User login
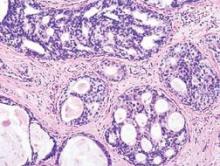
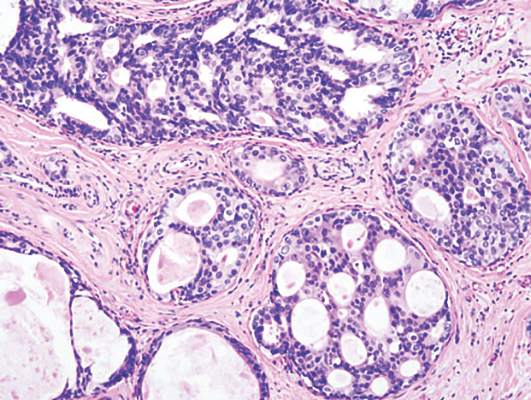
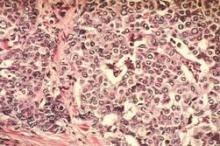
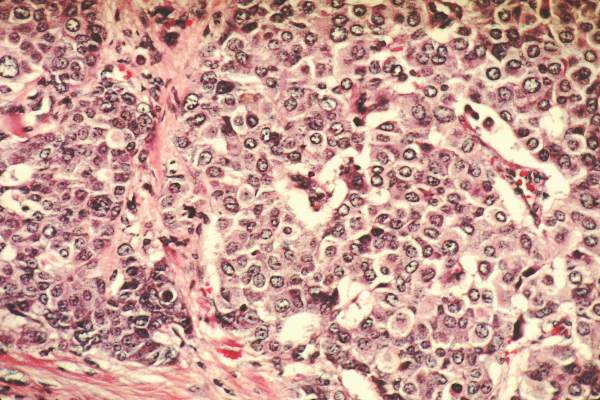
Radiation + lumpectomy tied to increased survival in older women with TNBC
Older women with triple-negative breast cancer appear to get an overall survival and disease-specific survival benefit with the addition of radiation to breast-conserving surgery, authors of a retrospective study said.
Among 974 women aged 70 and above with T1-2, N0, M0 triple-negative breast cancer (TNBC; lacking the Her2-neu, estrogen, and progesterone receptors), the addition of radiation to lumpectomy was associated at 23 months’ follow-up with an overall survival (OS) rate of 98.2%, compared with 85.6% for women who received lumpectomy alone (P less than .001). Respective rates of disease-specific survival (DSS) were 99% and 94% (P = .003).
“The use of adjuvant radiation therapy after lumpectomy for elderly women with early-stage TNBC was associated with improved OS and DSS. Noting the potential for selection bias in this study, future prospective study is required to define the management of early-stage triple-negative breast cancer,” wrote Dr. Sean Szjea and colleagues at the University of Texas, Galveston, in a meeting abstract. The study will be presented at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
It’s known that older women with estrogen-receptor positive disease can have good clinical outcomes with lumpectomy and adjuvant therapy alone, but whether adding radiation to breast-conserving surgery in older women with TNBC offers clinical benefit is less certain, the investigators said, prompting them to dive for data into the Surveillance, Epidemiology, and End Results database.
They collected information on 974 women aged 70 or older who underwent lumpectomy for early-stage TNBC with no nodal invasion or metastatic disease from 2010 through 2011. Of this group, 662 (68%) also received radiation therapy.
In addition to determining the OS and DSS rates in the overall population, the investigators conducted multivariate regression modeling controlling for confounding variables, including the use of neoadjuvant chemotherapy, the number of lymph nodes sampled, age, laterality, grade, T stage, the extent of surgery, and the existence of other cancers. They found that the survival benefit for radiation held for both OS (hazard ratio [HR], 0.14; P less than .001) and DSS (HR, 0.14; P = .01).
The authors reported having no relevant financial disclosures.
Older women with triple-negative breast cancer appear to get an overall survival and disease-specific survival benefit with the addition of radiation to breast-conserving surgery, authors of a retrospective study said.
Among 974 women aged 70 and above with T1-2, N0, M0 triple-negative breast cancer (TNBC; lacking the Her2-neu, estrogen, and progesterone receptors), the addition of radiation to lumpectomy was associated at 23 months’ follow-up with an overall survival (OS) rate of 98.2%, compared with 85.6% for women who received lumpectomy alone (P less than .001). Respective rates of disease-specific survival (DSS) were 99% and 94% (P = .003).
“The use of adjuvant radiation therapy after lumpectomy for elderly women with early-stage TNBC was associated with improved OS and DSS. Noting the potential for selection bias in this study, future prospective study is required to define the management of early-stage triple-negative breast cancer,” wrote Dr. Sean Szjea and colleagues at the University of Texas, Galveston, in a meeting abstract. The study will be presented at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
It’s known that older women with estrogen-receptor positive disease can have good clinical outcomes with lumpectomy and adjuvant therapy alone, but whether adding radiation to breast-conserving surgery in older women with TNBC offers clinical benefit is less certain, the investigators said, prompting them to dive for data into the Surveillance, Epidemiology, and End Results database.
They collected information on 974 women aged 70 or older who underwent lumpectomy for early-stage TNBC with no nodal invasion or metastatic disease from 2010 through 2011. Of this group, 662 (68%) also received radiation therapy.
In addition to determining the OS and DSS rates in the overall population, the investigators conducted multivariate regression modeling controlling for confounding variables, including the use of neoadjuvant chemotherapy, the number of lymph nodes sampled, age, laterality, grade, T stage, the extent of surgery, and the existence of other cancers. They found that the survival benefit for radiation held for both OS (hazard ratio [HR], 0.14; P less than .001) and DSS (HR, 0.14; P = .01).
The authors reported having no relevant financial disclosures.
Older women with triple-negative breast cancer appear to get an overall survival and disease-specific survival benefit with the addition of radiation to breast-conserving surgery, authors of a retrospective study said.
Among 974 women aged 70 and above with T1-2, N0, M0 triple-negative breast cancer (TNBC; lacking the Her2-neu, estrogen, and progesterone receptors), the addition of radiation to lumpectomy was associated at 23 months’ follow-up with an overall survival (OS) rate of 98.2%, compared with 85.6% for women who received lumpectomy alone (P less than .001). Respective rates of disease-specific survival (DSS) were 99% and 94% (P = .003).
“The use of adjuvant radiation therapy after lumpectomy for elderly women with early-stage TNBC was associated with improved OS and DSS. Noting the potential for selection bias in this study, future prospective study is required to define the management of early-stage triple-negative breast cancer,” wrote Dr. Sean Szjea and colleagues at the University of Texas, Galveston, in a meeting abstract. The study will be presented at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
It’s known that older women with estrogen-receptor positive disease can have good clinical outcomes with lumpectomy and adjuvant therapy alone, but whether adding radiation to breast-conserving surgery in older women with TNBC offers clinical benefit is less certain, the investigators said, prompting them to dive for data into the Surveillance, Epidemiology, and End Results database.
They collected information on 974 women aged 70 or older who underwent lumpectomy for early-stage TNBC with no nodal invasion or metastatic disease from 2010 through 2011. Of this group, 662 (68%) also received radiation therapy.
In addition to determining the OS and DSS rates in the overall population, the investigators conducted multivariate regression modeling controlling for confounding variables, including the use of neoadjuvant chemotherapy, the number of lymph nodes sampled, age, laterality, grade, T stage, the extent of surgery, and the existence of other cancers. They found that the survival benefit for radiation held for both OS (hazard ratio [HR], 0.14; P less than .001) and DSS (HR, 0.14; P = .01).
The authors reported having no relevant financial disclosures.
FROM THE 2015 ASCO BREAST CANCER SYMPOSIUM
Key clinical point: Radiation added to breast-conserving surgery in women 70 and older with early-stage triple-negative breast cancer was associated with improved survival.
Major finding: Overall survival with radiation and lumpectomy was 98.2%, compared with 85.6% for women who received lumpectomy alone (P less than .001)
Data source: A prospective database review of 974 women aged 70 and older treated from 2010 through 2011.
Disclosures: The authors reported having no relevant financial disclosures.
DCIS recurrences have declined significantly
Recurrence rates of ductal carcinoma in situ (DCIS ) have declined significantly over 3 decades, with the improvements likely because of better screening and pathologic assessment, investigators say.
A retrospective review of 2,996 women who underwent breast-conserving surgery (BCS) for DCIS at Memorial Sloan Kettering Cancer Center in New York from 1978 through 2010 showed that there were 363 recurrences within 5 years of treatment (12%). The 5-year recurrence rate for women treated from 1978 through 1998 was 13.6%, compared with 6.6% for women treated from 1999 through 2010.
The hazard ratio (HR) for more recent treatment was 0.62 (P less than .0001), report Dr. Kimberly J. Van Zee and colleagues at MSKCC in an oral abstract to be presented at the symposium sponsored by the American Society of Clinical Oncology.
The period of treatment remained a significant predictor of lower recurrence after multivariate analysis adjusting for age, family history, radiologic vs. clinical presentation, nuclear grade (nonhigh vs. high), necrosis, number of excisions (two or few vs. three or more), margin status (positive or close margins vs. negative), radiation, and use of endocrine therapy (HR 0.74, P = 0.02).
When the investigators stratified patients by whether they received radiation in addition to BCS, they found that recurrence rates dropped only for those patients who did not receive radiation (HR 0.62, P = 0.003). Among patients who received radiation, recurrence rates did not decline over time (HR 1.13, P = 0.6).
The authors conclude that the lower risk of recurrence over time is only partially explained by changes in clinical practice, such as screening, emphasis on negative surgical margins, and use of adjuvant therapies. The fact that the decline was seen only for women who did not receive radiation suggests that the improvements were not attributable to improvement in radiation therapy techniques. Rather, they may be due to improvements in mammography and in pathologic assessment, the investigators maintain.
“The lower recurrence risk observed for DCIS patients treated in more recent years is important for patient education, especially in view of the widely reported recent increase in use of mastectomy,” they write in the study abstract.
Recurrence rates of ductal carcinoma in situ (DCIS ) have declined significantly over 3 decades, with the improvements likely because of better screening and pathologic assessment, investigators say.
A retrospective review of 2,996 women who underwent breast-conserving surgery (BCS) for DCIS at Memorial Sloan Kettering Cancer Center in New York from 1978 through 2010 showed that there were 363 recurrences within 5 years of treatment (12%). The 5-year recurrence rate for women treated from 1978 through 1998 was 13.6%, compared with 6.6% for women treated from 1999 through 2010.
The hazard ratio (HR) for more recent treatment was 0.62 (P less than .0001), report Dr. Kimberly J. Van Zee and colleagues at MSKCC in an oral abstract to be presented at the symposium sponsored by the American Society of Clinical Oncology.
The period of treatment remained a significant predictor of lower recurrence after multivariate analysis adjusting for age, family history, radiologic vs. clinical presentation, nuclear grade (nonhigh vs. high), necrosis, number of excisions (two or few vs. three or more), margin status (positive or close margins vs. negative), radiation, and use of endocrine therapy (HR 0.74, P = 0.02).
When the investigators stratified patients by whether they received radiation in addition to BCS, they found that recurrence rates dropped only for those patients who did not receive radiation (HR 0.62, P = 0.003). Among patients who received radiation, recurrence rates did not decline over time (HR 1.13, P = 0.6).
The authors conclude that the lower risk of recurrence over time is only partially explained by changes in clinical practice, such as screening, emphasis on negative surgical margins, and use of adjuvant therapies. The fact that the decline was seen only for women who did not receive radiation suggests that the improvements were not attributable to improvement in radiation therapy techniques. Rather, they may be due to improvements in mammography and in pathologic assessment, the investigators maintain.
“The lower recurrence risk observed for DCIS patients treated in more recent years is important for patient education, especially in view of the widely reported recent increase in use of mastectomy,” they write in the study abstract.
Recurrence rates of ductal carcinoma in situ (DCIS ) have declined significantly over 3 decades, with the improvements likely because of better screening and pathologic assessment, investigators say.
A retrospective review of 2,996 women who underwent breast-conserving surgery (BCS) for DCIS at Memorial Sloan Kettering Cancer Center in New York from 1978 through 2010 showed that there were 363 recurrences within 5 years of treatment (12%). The 5-year recurrence rate for women treated from 1978 through 1998 was 13.6%, compared with 6.6% for women treated from 1999 through 2010.
The hazard ratio (HR) for more recent treatment was 0.62 (P less than .0001), report Dr. Kimberly J. Van Zee and colleagues at MSKCC in an oral abstract to be presented at the symposium sponsored by the American Society of Clinical Oncology.
The period of treatment remained a significant predictor of lower recurrence after multivariate analysis adjusting for age, family history, radiologic vs. clinical presentation, nuclear grade (nonhigh vs. high), necrosis, number of excisions (two or few vs. three or more), margin status (positive or close margins vs. negative), radiation, and use of endocrine therapy (HR 0.74, P = 0.02).
When the investigators stratified patients by whether they received radiation in addition to BCS, they found that recurrence rates dropped only for those patients who did not receive radiation (HR 0.62, P = 0.003). Among patients who received radiation, recurrence rates did not decline over time (HR 1.13, P = 0.6).
The authors conclude that the lower risk of recurrence over time is only partially explained by changes in clinical practice, such as screening, emphasis on negative surgical margins, and use of adjuvant therapies. The fact that the decline was seen only for women who did not receive radiation suggests that the improvements were not attributable to improvement in radiation therapy techniques. Rather, they may be due to improvements in mammography and in pathologic assessment, the investigators maintain.
“The lower recurrence risk observed for DCIS patients treated in more recent years is important for patient education, especially in view of the widely reported recent increase in use of mastectomy,” they write in the study abstract.
Key clinical point: Recurrence rates of DCIS in patients treated with breast-conserving surgery declined significantly from 1978 through 2010.
Major finding: The 5-year recurrence rate for women treated from 1978 through 1998 was 13.6%, compared with 6.6% for women treated from 1999 through 2010.
Data source: Retrospective case review of 2,996 women with DCIS treated with breast-conserving surgery with or without radiation.
Disclosures: The funding source was not disclosed. Coauthor Dr. Monica Morrow disclosed receiving honoraria from Genomic Health.
LCIS: 2% annual cancer risk, less than 1% with chemoprevention
Women with lobular carcinoma in situ (LCIS) showed a 2% annual risk of developing breast cancer, and women who chose chemoprevention reduced their annual cancer rate to less than 1%, in a single-center longitudinal study published online in Journal of Clinical Oncology.
LCIS is known to raise the risk of developing ductal carcinoma in situ or invasive carcinoma substantially, but estimates vary widely. In addition, no specific risk factors have been linked to disease progression. To determine the absolute risk of developing breast cancer over time and to explore the clinicopathological features that predispose patients to progression, researchers performed a longitudinal analysis of data concerning 1,060 patients diagnosed between 1980 and 2009 at Memorial Sloan Kettering Cancer Center, New York.
Most patients (831, 78%) chose to manage their condition with surveillance alone, 175 (17%) chose surveillance plus chemoprevention using a selective estrogen receptor modulator or an aromatase inhibitor, and the remaining 56 patients (5%) opted for bilateral prophylactic mastectomy. Overall, 150 of these women developed 168 breast cancers during a median follow-up of 81 months (range, 6-368 months), said Dr. Tari A. King and her associates at Memorial Sloan Kettering.
The annual incidence of breast cancer was 2% per year for the entire study population, with no indication that risk would plateau. Overall cumulative cancer incidence was 26%, similar to that reported in a National Surgical Adjuvant Breast and Bowel Project study. The use of chemoprevention was strongly associated with a reduction in breast cancer risk (HR, 0.27); the minority of women who chose this option reduced their annual cancer rate to less than 1%. This finding supports the current recommendation to use chemoprevention and highlights the significant impact of proper patient education and counseling, the researchers noted (J Clin Oncol. 2015 Sep 14. doi:10.1200/JCO.2015.61.4743).
Unexpectedly, none of the clinicopathological features commonly thought to influence cancer risk – age at diagnosis, family history, breast density, menopausal status, presence of synchronous atypical hyperplasia, or presence of bilateral LCIS – were risk factors. Only tumor volume, as measured by the ratio of the number of slides with LCIS to the total number of slides available, was significantly greater in women whose disease progressed (0.5 to 1) than in women whose disease did not (0.3 to 1).
The National Cancer Institute, the Walsh Family Fund, and the Cary Grossman Breast Research Fellowship supported the study. Dr. King reported having no conflicts of interest; one of her associates reported receiving honoraria from Genomic Health.
Women with lobular carcinoma in situ (LCIS) showed a 2% annual risk of developing breast cancer, and women who chose chemoprevention reduced their annual cancer rate to less than 1%, in a single-center longitudinal study published online in Journal of Clinical Oncology.
LCIS is known to raise the risk of developing ductal carcinoma in situ or invasive carcinoma substantially, but estimates vary widely. In addition, no specific risk factors have been linked to disease progression. To determine the absolute risk of developing breast cancer over time and to explore the clinicopathological features that predispose patients to progression, researchers performed a longitudinal analysis of data concerning 1,060 patients diagnosed between 1980 and 2009 at Memorial Sloan Kettering Cancer Center, New York.
Most patients (831, 78%) chose to manage their condition with surveillance alone, 175 (17%) chose surveillance plus chemoprevention using a selective estrogen receptor modulator or an aromatase inhibitor, and the remaining 56 patients (5%) opted for bilateral prophylactic mastectomy. Overall, 150 of these women developed 168 breast cancers during a median follow-up of 81 months (range, 6-368 months), said Dr. Tari A. King and her associates at Memorial Sloan Kettering.
The annual incidence of breast cancer was 2% per year for the entire study population, with no indication that risk would plateau. Overall cumulative cancer incidence was 26%, similar to that reported in a National Surgical Adjuvant Breast and Bowel Project study. The use of chemoprevention was strongly associated with a reduction in breast cancer risk (HR, 0.27); the minority of women who chose this option reduced their annual cancer rate to less than 1%. This finding supports the current recommendation to use chemoprevention and highlights the significant impact of proper patient education and counseling, the researchers noted (J Clin Oncol. 2015 Sep 14. doi:10.1200/JCO.2015.61.4743).
Unexpectedly, none of the clinicopathological features commonly thought to influence cancer risk – age at diagnosis, family history, breast density, menopausal status, presence of synchronous atypical hyperplasia, or presence of bilateral LCIS – were risk factors. Only tumor volume, as measured by the ratio of the number of slides with LCIS to the total number of slides available, was significantly greater in women whose disease progressed (0.5 to 1) than in women whose disease did not (0.3 to 1).
The National Cancer Institute, the Walsh Family Fund, and the Cary Grossman Breast Research Fellowship supported the study. Dr. King reported having no conflicts of interest; one of her associates reported receiving honoraria from Genomic Health.
Women with lobular carcinoma in situ (LCIS) showed a 2% annual risk of developing breast cancer, and women who chose chemoprevention reduced their annual cancer rate to less than 1%, in a single-center longitudinal study published online in Journal of Clinical Oncology.
LCIS is known to raise the risk of developing ductal carcinoma in situ or invasive carcinoma substantially, but estimates vary widely. In addition, no specific risk factors have been linked to disease progression. To determine the absolute risk of developing breast cancer over time and to explore the clinicopathological features that predispose patients to progression, researchers performed a longitudinal analysis of data concerning 1,060 patients diagnosed between 1980 and 2009 at Memorial Sloan Kettering Cancer Center, New York.
Most patients (831, 78%) chose to manage their condition with surveillance alone, 175 (17%) chose surveillance plus chemoprevention using a selective estrogen receptor modulator or an aromatase inhibitor, and the remaining 56 patients (5%) opted for bilateral prophylactic mastectomy. Overall, 150 of these women developed 168 breast cancers during a median follow-up of 81 months (range, 6-368 months), said Dr. Tari A. King and her associates at Memorial Sloan Kettering.
The annual incidence of breast cancer was 2% per year for the entire study population, with no indication that risk would plateau. Overall cumulative cancer incidence was 26%, similar to that reported in a National Surgical Adjuvant Breast and Bowel Project study. The use of chemoprevention was strongly associated with a reduction in breast cancer risk (HR, 0.27); the minority of women who chose this option reduced their annual cancer rate to less than 1%. This finding supports the current recommendation to use chemoprevention and highlights the significant impact of proper patient education and counseling, the researchers noted (J Clin Oncol. 2015 Sep 14. doi:10.1200/JCO.2015.61.4743).
Unexpectedly, none of the clinicopathological features commonly thought to influence cancer risk – age at diagnosis, family history, breast density, menopausal status, presence of synchronous atypical hyperplasia, or presence of bilateral LCIS – were risk factors. Only tumor volume, as measured by the ratio of the number of slides with LCIS to the total number of slides available, was significantly greater in women whose disease progressed (0.5 to 1) than in women whose disease did not (0.3 to 1).
The National Cancer Institute, the Walsh Family Fund, and the Cary Grossman Breast Research Fellowship supported the study. Dr. King reported having no conflicts of interest; one of her associates reported receiving honoraria from Genomic Health.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Women with lobular carcinoma in situ showed a 2% annual risk of developing breast cancer and a cumulative risk of 26% at 15 years, and chemoprevention lowers cancer risk.
Major finding: Chemoprevention reduced breast cancer risk (HR, 0.27), so that women who chose this option reduced their annual cancer rate to less than 1%.
Data source: A single-center longitudinal cohort study involving 1,060 patients diagnosed during 1980-2009 and followed for a median of 81 months.
Disclosures: The National Cancer Institute, the Walsh Family Fund, and the Cary Grossman Breast Research Fellowship supported the study. Dr. King reported having no conflicts of interest; one of her associates reported receiving honoraria from Genomic Health.
More genomic instability found in breast tumors of black women
Several differences between black and white women in the genotypic traits of their breast tumors have been identified and may explain, at least in part, the greater aggressiveness of breast cancer in black women, according to a report published online Sept. 14 in Journal of Clinical Oncology.
Researchers analyzed data from The Cancer Genome Atlas in what they described as the first study to systematically characterize the racial pattern of genomic and gene expression traits in primary breast tumors and to examine the association of these traits with tumor recurrence. They focused on 1,374 samples from white and 264 samples from black patients with stage I, II, or III breast cancer diagnosed in 1988-2013, reported Dr. Tanya Keenan of Massachusetts General Hospital and Harvard Medical School, Boston, and her associates.
The five most frequent mutations—in the TP53, PIK3CA, CDH1, GATA3, MLLT3, and MAP3K1 genes – were the same between black women and white women, but they occurred in different frequencies by race. Compared with white women, black women had significantly more TP53 mutations, more PAM50 basal tumors, and more TNBC basal-like 1 and mesenchymal stem–like tumors, all of which signal a more aggressive tumor biology. These differences also “might have implications as genotype-driven targeted therapies are developed,” the investigators said.
In addition, the breast cancers in black women had significantly greater intratumor genetic heterogeneity, which “may reflect either greater underlying genomic instability or more exposure to epigenetic or environmental agents of DNA damage. Either way, the greater genomic diversity within African American tumors suggests a greater capacity for clonal evolution that may contribute to aggressive or therapy-resistant disease,” they noted (J Clin Oncol. 2015 Sep 14. doi: 10.1200/JCO.2015.62.2126).
Black women had a higher risk of breast cancer recurrence than white women, which was greatly attenuated in multivariable analyses that controlled for their excess of TP53 mutations and PAM50 basal subtypes. This also suggests that differences in tumor genetics account, at least in part, for the known disparity in survival outcomes between the races, Dr. Keenan and her associates said.
Several differences between black and white women in the genotypic traits of their breast tumors have been identified and may explain, at least in part, the greater aggressiveness of breast cancer in black women, according to a report published online Sept. 14 in Journal of Clinical Oncology.
Researchers analyzed data from The Cancer Genome Atlas in what they described as the first study to systematically characterize the racial pattern of genomic and gene expression traits in primary breast tumors and to examine the association of these traits with tumor recurrence. They focused on 1,374 samples from white and 264 samples from black patients with stage I, II, or III breast cancer diagnosed in 1988-2013, reported Dr. Tanya Keenan of Massachusetts General Hospital and Harvard Medical School, Boston, and her associates.
The five most frequent mutations—in the TP53, PIK3CA, CDH1, GATA3, MLLT3, and MAP3K1 genes – were the same between black women and white women, but they occurred in different frequencies by race. Compared with white women, black women had significantly more TP53 mutations, more PAM50 basal tumors, and more TNBC basal-like 1 and mesenchymal stem–like tumors, all of which signal a more aggressive tumor biology. These differences also “might have implications as genotype-driven targeted therapies are developed,” the investigators said.
In addition, the breast cancers in black women had significantly greater intratumor genetic heterogeneity, which “may reflect either greater underlying genomic instability or more exposure to epigenetic or environmental agents of DNA damage. Either way, the greater genomic diversity within African American tumors suggests a greater capacity for clonal evolution that may contribute to aggressive or therapy-resistant disease,” they noted (J Clin Oncol. 2015 Sep 14. doi: 10.1200/JCO.2015.62.2126).
Black women had a higher risk of breast cancer recurrence than white women, which was greatly attenuated in multivariable analyses that controlled for their excess of TP53 mutations and PAM50 basal subtypes. This also suggests that differences in tumor genetics account, at least in part, for the known disparity in survival outcomes between the races, Dr. Keenan and her associates said.
Several differences between black and white women in the genotypic traits of their breast tumors have been identified and may explain, at least in part, the greater aggressiveness of breast cancer in black women, according to a report published online Sept. 14 in Journal of Clinical Oncology.
Researchers analyzed data from The Cancer Genome Atlas in what they described as the first study to systematically characterize the racial pattern of genomic and gene expression traits in primary breast tumors and to examine the association of these traits with tumor recurrence. They focused on 1,374 samples from white and 264 samples from black patients with stage I, II, or III breast cancer diagnosed in 1988-2013, reported Dr. Tanya Keenan of Massachusetts General Hospital and Harvard Medical School, Boston, and her associates.
The five most frequent mutations—in the TP53, PIK3CA, CDH1, GATA3, MLLT3, and MAP3K1 genes – were the same between black women and white women, but they occurred in different frequencies by race. Compared with white women, black women had significantly more TP53 mutations, more PAM50 basal tumors, and more TNBC basal-like 1 and mesenchymal stem–like tumors, all of which signal a more aggressive tumor biology. These differences also “might have implications as genotype-driven targeted therapies are developed,” the investigators said.
In addition, the breast cancers in black women had significantly greater intratumor genetic heterogeneity, which “may reflect either greater underlying genomic instability or more exposure to epigenetic or environmental agents of DNA damage. Either way, the greater genomic diversity within African American tumors suggests a greater capacity for clonal evolution that may contribute to aggressive or therapy-resistant disease,” they noted (J Clin Oncol. 2015 Sep 14. doi: 10.1200/JCO.2015.62.2126).
Black women had a higher risk of breast cancer recurrence than white women, which was greatly attenuated in multivariable analyses that controlled for their excess of TP53 mutations and PAM50 basal subtypes. This also suggests that differences in tumor genetics account, at least in part, for the known disparity in survival outcomes between the races, Dr. Keenan and her associates said.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Several differences in breast tumor genetics may explain the greater aggressiveness of breast cancer in black vs. white women.
Major finding: Compared with white women, black women had significantly more TP53 mutations, more PAM50 basal tumors, and more TNBC basal-like 1 and mesenchymal stem–like tumors, all of which signal a more aggressive tumor biology.
Data source: An analysis of tumor-specific genotypic traits in 1,374 breast cancer samples from white and 264 samples from black patients, collected in The Cancer Genome Atlas.
Disclosures: The sponsor of this study was not identified. Dr. Keenan reported having no relevant financial disclosures, and her associates reported ties to numerous industry sources.
Fulvestrant may best anastrozole as first-line therapy
Fulvestrant may extend survival longer than does anastrozole when used as first-line therapy for locally advanced or metastatic estrogen receptor–positive breast cancer, investigators reported online Sept. 14 in the Journal of Clinical Oncology.
Compared with anastrozole, fulvestrant reduced overall mortality risk by approximately 30% in a small international, open-label phase II clinical trial funded by the manufacturer. A double-blind phase III trial is now underway to confirm and extend these preliminary results, said Dr. Matthew J. Ellis of the Lester and Sue Smith Breast Center, Baylor University, Houston, and his associates.
In the phase II study, 102 postmenopausal women were randomly assigned to receive 500 mg fulvestrant and 103 to receive 1 mg anastrozole as first-line therapy at 62 medical centers in 9 countries. Median overall survival was 54.1 months with fulvestrant and 48.4 months with anastrozole, an extension of approximately 6 months (hazard ratio, 0.70). This difference persisted across all subgroup analyses and in a sensitivity analysis, the investigators said (J Clin Oncol. 2015 Sept. 14. doi: 10.1200/JCO.2015.61.5831).
No new safety or tolerability problems occurred. Two serious adverse events that were considered to be treatment related developed in the fulvestrant group: one case of hypertension and one case of pulmonary embolism.
It is relatively uncommon in countries with advanced health care for patients to present with advanced breast cancer that has never been treated with endocrine therapy. Given the high prevalence of the disease, however, this still “represents a numerically substantial patient population” in the West, and it continues to comprise a significant proportion of patients in developing countries, Dr. Ellis and his associates added.
This study was supported by AstraZeneca. Dr. Ellis reported ties to AstraZeneca, Bioclassifier, Pfizer, Novartis, and Celgene; his associates reported ties to numerous industry sources.
Fulvestrant may extend survival longer than does anastrozole when used as first-line therapy for locally advanced or metastatic estrogen receptor–positive breast cancer, investigators reported online Sept. 14 in the Journal of Clinical Oncology.
Compared with anastrozole, fulvestrant reduced overall mortality risk by approximately 30% in a small international, open-label phase II clinical trial funded by the manufacturer. A double-blind phase III trial is now underway to confirm and extend these preliminary results, said Dr. Matthew J. Ellis of the Lester and Sue Smith Breast Center, Baylor University, Houston, and his associates.
In the phase II study, 102 postmenopausal women were randomly assigned to receive 500 mg fulvestrant and 103 to receive 1 mg anastrozole as first-line therapy at 62 medical centers in 9 countries. Median overall survival was 54.1 months with fulvestrant and 48.4 months with anastrozole, an extension of approximately 6 months (hazard ratio, 0.70). This difference persisted across all subgroup analyses and in a sensitivity analysis, the investigators said (J Clin Oncol. 2015 Sept. 14. doi: 10.1200/JCO.2015.61.5831).
No new safety or tolerability problems occurred. Two serious adverse events that were considered to be treatment related developed in the fulvestrant group: one case of hypertension and one case of pulmonary embolism.
It is relatively uncommon in countries with advanced health care for patients to present with advanced breast cancer that has never been treated with endocrine therapy. Given the high prevalence of the disease, however, this still “represents a numerically substantial patient population” in the West, and it continues to comprise a significant proportion of patients in developing countries, Dr. Ellis and his associates added.
This study was supported by AstraZeneca. Dr. Ellis reported ties to AstraZeneca, Bioclassifier, Pfizer, Novartis, and Celgene; his associates reported ties to numerous industry sources.
Fulvestrant may extend survival longer than does anastrozole when used as first-line therapy for locally advanced or metastatic estrogen receptor–positive breast cancer, investigators reported online Sept. 14 in the Journal of Clinical Oncology.
Compared with anastrozole, fulvestrant reduced overall mortality risk by approximately 30% in a small international, open-label phase II clinical trial funded by the manufacturer. A double-blind phase III trial is now underway to confirm and extend these preliminary results, said Dr. Matthew J. Ellis of the Lester and Sue Smith Breast Center, Baylor University, Houston, and his associates.
In the phase II study, 102 postmenopausal women were randomly assigned to receive 500 mg fulvestrant and 103 to receive 1 mg anastrozole as first-line therapy at 62 medical centers in 9 countries. Median overall survival was 54.1 months with fulvestrant and 48.4 months with anastrozole, an extension of approximately 6 months (hazard ratio, 0.70). This difference persisted across all subgroup analyses and in a sensitivity analysis, the investigators said (J Clin Oncol. 2015 Sept. 14. doi: 10.1200/JCO.2015.61.5831).
No new safety or tolerability problems occurred. Two serious adverse events that were considered to be treatment related developed in the fulvestrant group: one case of hypertension and one case of pulmonary embolism.
It is relatively uncommon in countries with advanced health care for patients to present with advanced breast cancer that has never been treated with endocrine therapy. Given the high prevalence of the disease, however, this still “represents a numerically substantial patient population” in the West, and it continues to comprise a significant proportion of patients in developing countries, Dr. Ellis and his associates added.
This study was supported by AstraZeneca. Dr. Ellis reported ties to AstraZeneca, Bioclassifier, Pfizer, Novartis, and Celgene; his associates reported ties to numerous industry sources.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Fulvestrant may extend survival better than anastrozole does as first-line therapy for advanced ER-positive breast cancer.
Major finding: Median overall survival was 54.1 months with fulvestrant and 48.4 months with anastrozole, an extension of approximately 6 months (HR, 0.70).
Data source: An industry-sponsored, international, randomized, open-label phase-II trial involving 205 postmenopausal women with ER-positive locally advanced or metastatic breast cancer.
Disclosures: This study was supported by AstraZeneca. Dr. Ellis reported ties to AstraZeneca, Bioclassifier, Pfizer, Novartis, and Celgene; his associates reported ties to numerous industry sources.
DCIS: Recurrence risk rises after forgoing radiotherapy
The risk of recurrence continues to rise for at least 12 years among patients with low-risk DCIS who forgo radiotherapy after undergoing lumpectomy, investigators reported online Sept. 14 in Journal of Clinical Oncology.
These investigators performed an extended follow-up of 665 patients who participated in an Eastern Cooperative Oncology Group–American College of Radiology Imaging Network nonrandomized trial in 1997-2002. All the participants had either a single low- or intermediate-grade tumor sized 2.5 cm or smaller (cohort 1, 561 patients) or a high-grade tumor 1 cm or smaller (cohort 2, 104 patients), and lumpectomy without radiotherapy was considered a reasonable treatment option for all based on favorable clinical and pathological characteristics. The median patient age was 59 years, and 30% of the study population received tamoxifen during follow-up, said Dr. Lawrence J. Solin of Albert Einstein Healthcare Network, Philadelphia, and his associates.
During a median of 12.3 years of follow-up, there were 99 recurrences: 48 cases of DCIS and 51 cases of invasive cancer in the ipsilateral breast. The 12-year rates of developing any recurrence were 14.4% in cohort 1 and 24.6% in cohort 2, and the 12-year rates of developing an invasive cancer were 7.5% and 13.4%, respectively. Most important, the risk of any recurrence increased steadily over time, without any plateau, the investigators said (J Clin Oncol. 2015 Sep 14. doi: 10.1200/JCO.2015.60.8588).
“Individual patients and their physicians will need to decide if these 12-year risks are acceptable, and whether or not to forgo adding adjuvant treatment after surgical excision,” Dr. Solin and his associates said.
Most of the study participants were postmenopausal and had small, asymptomatic microcalcifications detected on routine screening mammography; most also had negative margins at resection. Since these traits are similar to those of patients with DCIS in population-based studies, the findings of this trial are relevant to contemporary practice, the researchers added.
“Not all patients and their physicians will agree on what is considered too high a risk of developing [a recurrence] or [an invasive recurrence] to recommend observation after surgical excision, or what risk is considered too low to justify adding radiation treatment. However, this study provides 12-year data to begin those discussions, and to help inform the treatment decision-making process,” Dr. Solin and his associates said.
The risk of recurrence continues to rise for at least 12 years among patients with low-risk DCIS who forgo radiotherapy after undergoing lumpectomy, investigators reported online Sept. 14 in Journal of Clinical Oncology.
These investigators performed an extended follow-up of 665 patients who participated in an Eastern Cooperative Oncology Group–American College of Radiology Imaging Network nonrandomized trial in 1997-2002. All the participants had either a single low- or intermediate-grade tumor sized 2.5 cm or smaller (cohort 1, 561 patients) or a high-grade tumor 1 cm or smaller (cohort 2, 104 patients), and lumpectomy without radiotherapy was considered a reasonable treatment option for all based on favorable clinical and pathological characteristics. The median patient age was 59 years, and 30% of the study population received tamoxifen during follow-up, said Dr. Lawrence J. Solin of Albert Einstein Healthcare Network, Philadelphia, and his associates.
During a median of 12.3 years of follow-up, there were 99 recurrences: 48 cases of DCIS and 51 cases of invasive cancer in the ipsilateral breast. The 12-year rates of developing any recurrence were 14.4% in cohort 1 and 24.6% in cohort 2, and the 12-year rates of developing an invasive cancer were 7.5% and 13.4%, respectively. Most important, the risk of any recurrence increased steadily over time, without any plateau, the investigators said (J Clin Oncol. 2015 Sep 14. doi: 10.1200/JCO.2015.60.8588).
“Individual patients and their physicians will need to decide if these 12-year risks are acceptable, and whether or not to forgo adding adjuvant treatment after surgical excision,” Dr. Solin and his associates said.
Most of the study participants were postmenopausal and had small, asymptomatic microcalcifications detected on routine screening mammography; most also had negative margins at resection. Since these traits are similar to those of patients with DCIS in population-based studies, the findings of this trial are relevant to contemporary practice, the researchers added.
“Not all patients and their physicians will agree on what is considered too high a risk of developing [a recurrence] or [an invasive recurrence] to recommend observation after surgical excision, or what risk is considered too low to justify adding radiation treatment. However, this study provides 12-year data to begin those discussions, and to help inform the treatment decision-making process,” Dr. Solin and his associates said.
The risk of recurrence continues to rise for at least 12 years among patients with low-risk DCIS who forgo radiotherapy after undergoing lumpectomy, investigators reported online Sept. 14 in Journal of Clinical Oncology.
These investigators performed an extended follow-up of 665 patients who participated in an Eastern Cooperative Oncology Group–American College of Radiology Imaging Network nonrandomized trial in 1997-2002. All the participants had either a single low- or intermediate-grade tumor sized 2.5 cm or smaller (cohort 1, 561 patients) or a high-grade tumor 1 cm or smaller (cohort 2, 104 patients), and lumpectomy without radiotherapy was considered a reasonable treatment option for all based on favorable clinical and pathological characteristics. The median patient age was 59 years, and 30% of the study population received tamoxifen during follow-up, said Dr. Lawrence J. Solin of Albert Einstein Healthcare Network, Philadelphia, and his associates.
During a median of 12.3 years of follow-up, there were 99 recurrences: 48 cases of DCIS and 51 cases of invasive cancer in the ipsilateral breast. The 12-year rates of developing any recurrence were 14.4% in cohort 1 and 24.6% in cohort 2, and the 12-year rates of developing an invasive cancer were 7.5% and 13.4%, respectively. Most important, the risk of any recurrence increased steadily over time, without any plateau, the investigators said (J Clin Oncol. 2015 Sep 14. doi: 10.1200/JCO.2015.60.8588).
“Individual patients and their physicians will need to decide if these 12-year risks are acceptable, and whether or not to forgo adding adjuvant treatment after surgical excision,” Dr. Solin and his associates said.
Most of the study participants were postmenopausal and had small, asymptomatic microcalcifications detected on routine screening mammography; most also had negative margins at resection. Since these traits are similar to those of patients with DCIS in population-based studies, the findings of this trial are relevant to contemporary practice, the researchers added.
“Not all patients and their physicians will agree on what is considered too high a risk of developing [a recurrence] or [an invasive recurrence] to recommend observation after surgical excision, or what risk is considered too low to justify adding radiation treatment. However, this study provides 12-year data to begin those discussions, and to help inform the treatment decision-making process,” Dr. Solin and his associates said.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The risk of recurrence continues to rise for at least 12 years in patients with DCIS who had lumpectomy without radiotherapy.
Major finding: During a median of 12.3 years of follow-up, there were 99 recurrences: 48 cases of DCIS and 51 cases of invasive cancer in the ipsilateral breast.
Data source: Extended follow-up of a prospective clinical trial involving 665 patients with low-risk DCIS who underwent lumpectomy without radiotherapy.
Disclosures: This study was supported in part by the National Cancer Institute and the Breast Cancer Research Foundation. Dr. Solin reported having no financial disclosures; his associates reported ties to numerous industry sources.
For metastatic breast cancer, adding pertuzumab not cost-effective
Adding pertuzumab to standard combination therapy with docetaxel plus trastuzumab is not cost-effective in patients with metastatic HER2-positive breast cancer, according to a report published online Sept. 8 in Journal of Clinical Oncology.
Adding pertuzumab in this setting is highly effective at extending survival, although patients usually succumb to their disease eventually. And this approach is recommended as the first-line treatment of choice by the National Comprehensive Cancer Network. But its cost-effectiveness has never been studied in the United States until now, said Dr. Ben Y. Durkee of the department of radiation oncology at Stanford (Calif.) University.
Dr. Durkee and his associates assessed the cost-effectiveness of adding pertuzumab to standard treatment by incorporating into their statistical model such existing data as the annual incidence of metastatic HER2-positive breast cancer (17,450 patients per year), the direct and indirect costs of standard combination therapy alone ($347,627 per patient per year), and the direct and indirect costs of adding pertuzumab therapy ($805,449 per patient per year) until disease progressed or toxic effects became unmanageable. The model assigned hypothetical patients to four possible health states based on data in the literature – stable disease, progressing disease, hospice, and dead – and tracked each one weekly for their remaining lifetimes.
This model produced outcomes consistent with those reported in the literature regarding overall survival, progression-free survival, major toxicities, and time spent in each health state. Median overall survival was 39.4 months for standard treatment and 56.9 months with the addition of pertuzumab.
In this statistical model, adding pertuzumab yielded an extra 1.82 life-years per patient and 0.64 QALYs at a per-patient cost of $457,821. “Taken together, the addition of pertuzumab to combined docetaxel plus trastuzumab cost $713,219 per QALY gained,” which is “well above any commonly used threshold and well above the de facto threshold of cost-effectiveness for interventions already in practice,” Dr. Durkee and his associates noted (J Clin Oncol. 2015 Sep 8. doi:10.1200/JCO.2015.62.9105).
Willingness-to-pay thresholds in most analyses of medical treatments range from $50,000 to $160,000 per QALY. A sensitivity analysis of the data demonstrated a 0% chance of cost-effectiveness at a willingness-to-pay level of $100,000 per QALY gained. Even when the willingness-to-pay level rose to $500,000 per QALY, this analysis showed a 0% chance of cost-effectiveness, the researchers noted.
“This analysis highlights the economic challenges of extending life for patients with noncurable disease. It also typifies the broader observation that half of our health care dollars are spent on 5% of the population,” the investigators said.
The Henry S. Kaplan Research Fund, the department of radiation oncology at Stanford University, and the National Institute on Aging supported the study. Dr. Durkee reported having no conflicts of interest. An associate reported stock or other ownership with ViewRay.
The high cost of treatment in the study by Durkee et al. is not entirely driven by the price of pertuzumab. Treatment overall is costly for patients with metastatic breast cancer; when it is highly effective and must be continued for the patient’s entire life span, it adds enormous costs in addition to its important benefits.
 |
Dr. Peter B. Bach |
We face this same problem with other cancer therapies for which the ongoing cost of life-maintaining treatment is substantial – far more than, for example, the average per-capita gross domestic product. By definition, we cannot economically afford a larger and larger pool of people receiving expensive maintenance treatments. Yet the last thing we want would be to deny lifesaving therapy to anyone who could benefit from it.
Dr. Peter B. Bach is at the Center for Health Policy and Outcomes at Memorial Sloan Kettering Cancer Center, New York. His financial disclosures are available at www.jco.org. Dr. Bach made these remarks in an editorial accompanying Dr. Durkee’s report (J Clin Oncol. 2015 Sep 8. doi:10.1200/JCO.2015.63.7397).
The high cost of treatment in the study by Durkee et al. is not entirely driven by the price of pertuzumab. Treatment overall is costly for patients with metastatic breast cancer; when it is highly effective and must be continued for the patient’s entire life span, it adds enormous costs in addition to its important benefits.
 |
Dr. Peter B. Bach |
We face this same problem with other cancer therapies for which the ongoing cost of life-maintaining treatment is substantial – far more than, for example, the average per-capita gross domestic product. By definition, we cannot economically afford a larger and larger pool of people receiving expensive maintenance treatments. Yet the last thing we want would be to deny lifesaving therapy to anyone who could benefit from it.
Dr. Peter B. Bach is at the Center for Health Policy and Outcomes at Memorial Sloan Kettering Cancer Center, New York. His financial disclosures are available at www.jco.org. Dr. Bach made these remarks in an editorial accompanying Dr. Durkee’s report (J Clin Oncol. 2015 Sep 8. doi:10.1200/JCO.2015.63.7397).
The high cost of treatment in the study by Durkee et al. is not entirely driven by the price of pertuzumab. Treatment overall is costly for patients with metastatic breast cancer; when it is highly effective and must be continued for the patient’s entire life span, it adds enormous costs in addition to its important benefits.
 |
Dr. Peter B. Bach |
We face this same problem with other cancer therapies for which the ongoing cost of life-maintaining treatment is substantial – far more than, for example, the average per-capita gross domestic product. By definition, we cannot economically afford a larger and larger pool of people receiving expensive maintenance treatments. Yet the last thing we want would be to deny lifesaving therapy to anyone who could benefit from it.
Dr. Peter B. Bach is at the Center for Health Policy and Outcomes at Memorial Sloan Kettering Cancer Center, New York. His financial disclosures are available at www.jco.org. Dr. Bach made these remarks in an editorial accompanying Dr. Durkee’s report (J Clin Oncol. 2015 Sep 8. doi:10.1200/JCO.2015.63.7397).
Adding pertuzumab to standard combination therapy with docetaxel plus trastuzumab is not cost-effective in patients with metastatic HER2-positive breast cancer, according to a report published online Sept. 8 in Journal of Clinical Oncology.
Adding pertuzumab in this setting is highly effective at extending survival, although patients usually succumb to their disease eventually. And this approach is recommended as the first-line treatment of choice by the National Comprehensive Cancer Network. But its cost-effectiveness has never been studied in the United States until now, said Dr. Ben Y. Durkee of the department of radiation oncology at Stanford (Calif.) University.
Dr. Durkee and his associates assessed the cost-effectiveness of adding pertuzumab to standard treatment by incorporating into their statistical model such existing data as the annual incidence of metastatic HER2-positive breast cancer (17,450 patients per year), the direct and indirect costs of standard combination therapy alone ($347,627 per patient per year), and the direct and indirect costs of adding pertuzumab therapy ($805,449 per patient per year) until disease progressed or toxic effects became unmanageable. The model assigned hypothetical patients to four possible health states based on data in the literature – stable disease, progressing disease, hospice, and dead – and tracked each one weekly for their remaining lifetimes.
This model produced outcomes consistent with those reported in the literature regarding overall survival, progression-free survival, major toxicities, and time spent in each health state. Median overall survival was 39.4 months for standard treatment and 56.9 months with the addition of pertuzumab.
In this statistical model, adding pertuzumab yielded an extra 1.82 life-years per patient and 0.64 QALYs at a per-patient cost of $457,821. “Taken together, the addition of pertuzumab to combined docetaxel plus trastuzumab cost $713,219 per QALY gained,” which is “well above any commonly used threshold and well above the de facto threshold of cost-effectiveness for interventions already in practice,” Dr. Durkee and his associates noted (J Clin Oncol. 2015 Sep 8. doi:10.1200/JCO.2015.62.9105).
Willingness-to-pay thresholds in most analyses of medical treatments range from $50,000 to $160,000 per QALY. A sensitivity analysis of the data demonstrated a 0% chance of cost-effectiveness at a willingness-to-pay level of $100,000 per QALY gained. Even when the willingness-to-pay level rose to $500,000 per QALY, this analysis showed a 0% chance of cost-effectiveness, the researchers noted.
“This analysis highlights the economic challenges of extending life for patients with noncurable disease. It also typifies the broader observation that half of our health care dollars are spent on 5% of the population,” the investigators said.
The Henry S. Kaplan Research Fund, the department of radiation oncology at Stanford University, and the National Institute on Aging supported the study. Dr. Durkee reported having no conflicts of interest. An associate reported stock or other ownership with ViewRay.
Adding pertuzumab to standard combination therapy with docetaxel plus trastuzumab is not cost-effective in patients with metastatic HER2-positive breast cancer, according to a report published online Sept. 8 in Journal of Clinical Oncology.
Adding pertuzumab in this setting is highly effective at extending survival, although patients usually succumb to their disease eventually. And this approach is recommended as the first-line treatment of choice by the National Comprehensive Cancer Network. But its cost-effectiveness has never been studied in the United States until now, said Dr. Ben Y. Durkee of the department of radiation oncology at Stanford (Calif.) University.
Dr. Durkee and his associates assessed the cost-effectiveness of adding pertuzumab to standard treatment by incorporating into their statistical model such existing data as the annual incidence of metastatic HER2-positive breast cancer (17,450 patients per year), the direct and indirect costs of standard combination therapy alone ($347,627 per patient per year), and the direct and indirect costs of adding pertuzumab therapy ($805,449 per patient per year) until disease progressed or toxic effects became unmanageable. The model assigned hypothetical patients to four possible health states based on data in the literature – stable disease, progressing disease, hospice, and dead – and tracked each one weekly for their remaining lifetimes.
This model produced outcomes consistent with those reported in the literature regarding overall survival, progression-free survival, major toxicities, and time spent in each health state. Median overall survival was 39.4 months for standard treatment and 56.9 months with the addition of pertuzumab.
In this statistical model, adding pertuzumab yielded an extra 1.82 life-years per patient and 0.64 QALYs at a per-patient cost of $457,821. “Taken together, the addition of pertuzumab to combined docetaxel plus trastuzumab cost $713,219 per QALY gained,” which is “well above any commonly used threshold and well above the de facto threshold of cost-effectiveness for interventions already in practice,” Dr. Durkee and his associates noted (J Clin Oncol. 2015 Sep 8. doi:10.1200/JCO.2015.62.9105).
Willingness-to-pay thresholds in most analyses of medical treatments range from $50,000 to $160,000 per QALY. A sensitivity analysis of the data demonstrated a 0% chance of cost-effectiveness at a willingness-to-pay level of $100,000 per QALY gained. Even when the willingness-to-pay level rose to $500,000 per QALY, this analysis showed a 0% chance of cost-effectiveness, the researchers noted.
“This analysis highlights the economic challenges of extending life for patients with noncurable disease. It also typifies the broader observation that half of our health care dollars are spent on 5% of the population,” the investigators said.
The Henry S. Kaplan Research Fund, the department of radiation oncology at Stanford University, and the National Institute on Aging supported the study. Dr. Durkee reported having no conflicts of interest. An associate reported stock or other ownership with ViewRay.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Adding pertuzumab to docetaxel plus trastuzumab combination therapy is not cost-effective for patients with metastatic HER2-positive breast cancer.
Major finding: Adding pertuzumab yielded an additional 1.82 life-years per patient and 0.64 QALYs at a per-patient cost of $457,821, for a total cost of $713,219 per QALY gained.
Data source: A cost-effectiveness analysis based on an incidence of 17,450 eligible patients per year in the United States, a $347,627 cost for trastuzumab plus docetaxel, and an $805,449 cost for adding pertuzumab to that combination.
Disclosures: The Henry S. Kaplan Research Fund, the department of radiation oncology at Stanford University, and the National Institute on Aging supported the study. Dr. Durkee reported having no conflicts of interest. An associate reported stock or other ownership with ViewRay.
Genetic biomarkers may be best bet for augmenting mammography screening
SEATTLE – Biomarkers may soon join other modalities for the early detection of breast cancer and identification of women at high risk for the disease, Susan L. Neuhausen, Ph.D., told attendees of the Global Biomarkers Consortium.
“There is an urgent need for biomarkers for breast cancer,” she maintained, citing its high prevalence and considerable mortality, coupled with better outcomes and lesser treatment requirements when it is caught early.
“I really think that for a lot of these biomarkers [in development], maybe their best use will be to augment mammographic screening, especially in the short term as they are being developed,” she speculated. Ultimately, “the hope – similar to PSA [prostate-specific antigen], where you can use it to detect cancer as well as use it as a marker of recurrence – is that these biomarkers of early detection will be developed to have the same attributes.”
Genetic biomarkers
“To me, the current best biomarker is actually a genetic biomarker,” said Dr. Neuhausen, who is the Morris and Horowitz Families Professor in Cancer Etiology and Outcomes Research, Population Sciences, and also coleader of the cancer control and population sciences program, Comprehensive Cancer Center, at the Beckman Research Institute, City of Hope, in Duarte, Calif.
Identifying the breast cancer susceptibility genes BRCA 1 and BRCA 2 paved the way for targeted chemoprevention and prophylactic surgery, which have been highly effective in reducing the incidence of breast cancer as well as ovarian cancer. However, mutations in these genes account for no more than approximately 5% of all breast cancers.
“There has been identification of additional moderate- to high-risk genes, and the strategies that work to prevent cancer in BRCA 1 and 2 carriers are the same that can used for these other high-risk genes,” Dr. Neuhausen noted. For example, mutations of partner and localizer of BRCA2 (PALB2) have been linked to heightened breast cancer risk (N Engl J Med. 2014;371:497-506). Additionally, many companies now offer multigene clinical risk panels.
“The problem or the issue at the current time is that we really don’t know what the risk of cancer in unaffected women who are carrying mutations in these genes is,” she commented. “The good news is there is a large European, United States, and Canadian study that is actually going to be screening for mutations in a total of about 25,000 women, so that similar to what’s been done in BRCA 1 and 2, we will actually have better risk estimates that one can then use for these women.”
A U.S. genome-wide association research effort, the Breast Cancer Surveillance Consortium (BCSC), has thus far identified about 100 loci linked to an increased risk of developing breast cancer, according to Dr. Neuhausen. “On an individual level, these really don’t account for much risk. However, if you combine them, they actually do,” she said.
Applied clinically, a polygenic risk score incorporating 77 single-nucleotide polymorphisms can stratify women into quintiles of risk (J Natl Cancer Inst. 2015;107(5):djv036). Among women without a family history of the disease, lifetime risk ranges from 5.2% for those in the lowest quintile to 16.6% for those in the highest.
Moreover, adding the polygenic risk score to the commonly used BCSC model increases the area under the curve from 0.66 to 0.69 for breast cancer risk prediction. It also results in reclassification of some women, in particular, identifying an additional 3% as having a greater than 3% risk of developing the disease over 5 years. “So this actually does have good discrimination and is something that can be useful moving forward,” Dr. Neuhausen commented.
“We have these low-, moderate-, and high-risk genes, and they really all need to be incorporated into models. There is research ongoing to try to incorporate all of them into models along with lifestyle factors and mammography, family history, that kind of thing,” she said. “But I really think that these genetic markers are very important because rather than early detection, if we can prevent breast cancer, that’s actually the real goal.”
Other biomarkers
A variety of other, nongenetic biomarkers for early breast cancer detection and risk stratification are generally less far along in development, but several have shown promise, according to Dr. Neuhausen.
In the realm of proteomics, a three–amino acid profile detectable in saliva that exploits the hypermetabolic and hypercatabolic nature of cancer was found to have an area under the curve of 0.916 for differentiating women with early breast cancer from unaffected peers (Clin Chim Acta. 2015;447:23-31). “It was only a very small study and they really need to look further, but I think it was an intriguing idea,” she commented.
Infrared spectroscopy of plasma identified a protein fingerprint that had roughly 90% sensitivity and 80% specificity for detecting breast cancer in a cohort that included women with the disease, women with benign breast conditions, and healthy women (BMC Cancer. 2015;15:408). And a pair of proteins in serum—HSP27 and 14-3-3 sigma—accurately distinguished breast cancer cases from controls in a Chinese population (Proteomics. 2003;3:433-9).
One study has suggested how proteomic biomarkers might be integrated with conventional modalities, showing that a five-protein signature in serum (dtectDx Breast, Provista) performed well among women younger than age 50 years for differentiating benign breast lesions from invasive breast cancer in those with a BI-RADS 3 or 4 mammogram (J Clin Oncol. 2014;32(26 Suppl 20). However, the signature had a fairly high false-positive rate, Dr. Neuhausen noted.
As for yet other types of biomarkers, a panel of circulating cell-free methylated DNA of eight tumor suppressor genes was found to have sensitivity and specificity exceeding 90% for differentiating between samples from breast cancer patients and from unaffected women (PLoS One. 2011;6:e16080).MicroRNA profiles of breast cancer have been identified, but findings have generally been inconsistent across studies (Breast Cancer. 2015;7:59-79). Somatic mutations in p53 and PI3KCA, present in about a third of breast cancers, currently have issues when applied to screening. “Those kinds of things are maybe better for determining response to treatment and that kind of thing. Are they ready for early detection? Not really,” Dr. Neuhausen said.
The situation is similar for DNA copy number, although intriguingly, a recent study of a prenatal test looking for fetal copy number aberrations in maternal plasma incidentally discovered maternal cell-free DNA that had copy number changes (JAMA Oncol. 2015 June 5.doi: 10.1001/jamaoncol.2015.1883). Imaging ultimately found various cancers in three women out of about 4,000 tested.
Dr. Neuhausen disclosed that she had no relevant conflicts of interest.
SEATTLE – Biomarkers may soon join other modalities for the early detection of breast cancer and identification of women at high risk for the disease, Susan L. Neuhausen, Ph.D., told attendees of the Global Biomarkers Consortium.
“There is an urgent need for biomarkers for breast cancer,” she maintained, citing its high prevalence and considerable mortality, coupled with better outcomes and lesser treatment requirements when it is caught early.
“I really think that for a lot of these biomarkers [in development], maybe their best use will be to augment mammographic screening, especially in the short term as they are being developed,” she speculated. Ultimately, “the hope – similar to PSA [prostate-specific antigen], where you can use it to detect cancer as well as use it as a marker of recurrence – is that these biomarkers of early detection will be developed to have the same attributes.”
Genetic biomarkers
“To me, the current best biomarker is actually a genetic biomarker,” said Dr. Neuhausen, who is the Morris and Horowitz Families Professor in Cancer Etiology and Outcomes Research, Population Sciences, and also coleader of the cancer control and population sciences program, Comprehensive Cancer Center, at the Beckman Research Institute, City of Hope, in Duarte, Calif.
Identifying the breast cancer susceptibility genes BRCA 1 and BRCA 2 paved the way for targeted chemoprevention and prophylactic surgery, which have been highly effective in reducing the incidence of breast cancer as well as ovarian cancer. However, mutations in these genes account for no more than approximately 5% of all breast cancers.
“There has been identification of additional moderate- to high-risk genes, and the strategies that work to prevent cancer in BRCA 1 and 2 carriers are the same that can used for these other high-risk genes,” Dr. Neuhausen noted. For example, mutations of partner and localizer of BRCA2 (PALB2) have been linked to heightened breast cancer risk (N Engl J Med. 2014;371:497-506). Additionally, many companies now offer multigene clinical risk panels.
“The problem or the issue at the current time is that we really don’t know what the risk of cancer in unaffected women who are carrying mutations in these genes is,” she commented. “The good news is there is a large European, United States, and Canadian study that is actually going to be screening for mutations in a total of about 25,000 women, so that similar to what’s been done in BRCA 1 and 2, we will actually have better risk estimates that one can then use for these women.”
A U.S. genome-wide association research effort, the Breast Cancer Surveillance Consortium (BCSC), has thus far identified about 100 loci linked to an increased risk of developing breast cancer, according to Dr. Neuhausen. “On an individual level, these really don’t account for much risk. However, if you combine them, they actually do,” she said.
Applied clinically, a polygenic risk score incorporating 77 single-nucleotide polymorphisms can stratify women into quintiles of risk (J Natl Cancer Inst. 2015;107(5):djv036). Among women without a family history of the disease, lifetime risk ranges from 5.2% for those in the lowest quintile to 16.6% for those in the highest.
Moreover, adding the polygenic risk score to the commonly used BCSC model increases the area under the curve from 0.66 to 0.69 for breast cancer risk prediction. It also results in reclassification of some women, in particular, identifying an additional 3% as having a greater than 3% risk of developing the disease over 5 years. “So this actually does have good discrimination and is something that can be useful moving forward,” Dr. Neuhausen commented.
“We have these low-, moderate-, and high-risk genes, and they really all need to be incorporated into models. There is research ongoing to try to incorporate all of them into models along with lifestyle factors and mammography, family history, that kind of thing,” she said. “But I really think that these genetic markers are very important because rather than early detection, if we can prevent breast cancer, that’s actually the real goal.”
Other biomarkers
A variety of other, nongenetic biomarkers for early breast cancer detection and risk stratification are generally less far along in development, but several have shown promise, according to Dr. Neuhausen.
In the realm of proteomics, a three–amino acid profile detectable in saliva that exploits the hypermetabolic and hypercatabolic nature of cancer was found to have an area under the curve of 0.916 for differentiating women with early breast cancer from unaffected peers (Clin Chim Acta. 2015;447:23-31). “It was only a very small study and they really need to look further, but I think it was an intriguing idea,” she commented.
Infrared spectroscopy of plasma identified a protein fingerprint that had roughly 90% sensitivity and 80% specificity for detecting breast cancer in a cohort that included women with the disease, women with benign breast conditions, and healthy women (BMC Cancer. 2015;15:408). And a pair of proteins in serum—HSP27 and 14-3-3 sigma—accurately distinguished breast cancer cases from controls in a Chinese population (Proteomics. 2003;3:433-9).
One study has suggested how proteomic biomarkers might be integrated with conventional modalities, showing that a five-protein signature in serum (dtectDx Breast, Provista) performed well among women younger than age 50 years for differentiating benign breast lesions from invasive breast cancer in those with a BI-RADS 3 or 4 mammogram (J Clin Oncol. 2014;32(26 Suppl 20). However, the signature had a fairly high false-positive rate, Dr. Neuhausen noted.
As for yet other types of biomarkers, a panel of circulating cell-free methylated DNA of eight tumor suppressor genes was found to have sensitivity and specificity exceeding 90% for differentiating between samples from breast cancer patients and from unaffected women (PLoS One. 2011;6:e16080).MicroRNA profiles of breast cancer have been identified, but findings have generally been inconsistent across studies (Breast Cancer. 2015;7:59-79). Somatic mutations in p53 and PI3KCA, present in about a third of breast cancers, currently have issues when applied to screening. “Those kinds of things are maybe better for determining response to treatment and that kind of thing. Are they ready for early detection? Not really,” Dr. Neuhausen said.
The situation is similar for DNA copy number, although intriguingly, a recent study of a prenatal test looking for fetal copy number aberrations in maternal plasma incidentally discovered maternal cell-free DNA that had copy number changes (JAMA Oncol. 2015 June 5.doi: 10.1001/jamaoncol.2015.1883). Imaging ultimately found various cancers in three women out of about 4,000 tested.
Dr. Neuhausen disclosed that she had no relevant conflicts of interest.
SEATTLE – Biomarkers may soon join other modalities for the early detection of breast cancer and identification of women at high risk for the disease, Susan L. Neuhausen, Ph.D., told attendees of the Global Biomarkers Consortium.
“There is an urgent need for biomarkers for breast cancer,” she maintained, citing its high prevalence and considerable mortality, coupled with better outcomes and lesser treatment requirements when it is caught early.
“I really think that for a lot of these biomarkers [in development], maybe their best use will be to augment mammographic screening, especially in the short term as they are being developed,” she speculated. Ultimately, “the hope – similar to PSA [prostate-specific antigen], where you can use it to detect cancer as well as use it as a marker of recurrence – is that these biomarkers of early detection will be developed to have the same attributes.”
Genetic biomarkers
“To me, the current best biomarker is actually a genetic biomarker,” said Dr. Neuhausen, who is the Morris and Horowitz Families Professor in Cancer Etiology and Outcomes Research, Population Sciences, and also coleader of the cancer control and population sciences program, Comprehensive Cancer Center, at the Beckman Research Institute, City of Hope, in Duarte, Calif.
Identifying the breast cancer susceptibility genes BRCA 1 and BRCA 2 paved the way for targeted chemoprevention and prophylactic surgery, which have been highly effective in reducing the incidence of breast cancer as well as ovarian cancer. However, mutations in these genes account for no more than approximately 5% of all breast cancers.
“There has been identification of additional moderate- to high-risk genes, and the strategies that work to prevent cancer in BRCA 1 and 2 carriers are the same that can used for these other high-risk genes,” Dr. Neuhausen noted. For example, mutations of partner and localizer of BRCA2 (PALB2) have been linked to heightened breast cancer risk (N Engl J Med. 2014;371:497-506). Additionally, many companies now offer multigene clinical risk panels.
“The problem or the issue at the current time is that we really don’t know what the risk of cancer in unaffected women who are carrying mutations in these genes is,” she commented. “The good news is there is a large European, United States, and Canadian study that is actually going to be screening for mutations in a total of about 25,000 women, so that similar to what’s been done in BRCA 1 and 2, we will actually have better risk estimates that one can then use for these women.”
A U.S. genome-wide association research effort, the Breast Cancer Surveillance Consortium (BCSC), has thus far identified about 100 loci linked to an increased risk of developing breast cancer, according to Dr. Neuhausen. “On an individual level, these really don’t account for much risk. However, if you combine them, they actually do,” she said.
Applied clinically, a polygenic risk score incorporating 77 single-nucleotide polymorphisms can stratify women into quintiles of risk (J Natl Cancer Inst. 2015;107(5):djv036). Among women without a family history of the disease, lifetime risk ranges from 5.2% for those in the lowest quintile to 16.6% for those in the highest.
Moreover, adding the polygenic risk score to the commonly used BCSC model increases the area under the curve from 0.66 to 0.69 for breast cancer risk prediction. It also results in reclassification of some women, in particular, identifying an additional 3% as having a greater than 3% risk of developing the disease over 5 years. “So this actually does have good discrimination and is something that can be useful moving forward,” Dr. Neuhausen commented.
“We have these low-, moderate-, and high-risk genes, and they really all need to be incorporated into models. There is research ongoing to try to incorporate all of them into models along with lifestyle factors and mammography, family history, that kind of thing,” she said. “But I really think that these genetic markers are very important because rather than early detection, if we can prevent breast cancer, that’s actually the real goal.”
Other biomarkers
A variety of other, nongenetic biomarkers for early breast cancer detection and risk stratification are generally less far along in development, but several have shown promise, according to Dr. Neuhausen.
In the realm of proteomics, a three–amino acid profile detectable in saliva that exploits the hypermetabolic and hypercatabolic nature of cancer was found to have an area under the curve of 0.916 for differentiating women with early breast cancer from unaffected peers (Clin Chim Acta. 2015;447:23-31). “It was only a very small study and they really need to look further, but I think it was an intriguing idea,” she commented.
Infrared spectroscopy of plasma identified a protein fingerprint that had roughly 90% sensitivity and 80% specificity for detecting breast cancer in a cohort that included women with the disease, women with benign breast conditions, and healthy women (BMC Cancer. 2015;15:408). And a pair of proteins in serum—HSP27 and 14-3-3 sigma—accurately distinguished breast cancer cases from controls in a Chinese population (Proteomics. 2003;3:433-9).
One study has suggested how proteomic biomarkers might be integrated with conventional modalities, showing that a five-protein signature in serum (dtectDx Breast, Provista) performed well among women younger than age 50 years for differentiating benign breast lesions from invasive breast cancer in those with a BI-RADS 3 or 4 mammogram (J Clin Oncol. 2014;32(26 Suppl 20). However, the signature had a fairly high false-positive rate, Dr. Neuhausen noted.
As for yet other types of biomarkers, a panel of circulating cell-free methylated DNA of eight tumor suppressor genes was found to have sensitivity and specificity exceeding 90% for differentiating between samples from breast cancer patients and from unaffected women (PLoS One. 2011;6:e16080).MicroRNA profiles of breast cancer have been identified, but findings have generally been inconsistent across studies (Breast Cancer. 2015;7:59-79). Somatic mutations in p53 and PI3KCA, present in about a third of breast cancers, currently have issues when applied to screening. “Those kinds of things are maybe better for determining response to treatment and that kind of thing. Are they ready for early detection? Not really,” Dr. Neuhausen said.
The situation is similar for DNA copy number, although intriguingly, a recent study of a prenatal test looking for fetal copy number aberrations in maternal plasma incidentally discovered maternal cell-free DNA that had copy number changes (JAMA Oncol. 2015 June 5.doi: 10.1001/jamaoncol.2015.1883). Imaging ultimately found various cancers in three women out of about 4,000 tested.
Dr. Neuhausen disclosed that she had no relevant conflicts of interest.
EXPERT ANALYSIS FROM THE GLOBAL BIOMARKERS CONSORTIUM
Recurrence score assay driving chemo decisions in unexpected ways
Adoption of the 21-gene recurrence score (RS) assay does not appear to have decreased chemotherapy use overall among Medicare beneficiaries diagnosed with breast cancer, investigators reported online in JAMA Oncology. However, use of the assay was associated with decreased chemotherapy use in high-risk patients and increased chemotherapy use in low-risk patients in the retrospective study.
“Contrary to our hypothesis, we did not observe a change in chemotherapy use in the overall study population after the adoption of the RS assay between 2005 and 2009 or a general association between receipt of chemotherapy and use of the assay in multivariable analyses or HRR [hospital referral region]–level analyses,” Dr. Michaela Dinan of the Duke Clinical Research Institute and her associates wrote (JAMA Oncol. doi: 10.1001/jamaoncol.2015.2722).
There was, however, an interaction between use of the Oncotype DX recurrence score (RS) assay and National Comprehensive Cancer Network (NCCN) risk category.
RS use was associated with lower chemotherapy use in women with high-risk disease (odds ratio, 0.34; 99% confidence interval, 0.20-0.57) and higher chemotherapy use in those with low-risk disease (OR, 4.46; 99% CI, 3.15-6.31).
NCCN guidelines recommend considering chemotherapy in estrogen receptor (ER)-positive, node-negative breast cancer for all but the smallest tumors.
The 21-gene Oncotype DX assay was one of the first gene expression profile tests to reach the market, and studies have suggested its use could save money by guiding allocation of chemotherapy to patients most likely to benefit.
An earlier multicenter NCCN database analysis reported an association between the progressive rise in RS testing and an overall decline in adjuvant chemotherapy in hormone-receptive breast cancers diagnosed between 2006 and 2008.
The current study took a wider look at use of the RS assay and chemotherapy using a nationally representative sample of 44,044 women, aged 65 years and older, diagnosed with early-stage, ER-positive breast cancer between 2005 and 2009 in the SEER (Surveillance, Epidemiology, and End Results) database linked with Medicare claims. Of these, 24% had low-risk, 51.3% intermediate-risk, and 24.6% high-risk disease based on NCCN criteria. Roughly a quarter were node positive.
Use of the RS assay was higher among patients 70 years or younger, patients with fewer comorbid conditions, and patients with T1c tumors, node-negative disease, and intermediate-risk disease, Dr. Dinan and associates reported.
In all, 14.3% of patients overall and 26.5% of patients 70 years or younger received chemotherapy within 12 months of diagnosis, “which supports a significant influence of age on chemotherapy use,” they said.
“Our data suggest that use of the RS assay may have decreased chemotherapy use in general practice among younger patients with high-risk disease in whom receipt of chemotherapy would have otherwise been likely but that it was associated with greater chemotherapy use in patients with low-risk disease. The impact of the RS assay on chemotherapy use is likely population dependent and is influenced by the population’s pretest likelihood of undergoing chemotherapy,” they concluded.
The authors acknowledge that the study had limitations as only RS assays paid for by Medicare were analyzed, HER2 status was unavailable, and patients in the SEER registry are more likely to be nonwhite and live in high-poverty and urban areas, which may affect generalizability.
The study was sponsored by a grant from the Agency for Healthcare Research and Quality. The authors reported having no conflicts of interests. Dr. Kurian and Dr. Friese reported receiving funding from a National Cancer Institute grant to the University of Michigan.
On Twitter @pwendl
The study by Dinan et al. adds a valuable population-based lens to our knowledge of RS adoption and chemotherapy use because prior utilization studies have been more limited in scope. However, many crucial details are missing from this bird’s-eye view.
Most critical are: (1) the exclusion of women younger than 65 years, who make up the majority of breast cancer patients; (2) the lack of RS assay results, which markedly limits evaluation of its impact on chemotherapy decision making; and (3) the absence of patient and physician perspectives, without which we cannot fully understand how use of the RS assay informs treatment choices. Only 14% of patients in this study received chemotherapy, compared with 40%-50% in studies with wider age ranges; this substantial difference in systemic therapy use suggests that the RS assay use patterns observed here may not generalize to younger women.
Getting “under the hood” of such complex medical decision making requires supplementing registry data not only with genomic test results but also with measures of the patient and physician experience. We still do not know why and under what circumstances physicians choose to order an RS assay; to what extent physicians’ treatment recommendations follow RS assay results; and what role, if any, patients play in the choice to order an RS assay and in subsequent chemotherapy decisions. Ultimately, we cannot assess the effects of RS assay use on breast cancer treatment without a deeper understanding of patients’ and physicians’ perspectives.
These comments were excerpted from an editorial accompanying the report by Dr. Dinan and her associates (JAMA Oncol. 2015. doi: 10.1001/jamaoncol.2015.2719).
Dr. Allison Kurian is an assistant professor of medicine (oncology) and of health research and policy at Stanford (Calif.) University Medical Center. Christopher Friese, Ph.D., R.N., is an assistant professor at the School of Nursing, University of Michigan, Ann Arbor.
The study by Dinan et al. adds a valuable population-based lens to our knowledge of RS adoption and chemotherapy use because prior utilization studies have been more limited in scope. However, many crucial details are missing from this bird’s-eye view.
Most critical are: (1) the exclusion of women younger than 65 years, who make up the majority of breast cancer patients; (2) the lack of RS assay results, which markedly limits evaluation of its impact on chemotherapy decision making; and (3) the absence of patient and physician perspectives, without which we cannot fully understand how use of the RS assay informs treatment choices. Only 14% of patients in this study received chemotherapy, compared with 40%-50% in studies with wider age ranges; this substantial difference in systemic therapy use suggests that the RS assay use patterns observed here may not generalize to younger women.
Getting “under the hood” of such complex medical decision making requires supplementing registry data not only with genomic test results but also with measures of the patient and physician experience. We still do not know why and under what circumstances physicians choose to order an RS assay; to what extent physicians’ treatment recommendations follow RS assay results; and what role, if any, patients play in the choice to order an RS assay and in subsequent chemotherapy decisions. Ultimately, we cannot assess the effects of RS assay use on breast cancer treatment without a deeper understanding of patients’ and physicians’ perspectives.
These comments were excerpted from an editorial accompanying the report by Dr. Dinan and her associates (JAMA Oncol. 2015. doi: 10.1001/jamaoncol.2015.2719).
Dr. Allison Kurian is an assistant professor of medicine (oncology) and of health research and policy at Stanford (Calif.) University Medical Center. Christopher Friese, Ph.D., R.N., is an assistant professor at the School of Nursing, University of Michigan, Ann Arbor.
The study by Dinan et al. adds a valuable population-based lens to our knowledge of RS adoption and chemotherapy use because prior utilization studies have been more limited in scope. However, many crucial details are missing from this bird’s-eye view.
Most critical are: (1) the exclusion of women younger than 65 years, who make up the majority of breast cancer patients; (2) the lack of RS assay results, which markedly limits evaluation of its impact on chemotherapy decision making; and (3) the absence of patient and physician perspectives, without which we cannot fully understand how use of the RS assay informs treatment choices. Only 14% of patients in this study received chemotherapy, compared with 40%-50% in studies with wider age ranges; this substantial difference in systemic therapy use suggests that the RS assay use patterns observed here may not generalize to younger women.
Getting “under the hood” of such complex medical decision making requires supplementing registry data not only with genomic test results but also with measures of the patient and physician experience. We still do not know why and under what circumstances physicians choose to order an RS assay; to what extent physicians’ treatment recommendations follow RS assay results; and what role, if any, patients play in the choice to order an RS assay and in subsequent chemotherapy decisions. Ultimately, we cannot assess the effects of RS assay use on breast cancer treatment without a deeper understanding of patients’ and physicians’ perspectives.
These comments were excerpted from an editorial accompanying the report by Dr. Dinan and her associates (JAMA Oncol. 2015. doi: 10.1001/jamaoncol.2015.2719).
Dr. Allison Kurian is an assistant professor of medicine (oncology) and of health research and policy at Stanford (Calif.) University Medical Center. Christopher Friese, Ph.D., R.N., is an assistant professor at the School of Nursing, University of Michigan, Ann Arbor.
Adoption of the 21-gene recurrence score (RS) assay does not appear to have decreased chemotherapy use overall among Medicare beneficiaries diagnosed with breast cancer, investigators reported online in JAMA Oncology. However, use of the assay was associated with decreased chemotherapy use in high-risk patients and increased chemotherapy use in low-risk patients in the retrospective study.
“Contrary to our hypothesis, we did not observe a change in chemotherapy use in the overall study population after the adoption of the RS assay between 2005 and 2009 or a general association between receipt of chemotherapy and use of the assay in multivariable analyses or HRR [hospital referral region]–level analyses,” Dr. Michaela Dinan of the Duke Clinical Research Institute and her associates wrote (JAMA Oncol. doi: 10.1001/jamaoncol.2015.2722).
There was, however, an interaction between use of the Oncotype DX recurrence score (RS) assay and National Comprehensive Cancer Network (NCCN) risk category.
RS use was associated with lower chemotherapy use in women with high-risk disease (odds ratio, 0.34; 99% confidence interval, 0.20-0.57) and higher chemotherapy use in those with low-risk disease (OR, 4.46; 99% CI, 3.15-6.31).
NCCN guidelines recommend considering chemotherapy in estrogen receptor (ER)-positive, node-negative breast cancer for all but the smallest tumors.
The 21-gene Oncotype DX assay was one of the first gene expression profile tests to reach the market, and studies have suggested its use could save money by guiding allocation of chemotherapy to patients most likely to benefit.
An earlier multicenter NCCN database analysis reported an association between the progressive rise in RS testing and an overall decline in adjuvant chemotherapy in hormone-receptive breast cancers diagnosed between 2006 and 2008.
The current study took a wider look at use of the RS assay and chemotherapy using a nationally representative sample of 44,044 women, aged 65 years and older, diagnosed with early-stage, ER-positive breast cancer between 2005 and 2009 in the SEER (Surveillance, Epidemiology, and End Results) database linked with Medicare claims. Of these, 24% had low-risk, 51.3% intermediate-risk, and 24.6% high-risk disease based on NCCN criteria. Roughly a quarter were node positive.
Use of the RS assay was higher among patients 70 years or younger, patients with fewer comorbid conditions, and patients with T1c tumors, node-negative disease, and intermediate-risk disease, Dr. Dinan and associates reported.
In all, 14.3% of patients overall and 26.5% of patients 70 years or younger received chemotherapy within 12 months of diagnosis, “which supports a significant influence of age on chemotherapy use,” they said.
“Our data suggest that use of the RS assay may have decreased chemotherapy use in general practice among younger patients with high-risk disease in whom receipt of chemotherapy would have otherwise been likely but that it was associated with greater chemotherapy use in patients with low-risk disease. The impact of the RS assay on chemotherapy use is likely population dependent and is influenced by the population’s pretest likelihood of undergoing chemotherapy,” they concluded.
The authors acknowledge that the study had limitations as only RS assays paid for by Medicare were analyzed, HER2 status was unavailable, and patients in the SEER registry are more likely to be nonwhite and live in high-poverty and urban areas, which may affect generalizability.
The study was sponsored by a grant from the Agency for Healthcare Research and Quality. The authors reported having no conflicts of interests. Dr. Kurian and Dr. Friese reported receiving funding from a National Cancer Institute grant to the University of Michigan.
On Twitter @pwendl
Adoption of the 21-gene recurrence score (RS) assay does not appear to have decreased chemotherapy use overall among Medicare beneficiaries diagnosed with breast cancer, investigators reported online in JAMA Oncology. However, use of the assay was associated with decreased chemotherapy use in high-risk patients and increased chemotherapy use in low-risk patients in the retrospective study.
“Contrary to our hypothesis, we did not observe a change in chemotherapy use in the overall study population after the adoption of the RS assay between 2005 and 2009 or a general association between receipt of chemotherapy and use of the assay in multivariable analyses or HRR [hospital referral region]–level analyses,” Dr. Michaela Dinan of the Duke Clinical Research Institute and her associates wrote (JAMA Oncol. doi: 10.1001/jamaoncol.2015.2722).
There was, however, an interaction between use of the Oncotype DX recurrence score (RS) assay and National Comprehensive Cancer Network (NCCN) risk category.
RS use was associated with lower chemotherapy use in women with high-risk disease (odds ratio, 0.34; 99% confidence interval, 0.20-0.57) and higher chemotherapy use in those with low-risk disease (OR, 4.46; 99% CI, 3.15-6.31).
NCCN guidelines recommend considering chemotherapy in estrogen receptor (ER)-positive, node-negative breast cancer for all but the smallest tumors.
The 21-gene Oncotype DX assay was one of the first gene expression profile tests to reach the market, and studies have suggested its use could save money by guiding allocation of chemotherapy to patients most likely to benefit.
An earlier multicenter NCCN database analysis reported an association between the progressive rise in RS testing and an overall decline in adjuvant chemotherapy in hormone-receptive breast cancers diagnosed between 2006 and 2008.
The current study took a wider look at use of the RS assay and chemotherapy using a nationally representative sample of 44,044 women, aged 65 years and older, diagnosed with early-stage, ER-positive breast cancer between 2005 and 2009 in the SEER (Surveillance, Epidemiology, and End Results) database linked with Medicare claims. Of these, 24% had low-risk, 51.3% intermediate-risk, and 24.6% high-risk disease based on NCCN criteria. Roughly a quarter were node positive.
Use of the RS assay was higher among patients 70 years or younger, patients with fewer comorbid conditions, and patients with T1c tumors, node-negative disease, and intermediate-risk disease, Dr. Dinan and associates reported.
In all, 14.3% of patients overall and 26.5% of patients 70 years or younger received chemotherapy within 12 months of diagnosis, “which supports a significant influence of age on chemotherapy use,” they said.
“Our data suggest that use of the RS assay may have decreased chemotherapy use in general practice among younger patients with high-risk disease in whom receipt of chemotherapy would have otherwise been likely but that it was associated with greater chemotherapy use in patients with low-risk disease. The impact of the RS assay on chemotherapy use is likely population dependent and is influenced by the population’s pretest likelihood of undergoing chemotherapy,” they concluded.
The authors acknowledge that the study had limitations as only RS assays paid for by Medicare were analyzed, HER2 status was unavailable, and patients in the SEER registry are more likely to be nonwhite and live in high-poverty and urban areas, which may affect generalizability.
The study was sponsored by a grant from the Agency for Healthcare Research and Quality. The authors reported having no conflicts of interests. Dr. Kurian and Dr. Friese reported receiving funding from a National Cancer Institute grant to the University of Michigan.
On Twitter @pwendl
FROM JAMA ONCOLOGY
Key clinical point: Use of the 21-gene recurrence score was associated with relatively lower chemotherapy use in women with high-risk disease and relatively higher chemotherapy use in patients with low-risk disease.
Major finding: RS use was associated with lower chemotherapy use in women with high-risk disease (OR, 0.34; 99% CI, 0.20-0.57) and higher chemotherapy use in those with low-risk disease (OR, 4.46; 99% CI, 3.15-6.31).
Data source: Retrospective cohort study in 44,044 Medicare beneficiaries with early-stage, ER-positive breast cancer.
Disclosures: The study was sponsored by a grant from the Agency for Healthcare Research and Quality. The authors reported having no conflicts of interests. Dr. Kurian and Dr. Friese reported receiving funding from a National Cancer Institute grant to the University of Michigan.
Genomic oncology: moving beyond the tip of the iceberg
In the 15 years since the first map of the human genome emerged, genetics has become an integral part of medical practice worldwide.1 Oncology is no exception; the genetic origins of cancer were suspected more than a century ago and it is now well understood that most cancers are driven by genetic alterations that disrupt key cellular pathways involved in tumor survival and progression.2
In the 15 years since the first map of the human genome emerged, genetics has become an integral part of medical practice worldwide.1 Oncology is no exception; the genetic origins of cancer were suspected more than a century ago and it is now well understood that most cancers are driven by genetic alterations that disrupt key cellular pathways involved in tumor survival and progression.2
In the 15 years since the first map of the human genome emerged, genetics has become an integral part of medical practice worldwide.1 Oncology is no exception; the genetic origins of cancer were suspected more than a century ago and it is now well understood that most cancers are driven by genetic alterations that disrupt key cellular pathways involved in tumor survival and progression.2