User login
A starting point for precision medicine in type 1 diabetes
MADRID – With type 1 diabetes, there can be great differences in terms of epidemiology, genetics, and possible constituent causes, as well as in the course of the disease before and after diagnosis. This point was made evident in the Can We Perform Precision Medicine in T1D? conference.
At the 63rd Congress of the Spanish Society of Endocrinology (SEEN), María José Redondo, MD, PhD, director of research in the division of diabetes and endocrinology at Texas Children’s Hospital Baylor College of Medicine in Houston, noted that delving into this evidence is the “clue” to implementing precision medicine strategies.
“Physiopathologically, there are different forms of type 1 diabetes that must be considered in the therapeutic approach. The objective is to describe this heterogeneity to discover the etiopathogenesis underlying it, so that endotypes can be defined and thus apply precision medicine. This is the paradigm followed by the European Association for the Study of Diabetes (EASD), the American Diabetes Association (ADA), and other organizations,” said Dr. Redondo.
She added that there have been significant advances in knowledge of factors that account for these epidemiologic and genetic variations. “For example, immunological processes appear to be different in children who develop type 1 diabetes at a young age, compared with those who present with the disease later in life.”
Metabolic factors are also involved in the development of type 1 diabetes in adolescents and adults, “and this metabolic heterogeneity is a very important aspect, since we currently use only glucose to diagnose diabetes and especially to classify it as type 1 when other factors should really be measured, such as C-peptide, since it has been seen that people with high levels of this peptide present a process that is closer to type 2 diabetes and have atypical characteristics for type 1 diabetes that are more like type 2 diabetes (obesity, older age, lack of typically genetic factors associated with type 1 diabetes),” noted Dr. Redondo.
Eluding classification
The specialist added that this evidence suggests a need to review the classification of the different types of diabetes. “The current general classification distinguishes type 1 diabetes, type 2 diabetes, gestational diabetes, monogenic (neonatal) diabetes, monogenic diabetes associated with cystic fibrosis, pancreatogenic, steroid-induced, and posttransplantation diabetes. However, in clinical practice, cases that are very difficult to diagnose and classify emerge, such as autoimmune diabetes, type 1 diabetes in people with insulin resistance, positive antibodies for type 2 diabetes, for example, in children with obesity (in which it is not known whether it is type 1 or type 2 diabetes), drug-induced diabetes in cases of insulin resistance, autoimmune type 1 diabetes with persistent C-peptide, or monogenic diabetes in people with obesity.
“Therefore, the current classification does not help to guide prevention or treatment, and the heterogeneity of the pathology is not as clear as we would like. Since, for example, insulin resistance affects both types of diabetes, inflammation exists in both cases, and the genes that give beta cell secretion defects exist in monogenic diabetes and probably in type 2 diabetes as well. It can be argued that type 2 diabetes is like a backdrop to a lot of diabetes that we know of so far and that it interacts with other factors that have happened to the particular person,” said Dr. Redondo.
“Furthermore, it has been shown that metformin can improve insulin resistance and cardiovascular events in patients with type 1 diabetes with obesity. On the other hand, most patients with type 2 diabetes do not need insulin after diagnosis, except for pediatric patients and those with positive antibodies who require insulin quickly. Added to this is the inability to differentiate between responders and nonresponders to immunomodulators in the prevention of type 1 diabetes, all of which highlights that there are pathogenic processes that can appear in different types of diabetes, which is why the current classification leaves out cases that do not clearly fit into a single disease type, while many people with the same diagnosis actually have very different diseases,” she pointed out.
Toward precision diagnostics
“Encapsulating” all these factors is the first step to applying precision medicine in type 1 diabetes, an area, Dr. Redondo explained, in which concrete actions are being carried out. “One of these actions is to determine BMI [body mass index], which has been incorporated into the diabetes prediction strategy that we use in clinical trials, since we know that people with a high BMI, along with other factors, clearly have a different risk. Likewise, we’ve seen that teplizumab could work better in the prevention of type 1 diabetes in individuals with anti-islet antibodies and that people who have the DR4 gene respond better than those who don’t have it and that those with the DR3 gene respond worse.”
Other recent advances along these lines involve the identification of treatments that can delay or even prevent the development of type 1 diabetes in people with positive antibodies, as well as the development of algorithms and models to predict who will develop the disease, thus placing preventive treatments within reach.
“The objective is to use all available information from each individual to understand the etiology and pathogenesis of the disease at a given moment, knowing that changes occur throughout life, and this also applies to other types of diabetes. The next step is to discover and test pathogenesis-focused therapeutic strategies with the most clinical impact in each patient at any given time,” said Dr. Redondo.
Technological tools
The specialist referred to recent advances in diabetes technology, especially semiclosed systems (such as a sensor/pump) that, in her opinion, have radically changed the control of the disease. “However, the main objective is to make type 1 diabetes preventable or reversible in people who have developed it,” she said.
Fernando Gómez-Peralta, MD, PhD, elected coordinator of the Diabetes Department at SEEN and head of the endocrinology and nutrition unit of General Hospital of Segovia, Spain, spoke about these technological advances in his presentation, “Technology and Diabetes: Clinical Experiences,” which was organized in collaboration with the Spanish Diabetes Society.
According to this expert, technological and digital tools are changing the daily lives of people with this disease. “Continuous glucose monitoring and new connected insulin pen and cap systems have increased the benefits for users of treatment with new insulins, for example,” said Dr. Gómez-Peralta.
He explained that most systems make it possible to access complete data regarding glycemic control and the treatment received and to share them with caregivers, professionals, and family members. “Some integrated insulin pump and sensor systems have self-adjusting insulin therapy algorithms that have been shown to greatly increase time-to-target glucose and reduce hypoglycemic events,” he said.
“Regarding glucose monitoring, there are devices with a longer duration (up to 2 weeks) and precision that are characterized by easier use for the patient, avoiding the need for calibration, with annoying capillary blood glucose levels.”
In the case of insulin administration, it is anticipated that in the future, some models will have very interesting features, Dr. Gómez-Peralta said. “Integrated closed-loop glucose sensor and insulin pump systems that have self-adjusting algorithms, regardless of the user, are highly effective and safe, and clearly improve glycemic control.
“For users of insulin injections, connected pens allow the integration of dynamic glucose information with doses, as well as the integration of user support tools for insulin adjustment,” Dr. Gómez-Peralta added.
The specialist stressed that a challenge for the future is to reduce the digital divide so as to increase the capacity and motivation to access these options. “In the coming years, health systems will have to face significant cost so that these systems are made available to all patients, and it is necessary to provide the systems with more material and human resources so that they can be integrated with our endocrinology and diabetes services and units.”
Dr. Redondo and Dr. Gómez-Peralta have disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish edition and a version appeared on Medscape.com.
MADRID – With type 1 diabetes, there can be great differences in terms of epidemiology, genetics, and possible constituent causes, as well as in the course of the disease before and after diagnosis. This point was made evident in the Can We Perform Precision Medicine in T1D? conference.
At the 63rd Congress of the Spanish Society of Endocrinology (SEEN), María José Redondo, MD, PhD, director of research in the division of diabetes and endocrinology at Texas Children’s Hospital Baylor College of Medicine in Houston, noted that delving into this evidence is the “clue” to implementing precision medicine strategies.
“Physiopathologically, there are different forms of type 1 diabetes that must be considered in the therapeutic approach. The objective is to describe this heterogeneity to discover the etiopathogenesis underlying it, so that endotypes can be defined and thus apply precision medicine. This is the paradigm followed by the European Association for the Study of Diabetes (EASD), the American Diabetes Association (ADA), and other organizations,” said Dr. Redondo.
She added that there have been significant advances in knowledge of factors that account for these epidemiologic and genetic variations. “For example, immunological processes appear to be different in children who develop type 1 diabetes at a young age, compared with those who present with the disease later in life.”
Metabolic factors are also involved in the development of type 1 diabetes in adolescents and adults, “and this metabolic heterogeneity is a very important aspect, since we currently use only glucose to diagnose diabetes and especially to classify it as type 1 when other factors should really be measured, such as C-peptide, since it has been seen that people with high levels of this peptide present a process that is closer to type 2 diabetes and have atypical characteristics for type 1 diabetes that are more like type 2 diabetes (obesity, older age, lack of typically genetic factors associated with type 1 diabetes),” noted Dr. Redondo.
Eluding classification
The specialist added that this evidence suggests a need to review the classification of the different types of diabetes. “The current general classification distinguishes type 1 diabetes, type 2 diabetes, gestational diabetes, monogenic (neonatal) diabetes, monogenic diabetes associated with cystic fibrosis, pancreatogenic, steroid-induced, and posttransplantation diabetes. However, in clinical practice, cases that are very difficult to diagnose and classify emerge, such as autoimmune diabetes, type 1 diabetes in people with insulin resistance, positive antibodies for type 2 diabetes, for example, in children with obesity (in which it is not known whether it is type 1 or type 2 diabetes), drug-induced diabetes in cases of insulin resistance, autoimmune type 1 diabetes with persistent C-peptide, or monogenic diabetes in people with obesity.
“Therefore, the current classification does not help to guide prevention or treatment, and the heterogeneity of the pathology is not as clear as we would like. Since, for example, insulin resistance affects both types of diabetes, inflammation exists in both cases, and the genes that give beta cell secretion defects exist in monogenic diabetes and probably in type 2 diabetes as well. It can be argued that type 2 diabetes is like a backdrop to a lot of diabetes that we know of so far and that it interacts with other factors that have happened to the particular person,” said Dr. Redondo.
“Furthermore, it has been shown that metformin can improve insulin resistance and cardiovascular events in patients with type 1 diabetes with obesity. On the other hand, most patients with type 2 diabetes do not need insulin after diagnosis, except for pediatric patients and those with positive antibodies who require insulin quickly. Added to this is the inability to differentiate between responders and nonresponders to immunomodulators in the prevention of type 1 diabetes, all of which highlights that there are pathogenic processes that can appear in different types of diabetes, which is why the current classification leaves out cases that do not clearly fit into a single disease type, while many people with the same diagnosis actually have very different diseases,” she pointed out.
Toward precision diagnostics
“Encapsulating” all these factors is the first step to applying precision medicine in type 1 diabetes, an area, Dr. Redondo explained, in which concrete actions are being carried out. “One of these actions is to determine BMI [body mass index], which has been incorporated into the diabetes prediction strategy that we use in clinical trials, since we know that people with a high BMI, along with other factors, clearly have a different risk. Likewise, we’ve seen that teplizumab could work better in the prevention of type 1 diabetes in individuals with anti-islet antibodies and that people who have the DR4 gene respond better than those who don’t have it and that those with the DR3 gene respond worse.”
Other recent advances along these lines involve the identification of treatments that can delay or even prevent the development of type 1 diabetes in people with positive antibodies, as well as the development of algorithms and models to predict who will develop the disease, thus placing preventive treatments within reach.
“The objective is to use all available information from each individual to understand the etiology and pathogenesis of the disease at a given moment, knowing that changes occur throughout life, and this also applies to other types of diabetes. The next step is to discover and test pathogenesis-focused therapeutic strategies with the most clinical impact in each patient at any given time,” said Dr. Redondo.
Technological tools
The specialist referred to recent advances in diabetes technology, especially semiclosed systems (such as a sensor/pump) that, in her opinion, have radically changed the control of the disease. “However, the main objective is to make type 1 diabetes preventable or reversible in people who have developed it,” she said.
Fernando Gómez-Peralta, MD, PhD, elected coordinator of the Diabetes Department at SEEN and head of the endocrinology and nutrition unit of General Hospital of Segovia, Spain, spoke about these technological advances in his presentation, “Technology and Diabetes: Clinical Experiences,” which was organized in collaboration with the Spanish Diabetes Society.
According to this expert, technological and digital tools are changing the daily lives of people with this disease. “Continuous glucose monitoring and new connected insulin pen and cap systems have increased the benefits for users of treatment with new insulins, for example,” said Dr. Gómez-Peralta.
He explained that most systems make it possible to access complete data regarding glycemic control and the treatment received and to share them with caregivers, professionals, and family members. “Some integrated insulin pump and sensor systems have self-adjusting insulin therapy algorithms that have been shown to greatly increase time-to-target glucose and reduce hypoglycemic events,” he said.
“Regarding glucose monitoring, there are devices with a longer duration (up to 2 weeks) and precision that are characterized by easier use for the patient, avoiding the need for calibration, with annoying capillary blood glucose levels.”
In the case of insulin administration, it is anticipated that in the future, some models will have very interesting features, Dr. Gómez-Peralta said. “Integrated closed-loop glucose sensor and insulin pump systems that have self-adjusting algorithms, regardless of the user, are highly effective and safe, and clearly improve glycemic control.
“For users of insulin injections, connected pens allow the integration of dynamic glucose information with doses, as well as the integration of user support tools for insulin adjustment,” Dr. Gómez-Peralta added.
The specialist stressed that a challenge for the future is to reduce the digital divide so as to increase the capacity and motivation to access these options. “In the coming years, health systems will have to face significant cost so that these systems are made available to all patients, and it is necessary to provide the systems with more material and human resources so that they can be integrated with our endocrinology and diabetes services and units.”
Dr. Redondo and Dr. Gómez-Peralta have disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish edition and a version appeared on Medscape.com.
MADRID – With type 1 diabetes, there can be great differences in terms of epidemiology, genetics, and possible constituent causes, as well as in the course of the disease before and after diagnosis. This point was made evident in the Can We Perform Precision Medicine in T1D? conference.
At the 63rd Congress of the Spanish Society of Endocrinology (SEEN), María José Redondo, MD, PhD, director of research in the division of diabetes and endocrinology at Texas Children’s Hospital Baylor College of Medicine in Houston, noted that delving into this evidence is the “clue” to implementing precision medicine strategies.
“Physiopathologically, there are different forms of type 1 diabetes that must be considered in the therapeutic approach. The objective is to describe this heterogeneity to discover the etiopathogenesis underlying it, so that endotypes can be defined and thus apply precision medicine. This is the paradigm followed by the European Association for the Study of Diabetes (EASD), the American Diabetes Association (ADA), and other organizations,” said Dr. Redondo.
She added that there have been significant advances in knowledge of factors that account for these epidemiologic and genetic variations. “For example, immunological processes appear to be different in children who develop type 1 diabetes at a young age, compared with those who present with the disease later in life.”
Metabolic factors are also involved in the development of type 1 diabetes in adolescents and adults, “and this metabolic heterogeneity is a very important aspect, since we currently use only glucose to diagnose diabetes and especially to classify it as type 1 when other factors should really be measured, such as C-peptide, since it has been seen that people with high levels of this peptide present a process that is closer to type 2 diabetes and have atypical characteristics for type 1 diabetes that are more like type 2 diabetes (obesity, older age, lack of typically genetic factors associated with type 1 diabetes),” noted Dr. Redondo.
Eluding classification
The specialist added that this evidence suggests a need to review the classification of the different types of diabetes. “The current general classification distinguishes type 1 diabetes, type 2 diabetes, gestational diabetes, monogenic (neonatal) diabetes, monogenic diabetes associated with cystic fibrosis, pancreatogenic, steroid-induced, and posttransplantation diabetes. However, in clinical practice, cases that are very difficult to diagnose and classify emerge, such as autoimmune diabetes, type 1 diabetes in people with insulin resistance, positive antibodies for type 2 diabetes, for example, in children with obesity (in which it is not known whether it is type 1 or type 2 diabetes), drug-induced diabetes in cases of insulin resistance, autoimmune type 1 diabetes with persistent C-peptide, or monogenic diabetes in people with obesity.
“Therefore, the current classification does not help to guide prevention or treatment, and the heterogeneity of the pathology is not as clear as we would like. Since, for example, insulin resistance affects both types of diabetes, inflammation exists in both cases, and the genes that give beta cell secretion defects exist in monogenic diabetes and probably in type 2 diabetes as well. It can be argued that type 2 diabetes is like a backdrop to a lot of diabetes that we know of so far and that it interacts with other factors that have happened to the particular person,” said Dr. Redondo.
“Furthermore, it has been shown that metformin can improve insulin resistance and cardiovascular events in patients with type 1 diabetes with obesity. On the other hand, most patients with type 2 diabetes do not need insulin after diagnosis, except for pediatric patients and those with positive antibodies who require insulin quickly. Added to this is the inability to differentiate between responders and nonresponders to immunomodulators in the prevention of type 1 diabetes, all of which highlights that there are pathogenic processes that can appear in different types of diabetes, which is why the current classification leaves out cases that do not clearly fit into a single disease type, while many people with the same diagnosis actually have very different diseases,” she pointed out.
Toward precision diagnostics
“Encapsulating” all these factors is the first step to applying precision medicine in type 1 diabetes, an area, Dr. Redondo explained, in which concrete actions are being carried out. “One of these actions is to determine BMI [body mass index], which has been incorporated into the diabetes prediction strategy that we use in clinical trials, since we know that people with a high BMI, along with other factors, clearly have a different risk. Likewise, we’ve seen that teplizumab could work better in the prevention of type 1 diabetes in individuals with anti-islet antibodies and that people who have the DR4 gene respond better than those who don’t have it and that those with the DR3 gene respond worse.”
Other recent advances along these lines involve the identification of treatments that can delay or even prevent the development of type 1 diabetes in people with positive antibodies, as well as the development of algorithms and models to predict who will develop the disease, thus placing preventive treatments within reach.
“The objective is to use all available information from each individual to understand the etiology and pathogenesis of the disease at a given moment, knowing that changes occur throughout life, and this also applies to other types of diabetes. The next step is to discover and test pathogenesis-focused therapeutic strategies with the most clinical impact in each patient at any given time,” said Dr. Redondo.
Technological tools
The specialist referred to recent advances in diabetes technology, especially semiclosed systems (such as a sensor/pump) that, in her opinion, have radically changed the control of the disease. “However, the main objective is to make type 1 diabetes preventable or reversible in people who have developed it,” she said.
Fernando Gómez-Peralta, MD, PhD, elected coordinator of the Diabetes Department at SEEN and head of the endocrinology and nutrition unit of General Hospital of Segovia, Spain, spoke about these technological advances in his presentation, “Technology and Diabetes: Clinical Experiences,” which was organized in collaboration with the Spanish Diabetes Society.
According to this expert, technological and digital tools are changing the daily lives of people with this disease. “Continuous glucose monitoring and new connected insulin pen and cap systems have increased the benefits for users of treatment with new insulins, for example,” said Dr. Gómez-Peralta.
He explained that most systems make it possible to access complete data regarding glycemic control and the treatment received and to share them with caregivers, professionals, and family members. “Some integrated insulin pump and sensor systems have self-adjusting insulin therapy algorithms that have been shown to greatly increase time-to-target glucose and reduce hypoglycemic events,” he said.
“Regarding glucose monitoring, there are devices with a longer duration (up to 2 weeks) and precision that are characterized by easier use for the patient, avoiding the need for calibration, with annoying capillary blood glucose levels.”
In the case of insulin administration, it is anticipated that in the future, some models will have very interesting features, Dr. Gómez-Peralta said. “Integrated closed-loop glucose sensor and insulin pump systems that have self-adjusting algorithms, regardless of the user, are highly effective and safe, and clearly improve glycemic control.
“For users of insulin injections, connected pens allow the integration of dynamic glucose information with doses, as well as the integration of user support tools for insulin adjustment,” Dr. Gómez-Peralta added.
The specialist stressed that a challenge for the future is to reduce the digital divide so as to increase the capacity and motivation to access these options. “In the coming years, health systems will have to face significant cost so that these systems are made available to all patients, and it is necessary to provide the systems with more material and human resources so that they can be integrated with our endocrinology and diabetes services and units.”
Dr. Redondo and Dr. Gómez-Peralta have disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish edition and a version appeared on Medscape.com.
Dubious diagnosis: Is there a better way to define ‘prediabetes’?
and subsequent complications, and therefore merit more intensive intervention.
“Prediabetes” is the term coined to refer to either “impaired fasting glucose (IFG)” or “impaired glucose tolerance (IGT),” both denoting levels of elevated glycemia that don’t meet the thresholds for diabetes. It’s a heterogeneous group overall, and despite its name, not everyone with prediabetes will progress to develop type 2 diabetes.
There have been major increases in prediabetes in the United States and globally over the past 2 decades, epidemiologist Elizabeth Selvin, PhD, said at the recent IDF World Diabetes Congress 2022.
She noted that the concept of “prediabetes” has been controversial, previously dubbed a “dubious diagnosis” and a “boon for Pharma” in a 2019 Science article.
Others have said it’s “not a medical condition” and that it’s “an artificial category with virtually zero clinical relevance” in a press statement issued for a 2014 BMJ article.
“I don’t agree with these statements entirely but I think they speak to the confusion and tremendous controversy around the concept of prediabetes ... I think instead of calling prediabetes a ‘dubious diagnosis’ we should think of it as an opportunity,” said Dr. Selvin, of Johns Hopkins University Bloomberg School of Public Health, Baltimore.
She proposes trying to home in on those with highest risk of developing type 2 diabetes, which she suggests could be achieved by using a combination of elevated fasting glucose and an elevated A1c, although she stresses that this isn’t in any official guidance.
With the appropriate definition, people who are truly at risk for progression to type 2 diabetes can be identified so that lifestyle factors and cardiovascular risk can be addressed, and weight loss efforts implemented.
“Prevention of weight gain is ... important. That message often gets lost. Even if we can’t get people to lose weight, preventing [further] weight gain is important,” she noted.
Asked to comment, Sue Kirkman, MD, told this news organization, “The term prediabetes – or IFG or IGT or any of the ‘intermediate’ terms – is pragmatic in a way. It helps clinicians and patients understand that they are in a higher-risk category and might need intervention and likely need ongoing monitoring. But like many other risk factors [such as] blood pressure, [high] BMI, etc., the risk is not dichotomous but a continuum.
“People at the low end of the ‘intermediate’ range are not going to have much more risk compared to people who are ‘normal,’ while those at the high end of the range have very high risk,” said Dr. Kirkman, of the University of North Carolina, Chapel Hill, and a coauthor of the American Diabetes Association’s diabetes and prediabetes classifications.
“So we lose information if we just lump everyone into a single category. For individual patients, we definitely need better ways to estimate and communicate their potential risk.”
Currently five definitions for prediabetes: Home in on risk
The problem, Dr. Selvin explained, is that currently there are five official definitions for “prediabetes” using cutoffs for hemoglobin A1c, fasting glucose, or an oral glucose tolerance test.
Each one identifies different numbers of people with differing risk levels, ranging from a prevalence of 4.3% of the middle-aged adult population with the International Expert Committee’s definition of A1c 6.0%-6.4% to 43.5% with the American Diabetes Association’s 100-125 mg/dL fasting glucose.
“That’s an enormous difference. No wonder people are confused about who has prediabetes and what we should do about it,” Dr. Selvin said, adding that the concern about overdiagnosing “prediabetes” is even greater for older populations, in whom “it’s incredibly common to have mildly elevated glucose.”
Hence her proposal of what she sees as an evidence-based, “really easy solution” that clinicians can use now to better identify which patients with “intermediate hyperglycemia” to be most concerned about: Use a combination of fasting glucose above 100 mg/dL and an A1c greater than 5.7%.
“If you have both fasting glucose and hemoglobin A1c, you can use them together ... This is not codified in any guidelines. You won’t see this mentioned anywhere. The guidelines are silent on what to do when some people have an elevated fasting glucose but not an elevated A1c ... but I think a simple message is that if people have both an elevated fasting glucose and an elevated A1c, that’s a very high-risk group,” she said.
On the other hand, Dr. Kirkman pointed out, “most discrepancies are near the margins, as in one test is slightly elevated and one isn’t, so those people probably are at low risk.
“It may be that both being elevated means higher risk because they have more hyperglycemia ... so it seems reasonable, but only if it changes what you tell people.”
For example, Dr. Kirkman said, “I’d tell someone with A1c of 5.8% and fasting glucose of 99 mg/dL the same thing I’d tell someone with that A1c and a glucose of 104 mg/dL – that their risk is still pretty low – and I’d recommend healthy lifestyle and weight loss if overweight either way.”
However, she also said, “Certainly people with higher glucose or A1c are at much higher risk, and same for those with both.”
Tie “prediabetes” definition to risk, as cardiology scores do?
Dr. Selvin also believes that risk-based definitions of prediabetes are needed. Ideally, these would incorporate demographics and clinical factors such as age and body mass index. Other biomarkers could potentially be developed and validated for inclusion in the definition, such as C-reactive protein (CRP), lipids, or even genetic/proteomic information.
Moreover, she thinks that the definition should be tied to clinical decision-making, as is the pooled cohort equation in cardiology.
“I think we could do something very similar in prediabetes,” she suggested, adding that even simply incorporating age and BMI into the definition could help further stratify the risk level until other predictors are validated.
Dr. Kirkman said, “The concept of risk scores a la cardiology is interesting, although we’d have to make them simple and also validate them against some outcome.”
Regarding the age issue, Dr. Kirkman noted that although age wasn’t a predictor of progression to type 2 diabetes in the placebo arm of the landmark Diabetes Prevention Program (DPP) trial, “I do agree that it’s a problem that many older folks have the label of prediabetes because of a mildly elevated A1c and we know that most will never get diabetes.”
And, she noted, in the DPP people with prediabetes who had a BMI over 35 kg/m2 did have significantly higher progression rates than those with lower BMI, while women with a history of gestational diabetes mellitus are also known to be at particularly high risk.
Whom should we throw the kitchen sink at?
Some of this discussion, Dr. Kirkman said, “is really a philosophical one, especially when you consider that lifestyle intervention has benefits for almost everyone on many short- and long-term outcomes.”
“The question is probably whom we should ‘throw the kitchen sink at,’ who should get more scalable advice that might apply to everyone regardless of glycemic levels, and whether there’s some more intermediate group that needs more of a [National Diabetes Prevention Program] approach.”
Dr. Selvin’s group is now working on gathering data to inform development of a risk-based prediabetes definition. “We have a whole research effort in this area. I hope that with some really strong data on risk in prediabetes, that can help to solve the heterogeneity issue. I’m focused on bringing evidence to bear to change the guidelines.”
In the meantime, she told this news organization, “I think there are things we can do now to provide more guidance. I get a lot of feedback from people saying things like ‘my physician told me I have prediabetes but now I don’t’ or ‘I saw in my labs that my blood sugar is elevated but my doctor never said anything.’ That’s a communications issue where we can do a better job.”
The meeting was sponsored by the International Diabetes Federation.
Dr. Selvin is deputy editor of Diabetes Care and on the editorial board of Diabetologia. She receives funding from the NIH and the Foundation for the NIH, and royalties from UpToDate for sections related to screening, diagnosis, and laboratory testing for diabetes. Dr. Kirkman reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
and subsequent complications, and therefore merit more intensive intervention.
“Prediabetes” is the term coined to refer to either “impaired fasting glucose (IFG)” or “impaired glucose tolerance (IGT),” both denoting levels of elevated glycemia that don’t meet the thresholds for diabetes. It’s a heterogeneous group overall, and despite its name, not everyone with prediabetes will progress to develop type 2 diabetes.
There have been major increases in prediabetes in the United States and globally over the past 2 decades, epidemiologist Elizabeth Selvin, PhD, said at the recent IDF World Diabetes Congress 2022.
She noted that the concept of “prediabetes” has been controversial, previously dubbed a “dubious diagnosis” and a “boon for Pharma” in a 2019 Science article.
Others have said it’s “not a medical condition” and that it’s “an artificial category with virtually zero clinical relevance” in a press statement issued for a 2014 BMJ article.
“I don’t agree with these statements entirely but I think they speak to the confusion and tremendous controversy around the concept of prediabetes ... I think instead of calling prediabetes a ‘dubious diagnosis’ we should think of it as an opportunity,” said Dr. Selvin, of Johns Hopkins University Bloomberg School of Public Health, Baltimore.
She proposes trying to home in on those with highest risk of developing type 2 diabetes, which she suggests could be achieved by using a combination of elevated fasting glucose and an elevated A1c, although she stresses that this isn’t in any official guidance.
With the appropriate definition, people who are truly at risk for progression to type 2 diabetes can be identified so that lifestyle factors and cardiovascular risk can be addressed, and weight loss efforts implemented.
“Prevention of weight gain is ... important. That message often gets lost. Even if we can’t get people to lose weight, preventing [further] weight gain is important,” she noted.
Asked to comment, Sue Kirkman, MD, told this news organization, “The term prediabetes – or IFG or IGT or any of the ‘intermediate’ terms – is pragmatic in a way. It helps clinicians and patients understand that they are in a higher-risk category and might need intervention and likely need ongoing monitoring. But like many other risk factors [such as] blood pressure, [high] BMI, etc., the risk is not dichotomous but a continuum.
“People at the low end of the ‘intermediate’ range are not going to have much more risk compared to people who are ‘normal,’ while those at the high end of the range have very high risk,” said Dr. Kirkman, of the University of North Carolina, Chapel Hill, and a coauthor of the American Diabetes Association’s diabetes and prediabetes classifications.
“So we lose information if we just lump everyone into a single category. For individual patients, we definitely need better ways to estimate and communicate their potential risk.”
Currently five definitions for prediabetes: Home in on risk
The problem, Dr. Selvin explained, is that currently there are five official definitions for “prediabetes” using cutoffs for hemoglobin A1c, fasting glucose, or an oral glucose tolerance test.
Each one identifies different numbers of people with differing risk levels, ranging from a prevalence of 4.3% of the middle-aged adult population with the International Expert Committee’s definition of A1c 6.0%-6.4% to 43.5% with the American Diabetes Association’s 100-125 mg/dL fasting glucose.
“That’s an enormous difference. No wonder people are confused about who has prediabetes and what we should do about it,” Dr. Selvin said, adding that the concern about overdiagnosing “prediabetes” is even greater for older populations, in whom “it’s incredibly common to have mildly elevated glucose.”
Hence her proposal of what she sees as an evidence-based, “really easy solution” that clinicians can use now to better identify which patients with “intermediate hyperglycemia” to be most concerned about: Use a combination of fasting glucose above 100 mg/dL and an A1c greater than 5.7%.
“If you have both fasting glucose and hemoglobin A1c, you can use them together ... This is not codified in any guidelines. You won’t see this mentioned anywhere. The guidelines are silent on what to do when some people have an elevated fasting glucose but not an elevated A1c ... but I think a simple message is that if people have both an elevated fasting glucose and an elevated A1c, that’s a very high-risk group,” she said.
On the other hand, Dr. Kirkman pointed out, “most discrepancies are near the margins, as in one test is slightly elevated and one isn’t, so those people probably are at low risk.
“It may be that both being elevated means higher risk because they have more hyperglycemia ... so it seems reasonable, but only if it changes what you tell people.”
For example, Dr. Kirkman said, “I’d tell someone with A1c of 5.8% and fasting glucose of 99 mg/dL the same thing I’d tell someone with that A1c and a glucose of 104 mg/dL – that their risk is still pretty low – and I’d recommend healthy lifestyle and weight loss if overweight either way.”
However, she also said, “Certainly people with higher glucose or A1c are at much higher risk, and same for those with both.”
Tie “prediabetes” definition to risk, as cardiology scores do?
Dr. Selvin also believes that risk-based definitions of prediabetes are needed. Ideally, these would incorporate demographics and clinical factors such as age and body mass index. Other biomarkers could potentially be developed and validated for inclusion in the definition, such as C-reactive protein (CRP), lipids, or even genetic/proteomic information.
Moreover, she thinks that the definition should be tied to clinical decision-making, as is the pooled cohort equation in cardiology.
“I think we could do something very similar in prediabetes,” she suggested, adding that even simply incorporating age and BMI into the definition could help further stratify the risk level until other predictors are validated.
Dr. Kirkman said, “The concept of risk scores a la cardiology is interesting, although we’d have to make them simple and also validate them against some outcome.”
Regarding the age issue, Dr. Kirkman noted that although age wasn’t a predictor of progression to type 2 diabetes in the placebo arm of the landmark Diabetes Prevention Program (DPP) trial, “I do agree that it’s a problem that many older folks have the label of prediabetes because of a mildly elevated A1c and we know that most will never get diabetes.”
And, she noted, in the DPP people with prediabetes who had a BMI over 35 kg/m2 did have significantly higher progression rates than those with lower BMI, while women with a history of gestational diabetes mellitus are also known to be at particularly high risk.
Whom should we throw the kitchen sink at?
Some of this discussion, Dr. Kirkman said, “is really a philosophical one, especially when you consider that lifestyle intervention has benefits for almost everyone on many short- and long-term outcomes.”
“The question is probably whom we should ‘throw the kitchen sink at,’ who should get more scalable advice that might apply to everyone regardless of glycemic levels, and whether there’s some more intermediate group that needs more of a [National Diabetes Prevention Program] approach.”
Dr. Selvin’s group is now working on gathering data to inform development of a risk-based prediabetes definition. “We have a whole research effort in this area. I hope that with some really strong data on risk in prediabetes, that can help to solve the heterogeneity issue. I’m focused on bringing evidence to bear to change the guidelines.”
In the meantime, she told this news organization, “I think there are things we can do now to provide more guidance. I get a lot of feedback from people saying things like ‘my physician told me I have prediabetes but now I don’t’ or ‘I saw in my labs that my blood sugar is elevated but my doctor never said anything.’ That’s a communications issue where we can do a better job.”
The meeting was sponsored by the International Diabetes Federation.
Dr. Selvin is deputy editor of Diabetes Care and on the editorial board of Diabetologia. She receives funding from the NIH and the Foundation for the NIH, and royalties from UpToDate for sections related to screening, diagnosis, and laboratory testing for diabetes. Dr. Kirkman reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
and subsequent complications, and therefore merit more intensive intervention.
“Prediabetes” is the term coined to refer to either “impaired fasting glucose (IFG)” or “impaired glucose tolerance (IGT),” both denoting levels of elevated glycemia that don’t meet the thresholds for diabetes. It’s a heterogeneous group overall, and despite its name, not everyone with prediabetes will progress to develop type 2 diabetes.
There have been major increases in prediabetes in the United States and globally over the past 2 decades, epidemiologist Elizabeth Selvin, PhD, said at the recent IDF World Diabetes Congress 2022.
She noted that the concept of “prediabetes” has been controversial, previously dubbed a “dubious diagnosis” and a “boon for Pharma” in a 2019 Science article.
Others have said it’s “not a medical condition” and that it’s “an artificial category with virtually zero clinical relevance” in a press statement issued for a 2014 BMJ article.
“I don’t agree with these statements entirely but I think they speak to the confusion and tremendous controversy around the concept of prediabetes ... I think instead of calling prediabetes a ‘dubious diagnosis’ we should think of it as an opportunity,” said Dr. Selvin, of Johns Hopkins University Bloomberg School of Public Health, Baltimore.
She proposes trying to home in on those with highest risk of developing type 2 diabetes, which she suggests could be achieved by using a combination of elevated fasting glucose and an elevated A1c, although she stresses that this isn’t in any official guidance.
With the appropriate definition, people who are truly at risk for progression to type 2 diabetes can be identified so that lifestyle factors and cardiovascular risk can be addressed, and weight loss efforts implemented.
“Prevention of weight gain is ... important. That message often gets lost. Even if we can’t get people to lose weight, preventing [further] weight gain is important,” she noted.
Asked to comment, Sue Kirkman, MD, told this news organization, “The term prediabetes – or IFG or IGT or any of the ‘intermediate’ terms – is pragmatic in a way. It helps clinicians and patients understand that they are in a higher-risk category and might need intervention and likely need ongoing monitoring. But like many other risk factors [such as] blood pressure, [high] BMI, etc., the risk is not dichotomous but a continuum.
“People at the low end of the ‘intermediate’ range are not going to have much more risk compared to people who are ‘normal,’ while those at the high end of the range have very high risk,” said Dr. Kirkman, of the University of North Carolina, Chapel Hill, and a coauthor of the American Diabetes Association’s diabetes and prediabetes classifications.
“So we lose information if we just lump everyone into a single category. For individual patients, we definitely need better ways to estimate and communicate their potential risk.”
Currently five definitions for prediabetes: Home in on risk
The problem, Dr. Selvin explained, is that currently there are five official definitions for “prediabetes” using cutoffs for hemoglobin A1c, fasting glucose, or an oral glucose tolerance test.
Each one identifies different numbers of people with differing risk levels, ranging from a prevalence of 4.3% of the middle-aged adult population with the International Expert Committee’s definition of A1c 6.0%-6.4% to 43.5% with the American Diabetes Association’s 100-125 mg/dL fasting glucose.
“That’s an enormous difference. No wonder people are confused about who has prediabetes and what we should do about it,” Dr. Selvin said, adding that the concern about overdiagnosing “prediabetes” is even greater for older populations, in whom “it’s incredibly common to have mildly elevated glucose.”
Hence her proposal of what she sees as an evidence-based, “really easy solution” that clinicians can use now to better identify which patients with “intermediate hyperglycemia” to be most concerned about: Use a combination of fasting glucose above 100 mg/dL and an A1c greater than 5.7%.
“If you have both fasting glucose and hemoglobin A1c, you can use them together ... This is not codified in any guidelines. You won’t see this mentioned anywhere. The guidelines are silent on what to do when some people have an elevated fasting glucose but not an elevated A1c ... but I think a simple message is that if people have both an elevated fasting glucose and an elevated A1c, that’s a very high-risk group,” she said.
On the other hand, Dr. Kirkman pointed out, “most discrepancies are near the margins, as in one test is slightly elevated and one isn’t, so those people probably are at low risk.
“It may be that both being elevated means higher risk because they have more hyperglycemia ... so it seems reasonable, but only if it changes what you tell people.”
For example, Dr. Kirkman said, “I’d tell someone with A1c of 5.8% and fasting glucose of 99 mg/dL the same thing I’d tell someone with that A1c and a glucose of 104 mg/dL – that their risk is still pretty low – and I’d recommend healthy lifestyle and weight loss if overweight either way.”
However, she also said, “Certainly people with higher glucose or A1c are at much higher risk, and same for those with both.”
Tie “prediabetes” definition to risk, as cardiology scores do?
Dr. Selvin also believes that risk-based definitions of prediabetes are needed. Ideally, these would incorporate demographics and clinical factors such as age and body mass index. Other biomarkers could potentially be developed and validated for inclusion in the definition, such as C-reactive protein (CRP), lipids, or even genetic/proteomic information.
Moreover, she thinks that the definition should be tied to clinical decision-making, as is the pooled cohort equation in cardiology.
“I think we could do something very similar in prediabetes,” she suggested, adding that even simply incorporating age and BMI into the definition could help further stratify the risk level until other predictors are validated.
Dr. Kirkman said, “The concept of risk scores a la cardiology is interesting, although we’d have to make them simple and also validate them against some outcome.”
Regarding the age issue, Dr. Kirkman noted that although age wasn’t a predictor of progression to type 2 diabetes in the placebo arm of the landmark Diabetes Prevention Program (DPP) trial, “I do agree that it’s a problem that many older folks have the label of prediabetes because of a mildly elevated A1c and we know that most will never get diabetes.”
And, she noted, in the DPP people with prediabetes who had a BMI over 35 kg/m2 did have significantly higher progression rates than those with lower BMI, while women with a history of gestational diabetes mellitus are also known to be at particularly high risk.
Whom should we throw the kitchen sink at?
Some of this discussion, Dr. Kirkman said, “is really a philosophical one, especially when you consider that lifestyle intervention has benefits for almost everyone on many short- and long-term outcomes.”
“The question is probably whom we should ‘throw the kitchen sink at,’ who should get more scalable advice that might apply to everyone regardless of glycemic levels, and whether there’s some more intermediate group that needs more of a [National Diabetes Prevention Program] approach.”
Dr. Selvin’s group is now working on gathering data to inform development of a risk-based prediabetes definition. “We have a whole research effort in this area. I hope that with some really strong data on risk in prediabetes, that can help to solve the heterogeneity issue. I’m focused on bringing evidence to bear to change the guidelines.”
In the meantime, she told this news organization, “I think there are things we can do now to provide more guidance. I get a lot of feedback from people saying things like ‘my physician told me I have prediabetes but now I don’t’ or ‘I saw in my labs that my blood sugar is elevated but my doctor never said anything.’ That’s a communications issue where we can do a better job.”
The meeting was sponsored by the International Diabetes Federation.
Dr. Selvin is deputy editor of Diabetes Care and on the editorial board of Diabetologia. She receives funding from the NIH and the Foundation for the NIH, and royalties from UpToDate for sections related to screening, diagnosis, and laboratory testing for diabetes. Dr. Kirkman reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT IDF WORLD DIABETES CONGRESS 2022
Principles and Process for Reducing the Need for Insulin in Patients With Type 2 Diabetes
For people living with type 2 diabetes mellitus (T2D), exogenous insulin, whether given early or later in T2D diagnosis, can provide many pharmacologically desirable effects. But it has always been clear, and is now more widely recognized, that insulin treatments are not completely risk-free for the patient. There are now newer, non-insulin therapy options that could be used, along with certain patient lifestyle changes in diet and activity levels, that have been shown to achieve desired glucose control—without the associated risks that insulin can bring.
But is it possible to markedly reduce the need for insulin in some 90% of T2D patients and to reduce the doses in the others? Yes—if patients have sufficient beta-cell function and are willing to change their lifestyle. This mode of treatment has been slowly gaining momentum as of late in the medical community because of the benefits it ultimately provides for the patient. In my practice, I personally have done this by using an evidence-based approach that includes thinking inside a larger box. It is a 2-way street, and each should drive the other: the right drugs (in the right doses), and in the right patients.
Why avoid early insulin therapy?
Is the requirement of early insulin therapy in many or most patients a myth?
Yes. It resulted from “old logic,” which was to use insulin early to reduce glucotoxicity and lipotoxicity. The American Diabetes Association guidelines recommend that glycated hemoglobin (HbA1c) should not exceed 8.0% and consider a fasting blood glucose level >250 mg/dL as high, with a need to start insulin treatment right away; other guidelines recommend initiating insulin immediately in patients with HbA1c >9% and postprandial glucose 300 mg/dL. But this was at a time when oral agents were not as effective and took time to titrate or engender good control. We now have agents that are more effective and start working right away.
However, the main problem in early insulin treatment is the significant risk of over-insulinization—a vicious cycle of insulin-caused increased appetite, hypoglycemia-resultant increased weight gain, insulin resistance (poorer control), increased circulating insulin, etc. Moreover, weight gain and individual hypoglycemic events can cause an increase in the risk of cardiovascular (CV) events.
I believe clinicians must start as early as possible in the natural history of T2D to prevent progressive beta-cell failure. Do not believe in “first-, second-, or third-line”; in other words, do not prioritize, so there is no competition between classes. The goal I have for my patients is to provide therapies that aim for the lowest HbA1c possible without hypoglycemia, provide the greatest CV benefit, and assist in weight reduction.
My protocol, “the egregious eleven,” involves using the least number of agents in combinations that treat the greatest number of mechanisms of hyperglycemia—without the use of sulfonylureas (which cause beta-cell apoptosis, hypoglycemia, and weight gain). Fortunately, newer agents, such as glucagon-like peptide 1 receptor agonist (GLP-1 RA) and sodium-glucose cotransporter 1 (SGLT-2) inhibitors, work right away, cause weight reduction, and have side benefits of CV risk reduction—as well as preserve beta-cell function. Metformin remains a valuable agent and has its own potential side benefits, and bromocriptine-QR and pioglitazone have CV side benefits. So, there is really no need for early insulin in true T2D patients (ie, those that are non-ketosis prone and have sufficient beta-cell reserve).
Why reduce insulin in patients who are already on insulin?
Prior protocols resulted in 40%-50% of T2D patients being placed on insulin unnecessarily. As discussed, the side effects of insulin are many; they include weight gain, insulin resistance, hypoglycemia, and CV complications—all of which have been associated with a decline in quality of life.
What is your approach to reduce or eliminate insulin in those already on it (unnecessarily)?
First, I pick the right patient. Physicians should use sound clinical judgment to identify patients with likely residual beta-cell function. It is not just the “insulin-resistant patient," as 30%-50% of type 1 diabetes mellitus patients also have insulin resistance.
It needs to be a definite T2D patient: not ketosis prone, a family history T2D, no islet cell antibodies (if one has any concerns, check for them). They were often started on insulin in the emergency department with no ketosis and never received non-insulin therapy.
Patients need to be willing to commit to my strict, no-concentrated-sweets diet, to perform careful glucose monitoring, and to check their ketones. Patients should be willing to contact me if their sugar level is >250 mg/dL for 2 measurements in a row, while testing 4 times a day or using a continuous glucose-monitoring (CGM) device.
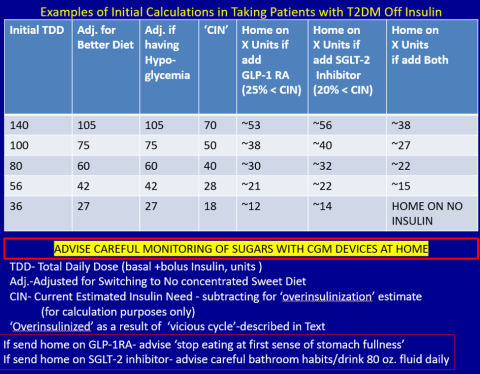
First, estimate a patient’s “current insulin need” (CIN), or the dose they might be on if they had not been subject to over-insulinization (ie, if they had not been subject to the “vicious cycle” discussed above). I do this by taking their total basal and bolus insulin dose, then reducing it by ~25% as the patient changes to a no-concentrated-sweets diet with an additional up-to-25% dose reduction if the patient has been experiencing symptomatic or asymptomatic hypoglycemia.
Next, I reduce this CIN number by ~25% upon starting a rapid-acting subcutaneous GLP-1 RA (liraglutide or oral semaglutide) and reduce the CIN another 20% as they start the SGLT-2 inhibitor. If patients come into my office on <40 U/d, I stop insulin as I start a GLP-1 RA and an SGLT-2 inhibitor and have them monitor home glucose levels to assure reasonable results as they go off the insulin and on their new therapy.
If patients come into my office on >40 U/d, they go home on a GLP-1 RA and an SGLT-2 inhibitor and ~30% of their presenting dose, apportioned between basal/bolus dosing based on when they are currently getting hypoglycemic.
The rapid initial reduction in their insulin doses, with initial adjustments in estimated insulin doses as needed based on home glucose monitoring, and rapid stabilization of glycemic levels by the effectiveness of these 2 agents give patients great motivation to keep up with the diet/program.
Then, as patients lose weight, they are told to report any glucose measurements <80 mg/dL, so that further reduction in insulin doses can be made. When patients achieve a new steady state of glycemia, weight, and GLP-1 RA and SGLT-2 inhibitor doses, you can add bromocriptine-QR, pioglitazone, and/or metformin as needed to allow for a further reduction of insulin. And, as you see the delayed effects of subsequently adding these new agents (eg, glucose <80 mg/dL), you can ultimately stop insulin when they get to <10-12 U/d. The process works very well, even in those starting on up to 300 units of insulin daily. Patients love the outcome and will greatly appreciate your care.
Feel free to contact Dr. Schwartz at stschwar@gmail.com with any questions about his protocol or diet.
For people living with type 2 diabetes mellitus (T2D), exogenous insulin, whether given early or later in T2D diagnosis, can provide many pharmacologically desirable effects. But it has always been clear, and is now more widely recognized, that insulin treatments are not completely risk-free for the patient. There are now newer, non-insulin therapy options that could be used, along with certain patient lifestyle changes in diet and activity levels, that have been shown to achieve desired glucose control—without the associated risks that insulin can bring.
But is it possible to markedly reduce the need for insulin in some 90% of T2D patients and to reduce the doses in the others? Yes—if patients have sufficient beta-cell function and are willing to change their lifestyle. This mode of treatment has been slowly gaining momentum as of late in the medical community because of the benefits it ultimately provides for the patient. In my practice, I personally have done this by using an evidence-based approach that includes thinking inside a larger box. It is a 2-way street, and each should drive the other: the right drugs (in the right doses), and in the right patients.
Why avoid early insulin therapy?
Is the requirement of early insulin therapy in many or most patients a myth?
Yes. It resulted from “old logic,” which was to use insulin early to reduce glucotoxicity and lipotoxicity. The American Diabetes Association guidelines recommend that glycated hemoglobin (HbA1c) should not exceed 8.0% and consider a fasting blood glucose level >250 mg/dL as high, with a need to start insulin treatment right away; other guidelines recommend initiating insulin immediately in patients with HbA1c >9% and postprandial glucose 300 mg/dL. But this was at a time when oral agents were not as effective and took time to titrate or engender good control. We now have agents that are more effective and start working right away.
However, the main problem in early insulin treatment is the significant risk of over-insulinization—a vicious cycle of insulin-caused increased appetite, hypoglycemia-resultant increased weight gain, insulin resistance (poorer control), increased circulating insulin, etc. Moreover, weight gain and individual hypoglycemic events can cause an increase in the risk of cardiovascular (CV) events.
I believe clinicians must start as early as possible in the natural history of T2D to prevent progressive beta-cell failure. Do not believe in “first-, second-, or third-line”; in other words, do not prioritize, so there is no competition between classes. The goal I have for my patients is to provide therapies that aim for the lowest HbA1c possible without hypoglycemia, provide the greatest CV benefit, and assist in weight reduction.
My protocol, “the egregious eleven,” involves using the least number of agents in combinations that treat the greatest number of mechanisms of hyperglycemia—without the use of sulfonylureas (which cause beta-cell apoptosis, hypoglycemia, and weight gain). Fortunately, newer agents, such as glucagon-like peptide 1 receptor agonist (GLP-1 RA) and sodium-glucose cotransporter 1 (SGLT-2) inhibitors, work right away, cause weight reduction, and have side benefits of CV risk reduction—as well as preserve beta-cell function. Metformin remains a valuable agent and has its own potential side benefits, and bromocriptine-QR and pioglitazone have CV side benefits. So, there is really no need for early insulin in true T2D patients (ie, those that are non-ketosis prone and have sufficient beta-cell reserve).
Why reduce insulin in patients who are already on insulin?
Prior protocols resulted in 40%-50% of T2D patients being placed on insulin unnecessarily. As discussed, the side effects of insulin are many; they include weight gain, insulin resistance, hypoglycemia, and CV complications—all of which have been associated with a decline in quality of life.
What is your approach to reduce or eliminate insulin in those already on it (unnecessarily)?
First, I pick the right patient. Physicians should use sound clinical judgment to identify patients with likely residual beta-cell function. It is not just the “insulin-resistant patient," as 30%-50% of type 1 diabetes mellitus patients also have insulin resistance.
It needs to be a definite T2D patient: not ketosis prone, a family history T2D, no islet cell antibodies (if one has any concerns, check for them). They were often started on insulin in the emergency department with no ketosis and never received non-insulin therapy.
Patients need to be willing to commit to my strict, no-concentrated-sweets diet, to perform careful glucose monitoring, and to check their ketones. Patients should be willing to contact me if their sugar level is >250 mg/dL for 2 measurements in a row, while testing 4 times a day or using a continuous glucose-monitoring (CGM) device.
First, estimate a patient’s “current insulin need” (CIN), or the dose they might be on if they had not been subject to over-insulinization (ie, if they had not been subject to the “vicious cycle” discussed above). I do this by taking their total basal and bolus insulin dose, then reducing it by ~25% as the patient changes to a no-concentrated-sweets diet with an additional up-to-25% dose reduction if the patient has been experiencing symptomatic or asymptomatic hypoglycemia.
Next, I reduce this CIN number by ~25% upon starting a rapid-acting subcutaneous GLP-1 RA (liraglutide or oral semaglutide) and reduce the CIN another 20% as they start the SGLT-2 inhibitor. If patients come into my office on <40 U/d, I stop insulin as I start a GLP-1 RA and an SGLT-2 inhibitor and have them monitor home glucose levels to assure reasonable results as they go off the insulin and on their new therapy.
If patients come into my office on >40 U/d, they go home on a GLP-1 RA and an SGLT-2 inhibitor and ~30% of their presenting dose, apportioned between basal/bolus dosing based on when they are currently getting hypoglycemic.
The rapid initial reduction in their insulin doses, with initial adjustments in estimated insulin doses as needed based on home glucose monitoring, and rapid stabilization of glycemic levels by the effectiveness of these 2 agents give patients great motivation to keep up with the diet/program.
Then, as patients lose weight, they are told to report any glucose measurements <80 mg/dL, so that further reduction in insulin doses can be made. When patients achieve a new steady state of glycemia, weight, and GLP-1 RA and SGLT-2 inhibitor doses, you can add bromocriptine-QR, pioglitazone, and/or metformin as needed to allow for a further reduction of insulin. And, as you see the delayed effects of subsequently adding these new agents (eg, glucose <80 mg/dL), you can ultimately stop insulin when they get to <10-12 U/d. The process works very well, even in those starting on up to 300 units of insulin daily. Patients love the outcome and will greatly appreciate your care.
Feel free to contact Dr. Schwartz at stschwar@gmail.com with any questions about his protocol or diet.
For people living with type 2 diabetes mellitus (T2D), exogenous insulin, whether given early or later in T2D diagnosis, can provide many pharmacologically desirable effects. But it has always been clear, and is now more widely recognized, that insulin treatments are not completely risk-free for the patient. There are now newer, non-insulin therapy options that could be used, along with certain patient lifestyle changes in diet and activity levels, that have been shown to achieve desired glucose control—without the associated risks that insulin can bring.
But is it possible to markedly reduce the need for insulin in some 90% of T2D patients and to reduce the doses in the others? Yes—if patients have sufficient beta-cell function and are willing to change their lifestyle. This mode of treatment has been slowly gaining momentum as of late in the medical community because of the benefits it ultimately provides for the patient. In my practice, I personally have done this by using an evidence-based approach that includes thinking inside a larger box. It is a 2-way street, and each should drive the other: the right drugs (in the right doses), and in the right patients.
Why avoid early insulin therapy?
Is the requirement of early insulin therapy in many or most patients a myth?
Yes. It resulted from “old logic,” which was to use insulin early to reduce glucotoxicity and lipotoxicity. The American Diabetes Association guidelines recommend that glycated hemoglobin (HbA1c) should not exceed 8.0% and consider a fasting blood glucose level >250 mg/dL as high, with a need to start insulin treatment right away; other guidelines recommend initiating insulin immediately in patients with HbA1c >9% and postprandial glucose 300 mg/dL. But this was at a time when oral agents were not as effective and took time to titrate or engender good control. We now have agents that are more effective and start working right away.
However, the main problem in early insulin treatment is the significant risk of over-insulinization—a vicious cycle of insulin-caused increased appetite, hypoglycemia-resultant increased weight gain, insulin resistance (poorer control), increased circulating insulin, etc. Moreover, weight gain and individual hypoglycemic events can cause an increase in the risk of cardiovascular (CV) events.
I believe clinicians must start as early as possible in the natural history of T2D to prevent progressive beta-cell failure. Do not believe in “first-, second-, or third-line”; in other words, do not prioritize, so there is no competition between classes. The goal I have for my patients is to provide therapies that aim for the lowest HbA1c possible without hypoglycemia, provide the greatest CV benefit, and assist in weight reduction.
My protocol, “the egregious eleven,” involves using the least number of agents in combinations that treat the greatest number of mechanisms of hyperglycemia—without the use of sulfonylureas (which cause beta-cell apoptosis, hypoglycemia, and weight gain). Fortunately, newer agents, such as glucagon-like peptide 1 receptor agonist (GLP-1 RA) and sodium-glucose cotransporter 1 (SGLT-2) inhibitors, work right away, cause weight reduction, and have side benefits of CV risk reduction—as well as preserve beta-cell function. Metformin remains a valuable agent and has its own potential side benefits, and bromocriptine-QR and pioglitazone have CV side benefits. So, there is really no need for early insulin in true T2D patients (ie, those that are non-ketosis prone and have sufficient beta-cell reserve).
Why reduce insulin in patients who are already on insulin?
Prior protocols resulted in 40%-50% of T2D patients being placed on insulin unnecessarily. As discussed, the side effects of insulin are many; they include weight gain, insulin resistance, hypoglycemia, and CV complications—all of which have been associated with a decline in quality of life.
What is your approach to reduce or eliminate insulin in those already on it (unnecessarily)?
First, I pick the right patient. Physicians should use sound clinical judgment to identify patients with likely residual beta-cell function. It is not just the “insulin-resistant patient," as 30%-50% of type 1 diabetes mellitus patients also have insulin resistance.
It needs to be a definite T2D patient: not ketosis prone, a family history T2D, no islet cell antibodies (if one has any concerns, check for them). They were often started on insulin in the emergency department with no ketosis and never received non-insulin therapy.
Patients need to be willing to commit to my strict, no-concentrated-sweets diet, to perform careful glucose monitoring, and to check their ketones. Patients should be willing to contact me if their sugar level is >250 mg/dL for 2 measurements in a row, while testing 4 times a day or using a continuous glucose-monitoring (CGM) device.
First, estimate a patient’s “current insulin need” (CIN), or the dose they might be on if they had not been subject to over-insulinization (ie, if they had not been subject to the “vicious cycle” discussed above). I do this by taking their total basal and bolus insulin dose, then reducing it by ~25% as the patient changes to a no-concentrated-sweets diet with an additional up-to-25% dose reduction if the patient has been experiencing symptomatic or asymptomatic hypoglycemia.
Next, I reduce this CIN number by ~25% upon starting a rapid-acting subcutaneous GLP-1 RA (liraglutide or oral semaglutide) and reduce the CIN another 20% as they start the SGLT-2 inhibitor. If patients come into my office on <40 U/d, I stop insulin as I start a GLP-1 RA and an SGLT-2 inhibitor and have them monitor home glucose levels to assure reasonable results as they go off the insulin and on their new therapy.
If patients come into my office on >40 U/d, they go home on a GLP-1 RA and an SGLT-2 inhibitor and ~30% of their presenting dose, apportioned between basal/bolus dosing based on when they are currently getting hypoglycemic.
The rapid initial reduction in their insulin doses, with initial adjustments in estimated insulin doses as needed based on home glucose monitoring, and rapid stabilization of glycemic levels by the effectiveness of these 2 agents give patients great motivation to keep up with the diet/program.
Then, as patients lose weight, they are told to report any glucose measurements <80 mg/dL, so that further reduction in insulin doses can be made. When patients achieve a new steady state of glycemia, weight, and GLP-1 RA and SGLT-2 inhibitor doses, you can add bromocriptine-QR, pioglitazone, and/or metformin as needed to allow for a further reduction of insulin. And, as you see the delayed effects of subsequently adding these new agents (eg, glucose <80 mg/dL), you can ultimately stop insulin when they get to <10-12 U/d. The process works very well, even in those starting on up to 300 units of insulin daily. Patients love the outcome and will greatly appreciate your care.
Feel free to contact Dr. Schwartz at stschwar@gmail.com with any questions about his protocol or diet.
Fitbit figures: More steps per day cut type 2 diabetes risk
The protective effect of daily step count on type 2 diabetes risk remained after adjusting for smoking and sedentary time.
Taking more steps per day was also associated with less risk of developing type 2 diabetes in different subgroups of physical activity intensity.
“Our data shows the importance of moving your body every day to lower your risk of [type 2] diabetes,” said the lead author of the research, Andrew S. Perry, MD. The findings were published online in the Journal of Clinical Endocrinology & Metabolism.
Despite low baseline risk, benefit from increased physical activity
The study was conducted in more than 5,000 participants in the National Institutes of Health’s All of Us research program who had a median age of 51 and were generally overweight (median BMI 27.8 kg/m2). Three quarters were women and 89% were White.
It used an innovative approach in a real-world population, said Dr. Perry, of Vanderbilt University Medical Center in Nashville, Tenn.
The individuals in this cohort had relatively few risk factors, so it was not surprising that the incidence of type 2 diabetes overall was low (2%), the researchers note. “Yet, despite being low risk, we still detected a signal of benefit from increased” physical activity, Dr. Perry and colleagues write.
The individuals had a median of 16 very active minutes/day, which corresponds to 112 very active minutes/week (ie, less than the guideline-recommended 150 minutes of physical activity/week).
“These results indicate that amounts of physical activity are correlated with lower risk of [type 2] diabetes, regardless of the intensity level, and even at amounts less than current guidelines recommend,” the researchers summarize.
Physical activity tracked over close to 4 years
Prior studies of the relationship between physical activity and type 2 diabetes risk relied primarily on questionnaires that asked people about physical activity at one point in time.
The researchers aimed to examine this association over time, in a contemporary cohort of Fitbit users who participated in the All of Us program.
From 12,781 participants with Fitbit data between 2010 and 2021, they identified 5,677 individuals who were at least 18 years old and had linked electronic health record data, no diabetes at baseline, at least 15 days of Fitbit data in the initial monitoring period, and at least 180 days of follow-up.
The Fitbit counts steps, and it also uses an algorithm to quantify physical activity intensity as lightly active (1.5-3 metabolic equivalent task (METs), fairly active (3-6 METs), and very active (> 6 METs).
During a median 3.8-year follow-up, participants made a median of 7,924 steps/day and were “fairly active” for a median of 16 minutes/day.
They found 97 new cases of type 2 diabetes over a follow-up of 4 years in the dataset.
The predicted cumulative incidence of type 2 diabetes at 5 years was 0.8% for individuals who walked 13,245 steps/day (90th percentile) vs. 2.3% for those who walked 4,301 steps/day (10th percentile).
“We hope to study more diverse populations in future studies to confirm the generalizability of these findings,” Dr. Perry said.
This study received funding from the National Heart, Lung, and Blood Institute. Dr. Perry reports no relevant financial relationships. Disclosures for the other authors are listed with the original article.
A version of this article first appeared on Medscape.com.
The protective effect of daily step count on type 2 diabetes risk remained after adjusting for smoking and sedentary time.
Taking more steps per day was also associated with less risk of developing type 2 diabetes in different subgroups of physical activity intensity.
“Our data shows the importance of moving your body every day to lower your risk of [type 2] diabetes,” said the lead author of the research, Andrew S. Perry, MD. The findings were published online in the Journal of Clinical Endocrinology & Metabolism.
Despite low baseline risk, benefit from increased physical activity
The study was conducted in more than 5,000 participants in the National Institutes of Health’s All of Us research program who had a median age of 51 and were generally overweight (median BMI 27.8 kg/m2). Three quarters were women and 89% were White.
It used an innovative approach in a real-world population, said Dr. Perry, of Vanderbilt University Medical Center in Nashville, Tenn.
The individuals in this cohort had relatively few risk factors, so it was not surprising that the incidence of type 2 diabetes overall was low (2%), the researchers note. “Yet, despite being low risk, we still detected a signal of benefit from increased” physical activity, Dr. Perry and colleagues write.
The individuals had a median of 16 very active minutes/day, which corresponds to 112 very active minutes/week (ie, less than the guideline-recommended 150 minutes of physical activity/week).
“These results indicate that amounts of physical activity are correlated with lower risk of [type 2] diabetes, regardless of the intensity level, and even at amounts less than current guidelines recommend,” the researchers summarize.
Physical activity tracked over close to 4 years
Prior studies of the relationship between physical activity and type 2 diabetes risk relied primarily on questionnaires that asked people about physical activity at one point in time.
The researchers aimed to examine this association over time, in a contemporary cohort of Fitbit users who participated in the All of Us program.
From 12,781 participants with Fitbit data between 2010 and 2021, they identified 5,677 individuals who were at least 18 years old and had linked electronic health record data, no diabetes at baseline, at least 15 days of Fitbit data in the initial monitoring period, and at least 180 days of follow-up.
The Fitbit counts steps, and it also uses an algorithm to quantify physical activity intensity as lightly active (1.5-3 metabolic equivalent task (METs), fairly active (3-6 METs), and very active (> 6 METs).
During a median 3.8-year follow-up, participants made a median of 7,924 steps/day and were “fairly active” for a median of 16 minutes/day.
They found 97 new cases of type 2 diabetes over a follow-up of 4 years in the dataset.
The predicted cumulative incidence of type 2 diabetes at 5 years was 0.8% for individuals who walked 13,245 steps/day (90th percentile) vs. 2.3% for those who walked 4,301 steps/day (10th percentile).
“We hope to study more diverse populations in future studies to confirm the generalizability of these findings,” Dr. Perry said.
This study received funding from the National Heart, Lung, and Blood Institute. Dr. Perry reports no relevant financial relationships. Disclosures for the other authors are listed with the original article.
A version of this article first appeared on Medscape.com.
The protective effect of daily step count on type 2 diabetes risk remained after adjusting for smoking and sedentary time.
Taking more steps per day was also associated with less risk of developing type 2 diabetes in different subgroups of physical activity intensity.
“Our data shows the importance of moving your body every day to lower your risk of [type 2] diabetes,” said the lead author of the research, Andrew S. Perry, MD. The findings were published online in the Journal of Clinical Endocrinology & Metabolism.
Despite low baseline risk, benefit from increased physical activity
The study was conducted in more than 5,000 participants in the National Institutes of Health’s All of Us research program who had a median age of 51 and were generally overweight (median BMI 27.8 kg/m2). Three quarters were women and 89% were White.
It used an innovative approach in a real-world population, said Dr. Perry, of Vanderbilt University Medical Center in Nashville, Tenn.
The individuals in this cohort had relatively few risk factors, so it was not surprising that the incidence of type 2 diabetes overall was low (2%), the researchers note. “Yet, despite being low risk, we still detected a signal of benefit from increased” physical activity, Dr. Perry and colleagues write.
The individuals had a median of 16 very active minutes/day, which corresponds to 112 very active minutes/week (ie, less than the guideline-recommended 150 minutes of physical activity/week).
“These results indicate that amounts of physical activity are correlated with lower risk of [type 2] diabetes, regardless of the intensity level, and even at amounts less than current guidelines recommend,” the researchers summarize.
Physical activity tracked over close to 4 years
Prior studies of the relationship between physical activity and type 2 diabetes risk relied primarily on questionnaires that asked people about physical activity at one point in time.
The researchers aimed to examine this association over time, in a contemporary cohort of Fitbit users who participated in the All of Us program.
From 12,781 participants with Fitbit data between 2010 and 2021, they identified 5,677 individuals who were at least 18 years old and had linked electronic health record data, no diabetes at baseline, at least 15 days of Fitbit data in the initial monitoring period, and at least 180 days of follow-up.
The Fitbit counts steps, and it also uses an algorithm to quantify physical activity intensity as lightly active (1.5-3 metabolic equivalent task (METs), fairly active (3-6 METs), and very active (> 6 METs).
During a median 3.8-year follow-up, participants made a median of 7,924 steps/day and were “fairly active” for a median of 16 minutes/day.
They found 97 new cases of type 2 diabetes over a follow-up of 4 years in the dataset.
The predicted cumulative incidence of type 2 diabetes at 5 years was 0.8% for individuals who walked 13,245 steps/day (90th percentile) vs. 2.3% for those who walked 4,301 steps/day (10th percentile).
“We hope to study more diverse populations in future studies to confirm the generalizability of these findings,” Dr. Perry said.
This study received funding from the National Heart, Lung, and Blood Institute. Dr. Perry reports no relevant financial relationships. Disclosures for the other authors are listed with the original article.
A version of this article first appeared on Medscape.com.
AI takes root in primary care. First stop: Diabetic retinopathy
At a routine doctor’s visit, a member of the clinic staff takes digital pictures of a patient’s retinas.
Within seconds, an artificial intelligence (AI) algorithm determines if the patient has diabetic retinopathy, a complication of diabetes that can lead to blindness.
If they do, the physician refers the patient to an eye care specialist for further evaluation and treatment.
This scene already is playing out in primary care clinics around the United States and in other countries, and it may become more common.
In May, OSF HealthCare, a network of medical facilities headquartered in Peoria, Ill., piloted an AI system to diagnose diabetic retinopathy, a condition that affects an estimated 4 million Americans. In 2023, the health care system plans to expand the technology to 34 locations.
Meanwhile, the Food and Drug Administration in November approved a new AI system to diagnose diabetic retinopathy, making AEYE-DS from AEYE Health the third such product on the market.
Roomasa Channa, MD, a clinician-scientist with the McPherson Eye Research Institute at the University of Wisconsin–Madison, has studied the use of AI in teenage patients with diabetes. She said she soon plans to use AI screening in federally qualified health centers to screen adults with diabetes.
Dr. Channa welcomed the latest regulatory clearance and said she hopes another Food and Drug Administration–cleared algorithm product will improve accessibility to the technology.
“It is good to see more players in the field: We need this technology to be readily available and affordable,” she said in an interview.
A mixed reception
Responses from physicians to this type of AI have been mixed. Some worry, for instance, that the algorithms might be programmed with unrecognized biases that could lead them to less accurately interpret images from certain patient groups. Researchers should be on the lookout out for this possibility, Dr. Channa said.
“We need more real-world studies in different settings,” she said. “We also need to keep collecting data on AI performance post approval,” like investigators do for newly approved drugs.
The first AI system to diagnose diabetic retinopathy, IDx-DR, was approved by the FDA in 2018 and rolled out in retail clinics soon after. A second system, EyeArt, gained clearance by the agency in 2020.
Adding AI algorithms into primary care practice has changed how patients with diabetes can receive a screening. It also has introduced a new way for certain medical conditions to be diagnosed in primary care.
The American Medical Association in 2021 released a new CPT code to allow clinicians to bill government and private insurers for use of these services. CPT code 92229 refers to imaging of the retina to detect disease with an automated analysis and report at the point of care.
Meeting a need
Health care clinics in underserved areas often do not have eye care providers onsite to conduct recommended screening exams, so AI could help patients receive screening who otherwise would not get it, Dr. Channa said.
Dr. Channa and colleagues successfully used one AI system, IDx-DR, to screen children at a pediatric diabetes clinic. Over a year, screening rates jumped from 49% to 95%.
This technology “can potentially help us in decreasing disparities in care and focusing our efforts on patients with the most severe diseases,” she said.
OSF HealthCare recently obtained an approximately $1 million grant from drug company Regeneron to expand the use of AI-based screening for diabetic retinopathy, following a successful pilot. Regeneron markets a treatment for diabetic retinopathy.
Without an AI option, recommended eye screening for patients with diabetes often falls through the cracks, according to Mark Meeker, DO, vice president of community medicine at OSF. Primary care physicians may refer patients elsewhere for their annual retinopathy screening exam.
“That often doesn’t get completed because it’s another trip, another appointment, another time away from work,” Dr. Meeker said.
All patients with diabetes should have their eyes screened each year, but between one- to two-thirds of patients nationwide do not, he said.
A member of the clinic staff takes digital pictures of the retina, almost always through undilated pupils.
If the result is normal, the patient is scheduled for another follow-up screening in a year. If early signs of diabetic retinopathy are spotted, patients are referred to an eye care specialist.
After 7 months of the pilot program, OSF had screened about 350 patients. Approximately 20% had diabetic retinopathy, according to OSF.
‘A huge impact’
OSF has about 66,000 patients with diabetes. About two-thirds do not receive annual screening, Dr. Meeker estimated. “This can have a huge impact on the quality of life in the coming years for our diabetic patients. It’s pretty profound.”
Eye care specialists typically treat diabetic retinopathy with lasers, surgery, or medication. For primary care clinicians, however, AI screening for retinopathy is an opportunity to emphasize how important it is to manage the disease and what its consequences can be.
AI screening is “another tool for us to use to get patients more engaged in their own care,” Dr. Meeker said. “This is probably the biggest advance in AI affecting our day-to-day interaction with patients that we’ve seen in primary care.”
A business opportunity, too?
The IDx-DR platform OSF is using in its clinics is owned by the company Digital Diagnostics. OSF Ventures, an investment arm of OSF HealthCare, has invested in the company, the health care system announced in August.
Other companies have had their products used in practice. In 2019, for example, Eyenuk described how its EyeArt system had been used to screen thousands of patients in Germany and in Italy.
And in 2021, Eyenuk reported that its customer base in the United States had expanded to more than 25 locations. The company credited a Centers for Medicare & Medicaid Services plan to cover CPT code 92229 with supporting this growth.
Zack Dvey-Aharon, PhD, the CEO of AEYE Health, said the company was motivated to enter this space when regulators decided that AI could be used to diagnose a condition — not just as a tool to help doctors arrive at a diagnosis.
With proper training, a person can diagnose diabetic retinopathy relatively easily if the image of the retina is of excellent quality. If image is dark or blurry, however, it’s a different story.
AI has its advantages in this scenario, according to Dr. Dvey-Aharon. “For AI, those darker, more blurry images are actually highly readable with fantastic accuracy.”
More to come?
The possibilities of AI in analyzing retinal images are vast.
New research shows that AI may be able to detect Alzheimer’s disease or predict a person’s risk for heart attack and stroke based on snapshots of the retina.
The retina may also shed light on kidney disease, control of blood glucose and blood pressure, hepatobiliary disease, and coronary artery calcium, according to Eric J. Topol, MD, director of Scripps Research Translational Institute in La Jolla, Calif.
Beyond retinas, interpretation of electrocardiograms (ECGs) may be another frontier for AI in primary care. In one trial, an AI-enhanced ECG reading facilitated early diagnosis of low ejection fraction, and some doctors now receive these reports routinely, Dr. Topol wrote.
The potential value of AI in medicine “extends to virtually all forms of medical images that have been assessed to date,” Dr. Topol wrote on his “Ground Truths” Substack.
Although much of the focus has been on what AI can see, researchers also are exploring what AI can do with what it hears. Early research suggests that algorithms may be able to diagnose disease by analyzing patients’ voices.
A version of this article first appeared on Medscape.com.
At a routine doctor’s visit, a member of the clinic staff takes digital pictures of a patient’s retinas.
Within seconds, an artificial intelligence (AI) algorithm determines if the patient has diabetic retinopathy, a complication of diabetes that can lead to blindness.
If they do, the physician refers the patient to an eye care specialist for further evaluation and treatment.
This scene already is playing out in primary care clinics around the United States and in other countries, and it may become more common.
In May, OSF HealthCare, a network of medical facilities headquartered in Peoria, Ill., piloted an AI system to diagnose diabetic retinopathy, a condition that affects an estimated 4 million Americans. In 2023, the health care system plans to expand the technology to 34 locations.
Meanwhile, the Food and Drug Administration in November approved a new AI system to diagnose diabetic retinopathy, making AEYE-DS from AEYE Health the third such product on the market.
Roomasa Channa, MD, a clinician-scientist with the McPherson Eye Research Institute at the University of Wisconsin–Madison, has studied the use of AI in teenage patients with diabetes. She said she soon plans to use AI screening in federally qualified health centers to screen adults with diabetes.
Dr. Channa welcomed the latest regulatory clearance and said she hopes another Food and Drug Administration–cleared algorithm product will improve accessibility to the technology.
“It is good to see more players in the field: We need this technology to be readily available and affordable,” she said in an interview.
A mixed reception
Responses from physicians to this type of AI have been mixed. Some worry, for instance, that the algorithms might be programmed with unrecognized biases that could lead them to less accurately interpret images from certain patient groups. Researchers should be on the lookout out for this possibility, Dr. Channa said.
“We need more real-world studies in different settings,” she said. “We also need to keep collecting data on AI performance post approval,” like investigators do for newly approved drugs.
The first AI system to diagnose diabetic retinopathy, IDx-DR, was approved by the FDA in 2018 and rolled out in retail clinics soon after. A second system, EyeArt, gained clearance by the agency in 2020.
Adding AI algorithms into primary care practice has changed how patients with diabetes can receive a screening. It also has introduced a new way for certain medical conditions to be diagnosed in primary care.
The American Medical Association in 2021 released a new CPT code to allow clinicians to bill government and private insurers for use of these services. CPT code 92229 refers to imaging of the retina to detect disease with an automated analysis and report at the point of care.
Meeting a need
Health care clinics in underserved areas often do not have eye care providers onsite to conduct recommended screening exams, so AI could help patients receive screening who otherwise would not get it, Dr. Channa said.
Dr. Channa and colleagues successfully used one AI system, IDx-DR, to screen children at a pediatric diabetes clinic. Over a year, screening rates jumped from 49% to 95%.
This technology “can potentially help us in decreasing disparities in care and focusing our efforts on patients with the most severe diseases,” she said.
OSF HealthCare recently obtained an approximately $1 million grant from drug company Regeneron to expand the use of AI-based screening for diabetic retinopathy, following a successful pilot. Regeneron markets a treatment for diabetic retinopathy.
Without an AI option, recommended eye screening for patients with diabetes often falls through the cracks, according to Mark Meeker, DO, vice president of community medicine at OSF. Primary care physicians may refer patients elsewhere for their annual retinopathy screening exam.
“That often doesn’t get completed because it’s another trip, another appointment, another time away from work,” Dr. Meeker said.
All patients with diabetes should have their eyes screened each year, but between one- to two-thirds of patients nationwide do not, he said.
A member of the clinic staff takes digital pictures of the retina, almost always through undilated pupils.
If the result is normal, the patient is scheduled for another follow-up screening in a year. If early signs of diabetic retinopathy are spotted, patients are referred to an eye care specialist.
After 7 months of the pilot program, OSF had screened about 350 patients. Approximately 20% had diabetic retinopathy, according to OSF.
‘A huge impact’
OSF has about 66,000 patients with diabetes. About two-thirds do not receive annual screening, Dr. Meeker estimated. “This can have a huge impact on the quality of life in the coming years for our diabetic patients. It’s pretty profound.”
Eye care specialists typically treat diabetic retinopathy with lasers, surgery, or medication. For primary care clinicians, however, AI screening for retinopathy is an opportunity to emphasize how important it is to manage the disease and what its consequences can be.
AI screening is “another tool for us to use to get patients more engaged in their own care,” Dr. Meeker said. “This is probably the biggest advance in AI affecting our day-to-day interaction with patients that we’ve seen in primary care.”
A business opportunity, too?
The IDx-DR platform OSF is using in its clinics is owned by the company Digital Diagnostics. OSF Ventures, an investment arm of OSF HealthCare, has invested in the company, the health care system announced in August.
Other companies have had their products used in practice. In 2019, for example, Eyenuk described how its EyeArt system had been used to screen thousands of patients in Germany and in Italy.
And in 2021, Eyenuk reported that its customer base in the United States had expanded to more than 25 locations. The company credited a Centers for Medicare & Medicaid Services plan to cover CPT code 92229 with supporting this growth.
Zack Dvey-Aharon, PhD, the CEO of AEYE Health, said the company was motivated to enter this space when regulators decided that AI could be used to diagnose a condition — not just as a tool to help doctors arrive at a diagnosis.
With proper training, a person can diagnose diabetic retinopathy relatively easily if the image of the retina is of excellent quality. If image is dark or blurry, however, it’s a different story.
AI has its advantages in this scenario, according to Dr. Dvey-Aharon. “For AI, those darker, more blurry images are actually highly readable with fantastic accuracy.”
More to come?
The possibilities of AI in analyzing retinal images are vast.
New research shows that AI may be able to detect Alzheimer’s disease or predict a person’s risk for heart attack and stroke based on snapshots of the retina.
The retina may also shed light on kidney disease, control of blood glucose and blood pressure, hepatobiliary disease, and coronary artery calcium, according to Eric J. Topol, MD, director of Scripps Research Translational Institute in La Jolla, Calif.
Beyond retinas, interpretation of electrocardiograms (ECGs) may be another frontier for AI in primary care. In one trial, an AI-enhanced ECG reading facilitated early diagnosis of low ejection fraction, and some doctors now receive these reports routinely, Dr. Topol wrote.
The potential value of AI in medicine “extends to virtually all forms of medical images that have been assessed to date,” Dr. Topol wrote on his “Ground Truths” Substack.
Although much of the focus has been on what AI can see, researchers also are exploring what AI can do with what it hears. Early research suggests that algorithms may be able to diagnose disease by analyzing patients’ voices.
A version of this article first appeared on Medscape.com.
At a routine doctor’s visit, a member of the clinic staff takes digital pictures of a patient’s retinas.
Within seconds, an artificial intelligence (AI) algorithm determines if the patient has diabetic retinopathy, a complication of diabetes that can lead to blindness.
If they do, the physician refers the patient to an eye care specialist for further evaluation and treatment.
This scene already is playing out in primary care clinics around the United States and in other countries, and it may become more common.
In May, OSF HealthCare, a network of medical facilities headquartered in Peoria, Ill., piloted an AI system to diagnose diabetic retinopathy, a condition that affects an estimated 4 million Americans. In 2023, the health care system plans to expand the technology to 34 locations.
Meanwhile, the Food and Drug Administration in November approved a new AI system to diagnose diabetic retinopathy, making AEYE-DS from AEYE Health the third such product on the market.
Roomasa Channa, MD, a clinician-scientist with the McPherson Eye Research Institute at the University of Wisconsin–Madison, has studied the use of AI in teenage patients with diabetes. She said she soon plans to use AI screening in federally qualified health centers to screen adults with diabetes.
Dr. Channa welcomed the latest regulatory clearance and said she hopes another Food and Drug Administration–cleared algorithm product will improve accessibility to the technology.
“It is good to see more players in the field: We need this technology to be readily available and affordable,” she said in an interview.
A mixed reception
Responses from physicians to this type of AI have been mixed. Some worry, for instance, that the algorithms might be programmed with unrecognized biases that could lead them to less accurately interpret images from certain patient groups. Researchers should be on the lookout out for this possibility, Dr. Channa said.
“We need more real-world studies in different settings,” she said. “We also need to keep collecting data on AI performance post approval,” like investigators do for newly approved drugs.
The first AI system to diagnose diabetic retinopathy, IDx-DR, was approved by the FDA in 2018 and rolled out in retail clinics soon after. A second system, EyeArt, gained clearance by the agency in 2020.
Adding AI algorithms into primary care practice has changed how patients with diabetes can receive a screening. It also has introduced a new way for certain medical conditions to be diagnosed in primary care.
The American Medical Association in 2021 released a new CPT code to allow clinicians to bill government and private insurers for use of these services. CPT code 92229 refers to imaging of the retina to detect disease with an automated analysis and report at the point of care.
Meeting a need
Health care clinics in underserved areas often do not have eye care providers onsite to conduct recommended screening exams, so AI could help patients receive screening who otherwise would not get it, Dr. Channa said.
Dr. Channa and colleagues successfully used one AI system, IDx-DR, to screen children at a pediatric diabetes clinic. Over a year, screening rates jumped from 49% to 95%.
This technology “can potentially help us in decreasing disparities in care and focusing our efforts on patients with the most severe diseases,” she said.
OSF HealthCare recently obtained an approximately $1 million grant from drug company Regeneron to expand the use of AI-based screening for diabetic retinopathy, following a successful pilot. Regeneron markets a treatment for diabetic retinopathy.
Without an AI option, recommended eye screening for patients with diabetes often falls through the cracks, according to Mark Meeker, DO, vice president of community medicine at OSF. Primary care physicians may refer patients elsewhere for their annual retinopathy screening exam.
“That often doesn’t get completed because it’s another trip, another appointment, another time away from work,” Dr. Meeker said.
All patients with diabetes should have their eyes screened each year, but between one- to two-thirds of patients nationwide do not, he said.
A member of the clinic staff takes digital pictures of the retina, almost always through undilated pupils.
If the result is normal, the patient is scheduled for another follow-up screening in a year. If early signs of diabetic retinopathy are spotted, patients are referred to an eye care specialist.
After 7 months of the pilot program, OSF had screened about 350 patients. Approximately 20% had diabetic retinopathy, according to OSF.
‘A huge impact’
OSF has about 66,000 patients with diabetes. About two-thirds do not receive annual screening, Dr. Meeker estimated. “This can have a huge impact on the quality of life in the coming years for our diabetic patients. It’s pretty profound.”
Eye care specialists typically treat diabetic retinopathy with lasers, surgery, or medication. For primary care clinicians, however, AI screening for retinopathy is an opportunity to emphasize how important it is to manage the disease and what its consequences can be.
AI screening is “another tool for us to use to get patients more engaged in their own care,” Dr. Meeker said. “This is probably the biggest advance in AI affecting our day-to-day interaction with patients that we’ve seen in primary care.”
A business opportunity, too?
The IDx-DR platform OSF is using in its clinics is owned by the company Digital Diagnostics. OSF Ventures, an investment arm of OSF HealthCare, has invested in the company, the health care system announced in August.
Other companies have had their products used in practice. In 2019, for example, Eyenuk described how its EyeArt system had been used to screen thousands of patients in Germany and in Italy.
And in 2021, Eyenuk reported that its customer base in the United States had expanded to more than 25 locations. The company credited a Centers for Medicare & Medicaid Services plan to cover CPT code 92229 with supporting this growth.
Zack Dvey-Aharon, PhD, the CEO of AEYE Health, said the company was motivated to enter this space when regulators decided that AI could be used to diagnose a condition — not just as a tool to help doctors arrive at a diagnosis.
With proper training, a person can diagnose diabetic retinopathy relatively easily if the image of the retina is of excellent quality. If image is dark or blurry, however, it’s a different story.
AI has its advantages in this scenario, according to Dr. Dvey-Aharon. “For AI, those darker, more blurry images are actually highly readable with fantastic accuracy.”
More to come?
The possibilities of AI in analyzing retinal images are vast.
New research shows that AI may be able to detect Alzheimer’s disease or predict a person’s risk for heart attack and stroke based on snapshots of the retina.
The retina may also shed light on kidney disease, control of blood glucose and blood pressure, hepatobiliary disease, and coronary artery calcium, according to Eric J. Topol, MD, director of Scripps Research Translational Institute in La Jolla, Calif.
Beyond retinas, interpretation of electrocardiograms (ECGs) may be another frontier for AI in primary care. In one trial, an AI-enhanced ECG reading facilitated early diagnosis of low ejection fraction, and some doctors now receive these reports routinely, Dr. Topol wrote.
The potential value of AI in medicine “extends to virtually all forms of medical images that have been assessed to date,” Dr. Topol wrote on his “Ground Truths” Substack.
Although much of the focus has been on what AI can see, researchers also are exploring what AI can do with what it hears. Early research suggests that algorithms may be able to diagnose disease by analyzing patients’ voices.
A version of this article first appeared on Medscape.com.
Not all children with type 2 diabetes have obesity
Obesity is not a universal phenotype in children with type 2 diabetes (T2D), a global systematic review and meta-analysis reported. In fact, the study found, as many as one in four children with T2D do not have obesity and some have normal reference-range body mass measurements. Further studies should consider other mechanisms beyond obesity in the genesis of pediatric diabetes, the authors of the international analysis concluded, writing for JAMA Network Open.
“We were aware that some children and adolescents with T2D did not have obesity, but we didn’t know the scale of obesity in T2D, or what variables may impact the occurrence of diabetes in this group,” endocrinologist M. Constantine Samaan, MD, MSc, associate professor of pediatrics at McMaster University in Hamilton, Ont., told this news organization. “So, the analysis did help us understand the body mass distribution of this group in more detail.”
The international investigators included in their meta-analysis 53 articles with 8,942 participants from multiple world regions and races/ethnicities. The overall prevalence of obesity in pediatric patients with T2D was 75.27% (95% confidence interval [CI], 70.47%-79.78%). The prevalence of obesity at time of diagnosis in 4,688 participants was 77.24% (95% CI, 70.55%-83.34%). Male participants had higher odds of obesity than females: odds ratio, 2.10 (95% CI, 1.33-3.31) – although girls are generally more likely to develop T2D. The highest prevalence of obesity occurred in Whites at 89.86% (95% CI, 71.50%-99.74%), while prevalence was lowest in Asian participants at 64.50% (95% CI, 53.28%-74.99%).
The authors noted that childhood obesity affects approximately 340 million children worldwide and is a major driver of pediatric T2D, an aggressive disease with a high treatment failure rate. Understanding the contribution of body mass to the evolution of insulin resistance, glucose intolerance, and T2D with its attendant comorbidities and complications, such as nonalcoholic fatty liver disease, remains crucial for developing personalized interventions.
Known risk factors for T2D include interactions between genetics and the environment, including lifestyle factors such as diet and low physical activity levels, Dr. Samaan noted. Certain ethnic groups have higher T2D risks, as do babies exposed in the womb to maternal obesity or diabetes, he said. “And there are likely many other factors that contribute to the risk of T2D, though these remain to be defined.”
Is “lean” T2D in children without obesity likely then to be hereditary, more severe, and harder to control with lifestyle modification? “That’s a great question, but the answer is we don’t know,” Dr. Samaan said.
Commenting on the study but not involved in it, Timothy J. Joos, MD, a pediatrician in Seattle affiliated with the Swedish Medical Center, said the findings raise the question of how many pediatric T2D patients are being missed because they don’t meet current screening criteria. “In nonobese T2D pediatric patients, genetics (and by proxy family history) obviously play a heavier role. In my practice, I often get parents asking me to screen their skinny teenager for diabetes because of diabetes in a family member. In the past I would begrudgingly comply with a smirk on my face. Now the smirk will be gone.”
Dr. Joos said it would be interesting to see what percentage of these T2D patients without obesity (body mass index < 95th percentile) would still meet the criteria for being overweight (BMI > 85th percentile) as this is the primary criterion for screening according to the American Diabetes Association guidelines.
Current guidelines generally look for elevated body mass measures as a main screening indication, Dr. Samaan’s group noted. But in their view, while factors such as ethnicity and in utero exposure to diabetes are already used in combination with BMI-based measures to justify screening, more sophisticated prediabetes and diabetes prediction models are needed to support a more comprehensive screening approach.
“Because being overweight is the initial criterion, children with multiple other criteria are not being screened,” Dr. Joos said. He agreed that more research is needed to sort out the other risk factors for pediatric T2D without obesity so these patients may be detected earlier.
New models may need to incorporate lifestyle factors, hormones, puberty, growth, and sex as well, the authors wrote. Markers of insulin resistance, insulin production capacity, and other markers are needed to refine the identification of those who should be screened.
Dr. Samaan’s group is planning to study the findings in more detail to clarify the effect of body mass on the comorbidities and complications of pediatric T2D.
In addition to the study limitation of significant interstudy heterogeneity, the authors acknowledged varying degrees of glycemic control and dyslipidemia among participants.
No specific funding was provided for this review and meta-analysis. The authors disclosed no conflicts of interest. Dr. Joos disclosed no competing interests with regard to his comments.
Obesity is not a universal phenotype in children with type 2 diabetes (T2D), a global systematic review and meta-analysis reported. In fact, the study found, as many as one in four children with T2D do not have obesity and some have normal reference-range body mass measurements. Further studies should consider other mechanisms beyond obesity in the genesis of pediatric diabetes, the authors of the international analysis concluded, writing for JAMA Network Open.
“We were aware that some children and adolescents with T2D did not have obesity, but we didn’t know the scale of obesity in T2D, or what variables may impact the occurrence of diabetes in this group,” endocrinologist M. Constantine Samaan, MD, MSc, associate professor of pediatrics at McMaster University in Hamilton, Ont., told this news organization. “So, the analysis did help us understand the body mass distribution of this group in more detail.”
The international investigators included in their meta-analysis 53 articles with 8,942 participants from multiple world regions and races/ethnicities. The overall prevalence of obesity in pediatric patients with T2D was 75.27% (95% confidence interval [CI], 70.47%-79.78%). The prevalence of obesity at time of diagnosis in 4,688 participants was 77.24% (95% CI, 70.55%-83.34%). Male participants had higher odds of obesity than females: odds ratio, 2.10 (95% CI, 1.33-3.31) – although girls are generally more likely to develop T2D. The highest prevalence of obesity occurred in Whites at 89.86% (95% CI, 71.50%-99.74%), while prevalence was lowest in Asian participants at 64.50% (95% CI, 53.28%-74.99%).
The authors noted that childhood obesity affects approximately 340 million children worldwide and is a major driver of pediatric T2D, an aggressive disease with a high treatment failure rate. Understanding the contribution of body mass to the evolution of insulin resistance, glucose intolerance, and T2D with its attendant comorbidities and complications, such as nonalcoholic fatty liver disease, remains crucial for developing personalized interventions.
Known risk factors for T2D include interactions between genetics and the environment, including lifestyle factors such as diet and low physical activity levels, Dr. Samaan noted. Certain ethnic groups have higher T2D risks, as do babies exposed in the womb to maternal obesity or diabetes, he said. “And there are likely many other factors that contribute to the risk of T2D, though these remain to be defined.”
Is “lean” T2D in children without obesity likely then to be hereditary, more severe, and harder to control with lifestyle modification? “That’s a great question, but the answer is we don’t know,” Dr. Samaan said.
Commenting on the study but not involved in it, Timothy J. Joos, MD, a pediatrician in Seattle affiliated with the Swedish Medical Center, said the findings raise the question of how many pediatric T2D patients are being missed because they don’t meet current screening criteria. “In nonobese T2D pediatric patients, genetics (and by proxy family history) obviously play a heavier role. In my practice, I often get parents asking me to screen their skinny teenager for diabetes because of diabetes in a family member. In the past I would begrudgingly comply with a smirk on my face. Now the smirk will be gone.”
Dr. Joos said it would be interesting to see what percentage of these T2D patients without obesity (body mass index < 95th percentile) would still meet the criteria for being overweight (BMI > 85th percentile) as this is the primary criterion for screening according to the American Diabetes Association guidelines.
Current guidelines generally look for elevated body mass measures as a main screening indication, Dr. Samaan’s group noted. But in their view, while factors such as ethnicity and in utero exposure to diabetes are already used in combination with BMI-based measures to justify screening, more sophisticated prediabetes and diabetes prediction models are needed to support a more comprehensive screening approach.
“Because being overweight is the initial criterion, children with multiple other criteria are not being screened,” Dr. Joos said. He agreed that more research is needed to sort out the other risk factors for pediatric T2D without obesity so these patients may be detected earlier.
New models may need to incorporate lifestyle factors, hormones, puberty, growth, and sex as well, the authors wrote. Markers of insulin resistance, insulin production capacity, and other markers are needed to refine the identification of those who should be screened.
Dr. Samaan’s group is planning to study the findings in more detail to clarify the effect of body mass on the comorbidities and complications of pediatric T2D.
In addition to the study limitation of significant interstudy heterogeneity, the authors acknowledged varying degrees of glycemic control and dyslipidemia among participants.
No specific funding was provided for this review and meta-analysis. The authors disclosed no conflicts of interest. Dr. Joos disclosed no competing interests with regard to his comments.
Obesity is not a universal phenotype in children with type 2 diabetes (T2D), a global systematic review and meta-analysis reported. In fact, the study found, as many as one in four children with T2D do not have obesity and some have normal reference-range body mass measurements. Further studies should consider other mechanisms beyond obesity in the genesis of pediatric diabetes, the authors of the international analysis concluded, writing for JAMA Network Open.
“We were aware that some children and adolescents with T2D did not have obesity, but we didn’t know the scale of obesity in T2D, or what variables may impact the occurrence of diabetes in this group,” endocrinologist M. Constantine Samaan, MD, MSc, associate professor of pediatrics at McMaster University in Hamilton, Ont., told this news organization. “So, the analysis did help us understand the body mass distribution of this group in more detail.”
The international investigators included in their meta-analysis 53 articles with 8,942 participants from multiple world regions and races/ethnicities. The overall prevalence of obesity in pediatric patients with T2D was 75.27% (95% confidence interval [CI], 70.47%-79.78%). The prevalence of obesity at time of diagnosis in 4,688 participants was 77.24% (95% CI, 70.55%-83.34%). Male participants had higher odds of obesity than females: odds ratio, 2.10 (95% CI, 1.33-3.31) – although girls are generally more likely to develop T2D. The highest prevalence of obesity occurred in Whites at 89.86% (95% CI, 71.50%-99.74%), while prevalence was lowest in Asian participants at 64.50% (95% CI, 53.28%-74.99%).
The authors noted that childhood obesity affects approximately 340 million children worldwide and is a major driver of pediatric T2D, an aggressive disease with a high treatment failure rate. Understanding the contribution of body mass to the evolution of insulin resistance, glucose intolerance, and T2D with its attendant comorbidities and complications, such as nonalcoholic fatty liver disease, remains crucial for developing personalized interventions.
Known risk factors for T2D include interactions between genetics and the environment, including lifestyle factors such as diet and low physical activity levels, Dr. Samaan noted. Certain ethnic groups have higher T2D risks, as do babies exposed in the womb to maternal obesity or diabetes, he said. “And there are likely many other factors that contribute to the risk of T2D, though these remain to be defined.”
Is “lean” T2D in children without obesity likely then to be hereditary, more severe, and harder to control with lifestyle modification? “That’s a great question, but the answer is we don’t know,” Dr. Samaan said.
Commenting on the study but not involved in it, Timothy J. Joos, MD, a pediatrician in Seattle affiliated with the Swedish Medical Center, said the findings raise the question of how many pediatric T2D patients are being missed because they don’t meet current screening criteria. “In nonobese T2D pediatric patients, genetics (and by proxy family history) obviously play a heavier role. In my practice, I often get parents asking me to screen their skinny teenager for diabetes because of diabetes in a family member. In the past I would begrudgingly comply with a smirk on my face. Now the smirk will be gone.”
Dr. Joos said it would be interesting to see what percentage of these T2D patients without obesity (body mass index < 95th percentile) would still meet the criteria for being overweight (BMI > 85th percentile) as this is the primary criterion for screening according to the American Diabetes Association guidelines.
Current guidelines generally look for elevated body mass measures as a main screening indication, Dr. Samaan’s group noted. But in their view, while factors such as ethnicity and in utero exposure to diabetes are already used in combination with BMI-based measures to justify screening, more sophisticated prediabetes and diabetes prediction models are needed to support a more comprehensive screening approach.
“Because being overweight is the initial criterion, children with multiple other criteria are not being screened,” Dr. Joos said. He agreed that more research is needed to sort out the other risk factors for pediatric T2D without obesity so these patients may be detected earlier.
New models may need to incorporate lifestyle factors, hormones, puberty, growth, and sex as well, the authors wrote. Markers of insulin resistance, insulin production capacity, and other markers are needed to refine the identification of those who should be screened.
Dr. Samaan’s group is planning to study the findings in more detail to clarify the effect of body mass on the comorbidities and complications of pediatric T2D.
In addition to the study limitation of significant interstudy heterogeneity, the authors acknowledged varying degrees of glycemic control and dyslipidemia among participants.
No specific funding was provided for this review and meta-analysis. The authors disclosed no conflicts of interest. Dr. Joos disclosed no competing interests with regard to his comments.
FROM JAMA NETWORK OPEN
Intermittent fasting can lead to type 2 diabetes remission
In a small randomized controlled trial of patients with type 2 diabetes in China, close to half of those who followed a novel intermittent fasting program for 3 months had diabetes remission (A1c less than 6.5% without taking antidiabetic drugs) that persisted for 1 year.
Importantly, “this study was performed under real-life conditions, and the intervention was delivered by trained nurses in primary care rather than by specialized staff at a research institute, making it a more practical and achievable way to manage” type 2 diabetes, the authors report.
Moreover, 65% of the patients in the intervention group who achieved diabetes remission had had diabetes for more than 6 years, which “suggests the possibility of remission for patients with longer duration” of diabetes, they note.
In addition, antidiabetic medication costs decreased by 77%, compared with baseline, in patients in the intermittent-fasting intervention group.
Although intermittent fasting has been studied for weight loss, it had not been investigated for effectiveness for diabetes remission.
These findings suggest that intermittent fasting “could be a paradigm shift in the management goals in diabetes care,” Xiao Yang and colleagues conclude in their study, published online in The Journal of Clinical Endocrinology & Metabolism.
“Type 2 diabetes is not necessarily a permanent, lifelong disease,” senior author Dongbo Liu, PhD, from the Hunan Agricultural University, Changsha, China, added in a press release from The Endocrine Society.
“Diabetes remission is possible if patients lose weight by changing their diet and exercise habits,” Dr. Liu said.
‘Excellent outcome’
Invited to comment, Amy E. Rothberg, MD, PhD, who was not involved with the research, agreed that the study indicates that intermittent fasting works for diabetes remission.
“We know that diabetes remission is possible with calorie restriction and subsequent weight loss, and intermittent fasting is just one of the many [dietary] approaches that may be suitable, appealing, and sustainable to some individuals, and usually results in calorie restriction and therefore weight loss,” she said.
The most studied types of intermittent fasting diets are alternate-day fasting, the 5:2 diet, and time-restricted consumption, Dr. Rothberg told this news organization.
This study presented a novel type of intermittent fasting, she noted. The intervention consisted of 6 cycles (3 months) of 5 fasting days followed by 10 ad libitum days, and then 3 months of follow-up (with no fasting days).
After 3 months of the intervention plus 3 months of follow-up, 47% of the 36 patients in the intervention group achieved diabetes remission (with a mean A1c of 5.66%), compared with only 2.8% of the 36 patients in the control group.
At 12 months, 44% of patients in the intervention group had sustained diabetes remission (with a mean A1c of 6.33%).
This was “an excellent outcome,” said Dr. Rothberg, professor of nutritional sciences, School of Public Health, University of Michigan, Ann Arbor, and a co-author of an international consensus statement that defined diabetes remission.
On average, patients in the intermittent fasting group lost 5.93 kg (13.0 lb) in 3 months, which was sustained over 12 months. “The large amount of weight reduction is key to continuing to achieve diabetes remission,” she noted.
This contrasted with an average weight loss of just 0.27 kg (0.6 lb) in the control group.
Participants who were prescribed fewer antidiabetic medications were more likely to achieve diabetes remission. The researchers acknowledge that the study was not blinded, and they did not record physical activity (although participants were encouraged to maintain their usual physical activity).
This was a small study, Dr. Rothberg acknowledged. The researchers did not specify which specific antidiabetic drugs patients were taking, and they did not determine waist or hip circumference or assess lipids.
The diet was culturally sensitive, appropriate, and feasible in this Chinese population and would not be generalizable to non-Asians.
Nevertheless, a similar approach could be used in any population if the diet is tailored to the individual, according to Dr. Rothberg. Importantly, patients would need to receive guidance from a dietician to make sure their diet comprises all the necessary micronutrients, vitamins, and minerals on fasting days, and they would need to maintain a relatively balanced diet and not gorge themselves on feast days.
“I think we should campaign widely about lifestyle approaches to achieve diabetes remission,” she urged.
72 patients with diabetes for an average of 6.6 years
“Despite a widespread public consensus that [type 2 diabetes] is irreversible and requires drug treatment escalation, there is some evidence of the possibility of remission,” Dr. Yang and colleagues write in their article.
They aimed to evaluate the effectiveness of intermittent fasting for diabetes remission and the durability of diabetes remission at 1 year.
Diabetes remission was defined having a stable A1c less than 6.5% for at least 3 months after discontinuing all antidiabetic medications, confirmed in at least annual A1c measurements (according to a 2021 consensus statement initiated by the American Diabetes Association).
Between 2019 and 2020, the researchers enrolled 72 participants aged 38-72 years who had had type 2 diabetes (duration 1 to 11 years) and a body mass index (BMI) of 19.1-30.4 kg/m2. Patients were randomized 1:1 to the intermittent fasting group or control group.
Baseline characteristics were similar in both groups. Patients were a mean age of 53 years and roughly 60% were men. They had a mean BMI of 24 kg/m2, a mean duration of diabetes of 6.6 years, and a mean A1c of 7.6%, and they were taking an average of 1.8 glucose-lowering medications.
On fasting days, patients in the intervention group received a Chinese Medical Nutrition Therapy kit that provided approximately 840 kcal/day (46% carbohydrates, 46% fat, 8% protein). The kit included a breakfast of a fruit and vegetable gruel, lunch of a solid beverage plus a nutritional rice composite, and dinner of a solid beverage and a meal replacement biscuit, which participants reconstituted by mixing with boiling water. They were allowed to consume noncaloric beverages.
On nonfasting days, patients chose foods ad libitum based on the 2017 Dietary Guidelines for Diabetes in China, which recommend approximately 50%-65% of total energy intake from carbohydrates, 15%-20% from protein, and 20%-30% from fat, and had greater than or equal to 5 g fiber per serving.
Patients in the control group chose foods ad libitum from the dietary guidelines during the entire study.
The study received funding from the National Natural Science Foundation of China. The authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
In a small randomized controlled trial of patients with type 2 diabetes in China, close to half of those who followed a novel intermittent fasting program for 3 months had diabetes remission (A1c less than 6.5% without taking antidiabetic drugs) that persisted for 1 year.
Importantly, “this study was performed under real-life conditions, and the intervention was delivered by trained nurses in primary care rather than by specialized staff at a research institute, making it a more practical and achievable way to manage” type 2 diabetes, the authors report.
Moreover, 65% of the patients in the intervention group who achieved diabetes remission had had diabetes for more than 6 years, which “suggests the possibility of remission for patients with longer duration” of diabetes, they note.
In addition, antidiabetic medication costs decreased by 77%, compared with baseline, in patients in the intermittent-fasting intervention group.
Although intermittent fasting has been studied for weight loss, it had not been investigated for effectiveness for diabetes remission.
These findings suggest that intermittent fasting “could be a paradigm shift in the management goals in diabetes care,” Xiao Yang and colleagues conclude in their study, published online in The Journal of Clinical Endocrinology & Metabolism.
“Type 2 diabetes is not necessarily a permanent, lifelong disease,” senior author Dongbo Liu, PhD, from the Hunan Agricultural University, Changsha, China, added in a press release from The Endocrine Society.
“Diabetes remission is possible if patients lose weight by changing their diet and exercise habits,” Dr. Liu said.
‘Excellent outcome’
Invited to comment, Amy E. Rothberg, MD, PhD, who was not involved with the research, agreed that the study indicates that intermittent fasting works for diabetes remission.
“We know that diabetes remission is possible with calorie restriction and subsequent weight loss, and intermittent fasting is just one of the many [dietary] approaches that may be suitable, appealing, and sustainable to some individuals, and usually results in calorie restriction and therefore weight loss,” she said.
The most studied types of intermittent fasting diets are alternate-day fasting, the 5:2 diet, and time-restricted consumption, Dr. Rothberg told this news organization.
This study presented a novel type of intermittent fasting, she noted. The intervention consisted of 6 cycles (3 months) of 5 fasting days followed by 10 ad libitum days, and then 3 months of follow-up (with no fasting days).
After 3 months of the intervention plus 3 months of follow-up, 47% of the 36 patients in the intervention group achieved diabetes remission (with a mean A1c of 5.66%), compared with only 2.8% of the 36 patients in the control group.
At 12 months, 44% of patients in the intervention group had sustained diabetes remission (with a mean A1c of 6.33%).
This was “an excellent outcome,” said Dr. Rothberg, professor of nutritional sciences, School of Public Health, University of Michigan, Ann Arbor, and a co-author of an international consensus statement that defined diabetes remission.
On average, patients in the intermittent fasting group lost 5.93 kg (13.0 lb) in 3 months, which was sustained over 12 months. “The large amount of weight reduction is key to continuing to achieve diabetes remission,” she noted.
This contrasted with an average weight loss of just 0.27 kg (0.6 lb) in the control group.
Participants who were prescribed fewer antidiabetic medications were more likely to achieve diabetes remission. The researchers acknowledge that the study was not blinded, and they did not record physical activity (although participants were encouraged to maintain their usual physical activity).
This was a small study, Dr. Rothberg acknowledged. The researchers did not specify which specific antidiabetic drugs patients were taking, and they did not determine waist or hip circumference or assess lipids.
The diet was culturally sensitive, appropriate, and feasible in this Chinese population and would not be generalizable to non-Asians.
Nevertheless, a similar approach could be used in any population if the diet is tailored to the individual, according to Dr. Rothberg. Importantly, patients would need to receive guidance from a dietician to make sure their diet comprises all the necessary micronutrients, vitamins, and minerals on fasting days, and they would need to maintain a relatively balanced diet and not gorge themselves on feast days.
“I think we should campaign widely about lifestyle approaches to achieve diabetes remission,” she urged.
72 patients with diabetes for an average of 6.6 years
“Despite a widespread public consensus that [type 2 diabetes] is irreversible and requires drug treatment escalation, there is some evidence of the possibility of remission,” Dr. Yang and colleagues write in their article.
They aimed to evaluate the effectiveness of intermittent fasting for diabetes remission and the durability of diabetes remission at 1 year.
Diabetes remission was defined having a stable A1c less than 6.5% for at least 3 months after discontinuing all antidiabetic medications, confirmed in at least annual A1c measurements (according to a 2021 consensus statement initiated by the American Diabetes Association).
Between 2019 and 2020, the researchers enrolled 72 participants aged 38-72 years who had had type 2 diabetes (duration 1 to 11 years) and a body mass index (BMI) of 19.1-30.4 kg/m2. Patients were randomized 1:1 to the intermittent fasting group or control group.
Baseline characteristics were similar in both groups. Patients were a mean age of 53 years and roughly 60% were men. They had a mean BMI of 24 kg/m2, a mean duration of diabetes of 6.6 years, and a mean A1c of 7.6%, and they were taking an average of 1.8 glucose-lowering medications.
On fasting days, patients in the intervention group received a Chinese Medical Nutrition Therapy kit that provided approximately 840 kcal/day (46% carbohydrates, 46% fat, 8% protein). The kit included a breakfast of a fruit and vegetable gruel, lunch of a solid beverage plus a nutritional rice composite, and dinner of a solid beverage and a meal replacement biscuit, which participants reconstituted by mixing with boiling water. They were allowed to consume noncaloric beverages.
On nonfasting days, patients chose foods ad libitum based on the 2017 Dietary Guidelines for Diabetes in China, which recommend approximately 50%-65% of total energy intake from carbohydrates, 15%-20% from protein, and 20%-30% from fat, and had greater than or equal to 5 g fiber per serving.
Patients in the control group chose foods ad libitum from the dietary guidelines during the entire study.
The study received funding from the National Natural Science Foundation of China. The authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
In a small randomized controlled trial of patients with type 2 diabetes in China, close to half of those who followed a novel intermittent fasting program for 3 months had diabetes remission (A1c less than 6.5% without taking antidiabetic drugs) that persisted for 1 year.
Importantly, “this study was performed under real-life conditions, and the intervention was delivered by trained nurses in primary care rather than by specialized staff at a research institute, making it a more practical and achievable way to manage” type 2 diabetes, the authors report.
Moreover, 65% of the patients in the intervention group who achieved diabetes remission had had diabetes for more than 6 years, which “suggests the possibility of remission for patients with longer duration” of diabetes, they note.
In addition, antidiabetic medication costs decreased by 77%, compared with baseline, in patients in the intermittent-fasting intervention group.
Although intermittent fasting has been studied for weight loss, it had not been investigated for effectiveness for diabetes remission.
These findings suggest that intermittent fasting “could be a paradigm shift in the management goals in diabetes care,” Xiao Yang and colleagues conclude in their study, published online in The Journal of Clinical Endocrinology & Metabolism.
“Type 2 diabetes is not necessarily a permanent, lifelong disease,” senior author Dongbo Liu, PhD, from the Hunan Agricultural University, Changsha, China, added in a press release from The Endocrine Society.
“Diabetes remission is possible if patients lose weight by changing their diet and exercise habits,” Dr. Liu said.
‘Excellent outcome’
Invited to comment, Amy E. Rothberg, MD, PhD, who was not involved with the research, agreed that the study indicates that intermittent fasting works for diabetes remission.
“We know that diabetes remission is possible with calorie restriction and subsequent weight loss, and intermittent fasting is just one of the many [dietary] approaches that may be suitable, appealing, and sustainable to some individuals, and usually results in calorie restriction and therefore weight loss,” she said.
The most studied types of intermittent fasting diets are alternate-day fasting, the 5:2 diet, and time-restricted consumption, Dr. Rothberg told this news organization.
This study presented a novel type of intermittent fasting, she noted. The intervention consisted of 6 cycles (3 months) of 5 fasting days followed by 10 ad libitum days, and then 3 months of follow-up (with no fasting days).
After 3 months of the intervention plus 3 months of follow-up, 47% of the 36 patients in the intervention group achieved diabetes remission (with a mean A1c of 5.66%), compared with only 2.8% of the 36 patients in the control group.
At 12 months, 44% of patients in the intervention group had sustained diabetes remission (with a mean A1c of 6.33%).
This was “an excellent outcome,” said Dr. Rothberg, professor of nutritional sciences, School of Public Health, University of Michigan, Ann Arbor, and a co-author of an international consensus statement that defined diabetes remission.
On average, patients in the intermittent fasting group lost 5.93 kg (13.0 lb) in 3 months, which was sustained over 12 months. “The large amount of weight reduction is key to continuing to achieve diabetes remission,” she noted.
This contrasted with an average weight loss of just 0.27 kg (0.6 lb) in the control group.
Participants who were prescribed fewer antidiabetic medications were more likely to achieve diabetes remission. The researchers acknowledge that the study was not blinded, and they did not record physical activity (although participants were encouraged to maintain their usual physical activity).
This was a small study, Dr. Rothberg acknowledged. The researchers did not specify which specific antidiabetic drugs patients were taking, and they did not determine waist or hip circumference or assess lipids.
The diet was culturally sensitive, appropriate, and feasible in this Chinese population and would not be generalizable to non-Asians.
Nevertheless, a similar approach could be used in any population if the diet is tailored to the individual, according to Dr. Rothberg. Importantly, patients would need to receive guidance from a dietician to make sure their diet comprises all the necessary micronutrients, vitamins, and minerals on fasting days, and they would need to maintain a relatively balanced diet and not gorge themselves on feast days.
“I think we should campaign widely about lifestyle approaches to achieve diabetes remission,” she urged.
72 patients with diabetes for an average of 6.6 years
“Despite a widespread public consensus that [type 2 diabetes] is irreversible and requires drug treatment escalation, there is some evidence of the possibility of remission,” Dr. Yang and colleagues write in their article.
They aimed to evaluate the effectiveness of intermittent fasting for diabetes remission and the durability of diabetes remission at 1 year.
Diabetes remission was defined having a stable A1c less than 6.5% for at least 3 months after discontinuing all antidiabetic medications, confirmed in at least annual A1c measurements (according to a 2021 consensus statement initiated by the American Diabetes Association).
Between 2019 and 2020, the researchers enrolled 72 participants aged 38-72 years who had had type 2 diabetes (duration 1 to 11 years) and a body mass index (BMI) of 19.1-30.4 kg/m2. Patients were randomized 1:1 to the intermittent fasting group or control group.
Baseline characteristics were similar in both groups. Patients were a mean age of 53 years and roughly 60% were men. They had a mean BMI of 24 kg/m2, a mean duration of diabetes of 6.6 years, and a mean A1c of 7.6%, and they were taking an average of 1.8 glucose-lowering medications.
On fasting days, patients in the intervention group received a Chinese Medical Nutrition Therapy kit that provided approximately 840 kcal/day (46% carbohydrates, 46% fat, 8% protein). The kit included a breakfast of a fruit and vegetable gruel, lunch of a solid beverage plus a nutritional rice composite, and dinner of a solid beverage and a meal replacement biscuit, which participants reconstituted by mixing with boiling water. They were allowed to consume noncaloric beverages.
On nonfasting days, patients chose foods ad libitum based on the 2017 Dietary Guidelines for Diabetes in China, which recommend approximately 50%-65% of total energy intake from carbohydrates, 15%-20% from protein, and 20%-30% from fat, and had greater than or equal to 5 g fiber per serving.
Patients in the control group chose foods ad libitum from the dietary guidelines during the entire study.
The study received funding from the National Natural Science Foundation of China. The authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
DELIVER subanalysis ‘seals deal’ for dapagliflozin in HF
A prespecified analysis of a large global trial of patients with symptomatic heart failure with mildly reduced and preserved ejection fraction “seals the deal” on the efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors to manage and improve their symptoms.
The prespecified analysis of the DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) trial included 5,795 patients with mildly reduced and preserved ejection fraction who completed the Kansas City Cardiomyopathy Questionnaire (KCCQ) after taking the SGLT2 inhibitor dapagliflozin or placebo. The results were published online in the Journal of the American College of Cardiology.
“We’ve known from studies prior to DELIVER that SGLT2 inhibitors have been shown to improve health status, patient symptoms and quality of life among those that are living with heart failure and mildly reduced [HFmrEF] and preserved [HFpEF] ejection fraction,” lead author Mikhail N. Kosiborod, MD, vice president for research at Saint Luke’s Health System, and codirector of the St. Luke’s Michael and Marly Haverty Cardiometabolic Center of Excellence at St. Luke’s Mid America Heart Institute, Kansas City, Mo., said in an interview. “But the picture was incomplete for a number of different reasons, partly because the previous studies were either relatively modest in size, geographically limited, or suggested potential attenuation of these benefits in patients with completely normal ejection fraction.”
Specifically, the study authors noted the EMPEROR-Preserved trial of the SGLT2 inhibitor empagliflozin showed improvement in health status vs. placebo across the range of EF except in those with normal EF of 65% or greater. The PRESERVED-HF trial of dapagliflozin demonstrated a more robust response than EMPEROR-Preserved or DELIVER, but PRESERVED-HF patients were recruited only in the United States and had more debilitating HF symptoms at baseline.
“Because of the results of the DELIVER trial and because of how large, extensive, and inclusive the trial was, it really seals the deal on the value of SGLT2 inhibitors in patients with heart failure,” said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri–Kansas City.
The DELIVER analysis found that the effects of dapagliflozin on reducing cardiovascular death and worsening HF were greatest in patients who had the most debilitating symptoms at baseline, measured as KCCQ total symptom score (TSS) as 63 or less, the lowest of three tertiles used in the analysis. At baseline, these patients had the highest rates of CV death or worsening HF than those in the other two tertiles: KCCQ-TSS of 63-84, and greater than 84.
Compared with placebo, treated patients in the lowest KCCQ-TSS quartile had a 30% reduction in risk for the primary composite outcome, which consisted of time to first CV death or HF event (hazard ratio, 0.70; 95% confidence interval, 0.58-0.84; P < .001). In the second tertile, the relative risk reduction was 19% (HR, 0.81; 95% CI, 0.65-1.01; P < .006), and the highest quartile showed no significant difference between treatment and placebo (HR, 1.07; 95% CI, 0.83-1.37; P < .62).
“The most important take home message is that the SGLT2 inhibitor dapagliflozin significantly improved patient symptoms as measured by the Kansas City Cardiomyopathy Questionnaire symptom score,” Dr. Kosiborod said. “It improved those symptoms within 1 month and those benefits were sustained out to 8 months.”
DELIVER patients also showed improvement in all other key KCCQ domains across the board, he added. “In addition, dapagliflozin also improved the proportion of patients who had small, moderate, and large improvements in a responder analysis. So really, by every measure that we had, dapagliflozin had a significant beneficial effect.”
The DELIVER results taken collectively with the EMPEROR-Preserved and PRESERVED-HF trials cinch the deal for SGLT2 inhibitors, Dr. Kosiborod said. “They deliver on the triple goal of care in patients with heart failure. They reduce the risk of cardiovascular death and worsening heart failure and they improve patient symptoms, function and quality of life – and they accomplish that across the entire continuum of heart failure regardless of ejection fraction, regardless of whether patients are hospitalized or in an ambulatory setting, regardless of age or background therapies or other comorbidities.”
He added: “It’s a pretty historic development because we haven’t had that before.”
AstraZeneca funded the DELIVER trial. Dr. Kosiborod disclosed financial relationships with Alnylam, Amgen, Applied Therapeutics, Bayer, Boehringer Ingelheim, Cytokinetics, Dexcom, Eli Lilly, Esperion Therapeutics, Janssen, Lexicon, Merck (Diabetes and Cardiovascular), Novo Nordisk, Sanofi, Pharmacosmos and Vifor Pharma.
A prespecified analysis of a large global trial of patients with symptomatic heart failure with mildly reduced and preserved ejection fraction “seals the deal” on the efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors to manage and improve their symptoms.
The prespecified analysis of the DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) trial included 5,795 patients with mildly reduced and preserved ejection fraction who completed the Kansas City Cardiomyopathy Questionnaire (KCCQ) after taking the SGLT2 inhibitor dapagliflozin or placebo. The results were published online in the Journal of the American College of Cardiology.
“We’ve known from studies prior to DELIVER that SGLT2 inhibitors have been shown to improve health status, patient symptoms and quality of life among those that are living with heart failure and mildly reduced [HFmrEF] and preserved [HFpEF] ejection fraction,” lead author Mikhail N. Kosiborod, MD, vice president for research at Saint Luke’s Health System, and codirector of the St. Luke’s Michael and Marly Haverty Cardiometabolic Center of Excellence at St. Luke’s Mid America Heart Institute, Kansas City, Mo., said in an interview. “But the picture was incomplete for a number of different reasons, partly because the previous studies were either relatively modest in size, geographically limited, or suggested potential attenuation of these benefits in patients with completely normal ejection fraction.”
Specifically, the study authors noted the EMPEROR-Preserved trial of the SGLT2 inhibitor empagliflozin showed improvement in health status vs. placebo across the range of EF except in those with normal EF of 65% or greater. The PRESERVED-HF trial of dapagliflozin demonstrated a more robust response than EMPEROR-Preserved or DELIVER, but PRESERVED-HF patients were recruited only in the United States and had more debilitating HF symptoms at baseline.
“Because of the results of the DELIVER trial and because of how large, extensive, and inclusive the trial was, it really seals the deal on the value of SGLT2 inhibitors in patients with heart failure,” said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri–Kansas City.
The DELIVER analysis found that the effects of dapagliflozin on reducing cardiovascular death and worsening HF were greatest in patients who had the most debilitating symptoms at baseline, measured as KCCQ total symptom score (TSS) as 63 or less, the lowest of three tertiles used in the analysis. At baseline, these patients had the highest rates of CV death or worsening HF than those in the other two tertiles: KCCQ-TSS of 63-84, and greater than 84.
Compared with placebo, treated patients in the lowest KCCQ-TSS quartile had a 30% reduction in risk for the primary composite outcome, which consisted of time to first CV death or HF event (hazard ratio, 0.70; 95% confidence interval, 0.58-0.84; P < .001). In the second tertile, the relative risk reduction was 19% (HR, 0.81; 95% CI, 0.65-1.01; P < .006), and the highest quartile showed no significant difference between treatment and placebo (HR, 1.07; 95% CI, 0.83-1.37; P < .62).
“The most important take home message is that the SGLT2 inhibitor dapagliflozin significantly improved patient symptoms as measured by the Kansas City Cardiomyopathy Questionnaire symptom score,” Dr. Kosiborod said. “It improved those symptoms within 1 month and those benefits were sustained out to 8 months.”
DELIVER patients also showed improvement in all other key KCCQ domains across the board, he added. “In addition, dapagliflozin also improved the proportion of patients who had small, moderate, and large improvements in a responder analysis. So really, by every measure that we had, dapagliflozin had a significant beneficial effect.”
The DELIVER results taken collectively with the EMPEROR-Preserved and PRESERVED-HF trials cinch the deal for SGLT2 inhibitors, Dr. Kosiborod said. “They deliver on the triple goal of care in patients with heart failure. They reduce the risk of cardiovascular death and worsening heart failure and they improve patient symptoms, function and quality of life – and they accomplish that across the entire continuum of heart failure regardless of ejection fraction, regardless of whether patients are hospitalized or in an ambulatory setting, regardless of age or background therapies or other comorbidities.”
He added: “It’s a pretty historic development because we haven’t had that before.”
AstraZeneca funded the DELIVER trial. Dr. Kosiborod disclosed financial relationships with Alnylam, Amgen, Applied Therapeutics, Bayer, Boehringer Ingelheim, Cytokinetics, Dexcom, Eli Lilly, Esperion Therapeutics, Janssen, Lexicon, Merck (Diabetes and Cardiovascular), Novo Nordisk, Sanofi, Pharmacosmos and Vifor Pharma.
A prespecified analysis of a large global trial of patients with symptomatic heart failure with mildly reduced and preserved ejection fraction “seals the deal” on the efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors to manage and improve their symptoms.
The prespecified analysis of the DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) trial included 5,795 patients with mildly reduced and preserved ejection fraction who completed the Kansas City Cardiomyopathy Questionnaire (KCCQ) after taking the SGLT2 inhibitor dapagliflozin or placebo. The results were published online in the Journal of the American College of Cardiology.
“We’ve known from studies prior to DELIVER that SGLT2 inhibitors have been shown to improve health status, patient symptoms and quality of life among those that are living with heart failure and mildly reduced [HFmrEF] and preserved [HFpEF] ejection fraction,” lead author Mikhail N. Kosiborod, MD, vice president for research at Saint Luke’s Health System, and codirector of the St. Luke’s Michael and Marly Haverty Cardiometabolic Center of Excellence at St. Luke’s Mid America Heart Institute, Kansas City, Mo., said in an interview. “But the picture was incomplete for a number of different reasons, partly because the previous studies were either relatively modest in size, geographically limited, or suggested potential attenuation of these benefits in patients with completely normal ejection fraction.”
Specifically, the study authors noted the EMPEROR-Preserved trial of the SGLT2 inhibitor empagliflozin showed improvement in health status vs. placebo across the range of EF except in those with normal EF of 65% or greater. The PRESERVED-HF trial of dapagliflozin demonstrated a more robust response than EMPEROR-Preserved or DELIVER, but PRESERVED-HF patients were recruited only in the United States and had more debilitating HF symptoms at baseline.
“Because of the results of the DELIVER trial and because of how large, extensive, and inclusive the trial was, it really seals the deal on the value of SGLT2 inhibitors in patients with heart failure,” said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri–Kansas City.
The DELIVER analysis found that the effects of dapagliflozin on reducing cardiovascular death and worsening HF were greatest in patients who had the most debilitating symptoms at baseline, measured as KCCQ total symptom score (TSS) as 63 or less, the lowest of three tertiles used in the analysis. At baseline, these patients had the highest rates of CV death or worsening HF than those in the other two tertiles: KCCQ-TSS of 63-84, and greater than 84.
Compared with placebo, treated patients in the lowest KCCQ-TSS quartile had a 30% reduction in risk for the primary composite outcome, which consisted of time to first CV death or HF event (hazard ratio, 0.70; 95% confidence interval, 0.58-0.84; P < .001). In the second tertile, the relative risk reduction was 19% (HR, 0.81; 95% CI, 0.65-1.01; P < .006), and the highest quartile showed no significant difference between treatment and placebo (HR, 1.07; 95% CI, 0.83-1.37; P < .62).
“The most important take home message is that the SGLT2 inhibitor dapagliflozin significantly improved patient symptoms as measured by the Kansas City Cardiomyopathy Questionnaire symptom score,” Dr. Kosiborod said. “It improved those symptoms within 1 month and those benefits were sustained out to 8 months.”
DELIVER patients also showed improvement in all other key KCCQ domains across the board, he added. “In addition, dapagliflozin also improved the proportion of patients who had small, moderate, and large improvements in a responder analysis. So really, by every measure that we had, dapagliflozin had a significant beneficial effect.”
The DELIVER results taken collectively with the EMPEROR-Preserved and PRESERVED-HF trials cinch the deal for SGLT2 inhibitors, Dr. Kosiborod said. “They deliver on the triple goal of care in patients with heart failure. They reduce the risk of cardiovascular death and worsening heart failure and they improve patient symptoms, function and quality of life – and they accomplish that across the entire continuum of heart failure regardless of ejection fraction, regardless of whether patients are hospitalized or in an ambulatory setting, regardless of age or background therapies or other comorbidities.”
He added: “It’s a pretty historic development because we haven’t had that before.”
AstraZeneca funded the DELIVER trial. Dr. Kosiborod disclosed financial relationships with Alnylam, Amgen, Applied Therapeutics, Bayer, Boehringer Ingelheim, Cytokinetics, Dexcom, Eli Lilly, Esperion Therapeutics, Janssen, Lexicon, Merck (Diabetes and Cardiovascular), Novo Nordisk, Sanofi, Pharmacosmos and Vifor Pharma.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
ADA issues 2023 ‘Standards of Care’ for diabetes: Focus on tight BP, lipids
New more aggressive targets for blood pressure and lipids are among the changes to the annual American Diabetes Association (ADA) Standards of Care in Diabetes – 2023.
The document, long considered the gold standard for care of the more than 100 million Americans living with diabetes and prediabetes, was published as a supplement in Diabetes Care. The guidelines are also accessible to doctors via an app; last year’s standards were accessed more than 4 million times.
The standards now advise a blood pressure target for people with diabetes of less than 130/80 mm Hg, and low-density lipoprotein (LDL) cholesterol targets of below 70 mg/dL or no greater than 55 mg/dL, depending on the individual’s cardiovascular risk.
“In this year’s version of the ADA Standards of Care – the longstanding guidelines for diabetes management globally – you’ll see information that really speaks to how we can more aggressively treat diabetes and reduce complications in a variety of different ways,” ADA Chief Scientific and Medical Officer Robert A. Gabbay, MD, PhD, said in an interview.
Other changes for 2023 include a new emphasis on weight loss as a goal of therapy for type 2 diabetes; guidance for screening and assessing peripheral arterial disease in an effort to prevent amputations; use of finerenone in people with diabetes and chronic kidney disease; use of approved point-of-care A1c tests; and guidance on screening for food insecurity, along with an elevated role for community health workers.
“The management of type 2 diabetes is not just about glucose,” Dr. Gabbay emphasized, noting that the ADA Standards have increasingly focused on cardiorenal risk as well as weight management. “We need to think about all those things, not just one. We have better tools now that have been helpful in being able to move forward with this.”
New targets in cardiovascular disease and risk management
As it has been for the past 6 years, the section on cardiovascular disease and risk management is also endorsed by the American College of Cardiology.
The new definition of hypertension in people with diabetes is ≥ 130 mm Hg systolic or ≥ 80 mm Hg diastolic blood pressure, repeated on two measurements at different times. Among individuals with established cardiovascular disease, hypertension can be diagnosed with one measurement of ≥ 180/110 mm Hg.
The goal of treatment is now less than 130/80 mm Hg if it can be reached safely.
In 2012, easing of the systolic target to 140 mm Hg by the ADA caused some controversy.
But, as Dr. Gabbay explained: “The evidence wasn’t there 10 years ago. We stuck to the evidence at that time, although there was a belief that lower was better. Over the past decade, a number of studies have made it quite clear that there is benefit to a lower target. That’s why we staked out the ground on this.”
The new Standards of Care also has new lipid targets. For people with diabetes aged 40-75 years at increased cardiovascular risk, including those with one or more atherosclerotic risk factors, high-intensity statin therapy is recommended to reduce LDL cholesterol by 50% or more from baseline and to a target of less than 70 mg/dL, in contrast to the previous target of 100 mg/dL.
To achieve that goal, the document advises to consider adding ezetimibe or a PCSK9 inhibitor to maximally tolerated statin therapy.
For people with diabetes aged 40-75 who have established cardiovascular disease, treatment with high-intensity statin therapy is recommended with the target of a 50% or greater reduction from baseline and an LDL cholesterol level of 55 mg/dL or lower, in contrast to the previous 70 mg/dL.
“That is a lower goal than previously recommended, and based on strong evidence in the literature,” Dr. Gabbay noted.
Here, a stronger recommendation is made for ezetimibe or a PCSK9 inhibitor added to maximal statins.
And for people with diabetes older than 75 years, those already on statins should continue taking them. For those who aren’t, it may be reasonable to initiate moderate-intensity statin therapy after discussion of the benefits and risks.
Another new recommendation based on recent trial data is use of a sodium–glucose cotransporter 2 (SGLT2) inhibitor in people with diabetes and heart failure with preserved, as well as reduced, ejection fraction.
Kidney disease guidance updated: SGLT2 inhibitors, finerenone
Another recommendation calls for the addition of finerenone for people with type 2 diabetes who have chronic kidney disease (CKD) with albuminuria and have been treated with the maximum tolerated doses of an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) to improve cardiovascular outcomes as well as reduce the risk of CKD progression.
The threshold for initiating an SGLT2 inhibitor for kidney protection has changed to an estimated glomerular filtration rate (eGFR) ≥ 20 mL/min/1.73 m2 and urinary albumin ≥ 200 mg/g creatinine (previously ≥ 25 mL/min/1.73 m2 and ≥ 300 mg/g, respectively). An SGLT2 inhibitor may also be beneficial in people with a urinary albumin of normal to ≥ 200 mg/g creatinine, but supporting data have not yet been published.
Referral to a nephrologist is advised for individuals with increasing urinary albumin levels or continued decreasing eGFR or eGFR < 30 mL/min/1.73 m2.
Weight loss, point-of-care testing, food insecurity assessment
Other changes for 2023 include fresh emphasis on supporting weight loss of up to 15% with the new twincretin tirzepatide (Mounjaro) – approved in the United States in May for type 2 diabetes – added as a glucose-lowering drug with weight loss potential.
A novel section was added with guidance for peripheral arterial disease screening.
And a new recommendation advises use of point-of-care A1c testing for diabetes screening and diagnosis using only tests approved by the Food and Drug Administration.
Also introduced for 2023 is guidance to use community health workers to support the management of diabetes and cardiovascular risk factors, particularly in underserved areas and health systems.
“Community health workers can be a link to help people navigate and engage with the health system for better outcomes,” said Dr. Gabbay.
He added that these professionals are among those who can also assist with screening for food insecurity, another new recommendation. “We talk about screening for food insecurity and tools to use. That shouldn’t be something only dietitians do.”
Dr. Gabbay said he’d like to see more clinicians partner with community health workers. “We’d like to see more of that ... They should be considered part of the health care team,” he said.
Dr. Gabbay has reported serving on advisory boards for Lark, Health Reveal, Sweetch, StartUp Health, Vida Health, and Onduo.
A version of this article first appeared on Medscape.com.
New more aggressive targets for blood pressure and lipids are among the changes to the annual American Diabetes Association (ADA) Standards of Care in Diabetes – 2023.
The document, long considered the gold standard for care of the more than 100 million Americans living with diabetes and prediabetes, was published as a supplement in Diabetes Care. The guidelines are also accessible to doctors via an app; last year’s standards were accessed more than 4 million times.
The standards now advise a blood pressure target for people with diabetes of less than 130/80 mm Hg, and low-density lipoprotein (LDL) cholesterol targets of below 70 mg/dL or no greater than 55 mg/dL, depending on the individual’s cardiovascular risk.
“In this year’s version of the ADA Standards of Care – the longstanding guidelines for diabetes management globally – you’ll see information that really speaks to how we can more aggressively treat diabetes and reduce complications in a variety of different ways,” ADA Chief Scientific and Medical Officer Robert A. Gabbay, MD, PhD, said in an interview.
Other changes for 2023 include a new emphasis on weight loss as a goal of therapy for type 2 diabetes; guidance for screening and assessing peripheral arterial disease in an effort to prevent amputations; use of finerenone in people with diabetes and chronic kidney disease; use of approved point-of-care A1c tests; and guidance on screening for food insecurity, along with an elevated role for community health workers.
“The management of type 2 diabetes is not just about glucose,” Dr. Gabbay emphasized, noting that the ADA Standards have increasingly focused on cardiorenal risk as well as weight management. “We need to think about all those things, not just one. We have better tools now that have been helpful in being able to move forward with this.”
New targets in cardiovascular disease and risk management
As it has been for the past 6 years, the section on cardiovascular disease and risk management is also endorsed by the American College of Cardiology.
The new definition of hypertension in people with diabetes is ≥ 130 mm Hg systolic or ≥ 80 mm Hg diastolic blood pressure, repeated on two measurements at different times. Among individuals with established cardiovascular disease, hypertension can be diagnosed with one measurement of ≥ 180/110 mm Hg.
The goal of treatment is now less than 130/80 mm Hg if it can be reached safely.
In 2012, easing of the systolic target to 140 mm Hg by the ADA caused some controversy.
But, as Dr. Gabbay explained: “The evidence wasn’t there 10 years ago. We stuck to the evidence at that time, although there was a belief that lower was better. Over the past decade, a number of studies have made it quite clear that there is benefit to a lower target. That’s why we staked out the ground on this.”
The new Standards of Care also has new lipid targets. For people with diabetes aged 40-75 years at increased cardiovascular risk, including those with one or more atherosclerotic risk factors, high-intensity statin therapy is recommended to reduce LDL cholesterol by 50% or more from baseline and to a target of less than 70 mg/dL, in contrast to the previous target of 100 mg/dL.
To achieve that goal, the document advises to consider adding ezetimibe or a PCSK9 inhibitor to maximally tolerated statin therapy.
For people with diabetes aged 40-75 who have established cardiovascular disease, treatment with high-intensity statin therapy is recommended with the target of a 50% or greater reduction from baseline and an LDL cholesterol level of 55 mg/dL or lower, in contrast to the previous 70 mg/dL.
“That is a lower goal than previously recommended, and based on strong evidence in the literature,” Dr. Gabbay noted.
Here, a stronger recommendation is made for ezetimibe or a PCSK9 inhibitor added to maximal statins.
And for people with diabetes older than 75 years, those already on statins should continue taking them. For those who aren’t, it may be reasonable to initiate moderate-intensity statin therapy after discussion of the benefits and risks.
Another new recommendation based on recent trial data is use of a sodium–glucose cotransporter 2 (SGLT2) inhibitor in people with diabetes and heart failure with preserved, as well as reduced, ejection fraction.
Kidney disease guidance updated: SGLT2 inhibitors, finerenone
Another recommendation calls for the addition of finerenone for people with type 2 diabetes who have chronic kidney disease (CKD) with albuminuria and have been treated with the maximum tolerated doses of an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) to improve cardiovascular outcomes as well as reduce the risk of CKD progression.
The threshold for initiating an SGLT2 inhibitor for kidney protection has changed to an estimated glomerular filtration rate (eGFR) ≥ 20 mL/min/1.73 m2 and urinary albumin ≥ 200 mg/g creatinine (previously ≥ 25 mL/min/1.73 m2 and ≥ 300 mg/g, respectively). An SGLT2 inhibitor may also be beneficial in people with a urinary albumin of normal to ≥ 200 mg/g creatinine, but supporting data have not yet been published.
Referral to a nephrologist is advised for individuals with increasing urinary albumin levels or continued decreasing eGFR or eGFR < 30 mL/min/1.73 m2.
Weight loss, point-of-care testing, food insecurity assessment
Other changes for 2023 include fresh emphasis on supporting weight loss of up to 15% with the new twincretin tirzepatide (Mounjaro) – approved in the United States in May for type 2 diabetes – added as a glucose-lowering drug with weight loss potential.
A novel section was added with guidance for peripheral arterial disease screening.
And a new recommendation advises use of point-of-care A1c testing for diabetes screening and diagnosis using only tests approved by the Food and Drug Administration.
Also introduced for 2023 is guidance to use community health workers to support the management of diabetes and cardiovascular risk factors, particularly in underserved areas and health systems.
“Community health workers can be a link to help people navigate and engage with the health system for better outcomes,” said Dr. Gabbay.
He added that these professionals are among those who can also assist with screening for food insecurity, another new recommendation. “We talk about screening for food insecurity and tools to use. That shouldn’t be something only dietitians do.”
Dr. Gabbay said he’d like to see more clinicians partner with community health workers. “We’d like to see more of that ... They should be considered part of the health care team,” he said.
Dr. Gabbay has reported serving on advisory boards for Lark, Health Reveal, Sweetch, StartUp Health, Vida Health, and Onduo.
A version of this article first appeared on Medscape.com.
New more aggressive targets for blood pressure and lipids are among the changes to the annual American Diabetes Association (ADA) Standards of Care in Diabetes – 2023.
The document, long considered the gold standard for care of the more than 100 million Americans living with diabetes and prediabetes, was published as a supplement in Diabetes Care. The guidelines are also accessible to doctors via an app; last year’s standards were accessed more than 4 million times.
The standards now advise a blood pressure target for people with diabetes of less than 130/80 mm Hg, and low-density lipoprotein (LDL) cholesterol targets of below 70 mg/dL or no greater than 55 mg/dL, depending on the individual’s cardiovascular risk.
“In this year’s version of the ADA Standards of Care – the longstanding guidelines for diabetes management globally – you’ll see information that really speaks to how we can more aggressively treat diabetes and reduce complications in a variety of different ways,” ADA Chief Scientific and Medical Officer Robert A. Gabbay, MD, PhD, said in an interview.
Other changes for 2023 include a new emphasis on weight loss as a goal of therapy for type 2 diabetes; guidance for screening and assessing peripheral arterial disease in an effort to prevent amputations; use of finerenone in people with diabetes and chronic kidney disease; use of approved point-of-care A1c tests; and guidance on screening for food insecurity, along with an elevated role for community health workers.
“The management of type 2 diabetes is not just about glucose,” Dr. Gabbay emphasized, noting that the ADA Standards have increasingly focused on cardiorenal risk as well as weight management. “We need to think about all those things, not just one. We have better tools now that have been helpful in being able to move forward with this.”
New targets in cardiovascular disease and risk management
As it has been for the past 6 years, the section on cardiovascular disease and risk management is also endorsed by the American College of Cardiology.
The new definition of hypertension in people with diabetes is ≥ 130 mm Hg systolic or ≥ 80 mm Hg diastolic blood pressure, repeated on two measurements at different times. Among individuals with established cardiovascular disease, hypertension can be diagnosed with one measurement of ≥ 180/110 mm Hg.
The goal of treatment is now less than 130/80 mm Hg if it can be reached safely.
In 2012, easing of the systolic target to 140 mm Hg by the ADA caused some controversy.
But, as Dr. Gabbay explained: “The evidence wasn’t there 10 years ago. We stuck to the evidence at that time, although there was a belief that lower was better. Over the past decade, a number of studies have made it quite clear that there is benefit to a lower target. That’s why we staked out the ground on this.”
The new Standards of Care also has new lipid targets. For people with diabetes aged 40-75 years at increased cardiovascular risk, including those with one or more atherosclerotic risk factors, high-intensity statin therapy is recommended to reduce LDL cholesterol by 50% or more from baseline and to a target of less than 70 mg/dL, in contrast to the previous target of 100 mg/dL.
To achieve that goal, the document advises to consider adding ezetimibe or a PCSK9 inhibitor to maximally tolerated statin therapy.
For people with diabetes aged 40-75 who have established cardiovascular disease, treatment with high-intensity statin therapy is recommended with the target of a 50% or greater reduction from baseline and an LDL cholesterol level of 55 mg/dL or lower, in contrast to the previous 70 mg/dL.
“That is a lower goal than previously recommended, and based on strong evidence in the literature,” Dr. Gabbay noted.
Here, a stronger recommendation is made for ezetimibe or a PCSK9 inhibitor added to maximal statins.
And for people with diabetes older than 75 years, those already on statins should continue taking them. For those who aren’t, it may be reasonable to initiate moderate-intensity statin therapy after discussion of the benefits and risks.
Another new recommendation based on recent trial data is use of a sodium–glucose cotransporter 2 (SGLT2) inhibitor in people with diabetes and heart failure with preserved, as well as reduced, ejection fraction.
Kidney disease guidance updated: SGLT2 inhibitors, finerenone
Another recommendation calls for the addition of finerenone for people with type 2 diabetes who have chronic kidney disease (CKD) with albuminuria and have been treated with the maximum tolerated doses of an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) to improve cardiovascular outcomes as well as reduce the risk of CKD progression.
The threshold for initiating an SGLT2 inhibitor for kidney protection has changed to an estimated glomerular filtration rate (eGFR) ≥ 20 mL/min/1.73 m2 and urinary albumin ≥ 200 mg/g creatinine (previously ≥ 25 mL/min/1.73 m2 and ≥ 300 mg/g, respectively). An SGLT2 inhibitor may also be beneficial in people with a urinary albumin of normal to ≥ 200 mg/g creatinine, but supporting data have not yet been published.
Referral to a nephrologist is advised for individuals with increasing urinary albumin levels or continued decreasing eGFR or eGFR < 30 mL/min/1.73 m2.
Weight loss, point-of-care testing, food insecurity assessment
Other changes for 2023 include fresh emphasis on supporting weight loss of up to 15% with the new twincretin tirzepatide (Mounjaro) – approved in the United States in May for type 2 diabetes – added as a glucose-lowering drug with weight loss potential.
A novel section was added with guidance for peripheral arterial disease screening.
And a new recommendation advises use of point-of-care A1c testing for diabetes screening and diagnosis using only tests approved by the Food and Drug Administration.
Also introduced for 2023 is guidance to use community health workers to support the management of diabetes and cardiovascular risk factors, particularly in underserved areas and health systems.
“Community health workers can be a link to help people navigate and engage with the health system for better outcomes,” said Dr. Gabbay.
He added that these professionals are among those who can also assist with screening for food insecurity, another new recommendation. “We talk about screening for food insecurity and tools to use. That shouldn’t be something only dietitians do.”
Dr. Gabbay said he’d like to see more clinicians partner with community health workers. “We’d like to see more of that ... They should be considered part of the health care team,” he said.
Dr. Gabbay has reported serving on advisory boards for Lark, Health Reveal, Sweetch, StartUp Health, Vida Health, and Onduo.
A version of this article first appeared on Medscape.com.
Low-carb, high-fat, calorie-unrestricted diet improves type 2 diabetes
This was true regardless of an individual’s calorie intake, in the randomized controlled trial published in the Annals of Internal Medicine.
Patients with T2D who ate a low-carb, high-fat diet (LCHF) lost more weight and saw greater improvements in both glycemic control and insulin resistance than those who ate a high-carb, low-fat diet (HCLF), reported lead author Camilla Dalby Hansen, MD, of University of Southern Denmark, Odense, and colleagues, suggesting that this is an effective, nonpharmaceutical treatment option for T2D.
The trial enrolled 185 patients with T2D, for whom low-calorie diets are often recommended to induce weight loss and improve glycemic control.
The trouble with this common recommendation, the investigators wrote, is that it induces hunger, so few patients stick to it.
“Therefore, calorie-unrestricted diets may be a better alternative to achieve long-term maintenance,” Dr. Hansen and colleagues wrote, noting that this approach “is not widely investigated.”
Study methods and results
In the new study, participants were randomized in a 2:1 ratio to follow the LCHF or HCLF diet for 6 months, with no restriction on calorie intake. Patients were evaluated at baseline, 3 months, 6 months, and 9 months (3 months after discontinuation). Parameters included glycemic control, serum lipid levels, and metabolic markers. The final analysis included 165 patients.
While patients in both groups lost weight, those in the LCHF group lost, on average, about 8 pounds more than the HCLF group, a significant difference. While the LCHF diet was associated with greater improvements in glycemic control (HbA1c) than the HCLF diet, it also led to slightly greater increases in LDL levels. In both groups, HDL levels increased, and triglycerides decreased, without significant differences between groups.
The above changes were not sustained 3 months after finishing the diet.
“I believe we have sufficient data to include LCHF as one of the diet options for people with type 2 diabetes,” Dr. Hansen said in a written comment, considering all available data.
Although the diet did lead to significant clinical benefits, she predicted that some patients would still struggle with adherence in the real world.
“The LCHF diet can be difficult for some people to follow,” Dr. Hansen said. “It is a bit more expensive, and it can be difficult to comply to in social gatherings, simply because our society is not suited for this type of diet.”
The magic of unrestricted calories
Jay H. Shubrook, DO, diabetologist and professor at Touro University of California, Vallejo, offered a similar view.
“When you start to fiddle with the diet, it affects not only the person, but all the people they eat with, because eating is a communal experience,” Dr. Shubrook said, in an interview.
Still, he said the present study is “a big deal,” because T2D is a “noncommunicable pandemic,” and “anything we could do that disrupts this process is very important.”
While some may struggle to follow the LCHF diet, Dr. Shubrook predicted better long-term adherence than the low-calorie diet usually recommended.
“What’s magic about this study is because it wasn’t calorie restricted, I think it made it a little bit more flexible for people to continue,” Dr. Shubrook said.
He added that he thinks patients will need a fair amount of coaching and education about food choices in order to lose weight on a diet without calorie restrictions.
Not the first study of its kind
In a written comment, Jeff Volek, PhD, RD, professor at the Ohio State University, Columbus, called the present study “another important piece of work, demonstrating yet again, that a low-carbohydrate eating pattern is superior to a high-carbohydrate approach in people with insulin resistance.”
Yet Dr. Volek, who has conducted numerous studies on low-carbohydrate diets, also said there is “little here that is new or surprising.”
He went on to admonish Dr. Hansen and colleagues for failing to recognize those who have already broken ground in this area.
“Unfortunately, these authors do not give credit to the many researchers who have published extensively on low-carbohydrate diets in the past, and instead make claims about being the first to study a calorie unrestricted low-carb diet in individuals with T2D, which is clearly not the case,” Dr. Volek said. “There is a large body of literature showing similar findings with better control over diet, larger cohorts, longer follow-up, and more comprehensive biomarker assessment.”
He noted that data supporting low-carb diets for T2D have been sufficient since at least 2019, when the American Diabetes Association updated their guidance on the subject.
Citing a paper published in Diabetes Care, he said, “Low-carbohydrate eating patterns, especially very-low-carbohydrate eating patterns, have been shown to reduce A1C and the need for antihyperglycemic medications.”
The study was funded by Novo Nordisk Foundation, Danish Diabetes Academy, Odense University Hospital, and others. The investigators disclosed additional relationships with Eli Lilly, Amgen, UCB, and others. Dr. Shubrook disclosed relationships with Abbot, AstraZeneca, Bayer, and others.
This was true regardless of an individual’s calorie intake, in the randomized controlled trial published in the Annals of Internal Medicine.
Patients with T2D who ate a low-carb, high-fat diet (LCHF) lost more weight and saw greater improvements in both glycemic control and insulin resistance than those who ate a high-carb, low-fat diet (HCLF), reported lead author Camilla Dalby Hansen, MD, of University of Southern Denmark, Odense, and colleagues, suggesting that this is an effective, nonpharmaceutical treatment option for T2D.
The trial enrolled 185 patients with T2D, for whom low-calorie diets are often recommended to induce weight loss and improve glycemic control.
The trouble with this common recommendation, the investigators wrote, is that it induces hunger, so few patients stick to it.
“Therefore, calorie-unrestricted diets may be a better alternative to achieve long-term maintenance,” Dr. Hansen and colleagues wrote, noting that this approach “is not widely investigated.”
Study methods and results
In the new study, participants were randomized in a 2:1 ratio to follow the LCHF or HCLF diet for 6 months, with no restriction on calorie intake. Patients were evaluated at baseline, 3 months, 6 months, and 9 months (3 months after discontinuation). Parameters included glycemic control, serum lipid levels, and metabolic markers. The final analysis included 165 patients.
While patients in both groups lost weight, those in the LCHF group lost, on average, about 8 pounds more than the HCLF group, a significant difference. While the LCHF diet was associated with greater improvements in glycemic control (HbA1c) than the HCLF diet, it also led to slightly greater increases in LDL levels. In both groups, HDL levels increased, and triglycerides decreased, without significant differences between groups.
The above changes were not sustained 3 months after finishing the diet.
“I believe we have sufficient data to include LCHF as one of the diet options for people with type 2 diabetes,” Dr. Hansen said in a written comment, considering all available data.
Although the diet did lead to significant clinical benefits, she predicted that some patients would still struggle with adherence in the real world.
“The LCHF diet can be difficult for some people to follow,” Dr. Hansen said. “It is a bit more expensive, and it can be difficult to comply to in social gatherings, simply because our society is not suited for this type of diet.”
The magic of unrestricted calories
Jay H. Shubrook, DO, diabetologist and professor at Touro University of California, Vallejo, offered a similar view.
“When you start to fiddle with the diet, it affects not only the person, but all the people they eat with, because eating is a communal experience,” Dr. Shubrook said, in an interview.
Still, he said the present study is “a big deal,” because T2D is a “noncommunicable pandemic,” and “anything we could do that disrupts this process is very important.”
While some may struggle to follow the LCHF diet, Dr. Shubrook predicted better long-term adherence than the low-calorie diet usually recommended.
“What’s magic about this study is because it wasn’t calorie restricted, I think it made it a little bit more flexible for people to continue,” Dr. Shubrook said.
He added that he thinks patients will need a fair amount of coaching and education about food choices in order to lose weight on a diet without calorie restrictions.
Not the first study of its kind
In a written comment, Jeff Volek, PhD, RD, professor at the Ohio State University, Columbus, called the present study “another important piece of work, demonstrating yet again, that a low-carbohydrate eating pattern is superior to a high-carbohydrate approach in people with insulin resistance.”
Yet Dr. Volek, who has conducted numerous studies on low-carbohydrate diets, also said there is “little here that is new or surprising.”
He went on to admonish Dr. Hansen and colleagues for failing to recognize those who have already broken ground in this area.
“Unfortunately, these authors do not give credit to the many researchers who have published extensively on low-carbohydrate diets in the past, and instead make claims about being the first to study a calorie unrestricted low-carb diet in individuals with T2D, which is clearly not the case,” Dr. Volek said. “There is a large body of literature showing similar findings with better control over diet, larger cohorts, longer follow-up, and more comprehensive biomarker assessment.”
He noted that data supporting low-carb diets for T2D have been sufficient since at least 2019, when the American Diabetes Association updated their guidance on the subject.
Citing a paper published in Diabetes Care, he said, “Low-carbohydrate eating patterns, especially very-low-carbohydrate eating patterns, have been shown to reduce A1C and the need for antihyperglycemic medications.”
The study was funded by Novo Nordisk Foundation, Danish Diabetes Academy, Odense University Hospital, and others. The investigators disclosed additional relationships with Eli Lilly, Amgen, UCB, and others. Dr. Shubrook disclosed relationships with Abbot, AstraZeneca, Bayer, and others.
This was true regardless of an individual’s calorie intake, in the randomized controlled trial published in the Annals of Internal Medicine.
Patients with T2D who ate a low-carb, high-fat diet (LCHF) lost more weight and saw greater improvements in both glycemic control and insulin resistance than those who ate a high-carb, low-fat diet (HCLF), reported lead author Camilla Dalby Hansen, MD, of University of Southern Denmark, Odense, and colleagues, suggesting that this is an effective, nonpharmaceutical treatment option for T2D.
The trial enrolled 185 patients with T2D, for whom low-calorie diets are often recommended to induce weight loss and improve glycemic control.
The trouble with this common recommendation, the investigators wrote, is that it induces hunger, so few patients stick to it.
“Therefore, calorie-unrestricted diets may be a better alternative to achieve long-term maintenance,” Dr. Hansen and colleagues wrote, noting that this approach “is not widely investigated.”
Study methods and results
In the new study, participants were randomized in a 2:1 ratio to follow the LCHF or HCLF diet for 6 months, with no restriction on calorie intake. Patients were evaluated at baseline, 3 months, 6 months, and 9 months (3 months after discontinuation). Parameters included glycemic control, serum lipid levels, and metabolic markers. The final analysis included 165 patients.
While patients in both groups lost weight, those in the LCHF group lost, on average, about 8 pounds more than the HCLF group, a significant difference. While the LCHF diet was associated with greater improvements in glycemic control (HbA1c) than the HCLF diet, it also led to slightly greater increases in LDL levels. In both groups, HDL levels increased, and triglycerides decreased, without significant differences between groups.
The above changes were not sustained 3 months after finishing the diet.
“I believe we have sufficient data to include LCHF as one of the diet options for people with type 2 diabetes,” Dr. Hansen said in a written comment, considering all available data.
Although the diet did lead to significant clinical benefits, she predicted that some patients would still struggle with adherence in the real world.
“The LCHF diet can be difficult for some people to follow,” Dr. Hansen said. “It is a bit more expensive, and it can be difficult to comply to in social gatherings, simply because our society is not suited for this type of diet.”
The magic of unrestricted calories
Jay H. Shubrook, DO, diabetologist and professor at Touro University of California, Vallejo, offered a similar view.
“When you start to fiddle with the diet, it affects not only the person, but all the people they eat with, because eating is a communal experience,” Dr. Shubrook said, in an interview.
Still, he said the present study is “a big deal,” because T2D is a “noncommunicable pandemic,” and “anything we could do that disrupts this process is very important.”
While some may struggle to follow the LCHF diet, Dr. Shubrook predicted better long-term adherence than the low-calorie diet usually recommended.
“What’s magic about this study is because it wasn’t calorie restricted, I think it made it a little bit more flexible for people to continue,” Dr. Shubrook said.
He added that he thinks patients will need a fair amount of coaching and education about food choices in order to lose weight on a diet without calorie restrictions.
Not the first study of its kind
In a written comment, Jeff Volek, PhD, RD, professor at the Ohio State University, Columbus, called the present study “another important piece of work, demonstrating yet again, that a low-carbohydrate eating pattern is superior to a high-carbohydrate approach in people with insulin resistance.”
Yet Dr. Volek, who has conducted numerous studies on low-carbohydrate diets, also said there is “little here that is new or surprising.”
He went on to admonish Dr. Hansen and colleagues for failing to recognize those who have already broken ground in this area.
“Unfortunately, these authors do not give credit to the many researchers who have published extensively on low-carbohydrate diets in the past, and instead make claims about being the first to study a calorie unrestricted low-carb diet in individuals with T2D, which is clearly not the case,” Dr. Volek said. “There is a large body of literature showing similar findings with better control over diet, larger cohorts, longer follow-up, and more comprehensive biomarker assessment.”
He noted that data supporting low-carb diets for T2D have been sufficient since at least 2019, when the American Diabetes Association updated their guidance on the subject.
Citing a paper published in Diabetes Care, he said, “Low-carbohydrate eating patterns, especially very-low-carbohydrate eating patterns, have been shown to reduce A1C and the need for antihyperglycemic medications.”
The study was funded by Novo Nordisk Foundation, Danish Diabetes Academy, Odense University Hospital, and others. The investigators disclosed additional relationships with Eli Lilly, Amgen, UCB, and others. Dr. Shubrook disclosed relationships with Abbot, AstraZeneca, Bayer, and others.
FROM ANNALS OF INTERNAL MEDICINE