User login
Evaluating patients with breast concerns: Lump, pain, and mastitis
The vast majority of symptomatic breast conditions are benign, with the most common symptoms being palpable mass and breast pain. Clinicians, including primary care clinicians and gynecologists, play a crucial role by performing the initial assessment and subsequent therapies and referrals and serve as the mediator between the specialists and by being the patient’s spokesperson. It is therefore important for clinicians to be aware of the various possible causes of these breast symptoms, to know which imaging tests to order, and also to understand the indications for biopsies and surgical referral.
Common types of breast lumps: Imaging workup and management
Accounting for 8% of women who present with breast symptoms, breast lump is the second most common symptom after breast pain.1 The positive likelihood ratio of finding breast cancer is highest among women with breast lumps compared with any other breast symptoms. Therefore, anxiety is related to this symptom, and a thorough evaluation is recommended.1 Cysts, fibroadenoma, and fat necrosis are 3 common benign causes of breast lumps.2
In this section, we review clinical presentation, imaging workup, and management strategies for common types of breast lumps.
CASE 1 Woman with tender breast lump
A 45-year-old woman presents with a breast lump of 6 months’ duration that is associated with a change in size with the menstrual cycle and pain. Clinical examination reveals a 4 x 4.5–cm mass in the right breast in the retroareolar region, which is smooth with some tenderness on palpation.
Breast cyst
According to the American College of Radiology appropriateness criteria for an adult woman 40 years of age or older who presents with a palpable breast mass, the initial imaging study is diagnostic mammography with or without digital tomosynthesis, usually followed by a directed ultrasound. If the mammogram is suspicious or highly suggestive of malignancy, or in cases where the mammogram does not show an abnormality, the next recommended step is breast ultrasonography. Any suspicious findings on ultrasound or mammogram should be followed by an image guided biopsy. Ultrasonography also may be appropriate if the mammogram findings are benign or probably benign.
For an adult woman younger than age 30 who presents with a palpable breast mass, breast ultrasonography is the appropriate initial imaging study. If the ultrasound is suspicious or highly suggestive of malignancy, then performing diagnostic mammography with or without digital tomosynthesis or ultrasound-guided core needle biopsy of the mass are both considered appropriate. However, no further imaging is recommended if the ultrasound is benign, probably benign, or negative. Breast ultrasonography or mammography is appropriate as the initial imaging test for adult women aged 30 to 39 years who present with a palpable breast mass.3,4
Approximately 50% of women after age 30 may develop fibrocystic breast disease, and 20% of them can present with pain or lump due to a macrocysts. Simple cysts must be distinguished from complex cysts with the help of ultrasound as the latter are associated with 23% to 31% increased risk of malignancy.
In this 45-year-old patient, the initial mammogram demonstrated a circumscribed mass underneath the area of palpable concern (FIGURE 1a, 1b). Targeted breast ultrasonography was performed for further assessment, which depicted the mass as a benign simple cyst (FIGURE 1c).
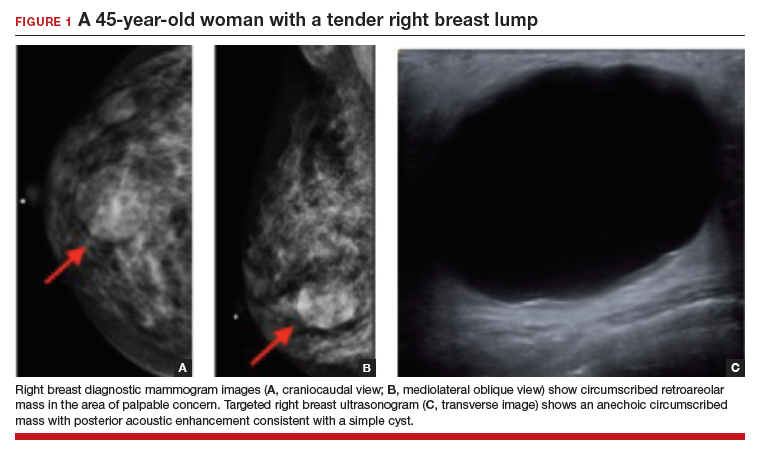
On ultrasound, a simple cyst is an anechoic, well-circumscribed mass with a thin capsule and with increased through transmission. Patients with small and asymptomatic simple cysts do not need imaging follow-up and can return for routine screening mammograms.
A breast surgeon, radiologist, or gynecologist can perform percutaneous aspiration if a cyst is large and symptomatic. A cyst with low-level internal echoes, fluid-fluid, or fluid-debris levels is considered a complicated cyst. Differential diagnosis also includes hematoma, fat necrosis, abscess, and galactocele, depending on the clinical presentation. Fine-needle aspiration or short-interval follow-up5,6 is appropriate for complicated cysts, while incision and drainage is indicated in patients with infected cysts and abscesses. A cyst with a solid component is considered a cystic, solid mass, and core needle biopsy is recommended. The differential diagnosis for cysts with solid components includes intracystic papilloma, papillary carcinoma, ductal carcinoma in situ, and necrotic cancers.5,6
Continue to: CASE 2 Painless breast mass in a young woman...
CASE 2 Painless breast mass in a young woman
A 22-year-old woman presents with a 2-month history of breast lump, which is not associated with pain or nipple discharge. On examination, there is a 2 x 2–cm mass in the right breast at 12 o’clock, 2 cm from the nipple, which is mobile, smooth, and nontender on palpation.
Fibroadenoma
In this 22-year-old, the initial imaging of choice is breast ultrasonography. Breast ultrasonography can differentiate a cystic mass from a solid mass, and it does not involve radiation. Right breast targeted ultrasound showed a circumscribed oval homogeneous hypoechoic mass that is wider than tall (FIGURE 2). The patient desired surgical removal, and a pre-lumpectomy core needle biopsy revealed a fibroadenoma.
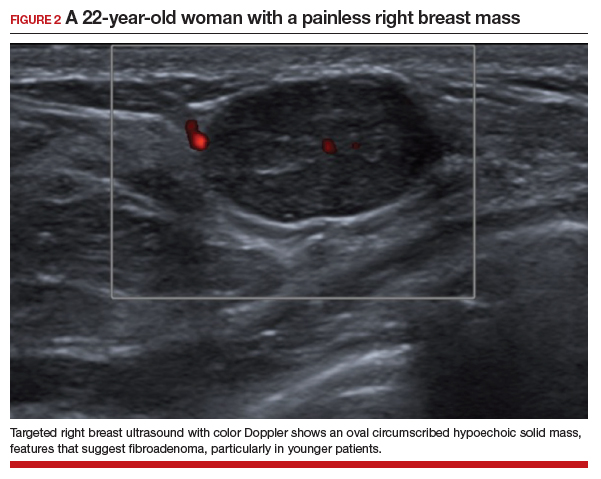
Fibroadenoma is the most common benign tumor of the breast. It is most often encountered in premenopausal women. Patients present with a painless breast lump, which is smooth and mobile on palpation. Fibroadenoma can be followed expectantly with repeat ultrasound (to assess over time for growth) if it is small and asymptomatic. No further action is needed if it remains stable. If a patient desires surgical excision, a core needle biopsy is usually performed before lumpectomy.
Excisional biopsy or removal of the mass is recommended if the mass is greater than 3 or 4 cm, is symptomatic, or if there is an increase in size that raises clinical concern for phyllodes tumor. Imaging features that are concerning for phyllodes tumors are size greater than 3 cm, indistinct or microlobulated margins, and heterogeneous echo pattern.7,8 In cases in which the imaging features are concerning for phyllodes tumor and a core needle biopsy is not definitive, wide surgical excision is recommended for definitive diagnosis.8
CASE 3 Patient develops breast mass post-surgery
A 45-year-old woman presents with a tender left breast mass that she noticed 2 months after breast reduction surgery. It has been increasing in size since. On clinical examination, a 4 x 4–cm mass is found at the surgical scar site, which is indurated on palpation and tender.
Fat necrosis
In this 45-year-old, the initial test of choice is diagnostic mammography, which showed a somewhat circumscribed area with fat under the palpable marker (FIGURE 3a). Breast ultrasonography was performed for further evaluation, which was inconclusive as the ultrasound showed ill-defined areas of mixed echogenicity (FIGURE 3b). Breast magnetic resonance imaging (MRI) clearly demonstrated fat necrosis in the area of the palpable lump (FIGURE 3c).

Fat necrosis of the breast is an inflammatory process that is seen after breast trauma or surgery. It can present as an incidental mammogram finding or a palpable mass. The patient may give a history of trauma, breast reduction surgery, or breast cancer surgery followed by radiation treatment. On clinical examination, fat necrosis occasionally can present as a firm mass with skin retraction or swelling concerning for cancer. Imaging features are variable depending on the stage of fat necrosis and inflammation.9-11
A mammogram may demonstrate a circumscribed fat-containing mass, an ill-defined mass, asymmetry or calcified oil cyst, and dystrophic calcifications. On ultrasound, fat necrosis can appear as anechoic or hypoechoic or as a complicated cyst or a mixed cystic, solid mass. MRI demonstrates a circumscribed or irregular fat-containing mass, with or without enhancement, and architectural distortion.
When the imaging features are clearly benign—for example, a circumscribed fat-containing mass on mammogram or on ultrasound or, on MRI, marked hypointensity of fat in the center of a circumscribed mass when compared with surrounding fat (keyhole sign)—no further follow-up is needed. When the imaging features are indeterminate, however, a short-interval follow-up can be considered. In cases with irregular fat-containing mass with enhancement, core needle biopsy is indicated to exclude cancer. If the workup remains inconclusive and the level of clinical suspicion is high, surgical excision can be performed for a definitive diagnosis.12
Continue to: Investigating breast pain: Imaging workup and management...
Investigating breast pain: Imaging workup and management
Breast pain, or mastalgia, is the most common concern of women presenting to a breast clinic and accounts for approximately half of such encounters.13 Causes of breast pain include hormonal changes, fibrocystic changes, musculoskeletal causes (such as costochondritis), lack of support, infection, and injury. While mastalgia often causes patient concern, the risk of malignancy in a woman presenting with breast pain alone is low. Still, it is essential to rule out other findings suspicious for cancer (mass, skin changes, or nipple discharge) with a thorough history and breast examination.
In this section, we review clinical presentation, imaging workup, and management for breast pain.
CASE 4 Woman with noncyclic breast pain
A 26-year-old woman presents to the clinic with mastalgia. The pain is noncyclic and primarily located in the upper outer quadrant of her left breast. There is no history of breast cancer in her family. She has no suspicious findings on the breast examination.
Mastalgia
The test of choice for this 26-year-old with focal left breast pain is targeted breast ultrasound. The patient’s ultrasound image showed no suspicious findings or solid or cystic mass (FIGURE 4).
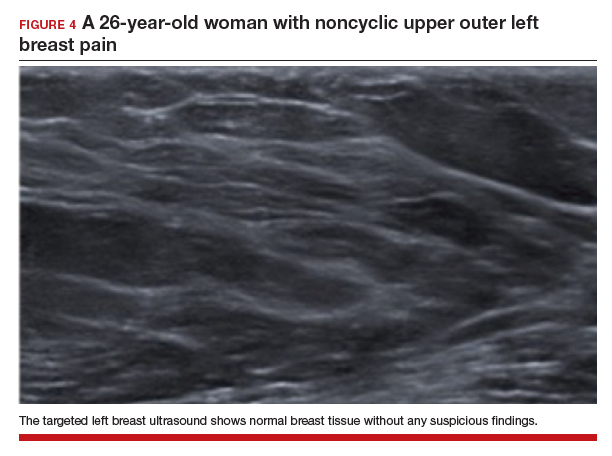
Two important characteristics of breast pain are whether it is noncyclical and whether it is focal. According to the American College of Radiology, no breast imaging is recommended for clinically insignificant cyclical, nonfocal (greater than 1 quadrant)/diffuse pain, as this type of mastalgia is not associated with malignancy.14
For patients age 40 or older, if they are not up to date with their annual screening mammogram, then a mammogram should be performed. An imaging workup is warranted for clinically significant mastalgia that is noncyclical and focal. Even then, no malignancy is identified in most patients with clinically significant mastalgia; in patients with breast pain as their only symptom, the prevalence of breast cancer is 0% to 3.0%.15-19
The initial imaging modality differs by patient age: younger than 30 years, ultrasonography; between 30 and 40 years, mammography or ultrasonography; and older than 40 years, mammography first followed by ultrasonography.14
Treatment of breast pain is primarily symptomatic, and evidence for specific treatments is generally lacking. Cyclical breast pain resolves spontaneously in 20% to 30% of women, while noncyclical pain responds poorly to treatment but resolves spontaneously in half of women.20 Reassurance is important and wearing a supportive bra often can alleviate breast pain. In addition, reducing caffeine intake can be helpful.
As a first-line treatment, both topical (diclofenac) and oral nonsteroidal anti-inflammatory drugs effectively can relieve breast pain. Supplements and herbal remedies (for example, evening primrose oil, vitamin E, flaxseed) have varying effectiveness and are of questionable benefit as few have trials to support their effectiveness.4 Danazol and tamoxifen have been shown to have some benefits but they also have adverse effects.20 Surgery does not play a role in the treatment of mastalgia.
CASE 5 Breastfeeding woman with breast pain
A 27-year-old postpartum woman presents with concerns for redness and pain in the upper inner left breast. She has been breastfeeding for the past few months. Breast examination demonstrates a 5-cm area of erythema and warmth but no fluctuance or masses.
Lactational mastitis
Targeted ultrasonography is the test of choice for this 27-year-old patient with focal breast pain, and the imaging revealed edema of subcutaneous tissues and ill-defined hypoechoic areas, likely inflamed fat lobules (FIGURE 5). These findings suggest uncomplicated lactational mastitis, which can be treated with antibiotics. Generally, the mastitis will improve within days of starting the antibiotics; if it does not improve, repeat examination and ultrasound should be performed to look for formation of an abscess that may require aspiration.
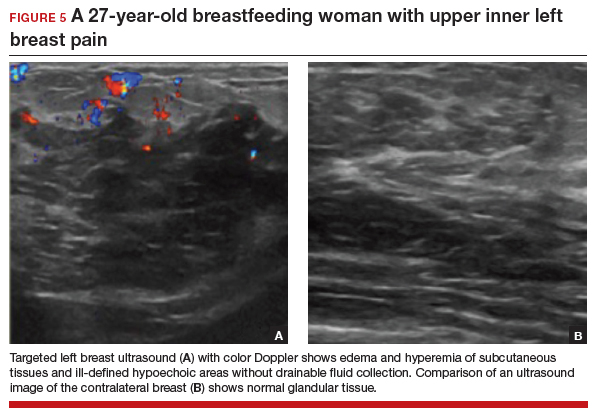
Continue to: CASE 6 Woman with painful periareolar mass...
CASE 6 Woman with painful periareolar mass
A 42-year-old perimenopausal woman describes having pain near the nipple of her right breast. She is a smoker and has no history of breast cancer in her family. Examination demonstrates a palpable, erythematous, painful, 3-cm periareolar fluctuant mass.
Nonpuerperal periareolar abscess
Appropriate initial imaging for this 42-year-old patient with focal pain is a diagnostic mammogram, which showed skin thickening and a retroareolar mass (FIGURE 6a). Further evaluation with targeted ultrasound showed a thick-walled anechoic collection with echoes compatible with an abscess (FIGURE 6b).
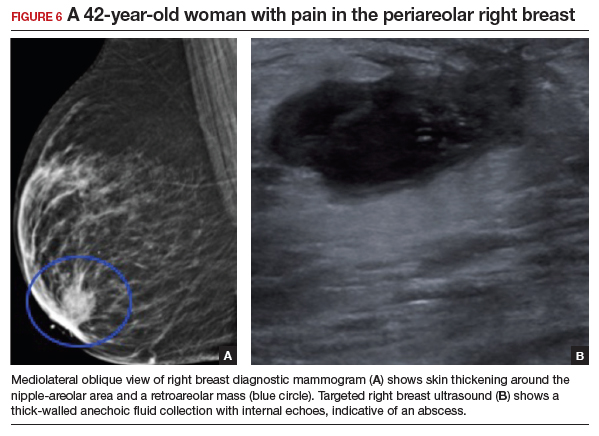
Mammographic findings in a patient with mastitis may be normal or demonstrate skin and trabecular thickening. Ultrasound imaging may show dilated ducts and heterogeneous tissue secondary to inflammation and edema without a discrete fluid collection. In cases with breast abscess, in addition to the mammographic findings described above, a mass, or an asymmetry, may be seen, most commonly in a subareolar location. On ultrasound, a hypoechoic collection with mobile debris, no internal flow on Doppler, and thick hypervascular walls can be seen with abscess, occasionally giving the appearance of a complicated cyst or a mixed cystic, solid mass.
The most important differential for mastitis is inflammatory breast cancer. Most cancers appear solid but can have central necrosis, mimicking a complicated cystic mass on ultrasound. The location for mastitis or abscess is most frequently subareolar. The presence of microcalcifications in a mass indicates the possibility of cancer.
Contrast-enhanced MRI can be helpful to differentiate between infection and cancer, with cancers showing initial early enhancement and washout kinetics compared with infected collections that show no enhancement or peripheral enhancement with a plateau or persistent enhancement curves. When clinical and imaging findings are unchanged after treatment of mastitis and abscesses, a core needle biopsy should be performed.21,22
There are 2 categories of mastitis and breast abscess: lactational and nonpuerperal (all mastitis that occurs outside the lactational period). The World Health Organization definition of puerperal mastitis includes pain, local redness, warmth and swelling of the breast (usually unilateral), fever, and malaise.4 Concerning etiology, epithelial lesions in the nipple area caused by breastfeeding can allow pathogens to enter and cause infection. The most common microorganism is Staphylococcus aureus.4 Continued emptying of the breast is important, combined with early antibiotic therapy (dicloxacillin is often the first line; if the patient is penicillin allergic, use a macrolide such as clindamycin). If no improvement is seen in 48 to 72 hours, imaging should be performed.
In most cases, continuation of breastfeeding is possible. If mastitis has evolved into an abscess in a lactating woman, it can be aspirated under ultrasound guidance. Incision and drainage should be avoided unless the abscess persists after multiple aspiration attempts, it is large, or if the overlying skin is thin or otherwise appears nonviable.
Nonpuerperal mastitis includes peripheral, periductal, and idiopathic granulomatous mastitis (IGM). Peripheral mastitis behaves like infections/abscesses in other soft tissues, responds well to treatment (antibiotics and percutaneous drainage), and is less likely to recur than periductal mastitis and IGM.21,23
Periductal mastitis and abscess, also known as Zuska disease, has a pathogenesis distinct from other forms of mastitis. Squamous metaplasia of the usual cuboidal epithelium of the breast ducts leads to keratin plugging that can cause infection.23 Risk factors include obesity, smoking, and macromastia. The typical presentation of Zuska disease is a woman with a history of chronic smoking and/or a congenital cleft in the central nipple.23 Periareolar signs of inflammation (redness, swelling, warmth) may be accompanied by an abscess. These can recur and lead to chronic fistula formation, especially if there is a history of intervention (such as aspiration, incision, and drainage).
Treatment of Zuska disease includes symptom relief and antibiotics. If S aureus is present, infection with methicillin-resistant S aureus is likely, and treatment with clindamycin or amoxicillin/clavulanic acid is preferred. If abscess is present, aspiration (preferred, often under ultrasound guidance) or incision and drainage (if the skin is compromised) may be required. If disease is recurrent or associated with a chronically draining fistula, surgical intervention may be warranted, in which resolution requires removing the keratin-plugged ducts in and immediately below the central core of the nipple. Given the association between Zuska disease and smoking, cessation should be encouraged, although there is no guarantee that this will resolve the issue.23
Continue to: CASE 7 Patient with breast pain and swelling...
CASE 7 Patient with breast pain and swelling
A 39-year-old woman presents with left breast swelling and pain of 1 month’s duration. On examination, there is a 6-cm area of edema, induration, and erythema.
Granulomatous mastitis
A diagnostic mammogram and ultrasound demonstrated an ill-defined hypoechoic mass (FIGURE 7a). Ultrasound-guided biopsy was performed, which showed granulomatous mastitis, negative for fungus and acid-fast bacilli. The patient was treated with prednisone and gradually improved (FIGURE 7b).
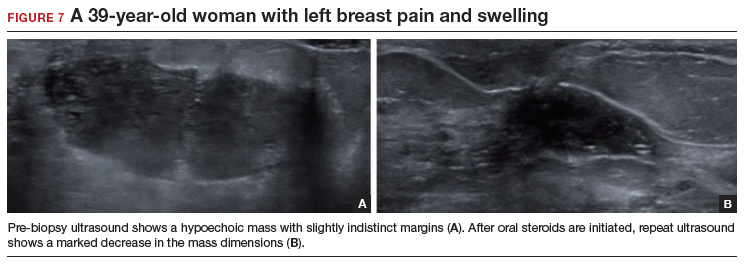
Granulomatous mastitis (GM) is a rare benign inflammatory process, with etiologies that include fungal infections, tuberculosis, Wegener granulomatosis, sarcoidosis, and idiopathic causes. Imaging can be nonspecific and show variable features. Mammograms can appear normal or show asymmetry or mass and skin thickening. Ultrasound can show heterogeneous parenchyma, ill-defined hypoechoic collection, or a mass with margins that can be circumscribed or indistinct or with tubular extensions, with or without overlying skin thickening, fistulas, and reactive lymph nodes.24
In this clinical setting, the differential diagnosis includes infectious mastitis, inflammatory breast cancer, foreign body injection granulomas, and diabetic mastopathy. Treatment involves drainage and fluid culture if there is a collection on imaging. A core biopsy is performed if imaging demonstrates a solid mass or fluid culture is negative and symptoms persist or recur. Oral steroids represent the mainstay of treatment if a core biopsy shows GM. However, immunosuppressants, including methotrexate, and surgery are options if initial treatment is not helpful.25,26
Conclusion
Breast symptoms are common reasons for patient visits to obstetricians and gynecologists. With a good understanding of the various symptomatic breast diseases and conditions, and by having a close collaboration with radiologists and breast surgeons, clinicians can provide excellent care to these patients and thereby improve patient outcomes and satisfaction. ●
- Eberl MM, Phillips RL Jr, Lamberts H, et al. Characterizing breast symptoms in family practice. Ann Fam Med. 2008;6:528-533.
- Malherbe F, Nel D, Molabe H, et al. Palpable breast lumps: an age-based approach to evaluation and diagnosis. S Afr Fam Pract (2022). 2022;64:e1-e5.
- Expert Panel on Breast Imaging; Klein KA, Kocher M, Lourenco AP, et al. American College of Radiology ACR appropriateness criteria: palpable breast masses. Accessed February 15, 2023. https://acsearch.acr.org/docs/69495/Narrative/
- Stachs A, Stubert J, Reimer T, et al. Benign breast disease in women. Dtsch Arztebl Int. 2019;116:565574.
- Hines N, Slanetz PJ, Eisenberg RL. Cystic masses of the breast. AJR Am J Roentgenol. 2010;194:W122133.
- Berg WA. Reducing unnecessary biopsy and follow-up of benign cystic breast lesions. Radiology. 2020;295:52-53.
- Duman L, Gezer NS, Balcı P, et al. Differentiation between phyllodes tumors and fibroadenomas based on mammographic sonographic and MRI features. Breast Care. 2016;11:123-127.
- Lerwill MF, Lee AHS, Tan PH. Fibroepithelial tumours of the breast—a review. Virchows Arch. 2022;480:45-63.
- Vasei N, Shishegar A, Ghalkhani F, et al. Fat necrosis in the breast: a systematic review of clinical. Lipids Health Dis. 2019;18:139.
- Kerridge WD, Kryvenko ON, Thompson A, et al. Fat necrosis of the breast: a pictorial review of the mammographic, ultrasound, CT, and MRI findings with histopathologic correlation. Radiol Res Pract. 2015;2015:613139.
- Taboada JL, Stephens TW, Krishnamurthy S, et al. The many faces of fat necrosis in the breast. AJR Am J Roentgenol. 2009;192:815-825.
- Tan PH, Lai LM, Carrington EV, et al. Fat necrosis of the breast—a review. Breast. 2006;15:313-318.
- Holbrook AI. Breast pain, a common grievance: guidance to radiologists. AJR Am J Roentgenol. 2020;214:259-264.
- Expert Panel on Breast Imaging; Moy L, Heller SL, Bailey L, et al. ACR appropriateness criteria: palpable breast masses. J Am Coll Radiol. 2017;14:S203-S224.
- Chetlen AL, Kapoor MM, Watts MR. Mastalgia: imaging workup appropriateness. Acad Radiol. 2017;24:345-349.
- Arslan M, Kucukerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
- Fariselli G, Lepera P, Viganotti G, et al. Localized mastalgia as presenting symptom in breast cancer. Eur J Surg Oncol. 1988;14:213-215.
- Leddy R, Irshad A, Zerwas E, et al. Role of breast ultrasound and mammography in evaluating patients presenting with focal breast pain in the absence of a palpable lump. Breast J. 2013;19:582-589.
- Leung JW, Kornguth PJ, Gotway MB. Utility of targeted sonography in the evaluation of focal breast pain. J Ultrasound Med. 2002;21:521-526.
- Goyal A. Breast pain. BMJ Clin Evid. 2011; 2011:0812.
- Kasales CJ, Han B, Smith Jr JS, et al. Nonpuerperal mastitis and subareolar abscess of the breast. AJR Am J Roentgenol. 2014;202:W133-W139.
- Mahoney MC, Ingram AD. Breast emergencies: types, imaging features, and management. AJR Am J Roentgenol. 2014;202:W390-W399.
- Snider HC. Management of mastitis, abscess, and fistula. Surg Clin North Am. 2022;102:1103-1116.
- Oztekin PS, Durhan G, Kosar PN, et al. Imaging findings in patients with granulomatous mastitis. Iran J Radiol. 2016;13:e33900.
- Pluguez-Turull CW, Nanyes JE, Quintero CJ, et al. Idiopathic granulomatous mastitis: manifestations at multimodality imaging and pitfalls. Radiographics. 2018;38:330-356.
- Hovanessian-Larsen LJ, Peyvandi B, Klipfel N, et al. Granulomatous lobular mastitis: imaging, diagnosis, and treatment. AJR Am J Roentgenol. 2009;193:574-581.
The vast majority of symptomatic breast conditions are benign, with the most common symptoms being palpable mass and breast pain. Clinicians, including primary care clinicians and gynecologists, play a crucial role by performing the initial assessment and subsequent therapies and referrals and serve as the mediator between the specialists and by being the patient’s spokesperson. It is therefore important for clinicians to be aware of the various possible causes of these breast symptoms, to know which imaging tests to order, and also to understand the indications for biopsies and surgical referral.
Common types of breast lumps: Imaging workup and management
Accounting for 8% of women who present with breast symptoms, breast lump is the second most common symptom after breast pain.1 The positive likelihood ratio of finding breast cancer is highest among women with breast lumps compared with any other breast symptoms. Therefore, anxiety is related to this symptom, and a thorough evaluation is recommended.1 Cysts, fibroadenoma, and fat necrosis are 3 common benign causes of breast lumps.2
In this section, we review clinical presentation, imaging workup, and management strategies for common types of breast lumps.
CASE 1 Woman with tender breast lump
A 45-year-old woman presents with a breast lump of 6 months’ duration that is associated with a change in size with the menstrual cycle and pain. Clinical examination reveals a 4 x 4.5–cm mass in the right breast in the retroareolar region, which is smooth with some tenderness on palpation.
Breast cyst
According to the American College of Radiology appropriateness criteria for an adult woman 40 years of age or older who presents with a palpable breast mass, the initial imaging study is diagnostic mammography with or without digital tomosynthesis, usually followed by a directed ultrasound. If the mammogram is suspicious or highly suggestive of malignancy, or in cases where the mammogram does not show an abnormality, the next recommended step is breast ultrasonography. Any suspicious findings on ultrasound or mammogram should be followed by an image guided biopsy. Ultrasonography also may be appropriate if the mammogram findings are benign or probably benign.
For an adult woman younger than age 30 who presents with a palpable breast mass, breast ultrasonography is the appropriate initial imaging study. If the ultrasound is suspicious or highly suggestive of malignancy, then performing diagnostic mammography with or without digital tomosynthesis or ultrasound-guided core needle biopsy of the mass are both considered appropriate. However, no further imaging is recommended if the ultrasound is benign, probably benign, or negative. Breast ultrasonography or mammography is appropriate as the initial imaging test for adult women aged 30 to 39 years who present with a palpable breast mass.3,4
Approximately 50% of women after age 30 may develop fibrocystic breast disease, and 20% of them can present with pain or lump due to a macrocysts. Simple cysts must be distinguished from complex cysts with the help of ultrasound as the latter are associated with 23% to 31% increased risk of malignancy.
In this 45-year-old patient, the initial mammogram demonstrated a circumscribed mass underneath the area of palpable concern (FIGURE 1a, 1b). Targeted breast ultrasonography was performed for further assessment, which depicted the mass as a benign simple cyst (FIGURE 1c).

On ultrasound, a simple cyst is an anechoic, well-circumscribed mass with a thin capsule and with increased through transmission. Patients with small and asymptomatic simple cysts do not need imaging follow-up and can return for routine screening mammograms.
A breast surgeon, radiologist, or gynecologist can perform percutaneous aspiration if a cyst is large and symptomatic. A cyst with low-level internal echoes, fluid-fluid, or fluid-debris levels is considered a complicated cyst. Differential diagnosis also includes hematoma, fat necrosis, abscess, and galactocele, depending on the clinical presentation. Fine-needle aspiration or short-interval follow-up5,6 is appropriate for complicated cysts, while incision and drainage is indicated in patients with infected cysts and abscesses. A cyst with a solid component is considered a cystic, solid mass, and core needle biopsy is recommended. The differential diagnosis for cysts with solid components includes intracystic papilloma, papillary carcinoma, ductal carcinoma in situ, and necrotic cancers.5,6
Continue to: CASE 2 Painless breast mass in a young woman...
CASE 2 Painless breast mass in a young woman
A 22-year-old woman presents with a 2-month history of breast lump, which is not associated with pain or nipple discharge. On examination, there is a 2 x 2–cm mass in the right breast at 12 o’clock, 2 cm from the nipple, which is mobile, smooth, and nontender on palpation.
Fibroadenoma
In this 22-year-old, the initial imaging of choice is breast ultrasonography. Breast ultrasonography can differentiate a cystic mass from a solid mass, and it does not involve radiation. Right breast targeted ultrasound showed a circumscribed oval homogeneous hypoechoic mass that is wider than tall (FIGURE 2). The patient desired surgical removal, and a pre-lumpectomy core needle biopsy revealed a fibroadenoma.

Fibroadenoma is the most common benign tumor of the breast. It is most often encountered in premenopausal women. Patients present with a painless breast lump, which is smooth and mobile on palpation. Fibroadenoma can be followed expectantly with repeat ultrasound (to assess over time for growth) if it is small and asymptomatic. No further action is needed if it remains stable. If a patient desires surgical excision, a core needle biopsy is usually performed before lumpectomy.
Excisional biopsy or removal of the mass is recommended if the mass is greater than 3 or 4 cm, is symptomatic, or if there is an increase in size that raises clinical concern for phyllodes tumor. Imaging features that are concerning for phyllodes tumors are size greater than 3 cm, indistinct or microlobulated margins, and heterogeneous echo pattern.7,8 In cases in which the imaging features are concerning for phyllodes tumor and a core needle biopsy is not definitive, wide surgical excision is recommended for definitive diagnosis.8
CASE 3 Patient develops breast mass post-surgery
A 45-year-old woman presents with a tender left breast mass that she noticed 2 months after breast reduction surgery. It has been increasing in size since. On clinical examination, a 4 x 4–cm mass is found at the surgical scar site, which is indurated on palpation and tender.
Fat necrosis
In this 45-year-old, the initial test of choice is diagnostic mammography, which showed a somewhat circumscribed area with fat under the palpable marker (FIGURE 3a). Breast ultrasonography was performed for further evaluation, which was inconclusive as the ultrasound showed ill-defined areas of mixed echogenicity (FIGURE 3b). Breast magnetic resonance imaging (MRI) clearly demonstrated fat necrosis in the area of the palpable lump (FIGURE 3c).

Fat necrosis of the breast is an inflammatory process that is seen after breast trauma or surgery. It can present as an incidental mammogram finding or a palpable mass. The patient may give a history of trauma, breast reduction surgery, or breast cancer surgery followed by radiation treatment. On clinical examination, fat necrosis occasionally can present as a firm mass with skin retraction or swelling concerning for cancer. Imaging features are variable depending on the stage of fat necrosis and inflammation.9-11
A mammogram may demonstrate a circumscribed fat-containing mass, an ill-defined mass, asymmetry or calcified oil cyst, and dystrophic calcifications. On ultrasound, fat necrosis can appear as anechoic or hypoechoic or as a complicated cyst or a mixed cystic, solid mass. MRI demonstrates a circumscribed or irregular fat-containing mass, with or without enhancement, and architectural distortion.
When the imaging features are clearly benign—for example, a circumscribed fat-containing mass on mammogram or on ultrasound or, on MRI, marked hypointensity of fat in the center of a circumscribed mass when compared with surrounding fat (keyhole sign)—no further follow-up is needed. When the imaging features are indeterminate, however, a short-interval follow-up can be considered. In cases with irregular fat-containing mass with enhancement, core needle biopsy is indicated to exclude cancer. If the workup remains inconclusive and the level of clinical suspicion is high, surgical excision can be performed for a definitive diagnosis.12
Continue to: Investigating breast pain: Imaging workup and management...
Investigating breast pain: Imaging workup and management
Breast pain, or mastalgia, is the most common concern of women presenting to a breast clinic and accounts for approximately half of such encounters.13 Causes of breast pain include hormonal changes, fibrocystic changes, musculoskeletal causes (such as costochondritis), lack of support, infection, and injury. While mastalgia often causes patient concern, the risk of malignancy in a woman presenting with breast pain alone is low. Still, it is essential to rule out other findings suspicious for cancer (mass, skin changes, or nipple discharge) with a thorough history and breast examination.
In this section, we review clinical presentation, imaging workup, and management for breast pain.
CASE 4 Woman with noncyclic breast pain
A 26-year-old woman presents to the clinic with mastalgia. The pain is noncyclic and primarily located in the upper outer quadrant of her left breast. There is no history of breast cancer in her family. She has no suspicious findings on the breast examination.
Mastalgia
The test of choice for this 26-year-old with focal left breast pain is targeted breast ultrasound. The patient’s ultrasound image showed no suspicious findings or solid or cystic mass (FIGURE 4).

Two important characteristics of breast pain are whether it is noncyclical and whether it is focal. According to the American College of Radiology, no breast imaging is recommended for clinically insignificant cyclical, nonfocal (greater than 1 quadrant)/diffuse pain, as this type of mastalgia is not associated with malignancy.14
For patients age 40 or older, if they are not up to date with their annual screening mammogram, then a mammogram should be performed. An imaging workup is warranted for clinically significant mastalgia that is noncyclical and focal. Even then, no malignancy is identified in most patients with clinically significant mastalgia; in patients with breast pain as their only symptom, the prevalence of breast cancer is 0% to 3.0%.15-19
The initial imaging modality differs by patient age: younger than 30 years, ultrasonography; between 30 and 40 years, mammography or ultrasonography; and older than 40 years, mammography first followed by ultrasonography.14
Treatment of breast pain is primarily symptomatic, and evidence for specific treatments is generally lacking. Cyclical breast pain resolves spontaneously in 20% to 30% of women, while noncyclical pain responds poorly to treatment but resolves spontaneously in half of women.20 Reassurance is important and wearing a supportive bra often can alleviate breast pain. In addition, reducing caffeine intake can be helpful.
As a first-line treatment, both topical (diclofenac) and oral nonsteroidal anti-inflammatory drugs effectively can relieve breast pain. Supplements and herbal remedies (for example, evening primrose oil, vitamin E, flaxseed) have varying effectiveness and are of questionable benefit as few have trials to support their effectiveness.4 Danazol and tamoxifen have been shown to have some benefits but they also have adverse effects.20 Surgery does not play a role in the treatment of mastalgia.
CASE 5 Breastfeeding woman with breast pain
A 27-year-old postpartum woman presents with concerns for redness and pain in the upper inner left breast. She has been breastfeeding for the past few months. Breast examination demonstrates a 5-cm area of erythema and warmth but no fluctuance or masses.
Lactational mastitis
Targeted ultrasonography is the test of choice for this 27-year-old patient with focal breast pain, and the imaging revealed edema of subcutaneous tissues and ill-defined hypoechoic areas, likely inflamed fat lobules (FIGURE 5). These findings suggest uncomplicated lactational mastitis, which can be treated with antibiotics. Generally, the mastitis will improve within days of starting the antibiotics; if it does not improve, repeat examination and ultrasound should be performed to look for formation of an abscess that may require aspiration.

Continue to: CASE 6 Woman with painful periareolar mass...
CASE 6 Woman with painful periareolar mass
A 42-year-old perimenopausal woman describes having pain near the nipple of her right breast. She is a smoker and has no history of breast cancer in her family. Examination demonstrates a palpable, erythematous, painful, 3-cm periareolar fluctuant mass.
Nonpuerperal periareolar abscess
Appropriate initial imaging for this 42-year-old patient with focal pain is a diagnostic mammogram, which showed skin thickening and a retroareolar mass (FIGURE 6a). Further evaluation with targeted ultrasound showed a thick-walled anechoic collection with echoes compatible with an abscess (FIGURE 6b).

Mammographic findings in a patient with mastitis may be normal or demonstrate skin and trabecular thickening. Ultrasound imaging may show dilated ducts and heterogeneous tissue secondary to inflammation and edema without a discrete fluid collection. In cases with breast abscess, in addition to the mammographic findings described above, a mass, or an asymmetry, may be seen, most commonly in a subareolar location. On ultrasound, a hypoechoic collection with mobile debris, no internal flow on Doppler, and thick hypervascular walls can be seen with abscess, occasionally giving the appearance of a complicated cyst or a mixed cystic, solid mass.
The most important differential for mastitis is inflammatory breast cancer. Most cancers appear solid but can have central necrosis, mimicking a complicated cystic mass on ultrasound. The location for mastitis or abscess is most frequently subareolar. The presence of microcalcifications in a mass indicates the possibility of cancer.
Contrast-enhanced MRI can be helpful to differentiate between infection and cancer, with cancers showing initial early enhancement and washout kinetics compared with infected collections that show no enhancement or peripheral enhancement with a plateau or persistent enhancement curves. When clinical and imaging findings are unchanged after treatment of mastitis and abscesses, a core needle biopsy should be performed.21,22
There are 2 categories of mastitis and breast abscess: lactational and nonpuerperal (all mastitis that occurs outside the lactational period). The World Health Organization definition of puerperal mastitis includes pain, local redness, warmth and swelling of the breast (usually unilateral), fever, and malaise.4 Concerning etiology, epithelial lesions in the nipple area caused by breastfeeding can allow pathogens to enter and cause infection. The most common microorganism is Staphylococcus aureus.4 Continued emptying of the breast is important, combined with early antibiotic therapy (dicloxacillin is often the first line; if the patient is penicillin allergic, use a macrolide such as clindamycin). If no improvement is seen in 48 to 72 hours, imaging should be performed.
In most cases, continuation of breastfeeding is possible. If mastitis has evolved into an abscess in a lactating woman, it can be aspirated under ultrasound guidance. Incision and drainage should be avoided unless the abscess persists after multiple aspiration attempts, it is large, or if the overlying skin is thin or otherwise appears nonviable.
Nonpuerperal mastitis includes peripheral, periductal, and idiopathic granulomatous mastitis (IGM). Peripheral mastitis behaves like infections/abscesses in other soft tissues, responds well to treatment (antibiotics and percutaneous drainage), and is less likely to recur than periductal mastitis and IGM.21,23
Periductal mastitis and abscess, also known as Zuska disease, has a pathogenesis distinct from other forms of mastitis. Squamous metaplasia of the usual cuboidal epithelium of the breast ducts leads to keratin plugging that can cause infection.23 Risk factors include obesity, smoking, and macromastia. The typical presentation of Zuska disease is a woman with a history of chronic smoking and/or a congenital cleft in the central nipple.23 Periareolar signs of inflammation (redness, swelling, warmth) may be accompanied by an abscess. These can recur and lead to chronic fistula formation, especially if there is a history of intervention (such as aspiration, incision, and drainage).
Treatment of Zuska disease includes symptom relief and antibiotics. If S aureus is present, infection with methicillin-resistant S aureus is likely, and treatment with clindamycin or amoxicillin/clavulanic acid is preferred. If abscess is present, aspiration (preferred, often under ultrasound guidance) or incision and drainage (if the skin is compromised) may be required. If disease is recurrent or associated with a chronically draining fistula, surgical intervention may be warranted, in which resolution requires removing the keratin-plugged ducts in and immediately below the central core of the nipple. Given the association between Zuska disease and smoking, cessation should be encouraged, although there is no guarantee that this will resolve the issue.23
Continue to: CASE 7 Patient with breast pain and swelling...
CASE 7 Patient with breast pain and swelling
A 39-year-old woman presents with left breast swelling and pain of 1 month’s duration. On examination, there is a 6-cm area of edema, induration, and erythema.
Granulomatous mastitis
A diagnostic mammogram and ultrasound demonstrated an ill-defined hypoechoic mass (FIGURE 7a). Ultrasound-guided biopsy was performed, which showed granulomatous mastitis, negative for fungus and acid-fast bacilli. The patient was treated with prednisone and gradually improved (FIGURE 7b).

Granulomatous mastitis (GM) is a rare benign inflammatory process, with etiologies that include fungal infections, tuberculosis, Wegener granulomatosis, sarcoidosis, and idiopathic causes. Imaging can be nonspecific and show variable features. Mammograms can appear normal or show asymmetry or mass and skin thickening. Ultrasound can show heterogeneous parenchyma, ill-defined hypoechoic collection, or a mass with margins that can be circumscribed or indistinct or with tubular extensions, with or without overlying skin thickening, fistulas, and reactive lymph nodes.24
In this clinical setting, the differential diagnosis includes infectious mastitis, inflammatory breast cancer, foreign body injection granulomas, and diabetic mastopathy. Treatment involves drainage and fluid culture if there is a collection on imaging. A core biopsy is performed if imaging demonstrates a solid mass or fluid culture is negative and symptoms persist or recur. Oral steroids represent the mainstay of treatment if a core biopsy shows GM. However, immunosuppressants, including methotrexate, and surgery are options if initial treatment is not helpful.25,26
Conclusion
Breast symptoms are common reasons for patient visits to obstetricians and gynecologists. With a good understanding of the various symptomatic breast diseases and conditions, and by having a close collaboration with radiologists and breast surgeons, clinicians can provide excellent care to these patients and thereby improve patient outcomes and satisfaction. ●
The vast majority of symptomatic breast conditions are benign, with the most common symptoms being palpable mass and breast pain. Clinicians, including primary care clinicians and gynecologists, play a crucial role by performing the initial assessment and subsequent therapies and referrals and serve as the mediator between the specialists and by being the patient’s spokesperson. It is therefore important for clinicians to be aware of the various possible causes of these breast symptoms, to know which imaging tests to order, and also to understand the indications for biopsies and surgical referral.
Common types of breast lumps: Imaging workup and management
Accounting for 8% of women who present with breast symptoms, breast lump is the second most common symptom after breast pain.1 The positive likelihood ratio of finding breast cancer is highest among women with breast lumps compared with any other breast symptoms. Therefore, anxiety is related to this symptom, and a thorough evaluation is recommended.1 Cysts, fibroadenoma, and fat necrosis are 3 common benign causes of breast lumps.2
In this section, we review clinical presentation, imaging workup, and management strategies for common types of breast lumps.
CASE 1 Woman with tender breast lump
A 45-year-old woman presents with a breast lump of 6 months’ duration that is associated with a change in size with the menstrual cycle and pain. Clinical examination reveals a 4 x 4.5–cm mass in the right breast in the retroareolar region, which is smooth with some tenderness on palpation.
Breast cyst
According to the American College of Radiology appropriateness criteria for an adult woman 40 years of age or older who presents with a palpable breast mass, the initial imaging study is diagnostic mammography with or without digital tomosynthesis, usually followed by a directed ultrasound. If the mammogram is suspicious or highly suggestive of malignancy, or in cases where the mammogram does not show an abnormality, the next recommended step is breast ultrasonography. Any suspicious findings on ultrasound or mammogram should be followed by an image guided biopsy. Ultrasonography also may be appropriate if the mammogram findings are benign or probably benign.
For an adult woman younger than age 30 who presents with a palpable breast mass, breast ultrasonography is the appropriate initial imaging study. If the ultrasound is suspicious or highly suggestive of malignancy, then performing diagnostic mammography with or without digital tomosynthesis or ultrasound-guided core needle biopsy of the mass are both considered appropriate. However, no further imaging is recommended if the ultrasound is benign, probably benign, or negative. Breast ultrasonography or mammography is appropriate as the initial imaging test for adult women aged 30 to 39 years who present with a palpable breast mass.3,4
Approximately 50% of women after age 30 may develop fibrocystic breast disease, and 20% of them can present with pain or lump due to a macrocysts. Simple cysts must be distinguished from complex cysts with the help of ultrasound as the latter are associated with 23% to 31% increased risk of malignancy.
In this 45-year-old patient, the initial mammogram demonstrated a circumscribed mass underneath the area of palpable concern (FIGURE 1a, 1b). Targeted breast ultrasonography was performed for further assessment, which depicted the mass as a benign simple cyst (FIGURE 1c).

On ultrasound, a simple cyst is an anechoic, well-circumscribed mass with a thin capsule and with increased through transmission. Patients with small and asymptomatic simple cysts do not need imaging follow-up and can return for routine screening mammograms.
A breast surgeon, radiologist, or gynecologist can perform percutaneous aspiration if a cyst is large and symptomatic. A cyst with low-level internal echoes, fluid-fluid, or fluid-debris levels is considered a complicated cyst. Differential diagnosis also includes hematoma, fat necrosis, abscess, and galactocele, depending on the clinical presentation. Fine-needle aspiration or short-interval follow-up5,6 is appropriate for complicated cysts, while incision and drainage is indicated in patients with infected cysts and abscesses. A cyst with a solid component is considered a cystic, solid mass, and core needle biopsy is recommended. The differential diagnosis for cysts with solid components includes intracystic papilloma, papillary carcinoma, ductal carcinoma in situ, and necrotic cancers.5,6
Continue to: CASE 2 Painless breast mass in a young woman...
CASE 2 Painless breast mass in a young woman
A 22-year-old woman presents with a 2-month history of breast lump, which is not associated with pain or nipple discharge. On examination, there is a 2 x 2–cm mass in the right breast at 12 o’clock, 2 cm from the nipple, which is mobile, smooth, and nontender on palpation.
Fibroadenoma
In this 22-year-old, the initial imaging of choice is breast ultrasonography. Breast ultrasonography can differentiate a cystic mass from a solid mass, and it does not involve radiation. Right breast targeted ultrasound showed a circumscribed oval homogeneous hypoechoic mass that is wider than tall (FIGURE 2). The patient desired surgical removal, and a pre-lumpectomy core needle biopsy revealed a fibroadenoma.

Fibroadenoma is the most common benign tumor of the breast. It is most often encountered in premenopausal women. Patients present with a painless breast lump, which is smooth and mobile on palpation. Fibroadenoma can be followed expectantly with repeat ultrasound (to assess over time for growth) if it is small and asymptomatic. No further action is needed if it remains stable. If a patient desires surgical excision, a core needle biopsy is usually performed before lumpectomy.
Excisional biopsy or removal of the mass is recommended if the mass is greater than 3 or 4 cm, is symptomatic, or if there is an increase in size that raises clinical concern for phyllodes tumor. Imaging features that are concerning for phyllodes tumors are size greater than 3 cm, indistinct or microlobulated margins, and heterogeneous echo pattern.7,8 In cases in which the imaging features are concerning for phyllodes tumor and a core needle biopsy is not definitive, wide surgical excision is recommended for definitive diagnosis.8
CASE 3 Patient develops breast mass post-surgery
A 45-year-old woman presents with a tender left breast mass that she noticed 2 months after breast reduction surgery. It has been increasing in size since. On clinical examination, a 4 x 4–cm mass is found at the surgical scar site, which is indurated on palpation and tender.
Fat necrosis
In this 45-year-old, the initial test of choice is diagnostic mammography, which showed a somewhat circumscribed area with fat under the palpable marker (FIGURE 3a). Breast ultrasonography was performed for further evaluation, which was inconclusive as the ultrasound showed ill-defined areas of mixed echogenicity (FIGURE 3b). Breast magnetic resonance imaging (MRI) clearly demonstrated fat necrosis in the area of the palpable lump (FIGURE 3c).

Fat necrosis of the breast is an inflammatory process that is seen after breast trauma or surgery. It can present as an incidental mammogram finding or a palpable mass. The patient may give a history of trauma, breast reduction surgery, or breast cancer surgery followed by radiation treatment. On clinical examination, fat necrosis occasionally can present as a firm mass with skin retraction or swelling concerning for cancer. Imaging features are variable depending on the stage of fat necrosis and inflammation.9-11
A mammogram may demonstrate a circumscribed fat-containing mass, an ill-defined mass, asymmetry or calcified oil cyst, and dystrophic calcifications. On ultrasound, fat necrosis can appear as anechoic or hypoechoic or as a complicated cyst or a mixed cystic, solid mass. MRI demonstrates a circumscribed or irregular fat-containing mass, with or without enhancement, and architectural distortion.
When the imaging features are clearly benign—for example, a circumscribed fat-containing mass on mammogram or on ultrasound or, on MRI, marked hypointensity of fat in the center of a circumscribed mass when compared with surrounding fat (keyhole sign)—no further follow-up is needed. When the imaging features are indeterminate, however, a short-interval follow-up can be considered. In cases with irregular fat-containing mass with enhancement, core needle biopsy is indicated to exclude cancer. If the workup remains inconclusive and the level of clinical suspicion is high, surgical excision can be performed for a definitive diagnosis.12
Continue to: Investigating breast pain: Imaging workup and management...
Investigating breast pain: Imaging workup and management
Breast pain, or mastalgia, is the most common concern of women presenting to a breast clinic and accounts for approximately half of such encounters.13 Causes of breast pain include hormonal changes, fibrocystic changes, musculoskeletal causes (such as costochondritis), lack of support, infection, and injury. While mastalgia often causes patient concern, the risk of malignancy in a woman presenting with breast pain alone is low. Still, it is essential to rule out other findings suspicious for cancer (mass, skin changes, or nipple discharge) with a thorough history and breast examination.
In this section, we review clinical presentation, imaging workup, and management for breast pain.
CASE 4 Woman with noncyclic breast pain
A 26-year-old woman presents to the clinic with mastalgia. The pain is noncyclic and primarily located in the upper outer quadrant of her left breast. There is no history of breast cancer in her family. She has no suspicious findings on the breast examination.
Mastalgia
The test of choice for this 26-year-old with focal left breast pain is targeted breast ultrasound. The patient’s ultrasound image showed no suspicious findings or solid or cystic mass (FIGURE 4).

Two important characteristics of breast pain are whether it is noncyclical and whether it is focal. According to the American College of Radiology, no breast imaging is recommended for clinically insignificant cyclical, nonfocal (greater than 1 quadrant)/diffuse pain, as this type of mastalgia is not associated with malignancy.14
For patients age 40 or older, if they are not up to date with their annual screening mammogram, then a mammogram should be performed. An imaging workup is warranted for clinically significant mastalgia that is noncyclical and focal. Even then, no malignancy is identified in most patients with clinically significant mastalgia; in patients with breast pain as their only symptom, the prevalence of breast cancer is 0% to 3.0%.15-19
The initial imaging modality differs by patient age: younger than 30 years, ultrasonography; between 30 and 40 years, mammography or ultrasonography; and older than 40 years, mammography first followed by ultrasonography.14
Treatment of breast pain is primarily symptomatic, and evidence for specific treatments is generally lacking. Cyclical breast pain resolves spontaneously in 20% to 30% of women, while noncyclical pain responds poorly to treatment but resolves spontaneously in half of women.20 Reassurance is important and wearing a supportive bra often can alleviate breast pain. In addition, reducing caffeine intake can be helpful.
As a first-line treatment, both topical (diclofenac) and oral nonsteroidal anti-inflammatory drugs effectively can relieve breast pain. Supplements and herbal remedies (for example, evening primrose oil, vitamin E, flaxseed) have varying effectiveness and are of questionable benefit as few have trials to support their effectiveness.4 Danazol and tamoxifen have been shown to have some benefits but they also have adverse effects.20 Surgery does not play a role in the treatment of mastalgia.
CASE 5 Breastfeeding woman with breast pain
A 27-year-old postpartum woman presents with concerns for redness and pain in the upper inner left breast. She has been breastfeeding for the past few months. Breast examination demonstrates a 5-cm area of erythema and warmth but no fluctuance or masses.
Lactational mastitis
Targeted ultrasonography is the test of choice for this 27-year-old patient with focal breast pain, and the imaging revealed edema of subcutaneous tissues and ill-defined hypoechoic areas, likely inflamed fat lobules (FIGURE 5). These findings suggest uncomplicated lactational mastitis, which can be treated with antibiotics. Generally, the mastitis will improve within days of starting the antibiotics; if it does not improve, repeat examination and ultrasound should be performed to look for formation of an abscess that may require aspiration.

Continue to: CASE 6 Woman with painful periareolar mass...
CASE 6 Woman with painful periareolar mass
A 42-year-old perimenopausal woman describes having pain near the nipple of her right breast. She is a smoker and has no history of breast cancer in her family. Examination demonstrates a palpable, erythematous, painful, 3-cm periareolar fluctuant mass.
Nonpuerperal periareolar abscess
Appropriate initial imaging for this 42-year-old patient with focal pain is a diagnostic mammogram, which showed skin thickening and a retroareolar mass (FIGURE 6a). Further evaluation with targeted ultrasound showed a thick-walled anechoic collection with echoes compatible with an abscess (FIGURE 6b).

Mammographic findings in a patient with mastitis may be normal or demonstrate skin and trabecular thickening. Ultrasound imaging may show dilated ducts and heterogeneous tissue secondary to inflammation and edema without a discrete fluid collection. In cases with breast abscess, in addition to the mammographic findings described above, a mass, or an asymmetry, may be seen, most commonly in a subareolar location. On ultrasound, a hypoechoic collection with mobile debris, no internal flow on Doppler, and thick hypervascular walls can be seen with abscess, occasionally giving the appearance of a complicated cyst or a mixed cystic, solid mass.
The most important differential for mastitis is inflammatory breast cancer. Most cancers appear solid but can have central necrosis, mimicking a complicated cystic mass on ultrasound. The location for mastitis or abscess is most frequently subareolar. The presence of microcalcifications in a mass indicates the possibility of cancer.
Contrast-enhanced MRI can be helpful to differentiate between infection and cancer, with cancers showing initial early enhancement and washout kinetics compared with infected collections that show no enhancement or peripheral enhancement with a plateau or persistent enhancement curves. When clinical and imaging findings are unchanged after treatment of mastitis and abscesses, a core needle biopsy should be performed.21,22
There are 2 categories of mastitis and breast abscess: lactational and nonpuerperal (all mastitis that occurs outside the lactational period). The World Health Organization definition of puerperal mastitis includes pain, local redness, warmth and swelling of the breast (usually unilateral), fever, and malaise.4 Concerning etiology, epithelial lesions in the nipple area caused by breastfeeding can allow pathogens to enter and cause infection. The most common microorganism is Staphylococcus aureus.4 Continued emptying of the breast is important, combined with early antibiotic therapy (dicloxacillin is often the first line; if the patient is penicillin allergic, use a macrolide such as clindamycin). If no improvement is seen in 48 to 72 hours, imaging should be performed.
In most cases, continuation of breastfeeding is possible. If mastitis has evolved into an abscess in a lactating woman, it can be aspirated under ultrasound guidance. Incision and drainage should be avoided unless the abscess persists after multiple aspiration attempts, it is large, or if the overlying skin is thin or otherwise appears nonviable.
Nonpuerperal mastitis includes peripheral, periductal, and idiopathic granulomatous mastitis (IGM). Peripheral mastitis behaves like infections/abscesses in other soft tissues, responds well to treatment (antibiotics and percutaneous drainage), and is less likely to recur than periductal mastitis and IGM.21,23
Periductal mastitis and abscess, also known as Zuska disease, has a pathogenesis distinct from other forms of mastitis. Squamous metaplasia of the usual cuboidal epithelium of the breast ducts leads to keratin plugging that can cause infection.23 Risk factors include obesity, smoking, and macromastia. The typical presentation of Zuska disease is a woman with a history of chronic smoking and/or a congenital cleft in the central nipple.23 Periareolar signs of inflammation (redness, swelling, warmth) may be accompanied by an abscess. These can recur and lead to chronic fistula formation, especially if there is a history of intervention (such as aspiration, incision, and drainage).
Treatment of Zuska disease includes symptom relief and antibiotics. If S aureus is present, infection with methicillin-resistant S aureus is likely, and treatment with clindamycin or amoxicillin/clavulanic acid is preferred. If abscess is present, aspiration (preferred, often under ultrasound guidance) or incision and drainage (if the skin is compromised) may be required. If disease is recurrent or associated with a chronically draining fistula, surgical intervention may be warranted, in which resolution requires removing the keratin-plugged ducts in and immediately below the central core of the nipple. Given the association between Zuska disease and smoking, cessation should be encouraged, although there is no guarantee that this will resolve the issue.23
Continue to: CASE 7 Patient with breast pain and swelling...
CASE 7 Patient with breast pain and swelling
A 39-year-old woman presents with left breast swelling and pain of 1 month’s duration. On examination, there is a 6-cm area of edema, induration, and erythema.
Granulomatous mastitis
A diagnostic mammogram and ultrasound demonstrated an ill-defined hypoechoic mass (FIGURE 7a). Ultrasound-guided biopsy was performed, which showed granulomatous mastitis, negative for fungus and acid-fast bacilli. The patient was treated with prednisone and gradually improved (FIGURE 7b).

Granulomatous mastitis (GM) is a rare benign inflammatory process, with etiologies that include fungal infections, tuberculosis, Wegener granulomatosis, sarcoidosis, and idiopathic causes. Imaging can be nonspecific and show variable features. Mammograms can appear normal or show asymmetry or mass and skin thickening. Ultrasound can show heterogeneous parenchyma, ill-defined hypoechoic collection, or a mass with margins that can be circumscribed or indistinct or with tubular extensions, with or without overlying skin thickening, fistulas, and reactive lymph nodes.24
In this clinical setting, the differential diagnosis includes infectious mastitis, inflammatory breast cancer, foreign body injection granulomas, and diabetic mastopathy. Treatment involves drainage and fluid culture if there is a collection on imaging. A core biopsy is performed if imaging demonstrates a solid mass or fluid culture is negative and symptoms persist or recur. Oral steroids represent the mainstay of treatment if a core biopsy shows GM. However, immunosuppressants, including methotrexate, and surgery are options if initial treatment is not helpful.25,26
Conclusion
Breast symptoms are common reasons for patient visits to obstetricians and gynecologists. With a good understanding of the various symptomatic breast diseases and conditions, and by having a close collaboration with radiologists and breast surgeons, clinicians can provide excellent care to these patients and thereby improve patient outcomes and satisfaction. ●
- Eberl MM, Phillips RL Jr, Lamberts H, et al. Characterizing breast symptoms in family practice. Ann Fam Med. 2008;6:528-533.
- Malherbe F, Nel D, Molabe H, et al. Palpable breast lumps: an age-based approach to evaluation and diagnosis. S Afr Fam Pract (2022). 2022;64:e1-e5.
- Expert Panel on Breast Imaging; Klein KA, Kocher M, Lourenco AP, et al. American College of Radiology ACR appropriateness criteria: palpable breast masses. Accessed February 15, 2023. https://acsearch.acr.org/docs/69495/Narrative/
- Stachs A, Stubert J, Reimer T, et al. Benign breast disease in women. Dtsch Arztebl Int. 2019;116:565574.
- Hines N, Slanetz PJ, Eisenberg RL. Cystic masses of the breast. AJR Am J Roentgenol. 2010;194:W122133.
- Berg WA. Reducing unnecessary biopsy and follow-up of benign cystic breast lesions. Radiology. 2020;295:52-53.
- Duman L, Gezer NS, Balcı P, et al. Differentiation between phyllodes tumors and fibroadenomas based on mammographic sonographic and MRI features. Breast Care. 2016;11:123-127.
- Lerwill MF, Lee AHS, Tan PH. Fibroepithelial tumours of the breast—a review. Virchows Arch. 2022;480:45-63.
- Vasei N, Shishegar A, Ghalkhani F, et al. Fat necrosis in the breast: a systematic review of clinical. Lipids Health Dis. 2019;18:139.
- Kerridge WD, Kryvenko ON, Thompson A, et al. Fat necrosis of the breast: a pictorial review of the mammographic, ultrasound, CT, and MRI findings with histopathologic correlation. Radiol Res Pract. 2015;2015:613139.
- Taboada JL, Stephens TW, Krishnamurthy S, et al. The many faces of fat necrosis in the breast. AJR Am J Roentgenol. 2009;192:815-825.
- Tan PH, Lai LM, Carrington EV, et al. Fat necrosis of the breast—a review. Breast. 2006;15:313-318.
- Holbrook AI. Breast pain, a common grievance: guidance to radiologists. AJR Am J Roentgenol. 2020;214:259-264.
- Expert Panel on Breast Imaging; Moy L, Heller SL, Bailey L, et al. ACR appropriateness criteria: palpable breast masses. J Am Coll Radiol. 2017;14:S203-S224.
- Chetlen AL, Kapoor MM, Watts MR. Mastalgia: imaging workup appropriateness. Acad Radiol. 2017;24:345-349.
- Arslan M, Kucukerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
- Fariselli G, Lepera P, Viganotti G, et al. Localized mastalgia as presenting symptom in breast cancer. Eur J Surg Oncol. 1988;14:213-215.
- Leddy R, Irshad A, Zerwas E, et al. Role of breast ultrasound and mammography in evaluating patients presenting with focal breast pain in the absence of a palpable lump. Breast J. 2013;19:582-589.
- Leung JW, Kornguth PJ, Gotway MB. Utility of targeted sonography in the evaluation of focal breast pain. J Ultrasound Med. 2002;21:521-526.
- Goyal A. Breast pain. BMJ Clin Evid. 2011; 2011:0812.
- Kasales CJ, Han B, Smith Jr JS, et al. Nonpuerperal mastitis and subareolar abscess of the breast. AJR Am J Roentgenol. 2014;202:W133-W139.
- Mahoney MC, Ingram AD. Breast emergencies: types, imaging features, and management. AJR Am J Roentgenol. 2014;202:W390-W399.
- Snider HC. Management of mastitis, abscess, and fistula. Surg Clin North Am. 2022;102:1103-1116.
- Oztekin PS, Durhan G, Kosar PN, et al. Imaging findings in patients with granulomatous mastitis. Iran J Radiol. 2016;13:e33900.
- Pluguez-Turull CW, Nanyes JE, Quintero CJ, et al. Idiopathic granulomatous mastitis: manifestations at multimodality imaging and pitfalls. Radiographics. 2018;38:330-356.
- Hovanessian-Larsen LJ, Peyvandi B, Klipfel N, et al. Granulomatous lobular mastitis: imaging, diagnosis, and treatment. AJR Am J Roentgenol. 2009;193:574-581.
- Eberl MM, Phillips RL Jr, Lamberts H, et al. Characterizing breast symptoms in family practice. Ann Fam Med. 2008;6:528-533.
- Malherbe F, Nel D, Molabe H, et al. Palpable breast lumps: an age-based approach to evaluation and diagnosis. S Afr Fam Pract (2022). 2022;64:e1-e5.
- Expert Panel on Breast Imaging; Klein KA, Kocher M, Lourenco AP, et al. American College of Radiology ACR appropriateness criteria: palpable breast masses. Accessed February 15, 2023. https://acsearch.acr.org/docs/69495/Narrative/
- Stachs A, Stubert J, Reimer T, et al. Benign breast disease in women. Dtsch Arztebl Int. 2019;116:565574.
- Hines N, Slanetz PJ, Eisenberg RL. Cystic masses of the breast. AJR Am J Roentgenol. 2010;194:W122133.
- Berg WA. Reducing unnecessary biopsy and follow-up of benign cystic breast lesions. Radiology. 2020;295:52-53.
- Duman L, Gezer NS, Balcı P, et al. Differentiation between phyllodes tumors and fibroadenomas based on mammographic sonographic and MRI features. Breast Care. 2016;11:123-127.
- Lerwill MF, Lee AHS, Tan PH. Fibroepithelial tumours of the breast—a review. Virchows Arch. 2022;480:45-63.
- Vasei N, Shishegar A, Ghalkhani F, et al. Fat necrosis in the breast: a systematic review of clinical. Lipids Health Dis. 2019;18:139.
- Kerridge WD, Kryvenko ON, Thompson A, et al. Fat necrosis of the breast: a pictorial review of the mammographic, ultrasound, CT, and MRI findings with histopathologic correlation. Radiol Res Pract. 2015;2015:613139.
- Taboada JL, Stephens TW, Krishnamurthy S, et al. The many faces of fat necrosis in the breast. AJR Am J Roentgenol. 2009;192:815-825.
- Tan PH, Lai LM, Carrington EV, et al. Fat necrosis of the breast—a review. Breast. 2006;15:313-318.
- Holbrook AI. Breast pain, a common grievance: guidance to radiologists. AJR Am J Roentgenol. 2020;214:259-264.
- Expert Panel on Breast Imaging; Moy L, Heller SL, Bailey L, et al. ACR appropriateness criteria: palpable breast masses. J Am Coll Radiol. 2017;14:S203-S224.
- Chetlen AL, Kapoor MM, Watts MR. Mastalgia: imaging workup appropriateness. Acad Radiol. 2017;24:345-349.
- Arslan M, Kucukerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
- Fariselli G, Lepera P, Viganotti G, et al. Localized mastalgia as presenting symptom in breast cancer. Eur J Surg Oncol. 1988;14:213-215.
- Leddy R, Irshad A, Zerwas E, et al. Role of breast ultrasound and mammography in evaluating patients presenting with focal breast pain in the absence of a palpable lump. Breast J. 2013;19:582-589.
- Leung JW, Kornguth PJ, Gotway MB. Utility of targeted sonography in the evaluation of focal breast pain. J Ultrasound Med. 2002;21:521-526.
- Goyal A. Breast pain. BMJ Clin Evid. 2011; 2011:0812.
- Kasales CJ, Han B, Smith Jr JS, et al. Nonpuerperal mastitis and subareolar abscess of the breast. AJR Am J Roentgenol. 2014;202:W133-W139.
- Mahoney MC, Ingram AD. Breast emergencies: types, imaging features, and management. AJR Am J Roentgenol. 2014;202:W390-W399.
- Snider HC. Management of mastitis, abscess, and fistula. Surg Clin North Am. 2022;102:1103-1116.
- Oztekin PS, Durhan G, Kosar PN, et al. Imaging findings in patients with granulomatous mastitis. Iran J Radiol. 2016;13:e33900.
- Pluguez-Turull CW, Nanyes JE, Quintero CJ, et al. Idiopathic granulomatous mastitis: manifestations at multimodality imaging and pitfalls. Radiographics. 2018;38:330-356.
- Hovanessian-Larsen LJ, Peyvandi B, Klipfel N, et al. Granulomatous lobular mastitis: imaging, diagnosis, and treatment. AJR Am J Roentgenol. 2009;193:574-581.
Breast cancer screening advice ‘dangerous’ for black women
The U.S. Preventive Services Task Force currently recommends that breast cancer screening start at age 50 years, regardless of race or ethnicity.
But
The current “one-size-fits-all” policy to screen the entire female population from a certain age may be “neither fair and equitable nor optimal,” noted the authors, led by Tianhui Chen, PhD, with Zhejiang Cancer Hospital, Hangzhou, China.
The study was published online in JAMA Network Open.
Laurie R. Margolies, MD, chief of breast imaging at the Dubin Breast Center of the Mount Sinai Tisch Cancer Center in New York City, agreed.
Black women get breast cancer at a much younger age, are less likely to be diagnosed with early breast cancer, and are more likely to die of breast cancer, explained Dr. Margolies, who was not involved in the study.
“That’s why the guidelines that say begin at age 50 are flawed and so dangerous,” she said in an interview with this news organization. “This study is really important to highlight that we’re missing an opportunity to detect and treat breast cancer early in the Black population.”
The current study explored the optimal race- and ethnicity-specific ages to initiate breast cancer screening to address racial disparities in breast cancer mortality.
Using a nationwide population-based cross-sectional study design, the team analyzed data on 415,277 women who died of breast cancer in the United States from 2011 to 2020.
The cohort was 75% White, 15% Black, 7% Hispanic, 3% Asian or Pacific Islander, and < 1% Native American or Alaska Native. A total of 115,214 women (28%) died before age 60. The team calculated the 10-year cumulative risk of breast cancer–specific death by age and by race and ethnicity.
For those aged 40-49, breast cancer mortality was highest among Black women (27 deaths per 100,000 person-years), followed by White women (15 deaths per 100,000 person-years) and American Indian/Alaska Native, Hispanic, and Asian/Pacific Islander women (11 deaths per 100,000 person-years).
If breast screening started at age 50 for the entire population, the mean 10-year cumulative risk of dying from breast cancer would be 0.329%. Black women reached this risk threshold level at age 42, whereas non-Hispanic White women reached the threshold at age 51, American Indian/Alaska Native and Hispanic women at age 57, and Asian/Pacific Islander women at age 61.
If screening started at age 45 for all women, the mean 10-year cumulative risk of breast cancer death would be 0.235%. Black women reached this risk threshold level at age 38, non-Hispanic White women at age 46, Hispanic women at age 49, Asian/Pacific Islander women at age 50, and American Indian/Alaska Native women at age 51.
If screening started at age 40 for all women, with a mean 10-year cumulative risk of 0.154%, Black women would reach this risk threshold at age 34, White women at age 41, Hispanic women at age 43, and American Indian/Alaska Native and Asian/Pacific Islander women at age 43.
Dr. Chen and colleagues concluded that failure to consider race and ethnicity in breast cancer screening guidelines “may pose a significant risk for greater harm to a group already at increased risk.
“Changing guidelines based on readily available risk factors, such as race and ethnicity, is possible and may be the first, yet important step toward a personalized and fair screening program,” the team explained.
Dr. Margolies said she believes individualized screening recommendations will likely come, but first, all women should start screening at age 40 instead of age 50.
“Most American women are starting in their 40s, or starting at 40, because we know what the current guidelines are,” she said. “The question that this study doesn’t answer is, is age 40 young enough for the Black population? Maybe it should be 35.”
The study was supported by grants from the National Key Research-Development Program of China and from the Ten-Thousand Talents Plan of Zhejiang Province and by Start-Up Funds for Recruited Talents in Zhejiang Cancer Hospital. Dr. Chen and Dr. Margolies have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The U.S. Preventive Services Task Force currently recommends that breast cancer screening start at age 50 years, regardless of race or ethnicity.
But
The current “one-size-fits-all” policy to screen the entire female population from a certain age may be “neither fair and equitable nor optimal,” noted the authors, led by Tianhui Chen, PhD, with Zhejiang Cancer Hospital, Hangzhou, China.
The study was published online in JAMA Network Open.
Laurie R. Margolies, MD, chief of breast imaging at the Dubin Breast Center of the Mount Sinai Tisch Cancer Center in New York City, agreed.
Black women get breast cancer at a much younger age, are less likely to be diagnosed with early breast cancer, and are more likely to die of breast cancer, explained Dr. Margolies, who was not involved in the study.
“That’s why the guidelines that say begin at age 50 are flawed and so dangerous,” she said in an interview with this news organization. “This study is really important to highlight that we’re missing an opportunity to detect and treat breast cancer early in the Black population.”
The current study explored the optimal race- and ethnicity-specific ages to initiate breast cancer screening to address racial disparities in breast cancer mortality.
Using a nationwide population-based cross-sectional study design, the team analyzed data on 415,277 women who died of breast cancer in the United States from 2011 to 2020.
The cohort was 75% White, 15% Black, 7% Hispanic, 3% Asian or Pacific Islander, and < 1% Native American or Alaska Native. A total of 115,214 women (28%) died before age 60. The team calculated the 10-year cumulative risk of breast cancer–specific death by age and by race and ethnicity.
For those aged 40-49, breast cancer mortality was highest among Black women (27 deaths per 100,000 person-years), followed by White women (15 deaths per 100,000 person-years) and American Indian/Alaska Native, Hispanic, and Asian/Pacific Islander women (11 deaths per 100,000 person-years).
If breast screening started at age 50 for the entire population, the mean 10-year cumulative risk of dying from breast cancer would be 0.329%. Black women reached this risk threshold level at age 42, whereas non-Hispanic White women reached the threshold at age 51, American Indian/Alaska Native and Hispanic women at age 57, and Asian/Pacific Islander women at age 61.
If screening started at age 45 for all women, the mean 10-year cumulative risk of breast cancer death would be 0.235%. Black women reached this risk threshold level at age 38, non-Hispanic White women at age 46, Hispanic women at age 49, Asian/Pacific Islander women at age 50, and American Indian/Alaska Native women at age 51.
If screening started at age 40 for all women, with a mean 10-year cumulative risk of 0.154%, Black women would reach this risk threshold at age 34, White women at age 41, Hispanic women at age 43, and American Indian/Alaska Native and Asian/Pacific Islander women at age 43.
Dr. Chen and colleagues concluded that failure to consider race and ethnicity in breast cancer screening guidelines “may pose a significant risk for greater harm to a group already at increased risk.
“Changing guidelines based on readily available risk factors, such as race and ethnicity, is possible and may be the first, yet important step toward a personalized and fair screening program,” the team explained.
Dr. Margolies said she believes individualized screening recommendations will likely come, but first, all women should start screening at age 40 instead of age 50.
“Most American women are starting in their 40s, or starting at 40, because we know what the current guidelines are,” she said. “The question that this study doesn’t answer is, is age 40 young enough for the Black population? Maybe it should be 35.”
The study was supported by grants from the National Key Research-Development Program of China and from the Ten-Thousand Talents Plan of Zhejiang Province and by Start-Up Funds for Recruited Talents in Zhejiang Cancer Hospital. Dr. Chen and Dr. Margolies have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The U.S. Preventive Services Task Force currently recommends that breast cancer screening start at age 50 years, regardless of race or ethnicity.
But
The current “one-size-fits-all” policy to screen the entire female population from a certain age may be “neither fair and equitable nor optimal,” noted the authors, led by Tianhui Chen, PhD, with Zhejiang Cancer Hospital, Hangzhou, China.
The study was published online in JAMA Network Open.
Laurie R. Margolies, MD, chief of breast imaging at the Dubin Breast Center of the Mount Sinai Tisch Cancer Center in New York City, agreed.
Black women get breast cancer at a much younger age, are less likely to be diagnosed with early breast cancer, and are more likely to die of breast cancer, explained Dr. Margolies, who was not involved in the study.
“That’s why the guidelines that say begin at age 50 are flawed and so dangerous,” she said in an interview with this news organization. “This study is really important to highlight that we’re missing an opportunity to detect and treat breast cancer early in the Black population.”
The current study explored the optimal race- and ethnicity-specific ages to initiate breast cancer screening to address racial disparities in breast cancer mortality.
Using a nationwide population-based cross-sectional study design, the team analyzed data on 415,277 women who died of breast cancer in the United States from 2011 to 2020.
The cohort was 75% White, 15% Black, 7% Hispanic, 3% Asian or Pacific Islander, and < 1% Native American or Alaska Native. A total of 115,214 women (28%) died before age 60. The team calculated the 10-year cumulative risk of breast cancer–specific death by age and by race and ethnicity.
For those aged 40-49, breast cancer mortality was highest among Black women (27 deaths per 100,000 person-years), followed by White women (15 deaths per 100,000 person-years) and American Indian/Alaska Native, Hispanic, and Asian/Pacific Islander women (11 deaths per 100,000 person-years).
If breast screening started at age 50 for the entire population, the mean 10-year cumulative risk of dying from breast cancer would be 0.329%. Black women reached this risk threshold level at age 42, whereas non-Hispanic White women reached the threshold at age 51, American Indian/Alaska Native and Hispanic women at age 57, and Asian/Pacific Islander women at age 61.
If screening started at age 45 for all women, the mean 10-year cumulative risk of breast cancer death would be 0.235%. Black women reached this risk threshold level at age 38, non-Hispanic White women at age 46, Hispanic women at age 49, Asian/Pacific Islander women at age 50, and American Indian/Alaska Native women at age 51.
If screening started at age 40 for all women, with a mean 10-year cumulative risk of 0.154%, Black women would reach this risk threshold at age 34, White women at age 41, Hispanic women at age 43, and American Indian/Alaska Native and Asian/Pacific Islander women at age 43.
Dr. Chen and colleagues concluded that failure to consider race and ethnicity in breast cancer screening guidelines “may pose a significant risk for greater harm to a group already at increased risk.
“Changing guidelines based on readily available risk factors, such as race and ethnicity, is possible and may be the first, yet important step toward a personalized and fair screening program,” the team explained.
Dr. Margolies said she believes individualized screening recommendations will likely come, but first, all women should start screening at age 40 instead of age 50.
“Most American women are starting in their 40s, or starting at 40, because we know what the current guidelines are,” she said. “The question that this study doesn’t answer is, is age 40 young enough for the Black population? Maybe it should be 35.”
The study was supported by grants from the National Key Research-Development Program of China and from the Ten-Thousand Talents Plan of Zhejiang Province and by Start-Up Funds for Recruited Talents in Zhejiang Cancer Hospital. Dr. Chen and Dr. Margolies have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Circulating DNA has promise for cancer detection, but faces challenges
Cancer screening remains challenging. There are screens available for a handful of solid tumors, but uptake is low caused in part by health care access barriers as well as the potential for unnecessary follow-up procedures, according to Phillip Febbo, MD.
These issues could threaten efforts like that of President Joe Biden’s Cancer Moonshot initiative, which aims to reduce cancer mortality by 50%. Advances in circulating tumor (ct) DNA analysis could help address these problems, but a lack of diversity among study participants needs to be addressed to ensure it has broad utility, continued Dr. Febbo, during his presentation at the annual meeting of the American Association for Cancer Research.
The problem is particularly acute among African American, Hispanic, and other underserved populations who often face health care barriers that can exacerbate the issue, said Dr. Febbo, who is chief medical officer for Illumina. The lack of access is compounded by the fact that there are only currently screens for lung, breast, colorectal, cervical, and prostate cancer, leaving a vast unmet need.
“We still do not have screening tests for 70% of the deaths that are due to cancer,” he said.
ctDNA released from dying cancer cells has the potential to reveal a wide range of cancer types and reduce barriers to access, because it is based on a blood test. It can be analyzed for various factors, including mutations, chromosomal rearrangements, methylation patterns, and other characteristics that hint at the presence of cancer. However, it can’t be successful without sufficient inclusion in research studies, Dr. Febbo explained.
“We have to ensure we have the right representation [of] populations when we develop these tests, when we go through the clinical trials, and as we bring these into communities,” he said.
During his presentation, Dr. Febbo shared a slide showing that about 78% of participants in published gene-association studies were White.
ctDNA showed promise in at least on recent study. Dr. Febbo discussed the ECLIPSE trial, which used the Guardant Health SHIELD assay for colorectal cancer (CRC). About 13% of its approximately 20,000 participants were Black or African American, 15% were Hispanic, and 7% were Asian Americans. It also included both urban and rural individuals. In results announced in December 2022, the researchers found a sensitivity of 83%, which was lower than the 92.3% found in standard CRC screening, but above the 74% threshold set by the Food and Drug Administration. The specificity was 90%.
One approach that could dramatically change the landscape of cancer screening is a multicancer early detection (MCED) test, according to Dr. Febbo. The CancerSeek MCED test, developed by Johns Hopkins Kimmel Cancer Center researchers, uses a series of genetic and protein biomarkers to detect all cancers, with the exception of leukemia, skin cancer, and central nervous system tumors. Among 10,006 women aged between 65 and 75 years with no history of cancer, it had a sensitivity of 27.1% and a specificity of 98.9%, with a positive predictive value of 19.4%. The study’s population was 95% non-Hispanic White.
He also discussed the Pathfinder study, sponsored by the Illumina subsidiary Grail, which included 6,662 individuals age 50 and over from seven sites in the United States, and grouped them into normal and increased risk; 92% were non-Hispanic White. It used the Galleri MCED test, which performed with a sensitivity of 29%, specificity of 99.1%, and a positive predictive value of 38.0%. False positives produced to limited burden, with 93% undergoing imaging, 28% nonsurgical invasive procedures, and 2% undergoing fruitless invasive surgical procedures.
Dr. Febbo touted the potential for such tests to greatly reduce cancer mortality, but only if there is adequate uptake of screening procedures, particularly in underserved groups. He put up a slide of a model showing that MCED has the potential to reduce cancer mortality by 20%, but only if the screen is widely accepted among all groups. “I’ve had my team model this. If we accept the current use of screening tests, and we don’t address disparities, and we don’t ensure everybody feels included and participates actively – not only in the research, but also in the testing and adoption, you would cut that potential benefit in half. That would be hundreds of thousands of lives lost because we didn’t address disparities.”
Successful recruiting of African Americans for research
Following Dr. Febbo’s talk, Karriem Watson, MS, spoke about some potential solutions to the issue, including his own experiences and success stories in recruiting African Americans to play active roles in research. He is chief engagement officer for the National Institute of Health’s All of Us Research Program, which aims to gather health data on at least 1 million residents of the United States. Mr. Watson has spent time reaching out to people living in communities in the Chicago area to encourage participation in breast cancer screening. An event at his church inspired his own sister to get a mammogram, and she was diagnosed with early-stage breast cancer.
“I’m a living witness that early engagement can lead to early detection,” said Mr. Watson during his talk.
He reported that the All of Us research program has succeeded in creating diversity within its data collection, as 46.7% of participants identify as racial and ethnic minorities.
Mr. Watson took issue with the common perception that underrepresented communities may be hard to reach.
“I want to challenge us to think outside the box and really ask ourselves: Are populations hard to reach, or are there opportunities for us to do better and more intentional engagement?” He went on to describe a program to recruit African American men as citizen scientists. He and his colleagues developed a network that included barbers, faith leaders, and fraternity and civic organization members to help recruit participants for a prostate cancer screening project. They exceeded their initial recruitment goal.
They went on to develop a network of barbers in the south and west sides of Chicago to recruit individuals to participate in studies of protein methylation and lung cancer screening, as well as a project that investigated associations between neighborhood of residence and lung cancer. The results of those efforts have also informed other projects, including a smoking cessation study. “We’ve not only included African American men in our research, but we’ve included them as part of our research team,” said Mr. Watson.
Dr. Febbo is also a stockholder of Illumina. Mr. Watson has no relevant financial disclosures.
From American Association for Cancer Research (AACR) Annual Meeting 2023: Improving cancer outcomes through equitable access to cfDNA tests. Presented Monday, April 17, 2023.
Cancer screening remains challenging. There are screens available for a handful of solid tumors, but uptake is low caused in part by health care access barriers as well as the potential for unnecessary follow-up procedures, according to Phillip Febbo, MD.
These issues could threaten efforts like that of President Joe Biden’s Cancer Moonshot initiative, which aims to reduce cancer mortality by 50%. Advances in circulating tumor (ct) DNA analysis could help address these problems, but a lack of diversity among study participants needs to be addressed to ensure it has broad utility, continued Dr. Febbo, during his presentation at the annual meeting of the American Association for Cancer Research.
The problem is particularly acute among African American, Hispanic, and other underserved populations who often face health care barriers that can exacerbate the issue, said Dr. Febbo, who is chief medical officer for Illumina. The lack of access is compounded by the fact that there are only currently screens for lung, breast, colorectal, cervical, and prostate cancer, leaving a vast unmet need.
“We still do not have screening tests for 70% of the deaths that are due to cancer,” he said.
ctDNA released from dying cancer cells has the potential to reveal a wide range of cancer types and reduce barriers to access, because it is based on a blood test. It can be analyzed for various factors, including mutations, chromosomal rearrangements, methylation patterns, and other characteristics that hint at the presence of cancer. However, it can’t be successful without sufficient inclusion in research studies, Dr. Febbo explained.
“We have to ensure we have the right representation [of] populations when we develop these tests, when we go through the clinical trials, and as we bring these into communities,” he said.
During his presentation, Dr. Febbo shared a slide showing that about 78% of participants in published gene-association studies were White.
ctDNA showed promise in at least on recent study. Dr. Febbo discussed the ECLIPSE trial, which used the Guardant Health SHIELD assay for colorectal cancer (CRC). About 13% of its approximately 20,000 participants were Black or African American, 15% were Hispanic, and 7% were Asian Americans. It also included both urban and rural individuals. In results announced in December 2022, the researchers found a sensitivity of 83%, which was lower than the 92.3% found in standard CRC screening, but above the 74% threshold set by the Food and Drug Administration. The specificity was 90%.
One approach that could dramatically change the landscape of cancer screening is a multicancer early detection (MCED) test, according to Dr. Febbo. The CancerSeek MCED test, developed by Johns Hopkins Kimmel Cancer Center researchers, uses a series of genetic and protein biomarkers to detect all cancers, with the exception of leukemia, skin cancer, and central nervous system tumors. Among 10,006 women aged between 65 and 75 years with no history of cancer, it had a sensitivity of 27.1% and a specificity of 98.9%, with a positive predictive value of 19.4%. The study’s population was 95% non-Hispanic White.
He also discussed the Pathfinder study, sponsored by the Illumina subsidiary Grail, which included 6,662 individuals age 50 and over from seven sites in the United States, and grouped them into normal and increased risk; 92% were non-Hispanic White. It used the Galleri MCED test, which performed with a sensitivity of 29%, specificity of 99.1%, and a positive predictive value of 38.0%. False positives produced to limited burden, with 93% undergoing imaging, 28% nonsurgical invasive procedures, and 2% undergoing fruitless invasive surgical procedures.
Dr. Febbo touted the potential for such tests to greatly reduce cancer mortality, but only if there is adequate uptake of screening procedures, particularly in underserved groups. He put up a slide of a model showing that MCED has the potential to reduce cancer mortality by 20%, but only if the screen is widely accepted among all groups. “I’ve had my team model this. If we accept the current use of screening tests, and we don’t address disparities, and we don’t ensure everybody feels included and participates actively – not only in the research, but also in the testing and adoption, you would cut that potential benefit in half. That would be hundreds of thousands of lives lost because we didn’t address disparities.”
Successful recruiting of African Americans for research
Following Dr. Febbo’s talk, Karriem Watson, MS, spoke about some potential solutions to the issue, including his own experiences and success stories in recruiting African Americans to play active roles in research. He is chief engagement officer for the National Institute of Health’s All of Us Research Program, which aims to gather health data on at least 1 million residents of the United States. Mr. Watson has spent time reaching out to people living in communities in the Chicago area to encourage participation in breast cancer screening. An event at his church inspired his own sister to get a mammogram, and she was diagnosed with early-stage breast cancer.
“I’m a living witness that early engagement can lead to early detection,” said Mr. Watson during his talk.
He reported that the All of Us research program has succeeded in creating diversity within its data collection, as 46.7% of participants identify as racial and ethnic minorities.
Mr. Watson took issue with the common perception that underrepresented communities may be hard to reach.
“I want to challenge us to think outside the box and really ask ourselves: Are populations hard to reach, or are there opportunities for us to do better and more intentional engagement?” He went on to describe a program to recruit African American men as citizen scientists. He and his colleagues developed a network that included barbers, faith leaders, and fraternity and civic organization members to help recruit participants for a prostate cancer screening project. They exceeded their initial recruitment goal.
They went on to develop a network of barbers in the south and west sides of Chicago to recruit individuals to participate in studies of protein methylation and lung cancer screening, as well as a project that investigated associations between neighborhood of residence and lung cancer. The results of those efforts have also informed other projects, including a smoking cessation study. “We’ve not only included African American men in our research, but we’ve included them as part of our research team,” said Mr. Watson.
Dr. Febbo is also a stockholder of Illumina. Mr. Watson has no relevant financial disclosures.
From American Association for Cancer Research (AACR) Annual Meeting 2023: Improving cancer outcomes through equitable access to cfDNA tests. Presented Monday, April 17, 2023.
Cancer screening remains challenging. There are screens available for a handful of solid tumors, but uptake is low caused in part by health care access barriers as well as the potential for unnecessary follow-up procedures, according to Phillip Febbo, MD.
These issues could threaten efforts like that of President Joe Biden’s Cancer Moonshot initiative, which aims to reduce cancer mortality by 50%. Advances in circulating tumor (ct) DNA analysis could help address these problems, but a lack of diversity among study participants needs to be addressed to ensure it has broad utility, continued Dr. Febbo, during his presentation at the annual meeting of the American Association for Cancer Research.
The problem is particularly acute among African American, Hispanic, and other underserved populations who often face health care barriers that can exacerbate the issue, said Dr. Febbo, who is chief medical officer for Illumina. The lack of access is compounded by the fact that there are only currently screens for lung, breast, colorectal, cervical, and prostate cancer, leaving a vast unmet need.
“We still do not have screening tests for 70% of the deaths that are due to cancer,” he said.
ctDNA released from dying cancer cells has the potential to reveal a wide range of cancer types and reduce barriers to access, because it is based on a blood test. It can be analyzed for various factors, including mutations, chromosomal rearrangements, methylation patterns, and other characteristics that hint at the presence of cancer. However, it can’t be successful without sufficient inclusion in research studies, Dr. Febbo explained.
“We have to ensure we have the right representation [of] populations when we develop these tests, when we go through the clinical trials, and as we bring these into communities,” he said.
During his presentation, Dr. Febbo shared a slide showing that about 78% of participants in published gene-association studies were White.
ctDNA showed promise in at least on recent study. Dr. Febbo discussed the ECLIPSE trial, which used the Guardant Health SHIELD assay for colorectal cancer (CRC). About 13% of its approximately 20,000 participants were Black or African American, 15% were Hispanic, and 7% were Asian Americans. It also included both urban and rural individuals. In results announced in December 2022, the researchers found a sensitivity of 83%, which was lower than the 92.3% found in standard CRC screening, but above the 74% threshold set by the Food and Drug Administration. The specificity was 90%.
One approach that could dramatically change the landscape of cancer screening is a multicancer early detection (MCED) test, according to Dr. Febbo. The CancerSeek MCED test, developed by Johns Hopkins Kimmel Cancer Center researchers, uses a series of genetic and protein biomarkers to detect all cancers, with the exception of leukemia, skin cancer, and central nervous system tumors. Among 10,006 women aged between 65 and 75 years with no history of cancer, it had a sensitivity of 27.1% and a specificity of 98.9%, with a positive predictive value of 19.4%. The study’s population was 95% non-Hispanic White.
He also discussed the Pathfinder study, sponsored by the Illumina subsidiary Grail, which included 6,662 individuals age 50 and over from seven sites in the United States, and grouped them into normal and increased risk; 92% were non-Hispanic White. It used the Galleri MCED test, which performed with a sensitivity of 29%, specificity of 99.1%, and a positive predictive value of 38.0%. False positives produced to limited burden, with 93% undergoing imaging, 28% nonsurgical invasive procedures, and 2% undergoing fruitless invasive surgical procedures.
Dr. Febbo touted the potential for such tests to greatly reduce cancer mortality, but only if there is adequate uptake of screening procedures, particularly in underserved groups. He put up a slide of a model showing that MCED has the potential to reduce cancer mortality by 20%, but only if the screen is widely accepted among all groups. “I’ve had my team model this. If we accept the current use of screening tests, and we don’t address disparities, and we don’t ensure everybody feels included and participates actively – not only in the research, but also in the testing and adoption, you would cut that potential benefit in half. That would be hundreds of thousands of lives lost because we didn’t address disparities.”
Successful recruiting of African Americans for research
Following Dr. Febbo’s talk, Karriem Watson, MS, spoke about some potential solutions to the issue, including his own experiences and success stories in recruiting African Americans to play active roles in research. He is chief engagement officer for the National Institute of Health’s All of Us Research Program, which aims to gather health data on at least 1 million residents of the United States. Mr. Watson has spent time reaching out to people living in communities in the Chicago area to encourage participation in breast cancer screening. An event at his church inspired his own sister to get a mammogram, and she was diagnosed with early-stage breast cancer.
“I’m a living witness that early engagement can lead to early detection,” said Mr. Watson during his talk.
He reported that the All of Us research program has succeeded in creating diversity within its data collection, as 46.7% of participants identify as racial and ethnic minorities.
Mr. Watson took issue with the common perception that underrepresented communities may be hard to reach.
“I want to challenge us to think outside the box and really ask ourselves: Are populations hard to reach, or are there opportunities for us to do better and more intentional engagement?” He went on to describe a program to recruit African American men as citizen scientists. He and his colleagues developed a network that included barbers, faith leaders, and fraternity and civic organization members to help recruit participants for a prostate cancer screening project. They exceeded their initial recruitment goal.
They went on to develop a network of barbers in the south and west sides of Chicago to recruit individuals to participate in studies of protein methylation and lung cancer screening, as well as a project that investigated associations between neighborhood of residence and lung cancer. The results of those efforts have also informed other projects, including a smoking cessation study. “We’ve not only included African American men in our research, but we’ve included them as part of our research team,” said Mr. Watson.
Dr. Febbo is also a stockholder of Illumina. Mr. Watson has no relevant financial disclosures.
From American Association for Cancer Research (AACR) Annual Meeting 2023: Improving cancer outcomes through equitable access to cfDNA tests. Presented Monday, April 17, 2023.
FROM AACR 2023
pCR and low residual tumor cellularity associated with better prognosis in BC
Key clinical point: Achievement of pathologic complete response (pCR) or residual tumor cellularity (RTC) <40% is associated with improved survival outcomes in patients with breast cancer (BC) who have received neoadjuvant chemotherapy (NAC).
Major finding: Early clinical stage (cT1-2), human epidermal growth factor receptor 2-positive BC subtype, and absence of vascular invasion predicted the achievement of pCR after NAC (all P = .001). Patients who achieved pCR vs pathologic partial response (pPR) had significantly longer overall survival (OS), disease-free survival (DFS), and distant disease-free survival (DDFS; all P < .001). However, among patients with pPR, RTC <40% vs ≥40% was associated with significantly longer DFS (P = .033) and DDFS (P = .015).
Study details: Findings are from a retrospective analysis including 495 patients with BC who underwent NAC, of which 29.9% of patients achieved pCR.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Gentile D et al. Pathologic response and residual tumor cellularity after neo-adjuvant chemotherapy predict prognosis in breast cancer patients. Breast. 2023;69:323-329 (Mar 27). Doi: 10.1016/j.breast.2023.03.016
Key clinical point: Achievement of pathologic complete response (pCR) or residual tumor cellularity (RTC) <40% is associated with improved survival outcomes in patients with breast cancer (BC) who have received neoadjuvant chemotherapy (NAC).
Major finding: Early clinical stage (cT1-2), human epidermal growth factor receptor 2-positive BC subtype, and absence of vascular invasion predicted the achievement of pCR after NAC (all P = .001). Patients who achieved pCR vs pathologic partial response (pPR) had significantly longer overall survival (OS), disease-free survival (DFS), and distant disease-free survival (DDFS; all P < .001). However, among patients with pPR, RTC <40% vs ≥40% was associated with significantly longer DFS (P = .033) and DDFS (P = .015).
Study details: Findings are from a retrospective analysis including 495 patients with BC who underwent NAC, of which 29.9% of patients achieved pCR.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Gentile D et al. Pathologic response and residual tumor cellularity after neo-adjuvant chemotherapy predict prognosis in breast cancer patients. Breast. 2023;69:323-329 (Mar 27). Doi: 10.1016/j.breast.2023.03.016
Key clinical point: Achievement of pathologic complete response (pCR) or residual tumor cellularity (RTC) <40% is associated with improved survival outcomes in patients with breast cancer (BC) who have received neoadjuvant chemotherapy (NAC).
Major finding: Early clinical stage (cT1-2), human epidermal growth factor receptor 2-positive BC subtype, and absence of vascular invasion predicted the achievement of pCR after NAC (all P = .001). Patients who achieved pCR vs pathologic partial response (pPR) had significantly longer overall survival (OS), disease-free survival (DFS), and distant disease-free survival (DDFS; all P < .001). However, among patients with pPR, RTC <40% vs ≥40% was associated with significantly longer DFS (P = .033) and DDFS (P = .015).
Study details: Findings are from a retrospective analysis including 495 patients with BC who underwent NAC, of which 29.9% of patients achieved pCR.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Gentile D et al. Pathologic response and residual tumor cellularity after neo-adjuvant chemotherapy predict prognosis in breast cancer patients. Breast. 2023;69:323-329 (Mar 27). Doi: 10.1016/j.breast.2023.03.016
Preterm birth, preeclampsia history, and premenopausal BC risk: Is there a link?
Key clinical point: Preterm birth was positively associated with the risk for incident premenopausal breast cancer (BC) in women aged <55 years who experienced preeclampsia or gestational hypertension.
Major finding: Overall, a history of preterm birth was not associated with the risk for premenopausal BC (hazard ratio [HR] 1.02; 95% CI 0.92-1.14). However, it was positively associated with the risk for premenopausal BC in women who had preeclampsia or gestational hypertension (HR 1.52; 95% CI 1.06-2.18). Preeclampsia on the other hand was inversely associated with premenopausal BC risk (HR 0.86; 95% CI 0.75-0.99).
Study details: Findings are from an analysis of six cohort studies including 184,866 premenopausal women aged <55 years, of which 3096 women were diagnosed with premenopausal BC.
Disclosures: This study was supported by the US National Institutes of Health and other sources. The authors declared no conflicts of interest.
Source: Nichols HB et al. Hypertensive conditions of pregnancy, preterm birth, and premenopausal breast cancer risk: A premenopausal breast cancer collaborative group analysis. Breast Cancer Res Treat. 2023 (Apr 5). Doi: 10.1007/s10549-023-06903-5
Key clinical point: Preterm birth was positively associated with the risk for incident premenopausal breast cancer (BC) in women aged <55 years who experienced preeclampsia or gestational hypertension.
Major finding: Overall, a history of preterm birth was not associated with the risk for premenopausal BC (hazard ratio [HR] 1.02; 95% CI 0.92-1.14). However, it was positively associated with the risk for premenopausal BC in women who had preeclampsia or gestational hypertension (HR 1.52; 95% CI 1.06-2.18). Preeclampsia on the other hand was inversely associated with premenopausal BC risk (HR 0.86; 95% CI 0.75-0.99).
Study details: Findings are from an analysis of six cohort studies including 184,866 premenopausal women aged <55 years, of which 3096 women were diagnosed with premenopausal BC.
Disclosures: This study was supported by the US National Institutes of Health and other sources. The authors declared no conflicts of interest.
Source: Nichols HB et al. Hypertensive conditions of pregnancy, preterm birth, and premenopausal breast cancer risk: A premenopausal breast cancer collaborative group analysis. Breast Cancer Res Treat. 2023 (Apr 5). Doi: 10.1007/s10549-023-06903-5
Key clinical point: Preterm birth was positively associated with the risk for incident premenopausal breast cancer (BC) in women aged <55 years who experienced preeclampsia or gestational hypertension.
Major finding: Overall, a history of preterm birth was not associated with the risk for premenopausal BC (hazard ratio [HR] 1.02; 95% CI 0.92-1.14). However, it was positively associated with the risk for premenopausal BC in women who had preeclampsia or gestational hypertension (HR 1.52; 95% CI 1.06-2.18). Preeclampsia on the other hand was inversely associated with premenopausal BC risk (HR 0.86; 95% CI 0.75-0.99).
Study details: Findings are from an analysis of six cohort studies including 184,866 premenopausal women aged <55 years, of which 3096 women were diagnosed with premenopausal BC.
Disclosures: This study was supported by the US National Institutes of Health and other sources. The authors declared no conflicts of interest.
Source: Nichols HB et al. Hypertensive conditions of pregnancy, preterm birth, and premenopausal breast cancer risk: A premenopausal breast cancer collaborative group analysis. Breast Cancer Res Treat. 2023 (Apr 5). Doi: 10.1007/s10549-023-06903-5
Spiculated morphology and antiparallel orientation on ultrasound may predict poor prognosis in breast cancer
Key clinical point: Sonography features, such as spiculated morphology and antiparallel orientation, were significantly associated with worsened survival outcomes in patients with primary breast cancer (BC; tumor size <20 mm).
Major finding: The presence of spiculated morphology and antiparallel orientation on ultrasound was a significant risk factor for poor breast cancer-specific survival (hazard ratio [HR] 7.45; P < .001) and disease-free survival (HR 6.42; P < .001), with age ≥55 years (P < .05) and positive lymph node metastases (P < .001) also being associated with worse prognosis.
Study details: Findings are from a retrospective study including 790 women with small primary BC (tumor size <20 mm).
Disclosures: This study was supported by the National Natural Science Foundation of China and other sources. The authors declared no conflicts of interest.
Source: Shao S et al. Ultrasound features for prediction of long-term outcomes of women with primary breast cancer <20 mm. Front Oncol. 2023;13:1103397 (Mar 16). Doi: 10.3389/fonc.2023.1103397
Key clinical point: Sonography features, such as spiculated morphology and antiparallel orientation, were significantly associated with worsened survival outcomes in patients with primary breast cancer (BC; tumor size <20 mm).
Major finding: The presence of spiculated morphology and antiparallel orientation on ultrasound was a significant risk factor for poor breast cancer-specific survival (hazard ratio [HR] 7.45; P < .001) and disease-free survival (HR 6.42; P < .001), with age ≥55 years (P < .05) and positive lymph node metastases (P < .001) also being associated with worse prognosis.
Study details: Findings are from a retrospective study including 790 women with small primary BC (tumor size <20 mm).
Disclosures: This study was supported by the National Natural Science Foundation of China and other sources. The authors declared no conflicts of interest.
Source: Shao S et al. Ultrasound features for prediction of long-term outcomes of women with primary breast cancer <20 mm. Front Oncol. 2023;13:1103397 (Mar 16). Doi: 10.3389/fonc.2023.1103397
Key clinical point: Sonography features, such as spiculated morphology and antiparallel orientation, were significantly associated with worsened survival outcomes in patients with primary breast cancer (BC; tumor size <20 mm).
Major finding: The presence of spiculated morphology and antiparallel orientation on ultrasound was a significant risk factor for poor breast cancer-specific survival (hazard ratio [HR] 7.45; P < .001) and disease-free survival (HR 6.42; P < .001), with age ≥55 years (P < .05) and positive lymph node metastases (P < .001) also being associated with worse prognosis.
Study details: Findings are from a retrospective study including 790 women with small primary BC (tumor size <20 mm).
Disclosures: This study was supported by the National Natural Science Foundation of China and other sources. The authors declared no conflicts of interest.
Source: Shao S et al. Ultrasound features for prediction of long-term outcomes of women with primary breast cancer <20 mm. Front Oncol. 2023;13:1103397 (Mar 16). Doi: 10.3389/fonc.2023.1103397
Breast-conserving surgery without axillary surgery and radiotherapy safe in elderly BC patients
Key clinical point: Breast-conserving surgery without axillary lymph node dissection as well as breast and axillary radiotherapy (BCSNR) was safe and resulted in survival rates comparable to mastectomy plus axillary lymph node dissection (MALND) in elderly patients with axillary lymph node-negative breast cancer (BC).
Major finding: At a median follow-up of 5 years, BCSNR vs MALND was not associated with significantly worsened distant recurrence-free survival (98.1% vs 93.2%; P = .990) and BC-specific survival (96.3% vs 99.3%; P = .076) rates but was associated with a significantly higher local recurrence rate (10.3% vs 2.2%; P = .001).
Study details: Findings are from a retrospective study including 541 patients aged ≥70 years with axillary lymph node-negative BC, of which 181 and 360 patients underwent MALND with negative axillary cleaning and BCSNR, respectively.
Disclosures: This study was supported by the CAMS Innovation Fund for Medical Sciences, China, and other sources. The authors declared no conflicts of interest.
Source: Zhong Y et al. Breast-conserving surgery without axillary surgery and radiation versus mastectomy plus axillary dissection in elderly breast cancer patients: A retrospective study. Front Oncol. 2023;13:1126104 (Mar 20). Doi: 10.3389/fonc.2023.1126104
Key clinical point: Breast-conserving surgery without axillary lymph node dissection as well as breast and axillary radiotherapy (BCSNR) was safe and resulted in survival rates comparable to mastectomy plus axillary lymph node dissection (MALND) in elderly patients with axillary lymph node-negative breast cancer (BC).
Major finding: At a median follow-up of 5 years, BCSNR vs MALND was not associated with significantly worsened distant recurrence-free survival (98.1% vs 93.2%; P = .990) and BC-specific survival (96.3% vs 99.3%; P = .076) rates but was associated with a significantly higher local recurrence rate (10.3% vs 2.2%; P = .001).
Study details: Findings are from a retrospective study including 541 patients aged ≥70 years with axillary lymph node-negative BC, of which 181 and 360 patients underwent MALND with negative axillary cleaning and BCSNR, respectively.
Disclosures: This study was supported by the CAMS Innovation Fund for Medical Sciences, China, and other sources. The authors declared no conflicts of interest.
Source: Zhong Y et al. Breast-conserving surgery without axillary surgery and radiation versus mastectomy plus axillary dissection in elderly breast cancer patients: A retrospective study. Front Oncol. 2023;13:1126104 (Mar 20). Doi: 10.3389/fonc.2023.1126104
Key clinical point: Breast-conserving surgery without axillary lymph node dissection as well as breast and axillary radiotherapy (BCSNR) was safe and resulted in survival rates comparable to mastectomy plus axillary lymph node dissection (MALND) in elderly patients with axillary lymph node-negative breast cancer (BC).
Major finding: At a median follow-up of 5 years, BCSNR vs MALND was not associated with significantly worsened distant recurrence-free survival (98.1% vs 93.2%; P = .990) and BC-specific survival (96.3% vs 99.3%; P = .076) rates but was associated with a significantly higher local recurrence rate (10.3% vs 2.2%; P = .001).
Study details: Findings are from a retrospective study including 541 patients aged ≥70 years with axillary lymph node-negative BC, of which 181 and 360 patients underwent MALND with negative axillary cleaning and BCSNR, respectively.
Disclosures: This study was supported by the CAMS Innovation Fund for Medical Sciences, China, and other sources. The authors declared no conflicts of interest.
Source: Zhong Y et al. Breast-conserving surgery without axillary surgery and radiation versus mastectomy plus axillary dissection in elderly breast cancer patients: A retrospective study. Front Oncol. 2023;13:1126104 (Mar 20). Doi: 10.3389/fonc.2023.1126104
Serum thymidine kinase 1 activity: A promising prognostic biomarker in advanced breast cancer
Key clinical point: Serum thymidine kinase 1 activity (sTKa) proved to be an excellent biomarker of progression risk in patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2–) advanced breast cancer (BC) receiving first-line ribociclib+letrozole.
Major finding: Disease progression risk was significantly higher in patients with high vs low sTKa levels at baseline (hazard ratio 2.21; P = .0002). Patients with high sTKa levels on day 1 of cycle 2 after initial decrease on day 15 of cycle 1 (C1D15; hazard ratio 2.89; P = .0006) or on C1D15 (hazard ratio 5.65; P < .0001) had worse prognosis than those with low sTKa levels at all time points.
Study details: This phase 3 BioItaLEE study included 287 postmenopausal women with HR+/HER2– advanced BC who received ribociclib+letrozole as first-line therapy.
Disclosures: This study was supported by Novartis Farma SpA, Italy. Some authors declared participating on advisory boards and receiving grants, fees, honoraria, or travel support from several sources, including Novartis.
Source: Malorni L et al. Serum thymidine kinase activity in patients with HR-positive/HER2-negative advanced breast cancer treated with ribociclib plus letrozole: Results from the prospective BioItaLEE trial. Eur J Cancer. 2023;186:1-11 (Mar 7). Doi: 10.1016/j.ejca.2023.03.001
Key clinical point: Serum thymidine kinase 1 activity (sTKa) proved to be an excellent biomarker of progression risk in patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2–) advanced breast cancer (BC) receiving first-line ribociclib+letrozole.
Major finding: Disease progression risk was significantly higher in patients with high vs low sTKa levels at baseline (hazard ratio 2.21; P = .0002). Patients with high sTKa levels on day 1 of cycle 2 after initial decrease on day 15 of cycle 1 (C1D15; hazard ratio 2.89; P = .0006) or on C1D15 (hazard ratio 5.65; P < .0001) had worse prognosis than those with low sTKa levels at all time points.
Study details: This phase 3 BioItaLEE study included 287 postmenopausal women with HR+/HER2– advanced BC who received ribociclib+letrozole as first-line therapy.
Disclosures: This study was supported by Novartis Farma SpA, Italy. Some authors declared participating on advisory boards and receiving grants, fees, honoraria, or travel support from several sources, including Novartis.
Source: Malorni L et al. Serum thymidine kinase activity in patients with HR-positive/HER2-negative advanced breast cancer treated with ribociclib plus letrozole: Results from the prospective BioItaLEE trial. Eur J Cancer. 2023;186:1-11 (Mar 7). Doi: 10.1016/j.ejca.2023.03.001
Key clinical point: Serum thymidine kinase 1 activity (sTKa) proved to be an excellent biomarker of progression risk in patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2–) advanced breast cancer (BC) receiving first-line ribociclib+letrozole.
Major finding: Disease progression risk was significantly higher in patients with high vs low sTKa levels at baseline (hazard ratio 2.21; P = .0002). Patients with high sTKa levels on day 1 of cycle 2 after initial decrease on day 15 of cycle 1 (C1D15; hazard ratio 2.89; P = .0006) or on C1D15 (hazard ratio 5.65; P < .0001) had worse prognosis than those with low sTKa levels at all time points.
Study details: This phase 3 BioItaLEE study included 287 postmenopausal women with HR+/HER2– advanced BC who received ribociclib+letrozole as first-line therapy.
Disclosures: This study was supported by Novartis Farma SpA, Italy. Some authors declared participating on advisory boards and receiving grants, fees, honoraria, or travel support from several sources, including Novartis.
Source: Malorni L et al. Serum thymidine kinase activity in patients with HR-positive/HER2-negative advanced breast cancer treated with ribociclib plus letrozole: Results from the prospective BioItaLEE trial. Eur J Cancer. 2023;186:1-11 (Mar 7). Doi: 10.1016/j.ejca.2023.03.001
Worse survival in BRCA1/2 germline mutation carriers receiving ET for HR+/HER2− BC
Key clinical point: In a pre-cyclin-dependent kinase 4 and 6 inhibitors era, patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) breast cancer (BC) who had BRCA1/2 germline mutation (gBRCAm) vs wild type (gBRCAwt) had worse survival outcomes, especially those who received first-line endocrine therapy (ET).
Major finding: Compared with gBRCAwt carriers, gBRCAm carriers had shorter overall survival (OS; adjusted hazard ratio [aHR] 1.26; P = .024) and progression-free survival (PFS; aHR 1.21; P = .017), with both OS (aHR 1.54; P = .037) and PFS (aHR 1.58; P = .003) being further attenuated in patients receiving first-line ET.
Study details: Findings are from a study including 13,776 patients with metastatic BC from the ESME (Épidémio-Stratégie Médico-Economique) metastatic BC database, of which 676 and 170 patients were gBRCAwt and gBRCAm carriers, respectively.
Disclosures: The ESME metastatic BC database received financial support from various sources. Some authors declared receiving grants, personal fees, or non-financial support, or having other ties with several sources.
Source: Frenel JS et al. Efficacy of front-line treatment for hormone receptor-positive HER2-negative metastatic breast cancer with germline BRCA1/2 mutation. Br J Cancer. 2023 (Apr 3). Doi: 10.1038/s41416-023-02248-4
Key clinical point: In a pre-cyclin-dependent kinase 4 and 6 inhibitors era, patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) breast cancer (BC) who had BRCA1/2 germline mutation (gBRCAm) vs wild type (gBRCAwt) had worse survival outcomes, especially those who received first-line endocrine therapy (ET).
Major finding: Compared with gBRCAwt carriers, gBRCAm carriers had shorter overall survival (OS; adjusted hazard ratio [aHR] 1.26; P = .024) and progression-free survival (PFS; aHR 1.21; P = .017), with both OS (aHR 1.54; P = .037) and PFS (aHR 1.58; P = .003) being further attenuated in patients receiving first-line ET.
Study details: Findings are from a study including 13,776 patients with metastatic BC from the ESME (Épidémio-Stratégie Médico-Economique) metastatic BC database, of which 676 and 170 patients were gBRCAwt and gBRCAm carriers, respectively.
Disclosures: The ESME metastatic BC database received financial support from various sources. Some authors declared receiving grants, personal fees, or non-financial support, or having other ties with several sources.
Source: Frenel JS et al. Efficacy of front-line treatment for hormone receptor-positive HER2-negative metastatic breast cancer with germline BRCA1/2 mutation. Br J Cancer. 2023 (Apr 3). Doi: 10.1038/s41416-023-02248-4
Key clinical point: In a pre-cyclin-dependent kinase 4 and 6 inhibitors era, patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) breast cancer (BC) who had BRCA1/2 germline mutation (gBRCAm) vs wild type (gBRCAwt) had worse survival outcomes, especially those who received first-line endocrine therapy (ET).
Major finding: Compared with gBRCAwt carriers, gBRCAm carriers had shorter overall survival (OS; adjusted hazard ratio [aHR] 1.26; P = .024) and progression-free survival (PFS; aHR 1.21; P = .017), with both OS (aHR 1.54; P = .037) and PFS (aHR 1.58; P = .003) being further attenuated in patients receiving first-line ET.
Study details: Findings are from a study including 13,776 patients with metastatic BC from the ESME (Épidémio-Stratégie Médico-Economique) metastatic BC database, of which 676 and 170 patients were gBRCAwt and gBRCAm carriers, respectively.
Disclosures: The ESME metastatic BC database received financial support from various sources. Some authors declared receiving grants, personal fees, or non-financial support, or having other ties with several sources.
Source: Frenel JS et al. Efficacy of front-line treatment for hormone receptor-positive HER2-negative metastatic breast cancer with germline BRCA1/2 mutation. Br J Cancer. 2023 (Apr 3). Doi: 10.1038/s41416-023-02248-4
Low-dose tamoxifen continues to prevent BC recurrence in breast noninvasive neoplasia
Key clinical point: In women with noninvasive neoplasia of the breast, treatment with low-dose tamoxifen for 3 years continued to prevent breast cancer (BC) recurrence, with an optimal safety profile, for at least 7 years after treatment cessation.
Major finding: After a median follow-up of 9.7 years, fewer cases of both invasive and in-situ BC (hazard ratio [HR] 0.58; log-rank P = .03) and contralateral BC (HR 0.36; P = .025) were reported in the tamoxifen vs placebo group. There was no increase in serious adverse events during tamoxifen therapy.
Study details: Findings are from a 10-year follow-up analysis of the phase 3 TAM-01 trial including 500 women with intraepithelial neoplasia of the breast who were randomly assigned to receive low-dose tamoxifen or placebo.
Disclosures: This study was supported by the Italian Ministry of Health and other sources. The authors declared serving as employees, consultants, or advisors, or receiving honoraria and travel and accommodation expenses from several sources.
Source: Lazzeroni M et al. Randomized placebo controlled trial of low-dose tamoxifen to prevent recurrence in breast noninvasive neoplasia: A 10-year follow-up of TAM-01 study. J Clin Oncol. 2023 (Mar 14). Doi: 10.1200/JCO.22.02900
Key clinical point: In women with noninvasive neoplasia of the breast, treatment with low-dose tamoxifen for 3 years continued to prevent breast cancer (BC) recurrence, with an optimal safety profile, for at least 7 years after treatment cessation.
Major finding: After a median follow-up of 9.7 years, fewer cases of both invasive and in-situ BC (hazard ratio [HR] 0.58; log-rank P = .03) and contralateral BC (HR 0.36; P = .025) were reported in the tamoxifen vs placebo group. There was no increase in serious adverse events during tamoxifen therapy.
Study details: Findings are from a 10-year follow-up analysis of the phase 3 TAM-01 trial including 500 women with intraepithelial neoplasia of the breast who were randomly assigned to receive low-dose tamoxifen or placebo.
Disclosures: This study was supported by the Italian Ministry of Health and other sources. The authors declared serving as employees, consultants, or advisors, or receiving honoraria and travel and accommodation expenses from several sources.
Source: Lazzeroni M et al. Randomized placebo controlled trial of low-dose tamoxifen to prevent recurrence in breast noninvasive neoplasia: A 10-year follow-up of TAM-01 study. J Clin Oncol. 2023 (Mar 14). Doi: 10.1200/JCO.22.02900
Key clinical point: In women with noninvasive neoplasia of the breast, treatment with low-dose tamoxifen for 3 years continued to prevent breast cancer (BC) recurrence, with an optimal safety profile, for at least 7 years after treatment cessation.
Major finding: After a median follow-up of 9.7 years, fewer cases of both invasive and in-situ BC (hazard ratio [HR] 0.58; log-rank P = .03) and contralateral BC (HR 0.36; P = .025) were reported in the tamoxifen vs placebo group. There was no increase in serious adverse events during tamoxifen therapy.
Study details: Findings are from a 10-year follow-up analysis of the phase 3 TAM-01 trial including 500 women with intraepithelial neoplasia of the breast who were randomly assigned to receive low-dose tamoxifen or placebo.
Disclosures: This study was supported by the Italian Ministry of Health and other sources. The authors declared serving as employees, consultants, or advisors, or receiving honoraria and travel and accommodation expenses from several sources.
Source: Lazzeroni M et al. Randomized placebo controlled trial of low-dose tamoxifen to prevent recurrence in breast noninvasive neoplasia: A 10-year follow-up of TAM-01 study. J Clin Oncol. 2023 (Mar 14). Doi: 10.1200/JCO.22.02900