User login
FDA issues stronger warning on neuropsychiatric event risk linked to montelukast
The Food and Drug Administration has issued , a prescription drug for asthma and allergy.
The new boxed warning advises health care providers to avoid prescribing montelukast for patients with mild symptoms, particularly those with allergic rhinitis, the FDA said in a press release. The drug was first approved in 1998, and the product labeling was updated in 2008 to include information about neuropsychiatric adverse events reported with usage of montelukast.
While the Sentinel study, along with other observational studies, did not find an increased risk of mental health side effects with montelukast treatment, compared with inhaled corticosteroids, those studies had limitations that may have affected results, the FDA said in the Drug Safety Communication. However, the FDA has continued to receive reports of neuropsychiatric events – including agitation, depression, sleeping problems, and suicidal thoughts and actions – in patients receiving the medication.
“The incidence of neuropsychiatric events associated with montelukast is unknown, but some reports are serious, and many patients and health care professionals are not fully aware of these risks,” Sally Seymour, MD, director of the division of pulmonary, allergy and rheumatology products in the FDA’s Center for Drug Evaluation and Research, said in the press release. “There are many other safe and effective medications to treat allergies with extensive history of use and safety, such that many products are available over the counter without a prescription.”
In addition to the boxed warning, the FDA now requires a new medication guide to be given to patients with each montelukast prescription, the FDA said.
The Food and Drug Administration has issued , a prescription drug for asthma and allergy.
The new boxed warning advises health care providers to avoid prescribing montelukast for patients with mild symptoms, particularly those with allergic rhinitis, the FDA said in a press release. The drug was first approved in 1998, and the product labeling was updated in 2008 to include information about neuropsychiatric adverse events reported with usage of montelukast.
While the Sentinel study, along with other observational studies, did not find an increased risk of mental health side effects with montelukast treatment, compared with inhaled corticosteroids, those studies had limitations that may have affected results, the FDA said in the Drug Safety Communication. However, the FDA has continued to receive reports of neuropsychiatric events – including agitation, depression, sleeping problems, and suicidal thoughts and actions – in patients receiving the medication.
“The incidence of neuropsychiatric events associated with montelukast is unknown, but some reports are serious, and many patients and health care professionals are not fully aware of these risks,” Sally Seymour, MD, director of the division of pulmonary, allergy and rheumatology products in the FDA’s Center for Drug Evaluation and Research, said in the press release. “There are many other safe and effective medications to treat allergies with extensive history of use and safety, such that many products are available over the counter without a prescription.”
In addition to the boxed warning, the FDA now requires a new medication guide to be given to patients with each montelukast prescription, the FDA said.
The Food and Drug Administration has issued , a prescription drug for asthma and allergy.
The new boxed warning advises health care providers to avoid prescribing montelukast for patients with mild symptoms, particularly those with allergic rhinitis, the FDA said in a press release. The drug was first approved in 1998, and the product labeling was updated in 2008 to include information about neuropsychiatric adverse events reported with usage of montelukast.
While the Sentinel study, along with other observational studies, did not find an increased risk of mental health side effects with montelukast treatment, compared with inhaled corticosteroids, those studies had limitations that may have affected results, the FDA said in the Drug Safety Communication. However, the FDA has continued to receive reports of neuropsychiatric events – including agitation, depression, sleeping problems, and suicidal thoughts and actions – in patients receiving the medication.
“The incidence of neuropsychiatric events associated with montelukast is unknown, but some reports are serious, and many patients and health care professionals are not fully aware of these risks,” Sally Seymour, MD, director of the division of pulmonary, allergy and rheumatology products in the FDA’s Center for Drug Evaluation and Research, said in the press release. “There are many other safe and effective medications to treat allergies with extensive history of use and safety, such that many products are available over the counter without a prescription.”
In addition to the boxed warning, the FDA now requires a new medication guide to be given to patients with each montelukast prescription, the FDA said.
FDA rules to ban ESDs for self-injurious, aggressive behavior
The Food and Drug Administration has banned all electrical stimulation devices used for self-injurious or aggressive behavior because of an unreasonable risk of illness or injury. This marks only the third time the FDA has banned a medical device since it gained the authority to do so.
Electrical stimulation devices (ESDs) administer electric shocks through electrodes attached to the skin during self-injurious or aggressive behavior in an attempt to condition the patient to stop engaging in that behavior, according to the FDA press release. Current evidence indicates that use of these devices can lead to worsening of underlying symptoms, depression, anxiety, PTSD, pain, burns, and tissue damage; in contrast, evidence supporting their use is weak. In addition, many patients exposed to ESDs have intellectual or developmental disabilities and might not be able to adequately communicate their level of pain.
“Since ESDs were first marketed more than 20 years ago, we have gained a better understanding of the danger these devices present to public health. Through advancements in medical science, there are now more treatment options available to reduce or stop self-injurious or aggressive behavior, thus avoiding the substantial risk ESDs present,” William H. Maisel, MD, MPH, director of the Office of Product Evaluation and Quality in the FDA’s Center for Devices and Radiological Health, said in the release.
The ruling follows a 2016 proposal to ban ESDs from the marketplace; the proposed rule received more than 1,500 comments from stakeholders, such as parents of people with intellectual and developmental disabilities, state agencies and their sister public-private organizations, the affected manufacturer and residential facility, some of the facility’s employees, and parents of individual residents, as well as from state and federal legislators and advocacy groups. Nearly all supported the ban.
The rule will go into effect 30 days after publication of the rule in the Federal Register, and compliance is required within 180 days.
The Food and Drug Administration has banned all electrical stimulation devices used for self-injurious or aggressive behavior because of an unreasonable risk of illness or injury. This marks only the third time the FDA has banned a medical device since it gained the authority to do so.
Electrical stimulation devices (ESDs) administer electric shocks through electrodes attached to the skin during self-injurious or aggressive behavior in an attempt to condition the patient to stop engaging in that behavior, according to the FDA press release. Current evidence indicates that use of these devices can lead to worsening of underlying symptoms, depression, anxiety, PTSD, pain, burns, and tissue damage; in contrast, evidence supporting their use is weak. In addition, many patients exposed to ESDs have intellectual or developmental disabilities and might not be able to adequately communicate their level of pain.
“Since ESDs were first marketed more than 20 years ago, we have gained a better understanding of the danger these devices present to public health. Through advancements in medical science, there are now more treatment options available to reduce or stop self-injurious or aggressive behavior, thus avoiding the substantial risk ESDs present,” William H. Maisel, MD, MPH, director of the Office of Product Evaluation and Quality in the FDA’s Center for Devices and Radiological Health, said in the release.
The ruling follows a 2016 proposal to ban ESDs from the marketplace; the proposed rule received more than 1,500 comments from stakeholders, such as parents of people with intellectual and developmental disabilities, state agencies and their sister public-private organizations, the affected manufacturer and residential facility, some of the facility’s employees, and parents of individual residents, as well as from state and federal legislators and advocacy groups. Nearly all supported the ban.
The rule will go into effect 30 days after publication of the rule in the Federal Register, and compliance is required within 180 days.
The Food and Drug Administration has banned all electrical stimulation devices used for self-injurious or aggressive behavior because of an unreasonable risk of illness or injury. This marks only the third time the FDA has banned a medical device since it gained the authority to do so.
Electrical stimulation devices (ESDs) administer electric shocks through electrodes attached to the skin during self-injurious or aggressive behavior in an attempt to condition the patient to stop engaging in that behavior, according to the FDA press release. Current evidence indicates that use of these devices can lead to worsening of underlying symptoms, depression, anxiety, PTSD, pain, burns, and tissue damage; in contrast, evidence supporting their use is weak. In addition, many patients exposed to ESDs have intellectual or developmental disabilities and might not be able to adequately communicate their level of pain.
“Since ESDs were first marketed more than 20 years ago, we have gained a better understanding of the danger these devices present to public health. Through advancements in medical science, there are now more treatment options available to reduce or stop self-injurious or aggressive behavior, thus avoiding the substantial risk ESDs present,” William H. Maisel, MD, MPH, director of the Office of Product Evaluation and Quality in the FDA’s Center for Devices and Radiological Health, said in the release.
The ruling follows a 2016 proposal to ban ESDs from the marketplace; the proposed rule received more than 1,500 comments from stakeholders, such as parents of people with intellectual and developmental disabilities, state agencies and their sister public-private organizations, the affected manufacturer and residential facility, some of the facility’s employees, and parents of individual residents, as well as from state and federal legislators and advocacy groups. Nearly all supported the ban.
The rule will go into effect 30 days after publication of the rule in the Federal Register, and compliance is required within 180 days.
rTMS for depression continues to evolve
LAS VEGAS – Repetitive transcranial magnetic stimulation methods for treatment-resistant depression continue to be refined.
“Original studies have relatively low response rates, but we’re seeing better response rates as we figure out the localization, the parameters, the wave form, and how frequently you can give it,” Alan F. Schatzberg, MD, said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
Repetitive transcranial magnetic stimulation (rTMS) involves the application of a magnetic field to a particular area of the brain, typically the dorsal lateral aspect of the prefrontal cortex. “It’s a weaker stimulant than electroconvulsive therapy, but it’s more focused and a lot safer,” said Dr. Schatzberg, professor of psychiatry and behavioral sciences at Stanford (Calif.) University. “It does not require anesthesia. In fact, it does seem to have some antidepressant effects.”
The original trial that applied this technology was conducted in 301 medication-free patients with major depression who had not benefited from prior treatment (Biol Psychiatry. 2007;62[11]:1208-16). Of the 301 patients, 155 received active rTMS, while 146 received sham rTMS. Treatment sessions were conducted five times per week for 4-6 weeks. The primary outcome was the symptom score change as assessed at week 4 with the Montgomery-Åsberg Depression Rating Scale (MADRS). Secondary outcomes included changes on the 17- and 24-item Hamilton Depression Rating Scale (HAMD), and response and remission rates with the MADRS and HAMD.
Response rates were significantly higher with active TMS on all three scales at weeks 4 and 6. Remission rates were approximately twofold higher with active TMS at week 6 and significant on the MADRS and HAMD24 scales (but not the HAMD17 scale). “The response rate for patients receiving active treatment was about 20%, and the remission at 6 weeks was about 18%,” said Dr. Schatzberg, who was an adviser to the study. “It was about twofold higher than in the sham group. It’s not dramatically effective, but it certainly is better than the sham control.” The MADRS score dropped about 6 points in the rTMS group, compared with about 2 points in the sham group, while the HAMD 24 score dropped about 7 points in the rTMS group, compared with about 3.5 points in the sham group.
In a separate, multisite, sham-controlled trial supported by the National Institutes of Health, researchers enrolled 199 antidepressant drug-free patients to determine whether daily left prefrontal rTMS safely and effectively treats major depressive disorder (Arch Gen Psychiatry. 2010;67[5]:507-16). Over the course of 3 weeks, the researchers delivered rTMS to the left prefrontal cortex for 37.5 minutes (3,000 pulses per session) using a figure-eight solid-core coil. Sham rTMS used a similar coil with a metal insert blocking the magnetic field and scalp electrodes that delivered matched somatosensory sensations. The retention rate was 88%, and no device-related serious adverse events were reported. A significantly greater proportion of patients treated with rTMS achieved remission, compared with those in the sham group (15% vs. 5%, respectively; P = .02). The odds of attaining remission were 4.2 times greater with active rTMS than with the sham treatment.
“These are not huge remission and response rates,” Dr. Schatzberg said of the results from this and other studies. “What can we do to start increasing efficacy? One thing you can do is design a better coil. You can alter the site of application, and you can change the pulse frequency and the pulse number. You can also change the brain wave focus. Theta seems to be mostly associated with hippocampal function around memory. Because of that, a number of groups starting giving theta waves.”
In one such study, researchers used accelerated, high-dose intermittent theta burst stimulation (iTBS) to treat highly treatment-resistant depression patients (Brain. 2018;141[3]:e18). The treatment lasted 5 days and consisted of 10 sessions per day, with 50 minutes between each session. “It’s a much more intensive system that delivers about 90,000 pulses,” said Dr. Schatzberg, who directs the Stanford Mood Disorders Center. Most patients remitted, but the durability of therapeutic response was weak, and all patients relapsed within 2 weeks post treatment.
“There’s more work to be done, but rTMS is really a good technology,” he concluded. “I think we will achieve much higher rates of success with this treatment once we push the envelope a little bit.”
Dr. Schatzberg disclosed that he has served a consultant to Alkermes, Avanir, Bracket, Compass, Delpor, Epiodyne, Janssen, Jazz, Lundbeck, McKinsey, Merck, Myriad Genetics, Owl, Neuronetics, Pfizer, Sage, and Sunovion. He has received research funding from Janssen and also holds an ownership interest in Corcept, Dermira, Delpor, Epiodyne, Incyte Genetics, Madrigal, Merck, Owl Analytics, Seattle Genetics, Titan, and Xhale.
LAS VEGAS – Repetitive transcranial magnetic stimulation methods for treatment-resistant depression continue to be refined.
“Original studies have relatively low response rates, but we’re seeing better response rates as we figure out the localization, the parameters, the wave form, and how frequently you can give it,” Alan F. Schatzberg, MD, said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
Repetitive transcranial magnetic stimulation (rTMS) involves the application of a magnetic field to a particular area of the brain, typically the dorsal lateral aspect of the prefrontal cortex. “It’s a weaker stimulant than electroconvulsive therapy, but it’s more focused and a lot safer,” said Dr. Schatzberg, professor of psychiatry and behavioral sciences at Stanford (Calif.) University. “It does not require anesthesia. In fact, it does seem to have some antidepressant effects.”
The original trial that applied this technology was conducted in 301 medication-free patients with major depression who had not benefited from prior treatment (Biol Psychiatry. 2007;62[11]:1208-16). Of the 301 patients, 155 received active rTMS, while 146 received sham rTMS. Treatment sessions were conducted five times per week for 4-6 weeks. The primary outcome was the symptom score change as assessed at week 4 with the Montgomery-Åsberg Depression Rating Scale (MADRS). Secondary outcomes included changes on the 17- and 24-item Hamilton Depression Rating Scale (HAMD), and response and remission rates with the MADRS and HAMD.
Response rates were significantly higher with active TMS on all three scales at weeks 4 and 6. Remission rates were approximately twofold higher with active TMS at week 6 and significant on the MADRS and HAMD24 scales (but not the HAMD17 scale). “The response rate for patients receiving active treatment was about 20%, and the remission at 6 weeks was about 18%,” said Dr. Schatzberg, who was an adviser to the study. “It was about twofold higher than in the sham group. It’s not dramatically effective, but it certainly is better than the sham control.” The MADRS score dropped about 6 points in the rTMS group, compared with about 2 points in the sham group, while the HAMD 24 score dropped about 7 points in the rTMS group, compared with about 3.5 points in the sham group.
In a separate, multisite, sham-controlled trial supported by the National Institutes of Health, researchers enrolled 199 antidepressant drug-free patients to determine whether daily left prefrontal rTMS safely and effectively treats major depressive disorder (Arch Gen Psychiatry. 2010;67[5]:507-16). Over the course of 3 weeks, the researchers delivered rTMS to the left prefrontal cortex for 37.5 minutes (3,000 pulses per session) using a figure-eight solid-core coil. Sham rTMS used a similar coil with a metal insert blocking the magnetic field and scalp electrodes that delivered matched somatosensory sensations. The retention rate was 88%, and no device-related serious adverse events were reported. A significantly greater proportion of patients treated with rTMS achieved remission, compared with those in the sham group (15% vs. 5%, respectively; P = .02). The odds of attaining remission were 4.2 times greater with active rTMS than with the sham treatment.
“These are not huge remission and response rates,” Dr. Schatzberg said of the results from this and other studies. “What can we do to start increasing efficacy? One thing you can do is design a better coil. You can alter the site of application, and you can change the pulse frequency and the pulse number. You can also change the brain wave focus. Theta seems to be mostly associated with hippocampal function around memory. Because of that, a number of groups starting giving theta waves.”
In one such study, researchers used accelerated, high-dose intermittent theta burst stimulation (iTBS) to treat highly treatment-resistant depression patients (Brain. 2018;141[3]:e18). The treatment lasted 5 days and consisted of 10 sessions per day, with 50 minutes between each session. “It’s a much more intensive system that delivers about 90,000 pulses,” said Dr. Schatzberg, who directs the Stanford Mood Disorders Center. Most patients remitted, but the durability of therapeutic response was weak, and all patients relapsed within 2 weeks post treatment.
“There’s more work to be done, but rTMS is really a good technology,” he concluded. “I think we will achieve much higher rates of success with this treatment once we push the envelope a little bit.”
Dr. Schatzberg disclosed that he has served a consultant to Alkermes, Avanir, Bracket, Compass, Delpor, Epiodyne, Janssen, Jazz, Lundbeck, McKinsey, Merck, Myriad Genetics, Owl, Neuronetics, Pfizer, Sage, and Sunovion. He has received research funding from Janssen and also holds an ownership interest in Corcept, Dermira, Delpor, Epiodyne, Incyte Genetics, Madrigal, Merck, Owl Analytics, Seattle Genetics, Titan, and Xhale.
LAS VEGAS – Repetitive transcranial magnetic stimulation methods for treatment-resistant depression continue to be refined.
“Original studies have relatively low response rates, but we’re seeing better response rates as we figure out the localization, the parameters, the wave form, and how frequently you can give it,” Alan F. Schatzberg, MD, said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
Repetitive transcranial magnetic stimulation (rTMS) involves the application of a magnetic field to a particular area of the brain, typically the dorsal lateral aspect of the prefrontal cortex. “It’s a weaker stimulant than electroconvulsive therapy, but it’s more focused and a lot safer,” said Dr. Schatzberg, professor of psychiatry and behavioral sciences at Stanford (Calif.) University. “It does not require anesthesia. In fact, it does seem to have some antidepressant effects.”
The original trial that applied this technology was conducted in 301 medication-free patients with major depression who had not benefited from prior treatment (Biol Psychiatry. 2007;62[11]:1208-16). Of the 301 patients, 155 received active rTMS, while 146 received sham rTMS. Treatment sessions were conducted five times per week for 4-6 weeks. The primary outcome was the symptom score change as assessed at week 4 with the Montgomery-Åsberg Depression Rating Scale (MADRS). Secondary outcomes included changes on the 17- and 24-item Hamilton Depression Rating Scale (HAMD), and response and remission rates with the MADRS and HAMD.
Response rates were significantly higher with active TMS on all three scales at weeks 4 and 6. Remission rates were approximately twofold higher with active TMS at week 6 and significant on the MADRS and HAMD24 scales (but not the HAMD17 scale). “The response rate for patients receiving active treatment was about 20%, and the remission at 6 weeks was about 18%,” said Dr. Schatzberg, who was an adviser to the study. “It was about twofold higher than in the sham group. It’s not dramatically effective, but it certainly is better than the sham control.” The MADRS score dropped about 6 points in the rTMS group, compared with about 2 points in the sham group, while the HAMD 24 score dropped about 7 points in the rTMS group, compared with about 3.5 points in the sham group.
In a separate, multisite, sham-controlled trial supported by the National Institutes of Health, researchers enrolled 199 antidepressant drug-free patients to determine whether daily left prefrontal rTMS safely and effectively treats major depressive disorder (Arch Gen Psychiatry. 2010;67[5]:507-16). Over the course of 3 weeks, the researchers delivered rTMS to the left prefrontal cortex for 37.5 minutes (3,000 pulses per session) using a figure-eight solid-core coil. Sham rTMS used a similar coil with a metal insert blocking the magnetic field and scalp electrodes that delivered matched somatosensory sensations. The retention rate was 88%, and no device-related serious adverse events were reported. A significantly greater proportion of patients treated with rTMS achieved remission, compared with those in the sham group (15% vs. 5%, respectively; P = .02). The odds of attaining remission were 4.2 times greater with active rTMS than with the sham treatment.
“These are not huge remission and response rates,” Dr. Schatzberg said of the results from this and other studies. “What can we do to start increasing efficacy? One thing you can do is design a better coil. You can alter the site of application, and you can change the pulse frequency and the pulse number. You can also change the brain wave focus. Theta seems to be mostly associated with hippocampal function around memory. Because of that, a number of groups starting giving theta waves.”
In one such study, researchers used accelerated, high-dose intermittent theta burst stimulation (iTBS) to treat highly treatment-resistant depression patients (Brain. 2018;141[3]:e18). The treatment lasted 5 days and consisted of 10 sessions per day, with 50 minutes between each session. “It’s a much more intensive system that delivers about 90,000 pulses,” said Dr. Schatzberg, who directs the Stanford Mood Disorders Center. Most patients remitted, but the durability of therapeutic response was weak, and all patients relapsed within 2 weeks post treatment.
“There’s more work to be done, but rTMS is really a good technology,” he concluded. “I think we will achieve much higher rates of success with this treatment once we push the envelope a little bit.”
Dr. Schatzberg disclosed that he has served a consultant to Alkermes, Avanir, Bracket, Compass, Delpor, Epiodyne, Janssen, Jazz, Lundbeck, McKinsey, Merck, Myriad Genetics, Owl, Neuronetics, Pfizer, Sage, and Sunovion. He has received research funding from Janssen and also holds an ownership interest in Corcept, Dermira, Delpor, Epiodyne, Incyte Genetics, Madrigal, Merck, Owl Analytics, Seattle Genetics, Titan, and Xhale.
REPORTING FROM NPA 2020
Ketamine and serotonin syndrome: A case report
Long utilized as a rapid anesthetic, ketamine has been increasingly used in sub-anesthetic doses for several psychiatric indications, including depression, suicidality, and chronic pain. Recently, an intranasal form of esketamine—the S-enantiomer of ketamine—was FDA-approved for treatment-resistant depression. Previously, researchers believed ketamine mediated its analgesic and psychotropic effects solely via N-methyl-
CASE REPORT
Ms. O, age 41, has a history of endometriosis, anticardiolipin antibody syndrome, major depressive disorder, and generalized anxiety disorder. She initially presented to an outside hospital and was admitted for chronic endometriosis pain. During that admission, her pain was treated with IV ketamine, 40 mg/hour, on hospital Days 1 through 4. While hospitalized, she continued to receive her home medications: fluoxetine, 40 mg/d, coumadin, 5 mg/d, and diphenhydramine, 25 mg/d. On Day 5, Ms. O experienced visual hallucinations and was diagnosed with ketamine-induced delirium. She was treated with haloperidol (dose unknown) with reportedly good effect. On Day 7, she was discharged home.
Upon returning home, she experienced persistent altered mental status. Her significant other brought her to our hospital for further workup. Ms. O’s body temperature was 37.6°C, and she was diaphoretic. Her blood pressure was 154/100 mm Hg, and her heart rate was 125 bpm. On physical examination, she had 4+ patellar and Achilles reflexes with left ankle clonus and crossed adductors. Her mental status exam showed increased latency of thought and speech, with bizarre affect as evidenced by illogical mannerisms and appearance. She said she was “not feeling myself” and would stare at walls for prolonged periods of time, appearing internally preoccupied and confused.
Ms. O was treated with IV lorazepam, 2 mg. Fourteen hours later, her temperature returned to normal, but she remained tachycardic, hypertensive, and altered. She received 2 additional doses of 2 mg and 1 mg. Seventeen hours after the initial dose of IV lorazepam was administered (and 3 hours after the second dose), Ms. O’s heart rate returned to normal. She was ultimately converted to oral lorazepam, 1 mg every 12 hours. Two hours later, Ms. O’s blood pressure returned to normal, and her physical exam showed normal reflexes.
Ms. O was given a presumptive diagnosis of ketamine-induced serotonin syndrome. She made a good recovery and was discharged home.
A suspected association
Serotonin syndrome is caused by increased levels of the neurotransmitter serotonin in the CNS. Clinical features of serotonin syndrome include agitation, restlessness, mydriasis, altered mental status or confusion, tachycardia, hypertension, muscle rigidity, diaphoresis, diarrhea, piloerection, headache, fasciculations, clonus, and shivering. Severe cases can be life-threatening and may present with high fever, seizures, arrhythmias, and loss of consciousness. Serotonin syndrome is a clinical diagnosis; the Hunter Serotonin Toxicity Criteria are often used to make the diagnosis. To meet these criteria, a patient must have received a serotonergic agent, and at least one of the following must be present4:
- spontaneous clonus
- inducible clonus and agitation or diaphoresis
- ocular clonus and agitation or diaphoresis
- tremor and hyperreflexia
- hypertonia, temperature >38°C, and ocular clonus or inducible clonus.
For Ms. O, we suspected that administration of ketamine in conjunction with fluoxetine, 40 mg/d, led to serotonin syndrome. Ms. O exhibited ocular clonus and diaphoresis, thus satisfying the Hunter Serotonin Toxicity Criteria, and she also had inducible clonus, altered mental status, hypertension, and tachycardia, which makes serotonin syndrome the most likely diagnosis. She improved after receiving lorazepam, which is often used to treat hypertonicity, decrease autonomic instability, and prevent seizures seen in serotonin syndrome.5
Continue to: There is sparse literature...
There is sparse literature describing serotonin syndrome related to ketamine use. Ketamine has been shown to increase levels of glutamate in the medial prefrontal cortex. Higher levels of glutamine in turn stimulate excitatory glutamatergic neurons that project to the dorsal raphe nucleus. When stimulated, the dorsal raphe nucleus releases serotonin.6 There is also evidence that ketamine inhibits uptake of serotonin in synapses.7 These mechanisms combine to create a net increase in CNS-wide serotonin.
Ketamine is being increasingly used to treat depression and other conditions. This case report underscores the importance of considering serotonin syndrome when treating patients receiving ketamine, especially when it is used in conjunction with selective serotonin reuptake inhibitors.
1. du Jardin KG, Müller HK, Elfving B, et al. Potential involvement of serotonergic signaling in ketamine’s antidepressant actions: A critical review. Prog Neuropsychopharmacol Biol Psychiatry. 2016;71:27-38.
2. Gigliucci V, O’Dowd G, Casey S, et al. Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl). 2013;228(1):157-166.
3. Warner ME, Naranjo J, Pollard EM, et al. Serotonergic medications, herbal supplements, and perioperative serotonin syndrome. Can J Anaesth. 2017;64(9):940-946.
4. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
5. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
6. López-Gil X, Jiménez-Sánchez L, Campa L, et al. Role of serotonin and noradrenaline in the rapid antidepressant action of ketamine. ACS Chem Neurosci. 2019;10(7):3318-3326.
7. Martin LL, Bouchal RL, Smith DJ. Ketamine inhibits serotonin uptake in vivo. Neuropharmacology. 1982;21(2):113-118.
Long utilized as a rapid anesthetic, ketamine has been increasingly used in sub-anesthetic doses for several psychiatric indications, including depression, suicidality, and chronic pain. Recently, an intranasal form of esketamine—the S-enantiomer of ketamine—was FDA-approved for treatment-resistant depression. Previously, researchers believed ketamine mediated its analgesic and psychotropic effects solely via N-methyl-
CASE REPORT
Ms. O, age 41, has a history of endometriosis, anticardiolipin antibody syndrome, major depressive disorder, and generalized anxiety disorder. She initially presented to an outside hospital and was admitted for chronic endometriosis pain. During that admission, her pain was treated with IV ketamine, 40 mg/hour, on hospital Days 1 through 4. While hospitalized, she continued to receive her home medications: fluoxetine, 40 mg/d, coumadin, 5 mg/d, and diphenhydramine, 25 mg/d. On Day 5, Ms. O experienced visual hallucinations and was diagnosed with ketamine-induced delirium. She was treated with haloperidol (dose unknown) with reportedly good effect. On Day 7, she was discharged home.
Upon returning home, she experienced persistent altered mental status. Her significant other brought her to our hospital for further workup. Ms. O’s body temperature was 37.6°C, and she was diaphoretic. Her blood pressure was 154/100 mm Hg, and her heart rate was 125 bpm. On physical examination, she had 4+ patellar and Achilles reflexes with left ankle clonus and crossed adductors. Her mental status exam showed increased latency of thought and speech, with bizarre affect as evidenced by illogical mannerisms and appearance. She said she was “not feeling myself” and would stare at walls for prolonged periods of time, appearing internally preoccupied and confused.
Ms. O was treated with IV lorazepam, 2 mg. Fourteen hours later, her temperature returned to normal, but she remained tachycardic, hypertensive, and altered. She received 2 additional doses of 2 mg and 1 mg. Seventeen hours after the initial dose of IV lorazepam was administered (and 3 hours after the second dose), Ms. O’s heart rate returned to normal. She was ultimately converted to oral lorazepam, 1 mg every 12 hours. Two hours later, Ms. O’s blood pressure returned to normal, and her physical exam showed normal reflexes.
Ms. O was given a presumptive diagnosis of ketamine-induced serotonin syndrome. She made a good recovery and was discharged home.
A suspected association
Serotonin syndrome is caused by increased levels of the neurotransmitter serotonin in the CNS. Clinical features of serotonin syndrome include agitation, restlessness, mydriasis, altered mental status or confusion, tachycardia, hypertension, muscle rigidity, diaphoresis, diarrhea, piloerection, headache, fasciculations, clonus, and shivering. Severe cases can be life-threatening and may present with high fever, seizures, arrhythmias, and loss of consciousness. Serotonin syndrome is a clinical diagnosis; the Hunter Serotonin Toxicity Criteria are often used to make the diagnosis. To meet these criteria, a patient must have received a serotonergic agent, and at least one of the following must be present4:
- spontaneous clonus
- inducible clonus and agitation or diaphoresis
- ocular clonus and agitation or diaphoresis
- tremor and hyperreflexia
- hypertonia, temperature >38°C, and ocular clonus or inducible clonus.
For Ms. O, we suspected that administration of ketamine in conjunction with fluoxetine, 40 mg/d, led to serotonin syndrome. Ms. O exhibited ocular clonus and diaphoresis, thus satisfying the Hunter Serotonin Toxicity Criteria, and she also had inducible clonus, altered mental status, hypertension, and tachycardia, which makes serotonin syndrome the most likely diagnosis. She improved after receiving lorazepam, which is often used to treat hypertonicity, decrease autonomic instability, and prevent seizures seen in serotonin syndrome.5
Continue to: There is sparse literature...
There is sparse literature describing serotonin syndrome related to ketamine use. Ketamine has been shown to increase levels of glutamate in the medial prefrontal cortex. Higher levels of glutamine in turn stimulate excitatory glutamatergic neurons that project to the dorsal raphe nucleus. When stimulated, the dorsal raphe nucleus releases serotonin.6 There is also evidence that ketamine inhibits uptake of serotonin in synapses.7 These mechanisms combine to create a net increase in CNS-wide serotonin.
Ketamine is being increasingly used to treat depression and other conditions. This case report underscores the importance of considering serotonin syndrome when treating patients receiving ketamine, especially when it is used in conjunction with selective serotonin reuptake inhibitors.
Long utilized as a rapid anesthetic, ketamine has been increasingly used in sub-anesthetic doses for several psychiatric indications, including depression, suicidality, and chronic pain. Recently, an intranasal form of esketamine—the S-enantiomer of ketamine—was FDA-approved for treatment-resistant depression. Previously, researchers believed ketamine mediated its analgesic and psychotropic effects solely via N-methyl-
CASE REPORT
Ms. O, age 41, has a history of endometriosis, anticardiolipin antibody syndrome, major depressive disorder, and generalized anxiety disorder. She initially presented to an outside hospital and was admitted for chronic endometriosis pain. During that admission, her pain was treated with IV ketamine, 40 mg/hour, on hospital Days 1 through 4. While hospitalized, she continued to receive her home medications: fluoxetine, 40 mg/d, coumadin, 5 mg/d, and diphenhydramine, 25 mg/d. On Day 5, Ms. O experienced visual hallucinations and was diagnosed with ketamine-induced delirium. She was treated with haloperidol (dose unknown) with reportedly good effect. On Day 7, she was discharged home.
Upon returning home, she experienced persistent altered mental status. Her significant other brought her to our hospital for further workup. Ms. O’s body temperature was 37.6°C, and she was diaphoretic. Her blood pressure was 154/100 mm Hg, and her heart rate was 125 bpm. On physical examination, she had 4+ patellar and Achilles reflexes with left ankle clonus and crossed adductors. Her mental status exam showed increased latency of thought and speech, with bizarre affect as evidenced by illogical mannerisms and appearance. She said she was “not feeling myself” and would stare at walls for prolonged periods of time, appearing internally preoccupied and confused.
Ms. O was treated with IV lorazepam, 2 mg. Fourteen hours later, her temperature returned to normal, but she remained tachycardic, hypertensive, and altered. She received 2 additional doses of 2 mg and 1 mg. Seventeen hours after the initial dose of IV lorazepam was administered (and 3 hours after the second dose), Ms. O’s heart rate returned to normal. She was ultimately converted to oral lorazepam, 1 mg every 12 hours. Two hours later, Ms. O’s blood pressure returned to normal, and her physical exam showed normal reflexes.
Ms. O was given a presumptive diagnosis of ketamine-induced serotonin syndrome. She made a good recovery and was discharged home.
A suspected association
Serotonin syndrome is caused by increased levels of the neurotransmitter serotonin in the CNS. Clinical features of serotonin syndrome include agitation, restlessness, mydriasis, altered mental status or confusion, tachycardia, hypertension, muscle rigidity, diaphoresis, diarrhea, piloerection, headache, fasciculations, clonus, and shivering. Severe cases can be life-threatening and may present with high fever, seizures, arrhythmias, and loss of consciousness. Serotonin syndrome is a clinical diagnosis; the Hunter Serotonin Toxicity Criteria are often used to make the diagnosis. To meet these criteria, a patient must have received a serotonergic agent, and at least one of the following must be present4:
- spontaneous clonus
- inducible clonus and agitation or diaphoresis
- ocular clonus and agitation or diaphoresis
- tremor and hyperreflexia
- hypertonia, temperature >38°C, and ocular clonus or inducible clonus.
For Ms. O, we suspected that administration of ketamine in conjunction with fluoxetine, 40 mg/d, led to serotonin syndrome. Ms. O exhibited ocular clonus and diaphoresis, thus satisfying the Hunter Serotonin Toxicity Criteria, and she also had inducible clonus, altered mental status, hypertension, and tachycardia, which makes serotonin syndrome the most likely diagnosis. She improved after receiving lorazepam, which is often used to treat hypertonicity, decrease autonomic instability, and prevent seizures seen in serotonin syndrome.5
Continue to: There is sparse literature...
There is sparse literature describing serotonin syndrome related to ketamine use. Ketamine has been shown to increase levels of glutamate in the medial prefrontal cortex. Higher levels of glutamine in turn stimulate excitatory glutamatergic neurons that project to the dorsal raphe nucleus. When stimulated, the dorsal raphe nucleus releases serotonin.6 There is also evidence that ketamine inhibits uptake of serotonin in synapses.7 These mechanisms combine to create a net increase in CNS-wide serotonin.
Ketamine is being increasingly used to treat depression and other conditions. This case report underscores the importance of considering serotonin syndrome when treating patients receiving ketamine, especially when it is used in conjunction with selective serotonin reuptake inhibitors.
1. du Jardin KG, Müller HK, Elfving B, et al. Potential involvement of serotonergic signaling in ketamine’s antidepressant actions: A critical review. Prog Neuropsychopharmacol Biol Psychiatry. 2016;71:27-38.
2. Gigliucci V, O’Dowd G, Casey S, et al. Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl). 2013;228(1):157-166.
3. Warner ME, Naranjo J, Pollard EM, et al. Serotonergic medications, herbal supplements, and perioperative serotonin syndrome. Can J Anaesth. 2017;64(9):940-946.
4. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
5. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
6. López-Gil X, Jiménez-Sánchez L, Campa L, et al. Role of serotonin and noradrenaline in the rapid antidepressant action of ketamine. ACS Chem Neurosci. 2019;10(7):3318-3326.
7. Martin LL, Bouchal RL, Smith DJ. Ketamine inhibits serotonin uptake in vivo. Neuropharmacology. 1982;21(2):113-118.
1. du Jardin KG, Müller HK, Elfving B, et al. Potential involvement of serotonergic signaling in ketamine’s antidepressant actions: A critical review. Prog Neuropsychopharmacol Biol Psychiatry. 2016;71:27-38.
2. Gigliucci V, O’Dowd G, Casey S, et al. Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl). 2013;228(1):157-166.
3. Warner ME, Naranjo J, Pollard EM, et al. Serotonergic medications, herbal supplements, and perioperative serotonin syndrome. Can J Anaesth. 2017;64(9):940-946.
4. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
5. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
6. López-Gil X, Jiménez-Sánchez L, Campa L, et al. Role of serotonin and noradrenaline in the rapid antidepressant action of ketamine. ACS Chem Neurosci. 2019;10(7):3318-3326.
7. Martin LL, Bouchal RL, Smith DJ. Ketamine inhibits serotonin uptake in vivo. Neuropharmacology. 1982;21(2):113-118.
Depression, or something else?
CASE Suicidal behavior, severe headaches
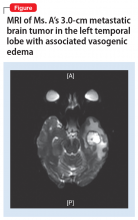
Ms. A, age 60, presents to the emergency department (ED) with depression, suicidal behavior, and 3 days of severe headaches. Neurology is consulted and an MRI is ordered, which shows a 3.0-cm mass lesion in the left temporal lobe with associated vasogenic edema that is suspicious for metastatic disease (Figure).
Ms. A is admitted to the hospital for further workup of her brain lesion. She is started on IV dexamethasone, 10 mg every 6 hours, a glucocorticosteroid, for brain edema, and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
Upon admission, in addition to oncology and neurosurgery, psychiatry is also consulted to evaluate Ms. A for depression and suicidality.
EVALUATION Mood changes and poor judgment
Ms. A has a psychiatric history of depression and alcohol use disorder but says she has not consumed any alcohol in years. Her medical history includes hypertension, diabetes, and stage 4 non-small–cell lung cancer, for which she received surgery and adjuvant chemoradiotherapy 1 year ago.
On initial intake, Ms. A reports that in addition to the headaches, she has also been experiencing worsening depression and suicidal behavior. For the past 2 months, she has had a severely depressed mood, with notable anhedonia, poor appetite, insomnia, low energy, and decreased concentration. The changes in her mental health were triggered by her mother’s death. Three days prior to admission, the patient planned to overdose on antihypertensive pills, but her suicide attempt was interrupted when her family called. She denies any current suicidal ideation, intent, or plan.
According to her family, Ms. A has been increasingly irritable and her personality has changed in the past month. She also has been repeatedly sorting through her neighbors’ garbage.
Ms. A’s current psychiatric medications are duloxetine, 30 mg/d; quetiapine, 50 mg every night at bedtime; and buspirone, 10 mg/d. However, it is unclear if she is consistently taking these medications.
Continue to: On mental status examination...
On mental status examination, Ms. A is calm and she has no abnormal movements. She says she is depressed. Her affect is reactive and labile. She is alert and oriented to person, place, and time. Her attention, registration, and recall are intact. Her executive function is not tested. However, Ms. A’s insight and judgment seem poor.
To address Ms. A’s worsening depression, the psychiatry team increases her duloxetine from 30 to 60 mg/d, and she continues quetiapine, 50 mg every night at bedtime, for mood lability. Buspirone is not continued because she was not taking a therapeutic dosage in the community.
Within 4 days, Ms. A shows improvement in sleep, appetite, and mood. She has no further suicidal ideation.
[polldaddy:10511743]
The authors’ observations
Ms. A had a recurrence of what was presumed to be major depressive disorder (MDD) in the context of her mother’s death. However, she also exhibited irritability, mood lability, and impulsivity, all of which could be part of her depression, or a separate problem related to her brain tumor. Because Ms. A had never displayed bizarre behavior before the past few weeks, it is likely that her CNS lesion was directly affecting her personality and possibly underlying her planned suicide attempt.
Fifty to 80% of patients with CNS tumors, either primary or metastatic, present with psychiatric symptoms.1 Table 11-3 lists common psychiatric symptoms of brain tumors. Unfortunately, there is little reliable evidence that directly correlates tumor location with specific psychiatric symptoms. A 2010 meta-analysis found a statistically significant link between anorexia nervosa and hypothalamic tumors.1 However, for other brain regions, there is only an increased likelihood that any given tumor location will produce psychiatric symptoms.1,4 For instance, compared to patients with tumors in other locations, those with temporal lobe tumors are more likely to present with mood disorders, personality changes, and memory problems.1 In contrast, patients with frontal lobe tumors have an increased likelihood of psychosis, mood disorders, and personality changes.1 Patients with tumors in the pituitary region often present with anxiety.1
Continue to: When considering treatment options...
When considering treatment options for Ms. A, alcohol withdrawal was unlikely given the remote history of alcohol use, low alcohol blood level, and lack of evidence of unstable vital signs or tremor. Although she might have benefited from inpatient psychiatric treatment, this needed to wait until there was a definitive treatment plan for her brain tumor. Finally, although a paraneoplastic syndrome, such as limbic encephalitis, could be causing her psychiatric symptoms, this scenario is less likely with non-small–cell lung cancer.
Although uncommon, CNS tumors can present with psychiatric symptoms as the only manifestation. This is more likely when a patient exhibits new-onset or atypical symptoms, or fails to respond to standard psychiatric treatment.4 Case reports have described patients with brain tumors being misdiagnosed as having a primary psychiatric condition, which delays treatment of their CNS cancer.2 Additionally, frontal and limbic tumors are more likely to present with psychiatric manifestations; up to 90% of patients exhibit altered mental status or personality changes, as did Ms. A.1,4 Clearly, it is easier to identify patients with psychiatric symptoms resulting from a brain tumor when they also present with focal neurologic deficits or systemic symptoms, such as headache or nausea and vomiting. Ms. A presented with severe headaches, which is what led to her early imaging and prompt diagnosis.
Numerous proposed mechanisms might account for the psychiatric symptoms that occur during the course of a brain tumor, including direct injury to neuronal cells, secretion of hormones or other tumor-derived substances, and peri-ictal phenomena.3
TREATMENT Tumor is removed, but memory is impaired
Ms. A is scheduled for craniotomy and surgical resection of the frontal mass. Prior to surgery, Ms. A shows interest in improving her health, cooperates with staff, and seeks her daughter’s input on treatment. One week after admission, Ms. A has her mass resected, which is confirmed on biopsy to be a lung metastasis. Post-surgery, Ms. A receives codeine, 30 mg every 6 hours as needed, for pain; she continues dexamethasone, 4 mg IV every 6 hours, for brain edema and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
On Day 2 after surgery, Ms. A attempts to elope. When she is approached by a psychiatrist on the treatment team, she does not recognize him. Although her long-term memory seems intact, she is unable to remember the details of recent events, including her medical and surgical treatments.
[polldaddy:10511745]
Continue to: The authors' observations
The authors’ observations
Ms. A’s memory impairment may be secondary to a surgically acquired neurocognitive deficit. In the United States, brain metastases represent a significant public health issue, affecting >100,000 patients per year.5 Metastatic lesions are the most common brain tumors. Lung cancer, breast cancer, and melanoma are the leading solid tumors to spread to the CNS.5 In cases of single brain metastasis, similar to Ms. A’s solitary left temporal lobe lesion, surgical resection plays a critical role in treatment. It provides histological confirmation of metastatic disease and can relieve mass effect if present. Studies have shown that combined surgical resection with radiation improves survival relative to patients who undergo radiation therapy alone.6,7
However, the benefits of surgical resection need to be balanced with preservation of neurologic function. Emerging evidence suggests that a majority of patients have surgically-acquired cognitive deficits due to damage of normal surrounding tissues, and these deficits are associated with reduced quality of life.8,9 Further, a study examining glioma surgical resections found that patients with left temporal lobe tumors exhibit more frequent and severe neurocognitive decline than patients with right temporal lobe tumors, especially in domains such as verbal memory.8 Ms. A’s memory impairment was persistent during her postoperative course, which suggests that it was not just an immediate post-surgical phenomenon, but a longer-lasting cognitive change directly related to the resection.
It is also possible that Ms. A had a prior neurocognitive disorder that manifested to a greater degree as a result of the CNS tumor. Ms. A might have had early-onset Alzheimer’s disease, although her intact memory before surgery makes this less likely. Alternatively, she could have had vascular dementia, especially given her long-standing hypertension and diabetes. This might have been missed in the initial evaluation because executive function was not tested. However, the relatively abrupt onset of memory problems after surgery suggests that she had no underlying neurocognitive disorder.
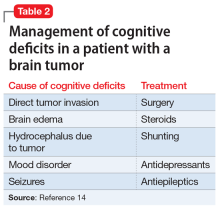
Ms. A’s presumed episode of MDD might also explain her memory changes. Major depressive disorder is increasingly common among geriatric patients, affecting approximately 5% of community-dwelling older adults.10 Its incidence increases with medical comorbidities, as suggested by depression rates of 5% to 10% in the primary care setting vs 37% in patients after critical-care hospitalizations.10 Late-life depression (LLD) occurs in adults age ≥60. Unlike depression in younger patients, LLD is more likely to be associated with cognitive impairment, specifically impairment of executive function and memory.11 The incidence of cognitive impairment in LLD is higher in patients with a history of depression, such as Ms. A.11,12 However, in general, patients who are depressed have memory complaints out of proportion to the clinical findings, and they show poor effort on cognitive testing. Ms. A exhibited neither of these, which makes it less likely that LLD was the exclusive cause of her memory loss.13 Table 214 outlines the management of cognitive deficits in a patient with a brain tumor.
EVALUATION Increasingly agitated and paranoid
After the tumor resection, Ms. A becomes increasingly irritable, uncooperative, and agitated. She repeatedly demands to be discharged. She insists she is fine and refuses medications and further laboratory workup. She becomes paranoid about the nursing staff and believes they are trying to kill her.
Continue to: On psychiatric re-evaluation...
On psychiatric re-evaluation, Ms. A demonstrates pressured speech, perseveration about going home, paranoid delusions, and anger at her family and physicians.
[polldaddy:10511747]
The authors’ observations
Ms. A’s refusal of medications and agitation may be explained by postoperative delirium, a surgical complication that is increasingly common among geriatric patients and is associated with poor clinical outcomes. Delirium is characterized by an acute onset and fluctuating course of symptoms that include inattention, motoric hypo- or hyperactivity, inappropriate behavior, emotional lability, cognitive dysfunction, and psychotic symptoms.15 Risk factors that contribute to postoperative delirium include older age, alcohol use, and poor baseline functional and cognitive status.16 The pathophysiology of delirium is not fully understood, but accumulating evidence suggests that different sets of interacting biologic factors (ie, neurotransmitters and inflammation) contribute to a disruption of large-scale neuronal networks in the brain, resulting in cognitive dysfunction.15 Patients who develop postoperative delirium are more likely to develop long-term cognitive dysfunction and have an increased risk of dementia.16
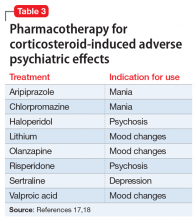
Another potential source of Ms. A’s agitation is steroid use. Ms. A received IV dexamethasone, 8 to 16 mg/d, around the time of her surgery. Steroids are commonly used to treat brain tumors, particularly when there is vasogenic edema. Steroid psychosis is a term loosely used to describe a wide range of psychiatric symptoms induced by corticosteroids that includes, but is not limited to, depression, mania, psychosis, delirium, and cognitive impairment.17 Steroid-induced psychiatric adverse effects occur in 5% to 18% of patients receiving corticosteroids and often happen early in treatment, although they can occur at any point.18 Corticosteroids influence brain activity via glucocorticoid and mineralocorticoid receptors. These receptors are widely distributed throughout the brain and affect neurotransmitter systems, such as the serotonergic system, that are associated with changes in mood, behavior, and cognition.17 While the adverse psychiatric manifestations of steroid use vary, higher dosages are associated with an increased risk of psychiatric complications; mania is more prevalent early in the course of treatment, and depression is more common with long-term use.17,19 Table 317,18 outlines the evidence-based treatment of corticosteroid-induced adverse psychiatric effects.
Although there are no clinical guidelines or FDA-approved medications for treating steroid-induced psychiatric adverse events, these are best managed by tapering and discontinuing steroids when possible and simultaneously using psychotropic medications to treat psychiatric symptoms. Case reports and limited evidence-based literature have demonstrated that steroid-induced mania responds to mood stabilizers or antipsychotics, while depression can be managed with antidepressants or lithium.17
Additionally, patients with CNS tumors are at risk for seizures and often are prescribed antiepileptics. Because it is easy to administer and does not need to be titrated, levetiracetam is a commonly used agent. However, levetiracetam can cause psychiatric adverse effects, including behavior changes and frank psychosis.20
Continue to: Finally, Ms. A's altered mental status...
Finally, Ms. A’s altered mental status could have been related to opioid intoxication. Opioids are used to manage postsurgical pain, and studies have shown these medications can be a precipitating factor for delirium in geriatric patients.21
TREATMENT Medication adjustments
At the request of the psychiatry team, levetiracetam is discontinued due to its potential for psychiatric adverse effects. The neurosurgery team replaces it with valproic acid, 500 mg every 12 hours. Ms. A is also tapered off steroids fairly rapidly because of the potential for steroid-induced psychiatric adverse effects. Her quetiapine is titrated from 50 to 150 mg every night at bedtime, and duloxetine is discontinued.
OUTCOME Agitation improves dramatically
Ms. A’s new medication regimen dramatically improves her agitation, which allows Ms. A, her family, and the medical team to work together to establish treatment goals. Ms. A ultimately returns home with the assistance of her family. She continues to have memory issues, but with improved emotion regulation. Several months later, Ms. A is readmitted to the hospital because her cancer has progressed despite treatment.
Bottom Line
Brain tumors may present with various psychiatric manifestations that can change during the course of the patient’s treatment. A comprehensive psychiatric evaluation should parse out the interplay between direct effects of the tumor and any adverse effects that are the result of medical and/or surgical interventions to determine the cause of psychiatric symptoms and their appropriate management.
Related Resource
Madhusoodanan S, Ting MB, Farah T, et al. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273-285.
Drug Brand Names
Aripiprazole • Abilify
Buspirone • Buspar
Chlorpromazine • Thorazine
Codeine • Codeine systemic
Dexamethasone • Decadron
Duloxetine • Cymbalta
Haloperidol • Haldol
Levetiracetam • Keppra
Lorazepam • Ativan
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakene
1. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
2. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
3. Pearl ML, Talgat G, Valea FA, et al. Psychiatric symptoms due to brain metastases. Med Update Psychiatr. 1998;3(4):91-94.
4. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343-349.
5. Ferguson SD, Wagner KM, Prabhu SS, et al. Neurosurgical management of brain metastases. Clin Exp Metastasis. 2017;34(6-7):377-389.
6. Husain ZA, Regine WF, Kwok Y, et al. Brain metastases: contemporary management and future directions. Eur J Clin Med Oncol. 2011;3(3):38-45.
7. Vecht CJ, Haaxmareiche H, Noordijk EM, et al. Treatment of single brain metastasis - radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993;33(6):583-590.
8. Barry RL, Byun NE, Tantawy MN, et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging Behav. 2018;12(1):87-95.
9. Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125.
10. Taylor WD. Depression in the elderly. N Engl J Med. 2014;371(13):1228-1236.
11. Liguori C, Pierantozzi M, Chiaravalloti A, et al. When cognitive decline and depression coexist in the elderly: CSF biomarkers analysis can differentiate Alzheimer’s disease from late-life depression. Front Aging Neurosci. 2018;10:38.
12. Luijendijk HJ, van den Berg JF, Dekker MJHJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394-1401.
13. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-117.
14. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168.
15. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
16. Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-323.
17. Kusljic S, Manias E, Gogos A. Corticosteroid-induced psychiatric disturbances: it is time for pharmacists to take notice. Res Soc Adm Pharm. 2016;12(2):355-360.
18. Cerullo MA. Corticosteroid-induced mania: prepare for the unpredictable. Current Psychiatry. 2006;5(6):43-50.
19. Dubovsky AN, Arvikar S, Stern TA, et al. Steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
20. Habets JGV, Leentjens AFG, Schijns OEMG. Serious and reversible levetiracetam-induced psychiatric symptoms after resection of frontal low-grade glioma: two case histories. Br J Neurosurg. 2017;31(4):471-473.
21
CASE Suicidal behavior, severe headaches
Ms. A, age 60, presents to the emergency department (ED) with depression, suicidal behavior, and 3 days of severe headaches. Neurology is consulted and an MRI is ordered, which shows a 3.0-cm mass lesion in the left temporal lobe with associated vasogenic edema that is suspicious for metastatic disease (Figure).
Ms. A is admitted to the hospital for further workup of her brain lesion. She is started on IV dexamethasone, 10 mg every 6 hours, a glucocorticosteroid, for brain edema, and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
Upon admission, in addition to oncology and neurosurgery, psychiatry is also consulted to evaluate Ms. A for depression and suicidality.
EVALUATION Mood changes and poor judgment
Ms. A has a psychiatric history of depression and alcohol use disorder but says she has not consumed any alcohol in years. Her medical history includes hypertension, diabetes, and stage 4 non-small–cell lung cancer, for which she received surgery and adjuvant chemoradiotherapy 1 year ago.
On initial intake, Ms. A reports that in addition to the headaches, she has also been experiencing worsening depression and suicidal behavior. For the past 2 months, she has had a severely depressed mood, with notable anhedonia, poor appetite, insomnia, low energy, and decreased concentration. The changes in her mental health were triggered by her mother’s death. Three days prior to admission, the patient planned to overdose on antihypertensive pills, but her suicide attempt was interrupted when her family called. She denies any current suicidal ideation, intent, or plan.
According to her family, Ms. A has been increasingly irritable and her personality has changed in the past month. She also has been repeatedly sorting through her neighbors’ garbage.
Ms. A’s current psychiatric medications are duloxetine, 30 mg/d; quetiapine, 50 mg every night at bedtime; and buspirone, 10 mg/d. However, it is unclear if she is consistently taking these medications.
Continue to: On mental status examination...
On mental status examination, Ms. A is calm and she has no abnormal movements. She says she is depressed. Her affect is reactive and labile. She is alert and oriented to person, place, and time. Her attention, registration, and recall are intact. Her executive function is not tested. However, Ms. A’s insight and judgment seem poor.
To address Ms. A’s worsening depression, the psychiatry team increases her duloxetine from 30 to 60 mg/d, and she continues quetiapine, 50 mg every night at bedtime, for mood lability. Buspirone is not continued because she was not taking a therapeutic dosage in the community.
Within 4 days, Ms. A shows improvement in sleep, appetite, and mood. She has no further suicidal ideation.
[polldaddy:10511743]
The authors’ observations
Ms. A had a recurrence of what was presumed to be major depressive disorder (MDD) in the context of her mother’s death. However, she also exhibited irritability, mood lability, and impulsivity, all of which could be part of her depression, or a separate problem related to her brain tumor. Because Ms. A had never displayed bizarre behavior before the past few weeks, it is likely that her CNS lesion was directly affecting her personality and possibly underlying her planned suicide attempt.
Fifty to 80% of patients with CNS tumors, either primary or metastatic, present with psychiatric symptoms.1 Table 11-3 lists common psychiatric symptoms of brain tumors. Unfortunately, there is little reliable evidence that directly correlates tumor location with specific psychiatric symptoms. A 2010 meta-analysis found a statistically significant link between anorexia nervosa and hypothalamic tumors.1 However, for other brain regions, there is only an increased likelihood that any given tumor location will produce psychiatric symptoms.1,4 For instance, compared to patients with tumors in other locations, those with temporal lobe tumors are more likely to present with mood disorders, personality changes, and memory problems.1 In contrast, patients with frontal lobe tumors have an increased likelihood of psychosis, mood disorders, and personality changes.1 Patients with tumors in the pituitary region often present with anxiety.1
Continue to: When considering treatment options...
When considering treatment options for Ms. A, alcohol withdrawal was unlikely given the remote history of alcohol use, low alcohol blood level, and lack of evidence of unstable vital signs or tremor. Although she might have benefited from inpatient psychiatric treatment, this needed to wait until there was a definitive treatment plan for her brain tumor. Finally, although a paraneoplastic syndrome, such as limbic encephalitis, could be causing her psychiatric symptoms, this scenario is less likely with non-small–cell lung cancer.
Although uncommon, CNS tumors can present with psychiatric symptoms as the only manifestation. This is more likely when a patient exhibits new-onset or atypical symptoms, or fails to respond to standard psychiatric treatment.4 Case reports have described patients with brain tumors being misdiagnosed as having a primary psychiatric condition, which delays treatment of their CNS cancer.2 Additionally, frontal and limbic tumors are more likely to present with psychiatric manifestations; up to 90% of patients exhibit altered mental status or personality changes, as did Ms. A.1,4 Clearly, it is easier to identify patients with psychiatric symptoms resulting from a brain tumor when they also present with focal neurologic deficits or systemic symptoms, such as headache or nausea and vomiting. Ms. A presented with severe headaches, which is what led to her early imaging and prompt diagnosis.
Numerous proposed mechanisms might account for the psychiatric symptoms that occur during the course of a brain tumor, including direct injury to neuronal cells, secretion of hormones or other tumor-derived substances, and peri-ictal phenomena.3
TREATMENT Tumor is removed, but memory is impaired
Ms. A is scheduled for craniotomy and surgical resection of the frontal mass. Prior to surgery, Ms. A shows interest in improving her health, cooperates with staff, and seeks her daughter’s input on treatment. One week after admission, Ms. A has her mass resected, which is confirmed on biopsy to be a lung metastasis. Post-surgery, Ms. A receives codeine, 30 mg every 6 hours as needed, for pain; she continues dexamethasone, 4 mg IV every 6 hours, for brain edema and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
On Day 2 after surgery, Ms. A attempts to elope. When she is approached by a psychiatrist on the treatment team, she does not recognize him. Although her long-term memory seems intact, she is unable to remember the details of recent events, including her medical and surgical treatments.
[polldaddy:10511745]
Continue to: The authors' observations
The authors’ observations
Ms. A’s memory impairment may be secondary to a surgically acquired neurocognitive deficit. In the United States, brain metastases represent a significant public health issue, affecting >100,000 patients per year.5 Metastatic lesions are the most common brain tumors. Lung cancer, breast cancer, and melanoma are the leading solid tumors to spread to the CNS.5 In cases of single brain metastasis, similar to Ms. A’s solitary left temporal lobe lesion, surgical resection plays a critical role in treatment. It provides histological confirmation of metastatic disease and can relieve mass effect if present. Studies have shown that combined surgical resection with radiation improves survival relative to patients who undergo radiation therapy alone.6,7
However, the benefits of surgical resection need to be balanced with preservation of neurologic function. Emerging evidence suggests that a majority of patients have surgically-acquired cognitive deficits due to damage of normal surrounding tissues, and these deficits are associated with reduced quality of life.8,9 Further, a study examining glioma surgical resections found that patients with left temporal lobe tumors exhibit more frequent and severe neurocognitive decline than patients with right temporal lobe tumors, especially in domains such as verbal memory.8 Ms. A’s memory impairment was persistent during her postoperative course, which suggests that it was not just an immediate post-surgical phenomenon, but a longer-lasting cognitive change directly related to the resection.
It is also possible that Ms. A had a prior neurocognitive disorder that manifested to a greater degree as a result of the CNS tumor. Ms. A might have had early-onset Alzheimer’s disease, although her intact memory before surgery makes this less likely. Alternatively, she could have had vascular dementia, especially given her long-standing hypertension and diabetes. This might have been missed in the initial evaluation because executive function was not tested. However, the relatively abrupt onset of memory problems after surgery suggests that she had no underlying neurocognitive disorder.
Ms. A’s presumed episode of MDD might also explain her memory changes. Major depressive disorder is increasingly common among geriatric patients, affecting approximately 5% of community-dwelling older adults.10 Its incidence increases with medical comorbidities, as suggested by depression rates of 5% to 10% in the primary care setting vs 37% in patients after critical-care hospitalizations.10 Late-life depression (LLD) occurs in adults age ≥60. Unlike depression in younger patients, LLD is more likely to be associated with cognitive impairment, specifically impairment of executive function and memory.11 The incidence of cognitive impairment in LLD is higher in patients with a history of depression, such as Ms. A.11,12 However, in general, patients who are depressed have memory complaints out of proportion to the clinical findings, and they show poor effort on cognitive testing. Ms. A exhibited neither of these, which makes it less likely that LLD was the exclusive cause of her memory loss.13 Table 214 outlines the management of cognitive deficits in a patient with a brain tumor.
EVALUATION Increasingly agitated and paranoid
After the tumor resection, Ms. A becomes increasingly irritable, uncooperative, and agitated. She repeatedly demands to be discharged. She insists she is fine and refuses medications and further laboratory workup. She becomes paranoid about the nursing staff and believes they are trying to kill her.
Continue to: On psychiatric re-evaluation...
On psychiatric re-evaluation, Ms. A demonstrates pressured speech, perseveration about going home, paranoid delusions, and anger at her family and physicians.
[polldaddy:10511747]
The authors’ observations
Ms. A’s refusal of medications and agitation may be explained by postoperative delirium, a surgical complication that is increasingly common among geriatric patients and is associated with poor clinical outcomes. Delirium is characterized by an acute onset and fluctuating course of symptoms that include inattention, motoric hypo- or hyperactivity, inappropriate behavior, emotional lability, cognitive dysfunction, and psychotic symptoms.15 Risk factors that contribute to postoperative delirium include older age, alcohol use, and poor baseline functional and cognitive status.16 The pathophysiology of delirium is not fully understood, but accumulating evidence suggests that different sets of interacting biologic factors (ie, neurotransmitters and inflammation) contribute to a disruption of large-scale neuronal networks in the brain, resulting in cognitive dysfunction.15 Patients who develop postoperative delirium are more likely to develop long-term cognitive dysfunction and have an increased risk of dementia.16
Another potential source of Ms. A’s agitation is steroid use. Ms. A received IV dexamethasone, 8 to 16 mg/d, around the time of her surgery. Steroids are commonly used to treat brain tumors, particularly when there is vasogenic edema. Steroid psychosis is a term loosely used to describe a wide range of psychiatric symptoms induced by corticosteroids that includes, but is not limited to, depression, mania, psychosis, delirium, and cognitive impairment.17 Steroid-induced psychiatric adverse effects occur in 5% to 18% of patients receiving corticosteroids and often happen early in treatment, although they can occur at any point.18 Corticosteroids influence brain activity via glucocorticoid and mineralocorticoid receptors. These receptors are widely distributed throughout the brain and affect neurotransmitter systems, such as the serotonergic system, that are associated with changes in mood, behavior, and cognition.17 While the adverse psychiatric manifestations of steroid use vary, higher dosages are associated with an increased risk of psychiatric complications; mania is more prevalent early in the course of treatment, and depression is more common with long-term use.17,19 Table 317,18 outlines the evidence-based treatment of corticosteroid-induced adverse psychiatric effects.
Although there are no clinical guidelines or FDA-approved medications for treating steroid-induced psychiatric adverse events, these are best managed by tapering and discontinuing steroids when possible and simultaneously using psychotropic medications to treat psychiatric symptoms. Case reports and limited evidence-based literature have demonstrated that steroid-induced mania responds to mood stabilizers or antipsychotics, while depression can be managed with antidepressants or lithium.17
Additionally, patients with CNS tumors are at risk for seizures and often are prescribed antiepileptics. Because it is easy to administer and does not need to be titrated, levetiracetam is a commonly used agent. However, levetiracetam can cause psychiatric adverse effects, including behavior changes and frank psychosis.20
Continue to: Finally, Ms. A's altered mental status...
Finally, Ms. A’s altered mental status could have been related to opioid intoxication. Opioids are used to manage postsurgical pain, and studies have shown these medications can be a precipitating factor for delirium in geriatric patients.21
TREATMENT Medication adjustments
At the request of the psychiatry team, levetiracetam is discontinued due to its potential for psychiatric adverse effects. The neurosurgery team replaces it with valproic acid, 500 mg every 12 hours. Ms. A is also tapered off steroids fairly rapidly because of the potential for steroid-induced psychiatric adverse effects. Her quetiapine is titrated from 50 to 150 mg every night at bedtime, and duloxetine is discontinued.
OUTCOME Agitation improves dramatically
Ms. A’s new medication regimen dramatically improves her agitation, which allows Ms. A, her family, and the medical team to work together to establish treatment goals. Ms. A ultimately returns home with the assistance of her family. She continues to have memory issues, but with improved emotion regulation. Several months later, Ms. A is readmitted to the hospital because her cancer has progressed despite treatment.
Bottom Line
Brain tumors may present with various psychiatric manifestations that can change during the course of the patient’s treatment. A comprehensive psychiatric evaluation should parse out the interplay between direct effects of the tumor and any adverse effects that are the result of medical and/or surgical interventions to determine the cause of psychiatric symptoms and their appropriate management.
Related Resource
Madhusoodanan S, Ting MB, Farah T, et al. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273-285.
Drug Brand Names
Aripiprazole • Abilify
Buspirone • Buspar
Chlorpromazine • Thorazine
Codeine • Codeine systemic
Dexamethasone • Decadron
Duloxetine • Cymbalta
Haloperidol • Haldol
Levetiracetam • Keppra
Lorazepam • Ativan
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakene
CASE Suicidal behavior, severe headaches
Ms. A, age 60, presents to the emergency department (ED) with depression, suicidal behavior, and 3 days of severe headaches. Neurology is consulted and an MRI is ordered, which shows a 3.0-cm mass lesion in the left temporal lobe with associated vasogenic edema that is suspicious for metastatic disease (Figure).
Ms. A is admitted to the hospital for further workup of her brain lesion. She is started on IV dexamethasone, 10 mg every 6 hours, a glucocorticosteroid, for brain edema, and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
Upon admission, in addition to oncology and neurosurgery, psychiatry is also consulted to evaluate Ms. A for depression and suicidality.
EVALUATION Mood changes and poor judgment
Ms. A has a psychiatric history of depression and alcohol use disorder but says she has not consumed any alcohol in years. Her medical history includes hypertension, diabetes, and stage 4 non-small–cell lung cancer, for which she received surgery and adjuvant chemoradiotherapy 1 year ago.
On initial intake, Ms. A reports that in addition to the headaches, she has also been experiencing worsening depression and suicidal behavior. For the past 2 months, she has had a severely depressed mood, with notable anhedonia, poor appetite, insomnia, low energy, and decreased concentration. The changes in her mental health were triggered by her mother’s death. Three days prior to admission, the patient planned to overdose on antihypertensive pills, but her suicide attempt was interrupted when her family called. She denies any current suicidal ideation, intent, or plan.
According to her family, Ms. A has been increasingly irritable and her personality has changed in the past month. She also has been repeatedly sorting through her neighbors’ garbage.
Ms. A’s current psychiatric medications are duloxetine, 30 mg/d; quetiapine, 50 mg every night at bedtime; and buspirone, 10 mg/d. However, it is unclear if she is consistently taking these medications.
Continue to: On mental status examination...
On mental status examination, Ms. A is calm and she has no abnormal movements. She says she is depressed. Her affect is reactive and labile. She is alert and oriented to person, place, and time. Her attention, registration, and recall are intact. Her executive function is not tested. However, Ms. A’s insight and judgment seem poor.
To address Ms. A’s worsening depression, the psychiatry team increases her duloxetine from 30 to 60 mg/d, and she continues quetiapine, 50 mg every night at bedtime, for mood lability. Buspirone is not continued because she was not taking a therapeutic dosage in the community.
Within 4 days, Ms. A shows improvement in sleep, appetite, and mood. She has no further suicidal ideation.
[polldaddy:10511743]
The authors’ observations
Ms. A had a recurrence of what was presumed to be major depressive disorder (MDD) in the context of her mother’s death. However, she also exhibited irritability, mood lability, and impulsivity, all of which could be part of her depression, or a separate problem related to her brain tumor. Because Ms. A had never displayed bizarre behavior before the past few weeks, it is likely that her CNS lesion was directly affecting her personality and possibly underlying her planned suicide attempt.
Fifty to 80% of patients with CNS tumors, either primary or metastatic, present with psychiatric symptoms.1 Table 11-3 lists common psychiatric symptoms of brain tumors. Unfortunately, there is little reliable evidence that directly correlates tumor location with specific psychiatric symptoms. A 2010 meta-analysis found a statistically significant link between anorexia nervosa and hypothalamic tumors.1 However, for other brain regions, there is only an increased likelihood that any given tumor location will produce psychiatric symptoms.1,4 For instance, compared to patients with tumors in other locations, those with temporal lobe tumors are more likely to present with mood disorders, personality changes, and memory problems.1 In contrast, patients with frontal lobe tumors have an increased likelihood of psychosis, mood disorders, and personality changes.1 Patients with tumors in the pituitary region often present with anxiety.1
Continue to: When considering treatment options...
When considering treatment options for Ms. A, alcohol withdrawal was unlikely given the remote history of alcohol use, low alcohol blood level, and lack of evidence of unstable vital signs or tremor. Although she might have benefited from inpatient psychiatric treatment, this needed to wait until there was a definitive treatment plan for her brain tumor. Finally, although a paraneoplastic syndrome, such as limbic encephalitis, could be causing her psychiatric symptoms, this scenario is less likely with non-small–cell lung cancer.
Although uncommon, CNS tumors can present with psychiatric symptoms as the only manifestation. This is more likely when a patient exhibits new-onset or atypical symptoms, or fails to respond to standard psychiatric treatment.4 Case reports have described patients with brain tumors being misdiagnosed as having a primary psychiatric condition, which delays treatment of their CNS cancer.2 Additionally, frontal and limbic tumors are more likely to present with psychiatric manifestations; up to 90% of patients exhibit altered mental status or personality changes, as did Ms. A.1,4 Clearly, it is easier to identify patients with psychiatric symptoms resulting from a brain tumor when they also present with focal neurologic deficits or systemic symptoms, such as headache or nausea and vomiting. Ms. A presented with severe headaches, which is what led to her early imaging and prompt diagnosis.
Numerous proposed mechanisms might account for the psychiatric symptoms that occur during the course of a brain tumor, including direct injury to neuronal cells, secretion of hormones or other tumor-derived substances, and peri-ictal phenomena.3
TREATMENT Tumor is removed, but memory is impaired
Ms. A is scheduled for craniotomy and surgical resection of the frontal mass. Prior to surgery, Ms. A shows interest in improving her health, cooperates with staff, and seeks her daughter’s input on treatment. One week after admission, Ms. A has her mass resected, which is confirmed on biopsy to be a lung metastasis. Post-surgery, Ms. A receives codeine, 30 mg every 6 hours as needed, for pain; she continues dexamethasone, 4 mg IV every 6 hours, for brain edema and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
On Day 2 after surgery, Ms. A attempts to elope. When she is approached by a psychiatrist on the treatment team, she does not recognize him. Although her long-term memory seems intact, she is unable to remember the details of recent events, including her medical and surgical treatments.
[polldaddy:10511745]
Continue to: The authors' observations
The authors’ observations
Ms. A’s memory impairment may be secondary to a surgically acquired neurocognitive deficit. In the United States, brain metastases represent a significant public health issue, affecting >100,000 patients per year.5 Metastatic lesions are the most common brain tumors. Lung cancer, breast cancer, and melanoma are the leading solid tumors to spread to the CNS.5 In cases of single brain metastasis, similar to Ms. A’s solitary left temporal lobe lesion, surgical resection plays a critical role in treatment. It provides histological confirmation of metastatic disease and can relieve mass effect if present. Studies have shown that combined surgical resection with radiation improves survival relative to patients who undergo radiation therapy alone.6,7
However, the benefits of surgical resection need to be balanced with preservation of neurologic function. Emerging evidence suggests that a majority of patients have surgically-acquired cognitive deficits due to damage of normal surrounding tissues, and these deficits are associated with reduced quality of life.8,9 Further, a study examining glioma surgical resections found that patients with left temporal lobe tumors exhibit more frequent and severe neurocognitive decline than patients with right temporal lobe tumors, especially in domains such as verbal memory.8 Ms. A’s memory impairment was persistent during her postoperative course, which suggests that it was not just an immediate post-surgical phenomenon, but a longer-lasting cognitive change directly related to the resection.
It is also possible that Ms. A had a prior neurocognitive disorder that manifested to a greater degree as a result of the CNS tumor. Ms. A might have had early-onset Alzheimer’s disease, although her intact memory before surgery makes this less likely. Alternatively, she could have had vascular dementia, especially given her long-standing hypertension and diabetes. This might have been missed in the initial evaluation because executive function was not tested. However, the relatively abrupt onset of memory problems after surgery suggests that she had no underlying neurocognitive disorder.
Ms. A’s presumed episode of MDD might also explain her memory changes. Major depressive disorder is increasingly common among geriatric patients, affecting approximately 5% of community-dwelling older adults.10 Its incidence increases with medical comorbidities, as suggested by depression rates of 5% to 10% in the primary care setting vs 37% in patients after critical-care hospitalizations.10 Late-life depression (LLD) occurs in adults age ≥60. Unlike depression in younger patients, LLD is more likely to be associated with cognitive impairment, specifically impairment of executive function and memory.11 The incidence of cognitive impairment in LLD is higher in patients with a history of depression, such as Ms. A.11,12 However, in general, patients who are depressed have memory complaints out of proportion to the clinical findings, and they show poor effort on cognitive testing. Ms. A exhibited neither of these, which makes it less likely that LLD was the exclusive cause of her memory loss.13 Table 214 outlines the management of cognitive deficits in a patient with a brain tumor.
EVALUATION Increasingly agitated and paranoid
After the tumor resection, Ms. A becomes increasingly irritable, uncooperative, and agitated. She repeatedly demands to be discharged. She insists she is fine and refuses medications and further laboratory workup. She becomes paranoid about the nursing staff and believes they are trying to kill her.
Continue to: On psychiatric re-evaluation...
On psychiatric re-evaluation, Ms. A demonstrates pressured speech, perseveration about going home, paranoid delusions, and anger at her family and physicians.
[polldaddy:10511747]
The authors’ observations
Ms. A’s refusal of medications and agitation may be explained by postoperative delirium, a surgical complication that is increasingly common among geriatric patients and is associated with poor clinical outcomes. Delirium is characterized by an acute onset and fluctuating course of symptoms that include inattention, motoric hypo- or hyperactivity, inappropriate behavior, emotional lability, cognitive dysfunction, and psychotic symptoms.15 Risk factors that contribute to postoperative delirium include older age, alcohol use, and poor baseline functional and cognitive status.16 The pathophysiology of delirium is not fully understood, but accumulating evidence suggests that different sets of interacting biologic factors (ie, neurotransmitters and inflammation) contribute to a disruption of large-scale neuronal networks in the brain, resulting in cognitive dysfunction.15 Patients who develop postoperative delirium are more likely to develop long-term cognitive dysfunction and have an increased risk of dementia.16
Another potential source of Ms. A’s agitation is steroid use. Ms. A received IV dexamethasone, 8 to 16 mg/d, around the time of her surgery. Steroids are commonly used to treat brain tumors, particularly when there is vasogenic edema. Steroid psychosis is a term loosely used to describe a wide range of psychiatric symptoms induced by corticosteroids that includes, but is not limited to, depression, mania, psychosis, delirium, and cognitive impairment.17 Steroid-induced psychiatric adverse effects occur in 5% to 18% of patients receiving corticosteroids and often happen early in treatment, although they can occur at any point.18 Corticosteroids influence brain activity via glucocorticoid and mineralocorticoid receptors. These receptors are widely distributed throughout the brain and affect neurotransmitter systems, such as the serotonergic system, that are associated with changes in mood, behavior, and cognition.17 While the adverse psychiatric manifestations of steroid use vary, higher dosages are associated with an increased risk of psychiatric complications; mania is more prevalent early in the course of treatment, and depression is more common with long-term use.17,19 Table 317,18 outlines the evidence-based treatment of corticosteroid-induced adverse psychiatric effects.
Although there are no clinical guidelines or FDA-approved medications for treating steroid-induced psychiatric adverse events, these are best managed by tapering and discontinuing steroids when possible and simultaneously using psychotropic medications to treat psychiatric symptoms. Case reports and limited evidence-based literature have demonstrated that steroid-induced mania responds to mood stabilizers or antipsychotics, while depression can be managed with antidepressants or lithium.17
Additionally, patients with CNS tumors are at risk for seizures and often are prescribed antiepileptics. Because it is easy to administer and does not need to be titrated, levetiracetam is a commonly used agent. However, levetiracetam can cause psychiatric adverse effects, including behavior changes and frank psychosis.20
Continue to: Finally, Ms. A's altered mental status...
Finally, Ms. A’s altered mental status could have been related to opioid intoxication. Opioids are used to manage postsurgical pain, and studies have shown these medications can be a precipitating factor for delirium in geriatric patients.21
TREATMENT Medication adjustments
At the request of the psychiatry team, levetiracetam is discontinued due to its potential for psychiatric adverse effects. The neurosurgery team replaces it with valproic acid, 500 mg every 12 hours. Ms. A is also tapered off steroids fairly rapidly because of the potential for steroid-induced psychiatric adverse effects. Her quetiapine is titrated from 50 to 150 mg every night at bedtime, and duloxetine is discontinued.
OUTCOME Agitation improves dramatically
Ms. A’s new medication regimen dramatically improves her agitation, which allows Ms. A, her family, and the medical team to work together to establish treatment goals. Ms. A ultimately returns home with the assistance of her family. She continues to have memory issues, but with improved emotion regulation. Several months later, Ms. A is readmitted to the hospital because her cancer has progressed despite treatment.
Bottom Line
Brain tumors may present with various psychiatric manifestations that can change during the course of the patient’s treatment. A comprehensive psychiatric evaluation should parse out the interplay between direct effects of the tumor and any adverse effects that are the result of medical and/or surgical interventions to determine the cause of psychiatric symptoms and their appropriate management.
Related Resource
Madhusoodanan S, Ting MB, Farah T, et al. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273-285.
Drug Brand Names
Aripiprazole • Abilify
Buspirone • Buspar
Chlorpromazine • Thorazine
Codeine • Codeine systemic
Dexamethasone • Decadron
Duloxetine • Cymbalta
Haloperidol • Haldol
Levetiracetam • Keppra
Lorazepam • Ativan
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakene
1. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
2. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
3. Pearl ML, Talgat G, Valea FA, et al. Psychiatric symptoms due to brain metastases. Med Update Psychiatr. 1998;3(4):91-94.
4. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343-349.
5. Ferguson SD, Wagner KM, Prabhu SS, et al. Neurosurgical management of brain metastases. Clin Exp Metastasis. 2017;34(6-7):377-389.
6. Husain ZA, Regine WF, Kwok Y, et al. Brain metastases: contemporary management and future directions. Eur J Clin Med Oncol. 2011;3(3):38-45.
7. Vecht CJ, Haaxmareiche H, Noordijk EM, et al. Treatment of single brain metastasis - radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993;33(6):583-590.
8. Barry RL, Byun NE, Tantawy MN, et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging Behav. 2018;12(1):87-95.
9. Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125.
10. Taylor WD. Depression in the elderly. N Engl J Med. 2014;371(13):1228-1236.
11. Liguori C, Pierantozzi M, Chiaravalloti A, et al. When cognitive decline and depression coexist in the elderly: CSF biomarkers analysis can differentiate Alzheimer’s disease from late-life depression. Front Aging Neurosci. 2018;10:38.
12. Luijendijk HJ, van den Berg JF, Dekker MJHJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394-1401.
13. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-117.
14. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168.
15. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
16. Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-323.
17. Kusljic S, Manias E, Gogos A. Corticosteroid-induced psychiatric disturbances: it is time for pharmacists to take notice. Res Soc Adm Pharm. 2016;12(2):355-360.
18. Cerullo MA. Corticosteroid-induced mania: prepare for the unpredictable. Current Psychiatry. 2006;5(6):43-50.
19. Dubovsky AN, Arvikar S, Stern TA, et al. Steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
20. Habets JGV, Leentjens AFG, Schijns OEMG. Serious and reversible levetiracetam-induced psychiatric symptoms after resection of frontal low-grade glioma: two case histories. Br J Neurosurg. 2017;31(4):471-473.
21
1. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
2. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
3. Pearl ML, Talgat G, Valea FA, et al. Psychiatric symptoms due to brain metastases. Med Update Psychiatr. 1998;3(4):91-94.
4. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343-349.
5. Ferguson SD, Wagner KM, Prabhu SS, et al. Neurosurgical management of brain metastases. Clin Exp Metastasis. 2017;34(6-7):377-389.
6. Husain ZA, Regine WF, Kwok Y, et al. Brain metastases: contemporary management and future directions. Eur J Clin Med Oncol. 2011;3(3):38-45.
7. Vecht CJ, Haaxmareiche H, Noordijk EM, et al. Treatment of single brain metastasis - radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993;33(6):583-590.
8. Barry RL, Byun NE, Tantawy MN, et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging Behav. 2018;12(1):87-95.
9. Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125.
10. Taylor WD. Depression in the elderly. N Engl J Med. 2014;371(13):1228-1236.
11. Liguori C, Pierantozzi M, Chiaravalloti A, et al. When cognitive decline and depression coexist in the elderly: CSF biomarkers analysis can differentiate Alzheimer’s disease from late-life depression. Front Aging Neurosci. 2018;10:38.
12. Luijendijk HJ, van den Berg JF, Dekker MJHJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394-1401.
13. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-117.
14. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168.
15. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
16. Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-323.
17. Kusljic S, Manias E, Gogos A. Corticosteroid-induced psychiatric disturbances: it is time for pharmacists to take notice. Res Soc Adm Pharm. 2016;12(2):355-360.
18. Cerullo MA. Corticosteroid-induced mania: prepare for the unpredictable. Current Psychiatry. 2006;5(6):43-50.
19. Dubovsky AN, Arvikar S, Stern TA, et al. Steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
20. Habets JGV, Leentjens AFG, Schijns OEMG. Serious and reversible levetiracetam-induced psychiatric symptoms after resection of frontal low-grade glioma: two case histories. Br J Neurosurg. 2017;31(4):471-473.
21
Depression in MS predicted worsening of neurologic function
WEST PALM BEACH, FLA. – Among patients with relapsing-remitting multiple sclerosis (MS), depression increases the likelihood of having worse neurologic function one year later, according to a study presented at ACTRIMS Forum 2020. Patients’ subjective descriptions of disease activity did not significantly change during that time, which “suggests that depression is not merely a reactive phenomenon, but rather an independent contributor to clinical worsening in the long term,” said Jenny Feng, MD, a neuroimmunology fellow at the Mellen Center for MS Treatment and Research at the Cleveland Clinic.
The researchers hypothesize that depression’s influence on psychomotor function may contribute to clinical worsening in MS.
More than half of patients with MS have depression, and there is a higher prevalence of depression in relapsing-remitting MS than in progressive disease. “Depression is associated with systemic inflammation,” Dr. Feng said at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “We know that depressed individuals tend to have slower walking speeds, slower processing speeds, and worse quality of life measures.” But neurologists do not know whether patients feel depressed because the disease is getting worse, or whether depression is an independent contributing factor to MS, Dr. Feng said.
To examine whether depression affects neurologic performance and disease activity in patients with MS, Dr. Feng and colleagues analyzed real-world data from about 2,400 patients in MS PATHS (Multiple Sclerosis Partners Advancing Technology and Health Solutions), a network of centers in the United States and Europe. The researchers assessed the longitudinal relationship between depression, measures of neurologic function, and MRI metrics.
The researchers included patients with relapsing-remitting MS who had clinical and imaging data available at baseline and about 1 year later. Patients completed tests of manual dexterity, walking speed, and processing speed that are based on the Multiple Sclerosis Functional Composite. A worsening of 20% on any measure is considered clinically significant.
Patients had a mean age of about 45 years and mean disease duration of about 14 years. Patients with a T score greater than 45 on the Neuro-QoL depression questionnaire were classified as having depression, and approximately half of the population had depression. Patients with depression were more likely to have an employment status of disabled and to receive infusion medications.
The investigators used propensity score analysis to adjust for baseline differences between patients with and without depression and evaluated the effect of depression on year 1 outcome measures using logistic regression for categorical variables and linear regression for continuous variables.
“After propensity weighting for baseline covariates including neuroperformance scores, individuals with depression continued to worsen,” Dr. Feng said. Patients with depression were more likely to have a 20% worsening in at least one measure of neurologic performance at year 1 (odds ratio, 1.31). “There was a trend for increased odds of interval relapses, increased T2 lesion burden, and contrast-enhancing lesions at year 1” in patients with depression, but the results were not statistically significant. “Despite worsening neuroperformance at year 1 in individuals with baseline depression, their [patient-reported outcomes] at year 1 were not significantly worse.”
The researcher lacked information about the date of depression onset and medication compliance, Dr. Feng said. In addition, propensity weighting does not account for potential bias due to missing data.
The findings support the existing practice of actively screening for and treating depression in patients with MS, Dr. Feng said.
Dr. Feng had no disclosures. Coauthors have consulted for and received research support from pharmaceutical companies. MS PATHS is supported by Biogen.
SOURCE: Feng JJ et al. ACTRIMS Forum 2020. Abstract P226.
WEST PALM BEACH, FLA. – Among patients with relapsing-remitting multiple sclerosis (MS), depression increases the likelihood of having worse neurologic function one year later, according to a study presented at ACTRIMS Forum 2020. Patients’ subjective descriptions of disease activity did not significantly change during that time, which “suggests that depression is not merely a reactive phenomenon, but rather an independent contributor to clinical worsening in the long term,” said Jenny Feng, MD, a neuroimmunology fellow at the Mellen Center for MS Treatment and Research at the Cleveland Clinic.
The researchers hypothesize that depression’s influence on psychomotor function may contribute to clinical worsening in MS.
More than half of patients with MS have depression, and there is a higher prevalence of depression in relapsing-remitting MS than in progressive disease. “Depression is associated with systemic inflammation,” Dr. Feng said at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “We know that depressed individuals tend to have slower walking speeds, slower processing speeds, and worse quality of life measures.” But neurologists do not know whether patients feel depressed because the disease is getting worse, or whether depression is an independent contributing factor to MS, Dr. Feng said.
To examine whether depression affects neurologic performance and disease activity in patients with MS, Dr. Feng and colleagues analyzed real-world data from about 2,400 patients in MS PATHS (Multiple Sclerosis Partners Advancing Technology and Health Solutions), a network of centers in the United States and Europe. The researchers assessed the longitudinal relationship between depression, measures of neurologic function, and MRI metrics.
The researchers included patients with relapsing-remitting MS who had clinical and imaging data available at baseline and about 1 year later. Patients completed tests of manual dexterity, walking speed, and processing speed that are based on the Multiple Sclerosis Functional Composite. A worsening of 20% on any measure is considered clinically significant.
Patients had a mean age of about 45 years and mean disease duration of about 14 years. Patients with a T score greater than 45 on the Neuro-QoL depression questionnaire were classified as having depression, and approximately half of the population had depression. Patients with depression were more likely to have an employment status of disabled and to receive infusion medications.
The investigators used propensity score analysis to adjust for baseline differences between patients with and without depression and evaluated the effect of depression on year 1 outcome measures using logistic regression for categorical variables and linear regression for continuous variables.
“After propensity weighting for baseline covariates including neuroperformance scores, individuals with depression continued to worsen,” Dr. Feng said. Patients with depression were more likely to have a 20% worsening in at least one measure of neurologic performance at year 1 (odds ratio, 1.31). “There was a trend for increased odds of interval relapses, increased T2 lesion burden, and contrast-enhancing lesions at year 1” in patients with depression, but the results were not statistically significant. “Despite worsening neuroperformance at year 1 in individuals with baseline depression, their [patient-reported outcomes] at year 1 were not significantly worse.”
The researcher lacked information about the date of depression onset and medication compliance, Dr. Feng said. In addition, propensity weighting does not account for potential bias due to missing data.
The findings support the existing practice of actively screening for and treating depression in patients with MS, Dr. Feng said.
Dr. Feng had no disclosures. Coauthors have consulted for and received research support from pharmaceutical companies. MS PATHS is supported by Biogen.
SOURCE: Feng JJ et al. ACTRIMS Forum 2020. Abstract P226.
WEST PALM BEACH, FLA. – Among patients with relapsing-remitting multiple sclerosis (MS), depression increases the likelihood of having worse neurologic function one year later, according to a study presented at ACTRIMS Forum 2020. Patients’ subjective descriptions of disease activity did not significantly change during that time, which “suggests that depression is not merely a reactive phenomenon, but rather an independent contributor to clinical worsening in the long term,” said Jenny Feng, MD, a neuroimmunology fellow at the Mellen Center for MS Treatment and Research at the Cleveland Clinic.
The researchers hypothesize that depression’s influence on psychomotor function may contribute to clinical worsening in MS.
More than half of patients with MS have depression, and there is a higher prevalence of depression in relapsing-remitting MS than in progressive disease. “Depression is associated with systemic inflammation,” Dr. Feng said at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “We know that depressed individuals tend to have slower walking speeds, slower processing speeds, and worse quality of life measures.” But neurologists do not know whether patients feel depressed because the disease is getting worse, or whether depression is an independent contributing factor to MS, Dr. Feng said.
To examine whether depression affects neurologic performance and disease activity in patients with MS, Dr. Feng and colleagues analyzed real-world data from about 2,400 patients in MS PATHS (Multiple Sclerosis Partners Advancing Technology and Health Solutions), a network of centers in the United States and Europe. The researchers assessed the longitudinal relationship between depression, measures of neurologic function, and MRI metrics.
The researchers included patients with relapsing-remitting MS who had clinical and imaging data available at baseline and about 1 year later. Patients completed tests of manual dexterity, walking speed, and processing speed that are based on the Multiple Sclerosis Functional Composite. A worsening of 20% on any measure is considered clinically significant.
Patients had a mean age of about 45 years and mean disease duration of about 14 years. Patients with a T score greater than 45 on the Neuro-QoL depression questionnaire were classified as having depression, and approximately half of the population had depression. Patients with depression were more likely to have an employment status of disabled and to receive infusion medications.
The investigators used propensity score analysis to adjust for baseline differences between patients with and without depression and evaluated the effect of depression on year 1 outcome measures using logistic regression for categorical variables and linear regression for continuous variables.
“After propensity weighting for baseline covariates including neuroperformance scores, individuals with depression continued to worsen,” Dr. Feng said. Patients with depression were more likely to have a 20% worsening in at least one measure of neurologic performance at year 1 (odds ratio, 1.31). “There was a trend for increased odds of interval relapses, increased T2 lesion burden, and contrast-enhancing lesions at year 1” in patients with depression, but the results were not statistically significant. “Despite worsening neuroperformance at year 1 in individuals with baseline depression, their [patient-reported outcomes] at year 1 were not significantly worse.”
The researcher lacked information about the date of depression onset and medication compliance, Dr. Feng said. In addition, propensity weighting does not account for potential bias due to missing data.
The findings support the existing practice of actively screening for and treating depression in patients with MS, Dr. Feng said.
Dr. Feng had no disclosures. Coauthors have consulted for and received research support from pharmaceutical companies. MS PATHS is supported by Biogen.
SOURCE: Feng JJ et al. ACTRIMS Forum 2020. Abstract P226.
REPORTING FROM ACTRIMS Forum 2020
How to beat bullying in the workplace
Cyberbullying can prove particularly insidious
Bullying happens to our patients and sometimes to the doctors in the medical community. As psychiatrists, we need to share information on how to spot it and deal with it in the workplace.
We can view bullying as the endpoint in a continuum with authority at one end and harassment at the other extreme. Discipline maintains order but those in charge can be misguided or mean spirited.
Bullying is bad and prevalent, but is it inevitable in the workplace? There are three categories: those who get bullied, those who bully, and those who witness bullying. Any one, two, or even all three can apply in a work environment. Some escape the problem, and for them, bullying remains theoretical, a phenomenon to understand.
How do we define bullying? You know it when you see it; bullying interferes with functioning. It includes harsh language, threats, snubbing, screaming, and undermining.
Case is illustrative
Helen, a medical consultant on a surgical unit, was reading a chart when another internist arrived for the same purpose. He introduced himself as a new full-time assistant to the head of medical consultations. Helen greeted him and said: “Since I started with this case, I will continue. There was probably an error in the referral process.” Bill looked concerned. “But he has uncontrolled diabetes.” Taken aback, Helen said: “I think I can handle it. I’ve been on the hospital staff for 25 years.”
Then the bullying began. On occasion, Bill and a resident consulted on patients Helen was treating already, as though her input were nonexistent. When Helen inquired about this, rather than attribute it to an error in communication within a large hospital, Bill diminished the value of her input. She asked, “How many medical consultants does a patient need?” She decided to confront Bill and tell him that he had no reason to treat her with disrespect. After that, Bill’s disparaging remarks intensified and he threatened her saying, “I’m not someone you want to go up against.” Bill sent her an email, “You are demeaning and harsh to the staff; if you want to retain your hospital credentials you must change your behavior.” In her response, Helen agreed to meet with Bill and she emailed, “It is not in my nature to mistreat anyone, staff or patient.” The meeting never happened.
Helen sought me out for psychiatric consultation and psychotherapy because she felt demoralized. Confused by Bill’s assault on her reputation, she needed a strategy and confirmation of her worth. We conceived a plan. Helen decided to get busy and get better. She redoubled her efforts to be cordial, and she remained effective with her patients. I suggested that she confide in a trusted senior attending at the hospital, which she did. She aired her insights to him. Excellence mattered and the threats disappeared. Bill had no power over Helen after all. She was a voluntary attending. She never succumbed to despair; rather she converted her response to the threats into useful energy.
When does authority become harassment?
A pecking order exists in every organization because, from the CEO to the janitor, it is necessary to maintain productivity. But when does this hierarchy become abusive? Discipline gets learned early. Those who are familiar with the comic strip “Calvin and Hobbes” by Bill Watterson may remember the 6-year-old boy asserting his intention to stay home from school – only to be forced to the bus stop by his mother. Call that authority, discipline, or even bullying, but it represents a child’s first encounter with obedience despite protest. When authority interferes rather than enhances effectiveness, question the methods used to attain order.
The vulnerable
If you do your job and you do it well, there should be no bullying. It is hard to know why a target gets chosen for harassment. However, some questions may need answering by the target. Does he or she avoid conflict even when there is bad behavior? Does past trauma immobilize him into passivity? Such issues necessitate self examination. Psychotherapy helps to uncover and clear up these issues.
Is bullying a fact of life?
In “Lord of the Flies,” William Golding portrays a fictional group of unsupervised boys abandoned on an island. An initial hierarchy descends into threats and eventual violence. Consider the animal world. In the wild, a wolf pack isolates a caribou from the herd to kill and devour. On a farm, llamas raised for yarn establish which llama is in charge. Those cases illustrate the hierarchy that exists because there is the need for food or reproductive prowess.
In the workplace, isolation of the target is common when authority extends to bullying. According to Robert Sapolsky, PhD, a neuroscientist and author, there are biological underpinnings for group conformity. This implies that colleagues who stand apart feel distress and get relief when they join the ganging up on a target. Those who watch harassment may hide from confronting it or even from pointing it out to protect themselves.
How do bullies think?
Challenge the bully at your own peril, because expecting a bully to change is futile. Recall Helen’s confrontation with Bill. It provoked him. His power to harass her came from his perceived position. Bullies regard the pleasant person as weak. Bullies can fall into two categories: Sadists who get pleasure from seeing others suffer, and opportunists. The latter focus only on their goals and disregard concerns expressed by others. Outside of the workplace, they may be reasonable. If workplace morale deteriorates along with productivity, the bully gets ousted. Otherwise, companies usually protect high performers at the expense of targets.
Is bullying different in medicine?
It can happen in a training program. The ingredients for bullying exist, including imbalance of power. Often, there are no witnesses, and there can be lack of accountability because of changing authorities. Just as technology can help make harassment possible, it also enables the target to spot and document inappropriate communications by saving emails and texts. Whenever a need for advancement exists along with changing authority, bullying gets tolerated. Who wants to be derailed by reporting? Those in training have the goal of completing a program. If they report bullying, they fear antagonism and retribution, a personal expense that can deter advancement.
Remedies
Let truth and fairness be your guide. That is easy to say and hard to do, but there are helpful personal and legal resources.
Personal capability
Get busy; get better. That became Helen’s method of choice. She focused on her role and productivity, not on her hurt. Helen shunned victimhood. With the help of psychotherapy and by confiding in a mentor, she prevailed. What works is recalling challenges that were mastered and the qualities that made for success. Acquire skills, build a good reputation, be assertive, not defensive.
The group is powerful, and that means it is important to build alliances above and below in the organizational hierarchy; cultivate friendship with trustworthy people. Occasionally, there is unwarranted ganging up on a manager, bullying from below. It is more likely to happen to a newly appointed supervisor. A way to avert that is to communicate with staff throughout the institution and remain accessible.
Legal options
What are legal options to confront bullying? Of note, workplace bullying is not necessarily illegal. According to one employment attorney, “There is no law that prohibits uncivil behavior on the job.” However, under Title VII of the federal law enacted in 1964, there are protected characteristics such as race, color, national origin, sex, age, and disability. The Equal Employment Opportunity Commission enforces Title VII. In cases of assault or stalking, harassment is illegal and criminal.
Some employees report to Human Resources or seek out their company’s employee assistance program. As useful as those options may be, they are part of the company and potentially partial to the administration. There can be incentives to protect those with power against a complainant. For assistance, it is preferable to enlist an outside attorney and a therapist in the community.
Advocacy exists. The Workplace Bullying Institute maintains a website, holds workshops, promotes literature, and offers information. The National Employment Lawyers Association can provide referrals or recommendations that come from other legal sources. Cases rarely reach court because of the expense of a trial; rather, the parties reach a financial settlement. When there is cause, an employment attorney can best pursue justice for the worker.
Conclusion
Get busy; get better is the solution to bullying. Avoid victimhood. That means prepare: Update the resume, seek opportunities, and identify allies. Bullies get beaten; as Abraham Lincoln said, “You can fool some of the people some of the time but you can’t fool all of the people all of the time.”
According to the Workplace Bullying Institute, 7 out of 10 bullied workers either resign or get fired. You should leave only when the leaving is better than the staying. Bullying brings out the worst in the workplace. Those who bully isolate the target. Coworkers often shun the target, fearing for their own position; they may even participate in the harassment. Psychiatrists need to remain sensitive to harassment in their own environment and for their patients. We have tools to address bullying in the workplace and a moral responsibility to combat it.
References
Workplace Bullying Institute (WBI)
National Employment Lawyers Association (NELA)
“Ozymandias” by Percy Bysshe Shelley
BY BEN HINDELL, PSY.D.
Cyberbullying is willful, repeated harm inflicted with the use of computers, cell phones, and other electronic devices. In some cases, a single message may be viewed by multiple people because the text and pictures are posted elsewhere.
Technology makes is possible to harass at any hour. The concept of willful harm is essential. Without interaction between sender and recipient, nuances are lost. Face to face might make a communication benign instead of malevolent or threatening. This has implications for the workplace, where colleagues increasingly communicate by email rather than discuss matters in person or by telephone.
Steps for survivors of cyberbullying
- Do not respond immediately to an inflammatory message, post, or email. Gather your thoughts and avoid responding in anger.
- Keep calm and rational, not emotional.
- Try to respond in person and work to avoid a conflict.
- Remember, your interpretation may differ from what was intended.
- Communicate openly and honestly and not defensively.
- Calmly indicate you were offended and you want the comments to stop.
- Move up the chain of command, if comments don’t cease.
- Save all messages and posts as evidence.
- Report the cyberbullying to your employer. Human resources may get involved.
- Detach from the cyberbully, if it continues. Block social media, cell phone messaging, and emails.
- Find support from friends, family, and a psychotherapist, if needed. As a last resort, it may become necessary to enlist an attorney.
- Take the high road; remain calm and professional at work. The bully may be seeking a reaction from the behavior. Prevent it.
All of the elements of workplace bullying apply to cyberbullying, but the latter can be more insidious. Psychiatrists and psychologists are able to support patients who deal with cyberbullying and help them cope successfully.
Dr. Cohen is in private practice and is a clinical assistant professor of psychiatry at Weill Cornell Medical Center of New York-Presbyterian Hospital, and psychiatric consultant at the Hospital for Special Surgery, also in New York. She made changes to the patient’s story to protect confidentiality.
Dr. Hindell is a psychologist with the Mental Health Service of Colorado College, Colorado Springs. He also practices psychotherapy in Denver. Dr. Hindell is the son of Dr. Cohen.
Cyberbullying can prove particularly insidious
Cyberbullying can prove particularly insidious
Bullying happens to our patients and sometimes to the doctors in the medical community. As psychiatrists, we need to share information on how to spot it and deal with it in the workplace.
We can view bullying as the endpoint in a continuum with authority at one end and harassment at the other extreme. Discipline maintains order but those in charge can be misguided or mean spirited.
Bullying is bad and prevalent, but is it inevitable in the workplace? There are three categories: those who get bullied, those who bully, and those who witness bullying. Any one, two, or even all three can apply in a work environment. Some escape the problem, and for them, bullying remains theoretical, a phenomenon to understand.
How do we define bullying? You know it when you see it; bullying interferes with functioning. It includes harsh language, threats, snubbing, screaming, and undermining.
Case is illustrative
Helen, a medical consultant on a surgical unit, was reading a chart when another internist arrived for the same purpose. He introduced himself as a new full-time assistant to the head of medical consultations. Helen greeted him and said: “Since I started with this case, I will continue. There was probably an error in the referral process.” Bill looked concerned. “But he has uncontrolled diabetes.” Taken aback, Helen said: “I think I can handle it. I’ve been on the hospital staff for 25 years.”
Then the bullying began. On occasion, Bill and a resident consulted on patients Helen was treating already, as though her input were nonexistent. When Helen inquired about this, rather than attribute it to an error in communication within a large hospital, Bill diminished the value of her input. She asked, “How many medical consultants does a patient need?” She decided to confront Bill and tell him that he had no reason to treat her with disrespect. After that, Bill’s disparaging remarks intensified and he threatened her saying, “I’m not someone you want to go up against.” Bill sent her an email, “You are demeaning and harsh to the staff; if you want to retain your hospital credentials you must change your behavior.” In her response, Helen agreed to meet with Bill and she emailed, “It is not in my nature to mistreat anyone, staff or patient.” The meeting never happened.
Helen sought me out for psychiatric consultation and psychotherapy because she felt demoralized. Confused by Bill’s assault on her reputation, she needed a strategy and confirmation of her worth. We conceived a plan. Helen decided to get busy and get better. She redoubled her efforts to be cordial, and she remained effective with her patients. I suggested that she confide in a trusted senior attending at the hospital, which she did. She aired her insights to him. Excellence mattered and the threats disappeared. Bill had no power over Helen after all. She was a voluntary attending. She never succumbed to despair; rather she converted her response to the threats into useful energy.
When does authority become harassment?
A pecking order exists in every organization because, from the CEO to the janitor, it is necessary to maintain productivity. But when does this hierarchy become abusive? Discipline gets learned early. Those who are familiar with the comic strip “Calvin and Hobbes” by Bill Watterson may remember the 6-year-old boy asserting his intention to stay home from school – only to be forced to the bus stop by his mother. Call that authority, discipline, or even bullying, but it represents a child’s first encounter with obedience despite protest. When authority interferes rather than enhances effectiveness, question the methods used to attain order.
The vulnerable
If you do your job and you do it well, there should be no bullying. It is hard to know why a target gets chosen for harassment. However, some questions may need answering by the target. Does he or she avoid conflict even when there is bad behavior? Does past trauma immobilize him into passivity? Such issues necessitate self examination. Psychotherapy helps to uncover and clear up these issues.
Is bullying a fact of life?
In “Lord of the Flies,” William Golding portrays a fictional group of unsupervised boys abandoned on an island. An initial hierarchy descends into threats and eventual violence. Consider the animal world. In the wild, a wolf pack isolates a caribou from the herd to kill and devour. On a farm, llamas raised for yarn establish which llama is in charge. Those cases illustrate the hierarchy that exists because there is the need for food or reproductive prowess.
In the workplace, isolation of the target is common when authority extends to bullying. According to Robert Sapolsky, PhD, a neuroscientist and author, there are biological underpinnings for group conformity. This implies that colleagues who stand apart feel distress and get relief when they join the ganging up on a target. Those who watch harassment may hide from confronting it or even from pointing it out to protect themselves.
How do bullies think?
Challenge the bully at your own peril, because expecting a bully to change is futile. Recall Helen’s confrontation with Bill. It provoked him. His power to harass her came from his perceived position. Bullies regard the pleasant person as weak. Bullies can fall into two categories: Sadists who get pleasure from seeing others suffer, and opportunists. The latter focus only on their goals and disregard concerns expressed by others. Outside of the workplace, they may be reasonable. If workplace morale deteriorates along with productivity, the bully gets ousted. Otherwise, companies usually protect high performers at the expense of targets.
Is bullying different in medicine?
It can happen in a training program. The ingredients for bullying exist, including imbalance of power. Often, there are no witnesses, and there can be lack of accountability because of changing authorities. Just as technology can help make harassment possible, it also enables the target to spot and document inappropriate communications by saving emails and texts. Whenever a need for advancement exists along with changing authority, bullying gets tolerated. Who wants to be derailed by reporting? Those in training have the goal of completing a program. If they report bullying, they fear antagonism and retribution, a personal expense that can deter advancement.
Remedies
Let truth and fairness be your guide. That is easy to say and hard to do, but there are helpful personal and legal resources.
Personal capability
Get busy; get better. That became Helen’s method of choice. She focused on her role and productivity, not on her hurt. Helen shunned victimhood. With the help of psychotherapy and by confiding in a mentor, she prevailed. What works is recalling challenges that were mastered and the qualities that made for success. Acquire skills, build a good reputation, be assertive, not defensive.
The group is powerful, and that means it is important to build alliances above and below in the organizational hierarchy; cultivate friendship with trustworthy people. Occasionally, there is unwarranted ganging up on a manager, bullying from below. It is more likely to happen to a newly appointed supervisor. A way to avert that is to communicate with staff throughout the institution and remain accessible.
Legal options
What are legal options to confront bullying? Of note, workplace bullying is not necessarily illegal. According to one employment attorney, “There is no law that prohibits uncivil behavior on the job.” However, under Title VII of the federal law enacted in 1964, there are protected characteristics such as race, color, national origin, sex, age, and disability. The Equal Employment Opportunity Commission enforces Title VII. In cases of assault or stalking, harassment is illegal and criminal.
Some employees report to Human Resources or seek out their company’s employee assistance program. As useful as those options may be, they are part of the company and potentially partial to the administration. There can be incentives to protect those with power against a complainant. For assistance, it is preferable to enlist an outside attorney and a therapist in the community.
Advocacy exists. The Workplace Bullying Institute maintains a website, holds workshops, promotes literature, and offers information. The National Employment Lawyers Association can provide referrals or recommendations that come from other legal sources. Cases rarely reach court because of the expense of a trial; rather, the parties reach a financial settlement. When there is cause, an employment attorney can best pursue justice for the worker.
Conclusion
Get busy; get better is the solution to bullying. Avoid victimhood. That means prepare: Update the resume, seek opportunities, and identify allies. Bullies get beaten; as Abraham Lincoln said, “You can fool some of the people some of the time but you can’t fool all of the people all of the time.”
According to the Workplace Bullying Institute, 7 out of 10 bullied workers either resign or get fired. You should leave only when the leaving is better than the staying. Bullying brings out the worst in the workplace. Those who bully isolate the target. Coworkers often shun the target, fearing for their own position; they may even participate in the harassment. Psychiatrists need to remain sensitive to harassment in their own environment and for their patients. We have tools to address bullying in the workplace and a moral responsibility to combat it.
References
Workplace Bullying Institute (WBI)
National Employment Lawyers Association (NELA)
“Ozymandias” by Percy Bysshe Shelley
BY BEN HINDELL, PSY.D.
Cyberbullying is willful, repeated harm inflicted with the use of computers, cell phones, and other electronic devices. In some cases, a single message may be viewed by multiple people because the text and pictures are posted elsewhere.
Technology makes is possible to harass at any hour. The concept of willful harm is essential. Without interaction between sender and recipient, nuances are lost. Face to face might make a communication benign instead of malevolent or threatening. This has implications for the workplace, where colleagues increasingly communicate by email rather than discuss matters in person or by telephone.
Steps for survivors of cyberbullying
- Do not respond immediately to an inflammatory message, post, or email. Gather your thoughts and avoid responding in anger.
- Keep calm and rational, not emotional.
- Try to respond in person and work to avoid a conflict.
- Remember, your interpretation may differ from what was intended.
- Communicate openly and honestly and not defensively.
- Calmly indicate you were offended and you want the comments to stop.
- Move up the chain of command, if comments don’t cease.
- Save all messages and posts as evidence.
- Report the cyberbullying to your employer. Human resources may get involved.
- Detach from the cyberbully, if it continues. Block social media, cell phone messaging, and emails.
- Find support from friends, family, and a psychotherapist, if needed. As a last resort, it may become necessary to enlist an attorney.
- Take the high road; remain calm and professional at work. The bully may be seeking a reaction from the behavior. Prevent it.
All of the elements of workplace bullying apply to cyberbullying, but the latter can be more insidious. Psychiatrists and psychologists are able to support patients who deal with cyberbullying and help them cope successfully.
Dr. Cohen is in private practice and is a clinical assistant professor of psychiatry at Weill Cornell Medical Center of New York-Presbyterian Hospital, and psychiatric consultant at the Hospital for Special Surgery, also in New York. She made changes to the patient’s story to protect confidentiality.
Dr. Hindell is a psychologist with the Mental Health Service of Colorado College, Colorado Springs. He also practices psychotherapy in Denver. Dr. Hindell is the son of Dr. Cohen.
Bullying happens to our patients and sometimes to the doctors in the medical community. As psychiatrists, we need to share information on how to spot it and deal with it in the workplace.
We can view bullying as the endpoint in a continuum with authority at one end and harassment at the other extreme. Discipline maintains order but those in charge can be misguided or mean spirited.
Bullying is bad and prevalent, but is it inevitable in the workplace? There are three categories: those who get bullied, those who bully, and those who witness bullying. Any one, two, or even all three can apply in a work environment. Some escape the problem, and for them, bullying remains theoretical, a phenomenon to understand.
How do we define bullying? You know it when you see it; bullying interferes with functioning. It includes harsh language, threats, snubbing, screaming, and undermining.
Case is illustrative
Helen, a medical consultant on a surgical unit, was reading a chart when another internist arrived for the same purpose. He introduced himself as a new full-time assistant to the head of medical consultations. Helen greeted him and said: “Since I started with this case, I will continue. There was probably an error in the referral process.” Bill looked concerned. “But he has uncontrolled diabetes.” Taken aback, Helen said: “I think I can handle it. I’ve been on the hospital staff for 25 years.”
Then the bullying began. On occasion, Bill and a resident consulted on patients Helen was treating already, as though her input were nonexistent. When Helen inquired about this, rather than attribute it to an error in communication within a large hospital, Bill diminished the value of her input. She asked, “How many medical consultants does a patient need?” She decided to confront Bill and tell him that he had no reason to treat her with disrespect. After that, Bill’s disparaging remarks intensified and he threatened her saying, “I’m not someone you want to go up against.” Bill sent her an email, “You are demeaning and harsh to the staff; if you want to retain your hospital credentials you must change your behavior.” In her response, Helen agreed to meet with Bill and she emailed, “It is not in my nature to mistreat anyone, staff or patient.” The meeting never happened.
Helen sought me out for psychiatric consultation and psychotherapy because she felt demoralized. Confused by Bill’s assault on her reputation, she needed a strategy and confirmation of her worth. We conceived a plan. Helen decided to get busy and get better. She redoubled her efforts to be cordial, and she remained effective with her patients. I suggested that she confide in a trusted senior attending at the hospital, which she did. She aired her insights to him. Excellence mattered and the threats disappeared. Bill had no power over Helen after all. She was a voluntary attending. She never succumbed to despair; rather she converted her response to the threats into useful energy.
When does authority become harassment?
A pecking order exists in every organization because, from the CEO to the janitor, it is necessary to maintain productivity. But when does this hierarchy become abusive? Discipline gets learned early. Those who are familiar with the comic strip “Calvin and Hobbes” by Bill Watterson may remember the 6-year-old boy asserting his intention to stay home from school – only to be forced to the bus stop by his mother. Call that authority, discipline, or even bullying, but it represents a child’s first encounter with obedience despite protest. When authority interferes rather than enhances effectiveness, question the methods used to attain order.
The vulnerable
If you do your job and you do it well, there should be no bullying. It is hard to know why a target gets chosen for harassment. However, some questions may need answering by the target. Does he or she avoid conflict even when there is bad behavior? Does past trauma immobilize him into passivity? Such issues necessitate self examination. Psychotherapy helps to uncover and clear up these issues.
Is bullying a fact of life?
In “Lord of the Flies,” William Golding portrays a fictional group of unsupervised boys abandoned on an island. An initial hierarchy descends into threats and eventual violence. Consider the animal world. In the wild, a wolf pack isolates a caribou from the herd to kill and devour. On a farm, llamas raised for yarn establish which llama is in charge. Those cases illustrate the hierarchy that exists because there is the need for food or reproductive prowess.
In the workplace, isolation of the target is common when authority extends to bullying. According to Robert Sapolsky, PhD, a neuroscientist and author, there are biological underpinnings for group conformity. This implies that colleagues who stand apart feel distress and get relief when they join the ganging up on a target. Those who watch harassment may hide from confronting it or even from pointing it out to protect themselves.
How do bullies think?
Challenge the bully at your own peril, because expecting a bully to change is futile. Recall Helen’s confrontation with Bill. It provoked him. His power to harass her came from his perceived position. Bullies regard the pleasant person as weak. Bullies can fall into two categories: Sadists who get pleasure from seeing others suffer, and opportunists. The latter focus only on their goals and disregard concerns expressed by others. Outside of the workplace, they may be reasonable. If workplace morale deteriorates along with productivity, the bully gets ousted. Otherwise, companies usually protect high performers at the expense of targets.
Is bullying different in medicine?
It can happen in a training program. The ingredients for bullying exist, including imbalance of power. Often, there are no witnesses, and there can be lack of accountability because of changing authorities. Just as technology can help make harassment possible, it also enables the target to spot and document inappropriate communications by saving emails and texts. Whenever a need for advancement exists along with changing authority, bullying gets tolerated. Who wants to be derailed by reporting? Those in training have the goal of completing a program. If they report bullying, they fear antagonism and retribution, a personal expense that can deter advancement.
Remedies
Let truth and fairness be your guide. That is easy to say and hard to do, but there are helpful personal and legal resources.
Personal capability
Get busy; get better. That became Helen’s method of choice. She focused on her role and productivity, not on her hurt. Helen shunned victimhood. With the help of psychotherapy and by confiding in a mentor, she prevailed. What works is recalling challenges that were mastered and the qualities that made for success. Acquire skills, build a good reputation, be assertive, not defensive.
The group is powerful, and that means it is important to build alliances above and below in the organizational hierarchy; cultivate friendship with trustworthy people. Occasionally, there is unwarranted ganging up on a manager, bullying from below. It is more likely to happen to a newly appointed supervisor. A way to avert that is to communicate with staff throughout the institution and remain accessible.
Legal options
What are legal options to confront bullying? Of note, workplace bullying is not necessarily illegal. According to one employment attorney, “There is no law that prohibits uncivil behavior on the job.” However, under Title VII of the federal law enacted in 1964, there are protected characteristics such as race, color, national origin, sex, age, and disability. The Equal Employment Opportunity Commission enforces Title VII. In cases of assault or stalking, harassment is illegal and criminal.
Some employees report to Human Resources or seek out their company’s employee assistance program. As useful as those options may be, they are part of the company and potentially partial to the administration. There can be incentives to protect those with power against a complainant. For assistance, it is preferable to enlist an outside attorney and a therapist in the community.
Advocacy exists. The Workplace Bullying Institute maintains a website, holds workshops, promotes literature, and offers information. The National Employment Lawyers Association can provide referrals or recommendations that come from other legal sources. Cases rarely reach court because of the expense of a trial; rather, the parties reach a financial settlement. When there is cause, an employment attorney can best pursue justice for the worker.
Conclusion
Get busy; get better is the solution to bullying. Avoid victimhood. That means prepare: Update the resume, seek opportunities, and identify allies. Bullies get beaten; as Abraham Lincoln said, “You can fool some of the people some of the time but you can’t fool all of the people all of the time.”
According to the Workplace Bullying Institute, 7 out of 10 bullied workers either resign or get fired. You should leave only when the leaving is better than the staying. Bullying brings out the worst in the workplace. Those who bully isolate the target. Coworkers often shun the target, fearing for their own position; they may even participate in the harassment. Psychiatrists need to remain sensitive to harassment in their own environment and for their patients. We have tools to address bullying in the workplace and a moral responsibility to combat it.
References
Workplace Bullying Institute (WBI)
National Employment Lawyers Association (NELA)
“Ozymandias” by Percy Bysshe Shelley
BY BEN HINDELL, PSY.D.
Cyberbullying is willful, repeated harm inflicted with the use of computers, cell phones, and other electronic devices. In some cases, a single message may be viewed by multiple people because the text and pictures are posted elsewhere.
Technology makes is possible to harass at any hour. The concept of willful harm is essential. Without interaction between sender and recipient, nuances are lost. Face to face might make a communication benign instead of malevolent or threatening. This has implications for the workplace, where colleagues increasingly communicate by email rather than discuss matters in person or by telephone.
Steps for survivors of cyberbullying
- Do not respond immediately to an inflammatory message, post, or email. Gather your thoughts and avoid responding in anger.
- Keep calm and rational, not emotional.
- Try to respond in person and work to avoid a conflict.
- Remember, your interpretation may differ from what was intended.
- Communicate openly and honestly and not defensively.
- Calmly indicate you were offended and you want the comments to stop.
- Move up the chain of command, if comments don’t cease.
- Save all messages and posts as evidence.
- Report the cyberbullying to your employer. Human resources may get involved.
- Detach from the cyberbully, if it continues. Block social media, cell phone messaging, and emails.
- Find support from friends, family, and a psychotherapist, if needed. As a last resort, it may become necessary to enlist an attorney.
- Take the high road; remain calm and professional at work. The bully may be seeking a reaction from the behavior. Prevent it.
All of the elements of workplace bullying apply to cyberbullying, but the latter can be more insidious. Psychiatrists and psychologists are able to support patients who deal with cyberbullying and help them cope successfully.
Dr. Cohen is in private practice and is a clinical assistant professor of psychiatry at Weill Cornell Medical Center of New York-Presbyterian Hospital, and psychiatric consultant at the Hospital for Special Surgery, also in New York. She made changes to the patient’s story to protect confidentiality.
Dr. Hindell is a psychologist with the Mental Health Service of Colorado College, Colorado Springs. He also practices psychotherapy in Denver. Dr. Hindell is the son of Dr. Cohen.
EEG signature predicts antidepressant response
Personalized treatment for depression may soon become a reality, thanks to an artificial intelligence (AI) algorithm that accurately predicts antidepressant efficacy in specific patients.
A landmark study of more than 300 patients with major depressive disorder (MDD) showed that a latent-space machine-learning algorithm tailored for resting-state EEG robustly predicted patient response to sertraline. The findings were generalizable across different study sites and EEG equipment.
“We found that the use of the artificial intelligence algorithm can identify the EEG signature for patients who do well on sertraline,” study investigator Madhukar H. Trivedi, MD, professor of psychiatry at the University of Texas Southwestern Medical Center in Dallas, said in an interview.
“Interestingly, when we looked further, it became clear that patients with that same EEG signature do not do well on placebo,” he added.
The study was published online Feb. 10 in Nature Biotechnology (doi: 10.1038/s41587-019-0397-3).
Pivotal study
Currently, major depression is defined using a range of clinical criteria. As such, it encompasses a heterogeneous mix of neurobiological phenotypes. Such heterogeneity may account for the modest superiority of antidepressant medication relative to placebo.
While recent research suggests that resting-state EEG may help identify treatment-predictive heterogeneity in depression, these studies have also been hindered by a lack of cross-validation and small sample sizes.
What’s more, these studies have either identified nonspecific predictors or failed to yield generalizable neural signatures that are predictive at the individual patient level (Am J Psychiatry. 2019 Jan 1;176[1]:44-56).
For these reasons, there is currently no robust neurobiological signature for an antidepressant-responsive phenotype that may help identify which patients would benefit from antidepressant medication. Nevertheless, said Dr. Trivedi, detailing such a signature would promote a neurobiological understanding of treatment response, with the potential for notable clinical implications.
“The idea behind this [National Institutes of Health]–funded study was to develop biomarkers that can distinguish treatment outcomes between drug and placebo,” he said. “To do so, we needed a randomized, placebo-controlled trial that has significant breadth in terms of biomarker evaluation and validation, and this study was designed specifically with this end in mind.
“There has not been a drug-placebo study that has looked at this in patients with depression,” Dr. Trivedi said. “So in that sense, this was really a pivotal study.”
To help address these challenges, the investigators developed a machine-learning algorithm they called SELSER (Sparse EEG Latent Space Regression).
Using data from four separate studies, they first established the resting-state EEG predictive signature by training SELSER on data from 309 patients from the EMBARC (Establishing Moderators and Biosignatures of Antidepressant Response in Clinic Care) study, a neuroimaging-coupled, placebo-controlled, randomized clinical study of antidepressant efficacy.
The generalizability of the antidepressant-predictive signature was then tested in a second independent sample of 72 depressed patients.
In a third independent sample of 24 depressed patients, the researchers assessed the convergent validity and neurobiological significance of the treatment-predictive, resting-state EEG signature.
Finally, a fourth sample of 152 depressed patients was used to test the generalizability of the results.
‘Fantastic’ result but validation needed
These combined efforts were aimed at revealing a treatment responsive phenotype in depression, dissociate between medication and placebo response, establish its mechanistic significance, and provide initial evidence regarding the potential for treatment selection on the basis of a resting-state EEG signature.
The study showed that improvement in patients’ symptoms was robustly predicted by the algorithm. These predictions were specific for sertraline relative to placebo.
When generalized to two depression samples, the researchers also found that the algorithm reflected general antidepressant medication responsivity and related differentially to a repetitive transcranial magnetic stimulation (TMS) treatment outcome.
“Although we only looked at sertraline,” Dr. Trivedi said, “we also applied the signature to a sample of patients who had been treated with transcranial magnetic stimulation. And we found that the signature for TMS [response] is different than the signature for sertraline.”
Interestingly, the antidepressant-predictive signature identified by SELSER was also superior to that of conventional machine-learning models or latent modeling methods, such as independent-component analysis or principal-component analysis. This SELSER signature was also superior to a model trained on clinical data alone and was able to predict outcome using resting-state EEG data acquired at a study site not included in the model training set.
The study also revealed evidence of multimodal convergent validity for the antidepressant-response signature by virtue of its correlation with expression of a task-based functional MRI signature in one of the four datasets.
The strength of the resting-state signature was also found to correlate with prefrontal neural responsivity, as indexed by direct stimulation with single-pulse TMS and EEG.
Given the ability of the algorithm to both predict outcome with sertraline and distinguish response between sertraline and placebo at the individual patient level, the investigators believe SELSER may one day support machine learning–driven personalized approaches to depression treatment.
“Our findings advance the neurobiological understanding of antidepressant treatment through an EEG-tailored computational model and provide a clinical avenue for personalized treatment of depression,” the authors wrote.
Yet, their work is far from over.
“Identifying this signature was fantastic, but you’ve got to be able to validate it as well,” Dr. Trivedi noted. “And luckily, we were able to validate it in the three additional studies.
“The next question is whether it can be broadened to other illnesses.”
Promising research
Commenting on the findings in an interview, Michele Ferrante, PhD, said he believes there may soon be a time during which algorithms such as this are used to personalize depression treatment.
“It’s well known that there are no good biological tests in psychiatry, but promising computational tools, biomarkers, and behavioral signatures for segregating patients according to treatment response are starting to emerge for depression,” said Dr. Ferrante, program chief of the Theoretical and Computational Neuroscience Program at the National Institute of Mental Health (NIMH).
“Precision in the ability to predict what patient will respond to each treatment will improve over time, I have no doubt,” added Dr. Ferrante, who was not involved with the current study.
However, he noted, such approaches are not without their potential drawbacks.
“The greatest challenge is to continuously validate these computational tools as they keep on learning from more heterogeneous groups. Another challenge will be to make sure that these computational tools become well-established, widely adopted, safe, and regulated by the [Food and Drug Administration] as Software as a Medical Device,” he said.
The current algorithm will also need to undergo further testing, said Dr. Ferrante.
“It has been validated on an external dataset,” he said, “but now we need to do rigorous prospective clinical trials where patients are selectively assigned by the AI to a treatment according to their biosignature, to see if these results hold true.
“Down the road, it would be important to implement computational models [that are] able to assign patients across the multiple treatments available for depression, including pharmaceuticals, psychosocial interventions, and neural devices.”
The study was funded directly and indirectly by the NIMH of the National Institutes of Health, the Stanford Neurosciences Institute, the Hersh Foundation, the National Key Research and Development Plan of China, and the National Natural Science Foundation of China.
Dr. Trivedi disclosed numerous financial relationships with pharmaceutical companies and device manufacturers. He has received grants/research support from the Agency for Healthcare Research and Quality, Cyberonic, the National Alliance for Research in Schizophrenia and Depression, the NIMH, and the National Institute on Drug Abuse.
A version of this article first appeared on Medscape.com.
Personalized treatment for depression may soon become a reality, thanks to an artificial intelligence (AI) algorithm that accurately predicts antidepressant efficacy in specific patients.
A landmark study of more than 300 patients with major depressive disorder (MDD) showed that a latent-space machine-learning algorithm tailored for resting-state EEG robustly predicted patient response to sertraline. The findings were generalizable across different study sites and EEG equipment.
“We found that the use of the artificial intelligence algorithm can identify the EEG signature for patients who do well on sertraline,” study investigator Madhukar H. Trivedi, MD, professor of psychiatry at the University of Texas Southwestern Medical Center in Dallas, said in an interview.
“Interestingly, when we looked further, it became clear that patients with that same EEG signature do not do well on placebo,” he added.
The study was published online Feb. 10 in Nature Biotechnology (doi: 10.1038/s41587-019-0397-3).
Pivotal study
Currently, major depression is defined using a range of clinical criteria. As such, it encompasses a heterogeneous mix of neurobiological phenotypes. Such heterogeneity may account for the modest superiority of antidepressant medication relative to placebo.
While recent research suggests that resting-state EEG may help identify treatment-predictive heterogeneity in depression, these studies have also been hindered by a lack of cross-validation and small sample sizes.
What’s more, these studies have either identified nonspecific predictors or failed to yield generalizable neural signatures that are predictive at the individual patient level (Am J Psychiatry. 2019 Jan 1;176[1]:44-56).
For these reasons, there is currently no robust neurobiological signature for an antidepressant-responsive phenotype that may help identify which patients would benefit from antidepressant medication. Nevertheless, said Dr. Trivedi, detailing such a signature would promote a neurobiological understanding of treatment response, with the potential for notable clinical implications.
“The idea behind this [National Institutes of Health]–funded study was to develop biomarkers that can distinguish treatment outcomes between drug and placebo,” he said. “To do so, we needed a randomized, placebo-controlled trial that has significant breadth in terms of biomarker evaluation and validation, and this study was designed specifically with this end in mind.
“There has not been a drug-placebo study that has looked at this in patients with depression,” Dr. Trivedi said. “So in that sense, this was really a pivotal study.”
To help address these challenges, the investigators developed a machine-learning algorithm they called SELSER (Sparse EEG Latent Space Regression).
Using data from four separate studies, they first established the resting-state EEG predictive signature by training SELSER on data from 309 patients from the EMBARC (Establishing Moderators and Biosignatures of Antidepressant Response in Clinic Care) study, a neuroimaging-coupled, placebo-controlled, randomized clinical study of antidepressant efficacy.
The generalizability of the antidepressant-predictive signature was then tested in a second independent sample of 72 depressed patients.
In a third independent sample of 24 depressed patients, the researchers assessed the convergent validity and neurobiological significance of the treatment-predictive, resting-state EEG signature.
Finally, a fourth sample of 152 depressed patients was used to test the generalizability of the results.
‘Fantastic’ result but validation needed
These combined efforts were aimed at revealing a treatment responsive phenotype in depression, dissociate between medication and placebo response, establish its mechanistic significance, and provide initial evidence regarding the potential for treatment selection on the basis of a resting-state EEG signature.
The study showed that improvement in patients’ symptoms was robustly predicted by the algorithm. These predictions were specific for sertraline relative to placebo.
When generalized to two depression samples, the researchers also found that the algorithm reflected general antidepressant medication responsivity and related differentially to a repetitive transcranial magnetic stimulation (TMS) treatment outcome.
“Although we only looked at sertraline,” Dr. Trivedi said, “we also applied the signature to a sample of patients who had been treated with transcranial magnetic stimulation. And we found that the signature for TMS [response] is different than the signature for sertraline.”
Interestingly, the antidepressant-predictive signature identified by SELSER was also superior to that of conventional machine-learning models or latent modeling methods, such as independent-component analysis or principal-component analysis. This SELSER signature was also superior to a model trained on clinical data alone and was able to predict outcome using resting-state EEG data acquired at a study site not included in the model training set.
The study also revealed evidence of multimodal convergent validity for the antidepressant-response signature by virtue of its correlation with expression of a task-based functional MRI signature in one of the four datasets.
The strength of the resting-state signature was also found to correlate with prefrontal neural responsivity, as indexed by direct stimulation with single-pulse TMS and EEG.
Given the ability of the algorithm to both predict outcome with sertraline and distinguish response between sertraline and placebo at the individual patient level, the investigators believe SELSER may one day support machine learning–driven personalized approaches to depression treatment.
“Our findings advance the neurobiological understanding of antidepressant treatment through an EEG-tailored computational model and provide a clinical avenue for personalized treatment of depression,” the authors wrote.
Yet, their work is far from over.
“Identifying this signature was fantastic, but you’ve got to be able to validate it as well,” Dr. Trivedi noted. “And luckily, we were able to validate it in the three additional studies.
“The next question is whether it can be broadened to other illnesses.”
Promising research
Commenting on the findings in an interview, Michele Ferrante, PhD, said he believes there may soon be a time during which algorithms such as this are used to personalize depression treatment.
“It’s well known that there are no good biological tests in psychiatry, but promising computational tools, biomarkers, and behavioral signatures for segregating patients according to treatment response are starting to emerge for depression,” said Dr. Ferrante, program chief of the Theoretical and Computational Neuroscience Program at the National Institute of Mental Health (NIMH).
“Precision in the ability to predict what patient will respond to each treatment will improve over time, I have no doubt,” added Dr. Ferrante, who was not involved with the current study.
However, he noted, such approaches are not without their potential drawbacks.
“The greatest challenge is to continuously validate these computational tools as they keep on learning from more heterogeneous groups. Another challenge will be to make sure that these computational tools become well-established, widely adopted, safe, and regulated by the [Food and Drug Administration] as Software as a Medical Device,” he said.
The current algorithm will also need to undergo further testing, said Dr. Ferrante.
“It has been validated on an external dataset,” he said, “but now we need to do rigorous prospective clinical trials where patients are selectively assigned by the AI to a treatment according to their biosignature, to see if these results hold true.
“Down the road, it would be important to implement computational models [that are] able to assign patients across the multiple treatments available for depression, including pharmaceuticals, psychosocial interventions, and neural devices.”
The study was funded directly and indirectly by the NIMH of the National Institutes of Health, the Stanford Neurosciences Institute, the Hersh Foundation, the National Key Research and Development Plan of China, and the National Natural Science Foundation of China.
Dr. Trivedi disclosed numerous financial relationships with pharmaceutical companies and device manufacturers. He has received grants/research support from the Agency for Healthcare Research and Quality, Cyberonic, the National Alliance for Research in Schizophrenia and Depression, the NIMH, and the National Institute on Drug Abuse.
A version of this article first appeared on Medscape.com.
Personalized treatment for depression may soon become a reality, thanks to an artificial intelligence (AI) algorithm that accurately predicts antidepressant efficacy in specific patients.
A landmark study of more than 300 patients with major depressive disorder (MDD) showed that a latent-space machine-learning algorithm tailored for resting-state EEG robustly predicted patient response to sertraline. The findings were generalizable across different study sites and EEG equipment.
“We found that the use of the artificial intelligence algorithm can identify the EEG signature for patients who do well on sertraline,” study investigator Madhukar H. Trivedi, MD, professor of psychiatry at the University of Texas Southwestern Medical Center in Dallas, said in an interview.
“Interestingly, when we looked further, it became clear that patients with that same EEG signature do not do well on placebo,” he added.
The study was published online Feb. 10 in Nature Biotechnology (doi: 10.1038/s41587-019-0397-3).
Pivotal study
Currently, major depression is defined using a range of clinical criteria. As such, it encompasses a heterogeneous mix of neurobiological phenotypes. Such heterogeneity may account for the modest superiority of antidepressant medication relative to placebo.
While recent research suggests that resting-state EEG may help identify treatment-predictive heterogeneity in depression, these studies have also been hindered by a lack of cross-validation and small sample sizes.
What’s more, these studies have either identified nonspecific predictors or failed to yield generalizable neural signatures that are predictive at the individual patient level (Am J Psychiatry. 2019 Jan 1;176[1]:44-56).
For these reasons, there is currently no robust neurobiological signature for an antidepressant-responsive phenotype that may help identify which patients would benefit from antidepressant medication. Nevertheless, said Dr. Trivedi, detailing such a signature would promote a neurobiological understanding of treatment response, with the potential for notable clinical implications.
“The idea behind this [National Institutes of Health]–funded study was to develop biomarkers that can distinguish treatment outcomes between drug and placebo,” he said. “To do so, we needed a randomized, placebo-controlled trial that has significant breadth in terms of biomarker evaluation and validation, and this study was designed specifically with this end in mind.
“There has not been a drug-placebo study that has looked at this in patients with depression,” Dr. Trivedi said. “So in that sense, this was really a pivotal study.”
To help address these challenges, the investigators developed a machine-learning algorithm they called SELSER (Sparse EEG Latent Space Regression).
Using data from four separate studies, they first established the resting-state EEG predictive signature by training SELSER on data from 309 patients from the EMBARC (Establishing Moderators and Biosignatures of Antidepressant Response in Clinic Care) study, a neuroimaging-coupled, placebo-controlled, randomized clinical study of antidepressant efficacy.
The generalizability of the antidepressant-predictive signature was then tested in a second independent sample of 72 depressed patients.
In a third independent sample of 24 depressed patients, the researchers assessed the convergent validity and neurobiological significance of the treatment-predictive, resting-state EEG signature.
Finally, a fourth sample of 152 depressed patients was used to test the generalizability of the results.
‘Fantastic’ result but validation needed
These combined efforts were aimed at revealing a treatment responsive phenotype in depression, dissociate between medication and placebo response, establish its mechanistic significance, and provide initial evidence regarding the potential for treatment selection on the basis of a resting-state EEG signature.
The study showed that improvement in patients’ symptoms was robustly predicted by the algorithm. These predictions were specific for sertraline relative to placebo.
When generalized to two depression samples, the researchers also found that the algorithm reflected general antidepressant medication responsivity and related differentially to a repetitive transcranial magnetic stimulation (TMS) treatment outcome.
“Although we only looked at sertraline,” Dr. Trivedi said, “we also applied the signature to a sample of patients who had been treated with transcranial magnetic stimulation. And we found that the signature for TMS [response] is different than the signature for sertraline.”
Interestingly, the antidepressant-predictive signature identified by SELSER was also superior to that of conventional machine-learning models or latent modeling methods, such as independent-component analysis or principal-component analysis. This SELSER signature was also superior to a model trained on clinical data alone and was able to predict outcome using resting-state EEG data acquired at a study site not included in the model training set.
The study also revealed evidence of multimodal convergent validity for the antidepressant-response signature by virtue of its correlation with expression of a task-based functional MRI signature in one of the four datasets.
The strength of the resting-state signature was also found to correlate with prefrontal neural responsivity, as indexed by direct stimulation with single-pulse TMS and EEG.
Given the ability of the algorithm to both predict outcome with sertraline and distinguish response between sertraline and placebo at the individual patient level, the investigators believe SELSER may one day support machine learning–driven personalized approaches to depression treatment.
“Our findings advance the neurobiological understanding of antidepressant treatment through an EEG-tailored computational model and provide a clinical avenue for personalized treatment of depression,” the authors wrote.
Yet, their work is far from over.
“Identifying this signature was fantastic, but you’ve got to be able to validate it as well,” Dr. Trivedi noted. “And luckily, we were able to validate it in the three additional studies.
“The next question is whether it can be broadened to other illnesses.”
Promising research
Commenting on the findings in an interview, Michele Ferrante, PhD, said he believes there may soon be a time during which algorithms such as this are used to personalize depression treatment.
“It’s well known that there are no good biological tests in psychiatry, but promising computational tools, biomarkers, and behavioral signatures for segregating patients according to treatment response are starting to emerge for depression,” said Dr. Ferrante, program chief of the Theoretical and Computational Neuroscience Program at the National Institute of Mental Health (NIMH).
“Precision in the ability to predict what patient will respond to each treatment will improve over time, I have no doubt,” added Dr. Ferrante, who was not involved with the current study.
However, he noted, such approaches are not without their potential drawbacks.
“The greatest challenge is to continuously validate these computational tools as they keep on learning from more heterogeneous groups. Another challenge will be to make sure that these computational tools become well-established, widely adopted, safe, and regulated by the [Food and Drug Administration] as Software as a Medical Device,” he said.
The current algorithm will also need to undergo further testing, said Dr. Ferrante.
“It has been validated on an external dataset,” he said, “but now we need to do rigorous prospective clinical trials where patients are selectively assigned by the AI to a treatment according to their biosignature, to see if these results hold true.
“Down the road, it would be important to implement computational models [that are] able to assign patients across the multiple treatments available for depression, including pharmaceuticals, psychosocial interventions, and neural devices.”
The study was funded directly and indirectly by the NIMH of the National Institutes of Health, the Stanford Neurosciences Institute, the Hersh Foundation, the National Key Research and Development Plan of China, and the National Natural Science Foundation of China.
Dr. Trivedi disclosed numerous financial relationships with pharmaceutical companies and device manufacturers. He has received grants/research support from the Agency for Healthcare Research and Quality, Cyberonic, the National Alliance for Research in Schizophrenia and Depression, the NIMH, and the National Institute on Drug Abuse.
A version of this article first appeared on Medscape.com.
FROM NATURE BIOTECHNOLOGY
Excessive masculinity linked to high suicide risk
Excessive masculinity is linked to a significantly increased risk for death by suicide in men, new research suggests.
In the first study to show this association, investigators found that men with high traditional masculinity (HTM) – a set of norms that includes competitiveness, emotional restriction, and aggression – were about two and half times more likely to die by suicide than their counterparts without HTM. The finding underscores the “central role” of gender in suicide death.
“We found that high-traditional-masculinity men were 2.4 times more likely to die by suicide than those who were not [of] high traditional masculinity. We feel this is a significant finding, and one that’s very rare to have evidence for,” study investigator Daniel Coleman, PhD, said in an interview.
“Our other findings are also important and interesting,” added Dr. Coleman, associate professor of social service at Fordham University, New York. “One was that high traditional masculinity was associated with a host of other significant risk factors for suicide death. So not only does high traditional masculinity add to the risk of suicide death, it also may have indirect effects through other variables, such as acting-out behavior.”
The study was published online Feb. 12 in JAMA Psychiatry (doi: 10.1001/jamapsychiatry.2019.4702).
First look
In the United States, death by suicide is 3.5 times more common in men than in women. Several potential drivers may explain this phenomenon; one plausible factor may be high levels of what the investigators describe as “traditional masculinity.”
Interestingly, previous studies suggest that HTM men experience suicidal thoughts to a greater degree than do other persons (Soc Psychiatry Psychiatr Epidemiol. 2017 Mar;52[3]:319-27). Nevertheless, the potential influence of HTM and suicide mortality has not been examined before now.
The study is a secondary analysis of the longitudinal Add Health (the National Longitudinal Study of Adolescent to Adult Health) study, which began in 1995 and followed 20,745 adolescents through young adulthood. Not only did that study show a direct association between measures of HTM and death by suicide, but it also corroborated the connection between HTM and other risk factors for suicide revealed in earlier research (Suicide Life Threat Behav. 2016 Apr;46[2]:191-205).
To tease out this relationship, Dr. Coleman and colleagues used data from the nationally representative Add Health study. That earlier research concluded that nine Add Health variables were associated with suicide; these included suicide by a family member, being expelled from school, running away from home, using a weapon, being of white race, a past history of smoking, being in a serious fight in the past year, delinquency, and fighting.
In the current study, the researchers hypothesized that HTM would be associated with these nine variables, in addition to suicide, depression, and gun access.
In the Add Health study, the adolescents were followed over time. In the current analysis, the researchers matched data from that study with death records from the National Death Index from 2014. Death by suicide was defined using National Death Index procedures.
The investigators then used an established procedure for scoring gender-typed attitudes and behaviors. As part of this, a single latent probability variable for identifying oneself as male was generated from 16 gender-discriminating variables.
Participants who were found to score at least a 73% probability of identifying as male (greater than 1 standard deviation above the mean) were classified as HTM.
“There’s been a lot of speculating about masculinity as a risk factor for male suicides,” Dr. Coleman said. “But it’s very difficult to study suicide death and something psychosocial like masculinity. So this was an attempt to fill that gap and test the hypothesis that’s being discussed quite a bit.”
A relevant risk factor
Twenty-two deaths occurred among the Add Health participants. Of those participants, 21 were men (odds ratio, 21.7; 95% confidence interval, 2.9-161; P less than .001).
The analysis showed that all nine risks for suicide that were highlighted in previous research were positively associated with HTM, with small to medium effect sizes. Of these, the most pronounced was family member suicide, with an OR of 1.89 (95% CI, 1.3-2.7).
Most tellingly, HTM men were 2.4 times more likely to end their lives by suicide than were men not defined as such (95% CI, 0.99-6.0; P less than .046). Nevertheless, HTM men were also 1.45 times less likely to report suicidal ideation (OR, 0.69; 95% CI, 0.60-0.81; P less than .001). There was no association between HTM and nonfatal suicide attempts.
Interestingly, HTM men were slightly more likely to report easy access to guns (OR, 1.1; 95% CI, 1.01-1.20; P less than .04), but they had lower levels of depression (Cohen’s d, 0.17; P less than .001).
HTM not only has a direct association with suicide but also with a web of indirect effects as well, thanks to its association with all the other risks identified in the previous study by another group of investigators.
HTM may be an underlying influence in male suicide that increases the probability of externalizing such behavioral risk factors as anger, violence, gun access, and school problems.
The finding that almost all of the people who died by suicide were men underscores the central role that gender plays in these tragedies. As such, the investigators hope that the study prompts more research, as well as intervention efforts aimed at the role of masculinity in suicide.
“There are already things going on around the world to try to address the risk factors of masculinity for suicide death,” Dr. Coleman said. “So even though we haven’t had the evidence that it’s a risk factor, people have been operating under that assumption anyway.
“Hopefully our research contributes to raising the profile that high traditional masculinity is a relevant risk factor that we can organize prevention and treatment around.”
An important contribution
Mark S. Kaplan, DrPH, commenting on the findings in an interview, said the study makes an important contribution to suicide research.
“Any study that tries to link a living sample with death data, as they did here, is important,” said Dr. Kaplan, professor of social welfare at the Luskin School of Public Affairs of the University of California, Los Angeles.
“It’s also important because it begins to scratch the surface of more proximal or distal factors that are associated with suicide, and masculinity is one of those factors,” Dr. Kaplan added.
“In an incremental way, it begins to add to the puzzle of why men have a higher mortality rate than their female counterparts. Because when it comes to suicide, men and women really are apples and oranges.”
Dr. Kaplan believes HTM is one of several traits that may lead men to take their own lives.
“There are all sorts of other issues. For example, masculinity might be interacting with some of the harsh socioeconomic conditions that many men face. I think all of this points to the real need to understand why men die from suicide,” he said.
The Add Health study is funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. No direct support was received from the grant for the current study. Dr. Coleman and Dr. Kaplan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Excessive masculinity is linked to a significantly increased risk for death by suicide in men, new research suggests.
In the first study to show this association, investigators found that men with high traditional masculinity (HTM) – a set of norms that includes competitiveness, emotional restriction, and aggression – were about two and half times more likely to die by suicide than their counterparts without HTM. The finding underscores the “central role” of gender in suicide death.
“We found that high-traditional-masculinity men were 2.4 times more likely to die by suicide than those who were not [of] high traditional masculinity. We feel this is a significant finding, and one that’s very rare to have evidence for,” study investigator Daniel Coleman, PhD, said in an interview.
“Our other findings are also important and interesting,” added Dr. Coleman, associate professor of social service at Fordham University, New York. “One was that high traditional masculinity was associated with a host of other significant risk factors for suicide death. So not only does high traditional masculinity add to the risk of suicide death, it also may have indirect effects through other variables, such as acting-out behavior.”
The study was published online Feb. 12 in JAMA Psychiatry (doi: 10.1001/jamapsychiatry.2019.4702).
First look
In the United States, death by suicide is 3.5 times more common in men than in women. Several potential drivers may explain this phenomenon; one plausible factor may be high levels of what the investigators describe as “traditional masculinity.”
Interestingly, previous studies suggest that HTM men experience suicidal thoughts to a greater degree than do other persons (Soc Psychiatry Psychiatr Epidemiol. 2017 Mar;52[3]:319-27). Nevertheless, the potential influence of HTM and suicide mortality has not been examined before now.
The study is a secondary analysis of the longitudinal Add Health (the National Longitudinal Study of Adolescent to Adult Health) study, which began in 1995 and followed 20,745 adolescents through young adulthood. Not only did that study show a direct association between measures of HTM and death by suicide, but it also corroborated the connection between HTM and other risk factors for suicide revealed in earlier research (Suicide Life Threat Behav. 2016 Apr;46[2]:191-205).
To tease out this relationship, Dr. Coleman and colleagues used data from the nationally representative Add Health study. That earlier research concluded that nine Add Health variables were associated with suicide; these included suicide by a family member, being expelled from school, running away from home, using a weapon, being of white race, a past history of smoking, being in a serious fight in the past year, delinquency, and fighting.
In the current study, the researchers hypothesized that HTM would be associated with these nine variables, in addition to suicide, depression, and gun access.
In the Add Health study, the adolescents were followed over time. In the current analysis, the researchers matched data from that study with death records from the National Death Index from 2014. Death by suicide was defined using National Death Index procedures.
The investigators then used an established procedure for scoring gender-typed attitudes and behaviors. As part of this, a single latent probability variable for identifying oneself as male was generated from 16 gender-discriminating variables.
Participants who were found to score at least a 73% probability of identifying as male (greater than 1 standard deviation above the mean) were classified as HTM.
“There’s been a lot of speculating about masculinity as a risk factor for male suicides,” Dr. Coleman said. “But it’s very difficult to study suicide death and something psychosocial like masculinity. So this was an attempt to fill that gap and test the hypothesis that’s being discussed quite a bit.”
A relevant risk factor
Twenty-two deaths occurred among the Add Health participants. Of those participants, 21 were men (odds ratio, 21.7; 95% confidence interval, 2.9-161; P less than .001).
The analysis showed that all nine risks for suicide that were highlighted in previous research were positively associated with HTM, with small to medium effect sizes. Of these, the most pronounced was family member suicide, with an OR of 1.89 (95% CI, 1.3-2.7).
Most tellingly, HTM men were 2.4 times more likely to end their lives by suicide than were men not defined as such (95% CI, 0.99-6.0; P less than .046). Nevertheless, HTM men were also 1.45 times less likely to report suicidal ideation (OR, 0.69; 95% CI, 0.60-0.81; P less than .001). There was no association between HTM and nonfatal suicide attempts.
Interestingly, HTM men were slightly more likely to report easy access to guns (OR, 1.1; 95% CI, 1.01-1.20; P less than .04), but they had lower levels of depression (Cohen’s d, 0.17; P less than .001).
HTM not only has a direct association with suicide but also with a web of indirect effects as well, thanks to its association with all the other risks identified in the previous study by another group of investigators.
HTM may be an underlying influence in male suicide that increases the probability of externalizing such behavioral risk factors as anger, violence, gun access, and school problems.
The finding that almost all of the people who died by suicide were men underscores the central role that gender plays in these tragedies. As such, the investigators hope that the study prompts more research, as well as intervention efforts aimed at the role of masculinity in suicide.
“There are already things going on around the world to try to address the risk factors of masculinity for suicide death,” Dr. Coleman said. “So even though we haven’t had the evidence that it’s a risk factor, people have been operating under that assumption anyway.
“Hopefully our research contributes to raising the profile that high traditional masculinity is a relevant risk factor that we can organize prevention and treatment around.”
An important contribution
Mark S. Kaplan, DrPH, commenting on the findings in an interview, said the study makes an important contribution to suicide research.
“Any study that tries to link a living sample with death data, as they did here, is important,” said Dr. Kaplan, professor of social welfare at the Luskin School of Public Affairs of the University of California, Los Angeles.
“It’s also important because it begins to scratch the surface of more proximal or distal factors that are associated with suicide, and masculinity is one of those factors,” Dr. Kaplan added.
“In an incremental way, it begins to add to the puzzle of why men have a higher mortality rate than their female counterparts. Because when it comes to suicide, men and women really are apples and oranges.”
Dr. Kaplan believes HTM is one of several traits that may lead men to take their own lives.
“There are all sorts of other issues. For example, masculinity might be interacting with some of the harsh socioeconomic conditions that many men face. I think all of this points to the real need to understand why men die from suicide,” he said.
The Add Health study is funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. No direct support was received from the grant for the current study. Dr. Coleman and Dr. Kaplan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Excessive masculinity is linked to a significantly increased risk for death by suicide in men, new research suggests.
In the first study to show this association, investigators found that men with high traditional masculinity (HTM) – a set of norms that includes competitiveness, emotional restriction, and aggression – were about two and half times more likely to die by suicide than their counterparts without HTM. The finding underscores the “central role” of gender in suicide death.
“We found that high-traditional-masculinity men were 2.4 times more likely to die by suicide than those who were not [of] high traditional masculinity. We feel this is a significant finding, and one that’s very rare to have evidence for,” study investigator Daniel Coleman, PhD, said in an interview.
“Our other findings are also important and interesting,” added Dr. Coleman, associate professor of social service at Fordham University, New York. “One was that high traditional masculinity was associated with a host of other significant risk factors for suicide death. So not only does high traditional masculinity add to the risk of suicide death, it also may have indirect effects through other variables, such as acting-out behavior.”
The study was published online Feb. 12 in JAMA Psychiatry (doi: 10.1001/jamapsychiatry.2019.4702).
First look
In the United States, death by suicide is 3.5 times more common in men than in women. Several potential drivers may explain this phenomenon; one plausible factor may be high levels of what the investigators describe as “traditional masculinity.”
Interestingly, previous studies suggest that HTM men experience suicidal thoughts to a greater degree than do other persons (Soc Psychiatry Psychiatr Epidemiol. 2017 Mar;52[3]:319-27). Nevertheless, the potential influence of HTM and suicide mortality has not been examined before now.
The study is a secondary analysis of the longitudinal Add Health (the National Longitudinal Study of Adolescent to Adult Health) study, which began in 1995 and followed 20,745 adolescents through young adulthood. Not only did that study show a direct association between measures of HTM and death by suicide, but it also corroborated the connection between HTM and other risk factors for suicide revealed in earlier research (Suicide Life Threat Behav. 2016 Apr;46[2]:191-205).
To tease out this relationship, Dr. Coleman and colleagues used data from the nationally representative Add Health study. That earlier research concluded that nine Add Health variables were associated with suicide; these included suicide by a family member, being expelled from school, running away from home, using a weapon, being of white race, a past history of smoking, being in a serious fight in the past year, delinquency, and fighting.
In the current study, the researchers hypothesized that HTM would be associated with these nine variables, in addition to suicide, depression, and gun access.
In the Add Health study, the adolescents were followed over time. In the current analysis, the researchers matched data from that study with death records from the National Death Index from 2014. Death by suicide was defined using National Death Index procedures.
The investigators then used an established procedure for scoring gender-typed attitudes and behaviors. As part of this, a single latent probability variable for identifying oneself as male was generated from 16 gender-discriminating variables.
Participants who were found to score at least a 73% probability of identifying as male (greater than 1 standard deviation above the mean) were classified as HTM.
“There’s been a lot of speculating about masculinity as a risk factor for male suicides,” Dr. Coleman said. “But it’s very difficult to study suicide death and something psychosocial like masculinity. So this was an attempt to fill that gap and test the hypothesis that’s being discussed quite a bit.”
A relevant risk factor
Twenty-two deaths occurred among the Add Health participants. Of those participants, 21 were men (odds ratio, 21.7; 95% confidence interval, 2.9-161; P less than .001).
The analysis showed that all nine risks for suicide that were highlighted in previous research were positively associated with HTM, with small to medium effect sizes. Of these, the most pronounced was family member suicide, with an OR of 1.89 (95% CI, 1.3-2.7).
Most tellingly, HTM men were 2.4 times more likely to end their lives by suicide than were men not defined as such (95% CI, 0.99-6.0; P less than .046). Nevertheless, HTM men were also 1.45 times less likely to report suicidal ideation (OR, 0.69; 95% CI, 0.60-0.81; P less than .001). There was no association between HTM and nonfatal suicide attempts.
Interestingly, HTM men were slightly more likely to report easy access to guns (OR, 1.1; 95% CI, 1.01-1.20; P less than .04), but they had lower levels of depression (Cohen’s d, 0.17; P less than .001).
HTM not only has a direct association with suicide but also with a web of indirect effects as well, thanks to its association with all the other risks identified in the previous study by another group of investigators.
HTM may be an underlying influence in male suicide that increases the probability of externalizing such behavioral risk factors as anger, violence, gun access, and school problems.
The finding that almost all of the people who died by suicide were men underscores the central role that gender plays in these tragedies. As such, the investigators hope that the study prompts more research, as well as intervention efforts aimed at the role of masculinity in suicide.
“There are already things going on around the world to try to address the risk factors of masculinity for suicide death,” Dr. Coleman said. “So even though we haven’t had the evidence that it’s a risk factor, people have been operating under that assumption anyway.
“Hopefully our research contributes to raising the profile that high traditional masculinity is a relevant risk factor that we can organize prevention and treatment around.”
An important contribution
Mark S. Kaplan, DrPH, commenting on the findings in an interview, said the study makes an important contribution to suicide research.
“Any study that tries to link a living sample with death data, as they did here, is important,” said Dr. Kaplan, professor of social welfare at the Luskin School of Public Affairs of the University of California, Los Angeles.
“It’s also important because it begins to scratch the surface of more proximal or distal factors that are associated with suicide, and masculinity is one of those factors,” Dr. Kaplan added.
“In an incremental way, it begins to add to the puzzle of why men have a higher mortality rate than their female counterparts. Because when it comes to suicide, men and women really are apples and oranges.”
Dr. Kaplan believes HTM is one of several traits that may lead men to take their own lives.
“There are all sorts of other issues. For example, masculinity might be interacting with some of the harsh socioeconomic conditions that many men face. I think all of this points to the real need to understand why men die from suicide,” he said.
The Add Health study is funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. No direct support was received from the grant for the current study. Dr. Coleman and Dr. Kaplan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Risk-benefit ratio on the radar as new antidepressant therapies emerge
LAS VEGAS – The risk-benefit ratio of new and emerging drugs for depression is going to be under the spotlight more than ever before, according to an expert in mood disorders.
“We’ve seen a shift in the landscape,” Alan F. Schatzberg, MD, said at an annual psychopharmacology update held by the Nevada Psychiatric Association. The risk-benefit ratio is going to become increasingly more of a focus in drug development, in terms of increasing risk.”
The development of the SSRIs, he continued, led to the introduction of agents that were generally effective and well tolerated.
“Particularly, they were well tolerated in terms of their wide safety margin,” said Dr. Schatzberg, who directs the Mood Disorders Center at Stanford (Calif.) University. “It’s difficult to [die by suicide] on an SSRI. Because of that, we had greater numbers of individuals treated than we did with the tricyclics.” The introduction of SSRIs led to “widening of the net in terms of the numbers of depressed patients,” he said. “Combine that with DSM-III, and DSM-IV having relatively easy criteria to obtain a diagnosis [of depression, and] you see a large group of subjects who are exposed to treatment. But a lot of those people may not respond to a particular agent. As new treatments are promulgated and tried, what you find is [that] a reasonable number of subjects are, in fact, truly resistant. That becomes a tough nut to crack for all of us who treat depression.”
Drugs for depression that clinicians commonly have in their armamentarium commonly revolve around monoaminergic function. New and emerging agents include hallucinatory serotonin 2a agonists, glutamatergic drugs such as ketamine, GABAergic neurosteroids, opioid modulators, and onabotulinumtoxinA.
In 2013, the Food and Drug Administration approved the multimodal agent vortioxetine for the treatment of major depressive disorder. The recommended dosing is 20 mg/day, and the drug appears to have a positive effect on cognition. A meta-analysis of vortioxetine, duloxetine, and placebo comparison trials evaluated the effect of each treatment on the Digit Symbol Substitution Test (Int J Neuropsychopharmacol. 2016 Jun 15;19[10]). Vortioxetine was superior to placebo in all three trials and was superior to duloxetine in two trials. Duloxetine was not superior to placebo in two trials.
In two double-blind studies, researchers evaluated the effects of psilocybin in cancer patients with comorbid depression and anxiety. Full doses of psilocybin were 0.3 mg/kg or 22-30 mg per 70 kg. Both studies demonstrated sustained responses at full doses (J Psychopharmacol. 2016 Nov 30;30[12]:1181-97 and J Psychopharmacol. 2016 Nov 30;30[12]:1165-80).
In an open label study of psilocybin in refractory major depression, 12 patients received 10 mg on day 1 and 25 mg on day 8. Eight of 12 patients responded at 1 week, and 7 of the 12 maintained response at 3 months (Lancet Psychiatry. 2016 Jul;3[7]:619-27). According to Dr. Schatzberg, this trial became the basis for a blinded study that Compass is conducting in the United States and in the United Kingdom in which refractory patients are going to be randomized to 1 mg, 10 mg, or 25 mg psilocybin using an independent rater. “These patients are accompanied during this experience by two therapists for up to 8 hours. It’s not quite a guided therapy; it’s kind of a safety net therapy if the patient needs [help]. We’ll see what happens.”
Another agent being studied is ketamine, which works on the glutamate system, an excitatory neurotransmitter. “Glutamate is the juice that keeps us going,” said Dr. Schatzberg, who is also the Kenneth T. Norris Jr. professor of psychiatry and behavioral sciences at the university. “We can’t live without glutamate.” An anesthetic agent that has been used for 50 years, ketamine is a N-methyl-d-aspartate antagonist that has mu opioid agonist effects and stimulant properties. “It causes a psychotomimetic dissociative kind of reaction,” he said. “The doses used in depression are subanesthetic. They may put the patient asleep, but they usually don’t. The problem with the antidepressant effect is that 70% of people who initially respond don’t continue to respond beyond 1 week.”
In a randomized, placebo-controlled, double-blind crossover study of patients with treatment-resistant depression, subjects who received ketamine showed significant improvement in depression, compared with subjects who received placebo within 110 minutes after injection, which remained significant throughout the following week (Arch Gen Psychiatry. 2006;63:856-64). Specifically, of the 17 subjects treated with ketamine, 71% met response and 29% met remission criteria the day following ketamine infusion. Thirty-five percent of subjects maintained response for at least 1 week.
In an effort to further evaluate the antidepressive effects of ketamine, researchers conducted a two-site, parallel-arm, randomized, controlled trial of a single infusion of ketamine, compared with an active placebo control condition, the anesthetic midazolam (Am J Psychiatry. 2013 Oct;170[10]:1134-42). After adjustment for baseline scores and site, the Montgomery-Åsberg Depression Rating Scale score was lower in the ketamine group than in the midazolam group by 7.95 points. The likelihood of response at 24 hours was greater with ketamine than with midazolam (odds ratio, 2.18), with response rates of 64% and 28%, respectively.
In 2019, the FDA approved esketamine nasal spray, in conjunction with an oral antidepressant, for the treatment of depression in adults with treatment-resistant depression. In three phase 3 double-blind studies of esketamine in treatment-refractory depression, one was positive, two were nearly positive, and the effect sizes were mild. One maintenance discontinuation trial was positive. Based on this data, Dr. Schatzberg said, there “is not much evidence” that patients get further gain beyond an antidepressant alone in the first 24-48 hours following ketamine administration. “It makes me wonder: Should we be continuing to give this drug intranasally beyond 48 hours?” he asked. “The reason I have concern is that in certain cultures, ketamine is a highly abusable drug.”
He and Gerard Sanacora, PhD, MD, addressed the topic in a 2015 opinion piece entitled, “Ketamine: Promising path or false prophecy in the development of novel therapeutics for mood disorders?”
“If we step back for a moment and look at where we are – an intravenously administered agent that is a street drug of abuse, works rapidly, and whose enantiomers are being studied by industry for intranasal use – we should be anxious, “ they wrote (Neuropsychopharmacology. 2015 Jan;40[2]:259-67). “We need to be as careful and conservative as possible and understand how it is acting and rule out the possibility of whether it acts as an opioid.”
Dr. Schatzberg disclosed that he has served a consultant to Alkermes, Avanir, Bracket, Compass, Delpor, Epiodyne, Janssen, Jazz, Lundbeck, McKinsey, Merck, Myriad Genetics, Owl, Neuronetics, Pfizer, Sage, and Sunovion. He has received research funding from Janssen and also holds an ownership interest in Corcept, Dermira, Delpor, Epiodyne, Incyte Genetics, Madrigal, Maerck, Owl Analytics, Seattle Genetics, Titan, and Xhale.
LAS VEGAS – The risk-benefit ratio of new and emerging drugs for depression is going to be under the spotlight more than ever before, according to an expert in mood disorders.
“We’ve seen a shift in the landscape,” Alan F. Schatzberg, MD, said at an annual psychopharmacology update held by the Nevada Psychiatric Association. The risk-benefit ratio is going to become increasingly more of a focus in drug development, in terms of increasing risk.”
The development of the SSRIs, he continued, led to the introduction of agents that were generally effective and well tolerated.
“Particularly, they were well tolerated in terms of their wide safety margin,” said Dr. Schatzberg, who directs the Mood Disorders Center at Stanford (Calif.) University. “It’s difficult to [die by suicide] on an SSRI. Because of that, we had greater numbers of individuals treated than we did with the tricyclics.” The introduction of SSRIs led to “widening of the net in terms of the numbers of depressed patients,” he said. “Combine that with DSM-III, and DSM-IV having relatively easy criteria to obtain a diagnosis [of depression, and] you see a large group of subjects who are exposed to treatment. But a lot of those people may not respond to a particular agent. As new treatments are promulgated and tried, what you find is [that] a reasonable number of subjects are, in fact, truly resistant. That becomes a tough nut to crack for all of us who treat depression.”
Drugs for depression that clinicians commonly have in their armamentarium commonly revolve around monoaminergic function. New and emerging agents include hallucinatory serotonin 2a agonists, glutamatergic drugs such as ketamine, GABAergic neurosteroids, opioid modulators, and onabotulinumtoxinA.
In 2013, the Food and Drug Administration approved the multimodal agent vortioxetine for the treatment of major depressive disorder. The recommended dosing is 20 mg/day, and the drug appears to have a positive effect on cognition. A meta-analysis of vortioxetine, duloxetine, and placebo comparison trials evaluated the effect of each treatment on the Digit Symbol Substitution Test (Int J Neuropsychopharmacol. 2016 Jun 15;19[10]). Vortioxetine was superior to placebo in all three trials and was superior to duloxetine in two trials. Duloxetine was not superior to placebo in two trials.
In two double-blind studies, researchers evaluated the effects of psilocybin in cancer patients with comorbid depression and anxiety. Full doses of psilocybin were 0.3 mg/kg or 22-30 mg per 70 kg. Both studies demonstrated sustained responses at full doses (J Psychopharmacol. 2016 Nov 30;30[12]:1181-97 and J Psychopharmacol. 2016 Nov 30;30[12]:1165-80).
In an open label study of psilocybin in refractory major depression, 12 patients received 10 mg on day 1 and 25 mg on day 8. Eight of 12 patients responded at 1 week, and 7 of the 12 maintained response at 3 months (Lancet Psychiatry. 2016 Jul;3[7]:619-27). According to Dr. Schatzberg, this trial became the basis for a blinded study that Compass is conducting in the United States and in the United Kingdom in which refractory patients are going to be randomized to 1 mg, 10 mg, or 25 mg psilocybin using an independent rater. “These patients are accompanied during this experience by two therapists for up to 8 hours. It’s not quite a guided therapy; it’s kind of a safety net therapy if the patient needs [help]. We’ll see what happens.”
Another agent being studied is ketamine, which works on the glutamate system, an excitatory neurotransmitter. “Glutamate is the juice that keeps us going,” said Dr. Schatzberg, who is also the Kenneth T. Norris Jr. professor of psychiatry and behavioral sciences at the university. “We can’t live without glutamate.” An anesthetic agent that has been used for 50 years, ketamine is a N-methyl-d-aspartate antagonist that has mu opioid agonist effects and stimulant properties. “It causes a psychotomimetic dissociative kind of reaction,” he said. “The doses used in depression are subanesthetic. They may put the patient asleep, but they usually don’t. The problem with the antidepressant effect is that 70% of people who initially respond don’t continue to respond beyond 1 week.”
In a randomized, placebo-controlled, double-blind crossover study of patients with treatment-resistant depression, subjects who received ketamine showed significant improvement in depression, compared with subjects who received placebo within 110 minutes after injection, which remained significant throughout the following week (Arch Gen Psychiatry. 2006;63:856-64). Specifically, of the 17 subjects treated with ketamine, 71% met response and 29% met remission criteria the day following ketamine infusion. Thirty-five percent of subjects maintained response for at least 1 week.
In an effort to further evaluate the antidepressive effects of ketamine, researchers conducted a two-site, parallel-arm, randomized, controlled trial of a single infusion of ketamine, compared with an active placebo control condition, the anesthetic midazolam (Am J Psychiatry. 2013 Oct;170[10]:1134-42). After adjustment for baseline scores and site, the Montgomery-Åsberg Depression Rating Scale score was lower in the ketamine group than in the midazolam group by 7.95 points. The likelihood of response at 24 hours was greater with ketamine than with midazolam (odds ratio, 2.18), with response rates of 64% and 28%, respectively.
In 2019, the FDA approved esketamine nasal spray, in conjunction with an oral antidepressant, for the treatment of depression in adults with treatment-resistant depression. In three phase 3 double-blind studies of esketamine in treatment-refractory depression, one was positive, two were nearly positive, and the effect sizes were mild. One maintenance discontinuation trial was positive. Based on this data, Dr. Schatzberg said, there “is not much evidence” that patients get further gain beyond an antidepressant alone in the first 24-48 hours following ketamine administration. “It makes me wonder: Should we be continuing to give this drug intranasally beyond 48 hours?” he asked. “The reason I have concern is that in certain cultures, ketamine is a highly abusable drug.”
He and Gerard Sanacora, PhD, MD, addressed the topic in a 2015 opinion piece entitled, “Ketamine: Promising path or false prophecy in the development of novel therapeutics for mood disorders?”
“If we step back for a moment and look at where we are – an intravenously administered agent that is a street drug of abuse, works rapidly, and whose enantiomers are being studied by industry for intranasal use – we should be anxious, “ they wrote (Neuropsychopharmacology. 2015 Jan;40[2]:259-67). “We need to be as careful and conservative as possible and understand how it is acting and rule out the possibility of whether it acts as an opioid.”
Dr. Schatzberg disclosed that he has served a consultant to Alkermes, Avanir, Bracket, Compass, Delpor, Epiodyne, Janssen, Jazz, Lundbeck, McKinsey, Merck, Myriad Genetics, Owl, Neuronetics, Pfizer, Sage, and Sunovion. He has received research funding from Janssen and also holds an ownership interest in Corcept, Dermira, Delpor, Epiodyne, Incyte Genetics, Madrigal, Maerck, Owl Analytics, Seattle Genetics, Titan, and Xhale.
LAS VEGAS – The risk-benefit ratio of new and emerging drugs for depression is going to be under the spotlight more than ever before, according to an expert in mood disorders.
“We’ve seen a shift in the landscape,” Alan F. Schatzberg, MD, said at an annual psychopharmacology update held by the Nevada Psychiatric Association. The risk-benefit ratio is going to become increasingly more of a focus in drug development, in terms of increasing risk.”
The development of the SSRIs, he continued, led to the introduction of agents that were generally effective and well tolerated.
“Particularly, they were well tolerated in terms of their wide safety margin,” said Dr. Schatzberg, who directs the Mood Disorders Center at Stanford (Calif.) University. “It’s difficult to [die by suicide] on an SSRI. Because of that, we had greater numbers of individuals treated than we did with the tricyclics.” The introduction of SSRIs led to “widening of the net in terms of the numbers of depressed patients,” he said. “Combine that with DSM-III, and DSM-IV having relatively easy criteria to obtain a diagnosis [of depression, and] you see a large group of subjects who are exposed to treatment. But a lot of those people may not respond to a particular agent. As new treatments are promulgated and tried, what you find is [that] a reasonable number of subjects are, in fact, truly resistant. That becomes a tough nut to crack for all of us who treat depression.”
Drugs for depression that clinicians commonly have in their armamentarium commonly revolve around monoaminergic function. New and emerging agents include hallucinatory serotonin 2a agonists, glutamatergic drugs such as ketamine, GABAergic neurosteroids, opioid modulators, and onabotulinumtoxinA.
In 2013, the Food and Drug Administration approved the multimodal agent vortioxetine for the treatment of major depressive disorder. The recommended dosing is 20 mg/day, and the drug appears to have a positive effect on cognition. A meta-analysis of vortioxetine, duloxetine, and placebo comparison trials evaluated the effect of each treatment on the Digit Symbol Substitution Test (Int J Neuropsychopharmacol. 2016 Jun 15;19[10]). Vortioxetine was superior to placebo in all three trials and was superior to duloxetine in two trials. Duloxetine was not superior to placebo in two trials.
In two double-blind studies, researchers evaluated the effects of psilocybin in cancer patients with comorbid depression and anxiety. Full doses of psilocybin were 0.3 mg/kg or 22-30 mg per 70 kg. Both studies demonstrated sustained responses at full doses (J Psychopharmacol. 2016 Nov 30;30[12]:1181-97 and J Psychopharmacol. 2016 Nov 30;30[12]:1165-80).
In an open label study of psilocybin in refractory major depression, 12 patients received 10 mg on day 1 and 25 mg on day 8. Eight of 12 patients responded at 1 week, and 7 of the 12 maintained response at 3 months (Lancet Psychiatry. 2016 Jul;3[7]:619-27). According to Dr. Schatzberg, this trial became the basis for a blinded study that Compass is conducting in the United States and in the United Kingdom in which refractory patients are going to be randomized to 1 mg, 10 mg, or 25 mg psilocybin using an independent rater. “These patients are accompanied during this experience by two therapists for up to 8 hours. It’s not quite a guided therapy; it’s kind of a safety net therapy if the patient needs [help]. We’ll see what happens.”
Another agent being studied is ketamine, which works on the glutamate system, an excitatory neurotransmitter. “Glutamate is the juice that keeps us going,” said Dr. Schatzberg, who is also the Kenneth T. Norris Jr. professor of psychiatry and behavioral sciences at the university. “We can’t live without glutamate.” An anesthetic agent that has been used for 50 years, ketamine is a N-methyl-d-aspartate antagonist that has mu opioid agonist effects and stimulant properties. “It causes a psychotomimetic dissociative kind of reaction,” he said. “The doses used in depression are subanesthetic. They may put the patient asleep, but they usually don’t. The problem with the antidepressant effect is that 70% of people who initially respond don’t continue to respond beyond 1 week.”
In a randomized, placebo-controlled, double-blind crossover study of patients with treatment-resistant depression, subjects who received ketamine showed significant improvement in depression, compared with subjects who received placebo within 110 minutes after injection, which remained significant throughout the following week (Arch Gen Psychiatry. 2006;63:856-64). Specifically, of the 17 subjects treated with ketamine, 71% met response and 29% met remission criteria the day following ketamine infusion. Thirty-five percent of subjects maintained response for at least 1 week.
In an effort to further evaluate the antidepressive effects of ketamine, researchers conducted a two-site, parallel-arm, randomized, controlled trial of a single infusion of ketamine, compared with an active placebo control condition, the anesthetic midazolam (Am J Psychiatry. 2013 Oct;170[10]:1134-42). After adjustment for baseline scores and site, the Montgomery-Åsberg Depression Rating Scale score was lower in the ketamine group than in the midazolam group by 7.95 points. The likelihood of response at 24 hours was greater with ketamine than with midazolam (odds ratio, 2.18), with response rates of 64% and 28%, respectively.
In 2019, the FDA approved esketamine nasal spray, in conjunction with an oral antidepressant, for the treatment of depression in adults with treatment-resistant depression. In three phase 3 double-blind studies of esketamine in treatment-refractory depression, one was positive, two were nearly positive, and the effect sizes were mild. One maintenance discontinuation trial was positive. Based on this data, Dr. Schatzberg said, there “is not much evidence” that patients get further gain beyond an antidepressant alone in the first 24-48 hours following ketamine administration. “It makes me wonder: Should we be continuing to give this drug intranasally beyond 48 hours?” he asked. “The reason I have concern is that in certain cultures, ketamine is a highly abusable drug.”
He and Gerard Sanacora, PhD, MD, addressed the topic in a 2015 opinion piece entitled, “Ketamine: Promising path or false prophecy in the development of novel therapeutics for mood disorders?”
“If we step back for a moment and look at where we are – an intravenously administered agent that is a street drug of abuse, works rapidly, and whose enantiomers are being studied by industry for intranasal use – we should be anxious, “ they wrote (Neuropsychopharmacology. 2015 Jan;40[2]:259-67). “We need to be as careful and conservative as possible and understand how it is acting and rule out the possibility of whether it acts as an opioid.”
Dr. Schatzberg disclosed that he has served a consultant to Alkermes, Avanir, Bracket, Compass, Delpor, Epiodyne, Janssen, Jazz, Lundbeck, McKinsey, Merck, Myriad Genetics, Owl, Neuronetics, Pfizer, Sage, and Sunovion. He has received research funding from Janssen and also holds an ownership interest in Corcept, Dermira, Delpor, Epiodyne, Incyte Genetics, Madrigal, Maerck, Owl Analytics, Seattle Genetics, Titan, and Xhale.
EXPERT ANALYSIS FROM NPA 2020