User login
Radiofrequency Ablation Advances as Radiation Alternative in Breast Cancer
Radiofrequency ablation could become an option for some patients facing adjuvant radiation after breast-conserving surgery for invasive breast cancer, the results of a phase II trial suggest.
Excision followed by radiofrequency ablation (eRFA) was at least as effective as radiation therapy following lumpectomy in preventing local tumor recurrence in the single-arm study of 73 patients who underwent breast conserving surgery, according to investigators. Only one patient had an in-site recurrence while three had recurrences, they reported at the annual meeting of the American Society of Breast Surgeons.
"For selected breast cancer patients undergoing breast-conservation therapy, eRFA is an attractive alternative to breast irradiation," said Dr. Misti Wilson at a press briefing.
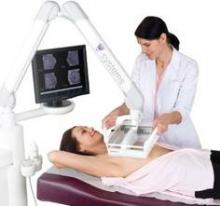
The intraoperative procedure of excision followed by radiofrequency ablation employs heat to create an additional tumor-free zone, approximating the zone treated by brachytherapy, around the lumpectomy cavity. Its benefits include reduced likelihood of the need for re-excision and for additional radiation; lower cost, compared with radiation; and good to excellent cosmetic results, said Dr. Wilson, a breast surgical oncology fellow at the University of Arkansas in Little Rock.
The study enrolled 73 patients with tumors of less than 3 cm. Median follow-up was 55 months. All underwent standard lumpectomy followed by radiofrequency ablation, in which the RFA probe was deployed 1 cm circumferentially into the walls of the lumpectomy cavity and maintained at 100 degrees C for 15 minutes.
None of the patients received subsequent radiation or chemotherapy. Only those with grossly positive margins or residual calcifications on postoperative mammography were re-resected.
Among 19 patients who had inadequate margins, RFA spared 16 (84%) from additional surgery. Only three patients (4%) had to return to the operating room for resection because of grossly positive margins. There was just one in-site recurrence out of 73 patients, and 3 had recurrences elsewhere, Dr. Wilson reported.
Of 40 patients who scored their cosmesis, 18 (45%) reported excellent cosmetic results, 18 (45%) reported good results, and 4 (10%) reported fair cosmetic results.
"These findings show that this is a safe procedure, patients can have less repeat surgery, they have good cosmetic outcomes, and RFA may replace radiation therapy in patients with small tumors and are node negative," Dr. Wilson said in an interview.
The cost of eRFA is around $2,000 dollars. In contrast, standard whole breast radiation costs approximately $11,000, and partial breast around $18,000 depending upon site and location, she said.
The ABLATE trial is investigating eRFA in a larger patient population. To date, the trial includes five centers (Columbia University, N.Y; University of Kansas, Lawrence; Comprehensive Breast Care of San Diego, University of Arizona, Tucson; and Rockefeller Cancer Institute in Little Rock) and is actively recruiting and training additional sites.
Early results were presented in a poster by the primary investigator of both studies, Dr. V. Suzanne Klimberg, professor of surgery and pathology and director of the breast program at the University of Arkansas.
Of 55 patients (mean age 65 years) with ductal carcinoma in situ or invasive breast cancer with average tumor size 0.9 cm who underwent eRFA, 20 had positive margins: 14 had close margins (less than 2 mm), 2 had focally positive margins, and 4 had grossly positive margins. Of those, 15 were spared re-excision. Morbidity at 30 days was 7.2% and there were no deaths.
The University of Arkansas sponsored the study in collaboration with AngioDynamics, maker of the RFA delivery system.Dr. Wilson stated that she has no disclosures. Dr. Klimberg received research grants from AngioDynamics and retailers Fashion Footwear Association of New York and QVC.
Radiofrequency ablation could become an option for some patients facing adjuvant radiation after breast-conserving surgery for invasive breast cancer, the results of a phase II trial suggest.
Excision followed by radiofrequency ablation (eRFA) was at least as effective as radiation therapy following lumpectomy in preventing local tumor recurrence in the single-arm study of 73 patients who underwent breast conserving surgery, according to investigators. Only one patient had an in-site recurrence while three had recurrences, they reported at the annual meeting of the American Society of Breast Surgeons.
"For selected breast cancer patients undergoing breast-conservation therapy, eRFA is an attractive alternative to breast irradiation," said Dr. Misti Wilson at a press briefing.
The intraoperative procedure of excision followed by radiofrequency ablation employs heat to create an additional tumor-free zone, approximating the zone treated by brachytherapy, around the lumpectomy cavity. Its benefits include reduced likelihood of the need for re-excision and for additional radiation; lower cost, compared with radiation; and good to excellent cosmetic results, said Dr. Wilson, a breast surgical oncology fellow at the University of Arkansas in Little Rock.
The study enrolled 73 patients with tumors of less than 3 cm. Median follow-up was 55 months. All underwent standard lumpectomy followed by radiofrequency ablation, in which the RFA probe was deployed 1 cm circumferentially into the walls of the lumpectomy cavity and maintained at 100 degrees C for 15 minutes.
None of the patients received subsequent radiation or chemotherapy. Only those with grossly positive margins or residual calcifications on postoperative mammography were re-resected.
Among 19 patients who had inadequate margins, RFA spared 16 (84%) from additional surgery. Only three patients (4%) had to return to the operating room for resection because of grossly positive margins. There was just one in-site recurrence out of 73 patients, and 3 had recurrences elsewhere, Dr. Wilson reported.
Of 40 patients who scored their cosmesis, 18 (45%) reported excellent cosmetic results, 18 (45%) reported good results, and 4 (10%) reported fair cosmetic results.
"These findings show that this is a safe procedure, patients can have less repeat surgery, they have good cosmetic outcomes, and RFA may replace radiation therapy in patients with small tumors and are node negative," Dr. Wilson said in an interview.
The cost of eRFA is around $2,000 dollars. In contrast, standard whole breast radiation costs approximately $11,000, and partial breast around $18,000 depending upon site and location, she said.
The ABLATE trial is investigating eRFA in a larger patient population. To date, the trial includes five centers (Columbia University, N.Y; University of Kansas, Lawrence; Comprehensive Breast Care of San Diego, University of Arizona, Tucson; and Rockefeller Cancer Institute in Little Rock) and is actively recruiting and training additional sites.
Early results were presented in a poster by the primary investigator of both studies, Dr. V. Suzanne Klimberg, professor of surgery and pathology and director of the breast program at the University of Arkansas.
Of 55 patients (mean age 65 years) with ductal carcinoma in situ or invasive breast cancer with average tumor size 0.9 cm who underwent eRFA, 20 had positive margins: 14 had close margins (less than 2 mm), 2 had focally positive margins, and 4 had grossly positive margins. Of those, 15 were spared re-excision. Morbidity at 30 days was 7.2% and there were no deaths.
The University of Arkansas sponsored the study in collaboration with AngioDynamics, maker of the RFA delivery system.Dr. Wilson stated that she has no disclosures. Dr. Klimberg received research grants from AngioDynamics and retailers Fashion Footwear Association of New York and QVC.
Radiofrequency ablation could become an option for some patients facing adjuvant radiation after breast-conserving surgery for invasive breast cancer, the results of a phase II trial suggest.
Excision followed by radiofrequency ablation (eRFA) was at least as effective as radiation therapy following lumpectomy in preventing local tumor recurrence in the single-arm study of 73 patients who underwent breast conserving surgery, according to investigators. Only one patient had an in-site recurrence while three had recurrences, they reported at the annual meeting of the American Society of Breast Surgeons.
"For selected breast cancer patients undergoing breast-conservation therapy, eRFA is an attractive alternative to breast irradiation," said Dr. Misti Wilson at a press briefing.
The intraoperative procedure of excision followed by radiofrequency ablation employs heat to create an additional tumor-free zone, approximating the zone treated by brachytherapy, around the lumpectomy cavity. Its benefits include reduced likelihood of the need for re-excision and for additional radiation; lower cost, compared with radiation; and good to excellent cosmetic results, said Dr. Wilson, a breast surgical oncology fellow at the University of Arkansas in Little Rock.
The study enrolled 73 patients with tumors of less than 3 cm. Median follow-up was 55 months. All underwent standard lumpectomy followed by radiofrequency ablation, in which the RFA probe was deployed 1 cm circumferentially into the walls of the lumpectomy cavity and maintained at 100 degrees C for 15 minutes.
None of the patients received subsequent radiation or chemotherapy. Only those with grossly positive margins or residual calcifications on postoperative mammography were re-resected.
Among 19 patients who had inadequate margins, RFA spared 16 (84%) from additional surgery. Only three patients (4%) had to return to the operating room for resection because of grossly positive margins. There was just one in-site recurrence out of 73 patients, and 3 had recurrences elsewhere, Dr. Wilson reported.
Of 40 patients who scored their cosmesis, 18 (45%) reported excellent cosmetic results, 18 (45%) reported good results, and 4 (10%) reported fair cosmetic results.
"These findings show that this is a safe procedure, patients can have less repeat surgery, they have good cosmetic outcomes, and RFA may replace radiation therapy in patients with small tumors and are node negative," Dr. Wilson said in an interview.
The cost of eRFA is around $2,000 dollars. In contrast, standard whole breast radiation costs approximately $11,000, and partial breast around $18,000 depending upon site and location, she said.
The ABLATE trial is investigating eRFA in a larger patient population. To date, the trial includes five centers (Columbia University, N.Y; University of Kansas, Lawrence; Comprehensive Breast Care of San Diego, University of Arizona, Tucson; and Rockefeller Cancer Institute in Little Rock) and is actively recruiting and training additional sites.
Early results were presented in a poster by the primary investigator of both studies, Dr. V. Suzanne Klimberg, professor of surgery and pathology and director of the breast program at the University of Arkansas.
Of 55 patients (mean age 65 years) with ductal carcinoma in situ or invasive breast cancer with average tumor size 0.9 cm who underwent eRFA, 20 had positive margins: 14 had close margins (less than 2 mm), 2 had focally positive margins, and 4 had grossly positive margins. Of those, 15 were spared re-excision. Morbidity at 30 days was 7.2% and there were no deaths.
The University of Arkansas sponsored the study in collaboration with AngioDynamics, maker of the RFA delivery system.Dr. Wilson stated that she has no disclosures. Dr. Klimberg received research grants from AngioDynamics and retailers Fashion Footwear Association of New York and QVC.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF BREAST SURGEONS
Major Finding: Only 1 of 73 patients treated with radiofrequency ablation had an in-site recurrence; 3 had recurrences elsewhere.
Data Source: The findings come from a single-arm, phase II trial in patients with invasive breast cancers treated with breast conserving surgery followed by immediate intraoperative eRFA.
Disclosures: The University of Arkansas sponsored the study in collaboration with AngioDynamics, maker of the RFA delivery system.Dr. Wilson stated that she has no disclosures. Dr. Klimberg received research grants from AngioDynamics and retailers Fashion Footwear Association of New York and QVC.
Surgery for DCIS Saves Lives
ORLANDO – Surgery for ductal carcinoma in situ, with or without adjuvant therapy, saves lives, asserted a breast cancer surgeon at a symposium sponsored by the Society of Surgical Oncology.
Following a surgical biopsy alone, about half of all cases of low-grade ductal carcinoma in situ (DCIS) will progress to invasive cancer within an average of 10-15 years, said Dr. Kimberly J. Van Zee, a surgical oncologist at Memorial Sloan-Kettering Cancer Center in New York.
Additionally, without intervention, low-grade DCIS will result in death from ipsilateral invasive recurrence of breast cancer in about 18% of patients, Dr. Van Zee said.
"With treatment of DCIS, whether it’s breast conservation or mastectomy, with or without radiation, breast cancer–specific survival is over 95%," she noted.
The incidence of DCIS has increased steadily since 1975, when the rate was slightly more than 5 in 100,000 women. In 2009, the rate had reached approximately 36 in 100,000, according to Surveillance, Epidemiology, and End Results (SEER) data. The increase is probably a result of the growing adoption of screening mammography over the same period, Dr. Van Zee commented.
Treatment trends for DCIS showed a gradual but steady decline in mastectomy – from 70% in 1983 to about 28% in 1999 – and a corresponding increase in breast-conserving treatment, which increased from about 25% to 68% over the same period.
Beginning around 2005, however, there was evidence that the trend was reversing, with upticks in both mastectomy for unilateral breast cancer (J. Clin. Oncol. 2010;28:3437-41) and contralateral prophylactic mastectomy, both among women with invasive cancers and DCIS (Ann. Surg. Oncol. 2010;17:2554-62). The trends paralleled the rise in screening mammography in the United States and elsewhere in the world.
The gradual but steady decline in breast cancer deaths that began in the early 1990s appears to be attributable to a combination of increased screening mammography and improvements in adjuvant therapy, Dr. Van Zee noted, citing a 2005 study (N. Engl. J. Med. 2005;353:1784-92).
"They dissected all the various effects of treatment, incidence of screening-detected diseases, etc., and all their analyses concluded that about half of the reduction in death rate was due to screening and the other half was due to adjuvant therapy. So I think this is good circumstantial evidence that screening, with its resultant increased incidence in DCIS and the resulting increased treatment of DCIS, does result in a lower death rate from breast cancer," she said.
Further evidence comes from studies in which pathologists reviewed thousands of slides of biopsy-acquired breast tissue originally reported as benign. In each study (Cancer 1980;46[4 Suppl]:919-25; Cancer 2005;103:2481-84), the investigator identified about 30 samples with evidence of low-grade, relatively low-volume DCIS that was not recognized or treated. After 20-30 years of follow-up, half of the women had developed a clinically apparent ipsilateral breast cancer recurrence. The majority of tumors were invasive. In the second study, the authors noted that 5 of the 28 women (18%) with previously undetected DCIS died of breast cancer.
Evidence from a meta-analysis (Cancer 1999;85:616-28) suggests that the risk for invasive recurrence following a mastectomy for DCIS is 1.1%, and that the risk for breast cancer death is less than 1.1%.
The risk for distant recurrence and/or death from breast-conserving surgery with or without adjuvant radiotherapy in prospective randomized trials of radiotherapy for DCIS was less than 5%. Among patients with invasive local failure in those trials, however, 18%-25% developed metastatic disease, indicating the importance of avoiding local recurrence.
Mastectomy and breast-conserving surgery combined with radiotherapy and/or endocrine therapy all provide excellent disease-specific and overall survival results, Dr. Van Zee said.
"The goal should be avoiding local recurrence and, in particular, invasive recurrence, minimizing morbidity, and perhaps individualizing the treatment to the disease. One could consider age, comorbidities, [and] life expectancy, and weigh those against the morbidity of the treatment and the risk of local recurrence," she said.
Dr. Van Zee reported no relevant financial disclosures.
ORLANDO – Surgery for ductal carcinoma in situ, with or without adjuvant therapy, saves lives, asserted a breast cancer surgeon at a symposium sponsored by the Society of Surgical Oncology.
Following a surgical biopsy alone, about half of all cases of low-grade ductal carcinoma in situ (DCIS) will progress to invasive cancer within an average of 10-15 years, said Dr. Kimberly J. Van Zee, a surgical oncologist at Memorial Sloan-Kettering Cancer Center in New York.
Additionally, without intervention, low-grade DCIS will result in death from ipsilateral invasive recurrence of breast cancer in about 18% of patients, Dr. Van Zee said.
"With treatment of DCIS, whether it’s breast conservation or mastectomy, with or without radiation, breast cancer–specific survival is over 95%," she noted.
The incidence of DCIS has increased steadily since 1975, when the rate was slightly more than 5 in 100,000 women. In 2009, the rate had reached approximately 36 in 100,000, according to Surveillance, Epidemiology, and End Results (SEER) data. The increase is probably a result of the growing adoption of screening mammography over the same period, Dr. Van Zee commented.
Treatment trends for DCIS showed a gradual but steady decline in mastectomy – from 70% in 1983 to about 28% in 1999 – and a corresponding increase in breast-conserving treatment, which increased from about 25% to 68% over the same period.
Beginning around 2005, however, there was evidence that the trend was reversing, with upticks in both mastectomy for unilateral breast cancer (J. Clin. Oncol. 2010;28:3437-41) and contralateral prophylactic mastectomy, both among women with invasive cancers and DCIS (Ann. Surg. Oncol. 2010;17:2554-62). The trends paralleled the rise in screening mammography in the United States and elsewhere in the world.
The gradual but steady decline in breast cancer deaths that began in the early 1990s appears to be attributable to a combination of increased screening mammography and improvements in adjuvant therapy, Dr. Van Zee noted, citing a 2005 study (N. Engl. J. Med. 2005;353:1784-92).
"They dissected all the various effects of treatment, incidence of screening-detected diseases, etc., and all their analyses concluded that about half of the reduction in death rate was due to screening and the other half was due to adjuvant therapy. So I think this is good circumstantial evidence that screening, with its resultant increased incidence in DCIS and the resulting increased treatment of DCIS, does result in a lower death rate from breast cancer," she said.
Further evidence comes from studies in which pathologists reviewed thousands of slides of biopsy-acquired breast tissue originally reported as benign. In each study (Cancer 1980;46[4 Suppl]:919-25; Cancer 2005;103:2481-84), the investigator identified about 30 samples with evidence of low-grade, relatively low-volume DCIS that was not recognized or treated. After 20-30 years of follow-up, half of the women had developed a clinically apparent ipsilateral breast cancer recurrence. The majority of tumors were invasive. In the second study, the authors noted that 5 of the 28 women (18%) with previously undetected DCIS died of breast cancer.
Evidence from a meta-analysis (Cancer 1999;85:616-28) suggests that the risk for invasive recurrence following a mastectomy for DCIS is 1.1%, and that the risk for breast cancer death is less than 1.1%.
The risk for distant recurrence and/or death from breast-conserving surgery with or without adjuvant radiotherapy in prospective randomized trials of radiotherapy for DCIS was less than 5%. Among patients with invasive local failure in those trials, however, 18%-25% developed metastatic disease, indicating the importance of avoiding local recurrence.
Mastectomy and breast-conserving surgery combined with radiotherapy and/or endocrine therapy all provide excellent disease-specific and overall survival results, Dr. Van Zee said.
"The goal should be avoiding local recurrence and, in particular, invasive recurrence, minimizing morbidity, and perhaps individualizing the treatment to the disease. One could consider age, comorbidities, [and] life expectancy, and weigh those against the morbidity of the treatment and the risk of local recurrence," she said.
Dr. Van Zee reported no relevant financial disclosures.
ORLANDO – Surgery for ductal carcinoma in situ, with or without adjuvant therapy, saves lives, asserted a breast cancer surgeon at a symposium sponsored by the Society of Surgical Oncology.
Following a surgical biopsy alone, about half of all cases of low-grade ductal carcinoma in situ (DCIS) will progress to invasive cancer within an average of 10-15 years, said Dr. Kimberly J. Van Zee, a surgical oncologist at Memorial Sloan-Kettering Cancer Center in New York.
Additionally, without intervention, low-grade DCIS will result in death from ipsilateral invasive recurrence of breast cancer in about 18% of patients, Dr. Van Zee said.
"With treatment of DCIS, whether it’s breast conservation or mastectomy, with or without radiation, breast cancer–specific survival is over 95%," she noted.
The incidence of DCIS has increased steadily since 1975, when the rate was slightly more than 5 in 100,000 women. In 2009, the rate had reached approximately 36 in 100,000, according to Surveillance, Epidemiology, and End Results (SEER) data. The increase is probably a result of the growing adoption of screening mammography over the same period, Dr. Van Zee commented.
Treatment trends for DCIS showed a gradual but steady decline in mastectomy – from 70% in 1983 to about 28% in 1999 – and a corresponding increase in breast-conserving treatment, which increased from about 25% to 68% over the same period.
Beginning around 2005, however, there was evidence that the trend was reversing, with upticks in both mastectomy for unilateral breast cancer (J. Clin. Oncol. 2010;28:3437-41) and contralateral prophylactic mastectomy, both among women with invasive cancers and DCIS (Ann. Surg. Oncol. 2010;17:2554-62). The trends paralleled the rise in screening mammography in the United States and elsewhere in the world.
The gradual but steady decline in breast cancer deaths that began in the early 1990s appears to be attributable to a combination of increased screening mammography and improvements in adjuvant therapy, Dr. Van Zee noted, citing a 2005 study (N. Engl. J. Med. 2005;353:1784-92).
"They dissected all the various effects of treatment, incidence of screening-detected diseases, etc., and all their analyses concluded that about half of the reduction in death rate was due to screening and the other half was due to adjuvant therapy. So I think this is good circumstantial evidence that screening, with its resultant increased incidence in DCIS and the resulting increased treatment of DCIS, does result in a lower death rate from breast cancer," she said.
Further evidence comes from studies in which pathologists reviewed thousands of slides of biopsy-acquired breast tissue originally reported as benign. In each study (Cancer 1980;46[4 Suppl]:919-25; Cancer 2005;103:2481-84), the investigator identified about 30 samples with evidence of low-grade, relatively low-volume DCIS that was not recognized or treated. After 20-30 years of follow-up, half of the women had developed a clinically apparent ipsilateral breast cancer recurrence. The majority of tumors were invasive. In the second study, the authors noted that 5 of the 28 women (18%) with previously undetected DCIS died of breast cancer.
Evidence from a meta-analysis (Cancer 1999;85:616-28) suggests that the risk for invasive recurrence following a mastectomy for DCIS is 1.1%, and that the risk for breast cancer death is less than 1.1%.
The risk for distant recurrence and/or death from breast-conserving surgery with or without adjuvant radiotherapy in prospective randomized trials of radiotherapy for DCIS was less than 5%. Among patients with invasive local failure in those trials, however, 18%-25% developed metastatic disease, indicating the importance of avoiding local recurrence.
Mastectomy and breast-conserving surgery combined with radiotherapy and/or endocrine therapy all provide excellent disease-specific and overall survival results, Dr. Van Zee said.
"The goal should be avoiding local recurrence and, in particular, invasive recurrence, minimizing morbidity, and perhaps individualizing the treatment to the disease. One could consider age, comorbidities, [and] life expectancy, and weigh those against the morbidity of the treatment and the risk of local recurrence," she said.
Dr. Van Zee reported no relevant financial disclosures.
EXPERT ANALYSIS FROM A SYMPOSIUM SPONSORED BY THE SOCIETY OF SURGICAL ONCOLOGY
Infrared Thermography Fails to Predict Breast Malignancy
Infrared thermography did not accurately predict malignancy and produced an unacceptably high false-positive rate in women with radiologic abnormalities requiring breast biopsy in a 2-year prospective study.
The No-Touch Breast Scan (NTBS) is a noninvasive, non–radiation-based imaging tool that measures and compares thermal abnormalities in breasts using dual infrared cameras and computer analysis. It generates a score that reflects blood flow patterns based on the theory of tumor angiogenesis.
The technology is being explored as an alternative to radiation-based imaging in women at risk for breast cancer and as a way to reduce the number of benign biopsies, Dr. Andrea V. Barrio said during a press briefing at the annual meeting of the American Society of Breast Surgeons.
This study evaluated NTBS screening as a predictor of breast cancer in patients undergoing minimally invasive breast biopsy for suspicious mammogram, ultrasound, or MRI findings.
But the results demonstrated that NTBS "cannot be used as a successful adjunct to mammography, nor can it replace any of the screening modalities that are standard practice. Mammography remains the gold standard for breast cancer screening," said Dr. Barrio, an attending breast surgeon at Bryn Mawr (Pa.) Hospital.
"I think the utility of NTBS remains unclear. For the purposes of our study, NTBS could not discriminate between benign and malignant lesions in the low-specificity mode, and the high-sensitivity mode resulted in an unacceptable number of false-positive results," she added in an interview.
A total of 181 women (median age at diagnosis 52.5 years) with 187 abnormal radiologic findings were evaluated from October 2009 to May 2011. Each patient had an NTBS prior to tissue biopsy, and final tissue pathologies were compared with the corresponding NTBS scan results. Each breast was interpreted as positive or negative based on computer analysis of thermal abnormalities. The contralateral breast was scanned in all patients.
Prior to Oct. 15, 2010, patients were initially scanned using a "high-specificity" NTBS mode termed NTBS1. Subsequently a "high-sensitivity mode (NTBS2)" was used to minimize false-negative results. Following initial data analysis, all patients were retrospectively re-evaluated in the NTBS2 mode.
Of the 181 patients initially evaluated, 3 were excluded due to a nonductal or lobular breast malignancy, leaving a total of 178 patients. Of those, 50 had 52 positive breast biopsies and 128 had 132 negative biopsies.
Of the 52 positive biopsies, only 26 had a positive NTBS, giving a sensitivity of just 50%. The sensitivity of NTBS was even lower in the 20 in situ cancers, compared with the 32 invasive cancers (35% vs. 59%, respectively), Dr. Barrio reported.
Of the 132 negative biopsies, 88 had negative NTBS scans, giving a 67% specificity. "The positive predictive value of NTBS was 37% and the negative predictive value was 77%," the study results showed.
Of 173 normal contralateral breasts that were scanned, 42 (24%) had a positive NTBS scan.
Among the 178 patients retrospectively evaluated using NTBS2, 22 were excluded because of an uninterpretable scan. Of the remaining 156 patients, 44 had 46 positive breast biopsies and 112 had 116 negative biopsies. Forty of the 46 positive biopsies matched a positive NTBS (sensitivity 87%).
Sensitivity was not appreciably different between in situ and invasive cancers in the NTBS2 mode (88% vs. 86%, respectively), she said.
Of the 116 negative biopsies, 55 had a negative NTBS, giving a specificity of just 48%. "The positive predictive value of NTBS2 was 40% and the negative predictive value was 90%," the study reported. Of the 151 normal contralateral breasts that were scanned, 72 (47%) had a positive reading.
In the interview, Dr. Barrio said that her group is not seeking a replacement for mammography, as it is a cost-efficient screening tool that has been proven to decrease mortality from breast cancer.
However, "we are looking for studies to supplement mammography, in order to address its limitations, i.e., dense breasts. I think molecular breast imaging in particular shows a lot of promise for the future in women with dense breasts."
This study was funded by the Humler Oncology fund. Dr. Barrio had no other disclosures.
Infrared thermography did not accurately predict malignancy and produced an unacceptably high false-positive rate in women with radiologic abnormalities requiring breast biopsy in a 2-year prospective study.
The No-Touch Breast Scan (NTBS) is a noninvasive, non–radiation-based imaging tool that measures and compares thermal abnormalities in breasts using dual infrared cameras and computer analysis. It generates a score that reflects blood flow patterns based on the theory of tumor angiogenesis.
The technology is being explored as an alternative to radiation-based imaging in women at risk for breast cancer and as a way to reduce the number of benign biopsies, Dr. Andrea V. Barrio said during a press briefing at the annual meeting of the American Society of Breast Surgeons.
This study evaluated NTBS screening as a predictor of breast cancer in patients undergoing minimally invasive breast biopsy for suspicious mammogram, ultrasound, or MRI findings.
But the results demonstrated that NTBS "cannot be used as a successful adjunct to mammography, nor can it replace any of the screening modalities that are standard practice. Mammography remains the gold standard for breast cancer screening," said Dr. Barrio, an attending breast surgeon at Bryn Mawr (Pa.) Hospital.
"I think the utility of NTBS remains unclear. For the purposes of our study, NTBS could not discriminate between benign and malignant lesions in the low-specificity mode, and the high-sensitivity mode resulted in an unacceptable number of false-positive results," she added in an interview.
A total of 181 women (median age at diagnosis 52.5 years) with 187 abnormal radiologic findings were evaluated from October 2009 to May 2011. Each patient had an NTBS prior to tissue biopsy, and final tissue pathologies were compared with the corresponding NTBS scan results. Each breast was interpreted as positive or negative based on computer analysis of thermal abnormalities. The contralateral breast was scanned in all patients.
Prior to Oct. 15, 2010, patients were initially scanned using a "high-specificity" NTBS mode termed NTBS1. Subsequently a "high-sensitivity mode (NTBS2)" was used to minimize false-negative results. Following initial data analysis, all patients were retrospectively re-evaluated in the NTBS2 mode.
Of the 181 patients initially evaluated, 3 were excluded due to a nonductal or lobular breast malignancy, leaving a total of 178 patients. Of those, 50 had 52 positive breast biopsies and 128 had 132 negative biopsies.
Of the 52 positive biopsies, only 26 had a positive NTBS, giving a sensitivity of just 50%. The sensitivity of NTBS was even lower in the 20 in situ cancers, compared with the 32 invasive cancers (35% vs. 59%, respectively), Dr. Barrio reported.
Of the 132 negative biopsies, 88 had negative NTBS scans, giving a 67% specificity. "The positive predictive value of NTBS was 37% and the negative predictive value was 77%," the study results showed.
Of 173 normal contralateral breasts that were scanned, 42 (24%) had a positive NTBS scan.
Among the 178 patients retrospectively evaluated using NTBS2, 22 were excluded because of an uninterpretable scan. Of the remaining 156 patients, 44 had 46 positive breast biopsies and 112 had 116 negative biopsies. Forty of the 46 positive biopsies matched a positive NTBS (sensitivity 87%).
Sensitivity was not appreciably different between in situ and invasive cancers in the NTBS2 mode (88% vs. 86%, respectively), she said.
Of the 116 negative biopsies, 55 had a negative NTBS, giving a specificity of just 48%. "The positive predictive value of NTBS2 was 40% and the negative predictive value was 90%," the study reported. Of the 151 normal contralateral breasts that were scanned, 72 (47%) had a positive reading.
In the interview, Dr. Barrio said that her group is not seeking a replacement for mammography, as it is a cost-efficient screening tool that has been proven to decrease mortality from breast cancer.
However, "we are looking for studies to supplement mammography, in order to address its limitations, i.e., dense breasts. I think molecular breast imaging in particular shows a lot of promise for the future in women with dense breasts."
This study was funded by the Humler Oncology fund. Dr. Barrio had no other disclosures.
Infrared thermography did not accurately predict malignancy and produced an unacceptably high false-positive rate in women with radiologic abnormalities requiring breast biopsy in a 2-year prospective study.
The No-Touch Breast Scan (NTBS) is a noninvasive, non–radiation-based imaging tool that measures and compares thermal abnormalities in breasts using dual infrared cameras and computer analysis. It generates a score that reflects blood flow patterns based on the theory of tumor angiogenesis.
The technology is being explored as an alternative to radiation-based imaging in women at risk for breast cancer and as a way to reduce the number of benign biopsies, Dr. Andrea V. Barrio said during a press briefing at the annual meeting of the American Society of Breast Surgeons.
This study evaluated NTBS screening as a predictor of breast cancer in patients undergoing minimally invasive breast biopsy for suspicious mammogram, ultrasound, or MRI findings.
But the results demonstrated that NTBS "cannot be used as a successful adjunct to mammography, nor can it replace any of the screening modalities that are standard practice. Mammography remains the gold standard for breast cancer screening," said Dr. Barrio, an attending breast surgeon at Bryn Mawr (Pa.) Hospital.
"I think the utility of NTBS remains unclear. For the purposes of our study, NTBS could not discriminate between benign and malignant lesions in the low-specificity mode, and the high-sensitivity mode resulted in an unacceptable number of false-positive results," she added in an interview.
A total of 181 women (median age at diagnosis 52.5 years) with 187 abnormal radiologic findings were evaluated from October 2009 to May 2011. Each patient had an NTBS prior to tissue biopsy, and final tissue pathologies were compared with the corresponding NTBS scan results. Each breast was interpreted as positive or negative based on computer analysis of thermal abnormalities. The contralateral breast was scanned in all patients.
Prior to Oct. 15, 2010, patients were initially scanned using a "high-specificity" NTBS mode termed NTBS1. Subsequently a "high-sensitivity mode (NTBS2)" was used to minimize false-negative results. Following initial data analysis, all patients were retrospectively re-evaluated in the NTBS2 mode.
Of the 181 patients initially evaluated, 3 were excluded due to a nonductal or lobular breast malignancy, leaving a total of 178 patients. Of those, 50 had 52 positive breast biopsies and 128 had 132 negative biopsies.
Of the 52 positive biopsies, only 26 had a positive NTBS, giving a sensitivity of just 50%. The sensitivity of NTBS was even lower in the 20 in situ cancers, compared with the 32 invasive cancers (35% vs. 59%, respectively), Dr. Barrio reported.
Of the 132 negative biopsies, 88 had negative NTBS scans, giving a 67% specificity. "The positive predictive value of NTBS was 37% and the negative predictive value was 77%," the study results showed.
Of 173 normal contralateral breasts that were scanned, 42 (24%) had a positive NTBS scan.
Among the 178 patients retrospectively evaluated using NTBS2, 22 were excluded because of an uninterpretable scan. Of the remaining 156 patients, 44 had 46 positive breast biopsies and 112 had 116 negative biopsies. Forty of the 46 positive biopsies matched a positive NTBS (sensitivity 87%).
Sensitivity was not appreciably different between in situ and invasive cancers in the NTBS2 mode (88% vs. 86%, respectively), she said.
Of the 116 negative biopsies, 55 had a negative NTBS, giving a specificity of just 48%. "The positive predictive value of NTBS2 was 40% and the negative predictive value was 90%," the study reported. Of the 151 normal contralateral breasts that were scanned, 72 (47%) had a positive reading.
In the interview, Dr. Barrio said that her group is not seeking a replacement for mammography, as it is a cost-efficient screening tool that has been proven to decrease mortality from breast cancer.
However, "we are looking for studies to supplement mammography, in order to address its limitations, i.e., dense breasts. I think molecular breast imaging in particular shows a lot of promise for the future in women with dense breasts."
This study was funded by the Humler Oncology fund. Dr. Barrio had no other disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF BREAST SURGEONS
Breast Cancer More Lethal in Men
Men with breast cancer died more than 2 years sooner than did women with the condition, in the largest-ever study of male breast cancer, investigators reported.
Male breast cancer patients presented with more advanced disease and had lower 5-year survival rates as well as shorter median overall survival than did women, Dr. Jon M. Greif said at the annual meeting of the American Society of Breast Surgeons.
They were less likely to have radiation therapy or partial mastectomy, but chemotherapy rates were not significantly different, said Dr. Greif, a breast surgeon who practices in Oakland, Calif.
The data come from an analysis of 13,457 men – representing 0.9% of all breast cancers – and 1,439,866 women with breast cancer in the National Cancer Data Base spanning the years 1998 through 2007. The explanation for the differences in overall survival is most likely multifactorial, according to Dr. Greif.
"Certainly, one reason is that with well accepted screening for female breast cancer, and heightened awareness amongst women, female breast cancer is detected earlier. Evidence from our study is that male breast cancer is larger and more likely to have spread to lymph nodes and beyond when first discovered," he said in an interview.
"However, male breast cancer was less likely to be low grade, and this would be a biological difference. And, finally, men were older, and more likely to die of other causes."
Men at particularly high risk should have careful clinical examinations annually, and consider annual screening mammography, advised Dr. Greif. Among those at high risk, he included men with known gene mutations that increase their risk (BRCA and Klinefelter’s syndrome, for example), men who have been treated or otherwise exposed to high levels of radiation to the chest, men with previous breast cancer, and men with strong family histories of male or female breast cancer.
"Currently, breast cancer in men is found as a palpable retro- or periareolar mass, a nipple discharge or crusting, skin erosion, or palpable lymph nodes. Examination of the retroareolar and periareolar tissues for lumps and/or skin changes should be a part of every man’s annual physical exam, and men should check occasionally themselves," Dr. Greif said.
Five-year overall survival was 83% for women with breast cancer (median survival 129 months) and 74% for men (median 101 months), a highly statistically significant difference (P less than. 0001), he reported.
A comparison of overall survival by stages showed significantly better outcomes for women with early disease, but similar outcomes in more advanced disease. Females had significantly better 5-year survival rates (P less than .0001) for stage 0 (94% vs. 90%), stage I (90% vs. 87%) and stage II (82% vs. 74%) breast cancer. No significant differences were seen in 5-year survival for stage III (56.9% vs. 56.5%, P = .99) or stage IV (19% vs. 16%, P = .20).
The following findings also were reported:
– Men with breast cancer were more often African American (11.7% vs. 9.9%, odds ratio 1.19), less often Hispanic (3.6% vs. 4.5%, OR 0.74), and older (63 vs. 59 years old).
– Men had larger tumors (median 20.0 vs. 15.0 mm), were less likely to have grade 1 tumors (16.0% vs. 20.7%), were more likely to have lymph node metastasis (41.9% vs. 33.2%, OR 1.45), and were more likely to have distant metastasis (4% vs. 3%, OR 1.39).
– Men were less likely to have lobular carcinoma (10% vs. 18%, OR 0.51) and more likely to be estrogen receptor positive (88.3% vs. 78.2%, OR 2.10) and progesterone receptor positive (76.8% vs. 67.0%, OR 1.63).
– Men were less likely to have been treated with a partial mastectomy (33% vs. 62%, OR 0.31) and less likely to have received radiation (35.9% vs. 50.4%, OR 0.55).
All of these differences were highly statistically significant, with P values less than .0001. However, the differences may not have been of clinical significance, the investigators said, citing the large numbers of cases.
The proportions of men and women receiving chemotherapy were similar (40.1% vs. 39.8%, OR 1.01, P = .40) and only small differences were seen in hormonal therapy rates (41.2% vs. 42.4%, OR 0.95, P = .006).
Treatment of male breast cancer is similar to that of female breast cancer, according to Dr. Greif. Nearly all male breast cancers are hormone receptor positive, so treatment with antiestrogenic endocrine therapy should be a part of the adjuvant treatment of nearly all male breast cancer.
Chemotherapy should be considered for tumors with higher risk of systemic return, he said. For tumors with risk of locoregional return, including those that are large and/or have lymph node involvement, adjuvant radiation should be part of the treatment.
The surgery for male breast cancer is almost always total mastectomy, he noted, and there is evidence that sentinel lymph node biopsy works for male breast cancer as well as in female breast cancer.
This study was funded in part by Alta Bates Summit Medical Center. None of the authors have any conflicts of interest or financial disclosures.
Men with breast cancer died more than 2 years sooner than did women with the condition, in the largest-ever study of male breast cancer, investigators reported.
Male breast cancer patients presented with more advanced disease and had lower 5-year survival rates as well as shorter median overall survival than did women, Dr. Jon M. Greif said at the annual meeting of the American Society of Breast Surgeons.
They were less likely to have radiation therapy or partial mastectomy, but chemotherapy rates were not significantly different, said Dr. Greif, a breast surgeon who practices in Oakland, Calif.
The data come from an analysis of 13,457 men – representing 0.9% of all breast cancers – and 1,439,866 women with breast cancer in the National Cancer Data Base spanning the years 1998 through 2007. The explanation for the differences in overall survival is most likely multifactorial, according to Dr. Greif.
"Certainly, one reason is that with well accepted screening for female breast cancer, and heightened awareness amongst women, female breast cancer is detected earlier. Evidence from our study is that male breast cancer is larger and more likely to have spread to lymph nodes and beyond when first discovered," he said in an interview.
"However, male breast cancer was less likely to be low grade, and this would be a biological difference. And, finally, men were older, and more likely to die of other causes."
Men at particularly high risk should have careful clinical examinations annually, and consider annual screening mammography, advised Dr. Greif. Among those at high risk, he included men with known gene mutations that increase their risk (BRCA and Klinefelter’s syndrome, for example), men who have been treated or otherwise exposed to high levels of radiation to the chest, men with previous breast cancer, and men with strong family histories of male or female breast cancer.
"Currently, breast cancer in men is found as a palpable retro- or periareolar mass, a nipple discharge or crusting, skin erosion, or palpable lymph nodes. Examination of the retroareolar and periareolar tissues for lumps and/or skin changes should be a part of every man’s annual physical exam, and men should check occasionally themselves," Dr. Greif said.
Five-year overall survival was 83% for women with breast cancer (median survival 129 months) and 74% for men (median 101 months), a highly statistically significant difference (P less than. 0001), he reported.
A comparison of overall survival by stages showed significantly better outcomes for women with early disease, but similar outcomes in more advanced disease. Females had significantly better 5-year survival rates (P less than .0001) for stage 0 (94% vs. 90%), stage I (90% vs. 87%) and stage II (82% vs. 74%) breast cancer. No significant differences were seen in 5-year survival for stage III (56.9% vs. 56.5%, P = .99) or stage IV (19% vs. 16%, P = .20).
The following findings also were reported:
– Men with breast cancer were more often African American (11.7% vs. 9.9%, odds ratio 1.19), less often Hispanic (3.6% vs. 4.5%, OR 0.74), and older (63 vs. 59 years old).
– Men had larger tumors (median 20.0 vs. 15.0 mm), were less likely to have grade 1 tumors (16.0% vs. 20.7%), were more likely to have lymph node metastasis (41.9% vs. 33.2%, OR 1.45), and were more likely to have distant metastasis (4% vs. 3%, OR 1.39).
– Men were less likely to have lobular carcinoma (10% vs. 18%, OR 0.51) and more likely to be estrogen receptor positive (88.3% vs. 78.2%, OR 2.10) and progesterone receptor positive (76.8% vs. 67.0%, OR 1.63).
– Men were less likely to have been treated with a partial mastectomy (33% vs. 62%, OR 0.31) and less likely to have received radiation (35.9% vs. 50.4%, OR 0.55).
All of these differences were highly statistically significant, with P values less than .0001. However, the differences may not have been of clinical significance, the investigators said, citing the large numbers of cases.
The proportions of men and women receiving chemotherapy were similar (40.1% vs. 39.8%, OR 1.01, P = .40) and only small differences were seen in hormonal therapy rates (41.2% vs. 42.4%, OR 0.95, P = .006).
Treatment of male breast cancer is similar to that of female breast cancer, according to Dr. Greif. Nearly all male breast cancers are hormone receptor positive, so treatment with antiestrogenic endocrine therapy should be a part of the adjuvant treatment of nearly all male breast cancer.
Chemotherapy should be considered for tumors with higher risk of systemic return, he said. For tumors with risk of locoregional return, including those that are large and/or have lymph node involvement, adjuvant radiation should be part of the treatment.
The surgery for male breast cancer is almost always total mastectomy, he noted, and there is evidence that sentinel lymph node biopsy works for male breast cancer as well as in female breast cancer.
This study was funded in part by Alta Bates Summit Medical Center. None of the authors have any conflicts of interest or financial disclosures.
Men with breast cancer died more than 2 years sooner than did women with the condition, in the largest-ever study of male breast cancer, investigators reported.
Male breast cancer patients presented with more advanced disease and had lower 5-year survival rates as well as shorter median overall survival than did women, Dr. Jon M. Greif said at the annual meeting of the American Society of Breast Surgeons.
They were less likely to have radiation therapy or partial mastectomy, but chemotherapy rates were not significantly different, said Dr. Greif, a breast surgeon who practices in Oakland, Calif.
The data come from an analysis of 13,457 men – representing 0.9% of all breast cancers – and 1,439,866 women with breast cancer in the National Cancer Data Base spanning the years 1998 through 2007. The explanation for the differences in overall survival is most likely multifactorial, according to Dr. Greif.
"Certainly, one reason is that with well accepted screening for female breast cancer, and heightened awareness amongst women, female breast cancer is detected earlier. Evidence from our study is that male breast cancer is larger and more likely to have spread to lymph nodes and beyond when first discovered," he said in an interview.
"However, male breast cancer was less likely to be low grade, and this would be a biological difference. And, finally, men were older, and more likely to die of other causes."
Men at particularly high risk should have careful clinical examinations annually, and consider annual screening mammography, advised Dr. Greif. Among those at high risk, he included men with known gene mutations that increase their risk (BRCA and Klinefelter’s syndrome, for example), men who have been treated or otherwise exposed to high levels of radiation to the chest, men with previous breast cancer, and men with strong family histories of male or female breast cancer.
"Currently, breast cancer in men is found as a palpable retro- or periareolar mass, a nipple discharge or crusting, skin erosion, or palpable lymph nodes. Examination of the retroareolar and periareolar tissues for lumps and/or skin changes should be a part of every man’s annual physical exam, and men should check occasionally themselves," Dr. Greif said.
Five-year overall survival was 83% for women with breast cancer (median survival 129 months) and 74% for men (median 101 months), a highly statistically significant difference (P less than. 0001), he reported.
A comparison of overall survival by stages showed significantly better outcomes for women with early disease, but similar outcomes in more advanced disease. Females had significantly better 5-year survival rates (P less than .0001) for stage 0 (94% vs. 90%), stage I (90% vs. 87%) and stage II (82% vs. 74%) breast cancer. No significant differences were seen in 5-year survival for stage III (56.9% vs. 56.5%, P = .99) or stage IV (19% vs. 16%, P = .20).
The following findings also were reported:
– Men with breast cancer were more often African American (11.7% vs. 9.9%, odds ratio 1.19), less often Hispanic (3.6% vs. 4.5%, OR 0.74), and older (63 vs. 59 years old).
– Men had larger tumors (median 20.0 vs. 15.0 mm), were less likely to have grade 1 tumors (16.0% vs. 20.7%), were more likely to have lymph node metastasis (41.9% vs. 33.2%, OR 1.45), and were more likely to have distant metastasis (4% vs. 3%, OR 1.39).
– Men were less likely to have lobular carcinoma (10% vs. 18%, OR 0.51) and more likely to be estrogen receptor positive (88.3% vs. 78.2%, OR 2.10) and progesterone receptor positive (76.8% vs. 67.0%, OR 1.63).
– Men were less likely to have been treated with a partial mastectomy (33% vs. 62%, OR 0.31) and less likely to have received radiation (35.9% vs. 50.4%, OR 0.55).
All of these differences were highly statistically significant, with P values less than .0001. However, the differences may not have been of clinical significance, the investigators said, citing the large numbers of cases.
The proportions of men and women receiving chemotherapy were similar (40.1% vs. 39.8%, OR 1.01, P = .40) and only small differences were seen in hormonal therapy rates (41.2% vs. 42.4%, OR 0.95, P = .006).
Treatment of male breast cancer is similar to that of female breast cancer, according to Dr. Greif. Nearly all male breast cancers are hormone receptor positive, so treatment with antiestrogenic endocrine therapy should be a part of the adjuvant treatment of nearly all male breast cancer.
Chemotherapy should be considered for tumors with higher risk of systemic return, he said. For tumors with risk of locoregional return, including those that are large and/or have lymph node involvement, adjuvant radiation should be part of the treatment.
The surgery for male breast cancer is almost always total mastectomy, he noted, and there is evidence that sentinel lymph node biopsy works for male breast cancer as well as in female breast cancer.
This study was funded in part by Alta Bates Summit Medical Center. None of the authors have any conflicts of interest or financial disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF BREAST SURGEONS
Major Finding: Five-year overall survival rates were 83% in women with breast cancer (median overall survival 129 months) and 74% in men (median 101 months), a highly significant statistical difference (P less than .0001).
Data Source: The data come from an analysis of 13,457 men and 1,439,866 women with breast cancer in the National Cancer Data Base spanning the years 1998 through 2007.
Disclosures: The study was funded in part by Alta Bates Summit Medical Centers. None of the authors have any conflicts of interest or financial disclosures.
Breast Brachytherapy Doubles Mastectomy Risk
A retrospective study of nearly 93,000 older women with invasive breast cancer suggests that brachytherapy after lumpectomy leads to more complications and more subsequent mastectomies than does postoperative whole-breast radiation.
The mastectomy rate 5 years later was about twice as high in women treated with brachytherapy – cumulative incidence 3.95% vs. 2.18% with whole-breast radiation (WBI) – and the difference persisted after a multivariate adjustment, with a hazard ratio of 2.19, according to a report published May 1, 2012 in JAMA.
Moreover, short-term and long-term complications, including breast pain, were significantly more common in women who had radiation delivered by brachytherapy. Overall survival was not significantly different, however, at about 87% in both groups studied.
What this means is that for every 56 women treated with brachytherapy, 1 woman was harmed with an unnecessary mastectomy (absolute excess risk, 1.77%), wrote Dr. Grace L. Smith of the University of Texas M.D. Anderson Cancer Center in Houston and her coauthors. At 1 year, 1 woman suffered an unnecessary postoperative complication for every 9 women treated with brachytherapy (absolute excess risk, 10.64%), and by 5 years, 1 woman for every 16 was harmed by an unnecessary postoperative radiation complication (absolute excess risk, 6.16%).
"Potential public health implications of these findings are substantial, given the high incidence of breast cancer, along with the recent rapid increase in breast brachytherapy use. Although these results await validation in the prospective setting, they also prompt caution over widespread application of breast brachytherapy outside the study setting," the authors concluded (JAMA 2012;307:1827-37).
An earlier version of the study stirred controversy when principal investigator Dr. Benjamin D. Smith, also of M.D. Anderson, presented it at the San Antonio Breast Cancer Symposium in December 2011. Three professional societies – the American Society for Radiation Oncology (ASTRO), American Society of Breast Surgeons, and American Brachytherapy Society – issued rebuttals soon after.
Among the objections raised were the retrospective nature of the study, limitations inherent in studies based on Medicare claims data, and the fact that the data did not take into account improvements in brachytherapy technology since the study years of 2000-2007. Definitive results from ongoing randomized trials comparing the safety and efficacy of brachytherapy and standard WBI are still years off, critics said, citing the ongoing phase III National Surgical Adjuvant Breast and Bowel Project (NSABP) B-39/Radiation Therapy Oncology Group (RTOG) 0413 trial.
For the current study, the investigators identified 92,735 women aged 67 years or older who had incident invasive breast cancer diagnosed between 2003 and 2007 and were followed through 2008. After lumpectomy, a large majority of the women studied, 85,783 (92.5%), underwent WBI, while 6,952 (7.5%) were treated with brachytherapy.
At 1 year, infectious skin or soft tissue infections were significantly more frequent with brachytherapy (16.20% vs. 10.33% with WBI), as were noninfectious postoperative complications (16.25% vs. 9.0%).
By 5 years, the cumulative incidence of breast pain reached 14.55% with brachytherapy, compared with 11.92% with WBI. Fat necrosis (8.26% vs. 4.05%) and rib fracture (4.53% vs. 3.62%) also occurred at higher rates in the brachytherapy group.
Dr. Smith was supported by a Multidisciplinary Postdoctoral Award from the Department of Defense. Coauthors Dr. Benjamin D. Smith and Dr. Sharon H. Giordano were supported by a grant from the Cancer Prevention and Research Institute of Texas. Dr. Ya-Chen Tina Shih was supported by grants from the Agency for Healthcare Research and Quality, the National Cancer Institute, and the University of Chicago Cancer Research Foundation Women’s Board. This study also was supported in part by grants from the National Cancer Institute and by a philanthropic gift from Ann and Clarence Cazalot.
A retrospective study of nearly 93,000 older women with invasive breast cancer suggests that brachytherapy after lumpectomy leads to more complications and more subsequent mastectomies than does postoperative whole-breast radiation.
The mastectomy rate 5 years later was about twice as high in women treated with brachytherapy – cumulative incidence 3.95% vs. 2.18% with whole-breast radiation (WBI) – and the difference persisted after a multivariate adjustment, with a hazard ratio of 2.19, according to a report published May 1, 2012 in JAMA.
Moreover, short-term and long-term complications, including breast pain, were significantly more common in women who had radiation delivered by brachytherapy. Overall survival was not significantly different, however, at about 87% in both groups studied.
What this means is that for every 56 women treated with brachytherapy, 1 woman was harmed with an unnecessary mastectomy (absolute excess risk, 1.77%), wrote Dr. Grace L. Smith of the University of Texas M.D. Anderson Cancer Center in Houston and her coauthors. At 1 year, 1 woman suffered an unnecessary postoperative complication for every 9 women treated with brachytherapy (absolute excess risk, 10.64%), and by 5 years, 1 woman for every 16 was harmed by an unnecessary postoperative radiation complication (absolute excess risk, 6.16%).
"Potential public health implications of these findings are substantial, given the high incidence of breast cancer, along with the recent rapid increase in breast brachytherapy use. Although these results await validation in the prospective setting, they also prompt caution over widespread application of breast brachytherapy outside the study setting," the authors concluded (JAMA 2012;307:1827-37).
An earlier version of the study stirred controversy when principal investigator Dr. Benjamin D. Smith, also of M.D. Anderson, presented it at the San Antonio Breast Cancer Symposium in December 2011. Three professional societies – the American Society for Radiation Oncology (ASTRO), American Society of Breast Surgeons, and American Brachytherapy Society – issued rebuttals soon after.
Among the objections raised were the retrospective nature of the study, limitations inherent in studies based on Medicare claims data, and the fact that the data did not take into account improvements in brachytherapy technology since the study years of 2000-2007. Definitive results from ongoing randomized trials comparing the safety and efficacy of brachytherapy and standard WBI are still years off, critics said, citing the ongoing phase III National Surgical Adjuvant Breast and Bowel Project (NSABP) B-39/Radiation Therapy Oncology Group (RTOG) 0413 trial.
For the current study, the investigators identified 92,735 women aged 67 years or older who had incident invasive breast cancer diagnosed between 2003 and 2007 and were followed through 2008. After lumpectomy, a large majority of the women studied, 85,783 (92.5%), underwent WBI, while 6,952 (7.5%) were treated with brachytherapy.
At 1 year, infectious skin or soft tissue infections were significantly more frequent with brachytherapy (16.20% vs. 10.33% with WBI), as were noninfectious postoperative complications (16.25% vs. 9.0%).
By 5 years, the cumulative incidence of breast pain reached 14.55% with brachytherapy, compared with 11.92% with WBI. Fat necrosis (8.26% vs. 4.05%) and rib fracture (4.53% vs. 3.62%) also occurred at higher rates in the brachytherapy group.
Dr. Smith was supported by a Multidisciplinary Postdoctoral Award from the Department of Defense. Coauthors Dr. Benjamin D. Smith and Dr. Sharon H. Giordano were supported by a grant from the Cancer Prevention and Research Institute of Texas. Dr. Ya-Chen Tina Shih was supported by grants from the Agency for Healthcare Research and Quality, the National Cancer Institute, and the University of Chicago Cancer Research Foundation Women’s Board. This study also was supported in part by grants from the National Cancer Institute and by a philanthropic gift from Ann and Clarence Cazalot.
A retrospective study of nearly 93,000 older women with invasive breast cancer suggests that brachytherapy after lumpectomy leads to more complications and more subsequent mastectomies than does postoperative whole-breast radiation.
The mastectomy rate 5 years later was about twice as high in women treated with brachytherapy – cumulative incidence 3.95% vs. 2.18% with whole-breast radiation (WBI) – and the difference persisted after a multivariate adjustment, with a hazard ratio of 2.19, according to a report published May 1, 2012 in JAMA.
Moreover, short-term and long-term complications, including breast pain, were significantly more common in women who had radiation delivered by brachytherapy. Overall survival was not significantly different, however, at about 87% in both groups studied.
What this means is that for every 56 women treated with brachytherapy, 1 woman was harmed with an unnecessary mastectomy (absolute excess risk, 1.77%), wrote Dr. Grace L. Smith of the University of Texas M.D. Anderson Cancer Center in Houston and her coauthors. At 1 year, 1 woman suffered an unnecessary postoperative complication for every 9 women treated with brachytherapy (absolute excess risk, 10.64%), and by 5 years, 1 woman for every 16 was harmed by an unnecessary postoperative radiation complication (absolute excess risk, 6.16%).
"Potential public health implications of these findings are substantial, given the high incidence of breast cancer, along with the recent rapid increase in breast brachytherapy use. Although these results await validation in the prospective setting, they also prompt caution over widespread application of breast brachytherapy outside the study setting," the authors concluded (JAMA 2012;307:1827-37).
An earlier version of the study stirred controversy when principal investigator Dr. Benjamin D. Smith, also of M.D. Anderson, presented it at the San Antonio Breast Cancer Symposium in December 2011. Three professional societies – the American Society for Radiation Oncology (ASTRO), American Society of Breast Surgeons, and American Brachytherapy Society – issued rebuttals soon after.
Among the objections raised were the retrospective nature of the study, limitations inherent in studies based on Medicare claims data, and the fact that the data did not take into account improvements in brachytherapy technology since the study years of 2000-2007. Definitive results from ongoing randomized trials comparing the safety and efficacy of brachytherapy and standard WBI are still years off, critics said, citing the ongoing phase III National Surgical Adjuvant Breast and Bowel Project (NSABP) B-39/Radiation Therapy Oncology Group (RTOG) 0413 trial.
For the current study, the investigators identified 92,735 women aged 67 years or older who had incident invasive breast cancer diagnosed between 2003 and 2007 and were followed through 2008. After lumpectomy, a large majority of the women studied, 85,783 (92.5%), underwent WBI, while 6,952 (7.5%) were treated with brachytherapy.
At 1 year, infectious skin or soft tissue infections were significantly more frequent with brachytherapy (16.20% vs. 10.33% with WBI), as were noninfectious postoperative complications (16.25% vs. 9.0%).
By 5 years, the cumulative incidence of breast pain reached 14.55% with brachytherapy, compared with 11.92% with WBI. Fat necrosis (8.26% vs. 4.05%) and rib fracture (4.53% vs. 3.62%) also occurred at higher rates in the brachytherapy group.
Dr. Smith was supported by a Multidisciplinary Postdoctoral Award from the Department of Defense. Coauthors Dr. Benjamin D. Smith and Dr. Sharon H. Giordano were supported by a grant from the Cancer Prevention and Research Institute of Texas. Dr. Ya-Chen Tina Shih was supported by grants from the Agency for Healthcare Research and Quality, the National Cancer Institute, and the University of Chicago Cancer Research Foundation Women’s Board. This study also was supported in part by grants from the National Cancer Institute and by a philanthropic gift from Ann and Clarence Cazalot.
FROM JAMA
More Counterfeit Bevacizumab Raises Legal Questions for Oncologists
The Food and Drug Administration has identified another batch of counterfeit bevacizumab in the United States, bringing with it concerns for physicians about their legal liability in the complex world of foreign-supplied drugs.
Agency lab tests confirmed that vials of Roche’s Altuzan 400 mg/16mL – a brand of bevacizumab approved in Turkey – contain no active ingredient, the FDA announced early in April. The only bevacizumab brand approved in the United States is Avastin, a product distributed by Roche-owned Genentech.
Subsequently, the agency sent letters to specific physicians in 13 states, who are believed to have purchased medications from foreign or unlicensed suppliers that sold illegal prescription medications. These medical practices "are putting patients at risk of exposure to medications that may be counterfeit, contaminated, improperly stored and transported, ineffective, and dangerous," the agency warned in the letters.
"Even if the identified drugs were not counterfeit, Altuzan is not approved by FDA for use in the United States. ... In virtually all cases, purchasing unapproved prescription drugs from foreign sources violates the Federal Food, Drug, and Cosmetic Act and is illegal," it advised the recipients.
Physicians Could Face Malpractice Suits
This language raises questions about physician liability when drugs are obtained from foreign distributors that have not been approved by the FDA. Not only do concerns about safety come into play, but intellectual property rights do as well, according to Dr. Maxwell Gregg Bloche, who is a physician, a professor of law, and codirector of the Georgetown–Johns Hopkins Joint Program in Law and Public Health.
Dr. Bloche emphasized a distinction between "the vast majority of prescriptions, which are not being supplied in the office" and those such as bevacizumab that are delivered in the context of a medical practice. In the former, the physician – acting as an enforcer of intellectual property law – could be acting against the interests of patients who might suffer terrible health consequences as a result of not being able to afford the drug. "However, when it comes to known counterfeit drugs that could be seriously dangerous, the physician’s role is to safeguard the patient," he said in an interview.
Adding complexity to the situation is an array of FDA-approved foreign suppliers, those foreign companies that knowingly supply dangerous or ineffective drugs, and a third category of suppliers that fall in between.
Outside the United States, there are reputable, high-quality pharmaceutical companies that offer generic drugs at much lower cost, noted Dr. Bloche. However, these companies often do not recognize U.S. patent laws. Only the name-brand drugs are legally available in the United States, until the FDA approves generic versions of those drugs.
"The physician is responsible for making medically sound judgments about risk," he said. If a patient mentions getting a prescribed drug from a foreign source, the physician can indicate that the source produces high-quality, generic versions. The patient may be breaking U.S. laws by doing so; the physician merely offered an opinion.
State malpractice laws come into play once the FDA has identified foreign suppliers that are selling counterfeit drugs – as in this round of counterfeit bevacizumab – and has warned physicians and the public, according to Dr. Bloche. Any physician who is still prescribing and/or using the drug for patients, or who tells the patient that the drug is safe, is then potentially liable.
"I think the medical malpractice law is the one that most physicians are going to be afraid of, which is a state tort law issue, said Dr. Bloche.
In recent months, one oncologist has been prosecuted on charges of distributing and receiving "misbranded and adulterated" prescription drugs in a case brought by the United States Attorney’s Office for the Eastern District of Missouri. Dr. Abid S. Nisar pleaded guilty to one count of "misbranding drugs," according to a government statement. The legal action was part of a larger case involving Neupogen, Herceptin, and Rituxan, but not Avastin, and a distributor known as Ban Dune Marketing Inc. (BDMI).
Buy From a Reputable Distributor
For practicing oncologist Dr. Patrick W. Cobb, "the main way that you know that you’re getting the right thing is to buy it from a reputable distributor. ... When we start looking outside of the large distributors, that’s when you run into problems," said Dr. Cobb, managing partner at Frontier Cancer Center in Billings, Montana, and former president of the Community Oncology Alliance.
When a drug is procured and given directly to a patient by a physician – as is the case with many oncology drugs – "the only safe move is for the physician to follow U.S. law, because then the physician is liable," Dr. Cobb said in an interview.
"When you’re an oncologist administering these kinds of drugs, you just can’t take that chance. You want to make sure that what you give the patient is exactly the drug and exactly the dosage."
In its first fake-bevacizumab warning, the FDA said that 19 U.S. medical practices obtained the counterfeit from Quality Specialty Products (QSP), a foreign supplier also known as Montana Health Care Solutions. QSP products are also distributed by Volunteer Distribution in Gainesboro, Tenn.
The agency specified medications purchased from a foreign distributor named Richards Pharma, also known as Richards Services, Warwick Healthcare Solutions, or Ban Dune Marketing Inc. (BDMI) in its more recent letter alerting oncologists to the second counterfeit.
"Packaging or vials found in the [United States] that claim to be Roche’s Altuzan with lot number B6021 should be considered counterfeit," the agency wrote. The counterfeit version of Altuzan contains "no active ingredient."
Other drugs already obtained from these sources are also suspect, according to the letter. "Many, if not all, of the products sold and distributed through this distributor have not been approved by the FDA," the agency said.
What the FDA Wants Physicians to Do
The FDA advised physicians to stop using these products and to contact the FDA. The products should be retained and securely stored until further notice by the FDA. The agency recommends that physicians take the following actions:
• Report adverse events related to the use of suspect injectable cancer medicines to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
• Verify that a supplier is licensed in a particular state by going to the Drug Integrity and Supply Chain Security section of www.fda.gov for a list of state websites and contacts where this information can be found.
• Report suspected counterfeit products to the FDA Office of Criminal Investigations (OCI) at 800-551-3989, or by e-mail to DrugSupplyChainIntegrity@fda.hhs.gov.
Dr. Bloche and Dr. Cobb said they have no relevant conflicts of interest.
The Food and Drug Administration has identified another batch of counterfeit bevacizumab in the United States, bringing with it concerns for physicians about their legal liability in the complex world of foreign-supplied drugs.
Agency lab tests confirmed that vials of Roche’s Altuzan 400 mg/16mL – a brand of bevacizumab approved in Turkey – contain no active ingredient, the FDA announced early in April. The only bevacizumab brand approved in the United States is Avastin, a product distributed by Roche-owned Genentech.
Subsequently, the agency sent letters to specific physicians in 13 states, who are believed to have purchased medications from foreign or unlicensed suppliers that sold illegal prescription medications. These medical practices "are putting patients at risk of exposure to medications that may be counterfeit, contaminated, improperly stored and transported, ineffective, and dangerous," the agency warned in the letters.
"Even if the identified drugs were not counterfeit, Altuzan is not approved by FDA for use in the United States. ... In virtually all cases, purchasing unapproved prescription drugs from foreign sources violates the Federal Food, Drug, and Cosmetic Act and is illegal," it advised the recipients.
Physicians Could Face Malpractice Suits
This language raises questions about physician liability when drugs are obtained from foreign distributors that have not been approved by the FDA. Not only do concerns about safety come into play, but intellectual property rights do as well, according to Dr. Maxwell Gregg Bloche, who is a physician, a professor of law, and codirector of the Georgetown–Johns Hopkins Joint Program in Law and Public Health.
Dr. Bloche emphasized a distinction between "the vast majority of prescriptions, which are not being supplied in the office" and those such as bevacizumab that are delivered in the context of a medical practice. In the former, the physician – acting as an enforcer of intellectual property law – could be acting against the interests of patients who might suffer terrible health consequences as a result of not being able to afford the drug. "However, when it comes to known counterfeit drugs that could be seriously dangerous, the physician’s role is to safeguard the patient," he said in an interview.
Adding complexity to the situation is an array of FDA-approved foreign suppliers, those foreign companies that knowingly supply dangerous or ineffective drugs, and a third category of suppliers that fall in between.
Outside the United States, there are reputable, high-quality pharmaceutical companies that offer generic drugs at much lower cost, noted Dr. Bloche. However, these companies often do not recognize U.S. patent laws. Only the name-brand drugs are legally available in the United States, until the FDA approves generic versions of those drugs.
"The physician is responsible for making medically sound judgments about risk," he said. If a patient mentions getting a prescribed drug from a foreign source, the physician can indicate that the source produces high-quality, generic versions. The patient may be breaking U.S. laws by doing so; the physician merely offered an opinion.
State malpractice laws come into play once the FDA has identified foreign suppliers that are selling counterfeit drugs – as in this round of counterfeit bevacizumab – and has warned physicians and the public, according to Dr. Bloche. Any physician who is still prescribing and/or using the drug for patients, or who tells the patient that the drug is safe, is then potentially liable.
"I think the medical malpractice law is the one that most physicians are going to be afraid of, which is a state tort law issue, said Dr. Bloche.
In recent months, one oncologist has been prosecuted on charges of distributing and receiving "misbranded and adulterated" prescription drugs in a case brought by the United States Attorney’s Office for the Eastern District of Missouri. Dr. Abid S. Nisar pleaded guilty to one count of "misbranding drugs," according to a government statement. The legal action was part of a larger case involving Neupogen, Herceptin, and Rituxan, but not Avastin, and a distributor known as Ban Dune Marketing Inc. (BDMI).
Buy From a Reputable Distributor
For practicing oncologist Dr. Patrick W. Cobb, "the main way that you know that you’re getting the right thing is to buy it from a reputable distributor. ... When we start looking outside of the large distributors, that’s when you run into problems," said Dr. Cobb, managing partner at Frontier Cancer Center in Billings, Montana, and former president of the Community Oncology Alliance.
When a drug is procured and given directly to a patient by a physician – as is the case with many oncology drugs – "the only safe move is for the physician to follow U.S. law, because then the physician is liable," Dr. Cobb said in an interview.
"When you’re an oncologist administering these kinds of drugs, you just can’t take that chance. You want to make sure that what you give the patient is exactly the drug and exactly the dosage."
In its first fake-bevacizumab warning, the FDA said that 19 U.S. medical practices obtained the counterfeit from Quality Specialty Products (QSP), a foreign supplier also known as Montana Health Care Solutions. QSP products are also distributed by Volunteer Distribution in Gainesboro, Tenn.
The agency specified medications purchased from a foreign distributor named Richards Pharma, also known as Richards Services, Warwick Healthcare Solutions, or Ban Dune Marketing Inc. (BDMI) in its more recent letter alerting oncologists to the second counterfeit.
"Packaging or vials found in the [United States] that claim to be Roche’s Altuzan with lot number B6021 should be considered counterfeit," the agency wrote. The counterfeit version of Altuzan contains "no active ingredient."
Other drugs already obtained from these sources are also suspect, according to the letter. "Many, if not all, of the products sold and distributed through this distributor have not been approved by the FDA," the agency said.
What the FDA Wants Physicians to Do
The FDA advised physicians to stop using these products and to contact the FDA. The products should be retained and securely stored until further notice by the FDA. The agency recommends that physicians take the following actions:
• Report adverse events related to the use of suspect injectable cancer medicines to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
• Verify that a supplier is licensed in a particular state by going to the Drug Integrity and Supply Chain Security section of www.fda.gov for a list of state websites and contacts where this information can be found.
• Report suspected counterfeit products to the FDA Office of Criminal Investigations (OCI) at 800-551-3989, or by e-mail to DrugSupplyChainIntegrity@fda.hhs.gov.
Dr. Bloche and Dr. Cobb said they have no relevant conflicts of interest.
The Food and Drug Administration has identified another batch of counterfeit bevacizumab in the United States, bringing with it concerns for physicians about their legal liability in the complex world of foreign-supplied drugs.
Agency lab tests confirmed that vials of Roche’s Altuzan 400 mg/16mL – a brand of bevacizumab approved in Turkey – contain no active ingredient, the FDA announced early in April. The only bevacizumab brand approved in the United States is Avastin, a product distributed by Roche-owned Genentech.
Subsequently, the agency sent letters to specific physicians in 13 states, who are believed to have purchased medications from foreign or unlicensed suppliers that sold illegal prescription medications. These medical practices "are putting patients at risk of exposure to medications that may be counterfeit, contaminated, improperly stored and transported, ineffective, and dangerous," the agency warned in the letters.
"Even if the identified drugs were not counterfeit, Altuzan is not approved by FDA for use in the United States. ... In virtually all cases, purchasing unapproved prescription drugs from foreign sources violates the Federal Food, Drug, and Cosmetic Act and is illegal," it advised the recipients.
Physicians Could Face Malpractice Suits
This language raises questions about physician liability when drugs are obtained from foreign distributors that have not been approved by the FDA. Not only do concerns about safety come into play, but intellectual property rights do as well, according to Dr. Maxwell Gregg Bloche, who is a physician, a professor of law, and codirector of the Georgetown–Johns Hopkins Joint Program in Law and Public Health.
Dr. Bloche emphasized a distinction between "the vast majority of prescriptions, which are not being supplied in the office" and those such as bevacizumab that are delivered in the context of a medical practice. In the former, the physician – acting as an enforcer of intellectual property law – could be acting against the interests of patients who might suffer terrible health consequences as a result of not being able to afford the drug. "However, when it comes to known counterfeit drugs that could be seriously dangerous, the physician’s role is to safeguard the patient," he said in an interview.
Adding complexity to the situation is an array of FDA-approved foreign suppliers, those foreign companies that knowingly supply dangerous or ineffective drugs, and a third category of suppliers that fall in between.
Outside the United States, there are reputable, high-quality pharmaceutical companies that offer generic drugs at much lower cost, noted Dr. Bloche. However, these companies often do not recognize U.S. patent laws. Only the name-brand drugs are legally available in the United States, until the FDA approves generic versions of those drugs.
"The physician is responsible for making medically sound judgments about risk," he said. If a patient mentions getting a prescribed drug from a foreign source, the physician can indicate that the source produces high-quality, generic versions. The patient may be breaking U.S. laws by doing so; the physician merely offered an opinion.
State malpractice laws come into play once the FDA has identified foreign suppliers that are selling counterfeit drugs – as in this round of counterfeit bevacizumab – and has warned physicians and the public, according to Dr. Bloche. Any physician who is still prescribing and/or using the drug for patients, or who tells the patient that the drug is safe, is then potentially liable.
"I think the medical malpractice law is the one that most physicians are going to be afraid of, which is a state tort law issue, said Dr. Bloche.
In recent months, one oncologist has been prosecuted on charges of distributing and receiving "misbranded and adulterated" prescription drugs in a case brought by the United States Attorney’s Office for the Eastern District of Missouri. Dr. Abid S. Nisar pleaded guilty to one count of "misbranding drugs," according to a government statement. The legal action was part of a larger case involving Neupogen, Herceptin, and Rituxan, but not Avastin, and a distributor known as Ban Dune Marketing Inc. (BDMI).
Buy From a Reputable Distributor
For practicing oncologist Dr. Patrick W. Cobb, "the main way that you know that you’re getting the right thing is to buy it from a reputable distributor. ... When we start looking outside of the large distributors, that’s when you run into problems," said Dr. Cobb, managing partner at Frontier Cancer Center in Billings, Montana, and former president of the Community Oncology Alliance.
When a drug is procured and given directly to a patient by a physician – as is the case with many oncology drugs – "the only safe move is for the physician to follow U.S. law, because then the physician is liable," Dr. Cobb said in an interview.
"When you’re an oncologist administering these kinds of drugs, you just can’t take that chance. You want to make sure that what you give the patient is exactly the drug and exactly the dosage."
In its first fake-bevacizumab warning, the FDA said that 19 U.S. medical practices obtained the counterfeit from Quality Specialty Products (QSP), a foreign supplier also known as Montana Health Care Solutions. QSP products are also distributed by Volunteer Distribution in Gainesboro, Tenn.
The agency specified medications purchased from a foreign distributor named Richards Pharma, also known as Richards Services, Warwick Healthcare Solutions, or Ban Dune Marketing Inc. (BDMI) in its more recent letter alerting oncologists to the second counterfeit.
"Packaging or vials found in the [United States] that claim to be Roche’s Altuzan with lot number B6021 should be considered counterfeit," the agency wrote. The counterfeit version of Altuzan contains "no active ingredient."
Other drugs already obtained from these sources are also suspect, according to the letter. "Many, if not all, of the products sold and distributed through this distributor have not been approved by the FDA," the agency said.
What the FDA Wants Physicians to Do
The FDA advised physicians to stop using these products and to contact the FDA. The products should be retained and securely stored until further notice by the FDA. The agency recommends that physicians take the following actions:
• Report adverse events related to the use of suspect injectable cancer medicines to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
• Verify that a supplier is licensed in a particular state by going to the Drug Integrity and Supply Chain Security section of www.fda.gov for a list of state websites and contacts where this information can be found.
• Report suspected counterfeit products to the FDA Office of Criminal Investigations (OCI) at 800-551-3989, or by e-mail to DrugSupplyChainIntegrity@fda.hhs.gov.
Dr. Bloche and Dr. Cobb said they have no relevant conflicts of interest.
FDA Panel Endorses Ultrasound System for Screening Dense Breasts
An automated breast ultrasound system is on track to become the first such device with an indication for use as an adjunct to mammography when screening asymptomatic women with dense breasts for breast cancer.
The Food and Drug Administration’s Radiological Devices Panel voted 13-0 that the benefits of U-Systems Inc.’s somo-v automated breast ultrasound system outweigh the risks for breast cancer screening.
During their meeting, the advisory group also voted unanimously that the noninvasive device is safe and effective, despite FDA concerns over whether the firm’s pivotal study data could be generalized to a broader population.
Approval is not guaranteed, but the agency usually follows the advice of its advisory panels.
U-Systems is seeking premarket approval to increase breast cancer detection in asymptomatic women with dense breasts who have not had a previous clinical breast intervention following a negative mammogram screening. Somo-v was 510(k) cleared in 2005 for diagnostic use as an adjunct to x-ray mammography.
A key question the FDA asked the panel was whether the proposed expanded indication – and the way it would be applied in practice – was appropriately reflected in the firm’s pivotal study design. The panel agreed that it was.
The proposed indication for use "is fine the way it’s written," said panel member Dr. Carl D’Orsi, professor of radiology and hematology/oncology at Emory University in Atlanta. "It should not be changed," Dr. D’Orsi said, reflecting the panel’s consensus.
Premarket approval would make somo-v the first ultrasound device available in the United States with a breast cancer screening indication, according to the company. The system is already the only ultrasound device approved for breast cancer screening in Canada and Europe.
Supporting the premarket approval application is a retrospective, multireader study that enrolled 200 subjects at 13 U.S. sites – culled from the firm’s larger SOMO-INSIGHT clinical trial – to evaluate whether digital mammography in combination with the somo-v automated breast ultrasound system (ABUS) is more sensitive in detecting breast cancer in women with dense breast tissue than x-ray mammography screening alone.
The primary end point of the study was to determine radiologist readers’ performance with and without ABUS using a statistical methodology called "receiver operator characteristic" curve analysis. The mean area under the curve (AUC) values were 0.604 for x-ray mammography alone and 0.747 for x-ray mammography plus ABUS.
"Results from our pivotal clinical retrospective reader study demonstrate that use of screening mammography with ABUS provides a substantial, statistically significant clinical improvement in a reader’s ability to detect mammographically negative breast cancers," said CEO Ronald Ho during the meeting.
"Our primary end point was met," added principal investigator Maryellen Giger, Ph.D., professor of radiology at the University of Chicago.
The difference of 0.143 in the AUC values "was statistically significant, given that the lower bound of an estimated 95% confidence interval for the difference in AUC is 0.074," the FDA agreed.
According to the company, mammography’s sensitivity for the detection of breast cancer is 85% in women overall, but the rate is reduced to 65% in women with dense breast tissue. "Dense breast tissue has been proven to conceal malignant lesions, limiting the effectiveness of mammography," Mr. Ho said. Dense breast tissue also increases a woman’s risk of breast cancer by up to four to six times, according to U-Systems.
"What differentiates our device is that we can detect lesions that are missed by mammography ... and we do it in a very fast work flow that’s reproducible and operator independent," the firm stated. The technology is designed to follow the same work flow as a traditional mammography procedure and provide an exam in 15 minutes.
Real-World Management Questioned
The FDA questioned whether the pivotal study’s results could be extrapolated to a real-world population of patients. One hundred sixty-four subjects were used in the study’s primary statistical analysis (133 normal cases chosen at random and 31 cancer cases), with 12 noncancer cases being evaluated by mammography alone.
Patients with prior clinical breast interventions, such as those who had a breast augmentation or breast cancer treatments including biopsy, radiation, lumpectomy, or mastectomy, were excluded from the study. U-Systems said it excluded these patients to eliminate potential bias and/or confounding effects.
But while the firm is seeking approval of somo-v as a screening tool for women with dense breasts "who have not had a previous clinical breast intervention," the FDA argued that in the real world, "patient management that includes an ABUS scan may be considered for patients who have had prior clinical breast intervention."
The panel discussed whether the exclusion of patients with a prior clinical breast intervention should be specified in the indication, and agreed that it should, given the pivotal study design. Additional studies would be needed to remove the restriction, the panel said.
Some panelists expressed concern over whether somo-v use on biopsy patients, for instance, might increase the device’s false-positive rate, given surgical scars’ similarity to malignancies on ultrasound.
Nevertheless, a majority did not seem overly concerned about potential off-label use on patients with prior interventions. "It seems to me that that’s not a big problem," said panel member Dr. Daniel Kopans, professor of radiology at Harvard Medical School, Boston. "If FDA approves the technology even with these limitations, those of us in breast imaging will use it as we see fit. ... I don’t think that [the indication] is a huge limitation."
Operator Training Concerns
The panel also discussed the sponsor’s proposed physician training program, concluding that it is adequate. However, after the panel voted on safety and effectiveness, some members debated whether potential use of the system by nonimaging specialists could adversely impact outcomes and whether controls were needed to restrict use to accredited radiologists.
According to the company, somo-v’s automation is intended to "reduce operator dependence," and the firm claims that "any medical staff member can be trained to operate the ABUS device consistently and reproducibly." But some panel members cautioned that the simplicity could lead to harm in clinical practice.
"Potentially, you could be in a situation where the mammogram is done by breast-imaging specialists, but the ultrasound is done by nonimaging specialists, which could significantly affect results," said panelist Dr. Robert Faulk, a diagnostic radiologist in private practice in Omaha, Neb.
Dr. Kopans agreed, citing the Mammography Quality Standards Act’s radiologist accreditation training and urging that "similar constraints" are needed for somo-v.
But in response, Janine Morris, the FDA’s acting radiologic devices division director, reminded the panelists that the FDA "doesn’t regulate [the] practice of medicine."
Somo-v relies on proprietary hardware and software, and incorporates a scan head that automatically acquires wide field-of-view breast images while the patient lies supine on a standard exam table.
The images are transferred to the system’s somo-VIEWer 3D workstation, which displays the images in a 3D reconstructed coronal view, as well as standard ultrasound formats, including transverse, radial, multislice, sagittal and antiradial views.
Elsevier Global Medical News and "The Gray Sheet" are owned by Elsevier.
An automated breast ultrasound system is on track to become the first such device with an indication for use as an adjunct to mammography when screening asymptomatic women with dense breasts for breast cancer.
The Food and Drug Administration’s Radiological Devices Panel voted 13-0 that the benefits of U-Systems Inc.’s somo-v automated breast ultrasound system outweigh the risks for breast cancer screening.
During their meeting, the advisory group also voted unanimously that the noninvasive device is safe and effective, despite FDA concerns over whether the firm’s pivotal study data could be generalized to a broader population.
Approval is not guaranteed, but the agency usually follows the advice of its advisory panels.
U-Systems is seeking premarket approval to increase breast cancer detection in asymptomatic women with dense breasts who have not had a previous clinical breast intervention following a negative mammogram screening. Somo-v was 510(k) cleared in 2005 for diagnostic use as an adjunct to x-ray mammography.
A key question the FDA asked the panel was whether the proposed expanded indication – and the way it would be applied in practice – was appropriately reflected in the firm’s pivotal study design. The panel agreed that it was.
The proposed indication for use "is fine the way it’s written," said panel member Dr. Carl D’Orsi, professor of radiology and hematology/oncology at Emory University in Atlanta. "It should not be changed," Dr. D’Orsi said, reflecting the panel’s consensus.
Premarket approval would make somo-v the first ultrasound device available in the United States with a breast cancer screening indication, according to the company. The system is already the only ultrasound device approved for breast cancer screening in Canada and Europe.
Supporting the premarket approval application is a retrospective, multireader study that enrolled 200 subjects at 13 U.S. sites – culled from the firm’s larger SOMO-INSIGHT clinical trial – to evaluate whether digital mammography in combination with the somo-v automated breast ultrasound system (ABUS) is more sensitive in detecting breast cancer in women with dense breast tissue than x-ray mammography screening alone.
The primary end point of the study was to determine radiologist readers’ performance with and without ABUS using a statistical methodology called "receiver operator characteristic" curve analysis. The mean area under the curve (AUC) values were 0.604 for x-ray mammography alone and 0.747 for x-ray mammography plus ABUS.
"Results from our pivotal clinical retrospective reader study demonstrate that use of screening mammography with ABUS provides a substantial, statistically significant clinical improvement in a reader’s ability to detect mammographically negative breast cancers," said CEO Ronald Ho during the meeting.
"Our primary end point was met," added principal investigator Maryellen Giger, Ph.D., professor of radiology at the University of Chicago.
The difference of 0.143 in the AUC values "was statistically significant, given that the lower bound of an estimated 95% confidence interval for the difference in AUC is 0.074," the FDA agreed.
According to the company, mammography’s sensitivity for the detection of breast cancer is 85% in women overall, but the rate is reduced to 65% in women with dense breast tissue. "Dense breast tissue has been proven to conceal malignant lesions, limiting the effectiveness of mammography," Mr. Ho said. Dense breast tissue also increases a woman’s risk of breast cancer by up to four to six times, according to U-Systems.
"What differentiates our device is that we can detect lesions that are missed by mammography ... and we do it in a very fast work flow that’s reproducible and operator independent," the firm stated. The technology is designed to follow the same work flow as a traditional mammography procedure and provide an exam in 15 minutes.
Real-World Management Questioned
The FDA questioned whether the pivotal study’s results could be extrapolated to a real-world population of patients. One hundred sixty-four subjects were used in the study’s primary statistical analysis (133 normal cases chosen at random and 31 cancer cases), with 12 noncancer cases being evaluated by mammography alone.
Patients with prior clinical breast interventions, such as those who had a breast augmentation or breast cancer treatments including biopsy, radiation, lumpectomy, or mastectomy, were excluded from the study. U-Systems said it excluded these patients to eliminate potential bias and/or confounding effects.
But while the firm is seeking approval of somo-v as a screening tool for women with dense breasts "who have not had a previous clinical breast intervention," the FDA argued that in the real world, "patient management that includes an ABUS scan may be considered for patients who have had prior clinical breast intervention."
The panel discussed whether the exclusion of patients with a prior clinical breast intervention should be specified in the indication, and agreed that it should, given the pivotal study design. Additional studies would be needed to remove the restriction, the panel said.
Some panelists expressed concern over whether somo-v use on biopsy patients, for instance, might increase the device’s false-positive rate, given surgical scars’ similarity to malignancies on ultrasound.
Nevertheless, a majority did not seem overly concerned about potential off-label use on patients with prior interventions. "It seems to me that that’s not a big problem," said panel member Dr. Daniel Kopans, professor of radiology at Harvard Medical School, Boston. "If FDA approves the technology even with these limitations, those of us in breast imaging will use it as we see fit. ... I don’t think that [the indication] is a huge limitation."
Operator Training Concerns
The panel also discussed the sponsor’s proposed physician training program, concluding that it is adequate. However, after the panel voted on safety and effectiveness, some members debated whether potential use of the system by nonimaging specialists could adversely impact outcomes and whether controls were needed to restrict use to accredited radiologists.
According to the company, somo-v’s automation is intended to "reduce operator dependence," and the firm claims that "any medical staff member can be trained to operate the ABUS device consistently and reproducibly." But some panel members cautioned that the simplicity could lead to harm in clinical practice.
"Potentially, you could be in a situation where the mammogram is done by breast-imaging specialists, but the ultrasound is done by nonimaging specialists, which could significantly affect results," said panelist Dr. Robert Faulk, a diagnostic radiologist in private practice in Omaha, Neb.
Dr. Kopans agreed, citing the Mammography Quality Standards Act’s radiologist accreditation training and urging that "similar constraints" are needed for somo-v.
But in response, Janine Morris, the FDA’s acting radiologic devices division director, reminded the panelists that the FDA "doesn’t regulate [the] practice of medicine."
Somo-v relies on proprietary hardware and software, and incorporates a scan head that automatically acquires wide field-of-view breast images while the patient lies supine on a standard exam table.
The images are transferred to the system’s somo-VIEWer 3D workstation, which displays the images in a 3D reconstructed coronal view, as well as standard ultrasound formats, including transverse, radial, multislice, sagittal and antiradial views.
Elsevier Global Medical News and "The Gray Sheet" are owned by Elsevier.
An automated breast ultrasound system is on track to become the first such device with an indication for use as an adjunct to mammography when screening asymptomatic women with dense breasts for breast cancer.
The Food and Drug Administration’s Radiological Devices Panel voted 13-0 that the benefits of U-Systems Inc.’s somo-v automated breast ultrasound system outweigh the risks for breast cancer screening.
During their meeting, the advisory group also voted unanimously that the noninvasive device is safe and effective, despite FDA concerns over whether the firm’s pivotal study data could be generalized to a broader population.
Approval is not guaranteed, but the agency usually follows the advice of its advisory panels.
U-Systems is seeking premarket approval to increase breast cancer detection in asymptomatic women with dense breasts who have not had a previous clinical breast intervention following a negative mammogram screening. Somo-v was 510(k) cleared in 2005 for diagnostic use as an adjunct to x-ray mammography.
A key question the FDA asked the panel was whether the proposed expanded indication – and the way it would be applied in practice – was appropriately reflected in the firm’s pivotal study design. The panel agreed that it was.
The proposed indication for use "is fine the way it’s written," said panel member Dr. Carl D’Orsi, professor of radiology and hematology/oncology at Emory University in Atlanta. "It should not be changed," Dr. D’Orsi said, reflecting the panel’s consensus.
Premarket approval would make somo-v the first ultrasound device available in the United States with a breast cancer screening indication, according to the company. The system is already the only ultrasound device approved for breast cancer screening in Canada and Europe.
Supporting the premarket approval application is a retrospective, multireader study that enrolled 200 subjects at 13 U.S. sites – culled from the firm’s larger SOMO-INSIGHT clinical trial – to evaluate whether digital mammography in combination with the somo-v automated breast ultrasound system (ABUS) is more sensitive in detecting breast cancer in women with dense breast tissue than x-ray mammography screening alone.
The primary end point of the study was to determine radiologist readers’ performance with and without ABUS using a statistical methodology called "receiver operator characteristic" curve analysis. The mean area under the curve (AUC) values were 0.604 for x-ray mammography alone and 0.747 for x-ray mammography plus ABUS.
"Results from our pivotal clinical retrospective reader study demonstrate that use of screening mammography with ABUS provides a substantial, statistically significant clinical improvement in a reader’s ability to detect mammographically negative breast cancers," said CEO Ronald Ho during the meeting.
"Our primary end point was met," added principal investigator Maryellen Giger, Ph.D., professor of radiology at the University of Chicago.
The difference of 0.143 in the AUC values "was statistically significant, given that the lower bound of an estimated 95% confidence interval for the difference in AUC is 0.074," the FDA agreed.
According to the company, mammography’s sensitivity for the detection of breast cancer is 85% in women overall, but the rate is reduced to 65% in women with dense breast tissue. "Dense breast tissue has been proven to conceal malignant lesions, limiting the effectiveness of mammography," Mr. Ho said. Dense breast tissue also increases a woman’s risk of breast cancer by up to four to six times, according to U-Systems.
"What differentiates our device is that we can detect lesions that are missed by mammography ... and we do it in a very fast work flow that’s reproducible and operator independent," the firm stated. The technology is designed to follow the same work flow as a traditional mammography procedure and provide an exam in 15 minutes.
Real-World Management Questioned
The FDA questioned whether the pivotal study’s results could be extrapolated to a real-world population of patients. One hundred sixty-four subjects were used in the study’s primary statistical analysis (133 normal cases chosen at random and 31 cancer cases), with 12 noncancer cases being evaluated by mammography alone.
Patients with prior clinical breast interventions, such as those who had a breast augmentation or breast cancer treatments including biopsy, radiation, lumpectomy, or mastectomy, were excluded from the study. U-Systems said it excluded these patients to eliminate potential bias and/or confounding effects.
But while the firm is seeking approval of somo-v as a screening tool for women with dense breasts "who have not had a previous clinical breast intervention," the FDA argued that in the real world, "patient management that includes an ABUS scan may be considered for patients who have had prior clinical breast intervention."
The panel discussed whether the exclusion of patients with a prior clinical breast intervention should be specified in the indication, and agreed that it should, given the pivotal study design. Additional studies would be needed to remove the restriction, the panel said.
Some panelists expressed concern over whether somo-v use on biopsy patients, for instance, might increase the device’s false-positive rate, given surgical scars’ similarity to malignancies on ultrasound.
Nevertheless, a majority did not seem overly concerned about potential off-label use on patients with prior interventions. "It seems to me that that’s not a big problem," said panel member Dr. Daniel Kopans, professor of radiology at Harvard Medical School, Boston. "If FDA approves the technology even with these limitations, those of us in breast imaging will use it as we see fit. ... I don’t think that [the indication] is a huge limitation."
Operator Training Concerns
The panel also discussed the sponsor’s proposed physician training program, concluding that it is adequate. However, after the panel voted on safety and effectiveness, some members debated whether potential use of the system by nonimaging specialists could adversely impact outcomes and whether controls were needed to restrict use to accredited radiologists.
According to the company, somo-v’s automation is intended to "reduce operator dependence," and the firm claims that "any medical staff member can be trained to operate the ABUS device consistently and reproducibly." But some panel members cautioned that the simplicity could lead to harm in clinical practice.
"Potentially, you could be in a situation where the mammogram is done by breast-imaging specialists, but the ultrasound is done by nonimaging specialists, which could significantly affect results," said panelist Dr. Robert Faulk, a diagnostic radiologist in private practice in Omaha, Neb.
Dr. Kopans agreed, citing the Mammography Quality Standards Act’s radiologist accreditation training and urging that "similar constraints" are needed for somo-v.
But in response, Janine Morris, the FDA’s acting radiologic devices division director, reminded the panelists that the FDA "doesn’t regulate [the] practice of medicine."
Somo-v relies on proprietary hardware and software, and incorporates a scan head that automatically acquires wide field-of-view breast images while the patient lies supine on a standard exam table.
The images are transferred to the system’s somo-VIEWer 3D workstation, which displays the images in a 3D reconstructed coronal view, as well as standard ultrasound formats, including transverse, radial, multislice, sagittal and antiradial views.
Elsevier Global Medical News and "The Gray Sheet" are owned by Elsevier.
Looks Aren’t Everything in Breast Reconstruction
Before-and-after photographs are the stock in trade of house painters, auto repair shops, and, yes, plastic and reconstructive surgeons. But a new study may make the last group pause, since it hints that far more is at play in breast cancer patients’ definition of “successful’’ breast reconstruction surgery than how their breasts appear.
The study from Liverpool, England (J Plast Reconstr Aesthet Surg (2012): doi:10.1016/j.bjps.2012.03.005) sidestepped traditional measures used to evaluate outcomes of aesthetic breast surgery and instead asked open-ended questions of survivors who had undergone reconstruction 1-8 years previously.
What the researchers discovered, not surprisingly, is that reconstruction patients are quite unlike cosmetic surgery patients in fundamental and important ways.
Of particular interest in the initial study cohort of 95 patients were 38 whose subjective evaluations of their surgical results completely contradicted objective ratings of cosmesis, the final appearance of the reconstructed breast(s) by surgeons and surgical nurses.
Incredibly, the association between women’s assessments and objective cosmesis ratings failed even to reach statistical significance.
In a structured data analysis of themes that arose in open-ended interviews with 27 of the survivors, the strongest link to women’s satisfaction with the procedure was the surgeon-patient relationship.
Next came the significance of reconstruction in what patients saw as the “completion of the cancer journey,” the authors wrote. “Patients who focused on this were positive about reconstruction that practitioners had rated negatively.”
A previous study asked patients about scarring, finding a correlation between scarring and dissatisfaction with reconstruction. But scarring wasn’t even a blip on the radar when, quoting from the Liverpool study, “we allowed patients to tell us what mattered to them rather than imposing our preconceptions.”
“It seems that surgeons and patients normally ‘talk different languages’; one technical and the other drawing more from relationships and patients’ sense of how normal they feel and appear and from their sense that reconstruction completes their cancer journey,” the investigators concluded. “In preoperative consultations, surgeons concentrate almost exclusively on the technical and cosmetic aspects of reconstruction: what can be achieved and what complications can occur.”
Of course, women who struggled with complications tended to factor that in to their assessments of their results, even if their final cosmetic outcome was considered by surgeons to be excellent.
Others were disappointed despite what seemed to surgeons to be excellent cosmetic results because, as one said, “I was expecting to feel feminine again, but I don’t, I don’t at all.”
What is perhaps even more interesting is to eavesdrop on the comments of women whose surgeons judged their cosmetic result to be poor.
Said one, “I had a really good relationship with (the surgeon) and I just found it so reassuring to see her. That was part of the whole thing really. She was just so positive, and so, well, just understanding I think … I was really glad that I had chosen that form of reconstruction because I had this regular contact with her.”
Said another, who felt “normal” despite what her surgeon considered to be a poor result: “If I didn’t have it done, I wouldn’t have felt normal at all. It would always remind me of what had happened.”
A highly complex patient-surgeon dance occurs when breast surgery is performed for more than cosmetic reasons, the study found.
One woman, disappointed with the way her reconstructed breast fit in a bra, could not bring herself to voice her concern with the surgeon she credited with saving her life.
“It’s very difficult to come face to face with somebody who says, ‘You’ve had cancer but we can get rid of it,’ and does their best… without seeming ungrateful,” she said, tearfully.
The study concludes with a fascinating discussion about the potential clinical implications of the findings.
Considering the profound influence of the patient-surgeon relationship on these particular patients, the investigators offer a cautionary suggestion to avoid being overly effusive about the cosmetic result they may see. Patients, they explain, may not necessarily share their enthusiasm, if they continue to struggle with the sense that cancer has marred their bodies, their sense of self, or their security in relationships.
“Both patient and surgeon have invested physically and emotionally in the procedure and it is difficult for either to admit to the other that it was “not worth it,” they note.
Women, on the other hand, who appear to be disproportionately pleased with the result of surgery that objectively achieved a poor result may simply be expressing relief and gratitude. “Their apparent satisfaction,” they wrote, “should not excuse poor surgical practice.” Rather, routine assessments of reconstructive practice should be made by objective sources, not simply patient report.
On the other hand, in individual patients, the objective in reconstructive surgery is patient satisfaction, they suggest. An unhappy patient might spur a conscientious surgeon to keep trying, perhaps through repeated procedures, to achieve a better result – a strategy that could be inappropriate and pointless considering that in some cases, “the reason for disappointment with reconstruction include many that the surgeon cannot influence surgically.”
It all suggests that communication between surgeons and reconstruction candidates and patients needs to be deep and candidly honest, informed by the emotional, sexual, and existential meaning the surgery holds.
In a word, it’s complicated.
Betsy Bates Freed, Psych.D., is a clinical psychologist in Santa Barbara, Calif., and a medical journalist.
Before-and-after photographs are the stock in trade of house painters, auto repair shops, and, yes, plastic and reconstructive surgeons. But a new study may make the last group pause, since it hints that far more is at play in breast cancer patients’ definition of “successful’’ breast reconstruction surgery than how their breasts appear.
The study from Liverpool, England (J Plast Reconstr Aesthet Surg (2012): doi:10.1016/j.bjps.2012.03.005) sidestepped traditional measures used to evaluate outcomes of aesthetic breast surgery and instead asked open-ended questions of survivors who had undergone reconstruction 1-8 years previously.
What the researchers discovered, not surprisingly, is that reconstruction patients are quite unlike cosmetic surgery patients in fundamental and important ways.
Of particular interest in the initial study cohort of 95 patients were 38 whose subjective evaluations of their surgical results completely contradicted objective ratings of cosmesis, the final appearance of the reconstructed breast(s) by surgeons and surgical nurses.
Incredibly, the association between women’s assessments and objective cosmesis ratings failed even to reach statistical significance.
In a structured data analysis of themes that arose in open-ended interviews with 27 of the survivors, the strongest link to women’s satisfaction with the procedure was the surgeon-patient relationship.
Next came the significance of reconstruction in what patients saw as the “completion of the cancer journey,” the authors wrote. “Patients who focused on this were positive about reconstruction that practitioners had rated negatively.”
A previous study asked patients about scarring, finding a correlation between scarring and dissatisfaction with reconstruction. But scarring wasn’t even a blip on the radar when, quoting from the Liverpool study, “we allowed patients to tell us what mattered to them rather than imposing our preconceptions.”
“It seems that surgeons and patients normally ‘talk different languages’; one technical and the other drawing more from relationships and patients’ sense of how normal they feel and appear and from their sense that reconstruction completes their cancer journey,” the investigators concluded. “In preoperative consultations, surgeons concentrate almost exclusively on the technical and cosmetic aspects of reconstruction: what can be achieved and what complications can occur.”
Of course, women who struggled with complications tended to factor that in to their assessments of their results, even if their final cosmetic outcome was considered by surgeons to be excellent.
Others were disappointed despite what seemed to surgeons to be excellent cosmetic results because, as one said, “I was expecting to feel feminine again, but I don’t, I don’t at all.”
What is perhaps even more interesting is to eavesdrop on the comments of women whose surgeons judged their cosmetic result to be poor.
Said one, “I had a really good relationship with (the surgeon) and I just found it so reassuring to see her. That was part of the whole thing really. She was just so positive, and so, well, just understanding I think … I was really glad that I had chosen that form of reconstruction because I had this regular contact with her.”
Said another, who felt “normal” despite what her surgeon considered to be a poor result: “If I didn’t have it done, I wouldn’t have felt normal at all. It would always remind me of what had happened.”
A highly complex patient-surgeon dance occurs when breast surgery is performed for more than cosmetic reasons, the study found.
One woman, disappointed with the way her reconstructed breast fit in a bra, could not bring herself to voice her concern with the surgeon she credited with saving her life.
“It’s very difficult to come face to face with somebody who says, ‘You’ve had cancer but we can get rid of it,’ and does their best… without seeming ungrateful,” she said, tearfully.
The study concludes with a fascinating discussion about the potential clinical implications of the findings.
Considering the profound influence of the patient-surgeon relationship on these particular patients, the investigators offer a cautionary suggestion to avoid being overly effusive about the cosmetic result they may see. Patients, they explain, may not necessarily share their enthusiasm, if they continue to struggle with the sense that cancer has marred their bodies, their sense of self, or their security in relationships.
“Both patient and surgeon have invested physically and emotionally in the procedure and it is difficult for either to admit to the other that it was “not worth it,” they note.
Women, on the other hand, who appear to be disproportionately pleased with the result of surgery that objectively achieved a poor result may simply be expressing relief and gratitude. “Their apparent satisfaction,” they wrote, “should not excuse poor surgical practice.” Rather, routine assessments of reconstructive practice should be made by objective sources, not simply patient report.
On the other hand, in individual patients, the objective in reconstructive surgery is patient satisfaction, they suggest. An unhappy patient might spur a conscientious surgeon to keep trying, perhaps through repeated procedures, to achieve a better result – a strategy that could be inappropriate and pointless considering that in some cases, “the reason for disappointment with reconstruction include many that the surgeon cannot influence surgically.”
It all suggests that communication between surgeons and reconstruction candidates and patients needs to be deep and candidly honest, informed by the emotional, sexual, and existential meaning the surgery holds.
In a word, it’s complicated.
Betsy Bates Freed, Psych.D., is a clinical psychologist in Santa Barbara, Calif., and a medical journalist.
Before-and-after photographs are the stock in trade of house painters, auto repair shops, and, yes, plastic and reconstructive surgeons. But a new study may make the last group pause, since it hints that far more is at play in breast cancer patients’ definition of “successful’’ breast reconstruction surgery than how their breasts appear.
The study from Liverpool, England (J Plast Reconstr Aesthet Surg (2012): doi:10.1016/j.bjps.2012.03.005) sidestepped traditional measures used to evaluate outcomes of aesthetic breast surgery and instead asked open-ended questions of survivors who had undergone reconstruction 1-8 years previously.
What the researchers discovered, not surprisingly, is that reconstruction patients are quite unlike cosmetic surgery patients in fundamental and important ways.
Of particular interest in the initial study cohort of 95 patients were 38 whose subjective evaluations of their surgical results completely contradicted objective ratings of cosmesis, the final appearance of the reconstructed breast(s) by surgeons and surgical nurses.
Incredibly, the association between women’s assessments and objective cosmesis ratings failed even to reach statistical significance.
In a structured data analysis of themes that arose in open-ended interviews with 27 of the survivors, the strongest link to women’s satisfaction with the procedure was the surgeon-patient relationship.
Next came the significance of reconstruction in what patients saw as the “completion of the cancer journey,” the authors wrote. “Patients who focused on this were positive about reconstruction that practitioners had rated negatively.”
A previous study asked patients about scarring, finding a correlation between scarring and dissatisfaction with reconstruction. But scarring wasn’t even a blip on the radar when, quoting from the Liverpool study, “we allowed patients to tell us what mattered to them rather than imposing our preconceptions.”
“It seems that surgeons and patients normally ‘talk different languages’; one technical and the other drawing more from relationships and patients’ sense of how normal they feel and appear and from their sense that reconstruction completes their cancer journey,” the investigators concluded. “In preoperative consultations, surgeons concentrate almost exclusively on the technical and cosmetic aspects of reconstruction: what can be achieved and what complications can occur.”
Of course, women who struggled with complications tended to factor that in to their assessments of their results, even if their final cosmetic outcome was considered by surgeons to be excellent.
Others were disappointed despite what seemed to surgeons to be excellent cosmetic results because, as one said, “I was expecting to feel feminine again, but I don’t, I don’t at all.”
What is perhaps even more interesting is to eavesdrop on the comments of women whose surgeons judged their cosmetic result to be poor.
Said one, “I had a really good relationship with (the surgeon) and I just found it so reassuring to see her. That was part of the whole thing really. She was just so positive, and so, well, just understanding I think … I was really glad that I had chosen that form of reconstruction because I had this regular contact with her.”
Said another, who felt “normal” despite what her surgeon considered to be a poor result: “If I didn’t have it done, I wouldn’t have felt normal at all. It would always remind me of what had happened.”
A highly complex patient-surgeon dance occurs when breast surgery is performed for more than cosmetic reasons, the study found.
One woman, disappointed with the way her reconstructed breast fit in a bra, could not bring herself to voice her concern with the surgeon she credited with saving her life.
“It’s very difficult to come face to face with somebody who says, ‘You’ve had cancer but we can get rid of it,’ and does their best… without seeming ungrateful,” she said, tearfully.
The study concludes with a fascinating discussion about the potential clinical implications of the findings.
Considering the profound influence of the patient-surgeon relationship on these particular patients, the investigators offer a cautionary suggestion to avoid being overly effusive about the cosmetic result they may see. Patients, they explain, may not necessarily share their enthusiasm, if they continue to struggle with the sense that cancer has marred their bodies, their sense of self, or their security in relationships.
“Both patient and surgeon have invested physically and emotionally in the procedure and it is difficult for either to admit to the other that it was “not worth it,” they note.
Women, on the other hand, who appear to be disproportionately pleased with the result of surgery that objectively achieved a poor result may simply be expressing relief and gratitude. “Their apparent satisfaction,” they wrote, “should not excuse poor surgical practice.” Rather, routine assessments of reconstructive practice should be made by objective sources, not simply patient report.
On the other hand, in individual patients, the objective in reconstructive surgery is patient satisfaction, they suggest. An unhappy patient might spur a conscientious surgeon to keep trying, perhaps through repeated procedures, to achieve a better result – a strategy that could be inappropriate and pointless considering that in some cases, “the reason for disappointment with reconstruction include many that the surgeon cannot influence surgically.”
It all suggests that communication between surgeons and reconstruction candidates and patients needs to be deep and candidly honest, informed by the emotional, sexual, and existential meaning the surgery holds.
In a word, it’s complicated.
Betsy Bates Freed, Psych.D., is a clinical psychologist in Santa Barbara, Calif., and a medical journalist.
Oncologists Favor Psychosocial Care, But Give It Short Shrift
MIAMI – Oncologists endorse the idea of connecting cancer patients to psychosocial care at the conclusion of active treatment. But practice doesn’t align with beliefs, perhaps because they are unfamiliar with where to refer their patients for care.
Among 57 oncologists who responded to a survey in the southeastern United States, 35, or 61%, considered psychosocial care to be beneficial. A majority thought it was "important" following cancer treatment, reported Laurie Freeman-Gibb at the annual conference of the American Psychosocial Oncology Society (APOS).
But the oncologists said they spent just 4.2 minutes, on average, discussing psychosocial care during consultations, according to Ms. Freeman-Gibb, a lecturer in the department of nursing at the University of Windsor in Ontario, and her colleague Dr. Andrew Hatchett, Ph.D., of the University of Louisiana at Lafayette’s department of kinesiology.
And since only about 1 in 6 oncologists responded to the survey – it was sent to 350 practitioners – the findings may present an overly optimistic picture of what happens in real-life practice when a patient leaves active treatment and returns to the community for care.
"I think it’s sometimes a time constraint," said Ms. Freeman-Gibb. "If you only have 20 minutes to see this person and you open the floodgates to what’s really going on, you might never get out the door ... especially if you don’t know whom to tell the patient to call."
Dr. Hatchett said the impetus for the study was a series of conversations he had with survivors, in which they seemed to indicate a "disconnect" in support after their active treatment ended. "It seemed as though after treatment the survivor was left to their own devices to acquire any additional help," he said.
Many oncologists told the researchers that they would like to refer survivors for follow-up psychosocial care, but they don’t know what’s available, the investigators said.
No comprehensive registry exists that would outline the locations and qualifications of therapists, exercise and rehabilitation specialists, and support agencies that specialize in the psychosocial needs of cancer survivors. In Ireland, a national registry does just that, detailing not only the services available but also their cost, said Ms. Freeman-Gibb.
The organization that sponsored the meeting, APOS, offers a free helpline intended to connect cancer patients and survivors with community counseling services and other sources of support. However, the oncologists in the survey were unaware of that resource, the investigators noted.
Development of a "network of resources" remains a goal of the researchers, who plan to conduct an expanded online survey of a larger pool of oncologists to build on the findings of their pilot questionnaire.
Having a better sense of available resources might make oncologists more comfortable bringing up survivors’ psychosocial adjustment, added Ms. Freeman-Gibb: "Their attitude is great. They say they would love to refer patients. But they don’t."
No outside funding was used to conduct the study.
MIAMI – Oncologists endorse the idea of connecting cancer patients to psychosocial care at the conclusion of active treatment. But practice doesn’t align with beliefs, perhaps because they are unfamiliar with where to refer their patients for care.
Among 57 oncologists who responded to a survey in the southeastern United States, 35, or 61%, considered psychosocial care to be beneficial. A majority thought it was "important" following cancer treatment, reported Laurie Freeman-Gibb at the annual conference of the American Psychosocial Oncology Society (APOS).
But the oncologists said they spent just 4.2 minutes, on average, discussing psychosocial care during consultations, according to Ms. Freeman-Gibb, a lecturer in the department of nursing at the University of Windsor in Ontario, and her colleague Dr. Andrew Hatchett, Ph.D., of the University of Louisiana at Lafayette’s department of kinesiology.
And since only about 1 in 6 oncologists responded to the survey – it was sent to 350 practitioners – the findings may present an overly optimistic picture of what happens in real-life practice when a patient leaves active treatment and returns to the community for care.
"I think it’s sometimes a time constraint," said Ms. Freeman-Gibb. "If you only have 20 minutes to see this person and you open the floodgates to what’s really going on, you might never get out the door ... especially if you don’t know whom to tell the patient to call."
Dr. Hatchett said the impetus for the study was a series of conversations he had with survivors, in which they seemed to indicate a "disconnect" in support after their active treatment ended. "It seemed as though after treatment the survivor was left to their own devices to acquire any additional help," he said.
Many oncologists told the researchers that they would like to refer survivors for follow-up psychosocial care, but they don’t know what’s available, the investigators said.
No comprehensive registry exists that would outline the locations and qualifications of therapists, exercise and rehabilitation specialists, and support agencies that specialize in the psychosocial needs of cancer survivors. In Ireland, a national registry does just that, detailing not only the services available but also their cost, said Ms. Freeman-Gibb.
The organization that sponsored the meeting, APOS, offers a free helpline intended to connect cancer patients and survivors with community counseling services and other sources of support. However, the oncologists in the survey were unaware of that resource, the investigators noted.
Development of a "network of resources" remains a goal of the researchers, who plan to conduct an expanded online survey of a larger pool of oncologists to build on the findings of their pilot questionnaire.
Having a better sense of available resources might make oncologists more comfortable bringing up survivors’ psychosocial adjustment, added Ms. Freeman-Gibb: "Their attitude is great. They say they would love to refer patients. But they don’t."
No outside funding was used to conduct the study.
MIAMI – Oncologists endorse the idea of connecting cancer patients to psychosocial care at the conclusion of active treatment. But practice doesn’t align with beliefs, perhaps because they are unfamiliar with where to refer their patients for care.
Among 57 oncologists who responded to a survey in the southeastern United States, 35, or 61%, considered psychosocial care to be beneficial. A majority thought it was "important" following cancer treatment, reported Laurie Freeman-Gibb at the annual conference of the American Psychosocial Oncology Society (APOS).
But the oncologists said they spent just 4.2 minutes, on average, discussing psychosocial care during consultations, according to Ms. Freeman-Gibb, a lecturer in the department of nursing at the University of Windsor in Ontario, and her colleague Dr. Andrew Hatchett, Ph.D., of the University of Louisiana at Lafayette’s department of kinesiology.
And since only about 1 in 6 oncologists responded to the survey – it was sent to 350 practitioners – the findings may present an overly optimistic picture of what happens in real-life practice when a patient leaves active treatment and returns to the community for care.
"I think it’s sometimes a time constraint," said Ms. Freeman-Gibb. "If you only have 20 minutes to see this person and you open the floodgates to what’s really going on, you might never get out the door ... especially if you don’t know whom to tell the patient to call."
Dr. Hatchett said the impetus for the study was a series of conversations he had with survivors, in which they seemed to indicate a "disconnect" in support after their active treatment ended. "It seemed as though after treatment the survivor was left to their own devices to acquire any additional help," he said.
Many oncologists told the researchers that they would like to refer survivors for follow-up psychosocial care, but they don’t know what’s available, the investigators said.
No comprehensive registry exists that would outline the locations and qualifications of therapists, exercise and rehabilitation specialists, and support agencies that specialize in the psychosocial needs of cancer survivors. In Ireland, a national registry does just that, detailing not only the services available but also their cost, said Ms. Freeman-Gibb.
The organization that sponsored the meeting, APOS, offers a free helpline intended to connect cancer patients and survivors with community counseling services and other sources of support. However, the oncologists in the survey were unaware of that resource, the investigators noted.
Development of a "network of resources" remains a goal of the researchers, who plan to conduct an expanded online survey of a larger pool of oncologists to build on the findings of their pilot questionnaire.
Having a better sense of available resources might make oncologists more comfortable bringing up survivors’ psychosocial adjustment, added Ms. Freeman-Gibb: "Their attitude is great. They say they would love to refer patients. But they don’t."
No outside funding was used to conduct the study.
FROM THE ANNUAL CONFERENCE OF THE AMERICAN PSYCHOSOCIAL ONCOLOGY SOCIETY
Maintenance Immunotherapy Extends Survival in Multiple Advanced Cancers
CHICAGO – Maintenance immunotherapy with low-dose interleukin-2 and 13-cis-retinoic acid demonstrated an "unexpected" survival benefit in a phase II trial among 500 patients who had derived clinical benefit from chemotherapy for a variety of stage IV cancers, investigators from Italy reported.
The 15-year disease-free survival and overall survival rates reached 32.6% and 36.8%, respectively, in the open, nonrandomized trial, and a breakdown by tumor type showed better outcomes than seen in historic controls, Dr. Francesco Recchia reported at the annual meeting of the American Association for Cancer Research.
Comparing their data with outcomes for the most commonly treated metastatic cancers in the Surveillance, Epidemiology, and End Results (SEER) database in the United States, the investigators found their 5-year overall survival rates were 42.7% vs. 23.3% for breast cancer, 26.4% vs. 3.6% for lung cancer, 43.6% vs. 11.7% for colorectal cancer, and 23% vs. 11% for renal cancer.
"The good and unexpected results of this immunotherapy regimen were seen in all types of cancer: ovarian cancer and recurrent ovarian cancer, non–small cell lung cancer, cardiac metastases of sarcoma, colorectal cancer, gastric cancer, renal cell carcinoma, melanoma, head and neck cancer, breast cancer, and pancreatic cancer," said Dr. Recchia, director of oncology at the Civilian Hospital in Avezzano (Italy).
"The best merit of our research is the low cost of this therapy," he added.
The impetus for the phase II trial was an observation, in 1995, that a patient, who could not tolerate standard high-dose (18 x 106 IU/m2) IL-2 therapy for metastatic melanoma, experienced a long-lasting response to low-dose subcutaneous IL-2. Dr. Recchia and his colleagues conducted a series of phase I and II randomized studies of low-dose IL-2 with and without retinoic acid, ultimately determining that the combination of the two agents was most effective in increasing natural killer (NK) cell populations and decreasing vascular endothelial growth factor (VEGF) expression.
In the current study, after a median follow-up of 60 months, Dr. Recchia and his colleagues observed a statistically significant increase in the number of NK cells – mediators of lytic activity against cancer cell lines – from a baseline mean of 309/mm3 to a mean of 579/mm3. They also found a statistically significant decrease in VEGF expression, from a baseline mean of 520 pg/mm3 to a mean of 150 pg/mm3 in the study participants.
"Both of these mechanisms are associated with slowing cancer growth," he said in an interview.
All of the patients enrolled in the study were in chemotherapy-induced remission, with good performance status, from stage IV cancer, and all (median age 61 years) were treated for 1 year with self-administered subcutaneous IL-2 (1.8 x 106 IU) and oral 13-cis-retinoic acid (0.5 mg/kg) 5 days per week for two consecutive 3-week cycles, with a 1-week rest. This was followed by continued intermittently scheduled therapy for 5 years or until disease progression, with assessment of NK cells, serum VEGF, tumor response, and toxicity every 4 months, Dr. Recchia said.
The study’s primary end point was NK cell and VEGF response, he added, noting that disease-free survival, overall survival, and toxicity in various tumor types were secondary end points.
The treatment was not associated with any World Health Organization grade 3 or 4 toxicities, Dr. Recchia reported. Grade 2 cutaneous toxicity (20%), fever (13%), mild hypothyroidism (5%), and triglyceride elevation (15%) were observed, he said; one patient discontinued treatment because of grade 2 urticaria.
Prior to using the maintenance regimen in clinical practice, the findings of the current study have to be validated in a blinded, controlled, randomized trial, said Dr. Recchia, noting that a phase III study is underway in patients with advanced breast cancer.
Dr. Recchia reported having no relevant financial conflicts of interest.
CHICAGO – Maintenance immunotherapy with low-dose interleukin-2 and 13-cis-retinoic acid demonstrated an "unexpected" survival benefit in a phase II trial among 500 patients who had derived clinical benefit from chemotherapy for a variety of stage IV cancers, investigators from Italy reported.
The 15-year disease-free survival and overall survival rates reached 32.6% and 36.8%, respectively, in the open, nonrandomized trial, and a breakdown by tumor type showed better outcomes than seen in historic controls, Dr. Francesco Recchia reported at the annual meeting of the American Association for Cancer Research.
Comparing their data with outcomes for the most commonly treated metastatic cancers in the Surveillance, Epidemiology, and End Results (SEER) database in the United States, the investigators found their 5-year overall survival rates were 42.7% vs. 23.3% for breast cancer, 26.4% vs. 3.6% for lung cancer, 43.6% vs. 11.7% for colorectal cancer, and 23% vs. 11% for renal cancer.
"The good and unexpected results of this immunotherapy regimen were seen in all types of cancer: ovarian cancer and recurrent ovarian cancer, non–small cell lung cancer, cardiac metastases of sarcoma, colorectal cancer, gastric cancer, renal cell carcinoma, melanoma, head and neck cancer, breast cancer, and pancreatic cancer," said Dr. Recchia, director of oncology at the Civilian Hospital in Avezzano (Italy).
"The best merit of our research is the low cost of this therapy," he added.
The impetus for the phase II trial was an observation, in 1995, that a patient, who could not tolerate standard high-dose (18 x 106 IU/m2) IL-2 therapy for metastatic melanoma, experienced a long-lasting response to low-dose subcutaneous IL-2. Dr. Recchia and his colleagues conducted a series of phase I and II randomized studies of low-dose IL-2 with and without retinoic acid, ultimately determining that the combination of the two agents was most effective in increasing natural killer (NK) cell populations and decreasing vascular endothelial growth factor (VEGF) expression.
In the current study, after a median follow-up of 60 months, Dr. Recchia and his colleagues observed a statistically significant increase in the number of NK cells – mediators of lytic activity against cancer cell lines – from a baseline mean of 309/mm3 to a mean of 579/mm3. They also found a statistically significant decrease in VEGF expression, from a baseline mean of 520 pg/mm3 to a mean of 150 pg/mm3 in the study participants.
"Both of these mechanisms are associated with slowing cancer growth," he said in an interview.
All of the patients enrolled in the study were in chemotherapy-induced remission, with good performance status, from stage IV cancer, and all (median age 61 years) were treated for 1 year with self-administered subcutaneous IL-2 (1.8 x 106 IU) and oral 13-cis-retinoic acid (0.5 mg/kg) 5 days per week for two consecutive 3-week cycles, with a 1-week rest. This was followed by continued intermittently scheduled therapy for 5 years or until disease progression, with assessment of NK cells, serum VEGF, tumor response, and toxicity every 4 months, Dr. Recchia said.
The study’s primary end point was NK cell and VEGF response, he added, noting that disease-free survival, overall survival, and toxicity in various tumor types were secondary end points.
The treatment was not associated with any World Health Organization grade 3 or 4 toxicities, Dr. Recchia reported. Grade 2 cutaneous toxicity (20%), fever (13%), mild hypothyroidism (5%), and triglyceride elevation (15%) were observed, he said; one patient discontinued treatment because of grade 2 urticaria.
Prior to using the maintenance regimen in clinical practice, the findings of the current study have to be validated in a blinded, controlled, randomized trial, said Dr. Recchia, noting that a phase III study is underway in patients with advanced breast cancer.
Dr. Recchia reported having no relevant financial conflicts of interest.
CHICAGO – Maintenance immunotherapy with low-dose interleukin-2 and 13-cis-retinoic acid demonstrated an "unexpected" survival benefit in a phase II trial among 500 patients who had derived clinical benefit from chemotherapy for a variety of stage IV cancers, investigators from Italy reported.
The 15-year disease-free survival and overall survival rates reached 32.6% and 36.8%, respectively, in the open, nonrandomized trial, and a breakdown by tumor type showed better outcomes than seen in historic controls, Dr. Francesco Recchia reported at the annual meeting of the American Association for Cancer Research.
Comparing their data with outcomes for the most commonly treated metastatic cancers in the Surveillance, Epidemiology, and End Results (SEER) database in the United States, the investigators found their 5-year overall survival rates were 42.7% vs. 23.3% for breast cancer, 26.4% vs. 3.6% for lung cancer, 43.6% vs. 11.7% for colorectal cancer, and 23% vs. 11% for renal cancer.
"The good and unexpected results of this immunotherapy regimen were seen in all types of cancer: ovarian cancer and recurrent ovarian cancer, non–small cell lung cancer, cardiac metastases of sarcoma, colorectal cancer, gastric cancer, renal cell carcinoma, melanoma, head and neck cancer, breast cancer, and pancreatic cancer," said Dr. Recchia, director of oncology at the Civilian Hospital in Avezzano (Italy).
"The best merit of our research is the low cost of this therapy," he added.
The impetus for the phase II trial was an observation, in 1995, that a patient, who could not tolerate standard high-dose (18 x 106 IU/m2) IL-2 therapy for metastatic melanoma, experienced a long-lasting response to low-dose subcutaneous IL-2. Dr. Recchia and his colleagues conducted a series of phase I and II randomized studies of low-dose IL-2 with and without retinoic acid, ultimately determining that the combination of the two agents was most effective in increasing natural killer (NK) cell populations and decreasing vascular endothelial growth factor (VEGF) expression.
In the current study, after a median follow-up of 60 months, Dr. Recchia and his colleagues observed a statistically significant increase in the number of NK cells – mediators of lytic activity against cancer cell lines – from a baseline mean of 309/mm3 to a mean of 579/mm3. They also found a statistically significant decrease in VEGF expression, from a baseline mean of 520 pg/mm3 to a mean of 150 pg/mm3 in the study participants.
"Both of these mechanisms are associated with slowing cancer growth," he said in an interview.
All of the patients enrolled in the study were in chemotherapy-induced remission, with good performance status, from stage IV cancer, and all (median age 61 years) were treated for 1 year with self-administered subcutaneous IL-2 (1.8 x 106 IU) and oral 13-cis-retinoic acid (0.5 mg/kg) 5 days per week for two consecutive 3-week cycles, with a 1-week rest. This was followed by continued intermittently scheduled therapy for 5 years or until disease progression, with assessment of NK cells, serum VEGF, tumor response, and toxicity every 4 months, Dr. Recchia said.
The study’s primary end point was NK cell and VEGF response, he added, noting that disease-free survival, overall survival, and toxicity in various tumor types were secondary end points.
The treatment was not associated with any World Health Organization grade 3 or 4 toxicities, Dr. Recchia reported. Grade 2 cutaneous toxicity (20%), fever (13%), mild hypothyroidism (5%), and triglyceride elevation (15%) were observed, he said; one patient discontinued treatment because of grade 2 urticaria.
Prior to using the maintenance regimen in clinical practice, the findings of the current study have to be validated in a blinded, controlled, randomized trial, said Dr. Recchia, noting that a phase III study is underway in patients with advanced breast cancer.
Dr. Recchia reported having no relevant financial conflicts of interest.
FROM THE ANNUAL MEETING OF THE AMERICAN ASSOCIATION FOR CANCER RESEARCH