User login
Fast Track to Abdominal Pain
ANSWER
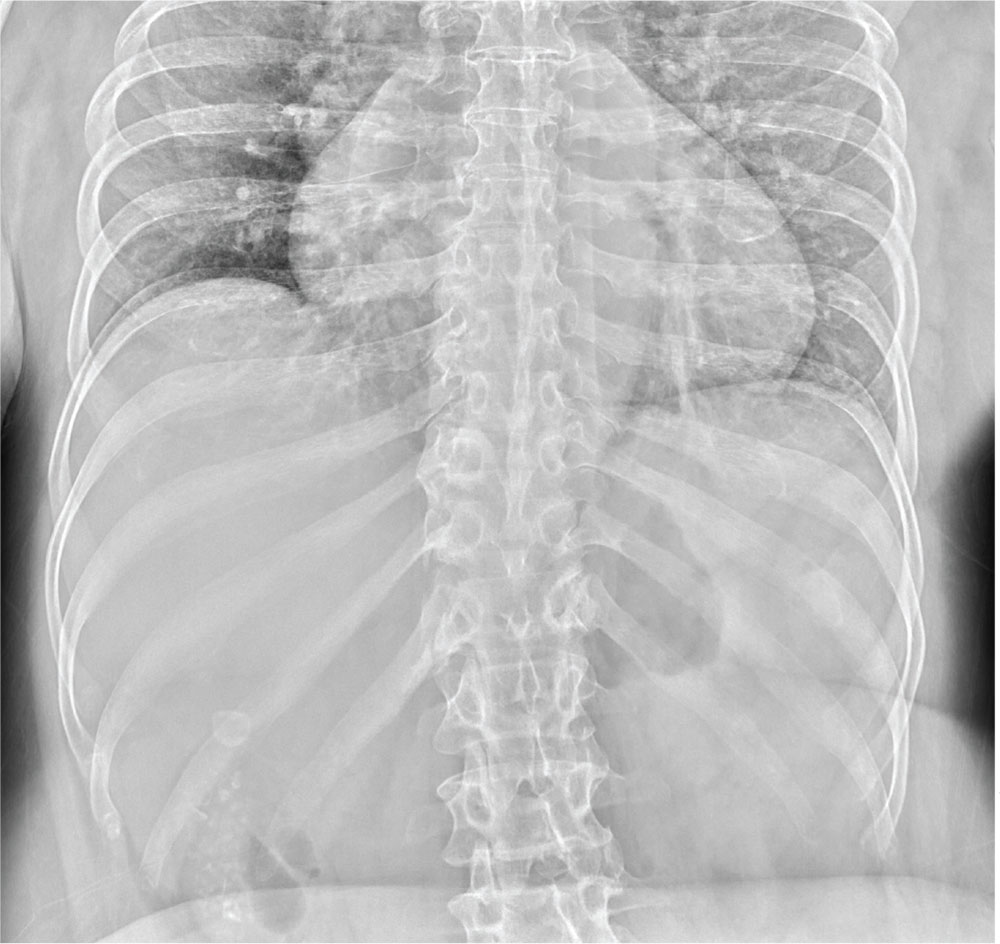
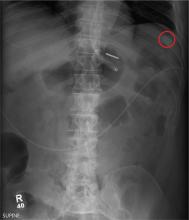
The radiograph illustrates a large calcification within the right upper quadrant—most likely a gallstone. Several smaller calcifications are clustered together in the same area, making the diagnosis cholelithiasis. The patient was referred to the general surgery clinic for further
evaluation.
For recent findings on gallstone disease and heart risk, see here.

ANSWER
The radiograph illustrates a large calcification within the right upper quadrant—most likely a gallstone. Several smaller calcifications are clustered together in the same area, making the diagnosis cholelithiasis. The patient was referred to the general surgery clinic for further
evaluation.
For recent findings on gallstone disease and heart risk, see here.

ANSWER
The radiograph illustrates a large calcification within the right upper quadrant—most likely a gallstone. Several smaller calcifications are clustered together in the same area, making the diagnosis cholelithiasis. The patient was referred to the general surgery clinic for further
evaluation.
For recent findings on gallstone disease and heart risk, see here.
An NP student you are precepting in the emergency department fast track area presents her patient to you: a 60-year-old woman with abdominal pain. The pain is chronic but has worsened slightly, prompting the patient, who does not have a primary care provider, to present today. She experiences occasional nausea but no fever, and she denies any bowel or bladder complaints other than constipation. Her medical history is significant for mild hypertension.
On exam, your student notes an obese female who is in no obvious distress. The patient’s vital signs are all within normal limits. The abdominal exam is unimpressive, revealing a soft abdomen with good bowel sounds. Although she does have mild diffuse tenderness, she has no rebound or guarding.
Although your student suspects the patient is just constipated, she orders blood work and urinalysis. An abdominal survey is obtained as well. What is your impression?
Woman, 36, With Fever and Malaise
IN THIS ARTICLE
- Clinical presentation and evaluation
- Terminology table
- Outcome for the case patient
A 36-year-old Bengali woman with a history of well-controlled diabetes presents to the emergency department with complaints of feeling “unwell” for about two weeks. She does not speak English, and a hospital-provided phone translator is used to obtain history and explain hospital course. The patient is vague regarding symptomatology, describing general malaise and tiredness. She says she became “much worse” two days ago and has shaking chills, sore throat, headache, and nonproductive cough, but she denies shortness of breath or chest pain. She also developed nausea and vomiting, stating, “I can’t keep anything down.”
She has not recently traveled out of the country and has no known sick contacts. Influenza activity is high in the area, and the patient has not received immunization. She had a “normal” menstrual period two weeks ago and firmly states, “There is no way I can be pregnant.” She admits to vaginal “spotting” off and on for the past two weeks without abdominal pain. She is married with six children and has no history of miscarriage, ectopic pregnancy, or induced abortion; she is not taking any form of birth control.
On exam, the patient is tachycardic, with a heart rate of 127 beats/min, and has a fever of 103.3°F. Blood pressure, respiratory rate, and pulse oximetry are normal. She appears unwell and dehydrated. Her mucous membranes are dry, but no skin rash is noted. There is no tonsillar swelling or exudate and no meningismus; the lung exam is clear, with no adventitious sounds. Abdominal exam demonstrates mild, generalized tenderness in the lower abdomen without peritoneal signs. No costovertebral angle tenderness is noted. Initial diagnostic considerations include sources of fever (eg, influenza, pneumonia, urinary tract infection, viral illness), or abdominal sources, such as appendicitis.
An upright anteroposterior chest x-ray shows no infiltrate, pleural effusion, or cardiomegaly. Laboratory results include a high white blood cell (WBC) count (16.9 k/mm3) with bandemia and normal electrolytes without anion gap. Rapid influenza A and B testing is negative. A urine pregnancy test is positive, and the urinalysis shows no infection but +2 ketones. Rh factor is positive. A serum quantitative β-hCG is 130,581 mIU/mL. Blood cultures are obtained, but results are not available.
Due to cultural differences, the patient is very reluctant to consent to a pelvic exam. After extensive counseling, she agrees to a bimanual exam only. The uterus is boggy and enlarged to about 12 weeks. There is exquisite uterine tenderness and purulent discharge on the gloved finger. The cervical os is closed, and there is scant bleeding.
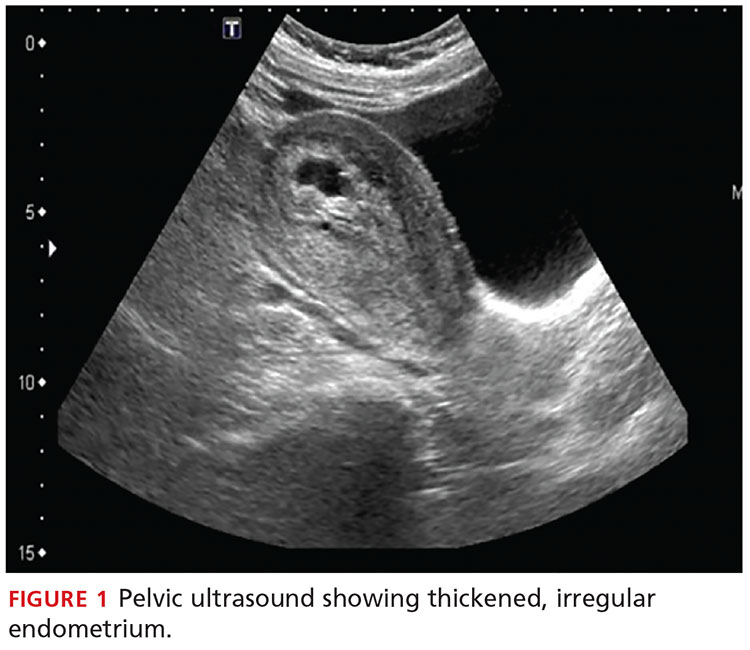
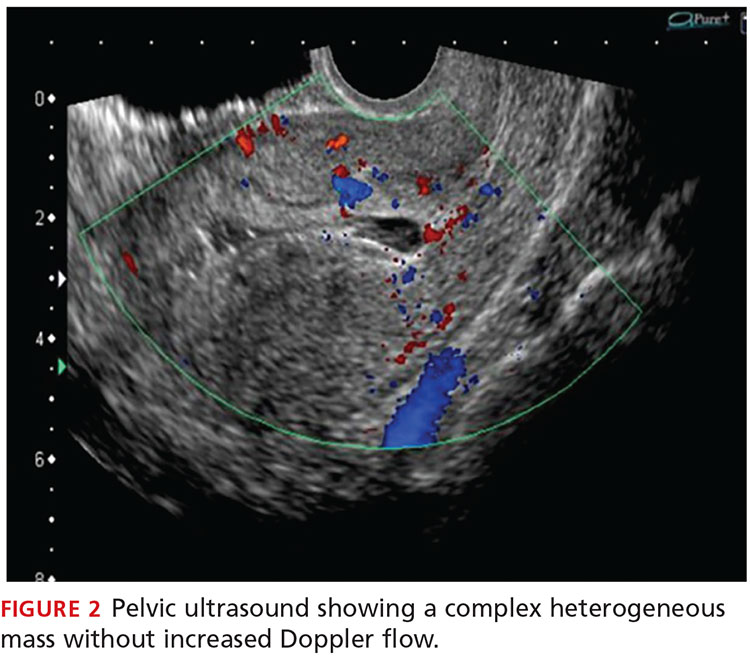
A transvaginal ultrasound is obtained; it reveals a thickened endometrium with echogenicity, without increased vascularity, and no identifiable intrauterine pregnancy. The adnexa have no masses, and there is no free fluid in the endometrium (see Figures 1 and 2).
The patient is given broad-spectrum antibiotics and urgently transported to the operating room by Ob-Gyn for uterine evacuation. She is found to have a septic abortion due to retained products of conception (RPOC) from an incomplete miscarriage.
Continue for discussion >>
DISCUSSIONIt is not uncommon for a woman to miscarry a very early pregnancy and not realize she had been pregnant.1 Many attribute it to a “heavy” or unusual period. In one study, 11% of patients who denied the possibility of pregnancy were, in fact, pregnant.2
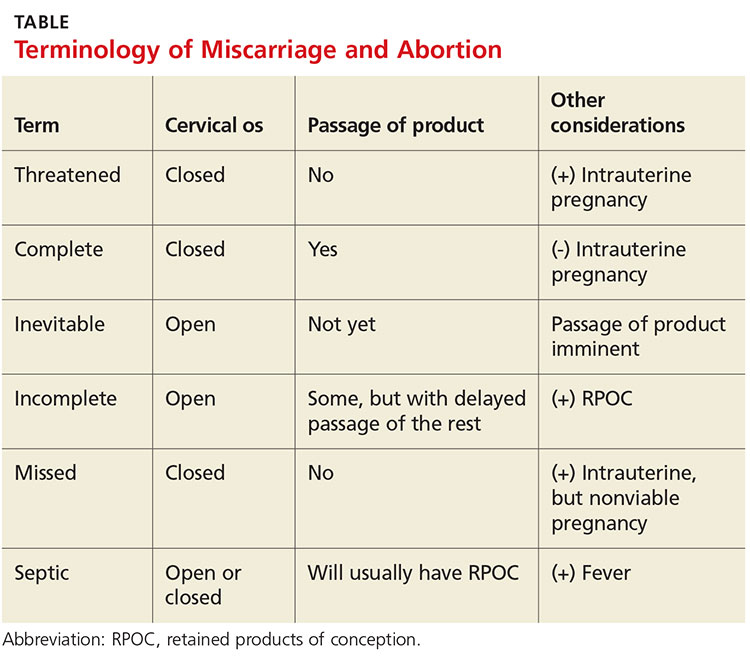
Miscarriage is a frequent outcome of early pregnancy; it is estimated that 11% to 20% of early pregnancies result in a spontaneous miscarriage.3-5 Most resolve without complications, but risk increases with gestational age. When they do occur, complications include RPOC, heavy prolonged bleeding, and endometritis. RPOC refers to placental or fetal tissue that remains in the uterus after a miscarriage, surgical abortion, or preterm/term delivery (see Table for additional terminology related to miscarriage and abortion). Because of increased morbidity, it is important to suspect RPOC after a known miscarriage or an induced abortion, or in a pregnant patient with bleeding.
Incidence and pathophysiologySeptic abortion is a relatively rare complication of miscarriage. It can refer to a spontaneous miscarriage complicated by a subsequent intrauterine infection, often caused by RPOC. Septic abortion is much more common after an induced abortion, in which there is instrumentation of the uterus.
The infection after a spontaneous miscarriage usually begins as endometritis. It involves the necrotic RPOC, which are prone to infection by the cervical and vaginal flora. It may spread further into the parametrium/myometrium and the peritoneal cavity. The infection may then progress to bacteremia and sepsis. Typical causative organisms include Escherichia coli, Enterobacter aerogenes, Proteus vulgaris, hemolytic streptococci, staphylococci, and some anaerobic organisms, including Clostridium perfringens.3
Death, although rare in developed countries, is usually secondary to the sequela of sepsis, including septic shock, renal failure, adult respiratory distress syndrome, and disseminated intravascular coagulation.3,6,7 Pelvic adhesions and hysterectomy are also possible outcomes of a septic abortion.
Continue for clinical presentation and evaluation >>
Clinical presentation and evaluationMany findings suggestive of septic abortion are nonspecific, such as bleeding, pain, uterine tenderness, and fever. A combination of historical risk, physical exam, and laboratory and ultrasound findings will often be needed to confirm the diagnosis.
Fever is never to be expected in an uncomplicated miscarriage. Vaginal bleeding and some cramping are common after miscarriage; women will bleed, on average, between eight and 11 days afterward.5 Women who fall outside the normal range and experience prolonged bleeding, heavy bleeding, or severe abdominal pain should be evaluated.
A workup for patients with a possible septic abortion should include a complete blood count, blood culture with additional laboratory investigation if there is concern for bacteremia/sepsis, and type and screen for Rh factor and for possible blood transfusion, if needed.
All patients with postabortion complications should be screened for Rh factor; Rho(D) immune globulin (RhoGAM) should be administered if results indicate that the patient is Rh-negative and unsensitized. A quantitative β-hCG level can be obtained to confirm pregnancy. A single measurement will not be helpful; β-hCG can remain positive for weeks after an uncomplicated miscarriage. On the other hand, a low level does not exclude RPOC—the RPOC, if necrotic, may remain in the uterus without secreting hormone. The trend of β-hCG over time can be helpful if the diagnosis is unclear.
A careful physical exam, including a pelvic exam, should be performed. Assess for uterine tenderness, peritoneal signs, and purulent discharge from the cervix. An open cervical os is suggestive of RPOC, as the cervix closes quickly after a complete miscarriage, but a closed cervical os does not exclude the possibility of RPOC or septic abortion. The amount of bleeding should be noted, along with any tissue or clots within the vaginal vault or cervix.
A pelvic ultrasound should be obtained in all patients concerning for a septic incomplete miscarriage. Ultrasound findings can be nonspecific, because small amounts of retained tissue can look like blood (a common finding after miscarriage). Ultrasound findings of heterogeneous, echogenic material within the uterus or a thick, irregular endometrium support a diagnosis of RPOC in patients considered at risk.8,9 Increased color Doppler flow is often seen with RPOC, but there may be decreased flow in the case of necrotic RPOC. Ultrasound findings consistent with RPOC in a febrile, ill patient suggest a septic abortion.
Continue for treatment and prognosis >>
Treatment and prognosisPatients with a septic abortion require immediate evacuation of the uterus to prevent deadly complications; antibiotics may not be able to perfuse to the necrotic source of infection.10 Suction curettage is less likely than sharp curettage to cause perforation.
Broad-spectrum antibiotics should be administered. The bacteria associated with a septic incomplete miscarriage are usually polymicrobial and represent the normal flora of the vagina and cervix. The choice of agents recommended is usually the same as for pelvic inflammatory disease.11
The treatment regimen typically includes clindamycin (900 mg IV q8h), plus gentamicin (5 mg/kg IV once a day), with or without ampicillin (2 g IV q4h).11,12 Alternatively, a combination of ampicillin, gentamicin, and metronidazole (500 mg IV q8h) can be used.
Further surgery, including laparotomy and possible hysterectomy, is indicated in patients who do not respond to uterine evacuation and parenteral antibiotics. Other possible complications requiring surgery include pelvic abscess, necrotizing Clostridium infections in the myometrium, and uterine perforation.
OUTCOME FOR THE CASE PATIENTThe patient was started on IV ampicillin, gentamicin, and clindamycin and taken promptly for a suction dilation and curettage. Pathology later showed a gestational sac with severe acute necrotizing chorioamnionitis and extensive bacterial growth. This confirmed the diagnosis of a septic, incomplete miscarriage.
Blood cultures remained without any growth, and the patient was afebrile on the second postop day. The WBC count and β-hCG level trended downward.
The patient was discharged on a 14-day course of oral doxycycline and metronidazole. She was then lost to further follow-up.
CONCLUSIONThe differential diagnosis in this ill, febrile patient was initially very broad. The importance of suspecting pregnancy in all women of childbearing age, especially those not using contraception, cannot be underestimated. The accuracy of patient history and recall of last menstrual period in determining the possibility of pregnancy is not sufficiently reliable.
1. Promislow JH, Baird DD, Wilcox AJ, et al. Bleeding following pregnancy loss prior to six weeks gestation. Hum Reprod. 2007;22(3):853-857.
2. Ramoska EA, Sacchetti AD, Nepp M. Reliability of patient history in determining the possibility of pregnancy. Ann Emerg Med. 1989;18(1):48-50.
3. Osazuwa H, Aziken M. Septic abortion: a review of social and demographic characteristics. Arch Gynecol Obstet. 2007;275(2):117-119.
4. Hure AJ, Powers JR, Mishra GD, et al. Miscarriage, preterm delivery, and stillbirth: large variations in rates within a cohort of Australian women. PLoS One. 2012;7(5):e37109.
5. Nielsen S, Hahlin M. Expectant management of first-trimester spontaneous abortion. Lancet. 1995;345(8942):84-86.
6. Eschenbach DA. Treating spontaneous and induced septic abortions. Obstet Gynecol. 2015;125(5):1042-1048.
7. Rana A, Pradhan N, Gurung G, Singh M. Induced septic abortion: a major factor in maternal mortality and morbidity. J Obstet Gynaecol Res. 2004;30(1):3-8.
8. Abbasi S, Jamal A, Eslamian L, Marsousi V. Role of clinical and ultrasound findings in the diagnosis of retained products of conception. Ultrasound Obstet Gynecol. 2008;32(5):704-707.
9. Esmaeillou H, Jamal A, Eslamian L, et al. Accurate detection of retained products of conception after first- and second-trimester abortion by color doppler sonography. J Med Ultrasound. 2015;23(7):34-38.
10. Finkielman JD, De Feo FD, Heller PG, Afessa B. The clinical course of patients with septic abortion admitted to an intensive care unit. Intensive Care Med. 2004;30(6):1097-1102.
11. CDC. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
12. Mackeen AD, Packard RE, Ota E, Speer L. Antibiotic regimens for postpartum endometritis. Cochrane Database Syst Rev. 2015;2:CD001067.
IN THIS ARTICLE
- Clinical presentation and evaluation
- Terminology table
- Outcome for the case patient
A 36-year-old Bengali woman with a history of well-controlled diabetes presents to the emergency department with complaints of feeling “unwell” for about two weeks. She does not speak English, and a hospital-provided phone translator is used to obtain history and explain hospital course. The patient is vague regarding symptomatology, describing general malaise and tiredness. She says she became “much worse” two days ago and has shaking chills, sore throat, headache, and nonproductive cough, but she denies shortness of breath or chest pain. She also developed nausea and vomiting, stating, “I can’t keep anything down.”
She has not recently traveled out of the country and has no known sick contacts. Influenza activity is high in the area, and the patient has not received immunization. She had a “normal” menstrual period two weeks ago and firmly states, “There is no way I can be pregnant.” She admits to vaginal “spotting” off and on for the past two weeks without abdominal pain. She is married with six children and has no history of miscarriage, ectopic pregnancy, or induced abortion; she is not taking any form of birth control.
On exam, the patient is tachycardic, with a heart rate of 127 beats/min, and has a fever of 103.3°F. Blood pressure, respiratory rate, and pulse oximetry are normal. She appears unwell and dehydrated. Her mucous membranes are dry, but no skin rash is noted. There is no tonsillar swelling or exudate and no meningismus; the lung exam is clear, with no adventitious sounds. Abdominal exam demonstrates mild, generalized tenderness in the lower abdomen without peritoneal signs. No costovertebral angle tenderness is noted. Initial diagnostic considerations include sources of fever (eg, influenza, pneumonia, urinary tract infection, viral illness), or abdominal sources, such as appendicitis.
An upright anteroposterior chest x-ray shows no infiltrate, pleural effusion, or cardiomegaly. Laboratory results include a high white blood cell (WBC) count (16.9 k/mm3) with bandemia and normal electrolytes without anion gap. Rapid influenza A and B testing is negative. A urine pregnancy test is positive, and the urinalysis shows no infection but +2 ketones. Rh factor is positive. A serum quantitative β-hCG is 130,581 mIU/mL. Blood cultures are obtained, but results are not available.
Due to cultural differences, the patient is very reluctant to consent to a pelvic exam. After extensive counseling, she agrees to a bimanual exam only. The uterus is boggy and enlarged to about 12 weeks. There is exquisite uterine tenderness and purulent discharge on the gloved finger. The cervical os is closed, and there is scant bleeding.
A transvaginal ultrasound is obtained; it reveals a thickened endometrium with echogenicity, without increased vascularity, and no identifiable intrauterine pregnancy. The adnexa have no masses, and there is no free fluid in the endometrium (see Figures 1 and 2).
The patient is given broad-spectrum antibiotics and urgently transported to the operating room by Ob-Gyn for uterine evacuation. She is found to have a septic abortion due to retained products of conception (RPOC) from an incomplete miscarriage.
Continue for discussion >>
DISCUSSIONIt is not uncommon for a woman to miscarry a very early pregnancy and not realize she had been pregnant.1 Many attribute it to a “heavy” or unusual period. In one study, 11% of patients who denied the possibility of pregnancy were, in fact, pregnant.2
Miscarriage is a frequent outcome of early pregnancy; it is estimated that 11% to 20% of early pregnancies result in a spontaneous miscarriage.3-5 Most resolve without complications, but risk increases with gestational age. When they do occur, complications include RPOC, heavy prolonged bleeding, and endometritis. RPOC refers to placental or fetal tissue that remains in the uterus after a miscarriage, surgical abortion, or preterm/term delivery (see Table for additional terminology related to miscarriage and abortion). Because of increased morbidity, it is important to suspect RPOC after a known miscarriage or an induced abortion, or in a pregnant patient with bleeding.
Incidence and pathophysiologySeptic abortion is a relatively rare complication of miscarriage. It can refer to a spontaneous miscarriage complicated by a subsequent intrauterine infection, often caused by RPOC. Septic abortion is much more common after an induced abortion, in which there is instrumentation of the uterus.
The infection after a spontaneous miscarriage usually begins as endometritis. It involves the necrotic RPOC, which are prone to infection by the cervical and vaginal flora. It may spread further into the parametrium/myometrium and the peritoneal cavity. The infection may then progress to bacteremia and sepsis. Typical causative organisms include Escherichia coli, Enterobacter aerogenes, Proteus vulgaris, hemolytic streptococci, staphylococci, and some anaerobic organisms, including Clostridium perfringens.3
Death, although rare in developed countries, is usually secondary to the sequela of sepsis, including septic shock, renal failure, adult respiratory distress syndrome, and disseminated intravascular coagulation.3,6,7 Pelvic adhesions and hysterectomy are also possible outcomes of a septic abortion.
Continue for clinical presentation and evaluation >>
Clinical presentation and evaluationMany findings suggestive of septic abortion are nonspecific, such as bleeding, pain, uterine tenderness, and fever. A combination of historical risk, physical exam, and laboratory and ultrasound findings will often be needed to confirm the diagnosis.
Fever is never to be expected in an uncomplicated miscarriage. Vaginal bleeding and some cramping are common after miscarriage; women will bleed, on average, between eight and 11 days afterward.5 Women who fall outside the normal range and experience prolonged bleeding, heavy bleeding, or severe abdominal pain should be evaluated.
A workup for patients with a possible septic abortion should include a complete blood count, blood culture with additional laboratory investigation if there is concern for bacteremia/sepsis, and type and screen for Rh factor and for possible blood transfusion, if needed.
All patients with postabortion complications should be screened for Rh factor; Rho(D) immune globulin (RhoGAM) should be administered if results indicate that the patient is Rh-negative and unsensitized. A quantitative β-hCG level can be obtained to confirm pregnancy. A single measurement will not be helpful; β-hCG can remain positive for weeks after an uncomplicated miscarriage. On the other hand, a low level does not exclude RPOC—the RPOC, if necrotic, may remain in the uterus without secreting hormone. The trend of β-hCG over time can be helpful if the diagnosis is unclear.
A careful physical exam, including a pelvic exam, should be performed. Assess for uterine tenderness, peritoneal signs, and purulent discharge from the cervix. An open cervical os is suggestive of RPOC, as the cervix closes quickly after a complete miscarriage, but a closed cervical os does not exclude the possibility of RPOC or septic abortion. The amount of bleeding should be noted, along with any tissue or clots within the vaginal vault or cervix.
A pelvic ultrasound should be obtained in all patients concerning for a septic incomplete miscarriage. Ultrasound findings can be nonspecific, because small amounts of retained tissue can look like blood (a common finding after miscarriage). Ultrasound findings of heterogeneous, echogenic material within the uterus or a thick, irregular endometrium support a diagnosis of RPOC in patients considered at risk.8,9 Increased color Doppler flow is often seen with RPOC, but there may be decreased flow in the case of necrotic RPOC. Ultrasound findings consistent with RPOC in a febrile, ill patient suggest a septic abortion.
Continue for treatment and prognosis >>
Treatment and prognosisPatients with a septic abortion require immediate evacuation of the uterus to prevent deadly complications; antibiotics may not be able to perfuse to the necrotic source of infection.10 Suction curettage is less likely than sharp curettage to cause perforation.
Broad-spectrum antibiotics should be administered. The bacteria associated with a septic incomplete miscarriage are usually polymicrobial and represent the normal flora of the vagina and cervix. The choice of agents recommended is usually the same as for pelvic inflammatory disease.11
The treatment regimen typically includes clindamycin (900 mg IV q8h), plus gentamicin (5 mg/kg IV once a day), with or without ampicillin (2 g IV q4h).11,12 Alternatively, a combination of ampicillin, gentamicin, and metronidazole (500 mg IV q8h) can be used.
Further surgery, including laparotomy and possible hysterectomy, is indicated in patients who do not respond to uterine evacuation and parenteral antibiotics. Other possible complications requiring surgery include pelvic abscess, necrotizing Clostridium infections in the myometrium, and uterine perforation.
OUTCOME FOR THE CASE PATIENTThe patient was started on IV ampicillin, gentamicin, and clindamycin and taken promptly for a suction dilation and curettage. Pathology later showed a gestational sac with severe acute necrotizing chorioamnionitis and extensive bacterial growth. This confirmed the diagnosis of a septic, incomplete miscarriage.
Blood cultures remained without any growth, and the patient was afebrile on the second postop day. The WBC count and β-hCG level trended downward.
The patient was discharged on a 14-day course of oral doxycycline and metronidazole. She was then lost to further follow-up.
CONCLUSIONThe differential diagnosis in this ill, febrile patient was initially very broad. The importance of suspecting pregnancy in all women of childbearing age, especially those not using contraception, cannot be underestimated. The accuracy of patient history and recall of last menstrual period in determining the possibility of pregnancy is not sufficiently reliable.
IN THIS ARTICLE
- Clinical presentation and evaluation
- Terminology table
- Outcome for the case patient
A 36-year-old Bengali woman with a history of well-controlled diabetes presents to the emergency department with complaints of feeling “unwell” for about two weeks. She does not speak English, and a hospital-provided phone translator is used to obtain history and explain hospital course. The patient is vague regarding symptomatology, describing general malaise and tiredness. She says she became “much worse” two days ago and has shaking chills, sore throat, headache, and nonproductive cough, but she denies shortness of breath or chest pain. She also developed nausea and vomiting, stating, “I can’t keep anything down.”
She has not recently traveled out of the country and has no known sick contacts. Influenza activity is high in the area, and the patient has not received immunization. She had a “normal” menstrual period two weeks ago and firmly states, “There is no way I can be pregnant.” She admits to vaginal “spotting” off and on for the past two weeks without abdominal pain. She is married with six children and has no history of miscarriage, ectopic pregnancy, or induced abortion; she is not taking any form of birth control.
On exam, the patient is tachycardic, with a heart rate of 127 beats/min, and has a fever of 103.3°F. Blood pressure, respiratory rate, and pulse oximetry are normal. She appears unwell and dehydrated. Her mucous membranes are dry, but no skin rash is noted. There is no tonsillar swelling or exudate and no meningismus; the lung exam is clear, with no adventitious sounds. Abdominal exam demonstrates mild, generalized tenderness in the lower abdomen without peritoneal signs. No costovertebral angle tenderness is noted. Initial diagnostic considerations include sources of fever (eg, influenza, pneumonia, urinary tract infection, viral illness), or abdominal sources, such as appendicitis.
An upright anteroposterior chest x-ray shows no infiltrate, pleural effusion, or cardiomegaly. Laboratory results include a high white blood cell (WBC) count (16.9 k/mm3) with bandemia and normal electrolytes without anion gap. Rapid influenza A and B testing is negative. A urine pregnancy test is positive, and the urinalysis shows no infection but +2 ketones. Rh factor is positive. A serum quantitative β-hCG is 130,581 mIU/mL. Blood cultures are obtained, but results are not available.
Due to cultural differences, the patient is very reluctant to consent to a pelvic exam. After extensive counseling, she agrees to a bimanual exam only. The uterus is boggy and enlarged to about 12 weeks. There is exquisite uterine tenderness and purulent discharge on the gloved finger. The cervical os is closed, and there is scant bleeding.
A transvaginal ultrasound is obtained; it reveals a thickened endometrium with echogenicity, without increased vascularity, and no identifiable intrauterine pregnancy. The adnexa have no masses, and there is no free fluid in the endometrium (see Figures 1 and 2).
The patient is given broad-spectrum antibiotics and urgently transported to the operating room by Ob-Gyn for uterine evacuation. She is found to have a septic abortion due to retained products of conception (RPOC) from an incomplete miscarriage.
Continue for discussion >>
DISCUSSIONIt is not uncommon for a woman to miscarry a very early pregnancy and not realize she had been pregnant.1 Many attribute it to a “heavy” or unusual period. In one study, 11% of patients who denied the possibility of pregnancy were, in fact, pregnant.2
Miscarriage is a frequent outcome of early pregnancy; it is estimated that 11% to 20% of early pregnancies result in a spontaneous miscarriage.3-5 Most resolve without complications, but risk increases with gestational age. When they do occur, complications include RPOC, heavy prolonged bleeding, and endometritis. RPOC refers to placental or fetal tissue that remains in the uterus after a miscarriage, surgical abortion, or preterm/term delivery (see Table for additional terminology related to miscarriage and abortion). Because of increased morbidity, it is important to suspect RPOC after a known miscarriage or an induced abortion, or in a pregnant patient with bleeding.
Incidence and pathophysiologySeptic abortion is a relatively rare complication of miscarriage. It can refer to a spontaneous miscarriage complicated by a subsequent intrauterine infection, often caused by RPOC. Septic abortion is much more common after an induced abortion, in which there is instrumentation of the uterus.
The infection after a spontaneous miscarriage usually begins as endometritis. It involves the necrotic RPOC, which are prone to infection by the cervical and vaginal flora. It may spread further into the parametrium/myometrium and the peritoneal cavity. The infection may then progress to bacteremia and sepsis. Typical causative organisms include Escherichia coli, Enterobacter aerogenes, Proteus vulgaris, hemolytic streptococci, staphylococci, and some anaerobic organisms, including Clostridium perfringens.3
Death, although rare in developed countries, is usually secondary to the sequela of sepsis, including septic shock, renal failure, adult respiratory distress syndrome, and disseminated intravascular coagulation.3,6,7 Pelvic adhesions and hysterectomy are also possible outcomes of a septic abortion.
Continue for clinical presentation and evaluation >>
Clinical presentation and evaluationMany findings suggestive of septic abortion are nonspecific, such as bleeding, pain, uterine tenderness, and fever. A combination of historical risk, physical exam, and laboratory and ultrasound findings will often be needed to confirm the diagnosis.
Fever is never to be expected in an uncomplicated miscarriage. Vaginal bleeding and some cramping are common after miscarriage; women will bleed, on average, between eight and 11 days afterward.5 Women who fall outside the normal range and experience prolonged bleeding, heavy bleeding, or severe abdominal pain should be evaluated.
A workup for patients with a possible septic abortion should include a complete blood count, blood culture with additional laboratory investigation if there is concern for bacteremia/sepsis, and type and screen for Rh factor and for possible blood transfusion, if needed.
All patients with postabortion complications should be screened for Rh factor; Rho(D) immune globulin (RhoGAM) should be administered if results indicate that the patient is Rh-negative and unsensitized. A quantitative β-hCG level can be obtained to confirm pregnancy. A single measurement will not be helpful; β-hCG can remain positive for weeks after an uncomplicated miscarriage. On the other hand, a low level does not exclude RPOC—the RPOC, if necrotic, may remain in the uterus without secreting hormone. The trend of β-hCG over time can be helpful if the diagnosis is unclear.
A careful physical exam, including a pelvic exam, should be performed. Assess for uterine tenderness, peritoneal signs, and purulent discharge from the cervix. An open cervical os is suggestive of RPOC, as the cervix closes quickly after a complete miscarriage, but a closed cervical os does not exclude the possibility of RPOC or septic abortion. The amount of bleeding should be noted, along with any tissue or clots within the vaginal vault or cervix.
A pelvic ultrasound should be obtained in all patients concerning for a septic incomplete miscarriage. Ultrasound findings can be nonspecific, because small amounts of retained tissue can look like blood (a common finding after miscarriage). Ultrasound findings of heterogeneous, echogenic material within the uterus or a thick, irregular endometrium support a diagnosis of RPOC in patients considered at risk.8,9 Increased color Doppler flow is often seen with RPOC, but there may be decreased flow in the case of necrotic RPOC. Ultrasound findings consistent with RPOC in a febrile, ill patient suggest a septic abortion.
Continue for treatment and prognosis >>
Treatment and prognosisPatients with a septic abortion require immediate evacuation of the uterus to prevent deadly complications; antibiotics may not be able to perfuse to the necrotic source of infection.10 Suction curettage is less likely than sharp curettage to cause perforation.
Broad-spectrum antibiotics should be administered. The bacteria associated with a septic incomplete miscarriage are usually polymicrobial and represent the normal flora of the vagina and cervix. The choice of agents recommended is usually the same as for pelvic inflammatory disease.11
The treatment regimen typically includes clindamycin (900 mg IV q8h), plus gentamicin (5 mg/kg IV once a day), with or without ampicillin (2 g IV q4h).11,12 Alternatively, a combination of ampicillin, gentamicin, and metronidazole (500 mg IV q8h) can be used.
Further surgery, including laparotomy and possible hysterectomy, is indicated in patients who do not respond to uterine evacuation and parenteral antibiotics. Other possible complications requiring surgery include pelvic abscess, necrotizing Clostridium infections in the myometrium, and uterine perforation.
OUTCOME FOR THE CASE PATIENTThe patient was started on IV ampicillin, gentamicin, and clindamycin and taken promptly for a suction dilation and curettage. Pathology later showed a gestational sac with severe acute necrotizing chorioamnionitis and extensive bacterial growth. This confirmed the diagnosis of a septic, incomplete miscarriage.
Blood cultures remained without any growth, and the patient was afebrile on the second postop day. The WBC count and β-hCG level trended downward.
The patient was discharged on a 14-day course of oral doxycycline and metronidazole. She was then lost to further follow-up.
CONCLUSIONThe differential diagnosis in this ill, febrile patient was initially very broad. The importance of suspecting pregnancy in all women of childbearing age, especially those not using contraception, cannot be underestimated. The accuracy of patient history and recall of last menstrual period in determining the possibility of pregnancy is not sufficiently reliable.
1. Promislow JH, Baird DD, Wilcox AJ, et al. Bleeding following pregnancy loss prior to six weeks gestation. Hum Reprod. 2007;22(3):853-857.
2. Ramoska EA, Sacchetti AD, Nepp M. Reliability of patient history in determining the possibility of pregnancy. Ann Emerg Med. 1989;18(1):48-50.
3. Osazuwa H, Aziken M. Septic abortion: a review of social and demographic characteristics. Arch Gynecol Obstet. 2007;275(2):117-119.
4. Hure AJ, Powers JR, Mishra GD, et al. Miscarriage, preterm delivery, and stillbirth: large variations in rates within a cohort of Australian women. PLoS One. 2012;7(5):e37109.
5. Nielsen S, Hahlin M. Expectant management of first-trimester spontaneous abortion. Lancet. 1995;345(8942):84-86.
6. Eschenbach DA. Treating spontaneous and induced septic abortions. Obstet Gynecol. 2015;125(5):1042-1048.
7. Rana A, Pradhan N, Gurung G, Singh M. Induced septic abortion: a major factor in maternal mortality and morbidity. J Obstet Gynaecol Res. 2004;30(1):3-8.
8. Abbasi S, Jamal A, Eslamian L, Marsousi V. Role of clinical and ultrasound findings in the diagnosis of retained products of conception. Ultrasound Obstet Gynecol. 2008;32(5):704-707.
9. Esmaeillou H, Jamal A, Eslamian L, et al. Accurate detection of retained products of conception after first- and second-trimester abortion by color doppler sonography. J Med Ultrasound. 2015;23(7):34-38.
10. Finkielman JD, De Feo FD, Heller PG, Afessa B. The clinical course of patients with septic abortion admitted to an intensive care unit. Intensive Care Med. 2004;30(6):1097-1102.
11. CDC. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
12. Mackeen AD, Packard RE, Ota E, Speer L. Antibiotic regimens for postpartum endometritis. Cochrane Database Syst Rev. 2015;2:CD001067.
1. Promislow JH, Baird DD, Wilcox AJ, et al. Bleeding following pregnancy loss prior to six weeks gestation. Hum Reprod. 2007;22(3):853-857.
2. Ramoska EA, Sacchetti AD, Nepp M. Reliability of patient history in determining the possibility of pregnancy. Ann Emerg Med. 1989;18(1):48-50.
3. Osazuwa H, Aziken M. Septic abortion: a review of social and demographic characteristics. Arch Gynecol Obstet. 2007;275(2):117-119.
4. Hure AJ, Powers JR, Mishra GD, et al. Miscarriage, preterm delivery, and stillbirth: large variations in rates within a cohort of Australian women. PLoS One. 2012;7(5):e37109.
5. Nielsen S, Hahlin M. Expectant management of first-trimester spontaneous abortion. Lancet. 1995;345(8942):84-86.
6. Eschenbach DA. Treating spontaneous and induced septic abortions. Obstet Gynecol. 2015;125(5):1042-1048.
7. Rana A, Pradhan N, Gurung G, Singh M. Induced septic abortion: a major factor in maternal mortality and morbidity. J Obstet Gynaecol Res. 2004;30(1):3-8.
8. Abbasi S, Jamal A, Eslamian L, Marsousi V. Role of clinical and ultrasound findings in the diagnosis of retained products of conception. Ultrasound Obstet Gynecol. 2008;32(5):704-707.
9. Esmaeillou H, Jamal A, Eslamian L, et al. Accurate detection of retained products of conception after first- and second-trimester abortion by color doppler sonography. J Med Ultrasound. 2015;23(7):34-38.
10. Finkielman JD, De Feo FD, Heller PG, Afessa B. The clinical course of patients with septic abortion admitted to an intensive care unit. Intensive Care Med. 2004;30(6):1097-1102.
11. CDC. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
12. Mackeen AD, Packard RE, Ota E, Speer L. Antibiotic regimens for postpartum endometritis. Cochrane Database Syst Rev. 2015;2:CD001067.
When Man’s Legs “Give Out,” His Buttocks Takes the Brunt
ANSWER

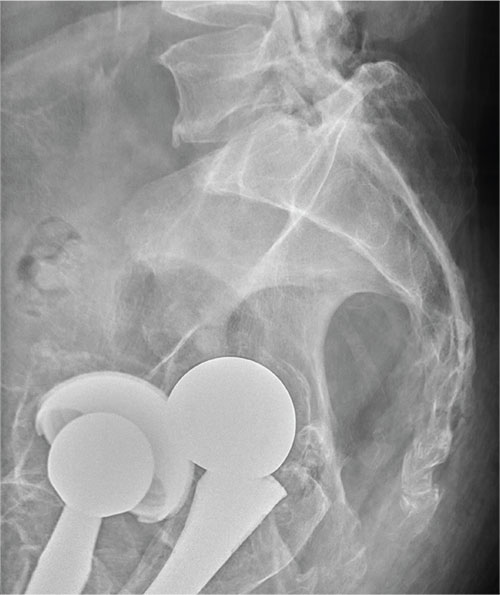
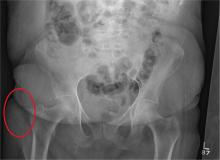
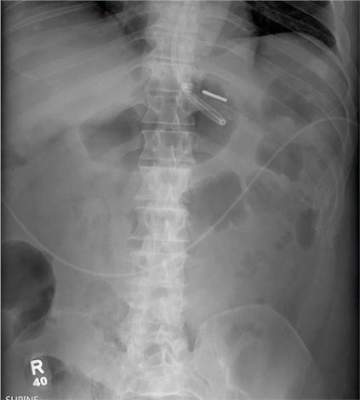
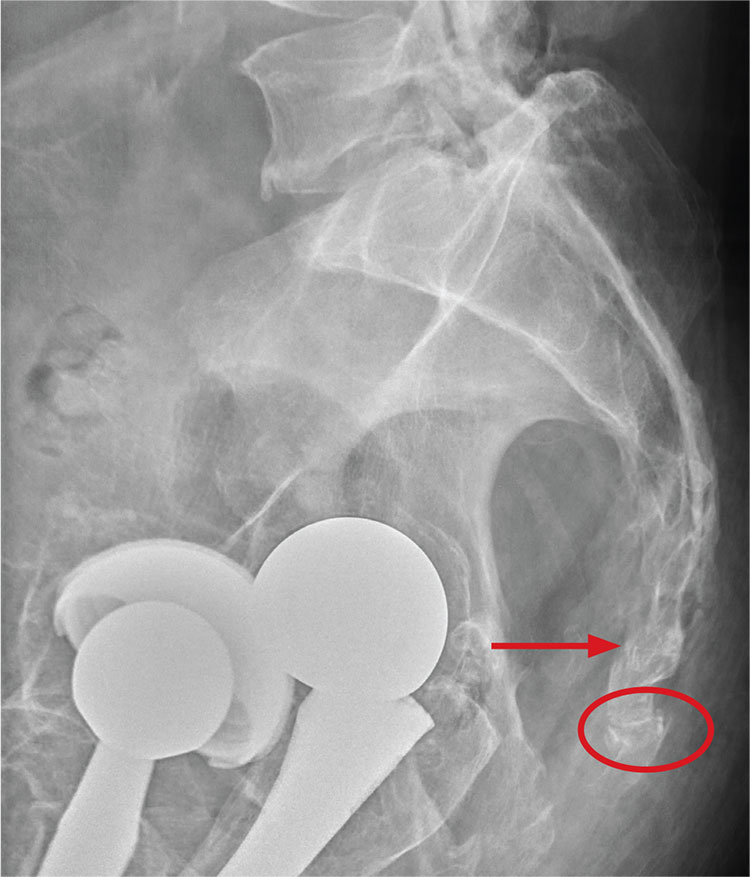
There are degenerative changes present. Bilateral hip prostheses are noted. Within the coccyx, there is bone remodeling and angulation that are likely chronic and related to remote trauma or injury (arrow). Below this, some cortical lucency (circled) is noted, most likely consistent with an acute fracture. The patient was prescribed a nonsteroidal medication and a mild narcotic pain medication.

ANSWER

There are degenerative changes present. Bilateral hip prostheses are noted. Within the coccyx, there is bone remodeling and angulation that are likely chronic and related to remote trauma or injury (arrow). Below this, some cortical lucency (circled) is noted, most likely consistent with an acute fracture. The patient was prescribed a nonsteroidal medication and a mild narcotic pain medication.

ANSWER

There are degenerative changes present. Bilateral hip prostheses are noted. Within the coccyx, there is bone remodeling and angulation that are likely chronic and related to remote trauma or injury (arrow). Below this, some cortical lucency (circled) is noted, most likely consistent with an acute fracture. The patient was prescribed a nonsteroidal medication and a mild narcotic pain medication.
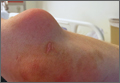
A 75-year-old man presents to the urgent care center for evaluation of pain in his buttocks after a fall. He states he was walking when his “legs gave out” and he hit the ground. He landed squarely on his buttocks, causing immediate pain. He was eventually able to get up with some assistance. He denies current weakness or any bowel or bladder complaints.
His medical/surgical history is significant for coronary artery disease, hypertension, and bilateral hip replacements. Physical exam reveals an elderly male who is uncomfortable but in no obvious distress. His vital signs are stable. He has moderate point tenderness over his sacrum but is able to move all his extremities well, with normal strength.
Radiograph of his sacrum/coccyx is shown. What is your impression?
A man with HIV and papules and nodules on the knees
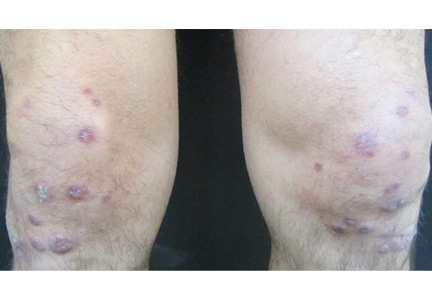
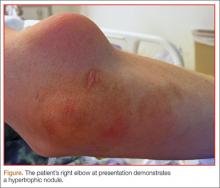
A 39-year-old man with human immunodeficiency virus (HIV) infection and a CD4 cell count of 528 × 106/L without treatment was referred for evaluation of periarticular, indurated, erythematous papules and nodules on the knees and elbows and purpuric lesions on the ankles (Figure 1). He has had recurrent fever, arthralgia, and mild constitutional symptoms during the past month. He also reported a diagnosis of polyclonal immunoglobulin A gammopathy.
Punch biopsy of the purpuric lesions was performed, and histologic study showed leukocytoclastic vasculitis with eosinophilic necrosis of the epithelium.
Treatment with a systemic corticosteroid was started. The purpuric lesions disappeared after 3 weeks of therapy, but the nodules over the extensor surface of both knees showed no improvement (Figure 2). Subsequent biopsy of late-stage lesions (3 months after the start of therapy) demonstrated perivascular fibrosis with small, persistent foci of vasculitis, and confirmed the diagnosis of HIV-associated nodular erythema elevatum diutinum (EED). Antiretroviral therapy was started in addition to intralesional corticosteroids and topical dapsone 5% gel, with resolution of the lesions 1 month later.
ERYTHEMA ELEVATUM DIUTINUM
EED is an uncommon chronic dermatosis, classified as a fibrosing form of leukocytoclastic vasculitis and characterized clinically by violaceous papules, plaques, and nodules, usually distributed acrally and symmetrically over extensor surfaces. The histopathologic picture depends on the stage of the lesion. Features of leukocytoclastic vasculitis are found in early-stage lesions, while a fibrotic replacement of the dermis with small persistent foci of vasculitis is typical of late-stage lesions.
EED has clinical and histopathologic similarities to Sweet syndrome, but EED is distinguished from neutrophilic dermatosis by vasculitis.
In HIV-infected patients, it is important to include pruritic papular eruption in the differential diagnosis. It is characterized by chronic bilaterally symmetric pruritic papules on the trunk and extremities and is the most common cutaneous noninfectious manifestation of HIV.
The clinical presentation of EED may also be easily confused with Kaposi sarcoma and bacillary angiomatosis.2
AN EMERGING HIV-RELATED DERMATOSIS
EED is emerging as a specific HIV-associated dermatosis, with 20 cases reported in the medical literature as of this writing.3
The cause of EED is not known, but it is often associated with streptococcal infection, monoclonal IgA gammopathy, hematologic malignancy, cryoglobulinemia, rheumatoid arthritis, and autoimmune disease.3 The stimulus could be immune-complex deposition in blood vessels triggered by HIV infection, or by another infection acting as an antigenic stimulus.4 The nodular variant of EED is even rarer, but it evolves most often in HIV-positive individuals.5,6
Oral dapsone is the treatment of choice but is less effective in late-stage fibrotic lesions.7 Treatment courses tend to be long and recurrence is common.8 Intralesional, topical, and oral corticosteroids, topical dapsone 5% gel,9 tetracycline and nicotinamide, sulfonamides, colchicine, chloroquine, and surgical excision are other options.
Our patient’s presentation reminds us to consider EED in HIV-infected patients and illustrates the importance of histologic diagnosis to differentiate EED from assumed Kaposi sarcoma in patients with HIV. EED can also be the first clinical sign of HIV infection. It is important to rule out underlying disorders such as HIV infection, because directed therapy is often the best management.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol 1992; 26:38–44.
- Requena L, Sánchez Yus E, Martín L, Barat A, Arias D. Erythema elevatum diutinum in a patient with acquired immunodeficiency syndrome. Another clinical simulator of Kaposi’s sarcoma. Arch Dermatol 1991; 127:1819–1822.
- Momen SE, Jorizzo J, Al-Niaimi F. Erythema elevatum diutinum: a review of presentation and treatment. J Eur Acad Dermatol Venereol 2014; 28:1594–1602.
- Muratori S, Carrera C, Gorani A, Alessi E. Erythema elevatum diutinum and HIV infection: a report of five cases. Br J Dermatol 1999; 141:335–338.
- LeBoit PE, Cockerell CJ. Nodular lesions of erythema elevatum diutinum in patients infected with the human immunodeficiency virus. J Am Acad Dermatol 1993; 28:919–922.
- Rover PA, Bittencourt C, Discacciati MP, Zaniboni MC, Arruda LH, Cintra ML. Erythema elevatum diutinum as a first clinical manifestation for diagnosing HIV infection: case history. Sao Paulo Med J 2005; 123:201–203.
- Fakheri A, Gupta SM, White SM, Don PC, Weinberg JM. Erythema elevatum diutinum in a patient with human immunodeficiency virus. Cutis 2001; 68:41–42.
- Katz SI, Gallin JL, Hertz KC, Fauci AS, Lawley TJ. Erythema elevatum diutinum: skin and systemic manifestations, immunologic studies, and successful treatment with dapsone. Medicine (Baltimore) 1977; 56:443–455.
- Frieling GW, Williams NL, Lim SJ, Rosenthal SI. Novel use of topical dapsone 5% gel for erythema elevatum diutinum: safer and effective. J Drugs Dermatol 2013; 12:481–484.
A 39-year-old man with human immunodeficiency virus (HIV) infection and a CD4 cell count of 528 × 106/L without treatment was referred for evaluation of periarticular, indurated, erythematous papules and nodules on the knees and elbows and purpuric lesions on the ankles (Figure 1). He has had recurrent fever, arthralgia, and mild constitutional symptoms during the past month. He also reported a diagnosis of polyclonal immunoglobulin A gammopathy.
Punch biopsy of the purpuric lesions was performed, and histologic study showed leukocytoclastic vasculitis with eosinophilic necrosis of the epithelium.
Treatment with a systemic corticosteroid was started. The purpuric lesions disappeared after 3 weeks of therapy, but the nodules over the extensor surface of both knees showed no improvement (Figure 2). Subsequent biopsy of late-stage lesions (3 months after the start of therapy) demonstrated perivascular fibrosis with small, persistent foci of vasculitis, and confirmed the diagnosis of HIV-associated nodular erythema elevatum diutinum (EED). Antiretroviral therapy was started in addition to intralesional corticosteroids and topical dapsone 5% gel, with resolution of the lesions 1 month later.
ERYTHEMA ELEVATUM DIUTINUM
EED is an uncommon chronic dermatosis, classified as a fibrosing form of leukocytoclastic vasculitis and characterized clinically by violaceous papules, plaques, and nodules, usually distributed acrally and symmetrically over extensor surfaces. The histopathologic picture depends on the stage of the lesion. Features of leukocytoclastic vasculitis are found in early-stage lesions, while a fibrotic replacement of the dermis with small persistent foci of vasculitis is typical of late-stage lesions.
EED has clinical and histopathologic similarities to Sweet syndrome, but EED is distinguished from neutrophilic dermatosis by vasculitis.
In HIV-infected patients, it is important to include pruritic papular eruption in the differential diagnosis. It is characterized by chronic bilaterally symmetric pruritic papules on the trunk and extremities and is the most common cutaneous noninfectious manifestation of HIV.
The clinical presentation of EED may also be easily confused with Kaposi sarcoma and bacillary angiomatosis.2
AN EMERGING HIV-RELATED DERMATOSIS
EED is emerging as a specific HIV-associated dermatosis, with 20 cases reported in the medical literature as of this writing.3
The cause of EED is not known, but it is often associated with streptococcal infection, monoclonal IgA gammopathy, hematologic malignancy, cryoglobulinemia, rheumatoid arthritis, and autoimmune disease.3 The stimulus could be immune-complex deposition in blood vessels triggered by HIV infection, or by another infection acting as an antigenic stimulus.4 The nodular variant of EED is even rarer, but it evolves most often in HIV-positive individuals.5,6
Oral dapsone is the treatment of choice but is less effective in late-stage fibrotic lesions.7 Treatment courses tend to be long and recurrence is common.8 Intralesional, topical, and oral corticosteroids, topical dapsone 5% gel,9 tetracycline and nicotinamide, sulfonamides, colchicine, chloroquine, and surgical excision are other options.
Our patient’s presentation reminds us to consider EED in HIV-infected patients and illustrates the importance of histologic diagnosis to differentiate EED from assumed Kaposi sarcoma in patients with HIV. EED can also be the first clinical sign of HIV infection. It is important to rule out underlying disorders such as HIV infection, because directed therapy is often the best management.
A 39-year-old man with human immunodeficiency virus (HIV) infection and a CD4 cell count of 528 × 106/L without treatment was referred for evaluation of periarticular, indurated, erythematous papules and nodules on the knees and elbows and purpuric lesions on the ankles (Figure 1). He has had recurrent fever, arthralgia, and mild constitutional symptoms during the past month. He also reported a diagnosis of polyclonal immunoglobulin A gammopathy.
Punch biopsy of the purpuric lesions was performed, and histologic study showed leukocytoclastic vasculitis with eosinophilic necrosis of the epithelium.
Treatment with a systemic corticosteroid was started. The purpuric lesions disappeared after 3 weeks of therapy, but the nodules over the extensor surface of both knees showed no improvement (Figure 2). Subsequent biopsy of late-stage lesions (3 months after the start of therapy) demonstrated perivascular fibrosis with small, persistent foci of vasculitis, and confirmed the diagnosis of HIV-associated nodular erythema elevatum diutinum (EED). Antiretroviral therapy was started in addition to intralesional corticosteroids and topical dapsone 5% gel, with resolution of the lesions 1 month later.
ERYTHEMA ELEVATUM DIUTINUM
EED is an uncommon chronic dermatosis, classified as a fibrosing form of leukocytoclastic vasculitis and characterized clinically by violaceous papules, plaques, and nodules, usually distributed acrally and symmetrically over extensor surfaces. The histopathologic picture depends on the stage of the lesion. Features of leukocytoclastic vasculitis are found in early-stage lesions, while a fibrotic replacement of the dermis with small persistent foci of vasculitis is typical of late-stage lesions.
EED has clinical and histopathologic similarities to Sweet syndrome, but EED is distinguished from neutrophilic dermatosis by vasculitis.
In HIV-infected patients, it is important to include pruritic papular eruption in the differential diagnosis. It is characterized by chronic bilaterally symmetric pruritic papules on the trunk and extremities and is the most common cutaneous noninfectious manifestation of HIV.
The clinical presentation of EED may also be easily confused with Kaposi sarcoma and bacillary angiomatosis.2
AN EMERGING HIV-RELATED DERMATOSIS
EED is emerging as a specific HIV-associated dermatosis, with 20 cases reported in the medical literature as of this writing.3
The cause of EED is not known, but it is often associated with streptococcal infection, monoclonal IgA gammopathy, hematologic malignancy, cryoglobulinemia, rheumatoid arthritis, and autoimmune disease.3 The stimulus could be immune-complex deposition in blood vessels triggered by HIV infection, or by another infection acting as an antigenic stimulus.4 The nodular variant of EED is even rarer, but it evolves most often in HIV-positive individuals.5,6
Oral dapsone is the treatment of choice but is less effective in late-stage fibrotic lesions.7 Treatment courses tend to be long and recurrence is common.8 Intralesional, topical, and oral corticosteroids, topical dapsone 5% gel,9 tetracycline and nicotinamide, sulfonamides, colchicine, chloroquine, and surgical excision are other options.
Our patient’s presentation reminds us to consider EED in HIV-infected patients and illustrates the importance of histologic diagnosis to differentiate EED from assumed Kaposi sarcoma in patients with HIV. EED can also be the first clinical sign of HIV infection. It is important to rule out underlying disorders such as HIV infection, because directed therapy is often the best management.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol 1992; 26:38–44.
- Requena L, Sánchez Yus E, Martín L, Barat A, Arias D. Erythema elevatum diutinum in a patient with acquired immunodeficiency syndrome. Another clinical simulator of Kaposi’s sarcoma. Arch Dermatol 1991; 127:1819–1822.
- Momen SE, Jorizzo J, Al-Niaimi F. Erythema elevatum diutinum: a review of presentation and treatment. J Eur Acad Dermatol Venereol 2014; 28:1594–1602.
- Muratori S, Carrera C, Gorani A, Alessi E. Erythema elevatum diutinum and HIV infection: a report of five cases. Br J Dermatol 1999; 141:335–338.
- LeBoit PE, Cockerell CJ. Nodular lesions of erythema elevatum diutinum in patients infected with the human immunodeficiency virus. J Am Acad Dermatol 1993; 28:919–922.
- Rover PA, Bittencourt C, Discacciati MP, Zaniboni MC, Arruda LH, Cintra ML. Erythema elevatum diutinum as a first clinical manifestation for diagnosing HIV infection: case history. Sao Paulo Med J 2005; 123:201–203.
- Fakheri A, Gupta SM, White SM, Don PC, Weinberg JM. Erythema elevatum diutinum in a patient with human immunodeficiency virus. Cutis 2001; 68:41–42.
- Katz SI, Gallin JL, Hertz KC, Fauci AS, Lawley TJ. Erythema elevatum diutinum: skin and systemic manifestations, immunologic studies, and successful treatment with dapsone. Medicine (Baltimore) 1977; 56:443–455.
- Frieling GW, Williams NL, Lim SJ, Rosenthal SI. Novel use of topical dapsone 5% gel for erythema elevatum diutinum: safer and effective. J Drugs Dermatol 2013; 12:481–484.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol 1992; 26:38–44.
- Requena L, Sánchez Yus E, Martín L, Barat A, Arias D. Erythema elevatum diutinum in a patient with acquired immunodeficiency syndrome. Another clinical simulator of Kaposi’s sarcoma. Arch Dermatol 1991; 127:1819–1822.
- Momen SE, Jorizzo J, Al-Niaimi F. Erythema elevatum diutinum: a review of presentation and treatment. J Eur Acad Dermatol Venereol 2014; 28:1594–1602.
- Muratori S, Carrera C, Gorani A, Alessi E. Erythema elevatum diutinum and HIV infection: a report of five cases. Br J Dermatol 1999; 141:335–338.
- LeBoit PE, Cockerell CJ. Nodular lesions of erythema elevatum diutinum in patients infected with the human immunodeficiency virus. J Am Acad Dermatol 1993; 28:919–922.
- Rover PA, Bittencourt C, Discacciati MP, Zaniboni MC, Arruda LH, Cintra ML. Erythema elevatum diutinum as a first clinical manifestation for diagnosing HIV infection: case history. Sao Paulo Med J 2005; 123:201–203.
- Fakheri A, Gupta SM, White SM, Don PC, Weinberg JM. Erythema elevatum diutinum in a patient with human immunodeficiency virus. Cutis 2001; 68:41–42.
- Katz SI, Gallin JL, Hertz KC, Fauci AS, Lawley TJ. Erythema elevatum diutinum: skin and systemic manifestations, immunologic studies, and successful treatment with dapsone. Medicine (Baltimore) 1977; 56:443–455.
- Frieling GW, Williams NL, Lim SJ, Rosenthal SI. Novel use of topical dapsone 5% gel for erythema elevatum diutinum: safer and effective. J Drugs Dermatol 2013; 12:481–484.
‘Air-raising’: An air-fluid level in the right subphrenic region
A 39-year-old Filipino man presented with nausea, vomiting, and abdominal pain of 2 weeks’ duration. He did not report trauma, and he had no history of medical illness or surgery.
On arrival, his blood pressure was 123/83 mm Hg, pulse 122 beats per minute, respiratory rate 18 breaths per minute, and temperature 100.7°F (38.1°C). On physical examination, he exhibited marked tenderness of the right upper quadrant on palpation. The abdomen was otherwise soft with no guarding or rebound tenderness.
Results of initial laboratory testing were as follows:
- Leukocyte count 17.0 × 109/L (reference range 4.5–11.0)
- Serum glucose 558 mg/dL without ketoacidosis
- Aspartate aminotransferase 109 U/L (2–40)
- Alanine aminotranferase 28 U/L (2–50)
- Total serum bilirubin 4.0 mg/dL (0.0–1.5).
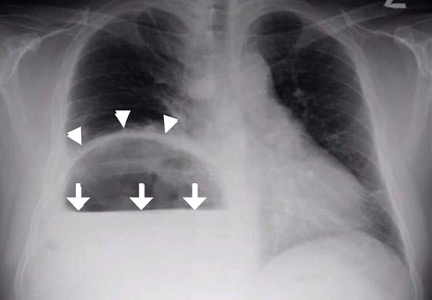
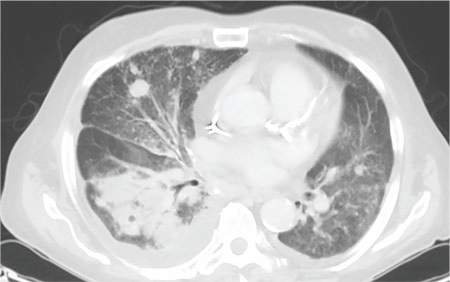
Plain chest radiography showed dramatic elevation of the right hemidiaphragm with a large subphrenic air-fluid level (Figure 1). Abdominal computed tomography (CT) demonstrated a multiloculated hepatic abscess 18 × 13.5 cm subjacent to the diaphragm (Figure 2). Cultures of blood and the abscess yielded Klebsiella pneumoniae. The patient recovered after percutaneous drainage and a course of ceftriaxone.
PRIMARY KLEBSIELLA LIVER ABSCESS
K pneumoniae, a gram-negative aerobic encapsulated bacillus of the normal human intestinal flora, is closely related to Escherichia coli, historically the most frequent bacterial cause of pyogenic liver abscess.1 Over the last 30 years, K pneumoniae has eclipsed E coli as the most common causative agent, with the epicenter of this trend being located in Taiwan and South Korea, perhaps because rates of fecal Klebsiella carriage in that region are particularly high.1,2
Concurrently, there has been increasing recognition—initially across Asia, but lately in Europe and the Western Hemisphere—of the so-called invasive Klebsiella liver abscess (KLA) syndrome, virtually unique to the hypervirulent K1 and K2 capsular serotypes of K pneumoniae prevalent in Asia.3–6 This community-acquired syndrome is characterized by hematogenous deposition of the organism at distant sites, such as the lung, soft tissues, central nervous system, and eyes. Impairment of phagocytic function, as occurs in diabetes mellitus, and the resistance to phagocytosis conferred by the K1 and K2 serotypes have been identified as predisposing factors for dissemination.7,8 The mucoid phenotype of K pneumoniae, very common in Asian isolates of the K1 and K2 serotypes, is also associated with hypervirulence and extrahepatic spread, presumably through evasion of phagocytosis and complement-mediated opsonization.2,9
Our patient’s risk factors for KLA were his Asian origin and uncontrolled diabetes. No evidence of remote infection was detected during his hospitalization.
HEMIDIAPHRAGM ELEVATION
Acquired hemidiaphragm elevation is most commonly unilateral and typically represents an incidental radiologic finding attributable to paralysis of the corresponding diaphragm after phrenic nerve injury caused by trauma, surgery, or infection. Unilateral diaphragmatic paralysis is classically confirmed by performing a fluoroscopic sniff test, which is positive if the affected hemidiaphragm is observed in real time to paradoxically move upward during forced inhalation.10 This condition is usually asymptomatic at rest but could cause exertional dyspnea and contribute to ventilatory failure when pulmonary disease coexists.11
Occasionally, as in our patient, hemidiaphragm elevation is part of the presentation of active abdominal pathology that displaces the corresponding hemidiaphragm cephalad by mass effect. Examples of such space-occupying abdominal lesions include infections, malignancy, hepatosplenomegaly, and pneumoperitoneum from a ruptured viscus. Pneumoperitoneum is suggested by the presence of an air crescent immediately subjacent to the affected hemidiaphragm on an upright radiograph accompanied by peritoneal signs.
Although there was subphrenic air on this patient’s initial chest radiograph, it was actually part of an air-fluid level without associated peritoneal signs. An air-fluid level is characterized by a sharp horizontal demarcation between the lighter gas component floating at the top and the heavier fluid component settling on the bottom (Figure 1). The subsequent CT excluded free intra-abdominal air while identifying a large hepatic abscess as the cause of hemidiaphragm elevation. In trauma victims, CT is also helpful in ruling out diaphragmatic rupture, which can have a similar radiographic appearance.12
Our patient’s presentation was a reminder that an elevated hemidiaphragm may reflect abdominal pathology and that subphrenic air in this context need not be either “free” or a surgical emergency. Drainage of the abscess restored the normal position of our patient’s right hemidiaphragm (Figure 3).
- Huang CJ, Pitt HA, Lipsett PA, et al. Pyogenic hepatic abscess: changing trends over 42 years. Ann Surg 1996; 223:600–607.
- Lin YT, Siu LK, Lin JC, et al. Seroepidemiology of Klebsiella pneumoniae colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol 2012; 12:13.
- Wang JH, Liu YC, Lee SS, et al. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis 1998; 26:1434–1438.
- Pastagia M, Arumugam V. Klebsiella pneumoniae liver abscesses in a public hospital in Queens, New York. Travel Med Infect Dis 2008; 6:228–233.
- Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis 2004; 39:1654–1659.
- Moore R, O’Shea D, Geoghegan T, Mallon PW, Sheehan G. Community-acquired Klebsiella pneumoniae liver abscess: an emerging infection in Ireland and Europe. Infection 2013; 41:681–686.
- Lecube A, Pachón G, Petriz J, Hernández C, Simó R. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLoS One 2011; 6:e23366.
- Lin JC, Siu LK, Fung CP, et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J Clin Endocrinol Metab 2006; 91:3084–3087.
- Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis 2012; 12:881–887.
- Gierada DS, Slone RM, Fleishman MJ. Imaging evaluation of the diaphragm. Chest Surg Clin North Am 1998; 8:237–280.
- Qureshi A. Diaphragm paralysis. Semin Respir Crit Care Med 2009; 30:315–320.
- Havens JM, Kelly E, Patel V. A 78-year-old man with an elevated hemidiaphragm following trauma. Chest 2008; 134:1336–1339.
A 39-year-old Filipino man presented with nausea, vomiting, and abdominal pain of 2 weeks’ duration. He did not report trauma, and he had no history of medical illness or surgery.
On arrival, his blood pressure was 123/83 mm Hg, pulse 122 beats per minute, respiratory rate 18 breaths per minute, and temperature 100.7°F (38.1°C). On physical examination, he exhibited marked tenderness of the right upper quadrant on palpation. The abdomen was otherwise soft with no guarding or rebound tenderness.
Results of initial laboratory testing were as follows:
- Leukocyte count 17.0 × 109/L (reference range 4.5–11.0)
- Serum glucose 558 mg/dL without ketoacidosis
- Aspartate aminotransferase 109 U/L (2–40)
- Alanine aminotranferase 28 U/L (2–50)
- Total serum bilirubin 4.0 mg/dL (0.0–1.5).
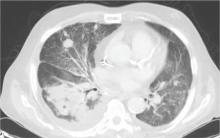
Plain chest radiography showed dramatic elevation of the right hemidiaphragm with a large subphrenic air-fluid level (Figure 1). Abdominal computed tomography (CT) demonstrated a multiloculated hepatic abscess 18 × 13.5 cm subjacent to the diaphragm (Figure 2). Cultures of blood and the abscess yielded Klebsiella pneumoniae. The patient recovered after percutaneous drainage and a course of ceftriaxone.
PRIMARY KLEBSIELLA LIVER ABSCESS
K pneumoniae, a gram-negative aerobic encapsulated bacillus of the normal human intestinal flora, is closely related to Escherichia coli, historically the most frequent bacterial cause of pyogenic liver abscess.1 Over the last 30 years, K pneumoniae has eclipsed E coli as the most common causative agent, with the epicenter of this trend being located in Taiwan and South Korea, perhaps because rates of fecal Klebsiella carriage in that region are particularly high.1,2
Concurrently, there has been increasing recognition—initially across Asia, but lately in Europe and the Western Hemisphere—of the so-called invasive Klebsiella liver abscess (KLA) syndrome, virtually unique to the hypervirulent K1 and K2 capsular serotypes of K pneumoniae prevalent in Asia.3–6 This community-acquired syndrome is characterized by hematogenous deposition of the organism at distant sites, such as the lung, soft tissues, central nervous system, and eyes. Impairment of phagocytic function, as occurs in diabetes mellitus, and the resistance to phagocytosis conferred by the K1 and K2 serotypes have been identified as predisposing factors for dissemination.7,8 The mucoid phenotype of K pneumoniae, very common in Asian isolates of the K1 and K2 serotypes, is also associated with hypervirulence and extrahepatic spread, presumably through evasion of phagocytosis and complement-mediated opsonization.2,9
Our patient’s risk factors for KLA were his Asian origin and uncontrolled diabetes. No evidence of remote infection was detected during his hospitalization.
HEMIDIAPHRAGM ELEVATION
Acquired hemidiaphragm elevation is most commonly unilateral and typically represents an incidental radiologic finding attributable to paralysis of the corresponding diaphragm after phrenic nerve injury caused by trauma, surgery, or infection. Unilateral diaphragmatic paralysis is classically confirmed by performing a fluoroscopic sniff test, which is positive if the affected hemidiaphragm is observed in real time to paradoxically move upward during forced inhalation.10 This condition is usually asymptomatic at rest but could cause exertional dyspnea and contribute to ventilatory failure when pulmonary disease coexists.11
Occasionally, as in our patient, hemidiaphragm elevation is part of the presentation of active abdominal pathology that displaces the corresponding hemidiaphragm cephalad by mass effect. Examples of such space-occupying abdominal lesions include infections, malignancy, hepatosplenomegaly, and pneumoperitoneum from a ruptured viscus. Pneumoperitoneum is suggested by the presence of an air crescent immediately subjacent to the affected hemidiaphragm on an upright radiograph accompanied by peritoneal signs.
Although there was subphrenic air on this patient’s initial chest radiograph, it was actually part of an air-fluid level without associated peritoneal signs. An air-fluid level is characterized by a sharp horizontal demarcation between the lighter gas component floating at the top and the heavier fluid component settling on the bottom (Figure 1). The subsequent CT excluded free intra-abdominal air while identifying a large hepatic abscess as the cause of hemidiaphragm elevation. In trauma victims, CT is also helpful in ruling out diaphragmatic rupture, which can have a similar radiographic appearance.12
Our patient’s presentation was a reminder that an elevated hemidiaphragm may reflect abdominal pathology and that subphrenic air in this context need not be either “free” or a surgical emergency. Drainage of the abscess restored the normal position of our patient’s right hemidiaphragm (Figure 3).
A 39-year-old Filipino man presented with nausea, vomiting, and abdominal pain of 2 weeks’ duration. He did not report trauma, and he had no history of medical illness or surgery.
On arrival, his blood pressure was 123/83 mm Hg, pulse 122 beats per minute, respiratory rate 18 breaths per minute, and temperature 100.7°F (38.1°C). On physical examination, he exhibited marked tenderness of the right upper quadrant on palpation. The abdomen was otherwise soft with no guarding or rebound tenderness.
Results of initial laboratory testing were as follows:
- Leukocyte count 17.0 × 109/L (reference range 4.5–11.0)
- Serum glucose 558 mg/dL without ketoacidosis
- Aspartate aminotransferase 109 U/L (2–40)
- Alanine aminotranferase 28 U/L (2–50)
- Total serum bilirubin 4.0 mg/dL (0.0–1.5).
Plain chest radiography showed dramatic elevation of the right hemidiaphragm with a large subphrenic air-fluid level (Figure 1). Abdominal computed tomography (CT) demonstrated a multiloculated hepatic abscess 18 × 13.5 cm subjacent to the diaphragm (Figure 2). Cultures of blood and the abscess yielded Klebsiella pneumoniae. The patient recovered after percutaneous drainage and a course of ceftriaxone.
PRIMARY KLEBSIELLA LIVER ABSCESS
K pneumoniae, a gram-negative aerobic encapsulated bacillus of the normal human intestinal flora, is closely related to Escherichia coli, historically the most frequent bacterial cause of pyogenic liver abscess.1 Over the last 30 years, K pneumoniae has eclipsed E coli as the most common causative agent, with the epicenter of this trend being located in Taiwan and South Korea, perhaps because rates of fecal Klebsiella carriage in that region are particularly high.1,2
Concurrently, there has been increasing recognition—initially across Asia, but lately in Europe and the Western Hemisphere—of the so-called invasive Klebsiella liver abscess (KLA) syndrome, virtually unique to the hypervirulent K1 and K2 capsular serotypes of K pneumoniae prevalent in Asia.3–6 This community-acquired syndrome is characterized by hematogenous deposition of the organism at distant sites, such as the lung, soft tissues, central nervous system, and eyes. Impairment of phagocytic function, as occurs in diabetes mellitus, and the resistance to phagocytosis conferred by the K1 and K2 serotypes have been identified as predisposing factors for dissemination.7,8 The mucoid phenotype of K pneumoniae, very common in Asian isolates of the K1 and K2 serotypes, is also associated with hypervirulence and extrahepatic spread, presumably through evasion of phagocytosis and complement-mediated opsonization.2,9
Our patient’s risk factors for KLA were his Asian origin and uncontrolled diabetes. No evidence of remote infection was detected during his hospitalization.
HEMIDIAPHRAGM ELEVATION
Acquired hemidiaphragm elevation is most commonly unilateral and typically represents an incidental radiologic finding attributable to paralysis of the corresponding diaphragm after phrenic nerve injury caused by trauma, surgery, or infection. Unilateral diaphragmatic paralysis is classically confirmed by performing a fluoroscopic sniff test, which is positive if the affected hemidiaphragm is observed in real time to paradoxically move upward during forced inhalation.10 This condition is usually asymptomatic at rest but could cause exertional dyspnea and contribute to ventilatory failure when pulmonary disease coexists.11
Occasionally, as in our patient, hemidiaphragm elevation is part of the presentation of active abdominal pathology that displaces the corresponding hemidiaphragm cephalad by mass effect. Examples of such space-occupying abdominal lesions include infections, malignancy, hepatosplenomegaly, and pneumoperitoneum from a ruptured viscus. Pneumoperitoneum is suggested by the presence of an air crescent immediately subjacent to the affected hemidiaphragm on an upright radiograph accompanied by peritoneal signs.
Although there was subphrenic air on this patient’s initial chest radiograph, it was actually part of an air-fluid level without associated peritoneal signs. An air-fluid level is characterized by a sharp horizontal demarcation between the lighter gas component floating at the top and the heavier fluid component settling on the bottom (Figure 1). The subsequent CT excluded free intra-abdominal air while identifying a large hepatic abscess as the cause of hemidiaphragm elevation. In trauma victims, CT is also helpful in ruling out diaphragmatic rupture, which can have a similar radiographic appearance.12
Our patient’s presentation was a reminder that an elevated hemidiaphragm may reflect abdominal pathology and that subphrenic air in this context need not be either “free” or a surgical emergency. Drainage of the abscess restored the normal position of our patient’s right hemidiaphragm (Figure 3).
- Huang CJ, Pitt HA, Lipsett PA, et al. Pyogenic hepatic abscess: changing trends over 42 years. Ann Surg 1996; 223:600–607.
- Lin YT, Siu LK, Lin JC, et al. Seroepidemiology of Klebsiella pneumoniae colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol 2012; 12:13.
- Wang JH, Liu YC, Lee SS, et al. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis 1998; 26:1434–1438.
- Pastagia M, Arumugam V. Klebsiella pneumoniae liver abscesses in a public hospital in Queens, New York. Travel Med Infect Dis 2008; 6:228–233.
- Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis 2004; 39:1654–1659.
- Moore R, O’Shea D, Geoghegan T, Mallon PW, Sheehan G. Community-acquired Klebsiella pneumoniae liver abscess: an emerging infection in Ireland and Europe. Infection 2013; 41:681–686.
- Lecube A, Pachón G, Petriz J, Hernández C, Simó R. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLoS One 2011; 6:e23366.
- Lin JC, Siu LK, Fung CP, et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J Clin Endocrinol Metab 2006; 91:3084–3087.
- Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis 2012; 12:881–887.
- Gierada DS, Slone RM, Fleishman MJ. Imaging evaluation of the diaphragm. Chest Surg Clin North Am 1998; 8:237–280.
- Qureshi A. Diaphragm paralysis. Semin Respir Crit Care Med 2009; 30:315–320.
- Havens JM, Kelly E, Patel V. A 78-year-old man with an elevated hemidiaphragm following trauma. Chest 2008; 134:1336–1339.
- Huang CJ, Pitt HA, Lipsett PA, et al. Pyogenic hepatic abscess: changing trends over 42 years. Ann Surg 1996; 223:600–607.
- Lin YT, Siu LK, Lin JC, et al. Seroepidemiology of Klebsiella pneumoniae colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol 2012; 12:13.
- Wang JH, Liu YC, Lee SS, et al. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis 1998; 26:1434–1438.
- Pastagia M, Arumugam V. Klebsiella pneumoniae liver abscesses in a public hospital in Queens, New York. Travel Med Infect Dis 2008; 6:228–233.
- Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis 2004; 39:1654–1659.
- Moore R, O’Shea D, Geoghegan T, Mallon PW, Sheehan G. Community-acquired Klebsiella pneumoniae liver abscess: an emerging infection in Ireland and Europe. Infection 2013; 41:681–686.
- Lecube A, Pachón G, Petriz J, Hernández C, Simó R. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLoS One 2011; 6:e23366.
- Lin JC, Siu LK, Fung CP, et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J Clin Endocrinol Metab 2006; 91:3084–3087.
- Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis 2012; 12:881–887.
- Gierada DS, Slone RM, Fleishman MJ. Imaging evaluation of the diaphragm. Chest Surg Clin North Am 1998; 8:237–280.
- Qureshi A. Diaphragm paralysis. Semin Respir Crit Care Med 2009; 30:315–320.
- Havens JM, Kelly E, Patel V. A 78-year-old man with an elevated hemidiaphragm following trauma. Chest 2008; 134:1336–1339.
An Alarming Slip of the Hip
After a fall, an 80-year-old woman is brought to the emergency department for evaluation of hip pain. She was getting out of bed when she slipped, fell, and landed on her right hip; bearing weight now is painful. She denies hitting her head. The patient’s vital signs are normal. Her medical history is significant for hypertension and diabetes. Inspection of the hip reveals no obvious deformity or shortening. The right lateral aspect of the hip exhibits mild swelling and decreased range of motion secondary to the pain. You order a pelvic radiograph, which is shown. What is your impression?
Extreme Athlete, 18, With Worsening Cough
IN THIS ARTICLE
- Adverse effects of ciprofloxacin
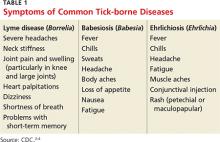
- Symptoms of common tick-borne diseases
- Symptoms of phase 1 and late-phase disease
- Additional resources
Jane, an 18-year-old college student, presents in early November with a three-week history of worsening cough and sinus congestion. Recently, the cough has been interrupting her sleep and yellow-green nasal drainage and sinus pressure have increased. Ordinarily very fit and athletic, she reports that since she arrived at college two months ago, her body has become “more fragile.”
Further questioning reveals that, over the past two months, the patient’s symptoms have included extreme fatigue, severe unremitting headache, blurred vision, shortness of breath, and a racing heart rate on exertion. Her symptoms make it impossible for her to maintain her demanding exercise routine, a development that compounds her frustration and sadness. She has also been forced to limit her participation in school activities, with significant academic decline as a result.
Aside from depression (well controlled with bupropion HCl extended release, 300 mg/d), Jane’s medical history is unremarkable. She reports having “excellent health” until she arrived at her mid-Atlantic urban college.
A complicated history
Born and raised in Connecticut, Jane is an avid runner who competes in extreme sports. This past summer, she trained for and participated in two “mud run” events (ie, endurance races of several miles with numerous challenges and obstacles) in Connecticut and New York. Training included endurance runs and sprints, as well as crawling through mud-laden fields and woods.
She also did a three-week summer internship on an oyster farm. There, she was required to shuck oysters and stand in brackish water for six-hour shifts to examine oyster beds. In the process, she sustained numerous cuts and bruises on her hands, arms, and legs.
A week or so after returning to college in late August, Jane developed blisters on both heels, which progressed to infected ulcerations. She was evaluated at the university hospital emergency department (ED) and treated with a 21-day course of ciprofloxacin. When left-sided unilateral knee swelling developed about two weeks later, she underwent arthrocentesis at the university health center, but joint aspirate was not sent for analysis. A two-week course of antibiotic therapy was initiated.
From October to her presentation in early November, Jane has experienced intermittent fevers and chills, with a temperature as high as 101°F. In addition, she complains of fasciculations and weakness in her lower limbs; dyspnea, tachycardia, and dizziness during or after any exertion; unremitting posterior neck pain; and a constant, severe headache located primarily in the bitemporal region. She developed bilateral conjunctivitis, which resolved spontaneously in about one week; persistent blurred vision; a transient petechial chest rash; recurring episodes of syncope; pyelonephritis; a persistent vaginal yeast infection; decreased appetite; and a 7-lb weight loss (5% of her total body weight).
Jane’s academic and athletic performance has been severely impaired. Once a long-distance runner, she can no longer walk any distance without frequent rest. In the four months since the mud runs, the patient reports, she has been seen in the student health center four times and in the ED twice. Additionally, she has undergone thorough examinations by clinicians specializing in infectious disease, pulmonology, neurology, and neuro-ophthalmology. She has undergone lab work, including
• Complete blood cell count with differential
• Comprehensive metabolic panel
• Urinalysis and urine culture
• Lyme antibody and blood polymerase chain reaction (PCR)
• HIV testing
• Rheumatoid factor
• Erythrocyte sedimentation rate (ESR)
• C-reactive protein (CRP)
• Epstein-Barr virus IgM
• Cytomegalovirus (CMV) IgM
• Human granulocytic ehrlichiosis (HGE) antibody and human anaplasma phagocytophilum (HGA)
• HGA PCR
• Rickettsia antibody panel
• Babesia microti antibodies
• Pregnancy testing
• Chest x-ray
• Lumbar puncture
All lab results were within normal range. In light of this, several clinicians have told Jane that her illness is “all in her head.”
Continue for the patient investigates >>
The patient investigates
In mid-December, after she has returned home from college, Jane’s symptoms abruptly worsen. She complains of feeling “shakier,” with weakness in her legs and what she calls “brain fog.” Her headache, blurred vision, and dizziness have worsened. Frightened and concerned, she returns to the ED. Results of a thorough evaluation, including lumbar puncture, reveal no abnormality.
Jane has become extremely frail. She is losing weight, her hair has lost its luster, and her nails are cracking and bleeding. She is unable to walk without concern for falling and cannot climb the 20 steps to her bedroom. Once a healthy and vibrant 18-year-old, she now spends most of her time in a lethargic state on a first-floor living room couch.
Frustrated by her unexplained declining health, she begins to research illnesses associated with extreme sports and prolonged marine exposure. She returns to ask about three possible explanations for her condition:
1. Adverse effects of ciprofloxacin use, which include fever or chills, dizziness, racing heartbeat, headache, and nausea.1
2. A tick-borne disease, possibly contracted during her practice runs in the Connecticut woods (see Table 1).2-4 Each year, she recalls, she has found and removed four or five embedded ticks. In the northeastern United States, the most common tick-borne diseases are borreliosis, babesiosis, and ehrlichiosis.5-7
3. Leptospirosis, contracted through the patient’s exposure to mud and brackish water during her summer activities. According to her research, more than 25 outbreaks and 600 cases of leptospirosis (between 1931 and 1998) have been associated with fresh pond, creek, or river water.8
Based on Jane’s symptoms and history, and in accord with her research, early-phase leptospirosis is identified as a diagnosis of exclusion (with a possible comorbid tick-borne zoonosis).
Continue for discussion >>
DISCUSSION
Leptospirosis develops when humans come into contact with animal urine infected by leptospires—that is, pathogenic spirochetes excreted via the renal tubules of infected host animals.9,10 While host animals include dogs, pigs, cattle, reptiles, and amphibians, the animal most commonly associated with human infection is the brown rat (Rattus norvegicus).11-15
Leptospires enter the human host through mucous membranes, cuts, or abrasions in the skin. Individuals at increased risk for infection include those whose work or other activities expose them “to animal reservoirs or contaminated environments”—including participants in water sports and similar recreation.11-14 As Mwachui et al explain, “recreational exposure to [Leptospira-]contaminated water has become more important for sport enthusiasts, swimmers and travellers from industrialized countries,” whereas flooding is usually involved in infection in undeveloped countries.16
The largest outbreak of leptospirosis reported in the US to date occurred in 1998, when heavy rains preceded a triathlon in Springfield, Illinois. When many participants became ill after the event, researchers from the National Center for Infectious Diseases were able to contact and test 834 of the 876 competing athletes; of these, 98 (12%) reported being ill and 52 (11%) tested positive for leptospirosis. Additionally, 14 of the 248 community residents who were sickened (6%) tested positive.17 According to CDC estimates, between 100 and 200 cases of leptospirosis develop annually in the US, with about half occurring in Hawaii.9
Onset of symptoms, which are described as protean and nonspecific, occurs two days to four weeks after exposure, making leptospirosis difficult to diagnosewithout a high degree of suspicion; zoonotic exposure (as with freshwater or mud sports) or a history of travel to Hawaii, Tahiti, Thailand, Indonesia, the Caribbean, and/or Costa Rica may raise suspicion.12-14,18 In early-phase leptospirosis, symptoms can mimic those of influenza, meningitis, malaria, dengue fever, scrub typhus, rickettsial disease, and typhoid fever (see Table 2).10 Thus, when a patient presents with these symptoms, it is imperative that the clinician consider leptospirosis.19Of note: Flu-like symptoms with conjunctival suffusion are considered pathognomonic for leptospirosis.18
About 10% of patients with early-phase leptospirosis will develop late-phase disease (ie, Weil’s disease), with severe symptoms that include jaundice, meningitis, pulmonary hemorrhage, and acute kidney injury (see Table 3 for a more detailed list).20 The case patient’s history and symptoms were consistent with a diagnosis of early-phase leptospirosis.
Epidemiology
In 2015, leptospirosis was estimated to affect more than 1 million persons worldwide, with 58,900 deaths attributed to the disease each year—making leptospirosis the leading cause of death attributable to zoonotic illness.11 Historically, leptospirosis-associated morbidity and mortality have been greatest in resource-poor countries with tropical climates (eg, southern and Southeast Asia, Central America and tropical Latin America, and East Sub-Saharan Africa).11,12
However, illness resulting from recreational exposures to contaminated water has been linked to increasing travel to exotic destinations, participation in adventure travel, and the growing popularity of extreme sports involving fresh water.9 Recreational mud run events, for example, involve swimming in potentially contaminated waters and crawling through flooded farm fields where animal urine can be present—an ideal environment for Leptospira to thrive and for participants to contract the disease.14,15
Continue for laboratory work-up >>
Laboratory work-up
Diagnosis of leptospirosis is challenging.21 Laboratory tests vary, depending on the timing and stage of infection, and are mostly unavailable in resource-poor countries. Test results for the patient with early-phase leptospirosis may demonstrate renal or hepatic abnormalities.18 However, laboratory confirmation of leptospirosis requires22
• A fourfold increase in antibody titer between acute and convalescent serum samples, as detected by microscopic agglutination testing (MAT) or
• A high MAT titer (> 1:400 to 1:800), in single or paired samples or
• Isolation of pathogenic Leptospira species from a normally sterile site or
• Detection of DNA from pathogenic Leptospira species by PCR
A positive laboratory result is, of course, confirmatory. However, negative laboratory findings must be viewed with healthy skepticism.12 A false-negative result may merely indicate the shortcoming of the testing method to accurately assess the presence of Leptospira.
Treatment options
The high mortality rate associated with severe leptospirosis makes early diagnosis and treatment essential.23 The World Health Organization warns that antibiotic treatment for leptospirosis must be instituted within five days of symptom onset.10
Treatment options for an ambulatory patient with mild symptoms and no organ involvement include oral doxycycline (100 mg bid for 5-7 d) or oral azithromycin (500 mg/d for 5-7 d). For patients with organ involvement, IV penicillin (1.5 million U every 6 h for 7 d), ceftriaxone (1 g/d for 7 d), or cefotaxime (1 g every 6 h for 7 d) may be considered.12,20
OUTCOME FOR THE CASE PATIENT
With leptospirosis as the diagnosis of exclusion, Jane was treated successfully with a 21-day course of oral doxycycline (100 mg bid). She has been symptom free since completing the regimen. After undergoing physical therapy and athletic training, she has been able to resume her full exercise regimen, and her recovery is considered complete.
CONCLUSION
The growing popularity of adventure travel and “extreme sports” events, particularly triathlons and mud runs, may precipitate an increase in associated infections with Leptospira and other zoonotic pathogens. For patients with flulike symptoms who routinely engage in such sports—especially those who present with conjunctival suffusion—leptospirosis should be considered in the differential diagnosis.
REFERENCES
1. Owens RC Jr, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(suppl 2):S144-S157.
2. CDC. Signs and symptoms of untreated Lyme disease (2015). www.cdc.gov/lyme/signs_symptoms/index.html. Accessed June 7, 2016.
3. CDC. Parasites: babesiosis (2014). www.cdc.gov/parasites/babesiosis/disease.html. Accessed June 7, 2016.
4. CDC. Ehrlichiosis: symptoms, diagnosis, and treatment (2013). www.cdc.gov/Ehrlichiosis/symptoms/index.html. Accessed June 7, 2016.
5. Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016 Feb 5. [Epub ahead of print]
6. Choi E, Pyzocha NJ, Maurer DM. Tick-borne illnesses. Curr Sports Med Rep. 2016;15(2):98-104.
7. Chomel B. Lyme disease. Rev Sci Tech. 2015;34(2):569-576.
8. Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296-326.
9. CDC. Leptospirosis: signs and symptoms (2016). www.cdc.gov/leptospirosis/symptoms/index.html. Accessed June 7, 2016.
10. World Health Organization, International Leptospirosis Society. Human Leptospirosis: Guidance for Diagnosis, Surveillance, and Control (2003). http://apps.who.int/iris/bitstream/10665/42667/1/WHO_CDS_CSR_EPH_2002.23.pdf. Accessed June 7, 2016.
11. Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898.
12. Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65-97.
13. Picardeau M. Diagnosis and epidemiology of leptospirosis. Médecine et Maladies Infectieuses. 2013;43(1):1-9.
14. Picardeau M. Leptospirosis: updating the global picture of an emerging neglected disease. PLoS Negl Trop Dis. 2015;9(9):e0004039.
15. Zavitsanou A, Babatsikou F. Leptospirosis: epidemiology and preventive measures. Health Sci J. 2008;2(2):75-82.
16. Mwachui MA, Crump L, Hartskeerl R, et al. Environmental and behavioural determinants of leptospirosis transmission: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003843.
17. Morgan J, Bornstein SL, Karpati AM, et al. Outbreak of leptospirosis among triathlon participants and community residents in Springfield, Illinois, 1998. Clin Infect Dis. 2002;34(12):1593-1599.
18. Katz AR, Ansdell VE, Effler PV, et al. Assessment of the clinical presentation and treatment of 353 cases of laboratory-confirmed leptospirosis in Hawaii, 1974-1998. Clin Infect Dis. 2001;33(11):1834-1841.
19. Yaakob Y, Rodrigues KF, John DV. Leptospirosis: recent incidents and available diagnostics—a review. Med J Malaysia. 2015;70(6):351-355.
20. Seguro AC, Andrade L. Pathophysiology of leptospirosis. Shock. 2013;39(suppl 1):17-23.
21. Musso D, La Scola B. Laboratory diagnosis of leptospirosis: a challenge. J Microbiol Immunol Infect. 2013;46(4):245-252.
22. Waggoner JJ, Balassiano I, Mohamed-Hadley A, et al. Reverse-transcriptase PCR detection of Leptospira: absence of agreement with single-specimen microscopic agglutination testing. PLoS One. 2015;10(7):e0132988.
23. Iwasaki H, Chagan-Yasutan H, Leano PS, et al. Combined antibody and DNA detection for early diagnosis of leptospirosis after a disaster. Diagn Microbiol Infect Dis. 2016;84(4):287-291
IN THIS ARTICLE
- Adverse effects of ciprofloxacin
- Symptoms of common tick-borne diseases
- Symptoms of phase 1 and late-phase disease
- Additional resources
Jane, an 18-year-old college student, presents in early November with a three-week history of worsening cough and sinus congestion. Recently, the cough has been interrupting her sleep and yellow-green nasal drainage and sinus pressure have increased. Ordinarily very fit and athletic, she reports that since she arrived at college two months ago, her body has become “more fragile.”
Further questioning reveals that, over the past two months, the patient’s symptoms have included extreme fatigue, severe unremitting headache, blurred vision, shortness of breath, and a racing heart rate on exertion. Her symptoms make it impossible for her to maintain her demanding exercise routine, a development that compounds her frustration and sadness. She has also been forced to limit her participation in school activities, with significant academic decline as a result.
Aside from depression (well controlled with bupropion HCl extended release, 300 mg/d), Jane’s medical history is unremarkable. She reports having “excellent health” until she arrived at her mid-Atlantic urban college.
A complicated history
Born and raised in Connecticut, Jane is an avid runner who competes in extreme sports. This past summer, she trained for and participated in two “mud run” events (ie, endurance races of several miles with numerous challenges and obstacles) in Connecticut and New York. Training included endurance runs and sprints, as well as crawling through mud-laden fields and woods.
She also did a three-week summer internship on an oyster farm. There, she was required to shuck oysters and stand in brackish water for six-hour shifts to examine oyster beds. In the process, she sustained numerous cuts and bruises on her hands, arms, and legs.
A week or so after returning to college in late August, Jane developed blisters on both heels, which progressed to infected ulcerations. She was evaluated at the university hospital emergency department (ED) and treated with a 21-day course of ciprofloxacin. When left-sided unilateral knee swelling developed about two weeks later, she underwent arthrocentesis at the university health center, but joint aspirate was not sent for analysis. A two-week course of antibiotic therapy was initiated.
From October to her presentation in early November, Jane has experienced intermittent fevers and chills, with a temperature as high as 101°F. In addition, she complains of fasciculations and weakness in her lower limbs; dyspnea, tachycardia, and dizziness during or after any exertion; unremitting posterior neck pain; and a constant, severe headache located primarily in the bitemporal region. She developed bilateral conjunctivitis, which resolved spontaneously in about one week; persistent blurred vision; a transient petechial chest rash; recurring episodes of syncope; pyelonephritis; a persistent vaginal yeast infection; decreased appetite; and a 7-lb weight loss (5% of her total body weight).
Jane’s academic and athletic performance has been severely impaired. Once a long-distance runner, she can no longer walk any distance without frequent rest. In the four months since the mud runs, the patient reports, she has been seen in the student health center four times and in the ED twice. Additionally, she has undergone thorough examinations by clinicians specializing in infectious disease, pulmonology, neurology, and neuro-ophthalmology. She has undergone lab work, including
• Complete blood cell count with differential
• Comprehensive metabolic panel
• Urinalysis and urine culture
• Lyme antibody and blood polymerase chain reaction (PCR)
• HIV testing
• Rheumatoid factor
• Erythrocyte sedimentation rate (ESR)
• C-reactive protein (CRP)
• Epstein-Barr virus IgM
• Cytomegalovirus (CMV) IgM
• Human granulocytic ehrlichiosis (HGE) antibody and human anaplasma phagocytophilum (HGA)
• HGA PCR
• Rickettsia antibody panel
• Babesia microti antibodies
• Pregnancy testing
• Chest x-ray
• Lumbar puncture
All lab results were within normal range. In light of this, several clinicians have told Jane that her illness is “all in her head.”
Continue for the patient investigates >>
The patient investigates
In mid-December, after she has returned home from college, Jane’s symptoms abruptly worsen. She complains of feeling “shakier,” with weakness in her legs and what she calls “brain fog.” Her headache, blurred vision, and dizziness have worsened. Frightened and concerned, she returns to the ED. Results of a thorough evaluation, including lumbar puncture, reveal no abnormality.
Jane has become extremely frail. She is losing weight, her hair has lost its luster, and her nails are cracking and bleeding. She is unable to walk without concern for falling and cannot climb the 20 steps to her bedroom. Once a healthy and vibrant 18-year-old, she now spends most of her time in a lethargic state on a first-floor living room couch.
Frustrated by her unexplained declining health, she begins to research illnesses associated with extreme sports and prolonged marine exposure. She returns to ask about three possible explanations for her condition:
1. Adverse effects of ciprofloxacin use, which include fever or chills, dizziness, racing heartbeat, headache, and nausea.1
2. A tick-borne disease, possibly contracted during her practice runs in the Connecticut woods (see Table 1).2-4 Each year, she recalls, she has found and removed four or five embedded ticks. In the northeastern United States, the most common tick-borne diseases are borreliosis, babesiosis, and ehrlichiosis.5-7
3. Leptospirosis, contracted through the patient’s exposure to mud and brackish water during her summer activities. According to her research, more than 25 outbreaks and 600 cases of leptospirosis (between 1931 and 1998) have been associated with fresh pond, creek, or river water.8
Based on Jane’s symptoms and history, and in accord with her research, early-phase leptospirosis is identified as a diagnosis of exclusion (with a possible comorbid tick-borne zoonosis).
Continue for discussion >>
DISCUSSION
Leptospirosis develops when humans come into contact with animal urine infected by leptospires—that is, pathogenic spirochetes excreted via the renal tubules of infected host animals.9,10 While host animals include dogs, pigs, cattle, reptiles, and amphibians, the animal most commonly associated with human infection is the brown rat (Rattus norvegicus).11-15
Leptospires enter the human host through mucous membranes, cuts, or abrasions in the skin. Individuals at increased risk for infection include those whose work or other activities expose them “to animal reservoirs or contaminated environments”—including participants in water sports and similar recreation.11-14 As Mwachui et al explain, “recreational exposure to [Leptospira-]contaminated water has become more important for sport enthusiasts, swimmers and travellers from industrialized countries,” whereas flooding is usually involved in infection in undeveloped countries.16
The largest outbreak of leptospirosis reported in the US to date occurred in 1998, when heavy rains preceded a triathlon in Springfield, Illinois. When many participants became ill after the event, researchers from the National Center for Infectious Diseases were able to contact and test 834 of the 876 competing athletes; of these, 98 (12%) reported being ill and 52 (11%) tested positive for leptospirosis. Additionally, 14 of the 248 community residents who were sickened (6%) tested positive.17 According to CDC estimates, between 100 and 200 cases of leptospirosis develop annually in the US, with about half occurring in Hawaii.9
Onset of symptoms, which are described as protean and nonspecific, occurs two days to four weeks after exposure, making leptospirosis difficult to diagnosewithout a high degree of suspicion; zoonotic exposure (as with freshwater or mud sports) or a history of travel to Hawaii, Tahiti, Thailand, Indonesia, the Caribbean, and/or Costa Rica may raise suspicion.12-14,18 In early-phase leptospirosis, symptoms can mimic those of influenza, meningitis, malaria, dengue fever, scrub typhus, rickettsial disease, and typhoid fever (see Table 2).10 Thus, when a patient presents with these symptoms, it is imperative that the clinician consider leptospirosis.19Of note: Flu-like symptoms with conjunctival suffusion are considered pathognomonic for leptospirosis.18
About 10% of patients with early-phase leptospirosis will develop late-phase disease (ie, Weil’s disease), with severe symptoms that include jaundice, meningitis, pulmonary hemorrhage, and acute kidney injury (see Table 3 for a more detailed list).20 The case patient’s history and symptoms were consistent with a diagnosis of early-phase leptospirosis.
Epidemiology
In 2015, leptospirosis was estimated to affect more than 1 million persons worldwide, with 58,900 deaths attributed to the disease each year—making leptospirosis the leading cause of death attributable to zoonotic illness.11 Historically, leptospirosis-associated morbidity and mortality have been greatest in resource-poor countries with tropical climates (eg, southern and Southeast Asia, Central America and tropical Latin America, and East Sub-Saharan Africa).11,12
However, illness resulting from recreational exposures to contaminated water has been linked to increasing travel to exotic destinations, participation in adventure travel, and the growing popularity of extreme sports involving fresh water.9 Recreational mud run events, for example, involve swimming in potentially contaminated waters and crawling through flooded farm fields where animal urine can be present—an ideal environment for Leptospira to thrive and for participants to contract the disease.14,15
Continue for laboratory work-up >>
Laboratory work-up
Diagnosis of leptospirosis is challenging.21 Laboratory tests vary, depending on the timing and stage of infection, and are mostly unavailable in resource-poor countries. Test results for the patient with early-phase leptospirosis may demonstrate renal or hepatic abnormalities.18 However, laboratory confirmation of leptospirosis requires22
• A fourfold increase in antibody titer between acute and convalescent serum samples, as detected by microscopic agglutination testing (MAT) or
• A high MAT titer (> 1:400 to 1:800), in single or paired samples or
• Isolation of pathogenic Leptospira species from a normally sterile site or
• Detection of DNA from pathogenic Leptospira species by PCR
A positive laboratory result is, of course, confirmatory. However, negative laboratory findings must be viewed with healthy skepticism.12 A false-negative result may merely indicate the shortcoming of the testing method to accurately assess the presence of Leptospira.
Treatment options
The high mortality rate associated with severe leptospirosis makes early diagnosis and treatment essential.23 The World Health Organization warns that antibiotic treatment for leptospirosis must be instituted within five days of symptom onset.10
Treatment options for an ambulatory patient with mild symptoms and no organ involvement include oral doxycycline (100 mg bid for 5-7 d) or oral azithromycin (500 mg/d for 5-7 d). For patients with organ involvement, IV penicillin (1.5 million U every 6 h for 7 d), ceftriaxone (1 g/d for 7 d), or cefotaxime (1 g every 6 h for 7 d) may be considered.12,20
OUTCOME FOR THE CASE PATIENT
With leptospirosis as the diagnosis of exclusion, Jane was treated successfully with a 21-day course of oral doxycycline (100 mg bid). She has been symptom free since completing the regimen. After undergoing physical therapy and athletic training, she has been able to resume her full exercise regimen, and her recovery is considered complete.
CONCLUSION
The growing popularity of adventure travel and “extreme sports” events, particularly triathlons and mud runs, may precipitate an increase in associated infections with Leptospira and other zoonotic pathogens. For patients with flulike symptoms who routinely engage in such sports—especially those who present with conjunctival suffusion—leptospirosis should be considered in the differential diagnosis.
REFERENCES
1. Owens RC Jr, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(suppl 2):S144-S157.
2. CDC. Signs and symptoms of untreated Lyme disease (2015). www.cdc.gov/lyme/signs_symptoms/index.html. Accessed June 7, 2016.
3. CDC. Parasites: babesiosis (2014). www.cdc.gov/parasites/babesiosis/disease.html. Accessed June 7, 2016.
4. CDC. Ehrlichiosis: symptoms, diagnosis, and treatment (2013). www.cdc.gov/Ehrlichiosis/symptoms/index.html. Accessed June 7, 2016.
5. Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016 Feb 5. [Epub ahead of print]
6. Choi E, Pyzocha NJ, Maurer DM. Tick-borne illnesses. Curr Sports Med Rep. 2016;15(2):98-104.
7. Chomel B. Lyme disease. Rev Sci Tech. 2015;34(2):569-576.
8. Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296-326.
9. CDC. Leptospirosis: signs and symptoms (2016). www.cdc.gov/leptospirosis/symptoms/index.html. Accessed June 7, 2016.
10. World Health Organization, International Leptospirosis Society. Human Leptospirosis: Guidance for Diagnosis, Surveillance, and Control (2003). http://apps.who.int/iris/bitstream/10665/42667/1/WHO_CDS_CSR_EPH_2002.23.pdf. Accessed June 7, 2016.
11. Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898.
12. Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65-97.
13. Picardeau M. Diagnosis and epidemiology of leptospirosis. Médecine et Maladies Infectieuses. 2013;43(1):1-9.
14. Picardeau M. Leptospirosis: updating the global picture of an emerging neglected disease. PLoS Negl Trop Dis. 2015;9(9):e0004039.
15. Zavitsanou A, Babatsikou F. Leptospirosis: epidemiology and preventive measures. Health Sci J. 2008;2(2):75-82.
16. Mwachui MA, Crump L, Hartskeerl R, et al. Environmental and behavioural determinants of leptospirosis transmission: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003843.
17. Morgan J, Bornstein SL, Karpati AM, et al. Outbreak of leptospirosis among triathlon participants and community residents in Springfield, Illinois, 1998. Clin Infect Dis. 2002;34(12):1593-1599.
18. Katz AR, Ansdell VE, Effler PV, et al. Assessment of the clinical presentation and treatment of 353 cases of laboratory-confirmed leptospirosis in Hawaii, 1974-1998. Clin Infect Dis. 2001;33(11):1834-1841.
19. Yaakob Y, Rodrigues KF, John DV. Leptospirosis: recent incidents and available diagnostics—a review. Med J Malaysia. 2015;70(6):351-355.
20. Seguro AC, Andrade L. Pathophysiology of leptospirosis. Shock. 2013;39(suppl 1):17-23.
21. Musso D, La Scola B. Laboratory diagnosis of leptospirosis: a challenge. J Microbiol Immunol Infect. 2013;46(4):245-252.
22. Waggoner JJ, Balassiano I, Mohamed-Hadley A, et al. Reverse-transcriptase PCR detection of Leptospira: absence of agreement with single-specimen microscopic agglutination testing. PLoS One. 2015;10(7):e0132988.
23. Iwasaki H, Chagan-Yasutan H, Leano PS, et al. Combined antibody and DNA detection for early diagnosis of leptospirosis after a disaster. Diagn Microbiol Infect Dis. 2016;84(4):287-291
IN THIS ARTICLE
- Adverse effects of ciprofloxacin
- Symptoms of common tick-borne diseases
- Symptoms of phase 1 and late-phase disease
- Additional resources
Jane, an 18-year-old college student, presents in early November with a three-week history of worsening cough and sinus congestion. Recently, the cough has been interrupting her sleep and yellow-green nasal drainage and sinus pressure have increased. Ordinarily very fit and athletic, she reports that since she arrived at college two months ago, her body has become “more fragile.”
Further questioning reveals that, over the past two months, the patient’s symptoms have included extreme fatigue, severe unremitting headache, blurred vision, shortness of breath, and a racing heart rate on exertion. Her symptoms make it impossible for her to maintain her demanding exercise routine, a development that compounds her frustration and sadness. She has also been forced to limit her participation in school activities, with significant academic decline as a result.
Aside from depression (well controlled with bupropion HCl extended release, 300 mg/d), Jane’s medical history is unremarkable. She reports having “excellent health” until she arrived at her mid-Atlantic urban college.
A complicated history
Born and raised in Connecticut, Jane is an avid runner who competes in extreme sports. This past summer, she trained for and participated in two “mud run” events (ie, endurance races of several miles with numerous challenges and obstacles) in Connecticut and New York. Training included endurance runs and sprints, as well as crawling through mud-laden fields and woods.
She also did a three-week summer internship on an oyster farm. There, she was required to shuck oysters and stand in brackish water for six-hour shifts to examine oyster beds. In the process, she sustained numerous cuts and bruises on her hands, arms, and legs.
A week or so after returning to college in late August, Jane developed blisters on both heels, which progressed to infected ulcerations. She was evaluated at the university hospital emergency department (ED) and treated with a 21-day course of ciprofloxacin. When left-sided unilateral knee swelling developed about two weeks later, she underwent arthrocentesis at the university health center, but joint aspirate was not sent for analysis. A two-week course of antibiotic therapy was initiated.
From October to her presentation in early November, Jane has experienced intermittent fevers and chills, with a temperature as high as 101°F. In addition, she complains of fasciculations and weakness in her lower limbs; dyspnea, tachycardia, and dizziness during or after any exertion; unremitting posterior neck pain; and a constant, severe headache located primarily in the bitemporal region. She developed bilateral conjunctivitis, which resolved spontaneously in about one week; persistent blurred vision; a transient petechial chest rash; recurring episodes of syncope; pyelonephritis; a persistent vaginal yeast infection; decreased appetite; and a 7-lb weight loss (5% of her total body weight).
Jane’s academic and athletic performance has been severely impaired. Once a long-distance runner, she can no longer walk any distance without frequent rest. In the four months since the mud runs, the patient reports, she has been seen in the student health center four times and in the ED twice. Additionally, she has undergone thorough examinations by clinicians specializing in infectious disease, pulmonology, neurology, and neuro-ophthalmology. She has undergone lab work, including
• Complete blood cell count with differential
• Comprehensive metabolic panel
• Urinalysis and urine culture
• Lyme antibody and blood polymerase chain reaction (PCR)
• HIV testing
• Rheumatoid factor
• Erythrocyte sedimentation rate (ESR)
• C-reactive protein (CRP)
• Epstein-Barr virus IgM
• Cytomegalovirus (CMV) IgM
• Human granulocytic ehrlichiosis (HGE) antibody and human anaplasma phagocytophilum (HGA)
• HGA PCR
• Rickettsia antibody panel
• Babesia microti antibodies
• Pregnancy testing
• Chest x-ray
• Lumbar puncture
All lab results were within normal range. In light of this, several clinicians have told Jane that her illness is “all in her head.”
Continue for the patient investigates >>
The patient investigates
In mid-December, after she has returned home from college, Jane’s symptoms abruptly worsen. She complains of feeling “shakier,” with weakness in her legs and what she calls “brain fog.” Her headache, blurred vision, and dizziness have worsened. Frightened and concerned, she returns to the ED. Results of a thorough evaluation, including lumbar puncture, reveal no abnormality.
Jane has become extremely frail. She is losing weight, her hair has lost its luster, and her nails are cracking and bleeding. She is unable to walk without concern for falling and cannot climb the 20 steps to her bedroom. Once a healthy and vibrant 18-year-old, she now spends most of her time in a lethargic state on a first-floor living room couch.
Frustrated by her unexplained declining health, she begins to research illnesses associated with extreme sports and prolonged marine exposure. She returns to ask about three possible explanations for her condition:
1. Adverse effects of ciprofloxacin use, which include fever or chills, dizziness, racing heartbeat, headache, and nausea.1
2. A tick-borne disease, possibly contracted during her practice runs in the Connecticut woods (see Table 1).2-4 Each year, she recalls, she has found and removed four or five embedded ticks. In the northeastern United States, the most common tick-borne diseases are borreliosis, babesiosis, and ehrlichiosis.5-7
3. Leptospirosis, contracted through the patient’s exposure to mud and brackish water during her summer activities. According to her research, more than 25 outbreaks and 600 cases of leptospirosis (between 1931 and 1998) have been associated with fresh pond, creek, or river water.8
Based on Jane’s symptoms and history, and in accord with her research, early-phase leptospirosis is identified as a diagnosis of exclusion (with a possible comorbid tick-borne zoonosis).
Continue for discussion >>
DISCUSSION
Leptospirosis develops when humans come into contact with animal urine infected by leptospires—that is, pathogenic spirochetes excreted via the renal tubules of infected host animals.9,10 While host animals include dogs, pigs, cattle, reptiles, and amphibians, the animal most commonly associated with human infection is the brown rat (Rattus norvegicus).11-15
Leptospires enter the human host through mucous membranes, cuts, or abrasions in the skin. Individuals at increased risk for infection include those whose work or other activities expose them “to animal reservoirs or contaminated environments”—including participants in water sports and similar recreation.11-14 As Mwachui et al explain, “recreational exposure to [Leptospira-]contaminated water has become more important for sport enthusiasts, swimmers and travellers from industrialized countries,” whereas flooding is usually involved in infection in undeveloped countries.16
The largest outbreak of leptospirosis reported in the US to date occurred in 1998, when heavy rains preceded a triathlon in Springfield, Illinois. When many participants became ill after the event, researchers from the National Center for Infectious Diseases were able to contact and test 834 of the 876 competing athletes; of these, 98 (12%) reported being ill and 52 (11%) tested positive for leptospirosis. Additionally, 14 of the 248 community residents who were sickened (6%) tested positive.17 According to CDC estimates, between 100 and 200 cases of leptospirosis develop annually in the US, with about half occurring in Hawaii.9
Onset of symptoms, which are described as protean and nonspecific, occurs two days to four weeks after exposure, making leptospirosis difficult to diagnosewithout a high degree of suspicion; zoonotic exposure (as with freshwater or mud sports) or a history of travel to Hawaii, Tahiti, Thailand, Indonesia, the Caribbean, and/or Costa Rica may raise suspicion.12-14,18 In early-phase leptospirosis, symptoms can mimic those of influenza, meningitis, malaria, dengue fever, scrub typhus, rickettsial disease, and typhoid fever (see Table 2).10 Thus, when a patient presents with these symptoms, it is imperative that the clinician consider leptospirosis.19Of note: Flu-like symptoms with conjunctival suffusion are considered pathognomonic for leptospirosis.18
About 10% of patients with early-phase leptospirosis will develop late-phase disease (ie, Weil’s disease), with severe symptoms that include jaundice, meningitis, pulmonary hemorrhage, and acute kidney injury (see Table 3 for a more detailed list).20 The case patient’s history and symptoms were consistent with a diagnosis of early-phase leptospirosis.
Epidemiology
In 2015, leptospirosis was estimated to affect more than 1 million persons worldwide, with 58,900 deaths attributed to the disease each year—making leptospirosis the leading cause of death attributable to zoonotic illness.11 Historically, leptospirosis-associated morbidity and mortality have been greatest in resource-poor countries with tropical climates (eg, southern and Southeast Asia, Central America and tropical Latin America, and East Sub-Saharan Africa).11,12
However, illness resulting from recreational exposures to contaminated water has been linked to increasing travel to exotic destinations, participation in adventure travel, and the growing popularity of extreme sports involving fresh water.9 Recreational mud run events, for example, involve swimming in potentially contaminated waters and crawling through flooded farm fields where animal urine can be present—an ideal environment for Leptospira to thrive and for participants to contract the disease.14,15
Continue for laboratory work-up >>
Laboratory work-up
Diagnosis of leptospirosis is challenging.21 Laboratory tests vary, depending on the timing and stage of infection, and are mostly unavailable in resource-poor countries. Test results for the patient with early-phase leptospirosis may demonstrate renal or hepatic abnormalities.18 However, laboratory confirmation of leptospirosis requires22
• A fourfold increase in antibody titer between acute and convalescent serum samples, as detected by microscopic agglutination testing (MAT) or
• A high MAT titer (> 1:400 to 1:800), in single or paired samples or
• Isolation of pathogenic Leptospira species from a normally sterile site or
• Detection of DNA from pathogenic Leptospira species by PCR
A positive laboratory result is, of course, confirmatory. However, negative laboratory findings must be viewed with healthy skepticism.12 A false-negative result may merely indicate the shortcoming of the testing method to accurately assess the presence of Leptospira.
Treatment options
The high mortality rate associated with severe leptospirosis makes early diagnosis and treatment essential.23 The World Health Organization warns that antibiotic treatment for leptospirosis must be instituted within five days of symptom onset.10
Treatment options for an ambulatory patient with mild symptoms and no organ involvement include oral doxycycline (100 mg bid for 5-7 d) or oral azithromycin (500 mg/d for 5-7 d). For patients with organ involvement, IV penicillin (1.5 million U every 6 h for 7 d), ceftriaxone (1 g/d for 7 d), or cefotaxime (1 g every 6 h for 7 d) may be considered.12,20
OUTCOME FOR THE CASE PATIENT
With leptospirosis as the diagnosis of exclusion, Jane was treated successfully with a 21-day course of oral doxycycline (100 mg bid). She has been symptom free since completing the regimen. After undergoing physical therapy and athletic training, she has been able to resume her full exercise regimen, and her recovery is considered complete.
CONCLUSION
The growing popularity of adventure travel and “extreme sports” events, particularly triathlons and mud runs, may precipitate an increase in associated infections with Leptospira and other zoonotic pathogens. For patients with flulike symptoms who routinely engage in such sports—especially those who present with conjunctival suffusion—leptospirosis should be considered in the differential diagnosis.
REFERENCES
1. Owens RC Jr, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(suppl 2):S144-S157.
2. CDC. Signs and symptoms of untreated Lyme disease (2015). www.cdc.gov/lyme/signs_symptoms/index.html. Accessed June 7, 2016.
3. CDC. Parasites: babesiosis (2014). www.cdc.gov/parasites/babesiosis/disease.html. Accessed June 7, 2016.
4. CDC. Ehrlichiosis: symptoms, diagnosis, and treatment (2013). www.cdc.gov/Ehrlichiosis/symptoms/index.html. Accessed June 7, 2016.
5. Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016 Feb 5. [Epub ahead of print]
6. Choi E, Pyzocha NJ, Maurer DM. Tick-borne illnesses. Curr Sports Med Rep. 2016;15(2):98-104.
7. Chomel B. Lyme disease. Rev Sci Tech. 2015;34(2):569-576.
8. Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296-326.
9. CDC. Leptospirosis: signs and symptoms (2016). www.cdc.gov/leptospirosis/symptoms/index.html. Accessed June 7, 2016.
10. World Health Organization, International Leptospirosis Society. Human Leptospirosis: Guidance for Diagnosis, Surveillance, and Control (2003). http://apps.who.int/iris/bitstream/10665/42667/1/WHO_CDS_CSR_EPH_2002.23.pdf. Accessed June 7, 2016.
11. Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898.
12. Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65-97.
13. Picardeau M. Diagnosis and epidemiology of leptospirosis. Médecine et Maladies Infectieuses. 2013;43(1):1-9.
14. Picardeau M. Leptospirosis: updating the global picture of an emerging neglected disease. PLoS Negl Trop Dis. 2015;9(9):e0004039.
15. Zavitsanou A, Babatsikou F. Leptospirosis: epidemiology and preventive measures. Health Sci J. 2008;2(2):75-82.
16. Mwachui MA, Crump L, Hartskeerl R, et al. Environmental and behavioural determinants of leptospirosis transmission: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003843.
17. Morgan J, Bornstein SL, Karpati AM, et al. Outbreak of leptospirosis among triathlon participants and community residents in Springfield, Illinois, 1998. Clin Infect Dis. 2002;34(12):1593-1599.
18. Katz AR, Ansdell VE, Effler PV, et al. Assessment of the clinical presentation and treatment of 353 cases of laboratory-confirmed leptospirosis in Hawaii, 1974-1998. Clin Infect Dis. 2001;33(11):1834-1841.
19. Yaakob Y, Rodrigues KF, John DV. Leptospirosis: recent incidents and available diagnostics—a review. Med J Malaysia. 2015;70(6):351-355.
20. Seguro AC, Andrade L. Pathophysiology of leptospirosis. Shock. 2013;39(suppl 1):17-23.
21. Musso D, La Scola B. Laboratory diagnosis of leptospirosis: a challenge. J Microbiol Immunol Infect. 2013;46(4):245-252.
22. Waggoner JJ, Balassiano I, Mohamed-Hadley A, et al. Reverse-transcriptase PCR detection of Leptospira: absence of agreement with single-specimen microscopic agglutination testing. PLoS One. 2015;10(7):e0132988.
23. Iwasaki H, Chagan-Yasutan H, Leano PS, et al. Combined antibody and DNA detection for early diagnosis of leptospirosis after a disaster. Diagn Microbiol Infect Dis. 2016;84(4):287-291
Febrile, Immunocompromised Man With Rash
IN THIS ARTICLE
- Conditions associated with increased risk for case disease
- Outcome for the case patient
- Differential diagnosis
A 78-year-old white man with chronic lymphocytic leukemia is admitted to the hospital with worsening cough, shortness of breath, and fever. His medical history is significant for pneumonia caused by Pneumocystis jirovecii in the past year. In the weeks preceding hospital admission, the patient developed an erythematous rash over his trunk (see photographs).
During the man’s hospital stay, this eruption becomes increasingly pruritic and spreads to his proximal extremities. His pulmonary symptoms improve slightly following the initiation of broad-spectrum antibiotic therapy (piperacillin/tazobactam and vancomycin), but CT performed one week after admission reveals worsening pulmonary disease (see image). The radiologist’s differential diagnosis includes neoplasm, fungal infection, Kaposi sarcoma, and autoimmune disease.
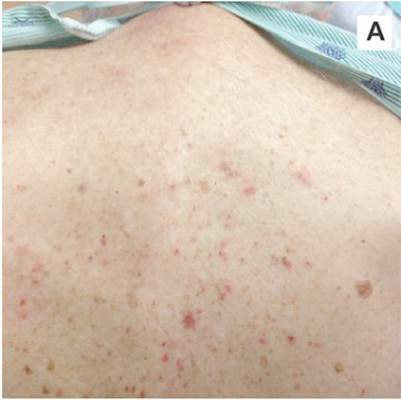 | 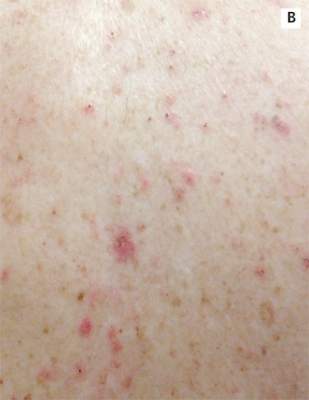 |
| A. The patient's back shows a distribution of lesions, with areas of excoriation caused by scratching. | B. A close-up reveals erythematous papules and keratotic papules. |
Suspecting that the progressive rash is related to the systemic process, the provider orders a punch biopsy in an effort to reach a diagnosis with minimally invasive studies. When the patient’s clinical status further declines, he undergoes video-assisted thoracoscopic surgery to obtain an excisional biopsy of one of the pulmonary nodules. Subsequent analysis reveals fungal organisms consistent with histoplasmosis. Interestingly, in the histologic review of the skin biopsy, focal acantholytic dyskeratosis—suggestive of Grover disease—is identified.
Continue for discussion >>
DISCUSSION
Grover disease (GD), also known as transient acantholytic dermatosis, is a skin condition of uncertain pathophysiology. Its clinical presentation can be difficult to distinguish from other dermopathies.1,2
Incidence
GD most commonly appears in fair-skinned persons of late middle age, with men affected at two to three times the rate seen in women.1,2 Although GD has been documented in patients ranging in age from 4 to 100, this dermopathy is rare in younger patients.1-3 Persons with a prior history of atopic dermatitis, contact dermatitis, or xerosis cutis are at increased risk for GD—likely due to an increased dermatologic sensitivity to irritants resulting from the aforementioned disorders.1,4 Risk for GD is also elevated in patients with chronic medical conditions, immunodeficiency, febrile illnesses, or malignancies (see Table 1).2-5
The true incidence of GD is not known; biopsy-proven GD is uncommon, and specific data on the incidence and prevalence of the condition are lacking. Swiss researchers who reviewed more than 30,000 skin biopsies in the late 1990s noted only 24 diagnosed cases of GD, and similar findings have been reported in the United States.1,6 However, the variable presentation and often mild nature of GD may result in cases of misdiagnosis, lack of diagnosis, or empiric treatment in the absence of a formal diagnosis.7
Causative factors
Although the pathophysiology of GD is uncertain, the most likely cause is an occlusion of the eccrine glands.3 This is followed by acantholysis, or separation of keratinocytes within the epidermis, which in turn leads to the development of vesicular lesions.
Though diagnosed most often in the winter, GD has also been associated with exposure to sunlight, heat, xerosis, and diaphoresis.1,3 Hospitalized or bedridden patients are at risk for occlusion of the eccrine glands and thus for GD. Use of certain therapies, including sulfadoxine/pyrimethamine (an antimalarial treatment), ionizing radiation, and interleukin-4, may also be precursors for the condition.2
Other exacerbating factors have been suggested, but reports are largely limited to case studies and other anecdotal publications.2 Concrete data regarding the etiology and pathophysiology of GD are still relatively scarce.
Clinical presentation
Patients with GD present with pruritic dermatitis on the trunk and proximal extremities, most classically on the anterior chest and mid back.2,3 The severity of the rash does not necessarily correlate to the degree of pruritus. Some patients report only mild pruritus, while others experience debilitating discomfort and pain. In most cases, erythematous and violaceous papules and vesicles appear first, followed by keratotic erosions.3
GD is a self-limited disorder that often resolves within a few weeks, although some cases will persist for several months.3,5 Severity and duration of symptoms appear to be correlated with increasing age; elderly patients experience worse pruritus for longer periods than do younger patients.2
Although the condition is sometimes referred to as transient acantholytic dermatosis, there are three typical presentations of GD: transient eruptive, persistent pruritic, and chronic asymptomatic.4 Transient eruptive GD presents suddenly, with intense pruritus, and tends to subside over several weeks. Persistent pruritic disease generally causes a milder pruritus, with symptoms that last for several months and are not well controlled by medication. Chronic asymptomatic GD can be difficult to treat medically, yet this form of the disease typically causes little to no irritation and requires minimal therapeutic intervention.4
Systemic symptoms of GD have not been observed. Pruritus and rash are the main features in most affected patients. However, pruritic papulovesicular eruptions are commonly seen in other conditions with similar characteristics (see Table 2,3,4), and GD is comparatively rare. While clinical appearance alone may suggest a diagnosis of GD, further testing may be needed to eliminate other conditions from the differential.
Treatment and prognosis
In the absence of randomized therapeutic trials for GD, there are no strict guidelines for treatment. When irritation, inflammation, and pruritus become bothersome, several interventions may be considered. The first step may consist of efforts to modify aggravating factors, such as dry skin, occlusion, excess heat, and rapid temperature changes. Indeed, for mild cases of GD, this may be all that is required.
The firstline pharmacotherapy for GD is medium- to high-potency topical corticosteroids, which reduce inflammation and pruritus in approximately half of affected patients.3,6,8 Topical emollients and oral antihistamines can also provide symptom relief. Vitamin D analogues are considered secondline therapy, and retinoids (both topical and systemic) have also been shown to reduce GD severity.3,4,8
Severe, refractory cases may require more aggressive systemic therapy with corticosteroids or retinoids. For pruritic relief, several weeks of oral corticosteroids may be necessary—and GD may rebound after treatment ceases.3,4 Therefore, oral corticosteroids should only be considered for severe or persistent cases, since the systemic adverse effects (eg, immunosuppression, weight gain, dysglycemia) of these drugs may outweigh the benefits in patients with GD. Other interventions, including phototherapy and immunosuppressive drugs (eg, etanercept) have also demonstrated benefit in select patients.4,9,10
The self-limited nature of GD, along with its lack of systemic symptoms, is associated with a generally benign course of disease and no long-term sequelae.3,5
Continue to outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
This case involved an immunocompromised patient with systemic symptoms, vasculitic cutaneous lesions, and significant pulmonary disease. The differential diagnosis was extensive, and diagnosis based on clinical grounds alone was extremely challenging. In these circumstances, diagnostic testing was essential to reach a final diagnosis.
In this case, the skin biopsy yielded a diagnosis of GD, and the rash was found to be unrelated to the patient’s systemic and pulmonary symptoms. The providers were then able to focus on the diagnosis of histoplasmosis, with only minimal intervention for the patient’s GD (ie, oral diphenhydramine prn for pruritus).
CONCLUSION
In many cases of GD, skin biopsy can guide providers when the history and physical examination do not yield a clear diagnosis. The histopathology of affected tissue can provide invaluable information about an underlying disease process, particularly in complex cases such as this patient’s. Skin biopsy provides a minimally invasive opportunity to obtain a diagnosis in patients with a condition that affects multiple organ systems, and its use should be considered in disease processes with cutaneous manifestations.
REFERENCES
1. Scheinfeld N, Mones J. Seasonal variation of transient acantholytic dyskeratosis (Grover’s disease). J Am Acad Dermatol. 2006;55(2): 263-268.
2. Parsons JM. Transient acantholytic dermatosis (Grover’s disease): a global perspective. J Am Acad Dermatol. 1996;35(5 part 1):653-666.
3. Weaver J, Bergfeld WF. Grover disease (transient acantholytic dermatosis). Arch Pathol Lab Med. 2009;133(9):1490-1494.
4. Quirk CJ, Heenan PJ. Grover’s disease: 34 years on. Australas J Dermatol. 2004;45(2):83-86.
5. Ippoliti G, Paulli M, Lucioni M, et al. Grover’s disease after heart transplantation: a case report. Case Rep Transplant. 2012;2012:126592.
6. Streit M, Paredes BE, Braathen LR, Brand CU. Transitory acantholytic dermatosis (Grover’s disease): an analysis of the clinical spectrum based on 21 histologically assessed cases [in German]. Hautarzt. 2000;51:244-249.
7. Joshi R, Taneja A. Grover’s disease with acrosyringeal acantholysis: a rare histological presentation of an uncommon disease. Indian J Dermatol. 2014;59(6):621-623.
8. Riemann H, High WA. Grover’s disease (transient and persistent acantholytic dermatosis). UpToDate. 2015. www.uptodate.com/contents/grovers-disease-transient-and-persistent-acantholytic-dermatosis. Accessed June 4, 2016.
9. Breuckmann F, Appelhans C, Altmeyer P, Kreuter A. Medium-dose ultraviolet A1 phototherapy in transient acantholytic dermatosis (Grover’s disease). J Am Acad Dermatol. 2005;52(1):169-170.
10. Norman R, Chau V. Use of etanercept in treating pruritus and preventing new lesions in Grover disease. J Am Acad Dermatol. 2011;64(4):796-798.
IN THIS ARTICLE
- Conditions associated with increased risk for case disease
- Outcome for the case patient
- Differential diagnosis
A 78-year-old white man with chronic lymphocytic leukemia is admitted to the hospital with worsening cough, shortness of breath, and fever. His medical history is significant for pneumonia caused by Pneumocystis jirovecii in the past year. In the weeks preceding hospital admission, the patient developed an erythematous rash over his trunk (see photographs).
During the man’s hospital stay, this eruption becomes increasingly pruritic and spreads to his proximal extremities. His pulmonary symptoms improve slightly following the initiation of broad-spectrum antibiotic therapy (piperacillin/tazobactam and vancomycin), but CT performed one week after admission reveals worsening pulmonary disease (see image). The radiologist’s differential diagnosis includes neoplasm, fungal infection, Kaposi sarcoma, and autoimmune disease.
 |  |
| A. The patient's back shows a distribution of lesions, with areas of excoriation caused by scratching. | B. A close-up reveals erythematous papules and keratotic papules. |
Suspecting that the progressive rash is related to the systemic process, the provider orders a punch biopsy in an effort to reach a diagnosis with minimally invasive studies. When the patient’s clinical status further declines, he undergoes video-assisted thoracoscopic surgery to obtain an excisional biopsy of one of the pulmonary nodules. Subsequent analysis reveals fungal organisms consistent with histoplasmosis. Interestingly, in the histologic review of the skin biopsy, focal acantholytic dyskeratosis—suggestive of Grover disease—is identified.
Continue for discussion >>
DISCUSSION
Grover disease (GD), also known as transient acantholytic dermatosis, is a skin condition of uncertain pathophysiology. Its clinical presentation can be difficult to distinguish from other dermopathies.1,2
Incidence
GD most commonly appears in fair-skinned persons of late middle age, with men affected at two to three times the rate seen in women.1,2 Although GD has been documented in patients ranging in age from 4 to 100, this dermopathy is rare in younger patients.1-3 Persons with a prior history of atopic dermatitis, contact dermatitis, or xerosis cutis are at increased risk for GD—likely due to an increased dermatologic sensitivity to irritants resulting from the aforementioned disorders.1,4 Risk for GD is also elevated in patients with chronic medical conditions, immunodeficiency, febrile illnesses, or malignancies (see Table 1).2-5
The true incidence of GD is not known; biopsy-proven GD is uncommon, and specific data on the incidence and prevalence of the condition are lacking. Swiss researchers who reviewed more than 30,000 skin biopsies in the late 1990s noted only 24 diagnosed cases of GD, and similar findings have been reported in the United States.1,6 However, the variable presentation and often mild nature of GD may result in cases of misdiagnosis, lack of diagnosis, or empiric treatment in the absence of a formal diagnosis.7
Causative factors
Although the pathophysiology of GD is uncertain, the most likely cause is an occlusion of the eccrine glands.3 This is followed by acantholysis, or separation of keratinocytes within the epidermis, which in turn leads to the development of vesicular lesions.
Though diagnosed most often in the winter, GD has also been associated with exposure to sunlight, heat, xerosis, and diaphoresis.1,3 Hospitalized or bedridden patients are at risk for occlusion of the eccrine glands and thus for GD. Use of certain therapies, including sulfadoxine/pyrimethamine (an antimalarial treatment), ionizing radiation, and interleukin-4, may also be precursors for the condition.2
Other exacerbating factors have been suggested, but reports are largely limited to case studies and other anecdotal publications.2 Concrete data regarding the etiology and pathophysiology of GD are still relatively scarce.
Clinical presentation
Patients with GD present with pruritic dermatitis on the trunk and proximal extremities, most classically on the anterior chest and mid back.2,3 The severity of the rash does not necessarily correlate to the degree of pruritus. Some patients report only mild pruritus, while others experience debilitating discomfort and pain. In most cases, erythematous and violaceous papules and vesicles appear first, followed by keratotic erosions.3
GD is a self-limited disorder that often resolves within a few weeks, although some cases will persist for several months.3,5 Severity and duration of symptoms appear to be correlated with increasing age; elderly patients experience worse pruritus for longer periods than do younger patients.2
Although the condition is sometimes referred to as transient acantholytic dermatosis, there are three typical presentations of GD: transient eruptive, persistent pruritic, and chronic asymptomatic.4 Transient eruptive GD presents suddenly, with intense pruritus, and tends to subside over several weeks. Persistent pruritic disease generally causes a milder pruritus, with symptoms that last for several months and are not well controlled by medication. Chronic asymptomatic GD can be difficult to treat medically, yet this form of the disease typically causes little to no irritation and requires minimal therapeutic intervention.4
Systemic symptoms of GD have not been observed. Pruritus and rash are the main features in most affected patients. However, pruritic papulovesicular eruptions are commonly seen in other conditions with similar characteristics (see Table 2,3,4), and GD is comparatively rare. While clinical appearance alone may suggest a diagnosis of GD, further testing may be needed to eliminate other conditions from the differential.
Treatment and prognosis
In the absence of randomized therapeutic trials for GD, there are no strict guidelines for treatment. When irritation, inflammation, and pruritus become bothersome, several interventions may be considered. The first step may consist of efforts to modify aggravating factors, such as dry skin, occlusion, excess heat, and rapid temperature changes. Indeed, for mild cases of GD, this may be all that is required.
The firstline pharmacotherapy for GD is medium- to high-potency topical corticosteroids, which reduce inflammation and pruritus in approximately half of affected patients.3,6,8 Topical emollients and oral antihistamines can also provide symptom relief. Vitamin D analogues are considered secondline therapy, and retinoids (both topical and systemic) have also been shown to reduce GD severity.3,4,8
Severe, refractory cases may require more aggressive systemic therapy with corticosteroids or retinoids. For pruritic relief, several weeks of oral corticosteroids may be necessary—and GD may rebound after treatment ceases.3,4 Therefore, oral corticosteroids should only be considered for severe or persistent cases, since the systemic adverse effects (eg, immunosuppression, weight gain, dysglycemia) of these drugs may outweigh the benefits in patients with GD. Other interventions, including phototherapy and immunosuppressive drugs (eg, etanercept) have also demonstrated benefit in select patients.4,9,10
The self-limited nature of GD, along with its lack of systemic symptoms, is associated with a generally benign course of disease and no long-term sequelae.3,5
Continue to outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
This case involved an immunocompromised patient with systemic symptoms, vasculitic cutaneous lesions, and significant pulmonary disease. The differential diagnosis was extensive, and diagnosis based on clinical grounds alone was extremely challenging. In these circumstances, diagnostic testing was essential to reach a final diagnosis.
In this case, the skin biopsy yielded a diagnosis of GD, and the rash was found to be unrelated to the patient’s systemic and pulmonary symptoms. The providers were then able to focus on the diagnosis of histoplasmosis, with only minimal intervention for the patient’s GD (ie, oral diphenhydramine prn for pruritus).
CONCLUSION
In many cases of GD, skin biopsy can guide providers when the history and physical examination do not yield a clear diagnosis. The histopathology of affected tissue can provide invaluable information about an underlying disease process, particularly in complex cases such as this patient’s. Skin biopsy provides a minimally invasive opportunity to obtain a diagnosis in patients with a condition that affects multiple organ systems, and its use should be considered in disease processes with cutaneous manifestations.
REFERENCES
1. Scheinfeld N, Mones J. Seasonal variation of transient acantholytic dyskeratosis (Grover’s disease). J Am Acad Dermatol. 2006;55(2): 263-268.
2. Parsons JM. Transient acantholytic dermatosis (Grover’s disease): a global perspective. J Am Acad Dermatol. 1996;35(5 part 1):653-666.
3. Weaver J, Bergfeld WF. Grover disease (transient acantholytic dermatosis). Arch Pathol Lab Med. 2009;133(9):1490-1494.
4. Quirk CJ, Heenan PJ. Grover’s disease: 34 years on. Australas J Dermatol. 2004;45(2):83-86.
5. Ippoliti G, Paulli M, Lucioni M, et al. Grover’s disease after heart transplantation: a case report. Case Rep Transplant. 2012;2012:126592.
6. Streit M, Paredes BE, Braathen LR, Brand CU. Transitory acantholytic dermatosis (Grover’s disease): an analysis of the clinical spectrum based on 21 histologically assessed cases [in German]. Hautarzt. 2000;51:244-249.
7. Joshi R, Taneja A. Grover’s disease with acrosyringeal acantholysis: a rare histological presentation of an uncommon disease. Indian J Dermatol. 2014;59(6):621-623.
8. Riemann H, High WA. Grover’s disease (transient and persistent acantholytic dermatosis). UpToDate. 2015. www.uptodate.com/contents/grovers-disease-transient-and-persistent-acantholytic-dermatosis. Accessed June 4, 2016.
9. Breuckmann F, Appelhans C, Altmeyer P, Kreuter A. Medium-dose ultraviolet A1 phototherapy in transient acantholytic dermatosis (Grover’s disease). J Am Acad Dermatol. 2005;52(1):169-170.
10. Norman R, Chau V. Use of etanercept in treating pruritus and preventing new lesions in Grover disease. J Am Acad Dermatol. 2011;64(4):796-798.
IN THIS ARTICLE
- Conditions associated with increased risk for case disease
- Outcome for the case patient
- Differential diagnosis
A 78-year-old white man with chronic lymphocytic leukemia is admitted to the hospital with worsening cough, shortness of breath, and fever. His medical history is significant for pneumonia caused by Pneumocystis jirovecii in the past year. In the weeks preceding hospital admission, the patient developed an erythematous rash over his trunk (see photographs).
During the man’s hospital stay, this eruption becomes increasingly pruritic and spreads to his proximal extremities. His pulmonary symptoms improve slightly following the initiation of broad-spectrum antibiotic therapy (piperacillin/tazobactam and vancomycin), but CT performed one week after admission reveals worsening pulmonary disease (see image). The radiologist’s differential diagnosis includes neoplasm, fungal infection, Kaposi sarcoma, and autoimmune disease.
 |  |
| A. The patient's back shows a distribution of lesions, with areas of excoriation caused by scratching. | B. A close-up reveals erythematous papules and keratotic papules. |
Suspecting that the progressive rash is related to the systemic process, the provider orders a punch biopsy in an effort to reach a diagnosis with minimally invasive studies. When the patient’s clinical status further declines, he undergoes video-assisted thoracoscopic surgery to obtain an excisional biopsy of one of the pulmonary nodules. Subsequent analysis reveals fungal organisms consistent with histoplasmosis. Interestingly, in the histologic review of the skin biopsy, focal acantholytic dyskeratosis—suggestive of Grover disease—is identified.
Continue for discussion >>
DISCUSSION
Grover disease (GD), also known as transient acantholytic dermatosis, is a skin condition of uncertain pathophysiology. Its clinical presentation can be difficult to distinguish from other dermopathies.1,2
Incidence
GD most commonly appears in fair-skinned persons of late middle age, with men affected at two to three times the rate seen in women.1,2 Although GD has been documented in patients ranging in age from 4 to 100, this dermopathy is rare in younger patients.1-3 Persons with a prior history of atopic dermatitis, contact dermatitis, or xerosis cutis are at increased risk for GD—likely due to an increased dermatologic sensitivity to irritants resulting from the aforementioned disorders.1,4 Risk for GD is also elevated in patients with chronic medical conditions, immunodeficiency, febrile illnesses, or malignancies (see Table 1).2-5
The true incidence of GD is not known; biopsy-proven GD is uncommon, and specific data on the incidence and prevalence of the condition are lacking. Swiss researchers who reviewed more than 30,000 skin biopsies in the late 1990s noted only 24 diagnosed cases of GD, and similar findings have been reported in the United States.1,6 However, the variable presentation and often mild nature of GD may result in cases of misdiagnosis, lack of diagnosis, or empiric treatment in the absence of a formal diagnosis.7
Causative factors
Although the pathophysiology of GD is uncertain, the most likely cause is an occlusion of the eccrine glands.3 This is followed by acantholysis, or separation of keratinocytes within the epidermis, which in turn leads to the development of vesicular lesions.
Though diagnosed most often in the winter, GD has also been associated with exposure to sunlight, heat, xerosis, and diaphoresis.1,3 Hospitalized or bedridden patients are at risk for occlusion of the eccrine glands and thus for GD. Use of certain therapies, including sulfadoxine/pyrimethamine (an antimalarial treatment), ionizing radiation, and interleukin-4, may also be precursors for the condition.2
Other exacerbating factors have been suggested, but reports are largely limited to case studies and other anecdotal publications.2 Concrete data regarding the etiology and pathophysiology of GD are still relatively scarce.
Clinical presentation
Patients with GD present with pruritic dermatitis on the trunk and proximal extremities, most classically on the anterior chest and mid back.2,3 The severity of the rash does not necessarily correlate to the degree of pruritus. Some patients report only mild pruritus, while others experience debilitating discomfort and pain. In most cases, erythematous and violaceous papules and vesicles appear first, followed by keratotic erosions.3
GD is a self-limited disorder that often resolves within a few weeks, although some cases will persist for several months.3,5 Severity and duration of symptoms appear to be correlated with increasing age; elderly patients experience worse pruritus for longer periods than do younger patients.2
Although the condition is sometimes referred to as transient acantholytic dermatosis, there are three typical presentations of GD: transient eruptive, persistent pruritic, and chronic asymptomatic.4 Transient eruptive GD presents suddenly, with intense pruritus, and tends to subside over several weeks. Persistent pruritic disease generally causes a milder pruritus, with symptoms that last for several months and are not well controlled by medication. Chronic asymptomatic GD can be difficult to treat medically, yet this form of the disease typically causes little to no irritation and requires minimal therapeutic intervention.4
Systemic symptoms of GD have not been observed. Pruritus and rash are the main features in most affected patients. However, pruritic papulovesicular eruptions are commonly seen in other conditions with similar characteristics (see Table 2,3,4), and GD is comparatively rare. While clinical appearance alone may suggest a diagnosis of GD, further testing may be needed to eliminate other conditions from the differential.
Treatment and prognosis
In the absence of randomized therapeutic trials for GD, there are no strict guidelines for treatment. When irritation, inflammation, and pruritus become bothersome, several interventions may be considered. The first step may consist of efforts to modify aggravating factors, such as dry skin, occlusion, excess heat, and rapid temperature changes. Indeed, for mild cases of GD, this may be all that is required.
The firstline pharmacotherapy for GD is medium- to high-potency topical corticosteroids, which reduce inflammation and pruritus in approximately half of affected patients.3,6,8 Topical emollients and oral antihistamines can also provide symptom relief. Vitamin D analogues are considered secondline therapy, and retinoids (both topical and systemic) have also been shown to reduce GD severity.3,4,8
Severe, refractory cases may require more aggressive systemic therapy with corticosteroids or retinoids. For pruritic relief, several weeks of oral corticosteroids may be necessary—and GD may rebound after treatment ceases.3,4 Therefore, oral corticosteroids should only be considered for severe or persistent cases, since the systemic adverse effects (eg, immunosuppression, weight gain, dysglycemia) of these drugs may outweigh the benefits in patients with GD. Other interventions, including phototherapy and immunosuppressive drugs (eg, etanercept) have also demonstrated benefit in select patients.4,9,10
The self-limited nature of GD, along with its lack of systemic symptoms, is associated with a generally benign course of disease and no long-term sequelae.3,5
Continue to outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
This case involved an immunocompromised patient with systemic symptoms, vasculitic cutaneous lesions, and significant pulmonary disease. The differential diagnosis was extensive, and diagnosis based on clinical grounds alone was extremely challenging. In these circumstances, diagnostic testing was essential to reach a final diagnosis.
In this case, the skin biopsy yielded a diagnosis of GD, and the rash was found to be unrelated to the patient’s systemic and pulmonary symptoms. The providers were then able to focus on the diagnosis of histoplasmosis, with only minimal intervention for the patient’s GD (ie, oral diphenhydramine prn for pruritus).
CONCLUSION
In many cases of GD, skin biopsy can guide providers when the history and physical examination do not yield a clear diagnosis. The histopathology of affected tissue can provide invaluable information about an underlying disease process, particularly in complex cases such as this patient’s. Skin biopsy provides a minimally invasive opportunity to obtain a diagnosis in patients with a condition that affects multiple organ systems, and its use should be considered in disease processes with cutaneous manifestations.
REFERENCES
1. Scheinfeld N, Mones J. Seasonal variation of transient acantholytic dyskeratosis (Grover’s disease). J Am Acad Dermatol. 2006;55(2): 263-268.
2. Parsons JM. Transient acantholytic dermatosis (Grover’s disease): a global perspective. J Am Acad Dermatol. 1996;35(5 part 1):653-666.
3. Weaver J, Bergfeld WF. Grover disease (transient acantholytic dermatosis). Arch Pathol Lab Med. 2009;133(9):1490-1494.
4. Quirk CJ, Heenan PJ. Grover’s disease: 34 years on. Australas J Dermatol. 2004;45(2):83-86.
5. Ippoliti G, Paulli M, Lucioni M, et al. Grover’s disease after heart transplantation: a case report. Case Rep Transplant. 2012;2012:126592.
6. Streit M, Paredes BE, Braathen LR, Brand CU. Transitory acantholytic dermatosis (Grover’s disease): an analysis of the clinical spectrum based on 21 histologically assessed cases [in German]. Hautarzt. 2000;51:244-249.
7. Joshi R, Taneja A. Grover’s disease with acrosyringeal acantholysis: a rare histological presentation of an uncommon disease. Indian J Dermatol. 2014;59(6):621-623.
8. Riemann H, High WA. Grover’s disease (transient and persistent acantholytic dermatosis). UpToDate. 2015. www.uptodate.com/contents/grovers-disease-transient-and-persistent-acantholytic-dermatosis. Accessed June 4, 2016.
9. Breuckmann F, Appelhans C, Altmeyer P, Kreuter A. Medium-dose ultraviolet A1 phototherapy in transient acantholytic dermatosis (Grover’s disease). J Am Acad Dermatol. 2005;52(1):169-170.
10. Norman R, Chau V. Use of etanercept in treating pruritus and preventing new lesions in Grover disease. J Am Acad Dermatol. 2011;64(4):796-798.
Altered Mental Status Demands Closer Look
Answer
The radiograph demonstrates a normal gas pattern with a properly placed enteric feeding tube, which appears to be within the stomach. Of note is a sclerotic-appearing lesion on the posterior aspect of the left eighth rib. In a patient with a possible tumor, this lesion poses concern for potential metastasis and warrants appropriate work-up.
Answer
The radiograph demonstrates a normal gas pattern with a properly placed enteric feeding tube, which appears to be within the stomach. Of note is a sclerotic-appearing lesion on the posterior aspect of the left eighth rib. In a patient with a possible tumor, this lesion poses concern for potential metastasis and warrants appropriate work-up.
Answer
The radiograph demonstrates a normal gas pattern with a properly placed enteric feeding tube, which appears to be within the stomach. Of note is a sclerotic-appearing lesion on the posterior aspect of the left eighth rib. In a patient with a possible tumor, this lesion poses concern for potential metastasis and warrants appropriate work-up.
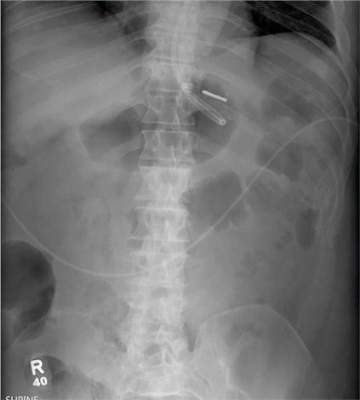
You receive a call from an ICU nurse regarding a patient your service is following—a 60-year-old man who was admitted for altered mental status and is being worked up for a possible brain mass. He has no other significant medical history. The nurse has placed a nasogastric feeding tube to facilitate nutrition and medication administration and has ordered a portable abdominal radiograph to confirm its placement. The completed radiograph is shown. What is your impression?
He Huffed and He Puffed and He Got Frostbite
Case
A 27-year-old man presented to an ED after experiencing a syncopal episode. His vital signs at presentation were normal. Physical examination was generally normal except that there were blisters on the patient’s abdomen, left hand, and right arm, as well as a hypertrophic nodule on the right elbow (Figure) and hard growths on the digits of the right hand. The patient stated the growths started 5 months ago and had been increasing in size. On further questioning, the patient admitted to “huffing” (ie, inhaling) at least six cans of pressurized dust-removal keyboard cleaning spray daily for the past 11 months.
Why do patients abuse keyboard cleaning spray?
The propellant used in certain liquefied compressed gas products is 1,1-difluoroethane (1,1-DFE), a fluorinated hydrocarbon. It is a member of a broad class of related compounds that are present in spray paints, glues, nail polish removers, fuels, hair sprays, and air-freshening products. These 1,1-DFE-containing products are abused for their rapid and short-acting central nervous system (CNS) depressant effects—not unlike that of ethanol. Typically, the vapor of a volatile hydrocarbon is inhaled directly from the open container (“sniffing”), from a bag (“bagging”), or from a soaked rag (huffing). Not only are such hydrocarbon-containing products easy to conceal, they are also highly accessible and inexpensive. Moreover, there are generally no direct legal consequences resulting from abuse of these substances.
All of the aforementioned factors make hydrocarbons a popular drug of abuse among adolescents. Approximately 75% of the population abusing hydrocarbons is younger than age 18 years, half of whom reported first use prior to age 13 years.1,2 Though inhalant abuse rarely continues into adulthood, 0.1% of individuals between the ages of 18 and 30 years report having an inhalant-use disorder.
Hydrocarbons and their halogenated derivatives are lipophilic compounds that are rapidly absorbed after inhalation and rapidly distributed to CNS and cardiac tissue. The brain concentration of 1,1-DFE likely peaks higher than concentrations in other organs and is cleared more rapidly.3 Hydrocarbons produce CNS depression secondary to multiple mechanisms, including gamma-aminobutyric acid agonism, dopamine modulation, and N-methyl-D-aspartate-receptor antagonism.4,5
What causes skin lesions on the abdomen and arms?
The lesions on the patient’s abdomen and extremities were consistent with frostbite. The liquefied compressed gas in computer-cleaning and related products is housed in a pressurized canister. The pressure is released when the spray nozzle is depressed; this causes the liquid to rapidly expand to a gas as it is released, resulting in a quick decrease in the temperature of the metal canister. This process, referred to as adiabatic cooling, demonstrates the first law of thermodynamics. The cold temperature of both the liquid and the canister can cause frostbite in the digits and other parts of the body with which the canister or liquid comes into contact.6
Why did the patient have syncope?
Halogenated hydrocarbons inhibit the cardiac delayed rectifier potassium channels involved in the repolarization of cardiac myocytes, causing a delay in repolarization that is manifested as prolongation of the QT interval on an electrocardiogram. This condition places patients at an increased risk of developing torsades de pointes (TdP).7 In most cases, TdP is self-terminating; however, if TdP persists, degeneration to ventricular fibrillation will result. Deaths caused in this fashion have been referred to as “sudden sniffing death syndrome,” and account for half of all hydrocarbon-related deaths.6,8 In addition to the cardiac effects, hydrocarbons are simple asphyxiants that act by displacing oxygen from inspired air, which also contributes to syncope.
It is important to note that epinephrine and other catecholamines increase the risk for dysrhythmias such as TdP in the setting of hydrocarbon abuse.9 For this reason, epinephrine should be used with caution in the setting of a hydrocarbon-induced arrhythmia. Beta-adrenergic antagonists such as esmolol and propranolol are preferable because they reduce the incidence of ectopia that may trigger TdP.10
What is the significance of the masses noted on the examination and radiograph?
Fluorosis is associated with abnormalities of skeletal and dental tissue. Skeletal fluorosis causes osteosclerosis of the axial skeleton, periosteal new bone formation, ligamentous and tendinous ossification, and osteophyte formation. Dental fluorosis causes a yellow/brown discoloration of the teeth with horizontal streaking (mottling), pitting, and chipping.11 Fluorosis is well-described in regions where water fluoride concentrations are high due to industrial exposure; from consumption of fluorinated wine or chronic overconsumption of tea (especially green or black tea); or from fluoridated toothpaste.12-14 More recently, fluorosis has been described in patients treated for an extended duration of time with voriconazole, a fluorinated antifungal agent.15 Unlike other hydrocarbon products, fluorinated hydrocarbons such as 1,1-DFE can significantly increase systemic fluoride concentrations with excessive use. Rapid skeletal fluorosis is not well described, but has been reported after chronic abuse of fluorinated hydrocarbons.16
How is fluorosis diagnosed and managed?
The lack of rapid laboratory testing available for serum, urine, and bone fluoride concentrations makes the initial diagnosis of fluorosis a clinical one. Imaging studies are generally highly suggestive of fluorosis and can be used to support the diagnosis. A dual energy X-ray absorptiometry scan of the spine, hip, femur, and distal portions of the radii can reveal elevated T-scores consistent with osteosclerosis.14 These findings, in conjunction with bone or joint pain, reduced range of motion, or kyphosis, should prompt clinicians to conduct further testing—even without a confirmed fluoride source. A serum fluoride (reference range, 0.2-3.2 mg/L) and 24-hour urine fluoride (reference range, 0.2-3.2 mg/dL) and creatinine evaluation can be used to diagnose fluorosis. However, a bone biopsy with quantitative bone ash fluoride analysis remains the gold standard for the diagnosis of skeletal fluorosis.16 Laboratory evaluation should also include an assessment of electrolytes, specifically calcium, 25-hydroxyvitamin D, and alkaline phosphatase. The differential diagnosis should include hemoglobinopathies, renal osteodystrophy, Paget disease, hypothyroidism, and skeletal metastases.16
Treatment of fluorosis is largely symptomatic and supportive, with identification and discontinuation of the fluoride source. Patients should be referred to an orthopedist for evaluation and management as needed. Evaluation by an endocrinologist should also be considered because patients may have chronic vitamin D and calcium deficiencies as a result of systemic fluorosis.
Case Conclusion
The patient’s laboratory assessment was notable for the following: alkaline phosphatase, 624 U/L (reference range, 44-147 IU/L); vitamin D, 10 ng/mL (reference range, 20-40 ng/mL); serum fluoride, 0.3 mg/L (reference range, 0.2-3.2 mg/L); urine fluoride, 52 mg/dL (0.2-3.2 mg/dL); and urine creatinine, 1 g/L (reference range, 0.3-3 g/L). Imaging studies noted periosteal bone formation on the lateral epicondyle of the distal right humerus, as well as similar osseous abnormalities in other locations. A bone biopsy was scheduled. The patient was treated with oral vitamin D and educated about the importance of discontinuing the huffing of all hydrocarbons.
1. Williams JF, Storck M; American Academy of Pediatrics Committee on Substance Abuse; American Academy of Pediatrics Committee on Native American Child Health. Inhalant abuse. Pediatrics. 2007;119(5):1009-1017.
2. Wu LT, Pilowsky DJ, Schlenger WE. Inhalant abuse and dependence among adolescents in the United States. J Am Acad Child Adolesc Psychiatry. 2004;43(10):1206-1214.
3. Avella J, Kunaparaju N, Kumar S, Lehrer M, Zito SW, Barletta M. Uptake and distribution of the abused inhalant 1,1-difluoroethane in the rat. J Anal Toxicol. 2010;34(7):381-388.
4. Tormoehlen LM, Tekulve KJ, Nañagas KA. Hydrocarbon toxicity: A review. Clin Toxicol (Phila). 2014;52(5):479-489.
5. Duncan JR, Lawrence AJ. Conventional concepts and new perspectives for understanding the addictive properties of inhalants. J Pharmacol Sci. 2013;122(4):237-243.
6. Sakai K, Maruyama-Maebashi K, Takatsu A, et al. Sudden death involving inhalation of 1,1-difluoroethane (HFC-152a) with spray cleaner: three case reports. Forensic Sci Int. 2011;206(1-3):e58-e61.
7. Himmel HM. Mechanisms involved in cardiac sensitization by volatile anesthetics: general applicability to halogenated hydrocarbons? Crit Rev Toxicol. 2008;38(9):773-803.
8. Avella J, Wilson JC, Lehrer M. Fatal cardiac arrhythmia after repeated exposure to 1,1-difluoroethane (DFE). Am J Forensic Med Pathol. 2006;27(1):58-60.
9. Nelson LS. Toxicologic myocardial sensitization. J Toxicol Clin Toxicol. 2002;40(7):867-879.
10. Mortiz F, de La Chapelle A, Bauer F, Leroy JP, Goullé JP, Bonmarchand G. Esmolol in the treatment of severe arrhythmia after acute trichloroethylene poisoning. Intensive Care Med. 2000;26(2):256.
11. Majumdar KK. Health impact of supplying safe drinking water containing fluoride below permissible level on flourosis patients in a fluoride-endemic rural area of West Bengal. Indian J Public Health. 2011;55(4):303-308.
12. Kakumanu N, Rao SD. Images in clinical medicine. Skeletal fluorosis due to excessive tea drinking. N Engl J Med 2013;368(12):1140.
13. Soriano M, Manchón F. Radiological aspects of a new type of bone fluorosis, periostitis deformans. Radiology 1966;87(6):1089-1094.
14. Tamer MN, Kale Köroğlu B, Arslan C, et al. Osteosclerosis due to endemic fluorosis. Sci Total Environ. 2007;373(1):43-48.
15. Bucknor MD, Gross AJ, Link TM. Voriconazole-induced periostitis in two post-transplant patients. J Radiol Case Rep. 2013;7(8):10-17.
16. Cohen E, Hsu RY, Evangelista P, Aaron R, Rubin LE. Rapid-onset diffuse skeletal fluorosis from inhalant abuse: a case report. JBJS Case Connector. 2014;4(4):e108.
Case
A 27-year-old man presented to an ED after experiencing a syncopal episode. His vital signs at presentation were normal. Physical examination was generally normal except that there were blisters on the patient’s abdomen, left hand, and right arm, as well as a hypertrophic nodule on the right elbow (Figure) and hard growths on the digits of the right hand. The patient stated the growths started 5 months ago and had been increasing in size. On further questioning, the patient admitted to “huffing” (ie, inhaling) at least six cans of pressurized dust-removal keyboard cleaning spray daily for the past 11 months.
Why do patients abuse keyboard cleaning spray?
The propellant used in certain liquefied compressed gas products is 1,1-difluoroethane (1,1-DFE), a fluorinated hydrocarbon. It is a member of a broad class of related compounds that are present in spray paints, glues, nail polish removers, fuels, hair sprays, and air-freshening products. These 1,1-DFE-containing products are abused for their rapid and short-acting central nervous system (CNS) depressant effects—not unlike that of ethanol. Typically, the vapor of a volatile hydrocarbon is inhaled directly from the open container (“sniffing”), from a bag (“bagging”), or from a soaked rag (huffing). Not only are such hydrocarbon-containing products easy to conceal, they are also highly accessible and inexpensive. Moreover, there are generally no direct legal consequences resulting from abuse of these substances.
All of the aforementioned factors make hydrocarbons a popular drug of abuse among adolescents. Approximately 75% of the population abusing hydrocarbons is younger than age 18 years, half of whom reported first use prior to age 13 years.1,2 Though inhalant abuse rarely continues into adulthood, 0.1% of individuals between the ages of 18 and 30 years report having an inhalant-use disorder.
Hydrocarbons and their halogenated derivatives are lipophilic compounds that are rapidly absorbed after inhalation and rapidly distributed to CNS and cardiac tissue. The brain concentration of 1,1-DFE likely peaks higher than concentrations in other organs and is cleared more rapidly.3 Hydrocarbons produce CNS depression secondary to multiple mechanisms, including gamma-aminobutyric acid agonism, dopamine modulation, and N-methyl-D-aspartate-receptor antagonism.4,5
What causes skin lesions on the abdomen and arms?
The lesions on the patient’s abdomen and extremities were consistent with frostbite. The liquefied compressed gas in computer-cleaning and related products is housed in a pressurized canister. The pressure is released when the spray nozzle is depressed; this causes the liquid to rapidly expand to a gas as it is released, resulting in a quick decrease in the temperature of the metal canister. This process, referred to as adiabatic cooling, demonstrates the first law of thermodynamics. The cold temperature of both the liquid and the canister can cause frostbite in the digits and other parts of the body with which the canister or liquid comes into contact.6
Why did the patient have syncope?
Halogenated hydrocarbons inhibit the cardiac delayed rectifier potassium channels involved in the repolarization of cardiac myocytes, causing a delay in repolarization that is manifested as prolongation of the QT interval on an electrocardiogram. This condition places patients at an increased risk of developing torsades de pointes (TdP).7 In most cases, TdP is self-terminating; however, if TdP persists, degeneration to ventricular fibrillation will result. Deaths caused in this fashion have been referred to as “sudden sniffing death syndrome,” and account for half of all hydrocarbon-related deaths.6,8 In addition to the cardiac effects, hydrocarbons are simple asphyxiants that act by displacing oxygen from inspired air, which also contributes to syncope.
It is important to note that epinephrine and other catecholamines increase the risk for dysrhythmias such as TdP in the setting of hydrocarbon abuse.9 For this reason, epinephrine should be used with caution in the setting of a hydrocarbon-induced arrhythmia. Beta-adrenergic antagonists such as esmolol and propranolol are preferable because they reduce the incidence of ectopia that may trigger TdP.10
What is the significance of the masses noted on the examination and radiograph?
Fluorosis is associated with abnormalities of skeletal and dental tissue. Skeletal fluorosis causes osteosclerosis of the axial skeleton, periosteal new bone formation, ligamentous and tendinous ossification, and osteophyte formation. Dental fluorosis causes a yellow/brown discoloration of the teeth with horizontal streaking (mottling), pitting, and chipping.11 Fluorosis is well-described in regions where water fluoride concentrations are high due to industrial exposure; from consumption of fluorinated wine or chronic overconsumption of tea (especially green or black tea); or from fluoridated toothpaste.12-14 More recently, fluorosis has been described in patients treated for an extended duration of time with voriconazole, a fluorinated antifungal agent.15 Unlike other hydrocarbon products, fluorinated hydrocarbons such as 1,1-DFE can significantly increase systemic fluoride concentrations with excessive use. Rapid skeletal fluorosis is not well described, but has been reported after chronic abuse of fluorinated hydrocarbons.16
How is fluorosis diagnosed and managed?
The lack of rapid laboratory testing available for serum, urine, and bone fluoride concentrations makes the initial diagnosis of fluorosis a clinical one. Imaging studies are generally highly suggestive of fluorosis and can be used to support the diagnosis. A dual energy X-ray absorptiometry scan of the spine, hip, femur, and distal portions of the radii can reveal elevated T-scores consistent with osteosclerosis.14 These findings, in conjunction with bone or joint pain, reduced range of motion, or kyphosis, should prompt clinicians to conduct further testing—even without a confirmed fluoride source. A serum fluoride (reference range, 0.2-3.2 mg/L) and 24-hour urine fluoride (reference range, 0.2-3.2 mg/dL) and creatinine evaluation can be used to diagnose fluorosis. However, a bone biopsy with quantitative bone ash fluoride analysis remains the gold standard for the diagnosis of skeletal fluorosis.16 Laboratory evaluation should also include an assessment of electrolytes, specifically calcium, 25-hydroxyvitamin D, and alkaline phosphatase. The differential diagnosis should include hemoglobinopathies, renal osteodystrophy, Paget disease, hypothyroidism, and skeletal metastases.16
Treatment of fluorosis is largely symptomatic and supportive, with identification and discontinuation of the fluoride source. Patients should be referred to an orthopedist for evaluation and management as needed. Evaluation by an endocrinologist should also be considered because patients may have chronic vitamin D and calcium deficiencies as a result of systemic fluorosis.
Case Conclusion
The patient’s laboratory assessment was notable for the following: alkaline phosphatase, 624 U/L (reference range, 44-147 IU/L); vitamin D, 10 ng/mL (reference range, 20-40 ng/mL); serum fluoride, 0.3 mg/L (reference range, 0.2-3.2 mg/L); urine fluoride, 52 mg/dL (0.2-3.2 mg/dL); and urine creatinine, 1 g/L (reference range, 0.3-3 g/L). Imaging studies noted periosteal bone formation on the lateral epicondyle of the distal right humerus, as well as similar osseous abnormalities in other locations. A bone biopsy was scheduled. The patient was treated with oral vitamin D and educated about the importance of discontinuing the huffing of all hydrocarbons.
Case
A 27-year-old man presented to an ED after experiencing a syncopal episode. His vital signs at presentation were normal. Physical examination was generally normal except that there were blisters on the patient’s abdomen, left hand, and right arm, as well as a hypertrophic nodule on the right elbow (Figure) and hard growths on the digits of the right hand. The patient stated the growths started 5 months ago and had been increasing in size. On further questioning, the patient admitted to “huffing” (ie, inhaling) at least six cans of pressurized dust-removal keyboard cleaning spray daily for the past 11 months.
Why do patients abuse keyboard cleaning spray?
The propellant used in certain liquefied compressed gas products is 1,1-difluoroethane (1,1-DFE), a fluorinated hydrocarbon. It is a member of a broad class of related compounds that are present in spray paints, glues, nail polish removers, fuels, hair sprays, and air-freshening products. These 1,1-DFE-containing products are abused for their rapid and short-acting central nervous system (CNS) depressant effects—not unlike that of ethanol. Typically, the vapor of a volatile hydrocarbon is inhaled directly from the open container (“sniffing”), from a bag (“bagging”), or from a soaked rag (huffing). Not only are such hydrocarbon-containing products easy to conceal, they are also highly accessible and inexpensive. Moreover, there are generally no direct legal consequences resulting from abuse of these substances.
All of the aforementioned factors make hydrocarbons a popular drug of abuse among adolescents. Approximately 75% of the population abusing hydrocarbons is younger than age 18 years, half of whom reported first use prior to age 13 years.1,2 Though inhalant abuse rarely continues into adulthood, 0.1% of individuals between the ages of 18 and 30 years report having an inhalant-use disorder.
Hydrocarbons and their halogenated derivatives are lipophilic compounds that are rapidly absorbed after inhalation and rapidly distributed to CNS and cardiac tissue. The brain concentration of 1,1-DFE likely peaks higher than concentrations in other organs and is cleared more rapidly.3 Hydrocarbons produce CNS depression secondary to multiple mechanisms, including gamma-aminobutyric acid agonism, dopamine modulation, and N-methyl-D-aspartate-receptor antagonism.4,5
What causes skin lesions on the abdomen and arms?
The lesions on the patient’s abdomen and extremities were consistent with frostbite. The liquefied compressed gas in computer-cleaning and related products is housed in a pressurized canister. The pressure is released when the spray nozzle is depressed; this causes the liquid to rapidly expand to a gas as it is released, resulting in a quick decrease in the temperature of the metal canister. This process, referred to as adiabatic cooling, demonstrates the first law of thermodynamics. The cold temperature of both the liquid and the canister can cause frostbite in the digits and other parts of the body with which the canister or liquid comes into contact.6
Why did the patient have syncope?
Halogenated hydrocarbons inhibit the cardiac delayed rectifier potassium channels involved in the repolarization of cardiac myocytes, causing a delay in repolarization that is manifested as prolongation of the QT interval on an electrocardiogram. This condition places patients at an increased risk of developing torsades de pointes (TdP).7 In most cases, TdP is self-terminating; however, if TdP persists, degeneration to ventricular fibrillation will result. Deaths caused in this fashion have been referred to as “sudden sniffing death syndrome,” and account for half of all hydrocarbon-related deaths.6,8 In addition to the cardiac effects, hydrocarbons are simple asphyxiants that act by displacing oxygen from inspired air, which also contributes to syncope.
It is important to note that epinephrine and other catecholamines increase the risk for dysrhythmias such as TdP in the setting of hydrocarbon abuse.9 For this reason, epinephrine should be used with caution in the setting of a hydrocarbon-induced arrhythmia. Beta-adrenergic antagonists such as esmolol and propranolol are preferable because they reduce the incidence of ectopia that may trigger TdP.10
What is the significance of the masses noted on the examination and radiograph?
Fluorosis is associated with abnormalities of skeletal and dental tissue. Skeletal fluorosis causes osteosclerosis of the axial skeleton, periosteal new bone formation, ligamentous and tendinous ossification, and osteophyte formation. Dental fluorosis causes a yellow/brown discoloration of the teeth with horizontal streaking (mottling), pitting, and chipping.11 Fluorosis is well-described in regions where water fluoride concentrations are high due to industrial exposure; from consumption of fluorinated wine or chronic overconsumption of tea (especially green or black tea); or from fluoridated toothpaste.12-14 More recently, fluorosis has been described in patients treated for an extended duration of time with voriconazole, a fluorinated antifungal agent.15 Unlike other hydrocarbon products, fluorinated hydrocarbons such as 1,1-DFE can significantly increase systemic fluoride concentrations with excessive use. Rapid skeletal fluorosis is not well described, but has been reported after chronic abuse of fluorinated hydrocarbons.16
How is fluorosis diagnosed and managed?
The lack of rapid laboratory testing available for serum, urine, and bone fluoride concentrations makes the initial diagnosis of fluorosis a clinical one. Imaging studies are generally highly suggestive of fluorosis and can be used to support the diagnosis. A dual energy X-ray absorptiometry scan of the spine, hip, femur, and distal portions of the radii can reveal elevated T-scores consistent with osteosclerosis.14 These findings, in conjunction with bone or joint pain, reduced range of motion, or kyphosis, should prompt clinicians to conduct further testing—even without a confirmed fluoride source. A serum fluoride (reference range, 0.2-3.2 mg/L) and 24-hour urine fluoride (reference range, 0.2-3.2 mg/dL) and creatinine evaluation can be used to diagnose fluorosis. However, a bone biopsy with quantitative bone ash fluoride analysis remains the gold standard for the diagnosis of skeletal fluorosis.16 Laboratory evaluation should also include an assessment of electrolytes, specifically calcium, 25-hydroxyvitamin D, and alkaline phosphatase. The differential diagnosis should include hemoglobinopathies, renal osteodystrophy, Paget disease, hypothyroidism, and skeletal metastases.16
Treatment of fluorosis is largely symptomatic and supportive, with identification and discontinuation of the fluoride source. Patients should be referred to an orthopedist for evaluation and management as needed. Evaluation by an endocrinologist should also be considered because patients may have chronic vitamin D and calcium deficiencies as a result of systemic fluorosis.
Case Conclusion
The patient’s laboratory assessment was notable for the following: alkaline phosphatase, 624 U/L (reference range, 44-147 IU/L); vitamin D, 10 ng/mL (reference range, 20-40 ng/mL); serum fluoride, 0.3 mg/L (reference range, 0.2-3.2 mg/L); urine fluoride, 52 mg/dL (0.2-3.2 mg/dL); and urine creatinine, 1 g/L (reference range, 0.3-3 g/L). Imaging studies noted periosteal bone formation on the lateral epicondyle of the distal right humerus, as well as similar osseous abnormalities in other locations. A bone biopsy was scheduled. The patient was treated with oral vitamin D and educated about the importance of discontinuing the huffing of all hydrocarbons.
1. Williams JF, Storck M; American Academy of Pediatrics Committee on Substance Abuse; American Academy of Pediatrics Committee on Native American Child Health. Inhalant abuse. Pediatrics. 2007;119(5):1009-1017.
2. Wu LT, Pilowsky DJ, Schlenger WE. Inhalant abuse and dependence among adolescents in the United States. J Am Acad Child Adolesc Psychiatry. 2004;43(10):1206-1214.
3. Avella J, Kunaparaju N, Kumar S, Lehrer M, Zito SW, Barletta M. Uptake and distribution of the abused inhalant 1,1-difluoroethane in the rat. J Anal Toxicol. 2010;34(7):381-388.
4. Tormoehlen LM, Tekulve KJ, Nañagas KA. Hydrocarbon toxicity: A review. Clin Toxicol (Phila). 2014;52(5):479-489.
5. Duncan JR, Lawrence AJ. Conventional concepts and new perspectives for understanding the addictive properties of inhalants. J Pharmacol Sci. 2013;122(4):237-243.
6. Sakai K, Maruyama-Maebashi K, Takatsu A, et al. Sudden death involving inhalation of 1,1-difluoroethane (HFC-152a) with spray cleaner: three case reports. Forensic Sci Int. 2011;206(1-3):e58-e61.
7. Himmel HM. Mechanisms involved in cardiac sensitization by volatile anesthetics: general applicability to halogenated hydrocarbons? Crit Rev Toxicol. 2008;38(9):773-803.
8. Avella J, Wilson JC, Lehrer M. Fatal cardiac arrhythmia after repeated exposure to 1,1-difluoroethane (DFE). Am J Forensic Med Pathol. 2006;27(1):58-60.
9. Nelson LS. Toxicologic myocardial sensitization. J Toxicol Clin Toxicol. 2002;40(7):867-879.
10. Mortiz F, de La Chapelle A, Bauer F, Leroy JP, Goullé JP, Bonmarchand G. Esmolol in the treatment of severe arrhythmia after acute trichloroethylene poisoning. Intensive Care Med. 2000;26(2):256.
11. Majumdar KK. Health impact of supplying safe drinking water containing fluoride below permissible level on flourosis patients in a fluoride-endemic rural area of West Bengal. Indian J Public Health. 2011;55(4):303-308.
12. Kakumanu N, Rao SD. Images in clinical medicine. Skeletal fluorosis due to excessive tea drinking. N Engl J Med 2013;368(12):1140.
13. Soriano M, Manchón F. Radiological aspects of a new type of bone fluorosis, periostitis deformans. Radiology 1966;87(6):1089-1094.
14. Tamer MN, Kale Köroğlu B, Arslan C, et al. Osteosclerosis due to endemic fluorosis. Sci Total Environ. 2007;373(1):43-48.
15. Bucknor MD, Gross AJ, Link TM. Voriconazole-induced periostitis in two post-transplant patients. J Radiol Case Rep. 2013;7(8):10-17.
16. Cohen E, Hsu RY, Evangelista P, Aaron R, Rubin LE. Rapid-onset diffuse skeletal fluorosis from inhalant abuse: a case report. JBJS Case Connector. 2014;4(4):e108.
1. Williams JF, Storck M; American Academy of Pediatrics Committee on Substance Abuse; American Academy of Pediatrics Committee on Native American Child Health. Inhalant abuse. Pediatrics. 2007;119(5):1009-1017.
2. Wu LT, Pilowsky DJ, Schlenger WE. Inhalant abuse and dependence among adolescents in the United States. J Am Acad Child Adolesc Psychiatry. 2004;43(10):1206-1214.
3. Avella J, Kunaparaju N, Kumar S, Lehrer M, Zito SW, Barletta M. Uptake and distribution of the abused inhalant 1,1-difluoroethane in the rat. J Anal Toxicol. 2010;34(7):381-388.
4. Tormoehlen LM, Tekulve KJ, Nañagas KA. Hydrocarbon toxicity: A review. Clin Toxicol (Phila). 2014;52(5):479-489.
5. Duncan JR, Lawrence AJ. Conventional concepts and new perspectives for understanding the addictive properties of inhalants. J Pharmacol Sci. 2013;122(4):237-243.
6. Sakai K, Maruyama-Maebashi K, Takatsu A, et al. Sudden death involving inhalation of 1,1-difluoroethane (HFC-152a) with spray cleaner: three case reports. Forensic Sci Int. 2011;206(1-3):e58-e61.
7. Himmel HM. Mechanisms involved in cardiac sensitization by volatile anesthetics: general applicability to halogenated hydrocarbons? Crit Rev Toxicol. 2008;38(9):773-803.
8. Avella J, Wilson JC, Lehrer M. Fatal cardiac arrhythmia after repeated exposure to 1,1-difluoroethane (DFE). Am J Forensic Med Pathol. 2006;27(1):58-60.
9. Nelson LS. Toxicologic myocardial sensitization. J Toxicol Clin Toxicol. 2002;40(7):867-879.
10. Mortiz F, de La Chapelle A, Bauer F, Leroy JP, Goullé JP, Bonmarchand G. Esmolol in the treatment of severe arrhythmia after acute trichloroethylene poisoning. Intensive Care Med. 2000;26(2):256.
11. Majumdar KK. Health impact of supplying safe drinking water containing fluoride below permissible level on flourosis patients in a fluoride-endemic rural area of West Bengal. Indian J Public Health. 2011;55(4):303-308.
12. Kakumanu N, Rao SD. Images in clinical medicine. Skeletal fluorosis due to excessive tea drinking. N Engl J Med 2013;368(12):1140.
13. Soriano M, Manchón F. Radiological aspects of a new type of bone fluorosis, periostitis deformans. Radiology 1966;87(6):1089-1094.
14. Tamer MN, Kale Köroğlu B, Arslan C, et al. Osteosclerosis due to endemic fluorosis. Sci Total Environ. 2007;373(1):43-48.
15. Bucknor MD, Gross AJ, Link TM. Voriconazole-induced periostitis in two post-transplant patients. J Radiol Case Rep. 2013;7(8):10-17.
16. Cohen E, Hsu RY, Evangelista P, Aaron R, Rubin LE. Rapid-onset diffuse skeletal fluorosis from inhalant abuse: a case report. JBJS Case Connector. 2014;4(4):e108.