User login
Posttraumatic stress may persist up to 9 months after pregnancy loss
new research suggests.
The outcomes of a prospective cohort study involving 737 women who had experienced miscarriage or ectopic pregnancy and 171 controls with healthy pregnancies were presented in a report in the American Journal of Obstetrics & Gynecology.
One month after their pregnancy loss, 29% of these women met the criteria for posttraumatic stress, 24% reported moderate to severe anxiety, and 11% reported moderate to severe depression. In comparison, just 13% of women in the control group met the criteria for anxiety, and 2% met the criteria for depression, which meant women who had experienced early pregnancy loss had a greater than twofold odds of anxiety and nearly fourfold (odds ratio, 3.88) greater odds of depression, reported Jessica Farren, PhD, of the Queen Charlotte’s and Chelsea Hospital, London, and coauthors.
The most common posttraumatic symptom, experienced by 91% of respondents with posttraumatic stress at 1 month after the pregnancy, was reexperiencing symptoms, while 60% experienced avoidance and hyperarousal symptoms. At 3 months after the loss, 50% of those with posttraumatic stress reported an interruption of their general satisfaction with life.
While the incidence of posttraumatic stress, anxiety, and depression decreased over time in the women who had early pregnancy loss, by the third month 21% still met the criteria for posttraumatic stress, and by 9 months, 18% still were experiencing posttraumatic stress. Similarly, moderate to severe anxiety was still present in 23% of women at 3 months and 17% at 9 months, and moderate to severe depression was still experienced by 8% of women at 3 months and 6% of women at 9 months.
Dr. Farren and coauthors wrote that, given the incidence of miscarriage and ectopic pregnancy in the population, the high proportion of women still experiencing posttraumatic stress, anxiety, and depression at 9 months pointed to a significant public health issue. “It is recognized that PTSD in other contexts can have a significant impact on work, social interaction, health care utilization, and risks in future pregnancies,” they wrote. “Work is needed to evaluate strategies to effectively identify and treat affected women with these specific psychopathologies.”
The investigators also looked at the differences in outcomes in women who experienced miscarriage, compared with those who experienced ectopic pregnancy.
Of the 363 women who had a miscarriage, 30% met criteria for posttraumatic stress at 1 month, 20% at 3 months, and 17% at 9 months. Moderate to severe anxiety was reported by 25% women at 1 month, 22% at 3 months, and 17% at 9 months. Moderate to severe depression was reported by 12% at 1 month, 7% at 3 months, and 5% at 9 months.
Of the 74 women who had an ectopic pregnancy, 23% met criteria for posttraumatic stress at 1 month, 28% at 3 months, and 21% at 9 months. Moderate to severe anxiety was reported by 21% at 1 month, 30% at 3 months, and 23% at 9 months. Moderate to severe depression was reported by 7% at 1 month, 12% at 3 months, and 11% at 9 months.
The authors noted that the incidence of posttraumatic stress, anxiety, and depression decreased more strongly over time in women who had experienced miscarriage, compared with those who experienced ectopic pregnancy, although they commented that the confidence intervals were wide.
One coauthor was supported by an Imperial Health Charity grant and another by the National Institute for Health Research Biomedical Research Centre. No conflicts of interest were declared.
SOURCE: Farren J et al. Amer J Obstet Gynecol. 2019 Dec 13. doi: 10.1016/j.ajog.2019.10.102.
new research suggests.
The outcomes of a prospective cohort study involving 737 women who had experienced miscarriage or ectopic pregnancy and 171 controls with healthy pregnancies were presented in a report in the American Journal of Obstetrics & Gynecology.
One month after their pregnancy loss, 29% of these women met the criteria for posttraumatic stress, 24% reported moderate to severe anxiety, and 11% reported moderate to severe depression. In comparison, just 13% of women in the control group met the criteria for anxiety, and 2% met the criteria for depression, which meant women who had experienced early pregnancy loss had a greater than twofold odds of anxiety and nearly fourfold (odds ratio, 3.88) greater odds of depression, reported Jessica Farren, PhD, of the Queen Charlotte’s and Chelsea Hospital, London, and coauthors.
The most common posttraumatic symptom, experienced by 91% of respondents with posttraumatic stress at 1 month after the pregnancy, was reexperiencing symptoms, while 60% experienced avoidance and hyperarousal symptoms. At 3 months after the loss, 50% of those with posttraumatic stress reported an interruption of their general satisfaction with life.
While the incidence of posttraumatic stress, anxiety, and depression decreased over time in the women who had early pregnancy loss, by the third month 21% still met the criteria for posttraumatic stress, and by 9 months, 18% still were experiencing posttraumatic stress. Similarly, moderate to severe anxiety was still present in 23% of women at 3 months and 17% at 9 months, and moderate to severe depression was still experienced by 8% of women at 3 months and 6% of women at 9 months.
Dr. Farren and coauthors wrote that, given the incidence of miscarriage and ectopic pregnancy in the population, the high proportion of women still experiencing posttraumatic stress, anxiety, and depression at 9 months pointed to a significant public health issue. “It is recognized that PTSD in other contexts can have a significant impact on work, social interaction, health care utilization, and risks in future pregnancies,” they wrote. “Work is needed to evaluate strategies to effectively identify and treat affected women with these specific psychopathologies.”
The investigators also looked at the differences in outcomes in women who experienced miscarriage, compared with those who experienced ectopic pregnancy.
Of the 363 women who had a miscarriage, 30% met criteria for posttraumatic stress at 1 month, 20% at 3 months, and 17% at 9 months. Moderate to severe anxiety was reported by 25% women at 1 month, 22% at 3 months, and 17% at 9 months. Moderate to severe depression was reported by 12% at 1 month, 7% at 3 months, and 5% at 9 months.
Of the 74 women who had an ectopic pregnancy, 23% met criteria for posttraumatic stress at 1 month, 28% at 3 months, and 21% at 9 months. Moderate to severe anxiety was reported by 21% at 1 month, 30% at 3 months, and 23% at 9 months. Moderate to severe depression was reported by 7% at 1 month, 12% at 3 months, and 11% at 9 months.
The authors noted that the incidence of posttraumatic stress, anxiety, and depression decreased more strongly over time in women who had experienced miscarriage, compared with those who experienced ectopic pregnancy, although they commented that the confidence intervals were wide.
One coauthor was supported by an Imperial Health Charity grant and another by the National Institute for Health Research Biomedical Research Centre. No conflicts of interest were declared.
SOURCE: Farren J et al. Amer J Obstet Gynecol. 2019 Dec 13. doi: 10.1016/j.ajog.2019.10.102.
new research suggests.
The outcomes of a prospective cohort study involving 737 women who had experienced miscarriage or ectopic pregnancy and 171 controls with healthy pregnancies were presented in a report in the American Journal of Obstetrics & Gynecology.
One month after their pregnancy loss, 29% of these women met the criteria for posttraumatic stress, 24% reported moderate to severe anxiety, and 11% reported moderate to severe depression. In comparison, just 13% of women in the control group met the criteria for anxiety, and 2% met the criteria for depression, which meant women who had experienced early pregnancy loss had a greater than twofold odds of anxiety and nearly fourfold (odds ratio, 3.88) greater odds of depression, reported Jessica Farren, PhD, of the Queen Charlotte’s and Chelsea Hospital, London, and coauthors.
The most common posttraumatic symptom, experienced by 91% of respondents with posttraumatic stress at 1 month after the pregnancy, was reexperiencing symptoms, while 60% experienced avoidance and hyperarousal symptoms. At 3 months after the loss, 50% of those with posttraumatic stress reported an interruption of their general satisfaction with life.
While the incidence of posttraumatic stress, anxiety, and depression decreased over time in the women who had early pregnancy loss, by the third month 21% still met the criteria for posttraumatic stress, and by 9 months, 18% still were experiencing posttraumatic stress. Similarly, moderate to severe anxiety was still present in 23% of women at 3 months and 17% at 9 months, and moderate to severe depression was still experienced by 8% of women at 3 months and 6% of women at 9 months.
Dr. Farren and coauthors wrote that, given the incidence of miscarriage and ectopic pregnancy in the population, the high proportion of women still experiencing posttraumatic stress, anxiety, and depression at 9 months pointed to a significant public health issue. “It is recognized that PTSD in other contexts can have a significant impact on work, social interaction, health care utilization, and risks in future pregnancies,” they wrote. “Work is needed to evaluate strategies to effectively identify and treat affected women with these specific psychopathologies.”
The investigators also looked at the differences in outcomes in women who experienced miscarriage, compared with those who experienced ectopic pregnancy.
Of the 363 women who had a miscarriage, 30% met criteria for posttraumatic stress at 1 month, 20% at 3 months, and 17% at 9 months. Moderate to severe anxiety was reported by 25% women at 1 month, 22% at 3 months, and 17% at 9 months. Moderate to severe depression was reported by 12% at 1 month, 7% at 3 months, and 5% at 9 months.
Of the 74 women who had an ectopic pregnancy, 23% met criteria for posttraumatic stress at 1 month, 28% at 3 months, and 21% at 9 months. Moderate to severe anxiety was reported by 21% at 1 month, 30% at 3 months, and 23% at 9 months. Moderate to severe depression was reported by 7% at 1 month, 12% at 3 months, and 11% at 9 months.
The authors noted that the incidence of posttraumatic stress, anxiety, and depression decreased more strongly over time in women who had experienced miscarriage, compared with those who experienced ectopic pregnancy, although they commented that the confidence intervals were wide.
One coauthor was supported by an Imperial Health Charity grant and another by the National Institute for Health Research Biomedical Research Centre. No conflicts of interest were declared.
SOURCE: Farren J et al. Amer J Obstet Gynecol. 2019 Dec 13. doi: 10.1016/j.ajog.2019.10.102.
FROM THE AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY
Are doctors really at highest risk for suicide?
In October 2012, Pamela Wible, MD, attended a memorial service in her town for a physician who had died by suicide. Sitting in the third row, she began to count all the colleagues she had lost to suicide, and the result shocked her: 3 in her small town alone, 10 if she expanded her scope to all the doctors she’d ever known.
And so she set out on a mission to document as many physician suicides as she could, in an attempt to understand why her fellow doctors were taking their lives. “I viewed this as a personal quest,” she said in an interview. “I wanted to find out why my friends were dying.” Over the course of 7 years, she documented more than 1,300 physician suicides in the United States with the help of individuals who have lost colleagues and loved ones. She maintains a suicide prevention hotline for medical students and doctors.
On her website, Dr. Wible calls high physician suicide rates a “public health crisis.” She states many conclusions from the stories she’s collected, among them that anesthesiologists are at highest risk for suicide among physicians.
The claim that doctors have a high suicide rate is a common one beyond Dr. Wible’s documentation project. Frequently cited papers contend that 300 physicians commit suicide per year, and that physicians’ suicide rate is higher than the general population. Researchers presenting at the American Psychiatric Association meeting in 2018 said physicians have the highest suicide rate of any profession – double that of the general population, with one completed suicide every day – and Medscape’s coverage of the talk has been widely referenced as supporting evidence.
A closer look at the data behind these claims, however, reveals the difficulty of establishing reliable statistics. Dr. Wible acknowledges that her data are limited. “We do not have accurate numbers. These [statistics] have come to me organically,” she said. Incorrectly coded death certificates are one reason it’s hard to get solid information. “When we’re trying to figure out how many doctors do die by suicide, it’s very hard to know.”
Similar claims have been made at various times about dentists, construction workers, and farmers, perhaps in an effort to call attention to difficult working conditions and inadequate mental health care. Overall, an associate professor at the University of Michigan, Ann Arbor, who researches physician wellness, mental health, and suicide. It’s critical to know the accurate numbers, she said, “so we can know if we’re making progress.”
Scrutinizing a statistic
The idea for the research presented at the APA meeting in 2018 came up a year earlier “when there were quite a number of physician deaths by suicide,” lead author Omotola T’Sarumi, MD, psychiatrist and chief resident at Columbia University’s Harlem Hospital in New York at the time of the presentation, said in an interview. The poster describes the methodology as a systematic review of research articles published in the last 10 years. Dr. T’Sarumi and colleagues concluded that the rate was 28-40 suicides per 100,000 doctors, compared with a rate of 12.3 per 100,000 for the general population. “That just stunned me,” she said. “We should be doing better.” A peer-reviewed article on the work has not been published.
The references on the poster show limited data to support the headline conclusion that physicians have the highest suicide rate of any profession: four papers and a book chapter. The poster itself does not describe the methodology used to arrive at the numbers stated, and Dr. T’Sarumi said that she was unable to gain access to her previous research since moving to a new institution. Dr. Gold, the first author on one of the papers the poster cites, said there are “huge issues” with the work. “In my paper that they’re citing, I was not looking at rates of suicide,” she said. “This is just picking a couple of studies and highlighting them.”
Dr. Gold’s paper uses data from the Centers for Disease Control and Prevention’s National Violent Death Reporting System (NVDRS) to identify differences in risk factors and suicide methods between physicians and others who died by suicide in 17 states. The researchers did not attempt to quantify a difference in overall rates, but found that physicians who end their own lives are more likely to have a known mental health disorder with lower rates of medication treatment than nonphysicians. “Inadequate treatment and increased problems related to job stress may be potentially modifiable risk factors to reduce suicidal death among physicians,” the authors conclude.
The second study referenced in the 2018 poster, “A History of Physician Suicide in America” by Rupinder Legha, MD, offers a narrative history of physician suicide, including a reference to an 1897 editorial in the Philadelphia Medical and Surgical Reporter that says: “Our profession is more prone to suicide than any other.” The study does not, however, attempt to quantify that risk.
The third study referenced does offer a quantitative analysis based on death and census data in 26 states, and concludes that the suicide rate for white female physicians was about two times higher than the general population. For white male physicians and dentists, however, the study found that the overall rate of suicide was lower than in the general population, but higher in male physicians and dentists older than 55 years.
In search of reliable data
With all of the popular but poorly substantiated claims about physician suicide, Dr. Gold argues that getting accurate numbers is critical. Without them, there is no way to know if rates are increasing or decreasing over time, or if attempts to help physicians in crisis are effective.
The CDC just released its own updated analysis of NVDRS data by major occupational groups across 32 states in 2016. It shows that males and females in the construction and extraction industries had the highest suicide rates: 49.4 per 100,000 and 25.5 per 100,000 respectively. Males in the “health care practitioners and technical” occupation group had a lower than average rate, while females in the same group had a higher than average rate.
The most reliable data that exist, according to Dr. Gold, are found in the CDC’s National Occupational Mortality Surveillance catalog, though it does not contain information from all states and is missing several years of records. Based on its data, the CDC provides a proportionate mortality ratio (PMR) that indicates whether the proportion of deaths tied to a given cause for a given occupation appears high or low, compared with all other occupations. But occupation data are often missing from the CDC’s records, which could make the PMRs unreliable. “You’re talking about relatively small numbers,” said Dr. Gold. “Even if we’re talking about 400 a year, the difference in one or two or five people being physicians could make a huge difference in the rate.”
The PMR for physicians who have died by intentional self-harm suggests that they are 2.5 times as likely as other populations to die by suicide. Filtering the data by race and gender, it appears black female physicians are at highest risk, more than five times as likely to die by suicide as other populations, while white males are twice as likely. Overall, the professionals with highest suicide risk in the database are hunters and trappers, followed by podiatrists, dentists, veterans, and nuclear engineers. Physicians follow with the fifth-highest rate.
The only way to get a true sense of physician suicide rates would be to collect all of the vital records data that states report to the federal government, according to Dr. Gold. “That would require 50 separate institutional review boards, so I doubt anyone is going to go to the effort to do that study,” she said.
Even without a reliable, exact number, it’s clear there are more physician suicides than there should be, Dr. Gold said. “This is a population that really should not be having a relatively high number of suicide deaths, whether it’s highest or not.”
As Dr. Legha wrote in his “History of Physician Suicide,” cited in the 2018 APA poster: “The problem of physician suicide is not solely a matter of whether or not it takes place at a rate higher than the general public. That a professional caregiver can fall ill and not receive adequate care and support, despite being surrounded by other caregivers, begs for a thoughtful assessment to determine why it happens at all.”
If you or someone you know is in need of support, the National Suicide Prevention Lifeline’s toll-free number is 1-800-273-TALK (8255). A version of this article first appeared on Medscape.com.
In October 2012, Pamela Wible, MD, attended a memorial service in her town for a physician who had died by suicide. Sitting in the third row, she began to count all the colleagues she had lost to suicide, and the result shocked her: 3 in her small town alone, 10 if she expanded her scope to all the doctors she’d ever known.
And so she set out on a mission to document as many physician suicides as she could, in an attempt to understand why her fellow doctors were taking their lives. “I viewed this as a personal quest,” she said in an interview. “I wanted to find out why my friends were dying.” Over the course of 7 years, she documented more than 1,300 physician suicides in the United States with the help of individuals who have lost colleagues and loved ones. She maintains a suicide prevention hotline for medical students and doctors.
On her website, Dr. Wible calls high physician suicide rates a “public health crisis.” She states many conclusions from the stories she’s collected, among them that anesthesiologists are at highest risk for suicide among physicians.
The claim that doctors have a high suicide rate is a common one beyond Dr. Wible’s documentation project. Frequently cited papers contend that 300 physicians commit suicide per year, and that physicians’ suicide rate is higher than the general population. Researchers presenting at the American Psychiatric Association meeting in 2018 said physicians have the highest suicide rate of any profession – double that of the general population, with one completed suicide every day – and Medscape’s coverage of the talk has been widely referenced as supporting evidence.
A closer look at the data behind these claims, however, reveals the difficulty of establishing reliable statistics. Dr. Wible acknowledges that her data are limited. “We do not have accurate numbers. These [statistics] have come to me organically,” she said. Incorrectly coded death certificates are one reason it’s hard to get solid information. “When we’re trying to figure out how many doctors do die by suicide, it’s very hard to know.”
Similar claims have been made at various times about dentists, construction workers, and farmers, perhaps in an effort to call attention to difficult working conditions and inadequate mental health care. Overall, an associate professor at the University of Michigan, Ann Arbor, who researches physician wellness, mental health, and suicide. It’s critical to know the accurate numbers, she said, “so we can know if we’re making progress.”
Scrutinizing a statistic
The idea for the research presented at the APA meeting in 2018 came up a year earlier “when there were quite a number of physician deaths by suicide,” lead author Omotola T’Sarumi, MD, psychiatrist and chief resident at Columbia University’s Harlem Hospital in New York at the time of the presentation, said in an interview. The poster describes the methodology as a systematic review of research articles published in the last 10 years. Dr. T’Sarumi and colleagues concluded that the rate was 28-40 suicides per 100,000 doctors, compared with a rate of 12.3 per 100,000 for the general population. “That just stunned me,” she said. “We should be doing better.” A peer-reviewed article on the work has not been published.
The references on the poster show limited data to support the headline conclusion that physicians have the highest suicide rate of any profession: four papers and a book chapter. The poster itself does not describe the methodology used to arrive at the numbers stated, and Dr. T’Sarumi said that she was unable to gain access to her previous research since moving to a new institution. Dr. Gold, the first author on one of the papers the poster cites, said there are “huge issues” with the work. “In my paper that they’re citing, I was not looking at rates of suicide,” she said. “This is just picking a couple of studies and highlighting them.”
Dr. Gold’s paper uses data from the Centers for Disease Control and Prevention’s National Violent Death Reporting System (NVDRS) to identify differences in risk factors and suicide methods between physicians and others who died by suicide in 17 states. The researchers did not attempt to quantify a difference in overall rates, but found that physicians who end their own lives are more likely to have a known mental health disorder with lower rates of medication treatment than nonphysicians. “Inadequate treatment and increased problems related to job stress may be potentially modifiable risk factors to reduce suicidal death among physicians,” the authors conclude.
The second study referenced in the 2018 poster, “A History of Physician Suicide in America” by Rupinder Legha, MD, offers a narrative history of physician suicide, including a reference to an 1897 editorial in the Philadelphia Medical and Surgical Reporter that says: “Our profession is more prone to suicide than any other.” The study does not, however, attempt to quantify that risk.
The third study referenced does offer a quantitative analysis based on death and census data in 26 states, and concludes that the suicide rate for white female physicians was about two times higher than the general population. For white male physicians and dentists, however, the study found that the overall rate of suicide was lower than in the general population, but higher in male physicians and dentists older than 55 years.
In search of reliable data
With all of the popular but poorly substantiated claims about physician suicide, Dr. Gold argues that getting accurate numbers is critical. Without them, there is no way to know if rates are increasing or decreasing over time, or if attempts to help physicians in crisis are effective.
The CDC just released its own updated analysis of NVDRS data by major occupational groups across 32 states in 2016. It shows that males and females in the construction and extraction industries had the highest suicide rates: 49.4 per 100,000 and 25.5 per 100,000 respectively. Males in the “health care practitioners and technical” occupation group had a lower than average rate, while females in the same group had a higher than average rate.
The most reliable data that exist, according to Dr. Gold, are found in the CDC’s National Occupational Mortality Surveillance catalog, though it does not contain information from all states and is missing several years of records. Based on its data, the CDC provides a proportionate mortality ratio (PMR) that indicates whether the proportion of deaths tied to a given cause for a given occupation appears high or low, compared with all other occupations. But occupation data are often missing from the CDC’s records, which could make the PMRs unreliable. “You’re talking about relatively small numbers,” said Dr. Gold. “Even if we’re talking about 400 a year, the difference in one or two or five people being physicians could make a huge difference in the rate.”
The PMR for physicians who have died by intentional self-harm suggests that they are 2.5 times as likely as other populations to die by suicide. Filtering the data by race and gender, it appears black female physicians are at highest risk, more than five times as likely to die by suicide as other populations, while white males are twice as likely. Overall, the professionals with highest suicide risk in the database are hunters and trappers, followed by podiatrists, dentists, veterans, and nuclear engineers. Physicians follow with the fifth-highest rate.
The only way to get a true sense of physician suicide rates would be to collect all of the vital records data that states report to the federal government, according to Dr. Gold. “That would require 50 separate institutional review boards, so I doubt anyone is going to go to the effort to do that study,” she said.
Even without a reliable, exact number, it’s clear there are more physician suicides than there should be, Dr. Gold said. “This is a population that really should not be having a relatively high number of suicide deaths, whether it’s highest or not.”
As Dr. Legha wrote in his “History of Physician Suicide,” cited in the 2018 APA poster: “The problem of physician suicide is not solely a matter of whether or not it takes place at a rate higher than the general public. That a professional caregiver can fall ill and not receive adequate care and support, despite being surrounded by other caregivers, begs for a thoughtful assessment to determine why it happens at all.”
If you or someone you know is in need of support, the National Suicide Prevention Lifeline’s toll-free number is 1-800-273-TALK (8255). A version of this article first appeared on Medscape.com.
In October 2012, Pamela Wible, MD, attended a memorial service in her town for a physician who had died by suicide. Sitting in the third row, she began to count all the colleagues she had lost to suicide, and the result shocked her: 3 in her small town alone, 10 if she expanded her scope to all the doctors she’d ever known.
And so she set out on a mission to document as many physician suicides as she could, in an attempt to understand why her fellow doctors were taking their lives. “I viewed this as a personal quest,” she said in an interview. “I wanted to find out why my friends were dying.” Over the course of 7 years, she documented more than 1,300 physician suicides in the United States with the help of individuals who have lost colleagues and loved ones. She maintains a suicide prevention hotline for medical students and doctors.
On her website, Dr. Wible calls high physician suicide rates a “public health crisis.” She states many conclusions from the stories she’s collected, among them that anesthesiologists are at highest risk for suicide among physicians.
The claim that doctors have a high suicide rate is a common one beyond Dr. Wible’s documentation project. Frequently cited papers contend that 300 physicians commit suicide per year, and that physicians’ suicide rate is higher than the general population. Researchers presenting at the American Psychiatric Association meeting in 2018 said physicians have the highest suicide rate of any profession – double that of the general population, with one completed suicide every day – and Medscape’s coverage of the talk has been widely referenced as supporting evidence.
A closer look at the data behind these claims, however, reveals the difficulty of establishing reliable statistics. Dr. Wible acknowledges that her data are limited. “We do not have accurate numbers. These [statistics] have come to me organically,” she said. Incorrectly coded death certificates are one reason it’s hard to get solid information. “When we’re trying to figure out how many doctors do die by suicide, it’s very hard to know.”
Similar claims have been made at various times about dentists, construction workers, and farmers, perhaps in an effort to call attention to difficult working conditions and inadequate mental health care. Overall, an associate professor at the University of Michigan, Ann Arbor, who researches physician wellness, mental health, and suicide. It’s critical to know the accurate numbers, she said, “so we can know if we’re making progress.”
Scrutinizing a statistic
The idea for the research presented at the APA meeting in 2018 came up a year earlier “when there were quite a number of physician deaths by suicide,” lead author Omotola T’Sarumi, MD, psychiatrist and chief resident at Columbia University’s Harlem Hospital in New York at the time of the presentation, said in an interview. The poster describes the methodology as a systematic review of research articles published in the last 10 years. Dr. T’Sarumi and colleagues concluded that the rate was 28-40 suicides per 100,000 doctors, compared with a rate of 12.3 per 100,000 for the general population. “That just stunned me,” she said. “We should be doing better.” A peer-reviewed article on the work has not been published.
The references on the poster show limited data to support the headline conclusion that physicians have the highest suicide rate of any profession: four papers and a book chapter. The poster itself does not describe the methodology used to arrive at the numbers stated, and Dr. T’Sarumi said that she was unable to gain access to her previous research since moving to a new institution. Dr. Gold, the first author on one of the papers the poster cites, said there are “huge issues” with the work. “In my paper that they’re citing, I was not looking at rates of suicide,” she said. “This is just picking a couple of studies and highlighting them.”
Dr. Gold’s paper uses data from the Centers for Disease Control and Prevention’s National Violent Death Reporting System (NVDRS) to identify differences in risk factors and suicide methods between physicians and others who died by suicide in 17 states. The researchers did not attempt to quantify a difference in overall rates, but found that physicians who end their own lives are more likely to have a known mental health disorder with lower rates of medication treatment than nonphysicians. “Inadequate treatment and increased problems related to job stress may be potentially modifiable risk factors to reduce suicidal death among physicians,” the authors conclude.
The second study referenced in the 2018 poster, “A History of Physician Suicide in America” by Rupinder Legha, MD, offers a narrative history of physician suicide, including a reference to an 1897 editorial in the Philadelphia Medical and Surgical Reporter that says: “Our profession is more prone to suicide than any other.” The study does not, however, attempt to quantify that risk.
The third study referenced does offer a quantitative analysis based on death and census data in 26 states, and concludes that the suicide rate for white female physicians was about two times higher than the general population. For white male physicians and dentists, however, the study found that the overall rate of suicide was lower than in the general population, but higher in male physicians and dentists older than 55 years.
In search of reliable data
With all of the popular but poorly substantiated claims about physician suicide, Dr. Gold argues that getting accurate numbers is critical. Without them, there is no way to know if rates are increasing or decreasing over time, or if attempts to help physicians in crisis are effective.
The CDC just released its own updated analysis of NVDRS data by major occupational groups across 32 states in 2016. It shows that males and females in the construction and extraction industries had the highest suicide rates: 49.4 per 100,000 and 25.5 per 100,000 respectively. Males in the “health care practitioners and technical” occupation group had a lower than average rate, while females in the same group had a higher than average rate.
The most reliable data that exist, according to Dr. Gold, are found in the CDC’s National Occupational Mortality Surveillance catalog, though it does not contain information from all states and is missing several years of records. Based on its data, the CDC provides a proportionate mortality ratio (PMR) that indicates whether the proportion of deaths tied to a given cause for a given occupation appears high or low, compared with all other occupations. But occupation data are often missing from the CDC’s records, which could make the PMRs unreliable. “You’re talking about relatively small numbers,” said Dr. Gold. “Even if we’re talking about 400 a year, the difference in one or two or five people being physicians could make a huge difference in the rate.”
The PMR for physicians who have died by intentional self-harm suggests that they are 2.5 times as likely as other populations to die by suicide. Filtering the data by race and gender, it appears black female physicians are at highest risk, more than five times as likely to die by suicide as other populations, while white males are twice as likely. Overall, the professionals with highest suicide risk in the database are hunters and trappers, followed by podiatrists, dentists, veterans, and nuclear engineers. Physicians follow with the fifth-highest rate.
The only way to get a true sense of physician suicide rates would be to collect all of the vital records data that states report to the federal government, according to Dr. Gold. “That would require 50 separate institutional review boards, so I doubt anyone is going to go to the effort to do that study,” she said.
Even without a reliable, exact number, it’s clear there are more physician suicides than there should be, Dr. Gold said. “This is a population that really should not be having a relatively high number of suicide deaths, whether it’s highest or not.”
As Dr. Legha wrote in his “History of Physician Suicide,” cited in the 2018 APA poster: “The problem of physician suicide is not solely a matter of whether or not it takes place at a rate higher than the general public. That a professional caregiver can fall ill and not receive adequate care and support, despite being surrounded by other caregivers, begs for a thoughtful assessment to determine why it happens at all.”
If you or someone you know is in need of support, the National Suicide Prevention Lifeline’s toll-free number is 1-800-273-TALK (8255). A version of this article first appeared on Medscape.com.
Top research findings of 2018-2019 for clinical practice
In Part 1 of this article, published in

1. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
In light of the association of major depressive disorder (MDD) with an increased risk of aging-related diseases, Han et al2 examined whether MDD was associated with higher epigenetic aging in blood as measured by DNA methylation patterns. They also studied whether clinical characteristics of MDD had a further impact on these patterns, and whether the findings replicated in brain tissue. Many differentially methylated regions of our DNA tend to change as we age. Han et al2 used these age-sensitive differentially methylated regions to estimate chronological age, using DNA extracted from various tissues, including blood and brain.
Study design
- As a part of the Netherlands Study of Depression and Anxiety (NESDA), this study included 811 patients with MDD and 319 control participants with no lifetime psychiatric disorders and low depressive symptoms (Inventory of Depressive Symptomatology score <14).
- Diagnosis of MDD and clinical characteristics were assessed by questionnaires and psychiatric interviews. Childhood trauma was assessed using the NEMESIS childhood trauma interview, which included a structured inventory of trauma exposure during childhood.
- DNA methylation age was estimated using all methylation sites in the blood of 811 patients with MDD and 319 control participants. The residuals of the DNA methylation age estimates regressed on chronological age were calculated to indicate epigenetic aging.
- Analyses were adjusted for sociodemographic characteristics, lifestyle, and health status.
- Postmortem brain samples of 74 patients with MDD and 64 control participants were used for replication.
Outcomes
- Significantly higher epigenetic aging was observed in patients with MDD compared with control participants (Cohen’s d = 0.18), which suggests that patients with MDD are biologically older than their corresponding chronological age. There was a significant dose effect with increasing symptom severity in the overall sample.
- In the MDD group, epigenetic aging was positively and significantly associated with childhood trauma.
- The case-control difference was replicated in an independent analysis of postmortem brain samples.
Conclusion
- These findings suggest that patients with MDD and people with a history of childhood trauma may biologically age relatively faster than those without MDD or childhood trauma. These findings may represent a biomarker of aging and might help identify patients who may benefit from early and intensive interventions to reduce the physical comorbidities of MDD.
- This study raises the possibility that MDD may be causally related to epigenetic age acceleration. However, it only points out the associations; there are other possible explanations for this correlation, including the possibility that a shared risk factor accounts for the observed association.
2. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
Delirium is common and often goes underdiagnosed. It is particularly prevalent among hospitalized geriatric patients. Several medications have been suggested to have a role in treating or preventing delirium. However, it remains uncertain which medications provide the best response rate, the lowest rate of delirium occurrence, and the best tolerability. In an attempt to find answers to these questions, Wu et al3 reviewed studies that evaluated the use of various medications used for delirium.
Study design
- Researchers conducted a systematic review and network meta-analysis of randomized controlled trials (RCTs) that investigated various pharmacologic agents used to treat or prevent delirium.
- Fifty-eight RCTs were included in the analyses. Of these, 20 RCTs with a total of 1,435 participants compared the outcomes of treatments of delirium, and 38 RCTs with a total of 8,168 participants examined prevention.
- A network meta-analysis was performed to determine if an agent or combinations of agents were superior to placebo or widely used medications.
Continue to: Outcomes
Outcomes
- Haloperidol plus lorazepam provided the best response rate for treating delirium compared with placebo/control.
- For delirium prevention, patients who received ramelteon, olanzapine, risperidone, or dexmedetomidine had significantly lower delirium occurrence rates than those receiving placebo/control.
- None of the pharmacologic treatments were significantly associated with a higher risk of all-cause mortality compared with placebo/control.
Conclusion
- Haloperidol plus lorazepam might be the best treatment and ramelteon the best preventive medicine for delirium. None of the pharmacologic interventions for treatment or prophylaxis increased all-cause mortality.
- However, network meta-analyses involve extrapolating treatment comparisons that are not made directly. As Blazer8 pointed out, both findings in this study (that haloperidol plus lorazepam is a unique intervention among the treatment trials and ramelteon is a unique intervention for prevention) seemed to be driven by 2 of the 58 studies that Wu et al3 examined.Wu et al3 also cautioned that both of these interventions needed to be further researched for efficacy.
3. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
While some evidence suggests that elevated brain noradrenergic activity is involved in the initiation and maintenance of alcohol use disorder,9 current medications used to treat alcohol use disorder do not target brain noradrenergic pathways. In an RCT, Simpson et al4 tested prazosin, an alpha-1 adrenergic receptor antagonist, for the treatment of alcohol use disorder.
Study design
- In this 12-week double-blind study, 92 participants with alcohol use disorder were randomly assigned to receive prazosin or placebo. Individuals with posttraumatic stress disorder were excluded.
- Prazosin was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of Week 2. The behavioral platform was medical management. Participants provided daily data on their alcohol consumption.
- Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Outcomes
- Among the 80 participants who completed the titration period and were included in the primary analyses, prazosin was associated with self-reported fewer heavy drinking days, and fewer drinks per week (Palatino LT Std−8 vs Palatino LT Std−1.5 with placebo). Drinking days per week and craving showed no group differences.
- The rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants receiving prazosin compared with those receiving placebo.
Continue to: Conclusion
Conclusion
- These findings of moderate reductions in heavy drinking days and drinks per week with prazosin suggest that prazosin may be a promising harm-reduction treatment for alcohol use disorder.
4. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
Postpartum depression is among the most common complications of childbirth. It can result in considerable suffering for mothers, children, and families. Gamma-aminobutyric acid (GABA) signaling has previously been reported to be involved in the pathophysiology of postpartum depression. Meltzer-Brody et al5 conducted 2 double-blind, randomized, placebo-controlled, phase 3 trials comparing brexanolone with placebo in women with postpartum depression at 30 clinical research centers and specialized psychiatric units in the United States.
Study design
- Participants were women age 18 to 45, Palatino LT Std≤6 months postpartum at screening, with postpartum depression as indicated by a qualifying 17-item Hamilton Depression Rating Scale (HAM-D) score of ≥26 for Study 1 or 20 to 25 for Study 2.
- Of the 375 women who were screened simultaneously across both studies, 138 were randomly assigned (1:1:1) to receive a single IV injection of brexanolone, 90 μg/kg per hour (BRX90) (n = 45), brexanolone, 60 μg/kg per hour (BRX60) (n = 47), or placebo (n = 46) for 60 hours in Study 1, and 108 were randomly assigned (1:1) to receive BRX90 (n = 54) or placebo (n = 54) for 60 hours in Study 2.
- The primary efficacy endpoint was change in total score on the HAM-D from baseline to 60 hours. Patients were followed until Day 30.
Outcomes
- In Study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (standard error [SE] 1.2) in the BRX60 group and 17.7 points (SE 1.2) in the BRX90 group, compared with 14.0 points (SE 1.1) in the placebo group.
- In Study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group.
- In Study 1, one patient in the BRX60 group had 2 serious adverse events (suicidal ideation and intentional overdose attempt during follow-up). In Study 2, one patient in the BRX90 group had 2 serious adverse events (altered state of consciousness and syncope), which were considered treatment-related.
Conclusion
- Administration of brexanolone injection for postpartum depression resulted in significant, clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with a rapid onset of action and durable treatment response during the study period. These results suggest that brexanolone injection has the potential to improve treatment options for women with this disorder.
Continue to: #5
5. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
In clinical practice, the use of multiple antipsychotic agents for the maintenance treatment of schizophrenia is common but generally not recommended. The effectiveness of antipsychotic polypharmacy in preventing relapse of schizophrenia has not been established, and whether specific antipsychotic combinations are superior to monotherapies for maintenance treatment of schizophrenia is unknown. Tiihonen et al6 investigated the association of specific antipsychotic combinations with psychiatric rehospitalization, which was used as a marker for relapse.
Study design
- This study included 62,250 patients with schizophrenia, treated between January 1, 1996 and December 31, 2015, in a comprehensive, nationwide cohort in Finland. Overall, 31,257 individuals (50.2%) were men, and the median age was 45.6 (interquartile range, 34.6 to 57.9).
- Patients were receiving 29 different antipsychotic monotherapy or polypharmacy regimens.
- Researchers analyzed data from April 24 to June 15, 2018 using psychiatric rehospitalization as a marker for relapse. To minimize selection bias, rehospitalization risks were investigated using within-individual analyses.
- The main outcome was the hazard ratio (HR) for psychiatric rehospitalization during use of polypharmacy vs monotherapy by the same patient.
Outcomes
- Clozapine plus aripiprazole was associated with the lowest risk of psychiatric rehospitalization, with a difference of 14% (HR, .86; CI, .79 to .94) compared with clozapine monotherapy in the analysis that included all polypharmacy periods, and 18% (HR, .82; CI, .75 to .89) in the conservatively defined polypharmacy analysis that excluded periods <90 days.
- Among patients experiencing their first episode of schizophrenia, the differences between clozapine plus aripiprazole vs clozapine monotherapy were greater, with a difference of 22% in the analysis that included all polypharmacy periods, and 23% in the conservatively defined polypharmacy analysis.
- At the aggregate level, any antipsychotic polypharmacy was associated with a 7% to 13% lower risk of psychiatric rehospitalization compared with any monotherapy.
- Clozapine was the only monotherapy among the 10 best treatments.
- Results on all-cause and somatic hospitalization, mortality, and other sensitivity analyses were in line with the primary outcomes.
Conclusion
- This study suggests that certain types of antipsychotic polypharmacy may reduce the risk of rehospitalization in patients with schizophrenia. Current treatment guidelines state that clinicians should prefer antipsychotic monotherapy and avoid polypharmacy. Tiihonen et al6 raise the question whether current treatment guidelines should continue to discourage antipsychotic polypharmacy in the maintenance treatment of schizophrenia.
- Despite the large administrative databases and sophisticated statistical methods used in this study, this approach has important limitations. As Goff10 points out, despite efforts to minimize bias, these results should be considered preliminary until confirmed by RCTs.
6. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
In routine clinical practice, patients with schizophrenia are often treated with combinations of antipsychotics and other psychotropic medications. However, there is little evidence about the comparative effectiveness of these adjunctive treatment strategies. Stroup et al7 investigated the comparative real-world effectiveness of adjunctive psychotropic treatments for patients with schizophrenia.
Continue to: Study design
Study design
- This comparative effectiveness study used US Medicaid data from January 1, 2001, to December 31, 2010. Data analysis was performed from January 1, 2017, to June 30, 2018.
- The study cohort included 81,921 adult outpatients diagnosed with schizophrenia with a mean age of 40.7 (range: 18 to 64), including 37,515 women (45.8%). All patients were stably treated with a single antipsychotic and then started on an adjunctive antidepressant (n = 31,117), benzodiazepine (n = 11,941), mood stabilizer (n = 12,849), or another antipsychotic (n = 26,014).
- Researchers used multinomial logistic regression models to estimate propensity scores to balance covariates across the 4 medication groups. Weighted Cox proportional hazards regression models were used to compare treatment outcomes during 365 days on an intention-to-treat basis.
- The main outcomes and measures included risk of hospitalization for a mental disorder (primary), emergency department (ED) visits for a mental disorder, and all-cause mortality.
Outcomes
- Compared with starting another antipsychotic, initiating use of an antidepressant was associated with a lower risk of psychiatric hospitalization, and initiating use of a benzodiazepine was associated with a higher risk. Initiating use of a mood stabilizer was not significantly different from initiating use of another antipsychotic.
- A similar pattern of associations was observed in psychiatric ED visits for initiating use of an antidepressant, benzodiazepine, or mood stabilizer.
- Initiating use of a mood stabilizer was associated with an increased risk of mortality.
Conclusion
- Compared with the addition of a second antipsychotic, adding an antidepressant was associated with substantially reduced rates of hospitalization, whereas adding a benzodiazepine was associated with a modest increase in the risk of hospitalization. While the addition of a mood stabilizer was not associated with a significant difference in the risk of hospitalization, it was associated with higher mortality.
- Despite the limitations associated with this study, the associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation.
Bottom Line
Significantly higher epigenetic aging has been observed in patients with major depressive disorder. Haloperidol plus lorazepam might be an effective treatment for delirium; and ramelteon may be effective for preventing delirium. Prazosin reduces heavy drinking in patients with alcohol use disorder. A 60-hour infusion of brexanolone can help alleviate postpartum depression. Clozapine plus aripiprazole reduces the risk of rehospitalization among patients with schizophrenia. Adding an antidepressant to an antipsychotic also can reduce the risk of rehospitalization among patients with schizophrenia.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Aripiprazole • Abilify
Brexanolone • Zulresso
Clozapine • Clozaril
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Ramelteon • Rozerem
Risperidone • Risperdal
1. Saeed SA, Stanley JB. Top research findings of 2018-2019. First of 2 parts. Current Psychiatry. 2020;19(1):13-18.
2. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
3. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
4. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
5. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
6. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
7. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
8. Blazer DG. Pharmacologic intervention for the treatment and prevention of delirium: looking beneath the modeling. JAMA Psychiatry. 2019;76(5):472-473.
9. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61-75.
10. Goff DC. Can adjunctive pharmacotherapy reduce hospitalization in schizophrenia? Insights from administrative databases. JAMA Psychiatry. 2019;76(5):468-469.
In Part 1 of this article, published in

1. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
In light of the association of major depressive disorder (MDD) with an increased risk of aging-related diseases, Han et al2 examined whether MDD was associated with higher epigenetic aging in blood as measured by DNA methylation patterns. They also studied whether clinical characteristics of MDD had a further impact on these patterns, and whether the findings replicated in brain tissue. Many differentially methylated regions of our DNA tend to change as we age. Han et al2 used these age-sensitive differentially methylated regions to estimate chronological age, using DNA extracted from various tissues, including blood and brain.
Study design
- As a part of the Netherlands Study of Depression and Anxiety (NESDA), this study included 811 patients with MDD and 319 control participants with no lifetime psychiatric disorders and low depressive symptoms (Inventory of Depressive Symptomatology score <14).
- Diagnosis of MDD and clinical characteristics were assessed by questionnaires and psychiatric interviews. Childhood trauma was assessed using the NEMESIS childhood trauma interview, which included a structured inventory of trauma exposure during childhood.
- DNA methylation age was estimated using all methylation sites in the blood of 811 patients with MDD and 319 control participants. The residuals of the DNA methylation age estimates regressed on chronological age were calculated to indicate epigenetic aging.
- Analyses were adjusted for sociodemographic characteristics, lifestyle, and health status.
- Postmortem brain samples of 74 patients with MDD and 64 control participants were used for replication.
Outcomes
- Significantly higher epigenetic aging was observed in patients with MDD compared with control participants (Cohen’s d = 0.18), which suggests that patients with MDD are biologically older than their corresponding chronological age. There was a significant dose effect with increasing symptom severity in the overall sample.
- In the MDD group, epigenetic aging was positively and significantly associated with childhood trauma.
- The case-control difference was replicated in an independent analysis of postmortem brain samples.
Conclusion
- These findings suggest that patients with MDD and people with a history of childhood trauma may biologically age relatively faster than those without MDD or childhood trauma. These findings may represent a biomarker of aging and might help identify patients who may benefit from early and intensive interventions to reduce the physical comorbidities of MDD.
- This study raises the possibility that MDD may be causally related to epigenetic age acceleration. However, it only points out the associations; there are other possible explanations for this correlation, including the possibility that a shared risk factor accounts for the observed association.
2. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
Delirium is common and often goes underdiagnosed. It is particularly prevalent among hospitalized geriatric patients. Several medications have been suggested to have a role in treating or preventing delirium. However, it remains uncertain which medications provide the best response rate, the lowest rate of delirium occurrence, and the best tolerability. In an attempt to find answers to these questions, Wu et al3 reviewed studies that evaluated the use of various medications used for delirium.
Study design
- Researchers conducted a systematic review and network meta-analysis of randomized controlled trials (RCTs) that investigated various pharmacologic agents used to treat or prevent delirium.
- Fifty-eight RCTs were included in the analyses. Of these, 20 RCTs with a total of 1,435 participants compared the outcomes of treatments of delirium, and 38 RCTs with a total of 8,168 participants examined prevention.
- A network meta-analysis was performed to determine if an agent or combinations of agents were superior to placebo or widely used medications.
Continue to: Outcomes
Outcomes
- Haloperidol plus lorazepam provided the best response rate for treating delirium compared with placebo/control.
- For delirium prevention, patients who received ramelteon, olanzapine, risperidone, or dexmedetomidine had significantly lower delirium occurrence rates than those receiving placebo/control.
- None of the pharmacologic treatments were significantly associated with a higher risk of all-cause mortality compared with placebo/control.
Conclusion
- Haloperidol plus lorazepam might be the best treatment and ramelteon the best preventive medicine for delirium. None of the pharmacologic interventions for treatment or prophylaxis increased all-cause mortality.
- However, network meta-analyses involve extrapolating treatment comparisons that are not made directly. As Blazer8 pointed out, both findings in this study (that haloperidol plus lorazepam is a unique intervention among the treatment trials and ramelteon is a unique intervention for prevention) seemed to be driven by 2 of the 58 studies that Wu et al3 examined.Wu et al3 also cautioned that both of these interventions needed to be further researched for efficacy.
3. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
While some evidence suggests that elevated brain noradrenergic activity is involved in the initiation and maintenance of alcohol use disorder,9 current medications used to treat alcohol use disorder do not target brain noradrenergic pathways. In an RCT, Simpson et al4 tested prazosin, an alpha-1 adrenergic receptor antagonist, for the treatment of alcohol use disorder.
Study design
- In this 12-week double-blind study, 92 participants with alcohol use disorder were randomly assigned to receive prazosin or placebo. Individuals with posttraumatic stress disorder were excluded.
- Prazosin was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of Week 2. The behavioral platform was medical management. Participants provided daily data on their alcohol consumption.
- Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Outcomes
- Among the 80 participants who completed the titration period and were included in the primary analyses, prazosin was associated with self-reported fewer heavy drinking days, and fewer drinks per week (Palatino LT Std−8 vs Palatino LT Std−1.5 with placebo). Drinking days per week and craving showed no group differences.
- The rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants receiving prazosin compared with those receiving placebo.
Continue to: Conclusion
Conclusion
- These findings of moderate reductions in heavy drinking days and drinks per week with prazosin suggest that prazosin may be a promising harm-reduction treatment for alcohol use disorder.
4. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
Postpartum depression is among the most common complications of childbirth. It can result in considerable suffering for mothers, children, and families. Gamma-aminobutyric acid (GABA) signaling has previously been reported to be involved in the pathophysiology of postpartum depression. Meltzer-Brody et al5 conducted 2 double-blind, randomized, placebo-controlled, phase 3 trials comparing brexanolone with placebo in women with postpartum depression at 30 clinical research centers and specialized psychiatric units in the United States.
Study design
- Participants were women age 18 to 45, Palatino LT Std≤6 months postpartum at screening, with postpartum depression as indicated by a qualifying 17-item Hamilton Depression Rating Scale (HAM-D) score of ≥26 for Study 1 or 20 to 25 for Study 2.
- Of the 375 women who were screened simultaneously across both studies, 138 were randomly assigned (1:1:1) to receive a single IV injection of brexanolone, 90 μg/kg per hour (BRX90) (n = 45), brexanolone, 60 μg/kg per hour (BRX60) (n = 47), or placebo (n = 46) for 60 hours in Study 1, and 108 were randomly assigned (1:1) to receive BRX90 (n = 54) or placebo (n = 54) for 60 hours in Study 2.
- The primary efficacy endpoint was change in total score on the HAM-D from baseline to 60 hours. Patients were followed until Day 30.
Outcomes
- In Study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (standard error [SE] 1.2) in the BRX60 group and 17.7 points (SE 1.2) in the BRX90 group, compared with 14.0 points (SE 1.1) in the placebo group.
- In Study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group.
- In Study 1, one patient in the BRX60 group had 2 serious adverse events (suicidal ideation and intentional overdose attempt during follow-up). In Study 2, one patient in the BRX90 group had 2 serious adverse events (altered state of consciousness and syncope), which were considered treatment-related.
Conclusion
- Administration of brexanolone injection for postpartum depression resulted in significant, clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with a rapid onset of action and durable treatment response during the study period. These results suggest that brexanolone injection has the potential to improve treatment options for women with this disorder.
Continue to: #5
5. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
In clinical practice, the use of multiple antipsychotic agents for the maintenance treatment of schizophrenia is common but generally not recommended. The effectiveness of antipsychotic polypharmacy in preventing relapse of schizophrenia has not been established, and whether specific antipsychotic combinations are superior to monotherapies for maintenance treatment of schizophrenia is unknown. Tiihonen et al6 investigated the association of specific antipsychotic combinations with psychiatric rehospitalization, which was used as a marker for relapse.
Study design
- This study included 62,250 patients with schizophrenia, treated between January 1, 1996 and December 31, 2015, in a comprehensive, nationwide cohort in Finland. Overall, 31,257 individuals (50.2%) were men, and the median age was 45.6 (interquartile range, 34.6 to 57.9).
- Patients were receiving 29 different antipsychotic monotherapy or polypharmacy regimens.
- Researchers analyzed data from April 24 to June 15, 2018 using psychiatric rehospitalization as a marker for relapse. To minimize selection bias, rehospitalization risks were investigated using within-individual analyses.
- The main outcome was the hazard ratio (HR) for psychiatric rehospitalization during use of polypharmacy vs monotherapy by the same patient.
Outcomes
- Clozapine plus aripiprazole was associated with the lowest risk of psychiatric rehospitalization, with a difference of 14% (HR, .86; CI, .79 to .94) compared with clozapine monotherapy in the analysis that included all polypharmacy periods, and 18% (HR, .82; CI, .75 to .89) in the conservatively defined polypharmacy analysis that excluded periods <90 days.
- Among patients experiencing their first episode of schizophrenia, the differences between clozapine plus aripiprazole vs clozapine monotherapy were greater, with a difference of 22% in the analysis that included all polypharmacy periods, and 23% in the conservatively defined polypharmacy analysis.
- At the aggregate level, any antipsychotic polypharmacy was associated with a 7% to 13% lower risk of psychiatric rehospitalization compared with any monotherapy.
- Clozapine was the only monotherapy among the 10 best treatments.
- Results on all-cause and somatic hospitalization, mortality, and other sensitivity analyses were in line with the primary outcomes.
Conclusion
- This study suggests that certain types of antipsychotic polypharmacy may reduce the risk of rehospitalization in patients with schizophrenia. Current treatment guidelines state that clinicians should prefer antipsychotic monotherapy and avoid polypharmacy. Tiihonen et al6 raise the question whether current treatment guidelines should continue to discourage antipsychotic polypharmacy in the maintenance treatment of schizophrenia.
- Despite the large administrative databases and sophisticated statistical methods used in this study, this approach has important limitations. As Goff10 points out, despite efforts to minimize bias, these results should be considered preliminary until confirmed by RCTs.
6. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
In routine clinical practice, patients with schizophrenia are often treated with combinations of antipsychotics and other psychotropic medications. However, there is little evidence about the comparative effectiveness of these adjunctive treatment strategies. Stroup et al7 investigated the comparative real-world effectiveness of adjunctive psychotropic treatments for patients with schizophrenia.
Continue to: Study design
Study design
- This comparative effectiveness study used US Medicaid data from January 1, 2001, to December 31, 2010. Data analysis was performed from January 1, 2017, to June 30, 2018.
- The study cohort included 81,921 adult outpatients diagnosed with schizophrenia with a mean age of 40.7 (range: 18 to 64), including 37,515 women (45.8%). All patients were stably treated with a single antipsychotic and then started on an adjunctive antidepressant (n = 31,117), benzodiazepine (n = 11,941), mood stabilizer (n = 12,849), or another antipsychotic (n = 26,014).
- Researchers used multinomial logistic regression models to estimate propensity scores to balance covariates across the 4 medication groups. Weighted Cox proportional hazards regression models were used to compare treatment outcomes during 365 days on an intention-to-treat basis.
- The main outcomes and measures included risk of hospitalization for a mental disorder (primary), emergency department (ED) visits for a mental disorder, and all-cause mortality.
Outcomes
- Compared with starting another antipsychotic, initiating use of an antidepressant was associated with a lower risk of psychiatric hospitalization, and initiating use of a benzodiazepine was associated with a higher risk. Initiating use of a mood stabilizer was not significantly different from initiating use of another antipsychotic.
- A similar pattern of associations was observed in psychiatric ED visits for initiating use of an antidepressant, benzodiazepine, or mood stabilizer.
- Initiating use of a mood stabilizer was associated with an increased risk of mortality.
Conclusion
- Compared with the addition of a second antipsychotic, adding an antidepressant was associated with substantially reduced rates of hospitalization, whereas adding a benzodiazepine was associated with a modest increase in the risk of hospitalization. While the addition of a mood stabilizer was not associated with a significant difference in the risk of hospitalization, it was associated with higher mortality.
- Despite the limitations associated with this study, the associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation.
Bottom Line
Significantly higher epigenetic aging has been observed in patients with major depressive disorder. Haloperidol plus lorazepam might be an effective treatment for delirium; and ramelteon may be effective for preventing delirium. Prazosin reduces heavy drinking in patients with alcohol use disorder. A 60-hour infusion of brexanolone can help alleviate postpartum depression. Clozapine plus aripiprazole reduces the risk of rehospitalization among patients with schizophrenia. Adding an antidepressant to an antipsychotic also can reduce the risk of rehospitalization among patients with schizophrenia.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Aripiprazole • Abilify
Brexanolone • Zulresso
Clozapine • Clozaril
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Ramelteon • Rozerem
Risperidone • Risperdal
In Part 1 of this article, published in

1. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
In light of the association of major depressive disorder (MDD) with an increased risk of aging-related diseases, Han et al2 examined whether MDD was associated with higher epigenetic aging in blood as measured by DNA methylation patterns. They also studied whether clinical characteristics of MDD had a further impact on these patterns, and whether the findings replicated in brain tissue. Many differentially methylated regions of our DNA tend to change as we age. Han et al2 used these age-sensitive differentially methylated regions to estimate chronological age, using DNA extracted from various tissues, including blood and brain.
Study design
- As a part of the Netherlands Study of Depression and Anxiety (NESDA), this study included 811 patients with MDD and 319 control participants with no lifetime psychiatric disorders and low depressive symptoms (Inventory of Depressive Symptomatology score <14).
- Diagnosis of MDD and clinical characteristics were assessed by questionnaires and psychiatric interviews. Childhood trauma was assessed using the NEMESIS childhood trauma interview, which included a structured inventory of trauma exposure during childhood.
- DNA methylation age was estimated using all methylation sites in the blood of 811 patients with MDD and 319 control participants. The residuals of the DNA methylation age estimates regressed on chronological age were calculated to indicate epigenetic aging.
- Analyses were adjusted for sociodemographic characteristics, lifestyle, and health status.
- Postmortem brain samples of 74 patients with MDD and 64 control participants were used for replication.
Outcomes
- Significantly higher epigenetic aging was observed in patients with MDD compared with control participants (Cohen’s d = 0.18), which suggests that patients with MDD are biologically older than their corresponding chronological age. There was a significant dose effect with increasing symptom severity in the overall sample.
- In the MDD group, epigenetic aging was positively and significantly associated with childhood trauma.
- The case-control difference was replicated in an independent analysis of postmortem brain samples.
Conclusion
- These findings suggest that patients with MDD and people with a history of childhood trauma may biologically age relatively faster than those without MDD or childhood trauma. These findings may represent a biomarker of aging and might help identify patients who may benefit from early and intensive interventions to reduce the physical comorbidities of MDD.
- This study raises the possibility that MDD may be causally related to epigenetic age acceleration. However, it only points out the associations; there are other possible explanations for this correlation, including the possibility that a shared risk factor accounts for the observed association.
2. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
Delirium is common and often goes underdiagnosed. It is particularly prevalent among hospitalized geriatric patients. Several medications have been suggested to have a role in treating or preventing delirium. However, it remains uncertain which medications provide the best response rate, the lowest rate of delirium occurrence, and the best tolerability. In an attempt to find answers to these questions, Wu et al3 reviewed studies that evaluated the use of various medications used for delirium.
Study design
- Researchers conducted a systematic review and network meta-analysis of randomized controlled trials (RCTs) that investigated various pharmacologic agents used to treat or prevent delirium.
- Fifty-eight RCTs were included in the analyses. Of these, 20 RCTs with a total of 1,435 participants compared the outcomes of treatments of delirium, and 38 RCTs with a total of 8,168 participants examined prevention.
- A network meta-analysis was performed to determine if an agent or combinations of agents were superior to placebo or widely used medications.
Continue to: Outcomes
Outcomes
- Haloperidol plus lorazepam provided the best response rate for treating delirium compared with placebo/control.
- For delirium prevention, patients who received ramelteon, olanzapine, risperidone, or dexmedetomidine had significantly lower delirium occurrence rates than those receiving placebo/control.
- None of the pharmacologic treatments were significantly associated with a higher risk of all-cause mortality compared with placebo/control.
Conclusion
- Haloperidol plus lorazepam might be the best treatment and ramelteon the best preventive medicine for delirium. None of the pharmacologic interventions for treatment or prophylaxis increased all-cause mortality.
- However, network meta-analyses involve extrapolating treatment comparisons that are not made directly. As Blazer8 pointed out, both findings in this study (that haloperidol plus lorazepam is a unique intervention among the treatment trials and ramelteon is a unique intervention for prevention) seemed to be driven by 2 of the 58 studies that Wu et al3 examined.Wu et al3 also cautioned that both of these interventions needed to be further researched for efficacy.
3. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
While some evidence suggests that elevated brain noradrenergic activity is involved in the initiation and maintenance of alcohol use disorder,9 current medications used to treat alcohol use disorder do not target brain noradrenergic pathways. In an RCT, Simpson et al4 tested prazosin, an alpha-1 adrenergic receptor antagonist, for the treatment of alcohol use disorder.
Study design
- In this 12-week double-blind study, 92 participants with alcohol use disorder were randomly assigned to receive prazosin or placebo. Individuals with posttraumatic stress disorder were excluded.
- Prazosin was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of Week 2. The behavioral platform was medical management. Participants provided daily data on their alcohol consumption.
- Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Outcomes
- Among the 80 participants who completed the titration period and were included in the primary analyses, prazosin was associated with self-reported fewer heavy drinking days, and fewer drinks per week (Palatino LT Std−8 vs Palatino LT Std−1.5 with placebo). Drinking days per week and craving showed no group differences.
- The rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants receiving prazosin compared with those receiving placebo.
Continue to: Conclusion
Conclusion
- These findings of moderate reductions in heavy drinking days and drinks per week with prazosin suggest that prazosin may be a promising harm-reduction treatment for alcohol use disorder.
4. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
Postpartum depression is among the most common complications of childbirth. It can result in considerable suffering for mothers, children, and families. Gamma-aminobutyric acid (GABA) signaling has previously been reported to be involved in the pathophysiology of postpartum depression. Meltzer-Brody et al5 conducted 2 double-blind, randomized, placebo-controlled, phase 3 trials comparing brexanolone with placebo in women with postpartum depression at 30 clinical research centers and specialized psychiatric units in the United States.
Study design
- Participants were women age 18 to 45, Palatino LT Std≤6 months postpartum at screening, with postpartum depression as indicated by a qualifying 17-item Hamilton Depression Rating Scale (HAM-D) score of ≥26 for Study 1 or 20 to 25 for Study 2.
- Of the 375 women who were screened simultaneously across both studies, 138 were randomly assigned (1:1:1) to receive a single IV injection of brexanolone, 90 μg/kg per hour (BRX90) (n = 45), brexanolone, 60 μg/kg per hour (BRX60) (n = 47), or placebo (n = 46) for 60 hours in Study 1, and 108 were randomly assigned (1:1) to receive BRX90 (n = 54) or placebo (n = 54) for 60 hours in Study 2.
- The primary efficacy endpoint was change in total score on the HAM-D from baseline to 60 hours. Patients were followed until Day 30.
Outcomes
- In Study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (standard error [SE] 1.2) in the BRX60 group and 17.7 points (SE 1.2) in the BRX90 group, compared with 14.0 points (SE 1.1) in the placebo group.
- In Study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group.
- In Study 1, one patient in the BRX60 group had 2 serious adverse events (suicidal ideation and intentional overdose attempt during follow-up). In Study 2, one patient in the BRX90 group had 2 serious adverse events (altered state of consciousness and syncope), which were considered treatment-related.
Conclusion
- Administration of brexanolone injection for postpartum depression resulted in significant, clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with a rapid onset of action and durable treatment response during the study period. These results suggest that brexanolone injection has the potential to improve treatment options for women with this disorder.
Continue to: #5
5. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
In clinical practice, the use of multiple antipsychotic agents for the maintenance treatment of schizophrenia is common but generally not recommended. The effectiveness of antipsychotic polypharmacy in preventing relapse of schizophrenia has not been established, and whether specific antipsychotic combinations are superior to monotherapies for maintenance treatment of schizophrenia is unknown. Tiihonen et al6 investigated the association of specific antipsychotic combinations with psychiatric rehospitalization, which was used as a marker for relapse.
Study design
- This study included 62,250 patients with schizophrenia, treated between January 1, 1996 and December 31, 2015, in a comprehensive, nationwide cohort in Finland. Overall, 31,257 individuals (50.2%) were men, and the median age was 45.6 (interquartile range, 34.6 to 57.9).
- Patients were receiving 29 different antipsychotic monotherapy or polypharmacy regimens.
- Researchers analyzed data from April 24 to June 15, 2018 using psychiatric rehospitalization as a marker for relapse. To minimize selection bias, rehospitalization risks were investigated using within-individual analyses.
- The main outcome was the hazard ratio (HR) for psychiatric rehospitalization during use of polypharmacy vs monotherapy by the same patient.
Outcomes
- Clozapine plus aripiprazole was associated with the lowest risk of psychiatric rehospitalization, with a difference of 14% (HR, .86; CI, .79 to .94) compared with clozapine monotherapy in the analysis that included all polypharmacy periods, and 18% (HR, .82; CI, .75 to .89) in the conservatively defined polypharmacy analysis that excluded periods <90 days.
- Among patients experiencing their first episode of schizophrenia, the differences between clozapine plus aripiprazole vs clozapine monotherapy were greater, with a difference of 22% in the analysis that included all polypharmacy periods, and 23% in the conservatively defined polypharmacy analysis.
- At the aggregate level, any antipsychotic polypharmacy was associated with a 7% to 13% lower risk of psychiatric rehospitalization compared with any monotherapy.
- Clozapine was the only monotherapy among the 10 best treatments.
- Results on all-cause and somatic hospitalization, mortality, and other sensitivity analyses were in line with the primary outcomes.
Conclusion
- This study suggests that certain types of antipsychotic polypharmacy may reduce the risk of rehospitalization in patients with schizophrenia. Current treatment guidelines state that clinicians should prefer antipsychotic monotherapy and avoid polypharmacy. Tiihonen et al6 raise the question whether current treatment guidelines should continue to discourage antipsychotic polypharmacy in the maintenance treatment of schizophrenia.
- Despite the large administrative databases and sophisticated statistical methods used in this study, this approach has important limitations. As Goff10 points out, despite efforts to minimize bias, these results should be considered preliminary until confirmed by RCTs.
6. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
In routine clinical practice, patients with schizophrenia are often treated with combinations of antipsychotics and other psychotropic medications. However, there is little evidence about the comparative effectiveness of these adjunctive treatment strategies. Stroup et al7 investigated the comparative real-world effectiveness of adjunctive psychotropic treatments for patients with schizophrenia.
Continue to: Study design
Study design
- This comparative effectiveness study used US Medicaid data from January 1, 2001, to December 31, 2010. Data analysis was performed from January 1, 2017, to June 30, 2018.
- The study cohort included 81,921 adult outpatients diagnosed with schizophrenia with a mean age of 40.7 (range: 18 to 64), including 37,515 women (45.8%). All patients were stably treated with a single antipsychotic and then started on an adjunctive antidepressant (n = 31,117), benzodiazepine (n = 11,941), mood stabilizer (n = 12,849), or another antipsychotic (n = 26,014).
- Researchers used multinomial logistic regression models to estimate propensity scores to balance covariates across the 4 medication groups. Weighted Cox proportional hazards regression models were used to compare treatment outcomes during 365 days on an intention-to-treat basis.
- The main outcomes and measures included risk of hospitalization for a mental disorder (primary), emergency department (ED) visits for a mental disorder, and all-cause mortality.
Outcomes
- Compared with starting another antipsychotic, initiating use of an antidepressant was associated with a lower risk of psychiatric hospitalization, and initiating use of a benzodiazepine was associated with a higher risk. Initiating use of a mood stabilizer was not significantly different from initiating use of another antipsychotic.
- A similar pattern of associations was observed in psychiatric ED visits for initiating use of an antidepressant, benzodiazepine, or mood stabilizer.
- Initiating use of a mood stabilizer was associated with an increased risk of mortality.
Conclusion
- Compared with the addition of a second antipsychotic, adding an antidepressant was associated with substantially reduced rates of hospitalization, whereas adding a benzodiazepine was associated with a modest increase in the risk of hospitalization. While the addition of a mood stabilizer was not associated with a significant difference in the risk of hospitalization, it was associated with higher mortality.
- Despite the limitations associated with this study, the associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation.
Bottom Line
Significantly higher epigenetic aging has been observed in patients with major depressive disorder. Haloperidol plus lorazepam might be an effective treatment for delirium; and ramelteon may be effective for preventing delirium. Prazosin reduces heavy drinking in patients with alcohol use disorder. A 60-hour infusion of brexanolone can help alleviate postpartum depression. Clozapine plus aripiprazole reduces the risk of rehospitalization among patients with schizophrenia. Adding an antidepressant to an antipsychotic also can reduce the risk of rehospitalization among patients with schizophrenia.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Aripiprazole • Abilify
Brexanolone • Zulresso
Clozapine • Clozaril
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Ramelteon • Rozerem
Risperidone • Risperdal
1. Saeed SA, Stanley JB. Top research findings of 2018-2019. First of 2 parts. Current Psychiatry. 2020;19(1):13-18.
2. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
3. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
4. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
5. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
6. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
7. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
8. Blazer DG. Pharmacologic intervention for the treatment and prevention of delirium: looking beneath the modeling. JAMA Psychiatry. 2019;76(5):472-473.
9. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61-75.
10. Goff DC. Can adjunctive pharmacotherapy reduce hospitalization in schizophrenia? Insights from administrative databases. JAMA Psychiatry. 2019;76(5):468-469.
1. Saeed SA, Stanley JB. Top research findings of 2018-2019. First of 2 parts. Current Psychiatry. 2020;19(1):13-18.
2. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
3. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
4. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
5. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
6. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
7. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
8. Blazer DG. Pharmacologic intervention for the treatment and prevention of delirium: looking beneath the modeling. JAMA Psychiatry. 2019;76(5):472-473.
9. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61-75.
10. Goff DC. Can adjunctive pharmacotherapy reduce hospitalization in schizophrenia? Insights from administrative databases. JAMA Psychiatry. 2019;76(5):468-469.
Suicidal while receiving treatment for breast cancer
CASE Worsening mood symptoms and suicidal ideation
On a recent visit to the oncology clinic, where she has been receiving treatment for breast cancer for 11 months, Mrs. L, age 46, reports the abrupt onset of sadness, irritability, difficulty sleeping, and negative self-thoughts.
Eleven months ago, Mrs. L was diagnosed with invasive lobular carcinoma of the right breast that was classified as T2N0MX, representing relatively early-stage disease. Shortly after her diagnosis, Mrs. L completed 4 cycles of neoadjuvant chemotherapy with doxorubicin and cyclophosphamide, followed by treatment with trastuzumab. Subsequently, she underwent a right segmental mastectomy with bilateral mastopexy and radiation therapy. Recently, Mrs. L’s oncology team prescribed tamoxifen, 20 mg/d, and trastuzumab, 420 mg IV every 3 weeks; however, within 3 weeks after starting tamoxifen, Mrs. L’s mood symptoms worsened to the point where she says she is considering suicide—with a plan to use her husband’s gun to kill herself.
Mrs. L has no other pertinent medical history and no reported history of psychiatric disease.
The primary oncology team discontinues tamoxifen (after 5 weeks of treatment) and refers Mrs. L to psychiatry for further mood evaluation.
[polldaddy:10497042]
The authors’ observations
The prevalence of depression is higher in patients with cancer than in the general population.1 The etiology of depression is often multifactorial.2 In Mrs. L’s case, we hypothesized that the possible cause of her depressive symptoms included concerns about her self-image after mastectomy and the adverse effects of chemotherapy and tamoxifen.
Among these possible causes, estrogen level is particularly important. Estrogen affects the brain in numerous ways, including by modulating different neurotransmitters,3,4 regulating neuroplasticity, providing neuroprotection by preventing formation of oxidative free radicals and of beta amyloid, and possibly avoiding inflammation. From a behavioral standpoint, estrogen acts as an antidepressant while enhancing memory and modulating maternal behavior.4 Therefore, decreased estrogen levels could result in depression and other neuropsychiatric problems. This is illustrated in Mrs. L’s case, where tamoxifen administered after breast cancer treatment coincided with the abrupt onset of depression with suicidal ideation.
Depression in patients receiving tamoxifen might be explained by the fact that tamoxifen is a selective estrogen receptor blocker with dual properties. Specifically, while it has antagonistic action in breast tissue, diminishing the growth-promoting action of estrogen on breast cancer cells, it additionally crosses the blood-brain barrier, so it may block the neuroprotective action of estrogen in the brain.
EXAMINATION Improvement in depression but slightly anxious
During her psychiatric examination, Mrs. L is fairly well-groomed and cooperative. Her speech is normal, thought process is organized, and she has fair insight into her medical situation, with fair judgment. She is alert, attentive, and oriented to time, place, as well as person. She confirms that she has no prior psychiatric history, including no prior suicide attempts. She lives with her husband, who has been supportive. Mrs. L has no children, and she continues to work.
Continue to: Mrs. L reports that per her oncology...
Mrs. L reports that per her oncology team’s instruction, she has not taken tamoxifen for almost 1 week, and notes improvement in her mood. She describes her mood as “fine now,” but appears slightly anxious. She adamantly denies suicidal ideation since stopping tamoxifen; however, she confirms that prior to stopping tamoxifen, she experienced low mood, suicidal thoughts, and a decreased interest in activities. Mrs. L’s Patient Health Questionnaire–9 score is 13, indicating moderate depression. She says she is constantly preoccupied with thoughts about the adverse effects of hormone therapy, and specifically about the oncology team’s suggestion of a retrial of tamoxifen. Due to her constant worry, she has difficulty relaxing; her Generalized Anxiety Disorder–7 item scale score is 12, indicating moderate anxiety. She has a history of cigarette smoking but stopped after her breast cancer diagnosis. She also reports gaining weight since beginning cancer treatment (body mass index: 28.0 kg/m2) and experiencing breast pain.
Mrs. L’s vital signs are normal. Results of her laboratory workup reveal a thyroid-stimulating hormone level of 1.40 µU/mL (reference range: 0.27 to 4.20 µU/mL); a follicle-stimulating hormone (FSH) level of 78.4 mIU/mL (postmenopausal reference range: 25.8 to 134.8 mIU/mL); and an estradiol level of <12.0 pg/mL (postmenopausal range: <55 pg/mL).
The authors’ observations
Studies investigating the effects of tamoxifen on mood have produced varying results (Table5-16). Some researchers have found a significant relationship between depression and tamoxifen in patients with breast cancer. In a case-control study, 42 postmenopausal women with breast cancer who received tamoxifen reported statistically significant elevated depression scores.5 Similarly, in a prospective trial that assessed mood symptoms in 21 pre- and postmenopausal women who developed estrogen deficiency during breast cancer treatment (including treatment with tamoxifen and chemotherapy), 38% of patients met the criteria for major depressive disorder (MDD) in the first 6 months of treatment. Sixty-six percent of these patients were postmenopausal, and 38% were premenopausal. Twenty-five percent of the premenopausal women who experienced MDD symptoms had been treated with tamoxifen and chemotherapy.6
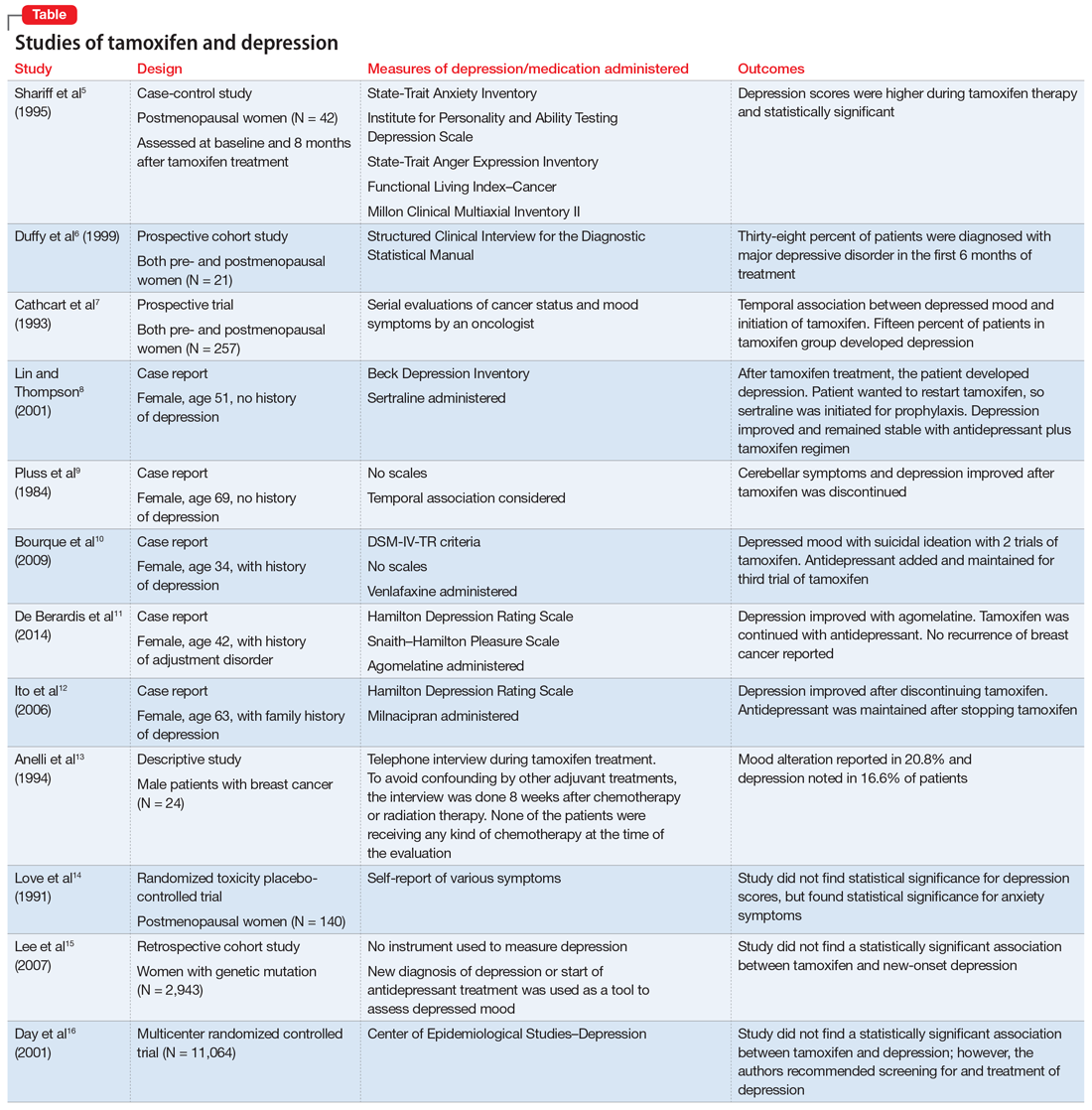
In a larger prospective trial (N = 257), an oncologist assessed mood symptoms in 2 groups of patients with breast cancer: individuals who received tamoxifen, and those who did not receive tamoxifen.7 They found that 15% of patients who received tamoxifen experienced depression, compared with 3% of patients who did not receive tamoxifen; this difference was statistically significant.7 Overall, 31% of the patients had “significant depression” and 27% discontinued tamoxifen because of adverse effects.7 There have been 2 case reports of tamoxifen use and severe depression in patients with no prior psychiatry history8,9 and 3 case reports of tamoxifen use and severe depression in patients who had a psychiatric history.10-12
One study that examined 24 men with breast cancer found that 62.5% of these patients experienced adverse effects related to tamoxifen, and 25% discontinued tamoxifen because of its adverse effects.13 Among the various adverse effects related to tamoxifen, mood alteration was reported in 20.8% of cases, and depressed feelings were reported in 16.6%.13
Continue to: Despite the evidence...
Despite this evidence, other studies have not found an association between tamoxifen and depressed mood in patients with breast cancer. One group of researchers who assessed various symptoms self-reported by postmenopausal women who were breast cancer survivors found that the depression scores were not significant.14 A retrospective cohort study assessed the onset of depression in patients with breast cancer with positive hormone receptor status (who received tamoxifen) vs negative hormone receptor status (who did not receive tamoxifen). These researchers did not find a statistically significant hazard ratio for “new-onset depression.”15 Unfortunately, the criteria for “new-onset depression” used in this study was the diagnosis of depression or use of an antidepressant given or ordered by a clinician, which is not a sensitive assessment of depressed mood.15
A multicenter randomized, placebo-controlled trial (the National Surgical Adjuvant Breast and Bowel Project) assessed the incidence of negative health outcomes, including depression, in a secondary outcome analysis.16 These researchers did not find a statistically significantassociation between tamoxifen and depression. However, in this study, assessment of depression was based on self-report using the Center of Epidemiologic Studies Depression (CES-D) scale, which does not clinically categorize depression. Furthermore, these researchers strongly recommended screening for mood disorders in routine clinical practice. In this study, 3 women completed suicide, 2 of whom were in the tamoxifen arm.16
[polldaddy:10497045]
The authors’ observations
Tamoxifen is a prodrug that converts to the active metabolite, endoxifen, via cytochrome P450 2D6 (CYP2D6) activity. Antidepressants with strong 2D6-inhibiting properties, such as fluoxetine, duloxetine, bupropion, and paroxetine, should be avoided in patients receiving tamoxifen because they interfere with the formation of the active metabolite and could reduce the effectiveness of tamoxifen and its ability to reduce the risk of cancer recurrence.17 Antidepressants can help treat psychological distress, especially depression, which is common in patients with cancer, and vasomotor symptoms, which may impair quality of life and adherence to long-term endocrine therapy. Because tamoxifen can decrease cancer recurrence and associated mortality,18 adherence with treatment is crucial.
TREATMENT Starting an antidepressant
The psychiatry team initiates venlafaxine, 37.5 mg/d, to treat Mrs. L’s anxiety and help prevent the recurrence of severe depression. They prescribe venlafaxine because they anticipate that, based on Mrs. L’s age, the oncology team might reconsider treatment with tamoxifen. Venlafaxine is preferred because it has a favorable pharmacodynamic profile and does not interfere with the metabolism of tamoxifen, as is the case with many selective serotonin reuptake inhibitors.17
Although Mrs. L’s depression had abated once she stopped receiving tamoxifen, she continues to experience anxiety and tearfulness, primarily due to fear of adverse effects of hormone therapy, and due to family as well as work stressors. Therefore, venlafaxine is gradually titrated up to 150 mg/d.
Continue to: The oncology team proposes...
The oncology team proposes a trial of leuprolide, a gonadotropin-releasing hormone agonist that downregulates pituitary receptors, subsequently suppressing female reproductive hormones, which in turn stops the ovaries from producing estrogen so there is a minimal amount of estrogen to promote the growth of estrogen–receptor-positive breast cancer. Mrs. L declines this agent because she is concerned that she will gain weight. Instead, Mrs. L expresses interest in undergoing an oophorectomy to reduce her estrogen level. In the meantime, based on her reproductive hormone levels (FSH and estradiol levels) which are indicative of postmenopausal status, the oncology team prescribes the aromatase inhibitor (AI) exemestane 25 mg/d. The AI helps to decrease the amount of estrogen the body makes peripherally, which is the main source of estrogen in postmenopausal women.
The authors’ observations
Estrogen originates in the ovaries in premenopausal women; it is also produced by peripheral conversion of androgens to estrogen in adipose tissues and muscle in postmenopausal women.19 Aromatase inhibitors block the enzyme aromatase that converts androgen to estrogen, which leads to estrogen deficiency in postmenopausal women and possibly to neuropsychiatric effects.19
The results of studies assessing the adverse psychiatric effects of AIs are mixed. When the results of studies evaluating tamoxifen are compared with those evaluating AIs, overall patients who received AIs had less severe or less frequent mood symptoms. One possible explanation could be that AIs are relatively new compared with tamoxifen. Second, AIs are more commonly used in postmenopausal women with breast cancer, and these patients’ overall estrogen level is significantly lower than that of premenopausal women with breast cancer. Therefore, the degree of hormone fluctuation is less intense in postmenopausal breast cancer survivors.
OUTCOME
After starting exemestane, and while still receiving venlafaxine, Mrs. L no longer experiences severe depressive symptoms. After 8 months, venlafaxine is discontinued. She continues to deny depressive symptoms but has intermittent anxiety, which she is able to manage without psychiatric medication. She continues to remain adherent with ongoing exemestane treatment, with no evidence of disease progression or recurrence.
The authors’ observations
For patients with estrogen-positive breast cancer, the decision to discontinue tamoxifen because of unacceptable adverse effects is an important one because it may increase the risk of cancer recurrence. Psychiatrists have an important role in supporting the patient through this process, helping patients understand alternatives, and working with the oncology team to formulate a plan that is acceptable to everyone.
Continue to: Bottom Line
Bottom Line
For patients with estrogen–positive breast cancer, anti-estrogen treatment can reduce the risk of cancer recurrence. However, it can cause adverse effects, including depression, that might impair quality of life and treatment adherence. For patients with severe depression, stopping estrogen blockers may be warranted. Initiating an antidepressant that does not interfere with the metabolism of tamoxifen may help treat depression and vasomotor symptoms.
Related Resource
- Agarwala P. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11):39-40,45-46,48-49.
Drug Brand Names
Agomelatine • Valdoxan
Bupropion • Wellbutrin, Zyban
Cyclophosphamide • Cytoxan
Doxorubicin • Adriamycin
Duloxetine • Cymbalta
Exemestane • Aromasin
Fluoxetine • Prozac
Leuprolide • Eligard, Lupron
Milnacipran • Savella
Paroxetine • Paxil
Sertraline • Zoloft
Tamoxifen • Soltamox
Trastuzumab • Herceptin
Venlafaxine • Effexor
1. Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
2. Thompson DS, Spanier CA, Vogel VG. The relationship between tamoxifen, estrogen, and depressive symptoms. Breast J. 1999;5(6):375-382.
3. Halbreich U. Role of estrogen in postmenopausal depression. Neurology. 1997;48(5 suppl 7):S16-S19.
4. Schiller CE, Johnson SL, Abate AC, et al. Reproductive steroid regulation of mood and behavior. Compr Physiol. 2016;6(3):1135-1160.
5. Shariff S, Cumming CE, Lees A, et al. Mood disorder in women with early breast cancer taking tamoxifen, an estradiol receptor antagonist. An expected or unexpected effect? Ann N Y Acad Sci. 1995;761:365-368.
6. Duffy LS, Greenberg DB, Younger J, et al. Iatrogenic acute estrogen deficiency and psychiatric syndromes in breast cancer patients. Psychosomatics. 1999;40(4):304-308.
7. Cathcart CK, Jones SE, Pumroy CS, et al. Clinical recognition and management of depression in node negative breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 1993;27(3):277-281.
8. Lin J, Thompson DS. Case report: tamoxifen-induced depression. Primary Care Update for Ob/Gyns. 2001;8(5):207-208.
9. Pluss JL, DiBella NJ. Reversible central nervous system dysfunction due to tamoxifen in a patient with breast cancer. Ann Intern Med. 1984;101(5):652.
10. Bourque F, Karama S, Looper K, et al. Acute tamoxifen-induced depression and its prevention with venlafaxine. Psychosomatics. 2009;50(2):162-165.
11. De Berardis D, Brucchi M, Serroni N, et al. Successful use of agomelatine in the treatment of major depression in a woman taking tamoxifen: a case report. Clin Neuropharmacol. 2014;37(1):31-33.
12. Ito M, Baba H, Kawashima R, et al. A case of prolonged depression with tamoxifen. Japan Med Assoc J. 2006;49(4):167-172.
13. Anelli TF, Anelli A, Tran KN, et al. Tamoxifen administration is associated with a high rate of treatment-limiting symptoms in male breast cancer patients. Cancer. 1994;74(1):74-77.
14. Love RR, Cameron L, Connell BL, et al. Symptoms associated with tamoxifen treatment in postmenopausal women. Arch Intern Med. 1991;151(9):1842-1847.
15. Lee KC, Ray GT, Hunkeler EM, et al. Tamoxifen treatment and new-onset depression in breast cancer patients. Psychosomatics. 2007;48(3):205-210.
16. Day R, Ganz PA, Costantino JP. Tamoxifen and depression: more evidence from the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention (P-1) Randomized Study. J Natl Cancer Inst. 2001;93(21):1615-1623.
17. Juurlink D. Revisiting the drug interaction between tamoxifen and SSRI antidepressants. BMJ. 2016;354:i5309.
18. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771-784.
19. Buijs C, de Vries EGE, Mourits MJE, et al. The influence of endocrine treatments for breast cancer on health-related quality of life. Cancer Treat Rev. 2008;34(7):640-655.
CASE Worsening mood symptoms and suicidal ideation
On a recent visit to the oncology clinic, where she has been receiving treatment for breast cancer for 11 months, Mrs. L, age 46, reports the abrupt onset of sadness, irritability, difficulty sleeping, and negative self-thoughts.
Eleven months ago, Mrs. L was diagnosed with invasive lobular carcinoma of the right breast that was classified as T2N0MX, representing relatively early-stage disease. Shortly after her diagnosis, Mrs. L completed 4 cycles of neoadjuvant chemotherapy with doxorubicin and cyclophosphamide, followed by treatment with trastuzumab. Subsequently, she underwent a right segmental mastectomy with bilateral mastopexy and radiation therapy. Recently, Mrs. L’s oncology team prescribed tamoxifen, 20 mg/d, and trastuzumab, 420 mg IV every 3 weeks; however, within 3 weeks after starting tamoxifen, Mrs. L’s mood symptoms worsened to the point where she says she is considering suicide—with a plan to use her husband’s gun to kill herself.
Mrs. L has no other pertinent medical history and no reported history of psychiatric disease.
The primary oncology team discontinues tamoxifen (after 5 weeks of treatment) and refers Mrs. L to psychiatry for further mood evaluation.
[polldaddy:10497042]
The authors’ observations
The prevalence of depression is higher in patients with cancer than in the general population.1 The etiology of depression is often multifactorial.2 In Mrs. L’s case, we hypothesized that the possible cause of her depressive symptoms included concerns about her self-image after mastectomy and the adverse effects of chemotherapy and tamoxifen.
Among these possible causes, estrogen level is particularly important. Estrogen affects the brain in numerous ways, including by modulating different neurotransmitters,3,4 regulating neuroplasticity, providing neuroprotection by preventing formation of oxidative free radicals and of beta amyloid, and possibly avoiding inflammation. From a behavioral standpoint, estrogen acts as an antidepressant while enhancing memory and modulating maternal behavior.4 Therefore, decreased estrogen levels could result in depression and other neuropsychiatric problems. This is illustrated in Mrs. L’s case, where tamoxifen administered after breast cancer treatment coincided with the abrupt onset of depression with suicidal ideation.
Depression in patients receiving tamoxifen might be explained by the fact that tamoxifen is a selective estrogen receptor blocker with dual properties. Specifically, while it has antagonistic action in breast tissue, diminishing the growth-promoting action of estrogen on breast cancer cells, it additionally crosses the blood-brain barrier, so it may block the neuroprotective action of estrogen in the brain.
EXAMINATION Improvement in depression but slightly anxious
During her psychiatric examination, Mrs. L is fairly well-groomed and cooperative. Her speech is normal, thought process is organized, and she has fair insight into her medical situation, with fair judgment. She is alert, attentive, and oriented to time, place, as well as person. She confirms that she has no prior psychiatric history, including no prior suicide attempts. She lives with her husband, who has been supportive. Mrs. L has no children, and she continues to work.
Continue to: Mrs. L reports that per her oncology...
Mrs. L reports that per her oncology team’s instruction, she has not taken tamoxifen for almost 1 week, and notes improvement in her mood. She describes her mood as “fine now,” but appears slightly anxious. She adamantly denies suicidal ideation since stopping tamoxifen; however, she confirms that prior to stopping tamoxifen, she experienced low mood, suicidal thoughts, and a decreased interest in activities. Mrs. L’s Patient Health Questionnaire–9 score is 13, indicating moderate depression. She says she is constantly preoccupied with thoughts about the adverse effects of hormone therapy, and specifically about the oncology team’s suggestion of a retrial of tamoxifen. Due to her constant worry, she has difficulty relaxing; her Generalized Anxiety Disorder–7 item scale score is 12, indicating moderate anxiety. She has a history of cigarette smoking but stopped after her breast cancer diagnosis. She also reports gaining weight since beginning cancer treatment (body mass index: 28.0 kg/m2) and experiencing breast pain.
Mrs. L’s vital signs are normal. Results of her laboratory workup reveal a thyroid-stimulating hormone level of 1.40 µU/mL (reference range: 0.27 to 4.20 µU/mL); a follicle-stimulating hormone (FSH) level of 78.4 mIU/mL (postmenopausal reference range: 25.8 to 134.8 mIU/mL); and an estradiol level of <12.0 pg/mL (postmenopausal range: <55 pg/mL).
The authors’ observations
Studies investigating the effects of tamoxifen on mood have produced varying results (Table5-16). Some researchers have found a significant relationship between depression and tamoxifen in patients with breast cancer. In a case-control study, 42 postmenopausal women with breast cancer who received tamoxifen reported statistically significant elevated depression scores.5 Similarly, in a prospective trial that assessed mood symptoms in 21 pre- and postmenopausal women who developed estrogen deficiency during breast cancer treatment (including treatment with tamoxifen and chemotherapy), 38% of patients met the criteria for major depressive disorder (MDD) in the first 6 months of treatment. Sixty-six percent of these patients were postmenopausal, and 38% were premenopausal. Twenty-five percent of the premenopausal women who experienced MDD symptoms had been treated with tamoxifen and chemotherapy.6

In a larger prospective trial (N = 257), an oncologist assessed mood symptoms in 2 groups of patients with breast cancer: individuals who received tamoxifen, and those who did not receive tamoxifen.7 They found that 15% of patients who received tamoxifen experienced depression, compared with 3% of patients who did not receive tamoxifen; this difference was statistically significant.7 Overall, 31% of the patients had “significant depression” and 27% discontinued tamoxifen because of adverse effects.7 There have been 2 case reports of tamoxifen use and severe depression in patients with no prior psychiatry history8,9 and 3 case reports of tamoxifen use and severe depression in patients who had a psychiatric history.10-12
One study that examined 24 men with breast cancer found that 62.5% of these patients experienced adverse effects related to tamoxifen, and 25% discontinued tamoxifen because of its adverse effects.13 Among the various adverse effects related to tamoxifen, mood alteration was reported in 20.8% of cases, and depressed feelings were reported in 16.6%.13
Continue to: Despite the evidence...
Despite this evidence, other studies have not found an association between tamoxifen and depressed mood in patients with breast cancer. One group of researchers who assessed various symptoms self-reported by postmenopausal women who were breast cancer survivors found that the depression scores were not significant.14 A retrospective cohort study assessed the onset of depression in patients with breast cancer with positive hormone receptor status (who received tamoxifen) vs negative hormone receptor status (who did not receive tamoxifen). These researchers did not find a statistically significant hazard ratio for “new-onset depression.”15 Unfortunately, the criteria for “new-onset depression” used in this study was the diagnosis of depression or use of an antidepressant given or ordered by a clinician, which is not a sensitive assessment of depressed mood.15
A multicenter randomized, placebo-controlled trial (the National Surgical Adjuvant Breast and Bowel Project) assessed the incidence of negative health outcomes, including depression, in a secondary outcome analysis.16 These researchers did not find a statistically significantassociation between tamoxifen and depression. However, in this study, assessment of depression was based on self-report using the Center of Epidemiologic Studies Depression (CES-D) scale, which does not clinically categorize depression. Furthermore, these researchers strongly recommended screening for mood disorders in routine clinical practice. In this study, 3 women completed suicide, 2 of whom were in the tamoxifen arm.16
[polldaddy:10497045]
The authors’ observations
Tamoxifen is a prodrug that converts to the active metabolite, endoxifen, via cytochrome P450 2D6 (CYP2D6) activity. Antidepressants with strong 2D6-inhibiting properties, such as fluoxetine, duloxetine, bupropion, and paroxetine, should be avoided in patients receiving tamoxifen because they interfere with the formation of the active metabolite and could reduce the effectiveness of tamoxifen and its ability to reduce the risk of cancer recurrence.17 Antidepressants can help treat psychological distress, especially depression, which is common in patients with cancer, and vasomotor symptoms, which may impair quality of life and adherence to long-term endocrine therapy. Because tamoxifen can decrease cancer recurrence and associated mortality,18 adherence with treatment is crucial.
TREATMENT Starting an antidepressant
The psychiatry team initiates venlafaxine, 37.5 mg/d, to treat Mrs. L’s anxiety and help prevent the recurrence of severe depression. They prescribe venlafaxine because they anticipate that, based on Mrs. L’s age, the oncology team might reconsider treatment with tamoxifen. Venlafaxine is preferred because it has a favorable pharmacodynamic profile and does not interfere with the metabolism of tamoxifen, as is the case with many selective serotonin reuptake inhibitors.17
Although Mrs. L’s depression had abated once she stopped receiving tamoxifen, she continues to experience anxiety and tearfulness, primarily due to fear of adverse effects of hormone therapy, and due to family as well as work stressors. Therefore, venlafaxine is gradually titrated up to 150 mg/d.
Continue to: The oncology team proposes...
The oncology team proposes a trial of leuprolide, a gonadotropin-releasing hormone agonist that downregulates pituitary receptors, subsequently suppressing female reproductive hormones, which in turn stops the ovaries from producing estrogen so there is a minimal amount of estrogen to promote the growth of estrogen–receptor-positive breast cancer. Mrs. L declines this agent because she is concerned that she will gain weight. Instead, Mrs. L expresses interest in undergoing an oophorectomy to reduce her estrogen level. In the meantime, based on her reproductive hormone levels (FSH and estradiol levels) which are indicative of postmenopausal status, the oncology team prescribes the aromatase inhibitor (AI) exemestane 25 mg/d. The AI helps to decrease the amount of estrogen the body makes peripherally, which is the main source of estrogen in postmenopausal women.
The authors’ observations
Estrogen originates in the ovaries in premenopausal women; it is also produced by peripheral conversion of androgens to estrogen in adipose tissues and muscle in postmenopausal women.19 Aromatase inhibitors block the enzyme aromatase that converts androgen to estrogen, which leads to estrogen deficiency in postmenopausal women and possibly to neuropsychiatric effects.19
The results of studies assessing the adverse psychiatric effects of AIs are mixed. When the results of studies evaluating tamoxifen are compared with those evaluating AIs, overall patients who received AIs had less severe or less frequent mood symptoms. One possible explanation could be that AIs are relatively new compared with tamoxifen. Second, AIs are more commonly used in postmenopausal women with breast cancer, and these patients’ overall estrogen level is significantly lower than that of premenopausal women with breast cancer. Therefore, the degree of hormone fluctuation is less intense in postmenopausal breast cancer survivors.
OUTCOME
After starting exemestane, and while still receiving venlafaxine, Mrs. L no longer experiences severe depressive symptoms. After 8 months, venlafaxine is discontinued. She continues to deny depressive symptoms but has intermittent anxiety, which she is able to manage without psychiatric medication. She continues to remain adherent with ongoing exemestane treatment, with no evidence of disease progression or recurrence.
The authors’ observations
For patients with estrogen-positive breast cancer, the decision to discontinue tamoxifen because of unacceptable adverse effects is an important one because it may increase the risk of cancer recurrence. Psychiatrists have an important role in supporting the patient through this process, helping patients understand alternatives, and working with the oncology team to formulate a plan that is acceptable to everyone.
Continue to: Bottom Line
Bottom Line
For patients with estrogen–positive breast cancer, anti-estrogen treatment can reduce the risk of cancer recurrence. However, it can cause adverse effects, including depression, that might impair quality of life and treatment adherence. For patients with severe depression, stopping estrogen blockers may be warranted. Initiating an antidepressant that does not interfere with the metabolism of tamoxifen may help treat depression and vasomotor symptoms.
Related Resource
- Agarwala P. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11):39-40,45-46,48-49.
Drug Brand Names
Agomelatine • Valdoxan
Bupropion • Wellbutrin, Zyban
Cyclophosphamide • Cytoxan
Doxorubicin • Adriamycin
Duloxetine • Cymbalta
Exemestane • Aromasin
Fluoxetine • Prozac
Leuprolide • Eligard, Lupron
Milnacipran • Savella
Paroxetine • Paxil
Sertraline • Zoloft
Tamoxifen • Soltamox
Trastuzumab • Herceptin
Venlafaxine • Effexor
CASE Worsening mood symptoms and suicidal ideation
On a recent visit to the oncology clinic, where she has been receiving treatment for breast cancer for 11 months, Mrs. L, age 46, reports the abrupt onset of sadness, irritability, difficulty sleeping, and negative self-thoughts.
Eleven months ago, Mrs. L was diagnosed with invasive lobular carcinoma of the right breast that was classified as T2N0MX, representing relatively early-stage disease. Shortly after her diagnosis, Mrs. L completed 4 cycles of neoadjuvant chemotherapy with doxorubicin and cyclophosphamide, followed by treatment with trastuzumab. Subsequently, she underwent a right segmental mastectomy with bilateral mastopexy and radiation therapy. Recently, Mrs. L’s oncology team prescribed tamoxifen, 20 mg/d, and trastuzumab, 420 mg IV every 3 weeks; however, within 3 weeks after starting tamoxifen, Mrs. L’s mood symptoms worsened to the point where she says she is considering suicide—with a plan to use her husband’s gun to kill herself.
Mrs. L has no other pertinent medical history and no reported history of psychiatric disease.
The primary oncology team discontinues tamoxifen (after 5 weeks of treatment) and refers Mrs. L to psychiatry for further mood evaluation.
[polldaddy:10497042]
The authors’ observations
The prevalence of depression is higher in patients with cancer than in the general population.1 The etiology of depression is often multifactorial.2 In Mrs. L’s case, we hypothesized that the possible cause of her depressive symptoms included concerns about her self-image after mastectomy and the adverse effects of chemotherapy and tamoxifen.
Among these possible causes, estrogen level is particularly important. Estrogen affects the brain in numerous ways, including by modulating different neurotransmitters,3,4 regulating neuroplasticity, providing neuroprotection by preventing formation of oxidative free radicals and of beta amyloid, and possibly avoiding inflammation. From a behavioral standpoint, estrogen acts as an antidepressant while enhancing memory and modulating maternal behavior.4 Therefore, decreased estrogen levels could result in depression and other neuropsychiatric problems. This is illustrated in Mrs. L’s case, where tamoxifen administered after breast cancer treatment coincided with the abrupt onset of depression with suicidal ideation.
Depression in patients receiving tamoxifen might be explained by the fact that tamoxifen is a selective estrogen receptor blocker with dual properties. Specifically, while it has antagonistic action in breast tissue, diminishing the growth-promoting action of estrogen on breast cancer cells, it additionally crosses the blood-brain barrier, so it may block the neuroprotective action of estrogen in the brain.
EXAMINATION Improvement in depression but slightly anxious
During her psychiatric examination, Mrs. L is fairly well-groomed and cooperative. Her speech is normal, thought process is organized, and she has fair insight into her medical situation, with fair judgment. She is alert, attentive, and oriented to time, place, as well as person. She confirms that she has no prior psychiatric history, including no prior suicide attempts. She lives with her husband, who has been supportive. Mrs. L has no children, and she continues to work.
Continue to: Mrs. L reports that per her oncology...
Mrs. L reports that per her oncology team’s instruction, she has not taken tamoxifen for almost 1 week, and notes improvement in her mood. She describes her mood as “fine now,” but appears slightly anxious. She adamantly denies suicidal ideation since stopping tamoxifen; however, she confirms that prior to stopping tamoxifen, she experienced low mood, suicidal thoughts, and a decreased interest in activities. Mrs. L’s Patient Health Questionnaire–9 score is 13, indicating moderate depression. She says she is constantly preoccupied with thoughts about the adverse effects of hormone therapy, and specifically about the oncology team’s suggestion of a retrial of tamoxifen. Due to her constant worry, she has difficulty relaxing; her Generalized Anxiety Disorder–7 item scale score is 12, indicating moderate anxiety. She has a history of cigarette smoking but stopped after her breast cancer diagnosis. She also reports gaining weight since beginning cancer treatment (body mass index: 28.0 kg/m2) and experiencing breast pain.
Mrs. L’s vital signs are normal. Results of her laboratory workup reveal a thyroid-stimulating hormone level of 1.40 µU/mL (reference range: 0.27 to 4.20 µU/mL); a follicle-stimulating hormone (FSH) level of 78.4 mIU/mL (postmenopausal reference range: 25.8 to 134.8 mIU/mL); and an estradiol level of <12.0 pg/mL (postmenopausal range: <55 pg/mL).
The authors’ observations
Studies investigating the effects of tamoxifen on mood have produced varying results (Table5-16). Some researchers have found a significant relationship between depression and tamoxifen in patients with breast cancer. In a case-control study, 42 postmenopausal women with breast cancer who received tamoxifen reported statistically significant elevated depression scores.5 Similarly, in a prospective trial that assessed mood symptoms in 21 pre- and postmenopausal women who developed estrogen deficiency during breast cancer treatment (including treatment with tamoxifen and chemotherapy), 38% of patients met the criteria for major depressive disorder (MDD) in the first 6 months of treatment. Sixty-six percent of these patients were postmenopausal, and 38% were premenopausal. Twenty-five percent of the premenopausal women who experienced MDD symptoms had been treated with tamoxifen and chemotherapy.6

In a larger prospective trial (N = 257), an oncologist assessed mood symptoms in 2 groups of patients with breast cancer: individuals who received tamoxifen, and those who did not receive tamoxifen.7 They found that 15% of patients who received tamoxifen experienced depression, compared with 3% of patients who did not receive tamoxifen; this difference was statistically significant.7 Overall, 31% of the patients had “significant depression” and 27% discontinued tamoxifen because of adverse effects.7 There have been 2 case reports of tamoxifen use and severe depression in patients with no prior psychiatry history8,9 and 3 case reports of tamoxifen use and severe depression in patients who had a psychiatric history.10-12
One study that examined 24 men with breast cancer found that 62.5% of these patients experienced adverse effects related to tamoxifen, and 25% discontinued tamoxifen because of its adverse effects.13 Among the various adverse effects related to tamoxifen, mood alteration was reported in 20.8% of cases, and depressed feelings were reported in 16.6%.13
Continue to: Despite the evidence...
Despite this evidence, other studies have not found an association between tamoxifen and depressed mood in patients with breast cancer. One group of researchers who assessed various symptoms self-reported by postmenopausal women who were breast cancer survivors found that the depression scores were not significant.14 A retrospective cohort study assessed the onset of depression in patients with breast cancer with positive hormone receptor status (who received tamoxifen) vs negative hormone receptor status (who did not receive tamoxifen). These researchers did not find a statistically significant hazard ratio for “new-onset depression.”15 Unfortunately, the criteria for “new-onset depression” used in this study was the diagnosis of depression or use of an antidepressant given or ordered by a clinician, which is not a sensitive assessment of depressed mood.15
A multicenter randomized, placebo-controlled trial (the National Surgical Adjuvant Breast and Bowel Project) assessed the incidence of negative health outcomes, including depression, in a secondary outcome analysis.16 These researchers did not find a statistically significantassociation between tamoxifen and depression. However, in this study, assessment of depression was based on self-report using the Center of Epidemiologic Studies Depression (CES-D) scale, which does not clinically categorize depression. Furthermore, these researchers strongly recommended screening for mood disorders in routine clinical practice. In this study, 3 women completed suicide, 2 of whom were in the tamoxifen arm.16
[polldaddy:10497045]
The authors’ observations
Tamoxifen is a prodrug that converts to the active metabolite, endoxifen, via cytochrome P450 2D6 (CYP2D6) activity. Antidepressants with strong 2D6-inhibiting properties, such as fluoxetine, duloxetine, bupropion, and paroxetine, should be avoided in patients receiving tamoxifen because they interfere with the formation of the active metabolite and could reduce the effectiveness of tamoxifen and its ability to reduce the risk of cancer recurrence.17 Antidepressants can help treat psychological distress, especially depression, which is common in patients with cancer, and vasomotor symptoms, which may impair quality of life and adherence to long-term endocrine therapy. Because tamoxifen can decrease cancer recurrence and associated mortality,18 adherence with treatment is crucial.
TREATMENT Starting an antidepressant
The psychiatry team initiates venlafaxine, 37.5 mg/d, to treat Mrs. L’s anxiety and help prevent the recurrence of severe depression. They prescribe venlafaxine because they anticipate that, based on Mrs. L’s age, the oncology team might reconsider treatment with tamoxifen. Venlafaxine is preferred because it has a favorable pharmacodynamic profile and does not interfere with the metabolism of tamoxifen, as is the case with many selective serotonin reuptake inhibitors.17
Although Mrs. L’s depression had abated once she stopped receiving tamoxifen, she continues to experience anxiety and tearfulness, primarily due to fear of adverse effects of hormone therapy, and due to family as well as work stressors. Therefore, venlafaxine is gradually titrated up to 150 mg/d.
Continue to: The oncology team proposes...
The oncology team proposes a trial of leuprolide, a gonadotropin-releasing hormone agonist that downregulates pituitary receptors, subsequently suppressing female reproductive hormones, which in turn stops the ovaries from producing estrogen so there is a minimal amount of estrogen to promote the growth of estrogen–receptor-positive breast cancer. Mrs. L declines this agent because she is concerned that she will gain weight. Instead, Mrs. L expresses interest in undergoing an oophorectomy to reduce her estrogen level. In the meantime, based on her reproductive hormone levels (FSH and estradiol levels) which are indicative of postmenopausal status, the oncology team prescribes the aromatase inhibitor (AI) exemestane 25 mg/d. The AI helps to decrease the amount of estrogen the body makes peripherally, which is the main source of estrogen in postmenopausal women.
The authors’ observations
Estrogen originates in the ovaries in premenopausal women; it is also produced by peripheral conversion of androgens to estrogen in adipose tissues and muscle in postmenopausal women.19 Aromatase inhibitors block the enzyme aromatase that converts androgen to estrogen, which leads to estrogen deficiency in postmenopausal women and possibly to neuropsychiatric effects.19
The results of studies assessing the adverse psychiatric effects of AIs are mixed. When the results of studies evaluating tamoxifen are compared with those evaluating AIs, overall patients who received AIs had less severe or less frequent mood symptoms. One possible explanation could be that AIs are relatively new compared with tamoxifen. Second, AIs are more commonly used in postmenopausal women with breast cancer, and these patients’ overall estrogen level is significantly lower than that of premenopausal women with breast cancer. Therefore, the degree of hormone fluctuation is less intense in postmenopausal breast cancer survivors.
OUTCOME
After starting exemestane, and while still receiving venlafaxine, Mrs. L no longer experiences severe depressive symptoms. After 8 months, venlafaxine is discontinued. She continues to deny depressive symptoms but has intermittent anxiety, which she is able to manage without psychiatric medication. She continues to remain adherent with ongoing exemestane treatment, with no evidence of disease progression or recurrence.
The authors’ observations
For patients with estrogen-positive breast cancer, the decision to discontinue tamoxifen because of unacceptable adverse effects is an important one because it may increase the risk of cancer recurrence. Psychiatrists have an important role in supporting the patient through this process, helping patients understand alternatives, and working with the oncology team to formulate a plan that is acceptable to everyone.
Continue to: Bottom Line
Bottom Line
For patients with estrogen–positive breast cancer, anti-estrogen treatment can reduce the risk of cancer recurrence. However, it can cause adverse effects, including depression, that might impair quality of life and treatment adherence. For patients with severe depression, stopping estrogen blockers may be warranted. Initiating an antidepressant that does not interfere with the metabolism of tamoxifen may help treat depression and vasomotor symptoms.
Related Resource
- Agarwala P. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11):39-40,45-46,48-49.
Drug Brand Names
Agomelatine • Valdoxan
Bupropion • Wellbutrin, Zyban
Cyclophosphamide • Cytoxan
Doxorubicin • Adriamycin
Duloxetine • Cymbalta
Exemestane • Aromasin
Fluoxetine • Prozac
Leuprolide • Eligard, Lupron
Milnacipran • Savella
Paroxetine • Paxil
Sertraline • Zoloft
Tamoxifen • Soltamox
Trastuzumab • Herceptin
Venlafaxine • Effexor
1. Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
2. Thompson DS, Spanier CA, Vogel VG. The relationship between tamoxifen, estrogen, and depressive symptoms. Breast J. 1999;5(6):375-382.
3. Halbreich U. Role of estrogen in postmenopausal depression. Neurology. 1997;48(5 suppl 7):S16-S19.
4. Schiller CE, Johnson SL, Abate AC, et al. Reproductive steroid regulation of mood and behavior. Compr Physiol. 2016;6(3):1135-1160.
5. Shariff S, Cumming CE, Lees A, et al. Mood disorder in women with early breast cancer taking tamoxifen, an estradiol receptor antagonist. An expected or unexpected effect? Ann N Y Acad Sci. 1995;761:365-368.
6. Duffy LS, Greenberg DB, Younger J, et al. Iatrogenic acute estrogen deficiency and psychiatric syndromes in breast cancer patients. Psychosomatics. 1999;40(4):304-308.
7. Cathcart CK, Jones SE, Pumroy CS, et al. Clinical recognition and management of depression in node negative breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 1993;27(3):277-281.
8. Lin J, Thompson DS. Case report: tamoxifen-induced depression. Primary Care Update for Ob/Gyns. 2001;8(5):207-208.
9. Pluss JL, DiBella NJ. Reversible central nervous system dysfunction due to tamoxifen in a patient with breast cancer. Ann Intern Med. 1984;101(5):652.
10. Bourque F, Karama S, Looper K, et al. Acute tamoxifen-induced depression and its prevention with venlafaxine. Psychosomatics. 2009;50(2):162-165.
11. De Berardis D, Brucchi M, Serroni N, et al. Successful use of agomelatine in the treatment of major depression in a woman taking tamoxifen: a case report. Clin Neuropharmacol. 2014;37(1):31-33.
12. Ito M, Baba H, Kawashima R, et al. A case of prolonged depression with tamoxifen. Japan Med Assoc J. 2006;49(4):167-172.
13. Anelli TF, Anelli A, Tran KN, et al. Tamoxifen administration is associated with a high rate of treatment-limiting symptoms in male breast cancer patients. Cancer. 1994;74(1):74-77.
14. Love RR, Cameron L, Connell BL, et al. Symptoms associated with tamoxifen treatment in postmenopausal women. Arch Intern Med. 1991;151(9):1842-1847.
15. Lee KC, Ray GT, Hunkeler EM, et al. Tamoxifen treatment and new-onset depression in breast cancer patients. Psychosomatics. 2007;48(3):205-210.
16. Day R, Ganz PA, Costantino JP. Tamoxifen and depression: more evidence from the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention (P-1) Randomized Study. J Natl Cancer Inst. 2001;93(21):1615-1623.
17. Juurlink D. Revisiting the drug interaction between tamoxifen and SSRI antidepressants. BMJ. 2016;354:i5309.
18. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771-784.
19. Buijs C, de Vries EGE, Mourits MJE, et al. The influence of endocrine treatments for breast cancer on health-related quality of life. Cancer Treat Rev. 2008;34(7):640-655.
1. Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
2. Thompson DS, Spanier CA, Vogel VG. The relationship between tamoxifen, estrogen, and depressive symptoms. Breast J. 1999;5(6):375-382.
3. Halbreich U. Role of estrogen in postmenopausal depression. Neurology. 1997;48(5 suppl 7):S16-S19.
4. Schiller CE, Johnson SL, Abate AC, et al. Reproductive steroid regulation of mood and behavior. Compr Physiol. 2016;6(3):1135-1160.
5. Shariff S, Cumming CE, Lees A, et al. Mood disorder in women with early breast cancer taking tamoxifen, an estradiol receptor antagonist. An expected or unexpected effect? Ann N Y Acad Sci. 1995;761:365-368.
6. Duffy LS, Greenberg DB, Younger J, et al. Iatrogenic acute estrogen deficiency and psychiatric syndromes in breast cancer patients. Psychosomatics. 1999;40(4):304-308.
7. Cathcart CK, Jones SE, Pumroy CS, et al. Clinical recognition and management of depression in node negative breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 1993;27(3):277-281.
8. Lin J, Thompson DS. Case report: tamoxifen-induced depression. Primary Care Update for Ob/Gyns. 2001;8(5):207-208.
9. Pluss JL, DiBella NJ. Reversible central nervous system dysfunction due to tamoxifen in a patient with breast cancer. Ann Intern Med. 1984;101(5):652.
10. Bourque F, Karama S, Looper K, et al. Acute tamoxifen-induced depression and its prevention with venlafaxine. Psychosomatics. 2009;50(2):162-165.
11. De Berardis D, Brucchi M, Serroni N, et al. Successful use of agomelatine in the treatment of major depression in a woman taking tamoxifen: a case report. Clin Neuropharmacol. 2014;37(1):31-33.
12. Ito M, Baba H, Kawashima R, et al. A case of prolonged depression with tamoxifen. Japan Med Assoc J. 2006;49(4):167-172.
13. Anelli TF, Anelli A, Tran KN, et al. Tamoxifen administration is associated with a high rate of treatment-limiting symptoms in male breast cancer patients. Cancer. 1994;74(1):74-77.
14. Love RR, Cameron L, Connell BL, et al. Symptoms associated with tamoxifen treatment in postmenopausal women. Arch Intern Med. 1991;151(9):1842-1847.
15. Lee KC, Ray GT, Hunkeler EM, et al. Tamoxifen treatment and new-onset depression in breast cancer patients. Psychosomatics. 2007;48(3):205-210.
16. Day R, Ganz PA, Costantino JP. Tamoxifen and depression: more evidence from the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention (P-1) Randomized Study. J Natl Cancer Inst. 2001;93(21):1615-1623.
17. Juurlink D. Revisiting the drug interaction between tamoxifen and SSRI antidepressants. BMJ. 2016;354:i5309.
18. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771-784.
19. Buijs C, de Vries EGE, Mourits MJE, et al. The influence of endocrine treatments for breast cancer on health-related quality of life. Cancer Treat Rev. 2008;34(7):640-655.
Depression after miscarriage: Follow-up care is key
A Washington Post article on depression after miscarriage is a reminder that, although couples can suffer deeply from such a loss, there still are ways to provide them with meaningful support (“After miscarriage, I was rocked by depression. Like many other women, I didn’t get follow-up care for this loss,” by Katie C. Reilly, Nov 30, 2019).
Psychiatrists who focus on reproductive psychiatry and collaborative care are trying to change the current therapeutic landscape and improve practitioner awareness and treatment. Ob.gyns. managing patients who have experienced reproductive loss, especially early-term loss, may not immediately refer couples to a therapist or psychiatrist, but we can change this. Practitioners who focus on reproductive health – both physical and mental – are trying to better understand such couples’ experiences, increase their access to care, develop preventative care strategies, and improve provider education.
At the outset, providers who treat patients who have experienced a perinatal loss must recognize that not all individuals will feel that a loss is tragic. Instead, patient reactions occur along a spectrum, and there is no “correct” way to process a loss. A couple’s reaction may depend on a variety of factors, including how late or early in pregnancy the loss occurs, whether the pregnancy is planned or unplanned, and what other psychosocial stressors, such as unstable housing, limited income, and few social supports, may exist. Not every patient experiencing grief, even profoundly, will shed tears; we need to be open to all potential reactions and be mindful when a person may need additional support.
According to the Washington Post article, even though 50% of miscarriages are due to chromosomal abnormalities, women still feel ultimately responsible for the loss. As a society we are bombarded with “experts” in the media telling us the best way, the right way, the healthiest way to live. This barrage of advice distorts our views of what it really means to be a good parent and subtly conveys the idea that mothers are solely responsible for any bad pregnancy outcomes. I remember being fearful of causing unintentional harm to my unborn baby during my own pregnancy. What if I accidentally ate something that would affect her development? Is exposure to second-hand smoke as I walk down the street harming her? How bad would it be if I just had one cup of coffee? My doubts caused quite a bit of distress for me, which is a mild form of the distress I see when counseling couples after their miscarriages.
The article’s author also expressed concern about the emotional sterility of the environment in which miscarriages usually occur: a hospital ED. EDs are designed to promote a level of detachment and to quell any stress for the clinicians so that they can calmly handle unexpected health crises. EDs are not primarily designed to provide patients with emotional support, nor should they be. However, we still can make some improvements to existing ED design to better address couples’ emotional needs. For example, some EDs have placed mental health clinicians on staff, others call patients post discharge to address concerns, and some EDs even provide patients access to mental health trauma teams. Such services are not found in all EDs, and even those that exist may just scratch the surface of what is needed, but they are a step in the right direction. Providing this level of auxiliary care directly from the ED increases patients’ ability to access mental health support in the place where miscarriages are most likely to be first diagnosed and managed.
The American College of Obstetricians and Gynecologists already is trying to fill in the missing pieces when it comes to identifying mood symptoms following miscarriage. One of the key recommendations from the May 2018 Committee Opinion on Redefining the Postpartum Visit is that every woman who has experienced a miscarriage, stillbirth, or neonatal death should receive follow-up care. Mental health is a suggested component of the postpartum care plan. Some outpatient ob.gyn. practices and inpatient units are using screening tools to identify postpartum depression. For example, the Edinburgh Postnatal Depression Scale can be utilized following a miscarriage to help providers identify symptoms of depression and anxiety.
However, The trend in psychiatry over the past decade has been toward collaborative care, models that embed psychiatrists and other mental health clinicians in ob.gyn. practices to help guide the diagnosis and treatment of mental health problems. Some psychiatrists practice a co-located model in which they see patients alongside their ob.gyn. colleagues, whereas other psychiatrists treat a larger number of patients by using chart reviews for medication management while relying on behavioral health care managers for counseling and monitoring. Using this model of mental health care, more patients have access to services that are provided in a location familiar to them.
Another step in the right direction is the October 2019 launch of The National Curriculum in Reproductive Psychiatry (NCRP), which provides free educational material for psychiatry faculty and residents to enhance education on topics related to reproductive psychiatry, including miscarriage, loss, and development of trauma disorders. NCRP aspires to develop educational materials for ob.gyn. residents.
In the past we may have missed the mark in recognizing and treating the trauma that prenatal loss can cause, but we are trying to improve our approaches. More and more couples are sharing their experiences and advocating for themselves and others, often creating change in medical practice, and doctors are starting to listen. As any clinician knows, changes to standards of care can take several years to disseminate into general practice, but this gap between knowledge and treatment is now in the forefront of our minds. I am hopeful that we will continue to make advances and provide better care to our patients who have endured the loss of a pregnancy.
Dr. Latorre is an assistant professor in the department of psychiatry at the University of Maryland School of Medicine. She has reported no relevant financial disclosures. Email her at obnews@mdedge.com.
A Washington Post article on depression after miscarriage is a reminder that, although couples can suffer deeply from such a loss, there still are ways to provide them with meaningful support (“After miscarriage, I was rocked by depression. Like many other women, I didn’t get follow-up care for this loss,” by Katie C. Reilly, Nov 30, 2019).
Psychiatrists who focus on reproductive psychiatry and collaborative care are trying to change the current therapeutic landscape and improve practitioner awareness and treatment. Ob.gyns. managing patients who have experienced reproductive loss, especially early-term loss, may not immediately refer couples to a therapist or psychiatrist, but we can change this. Practitioners who focus on reproductive health – both physical and mental – are trying to better understand such couples’ experiences, increase their access to care, develop preventative care strategies, and improve provider education.
At the outset, providers who treat patients who have experienced a perinatal loss must recognize that not all individuals will feel that a loss is tragic. Instead, patient reactions occur along a spectrum, and there is no “correct” way to process a loss. A couple’s reaction may depend on a variety of factors, including how late or early in pregnancy the loss occurs, whether the pregnancy is planned or unplanned, and what other psychosocial stressors, such as unstable housing, limited income, and few social supports, may exist. Not every patient experiencing grief, even profoundly, will shed tears; we need to be open to all potential reactions and be mindful when a person may need additional support.
According to the Washington Post article, even though 50% of miscarriages are due to chromosomal abnormalities, women still feel ultimately responsible for the loss. As a society we are bombarded with “experts” in the media telling us the best way, the right way, the healthiest way to live. This barrage of advice distorts our views of what it really means to be a good parent and subtly conveys the idea that mothers are solely responsible for any bad pregnancy outcomes. I remember being fearful of causing unintentional harm to my unborn baby during my own pregnancy. What if I accidentally ate something that would affect her development? Is exposure to second-hand smoke as I walk down the street harming her? How bad would it be if I just had one cup of coffee? My doubts caused quite a bit of distress for me, which is a mild form of the distress I see when counseling couples after their miscarriages.
The article’s author also expressed concern about the emotional sterility of the environment in which miscarriages usually occur: a hospital ED. EDs are designed to promote a level of detachment and to quell any stress for the clinicians so that they can calmly handle unexpected health crises. EDs are not primarily designed to provide patients with emotional support, nor should they be. However, we still can make some improvements to existing ED design to better address couples’ emotional needs. For example, some EDs have placed mental health clinicians on staff, others call patients post discharge to address concerns, and some EDs even provide patients access to mental health trauma teams. Such services are not found in all EDs, and even those that exist may just scratch the surface of what is needed, but they are a step in the right direction. Providing this level of auxiliary care directly from the ED increases patients’ ability to access mental health support in the place where miscarriages are most likely to be first diagnosed and managed.
The American College of Obstetricians and Gynecologists already is trying to fill in the missing pieces when it comes to identifying mood symptoms following miscarriage. One of the key recommendations from the May 2018 Committee Opinion on Redefining the Postpartum Visit is that every woman who has experienced a miscarriage, stillbirth, or neonatal death should receive follow-up care. Mental health is a suggested component of the postpartum care plan. Some outpatient ob.gyn. practices and inpatient units are using screening tools to identify postpartum depression. For example, the Edinburgh Postnatal Depression Scale can be utilized following a miscarriage to help providers identify symptoms of depression and anxiety.
However, The trend in psychiatry over the past decade has been toward collaborative care, models that embed psychiatrists and other mental health clinicians in ob.gyn. practices to help guide the diagnosis and treatment of mental health problems. Some psychiatrists practice a co-located model in which they see patients alongside their ob.gyn. colleagues, whereas other psychiatrists treat a larger number of patients by using chart reviews for medication management while relying on behavioral health care managers for counseling and monitoring. Using this model of mental health care, more patients have access to services that are provided in a location familiar to them.
Another step in the right direction is the October 2019 launch of The National Curriculum in Reproductive Psychiatry (NCRP), which provides free educational material for psychiatry faculty and residents to enhance education on topics related to reproductive psychiatry, including miscarriage, loss, and development of trauma disorders. NCRP aspires to develop educational materials for ob.gyn. residents.
In the past we may have missed the mark in recognizing and treating the trauma that prenatal loss can cause, but we are trying to improve our approaches. More and more couples are sharing their experiences and advocating for themselves and others, often creating change in medical practice, and doctors are starting to listen. As any clinician knows, changes to standards of care can take several years to disseminate into general practice, but this gap between knowledge and treatment is now in the forefront of our minds. I am hopeful that we will continue to make advances and provide better care to our patients who have endured the loss of a pregnancy.
Dr. Latorre is an assistant professor in the department of psychiatry at the University of Maryland School of Medicine. She has reported no relevant financial disclosures. Email her at obnews@mdedge.com.
A Washington Post article on depression after miscarriage is a reminder that, although couples can suffer deeply from such a loss, there still are ways to provide them with meaningful support (“After miscarriage, I was rocked by depression. Like many other women, I didn’t get follow-up care for this loss,” by Katie C. Reilly, Nov 30, 2019).
Psychiatrists who focus on reproductive psychiatry and collaborative care are trying to change the current therapeutic landscape and improve practitioner awareness and treatment. Ob.gyns. managing patients who have experienced reproductive loss, especially early-term loss, may not immediately refer couples to a therapist or psychiatrist, but we can change this. Practitioners who focus on reproductive health – both physical and mental – are trying to better understand such couples’ experiences, increase their access to care, develop preventative care strategies, and improve provider education.
At the outset, providers who treat patients who have experienced a perinatal loss must recognize that not all individuals will feel that a loss is tragic. Instead, patient reactions occur along a spectrum, and there is no “correct” way to process a loss. A couple’s reaction may depend on a variety of factors, including how late or early in pregnancy the loss occurs, whether the pregnancy is planned or unplanned, and what other psychosocial stressors, such as unstable housing, limited income, and few social supports, may exist. Not every patient experiencing grief, even profoundly, will shed tears; we need to be open to all potential reactions and be mindful when a person may need additional support.
According to the Washington Post article, even though 50% of miscarriages are due to chromosomal abnormalities, women still feel ultimately responsible for the loss. As a society we are bombarded with “experts” in the media telling us the best way, the right way, the healthiest way to live. This barrage of advice distorts our views of what it really means to be a good parent and subtly conveys the idea that mothers are solely responsible for any bad pregnancy outcomes. I remember being fearful of causing unintentional harm to my unborn baby during my own pregnancy. What if I accidentally ate something that would affect her development? Is exposure to second-hand smoke as I walk down the street harming her? How bad would it be if I just had one cup of coffee? My doubts caused quite a bit of distress for me, which is a mild form of the distress I see when counseling couples after their miscarriages.
The article’s author also expressed concern about the emotional sterility of the environment in which miscarriages usually occur: a hospital ED. EDs are designed to promote a level of detachment and to quell any stress for the clinicians so that they can calmly handle unexpected health crises. EDs are not primarily designed to provide patients with emotional support, nor should they be. However, we still can make some improvements to existing ED design to better address couples’ emotional needs. For example, some EDs have placed mental health clinicians on staff, others call patients post discharge to address concerns, and some EDs even provide patients access to mental health trauma teams. Such services are not found in all EDs, and even those that exist may just scratch the surface of what is needed, but they are a step in the right direction. Providing this level of auxiliary care directly from the ED increases patients’ ability to access mental health support in the place where miscarriages are most likely to be first diagnosed and managed.
The American College of Obstetricians and Gynecologists already is trying to fill in the missing pieces when it comes to identifying mood symptoms following miscarriage. One of the key recommendations from the May 2018 Committee Opinion on Redefining the Postpartum Visit is that every woman who has experienced a miscarriage, stillbirth, or neonatal death should receive follow-up care. Mental health is a suggested component of the postpartum care plan. Some outpatient ob.gyn. practices and inpatient units are using screening tools to identify postpartum depression. For example, the Edinburgh Postnatal Depression Scale can be utilized following a miscarriage to help providers identify symptoms of depression and anxiety.
However, The trend in psychiatry over the past decade has been toward collaborative care, models that embed psychiatrists and other mental health clinicians in ob.gyn. practices to help guide the diagnosis and treatment of mental health problems. Some psychiatrists practice a co-located model in which they see patients alongside their ob.gyn. colleagues, whereas other psychiatrists treat a larger number of patients by using chart reviews for medication management while relying on behavioral health care managers for counseling and monitoring. Using this model of mental health care, more patients have access to services that are provided in a location familiar to them.
Another step in the right direction is the October 2019 launch of The National Curriculum in Reproductive Psychiatry (NCRP), which provides free educational material for psychiatry faculty and residents to enhance education on topics related to reproductive psychiatry, including miscarriage, loss, and development of trauma disorders. NCRP aspires to develop educational materials for ob.gyn. residents.
In the past we may have missed the mark in recognizing and treating the trauma that prenatal loss can cause, but we are trying to improve our approaches. More and more couples are sharing their experiences and advocating for themselves and others, often creating change in medical practice, and doctors are starting to listen. As any clinician knows, changes to standards of care can take several years to disseminate into general practice, but this gap between knowledge and treatment is now in the forefront of our minds. I am hopeful that we will continue to make advances and provide better care to our patients who have endured the loss of a pregnancy.
Dr. Latorre is an assistant professor in the department of psychiatry at the University of Maryland School of Medicine. She has reported no relevant financial disclosures. Email her at obnews@mdedge.com.
Suicide rate higher than average for female clinicians
The suicide rate for women who provide health care is higher than that of all women of working age, while male health care practitioners are less likely to end their lives than working-age men as a whole, according to the Centers for Disease Control and Prevention.
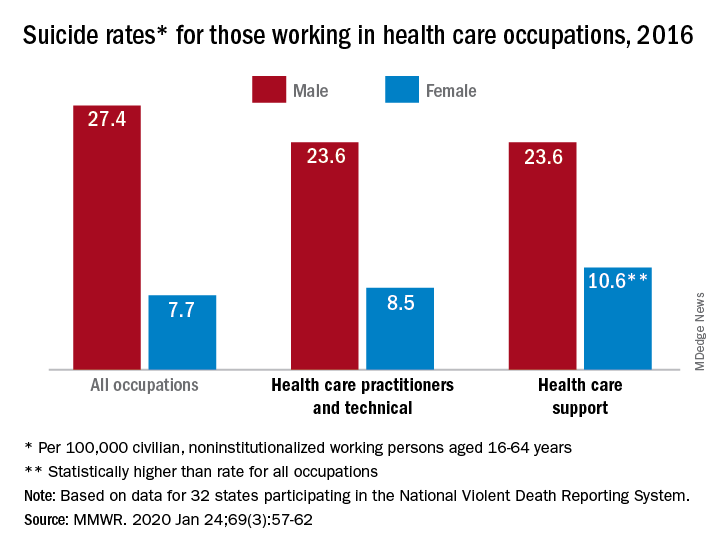
In 2016, the suicide rate for women classified as “healthcare practitioners and technical” – a category that includes physicians and surgeons, as well as chiropractors, physician assistants, and nurse practitioners – was 8.5 per 100,000 population, compared with 7.7 per 100,000 for all working women aged 16-64 years. That difference, however, was not statistically significant, Cora Peterson, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
For females classified as “healthcare support” – medical assistants and transcriptionists, phlebotomists, and pharmacy aides – the suicide rate of 10.6 per 100,000 was significantly higher than that of all working women, the investigators noted.
The suicide rate for males in each of the two occupation categories was 23.6 per 100,000 population in 2016, lower than the rate of 27.4 per 100,000 for males of all occupations, they said, based on data from 32 states that participated in the 2016 National Violent Death Reporting System.
For males, the highest suicide rates in occupations meeting criteria for sample size were “construction and extraction” (49.4 per 100,000); “installation, maintenance, and repair” (36.9); and “arts, design, entertainment, sports, and media” (32.0). Among females, the highest rates were seen in “construction and extraction” (25.5 per 100,000), “protective service” (14.0), and “transportation and material moving” (12.5), with healthcare support next, Dr. Peterson and associates reported.
“Although relative comparisons of suicide rates in this manner are useful for prevention purposes, Therefore, all industry sectors and occupational groups can contribute to reducing suicide incidence,” they wrote.
SOURCE: Peterson C et al. MMWR. 2020 Jan 24;69(3):57-62.
The suicide rate for women who provide health care is higher than that of all women of working age, while male health care practitioners are less likely to end their lives than working-age men as a whole, according to the Centers for Disease Control and Prevention.

In 2016, the suicide rate for women classified as “healthcare practitioners and technical” – a category that includes physicians and surgeons, as well as chiropractors, physician assistants, and nurse practitioners – was 8.5 per 100,000 population, compared with 7.7 per 100,000 for all working women aged 16-64 years. That difference, however, was not statistically significant, Cora Peterson, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
For females classified as “healthcare support” – medical assistants and transcriptionists, phlebotomists, and pharmacy aides – the suicide rate of 10.6 per 100,000 was significantly higher than that of all working women, the investigators noted.
The suicide rate for males in each of the two occupation categories was 23.6 per 100,000 population in 2016, lower than the rate of 27.4 per 100,000 for males of all occupations, they said, based on data from 32 states that participated in the 2016 National Violent Death Reporting System.
For males, the highest suicide rates in occupations meeting criteria for sample size were “construction and extraction” (49.4 per 100,000); “installation, maintenance, and repair” (36.9); and “arts, design, entertainment, sports, and media” (32.0). Among females, the highest rates were seen in “construction and extraction” (25.5 per 100,000), “protective service” (14.0), and “transportation and material moving” (12.5), with healthcare support next, Dr. Peterson and associates reported.
“Although relative comparisons of suicide rates in this manner are useful for prevention purposes, Therefore, all industry sectors and occupational groups can contribute to reducing suicide incidence,” they wrote.
SOURCE: Peterson C et al. MMWR. 2020 Jan 24;69(3):57-62.
The suicide rate for women who provide health care is higher than that of all women of working age, while male health care practitioners are less likely to end their lives than working-age men as a whole, according to the Centers for Disease Control and Prevention.

In 2016, the suicide rate for women classified as “healthcare practitioners and technical” – a category that includes physicians and surgeons, as well as chiropractors, physician assistants, and nurse practitioners – was 8.5 per 100,000 population, compared with 7.7 per 100,000 for all working women aged 16-64 years. That difference, however, was not statistically significant, Cora Peterson, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
For females classified as “healthcare support” – medical assistants and transcriptionists, phlebotomists, and pharmacy aides – the suicide rate of 10.6 per 100,000 was significantly higher than that of all working women, the investigators noted.
The suicide rate for males in each of the two occupation categories was 23.6 per 100,000 population in 2016, lower than the rate of 27.4 per 100,000 for males of all occupations, they said, based on data from 32 states that participated in the 2016 National Violent Death Reporting System.
For males, the highest suicide rates in occupations meeting criteria for sample size were “construction and extraction” (49.4 per 100,000); “installation, maintenance, and repair” (36.9); and “arts, design, entertainment, sports, and media” (32.0). Among females, the highest rates were seen in “construction and extraction” (25.5 per 100,000), “protective service” (14.0), and “transportation and material moving” (12.5), with healthcare support next, Dr. Peterson and associates reported.
“Although relative comparisons of suicide rates in this manner are useful for prevention purposes, Therefore, all industry sectors and occupational groups can contribute to reducing suicide incidence,” they wrote.
SOURCE: Peterson C et al. MMWR. 2020 Jan 24;69(3):57-62.
FROM MMWR
The suicide wars
Topic of suicide prevention causing divisions within psychiatry
At every swipe through my social media feeds, I’m greeted with another topic that has advocates clustered at the extremes. People align, and they align quickly in our strangely polarized world in which anyone who might sit in the middle lies low.
It seems we’re divided: On the left you are a CNN fan or you’re one of those soulless monsters who tunes in to Fox News. You’re pro-life or you’re a baby killer, advocating for late-term abortions or even the execution of live infants. When it comes to firearm regulation, one side says you’re a threat to the Constitution, while the other says that those opposed are responsible for the death of every person who was ever the victim of a discharged firearm. And those who feel strongly about a given topic often justify their attacks on those who disagree. Psychiatry is no stranger to this thinking, and we are the only medical specialty with organized “antipsychiatry” groups who oppose our work. I have been a bit surprised, however, that the topic of suicide prevention is one that has us divided within our own specialty.
Amy Barnhorst, MD, is a psychiatrist at the University of California, Davis, and the author of “The empty promise of suicide prevention: Many of the problems that lead people to kill themselves cannot be fixed with a little serotonin,” an op-ed piece that appeared in the New York Times on April 26, 2019. Dr. Barnhorst began her essay with the story of a patient who was hospitalized after a relative realized she was planning her suicide. That story had an ending that psychiatrists relish: A person with previously unrecognized and untreated bipolar disorder received care, including medication, and got better. This suicide was preventable, a life was saved, and this story followed a model we all hope is being replicated over and over.
Dr. Barnhorst went on to say that this was an outlier in her career, that most of the suicidal patients she sees are impoverished, homeless, addicted, and she wrote about how little the treatment setting has to offer: The idea that a pill would fix these problems is almost laughable. She suggests that there is more to suicide prevention than identifying prospective patients and getting them acute psychiatric care.
The decision to stop living is one that people arrive at by different paths, some over months, but many in a matter of minutes. Those people won’t be intercepted by the mental health system. We certainly need more psychiatric services and more research into better, faster-acting treatments for severe depression and suicidal thoughts, but that will never be enough.
We need to address the root causes of our nation’s suicide problem – poverty, homelessness, and the accompanying exposure to trauma, crime, and drugs. That means better alcohol and drug treatment, family counseling, low-income housing resources, job training, and individual therapy. And for those at risk who still slip past all the checkpoints, we need to make sure they don’t have access to guns and lethal medications.
Psychological autopsies done after suicides have indicated that more than 90% of people who die from suicide suffered from a mental illness, yet 54% of those who ended their own lives had never received a psychiatric diagnosis. There is a hopefulness that, if only we had more – more services, more therapy, more medication – then we could prevent suicide. Unfortunately, this line of thinking, with a “Zero Suicide” initiative, points a finger at those who survive: Suicide is preventable, so someone is to blame, if not a family member for missing the warning signs then the clinician who offered treatment that wasn’t good enough.
Along this line, the New York Times printed another opinion piece on Jan. 6 by Richard A. Friedman, MD, titled, “Why are young Americans killing themselves?” Dr. Friedman’s conclusion was more along the psychiatrist party line: “The good news is that we don’t have to wait for all the answers to know what to do. We know that various psychotherapies and medication are highly effective in treating depression. We just need to do a better job of identifying, reaching out to and providing resources for at-risk youths.”
Dr. Friedman goes on to propose universal screening at school, among other measures to identify those at risk. It is no surprise that Dr. Friedman’s article had more than 1,700 comments before commenting was closed by the Times. I have written about the pros and cons of screening adolescents for depression in a primary care setting, so putting the responsibility of identifying suicidal teenagers on school teachers seems like an ominous responsibility to add to a teacher’s obligations.
I did not read Dr. Barnhorst’s earlier op-ed piece as a condemnation of psychiatric care, but rather as a call to action and a reality check on the idea that psychiatry is the only answer to our suicide epidemic. More people than ever get treatment – from psychiatrists, from primary care doctors, from nonphysician prescribing clinicians, and from so many varieties of psychotherapists, and yet our suicide rates continue to rise.
In a post on the Psychology Today website, Sara Gorman, PhD, and Jack M. Gorman, MD, discussed Dr. Barnhorst’s article. “In the process of making her point, Barnhorst also manages to seriously trivialize the role of antidepressant medication in the treatment of depression and to imply that, given societal woes, there isn’t much we can do to try to prevent suicides – aside from limiting access to lethal means,” they wrote.
The Gormans were not alone in their objections; the day after the op-ed appeared in the New York Times, a well-respected psychiatry department chairman took on not just the content of the op-ed, but also the author, in his Twitter feed. He wrote, “@amybarnhorst doesn’t read scientific literature or skipped training. this article is wrong. #suicide is largely preventable, if proper measures taken n Rx provided. @nytimes please vet authors better @APAPsychiatric.” Dr. Barnhorst, also a voice on Twitter, added the wry response, “I skipped training.” When Twitter users responded that initial Twitter comment conveyed a lack of civility toward a colleague, the original Tweeter – I’m withholding his name with the hope that even writing about these interactions won’t put me on anyone’s enemy list – like many others sitting on the poles of these contentious topics, responded with the following, “All for civility except in the case of misinformation that puts lives at risk, especially when purveyed by a professional who wears the patina of credibility.”
If it’s not yet obvious, I don’t believe there is a simple answer to our suicide problem, nor do I think it puts lives at risk to point out that, so far, our treatments have not lowered suicide rates. The issue is complex and we have no perfect explanation as to why countries differ so greatly with regard to suicide. There are impoverished, war-torn countries with remarkably lower suicide rates, and nations with much stricter gun laws that have higher statistics. Honduras, deemed “the murder capital of the world,” has an enviable suicide rate of only 2.9 per 100,000.
If the solution were as simple as making medications more accessible, the answer might be an easy one (or at least worth trying) – make antidepressants available over-the-counter, a move that would both increase access and decrease stigma.
Some people are determined to end their own lives. They aren’t looking to see psychiatrists or to call hotlines, and they may well resort to an alternate method if any given one is not readily available. For these individuals, suicide may not be preventable, and we may be left to say that this tragic phenomena with its diverse causes should also lead us to explore the root causes of human misery and our cultural features that lead some people to end their own lives while others endure.
Clearly, there are those who have untreated psychiatric illnesses and who make impulsive and lethal decisions – access to care and means restrictions certainly save some lives. And while it is obvious to us as psychiatrists that anyone who is depressed or is having suicidal thoughts is deserving of a psychiatric evaluation and intervention, the truth remains that access to treatment in this country is limited by finances, by the availability of mental health professionals, and by stigma and shame. In the end, The one thing I am certain of is that our efforts to prevent suicide should unite, and not fracture, our profession.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Topic of suicide prevention causing divisions within psychiatry
Topic of suicide prevention causing divisions within psychiatry
At every swipe through my social media feeds, I’m greeted with another topic that has advocates clustered at the extremes. People align, and they align quickly in our strangely polarized world in which anyone who might sit in the middle lies low.
It seems we’re divided: On the left you are a CNN fan or you’re one of those soulless monsters who tunes in to Fox News. You’re pro-life or you’re a baby killer, advocating for late-term abortions or even the execution of live infants. When it comes to firearm regulation, one side says you’re a threat to the Constitution, while the other says that those opposed are responsible for the death of every person who was ever the victim of a discharged firearm. And those who feel strongly about a given topic often justify their attacks on those who disagree. Psychiatry is no stranger to this thinking, and we are the only medical specialty with organized “antipsychiatry” groups who oppose our work. I have been a bit surprised, however, that the topic of suicide prevention is one that has us divided within our own specialty.
Amy Barnhorst, MD, is a psychiatrist at the University of California, Davis, and the author of “The empty promise of suicide prevention: Many of the problems that lead people to kill themselves cannot be fixed with a little serotonin,” an op-ed piece that appeared in the New York Times on April 26, 2019. Dr. Barnhorst began her essay with the story of a patient who was hospitalized after a relative realized she was planning her suicide. That story had an ending that psychiatrists relish: A person with previously unrecognized and untreated bipolar disorder received care, including medication, and got better. This suicide was preventable, a life was saved, and this story followed a model we all hope is being replicated over and over.
Dr. Barnhorst went on to say that this was an outlier in her career, that most of the suicidal patients she sees are impoverished, homeless, addicted, and she wrote about how little the treatment setting has to offer: The idea that a pill would fix these problems is almost laughable. She suggests that there is more to suicide prevention than identifying prospective patients and getting them acute psychiatric care.
The decision to stop living is one that people arrive at by different paths, some over months, but many in a matter of minutes. Those people won’t be intercepted by the mental health system. We certainly need more psychiatric services and more research into better, faster-acting treatments for severe depression and suicidal thoughts, but that will never be enough.
We need to address the root causes of our nation’s suicide problem – poverty, homelessness, and the accompanying exposure to trauma, crime, and drugs. That means better alcohol and drug treatment, family counseling, low-income housing resources, job training, and individual therapy. And for those at risk who still slip past all the checkpoints, we need to make sure they don’t have access to guns and lethal medications.
Psychological autopsies done after suicides have indicated that more than 90% of people who die from suicide suffered from a mental illness, yet 54% of those who ended their own lives had never received a psychiatric diagnosis. There is a hopefulness that, if only we had more – more services, more therapy, more medication – then we could prevent suicide. Unfortunately, this line of thinking, with a “Zero Suicide” initiative, points a finger at those who survive: Suicide is preventable, so someone is to blame, if not a family member for missing the warning signs then the clinician who offered treatment that wasn’t good enough.
Along this line, the New York Times printed another opinion piece on Jan. 6 by Richard A. Friedman, MD, titled, “Why are young Americans killing themselves?” Dr. Friedman’s conclusion was more along the psychiatrist party line: “The good news is that we don’t have to wait for all the answers to know what to do. We know that various psychotherapies and medication are highly effective in treating depression. We just need to do a better job of identifying, reaching out to and providing resources for at-risk youths.”
Dr. Friedman goes on to propose universal screening at school, among other measures to identify those at risk. It is no surprise that Dr. Friedman’s article had more than 1,700 comments before commenting was closed by the Times. I have written about the pros and cons of screening adolescents for depression in a primary care setting, so putting the responsibility of identifying suicidal teenagers on school teachers seems like an ominous responsibility to add to a teacher’s obligations.
I did not read Dr. Barnhorst’s earlier op-ed piece as a condemnation of psychiatric care, but rather as a call to action and a reality check on the idea that psychiatry is the only answer to our suicide epidemic. More people than ever get treatment – from psychiatrists, from primary care doctors, from nonphysician prescribing clinicians, and from so many varieties of psychotherapists, and yet our suicide rates continue to rise.
In a post on the Psychology Today website, Sara Gorman, PhD, and Jack M. Gorman, MD, discussed Dr. Barnhorst’s article. “In the process of making her point, Barnhorst also manages to seriously trivialize the role of antidepressant medication in the treatment of depression and to imply that, given societal woes, there isn’t much we can do to try to prevent suicides – aside from limiting access to lethal means,” they wrote.
The Gormans were not alone in their objections; the day after the op-ed appeared in the New York Times, a well-respected psychiatry department chairman took on not just the content of the op-ed, but also the author, in his Twitter feed. He wrote, “@amybarnhorst doesn’t read scientific literature or skipped training. this article is wrong. #suicide is largely preventable, if proper measures taken n Rx provided. @nytimes please vet authors better @APAPsychiatric.” Dr. Barnhorst, also a voice on Twitter, added the wry response, “I skipped training.” When Twitter users responded that initial Twitter comment conveyed a lack of civility toward a colleague, the original Tweeter – I’m withholding his name with the hope that even writing about these interactions won’t put me on anyone’s enemy list – like many others sitting on the poles of these contentious topics, responded with the following, “All for civility except in the case of misinformation that puts lives at risk, especially when purveyed by a professional who wears the patina of credibility.”
If it’s not yet obvious, I don’t believe there is a simple answer to our suicide problem, nor do I think it puts lives at risk to point out that, so far, our treatments have not lowered suicide rates. The issue is complex and we have no perfect explanation as to why countries differ so greatly with regard to suicide. There are impoverished, war-torn countries with remarkably lower suicide rates, and nations with much stricter gun laws that have higher statistics. Honduras, deemed “the murder capital of the world,” has an enviable suicide rate of only 2.9 per 100,000.
If the solution were as simple as making medications more accessible, the answer might be an easy one (or at least worth trying) – make antidepressants available over-the-counter, a move that would both increase access and decrease stigma.
Some people are determined to end their own lives. They aren’t looking to see psychiatrists or to call hotlines, and they may well resort to an alternate method if any given one is not readily available. For these individuals, suicide may not be preventable, and we may be left to say that this tragic phenomena with its diverse causes should also lead us to explore the root causes of human misery and our cultural features that lead some people to end their own lives while others endure.
Clearly, there are those who have untreated psychiatric illnesses and who make impulsive and lethal decisions – access to care and means restrictions certainly save some lives. And while it is obvious to us as psychiatrists that anyone who is depressed or is having suicidal thoughts is deserving of a psychiatric evaluation and intervention, the truth remains that access to treatment in this country is limited by finances, by the availability of mental health professionals, and by stigma and shame. In the end, The one thing I am certain of is that our efforts to prevent suicide should unite, and not fracture, our profession.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
At every swipe through my social media feeds, I’m greeted with another topic that has advocates clustered at the extremes. People align, and they align quickly in our strangely polarized world in which anyone who might sit in the middle lies low.
It seems we’re divided: On the left you are a CNN fan or you’re one of those soulless monsters who tunes in to Fox News. You’re pro-life or you’re a baby killer, advocating for late-term abortions or even the execution of live infants. When it comes to firearm regulation, one side says you’re a threat to the Constitution, while the other says that those opposed are responsible for the death of every person who was ever the victim of a discharged firearm. And those who feel strongly about a given topic often justify their attacks on those who disagree. Psychiatry is no stranger to this thinking, and we are the only medical specialty with organized “antipsychiatry” groups who oppose our work. I have been a bit surprised, however, that the topic of suicide prevention is one that has us divided within our own specialty.
Amy Barnhorst, MD, is a psychiatrist at the University of California, Davis, and the author of “The empty promise of suicide prevention: Many of the problems that lead people to kill themselves cannot be fixed with a little serotonin,” an op-ed piece that appeared in the New York Times on April 26, 2019. Dr. Barnhorst began her essay with the story of a patient who was hospitalized after a relative realized she was planning her suicide. That story had an ending that psychiatrists relish: A person with previously unrecognized and untreated bipolar disorder received care, including medication, and got better. This suicide was preventable, a life was saved, and this story followed a model we all hope is being replicated over and over.
Dr. Barnhorst went on to say that this was an outlier in her career, that most of the suicidal patients she sees are impoverished, homeless, addicted, and she wrote about how little the treatment setting has to offer: The idea that a pill would fix these problems is almost laughable. She suggests that there is more to suicide prevention than identifying prospective patients and getting them acute psychiatric care.
The decision to stop living is one that people arrive at by different paths, some over months, but many in a matter of minutes. Those people won’t be intercepted by the mental health system. We certainly need more psychiatric services and more research into better, faster-acting treatments for severe depression and suicidal thoughts, but that will never be enough.
We need to address the root causes of our nation’s suicide problem – poverty, homelessness, and the accompanying exposure to trauma, crime, and drugs. That means better alcohol and drug treatment, family counseling, low-income housing resources, job training, and individual therapy. And for those at risk who still slip past all the checkpoints, we need to make sure they don’t have access to guns and lethal medications.
Psychological autopsies done after suicides have indicated that more than 90% of people who die from suicide suffered from a mental illness, yet 54% of those who ended their own lives had never received a psychiatric diagnosis. There is a hopefulness that, if only we had more – more services, more therapy, more medication – then we could prevent suicide. Unfortunately, this line of thinking, with a “Zero Suicide” initiative, points a finger at those who survive: Suicide is preventable, so someone is to blame, if not a family member for missing the warning signs then the clinician who offered treatment that wasn’t good enough.
Along this line, the New York Times printed another opinion piece on Jan. 6 by Richard A. Friedman, MD, titled, “Why are young Americans killing themselves?” Dr. Friedman’s conclusion was more along the psychiatrist party line: “The good news is that we don’t have to wait for all the answers to know what to do. We know that various psychotherapies and medication are highly effective in treating depression. We just need to do a better job of identifying, reaching out to and providing resources for at-risk youths.”
Dr. Friedman goes on to propose universal screening at school, among other measures to identify those at risk. It is no surprise that Dr. Friedman’s article had more than 1,700 comments before commenting was closed by the Times. I have written about the pros and cons of screening adolescents for depression in a primary care setting, so putting the responsibility of identifying suicidal teenagers on school teachers seems like an ominous responsibility to add to a teacher’s obligations.
I did not read Dr. Barnhorst’s earlier op-ed piece as a condemnation of psychiatric care, but rather as a call to action and a reality check on the idea that psychiatry is the only answer to our suicide epidemic. More people than ever get treatment – from psychiatrists, from primary care doctors, from nonphysician prescribing clinicians, and from so many varieties of psychotherapists, and yet our suicide rates continue to rise.
In a post on the Psychology Today website, Sara Gorman, PhD, and Jack M. Gorman, MD, discussed Dr. Barnhorst’s article. “In the process of making her point, Barnhorst also manages to seriously trivialize the role of antidepressant medication in the treatment of depression and to imply that, given societal woes, there isn’t much we can do to try to prevent suicides – aside from limiting access to lethal means,” they wrote.
The Gormans were not alone in their objections; the day after the op-ed appeared in the New York Times, a well-respected psychiatry department chairman took on not just the content of the op-ed, but also the author, in his Twitter feed. He wrote, “@amybarnhorst doesn’t read scientific literature or skipped training. this article is wrong. #suicide is largely preventable, if proper measures taken n Rx provided. @nytimes please vet authors better @APAPsychiatric.” Dr. Barnhorst, also a voice on Twitter, added the wry response, “I skipped training.” When Twitter users responded that initial Twitter comment conveyed a lack of civility toward a colleague, the original Tweeter – I’m withholding his name with the hope that even writing about these interactions won’t put me on anyone’s enemy list – like many others sitting on the poles of these contentious topics, responded with the following, “All for civility except in the case of misinformation that puts lives at risk, especially when purveyed by a professional who wears the patina of credibility.”
If it’s not yet obvious, I don’t believe there is a simple answer to our suicide problem, nor do I think it puts lives at risk to point out that, so far, our treatments have not lowered suicide rates. The issue is complex and we have no perfect explanation as to why countries differ so greatly with regard to suicide. There are impoverished, war-torn countries with remarkably lower suicide rates, and nations with much stricter gun laws that have higher statistics. Honduras, deemed “the murder capital of the world,” has an enviable suicide rate of only 2.9 per 100,000.
If the solution were as simple as making medications more accessible, the answer might be an easy one (or at least worth trying) – make antidepressants available over-the-counter, a move that would both increase access and decrease stigma.
Some people are determined to end their own lives. They aren’t looking to see psychiatrists or to call hotlines, and they may well resort to an alternate method if any given one is not readily available. For these individuals, suicide may not be preventable, and we may be left to say that this tragic phenomena with its diverse causes should also lead us to explore the root causes of human misery and our cultural features that lead some people to end their own lives while others endure.
Clearly, there are those who have untreated psychiatric illnesses and who make impulsive and lethal decisions – access to care and means restrictions certainly save some lives. And while it is obvious to us as psychiatrists that anyone who is depressed or is having suicidal thoughts is deserving of a psychiatric evaluation and intervention, the truth remains that access to treatment in this country is limited by finances, by the availability of mental health professionals, and by stigma and shame. In the end, The one thing I am certain of is that our efforts to prevent suicide should unite, and not fracture, our profession.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Common drug with lots of surprising side effects
A 55-year-old woman comes to clinic for follow-up. She reports her family is worried that she isn’t getting enough sleep and is more tired than usual. The patient reports she is sleeping 8 hours a night and wakes up feeling rested, but she has noticed she has been yawning much more frequently than she remembers in the past.
Past medical history: gastroesophageal reflux disease, hypertension, generalized anxiety disorder, hypothyroidism, and osteoporosis. Medications: amlodipine, lansoprazole, irbesartan, escitalopram, levothyroxine, and alendronate. Physical examination: blood pressure 110/70 mm Hg, pulse 60 bpm. Lower extremities: 1+ edema.
What is the likely cause of her increased yawning?
A. Amlodipine.
B. Alendronate.
C. Irbesartan.
D. Escitalopram.
E. Lansoprazole.
The correct answer here is escitalopram. Selective serotonin reuptake inhibitors in general are well tolerated. Given how commonly these drugs are used, however, there are a number of lesser-known side effects that you are likely to see.
In the above case, this patient has yawning caused by her SSRI. Roncero et al. described a case of yawning in a patient on escitalopram that resolved when the dose of escitalopram was reduced.1 Paroxetine has been reported to cause yawning at both low and high doses.2
In a review of drug-induced yawning, SSRIs as a class were most frequently involved, and sertraline and fluoxetine were implicated in addition to paroxetine.3 The serotonin-norepinephrine reuptake inhibitors duloxetine and venlafaxine have also been associated with yawning.4,5
Hyperhydrosis has also been linked to SSRIs and SNRIs, and both yawning and hyperhidrosis may occur because of an underlying thermoregulatory dysfunction.6
SSRIs have been linked to increased bleeding risk, especially increased risk of upper gastrointestinal hemorrhage. Laporte and colleagues showed an association of SSRI use and risk of bleeding in a meta-analysis of 42 observational studies, with an odds ratio of 1.41 (95% confidence interval, 1.27-1.57; P less than .0001).7 The risk of upper gastrointestinal (UGI) bleeding is further increased if patients are also taking NSAIDs.
Anglin et al. looked at 15 case-control studies and 4 cohort studies and found an OR of 1.66 for UGI bleeding with SSRI use, and an OR of 4.25 for UGI bleeding if SSRI use was combined with NSAID use.8 The number needed to harm is 3,177 for NSAID use in populations at low risk for GI bleeding, but it is much lower (881) in higher-risk populations.8 Make sure to think about patients’ bleeding risks when starting SSRIs.
An issue that comes up frequently is: What is the risk of bleeding in patients on SSRIs who are also on anticoagulants? Dr. Quinn and colleagues looked at the bleeding risk of anticoagulated patients also taking SSRIs in the ROCKET AF trial.9 They found 737 patients who received SSRIs and matched them with other patients not on SSRIs in the trial. All patients in the trial were either receiving rivaroxaban or warfarin for stroke prophylaxis. They found no significant increase risk in bleeding in the patients on SSRIs and anticoagulants.
Take-home points:
- Yawning and hyperhidrosis are interesting side effects of SSRIs.
- Bleeding risk is increased in patients on SSRIs, especially when combined with NSAIDs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. Neurologia. 2013 Nov-Dec;28(9):589-90.
2. Psychiatry Clin Neurosci. 2006 Apr;60(2):260.
3. Presse Med. 2014 Oct;43(10 Pt 1):1135-6.
4. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Jun 15;33(4):747.
5. Ann Pharmacother. 2011 Oct;45(10):1297-301.
6. Depress Anxiety. 2017 Dec;34(12):1134-46.
7. Pharmacol Res. 2017 Apr;118:19-32.
8. Am J Gastroenterol. 2014 Jun;109(6):811-9.
9. J Am Heart Assoc. 2018 Aug 7;7(15):e008755.
A 55-year-old woman comes to clinic for follow-up. She reports her family is worried that she isn’t getting enough sleep and is more tired than usual. The patient reports she is sleeping 8 hours a night and wakes up feeling rested, but she has noticed she has been yawning much more frequently than she remembers in the past.
Past medical history: gastroesophageal reflux disease, hypertension, generalized anxiety disorder, hypothyroidism, and osteoporosis. Medications: amlodipine, lansoprazole, irbesartan, escitalopram, levothyroxine, and alendronate. Physical examination: blood pressure 110/70 mm Hg, pulse 60 bpm. Lower extremities: 1+ edema.
What is the likely cause of her increased yawning?
A. Amlodipine.
B. Alendronate.
C. Irbesartan.
D. Escitalopram.
E. Lansoprazole.
The correct answer here is escitalopram. Selective serotonin reuptake inhibitors in general are well tolerated. Given how commonly these drugs are used, however, there are a number of lesser-known side effects that you are likely to see.
In the above case, this patient has yawning caused by her SSRI. Roncero et al. described a case of yawning in a patient on escitalopram that resolved when the dose of escitalopram was reduced.1 Paroxetine has been reported to cause yawning at both low and high doses.2
In a review of drug-induced yawning, SSRIs as a class were most frequently involved, and sertraline and fluoxetine were implicated in addition to paroxetine.3 The serotonin-norepinephrine reuptake inhibitors duloxetine and venlafaxine have also been associated with yawning.4,5
Hyperhydrosis has also been linked to SSRIs and SNRIs, and both yawning and hyperhidrosis may occur because of an underlying thermoregulatory dysfunction.6
SSRIs have been linked to increased bleeding risk, especially increased risk of upper gastrointestinal hemorrhage. Laporte and colleagues showed an association of SSRI use and risk of bleeding in a meta-analysis of 42 observational studies, with an odds ratio of 1.41 (95% confidence interval, 1.27-1.57; P less than .0001).7 The risk of upper gastrointestinal (UGI) bleeding is further increased if patients are also taking NSAIDs.
Anglin et al. looked at 15 case-control studies and 4 cohort studies and found an OR of 1.66 for UGI bleeding with SSRI use, and an OR of 4.25 for UGI bleeding if SSRI use was combined with NSAID use.8 The number needed to harm is 3,177 for NSAID use in populations at low risk for GI bleeding, but it is much lower (881) in higher-risk populations.8 Make sure to think about patients’ bleeding risks when starting SSRIs.
An issue that comes up frequently is: What is the risk of bleeding in patients on SSRIs who are also on anticoagulants? Dr. Quinn and colleagues looked at the bleeding risk of anticoagulated patients also taking SSRIs in the ROCKET AF trial.9 They found 737 patients who received SSRIs and matched them with other patients not on SSRIs in the trial. All patients in the trial were either receiving rivaroxaban or warfarin for stroke prophylaxis. They found no significant increase risk in bleeding in the patients on SSRIs and anticoagulants.
Take-home points:
- Yawning and hyperhidrosis are interesting side effects of SSRIs.
- Bleeding risk is increased in patients on SSRIs, especially when combined with NSAIDs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. Neurologia. 2013 Nov-Dec;28(9):589-90.
2. Psychiatry Clin Neurosci. 2006 Apr;60(2):260.
3. Presse Med. 2014 Oct;43(10 Pt 1):1135-6.
4. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Jun 15;33(4):747.
5. Ann Pharmacother. 2011 Oct;45(10):1297-301.
6. Depress Anxiety. 2017 Dec;34(12):1134-46.
7. Pharmacol Res. 2017 Apr;118:19-32.
8. Am J Gastroenterol. 2014 Jun;109(6):811-9.
9. J Am Heart Assoc. 2018 Aug 7;7(15):e008755.
A 55-year-old woman comes to clinic for follow-up. She reports her family is worried that she isn’t getting enough sleep and is more tired than usual. The patient reports she is sleeping 8 hours a night and wakes up feeling rested, but she has noticed she has been yawning much more frequently than she remembers in the past.
Past medical history: gastroesophageal reflux disease, hypertension, generalized anxiety disorder, hypothyroidism, and osteoporosis. Medications: amlodipine, lansoprazole, irbesartan, escitalopram, levothyroxine, and alendronate. Physical examination: blood pressure 110/70 mm Hg, pulse 60 bpm. Lower extremities: 1+ edema.
What is the likely cause of her increased yawning?
A. Amlodipine.
B. Alendronate.
C. Irbesartan.
D. Escitalopram.
E. Lansoprazole.
The correct answer here is escitalopram. Selective serotonin reuptake inhibitors in general are well tolerated. Given how commonly these drugs are used, however, there are a number of lesser-known side effects that you are likely to see.
In the above case, this patient has yawning caused by her SSRI. Roncero et al. described a case of yawning in a patient on escitalopram that resolved when the dose of escitalopram was reduced.1 Paroxetine has been reported to cause yawning at both low and high doses.2
In a review of drug-induced yawning, SSRIs as a class were most frequently involved, and sertraline and fluoxetine were implicated in addition to paroxetine.3 The serotonin-norepinephrine reuptake inhibitors duloxetine and venlafaxine have also been associated with yawning.4,5
Hyperhydrosis has also been linked to SSRIs and SNRIs, and both yawning and hyperhidrosis may occur because of an underlying thermoregulatory dysfunction.6
SSRIs have been linked to increased bleeding risk, especially increased risk of upper gastrointestinal hemorrhage. Laporte and colleagues showed an association of SSRI use and risk of bleeding in a meta-analysis of 42 observational studies, with an odds ratio of 1.41 (95% confidence interval, 1.27-1.57; P less than .0001).7 The risk of upper gastrointestinal (UGI) bleeding is further increased if patients are also taking NSAIDs.
Anglin et al. looked at 15 case-control studies and 4 cohort studies and found an OR of 1.66 for UGI bleeding with SSRI use, and an OR of 4.25 for UGI bleeding if SSRI use was combined with NSAID use.8 The number needed to harm is 3,177 for NSAID use in populations at low risk for GI bleeding, but it is much lower (881) in higher-risk populations.8 Make sure to think about patients’ bleeding risks when starting SSRIs.
An issue that comes up frequently is: What is the risk of bleeding in patients on SSRIs who are also on anticoagulants? Dr. Quinn and colleagues looked at the bleeding risk of anticoagulated patients also taking SSRIs in the ROCKET AF trial.9 They found 737 patients who received SSRIs and matched them with other patients not on SSRIs in the trial. All patients in the trial were either receiving rivaroxaban or warfarin for stroke prophylaxis. They found no significant increase risk in bleeding in the patients on SSRIs and anticoagulants.
Take-home points:
- Yawning and hyperhidrosis are interesting side effects of SSRIs.
- Bleeding risk is increased in patients on SSRIs, especially when combined with NSAIDs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. Neurologia. 2013 Nov-Dec;28(9):589-90.
2. Psychiatry Clin Neurosci. 2006 Apr;60(2):260.
3. Presse Med. 2014 Oct;43(10 Pt 1):1135-6.
4. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Jun 15;33(4):747.
5. Ann Pharmacother. 2011 Oct;45(10):1297-301.
6. Depress Anxiety. 2017 Dec;34(12):1134-46.
7. Pharmacol Res. 2017 Apr;118:19-32.
8. Am J Gastroenterol. 2014 Jun;109(6):811-9.
9. J Am Heart Assoc. 2018 Aug 7;7(15):e008755.
Elizabeth Wurtzel helped clear the air on stigma
When Elizabeth Wurtzel wrote “Prozac Nation,” an autobiographical account published in 1994 of her experience with depression and psychiatric medications, she helped shift the public dialogue about mental illness, and in doing so chipped away at the stigma that continues to haunt many of our patients.
Ms. Wurtzel, who died recently at age 52, wrote about depression passionately and matter-of-factly.
As she stated in “Prozac Nation”: “Depression was the loneliest &*%$ thing on earth. There were no halfway houses for depressives, no Depression Anonymous meetings that I knew of. Yes, of course, there were mental hospitals like McLean and Bellevue and Payne Whitney and the Menninger Clinic, but I couldn’t hope to end up in one of those places unless I made a suicide attempt serious enough to warrant oxygen or stitches or a stomach pump.
“I used to wish – to pray to God for the courage and strength – that I’d have the guts not to get better, but to slit my wrists and get a whole lot worse so I could land in some mental ward, where real help might have been possible.”
Think of where the public consciousness was in 1994. Peter D. Kramer, MD, had started the conversation on Prozac a year earlier with his book, “Listening to Prozac,” Kurt Cobain died by suicide in 1994, and 2 years later President Bill Clinton signed the Personal Responsibility and Work Opportunity Reconciliation Act, a law that “reformed” welfare and some say made it more difficult for low-income Americans to secure psychiatric and addiction services (Milbank Q. 2005 Mar;83[1]:65-99).
Prozac, as we know, was the first SSRI on the market in the late 1980s and was hailed as a major medication breakthrough in the treatment of depression. It lacked the side effects of the tricyclic antidepressants of previous years and did not have the potentially dangerous food restrictions associated with monoamine oxidase inhibitors.
Interestingly, the major reviews of Ms. Wurtzel’s book, mainly written by men, were negative. Those reviews focused more on the lifestyle of Ms. Wurtzel, her introspection, and how difficult life was for her, rather than the importance of the book. To me, her writing skills were exceptional, as was her willingness to put her lifestyle and suffering on the line.
The literary critics failed to recognize the book’s importance in unmasking the massive denial of mental illnesses and what Ms. Wurtzel was trying to get across. There have been many successful male writers over the years whose lives were difficult and replete with emotional pain and suffering, and their work was lauded. Regardless of the reviews’ negativity, readers found her book open and enlightening, making it a bestseller – thus paving the way for better and more-open discussion of mental disorders. It also became a touchstone in discussions of antidepressants in the psychiatric literature (Lancet. 1998 Sep 26. doi: 10.1016/S0140-6736(98)08418-9; Lancet. 2015 Oct 1. doi: 10.1016/S2215-0366[15]00430-7; and Biol Psychiatry. 2018 Dec 1;84[11]:e73-5).
However, unfortunately, the stigma still exists on many levels, often starting with the medical profession itself. In my experience over the years in teaching and supervising medical students, many of those not interested in becoming a psychiatrist all too often could not wait for their psych rotation to be over. Generally, they did not take the rotation seriously. I’ve even heard students making light of the delusions and paranoia seen in the suffering of acutely ill patients.
We can take this even further within the profession. I have had many referrals from far too many extremely competent physicians, across many medical specialties, who would refer to their patient as “sort of crazy.” Those physicians want the best for their patients, clearly, in making the referral, but they need to change their thinking and, therefore, their vocabulary about mental disorders. I’d like to see these physicians be more respectful of our patients – just as I would be if I were referring a patient complaining of fatigue and joint pain to a general internist or rheumatologist.
I once knew a brilliant orthopedic surgeon who, when he made a referral, would sit down with the patient and clearly explain why they were not crazy but had an anxiety or a mood problem that he didn’t treat but had a person to refer to who could help. Likewise, I know an ophthalmologist who tells his patients with some emotional symptoms that they are experiencing a difficult situation and would benefit from help that he is not able to provide but could be resolved with another type of specialist who works with their “difficulties.” We clearly need more docs like this who go out of their specialty to explain what patients might need – despite the administrative burden exacted by EMRs on doctors’ time and energy.
As we grow more tolerant in our culture and eliminate distasteful words about people and groups, maybe we should try and avoid the word crazy – even in our general vocabulary. Furthermore, in social situations, while out to dinner with friends, at the gym, or even while in the workplace, just as we may refer to our primary care doc as the best or report we have the best cardiologist or dermatologist, we rarely hear someone being open about the best psychiatrist, psychologist, or therapist in the same manner.
“If ‘Prozac Nation’ has any particular purpose,” she wrote in the afterword, “it would be to come out and say that clinical depression is a real problem, that it ruins lives, that it ends lives, that it very nearly ended my life, that it afflicts many, many people, many very bright and worthy and thoughtful and caring people, people who could probably save the world or at the very least do it some real good.” Those people are our patients, and medicine should take the lead in working further to destigmatize mental illness.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
When Elizabeth Wurtzel wrote “Prozac Nation,” an autobiographical account published in 1994 of her experience with depression and psychiatric medications, she helped shift the public dialogue about mental illness, and in doing so chipped away at the stigma that continues to haunt many of our patients.
Ms. Wurtzel, who died recently at age 52, wrote about depression passionately and matter-of-factly.
As she stated in “Prozac Nation”: “Depression was the loneliest &*%$ thing on earth. There were no halfway houses for depressives, no Depression Anonymous meetings that I knew of. Yes, of course, there were mental hospitals like McLean and Bellevue and Payne Whitney and the Menninger Clinic, but I couldn’t hope to end up in one of those places unless I made a suicide attempt serious enough to warrant oxygen or stitches or a stomach pump.
“I used to wish – to pray to God for the courage and strength – that I’d have the guts not to get better, but to slit my wrists and get a whole lot worse so I could land in some mental ward, where real help might have been possible.”
Think of where the public consciousness was in 1994. Peter D. Kramer, MD, had started the conversation on Prozac a year earlier with his book, “Listening to Prozac,” Kurt Cobain died by suicide in 1994, and 2 years later President Bill Clinton signed the Personal Responsibility and Work Opportunity Reconciliation Act, a law that “reformed” welfare and some say made it more difficult for low-income Americans to secure psychiatric and addiction services (Milbank Q. 2005 Mar;83[1]:65-99).
Prozac, as we know, was the first SSRI on the market in the late 1980s and was hailed as a major medication breakthrough in the treatment of depression. It lacked the side effects of the tricyclic antidepressants of previous years and did not have the potentially dangerous food restrictions associated with monoamine oxidase inhibitors.
Interestingly, the major reviews of Ms. Wurtzel’s book, mainly written by men, were negative. Those reviews focused more on the lifestyle of Ms. Wurtzel, her introspection, and how difficult life was for her, rather than the importance of the book. To me, her writing skills were exceptional, as was her willingness to put her lifestyle and suffering on the line.
The literary critics failed to recognize the book’s importance in unmasking the massive denial of mental illnesses and what Ms. Wurtzel was trying to get across. There have been many successful male writers over the years whose lives were difficult and replete with emotional pain and suffering, and their work was lauded. Regardless of the reviews’ negativity, readers found her book open and enlightening, making it a bestseller – thus paving the way for better and more-open discussion of mental disorders. It also became a touchstone in discussions of antidepressants in the psychiatric literature (Lancet. 1998 Sep 26. doi: 10.1016/S0140-6736(98)08418-9; Lancet. 2015 Oct 1. doi: 10.1016/S2215-0366[15]00430-7; and Biol Psychiatry. 2018 Dec 1;84[11]:e73-5).
However, unfortunately, the stigma still exists on many levels, often starting with the medical profession itself. In my experience over the years in teaching and supervising medical students, many of those not interested in becoming a psychiatrist all too often could not wait for their psych rotation to be over. Generally, they did not take the rotation seriously. I’ve even heard students making light of the delusions and paranoia seen in the suffering of acutely ill patients.
We can take this even further within the profession. I have had many referrals from far too many extremely competent physicians, across many medical specialties, who would refer to their patient as “sort of crazy.” Those physicians want the best for their patients, clearly, in making the referral, but they need to change their thinking and, therefore, their vocabulary about mental disorders. I’d like to see these physicians be more respectful of our patients – just as I would be if I were referring a patient complaining of fatigue and joint pain to a general internist or rheumatologist.
I once knew a brilliant orthopedic surgeon who, when he made a referral, would sit down with the patient and clearly explain why they were not crazy but had an anxiety or a mood problem that he didn’t treat but had a person to refer to who could help. Likewise, I know an ophthalmologist who tells his patients with some emotional symptoms that they are experiencing a difficult situation and would benefit from help that he is not able to provide but could be resolved with another type of specialist who works with their “difficulties.” We clearly need more docs like this who go out of their specialty to explain what patients might need – despite the administrative burden exacted by EMRs on doctors’ time and energy.
As we grow more tolerant in our culture and eliminate distasteful words about people and groups, maybe we should try and avoid the word crazy – even in our general vocabulary. Furthermore, in social situations, while out to dinner with friends, at the gym, or even while in the workplace, just as we may refer to our primary care doc as the best or report we have the best cardiologist or dermatologist, we rarely hear someone being open about the best psychiatrist, psychologist, or therapist in the same manner.
“If ‘Prozac Nation’ has any particular purpose,” she wrote in the afterword, “it would be to come out and say that clinical depression is a real problem, that it ruins lives, that it ends lives, that it very nearly ended my life, that it afflicts many, many people, many very bright and worthy and thoughtful and caring people, people who could probably save the world or at the very least do it some real good.” Those people are our patients, and medicine should take the lead in working further to destigmatize mental illness.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
When Elizabeth Wurtzel wrote “Prozac Nation,” an autobiographical account published in 1994 of her experience with depression and psychiatric medications, she helped shift the public dialogue about mental illness, and in doing so chipped away at the stigma that continues to haunt many of our patients.
Ms. Wurtzel, who died recently at age 52, wrote about depression passionately and matter-of-factly.
As she stated in “Prozac Nation”: “Depression was the loneliest &*%$ thing on earth. There were no halfway houses for depressives, no Depression Anonymous meetings that I knew of. Yes, of course, there were mental hospitals like McLean and Bellevue and Payne Whitney and the Menninger Clinic, but I couldn’t hope to end up in one of those places unless I made a suicide attempt serious enough to warrant oxygen or stitches or a stomach pump.
“I used to wish – to pray to God for the courage and strength – that I’d have the guts not to get better, but to slit my wrists and get a whole lot worse so I could land in some mental ward, where real help might have been possible.”
Think of where the public consciousness was in 1994. Peter D. Kramer, MD, had started the conversation on Prozac a year earlier with his book, “Listening to Prozac,” Kurt Cobain died by suicide in 1994, and 2 years later President Bill Clinton signed the Personal Responsibility and Work Opportunity Reconciliation Act, a law that “reformed” welfare and some say made it more difficult for low-income Americans to secure psychiatric and addiction services (Milbank Q. 2005 Mar;83[1]:65-99).
Prozac, as we know, was the first SSRI on the market in the late 1980s and was hailed as a major medication breakthrough in the treatment of depression. It lacked the side effects of the tricyclic antidepressants of previous years and did not have the potentially dangerous food restrictions associated with monoamine oxidase inhibitors.
Interestingly, the major reviews of Ms. Wurtzel’s book, mainly written by men, were negative. Those reviews focused more on the lifestyle of Ms. Wurtzel, her introspection, and how difficult life was for her, rather than the importance of the book. To me, her writing skills were exceptional, as was her willingness to put her lifestyle and suffering on the line.
The literary critics failed to recognize the book’s importance in unmasking the massive denial of mental illnesses and what Ms. Wurtzel was trying to get across. There have been many successful male writers over the years whose lives were difficult and replete with emotional pain and suffering, and their work was lauded. Regardless of the reviews’ negativity, readers found her book open and enlightening, making it a bestseller – thus paving the way for better and more-open discussion of mental disorders. It also became a touchstone in discussions of antidepressants in the psychiatric literature (Lancet. 1998 Sep 26. doi: 10.1016/S0140-6736(98)08418-9; Lancet. 2015 Oct 1. doi: 10.1016/S2215-0366[15]00430-7; and Biol Psychiatry. 2018 Dec 1;84[11]:e73-5).
However, unfortunately, the stigma still exists on many levels, often starting with the medical profession itself. In my experience over the years in teaching and supervising medical students, many of those not interested in becoming a psychiatrist all too often could not wait for their psych rotation to be over. Generally, they did not take the rotation seriously. I’ve even heard students making light of the delusions and paranoia seen in the suffering of acutely ill patients.
We can take this even further within the profession. I have had many referrals from far too many extremely competent physicians, across many medical specialties, who would refer to their patient as “sort of crazy.” Those physicians want the best for their patients, clearly, in making the referral, but they need to change their thinking and, therefore, their vocabulary about mental disorders. I’d like to see these physicians be more respectful of our patients – just as I would be if I were referring a patient complaining of fatigue and joint pain to a general internist or rheumatologist.
I once knew a brilliant orthopedic surgeon who, when he made a referral, would sit down with the patient and clearly explain why they were not crazy but had an anxiety or a mood problem that he didn’t treat but had a person to refer to who could help. Likewise, I know an ophthalmologist who tells his patients with some emotional symptoms that they are experiencing a difficult situation and would benefit from help that he is not able to provide but could be resolved with another type of specialist who works with their “difficulties.” We clearly need more docs like this who go out of their specialty to explain what patients might need – despite the administrative burden exacted by EMRs on doctors’ time and energy.
As we grow more tolerant in our culture and eliminate distasteful words about people and groups, maybe we should try and avoid the word crazy – even in our general vocabulary. Furthermore, in social situations, while out to dinner with friends, at the gym, or even while in the workplace, just as we may refer to our primary care doc as the best or report we have the best cardiologist or dermatologist, we rarely hear someone being open about the best psychiatrist, psychologist, or therapist in the same manner.
“If ‘Prozac Nation’ has any particular purpose,” she wrote in the afterword, “it would be to come out and say that clinical depression is a real problem, that it ruins lives, that it ends lives, that it very nearly ended my life, that it afflicts many, many people, many very bright and worthy and thoughtful and caring people, people who could probably save the world or at the very least do it some real good.” Those people are our patients, and medicine should take the lead in working further to destigmatize mental illness.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
Your colleague appears depressed. Now what?
Encountering a colleague who appears depressed or suicidal can be challenging, but experts say the worst thing you can do is turn away.
, professor of clinical psychiatry at State University of New York, Brooklyn. “Don’t leave it to ‘the other guy.’ ”
Below, Dr. Myers and other experts offer advice on how to approach a fellow physician who seems depressed or might be in need of professional help.
1. Reach out to the colleague, and find a private, quiet space to talk.
2. Let them know you’re there for them, and do not pass judgment.
3. Share a short list of reasons for your concerns. For example, “It seems you have lost some weight,” or “You seem sad a lot of the time.”
4. Encourage them to accept help. Refrain from giving them a list of names for professional help. Instead, make the phone call for them or set up the appointment, if necessary.
5. Ask specifically about suicidal ideation. For instance, “Are you having thoughts of hurting yourself?” or “Are you having thoughts of ending your life?” People who are suicidal often want someone to ask and help.
6. Stay with the colleague until he or she has worked out a plan of safety – for example, returned to family/loved ones, visited a physician or mental health professional, gone to an emergency department, or called for an ambulance or other assistance.
7. Accompany the colleague to the emergency department or call 911.
8. Remember these two goals: Inquire about safety/danger. Ensure safety of the individual.
Sources: Dr. Myers, Dr. Zisook, and Dr. Yellowlees.
For more information, contact the American Foundation for Suicide Prevention (www.afsp.org), and the 24-hour crisis line: 1-800-273-TALK (8255). Late last year, the Federal Communications Commission approved plans to designate 988 as a suicide prevention hotline number. Implementation of the proposal is expected to take several months.
Encountering a colleague who appears depressed or suicidal can be challenging, but experts say the worst thing you can do is turn away.
, professor of clinical psychiatry at State University of New York, Brooklyn. “Don’t leave it to ‘the other guy.’ ”
Below, Dr. Myers and other experts offer advice on how to approach a fellow physician who seems depressed or might be in need of professional help.
1. Reach out to the colleague, and find a private, quiet space to talk.
2. Let them know you’re there for them, and do not pass judgment.
3. Share a short list of reasons for your concerns. For example, “It seems you have lost some weight,” or “You seem sad a lot of the time.”
4. Encourage them to accept help. Refrain from giving them a list of names for professional help. Instead, make the phone call for them or set up the appointment, if necessary.
5. Ask specifically about suicidal ideation. For instance, “Are you having thoughts of hurting yourself?” or “Are you having thoughts of ending your life?” People who are suicidal often want someone to ask and help.
6. Stay with the colleague until he or she has worked out a plan of safety – for example, returned to family/loved ones, visited a physician or mental health professional, gone to an emergency department, or called for an ambulance or other assistance.
7. Accompany the colleague to the emergency department or call 911.
8. Remember these two goals: Inquire about safety/danger. Ensure safety of the individual.
Sources: Dr. Myers, Dr. Zisook, and Dr. Yellowlees.
For more information, contact the American Foundation for Suicide Prevention (www.afsp.org), and the 24-hour crisis line: 1-800-273-TALK (8255). Late last year, the Federal Communications Commission approved plans to designate 988 as a suicide prevention hotline number. Implementation of the proposal is expected to take several months.
Encountering a colleague who appears depressed or suicidal can be challenging, but experts say the worst thing you can do is turn away.
, professor of clinical psychiatry at State University of New York, Brooklyn. “Don’t leave it to ‘the other guy.’ ”
Below, Dr. Myers and other experts offer advice on how to approach a fellow physician who seems depressed or might be in need of professional help.
1. Reach out to the colleague, and find a private, quiet space to talk.
2. Let them know you’re there for them, and do not pass judgment.
3. Share a short list of reasons for your concerns. For example, “It seems you have lost some weight,” or “You seem sad a lot of the time.”
4. Encourage them to accept help. Refrain from giving them a list of names for professional help. Instead, make the phone call for them or set up the appointment, if necessary.
5. Ask specifically about suicidal ideation. For instance, “Are you having thoughts of hurting yourself?” or “Are you having thoughts of ending your life?” People who are suicidal often want someone to ask and help.
6. Stay with the colleague until he or she has worked out a plan of safety – for example, returned to family/loved ones, visited a physician or mental health professional, gone to an emergency department, or called for an ambulance or other assistance.
7. Accompany the colleague to the emergency department or call 911.
8. Remember these two goals: Inquire about safety/danger. Ensure safety of the individual.
Sources: Dr. Myers, Dr. Zisook, and Dr. Yellowlees.
For more information, contact the American Foundation for Suicide Prevention (www.afsp.org), and the 24-hour crisis line: 1-800-273-TALK (8255). Late last year, the Federal Communications Commission approved plans to designate 988 as a suicide prevention hotline number. Implementation of the proposal is expected to take several months.












