User login
Methotrexate assay useful in severe pediatric dermatitis, psoriasis
MILWAUKEE – In children with severe atopic dermatitis or psoriasis, the methotrexate polyglutamate assay appears useful in guiding dose modification in those failing to respond to methotrexate at 12 weeks, a retrospective study has shown.
The methotrexate polyglutamate 3 assay (MTX PG3) measures concentrations of methotrexate polyglutamates, the active metabolites of methotrexate, Syed Rahman said at the annual meeting of the Society for Pediatric Dermatology.
Methotrexate, a prodrug, requires enzymatic conversion via sequential addition of glutamic acid residues. Those residues form a glutamate "tail" that enhances intracellular methotrexate retention. The 3-tail metabolite, MTX PG3, is the predominant species in most patients, explained Mr. Rahman of St. Louis University.
"This study supports the usefulness of a commercially available tool for customizing optimal methotrexate dosing, coauthor Dr. Elaine Siegfried, professor of pediatrics and dermatology at the university, said in an interview.
The MTX PG3 assay has been validated in adults with rheumatoid arthritis where patients with a therapeutic methotrexate polyglutamate level of more than 60 nmol/L were fivefold more likely to have a good response to methotrexate than those with levels below this threshold, according to poster results presented at the 2008 American College of Rheumatology annual meeting (cited on the company website). However, the test was not predictive of response in 36% of patients whose arthritis improved with treatment despite subtherapeutic levels.
For the current analysis, the investigators reviewed the charts of 46 children with severe atopic dermatitis, psoriasis, or atopic dermatitis–psoriasis overlap, who were treated with a starting dose of oral methotrexate 0.5 mg/kg per week (maximum, 15 mg) and assessed by MTX PG3 assay at week 12. In a subset of children who failed to respond, the dose was modified and the level rechecked at 8 weeks. Disease severity was rated using a 5-point physician global assessment scale, with a good to excellent response defined by a 2-point improvement.
In all, 83% of children achieved a good to excellent response, with 27 patients doing so within 12 weeks and 11 responding after dose adjustment involving either a higher oral dose or switching to subcutaneous administration, Mr. Rahman reported.
Response rates to methotrexate were slightly higher in children with psoriasis or overlap (the two groups were combined) than those with atopic dermatitis (94% vs. 77%; no P value given). Overall, 8 children failed to respond.
The mean MTX PG3 level significantly correlated with efficacy in patients with atopic dermatitis (responders, 34.8 nmol/L, vs. 17.8 nmol/L, nonresponders; P = .018), but not in those with psoriasis–atopic dermatitis overlap (responders, 26.5 nmol/L, vs. 20.7, nonresponders; P = .724), Mr. Rahman reported.
The data suggest that compared with the therapeutic level of 60 nmol/L identified in adults, "pediatric patients have a lower therapeutic value," he said.
A subset analysis of MTX PG3 levels based on response identified a significantly higher mean maximum MTX PG3 level for responders than nonresponders (31.5 vs. 18.1 nmol/L; P = .035).
More importantly, late responders had the highest methotrexate levels. All late responders achieved levels greater than 30 nmol/L, and mean levels were significantly higher than for early responders (41.9 vs. 27.3 nmol/L; P = .024), suggesting that the test is most valuable for the subset of children requiring dose adjustment, Mr. Rahman said.
"Patients who respond won’t even need the test, but for those at the 12-week juncture who do not respond and their levels are less than 30 nmol/L, this [study] suggests that if we increase the PO [dose] or change to subcutaneous [administration], we will eventually have a response," he said.
During a discussion of the results, an audience member questioned whether the test, with its added expense, really enhances clinical judgment because most clinicians would already adjust the dose in their patients failing to respond at 12 weeks.
Mr. Rahman replied that not all patients will respond to continued treatment with methotrexate, and that the test could differentiate between responders and nonresponders, thereby directing the patient to alternative therapy earlier.
Session moderator Dr. Amy Paller, chair of dermatology and professor of pediatrics and dermatology at Northwestern University in Chicago, agreed that the test is most valuable for the subset of children who are nonresponders at 12 weeks.
"In these children, this test can guide dose modification," she said in an interview. "Pharmacogenetic variation can require relatively higher oral doses or subcutaneous administration."
In contrast to a therapeutic level of more than 60 nmol/L established for adults, a level of 30 nmol/L may be sufficient in children, Dr. Paller said.
"A MTX PG3 less than 30 nmol/L in a child who has failed to respond within 12 weeks suggests that an increase in oral dose, or even a change to subcutaneous [administration], will boost efficacy," she commented.
Mr. Rahman, Dr. Siegfried, and Dr. Paller reported having no relevant financial disclosures.
MILWAUKEE – In children with severe atopic dermatitis or psoriasis, the methotrexate polyglutamate assay appears useful in guiding dose modification in those failing to respond to methotrexate at 12 weeks, a retrospective study has shown.
The methotrexate polyglutamate 3 assay (MTX PG3) measures concentrations of methotrexate polyglutamates, the active metabolites of methotrexate, Syed Rahman said at the annual meeting of the Society for Pediatric Dermatology.
Methotrexate, a prodrug, requires enzymatic conversion via sequential addition of glutamic acid residues. Those residues form a glutamate "tail" that enhances intracellular methotrexate retention. The 3-tail metabolite, MTX PG3, is the predominant species in most patients, explained Mr. Rahman of St. Louis University.
"This study supports the usefulness of a commercially available tool for customizing optimal methotrexate dosing, coauthor Dr. Elaine Siegfried, professor of pediatrics and dermatology at the university, said in an interview.
The MTX PG3 assay has been validated in adults with rheumatoid arthritis where patients with a therapeutic methotrexate polyglutamate level of more than 60 nmol/L were fivefold more likely to have a good response to methotrexate than those with levels below this threshold, according to poster results presented at the 2008 American College of Rheumatology annual meeting (cited on the company website). However, the test was not predictive of response in 36% of patients whose arthritis improved with treatment despite subtherapeutic levels.
For the current analysis, the investigators reviewed the charts of 46 children with severe atopic dermatitis, psoriasis, or atopic dermatitis–psoriasis overlap, who were treated with a starting dose of oral methotrexate 0.5 mg/kg per week (maximum, 15 mg) and assessed by MTX PG3 assay at week 12. In a subset of children who failed to respond, the dose was modified and the level rechecked at 8 weeks. Disease severity was rated using a 5-point physician global assessment scale, with a good to excellent response defined by a 2-point improvement.
In all, 83% of children achieved a good to excellent response, with 27 patients doing so within 12 weeks and 11 responding after dose adjustment involving either a higher oral dose or switching to subcutaneous administration, Mr. Rahman reported.
Response rates to methotrexate were slightly higher in children with psoriasis or overlap (the two groups were combined) than those with atopic dermatitis (94% vs. 77%; no P value given). Overall, 8 children failed to respond.
The mean MTX PG3 level significantly correlated with efficacy in patients with atopic dermatitis (responders, 34.8 nmol/L, vs. 17.8 nmol/L, nonresponders; P = .018), but not in those with psoriasis–atopic dermatitis overlap (responders, 26.5 nmol/L, vs. 20.7, nonresponders; P = .724), Mr. Rahman reported.
The data suggest that compared with the therapeutic level of 60 nmol/L identified in adults, "pediatric patients have a lower therapeutic value," he said.
A subset analysis of MTX PG3 levels based on response identified a significantly higher mean maximum MTX PG3 level for responders than nonresponders (31.5 vs. 18.1 nmol/L; P = .035).
More importantly, late responders had the highest methotrexate levels. All late responders achieved levels greater than 30 nmol/L, and mean levels were significantly higher than for early responders (41.9 vs. 27.3 nmol/L; P = .024), suggesting that the test is most valuable for the subset of children requiring dose adjustment, Mr. Rahman said.
"Patients who respond won’t even need the test, but for those at the 12-week juncture who do not respond and their levels are less than 30 nmol/L, this [study] suggests that if we increase the PO [dose] or change to subcutaneous [administration], we will eventually have a response," he said.
During a discussion of the results, an audience member questioned whether the test, with its added expense, really enhances clinical judgment because most clinicians would already adjust the dose in their patients failing to respond at 12 weeks.
Mr. Rahman replied that not all patients will respond to continued treatment with methotrexate, and that the test could differentiate between responders and nonresponders, thereby directing the patient to alternative therapy earlier.
Session moderator Dr. Amy Paller, chair of dermatology and professor of pediatrics and dermatology at Northwestern University in Chicago, agreed that the test is most valuable for the subset of children who are nonresponders at 12 weeks.
"In these children, this test can guide dose modification," she said in an interview. "Pharmacogenetic variation can require relatively higher oral doses or subcutaneous administration."
In contrast to a therapeutic level of more than 60 nmol/L established for adults, a level of 30 nmol/L may be sufficient in children, Dr. Paller said.
"A MTX PG3 less than 30 nmol/L in a child who has failed to respond within 12 weeks suggests that an increase in oral dose, or even a change to subcutaneous [administration], will boost efficacy," she commented.
Mr. Rahman, Dr. Siegfried, and Dr. Paller reported having no relevant financial disclosures.
MILWAUKEE – In children with severe atopic dermatitis or psoriasis, the methotrexate polyglutamate assay appears useful in guiding dose modification in those failing to respond to methotrexate at 12 weeks, a retrospective study has shown.
The methotrexate polyglutamate 3 assay (MTX PG3) measures concentrations of methotrexate polyglutamates, the active metabolites of methotrexate, Syed Rahman said at the annual meeting of the Society for Pediatric Dermatology.
Methotrexate, a prodrug, requires enzymatic conversion via sequential addition of glutamic acid residues. Those residues form a glutamate "tail" that enhances intracellular methotrexate retention. The 3-tail metabolite, MTX PG3, is the predominant species in most patients, explained Mr. Rahman of St. Louis University.
"This study supports the usefulness of a commercially available tool for customizing optimal methotrexate dosing, coauthor Dr. Elaine Siegfried, professor of pediatrics and dermatology at the university, said in an interview.
The MTX PG3 assay has been validated in adults with rheumatoid arthritis where patients with a therapeutic methotrexate polyglutamate level of more than 60 nmol/L were fivefold more likely to have a good response to methotrexate than those with levels below this threshold, according to poster results presented at the 2008 American College of Rheumatology annual meeting (cited on the company website). However, the test was not predictive of response in 36% of patients whose arthritis improved with treatment despite subtherapeutic levels.
For the current analysis, the investigators reviewed the charts of 46 children with severe atopic dermatitis, psoriasis, or atopic dermatitis–psoriasis overlap, who were treated with a starting dose of oral methotrexate 0.5 mg/kg per week (maximum, 15 mg) and assessed by MTX PG3 assay at week 12. In a subset of children who failed to respond, the dose was modified and the level rechecked at 8 weeks. Disease severity was rated using a 5-point physician global assessment scale, with a good to excellent response defined by a 2-point improvement.
In all, 83% of children achieved a good to excellent response, with 27 patients doing so within 12 weeks and 11 responding after dose adjustment involving either a higher oral dose or switching to subcutaneous administration, Mr. Rahman reported.
Response rates to methotrexate were slightly higher in children with psoriasis or overlap (the two groups were combined) than those with atopic dermatitis (94% vs. 77%; no P value given). Overall, 8 children failed to respond.
The mean MTX PG3 level significantly correlated with efficacy in patients with atopic dermatitis (responders, 34.8 nmol/L, vs. 17.8 nmol/L, nonresponders; P = .018), but not in those with psoriasis–atopic dermatitis overlap (responders, 26.5 nmol/L, vs. 20.7, nonresponders; P = .724), Mr. Rahman reported.
The data suggest that compared with the therapeutic level of 60 nmol/L identified in adults, "pediatric patients have a lower therapeutic value," he said.
A subset analysis of MTX PG3 levels based on response identified a significantly higher mean maximum MTX PG3 level for responders than nonresponders (31.5 vs. 18.1 nmol/L; P = .035).
More importantly, late responders had the highest methotrexate levels. All late responders achieved levels greater than 30 nmol/L, and mean levels were significantly higher than for early responders (41.9 vs. 27.3 nmol/L; P = .024), suggesting that the test is most valuable for the subset of children requiring dose adjustment, Mr. Rahman said.
"Patients who respond won’t even need the test, but for those at the 12-week juncture who do not respond and their levels are less than 30 nmol/L, this [study] suggests that if we increase the PO [dose] or change to subcutaneous [administration], we will eventually have a response," he said.
During a discussion of the results, an audience member questioned whether the test, with its added expense, really enhances clinical judgment because most clinicians would already adjust the dose in their patients failing to respond at 12 weeks.
Mr. Rahman replied that not all patients will respond to continued treatment with methotrexate, and that the test could differentiate between responders and nonresponders, thereby directing the patient to alternative therapy earlier.
Session moderator Dr. Amy Paller, chair of dermatology and professor of pediatrics and dermatology at Northwestern University in Chicago, agreed that the test is most valuable for the subset of children who are nonresponders at 12 weeks.
"In these children, this test can guide dose modification," she said in an interview. "Pharmacogenetic variation can require relatively higher oral doses or subcutaneous administration."
In contrast to a therapeutic level of more than 60 nmol/L established for adults, a level of 30 nmol/L may be sufficient in children, Dr. Paller said.
"A MTX PG3 less than 30 nmol/L in a child who has failed to respond within 12 weeks suggests that an increase in oral dose, or even a change to subcutaneous [administration], will boost efficacy," she commented.
Mr. Rahman, Dr. Siegfried, and Dr. Paller reported having no relevant financial disclosures.
AT THE ANNUAL MEETING OF THE SOCIETY FOR PEDIATRIC DERMATOLOGY
Major finding: Late responders had the highest methotrexate levels (41.9 nmol/L vs. 27.3 nmol/L in early responders; P = .024) on the MTX PG3 assay.
Data source: A retrospective study of 46 children on methotrexate for atopic dermatitis, psoriasis, or atopic dermatitis–psoriasis overlap who were assessed by MTX PG3 assay.
Disclosures: Mr. Rahman, Dr. Siegfried, and Dr. Paller reported having no relevant financial disclosures.
Modern Moisturizer Myths, Misconceptions, and Truths
Reduced Burning and Stinging Associated With Topical Application of Lactic Acid 10% With Strontium Versus Ammonium Lactate 12%
Dupilumab cuts moderate, severe asthma exacerbations
Dupilumab reduced exacerbations of moderate to severe asthma by 87% in adults with poorly controlled disease, and induced rapid and sustained improvements in numerous other measures of asthma severity in an industry-sponsored phase II study published online May 21 in the New England Journal of Medicine.
Dupilumab, a monoclonal antibody that inhibits interleukin-4 and interleukin-13 signaling, "showed substantial efficacy with regard to both objective and patient-reported end points" when it was used concomitantly with inhaled glucocorticoids and long-acting beta-agonists (LABAs), as well as when those background therapies were withdrawn, reported Dr. Sally Wenzel of the University of Pittsburgh and her associates.
"The magnitude and breadth of efficacy that we observed exceed those in other studies of Th2 [type 2 helper T-cell] cytokine inhibition," the researchers noted in the online report, which was presented simultaneously at the annual meeting of the American Thoracic Society.
Dupilumab currently is being assessed for the treatment of several diseases mediated by Th2 pathways. The goal of this phase II trial was to evaluate its safety and efficacy in adults with persistent moderate to severe asthma and elevated eosinophil levels whose symptoms were not well controlled with medium- to high-dose inhaled glucocorticoids plus LABAs (usually fluticasone and salmeterol).
The 104 participants were treated at 28 sites across the United States for 12 weeks, and then followed for another 8 weeks. During the intervention phase of the study, approximately half were randomized to receive once-weekly subcutaneous injections of dupilumab (300 mg) and half to receive matching placebo injections, in addition to the background asthma medications.
At week 4, the study subjects discontinued LABAs, and at weeks 6-9 they tapered off inhaled glucocorticoids. "This approach enabled us to observe the effects of dupilumab when added to background therapy, after LABA discontinuation, during the tapering of inhaled glucocorticoids, and as monotherapy," the researchers said.
The primary endpoint of the study was an asthma exacerbation during the 12-week intervention period. Exacerbations occurred in 3 patients receiving dupilumab (6%), compared with 23 receiving placebo (44%), a highly significant difference.
In addition, the time to an asthma exacerbation was significantly longer with dupilumab than placebo. And forced expiratory volume in 1 second (FEV1) "improved by more than 200 mL when dupilumab, as compared with placebo, was added to inhaled glucocorticoids and LABAs, an increase sustained during their tapering and discontinuation," the researchers noted (N. Engl. J. Med. 2013 May 21 [doi: 10.1056/NEJMoa1304048]).
Several other secondary endpoints also favored dupilumab over placebo, including morning peak expiratory flow values, scores on the Asthma Control Questionnaire (ACQ5), morning and evening asthma symptom scores, and the number of albuterol or levalbuterol inhalations needed per day. These measures improved at the beginning of the intervention in both study groups, then quickly returned to baseline levels in the placebo group while remaining constant in the dupilumab group.
The percentage of patients reporting adverse events was similar between the dupilumab and placebo groups (81% vs. 77%). These events tended to be nonspecific and mild, and included nasopharyngitis, nausea, and headache. One patient developed a progressive papular rash, urticaria, and edema after his ninth injection of dupilumab, which responded to nonurgent treatment of the symptoms and did not recur once the drug was withdrawn.
No serious adverse events were attributed to the study drug, and no patient showed clinically significant changes in vital signs, findings on physical examination, laboratory tests, or ECGs.
"Further studies are needed to confirm these observations and better define the target population, dosing regimen, and long-term efficacy and safety," Dr. Wenzel noted.
However, the study findings support the theory that the Th2 cytokines interleukin-4 and interleukin-13 play a pathogenic role in persistent, moderate-to-severe asthma, she added.
This study was funded by Sanofi and Regeneron Pharmaceuticals. Dr. Wenzel reported ties to Sanofi, Regeneron, Amgen, Merck, and other companies. Her associates reported ties to numerous industry sources.
Dupilumab reduced exacerbations of moderate to severe asthma by 87% in adults with poorly controlled disease, and induced rapid and sustained improvements in numerous other measures of asthma severity in an industry-sponsored phase II study published online May 21 in the New England Journal of Medicine.
Dupilumab, a monoclonal antibody that inhibits interleukin-4 and interleukin-13 signaling, "showed substantial efficacy with regard to both objective and patient-reported end points" when it was used concomitantly with inhaled glucocorticoids and long-acting beta-agonists (LABAs), as well as when those background therapies were withdrawn, reported Dr. Sally Wenzel of the University of Pittsburgh and her associates.
"The magnitude and breadth of efficacy that we observed exceed those in other studies of Th2 [type 2 helper T-cell] cytokine inhibition," the researchers noted in the online report, which was presented simultaneously at the annual meeting of the American Thoracic Society.
Dupilumab currently is being assessed for the treatment of several diseases mediated by Th2 pathways. The goal of this phase II trial was to evaluate its safety and efficacy in adults with persistent moderate to severe asthma and elevated eosinophil levels whose symptoms were not well controlled with medium- to high-dose inhaled glucocorticoids plus LABAs (usually fluticasone and salmeterol).
The 104 participants were treated at 28 sites across the United States for 12 weeks, and then followed for another 8 weeks. During the intervention phase of the study, approximately half were randomized to receive once-weekly subcutaneous injections of dupilumab (300 mg) and half to receive matching placebo injections, in addition to the background asthma medications.
At week 4, the study subjects discontinued LABAs, and at weeks 6-9 they tapered off inhaled glucocorticoids. "This approach enabled us to observe the effects of dupilumab when added to background therapy, after LABA discontinuation, during the tapering of inhaled glucocorticoids, and as monotherapy," the researchers said.
The primary endpoint of the study was an asthma exacerbation during the 12-week intervention period. Exacerbations occurred in 3 patients receiving dupilumab (6%), compared with 23 receiving placebo (44%), a highly significant difference.
In addition, the time to an asthma exacerbation was significantly longer with dupilumab than placebo. And forced expiratory volume in 1 second (FEV1) "improved by more than 200 mL when dupilumab, as compared with placebo, was added to inhaled glucocorticoids and LABAs, an increase sustained during their tapering and discontinuation," the researchers noted (N. Engl. J. Med. 2013 May 21 [doi: 10.1056/NEJMoa1304048]).
Several other secondary endpoints also favored dupilumab over placebo, including morning peak expiratory flow values, scores on the Asthma Control Questionnaire (ACQ5), morning and evening asthma symptom scores, and the number of albuterol or levalbuterol inhalations needed per day. These measures improved at the beginning of the intervention in both study groups, then quickly returned to baseline levels in the placebo group while remaining constant in the dupilumab group.
The percentage of patients reporting adverse events was similar between the dupilumab and placebo groups (81% vs. 77%). These events tended to be nonspecific and mild, and included nasopharyngitis, nausea, and headache. One patient developed a progressive papular rash, urticaria, and edema after his ninth injection of dupilumab, which responded to nonurgent treatment of the symptoms and did not recur once the drug was withdrawn.
No serious adverse events were attributed to the study drug, and no patient showed clinically significant changes in vital signs, findings on physical examination, laboratory tests, or ECGs.
"Further studies are needed to confirm these observations and better define the target population, dosing regimen, and long-term efficacy and safety," Dr. Wenzel noted.
However, the study findings support the theory that the Th2 cytokines interleukin-4 and interleukin-13 play a pathogenic role in persistent, moderate-to-severe asthma, she added.
This study was funded by Sanofi and Regeneron Pharmaceuticals. Dr. Wenzel reported ties to Sanofi, Regeneron, Amgen, Merck, and other companies. Her associates reported ties to numerous industry sources.
Dupilumab reduced exacerbations of moderate to severe asthma by 87% in adults with poorly controlled disease, and induced rapid and sustained improvements in numerous other measures of asthma severity in an industry-sponsored phase II study published online May 21 in the New England Journal of Medicine.
Dupilumab, a monoclonal antibody that inhibits interleukin-4 and interleukin-13 signaling, "showed substantial efficacy with regard to both objective and patient-reported end points" when it was used concomitantly with inhaled glucocorticoids and long-acting beta-agonists (LABAs), as well as when those background therapies were withdrawn, reported Dr. Sally Wenzel of the University of Pittsburgh and her associates.
"The magnitude and breadth of efficacy that we observed exceed those in other studies of Th2 [type 2 helper T-cell] cytokine inhibition," the researchers noted in the online report, which was presented simultaneously at the annual meeting of the American Thoracic Society.
Dupilumab currently is being assessed for the treatment of several diseases mediated by Th2 pathways. The goal of this phase II trial was to evaluate its safety and efficacy in adults with persistent moderate to severe asthma and elevated eosinophil levels whose symptoms were not well controlled with medium- to high-dose inhaled glucocorticoids plus LABAs (usually fluticasone and salmeterol).
The 104 participants were treated at 28 sites across the United States for 12 weeks, and then followed for another 8 weeks. During the intervention phase of the study, approximately half were randomized to receive once-weekly subcutaneous injections of dupilumab (300 mg) and half to receive matching placebo injections, in addition to the background asthma medications.
At week 4, the study subjects discontinued LABAs, and at weeks 6-9 they tapered off inhaled glucocorticoids. "This approach enabled us to observe the effects of dupilumab when added to background therapy, after LABA discontinuation, during the tapering of inhaled glucocorticoids, and as monotherapy," the researchers said.
The primary endpoint of the study was an asthma exacerbation during the 12-week intervention period. Exacerbations occurred in 3 patients receiving dupilumab (6%), compared with 23 receiving placebo (44%), a highly significant difference.
In addition, the time to an asthma exacerbation was significantly longer with dupilumab than placebo. And forced expiratory volume in 1 second (FEV1) "improved by more than 200 mL when dupilumab, as compared with placebo, was added to inhaled glucocorticoids and LABAs, an increase sustained during their tapering and discontinuation," the researchers noted (N. Engl. J. Med. 2013 May 21 [doi: 10.1056/NEJMoa1304048]).
Several other secondary endpoints also favored dupilumab over placebo, including morning peak expiratory flow values, scores on the Asthma Control Questionnaire (ACQ5), morning and evening asthma symptom scores, and the number of albuterol or levalbuterol inhalations needed per day. These measures improved at the beginning of the intervention in both study groups, then quickly returned to baseline levels in the placebo group while remaining constant in the dupilumab group.
The percentage of patients reporting adverse events was similar between the dupilumab and placebo groups (81% vs. 77%). These events tended to be nonspecific and mild, and included nasopharyngitis, nausea, and headache. One patient developed a progressive papular rash, urticaria, and edema after his ninth injection of dupilumab, which responded to nonurgent treatment of the symptoms and did not recur once the drug was withdrawn.
No serious adverse events were attributed to the study drug, and no patient showed clinically significant changes in vital signs, findings on physical examination, laboratory tests, or ECGs.
"Further studies are needed to confirm these observations and better define the target population, dosing regimen, and long-term efficacy and safety," Dr. Wenzel noted.
However, the study findings support the theory that the Th2 cytokines interleukin-4 and interleukin-13 play a pathogenic role in persistent, moderate-to-severe asthma, she added.
This study was funded by Sanofi and Regeneron Pharmaceuticals. Dr. Wenzel reported ties to Sanofi, Regeneron, Amgen, Merck, and other companies. Her associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Major Finding: Dupilumab was associated with an 87% reduction in asthma exacerbation; three patients receiving dupilumab (6%) had an exacerbation during the study, compared with 23 receiving placebo (44%).
Data Source: A randomized, double-blind, phase II clinical trial including 104 adults with persistent moderate to severe asthma.
Disclosures: This study was funded by Sanofi and Regeneron Pharmaceuticals. Dr. Wenzel reported ties to Sanofi, Regeneron, Amgen, Merck, and other companies. Her associates reported ties to numerous industry sources.
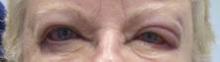
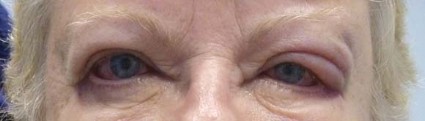
Eczema of the eyelids? Think chemical allergy
LAS VEGAS – If a patient presents with eczema of the eyelids, or swollen eyelids that don’t respond to topical steroids, think about sending them for chemical testing, advised Dr. Janet M. Neigel.
"The eyelids are red and scaly, a little swollen, and it just never goes away," she said in an interview at the annual meeting of the American Academy of Cosmetic Surgery.
Dr. Neigel, a cosmetic surgeon in West Orange, N.J., said that over the past 5 years, she has seen increasing numbers of patients present with eczema localized to the eyelids or eyeball area that recurs like pesky crabgrass.
"I treat them with topical steroids," Dr. Neigel said. "It will get better, but it always comes back. Some of this is seasonal. It may only happen in the winter, when the air is drier and their skin tends to get drier. In others it can be all year long," she said. "It seems to be more common in women, but I see men with this condition, too. In men, it tends to present as a reddish eye and tearing," she noted.
In the majority of cases, the culprit turns out to be an allergy to chemicals including gold, nickel, tin, rubber, preservatives in shampoos and laundry detergent, and formaldehyde resin, which is used in nail polish. "There was one patient who was allergic to the preservative in eyedrops," Dr. Neigel recalled. "She was on several different eyedrops trying to treat the swollen eye area, and it was just making the condition worse."
Another patient’s eczema cleared only after she removed her wedding ring, Dr. Neigel said. "So she couldn’t wear any gold jewelry. In somebody else it was tin and nickel, so she couldn’t wear any cheap jewelry."
Ointments commonly used for cosmetic procedures also can cause trouble. "There is cross-reactivity between neomycin, tobramycin, and Neosporin," Dr. Neigel said. "One patient was applying Neosporin every time she bumped herself on different parts of her body, and her eyelids were the only things flaring up."
Dr. Neigel speculated that the reaction in such cases is localized to the eyelid because "it’s the thinnest skin in the body. It’s the most sensitive, and for some reason, the patients I’m seeing only have reactions there," she noted. So, for patients with allergic conjunctivitis or tearing for a contact dermatitis–type presentation around the eyeball or the eyelids, send them for chemical testing, she advised. "There’s a good chance you might clear things up and figure out what they’re truly reacting to – get to the source instead of just treating the problem symptomatically," she said.
Dr. Neigel said she had no relevant financial disclosures.
LAS VEGAS – If a patient presents with eczema of the eyelids, or swollen eyelids that don’t respond to topical steroids, think about sending them for chemical testing, advised Dr. Janet M. Neigel.
"The eyelids are red and scaly, a little swollen, and it just never goes away," she said in an interview at the annual meeting of the American Academy of Cosmetic Surgery.
Dr. Neigel, a cosmetic surgeon in West Orange, N.J., said that over the past 5 years, she has seen increasing numbers of patients present with eczema localized to the eyelids or eyeball area that recurs like pesky crabgrass.
"I treat them with topical steroids," Dr. Neigel said. "It will get better, but it always comes back. Some of this is seasonal. It may only happen in the winter, when the air is drier and their skin tends to get drier. In others it can be all year long," she said. "It seems to be more common in women, but I see men with this condition, too. In men, it tends to present as a reddish eye and tearing," she noted.
In the majority of cases, the culprit turns out to be an allergy to chemicals including gold, nickel, tin, rubber, preservatives in shampoos and laundry detergent, and formaldehyde resin, which is used in nail polish. "There was one patient who was allergic to the preservative in eyedrops," Dr. Neigel recalled. "She was on several different eyedrops trying to treat the swollen eye area, and it was just making the condition worse."
Another patient’s eczema cleared only after she removed her wedding ring, Dr. Neigel said. "So she couldn’t wear any gold jewelry. In somebody else it was tin and nickel, so she couldn’t wear any cheap jewelry."
Ointments commonly used for cosmetic procedures also can cause trouble. "There is cross-reactivity between neomycin, tobramycin, and Neosporin," Dr. Neigel said. "One patient was applying Neosporin every time she bumped herself on different parts of her body, and her eyelids were the only things flaring up."
Dr. Neigel speculated that the reaction in such cases is localized to the eyelid because "it’s the thinnest skin in the body. It’s the most sensitive, and for some reason, the patients I’m seeing only have reactions there," she noted. So, for patients with allergic conjunctivitis or tearing for a contact dermatitis–type presentation around the eyeball or the eyelids, send them for chemical testing, she advised. "There’s a good chance you might clear things up and figure out what they’re truly reacting to – get to the source instead of just treating the problem symptomatically," she said.
Dr. Neigel said she had no relevant financial disclosures.
LAS VEGAS – If a patient presents with eczema of the eyelids, or swollen eyelids that don’t respond to topical steroids, think about sending them for chemical testing, advised Dr. Janet M. Neigel.
"The eyelids are red and scaly, a little swollen, and it just never goes away," she said in an interview at the annual meeting of the American Academy of Cosmetic Surgery.
Dr. Neigel, a cosmetic surgeon in West Orange, N.J., said that over the past 5 years, she has seen increasing numbers of patients present with eczema localized to the eyelids or eyeball area that recurs like pesky crabgrass.
"I treat them with topical steroids," Dr. Neigel said. "It will get better, but it always comes back. Some of this is seasonal. It may only happen in the winter, when the air is drier and their skin tends to get drier. In others it can be all year long," she said. "It seems to be more common in women, but I see men with this condition, too. In men, it tends to present as a reddish eye and tearing," she noted.
In the majority of cases, the culprit turns out to be an allergy to chemicals including gold, nickel, tin, rubber, preservatives in shampoos and laundry detergent, and formaldehyde resin, which is used in nail polish. "There was one patient who was allergic to the preservative in eyedrops," Dr. Neigel recalled. "She was on several different eyedrops trying to treat the swollen eye area, and it was just making the condition worse."
Another patient’s eczema cleared only after she removed her wedding ring, Dr. Neigel said. "So she couldn’t wear any gold jewelry. In somebody else it was tin and nickel, so she couldn’t wear any cheap jewelry."
Ointments commonly used for cosmetic procedures also can cause trouble. "There is cross-reactivity between neomycin, tobramycin, and Neosporin," Dr. Neigel said. "One patient was applying Neosporin every time she bumped herself on different parts of her body, and her eyelids were the only things flaring up."
Dr. Neigel speculated that the reaction in such cases is localized to the eyelid because "it’s the thinnest skin in the body. It’s the most sensitive, and for some reason, the patients I’m seeing only have reactions there," she noted. So, for patients with allergic conjunctivitis or tearing for a contact dermatitis–type presentation around the eyeball or the eyelids, send them for chemical testing, she advised. "There’s a good chance you might clear things up and figure out what they’re truly reacting to – get to the source instead of just treating the problem symptomatically," she said.
Dr. Neigel said she had no relevant financial disclosures.
AT THE AACS ANNUAL MEETING
Nickel, cobalt sensitivity increases with number of body piercings
MIAMI BEACH – The risk of nickel and cobalt sensitivity increases in tandem with the number of body piercings, according to findings from a study involving nearly 9,400 patch-tested patients.
Overall, 3,907 (41.6%) of the 9,388 subjects had no piercings, 131 (1.3%) had one piercing, 3,987 (42.5%) had two piercings, 934 (9.9%) had three to four piercings, and 429 (4.6%) had five or more piercings, Jaime L. Loso reported at the annual meeting of the American Contact Dermatitis Society.
Nickel and cobalt sensitivity both were significantly associated with piercing (relative risks of 2.52 and 1.63, respectively); chromate sensitivity had an inverse relationship with piercing (relative risk, 0.60), said Ms. Loso, a third-year medical student at the University of Minnesota, Minneapolis.
Overall, 1,651 patients (17.7%) had nickel sensitivity and 685 (7.3%) had cobalt sensitivity. The sensitivity rates increased with the number of piercings. The rates of sensitivity among those with zero, one, two, three to four, and five or more piercings were 9.4%, 16%, 22.6%, 25.1%, and 32.4%, respectively, for nickel and 5.3%, 7.6%, 8.2%, 9.5%, and 11.7% for cobalt.
Chromate sensitivity was less common, occurring in only 306 patients (3.26%). Less than 4.3% of patients in all piercing groups had chromate sensitivity, Ms. Loso noted.
"Nickel is the most common allergen for patch-tested patients, and body piercing has been directly correlated with the development of nickel allergy," she said, adding that cobalt content is often associated with nickel content in consumer products, although recent studies suggest that only a small amount of jewelry – mainly earrings – releases cobalt.
In the current study, which involved patients identified using North American Contact Dermatitis Group data from 2007 to 2010, younger patients were affected more often than older patients, females were affected more often than males, and – surprisingly – piercing was associated with allergy in males more often than in females, she reported.
The findings, though limited by the fact that the referral population may not be representative of the general population and by a lack of information regarding age at the time of piercing and the body sites pierced, help characterize metal allergy associated with body piercing, Ms. Loso said.
She noted that nickel allergy has been well studied in Europe – resulting in regulation of the nickel content in consumer goods and a subsequent significant decline in nickel sensitivity among young females there. The same is not true for the United States, where nickel sensitivity remains an important problem.
Ms. Loso reported having no disclosures.
MIAMI BEACH – The risk of nickel and cobalt sensitivity increases in tandem with the number of body piercings, according to findings from a study involving nearly 9,400 patch-tested patients.
Overall, 3,907 (41.6%) of the 9,388 subjects had no piercings, 131 (1.3%) had one piercing, 3,987 (42.5%) had two piercings, 934 (9.9%) had three to four piercings, and 429 (4.6%) had five or more piercings, Jaime L. Loso reported at the annual meeting of the American Contact Dermatitis Society.
Nickel and cobalt sensitivity both were significantly associated with piercing (relative risks of 2.52 and 1.63, respectively); chromate sensitivity had an inverse relationship with piercing (relative risk, 0.60), said Ms. Loso, a third-year medical student at the University of Minnesota, Minneapolis.
Overall, 1,651 patients (17.7%) had nickel sensitivity and 685 (7.3%) had cobalt sensitivity. The sensitivity rates increased with the number of piercings. The rates of sensitivity among those with zero, one, two, three to four, and five or more piercings were 9.4%, 16%, 22.6%, 25.1%, and 32.4%, respectively, for nickel and 5.3%, 7.6%, 8.2%, 9.5%, and 11.7% for cobalt.
Chromate sensitivity was less common, occurring in only 306 patients (3.26%). Less than 4.3% of patients in all piercing groups had chromate sensitivity, Ms. Loso noted.
"Nickel is the most common allergen for patch-tested patients, and body piercing has been directly correlated with the development of nickel allergy," she said, adding that cobalt content is often associated with nickel content in consumer products, although recent studies suggest that only a small amount of jewelry – mainly earrings – releases cobalt.
In the current study, which involved patients identified using North American Contact Dermatitis Group data from 2007 to 2010, younger patients were affected more often than older patients, females were affected more often than males, and – surprisingly – piercing was associated with allergy in males more often than in females, she reported.
The findings, though limited by the fact that the referral population may not be representative of the general population and by a lack of information regarding age at the time of piercing and the body sites pierced, help characterize metal allergy associated with body piercing, Ms. Loso said.
She noted that nickel allergy has been well studied in Europe – resulting in regulation of the nickel content in consumer goods and a subsequent significant decline in nickel sensitivity among young females there. The same is not true for the United States, where nickel sensitivity remains an important problem.
Ms. Loso reported having no disclosures.
MIAMI BEACH – The risk of nickel and cobalt sensitivity increases in tandem with the number of body piercings, according to findings from a study involving nearly 9,400 patch-tested patients.
Overall, 3,907 (41.6%) of the 9,388 subjects had no piercings, 131 (1.3%) had one piercing, 3,987 (42.5%) had two piercings, 934 (9.9%) had three to four piercings, and 429 (4.6%) had five or more piercings, Jaime L. Loso reported at the annual meeting of the American Contact Dermatitis Society.
Nickel and cobalt sensitivity both were significantly associated with piercing (relative risks of 2.52 and 1.63, respectively); chromate sensitivity had an inverse relationship with piercing (relative risk, 0.60), said Ms. Loso, a third-year medical student at the University of Minnesota, Minneapolis.
Overall, 1,651 patients (17.7%) had nickel sensitivity and 685 (7.3%) had cobalt sensitivity. The sensitivity rates increased with the number of piercings. The rates of sensitivity among those with zero, one, two, three to four, and five or more piercings were 9.4%, 16%, 22.6%, 25.1%, and 32.4%, respectively, for nickel and 5.3%, 7.6%, 8.2%, 9.5%, and 11.7% for cobalt.
Chromate sensitivity was less common, occurring in only 306 patients (3.26%). Less than 4.3% of patients in all piercing groups had chromate sensitivity, Ms. Loso noted.
"Nickel is the most common allergen for patch-tested patients, and body piercing has been directly correlated with the development of nickel allergy," she said, adding that cobalt content is often associated with nickel content in consumer products, although recent studies suggest that only a small amount of jewelry – mainly earrings – releases cobalt.
In the current study, which involved patients identified using North American Contact Dermatitis Group data from 2007 to 2010, younger patients were affected more often than older patients, females were affected more often than males, and – surprisingly – piercing was associated with allergy in males more often than in females, she reported.
The findings, though limited by the fact that the referral population may not be representative of the general population and by a lack of information regarding age at the time of piercing and the body sites pierced, help characterize metal allergy associated with body piercing, Ms. Loso said.
She noted that nickel allergy has been well studied in Europe – resulting in regulation of the nickel content in consumer goods and a subsequent significant decline in nickel sensitivity among young females there. The same is not true for the United States, where nickel sensitivity remains an important problem.
Ms. Loso reported having no disclosures.
AT THE ACDS ANNUAL MEETING
Major finding: Sensitivity to nickel and cobalt (relative risk of 2.52, 1.63, respectively) was significantly associated with body piercing.
Data source: A retrospective cross-sectional analysis.
Disclosures: Dr. Loso reported having no disclosures.
Cat allergy vaccine effects persist at 2 years
A short course of treatment with an investigational synthetic cat-peptide antigen desensitizing vaccine, or Cat-PAD, results in a substantial and persistent reduction in cat allergy symptom scores, according to two-year findings from a randomized, placebo-controlled phase II clinical study involving 202 adult patients.
Study participants were initially randomized to receive either eight 3-nmol intradermal doses at 2-week intervals, four 6 nmol-doses at 4-week intervals, or placebo. At 1-year follow-up, the improvement in Total Rhinoconjunctivitis Symptom Score (TRSS) was significantly greater in the patients who received four doses of Cat-PAD (ToleroMune Cat, Circassia Limited, Oxford, England), compared with those who received placebo (–7.1 points vs. –2.99 points), according to findings published online in the Journal of Allergy and Clinical Immunology (doi:10.1016/j.jaci.2012.12.1185).
Data from a 2-year follow-up study were reported by Rod P. Hafner, Ph.D., and his colleagues in a poster at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Of 89 patients enrolled into the follow-up study, 50 returned at 2-years after the start of treatment, having received no additional treatment, for an environmental exposure chamber challenge. The magnitude of difference from baseline in TRSS seen at 1 year in those who received 4 doses and those who received placebo was maintained at 2 years (–5.87 vs. –2.02) among those who initially received the 4-dose regimen.
"Cat-PAD is the first in a new class of synthetic peptide immune-regulatory epitopes. The results from this study provide the first evidence that four doses of 6 nmol Cat-PAD over a 12-week period has a disease-modifying effect with subjects showing sustained improvement at 2 years," the investigators wrote.
Study participants were adults aged 18-65 years with cat allergy who underwent a baseline environmental exposure chamber (EEC) challenge, as well as follow-up EEC challenges at 18-22 weeks and at 100-104 weeks.
"Cat allergen was dispersed into the EEC to achieve a consistent mean level of approx. 50 ng Fel d1/m3, using a validated method," they explained, noting that TRSS was calculated at each EEC challenge based on self-scoring of four nasal symptoms (running nose, sneezing, blocked nose, itchy nose), and four ocular symptoms (itchy eyes, watery eyes, red eyes, sore eyes) on a scale of 0-3, every 30 minutes during the challenge.
Cat-PAD is a "potentially exciting new approach to cat allergy immunotherapy," the investigators said, noting that improvements in the TRSS seen in the initial phase II study and follow-up study represent a substantial improvement over numerous therapies investigated in the past, in some cases reaching a threefold improvement in symptom reduction.
"For example, studies of similar design in an EEC reported TRSS changes of approximately –1.5 units after 16 weeks treatment with a sublingual cat allergy tablet, while a single 180-mg dose of the antihistamine fexofenadine achieved a mean difference in TRSS of –1.3 in a cat allergen study. Moreover, the change in TRSS of –3.8 units reported here was observed after four administrations of Cat-PAD over a 12-week period and persisted 21 months after the end of treatment," they concluded.
In late 2012 the investigators began enrolling a phase III study, which will include individuals aged 12-65 years.
This study was funded by Circassia Limited. Dr. Hafner is employed by Circassia.
A short course of treatment with an investigational synthetic cat-peptide antigen desensitizing vaccine, or Cat-PAD, results in a substantial and persistent reduction in cat allergy symptom scores, according to two-year findings from a randomized, placebo-controlled phase II clinical study involving 202 adult patients.
Study participants were initially randomized to receive either eight 3-nmol intradermal doses at 2-week intervals, four 6 nmol-doses at 4-week intervals, or placebo. At 1-year follow-up, the improvement in Total Rhinoconjunctivitis Symptom Score (TRSS) was significantly greater in the patients who received four doses of Cat-PAD (ToleroMune Cat, Circassia Limited, Oxford, England), compared with those who received placebo (–7.1 points vs. –2.99 points), according to findings published online in the Journal of Allergy and Clinical Immunology (doi:10.1016/j.jaci.2012.12.1185).
Data from a 2-year follow-up study were reported by Rod P. Hafner, Ph.D., and his colleagues in a poster at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Of 89 patients enrolled into the follow-up study, 50 returned at 2-years after the start of treatment, having received no additional treatment, for an environmental exposure chamber challenge. The magnitude of difference from baseline in TRSS seen at 1 year in those who received 4 doses and those who received placebo was maintained at 2 years (–5.87 vs. –2.02) among those who initially received the 4-dose regimen.
"Cat-PAD is the first in a new class of synthetic peptide immune-regulatory epitopes. The results from this study provide the first evidence that four doses of 6 nmol Cat-PAD over a 12-week period has a disease-modifying effect with subjects showing sustained improvement at 2 years," the investigators wrote.
Study participants were adults aged 18-65 years with cat allergy who underwent a baseline environmental exposure chamber (EEC) challenge, as well as follow-up EEC challenges at 18-22 weeks and at 100-104 weeks.
"Cat allergen was dispersed into the EEC to achieve a consistent mean level of approx. 50 ng Fel d1/m3, using a validated method," they explained, noting that TRSS was calculated at each EEC challenge based on self-scoring of four nasal symptoms (running nose, sneezing, blocked nose, itchy nose), and four ocular symptoms (itchy eyes, watery eyes, red eyes, sore eyes) on a scale of 0-3, every 30 minutes during the challenge.
Cat-PAD is a "potentially exciting new approach to cat allergy immunotherapy," the investigators said, noting that improvements in the TRSS seen in the initial phase II study and follow-up study represent a substantial improvement over numerous therapies investigated in the past, in some cases reaching a threefold improvement in symptom reduction.
"For example, studies of similar design in an EEC reported TRSS changes of approximately –1.5 units after 16 weeks treatment with a sublingual cat allergy tablet, while a single 180-mg dose of the antihistamine fexofenadine achieved a mean difference in TRSS of –1.3 in a cat allergen study. Moreover, the change in TRSS of –3.8 units reported here was observed after four administrations of Cat-PAD over a 12-week period and persisted 21 months after the end of treatment," they concluded.
In late 2012 the investigators began enrolling a phase III study, which will include individuals aged 12-65 years.
This study was funded by Circassia Limited. Dr. Hafner is employed by Circassia.
A short course of treatment with an investigational synthetic cat-peptide antigen desensitizing vaccine, or Cat-PAD, results in a substantial and persistent reduction in cat allergy symptom scores, according to two-year findings from a randomized, placebo-controlled phase II clinical study involving 202 adult patients.
Study participants were initially randomized to receive either eight 3-nmol intradermal doses at 2-week intervals, four 6 nmol-doses at 4-week intervals, or placebo. At 1-year follow-up, the improvement in Total Rhinoconjunctivitis Symptom Score (TRSS) was significantly greater in the patients who received four doses of Cat-PAD (ToleroMune Cat, Circassia Limited, Oxford, England), compared with those who received placebo (–7.1 points vs. –2.99 points), according to findings published online in the Journal of Allergy and Clinical Immunology (doi:10.1016/j.jaci.2012.12.1185).
Data from a 2-year follow-up study were reported by Rod P. Hafner, Ph.D., and his colleagues in a poster at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Of 89 patients enrolled into the follow-up study, 50 returned at 2-years after the start of treatment, having received no additional treatment, for an environmental exposure chamber challenge. The magnitude of difference from baseline in TRSS seen at 1 year in those who received 4 doses and those who received placebo was maintained at 2 years (–5.87 vs. –2.02) among those who initially received the 4-dose regimen.
"Cat-PAD is the first in a new class of synthetic peptide immune-regulatory epitopes. The results from this study provide the first evidence that four doses of 6 nmol Cat-PAD over a 12-week period has a disease-modifying effect with subjects showing sustained improvement at 2 years," the investigators wrote.
Study participants were adults aged 18-65 years with cat allergy who underwent a baseline environmental exposure chamber (EEC) challenge, as well as follow-up EEC challenges at 18-22 weeks and at 100-104 weeks.
"Cat allergen was dispersed into the EEC to achieve a consistent mean level of approx. 50 ng Fel d1/m3, using a validated method," they explained, noting that TRSS was calculated at each EEC challenge based on self-scoring of four nasal symptoms (running nose, sneezing, blocked nose, itchy nose), and four ocular symptoms (itchy eyes, watery eyes, red eyes, sore eyes) on a scale of 0-3, every 30 minutes during the challenge.
Cat-PAD is a "potentially exciting new approach to cat allergy immunotherapy," the investigators said, noting that improvements in the TRSS seen in the initial phase II study and follow-up study represent a substantial improvement over numerous therapies investigated in the past, in some cases reaching a threefold improvement in symptom reduction.
"For example, studies of similar design in an EEC reported TRSS changes of approximately –1.5 units after 16 weeks treatment with a sublingual cat allergy tablet, while a single 180-mg dose of the antihistamine fexofenadine achieved a mean difference in TRSS of –1.3 in a cat allergen study. Moreover, the change in TRSS of –3.8 units reported here was observed after four administrations of Cat-PAD over a 12-week period and persisted 21 months after the end of treatment," they concluded.
In late 2012 the investigators began enrolling a phase III study, which will include individuals aged 12-65 years.
This study was funded by Circassia Limited. Dr. Hafner is employed by Circassia.
AT THE AAAAI ANNUAL MEETING
Major finding: Improvements in TRSS in treated vs. placebo patients were similar at 1 year (–7.1 points vs. –2.99 points), and 2 years (–5.87 vs. –2.02) follow-up.
Data source: A randomized placebo-controlled study and follow-up study involving 202 adults.
Disclosures: This study was funded by Circassia Limited. Dr. Hafner is employed by Circassia.
Atopic dermatitis linked to psychiatric comorbidities
Emerging data strongly suggest that children with atopic dermatitis are at increased risk for attention-deficit/hyperactivity disorder.
"The first paper was actually a really good paper, but it was just one study and so we did not quite believe it. But now there’s a confirmatory study by a separate group with really big data," Dr. Lawrence K. Eichenfield said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
In addition, the second study pointed to a possible higher risk of autism in children with atopic dermatitis, and it also confirmed other reports of increased rates of depression and anxiety, noted Dr. Eichenfield, professor of clinical pediatrics and medicine at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital, also in San Diego.
Atopic dermatitis is the most common chronic inflammatory condition in childhood. Attention-deficit/hyperactivity disorder is the most common psychiatric disorder. The first study to report an association between the two was a German, case-control, population-based study involving 1,436 atopic dermatitis patients aged 6-17 years and an equal number of age- and gender-matched controls without the skin disease.
The prevalence of diagnosed ADHD was 5.2% in the atopic dermatitis patients and 3.4% in controls (P = 0.02). In a multivariate logistic regression analysis, atopic dermatitis was an independent predictor of prevalent ADHD, with an associated 47% increased risk of the psychiatric disorder compared with youths without atopic dermatitis. Moreover, there was a suggestion that the more severe a patient’s atopic dermatitis, the greater the likelihood of comorbid ADHD, since the likelihood of ADHD increased with each additional physician visit for atopic dermatitis (JAMA 2009;301:724-6).
More recently, in a study presented at a major meeting but not yet published, Dr. Eric L. Simpson and his coinvestigators at Oregon Health and Science University, Portland, confirmed the initial German finding that pediatric atopic dermatitis is associated with ADHD, this time in a database of 90,000 U.S. children. Once again, the more severe the skin disease, the greater the risk of ADHD. In this study, atopic dermatitis was also associated with increased risks of depression, anxiety, and autism.
Possible mechanisms underlying the observed association between atopic dermatitis and comorbid psychiatric conditions include sleep disturbance – a prominent feature in atopic dermatitis – and systemic inflammation, according to Dr. Eichenfield.
The well-established atopic comorbidities associated with atopic dermatitis are asthma, allergic rhinitis, and food allergy. Dr. Eichenfield said that he welcomes the evolving evidence of psychologic comorbidities for the opportunity it provides to encourage treatment adherence. Like other treating physicians, he finds topical steroid phobia to be a huge problem.
"I actually use this information in my clinical practice as a way to get my families to adequately treat atopic dermatitis," he said. "If I have families that are very steroid phobic, I’ll say, ‘You know, you really want to treat your kid with appropriate topical therapy to minimize the disease impact: to minimize the sleep disturbance and potentially minimize the neurobehavioral impact of atopic dermatitis.’ I use that as a tool to stay with our regimens of care."
Steroid phobia is a problem worldwide, not just in the United States. For example, Dr. Eichenfield noted that when French dermatologists recently surveyed 208 consecutive atopic dermatitis patients or their parents using a detailed 69-item questionnaire, 81% reported having fears about topical corticosteroids – the mainstay of atopic dermatitis treatment – and 36% admitted to treatment nonadherence as a result of their worries. Fears did not correlate with disease severity (Br. J. Dermatol. 2011;165:808-14).
"I think that in all of dermatology there’s probably no greater chasm between where our families are at in terms of concerns about a medication and where we are as physicians in terms of not having concerns than in the area of topical steroids for atopic dermatitis," Dr. Eichenfield said.
He noted that he makes extensive use of parental educational handouts and written action plans in addition to face-to-face discussion of safety concerns at the time he prescribes topical steroids. One strategy that he’s recently realized makes a big difference in terms of adherence is to explain carefully that the midpotency topical steroids used in treating atopic dermatitis – as distinct from potent and superpotent topical steroids – do not have the side effects and systemic absorption issues steroid-phobic parents are worried about.
Another effective strategy is to be very specific about the quantity of topical medication he wants the patient to use for flare control.
"Instead of saying, ‘Twice a day application of a midstrength topical steroid for 2 weeks,’ now I’ll say, "I would like you to use up this 80-g tube in the next 2 weeks and then I’ll see you back – and it’s safe to use this amount for this particular length of time.’ I find that makes a huge difference in terms of outcomes if I explain it that way," Dr. Eichenfield said.
He reported serving as a consultant to Bayer, Galderma, GlaxoSmithKline, and Valeant.
SDEF and this news organization are owned by the same parent company.
Emerging data strongly suggest that children with atopic dermatitis are at increased risk for attention-deficit/hyperactivity disorder.
"The first paper was actually a really good paper, but it was just one study and so we did not quite believe it. But now there’s a confirmatory study by a separate group with really big data," Dr. Lawrence K. Eichenfield said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
In addition, the second study pointed to a possible higher risk of autism in children with atopic dermatitis, and it also confirmed other reports of increased rates of depression and anxiety, noted Dr. Eichenfield, professor of clinical pediatrics and medicine at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital, also in San Diego.
Atopic dermatitis is the most common chronic inflammatory condition in childhood. Attention-deficit/hyperactivity disorder is the most common psychiatric disorder. The first study to report an association between the two was a German, case-control, population-based study involving 1,436 atopic dermatitis patients aged 6-17 years and an equal number of age- and gender-matched controls without the skin disease.
The prevalence of diagnosed ADHD was 5.2% in the atopic dermatitis patients and 3.4% in controls (P = 0.02). In a multivariate logistic regression analysis, atopic dermatitis was an independent predictor of prevalent ADHD, with an associated 47% increased risk of the psychiatric disorder compared with youths without atopic dermatitis. Moreover, there was a suggestion that the more severe a patient’s atopic dermatitis, the greater the likelihood of comorbid ADHD, since the likelihood of ADHD increased with each additional physician visit for atopic dermatitis (JAMA 2009;301:724-6).
More recently, in a study presented at a major meeting but not yet published, Dr. Eric L. Simpson and his coinvestigators at Oregon Health and Science University, Portland, confirmed the initial German finding that pediatric atopic dermatitis is associated with ADHD, this time in a database of 90,000 U.S. children. Once again, the more severe the skin disease, the greater the risk of ADHD. In this study, atopic dermatitis was also associated with increased risks of depression, anxiety, and autism.
Possible mechanisms underlying the observed association between atopic dermatitis and comorbid psychiatric conditions include sleep disturbance – a prominent feature in atopic dermatitis – and systemic inflammation, according to Dr. Eichenfield.
The well-established atopic comorbidities associated with atopic dermatitis are asthma, allergic rhinitis, and food allergy. Dr. Eichenfield said that he welcomes the evolving evidence of psychologic comorbidities for the opportunity it provides to encourage treatment adherence. Like other treating physicians, he finds topical steroid phobia to be a huge problem.
"I actually use this information in my clinical practice as a way to get my families to adequately treat atopic dermatitis," he said. "If I have families that are very steroid phobic, I’ll say, ‘You know, you really want to treat your kid with appropriate topical therapy to minimize the disease impact: to minimize the sleep disturbance and potentially minimize the neurobehavioral impact of atopic dermatitis.’ I use that as a tool to stay with our regimens of care."
Steroid phobia is a problem worldwide, not just in the United States. For example, Dr. Eichenfield noted that when French dermatologists recently surveyed 208 consecutive atopic dermatitis patients or their parents using a detailed 69-item questionnaire, 81% reported having fears about topical corticosteroids – the mainstay of atopic dermatitis treatment – and 36% admitted to treatment nonadherence as a result of their worries. Fears did not correlate with disease severity (Br. J. Dermatol. 2011;165:808-14).
"I think that in all of dermatology there’s probably no greater chasm between where our families are at in terms of concerns about a medication and where we are as physicians in terms of not having concerns than in the area of topical steroids for atopic dermatitis," Dr. Eichenfield said.
He noted that he makes extensive use of parental educational handouts and written action plans in addition to face-to-face discussion of safety concerns at the time he prescribes topical steroids. One strategy that he’s recently realized makes a big difference in terms of adherence is to explain carefully that the midpotency topical steroids used in treating atopic dermatitis – as distinct from potent and superpotent topical steroids – do not have the side effects and systemic absorption issues steroid-phobic parents are worried about.
Another effective strategy is to be very specific about the quantity of topical medication he wants the patient to use for flare control.
"Instead of saying, ‘Twice a day application of a midstrength topical steroid for 2 weeks,’ now I’ll say, "I would like you to use up this 80-g tube in the next 2 weeks and then I’ll see you back – and it’s safe to use this amount for this particular length of time.’ I find that makes a huge difference in terms of outcomes if I explain it that way," Dr. Eichenfield said.
He reported serving as a consultant to Bayer, Galderma, GlaxoSmithKline, and Valeant.
SDEF and this news organization are owned by the same parent company.
Emerging data strongly suggest that children with atopic dermatitis are at increased risk for attention-deficit/hyperactivity disorder.
"The first paper was actually a really good paper, but it was just one study and so we did not quite believe it. But now there’s a confirmatory study by a separate group with really big data," Dr. Lawrence K. Eichenfield said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
In addition, the second study pointed to a possible higher risk of autism in children with atopic dermatitis, and it also confirmed other reports of increased rates of depression and anxiety, noted Dr. Eichenfield, professor of clinical pediatrics and medicine at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital, also in San Diego.
Atopic dermatitis is the most common chronic inflammatory condition in childhood. Attention-deficit/hyperactivity disorder is the most common psychiatric disorder. The first study to report an association between the two was a German, case-control, population-based study involving 1,436 atopic dermatitis patients aged 6-17 years and an equal number of age- and gender-matched controls without the skin disease.
The prevalence of diagnosed ADHD was 5.2% in the atopic dermatitis patients and 3.4% in controls (P = 0.02). In a multivariate logistic regression analysis, atopic dermatitis was an independent predictor of prevalent ADHD, with an associated 47% increased risk of the psychiatric disorder compared with youths without atopic dermatitis. Moreover, there was a suggestion that the more severe a patient’s atopic dermatitis, the greater the likelihood of comorbid ADHD, since the likelihood of ADHD increased with each additional physician visit for atopic dermatitis (JAMA 2009;301:724-6).
More recently, in a study presented at a major meeting but not yet published, Dr. Eric L. Simpson and his coinvestigators at Oregon Health and Science University, Portland, confirmed the initial German finding that pediatric atopic dermatitis is associated with ADHD, this time in a database of 90,000 U.S. children. Once again, the more severe the skin disease, the greater the risk of ADHD. In this study, atopic dermatitis was also associated with increased risks of depression, anxiety, and autism.
Possible mechanisms underlying the observed association between atopic dermatitis and comorbid psychiatric conditions include sleep disturbance – a prominent feature in atopic dermatitis – and systemic inflammation, according to Dr. Eichenfield.
The well-established atopic comorbidities associated with atopic dermatitis are asthma, allergic rhinitis, and food allergy. Dr. Eichenfield said that he welcomes the evolving evidence of psychologic comorbidities for the opportunity it provides to encourage treatment adherence. Like other treating physicians, he finds topical steroid phobia to be a huge problem.
"I actually use this information in my clinical practice as a way to get my families to adequately treat atopic dermatitis," he said. "If I have families that are very steroid phobic, I’ll say, ‘You know, you really want to treat your kid with appropriate topical therapy to minimize the disease impact: to minimize the sleep disturbance and potentially minimize the neurobehavioral impact of atopic dermatitis.’ I use that as a tool to stay with our regimens of care."
Steroid phobia is a problem worldwide, not just in the United States. For example, Dr. Eichenfield noted that when French dermatologists recently surveyed 208 consecutive atopic dermatitis patients or their parents using a detailed 69-item questionnaire, 81% reported having fears about topical corticosteroids – the mainstay of atopic dermatitis treatment – and 36% admitted to treatment nonadherence as a result of their worries. Fears did not correlate with disease severity (Br. J. Dermatol. 2011;165:808-14).
"I think that in all of dermatology there’s probably no greater chasm between where our families are at in terms of concerns about a medication and where we are as physicians in terms of not having concerns than in the area of topical steroids for atopic dermatitis," Dr. Eichenfield said.
He noted that he makes extensive use of parental educational handouts and written action plans in addition to face-to-face discussion of safety concerns at the time he prescribes topical steroids. One strategy that he’s recently realized makes a big difference in terms of adherence is to explain carefully that the midpotency topical steroids used in treating atopic dermatitis – as distinct from potent and superpotent topical steroids – do not have the side effects and systemic absorption issues steroid-phobic parents are worried about.
Another effective strategy is to be very specific about the quantity of topical medication he wants the patient to use for flare control.
"Instead of saying, ‘Twice a day application of a midstrength topical steroid for 2 weeks,’ now I’ll say, "I would like you to use up this 80-g tube in the next 2 weeks and then I’ll see you back – and it’s safe to use this amount for this particular length of time.’ I find that makes a huge difference in terms of outcomes if I explain it that way," Dr. Eichenfield said.
He reported serving as a consultant to Bayer, Galderma, GlaxoSmithKline, and Valeant.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Methylisothiazolinone named contact allergen of the year
The increasing use of methylisothiazolinone by itself in cosmetic and toiletry products has earned the preservative the title of contact allergen of the year from the American Contact Dermatitis Society.
Methylisothiazolinone (MI), a moderate-strong sensitizer, is important to know about, because a few years ago it was introduced into the market as a stand-alone preservative at up to 100 parts per million, Dr. Donald V. Belsito, professor of clinical dermatology at Columbia University, N.Y., said at the society’s annual meeting.
Previously, MI was used only in combination with methylchloroisothiazolinone (MCI), and the concentration limit of MI in that combination was 3.75 ppm for rinse-off products, and 1.8 ppm for leave-on products. Even at those lower limits, the MCI/MI combination in 2009-2010 was considered the fifth most common cause of preservative allergy.
The new 100-ppm limit (instituted based on the belief that MI was a weaker sensitizer than MCI) represents a 25-fold increase in the permitted concentration in cosmetics, Dr. Belsito noted.
At least one study, however, has shown that the eliciting concentration for MI reactivity can be as low as 5 ppm, Dr. Mari Paz Castanedo-Tardana of the Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and Dr. Kathryn A. Zug of Geisel School of Medicine at Dartmouth, Hanover, N.H., noted in an article in the January/February issue of Dermatitis, which granted MI its dubious 2013 title.
Dr. Castanedo-Tardana and Dr. Zug noted that in 2010, data suggested that more than 2,400 U.S. cosmetic products contained MI, which represents a doubling since 2007. The preservative is also used in industrial products without limit, thus providing other opportunities for exposure, such as occupational exposure (Dermatitis 2013;24:1-6).
An important problem is that few data exist concerning the cross-reaction patterns of MCI and MI, the authors noted.
"Bruze et al. showed that a small proportion of patch test subjects sensitized to MCI/MI also reacted to MI. Isaksson et al. also suggested that patients with high patch test reactivity to MCI may also react to high concentrations of MI (1,000 ppm). The percentage of concomitant reactions between MCI/MI and MI in the patch test population is relevant with regard to the diagnosis of MI contact allergy," according to the authors. They also noted that in a Danish study, a Finnish study, and a German study, only 40%, 66%, and 67% of patients with a positive reaction to MI, respectively, also had a positive reaction to MCI/MI.
The limited experience with MI-only patch tests concentrations also helped the allergen gain its newly bestowed title.
Concentrations of 300 ppm (0.03% aq), 500 ppm (0.05% aq), 1,000 ppm (0.1% aq), and 2,000 ppm (0.2% aq) have been tested, and all have yielded relatively similar percentages of positive test reactions.
"As with any other allergen, the ideal patch test concentration should be able to detect as many cases of contact allergy as possible without causing irritant reactions or active sensitization," the authors said, noting that preliminary results from Denmark suggest that 2,000 ppm (0.2% aq) is an appropriate concentration.
For now, however, it is likely that since MI is not routinely tested in any standard screening series, allergy to this preservative is being widely overlooked.
In fact, reports from Europe show that the frequency of allergy to MI is already at the same level as other preservatives that have been available for many years.
"Methylisothiazolinone should be considered as a potential suspect allergen among patients with suspected cosmetic dermatitis, facial dermatitis, and sunscreen allergy," the authors said, concluding that the addition of MI to an allergen screening series "will likely uncover otherwise undiagnosed cases of preservative contact allergy."
The presenter and authors reported having no disclosures.
The increasing use of methylisothiazolinone by itself in cosmetic and toiletry products has earned the preservative the title of contact allergen of the year from the American Contact Dermatitis Society.
Methylisothiazolinone (MI), a moderate-strong sensitizer, is important to know about, because a few years ago it was introduced into the market as a stand-alone preservative at up to 100 parts per million, Dr. Donald V. Belsito, professor of clinical dermatology at Columbia University, N.Y., said at the society’s annual meeting.
Previously, MI was used only in combination with methylchloroisothiazolinone (MCI), and the concentration limit of MI in that combination was 3.75 ppm for rinse-off products, and 1.8 ppm for leave-on products. Even at those lower limits, the MCI/MI combination in 2009-2010 was considered the fifth most common cause of preservative allergy.
The new 100-ppm limit (instituted based on the belief that MI was a weaker sensitizer than MCI) represents a 25-fold increase in the permitted concentration in cosmetics, Dr. Belsito noted.
At least one study, however, has shown that the eliciting concentration for MI reactivity can be as low as 5 ppm, Dr. Mari Paz Castanedo-Tardana of the Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and Dr. Kathryn A. Zug of Geisel School of Medicine at Dartmouth, Hanover, N.H., noted in an article in the January/February issue of Dermatitis, which granted MI its dubious 2013 title.
Dr. Castanedo-Tardana and Dr. Zug noted that in 2010, data suggested that more than 2,400 U.S. cosmetic products contained MI, which represents a doubling since 2007. The preservative is also used in industrial products without limit, thus providing other opportunities for exposure, such as occupational exposure (Dermatitis 2013;24:1-6).
An important problem is that few data exist concerning the cross-reaction patterns of MCI and MI, the authors noted.
"Bruze et al. showed that a small proportion of patch test subjects sensitized to MCI/MI also reacted to MI. Isaksson et al. also suggested that patients with high patch test reactivity to MCI may also react to high concentrations of MI (1,000 ppm). The percentage of concomitant reactions between MCI/MI and MI in the patch test population is relevant with regard to the diagnosis of MI contact allergy," according to the authors. They also noted that in a Danish study, a Finnish study, and a German study, only 40%, 66%, and 67% of patients with a positive reaction to MI, respectively, also had a positive reaction to MCI/MI.
The limited experience with MI-only patch tests concentrations also helped the allergen gain its newly bestowed title.
Concentrations of 300 ppm (0.03% aq), 500 ppm (0.05% aq), 1,000 ppm (0.1% aq), and 2,000 ppm (0.2% aq) have been tested, and all have yielded relatively similar percentages of positive test reactions.
"As with any other allergen, the ideal patch test concentration should be able to detect as many cases of contact allergy as possible without causing irritant reactions or active sensitization," the authors said, noting that preliminary results from Denmark suggest that 2,000 ppm (0.2% aq) is an appropriate concentration.
For now, however, it is likely that since MI is not routinely tested in any standard screening series, allergy to this preservative is being widely overlooked.
In fact, reports from Europe show that the frequency of allergy to MI is already at the same level as other preservatives that have been available for many years.
"Methylisothiazolinone should be considered as a potential suspect allergen among patients with suspected cosmetic dermatitis, facial dermatitis, and sunscreen allergy," the authors said, concluding that the addition of MI to an allergen screening series "will likely uncover otherwise undiagnosed cases of preservative contact allergy."
The presenter and authors reported having no disclosures.
The increasing use of methylisothiazolinone by itself in cosmetic and toiletry products has earned the preservative the title of contact allergen of the year from the American Contact Dermatitis Society.
Methylisothiazolinone (MI), a moderate-strong sensitizer, is important to know about, because a few years ago it was introduced into the market as a stand-alone preservative at up to 100 parts per million, Dr. Donald V. Belsito, professor of clinical dermatology at Columbia University, N.Y., said at the society’s annual meeting.
Previously, MI was used only in combination with methylchloroisothiazolinone (MCI), and the concentration limit of MI in that combination was 3.75 ppm for rinse-off products, and 1.8 ppm for leave-on products. Even at those lower limits, the MCI/MI combination in 2009-2010 was considered the fifth most common cause of preservative allergy.
The new 100-ppm limit (instituted based on the belief that MI was a weaker sensitizer than MCI) represents a 25-fold increase in the permitted concentration in cosmetics, Dr. Belsito noted.
At least one study, however, has shown that the eliciting concentration for MI reactivity can be as low as 5 ppm, Dr. Mari Paz Castanedo-Tardana of the Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and Dr. Kathryn A. Zug of Geisel School of Medicine at Dartmouth, Hanover, N.H., noted in an article in the January/February issue of Dermatitis, which granted MI its dubious 2013 title.
Dr. Castanedo-Tardana and Dr. Zug noted that in 2010, data suggested that more than 2,400 U.S. cosmetic products contained MI, which represents a doubling since 2007. The preservative is also used in industrial products without limit, thus providing other opportunities for exposure, such as occupational exposure (Dermatitis 2013;24:1-6).
An important problem is that few data exist concerning the cross-reaction patterns of MCI and MI, the authors noted.
"Bruze et al. showed that a small proportion of patch test subjects sensitized to MCI/MI also reacted to MI. Isaksson et al. also suggested that patients with high patch test reactivity to MCI may also react to high concentrations of MI (1,000 ppm). The percentage of concomitant reactions between MCI/MI and MI in the patch test population is relevant with regard to the diagnosis of MI contact allergy," according to the authors. They also noted that in a Danish study, a Finnish study, and a German study, only 40%, 66%, and 67% of patients with a positive reaction to MI, respectively, also had a positive reaction to MCI/MI.
The limited experience with MI-only patch tests concentrations also helped the allergen gain its newly bestowed title.
Concentrations of 300 ppm (0.03% aq), 500 ppm (0.05% aq), 1,000 ppm (0.1% aq), and 2,000 ppm (0.2% aq) have been tested, and all have yielded relatively similar percentages of positive test reactions.
"As with any other allergen, the ideal patch test concentration should be able to detect as many cases of contact allergy as possible without causing irritant reactions or active sensitization," the authors said, noting that preliminary results from Denmark suggest that 2,000 ppm (0.2% aq) is an appropriate concentration.
For now, however, it is likely that since MI is not routinely tested in any standard screening series, allergy to this preservative is being widely overlooked.
In fact, reports from Europe show that the frequency of allergy to MI is already at the same level as other preservatives that have been available for many years.
"Methylisothiazolinone should be considered as a potential suspect allergen among patients with suspected cosmetic dermatitis, facial dermatitis, and sunscreen allergy," the authors said, concluding that the addition of MI to an allergen screening series "will likely uncover otherwise undiagnosed cases of preservative contact allergy."
The presenter and authors reported having no disclosures.
AT THE ACDS ANNUAL MEETING
Omalizumab shows efficacy for refractory chronic idiopathic urticaria
SAN ANTONIO – Omalizumab diminished the signs and symptoms of chronic idiopathic urticaria in a dose-dependent fashion in a phase III study of 323 patients who failed to respond adequately to H1-antihistamines.
After 12 weeks of treatment with the recombinant humanized monoclonal antibody, improvements from baseline in weekly itch-severity scores were significantly greater in patients randomized to receive three doses of either 300 mg or 150 mg every 4 weeks, compared with placebo (score change of -9.8 and -8.1 vs. -5.1, respectively). The itch-severity score in patients randomized to receive a 75-mg dose changed by -5.9 points, but this did not differ significantly from the change in the placebo group, according to Dr. Marcus Maurer of Charite-Universitatsmedizin, Berlin. The report was published online on Feb. 24 in the New England Journal of Medicine.
The findings from this international double-blind trial were reported simultaneously at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
In addition to meeting the primary 12-week response endpoint of change in weekly itch-severity score, patients receiving the 300-mg and 150-mg doses of omalizumab also experienced significant improvements, compared with patients given placebo, on all but one secondary endpoint, including change in the 7-day urticaria activity score (UAS7), change in the score for the weekly number of hives, time until a reduction from baseline of at least 5 points in the weekly itch-severity score (the MID), the proportions of patients with a UAS7 of 6 or less, the number of patients with a weekly MID response in the itch-severity score, the change from baseline in the score for the size of the largest hive, and change from baseline in the overall score on the Dermatology Life Quality Index. Those receiving 300 mg, but not those receiving 150 mg, also experienced significant improvement in the proportion of angioedema-free days from weeks 4-12 (N. Engl. J. Med. 2013 Feb. 24 [doi:10.1056/NEJMoa1215372]).
In a post hoc analysis at 12 weeks, 53%, 23%, 18%, and 10%, of those in the 300-mg, 150-mg, 75-mg, and placebo groups, respectively, were completely free of hives, and 44%, 22%, 16%, and 5%, respectively, were free of both hives and itching.
Of note, many patients experienced extremely rapid improvement, raising questions about a potential, as-yet unidentified and fundamental characteristic of this disease, study coauthors Dr. Thomas B. Casale, professor of medicine and medical microbiology and immunology and chief of allergy/immunology at Creighton University Medical Center, Omaha, Neb., and Dr. Allen P. Kaplan, clinical professor of medicine at the Medical University of South Carolina, Charleston, reported during a press briefing at AAAAI.
They explained that omalizumab, currently approved as an add-on therapy for moderate to severe persistent allergic asthma, is known to bind the allergic antibody immunoglobulin E (IgE). Many patients with chronic urticaria have an antibody that binds to a protein on the surface of histamine-containing cells, and that protein binds IgE.
"Just one injection drops one’s IgE level pretty close to zero ... and we learned that when we drop IgE to rock bottom, the protein on the cell to which it is attached also drops – and that’s the protein that the circulating antibody interacts with. The thinking was that if we drop the surface protein low enough, there’s nothing for the antibody to react with, and the hives would improve," he said.
This was, in fact, the case, as demonstrated in a phase I study of 12 patients, in which 7 patients responded dramatically, 4 responded partially, and 1 had no response. These findings led to the current phase III study, which is one of three such studies that investigators hope will lead to the drug’s approval for chronic idiopathic urticaria, because the high cost of the drug is prohibitive with respect to off-label use.
The same effect was seen in the current study.
This effect, however, takes a couple weeks to become apparent, so the rapid responses that occur in numerous patients suggest there is something more at play.
"This is working even faster than we thought, and probably there is a function of the allergic antibody that we do not yet understand," Dr. Kaplan said. "So this is going to be not only a terrific therapy for the disorder, but will lead to research in the future that probably – we hope – will elucidate the underlying abnormality of chronic urticaria beyond our current understanding of it. It’s very exciting, because it might elucidate some fundamental analogy that we don’t appreciate, and would have implications for allergic disease across the board."
The current study comprised patients aged 12-75 years who had a 6-month or longer history of chronic idiopathic urticaria, the presence of hives associated with itching for at least 8 consecutive weeks at any time prior to enrollment (despite H1-antihistamine use), a UAS7 of 16 or more during a 7-day period, a weekly itch-severity score of at least 8, a score of at least 4 on the UAS on at least one of the screening-visit days, and receipt of a licensed dose of a second-generation H1-antihistamine for at least 3 consecutive days immediately prior to the screening visit. Those with a clearly defined underlying cause for their symptoms were excluded. The doses evaluated in this study were based on a prior dose-ranging study, which showed no additional benefit with doses over 300 mg, the investigators said.
Patients continued to receive stable doses of H1-antihistamines throughout the 12-week treatment period, and were permitted to use diphenhydramine as a rescue medication.
Omalizumab was well tolerated in this study, with a similar number of adverse events occurring across the treatment groups. Serious adverse events occurred more often in the 300-mg group, with 6% of patients in that group experiencing a serious adverse event, compared with 3% of patients in the placebo group, and 1% of patients in the 150-mg and 75-mg groups, but the events were not considered to be related to the study drug.
The duration of response, however, was limited, as noted during a 16-week observation period following the initial 12-week treatment period.
Although the weekly itch-severity scores did not return to baseline during follow-up, they did increase to the levels seen in the placebo group.
The findings are nonetheless encouraging, Dr. Kaplan said, noting that this disease, which can have dramatic adverse effects on quality of life, is not uncommon, and can be difficult to treat, with about half of all patients failing to respond to standard therapy with high dose antihistamines. Alternate treatments used to treat the disease, including steroids and cyclosporine, can be effective, but can be highly toxic, he said.
"For refractory patients we have nothing that matches (omalizumab’s) combination of this kind of efficacy with low side effects, so many of us in the field kind of view this as a game changer for the patients," he said.
This study was sponsored by Genentech and Novartis Pharma. Several study authors made disclosures. A complete list of these disclosures is available with the full text of the article at NEJM.org.
recombinant humanized monoclonal antibody, itch-severity scores, Dr. Marcus Maurer, New England Journal of Medicine, American Academy of Allergy, Asthma, and Immunology, 7-day urticaria activity score,
SAN ANTONIO – Omalizumab diminished the signs and symptoms of chronic idiopathic urticaria in a dose-dependent fashion in a phase III study of 323 patients who failed to respond adequately to H1-antihistamines.
After 12 weeks of treatment with the recombinant humanized monoclonal antibody, improvements from baseline in weekly itch-severity scores were significantly greater in patients randomized to receive three doses of either 300 mg or 150 mg every 4 weeks, compared with placebo (score change of -9.8 and -8.1 vs. -5.1, respectively). The itch-severity score in patients randomized to receive a 75-mg dose changed by -5.9 points, but this did not differ significantly from the change in the placebo group, according to Dr. Marcus Maurer of Charite-Universitatsmedizin, Berlin. The report was published online on Feb. 24 in the New England Journal of Medicine.
The findings from this international double-blind trial were reported simultaneously at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
In addition to meeting the primary 12-week response endpoint of change in weekly itch-severity score, patients receiving the 300-mg and 150-mg doses of omalizumab also experienced significant improvements, compared with patients given placebo, on all but one secondary endpoint, including change in the 7-day urticaria activity score (UAS7), change in the score for the weekly number of hives, time until a reduction from baseline of at least 5 points in the weekly itch-severity score (the MID), the proportions of patients with a UAS7 of 6 or less, the number of patients with a weekly MID response in the itch-severity score, the change from baseline in the score for the size of the largest hive, and change from baseline in the overall score on the Dermatology Life Quality Index. Those receiving 300 mg, but not those receiving 150 mg, also experienced significant improvement in the proportion of angioedema-free days from weeks 4-12 (N. Engl. J. Med. 2013 Feb. 24 [doi:10.1056/NEJMoa1215372]).
In a post hoc analysis at 12 weeks, 53%, 23%, 18%, and 10%, of those in the 300-mg, 150-mg, 75-mg, and placebo groups, respectively, were completely free of hives, and 44%, 22%, 16%, and 5%, respectively, were free of both hives and itching.
Of note, many patients experienced extremely rapid improvement, raising questions about a potential, as-yet unidentified and fundamental characteristic of this disease, study coauthors Dr. Thomas B. Casale, professor of medicine and medical microbiology and immunology and chief of allergy/immunology at Creighton University Medical Center, Omaha, Neb., and Dr. Allen P. Kaplan, clinical professor of medicine at the Medical University of South Carolina, Charleston, reported during a press briefing at AAAAI.
They explained that omalizumab, currently approved as an add-on therapy for moderate to severe persistent allergic asthma, is known to bind the allergic antibody immunoglobulin E (IgE). Many patients with chronic urticaria have an antibody that binds to a protein on the surface of histamine-containing cells, and that protein binds IgE.
"Just one injection drops one’s IgE level pretty close to zero ... and we learned that when we drop IgE to rock bottom, the protein on the cell to which it is attached also drops – and that’s the protein that the circulating antibody interacts with. The thinking was that if we drop the surface protein low enough, there’s nothing for the antibody to react with, and the hives would improve," he said.
This was, in fact, the case, as demonstrated in a phase I study of 12 patients, in which 7 patients responded dramatically, 4 responded partially, and 1 had no response. These findings led to the current phase III study, which is one of three such studies that investigators hope will lead to the drug’s approval for chronic idiopathic urticaria, because the high cost of the drug is prohibitive with respect to off-label use.
The same effect was seen in the current study.
This effect, however, takes a couple weeks to become apparent, so the rapid responses that occur in numerous patients suggest there is something more at play.
"This is working even faster than we thought, and probably there is a function of the allergic antibody that we do not yet understand," Dr. Kaplan said. "So this is going to be not only a terrific therapy for the disorder, but will lead to research in the future that probably – we hope – will elucidate the underlying abnormality of chronic urticaria beyond our current understanding of it. It’s very exciting, because it might elucidate some fundamental analogy that we don’t appreciate, and would have implications for allergic disease across the board."
The current study comprised patients aged 12-75 years who had a 6-month or longer history of chronic idiopathic urticaria, the presence of hives associated with itching for at least 8 consecutive weeks at any time prior to enrollment (despite H1-antihistamine use), a UAS7 of 16 or more during a 7-day period, a weekly itch-severity score of at least 8, a score of at least 4 on the UAS on at least one of the screening-visit days, and receipt of a licensed dose of a second-generation H1-antihistamine for at least 3 consecutive days immediately prior to the screening visit. Those with a clearly defined underlying cause for their symptoms were excluded. The doses evaluated in this study were based on a prior dose-ranging study, which showed no additional benefit with doses over 300 mg, the investigators said.
Patients continued to receive stable doses of H1-antihistamines throughout the 12-week treatment period, and were permitted to use diphenhydramine as a rescue medication.
Omalizumab was well tolerated in this study, with a similar number of adverse events occurring across the treatment groups. Serious adverse events occurred more often in the 300-mg group, with 6% of patients in that group experiencing a serious adverse event, compared with 3% of patients in the placebo group, and 1% of patients in the 150-mg and 75-mg groups, but the events were not considered to be related to the study drug.
The duration of response, however, was limited, as noted during a 16-week observation period following the initial 12-week treatment period.
Although the weekly itch-severity scores did not return to baseline during follow-up, they did increase to the levels seen in the placebo group.
The findings are nonetheless encouraging, Dr. Kaplan said, noting that this disease, which can have dramatic adverse effects on quality of life, is not uncommon, and can be difficult to treat, with about half of all patients failing to respond to standard therapy with high dose antihistamines. Alternate treatments used to treat the disease, including steroids and cyclosporine, can be effective, but can be highly toxic, he said.
"For refractory patients we have nothing that matches (omalizumab’s) combination of this kind of efficacy with low side effects, so many of us in the field kind of view this as a game changer for the patients," he said.
This study was sponsored by Genentech and Novartis Pharma. Several study authors made disclosures. A complete list of these disclosures is available with the full text of the article at NEJM.org.
SAN ANTONIO – Omalizumab diminished the signs and symptoms of chronic idiopathic urticaria in a dose-dependent fashion in a phase III study of 323 patients who failed to respond adequately to H1-antihistamines.
After 12 weeks of treatment with the recombinant humanized monoclonal antibody, improvements from baseline in weekly itch-severity scores were significantly greater in patients randomized to receive three doses of either 300 mg or 150 mg every 4 weeks, compared with placebo (score change of -9.8 and -8.1 vs. -5.1, respectively). The itch-severity score in patients randomized to receive a 75-mg dose changed by -5.9 points, but this did not differ significantly from the change in the placebo group, according to Dr. Marcus Maurer of Charite-Universitatsmedizin, Berlin. The report was published online on Feb. 24 in the New England Journal of Medicine.
The findings from this international double-blind trial were reported simultaneously at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
In addition to meeting the primary 12-week response endpoint of change in weekly itch-severity score, patients receiving the 300-mg and 150-mg doses of omalizumab also experienced significant improvements, compared with patients given placebo, on all but one secondary endpoint, including change in the 7-day urticaria activity score (UAS7), change in the score for the weekly number of hives, time until a reduction from baseline of at least 5 points in the weekly itch-severity score (the MID), the proportions of patients with a UAS7 of 6 or less, the number of patients with a weekly MID response in the itch-severity score, the change from baseline in the score for the size of the largest hive, and change from baseline in the overall score on the Dermatology Life Quality Index. Those receiving 300 mg, but not those receiving 150 mg, also experienced significant improvement in the proportion of angioedema-free days from weeks 4-12 (N. Engl. J. Med. 2013 Feb. 24 [doi:10.1056/NEJMoa1215372]).
In a post hoc analysis at 12 weeks, 53%, 23%, 18%, and 10%, of those in the 300-mg, 150-mg, 75-mg, and placebo groups, respectively, were completely free of hives, and 44%, 22%, 16%, and 5%, respectively, were free of both hives and itching.
Of note, many patients experienced extremely rapid improvement, raising questions about a potential, as-yet unidentified and fundamental characteristic of this disease, study coauthors Dr. Thomas B. Casale, professor of medicine and medical microbiology and immunology and chief of allergy/immunology at Creighton University Medical Center, Omaha, Neb., and Dr. Allen P. Kaplan, clinical professor of medicine at the Medical University of South Carolina, Charleston, reported during a press briefing at AAAAI.
They explained that omalizumab, currently approved as an add-on therapy for moderate to severe persistent allergic asthma, is known to bind the allergic antibody immunoglobulin E (IgE). Many patients with chronic urticaria have an antibody that binds to a protein on the surface of histamine-containing cells, and that protein binds IgE.
"Just one injection drops one’s IgE level pretty close to zero ... and we learned that when we drop IgE to rock bottom, the protein on the cell to which it is attached also drops – and that’s the protein that the circulating antibody interacts with. The thinking was that if we drop the surface protein low enough, there’s nothing for the antibody to react with, and the hives would improve," he said.
This was, in fact, the case, as demonstrated in a phase I study of 12 patients, in which 7 patients responded dramatically, 4 responded partially, and 1 had no response. These findings led to the current phase III study, which is one of three such studies that investigators hope will lead to the drug’s approval for chronic idiopathic urticaria, because the high cost of the drug is prohibitive with respect to off-label use.
The same effect was seen in the current study.
This effect, however, takes a couple weeks to become apparent, so the rapid responses that occur in numerous patients suggest there is something more at play.
"This is working even faster than we thought, and probably there is a function of the allergic antibody that we do not yet understand," Dr. Kaplan said. "So this is going to be not only a terrific therapy for the disorder, but will lead to research in the future that probably – we hope – will elucidate the underlying abnormality of chronic urticaria beyond our current understanding of it. It’s very exciting, because it might elucidate some fundamental analogy that we don’t appreciate, and would have implications for allergic disease across the board."
The current study comprised patients aged 12-75 years who had a 6-month or longer history of chronic idiopathic urticaria, the presence of hives associated with itching for at least 8 consecutive weeks at any time prior to enrollment (despite H1-antihistamine use), a UAS7 of 16 or more during a 7-day period, a weekly itch-severity score of at least 8, a score of at least 4 on the UAS on at least one of the screening-visit days, and receipt of a licensed dose of a second-generation H1-antihistamine for at least 3 consecutive days immediately prior to the screening visit. Those with a clearly defined underlying cause for their symptoms were excluded. The doses evaluated in this study were based on a prior dose-ranging study, which showed no additional benefit with doses over 300 mg, the investigators said.
Patients continued to receive stable doses of H1-antihistamines throughout the 12-week treatment period, and were permitted to use diphenhydramine as a rescue medication.
Omalizumab was well tolerated in this study, with a similar number of adverse events occurring across the treatment groups. Serious adverse events occurred more often in the 300-mg group, with 6% of patients in that group experiencing a serious adverse event, compared with 3% of patients in the placebo group, and 1% of patients in the 150-mg and 75-mg groups, but the events were not considered to be related to the study drug.
The duration of response, however, was limited, as noted during a 16-week observation period following the initial 12-week treatment period.
Although the weekly itch-severity scores did not return to baseline during follow-up, they did increase to the levels seen in the placebo group.
The findings are nonetheless encouraging, Dr. Kaplan said, noting that this disease, which can have dramatic adverse effects on quality of life, is not uncommon, and can be difficult to treat, with about half of all patients failing to respond to standard therapy with high dose antihistamines. Alternate treatments used to treat the disease, including steroids and cyclosporine, can be effective, but can be highly toxic, he said.
"For refractory patients we have nothing that matches (omalizumab’s) combination of this kind of efficacy with low side effects, so many of us in the field kind of view this as a game changer for the patients," he said.
This study was sponsored by Genentech and Novartis Pharma. Several study authors made disclosures. A complete list of these disclosures is available with the full text of the article at NEJM.org.
recombinant humanized monoclonal antibody, itch-severity scores, Dr. Marcus Maurer, New England Journal of Medicine, American Academy of Allergy, Asthma, and Immunology, 7-day urticaria activity score,
recombinant humanized monoclonal antibody, itch-severity scores, Dr. Marcus Maurer, New England Journal of Medicine, American Academy of Allergy, Asthma, and Immunology, 7-day urticaria activity score,
AT THE AAAAI ANNUAL MEETING
Major finding: Treatment significantly improved weekly itch-severity scores after 12 weeks.
Data source: Randomized controlled phase III trial involving 323 patients.
Disclosures: This study was sponsored by Genentech and Novartis Pharma. Several study authors reported having disclosures. A complete list of these disclosures is available with the full text of the article at NEJM.org.