User login
Cosmetic Corner: Dermatologists Weigh in on Products for Sensitive Skin
To improve patient care and outcomes, leading dermatologists offered their recommendations on products for sensitive skin. Consideration must be given to:
- Avène Cicalfate Restorative Skin Cream
Pierre Fabre Dermo-Cosmetique USA
“Sucralfate for speeding up skin repair and the soothing thermal spring waters found in this product make it perfect postprocedure for immediately cooling and calming the skin.”—Jeannette Graf, MD, New York, New York
- Cetaphil RestoraDerm Eczema Calming Body Moisturizer
Galderma Laboratories, LP
“This product is formulated for atopic skin. I personally use it on my face as a moisturizer during the cold New York City winter.”—Anthony M. Rossi, MD, New York, New York
- Vanicream
Pharmaceutical Specialties, Inc
“I recommend Vanicream brand products to patients with sensitive skin or eczema. These products are fragrance free and have minimal ingredients.”— Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Athlete's foot treatments, cleansing devices, redness-reducing products, and face scrubs will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on products for sensitive skin. Consideration must be given to:
- Avène Cicalfate Restorative Skin Cream
Pierre Fabre Dermo-Cosmetique USA
“Sucralfate for speeding up skin repair and the soothing thermal spring waters found in this product make it perfect postprocedure for immediately cooling and calming the skin.”—Jeannette Graf, MD, New York, New York
- Cetaphil RestoraDerm Eczema Calming Body Moisturizer
Galderma Laboratories, LP
“This product is formulated for atopic skin. I personally use it on my face as a moisturizer during the cold New York City winter.”—Anthony M. Rossi, MD, New York, New York
- Vanicream
Pharmaceutical Specialties, Inc
“I recommend Vanicream brand products to patients with sensitive skin or eczema. These products are fragrance free and have minimal ingredients.”— Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Athlete's foot treatments, cleansing devices, redness-reducing products, and face scrubs will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on products for sensitive skin. Consideration must be given to:
- Avène Cicalfate Restorative Skin Cream
Pierre Fabre Dermo-Cosmetique USA
“Sucralfate for speeding up skin repair and the soothing thermal spring waters found in this product make it perfect postprocedure for immediately cooling and calming the skin.”—Jeannette Graf, MD, New York, New York
- Cetaphil RestoraDerm Eczema Calming Body Moisturizer
Galderma Laboratories, LP
“This product is formulated for atopic skin. I personally use it on my face as a moisturizer during the cold New York City winter.”—Anthony M. Rossi, MD, New York, New York
- Vanicream
Pharmaceutical Specialties, Inc
“I recommend Vanicream brand products to patients with sensitive skin or eczema. These products are fragrance free and have minimal ingredients.”— Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Athlete's foot treatments, cleansing devices, redness-reducing products, and face scrubs will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
Vitamin D3 supplementation beat placebo in atopic dermatitis trial
PORTLAND – Short-term daily oral supplementation with 5000 IU of vitamin D3 was safe and significantly outperformed placebo for lessening the signs and symptoms of atopic dermatitis (AD), according to the results of a randomized, double-blind trial of 65 patients with moderate to severe disease.
At week 12, average improvements on the Scoring Atopic Dermatitis (SCORAD) index (standard deviation, 11.1 points) were 21.2 points in the vitamin D3 group and 13.9 points (standard deviation, 12.3 points) in the placebo group (P = .02), Karen Sanchez-Armendariz, MD, reported at the annual meeting of the Society for Investigative Dermatology. “Vitamin D3 should be considered a valuable adjuvant in the treatment of AD, especially in patients with relapses,” she said.
Past studies have reported an inverse relationship between 25(OH)D (vitamin D) serum levels and the severity of AD, noted Dr. Sanchez-Armendariz, who is with the National Autonomous University of Mexico in Mexico City. This finding makes sense, as vitamin D3 induces the topical production of antimicrobial peptides, which have direct antimicrobial activity and induce a host response characterized by cytokine release, chemotaxis, inflammation, angiogenesis, and re-epithelialization, she added.
Consequently, 25(OH)D deficiency has been posited as a culprit in the pathophysiology of AD. However, scientific and endocrine societies have published differing recommendations about safe and optimal doses for vitamin D3 supplementation, while experts have noted that 5000 IU per day is needed to achieve an increase of 10 ng 25(OH)D per mL serum (J Am Board Fam Med. 2014 Jul-Aug;27[4]:495-509).
For the study, therefore, Dr. Sanchez-Armendariz and her associates evaluated this dose as adjuvant therapy in patients with moderate to severe AD despite topical hydrocortisone aceponate, soap substitute, and emollient. Patients continued this usual care and received either a cellulose capsule placebo or 5000 IU oral vitamin D3 per day. Based on previously recommended cutoff values, the researchers considered serum 25(OH)D levels sufficient if they exceeded 30 ng per mL, insufficient if they were between 20 and 30 ng per mL, and deficient if they were less than 20 ng per mL (J Bone Miner Res. 2011 Mar;26[3]:455-7).
At baseline, the placebo and intervention groups resembled each other in terms of baseline levels of 25(OH)D, calcium, and phosphorus, pruritus, and scores on SCORAD and the six-area Total Body Severity Assessment. A total of 59% of patients had insufficient serum 25(OH)D, and 39% were deficient.
Patients in both groups improved on the Total Body Severity Assessment, but the extent of improvement did not statistically differ between groups, Dr. Sanchez-Armendariz reported. Supplementation did significantly increase serum 25(OH)D levels, which at week 12 averaged 58.3 ng per mL (SD, 18.5 ng per mL), compared with 22.2 (SD, 7.6 ng per mL) with placebo (P less than .001).
Interestingly, at week 12, all patients in the vitamin D3 group achieved serum 25(OH)D levels of at least 30 ng per mL, as did 41% of patients in the placebo arm. Patients in both groups who achieved this “sufficient” serum 25(OH)D level averaged a nearly 21-point improvement on the SCORAD (SD, 11.1 points), compared with 7.5 points (SD, 9.9 points) among patients who remained deficient, and 17.4 points (SD, 12.7 points) among patients who remained insufficient (P = .004 for difference among groups; P = .02 for difference between sufficient and insufficient groups).
An analysis of variance confirmed this finding, showing that, regardless of treatment group, patients whose final serum 25(OH)D level was at least 20 ng per mL scored an average of 20 points on the SCORAD, compared with 38 points for patients whose 25(OH)D level remained below 20 ng per mL. Patients whose final 25(OH)D level exceeded 20 ng per mL had fivefold greater odds of having a SCORAD below 25 than did patients with lower serum 25(OH)D levels (odds ratio, 5.1; 95% confidence interval, 1.2-22.6; P = .03).
The investigators identified no adverse effects of vitamin D3 supplementation, Dr. Sanchez-Armendariz said. “Attaining 25(OH)D levels above 20 ng per mL in addition to baseline therapy reduced the SCORAD of patients with moderate to severe atopic dermatitis,” she added. Clinicians should consider vitamin D3 deficiency and supplementation in patients with relapsing or refractory AD, she emphasized.
Dr. Sanchez-Armendariz reported no external funding sources and no conflicts of interest.
PORTLAND – Short-term daily oral supplementation with 5000 IU of vitamin D3 was safe and significantly outperformed placebo for lessening the signs and symptoms of atopic dermatitis (AD), according to the results of a randomized, double-blind trial of 65 patients with moderate to severe disease.
At week 12, average improvements on the Scoring Atopic Dermatitis (SCORAD) index (standard deviation, 11.1 points) were 21.2 points in the vitamin D3 group and 13.9 points (standard deviation, 12.3 points) in the placebo group (P = .02), Karen Sanchez-Armendariz, MD, reported at the annual meeting of the Society for Investigative Dermatology. “Vitamin D3 should be considered a valuable adjuvant in the treatment of AD, especially in patients with relapses,” she said.
Past studies have reported an inverse relationship between 25(OH)D (vitamin D) serum levels and the severity of AD, noted Dr. Sanchez-Armendariz, who is with the National Autonomous University of Mexico in Mexico City. This finding makes sense, as vitamin D3 induces the topical production of antimicrobial peptides, which have direct antimicrobial activity and induce a host response characterized by cytokine release, chemotaxis, inflammation, angiogenesis, and re-epithelialization, she added.
Consequently, 25(OH)D deficiency has been posited as a culprit in the pathophysiology of AD. However, scientific and endocrine societies have published differing recommendations about safe and optimal doses for vitamin D3 supplementation, while experts have noted that 5000 IU per day is needed to achieve an increase of 10 ng 25(OH)D per mL serum (J Am Board Fam Med. 2014 Jul-Aug;27[4]:495-509).
For the study, therefore, Dr. Sanchez-Armendariz and her associates evaluated this dose as adjuvant therapy in patients with moderate to severe AD despite topical hydrocortisone aceponate, soap substitute, and emollient. Patients continued this usual care and received either a cellulose capsule placebo or 5000 IU oral vitamin D3 per day. Based on previously recommended cutoff values, the researchers considered serum 25(OH)D levels sufficient if they exceeded 30 ng per mL, insufficient if they were between 20 and 30 ng per mL, and deficient if they were less than 20 ng per mL (J Bone Miner Res. 2011 Mar;26[3]:455-7).
At baseline, the placebo and intervention groups resembled each other in terms of baseline levels of 25(OH)D, calcium, and phosphorus, pruritus, and scores on SCORAD and the six-area Total Body Severity Assessment. A total of 59% of patients had insufficient serum 25(OH)D, and 39% were deficient.
Patients in both groups improved on the Total Body Severity Assessment, but the extent of improvement did not statistically differ between groups, Dr. Sanchez-Armendariz reported. Supplementation did significantly increase serum 25(OH)D levels, which at week 12 averaged 58.3 ng per mL (SD, 18.5 ng per mL), compared with 22.2 (SD, 7.6 ng per mL) with placebo (P less than .001).
Interestingly, at week 12, all patients in the vitamin D3 group achieved serum 25(OH)D levels of at least 30 ng per mL, as did 41% of patients in the placebo arm. Patients in both groups who achieved this “sufficient” serum 25(OH)D level averaged a nearly 21-point improvement on the SCORAD (SD, 11.1 points), compared with 7.5 points (SD, 9.9 points) among patients who remained deficient, and 17.4 points (SD, 12.7 points) among patients who remained insufficient (P = .004 for difference among groups; P = .02 for difference between sufficient and insufficient groups).
An analysis of variance confirmed this finding, showing that, regardless of treatment group, patients whose final serum 25(OH)D level was at least 20 ng per mL scored an average of 20 points on the SCORAD, compared with 38 points for patients whose 25(OH)D level remained below 20 ng per mL. Patients whose final 25(OH)D level exceeded 20 ng per mL had fivefold greater odds of having a SCORAD below 25 than did patients with lower serum 25(OH)D levels (odds ratio, 5.1; 95% confidence interval, 1.2-22.6; P = .03).
The investigators identified no adverse effects of vitamin D3 supplementation, Dr. Sanchez-Armendariz said. “Attaining 25(OH)D levels above 20 ng per mL in addition to baseline therapy reduced the SCORAD of patients with moderate to severe atopic dermatitis,” she added. Clinicians should consider vitamin D3 deficiency and supplementation in patients with relapsing or refractory AD, she emphasized.
Dr. Sanchez-Armendariz reported no external funding sources and no conflicts of interest.
PORTLAND – Short-term daily oral supplementation with 5000 IU of vitamin D3 was safe and significantly outperformed placebo for lessening the signs and symptoms of atopic dermatitis (AD), according to the results of a randomized, double-blind trial of 65 patients with moderate to severe disease.
At week 12, average improvements on the Scoring Atopic Dermatitis (SCORAD) index (standard deviation, 11.1 points) were 21.2 points in the vitamin D3 group and 13.9 points (standard deviation, 12.3 points) in the placebo group (P = .02), Karen Sanchez-Armendariz, MD, reported at the annual meeting of the Society for Investigative Dermatology. “Vitamin D3 should be considered a valuable adjuvant in the treatment of AD, especially in patients with relapses,” she said.
Past studies have reported an inverse relationship between 25(OH)D (vitamin D) serum levels and the severity of AD, noted Dr. Sanchez-Armendariz, who is with the National Autonomous University of Mexico in Mexico City. This finding makes sense, as vitamin D3 induces the topical production of antimicrobial peptides, which have direct antimicrobial activity and induce a host response characterized by cytokine release, chemotaxis, inflammation, angiogenesis, and re-epithelialization, she added.
Consequently, 25(OH)D deficiency has been posited as a culprit in the pathophysiology of AD. However, scientific and endocrine societies have published differing recommendations about safe and optimal doses for vitamin D3 supplementation, while experts have noted that 5000 IU per day is needed to achieve an increase of 10 ng 25(OH)D per mL serum (J Am Board Fam Med. 2014 Jul-Aug;27[4]:495-509).
For the study, therefore, Dr. Sanchez-Armendariz and her associates evaluated this dose as adjuvant therapy in patients with moderate to severe AD despite topical hydrocortisone aceponate, soap substitute, and emollient. Patients continued this usual care and received either a cellulose capsule placebo or 5000 IU oral vitamin D3 per day. Based on previously recommended cutoff values, the researchers considered serum 25(OH)D levels sufficient if they exceeded 30 ng per mL, insufficient if they were between 20 and 30 ng per mL, and deficient if they were less than 20 ng per mL (J Bone Miner Res. 2011 Mar;26[3]:455-7).
At baseline, the placebo and intervention groups resembled each other in terms of baseline levels of 25(OH)D, calcium, and phosphorus, pruritus, and scores on SCORAD and the six-area Total Body Severity Assessment. A total of 59% of patients had insufficient serum 25(OH)D, and 39% were deficient.
Patients in both groups improved on the Total Body Severity Assessment, but the extent of improvement did not statistically differ between groups, Dr. Sanchez-Armendariz reported. Supplementation did significantly increase serum 25(OH)D levels, which at week 12 averaged 58.3 ng per mL (SD, 18.5 ng per mL), compared with 22.2 (SD, 7.6 ng per mL) with placebo (P less than .001).
Interestingly, at week 12, all patients in the vitamin D3 group achieved serum 25(OH)D levels of at least 30 ng per mL, as did 41% of patients in the placebo arm. Patients in both groups who achieved this “sufficient” serum 25(OH)D level averaged a nearly 21-point improvement on the SCORAD (SD, 11.1 points), compared with 7.5 points (SD, 9.9 points) among patients who remained deficient, and 17.4 points (SD, 12.7 points) among patients who remained insufficient (P = .004 for difference among groups; P = .02 for difference between sufficient and insufficient groups).
An analysis of variance confirmed this finding, showing that, regardless of treatment group, patients whose final serum 25(OH)D level was at least 20 ng per mL scored an average of 20 points on the SCORAD, compared with 38 points for patients whose 25(OH)D level remained below 20 ng per mL. Patients whose final 25(OH)D level exceeded 20 ng per mL had fivefold greater odds of having a SCORAD below 25 than did patients with lower serum 25(OH)D levels (odds ratio, 5.1; 95% confidence interval, 1.2-22.6; P = .03).
The investigators identified no adverse effects of vitamin D3 supplementation, Dr. Sanchez-Armendariz said. “Attaining 25(OH)D levels above 20 ng per mL in addition to baseline therapy reduced the SCORAD of patients with moderate to severe atopic dermatitis,” she added. Clinicians should consider vitamin D3 deficiency and supplementation in patients with relapsing or refractory AD, she emphasized.
Dr. Sanchez-Armendariz reported no external funding sources and no conflicts of interest.
Key clinical point:
Major finding: At week 12, average improvements on the SCORAD index were 21.2 points with vitamin D3 and 13.9 points (SD, 12.3 points) with placebo (P = .02).
Data source: A double-blind, placebo-controlled, randomized trial of 65 patients with moderate to severe atopic dermatitis despite usual therapy with topical steroids, soap substitute, and topical emollient.
Disclosures: Dr. Sanchez-Armendariz did not acknowledge external funding sources. She reported having no conflicts of interest.
Severe Refractory Atopic Dermatitis With Elevated Serum IgE Treated With Omalizumab
To the Editor:
Atopic dermatitis (AD) is a common skin condition with an increasing prevalence, affecting up to 20% of children and 3% of adults.1,2 More than 80% of patients with AD have elevated IgE levels.3,4 IgE modulates the inflammatory response in AD in several ways including “a biphasic immediate/late phase reaction, allergen presentation by IgE-bearing Langerhans cells, allergen-induced activation of IgE-bearing macrophages, and IgE autoreactivity to human proteins.”5 Historically, most therapies have focused on mitigating the allergic symptoms caused by degranulated effector cells, such as antihistamines. However, a new class of biologically engineered medications (eg, anti-IgE [omalizumab]) aim to prevent the initiation of the allergic response.6 Variable success has been reported using omalizumab in the treatment of AD, though the majority of studies have shown improvement, especially when used in combination with conventional therapies.4,5,7-22 Omalizumab dosage is determined by body weight and pretreatment serum total IgE levels and is administered via subcutaneous injections every 2 to 4 weeks.6,7,23-26 However, the dosing tables are based on asthma therapy, in which serum IgE levels may be much lower than chronic AD,7,24 and the appropriate dosage in AD patients with markedly elevated IgE is unclear. We report an interesting case of a 57-year-old man with erythroderma from long-standing severe chronic AD that was unresponsive to conventional therapy as well as an associated serum IgE level of 17,183 IU/mL who dramatically improved when omalizumab was added to his treatment regimen.
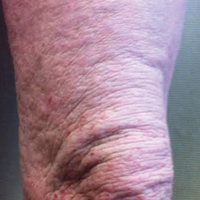
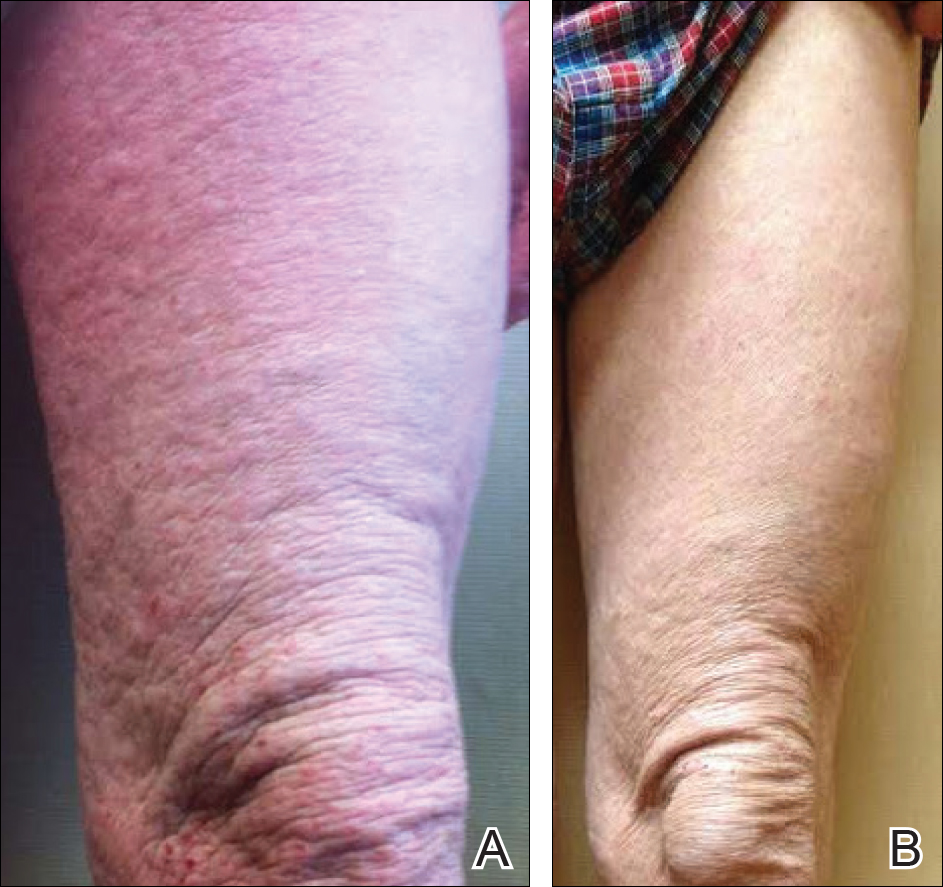
A 57-year-old man presented with essentially 100% body surface area involvement of AD with erythroderma and pruritus. Severe AD developed at infancy and cleared at 5 years of age; childhood onset of asthma was responsive to theophylline and oral inhalers. He developed recurrent AD and asthma at 38 years of age, which was progressive and developed into severe recalcitrant erythroderma by 50 years of age. His AD was unresponsive to multiple therapies, including topical steroids, antibiotics, tacrolimus, bleach baths, antihistamines, methotrexate (15 mg weekly for 1 year, then 12.5 mg weekly for 6 months), UVB phototherapy, and psoralen plus UVA photochemotherapy. He had minimal improvement with cyclosporine (200 mg daily for 4 weeks) and mycophenolate mofetil (3 g daily), and required systemic steroids for relief. The skin was violaceous and lichenified (Figure, A). Laboratory studies were normal, except for a serum IgE level of 17,183 IU/mL (reference range, <150 IU/mL) and peripheral blood eosinophilia up to 29.8% (reference range, 1%–5%) of the differential. Skin biopsies showed AD progressing to lichen simplex chronicus. Omalizumab was added to the therapeutic regimen at a dose of 375 mg every 2 weeks, with noticeable improvement after 3 months. The patient had approximately 80% to 90% clearing with resolution of erythroderma and pruritus, and only limited residual lichenification (Figure, B). The mycophenolate was tapered slowly, and the patient experienced a mild flare at 1 g daily. He is presently on 1 g of mycophenolate daily and omalizumab (375 mg every 2 weeks) and remains remarkably improved. His IgE level decreased to 11,983 IU/mL.
Omalizumab is a monoclonal IgG1 antibody that specifically binds to the FcεRI domain of serum IgE. It blocks binding to high-affinity receptors on effector cells, primarily mast cells, basophils, macrophages, and dendritic cells; it also decreases free IgE serum levels and downregulates the IgE receptor.4,6-10,23-25,27,28 Currently, omalizumab is US Food and Drug Administration approved for moderate to severe persistent asthma in patients 6 years or older with a positive aeroallergen skin test and IgE levels up to 700 IU/mL.6,7,23-25,27,28
However, scattered case reports and small case series have described variable success in the treatment of severe AD that is unresponsive to conventional therapy in patients with markedly elevated serum IgE levels.4,5,7-22 The majority of patients (approximately 80% of published cases yielded by a PubMed search of articles indexed for MEDLINE using the search terms omalizumab and atopic dermatitis) showed improvement when measured by clinical severity scores and quality of life improvement, especially when used in conjunction with conventional therapy. Possible reasons for reported treatment failure include insufficient dosage, lack of established treatment guidelines for markedly elevated serum IgE levels, severity of disease, or variable response with failure to lower IgE level below a required threshold.7,9,23,24,27
Krathen and Hsu9 reported treatment failure with omalizumab for AD in 3 patients with serum IgE levels ranging from 5440 and 24,400 IU/mL, and one review indicated omalizumab may work best in patients with only moderately elevated serum IgE levels.21 However, Toledo et al18 reported efficacy of low-dose omalizumab for pretreatment IgE levels up to 30,000 IU/mL in 6 of 11 reported cases. The pretreatment serum IgE level is not predictive of response, and lowering the serum IgE level without normalization can be efficacious,12,23 as in the current case. Serum IgE levels are not used for monitoring therapeutic response or calculating future dosing, given potential increases in serum IgE levels during and after therapy (for up to 12 months) secondary to the formation of anti-IgE:IgE complexes.6,28 Omalizumab appears most effective when used in combination with conventional therapies. Hopefully ongoing studies will further elucidate the role of omalizumab in recalcitrant AD with elevated serum IgE levels.
- Schultz-Larsen F, Diepgen T, Svennson A. The occurrence of atopic dermatitis in north Europe: an international questionnaire study. J Am Acad Dermatol. 1996;34:760-764.
- Laughter D, Istvan JA, Tofte SJ, et al. The presence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43:649-655.
- Jones HE, Inouye JC, McGerity JL, et al. Atopic disease and serum immunoglobulin-E. Br J Dermatol. 1975;92:17-25.
- Abramovits W. A clinician’s paradigm in the treatment of atopic dermatitis. J Am Acad Dermatol. 2005;53(1, suppl 1):570-577.
- Leung D, Soter N. Cellular and immunologic mechanisms in atopic dermatitis. J Am Acad Dermatol. 2001;44(suppl):S1-S12.
- US Food and Drug Administration. Briefing document on safety. Omalizumab (Xolair) (recombinant humanized monoclonal antibody to IgE) for treatment of allergic asthma. http://www.fda.gov/ohrms/dockets/ac/03/briefing/3952B1_02_FDA-Xolair-Safety.pdf. Published April 18, 2003. Accessed June 23, 2014.
- Lane JE, Cheyney JM, Lane TN, et al. Treatment of recalcitrant atopic dermatitis with omalizumab. J Am Acad Dermatol. 2006;54:68-72.
- Caruso C, Gaeta F, Valluzzi RL, et al. Omalizumab efficacy in a girl with atopic eczema. Allergy. 2010;65:278-279.
- Krathen RA, Hsu S. Failure of omalizumab for the treatment of severe atopic dermatitis. J Am Dermatol. 2005;53:338-340.
- Fernández-Antón Martínez MC, Leis-Dosil V, Alfageme-Roldán F, et al. Omalizumab for the treatment of Atopic Dermatitis. Actas Dermosifiliogr. 2012;103:624-628.
- Pelaia G, Gallelli L, Romeo P, et al. Omalizumab decreases exacerbation frequency, oral intake of corticosteroids and peripheral blood eosinophils in atopic patients with uncontrolled asthma. Int J Clin Pharmacol Ther. 2011;49:713-721.
- Belloni B, Ziai M, Lim A, et al. Low-dose anti-IgE therapy in patients with atopic eczema with high serum IgE levels. J Allergy Clin Immunol. 2007;120:1223-1225.
- Park SY, Choi MR, Na JI, et al. Recalcitrant atopic dermatitis treated with omalizumab. Ann Dermatol. 2010;22:349-352.
- Velling P, Skowasch D, Pabst S. Improvement of quality of life in patients with concomitant allergic asthma and atopic dermatitis: one year follow-up of omalizumab therapy. Eur J Med Res. 2011;15:407-410.
- Amrol D. Anti-immunoglobulin E in the treatment of refractory atopic dermatitis. South Med J. 2010;103:554-558.
- Heil PM, Maurer D, Klein B, et al. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course-a randomized, placebo-controlled and double blind study. J Dtsch Dermatol Ges. 2010;8:990-998.
- Ramírez del Pozo ME, Contreras Contreras E, López Tiro J, et al. Omalizumab (anti-IgE antibody) in the treatment of severe atopic dermatitis. J Investig Allergol Clin Immunol. 2011;21:416-417.
- Toledo F, Silvestre JF, Muñoz C. Combined therapy with low-dose omalizumab and intravenous immunoglobulin for severe atopic dermatitis: report of four cases. J Eur Acad Dermatol Venereol. 2012;26:1325-1327.
- Sheinkopf LE, Rafi AW, Katz RM. Efficacy of omalizumab in the treatment of atopic dermatitis: a pilot study. Allergy Asthma Proc. 2008;29:530-537.
- Incorvia C, Pravettoni C, Mauro M, et al. Effectiveness of omalizumab in a patient with severe asthma and atopic dermatitis. Monaldi Arch Chest Dis. 2008:69:78-80.
- Schmitt J, Schäkel K. Omalizumab as a therapeutic option in atopic eczema. Current evidence and potential benefit [in German]. Hautarzt. 2007;58:130-132.
- Thaiwat S, Sangasapaviliya A. Omalizumab treatment in severe atopic dermatitis. Asian Pac J Allergy Immunol. 2011;29:357-360.
- Kopp MV. Omalizumab: anti-IgE therapy in allergy. Curr Allergy Asthma Rep. 2011;11:101-106.
- Vichyanond P. Omalizumab in allergic diseases, a recent review. Asian Pc J Allergy Immunol. 2011;29:209-219.
- Scheinfeld N. Omalizumab. A recombinant humanized monoclonal IgE-blocking antibody. Dermatol Online J. 2005;11:2.
- Lowe PJ, Georgiou P, Canvin J. Revision of omalizumab dosing table for dosing every 4 instead of 2 weeks for specific ranges of bodyweight and baseline IgE [published online ahead of print December 8, 2014]. Regul Toxicol Pharmacol. 2015;71:68-77.
- Vigo PG, Girgis KR, Pfuetze BL, et al. Efficacy of anti-IgE therapy in patients with atopic dermatitis. J Am Acad Dermatol. 2006;55:168-170.
- Hanifin J, Chan S. Biochemical and immunologic mechanisms in atopic dermatitis: new targets for emerging therapies. J Am Acad Dermatol. 1999;41:72-77.
To the Editor:
Atopic dermatitis (AD) is a common skin condition with an increasing prevalence, affecting up to 20% of children and 3% of adults.1,2 More than 80% of patients with AD have elevated IgE levels.3,4 IgE modulates the inflammatory response in AD in several ways including “a biphasic immediate/late phase reaction, allergen presentation by IgE-bearing Langerhans cells, allergen-induced activation of IgE-bearing macrophages, and IgE autoreactivity to human proteins.”5 Historically, most therapies have focused on mitigating the allergic symptoms caused by degranulated effector cells, such as antihistamines. However, a new class of biologically engineered medications (eg, anti-IgE [omalizumab]) aim to prevent the initiation of the allergic response.6 Variable success has been reported using omalizumab in the treatment of AD, though the majority of studies have shown improvement, especially when used in combination with conventional therapies.4,5,7-22 Omalizumab dosage is determined by body weight and pretreatment serum total IgE levels and is administered via subcutaneous injections every 2 to 4 weeks.6,7,23-26 However, the dosing tables are based on asthma therapy, in which serum IgE levels may be much lower than chronic AD,7,24 and the appropriate dosage in AD patients with markedly elevated IgE is unclear. We report an interesting case of a 57-year-old man with erythroderma from long-standing severe chronic AD that was unresponsive to conventional therapy as well as an associated serum IgE level of 17,183 IU/mL who dramatically improved when omalizumab was added to his treatment regimen.
A 57-year-old man presented with essentially 100% body surface area involvement of AD with erythroderma and pruritus. Severe AD developed at infancy and cleared at 5 years of age; childhood onset of asthma was responsive to theophylline and oral inhalers. He developed recurrent AD and asthma at 38 years of age, which was progressive and developed into severe recalcitrant erythroderma by 50 years of age. His AD was unresponsive to multiple therapies, including topical steroids, antibiotics, tacrolimus, bleach baths, antihistamines, methotrexate (15 mg weekly for 1 year, then 12.5 mg weekly for 6 months), UVB phototherapy, and psoralen plus UVA photochemotherapy. He had minimal improvement with cyclosporine (200 mg daily for 4 weeks) and mycophenolate mofetil (3 g daily), and required systemic steroids for relief. The skin was violaceous and lichenified (Figure, A). Laboratory studies were normal, except for a serum IgE level of 17,183 IU/mL (reference range, <150 IU/mL) and peripheral blood eosinophilia up to 29.8% (reference range, 1%–5%) of the differential. Skin biopsies showed AD progressing to lichen simplex chronicus. Omalizumab was added to the therapeutic regimen at a dose of 375 mg every 2 weeks, with noticeable improvement after 3 months. The patient had approximately 80% to 90% clearing with resolution of erythroderma and pruritus, and only limited residual lichenification (Figure, B). The mycophenolate was tapered slowly, and the patient experienced a mild flare at 1 g daily. He is presently on 1 g of mycophenolate daily and omalizumab (375 mg every 2 weeks) and remains remarkably improved. His IgE level decreased to 11,983 IU/mL.

Omalizumab is a monoclonal IgG1 antibody that specifically binds to the FcεRI domain of serum IgE. It blocks binding to high-affinity receptors on effector cells, primarily mast cells, basophils, macrophages, and dendritic cells; it also decreases free IgE serum levels and downregulates the IgE receptor.4,6-10,23-25,27,28 Currently, omalizumab is US Food and Drug Administration approved for moderate to severe persistent asthma in patients 6 years or older with a positive aeroallergen skin test and IgE levels up to 700 IU/mL.6,7,23-25,27,28
However, scattered case reports and small case series have described variable success in the treatment of severe AD that is unresponsive to conventional therapy in patients with markedly elevated serum IgE levels.4,5,7-22 The majority of patients (approximately 80% of published cases yielded by a PubMed search of articles indexed for MEDLINE using the search terms omalizumab and atopic dermatitis) showed improvement when measured by clinical severity scores and quality of life improvement, especially when used in conjunction with conventional therapy. Possible reasons for reported treatment failure include insufficient dosage, lack of established treatment guidelines for markedly elevated serum IgE levels, severity of disease, or variable response with failure to lower IgE level below a required threshold.7,9,23,24,27
Krathen and Hsu9 reported treatment failure with omalizumab for AD in 3 patients with serum IgE levels ranging from 5440 and 24,400 IU/mL, and one review indicated omalizumab may work best in patients with only moderately elevated serum IgE levels.21 However, Toledo et al18 reported efficacy of low-dose omalizumab for pretreatment IgE levels up to 30,000 IU/mL in 6 of 11 reported cases. The pretreatment serum IgE level is not predictive of response, and lowering the serum IgE level without normalization can be efficacious,12,23 as in the current case. Serum IgE levels are not used for monitoring therapeutic response or calculating future dosing, given potential increases in serum IgE levels during and after therapy (for up to 12 months) secondary to the formation of anti-IgE:IgE complexes.6,28 Omalizumab appears most effective when used in combination with conventional therapies. Hopefully ongoing studies will further elucidate the role of omalizumab in recalcitrant AD with elevated serum IgE levels.
To the Editor:
Atopic dermatitis (AD) is a common skin condition with an increasing prevalence, affecting up to 20% of children and 3% of adults.1,2 More than 80% of patients with AD have elevated IgE levels.3,4 IgE modulates the inflammatory response in AD in several ways including “a biphasic immediate/late phase reaction, allergen presentation by IgE-bearing Langerhans cells, allergen-induced activation of IgE-bearing macrophages, and IgE autoreactivity to human proteins.”5 Historically, most therapies have focused on mitigating the allergic symptoms caused by degranulated effector cells, such as antihistamines. However, a new class of biologically engineered medications (eg, anti-IgE [omalizumab]) aim to prevent the initiation of the allergic response.6 Variable success has been reported using omalizumab in the treatment of AD, though the majority of studies have shown improvement, especially when used in combination with conventional therapies.4,5,7-22 Omalizumab dosage is determined by body weight and pretreatment serum total IgE levels and is administered via subcutaneous injections every 2 to 4 weeks.6,7,23-26 However, the dosing tables are based on asthma therapy, in which serum IgE levels may be much lower than chronic AD,7,24 and the appropriate dosage in AD patients with markedly elevated IgE is unclear. We report an interesting case of a 57-year-old man with erythroderma from long-standing severe chronic AD that was unresponsive to conventional therapy as well as an associated serum IgE level of 17,183 IU/mL who dramatically improved when omalizumab was added to his treatment regimen.
A 57-year-old man presented with essentially 100% body surface area involvement of AD with erythroderma and pruritus. Severe AD developed at infancy and cleared at 5 years of age; childhood onset of asthma was responsive to theophylline and oral inhalers. He developed recurrent AD and asthma at 38 years of age, which was progressive and developed into severe recalcitrant erythroderma by 50 years of age. His AD was unresponsive to multiple therapies, including topical steroids, antibiotics, tacrolimus, bleach baths, antihistamines, methotrexate (15 mg weekly for 1 year, then 12.5 mg weekly for 6 months), UVB phototherapy, and psoralen plus UVA photochemotherapy. He had minimal improvement with cyclosporine (200 mg daily for 4 weeks) and mycophenolate mofetil (3 g daily), and required systemic steroids for relief. The skin was violaceous and lichenified (Figure, A). Laboratory studies were normal, except for a serum IgE level of 17,183 IU/mL (reference range, <150 IU/mL) and peripheral blood eosinophilia up to 29.8% (reference range, 1%–5%) of the differential. Skin biopsies showed AD progressing to lichen simplex chronicus. Omalizumab was added to the therapeutic regimen at a dose of 375 mg every 2 weeks, with noticeable improvement after 3 months. The patient had approximately 80% to 90% clearing with resolution of erythroderma and pruritus, and only limited residual lichenification (Figure, B). The mycophenolate was tapered slowly, and the patient experienced a mild flare at 1 g daily. He is presently on 1 g of mycophenolate daily and omalizumab (375 mg every 2 weeks) and remains remarkably improved. His IgE level decreased to 11,983 IU/mL.

Omalizumab is a monoclonal IgG1 antibody that specifically binds to the FcεRI domain of serum IgE. It blocks binding to high-affinity receptors on effector cells, primarily mast cells, basophils, macrophages, and dendritic cells; it also decreases free IgE serum levels and downregulates the IgE receptor.4,6-10,23-25,27,28 Currently, omalizumab is US Food and Drug Administration approved for moderate to severe persistent asthma in patients 6 years or older with a positive aeroallergen skin test and IgE levels up to 700 IU/mL.6,7,23-25,27,28
However, scattered case reports and small case series have described variable success in the treatment of severe AD that is unresponsive to conventional therapy in patients with markedly elevated serum IgE levels.4,5,7-22 The majority of patients (approximately 80% of published cases yielded by a PubMed search of articles indexed for MEDLINE using the search terms omalizumab and atopic dermatitis) showed improvement when measured by clinical severity scores and quality of life improvement, especially when used in conjunction with conventional therapy. Possible reasons for reported treatment failure include insufficient dosage, lack of established treatment guidelines for markedly elevated serum IgE levels, severity of disease, or variable response with failure to lower IgE level below a required threshold.7,9,23,24,27
Krathen and Hsu9 reported treatment failure with omalizumab for AD in 3 patients with serum IgE levels ranging from 5440 and 24,400 IU/mL, and one review indicated omalizumab may work best in patients with only moderately elevated serum IgE levels.21 However, Toledo et al18 reported efficacy of low-dose omalizumab for pretreatment IgE levels up to 30,000 IU/mL in 6 of 11 reported cases. The pretreatment serum IgE level is not predictive of response, and lowering the serum IgE level without normalization can be efficacious,12,23 as in the current case. Serum IgE levels are not used for monitoring therapeutic response or calculating future dosing, given potential increases in serum IgE levels during and after therapy (for up to 12 months) secondary to the formation of anti-IgE:IgE complexes.6,28 Omalizumab appears most effective when used in combination with conventional therapies. Hopefully ongoing studies will further elucidate the role of omalizumab in recalcitrant AD with elevated serum IgE levels.
- Schultz-Larsen F, Diepgen T, Svennson A. The occurrence of atopic dermatitis in north Europe: an international questionnaire study. J Am Acad Dermatol. 1996;34:760-764.
- Laughter D, Istvan JA, Tofte SJ, et al. The presence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43:649-655.
- Jones HE, Inouye JC, McGerity JL, et al. Atopic disease and serum immunoglobulin-E. Br J Dermatol. 1975;92:17-25.
- Abramovits W. A clinician’s paradigm in the treatment of atopic dermatitis. J Am Acad Dermatol. 2005;53(1, suppl 1):570-577.
- Leung D, Soter N. Cellular and immunologic mechanisms in atopic dermatitis. J Am Acad Dermatol. 2001;44(suppl):S1-S12.
- US Food and Drug Administration. Briefing document on safety. Omalizumab (Xolair) (recombinant humanized monoclonal antibody to IgE) for treatment of allergic asthma. http://www.fda.gov/ohrms/dockets/ac/03/briefing/3952B1_02_FDA-Xolair-Safety.pdf. Published April 18, 2003. Accessed June 23, 2014.
- Lane JE, Cheyney JM, Lane TN, et al. Treatment of recalcitrant atopic dermatitis with omalizumab. J Am Acad Dermatol. 2006;54:68-72.
- Caruso C, Gaeta F, Valluzzi RL, et al. Omalizumab efficacy in a girl with atopic eczema. Allergy. 2010;65:278-279.
- Krathen RA, Hsu S. Failure of omalizumab for the treatment of severe atopic dermatitis. J Am Dermatol. 2005;53:338-340.
- Fernández-Antón Martínez MC, Leis-Dosil V, Alfageme-Roldán F, et al. Omalizumab for the treatment of Atopic Dermatitis. Actas Dermosifiliogr. 2012;103:624-628.
- Pelaia G, Gallelli L, Romeo P, et al. Omalizumab decreases exacerbation frequency, oral intake of corticosteroids and peripheral blood eosinophils in atopic patients with uncontrolled asthma. Int J Clin Pharmacol Ther. 2011;49:713-721.
- Belloni B, Ziai M, Lim A, et al. Low-dose anti-IgE therapy in patients with atopic eczema with high serum IgE levels. J Allergy Clin Immunol. 2007;120:1223-1225.
- Park SY, Choi MR, Na JI, et al. Recalcitrant atopic dermatitis treated with omalizumab. Ann Dermatol. 2010;22:349-352.
- Velling P, Skowasch D, Pabst S. Improvement of quality of life in patients with concomitant allergic asthma and atopic dermatitis: one year follow-up of omalizumab therapy. Eur J Med Res. 2011;15:407-410.
- Amrol D. Anti-immunoglobulin E in the treatment of refractory atopic dermatitis. South Med J. 2010;103:554-558.
- Heil PM, Maurer D, Klein B, et al. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course-a randomized, placebo-controlled and double blind study. J Dtsch Dermatol Ges. 2010;8:990-998.
- Ramírez del Pozo ME, Contreras Contreras E, López Tiro J, et al. Omalizumab (anti-IgE antibody) in the treatment of severe atopic dermatitis. J Investig Allergol Clin Immunol. 2011;21:416-417.
- Toledo F, Silvestre JF, Muñoz C. Combined therapy with low-dose omalizumab and intravenous immunoglobulin for severe atopic dermatitis: report of four cases. J Eur Acad Dermatol Venereol. 2012;26:1325-1327.
- Sheinkopf LE, Rafi AW, Katz RM. Efficacy of omalizumab in the treatment of atopic dermatitis: a pilot study. Allergy Asthma Proc. 2008;29:530-537.
- Incorvia C, Pravettoni C, Mauro M, et al. Effectiveness of omalizumab in a patient with severe asthma and atopic dermatitis. Monaldi Arch Chest Dis. 2008:69:78-80.
- Schmitt J, Schäkel K. Omalizumab as a therapeutic option in atopic eczema. Current evidence and potential benefit [in German]. Hautarzt. 2007;58:130-132.
- Thaiwat S, Sangasapaviliya A. Omalizumab treatment in severe atopic dermatitis. Asian Pac J Allergy Immunol. 2011;29:357-360.
- Kopp MV. Omalizumab: anti-IgE therapy in allergy. Curr Allergy Asthma Rep. 2011;11:101-106.
- Vichyanond P. Omalizumab in allergic diseases, a recent review. Asian Pc J Allergy Immunol. 2011;29:209-219.
- Scheinfeld N. Omalizumab. A recombinant humanized monoclonal IgE-blocking antibody. Dermatol Online J. 2005;11:2.
- Lowe PJ, Georgiou P, Canvin J. Revision of omalizumab dosing table for dosing every 4 instead of 2 weeks for specific ranges of bodyweight and baseline IgE [published online ahead of print December 8, 2014]. Regul Toxicol Pharmacol. 2015;71:68-77.
- Vigo PG, Girgis KR, Pfuetze BL, et al. Efficacy of anti-IgE therapy in patients with atopic dermatitis. J Am Acad Dermatol. 2006;55:168-170.
- Hanifin J, Chan S. Biochemical and immunologic mechanisms in atopic dermatitis: new targets for emerging therapies. J Am Acad Dermatol. 1999;41:72-77.
- Schultz-Larsen F, Diepgen T, Svennson A. The occurrence of atopic dermatitis in north Europe: an international questionnaire study. J Am Acad Dermatol. 1996;34:760-764.
- Laughter D, Istvan JA, Tofte SJ, et al. The presence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43:649-655.
- Jones HE, Inouye JC, McGerity JL, et al. Atopic disease and serum immunoglobulin-E. Br J Dermatol. 1975;92:17-25.
- Abramovits W. A clinician’s paradigm in the treatment of atopic dermatitis. J Am Acad Dermatol. 2005;53(1, suppl 1):570-577.
- Leung D, Soter N. Cellular and immunologic mechanisms in atopic dermatitis. J Am Acad Dermatol. 2001;44(suppl):S1-S12.
- US Food and Drug Administration. Briefing document on safety. Omalizumab (Xolair) (recombinant humanized monoclonal antibody to IgE) for treatment of allergic asthma. http://www.fda.gov/ohrms/dockets/ac/03/briefing/3952B1_02_FDA-Xolair-Safety.pdf. Published April 18, 2003. Accessed June 23, 2014.
- Lane JE, Cheyney JM, Lane TN, et al. Treatment of recalcitrant atopic dermatitis with omalizumab. J Am Acad Dermatol. 2006;54:68-72.
- Caruso C, Gaeta F, Valluzzi RL, et al. Omalizumab efficacy in a girl with atopic eczema. Allergy. 2010;65:278-279.
- Krathen RA, Hsu S. Failure of omalizumab for the treatment of severe atopic dermatitis. J Am Dermatol. 2005;53:338-340.
- Fernández-Antón Martínez MC, Leis-Dosil V, Alfageme-Roldán F, et al. Omalizumab for the treatment of Atopic Dermatitis. Actas Dermosifiliogr. 2012;103:624-628.
- Pelaia G, Gallelli L, Romeo P, et al. Omalizumab decreases exacerbation frequency, oral intake of corticosteroids and peripheral blood eosinophils in atopic patients with uncontrolled asthma. Int J Clin Pharmacol Ther. 2011;49:713-721.
- Belloni B, Ziai M, Lim A, et al. Low-dose anti-IgE therapy in patients with atopic eczema with high serum IgE levels. J Allergy Clin Immunol. 2007;120:1223-1225.
- Park SY, Choi MR, Na JI, et al. Recalcitrant atopic dermatitis treated with omalizumab. Ann Dermatol. 2010;22:349-352.
- Velling P, Skowasch D, Pabst S. Improvement of quality of life in patients with concomitant allergic asthma and atopic dermatitis: one year follow-up of omalizumab therapy. Eur J Med Res. 2011;15:407-410.
- Amrol D. Anti-immunoglobulin E in the treatment of refractory atopic dermatitis. South Med J. 2010;103:554-558.
- Heil PM, Maurer D, Klein B, et al. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course-a randomized, placebo-controlled and double blind study. J Dtsch Dermatol Ges. 2010;8:990-998.
- Ramírez del Pozo ME, Contreras Contreras E, López Tiro J, et al. Omalizumab (anti-IgE antibody) in the treatment of severe atopic dermatitis. J Investig Allergol Clin Immunol. 2011;21:416-417.
- Toledo F, Silvestre JF, Muñoz C. Combined therapy with low-dose omalizumab and intravenous immunoglobulin for severe atopic dermatitis: report of four cases. J Eur Acad Dermatol Venereol. 2012;26:1325-1327.
- Sheinkopf LE, Rafi AW, Katz RM. Efficacy of omalizumab in the treatment of atopic dermatitis: a pilot study. Allergy Asthma Proc. 2008;29:530-537.
- Incorvia C, Pravettoni C, Mauro M, et al. Effectiveness of omalizumab in a patient with severe asthma and atopic dermatitis. Monaldi Arch Chest Dis. 2008:69:78-80.
- Schmitt J, Schäkel K. Omalizumab as a therapeutic option in atopic eczema. Current evidence and potential benefit [in German]. Hautarzt. 2007;58:130-132.
- Thaiwat S, Sangasapaviliya A. Omalizumab treatment in severe atopic dermatitis. Asian Pac J Allergy Immunol. 2011;29:357-360.
- Kopp MV. Omalizumab: anti-IgE therapy in allergy. Curr Allergy Asthma Rep. 2011;11:101-106.
- Vichyanond P. Omalizumab in allergic diseases, a recent review. Asian Pc J Allergy Immunol. 2011;29:209-219.
- Scheinfeld N. Omalizumab. A recombinant humanized monoclonal IgE-blocking antibody. Dermatol Online J. 2005;11:2.
- Lowe PJ, Georgiou P, Canvin J. Revision of omalizumab dosing table for dosing every 4 instead of 2 weeks for specific ranges of bodyweight and baseline IgE [published online ahead of print December 8, 2014]. Regul Toxicol Pharmacol. 2015;71:68-77.
- Vigo PG, Girgis KR, Pfuetze BL, et al. Efficacy of anti-IgE therapy in patients with atopic dermatitis. J Am Acad Dermatol. 2006;55:168-170.
- Hanifin J, Chan S. Biochemical and immunologic mechanisms in atopic dermatitis: new targets for emerging therapies. J Am Acad Dermatol. 1999;41:72-77.
Practice Points
- Omalizumab can be effective in treating patients with severe recalcitrant atopic dermatitis with markedly elevated serum IgE.
- Omalizumab appears most effective when used in combination with conventional therapies.
Dupilumab approval marks the first targeted treatment for AD
The approval of dupilumab, the first targeted biologic therapy approved in the United States for treatment of atopic dermatitis, provides a welcome, long-awaited alternative to currently available therapies for moderate to severe disease, according to two of the pivotal trial investigators.
In late March, the Food and Drug Administration approved dupilumab, a monoclonal antibody that inhibits signaling of both interleukin-4 and interleukin-13, for the treatment of moderate to severe AD in adults “whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.” It is administered subcutaneously, with an initial 600-mg dose, followed by 300 mg every other week, according to the prescribing information.
Dupilumab (Dupixent) was the second novel treatment approved for AD in less than 4 months – after years of no approvals of new therapies for this indication. In December, crisaborole ointment (Eucrisa) was approved for treatment of mild to moderate AD in patients aged 2 years and older. Crisaborole is a topical phosphodiesterase-4 inhibitor.
Dupilumab’s approval was based on three phase III studies of adults with moderate to severe AD: SOLO-1 and SOLO-2, which evaluated dupilumab as monotherapy, and the CHRONOS study, which compared dupilumab with topical corticosteroids to treatment with topical corticosteroids alone.
At 16 weeks in the SOLO trials, those treated with dupilumab had improvements in signs and symptoms of AD, including pruritus, anxiety and depression, and quality of life, compared with placebo. Almost 40% of those treated with dupilumab met the primary outcome – a score of clear or almost clear on the Investigator’s Global Assessment and a reduction of 2 points or more in that score from baseline – compared with 8%-10% of those on placebo (P less than .001). Injection-site reactions and conjunctivitis were more common among those treated with dupilumab (N Engl J Med. 2016;375:2335-48).
Emma Guttman-Yassky, MD, PhD, also an investigator in the SOLO 1 and SOLO 2 trials, said that prior to the approval, “we had nothing to treat our patients safely long term.”
“With dupilumab, because it’s specific, it provides the safety that we need,” while providing efficacy that is similar to or better than that of cyclosporine, with the caveat that dupilumab and cyclosporine have not been evaluated in a head-to-head study, Dr. Guttman-Yassky, professor and vice-chair of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
About 5%-10% of those treated at her site had allergic conjunctivitis; most cases resolved with eye drops. So far, “it looks very safe but we need to have long-term data,” she added.
Dr. Simpson said that he was pleased to see the maintenance of therapeutic effects with no emergence of new side effects with the report of 52-week CHRONOS data in March at the American Academy of Dermatology annual meeting.
“We’re entering the era and the decade of atopic dermatitis,” he said. “Dermatologists should look out for many new topical and systemic therapies now that we’re uncovering and figuring out the pathophysiology of atopic dermatitis.”
Dupilumab, which costs $37,000 per year, is marketed by Regeneron and Sanofi. Studies in children and adolescents with AD are underway.
Dr. Guttman-Yassky has received funding from Regeneron for mechanistic studies and is working with most companies developing AD treatments. Dr. Simpson has received research grants from and serves as a consultant to Regeneron, as well as other pharmaceutical companies.
The approval of dupilumab, the first targeted biologic therapy approved in the United States for treatment of atopic dermatitis, provides a welcome, long-awaited alternative to currently available therapies for moderate to severe disease, according to two of the pivotal trial investigators.
In late March, the Food and Drug Administration approved dupilumab, a monoclonal antibody that inhibits signaling of both interleukin-4 and interleukin-13, for the treatment of moderate to severe AD in adults “whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.” It is administered subcutaneously, with an initial 600-mg dose, followed by 300 mg every other week, according to the prescribing information.
Dupilumab (Dupixent) was the second novel treatment approved for AD in less than 4 months – after years of no approvals of new therapies for this indication. In December, crisaborole ointment (Eucrisa) was approved for treatment of mild to moderate AD in patients aged 2 years and older. Crisaborole is a topical phosphodiesterase-4 inhibitor.
Dupilumab’s approval was based on three phase III studies of adults with moderate to severe AD: SOLO-1 and SOLO-2, which evaluated dupilumab as monotherapy, and the CHRONOS study, which compared dupilumab with topical corticosteroids to treatment with topical corticosteroids alone.
At 16 weeks in the SOLO trials, those treated with dupilumab had improvements in signs and symptoms of AD, including pruritus, anxiety and depression, and quality of life, compared with placebo. Almost 40% of those treated with dupilumab met the primary outcome – a score of clear or almost clear on the Investigator’s Global Assessment and a reduction of 2 points or more in that score from baseline – compared with 8%-10% of those on placebo (P less than .001). Injection-site reactions and conjunctivitis were more common among those treated with dupilumab (N Engl J Med. 2016;375:2335-48).
Emma Guttman-Yassky, MD, PhD, also an investigator in the SOLO 1 and SOLO 2 trials, said that prior to the approval, “we had nothing to treat our patients safely long term.”
“With dupilumab, because it’s specific, it provides the safety that we need,” while providing efficacy that is similar to or better than that of cyclosporine, with the caveat that dupilumab and cyclosporine have not been evaluated in a head-to-head study, Dr. Guttman-Yassky, professor and vice-chair of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
About 5%-10% of those treated at her site had allergic conjunctivitis; most cases resolved with eye drops. So far, “it looks very safe but we need to have long-term data,” she added.
Dr. Simpson said that he was pleased to see the maintenance of therapeutic effects with no emergence of new side effects with the report of 52-week CHRONOS data in March at the American Academy of Dermatology annual meeting.
“We’re entering the era and the decade of atopic dermatitis,” he said. “Dermatologists should look out for many new topical and systemic therapies now that we’re uncovering and figuring out the pathophysiology of atopic dermatitis.”
Dupilumab, which costs $37,000 per year, is marketed by Regeneron and Sanofi. Studies in children and adolescents with AD are underway.
Dr. Guttman-Yassky has received funding from Regeneron for mechanistic studies and is working with most companies developing AD treatments. Dr. Simpson has received research grants from and serves as a consultant to Regeneron, as well as other pharmaceutical companies.
The approval of dupilumab, the first targeted biologic therapy approved in the United States for treatment of atopic dermatitis, provides a welcome, long-awaited alternative to currently available therapies for moderate to severe disease, according to two of the pivotal trial investigators.
In late March, the Food and Drug Administration approved dupilumab, a monoclonal antibody that inhibits signaling of both interleukin-4 and interleukin-13, for the treatment of moderate to severe AD in adults “whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.” It is administered subcutaneously, with an initial 600-mg dose, followed by 300 mg every other week, according to the prescribing information.
Dupilumab (Dupixent) was the second novel treatment approved for AD in less than 4 months – after years of no approvals of new therapies for this indication. In December, crisaborole ointment (Eucrisa) was approved for treatment of mild to moderate AD in patients aged 2 years and older. Crisaborole is a topical phosphodiesterase-4 inhibitor.
Dupilumab’s approval was based on three phase III studies of adults with moderate to severe AD: SOLO-1 and SOLO-2, which evaluated dupilumab as monotherapy, and the CHRONOS study, which compared dupilumab with topical corticosteroids to treatment with topical corticosteroids alone.
At 16 weeks in the SOLO trials, those treated with dupilumab had improvements in signs and symptoms of AD, including pruritus, anxiety and depression, and quality of life, compared with placebo. Almost 40% of those treated with dupilumab met the primary outcome – a score of clear or almost clear on the Investigator’s Global Assessment and a reduction of 2 points or more in that score from baseline – compared with 8%-10% of those on placebo (P less than .001). Injection-site reactions and conjunctivitis were more common among those treated with dupilumab (N Engl J Med. 2016;375:2335-48).
Emma Guttman-Yassky, MD, PhD, also an investigator in the SOLO 1 and SOLO 2 trials, said that prior to the approval, “we had nothing to treat our patients safely long term.”
“With dupilumab, because it’s specific, it provides the safety that we need,” while providing efficacy that is similar to or better than that of cyclosporine, with the caveat that dupilumab and cyclosporine have not been evaluated in a head-to-head study, Dr. Guttman-Yassky, professor and vice-chair of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
About 5%-10% of those treated at her site had allergic conjunctivitis; most cases resolved with eye drops. So far, “it looks very safe but we need to have long-term data,” she added.
Dr. Simpson said that he was pleased to see the maintenance of therapeutic effects with no emergence of new side effects with the report of 52-week CHRONOS data in March at the American Academy of Dermatology annual meeting.
“We’re entering the era and the decade of atopic dermatitis,” he said. “Dermatologists should look out for many new topical and systemic therapies now that we’re uncovering and figuring out the pathophysiology of atopic dermatitis.”
Dupilumab, which costs $37,000 per year, is marketed by Regeneron and Sanofi. Studies in children and adolescents with AD are underway.
Dr. Guttman-Yassky has received funding from Regeneron for mechanistic studies and is working with most companies developing AD treatments. Dr. Simpson has received research grants from and serves as a consultant to Regeneron, as well as other pharmaceutical companies.
Inpatient cost of atopic dermatitis called ‘substantial’
Inpatient care for atopic dermatitis in the U.S. totaled almost $128 million between 2002 and 2012, indicating a rising financial burden, according to a study funded by the Agency for Healthcare Research and Quality (AHRQ).
Cost of care averaged $8.3 million per year for adults and $3.3 million per year for children, with per-day costs for adult care increasing from $3,200 in 2002 to $3,783 in 2012 and per-day costs for pediatric care increasing from $2,430 to $2,914 in the same period, according to Shanthi Narla, a doctoral research fellow at Northwestern University, Chicago, and her colleagues (J Invest Dermatol. 2017 Mar 1. doi: 10.1016/j.jid.2017.02.975)
Growth in hospitalizations was seen especially in adult AD patients, which rose from 58 million to 76 million over the decade.
Despite such high comparative rates of hospitalization, most adult (80%) and child (97%) AD patients had a shorter average length of stay than those without AD (adults: 2.7 vs. 3.5 days; children: 2.4 vs. 2.7 days; P = .0004),
Growing prevalence of AD hospitalization contributed to the larger financial burden for AD patients, compared with psoriasis and pemphigus, the researchers noted, considering length of stay per AD hospitalization averaged 62.5% and 50% shorter than those for pemphigus or psoriasis.
“The acute signs and symptoms of [AD or eczema]... erythema, oozing/weeping, scaling and pruritus, may resolve faster with optimized treatment than vesicobullae and erosions in pemphigus or ‘lakes of pus’ and skin sloughing in generalized pustular psoriasis,” they wrote.
Additionally, hospitalization costs for AD were 60% and 33% of the cost per psoriasis and pemphigus hospitalizations, respectively, according to Ms. Narla and her colleagues.
The researchers said they were concerned that the prevalence of AD, and subsequently the inpatient financial burden, will continue to increase if left unchecked.
“Prevalences and costs of hospitalization for AD significantly increased during the study period without plateauing, indicating that the total cost of inpatient care for AD may continue to increase,” the researchers asserted.
This study was limited by a lack of information on the severity of patients’ symptoms, as well as whether a dermatologist or other physician diagnosed the patients and by which criteria.
ezimmerman@frontlinemedcom.com
On Twitter @EAZTweets
Inpatient care for atopic dermatitis in the U.S. totaled almost $128 million between 2002 and 2012, indicating a rising financial burden, according to a study funded by the Agency for Healthcare Research and Quality (AHRQ).
Cost of care averaged $8.3 million per year for adults and $3.3 million per year for children, with per-day costs for adult care increasing from $3,200 in 2002 to $3,783 in 2012 and per-day costs for pediatric care increasing from $2,430 to $2,914 in the same period, according to Shanthi Narla, a doctoral research fellow at Northwestern University, Chicago, and her colleagues (J Invest Dermatol. 2017 Mar 1. doi: 10.1016/j.jid.2017.02.975)
Growth in hospitalizations was seen especially in adult AD patients, which rose from 58 million to 76 million over the decade.
Despite such high comparative rates of hospitalization, most adult (80%) and child (97%) AD patients had a shorter average length of stay than those without AD (adults: 2.7 vs. 3.5 days; children: 2.4 vs. 2.7 days; P = .0004),
Growing prevalence of AD hospitalization contributed to the larger financial burden for AD patients, compared with psoriasis and pemphigus, the researchers noted, considering length of stay per AD hospitalization averaged 62.5% and 50% shorter than those for pemphigus or psoriasis.
“The acute signs and symptoms of [AD or eczema]... erythema, oozing/weeping, scaling and pruritus, may resolve faster with optimized treatment than vesicobullae and erosions in pemphigus or ‘lakes of pus’ and skin sloughing in generalized pustular psoriasis,” they wrote.
Additionally, hospitalization costs for AD were 60% and 33% of the cost per psoriasis and pemphigus hospitalizations, respectively, according to Ms. Narla and her colleagues.
The researchers said they were concerned that the prevalence of AD, and subsequently the inpatient financial burden, will continue to increase if left unchecked.
“Prevalences and costs of hospitalization for AD significantly increased during the study period without plateauing, indicating that the total cost of inpatient care for AD may continue to increase,” the researchers asserted.
This study was limited by a lack of information on the severity of patients’ symptoms, as well as whether a dermatologist or other physician diagnosed the patients and by which criteria.
ezimmerman@frontlinemedcom.com
On Twitter @EAZTweets
Inpatient care for atopic dermatitis in the U.S. totaled almost $128 million between 2002 and 2012, indicating a rising financial burden, according to a study funded by the Agency for Healthcare Research and Quality (AHRQ).
Cost of care averaged $8.3 million per year for adults and $3.3 million per year for children, with per-day costs for adult care increasing from $3,200 in 2002 to $3,783 in 2012 and per-day costs for pediatric care increasing from $2,430 to $2,914 in the same period, according to Shanthi Narla, a doctoral research fellow at Northwestern University, Chicago, and her colleagues (J Invest Dermatol. 2017 Mar 1. doi: 10.1016/j.jid.2017.02.975)
Growth in hospitalizations was seen especially in adult AD patients, which rose from 58 million to 76 million over the decade.
Despite such high comparative rates of hospitalization, most adult (80%) and child (97%) AD patients had a shorter average length of stay than those without AD (adults: 2.7 vs. 3.5 days; children: 2.4 vs. 2.7 days; P = .0004),
Growing prevalence of AD hospitalization contributed to the larger financial burden for AD patients, compared with psoriasis and pemphigus, the researchers noted, considering length of stay per AD hospitalization averaged 62.5% and 50% shorter than those for pemphigus or psoriasis.
“The acute signs and symptoms of [AD or eczema]... erythema, oozing/weeping, scaling and pruritus, may resolve faster with optimized treatment than vesicobullae and erosions in pemphigus or ‘lakes of pus’ and skin sloughing in generalized pustular psoriasis,” they wrote.
Additionally, hospitalization costs for AD were 60% and 33% of the cost per psoriasis and pemphigus hospitalizations, respectively, according to Ms. Narla and her colleagues.
The researchers said they were concerned that the prevalence of AD, and subsequently the inpatient financial burden, will continue to increase if left unchecked.
“Prevalences and costs of hospitalization for AD significantly increased during the study period without plateauing, indicating that the total cost of inpatient care for AD may continue to increase,” the researchers asserted.
This study was limited by a lack of information on the severity of patients’ symptoms, as well as whether a dermatologist or other physician diagnosed the patients and by which criteria.
ezimmerman@frontlinemedcom.com
On Twitter @EAZTweets
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Key clinical point:
Major finding: Hospitalization costs for atopic dermatitis or eczema in adults and children in the U.S. totaled $127.8 million between 2002 and 2012.
Data source: Retrospective analysis of 87 million patient records from the 2002-2012 National Inpatient Sample, with cost of care adjusted for inflation.
Disclosures: Study was sponsored by the Agency for Healthcare Research and Quality (AHRQ), the Dermatology Foundation, and American Medical Association Foundation. The investigators report no relevant financial disclosures.
Strontium, ketamine target troublesome itch
ORLANDO – Two drugs that target ion channels in nerves are being used to quiet neurogenic itch.
The powerful anesthetic ketamine and the element strontium have both been formulated into topical compounds that do very well in quelling itches that have been stubbornly resistant to other therapies, Gil Yosipovitch, MD, said at the annual meeting of the American Academy of Dermatology.
Both studies employed a 4% strontium hydrogel that is available over the counter (TriCalm). The product is designed to alleviate skin irritation (itching, burning, or stinging sensations), according to the manufacturer’s website.
The first study, published in 2013, comprised 32 healthy subjects in whom itch was induced with cowhage before and after skin treatment with the strontium gel, a control vehicle, topical 1% hydrocortisone, and topical 2% diphenhydramine (Acta Derm Venereol. 2013 Sep 4;93[5]:520-6).
Strontium significantly reduced the peak intensity and duration of itch relative to all three of the comparators.
A confirmatory study was published in 2015. The vehicle-controlled, randomized, crossover study recorded cowhage-induced itch intensity and duration in 48 healthy subjects before and after skin treatment with TriCalm, 2% diphenhydramine, 1% hydrocortisone, and hydrogel vehicle (Clin Cosmet Investig Dermatol. 2015 Apr 24;8:223-9). The results were similar, Dr. Yosipovitch said.
TriCalm effectively reduced peak itch intensity by about 3 points on a visual analog scale – a 41% reduction. Itch duration was reduced by 40%. These results were both clinically and statistically significantly better than those achieved by the other active comparators and the vehicle control.
Dr. Yosipovitch said the gel is most effective on nonhistaminergic itches, including those with a neurogenic component, nummular eczema, and facial itch.
“The most powerful antipruritic we have seen in the last 3 years, however, is topical ketamine,” Dr. Yosipovitch said. Typically formulated in 2%-10% creams, the anesthetic is usually combined with amitriptyline and lidocaine. “I see this as the most effective topical antipruritic and antinociceptive we have been using.”
Ketamine is an antagonist of the n-methyl-D-aspartate (NMDA) glutamate receptor and an ion channel protein. Amitriptyline serves primarily as a voltage-gated sodium channel antagonist, and lidocaine as a local anesthetic.
Mark Davis, MD, professor of dermatology at the Mayo Clinic, Rochester, Minn., and associates initially investigated 0.5% ketamine in a topical combination with amitriptyline 1% in a cream. The compound was remarkably effective for a 41-year-old man with a recalcitrant case of brachioradial pruritus – a neuropathic condition characterized by upper-extremity itching (JAMA Dermatol. 2013;149[2]:148-50). The patient had already failed treatment with halobetasol propionate, pimecrolimus, capsaicin, doxepin hydrochloride creams, and oral hydroxyzine hydrochloride and desloratadine. However, he had complete resolution of the itch soon after using the combination cream two to three times daily. At last follow-up, 4 years later, he was still using it at least once daily and continued to obtain complete relief.
Dr. Yosipovitch said this case was followed by a retrospective study of 16 patients who had used the 0.5% ketamine cream with either 1% or 2% amitriptyline for recalcitrant pruritus. The etiologies included neurodermatitis, pruritus caused by postherpetic neuralgia, nostalgia paresthetica, anesthesia dolorosa, nasal pruritus, and diabetic neuropathy (J Am Acad Dermatol. 2013 Aug;69[2]:320-1).
They used the medication one to five times per day for a mean duration of 10 months. Of the 16 patients, two had complete relief; two had substantial relief; six had some relief; five had no relief; and one reported increased itching.
Most recently, Dr. Yosipovitch and associates reported the results of a retrospective case review of 96 patients with a variety of pruritic conditions. The most frequent indications were neuropathic conditions (29%) and prurigo nodularis (19%). Most patients got a compounded cream of 10% ketamine, 5% amitriptyline, and 2% lidocaine;16 patients got a compound with 5% ketamine. The medication worked quickly, providing itch relief within a median of about 4 minutes, with an average of about a 50% decrease in itch rating (J Am Acad Dermatol. 2017 Apr;76[4]:760-1).
Forty patients participated in a pharmacy-administered telephone survey that assessed medication tolerability and efficacy. Of these, 23 patients (58%) had relief “to a great extent” and 14 (35%) “to a moderate extent.”
There were mild side effects (burning and redness at the application site) in 16 patients. “We attributed this mainly to the lidocaine component,” Dr. Yosipovitch said. “Itch reduction lasted from 30 minutes to 7 hours, so we think this is quite a powerful tool. I now often use this topical for patients with severe intractable itch.”
He added that a case report of encephalopathy associated with the cream has recently surfaced. The patient was an elderly man with Parkinson’s disease who had been using 10% ketamine compounded with amitriptyline and lidocaine for 4 days. He gradually increased the use until he was applying it onto almost all of his upper body. The day after this extensive application, the patient presented to an emergency department with slurred speech, ataxia, and altered mental status (JAMA Dermatol. 2016;152[12]:1390-1).
“So a word of warning here: I don’t recommend using it all over the body,” Dr. Yosipovitch said.
Dr. Yosipovitch has financial relationships with numerous companies that are investigating antipruritic compounds, including strontium.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
ORLANDO – Two drugs that target ion channels in nerves are being used to quiet neurogenic itch.
The powerful anesthetic ketamine and the element strontium have both been formulated into topical compounds that do very well in quelling itches that have been stubbornly resistant to other therapies, Gil Yosipovitch, MD, said at the annual meeting of the American Academy of Dermatology.
Both studies employed a 4% strontium hydrogel that is available over the counter (TriCalm). The product is designed to alleviate skin irritation (itching, burning, or stinging sensations), according to the manufacturer’s website.
The first study, published in 2013, comprised 32 healthy subjects in whom itch was induced with cowhage before and after skin treatment with the strontium gel, a control vehicle, topical 1% hydrocortisone, and topical 2% diphenhydramine (Acta Derm Venereol. 2013 Sep 4;93[5]:520-6).
Strontium significantly reduced the peak intensity and duration of itch relative to all three of the comparators.
A confirmatory study was published in 2015. The vehicle-controlled, randomized, crossover study recorded cowhage-induced itch intensity and duration in 48 healthy subjects before and after skin treatment with TriCalm, 2% diphenhydramine, 1% hydrocortisone, and hydrogel vehicle (Clin Cosmet Investig Dermatol. 2015 Apr 24;8:223-9). The results were similar, Dr. Yosipovitch said.
TriCalm effectively reduced peak itch intensity by about 3 points on a visual analog scale – a 41% reduction. Itch duration was reduced by 40%. These results were both clinically and statistically significantly better than those achieved by the other active comparators and the vehicle control.
Dr. Yosipovitch said the gel is most effective on nonhistaminergic itches, including those with a neurogenic component, nummular eczema, and facial itch.
“The most powerful antipruritic we have seen in the last 3 years, however, is topical ketamine,” Dr. Yosipovitch said. Typically formulated in 2%-10% creams, the anesthetic is usually combined with amitriptyline and lidocaine. “I see this as the most effective topical antipruritic and antinociceptive we have been using.”
Ketamine is an antagonist of the n-methyl-D-aspartate (NMDA) glutamate receptor and an ion channel protein. Amitriptyline serves primarily as a voltage-gated sodium channel antagonist, and lidocaine as a local anesthetic.
Mark Davis, MD, professor of dermatology at the Mayo Clinic, Rochester, Minn., and associates initially investigated 0.5% ketamine in a topical combination with amitriptyline 1% in a cream. The compound was remarkably effective for a 41-year-old man with a recalcitrant case of brachioradial pruritus – a neuropathic condition characterized by upper-extremity itching (JAMA Dermatol. 2013;149[2]:148-50). The patient had already failed treatment with halobetasol propionate, pimecrolimus, capsaicin, doxepin hydrochloride creams, and oral hydroxyzine hydrochloride and desloratadine. However, he had complete resolution of the itch soon after using the combination cream two to three times daily. At last follow-up, 4 years later, he was still using it at least once daily and continued to obtain complete relief.
Dr. Yosipovitch said this case was followed by a retrospective study of 16 patients who had used the 0.5% ketamine cream with either 1% or 2% amitriptyline for recalcitrant pruritus. The etiologies included neurodermatitis, pruritus caused by postherpetic neuralgia, nostalgia paresthetica, anesthesia dolorosa, nasal pruritus, and diabetic neuropathy (J Am Acad Dermatol. 2013 Aug;69[2]:320-1).
They used the medication one to five times per day for a mean duration of 10 months. Of the 16 patients, two had complete relief; two had substantial relief; six had some relief; five had no relief; and one reported increased itching.
Most recently, Dr. Yosipovitch and associates reported the results of a retrospective case review of 96 patients with a variety of pruritic conditions. The most frequent indications were neuropathic conditions (29%) and prurigo nodularis (19%). Most patients got a compounded cream of 10% ketamine, 5% amitriptyline, and 2% lidocaine;16 patients got a compound with 5% ketamine. The medication worked quickly, providing itch relief within a median of about 4 minutes, with an average of about a 50% decrease in itch rating (J Am Acad Dermatol. 2017 Apr;76[4]:760-1).
Forty patients participated in a pharmacy-administered telephone survey that assessed medication tolerability and efficacy. Of these, 23 patients (58%) had relief “to a great extent” and 14 (35%) “to a moderate extent.”
There were mild side effects (burning and redness at the application site) in 16 patients. “We attributed this mainly to the lidocaine component,” Dr. Yosipovitch said. “Itch reduction lasted from 30 minutes to 7 hours, so we think this is quite a powerful tool. I now often use this topical for patients with severe intractable itch.”
He added that a case report of encephalopathy associated with the cream has recently surfaced. The patient was an elderly man with Parkinson’s disease who had been using 10% ketamine compounded with amitriptyline and lidocaine for 4 days. He gradually increased the use until he was applying it onto almost all of his upper body. The day after this extensive application, the patient presented to an emergency department with slurred speech, ataxia, and altered mental status (JAMA Dermatol. 2016;152[12]:1390-1).
“So a word of warning here: I don’t recommend using it all over the body,” Dr. Yosipovitch said.
Dr. Yosipovitch has financial relationships with numerous companies that are investigating antipruritic compounds, including strontium.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
ORLANDO – Two drugs that target ion channels in nerves are being used to quiet neurogenic itch.
The powerful anesthetic ketamine and the element strontium have both been formulated into topical compounds that do very well in quelling itches that have been stubbornly resistant to other therapies, Gil Yosipovitch, MD, said at the annual meeting of the American Academy of Dermatology.
Both studies employed a 4% strontium hydrogel that is available over the counter (TriCalm). The product is designed to alleviate skin irritation (itching, burning, or stinging sensations), according to the manufacturer’s website.
The first study, published in 2013, comprised 32 healthy subjects in whom itch was induced with cowhage before and after skin treatment with the strontium gel, a control vehicle, topical 1% hydrocortisone, and topical 2% diphenhydramine (Acta Derm Venereol. 2013 Sep 4;93[5]:520-6).
Strontium significantly reduced the peak intensity and duration of itch relative to all three of the comparators.
A confirmatory study was published in 2015. The vehicle-controlled, randomized, crossover study recorded cowhage-induced itch intensity and duration in 48 healthy subjects before and after skin treatment with TriCalm, 2% diphenhydramine, 1% hydrocortisone, and hydrogel vehicle (Clin Cosmet Investig Dermatol. 2015 Apr 24;8:223-9). The results were similar, Dr. Yosipovitch said.
TriCalm effectively reduced peak itch intensity by about 3 points on a visual analog scale – a 41% reduction. Itch duration was reduced by 40%. These results were both clinically and statistically significantly better than those achieved by the other active comparators and the vehicle control.
Dr. Yosipovitch said the gel is most effective on nonhistaminergic itches, including those with a neurogenic component, nummular eczema, and facial itch.
“The most powerful antipruritic we have seen in the last 3 years, however, is topical ketamine,” Dr. Yosipovitch said. Typically formulated in 2%-10% creams, the anesthetic is usually combined with amitriptyline and lidocaine. “I see this as the most effective topical antipruritic and antinociceptive we have been using.”
Ketamine is an antagonist of the n-methyl-D-aspartate (NMDA) glutamate receptor and an ion channel protein. Amitriptyline serves primarily as a voltage-gated sodium channel antagonist, and lidocaine as a local anesthetic.
Mark Davis, MD, professor of dermatology at the Mayo Clinic, Rochester, Minn., and associates initially investigated 0.5% ketamine in a topical combination with amitriptyline 1% in a cream. The compound was remarkably effective for a 41-year-old man with a recalcitrant case of brachioradial pruritus – a neuropathic condition characterized by upper-extremity itching (JAMA Dermatol. 2013;149[2]:148-50). The patient had already failed treatment with halobetasol propionate, pimecrolimus, capsaicin, doxepin hydrochloride creams, and oral hydroxyzine hydrochloride and desloratadine. However, he had complete resolution of the itch soon after using the combination cream two to three times daily. At last follow-up, 4 years later, he was still using it at least once daily and continued to obtain complete relief.
Dr. Yosipovitch said this case was followed by a retrospective study of 16 patients who had used the 0.5% ketamine cream with either 1% or 2% amitriptyline for recalcitrant pruritus. The etiologies included neurodermatitis, pruritus caused by postherpetic neuralgia, nostalgia paresthetica, anesthesia dolorosa, nasal pruritus, and diabetic neuropathy (J Am Acad Dermatol. 2013 Aug;69[2]:320-1).
They used the medication one to five times per day for a mean duration of 10 months. Of the 16 patients, two had complete relief; two had substantial relief; six had some relief; five had no relief; and one reported increased itching.
Most recently, Dr. Yosipovitch and associates reported the results of a retrospective case review of 96 patients with a variety of pruritic conditions. The most frequent indications were neuropathic conditions (29%) and prurigo nodularis (19%). Most patients got a compounded cream of 10% ketamine, 5% amitriptyline, and 2% lidocaine;16 patients got a compound with 5% ketamine. The medication worked quickly, providing itch relief within a median of about 4 minutes, with an average of about a 50% decrease in itch rating (J Am Acad Dermatol. 2017 Apr;76[4]:760-1).
Forty patients participated in a pharmacy-administered telephone survey that assessed medication tolerability and efficacy. Of these, 23 patients (58%) had relief “to a great extent” and 14 (35%) “to a moderate extent.”
There were mild side effects (burning and redness at the application site) in 16 patients. “We attributed this mainly to the lidocaine component,” Dr. Yosipovitch said. “Itch reduction lasted from 30 minutes to 7 hours, so we think this is quite a powerful tool. I now often use this topical for patients with severe intractable itch.”
He added that a case report of encephalopathy associated with the cream has recently surfaced. The patient was an elderly man with Parkinson’s disease who had been using 10% ketamine compounded with amitriptyline and lidocaine for 4 days. He gradually increased the use until he was applying it onto almost all of his upper body. The day after this extensive application, the patient presented to an emergency department with slurred speech, ataxia, and altered mental status (JAMA Dermatol. 2016;152[12]:1390-1).
“So a word of warning here: I don’t recommend using it all over the body,” Dr. Yosipovitch said.
Dr. Yosipovitch has financial relationships with numerous companies that are investigating antipruritic compounds, including strontium.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
EXPERT ANALYSIS FROM AAD 17
Dupilumab: FDA approves first biologic for atopic dermatitis
Dupilumab, a monoclonal antibody that targets both interleukin-4 and interleukin-13, has been approved for the treatment of moderate to severe atopic dermatitis in adults not adequately controlled with topical prescription therapies or for whom topicals are not appropriate.
The approval marks the first biologic approved for treating AD, according to a March 28 announcement from the Food and Drug Administration.
Dupilumab “inhibits signaling of IL-4 and IL-13, two key cytokines required for the type 2 (including Th2) immune response, which is believed to be a major driver in the pathogenesis of the disease,” according to Regeneron, which will market dupilumab.
Approval was based on three phase III pivotal studies of adults with moderate to severe AD whose disease was not adequately controlled with topical prescription treatments: SOLO-1 and SOLO-2, which evaluated dupilumab as monotherapy, and the CHRONOS study, which compared dupilumab with topical corticosteroids to treatment with topical corticosteroids alone.
The 16-week data from the SOLO-1 and -2 studies were presented at the 2016 annual congress of the European Academy for Dermatology and Venereology.
In two phase III trials of identical design involving patients with atopic dermatitis, dupilumab, administered weekly or every 2 weeks, improved the signs and symptoms of atopic dermatitis, including pruritus, symptoms of anxiety and depression, and quality of life, as compared with placebo. Eczema Area and Severity Index was reported in significantly more patients who received each regimen of dupilumab than in patients who received placebo (P less than .001 for all comparisons).
Dupilumab also was associated with improvement in other clinical endpoints, including reductions in pruritus and symptoms of anxiety or depression, and an improvement in quality of life. Injection-site reactions and conjunctivitis were more frequent in the dupilumab groups than in the placebo groups. The results were published in the New England Journal of Medicine (2016;375:2335-48).
More recently, 52-week data from the CHRONOS study were reported at the annual meeting of the American Academy of Dermatology in March. In that study of 740 adults with moderate to severe AD that was not controlled with topical medications – including corticosteroids with or without calcineurin inhibitors – those randomized to 300 mg of dupilumab once a week, plus topical corticosteroids, showed significantly greater improvements in measures of overall disease severity at 16 weeks and at 52 weeks, compared with those treated with steroids alone. Measures used included Eczema Area and Severity Index and the Pruritus Numerical Rating Scale, Patient Oriented Eczema Measure, Dermatology Life Quality Index.
Adverse events experienced with dupilumab included injection site reactions, eye and eyelid inflammation, and cold sores on the mouth or lips, according to a Regeneron statement.
Dupilumab will be available “later this week,” according to the statement, which noted the wholesale acquisition cost of the medication is expected to be $37,000 annually.
The FDA approval announcement noted that the safety and efficacy of dupilumab had not been established in patients with asthma.
Dupilumab currently is being studied for children with AD in phase II studies and is being studied for other indications: eosinophilic esophagitis in phase II studies, and asthma and nasal polyps in phase III studies.
Dupilumab will be marketed as Dupixent by Regeneron.
Dupilumab, a monoclonal antibody that targets both interleukin-4 and interleukin-13, has been approved for the treatment of moderate to severe atopic dermatitis in adults not adequately controlled with topical prescription therapies or for whom topicals are not appropriate.
The approval marks the first biologic approved for treating AD, according to a March 28 announcement from the Food and Drug Administration.
Dupilumab “inhibits signaling of IL-4 and IL-13, two key cytokines required for the type 2 (including Th2) immune response, which is believed to be a major driver in the pathogenesis of the disease,” according to Regeneron, which will market dupilumab.
Approval was based on three phase III pivotal studies of adults with moderate to severe AD whose disease was not adequately controlled with topical prescription treatments: SOLO-1 and SOLO-2, which evaluated dupilumab as monotherapy, and the CHRONOS study, which compared dupilumab with topical corticosteroids to treatment with topical corticosteroids alone.
The 16-week data from the SOLO-1 and -2 studies were presented at the 2016 annual congress of the European Academy for Dermatology and Venereology.
In two phase III trials of identical design involving patients with atopic dermatitis, dupilumab, administered weekly or every 2 weeks, improved the signs and symptoms of atopic dermatitis, including pruritus, symptoms of anxiety and depression, and quality of life, as compared with placebo. Eczema Area and Severity Index was reported in significantly more patients who received each regimen of dupilumab than in patients who received placebo (P less than .001 for all comparisons).
Dupilumab also was associated with improvement in other clinical endpoints, including reductions in pruritus and symptoms of anxiety or depression, and an improvement in quality of life. Injection-site reactions and conjunctivitis were more frequent in the dupilumab groups than in the placebo groups. The results were published in the New England Journal of Medicine (2016;375:2335-48).
More recently, 52-week data from the CHRONOS study were reported at the annual meeting of the American Academy of Dermatology in March. In that study of 740 adults with moderate to severe AD that was not controlled with topical medications – including corticosteroids with or without calcineurin inhibitors – those randomized to 300 mg of dupilumab once a week, plus topical corticosteroids, showed significantly greater improvements in measures of overall disease severity at 16 weeks and at 52 weeks, compared with those treated with steroids alone. Measures used included Eczema Area and Severity Index and the Pruritus Numerical Rating Scale, Patient Oriented Eczema Measure, Dermatology Life Quality Index.
Adverse events experienced with dupilumab included injection site reactions, eye and eyelid inflammation, and cold sores on the mouth or lips, according to a Regeneron statement.
Dupilumab will be available “later this week,” according to the statement, which noted the wholesale acquisition cost of the medication is expected to be $37,000 annually.
The FDA approval announcement noted that the safety and efficacy of dupilumab had not been established in patients with asthma.
Dupilumab currently is being studied for children with AD in phase II studies and is being studied for other indications: eosinophilic esophagitis in phase II studies, and asthma and nasal polyps in phase III studies.
Dupilumab will be marketed as Dupixent by Regeneron.
Dupilumab, a monoclonal antibody that targets both interleukin-4 and interleukin-13, has been approved for the treatment of moderate to severe atopic dermatitis in adults not adequately controlled with topical prescription therapies or for whom topicals are not appropriate.
The approval marks the first biologic approved for treating AD, according to a March 28 announcement from the Food and Drug Administration.
Dupilumab “inhibits signaling of IL-4 and IL-13, two key cytokines required for the type 2 (including Th2) immune response, which is believed to be a major driver in the pathogenesis of the disease,” according to Regeneron, which will market dupilumab.
Approval was based on three phase III pivotal studies of adults with moderate to severe AD whose disease was not adequately controlled with topical prescription treatments: SOLO-1 and SOLO-2, which evaluated dupilumab as monotherapy, and the CHRONOS study, which compared dupilumab with topical corticosteroids to treatment with topical corticosteroids alone.
The 16-week data from the SOLO-1 and -2 studies were presented at the 2016 annual congress of the European Academy for Dermatology and Venereology.
In two phase III trials of identical design involving patients with atopic dermatitis, dupilumab, administered weekly or every 2 weeks, improved the signs and symptoms of atopic dermatitis, including pruritus, symptoms of anxiety and depression, and quality of life, as compared with placebo. Eczema Area and Severity Index was reported in significantly more patients who received each regimen of dupilumab than in patients who received placebo (P less than .001 for all comparisons).
Dupilumab also was associated with improvement in other clinical endpoints, including reductions in pruritus and symptoms of anxiety or depression, and an improvement in quality of life. Injection-site reactions and conjunctivitis were more frequent in the dupilumab groups than in the placebo groups. The results were published in the New England Journal of Medicine (2016;375:2335-48).
More recently, 52-week data from the CHRONOS study were reported at the annual meeting of the American Academy of Dermatology in March. In that study of 740 adults with moderate to severe AD that was not controlled with topical medications – including corticosteroids with or without calcineurin inhibitors – those randomized to 300 mg of dupilumab once a week, plus topical corticosteroids, showed significantly greater improvements in measures of overall disease severity at 16 weeks and at 52 weeks, compared with those treated with steroids alone. Measures used included Eczema Area and Severity Index and the Pruritus Numerical Rating Scale, Patient Oriented Eczema Measure, Dermatology Life Quality Index.
Adverse events experienced with dupilumab included injection site reactions, eye and eyelid inflammation, and cold sores on the mouth or lips, according to a Regeneron statement.
Dupilumab will be available “later this week,” according to the statement, which noted the wholesale acquisition cost of the medication is expected to be $37,000 annually.
The FDA approval announcement noted that the safety and efficacy of dupilumab had not been established in patients with asthma.
Dupilumab currently is being studied for children with AD in phase II studies and is being studied for other indications: eosinophilic esophagitis in phase II studies, and asthma and nasal polyps in phase III studies.
Dupilumab will be marketed as Dupixent by Regeneron.
Dupilumab improved eczema scores in children in open label trial
ORLANDO – Treatment with dupilumab in children and adolescents with moderate to severe atopic dermatitis (AD) reduced severity and pruritus scores from baseline and was well tolerated, in a multicenter, open-label trial of 78 children and adolescents.
Dupilumab’s “powerful ability” to block interleukin-4 and interleukin-13 pathways of inflammation is “especially exciting because AD is even more Th-2 cell driven in children,” said Michael J. Cork, MD, PhD, head of dermatologic research at the University of Sheffield (England), who presented the findings during a late-breaking research session at the annual meeting of the American Academy of Dermatology.
The study assessed the pharmacokinetics, safety, and efficacy of dupilumab and was conducted with 38 children, aged 6-11 years, and 40 adolescents, aged 12-17 years, with moderate to severe AD. All had failed topical corticosteroid therapy. Some of the children (16%) and adolescents (22.5%) had failed at least one systemic therapy.
Both age groups were given either a 2 mg/kg or a 4 mg/kg dose of dupilumab (administered subcutaneously), nothing for 8 weeks, followed by 4 weekly doses of their respective regimens. Mean Eczema Area and Severity Index (EASI) scores at baseline were 31.7 among the adolescents and 35.9 among the children.
At week 12, mean scores in the younger cohort given either 2 mg/kg or 4 mg/kg had improved by 76.2% and 63.4%, respectively, from baseline. In the adolescents, EASI scores at week 12 had improved by a mean of 66.4% in the 2-mg/kg group and 69.7% in the 4-mg/kg group.
Itch also improved “dramatically,” according to Dr. Cork. In the younger children, peak pruritus Numerical Rating Scale scores improved from baseline by a mean of 41.6% in the lower-dose group and 39.6% in the higher-dose group. In the older cohort, pruritus scores improved from baseline by a mean of 30.8% in the lower-dose group and 37.6% in the higher-dose group.
Treatment was well tolerated across the study, and adverse events were “mild, transient, and unrelated,” Dr. Cork said. “I would like to emphasize that these were not related to dupilumab, as they occurred during the period of time after the first dose, in weeks 6 and 7,” he commented. He attributed AD flares experienced in the study to the quick clearance of the drug in the first few weeks.
Dr. Cork reported numerous disclosures, including serving as an adviser, consultant, and investigator for Regeneron, the sponsor of the trial, and Sanofi; the companies developing dupilumab.
ORLANDO – Treatment with dupilumab in children and adolescents with moderate to severe atopic dermatitis (AD) reduced severity and pruritus scores from baseline and was well tolerated, in a multicenter, open-label trial of 78 children and adolescents.
Dupilumab’s “powerful ability” to block interleukin-4 and interleukin-13 pathways of inflammation is “especially exciting because AD is even more Th-2 cell driven in children,” said Michael J. Cork, MD, PhD, head of dermatologic research at the University of Sheffield (England), who presented the findings during a late-breaking research session at the annual meeting of the American Academy of Dermatology.
The study assessed the pharmacokinetics, safety, and efficacy of dupilumab and was conducted with 38 children, aged 6-11 years, and 40 adolescents, aged 12-17 years, with moderate to severe AD. All had failed topical corticosteroid therapy. Some of the children (16%) and adolescents (22.5%) had failed at least one systemic therapy.
Both age groups were given either a 2 mg/kg or a 4 mg/kg dose of dupilumab (administered subcutaneously), nothing for 8 weeks, followed by 4 weekly doses of their respective regimens. Mean Eczema Area and Severity Index (EASI) scores at baseline were 31.7 among the adolescents and 35.9 among the children.
At week 12, mean scores in the younger cohort given either 2 mg/kg or 4 mg/kg had improved by 76.2% and 63.4%, respectively, from baseline. In the adolescents, EASI scores at week 12 had improved by a mean of 66.4% in the 2-mg/kg group and 69.7% in the 4-mg/kg group.
Itch also improved “dramatically,” according to Dr. Cork. In the younger children, peak pruritus Numerical Rating Scale scores improved from baseline by a mean of 41.6% in the lower-dose group and 39.6% in the higher-dose group. In the older cohort, pruritus scores improved from baseline by a mean of 30.8% in the lower-dose group and 37.6% in the higher-dose group.
Treatment was well tolerated across the study, and adverse events were “mild, transient, and unrelated,” Dr. Cork said. “I would like to emphasize that these were not related to dupilumab, as they occurred during the period of time after the first dose, in weeks 6 and 7,” he commented. He attributed AD flares experienced in the study to the quick clearance of the drug in the first few weeks.
Dr. Cork reported numerous disclosures, including serving as an adviser, consultant, and investigator for Regeneron, the sponsor of the trial, and Sanofi; the companies developing dupilumab.
ORLANDO – Treatment with dupilumab in children and adolescents with moderate to severe atopic dermatitis (AD) reduced severity and pruritus scores from baseline and was well tolerated, in a multicenter, open-label trial of 78 children and adolescents.
Dupilumab’s “powerful ability” to block interleukin-4 and interleukin-13 pathways of inflammation is “especially exciting because AD is even more Th-2 cell driven in children,” said Michael J. Cork, MD, PhD, head of dermatologic research at the University of Sheffield (England), who presented the findings during a late-breaking research session at the annual meeting of the American Academy of Dermatology.
The study assessed the pharmacokinetics, safety, and efficacy of dupilumab and was conducted with 38 children, aged 6-11 years, and 40 adolescents, aged 12-17 years, with moderate to severe AD. All had failed topical corticosteroid therapy. Some of the children (16%) and adolescents (22.5%) had failed at least one systemic therapy.
Both age groups were given either a 2 mg/kg or a 4 mg/kg dose of dupilumab (administered subcutaneously), nothing for 8 weeks, followed by 4 weekly doses of their respective regimens. Mean Eczema Area and Severity Index (EASI) scores at baseline were 31.7 among the adolescents and 35.9 among the children.
At week 12, mean scores in the younger cohort given either 2 mg/kg or 4 mg/kg had improved by 76.2% and 63.4%, respectively, from baseline. In the adolescents, EASI scores at week 12 had improved by a mean of 66.4% in the 2-mg/kg group and 69.7% in the 4-mg/kg group.
Itch also improved “dramatically,” according to Dr. Cork. In the younger children, peak pruritus Numerical Rating Scale scores improved from baseline by a mean of 41.6% in the lower-dose group and 39.6% in the higher-dose group. In the older cohort, pruritus scores improved from baseline by a mean of 30.8% in the lower-dose group and 37.6% in the higher-dose group.
Treatment was well tolerated across the study, and adverse events were “mild, transient, and unrelated,” Dr. Cork said. “I would like to emphasize that these were not related to dupilumab, as they occurred during the period of time after the first dose, in weeks 6 and 7,” he commented. He attributed AD flares experienced in the study to the quick clearance of the drug in the first few weeks.
Dr. Cork reported numerous disclosures, including serving as an adviser, consultant, and investigator for Regeneron, the sponsor of the trial, and Sanofi; the companies developing dupilumab.
AT AAD 17
Key clinical point: Data on dupilumab for treating moderate to severe atopic dermatitis in children and adolescents are promising.
Major finding: At week 12, treatment with dupilumab at 2 mg/kg and 4 mg/kg doses, across 8 weeks in two pediatric cohorts improved baseline EASI scores by 69.7% and pruritus scores by a third and was well tolerated.
Data source: A phase IIa, multicenter, open label pharmacokinetics, safety, and efficacy trial evaluating two dosing regimens of dupilumab in 78 children with moderate to severe AD.
Disclosures: Dr. Cork reported numerous disclosures, including serving as an adviser, consultant, and investigator for Regeneron, the sponsor of the trial, and Sanofi – the two companies developing dupilumab.
How will crisaborole for atopic dermatitis fit into clinical practice?
WAILEA, HAWAII – With the recent approval of crisaborole ointment 2% for the treatment of atopic dermatitis, the question of how to best utilize this novel drug in daily clinical practice was put to two atopic dermatitis experts at the Hawaii Dermatology Seminar sponsored by the Global Academy for Medical Education/Skin Disease Research Foundation.
Two of these experts, Lawrence F. Eichenfield, MD, and Wynnis L. Tom, MD, have garnered extensive clinical trials experience with crisaborole (Eucrisa), which, in December 2016, became the first prescription drug approved by the Food and Drug Administration for atopic dermatitis (AD) in more than 10 years. The topical phosphodiesterase-4 inhibitor is indicated for twice-daily treatment of patients aged 2 years and older with mild to moderate AD.
“Topical steroids are the most cost-effective medicine for the management of acute flares of atopic dermatitis. And if I can then take patients down to only intermittent use of topical corticosteroids of mid-strength or lower, depending on patient age, I’ll probably hang in and do just that,” said Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego.
“But, when I have patients with more persistent disease, frequent flaring where I can’t get by with just twice-a-week topical steroids, or ... sensitive areas where I need to avoid steroid atrophy, that’s when I’m likely to incorporate a nonsteroidal agent into regimens of care. Traditionally, over the past 15-18 years, that’s been either with topical tacrolimus [Protopic] or pimecrolimus [Elidel]. And now crisaborole will fit right in there,” he continued.
As for how long to keep patients on crisaborole, Dr. Eichenfield noted that the package labeling “is pretty open.” It doesn’t address the question of treatment duration, and the patient handout that accompanies the tube of ointment simply states, “Use [crisaborole] Eucrisa exactly as your health care provider tells you to use it.”
“In a no-data situation such as this, I’ll probably try to see if use a few times a week or in a mix/match between corticosteroids, a topical calcineurin inhibitor, and crisaborole will provide decent long-term disease control,” Dr. Eichenfield said.
The important, remaining questions regarding crisaborole include, Is it comparatively efficient? – a question that will require head-to-head clinical trials. Is it cost effective? Will it be effective and safe in children younger than 2 years of age? Is it safe beyond 1 year of use? – a particularly important question, given that the phase III clinical trials were only a month long, the dermatologist added.
Dr. Eichenfield emphasized that he was offering only his personal opinion on the use of crisaborole in response to audience questions. Eventually, guidelines committees will formally address how to incorporate the new drug into a sound treatment strategy.
Dr. Tom, like Dr. Eichenfield, was a coauthor of the two pivotal phase III randomized trials of crisaborole, which found infrequent and minimal side effects along with significant improvement of formal quality of life measures (J Am Acad Dermatol. 2016 Sep;75[3]:494-503.e4).
She said that one way she’ll immediately put the new topical agent to work is in treating disease on the face and other sensitive skin areas. The drug’s steroid-sparing ability is also a major asset, given how many parents of children with AD are steroid phobic.
“I’m certainly not going to give up on topical steroids, but anything that enables me to lessen their use is a comfort for parents. That’s really important,” observed Dr. Tom of the University of California, San Diego, and Rady Children’s Hospital.
Both pediatric dermatologists reported having received research grants from or serving as consultants to roughly half-a-dozen pharmaceutical companies, including Anacor/Pfizer, which markets crisaborole.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – With the recent approval of crisaborole ointment 2% for the treatment of atopic dermatitis, the question of how to best utilize this novel drug in daily clinical practice was put to two atopic dermatitis experts at the Hawaii Dermatology Seminar sponsored by the Global Academy for Medical Education/Skin Disease Research Foundation.
Two of these experts, Lawrence F. Eichenfield, MD, and Wynnis L. Tom, MD, have garnered extensive clinical trials experience with crisaborole (Eucrisa), which, in December 2016, became the first prescription drug approved by the Food and Drug Administration for atopic dermatitis (AD) in more than 10 years. The topical phosphodiesterase-4 inhibitor is indicated for twice-daily treatment of patients aged 2 years and older with mild to moderate AD.
“Topical steroids are the most cost-effective medicine for the management of acute flares of atopic dermatitis. And if I can then take patients down to only intermittent use of topical corticosteroids of mid-strength or lower, depending on patient age, I’ll probably hang in and do just that,” said Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego.
“But, when I have patients with more persistent disease, frequent flaring where I can’t get by with just twice-a-week topical steroids, or ... sensitive areas where I need to avoid steroid atrophy, that’s when I’m likely to incorporate a nonsteroidal agent into regimens of care. Traditionally, over the past 15-18 years, that’s been either with topical tacrolimus [Protopic] or pimecrolimus [Elidel]. And now crisaborole will fit right in there,” he continued.
As for how long to keep patients on crisaborole, Dr. Eichenfield noted that the package labeling “is pretty open.” It doesn’t address the question of treatment duration, and the patient handout that accompanies the tube of ointment simply states, “Use [crisaborole] Eucrisa exactly as your health care provider tells you to use it.”
“In a no-data situation such as this, I’ll probably try to see if use a few times a week or in a mix/match between corticosteroids, a topical calcineurin inhibitor, and crisaborole will provide decent long-term disease control,” Dr. Eichenfield said.
The important, remaining questions regarding crisaborole include, Is it comparatively efficient? – a question that will require head-to-head clinical trials. Is it cost effective? Will it be effective and safe in children younger than 2 years of age? Is it safe beyond 1 year of use? – a particularly important question, given that the phase III clinical trials were only a month long, the dermatologist added.
Dr. Eichenfield emphasized that he was offering only his personal opinion on the use of crisaborole in response to audience questions. Eventually, guidelines committees will formally address how to incorporate the new drug into a sound treatment strategy.
Dr. Tom, like Dr. Eichenfield, was a coauthor of the two pivotal phase III randomized trials of crisaborole, which found infrequent and minimal side effects along with significant improvement of formal quality of life measures (J Am Acad Dermatol. 2016 Sep;75[3]:494-503.e4).
She said that one way she’ll immediately put the new topical agent to work is in treating disease on the face and other sensitive skin areas. The drug’s steroid-sparing ability is also a major asset, given how many parents of children with AD are steroid phobic.
“I’m certainly not going to give up on topical steroids, but anything that enables me to lessen their use is a comfort for parents. That’s really important,” observed Dr. Tom of the University of California, San Diego, and Rady Children’s Hospital.
Both pediatric dermatologists reported having received research grants from or serving as consultants to roughly half-a-dozen pharmaceutical companies, including Anacor/Pfizer, which markets crisaborole.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – With the recent approval of crisaborole ointment 2% for the treatment of atopic dermatitis, the question of how to best utilize this novel drug in daily clinical practice was put to two atopic dermatitis experts at the Hawaii Dermatology Seminar sponsored by the Global Academy for Medical Education/Skin Disease Research Foundation.
Two of these experts, Lawrence F. Eichenfield, MD, and Wynnis L. Tom, MD, have garnered extensive clinical trials experience with crisaborole (Eucrisa), which, in December 2016, became the first prescription drug approved by the Food and Drug Administration for atopic dermatitis (AD) in more than 10 years. The topical phosphodiesterase-4 inhibitor is indicated for twice-daily treatment of patients aged 2 years and older with mild to moderate AD.
“Topical steroids are the most cost-effective medicine for the management of acute flares of atopic dermatitis. And if I can then take patients down to only intermittent use of topical corticosteroids of mid-strength or lower, depending on patient age, I’ll probably hang in and do just that,” said Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego.
“But, when I have patients with more persistent disease, frequent flaring where I can’t get by with just twice-a-week topical steroids, or ... sensitive areas where I need to avoid steroid atrophy, that’s when I’m likely to incorporate a nonsteroidal agent into regimens of care. Traditionally, over the past 15-18 years, that’s been either with topical tacrolimus [Protopic] or pimecrolimus [Elidel]. And now crisaborole will fit right in there,” he continued.
As for how long to keep patients on crisaborole, Dr. Eichenfield noted that the package labeling “is pretty open.” It doesn’t address the question of treatment duration, and the patient handout that accompanies the tube of ointment simply states, “Use [crisaborole] Eucrisa exactly as your health care provider tells you to use it.”
“In a no-data situation such as this, I’ll probably try to see if use a few times a week or in a mix/match between corticosteroids, a topical calcineurin inhibitor, and crisaborole will provide decent long-term disease control,” Dr. Eichenfield said.
The important, remaining questions regarding crisaborole include, Is it comparatively efficient? – a question that will require head-to-head clinical trials. Is it cost effective? Will it be effective and safe in children younger than 2 years of age? Is it safe beyond 1 year of use? – a particularly important question, given that the phase III clinical trials were only a month long, the dermatologist added.
Dr. Eichenfield emphasized that he was offering only his personal opinion on the use of crisaborole in response to audience questions. Eventually, guidelines committees will formally address how to incorporate the new drug into a sound treatment strategy.
Dr. Tom, like Dr. Eichenfield, was a coauthor of the two pivotal phase III randomized trials of crisaborole, which found infrequent and minimal side effects along with significant improvement of formal quality of life measures (J Am Acad Dermatol. 2016 Sep;75[3]:494-503.e4).
She said that one way she’ll immediately put the new topical agent to work is in treating disease on the face and other sensitive skin areas. The drug’s steroid-sparing ability is also a major asset, given how many parents of children with AD are steroid phobic.
“I’m certainly not going to give up on topical steroids, but anything that enables me to lessen their use is a comfort for parents. That’s really important,” observed Dr. Tom of the University of California, San Diego, and Rady Children’s Hospital.
Both pediatric dermatologists reported having received research grants from or serving as consultants to roughly half-a-dozen pharmaceutical companies, including Anacor/Pfizer, which markets crisaborole.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
Gender impacts risk of atopic diseases
ATLANTA – Obese females who live in urban settings are significantly more likely to develop atopic diseases, compared with their male counterparts, results from a single-center study showed.
“Some research has shown that obese girls are much more likely to be atopic, but many of them only look at one disease alone, such as atopic dermatitis or food allergies,” lead study author Sairaman Nagarajan, MD, MPH, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “Most of the studies are in asthma.”
The mean age of patients was 9 years, 23% were obese, and 55% were male. The researchers observed no differences in laboratory biomarkers nor in the prevalence of individual/or cumulative atopic disease in obese children, compared with controls. When stratified by gender, obese females had a significantly higher mean atopic disease score, compared with controls (4.00 vs. 2.62, respectively; P less than .001), while males had a significantly lower mean atopic disease score, compared with controls (3 vs. 3.42; P less than .001). Regression models yielded similar results; obese females had a significantly higher mean atopic disease score, compared with controls (by a mean elevation of 1.37 points; P less than .005), while males had a significantly lower mean atopic disease score (by a mean decline of -0.42 points; P less than .006). “From this we can say that female gender is a positive risk factor for atopy, and urban obese females may be particularly likely to benefit from lifestyle modification therapy like exercise and diet in controlling weight, and thereby allergies,” Dr. Nagarajan said.
He noted that it remains unclear whether the findings would apply to children who live in nonurban settings. “It’s difficult to say because there are some variables which are unique to urban minority populations like the housing that they live in and the kind of stores they buy food from,” he said.
Dr. Nagarajan reported having no financial disclosures.
ATLANTA – Obese females who live in urban settings are significantly more likely to develop atopic diseases, compared with their male counterparts, results from a single-center study showed.
“Some research has shown that obese girls are much more likely to be atopic, but many of them only look at one disease alone, such as atopic dermatitis or food allergies,” lead study author Sairaman Nagarajan, MD, MPH, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “Most of the studies are in asthma.”
The mean age of patients was 9 years, 23% were obese, and 55% were male. The researchers observed no differences in laboratory biomarkers nor in the prevalence of individual/or cumulative atopic disease in obese children, compared with controls. When stratified by gender, obese females had a significantly higher mean atopic disease score, compared with controls (4.00 vs. 2.62, respectively; P less than .001), while males had a significantly lower mean atopic disease score, compared with controls (3 vs. 3.42; P less than .001). Regression models yielded similar results; obese females had a significantly higher mean atopic disease score, compared with controls (by a mean elevation of 1.37 points; P less than .005), while males had a significantly lower mean atopic disease score (by a mean decline of -0.42 points; P less than .006). “From this we can say that female gender is a positive risk factor for atopy, and urban obese females may be particularly likely to benefit from lifestyle modification therapy like exercise and diet in controlling weight, and thereby allergies,” Dr. Nagarajan said.
He noted that it remains unclear whether the findings would apply to children who live in nonurban settings. “It’s difficult to say because there are some variables which are unique to urban minority populations like the housing that they live in and the kind of stores they buy food from,” he said.
Dr. Nagarajan reported having no financial disclosures.
ATLANTA – Obese females who live in urban settings are significantly more likely to develop atopic diseases, compared with their male counterparts, results from a single-center study showed.
“Some research has shown that obese girls are much more likely to be atopic, but many of them only look at one disease alone, such as atopic dermatitis or food allergies,” lead study author Sairaman Nagarajan, MD, MPH, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “Most of the studies are in asthma.”
The mean age of patients was 9 years, 23% were obese, and 55% were male. The researchers observed no differences in laboratory biomarkers nor in the prevalence of individual/or cumulative atopic disease in obese children, compared with controls. When stratified by gender, obese females had a significantly higher mean atopic disease score, compared with controls (4.00 vs. 2.62, respectively; P less than .001), while males had a significantly lower mean atopic disease score, compared with controls (3 vs. 3.42; P less than .001). Regression models yielded similar results; obese females had a significantly higher mean atopic disease score, compared with controls (by a mean elevation of 1.37 points; P less than .005), while males had a significantly lower mean atopic disease score (by a mean decline of -0.42 points; P less than .006). “From this we can say that female gender is a positive risk factor for atopy, and urban obese females may be particularly likely to benefit from lifestyle modification therapy like exercise and diet in controlling weight, and thereby allergies,” Dr. Nagarajan said.
He noted that it remains unclear whether the findings would apply to children who live in nonurban settings. “It’s difficult to say because there are some variables which are unique to urban minority populations like the housing that they live in and the kind of stores they buy food from,” he said.
Dr. Nagarajan reported having no financial disclosures.
AT 2017 AAAAI ANNUAL MEETING
Key clinical point:
Major finding: When stratified by gender, obese females had a significantly higher mean atopic disease score, compared with controls (4 vs. 2.62, respectively; P less than .001), while males had a significantly lower mean atopic disease score, compared with controls (3 vs. 3.42; P less than .001).
Data source: A retrospective review of 113 children who were evaluated for a history of allergic rhinitis, eczema, asthma, food allergies, and IgE, the percentage of eosinophils, and absolute eosinophil counts.
Disclosures: Dr. Nagarajan reported having no financial disclosures.