
"Early exposure to animal fur could be a simple, cheap, and effective way to resemble an environment with higher microbial exposure," said Dr. Tischer, a researcher at the German Institute for Environmental Health in Neuherberg, Germany. "It might follow similar protective mechanisms in relation to asthma and allergy as it has been observed in farm and rural environments."
The investigators studied 2,441 children in Germany who were up to 10 years old; parents answered a series of questionnaires about asthma and respiratory risk factors and health outcomes. In all, 55% of the children slept on animal skins or animal furs during their first 3 months of life, Dr. Tischer and her associates reported.
By age 10 years, children who slept on animal skins or animal fur as infants had a 25% lower odds of ever having wheezed (adjusted odds ratio, 0.75), a 38% lower odds of having been diagnosed with asthma (aOR, 0.62), and a 35% lower odds of having been diagnosed with hay fever (aOR, 0.65) compared with children who did not sleep on animals skins or furs as infants, the investigators reported.
Funding information for the study was not available. Dr. Tischer reported no conflicts of interest.
FROM THE EUROPEAN RESPIRATORY SOCIETY INTERNATIONAL CONGRESS
Key clinical point: Sleeping on animals skins during infancy might protect against wheezing, asthma, and hay fever.
Major finding: Children who slept on animal skins during the first 3 months of life had 25% lower odds of wheezing, 38% lower odds of asthma, and 35% lower odds of hay fever at up to 10 years of age, compared with children who did not sleep on skins as infants.
Data source: Population-based cohort study of 2,441 children up to age 10 years in Germany.
Disclosures: Funding information for the study was not available. Dr. Tischer reported no conflicts of interest.
Gold and nickel lead list of eyelid irritants
CHICAGO – Chances are good that if your patient presents with eyelid dermatitis, allergic contact with gold or nickel is the culprit.
"Gold is not thought to be easily released from jewelry, which would be the typical exposure with eyelids, unless it comes into contact with sweat, friction, or abrasives," Dr. Amber Reck Atwater told attendees of a session on facial dermatoses at the American Academy of Dermatology summer meeting.
However, when gold comes into contact with titanium dioxide, a common active ingredient in many cosmetics such as eye shadow, patients are at risk for eyelid irritation.
"If I am wearing a gold ring, and I put this on my eyelids using my finger, the gold will be more easily released, and I will be more likely to get a reaction on my lids," said Dr. Atwater, of the department of dermatology at Duke University, Durham, N.C.
Nickel is another leading cause of eyelid dermatitis, Dr. Atwater said. She warned of the metal’s pernicious tendency to hide in personal care products such as eyelash curlers that do not list it as an active or inactive ingredient.
A simple and relatively inexpensive dimethylglyoxime test, which Dr. Atwater said can be purchased on the consumer market, can help identify items that may contain nickel. Rub a drop of dimethylglyoxime onto an item, such as a house key, with a cotton swab. If the key turns bright pink, then you know it has nickel in it.
"So you can imagine that if I am holding my keys in my hands, I could be transferring nickel from the keys to my eyelids," said Dr. Atwater.
Other potential sources of nickel include faucets, sunglasses with metal frames, barbells, and other weight-lifting equipment.
Other common causes of eyelid dermatitis are products that contain fragrance, including balsam of Peru, neomycin (typically found in antibacterial eye drops), formaldehyde and bronopol (preservatives that are found in certain cosmetics), skin care products, and topicals.
Allergic contact dermatitis in the eyelid can present in an upper, lower, unilateral, or bilateral fashion on the eyelids alone, but typically presents in combination with dermatitis on other areas of the face, or even other areas of the body, according to Dr. Atwater.
"You should be highly suspicious that it’s contact dermatitis when you see eyelid dermatitis with other parts of the body involved," she said.
When the dermatitis presents in the eyelids alone, other factors such as seborrheic dermatitis or aspecific xerotic dermatitis could be the cause.
"Still, a good 30%-50% of our patients will have allergic contact dermatitis when we see them with eyelid dermatitis alone," Dr. Atwater said.
The eyelids are particularly susceptible to irritation in part because the skin is extremely thin – 0.55 mm – compared with other facial areas where the average skin thickness is about 2 mm, Dr. Atwater explained.
"And it’s really easy to transfer substances from our hands to our eyes. People rub their eyes and their faces a lot throughout the day," she noted.
Dr. Atwater had no financial conflicts to disclose.
On Twitter @whitneymcknight
CHICAGO – Chances are good that if your patient presents with eyelid dermatitis, allergic contact with gold or nickel is the culprit.
"Gold is not thought to be easily released from jewelry, which would be the typical exposure with eyelids, unless it comes into contact with sweat, friction, or abrasives," Dr. Amber Reck Atwater told attendees of a session on facial dermatoses at the American Academy of Dermatology summer meeting.
However, when gold comes into contact with titanium dioxide, a common active ingredient in many cosmetics such as eye shadow, patients are at risk for eyelid irritation.
"If I am wearing a gold ring, and I put this on my eyelids using my finger, the gold will be more easily released, and I will be more likely to get a reaction on my lids," said Dr. Atwater, of the department of dermatology at Duke University, Durham, N.C.
Nickel is another leading cause of eyelid dermatitis, Dr. Atwater said. She warned of the metal’s pernicious tendency to hide in personal care products such as eyelash curlers that do not list it as an active or inactive ingredient.
A simple and relatively inexpensive dimethylglyoxime test, which Dr. Atwater said can be purchased on the consumer market, can help identify items that may contain nickel. Rub a drop of dimethylglyoxime onto an item, such as a house key, with a cotton swab. If the key turns bright pink, then you know it has nickel in it.
"So you can imagine that if I am holding my keys in my hands, I could be transferring nickel from the keys to my eyelids," said Dr. Atwater.
Other potential sources of nickel include faucets, sunglasses with metal frames, barbells, and other weight-lifting equipment.
Other common causes of eyelid dermatitis are products that contain fragrance, including balsam of Peru, neomycin (typically found in antibacterial eye drops), formaldehyde and bronopol (preservatives that are found in certain cosmetics), skin care products, and topicals.
Allergic contact dermatitis in the eyelid can present in an upper, lower, unilateral, or bilateral fashion on the eyelids alone, but typically presents in combination with dermatitis on other areas of the face, or even other areas of the body, according to Dr. Atwater.
"You should be highly suspicious that it’s contact dermatitis when you see eyelid dermatitis with other parts of the body involved," she said.
When the dermatitis presents in the eyelids alone, other factors such as seborrheic dermatitis or aspecific xerotic dermatitis could be the cause.
"Still, a good 30%-50% of our patients will have allergic contact dermatitis when we see them with eyelid dermatitis alone," Dr. Atwater said.
The eyelids are particularly susceptible to irritation in part because the skin is extremely thin – 0.55 mm – compared with other facial areas where the average skin thickness is about 2 mm, Dr. Atwater explained.
"And it’s really easy to transfer substances from our hands to our eyes. People rub their eyes and their faces a lot throughout the day," she noted.
Dr. Atwater had no financial conflicts to disclose.
On Twitter @whitneymcknight
CHICAGO – Chances are good that if your patient presents with eyelid dermatitis, allergic contact with gold or nickel is the culprit.
"Gold is not thought to be easily released from jewelry, which would be the typical exposure with eyelids, unless it comes into contact with sweat, friction, or abrasives," Dr. Amber Reck Atwater told attendees of a session on facial dermatoses at the American Academy of Dermatology summer meeting.
However, when gold comes into contact with titanium dioxide, a common active ingredient in many cosmetics such as eye shadow, patients are at risk for eyelid irritation.
"If I am wearing a gold ring, and I put this on my eyelids using my finger, the gold will be more easily released, and I will be more likely to get a reaction on my lids," said Dr. Atwater, of the department of dermatology at Duke University, Durham, N.C.
Nickel is another leading cause of eyelid dermatitis, Dr. Atwater said. She warned of the metal’s pernicious tendency to hide in personal care products such as eyelash curlers that do not list it as an active or inactive ingredient.
A simple and relatively inexpensive dimethylglyoxime test, which Dr. Atwater said can be purchased on the consumer market, can help identify items that may contain nickel. Rub a drop of dimethylglyoxime onto an item, such as a house key, with a cotton swab. If the key turns bright pink, then you know it has nickel in it.
"So you can imagine that if I am holding my keys in my hands, I could be transferring nickel from the keys to my eyelids," said Dr. Atwater.
Other potential sources of nickel include faucets, sunglasses with metal frames, barbells, and other weight-lifting equipment.
Other common causes of eyelid dermatitis are products that contain fragrance, including balsam of Peru, neomycin (typically found in antibacterial eye drops), formaldehyde and bronopol (preservatives that are found in certain cosmetics), skin care products, and topicals.
Allergic contact dermatitis in the eyelid can present in an upper, lower, unilateral, or bilateral fashion on the eyelids alone, but typically presents in combination with dermatitis on other areas of the face, or even other areas of the body, according to Dr. Atwater.
"You should be highly suspicious that it’s contact dermatitis when you see eyelid dermatitis with other parts of the body involved," she said.
When the dermatitis presents in the eyelids alone, other factors such as seborrheic dermatitis or aspecific xerotic dermatitis could be the cause.
"Still, a good 30%-50% of our patients will have allergic contact dermatitis when we see them with eyelid dermatitis alone," Dr. Atwater said.
The eyelids are particularly susceptible to irritation in part because the skin is extremely thin – 0.55 mm – compared with other facial areas where the average skin thickness is about 2 mm, Dr. Atwater explained.
"And it’s really easy to transfer substances from our hands to our eyes. People rub their eyes and their faces a lot throughout the day," she noted.
Dr. Atwater had no financial conflicts to disclose.
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2014
Consider a zero therapy approach to periorificial dermatitis
CHICAGO – For periorificial dermatitis, don’t do a thing. That was the first-line therapy suggested at this year’s American Academy of Dermatology summer meeting.
"Sometimes you have to be brave to tell a patient you’re sending them home with nothing," said Dr. Sarah Wolfe of Duke University in Durham, N.C. "But ‘zero’ therapy is important. It’s simply counseling your patient to recognize the disease and avoid anything that could be exacerbating or precipitating it."
In theory, this advice includes abstinence from using any cosmetics for about 2 months, but "I am not sure whom that will work for," said Dr. Wolfe.
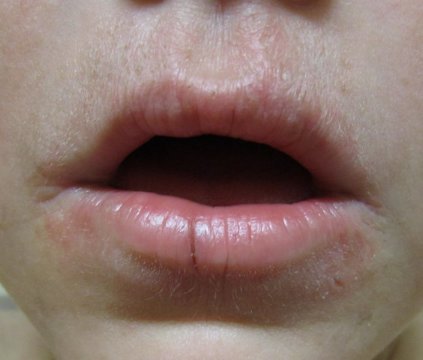
Periorificial dermatitis can affect anyone, but typically occurs in women aged 20-45 years across all ethnicities; it generally presents as erythematous pustules up to 2 mm in size near the nose and mouth, and patients report a burning sensation.
In children, the condition occurs equally in both genders, and tends to peak by age 5 years. Some children develop a distinctive form of the disease – childhood granulomatous periorificial dermatitis – that features persistent, inflammatory papules and pustules that appear symmetrically around the mouth. Although this form tends to be harder to treat and lasts longer, reassure patients that the condition will heal without scarring, Dr. Wolfe said.
Role of steroids
The minimalist approach to treating periorificial dermatitis is particularly important if the condition is related to steroid use, Dr. Wolfe said. Although the prevailing wisdom has been that corticosteroids caused periorificial dermatitis, enough evidence exists to show that corticosteroids likely exacerbate, rather than cause the problem, she explained.
Sometimes patients will use topical steroids (often medications prescribed for other family members or for other ailments) to help calm the burning, unaware of steroids’ potential to make their symptoms worse, she added.
If steroids are implicated, prepare your patient for the possibility of a steroid rebound that lasts a couple of weeks once they stop using the topicals.
"You have to either counsel them through it, or consider using a lower-potency steroid such as a hydrocortisone 2.5% or 1% to get them through that period," Dr. Wolfe said.
Inhaled steroids also have been associated with periorificial dermatitis, occurring where the inhalant mask touches the skin and lips. Intranasal steroids, particularly mometasone furoate and fluticasone furoate, have similarly been associated with the condition in cases of pustules starting near the nose and then spreading to the mouth and chin (Allergy 2001;56:944-8), (Cutan. Ocul. Toxicol. 2012;31:160-3).
Systemic steroids such as prednisone have been associated with the development of periorificial dermatitis upon tapering them, although this association has not been well studied. Because of the risk for the unintentional transfer of topical steroids to the face from other parts of the body being treated, counsel patients to be scrupulous about handwashing after applying their medications, Dr. Wolf emphasized.
Similarly, unintentional corticosteroid use can occur with the use of over the counter skin-lightening products marketed as "herbal" or "all natural," she said.
Oral, topical options
If you and your patient prefer to actively treat the condition, Dr. Wolfe recommended using the most evidence-based therapy available: Oral tetracycline 250-500 mg twice per day, a therapy which showed clearance in 4 to 8 weeks when compared to placebo (G. Ital. Dermatol. Venereol. 2010 Aug;145:433-44).
She did not recommend using this therapy in children younger than 8 years, because of the drug’s association with dental abnormalities. Other antibiotics such as doxycycline, minocycline, and azithromycin (in children) have not been well studied, Dr. Wolfe said, although she noted anecdotal reports of pediatric dermatologists prescribing azithromycin if the patient’s presentation is "slightly disfiguring."
Although erythromycin may be easier to use when controlling costs, both it and pimecrolimus are viable options for topical therapy, Dr. Wolfe said. Pimecrolimus 1% cream has been shown to reduce disease severity in 2 weeks, while erythromycin 2% solution, in ointment or gel form twice daily, brought clearance in 3-7 weeks in a randomized, placebo-controlled trial. She emphasized the importance of discussing all topical exposures with patients, regardless of the treatment option chosen.
Dr. Wolfe had no financial conflicts to disclose.
On Twitter @whitneymcknight
CHICAGO – For periorificial dermatitis, don’t do a thing. That was the first-line therapy suggested at this year’s American Academy of Dermatology summer meeting.
"Sometimes you have to be brave to tell a patient you’re sending them home with nothing," said Dr. Sarah Wolfe of Duke University in Durham, N.C. "But ‘zero’ therapy is important. It’s simply counseling your patient to recognize the disease and avoid anything that could be exacerbating or precipitating it."
In theory, this advice includes abstinence from using any cosmetics for about 2 months, but "I am not sure whom that will work for," said Dr. Wolfe.
Periorificial dermatitis can affect anyone, but typically occurs in women aged 20-45 years across all ethnicities; it generally presents as erythematous pustules up to 2 mm in size near the nose and mouth, and patients report a burning sensation.
In children, the condition occurs equally in both genders, and tends to peak by age 5 years. Some children develop a distinctive form of the disease – childhood granulomatous periorificial dermatitis – that features persistent, inflammatory papules and pustules that appear symmetrically around the mouth. Although this form tends to be harder to treat and lasts longer, reassure patients that the condition will heal without scarring, Dr. Wolfe said.
Role of steroids
The minimalist approach to treating periorificial dermatitis is particularly important if the condition is related to steroid use, Dr. Wolfe said. Although the prevailing wisdom has been that corticosteroids caused periorificial dermatitis, enough evidence exists to show that corticosteroids likely exacerbate, rather than cause the problem, she explained.
Sometimes patients will use topical steroids (often medications prescribed for other family members or for other ailments) to help calm the burning, unaware of steroids’ potential to make their symptoms worse, she added.
If steroids are implicated, prepare your patient for the possibility of a steroid rebound that lasts a couple of weeks once they stop using the topicals.
"You have to either counsel them through it, or consider using a lower-potency steroid such as a hydrocortisone 2.5% or 1% to get them through that period," Dr. Wolfe said.
Inhaled steroids also have been associated with periorificial dermatitis, occurring where the inhalant mask touches the skin and lips. Intranasal steroids, particularly mometasone furoate and fluticasone furoate, have similarly been associated with the condition in cases of pustules starting near the nose and then spreading to the mouth and chin (Allergy 2001;56:944-8), (Cutan. Ocul. Toxicol. 2012;31:160-3).
Systemic steroids such as prednisone have been associated with the development of periorificial dermatitis upon tapering them, although this association has not been well studied. Because of the risk for the unintentional transfer of topical steroids to the face from other parts of the body being treated, counsel patients to be scrupulous about handwashing after applying their medications, Dr. Wolf emphasized.
Similarly, unintentional corticosteroid use can occur with the use of over the counter skin-lightening products marketed as "herbal" or "all natural," she said.
Oral, topical options
If you and your patient prefer to actively treat the condition, Dr. Wolfe recommended using the most evidence-based therapy available: Oral tetracycline 250-500 mg twice per day, a therapy which showed clearance in 4 to 8 weeks when compared to placebo (G. Ital. Dermatol. Venereol. 2010 Aug;145:433-44).
She did not recommend using this therapy in children younger than 8 years, because of the drug’s association with dental abnormalities. Other antibiotics such as doxycycline, minocycline, and azithromycin (in children) have not been well studied, Dr. Wolfe said, although she noted anecdotal reports of pediatric dermatologists prescribing azithromycin if the patient’s presentation is "slightly disfiguring."
Although erythromycin may be easier to use when controlling costs, both it and pimecrolimus are viable options for topical therapy, Dr. Wolfe said. Pimecrolimus 1% cream has been shown to reduce disease severity in 2 weeks, while erythromycin 2% solution, in ointment or gel form twice daily, brought clearance in 3-7 weeks in a randomized, placebo-controlled trial. She emphasized the importance of discussing all topical exposures with patients, regardless of the treatment option chosen.
Dr. Wolfe had no financial conflicts to disclose.
On Twitter @whitneymcknight
CHICAGO – For periorificial dermatitis, don’t do a thing. That was the first-line therapy suggested at this year’s American Academy of Dermatology summer meeting.
"Sometimes you have to be brave to tell a patient you’re sending them home with nothing," said Dr. Sarah Wolfe of Duke University in Durham, N.C. "But ‘zero’ therapy is important. It’s simply counseling your patient to recognize the disease and avoid anything that could be exacerbating or precipitating it."
In theory, this advice includes abstinence from using any cosmetics for about 2 months, but "I am not sure whom that will work for," said Dr. Wolfe.
Periorificial dermatitis can affect anyone, but typically occurs in women aged 20-45 years across all ethnicities; it generally presents as erythematous pustules up to 2 mm in size near the nose and mouth, and patients report a burning sensation.
In children, the condition occurs equally in both genders, and tends to peak by age 5 years. Some children develop a distinctive form of the disease – childhood granulomatous periorificial dermatitis – that features persistent, inflammatory papules and pustules that appear symmetrically around the mouth. Although this form tends to be harder to treat and lasts longer, reassure patients that the condition will heal without scarring, Dr. Wolfe said.
Role of steroids
The minimalist approach to treating periorificial dermatitis is particularly important if the condition is related to steroid use, Dr. Wolfe said. Although the prevailing wisdom has been that corticosteroids caused periorificial dermatitis, enough evidence exists to show that corticosteroids likely exacerbate, rather than cause the problem, she explained.
Sometimes patients will use topical steroids (often medications prescribed for other family members or for other ailments) to help calm the burning, unaware of steroids’ potential to make their symptoms worse, she added.
If steroids are implicated, prepare your patient for the possibility of a steroid rebound that lasts a couple of weeks once they stop using the topicals.
"You have to either counsel them through it, or consider using a lower-potency steroid such as a hydrocortisone 2.5% or 1% to get them through that period," Dr. Wolfe said.
Inhaled steroids also have been associated with periorificial dermatitis, occurring where the inhalant mask touches the skin and lips. Intranasal steroids, particularly mometasone furoate and fluticasone furoate, have similarly been associated with the condition in cases of pustules starting near the nose and then spreading to the mouth and chin (Allergy 2001;56:944-8), (Cutan. Ocul. Toxicol. 2012;31:160-3).
Systemic steroids such as prednisone have been associated with the development of periorificial dermatitis upon tapering them, although this association has not been well studied. Because of the risk for the unintentional transfer of topical steroids to the face from other parts of the body being treated, counsel patients to be scrupulous about handwashing after applying their medications, Dr. Wolf emphasized.
Similarly, unintentional corticosteroid use can occur with the use of over the counter skin-lightening products marketed as "herbal" or "all natural," she said.
Oral, topical options
If you and your patient prefer to actively treat the condition, Dr. Wolfe recommended using the most evidence-based therapy available: Oral tetracycline 250-500 mg twice per day, a therapy which showed clearance in 4 to 8 weeks when compared to placebo (G. Ital. Dermatol. Venereol. 2010 Aug;145:433-44).
She did not recommend using this therapy in children younger than 8 years, because of the drug’s association with dental abnormalities. Other antibiotics such as doxycycline, minocycline, and azithromycin (in children) have not been well studied, Dr. Wolfe said, although she noted anecdotal reports of pediatric dermatologists prescribing azithromycin if the patient’s presentation is "slightly disfiguring."
Although erythromycin may be easier to use when controlling costs, both it and pimecrolimus are viable options for topical therapy, Dr. Wolfe said. Pimecrolimus 1% cream has been shown to reduce disease severity in 2 weeks, while erythromycin 2% solution, in ointment or gel form twice daily, brought clearance in 3-7 weeks in a randomized, placebo-controlled trial. She emphasized the importance of discussing all topical exposures with patients, regardless of the treatment option chosen.
Dr. Wolfe had no financial conflicts to disclose.
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2014
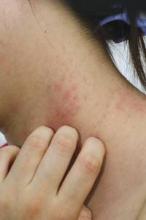
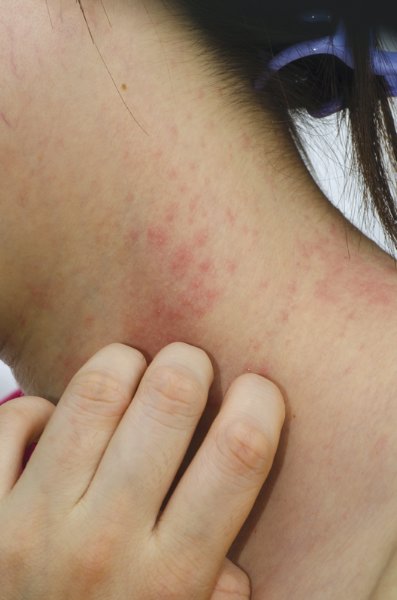
Patch testing tricks in atopic dermatitis with concomitant contact dermatitis
COEUR D’ALENE, IDAHO– Contact dermatitis goes together with moderate-to-severe atopic dermatitis like ham and eggs. Reading patch test results in such patients poses unique challenges, because of the impaired skin barrier function intrinsic to atopic dermatitis, coupled with the moist environment created under the occlusive patches, which predisposes to Staphylococcus aureus colonization and superinfection.
All of this makes the evaluation of patch test results in atopic dermatitis more complicated than in patients without contact dermatitis. But there are tricks that greatly reduce the difficulty.
"Patch testing can play a crucial role in the work-up and management of patients with refractory atopic dermatitis. Prior to patch testing, measures should be taken to improve the skin barrier and reduce bacterial overload," Dr. Sharon E. Jacob advised at the annual meeting of the Society for Pediatric Dermatology.
Her recommended preparation program starts 3 weeks prior to the scheduled patch test. The emphasis is on beefing up the disrupted skin barrier through the use of lipid-replenishing emollients and nonalkaline soaps, avoidance of fragrances and other irritant allergens, and preemptive treatment of S. aureus colonization or superinfection, explained Dr. Jacob of Loma Linda (Calif.) University.
At the start of the 3-week countdown, a patient with no clinical signs of skin infection should be checked for nasal carriage of S. aureus. If positive, or if the patient has a remote history of S. aureus colonization or superinfection but no current signs of colonization, such as oozing or crusting, it’s appropriate to begin intranasal, perianal, and umbilical topical mupirocin twice daily for 5 days.
It’s also time to eliminate irritant allergens, start a regimen of dilute bleach baths, embrace the special emollients and soaps, and make sure areas of dermatitis are getting adequately treated with topical steroids, except for the planned patch test area, which must remain steroid free for 7 days prior to the test day.
The lipid-containing emollients, which should contain ceramides or filaggrin degradation products, are to be used all over the body, including the back, until the day before the test.
Examples of the nonalkaline soaps, which are employed to maintain an acidic skin pH, include Aveeno Moisturizing Bar soap, Cetaphil Gentle Cleansing Bar, and Dove Sensitive Skin Unscented Beauty Bar, the pediatric dermatologist continued.
Patients on UV phototherapy for their atopic dermatitis can continue except at the planned patch test site, where it should be avoided for 2 weeks beforehand.
In an adolescent with head and neck atopic dermatitis, a pre–patch test course of oral antifungal therapy is worth considering, according to Dr. Jacob.
For the atopic dermatitis patient with clinical signs of bacterial infection at the pre–patch test office visit, culture the lesions and start a preemptive 10-day course of oral cephalexin at 25-50 mg/kg per day three times daily beginning 3 days prior to patch testing, with the choice of antimicrobial adjusted as warranted by the culture results. These patients also go on the emollients, soaps, dilute bleach baths, and irritant allergen avoidance regimen.
A patient with no signs of skin infection when the test patches are removed can continue with the test readings as scheduled, with no further intervention. However, if signs of infection are present, the patient should immediately go on oral cephalexin if not already on it and take a single bleach bath the same day the patches come off. The bath should be at one-tenth to one-half the customary concentration of 0.005% sodium hypochlorite and should be followed by a fresh water rinse.
The usual patch test reading schedule in atopic dermatitis patients is at 24-48 hours, again at 72-96 hours, and once again at 120 hours. That last reading is vital because patients with atopic dermatitis can have a low irritant threshold; the delayed reading lessens the possibility of reading irritant reactions as positives.
The reading at 72-96 hours is the time for induration testing.
"When evaluating patch test reactions, palpation for induration is absolutely necessary," Dr. Jacob emphasized. "First the evaluator palpates the baseline dermatitis, and then the patch application squares, looking for areas of increased induration and effectively subtracting background."
She reported serving as a consultant to Johnson & Johnson and Medimetriks and as a clinical investigator for SmartPractice, which manufactures patch test kits, the use of which remains nonapproved by the Food and Drug Administration in children.
COEUR D’ALENE, IDAHO– Contact dermatitis goes together with moderate-to-severe atopic dermatitis like ham and eggs. Reading patch test results in such patients poses unique challenges, because of the impaired skin barrier function intrinsic to atopic dermatitis, coupled with the moist environment created under the occlusive patches, which predisposes to Staphylococcus aureus colonization and superinfection.
All of this makes the evaluation of patch test results in atopic dermatitis more complicated than in patients without contact dermatitis. But there are tricks that greatly reduce the difficulty.
"Patch testing can play a crucial role in the work-up and management of patients with refractory atopic dermatitis. Prior to patch testing, measures should be taken to improve the skin barrier and reduce bacterial overload," Dr. Sharon E. Jacob advised at the annual meeting of the Society for Pediatric Dermatology.
Her recommended preparation program starts 3 weeks prior to the scheduled patch test. The emphasis is on beefing up the disrupted skin barrier through the use of lipid-replenishing emollients and nonalkaline soaps, avoidance of fragrances and other irritant allergens, and preemptive treatment of S. aureus colonization or superinfection, explained Dr. Jacob of Loma Linda (Calif.) University.
At the start of the 3-week countdown, a patient with no clinical signs of skin infection should be checked for nasal carriage of S. aureus. If positive, or if the patient has a remote history of S. aureus colonization or superinfection but no current signs of colonization, such as oozing or crusting, it’s appropriate to begin intranasal, perianal, and umbilical topical mupirocin twice daily for 5 days.
It’s also time to eliminate irritant allergens, start a regimen of dilute bleach baths, embrace the special emollients and soaps, and make sure areas of dermatitis are getting adequately treated with topical steroids, except for the planned patch test area, which must remain steroid free for 7 days prior to the test day.
The lipid-containing emollients, which should contain ceramides or filaggrin degradation products, are to be used all over the body, including the back, until the day before the test.
Examples of the nonalkaline soaps, which are employed to maintain an acidic skin pH, include Aveeno Moisturizing Bar soap, Cetaphil Gentle Cleansing Bar, and Dove Sensitive Skin Unscented Beauty Bar, the pediatric dermatologist continued.
Patients on UV phototherapy for their atopic dermatitis can continue except at the planned patch test site, where it should be avoided for 2 weeks beforehand.
In an adolescent with head and neck atopic dermatitis, a pre–patch test course of oral antifungal therapy is worth considering, according to Dr. Jacob.
For the atopic dermatitis patient with clinical signs of bacterial infection at the pre–patch test office visit, culture the lesions and start a preemptive 10-day course of oral cephalexin at 25-50 mg/kg per day three times daily beginning 3 days prior to patch testing, with the choice of antimicrobial adjusted as warranted by the culture results. These patients also go on the emollients, soaps, dilute bleach baths, and irritant allergen avoidance regimen.
A patient with no signs of skin infection when the test patches are removed can continue with the test readings as scheduled, with no further intervention. However, if signs of infection are present, the patient should immediately go on oral cephalexin if not already on it and take a single bleach bath the same day the patches come off. The bath should be at one-tenth to one-half the customary concentration of 0.005% sodium hypochlorite and should be followed by a fresh water rinse.
The usual patch test reading schedule in atopic dermatitis patients is at 24-48 hours, again at 72-96 hours, and once again at 120 hours. That last reading is vital because patients with atopic dermatitis can have a low irritant threshold; the delayed reading lessens the possibility of reading irritant reactions as positives.
The reading at 72-96 hours is the time for induration testing.
"When evaluating patch test reactions, palpation for induration is absolutely necessary," Dr. Jacob emphasized. "First the evaluator palpates the baseline dermatitis, and then the patch application squares, looking for areas of increased induration and effectively subtracting background."
She reported serving as a consultant to Johnson & Johnson and Medimetriks and as a clinical investigator for SmartPractice, which manufactures patch test kits, the use of which remains nonapproved by the Food and Drug Administration in children.
COEUR D’ALENE, IDAHO– Contact dermatitis goes together with moderate-to-severe atopic dermatitis like ham and eggs. Reading patch test results in such patients poses unique challenges, because of the impaired skin barrier function intrinsic to atopic dermatitis, coupled with the moist environment created under the occlusive patches, which predisposes to Staphylococcus aureus colonization and superinfection.
All of this makes the evaluation of patch test results in atopic dermatitis more complicated than in patients without contact dermatitis. But there are tricks that greatly reduce the difficulty.
"Patch testing can play a crucial role in the work-up and management of patients with refractory atopic dermatitis. Prior to patch testing, measures should be taken to improve the skin barrier and reduce bacterial overload," Dr. Sharon E. Jacob advised at the annual meeting of the Society for Pediatric Dermatology.
Her recommended preparation program starts 3 weeks prior to the scheduled patch test. The emphasis is on beefing up the disrupted skin barrier through the use of lipid-replenishing emollients and nonalkaline soaps, avoidance of fragrances and other irritant allergens, and preemptive treatment of S. aureus colonization or superinfection, explained Dr. Jacob of Loma Linda (Calif.) University.
At the start of the 3-week countdown, a patient with no clinical signs of skin infection should be checked for nasal carriage of S. aureus. If positive, or if the patient has a remote history of S. aureus colonization or superinfection but no current signs of colonization, such as oozing or crusting, it’s appropriate to begin intranasal, perianal, and umbilical topical mupirocin twice daily for 5 days.
It’s also time to eliminate irritant allergens, start a regimen of dilute bleach baths, embrace the special emollients and soaps, and make sure areas of dermatitis are getting adequately treated with topical steroids, except for the planned patch test area, which must remain steroid free for 7 days prior to the test day.
The lipid-containing emollients, which should contain ceramides or filaggrin degradation products, are to be used all over the body, including the back, until the day before the test.
Examples of the nonalkaline soaps, which are employed to maintain an acidic skin pH, include Aveeno Moisturizing Bar soap, Cetaphil Gentle Cleansing Bar, and Dove Sensitive Skin Unscented Beauty Bar, the pediatric dermatologist continued.
Patients on UV phototherapy for their atopic dermatitis can continue except at the planned patch test site, where it should be avoided for 2 weeks beforehand.
In an adolescent with head and neck atopic dermatitis, a pre–patch test course of oral antifungal therapy is worth considering, according to Dr. Jacob.
For the atopic dermatitis patient with clinical signs of bacterial infection at the pre–patch test office visit, culture the lesions and start a preemptive 10-day course of oral cephalexin at 25-50 mg/kg per day three times daily beginning 3 days prior to patch testing, with the choice of antimicrobial adjusted as warranted by the culture results. These patients also go on the emollients, soaps, dilute bleach baths, and irritant allergen avoidance regimen.
A patient with no signs of skin infection when the test patches are removed can continue with the test readings as scheduled, with no further intervention. However, if signs of infection are present, the patient should immediately go on oral cephalexin if not already on it and take a single bleach bath the same day the patches come off. The bath should be at one-tenth to one-half the customary concentration of 0.005% sodium hypochlorite and should be followed by a fresh water rinse.
The usual patch test reading schedule in atopic dermatitis patients is at 24-48 hours, again at 72-96 hours, and once again at 120 hours. That last reading is vital because patients with atopic dermatitis can have a low irritant threshold; the delayed reading lessens the possibility of reading irritant reactions as positives.
The reading at 72-96 hours is the time for induration testing.
"When evaluating patch test reactions, palpation for induration is absolutely necessary," Dr. Jacob emphasized. "First the evaluator palpates the baseline dermatitis, and then the patch application squares, looking for areas of increased induration and effectively subtracting background."
She reported serving as a consultant to Johnson & Johnson and Medimetriks and as a clinical investigator for SmartPractice, which manufactures patch test kits, the use of which remains nonapproved by the Food and Drug Administration in children.
EXPERT ANALYSIS FROM THE SPD 2014
What do the guidelines say?
Atopic dermatitis remains a challenging condition.
The 2014 guidelines of care for the management of atopic dermatitis (AD) are being published by the American Academy of Dermatology in a series of four parts. Each part begins with a disclaimer stating that, "the ultimate judgment regarding the propriety of any specific therapy must be made by the physician and the patient in light of all the circumstances presented by the individual patient and the known variability and biologic behavior of the disease." The disclaimer continues, "This guideline reflects the best available data at the time the guideline was prepared. The results of future studies may require revisions to the recommendations in this guideline to reflect new data."
• Section 1: Diagnosis and assessment of atopic dermatitis. This section includes risk factors for the development of AD, diagnostic and monitoring techniques, assessment and outcomes, and clinical associations in AD patients (J. Am. Acad. Dermatol. 2014;70:338-51).
• Section 2: Management and treatment of atopic dermatitis with topical therapies. This section focuses on recommendations for the use of nonpharmacologic and topical therapies in the management of AD (J. Am. Acad. Dermatol. 2014;71:116-32).
• Section 3: Management and treatment with phototherapy and systemic agents. This section reviews indications for the use of phototherapy and systemic immunomodulators for treating AD, including side-effect profiles and clinical considerations for treating children (J. Am. Acad. Dermatol. 2014;71:327-49).
• Section 4: The fourth and final section of the guidelines is expected to be published in the September 2014 issue of the Journal of the American Academy of Dermatology.
No outside funding sources were involved in the creation of the guidelines. Disclosures of members of the guidelines committee are available following full text of each guidelines section in print and online.
Atopic dermatitis remains a challenging condition.
The 2014 guidelines of care for the management of atopic dermatitis (AD) are being published by the American Academy of Dermatology in a series of four parts. Each part begins with a disclaimer stating that, "the ultimate judgment regarding the propriety of any specific therapy must be made by the physician and the patient in light of all the circumstances presented by the individual patient and the known variability and biologic behavior of the disease." The disclaimer continues, "This guideline reflects the best available data at the time the guideline was prepared. The results of future studies may require revisions to the recommendations in this guideline to reflect new data."
• Section 1: Diagnosis and assessment of atopic dermatitis. This section includes risk factors for the development of AD, diagnostic and monitoring techniques, assessment and outcomes, and clinical associations in AD patients (J. Am. Acad. Dermatol. 2014;70:338-51).
• Section 2: Management and treatment of atopic dermatitis with topical therapies. This section focuses on recommendations for the use of nonpharmacologic and topical therapies in the management of AD (J. Am. Acad. Dermatol. 2014;71:116-32).
• Section 3: Management and treatment with phototherapy and systemic agents. This section reviews indications for the use of phototherapy and systemic immunomodulators for treating AD, including side-effect profiles and clinical considerations for treating children (J. Am. Acad. Dermatol. 2014;71:327-49).
• Section 4: The fourth and final section of the guidelines is expected to be published in the September 2014 issue of the Journal of the American Academy of Dermatology.
No outside funding sources were involved in the creation of the guidelines. Disclosures of members of the guidelines committee are available following full text of each guidelines section in print and online.
Atopic dermatitis remains a challenging condition.
The 2014 guidelines of care for the management of atopic dermatitis (AD) are being published by the American Academy of Dermatology in a series of four parts. Each part begins with a disclaimer stating that, "the ultimate judgment regarding the propriety of any specific therapy must be made by the physician and the patient in light of all the circumstances presented by the individual patient and the known variability and biologic behavior of the disease." The disclaimer continues, "This guideline reflects the best available data at the time the guideline was prepared. The results of future studies may require revisions to the recommendations in this guideline to reflect new data."
• Section 1: Diagnosis and assessment of atopic dermatitis. This section includes risk factors for the development of AD, diagnostic and monitoring techniques, assessment and outcomes, and clinical associations in AD patients (J. Am. Acad. Dermatol. 2014;70:338-51).
• Section 2: Management and treatment of atopic dermatitis with topical therapies. This section focuses on recommendations for the use of nonpharmacologic and topical therapies in the management of AD (J. Am. Acad. Dermatol. 2014;71:116-32).
• Section 3: Management and treatment with phototherapy and systemic agents. This section reviews indications for the use of phototherapy and systemic immunomodulators for treating AD, including side-effect profiles and clinical considerations for treating children (J. Am. Acad. Dermatol. 2014;71:327-49).
• Section 4: The fourth and final section of the guidelines is expected to be published in the September 2014 issue of the Journal of the American Academy of Dermatology.
No outside funding sources were involved in the creation of the guidelines. Disclosures of members of the guidelines committee are available following full text of each guidelines section in print and online.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis
Note from the National Guideline Clearinghouse (NGC): This document is the first section in a series of four and covers methods for diagnosis and assessment of atopic dermatitis (AD). The second guideline in the series will address the management and treatment of AD with pharmacologic and nonpharmacologic topical modalities; the third section will cover phototherapy and systemic treatment options; and the fourth section will address the minimization of disease flares, educational interventions, and use of adjunctive approaches.
Features to Be Considered in the Diagnosis of Patients with AD
| Essential Features—Must be present:
*Patterns Include:
Important Features—Seen in most cases, adding support to the diagnosis:
Associated Features—These clinical associations help to suggest the diagnosis of AD but are too nonspecific to be used for defining or detecting AD for research and epidemiologic studies:
Exclusionary Conditions—It should be noted that a diagnosis of AD depends on excluding conditions, such as:
Adapted from Eichenfield LF, Hanifin JM, Luger TA, Stevens SR, Pride HB. Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol 2003;49:1088-95. Used with permission of the American Academy of Dermatology. |
Recommendation for the Diagnosis of AD
Patients with presumed AD should have their diagnosis based on the criteria summarized in the box above. On occasion, skin biopsy specimens or other tests (such as serum immunoglobulin E, potassium hydroxide preparation, patch testing, and/or genetic testing) may be helpful to rule out other or associated skin conditions.
Strength of Recommendations for the Diagnosis and Assessment of AD
| Recommendation | Strength of Recommendation | Level of Evidence | References |
|---|---|---|---|
| Diagnosis made using criteria in the box above | C | III | Mevorah et al., 1988; Gu et al., 2001; Lan et al., 2009; Diepgen, Sauerbrei, & Fartasch, 1996; De, Kanwar, & Handa, 2006; Loden, Andersson, & Lindberg, 1998; Samochocki & Dejewska, 2012; Samochocki, Paulochowska, & Zabielski, 2000; Chalmers et al., 2007; Firooz et al., 1999; Saeki et al., 2007; Firooz & Kashani, 2008; Hamada et al., 2005; Williams et al., 1994; Williams et al., 1996 |
| No specific biomarkers for diagnosis or severity assessment | B | II | Murat-Susic et al., 2006; Schulte-Herbruggen et al., 2007; Amon et al., 2000; Dhar et al., 2005; Gerdes, Kurrat, & Mrowietz, 2009; Aral et al, 2006; Di Lorenzo et al., 2003; El Mongy et al., 2008; Ezzat, Hasan, & Shaheen, 2011; Jahnz-Rozyk et al., 2005; Nakazato et al., 2008; Belloni Fortina et al., 2006; Gutgesell et al., 2002; Hirai et al., 1996; Hon et al., 2007; Horikawa et al., 2002; Kakinuma et al., 2003; La Grutta et al., 2005; Leung et al., 2003; Mostafa et al., 2008; Oflazoglu et al., "CD30 expression," 2008; Oflazoglu et al., "CD40 expression," 2008; Ott et al., 2010; Raap et al., 2006; Song et al., 2006; Wolkerstorfer et al., 1998 |
| Immunoglobulin E levels not routinely recommended | A | I | Schneider et al., 2013; Murat-Susic et al., 2006; Schulte-Herbruggen et al., 2007; Gerdes, Kurrat, & Mrowietz, 2009; Aral et al., 2006; Vakirlis et al., 2011; Wu et al., 2011 |
| Available disease severity scales not for routine clinical use | C | II | Schmitt, Langan, & Williams, 2007; Schram et al., 2012; Sprikkelman et al., 1997; Angelova-Fischer et al., 2005; Wolkerstorfer et al., 1999; Linnet & Jemec, 1999; Hon et al., 2006; Barbier et al., 2004; Charman, Venn, & Williams, 2002; Charman, Venn, & Williams, 2004; Charman et al., 1999; Cosickic et al., 2010; Emerson, Charman, & Williams, 2000; Hanifin et al., 2001; Holm et al., 2007; Oranje et al., 1997; Rullo et al., 2008 |
| Available quality of life severity scales not for routine clinical use | C | II | Chamlin et al., 2007; Augustin et al., 2004; Hon et al, 2006; Misery et al., 2007 |
| Should query itch, sleep, impact on daily activity, and disease persistence | C | III | Chamlin et al., 2005; Hon et al., 2008; Dawn et al., 2009; Lewis-Jones, 2006; Weisshaar et al., 2008; Ricci et al., 2007; Bender et al., 2008; Ben-Gashir, Seed, & Hay, 2002 |
| Awareness and discussion of common associations | C | I and II | Chamlin et al., 2005; Hon et al., 2008; Batlles-Garrido et al., 2010; Chawes et al., 2010; Sultesz et al., 2010; Kyllonen et al., 2006; Hwang et al., 2010; Hyvarinen et al., 2005; Eller et al., 2009; Horwitz, Hossain, & Yousef, 2009; Bashir, Dar, & Rao, 2010; Schmitt et al., "Psychiatric comorbidity," 2009; Schmitt et al., "Atopic eczema," 2009; Yaghmaie, Koudelka, & Simpson, 2013; Harding et al., 2008; Synnerstad et al., 2008; Vajdic et al., 2009; Kajbaf, Asar, & Alipoor, 2011; Vlaski et al., 2006 |
| Integrated, multidisciplinary approach to care | C | III | Boguniewicz et al., 2008; Ricci et al., 2009 |
Recommendations for the Use of Biomarkers in the Assessment of AD
- For patients with presumed AD, there are no specific biomarkers that can be recommended for diagnosis and/or assessment of disease severity.
- Monitoring of immunoglobulin E levels is not recommended for the routine assessment of disease severity.
Recommendations for Disease Severity and Clinical Outcomes Assessment
- For the general management of patients with AD, available disease severity measurement scales are not recommended for routine clinical practice, because they were not usually designed for this purpose.
- For the general management of patients with AD, available patient quality of life measurement scales are not recommended for routine clinical practice.
- It is recommended that clinicians ask general questions about itch, sleep, impact on daily activity, and persistence of disease, and currently available scales be used mainly when practical.
Recommendations for the Assessment of Clinical Associations of AD
- Physicians should be aware of and assess for conditions associated with AD, such as rhinitis/rhinoconjunctivitis, asthma, food allergy, sleep disturbance, depression, and other neuropsychiatric conditions, and it is recommended that physicians discuss them with the patient as part of the treatment/management plan, when appropriate.
- An integrated, multidisciplinary approach to care may be valuable and is suggested for AD patients who present with common associations.
Definitions:
Levels of Evidence
- Good-quality patient-oriented evidence (i.e., evidence measuring outcomes that matter to patients: morbidity, mortality, symptom improvement, cost reduction, and quality of life)
- Limited-quality patient-oriented evidence
- Other evidence including consensus guidelines, opinion, case studies, or disease-oriented evidence (i.e., evidence measuring intermediate, physiologic, or surrogate end points that may or may not reflect improvements in patient outcomes)
Grades of Recommendation
- Recommendation based on consistent and good quality patient-oriented evidence
- Recommendation based on inconsistent or limited quality patient-oriented evidence
- Recommendation based on consensus, opinion, case studies, or disease-oriented evidence
Note from the National Guideline Clearinghouse (NGC): This document is the first section in a series of four and covers methods for diagnosis and assessment of atopic dermatitis (AD). The second guideline in the series will address the management and treatment of AD with pharmacologic and nonpharmacologic topical modalities; the third section will cover phototherapy and systemic treatment options; and the fourth section will address the minimization of disease flares, educational interventions, and use of adjunctive approaches.
Features to Be Considered in the Diagnosis of Patients with AD
| Essential Features—Must be present:
*Patterns Include:
Important Features—Seen in most cases, adding support to the diagnosis:
Associated Features—These clinical associations help to suggest the diagnosis of AD but are too nonspecific to be used for defining or detecting AD for research and epidemiologic studies:
Exclusionary Conditions—It should be noted that a diagnosis of AD depends on excluding conditions, such as:
Adapted from Eichenfield LF, Hanifin JM, Luger TA, Stevens SR, Pride HB. Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol 2003;49:1088-95. Used with permission of the American Academy of Dermatology. |
Recommendation for the Diagnosis of AD
Patients with presumed AD should have their diagnosis based on the criteria summarized in the box above. On occasion, skin biopsy specimens or other tests (such as serum immunoglobulin E, potassium hydroxide preparation, patch testing, and/or genetic testing) may be helpful to rule out other or associated skin conditions.
Strength of Recommendations for the Diagnosis and Assessment of AD
| Recommendation | Strength of Recommendation | Level of Evidence | References |
|---|---|---|---|
| Diagnosis made using criteria in the box above | C | III | Mevorah et al., 1988; Gu et al., 2001; Lan et al., 2009; Diepgen, Sauerbrei, & Fartasch, 1996; De, Kanwar, & Handa, 2006; Loden, Andersson, & Lindberg, 1998; Samochocki & Dejewska, 2012; Samochocki, Paulochowska, & Zabielski, 2000; Chalmers et al., 2007; Firooz et al., 1999; Saeki et al., 2007; Firooz & Kashani, 2008; Hamada et al., 2005; Williams et al., 1994; Williams et al., 1996 |
| No specific biomarkers for diagnosis or severity assessment | B | II | Murat-Susic et al., 2006; Schulte-Herbruggen et al., 2007; Amon et al., 2000; Dhar et al., 2005; Gerdes, Kurrat, & Mrowietz, 2009; Aral et al, 2006; Di Lorenzo et al., 2003; El Mongy et al., 2008; Ezzat, Hasan, & Shaheen, 2011; Jahnz-Rozyk et al., 2005; Nakazato et al., 2008; Belloni Fortina et al., 2006; Gutgesell et al., 2002; Hirai et al., 1996; Hon et al., 2007; Horikawa et al., 2002; Kakinuma et al., 2003; La Grutta et al., 2005; Leung et al., 2003; Mostafa et al., 2008; Oflazoglu et al., "CD30 expression," 2008; Oflazoglu et al., "CD40 expression," 2008; Ott et al., 2010; Raap et al., 2006; Song et al., 2006; Wolkerstorfer et al., 1998 |
| Immunoglobulin E levels not routinely recommended | A | I | Schneider et al., 2013; Murat-Susic et al., 2006; Schulte-Herbruggen et al., 2007; Gerdes, Kurrat, & Mrowietz, 2009; Aral et al., 2006; Vakirlis et al., 2011; Wu et al., 2011 |
| Available disease severity scales not for routine clinical use | C | II | Schmitt, Langan, & Williams, 2007; Schram et al., 2012; Sprikkelman et al., 1997; Angelova-Fischer et al., 2005; Wolkerstorfer et al., 1999; Linnet & Jemec, 1999; Hon et al., 2006; Barbier et al., 2004; Charman, Venn, & Williams, 2002; Charman, Venn, & Williams, 2004; Charman et al., 1999; Cosickic et al., 2010; Emerson, Charman, & Williams, 2000; Hanifin et al., 2001; Holm et al., 2007; Oranje et al., 1997; Rullo et al., 2008 |
| Available quality of life severity scales not for routine clinical use | C | II | Chamlin et al., 2007; Augustin et al., 2004; Hon et al, 2006; Misery et al., 2007 |
| Should query itch, sleep, impact on daily activity, and disease persistence | C | III | Chamlin et al., 2005; Hon et al., 2008; Dawn et al., 2009; Lewis-Jones, 2006; Weisshaar et al., 2008; Ricci et al., 2007; Bender et al., 2008; Ben-Gashir, Seed, & Hay, 2002 |
| Awareness and discussion of common associations | C | I and II | Chamlin et al., 2005; Hon et al., 2008; Batlles-Garrido et al., 2010; Chawes et al., 2010; Sultesz et al., 2010; Kyllonen et al., 2006; Hwang et al., 2010; Hyvarinen et al., 2005; Eller et al., 2009; Horwitz, Hossain, & Yousef, 2009; Bashir, Dar, & Rao, 2010; Schmitt et al., "Psychiatric comorbidity," 2009; Schmitt et al., "Atopic eczema," 2009; Yaghmaie, Koudelka, & Simpson, 2013; Harding et al., 2008; Synnerstad et al., 2008; Vajdic et al., 2009; Kajbaf, Asar, & Alipoor, 2011; Vlaski et al., 2006 |
| Integrated, multidisciplinary approach to care | C | III | Boguniewicz et al., 2008; Ricci et al., 2009 |
Recommendations for the Use of Biomarkers in the Assessment of AD
- For patients with presumed AD, there are no specific biomarkers that can be recommended for diagnosis and/or assessment of disease severity.
- Monitoring of immunoglobulin E levels is not recommended for the routine assessment of disease severity.
Recommendations for Disease Severity and Clinical Outcomes Assessment
- For the general management of patients with AD, available disease severity measurement scales are not recommended for routine clinical practice, because they were not usually designed for this purpose.
- For the general management of patients with AD, available patient quality of life measurement scales are not recommended for routine clinical practice.
- It is recommended that clinicians ask general questions about itch, sleep, impact on daily activity, and persistence of disease, and currently available scales be used mainly when practical.
Recommendations for the Assessment of Clinical Associations of AD
- Physicians should be aware of and assess for conditions associated with AD, such as rhinitis/rhinoconjunctivitis, asthma, food allergy, sleep disturbance, depression, and other neuropsychiatric conditions, and it is recommended that physicians discuss them with the patient as part of the treatment/management plan, when appropriate.
- An integrated, multidisciplinary approach to care may be valuable and is suggested for AD patients who present with common associations.
Definitions:
Levels of Evidence
- Good-quality patient-oriented evidence (i.e., evidence measuring outcomes that matter to patients: morbidity, mortality, symptom improvement, cost reduction, and quality of life)
- Limited-quality patient-oriented evidence
- Other evidence including consensus guidelines, opinion, case studies, or disease-oriented evidence (i.e., evidence measuring intermediate, physiologic, or surrogate end points that may or may not reflect improvements in patient outcomes)
Grades of Recommendation
- Recommendation based on consistent and good quality patient-oriented evidence
- Recommendation based on inconsistent or limited quality patient-oriented evidence
- Recommendation based on consensus, opinion, case studies, or disease-oriented evidence
Note from the National Guideline Clearinghouse (NGC): This document is the first section in a series of four and covers methods for diagnosis and assessment of atopic dermatitis (AD). The second guideline in the series will address the management and treatment of AD with pharmacologic and nonpharmacologic topical modalities; the third section will cover phototherapy and systemic treatment options; and the fourth section will address the minimization of disease flares, educational interventions, and use of adjunctive approaches.
Features to Be Considered in the Diagnosis of Patients with AD
| Essential Features—Must be present:
*Patterns Include:
Important Features—Seen in most cases, adding support to the diagnosis:
Associated Features—These clinical associations help to suggest the diagnosis of AD but are too nonspecific to be used for defining or detecting AD for research and epidemiologic studies:
Exclusionary Conditions—It should be noted that a diagnosis of AD depends on excluding conditions, such as:
Adapted from Eichenfield LF, Hanifin JM, Luger TA, Stevens SR, Pride HB. Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol 2003;49:1088-95. Used with permission of the American Academy of Dermatology. |
Recommendation for the Diagnosis of AD
Patients with presumed AD should have their diagnosis based on the criteria summarized in the box above. On occasion, skin biopsy specimens or other tests (such as serum immunoglobulin E, potassium hydroxide preparation, patch testing, and/or genetic testing) may be helpful to rule out other or associated skin conditions.
Strength of Recommendations for the Diagnosis and Assessment of AD
| Recommendation | Strength of Recommendation | Level of Evidence | References |
|---|---|---|---|
| Diagnosis made using criteria in the box above | C | III | Mevorah et al., 1988; Gu et al., 2001; Lan et al., 2009; Diepgen, Sauerbrei, & Fartasch, 1996; De, Kanwar, & Handa, 2006; Loden, Andersson, & Lindberg, 1998; Samochocki & Dejewska, 2012; Samochocki, Paulochowska, & Zabielski, 2000; Chalmers et al., 2007; Firooz et al., 1999; Saeki et al., 2007; Firooz & Kashani, 2008; Hamada et al., 2005; Williams et al., 1994; Williams et al., 1996 |
| No specific biomarkers for diagnosis or severity assessment | B | II | Murat-Susic et al., 2006; Schulte-Herbruggen et al., 2007; Amon et al., 2000; Dhar et al., 2005; Gerdes, Kurrat, & Mrowietz, 2009; Aral et al, 2006; Di Lorenzo et al., 2003; El Mongy et al., 2008; Ezzat, Hasan, & Shaheen, 2011; Jahnz-Rozyk et al., 2005; Nakazato et al., 2008; Belloni Fortina et al., 2006; Gutgesell et al., 2002; Hirai et al., 1996; Hon et al., 2007; Horikawa et al., 2002; Kakinuma et al., 2003; La Grutta et al., 2005; Leung et al., 2003; Mostafa et al., 2008; Oflazoglu et al., "CD30 expression," 2008; Oflazoglu et al., "CD40 expression," 2008; Ott et al., 2010; Raap et al., 2006; Song et al., 2006; Wolkerstorfer et al., 1998 |
| Immunoglobulin E levels not routinely recommended | A | I | Schneider et al., 2013; Murat-Susic et al., 2006; Schulte-Herbruggen et al., 2007; Gerdes, Kurrat, & Mrowietz, 2009; Aral et al., 2006; Vakirlis et al., 2011; Wu et al., 2011 |
| Available disease severity scales not for routine clinical use | C | II | Schmitt, Langan, & Williams, 2007; Schram et al., 2012; Sprikkelman et al., 1997; Angelova-Fischer et al., 2005; Wolkerstorfer et al., 1999; Linnet & Jemec, 1999; Hon et al., 2006; Barbier et al., 2004; Charman, Venn, & Williams, 2002; Charman, Venn, & Williams, 2004; Charman et al., 1999; Cosickic et al., 2010; Emerson, Charman, & Williams, 2000; Hanifin et al., 2001; Holm et al., 2007; Oranje et al., 1997; Rullo et al., 2008 |
| Available quality of life severity scales not for routine clinical use | C | II | Chamlin et al., 2007; Augustin et al., 2004; Hon et al, 2006; Misery et al., 2007 |
| Should query itch, sleep, impact on daily activity, and disease persistence | C | III | Chamlin et al., 2005; Hon et al., 2008; Dawn et al., 2009; Lewis-Jones, 2006; Weisshaar et al., 2008; Ricci et al., 2007; Bender et al., 2008; Ben-Gashir, Seed, & Hay, 2002 |
| Awareness and discussion of common associations | C | I and II | Chamlin et al., 2005; Hon et al., 2008; Batlles-Garrido et al., 2010; Chawes et al., 2010; Sultesz et al., 2010; Kyllonen et al., 2006; Hwang et al., 2010; Hyvarinen et al., 2005; Eller et al., 2009; Horwitz, Hossain, & Yousef, 2009; Bashir, Dar, & Rao, 2010; Schmitt et al., "Psychiatric comorbidity," 2009; Schmitt et al., "Atopic eczema," 2009; Yaghmaie, Koudelka, & Simpson, 2013; Harding et al., 2008; Synnerstad et al., 2008; Vajdic et al., 2009; Kajbaf, Asar, & Alipoor, 2011; Vlaski et al., 2006 |
| Integrated, multidisciplinary approach to care | C | III | Boguniewicz et al., 2008; Ricci et al., 2009 |
Recommendations for the Use of Biomarkers in the Assessment of AD
- For patients with presumed AD, there are no specific biomarkers that can be recommended for diagnosis and/or assessment of disease severity.
- Monitoring of immunoglobulin E levels is not recommended for the routine assessment of disease severity.
Recommendations for Disease Severity and Clinical Outcomes Assessment
- For the general management of patients with AD, available disease severity measurement scales are not recommended for routine clinical practice, because they were not usually designed for this purpose.
- For the general management of patients with AD, available patient quality of life measurement scales are not recommended for routine clinical practice.
- It is recommended that clinicians ask general questions about itch, sleep, impact on daily activity, and persistence of disease, and currently available scales be used mainly when practical.
Recommendations for the Assessment of Clinical Associations of AD
- Physicians should be aware of and assess for conditions associated with AD, such as rhinitis/rhinoconjunctivitis, asthma, food allergy, sleep disturbance, depression, and other neuropsychiatric conditions, and it is recommended that physicians discuss them with the patient as part of the treatment/management plan, when appropriate.
- An integrated, multidisciplinary approach to care may be valuable and is suggested for AD patients who present with common associations.
Definitions:
Levels of Evidence
- Good-quality patient-oriented evidence (i.e., evidence measuring outcomes that matter to patients: morbidity, mortality, symptom improvement, cost reduction, and quality of life)
- Limited-quality patient-oriented evidence
- Other evidence including consensus guidelines, opinion, case studies, or disease-oriented evidence (i.e., evidence measuring intermediate, physiologic, or surrogate end points that may or may not reflect improvements in patient outcomes)
Grades of Recommendation
- Recommendation based on consistent and good quality patient-oriented evidence
- Recommendation based on inconsistent or limited quality patient-oriented evidence
- Recommendation based on consensus, opinion, case studies, or disease-oriented evidence
Atopic dermatitis affects up to 25% of children and 2% to 3% of adults. This guideline addresses methods for the diagnosis and monitoring of disease, outcomes measures for assessment, and common clinical associations that affect patients with AD are discussed.
Guidelines are copyright © 2013 American Academy of Dermatology, Inc. Published by Mosby, Inc. All rights reserved. The summary is provided by the Agency for Healthcare Research and Quality
Mutations identified for phenytoin-related severe skin reactions
Mutations in the CYP2C genes on chromosome 10 appear to predispose carriers to severe adverse cutaneous reactions to the antiepileptic drug phenytoin, according to a report published Aug. 5 in JAMA.
Researchers performed a genome-wide association study of more than 850,000 single-nucleotide polymorphisms (SNPs), followed by direct sequencing of the genes identified as suspicious, to investigate possible genetic factors associated with severe phenytoin-related cutaneous reactions. Phenytoin – the most frequently used first-line antiepileptic agent in hospitalized patients, which is also effective for other neurologic disorders – is known to cause cutaneous reactions ranging from mild rash to life-threatening eosinophilia, Stevens-Johnson syndrome, and toxic epidermal necrolysis, said Dr. Wen-Hung Chung, of the department of dermatology at Chang Gung Memorial Hospital, Taiwan, and his associates.
The study participants were 168 Taiwanese patients taking the drug who developed cutaneous reactions, including 13 who died from those adverse events; 130 Taiwanese patients who were tolerant of phenytoin; and 412 controls from the general Taiwanese population. The genome-wide association study identified a cluster of 16 SNPs on chromosome 10 that showed some association with the adverse cutaneous reactions, including 8 SNPs on CYP2C genes. Direct sequencing of the CYP2C genes found another two variants that were significantly associated with phenytoin-related severe cutaneous adverse reactions. The findings were replicated in an independent set of 30 cases of phenytoin-related severe cutaneous adverse reaction who were recruited from the Taiwan Severe Cutaneous Adverse Reactions Consortium and compared against the 130 phenytoin-tolerant controls (JAMA 2014;312:525-34).
One of the identified mutations, CYP2C9*3, is known from previous studies to impair clearance of phenytoin from the body; in this study it also was linked to extremely slow metabolism, and thus high plasma concentrations, of the drug. The association between CYP2C9*3 and severe cutaneous adverse reactions findings was then validated in additional population-based samples from Taiwan, Japan, and Malaysia. A meta-analysis of the data from all the study populations showed that, overall, CYP2C9*3 carriers were at markedly increased risk for severe cutaneous adverse reactions, with an odds ratio of 11.
"We propose that delayed clearance and accumulation of reactive metabolites caused by genetic variants of drug-metabolizing enzymes may be the primary factor, and that immunogenicity, such as the presence of risk HLA alleles and specific T-cell receptor clonotypes in susceptible individuals, may facilitate the development and guide the different types of cutaneous adverse reactions," Dr. Chung and his associates wrote.
However, delayed clearance was also noted in severely affected patients who did not carry the CYP2C9*3 mutation, "suggesting that nongenetic factors such as renal insufficiency, hepatic dysfunction, and concurrent use of substances that compete with or inhibit the enzymes may also affect phenytoin metabolism and contribute to severe cutaneous adverse reactions," they said.
If these findings are corroborated in future studies, it is possible that patients might be tested for these genetic mutations before they take phenytoin, to prevent these severe and sometimes fatal reactions, the investigators added.
This study was supported by the National Science Council, Taiwan; the National Core Facility Program for Biotechnology, Taiwan; and Chang Gung Memorial Hospital, also in Taiwan. Dr. Chung and a coauthor reported having a patent pending for risk assessment of phenytoin-induced adverse reactions.
Mutations in the CYP2C genes on chromosome 10 appear to predispose carriers to severe adverse cutaneous reactions to the antiepileptic drug phenytoin, according to a report published Aug. 5 in JAMA.
Researchers performed a genome-wide association study of more than 850,000 single-nucleotide polymorphisms (SNPs), followed by direct sequencing of the genes identified as suspicious, to investigate possible genetic factors associated with severe phenytoin-related cutaneous reactions. Phenytoin – the most frequently used first-line antiepileptic agent in hospitalized patients, which is also effective for other neurologic disorders – is known to cause cutaneous reactions ranging from mild rash to life-threatening eosinophilia, Stevens-Johnson syndrome, and toxic epidermal necrolysis, said Dr. Wen-Hung Chung, of the department of dermatology at Chang Gung Memorial Hospital, Taiwan, and his associates.
The study participants were 168 Taiwanese patients taking the drug who developed cutaneous reactions, including 13 who died from those adverse events; 130 Taiwanese patients who were tolerant of phenytoin; and 412 controls from the general Taiwanese population. The genome-wide association study identified a cluster of 16 SNPs on chromosome 10 that showed some association with the adverse cutaneous reactions, including 8 SNPs on CYP2C genes. Direct sequencing of the CYP2C genes found another two variants that were significantly associated with phenytoin-related severe cutaneous adverse reactions. The findings were replicated in an independent set of 30 cases of phenytoin-related severe cutaneous adverse reaction who were recruited from the Taiwan Severe Cutaneous Adverse Reactions Consortium and compared against the 130 phenytoin-tolerant controls (JAMA 2014;312:525-34).
One of the identified mutations, CYP2C9*3, is known from previous studies to impair clearance of phenytoin from the body; in this study it also was linked to extremely slow metabolism, and thus high plasma concentrations, of the drug. The association between CYP2C9*3 and severe cutaneous adverse reactions findings was then validated in additional population-based samples from Taiwan, Japan, and Malaysia. A meta-analysis of the data from all the study populations showed that, overall, CYP2C9*3 carriers were at markedly increased risk for severe cutaneous adverse reactions, with an odds ratio of 11.
"We propose that delayed clearance and accumulation of reactive metabolites caused by genetic variants of drug-metabolizing enzymes may be the primary factor, and that immunogenicity, such as the presence of risk HLA alleles and specific T-cell receptor clonotypes in susceptible individuals, may facilitate the development and guide the different types of cutaneous adverse reactions," Dr. Chung and his associates wrote.
However, delayed clearance was also noted in severely affected patients who did not carry the CYP2C9*3 mutation, "suggesting that nongenetic factors such as renal insufficiency, hepatic dysfunction, and concurrent use of substances that compete with or inhibit the enzymes may also affect phenytoin metabolism and contribute to severe cutaneous adverse reactions," they said.
If these findings are corroborated in future studies, it is possible that patients might be tested for these genetic mutations before they take phenytoin, to prevent these severe and sometimes fatal reactions, the investigators added.
This study was supported by the National Science Council, Taiwan; the National Core Facility Program for Biotechnology, Taiwan; and Chang Gung Memorial Hospital, also in Taiwan. Dr. Chung and a coauthor reported having a patent pending for risk assessment of phenytoin-induced adverse reactions.
Mutations in the CYP2C genes on chromosome 10 appear to predispose carriers to severe adverse cutaneous reactions to the antiepileptic drug phenytoin, according to a report published Aug. 5 in JAMA.
Researchers performed a genome-wide association study of more than 850,000 single-nucleotide polymorphisms (SNPs), followed by direct sequencing of the genes identified as suspicious, to investigate possible genetic factors associated with severe phenytoin-related cutaneous reactions. Phenytoin – the most frequently used first-line antiepileptic agent in hospitalized patients, which is also effective for other neurologic disorders – is known to cause cutaneous reactions ranging from mild rash to life-threatening eosinophilia, Stevens-Johnson syndrome, and toxic epidermal necrolysis, said Dr. Wen-Hung Chung, of the department of dermatology at Chang Gung Memorial Hospital, Taiwan, and his associates.
The study participants were 168 Taiwanese patients taking the drug who developed cutaneous reactions, including 13 who died from those adverse events; 130 Taiwanese patients who were tolerant of phenytoin; and 412 controls from the general Taiwanese population. The genome-wide association study identified a cluster of 16 SNPs on chromosome 10 that showed some association with the adverse cutaneous reactions, including 8 SNPs on CYP2C genes. Direct sequencing of the CYP2C genes found another two variants that were significantly associated with phenytoin-related severe cutaneous adverse reactions. The findings were replicated in an independent set of 30 cases of phenytoin-related severe cutaneous adverse reaction who were recruited from the Taiwan Severe Cutaneous Adverse Reactions Consortium and compared against the 130 phenytoin-tolerant controls (JAMA 2014;312:525-34).
One of the identified mutations, CYP2C9*3, is known from previous studies to impair clearance of phenytoin from the body; in this study it also was linked to extremely slow metabolism, and thus high plasma concentrations, of the drug. The association between CYP2C9*3 and severe cutaneous adverse reactions findings was then validated in additional population-based samples from Taiwan, Japan, and Malaysia. A meta-analysis of the data from all the study populations showed that, overall, CYP2C9*3 carriers were at markedly increased risk for severe cutaneous adverse reactions, with an odds ratio of 11.
"We propose that delayed clearance and accumulation of reactive metabolites caused by genetic variants of drug-metabolizing enzymes may be the primary factor, and that immunogenicity, such as the presence of risk HLA alleles and specific T-cell receptor clonotypes in susceptible individuals, may facilitate the development and guide the different types of cutaneous adverse reactions," Dr. Chung and his associates wrote.
However, delayed clearance was also noted in severely affected patients who did not carry the CYP2C9*3 mutation, "suggesting that nongenetic factors such as renal insufficiency, hepatic dysfunction, and concurrent use of substances that compete with or inhibit the enzymes may also affect phenytoin metabolism and contribute to severe cutaneous adverse reactions," they said.
If these findings are corroborated in future studies, it is possible that patients might be tested for these genetic mutations before they take phenytoin, to prevent these severe and sometimes fatal reactions, the investigators added.
This study was supported by the National Science Council, Taiwan; the National Core Facility Program for Biotechnology, Taiwan; and Chang Gung Memorial Hospital, also in Taiwan. Dr. Chung and a coauthor reported having a patent pending for risk assessment of phenytoin-induced adverse reactions.
FROM JAMA
Key clinical point: If the findings are corroborated in future studies, patients might be tested for genetic mutations in CYP2C genes before they take phenytoin.
Major finding: Carriers of the CYP2C9*3 variant were at markedly increased risk for developing severe cutaneous adverse reactions when treated with phenytoin, with an OR of 11.
Data source: A genome-wide association study involving 168 cases of phenytoin-induced severe cutaneous reactions, 130 phenytoin-tolerant controls, and 412 controls from the general Taiwanese population, with additional analyses in further samples from patients in Taiwan, Japan, and Malaysia.
Disclosures: This study was supported by the National Science Council, Taiwan; the National Core Facility Program for Biotechnology, Taiwan; and Chang Gung Memorial Hospital, also in Taiwan. Dr. Chung and a coauthor reported having a patent pending for risk assessment of phenytoin-induced adverse reactions.
Childhood eczema takes financial, emotional toll on families
COEUR D’ALENE, ID. – A new study puts a price on the financial and emotional costs of childhood atopic dermatitis – and concludes both are much steeper than generally recognized.
Moreover, among low-income families, a significant correlation was documented between the monthly financial cost of atopic dermatitis care and the emotional burden imposed by the disease as reflected in higher CADIS (Childhood Atopic Dermatitis Impact Scale) scores, Ms. Michelle G. Filanovsky reported at the annual meeting of the Society for Pediatric Dermatology.
"Our study is the first to correlate financial burden with emotional impact of atopic dermatitis for patients of lower socioeconomic status. This has great implications for how practitioners can help lessen the burden of the disease: Perhaps by helping families lower the cost of disease, we can help lower the emotional burden," observed Ms. Filanovsky, a medical student at Case Western Reserve University, Cleveland.
She and her coinvestigators surveyed parents or other caretakers of 79 children aged 6 months to 12 years with moderate to severe atopic dermatitis who presented to Cleveland dermatology clinics, typically because of a disease flare. Forty-five of the children were covered by Medicaid.
Subjects were queried regarding their total direct and indirect costs for atopic dermatitis care during the past 4 weeks. Direct costs include physician office and emergency department visits, prescription medications, complementary and alternative medicine, and – most importantly – over-the-counter (OTC) products, which are used extensively in atopic dermatitis care. Indirect costs included time missed from work or school and additional child-care expenses.
The disease’s emotional impact was assessed by CADIS on a 1-10 scale. CADIS addresses issues including caregiver sleep disruption and other caregiver concerns, as well as any difficulties the child is experiencing with sleep, school, socialization and conduct, self-esteem, and activity limitations.
The mean personal cost of atopic dermatitis in the month prior to the office visit was $273.78, representing on average more than one-third of a family’s available monthly money, Ms. Filanovsky said. The breakdown was $75.12 in direct costs and $198.66 for indirect costs. Families with commercial health care insurance averaged $130.58 in direct and $436.16 in indirect costs, with much lower costs in the Medicaid group.
OTC products made up the largest portion of direct costs. An average of $15.28 was spent on moisturizers, $9.42 on OTC topical steroids, $8.46 on bath products, and $4.66 on antihistamines. Time missed from work accounted for the bulk of the indirect costs.
Medicaid patients showed a significant linear correlation between CADIS scores and both total monthly costs of atopic dermatitis care and monthly costs adjusted by family size and income. However, commercially insured families did not.
Ms. Filanovsky proposed several measures as worthy of further study to help reduce the financial and emotional burden of atopic dermatitis, including insurance coverage of moisturizers, physician guidance regarding the most cost-effective OTC products, and implementation of afterwork office hours or nurse on-call visits during flares to minimize the indirect cost of care.
Survey respondents received $25 for their participation, with the funds provided by Nestle. Ms. Filanovsky reported no financial conflicts regarding this study.
COEUR D’ALENE, ID. – A new study puts a price on the financial and emotional costs of childhood atopic dermatitis – and concludes both are much steeper than generally recognized.
Moreover, among low-income families, a significant correlation was documented between the monthly financial cost of atopic dermatitis care and the emotional burden imposed by the disease as reflected in higher CADIS (Childhood Atopic Dermatitis Impact Scale) scores, Ms. Michelle G. Filanovsky reported at the annual meeting of the Society for Pediatric Dermatology.
"Our study is the first to correlate financial burden with emotional impact of atopic dermatitis for patients of lower socioeconomic status. This has great implications for how practitioners can help lessen the burden of the disease: Perhaps by helping families lower the cost of disease, we can help lower the emotional burden," observed Ms. Filanovsky, a medical student at Case Western Reserve University, Cleveland.
She and her coinvestigators surveyed parents or other caretakers of 79 children aged 6 months to 12 years with moderate to severe atopic dermatitis who presented to Cleveland dermatology clinics, typically because of a disease flare. Forty-five of the children were covered by Medicaid.
Subjects were queried regarding their total direct and indirect costs for atopic dermatitis care during the past 4 weeks. Direct costs include physician office and emergency department visits, prescription medications, complementary and alternative medicine, and – most importantly – over-the-counter (OTC) products, which are used extensively in atopic dermatitis care. Indirect costs included time missed from work or school and additional child-care expenses.
The disease’s emotional impact was assessed by CADIS on a 1-10 scale. CADIS addresses issues including caregiver sleep disruption and other caregiver concerns, as well as any difficulties the child is experiencing with sleep, school, socialization and conduct, self-esteem, and activity limitations.
The mean personal cost of atopic dermatitis in the month prior to the office visit was $273.78, representing on average more than one-third of a family’s available monthly money, Ms. Filanovsky said. The breakdown was $75.12 in direct costs and $198.66 for indirect costs. Families with commercial health care insurance averaged $130.58 in direct and $436.16 in indirect costs, with much lower costs in the Medicaid group.
OTC products made up the largest portion of direct costs. An average of $15.28 was spent on moisturizers, $9.42 on OTC topical steroids, $8.46 on bath products, and $4.66 on antihistamines. Time missed from work accounted for the bulk of the indirect costs.
Medicaid patients showed a significant linear correlation between CADIS scores and both total monthly costs of atopic dermatitis care and monthly costs adjusted by family size and income. However, commercially insured families did not.
Ms. Filanovsky proposed several measures as worthy of further study to help reduce the financial and emotional burden of atopic dermatitis, including insurance coverage of moisturizers, physician guidance regarding the most cost-effective OTC products, and implementation of afterwork office hours or nurse on-call visits during flares to minimize the indirect cost of care.
Survey respondents received $25 for their participation, with the funds provided by Nestle. Ms. Filanovsky reported no financial conflicts regarding this study.
COEUR D’ALENE, ID. – A new study puts a price on the financial and emotional costs of childhood atopic dermatitis – and concludes both are much steeper than generally recognized.
Moreover, among low-income families, a significant correlation was documented between the monthly financial cost of atopic dermatitis care and the emotional burden imposed by the disease as reflected in higher CADIS (Childhood Atopic Dermatitis Impact Scale) scores, Ms. Michelle G. Filanovsky reported at the annual meeting of the Society for Pediatric Dermatology.
"Our study is the first to correlate financial burden with emotional impact of atopic dermatitis for patients of lower socioeconomic status. This has great implications for how practitioners can help lessen the burden of the disease: Perhaps by helping families lower the cost of disease, we can help lower the emotional burden," observed Ms. Filanovsky, a medical student at Case Western Reserve University, Cleveland.
She and her coinvestigators surveyed parents or other caretakers of 79 children aged 6 months to 12 years with moderate to severe atopic dermatitis who presented to Cleveland dermatology clinics, typically because of a disease flare. Forty-five of the children were covered by Medicaid.
Subjects were queried regarding their total direct and indirect costs for atopic dermatitis care during the past 4 weeks. Direct costs include physician office and emergency department visits, prescription medications, complementary and alternative medicine, and – most importantly – over-the-counter (OTC) products, which are used extensively in atopic dermatitis care. Indirect costs included time missed from work or school and additional child-care expenses.
The disease’s emotional impact was assessed by CADIS on a 1-10 scale. CADIS addresses issues including caregiver sleep disruption and other caregiver concerns, as well as any difficulties the child is experiencing with sleep, school, socialization and conduct, self-esteem, and activity limitations.
The mean personal cost of atopic dermatitis in the month prior to the office visit was $273.78, representing on average more than one-third of a family’s available monthly money, Ms. Filanovsky said. The breakdown was $75.12 in direct costs and $198.66 for indirect costs. Families with commercial health care insurance averaged $130.58 in direct and $436.16 in indirect costs, with much lower costs in the Medicaid group.
OTC products made up the largest portion of direct costs. An average of $15.28 was spent on moisturizers, $9.42 on OTC topical steroids, $8.46 on bath products, and $4.66 on antihistamines. Time missed from work accounted for the bulk of the indirect costs.
Medicaid patients showed a significant linear correlation between CADIS scores and both total monthly costs of atopic dermatitis care and monthly costs adjusted by family size and income. However, commercially insured families did not.
Ms. Filanovsky proposed several measures as worthy of further study to help reduce the financial and emotional burden of atopic dermatitis, including insurance coverage of moisturizers, physician guidance regarding the most cost-effective OTC products, and implementation of afterwork office hours or nurse on-call visits during flares to minimize the indirect cost of care.
Survey respondents received $25 for their participation, with the funds provided by Nestle. Ms. Filanovsky reported no financial conflicts regarding this study.
AT THE SPD ANNUAL MEETING
Key clinical point: Managing the financial cost of a child’s atopic dermatitis care may mitigate the emotional burden on these children and their families.
Major finding: Families spent an average of $273.78 in direct and indirect costs, including moisturizers and other OTC products, on the care of their child with moderate to severe atopic dermatitis during the 4 weeks prior to a dermatologic office visit.
Data source: A survey of the parents or caregivers of 79 children with moderate to severe atopic dermatitis seen at Cleveland dermatology clinics.
Disclosures: Survey respondents received $25 for their participation, with the funds provided by Nestle. Ms. Filanovsky reported no financial conflicts regarding this study.
‘Soak and smear’ not superior for kids’ atopic dermatitis
COEUR D’ALENE, IDAHO – Topical corticosteroids applied to the dry skin of children with atopic dermatitis proved as effective for clinical improvement as the soak and smear technique favored by many physicians, according to a randomized, investigator-blinded clinical trial.
"The use of corticosteroid application to prehydrated, wet skin is not more efficacious than corticosteroid application to dry skin in pediatric patients with atopic dermatitis," Dr. Richard J. Antaya reported at the annual meeting of the Society for Pediatric Dermatology. "This study suggests that 2 weeks of using either soak and smear or standard topical corticosteroid application techniques results in considerable improvement in atopic dermatitis severity," he said.
Eczema Area and Severity Index (EASI) scores improved to a similarly impressive extent – close to 85% after 2 weeks – regardless of which application method was used, added Dr. Antaya, professor of dermatology, pediatrics, and nursing and director of pediatric dermatology at Yale University in New Haven, Conn.
The study included 47 patients aged 4 months to 16 years with atopic dermatitis and a mean baseline EASI score of 15.5. All were assigned to 2 weeks of twice-daily topical steroid therapy. Those younger than age 2 years received a prescription for a 1-lb jar of hydrocortisone 2.5% ointment; older patients received 1-lb jars of triamcinolone 0.1% ointment, and, for the more sensitive face and intertriginous areas, hydrocortisone 2.5% ointment. Patients were randomized to twice-daily application of their medication to affected dry skin or to a single daily soak and smear session and one application of the medication to dry skin.
Soak and smear entails a 10-minute soak in lukewarm plain water to boost skin hydration, followed by steroid application to the wet skin. Data from several studies conducted in adults concluded that soak and smear is more effective than was conventional steroid application to dry skin. For example, a retrospective study of 28 adults referred to a tertiary dermatologic center for highly refractory atopic dermatitis or other eczematous dermatoses showed 26 of the 28 were clear or at least 90% improved after several days to 2 weeks of soak and smear sessions (Arch. Dermatol. 2005;141:1556-9).
In Dr. Antaya’s pediatric atopic dermatitis study, assessment of EASI scores was performed by a dermatologist blinded to the treatment arm. The profound and similar improvement in EASI scores in the two treatment groups was accompanied by essentially equal improvements in measures of sleep quality, itch, and overall disease impact.
Also, there was no difference in EASI score improvement between the two groups when patients were stratified according to baseline atopic dermatitis severity.
No differences between the groups were noted in treatment days missed or development of folliculitis. Neither group showed any evidence of hypothalamic-pituitary-adrenal axis suppression, he added.
Dr. Antaya attributed the marked improvement in atopic dermatitis seen in both study groups to the fact that, at the study outset, caregivers received education aimed at alleviating steroid phobia. Also, the 1-lb jars of medication encouraged treatment compliance, he noted.
Dr. Antaya reported having no financial conflicts with regard to this study.
COEUR D’ALENE, IDAHO – Topical corticosteroids applied to the dry skin of children with atopic dermatitis proved as effective for clinical improvement as the soak and smear technique favored by many physicians, according to a randomized, investigator-blinded clinical trial.
"The use of corticosteroid application to prehydrated, wet skin is not more efficacious than corticosteroid application to dry skin in pediatric patients with atopic dermatitis," Dr. Richard J. Antaya reported at the annual meeting of the Society for Pediatric Dermatology. "This study suggests that 2 weeks of using either soak and smear or standard topical corticosteroid application techniques results in considerable improvement in atopic dermatitis severity," he said.
Eczema Area and Severity Index (EASI) scores improved to a similarly impressive extent – close to 85% after 2 weeks – regardless of which application method was used, added Dr. Antaya, professor of dermatology, pediatrics, and nursing and director of pediatric dermatology at Yale University in New Haven, Conn.
The study included 47 patients aged 4 months to 16 years with atopic dermatitis and a mean baseline EASI score of 15.5. All were assigned to 2 weeks of twice-daily topical steroid therapy. Those younger than age 2 years received a prescription for a 1-lb jar of hydrocortisone 2.5% ointment; older patients received 1-lb jars of triamcinolone 0.1% ointment, and, for the more sensitive face and intertriginous areas, hydrocortisone 2.5% ointment. Patients were randomized to twice-daily application of their medication to affected dry skin or to a single daily soak and smear session and one application of the medication to dry skin.
Soak and smear entails a 10-minute soak in lukewarm plain water to boost skin hydration, followed by steroid application to the wet skin. Data from several studies conducted in adults concluded that soak and smear is more effective than was conventional steroid application to dry skin. For example, a retrospective study of 28 adults referred to a tertiary dermatologic center for highly refractory atopic dermatitis or other eczematous dermatoses showed 26 of the 28 were clear or at least 90% improved after several days to 2 weeks of soak and smear sessions (Arch. Dermatol. 2005;141:1556-9).
In Dr. Antaya’s pediatric atopic dermatitis study, assessment of EASI scores was performed by a dermatologist blinded to the treatment arm. The profound and similar improvement in EASI scores in the two treatment groups was accompanied by essentially equal improvements in measures of sleep quality, itch, and overall disease impact.
Also, there was no difference in EASI score improvement between the two groups when patients were stratified according to baseline atopic dermatitis severity.
No differences between the groups were noted in treatment days missed or development of folliculitis. Neither group showed any evidence of hypothalamic-pituitary-adrenal axis suppression, he added.
Dr. Antaya attributed the marked improvement in atopic dermatitis seen in both study groups to the fact that, at the study outset, caregivers received education aimed at alleviating steroid phobia. Also, the 1-lb jars of medication encouraged treatment compliance, he noted.
Dr. Antaya reported having no financial conflicts with regard to this study.
COEUR D’ALENE, IDAHO – Topical corticosteroids applied to the dry skin of children with atopic dermatitis proved as effective for clinical improvement as the soak and smear technique favored by many physicians, according to a randomized, investigator-blinded clinical trial.
"The use of corticosteroid application to prehydrated, wet skin is not more efficacious than corticosteroid application to dry skin in pediatric patients with atopic dermatitis," Dr. Richard J. Antaya reported at the annual meeting of the Society for Pediatric Dermatology. "This study suggests that 2 weeks of using either soak and smear or standard topical corticosteroid application techniques results in considerable improvement in atopic dermatitis severity," he said.
Eczema Area and Severity Index (EASI) scores improved to a similarly impressive extent – close to 85% after 2 weeks – regardless of which application method was used, added Dr. Antaya, professor of dermatology, pediatrics, and nursing and director of pediatric dermatology at Yale University in New Haven, Conn.
The study included 47 patients aged 4 months to 16 years with atopic dermatitis and a mean baseline EASI score of 15.5. All were assigned to 2 weeks of twice-daily topical steroid therapy. Those younger than age 2 years received a prescription for a 1-lb jar of hydrocortisone 2.5% ointment; older patients received 1-lb jars of triamcinolone 0.1% ointment, and, for the more sensitive face and intertriginous areas, hydrocortisone 2.5% ointment. Patients were randomized to twice-daily application of their medication to affected dry skin or to a single daily soak and smear session and one application of the medication to dry skin.
Soak and smear entails a 10-minute soak in lukewarm plain water to boost skin hydration, followed by steroid application to the wet skin. Data from several studies conducted in adults concluded that soak and smear is more effective than was conventional steroid application to dry skin. For example, a retrospective study of 28 adults referred to a tertiary dermatologic center for highly refractory atopic dermatitis or other eczematous dermatoses showed 26 of the 28 were clear or at least 90% improved after several days to 2 weeks of soak and smear sessions (Arch. Dermatol. 2005;141:1556-9).
In Dr. Antaya’s pediatric atopic dermatitis study, assessment of EASI scores was performed by a dermatologist blinded to the treatment arm. The profound and similar improvement in EASI scores in the two treatment groups was accompanied by essentially equal improvements in measures of sleep quality, itch, and overall disease impact.
Also, there was no difference in EASI score improvement between the two groups when patients were stratified according to baseline atopic dermatitis severity.
No differences between the groups were noted in treatment days missed or development of folliculitis. Neither group showed any evidence of hypothalamic-pituitary-adrenal axis suppression, he added.
Dr. Antaya attributed the marked improvement in atopic dermatitis seen in both study groups to the fact that, at the study outset, caregivers received education aimed at alleviating steroid phobia. Also, the 1-lb jars of medication encouraged treatment compliance, he noted.
Dr. Antaya reported having no financial conflicts with regard to this study.
AT THE SPD ANNUAL MEETING
Key clinical point: Physicians can anticipate equally profound improvement in the severity of pediatric atopic dermatitis after 2 weeks of twice-daily topical corticosteroid therapy, regardless of whether the medication is applied to prehydrated or dry skin.
Major finding: After 2 weeks of twice-daily topical corticosteroid therapy, pediatric atopic dermatitis patients showed roughly an 85% improvement in disease severity, regardless of whether the medication was applied to dry skin or using the soak and smear method.
Data source: A 2-week, randomized, investigator-blinded clinical trial of twice-daily topical corticosteroid therapy in 47 children and teens with atopic dermatitis.
Disclosures: The presenter reported having no financial conflicts regarding this study, conducted free of commercial support.
An insider’s look at the 2014 atopic dermatitis guidelines
COEUR D’ALENE, IDAHO – The 2014 American Academy of Dermatology atopic dermatitis guidelines may already need an update, according to the cochair of the guidelines panel.
The guidelines were based upon studies published through 2012. Since then, new evidence has emerged that raises the level of uncertainty regarding several key questions the panel addressed, Dr. Robert Sidbury observed at the annual meeting of the Society for Pediatric Dermatology. Among these questions: To bathe or not to bathe? Will a child outgrow atopic dermatitis?
Serving as cochair of the guidelines committee was both a reassuring and daunting experience, according to Dr. Sidbury, chief of the division of dermatology at Seattle Children’s Hospital.
It was reassuring to note that the committee members, who included 17 atopic dermatitis experts from three countries, were free from financial conflicts as they sifted through the published data for evidence-based recommendations to inform practice. But it was daunting to learn how sketchy the supporting evidence is for some of the conventional wisdom regarding atopic dermatitis management, he explained.
By way of providing what he called "a peek behind the curtains" of the guidelines-development process, Dr. Sidbury highlighted the issue of daily bathing followed by application of emollients and moisturizers. This was among the topics the panel struggled with the most, which might come as a surprise to outsiders who consider this to be standard practice, he noted.
"It seems like a very straightforward thing. Almost everyone in this room, to a person, recommends a daily bath followed by moisturizers, yet when we examined the studies we realized that recommendation isn’t based upon much evidence," he said.
Thus, the panel concluded that bathing is "suggested" for atopic dermatitis patients, while adding that "there is no standard" for the duration or frequency of bathing. The panel rated the strength of their recommendation as C, and the level of evidence as III.
"That’s a fairly weak recommendation based upon fairly week evidence," Dr. Sidbury commented.
Moreover, since publication of the guidelines, two new studies have come forth that address the question of whether bathing plus moisturizers is beneficial in atopic dermatitis. The results conflict with each other, making recommendations even more difficult.
Data from a retrospective study of 75 patients with moderate or severe atopic dermatitis suggested that a daily 15- to 20-minute bath followed by a mid-potency topical steroid and moisturizer was indeed beneficial: 79% of subjects showed marked improvement based on Investigator’s Global Assessment at week 3, and 4% were clear (Dermatitis 2014;25:56-9).
By contrast, data from a prospective trial in which 28 children with atopic dermatitis were randomized to a daily vs. twice-weekly bath followed by appropriate care indicated that, while hydration with emollients was important, bathing frequency wasn’t (Clin. Pediatr. 2014;53:677-81).
"This paper makes me feel better about the guidelines not saying, ‘You should bathe every day,’ although that’s still my own recommendation to patients," Dr. Sidbury said.
This year also has brought two conflicting studies regarding the natural history of atopic dermatitis. A large national Taiwanese population-based cohort study of children diagnosed with atopic dermatitis within the first 2 years of life and followed from birth to age 10 years concluded that 70% of these early-onset patients eventually went into remission. A total of 19% of patients did so within the first year, and 49% in less than 4 years. The median disease duration was 4.2 years (Br. J. Dermatol. 2014;170:130-5).
On the other hand, a report from the 7,157-patient, cross-sectional, longitudinal Pediatric Eczema Elective Registry (PEER) found that by age 20, only 50% of the patients had experienced at least one symptom-free period lasting 6 months or more. The investigators concluded that atopic dermatitis is probably a lifelong disease (JAMA Dermatology 2014;150:593-600).
"That’s a provocative conclusion, and a tough thing to tell a parent," Dr. Sidbury observed. "I offer parents realistic but optimistic counsel. I tell them the tendency toward xerosis, irritancy, and infection will persist – the patient in front of you is never going to want to wear a wool sweater for the rest of their life. But the incessant itch, the need for treatment, the impact on quality of life – which is really the issue at hand – hopefully will not persist."
Since the release earlier this year of the first three of the four sections of the atopic dermatitis guidelines, Dr. Sidbury and the other panelists have received considerable feedback that the guidelines didn’t adequately address the topic of topical steroid addiction.
"Some say we missed the boat in not making coherent recommendations to parents about it. We got some very pointed comments," he conceded.
He noted that a systematic review presented at last year’s International Symposium on Atopic Dermatitis concluded that topical steroid withdrawal is a real phenomenon distinct from other topical steroid side effects. It comes in two rosacea-like variants: an erythroedematous form and a papulopustular form. An atopic dermatitis patient’s report of a burning sensation upon cessation of topical steroid therapy is a red flag.
Despite the occasional missed opportunity in drawing up the first AAD atopic guidelines in 10 years, the process was richly rewarding, Dr. Sidbury said. And although experts will continue to debate the unresolved controversies in atopic dermatitis, for him the most important lesson to emerge from the panel’s comprehensive review of the evidence was strikingly clear: "Time and time again, education trumps all. Education of patients and families leads to the best outcomes. I think that’s an important lesson to take home," he said.
Dr. Sidbury had no financial conflicts to disclose.
COEUR D’ALENE, IDAHO – The 2014 American Academy of Dermatology atopic dermatitis guidelines may already need an update, according to the cochair of the guidelines panel.
The guidelines were based upon studies published through 2012. Since then, new evidence has emerged that raises the level of uncertainty regarding several key questions the panel addressed, Dr. Robert Sidbury observed at the annual meeting of the Society for Pediatric Dermatology. Among these questions: To bathe or not to bathe? Will a child outgrow atopic dermatitis?
Serving as cochair of the guidelines committee was both a reassuring and daunting experience, according to Dr. Sidbury, chief of the division of dermatology at Seattle Children’s Hospital.
It was reassuring to note that the committee members, who included 17 atopic dermatitis experts from three countries, were free from financial conflicts as they sifted through the published data for evidence-based recommendations to inform practice. But it was daunting to learn how sketchy the supporting evidence is for some of the conventional wisdom regarding atopic dermatitis management, he explained.
By way of providing what he called "a peek behind the curtains" of the guidelines-development process, Dr. Sidbury highlighted the issue of daily bathing followed by application of emollients and moisturizers. This was among the topics the panel struggled with the most, which might come as a surprise to outsiders who consider this to be standard practice, he noted.
"It seems like a very straightforward thing. Almost everyone in this room, to a person, recommends a daily bath followed by moisturizers, yet when we examined the studies we realized that recommendation isn’t based upon much evidence," he said.
Thus, the panel concluded that bathing is "suggested" for atopic dermatitis patients, while adding that "there is no standard" for the duration or frequency of bathing. The panel rated the strength of their recommendation as C, and the level of evidence as III.
"That’s a fairly weak recommendation based upon fairly week evidence," Dr. Sidbury commented.
Moreover, since publication of the guidelines, two new studies have come forth that address the question of whether bathing plus moisturizers is beneficial in atopic dermatitis. The results conflict with each other, making recommendations even more difficult.
Data from a retrospective study of 75 patients with moderate or severe atopic dermatitis suggested that a daily 15- to 20-minute bath followed by a mid-potency topical steroid and moisturizer was indeed beneficial: 79% of subjects showed marked improvement based on Investigator’s Global Assessment at week 3, and 4% were clear (Dermatitis 2014;25:56-9).
By contrast, data from a prospective trial in which 28 children with atopic dermatitis were randomized to a daily vs. twice-weekly bath followed by appropriate care indicated that, while hydration with emollients was important, bathing frequency wasn’t (Clin. Pediatr. 2014;53:677-81).
"This paper makes me feel better about the guidelines not saying, ‘You should bathe every day,’ although that’s still my own recommendation to patients," Dr. Sidbury said.
This year also has brought two conflicting studies regarding the natural history of atopic dermatitis. A large national Taiwanese population-based cohort study of children diagnosed with atopic dermatitis within the first 2 years of life and followed from birth to age 10 years concluded that 70% of these early-onset patients eventually went into remission. A total of 19% of patients did so within the first year, and 49% in less than 4 years. The median disease duration was 4.2 years (Br. J. Dermatol. 2014;170:130-5).
On the other hand, a report from the 7,157-patient, cross-sectional, longitudinal Pediatric Eczema Elective Registry (PEER) found that by age 20, only 50% of the patients had experienced at least one symptom-free period lasting 6 months or more. The investigators concluded that atopic dermatitis is probably a lifelong disease (JAMA Dermatology 2014;150:593-600).
"That’s a provocative conclusion, and a tough thing to tell a parent," Dr. Sidbury observed. "I offer parents realistic but optimistic counsel. I tell them the tendency toward xerosis, irritancy, and infection will persist – the patient in front of you is never going to want to wear a wool sweater for the rest of their life. But the incessant itch, the need for treatment, the impact on quality of life – which is really the issue at hand – hopefully will not persist."
Since the release earlier this year of the first three of the four sections of the atopic dermatitis guidelines, Dr. Sidbury and the other panelists have received considerable feedback that the guidelines didn’t adequately address the topic of topical steroid addiction.
"Some say we missed the boat in not making coherent recommendations to parents about it. We got some very pointed comments," he conceded.
He noted that a systematic review presented at last year’s International Symposium on Atopic Dermatitis concluded that topical steroid withdrawal is a real phenomenon distinct from other topical steroid side effects. It comes in two rosacea-like variants: an erythroedematous form and a papulopustular form. An atopic dermatitis patient’s report of a burning sensation upon cessation of topical steroid therapy is a red flag.
Despite the occasional missed opportunity in drawing up the first AAD atopic guidelines in 10 years, the process was richly rewarding, Dr. Sidbury said. And although experts will continue to debate the unresolved controversies in atopic dermatitis, for him the most important lesson to emerge from the panel’s comprehensive review of the evidence was strikingly clear: "Time and time again, education trumps all. Education of patients and families leads to the best outcomes. I think that’s an important lesson to take home," he said.
Dr. Sidbury had no financial conflicts to disclose.
COEUR D’ALENE, IDAHO – The 2014 American Academy of Dermatology atopic dermatitis guidelines may already need an update, according to the cochair of the guidelines panel.
The guidelines were based upon studies published through 2012. Since then, new evidence has emerged that raises the level of uncertainty regarding several key questions the panel addressed, Dr. Robert Sidbury observed at the annual meeting of the Society for Pediatric Dermatology. Among these questions: To bathe or not to bathe? Will a child outgrow atopic dermatitis?
Serving as cochair of the guidelines committee was both a reassuring and daunting experience, according to Dr. Sidbury, chief of the division of dermatology at Seattle Children’s Hospital.
It was reassuring to note that the committee members, who included 17 atopic dermatitis experts from three countries, were free from financial conflicts as they sifted through the published data for evidence-based recommendations to inform practice. But it was daunting to learn how sketchy the supporting evidence is for some of the conventional wisdom regarding atopic dermatitis management, he explained.
By way of providing what he called "a peek behind the curtains" of the guidelines-development process, Dr. Sidbury highlighted the issue of daily bathing followed by application of emollients and moisturizers. This was among the topics the panel struggled with the most, which might come as a surprise to outsiders who consider this to be standard practice, he noted.
"It seems like a very straightforward thing. Almost everyone in this room, to a person, recommends a daily bath followed by moisturizers, yet when we examined the studies we realized that recommendation isn’t based upon much evidence," he said.
Thus, the panel concluded that bathing is "suggested" for atopic dermatitis patients, while adding that "there is no standard" for the duration or frequency of bathing. The panel rated the strength of their recommendation as C, and the level of evidence as III.
"That’s a fairly weak recommendation based upon fairly week evidence," Dr. Sidbury commented.
Moreover, since publication of the guidelines, two new studies have come forth that address the question of whether bathing plus moisturizers is beneficial in atopic dermatitis. The results conflict with each other, making recommendations even more difficult.
Data from a retrospective study of 75 patients with moderate or severe atopic dermatitis suggested that a daily 15- to 20-minute bath followed by a mid-potency topical steroid and moisturizer was indeed beneficial: 79% of subjects showed marked improvement based on Investigator’s Global Assessment at week 3, and 4% were clear (Dermatitis 2014;25:56-9).
By contrast, data from a prospective trial in which 28 children with atopic dermatitis were randomized to a daily vs. twice-weekly bath followed by appropriate care indicated that, while hydration with emollients was important, bathing frequency wasn’t (Clin. Pediatr. 2014;53:677-81).
"This paper makes me feel better about the guidelines not saying, ‘You should bathe every day,’ although that’s still my own recommendation to patients," Dr. Sidbury said.
This year also has brought two conflicting studies regarding the natural history of atopic dermatitis. A large national Taiwanese population-based cohort study of children diagnosed with atopic dermatitis within the first 2 years of life and followed from birth to age 10 years concluded that 70% of these early-onset patients eventually went into remission. A total of 19% of patients did so within the first year, and 49% in less than 4 years. The median disease duration was 4.2 years (Br. J. Dermatol. 2014;170:130-5).
On the other hand, a report from the 7,157-patient, cross-sectional, longitudinal Pediatric Eczema Elective Registry (PEER) found that by age 20, only 50% of the patients had experienced at least one symptom-free period lasting 6 months or more. The investigators concluded that atopic dermatitis is probably a lifelong disease (JAMA Dermatology 2014;150:593-600).
"That’s a provocative conclusion, and a tough thing to tell a parent," Dr. Sidbury observed. "I offer parents realistic but optimistic counsel. I tell them the tendency toward xerosis, irritancy, and infection will persist – the patient in front of you is never going to want to wear a wool sweater for the rest of their life. But the incessant itch, the need for treatment, the impact on quality of life – which is really the issue at hand – hopefully will not persist."
Since the release earlier this year of the first three of the four sections of the atopic dermatitis guidelines, Dr. Sidbury and the other panelists have received considerable feedback that the guidelines didn’t adequately address the topic of topical steroid addiction.
"Some say we missed the boat in not making coherent recommendations to parents about it. We got some very pointed comments," he conceded.
He noted that a systematic review presented at last year’s International Symposium on Atopic Dermatitis concluded that topical steroid withdrawal is a real phenomenon distinct from other topical steroid side effects. It comes in two rosacea-like variants: an erythroedematous form and a papulopustular form. An atopic dermatitis patient’s report of a burning sensation upon cessation of topical steroid therapy is a red flag.
Despite the occasional missed opportunity in drawing up the first AAD atopic guidelines in 10 years, the process was richly rewarding, Dr. Sidbury said. And although experts will continue to debate the unresolved controversies in atopic dermatitis, for him the most important lesson to emerge from the panel’s comprehensive review of the evidence was strikingly clear: "Time and time again, education trumps all. Education of patients and families leads to the best outcomes. I think that’s an important lesson to take home," he said.
Dr. Sidbury had no financial conflicts to disclose.
EXPERT OPINION FROM THE SPD ANNUAL MEETING